User login
Cochrane review: Topical steroid–vitamin D combination best for scalp psoriasis
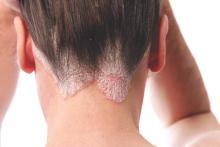
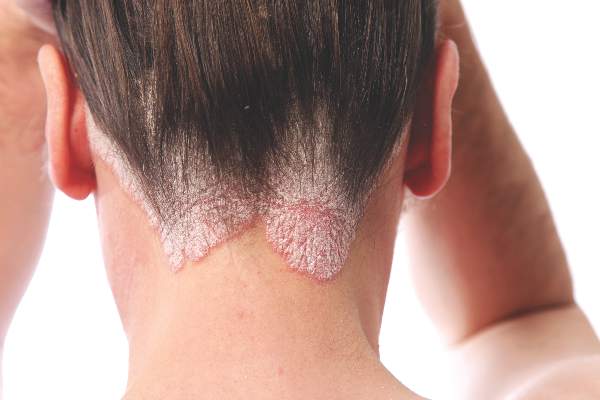
As a treatment for scalp psoriasis, the combination of a topical steroid and topical vitamin D was marginally better than topical steroids alone, but both approaches have a similar safety profile, according to a Cochrane review.
The systematic review included 59 randomized controlled trials of topical treatments for scalp psoriasis, representing a total of 11,561 participants of all ages, most of whom were followed for less than 6 months, according to Justin Schlager, MD, of the Charité – Universitätsmedizin Berlin, and coauthors (Cochrane Database of Systematic Reviews 2016. Issue 2. doi: 10.1002/14651858.CD009687.pub2).
In the analysis, topical steroid monotherapy was significantly better than topical vitamin D for clearance, as assessed by the Investigator’s Global Assessment of Disease Severity (risk ratio, 1.82; 95% confidence interval, 1.52-2.18). But the combination of a topical steroid and vitamin D showed a small but statistically significant advantage over steroids alone (RR, 1.22; 95% CI, 1.08-1.36) and an even greater advantage over vitamin D alone (RR, 2.28; 95% CI, 1.87-2.78).
Similarly, for treatment response, the combination of a topical steroid and vitamin D showed the greatest benefit in terms of treatment response when compared with steroid monotherapy (RR, 1.15; 95% CI, 1.06-1.25) – an additional benefit the authors observed was small – and when compared with vitamin D alone (RR, 2.31; 95% CI, 1.75-3.04).
But for monotherapy, corticosteroids were more than twice as effective as vitamin D alone for treatment response (RR, 2.09; 95% CI, 1.80-2.41). The analysis also showed that corticosteroids of moderate, high, and very high potency were similarly effective.
Steroids were associated with a significantly lower risk of withdrawal because of adverse events than vitamin D (RR, 0.22; 95% CI, 0.11-0.42). Patients using topical steroids reported adverse events such as a burning sensation or irritation at the site of application, while patients taking vitamin D reported side effects such as pruritus, candidiasis, dermatitis, and erythema, although in both cases these were mostly limited to the application site.
The combination of vitamin D and topical steroid had a lower risk of withdrawals because of adverse events than vitamin D alone, but showed a similar rate to topical steroids alone.
“Given the similar safety profile and only slim benefit of the two-compound combination over the steroid alone, monotherapy with generic topical corticosteroids may be fully acceptable for short-term therapy,” the authors wrote. The authors noted that data on patients’ quality of life was poor across all the studies included in the analysis and called for future trials to address this gap, and more long-term assessments.
“Regardless of the type of psoriasis, up to 79% of people with the condition present with scalp involvement, which has frequently been the first site to show symptoms of the disease,” they pointed out.
Thirty of the 59 studies were either conducted or sponsored by the manufacturer of the study medication, and the authors described the overall quality of the studies as “moderate.” Thirty-three studies were double blind, 14 were single blind, two had “third-party” blinding, six were open-label, and four did not report blinding information.
The study was supported by the Universidade Federal de São Paulo, Brazil; the Universidade Federal do Rio Grande do Norte, Brazil; and the National Institute for Health Research, United Kingdom. Six authors and one clinical referee declared speakers’ fees, research grants, and funding from the pharmaceutical industry. One author had no conflicts of interest to disclose.
As a treatment for scalp psoriasis, the combination of a topical steroid and topical vitamin D was marginally better than topical steroids alone, but both approaches have a similar safety profile, according to a Cochrane review.
The systematic review included 59 randomized controlled trials of topical treatments for scalp psoriasis, representing a total of 11,561 participants of all ages, most of whom were followed for less than 6 months, according to Justin Schlager, MD, of the Charité – Universitätsmedizin Berlin, and coauthors (Cochrane Database of Systematic Reviews 2016. Issue 2. doi: 10.1002/14651858.CD009687.pub2).
In the analysis, topical steroid monotherapy was significantly better than topical vitamin D for clearance, as assessed by the Investigator’s Global Assessment of Disease Severity (risk ratio, 1.82; 95% confidence interval, 1.52-2.18). But the combination of a topical steroid and vitamin D showed a small but statistically significant advantage over steroids alone (RR, 1.22; 95% CI, 1.08-1.36) and an even greater advantage over vitamin D alone (RR, 2.28; 95% CI, 1.87-2.78).
Similarly, for treatment response, the combination of a topical steroid and vitamin D showed the greatest benefit in terms of treatment response when compared with steroid monotherapy (RR, 1.15; 95% CI, 1.06-1.25) – an additional benefit the authors observed was small – and when compared with vitamin D alone (RR, 2.31; 95% CI, 1.75-3.04).
But for monotherapy, corticosteroids were more than twice as effective as vitamin D alone for treatment response (RR, 2.09; 95% CI, 1.80-2.41). The analysis also showed that corticosteroids of moderate, high, and very high potency were similarly effective.
Steroids were associated with a significantly lower risk of withdrawal because of adverse events than vitamin D (RR, 0.22; 95% CI, 0.11-0.42). Patients using topical steroids reported adverse events such as a burning sensation or irritation at the site of application, while patients taking vitamin D reported side effects such as pruritus, candidiasis, dermatitis, and erythema, although in both cases these were mostly limited to the application site.
The combination of vitamin D and topical steroid had a lower risk of withdrawals because of adverse events than vitamin D alone, but showed a similar rate to topical steroids alone.
“Given the similar safety profile and only slim benefit of the two-compound combination over the steroid alone, monotherapy with generic topical corticosteroids may be fully acceptable for short-term therapy,” the authors wrote. The authors noted that data on patients’ quality of life was poor across all the studies included in the analysis and called for future trials to address this gap, and more long-term assessments.
“Regardless of the type of psoriasis, up to 79% of people with the condition present with scalp involvement, which has frequently been the first site to show symptoms of the disease,” they pointed out.
Thirty of the 59 studies were either conducted or sponsored by the manufacturer of the study medication, and the authors described the overall quality of the studies as “moderate.” Thirty-three studies were double blind, 14 were single blind, two had “third-party” blinding, six were open-label, and four did not report blinding information.
The study was supported by the Universidade Federal de São Paulo, Brazil; the Universidade Federal do Rio Grande do Norte, Brazil; and the National Institute for Health Research, United Kingdom. Six authors and one clinical referee declared speakers’ fees, research grants, and funding from the pharmaceutical industry. One author had no conflicts of interest to disclose.
As a treatment for scalp psoriasis, the combination of a topical steroid and topical vitamin D was marginally better than topical steroids alone, but both approaches have a similar safety profile, according to a Cochrane review.
The systematic review included 59 randomized controlled trials of topical treatments for scalp psoriasis, representing a total of 11,561 participants of all ages, most of whom were followed for less than 6 months, according to Justin Schlager, MD, of the Charité – Universitätsmedizin Berlin, and coauthors (Cochrane Database of Systematic Reviews 2016. Issue 2. doi: 10.1002/14651858.CD009687.pub2).
In the analysis, topical steroid monotherapy was significantly better than topical vitamin D for clearance, as assessed by the Investigator’s Global Assessment of Disease Severity (risk ratio, 1.82; 95% confidence interval, 1.52-2.18). But the combination of a topical steroid and vitamin D showed a small but statistically significant advantage over steroids alone (RR, 1.22; 95% CI, 1.08-1.36) and an even greater advantage over vitamin D alone (RR, 2.28; 95% CI, 1.87-2.78).
Similarly, for treatment response, the combination of a topical steroid and vitamin D showed the greatest benefit in terms of treatment response when compared with steroid monotherapy (RR, 1.15; 95% CI, 1.06-1.25) – an additional benefit the authors observed was small – and when compared with vitamin D alone (RR, 2.31; 95% CI, 1.75-3.04).
But for monotherapy, corticosteroids were more than twice as effective as vitamin D alone for treatment response (RR, 2.09; 95% CI, 1.80-2.41). The analysis also showed that corticosteroids of moderate, high, and very high potency were similarly effective.
Steroids were associated with a significantly lower risk of withdrawal because of adverse events than vitamin D (RR, 0.22; 95% CI, 0.11-0.42). Patients using topical steroids reported adverse events such as a burning sensation or irritation at the site of application, while patients taking vitamin D reported side effects such as pruritus, candidiasis, dermatitis, and erythema, although in both cases these were mostly limited to the application site.
The combination of vitamin D and topical steroid had a lower risk of withdrawals because of adverse events than vitamin D alone, but showed a similar rate to topical steroids alone.
“Given the similar safety profile and only slim benefit of the two-compound combination over the steroid alone, monotherapy with generic topical corticosteroids may be fully acceptable for short-term therapy,” the authors wrote. The authors noted that data on patients’ quality of life was poor across all the studies included in the analysis and called for future trials to address this gap, and more long-term assessments.
“Regardless of the type of psoriasis, up to 79% of people with the condition present with scalp involvement, which has frequently been the first site to show symptoms of the disease,” they pointed out.
Thirty of the 59 studies were either conducted or sponsored by the manufacturer of the study medication, and the authors described the overall quality of the studies as “moderate.” Thirty-three studies were double blind, 14 were single blind, two had “third-party” blinding, six were open-label, and four did not report blinding information.
The study was supported by the Universidade Federal de São Paulo, Brazil; the Universidade Federal do Rio Grande do Norte, Brazil; and the National Institute for Health Research, United Kingdom. Six authors and one clinical referee declared speakers’ fees, research grants, and funding from the pharmaceutical industry. One author had no conflicts of interest to disclose.
FROM THE COCHRANE DATABASE OF SYSTEMATIC REVIEWS
Key clinical point: The combination of a topical steroid and topical vitamin D is marginally better but with a similar safety profile to steroids alone as a treatment for psoriasis on the scalp.
Major finding: The combination of a topical steroid and vitamin D showed a small but statistically significant advantage over steroids alone, and a greater advantage over vitamin D alone.
Data source: A systematic review of 59 randomized controlled studies in 11,561 patients.
Disclosures: The study was supported by the Universidade Federal de São Paulo, Brazil; the Universidade Federal do Rio Grande do Norte, Brazil; and the National Institute for Health Research, United Kingdom. Six authors and one clinical referee declared speakers’ fees, research grants, and funding from the pharmaceutical industry. One author had no conflicts of interest to disclose.
Lasers and topicals both lighten solar lentigines
Quality-switched (QS) laser therapy with a ruby laser and topical triple-combination therapy (TCT) were similarly effective in lightening solar lentigines, based on data from a prospective, open label study of 15 adults. The findings were published in Dermatologic Surgery.
The QS ruby laser (QSRL) delivered longer-lasting skin-lightening results in less time, but the topical therapy is less expensive and has a lower risk of adverse events, wrote Dr. Laurence Imhof of the department of dermatology, University Hospital Zürich, and colleagues (Dermatol Surg. 2016;42:853-57. doi: 10.1097/DSS.0000000000000793). “Although therapy [for solar lentigines] is not medically indicated, there is a rising treatment demand for aesthetic reasons. Therefore, the treatment should be very safe and affordable,” they wrote.
The researchers compared the safety and efficacy of treating solar lentigines with a 694-nm QSRL and TCT (hydroquinone 5%, tretinoin 0.03%, and dexamethasone 0.03%). The patients included 14 women and 1 man with evenly distributed solar lentigines on the backs of both hands. Each patient was treated with 1-2 QSRL sessions on the back of the right hand and 7 weeks of once-daily application of TCT on the back of the left hand. The average age of the patients was 61 years; 13 were Fitzpatrick Skin Types II and III. Adverse events were mild and transient with both treatments, although the QSRL caused significantly more crusting and hyperpigmentation than the TCT.
Both treatments significantly reduced pigment at the end of treatment (day 56) and 12 weeks’ post treatment (140 days), compared with baseline. Treatment areas were evaluated at days 28, 56, and 140 by the treating physician, the patient, and a blinded physician (based on photos).The 6-point grading scale rated the degree of lightening/percentage of clearing, ranging from worse (less than 0%) to excellent (76%-100%).
At 56 days, the mean macroscopic improvement with QSRL laser was rated 3.5 by the treating physician and patient and 3 by a blinded physician; the mean macroscopic improvement with TCT was rated 2.4 by the treating physician, 2.2 by the patient, and 1.7 by the blinded physician. At 140 days, the mean macroscopic improvements with the laser were rated 2.9 by the treating physician, 2.7 by the patient, and 2.8 by the blinded physician, compared with 1.2, 1.1, and 0.7, respectively, with TCT.
The results were limited by the small size, short follow-up period, and open-label design, the researchers noted. However, the study is the first known to compare a laser and a topical treatment for the management of solar lentigines, and the findings suggest that both therapies can be recommended for the aesthetic treatment of solar lentigines, they said.
The TCT cream used in the study was provided by Louis Widmer SA, which provided a grant to the University of Zürich. The researchers had no financial conflicts to disclose.

|
Dr. Nazanin Saedi |
“We commend Imhof and colleagues for providing a long-awaited direct comparison of the Q-switched laser and topical triple-combination therapy,” wrote Dr. Laura M. Schilling and Dr. Nazanin Saedi, in an accompanying editorial (Dermatol Surg. 2016;42:8580859. doi: 10.1097/DSS.0000000000000792). Both treatments are viable options for patients, they emphasized. “It is fundamental, especially in this era of patient-centered care, to recognize that treatment options must be individualized. There are numerous variables to be considered when choosing the appropriate treatment for a patient including efficacy, skin type, cost, availability, and patient preference, among others.”
Dr. Schilling and Dr. Saedi are with the department of dermatology and cutaneous biology, Thomas Jefferson University, Philadelphia. They had no financial conflicts to disclose.

|
Dr. Nazanin Saedi |
“We commend Imhof and colleagues for providing a long-awaited direct comparison of the Q-switched laser and topical triple-combination therapy,” wrote Dr. Laura M. Schilling and Dr. Nazanin Saedi, in an accompanying editorial (Dermatol Surg. 2016;42:8580859. doi: 10.1097/DSS.0000000000000792). Both treatments are viable options for patients, they emphasized. “It is fundamental, especially in this era of patient-centered care, to recognize that treatment options must be individualized. There are numerous variables to be considered when choosing the appropriate treatment for a patient including efficacy, skin type, cost, availability, and patient preference, among others.”
Dr. Schilling and Dr. Saedi are with the department of dermatology and cutaneous biology, Thomas Jefferson University, Philadelphia. They had no financial conflicts to disclose.

|
Dr. Nazanin Saedi |
“We commend Imhof and colleagues for providing a long-awaited direct comparison of the Q-switched laser and topical triple-combination therapy,” wrote Dr. Laura M. Schilling and Dr. Nazanin Saedi, in an accompanying editorial (Dermatol Surg. 2016;42:8580859. doi: 10.1097/DSS.0000000000000792). Both treatments are viable options for patients, they emphasized. “It is fundamental, especially in this era of patient-centered care, to recognize that treatment options must be individualized. There are numerous variables to be considered when choosing the appropriate treatment for a patient including efficacy, skin type, cost, availability, and patient preference, among others.”
Dr. Schilling and Dr. Saedi are with the department of dermatology and cutaneous biology, Thomas Jefferson University, Philadelphia. They had no financial conflicts to disclose.
Quality-switched (QS) laser therapy with a ruby laser and topical triple-combination therapy (TCT) were similarly effective in lightening solar lentigines, based on data from a prospective, open label study of 15 adults. The findings were published in Dermatologic Surgery.
The QS ruby laser (QSRL) delivered longer-lasting skin-lightening results in less time, but the topical therapy is less expensive and has a lower risk of adverse events, wrote Dr. Laurence Imhof of the department of dermatology, University Hospital Zürich, and colleagues (Dermatol Surg. 2016;42:853-57. doi: 10.1097/DSS.0000000000000793). “Although therapy [for solar lentigines] is not medically indicated, there is a rising treatment demand for aesthetic reasons. Therefore, the treatment should be very safe and affordable,” they wrote.
The researchers compared the safety and efficacy of treating solar lentigines with a 694-nm QSRL and TCT (hydroquinone 5%, tretinoin 0.03%, and dexamethasone 0.03%). The patients included 14 women and 1 man with evenly distributed solar lentigines on the backs of both hands. Each patient was treated with 1-2 QSRL sessions on the back of the right hand and 7 weeks of once-daily application of TCT on the back of the left hand. The average age of the patients was 61 years; 13 were Fitzpatrick Skin Types II and III. Adverse events were mild and transient with both treatments, although the QSRL caused significantly more crusting and hyperpigmentation than the TCT.
Both treatments significantly reduced pigment at the end of treatment (day 56) and 12 weeks’ post treatment (140 days), compared with baseline. Treatment areas were evaluated at days 28, 56, and 140 by the treating physician, the patient, and a blinded physician (based on photos).The 6-point grading scale rated the degree of lightening/percentage of clearing, ranging from worse (less than 0%) to excellent (76%-100%).
At 56 days, the mean macroscopic improvement with QSRL laser was rated 3.5 by the treating physician and patient and 3 by a blinded physician; the mean macroscopic improvement with TCT was rated 2.4 by the treating physician, 2.2 by the patient, and 1.7 by the blinded physician. At 140 days, the mean macroscopic improvements with the laser were rated 2.9 by the treating physician, 2.7 by the patient, and 2.8 by the blinded physician, compared with 1.2, 1.1, and 0.7, respectively, with TCT.
The results were limited by the small size, short follow-up period, and open-label design, the researchers noted. However, the study is the first known to compare a laser and a topical treatment for the management of solar lentigines, and the findings suggest that both therapies can be recommended for the aesthetic treatment of solar lentigines, they said.
The TCT cream used in the study was provided by Louis Widmer SA, which provided a grant to the University of Zürich. The researchers had no financial conflicts to disclose.
Quality-switched (QS) laser therapy with a ruby laser and topical triple-combination therapy (TCT) were similarly effective in lightening solar lentigines, based on data from a prospective, open label study of 15 adults. The findings were published in Dermatologic Surgery.
The QS ruby laser (QSRL) delivered longer-lasting skin-lightening results in less time, but the topical therapy is less expensive and has a lower risk of adverse events, wrote Dr. Laurence Imhof of the department of dermatology, University Hospital Zürich, and colleagues (Dermatol Surg. 2016;42:853-57. doi: 10.1097/DSS.0000000000000793). “Although therapy [for solar lentigines] is not medically indicated, there is a rising treatment demand for aesthetic reasons. Therefore, the treatment should be very safe and affordable,” they wrote.
The researchers compared the safety and efficacy of treating solar lentigines with a 694-nm QSRL and TCT (hydroquinone 5%, tretinoin 0.03%, and dexamethasone 0.03%). The patients included 14 women and 1 man with evenly distributed solar lentigines on the backs of both hands. Each patient was treated with 1-2 QSRL sessions on the back of the right hand and 7 weeks of once-daily application of TCT on the back of the left hand. The average age of the patients was 61 years; 13 were Fitzpatrick Skin Types II and III. Adverse events were mild and transient with both treatments, although the QSRL caused significantly more crusting and hyperpigmentation than the TCT.
Both treatments significantly reduced pigment at the end of treatment (day 56) and 12 weeks’ post treatment (140 days), compared with baseline. Treatment areas were evaluated at days 28, 56, and 140 by the treating physician, the patient, and a blinded physician (based on photos).The 6-point grading scale rated the degree of lightening/percentage of clearing, ranging from worse (less than 0%) to excellent (76%-100%).
At 56 days, the mean macroscopic improvement with QSRL laser was rated 3.5 by the treating physician and patient and 3 by a blinded physician; the mean macroscopic improvement with TCT was rated 2.4 by the treating physician, 2.2 by the patient, and 1.7 by the blinded physician. At 140 days, the mean macroscopic improvements with the laser were rated 2.9 by the treating physician, 2.7 by the patient, and 2.8 by the blinded physician, compared with 1.2, 1.1, and 0.7, respectively, with TCT.
The results were limited by the small size, short follow-up period, and open-label design, the researchers noted. However, the study is the first known to compare a laser and a topical treatment for the management of solar lentigines, and the findings suggest that both therapies can be recommended for the aesthetic treatment of solar lentigines, they said.
The TCT cream used in the study was provided by Louis Widmer SA, which provided a grant to the University of Zürich. The researchers had no financial conflicts to disclose.
FROM DERMATOLOGIC SURGERY
Key clinical point: Both treatments significantly reduced pigment at the study conclusion (day 56) and at 12 weeks post treatment, compared with baseline.
Major finding: The mean macroscopic improvement with QSRL laser at 56 days was rated 3.5 by the treating physician and patient and 3 by a blinded physician; the mean macroscopic improvement with TCT was rated 2.4 by the treating physician, 2.2 by the patient, and 1.7 by the blinded physician.
Data source: A prospective, open-label 20-week study of 15 patients with solar lentigines.
Disclosures: The TCT cream used in the study was provided by Louis Widmer SA, which provided a grant to the University of Zürich. The researchers had no financial conflicts to disclose.
What is the VA? The Largest Educator of Health Care Professionals in the U.S.
This July, medical students, residents, and fellows in almost every medical and surgical specialty will join nurses at all levels of training, undergraduate and graduate pharmacists, dentists, and allied health students to train at a VA hospital. The VA Office of Academic Affiliations (OAA), which coordinates this massive educational effort, reported that in 2015, the last year for which data are available, 123,552 health care trainees were enrolled in VA programs.1 That’s in addition to the hundreds of health care professionals trained in the 4 branches of the armed services, including students at the Uniformed Services University of the Health Sciences and those receiving a health care education through the PHS. Federal institutions are easily the largest contributors to health care education in the nation and very likely in the world.
This mission to educate the U.S. health care workforce is not new. This year marks the 70th anniversary of the collaboration between the VA and academic affiliates across the country to ensure a highly qualified cadre of health care professionals care, not only for veterans, but also for the public. Hence, the OAA motto: To educate for VA and for the nation.
When allopathic and osteopathic medical schools are combined, there are partnerships between the VA and 90% of U.S. medical schools. More than 70% of all U.S. practicing physicians trained at a VA facility at some time.2 Currently, the VA has more than 40 different health care professional training programs under its auspices.
This educational mission is a core VA function that is enshrined in law as are VA’s other 3 core charges. According to the statute, the VA secretary shall “to the extent feasible without interfering with the medical care and treatment of veterans, develop and carry out a program of education and training of health personnel.” The primary clinical care, education, and research functions of the VA are inseparable, and none can be carried out without an adequate number of qualified staff.
Government reports and the media have identified the shortage of VA health care professionals as a major contributor to the wait times crisis of the past several years.3 Section 301 of the Veterans Access, Choice, and Accountability Act of 2014 actually requires the VA Office of Inspector General (OIG) to conduct assessments of the staffing shortages in the department. Reports from the OIG have identified 5 critical need occupations: medical officer, nurse, psychologist, physician assistant, and physical therapist.4
From my perspective as a medical officer, I am certain that the reason I went straight to the VA after my residency in psychiatry and have never left is my overwhelmingly positive experience as a medical student and resident. The VA had many of the best teachers in my training programs. The patients were—and still are nearly 20 years later—among the most respectful and appreciative of any I have treated.
Many VA patients considered us, even as trainees, their doctors and often asked us when we were residents whether they could “keep us,” although they knew that as former members of the military, most of us would rotate out of their lives. Yet they also knew that because of the strength of the training programs, a new young doctor would come to take care of them. Even now when the occasional angry patient says, “all you doctors care about is money,” I am proud to say that I could probably make more money in the private sector, but I choose to work at the VA.
Many of my fine colleagues in medicine, nursing, psychology, and allied health also remained at the VA after their training, inspired to provide public health to those who served and were underserved. Those who entered military medicine or the PHS had similar ideals borne of the role models who taught them in those federal institutions. One of the often unappreciated negative consequences of the VA scandal is that it may discouarge students in the health care professions from rotating through or seriously considering careers in the VA.
The VA and the military often do not receive the recognition they deserve as academic medical institutions. Some of the most renowned and accomplished faculty of prestigious medical universities also work at VA facilities. The ability to simultaneously teach gifted students, conduct cutting-edge research, and practice high-quality medicine all in a public health setting are what attracted me and many other idealistic health care professionals to the VA.
The VA, however, has taken active steps to restore its reputation as one of the best places to learn and work. Three outstanding initiatives deserve special attention. The first being a series of visits to medical schools that Carolyn M. Clancy, MD, made when she was the interim under secretary for health. Fortunately for me, she spoke at the academic affiliate of my VAMC (the University of New Mexico School of Medicine), where she talked about the excitement and rewards of VA clinical care and research.5
The VA Nursing Academic Partnerships (VANAP) is another initiative to promote VA as an educator and employer of health professionals. Comprised of 18 competitively selected nursing schools in the nation and the VA, VANAP’s objective is “increasing recruitment and retention of VA nurses as a result of enhanced roles in nursing education.” The New Mexico VA Health Care System, the hospital I practice at, had the honor of being awarded one of these partnerships, and I have been encouraged to see many student nurses choose the training track at the VA and express interest in employment.
According to a nurse at the Oregon Health & Science University VA partnership, “One thing I learned that I did not expect was about the wars the clients had served in. I gained a greater respect for our men and women in the service past and present…I have now an understanding of not only the physical, but also the mental and emotional effects war has on an individual.”6 It is important to realize that even if physicians and nurses in training do not ultimately enter the VA workforce, they still leave their educational experience with a more empathic understanding of the health care needs of veterans.
The salience of the third endeavor, however, has not been widely recognized. In March, Secretary Robert McDonald spoke at a meeting of the Association of American Medical Colleges Council of Deans. His speech traced the history of academic collaboration with the VA; acknowledged the bureaucratic, information technology, and other challenges faced by the VA and its academic affiliates; and reaffirmed the VA’s commitment to academic partnerships. He recognized the significant and lasting contributions the relationship with academic medical centers has had on the care of veterans and the community for decades. His remarks concluded with a vision of the potential the partnership has to transform health education and the delivery of care in the years to come. But perhaps the most hopeful remarks in the speech came not from Secretary McDonald but from the comments of medical students who had rotated at the San Diego VAMC, which he shared:
“The emphasis on teaching was fantastic, and far superior to most other rotations.”
“The vets were a wonderful patient population who really allowed us a great opportunity to learn.”
“The VA is the best place for medical students to work.”7
1. U.S. Veterans Health Administration Office of Academic Affiliations. 2015 statistics: health professions traineees. U.S. Department of Veterans Affairs website. http://www.va.gov/oaa/docs/OAA_Statistics.pdf. Accessed June 14, 2016.
2. Office of Academic Affiliations. 70th anniversary of academic affiliations. U.S. Department of Veterans Affairs website. http://www.va.gov/OAA/OAA_70th_Anniversary.asp. Update February 18, 2016. Accessed June 4, 2016.
3. Oppel RA, Goodnough A. Doctor shortage is cited in delays at V.A. hospitals. The New York Times. May 29, 2014.
4. Zonana HV, Wells JA, Getz MA, Buchanan J. Part I: The NGRI Registry: initial analyses of data collected on Connecticut insanity acquittees. Bull Am Acad Psychiatry Law. 1990;18(2):115-128.
5. Foster C. V.A. official visits HSC, as agency seeks to hire health care workers. UNM HSC Newsbeat. November 11, 2014.
6. VA Nursing Academic Partnerships. Oregon Health and Science University website. http://www.ohsu.edu/xd/education/schools/school-of-nursing/about/loader.cfm?csModule=security/getfile&pageid=2301310. Accessed June 14, 2016.
7. McDonald R. McDonald: Academic affiliations a source of strength for VA, medical schools. U.S. Department of Veterans Affairs website. http://www.blogs.va.gov/VAntage/18655/mcdonald-academic-affiliations-a-source-of-strength-for-the-va-medical-schools. Updated March 30, 2015. Accessed June 14, 2016.
This July, medical students, residents, and fellows in almost every medical and surgical specialty will join nurses at all levels of training, undergraduate and graduate pharmacists, dentists, and allied health students to train at a VA hospital. The VA Office of Academic Affiliations (OAA), which coordinates this massive educational effort, reported that in 2015, the last year for which data are available, 123,552 health care trainees were enrolled in VA programs.1 That’s in addition to the hundreds of health care professionals trained in the 4 branches of the armed services, including students at the Uniformed Services University of the Health Sciences and those receiving a health care education through the PHS. Federal institutions are easily the largest contributors to health care education in the nation and very likely in the world.
This mission to educate the U.S. health care workforce is not new. This year marks the 70th anniversary of the collaboration between the VA and academic affiliates across the country to ensure a highly qualified cadre of health care professionals care, not only for veterans, but also for the public. Hence, the OAA motto: To educate for VA and for the nation.
When allopathic and osteopathic medical schools are combined, there are partnerships between the VA and 90% of U.S. medical schools. More than 70% of all U.S. practicing physicians trained at a VA facility at some time.2 Currently, the VA has more than 40 different health care professional training programs under its auspices.
This educational mission is a core VA function that is enshrined in law as are VA’s other 3 core charges. According to the statute, the VA secretary shall “to the extent feasible without interfering with the medical care and treatment of veterans, develop and carry out a program of education and training of health personnel.” The primary clinical care, education, and research functions of the VA are inseparable, and none can be carried out without an adequate number of qualified staff.
Government reports and the media have identified the shortage of VA health care professionals as a major contributor to the wait times crisis of the past several years.3 Section 301 of the Veterans Access, Choice, and Accountability Act of 2014 actually requires the VA Office of Inspector General (OIG) to conduct assessments of the staffing shortages in the department. Reports from the OIG have identified 5 critical need occupations: medical officer, nurse, psychologist, physician assistant, and physical therapist.4
From my perspective as a medical officer, I am certain that the reason I went straight to the VA after my residency in psychiatry and have never left is my overwhelmingly positive experience as a medical student and resident. The VA had many of the best teachers in my training programs. The patients were—and still are nearly 20 years later—among the most respectful and appreciative of any I have treated.
Many VA patients considered us, even as trainees, their doctors and often asked us when we were residents whether they could “keep us,” although they knew that as former members of the military, most of us would rotate out of their lives. Yet they also knew that because of the strength of the training programs, a new young doctor would come to take care of them. Even now when the occasional angry patient says, “all you doctors care about is money,” I am proud to say that I could probably make more money in the private sector, but I choose to work at the VA.
Many of my fine colleagues in medicine, nursing, psychology, and allied health also remained at the VA after their training, inspired to provide public health to those who served and were underserved. Those who entered military medicine or the PHS had similar ideals borne of the role models who taught them in those federal institutions. One of the often unappreciated negative consequences of the VA scandal is that it may discouarge students in the health care professions from rotating through or seriously considering careers in the VA.
The VA and the military often do not receive the recognition they deserve as academic medical institutions. Some of the most renowned and accomplished faculty of prestigious medical universities also work at VA facilities. The ability to simultaneously teach gifted students, conduct cutting-edge research, and practice high-quality medicine all in a public health setting are what attracted me and many other idealistic health care professionals to the VA.
The VA, however, has taken active steps to restore its reputation as one of the best places to learn and work. Three outstanding initiatives deserve special attention. The first being a series of visits to medical schools that Carolyn M. Clancy, MD, made when she was the interim under secretary for health. Fortunately for me, she spoke at the academic affiliate of my VAMC (the University of New Mexico School of Medicine), where she talked about the excitement and rewards of VA clinical care and research.5
The VA Nursing Academic Partnerships (VANAP) is another initiative to promote VA as an educator and employer of health professionals. Comprised of 18 competitively selected nursing schools in the nation and the VA, VANAP’s objective is “increasing recruitment and retention of VA nurses as a result of enhanced roles in nursing education.” The New Mexico VA Health Care System, the hospital I practice at, had the honor of being awarded one of these partnerships, and I have been encouraged to see many student nurses choose the training track at the VA and express interest in employment.
According to a nurse at the Oregon Health & Science University VA partnership, “One thing I learned that I did not expect was about the wars the clients had served in. I gained a greater respect for our men and women in the service past and present…I have now an understanding of not only the physical, but also the mental and emotional effects war has on an individual.”6 It is important to realize that even if physicians and nurses in training do not ultimately enter the VA workforce, they still leave their educational experience with a more empathic understanding of the health care needs of veterans.
The salience of the third endeavor, however, has not been widely recognized. In March, Secretary Robert McDonald spoke at a meeting of the Association of American Medical Colleges Council of Deans. His speech traced the history of academic collaboration with the VA; acknowledged the bureaucratic, information technology, and other challenges faced by the VA and its academic affiliates; and reaffirmed the VA’s commitment to academic partnerships. He recognized the significant and lasting contributions the relationship with academic medical centers has had on the care of veterans and the community for decades. His remarks concluded with a vision of the potential the partnership has to transform health education and the delivery of care in the years to come. But perhaps the most hopeful remarks in the speech came not from Secretary McDonald but from the comments of medical students who had rotated at the San Diego VAMC, which he shared:
“The emphasis on teaching was fantastic, and far superior to most other rotations.”
“The vets were a wonderful patient population who really allowed us a great opportunity to learn.”
“The VA is the best place for medical students to work.”7
This July, medical students, residents, and fellows in almost every medical and surgical specialty will join nurses at all levels of training, undergraduate and graduate pharmacists, dentists, and allied health students to train at a VA hospital. The VA Office of Academic Affiliations (OAA), which coordinates this massive educational effort, reported that in 2015, the last year for which data are available, 123,552 health care trainees were enrolled in VA programs.1 That’s in addition to the hundreds of health care professionals trained in the 4 branches of the armed services, including students at the Uniformed Services University of the Health Sciences and those receiving a health care education through the PHS. Federal institutions are easily the largest contributors to health care education in the nation and very likely in the world.
This mission to educate the U.S. health care workforce is not new. This year marks the 70th anniversary of the collaboration between the VA and academic affiliates across the country to ensure a highly qualified cadre of health care professionals care, not only for veterans, but also for the public. Hence, the OAA motto: To educate for VA and for the nation.
When allopathic and osteopathic medical schools are combined, there are partnerships between the VA and 90% of U.S. medical schools. More than 70% of all U.S. practicing physicians trained at a VA facility at some time.2 Currently, the VA has more than 40 different health care professional training programs under its auspices.
This educational mission is a core VA function that is enshrined in law as are VA’s other 3 core charges. According to the statute, the VA secretary shall “to the extent feasible without interfering with the medical care and treatment of veterans, develop and carry out a program of education and training of health personnel.” The primary clinical care, education, and research functions of the VA are inseparable, and none can be carried out without an adequate number of qualified staff.
Government reports and the media have identified the shortage of VA health care professionals as a major contributor to the wait times crisis of the past several years.3 Section 301 of the Veterans Access, Choice, and Accountability Act of 2014 actually requires the VA Office of Inspector General (OIG) to conduct assessments of the staffing shortages in the department. Reports from the OIG have identified 5 critical need occupations: medical officer, nurse, psychologist, physician assistant, and physical therapist.4
From my perspective as a medical officer, I am certain that the reason I went straight to the VA after my residency in psychiatry and have never left is my overwhelmingly positive experience as a medical student and resident. The VA had many of the best teachers in my training programs. The patients were—and still are nearly 20 years later—among the most respectful and appreciative of any I have treated.
Many VA patients considered us, even as trainees, their doctors and often asked us when we were residents whether they could “keep us,” although they knew that as former members of the military, most of us would rotate out of their lives. Yet they also knew that because of the strength of the training programs, a new young doctor would come to take care of them. Even now when the occasional angry patient says, “all you doctors care about is money,” I am proud to say that I could probably make more money in the private sector, but I choose to work at the VA.
Many of my fine colleagues in medicine, nursing, psychology, and allied health also remained at the VA after their training, inspired to provide public health to those who served and were underserved. Those who entered military medicine or the PHS had similar ideals borne of the role models who taught them in those federal institutions. One of the often unappreciated negative consequences of the VA scandal is that it may discouarge students in the health care professions from rotating through or seriously considering careers in the VA.
The VA and the military often do not receive the recognition they deserve as academic medical institutions. Some of the most renowned and accomplished faculty of prestigious medical universities also work at VA facilities. The ability to simultaneously teach gifted students, conduct cutting-edge research, and practice high-quality medicine all in a public health setting are what attracted me and many other idealistic health care professionals to the VA.
The VA, however, has taken active steps to restore its reputation as one of the best places to learn and work. Three outstanding initiatives deserve special attention. The first being a series of visits to medical schools that Carolyn M. Clancy, MD, made when she was the interim under secretary for health. Fortunately for me, she spoke at the academic affiliate of my VAMC (the University of New Mexico School of Medicine), where she talked about the excitement and rewards of VA clinical care and research.5
The VA Nursing Academic Partnerships (VANAP) is another initiative to promote VA as an educator and employer of health professionals. Comprised of 18 competitively selected nursing schools in the nation and the VA, VANAP’s objective is “increasing recruitment and retention of VA nurses as a result of enhanced roles in nursing education.” The New Mexico VA Health Care System, the hospital I practice at, had the honor of being awarded one of these partnerships, and I have been encouraged to see many student nurses choose the training track at the VA and express interest in employment.
According to a nurse at the Oregon Health & Science University VA partnership, “One thing I learned that I did not expect was about the wars the clients had served in. I gained a greater respect for our men and women in the service past and present…I have now an understanding of not only the physical, but also the mental and emotional effects war has on an individual.”6 It is important to realize that even if physicians and nurses in training do not ultimately enter the VA workforce, they still leave their educational experience with a more empathic understanding of the health care needs of veterans.
The salience of the third endeavor, however, has not been widely recognized. In March, Secretary Robert McDonald spoke at a meeting of the Association of American Medical Colleges Council of Deans. His speech traced the history of academic collaboration with the VA; acknowledged the bureaucratic, information technology, and other challenges faced by the VA and its academic affiliates; and reaffirmed the VA’s commitment to academic partnerships. He recognized the significant and lasting contributions the relationship with academic medical centers has had on the care of veterans and the community for decades. His remarks concluded with a vision of the potential the partnership has to transform health education and the delivery of care in the years to come. But perhaps the most hopeful remarks in the speech came not from Secretary McDonald but from the comments of medical students who had rotated at the San Diego VAMC, which he shared:
“The emphasis on teaching was fantastic, and far superior to most other rotations.”
“The vets were a wonderful patient population who really allowed us a great opportunity to learn.”
“The VA is the best place for medical students to work.”7
1. U.S. Veterans Health Administration Office of Academic Affiliations. 2015 statistics: health professions traineees. U.S. Department of Veterans Affairs website. http://www.va.gov/oaa/docs/OAA_Statistics.pdf. Accessed June 14, 2016.
2. Office of Academic Affiliations. 70th anniversary of academic affiliations. U.S. Department of Veterans Affairs website. http://www.va.gov/OAA/OAA_70th_Anniversary.asp. Update February 18, 2016. Accessed June 4, 2016.
3. Oppel RA, Goodnough A. Doctor shortage is cited in delays at V.A. hospitals. The New York Times. May 29, 2014.
4. Zonana HV, Wells JA, Getz MA, Buchanan J. Part I: The NGRI Registry: initial analyses of data collected on Connecticut insanity acquittees. Bull Am Acad Psychiatry Law. 1990;18(2):115-128.
5. Foster C. V.A. official visits HSC, as agency seeks to hire health care workers. UNM HSC Newsbeat. November 11, 2014.
6. VA Nursing Academic Partnerships. Oregon Health and Science University website. http://www.ohsu.edu/xd/education/schools/school-of-nursing/about/loader.cfm?csModule=security/getfile&pageid=2301310. Accessed June 14, 2016.
7. McDonald R. McDonald: Academic affiliations a source of strength for VA, medical schools. U.S. Department of Veterans Affairs website. http://www.blogs.va.gov/VAntage/18655/mcdonald-academic-affiliations-a-source-of-strength-for-the-va-medical-schools. Updated March 30, 2015. Accessed June 14, 2016.
1. U.S. Veterans Health Administration Office of Academic Affiliations. 2015 statistics: health professions traineees. U.S. Department of Veterans Affairs website. http://www.va.gov/oaa/docs/OAA_Statistics.pdf. Accessed June 14, 2016.
2. Office of Academic Affiliations. 70th anniversary of academic affiliations. U.S. Department of Veterans Affairs website. http://www.va.gov/OAA/OAA_70th_Anniversary.asp. Update February 18, 2016. Accessed June 4, 2016.
3. Oppel RA, Goodnough A. Doctor shortage is cited in delays at V.A. hospitals. The New York Times. May 29, 2014.
4. Zonana HV, Wells JA, Getz MA, Buchanan J. Part I: The NGRI Registry: initial analyses of data collected on Connecticut insanity acquittees. Bull Am Acad Psychiatry Law. 1990;18(2):115-128.
5. Foster C. V.A. official visits HSC, as agency seeks to hire health care workers. UNM HSC Newsbeat. November 11, 2014.
6. VA Nursing Academic Partnerships. Oregon Health and Science University website. http://www.ohsu.edu/xd/education/schools/school-of-nursing/about/loader.cfm?csModule=security/getfile&pageid=2301310. Accessed June 14, 2016.
7. McDonald R. McDonald: Academic affiliations a source of strength for VA, medical schools. U.S. Department of Veterans Affairs website. http://www.blogs.va.gov/VAntage/18655/mcdonald-academic-affiliations-a-source-of-strength-for-the-va-medical-schools. Updated March 30, 2015. Accessed June 14, 2016.
FDA lifts hold on phase 2 JCAR015 trial

Image from NIAID
The US Food and Drug Administration (FDA) has removed the clinical hold on the phase 2 ROCKET trial, a study of the chimeric antigen receptor (CAR) T-cell therapy JCAR015 in adults with relapsed or refractory B-cell acute lymphoblastic leukemia.
The trial will continue with a revised protocol, under which patients will receive only cyclophosphamide as conditioning.
The ROCKET trial was placed on hold after 3 patients died of cerebral edema.
Juno Therapeutics, the company developing JCAR015, believes the deaths were likely a result of adding fludarabine to the conditioning regimen.
Patients initially received conditioning with cyclophosphamide alone, but investigators decided to add fludarabine in hopes of increasing efficacy. Adding fludarabine to conditioning had been shown to increase the efficacy of 2 other CAR T-cell therapies, JCAR014 and JCAR017, in phase 1/2 trials.
However, in the ROCKET trial, the addition of fludarabine was associated with an increase in the incidence of severe neurotoxicity and the 3 deaths from cerebral edema.
Although other factors may have contributed to the deaths, Juno said fludarabine was the most likely culprit. So the company asked the FDA if it could continue the ROCKET trial using conditioning with cyclophosphamide alone.
In response, the FDA requested that Juno submit a revised patient informed consent form, revised investigator brochure, revised trial protocol, and a copy of a presentation the company made to the FDA.
The FDA said it would review these documents within 30 days of receiving them, but the review only took a few days. The agency agreed to lift the clinical hold and allow the trial to proceed with the revised protocol.
ROCKET is not the first trial of JCAR015 to be placed on clinical hold. The phase 1 trial of the therapy was placed on hold in 2014, after 2 patients died of cytokine release syndrome.
That hold was lifted following changes to enrollment criteria and dosing. Results from this trial were presented at ASCO 2015 and ASCO 2016. ![]()

Image from NIAID
The US Food and Drug Administration (FDA) has removed the clinical hold on the phase 2 ROCKET trial, a study of the chimeric antigen receptor (CAR) T-cell therapy JCAR015 in adults with relapsed or refractory B-cell acute lymphoblastic leukemia.
The trial will continue with a revised protocol, under which patients will receive only cyclophosphamide as conditioning.
The ROCKET trial was placed on hold after 3 patients died of cerebral edema.
Juno Therapeutics, the company developing JCAR015, believes the deaths were likely a result of adding fludarabine to the conditioning regimen.
Patients initially received conditioning with cyclophosphamide alone, but investigators decided to add fludarabine in hopes of increasing efficacy. Adding fludarabine to conditioning had been shown to increase the efficacy of 2 other CAR T-cell therapies, JCAR014 and JCAR017, in phase 1/2 trials.
However, in the ROCKET trial, the addition of fludarabine was associated with an increase in the incidence of severe neurotoxicity and the 3 deaths from cerebral edema.
Although other factors may have contributed to the deaths, Juno said fludarabine was the most likely culprit. So the company asked the FDA if it could continue the ROCKET trial using conditioning with cyclophosphamide alone.
In response, the FDA requested that Juno submit a revised patient informed consent form, revised investigator brochure, revised trial protocol, and a copy of a presentation the company made to the FDA.
The FDA said it would review these documents within 30 days of receiving them, but the review only took a few days. The agency agreed to lift the clinical hold and allow the trial to proceed with the revised protocol.
ROCKET is not the first trial of JCAR015 to be placed on clinical hold. The phase 1 trial of the therapy was placed on hold in 2014, after 2 patients died of cytokine release syndrome.
That hold was lifted following changes to enrollment criteria and dosing. Results from this trial were presented at ASCO 2015 and ASCO 2016. ![]()

Image from NIAID
The US Food and Drug Administration (FDA) has removed the clinical hold on the phase 2 ROCKET trial, a study of the chimeric antigen receptor (CAR) T-cell therapy JCAR015 in adults with relapsed or refractory B-cell acute lymphoblastic leukemia.
The trial will continue with a revised protocol, under which patients will receive only cyclophosphamide as conditioning.
The ROCKET trial was placed on hold after 3 patients died of cerebral edema.
Juno Therapeutics, the company developing JCAR015, believes the deaths were likely a result of adding fludarabine to the conditioning regimen.
Patients initially received conditioning with cyclophosphamide alone, but investigators decided to add fludarabine in hopes of increasing efficacy. Adding fludarabine to conditioning had been shown to increase the efficacy of 2 other CAR T-cell therapies, JCAR014 and JCAR017, in phase 1/2 trials.
However, in the ROCKET trial, the addition of fludarabine was associated with an increase in the incidence of severe neurotoxicity and the 3 deaths from cerebral edema.
Although other factors may have contributed to the deaths, Juno said fludarabine was the most likely culprit. So the company asked the FDA if it could continue the ROCKET trial using conditioning with cyclophosphamide alone.
In response, the FDA requested that Juno submit a revised patient informed consent form, revised investigator brochure, revised trial protocol, and a copy of a presentation the company made to the FDA.
The FDA said it would review these documents within 30 days of receiving them, but the review only took a few days. The agency agreed to lift the clinical hold and allow the trial to proceed with the revised protocol.
ROCKET is not the first trial of JCAR015 to be placed on clinical hold. The phase 1 trial of the therapy was placed on hold in 2014, after 2 patients died of cytokine release syndrome.
That hold was lifted following changes to enrollment criteria and dosing. Results from this trial were presented at ASCO 2015 and ASCO 2016. ![]()
Cell of origin impacts aggressiveness of AML

New research suggests the cell of origin controls the aggressiveness and outcome of acute myeloid leukemia (AML) driven by MLL-AF9.
Using a mouse model, investigators found that MLL-AF9 expression in long-term hematopoietic stem cells (HSCs) causes invasive, chemoresistant AML that expresses genes related to epithelial-mesenchymal transition (EMT).
The researchers also found these genes were associated with poor survival in humans with AML.
“The prognosis thus depends on the particular hematopoietic stem or precursor cells in which the genetic alteration occurs and what genes are expressed,” said Antoine Peters, PhD, of Friedrich Miescher Institute for Biomedical Research in Basel, Switzerland.
Dr Peters and his colleagues related these findings in Cancer Cell.
The investigators compared the results of MLL-AF9 expression in long-term HSCs and granulocyte-macrophage progenitors (GMPs) in vitro. They found that colonies derived from long-term HSCs exhibited a greater capacity to migrate and expressed genes known to be involved in cell migration and tissue invasion.
With in vivo experiments, the researchers found that long-term HSCs were more potent in inducing AML than GMPs. Twenty percent of long-term HSC-derived AMLs proved especially aggressive, exhibiting extensive tissue infiltration, resistance to chemotherapy, and the expression of EMT genes such as EVI1, ERG, and ZEB1.
The investigators then tested the function of EMT-related transcription factors in leukemic cell migration and invasion. And they found that knocking down ZEB1 significantly reduced leukemic blast invasion.
Next, the researchers classified mouse and human leukemias according to EVI1/Evi1 and ERG/Erg expression and compared transcriptional profiles between species.
This revealed 111 genes that were more highly expressed in EVI1highERGhigh AML and Evi1highErghigh iMLL-AF9 long-term-HSC-early AML. These genes were dubbed ‘‘cluster I.’’
The investigators also identified 40 genes that were more highly expressed in EVI1lowERGlow AML samples and in iMLL-AF9 GMP-derived AML. These were called ‘‘cluster II.’’
Finally, the team evaluated how the 2 gene clusters related to overall survival in patients with 11q23+ AML.
Patients with high expression of cluster I genes tended to have poor survival rates, while patients with high levels of cluster II genes had variable survival rates and a propensity for poor outcomes if they had co-expression of a “substantial fraction” of cluster I genes.
The researchers noted that many cluster I genes are implicated in cell migration, invasion, EMT, and inflammation.
“The expression of genes such as EVI1, ERG, or ZEB1 now allows us to classify patients into different groups according to prognosis and, if necessary, to adapt treatment,” said Jürg Schwaller, MD, of University Children’s Hospital and University of Basel in Switzerland.
“Our findings should also enable us to develop new, more personalized therapies for these patients.” ![]()

New research suggests the cell of origin controls the aggressiveness and outcome of acute myeloid leukemia (AML) driven by MLL-AF9.
Using a mouse model, investigators found that MLL-AF9 expression in long-term hematopoietic stem cells (HSCs) causes invasive, chemoresistant AML that expresses genes related to epithelial-mesenchymal transition (EMT).
The researchers also found these genes were associated with poor survival in humans with AML.
“The prognosis thus depends on the particular hematopoietic stem or precursor cells in which the genetic alteration occurs and what genes are expressed,” said Antoine Peters, PhD, of Friedrich Miescher Institute for Biomedical Research in Basel, Switzerland.
Dr Peters and his colleagues related these findings in Cancer Cell.
The investigators compared the results of MLL-AF9 expression in long-term HSCs and granulocyte-macrophage progenitors (GMPs) in vitro. They found that colonies derived from long-term HSCs exhibited a greater capacity to migrate and expressed genes known to be involved in cell migration and tissue invasion.
With in vivo experiments, the researchers found that long-term HSCs were more potent in inducing AML than GMPs. Twenty percent of long-term HSC-derived AMLs proved especially aggressive, exhibiting extensive tissue infiltration, resistance to chemotherapy, and the expression of EMT genes such as EVI1, ERG, and ZEB1.
The investigators then tested the function of EMT-related transcription factors in leukemic cell migration and invasion. And they found that knocking down ZEB1 significantly reduced leukemic blast invasion.
Next, the researchers classified mouse and human leukemias according to EVI1/Evi1 and ERG/Erg expression and compared transcriptional profiles between species.
This revealed 111 genes that were more highly expressed in EVI1highERGhigh AML and Evi1highErghigh iMLL-AF9 long-term-HSC-early AML. These genes were dubbed ‘‘cluster I.’’
The investigators also identified 40 genes that were more highly expressed in EVI1lowERGlow AML samples and in iMLL-AF9 GMP-derived AML. These were called ‘‘cluster II.’’
Finally, the team evaluated how the 2 gene clusters related to overall survival in patients with 11q23+ AML.
Patients with high expression of cluster I genes tended to have poor survival rates, while patients with high levels of cluster II genes had variable survival rates and a propensity for poor outcomes if they had co-expression of a “substantial fraction” of cluster I genes.
The researchers noted that many cluster I genes are implicated in cell migration, invasion, EMT, and inflammation.
“The expression of genes such as EVI1, ERG, or ZEB1 now allows us to classify patients into different groups according to prognosis and, if necessary, to adapt treatment,” said Jürg Schwaller, MD, of University Children’s Hospital and University of Basel in Switzerland.
“Our findings should also enable us to develop new, more personalized therapies for these patients.” ![]()

New research suggests the cell of origin controls the aggressiveness and outcome of acute myeloid leukemia (AML) driven by MLL-AF9.
Using a mouse model, investigators found that MLL-AF9 expression in long-term hematopoietic stem cells (HSCs) causes invasive, chemoresistant AML that expresses genes related to epithelial-mesenchymal transition (EMT).
The researchers also found these genes were associated with poor survival in humans with AML.
“The prognosis thus depends on the particular hematopoietic stem or precursor cells in which the genetic alteration occurs and what genes are expressed,” said Antoine Peters, PhD, of Friedrich Miescher Institute for Biomedical Research in Basel, Switzerland.
Dr Peters and his colleagues related these findings in Cancer Cell.
The investigators compared the results of MLL-AF9 expression in long-term HSCs and granulocyte-macrophage progenitors (GMPs) in vitro. They found that colonies derived from long-term HSCs exhibited a greater capacity to migrate and expressed genes known to be involved in cell migration and tissue invasion.
With in vivo experiments, the researchers found that long-term HSCs were more potent in inducing AML than GMPs. Twenty percent of long-term HSC-derived AMLs proved especially aggressive, exhibiting extensive tissue infiltration, resistance to chemotherapy, and the expression of EMT genes such as EVI1, ERG, and ZEB1.
The investigators then tested the function of EMT-related transcription factors in leukemic cell migration and invasion. And they found that knocking down ZEB1 significantly reduced leukemic blast invasion.
Next, the researchers classified mouse and human leukemias according to EVI1/Evi1 and ERG/Erg expression and compared transcriptional profiles between species.
This revealed 111 genes that were more highly expressed in EVI1highERGhigh AML and Evi1highErghigh iMLL-AF9 long-term-HSC-early AML. These genes were dubbed ‘‘cluster I.’’
The investigators also identified 40 genes that were more highly expressed in EVI1lowERGlow AML samples and in iMLL-AF9 GMP-derived AML. These were called ‘‘cluster II.’’
Finally, the team evaluated how the 2 gene clusters related to overall survival in patients with 11q23+ AML.
Patients with high expression of cluster I genes tended to have poor survival rates, while patients with high levels of cluster II genes had variable survival rates and a propensity for poor outcomes if they had co-expression of a “substantial fraction” of cluster I genes.
The researchers noted that many cluster I genes are implicated in cell migration, invasion, EMT, and inflammation.
“The expression of genes such as EVI1, ERG, or ZEB1 now allows us to classify patients into different groups according to prognosis and, if necessary, to adapt treatment,” said Jürg Schwaller, MD, of University Children’s Hospital and University of Basel in Switzerland.
“Our findings should also enable us to develop new, more personalized therapies for these patients.” ![]()
Study reveals increase in susceptibility to malaria

Photo by Gabrielle Tenenbaum
Progress in reducing the malaria burden in Africa may have had the paradoxical effect of increasing malaria transmission among older children in recent years, according to research published in PLOS Medicine.
Researchers analyzed data on children admitted to a hospital in Kenya over a 25-year period and found that malaria transmission decreased from 1998 to 2009 but began to rise after that, and older children accounted for most of this increase.
The researchers believe this may be a result of a decrease in acquired immunity among the older children.
Philip Bejon, PhD, of the University of Oxford in the UK, and his colleagues conducted this study. The team analyzed malaria screening data from pediatric emergency admissions to Kilifi County Hospital between 1990 and 2014 and linked it to data on residence and insecticide-treated bednet (ITN) use.
During the period studied, 69,104 children, ages 3 months to 13 years, had malaria screening data available.
The proportion of hospital admissions found positive for malaria decreased from a high of 56% in 1998 to a low of 7% in 2009, but then rose again to 24% in 2014.
The researchers said they were unable to determine the cause of the decline in malaria infections between 1998 and 2009, which pre-dated the dramatic scale-up in ITN distribution and use in the area.
However, they noted that the subsequent increases in malaria infections coincided with a shift in burden from younger to older children, and children who lived in areas with high usage of ITNs were less likely to present with malaria than children who lived in areas with low usage of ITNs.
The researchers said the upswing in malaria transmission among older children following a period of low malaria transmission may be due to decreased acquired immunity because these children were less likely to have been exposed to malaria early in life.
The team also noted that varying levels of access to care and ITN use may have introduced bias in this study, and these data were taken from a single geographic setting. However, they still believe the findings suggest malaria elimination will require continued—and increasing—vigilance. ![]()

Photo by Gabrielle Tenenbaum
Progress in reducing the malaria burden in Africa may have had the paradoxical effect of increasing malaria transmission among older children in recent years, according to research published in PLOS Medicine.
Researchers analyzed data on children admitted to a hospital in Kenya over a 25-year period and found that malaria transmission decreased from 1998 to 2009 but began to rise after that, and older children accounted for most of this increase.
The researchers believe this may be a result of a decrease in acquired immunity among the older children.
Philip Bejon, PhD, of the University of Oxford in the UK, and his colleagues conducted this study. The team analyzed malaria screening data from pediatric emergency admissions to Kilifi County Hospital between 1990 and 2014 and linked it to data on residence and insecticide-treated bednet (ITN) use.
During the period studied, 69,104 children, ages 3 months to 13 years, had malaria screening data available.
The proportion of hospital admissions found positive for malaria decreased from a high of 56% in 1998 to a low of 7% in 2009, but then rose again to 24% in 2014.
The researchers said they were unable to determine the cause of the decline in malaria infections between 1998 and 2009, which pre-dated the dramatic scale-up in ITN distribution and use in the area.
However, they noted that the subsequent increases in malaria infections coincided with a shift in burden from younger to older children, and children who lived in areas with high usage of ITNs were less likely to present with malaria than children who lived in areas with low usage of ITNs.
The researchers said the upswing in malaria transmission among older children following a period of low malaria transmission may be due to decreased acquired immunity because these children were less likely to have been exposed to malaria early in life.
The team also noted that varying levels of access to care and ITN use may have introduced bias in this study, and these data were taken from a single geographic setting. However, they still believe the findings suggest malaria elimination will require continued—and increasing—vigilance. ![]()

Photo by Gabrielle Tenenbaum
Progress in reducing the malaria burden in Africa may have had the paradoxical effect of increasing malaria transmission among older children in recent years, according to research published in PLOS Medicine.
Researchers analyzed data on children admitted to a hospital in Kenya over a 25-year period and found that malaria transmission decreased from 1998 to 2009 but began to rise after that, and older children accounted for most of this increase.
The researchers believe this may be a result of a decrease in acquired immunity among the older children.
Philip Bejon, PhD, of the University of Oxford in the UK, and his colleagues conducted this study. The team analyzed malaria screening data from pediatric emergency admissions to Kilifi County Hospital between 1990 and 2014 and linked it to data on residence and insecticide-treated bednet (ITN) use.
During the period studied, 69,104 children, ages 3 months to 13 years, had malaria screening data available.
The proportion of hospital admissions found positive for malaria decreased from a high of 56% in 1998 to a low of 7% in 2009, but then rose again to 24% in 2014.
The researchers said they were unable to determine the cause of the decline in malaria infections between 1998 and 2009, which pre-dated the dramatic scale-up in ITN distribution and use in the area.
However, they noted that the subsequent increases in malaria infections coincided with a shift in burden from younger to older children, and children who lived in areas with high usage of ITNs were less likely to present with malaria than children who lived in areas with low usage of ITNs.
The researchers said the upswing in malaria transmission among older children following a period of low malaria transmission may be due to decreased acquired immunity because these children were less likely to have been exposed to malaria early in life.
The team also noted that varying levels of access to care and ITN use may have introduced bias in this study, and these data were taken from a single geographic setting. However, they still believe the findings suggest malaria elimination will require continued—and increasing—vigilance. ![]()
Immunotherapy may benefit relapsed HSCT recipients

Photo from Business Wire
Results of a phase 1 study suggest that repeated doses of the immunotherapy drug ipilimumab is a feasible treatment option for patients with hematologic diseases who relapse after allogeneic hematopoietic stem cell transplant (HSCT).
Seven of the 28 patients studied responded to the treatment, but immune-mediated toxic effects and graft-vs-host disease (GVHD) occurred as well.
These results were published in NEJM.
Ipilimumab, which is already approved to treat unresectable or metastatic melanoma, works by blocking the immune checkpoint CTLA-4. Blockade of CTLA-4 has been shown to augment T-cell activation and proliferation.
“We believe [,in the case of relapse after HSCT,] the donor immune cells are present but can’t recognize the tumor cells because of inhibitory signals that disguise them,” said study author Matthew Davids, MD, of the Dana-Farber Cancer Institute in Boston, Massachusetts.
“By blocking the checkpoint, you allow the donor cells to see the cancer cells.”
Dr Davids and his colleagues tested this theory in 28 patients who had relapsed after allogeneic HSCT. The patients had acute myeloid leukemia (AML, n=12), Hodgkin lymphoma (n=7), non-Hodgkin lymphoma (n=4), myelodysplastic syndromes (MDS, n=2), multiple myeloma (n=1), myeloproliferative neoplasm (n=1), or acute lymphoblastic leukemia (n=1).
Patients had received a median of 3 prior treatment regimens, excluding HSCT (range, 1 to 14), and 20 patients (71%) had received treatment for relapse after transplant. Eight patients (29%) previously had grade 1/2 acute GVHD, and 16 (57%) previously had chronic GVHD.
The median time from transplant to initial treatment with ipilimumab was 675 days (range, 198 to 1830), and the median time from relapse to initial treatment with ipilimumab was 97 days (range, 0 to
1415).
Patients received induction therapy with ipilimumab at a dose of 3 mg/kg or 10 mg/kg every 3 weeks for a total of 4 doses. Those who had a clinical benefit received additional doses every 12 weeks for up to 60 weeks.
Safety
Five patients discontinued ipilimumab due to dose-limiting toxic effects. Four of these patients had GVHD, and 1 had severe immune-related adverse events.
Dose-limiting GVHD presented as chronic GVHD of the liver in 3 patients and acute GVHD of the gut in 1 patient.
Immune-related adverse events included death (n=1), pneumonitis (2 grade 2 events, 1 grade 4 event), colitis (1 grade 3 event), immune thrombocytopenia (1 grade 2 event), and diarrhea (1 grade 2 event).
Efficacy
There were no responses in patients who received ipilimumab at 3 mg/kg. Among the 22 patients who received ipilimumab at 10 mg/kg, 5 had a complete response, and 2 had a partial response.
Six other patients did not qualify as having responses but had a decrease in their tumor burden. Altogether, ipilimumab reduced tumor burden in 59% of patients.
The complete responses occurred in 4 patients with extramedullary AML and 1 patient with MDS developing into AML. Two of the AML patients remained in complete response at 12 and 15 months, and the patient with MDS remained in complete response at 16 months.
At a median follow-up of 15 months (range, 8 to 27), the median duration of response had not been reached. Responses were associated with in situ infiltration of cytotoxic CD8+ T cells, decreased activation of regulatory T cells, and expansion of subpopulations of effector T cells.
The 1-year overall survival rate was 49%.
The investigators said these encouraging results have set the stage for larger trials of checkpoint blockade in this patient population. Further research is planned to determine whether immunotherapy drugs could be given to high-risk patients to prevent relapse. ![]()

Photo from Business Wire
Results of a phase 1 study suggest that repeated doses of the immunotherapy drug ipilimumab is a feasible treatment option for patients with hematologic diseases who relapse after allogeneic hematopoietic stem cell transplant (HSCT).
Seven of the 28 patients studied responded to the treatment, but immune-mediated toxic effects and graft-vs-host disease (GVHD) occurred as well.
These results were published in NEJM.
Ipilimumab, which is already approved to treat unresectable or metastatic melanoma, works by blocking the immune checkpoint CTLA-4. Blockade of CTLA-4 has been shown to augment T-cell activation and proliferation.
“We believe [,in the case of relapse after HSCT,] the donor immune cells are present but can’t recognize the tumor cells because of inhibitory signals that disguise them,” said study author Matthew Davids, MD, of the Dana-Farber Cancer Institute in Boston, Massachusetts.
“By blocking the checkpoint, you allow the donor cells to see the cancer cells.”
Dr Davids and his colleagues tested this theory in 28 patients who had relapsed after allogeneic HSCT. The patients had acute myeloid leukemia (AML, n=12), Hodgkin lymphoma (n=7), non-Hodgkin lymphoma (n=4), myelodysplastic syndromes (MDS, n=2), multiple myeloma (n=1), myeloproliferative neoplasm (n=1), or acute lymphoblastic leukemia (n=1).
Patients had received a median of 3 prior treatment regimens, excluding HSCT (range, 1 to 14), and 20 patients (71%) had received treatment for relapse after transplant. Eight patients (29%) previously had grade 1/2 acute GVHD, and 16 (57%) previously had chronic GVHD.
The median time from transplant to initial treatment with ipilimumab was 675 days (range, 198 to 1830), and the median time from relapse to initial treatment with ipilimumab was 97 days (range, 0 to
1415).
Patients received induction therapy with ipilimumab at a dose of 3 mg/kg or 10 mg/kg every 3 weeks for a total of 4 doses. Those who had a clinical benefit received additional doses every 12 weeks for up to 60 weeks.
Safety
Five patients discontinued ipilimumab due to dose-limiting toxic effects. Four of these patients had GVHD, and 1 had severe immune-related adverse events.
Dose-limiting GVHD presented as chronic GVHD of the liver in 3 patients and acute GVHD of the gut in 1 patient.
Immune-related adverse events included death (n=1), pneumonitis (2 grade 2 events, 1 grade 4 event), colitis (1 grade 3 event), immune thrombocytopenia (1 grade 2 event), and diarrhea (1 grade 2 event).
Efficacy
There were no responses in patients who received ipilimumab at 3 mg/kg. Among the 22 patients who received ipilimumab at 10 mg/kg, 5 had a complete response, and 2 had a partial response.
Six other patients did not qualify as having responses but had a decrease in their tumor burden. Altogether, ipilimumab reduced tumor burden in 59% of patients.
The complete responses occurred in 4 patients with extramedullary AML and 1 patient with MDS developing into AML. Two of the AML patients remained in complete response at 12 and 15 months, and the patient with MDS remained in complete response at 16 months.
At a median follow-up of 15 months (range, 8 to 27), the median duration of response had not been reached. Responses were associated with in situ infiltration of cytotoxic CD8+ T cells, decreased activation of regulatory T cells, and expansion of subpopulations of effector T cells.
The 1-year overall survival rate was 49%.
The investigators said these encouraging results have set the stage for larger trials of checkpoint blockade in this patient population. Further research is planned to determine whether immunotherapy drugs could be given to high-risk patients to prevent relapse. ![]()

Photo from Business Wire
Results of a phase 1 study suggest that repeated doses of the immunotherapy drug ipilimumab is a feasible treatment option for patients with hematologic diseases who relapse after allogeneic hematopoietic stem cell transplant (HSCT).
Seven of the 28 patients studied responded to the treatment, but immune-mediated toxic effects and graft-vs-host disease (GVHD) occurred as well.
These results were published in NEJM.
Ipilimumab, which is already approved to treat unresectable or metastatic melanoma, works by blocking the immune checkpoint CTLA-4. Blockade of CTLA-4 has been shown to augment T-cell activation and proliferation.
“We believe [,in the case of relapse after HSCT,] the donor immune cells are present but can’t recognize the tumor cells because of inhibitory signals that disguise them,” said study author Matthew Davids, MD, of the Dana-Farber Cancer Institute in Boston, Massachusetts.
“By blocking the checkpoint, you allow the donor cells to see the cancer cells.”
Dr Davids and his colleagues tested this theory in 28 patients who had relapsed after allogeneic HSCT. The patients had acute myeloid leukemia (AML, n=12), Hodgkin lymphoma (n=7), non-Hodgkin lymphoma (n=4), myelodysplastic syndromes (MDS, n=2), multiple myeloma (n=1), myeloproliferative neoplasm (n=1), or acute lymphoblastic leukemia (n=1).
Patients had received a median of 3 prior treatment regimens, excluding HSCT (range, 1 to 14), and 20 patients (71%) had received treatment for relapse after transplant. Eight patients (29%) previously had grade 1/2 acute GVHD, and 16 (57%) previously had chronic GVHD.
The median time from transplant to initial treatment with ipilimumab was 675 days (range, 198 to 1830), and the median time from relapse to initial treatment with ipilimumab was 97 days (range, 0 to
1415).
Patients received induction therapy with ipilimumab at a dose of 3 mg/kg or 10 mg/kg every 3 weeks for a total of 4 doses. Those who had a clinical benefit received additional doses every 12 weeks for up to 60 weeks.
Safety
Five patients discontinued ipilimumab due to dose-limiting toxic effects. Four of these patients had GVHD, and 1 had severe immune-related adverse events.
Dose-limiting GVHD presented as chronic GVHD of the liver in 3 patients and acute GVHD of the gut in 1 patient.
Immune-related adverse events included death (n=1), pneumonitis (2 grade 2 events, 1 grade 4 event), colitis (1 grade 3 event), immune thrombocytopenia (1 grade 2 event), and diarrhea (1 grade 2 event).
Efficacy
There were no responses in patients who received ipilimumab at 3 mg/kg. Among the 22 patients who received ipilimumab at 10 mg/kg, 5 had a complete response, and 2 had a partial response.
Six other patients did not qualify as having responses but had a decrease in their tumor burden. Altogether, ipilimumab reduced tumor burden in 59% of patients.
The complete responses occurred in 4 patients with extramedullary AML and 1 patient with MDS developing into AML. Two of the AML patients remained in complete response at 12 and 15 months, and the patient with MDS remained in complete response at 16 months.
At a median follow-up of 15 months (range, 8 to 27), the median duration of response had not been reached. Responses were associated with in situ infiltration of cytotoxic CD8+ T cells, decreased activation of regulatory T cells, and expansion of subpopulations of effector T cells.
The 1-year overall survival rate was 49%.
The investigators said these encouraging results have set the stage for larger trials of checkpoint blockade in this patient population. Further research is planned to determine whether immunotherapy drugs could be given to high-risk patients to prevent relapse. ![]()
Pushing the Limits
Deciding when a hospitalized child's vital signs are acceptably within range and when they should generate alerts, alarms, and escalations of care is critically important yet surprisingly complicated. Many patients in the hospital who are recovering appropriately exhibit vital signs that fall outside normal ranges for well children. In a technology‐focused hospital environment, these out‐of‐range vital signs often generate alerts in the electronic health record (EHR) and alarms on physiologic monitors that can disrupt patients' sleep, generate concern in parents, lead to unnecessary testing and treatment by physicians, interrupt nurses during important patient care tasks, and lead to alarm fatigue. It is this last area, the problem of alarm fatigue, that Goel and colleagues[1] have used to frame the rationale and results of their study reported in this issue of the Journal of Hospital Medicine.
Goel and colleagues correctly point out that physiologic monitor alarm rates are high in children's hospitals, and alarms warranting intervention or action are rare.[2, 3, 4, 5, 6] Few studies have rigorously examined interventions to reduce unnecessary hospital physiologic monitor alarms, especially in pediatric settings. Of all the potential interventions, widening parameters has the most face validity: if you set wide enough alarm parameters, fewer alarms will be triggered. However, it comes with a potential safety tradeoff of missed actionable alarms.
Before EHR data became widely available for research, normal (or perhaps more appropriate for the hospital setting, expected) vital sign ranges were defined using expert opinion. The first publication describing the distribution of EHR‐documented vital signs in hospitalized children was published in 2013.[7] Goel and colleagues have built upon this prior work in their article, in which they present percentiles of EHR‐documented heart rate (HR) and respiratory rate (RR) developed using data from more than 7000 children hospitalized at an academic children's hospital. In a separate validation dataset, they then compared the performance of their proposed physiologic monitor alarm parametersthe 5th and 95th percentiles for HR and RR from this studyto the 2004 National Institutes of Health (NIH) vital sign reference ranges[8] that were the basis of default alarm parameters at their hospital. They also compared their percentiles to the 2013 study.[7]
The 2 main findings of Goel and colleagues' study were: (1) using their separate validation dataset, 55.6% fewer HR and RR observations were out of range based on their newly developed percentiles as compared to the NIH vital sign reference ranges; and (2) the HR and RR percentiles they developed were very similar to those reported in the 2013 study,[7] which used data from 2 other institutions, externally validating their findings.
The team then pushed the data a step further in a safety analysis and evaluated the sensitivity of the 5th and 95th percentiles for HR and RR from this study for detecting deterioration in 148 patients in the 12 hours before either a rapid response team activation or a cardiorespiratory arrest. The overall sensitivity for having either a HR or RR value out of range was 93% for Goel and colleagues' percentiles and 97% for the NIH ranges. Goel and colleagues concluded that using the 5th and 95th HR and RR percentiles provides a potentially safe means by which to modify physiologic bedside monitor alarm limits.
There are 2 important limitations to this work. The first is that the study uses EHR‐documented data to estimate the performance of new physiologic monitor settings. Although there are few published reports of differences between nurse‐charted vital signs and monitor data, those that do exist suggest that nurse charting favors more stable vital signs,[9, 10] even when charting oxygen saturation in patients with true, prolonged desaturation.[9] We agree with the authors of 1 report, who speculated that nurses recognize that temporary changes in vital signs are untypical for that patient and might choose to ignore them and either await a period of stability or make an educated estimate for that hour.[9] When using Goel and colleagues' 5th and 95th percentiles as alarm parameters, the expected scenario is that monitors will generate alarms for 10% of HR values and 10% of RR values. Because of the differences between nurse‐charted vital signs and monitor data, the monitors will probably generate many more alarms.
The second limitation is the approach Goel and colleagues took in performing a safety analysis using chart review. Unfortunately, it is nearly impossible for a retrospective chart review to form the basis of a convincing scientific argument for the safety of different alarm parameters. It requires balancing the complex and sometimes competing nurse‐level, patient‐level, and alarm‐level factors that determine nurse response time to alarms. It is possible to do prospectively, and we hope Goel's team will follow up this article with a description of the implementation and safety of these parameters in clinical practice.
In addition, the clinical implications of HR and RR at the 95th percentile might be considered less immediately life threatening than HR and RR at the 5th percentile, even though statistically they are equally abnormal. When choosing percentile‐based alarm parameters, statistical symmetry might be less important than the potential immediate consequences of missing bradycardia or bradypnea. It would be reasonable to consider setting high HR and RR at the 99th percentile or higher, because elevated HR or RR alone is rarely immediately actionable, and set the low HR and RR at the 5th or 10th percentile.
Despite these caveats, should the percentiles proposed by Goel and colleagues be used to inform pediatric vital sign clinical decision support throughout the world? When faced with the alternative of using vital sign parameters that are not based on data from hospitalized children, these percentiles offer a clear advantage, especially for hospitals similar to Goel's. The most obvious immediate use for these percentiles is to improve noninterruptive[11] vital sign clinical decision support in the EHR, the actual source of the data in this study.
The question of whether to implement Goel's 5th and 95th percentiles as physiologic monitor alarm parameters is more complex. In contrast to EHR decision support, there are much clearer downstream consequences of sounding unnecessary alarms as well as failing to sound important alarms for a child in extremis. Because their percentiles are not based on monitor data, the projected number of alarms generated at different percentile thresholds cannot be accurately estimated, although using their 5th and 95th percentiles should result in fewer alarms than the NIH parameters.
In conclusion, the work by Goel and colleagues represents an important contribution to knowledge about the ranges of expected vital signs in hospitalized children. Their findings can be immediately used to guide EHR decision support. Their percentiles are also relevant to physiologic monitor alarm parameters, although the performance and safety of using the 5th and 95th percentiles remain in question. Hospitals aiming to implement these data‐driven parameters should first evaluate the performance of different percentiles from this article using data obtained from their own monitor system and, if proceeding with clinical implementation, pilot the parameters to accurately gauge alarm rates and assess safety before spreading hospital wide.
Disclosures
Dr. Bonafide is supported by a Mentored Patient‐Oriented Research Career Development Award from the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number K23HL116427. Dr. Brady is supported by a Patient‐Centered Outcomes Research Mentored Clinical Investigator Award from the Agency for Healthcare Research and Quality under award number K08HS023827. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding organizations. The funding organizations had no role in the design, preparation, review, or approval of this article; nor the decision to submit the article for publication. The authors have no financial relationships relevant to this article or conflicts of interest to disclose.
- , , , et al. Safety analysis of proposed data‐driven physiologic alarm parameters for hospitalized children. J Hosp Med. 2016;11(12):817–823.
- , , , et al. Association between exposure to nonactionable physiologic monitor alarms and response time in a children's hospital. J Hosp Med. 2015;10(6):345–351.
- . Crying wolf: false alarms in a pediatric intensive care unit. Crit Care Med. 1994;22(6):981–985.
- , , , , . What are we missing? Arrhythmia detection in the pediatric intensive care unit. J Pediatr. 2013;163(2):511–514.
- , , , et al. Cardiopulmonary monitors and clinically significant events in critically ill children. Biomed Instrum Technol. 2011;(suppl):38–45.
- , . Poor prognosis for existing monitors in the intensive care unit. Crit Care Med. 1997;25(4):614–619.
- , , , , , . Development of heart and respiratory rate percentile curves for hospitalized children. Pediatrics. 2013;131:e1150–e1157.
- NIH Clinical Center. Pediatric services: age‐appropriate vital signs. Available at: https://web.archive.org/web/20041101222327/http://www.cc. nih.gov/ccc/pedweb/pedsstaff/age.html. Published November 1, 2004. Accessed June 9, 2016.
- , , , , . A comparison of oxygen saturation data in inpatients with low oxygen saturation using automated continuous monitoring and intermittent manual data charting. Anesth Analg. 2014;118(2):326–331.
- , , , . Comparison of nurse and computer charting of physiological variables in an intensive care unit. Int J Clin Monit Comput. 1996;13(4):235–241.
- , , , et al. Drug‐drug interactions that should be non‐interruptive in order to reduce alert fatigue in electronic health records. J Am Med Inform Assoc. 2013;20(3):489–493.
Deciding when a hospitalized child's vital signs are acceptably within range and when they should generate alerts, alarms, and escalations of care is critically important yet surprisingly complicated. Many patients in the hospital who are recovering appropriately exhibit vital signs that fall outside normal ranges for well children. In a technology‐focused hospital environment, these out‐of‐range vital signs often generate alerts in the electronic health record (EHR) and alarms on physiologic monitors that can disrupt patients' sleep, generate concern in parents, lead to unnecessary testing and treatment by physicians, interrupt nurses during important patient care tasks, and lead to alarm fatigue. It is this last area, the problem of alarm fatigue, that Goel and colleagues[1] have used to frame the rationale and results of their study reported in this issue of the Journal of Hospital Medicine.
Goel and colleagues correctly point out that physiologic monitor alarm rates are high in children's hospitals, and alarms warranting intervention or action are rare.[2, 3, 4, 5, 6] Few studies have rigorously examined interventions to reduce unnecessary hospital physiologic monitor alarms, especially in pediatric settings. Of all the potential interventions, widening parameters has the most face validity: if you set wide enough alarm parameters, fewer alarms will be triggered. However, it comes with a potential safety tradeoff of missed actionable alarms.
Before EHR data became widely available for research, normal (or perhaps more appropriate for the hospital setting, expected) vital sign ranges were defined using expert opinion. The first publication describing the distribution of EHR‐documented vital signs in hospitalized children was published in 2013.[7] Goel and colleagues have built upon this prior work in their article, in which they present percentiles of EHR‐documented heart rate (HR) and respiratory rate (RR) developed using data from more than 7000 children hospitalized at an academic children's hospital. In a separate validation dataset, they then compared the performance of their proposed physiologic monitor alarm parametersthe 5th and 95th percentiles for HR and RR from this studyto the 2004 National Institutes of Health (NIH) vital sign reference ranges[8] that were the basis of default alarm parameters at their hospital. They also compared their percentiles to the 2013 study.[7]
The 2 main findings of Goel and colleagues' study were: (1) using their separate validation dataset, 55.6% fewer HR and RR observations were out of range based on their newly developed percentiles as compared to the NIH vital sign reference ranges; and (2) the HR and RR percentiles they developed were very similar to those reported in the 2013 study,[7] which used data from 2 other institutions, externally validating their findings.
The team then pushed the data a step further in a safety analysis and evaluated the sensitivity of the 5th and 95th percentiles for HR and RR from this study for detecting deterioration in 148 patients in the 12 hours before either a rapid response team activation or a cardiorespiratory arrest. The overall sensitivity for having either a HR or RR value out of range was 93% for Goel and colleagues' percentiles and 97% for the NIH ranges. Goel and colleagues concluded that using the 5th and 95th HR and RR percentiles provides a potentially safe means by which to modify physiologic bedside monitor alarm limits.
There are 2 important limitations to this work. The first is that the study uses EHR‐documented data to estimate the performance of new physiologic monitor settings. Although there are few published reports of differences between nurse‐charted vital signs and monitor data, those that do exist suggest that nurse charting favors more stable vital signs,[9, 10] even when charting oxygen saturation in patients with true, prolonged desaturation.[9] We agree with the authors of 1 report, who speculated that nurses recognize that temporary changes in vital signs are untypical for that patient and might choose to ignore them and either await a period of stability or make an educated estimate for that hour.[9] When using Goel and colleagues' 5th and 95th percentiles as alarm parameters, the expected scenario is that monitors will generate alarms for 10% of HR values and 10% of RR values. Because of the differences between nurse‐charted vital signs and monitor data, the monitors will probably generate many more alarms.
The second limitation is the approach Goel and colleagues took in performing a safety analysis using chart review. Unfortunately, it is nearly impossible for a retrospective chart review to form the basis of a convincing scientific argument for the safety of different alarm parameters. It requires balancing the complex and sometimes competing nurse‐level, patient‐level, and alarm‐level factors that determine nurse response time to alarms. It is possible to do prospectively, and we hope Goel's team will follow up this article with a description of the implementation and safety of these parameters in clinical practice.
In addition, the clinical implications of HR and RR at the 95th percentile might be considered less immediately life threatening than HR and RR at the 5th percentile, even though statistically they are equally abnormal. When choosing percentile‐based alarm parameters, statistical symmetry might be less important than the potential immediate consequences of missing bradycardia or bradypnea. It would be reasonable to consider setting high HR and RR at the 99th percentile or higher, because elevated HR or RR alone is rarely immediately actionable, and set the low HR and RR at the 5th or 10th percentile.
Despite these caveats, should the percentiles proposed by Goel and colleagues be used to inform pediatric vital sign clinical decision support throughout the world? When faced with the alternative of using vital sign parameters that are not based on data from hospitalized children, these percentiles offer a clear advantage, especially for hospitals similar to Goel's. The most obvious immediate use for these percentiles is to improve noninterruptive[11] vital sign clinical decision support in the EHR, the actual source of the data in this study.
The question of whether to implement Goel's 5th and 95th percentiles as physiologic monitor alarm parameters is more complex. In contrast to EHR decision support, there are much clearer downstream consequences of sounding unnecessary alarms as well as failing to sound important alarms for a child in extremis. Because their percentiles are not based on monitor data, the projected number of alarms generated at different percentile thresholds cannot be accurately estimated, although using their 5th and 95th percentiles should result in fewer alarms than the NIH parameters.
In conclusion, the work by Goel and colleagues represents an important contribution to knowledge about the ranges of expected vital signs in hospitalized children. Their findings can be immediately used to guide EHR decision support. Their percentiles are also relevant to physiologic monitor alarm parameters, although the performance and safety of using the 5th and 95th percentiles remain in question. Hospitals aiming to implement these data‐driven parameters should first evaluate the performance of different percentiles from this article using data obtained from their own monitor system and, if proceeding with clinical implementation, pilot the parameters to accurately gauge alarm rates and assess safety before spreading hospital wide.
Disclosures
Dr. Bonafide is supported by a Mentored Patient‐Oriented Research Career Development Award from the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number K23HL116427. Dr. Brady is supported by a Patient‐Centered Outcomes Research Mentored Clinical Investigator Award from the Agency for Healthcare Research and Quality under award number K08HS023827. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding organizations. The funding organizations had no role in the design, preparation, review, or approval of this article; nor the decision to submit the article for publication. The authors have no financial relationships relevant to this article or conflicts of interest to disclose.
Deciding when a hospitalized child's vital signs are acceptably within range and when they should generate alerts, alarms, and escalations of care is critically important yet surprisingly complicated. Many patients in the hospital who are recovering appropriately exhibit vital signs that fall outside normal ranges for well children. In a technology‐focused hospital environment, these out‐of‐range vital signs often generate alerts in the electronic health record (EHR) and alarms on physiologic monitors that can disrupt patients' sleep, generate concern in parents, lead to unnecessary testing and treatment by physicians, interrupt nurses during important patient care tasks, and lead to alarm fatigue. It is this last area, the problem of alarm fatigue, that Goel and colleagues[1] have used to frame the rationale and results of their study reported in this issue of the Journal of Hospital Medicine.
Goel and colleagues correctly point out that physiologic monitor alarm rates are high in children's hospitals, and alarms warranting intervention or action are rare.[2, 3, 4, 5, 6] Few studies have rigorously examined interventions to reduce unnecessary hospital physiologic monitor alarms, especially in pediatric settings. Of all the potential interventions, widening parameters has the most face validity: if you set wide enough alarm parameters, fewer alarms will be triggered. However, it comes with a potential safety tradeoff of missed actionable alarms.
Before EHR data became widely available for research, normal (or perhaps more appropriate for the hospital setting, expected) vital sign ranges were defined using expert opinion. The first publication describing the distribution of EHR‐documented vital signs in hospitalized children was published in 2013.[7] Goel and colleagues have built upon this prior work in their article, in which they present percentiles of EHR‐documented heart rate (HR) and respiratory rate (RR) developed using data from more than 7000 children hospitalized at an academic children's hospital. In a separate validation dataset, they then compared the performance of their proposed physiologic monitor alarm parametersthe 5th and 95th percentiles for HR and RR from this studyto the 2004 National Institutes of Health (NIH) vital sign reference ranges[8] that were the basis of default alarm parameters at their hospital. They also compared their percentiles to the 2013 study.[7]
The 2 main findings of Goel and colleagues' study were: (1) using their separate validation dataset, 55.6% fewer HR and RR observations were out of range based on their newly developed percentiles as compared to the NIH vital sign reference ranges; and (2) the HR and RR percentiles they developed were very similar to those reported in the 2013 study,[7] which used data from 2 other institutions, externally validating their findings.
The team then pushed the data a step further in a safety analysis and evaluated the sensitivity of the 5th and 95th percentiles for HR and RR from this study for detecting deterioration in 148 patients in the 12 hours before either a rapid response team activation or a cardiorespiratory arrest. The overall sensitivity for having either a HR or RR value out of range was 93% for Goel and colleagues' percentiles and 97% for the NIH ranges. Goel and colleagues concluded that using the 5th and 95th HR and RR percentiles provides a potentially safe means by which to modify physiologic bedside monitor alarm limits.
There are 2 important limitations to this work. The first is that the study uses EHR‐documented data to estimate the performance of new physiologic monitor settings. Although there are few published reports of differences between nurse‐charted vital signs and monitor data, those that do exist suggest that nurse charting favors more stable vital signs,[9, 10] even when charting oxygen saturation in patients with true, prolonged desaturation.[9] We agree with the authors of 1 report, who speculated that nurses recognize that temporary changes in vital signs are untypical for that patient and might choose to ignore them and either await a period of stability or make an educated estimate for that hour.[9] When using Goel and colleagues' 5th and 95th percentiles as alarm parameters, the expected scenario is that monitors will generate alarms for 10% of HR values and 10% of RR values. Because of the differences between nurse‐charted vital signs and monitor data, the monitors will probably generate many more alarms.
The second limitation is the approach Goel and colleagues took in performing a safety analysis using chart review. Unfortunately, it is nearly impossible for a retrospective chart review to form the basis of a convincing scientific argument for the safety of different alarm parameters. It requires balancing the complex and sometimes competing nurse‐level, patient‐level, and alarm‐level factors that determine nurse response time to alarms. It is possible to do prospectively, and we hope Goel's team will follow up this article with a description of the implementation and safety of these parameters in clinical practice.
In addition, the clinical implications of HR and RR at the 95th percentile might be considered less immediately life threatening than HR and RR at the 5th percentile, even though statistically they are equally abnormal. When choosing percentile‐based alarm parameters, statistical symmetry might be less important than the potential immediate consequences of missing bradycardia or bradypnea. It would be reasonable to consider setting high HR and RR at the 99th percentile or higher, because elevated HR or RR alone is rarely immediately actionable, and set the low HR and RR at the 5th or 10th percentile.
Despite these caveats, should the percentiles proposed by Goel and colleagues be used to inform pediatric vital sign clinical decision support throughout the world? When faced with the alternative of using vital sign parameters that are not based on data from hospitalized children, these percentiles offer a clear advantage, especially for hospitals similar to Goel's. The most obvious immediate use for these percentiles is to improve noninterruptive[11] vital sign clinical decision support in the EHR, the actual source of the data in this study.
The question of whether to implement Goel's 5th and 95th percentiles as physiologic monitor alarm parameters is more complex. In contrast to EHR decision support, there are much clearer downstream consequences of sounding unnecessary alarms as well as failing to sound important alarms for a child in extremis. Because their percentiles are not based on monitor data, the projected number of alarms generated at different percentile thresholds cannot be accurately estimated, although using their 5th and 95th percentiles should result in fewer alarms than the NIH parameters.
In conclusion, the work by Goel and colleagues represents an important contribution to knowledge about the ranges of expected vital signs in hospitalized children. Their findings can be immediately used to guide EHR decision support. Their percentiles are also relevant to physiologic monitor alarm parameters, although the performance and safety of using the 5th and 95th percentiles remain in question. Hospitals aiming to implement these data‐driven parameters should first evaluate the performance of different percentiles from this article using data obtained from their own monitor system and, if proceeding with clinical implementation, pilot the parameters to accurately gauge alarm rates and assess safety before spreading hospital wide.
Disclosures
Dr. Bonafide is supported by a Mentored Patient‐Oriented Research Career Development Award from the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number K23HL116427. Dr. Brady is supported by a Patient‐Centered Outcomes Research Mentored Clinical Investigator Award from the Agency for Healthcare Research and Quality under award number K08HS023827. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding organizations. The funding organizations had no role in the design, preparation, review, or approval of this article; nor the decision to submit the article for publication. The authors have no financial relationships relevant to this article or conflicts of interest to disclose.
- , , , et al. Safety analysis of proposed data‐driven physiologic alarm parameters for hospitalized children. J Hosp Med. 2016;11(12):817–823.
- , , , et al. Association between exposure to nonactionable physiologic monitor alarms and response time in a children's hospital. J Hosp Med. 2015;10(6):345–351.
- . Crying wolf: false alarms in a pediatric intensive care unit. Crit Care Med. 1994;22(6):981–985.
- , , , , . What are we missing? Arrhythmia detection in the pediatric intensive care unit. J Pediatr. 2013;163(2):511–514.
- , , , et al. Cardiopulmonary monitors and clinically significant events in critically ill children. Biomed Instrum Technol. 2011;(suppl):38–45.
- , . Poor prognosis for existing monitors in the intensive care unit. Crit Care Med. 1997;25(4):614–619.
- , , , , , . Development of heart and respiratory rate percentile curves for hospitalized children. Pediatrics. 2013;131:e1150–e1157.
- NIH Clinical Center. Pediatric services: age‐appropriate vital signs. Available at: https://web.archive.org/web/20041101222327/http://www.cc. nih.gov/ccc/pedweb/pedsstaff/age.html. Published November 1, 2004. Accessed June 9, 2016.
- , , , , . A comparison of oxygen saturation data in inpatients with low oxygen saturation using automated continuous monitoring and intermittent manual data charting. Anesth Analg. 2014;118(2):326–331.
- , , , . Comparison of nurse and computer charting of physiological variables in an intensive care unit. Int J Clin Monit Comput. 1996;13(4):235–241.
- , , , et al. Drug‐drug interactions that should be non‐interruptive in order to reduce alert fatigue in electronic health records. J Am Med Inform Assoc. 2013;20(3):489–493.
- , , , et al. Safety analysis of proposed data‐driven physiologic alarm parameters for hospitalized children. J Hosp Med. 2016;11(12):817–823.
- , , , et al. Association between exposure to nonactionable physiologic monitor alarms and response time in a children's hospital. J Hosp Med. 2015;10(6):345–351.
- . Crying wolf: false alarms in a pediatric intensive care unit. Crit Care Med. 1994;22(6):981–985.
- , , , , . What are we missing? Arrhythmia detection in the pediatric intensive care unit. J Pediatr. 2013;163(2):511–514.
- , , , et al. Cardiopulmonary monitors and clinically significant events in critically ill children. Biomed Instrum Technol. 2011;(suppl):38–45.
- , . Poor prognosis for existing monitors in the intensive care unit. Crit Care Med. 1997;25(4):614–619.
- , , , , , . Development of heart and respiratory rate percentile curves for hospitalized children. Pediatrics. 2013;131:e1150–e1157.
- NIH Clinical Center. Pediatric services: age‐appropriate vital signs. Available at: https://web.archive.org/web/20041101222327/http://www.cc. nih.gov/ccc/pedweb/pedsstaff/age.html. Published November 1, 2004. Accessed June 9, 2016.
- , , , , . A comparison of oxygen saturation data in inpatients with low oxygen saturation using automated continuous monitoring and intermittent manual data charting. Anesth Analg. 2014;118(2):326–331.
- , , , . Comparison of nurse and computer charting of physiological variables in an intensive care unit. Int J Clin Monit Comput. 1996;13(4):235–241.
- , , , et al. Drug‐drug interactions that should be non‐interruptive in order to reduce alert fatigue in electronic health records. J Am Med Inform Assoc. 2013;20(3):489–493.
Data‐Driven Pediatric Alarm Limits
The management of alarms in the hospital setting is a significant patient safety issue. In 2013, the Joint Commission issued Sentinel Event Alert #50 to draw attention to the fact that tens of thousands of alarms occur daily throughout individual hospitals, and 85% to 99% are false or not clinically actionable.[1] These alarms, designed to be a safety net in patient care, have the unintended consequence of causing provider desensitization, also known as alarm fatigue, which contributes to adverse events as severe as patient mortality.[1, 2] For this reason, a 2014 Joint Commission National Patient Safety Goal urged hospitals to prioritize alarm system safety and to develop policies and procedures to manage alarms and alarm fatigue.[3]
Multiple efforts have been made to address alarm fatigue in hospitalized adults. Studies have quantified the frequency and types of medical device alarms,[4, 5, 6, 7, 8, 9] and some proposed solutions to decrease excess alarms.[10, 11, 12, 13, 14, 15] One such solution is to change alarm limit settings, an intervention shown to be efficacious in the literature.[5, 6, 16, 17] Although no adverse patient outcomes are reported in these studies, none of them included a formal safety evaluation to evaluate whether alarm rate reduction occurred at the expense of clinically significant alarms.
Specific to pediatrics, frameworks to address alarm fatigue have been proposed,[18] and the relationship between nurse response time and frequency of exposure to nonactionable alarms has been reported.[19] However, efforts to address alarm fatigue in the pediatric setting are less well studied overall, and there is little guidance regarding optimization of pediatric alarm parameters. Although multiple established reference ranges exist for pediatric vital signs,[20, 21, 22] a systematic review in 2011 found that only 2 of 5 published heart rate (HR) and 6 respiratory rate (RR) guidelines cited any references, and even these had weak underpinning evidence.[23] Consequently, ranges defining normal pediatric vital signs are derived either from small sample observational data in healthy outpatient children or consensus opinion. In a 2013 study by Bonafide et al.,[24] charted vital sign data from hospitalized children were used to develop percentile curves for HR and RR, and from these it was estimated that 54% of vital sign measurements in hospitalized children are out of range using currently accepted normal vital sign parameters.[24] Although these calculated vital sign parameters were not implemented clinically, they called into question reference ranges that are currently widely accepted and used as parameters for electronic health record (EHR) alerts, early warning scoring systems, and physiologic monitor alarms.
With the goal of safely decreasing the number of out‐of‐range vital sign measurements that result from current, often nonevidence‐based pediatric vital sign reference ranges, we used data from noncritically ill pediatric inpatients to derive HR and RR percentile charts for hospitalized children. In anticipation of local implementation of these data‐driven vital sign ranges as physiologic monitor parameters, we performed a retrospective safety analysis by evaluating the effect of data‐driven alarm limit modification on identification of cardiorespiratory arrests (CRA) and rapid response team (RRT) activations.
METHODS
We performed a cross‐sectional study of children less than 18 years of age hospitalized on general medical and surgical units at Lucile Packard Children's Hospital Stanford, a 311‐bed quaternary‐care academic hospital with a full complement of pediatric medical and surgical subspecialties and transplant programs. During the study period, the hospital used the Cerner EHR (Millennium; Cerner, Kansas City, MO) and Philips IntelliVue bedside monitors (Koninklijke Philips N.V., Amsterdam, the Netherlands). The Stanford University Institutional Review Board approved this study.
Establishing Data‐Driven HR and RR Parameters
Vital sign documentation in the EHR at our institution is performed primarily by nurses and facilitated by bedside monitor biomedical device integration. We extracted vital signs data from the institution's EHR for all general medical and surgical patients discharged between January 1, 2013 and May 3, 2014. To be most conservative in the definition of normal vital sign ranges for pediatric inpatients, we excluded critically ill children (those who spent any part of their hospitalization in an intensive care unit [ICU]). Physiologically implausible vital sign values were excluded as per the methods of Bonafide et al.[24] The data were separated into 2 different sets: a training set (patients discharged between January 1, 2013 and December 31, 2013) and a test set for validation (patients discharged between January 1, 2014 and May 3, 2014). To avoid oversampling from both particular time periods and individual patients in the training set, we randomly selected 1 HR and RR pair from each 4‐hour interval during a hospitalization, and then randomly sampled a maximum of 10 HR and RR pairs per patient. Using these vital sign measurements, we calculated age‐stratified 1st, 5th, 10th, 50th, 90th, 95th, and 99th percentiles for both HR and RR.
Based on a combination of expert opinion and local consensus from our Medical Executive and Patient Safety Committees, we selected the 5th and 95th percentile values as proposed data‐driven parameter limits and compared them to the 5th and 95th percentile values generated in the 2013 study[24] and to the 2004 National Institutes of Health (NIH)adapted vital sign reference ranges currently used at our hospital.[25] Using 1 randomly selected HR and RR pair from every 4‐hour interval in the validation set, we compared the proportion of out‐of‐range HR and RR observations with the proposed 5th and 95th percentile data‐driven parameters versus the current NIH reference ranges. We also calculated average differences between our data‐driven 5th and 95th percentile values and the calculated HR and RR values in the 2013 study.[24]
Safety Analysis
To assess the safety of the newly created 5th and 95th percentile HR and RR parameters prior to clinical adoption, we retrospectively reviewed data associated with all RRT and CRA events on the hospital's medical/surgical units from March 4, 2013 until March 3, 2014. The RRT/CRA event data were obtained from logs kept by the hospital's code committee. We excluded events that lacked a documented patient identifier, occurred in locations other than the acute medical/surgical units, or occurred in patients >18 years old. The resulting charts were manually reviewed to determine the date and time of RRT or CRA event activation. Because evidence exists that hospitalized pediatric patients with CRA show signs of vital sign decompensation as early as 12 hours prior to the event,[26, 27, 28, 29] we extracted all EHR‐charted HR and RR data in the 12 hours preceding RRT and CRA events from the institution's clinical data warehouse for analysis, excluding patients without charted vital sign data in this time period. The sets of patients with any out‐of‐range HR or RR measurements in the 12‐hours prior to an event were compared according to the current NIH reference ranges[25] versus data‐driven parameters. Additionally, manual chart review was performed to assess the reason for code or RRT activation, and to determine the role that out‐of‐range vital signs played in alerting clinical staff of patient decompensation.
Statistical Analysis
All analysis was performed using R statistical package software (version 0.98.1062 for Mac OS X 10_9_5; The R Foundation for Statistical Computing, Vienna, Austria) with an SQL database (MySQL 2015; Oracle Corp., Redwood City, CA).
RESULTS
Data‐Driven HR and RR Parameters
We established a training set of 62,508 vital sign measurements for 7202 unique patients to calculate 1st, 5th, 10th, 50th, 90th, 95th, and 99th percentiles for HR and RR among the 14 age groups (see Supporting Information, Appendix 1, in the online version of this article). Figures 1 and 2 compare the proposed data‐driven vital sign ranges with (1) our current HR and RR reference ranges and (2) the 5th and 95th percentile values created in the similar 2013 study.[24] The greatest difference between our study and the 2013 study was across data‐driven 95th percentile RR parameters, which were an average of 4.8 points lower in our study.
Our validation set consisted of 82,993 vital sign measurements for 2287 unique patients. Application of data‐driven HR and RR 5th and 95th percentile limits resulted in 24,045 (55.6%) fewer out‐of‐range measurements compared to current NIH reference ranges (19,240 vs 43,285). Forty‐five percent fewer HR values and 61% fewer RR values were considered out of range using the proposed data‐driven parameters (see Supporting Information, Appendix 2, in the online version of this article).
Safety
Of the 218 unique out‐of‐ICU RRT and CRA events logged from March 4, 2013 to March 3, 2014, 63 patients were excluded from analysis: 10 lacked identifying information, 33 occurred outside of medical/surgical units, and 20 occurred in patients >18 years of age. The remaining 155 patient charts were reviewed. Seven patients were subsequently excluded because they lacked EHR‐documented vital signs data in the 12 hours prior to RRT or CRA team activation, yielding a cohort of 148 patients (128 RRT events, 20 CRA events).
Table 1 describes the analysis of vital signs in the 12 hours leading up to the 148 RRT and CRA events. All 121 patients with out‐of‐range HR values using NIH reference ranges also had out‐of‐range HR values with the proposed data‐driven parameters; an additional 8 patients had low HR values using the data‐driven parameters. Of the 137 patients with an out‐of‐range RR value using NIH reference ranges, 33 (24.1%) were not considered out of range by the data‐driven parameters. Of these, 28 had high RR and 5 had low RR according to NIH reference ranges.
| No. Patients With HR Out of Range* | No. Patients With RR Out of Range* | No. Patients With HR or RR Out of Range* | |
|---|---|---|---|
| |||
| NIH ranges | 121 | 137 | 144 |
| Data‐driven ranges | 129 | 104 | 138 |
| Difference (causal threshold) | +8 (low HR) | 28 (high RR), 5 (low RR) | +2 (low HR), 8 (high RR) |
After evaluating out‐of‐range HR and RR individually, the 148 RRT and CRA events were analyzed for either out‐of‐range HR values or RR values. In doing so, 144 (97.3%) patients had either HR or RR measurements that were considered out of range using our current NIH reference ranges. One hundred thirty‐eight (93.2%) had either HR or RR measurements that were considered out of range with the proposed parameters. One hundred thirty‐six (94.4%) of the 144 patients with out‐of‐range HR or RR measurements according to NIH reference ranges were also considered out of range using proposed parameters. The data‐driven parameters identified 2 additional patients with low HR who did not have out‐of‐range HR or RR values using the current NIH reference ranges. Manual chart review of the RRT/CRA events in the 8 patients who had normal HR or RR using the data‐driven parameters revealed that RRT or CRA team interventions occurred for clinical indications that did not rely upon HR or RR measurement (eg, laboratory testing abnormalities, desaturation events) (Table 2).
| Indication for event | Patient Age |
|---|---|
| |
| 1. Desaturation and apnea | 10 months |
| 2. Hyperammonemia (abnormal lab result) | 5 years |
| 3. Acute hematemesis | 16 years |
| 4. Lightheadedness, feeling faint | 17 years |
| 5. Desaturation with significant oxygen requirement | 17 years |
| 6. Desaturation with significant oxygen requirement | 17 years |
| 7. Patient stated difficulty breathing | 18 years |
| 8. Difficulty breathing (anaphylactic shock)* | 18 years |
DISCUSSION
This is the first published study to analyze the safety of implementing data‐driven HR and RR parameters in hospitalized children. Based on retrospective analysis of a 12‐month cohort of patients requiring RRT or CRA team activation, our data‐driven HR and RR parameters were at least as safe as the NIH‐published reference ranges employed at our children's hospital. In addition to maintaining sensitivity to RRT and CRA events, the data‐driven parameters resulted in an estimated 55.6% fewer out‐of‐range measurements among medical/surgical pediatric inpatients.
Improper alarm settings are 1 of 4 major contributing factors to reported alarm‐related events,[1] and data‐driven HR and RR parameters provide a means by which to address the Joint Commission Sentinel Event Alert[1] and National Patient Safety Goal[3] regarding alarm management safety for hospitalized pediatric patients. Our results suggest that this evidence‐based approach may reduce the frequency of false alarms (thereby mitigating alarm fatigue), and should be studied prospectively for implementation in the clinical setting.
The selection of percentile values to define the new data‐driven parameter ranges involved various considerations. In an effort to minimize alarm fatigue, we considered using the 1st and 99th percentile values. However, our Medical Executive and Patient Safety Committees determined that the 99th percentile values for HR and RR for many of the age groups exceeded those that would raise clinical concern. A more conservative approach, applying the 5th and 95th percentile values, was deemed clinically appropriate and consistent with recommendations from the only other study to calculate data‐driven HR and RR parameters for hospitalized children.[24]
When taken in total, Bonafide et al.'s 2013 study demonstrated that up to 54% of vital sign values were abnormal according to textbook reference ranges.[24] Similarly, we estimated 55.6% fewer out‐of‐range HR and RR measurements with our data‐driven parameters. Although our 5th and 95th HR percentile and 5th percentile RR values are strikingly similar to those developed in the 2013 study,[24] the difference in 95th percentile RR values between the studies was potentially clinically significant, with our data‐driven upper RR values being 4.8 breaths per minute lower (more conservative) on average. Bonafide et al. transformed the RR values to fit a normal distribution, which might account for this difference. Ultimately, our safety analysis demonstrated that 24% fewer patients were considered out of range for high RR prior to RRT/CRA events with the data‐driven parameters compared to NIH norms. Even fewer RRT/CRA patients would have been considered out of range per Bonafide's less conservative 95% RR limits.
Importantly, all 8 patients in our safety analysis without abnormal vital sign measurements in the 12 hours preceding their clinical events according to the proposed data‐driven parameters (but identified as having high RR per current reference ranges) had RRT or CRA events triggered due to other significant clinical manifestations or vital sign abnormalities (eg, hypoxia). This finding is supported by the literature, which suggests that RRTs are rarely activated due to single vital sign abnormality alone. Prior analysis of RRT activations in our pediatric hospital demonstrated that only approximately 10% of RRTs were activated primarily on the basis of HR or RR vital sign abnormalities (5.6% tachycardia, 2.8% tachypnea, 1.4% bradycardia), whereas 36% were activated due to respiratory distress.[30] The clinical relevance of high RR in isolation is questionable given a recent pediatric study that raised all RR limits and decreased alarm frequency without adverse patient safety consequences.[31] Our results suggest that modifying HR and RR alarm parameters using data‐driven 5th and 95th percentile limits to decrease alarm frequency does not pose additional safety risk related to identification of RRT and CRA events. We encourage continued work toward development of multivariate or smart alarms that analyze multiple simultaneous vital sign measurements and trends to determine whether an alarm should be triggered.[32, 33]
The ability to demonstrate the safety of data‐driven HR and RR parameters is a precursor to hospital‐wide implementation. We believe it is crucial to perform a safety analysis prior to implementation due to the role vital signs play in clinical assessment and detection of patient deterioration.[30, 34, 35, 36, 37] Though a few studies have shown that modification of alarm parameters decreases alarm frequency,[5, 6, 10, 16, 17] to our knowledge no formal safety evaluations have ever been published. This study provides the first published safety evaluation of data‐driven HR and RR parameters.
By decreasing the quantity of out‐of‐range vital sign values while preserving the ability to detect patient deterioration, data‐driven vital sign alarm limits have the potential to decrease false monitor alarms, alarm‐generated noise, and alarm fatigue. Future work includes prospectively studying the impact of adoption of data‐driven vital sign parameters on monitor alarm burden and monitoring the safety of the changes. Additional safety analysis could include comparing the sensitivity and specificity of early warning score systems when data‐driven vital sign ranges are substituted for traditional physiologic parameters. Further personalization of vital sign parameters will involve incorporating patient‐specific characteristics (eg, demographics, diagnoses) into the data‐driven analysis to further decrease alarm burden while enhancing patient safety. Ultimately, using a patient's own physiologic data to define highly personalized vital sign parameter limits represents a truly precision approach, and could revolutionize the way hospitalized patients are monitored.
Numerous relevant issues are not yet addressed in this initial, single‐institution study. First, although the biomedical device integration facilitated the direct import of monitor data into the EHR (decreasing transcription errors), our analysis was performed using EHR‐charted data. As such, the effect on bedside monitor alarms was not directly evaluated in our study, including those due to technical alarms or patient artifact. Second, our overall sample size for the training set was quite large; however, in some cases the number of patients per age category was limited. Third, although we evaluated the identification of severe deterioration leading to RRT or CRA events, the sensitivity of the new limits to the need for other interventions (eg, fluid bolus for dehydration or escalation of respiratory support for asthma exacerbation) or unplanned transfers to the ICU was not assessed. Fourth, the analysis was retrospective, and so the impact of data‐driven alarm limits on length of stay and readmission could not be defined. Fifth, excluding all vital sign measurements from patients who spent any time in the ICU setting decreased the amount of data available for analysis. However, excluding sicker patients probably resulted in narrower data‐driven HR and RR ranges, leading to more conservative proposed parameters that are more likely to identify patient decompensation in our safety analysis. Finally, this was a single‐site study. We believe our data‐driven limits are applicable to other tertiary or quaternary care facilities given the similarity to those generated in a study performed in a comparable setting,[24] but generalizability to other settings may be limited if the local population is sufficiently different. Furthermore, because institutional policies (eg, indications for care escalation) differ, individual institutions should determine whether our analysis is applicable to their setting or if local safety evaluation is necessary.
CONCLUSION
A large proportion of HR and RR values for hospitalized children at our institution are out of range according to current vital sign reference ranges. Our new data‐driven alarm parameters for hospitalized children provide a potentially safe means by which to modify physiologic bedside monitor alarm limits, a first step toward customization of alarm limit settings in an effort to mitigate alarm fatigue.
Acknowledgements
The authors thank Debby Huang and Joshua Glandorf in the Information Services Department at Stanford Children's Health for assistance with data acquisition. No compensation was received for their contributions.
Disclosures: All authors gave approval of the final manuscript version submitted for publication and agreed to be accountable for all aspects of the work. Dr. Veena V. Goel conceptualized and designed the study; collected, managed, analyzed and interpreted the data; prepared and reviewed the initial manuscript; and approved the final manuscript as submitted. Ms. Sarah F. Poole contributed to the design of the study and performed the primary data analysis for the study. Ms. Poole critically revised the manuscript for important intellectual content and approved the final manuscript as submitted. Dr. Goel and Ms. Poole had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Dr. Paul J. Sharek and Dr. Jonathan P. Palma contributed to the study design and data interpretation. Drs. Sharek and Palma critically revised the manuscript for important intellectual content and approved the final manuscript as submitted. Dr. Terry S. Platchek, Dr. Natalie M. Pageler, and Dr. Christopher A. Longhurst contributed to the study design. Drs. Platchek, Pageler, and Longhurst critically revised the manuscript for important intellectual content and approved the final manuscript as submitted. Ms. Poole is supported by the Stanford Biosciences Graduate Program through a Fulbright New Zealand Science and Innovation Graduate Award and through the J.R. Templin Trust Scholarship. The authors report no conflicts of interest.
- The Joint Commission. Medical device alarm safety in hospitals. Sentinel Event Alert. 2013;(50):1–3. Available at: https://www.jointcommission.org/sea_issue_50/. Accessed October 12, 2013.
- . “Alarm fatigue” a factor in 2d death: UMass hospital cited for violations. The Boston Globe. September 21, 2011. Available at: https://www.bostonglobe.com/2011/09/20/umass/qSOhm8dYmmaq4uTHZb7FNM/story.html. Accessed December 19, 2014
- The Joint Commission. Alarm system safety. Available at: https://www.jointcommission.org/assets/1/18/R3_Report_Issue_5_12_2_13_Final.pdf. Published December 11, 2013. Accessed October 12, 2013.
- , , , , . ALARMED: adverse events in low‐risk patients with chest pain receiving continuous electrocardiographic monitoring in the emergency department. A pilot study. Am J Emerg Med. 2006;24(1):62–67.
- , . Monitor alarm fatigue: standardizing use of physiological monitors and decreasing nuisance alarms. Am J Crit Care. 2010;19(1):28–34; quiz 35.
- , , . Physiologic monitoring alarm load on medical/surgical floors of a community hospital. Biomed Instrum Technol. 2011;(suppl):29–36.
- , , , , , . Multicentric study of monitoring alarms in the adult intensive care unit (ICU): a descriptive analysis. Intensive Care Med. 1999;25(12):1360–1366.
- . Crying wolf: false alarms in a pediatric intensive care unit. Crit Care Med. 1994;22(6):981–985.
- , , , et al. Cardiopulmonary monitors and clinically significant events in critically ill children. Biomed Instrum Technol. 2011;(suppl):38–45.
- , , , et al. Systematic review of physiologic monitor alarm characteristics and pragmatic interventions to reduce alarm frequency. J Hosp Med. 2016;11(2):136–144.
- . Alarm fatigue. Nurs Clin North Am. 2012;47(3):375–382.
- . Monitor alarm fatigue: an integrative review. Biomed Instrum Technol. 2012;46(4):268–277.
- , , , . Use of pagers with an alarm escalation system to reduce cardiac monitor alarm signals. J Nurs Care Qual. 2014;29(1):9–18.
- . An evidence‐based approach to reduce nuisance alarms and alarm fatigue. Biomed Instrum Technol. 2011;(suppl):46–52.
- , , , et al. Insights into the problem of alarm fatigue with physiologic monitor devices: a comprehensive observational study of consecutive intensive care unit patients. PLoS One. 2014;9(10):e110274.
- , , , , , . Effect of altering alarm settings: a randomized controlled study. Biomed Instrum Technol. 2015;49(3):214–222.
- , , , , . Alarm limit settings for early warning systems to identify at‐risk patients. J Adv Nurs. 2009;65(9):1844–1852.
- , . A framework for reducing alarm fatigue on pediatric inpatient units. Hosp Pediatr. 2015;5(3):160–163.
- , , , et al. Association between exposure to nonactionable physiologic monitor alarms and response time in a children's hospital. J Hosp Med. 2015;10(6):345–351.
- The Johns Hopkins Hospital, , . The Harriet Lane Handbook. 20th ed. Philadelphia, PA: Elsevier Saunders; 2014.
- , . Nelson Textbook of Pediatrics. 19th ed. Philadelphia, PA.: Elsevier Saunders; 2011.
- , , , . Pediatric assessment. In: Pediatric Advanced Life Support: Provider Manual. Dallas, TX: American Heart Association; 2006:9–16.
- , , , et al. Normal ranges of heart rate and respiratory rate in children from birth to 18 years of age: a systematic review of observational studies. Lancet. 2011;377(9770):1011–1018.
- , , , , , . Development of heart and respiratory rate percentile curves for hospitalized children. Pediatrics. 2013;131(4):e1150–e1157.
- National Institutes of Health. Age‐appropriate vital signs. Available at: https://web.archive.org/web/20041101222327/http://www.cc.nih.gov/ccc/pedweb/pedsstaff/age.html. Accessed July 26, 2015.
- Guidelines 2000 for cardiopulmonary resuscitation and emergency cardiovascular care. Part 9: pediatric basic life support. The American Heart Association in collaboration with the International Liaison Committee on Resuscitation. Circulation. 2000;102(8 suppl):I253–I290.
- , , , , , . Recognising clinical instability in hospital patients before cardiac arrest or unplanned admission to intensive care. A pilot study in a tertiary‐care hospital. Med J Aust. 1999;171(1):22–25.
- , , , et al. Duration of life‐threatening antecedents prior to intensive care admission. Intensive Care Med. 2002;28(11):1629–1634.
- , . Pediatric cardiopulmonary resuscitation: a collective review. Ann Emerg Med. 1999;33(2):195–205.
- , , , et al. Effect of a rapid response team on hospital‐wide mortality and code rates outside the ICU in a Children's Hospital. JAMA. 2007;298(19):2267–2274.
- , , , et al. A team‐based approach to reducing cardiac monitor alarms. Pediatrics. 2014;134(6):e1686–e1694.
- , , , et al. Collection of annotated data in a clinical validation study for alarm algorithms in intensive care—a methodologic framework. J Crit Care. 2010;25(1):128–135.
- , , . Making ICU alarms meaningful: a comparison of traditional vs. trend‐based algorithms. Proc AMIA Symp. 1999:379–383.
- , , , et al. Implementation of a medical emergency team in a large pediatric teaching hospital prevents respiratory and cardiopulmonary arrests outside the intensive care unit. Pediatr Crit Care Med. 2007;8(3):236–246; quiz 247.
- . Centile‐based Early Warning Scores derived from statistical distributions of vital signs. Resuscitation. 2011;82(8):969–970.
- , , , , , . Centile‐based early warning scores derived from statistical distributions of vital signs. Resuscitation. 2011;82(8):1013–1018.
- , , , , . Reduction of paediatric in‐patient cardiac arrest and death with a medical emergency team: preliminary results. Arch Dis Child. 2005;90(11):1148–1152.
The management of alarms in the hospital setting is a significant patient safety issue. In 2013, the Joint Commission issued Sentinel Event Alert #50 to draw attention to the fact that tens of thousands of alarms occur daily throughout individual hospitals, and 85% to 99% are false or not clinically actionable.[1] These alarms, designed to be a safety net in patient care, have the unintended consequence of causing provider desensitization, also known as alarm fatigue, which contributes to adverse events as severe as patient mortality.[1, 2] For this reason, a 2014 Joint Commission National Patient Safety Goal urged hospitals to prioritize alarm system safety and to develop policies and procedures to manage alarms and alarm fatigue.[3]
Multiple efforts have been made to address alarm fatigue in hospitalized adults. Studies have quantified the frequency and types of medical device alarms,[4, 5, 6, 7, 8, 9] and some proposed solutions to decrease excess alarms.[10, 11, 12, 13, 14, 15] One such solution is to change alarm limit settings, an intervention shown to be efficacious in the literature.[5, 6, 16, 17] Although no adverse patient outcomes are reported in these studies, none of them included a formal safety evaluation to evaluate whether alarm rate reduction occurred at the expense of clinically significant alarms.
Specific to pediatrics, frameworks to address alarm fatigue have been proposed,[18] and the relationship between nurse response time and frequency of exposure to nonactionable alarms has been reported.[19] However, efforts to address alarm fatigue in the pediatric setting are less well studied overall, and there is little guidance regarding optimization of pediatric alarm parameters. Although multiple established reference ranges exist for pediatric vital signs,[20, 21, 22] a systematic review in 2011 found that only 2 of 5 published heart rate (HR) and 6 respiratory rate (RR) guidelines cited any references, and even these had weak underpinning evidence.[23] Consequently, ranges defining normal pediatric vital signs are derived either from small sample observational data in healthy outpatient children or consensus opinion. In a 2013 study by Bonafide et al.,[24] charted vital sign data from hospitalized children were used to develop percentile curves for HR and RR, and from these it was estimated that 54% of vital sign measurements in hospitalized children are out of range using currently accepted normal vital sign parameters.[24] Although these calculated vital sign parameters were not implemented clinically, they called into question reference ranges that are currently widely accepted and used as parameters for electronic health record (EHR) alerts, early warning scoring systems, and physiologic monitor alarms.
With the goal of safely decreasing the number of out‐of‐range vital sign measurements that result from current, often nonevidence‐based pediatric vital sign reference ranges, we used data from noncritically ill pediatric inpatients to derive HR and RR percentile charts for hospitalized children. In anticipation of local implementation of these data‐driven vital sign ranges as physiologic monitor parameters, we performed a retrospective safety analysis by evaluating the effect of data‐driven alarm limit modification on identification of cardiorespiratory arrests (CRA) and rapid response team (RRT) activations.
METHODS
We performed a cross‐sectional study of children less than 18 years of age hospitalized on general medical and surgical units at Lucile Packard Children's Hospital Stanford, a 311‐bed quaternary‐care academic hospital with a full complement of pediatric medical and surgical subspecialties and transplant programs. During the study period, the hospital used the Cerner EHR (Millennium; Cerner, Kansas City, MO) and Philips IntelliVue bedside monitors (Koninklijke Philips N.V., Amsterdam, the Netherlands). The Stanford University Institutional Review Board approved this study.
Establishing Data‐Driven HR and RR Parameters
Vital sign documentation in the EHR at our institution is performed primarily by nurses and facilitated by bedside monitor biomedical device integration. We extracted vital signs data from the institution's EHR for all general medical and surgical patients discharged between January 1, 2013 and May 3, 2014. To be most conservative in the definition of normal vital sign ranges for pediatric inpatients, we excluded critically ill children (those who spent any part of their hospitalization in an intensive care unit [ICU]). Physiologically implausible vital sign values were excluded as per the methods of Bonafide et al.[24] The data were separated into 2 different sets: a training set (patients discharged between January 1, 2013 and December 31, 2013) and a test set for validation (patients discharged between January 1, 2014 and May 3, 2014). To avoid oversampling from both particular time periods and individual patients in the training set, we randomly selected 1 HR and RR pair from each 4‐hour interval during a hospitalization, and then randomly sampled a maximum of 10 HR and RR pairs per patient. Using these vital sign measurements, we calculated age‐stratified 1st, 5th, 10th, 50th, 90th, 95th, and 99th percentiles for both HR and RR.
Based on a combination of expert opinion and local consensus from our Medical Executive and Patient Safety Committees, we selected the 5th and 95th percentile values as proposed data‐driven parameter limits and compared them to the 5th and 95th percentile values generated in the 2013 study[24] and to the 2004 National Institutes of Health (NIH)adapted vital sign reference ranges currently used at our hospital.[25] Using 1 randomly selected HR and RR pair from every 4‐hour interval in the validation set, we compared the proportion of out‐of‐range HR and RR observations with the proposed 5th and 95th percentile data‐driven parameters versus the current NIH reference ranges. We also calculated average differences between our data‐driven 5th and 95th percentile values and the calculated HR and RR values in the 2013 study.[24]
Safety Analysis
To assess the safety of the newly created 5th and 95th percentile HR and RR parameters prior to clinical adoption, we retrospectively reviewed data associated with all RRT and CRA events on the hospital's medical/surgical units from March 4, 2013 until March 3, 2014. The RRT/CRA event data were obtained from logs kept by the hospital's code committee. We excluded events that lacked a documented patient identifier, occurred in locations other than the acute medical/surgical units, or occurred in patients >18 years old. The resulting charts were manually reviewed to determine the date and time of RRT or CRA event activation. Because evidence exists that hospitalized pediatric patients with CRA show signs of vital sign decompensation as early as 12 hours prior to the event,[26, 27, 28, 29] we extracted all EHR‐charted HR and RR data in the 12 hours preceding RRT and CRA events from the institution's clinical data warehouse for analysis, excluding patients without charted vital sign data in this time period. The sets of patients with any out‐of‐range HR or RR measurements in the 12‐hours prior to an event were compared according to the current NIH reference ranges[25] versus data‐driven parameters. Additionally, manual chart review was performed to assess the reason for code or RRT activation, and to determine the role that out‐of‐range vital signs played in alerting clinical staff of patient decompensation.
Statistical Analysis
All analysis was performed using R statistical package software (version 0.98.1062 for Mac OS X 10_9_5; The R Foundation for Statistical Computing, Vienna, Austria) with an SQL database (MySQL 2015; Oracle Corp., Redwood City, CA).
RESULTS
Data‐Driven HR and RR Parameters
We established a training set of 62,508 vital sign measurements for 7202 unique patients to calculate 1st, 5th, 10th, 50th, 90th, 95th, and 99th percentiles for HR and RR among the 14 age groups (see Supporting Information, Appendix 1, in the online version of this article). Figures 1 and 2 compare the proposed data‐driven vital sign ranges with (1) our current HR and RR reference ranges and (2) the 5th and 95th percentile values created in the similar 2013 study.[24] The greatest difference between our study and the 2013 study was across data‐driven 95th percentile RR parameters, which were an average of 4.8 points lower in our study.


Our validation set consisted of 82,993 vital sign measurements for 2287 unique patients. Application of data‐driven HR and RR 5th and 95th percentile limits resulted in 24,045 (55.6%) fewer out‐of‐range measurements compared to current NIH reference ranges (19,240 vs 43,285). Forty‐five percent fewer HR values and 61% fewer RR values were considered out of range using the proposed data‐driven parameters (see Supporting Information, Appendix 2, in the online version of this article).
Safety
Of the 218 unique out‐of‐ICU RRT and CRA events logged from March 4, 2013 to March 3, 2014, 63 patients were excluded from analysis: 10 lacked identifying information, 33 occurred outside of medical/surgical units, and 20 occurred in patients >18 years of age. The remaining 155 patient charts were reviewed. Seven patients were subsequently excluded because they lacked EHR‐documented vital signs data in the 12 hours prior to RRT or CRA team activation, yielding a cohort of 148 patients (128 RRT events, 20 CRA events).
Table 1 describes the analysis of vital signs in the 12 hours leading up to the 148 RRT and CRA events. All 121 patients with out‐of‐range HR values using NIH reference ranges also had out‐of‐range HR values with the proposed data‐driven parameters; an additional 8 patients had low HR values using the data‐driven parameters. Of the 137 patients with an out‐of‐range RR value using NIH reference ranges, 33 (24.1%) were not considered out of range by the data‐driven parameters. Of these, 28 had high RR and 5 had low RR according to NIH reference ranges.
| No. Patients With HR Out of Range* | No. Patients With RR Out of Range* | No. Patients With HR or RR Out of Range* | |
|---|---|---|---|
| |||
| NIH ranges | 121 | 137 | 144 |
| Data‐driven ranges | 129 | 104 | 138 |
| Difference (causal threshold) | +8 (low HR) | 28 (high RR), 5 (low RR) | +2 (low HR), 8 (high RR) |
After evaluating out‐of‐range HR and RR individually, the 148 RRT and CRA events were analyzed for either out‐of‐range HR values or RR values. In doing so, 144 (97.3%) patients had either HR or RR measurements that were considered out of range using our current NIH reference ranges. One hundred thirty‐eight (93.2%) had either HR or RR measurements that were considered out of range with the proposed parameters. One hundred thirty‐six (94.4%) of the 144 patients with out‐of‐range HR or RR measurements according to NIH reference ranges were also considered out of range using proposed parameters. The data‐driven parameters identified 2 additional patients with low HR who did not have out‐of‐range HR or RR values using the current NIH reference ranges. Manual chart review of the RRT/CRA events in the 8 patients who had normal HR or RR using the data‐driven parameters revealed that RRT or CRA team interventions occurred for clinical indications that did not rely upon HR or RR measurement (eg, laboratory testing abnormalities, desaturation events) (Table 2).
| Indication for event | Patient Age |
|---|---|
| |
| 1. Desaturation and apnea | 10 months |
| 2. Hyperammonemia (abnormal lab result) | 5 years |
| 3. Acute hematemesis | 16 years |
| 4. Lightheadedness, feeling faint | 17 years |
| 5. Desaturation with significant oxygen requirement | 17 years |
| 6. Desaturation with significant oxygen requirement | 17 years |
| 7. Patient stated difficulty breathing | 18 years |
| 8. Difficulty breathing (anaphylactic shock)* | 18 years |
DISCUSSION
This is the first published study to analyze the safety of implementing data‐driven HR and RR parameters in hospitalized children. Based on retrospective analysis of a 12‐month cohort of patients requiring RRT or CRA team activation, our data‐driven HR and RR parameters were at least as safe as the NIH‐published reference ranges employed at our children's hospital. In addition to maintaining sensitivity to RRT and CRA events, the data‐driven parameters resulted in an estimated 55.6% fewer out‐of‐range measurements among medical/surgical pediatric inpatients.
Improper alarm settings are 1 of 4 major contributing factors to reported alarm‐related events,[1] and data‐driven HR and RR parameters provide a means by which to address the Joint Commission Sentinel Event Alert[1] and National Patient Safety Goal[3] regarding alarm management safety for hospitalized pediatric patients. Our results suggest that this evidence‐based approach may reduce the frequency of false alarms (thereby mitigating alarm fatigue), and should be studied prospectively for implementation in the clinical setting.
The selection of percentile values to define the new data‐driven parameter ranges involved various considerations. In an effort to minimize alarm fatigue, we considered using the 1st and 99th percentile values. However, our Medical Executive and Patient Safety Committees determined that the 99th percentile values for HR and RR for many of the age groups exceeded those that would raise clinical concern. A more conservative approach, applying the 5th and 95th percentile values, was deemed clinically appropriate and consistent with recommendations from the only other study to calculate data‐driven HR and RR parameters for hospitalized children.[24]
When taken in total, Bonafide et al.'s 2013 study demonstrated that up to 54% of vital sign values were abnormal according to textbook reference ranges.[24] Similarly, we estimated 55.6% fewer out‐of‐range HR and RR measurements with our data‐driven parameters. Although our 5th and 95th HR percentile and 5th percentile RR values are strikingly similar to those developed in the 2013 study,[24] the difference in 95th percentile RR values between the studies was potentially clinically significant, with our data‐driven upper RR values being 4.8 breaths per minute lower (more conservative) on average. Bonafide et al. transformed the RR values to fit a normal distribution, which might account for this difference. Ultimately, our safety analysis demonstrated that 24% fewer patients were considered out of range for high RR prior to RRT/CRA events with the data‐driven parameters compared to NIH norms. Even fewer RRT/CRA patients would have been considered out of range per Bonafide's less conservative 95% RR limits.
Importantly, all 8 patients in our safety analysis without abnormal vital sign measurements in the 12 hours preceding their clinical events according to the proposed data‐driven parameters (but identified as having high RR per current reference ranges) had RRT or CRA events triggered due to other significant clinical manifestations or vital sign abnormalities (eg, hypoxia). This finding is supported by the literature, which suggests that RRTs are rarely activated due to single vital sign abnormality alone. Prior analysis of RRT activations in our pediatric hospital demonstrated that only approximately 10% of RRTs were activated primarily on the basis of HR or RR vital sign abnormalities (5.6% tachycardia, 2.8% tachypnea, 1.4% bradycardia), whereas 36% were activated due to respiratory distress.[30] The clinical relevance of high RR in isolation is questionable given a recent pediatric study that raised all RR limits and decreased alarm frequency without adverse patient safety consequences.[31] Our results suggest that modifying HR and RR alarm parameters using data‐driven 5th and 95th percentile limits to decrease alarm frequency does not pose additional safety risk related to identification of RRT and CRA events. We encourage continued work toward development of multivariate or smart alarms that analyze multiple simultaneous vital sign measurements and trends to determine whether an alarm should be triggered.[32, 33]
The ability to demonstrate the safety of data‐driven HR and RR parameters is a precursor to hospital‐wide implementation. We believe it is crucial to perform a safety analysis prior to implementation due to the role vital signs play in clinical assessment and detection of patient deterioration.[30, 34, 35, 36, 37] Though a few studies have shown that modification of alarm parameters decreases alarm frequency,[5, 6, 10, 16, 17] to our knowledge no formal safety evaluations have ever been published. This study provides the first published safety evaluation of data‐driven HR and RR parameters.
By decreasing the quantity of out‐of‐range vital sign values while preserving the ability to detect patient deterioration, data‐driven vital sign alarm limits have the potential to decrease false monitor alarms, alarm‐generated noise, and alarm fatigue. Future work includes prospectively studying the impact of adoption of data‐driven vital sign parameters on monitor alarm burden and monitoring the safety of the changes. Additional safety analysis could include comparing the sensitivity and specificity of early warning score systems when data‐driven vital sign ranges are substituted for traditional physiologic parameters. Further personalization of vital sign parameters will involve incorporating patient‐specific characteristics (eg, demographics, diagnoses) into the data‐driven analysis to further decrease alarm burden while enhancing patient safety. Ultimately, using a patient's own physiologic data to define highly personalized vital sign parameter limits represents a truly precision approach, and could revolutionize the way hospitalized patients are monitored.
Numerous relevant issues are not yet addressed in this initial, single‐institution study. First, although the biomedical device integration facilitated the direct import of monitor data into the EHR (decreasing transcription errors), our analysis was performed using EHR‐charted data. As such, the effect on bedside monitor alarms was not directly evaluated in our study, including those due to technical alarms or patient artifact. Second, our overall sample size for the training set was quite large; however, in some cases the number of patients per age category was limited. Third, although we evaluated the identification of severe deterioration leading to RRT or CRA events, the sensitivity of the new limits to the need for other interventions (eg, fluid bolus for dehydration or escalation of respiratory support for asthma exacerbation) or unplanned transfers to the ICU was not assessed. Fourth, the analysis was retrospective, and so the impact of data‐driven alarm limits on length of stay and readmission could not be defined. Fifth, excluding all vital sign measurements from patients who spent any time in the ICU setting decreased the amount of data available for analysis. However, excluding sicker patients probably resulted in narrower data‐driven HR and RR ranges, leading to more conservative proposed parameters that are more likely to identify patient decompensation in our safety analysis. Finally, this was a single‐site study. We believe our data‐driven limits are applicable to other tertiary or quaternary care facilities given the similarity to those generated in a study performed in a comparable setting,[24] but generalizability to other settings may be limited if the local population is sufficiently different. Furthermore, because institutional policies (eg, indications for care escalation) differ, individual institutions should determine whether our analysis is applicable to their setting or if local safety evaluation is necessary.
CONCLUSION
A large proportion of HR and RR values for hospitalized children at our institution are out of range according to current vital sign reference ranges. Our new data‐driven alarm parameters for hospitalized children provide a potentially safe means by which to modify physiologic bedside monitor alarm limits, a first step toward customization of alarm limit settings in an effort to mitigate alarm fatigue.
Acknowledgements
The authors thank Debby Huang and Joshua Glandorf in the Information Services Department at Stanford Children's Health for assistance with data acquisition. No compensation was received for their contributions.
Disclosures: All authors gave approval of the final manuscript version submitted for publication and agreed to be accountable for all aspects of the work. Dr. Veena V. Goel conceptualized and designed the study; collected, managed, analyzed and interpreted the data; prepared and reviewed the initial manuscript; and approved the final manuscript as submitted. Ms. Sarah F. Poole contributed to the design of the study and performed the primary data analysis for the study. Ms. Poole critically revised the manuscript for important intellectual content and approved the final manuscript as submitted. Dr. Goel and Ms. Poole had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Dr. Paul J. Sharek and Dr. Jonathan P. Palma contributed to the study design and data interpretation. Drs. Sharek and Palma critically revised the manuscript for important intellectual content and approved the final manuscript as submitted. Dr. Terry S. Platchek, Dr. Natalie M. Pageler, and Dr. Christopher A. Longhurst contributed to the study design. Drs. Platchek, Pageler, and Longhurst critically revised the manuscript for important intellectual content and approved the final manuscript as submitted. Ms. Poole is supported by the Stanford Biosciences Graduate Program through a Fulbright New Zealand Science and Innovation Graduate Award and through the J.R. Templin Trust Scholarship. The authors report no conflicts of interest.
The management of alarms in the hospital setting is a significant patient safety issue. In 2013, the Joint Commission issued Sentinel Event Alert #50 to draw attention to the fact that tens of thousands of alarms occur daily throughout individual hospitals, and 85% to 99% are false or not clinically actionable.[1] These alarms, designed to be a safety net in patient care, have the unintended consequence of causing provider desensitization, also known as alarm fatigue, which contributes to adverse events as severe as patient mortality.[1, 2] For this reason, a 2014 Joint Commission National Patient Safety Goal urged hospitals to prioritize alarm system safety and to develop policies and procedures to manage alarms and alarm fatigue.[3]
Multiple efforts have been made to address alarm fatigue in hospitalized adults. Studies have quantified the frequency and types of medical device alarms,[4, 5, 6, 7, 8, 9] and some proposed solutions to decrease excess alarms.[10, 11, 12, 13, 14, 15] One such solution is to change alarm limit settings, an intervention shown to be efficacious in the literature.[5, 6, 16, 17] Although no adverse patient outcomes are reported in these studies, none of them included a formal safety evaluation to evaluate whether alarm rate reduction occurred at the expense of clinically significant alarms.
Specific to pediatrics, frameworks to address alarm fatigue have been proposed,[18] and the relationship between nurse response time and frequency of exposure to nonactionable alarms has been reported.[19] However, efforts to address alarm fatigue in the pediatric setting are less well studied overall, and there is little guidance regarding optimization of pediatric alarm parameters. Although multiple established reference ranges exist for pediatric vital signs,[20, 21, 22] a systematic review in 2011 found that only 2 of 5 published heart rate (HR) and 6 respiratory rate (RR) guidelines cited any references, and even these had weak underpinning evidence.[23] Consequently, ranges defining normal pediatric vital signs are derived either from small sample observational data in healthy outpatient children or consensus opinion. In a 2013 study by Bonafide et al.,[24] charted vital sign data from hospitalized children were used to develop percentile curves for HR and RR, and from these it was estimated that 54% of vital sign measurements in hospitalized children are out of range using currently accepted normal vital sign parameters.[24] Although these calculated vital sign parameters were not implemented clinically, they called into question reference ranges that are currently widely accepted and used as parameters for electronic health record (EHR) alerts, early warning scoring systems, and physiologic monitor alarms.
With the goal of safely decreasing the number of out‐of‐range vital sign measurements that result from current, often nonevidence‐based pediatric vital sign reference ranges, we used data from noncritically ill pediatric inpatients to derive HR and RR percentile charts for hospitalized children. In anticipation of local implementation of these data‐driven vital sign ranges as physiologic monitor parameters, we performed a retrospective safety analysis by evaluating the effect of data‐driven alarm limit modification on identification of cardiorespiratory arrests (CRA) and rapid response team (RRT) activations.
METHODS
We performed a cross‐sectional study of children less than 18 years of age hospitalized on general medical and surgical units at Lucile Packard Children's Hospital Stanford, a 311‐bed quaternary‐care academic hospital with a full complement of pediatric medical and surgical subspecialties and transplant programs. During the study period, the hospital used the Cerner EHR (Millennium; Cerner, Kansas City, MO) and Philips IntelliVue bedside monitors (Koninklijke Philips N.V., Amsterdam, the Netherlands). The Stanford University Institutional Review Board approved this study.
Establishing Data‐Driven HR and RR Parameters
Vital sign documentation in the EHR at our institution is performed primarily by nurses and facilitated by bedside monitor biomedical device integration. We extracted vital signs data from the institution's EHR for all general medical and surgical patients discharged between January 1, 2013 and May 3, 2014. To be most conservative in the definition of normal vital sign ranges for pediatric inpatients, we excluded critically ill children (those who spent any part of their hospitalization in an intensive care unit [ICU]). Physiologically implausible vital sign values were excluded as per the methods of Bonafide et al.[24] The data were separated into 2 different sets: a training set (patients discharged between January 1, 2013 and December 31, 2013) and a test set for validation (patients discharged between January 1, 2014 and May 3, 2014). To avoid oversampling from both particular time periods and individual patients in the training set, we randomly selected 1 HR and RR pair from each 4‐hour interval during a hospitalization, and then randomly sampled a maximum of 10 HR and RR pairs per patient. Using these vital sign measurements, we calculated age‐stratified 1st, 5th, 10th, 50th, 90th, 95th, and 99th percentiles for both HR and RR.
Based on a combination of expert opinion and local consensus from our Medical Executive and Patient Safety Committees, we selected the 5th and 95th percentile values as proposed data‐driven parameter limits and compared them to the 5th and 95th percentile values generated in the 2013 study[24] and to the 2004 National Institutes of Health (NIH)adapted vital sign reference ranges currently used at our hospital.[25] Using 1 randomly selected HR and RR pair from every 4‐hour interval in the validation set, we compared the proportion of out‐of‐range HR and RR observations with the proposed 5th and 95th percentile data‐driven parameters versus the current NIH reference ranges. We also calculated average differences between our data‐driven 5th and 95th percentile values and the calculated HR and RR values in the 2013 study.[24]
Safety Analysis
To assess the safety of the newly created 5th and 95th percentile HR and RR parameters prior to clinical adoption, we retrospectively reviewed data associated with all RRT and CRA events on the hospital's medical/surgical units from March 4, 2013 until March 3, 2014. The RRT/CRA event data were obtained from logs kept by the hospital's code committee. We excluded events that lacked a documented patient identifier, occurred in locations other than the acute medical/surgical units, or occurred in patients >18 years old. The resulting charts were manually reviewed to determine the date and time of RRT or CRA event activation. Because evidence exists that hospitalized pediatric patients with CRA show signs of vital sign decompensation as early as 12 hours prior to the event,[26, 27, 28, 29] we extracted all EHR‐charted HR and RR data in the 12 hours preceding RRT and CRA events from the institution's clinical data warehouse for analysis, excluding patients without charted vital sign data in this time period. The sets of patients with any out‐of‐range HR or RR measurements in the 12‐hours prior to an event were compared according to the current NIH reference ranges[25] versus data‐driven parameters. Additionally, manual chart review was performed to assess the reason for code or RRT activation, and to determine the role that out‐of‐range vital signs played in alerting clinical staff of patient decompensation.
Statistical Analysis
All analysis was performed using R statistical package software (version 0.98.1062 for Mac OS X 10_9_5; The R Foundation for Statistical Computing, Vienna, Austria) with an SQL database (MySQL 2015; Oracle Corp., Redwood City, CA).
RESULTS
Data‐Driven HR and RR Parameters
We established a training set of 62,508 vital sign measurements for 7202 unique patients to calculate 1st, 5th, 10th, 50th, 90th, 95th, and 99th percentiles for HR and RR among the 14 age groups (see Supporting Information, Appendix 1, in the online version of this article). Figures 1 and 2 compare the proposed data‐driven vital sign ranges with (1) our current HR and RR reference ranges and (2) the 5th and 95th percentile values created in the similar 2013 study.[24] The greatest difference between our study and the 2013 study was across data‐driven 95th percentile RR parameters, which were an average of 4.8 points lower in our study.


Our validation set consisted of 82,993 vital sign measurements for 2287 unique patients. Application of data‐driven HR and RR 5th and 95th percentile limits resulted in 24,045 (55.6%) fewer out‐of‐range measurements compared to current NIH reference ranges (19,240 vs 43,285). Forty‐five percent fewer HR values and 61% fewer RR values were considered out of range using the proposed data‐driven parameters (see Supporting Information, Appendix 2, in the online version of this article).
Safety
Of the 218 unique out‐of‐ICU RRT and CRA events logged from March 4, 2013 to March 3, 2014, 63 patients were excluded from analysis: 10 lacked identifying information, 33 occurred outside of medical/surgical units, and 20 occurred in patients >18 years of age. The remaining 155 patient charts were reviewed. Seven patients were subsequently excluded because they lacked EHR‐documented vital signs data in the 12 hours prior to RRT or CRA team activation, yielding a cohort of 148 patients (128 RRT events, 20 CRA events).
Table 1 describes the analysis of vital signs in the 12 hours leading up to the 148 RRT and CRA events. All 121 patients with out‐of‐range HR values using NIH reference ranges also had out‐of‐range HR values with the proposed data‐driven parameters; an additional 8 patients had low HR values using the data‐driven parameters. Of the 137 patients with an out‐of‐range RR value using NIH reference ranges, 33 (24.1%) were not considered out of range by the data‐driven parameters. Of these, 28 had high RR and 5 had low RR according to NIH reference ranges.
| No. Patients With HR Out of Range* | No. Patients With RR Out of Range* | No. Patients With HR or RR Out of Range* | |
|---|---|---|---|
| |||
| NIH ranges | 121 | 137 | 144 |
| Data‐driven ranges | 129 | 104 | 138 |
| Difference (causal threshold) | +8 (low HR) | 28 (high RR), 5 (low RR) | +2 (low HR), 8 (high RR) |
After evaluating out‐of‐range HR and RR individually, the 148 RRT and CRA events were analyzed for either out‐of‐range HR values or RR values. In doing so, 144 (97.3%) patients had either HR or RR measurements that were considered out of range using our current NIH reference ranges. One hundred thirty‐eight (93.2%) had either HR or RR measurements that were considered out of range with the proposed parameters. One hundred thirty‐six (94.4%) of the 144 patients with out‐of‐range HR or RR measurements according to NIH reference ranges were also considered out of range using proposed parameters. The data‐driven parameters identified 2 additional patients with low HR who did not have out‐of‐range HR or RR values using the current NIH reference ranges. Manual chart review of the RRT/CRA events in the 8 patients who had normal HR or RR using the data‐driven parameters revealed that RRT or CRA team interventions occurred for clinical indications that did not rely upon HR or RR measurement (eg, laboratory testing abnormalities, desaturation events) (Table 2).
| Indication for event | Patient Age |
|---|---|
| |
| 1. Desaturation and apnea | 10 months |
| 2. Hyperammonemia (abnormal lab result) | 5 years |
| 3. Acute hematemesis | 16 years |
| 4. Lightheadedness, feeling faint | 17 years |
| 5. Desaturation with significant oxygen requirement | 17 years |
| 6. Desaturation with significant oxygen requirement | 17 years |
| 7. Patient stated difficulty breathing | 18 years |
| 8. Difficulty breathing (anaphylactic shock)* | 18 years |
DISCUSSION
This is the first published study to analyze the safety of implementing data‐driven HR and RR parameters in hospitalized children. Based on retrospective analysis of a 12‐month cohort of patients requiring RRT or CRA team activation, our data‐driven HR and RR parameters were at least as safe as the NIH‐published reference ranges employed at our children's hospital. In addition to maintaining sensitivity to RRT and CRA events, the data‐driven parameters resulted in an estimated 55.6% fewer out‐of‐range measurements among medical/surgical pediatric inpatients.
Improper alarm settings are 1 of 4 major contributing factors to reported alarm‐related events,[1] and data‐driven HR and RR parameters provide a means by which to address the Joint Commission Sentinel Event Alert[1] and National Patient Safety Goal[3] regarding alarm management safety for hospitalized pediatric patients. Our results suggest that this evidence‐based approach may reduce the frequency of false alarms (thereby mitigating alarm fatigue), and should be studied prospectively for implementation in the clinical setting.
The selection of percentile values to define the new data‐driven parameter ranges involved various considerations. In an effort to minimize alarm fatigue, we considered using the 1st and 99th percentile values. However, our Medical Executive and Patient Safety Committees determined that the 99th percentile values for HR and RR for many of the age groups exceeded those that would raise clinical concern. A more conservative approach, applying the 5th and 95th percentile values, was deemed clinically appropriate and consistent with recommendations from the only other study to calculate data‐driven HR and RR parameters for hospitalized children.[24]
When taken in total, Bonafide et al.'s 2013 study demonstrated that up to 54% of vital sign values were abnormal according to textbook reference ranges.[24] Similarly, we estimated 55.6% fewer out‐of‐range HR and RR measurements with our data‐driven parameters. Although our 5th and 95th HR percentile and 5th percentile RR values are strikingly similar to those developed in the 2013 study,[24] the difference in 95th percentile RR values between the studies was potentially clinically significant, with our data‐driven upper RR values being 4.8 breaths per minute lower (more conservative) on average. Bonafide et al. transformed the RR values to fit a normal distribution, which might account for this difference. Ultimately, our safety analysis demonstrated that 24% fewer patients were considered out of range for high RR prior to RRT/CRA events with the data‐driven parameters compared to NIH norms. Even fewer RRT/CRA patients would have been considered out of range per Bonafide's less conservative 95% RR limits.
Importantly, all 8 patients in our safety analysis without abnormal vital sign measurements in the 12 hours preceding their clinical events according to the proposed data‐driven parameters (but identified as having high RR per current reference ranges) had RRT or CRA events triggered due to other significant clinical manifestations or vital sign abnormalities (eg, hypoxia). This finding is supported by the literature, which suggests that RRTs are rarely activated due to single vital sign abnormality alone. Prior analysis of RRT activations in our pediatric hospital demonstrated that only approximately 10% of RRTs were activated primarily on the basis of HR or RR vital sign abnormalities (5.6% tachycardia, 2.8% tachypnea, 1.4% bradycardia), whereas 36% were activated due to respiratory distress.[30] The clinical relevance of high RR in isolation is questionable given a recent pediatric study that raised all RR limits and decreased alarm frequency without adverse patient safety consequences.[31] Our results suggest that modifying HR and RR alarm parameters using data‐driven 5th and 95th percentile limits to decrease alarm frequency does not pose additional safety risk related to identification of RRT and CRA events. We encourage continued work toward development of multivariate or smart alarms that analyze multiple simultaneous vital sign measurements and trends to determine whether an alarm should be triggered.[32, 33]
The ability to demonstrate the safety of data‐driven HR and RR parameters is a precursor to hospital‐wide implementation. We believe it is crucial to perform a safety analysis prior to implementation due to the role vital signs play in clinical assessment and detection of patient deterioration.[30, 34, 35, 36, 37] Though a few studies have shown that modification of alarm parameters decreases alarm frequency,[5, 6, 10, 16, 17] to our knowledge no formal safety evaluations have ever been published. This study provides the first published safety evaluation of data‐driven HR and RR parameters.
By decreasing the quantity of out‐of‐range vital sign values while preserving the ability to detect patient deterioration, data‐driven vital sign alarm limits have the potential to decrease false monitor alarms, alarm‐generated noise, and alarm fatigue. Future work includes prospectively studying the impact of adoption of data‐driven vital sign parameters on monitor alarm burden and monitoring the safety of the changes. Additional safety analysis could include comparing the sensitivity and specificity of early warning score systems when data‐driven vital sign ranges are substituted for traditional physiologic parameters. Further personalization of vital sign parameters will involve incorporating patient‐specific characteristics (eg, demographics, diagnoses) into the data‐driven analysis to further decrease alarm burden while enhancing patient safety. Ultimately, using a patient's own physiologic data to define highly personalized vital sign parameter limits represents a truly precision approach, and could revolutionize the way hospitalized patients are monitored.
Numerous relevant issues are not yet addressed in this initial, single‐institution study. First, although the biomedical device integration facilitated the direct import of monitor data into the EHR (decreasing transcription errors), our analysis was performed using EHR‐charted data. As such, the effect on bedside monitor alarms was not directly evaluated in our study, including those due to technical alarms or patient artifact. Second, our overall sample size for the training set was quite large; however, in some cases the number of patients per age category was limited. Third, although we evaluated the identification of severe deterioration leading to RRT or CRA events, the sensitivity of the new limits to the need for other interventions (eg, fluid bolus for dehydration or escalation of respiratory support for asthma exacerbation) or unplanned transfers to the ICU was not assessed. Fourth, the analysis was retrospective, and so the impact of data‐driven alarm limits on length of stay and readmission could not be defined. Fifth, excluding all vital sign measurements from patients who spent any time in the ICU setting decreased the amount of data available for analysis. However, excluding sicker patients probably resulted in narrower data‐driven HR and RR ranges, leading to more conservative proposed parameters that are more likely to identify patient decompensation in our safety analysis. Finally, this was a single‐site study. We believe our data‐driven limits are applicable to other tertiary or quaternary care facilities given the similarity to those generated in a study performed in a comparable setting,[24] but generalizability to other settings may be limited if the local population is sufficiently different. Furthermore, because institutional policies (eg, indications for care escalation) differ, individual institutions should determine whether our analysis is applicable to their setting or if local safety evaluation is necessary.
CONCLUSION
A large proportion of HR and RR values for hospitalized children at our institution are out of range according to current vital sign reference ranges. Our new data‐driven alarm parameters for hospitalized children provide a potentially safe means by which to modify physiologic bedside monitor alarm limits, a first step toward customization of alarm limit settings in an effort to mitigate alarm fatigue.
Acknowledgements
The authors thank Debby Huang and Joshua Glandorf in the Information Services Department at Stanford Children's Health for assistance with data acquisition. No compensation was received for their contributions.
Disclosures: All authors gave approval of the final manuscript version submitted for publication and agreed to be accountable for all aspects of the work. Dr. Veena V. Goel conceptualized and designed the study; collected, managed, analyzed and interpreted the data; prepared and reviewed the initial manuscript; and approved the final manuscript as submitted. Ms. Sarah F. Poole contributed to the design of the study and performed the primary data analysis for the study. Ms. Poole critically revised the manuscript for important intellectual content and approved the final manuscript as submitted. Dr. Goel and Ms. Poole had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Dr. Paul J. Sharek and Dr. Jonathan P. Palma contributed to the study design and data interpretation. Drs. Sharek and Palma critically revised the manuscript for important intellectual content and approved the final manuscript as submitted. Dr. Terry S. Platchek, Dr. Natalie M. Pageler, and Dr. Christopher A. Longhurst contributed to the study design. Drs. Platchek, Pageler, and Longhurst critically revised the manuscript for important intellectual content and approved the final manuscript as submitted. Ms. Poole is supported by the Stanford Biosciences Graduate Program through a Fulbright New Zealand Science and Innovation Graduate Award and through the J.R. Templin Trust Scholarship. The authors report no conflicts of interest.
- The Joint Commission. Medical device alarm safety in hospitals. Sentinel Event Alert. 2013;(50):1–3. Available at: https://www.jointcommission.org/sea_issue_50/. Accessed October 12, 2013.
- . “Alarm fatigue” a factor in 2d death: UMass hospital cited for violations. The Boston Globe. September 21, 2011. Available at: https://www.bostonglobe.com/2011/09/20/umass/qSOhm8dYmmaq4uTHZb7FNM/story.html. Accessed December 19, 2014
- The Joint Commission. Alarm system safety. Available at: https://www.jointcommission.org/assets/1/18/R3_Report_Issue_5_12_2_13_Final.pdf. Published December 11, 2013. Accessed October 12, 2013.
- , , , , . ALARMED: adverse events in low‐risk patients with chest pain receiving continuous electrocardiographic monitoring in the emergency department. A pilot study. Am J Emerg Med. 2006;24(1):62–67.
- , . Monitor alarm fatigue: standardizing use of physiological monitors and decreasing nuisance alarms. Am J Crit Care. 2010;19(1):28–34; quiz 35.
- , , . Physiologic monitoring alarm load on medical/surgical floors of a community hospital. Biomed Instrum Technol. 2011;(suppl):29–36.
- , , , , , . Multicentric study of monitoring alarms in the adult intensive care unit (ICU): a descriptive analysis. Intensive Care Med. 1999;25(12):1360–1366.
- . Crying wolf: false alarms in a pediatric intensive care unit. Crit Care Med. 1994;22(6):981–985.
- , , , et al. Cardiopulmonary monitors and clinically significant events in critically ill children. Biomed Instrum Technol. 2011;(suppl):38–45.
- , , , et al. Systematic review of physiologic monitor alarm characteristics and pragmatic interventions to reduce alarm frequency. J Hosp Med. 2016;11(2):136–144.
- . Alarm fatigue. Nurs Clin North Am. 2012;47(3):375–382.
- . Monitor alarm fatigue: an integrative review. Biomed Instrum Technol. 2012;46(4):268–277.
- , , , . Use of pagers with an alarm escalation system to reduce cardiac monitor alarm signals. J Nurs Care Qual. 2014;29(1):9–18.
- . An evidence‐based approach to reduce nuisance alarms and alarm fatigue. Biomed Instrum Technol. 2011;(suppl):46–52.
- , , , et al. Insights into the problem of alarm fatigue with physiologic monitor devices: a comprehensive observational study of consecutive intensive care unit patients. PLoS One. 2014;9(10):e110274.
- , , , , , . Effect of altering alarm settings: a randomized controlled study. Biomed Instrum Technol. 2015;49(3):214–222.
- , , , , . Alarm limit settings for early warning systems to identify at‐risk patients. J Adv Nurs. 2009;65(9):1844–1852.
- , . A framework for reducing alarm fatigue on pediatric inpatient units. Hosp Pediatr. 2015;5(3):160–163.
- , , , et al. Association between exposure to nonactionable physiologic monitor alarms and response time in a children's hospital. J Hosp Med. 2015;10(6):345–351.
- The Johns Hopkins Hospital, , . The Harriet Lane Handbook. 20th ed. Philadelphia, PA: Elsevier Saunders; 2014.
- , . Nelson Textbook of Pediatrics. 19th ed. Philadelphia, PA.: Elsevier Saunders; 2011.
- , , , . Pediatric assessment. In: Pediatric Advanced Life Support: Provider Manual. Dallas, TX: American Heart Association; 2006:9–16.
- , , , et al. Normal ranges of heart rate and respiratory rate in children from birth to 18 years of age: a systematic review of observational studies. Lancet. 2011;377(9770):1011–1018.
- , , , , , . Development of heart and respiratory rate percentile curves for hospitalized children. Pediatrics. 2013;131(4):e1150–e1157.
- National Institutes of Health. Age‐appropriate vital signs. Available at: https://web.archive.org/web/20041101222327/http://www.cc.nih.gov/ccc/pedweb/pedsstaff/age.html. Accessed July 26, 2015.
- Guidelines 2000 for cardiopulmonary resuscitation and emergency cardiovascular care. Part 9: pediatric basic life support. The American Heart Association in collaboration with the International Liaison Committee on Resuscitation. Circulation. 2000;102(8 suppl):I253–I290.
- , , , , , . Recognising clinical instability in hospital patients before cardiac arrest or unplanned admission to intensive care. A pilot study in a tertiary‐care hospital. Med J Aust. 1999;171(1):22–25.
- , , , et al. Duration of life‐threatening antecedents prior to intensive care admission. Intensive Care Med. 2002;28(11):1629–1634.
- , . Pediatric cardiopulmonary resuscitation: a collective review. Ann Emerg Med. 1999;33(2):195–205.
- , , , et al. Effect of a rapid response team on hospital‐wide mortality and code rates outside the ICU in a Children's Hospital. JAMA. 2007;298(19):2267–2274.
- , , , et al. A team‐based approach to reducing cardiac monitor alarms. Pediatrics. 2014;134(6):e1686–e1694.
- , , , et al. Collection of annotated data in a clinical validation study for alarm algorithms in intensive care—a methodologic framework. J Crit Care. 2010;25(1):128–135.
- , , . Making ICU alarms meaningful: a comparison of traditional vs. trend‐based algorithms. Proc AMIA Symp. 1999:379–383.
- , , , et al. Implementation of a medical emergency team in a large pediatric teaching hospital prevents respiratory and cardiopulmonary arrests outside the intensive care unit. Pediatr Crit Care Med. 2007;8(3):236–246; quiz 247.
- . Centile‐based Early Warning Scores derived from statistical distributions of vital signs. Resuscitation. 2011;82(8):969–970.
- , , , , , . Centile‐based early warning scores derived from statistical distributions of vital signs. Resuscitation. 2011;82(8):1013–1018.
- , , , , . Reduction of paediatric in‐patient cardiac arrest and death with a medical emergency team: preliminary results. Arch Dis Child. 2005;90(11):1148–1152.
- The Joint Commission. Medical device alarm safety in hospitals. Sentinel Event Alert. 2013;(50):1–3. Available at: https://www.jointcommission.org/sea_issue_50/. Accessed October 12, 2013.
- . “Alarm fatigue” a factor in 2d death: UMass hospital cited for violations. The Boston Globe. September 21, 2011. Available at: https://www.bostonglobe.com/2011/09/20/umass/qSOhm8dYmmaq4uTHZb7FNM/story.html. Accessed December 19, 2014
- The Joint Commission. Alarm system safety. Available at: https://www.jointcommission.org/assets/1/18/R3_Report_Issue_5_12_2_13_Final.pdf. Published December 11, 2013. Accessed October 12, 2013.
- , , , , . ALARMED: adverse events in low‐risk patients with chest pain receiving continuous electrocardiographic monitoring in the emergency department. A pilot study. Am J Emerg Med. 2006;24(1):62–67.
- , . Monitor alarm fatigue: standardizing use of physiological monitors and decreasing nuisance alarms. Am J Crit Care. 2010;19(1):28–34; quiz 35.
- , , . Physiologic monitoring alarm load on medical/surgical floors of a community hospital. Biomed Instrum Technol. 2011;(suppl):29–36.
- , , , , , . Multicentric study of monitoring alarms in the adult intensive care unit (ICU): a descriptive analysis. Intensive Care Med. 1999;25(12):1360–1366.
- . Crying wolf: false alarms in a pediatric intensive care unit. Crit Care Med. 1994;22(6):981–985.
- , , , et al. Cardiopulmonary monitors and clinically significant events in critically ill children. Biomed Instrum Technol. 2011;(suppl):38–45.
- , , , et al. Systematic review of physiologic monitor alarm characteristics and pragmatic interventions to reduce alarm frequency. J Hosp Med. 2016;11(2):136–144.
- . Alarm fatigue. Nurs Clin North Am. 2012;47(3):375–382.
- . Monitor alarm fatigue: an integrative review. Biomed Instrum Technol. 2012;46(4):268–277.
- , , , . Use of pagers with an alarm escalation system to reduce cardiac monitor alarm signals. J Nurs Care Qual. 2014;29(1):9–18.
- . An evidence‐based approach to reduce nuisance alarms and alarm fatigue. Biomed Instrum Technol. 2011;(suppl):46–52.
- , , , et al. Insights into the problem of alarm fatigue with physiologic monitor devices: a comprehensive observational study of consecutive intensive care unit patients. PLoS One. 2014;9(10):e110274.
- , , , , , . Effect of altering alarm settings: a randomized controlled study. Biomed Instrum Technol. 2015;49(3):214–222.
- , , , , . Alarm limit settings for early warning systems to identify at‐risk patients. J Adv Nurs. 2009;65(9):1844–1852.
- , . A framework for reducing alarm fatigue on pediatric inpatient units. Hosp Pediatr. 2015;5(3):160–163.
- , , , et al. Association between exposure to nonactionable physiologic monitor alarms and response time in a children's hospital. J Hosp Med. 2015;10(6):345–351.
- The Johns Hopkins Hospital, , . The Harriet Lane Handbook. 20th ed. Philadelphia, PA: Elsevier Saunders; 2014.
- , . Nelson Textbook of Pediatrics. 19th ed. Philadelphia, PA.: Elsevier Saunders; 2011.
- , , , . Pediatric assessment. In: Pediatric Advanced Life Support: Provider Manual. Dallas, TX: American Heart Association; 2006:9–16.
- , , , et al. Normal ranges of heart rate and respiratory rate in children from birth to 18 years of age: a systematic review of observational studies. Lancet. 2011;377(9770):1011–1018.
- , , , , , . Development of heart and respiratory rate percentile curves for hospitalized children. Pediatrics. 2013;131(4):e1150–e1157.
- National Institutes of Health. Age‐appropriate vital signs. Available at: https://web.archive.org/web/20041101222327/http://www.cc.nih.gov/ccc/pedweb/pedsstaff/age.html. Accessed July 26, 2015.
- Guidelines 2000 for cardiopulmonary resuscitation and emergency cardiovascular care. Part 9: pediatric basic life support. The American Heart Association in collaboration with the International Liaison Committee on Resuscitation. Circulation. 2000;102(8 suppl):I253–I290.
- , , , , , . Recognising clinical instability in hospital patients before cardiac arrest or unplanned admission to intensive care. A pilot study in a tertiary‐care hospital. Med J Aust. 1999;171(1):22–25.
- , , , et al. Duration of life‐threatening antecedents prior to intensive care admission. Intensive Care Med. 2002;28(11):1629–1634.
- , . Pediatric cardiopulmonary resuscitation: a collective review. Ann Emerg Med. 1999;33(2):195–205.
- , , , et al. Effect of a rapid response team on hospital‐wide mortality and code rates outside the ICU in a Children's Hospital. JAMA. 2007;298(19):2267–2274.
- , , , et al. A team‐based approach to reducing cardiac monitor alarms. Pediatrics. 2014;134(6):e1686–e1694.
- , , , et al. Collection of annotated data in a clinical validation study for alarm algorithms in intensive care—a methodologic framework. J Crit Care. 2010;25(1):128–135.
- , , . Making ICU alarms meaningful: a comparison of traditional vs. trend‐based algorithms. Proc AMIA Symp. 1999:379–383.
- , , , et al. Implementation of a medical emergency team in a large pediatric teaching hospital prevents respiratory and cardiopulmonary arrests outside the intensive care unit. Pediatr Crit Care Med. 2007;8(3):236–246; quiz 247.
- . Centile‐based Early Warning Scores derived from statistical distributions of vital signs. Resuscitation. 2011;82(8):969–970.
- , , , , , . Centile‐based early warning scores derived from statistical distributions of vital signs. Resuscitation. 2011;82(8):1013–1018.
- , , , , . Reduction of paediatric in‐patient cardiac arrest and death with a medical emergency team: preliminary results. Arch Dis Child. 2005;90(11):1148–1152.
FDA Advisory Panel Unanimously Backs Biosimilars for Humira, Enbrel
The Food and Drug Administration’s Arthritis Advisory Committee, together with an added complement of dermatologists and gastroenterologists, unanimously recommended during meetings on July 12 and 13 that the agency license a biosimilar Humira (adalimumab) that is made by Amgen and a biosimilar Enbrel (etanercept) that is made by Sandoz for many of the same indications held by the reference drugs.
The FDA advisory panel that endorsed biosimilar Humira recommended the agent’s approval in a 26-0 vote for many, but not all of the indications currently assigned to Humira itself: rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, juvenile idiopathic arthritis in patients at least 4 years old, plaque psoriasis, adult Crohn’s disease, and adult ulcerative colitis.
A slightly different group of 20 advisory panel members (without any gastroenterologists) voted 20-0 in favor of the FDA granting biosimilar Enbrel all five of the indications now held by Enbrel: rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, juvenile idiopathic arthritis, and plaque psoriasis.
The biosimilar Humira and the biosimilar Enbrel are, respectively, the third and fourth candidate biosimilars to emerge from the FDA’s development program and receive advisory committee scrutiny and support. The first agent through the process, biosimilar filgrastim (Zarxio) received FDA approval in 2015 and is available in the United States. Although the second biosimilar through the process, the tumor necrosis factor inhibitor Inflectra that is biosimilar Remicade (infliximab), received FDA approval in April of this year, it has not yet become available for sale, although a spokeswoman for the company that will market it, Pfizer, said that the company expects to start U.S. sales of Inflectra before the end of 2016.
While the Arthritis Advisory Committee ended each of its daylong deliberations for each of the two candidate biosimilars with unanimous support, the panelists’ discussions among themselves and with FDA staffers reflected some uncertainty with the biosimilar concept, especially during the first day when they focused on biosimilar Humira. The major sticking point revolved around the regulatory pathway to approval first established by the Biologics Price Competition and Innovation Act of 2009 and subsequently refined by the FDA that allows a candidate biosimilar to establish its biosimilarity primarily though the results of analytical studies that establish that the candidate molecule is highly similar to the reference molecule. This approval scheme uses clinical trials in a confirmatory role to establish biosimilarity rather than as the linchpin of approval.
It also means that the FDA can grant clinical indications to the biosimilar drug based not on the results from clinical trials, but based entirely on what have already been demonstrated as safe and effective clinical applications for the reference drug. For example, the biosimilar Humira underwent testing in two clinical studies showing similar efficacy and safety as Humira in patients with rheumatoid arthritis and in patients with plaque psoriasis, but received endorsements based on extrapolations for an additional five indications. Biosimilar Enbrel was compared with Enbrel in patients with plaque psoriasis only and still received extrapolated indications for the additional four rheumatologic conditions.
“This is a new level of extrapolation, across indications,” noted Sarah E. Streett, MD, a gastroenterologist at Stanford (Calif.) University, one of several panelists who initially voiced uncertainty about the concept.
But FDA staffer Nikolay P. Nikolov, MD, who led the agency’s presentation, assured the panelists that the concept of extrapolation was at the heart of biosimilar development and regulatory assessment.
“We have confidence from the data that the two molecules [the reference drug and biosimilar drug] are so similar that we can rely on the safety and efficacy of the reference product. The premise of our approach to biosimilars is that this is not a new molecule that we know nothing about.”
The other uncertainty about biosimilar Humira and biosimilar Enbrel that raised concerns of many panelists were the prospects for nonmedical switching once these drugs reach the market. Nonmedical switching refers to when an insurance company or pharmacy benefit manager substitutes a biosimilar for a reference drug without approval from or even the knowledge of the prescribing physician or the patient. Many of the people who spoke during the public forum period on both days of hearings voiced their concerns about this prospect.
“Nonmedical switching is a major concern of clinicians and policy makers, and we need greater clarification from the FDA,” said committee chair Daniel H. Solomon, MD, a rheumatologist and professor of medicine at Harvard Medical School in Boston.
“I see a remarkable disconnect between the public’s concerns [about nonmedical switching] and the charge to the committee. These are essential issues that need a forum to be aired out,” said panelist Steven F. Solga, MD, chief of gastroenterology at St. Luke’s Hospital in Bethlehem, Pa.
Dr. Nikolov assured committee members that the FDA recognized this concern and was working on it. “We appreciate the disconnect between the charge and the concerns of the community. I assure you that the issues brought up will be part of our discussions so we can get this [biosimilar pathway] implemented the right way.”
According to the FDA’s regulations, a biosimilar designation does not allow for nonmedical switching, something that could only happen under a related but distinct designation known as interchangeability. During the committee meeting on July 13, a FDA staffer said that the agency is currently developing guidance for an “interchangeable” designation and plans to have it available before then end of 2016.
The Food and Drug Administration’s Arthritis Advisory Committee, together with an added complement of dermatologists and gastroenterologists, unanimously recommended during meetings on July 12 and 13 that the agency license a biosimilar Humira (adalimumab) that is made by Amgen and a biosimilar Enbrel (etanercept) that is made by Sandoz for many of the same indications held by the reference drugs.
The FDA advisory panel that endorsed biosimilar Humira recommended the agent’s approval in a 26-0 vote for many, but not all of the indications currently assigned to Humira itself: rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, juvenile idiopathic arthritis in patients at least 4 years old, plaque psoriasis, adult Crohn’s disease, and adult ulcerative colitis.
A slightly different group of 20 advisory panel members (without any gastroenterologists) voted 20-0 in favor of the FDA granting biosimilar Enbrel all five of the indications now held by Enbrel: rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, juvenile idiopathic arthritis, and plaque psoriasis.
The biosimilar Humira and the biosimilar Enbrel are, respectively, the third and fourth candidate biosimilars to emerge from the FDA’s development program and receive advisory committee scrutiny and support. The first agent through the process, biosimilar filgrastim (Zarxio) received FDA approval in 2015 and is available in the United States. Although the second biosimilar through the process, the tumor necrosis factor inhibitor Inflectra that is biosimilar Remicade (infliximab), received FDA approval in April of this year, it has not yet become available for sale, although a spokeswoman for the company that will market it, Pfizer, said that the company expects to start U.S. sales of Inflectra before the end of 2016.
While the Arthritis Advisory Committee ended each of its daylong deliberations for each of the two candidate biosimilars with unanimous support, the panelists’ discussions among themselves and with FDA staffers reflected some uncertainty with the biosimilar concept, especially during the first day when they focused on biosimilar Humira. The major sticking point revolved around the regulatory pathway to approval first established by the Biologics Price Competition and Innovation Act of 2009 and subsequently refined by the FDA that allows a candidate biosimilar to establish its biosimilarity primarily though the results of analytical studies that establish that the candidate molecule is highly similar to the reference molecule. This approval scheme uses clinical trials in a confirmatory role to establish biosimilarity rather than as the linchpin of approval.
It also means that the FDA can grant clinical indications to the biosimilar drug based not on the results from clinical trials, but based entirely on what have already been demonstrated as safe and effective clinical applications for the reference drug. For example, the biosimilar Humira underwent testing in two clinical studies showing similar efficacy and safety as Humira in patients with rheumatoid arthritis and in patients with plaque psoriasis, but received endorsements based on extrapolations for an additional five indications. Biosimilar Enbrel was compared with Enbrel in patients with plaque psoriasis only and still received extrapolated indications for the additional four rheumatologic conditions.
“This is a new level of extrapolation, across indications,” noted Sarah E. Streett, MD, a gastroenterologist at Stanford (Calif.) University, one of several panelists who initially voiced uncertainty about the concept.
But FDA staffer Nikolay P. Nikolov, MD, who led the agency’s presentation, assured the panelists that the concept of extrapolation was at the heart of biosimilar development and regulatory assessment.
“We have confidence from the data that the two molecules [the reference drug and biosimilar drug] are so similar that we can rely on the safety and efficacy of the reference product. The premise of our approach to biosimilars is that this is not a new molecule that we know nothing about.”
The other uncertainty about biosimilar Humira and biosimilar Enbrel that raised concerns of many panelists were the prospects for nonmedical switching once these drugs reach the market. Nonmedical switching refers to when an insurance company or pharmacy benefit manager substitutes a biosimilar for a reference drug without approval from or even the knowledge of the prescribing physician or the patient. Many of the people who spoke during the public forum period on both days of hearings voiced their concerns about this prospect.
“Nonmedical switching is a major concern of clinicians and policy makers, and we need greater clarification from the FDA,” said committee chair Daniel H. Solomon, MD, a rheumatologist and professor of medicine at Harvard Medical School in Boston.
“I see a remarkable disconnect between the public’s concerns [about nonmedical switching] and the charge to the committee. These are essential issues that need a forum to be aired out,” said panelist Steven F. Solga, MD, chief of gastroenterology at St. Luke’s Hospital in Bethlehem, Pa.
Dr. Nikolov assured committee members that the FDA recognized this concern and was working on it. “We appreciate the disconnect between the charge and the concerns of the community. I assure you that the issues brought up will be part of our discussions so we can get this [biosimilar pathway] implemented the right way.”
According to the FDA’s regulations, a biosimilar designation does not allow for nonmedical switching, something that could only happen under a related but distinct designation known as interchangeability. During the committee meeting on July 13, a FDA staffer said that the agency is currently developing guidance for an “interchangeable” designation and plans to have it available before then end of 2016.
The Food and Drug Administration’s Arthritis Advisory Committee, together with an added complement of dermatologists and gastroenterologists, unanimously recommended during meetings on July 12 and 13 that the agency license a biosimilar Humira (adalimumab) that is made by Amgen and a biosimilar Enbrel (etanercept) that is made by Sandoz for many of the same indications held by the reference drugs.
The FDA advisory panel that endorsed biosimilar Humira recommended the agent’s approval in a 26-0 vote for many, but not all of the indications currently assigned to Humira itself: rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, juvenile idiopathic arthritis in patients at least 4 years old, plaque psoriasis, adult Crohn’s disease, and adult ulcerative colitis.
A slightly different group of 20 advisory panel members (without any gastroenterologists) voted 20-0 in favor of the FDA granting biosimilar Enbrel all five of the indications now held by Enbrel: rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, juvenile idiopathic arthritis, and plaque psoriasis.
The biosimilar Humira and the biosimilar Enbrel are, respectively, the third and fourth candidate biosimilars to emerge from the FDA’s development program and receive advisory committee scrutiny and support. The first agent through the process, biosimilar filgrastim (Zarxio) received FDA approval in 2015 and is available in the United States. Although the second biosimilar through the process, the tumor necrosis factor inhibitor Inflectra that is biosimilar Remicade (infliximab), received FDA approval in April of this year, it has not yet become available for sale, although a spokeswoman for the company that will market it, Pfizer, said that the company expects to start U.S. sales of Inflectra before the end of 2016.
While the Arthritis Advisory Committee ended each of its daylong deliberations for each of the two candidate biosimilars with unanimous support, the panelists’ discussions among themselves and with FDA staffers reflected some uncertainty with the biosimilar concept, especially during the first day when they focused on biosimilar Humira. The major sticking point revolved around the regulatory pathway to approval first established by the Biologics Price Competition and Innovation Act of 2009 and subsequently refined by the FDA that allows a candidate biosimilar to establish its biosimilarity primarily though the results of analytical studies that establish that the candidate molecule is highly similar to the reference molecule. This approval scheme uses clinical trials in a confirmatory role to establish biosimilarity rather than as the linchpin of approval.
It also means that the FDA can grant clinical indications to the biosimilar drug based not on the results from clinical trials, but based entirely on what have already been demonstrated as safe and effective clinical applications for the reference drug. For example, the biosimilar Humira underwent testing in two clinical studies showing similar efficacy and safety as Humira in patients with rheumatoid arthritis and in patients with plaque psoriasis, but received endorsements based on extrapolations for an additional five indications. Biosimilar Enbrel was compared with Enbrel in patients with plaque psoriasis only and still received extrapolated indications for the additional four rheumatologic conditions.
“This is a new level of extrapolation, across indications,” noted Sarah E. Streett, MD, a gastroenterologist at Stanford (Calif.) University, one of several panelists who initially voiced uncertainty about the concept.
But FDA staffer Nikolay P. Nikolov, MD, who led the agency’s presentation, assured the panelists that the concept of extrapolation was at the heart of biosimilar development and regulatory assessment.
“We have confidence from the data that the two molecules [the reference drug and biosimilar drug] are so similar that we can rely on the safety and efficacy of the reference product. The premise of our approach to biosimilars is that this is not a new molecule that we know nothing about.”
The other uncertainty about biosimilar Humira and biosimilar Enbrel that raised concerns of many panelists were the prospects for nonmedical switching once these drugs reach the market. Nonmedical switching refers to when an insurance company or pharmacy benefit manager substitutes a biosimilar for a reference drug without approval from or even the knowledge of the prescribing physician or the patient. Many of the people who spoke during the public forum period on both days of hearings voiced their concerns about this prospect.
“Nonmedical switching is a major concern of clinicians and policy makers, and we need greater clarification from the FDA,” said committee chair Daniel H. Solomon, MD, a rheumatologist and professor of medicine at Harvard Medical School in Boston.
“I see a remarkable disconnect between the public’s concerns [about nonmedical switching] and the charge to the committee. These are essential issues that need a forum to be aired out,” said panelist Steven F. Solga, MD, chief of gastroenterology at St. Luke’s Hospital in Bethlehem, Pa.
Dr. Nikolov assured committee members that the FDA recognized this concern and was working on it. “We appreciate the disconnect between the charge and the concerns of the community. I assure you that the issues brought up will be part of our discussions so we can get this [biosimilar pathway] implemented the right way.”
According to the FDA’s regulations, a biosimilar designation does not allow for nonmedical switching, something that could only happen under a related but distinct designation known as interchangeability. During the committee meeting on July 13, a FDA staffer said that the agency is currently developing guidance for an “interchangeable” designation and plans to have it available before then end of 2016.