User login
HHS issues guidance on ransomware attacks
The U.S. Department of Health & Human Services’ Office of Civil Rights has issued new guidance to help physicians and their practices combat a ransomware attack.
Ransomware – a type of malicious software designed to block access to a computer system until a sum of money is paid – is becoming a bigger problem for U.S. businesses in general. Daily ransomware attacks against all types of computer systems increased 300% in early 2016 to 4,000, from 1,000 daily attacks in 2015, according to the Department of Justice.
The HHS fact sheet offers information on how HIPAA compliance can help protect and recover infected systems; how to detect if systems are infected; and what to do if a system becomes infected, including what is reportable.
There are “measures known to be effective to prevent the introduction of ransomware and to recover from a ransomware attack,” according to HHS.
The U.S. Department of Health & Human Services’ Office of Civil Rights has issued new guidance to help physicians and their practices combat a ransomware attack.
Ransomware – a type of malicious software designed to block access to a computer system until a sum of money is paid – is becoming a bigger problem for U.S. businesses in general. Daily ransomware attacks against all types of computer systems increased 300% in early 2016 to 4,000, from 1,000 daily attacks in 2015, according to the Department of Justice.
The HHS fact sheet offers information on how HIPAA compliance can help protect and recover infected systems; how to detect if systems are infected; and what to do if a system becomes infected, including what is reportable.
There are “measures known to be effective to prevent the introduction of ransomware and to recover from a ransomware attack,” according to HHS.
The U.S. Department of Health & Human Services’ Office of Civil Rights has issued new guidance to help physicians and their practices combat a ransomware attack.
Ransomware – a type of malicious software designed to block access to a computer system until a sum of money is paid – is becoming a bigger problem for U.S. businesses in general. Daily ransomware attacks against all types of computer systems increased 300% in early 2016 to 4,000, from 1,000 daily attacks in 2015, according to the Department of Justice.
The HHS fact sheet offers information on how HIPAA compliance can help protect and recover infected systems; how to detect if systems are infected; and what to do if a system becomes infected, including what is reportable.
There are “measures known to be effective to prevent the introduction of ransomware and to recover from a ransomware attack,” according to HHS.
Dexlansoprazole Approved to Treat Heartburn in Younger Patients
The Food and Drug Administration has approved dexlansoprazole (Dexilant) for gastroesophageal reflux disease patients aged 12-17 years, according to a press release from Takeda Pharmaceuticals.
FDA approval of dexlansoprazole for this age group is based on adequate and well controlled studies proving its efficacy in adults, as well data on safety, pharmacokinetics, and efficacy in children 12-17 years. Dexlansoprazole is approved for younger patients to maintain healing of erosive esophagus and heartburn relief for 16 weeks, healing all grades of EE for 8 weeks, and treating heartburn associated with GERD for 4 weeks.
Ddexlansoprazole is a proton pump inhibitor, prescribed to relieve heartburn associated with GERD and maintain healed erosive esophagus. The most common side effects seen in individuals aged 12-17 were headache, abdominal pain, diarrhea, nasopharyngitis, and oropharyngeal pain.
“Takeda is pleased with the FDA’s approval to expand the access of Dexilant to younger patients with GERD. This new approval provides an alternative treatment option for appropriate patients with this condition. With our more than 20 years of experience in gastroenterology, we remain dedicated to all patients living with these conditions,” noted Thomas Gibbs, senior vice president of general medicine at Takeda.
Find the full press release on the Takeda Pharmaceuticals website.
The Food and Drug Administration has approved dexlansoprazole (Dexilant) for gastroesophageal reflux disease patients aged 12-17 years, according to a press release from Takeda Pharmaceuticals.
FDA approval of dexlansoprazole for this age group is based on adequate and well controlled studies proving its efficacy in adults, as well data on safety, pharmacokinetics, and efficacy in children 12-17 years. Dexlansoprazole is approved for younger patients to maintain healing of erosive esophagus and heartburn relief for 16 weeks, healing all grades of EE for 8 weeks, and treating heartburn associated with GERD for 4 weeks.
Ddexlansoprazole is a proton pump inhibitor, prescribed to relieve heartburn associated with GERD and maintain healed erosive esophagus. The most common side effects seen in individuals aged 12-17 were headache, abdominal pain, diarrhea, nasopharyngitis, and oropharyngeal pain.
“Takeda is pleased with the FDA’s approval to expand the access of Dexilant to younger patients with GERD. This new approval provides an alternative treatment option for appropriate patients with this condition. With our more than 20 years of experience in gastroenterology, we remain dedicated to all patients living with these conditions,” noted Thomas Gibbs, senior vice president of general medicine at Takeda.
Find the full press release on the Takeda Pharmaceuticals website.
The Food and Drug Administration has approved dexlansoprazole (Dexilant) for gastroesophageal reflux disease patients aged 12-17 years, according to a press release from Takeda Pharmaceuticals.
FDA approval of dexlansoprazole for this age group is based on adequate and well controlled studies proving its efficacy in adults, as well data on safety, pharmacokinetics, and efficacy in children 12-17 years. Dexlansoprazole is approved for younger patients to maintain healing of erosive esophagus and heartburn relief for 16 weeks, healing all grades of EE for 8 weeks, and treating heartburn associated with GERD for 4 weeks.
Ddexlansoprazole is a proton pump inhibitor, prescribed to relieve heartburn associated with GERD and maintain healed erosive esophagus. The most common side effects seen in individuals aged 12-17 were headache, abdominal pain, diarrhea, nasopharyngitis, and oropharyngeal pain.
“Takeda is pleased with the FDA’s approval to expand the access of Dexilant to younger patients with GERD. This new approval provides an alternative treatment option for appropriate patients with this condition. With our more than 20 years of experience in gastroenterology, we remain dedicated to all patients living with these conditions,” noted Thomas Gibbs, senior vice president of general medicine at Takeda.
Find the full press release on the Takeda Pharmaceuticals website.
CDC forecasts low chance of mosquito-borne Zika infection at Olympics
With only a few weeks left until the 2016 Olympic Games get underway in Rio de Janeiro, officials at the Centers for Disease Control and Prevention are urging anyone traveling to the Olympics to take precautions to avoid contracting Zika virus infection or spreading it when they return home.
But the CDC estimates that there is a low probability of mosquito-borne Zika virus infections during the Olympics because Rio will be experiencing cooler, drier weather then, which typically reduces the mosquito population.
Along with lower mosquito activity, the CDC said that the number of visitors expected in Brazil for the Olympics represents only a fraction of the total travel volume to Zika-affected countries during 2015. The Brazilian Tourism Board is expecting anywhere between 350,000 and 500,000 visitors for the Olympic Games, coming from 207 countries. That represents less than 0.25% of the total travel volume to Zika-affected countries during the entirety of 2015, according to the CDC.
There are 19 countries that the CDC deems susceptible to sustained mosquito-borne transmission of the Zika virus, should the virus enter the country via an attendee of the Olympics (MMWR. 2016 Jul 13. doi: 10.15585/mmwr.mm6528e1).
Of these 19 – none of which are currently experiencing a Zika outbreak – 15 are “not estimated to increase substantially the level of risk above that incurred by the usual aviation travel baseline for these countries.” This leaves Chad, Djibouti, Eritrea, and Yemen at an elevated risk for a Zika outbreak. These four countries “are unique in that they do not have a substantial number of travelers to any country with local Zika virus transmission, except for anticipated travel to the Games,” according to the CDC.
The CDC is urging travelers to take protective measures for their entire stay in Rio and for at least 3 weeks after returning home. The measures include applying mosquito repellent, wearing long-sleeved shirts and long pants, staying in rooms that are air conditioned, and using either screen doors or a mosquito net for additional protection. Additionally, all travelers should take measures to prevent sexual transmission. The CDC continues to advise pregnant women not to travel to the Olympics.
With only a few weeks left until the 2016 Olympic Games get underway in Rio de Janeiro, officials at the Centers for Disease Control and Prevention are urging anyone traveling to the Olympics to take precautions to avoid contracting Zika virus infection or spreading it when they return home.
But the CDC estimates that there is a low probability of mosquito-borne Zika virus infections during the Olympics because Rio will be experiencing cooler, drier weather then, which typically reduces the mosquito population.
Along with lower mosquito activity, the CDC said that the number of visitors expected in Brazil for the Olympics represents only a fraction of the total travel volume to Zika-affected countries during 2015. The Brazilian Tourism Board is expecting anywhere between 350,000 and 500,000 visitors for the Olympic Games, coming from 207 countries. That represents less than 0.25% of the total travel volume to Zika-affected countries during the entirety of 2015, according to the CDC.
There are 19 countries that the CDC deems susceptible to sustained mosquito-borne transmission of the Zika virus, should the virus enter the country via an attendee of the Olympics (MMWR. 2016 Jul 13. doi: 10.15585/mmwr.mm6528e1).
Of these 19 – none of which are currently experiencing a Zika outbreak – 15 are “not estimated to increase substantially the level of risk above that incurred by the usual aviation travel baseline for these countries.” This leaves Chad, Djibouti, Eritrea, and Yemen at an elevated risk for a Zika outbreak. These four countries “are unique in that they do not have a substantial number of travelers to any country with local Zika virus transmission, except for anticipated travel to the Games,” according to the CDC.
The CDC is urging travelers to take protective measures for their entire stay in Rio and for at least 3 weeks after returning home. The measures include applying mosquito repellent, wearing long-sleeved shirts and long pants, staying in rooms that are air conditioned, and using either screen doors or a mosquito net for additional protection. Additionally, all travelers should take measures to prevent sexual transmission. The CDC continues to advise pregnant women not to travel to the Olympics.
With only a few weeks left until the 2016 Olympic Games get underway in Rio de Janeiro, officials at the Centers for Disease Control and Prevention are urging anyone traveling to the Olympics to take precautions to avoid contracting Zika virus infection or spreading it when they return home.
But the CDC estimates that there is a low probability of mosquito-borne Zika virus infections during the Olympics because Rio will be experiencing cooler, drier weather then, which typically reduces the mosquito population.
Along with lower mosquito activity, the CDC said that the number of visitors expected in Brazil for the Olympics represents only a fraction of the total travel volume to Zika-affected countries during 2015. The Brazilian Tourism Board is expecting anywhere between 350,000 and 500,000 visitors for the Olympic Games, coming from 207 countries. That represents less than 0.25% of the total travel volume to Zika-affected countries during the entirety of 2015, according to the CDC.
There are 19 countries that the CDC deems susceptible to sustained mosquito-borne transmission of the Zika virus, should the virus enter the country via an attendee of the Olympics (MMWR. 2016 Jul 13. doi: 10.15585/mmwr.mm6528e1).
Of these 19 – none of which are currently experiencing a Zika outbreak – 15 are “not estimated to increase substantially the level of risk above that incurred by the usual aviation travel baseline for these countries.” This leaves Chad, Djibouti, Eritrea, and Yemen at an elevated risk for a Zika outbreak. These four countries “are unique in that they do not have a substantial number of travelers to any country with local Zika virus transmission, except for anticipated travel to the Games,” according to the CDC.
The CDC is urging travelers to take protective measures for their entire stay in Rio and for at least 3 weeks after returning home. The measures include applying mosquito repellent, wearing long-sleeved shirts and long pants, staying in rooms that are air conditioned, and using either screen doors or a mosquito net for additional protection. Additionally, all travelers should take measures to prevent sexual transmission. The CDC continues to advise pregnant women not to travel to the Olympics.
FROM MMWR
White House to Advance Obama's Precision Medicine Initiative
WASHINGTON - The White House announced on Wednesday measures aimed at advancing President Barack Obama's precision medicine initiative, including plans to speed the development of tests used to identify genetic mutations and guide medical treatment.
The U.S. Food and Drug Administration said it planned to issue a proposal to create performance standards to guide development of next generation sequencing (NGS) tests. These tests scan a person's DNA and identify genetic differences that could be responsible for a patient's symptoms.
The standards would be designed to assess how accurately a test identifies a genetic variant. The developer would certify that it had met those standards. Currently the FDA itself determines the test's accuracy.
"We believe that the use of standards is the best way to allow regulation to keep pace with the evolution of NGS technology," Dr. Robert Califf told reporters on a conference call.
A second FDA proposal would allow test developers to use data from publicly accessible genetic databases, not just their own data, to demonstrate that the test accurately predicts disease. Califf said the approach could potentially get rid of the need for the FDA to review the tests before they reach the market.
"Taken together, these guidances will foster innovation, assure the quality and reliability of NGS-based tests and promote their adoption into clinical practice," he said.
The FDA's action is part of a broader government initiative to promote the development of individually tailored medicines. Obama introduced the initiative in his State of the Union address last year, saying he wanted the United States to lead a new era of medicine, "one that delivers the right treatment at the right time."
As part of the project, the National Institutes of Health will invest $55 million to build the infrastructure needed to collect genetic data from more than 1 million volunteers, its director, Dr. Francis Collins, said on the conference call.
Collins said it will take three to four years to assemble the desired amount of genetic material, which will then be available to researchers to help develop drugs for cancer and other disease. Anyone, he said, can participate.
"This is about all of us," he said. "Participants will be true partners, not subjects, not patients." Data sharing, he added, will be "swift."
WASHINGTON - The White House announced on Wednesday measures aimed at advancing President Barack Obama's precision medicine initiative, including plans to speed the development of tests used to identify genetic mutations and guide medical treatment.
The U.S. Food and Drug Administration said it planned to issue a proposal to create performance standards to guide development of next generation sequencing (NGS) tests. These tests scan a person's DNA and identify genetic differences that could be responsible for a patient's symptoms.
The standards would be designed to assess how accurately a test identifies a genetic variant. The developer would certify that it had met those standards. Currently the FDA itself determines the test's accuracy.
"We believe that the use of standards is the best way to allow regulation to keep pace with the evolution of NGS technology," Dr. Robert Califf told reporters on a conference call.
A second FDA proposal would allow test developers to use data from publicly accessible genetic databases, not just their own data, to demonstrate that the test accurately predicts disease. Califf said the approach could potentially get rid of the need for the FDA to review the tests before they reach the market.
"Taken together, these guidances will foster innovation, assure the quality and reliability of NGS-based tests and promote their adoption into clinical practice," he said.
The FDA's action is part of a broader government initiative to promote the development of individually tailored medicines. Obama introduced the initiative in his State of the Union address last year, saying he wanted the United States to lead a new era of medicine, "one that delivers the right treatment at the right time."
As part of the project, the National Institutes of Health will invest $55 million to build the infrastructure needed to collect genetic data from more than 1 million volunteers, its director, Dr. Francis Collins, said on the conference call.
Collins said it will take three to four years to assemble the desired amount of genetic material, which will then be available to researchers to help develop drugs for cancer and other disease. Anyone, he said, can participate.
"This is about all of us," he said. "Participants will be true partners, not subjects, not patients." Data sharing, he added, will be "swift."
WASHINGTON - The White House announced on Wednesday measures aimed at advancing President Barack Obama's precision medicine initiative, including plans to speed the development of tests used to identify genetic mutations and guide medical treatment.
The U.S. Food and Drug Administration said it planned to issue a proposal to create performance standards to guide development of next generation sequencing (NGS) tests. These tests scan a person's DNA and identify genetic differences that could be responsible for a patient's symptoms.
The standards would be designed to assess how accurately a test identifies a genetic variant. The developer would certify that it had met those standards. Currently the FDA itself determines the test's accuracy.
"We believe that the use of standards is the best way to allow regulation to keep pace with the evolution of NGS technology," Dr. Robert Califf told reporters on a conference call.
A second FDA proposal would allow test developers to use data from publicly accessible genetic databases, not just their own data, to demonstrate that the test accurately predicts disease. Califf said the approach could potentially get rid of the need for the FDA to review the tests before they reach the market.
"Taken together, these guidances will foster innovation, assure the quality and reliability of NGS-based tests and promote their adoption into clinical practice," he said.
The FDA's action is part of a broader government initiative to promote the development of individually tailored medicines. Obama introduced the initiative in his State of the Union address last year, saying he wanted the United States to lead a new era of medicine, "one that delivers the right treatment at the right time."
As part of the project, the National Institutes of Health will invest $55 million to build the infrastructure needed to collect genetic data from more than 1 million volunteers, its director, Dr. Francis Collins, said on the conference call.
Collins said it will take three to four years to assemble the desired amount of genetic material, which will then be available to researchers to help develop drugs for cancer and other disease. Anyone, he said, can participate.
"This is about all of us," he said. "Participants will be true partners, not subjects, not patients." Data sharing, he added, will be "swift."
Looking for a grant? Now accepting 2016 applications
Four $1,000 grants will be awarded to help awardees develop mentorship programs, course or curricular materials, or teaching tools related to gastroenterology and hepatology. The grants also may be used to help develop innovative assessment tools or to do research projects in GI and hepatology education.
The deadline to apply for the 2016 awards is Aug. 31, 2016, and the awardees will be notified by Oct. 10, 2016. Members of the Academy of Educators are invited to submit applications to [email protected]. Not a member yet? Join today for consideration.
Learn more and apply today at www.gastro.org/about/initiatives/aga-academy-of-educators. Please contact Paula Dorfman with any questions [email protected].
Four $1,000 grants will be awarded to help awardees develop mentorship programs, course or curricular materials, or teaching tools related to gastroenterology and hepatology. The grants also may be used to help develop innovative assessment tools or to do research projects in GI and hepatology education.
The deadline to apply for the 2016 awards is Aug. 31, 2016, and the awardees will be notified by Oct. 10, 2016. Members of the Academy of Educators are invited to submit applications to [email protected]. Not a member yet? Join today for consideration.
Learn more and apply today at www.gastro.org/about/initiatives/aga-academy-of-educators. Please contact Paula Dorfman with any questions [email protected].
Four $1,000 grants will be awarded to help awardees develop mentorship programs, course or curricular materials, or teaching tools related to gastroenterology and hepatology. The grants also may be used to help develop innovative assessment tools or to do research projects in GI and hepatology education.
The deadline to apply for the 2016 awards is Aug. 31, 2016, and the awardees will be notified by Oct. 10, 2016. Members of the Academy of Educators are invited to submit applications to [email protected]. Not a member yet? Join today for consideration.
Learn more and apply today at www.gastro.org/about/initiatives/aga-academy-of-educators. Please contact Paula Dorfman with any questions [email protected].
AGA and Takeda Pharmaceuticals announce new grants for IBD researchers
Thanks to a generous grant from Takeda Pharmaceuticals U.S.A., the AGA Research Foundation is thrilled to announce three new research grants to fund young investigators working on inflammatory bowel disease (IBD) projects. This funding will provide qualified scientists with the opportunity to make discoveries that will lead to improvements in patient care.
The AGA-Takeda Pharmaceuticals Research Scholar Awards in Inflammatory Bowel Disease will provide $90,000 per year for 3 years (total $270,000) to three young investigators working toward independent research careers with a focus on IBD.
“The AGA Research Foundation is very grateful to have Takeda’s support for promising young researchers at a very vulnerable stage in their careers,” said Robert S. Sandler, M.D., MPH, AGAF, chair of the AGA Research Foundation. “Inflammatory bowel disease offers exciting opportunities for research, and we look forward to seeing how these three award recipients will advance our understanding of this serious digestive disease.”
“Takeda is proud to partner with the AGA Research Foundation to present three research scholar awards in IBD,” said Karen Lasch, MD, executive medical director, gastroenterology, Takeda. “As we look to the future, there is great promise with significant IBD research underway, but a strong need still exists for further scientific and clinical understanding. Providing young research investigators with the support they need to drive innovation and discovery is critical.”
Interested researchers can learn more by visiting the AGA website. The deadline for applications is Aug. 26, 2016, for funding beginning July 1, 2017. The AGA Research Awards Panel is looking for individuals in the beginning years of their careers who have demonstrated exceptional promise and have some record of accomplishment in research.
The overall objective of the AGA Research Scholar Award (RSA) is to enable young investigators, instructors, research associates or equivalents to develop independent and productive research careers in digestive diseases by ensuring that a major proportion of their time is protected for research.
Thanks to a generous grant from Takeda Pharmaceuticals U.S.A., the AGA Research Foundation is thrilled to announce three new research grants to fund young investigators working on inflammatory bowel disease (IBD) projects. This funding will provide qualified scientists with the opportunity to make discoveries that will lead to improvements in patient care.
The AGA-Takeda Pharmaceuticals Research Scholar Awards in Inflammatory Bowel Disease will provide $90,000 per year for 3 years (total $270,000) to three young investigators working toward independent research careers with a focus on IBD.
“The AGA Research Foundation is very grateful to have Takeda’s support for promising young researchers at a very vulnerable stage in their careers,” said Robert S. Sandler, M.D., MPH, AGAF, chair of the AGA Research Foundation. “Inflammatory bowel disease offers exciting opportunities for research, and we look forward to seeing how these three award recipients will advance our understanding of this serious digestive disease.”
“Takeda is proud to partner with the AGA Research Foundation to present three research scholar awards in IBD,” said Karen Lasch, MD, executive medical director, gastroenterology, Takeda. “As we look to the future, there is great promise with significant IBD research underway, but a strong need still exists for further scientific and clinical understanding. Providing young research investigators with the support they need to drive innovation and discovery is critical.”
Interested researchers can learn more by visiting the AGA website. The deadline for applications is Aug. 26, 2016, for funding beginning July 1, 2017. The AGA Research Awards Panel is looking for individuals in the beginning years of their careers who have demonstrated exceptional promise and have some record of accomplishment in research.
The overall objective of the AGA Research Scholar Award (RSA) is to enable young investigators, instructors, research associates or equivalents to develop independent and productive research careers in digestive diseases by ensuring that a major proportion of their time is protected for research.
Thanks to a generous grant from Takeda Pharmaceuticals U.S.A., the AGA Research Foundation is thrilled to announce three new research grants to fund young investigators working on inflammatory bowel disease (IBD) projects. This funding will provide qualified scientists with the opportunity to make discoveries that will lead to improvements in patient care.
The AGA-Takeda Pharmaceuticals Research Scholar Awards in Inflammatory Bowel Disease will provide $90,000 per year for 3 years (total $270,000) to three young investigators working toward independent research careers with a focus on IBD.
“The AGA Research Foundation is very grateful to have Takeda’s support for promising young researchers at a very vulnerable stage in their careers,” said Robert S. Sandler, M.D., MPH, AGAF, chair of the AGA Research Foundation. “Inflammatory bowel disease offers exciting opportunities for research, and we look forward to seeing how these three award recipients will advance our understanding of this serious digestive disease.”
“Takeda is proud to partner with the AGA Research Foundation to present three research scholar awards in IBD,” said Karen Lasch, MD, executive medical director, gastroenterology, Takeda. “As we look to the future, there is great promise with significant IBD research underway, but a strong need still exists for further scientific and clinical understanding. Providing young research investigators with the support they need to drive innovation and discovery is critical.”
Interested researchers can learn more by visiting the AGA website. The deadline for applications is Aug. 26, 2016, for funding beginning July 1, 2017. The AGA Research Awards Panel is looking for individuals in the beginning years of their careers who have demonstrated exceptional promise and have some record of accomplishment in research.
The overall objective of the AGA Research Scholar Award (RSA) is to enable young investigators, instructors, research associates or equivalents to develop independent and productive research careers in digestive diseases by ensuring that a major proportion of their time is protected for research.
Experts emphasize scrupulous procedures for duodenoscopes, ERCP
Contaminated duodenoscopes have caused multiple outbreaks of multidrug-resistant infections with sometimes lethal consequences; until these instruments become easier to clean, personnel must strictly follow recommendations for sterilization, surveillance, and unit-by-unit quality control, according to an extensive commentary accompanying an American Gastroenterological Association Clinical Practice Update.
“Patients and physicians want and expect no transmission of infections by any medical instrument,” wrote Bret Petersen, M.D., of the Mayo Clinic, Rochester, Minn., Johannes Koch, M.D., of Virginia Mason Medical Center, Seattle, and Gregory Ginsberg, M.D., of the University of Pennsylvania, Philadelphia. It is the collective responsibility of endoscope manufacturers, health systems, and providers to ensure endoscope reprocessing is mistake proof, establishing systems to identify and eliminate the risk of infection for patients undergoing flexible endoscopy.”
More than 650,000 endoscopic retrograde cholangiopancreatographies (ERCPs) occur in the United States annually, and “even the lowest reported defect rate of 0.7% will expose 4,500 patients to a preventable risk,” the experts noted. Carbapenem-resistant Enterobacteriaceae (CRE) are becoming more prevalent and have been transmitted during ERCP, even when personnel seem to have followed sterilization protocols to the letter. Clinical CRE infections have a fatality rate of at least 50%, months may elapse between exposure and symptom onset, and infections may involve distant organs. These factors, along with the phenomenon of “silent carriers,” have linked duodenoscopes to at least 250 multidrug-resistant infections and at least 20 fatalities worldwide, the experts wrote (Gastroenterology 2016 May 27. doi: 10.1053/j.gastro.2016.05.040).
Current duodenoscopes can be tough to sterilize. Between 1 billion and 1 trillion organisms typically cover a used instrument. Bedside cleaning cuts this number about 1,000-fold, and manual washing kills about another million organisms, leaving up to 1 billion bugs to be killed by high-level disinfection. That’s “a tall order” that can strain space, time, and staffing resources, especially given the fact that duodenoscopes have “tight crevices and mechanical joints that are exposed repeatedly to highly infectious bioburden,” the experts wrote. Furthermore, slips in processing enable the formation of biofilms that resist both cleaning and high-level disinfection.
The key to stopping duodenoscopes from transmitting dangerous pathogens is manual cleaning, including wiping the outside of the duodenoscope, flushing its channels, and brushing the elevator lever “immediately after use and before the surfaces have become dried,” the experts stressed. Disinfectants should be used at the right concentration and temperature, and for the intended amount of time. Biofilms form on moist surfaces only, so channels should be flushed with alcohol (a desiccant), dried with forced air, and stored in a dry environment.
But recent outbreaks spurred the Food and Drug Administration to recommend further steps – including better oversight and training of reprocessing staff and closer attention to precleaning, manual cleaning, and manufacturer recommendations for use, including determining whether the company used its own “proprietary” cleaning brushes in its validation studies, the experts noted. “Optional supplemental measures” include surveillance cultures of duodenoscopes, ethylene oxide sterilization, and double reprocessing, in which each scope undergoes two cycles of manual cleaning and high-intensity sterilization between patients. Double reprocessing might be the simplest and most easily adopted of these measures, the experts said. The AGA, for its part, recommends active surveillance of patients who undergo ERCP, surveillance cultures of scopes, and recording of the serial number of every scope used in every procedure.
Surveillance culture makes sense, but can be costly and hard to conduct and interpret because sampling detects vast numbers of nonpathogenic organisms in addition to any pathogens, the experts noted. The Centers for Disease Control and Prevention recommends that each institution follow its own complex outbreak sampling protocol and quarantine duodenoscopes for 2-3 days, pending negative results. That may mean buying more duodenoscopes. A less costly option is to culture a subset of scopes at the end of every workweek, the experts said. Real-time tests that reliably reflect bacterial culture results remain “elusive,” but testing for adenosine triphosphate after manual washing is easiest and best studied, they added.
Clearly, industry is responsible for making endoscopes that can be reliably disinfected. “Recent submissions by all three manufacturers (Olympus, Pentax, and Fujinon) have validated current reprocessing outcomes in test environments, and the FDA has ruled that postmarket studies of reprocessing in clinical settings are expected, but these results will not be forthcoming for several years,” the experts wrote. Redesigning duodenoscopes may be “the ultimate solution,” but in the meantime, endoscopists should carefully review indications for ERCP and ensure thorough informed consent. Doing so “will uphold the trust that we must achieve and maintain with our patients,” the authors said.
They had no funding sources. Dr. Koch has consulted for Sedasys, and Dr. Ginsberg has consulted for Olympus.
Contaminated duodenoscopes have caused multiple outbreaks of multidrug-resistant infections with sometimes lethal consequences; until these instruments become easier to clean, personnel must strictly follow recommendations for sterilization, surveillance, and unit-by-unit quality control, according to an extensive commentary accompanying an American Gastroenterological Association Clinical Practice Update.
“Patients and physicians want and expect no transmission of infections by any medical instrument,” wrote Bret Petersen, M.D., of the Mayo Clinic, Rochester, Minn., Johannes Koch, M.D., of Virginia Mason Medical Center, Seattle, and Gregory Ginsberg, M.D., of the University of Pennsylvania, Philadelphia. It is the collective responsibility of endoscope manufacturers, health systems, and providers to ensure endoscope reprocessing is mistake proof, establishing systems to identify and eliminate the risk of infection for patients undergoing flexible endoscopy.”
More than 650,000 endoscopic retrograde cholangiopancreatographies (ERCPs) occur in the United States annually, and “even the lowest reported defect rate of 0.7% will expose 4,500 patients to a preventable risk,” the experts noted. Carbapenem-resistant Enterobacteriaceae (CRE) are becoming more prevalent and have been transmitted during ERCP, even when personnel seem to have followed sterilization protocols to the letter. Clinical CRE infections have a fatality rate of at least 50%, months may elapse between exposure and symptom onset, and infections may involve distant organs. These factors, along with the phenomenon of “silent carriers,” have linked duodenoscopes to at least 250 multidrug-resistant infections and at least 20 fatalities worldwide, the experts wrote (Gastroenterology 2016 May 27. doi: 10.1053/j.gastro.2016.05.040).
Current duodenoscopes can be tough to sterilize. Between 1 billion and 1 trillion organisms typically cover a used instrument. Bedside cleaning cuts this number about 1,000-fold, and manual washing kills about another million organisms, leaving up to 1 billion bugs to be killed by high-level disinfection. That’s “a tall order” that can strain space, time, and staffing resources, especially given the fact that duodenoscopes have “tight crevices and mechanical joints that are exposed repeatedly to highly infectious bioburden,” the experts wrote. Furthermore, slips in processing enable the formation of biofilms that resist both cleaning and high-level disinfection.
The key to stopping duodenoscopes from transmitting dangerous pathogens is manual cleaning, including wiping the outside of the duodenoscope, flushing its channels, and brushing the elevator lever “immediately after use and before the surfaces have become dried,” the experts stressed. Disinfectants should be used at the right concentration and temperature, and for the intended amount of time. Biofilms form on moist surfaces only, so channels should be flushed with alcohol (a desiccant), dried with forced air, and stored in a dry environment.
But recent outbreaks spurred the Food and Drug Administration to recommend further steps – including better oversight and training of reprocessing staff and closer attention to precleaning, manual cleaning, and manufacturer recommendations for use, including determining whether the company used its own “proprietary” cleaning brushes in its validation studies, the experts noted. “Optional supplemental measures” include surveillance cultures of duodenoscopes, ethylene oxide sterilization, and double reprocessing, in which each scope undergoes two cycles of manual cleaning and high-intensity sterilization between patients. Double reprocessing might be the simplest and most easily adopted of these measures, the experts said. The AGA, for its part, recommends active surveillance of patients who undergo ERCP, surveillance cultures of scopes, and recording of the serial number of every scope used in every procedure.
Surveillance culture makes sense, but can be costly and hard to conduct and interpret because sampling detects vast numbers of nonpathogenic organisms in addition to any pathogens, the experts noted. The Centers for Disease Control and Prevention recommends that each institution follow its own complex outbreak sampling protocol and quarantine duodenoscopes for 2-3 days, pending negative results. That may mean buying more duodenoscopes. A less costly option is to culture a subset of scopes at the end of every workweek, the experts said. Real-time tests that reliably reflect bacterial culture results remain “elusive,” but testing for adenosine triphosphate after manual washing is easiest and best studied, they added.
Clearly, industry is responsible for making endoscopes that can be reliably disinfected. “Recent submissions by all three manufacturers (Olympus, Pentax, and Fujinon) have validated current reprocessing outcomes in test environments, and the FDA has ruled that postmarket studies of reprocessing in clinical settings are expected, but these results will not be forthcoming for several years,” the experts wrote. Redesigning duodenoscopes may be “the ultimate solution,” but in the meantime, endoscopists should carefully review indications for ERCP and ensure thorough informed consent. Doing so “will uphold the trust that we must achieve and maintain with our patients,” the authors said.
They had no funding sources. Dr. Koch has consulted for Sedasys, and Dr. Ginsberg has consulted for Olympus.
Contaminated duodenoscopes have caused multiple outbreaks of multidrug-resistant infections with sometimes lethal consequences; until these instruments become easier to clean, personnel must strictly follow recommendations for sterilization, surveillance, and unit-by-unit quality control, according to an extensive commentary accompanying an American Gastroenterological Association Clinical Practice Update.
“Patients and physicians want and expect no transmission of infections by any medical instrument,” wrote Bret Petersen, M.D., of the Mayo Clinic, Rochester, Minn., Johannes Koch, M.D., of Virginia Mason Medical Center, Seattle, and Gregory Ginsberg, M.D., of the University of Pennsylvania, Philadelphia. It is the collective responsibility of endoscope manufacturers, health systems, and providers to ensure endoscope reprocessing is mistake proof, establishing systems to identify and eliminate the risk of infection for patients undergoing flexible endoscopy.”
More than 650,000 endoscopic retrograde cholangiopancreatographies (ERCPs) occur in the United States annually, and “even the lowest reported defect rate of 0.7% will expose 4,500 patients to a preventable risk,” the experts noted. Carbapenem-resistant Enterobacteriaceae (CRE) are becoming more prevalent and have been transmitted during ERCP, even when personnel seem to have followed sterilization protocols to the letter. Clinical CRE infections have a fatality rate of at least 50%, months may elapse between exposure and symptom onset, and infections may involve distant organs. These factors, along with the phenomenon of “silent carriers,” have linked duodenoscopes to at least 250 multidrug-resistant infections and at least 20 fatalities worldwide, the experts wrote (Gastroenterology 2016 May 27. doi: 10.1053/j.gastro.2016.05.040).
Current duodenoscopes can be tough to sterilize. Between 1 billion and 1 trillion organisms typically cover a used instrument. Bedside cleaning cuts this number about 1,000-fold, and manual washing kills about another million organisms, leaving up to 1 billion bugs to be killed by high-level disinfection. That’s “a tall order” that can strain space, time, and staffing resources, especially given the fact that duodenoscopes have “tight crevices and mechanical joints that are exposed repeatedly to highly infectious bioburden,” the experts wrote. Furthermore, slips in processing enable the formation of biofilms that resist both cleaning and high-level disinfection.
The key to stopping duodenoscopes from transmitting dangerous pathogens is manual cleaning, including wiping the outside of the duodenoscope, flushing its channels, and brushing the elevator lever “immediately after use and before the surfaces have become dried,” the experts stressed. Disinfectants should be used at the right concentration and temperature, and for the intended amount of time. Biofilms form on moist surfaces only, so channels should be flushed with alcohol (a desiccant), dried with forced air, and stored in a dry environment.
But recent outbreaks spurred the Food and Drug Administration to recommend further steps – including better oversight and training of reprocessing staff and closer attention to precleaning, manual cleaning, and manufacturer recommendations for use, including determining whether the company used its own “proprietary” cleaning brushes in its validation studies, the experts noted. “Optional supplemental measures” include surveillance cultures of duodenoscopes, ethylene oxide sterilization, and double reprocessing, in which each scope undergoes two cycles of manual cleaning and high-intensity sterilization between patients. Double reprocessing might be the simplest and most easily adopted of these measures, the experts said. The AGA, for its part, recommends active surveillance of patients who undergo ERCP, surveillance cultures of scopes, and recording of the serial number of every scope used in every procedure.
Surveillance culture makes sense, but can be costly and hard to conduct and interpret because sampling detects vast numbers of nonpathogenic organisms in addition to any pathogens, the experts noted. The Centers for Disease Control and Prevention recommends that each institution follow its own complex outbreak sampling protocol and quarantine duodenoscopes for 2-3 days, pending negative results. That may mean buying more duodenoscopes. A less costly option is to culture a subset of scopes at the end of every workweek, the experts said. Real-time tests that reliably reflect bacterial culture results remain “elusive,” but testing for adenosine triphosphate after manual washing is easiest and best studied, they added.
Clearly, industry is responsible for making endoscopes that can be reliably disinfected. “Recent submissions by all three manufacturers (Olympus, Pentax, and Fujinon) have validated current reprocessing outcomes in test environments, and the FDA has ruled that postmarket studies of reprocessing in clinical settings are expected, but these results will not be forthcoming for several years,” the experts wrote. Redesigning duodenoscopes may be “the ultimate solution,” but in the meantime, endoscopists should carefully review indications for ERCP and ensure thorough informed consent. Doing so “will uphold the trust that we must achieve and maintain with our patients,” the authors said.
They had no funding sources. Dr. Koch has consulted for Sedasys, and Dr. Ginsberg has consulted for Olympus.
FROM GASTROENTEROLOGY
Medicaid expansion linked to lower uninsured rates
The overall uninsured rate for nonelderly adults took a significant drop from 18.8% in 2013 to 14.4% in 2014, with rates by age lower and declines generally larger among states that expanded Medicaid coverage, according to the Agency for Healthcare Research and Quality.
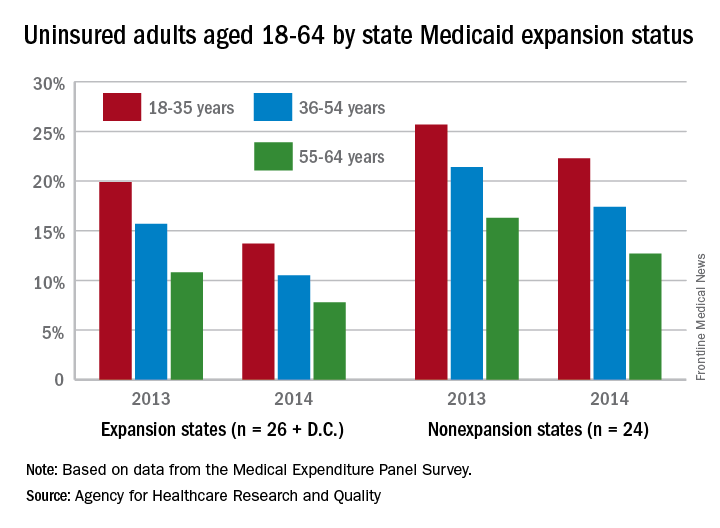
Among adults aged 18-35 years, states (and the District of Columbia) that expanded Medicaid had a larger drop in the percentage who were uninsured for the entire calendar year, going from 19.9% in 2013 to 13.7% in 2014 (6.2 percentage points), than did states that did not expand Medicaid, which dropped from 25.7% to 22.3% (3.4 percentage points), the AHRQ reported.
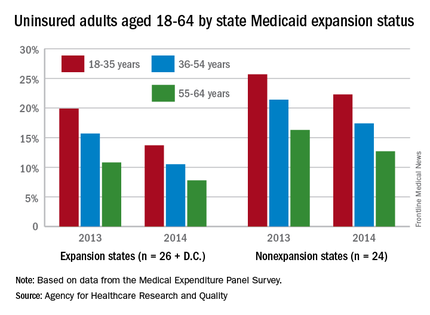
For adults aged 36-54 years, the situation was similar: The uninsured rate in states that expanded Medicaid went from 15.7% in 2013 to 10.5% in 2014, or 5.2 percentage points, while the rate dropped from 21.4% to 17.4%, or 4 percentage points, in states that did not expand Medicaid, the report noted.
Uninsured rates were lower among adults aged 56-64 in states that did expand Medicaid, but the absolute decrease was actually larger among states that did not expand it. The rate in nonexpanding states decreased by 3.6 percentage points, 16.3% to 12.7%, while expanding states saw a drop from 10.8% to 7.8%, 3 percentage points, according to data from the Medical Expenditure Panel Survey.
The overall uninsured rate for nonelderly adults took a significant drop from 18.8% in 2013 to 14.4% in 2014, with rates by age lower and declines generally larger among states that expanded Medicaid coverage, according to the Agency for Healthcare Research and Quality.
Among adults aged 18-35 years, states (and the District of Columbia) that expanded Medicaid had a larger drop in the percentage who were uninsured for the entire calendar year, going from 19.9% in 2013 to 13.7% in 2014 (6.2 percentage points), than did states that did not expand Medicaid, which dropped from 25.7% to 22.3% (3.4 percentage points), the AHRQ reported.

For adults aged 36-54 years, the situation was similar: The uninsured rate in states that expanded Medicaid went from 15.7% in 2013 to 10.5% in 2014, or 5.2 percentage points, while the rate dropped from 21.4% to 17.4%, or 4 percentage points, in states that did not expand Medicaid, the report noted.
Uninsured rates were lower among adults aged 56-64 in states that did expand Medicaid, but the absolute decrease was actually larger among states that did not expand it. The rate in nonexpanding states decreased by 3.6 percentage points, 16.3% to 12.7%, while expanding states saw a drop from 10.8% to 7.8%, 3 percentage points, according to data from the Medical Expenditure Panel Survey.
The overall uninsured rate for nonelderly adults took a significant drop from 18.8% in 2013 to 14.4% in 2014, with rates by age lower and declines generally larger among states that expanded Medicaid coverage, according to the Agency for Healthcare Research and Quality.
Among adults aged 18-35 years, states (and the District of Columbia) that expanded Medicaid had a larger drop in the percentage who were uninsured for the entire calendar year, going from 19.9% in 2013 to 13.7% in 2014 (6.2 percentage points), than did states that did not expand Medicaid, which dropped from 25.7% to 22.3% (3.4 percentage points), the AHRQ reported.

For adults aged 36-54 years, the situation was similar: The uninsured rate in states that expanded Medicaid went from 15.7% in 2013 to 10.5% in 2014, or 5.2 percentage points, while the rate dropped from 21.4% to 17.4%, or 4 percentage points, in states that did not expand Medicaid, the report noted.
Uninsured rates were lower among adults aged 56-64 in states that did expand Medicaid, but the absolute decrease was actually larger among states that did not expand it. The rate in nonexpanding states decreased by 3.6 percentage points, 16.3% to 12.7%, while expanding states saw a drop from 10.8% to 7.8%, 3 percentage points, according to data from the Medical Expenditure Panel Survey.
Hepatitis disease burden continues to rise
Although the disease burden of most other prevalent communicable diseases has gone down considerably over the last 25 years, viral hepatitis continues to be a challenge for health care professionals around the world, as incidences of the disease have climbed steadily between 1990 and 2013.
Advances in treating hepatitis A, B, C, and E viruses over the last 25 years have helped to “overcome many barriers to the control and treatment of viral hepatitis in low-income countries and are set to be important components of a new global health strategy,” wrote the study investigators, led by Jeffrey D. Stanaway, MD, of the University of Washington, Seattle. “However, a better understanding of the burden of disease is required to guide these efforts.”
Dr. Stanaway and his coauthors looked at the Global Burden of Disease (GBD) study for data on worldwide morbidity and mortality associated with hepatitis A, B, C, and E viruses, as well as cirrhosis and liver cancer secondary to hepatitis B or C virus. Data collected for the GBD was used to determine disability-adjusted life-years (DALYs), which is a metric calculated by adding up years of life lost (YLL) and years lived with disability (YLD), both of which were also measured by the GBD.
Data showed that worldwide deaths related to viral hepatitis numbered 0.89 million in 1990 (95% uncertainty interval [UI]: 0.86-0.94), but jumped up dramatically to 1.45 million in 2013 (95% UI: 1.38-1.54). Over the same time period, YLLs and YLDs also increased, going from 31.0 million (95% UI: 29.6-32.6) and 0.65 million (95% UI: 0.45-0.89), respectively, in 1990, to 41.6 million (95% UI: 39.1-44.7), and 0.87 million (95% UI: 0.61-1.18), respectively, in 2013. Consequently, DALYs increased from 31.7 million in 1990 (95% UI: 30.2-33.3) to 42.5 million (95% UI: 39.9-45.6).
These figures represent a 34% increase in viral hepatitis disease burden over that period of time. Furthermore, viral hepatitis went from being the 10th leading cause of death in the world in 1990 (95% UI: 10-12) to the seventh leading cause in 2013 (95% UI: 7-8). However, analysis without the data’s demographic trends showed that YLL and YLD rates declined by 20% and 13%, respectively (95% UI: 8-30 and 8-18), while DALY rates dropped by 20% (95% UI: 8-30) with no significant trend detected in age-specific mortality rates, indicating that demographic changes such as population growth may be the biggest factor contributing to viral hepatitis’ growing disease burden.
“HAV is the only hepatitis virus for which DALYs have declined significantly between 1990 and 2013. Some of this decline has been driven by changing population age structures, but most is due to declines in age-specific rates, most likely as a result of vaccination and improvements in water supply and sanitation,” the authors noted.
Dr. Stanaway and his coauthors also urged public health institutions around the world to devote more funding to targeting viral hepatitis, noting that the current state of funding is “disproportionate to [viral hepatitis’] importance as a major cause of death and disability.”
The Bill & Melinda Gates Foundation funded the study. Coauthor Graham S. Cooke, MD, reported being an investigator on trials of hepatitis C virus therapy sponsored by Boehringer Ingelheim, Gilead, Merck, and Bristol-Myers Squibb, and has acted in an advisory role to Merck, Boehringer Ingelheim, Gilead, Janssen, and WHO in relation to viral hepatitis and clinical trials unrelated to this work. Dr. Stanaway and other coauthors did not report any relevant financial disclosures.
Although the disease burden of most other prevalent communicable diseases has gone down considerably over the last 25 years, viral hepatitis continues to be a challenge for health care professionals around the world, as incidences of the disease have climbed steadily between 1990 and 2013.
Advances in treating hepatitis A, B, C, and E viruses over the last 25 years have helped to “overcome many barriers to the control and treatment of viral hepatitis in low-income countries and are set to be important components of a new global health strategy,” wrote the study investigators, led by Jeffrey D. Stanaway, MD, of the University of Washington, Seattle. “However, a better understanding of the burden of disease is required to guide these efforts.”
Dr. Stanaway and his coauthors looked at the Global Burden of Disease (GBD) study for data on worldwide morbidity and mortality associated with hepatitis A, B, C, and E viruses, as well as cirrhosis and liver cancer secondary to hepatitis B or C virus. Data collected for the GBD was used to determine disability-adjusted life-years (DALYs), which is a metric calculated by adding up years of life lost (YLL) and years lived with disability (YLD), both of which were also measured by the GBD.
Data showed that worldwide deaths related to viral hepatitis numbered 0.89 million in 1990 (95% uncertainty interval [UI]: 0.86-0.94), but jumped up dramatically to 1.45 million in 2013 (95% UI: 1.38-1.54). Over the same time period, YLLs and YLDs also increased, going from 31.0 million (95% UI: 29.6-32.6) and 0.65 million (95% UI: 0.45-0.89), respectively, in 1990, to 41.6 million (95% UI: 39.1-44.7), and 0.87 million (95% UI: 0.61-1.18), respectively, in 2013. Consequently, DALYs increased from 31.7 million in 1990 (95% UI: 30.2-33.3) to 42.5 million (95% UI: 39.9-45.6).
These figures represent a 34% increase in viral hepatitis disease burden over that period of time. Furthermore, viral hepatitis went from being the 10th leading cause of death in the world in 1990 (95% UI: 10-12) to the seventh leading cause in 2013 (95% UI: 7-8). However, analysis without the data’s demographic trends showed that YLL and YLD rates declined by 20% and 13%, respectively (95% UI: 8-30 and 8-18), while DALY rates dropped by 20% (95% UI: 8-30) with no significant trend detected in age-specific mortality rates, indicating that demographic changes such as population growth may be the biggest factor contributing to viral hepatitis’ growing disease burden.
“HAV is the only hepatitis virus for which DALYs have declined significantly between 1990 and 2013. Some of this decline has been driven by changing population age structures, but most is due to declines in age-specific rates, most likely as a result of vaccination and improvements in water supply and sanitation,” the authors noted.
Dr. Stanaway and his coauthors also urged public health institutions around the world to devote more funding to targeting viral hepatitis, noting that the current state of funding is “disproportionate to [viral hepatitis’] importance as a major cause of death and disability.”
The Bill & Melinda Gates Foundation funded the study. Coauthor Graham S. Cooke, MD, reported being an investigator on trials of hepatitis C virus therapy sponsored by Boehringer Ingelheim, Gilead, Merck, and Bristol-Myers Squibb, and has acted in an advisory role to Merck, Boehringer Ingelheim, Gilead, Janssen, and WHO in relation to viral hepatitis and clinical trials unrelated to this work. Dr. Stanaway and other coauthors did not report any relevant financial disclosures.
Although the disease burden of most other prevalent communicable diseases has gone down considerably over the last 25 years, viral hepatitis continues to be a challenge for health care professionals around the world, as incidences of the disease have climbed steadily between 1990 and 2013.
Advances in treating hepatitis A, B, C, and E viruses over the last 25 years have helped to “overcome many barriers to the control and treatment of viral hepatitis in low-income countries and are set to be important components of a new global health strategy,” wrote the study investigators, led by Jeffrey D. Stanaway, MD, of the University of Washington, Seattle. “However, a better understanding of the burden of disease is required to guide these efforts.”
Dr. Stanaway and his coauthors looked at the Global Burden of Disease (GBD) study for data on worldwide morbidity and mortality associated with hepatitis A, B, C, and E viruses, as well as cirrhosis and liver cancer secondary to hepatitis B or C virus. Data collected for the GBD was used to determine disability-adjusted life-years (DALYs), which is a metric calculated by adding up years of life lost (YLL) and years lived with disability (YLD), both of which were also measured by the GBD.
Data showed that worldwide deaths related to viral hepatitis numbered 0.89 million in 1990 (95% uncertainty interval [UI]: 0.86-0.94), but jumped up dramatically to 1.45 million in 2013 (95% UI: 1.38-1.54). Over the same time period, YLLs and YLDs also increased, going from 31.0 million (95% UI: 29.6-32.6) and 0.65 million (95% UI: 0.45-0.89), respectively, in 1990, to 41.6 million (95% UI: 39.1-44.7), and 0.87 million (95% UI: 0.61-1.18), respectively, in 2013. Consequently, DALYs increased from 31.7 million in 1990 (95% UI: 30.2-33.3) to 42.5 million (95% UI: 39.9-45.6).
These figures represent a 34% increase in viral hepatitis disease burden over that period of time. Furthermore, viral hepatitis went from being the 10th leading cause of death in the world in 1990 (95% UI: 10-12) to the seventh leading cause in 2013 (95% UI: 7-8). However, analysis without the data’s demographic trends showed that YLL and YLD rates declined by 20% and 13%, respectively (95% UI: 8-30 and 8-18), while DALY rates dropped by 20% (95% UI: 8-30) with no significant trend detected in age-specific mortality rates, indicating that demographic changes such as population growth may be the biggest factor contributing to viral hepatitis’ growing disease burden.
“HAV is the only hepatitis virus for which DALYs have declined significantly between 1990 and 2013. Some of this decline has been driven by changing population age structures, but most is due to declines in age-specific rates, most likely as a result of vaccination and improvements in water supply and sanitation,” the authors noted.
Dr. Stanaway and his coauthors also urged public health institutions around the world to devote more funding to targeting viral hepatitis, noting that the current state of funding is “disproportionate to [viral hepatitis’] importance as a major cause of death and disability.”
The Bill & Melinda Gates Foundation funded the study. Coauthor Graham S. Cooke, MD, reported being an investigator on trials of hepatitis C virus therapy sponsored by Boehringer Ingelheim, Gilead, Merck, and Bristol-Myers Squibb, and has acted in an advisory role to Merck, Boehringer Ingelheim, Gilead, Janssen, and WHO in relation to viral hepatitis and clinical trials unrelated to this work. Dr. Stanaway and other coauthors did not report any relevant financial disclosures.
FROM THE LANCET
Key clinical point: Viral hepatitis increased from 1990 through 2013 despite other communicable diseases decreasing in prevalence, necessitating more effort from the health care community to mitigate the disease burden.
Major finding: Viral hepatitis deaths worldwide were 0.89 million in 1990, and 1.45 million in 2013 (95% uncertainty interval: 0.86-0.94 and 1.38-1.54, respectively).
Data source: Retrospective analysis of data from the Global Burden of Disease study on acute viral hepatitis, cirrhosis, and liver cancer caused by viral hepatitis.
Disclosures: The Bill & Melinda Gates Foundation funded the study. Some coauthors disclosed potential conflicts of interest.
Violent experiences increase risk of violent behavior in patients, controls
Exposure to violence significantly increases the chance that a person will commit a violent crime in the subsequent week – whether or not that person has an existing mental illness.
Absolute risk was highest among people with schizophrenia, with a violent crime rate of 177 per 10,000 individuals, compared with 22 per 10,000 before the trigger event. But although that was significantly higher than the rate seen among patients with bipolar disorder and normal controls, those groups also experienced significant increases in violent behavior after a violent experience (83 vs. 13 per 10,000 and 70 vs. 9 per 10,000, respectively), Amir Sariaslan, PhD, wrote July 13 online in JAMA Psychiatry (doi: 10.1001/jamapsychiatry.2016.1349).
The Swedish national study, which comprised more than 3 million people, found other triggers for transient violent criminality among both patients and controls. These included traumatic brain injuries, unintentional injuries, self-harm, substance intoxication, and parental bereavement, wrote Dr. Sariaslan of the University of Oxford (England), and his colleagues.
“We also observed that the triggers had less effect as follow-up time increased,” the team noted. “These findings support the hypothesis that recent exposure to a stressful life event, an intentional or unintentional injury, or having been diagnosed with substance intoxication increases the short-term risk of interpersonal violence.”
The study comprised 64,595 patients diagnosed with psychotic disorders and in 2.76 million controls. Since it spanned 13 years, each person also was able to serve as his own individual control as well. The model examined both absolute and relative risks, and controlled for a large number of sociodemographic, clinical, and psychosocial variables.
Exposure to violence was the strongest precipitating factor for violent crime, exerting an increased relative risk of almost 13-fold among patients with schizophrenia, and 8-fold among both patients with bipolar disorder and controls.
The increased risks associated with traumatic brain injury were 6.7 for those with schizophrenia, 4.3 for those with bipolar disorder and almost 8 for controls. For self-harm, the risk hovered around fourfold for all groups.
The risks for unintentional injuries ranged from 3.5-4.8; and for substance intoxication, from 3.0-4.0.
The increased risks associated with parental bereavement showed a slightly different pattern, being sharply increased among those with schizophrenia, compared with controls (5.0 vs. 1.7), the investigators wrote.
“An explanation for this finding is that elevated levels of social support from family members and close friends in the controls may be protective against violence,” they suggested.
The study also determined that the effects of these triggers weakened over time. Again, this observation was most obvious with exposure to violence, and in the group with schizophrenia. The increased risk of committing a violent crime dropped from a high of 12.7 in the first week to baseline by the second week.
The finding of an increased risk of violent behavior after an incident of self-harm is a novel one, the investigators added.
“Our findings suggest that self-harming patients, particularly those with psychoses, are an important group to be assessed for interpersonal violence in addition to the routinely examined risk of suicide,” they wrote.
The study was supported by the Wellcome Trust, and grants from Swedish governmental agencies. None of the authors had any financial disclosures.
Acting with violence after being exposed to violence seems to be a “universal phenomenon,” Jan Volavka, MD, PhD, wrote in an accompanying editorial – and this intriguing observation should spark clinicians to assess and intervene early.
The innate stress response system probably mediates the link between experiencing and perpetrating violence. This has been proven in rat models, he said, “where stressors activate the hypothalamic-pituitary-adrenal axis and glucocorticoids are released, which leads to increased sensitivity to aggression-promoting stimuli.”
The findings of this strong – albeit transitory – increase in the propensity for violent action are a very strong argument for proactively assessing patients who experience a violent incident.
“Clinically, these findings imply that patients with schizophrenia or bipolar disorder should receive a psychiatric assessment for the risk of violence if they sustain an experience similar to one of the triggers tested in this study. The need for assessment is particularly pressing for young patients who have been targets of violence.”
Because of the time-bound nature of the reaction, “the assessment should occur as soon as possible after the event; certainly, within the first week. Depending on the results, the patient may need supportive psychotherapy, medication adjustment, or hospitalization. In general, the findings raise the need to treat comorbid substance use disorders in individuals with schizophrenia and bipolar disorder.”
Dr. Volavka is a professor of psychiatry emeritus at the New York University.
Acting with violence after being exposed to violence seems to be a “universal phenomenon,” Jan Volavka, MD, PhD, wrote in an accompanying editorial – and this intriguing observation should spark clinicians to assess and intervene early.
The innate stress response system probably mediates the link between experiencing and perpetrating violence. This has been proven in rat models, he said, “where stressors activate the hypothalamic-pituitary-adrenal axis and glucocorticoids are released, which leads to increased sensitivity to aggression-promoting stimuli.”
The findings of this strong – albeit transitory – increase in the propensity for violent action are a very strong argument for proactively assessing patients who experience a violent incident.
“Clinically, these findings imply that patients with schizophrenia or bipolar disorder should receive a psychiatric assessment for the risk of violence if they sustain an experience similar to one of the triggers tested in this study. The need for assessment is particularly pressing for young patients who have been targets of violence.”
Because of the time-bound nature of the reaction, “the assessment should occur as soon as possible after the event; certainly, within the first week. Depending on the results, the patient may need supportive psychotherapy, medication adjustment, or hospitalization. In general, the findings raise the need to treat comorbid substance use disorders in individuals with schizophrenia and bipolar disorder.”
Dr. Volavka is a professor of psychiatry emeritus at the New York University.
Acting with violence after being exposed to violence seems to be a “universal phenomenon,” Jan Volavka, MD, PhD, wrote in an accompanying editorial – and this intriguing observation should spark clinicians to assess and intervene early.
The innate stress response system probably mediates the link between experiencing and perpetrating violence. This has been proven in rat models, he said, “where stressors activate the hypothalamic-pituitary-adrenal axis and glucocorticoids are released, which leads to increased sensitivity to aggression-promoting stimuli.”
The findings of this strong – albeit transitory – increase in the propensity for violent action are a very strong argument for proactively assessing patients who experience a violent incident.
“Clinically, these findings imply that patients with schizophrenia or bipolar disorder should receive a psychiatric assessment for the risk of violence if they sustain an experience similar to one of the triggers tested in this study. The need for assessment is particularly pressing for young patients who have been targets of violence.”
Because of the time-bound nature of the reaction, “the assessment should occur as soon as possible after the event; certainly, within the first week. Depending on the results, the patient may need supportive psychotherapy, medication adjustment, or hospitalization. In general, the findings raise the need to treat comorbid substance use disorders in individuals with schizophrenia and bipolar disorder.”
Dr. Volavka is a professor of psychiatry emeritus at the New York University.
Exposure to violence significantly increases the chance that a person will commit a violent crime in the subsequent week – whether or not that person has an existing mental illness.
Absolute risk was highest among people with schizophrenia, with a violent crime rate of 177 per 10,000 individuals, compared with 22 per 10,000 before the trigger event. But although that was significantly higher than the rate seen among patients with bipolar disorder and normal controls, those groups also experienced significant increases in violent behavior after a violent experience (83 vs. 13 per 10,000 and 70 vs. 9 per 10,000, respectively), Amir Sariaslan, PhD, wrote July 13 online in JAMA Psychiatry (doi: 10.1001/jamapsychiatry.2016.1349).
The Swedish national study, which comprised more than 3 million people, found other triggers for transient violent criminality among both patients and controls. These included traumatic brain injuries, unintentional injuries, self-harm, substance intoxication, and parental bereavement, wrote Dr. Sariaslan of the University of Oxford (England), and his colleagues.
“We also observed that the triggers had less effect as follow-up time increased,” the team noted. “These findings support the hypothesis that recent exposure to a stressful life event, an intentional or unintentional injury, or having been diagnosed with substance intoxication increases the short-term risk of interpersonal violence.”
The study comprised 64,595 patients diagnosed with psychotic disorders and in 2.76 million controls. Since it spanned 13 years, each person also was able to serve as his own individual control as well. The model examined both absolute and relative risks, and controlled for a large number of sociodemographic, clinical, and psychosocial variables.
Exposure to violence was the strongest precipitating factor for violent crime, exerting an increased relative risk of almost 13-fold among patients with schizophrenia, and 8-fold among both patients with bipolar disorder and controls.
The increased risks associated with traumatic brain injury were 6.7 for those with schizophrenia, 4.3 for those with bipolar disorder and almost 8 for controls. For self-harm, the risk hovered around fourfold for all groups.
The risks for unintentional injuries ranged from 3.5-4.8; and for substance intoxication, from 3.0-4.0.
The increased risks associated with parental bereavement showed a slightly different pattern, being sharply increased among those with schizophrenia, compared with controls (5.0 vs. 1.7), the investigators wrote.
“An explanation for this finding is that elevated levels of social support from family members and close friends in the controls may be protective against violence,” they suggested.
The study also determined that the effects of these triggers weakened over time. Again, this observation was most obvious with exposure to violence, and in the group with schizophrenia. The increased risk of committing a violent crime dropped from a high of 12.7 in the first week to baseline by the second week.
The finding of an increased risk of violent behavior after an incident of self-harm is a novel one, the investigators added.
“Our findings suggest that self-harming patients, particularly those with psychoses, are an important group to be assessed for interpersonal violence in addition to the routinely examined risk of suicide,” they wrote.
The study was supported by the Wellcome Trust, and grants from Swedish governmental agencies. None of the authors had any financial disclosures.
Exposure to violence significantly increases the chance that a person will commit a violent crime in the subsequent week – whether or not that person has an existing mental illness.
Absolute risk was highest among people with schizophrenia, with a violent crime rate of 177 per 10,000 individuals, compared with 22 per 10,000 before the trigger event. But although that was significantly higher than the rate seen among patients with bipolar disorder and normal controls, those groups also experienced significant increases in violent behavior after a violent experience (83 vs. 13 per 10,000 and 70 vs. 9 per 10,000, respectively), Amir Sariaslan, PhD, wrote July 13 online in JAMA Psychiatry (doi: 10.1001/jamapsychiatry.2016.1349).
The Swedish national study, which comprised more than 3 million people, found other triggers for transient violent criminality among both patients and controls. These included traumatic brain injuries, unintentional injuries, self-harm, substance intoxication, and parental bereavement, wrote Dr. Sariaslan of the University of Oxford (England), and his colleagues.
“We also observed that the triggers had less effect as follow-up time increased,” the team noted. “These findings support the hypothesis that recent exposure to a stressful life event, an intentional or unintentional injury, or having been diagnosed with substance intoxication increases the short-term risk of interpersonal violence.”
The study comprised 64,595 patients diagnosed with psychotic disorders and in 2.76 million controls. Since it spanned 13 years, each person also was able to serve as his own individual control as well. The model examined both absolute and relative risks, and controlled for a large number of sociodemographic, clinical, and psychosocial variables.
Exposure to violence was the strongest precipitating factor for violent crime, exerting an increased relative risk of almost 13-fold among patients with schizophrenia, and 8-fold among both patients with bipolar disorder and controls.
The increased risks associated with traumatic brain injury were 6.7 for those with schizophrenia, 4.3 for those with bipolar disorder and almost 8 for controls. For self-harm, the risk hovered around fourfold for all groups.
The risks for unintentional injuries ranged from 3.5-4.8; and for substance intoxication, from 3.0-4.0.
The increased risks associated with parental bereavement showed a slightly different pattern, being sharply increased among those with schizophrenia, compared with controls (5.0 vs. 1.7), the investigators wrote.
“An explanation for this finding is that elevated levels of social support from family members and close friends in the controls may be protective against violence,” they suggested.
The study also determined that the effects of these triggers weakened over time. Again, this observation was most obvious with exposure to violence, and in the group with schizophrenia. The increased risk of committing a violent crime dropped from a high of 12.7 in the first week to baseline by the second week.
The finding of an increased risk of violent behavior after an incident of self-harm is a novel one, the investigators added.
“Our findings suggest that self-harming patients, particularly those with psychoses, are an important group to be assessed for interpersonal violence in addition to the routinely examined risk of suicide,” they wrote.
The study was supported by the Wellcome Trust, and grants from Swedish governmental agencies. None of the authors had any financial disclosures.
FROM JAMA PSYCHIATRY
Key clinical point: Exposure to violence transiently increases the risk of perpetrating violence, whether or not a mental illness is present.
Major finding: A violent experience increased the risk of committing violent crime by almost 13-fold among patients with schizophrenia and by 8-fold among both patients with bipolar disorder and controls.
Data source: The Swedish national population study comprised almost 3.5 million people.
Disclosures: The study was supported by the Wellcome Trust, and grants from Swedish governmental agencies. None of the authors had any financial disclosures.