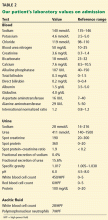
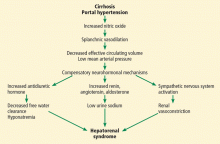
User login
Is there a time limit for systemic menopausal hormone therapy?
The duration of hormone therapy needs to be an individualized decision, shared between the patient and her physician and assessed annually. Quality of life, vasomotor symptoms, current age, time since menopause, hysterectomy status, personal risks (of osteoporosis, breast cancer, heart disease, stroke, venous thromboembolism), and patient preferences need to be considered.
The North American Menopause Society (NAMS) and other organizations recommend that the lowest dose of hormone therapy be used for the shortest duration needed to manage menopausal symptoms.1–4 However, NAMS states that extending the duration of hormone therapy may be appropriate in women who have persistent symptoms or to prevent osteoporosis if the patient cannot tolerate alternative therapies.1
Forty-two percent of postmenopausal women continue to experience vasomotor symptoms at age 60 to 65.5 The median total duration of vasomotor symptoms is 7.4 years, and in black women and women with moderate or severe hot flashes the symptoms typically last 10 years.6 Vasomotor symptoms recur in 50% of women who discontinue hormone therapy, regardless of whether it is stopped abruptly or tapered.1
FACTORS TO CONSIDER WHEN PRESCRIBING HORMONE THERAPY
Bone health
A statement issued in 2013 by seven medical societies said that hormone therapy is effective and appropriate for preventing osteoporosis-related fracture in at-risk women under age 60 or within 10 years of menopause.7
The Women’s Health Initiative,8 a randomized placebo-controlled trial, showed a statistically significant lower risk of vertebral and nonvertebral fracture after 3 years of use of conjugated equine estrogen with medroxyprogesterone acetate than with placebo:
- Hazard ratio 0.76, 95% confidence interval (CI) 0.69–0.83.
It also showed a mean increase of 3.7% (P < .001) in total hip bone mineral density. By the end of the trial intervention, women receiving either this combined therapy or conjugated equine estrogen alone saw a 33% overall reduction in hip fracture risk. The absolute risk reduction was 5 per 10,000 years of use.9
Karim et al,10 in a large observational study that followed initial hormone therapy users over 6.5 years, found that those who stopped it had a 55% greater risk of hip fracture and experienced significant bone loss as measured by bone mineral density compared with women who continued hormone therapy, and that the protective effects of hormone therapy disappeared as early as 2 years after stopping treatment.10
NAMS also recommends that women with premature menopause (before age 40) be offered and encouraged to use hormone therapy to preserve bone density and manage vasomotor symptoms until the age of natural menopause (age 51).1,11
Cardiovascular health
Large observational studies have found that hormone therapy is associated with a 30% to 50% lower cardiovascular risk.12 Randomized controlled trials of hormone therapy for 7 to 11 years suggest that coronary heart disease risk is modified by age and time since menopause.13,14
The Women’s Health Initiative and other randomized controlled trials suggest a lower risk of coronary heart disease in women who begin hormone therapy before age 60 and within 10 years of the onset of menopause, but an increased risk for women over age 60 and more than 10 years since menopause. However, several of these trends have not reached statistical significance (Table 1).13–15
The Women’s Health Initiative9 published its long-term follow-up results in 2013, with data on both the intervention phase (median of 7.2 years for estrogen-only therapy and 5.6 years for estrogen-progestin therapy) and the post-stopping phase (median 6.6 years for the estrogen-only group and 8.2 years for the estrogen-progestin group), with a total cumulative follow-up of 13 years. The overall 13-year cumulative absolute risk of coronary heart disease was 4 fewer events per 10,000 years of estrogen-only therapy and 3 additional events per 10,000 years of estrogen-progestin therapy. Neither result was statistically significant:
- Hazard ratio with estrogen-only use 0.94, 95% CI 0.82–1.09
- Hazard ratio with estrogen-progestin use 1.09, 95% CI 0.92–1.24.
The Danish Osteoporosis Study was the first randomized controlled trial of hormone therapy in women ages 45 through 58 who were recently menopausal (average within 7 months of menopause).15 Women assigned to hormone therapy in the form of oral estradiol with or without norethisterone (known as norethindrone in the United States) had a statistically significant lower risk of the primary composite end point of heart failure and myocardial infarction after 11 years of hormone therapy, and this finding persisted through 16 years of follow-up (Table 1).
Stroke
Overall stroke risk was significantly increased with hormone therapy in the Women’s Health Initiative trial (hazard ratio 1.32, 95% CI 1.12–1.56); however, the absolute increase in risk was small in both estrogen-alone and estrogen-progestin therapy users, 11 and 8 events, respectively, among 10,000 users. Younger women (ages 50–59) saw a nonsignificantly lower risk (2 fewer cases per 10,000 years of use).14 After 13 years of cumulative follow-up (combined intervention and follow-up phase), the risk of stroke persisted at 5 cases per 10,000 users for both arms, but only the estrogen-progestin results were statistically significant.9
The Danish Osteoporosis Study15 found no increased risk of stroke after 16 years of follow-up in recently menopausal women:
- Hazard ratio 0.89, 95% CI 0.48–1.65.
Venous thromboembolism
Data from both observational and randomized controlled trials demonstrate an increased risk of venous thromboembolism with oral hormone therapy, and the risk appears to be highest during the first few years of use.1 The pooled cohort from the Women’s Health Initiative had 18 additional cases of venous thromboembolism per 10,000 women in estrogen-progestin users compared with nonusers, and 7 additional cases in those using estrogen-only therapy.
Breast health
Observational studies and randomized controlled trials have provided data on longer use of hormone therapy and breast cancer risk, but the true magnitude of this risk is unclear.
The Danish Osteoporosis Study,15 in a younger cohort of women, showed no increased risk of breast cancer after 16 years of follow-up:
- Hazard ratio 0.90, 95% CI 0.52–1.57.
The Women’s Health Initiative9 showed a statistically nonsignificant lower risk of breast cancer in women of all ages exposed to conjugated equine estrogen alone for 7.1 years (6 fewer cases per 10,000 women-years of use), and after 6 years of follow-up this developed statistical significance:
- Hazard ratio 0.79, 95% CI 0.65–0.97.
In contrast, those using conjugated equine estrogen plus medroxyprogesterone acetate had a statistically nonsignificant increase in the risk of new breast cancer after 3 to 5 years:
- 3-year relative risk 1.26, 95% CI 0.73–2.20
- 5-year relative risk 1.99, 95% CI 1.18–3.35
- Absolute risk 8 cases per 10,000 women-years of use.
The increased risk of breast cancer significantly declined within 3 years after stopping hormone therapy.
However, even after stopping hormone therapy, there remains a statistically small but significant increased risk of breast cancer, as demonstrated in the postintervention 13-year follow-up data on breast cancer risk and estrogen-progestin use from the Women’s Health Initiative9:
- Hazard ratio 1.28, 95% CI 1.11–1.48
- Absolute cumulative risk 9 cases per 10,000 women-years of use.
The Nurses’ Health Study, an observational study, prospectively followed 11,508 hysterectomized women on estrogen therapy and found that breast cancer risk increased with longer duration of use. An analysis by Chen et al16 found a trend toward increased breast cancer risk after 10 years of estrogen therapy, but this did not become statistically significant until 20 years of ongoing estrogen use. The risk of estrogen receptor-positive and progesterone receptor-positive breast cancer became statistically significant earlier, after 15 years. The relative risk associated with using estrogen for more than 15 years was 1.18, and the risk with using it for more than 20 years was 1.42.16
To put this in perspective, Chen et al17 found a similar breast cancer risk with alcohol consumption. The relative risk of invasive breast cancer was 1.15 in women who drank 3 to 6 servings of alcohol per week, 1 serving being equivalent to 4 oz of wine, which contains 11 g of alcohol.
Mortality
Studies have suggested that hormone therapy users have a lower mortality rate, even with long-term use.
A meta-analysis18 of 8 observational trials and 19 randomized controlled trials found that younger women (average age 54) on hormone therapy had a 28% lower total mortality rate compared with women not taking hormone therapy:
- Relative risk 0.72, 95% credible interval 0.62–0.82.
The Women’s Health Initiative19 suggested that the mortality rate was 30% lower in hormone therapy users younger than age 60 than in similar nonusers, though this difference did not reach statistical significance.
- Relative risk with estrogen-only therapy: 0.71, 95% CI 0.46–1.11
- Relative risk with combined estrogen-progestin therapy 0.69, 95% CI 0.44–1.07.
The Danish Osteoporosis Study,15 at 16 years of follow-up, similarly demonstrated a 34% lower mortality rate in hormone therapy users, which was not statistically significant:
- Relative risk 0.66, 95% CI 0.41–1.08.
A Cochrane review20 in 2015 found that the subgroup of women who started hormone therapy before age 60 or within 10 years of menopause saw an overall benefit in terms of survival and lower risk of coronary heart disease: RR 0.70, 95% CI 0.52–0.95 (moderate-quality evidence).
TYPE OF FORMULATION
Compared with estrogen-progestin therapy, estrogen-only therapy has a more favorable risk profile in terms of coronary heart disease and breast cancer, although stroke risk remains elevated in users of conjugated equine estrogen with or without medroxyprogesterone acetate.
There is limited evidence directly comparing different formulations of hormone therapy, although they all effectively treat vasomotor symptoms.1
Oral vs transdermal formulations
Canonico et al,21 in a meta-analysis of observational studies, found that oral estrogen was associated with a higher risk of venous thromboembolism than transdermal estrogen:
- Relative risk with oral estrogen 2.5, 95% CI 1.9–3.4
- Relative risk with transdermal estrogen 1.2, 95% CI 0.9–1.7.
The Estrogen and Thromboembolism Risk (ESTHER) study22 was a multicenter case-control study of women ages 45 to 70 that assessed risk of venous thromboembolism in oral vs transdermal estrogen users. Compared with women not taking hormone therapy, current users of oral estrogen had a significantly higher risk of venous thromboembolism, while transdermal estrogen users did not:
- Odds ratio with oral estrogen 4.2, 95% CI 1.5–11.6
- Odds ratio with transdermal estrogen 0.9, 95% CI 0.4–2.1.
The Kronos Early Estrogen Prevention Study (KEEPS)23 did not support these findings. This 4-year randomized controlled trial, published in 2014, was designed to assess the risk of atherosclerosis progression with early menopause initiation of placebo vs low-dose oral hormone therapy (conjugated equine estrogen 0.45 mg daily with cyclical micronized progesterone) or transdermal hormone therapy (estradiol 50 µg/week with cyclical micronized progesterone).
In the 727 women in the study, there was one transient ischemic attack in the oral hormone therapy group, one unconfirmed stroke in the transdermal hormone therapy group, and one case of venous thromboembolism in each group, findings that were underpowered for statistical significance. Both oral and transdermal hormonal therapy had neutral effects on atherosclerosis progression, as assessed by arterial imaging. Transdermal hormone therapy was associated with improvements in markers of insulin resistance and was not associated with an increase in triglycerides, C-reactive protein, or sex hormone-binding globulin, as would be expected with transdermal circumvention of the first-pass hepatic effect.
BALANCING THE RISKS AND BENEFITS FOR THE PATIENT
The most effective treatment for vasomotor symptoms in women at any age is hormone therapy, and the benefits are more likely to outweigh risks when initiated before age 60 or within 10 years of menopause.7 The Women’s Health Initiative randomized study was limited to 5.6 to 7.2 years of hormone therapy (13 years of cumulative follow-up), and the Danish Osteoporosis Study was limited to 11 years of use (16 years cumulative follow-up).
The coronary heart disease outcomes for longer durations of therapy remain uncertain. There is a small but statistically significant increased risk of stroke and venous thromboembolism with oral hormone therapy, and breast cancer risk is associated with long-term estrogen-progestin use.
Patients on hormone therapy should be evaluated annually regarding the need for ongoing therapy. Persistent moderate-severe vasomotor symptoms, quality of life benefits of hormone therapy, contraindications to its use (Table 2), and patient preference need to be assessed as well as baseline risks of cardiovascular disease, breast cancer, and fracture.
Risk calculators may facilitate the shared decision-making process. Examples are:
- The American College of Cardiology/American Heart association risk calculator for cardiovascular disease24 (www.cvriskcalculator.com)
- The World Health Organization Fracture Risk Assessment Tool (FRAX)25
(www.shef.ac.uk/FRAX/tool.jsp)
- The Gail model for breast cancer risk26 (www.cancer.gov/bcrisktool/).
- MenoPro, a menopause decision-support algorithm and companion mobile app developed by NAMS to help direct treatment decisions based on the 10-year risk of atherosclerotic cardiovascular disease (www.menopause.org/for-professionals/-i-menopro-i-mobile-app).27
The discussion of the risks of hormone therapy with patients should incorporate the perspective of absolute risk. For example, a woman wishing to continue estrogen-progestin therapy should be told that the Women’s Health Initiative data suggest that, after 5 years of use, breast cancer risk may be increased by 8 additional cases per 10,000 users per year. According to the World Health Organization, this magnitude of risk is defined as rare (less than 1 event per 1,000 women).28
A strategy of prescribing the lowest dose to achieve the desired clinical benefits is prudent and recommended.1–3 Table 3 outlines the estrogen formulations now available in the United States, with their doses and formulations.
Unless contraindications develop (Table 2), patients may elect to continue hormone therapy if its benefits outweigh its risks. The American College of Obstetricians and Gynecologists (ACOG) 2014 practice recommendations for management of menopausal symptoms31 and the 2015 NAMS statement both recommend that hormone therapy not be discontinued based solely on a woman’s age.29
Hormone therapy is on the Beer’s list of potentially inappropriate medications for older adults,30 which remains a hurdle to its long-term use and seems to be at odds with these ACOG and NAMS statements.
Patients who choose to discontinue hormone therapy need to be monitored for persistent bothersome vasomotor symptoms, bone loss, osteoporosis, and the genitourinary syndrome of menopause (previously referred to as vulvovaginal atrophy)31 and offered alternative therapies if needed.
- North American Menopause Society. The 2012 hormone therapy position statement of: The North American Menopause Society. Menopause 2012; 19:257–271.
- American College of Obstetricians and Gynecologists. Practice Bulletin No. 141: Management of menopausal symptoms. Obstet Gynecol 2014; 123:202–216.
- Stuenkel CA, Davis SR, Gompel A, et al. Treatment of symptoms of the menopause: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2015; 100:3975–4011.
- de Villiers TJ, Pines A, Panay N, et al; International Menopause Society. Updated 2013 International Menopause Society recommendations on menopausal hormone therapy and preventive strategies for midlife health. Climacteric 2013; 16:316–337.
- Gartoulla P, Worsley R, Robin J, Davis S. Moderate to severe vasomotor and sexual symptoms remain problematic for women aged 60 to 65 years. Menopause 2015; 22:694–701.
- Avis NE, Crawford SL, Greendale G, et al. Duration of menopausal vasomotor symptoms across the menopause transition. JAMA Intern Med 2015; 175:531–539.
- de Villiers TJ, Gass ML, Haines CJ, et al. Global consensus statement on menopausal hormone therapy. Climacteric 2013; 16:203–204.
- Cauley J, Robbins J, Chen Z, et al. Effects of estrogen plus progestin on risk of fracture and bone mineral density: the Women’s Health Initiative randomized trial. JAMA 2003; 290:1729–1738.
- Manson J, Chlebowski R, Stefanick M, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA 2013; 310:1353–1368.
- Karim R, Dell RM, Greene DF, et al. Hip fracture in postmenopausal women after cessation of hormone therapy: results from a prospective study in a large health management organization. Menopause 2011; 18:1172–1177.
- Shifren J, Gass M, and the NAMS Recommendations for Clinical Care of Midlife Women Working Group. The North American Menopause Society recommendations for clinical care of midlife women. Menopause 2014; 21:1038–1062.
- Hodis HN, Mack WJ. Hormone replacement therapy and the association with coronary heart disease and overall mortality: clinical application of the timing hypothesis. J Steroid Biochem Mol Biol 2014; 142:68–75.
- Salpeter SR, Walsh JM, Greyber E, et al. Brief report: coronary heart disease events associated with hormone therapy in younger and older women. A meta-analysis. J Gen Intern Med 2006; 21:363–366.
- Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA 2007; 297:1465–1477.
- Schierbeck LL, Rejnmark L, Tofteng CL, et al. Effect of hormone replacement therapy on cardiovascular events in recently postmenopausal women: randomised trial. BMJ 2012; 345:e6409.
- Chen WY, Manson JE, Hankinson SE, et al. Unopposed estrogen therapy and the risk of breast cancer. Arch Intern Med 2006; 166:1027–1032.
- Chen W, Rosner B, Hankinson SE, et al. Moderate alcohol consumption during adult life, drinking patterns, and breast cancer risk. JAMA 2011; 306:1884–1890.
- Salpeter SR, Cheng J, Thabane L, et al. Bayesian meta-analysis of hormone therapy and mortality in younger postmenopausal women. Am J Med 2009; 122:1016–1022.
- Hodis HN, Collins P, Mack WJ, Schierbeck LL. The timing hypothesis for coronary heart disease prevention with hormone therapy: past, present and future in perspective. Climacteric 2012; 15:217–228.
- Boardman HM, Hartley L, Eisinga A, et al. Hormone therapy for preventing cardiovascular disease in post-menopausal women. Cochrane Database Syst Rev 2015;3:CD002229.
- Canonico M, Plu-Bureau G, Lowe GD, et al. Hormone replacement therapy and risk of venous thromboembolism in postmenopausal women: systemic review and meta-analysis. BMJ 2008; 336:1227–1231.
- Canonico M, Oger E, Plu-Bureau G, et al; Estrogen and Thromboembolism Risk (ESTHER) Study Group. Hormone therapy and venous thromboembolism among postmenopausal women: impact of the route of estrogen administration and progestogens: the ESTHER study. Circulation 2007; 115:840–845.
- Harman S, Black D, Naftolin F, et al. Arterial imaging outcomes and cardiovascular risk factors in recently menopausal women. Ann Intern Med 2014; 161:249–260.
- Goff DC Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 63:2935–2959.
- World Health Organization Collaborating Centre for Metabolic Bone Diseases. FRAX WHO fracture risk assessment tool. www.shef.ac.uk/FRAX/. Accessed May 27, 2016.
- Gail M, Brinton L, Byar D, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst 1989; 81:1879–1886.
- Manson J, Ames J, Shapiro M, et al. Algorithm and mobile app for menopausal symptom management and hormonal/non-hormonal therapy decision making: a clinical decision-support tool from the North American Menopause Society. Menopause 2015; 22:247–253.
- Hodis HN, Mack WJ. Postmenopausal hormone therapy in clinical perspective. Menopause 2007; 14:944–957.
- North American Menopause Society. The North American Menopause Society statement on continuing use of systemic hormone therapy after the age of 65. Menopause 2015; 22:693.
- American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2015; 63:2227–2246.
- Portman DJ, Gass ML; Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause 2014; 21:1063–1068.
The duration of hormone therapy needs to be an individualized decision, shared between the patient and her physician and assessed annually. Quality of life, vasomotor symptoms, current age, time since menopause, hysterectomy status, personal risks (of osteoporosis, breast cancer, heart disease, stroke, venous thromboembolism), and patient preferences need to be considered.
The North American Menopause Society (NAMS) and other organizations recommend that the lowest dose of hormone therapy be used for the shortest duration needed to manage menopausal symptoms.1–4 However, NAMS states that extending the duration of hormone therapy may be appropriate in women who have persistent symptoms or to prevent osteoporosis if the patient cannot tolerate alternative therapies.1
Forty-two percent of postmenopausal women continue to experience vasomotor symptoms at age 60 to 65.5 The median total duration of vasomotor symptoms is 7.4 years, and in black women and women with moderate or severe hot flashes the symptoms typically last 10 years.6 Vasomotor symptoms recur in 50% of women who discontinue hormone therapy, regardless of whether it is stopped abruptly or tapered.1
FACTORS TO CONSIDER WHEN PRESCRIBING HORMONE THERAPY
Bone health
A statement issued in 2013 by seven medical societies said that hormone therapy is effective and appropriate for preventing osteoporosis-related fracture in at-risk women under age 60 or within 10 years of menopause.7
The Women’s Health Initiative,8 a randomized placebo-controlled trial, showed a statistically significant lower risk of vertebral and nonvertebral fracture after 3 years of use of conjugated equine estrogen with medroxyprogesterone acetate than with placebo:
- Hazard ratio 0.76, 95% confidence interval (CI) 0.69–0.83.
It also showed a mean increase of 3.7% (P < .001) in total hip bone mineral density. By the end of the trial intervention, women receiving either this combined therapy or conjugated equine estrogen alone saw a 33% overall reduction in hip fracture risk. The absolute risk reduction was 5 per 10,000 years of use.9
Karim et al,10 in a large observational study that followed initial hormone therapy users over 6.5 years, found that those who stopped it had a 55% greater risk of hip fracture and experienced significant bone loss as measured by bone mineral density compared with women who continued hormone therapy, and that the protective effects of hormone therapy disappeared as early as 2 years after stopping treatment.10
NAMS also recommends that women with premature menopause (before age 40) be offered and encouraged to use hormone therapy to preserve bone density and manage vasomotor symptoms until the age of natural menopause (age 51).1,11
Cardiovascular health
Large observational studies have found that hormone therapy is associated with a 30% to 50% lower cardiovascular risk.12 Randomized controlled trials of hormone therapy for 7 to 11 years suggest that coronary heart disease risk is modified by age and time since menopause.13,14
The Women’s Health Initiative and other randomized controlled trials suggest a lower risk of coronary heart disease in women who begin hormone therapy before age 60 and within 10 years of the onset of menopause, but an increased risk for women over age 60 and more than 10 years since menopause. However, several of these trends have not reached statistical significance (Table 1).13–15
The Women’s Health Initiative9 published its long-term follow-up results in 2013, with data on both the intervention phase (median of 7.2 years for estrogen-only therapy and 5.6 years for estrogen-progestin therapy) and the post-stopping phase (median 6.6 years for the estrogen-only group and 8.2 years for the estrogen-progestin group), with a total cumulative follow-up of 13 years. The overall 13-year cumulative absolute risk of coronary heart disease was 4 fewer events per 10,000 years of estrogen-only therapy and 3 additional events per 10,000 years of estrogen-progestin therapy. Neither result was statistically significant:
- Hazard ratio with estrogen-only use 0.94, 95% CI 0.82–1.09
- Hazard ratio with estrogen-progestin use 1.09, 95% CI 0.92–1.24.
The Danish Osteoporosis Study was the first randomized controlled trial of hormone therapy in women ages 45 through 58 who were recently menopausal (average within 7 months of menopause).15 Women assigned to hormone therapy in the form of oral estradiol with or without norethisterone (known as norethindrone in the United States) had a statistically significant lower risk of the primary composite end point of heart failure and myocardial infarction after 11 years of hormone therapy, and this finding persisted through 16 years of follow-up (Table 1).
Stroke
Overall stroke risk was significantly increased with hormone therapy in the Women’s Health Initiative trial (hazard ratio 1.32, 95% CI 1.12–1.56); however, the absolute increase in risk was small in both estrogen-alone and estrogen-progestin therapy users, 11 and 8 events, respectively, among 10,000 users. Younger women (ages 50–59) saw a nonsignificantly lower risk (2 fewer cases per 10,000 years of use).14 After 13 years of cumulative follow-up (combined intervention and follow-up phase), the risk of stroke persisted at 5 cases per 10,000 users for both arms, but only the estrogen-progestin results were statistically significant.9
The Danish Osteoporosis Study15 found no increased risk of stroke after 16 years of follow-up in recently menopausal women:
- Hazard ratio 0.89, 95% CI 0.48–1.65.
Venous thromboembolism
Data from both observational and randomized controlled trials demonstrate an increased risk of venous thromboembolism with oral hormone therapy, and the risk appears to be highest during the first few years of use.1 The pooled cohort from the Women’s Health Initiative had 18 additional cases of venous thromboembolism per 10,000 women in estrogen-progestin users compared with nonusers, and 7 additional cases in those using estrogen-only therapy.
Breast health
Observational studies and randomized controlled trials have provided data on longer use of hormone therapy and breast cancer risk, but the true magnitude of this risk is unclear.
The Danish Osteoporosis Study,15 in a younger cohort of women, showed no increased risk of breast cancer after 16 years of follow-up:
- Hazard ratio 0.90, 95% CI 0.52–1.57.
The Women’s Health Initiative9 showed a statistically nonsignificant lower risk of breast cancer in women of all ages exposed to conjugated equine estrogen alone for 7.1 years (6 fewer cases per 10,000 women-years of use), and after 6 years of follow-up this developed statistical significance:
- Hazard ratio 0.79, 95% CI 0.65–0.97.
In contrast, those using conjugated equine estrogen plus medroxyprogesterone acetate had a statistically nonsignificant increase in the risk of new breast cancer after 3 to 5 years:
- 3-year relative risk 1.26, 95% CI 0.73–2.20
- 5-year relative risk 1.99, 95% CI 1.18–3.35
- Absolute risk 8 cases per 10,000 women-years of use.
The increased risk of breast cancer significantly declined within 3 years after stopping hormone therapy.
However, even after stopping hormone therapy, there remains a statistically small but significant increased risk of breast cancer, as demonstrated in the postintervention 13-year follow-up data on breast cancer risk and estrogen-progestin use from the Women’s Health Initiative9:
- Hazard ratio 1.28, 95% CI 1.11–1.48
- Absolute cumulative risk 9 cases per 10,000 women-years of use.
The Nurses’ Health Study, an observational study, prospectively followed 11,508 hysterectomized women on estrogen therapy and found that breast cancer risk increased with longer duration of use. An analysis by Chen et al16 found a trend toward increased breast cancer risk after 10 years of estrogen therapy, but this did not become statistically significant until 20 years of ongoing estrogen use. The risk of estrogen receptor-positive and progesterone receptor-positive breast cancer became statistically significant earlier, after 15 years. The relative risk associated with using estrogen for more than 15 years was 1.18, and the risk with using it for more than 20 years was 1.42.16
To put this in perspective, Chen et al17 found a similar breast cancer risk with alcohol consumption. The relative risk of invasive breast cancer was 1.15 in women who drank 3 to 6 servings of alcohol per week, 1 serving being equivalent to 4 oz of wine, which contains 11 g of alcohol.
Mortality
Studies have suggested that hormone therapy users have a lower mortality rate, even with long-term use.
A meta-analysis18 of 8 observational trials and 19 randomized controlled trials found that younger women (average age 54) on hormone therapy had a 28% lower total mortality rate compared with women not taking hormone therapy:
- Relative risk 0.72, 95% credible interval 0.62–0.82.
The Women’s Health Initiative19 suggested that the mortality rate was 30% lower in hormone therapy users younger than age 60 than in similar nonusers, though this difference did not reach statistical significance.
- Relative risk with estrogen-only therapy: 0.71, 95% CI 0.46–1.11
- Relative risk with combined estrogen-progestin therapy 0.69, 95% CI 0.44–1.07.
The Danish Osteoporosis Study,15 at 16 years of follow-up, similarly demonstrated a 34% lower mortality rate in hormone therapy users, which was not statistically significant:
- Relative risk 0.66, 95% CI 0.41–1.08.
A Cochrane review20 in 2015 found that the subgroup of women who started hormone therapy before age 60 or within 10 years of menopause saw an overall benefit in terms of survival and lower risk of coronary heart disease: RR 0.70, 95% CI 0.52–0.95 (moderate-quality evidence).
TYPE OF FORMULATION
Compared with estrogen-progestin therapy, estrogen-only therapy has a more favorable risk profile in terms of coronary heart disease and breast cancer, although stroke risk remains elevated in users of conjugated equine estrogen with or without medroxyprogesterone acetate.
There is limited evidence directly comparing different formulations of hormone therapy, although they all effectively treat vasomotor symptoms.1
Oral vs transdermal formulations
Canonico et al,21 in a meta-analysis of observational studies, found that oral estrogen was associated with a higher risk of venous thromboembolism than transdermal estrogen:
- Relative risk with oral estrogen 2.5, 95% CI 1.9–3.4
- Relative risk with transdermal estrogen 1.2, 95% CI 0.9–1.7.
The Estrogen and Thromboembolism Risk (ESTHER) study22 was a multicenter case-control study of women ages 45 to 70 that assessed risk of venous thromboembolism in oral vs transdermal estrogen users. Compared with women not taking hormone therapy, current users of oral estrogen had a significantly higher risk of venous thromboembolism, while transdermal estrogen users did not:
- Odds ratio with oral estrogen 4.2, 95% CI 1.5–11.6
- Odds ratio with transdermal estrogen 0.9, 95% CI 0.4–2.1.
The Kronos Early Estrogen Prevention Study (KEEPS)23 did not support these findings. This 4-year randomized controlled trial, published in 2014, was designed to assess the risk of atherosclerosis progression with early menopause initiation of placebo vs low-dose oral hormone therapy (conjugated equine estrogen 0.45 mg daily with cyclical micronized progesterone) or transdermal hormone therapy (estradiol 50 µg/week with cyclical micronized progesterone).
In the 727 women in the study, there was one transient ischemic attack in the oral hormone therapy group, one unconfirmed stroke in the transdermal hormone therapy group, and one case of venous thromboembolism in each group, findings that were underpowered for statistical significance. Both oral and transdermal hormonal therapy had neutral effects on atherosclerosis progression, as assessed by arterial imaging. Transdermal hormone therapy was associated with improvements in markers of insulin resistance and was not associated with an increase in triglycerides, C-reactive protein, or sex hormone-binding globulin, as would be expected with transdermal circumvention of the first-pass hepatic effect.
BALANCING THE RISKS AND BENEFITS FOR THE PATIENT
The most effective treatment for vasomotor symptoms in women at any age is hormone therapy, and the benefits are more likely to outweigh risks when initiated before age 60 or within 10 years of menopause.7 The Women’s Health Initiative randomized study was limited to 5.6 to 7.2 years of hormone therapy (13 years of cumulative follow-up), and the Danish Osteoporosis Study was limited to 11 years of use (16 years cumulative follow-up).
The coronary heart disease outcomes for longer durations of therapy remain uncertain. There is a small but statistically significant increased risk of stroke and venous thromboembolism with oral hormone therapy, and breast cancer risk is associated with long-term estrogen-progestin use.
Patients on hormone therapy should be evaluated annually regarding the need for ongoing therapy. Persistent moderate-severe vasomotor symptoms, quality of life benefits of hormone therapy, contraindications to its use (Table 2), and patient preference need to be assessed as well as baseline risks of cardiovascular disease, breast cancer, and fracture.
Risk calculators may facilitate the shared decision-making process. Examples are:
- The American College of Cardiology/American Heart association risk calculator for cardiovascular disease24 (www.cvriskcalculator.com)
- The World Health Organization Fracture Risk Assessment Tool (FRAX)25
(www.shef.ac.uk/FRAX/tool.jsp)
- The Gail model for breast cancer risk26 (www.cancer.gov/bcrisktool/).
- MenoPro, a menopause decision-support algorithm and companion mobile app developed by NAMS to help direct treatment decisions based on the 10-year risk of atherosclerotic cardiovascular disease (www.menopause.org/for-professionals/-i-menopro-i-mobile-app).27
The discussion of the risks of hormone therapy with patients should incorporate the perspective of absolute risk. For example, a woman wishing to continue estrogen-progestin therapy should be told that the Women’s Health Initiative data suggest that, after 5 years of use, breast cancer risk may be increased by 8 additional cases per 10,000 users per year. According to the World Health Organization, this magnitude of risk is defined as rare (less than 1 event per 1,000 women).28
A strategy of prescribing the lowest dose to achieve the desired clinical benefits is prudent and recommended.1–3 Table 3 outlines the estrogen formulations now available in the United States, with their doses and formulations.
Unless contraindications develop (Table 2), patients may elect to continue hormone therapy if its benefits outweigh its risks. The American College of Obstetricians and Gynecologists (ACOG) 2014 practice recommendations for management of menopausal symptoms31 and the 2015 NAMS statement both recommend that hormone therapy not be discontinued based solely on a woman’s age.29
Hormone therapy is on the Beer’s list of potentially inappropriate medications for older adults,30 which remains a hurdle to its long-term use and seems to be at odds with these ACOG and NAMS statements.
Patients who choose to discontinue hormone therapy need to be monitored for persistent bothersome vasomotor symptoms, bone loss, osteoporosis, and the genitourinary syndrome of menopause (previously referred to as vulvovaginal atrophy)31 and offered alternative therapies if needed.
The duration of hormone therapy needs to be an individualized decision, shared between the patient and her physician and assessed annually. Quality of life, vasomotor symptoms, current age, time since menopause, hysterectomy status, personal risks (of osteoporosis, breast cancer, heart disease, stroke, venous thromboembolism), and patient preferences need to be considered.
The North American Menopause Society (NAMS) and other organizations recommend that the lowest dose of hormone therapy be used for the shortest duration needed to manage menopausal symptoms.1–4 However, NAMS states that extending the duration of hormone therapy may be appropriate in women who have persistent symptoms or to prevent osteoporosis if the patient cannot tolerate alternative therapies.1
Forty-two percent of postmenopausal women continue to experience vasomotor symptoms at age 60 to 65.5 The median total duration of vasomotor symptoms is 7.4 years, and in black women and women with moderate or severe hot flashes the symptoms typically last 10 years.6 Vasomotor symptoms recur in 50% of women who discontinue hormone therapy, regardless of whether it is stopped abruptly or tapered.1
FACTORS TO CONSIDER WHEN PRESCRIBING HORMONE THERAPY
Bone health
A statement issued in 2013 by seven medical societies said that hormone therapy is effective and appropriate for preventing osteoporosis-related fracture in at-risk women under age 60 or within 10 years of menopause.7
The Women’s Health Initiative,8 a randomized placebo-controlled trial, showed a statistically significant lower risk of vertebral and nonvertebral fracture after 3 years of use of conjugated equine estrogen with medroxyprogesterone acetate than with placebo:
- Hazard ratio 0.76, 95% confidence interval (CI) 0.69–0.83.
It also showed a mean increase of 3.7% (P < .001) in total hip bone mineral density. By the end of the trial intervention, women receiving either this combined therapy or conjugated equine estrogen alone saw a 33% overall reduction in hip fracture risk. The absolute risk reduction was 5 per 10,000 years of use.9
Karim et al,10 in a large observational study that followed initial hormone therapy users over 6.5 years, found that those who stopped it had a 55% greater risk of hip fracture and experienced significant bone loss as measured by bone mineral density compared with women who continued hormone therapy, and that the protective effects of hormone therapy disappeared as early as 2 years after stopping treatment.10
NAMS also recommends that women with premature menopause (before age 40) be offered and encouraged to use hormone therapy to preserve bone density and manage vasomotor symptoms until the age of natural menopause (age 51).1,11
Cardiovascular health
Large observational studies have found that hormone therapy is associated with a 30% to 50% lower cardiovascular risk.12 Randomized controlled trials of hormone therapy for 7 to 11 years suggest that coronary heart disease risk is modified by age and time since menopause.13,14
The Women’s Health Initiative and other randomized controlled trials suggest a lower risk of coronary heart disease in women who begin hormone therapy before age 60 and within 10 years of the onset of menopause, but an increased risk for women over age 60 and more than 10 years since menopause. However, several of these trends have not reached statistical significance (Table 1).13–15
The Women’s Health Initiative9 published its long-term follow-up results in 2013, with data on both the intervention phase (median of 7.2 years for estrogen-only therapy and 5.6 years for estrogen-progestin therapy) and the post-stopping phase (median 6.6 years for the estrogen-only group and 8.2 years for the estrogen-progestin group), with a total cumulative follow-up of 13 years. The overall 13-year cumulative absolute risk of coronary heart disease was 4 fewer events per 10,000 years of estrogen-only therapy and 3 additional events per 10,000 years of estrogen-progestin therapy. Neither result was statistically significant:
- Hazard ratio with estrogen-only use 0.94, 95% CI 0.82–1.09
- Hazard ratio with estrogen-progestin use 1.09, 95% CI 0.92–1.24.
The Danish Osteoporosis Study was the first randomized controlled trial of hormone therapy in women ages 45 through 58 who were recently menopausal (average within 7 months of menopause).15 Women assigned to hormone therapy in the form of oral estradiol with or without norethisterone (known as norethindrone in the United States) had a statistically significant lower risk of the primary composite end point of heart failure and myocardial infarction after 11 years of hormone therapy, and this finding persisted through 16 years of follow-up (Table 1).
Stroke
Overall stroke risk was significantly increased with hormone therapy in the Women’s Health Initiative trial (hazard ratio 1.32, 95% CI 1.12–1.56); however, the absolute increase in risk was small in both estrogen-alone and estrogen-progestin therapy users, 11 and 8 events, respectively, among 10,000 users. Younger women (ages 50–59) saw a nonsignificantly lower risk (2 fewer cases per 10,000 years of use).14 After 13 years of cumulative follow-up (combined intervention and follow-up phase), the risk of stroke persisted at 5 cases per 10,000 users for both arms, but only the estrogen-progestin results were statistically significant.9
The Danish Osteoporosis Study15 found no increased risk of stroke after 16 years of follow-up in recently menopausal women:
- Hazard ratio 0.89, 95% CI 0.48–1.65.
Venous thromboembolism
Data from both observational and randomized controlled trials demonstrate an increased risk of venous thromboembolism with oral hormone therapy, and the risk appears to be highest during the first few years of use.1 The pooled cohort from the Women’s Health Initiative had 18 additional cases of venous thromboembolism per 10,000 women in estrogen-progestin users compared with nonusers, and 7 additional cases in those using estrogen-only therapy.
Breast health
Observational studies and randomized controlled trials have provided data on longer use of hormone therapy and breast cancer risk, but the true magnitude of this risk is unclear.
The Danish Osteoporosis Study,15 in a younger cohort of women, showed no increased risk of breast cancer after 16 years of follow-up:
- Hazard ratio 0.90, 95% CI 0.52–1.57.
The Women’s Health Initiative9 showed a statistically nonsignificant lower risk of breast cancer in women of all ages exposed to conjugated equine estrogen alone for 7.1 years (6 fewer cases per 10,000 women-years of use), and after 6 years of follow-up this developed statistical significance:
- Hazard ratio 0.79, 95% CI 0.65–0.97.
In contrast, those using conjugated equine estrogen plus medroxyprogesterone acetate had a statistically nonsignificant increase in the risk of new breast cancer after 3 to 5 years:
- 3-year relative risk 1.26, 95% CI 0.73–2.20
- 5-year relative risk 1.99, 95% CI 1.18–3.35
- Absolute risk 8 cases per 10,000 women-years of use.
The increased risk of breast cancer significantly declined within 3 years after stopping hormone therapy.
However, even after stopping hormone therapy, there remains a statistically small but significant increased risk of breast cancer, as demonstrated in the postintervention 13-year follow-up data on breast cancer risk and estrogen-progestin use from the Women’s Health Initiative9:
- Hazard ratio 1.28, 95% CI 1.11–1.48
- Absolute cumulative risk 9 cases per 10,000 women-years of use.
The Nurses’ Health Study, an observational study, prospectively followed 11,508 hysterectomized women on estrogen therapy and found that breast cancer risk increased with longer duration of use. An analysis by Chen et al16 found a trend toward increased breast cancer risk after 10 years of estrogen therapy, but this did not become statistically significant until 20 years of ongoing estrogen use. The risk of estrogen receptor-positive and progesterone receptor-positive breast cancer became statistically significant earlier, after 15 years. The relative risk associated with using estrogen for more than 15 years was 1.18, and the risk with using it for more than 20 years was 1.42.16
To put this in perspective, Chen et al17 found a similar breast cancer risk with alcohol consumption. The relative risk of invasive breast cancer was 1.15 in women who drank 3 to 6 servings of alcohol per week, 1 serving being equivalent to 4 oz of wine, which contains 11 g of alcohol.
Mortality
Studies have suggested that hormone therapy users have a lower mortality rate, even with long-term use.
A meta-analysis18 of 8 observational trials and 19 randomized controlled trials found that younger women (average age 54) on hormone therapy had a 28% lower total mortality rate compared with women not taking hormone therapy:
- Relative risk 0.72, 95% credible interval 0.62–0.82.
The Women’s Health Initiative19 suggested that the mortality rate was 30% lower in hormone therapy users younger than age 60 than in similar nonusers, though this difference did not reach statistical significance.
- Relative risk with estrogen-only therapy: 0.71, 95% CI 0.46–1.11
- Relative risk with combined estrogen-progestin therapy 0.69, 95% CI 0.44–1.07.
The Danish Osteoporosis Study,15 at 16 years of follow-up, similarly demonstrated a 34% lower mortality rate in hormone therapy users, which was not statistically significant:
- Relative risk 0.66, 95% CI 0.41–1.08.
A Cochrane review20 in 2015 found that the subgroup of women who started hormone therapy before age 60 or within 10 years of menopause saw an overall benefit in terms of survival and lower risk of coronary heart disease: RR 0.70, 95% CI 0.52–0.95 (moderate-quality evidence).
TYPE OF FORMULATION
Compared with estrogen-progestin therapy, estrogen-only therapy has a more favorable risk profile in terms of coronary heart disease and breast cancer, although stroke risk remains elevated in users of conjugated equine estrogen with or without medroxyprogesterone acetate.
There is limited evidence directly comparing different formulations of hormone therapy, although they all effectively treat vasomotor symptoms.1
Oral vs transdermal formulations
Canonico et al,21 in a meta-analysis of observational studies, found that oral estrogen was associated with a higher risk of venous thromboembolism than transdermal estrogen:
- Relative risk with oral estrogen 2.5, 95% CI 1.9–3.4
- Relative risk with transdermal estrogen 1.2, 95% CI 0.9–1.7.
The Estrogen and Thromboembolism Risk (ESTHER) study22 was a multicenter case-control study of women ages 45 to 70 that assessed risk of venous thromboembolism in oral vs transdermal estrogen users. Compared with women not taking hormone therapy, current users of oral estrogen had a significantly higher risk of venous thromboembolism, while transdermal estrogen users did not:
- Odds ratio with oral estrogen 4.2, 95% CI 1.5–11.6
- Odds ratio with transdermal estrogen 0.9, 95% CI 0.4–2.1.
The Kronos Early Estrogen Prevention Study (KEEPS)23 did not support these findings. This 4-year randomized controlled trial, published in 2014, was designed to assess the risk of atherosclerosis progression with early menopause initiation of placebo vs low-dose oral hormone therapy (conjugated equine estrogen 0.45 mg daily with cyclical micronized progesterone) or transdermal hormone therapy (estradiol 50 µg/week with cyclical micronized progesterone).
In the 727 women in the study, there was one transient ischemic attack in the oral hormone therapy group, one unconfirmed stroke in the transdermal hormone therapy group, and one case of venous thromboembolism in each group, findings that were underpowered for statistical significance. Both oral and transdermal hormonal therapy had neutral effects on atherosclerosis progression, as assessed by arterial imaging. Transdermal hormone therapy was associated with improvements in markers of insulin resistance and was not associated with an increase in triglycerides, C-reactive protein, or sex hormone-binding globulin, as would be expected with transdermal circumvention of the first-pass hepatic effect.
BALANCING THE RISKS AND BENEFITS FOR THE PATIENT
The most effective treatment for vasomotor symptoms in women at any age is hormone therapy, and the benefits are more likely to outweigh risks when initiated before age 60 or within 10 years of menopause.7 The Women’s Health Initiative randomized study was limited to 5.6 to 7.2 years of hormone therapy (13 years of cumulative follow-up), and the Danish Osteoporosis Study was limited to 11 years of use (16 years cumulative follow-up).
The coronary heart disease outcomes for longer durations of therapy remain uncertain. There is a small but statistically significant increased risk of stroke and venous thromboembolism with oral hormone therapy, and breast cancer risk is associated with long-term estrogen-progestin use.
Patients on hormone therapy should be evaluated annually regarding the need for ongoing therapy. Persistent moderate-severe vasomotor symptoms, quality of life benefits of hormone therapy, contraindications to its use (Table 2), and patient preference need to be assessed as well as baseline risks of cardiovascular disease, breast cancer, and fracture.
Risk calculators may facilitate the shared decision-making process. Examples are:
- The American College of Cardiology/American Heart association risk calculator for cardiovascular disease24 (www.cvriskcalculator.com)
- The World Health Organization Fracture Risk Assessment Tool (FRAX)25
(www.shef.ac.uk/FRAX/tool.jsp)
- The Gail model for breast cancer risk26 (www.cancer.gov/bcrisktool/).
- MenoPro, a menopause decision-support algorithm and companion mobile app developed by NAMS to help direct treatment decisions based on the 10-year risk of atherosclerotic cardiovascular disease (www.menopause.org/for-professionals/-i-menopro-i-mobile-app).27
The discussion of the risks of hormone therapy with patients should incorporate the perspective of absolute risk. For example, a woman wishing to continue estrogen-progestin therapy should be told that the Women’s Health Initiative data suggest that, after 5 years of use, breast cancer risk may be increased by 8 additional cases per 10,000 users per year. According to the World Health Organization, this magnitude of risk is defined as rare (less than 1 event per 1,000 women).28
A strategy of prescribing the lowest dose to achieve the desired clinical benefits is prudent and recommended.1–3 Table 3 outlines the estrogen formulations now available in the United States, with their doses and formulations.
Unless contraindications develop (Table 2), patients may elect to continue hormone therapy if its benefits outweigh its risks. The American College of Obstetricians and Gynecologists (ACOG) 2014 practice recommendations for management of menopausal symptoms31 and the 2015 NAMS statement both recommend that hormone therapy not be discontinued based solely on a woman’s age.29
Hormone therapy is on the Beer’s list of potentially inappropriate medications for older adults,30 which remains a hurdle to its long-term use and seems to be at odds with these ACOG and NAMS statements.
Patients who choose to discontinue hormone therapy need to be monitored for persistent bothersome vasomotor symptoms, bone loss, osteoporosis, and the genitourinary syndrome of menopause (previously referred to as vulvovaginal atrophy)31 and offered alternative therapies if needed.
- North American Menopause Society. The 2012 hormone therapy position statement of: The North American Menopause Society. Menopause 2012; 19:257–271.
- American College of Obstetricians and Gynecologists. Practice Bulletin No. 141: Management of menopausal symptoms. Obstet Gynecol 2014; 123:202–216.
- Stuenkel CA, Davis SR, Gompel A, et al. Treatment of symptoms of the menopause: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2015; 100:3975–4011.
- de Villiers TJ, Pines A, Panay N, et al; International Menopause Society. Updated 2013 International Menopause Society recommendations on menopausal hormone therapy and preventive strategies for midlife health. Climacteric 2013; 16:316–337.
- Gartoulla P, Worsley R, Robin J, Davis S. Moderate to severe vasomotor and sexual symptoms remain problematic for women aged 60 to 65 years. Menopause 2015; 22:694–701.
- Avis NE, Crawford SL, Greendale G, et al. Duration of menopausal vasomotor symptoms across the menopause transition. JAMA Intern Med 2015; 175:531–539.
- de Villiers TJ, Gass ML, Haines CJ, et al. Global consensus statement on menopausal hormone therapy. Climacteric 2013; 16:203–204.
- Cauley J, Robbins J, Chen Z, et al. Effects of estrogen plus progestin on risk of fracture and bone mineral density: the Women’s Health Initiative randomized trial. JAMA 2003; 290:1729–1738.
- Manson J, Chlebowski R, Stefanick M, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA 2013; 310:1353–1368.
- Karim R, Dell RM, Greene DF, et al. Hip fracture in postmenopausal women after cessation of hormone therapy: results from a prospective study in a large health management organization. Menopause 2011; 18:1172–1177.
- Shifren J, Gass M, and the NAMS Recommendations for Clinical Care of Midlife Women Working Group. The North American Menopause Society recommendations for clinical care of midlife women. Menopause 2014; 21:1038–1062.
- Hodis HN, Mack WJ. Hormone replacement therapy and the association with coronary heart disease and overall mortality: clinical application of the timing hypothesis. J Steroid Biochem Mol Biol 2014; 142:68–75.
- Salpeter SR, Walsh JM, Greyber E, et al. Brief report: coronary heart disease events associated with hormone therapy in younger and older women. A meta-analysis. J Gen Intern Med 2006; 21:363–366.
- Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA 2007; 297:1465–1477.
- Schierbeck LL, Rejnmark L, Tofteng CL, et al. Effect of hormone replacement therapy on cardiovascular events in recently postmenopausal women: randomised trial. BMJ 2012; 345:e6409.
- Chen WY, Manson JE, Hankinson SE, et al. Unopposed estrogen therapy and the risk of breast cancer. Arch Intern Med 2006; 166:1027–1032.
- Chen W, Rosner B, Hankinson SE, et al. Moderate alcohol consumption during adult life, drinking patterns, and breast cancer risk. JAMA 2011; 306:1884–1890.
- Salpeter SR, Cheng J, Thabane L, et al. Bayesian meta-analysis of hormone therapy and mortality in younger postmenopausal women. Am J Med 2009; 122:1016–1022.
- Hodis HN, Collins P, Mack WJ, Schierbeck LL. The timing hypothesis for coronary heart disease prevention with hormone therapy: past, present and future in perspective. Climacteric 2012; 15:217–228.
- Boardman HM, Hartley L, Eisinga A, et al. Hormone therapy for preventing cardiovascular disease in post-menopausal women. Cochrane Database Syst Rev 2015;3:CD002229.
- Canonico M, Plu-Bureau G, Lowe GD, et al. Hormone replacement therapy and risk of venous thromboembolism in postmenopausal women: systemic review and meta-analysis. BMJ 2008; 336:1227–1231.
- Canonico M, Oger E, Plu-Bureau G, et al; Estrogen and Thromboembolism Risk (ESTHER) Study Group. Hormone therapy and venous thromboembolism among postmenopausal women: impact of the route of estrogen administration and progestogens: the ESTHER study. Circulation 2007; 115:840–845.
- Harman S, Black D, Naftolin F, et al. Arterial imaging outcomes and cardiovascular risk factors in recently menopausal women. Ann Intern Med 2014; 161:249–260.
- Goff DC Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 63:2935–2959.
- World Health Organization Collaborating Centre for Metabolic Bone Diseases. FRAX WHO fracture risk assessment tool. www.shef.ac.uk/FRAX/. Accessed May 27, 2016.
- Gail M, Brinton L, Byar D, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst 1989; 81:1879–1886.
- Manson J, Ames J, Shapiro M, et al. Algorithm and mobile app for menopausal symptom management and hormonal/non-hormonal therapy decision making: a clinical decision-support tool from the North American Menopause Society. Menopause 2015; 22:247–253.
- Hodis HN, Mack WJ. Postmenopausal hormone therapy in clinical perspective. Menopause 2007; 14:944–957.
- North American Menopause Society. The North American Menopause Society statement on continuing use of systemic hormone therapy after the age of 65. Menopause 2015; 22:693.
- American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2015; 63:2227–2246.
- Portman DJ, Gass ML; Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause 2014; 21:1063–1068.
- North American Menopause Society. The 2012 hormone therapy position statement of: The North American Menopause Society. Menopause 2012; 19:257–271.
- American College of Obstetricians and Gynecologists. Practice Bulletin No. 141: Management of menopausal symptoms. Obstet Gynecol 2014; 123:202–216.
- Stuenkel CA, Davis SR, Gompel A, et al. Treatment of symptoms of the menopause: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2015; 100:3975–4011.
- de Villiers TJ, Pines A, Panay N, et al; International Menopause Society. Updated 2013 International Menopause Society recommendations on menopausal hormone therapy and preventive strategies for midlife health. Climacteric 2013; 16:316–337.
- Gartoulla P, Worsley R, Robin J, Davis S. Moderate to severe vasomotor and sexual symptoms remain problematic for women aged 60 to 65 years. Menopause 2015; 22:694–701.
- Avis NE, Crawford SL, Greendale G, et al. Duration of menopausal vasomotor symptoms across the menopause transition. JAMA Intern Med 2015; 175:531–539.
- de Villiers TJ, Gass ML, Haines CJ, et al. Global consensus statement on menopausal hormone therapy. Climacteric 2013; 16:203–204.
- Cauley J, Robbins J, Chen Z, et al. Effects of estrogen plus progestin on risk of fracture and bone mineral density: the Women’s Health Initiative randomized trial. JAMA 2003; 290:1729–1738.
- Manson J, Chlebowski R, Stefanick M, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA 2013; 310:1353–1368.
- Karim R, Dell RM, Greene DF, et al. Hip fracture in postmenopausal women after cessation of hormone therapy: results from a prospective study in a large health management organization. Menopause 2011; 18:1172–1177.
- Shifren J, Gass M, and the NAMS Recommendations for Clinical Care of Midlife Women Working Group. The North American Menopause Society recommendations for clinical care of midlife women. Menopause 2014; 21:1038–1062.
- Hodis HN, Mack WJ. Hormone replacement therapy and the association with coronary heart disease and overall mortality: clinical application of the timing hypothesis. J Steroid Biochem Mol Biol 2014; 142:68–75.
- Salpeter SR, Walsh JM, Greyber E, et al. Brief report: coronary heart disease events associated with hormone therapy in younger and older women. A meta-analysis. J Gen Intern Med 2006; 21:363–366.
- Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA 2007; 297:1465–1477.
- Schierbeck LL, Rejnmark L, Tofteng CL, et al. Effect of hormone replacement therapy on cardiovascular events in recently postmenopausal women: randomised trial. BMJ 2012; 345:e6409.
- Chen WY, Manson JE, Hankinson SE, et al. Unopposed estrogen therapy and the risk of breast cancer. Arch Intern Med 2006; 166:1027–1032.
- Chen W, Rosner B, Hankinson SE, et al. Moderate alcohol consumption during adult life, drinking patterns, and breast cancer risk. JAMA 2011; 306:1884–1890.
- Salpeter SR, Cheng J, Thabane L, et al. Bayesian meta-analysis of hormone therapy and mortality in younger postmenopausal women. Am J Med 2009; 122:1016–1022.
- Hodis HN, Collins P, Mack WJ, Schierbeck LL. The timing hypothesis for coronary heart disease prevention with hormone therapy: past, present and future in perspective. Climacteric 2012; 15:217–228.
- Boardman HM, Hartley L, Eisinga A, et al. Hormone therapy for preventing cardiovascular disease in post-menopausal women. Cochrane Database Syst Rev 2015;3:CD002229.
- Canonico M, Plu-Bureau G, Lowe GD, et al. Hormone replacement therapy and risk of venous thromboembolism in postmenopausal women: systemic review and meta-analysis. BMJ 2008; 336:1227–1231.
- Canonico M, Oger E, Plu-Bureau G, et al; Estrogen and Thromboembolism Risk (ESTHER) Study Group. Hormone therapy and venous thromboembolism among postmenopausal women: impact of the route of estrogen administration and progestogens: the ESTHER study. Circulation 2007; 115:840–845.
- Harman S, Black D, Naftolin F, et al. Arterial imaging outcomes and cardiovascular risk factors in recently menopausal women. Ann Intern Med 2014; 161:249–260.
- Goff DC Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 63:2935–2959.
- World Health Organization Collaborating Centre for Metabolic Bone Diseases. FRAX WHO fracture risk assessment tool. www.shef.ac.uk/FRAX/. Accessed May 27, 2016.
- Gail M, Brinton L, Byar D, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst 1989; 81:1879–1886.
- Manson J, Ames J, Shapiro M, et al. Algorithm and mobile app for menopausal symptom management and hormonal/non-hormonal therapy decision making: a clinical decision-support tool from the North American Menopause Society. Menopause 2015; 22:247–253.
- Hodis HN, Mack WJ. Postmenopausal hormone therapy in clinical perspective. Menopause 2007; 14:944–957.
- North American Menopause Society. The North American Menopause Society statement on continuing use of systemic hormone therapy after the age of 65. Menopause 2015; 22:693.
- American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2015; 63:2227–2246.
- Portman DJ, Gass ML; Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause 2014; 21:1063–1068.
KEY POINTS
- Hormone therapy is the most effective treatment available for the vasomotor symptoms of menopause, and it also is effective and appropriate for preventing osteoporosis-related fracture in at-risk women under age 60 or within 10 years of menopause.
- Oral hormone therapy is associated with a small but statistically significant increase in the risk of stroke and venous thromboembolism and breast cancer risk with combination therapy only.
- Extended hormone therapy may be appropriate to treat vasomotor symptoms or prevent osteoporosis when alternative therapies are not an option.
- The decision whether to continue hormone therapy should be revisited every year. Discussions with patients should include the perspective of absolute risk.
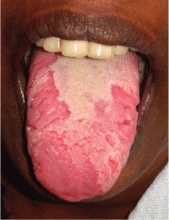
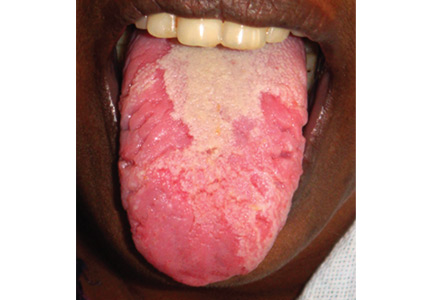
Geographic tongue
A previously healthy 35-year-old woman presented with reddish discoloration of her tongue for the past 7 days, accompanied by mild soreness over the area when eating spicy foods. The lesion had also changed shape repeatedly. She denied any other local or systemic symptoms.
Lingual examination showed clearly delineated areas of shiny, erythematous mucosa on the dorsal and lateral aspects of the tongue, surrounded by white borders (Figure 1). Examination of the throat and oral cavity were unremarkable. All other systemic examinations were normal. Laboratory testing showed a normal hemogram, blood glucose, and metabolic profile.
These findings were suggestive of geographic tongue, a benign, self-limiting inflammation. The patient was reassured of the benign nature of the condition and was advised to avoid spicy food until resolution of the lesion. A follow-up examination 1 month later showed complete healing of the lesion.
A COMMON, BENIGN, SELF-LIMITING MUCOSAL CONDITION
Geographic tongue—also known as benign migratory glossitis and lingual erythema migrans—is commonly seen in daily practice, with a prevalence of 2% to 3% in the general population.1 In the United States, the condition is more prevalent in whites and blacks than in Hispanics, but has no association with age or sex.
This condition is characterized by circinate, maplike areas of erythema surrounded by well-demarcated scalloped white borders, typically on the dorsum and the lateral borders of the tongue.2 The appearance, which represents loss of filiform papillae (depapillation) from the lingual mucosa, can change in size, shape, or location in a matter of minutes or hours. The name “lingual erythema migrans” reflects the changing clinical picture.3 Rarely, the labial or palatal mucosa is affected.
POSTULATED TO BE AN INTRAORAL FORM OF PSORIASIS
The precise etiology remains obscure.2 Histopathologically, geographic tongue is characterized by hyperparakeratosis and acanthosis resembling psoriasis. Hence, it has been postulated that it represents a form of intraoral psoriasis.2,4 The condition is also associated with allergy, stress, diabetes mellitus, and anemia. Triggers include hot, spicy, and acidic foods and alcohol. Contrary to previous belief, geographic tongue has been found to have an inverse association with smoking.5 Although striking, the lesion rarely warrants further investigation.
REASSURANCE IS THE MAIN TREATMENT
Geographic tongue has a remitting and relapsing course with no complications or permanent sequelae.3 The differential diagnosis includes oral candidiasis, leukoplakia, vitamin deficiency glossitis, lichen planus, systemic lupus erythematosus, drug reaction, and recurrent aphthous stomatitis. The condition is differentiated from oral candidiasis by its presence in an otherwise healthy person and by the changing pattern of the lesions over time. Also, candidal pseudomembranes can be easily removed, leaving a painless red base. Evaluations to rule out anemia, nutritional deficiencies, and diabetes mellitus can be done if these conditions are suspected, as they are associated with geographic tongue.
Reassurance is the main treatment. Topical corticosteroids and local anesthetics may provide symptomatic relief in mild forms of the disease. Topical tacrolimus and systemic cyclosporine have been reported as useful in severe cases.6
- Masferrer E, Jucgla A. Images in clinical medicine. Geographic tongue. N Engl J Med 2009; 361:e44.
- Assimakopoulos D, Patrikakos G, Fotika C, Elisaf M. Benign migratory glossitis or geographic tongue: an enigmatic oral lesion. Am J Med 2002; 113:751–755.
- Scully C, Hegarty A. The oral cavity and lips. In: Burns T, Breathnach S, Cox N, Griffiths C, editors. Rook's Textbook of Dermatology. 8th ed. West Sussex, UK: Wiley-Blackwell; 2010:69–100.
- Zargari O. The prevalence and significance of fissured tongue and geographical tongue in psoriatic patients. Clin Exp Dermatol 2006; 31:192–195.
- Shulman JD, Carpenter WM. Prevalence and risk factors associated with geographic tongue among US adults. Oral Dis 2006; 12:381–386.
- Ishibashi M, Tojo G, Watanabe M, Tamabuchi T, Masu T, Aiba S. Geographic tongue treated with topical tacrolimus. J Dermatol Case Rep 2010; 4:57–59.
A previously healthy 35-year-old woman presented with reddish discoloration of her tongue for the past 7 days, accompanied by mild soreness over the area when eating spicy foods. The lesion had also changed shape repeatedly. She denied any other local or systemic symptoms.
Lingual examination showed clearly delineated areas of shiny, erythematous mucosa on the dorsal and lateral aspects of the tongue, surrounded by white borders (Figure 1). Examination of the throat and oral cavity were unremarkable. All other systemic examinations were normal. Laboratory testing showed a normal hemogram, blood glucose, and metabolic profile.
These findings were suggestive of geographic tongue, a benign, self-limiting inflammation. The patient was reassured of the benign nature of the condition and was advised to avoid spicy food until resolution of the lesion. A follow-up examination 1 month later showed complete healing of the lesion.
A COMMON, BENIGN, SELF-LIMITING MUCOSAL CONDITION
Geographic tongue—also known as benign migratory glossitis and lingual erythema migrans—is commonly seen in daily practice, with a prevalence of 2% to 3% in the general population.1 In the United States, the condition is more prevalent in whites and blacks than in Hispanics, but has no association with age or sex.
This condition is characterized by circinate, maplike areas of erythema surrounded by well-demarcated scalloped white borders, typically on the dorsum and the lateral borders of the tongue.2 The appearance, which represents loss of filiform papillae (depapillation) from the lingual mucosa, can change in size, shape, or location in a matter of minutes or hours. The name “lingual erythema migrans” reflects the changing clinical picture.3 Rarely, the labial or palatal mucosa is affected.
POSTULATED TO BE AN INTRAORAL FORM OF PSORIASIS
The precise etiology remains obscure.2 Histopathologically, geographic tongue is characterized by hyperparakeratosis and acanthosis resembling psoriasis. Hence, it has been postulated that it represents a form of intraoral psoriasis.2,4 The condition is also associated with allergy, stress, diabetes mellitus, and anemia. Triggers include hot, spicy, and acidic foods and alcohol. Contrary to previous belief, geographic tongue has been found to have an inverse association with smoking.5 Although striking, the lesion rarely warrants further investigation.
REASSURANCE IS THE MAIN TREATMENT
Geographic tongue has a remitting and relapsing course with no complications or permanent sequelae.3 The differential diagnosis includes oral candidiasis, leukoplakia, vitamin deficiency glossitis, lichen planus, systemic lupus erythematosus, drug reaction, and recurrent aphthous stomatitis. The condition is differentiated from oral candidiasis by its presence in an otherwise healthy person and by the changing pattern of the lesions over time. Also, candidal pseudomembranes can be easily removed, leaving a painless red base. Evaluations to rule out anemia, nutritional deficiencies, and diabetes mellitus can be done if these conditions are suspected, as they are associated with geographic tongue.
Reassurance is the main treatment. Topical corticosteroids and local anesthetics may provide symptomatic relief in mild forms of the disease. Topical tacrolimus and systemic cyclosporine have been reported as useful in severe cases.6
A previously healthy 35-year-old woman presented with reddish discoloration of her tongue for the past 7 days, accompanied by mild soreness over the area when eating spicy foods. The lesion had also changed shape repeatedly. She denied any other local or systemic symptoms.
Lingual examination showed clearly delineated areas of shiny, erythematous mucosa on the dorsal and lateral aspects of the tongue, surrounded by white borders (Figure 1). Examination of the throat and oral cavity were unremarkable. All other systemic examinations were normal. Laboratory testing showed a normal hemogram, blood glucose, and metabolic profile.
These findings were suggestive of geographic tongue, a benign, self-limiting inflammation. The patient was reassured of the benign nature of the condition and was advised to avoid spicy food until resolution of the lesion. A follow-up examination 1 month later showed complete healing of the lesion.
A COMMON, BENIGN, SELF-LIMITING MUCOSAL CONDITION
Geographic tongue—also known as benign migratory glossitis and lingual erythema migrans—is commonly seen in daily practice, with a prevalence of 2% to 3% in the general population.1 In the United States, the condition is more prevalent in whites and blacks than in Hispanics, but has no association with age or sex.
This condition is characterized by circinate, maplike areas of erythema surrounded by well-demarcated scalloped white borders, typically on the dorsum and the lateral borders of the tongue.2 The appearance, which represents loss of filiform papillae (depapillation) from the lingual mucosa, can change in size, shape, or location in a matter of minutes or hours. The name “lingual erythema migrans” reflects the changing clinical picture.3 Rarely, the labial or palatal mucosa is affected.
POSTULATED TO BE AN INTRAORAL FORM OF PSORIASIS
The precise etiology remains obscure.2 Histopathologically, geographic tongue is characterized by hyperparakeratosis and acanthosis resembling psoriasis. Hence, it has been postulated that it represents a form of intraoral psoriasis.2,4 The condition is also associated with allergy, stress, diabetes mellitus, and anemia. Triggers include hot, spicy, and acidic foods and alcohol. Contrary to previous belief, geographic tongue has been found to have an inverse association with smoking.5 Although striking, the lesion rarely warrants further investigation.
REASSURANCE IS THE MAIN TREATMENT
Geographic tongue has a remitting and relapsing course with no complications or permanent sequelae.3 The differential diagnosis includes oral candidiasis, leukoplakia, vitamin deficiency glossitis, lichen planus, systemic lupus erythematosus, drug reaction, and recurrent aphthous stomatitis. The condition is differentiated from oral candidiasis by its presence in an otherwise healthy person and by the changing pattern of the lesions over time. Also, candidal pseudomembranes can be easily removed, leaving a painless red base. Evaluations to rule out anemia, nutritional deficiencies, and diabetes mellitus can be done if these conditions are suspected, as they are associated with geographic tongue.
Reassurance is the main treatment. Topical corticosteroids and local anesthetics may provide symptomatic relief in mild forms of the disease. Topical tacrolimus and systemic cyclosporine have been reported as useful in severe cases.6
- Masferrer E, Jucgla A. Images in clinical medicine. Geographic tongue. N Engl J Med 2009; 361:e44.
- Assimakopoulos D, Patrikakos G, Fotika C, Elisaf M. Benign migratory glossitis or geographic tongue: an enigmatic oral lesion. Am J Med 2002; 113:751–755.
- Scully C, Hegarty A. The oral cavity and lips. In: Burns T, Breathnach S, Cox N, Griffiths C, editors. Rook's Textbook of Dermatology. 8th ed. West Sussex, UK: Wiley-Blackwell; 2010:69–100.
- Zargari O. The prevalence and significance of fissured tongue and geographical tongue in psoriatic patients. Clin Exp Dermatol 2006; 31:192–195.
- Shulman JD, Carpenter WM. Prevalence and risk factors associated with geographic tongue among US adults. Oral Dis 2006; 12:381–386.
- Ishibashi M, Tojo G, Watanabe M, Tamabuchi T, Masu T, Aiba S. Geographic tongue treated with topical tacrolimus. J Dermatol Case Rep 2010; 4:57–59.
- Masferrer E, Jucgla A. Images in clinical medicine. Geographic tongue. N Engl J Med 2009; 361:e44.
- Assimakopoulos D, Patrikakos G, Fotika C, Elisaf M. Benign migratory glossitis or geographic tongue: an enigmatic oral lesion. Am J Med 2002; 113:751–755.
- Scully C, Hegarty A. The oral cavity and lips. In: Burns T, Breathnach S, Cox N, Griffiths C, editors. Rook's Textbook of Dermatology. 8th ed. West Sussex, UK: Wiley-Blackwell; 2010:69–100.
- Zargari O. The prevalence and significance of fissured tongue and geographical tongue in psoriatic patients. Clin Exp Dermatol 2006; 31:192–195.
- Shulman JD, Carpenter WM. Prevalence and risk factors associated with geographic tongue among US adults. Oral Dis 2006; 12:381–386.
- Ishibashi M, Tojo G, Watanabe M, Tamabuchi T, Masu T, Aiba S. Geographic tongue treated with topical tacrolimus. J Dermatol Case Rep 2010; 4:57–59.
Thrombotic thrombocytopenic purpura: The role of ADAMTS13
A breakthrough in understanding the pathogenesis of thrombotic thrombocytopenic purpura (TTP) came with the discovery of ADAMTS13 (an abbreviation for “a disintegrin and metalloproteinase with thrombospondin type 1 motif, member 13”), a plasma protein that cleaves von Willebrand factor, which interacts with platelets to promote blood clotting. If ADAMTS13 is lacking, unusually large multimers of von Willebrand factor can accumulate and trigger intravascular platelet aggregation and microthrombosis, causing the signs and symptoms of TTP.1–3
This knowledge has practical applications: we can now measure ADAMTS13 activity, ADAMTS13 inhibitor, and antibodies against ADAMTS13 to help us diagnose TTP and distinguish it from other forms of thrombotic microangiopathy, such as hemolytic-uremic syndrome, that have similar symptoms but require different treatment.
Using case studies, this article describes typical presentations of acute and relapsing TTP; the role of laboratory testing, including the ADAMTS13 assay; how to distinguish TTP from other conditions that present similarly; and how to manage this condition.
A HIGH RISK OF DEATH WITHOUT PLASMA EXCHANGE
TTP is characterized by disseminated microthrombi composed of agglutinated platelets and von Willebrand factor in small vessels. Tissue damage by microthrombi can cause thrombocytopenia (platelet deficiency), microangiopathic hemolytic anemia (loss of red blood cells caused by destructive conditions in small vessels), and multiorgan failure.1
Untreated TTP has a mortality rate of about 90%.1 As shown in Case 1, Case 2, and Table 1, rapid diagnosis and prompt initiation of daily therapeutic plasma exchange can improve this grave outlook.4

ADAMTS13 DEFICIENCY CAN BE ACQUIRED OR CONGENITAL
Two major forms of TTP with ADAMTS13 deficiency and microvascular thrombosis are recognized:
Acquired TTP, the more common form, peaks in incidence between ages 30 and 50.2,5 It more often affects women, particularly during and after pregnancy (its estimated prevalence is 1 in 25,000 pregnancies), and African Americans.6 Acquired TTP may be:
- Primary (idiopathic or autoantibody-mediated), associated with severely decreased ADAMTS13 and the presence of ultra-large von Willebrand factor multimers, or
- Secondary (23%–67% of cases), arising from a variety of conditions, including autoimmune disorders (eg, systemic lupus erythematosus, rheumatoid arthritis), solid organ or hematopoietic cell transplant, malignancy, drugs, and pregnancy (Table 2).1,5–8 Secondary TTP has a worse prognosis than idiopathic TTP.5,9
Congenital TTP (Upshaw-Shulman syndrome) is a rare autosomal-recessive disease caused by compound heterozygous or homozygous mutations of the ADAMTS13 gene, producing nonfunctional ADAMTS13 protein. Patients have severely deficient ADAMTS13 activity but usually do not develop autoantibodies. There is a high risk of chronic, relapsing episodes; identified triggers include pregnancy and heavy alcohol intake.2,10 About half of patients with congenital TTP have an early onset, usually presenting with acute TTP between birth and age 5, and about half have a late onset, usually remaining without symptoms until age 20 to 40.
THE CLINICAL PICTURE OF TTP IS NOT ALWAYS CLASSIC
TTP is primarily diagnosed clinically, but diagnosis is often difficult because of various nonspecific symptoms. Typical TTP presents with the “classic pentad”:
- Severe thrombocytopenia (70%–100% of patients)
- Microangiopathic hemolytic anemia with multiple schistocytes (70%–100%) (Figure 1)
- Neurologic involvement (50%–90%)
- Renal abnormalities (about 50%)
- Fever (25%).
However, the entire picture often does not emerge in a single patient.2,6 Waiting for the entire pentad to develop before diagnosing TTP can have grave clinical consequences,1,2,5 and the presence of thrombocytopenia and unexplained microangiopathic hemolytic anemia are considered clinically sufficient to suspect TTP.5
Neurologic symptoms usually fluctuate. They can include mild abnormalities such as weakness, dizziness, headache, blurred vision, ataxia, and transient mental status changes, as well as severe abnormalities including stroke, seizure, and coma.2,6
Most patients have normal findings on computed tomography and magnetic resonance imaging at the onset of neurologic symptoms or with a history of TTP. Some patients (8%–39%) show reversible acute brain lesions, including ischemic changes.11–13
Other signs and symptoms may result from multiorgan failure due to microthrombosis; ischemia in retinal, coronary, and abdominal circulations; and unconjugated hyperbilirubinemia.2
Atypical presentations. About 18% of patients have cardiac involvement from microvascular occlusion, with arrhythmia, angina, or congestive heart failure. Abdominal pain and pancreatitis occur in 5% to 13%, and visual disturbances in 8% to 10%.
Patients with an atypical presentation may not have laboratory evidence of microangiopathic hemolytic anemia, but an ADAMTS13 assay will show severely decreased activity. Therapeutic plasma exchange can improve atypical symptoms.2,3,10,14,15
ADAMTS13 ASSAY IS KEY TO DIAGNOSIS
Laboratory evidence typically includes hemolytic anemia (reticulocytosis, schistocytes, elevated indirect bilirubin, reduced haptoglobin, elevated lactate dehydrogenase) and thrombocytopenia.3 There are no significant abnormalities in prothrombin time, international normalized ratio, activated partial thromboplastin time, fibrinogen, or D-dimer level.
Measuring the levels of ADAMTS13 activity, ADAMTS13 inhibitor, and ADAMTS13 antibody is becoming standard to confirm the diagnosis of TTP, to determine if it is congenital or acquired, and to distinguish it from thrombocytopenic conditions such as hemolytic-uremic syndrome, idiopathic thrombocytopenic purpura, and heparin-induced thrombocytopenia.4,5 A newer ADAMTS13 assay based on fluorescence energy transfer (FRET) technology with a synthetic amino acid-von Willebrand factor peptide substrate has a faster turnaround time and less test variability.6,16,17 This FRET assay can give the result of ADAMTS13 activity within 2 hours. In comparison, the assay based on multimeric von Willebrand factor takes 2 to 3 days, and mass spectrometry to measure the cleavage products of a synthetic von Willebrand factor molecule takes about 4 hours.3,10,16
About two-thirds of patients with the clinical diagnosis of idiopathic TTP have ADAMTS13 activity levels lower than 10%.5,14,18 In the appropriate clinical setting, this threshold level is highly sensitive (89%–100%) and specific (99%–100%) in differentiating TTP from other thrombotic angiopathies.2,3,18
Note: The ADAMTS13 assay was needed for early correct diagnosis in Case 1 and Case 2.
Inhibitors provide more clues
Autoantibodies can be classified according to whether they inhibit ADAMTS13 activity.
Neutralizing inhibitors. Most cases of acquired, idiopathic TTP with severe ADAMTS13 deficiency are related to circulating autoantibodies that neutralize ADAMTS13 activity. This ADAMTS13 inhibitor level is obtained by measuring residual ADAMTS13 activity after mixing equal amounts of patient plasma with normal pooled plasma. ADAMTS13 inhibitor is detectable in 44% to 93% of patients with severely deficient ADAMTS13 activity.3,6,19
Nonneutralizing inhibitors. From 10% to 15% of patients with TTP with severe ADAMTS13 deficiency lack ADAMTS13 autoantibodies measured by enzyme immunoassay but have nonneutralizing immunoglobulin G (IgG) or IgM autoantibodies. In such cases, ADAMTS13 deficiency may be related to increased antibody-mediated clearance or other unknown mechanisms.
Neutralizing inhibitors and nonneutralizing inhibitors may be present simultaneously in some patients.3,10,19,20
Blood factors affect ADAMTS13 activity
Specimen factors can affect ADAMTS13 activity and antibody levels.
Hemoglobin is a potent inhibitor of ADAMTS13, so an elevated plasma level of free hemoglobin (> 2 g/dL) can reduce ADAMTS13 activity, as can hyperbilirubinemia (> 15 mg/dL).
High levels of endogenous von Willebrand factor, lipids, thrombin, or other proteases that may cleave ADAMTS13 can also reduce ADAMTS13 activity.3 Conversely, recent plasma exchange or transfusion can mask the diagnosis of TTP because of false normalization of ADAMTS13 activity. In addition, ADAMTS13 autoantibody can be detected in other immune-mediated disorders (eg, systemic lupus erythematosus, antiphospholipid syndrome), and hypergammaglobulinemia, as well as in 10% to 15% of healthy individuals.19
CONSIDER OTHER CONDITIONS
Before diagnosing TTP, other conditions causing thrombocytopenia and hemolytic anemia should be excluded by taking a careful clinical, laboratory, and medication history (Table 2). Of these conditions, the most challenging to differentiate from TTP—and often indistinguishable from it at presentation—is hemolytic-uremic syndrome (Table 3).
Hemolytic-uremic syndrome
Hemolytic-uremic syndrome presents with a triad of thrombocytopenia, acute renal failure, and microangiopathic hemolytic anemia, with increased lactate dehydrogenase levels. Renal dysfunction from ischemia or tissue injury by microvascular thrombi predominates. Hemolytic-uremic syndrome most often occurs in children and is often related to hemorrhagic enterocolitis caused by infection with Escherichia coli O157:H7 or Shigella species (90%–95% of cases).1,2,5
From 5% to 10% of cases of hemolytic- uremic syndrome are atypical. These cases are not associated with diarrhea, and many are caused by genetic mutations that result in chronic excessive complement activation. Implicated genes regulate complement regulator factor H (20%–30% of cases) or CD46 (10%) and other cofactors, or autoantibodies against factor H (10%), which affect the alternate complement pathway.6,21–23
Initial therapeutic plasma exchange is commonly undertaken for atypical hemolytic- uremic syndrome, particularly for patients at risk of rapid progression to end-stage renal failure. But despite such treatment, about 60% of these patients die or develop permanent renal damage within 1 year.2,3,24
Eculizumab, a monoclonal antibody against complement component C5, has been approved by the US Food and Drug Administration for atypical hemolytic-uremic syndrome and may improve quality of life.25–27
PLASMA EXCHANGE IS THE MAINSTAY OF THERAPY
In 2012, the British Society for Haematology published revised guidelines for managing TTP and other thrombotic microangiopathies.28
Acquired idiopathic TTP with reduced ADAMTS13 activity requires immediate therapeutic plasma exchange. Daily plasma exchange combines plasmapheresis to remove circulating ultralarge von Willebrand factor-platelet strings and autoantibodies against ADAMTS13, and infusion of fresh-frozen plasma to replace ADAMTS13.18 This procedure is the mainstay of therapy and brings 70% to 90% of patients with idiopathic TTP to remission.1,2,5,6 However, the optimal duration of daily plasma exchange and the number of procedures required is highly variable according to clinical condition. Therapeutic plasma exchange can also cause plasma-related adverse reactions.9,28 Congenital TTP requires plasma infusion or exchange depending on the patient’s severity of ADAMTS13 deficiency.
Corticosteroids are used in combination with daily therapeutic plasma exchange, although evidence from controlled trials of their efficacy in this setting is lacking. Patients with severely decreased ADAMTS13 activity or low titers of ADAMTS13 autoantibodies tend to respond to the therapy.5,8,29
An ADAMTS13 assay with a short turn-around time can help guide the decision to initiate therapeutic plasma exchange. However, if there is a strong clinical suspicion of TTP, plasma exchange should be initiated immediately without waiting for test results.5,30 Monitoring ADAMTS13 activity or inhibitor during initial plasma exchange therapy has had conflicting results in several studies and is generally not recommended for patients with acquired TTP.8,30,31
RELAPSE IS COMMON
About 20% to 50% of patients with idiopathic TTP experience a relapse (Case 2). Most relapses occur within the first 2 years after the initial episode, with an estimated risk of 43% for relapse at 7.5 years.5,9
Factors that predict a higher risk of relapse include persistently severely decreased ADAMTS13 activity, positive inhibitor, and high titers of autoantibodies to ADAMTS13 during symptomatic TTP. During clinical remission, persistence of autoantibodies also indicates increased risk.1,3,5,6,9
Patients who have a relapse and whose disease is refractory to therapeutic plasma exchange (10%–20% of cases) have been treated with corticosteroids, splenectomy, or immunosuppressive agents (cyclosporine, azathioprine, or cyclophosphamide) with varying rates of success. Rituximab (monoclonal anti-CD20) has recently been used as second-line therapy in refractory or relapsing immune-mediated TTP or idiopathic TTP with neurologic or cardiac symptoms associated with a poor prognosis. Therapy including rituximab results in improved response and progression-free survival.32 Other potential therapies, including recombinant active ADAMTS13, are under investigation.9,23,28,30,33,34
- Sadler JE, Moake JL, Miyata T, George JN. Recent advances in thrombotic thrombocytopenic purpura. Hematology Am Soc Hematol Educ Program 2004; 1:407–423.
- Shenkman B, Einav Y. Thrombotic thrombocytopenic purpura and other thrombotic microangiopathic hemolytic anemias: diagnosis and classification. Autoimmun Rev 2014; 13:584–586.
- Shah N, Sarode R. Thrombotic thrombocytopenic purpura-what is new? J Clin Apher 2013; 28:30–35.
- Imanirad I, Rajasekhar A, Zumberg M. A case series of atypical presentations of thrombotic thrombocytopenic purpura. J Clin Apher 2012; 27:221–226.
- George JN, Al-Nouri ZL. Diagnostic and therapeutic challenges in the thrombotic thrombocytopenic purpura and hemolytic uremic syndromes. Hematology Am Soc Hematol Educ Program 2012; 1:604–609.
- Shah N, Rutherford C, Matevosyan K, Shen YM, Sarode R. Role of ADAMTS13 in the management of thrombotic microangiopathies including thrombotic thrombocytopenic purpura (TTP). Br J Haematol 2013; 163:514–519.
- Cataland SR, Yang S, Wu HM. The use of ADAMTS13 activity, platelet count, and serum creatinine to differentiate acquired thrombotic thrombocytopenic purpura from other thrombotic microangiopathies. Br J Haematol 2012; 157:501–503.
- Mannucci PM, Peyvandi F. TTP and ADAMTS13: when Is testing appropriate? Hematology Am Soc Hematol Educ Program 2007; 1:121–126.
- Chaturved S, Carcioppolo D, Zhang L, McCar KR. Management and outcomes of patients with TTP: analysis of 100 cases at a single institution. Am J Hematol 2013; 88:560–565.
- Peyvandi F, Palla R, Lotta LA, Mackie I, Scully MA, Machin SJ. ADAMTS-13 assays in thrombotic thrombocytopenic purpura. J Thromb Haemost 2010; 8:631–640.
- Cataland SR, Scully MA, Paskavitz J, et al. Evidence of persistent neurologic injury following thrombotic thrombocytopenic purpura. Am J Hematol 2011; 86:87–89.
- Meloni G, Proia A, Antonini G, et al. Thrombotic thrombocytopenic purpura: prospective neurologic, neuroimaging and neurophysiologic evaluation. Haematologica 2001; 86:1194–1199.
- Kwaan HC, Boggio LN. The clinical spectrum of thrombotic thrombocytopenic purpura. Semin Thromb Hemost 2005; 31:673–680.
- Sarode R. Atypical presentations of thrombotic thrombocytopenic purpura: a review. J Clin Apher 2009; 24:47–52.
- Volcy J, Nzerue CM, Oderinde A, Hewan-Iowe K. Cocaine-induced acute renal failure, hemolysis, and thrombocytopenia mimicking thrombotic thrombocytopenic purpura. Am J Kidney Dis 2000; 35:E3.
- Kremer Hovinga JA, Mottini M, Lammle B. Measurement of ADAMTS-13 activity in plasma by the FRETS-VWF73 assay: comparison with other assay methods. J Thromb Haemost 2006; 4:1146–1148.
- Groot E, Hulstein JJ, Rison CN, de Groot PG, Fijnheer R. FRETS-VWF73: a rapid and predictive tool for thrombotic thrombocytopenic purpura. J Thromb Haemost 2006; 4:698–699.
- Barrows BD, Teruya J. Use of the ADAMTS13 activity assay improved the accuracy and efficiency of the diagnosis and treatment of suspected acquired thrombotic thrombocytopenic purpura. Arch Pathol Lab Med 2014; 138:546–549.
- Rieger M, Mannucci PM, Kremer Hovinga JA, et al. ADAMTS13 autoantibodies in patients with thrombotic microangiopathies and other immunomediated diseases. Blood 2005; 106:1262–1267.
- Rogers HJ, Kottke-Marchant K. ADAMTS13 evaluation for thrombotic thrombocytopenic purpura. Pathology Innovations, Pathology and Laboratory Medicine Institute. Cleveland Clinic, Fall 2014:6–9.
- Józsi M, Licht C, Strobel S, et al. Factor H autoantibodies in atypical hemolytic uremic syndrome correlate with CFHR1/CFHR3 deficiency. Blood 2008; 111:1512–1514.
- Diamante Chiodini B, Davin JC, Corazza F, et al. Eculizumab in anti-factor H antibodies associated with atypical hemolytic uremic syndrome. Pediatrics 2014; 133:e1764–e1768.
- Taylor CM, Machin S, Wigmore SJ, Goodship TH; working party from the Renal Association, the British Committee for Standards in Haematology and the British Transplantation Society. Clinical practice guidelines for the management of atypical haemolytic uraemic syndrome in the United Kingdom. Br J Haematol 2009; 148:37–47.
- Loirat C, Garnier A, Sellier-Leclerc AL, Kwon T. Plasmatherapy in atypical hemolytic uremic syndrome. Semin Thromb Hemost 2010; 36:673–681.
- Tsai HM, Kuo E. Eculizumab therapy leads to rapid resolution of thrombocytopenia in atypical hemolytic uremic syndrome. Adv Hematol 2014; 295323:1–7.
- Lapeyraque AL, Frémeaux-Bacchi V, Robitaille P. Efficacy of eculizumab in a patient with factor-H-associated atypical hemolytic uremic syndrome. Pediatr Nephrol 2011; 26:621–624.
- Baskin E, Gulleroglu K, Kantar A, Bayrakci U, Ozkaya O. Success of eculizumab in the treatment of atypical hemolytic uremic syndrome. Pediatr Nephrol 2015; 30:783–789.
- Scully M, Hunt BJ, Benjamin S, et al; British Committee for Standards in Haematology. Guidelines on the diagnosis and management of thrombotic thrombocytopenic purpura and other thrombotic microangiopathies. Br J Haematol 2012; 158:323–325.
- Abassi E, Yawn D, Leveque E, Nolasco L, Lopez J, Moake J. Correlation of ADAMTS-13 activity with response to plasma exchange in patients diagnosed with thrombotic thrombocytopenic purpura (Abstract #3921). Blood 2004; 104:242a.
- Blombery P, Scully M. Management of thrombocytic thrombocytopenic purpura: current perspectives. J Blood Med 2014; 5:15–23.
- Wu N, Liu J, Yang S, et al. Diagnostic and prognostic values of ADAMTS13 activity measured during daily plasma exchange therapy in patients with acquired thrombotic thrombocytopenic purpura. Transfusion 2015; 55:18–24.
- Cuker A. Adjuvant rituximab to prevent TTP relapse. Blood 2016; 127:2952–2953.
- Chapman K, Yuen S. Therapy for thrombotic thrombocytopenic purpura: past, present and future. Semin Thromb Hemost 2014; 40:34–40.
- Heidel F, Lipka DB, von Auer C, Huber C, Schrarrer I, Hess G. Addition of rituximab to standard therapy improves response rate and progression-free survival in relapsed or refractory thrombotic thrombocytopenic purpura and autoimmune haemolytic anaemia. Thromb Haemost 2007; 97:228–233.
A breakthrough in understanding the pathogenesis of thrombotic thrombocytopenic purpura (TTP) came with the discovery of ADAMTS13 (an abbreviation for “a disintegrin and metalloproteinase with thrombospondin type 1 motif, member 13”), a plasma protein that cleaves von Willebrand factor, which interacts with platelets to promote blood clotting. If ADAMTS13 is lacking, unusually large multimers of von Willebrand factor can accumulate and trigger intravascular platelet aggregation and microthrombosis, causing the signs and symptoms of TTP.1–3
This knowledge has practical applications: we can now measure ADAMTS13 activity, ADAMTS13 inhibitor, and antibodies against ADAMTS13 to help us diagnose TTP and distinguish it from other forms of thrombotic microangiopathy, such as hemolytic-uremic syndrome, that have similar symptoms but require different treatment.
Using case studies, this article describes typical presentations of acute and relapsing TTP; the role of laboratory testing, including the ADAMTS13 assay; how to distinguish TTP from other conditions that present similarly; and how to manage this condition.
A HIGH RISK OF DEATH WITHOUT PLASMA EXCHANGE
TTP is characterized by disseminated microthrombi composed of agglutinated platelets and von Willebrand factor in small vessels. Tissue damage by microthrombi can cause thrombocytopenia (platelet deficiency), microangiopathic hemolytic anemia (loss of red blood cells caused by destructive conditions in small vessels), and multiorgan failure.1
Untreated TTP has a mortality rate of about 90%.1 As shown in Case 1, Case 2, and Table 1, rapid diagnosis and prompt initiation of daily therapeutic plasma exchange can improve this grave outlook.4


ADAMTS13 DEFICIENCY CAN BE ACQUIRED OR CONGENITAL
Two major forms of TTP with ADAMTS13 deficiency and microvascular thrombosis are recognized:
Acquired TTP, the more common form, peaks in incidence between ages 30 and 50.2,5 It more often affects women, particularly during and after pregnancy (its estimated prevalence is 1 in 25,000 pregnancies), and African Americans.6 Acquired TTP may be:
- Primary (idiopathic or autoantibody-mediated), associated with severely decreased ADAMTS13 and the presence of ultra-large von Willebrand factor multimers, or
- Secondary (23%–67% of cases), arising from a variety of conditions, including autoimmune disorders (eg, systemic lupus erythematosus, rheumatoid arthritis), solid organ or hematopoietic cell transplant, malignancy, drugs, and pregnancy (Table 2).1,5–8 Secondary TTP has a worse prognosis than idiopathic TTP.5,9
Congenital TTP (Upshaw-Shulman syndrome) is a rare autosomal-recessive disease caused by compound heterozygous or homozygous mutations of the ADAMTS13 gene, producing nonfunctional ADAMTS13 protein. Patients have severely deficient ADAMTS13 activity but usually do not develop autoantibodies. There is a high risk of chronic, relapsing episodes; identified triggers include pregnancy and heavy alcohol intake.2,10 About half of patients with congenital TTP have an early onset, usually presenting with acute TTP between birth and age 5, and about half have a late onset, usually remaining without symptoms until age 20 to 40.
THE CLINICAL PICTURE OF TTP IS NOT ALWAYS CLASSIC
TTP is primarily diagnosed clinically, but diagnosis is often difficult because of various nonspecific symptoms. Typical TTP presents with the “classic pentad”:
- Severe thrombocytopenia (70%–100% of patients)
- Microangiopathic hemolytic anemia with multiple schistocytes (70%–100%) (Figure 1)
- Neurologic involvement (50%–90%)
- Renal abnormalities (about 50%)
- Fever (25%).
However, the entire picture often does not emerge in a single patient.2,6 Waiting for the entire pentad to develop before diagnosing TTP can have grave clinical consequences,1,2,5 and the presence of thrombocytopenia and unexplained microangiopathic hemolytic anemia are considered clinically sufficient to suspect TTP.5
Neurologic symptoms usually fluctuate. They can include mild abnormalities such as weakness, dizziness, headache, blurred vision, ataxia, and transient mental status changes, as well as severe abnormalities including stroke, seizure, and coma.2,6
Most patients have normal findings on computed tomography and magnetic resonance imaging at the onset of neurologic symptoms or with a history of TTP. Some patients (8%–39%) show reversible acute brain lesions, including ischemic changes.11–13
Other signs and symptoms may result from multiorgan failure due to microthrombosis; ischemia in retinal, coronary, and abdominal circulations; and unconjugated hyperbilirubinemia.2
Atypical presentations. About 18% of patients have cardiac involvement from microvascular occlusion, with arrhythmia, angina, or congestive heart failure. Abdominal pain and pancreatitis occur in 5% to 13%, and visual disturbances in 8% to 10%.
Patients with an atypical presentation may not have laboratory evidence of microangiopathic hemolytic anemia, but an ADAMTS13 assay will show severely decreased activity. Therapeutic plasma exchange can improve atypical symptoms.2,3,10,14,15
ADAMTS13 ASSAY IS KEY TO DIAGNOSIS
Laboratory evidence typically includes hemolytic anemia (reticulocytosis, schistocytes, elevated indirect bilirubin, reduced haptoglobin, elevated lactate dehydrogenase) and thrombocytopenia.3 There are no significant abnormalities in prothrombin time, international normalized ratio, activated partial thromboplastin time, fibrinogen, or D-dimer level.
Measuring the levels of ADAMTS13 activity, ADAMTS13 inhibitor, and ADAMTS13 antibody is becoming standard to confirm the diagnosis of TTP, to determine if it is congenital or acquired, and to distinguish it from thrombocytopenic conditions such as hemolytic-uremic syndrome, idiopathic thrombocytopenic purpura, and heparin-induced thrombocytopenia.4,5 A newer ADAMTS13 assay based on fluorescence energy transfer (FRET) technology with a synthetic amino acid-von Willebrand factor peptide substrate has a faster turnaround time and less test variability.6,16,17 This FRET assay can give the result of ADAMTS13 activity within 2 hours. In comparison, the assay based on multimeric von Willebrand factor takes 2 to 3 days, and mass spectrometry to measure the cleavage products of a synthetic von Willebrand factor molecule takes about 4 hours.3,10,16
About two-thirds of patients with the clinical diagnosis of idiopathic TTP have ADAMTS13 activity levels lower than 10%.5,14,18 In the appropriate clinical setting, this threshold level is highly sensitive (89%–100%) and specific (99%–100%) in differentiating TTP from other thrombotic angiopathies.2,3,18
Note: The ADAMTS13 assay was needed for early correct diagnosis in Case 1 and Case 2.
Inhibitors provide more clues
Autoantibodies can be classified according to whether they inhibit ADAMTS13 activity.
Neutralizing inhibitors. Most cases of acquired, idiopathic TTP with severe ADAMTS13 deficiency are related to circulating autoantibodies that neutralize ADAMTS13 activity. This ADAMTS13 inhibitor level is obtained by measuring residual ADAMTS13 activity after mixing equal amounts of patient plasma with normal pooled plasma. ADAMTS13 inhibitor is detectable in 44% to 93% of patients with severely deficient ADAMTS13 activity.3,6,19
Nonneutralizing inhibitors. From 10% to 15% of patients with TTP with severe ADAMTS13 deficiency lack ADAMTS13 autoantibodies measured by enzyme immunoassay but have nonneutralizing immunoglobulin G (IgG) or IgM autoantibodies. In such cases, ADAMTS13 deficiency may be related to increased antibody-mediated clearance or other unknown mechanisms.
Neutralizing inhibitors and nonneutralizing inhibitors may be present simultaneously in some patients.3,10,19,20
Blood factors affect ADAMTS13 activity
Specimen factors can affect ADAMTS13 activity and antibody levels.
Hemoglobin is a potent inhibitor of ADAMTS13, so an elevated plasma level of free hemoglobin (> 2 g/dL) can reduce ADAMTS13 activity, as can hyperbilirubinemia (> 15 mg/dL).
High levels of endogenous von Willebrand factor, lipids, thrombin, or other proteases that may cleave ADAMTS13 can also reduce ADAMTS13 activity.3 Conversely, recent plasma exchange or transfusion can mask the diagnosis of TTP because of false normalization of ADAMTS13 activity. In addition, ADAMTS13 autoantibody can be detected in other immune-mediated disorders (eg, systemic lupus erythematosus, antiphospholipid syndrome), and hypergammaglobulinemia, as well as in 10% to 15% of healthy individuals.19
CONSIDER OTHER CONDITIONS
Before diagnosing TTP, other conditions causing thrombocytopenia and hemolytic anemia should be excluded by taking a careful clinical, laboratory, and medication history (Table 2). Of these conditions, the most challenging to differentiate from TTP—and often indistinguishable from it at presentation—is hemolytic-uremic syndrome (Table 3).
Hemolytic-uremic syndrome
Hemolytic-uremic syndrome presents with a triad of thrombocytopenia, acute renal failure, and microangiopathic hemolytic anemia, with increased lactate dehydrogenase levels. Renal dysfunction from ischemia or tissue injury by microvascular thrombi predominates. Hemolytic-uremic syndrome most often occurs in children and is often related to hemorrhagic enterocolitis caused by infection with Escherichia coli O157:H7 or Shigella species (90%–95% of cases).1,2,5
From 5% to 10% of cases of hemolytic- uremic syndrome are atypical. These cases are not associated with diarrhea, and many are caused by genetic mutations that result in chronic excessive complement activation. Implicated genes regulate complement regulator factor H (20%–30% of cases) or CD46 (10%) and other cofactors, or autoantibodies against factor H (10%), which affect the alternate complement pathway.6,21–23
Initial therapeutic plasma exchange is commonly undertaken for atypical hemolytic- uremic syndrome, particularly for patients at risk of rapid progression to end-stage renal failure. But despite such treatment, about 60% of these patients die or develop permanent renal damage within 1 year.2,3,24
Eculizumab, a monoclonal antibody against complement component C5, has been approved by the US Food and Drug Administration for atypical hemolytic-uremic syndrome and may improve quality of life.25–27
PLASMA EXCHANGE IS THE MAINSTAY OF THERAPY
In 2012, the British Society for Haematology published revised guidelines for managing TTP and other thrombotic microangiopathies.28
Acquired idiopathic TTP with reduced ADAMTS13 activity requires immediate therapeutic plasma exchange. Daily plasma exchange combines plasmapheresis to remove circulating ultralarge von Willebrand factor-platelet strings and autoantibodies against ADAMTS13, and infusion of fresh-frozen plasma to replace ADAMTS13.18 This procedure is the mainstay of therapy and brings 70% to 90% of patients with idiopathic TTP to remission.1,2,5,6 However, the optimal duration of daily plasma exchange and the number of procedures required is highly variable according to clinical condition. Therapeutic plasma exchange can also cause plasma-related adverse reactions.9,28 Congenital TTP requires plasma infusion or exchange depending on the patient’s severity of ADAMTS13 deficiency.
Corticosteroids are used in combination with daily therapeutic plasma exchange, although evidence from controlled trials of their efficacy in this setting is lacking. Patients with severely decreased ADAMTS13 activity or low titers of ADAMTS13 autoantibodies tend to respond to the therapy.5,8,29
An ADAMTS13 assay with a short turn-around time can help guide the decision to initiate therapeutic plasma exchange. However, if there is a strong clinical suspicion of TTP, plasma exchange should be initiated immediately without waiting for test results.5,30 Monitoring ADAMTS13 activity or inhibitor during initial plasma exchange therapy has had conflicting results in several studies and is generally not recommended for patients with acquired TTP.8,30,31
RELAPSE IS COMMON
About 20% to 50% of patients with idiopathic TTP experience a relapse (Case 2). Most relapses occur within the first 2 years after the initial episode, with an estimated risk of 43% for relapse at 7.5 years.5,9
Factors that predict a higher risk of relapse include persistently severely decreased ADAMTS13 activity, positive inhibitor, and high titers of autoantibodies to ADAMTS13 during symptomatic TTP. During clinical remission, persistence of autoantibodies also indicates increased risk.1,3,5,6,9
Patients who have a relapse and whose disease is refractory to therapeutic plasma exchange (10%–20% of cases) have been treated with corticosteroids, splenectomy, or immunosuppressive agents (cyclosporine, azathioprine, or cyclophosphamide) with varying rates of success. Rituximab (monoclonal anti-CD20) has recently been used as second-line therapy in refractory or relapsing immune-mediated TTP or idiopathic TTP with neurologic or cardiac symptoms associated with a poor prognosis. Therapy including rituximab results in improved response and progression-free survival.32 Other potential therapies, including recombinant active ADAMTS13, are under investigation.9,23,28,30,33,34
A breakthrough in understanding the pathogenesis of thrombotic thrombocytopenic purpura (TTP) came with the discovery of ADAMTS13 (an abbreviation for “a disintegrin and metalloproteinase with thrombospondin type 1 motif, member 13”), a plasma protein that cleaves von Willebrand factor, which interacts with platelets to promote blood clotting. If ADAMTS13 is lacking, unusually large multimers of von Willebrand factor can accumulate and trigger intravascular platelet aggregation and microthrombosis, causing the signs and symptoms of TTP.1–3
This knowledge has practical applications: we can now measure ADAMTS13 activity, ADAMTS13 inhibitor, and antibodies against ADAMTS13 to help us diagnose TTP and distinguish it from other forms of thrombotic microangiopathy, such as hemolytic-uremic syndrome, that have similar symptoms but require different treatment.
Using case studies, this article describes typical presentations of acute and relapsing TTP; the role of laboratory testing, including the ADAMTS13 assay; how to distinguish TTP from other conditions that present similarly; and how to manage this condition.
A HIGH RISK OF DEATH WITHOUT PLASMA EXCHANGE
TTP is characterized by disseminated microthrombi composed of agglutinated platelets and von Willebrand factor in small vessels. Tissue damage by microthrombi can cause thrombocytopenia (platelet deficiency), microangiopathic hemolytic anemia (loss of red blood cells caused by destructive conditions in small vessels), and multiorgan failure.1
Untreated TTP has a mortality rate of about 90%.1 As shown in Case 1, Case 2, and Table 1, rapid diagnosis and prompt initiation of daily therapeutic plasma exchange can improve this grave outlook.4


ADAMTS13 DEFICIENCY CAN BE ACQUIRED OR CONGENITAL
Two major forms of TTP with ADAMTS13 deficiency and microvascular thrombosis are recognized:
Acquired TTP, the more common form, peaks in incidence between ages 30 and 50.2,5 It more often affects women, particularly during and after pregnancy (its estimated prevalence is 1 in 25,000 pregnancies), and African Americans.6 Acquired TTP may be:
- Primary (idiopathic or autoantibody-mediated), associated with severely decreased ADAMTS13 and the presence of ultra-large von Willebrand factor multimers, or
- Secondary (23%–67% of cases), arising from a variety of conditions, including autoimmune disorders (eg, systemic lupus erythematosus, rheumatoid arthritis), solid organ or hematopoietic cell transplant, malignancy, drugs, and pregnancy (Table 2).1,5–8 Secondary TTP has a worse prognosis than idiopathic TTP.5,9
Congenital TTP (Upshaw-Shulman syndrome) is a rare autosomal-recessive disease caused by compound heterozygous or homozygous mutations of the ADAMTS13 gene, producing nonfunctional ADAMTS13 protein. Patients have severely deficient ADAMTS13 activity but usually do not develop autoantibodies. There is a high risk of chronic, relapsing episodes; identified triggers include pregnancy and heavy alcohol intake.2,10 About half of patients with congenital TTP have an early onset, usually presenting with acute TTP between birth and age 5, and about half have a late onset, usually remaining without symptoms until age 20 to 40.
THE CLINICAL PICTURE OF TTP IS NOT ALWAYS CLASSIC
TTP is primarily diagnosed clinically, but diagnosis is often difficult because of various nonspecific symptoms. Typical TTP presents with the “classic pentad”:
- Severe thrombocytopenia (70%–100% of patients)
- Microangiopathic hemolytic anemia with multiple schistocytes (70%–100%) (Figure 1)
- Neurologic involvement (50%–90%)
- Renal abnormalities (about 50%)
- Fever (25%).
However, the entire picture often does not emerge in a single patient.2,6 Waiting for the entire pentad to develop before diagnosing TTP can have grave clinical consequences,1,2,5 and the presence of thrombocytopenia and unexplained microangiopathic hemolytic anemia are considered clinically sufficient to suspect TTP.5
Neurologic symptoms usually fluctuate. They can include mild abnormalities such as weakness, dizziness, headache, blurred vision, ataxia, and transient mental status changes, as well as severe abnormalities including stroke, seizure, and coma.2,6
Most patients have normal findings on computed tomography and magnetic resonance imaging at the onset of neurologic symptoms or with a history of TTP. Some patients (8%–39%) show reversible acute brain lesions, including ischemic changes.11–13
Other signs and symptoms may result from multiorgan failure due to microthrombosis; ischemia in retinal, coronary, and abdominal circulations; and unconjugated hyperbilirubinemia.2
Atypical presentations. About 18% of patients have cardiac involvement from microvascular occlusion, with arrhythmia, angina, or congestive heart failure. Abdominal pain and pancreatitis occur in 5% to 13%, and visual disturbances in 8% to 10%.
Patients with an atypical presentation may not have laboratory evidence of microangiopathic hemolytic anemia, but an ADAMTS13 assay will show severely decreased activity. Therapeutic plasma exchange can improve atypical symptoms.2,3,10,14,15
ADAMTS13 ASSAY IS KEY TO DIAGNOSIS
Laboratory evidence typically includes hemolytic anemia (reticulocytosis, schistocytes, elevated indirect bilirubin, reduced haptoglobin, elevated lactate dehydrogenase) and thrombocytopenia.3 There are no significant abnormalities in prothrombin time, international normalized ratio, activated partial thromboplastin time, fibrinogen, or D-dimer level.
Measuring the levels of ADAMTS13 activity, ADAMTS13 inhibitor, and ADAMTS13 antibody is becoming standard to confirm the diagnosis of TTP, to determine if it is congenital or acquired, and to distinguish it from thrombocytopenic conditions such as hemolytic-uremic syndrome, idiopathic thrombocytopenic purpura, and heparin-induced thrombocytopenia.4,5 A newer ADAMTS13 assay based on fluorescence energy transfer (FRET) technology with a synthetic amino acid-von Willebrand factor peptide substrate has a faster turnaround time and less test variability.6,16,17 This FRET assay can give the result of ADAMTS13 activity within 2 hours. In comparison, the assay based on multimeric von Willebrand factor takes 2 to 3 days, and mass spectrometry to measure the cleavage products of a synthetic von Willebrand factor molecule takes about 4 hours.3,10,16
About two-thirds of patients with the clinical diagnosis of idiopathic TTP have ADAMTS13 activity levels lower than 10%.5,14,18 In the appropriate clinical setting, this threshold level is highly sensitive (89%–100%) and specific (99%–100%) in differentiating TTP from other thrombotic angiopathies.2,3,18
Note: The ADAMTS13 assay was needed for early correct diagnosis in Case 1 and Case 2.
Inhibitors provide more clues
Autoantibodies can be classified according to whether they inhibit ADAMTS13 activity.
Neutralizing inhibitors. Most cases of acquired, idiopathic TTP with severe ADAMTS13 deficiency are related to circulating autoantibodies that neutralize ADAMTS13 activity. This ADAMTS13 inhibitor level is obtained by measuring residual ADAMTS13 activity after mixing equal amounts of patient plasma with normal pooled plasma. ADAMTS13 inhibitor is detectable in 44% to 93% of patients with severely deficient ADAMTS13 activity.3,6,19
Nonneutralizing inhibitors. From 10% to 15% of patients with TTP with severe ADAMTS13 deficiency lack ADAMTS13 autoantibodies measured by enzyme immunoassay but have nonneutralizing immunoglobulin G (IgG) or IgM autoantibodies. In such cases, ADAMTS13 deficiency may be related to increased antibody-mediated clearance or other unknown mechanisms.
Neutralizing inhibitors and nonneutralizing inhibitors may be present simultaneously in some patients.3,10,19,20
Blood factors affect ADAMTS13 activity
Specimen factors can affect ADAMTS13 activity and antibody levels.
Hemoglobin is a potent inhibitor of ADAMTS13, so an elevated plasma level of free hemoglobin (> 2 g/dL) can reduce ADAMTS13 activity, as can hyperbilirubinemia (> 15 mg/dL).
High levels of endogenous von Willebrand factor, lipids, thrombin, or other proteases that may cleave ADAMTS13 can also reduce ADAMTS13 activity.3 Conversely, recent plasma exchange or transfusion can mask the diagnosis of TTP because of false normalization of ADAMTS13 activity. In addition, ADAMTS13 autoantibody can be detected in other immune-mediated disorders (eg, systemic lupus erythematosus, antiphospholipid syndrome), and hypergammaglobulinemia, as well as in 10% to 15% of healthy individuals.19
CONSIDER OTHER CONDITIONS
Before diagnosing TTP, other conditions causing thrombocytopenia and hemolytic anemia should be excluded by taking a careful clinical, laboratory, and medication history (Table 2). Of these conditions, the most challenging to differentiate from TTP—and often indistinguishable from it at presentation—is hemolytic-uremic syndrome (Table 3).
Hemolytic-uremic syndrome
Hemolytic-uremic syndrome presents with a triad of thrombocytopenia, acute renal failure, and microangiopathic hemolytic anemia, with increased lactate dehydrogenase levels. Renal dysfunction from ischemia or tissue injury by microvascular thrombi predominates. Hemolytic-uremic syndrome most often occurs in children and is often related to hemorrhagic enterocolitis caused by infection with Escherichia coli O157:H7 or Shigella species (90%–95% of cases).1,2,5
From 5% to 10% of cases of hemolytic- uremic syndrome are atypical. These cases are not associated with diarrhea, and many are caused by genetic mutations that result in chronic excessive complement activation. Implicated genes regulate complement regulator factor H (20%–30% of cases) or CD46 (10%) and other cofactors, or autoantibodies against factor H (10%), which affect the alternate complement pathway.6,21–23
Initial therapeutic plasma exchange is commonly undertaken for atypical hemolytic- uremic syndrome, particularly for patients at risk of rapid progression to end-stage renal failure. But despite such treatment, about 60% of these patients die or develop permanent renal damage within 1 year.2,3,24
Eculizumab, a monoclonal antibody against complement component C5, has been approved by the US Food and Drug Administration for atypical hemolytic-uremic syndrome and may improve quality of life.25–27
PLASMA EXCHANGE IS THE MAINSTAY OF THERAPY
In 2012, the British Society for Haematology published revised guidelines for managing TTP and other thrombotic microangiopathies.28
Acquired idiopathic TTP with reduced ADAMTS13 activity requires immediate therapeutic plasma exchange. Daily plasma exchange combines plasmapheresis to remove circulating ultralarge von Willebrand factor-platelet strings and autoantibodies against ADAMTS13, and infusion of fresh-frozen plasma to replace ADAMTS13.18 This procedure is the mainstay of therapy and brings 70% to 90% of patients with idiopathic TTP to remission.1,2,5,6 However, the optimal duration of daily plasma exchange and the number of procedures required is highly variable according to clinical condition. Therapeutic plasma exchange can also cause plasma-related adverse reactions.9,28 Congenital TTP requires plasma infusion or exchange depending on the patient’s severity of ADAMTS13 deficiency.
Corticosteroids are used in combination with daily therapeutic plasma exchange, although evidence from controlled trials of their efficacy in this setting is lacking. Patients with severely decreased ADAMTS13 activity or low titers of ADAMTS13 autoantibodies tend to respond to the therapy.5,8,29
An ADAMTS13 assay with a short turn-around time can help guide the decision to initiate therapeutic plasma exchange. However, if there is a strong clinical suspicion of TTP, plasma exchange should be initiated immediately without waiting for test results.5,30 Monitoring ADAMTS13 activity or inhibitor during initial plasma exchange therapy has had conflicting results in several studies and is generally not recommended for patients with acquired TTP.8,30,31
RELAPSE IS COMMON
About 20% to 50% of patients with idiopathic TTP experience a relapse (Case 2). Most relapses occur within the first 2 years after the initial episode, with an estimated risk of 43% for relapse at 7.5 years.5,9
Factors that predict a higher risk of relapse include persistently severely decreased ADAMTS13 activity, positive inhibitor, and high titers of autoantibodies to ADAMTS13 during symptomatic TTP. During clinical remission, persistence of autoantibodies also indicates increased risk.1,3,5,6,9
Patients who have a relapse and whose disease is refractory to therapeutic plasma exchange (10%–20% of cases) have been treated with corticosteroids, splenectomy, or immunosuppressive agents (cyclosporine, azathioprine, or cyclophosphamide) with varying rates of success. Rituximab (monoclonal anti-CD20) has recently been used as second-line therapy in refractory or relapsing immune-mediated TTP or idiopathic TTP with neurologic or cardiac symptoms associated with a poor prognosis. Therapy including rituximab results in improved response and progression-free survival.32 Other potential therapies, including recombinant active ADAMTS13, are under investigation.9,23,28,30,33,34
- Sadler JE, Moake JL, Miyata T, George JN. Recent advances in thrombotic thrombocytopenic purpura. Hematology Am Soc Hematol Educ Program 2004; 1:407–423.
- Shenkman B, Einav Y. Thrombotic thrombocytopenic purpura and other thrombotic microangiopathic hemolytic anemias: diagnosis and classification. Autoimmun Rev 2014; 13:584–586.
- Shah N, Sarode R. Thrombotic thrombocytopenic purpura-what is new? J Clin Apher 2013; 28:30–35.
- Imanirad I, Rajasekhar A, Zumberg M. A case series of atypical presentations of thrombotic thrombocytopenic purpura. J Clin Apher 2012; 27:221–226.
- George JN, Al-Nouri ZL. Diagnostic and therapeutic challenges in the thrombotic thrombocytopenic purpura and hemolytic uremic syndromes. Hematology Am Soc Hematol Educ Program 2012; 1:604–609.
- Shah N, Rutherford C, Matevosyan K, Shen YM, Sarode R. Role of ADAMTS13 in the management of thrombotic microangiopathies including thrombotic thrombocytopenic purpura (TTP). Br J Haematol 2013; 163:514–519.
- Cataland SR, Yang S, Wu HM. The use of ADAMTS13 activity, platelet count, and serum creatinine to differentiate acquired thrombotic thrombocytopenic purpura from other thrombotic microangiopathies. Br J Haematol 2012; 157:501–503.
- Mannucci PM, Peyvandi F. TTP and ADAMTS13: when Is testing appropriate? Hematology Am Soc Hematol Educ Program 2007; 1:121–126.
- Chaturved S, Carcioppolo D, Zhang L, McCar KR. Management and outcomes of patients with TTP: analysis of 100 cases at a single institution. Am J Hematol 2013; 88:560–565.
- Peyvandi F, Palla R, Lotta LA, Mackie I, Scully MA, Machin SJ. ADAMTS-13 assays in thrombotic thrombocytopenic purpura. J Thromb Haemost 2010; 8:631–640.
- Cataland SR, Scully MA, Paskavitz J, et al. Evidence of persistent neurologic injury following thrombotic thrombocytopenic purpura. Am J Hematol 2011; 86:87–89.
- Meloni G, Proia A, Antonini G, et al. Thrombotic thrombocytopenic purpura: prospective neurologic, neuroimaging and neurophysiologic evaluation. Haematologica 2001; 86:1194–1199.
- Kwaan HC, Boggio LN. The clinical spectrum of thrombotic thrombocytopenic purpura. Semin Thromb Hemost 2005; 31:673–680.
- Sarode R. Atypical presentations of thrombotic thrombocytopenic purpura: a review. J Clin Apher 2009; 24:47–52.
- Volcy J, Nzerue CM, Oderinde A, Hewan-Iowe K. Cocaine-induced acute renal failure, hemolysis, and thrombocytopenia mimicking thrombotic thrombocytopenic purpura. Am J Kidney Dis 2000; 35:E3.
- Kremer Hovinga JA, Mottini M, Lammle B. Measurement of ADAMTS-13 activity in plasma by the FRETS-VWF73 assay: comparison with other assay methods. J Thromb Haemost 2006; 4:1146–1148.
- Groot E, Hulstein JJ, Rison CN, de Groot PG, Fijnheer R. FRETS-VWF73: a rapid and predictive tool for thrombotic thrombocytopenic purpura. J Thromb Haemost 2006; 4:698–699.
- Barrows BD, Teruya J. Use of the ADAMTS13 activity assay improved the accuracy and efficiency of the diagnosis and treatment of suspected acquired thrombotic thrombocytopenic purpura. Arch Pathol Lab Med 2014; 138:546–549.
- Rieger M, Mannucci PM, Kremer Hovinga JA, et al. ADAMTS13 autoantibodies in patients with thrombotic microangiopathies and other immunomediated diseases. Blood 2005; 106:1262–1267.
- Rogers HJ, Kottke-Marchant K. ADAMTS13 evaluation for thrombotic thrombocytopenic purpura. Pathology Innovations, Pathology and Laboratory Medicine Institute. Cleveland Clinic, Fall 2014:6–9.
- Józsi M, Licht C, Strobel S, et al. Factor H autoantibodies in atypical hemolytic uremic syndrome correlate with CFHR1/CFHR3 deficiency. Blood 2008; 111:1512–1514.
- Diamante Chiodini B, Davin JC, Corazza F, et al. Eculizumab in anti-factor H antibodies associated with atypical hemolytic uremic syndrome. Pediatrics 2014; 133:e1764–e1768.
- Taylor CM, Machin S, Wigmore SJ, Goodship TH; working party from the Renal Association, the British Committee for Standards in Haematology and the British Transplantation Society. Clinical practice guidelines for the management of atypical haemolytic uraemic syndrome in the United Kingdom. Br J Haematol 2009; 148:37–47.
- Loirat C, Garnier A, Sellier-Leclerc AL, Kwon T. Plasmatherapy in atypical hemolytic uremic syndrome. Semin Thromb Hemost 2010; 36:673–681.
- Tsai HM, Kuo E. Eculizumab therapy leads to rapid resolution of thrombocytopenia in atypical hemolytic uremic syndrome. Adv Hematol 2014; 295323:1–7.
- Lapeyraque AL, Frémeaux-Bacchi V, Robitaille P. Efficacy of eculizumab in a patient with factor-H-associated atypical hemolytic uremic syndrome. Pediatr Nephrol 2011; 26:621–624.
- Baskin E, Gulleroglu K, Kantar A, Bayrakci U, Ozkaya O. Success of eculizumab in the treatment of atypical hemolytic uremic syndrome. Pediatr Nephrol 2015; 30:783–789.
- Scully M, Hunt BJ, Benjamin S, et al; British Committee for Standards in Haematology. Guidelines on the diagnosis and management of thrombotic thrombocytopenic purpura and other thrombotic microangiopathies. Br J Haematol 2012; 158:323–325.
- Abassi E, Yawn D, Leveque E, Nolasco L, Lopez J, Moake J. Correlation of ADAMTS-13 activity with response to plasma exchange in patients diagnosed with thrombotic thrombocytopenic purpura (Abstract #3921). Blood 2004; 104:242a.
- Blombery P, Scully M. Management of thrombocytic thrombocytopenic purpura: current perspectives. J Blood Med 2014; 5:15–23.
- Wu N, Liu J, Yang S, et al. Diagnostic and prognostic values of ADAMTS13 activity measured during daily plasma exchange therapy in patients with acquired thrombotic thrombocytopenic purpura. Transfusion 2015; 55:18–24.
- Cuker A. Adjuvant rituximab to prevent TTP relapse. Blood 2016; 127:2952–2953.
- Chapman K, Yuen S. Therapy for thrombotic thrombocytopenic purpura: past, present and future. Semin Thromb Hemost 2014; 40:34–40.
- Heidel F, Lipka DB, von Auer C, Huber C, Schrarrer I, Hess G. Addition of rituximab to standard therapy improves response rate and progression-free survival in relapsed or refractory thrombotic thrombocytopenic purpura and autoimmune haemolytic anaemia. Thromb Haemost 2007; 97:228–233.
- Sadler JE, Moake JL, Miyata T, George JN. Recent advances in thrombotic thrombocytopenic purpura. Hematology Am Soc Hematol Educ Program 2004; 1:407–423.
- Shenkman B, Einav Y. Thrombotic thrombocytopenic purpura and other thrombotic microangiopathic hemolytic anemias: diagnosis and classification. Autoimmun Rev 2014; 13:584–586.
- Shah N, Sarode R. Thrombotic thrombocytopenic purpura-what is new? J Clin Apher 2013; 28:30–35.
- Imanirad I, Rajasekhar A, Zumberg M. A case series of atypical presentations of thrombotic thrombocytopenic purpura. J Clin Apher 2012; 27:221–226.
- George JN, Al-Nouri ZL. Diagnostic and therapeutic challenges in the thrombotic thrombocytopenic purpura and hemolytic uremic syndromes. Hematology Am Soc Hematol Educ Program 2012; 1:604–609.
- Shah N, Rutherford C, Matevosyan K, Shen YM, Sarode R. Role of ADAMTS13 in the management of thrombotic microangiopathies including thrombotic thrombocytopenic purpura (TTP). Br J Haematol 2013; 163:514–519.
- Cataland SR, Yang S, Wu HM. The use of ADAMTS13 activity, platelet count, and serum creatinine to differentiate acquired thrombotic thrombocytopenic purpura from other thrombotic microangiopathies. Br J Haematol 2012; 157:501–503.
- Mannucci PM, Peyvandi F. TTP and ADAMTS13: when Is testing appropriate? Hematology Am Soc Hematol Educ Program 2007; 1:121–126.
- Chaturved S, Carcioppolo D, Zhang L, McCar KR. Management and outcomes of patients with TTP: analysis of 100 cases at a single institution. Am J Hematol 2013; 88:560–565.
- Peyvandi F, Palla R, Lotta LA, Mackie I, Scully MA, Machin SJ. ADAMTS-13 assays in thrombotic thrombocytopenic purpura. J Thromb Haemost 2010; 8:631–640.
- Cataland SR, Scully MA, Paskavitz J, et al. Evidence of persistent neurologic injury following thrombotic thrombocytopenic purpura. Am J Hematol 2011; 86:87–89.
- Meloni G, Proia A, Antonini G, et al. Thrombotic thrombocytopenic purpura: prospective neurologic, neuroimaging and neurophysiologic evaluation. Haematologica 2001; 86:1194–1199.
- Kwaan HC, Boggio LN. The clinical spectrum of thrombotic thrombocytopenic purpura. Semin Thromb Hemost 2005; 31:673–680.
- Sarode R. Atypical presentations of thrombotic thrombocytopenic purpura: a review. J Clin Apher 2009; 24:47–52.
- Volcy J, Nzerue CM, Oderinde A, Hewan-Iowe K. Cocaine-induced acute renal failure, hemolysis, and thrombocytopenia mimicking thrombotic thrombocytopenic purpura. Am J Kidney Dis 2000; 35:E3.
- Kremer Hovinga JA, Mottini M, Lammle B. Measurement of ADAMTS-13 activity in plasma by the FRETS-VWF73 assay: comparison with other assay methods. J Thromb Haemost 2006; 4:1146–1148.
- Groot E, Hulstein JJ, Rison CN, de Groot PG, Fijnheer R. FRETS-VWF73: a rapid and predictive tool for thrombotic thrombocytopenic purpura. J Thromb Haemost 2006; 4:698–699.
- Barrows BD, Teruya J. Use of the ADAMTS13 activity assay improved the accuracy and efficiency of the diagnosis and treatment of suspected acquired thrombotic thrombocytopenic purpura. Arch Pathol Lab Med 2014; 138:546–549.
- Rieger M, Mannucci PM, Kremer Hovinga JA, et al. ADAMTS13 autoantibodies in patients with thrombotic microangiopathies and other immunomediated diseases. Blood 2005; 106:1262–1267.
- Rogers HJ, Kottke-Marchant K. ADAMTS13 evaluation for thrombotic thrombocytopenic purpura. Pathology Innovations, Pathology and Laboratory Medicine Institute. Cleveland Clinic, Fall 2014:6–9.
- Józsi M, Licht C, Strobel S, et al. Factor H autoantibodies in atypical hemolytic uremic syndrome correlate with CFHR1/CFHR3 deficiency. Blood 2008; 111:1512–1514.
- Diamante Chiodini B, Davin JC, Corazza F, et al. Eculizumab in anti-factor H antibodies associated with atypical hemolytic uremic syndrome. Pediatrics 2014; 133:e1764–e1768.
- Taylor CM, Machin S, Wigmore SJ, Goodship TH; working party from the Renal Association, the British Committee for Standards in Haematology and the British Transplantation Society. Clinical practice guidelines for the management of atypical haemolytic uraemic syndrome in the United Kingdom. Br J Haematol 2009; 148:37–47.
- Loirat C, Garnier A, Sellier-Leclerc AL, Kwon T. Plasmatherapy in atypical hemolytic uremic syndrome. Semin Thromb Hemost 2010; 36:673–681.
- Tsai HM, Kuo E. Eculizumab therapy leads to rapid resolution of thrombocytopenia in atypical hemolytic uremic syndrome. Adv Hematol 2014; 295323:1–7.
- Lapeyraque AL, Frémeaux-Bacchi V, Robitaille P. Efficacy of eculizumab in a patient with factor-H-associated atypical hemolytic uremic syndrome. Pediatr Nephrol 2011; 26:621–624.
- Baskin E, Gulleroglu K, Kantar A, Bayrakci U, Ozkaya O. Success of eculizumab in the treatment of atypical hemolytic uremic syndrome. Pediatr Nephrol 2015; 30:783–789.
- Scully M, Hunt BJ, Benjamin S, et al; British Committee for Standards in Haematology. Guidelines on the diagnosis and management of thrombotic thrombocytopenic purpura and other thrombotic microangiopathies. Br J Haematol 2012; 158:323–325.
- Abassi E, Yawn D, Leveque E, Nolasco L, Lopez J, Moake J. Correlation of ADAMTS-13 activity with response to plasma exchange in patients diagnosed with thrombotic thrombocytopenic purpura (Abstract #3921). Blood 2004; 104:242a.
- Blombery P, Scully M. Management of thrombocytic thrombocytopenic purpura: current perspectives. J Blood Med 2014; 5:15–23.
- Wu N, Liu J, Yang S, et al. Diagnostic and prognostic values of ADAMTS13 activity measured during daily plasma exchange therapy in patients with acquired thrombotic thrombocytopenic purpura. Transfusion 2015; 55:18–24.
- Cuker A. Adjuvant rituximab to prevent TTP relapse. Blood 2016; 127:2952–2953.
- Chapman K, Yuen S. Therapy for thrombotic thrombocytopenic purpura: past, present and future. Semin Thromb Hemost 2014; 40:34–40.
- Heidel F, Lipka DB, von Auer C, Huber C, Schrarrer I, Hess G. Addition of rituximab to standard therapy improves response rate and progression-free survival in relapsed or refractory thrombotic thrombocytopenic purpura and autoimmune haemolytic anaemia. Thromb Haemost 2007; 97:228–233.
KEY POINTS
- Symptoms of TTP are usually neurologic but can also be cardiac or abdominal. Thrombocytopenia and unexplained microangiopathic hemolytic anemia are sufficient to highly suspect the disease.
- In the appropriate clinical setting, an ADAMTS13 activity level lower than 10% is highly indicative of TTP.
- ADAMTS13 inhibitor and ADAMTS13 antibody assays provide more diagnostic clues. ADAMTS13 antibody is generally absent in the congenital form.
- The ADAMTS13 assay can help distinguish TTP from hemolytic-uremic syndrome, which presents similarly but typically involves normal or only mildly reduced ADAMTS13 activity.
- A strong clinical suspicion of TTP warrants immediate initiation of therapeutic plasma exchange without waiting for ADAMTS13 test results.
Advanced-stage calciphylaxis: Think before you punch
A 53-year-old woman presented with extensive, nonulcerated, painful plaques on both calves. She had long-standing diabetes mellitus and had recently started hemodialysis. She had no fever or trauma and did not appear to be in shock.
On physical examination, she had extensive, well-demarcated, nonulcerated, indurated dark eschar over the right calf (Figure 1). Her left calf had similar lesions that appeared as focal, discrete, nonulcerated, violaceous plaques, with associated tenderness. No significant erythema, edema, drainage, or fluctuance was noted.
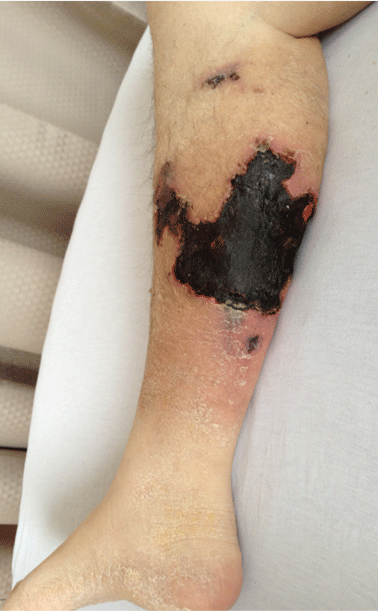
A broad-spectrum antibiotic was started empirically but was discontinued when routine blood testing and magnetic resonance imaging showed no evidence of infection. Histologic study of a full-thickness skin biopsy specimen (Figure 2) showed tissue necrosis, ulceration, and concentric calcification of small and medium-sized blood vessels, many with luminal thrombi, all of which together were diagnostic for calciphylaxis.
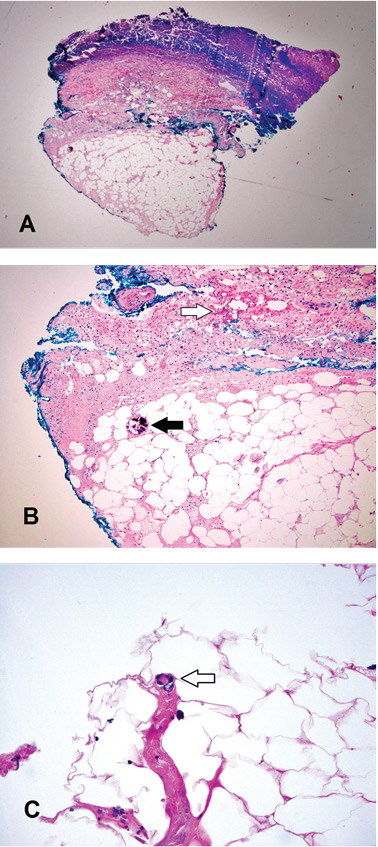
Treatment was started with cinacalcet, low-calcium dialysis baths, phosphate binders, and sodium thiosulfate. However, within a few days of the biopsy procedure, an infection developed at the biopsy site, and the patient developed sepsis and septic shock. She received broad-spectrum antibiotics and underwent extensive debridement with wound care. After a protracted hospital course, the infection resolved.
CALCIPHYLAXIS RISK FACTORS
Calciphylaxis, also referred to as calcific uremic arteriolopathy, is a rare and often fatal condition in patients with end-stage renal disease who are on hemodialysis (1% to 4% of dialysis patients).1–3 It is also seen in patients who have undergone renal transplant and in patients with chronic kidney disease who have a chronic inflammatory disease or who have been exposed to corticosteroids or warfarin. However, it can also occur in patients without chronic kidney disease or end-stage renal disease.
The term “calcific uremic arteriolopathy” is a misnomer, as this condition can occur in patients with normal renal function (nonuremic calciphylaxis). Also, despite what the term calciphylaxis implies, there is no systemic anaphylaxis.3–5
Documented risk factors include obesity; female sex; use of warfarin, corticosteroids, or vitamin D analogues; low serum albumin; hypercoagulable states; hyperparathyroidism; alcoholic liver disease; elevated calcium-phosphorus product; inflammation; connective tissue disease; and cancer.4–6
DIAGNOSTIC CLUES
There are no strict guidelines for the diagnosis of calciphylaxis, and the exact pathophysiology of calciphylaxis is not understood.1–4
Ulceration is considered the clinical hallmark, but there are increasing reports of patients presenting with nonulcerated plaques, as in our patient. The literature suggests a mortality rate of 33% at 6 months in these patients, but ulceration increases the risk of death to over 80%, and sepsis is the leading cause of death.7,8
Histologic features identified on full-thickness biopsy specimens are intravascular deposition of calcium in the media of the blood vessels, as well as fibrin thrombi formation, intimal proliferation, tissue necrosis, and resultant ischemia. However, as in our patient and as discussed below, the biopsy procedure can induce or exacerbate ulceration, increasing the risk of sepsis, and is thus controversial.7
In the early stages, lesions of calciphylaxis are focal and appear as erythema or livedo reticularis with or without subcutaneous plaques or ulcers. As the disease progresses, the ischemic changes coalesce to form denser violaceous, painful, plaquelike subcutaneous nodules with eschar. In the advanced stages, the eschar or ulceration involves an extensive area.
Diagnosis in the early stages is challenging because of the focal nature of involvement. The differential diagnosis includes potentially fatal conditions such as systemic vasculitis, nephrogenic systemic fibrosis, pyoderma gangrenosum, gangrene from peripheral arterial disease, cholesterol embolization, warfarin-induced necrosis, purpura fulminans, and oxalate vasculopathy.7
In the advanced stages, the diagnosis of calciphylaxis is clinically more evident, and the differential diagnosis usually narrows. Well-demarcated, necrotic, indurated lesions that are bilateral in a patient with end-stage renal disease without shock makes the diagnosis very likely.
The dangers of biopsy
As seen in our patient, biopsy for histologic confirmation of calciphylaxis can increase the risk of infection and sepsis.7 Also, the efficacy and clinical utility are uncertain because the quantity or depth of tissue obtained may not be enough for diagnosis. Deep incisional cutaneous biopsy is needed rather than punch biopsy to provide ample subcutaneous tissue for histologic study.3
Further, the biopsy procedure induces ulceration in the region of the incision, increasing the risk of infection and poor healing and escalating the risk of sepsis and death.7–9 Since extensive necrosis predisposes to a negative biopsy, a high clinical suspicion should drive early treatment of calciphylaxis.10 Noninvasive imaging studies such as plain radiography and bone scintigraphy can aid the diagnosis by detecting moderate to severe soft-tissue vascular calcification in these areas.7–11
DEBRIDEMENT IS CONTROVERSIAL
Conservative measures are the mainstay of care and include dietary alterations, noncalcium and nonaluminum phosphate binders, and low-calcium bath dialysis. There is mounting evidence for the use of calcimimetics and sodium thiosulfate.7,12–14
The role of wound debridement is controversial, as concomitant poor peripheral vascular perfusion can delay wound healing and, if ulceration ensues, there is a dramatic escalation of mortality risk. The decision for wound debridement is determined case by case, based on an assessment of the comorbidities, vascular perfusion, and status of the eschar.
Extensive wound debridement should be considered immediately after biopsy or with any signs of ulceration or infection—this in addition to meticulous wound care, which will promote healing and prevent serious complications secondary to infection.15
A TEAM APPROACH IMPROVES OUTCOMES
A multidisciplinary approach involving surgeons, nephrologists, dermatologists, dermatopathologists, wound or burn care team, nutrition team, pain management team, and infectious disease team is important to improve outcomes.7
Management mainly involves controlling pain; avoiding local trauma; treating and preventing infection; stopping causative agents such as warfarin and corticosteroids; intensive hemodialysis with an increase in both frequency and duration; intravenous sodium thiosulphate; non-calcium-phosphorus binders and cinacalcet in patients with elevated parathyroid hormone; and hyperbaric oxygen.12–14 There are also reports of success with oral etidronate and intravenous pamidronate.16,17
- Spanakis EK, Sellmeyer DE. Nonuremic calciphylaxis precipitated by teriparatide [rhPTH(1-34)] therapy in the setting of chronic warfarin and glucocorticoid treatment. Osteoporos Int 2014; 25:1411–1414.
- Brandenburg VM, Cozzolino M, Ketteler M. Calciphylaxis: a still unmet challenge. J Nephrol 2011; 24:142–148.
- Wilmer WA, Magro CM. Calciphylaxis: emerging concepts in prevention, diagnosis, and treatment. Semin Dial 2002; 15:172–186.
- Rimtepathip P, Cohen D. A rare presentation of calciphylaxis in normal renal function. Int J Case Rep Images 2015; 6:366–369.
- Lonowski S, Martin S, Worswick S. Widespread calciphylaxis and normal renal function: no improvement with sodium thiosulfate. Dermatol Online J 2015; 21:13030/qt76845802.
- Zhou Q, Neubauer J, Kern JS, Grotz W, Walz G, Huber TB. Calciphylaxis. Lancet 2014; 383:1067.
- Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis 2015; 66:133–146.
- Fine A, Zacharias J. Calciphylaxis is usually non-ulcerating: risk factors, outcome and therapy. Kidney Int 2002; 61:2210–2217.
- Hayashi M. Calciphylaxis: diagnosis and clinical features. Clin Exp Nephrol 2013; 17:498–503.
- Stavros K, Motiwala R, Zhou L, Sejdiu F, Shin S. Calciphylaxis in a dialysis patient diagnosed by muscle biopsy. J Clin Neuromuscul Dis 2014; 15:108–111.
- Bonchak JG, Park KK, Vethanayagamony T, Sheikh MM, Winterfield LS. Calciphylaxis: a case series and the role of radiology in diagnosis. Int J Dermatol 2015. [Epub ahead of print]
- Ross EA. Evolution of treatment strategies for calciphylaxis. Am J Nephrol 2011; 34:460–467.
- Cicone JS, Petronis JB, Embert CD, Spector DA. Successful treatment of calciphylaxis with intravenous sodium thiosulfate. Am J Kidney Dis 2004; 43:1104–1108.
- Brandenburg VM, Kramann R, Specht P, Ketteler M. Calciphylaxis in CKD and beyond. Nephrol Dial Transplant 2012; 27:1314–1318.
- Martin R. Mysterious calciphylaxis: wounds with eschar—to debride or not to debride? Ostomy Wound Manage 2004; 50:64–66.
- Shiraishi N, Kitamura K, Miyoshi T, et al. Successful treatment of a patient with severe calcific uremic arteriolopathy (calciphylaxis) by etidronate disodium. Am J Kidney Dis 2006; 48:151–154.
- Hanafusa T, Yamaguchi Y, Tani M, Umegaki N, Nishimura Y, Katayama I. Intractable wounds caused by calcific uremic arteriolopathy treated with bisphosphonates. J Am Acad Dermatol 2007; 57:1021–1025.
A 53-year-old woman presented with extensive, nonulcerated, painful plaques on both calves. She had long-standing diabetes mellitus and had recently started hemodialysis. She had no fever or trauma and did not appear to be in shock.
On physical examination, she had extensive, well-demarcated, nonulcerated, indurated dark eschar over the right calf (Figure 1). Her left calf had similar lesions that appeared as focal, discrete, nonulcerated, violaceous plaques, with associated tenderness. No significant erythema, edema, drainage, or fluctuance was noted.

A broad-spectrum antibiotic was started empirically but was discontinued when routine blood testing and magnetic resonance imaging showed no evidence of infection. Histologic study of a full-thickness skin biopsy specimen (Figure 2) showed tissue necrosis, ulceration, and concentric calcification of small and medium-sized blood vessels, many with luminal thrombi, all of which together were diagnostic for calciphylaxis.

Treatment was started with cinacalcet, low-calcium dialysis baths, phosphate binders, and sodium thiosulfate. However, within a few days of the biopsy procedure, an infection developed at the biopsy site, and the patient developed sepsis and septic shock. She received broad-spectrum antibiotics and underwent extensive debridement with wound care. After a protracted hospital course, the infection resolved.
CALCIPHYLAXIS RISK FACTORS
Calciphylaxis, also referred to as calcific uremic arteriolopathy, is a rare and often fatal condition in patients with end-stage renal disease who are on hemodialysis (1% to 4% of dialysis patients).1–3 It is also seen in patients who have undergone renal transplant and in patients with chronic kidney disease who have a chronic inflammatory disease or who have been exposed to corticosteroids or warfarin. However, it can also occur in patients without chronic kidney disease or end-stage renal disease.
The term “calcific uremic arteriolopathy” is a misnomer, as this condition can occur in patients with normal renal function (nonuremic calciphylaxis). Also, despite what the term calciphylaxis implies, there is no systemic anaphylaxis.3–5
Documented risk factors include obesity; female sex; use of warfarin, corticosteroids, or vitamin D analogues; low serum albumin; hypercoagulable states; hyperparathyroidism; alcoholic liver disease; elevated calcium-phosphorus product; inflammation; connective tissue disease; and cancer.4–6
DIAGNOSTIC CLUES
There are no strict guidelines for the diagnosis of calciphylaxis, and the exact pathophysiology of calciphylaxis is not understood.1–4
Ulceration is considered the clinical hallmark, but there are increasing reports of patients presenting with nonulcerated plaques, as in our patient. The literature suggests a mortality rate of 33% at 6 months in these patients, but ulceration increases the risk of death to over 80%, and sepsis is the leading cause of death.7,8
Histologic features identified on full-thickness biopsy specimens are intravascular deposition of calcium in the media of the blood vessels, as well as fibrin thrombi formation, intimal proliferation, tissue necrosis, and resultant ischemia. However, as in our patient and as discussed below, the biopsy procedure can induce or exacerbate ulceration, increasing the risk of sepsis, and is thus controversial.7
In the early stages, lesions of calciphylaxis are focal and appear as erythema or livedo reticularis with or without subcutaneous plaques or ulcers. As the disease progresses, the ischemic changes coalesce to form denser violaceous, painful, plaquelike subcutaneous nodules with eschar. In the advanced stages, the eschar or ulceration involves an extensive area.
Diagnosis in the early stages is challenging because of the focal nature of involvement. The differential diagnosis includes potentially fatal conditions such as systemic vasculitis, nephrogenic systemic fibrosis, pyoderma gangrenosum, gangrene from peripheral arterial disease, cholesterol embolization, warfarin-induced necrosis, purpura fulminans, and oxalate vasculopathy.7
In the advanced stages, the diagnosis of calciphylaxis is clinically more evident, and the differential diagnosis usually narrows. Well-demarcated, necrotic, indurated lesions that are bilateral in a patient with end-stage renal disease without shock makes the diagnosis very likely.
The dangers of biopsy
As seen in our patient, biopsy for histologic confirmation of calciphylaxis can increase the risk of infection and sepsis.7 Also, the efficacy and clinical utility are uncertain because the quantity or depth of tissue obtained may not be enough for diagnosis. Deep incisional cutaneous biopsy is needed rather than punch biopsy to provide ample subcutaneous tissue for histologic study.3
Further, the biopsy procedure induces ulceration in the region of the incision, increasing the risk of infection and poor healing and escalating the risk of sepsis and death.7–9 Since extensive necrosis predisposes to a negative biopsy, a high clinical suspicion should drive early treatment of calciphylaxis.10 Noninvasive imaging studies such as plain radiography and bone scintigraphy can aid the diagnosis by detecting moderate to severe soft-tissue vascular calcification in these areas.7–11
DEBRIDEMENT IS CONTROVERSIAL
Conservative measures are the mainstay of care and include dietary alterations, noncalcium and nonaluminum phosphate binders, and low-calcium bath dialysis. There is mounting evidence for the use of calcimimetics and sodium thiosulfate.7,12–14
The role of wound debridement is controversial, as concomitant poor peripheral vascular perfusion can delay wound healing and, if ulceration ensues, there is a dramatic escalation of mortality risk. The decision for wound debridement is determined case by case, based on an assessment of the comorbidities, vascular perfusion, and status of the eschar.
Extensive wound debridement should be considered immediately after biopsy or with any signs of ulceration or infection—this in addition to meticulous wound care, which will promote healing and prevent serious complications secondary to infection.15
A TEAM APPROACH IMPROVES OUTCOMES
A multidisciplinary approach involving surgeons, nephrologists, dermatologists, dermatopathologists, wound or burn care team, nutrition team, pain management team, and infectious disease team is important to improve outcomes.7
Management mainly involves controlling pain; avoiding local trauma; treating and preventing infection; stopping causative agents such as warfarin and corticosteroids; intensive hemodialysis with an increase in both frequency and duration; intravenous sodium thiosulphate; non-calcium-phosphorus binders and cinacalcet in patients with elevated parathyroid hormone; and hyperbaric oxygen.12–14 There are also reports of success with oral etidronate and intravenous pamidronate.16,17
A 53-year-old woman presented with extensive, nonulcerated, painful plaques on both calves. She had long-standing diabetes mellitus and had recently started hemodialysis. She had no fever or trauma and did not appear to be in shock.
On physical examination, she had extensive, well-demarcated, nonulcerated, indurated dark eschar over the right calf (Figure 1). Her left calf had similar lesions that appeared as focal, discrete, nonulcerated, violaceous plaques, with associated tenderness. No significant erythema, edema, drainage, or fluctuance was noted.

A broad-spectrum antibiotic was started empirically but was discontinued when routine blood testing and magnetic resonance imaging showed no evidence of infection. Histologic study of a full-thickness skin biopsy specimen (Figure 2) showed tissue necrosis, ulceration, and concentric calcification of small and medium-sized blood vessels, many with luminal thrombi, all of which together were diagnostic for calciphylaxis.

Treatment was started with cinacalcet, low-calcium dialysis baths, phosphate binders, and sodium thiosulfate. However, within a few days of the biopsy procedure, an infection developed at the biopsy site, and the patient developed sepsis and septic shock. She received broad-spectrum antibiotics and underwent extensive debridement with wound care. After a protracted hospital course, the infection resolved.
CALCIPHYLAXIS RISK FACTORS
Calciphylaxis, also referred to as calcific uremic arteriolopathy, is a rare and often fatal condition in patients with end-stage renal disease who are on hemodialysis (1% to 4% of dialysis patients).1–3 It is also seen in patients who have undergone renal transplant and in patients with chronic kidney disease who have a chronic inflammatory disease or who have been exposed to corticosteroids or warfarin. However, it can also occur in patients without chronic kidney disease or end-stage renal disease.
The term “calcific uremic arteriolopathy” is a misnomer, as this condition can occur in patients with normal renal function (nonuremic calciphylaxis). Also, despite what the term calciphylaxis implies, there is no systemic anaphylaxis.3–5
Documented risk factors include obesity; female sex; use of warfarin, corticosteroids, or vitamin D analogues; low serum albumin; hypercoagulable states; hyperparathyroidism; alcoholic liver disease; elevated calcium-phosphorus product; inflammation; connective tissue disease; and cancer.4–6
DIAGNOSTIC CLUES
There are no strict guidelines for the diagnosis of calciphylaxis, and the exact pathophysiology of calciphylaxis is not understood.1–4
Ulceration is considered the clinical hallmark, but there are increasing reports of patients presenting with nonulcerated plaques, as in our patient. The literature suggests a mortality rate of 33% at 6 months in these patients, but ulceration increases the risk of death to over 80%, and sepsis is the leading cause of death.7,8
Histologic features identified on full-thickness biopsy specimens are intravascular deposition of calcium in the media of the blood vessels, as well as fibrin thrombi formation, intimal proliferation, tissue necrosis, and resultant ischemia. However, as in our patient and as discussed below, the biopsy procedure can induce or exacerbate ulceration, increasing the risk of sepsis, and is thus controversial.7
In the early stages, lesions of calciphylaxis are focal and appear as erythema or livedo reticularis with or without subcutaneous plaques or ulcers. As the disease progresses, the ischemic changes coalesce to form denser violaceous, painful, plaquelike subcutaneous nodules with eschar. In the advanced stages, the eschar or ulceration involves an extensive area.
Diagnosis in the early stages is challenging because of the focal nature of involvement. The differential diagnosis includes potentially fatal conditions such as systemic vasculitis, nephrogenic systemic fibrosis, pyoderma gangrenosum, gangrene from peripheral arterial disease, cholesterol embolization, warfarin-induced necrosis, purpura fulminans, and oxalate vasculopathy.7
In the advanced stages, the diagnosis of calciphylaxis is clinically more evident, and the differential diagnosis usually narrows. Well-demarcated, necrotic, indurated lesions that are bilateral in a patient with end-stage renal disease without shock makes the diagnosis very likely.
The dangers of biopsy
As seen in our patient, biopsy for histologic confirmation of calciphylaxis can increase the risk of infection and sepsis.7 Also, the efficacy and clinical utility are uncertain because the quantity or depth of tissue obtained may not be enough for diagnosis. Deep incisional cutaneous biopsy is needed rather than punch biopsy to provide ample subcutaneous tissue for histologic study.3
Further, the biopsy procedure induces ulceration in the region of the incision, increasing the risk of infection and poor healing and escalating the risk of sepsis and death.7–9 Since extensive necrosis predisposes to a negative biopsy, a high clinical suspicion should drive early treatment of calciphylaxis.10 Noninvasive imaging studies such as plain radiography and bone scintigraphy can aid the diagnosis by detecting moderate to severe soft-tissue vascular calcification in these areas.7–11
DEBRIDEMENT IS CONTROVERSIAL
Conservative measures are the mainstay of care and include dietary alterations, noncalcium and nonaluminum phosphate binders, and low-calcium bath dialysis. There is mounting evidence for the use of calcimimetics and sodium thiosulfate.7,12–14
The role of wound debridement is controversial, as concomitant poor peripheral vascular perfusion can delay wound healing and, if ulceration ensues, there is a dramatic escalation of mortality risk. The decision for wound debridement is determined case by case, based on an assessment of the comorbidities, vascular perfusion, and status of the eschar.
Extensive wound debridement should be considered immediately after biopsy or with any signs of ulceration or infection—this in addition to meticulous wound care, which will promote healing and prevent serious complications secondary to infection.15
A TEAM APPROACH IMPROVES OUTCOMES
A multidisciplinary approach involving surgeons, nephrologists, dermatologists, dermatopathologists, wound or burn care team, nutrition team, pain management team, and infectious disease team is important to improve outcomes.7
Management mainly involves controlling pain; avoiding local trauma; treating and preventing infection; stopping causative agents such as warfarin and corticosteroids; intensive hemodialysis with an increase in both frequency and duration; intravenous sodium thiosulphate; non-calcium-phosphorus binders and cinacalcet in patients with elevated parathyroid hormone; and hyperbaric oxygen.12–14 There are also reports of success with oral etidronate and intravenous pamidronate.16,17
- Spanakis EK, Sellmeyer DE. Nonuremic calciphylaxis precipitated by teriparatide [rhPTH(1-34)] therapy in the setting of chronic warfarin and glucocorticoid treatment. Osteoporos Int 2014; 25:1411–1414.
- Brandenburg VM, Cozzolino M, Ketteler M. Calciphylaxis: a still unmet challenge. J Nephrol 2011; 24:142–148.
- Wilmer WA, Magro CM. Calciphylaxis: emerging concepts in prevention, diagnosis, and treatment. Semin Dial 2002; 15:172–186.
- Rimtepathip P, Cohen D. A rare presentation of calciphylaxis in normal renal function. Int J Case Rep Images 2015; 6:366–369.
- Lonowski S, Martin S, Worswick S. Widespread calciphylaxis and normal renal function: no improvement with sodium thiosulfate. Dermatol Online J 2015; 21:13030/qt76845802.
- Zhou Q, Neubauer J, Kern JS, Grotz W, Walz G, Huber TB. Calciphylaxis. Lancet 2014; 383:1067.
- Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis 2015; 66:133–146.
- Fine A, Zacharias J. Calciphylaxis is usually non-ulcerating: risk factors, outcome and therapy. Kidney Int 2002; 61:2210–2217.
- Hayashi M. Calciphylaxis: diagnosis and clinical features. Clin Exp Nephrol 2013; 17:498–503.
- Stavros K, Motiwala R, Zhou L, Sejdiu F, Shin S. Calciphylaxis in a dialysis patient diagnosed by muscle biopsy. J Clin Neuromuscul Dis 2014; 15:108–111.
- Bonchak JG, Park KK, Vethanayagamony T, Sheikh MM, Winterfield LS. Calciphylaxis: a case series and the role of radiology in diagnosis. Int J Dermatol 2015. [Epub ahead of print]
- Ross EA. Evolution of treatment strategies for calciphylaxis. Am J Nephrol 2011; 34:460–467.
- Cicone JS, Petronis JB, Embert CD, Spector DA. Successful treatment of calciphylaxis with intravenous sodium thiosulfate. Am J Kidney Dis 2004; 43:1104–1108.
- Brandenburg VM, Kramann R, Specht P, Ketteler M. Calciphylaxis in CKD and beyond. Nephrol Dial Transplant 2012; 27:1314–1318.
- Martin R. Mysterious calciphylaxis: wounds with eschar—to debride or not to debride? Ostomy Wound Manage 2004; 50:64–66.
- Shiraishi N, Kitamura K, Miyoshi T, et al. Successful treatment of a patient with severe calcific uremic arteriolopathy (calciphylaxis) by etidronate disodium. Am J Kidney Dis 2006; 48:151–154.
- Hanafusa T, Yamaguchi Y, Tani M, Umegaki N, Nishimura Y, Katayama I. Intractable wounds caused by calcific uremic arteriolopathy treated with bisphosphonates. J Am Acad Dermatol 2007; 57:1021–1025.
- Spanakis EK, Sellmeyer DE. Nonuremic calciphylaxis precipitated by teriparatide [rhPTH(1-34)] therapy in the setting of chronic warfarin and glucocorticoid treatment. Osteoporos Int 2014; 25:1411–1414.
- Brandenburg VM, Cozzolino M, Ketteler M. Calciphylaxis: a still unmet challenge. J Nephrol 2011; 24:142–148.
- Wilmer WA, Magro CM. Calciphylaxis: emerging concepts in prevention, diagnosis, and treatment. Semin Dial 2002; 15:172–186.
- Rimtepathip P, Cohen D. A rare presentation of calciphylaxis in normal renal function. Int J Case Rep Images 2015; 6:366–369.
- Lonowski S, Martin S, Worswick S. Widespread calciphylaxis and normal renal function: no improvement with sodium thiosulfate. Dermatol Online J 2015; 21:13030/qt76845802.
- Zhou Q, Neubauer J, Kern JS, Grotz W, Walz G, Huber TB. Calciphylaxis. Lancet 2014; 383:1067.
- Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis 2015; 66:133–146.
- Fine A, Zacharias J. Calciphylaxis is usually non-ulcerating: risk factors, outcome and therapy. Kidney Int 2002; 61:2210–2217.
- Hayashi M. Calciphylaxis: diagnosis and clinical features. Clin Exp Nephrol 2013; 17:498–503.
- Stavros K, Motiwala R, Zhou L, Sejdiu F, Shin S. Calciphylaxis in a dialysis patient diagnosed by muscle biopsy. J Clin Neuromuscul Dis 2014; 15:108–111.
- Bonchak JG, Park KK, Vethanayagamony T, Sheikh MM, Winterfield LS. Calciphylaxis: a case series and the role of radiology in diagnosis. Int J Dermatol 2015. [Epub ahead of print]
- Ross EA. Evolution of treatment strategies for calciphylaxis. Am J Nephrol 2011; 34:460–467.
- Cicone JS, Petronis JB, Embert CD, Spector DA. Successful treatment of calciphylaxis with intravenous sodium thiosulfate. Am J Kidney Dis 2004; 43:1104–1108.
- Brandenburg VM, Kramann R, Specht P, Ketteler M. Calciphylaxis in CKD and beyond. Nephrol Dial Transplant 2012; 27:1314–1318.
- Martin R. Mysterious calciphylaxis: wounds with eschar—to debride or not to debride? Ostomy Wound Manage 2004; 50:64–66.
- Shiraishi N, Kitamura K, Miyoshi T, et al. Successful treatment of a patient with severe calcific uremic arteriolopathy (calciphylaxis) by etidronate disodium. Am J Kidney Dis 2006; 48:151–154.
- Hanafusa T, Yamaguchi Y, Tani M, Umegaki N, Nishimura Y, Katayama I. Intractable wounds caused by calcific uremic arteriolopathy treated with bisphosphonates. J Am Acad Dermatol 2007; 57:1021–1025.
Renal failure in HCV cirrhosis
A 54-year-old man with a history of cirrhosis secondary to hepatitis C virus (HCV) infection has had a progressive decline in kidney function. He was diagnosed with hepatitis C 15 years ago; he tried interferon treatment, but this failed. He received a transjugular intrahepatic shunt 10 years ago after an episode of esophageal variceal bleeding. He has since been taking furosemide and spironolactone as maintenance treatment for ascites, and he has no other medical concerns such as hypertension or diabetes.
Two weeks ago, routine laboratory tests in the clinic showed that his serum creatinine level had increased from baseline. He was asked to stop his diuretics and increase his fluid intake. Nevertheless, his kidney function continued to decline (Table 1), and he was admitted to the hospital for further evaluation.
On admission, he appeared comfortable. He denied recent use of any medications, including nonsteroidal anti-inflammatory drugs, antibiotics, and diuretics, and he had no genitourinary symptoms. His temperature was normal, blood pressure 170/90 mm Hg, pulse rate 72 per minute, and respiratory rate 16. His skin and sclerae were not jaundiced; his abdomen was not tender, but it was grossly distended with ascites. He also had +3 pedal edema (on a scale of 4) extending to both knees. The rest of his physical examination was unremarkable. Results of further laboratory tests are shown in in Table 2.
Ultrasonography of the liver demonstrated cirrhosis with patent flow through the shunt, and ultrasonography of the kidneys showed that both were slightly enlarged with increased cortical echogenicity but no hydronephrosis or obstruction.
EXPLORING THE CAUSE OF RENAL FAILURE
1. Given this information, what is the likely cause of our patient’s renal failure?
- Volume depletion
- Acute tubular necrosis
- Hepatorenal syndrome
- HCV glomerulopathy
Renal failure is a common complication in cirrhosis and portends a higher risk of death.1 The differential diagnosis is broad, but a systematic approach incorporating data from the history, physical examination, and laboratory tests can help identify the cause and is essential in determining the prognosis and proper treatment.
Volume depletion
Volume depletion is a common cause of renal failure in cirrhotic patients. Common precipitants are excessive diuresis and gastrointestinal fluid loss from bleeding, vomiting, and diarrhea. Despite having ascites and edema, patients may have low fluid volume in the vascular space. Therefore, the first step in a patient with acute kidney injury is to withhold diuretics and give fluids. The renal failure usually rapidly reverses if the patient does not have renal parenchymal disease.2
Our patient did not present with any fluid losses, and his high blood pressure and normal heart rate did not suggest volume depletion. And most importantly, withholding his diuretics and giving fluids did not reverse his renal failure. Thus, volume depletion was an unlikely cause.
Acute tubular necrosis
The altered hemodynamics caused by cirrhosis predispose patients to acute tubular necrosis. Classically, this presents as muddy brown casts and renal tubular epithelial cells on urinalysis and as a fractional excretion of sodium greater than 2%.1 However, these microscopic findings lack sensitivity, and patients with cirrhosis may have marked sodium avidity and low urine sodium excretion despite tubular injury.3
This diagnosis must still be considered in patients with renal failure, especially after an insult such as hemorrhagic or septic shock or intake of nephrotoxins. However, because our patient did not have a history of any of these and because his renal failure had been progressing over weeks, acute tubular necrosis was considered unlikely.
Hepatorenal syndrome
Hepatorenal syndrome is characterized by progressive renal failure in the absence of renal parenchymal disease. It is a functional disorder, ie, the decreased glomerular filtration rate results from renal vasoconstriction, which in turn is due to decreased systemic vascular resistance and increased compensatory activity of the renin-angiotensin-aldosterone axis and of antiduretic hormone release (Figure 1).
Hepatorenal syndrome often occurs in patients with advanced liver disease. These patients typically have a hyperdynamic circulation (systemic vasodilation, low blood pressure, and increased blood volume) with a low mean arterial pressure and increased renin and norepinephrine levels. Other frequent findings include hyponatremia, low urinary sodium excretion (< 2 mmol/day), and low free water clearance,4 all of which mark the high systemic levels of antidiuretic hormone and aldosterone.
Importantly, while hepatorenal syndrome is always considered in the differential diagnosis because of its unique prognosis and therapy, it remains a diagnosis of exclusion. The International Ascites Club5 has provided diagnostic criteria for hepatorenal syndrome:
- Cirrhosis and ascites
- Serum creatinine greater than 1.5 mg/dL
- Failure of serum creatinine to fall to less than 1.5 mg/dL after at least 48 hours of diuretic withdrawal and volume expansion with albumin (recommended dose 1 g/kg body weight per day up to a maximum of 100 g per day)
- Absence of shock
- No current or recent treatment with nephrotoxic drugs
- No signs of parenchymal kidney disease such as proteinuria (protein excretion > 500 mg/day), microhematuria (> 50 red blood cells per high-power field), or abnormalities on renal ultrasonography.
While these criteria are not perfect,6 they remind clinicians that there are other important causes of renal insufficiency in cirrhosis.
Clinically, our patient had no evidence of a hyperdynamic circulation and was instead hypertensive. He was eunatremic and did not have marked renal sodium avidity. His pyuria, proteinuria (his protein excretion was approximately 1.9 g/day as determined by urine spot protein-to-creatinine ratio), and results of ultrasonography also suggested underlying renal parenchymal disease. Therefore, hepatorenal syndrome was not the likely diagnosis.
HCV glomerulopathy
Intrinsic renal disease is likely, given our patient’s proteinuria, active urine sediment (ie, containing red blood cells, white blood cells, and protein), and abnormal findings on ultrasonography. In patients with HCV infection and no other cause of intrinsic kidney disease, immune complex deposition leading to glomerulonephritis is the most common pattern.7 Despite the intrinsic renal disease, fractional excretion of sodium may be less than 1% in glomerulonephritis. Hypertension in a patient such as ours with cirrhosis and renal insufficiency raises suspicion for glomerular disease, as hypertension is unlikely in advanced cirrhosis.8
Glomerulonephritis in patients with cirrhosis is often clinically silent and may be highly prevalent; some studies have shown glomerular involvement in 55% to 83% of patients with cirrhosis.9,10 This increases the risk of end-stage renal disease, and the Kidney Disease Improving Global Outcomes guideline recommends that HCV-infected patients be tested at least once a year for proteinuria, hematuria, and estimated glomerular filtration rate to detect possible HCV-associated kidney disease.11 According to current guidelines of the Infectious Diseases Society of America (IDSA) and American Association for the Study of Liver Diseases (AASLD) , detection of glomerulonephritis in HCV patients puts them in the highest priority class for treatment of HCV.12
HISTOLOGIC FINDINGS
Because of the high likelihood of glomerulopathy, our patient underwent renal biopsy.
2. What is the classic pathologic finding in HCV kidney disease?
- Focal segmental glomerulosclerosis
- Crescentic glomerulonephritis
- Membranoproliferative glomerulonephritis
- Membranous glomerulonephritis
A number of pathologic patterns have been described in HCV kidney disease, including membranous glomerulonephritis, immunoglobulin A nephropathy, and focal segmental glomerulosclerosis. However, by far the most common pattern is type 1 membranoproliferative glomerulonephritis.13 (Types 2 and 3 are much less common, and we will not discuss them here.) In type 1, light microscopy shows increased mesangial cells and thickened capillary walls (lobular glomeruli), staining of the basement membrane reveals double contours (“tram tracking”) or splitting due to mesangial deposition, and immunofluorescence demonstrates immunoglobulin G and complement C3 deposition. All of these findings were seen in our patient (Figure 2, Figure 3).
Membranoproliferative glomerulonephritis in patients with HCV is most commonly associated with cryoglobulins, a mixture of monoclonal or polyclonal immunoglobulin (Ig) M that have antiglobulin (rheumatoid factor) activity and bind to polyclonal IgG. They reversibly precipitate at less than 37°C, (98.6°F), hence their name. Only 50% to 70% of patients with cryoglobulinemic membranoproliferative glomerulonephritis have detectable serum cryoglobulins; however, kidney biopsy may show globular accumulations of eosinophilic material and prominent hypercellularity due to infiltration of glomerular capillaries with mononuclear and polymorphonuclear leukocytes.
Noncryoglobulinemic membranoproliferative glomerulonephritis is also found in patients with HCV infection. Its histologic features are similar, but on biopsy, there is less prominent leukocytic infiltration and no eosinophilic material. Although the pathogenesis of glomerulonephritis in HCV infection is poorly understood, it is thought to result from deposition of circulating immune complexes of HCV, anti-HCV, and rheumatoid factor in the glomeruli.
3. What laboratory finding is often seen in membranoproliferative glomerulonephritis?
- Positive cytoplasmic antineutrophil cytoplasmic antibody
- serum complement Low levels
- Antiphospholipase A2 receptor antibodies
Cytoplasmic antineutrophil cytoplasmic antibody is seen in granulomatosis with polyangiitis, while antiphospholipid A2 receptor antibodies are seen in idiopathic membranous nephritis.
Low serum complement levels are frequently found in membranoproliferative glomerulonephritis. It is believed that immune complex deposition leads to glomerular damage through activation of the complement pathway and the subsequent influx of inflammatory cells, release of cytokines and proteases, and damage to capillary walls. When repair ensues, new mesangial matrix and basement membrane are deposited, leading to mesangial expansion and duplicated basement membrane.14
In cryoglobulinemic membranoproliferative glomerulonephritis, the complement C4 level is often much lower than C3, but in noncryoglobulinemic forms C3 is lower. A mnemonic to remember nephritic syndromes with low complement levels is “hy-PO-CO-MP-L-EM-ents”; PO for postinfectious, CO for cryoglobulins, MP for membranoproliferative glomerulonephritis, L for lupus, and EM for embolic.
BACK TO OUR PATIENT
In addition to kidney biopsy, we tested our patient for serum cryoglobulins, rheumatoid factor, and serum complements. Results from these tests (Table 3), in addition to the lack of cryoglobulins on his biopsy, led to the conclusion that he had noncryoglobulinemic membranoproliferative glomerulonephritis.
WHO SHOULD RECEIVE TREATMENT FOR HCV?
4. According to the current IDSA/AASLD guidelines, which of the following patients should not receive direct-acting antiviral therapy for HCV?
- Patients with HCV and only low-stage fibrosis
- Patients with decompensated cirrhosis
- Patients with a glomerular filtration rate less than 30 mL/minute
- None of the above—nearly all patients with HCV infection should receive treatment for it
While certain patients have compelling indications for HCV treatment, such as advanced fibrosis, severe extrahepatic manifestations of HCV (eg, glomerulonephritis, cryoglobulinemia), and posttransplant status, current guidelines recommend treatment for nearly all patients with HCV, including those with low-stage fibrosis.12
Patients with Child-Pugh grade B or C decompensated cirrhosis, even with hepatocellular carcinoma, may be considered for treatment. Multiple studies have demonstrated the efficacy and safety of direct-acting antiviral drugs in this patient population. In one randomized controlled trial,15 the combination of ledipasvir, sofosbuvir, and ribavirin resulted in high sustained virologic response rates at 12 weeks in patients infected with HCV genotype 1 or 4 with advanced liver disease, irrespective of transplant status (86% to 89% of patients were pretransplant). Sustained virologic response was associated with improvements in Model for End-Stage Liver Disease and Child-Pugh scores largely due to decreases in bilirubin and improvement in synthetic function (ie, albumin).
Similarly, even patients with a glomerular filtration rate less than 30 mL/min are candidates for treatment. Those with a glomerular filtration rate above 30 mL/min need no dosage adjustments for the most common regimens, while regimens are also available for those with a rate less than 30 mL/min. Although patients with low baseline renal function have a higher frequency of anemia (especially with ribavirin), worsening renal dysfunction, and more severe adverse events, treatment responses remain high and comparable to those without renal impairment.
The Hepatitis C Therapeutic Registry and Research Network (HCV-TARGET) is conducting an ongoing prospective study evaluating real-world use of direct-acting antiviral agents. The study has reported the safety and efficacy of sofosbuvir-containing regimens in patients with varying severities of kidney disease, including glomerular filtration rates less than 30 mL/min). The patients received different regimens that included sofosbuvir. The regimens were reportedly tolerated, and the rate of sustained viral response at 12 weeks remained high.16
The efficacy of direct-acting antiviral agents for HCV-associated glomerulonephritis remains to be studied but is promising. Earlier studies found that antiviral therapy based on interferon alfa with or without ribavirin can significantly decrease proteinuria and stabilize renal function.17–20 HCV RNA clearance has been found to best predict renal improvement.
OUR PATIENT’S COURSE
Unfortunately, our patient’s kidney function declined further over the next 3 months, and he is currently on dialysis awaiting simultaneous liver and kidney transplant.
- Ginès P, Schrier RW. Renal failure in cirrhosis. N Engl J Med 2009; 361:1279–1290.
- Mackelaite L, Alsauskas ZC, Ranganna K. Renal failure in patients with cirrhosis. Med Clin North Am 2009; 93:855–869.
- Wadei HM, Mai ML, Ahsan N, Gonwa TA. Hepatorenal syndrome: pathophysiology and management. Clin J Am Soc Nephrol 2006; 1:1066–1079.
- Gines A, Escorsell A, Gines P, et al. Incidence, predictive factors, and prognosis of the hepatorenal syndrome in cirrhosis with ascites. Gastroenterology 1993; 105:229–236.
- Salerno F, Gerbes A, Ginès P, Wong F, Arroyo V. Diagnosis, prevention and treatment of hepatorenal syndrome in cirrhosis. Gut 2007; 56:1310–1318.
- Watt K, Uhanova J, Minuk GY. Hepatorenal syndrome: diagnostic accuracy, clinical features, and outcome in a tertiary care center. Am J Gastroenterol 2002; 97:2046–2050.
- Graupera I, Cardenas A. Diagnostic approach to renal failure in cirrhosis. Clin Liver Dis 2013; 2:128–131.
- Dash SC, Bhowmik D. Glomerulopathy with liver disease: patterns and management. Saudi J Kidney Dis Transpl 2000; 11:414–420.
- Arase Y, Ikeda K, Murashima N, et al. Glomerulonephritis in autopsy cases with hepatitis C virus infection. Intern Med 1998; 37:836–840.
- McGuire BM, Julian BA, Bynon JS, et al. Brief communication: glomerulonephritis in patients with hepatitis C cirrhosis undergoing liver transplantation. Ann Intern Med 2006; 144:735–741.
- Kidney Disease: Improving Global Outcomes (KDIGO). KDIGO clinical practice guidelines for the prevention, diagnosis, evaluation, and treatment of hepatitis C in chronic kidney disease. Kidney Int Suppl 2008; 109:S1–S99.
- American Association for the Study of Liver Diseases (AASLD) and the Infectious Diseases Society of America (IDSA). HCV guidance: recommendations for testing, managing, and treating hepatitis C. www.hcvguidelines.org/. Accessed July 10, 2016.
- Lai KN. Hepatitis-related renal disease. Future Virology 2011; 6:1361–1376.
- Sethi S, Fervenza FC. Membranoproliferative glomerulonephritis—a new look at an old entity. N Engl J Med 2012; 366:1119–1131.
- Charlton M, Everson GT, Flamm SL, et al; SOLAR-1 Investigators. Ledipasvir and sofosbuvir plus ribavirin for treatment of HCV infection in patients with advanced liver disease. Gastroenterology 2015; 149:649–659.
- Saxena V, Koraishy FM, Sise ME, et al; HCV-TARGET. Safety and efficacy of sofosbuvir-containing regimens in hepatitis C-infected patients with impaired renal function. Liver Int 2016; 36:807–816.
- Feng B, Eknoyan G, Guo ZS, et al. Effect of interferon alpha-based antiviral therapy on hepatitis C virus-associated glomerulonephritis: a meta-analysis. Nephrol Dial Transplant 2012; 27:640–646.
- Bruchfeld A, Lindahl K, Ståhle L, Söderberg M, Schvarcz R. Interferon and ribavirin treatment in patients with hepatitis C-associated renal disease and renal insufficiency. Nephrol Dial Transplant 2003; 18:1573–1580.
- Rossi P, Bertani T, Baio P, et al. Hepatitis C virus-related cryoglobulinemic glomerulonephritis. Long-term remission after antiviral therapy. Kidney Int 2003; 63:2236–2241.
- Alric L, Plaisier E, Thebault S, et al. Influence of antiviral therapy in hepatitis C virus associated cryoglobulinemic MPGN. Am J Kidney Dis 2004; 43:617–623.
A 54-year-old man with a history of cirrhosis secondary to hepatitis C virus (HCV) infection has had a progressive decline in kidney function. He was diagnosed with hepatitis C 15 years ago; he tried interferon treatment, but this failed. He received a transjugular intrahepatic shunt 10 years ago after an episode of esophageal variceal bleeding. He has since been taking furosemide and spironolactone as maintenance treatment for ascites, and he has no other medical concerns such as hypertension or diabetes.
Two weeks ago, routine laboratory tests in the clinic showed that his serum creatinine level had increased from baseline. He was asked to stop his diuretics and increase his fluid intake. Nevertheless, his kidney function continued to decline (Table 1), and he was admitted to the hospital for further evaluation.
On admission, he appeared comfortable. He denied recent use of any medications, including nonsteroidal anti-inflammatory drugs, antibiotics, and diuretics, and he had no genitourinary symptoms. His temperature was normal, blood pressure 170/90 mm Hg, pulse rate 72 per minute, and respiratory rate 16. His skin and sclerae were not jaundiced; his abdomen was not tender, but it was grossly distended with ascites. He also had +3 pedal edema (on a scale of 4) extending to both knees. The rest of his physical examination was unremarkable. Results of further laboratory tests are shown in in Table 2.
Ultrasonography of the liver demonstrated cirrhosis with patent flow through the shunt, and ultrasonography of the kidneys showed that both were slightly enlarged with increased cortical echogenicity but no hydronephrosis or obstruction.
EXPLORING THE CAUSE OF RENAL FAILURE
1. Given this information, what is the likely cause of our patient’s renal failure?
- Volume depletion
- Acute tubular necrosis
- Hepatorenal syndrome
- HCV glomerulopathy
Renal failure is a common complication in cirrhosis and portends a higher risk of death.1 The differential diagnosis is broad, but a systematic approach incorporating data from the history, physical examination, and laboratory tests can help identify the cause and is essential in determining the prognosis and proper treatment.
Volume depletion
Volume depletion is a common cause of renal failure in cirrhotic patients. Common precipitants are excessive diuresis and gastrointestinal fluid loss from bleeding, vomiting, and diarrhea. Despite having ascites and edema, patients may have low fluid volume in the vascular space. Therefore, the first step in a patient with acute kidney injury is to withhold diuretics and give fluids. The renal failure usually rapidly reverses if the patient does not have renal parenchymal disease.2
Our patient did not present with any fluid losses, and his high blood pressure and normal heart rate did not suggest volume depletion. And most importantly, withholding his diuretics and giving fluids did not reverse his renal failure. Thus, volume depletion was an unlikely cause.
Acute tubular necrosis
The altered hemodynamics caused by cirrhosis predispose patients to acute tubular necrosis. Classically, this presents as muddy brown casts and renal tubular epithelial cells on urinalysis and as a fractional excretion of sodium greater than 2%.1 However, these microscopic findings lack sensitivity, and patients with cirrhosis may have marked sodium avidity and low urine sodium excretion despite tubular injury.3
This diagnosis must still be considered in patients with renal failure, especially after an insult such as hemorrhagic or septic shock or intake of nephrotoxins. However, because our patient did not have a history of any of these and because his renal failure had been progressing over weeks, acute tubular necrosis was considered unlikely.
Hepatorenal syndrome
Hepatorenal syndrome is characterized by progressive renal failure in the absence of renal parenchymal disease. It is a functional disorder, ie, the decreased glomerular filtration rate results from renal vasoconstriction, which in turn is due to decreased systemic vascular resistance and increased compensatory activity of the renin-angiotensin-aldosterone axis and of antiduretic hormone release (Figure 1).
Hepatorenal syndrome often occurs in patients with advanced liver disease. These patients typically have a hyperdynamic circulation (systemic vasodilation, low blood pressure, and increased blood volume) with a low mean arterial pressure and increased renin and norepinephrine levels. Other frequent findings include hyponatremia, low urinary sodium excretion (< 2 mmol/day), and low free water clearance,4 all of which mark the high systemic levels of antidiuretic hormone and aldosterone.
Importantly, while hepatorenal syndrome is always considered in the differential diagnosis because of its unique prognosis and therapy, it remains a diagnosis of exclusion. The International Ascites Club5 has provided diagnostic criteria for hepatorenal syndrome:
- Cirrhosis and ascites
- Serum creatinine greater than 1.5 mg/dL
- Failure of serum creatinine to fall to less than 1.5 mg/dL after at least 48 hours of diuretic withdrawal and volume expansion with albumin (recommended dose 1 g/kg body weight per day up to a maximum of 100 g per day)
- Absence of shock
- No current or recent treatment with nephrotoxic drugs
- No signs of parenchymal kidney disease such as proteinuria (protein excretion > 500 mg/day), microhematuria (> 50 red blood cells per high-power field), or abnormalities on renal ultrasonography.
While these criteria are not perfect,6 they remind clinicians that there are other important causes of renal insufficiency in cirrhosis.
Clinically, our patient had no evidence of a hyperdynamic circulation and was instead hypertensive. He was eunatremic and did not have marked renal sodium avidity. His pyuria, proteinuria (his protein excretion was approximately 1.9 g/day as determined by urine spot protein-to-creatinine ratio), and results of ultrasonography also suggested underlying renal parenchymal disease. Therefore, hepatorenal syndrome was not the likely diagnosis.
HCV glomerulopathy
Intrinsic renal disease is likely, given our patient’s proteinuria, active urine sediment (ie, containing red blood cells, white blood cells, and protein), and abnormal findings on ultrasonography. In patients with HCV infection and no other cause of intrinsic kidney disease, immune complex deposition leading to glomerulonephritis is the most common pattern.7 Despite the intrinsic renal disease, fractional excretion of sodium may be less than 1% in glomerulonephritis. Hypertension in a patient such as ours with cirrhosis and renal insufficiency raises suspicion for glomerular disease, as hypertension is unlikely in advanced cirrhosis.8
Glomerulonephritis in patients with cirrhosis is often clinically silent and may be highly prevalent; some studies have shown glomerular involvement in 55% to 83% of patients with cirrhosis.9,10 This increases the risk of end-stage renal disease, and the Kidney Disease Improving Global Outcomes guideline recommends that HCV-infected patients be tested at least once a year for proteinuria, hematuria, and estimated glomerular filtration rate to detect possible HCV-associated kidney disease.11 According to current guidelines of the Infectious Diseases Society of America (IDSA) and American Association for the Study of Liver Diseases (AASLD) , detection of glomerulonephritis in HCV patients puts them in the highest priority class for treatment of HCV.12
HISTOLOGIC FINDINGS
Because of the high likelihood of glomerulopathy, our patient underwent renal biopsy.
2. What is the classic pathologic finding in HCV kidney disease?
- Focal segmental glomerulosclerosis
- Crescentic glomerulonephritis
- Membranoproliferative glomerulonephritis
- Membranous glomerulonephritis
A number of pathologic patterns have been described in HCV kidney disease, including membranous glomerulonephritis, immunoglobulin A nephropathy, and focal segmental glomerulosclerosis. However, by far the most common pattern is type 1 membranoproliferative glomerulonephritis.13 (Types 2 and 3 are much less common, and we will not discuss them here.) In type 1, light microscopy shows increased mesangial cells and thickened capillary walls (lobular glomeruli), staining of the basement membrane reveals double contours (“tram tracking”) or splitting due to mesangial deposition, and immunofluorescence demonstrates immunoglobulin G and complement C3 deposition. All of these findings were seen in our patient (Figure 2, Figure 3).
Membranoproliferative glomerulonephritis in patients with HCV is most commonly associated with cryoglobulins, a mixture of monoclonal or polyclonal immunoglobulin (Ig) M that have antiglobulin (rheumatoid factor) activity and bind to polyclonal IgG. They reversibly precipitate at less than 37°C, (98.6°F), hence their name. Only 50% to 70% of patients with cryoglobulinemic membranoproliferative glomerulonephritis have detectable serum cryoglobulins; however, kidney biopsy may show globular accumulations of eosinophilic material and prominent hypercellularity due to infiltration of glomerular capillaries with mononuclear and polymorphonuclear leukocytes.
Noncryoglobulinemic membranoproliferative glomerulonephritis is also found in patients with HCV infection. Its histologic features are similar, but on biopsy, there is less prominent leukocytic infiltration and no eosinophilic material. Although the pathogenesis of glomerulonephritis in HCV infection is poorly understood, it is thought to result from deposition of circulating immune complexes of HCV, anti-HCV, and rheumatoid factor in the glomeruli.
3. What laboratory finding is often seen in membranoproliferative glomerulonephritis?
- Positive cytoplasmic antineutrophil cytoplasmic antibody
- serum complement Low levels
- Antiphospholipase A2 receptor antibodies
Cytoplasmic antineutrophil cytoplasmic antibody is seen in granulomatosis with polyangiitis, while antiphospholipid A2 receptor antibodies are seen in idiopathic membranous nephritis.
Low serum complement levels are frequently found in membranoproliferative glomerulonephritis. It is believed that immune complex deposition leads to glomerular damage through activation of the complement pathway and the subsequent influx of inflammatory cells, release of cytokines and proteases, and damage to capillary walls. When repair ensues, new mesangial matrix and basement membrane are deposited, leading to mesangial expansion and duplicated basement membrane.14
In cryoglobulinemic membranoproliferative glomerulonephritis, the complement C4 level is often much lower than C3, but in noncryoglobulinemic forms C3 is lower. A mnemonic to remember nephritic syndromes with low complement levels is “hy-PO-CO-MP-L-EM-ents”; PO for postinfectious, CO for cryoglobulins, MP for membranoproliferative glomerulonephritis, L for lupus, and EM for embolic.
BACK TO OUR PATIENT
In addition to kidney biopsy, we tested our patient for serum cryoglobulins, rheumatoid factor, and serum complements. Results from these tests (Table 3), in addition to the lack of cryoglobulins on his biopsy, led to the conclusion that he had noncryoglobulinemic membranoproliferative glomerulonephritis.
WHO SHOULD RECEIVE TREATMENT FOR HCV?
4. According to the current IDSA/AASLD guidelines, which of the following patients should not receive direct-acting antiviral therapy for HCV?
- Patients with HCV and only low-stage fibrosis
- Patients with decompensated cirrhosis
- Patients with a glomerular filtration rate less than 30 mL/minute
- None of the above—nearly all patients with HCV infection should receive treatment for it
While certain patients have compelling indications for HCV treatment, such as advanced fibrosis, severe extrahepatic manifestations of HCV (eg, glomerulonephritis, cryoglobulinemia), and posttransplant status, current guidelines recommend treatment for nearly all patients with HCV, including those with low-stage fibrosis.12
Patients with Child-Pugh grade B or C decompensated cirrhosis, even with hepatocellular carcinoma, may be considered for treatment. Multiple studies have demonstrated the efficacy and safety of direct-acting antiviral drugs in this patient population. In one randomized controlled trial,15 the combination of ledipasvir, sofosbuvir, and ribavirin resulted in high sustained virologic response rates at 12 weeks in patients infected with HCV genotype 1 or 4 with advanced liver disease, irrespective of transplant status (86% to 89% of patients were pretransplant). Sustained virologic response was associated with improvements in Model for End-Stage Liver Disease and Child-Pugh scores largely due to decreases in bilirubin and improvement in synthetic function (ie, albumin).
Similarly, even patients with a glomerular filtration rate less than 30 mL/min are candidates for treatment. Those with a glomerular filtration rate above 30 mL/min need no dosage adjustments for the most common regimens, while regimens are also available for those with a rate less than 30 mL/min. Although patients with low baseline renal function have a higher frequency of anemia (especially with ribavirin), worsening renal dysfunction, and more severe adverse events, treatment responses remain high and comparable to those without renal impairment.
The Hepatitis C Therapeutic Registry and Research Network (HCV-TARGET) is conducting an ongoing prospective study evaluating real-world use of direct-acting antiviral agents. The study has reported the safety and efficacy of sofosbuvir-containing regimens in patients with varying severities of kidney disease, including glomerular filtration rates less than 30 mL/min). The patients received different regimens that included sofosbuvir. The regimens were reportedly tolerated, and the rate of sustained viral response at 12 weeks remained high.16
The efficacy of direct-acting antiviral agents for HCV-associated glomerulonephritis remains to be studied but is promising. Earlier studies found that antiviral therapy based on interferon alfa with or without ribavirin can significantly decrease proteinuria and stabilize renal function.17–20 HCV RNA clearance has been found to best predict renal improvement.
OUR PATIENT’S COURSE
Unfortunately, our patient’s kidney function declined further over the next 3 months, and he is currently on dialysis awaiting simultaneous liver and kidney transplant.
A 54-year-old man with a history of cirrhosis secondary to hepatitis C virus (HCV) infection has had a progressive decline in kidney function. He was diagnosed with hepatitis C 15 years ago; he tried interferon treatment, but this failed. He received a transjugular intrahepatic shunt 10 years ago after an episode of esophageal variceal bleeding. He has since been taking furosemide and spironolactone as maintenance treatment for ascites, and he has no other medical concerns such as hypertension or diabetes.
Two weeks ago, routine laboratory tests in the clinic showed that his serum creatinine level had increased from baseline. He was asked to stop his diuretics and increase his fluid intake. Nevertheless, his kidney function continued to decline (Table 1), and he was admitted to the hospital for further evaluation.
On admission, he appeared comfortable. He denied recent use of any medications, including nonsteroidal anti-inflammatory drugs, antibiotics, and diuretics, and he had no genitourinary symptoms. His temperature was normal, blood pressure 170/90 mm Hg, pulse rate 72 per minute, and respiratory rate 16. His skin and sclerae were not jaundiced; his abdomen was not tender, but it was grossly distended with ascites. He also had +3 pedal edema (on a scale of 4) extending to both knees. The rest of his physical examination was unremarkable. Results of further laboratory tests are shown in in Table 2.
Ultrasonography of the liver demonstrated cirrhosis with patent flow through the shunt, and ultrasonography of the kidneys showed that both were slightly enlarged with increased cortical echogenicity but no hydronephrosis or obstruction.
EXPLORING THE CAUSE OF RENAL FAILURE
1. Given this information, what is the likely cause of our patient’s renal failure?
- Volume depletion
- Acute tubular necrosis
- Hepatorenal syndrome
- HCV glomerulopathy
Renal failure is a common complication in cirrhosis and portends a higher risk of death.1 The differential diagnosis is broad, but a systematic approach incorporating data from the history, physical examination, and laboratory tests can help identify the cause and is essential in determining the prognosis and proper treatment.
Volume depletion
Volume depletion is a common cause of renal failure in cirrhotic patients. Common precipitants are excessive diuresis and gastrointestinal fluid loss from bleeding, vomiting, and diarrhea. Despite having ascites and edema, patients may have low fluid volume in the vascular space. Therefore, the first step in a patient with acute kidney injury is to withhold diuretics and give fluids. The renal failure usually rapidly reverses if the patient does not have renal parenchymal disease.2
Our patient did not present with any fluid losses, and his high blood pressure and normal heart rate did not suggest volume depletion. And most importantly, withholding his diuretics and giving fluids did not reverse his renal failure. Thus, volume depletion was an unlikely cause.
Acute tubular necrosis
The altered hemodynamics caused by cirrhosis predispose patients to acute tubular necrosis. Classically, this presents as muddy brown casts and renal tubular epithelial cells on urinalysis and as a fractional excretion of sodium greater than 2%.1 However, these microscopic findings lack sensitivity, and patients with cirrhosis may have marked sodium avidity and low urine sodium excretion despite tubular injury.3
This diagnosis must still be considered in patients with renal failure, especially after an insult such as hemorrhagic or septic shock or intake of nephrotoxins. However, because our patient did not have a history of any of these and because his renal failure had been progressing over weeks, acute tubular necrosis was considered unlikely.
Hepatorenal syndrome
Hepatorenal syndrome is characterized by progressive renal failure in the absence of renal parenchymal disease. It is a functional disorder, ie, the decreased glomerular filtration rate results from renal vasoconstriction, which in turn is due to decreased systemic vascular resistance and increased compensatory activity of the renin-angiotensin-aldosterone axis and of antiduretic hormone release (Figure 1).
Hepatorenal syndrome often occurs in patients with advanced liver disease. These patients typically have a hyperdynamic circulation (systemic vasodilation, low blood pressure, and increased blood volume) with a low mean arterial pressure and increased renin and norepinephrine levels. Other frequent findings include hyponatremia, low urinary sodium excretion (< 2 mmol/day), and low free water clearance,4 all of which mark the high systemic levels of antidiuretic hormone and aldosterone.
Importantly, while hepatorenal syndrome is always considered in the differential diagnosis because of its unique prognosis and therapy, it remains a diagnosis of exclusion. The International Ascites Club5 has provided diagnostic criteria for hepatorenal syndrome:
- Cirrhosis and ascites
- Serum creatinine greater than 1.5 mg/dL
- Failure of serum creatinine to fall to less than 1.5 mg/dL after at least 48 hours of diuretic withdrawal and volume expansion with albumin (recommended dose 1 g/kg body weight per day up to a maximum of 100 g per day)
- Absence of shock
- No current or recent treatment with nephrotoxic drugs
- No signs of parenchymal kidney disease such as proteinuria (protein excretion > 500 mg/day), microhematuria (> 50 red blood cells per high-power field), or abnormalities on renal ultrasonography.
While these criteria are not perfect,6 they remind clinicians that there are other important causes of renal insufficiency in cirrhosis.
Clinically, our patient had no evidence of a hyperdynamic circulation and was instead hypertensive. He was eunatremic and did not have marked renal sodium avidity. His pyuria, proteinuria (his protein excretion was approximately 1.9 g/day as determined by urine spot protein-to-creatinine ratio), and results of ultrasonography also suggested underlying renal parenchymal disease. Therefore, hepatorenal syndrome was not the likely diagnosis.
HCV glomerulopathy
Intrinsic renal disease is likely, given our patient’s proteinuria, active urine sediment (ie, containing red blood cells, white blood cells, and protein), and abnormal findings on ultrasonography. In patients with HCV infection and no other cause of intrinsic kidney disease, immune complex deposition leading to glomerulonephritis is the most common pattern.7 Despite the intrinsic renal disease, fractional excretion of sodium may be less than 1% in glomerulonephritis. Hypertension in a patient such as ours with cirrhosis and renal insufficiency raises suspicion for glomerular disease, as hypertension is unlikely in advanced cirrhosis.8
Glomerulonephritis in patients with cirrhosis is often clinically silent and may be highly prevalent; some studies have shown glomerular involvement in 55% to 83% of patients with cirrhosis.9,10 This increases the risk of end-stage renal disease, and the Kidney Disease Improving Global Outcomes guideline recommends that HCV-infected patients be tested at least once a year for proteinuria, hematuria, and estimated glomerular filtration rate to detect possible HCV-associated kidney disease.11 According to current guidelines of the Infectious Diseases Society of America (IDSA) and American Association for the Study of Liver Diseases (AASLD) , detection of glomerulonephritis in HCV patients puts them in the highest priority class for treatment of HCV.12
HISTOLOGIC FINDINGS
Because of the high likelihood of glomerulopathy, our patient underwent renal biopsy.
2. What is the classic pathologic finding in HCV kidney disease?
- Focal segmental glomerulosclerosis
- Crescentic glomerulonephritis
- Membranoproliferative glomerulonephritis
- Membranous glomerulonephritis
A number of pathologic patterns have been described in HCV kidney disease, including membranous glomerulonephritis, immunoglobulin A nephropathy, and focal segmental glomerulosclerosis. However, by far the most common pattern is type 1 membranoproliferative glomerulonephritis.13 (Types 2 and 3 are much less common, and we will not discuss them here.) In type 1, light microscopy shows increased mesangial cells and thickened capillary walls (lobular glomeruli), staining of the basement membrane reveals double contours (“tram tracking”) or splitting due to mesangial deposition, and immunofluorescence demonstrates immunoglobulin G and complement C3 deposition. All of these findings were seen in our patient (Figure 2, Figure 3).
Membranoproliferative glomerulonephritis in patients with HCV is most commonly associated with cryoglobulins, a mixture of monoclonal or polyclonal immunoglobulin (Ig) M that have antiglobulin (rheumatoid factor) activity and bind to polyclonal IgG. They reversibly precipitate at less than 37°C, (98.6°F), hence their name. Only 50% to 70% of patients with cryoglobulinemic membranoproliferative glomerulonephritis have detectable serum cryoglobulins; however, kidney biopsy may show globular accumulations of eosinophilic material and prominent hypercellularity due to infiltration of glomerular capillaries with mononuclear and polymorphonuclear leukocytes.
Noncryoglobulinemic membranoproliferative glomerulonephritis is also found in patients with HCV infection. Its histologic features are similar, but on biopsy, there is less prominent leukocytic infiltration and no eosinophilic material. Although the pathogenesis of glomerulonephritis in HCV infection is poorly understood, it is thought to result from deposition of circulating immune complexes of HCV, anti-HCV, and rheumatoid factor in the glomeruli.
3. What laboratory finding is often seen in membranoproliferative glomerulonephritis?
- Positive cytoplasmic antineutrophil cytoplasmic antibody
- serum complement Low levels
- Antiphospholipase A2 receptor antibodies
Cytoplasmic antineutrophil cytoplasmic antibody is seen in granulomatosis with polyangiitis, while antiphospholipid A2 receptor antibodies are seen in idiopathic membranous nephritis.
Low serum complement levels are frequently found in membranoproliferative glomerulonephritis. It is believed that immune complex deposition leads to glomerular damage through activation of the complement pathway and the subsequent influx of inflammatory cells, release of cytokines and proteases, and damage to capillary walls. When repair ensues, new mesangial matrix and basement membrane are deposited, leading to mesangial expansion and duplicated basement membrane.14
In cryoglobulinemic membranoproliferative glomerulonephritis, the complement C4 level is often much lower than C3, but in noncryoglobulinemic forms C3 is lower. A mnemonic to remember nephritic syndromes with low complement levels is “hy-PO-CO-MP-L-EM-ents”; PO for postinfectious, CO for cryoglobulins, MP for membranoproliferative glomerulonephritis, L for lupus, and EM for embolic.
BACK TO OUR PATIENT
In addition to kidney biopsy, we tested our patient for serum cryoglobulins, rheumatoid factor, and serum complements. Results from these tests (Table 3), in addition to the lack of cryoglobulins on his biopsy, led to the conclusion that he had noncryoglobulinemic membranoproliferative glomerulonephritis.
WHO SHOULD RECEIVE TREATMENT FOR HCV?
4. According to the current IDSA/AASLD guidelines, which of the following patients should not receive direct-acting antiviral therapy for HCV?
- Patients with HCV and only low-stage fibrosis
- Patients with decompensated cirrhosis
- Patients with a glomerular filtration rate less than 30 mL/minute
- None of the above—nearly all patients with HCV infection should receive treatment for it
While certain patients have compelling indications for HCV treatment, such as advanced fibrosis, severe extrahepatic manifestations of HCV (eg, glomerulonephritis, cryoglobulinemia), and posttransplant status, current guidelines recommend treatment for nearly all patients with HCV, including those with low-stage fibrosis.12
Patients with Child-Pugh grade B or C decompensated cirrhosis, even with hepatocellular carcinoma, may be considered for treatment. Multiple studies have demonstrated the efficacy and safety of direct-acting antiviral drugs in this patient population. In one randomized controlled trial,15 the combination of ledipasvir, sofosbuvir, and ribavirin resulted in high sustained virologic response rates at 12 weeks in patients infected with HCV genotype 1 or 4 with advanced liver disease, irrespective of transplant status (86% to 89% of patients were pretransplant). Sustained virologic response was associated with improvements in Model for End-Stage Liver Disease and Child-Pugh scores largely due to decreases in bilirubin and improvement in synthetic function (ie, albumin).
Similarly, even patients with a glomerular filtration rate less than 30 mL/min are candidates for treatment. Those with a glomerular filtration rate above 30 mL/min need no dosage adjustments for the most common regimens, while regimens are also available for those with a rate less than 30 mL/min. Although patients with low baseline renal function have a higher frequency of anemia (especially with ribavirin), worsening renal dysfunction, and more severe adverse events, treatment responses remain high and comparable to those without renal impairment.
The Hepatitis C Therapeutic Registry and Research Network (HCV-TARGET) is conducting an ongoing prospective study evaluating real-world use of direct-acting antiviral agents. The study has reported the safety and efficacy of sofosbuvir-containing regimens in patients with varying severities of kidney disease, including glomerular filtration rates less than 30 mL/min). The patients received different regimens that included sofosbuvir. The regimens were reportedly tolerated, and the rate of sustained viral response at 12 weeks remained high.16
The efficacy of direct-acting antiviral agents for HCV-associated glomerulonephritis remains to be studied but is promising. Earlier studies found that antiviral therapy based on interferon alfa with or without ribavirin can significantly decrease proteinuria and stabilize renal function.17–20 HCV RNA clearance has been found to best predict renal improvement.
OUR PATIENT’S COURSE
Unfortunately, our patient’s kidney function declined further over the next 3 months, and he is currently on dialysis awaiting simultaneous liver and kidney transplant.
- Ginès P, Schrier RW. Renal failure in cirrhosis. N Engl J Med 2009; 361:1279–1290.
- Mackelaite L, Alsauskas ZC, Ranganna K. Renal failure in patients with cirrhosis. Med Clin North Am 2009; 93:855–869.
- Wadei HM, Mai ML, Ahsan N, Gonwa TA. Hepatorenal syndrome: pathophysiology and management. Clin J Am Soc Nephrol 2006; 1:1066–1079.
- Gines A, Escorsell A, Gines P, et al. Incidence, predictive factors, and prognosis of the hepatorenal syndrome in cirrhosis with ascites. Gastroenterology 1993; 105:229–236.
- Salerno F, Gerbes A, Ginès P, Wong F, Arroyo V. Diagnosis, prevention and treatment of hepatorenal syndrome in cirrhosis. Gut 2007; 56:1310–1318.
- Watt K, Uhanova J, Minuk GY. Hepatorenal syndrome: diagnostic accuracy, clinical features, and outcome in a tertiary care center. Am J Gastroenterol 2002; 97:2046–2050.
- Graupera I, Cardenas A. Diagnostic approach to renal failure in cirrhosis. Clin Liver Dis 2013; 2:128–131.
- Dash SC, Bhowmik D. Glomerulopathy with liver disease: patterns and management. Saudi J Kidney Dis Transpl 2000; 11:414–420.
- Arase Y, Ikeda K, Murashima N, et al. Glomerulonephritis in autopsy cases with hepatitis C virus infection. Intern Med 1998; 37:836–840.
- McGuire BM, Julian BA, Bynon JS, et al. Brief communication: glomerulonephritis in patients with hepatitis C cirrhosis undergoing liver transplantation. Ann Intern Med 2006; 144:735–741.
- Kidney Disease: Improving Global Outcomes (KDIGO). KDIGO clinical practice guidelines for the prevention, diagnosis, evaluation, and treatment of hepatitis C in chronic kidney disease. Kidney Int Suppl 2008; 109:S1–S99.
- American Association for the Study of Liver Diseases (AASLD) and the Infectious Diseases Society of America (IDSA). HCV guidance: recommendations for testing, managing, and treating hepatitis C. www.hcvguidelines.org/. Accessed July 10, 2016.
- Lai KN. Hepatitis-related renal disease. Future Virology 2011; 6:1361–1376.
- Sethi S, Fervenza FC. Membranoproliferative glomerulonephritis—a new look at an old entity. N Engl J Med 2012; 366:1119–1131.
- Charlton M, Everson GT, Flamm SL, et al; SOLAR-1 Investigators. Ledipasvir and sofosbuvir plus ribavirin for treatment of HCV infection in patients with advanced liver disease. Gastroenterology 2015; 149:649–659.
- Saxena V, Koraishy FM, Sise ME, et al; HCV-TARGET. Safety and efficacy of sofosbuvir-containing regimens in hepatitis C-infected patients with impaired renal function. Liver Int 2016; 36:807–816.
- Feng B, Eknoyan G, Guo ZS, et al. Effect of interferon alpha-based antiviral therapy on hepatitis C virus-associated glomerulonephritis: a meta-analysis. Nephrol Dial Transplant 2012; 27:640–646.
- Bruchfeld A, Lindahl K, Ståhle L, Söderberg M, Schvarcz R. Interferon and ribavirin treatment in patients with hepatitis C-associated renal disease and renal insufficiency. Nephrol Dial Transplant 2003; 18:1573–1580.
- Rossi P, Bertani T, Baio P, et al. Hepatitis C virus-related cryoglobulinemic glomerulonephritis. Long-term remission after antiviral therapy. Kidney Int 2003; 63:2236–2241.
- Alric L, Plaisier E, Thebault S, et al. Influence of antiviral therapy in hepatitis C virus associated cryoglobulinemic MPGN. Am J Kidney Dis 2004; 43:617–623.
- Ginès P, Schrier RW. Renal failure in cirrhosis. N Engl J Med 2009; 361:1279–1290.
- Mackelaite L, Alsauskas ZC, Ranganna K. Renal failure in patients with cirrhosis. Med Clin North Am 2009; 93:855–869.
- Wadei HM, Mai ML, Ahsan N, Gonwa TA. Hepatorenal syndrome: pathophysiology and management. Clin J Am Soc Nephrol 2006; 1:1066–1079.
- Gines A, Escorsell A, Gines P, et al. Incidence, predictive factors, and prognosis of the hepatorenal syndrome in cirrhosis with ascites. Gastroenterology 1993; 105:229–236.
- Salerno F, Gerbes A, Ginès P, Wong F, Arroyo V. Diagnosis, prevention and treatment of hepatorenal syndrome in cirrhosis. Gut 2007; 56:1310–1318.
- Watt K, Uhanova J, Minuk GY. Hepatorenal syndrome: diagnostic accuracy, clinical features, and outcome in a tertiary care center. Am J Gastroenterol 2002; 97:2046–2050.
- Graupera I, Cardenas A. Diagnostic approach to renal failure in cirrhosis. Clin Liver Dis 2013; 2:128–131.
- Dash SC, Bhowmik D. Glomerulopathy with liver disease: patterns and management. Saudi J Kidney Dis Transpl 2000; 11:414–420.
- Arase Y, Ikeda K, Murashima N, et al. Glomerulonephritis in autopsy cases with hepatitis C virus infection. Intern Med 1998; 37:836–840.
- McGuire BM, Julian BA, Bynon JS, et al. Brief communication: glomerulonephritis in patients with hepatitis C cirrhosis undergoing liver transplantation. Ann Intern Med 2006; 144:735–741.
- Kidney Disease: Improving Global Outcomes (KDIGO). KDIGO clinical practice guidelines for the prevention, diagnosis, evaluation, and treatment of hepatitis C in chronic kidney disease. Kidney Int Suppl 2008; 109:S1–S99.
- American Association for the Study of Liver Diseases (AASLD) and the Infectious Diseases Society of America (IDSA). HCV guidance: recommendations for testing, managing, and treating hepatitis C. www.hcvguidelines.org/. Accessed July 10, 2016.
- Lai KN. Hepatitis-related renal disease. Future Virology 2011; 6:1361–1376.
- Sethi S, Fervenza FC. Membranoproliferative glomerulonephritis—a new look at an old entity. N Engl J Med 2012; 366:1119–1131.
- Charlton M, Everson GT, Flamm SL, et al; SOLAR-1 Investigators. Ledipasvir and sofosbuvir plus ribavirin for treatment of HCV infection in patients with advanced liver disease. Gastroenterology 2015; 149:649–659.
- Saxena V, Koraishy FM, Sise ME, et al; HCV-TARGET. Safety and efficacy of sofosbuvir-containing regimens in hepatitis C-infected patients with impaired renal function. Liver Int 2016; 36:807–816.
- Feng B, Eknoyan G, Guo ZS, et al. Effect of interferon alpha-based antiviral therapy on hepatitis C virus-associated glomerulonephritis: a meta-analysis. Nephrol Dial Transplant 2012; 27:640–646.
- Bruchfeld A, Lindahl K, Ståhle L, Söderberg M, Schvarcz R. Interferon and ribavirin treatment in patients with hepatitis C-associated renal disease and renal insufficiency. Nephrol Dial Transplant 2003; 18:1573–1580.
- Rossi P, Bertani T, Baio P, et al. Hepatitis C virus-related cryoglobulinemic glomerulonephritis. Long-term remission after antiviral therapy. Kidney Int 2003; 63:2236–2241.
- Alric L, Plaisier E, Thebault S, et al. Influence of antiviral therapy in hepatitis C virus associated cryoglobulinemic MPGN. Am J Kidney Dis 2004; 43:617–623.
Trust the thyroid thermostat
Primary hypothyroidism is common. Most patients acquire it when the thyroid gland is damaged by autoimmune inflammation. It is readily and reliably treated with the orally administered synthetic hormone levothyroxine, or less reliably with thyroid gland extracts. Absorption of either medication is significantly influenced by food, so patients need to pay attention to the timing of ingestion. But occasional blood testing can be used to easily monitor the sufficiency of replacement therapy.
The predominant active thyroid hormone is triiodothyronine (T3), most of which is converted from thyroxine (T4) by deiodination outside of the thyroid gland. Circulating thyroid-binding globulins tie up significant amounts of these hormones in the blood, and this protein binding is affected by a number of factors. Free T3 and T4—not bound—are the substances that exert physiologic effects on target organs and also give feedback information to the pituitary gland which, completing the loop, releases thyroid-stimulating hormone (TSH) and ultimately controls the healthy thyroid gland’s production and release of its hormones. Hence, the total circulating thyroid hormone levels are not as biologically relevant as the free T3 and T4 levels. Even in the absence of a functioning thyroid gland, the TSH level reliably reflects the bioactivity of circulating thyroid hormones so long as the pituitary gland is functioning normally.
Routine tracking of the biologic effects of thyroid hormone, such as the metabolic rate, is unreasonable, and other biologic effects such as the cholesterol level are influenced by so many factors in addition to T3 as to be unreliable indicators of thyroid hormone levels. Assuming the patient’s hypothalamic-pituitary axis is normal, the most reasonable and reliable way to track the biologic effect of thyroid hormone is to follow the TSH level. Although the exact relationship between free thyroid hormone and TSH levels is slightly different between patients with normal thyroid glands and those with damaged glands receiving replacement therapy, TSH measurement is an excellent indicator of the level that the brain wants thyroid function to be. Other than in specific nonhomeostatic circumstances, the pituitary gland is usually a superb thermostat for thyroid hormone activity.
In this issue of the Journal, Dr. Christian Nasr discusses the rationale for routinely using TSH measurement alone to direct exogenous thyroid replacement, explaining why it is cost-effective and clinically appropriate.
While following T3 and T4 may occasionally be useful in a few patients, the wealth of clinical data does not support this practice. As a routine practice it is certainly financially wasteful, and may lead to inappropriate clinical decisions.
Why, then, do some physicians persist in regularly following T3 and T4 levels in addition to TSH? There is no single answer. Although some patients may feel “better” if they take a little more rather than a little less levothyroxine, whether this benefit outweighs the metabolic price in the long run is not at all clear. Plus, in the published experience with treating subclinical hypothyroidism,1 patients did not generally feel better or experience desired weight loss when they received slightly more exogenous thyroid hormone. Somewhat analogously, if the TSH level is already normal, increasing thyroid replacement to attain a free T3 or T4 level in the high-normal range is unlikely to improve clinical outcomes in a meaningful way and may well be detrimental in the long term.
Despite a lot of chatter on Internet blogs regarding the multiple benefits of selective T3 replacement and higher T3 levels, akin to testosterone supplementation above what the (normal functioning) hypothalamic-pituitary axis has determined to be biologically appropriate, there is limited clinical evidence to support this practice. When replacing the output of a diseased or absent thyroid gland, it is reasonable clinical practice to trust the pituitary readings of the thyroid thermostat.
- Rugge JB, Bougatsos C, Chou R. Screening and treatment of thyroid dysfunction: an evidence review for the U.S. Preventive Services Task Force. Ann Intern Med 2015; 162:35–45.
Primary hypothyroidism is common. Most patients acquire it when the thyroid gland is damaged by autoimmune inflammation. It is readily and reliably treated with the orally administered synthetic hormone levothyroxine, or less reliably with thyroid gland extracts. Absorption of either medication is significantly influenced by food, so patients need to pay attention to the timing of ingestion. But occasional blood testing can be used to easily monitor the sufficiency of replacement therapy.
The predominant active thyroid hormone is triiodothyronine (T3), most of which is converted from thyroxine (T4) by deiodination outside of the thyroid gland. Circulating thyroid-binding globulins tie up significant amounts of these hormones in the blood, and this protein binding is affected by a number of factors. Free T3 and T4—not bound—are the substances that exert physiologic effects on target organs and also give feedback information to the pituitary gland which, completing the loop, releases thyroid-stimulating hormone (TSH) and ultimately controls the healthy thyroid gland’s production and release of its hormones. Hence, the total circulating thyroid hormone levels are not as biologically relevant as the free T3 and T4 levels. Even in the absence of a functioning thyroid gland, the TSH level reliably reflects the bioactivity of circulating thyroid hormones so long as the pituitary gland is functioning normally.
Routine tracking of the biologic effects of thyroid hormone, such as the metabolic rate, is unreasonable, and other biologic effects such as the cholesterol level are influenced by so many factors in addition to T3 as to be unreliable indicators of thyroid hormone levels. Assuming the patient’s hypothalamic-pituitary axis is normal, the most reasonable and reliable way to track the biologic effect of thyroid hormone is to follow the TSH level. Although the exact relationship between free thyroid hormone and TSH levels is slightly different between patients with normal thyroid glands and those with damaged glands receiving replacement therapy, TSH measurement is an excellent indicator of the level that the brain wants thyroid function to be. Other than in specific nonhomeostatic circumstances, the pituitary gland is usually a superb thermostat for thyroid hormone activity.
In this issue of the Journal, Dr. Christian Nasr discusses the rationale for routinely using TSH measurement alone to direct exogenous thyroid replacement, explaining why it is cost-effective and clinically appropriate.
While following T3 and T4 may occasionally be useful in a few patients, the wealth of clinical data does not support this practice. As a routine practice it is certainly financially wasteful, and may lead to inappropriate clinical decisions.
Why, then, do some physicians persist in regularly following T3 and T4 levels in addition to TSH? There is no single answer. Although some patients may feel “better” if they take a little more rather than a little less levothyroxine, whether this benefit outweighs the metabolic price in the long run is not at all clear. Plus, in the published experience with treating subclinical hypothyroidism,1 patients did not generally feel better or experience desired weight loss when they received slightly more exogenous thyroid hormone. Somewhat analogously, if the TSH level is already normal, increasing thyroid replacement to attain a free T3 or T4 level in the high-normal range is unlikely to improve clinical outcomes in a meaningful way and may well be detrimental in the long term.
Despite a lot of chatter on Internet blogs regarding the multiple benefits of selective T3 replacement and higher T3 levels, akin to testosterone supplementation above what the (normal functioning) hypothalamic-pituitary axis has determined to be biologically appropriate, there is limited clinical evidence to support this practice. When replacing the output of a diseased or absent thyroid gland, it is reasonable clinical practice to trust the pituitary readings of the thyroid thermostat.
Primary hypothyroidism is common. Most patients acquire it when the thyroid gland is damaged by autoimmune inflammation. It is readily and reliably treated with the orally administered synthetic hormone levothyroxine, or less reliably with thyroid gland extracts. Absorption of either medication is significantly influenced by food, so patients need to pay attention to the timing of ingestion. But occasional blood testing can be used to easily monitor the sufficiency of replacement therapy.
The predominant active thyroid hormone is triiodothyronine (T3), most of which is converted from thyroxine (T4) by deiodination outside of the thyroid gland. Circulating thyroid-binding globulins tie up significant amounts of these hormones in the blood, and this protein binding is affected by a number of factors. Free T3 and T4—not bound—are the substances that exert physiologic effects on target organs and also give feedback information to the pituitary gland which, completing the loop, releases thyroid-stimulating hormone (TSH) and ultimately controls the healthy thyroid gland’s production and release of its hormones. Hence, the total circulating thyroid hormone levels are not as biologically relevant as the free T3 and T4 levels. Even in the absence of a functioning thyroid gland, the TSH level reliably reflects the bioactivity of circulating thyroid hormones so long as the pituitary gland is functioning normally.
Routine tracking of the biologic effects of thyroid hormone, such as the metabolic rate, is unreasonable, and other biologic effects such as the cholesterol level are influenced by so many factors in addition to T3 as to be unreliable indicators of thyroid hormone levels. Assuming the patient’s hypothalamic-pituitary axis is normal, the most reasonable and reliable way to track the biologic effect of thyroid hormone is to follow the TSH level. Although the exact relationship between free thyroid hormone and TSH levels is slightly different between patients with normal thyroid glands and those with damaged glands receiving replacement therapy, TSH measurement is an excellent indicator of the level that the brain wants thyroid function to be. Other than in specific nonhomeostatic circumstances, the pituitary gland is usually a superb thermostat for thyroid hormone activity.
In this issue of the Journal, Dr. Christian Nasr discusses the rationale for routinely using TSH measurement alone to direct exogenous thyroid replacement, explaining why it is cost-effective and clinically appropriate.
While following T3 and T4 may occasionally be useful in a few patients, the wealth of clinical data does not support this practice. As a routine practice it is certainly financially wasteful, and may lead to inappropriate clinical decisions.
Why, then, do some physicians persist in regularly following T3 and T4 levels in addition to TSH? There is no single answer. Although some patients may feel “better” if they take a little more rather than a little less levothyroxine, whether this benefit outweighs the metabolic price in the long run is not at all clear. Plus, in the published experience with treating subclinical hypothyroidism,1 patients did not generally feel better or experience desired weight loss when they received slightly more exogenous thyroid hormone. Somewhat analogously, if the TSH level is already normal, increasing thyroid replacement to attain a free T3 or T4 level in the high-normal range is unlikely to improve clinical outcomes in a meaningful way and may well be detrimental in the long term.
Despite a lot of chatter on Internet blogs regarding the multiple benefits of selective T3 replacement and higher T3 levels, akin to testosterone supplementation above what the (normal functioning) hypothalamic-pituitary axis has determined to be biologically appropriate, there is limited clinical evidence to support this practice. When replacing the output of a diseased or absent thyroid gland, it is reasonable clinical practice to trust the pituitary readings of the thyroid thermostat.
- Rugge JB, Bougatsos C, Chou R. Screening and treatment of thyroid dysfunction: an evidence review for the U.S. Preventive Services Task Force. Ann Intern Med 2015; 162:35–45.
- Rugge JB, Bougatsos C, Chou R. Screening and treatment of thyroid dysfunction: an evidence review for the U.S. Preventive Services Task Force. Ann Intern Med 2015; 162:35–45.
Is a serum TSH measurement sufficient to monitor the treatment of primary hypothyroidism?
A 28-year-old woman returns for follow-up of her hypothyroidism. She was diagnosed 4 years ago when she presented with fatigue, “foggy” thinking, poor concentration, cold intolerance, and constipation. Her thyroid-stimulating hormone (TSH) level at that time was elevated at 15 mIU/L (reference range 0.4–4). She was started on 50 µg of levothyroxine daily, which helped her symptoms, but she continued to complain of tiredness and the inability to lose weight. She has been on 100 µg of levothyroxine daily since her last visit 1 year ago.
On examination, she has a small, diffuse, and firm goiter; she has no Cushing-like features, visual field abnormalities, or signs of hypothyroidism.
Her TSH level today is 1.2 mIU/L. Based on this, you recommend no change in her daily levothyroxine dose. She expresses dissatisfaction that you had ordered only a TSH, and she asks you to order thyroxine (T4) and triiodothyronine (T3) measurements because she read on the Internet that those were needed to determine the appropriateness of the levothyroxine dose.
Should T4 or T3 be routinely measured when adjusting thyroid replacement therapy?
IN PRIMARY HYPOTHYROIDISM, TSH IS ENOUGH
In a patient with primary hypothyroidism and no suspicion of pituitary abnormality, a serum TSH is sufficient for monitoring thyroid status and adjusting the dose of thyroid hormone.
Hypothyroidism is one of the most common endocrine disorders, affecting about 4% of the adult US population.1 In areas of iodine sufficiency, primary hypothyroidism is due predominantly to Hashimoto thyroiditis.
The role of the lack of thyroid hormone in the pathogenesis of myxedema was recognized in the late 19th century through the observation of a “cretinoid” state occurring in middle-aged women, associated with atrophy of the thyroid gland and a similar severe state noted after total thyroidectomy.2
In 1891, George R. Murray was able to “cure” myxedema in a patient by injecting sheep thyroid extract subcutaneously. Thyroid extracts continued to be the only treatment for hypothyroidism until 1950, when levothyroxine was introduced and later became the main treatment. Around that time, T3 was discovered and was described as being the physiologically active thyroid hormone. Later, it was noted that 80% to 90% of circulating T3 is generated through peripheral deiodination of T4, the latter being considered a prohormone.2
The pituitary-thyroid axis is regulated through negative feedback. At concentrations of free T4 below normal, plasma TSH rises rapidly with small decrements in T4 levels.3 The opposite phenomenon occurs with free T4 concentrations above normal. Since T4 has a long disappearance half-time—about 7 days—a normal TSH tends to stay relatively stable in the same individual.4 The relationship between TSH and T4 was long thought to be inverse log-linear, but Hadlow et al5 found that it is complex and nonlinear and differs by age and sex. TSH and T4 concentrations have narrower within-individual variability than inter-individual variability. Although environmental factors may affect this hypothalamic-pituitary set-point, there is evidence that heritability is a major determinant of individual variability.6
GUIDELINES AND CHOOSING WISELY
In 2014, the American Thyroid Association published comprehensive, evidence-based guidelines for the treatment of hypothyroidism.7 The guidelines state that the goal of thyroid hormone replacement is to achieve clinical and biochemical euthyroidism.7 TSH continues to be the most reliable marker of adequacy of thyroid hormone replacement in primary hypothyroidism. The guidelines recommend aiming for a TSH in the normal range (generally 0.4–4 mIU/L).
Most studies of the risks associated with hypothyroidism or thyrotoxicosis have looked at TSH levels. Significantly increased risk of cardiovascular mortality and morbidity is seen in individuals with TSH levels higher than 10 mIU/L.8 On the other hand, excess thyroid hormone leading to a TSH level lower than 0.1 mIU/L has been associated with an increased risk of atrial fibrillation in older persons and osteoporosis in postmenopausal women.
The classic symptoms and signs of hypothyroidism correlate with biochemical hypothyroidism and usually improve with the restoration of euthyroidism. Some of these symptoms, however, lack sensitivity and specificity, especially with modest degrees of hypothyroidism.7 A randomized controlled trial showed that patients were unable to detect any difference in symptoms when the levothyroxine dose was changed by about 20%.9
HARMS ASSOCIATED WITH ORDERING T4 AND T3
Other than the financial burden to the patient and society, there is no major morbidity caused by obtaining T4 or T3 levels, or both. However, knowing the T4 or T3 level does not help with management beyond the information offered by the TSH value. Hypothyroid patients treated with levothyroxine to maintain a normal TSH generally have higher free T4 levels and lower free T3 levels than euthyroid patients with similar TSH values.10 Therefore, reacting to a high T4 level or a low T3 level in a treated hypothyroid patient with a normal TSH may lead to inappropriate dose adjustment. On the other hand, increasing the dose of thyroid hormone in a patient with a low TSH whose T3 level is low-normal may lead to morbidity.
SPECIAL SCENARIO: PITUITARY COMPROMISE
We assume that the patient described above has primary hypothyroidism and that her pituitary-thyroid axis is intact. Primary hypothyroidism is diagnosed by a high TSH along with a low or low-normal T4. In this typical case, TSH can be used to guide therapy without the need for other tests.
However, when there is pituitary compromise (hypopituitarism, congenital central hypothyroidism), the TSH will not be reliable to monitor the adequacy of thyroid hormone replacement therapy. The aim of levothyroxine management in these patients is to maintain a free T4 concentration in the upper half of the normal range. If the free T3 concentration is followed and is found to be elevated, the dose of levothyroxine should be reduced.11
CLINICAL BOTTOM LINE
Since our patient’s dose of levothyroxine has been stable and her TSH is not elevated, measuring serum levels of T4 and T3 would not contribute to her management. For such a patient, if the TSH were less than 3 mIU/L, increasing the dose would be unlikely to offer clinical benefit.
On the other hand, if her TSH was higher than 4 mIU/L, then it would be legitimate to tweak the dose upward and reassess her thyroid state clinically and biochemically 6 to 8 weeks later. One would need to be careful not to induce thyrotoxicosis through such an intervention because of the potential morbidity.
The TSH level is typically monitored every 6 to 12 months when the patient is clinically stable. It should be measured sooner in circumstances that include the following:
- Symptoms of hypothyroidism or thyrotoxicosis
- Starting a new medication known to affect thyroid hormone levels
- Significant weight change
- Hospitalization
- Pregnancy.
- Aoki Y, Belin RM, Clickner R, Jeffries R, Phillips L, Mahaffey KR. Serum TSH and total T4 in the United States population and their association with participant characteristics: National Health and Nutrition Examination Survey (NHANES 1999–2002). Thyroid 2007; 17:1211–1223.
- Kopp PE. Commentary on: guidelines for the treatment of hypothyroidism. Thyroid 2014; 24:1667–1669.
- Reichlin S, Utiger RD. Regulation of the pituitary-thyroid axis in man: relationship of TSH concentration to concentration of free and total thyroxine in plasma. J Clin Endocrinol Metab 1967; 27:251–255.
- Azukizawa M, Pekary AE, Hershman JM, Parker DC. Plasma thyrotropin, thyroxine, and triiodothyronine relationships in man. J Clin Endocrinol Metab 1976; 43:533–542.
- Hadlow NC, Rothacker KM, Wardrop R, Brown SJ, Mun Lim E, Walsh JP. The relationship between TSH and free T4 in a large population is complex and nonlinear and differs by age and sex. J Clin Endocrinol Metab 2013; 98:2936–2943.
- Clark PM, Holder RL, Haque SM, Hobbs FDR, Roberts LM, Franklyn JA. The relationship between serum TSH and free T4 in older people. J Clin Pathol 2012; 65:463–465.
- Jonklaas J, Bianco AC, Bauer AJ, et al; American Thyroid Association Task Force on Thyroid Hormone Replacement. Guidelines for the treatment of hypothyroidism. Thyroid 2014; 24:1670–1751.
- Rodondi N, den Elzen WP, Bauer DC, et al; Thyroid Studies Collaboration. Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA 2010; 304:1365–1374.
- Walsh JP, Ward LC, Burke V, et al. Small changes in thyroxine dosage do not produce measurable changes in hypothyroid symptoms, well-being, or quality of life: results of a double-blind, randomized clinical trial. J Clin Endocrinol Metab 2006; 91:2624–2630.
- Woeber KA. Levothyroxine therapy and serum free thyroxine and free triiodothyronine concentrations. J Endocrinol Invest 2002; 25:106–109.
- Grunenwald S, Caron P. Central hypothyroidism in adults: better understanding for better care. Pituitary 2015; 18:169–175.
A 28-year-old woman returns for follow-up of her hypothyroidism. She was diagnosed 4 years ago when she presented with fatigue, “foggy” thinking, poor concentration, cold intolerance, and constipation. Her thyroid-stimulating hormone (TSH) level at that time was elevated at 15 mIU/L (reference range 0.4–4). She was started on 50 µg of levothyroxine daily, which helped her symptoms, but she continued to complain of tiredness and the inability to lose weight. She has been on 100 µg of levothyroxine daily since her last visit 1 year ago.
On examination, she has a small, diffuse, and firm goiter; she has no Cushing-like features, visual field abnormalities, or signs of hypothyroidism.
Her TSH level today is 1.2 mIU/L. Based on this, you recommend no change in her daily levothyroxine dose. She expresses dissatisfaction that you had ordered only a TSH, and she asks you to order thyroxine (T4) and triiodothyronine (T3) measurements because she read on the Internet that those were needed to determine the appropriateness of the levothyroxine dose.
Should T4 or T3 be routinely measured when adjusting thyroid replacement therapy?
IN PRIMARY HYPOTHYROIDISM, TSH IS ENOUGH
In a patient with primary hypothyroidism and no suspicion of pituitary abnormality, a serum TSH is sufficient for monitoring thyroid status and adjusting the dose of thyroid hormone.
Hypothyroidism is one of the most common endocrine disorders, affecting about 4% of the adult US population.1 In areas of iodine sufficiency, primary hypothyroidism is due predominantly to Hashimoto thyroiditis.
The role of the lack of thyroid hormone in the pathogenesis of myxedema was recognized in the late 19th century through the observation of a “cretinoid” state occurring in middle-aged women, associated with atrophy of the thyroid gland and a similar severe state noted after total thyroidectomy.2
In 1891, George R. Murray was able to “cure” myxedema in a patient by injecting sheep thyroid extract subcutaneously. Thyroid extracts continued to be the only treatment for hypothyroidism until 1950, when levothyroxine was introduced and later became the main treatment. Around that time, T3 was discovered and was described as being the physiologically active thyroid hormone. Later, it was noted that 80% to 90% of circulating T3 is generated through peripheral deiodination of T4, the latter being considered a prohormone.2
The pituitary-thyroid axis is regulated through negative feedback. At concentrations of free T4 below normal, plasma TSH rises rapidly with small decrements in T4 levels.3 The opposite phenomenon occurs with free T4 concentrations above normal. Since T4 has a long disappearance half-time—about 7 days—a normal TSH tends to stay relatively stable in the same individual.4 The relationship between TSH and T4 was long thought to be inverse log-linear, but Hadlow et al5 found that it is complex and nonlinear and differs by age and sex. TSH and T4 concentrations have narrower within-individual variability than inter-individual variability. Although environmental factors may affect this hypothalamic-pituitary set-point, there is evidence that heritability is a major determinant of individual variability.6
GUIDELINES AND CHOOSING WISELY
In 2014, the American Thyroid Association published comprehensive, evidence-based guidelines for the treatment of hypothyroidism.7 The guidelines state that the goal of thyroid hormone replacement is to achieve clinical and biochemical euthyroidism.7 TSH continues to be the most reliable marker of adequacy of thyroid hormone replacement in primary hypothyroidism. The guidelines recommend aiming for a TSH in the normal range (generally 0.4–4 mIU/L).
Most studies of the risks associated with hypothyroidism or thyrotoxicosis have looked at TSH levels. Significantly increased risk of cardiovascular mortality and morbidity is seen in individuals with TSH levels higher than 10 mIU/L.8 On the other hand, excess thyroid hormone leading to a TSH level lower than 0.1 mIU/L has been associated with an increased risk of atrial fibrillation in older persons and osteoporosis in postmenopausal women.
The classic symptoms and signs of hypothyroidism correlate with biochemical hypothyroidism and usually improve with the restoration of euthyroidism. Some of these symptoms, however, lack sensitivity and specificity, especially with modest degrees of hypothyroidism.7 A randomized controlled trial showed that patients were unable to detect any difference in symptoms when the levothyroxine dose was changed by about 20%.9
HARMS ASSOCIATED WITH ORDERING T4 AND T3
Other than the financial burden to the patient and society, there is no major morbidity caused by obtaining T4 or T3 levels, or both. However, knowing the T4 or T3 level does not help with management beyond the information offered by the TSH value. Hypothyroid patients treated with levothyroxine to maintain a normal TSH generally have higher free T4 levels and lower free T3 levels than euthyroid patients with similar TSH values.10 Therefore, reacting to a high T4 level or a low T3 level in a treated hypothyroid patient with a normal TSH may lead to inappropriate dose adjustment. On the other hand, increasing the dose of thyroid hormone in a patient with a low TSH whose T3 level is low-normal may lead to morbidity.
SPECIAL SCENARIO: PITUITARY COMPROMISE
We assume that the patient described above has primary hypothyroidism and that her pituitary-thyroid axis is intact. Primary hypothyroidism is diagnosed by a high TSH along with a low or low-normal T4. In this typical case, TSH can be used to guide therapy without the need for other tests.
However, when there is pituitary compromise (hypopituitarism, congenital central hypothyroidism), the TSH will not be reliable to monitor the adequacy of thyroid hormone replacement therapy. The aim of levothyroxine management in these patients is to maintain a free T4 concentration in the upper half of the normal range. If the free T3 concentration is followed and is found to be elevated, the dose of levothyroxine should be reduced.11
CLINICAL BOTTOM LINE
Since our patient’s dose of levothyroxine has been stable and her TSH is not elevated, measuring serum levels of T4 and T3 would not contribute to her management. For such a patient, if the TSH were less than 3 mIU/L, increasing the dose would be unlikely to offer clinical benefit.
On the other hand, if her TSH was higher than 4 mIU/L, then it would be legitimate to tweak the dose upward and reassess her thyroid state clinically and biochemically 6 to 8 weeks later. One would need to be careful not to induce thyrotoxicosis through such an intervention because of the potential morbidity.
The TSH level is typically monitored every 6 to 12 months when the patient is clinically stable. It should be measured sooner in circumstances that include the following:
- Symptoms of hypothyroidism or thyrotoxicosis
- Starting a new medication known to affect thyroid hormone levels
- Significant weight change
- Hospitalization
- Pregnancy.
A 28-year-old woman returns for follow-up of her hypothyroidism. She was diagnosed 4 years ago when she presented with fatigue, “foggy” thinking, poor concentration, cold intolerance, and constipation. Her thyroid-stimulating hormone (TSH) level at that time was elevated at 15 mIU/L (reference range 0.4–4). She was started on 50 µg of levothyroxine daily, which helped her symptoms, but she continued to complain of tiredness and the inability to lose weight. She has been on 100 µg of levothyroxine daily since her last visit 1 year ago.
On examination, she has a small, diffuse, and firm goiter; she has no Cushing-like features, visual field abnormalities, or signs of hypothyroidism.
Her TSH level today is 1.2 mIU/L. Based on this, you recommend no change in her daily levothyroxine dose. She expresses dissatisfaction that you had ordered only a TSH, and she asks you to order thyroxine (T4) and triiodothyronine (T3) measurements because she read on the Internet that those were needed to determine the appropriateness of the levothyroxine dose.
Should T4 or T3 be routinely measured when adjusting thyroid replacement therapy?
IN PRIMARY HYPOTHYROIDISM, TSH IS ENOUGH
In a patient with primary hypothyroidism and no suspicion of pituitary abnormality, a serum TSH is sufficient for monitoring thyroid status and adjusting the dose of thyroid hormone.
Hypothyroidism is one of the most common endocrine disorders, affecting about 4% of the adult US population.1 In areas of iodine sufficiency, primary hypothyroidism is due predominantly to Hashimoto thyroiditis.
The role of the lack of thyroid hormone in the pathogenesis of myxedema was recognized in the late 19th century through the observation of a “cretinoid” state occurring in middle-aged women, associated with atrophy of the thyroid gland and a similar severe state noted after total thyroidectomy.2
In 1891, George R. Murray was able to “cure” myxedema in a patient by injecting sheep thyroid extract subcutaneously. Thyroid extracts continued to be the only treatment for hypothyroidism until 1950, when levothyroxine was introduced and later became the main treatment. Around that time, T3 was discovered and was described as being the physiologically active thyroid hormone. Later, it was noted that 80% to 90% of circulating T3 is generated through peripheral deiodination of T4, the latter being considered a prohormone.2
The pituitary-thyroid axis is regulated through negative feedback. At concentrations of free T4 below normal, plasma TSH rises rapidly with small decrements in T4 levels.3 The opposite phenomenon occurs with free T4 concentrations above normal. Since T4 has a long disappearance half-time—about 7 days—a normal TSH tends to stay relatively stable in the same individual.4 The relationship between TSH and T4 was long thought to be inverse log-linear, but Hadlow et al5 found that it is complex and nonlinear and differs by age and sex. TSH and T4 concentrations have narrower within-individual variability than inter-individual variability. Although environmental factors may affect this hypothalamic-pituitary set-point, there is evidence that heritability is a major determinant of individual variability.6
GUIDELINES AND CHOOSING WISELY
In 2014, the American Thyroid Association published comprehensive, evidence-based guidelines for the treatment of hypothyroidism.7 The guidelines state that the goal of thyroid hormone replacement is to achieve clinical and biochemical euthyroidism.7 TSH continues to be the most reliable marker of adequacy of thyroid hormone replacement in primary hypothyroidism. The guidelines recommend aiming for a TSH in the normal range (generally 0.4–4 mIU/L).
Most studies of the risks associated with hypothyroidism or thyrotoxicosis have looked at TSH levels. Significantly increased risk of cardiovascular mortality and morbidity is seen in individuals with TSH levels higher than 10 mIU/L.8 On the other hand, excess thyroid hormone leading to a TSH level lower than 0.1 mIU/L has been associated with an increased risk of atrial fibrillation in older persons and osteoporosis in postmenopausal women.
The classic symptoms and signs of hypothyroidism correlate with biochemical hypothyroidism and usually improve with the restoration of euthyroidism. Some of these symptoms, however, lack sensitivity and specificity, especially with modest degrees of hypothyroidism.7 A randomized controlled trial showed that patients were unable to detect any difference in symptoms when the levothyroxine dose was changed by about 20%.9
HARMS ASSOCIATED WITH ORDERING T4 AND T3
Other than the financial burden to the patient and society, there is no major morbidity caused by obtaining T4 or T3 levels, or both. However, knowing the T4 or T3 level does not help with management beyond the information offered by the TSH value. Hypothyroid patients treated with levothyroxine to maintain a normal TSH generally have higher free T4 levels and lower free T3 levels than euthyroid patients with similar TSH values.10 Therefore, reacting to a high T4 level or a low T3 level in a treated hypothyroid patient with a normal TSH may lead to inappropriate dose adjustment. On the other hand, increasing the dose of thyroid hormone in a patient with a low TSH whose T3 level is low-normal may lead to morbidity.
SPECIAL SCENARIO: PITUITARY COMPROMISE
We assume that the patient described above has primary hypothyroidism and that her pituitary-thyroid axis is intact. Primary hypothyroidism is diagnosed by a high TSH along with a low or low-normal T4. In this typical case, TSH can be used to guide therapy without the need for other tests.
However, when there is pituitary compromise (hypopituitarism, congenital central hypothyroidism), the TSH will not be reliable to monitor the adequacy of thyroid hormone replacement therapy. The aim of levothyroxine management in these patients is to maintain a free T4 concentration in the upper half of the normal range. If the free T3 concentration is followed and is found to be elevated, the dose of levothyroxine should be reduced.11
CLINICAL BOTTOM LINE
Since our patient’s dose of levothyroxine has been stable and her TSH is not elevated, measuring serum levels of T4 and T3 would not contribute to her management. For such a patient, if the TSH were less than 3 mIU/L, increasing the dose would be unlikely to offer clinical benefit.
On the other hand, if her TSH was higher than 4 mIU/L, then it would be legitimate to tweak the dose upward and reassess her thyroid state clinically and biochemically 6 to 8 weeks later. One would need to be careful not to induce thyrotoxicosis through such an intervention because of the potential morbidity.
The TSH level is typically monitored every 6 to 12 months when the patient is clinically stable. It should be measured sooner in circumstances that include the following:
- Symptoms of hypothyroidism or thyrotoxicosis
- Starting a new medication known to affect thyroid hormone levels
- Significant weight change
- Hospitalization
- Pregnancy.
- Aoki Y, Belin RM, Clickner R, Jeffries R, Phillips L, Mahaffey KR. Serum TSH and total T4 in the United States population and their association with participant characteristics: National Health and Nutrition Examination Survey (NHANES 1999–2002). Thyroid 2007; 17:1211–1223.
- Kopp PE. Commentary on: guidelines for the treatment of hypothyroidism. Thyroid 2014; 24:1667–1669.
- Reichlin S, Utiger RD. Regulation of the pituitary-thyroid axis in man: relationship of TSH concentration to concentration of free and total thyroxine in plasma. J Clin Endocrinol Metab 1967; 27:251–255.
- Azukizawa M, Pekary AE, Hershman JM, Parker DC. Plasma thyrotropin, thyroxine, and triiodothyronine relationships in man. J Clin Endocrinol Metab 1976; 43:533–542.
- Hadlow NC, Rothacker KM, Wardrop R, Brown SJ, Mun Lim E, Walsh JP. The relationship between TSH and free T4 in a large population is complex and nonlinear and differs by age and sex. J Clin Endocrinol Metab 2013; 98:2936–2943.
- Clark PM, Holder RL, Haque SM, Hobbs FDR, Roberts LM, Franklyn JA. The relationship between serum TSH and free T4 in older people. J Clin Pathol 2012; 65:463–465.
- Jonklaas J, Bianco AC, Bauer AJ, et al; American Thyroid Association Task Force on Thyroid Hormone Replacement. Guidelines for the treatment of hypothyroidism. Thyroid 2014; 24:1670–1751.
- Rodondi N, den Elzen WP, Bauer DC, et al; Thyroid Studies Collaboration. Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA 2010; 304:1365–1374.
- Walsh JP, Ward LC, Burke V, et al. Small changes in thyroxine dosage do not produce measurable changes in hypothyroid symptoms, well-being, or quality of life: results of a double-blind, randomized clinical trial. J Clin Endocrinol Metab 2006; 91:2624–2630.
- Woeber KA. Levothyroxine therapy and serum free thyroxine and free triiodothyronine concentrations. J Endocrinol Invest 2002; 25:106–109.
- Grunenwald S, Caron P. Central hypothyroidism in adults: better understanding for better care. Pituitary 2015; 18:169–175.
- Aoki Y, Belin RM, Clickner R, Jeffries R, Phillips L, Mahaffey KR. Serum TSH and total T4 in the United States population and their association with participant characteristics: National Health and Nutrition Examination Survey (NHANES 1999–2002). Thyroid 2007; 17:1211–1223.
- Kopp PE. Commentary on: guidelines for the treatment of hypothyroidism. Thyroid 2014; 24:1667–1669.
- Reichlin S, Utiger RD. Regulation of the pituitary-thyroid axis in man: relationship of TSH concentration to concentration of free and total thyroxine in plasma. J Clin Endocrinol Metab 1967; 27:251–255.
- Azukizawa M, Pekary AE, Hershman JM, Parker DC. Plasma thyrotropin, thyroxine, and triiodothyronine relationships in man. J Clin Endocrinol Metab 1976; 43:533–542.
- Hadlow NC, Rothacker KM, Wardrop R, Brown SJ, Mun Lim E, Walsh JP. The relationship between TSH and free T4 in a large population is complex and nonlinear and differs by age and sex. J Clin Endocrinol Metab 2013; 98:2936–2943.
- Clark PM, Holder RL, Haque SM, Hobbs FDR, Roberts LM, Franklyn JA. The relationship between serum TSH and free T4 in older people. J Clin Pathol 2012; 65:463–465.
- Jonklaas J, Bianco AC, Bauer AJ, et al; American Thyroid Association Task Force on Thyroid Hormone Replacement. Guidelines for the treatment of hypothyroidism. Thyroid 2014; 24:1670–1751.
- Rodondi N, den Elzen WP, Bauer DC, et al; Thyroid Studies Collaboration. Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA 2010; 304:1365–1374.
- Walsh JP, Ward LC, Burke V, et al. Small changes in thyroxine dosage do not produce measurable changes in hypothyroid symptoms, well-being, or quality of life: results of a double-blind, randomized clinical trial. J Clin Endocrinol Metab 2006; 91:2624–2630.
- Woeber KA. Levothyroxine therapy and serum free thyroxine and free triiodothyronine concentrations. J Endocrinol Invest 2002; 25:106–109.
- Grunenwald S, Caron P. Central hypothyroidism in adults: better understanding for better care. Pituitary 2015; 18:169–175.
Your patient has chronic leukemia: Now what?
The advent of targeted therapies has dramatically changed the management of chronic leukemia. Chemotherapy—highly toxic, nonspecific drugs that can be dangerous to patients and providers and result in only modest success—is gradually being replaced by biologic targeting of malignancy. Scientists are rapidly identifying extracellular and intracellular targets on tumor cells and are developing and testing promising new therapies aimed at these targets. Survival of cancer patients has become so common that clinicians outside the specialties of hematology and oncology are now caring for them.
This article describes new biologic therapies for chronic myelogenous leukemia (CML) and chronic lymphocytic leukemia (CLL), along with the diagnosis of these diseases and management of survivors in the primary care setting.
CHRONIC MYELOGENOUS LEUKEMIA
A seemingly healthy person needs laboratory blood work, perhaps for an insurance physical examination or for a preoperative workup. Or a patient comes to the emergency department with a sore throat and routine blood tests are ordered. Their laboratory values:
- White blood cell count 250 × 109/L (reference range 3–11)
- Neutrophils 70% (40%–70%)
- Blasts 1% (0)
- Metacytes and myelocytes 5% (0)
- Bands 5% (0)
- Lymphocytes 10% (22%–40%)
- Monocytes 5% (0–7%)
- Basophils 3% (0–1%)
- Eosinophils 1% (0–4%)
- Hemoglobin 12.1 g/dL (11.5–15.5 in women, 13.0–17.0 in men)
- Platelet count 525 × 109/L (150–400).
Leukocytosis and a ‘left shift’
Although this scenario often raises concern for acute leukemia, a careful look shows evidence of a chronic myeloproliferative disorder instead. Specifically, this patient’s laboratory values show a “left shift”—an increase in immature neutrophils, ie, blasts, myelocytes, and bands.
This picture is characteristic of CML, an uncommon leukemia with about 4,500 new cases annually in the United States. Patients can present at any age, but the disease occurs more often in older people, with a median age of 66.1
The presentation is usually subtle: about half of cases are detected by routine laboratory testing, which typically reveals a left-shifted leukocytosis with basophilia and a few blasts. Mild anemia is common. The platelet count is elevated in 30% to 50% of patients at diagnosis. Bone marrow aspirate shows significant myeloid hyperplasia without dysplasia, and sometimes shows mild fibrosis.
Philadelphia chromosome is diagnostic
A definitive diagnosis is made by demonstration of an abnormally short chromosome 22. Described in 1960 by Peter Nowell of the University of Pennsylvania and David Hugerford of the Institute for Cancer Research,2 this abnormality, called the Philadelphia chromosome, was the first specific genetic abnormality associated with a human cancer. Later, researchers used banding techniques to find that the Philadelphia chromosome results from a reciprocal translocation of genetic material between the BCR gene on chromosome 22 and the ABL1 gene on chromosome 9, t(9:22).3,4 The resulting chimeric gene, called BCR-ABL, codes for an oncogenic protein, a tyrosine kinase with constitutive activity.
The Philadelphia chromosome is present in 95% of patients with CML and can be found in all myeloid cell lineages, including erythrocytes, granulocytes, monocytes, and megakaryocytes as well as some cells of lymphocytic lineage, indicating that malignant transformation to CML takes place at the stem cell level.
The mutation causes several problems: the abnormal tyrosine kinase increases cell proliferation, inhibits apoptosis, and alters adhesion molecules in the stroma of the bone marrow, allowing immature cells to leak into the bloodstream. Most important, the mutation increases genomic instability so that additional mutations are likelier to occur over time, making it inevitable that, without treatment, the disease will progress to a fatal blast crisis within an average of 5 years of diagnosis.
CML has three clinical phases
Untreated, CML progresses through three distinct phases: chronic, accelerated, and blast crisis, defined by abnormalities in the blood smear and bone marrow (Table 1).5,6 Most patients (85%) are diagnosed during the chronic phase. The accelerated and blastic phases resemble acute leukemia.
Chronic phase management
Therapies over the years have included arsenic (Fowler solution), splenic radiotherapy, busulfan, hydroxyurea, cytarabine, and interferon. All had some palliative success, but usually did not suppress leukemic progression.7
In contrast, patients undergoing allogeneic bone marrow transplant had a 5-year survival rate of 60% to 80% during the chronic phase of CML, 40% to 60% during the accelerated phase, and 10% to 20% during a blast crisis.8 Long-term survival confirmed the ability of transplant to cure CML, and bone marrow transplant with matched donors was the standard of care for younger patients until the end of the 20th century.
Tyrosine kinase inhibition
A new paradigm in treatment began with the development of imatinib, a tyrosine kinase inhibitor that directly interferes with the product of the chimeric BCR-ABL gene.9
Patients treated with imatinib during the chronic phase of CML have survival rates similar to those of people without the disease, and they usually do not progress to the accelerated and blast phases. As a result of this success, the number of transplants for CML has fallen precipitously.
Other tyrosine kinase inhibitors (dasatinib, nilotinib) that have since been developed have shown even better results in achieving remission and preventing progression. Improved survival is more difficult to demonstrate because the control groups in studies receive imatinib and have 10-year survival rates of about 90%.10–12
With the tyrosine kinase inhibitors, CML can be regarded as functionally cured.13 Patients take these drugs for life and usually experience a relapse if they stop. Patients with CML are now more likely to die of a comorbidity than of CML.
Choose therapy by tolerability
Which tyrosine kinase inhibitor to use depends more on the side-effect profile of the drug than on its efficacy. Nilotinib should be avoided in patients with vascular disease, and dasatinib avoided in patients with pulmonary disease. Each drug may be associated with some degree of nausea, diarrhea, cramps, rash, and edema.10–12
CML is not an immunosuppressive disease, nor are the drugs used to treat it. Patients with CML have an intact immune system. Therefore, precautions taken for patients with acute leukemia or lymphoid malignancy are not required for patients with CML.
Managing survivors
Since imatinib was introduced in 2000, the US Food and Drug Administration (FDA) has approved approximately 20 tyrosine kinase inhibitors for various cancers. These drugs are improving survival rates so well that patients with cancer are increasingly being seen by their primary care doctors for their medical problems.
Some problems have emerged that are consequences of this successful therapy. Angiogenesis inhibitors such as bevacizumab affect vascular endothelial growth factors, which injure endothelial cells. These effects may result in high blood pressure and arterial occlusive disease. Algorithms have been proposed for managing cardiovascular complications for patients taking tyrosine kinase inhibitors.14 Further, cardiovascular risk factors such as hyperlipidemia, diabetes, and obesity must be aggressively managed in patients taking tyrosine kinase inhibitors.
Vascular effects, rashes, and drug interactions may best be managed by primary care physicians, cardiologists, and nephrologists, who deal with such problems regularly.
CHRONIC LYMPHOCYTIC LEUKEMIA
A patient undergoes routine laboratory blood work in the emergency department or clinic, with these results:
- White blood cell count 250 × 109/L
- Neutrophils 1%
- Lymphocytes 99%
- Hemoglobin 12.1 g/dL
- Platelet count 160 × 109/L.
Like patients with CML, those with CLL usually present with no symptoms. The complete blood cell count reveals numerous white blood cells and lymphocytosis. Patients may have painless lymphadenopathy, anemia, and thrombocytopenia, but they do not typically have fever, sweats, or weight loss.
The disease is characterized by clonal proliferation and accumulation of mature-appearing neoplastic B lymphocytes in the blood, bone marrow, lymph nodes, and spleen. The peripheral blood smear shows “smudge cells,” indicating fragile lymphocytes.
The median age at diagnosis is about 70, with fewer than 15% of newly diagnosed patients under age 50.
CLL is the most common leukemia in the Western world, accounting for about 30% of cases of leukemia in adults. It is rare in Asians, probably because of genetic differences.
Monoclonal B-cell lymphocytosis precedes CLL
Monoclonal B-cell lymphocytosis is related to CLL and always precedes it. It is a common condition, detectable in up to 5% of older adults. The differential count shows a less severe lymphocytosis than in CLL.
Because monoclonal B-cell lymphocytosis does not always convert to leukemia, it is important for insurance coverage purposes not to diagnose it as a leukemia. Treatment-free survival of patients diagnosed with monoclonal B-cell lymphocytosis is 87% at 5 years.15,16
Diagnosing CLL
Lymphocytosis can indicate other low-grade lymphoproliferative diseases and malignancies, so further evaluation is critical. To diagnose CLL, the B-cell count by flow cytometry (not the absolute lymphocyte count from the complete blood cell count) must be at least 5 × 109/L. Below that threshold, monoclonal B-cell lymphocytosis is diagnosed unless lymphadenopathy is present, indicating small lymphocytic lymphoma. Unlike in benign lymphoproliferations, CLL lymphocytes coexpress the B-cell marker CD19 and the T-cell marker CD5.17 Bone marrow examination is rarely needed for the diagnosis of CLL.
Two types of CLL can be defined, depending on whether the B cells carry V genes that are mutated or unmutated. B cells expressing ZAP-70 and CD38 tend to carry the unmutated gene, which is associated with a worse prognosis.18 Regardless of which type a patient has, treatments and the indications for treatment are the same.
Increasing immune dysfunction
CLL is staged according to effects on lymph tissue and hematopoiesis. The Rai system for clinical staging of CLL has been used since 1975 with little alteration (Table 2).19
CLL is often an indolent lymphoproliferative malignancy and does not always progress to a fatal end stage. Therefore, treatment may be deferred, with a watch-and-wait approach until symptoms develop or the disease progresses. Approximately half of patients never require treatment.20 Progression involves increasing bone marrow impairment with greater susceptibility to infection (due to intrinsic features of CLL and its therapy) and hypogammaglobulinemia in advanced disease.21,22 Systemic infection is the cause of death for most patients.
Because CLL is a disease of the immune system, the development of autoantibodies is a cardinal feature. Autoimmune complications are almost exclusively limited to blood and can include hemolytic anemia, pure red cell aplasia, immune-mediated thrombocytopenia, and granulocytopenia. Other autoimmune diseases, such as rheumatoid arthritis, thyroiditis, and Addison disease, are uncommon.23,24
Other complications may occur in patients who have been treated with chemotherapy, and these are usually fatal. The Richter transformation (to an aggressive lymphoma) occurs in about 15%. Other less common complications include prolymphocytoid transformation and secondary malignancies, particularly carcinomas of the lung and gastrointestinal tract and acute (myeloid) leukemia.25
Survival rates in CLL have improved substantially over the past decades,26–28 with significant gains following the introduction of antibiotics and, to a lesser extent, transfusions. Median survival is generally between 6 and 9 years, but many patients live for years without requiring therapy.
Chemotherapy: The mainstay of treatment
When to begin therapy remains one of the most challenging issues of patient management. Unlike in CML, there is no advantage to starting at diagnosis when most patients are asymptomatic.29
In 1996, the National Cancer Institute issued guidelines for starting treatment, which were updated in 2008 with very little change (Table 3).30 In general, the onset of symptoms and evidence of impaired marrow function, including an abnormal hemoglobin level and platelet count, are indications. The white blood cell count continuously increases during the disease course but is not usually an important factor for initiating treatment.
The therapeutic goal for most patients who require treatment has historically been palliation of symptoms. Therapy must be individualized to a patient’s age and clinical status, with a heavier reliance on chemotherapeutic agents for patients who can tolerate it and on immunotherapy for others. General strategies are as follows:
- “Go-Go” patients—young, fit, with few comorbidities, good renal function—are the minority. Recommendation: combination chemotherapy with fludarabine, cyclophosphamide, and rituximab (FCR).
- “Slo-Go” patients are reasonably fit and can tolerate chemotherapy but not FCR. Recommendation: combination therapy with either bendamustine and rituximab or chlorambucil and rituximab (for less fit patients). Recent evidence indicates ibrutinib may be useful for such patients.31
- “No-Go” patients are frail with short life expectancy. Recommendation: rituximab or observation (see below)
All CLL treatments are potentially toxic. Chemotherapy damages DNA and often causes blood cell counts to fall. Immunosuppression worsens with almost any treatment, involving a substantial risk of secondary malignancy. Although survival improves with therapy, relapse is universal.
Targeting CLL pathways
The new paradigm for cancer therapy is to identify a cellular pathway that drives oncogenesis or proliferation and interfere with it. The B-cell receptor pathway is enormously complex with numerous complex factors, making it difficult to discern the critical mutation that drives the proliferation of lymphocytes.
Bruton tyrosine kinase (Btk) is one factor that is critical for CLL proliferation. Patients with congenitally mutated or dysfunctional Btk have lymphopenia and agammaglobulinemia, making it a promising target for patients with B-cell disorders. Other experimental therapies are based on other such identified factors.
In 2014, the FDA approved two drugs for CLL—ibrutinib, a Btk inhibitor, and idelalisib, an inhibitor of phosphoinositide 3-kinase—after they were shown in clinical trials to dramatically improve outcomes in patients with relapsed CLL.32,33 Trials with these drugs are ongoing. These drugs also inhibit tyrosine kinase and so have vascular side effects in addition to their own idiosyncratic effects.
Ibrutinib has anticoagulant effects and should be stopped before surgery. It also can cause or exacerbate atrial fibrillation, making management of CLL difficult. It is associated with hypogammaglobulinemia, often requiring ongoing immunoglobulin replacement.
Idelalisib tends to cause systemic autoimmune phenomena such as pneumonitis and colitis.
Using T cells as therapy
It has long been observed that patients who undergo bone marrow transplant for leukemia have lower relapse rates if the transplant is allogeneic rather than from a twin. Further, if T cells are removed from the donor graft, graft-vs-host disease may be prevented but the risk of relapses increases. Finally, the presence of graft-vs-host disease tends to reduce the risk of relapse.34 Therefore, T cells clearly are key ingredients for success in the setting of bone marrow transplant. In fact, merely providing T cells for a relapse after allogeneic transplant can induce remission. However, because donor T cells are not targeted, acute and chronic graft-vs-host disease often can ensue.
‘Designer’ monoclonal antibodies
The B lymphocyte has multiple potential targets for new therapies for CLL as well as other cancers involving B cells. CD20 was identified on the surface of B cells in 1988 and is the target protein of the monoclonal antibody drug rituximab. Monoclonal antibodies can be modified to target other surface antigens, to link radioisotopes to deliver radiation therapy, and to deliver drugs that would otherwise be too toxic to be given systemically.35 Monoclonal antibodies can also be modified to enhance function.
Antibodies alone, however, must often rely on the host T cells for cytotoxicity and they are often compromised by either the underlying disease or treatment. Adapting the targeting function of antibodies to enhance or genetically alter T cells to recognize cancer-specific antigens is now being explored for leukemias.36
In 2014, the FDA approved blinatumomab for the treatment of relapsed or refractory acute lymphoblastic leukemia. This biopharmaceutical agent recruits T cells with one antibody-like moiety and targets the CD19 receptor of B cells with another. Given as a single intravenous treatment without chemotherapy, it has an almost 50% response rate, and those who respond tend to stay in remission. Other similar drugs are being developed, and using them earlier in treatment and for other B-cell leukemias is being explored.
New B-cell targeted therapy with CAR-Ts
Newer treatments are being developed based on chimeric antigen receptor T (CAR-T) cells. These engineered T cells express an anti-CD19 moiety that targets B cells, but also activate upon binding to them.37 CAR-T technology is being refined and shows great promise for cancer treatment.
Multiple clinical trials are currently under way in which the investigators collect autologous T cells by leukopheresis from a patient with a relapsed or refractory B-cell malignancy, transduce the T cells with retroviral vectors into anti-CD19 CAR-T cells, and then reinfuse them into the patient following modest chemotherapy.38
Study results from a small number of patients with relapsing or refractory CLL showed that some patients achieved long-term, progression-free survival.39 The most success with this therapy, however, has been in acute lymphoblastic leukemia.40 Possibly, this treatment could be applied to other lymphoid malignancies that also express CD19.
More advances
CAR-T cell therapy has drawbacks. The cells attack only the target antigen, which currently limits their use mostly to hematologic malignancies. In addition, autologous T cells are not robust. Also, the use of allogeneic T cells is restricted by their major histocompatibility complex, and the cells will be rejected by the recipient if not matched.
An attempt to overcome some of these drawbacks is to develop T cells redirected for universal cytokine killing. CAR-T cells are modified with a gene that causes them to excrete interleukin 12, which attracts macrophages and natural killer cells to the environment to better fight the tumor.41
Other modifications include editing out certain genes including the major histocompatibility complex, which avoids the problem of rejection. Another modification is to insert a “suicide gene” that allows the engineered T cells to be killed with an antidote if they do not work as planned.
Such gene-editing techniques hold great promise for curing cancers without chemotherapy in the not so distant future.
- National Cancer Institute Surveillance, Epidemiology, and End Results Program. SEER Stat Fact Sheets: Chronic Myeloid Leukemia. http://seer.cancer.gov/statfacts/html/cmyl.html. Accessed July 1, 2016.
- Nowell PC, Hungerford DA. A minute chromosome in human chronic granulocytic leukemia. Science 1960; 132:1497.
- Melo JV. The diversity of BCR-ABL fusion proteins and their relationship to leukemia phenotype. Blood 1996; 88:2375–2384.
- Pasternak G, Hochhaus A, Schultheis B, Hehlmann R. Chronic myelogenous leukemia: molecular and cellular aspects. J Cancer Res Clin Oncol 1998; 124:643–660.
- Faderl S, Kantarjian HM, Talpaz M. Chronic myelogenous leukemia: update on biology and treatment. Oncology (Williston Park) 1999; 13:169–184.
- Sawyers CL. Chronic myeloid leukemia. N Engl J Med 1999; 340:1330–1340.
- Hehlmann R, Heimpel H, Hasford J, et al. Randomized comparison of interferon-alpha with busulfan and hydroxyurea in chronic myelogenous leukemia. The German CML Study Group. Blood 1994; 84:4064–4077.
- Radich JP, Olavarria E, Apperley JF. Allogeneic hematopoietic stem cell transplantation for chronic myeloid leukemia. Hematol Oncol Clin North Am 2004; 18:685–702.
- Druker BJ. Translation of the Philadelphia chromosome into therapy for CML. Blood 2008; 112:4808–4817.
- O’Brien SG, Guilhot F, Larson RA, et al; IRIS Investigators. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med 2003; 348:994-1004.
- Kantarjian H, Shah NP, Hochhaus A, et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med 2010; 362:2260–2270.
- Saglio G, Kim DW, Issaragrisil S, et al; ENESTnd Investigators. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med 2010; 362:2251–2259.
- Pfirrmann M, Baccarani M, Saussele S, et al. Prognosis of long-term survival considering disease-specific death in patients with chronic myeloid leukemia. Leukemia 2016; 30:48-56.
- Li W, Croce K, Steensma DP, McDermott DF, Ben-Yehuda O, Moslehi J. Vascular and metabolic implications of novel targeted cancer therapies: focus on kinase inhibitors. J Am Coll Cardiol 2015; 66:1160–1178.
- Rawstron AC, Bennett F, Hillmen P. The biological and clinical relationship between CD5+23+ monoclonal B-cell lymphocytosis and chronic lymphocytic leukaemia. Br J Haematol 2007; 139:724–729.
- Rawstron AC, Bennett FL, O’Connor SJ, et al. Monoclonal B-cell lymphocytosis and chronic lymphocytic leukemia. N Engl J Med 2008; 359:575–583.
- Hallek M, Cheson BD, Catovsky D, et al; International Workshop on Chronic Lymphocytic Leukemia. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood 2008; 111:5446–5456.
- Chiorazzi N, Rai KR, Ferrarini M. Chronic lymphocytic leukemia. N Engl J Med 2005; 352:804–815.
- Rai KR, Sawitsky A, Cronkite EP, Chanana AD, Levy RN, Pasternack BS. Clinical staging of chronic lymphocytic leukemia. Blood 1975; 46:219–234.
- Dierlamm J, Michaux L, Criel A, Wlodarska I, Van den Berghe H, Hossfeld DK. Genetic abnormalities in chronic lymphocytic leukemia and their clinical and prognostic implications. Cancer Genet Cytogenet 1997; 94:27–35.
- Rozman C, Montserrat E. Chronic lymphocytic leukemia. N Engl J Med 1995; 333:1052–1057. Erratum in: N Engl J Med 1995; 333:1515.
- Jemal A, Thomas A, Murray T, Thun M. Cancer statistics, 2002. CA Cancer J Clin 2002; 52:23-47. Errata in: CA Cancer J Clin 2002; 52:119. CA Cancer J Clin 2002; 52:181–182.
- Caligaris-Cappio F, Hamblin TJ. B-cell chronic lymphocytic leukemia: a bird of a different feather. J Clin Oncol 1999; 17:399–408.
- Keating MJ. Chronic lymphocytic leukemia. Semin Oncol 1999; 26(suppl 14):107–114.
- Kalil N, Cheson BD. Management of chronic lymphocytic leukaemia. Drugs Aging 2000; 16:9–27.
- Minot GR, Buckman TE, Isaacs R. Chronic myelogenous leukemia: age incidence, duration, and benefit derived from irradiation. JAMA 1924; 82:1489–1494.
- Reinhard EH, Neely CL, Samples DM. Radioactive phosphorus in the treatment of chronic leukemias: long-term results over a period of 15 years. Cancer 1959; 50:942–958.
- Diehl LF, Karnell LH, Menck HR. The American College of Surgeons Commission on Cancer and the American Cancer Society. The National Cancer Data Base report on age, gender, treatment, and outcomes of patients with chronic lymphocytic leukemia. Cancer 1999; 86:2684–2692.
- Chemotherapeutic options in chronic lymphocytic leukemia: a meta-analysis of the randomized trials. CLL Trialists’ Collaborative Group. J Natl Cancer Inst 1999; 91:861–868.
- Cheson BD, Bennett JM, Grever M, et al. National Cancer Institute-sponsored working group guidelines for chronic lymphocytic leukemia: revised guidelines for diagnosis and treatment. Blood 1996; 87:4990–4997.
- Burger JA, Tedeschi A, Barr PM, et al; RESONATE-2 Investigators. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med 2015; 373:2425–2437.
- Byrd JC, Brown JR, O’Brien S, et al; RESONATE Investigators. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med 2014; 371:213–223.
- Furman RR, Sharman JP, Coutre SE, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med 2014; 370:997–1007.
- Horowitz MM, Gale RP, Sondel PM, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood 1990; 75:555–562.
- Weiner GJ. Building better monoclonal antibody-based therapeutics. Nat Rev Cancer 2015; 15:361–370.
- Kershaw MH, Westwood JA, Darcy PK. Gene-engineered T cells for cancer therapy. Nat Rev Cancer 2013; 13:525–541.
- Urba WJ, Longo DL. Redirecting T cells. N Engl J Med 2011; 365:754–757.
- Klebanoff CA, Yamamoto TN, Restifo NP. Immunotherapy: treatment of aggressive lymphomas with anti-CD19 CAR T cells. Nat Rev Clin Oncol 2014; 11:685-686.
- Porter DL, Hwang WT, Frey NV, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med 2015; 7:303ra139.
- Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet 2015; 385:517–528.
- Chmielewski M, Hombach AA, Abken H. Of CARs and TRUCKs: chimeric antigen receptor (CAR) T cells engineered with an inducible cytokine to modulate the tumor stroma. Immunol Rev 2014; 257:83–90.
The advent of targeted therapies has dramatically changed the management of chronic leukemia. Chemotherapy—highly toxic, nonspecific drugs that can be dangerous to patients and providers and result in only modest success—is gradually being replaced by biologic targeting of malignancy. Scientists are rapidly identifying extracellular and intracellular targets on tumor cells and are developing and testing promising new therapies aimed at these targets. Survival of cancer patients has become so common that clinicians outside the specialties of hematology and oncology are now caring for them.
This article describes new biologic therapies for chronic myelogenous leukemia (CML) and chronic lymphocytic leukemia (CLL), along with the diagnosis of these diseases and management of survivors in the primary care setting.
CHRONIC MYELOGENOUS LEUKEMIA
A seemingly healthy person needs laboratory blood work, perhaps for an insurance physical examination or for a preoperative workup. Or a patient comes to the emergency department with a sore throat and routine blood tests are ordered. Their laboratory values:
- White blood cell count 250 × 109/L (reference range 3–11)
- Neutrophils 70% (40%–70%)
- Blasts 1% (0)
- Metacytes and myelocytes 5% (0)
- Bands 5% (0)
- Lymphocytes 10% (22%–40%)
- Monocytes 5% (0–7%)
- Basophils 3% (0–1%)
- Eosinophils 1% (0–4%)
- Hemoglobin 12.1 g/dL (11.5–15.5 in women, 13.0–17.0 in men)
- Platelet count 525 × 109/L (150–400).
Leukocytosis and a ‘left shift’
Although this scenario often raises concern for acute leukemia, a careful look shows evidence of a chronic myeloproliferative disorder instead. Specifically, this patient’s laboratory values show a “left shift”—an increase in immature neutrophils, ie, blasts, myelocytes, and bands.
This picture is characteristic of CML, an uncommon leukemia with about 4,500 new cases annually in the United States. Patients can present at any age, but the disease occurs more often in older people, with a median age of 66.1
The presentation is usually subtle: about half of cases are detected by routine laboratory testing, which typically reveals a left-shifted leukocytosis with basophilia and a few blasts. Mild anemia is common. The platelet count is elevated in 30% to 50% of patients at diagnosis. Bone marrow aspirate shows significant myeloid hyperplasia without dysplasia, and sometimes shows mild fibrosis.
Philadelphia chromosome is diagnostic
A definitive diagnosis is made by demonstration of an abnormally short chromosome 22. Described in 1960 by Peter Nowell of the University of Pennsylvania and David Hugerford of the Institute for Cancer Research,2 this abnormality, called the Philadelphia chromosome, was the first specific genetic abnormality associated with a human cancer. Later, researchers used banding techniques to find that the Philadelphia chromosome results from a reciprocal translocation of genetic material between the BCR gene on chromosome 22 and the ABL1 gene on chromosome 9, t(9:22).3,4 The resulting chimeric gene, called BCR-ABL, codes for an oncogenic protein, a tyrosine kinase with constitutive activity.
The Philadelphia chromosome is present in 95% of patients with CML and can be found in all myeloid cell lineages, including erythrocytes, granulocytes, monocytes, and megakaryocytes as well as some cells of lymphocytic lineage, indicating that malignant transformation to CML takes place at the stem cell level.
The mutation causes several problems: the abnormal tyrosine kinase increases cell proliferation, inhibits apoptosis, and alters adhesion molecules in the stroma of the bone marrow, allowing immature cells to leak into the bloodstream. Most important, the mutation increases genomic instability so that additional mutations are likelier to occur over time, making it inevitable that, without treatment, the disease will progress to a fatal blast crisis within an average of 5 years of diagnosis.
CML has three clinical phases
Untreated, CML progresses through three distinct phases: chronic, accelerated, and blast crisis, defined by abnormalities in the blood smear and bone marrow (Table 1).5,6 Most patients (85%) are diagnosed during the chronic phase. The accelerated and blastic phases resemble acute leukemia.
Chronic phase management
Therapies over the years have included arsenic (Fowler solution), splenic radiotherapy, busulfan, hydroxyurea, cytarabine, and interferon. All had some palliative success, but usually did not suppress leukemic progression.7
In contrast, patients undergoing allogeneic bone marrow transplant had a 5-year survival rate of 60% to 80% during the chronic phase of CML, 40% to 60% during the accelerated phase, and 10% to 20% during a blast crisis.8 Long-term survival confirmed the ability of transplant to cure CML, and bone marrow transplant with matched donors was the standard of care for younger patients until the end of the 20th century.
Tyrosine kinase inhibition
A new paradigm in treatment began with the development of imatinib, a tyrosine kinase inhibitor that directly interferes with the product of the chimeric BCR-ABL gene.9
Patients treated with imatinib during the chronic phase of CML have survival rates similar to those of people without the disease, and they usually do not progress to the accelerated and blast phases. As a result of this success, the number of transplants for CML has fallen precipitously.
Other tyrosine kinase inhibitors (dasatinib, nilotinib) that have since been developed have shown even better results in achieving remission and preventing progression. Improved survival is more difficult to demonstrate because the control groups in studies receive imatinib and have 10-year survival rates of about 90%.10–12
With the tyrosine kinase inhibitors, CML can be regarded as functionally cured.13 Patients take these drugs for life and usually experience a relapse if they stop. Patients with CML are now more likely to die of a comorbidity than of CML.
Choose therapy by tolerability
Which tyrosine kinase inhibitor to use depends more on the side-effect profile of the drug than on its efficacy. Nilotinib should be avoided in patients with vascular disease, and dasatinib avoided in patients with pulmonary disease. Each drug may be associated with some degree of nausea, diarrhea, cramps, rash, and edema.10–12
CML is not an immunosuppressive disease, nor are the drugs used to treat it. Patients with CML have an intact immune system. Therefore, precautions taken for patients with acute leukemia or lymphoid malignancy are not required for patients with CML.
Managing survivors
Since imatinib was introduced in 2000, the US Food and Drug Administration (FDA) has approved approximately 20 tyrosine kinase inhibitors for various cancers. These drugs are improving survival rates so well that patients with cancer are increasingly being seen by their primary care doctors for their medical problems.
Some problems have emerged that are consequences of this successful therapy. Angiogenesis inhibitors such as bevacizumab affect vascular endothelial growth factors, which injure endothelial cells. These effects may result in high blood pressure and arterial occlusive disease. Algorithms have been proposed for managing cardiovascular complications for patients taking tyrosine kinase inhibitors.14 Further, cardiovascular risk factors such as hyperlipidemia, diabetes, and obesity must be aggressively managed in patients taking tyrosine kinase inhibitors.
Vascular effects, rashes, and drug interactions may best be managed by primary care physicians, cardiologists, and nephrologists, who deal with such problems regularly.
CHRONIC LYMPHOCYTIC LEUKEMIA
A patient undergoes routine laboratory blood work in the emergency department or clinic, with these results:
- White blood cell count 250 × 109/L
- Neutrophils 1%
- Lymphocytes 99%
- Hemoglobin 12.1 g/dL
- Platelet count 160 × 109/L.
Like patients with CML, those with CLL usually present with no symptoms. The complete blood cell count reveals numerous white blood cells and lymphocytosis. Patients may have painless lymphadenopathy, anemia, and thrombocytopenia, but they do not typically have fever, sweats, or weight loss.
The disease is characterized by clonal proliferation and accumulation of mature-appearing neoplastic B lymphocytes in the blood, bone marrow, lymph nodes, and spleen. The peripheral blood smear shows “smudge cells,” indicating fragile lymphocytes.
The median age at diagnosis is about 70, with fewer than 15% of newly diagnosed patients under age 50.
CLL is the most common leukemia in the Western world, accounting for about 30% of cases of leukemia in adults. It is rare in Asians, probably because of genetic differences.
Monoclonal B-cell lymphocytosis precedes CLL
Monoclonal B-cell lymphocytosis is related to CLL and always precedes it. It is a common condition, detectable in up to 5% of older adults. The differential count shows a less severe lymphocytosis than in CLL.
Because monoclonal B-cell lymphocytosis does not always convert to leukemia, it is important for insurance coverage purposes not to diagnose it as a leukemia. Treatment-free survival of patients diagnosed with monoclonal B-cell lymphocytosis is 87% at 5 years.15,16
Diagnosing CLL
Lymphocytosis can indicate other low-grade lymphoproliferative diseases and malignancies, so further evaluation is critical. To diagnose CLL, the B-cell count by flow cytometry (not the absolute lymphocyte count from the complete blood cell count) must be at least 5 × 109/L. Below that threshold, monoclonal B-cell lymphocytosis is diagnosed unless lymphadenopathy is present, indicating small lymphocytic lymphoma. Unlike in benign lymphoproliferations, CLL lymphocytes coexpress the B-cell marker CD19 and the T-cell marker CD5.17 Bone marrow examination is rarely needed for the diagnosis of CLL.
Two types of CLL can be defined, depending on whether the B cells carry V genes that are mutated or unmutated. B cells expressing ZAP-70 and CD38 tend to carry the unmutated gene, which is associated with a worse prognosis.18 Regardless of which type a patient has, treatments and the indications for treatment are the same.
Increasing immune dysfunction
CLL is staged according to effects on lymph tissue and hematopoiesis. The Rai system for clinical staging of CLL has been used since 1975 with little alteration (Table 2).19
CLL is often an indolent lymphoproliferative malignancy and does not always progress to a fatal end stage. Therefore, treatment may be deferred, with a watch-and-wait approach until symptoms develop or the disease progresses. Approximately half of patients never require treatment.20 Progression involves increasing bone marrow impairment with greater susceptibility to infection (due to intrinsic features of CLL and its therapy) and hypogammaglobulinemia in advanced disease.21,22 Systemic infection is the cause of death for most patients.
Because CLL is a disease of the immune system, the development of autoantibodies is a cardinal feature. Autoimmune complications are almost exclusively limited to blood and can include hemolytic anemia, pure red cell aplasia, immune-mediated thrombocytopenia, and granulocytopenia. Other autoimmune diseases, such as rheumatoid arthritis, thyroiditis, and Addison disease, are uncommon.23,24
Other complications may occur in patients who have been treated with chemotherapy, and these are usually fatal. The Richter transformation (to an aggressive lymphoma) occurs in about 15%. Other less common complications include prolymphocytoid transformation and secondary malignancies, particularly carcinomas of the lung and gastrointestinal tract and acute (myeloid) leukemia.25
Survival rates in CLL have improved substantially over the past decades,26–28 with significant gains following the introduction of antibiotics and, to a lesser extent, transfusions. Median survival is generally between 6 and 9 years, but many patients live for years without requiring therapy.
Chemotherapy: The mainstay of treatment
When to begin therapy remains one of the most challenging issues of patient management. Unlike in CML, there is no advantage to starting at diagnosis when most patients are asymptomatic.29
In 1996, the National Cancer Institute issued guidelines for starting treatment, which were updated in 2008 with very little change (Table 3).30 In general, the onset of symptoms and evidence of impaired marrow function, including an abnormal hemoglobin level and platelet count, are indications. The white blood cell count continuously increases during the disease course but is not usually an important factor for initiating treatment.
The therapeutic goal for most patients who require treatment has historically been palliation of symptoms. Therapy must be individualized to a patient’s age and clinical status, with a heavier reliance on chemotherapeutic agents for patients who can tolerate it and on immunotherapy for others. General strategies are as follows:
- “Go-Go” patients—young, fit, with few comorbidities, good renal function—are the minority. Recommendation: combination chemotherapy with fludarabine, cyclophosphamide, and rituximab (FCR).
- “Slo-Go” patients are reasonably fit and can tolerate chemotherapy but not FCR. Recommendation: combination therapy with either bendamustine and rituximab or chlorambucil and rituximab (for less fit patients). Recent evidence indicates ibrutinib may be useful for such patients.31
- “No-Go” patients are frail with short life expectancy. Recommendation: rituximab or observation (see below)
All CLL treatments are potentially toxic. Chemotherapy damages DNA and often causes blood cell counts to fall. Immunosuppression worsens with almost any treatment, involving a substantial risk of secondary malignancy. Although survival improves with therapy, relapse is universal.
Targeting CLL pathways
The new paradigm for cancer therapy is to identify a cellular pathway that drives oncogenesis or proliferation and interfere with it. The B-cell receptor pathway is enormously complex with numerous complex factors, making it difficult to discern the critical mutation that drives the proliferation of lymphocytes.
Bruton tyrosine kinase (Btk) is one factor that is critical for CLL proliferation. Patients with congenitally mutated or dysfunctional Btk have lymphopenia and agammaglobulinemia, making it a promising target for patients with B-cell disorders. Other experimental therapies are based on other such identified factors.
In 2014, the FDA approved two drugs for CLL—ibrutinib, a Btk inhibitor, and idelalisib, an inhibitor of phosphoinositide 3-kinase—after they were shown in clinical trials to dramatically improve outcomes in patients with relapsed CLL.32,33 Trials with these drugs are ongoing. These drugs also inhibit tyrosine kinase and so have vascular side effects in addition to their own idiosyncratic effects.
Ibrutinib has anticoagulant effects and should be stopped before surgery. It also can cause or exacerbate atrial fibrillation, making management of CLL difficult. It is associated with hypogammaglobulinemia, often requiring ongoing immunoglobulin replacement.
Idelalisib tends to cause systemic autoimmune phenomena such as pneumonitis and colitis.
Using T cells as therapy
It has long been observed that patients who undergo bone marrow transplant for leukemia have lower relapse rates if the transplant is allogeneic rather than from a twin. Further, if T cells are removed from the donor graft, graft-vs-host disease may be prevented but the risk of relapses increases. Finally, the presence of graft-vs-host disease tends to reduce the risk of relapse.34 Therefore, T cells clearly are key ingredients for success in the setting of bone marrow transplant. In fact, merely providing T cells for a relapse after allogeneic transplant can induce remission. However, because donor T cells are not targeted, acute and chronic graft-vs-host disease often can ensue.
‘Designer’ monoclonal antibodies
The B lymphocyte has multiple potential targets for new therapies for CLL as well as other cancers involving B cells. CD20 was identified on the surface of B cells in 1988 and is the target protein of the monoclonal antibody drug rituximab. Monoclonal antibodies can be modified to target other surface antigens, to link radioisotopes to deliver radiation therapy, and to deliver drugs that would otherwise be too toxic to be given systemically.35 Monoclonal antibodies can also be modified to enhance function.
Antibodies alone, however, must often rely on the host T cells for cytotoxicity and they are often compromised by either the underlying disease or treatment. Adapting the targeting function of antibodies to enhance or genetically alter T cells to recognize cancer-specific antigens is now being explored for leukemias.36
In 2014, the FDA approved blinatumomab for the treatment of relapsed or refractory acute lymphoblastic leukemia. This biopharmaceutical agent recruits T cells with one antibody-like moiety and targets the CD19 receptor of B cells with another. Given as a single intravenous treatment without chemotherapy, it has an almost 50% response rate, and those who respond tend to stay in remission. Other similar drugs are being developed, and using them earlier in treatment and for other B-cell leukemias is being explored.
New B-cell targeted therapy with CAR-Ts
Newer treatments are being developed based on chimeric antigen receptor T (CAR-T) cells. These engineered T cells express an anti-CD19 moiety that targets B cells, but also activate upon binding to them.37 CAR-T technology is being refined and shows great promise for cancer treatment.
Multiple clinical trials are currently under way in which the investigators collect autologous T cells by leukopheresis from a patient with a relapsed or refractory B-cell malignancy, transduce the T cells with retroviral vectors into anti-CD19 CAR-T cells, and then reinfuse them into the patient following modest chemotherapy.38
Study results from a small number of patients with relapsing or refractory CLL showed that some patients achieved long-term, progression-free survival.39 The most success with this therapy, however, has been in acute lymphoblastic leukemia.40 Possibly, this treatment could be applied to other lymphoid malignancies that also express CD19.
More advances
CAR-T cell therapy has drawbacks. The cells attack only the target antigen, which currently limits their use mostly to hematologic malignancies. In addition, autologous T cells are not robust. Also, the use of allogeneic T cells is restricted by their major histocompatibility complex, and the cells will be rejected by the recipient if not matched.
An attempt to overcome some of these drawbacks is to develop T cells redirected for universal cytokine killing. CAR-T cells are modified with a gene that causes them to excrete interleukin 12, which attracts macrophages and natural killer cells to the environment to better fight the tumor.41
Other modifications include editing out certain genes including the major histocompatibility complex, which avoids the problem of rejection. Another modification is to insert a “suicide gene” that allows the engineered T cells to be killed with an antidote if they do not work as planned.
Such gene-editing techniques hold great promise for curing cancers without chemotherapy in the not so distant future.
The advent of targeted therapies has dramatically changed the management of chronic leukemia. Chemotherapy—highly toxic, nonspecific drugs that can be dangerous to patients and providers and result in only modest success—is gradually being replaced by biologic targeting of malignancy. Scientists are rapidly identifying extracellular and intracellular targets on tumor cells and are developing and testing promising new therapies aimed at these targets. Survival of cancer patients has become so common that clinicians outside the specialties of hematology and oncology are now caring for them.
This article describes new biologic therapies for chronic myelogenous leukemia (CML) and chronic lymphocytic leukemia (CLL), along with the diagnosis of these diseases and management of survivors in the primary care setting.
CHRONIC MYELOGENOUS LEUKEMIA
A seemingly healthy person needs laboratory blood work, perhaps for an insurance physical examination or for a preoperative workup. Or a patient comes to the emergency department with a sore throat and routine blood tests are ordered. Their laboratory values:
- White blood cell count 250 × 109/L (reference range 3–11)
- Neutrophils 70% (40%–70%)
- Blasts 1% (0)
- Metacytes and myelocytes 5% (0)
- Bands 5% (0)
- Lymphocytes 10% (22%–40%)
- Monocytes 5% (0–7%)
- Basophils 3% (0–1%)
- Eosinophils 1% (0–4%)
- Hemoglobin 12.1 g/dL (11.5–15.5 in women, 13.0–17.0 in men)
- Platelet count 525 × 109/L (150–400).
Leukocytosis and a ‘left shift’
Although this scenario often raises concern for acute leukemia, a careful look shows evidence of a chronic myeloproliferative disorder instead. Specifically, this patient’s laboratory values show a “left shift”—an increase in immature neutrophils, ie, blasts, myelocytes, and bands.
This picture is characteristic of CML, an uncommon leukemia with about 4,500 new cases annually in the United States. Patients can present at any age, but the disease occurs more often in older people, with a median age of 66.1
The presentation is usually subtle: about half of cases are detected by routine laboratory testing, which typically reveals a left-shifted leukocytosis with basophilia and a few blasts. Mild anemia is common. The platelet count is elevated in 30% to 50% of patients at diagnosis. Bone marrow aspirate shows significant myeloid hyperplasia without dysplasia, and sometimes shows mild fibrosis.
Philadelphia chromosome is diagnostic
A definitive diagnosis is made by demonstration of an abnormally short chromosome 22. Described in 1960 by Peter Nowell of the University of Pennsylvania and David Hugerford of the Institute for Cancer Research,2 this abnormality, called the Philadelphia chromosome, was the first specific genetic abnormality associated with a human cancer. Later, researchers used banding techniques to find that the Philadelphia chromosome results from a reciprocal translocation of genetic material between the BCR gene on chromosome 22 and the ABL1 gene on chromosome 9, t(9:22).3,4 The resulting chimeric gene, called BCR-ABL, codes for an oncogenic protein, a tyrosine kinase with constitutive activity.
The Philadelphia chromosome is present in 95% of patients with CML and can be found in all myeloid cell lineages, including erythrocytes, granulocytes, monocytes, and megakaryocytes as well as some cells of lymphocytic lineage, indicating that malignant transformation to CML takes place at the stem cell level.
The mutation causes several problems: the abnormal tyrosine kinase increases cell proliferation, inhibits apoptosis, and alters adhesion molecules in the stroma of the bone marrow, allowing immature cells to leak into the bloodstream. Most important, the mutation increases genomic instability so that additional mutations are likelier to occur over time, making it inevitable that, without treatment, the disease will progress to a fatal blast crisis within an average of 5 years of diagnosis.
CML has three clinical phases
Untreated, CML progresses through three distinct phases: chronic, accelerated, and blast crisis, defined by abnormalities in the blood smear and bone marrow (Table 1).5,6 Most patients (85%) are diagnosed during the chronic phase. The accelerated and blastic phases resemble acute leukemia.
Chronic phase management
Therapies over the years have included arsenic (Fowler solution), splenic radiotherapy, busulfan, hydroxyurea, cytarabine, and interferon. All had some palliative success, but usually did not suppress leukemic progression.7
In contrast, patients undergoing allogeneic bone marrow transplant had a 5-year survival rate of 60% to 80% during the chronic phase of CML, 40% to 60% during the accelerated phase, and 10% to 20% during a blast crisis.8 Long-term survival confirmed the ability of transplant to cure CML, and bone marrow transplant with matched donors was the standard of care for younger patients until the end of the 20th century.
Tyrosine kinase inhibition
A new paradigm in treatment began with the development of imatinib, a tyrosine kinase inhibitor that directly interferes with the product of the chimeric BCR-ABL gene.9
Patients treated with imatinib during the chronic phase of CML have survival rates similar to those of people without the disease, and they usually do not progress to the accelerated and blast phases. As a result of this success, the number of transplants for CML has fallen precipitously.
Other tyrosine kinase inhibitors (dasatinib, nilotinib) that have since been developed have shown even better results in achieving remission and preventing progression. Improved survival is more difficult to demonstrate because the control groups in studies receive imatinib and have 10-year survival rates of about 90%.10–12
With the tyrosine kinase inhibitors, CML can be regarded as functionally cured.13 Patients take these drugs for life and usually experience a relapse if they stop. Patients with CML are now more likely to die of a comorbidity than of CML.
Choose therapy by tolerability
Which tyrosine kinase inhibitor to use depends more on the side-effect profile of the drug than on its efficacy. Nilotinib should be avoided in patients with vascular disease, and dasatinib avoided in patients with pulmonary disease. Each drug may be associated with some degree of nausea, diarrhea, cramps, rash, and edema.10–12
CML is not an immunosuppressive disease, nor are the drugs used to treat it. Patients with CML have an intact immune system. Therefore, precautions taken for patients with acute leukemia or lymphoid malignancy are not required for patients with CML.
Managing survivors
Since imatinib was introduced in 2000, the US Food and Drug Administration (FDA) has approved approximately 20 tyrosine kinase inhibitors for various cancers. These drugs are improving survival rates so well that patients with cancer are increasingly being seen by their primary care doctors for their medical problems.
Some problems have emerged that are consequences of this successful therapy. Angiogenesis inhibitors such as bevacizumab affect vascular endothelial growth factors, which injure endothelial cells. These effects may result in high blood pressure and arterial occlusive disease. Algorithms have been proposed for managing cardiovascular complications for patients taking tyrosine kinase inhibitors.14 Further, cardiovascular risk factors such as hyperlipidemia, diabetes, and obesity must be aggressively managed in patients taking tyrosine kinase inhibitors.
Vascular effects, rashes, and drug interactions may best be managed by primary care physicians, cardiologists, and nephrologists, who deal with such problems regularly.
CHRONIC LYMPHOCYTIC LEUKEMIA
A patient undergoes routine laboratory blood work in the emergency department or clinic, with these results:
- White blood cell count 250 × 109/L
- Neutrophils 1%
- Lymphocytes 99%
- Hemoglobin 12.1 g/dL
- Platelet count 160 × 109/L.
Like patients with CML, those with CLL usually present with no symptoms. The complete blood cell count reveals numerous white blood cells and lymphocytosis. Patients may have painless lymphadenopathy, anemia, and thrombocytopenia, but they do not typically have fever, sweats, or weight loss.
The disease is characterized by clonal proliferation and accumulation of mature-appearing neoplastic B lymphocytes in the blood, bone marrow, lymph nodes, and spleen. The peripheral blood smear shows “smudge cells,” indicating fragile lymphocytes.
The median age at diagnosis is about 70, with fewer than 15% of newly diagnosed patients under age 50.
CLL is the most common leukemia in the Western world, accounting for about 30% of cases of leukemia in adults. It is rare in Asians, probably because of genetic differences.
Monoclonal B-cell lymphocytosis precedes CLL
Monoclonal B-cell lymphocytosis is related to CLL and always precedes it. It is a common condition, detectable in up to 5% of older adults. The differential count shows a less severe lymphocytosis than in CLL.
Because monoclonal B-cell lymphocytosis does not always convert to leukemia, it is important for insurance coverage purposes not to diagnose it as a leukemia. Treatment-free survival of patients diagnosed with monoclonal B-cell lymphocytosis is 87% at 5 years.15,16
Diagnosing CLL
Lymphocytosis can indicate other low-grade lymphoproliferative diseases and malignancies, so further evaluation is critical. To diagnose CLL, the B-cell count by flow cytometry (not the absolute lymphocyte count from the complete blood cell count) must be at least 5 × 109/L. Below that threshold, monoclonal B-cell lymphocytosis is diagnosed unless lymphadenopathy is present, indicating small lymphocytic lymphoma. Unlike in benign lymphoproliferations, CLL lymphocytes coexpress the B-cell marker CD19 and the T-cell marker CD5.17 Bone marrow examination is rarely needed for the diagnosis of CLL.
Two types of CLL can be defined, depending on whether the B cells carry V genes that are mutated or unmutated. B cells expressing ZAP-70 and CD38 tend to carry the unmutated gene, which is associated with a worse prognosis.18 Regardless of which type a patient has, treatments and the indications for treatment are the same.
Increasing immune dysfunction
CLL is staged according to effects on lymph tissue and hematopoiesis. The Rai system for clinical staging of CLL has been used since 1975 with little alteration (Table 2).19
CLL is often an indolent lymphoproliferative malignancy and does not always progress to a fatal end stage. Therefore, treatment may be deferred, with a watch-and-wait approach until symptoms develop or the disease progresses. Approximately half of patients never require treatment.20 Progression involves increasing bone marrow impairment with greater susceptibility to infection (due to intrinsic features of CLL and its therapy) and hypogammaglobulinemia in advanced disease.21,22 Systemic infection is the cause of death for most patients.
Because CLL is a disease of the immune system, the development of autoantibodies is a cardinal feature. Autoimmune complications are almost exclusively limited to blood and can include hemolytic anemia, pure red cell aplasia, immune-mediated thrombocytopenia, and granulocytopenia. Other autoimmune diseases, such as rheumatoid arthritis, thyroiditis, and Addison disease, are uncommon.23,24
Other complications may occur in patients who have been treated with chemotherapy, and these are usually fatal. The Richter transformation (to an aggressive lymphoma) occurs in about 15%. Other less common complications include prolymphocytoid transformation and secondary malignancies, particularly carcinomas of the lung and gastrointestinal tract and acute (myeloid) leukemia.25
Survival rates in CLL have improved substantially over the past decades,26–28 with significant gains following the introduction of antibiotics and, to a lesser extent, transfusions. Median survival is generally between 6 and 9 years, but many patients live for years without requiring therapy.
Chemotherapy: The mainstay of treatment
When to begin therapy remains one of the most challenging issues of patient management. Unlike in CML, there is no advantage to starting at diagnosis when most patients are asymptomatic.29
In 1996, the National Cancer Institute issued guidelines for starting treatment, which were updated in 2008 with very little change (Table 3).30 In general, the onset of symptoms and evidence of impaired marrow function, including an abnormal hemoglobin level and platelet count, are indications. The white blood cell count continuously increases during the disease course but is not usually an important factor for initiating treatment.
The therapeutic goal for most patients who require treatment has historically been palliation of symptoms. Therapy must be individualized to a patient’s age and clinical status, with a heavier reliance on chemotherapeutic agents for patients who can tolerate it and on immunotherapy for others. General strategies are as follows:
- “Go-Go” patients—young, fit, with few comorbidities, good renal function—are the minority. Recommendation: combination chemotherapy with fludarabine, cyclophosphamide, and rituximab (FCR).
- “Slo-Go” patients are reasonably fit and can tolerate chemotherapy but not FCR. Recommendation: combination therapy with either bendamustine and rituximab or chlorambucil and rituximab (for less fit patients). Recent evidence indicates ibrutinib may be useful for such patients.31
- “No-Go” patients are frail with short life expectancy. Recommendation: rituximab or observation (see below)
All CLL treatments are potentially toxic. Chemotherapy damages DNA and often causes blood cell counts to fall. Immunosuppression worsens with almost any treatment, involving a substantial risk of secondary malignancy. Although survival improves with therapy, relapse is universal.
Targeting CLL pathways
The new paradigm for cancer therapy is to identify a cellular pathway that drives oncogenesis or proliferation and interfere with it. The B-cell receptor pathway is enormously complex with numerous complex factors, making it difficult to discern the critical mutation that drives the proliferation of lymphocytes.
Bruton tyrosine kinase (Btk) is one factor that is critical for CLL proliferation. Patients with congenitally mutated or dysfunctional Btk have lymphopenia and agammaglobulinemia, making it a promising target for patients with B-cell disorders. Other experimental therapies are based on other such identified factors.
In 2014, the FDA approved two drugs for CLL—ibrutinib, a Btk inhibitor, and idelalisib, an inhibitor of phosphoinositide 3-kinase—after they were shown in clinical trials to dramatically improve outcomes in patients with relapsed CLL.32,33 Trials with these drugs are ongoing. These drugs also inhibit tyrosine kinase and so have vascular side effects in addition to their own idiosyncratic effects.
Ibrutinib has anticoagulant effects and should be stopped before surgery. It also can cause or exacerbate atrial fibrillation, making management of CLL difficult. It is associated with hypogammaglobulinemia, often requiring ongoing immunoglobulin replacement.
Idelalisib tends to cause systemic autoimmune phenomena such as pneumonitis and colitis.
Using T cells as therapy
It has long been observed that patients who undergo bone marrow transplant for leukemia have lower relapse rates if the transplant is allogeneic rather than from a twin. Further, if T cells are removed from the donor graft, graft-vs-host disease may be prevented but the risk of relapses increases. Finally, the presence of graft-vs-host disease tends to reduce the risk of relapse.34 Therefore, T cells clearly are key ingredients for success in the setting of bone marrow transplant. In fact, merely providing T cells for a relapse after allogeneic transplant can induce remission. However, because donor T cells are not targeted, acute and chronic graft-vs-host disease often can ensue.
‘Designer’ monoclonal antibodies
The B lymphocyte has multiple potential targets for new therapies for CLL as well as other cancers involving B cells. CD20 was identified on the surface of B cells in 1988 and is the target protein of the monoclonal antibody drug rituximab. Monoclonal antibodies can be modified to target other surface antigens, to link radioisotopes to deliver radiation therapy, and to deliver drugs that would otherwise be too toxic to be given systemically.35 Monoclonal antibodies can also be modified to enhance function.
Antibodies alone, however, must often rely on the host T cells for cytotoxicity and they are often compromised by either the underlying disease or treatment. Adapting the targeting function of antibodies to enhance or genetically alter T cells to recognize cancer-specific antigens is now being explored for leukemias.36
In 2014, the FDA approved blinatumomab for the treatment of relapsed or refractory acute lymphoblastic leukemia. This biopharmaceutical agent recruits T cells with one antibody-like moiety and targets the CD19 receptor of B cells with another. Given as a single intravenous treatment without chemotherapy, it has an almost 50% response rate, and those who respond tend to stay in remission. Other similar drugs are being developed, and using them earlier in treatment and for other B-cell leukemias is being explored.
New B-cell targeted therapy with CAR-Ts
Newer treatments are being developed based on chimeric antigen receptor T (CAR-T) cells. These engineered T cells express an anti-CD19 moiety that targets B cells, but also activate upon binding to them.37 CAR-T technology is being refined and shows great promise for cancer treatment.
Multiple clinical trials are currently under way in which the investigators collect autologous T cells by leukopheresis from a patient with a relapsed or refractory B-cell malignancy, transduce the T cells with retroviral vectors into anti-CD19 CAR-T cells, and then reinfuse them into the patient following modest chemotherapy.38
Study results from a small number of patients with relapsing or refractory CLL showed that some patients achieved long-term, progression-free survival.39 The most success with this therapy, however, has been in acute lymphoblastic leukemia.40 Possibly, this treatment could be applied to other lymphoid malignancies that also express CD19.
More advances
CAR-T cell therapy has drawbacks. The cells attack only the target antigen, which currently limits their use mostly to hematologic malignancies. In addition, autologous T cells are not robust. Also, the use of allogeneic T cells is restricted by their major histocompatibility complex, and the cells will be rejected by the recipient if not matched.
An attempt to overcome some of these drawbacks is to develop T cells redirected for universal cytokine killing. CAR-T cells are modified with a gene that causes them to excrete interleukin 12, which attracts macrophages and natural killer cells to the environment to better fight the tumor.41
Other modifications include editing out certain genes including the major histocompatibility complex, which avoids the problem of rejection. Another modification is to insert a “suicide gene” that allows the engineered T cells to be killed with an antidote if they do not work as planned.
Such gene-editing techniques hold great promise for curing cancers without chemotherapy in the not so distant future.
- National Cancer Institute Surveillance, Epidemiology, and End Results Program. SEER Stat Fact Sheets: Chronic Myeloid Leukemia. http://seer.cancer.gov/statfacts/html/cmyl.html. Accessed July 1, 2016.
- Nowell PC, Hungerford DA. A minute chromosome in human chronic granulocytic leukemia. Science 1960; 132:1497.
- Melo JV. The diversity of BCR-ABL fusion proteins and their relationship to leukemia phenotype. Blood 1996; 88:2375–2384.
- Pasternak G, Hochhaus A, Schultheis B, Hehlmann R. Chronic myelogenous leukemia: molecular and cellular aspects. J Cancer Res Clin Oncol 1998; 124:643–660.
- Faderl S, Kantarjian HM, Talpaz M. Chronic myelogenous leukemia: update on biology and treatment. Oncology (Williston Park) 1999; 13:169–184.
- Sawyers CL. Chronic myeloid leukemia. N Engl J Med 1999; 340:1330–1340.
- Hehlmann R, Heimpel H, Hasford J, et al. Randomized comparison of interferon-alpha with busulfan and hydroxyurea in chronic myelogenous leukemia. The German CML Study Group. Blood 1994; 84:4064–4077.
- Radich JP, Olavarria E, Apperley JF. Allogeneic hematopoietic stem cell transplantation for chronic myeloid leukemia. Hematol Oncol Clin North Am 2004; 18:685–702.
- Druker BJ. Translation of the Philadelphia chromosome into therapy for CML. Blood 2008; 112:4808–4817.
- O’Brien SG, Guilhot F, Larson RA, et al; IRIS Investigators. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med 2003; 348:994-1004.
- Kantarjian H, Shah NP, Hochhaus A, et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med 2010; 362:2260–2270.
- Saglio G, Kim DW, Issaragrisil S, et al; ENESTnd Investigators. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med 2010; 362:2251–2259.
- Pfirrmann M, Baccarani M, Saussele S, et al. Prognosis of long-term survival considering disease-specific death in patients with chronic myeloid leukemia. Leukemia 2016; 30:48-56.
- Li W, Croce K, Steensma DP, McDermott DF, Ben-Yehuda O, Moslehi J. Vascular and metabolic implications of novel targeted cancer therapies: focus on kinase inhibitors. J Am Coll Cardiol 2015; 66:1160–1178.
- Rawstron AC, Bennett F, Hillmen P. The biological and clinical relationship between CD5+23+ monoclonal B-cell lymphocytosis and chronic lymphocytic leukaemia. Br J Haematol 2007; 139:724–729.
- Rawstron AC, Bennett FL, O’Connor SJ, et al. Monoclonal B-cell lymphocytosis and chronic lymphocytic leukemia. N Engl J Med 2008; 359:575–583.
- Hallek M, Cheson BD, Catovsky D, et al; International Workshop on Chronic Lymphocytic Leukemia. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood 2008; 111:5446–5456.
- Chiorazzi N, Rai KR, Ferrarini M. Chronic lymphocytic leukemia. N Engl J Med 2005; 352:804–815.
- Rai KR, Sawitsky A, Cronkite EP, Chanana AD, Levy RN, Pasternack BS. Clinical staging of chronic lymphocytic leukemia. Blood 1975; 46:219–234.
- Dierlamm J, Michaux L, Criel A, Wlodarska I, Van den Berghe H, Hossfeld DK. Genetic abnormalities in chronic lymphocytic leukemia and their clinical and prognostic implications. Cancer Genet Cytogenet 1997; 94:27–35.
- Rozman C, Montserrat E. Chronic lymphocytic leukemia. N Engl J Med 1995; 333:1052–1057. Erratum in: N Engl J Med 1995; 333:1515.
- Jemal A, Thomas A, Murray T, Thun M. Cancer statistics, 2002. CA Cancer J Clin 2002; 52:23-47. Errata in: CA Cancer J Clin 2002; 52:119. CA Cancer J Clin 2002; 52:181–182.
- Caligaris-Cappio F, Hamblin TJ. B-cell chronic lymphocytic leukemia: a bird of a different feather. J Clin Oncol 1999; 17:399–408.
- Keating MJ. Chronic lymphocytic leukemia. Semin Oncol 1999; 26(suppl 14):107–114.
- Kalil N, Cheson BD. Management of chronic lymphocytic leukaemia. Drugs Aging 2000; 16:9–27.
- Minot GR, Buckman TE, Isaacs R. Chronic myelogenous leukemia: age incidence, duration, and benefit derived from irradiation. JAMA 1924; 82:1489–1494.
- Reinhard EH, Neely CL, Samples DM. Radioactive phosphorus in the treatment of chronic leukemias: long-term results over a period of 15 years. Cancer 1959; 50:942–958.
- Diehl LF, Karnell LH, Menck HR. The American College of Surgeons Commission on Cancer and the American Cancer Society. The National Cancer Data Base report on age, gender, treatment, and outcomes of patients with chronic lymphocytic leukemia. Cancer 1999; 86:2684–2692.
- Chemotherapeutic options in chronic lymphocytic leukemia: a meta-analysis of the randomized trials. CLL Trialists’ Collaborative Group. J Natl Cancer Inst 1999; 91:861–868.
- Cheson BD, Bennett JM, Grever M, et al. National Cancer Institute-sponsored working group guidelines for chronic lymphocytic leukemia: revised guidelines for diagnosis and treatment. Blood 1996; 87:4990–4997.
- Burger JA, Tedeschi A, Barr PM, et al; RESONATE-2 Investigators. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med 2015; 373:2425–2437.
- Byrd JC, Brown JR, O’Brien S, et al; RESONATE Investigators. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med 2014; 371:213–223.
- Furman RR, Sharman JP, Coutre SE, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med 2014; 370:997–1007.
- Horowitz MM, Gale RP, Sondel PM, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood 1990; 75:555–562.
- Weiner GJ. Building better monoclonal antibody-based therapeutics. Nat Rev Cancer 2015; 15:361–370.
- Kershaw MH, Westwood JA, Darcy PK. Gene-engineered T cells for cancer therapy. Nat Rev Cancer 2013; 13:525–541.
- Urba WJ, Longo DL. Redirecting T cells. N Engl J Med 2011; 365:754–757.
- Klebanoff CA, Yamamoto TN, Restifo NP. Immunotherapy: treatment of aggressive lymphomas with anti-CD19 CAR T cells. Nat Rev Clin Oncol 2014; 11:685-686.
- Porter DL, Hwang WT, Frey NV, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med 2015; 7:303ra139.
- Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet 2015; 385:517–528.
- Chmielewski M, Hombach AA, Abken H. Of CARs and TRUCKs: chimeric antigen receptor (CAR) T cells engineered with an inducible cytokine to modulate the tumor stroma. Immunol Rev 2014; 257:83–90.
- National Cancer Institute Surveillance, Epidemiology, and End Results Program. SEER Stat Fact Sheets: Chronic Myeloid Leukemia. http://seer.cancer.gov/statfacts/html/cmyl.html. Accessed July 1, 2016.
- Nowell PC, Hungerford DA. A minute chromosome in human chronic granulocytic leukemia. Science 1960; 132:1497.
- Melo JV. The diversity of BCR-ABL fusion proteins and their relationship to leukemia phenotype. Blood 1996; 88:2375–2384.
- Pasternak G, Hochhaus A, Schultheis B, Hehlmann R. Chronic myelogenous leukemia: molecular and cellular aspects. J Cancer Res Clin Oncol 1998; 124:643–660.
- Faderl S, Kantarjian HM, Talpaz M. Chronic myelogenous leukemia: update on biology and treatment. Oncology (Williston Park) 1999; 13:169–184.
- Sawyers CL. Chronic myeloid leukemia. N Engl J Med 1999; 340:1330–1340.
- Hehlmann R, Heimpel H, Hasford J, et al. Randomized comparison of interferon-alpha with busulfan and hydroxyurea in chronic myelogenous leukemia. The German CML Study Group. Blood 1994; 84:4064–4077.
- Radich JP, Olavarria E, Apperley JF. Allogeneic hematopoietic stem cell transplantation for chronic myeloid leukemia. Hematol Oncol Clin North Am 2004; 18:685–702.
- Druker BJ. Translation of the Philadelphia chromosome into therapy for CML. Blood 2008; 112:4808–4817.
- O’Brien SG, Guilhot F, Larson RA, et al; IRIS Investigators. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med 2003; 348:994-1004.
- Kantarjian H, Shah NP, Hochhaus A, et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med 2010; 362:2260–2270.
- Saglio G, Kim DW, Issaragrisil S, et al; ENESTnd Investigators. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med 2010; 362:2251–2259.
- Pfirrmann M, Baccarani M, Saussele S, et al. Prognosis of long-term survival considering disease-specific death in patients with chronic myeloid leukemia. Leukemia 2016; 30:48-56.
- Li W, Croce K, Steensma DP, McDermott DF, Ben-Yehuda O, Moslehi J. Vascular and metabolic implications of novel targeted cancer therapies: focus on kinase inhibitors. J Am Coll Cardiol 2015; 66:1160–1178.
- Rawstron AC, Bennett F, Hillmen P. The biological and clinical relationship between CD5+23+ monoclonal B-cell lymphocytosis and chronic lymphocytic leukaemia. Br J Haematol 2007; 139:724–729.
- Rawstron AC, Bennett FL, O’Connor SJ, et al. Monoclonal B-cell lymphocytosis and chronic lymphocytic leukemia. N Engl J Med 2008; 359:575–583.
- Hallek M, Cheson BD, Catovsky D, et al; International Workshop on Chronic Lymphocytic Leukemia. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood 2008; 111:5446–5456.
- Chiorazzi N, Rai KR, Ferrarini M. Chronic lymphocytic leukemia. N Engl J Med 2005; 352:804–815.
- Rai KR, Sawitsky A, Cronkite EP, Chanana AD, Levy RN, Pasternack BS. Clinical staging of chronic lymphocytic leukemia. Blood 1975; 46:219–234.
- Dierlamm J, Michaux L, Criel A, Wlodarska I, Van den Berghe H, Hossfeld DK. Genetic abnormalities in chronic lymphocytic leukemia and their clinical and prognostic implications. Cancer Genet Cytogenet 1997; 94:27–35.
- Rozman C, Montserrat E. Chronic lymphocytic leukemia. N Engl J Med 1995; 333:1052–1057. Erratum in: N Engl J Med 1995; 333:1515.
- Jemal A, Thomas A, Murray T, Thun M. Cancer statistics, 2002. CA Cancer J Clin 2002; 52:23-47. Errata in: CA Cancer J Clin 2002; 52:119. CA Cancer J Clin 2002; 52:181–182.
- Caligaris-Cappio F, Hamblin TJ. B-cell chronic lymphocytic leukemia: a bird of a different feather. J Clin Oncol 1999; 17:399–408.
- Keating MJ. Chronic lymphocytic leukemia. Semin Oncol 1999; 26(suppl 14):107–114.
- Kalil N, Cheson BD. Management of chronic lymphocytic leukaemia. Drugs Aging 2000; 16:9–27.
- Minot GR, Buckman TE, Isaacs R. Chronic myelogenous leukemia: age incidence, duration, and benefit derived from irradiation. JAMA 1924; 82:1489–1494.
- Reinhard EH, Neely CL, Samples DM. Radioactive phosphorus in the treatment of chronic leukemias: long-term results over a period of 15 years. Cancer 1959; 50:942–958.
- Diehl LF, Karnell LH, Menck HR. The American College of Surgeons Commission on Cancer and the American Cancer Society. The National Cancer Data Base report on age, gender, treatment, and outcomes of patients with chronic lymphocytic leukemia. Cancer 1999; 86:2684–2692.
- Chemotherapeutic options in chronic lymphocytic leukemia: a meta-analysis of the randomized trials. CLL Trialists’ Collaborative Group. J Natl Cancer Inst 1999; 91:861–868.
- Cheson BD, Bennett JM, Grever M, et al. National Cancer Institute-sponsored working group guidelines for chronic lymphocytic leukemia: revised guidelines for diagnosis and treatment. Blood 1996; 87:4990–4997.
- Burger JA, Tedeschi A, Barr PM, et al; RESONATE-2 Investigators. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med 2015; 373:2425–2437.
- Byrd JC, Brown JR, O’Brien S, et al; RESONATE Investigators. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med 2014; 371:213–223.
- Furman RR, Sharman JP, Coutre SE, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med 2014; 370:997–1007.
- Horowitz MM, Gale RP, Sondel PM, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood 1990; 75:555–562.
- Weiner GJ. Building better monoclonal antibody-based therapeutics. Nat Rev Cancer 2015; 15:361–370.
- Kershaw MH, Westwood JA, Darcy PK. Gene-engineered T cells for cancer therapy. Nat Rev Cancer 2013; 13:525–541.
- Urba WJ, Longo DL. Redirecting T cells. N Engl J Med 2011; 365:754–757.
- Klebanoff CA, Yamamoto TN, Restifo NP. Immunotherapy: treatment of aggressive lymphomas with anti-CD19 CAR T cells. Nat Rev Clin Oncol 2014; 11:685-686.
- Porter DL, Hwang WT, Frey NV, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med 2015; 7:303ra139.
- Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet 2015; 385:517–528.
- Chmielewski M, Hombach AA, Abken H. Of CARs and TRUCKs: chimeric antigen receptor (CAR) T cells engineered with an inducible cytokine to modulate the tumor stroma. Immunol Rev 2014; 257:83–90.
KEY POINTS
- Chronic myelogenous leukemia (CML) can now be functionally cured with tyrosine kinase inhibitors, which interfere with the product of the oncogene causing the disease.
- Patients diagnosed with CML should begin therapy immediately even if they have no symptoms.
- Tyrosine kinase inhibitors have side effects that increase cardiovascular risk.
- Chronic lymphocytic leukemia (CLL) is an immunologic disease involving clonal proliferation of B cells. Chemotherapy for CLL should begin only when symptoms or indicators of impaired marrow function reach a certain threshold.
- New treatments for CLL increase the risk of atrial fibrillation and autoimmunity.
- Experimental B-cell–targeted therapies have demonstrated encouraging results even when chemotherapy fails in CLL and other B-cell cancers.
Support Our Veterans and PA History!
If you are a veteran PA or a PA on active duty in the uniformed services, you may want to take advantage of the PA History Society’s plans to upgrade the Veteran Memorial Garden at the Eugene A. Stead, Jr, Center for Physician Assistants in Durham, North Carolina, into a “place of remembrance.”
The Society is selling 9 x 9-in engraved brick pavers for $100 each. For those interested in purchasing more than one paver, they are offering a sliding scale: 1 for $100, 2 for $175, 3 for $250, 4 for $325, and 5 for $400. The engraved paver will include the appropriate uniformed service logo and 3 lines for name, branch, and years of service. The pavers will be embedded in the wheelchair accessible walkway and in the patio area surrounding a life-size bronze combat medic statue— the centerpiece of the garden.
This is a chance to honor yourself and other PA colleagues who have served or are currently serving their country. Construction and landscaping is to begin in October 2016, with a dedication ceremony scheduled for April 2017.
Order now via the Society’s website at http://pahx.org/ to make sure that your paver is displayed prominently in the garden.
Reginald Carter, PhD, PA
Historian Emeritus
PA History Society
If you are a veteran PA or a PA on active duty in the uniformed services, you may want to take advantage of the PA History Society’s plans to upgrade the Veteran Memorial Garden at the Eugene A. Stead, Jr, Center for Physician Assistants in Durham, North Carolina, into a “place of remembrance.”
The Society is selling 9 x 9-in engraved brick pavers for $100 each. For those interested in purchasing more than one paver, they are offering a sliding scale: 1 for $100, 2 for $175, 3 for $250, 4 for $325, and 5 for $400. The engraved paver will include the appropriate uniformed service logo and 3 lines for name, branch, and years of service. The pavers will be embedded in the wheelchair accessible walkway and in the patio area surrounding a life-size bronze combat medic statue— the centerpiece of the garden.
This is a chance to honor yourself and other PA colleagues who have served or are currently serving their country. Construction and landscaping is to begin in October 2016, with a dedication ceremony scheduled for April 2017.
Order now via the Society’s website at http://pahx.org/ to make sure that your paver is displayed prominently in the garden.
Reginald Carter, PhD, PA
Historian Emeritus
PA History Society
If you are a veteran PA or a PA on active duty in the uniformed services, you may want to take advantage of the PA History Society’s plans to upgrade the Veteran Memorial Garden at the Eugene A. Stead, Jr, Center for Physician Assistants in Durham, North Carolina, into a “place of remembrance.”
The Society is selling 9 x 9-in engraved brick pavers for $100 each. For those interested in purchasing more than one paver, they are offering a sliding scale: 1 for $100, 2 for $175, 3 for $250, 4 for $325, and 5 for $400. The engraved paver will include the appropriate uniformed service logo and 3 lines for name, branch, and years of service. The pavers will be embedded in the wheelchair accessible walkway and in the patio area surrounding a life-size bronze combat medic statue— the centerpiece of the garden.
This is a chance to honor yourself and other PA colleagues who have served or are currently serving their country. Construction and landscaping is to begin in October 2016, with a dedication ceremony scheduled for April 2017.
Order now via the Society’s website at http://pahx.org/ to make sure that your paver is displayed prominently in the garden.
Reginald Carter, PhD, PA
Historian Emeritus
PA History Society
Who benefits most from immediate HIV therapy?
DURBAN, SOUTH AFRICA – Immediate initiation of antiretroviral therapy in asymptomatic treatment-naive HIV-infected adults with a CD4+ cell count greater than 500/mL brings considerably more bang for the buck in selected patient subgroups, according to a secondary analysis from the landmark START trial.
Four subgroups in START stood out as having larger absolute risk reductions and lower numbers-needed-to-treat with a strategy of immediate treatment: patients above age 50, those with a baseline Framingham Risk Score in excess of 10%, individuals whose plasma HIV RNA level exceeds 50,000 copies/mL, and patients with a CD4:CD8 ratio below 0.5, Dr. Jean-Michel Molina reported at the 21st International AIDS Conference.
“These patients might be prioritized for immediate access to ART,” observed Dr. Molina, professor of infectious diseases at the University of Paris-Diderot and head of the infectious diseases department at Saint-Louis Hospital, also in Paris.
The START (Strategic Timing of AntiRetroviral Treatment) study was a major clinical trial conducted in 35 countries. Investigators randomized 4,685 treatment-naive HIV-infected men and women with CD4+ cell counts in the normal range to immediate antiretroviral therapy or to deferral of treatment until their CD4+ cell count dropped to 350 cells/mL. After 3 years of prospective follow-up, the immediate-treatment strategy was associated with a 47% reduction in risk for the primary endpoint, a composite of AIDS, major cardiovascular or other non-AIDS events, and death. The number needed to treat immediately for 1 year in order to prevent one major event was 128 (N Engl J Med. 2015;373:795-807).
The START findings prompted a revision in World Health Organization guidelines, which now recommend universal antiretroviral treatment (ART) in patients with HIV infection regardless of their CD4+ cell count.
But some patients are reluctant to go on lifetime ART, particularly since they still feel normal while in the initial phases of HIV infection. In such cases, these new subgroup data may tip the balance in decision-making. Moreover, the new START findings should help physicians and policy makers in prioritizing access to immediate ART in settings where it isn’t universally available, according to Dr. Molina.
In the prespecified subgroup analysis, patients aged 50 and up at enrollment had a 2.9% incidence of the primary composite endpoint at 3 years if randomized to immediate ART and an 11.7% rate if they were assigned to deferred ART. The number of 50-plus-year-olds needed to treat (NNT) immediately for 1 year in order to prevent one additional case of AIDS, a major non-AIDS event, or death was just 45, compared to NNTs of 151 and 206 in patients aged 30-49 and younger than 30, respectively. Patients aged 50 and older accounted for nearly 12% of the overall study population.
For the roughly 28% of START participants whose baseline CD4:CD8 ratio was less than 0.5, the NNT for immediate rather than deferred therapy was 60, substantially more favorable than the NNTs of 214 in patients with a baseline ratio of 0.5-0.8 and 248 in patients with a CD4:CD8 ratio greater than 0.8. The incidence of the primary endpoint at 3 years of follow-up in patients with a CD4:CD8 ratio of less than 0.5 was 0.5% in the immediate ART group and 6.3% with deferred therapy.
Similarly, patients with a baseline 10-year Framingham Risk Score (FRS) of 10% or higher had an NNT of 69, compared with NNTs of 111 in subjects with an FRS of 1%-9.9% and 276 in those with an FRS of less than 1%. Patients with an FRS of 10% or more had a 2.4% incidence of the primary endpoint at 3 years if assigned to immediate ART and a 10.1% rate with deferred therapy. Patients with an FRS of 10% or more comprised only 9.6% of the study population, Dr. Molina continued.
Patients with a heavy baseline viral load as evidenced by a plasma HIV RNA level of at least 50,000 copies/mL accounted for roughly 22% of the total study sample. Their 3-year rate of the primary outcome was 2.1% with immediate ART and 6.9% with deferred treatment. The NNT was 67, compared to an NNT of 122 in patients with 3,000-49,999 copies/mL and 992 in the one-quarter of START participants with a baseline plasma HIV RNA level of less than 3,000 copies/mL.
Variables that weren’t related to the magnitude of absolute risk reduction and NNT in the START subgroup analysis were race, gender, geographic region, baseline CD4 cell count, and whether an individual resided in a high- or lower-income country.
Several audience members rose to assert that the CD4:CD8 ratio and viral load might very well be redundant predictors measuring the same thing, since they are typically tightly correlated. Dr. Molina replied that the START investigators are planning to conduct a multivariate analysis of the data in the near future, which should provide a definitive answer.
Another audience member expressed surprise at what struck him as a low cardiovascular event rate in the START study, given that HIV infection is known to be associated with accelerated atherosclerosis. Dr. Molina said the explanation for the low number of cardiovascular events lies in the fact that cardiovascular risk is so heavily age-dependent, and START participants were relatively young, with a median age of 36 years.
The START trial was carried out by the International Network for Strategic Initiatives in Global HIV Trials (INSIGHT) with funding provided mainly by the National Institutes of Health. Dr. Molina reported having no financial conflicts of interest.
DURBAN, SOUTH AFRICA – Immediate initiation of antiretroviral therapy in asymptomatic treatment-naive HIV-infected adults with a CD4+ cell count greater than 500/mL brings considerably more bang for the buck in selected patient subgroups, according to a secondary analysis from the landmark START trial.
Four subgroups in START stood out as having larger absolute risk reductions and lower numbers-needed-to-treat with a strategy of immediate treatment: patients above age 50, those with a baseline Framingham Risk Score in excess of 10%, individuals whose plasma HIV RNA level exceeds 50,000 copies/mL, and patients with a CD4:CD8 ratio below 0.5, Dr. Jean-Michel Molina reported at the 21st International AIDS Conference.
“These patients might be prioritized for immediate access to ART,” observed Dr. Molina, professor of infectious diseases at the University of Paris-Diderot and head of the infectious diseases department at Saint-Louis Hospital, also in Paris.
The START (Strategic Timing of AntiRetroviral Treatment) study was a major clinical trial conducted in 35 countries. Investigators randomized 4,685 treatment-naive HIV-infected men and women with CD4+ cell counts in the normal range to immediate antiretroviral therapy or to deferral of treatment until their CD4+ cell count dropped to 350 cells/mL. After 3 years of prospective follow-up, the immediate-treatment strategy was associated with a 47% reduction in risk for the primary endpoint, a composite of AIDS, major cardiovascular or other non-AIDS events, and death. The number needed to treat immediately for 1 year in order to prevent one major event was 128 (N Engl J Med. 2015;373:795-807).
The START findings prompted a revision in World Health Organization guidelines, which now recommend universal antiretroviral treatment (ART) in patients with HIV infection regardless of their CD4+ cell count.
But some patients are reluctant to go on lifetime ART, particularly since they still feel normal while in the initial phases of HIV infection. In such cases, these new subgroup data may tip the balance in decision-making. Moreover, the new START findings should help physicians and policy makers in prioritizing access to immediate ART in settings where it isn’t universally available, according to Dr. Molina.
In the prespecified subgroup analysis, patients aged 50 and up at enrollment had a 2.9% incidence of the primary composite endpoint at 3 years if randomized to immediate ART and an 11.7% rate if they were assigned to deferred ART. The number of 50-plus-year-olds needed to treat (NNT) immediately for 1 year in order to prevent one additional case of AIDS, a major non-AIDS event, or death was just 45, compared to NNTs of 151 and 206 in patients aged 30-49 and younger than 30, respectively. Patients aged 50 and older accounted for nearly 12% of the overall study population.
For the roughly 28% of START participants whose baseline CD4:CD8 ratio was less than 0.5, the NNT for immediate rather than deferred therapy was 60, substantially more favorable than the NNTs of 214 in patients with a baseline ratio of 0.5-0.8 and 248 in patients with a CD4:CD8 ratio greater than 0.8. The incidence of the primary endpoint at 3 years of follow-up in patients with a CD4:CD8 ratio of less than 0.5 was 0.5% in the immediate ART group and 6.3% with deferred therapy.
Similarly, patients with a baseline 10-year Framingham Risk Score (FRS) of 10% or higher had an NNT of 69, compared with NNTs of 111 in subjects with an FRS of 1%-9.9% and 276 in those with an FRS of less than 1%. Patients with an FRS of 10% or more had a 2.4% incidence of the primary endpoint at 3 years if assigned to immediate ART and a 10.1% rate with deferred therapy. Patients with an FRS of 10% or more comprised only 9.6% of the study population, Dr. Molina continued.
Patients with a heavy baseline viral load as evidenced by a plasma HIV RNA level of at least 50,000 copies/mL accounted for roughly 22% of the total study sample. Their 3-year rate of the primary outcome was 2.1% with immediate ART and 6.9% with deferred treatment. The NNT was 67, compared to an NNT of 122 in patients with 3,000-49,999 copies/mL and 992 in the one-quarter of START participants with a baseline plasma HIV RNA level of less than 3,000 copies/mL.
Variables that weren’t related to the magnitude of absolute risk reduction and NNT in the START subgroup analysis were race, gender, geographic region, baseline CD4 cell count, and whether an individual resided in a high- or lower-income country.
Several audience members rose to assert that the CD4:CD8 ratio and viral load might very well be redundant predictors measuring the same thing, since they are typically tightly correlated. Dr. Molina replied that the START investigators are planning to conduct a multivariate analysis of the data in the near future, which should provide a definitive answer.
Another audience member expressed surprise at what struck him as a low cardiovascular event rate in the START study, given that HIV infection is known to be associated with accelerated atherosclerosis. Dr. Molina said the explanation for the low number of cardiovascular events lies in the fact that cardiovascular risk is so heavily age-dependent, and START participants were relatively young, with a median age of 36 years.
The START trial was carried out by the International Network for Strategic Initiatives in Global HIV Trials (INSIGHT) with funding provided mainly by the National Institutes of Health. Dr. Molina reported having no financial conflicts of interest.
DURBAN, SOUTH AFRICA – Immediate initiation of antiretroviral therapy in asymptomatic treatment-naive HIV-infected adults with a CD4+ cell count greater than 500/mL brings considerably more bang for the buck in selected patient subgroups, according to a secondary analysis from the landmark START trial.
Four subgroups in START stood out as having larger absolute risk reductions and lower numbers-needed-to-treat with a strategy of immediate treatment: patients above age 50, those with a baseline Framingham Risk Score in excess of 10%, individuals whose plasma HIV RNA level exceeds 50,000 copies/mL, and patients with a CD4:CD8 ratio below 0.5, Dr. Jean-Michel Molina reported at the 21st International AIDS Conference.
“These patients might be prioritized for immediate access to ART,” observed Dr. Molina, professor of infectious diseases at the University of Paris-Diderot and head of the infectious diseases department at Saint-Louis Hospital, also in Paris.
The START (Strategic Timing of AntiRetroviral Treatment) study was a major clinical trial conducted in 35 countries. Investigators randomized 4,685 treatment-naive HIV-infected men and women with CD4+ cell counts in the normal range to immediate antiretroviral therapy or to deferral of treatment until their CD4+ cell count dropped to 350 cells/mL. After 3 years of prospective follow-up, the immediate-treatment strategy was associated with a 47% reduction in risk for the primary endpoint, a composite of AIDS, major cardiovascular or other non-AIDS events, and death. The number needed to treat immediately for 1 year in order to prevent one major event was 128 (N Engl J Med. 2015;373:795-807).
The START findings prompted a revision in World Health Organization guidelines, which now recommend universal antiretroviral treatment (ART) in patients with HIV infection regardless of their CD4+ cell count.
But some patients are reluctant to go on lifetime ART, particularly since they still feel normal while in the initial phases of HIV infection. In such cases, these new subgroup data may tip the balance in decision-making. Moreover, the new START findings should help physicians and policy makers in prioritizing access to immediate ART in settings where it isn’t universally available, according to Dr. Molina.
In the prespecified subgroup analysis, patients aged 50 and up at enrollment had a 2.9% incidence of the primary composite endpoint at 3 years if randomized to immediate ART and an 11.7% rate if they were assigned to deferred ART. The number of 50-plus-year-olds needed to treat (NNT) immediately for 1 year in order to prevent one additional case of AIDS, a major non-AIDS event, or death was just 45, compared to NNTs of 151 and 206 in patients aged 30-49 and younger than 30, respectively. Patients aged 50 and older accounted for nearly 12% of the overall study population.
For the roughly 28% of START participants whose baseline CD4:CD8 ratio was less than 0.5, the NNT for immediate rather than deferred therapy was 60, substantially more favorable than the NNTs of 214 in patients with a baseline ratio of 0.5-0.8 and 248 in patients with a CD4:CD8 ratio greater than 0.8. The incidence of the primary endpoint at 3 years of follow-up in patients with a CD4:CD8 ratio of less than 0.5 was 0.5% in the immediate ART group and 6.3% with deferred therapy.
Similarly, patients with a baseline 10-year Framingham Risk Score (FRS) of 10% or higher had an NNT of 69, compared with NNTs of 111 in subjects with an FRS of 1%-9.9% and 276 in those with an FRS of less than 1%. Patients with an FRS of 10% or more had a 2.4% incidence of the primary endpoint at 3 years if assigned to immediate ART and a 10.1% rate with deferred therapy. Patients with an FRS of 10% or more comprised only 9.6% of the study population, Dr. Molina continued.
Patients with a heavy baseline viral load as evidenced by a plasma HIV RNA level of at least 50,000 copies/mL accounted for roughly 22% of the total study sample. Their 3-year rate of the primary outcome was 2.1% with immediate ART and 6.9% with deferred treatment. The NNT was 67, compared to an NNT of 122 in patients with 3,000-49,999 copies/mL and 992 in the one-quarter of START participants with a baseline plasma HIV RNA level of less than 3,000 copies/mL.
Variables that weren’t related to the magnitude of absolute risk reduction and NNT in the START subgroup analysis were race, gender, geographic region, baseline CD4 cell count, and whether an individual resided in a high- or lower-income country.
Several audience members rose to assert that the CD4:CD8 ratio and viral load might very well be redundant predictors measuring the same thing, since they are typically tightly correlated. Dr. Molina replied that the START investigators are planning to conduct a multivariate analysis of the data in the near future, which should provide a definitive answer.
Another audience member expressed surprise at what struck him as a low cardiovascular event rate in the START study, given that HIV infection is known to be associated with accelerated atherosclerosis. Dr. Molina said the explanation for the low number of cardiovascular events lies in the fact that cardiovascular risk is so heavily age-dependent, and START participants were relatively young, with a median age of 36 years.
The START trial was carried out by the International Network for Strategic Initiatives in Global HIV Trials (INSIGHT) with funding provided mainly by the National Institutes of Health. Dr. Molina reported having no financial conflicts of interest.
AT AIDS 2016
Key clinical point: Four specific subgroups of asymptomatic HIV-infected adults who obtain the most clinical benefit from immediate rather than deferred antiretroviral therapy have been identified.
Major finding: The number of asymptomatic HIV-infected adults needed to treat immediately with antiretroviral therapy for 1 year instead of deferring treatment in order to avoid one case of AIDS or serious non-AIDS illness is 45 in patients aged 50 and older, compared with NNTs of 151 in 30- to 49-year-olds and 206 in patients younger than age 30.
Data source: This was a prespecified subgroup analysis of the landmark START trial, in which 4,685 treatment-naive asymptomatic HIV-infected adults with more than 500 CD4+ cells/mL were randomized to immediate or deferred antiretroviral therapy.
Disclosures: The START trial was funded chiefly by the National Institutes of Health. The presenter reported having no financial conflicts of interest.