User login
H. pylori’s relationship with gastric cancer? It’s complicated
CHICAGO – Does eradicating Helicobacter pylori prevent gastric cancer?
The answer is yes, sometimes, but it depends on where you live, and what other bacteria coexist in your gut microbiome.
The overall view is a positive one, Richard M. Peek Jr., MD, said at the at the meeting sponsored by the American Gastroenterological Association. A very large, recent meta-analysis confirms it (Gastroenterology. 2016. doi:10.1053/j.gastro.2016.01.028). Comprising 24 studies and 48,000 subjects, the meta-analysis determined that eradicating the bacteria in infected people cut gastric cancer incidence significantly.
That’s great news – but there’s a big caveat, said Dr. Peek of Vanderbilt University, Nashville. “The benefit was dependent on what your baseline risk was. For those with a high baseline risk, the benefit was tremendous. For those with a low baseline risk, it was not statistically significant.”
There are long-term data suggesting that treating H. pylori sooner rather than later is the way to go. A 2005 study followed more than 700 patients with preneoplastic gastric lesions for 12 years. It found that the treatment effect was cumulative: The longer the patient was free of H. pylori, the more reliably healing occurred (Gut. 2005. doi:10.1136/gut.2005.072009).
At baseline, the patients were randomized to nutritional supplements or to a combination of amoxicillin, metronidazole, and bismuth subsalicylate. At 6 years, the trial was unblinded and all patients were offered treatment. Patients were followed for another 6 years. Those who were H. pylori negative at 12 years had 15% more regression and 14% less progression than subjects who were positive at 12 years. Among those who received anti–H. pylori treatment at the 6-year mark, the effect was smaller and nonsignificant.
Perhaps surprisingly, though, the biggest bang for H. pylori treatment is seen in the antrum of the stomach, not in the corpus. Another meta-analysis, this one of 16 studies, found very consistent reductions in the severity of intestinal metaplasia in the antrum after antibiotic treatment – but no difference at all in corpus metaplasia. The reason for that finding isn’t at all clear, the authors of that paper noted (World J Gastro. 2014. doi:10.3748/wjg.v20.i19.5903).
The bacteria-metaplasia cancer link gets even more complicated when H. pylori is viewed as a contributing member of society, rather than a hermit. The bacterium seems to be a bully in the neighborhood, radically altering the normal gastric microbiome, Dr. Peek said.
In the absence of H. pylori, the gastric microbiome is much more diverse, consisting of about 50% Actinobacteria and 25% Firmicutes species. Bacteroides and Proteobacteria species make up the remainder, with a small population of Cyanobacteria as well. In its presence, Proteobacteria – a gram-negative genus that includes a wide variety of pathogens – almost completely subsume beneficial bacteria.
Researchers saw this change in action in 2011, when a group at the Massachusetts Institute of Technology, Cambridge, inoculated two mouse populations with H. pylori and followed them for gastric neoplasms (Gastroenterology. 2011. doi:10.1053/j.gastro.2010.09.048). All the mice were genetically engineered to overexpress human gastrin, a characteristic that invariably leads them to develop gastric cancers.
One group comprised germ-free mice raised in sterile environments. The control group was free of pathogens, but lived in a conventional environment and so had normal gastric flora. Both groups were inoculated with H. pylori.
By 11 months, the microbiome of the control group was strikingly different. It showed a significant increase in the number of Firmicutes bacteria in the stomach, with an associated decrease in the number and variety of other bacteria including Bacteroides. This was especially interesting when viewed in relation to the rate of gastric neoplasia, Dr. Peek said.
These mice are programmed to develop gastric cancer by 6 months of age – and this is what happened in the control mice, which had H. pylori plus other gastric microbes. But the germ-free mice who were monoinfected with H. pylori showed a much different progression of disease. At 7 months, most showed only a mild hypergastrinemia. Conversely, at 7 months, all of the H. pylori–infected control mice had developed gastric intraepithelial neoplasia, 80% of it high grade. Only 10% of the monoinfected mice developed cancer, and all of it was low grade.
“It looks like there is active collaboration between H. pylori and other bacteria in the stomach,” resulting in this increased cancer risk, Dr. Peek said.
It’s a collaboration that reaches deep into the tumors themselves, he said. “A very interesting study a couple of years ago searched cancer genomes for the presence of bacterial DNA, and found that gastric cancers incorporated the second-highest amount of microbial DNA into their cancer genomes. But it wasn’t just H. pylori. Many other species had integrated their DNA into these tumors.”
That study, published in 2013, was the first to prove that bacterial DNA can impact carcinogenesis. Acute myeloid leukemia showed the highest integration of bacterial DNA, but gastric adenocarcinoma was a close second. Most of the species were of the Proteobacteria lineages (83%), with a third of that represented by Pseudomonas, particularly P. fluorescens and P. aeruginosa. Both of those species have been shown to promote gastric tumorigenesis in rats. All of the DNA integrations occurred in five genes; four of these are already known to be upregulated in gastric cancer (PLOS Comp Biol. 2013;9[6]:e1003107).
Interestingly, only a few of the sample reads turned up DNA integration with H. pylori.
This reduction in gastric microbial diversity could be an important key to H. pylori’s relation to gastric cancer, Dr. Peek said. He examined this in residents of two towns in Colombia, South America: Tumaco, where the risk of gastric cancer is low, and Tuquerres, where it’s 25 times higher (Sci Rep. 2016. doi:10.1038/srep18594).
What was different was the gastric microbiome of residents. Those living in low-risk Tumaco had much more microbial diversity: 361 varieties, compared with 194 in Tuquerres. And 16 of these groups – representative of what’s usually considered a healthy microbiome – were absent in the high-risk subjects. But Tuquerres residents had two bacteria that weren’t found in Tumaco residents, including Leptorichia wadei, which has been associated with necrotizing enterocolitis.
There was no difference, however, in the prevalence of H. pylori between these high- and low-risk groups.
These new findings illustrate an increasingly complicated interplay of bacteria and gastric cancer, Dr. Peek said. But they also provide a new direction for research.
“We have a framework now where we can move forward and try to understand how some of these other strains impact gastric cancer risk,” he said.
Dr. Peek had no relevant financial disclosures.
On Twitter @Alz_Gal
CHICAGO – Does eradicating Helicobacter pylori prevent gastric cancer?
The answer is yes, sometimes, but it depends on where you live, and what other bacteria coexist in your gut microbiome.
The overall view is a positive one, Richard M. Peek Jr., MD, said at the at the meeting sponsored by the American Gastroenterological Association. A very large, recent meta-analysis confirms it (Gastroenterology. 2016. doi:10.1053/j.gastro.2016.01.028). Comprising 24 studies and 48,000 subjects, the meta-analysis determined that eradicating the bacteria in infected people cut gastric cancer incidence significantly.
That’s great news – but there’s a big caveat, said Dr. Peek of Vanderbilt University, Nashville. “The benefit was dependent on what your baseline risk was. For those with a high baseline risk, the benefit was tremendous. For those with a low baseline risk, it was not statistically significant.”
There are long-term data suggesting that treating H. pylori sooner rather than later is the way to go. A 2005 study followed more than 700 patients with preneoplastic gastric lesions for 12 years. It found that the treatment effect was cumulative: The longer the patient was free of H. pylori, the more reliably healing occurred (Gut. 2005. doi:10.1136/gut.2005.072009).
At baseline, the patients were randomized to nutritional supplements or to a combination of amoxicillin, metronidazole, and bismuth subsalicylate. At 6 years, the trial was unblinded and all patients were offered treatment. Patients were followed for another 6 years. Those who were H. pylori negative at 12 years had 15% more regression and 14% less progression than subjects who were positive at 12 years. Among those who received anti–H. pylori treatment at the 6-year mark, the effect was smaller and nonsignificant.
Perhaps surprisingly, though, the biggest bang for H. pylori treatment is seen in the antrum of the stomach, not in the corpus. Another meta-analysis, this one of 16 studies, found very consistent reductions in the severity of intestinal metaplasia in the antrum after antibiotic treatment – but no difference at all in corpus metaplasia. The reason for that finding isn’t at all clear, the authors of that paper noted (World J Gastro. 2014. doi:10.3748/wjg.v20.i19.5903).
The bacteria-metaplasia cancer link gets even more complicated when H. pylori is viewed as a contributing member of society, rather than a hermit. The bacterium seems to be a bully in the neighborhood, radically altering the normal gastric microbiome, Dr. Peek said.
In the absence of H. pylori, the gastric microbiome is much more diverse, consisting of about 50% Actinobacteria and 25% Firmicutes species. Bacteroides and Proteobacteria species make up the remainder, with a small population of Cyanobacteria as well. In its presence, Proteobacteria – a gram-negative genus that includes a wide variety of pathogens – almost completely subsume beneficial bacteria.
Researchers saw this change in action in 2011, when a group at the Massachusetts Institute of Technology, Cambridge, inoculated two mouse populations with H. pylori and followed them for gastric neoplasms (Gastroenterology. 2011. doi:10.1053/j.gastro.2010.09.048). All the mice were genetically engineered to overexpress human gastrin, a characteristic that invariably leads them to develop gastric cancers.
One group comprised germ-free mice raised in sterile environments. The control group was free of pathogens, but lived in a conventional environment and so had normal gastric flora. Both groups were inoculated with H. pylori.
By 11 months, the microbiome of the control group was strikingly different. It showed a significant increase in the number of Firmicutes bacteria in the stomach, with an associated decrease in the number and variety of other bacteria including Bacteroides. This was especially interesting when viewed in relation to the rate of gastric neoplasia, Dr. Peek said.
These mice are programmed to develop gastric cancer by 6 months of age – and this is what happened in the control mice, which had H. pylori plus other gastric microbes. But the germ-free mice who were monoinfected with H. pylori showed a much different progression of disease. At 7 months, most showed only a mild hypergastrinemia. Conversely, at 7 months, all of the H. pylori–infected control mice had developed gastric intraepithelial neoplasia, 80% of it high grade. Only 10% of the monoinfected mice developed cancer, and all of it was low grade.
“It looks like there is active collaboration between H. pylori and other bacteria in the stomach,” resulting in this increased cancer risk, Dr. Peek said.
It’s a collaboration that reaches deep into the tumors themselves, he said. “A very interesting study a couple of years ago searched cancer genomes for the presence of bacterial DNA, and found that gastric cancers incorporated the second-highest amount of microbial DNA into their cancer genomes. But it wasn’t just H. pylori. Many other species had integrated their DNA into these tumors.”
That study, published in 2013, was the first to prove that bacterial DNA can impact carcinogenesis. Acute myeloid leukemia showed the highest integration of bacterial DNA, but gastric adenocarcinoma was a close second. Most of the species were of the Proteobacteria lineages (83%), with a third of that represented by Pseudomonas, particularly P. fluorescens and P. aeruginosa. Both of those species have been shown to promote gastric tumorigenesis in rats. All of the DNA integrations occurred in five genes; four of these are already known to be upregulated in gastric cancer (PLOS Comp Biol. 2013;9[6]:e1003107).
Interestingly, only a few of the sample reads turned up DNA integration with H. pylori.
This reduction in gastric microbial diversity could be an important key to H. pylori’s relation to gastric cancer, Dr. Peek said. He examined this in residents of two towns in Colombia, South America: Tumaco, where the risk of gastric cancer is low, and Tuquerres, where it’s 25 times higher (Sci Rep. 2016. doi:10.1038/srep18594).
What was different was the gastric microbiome of residents. Those living in low-risk Tumaco had much more microbial diversity: 361 varieties, compared with 194 in Tuquerres. And 16 of these groups – representative of what’s usually considered a healthy microbiome – were absent in the high-risk subjects. But Tuquerres residents had two bacteria that weren’t found in Tumaco residents, including Leptorichia wadei, which has been associated with necrotizing enterocolitis.
There was no difference, however, in the prevalence of H. pylori between these high- and low-risk groups.
These new findings illustrate an increasingly complicated interplay of bacteria and gastric cancer, Dr. Peek said. But they also provide a new direction for research.
“We have a framework now where we can move forward and try to understand how some of these other strains impact gastric cancer risk,” he said.
Dr. Peek had no relevant financial disclosures.
On Twitter @Alz_Gal
CHICAGO – Does eradicating Helicobacter pylori prevent gastric cancer?
The answer is yes, sometimes, but it depends on where you live, and what other bacteria coexist in your gut microbiome.
The overall view is a positive one, Richard M. Peek Jr., MD, said at the at the meeting sponsored by the American Gastroenterological Association. A very large, recent meta-analysis confirms it (Gastroenterology. 2016. doi:10.1053/j.gastro.2016.01.028). Comprising 24 studies and 48,000 subjects, the meta-analysis determined that eradicating the bacteria in infected people cut gastric cancer incidence significantly.
That’s great news – but there’s a big caveat, said Dr. Peek of Vanderbilt University, Nashville. “The benefit was dependent on what your baseline risk was. For those with a high baseline risk, the benefit was tremendous. For those with a low baseline risk, it was not statistically significant.”
There are long-term data suggesting that treating H. pylori sooner rather than later is the way to go. A 2005 study followed more than 700 patients with preneoplastic gastric lesions for 12 years. It found that the treatment effect was cumulative: The longer the patient was free of H. pylori, the more reliably healing occurred (Gut. 2005. doi:10.1136/gut.2005.072009).
At baseline, the patients were randomized to nutritional supplements or to a combination of amoxicillin, metronidazole, and bismuth subsalicylate. At 6 years, the trial was unblinded and all patients were offered treatment. Patients were followed for another 6 years. Those who were H. pylori negative at 12 years had 15% more regression and 14% less progression than subjects who were positive at 12 years. Among those who received anti–H. pylori treatment at the 6-year mark, the effect was smaller and nonsignificant.
Perhaps surprisingly, though, the biggest bang for H. pylori treatment is seen in the antrum of the stomach, not in the corpus. Another meta-analysis, this one of 16 studies, found very consistent reductions in the severity of intestinal metaplasia in the antrum after antibiotic treatment – but no difference at all in corpus metaplasia. The reason for that finding isn’t at all clear, the authors of that paper noted (World J Gastro. 2014. doi:10.3748/wjg.v20.i19.5903).
The bacteria-metaplasia cancer link gets even more complicated when H. pylori is viewed as a contributing member of society, rather than a hermit. The bacterium seems to be a bully in the neighborhood, radically altering the normal gastric microbiome, Dr. Peek said.
In the absence of H. pylori, the gastric microbiome is much more diverse, consisting of about 50% Actinobacteria and 25% Firmicutes species. Bacteroides and Proteobacteria species make up the remainder, with a small population of Cyanobacteria as well. In its presence, Proteobacteria – a gram-negative genus that includes a wide variety of pathogens – almost completely subsume beneficial bacteria.
Researchers saw this change in action in 2011, when a group at the Massachusetts Institute of Technology, Cambridge, inoculated two mouse populations with H. pylori and followed them for gastric neoplasms (Gastroenterology. 2011. doi:10.1053/j.gastro.2010.09.048). All the mice were genetically engineered to overexpress human gastrin, a characteristic that invariably leads them to develop gastric cancers.
One group comprised germ-free mice raised in sterile environments. The control group was free of pathogens, but lived in a conventional environment and so had normal gastric flora. Both groups were inoculated with H. pylori.
By 11 months, the microbiome of the control group was strikingly different. It showed a significant increase in the number of Firmicutes bacteria in the stomach, with an associated decrease in the number and variety of other bacteria including Bacteroides. This was especially interesting when viewed in relation to the rate of gastric neoplasia, Dr. Peek said.
These mice are programmed to develop gastric cancer by 6 months of age – and this is what happened in the control mice, which had H. pylori plus other gastric microbes. But the germ-free mice who were monoinfected with H. pylori showed a much different progression of disease. At 7 months, most showed only a mild hypergastrinemia. Conversely, at 7 months, all of the H. pylori–infected control mice had developed gastric intraepithelial neoplasia, 80% of it high grade. Only 10% of the monoinfected mice developed cancer, and all of it was low grade.
“It looks like there is active collaboration between H. pylori and other bacteria in the stomach,” resulting in this increased cancer risk, Dr. Peek said.
It’s a collaboration that reaches deep into the tumors themselves, he said. “A very interesting study a couple of years ago searched cancer genomes for the presence of bacterial DNA, and found that gastric cancers incorporated the second-highest amount of microbial DNA into their cancer genomes. But it wasn’t just H. pylori. Many other species had integrated their DNA into these tumors.”
That study, published in 2013, was the first to prove that bacterial DNA can impact carcinogenesis. Acute myeloid leukemia showed the highest integration of bacterial DNA, but gastric adenocarcinoma was a close second. Most of the species were of the Proteobacteria lineages (83%), with a third of that represented by Pseudomonas, particularly P. fluorescens and P. aeruginosa. Both of those species have been shown to promote gastric tumorigenesis in rats. All of the DNA integrations occurred in five genes; four of these are already known to be upregulated in gastric cancer (PLOS Comp Biol. 2013;9[6]:e1003107).
Interestingly, only a few of the sample reads turned up DNA integration with H. pylori.
This reduction in gastric microbial diversity could be an important key to H. pylori’s relation to gastric cancer, Dr. Peek said. He examined this in residents of two towns in Colombia, South America: Tumaco, where the risk of gastric cancer is low, and Tuquerres, where it’s 25 times higher (Sci Rep. 2016. doi:10.1038/srep18594).
What was different was the gastric microbiome of residents. Those living in low-risk Tumaco had much more microbial diversity: 361 varieties, compared with 194 in Tuquerres. And 16 of these groups – representative of what’s usually considered a healthy microbiome – were absent in the high-risk subjects. But Tuquerres residents had two bacteria that weren’t found in Tumaco residents, including Leptorichia wadei, which has been associated with necrotizing enterocolitis.
There was no difference, however, in the prevalence of H. pylori between these high- and low-risk groups.
These new findings illustrate an increasingly complicated interplay of bacteria and gastric cancer, Dr. Peek said. But they also provide a new direction for research.
“We have a framework now where we can move forward and try to understand how some of these other strains impact gastric cancer risk,” he said.
Dr. Peek had no relevant financial disclosures.
On Twitter @Alz_Gal
AT THE 2016 JAMES W. FRESTON CONFERENCE
Strange bedfellows: FMT and esophageal disease
Fecal microbiota transplant (FMT) and esophageal disease were strange bedfellows in a series of informative lectures by world experts in their respective fields.
FMT breaks the cycle of resistant Clostridium difficile infection, where resistant bacteria overwhelm innate gut flora following antibiotic use. FMT introduces competition for nutrients and microbiota-derived bacteriocins targeting resistant bacteria, restores secondary bile salt metabolism, and stimulates mucosal immunity against resistant bacteria. The potential for FMT to benefit diseases other than recurrent C. difficile infection continues to be explored.
Eosinophilic esophagitis (EoE) is characterized clinically by dysphagia and food impaction, and pathologically by eosinophil-dominant inflammation (at least 15 eosinophils/high-power field on biopsy). Esophageal subepithelial fibrosis contributes to a narrow caliber, poorly distensible esophagus. Alternative causes of esophageal eosinophilia, particularly that induced by reflux, need to be excluded. Proton pump inhibitor (PPI) response does not distinguish EoE from reflux disease, but management starts with these drugs. Swallowed topical steroids, and the six-food elimination diet are alternative effective therapies. Biologic agents are being evaluated as future therapeutic options.
High-resolution manometry (HRM) has improved acquisition and display of esophageal pressure data, simplifying interpretation using three software tools, integrated relaxation pressure, distal contractile integral, and distal latency. Stationary impedance with HRM (high-resolution impedance manometry, HRIM) provides further gains in esophageal bolus transit assessment. Automated impedance manometry analysis, esophageal impedance integral ratio, bolus flow time, and functional lumen imaging probe–derived metrics add to esophageal physiologic assessments and esophageal function testing.
Rare disorders such as esophageal lichen planus can manifest with dysphagia and esophageal inflammation and strictures; these disorders are managed with immunosuppressive agents and cautious endoscopic dilation. Rumination (regurgitation of recently ingested food) and belching (with aerophagy) mimic reflux disease. These are distinguished using HRIM and pH-impedance monitoring, and treated with diaphragmatic breathing. Early postfundoplication dysphagia is common and responds to dilation; peptic strictures or slipped fundoplication needing wrap revision can cause late dysphagia. Scleroderma esophagus can be difficult to differentiate from advanced achalasia. Pneumatic dilation can benefit postmyotomy dysphagia.
Low-grade dysplasia within Barrett’s esophagus has a variable natural history, primarily from overdiagnosis and interobserver variation; many patients are down-staged upon review. Progression to high-grade dysplasia or intramucosal cancer (0.5%-1.7% per annum) is higher with confirmed low-grade dysplasia. Consequently, guidelines recommend review by expert pathologists and surveillance after 6-12 months. Evidence for radiofrequency ablation in carefully selected patients continues to grow. Radiofrequency ablation eliminates dysplasia and reduces the risk of progression to high-grade dysplasia. Better markers for diagnosis and prognosis continue to be studied.
Dr. Gyawali is professor of medicine, division of gastroenterology, Washington University in St. Louis. He has consulted for and received speaking fees or research funding from Medtronic, Torax, Ironwood, and Allergan.
This is a summary provided by the moderator of one of the spring postgraduate course sessions held at DDW 2016.
Fecal microbiota transplant (FMT) and esophageal disease were strange bedfellows in a series of informative lectures by world experts in their respective fields.
FMT breaks the cycle of resistant Clostridium difficile infection, where resistant bacteria overwhelm innate gut flora following antibiotic use. FMT introduces competition for nutrients and microbiota-derived bacteriocins targeting resistant bacteria, restores secondary bile salt metabolism, and stimulates mucosal immunity against resistant bacteria. The potential for FMT to benefit diseases other than recurrent C. difficile infection continues to be explored.
Eosinophilic esophagitis (EoE) is characterized clinically by dysphagia and food impaction, and pathologically by eosinophil-dominant inflammation (at least 15 eosinophils/high-power field on biopsy). Esophageal subepithelial fibrosis contributes to a narrow caliber, poorly distensible esophagus. Alternative causes of esophageal eosinophilia, particularly that induced by reflux, need to be excluded. Proton pump inhibitor (PPI) response does not distinguish EoE from reflux disease, but management starts with these drugs. Swallowed topical steroids, and the six-food elimination diet are alternative effective therapies. Biologic agents are being evaluated as future therapeutic options.
High-resolution manometry (HRM) has improved acquisition and display of esophageal pressure data, simplifying interpretation using three software tools, integrated relaxation pressure, distal contractile integral, and distal latency. Stationary impedance with HRM (high-resolution impedance manometry, HRIM) provides further gains in esophageal bolus transit assessment. Automated impedance manometry analysis, esophageal impedance integral ratio, bolus flow time, and functional lumen imaging probe–derived metrics add to esophageal physiologic assessments and esophageal function testing.
Rare disorders such as esophageal lichen planus can manifest with dysphagia and esophageal inflammation and strictures; these disorders are managed with immunosuppressive agents and cautious endoscopic dilation. Rumination (regurgitation of recently ingested food) and belching (with aerophagy) mimic reflux disease. These are distinguished using HRIM and pH-impedance monitoring, and treated with diaphragmatic breathing. Early postfundoplication dysphagia is common and responds to dilation; peptic strictures or slipped fundoplication needing wrap revision can cause late dysphagia. Scleroderma esophagus can be difficult to differentiate from advanced achalasia. Pneumatic dilation can benefit postmyotomy dysphagia.
Low-grade dysplasia within Barrett’s esophagus has a variable natural history, primarily from overdiagnosis and interobserver variation; many patients are down-staged upon review. Progression to high-grade dysplasia or intramucosal cancer (0.5%-1.7% per annum) is higher with confirmed low-grade dysplasia. Consequently, guidelines recommend review by expert pathologists and surveillance after 6-12 months. Evidence for radiofrequency ablation in carefully selected patients continues to grow. Radiofrequency ablation eliminates dysplasia and reduces the risk of progression to high-grade dysplasia. Better markers for diagnosis and prognosis continue to be studied.
Dr. Gyawali is professor of medicine, division of gastroenterology, Washington University in St. Louis. He has consulted for and received speaking fees or research funding from Medtronic, Torax, Ironwood, and Allergan.
This is a summary provided by the moderator of one of the spring postgraduate course sessions held at DDW 2016.
Fecal microbiota transplant (FMT) and esophageal disease were strange bedfellows in a series of informative lectures by world experts in their respective fields.
FMT breaks the cycle of resistant Clostridium difficile infection, where resistant bacteria overwhelm innate gut flora following antibiotic use. FMT introduces competition for nutrients and microbiota-derived bacteriocins targeting resistant bacteria, restores secondary bile salt metabolism, and stimulates mucosal immunity against resistant bacteria. The potential for FMT to benefit diseases other than recurrent C. difficile infection continues to be explored.
Eosinophilic esophagitis (EoE) is characterized clinically by dysphagia and food impaction, and pathologically by eosinophil-dominant inflammation (at least 15 eosinophils/high-power field on biopsy). Esophageal subepithelial fibrosis contributes to a narrow caliber, poorly distensible esophagus. Alternative causes of esophageal eosinophilia, particularly that induced by reflux, need to be excluded. Proton pump inhibitor (PPI) response does not distinguish EoE from reflux disease, but management starts with these drugs. Swallowed topical steroids, and the six-food elimination diet are alternative effective therapies. Biologic agents are being evaluated as future therapeutic options.
High-resolution manometry (HRM) has improved acquisition and display of esophageal pressure data, simplifying interpretation using three software tools, integrated relaxation pressure, distal contractile integral, and distal latency. Stationary impedance with HRM (high-resolution impedance manometry, HRIM) provides further gains in esophageal bolus transit assessment. Automated impedance manometry analysis, esophageal impedance integral ratio, bolus flow time, and functional lumen imaging probe–derived metrics add to esophageal physiologic assessments and esophageal function testing.
Rare disorders such as esophageal lichen planus can manifest with dysphagia and esophageal inflammation and strictures; these disorders are managed with immunosuppressive agents and cautious endoscopic dilation. Rumination (regurgitation of recently ingested food) and belching (with aerophagy) mimic reflux disease. These are distinguished using HRIM and pH-impedance monitoring, and treated with diaphragmatic breathing. Early postfundoplication dysphagia is common and responds to dilation; peptic strictures or slipped fundoplication needing wrap revision can cause late dysphagia. Scleroderma esophagus can be difficult to differentiate from advanced achalasia. Pneumatic dilation can benefit postmyotomy dysphagia.
Low-grade dysplasia within Barrett’s esophagus has a variable natural history, primarily from overdiagnosis and interobserver variation; many patients are down-staged upon review. Progression to high-grade dysplasia or intramucosal cancer (0.5%-1.7% per annum) is higher with confirmed low-grade dysplasia. Consequently, guidelines recommend review by expert pathologists and surveillance after 6-12 months. Evidence for radiofrequency ablation in carefully selected patients continues to grow. Radiofrequency ablation eliminates dysplasia and reduces the risk of progression to high-grade dysplasia. Better markers for diagnosis and prognosis continue to be studied.
Dr. Gyawali is professor of medicine, division of gastroenterology, Washington University in St. Louis. He has consulted for and received speaking fees or research funding from Medtronic, Torax, Ironwood, and Allergan.
This is a summary provided by the moderator of one of the spring postgraduate course sessions held at DDW 2016.
Eliminating the anxiety of managing functional GI disorders
Kicking off the 2016 AGA Spring Postgraduate Course, this symposium encouraged attendees to embrace multidisciplinary approaches to managing patients with common gastrointestinal symptoms of nonstructural origin. My talk on “Managing the Big Four – Dyspepsia, Constipation, Diarrhea, and Abdominal Pain” reviewed the pathophysiology and management of these conditions. Thereafter, Sheila Crowe, MD, AGAF, Laurie Keefer, PhD, and Michael Camilleri, MD, AGAF, respectively, reviewed dietary approaches, psychological, and behavioral therapies, and overlooked, overused, and emerging pharmacotherapy for managing these conditions.
The clinical evaluation enables a precise symptom-based diagnosis of these conditions (e.g., dyspepsia, diarrhea-predominant irritable bowel syndrome [IBS], chronic constipation, defecatory disorders, and chronic abdominal pain). Dr. Keefer emphasized the importance of setting a pro-solution agenda early in the interview as well as listening, understanding, and believing symptoms. Empathy is essential. At the same time, patients need to assume personal responsibility and contribute to their own wellness. Expectations and a treatment plan should be negotiated. Bowel symptom questionnaires and, if necessary, bowel diaries ensure that symptoms are addressed comprehensively and save time. A meticulous digital rectal exam is essential since defecatory disorders are associated with not only lower but also upper GI symptoms. Only selected diagnostic tests, guided by the clinical features, should be performed.
Our understanding of the pathophysiology is evolving. Functional dyspepsia is implicated to impaired gastric accommodation, delayed gastric emptying, and increased gastric as well as duodenal sensitivity. Peripheral irritation (e.g., due to persistent low-grade inflammation after resolution of acute gastroenteritis or bile acids) and central dysfunctions (e.g., resulting from anxiety or depression) can alter GI transit and sensitivity resulting in IBS. Slow colon transit and impaired defecation (i.e., defecatory disorders) can cause chronic constipation.
Initially, therapy should utilize inexpensive, over-the-counter agents (loperamide for diarrhea). Dr. Camilleri also highlighted the utility of bile acid binding agents (e.g., cholestyramine and colesevelam) and when necessary, alosteron for diarrhea and cautioned attendees to use rifaximin as recommended by the Food and Drug Administration (i.e., up to three courses of 2 weeks) and not for long-term therapy. Several newer agents for these disorders are being developed. Dr. Crowe reminded the audience that foods often induce symptoms. In a recent study, a “common-sense” IBS diet was as effective as was a low-FODMAP diet for IBS. Eliminating gluten or wheat starch may benefit some patients with IBS without celiac disease but more evidence is required. Dr. Keefer highlighted the utility of diaphragmatic breathing for rumination, pelvic floor biofeedback therapy for defecatory disorders, and psychological therapies, especially cognitive behavioral therapy, for patients with a variety of GI symptoms.
Dr. Bharucha is with the division of gastroenterology and hepatology at Mayo Clinic, Rochester, Minn.
This is a summary provided by the moderator of one of the spring postgraduate course sessions held at DDW 2016.
Kicking off the 2016 AGA Spring Postgraduate Course, this symposium encouraged attendees to embrace multidisciplinary approaches to managing patients with common gastrointestinal symptoms of nonstructural origin. My talk on “Managing the Big Four – Dyspepsia, Constipation, Diarrhea, and Abdominal Pain” reviewed the pathophysiology and management of these conditions. Thereafter, Sheila Crowe, MD, AGAF, Laurie Keefer, PhD, and Michael Camilleri, MD, AGAF, respectively, reviewed dietary approaches, psychological, and behavioral therapies, and overlooked, overused, and emerging pharmacotherapy for managing these conditions.
The clinical evaluation enables a precise symptom-based diagnosis of these conditions (e.g., dyspepsia, diarrhea-predominant irritable bowel syndrome [IBS], chronic constipation, defecatory disorders, and chronic abdominal pain). Dr. Keefer emphasized the importance of setting a pro-solution agenda early in the interview as well as listening, understanding, and believing symptoms. Empathy is essential. At the same time, patients need to assume personal responsibility and contribute to their own wellness. Expectations and a treatment plan should be negotiated. Bowel symptom questionnaires and, if necessary, bowel diaries ensure that symptoms are addressed comprehensively and save time. A meticulous digital rectal exam is essential since defecatory disorders are associated with not only lower but also upper GI symptoms. Only selected diagnostic tests, guided by the clinical features, should be performed.
Our understanding of the pathophysiology is evolving. Functional dyspepsia is implicated to impaired gastric accommodation, delayed gastric emptying, and increased gastric as well as duodenal sensitivity. Peripheral irritation (e.g., due to persistent low-grade inflammation after resolution of acute gastroenteritis or bile acids) and central dysfunctions (e.g., resulting from anxiety or depression) can alter GI transit and sensitivity resulting in IBS. Slow colon transit and impaired defecation (i.e., defecatory disorders) can cause chronic constipation.
Initially, therapy should utilize inexpensive, over-the-counter agents (loperamide for diarrhea). Dr. Camilleri also highlighted the utility of bile acid binding agents (e.g., cholestyramine and colesevelam) and when necessary, alosteron for diarrhea and cautioned attendees to use rifaximin as recommended by the Food and Drug Administration (i.e., up to three courses of 2 weeks) and not for long-term therapy. Several newer agents for these disorders are being developed. Dr. Crowe reminded the audience that foods often induce symptoms. In a recent study, a “common-sense” IBS diet was as effective as was a low-FODMAP diet for IBS. Eliminating gluten or wheat starch may benefit some patients with IBS without celiac disease but more evidence is required. Dr. Keefer highlighted the utility of diaphragmatic breathing for rumination, pelvic floor biofeedback therapy for defecatory disorders, and psychological therapies, especially cognitive behavioral therapy, for patients with a variety of GI symptoms.
Dr. Bharucha is with the division of gastroenterology and hepatology at Mayo Clinic, Rochester, Minn.
This is a summary provided by the moderator of one of the spring postgraduate course sessions held at DDW 2016.
Kicking off the 2016 AGA Spring Postgraduate Course, this symposium encouraged attendees to embrace multidisciplinary approaches to managing patients with common gastrointestinal symptoms of nonstructural origin. My talk on “Managing the Big Four – Dyspepsia, Constipation, Diarrhea, and Abdominal Pain” reviewed the pathophysiology and management of these conditions. Thereafter, Sheila Crowe, MD, AGAF, Laurie Keefer, PhD, and Michael Camilleri, MD, AGAF, respectively, reviewed dietary approaches, psychological, and behavioral therapies, and overlooked, overused, and emerging pharmacotherapy for managing these conditions.
The clinical evaluation enables a precise symptom-based diagnosis of these conditions (e.g., dyspepsia, diarrhea-predominant irritable bowel syndrome [IBS], chronic constipation, defecatory disorders, and chronic abdominal pain). Dr. Keefer emphasized the importance of setting a pro-solution agenda early in the interview as well as listening, understanding, and believing symptoms. Empathy is essential. At the same time, patients need to assume personal responsibility and contribute to their own wellness. Expectations and a treatment plan should be negotiated. Bowel symptom questionnaires and, if necessary, bowel diaries ensure that symptoms are addressed comprehensively and save time. A meticulous digital rectal exam is essential since defecatory disorders are associated with not only lower but also upper GI symptoms. Only selected diagnostic tests, guided by the clinical features, should be performed.
Our understanding of the pathophysiology is evolving. Functional dyspepsia is implicated to impaired gastric accommodation, delayed gastric emptying, and increased gastric as well as duodenal sensitivity. Peripheral irritation (e.g., due to persistent low-grade inflammation after resolution of acute gastroenteritis or bile acids) and central dysfunctions (e.g., resulting from anxiety or depression) can alter GI transit and sensitivity resulting in IBS. Slow colon transit and impaired defecation (i.e., defecatory disorders) can cause chronic constipation.
Initially, therapy should utilize inexpensive, over-the-counter agents (loperamide for diarrhea). Dr. Camilleri also highlighted the utility of bile acid binding agents (e.g., cholestyramine and colesevelam) and when necessary, alosteron for diarrhea and cautioned attendees to use rifaximin as recommended by the Food and Drug Administration (i.e., up to three courses of 2 weeks) and not for long-term therapy. Several newer agents for these disorders are being developed. Dr. Crowe reminded the audience that foods often induce symptoms. In a recent study, a “common-sense” IBS diet was as effective as was a low-FODMAP diet for IBS. Eliminating gluten or wheat starch may benefit some patients with IBS without celiac disease but more evidence is required. Dr. Keefer highlighted the utility of diaphragmatic breathing for rumination, pelvic floor biofeedback therapy for defecatory disorders, and psychological therapies, especially cognitive behavioral therapy, for patients with a variety of GI symptoms.
Dr. Bharucha is with the division of gastroenterology and hepatology at Mayo Clinic, Rochester, Minn.
This is a summary provided by the moderator of one of the spring postgraduate course sessions held at DDW 2016.
The old order changeth, yielding place to new ...
The September 2016 edition of GI & Hepatology News will be the last under the current editorial team. After 5 years, it is time for us to step aside and make way for a new group of editors. I am personally delighted that editorship of the paper will be in the safe hands of John I. Allen, MD, MBA, AGAF, who served as the AGA Institute’s president from 2014 to 2015. The Associate Editors and I wish him and his team every success. My own involvement with GI & Hepatology News actually precedes my time as Editor in Chief (EIC). I was privileged to serve on the selection committee that recommended the appointment of Charles J. Lightdale, MD, AGAF as the inaugural editor of the paper. He did an outstanding job and was a very tough act to follow. Now, it is time for Dr. Allen to take control of the paper, and I am confident that he will do a similarly excellent job.
The title of this piece is taken from a longer quote of Alfred Lord Tennyson. Although a bit of a cliché, it initially seemed an appropriate choice. However, if you look it up in full, it might seem inordinately gloomy, which was not my intention since there is certainly no need for pessimism. (After all, change is inevitable – except from a vending machine, of course.) The newspaper has been highly rated by its readers in the various surveys conducted by AGA, our publisher, and others. The Associate Editors and I have tried to feature articles on a broad range of issues that we hoped would have been among the most relevant for our predominantly clinical readership. It has been an exciting time in gastroenterology; serving as EIC of GI & Hepatology News has brought a number of issues to my attention that I might have otherwise missed. I would like to take this opportunity to thank Lora T. McGlade and the contributing writers from Frontline Medical Communications who prepared our news coverage. Sometimes it was difficult to rank the priority of the articles on offer but I hope that we chose many that were newsworthy and relevant.
I am extremely grateful to the Associate Editors; all did an outstanding job and managed to keep to necessarily strict deadlines. Joel V. Brill, MD, AGAF, has been a terrific source of information about practice management and legislative issues and has made enormous contributions to the newspaper. Barbara H. Jung, MD, AGAF, who had responsibility for the broad topic of gastrointestinal oncology, frequently provided expert commentary and was extremely helpful in selecting articles to publish and in attracting commentary and perspective from other experts within AGA. John A. Martin, MD, took responsibility for endoscopy, obesity management, and pancreatic/biliary disease and frequently produced useful and succinct commentaries on some of the articles we published. Hepatology was the bailiwick of Kevin D. Mullen, MD, FRCPI, who helped select the most relevant articles and suggested experts to comment on the most important ones. David T. Rubin, MD, AGAF, handled IBD and intestinal disorders. Clearly there have been major advances in IBD management in recent years, with numerous new and emerging treatments. Dr. Rubin skillfully steered us through this maze, and gave much thoughtful guidance and expert input. With the Associate Editors’ help, I hope you will agree that we managed to keep the information in the paper as topical as possible for a monthly publication.
Thanks are also due to the team at AGA. Brook A. Simpson, as the lead staff member, has been responsible for managing all of the operations of GI & Hepatology News. Working with Brook was a distinct pleasure; she always provided excellent advice about AGA matters and its positions on key issues. I am also grateful to Erin C. Dubnansky, Lindsey M. Brounstein, and Jillian L. Schweitzer for their support.
In conclusion, it has been a privilege to serve as EIC of GI & Hepatology News over the past 5 years. I know that the paper is in good hands with Dr. Allen. I look forward to seeing the improvements and changes that he and his team will introduce.
Dr. Howden is Hyman Professor of Medicine, chief, division of gastroenterology, University of Tennessee Health Science Center, Memphis, and Editor in Chief, GI & Hepatology News.
The September 2016 edition of GI & Hepatology News will be the last under the current editorial team. After 5 years, it is time for us to step aside and make way for a new group of editors. I am personally delighted that editorship of the paper will be in the safe hands of John I. Allen, MD, MBA, AGAF, who served as the AGA Institute’s president from 2014 to 2015. The Associate Editors and I wish him and his team every success. My own involvement with GI & Hepatology News actually precedes my time as Editor in Chief (EIC). I was privileged to serve on the selection committee that recommended the appointment of Charles J. Lightdale, MD, AGAF as the inaugural editor of the paper. He did an outstanding job and was a very tough act to follow. Now, it is time for Dr. Allen to take control of the paper, and I am confident that he will do a similarly excellent job.
The title of this piece is taken from a longer quote of Alfred Lord Tennyson. Although a bit of a cliché, it initially seemed an appropriate choice. However, if you look it up in full, it might seem inordinately gloomy, which was not my intention since there is certainly no need for pessimism. (After all, change is inevitable – except from a vending machine, of course.) The newspaper has been highly rated by its readers in the various surveys conducted by AGA, our publisher, and others. The Associate Editors and I have tried to feature articles on a broad range of issues that we hoped would have been among the most relevant for our predominantly clinical readership. It has been an exciting time in gastroenterology; serving as EIC of GI & Hepatology News has brought a number of issues to my attention that I might have otherwise missed. I would like to take this opportunity to thank Lora T. McGlade and the contributing writers from Frontline Medical Communications who prepared our news coverage. Sometimes it was difficult to rank the priority of the articles on offer but I hope that we chose many that were newsworthy and relevant.
I am extremely grateful to the Associate Editors; all did an outstanding job and managed to keep to necessarily strict deadlines. Joel V. Brill, MD, AGAF, has been a terrific source of information about practice management and legislative issues and has made enormous contributions to the newspaper. Barbara H. Jung, MD, AGAF, who had responsibility for the broad topic of gastrointestinal oncology, frequently provided expert commentary and was extremely helpful in selecting articles to publish and in attracting commentary and perspective from other experts within AGA. John A. Martin, MD, took responsibility for endoscopy, obesity management, and pancreatic/biliary disease and frequently produced useful and succinct commentaries on some of the articles we published. Hepatology was the bailiwick of Kevin D. Mullen, MD, FRCPI, who helped select the most relevant articles and suggested experts to comment on the most important ones. David T. Rubin, MD, AGAF, handled IBD and intestinal disorders. Clearly there have been major advances in IBD management in recent years, with numerous new and emerging treatments. Dr. Rubin skillfully steered us through this maze, and gave much thoughtful guidance and expert input. With the Associate Editors’ help, I hope you will agree that we managed to keep the information in the paper as topical as possible for a monthly publication.
Thanks are also due to the team at AGA. Brook A. Simpson, as the lead staff member, has been responsible for managing all of the operations of GI & Hepatology News. Working with Brook was a distinct pleasure; she always provided excellent advice about AGA matters and its positions on key issues. I am also grateful to Erin C. Dubnansky, Lindsey M. Brounstein, and Jillian L. Schweitzer for their support.
In conclusion, it has been a privilege to serve as EIC of GI & Hepatology News over the past 5 years. I know that the paper is in good hands with Dr. Allen. I look forward to seeing the improvements and changes that he and his team will introduce.
Dr. Howden is Hyman Professor of Medicine, chief, division of gastroenterology, University of Tennessee Health Science Center, Memphis, and Editor in Chief, GI & Hepatology News.
The September 2016 edition of GI & Hepatology News will be the last under the current editorial team. After 5 years, it is time for us to step aside and make way for a new group of editors. I am personally delighted that editorship of the paper will be in the safe hands of John I. Allen, MD, MBA, AGAF, who served as the AGA Institute’s president from 2014 to 2015. The Associate Editors and I wish him and his team every success. My own involvement with GI & Hepatology News actually precedes my time as Editor in Chief (EIC). I was privileged to serve on the selection committee that recommended the appointment of Charles J. Lightdale, MD, AGAF as the inaugural editor of the paper. He did an outstanding job and was a very tough act to follow. Now, it is time for Dr. Allen to take control of the paper, and I am confident that he will do a similarly excellent job.
The title of this piece is taken from a longer quote of Alfred Lord Tennyson. Although a bit of a cliché, it initially seemed an appropriate choice. However, if you look it up in full, it might seem inordinately gloomy, which was not my intention since there is certainly no need for pessimism. (After all, change is inevitable – except from a vending machine, of course.) The newspaper has been highly rated by its readers in the various surveys conducted by AGA, our publisher, and others. The Associate Editors and I have tried to feature articles on a broad range of issues that we hoped would have been among the most relevant for our predominantly clinical readership. It has been an exciting time in gastroenterology; serving as EIC of GI & Hepatology News has brought a number of issues to my attention that I might have otherwise missed. I would like to take this opportunity to thank Lora T. McGlade and the contributing writers from Frontline Medical Communications who prepared our news coverage. Sometimes it was difficult to rank the priority of the articles on offer but I hope that we chose many that were newsworthy and relevant.
I am extremely grateful to the Associate Editors; all did an outstanding job and managed to keep to necessarily strict deadlines. Joel V. Brill, MD, AGAF, has been a terrific source of information about practice management and legislative issues and has made enormous contributions to the newspaper. Barbara H. Jung, MD, AGAF, who had responsibility for the broad topic of gastrointestinal oncology, frequently provided expert commentary and was extremely helpful in selecting articles to publish and in attracting commentary and perspective from other experts within AGA. John A. Martin, MD, took responsibility for endoscopy, obesity management, and pancreatic/biliary disease and frequently produced useful and succinct commentaries on some of the articles we published. Hepatology was the bailiwick of Kevin D. Mullen, MD, FRCPI, who helped select the most relevant articles and suggested experts to comment on the most important ones. David T. Rubin, MD, AGAF, handled IBD and intestinal disorders. Clearly there have been major advances in IBD management in recent years, with numerous new and emerging treatments. Dr. Rubin skillfully steered us through this maze, and gave much thoughtful guidance and expert input. With the Associate Editors’ help, I hope you will agree that we managed to keep the information in the paper as topical as possible for a monthly publication.
Thanks are also due to the team at AGA. Brook A. Simpson, as the lead staff member, has been responsible for managing all of the operations of GI & Hepatology News. Working with Brook was a distinct pleasure; she always provided excellent advice about AGA matters and its positions on key issues. I am also grateful to Erin C. Dubnansky, Lindsey M. Brounstein, and Jillian L. Schweitzer for their support.
In conclusion, it has been a privilege to serve as EIC of GI & Hepatology News over the past 5 years. I know that the paper is in good hands with Dr. Allen. I look forward to seeing the improvements and changes that he and his team will introduce.
Dr. Howden is Hyman Professor of Medicine, chief, division of gastroenterology, University of Tennessee Health Science Center, Memphis, and Editor in Chief, GI & Hepatology News.
New and Noteworthy Information—September 2016
Hospitalization of patients with stroke in primary stroke centers, compared with noncertified hospitals, is associated with decreased seven-day and 30-day case fatality, according to a study published online ahead of print July 25 in JAMA Internal Medicine. Among 865,184 elderly patients with stroke (mean age, 78.9; 55.5% female), 53.9% were treated in primary stroke centers. Admission to primary stroke centers was associated with 1.8% lower seven-day and 1.8% lower 30-day case fatality. Fifty-six patients with stroke needed to be treated in primary stroke centers to save one life at 30 days. Overall, receiving treatment in primary stroke centers was associated with a 30-day survival benefit for patients traveling less than 90 minutes, but traveling at least 90 minutes offset any benefit of care in primary stroke centers.
Obesity may increase the risk of neurodegeneration, according to a study published online ahead of print July 27 in Neurobiology of Aging. Participants were a population-based cohort of cognitively healthy adults recruited over a five-year period. In all, 527 subjects with an age range of 20 to 87 were included. Researchers performed a cross-sectional analysis of MRI-based brain structure and found a statistically significant interaction between age and BMI. Cortical reconstruction techniques were used to generate measures of whole brain cerebral white matter volume, cortical thickness, and surface area. Cerebral white matter volume in overweight and obese individuals was associated with a greater degree of atrophy, with maximal effects in middle age corresponding to an estimated increase in brain age of 10 years.
Thymectomy improves clinical outcomes over a three-year period in patients with nonthymomatous myasthenia gravis, according to a study published August 11 in the New England Journal of Medicine. Researchers randomized 126 patients to thymectomy plus alternate-day prednisone or alternate-day prednisone alone. Patients who underwent thymectomy had a lower time-weighted average Quantitative Myasthenia Gravis score over a three-year period than those who received prednisone alone. Patients in the thymectomy group also had a lower average requirement for alternate-day prednisone. Fewer patients in the thymectomy group than in the prednisone-only group required immunosuppression with azathioprine or were hospitalized for exacerbations. The number of patients with treatment-associated complications did not differ significantly between groups. However, patients in the thymectomy group had fewer treatment-associated symptoms related to immunosuppressive medications.
Calcium supplementation may increase the risk of developing dementia in elderly women with cerebrovascular disease, according to a study published online ahead of print August 17 in Neurology. This longitudinal population-based study included 700 women without dementia between ages 70 and 92. At baseline and at five-year follow-up, the women underwent comprehensive neuropsychiatric and somatic examinations. A CT scan also was performed in 447 participants at baseline. Information on the use and dosage of calcium supplements was collected. Women treated with calcium supplements had a higher risk of developing dementia and the subtype of stroke-related dementia. Calcium supplementation was associated with the development of dementia in groups with a history of stroke or presence of white matter lesions, but not in groups without these conditions.
Exposure to bright light during the day may help combat sleep disturbances associated with the evening use of electronic devices emitting blue light, according to a study published online ahead of print June 16 in Sleep Medicine. Following a constant bright light exposure over 6.5 hours, 14 participants read a novel either on a tablet or as a physical book for two hours. Evening concentrations of saliva melatonin were measured repeatedly. Sleepiness was assessed before and after nocturnal sleep. About one week later, experiments were repeated. Participants who had read the novel on a tablet in the first experimental session continued reading the same novel as a physical book, and vice versa. There were no differences in sleep parameters and presleep saliva melatonin levels between the tablet reading and physical book reading conditions.
Treatment immediately after clinically isolated syndrome (CIS) is more beneficial than delayed treatment, according to a study published online ahead of print August 10 in Neurology. Researchers randomized 278 people with CIS to interferon beta-1b or placebo. After two years or a diagnosis of multiple sclerosis (MS), patients receiving placebo could receive treatment. After 11 years, risk of clinically definite MS remained lower in the early-treatment arm, compared with the delayed-treatment arm, with longer time to first relapse and lower overall annualized relapse rate. Twenty-five patients converted to secondary progressive MS. Expanded Disability Status Scale scores remained low and stable, with no difference between treatment arms. The early-treatment group had better Paced Auditory Serial Addition Task-3 total scores. Health resource utilization was low in both groups.
Patients with anemia have increased mortality after stroke, according to a study published online ahead of print August 17 in the Journal of the American Heart Association. Researchers analyzed data from a cohort of 8,013 patients with stroke who were consecutively admitted over 11 years. Anemia was present in 24.5% of the cohort on admission and was associated with increased odds of mortality at most of the time points examined up to one year following stroke. Elevated hemoglobin also was associated with increased mortality. In addition, investigators conducted a systematic review using various databases. When combined with the cohort from the current study, the pooled population had 29,943 patients with stroke. Anemia on admission was associated with an increased risk of mortality in ischemic stroke and hemorrhagic stroke.
Bedside EEG methods may indicate the level of awareness of patients in a vegetative state, according to a study published online ahead of print August 4 in Annals of Neurology. Fourteen patients with severe brain injuries were evaluated with an EEG vibrotactile attention task designed to identify a hierarchy of residual somatosensory and cognitive abilities. Each patient also was assessed with a clinical behavioral scale and two fMRI assessments of covert command following. Six patients produced only sensory responses, with no evidence of cognitive event-related potentials. Furthermore, eight patients demonstrated reliable bottom-up attention-orienting responses. No patient showed evidence of top-down attention. Only patients who followed commands, whether overtly with behavior or covertly with functional neuroimaging, also demonstrated event-related potential evidence of attentional orienting.
The PET tracer [18F]-AV-1451 may help identify the stages of the preclinical and clinical phases of Alzheimer's disease, according to a study published online ahead of print July 25 in JAMA Neurology. In all, 59 participants (64% male; mean age, 74) underwent PET imaging. The [18F]-AV-1451 standardized uptake value ratio (SUVR) in the hippocampus and Alzheimer's disease cortical signature regions distinguished participants with Alzheimer's disease from cognitively normal participants. A SUVR cutoff value of 1.19 from Alzheimer's disease cortical signature regions best distinguished these groups. Amyloid β-positivity was associated with an elevated [18F]-AV-1451 SUVR in Alzheimer's disease cortical signature regions, but not in the hippocampus. Amyloid β-positivity alone was not related to hippocampal volume or Alzheimer's disease signature cortical thickness. An elevated [18F]-AV-1451 SUVR was associated with brain volumetric loss.
Symptom exacerbations after concussion are common among children and may not impede recovery, according to a study published online ahead of print August 1 in JAMA Pediatrics. Eligible participants were between ages 11 and 18 and had sustained a concussion that did not result in an abnormal CT scan or require hospital admission. The mean age of the 63 participants (34.9% girls) was 13.8. Symptom spikes occurred in 31.7% of the sample. An abrupt increase in mental activity from one day to the next increased the risk of a symptom spike. Patients with symptom spikes were initially more symptomatic in the emergency department and throughout the observation period, but did not differ from the group without symptom spikes on cognition or balance 10 days following injury.
The FDA has approved the supplemental Biologics License Application from Ipsen Biopharmaceuticals for Dysport (abobotulinumtoxinA) for injection in the treatment of lower limb spasticity in pediatric patients age 2 and older. This approval is based on a phase III pivotal study of 235 pediatric patients ages 2 to 17 with lower limb spasticity because of cerebral palsy causing dynamic equinus foot deformity. Patients treated with Dysport showed statistically significant improvement in ankle plantar flexor muscle tone. Like all botulinum toxin products, Dysport has a boxed warning stating that the effects of the botulinum toxin may spread from the area of injection to other areas of the body, causing symptoms similar to those of botulism. Ipsen Biopharmaceuticals is headquartered in Basking Ridge, New Jersey.
Lower BMI in late life is associated with greater cortical amyloid burden, according to a study published June 18 in the Journal of Alzheimer's Disease. The study entailed cross-sectional analyses that were completed using baseline data from the Harvard Aging Brain Study, which included 280 cognitively normal adults ages 62 to 90. Assessments included medical histories and physical exams, Pittsburgh compound B (PiB) PET amyloid imaging, and APOE4 genotyping. In the primary analysis, greater PiB retention was associated with lower BMI. In the secondary analyses, APOE4 carrier status and normal BMI, as opposed to overweight or obese BMI, were associated with greater PiB retention. The interaction between BMI and APOE4 also was significant. Future studies should seek to clarify the mechanism of this association, said the researchers.
Sleep-disordered breathing (SDB) and sleep-wake disturbances (SWD) increase the risk of stroke in the general population and affect short- and long-term stroke recovery and outcome, according to a literature review published online ahead of print August 3 in Neurology. Several studies have proven SDB to represent an independent risk factor for stroke. Sleep studies in patients with transient ischemic attack or stroke are recommended in view of the high prevalence of SDB, said the researchers. Treatment of obstructive SDB with continuous positive airway pressure is recommended, given the strength of the evidence that supports the treatment's benefit. Oxygen, biphasic positive airway pressure, and adaptive servoventilation may be considered in patients with central SDB, said the researchers. Experimental studies found that SWD may impair neuroplasticity and functional stroke recovery.
—Kimberly Williams
Hospitalization of patients with stroke in primary stroke centers, compared with noncertified hospitals, is associated with decreased seven-day and 30-day case fatality, according to a study published online ahead of print July 25 in JAMA Internal Medicine. Among 865,184 elderly patients with stroke (mean age, 78.9; 55.5% female), 53.9% were treated in primary stroke centers. Admission to primary stroke centers was associated with 1.8% lower seven-day and 1.8% lower 30-day case fatality. Fifty-six patients with stroke needed to be treated in primary stroke centers to save one life at 30 days. Overall, receiving treatment in primary stroke centers was associated with a 30-day survival benefit for patients traveling less than 90 minutes, but traveling at least 90 minutes offset any benefit of care in primary stroke centers.
Obesity may increase the risk of neurodegeneration, according to a study published online ahead of print July 27 in Neurobiology of Aging. Participants were a population-based cohort of cognitively healthy adults recruited over a five-year period. In all, 527 subjects with an age range of 20 to 87 were included. Researchers performed a cross-sectional analysis of MRI-based brain structure and found a statistically significant interaction between age and BMI. Cortical reconstruction techniques were used to generate measures of whole brain cerebral white matter volume, cortical thickness, and surface area. Cerebral white matter volume in overweight and obese individuals was associated with a greater degree of atrophy, with maximal effects in middle age corresponding to an estimated increase in brain age of 10 years.
Thymectomy improves clinical outcomes over a three-year period in patients with nonthymomatous myasthenia gravis, according to a study published August 11 in the New England Journal of Medicine. Researchers randomized 126 patients to thymectomy plus alternate-day prednisone or alternate-day prednisone alone. Patients who underwent thymectomy had a lower time-weighted average Quantitative Myasthenia Gravis score over a three-year period than those who received prednisone alone. Patients in the thymectomy group also had a lower average requirement for alternate-day prednisone. Fewer patients in the thymectomy group than in the prednisone-only group required immunosuppression with azathioprine or were hospitalized for exacerbations. The number of patients with treatment-associated complications did not differ significantly between groups. However, patients in the thymectomy group had fewer treatment-associated symptoms related to immunosuppressive medications.
Calcium supplementation may increase the risk of developing dementia in elderly women with cerebrovascular disease, according to a study published online ahead of print August 17 in Neurology. This longitudinal population-based study included 700 women without dementia between ages 70 and 92. At baseline and at five-year follow-up, the women underwent comprehensive neuropsychiatric and somatic examinations. A CT scan also was performed in 447 participants at baseline. Information on the use and dosage of calcium supplements was collected. Women treated with calcium supplements had a higher risk of developing dementia and the subtype of stroke-related dementia. Calcium supplementation was associated with the development of dementia in groups with a history of stroke or presence of white matter lesions, but not in groups without these conditions.
Exposure to bright light during the day may help combat sleep disturbances associated with the evening use of electronic devices emitting blue light, according to a study published online ahead of print June 16 in Sleep Medicine. Following a constant bright light exposure over 6.5 hours, 14 participants read a novel either on a tablet or as a physical book for two hours. Evening concentrations of saliva melatonin were measured repeatedly. Sleepiness was assessed before and after nocturnal sleep. About one week later, experiments were repeated. Participants who had read the novel on a tablet in the first experimental session continued reading the same novel as a physical book, and vice versa. There were no differences in sleep parameters and presleep saliva melatonin levels between the tablet reading and physical book reading conditions.
Treatment immediately after clinically isolated syndrome (CIS) is more beneficial than delayed treatment, according to a study published online ahead of print August 10 in Neurology. Researchers randomized 278 people with CIS to interferon beta-1b or placebo. After two years or a diagnosis of multiple sclerosis (MS), patients receiving placebo could receive treatment. After 11 years, risk of clinically definite MS remained lower in the early-treatment arm, compared with the delayed-treatment arm, with longer time to first relapse and lower overall annualized relapse rate. Twenty-five patients converted to secondary progressive MS. Expanded Disability Status Scale scores remained low and stable, with no difference between treatment arms. The early-treatment group had better Paced Auditory Serial Addition Task-3 total scores. Health resource utilization was low in both groups.
Patients with anemia have increased mortality after stroke, according to a study published online ahead of print August 17 in the Journal of the American Heart Association. Researchers analyzed data from a cohort of 8,013 patients with stroke who were consecutively admitted over 11 years. Anemia was present in 24.5% of the cohort on admission and was associated with increased odds of mortality at most of the time points examined up to one year following stroke. Elevated hemoglobin also was associated with increased mortality. In addition, investigators conducted a systematic review using various databases. When combined with the cohort from the current study, the pooled population had 29,943 patients with stroke. Anemia on admission was associated with an increased risk of mortality in ischemic stroke and hemorrhagic stroke.
Bedside EEG methods may indicate the level of awareness of patients in a vegetative state, according to a study published online ahead of print August 4 in Annals of Neurology. Fourteen patients with severe brain injuries were evaluated with an EEG vibrotactile attention task designed to identify a hierarchy of residual somatosensory and cognitive abilities. Each patient also was assessed with a clinical behavioral scale and two fMRI assessments of covert command following. Six patients produced only sensory responses, with no evidence of cognitive event-related potentials. Furthermore, eight patients demonstrated reliable bottom-up attention-orienting responses. No patient showed evidence of top-down attention. Only patients who followed commands, whether overtly with behavior or covertly with functional neuroimaging, also demonstrated event-related potential evidence of attentional orienting.
The PET tracer [18F]-AV-1451 may help identify the stages of the preclinical and clinical phases of Alzheimer's disease, according to a study published online ahead of print July 25 in JAMA Neurology. In all, 59 participants (64% male; mean age, 74) underwent PET imaging. The [18F]-AV-1451 standardized uptake value ratio (SUVR) in the hippocampus and Alzheimer's disease cortical signature regions distinguished participants with Alzheimer's disease from cognitively normal participants. A SUVR cutoff value of 1.19 from Alzheimer's disease cortical signature regions best distinguished these groups. Amyloid β-positivity was associated with an elevated [18F]-AV-1451 SUVR in Alzheimer's disease cortical signature regions, but not in the hippocampus. Amyloid β-positivity alone was not related to hippocampal volume or Alzheimer's disease signature cortical thickness. An elevated [18F]-AV-1451 SUVR was associated with brain volumetric loss.
Symptom exacerbations after concussion are common among children and may not impede recovery, according to a study published online ahead of print August 1 in JAMA Pediatrics. Eligible participants were between ages 11 and 18 and had sustained a concussion that did not result in an abnormal CT scan or require hospital admission. The mean age of the 63 participants (34.9% girls) was 13.8. Symptom spikes occurred in 31.7% of the sample. An abrupt increase in mental activity from one day to the next increased the risk of a symptom spike. Patients with symptom spikes were initially more symptomatic in the emergency department and throughout the observation period, but did not differ from the group without symptom spikes on cognition or balance 10 days following injury.
The FDA has approved the supplemental Biologics License Application from Ipsen Biopharmaceuticals for Dysport (abobotulinumtoxinA) for injection in the treatment of lower limb spasticity in pediatric patients age 2 and older. This approval is based on a phase III pivotal study of 235 pediatric patients ages 2 to 17 with lower limb spasticity because of cerebral palsy causing dynamic equinus foot deformity. Patients treated with Dysport showed statistically significant improvement in ankle plantar flexor muscle tone. Like all botulinum toxin products, Dysport has a boxed warning stating that the effects of the botulinum toxin may spread from the area of injection to other areas of the body, causing symptoms similar to those of botulism. Ipsen Biopharmaceuticals is headquartered in Basking Ridge, New Jersey.
Lower BMI in late life is associated with greater cortical amyloid burden, according to a study published June 18 in the Journal of Alzheimer's Disease. The study entailed cross-sectional analyses that were completed using baseline data from the Harvard Aging Brain Study, which included 280 cognitively normal adults ages 62 to 90. Assessments included medical histories and physical exams, Pittsburgh compound B (PiB) PET amyloid imaging, and APOE4 genotyping. In the primary analysis, greater PiB retention was associated with lower BMI. In the secondary analyses, APOE4 carrier status and normal BMI, as opposed to overweight or obese BMI, were associated with greater PiB retention. The interaction between BMI and APOE4 also was significant. Future studies should seek to clarify the mechanism of this association, said the researchers.
Sleep-disordered breathing (SDB) and sleep-wake disturbances (SWD) increase the risk of stroke in the general population and affect short- and long-term stroke recovery and outcome, according to a literature review published online ahead of print August 3 in Neurology. Several studies have proven SDB to represent an independent risk factor for stroke. Sleep studies in patients with transient ischemic attack or stroke are recommended in view of the high prevalence of SDB, said the researchers. Treatment of obstructive SDB with continuous positive airway pressure is recommended, given the strength of the evidence that supports the treatment's benefit. Oxygen, biphasic positive airway pressure, and adaptive servoventilation may be considered in patients with central SDB, said the researchers. Experimental studies found that SWD may impair neuroplasticity and functional stroke recovery.
—Kimberly Williams
Hospitalization of patients with stroke in primary stroke centers, compared with noncertified hospitals, is associated with decreased seven-day and 30-day case fatality, according to a study published online ahead of print July 25 in JAMA Internal Medicine. Among 865,184 elderly patients with stroke (mean age, 78.9; 55.5% female), 53.9% were treated in primary stroke centers. Admission to primary stroke centers was associated with 1.8% lower seven-day and 1.8% lower 30-day case fatality. Fifty-six patients with stroke needed to be treated in primary stroke centers to save one life at 30 days. Overall, receiving treatment in primary stroke centers was associated with a 30-day survival benefit for patients traveling less than 90 minutes, but traveling at least 90 minutes offset any benefit of care in primary stroke centers.
Obesity may increase the risk of neurodegeneration, according to a study published online ahead of print July 27 in Neurobiology of Aging. Participants were a population-based cohort of cognitively healthy adults recruited over a five-year period. In all, 527 subjects with an age range of 20 to 87 were included. Researchers performed a cross-sectional analysis of MRI-based brain structure and found a statistically significant interaction between age and BMI. Cortical reconstruction techniques were used to generate measures of whole brain cerebral white matter volume, cortical thickness, and surface area. Cerebral white matter volume in overweight and obese individuals was associated with a greater degree of atrophy, with maximal effects in middle age corresponding to an estimated increase in brain age of 10 years.
Thymectomy improves clinical outcomes over a three-year period in patients with nonthymomatous myasthenia gravis, according to a study published August 11 in the New England Journal of Medicine. Researchers randomized 126 patients to thymectomy plus alternate-day prednisone or alternate-day prednisone alone. Patients who underwent thymectomy had a lower time-weighted average Quantitative Myasthenia Gravis score over a three-year period than those who received prednisone alone. Patients in the thymectomy group also had a lower average requirement for alternate-day prednisone. Fewer patients in the thymectomy group than in the prednisone-only group required immunosuppression with azathioprine or were hospitalized for exacerbations. The number of patients with treatment-associated complications did not differ significantly between groups. However, patients in the thymectomy group had fewer treatment-associated symptoms related to immunosuppressive medications.
Calcium supplementation may increase the risk of developing dementia in elderly women with cerebrovascular disease, according to a study published online ahead of print August 17 in Neurology. This longitudinal population-based study included 700 women without dementia between ages 70 and 92. At baseline and at five-year follow-up, the women underwent comprehensive neuropsychiatric and somatic examinations. A CT scan also was performed in 447 participants at baseline. Information on the use and dosage of calcium supplements was collected. Women treated with calcium supplements had a higher risk of developing dementia and the subtype of stroke-related dementia. Calcium supplementation was associated with the development of dementia in groups with a history of stroke or presence of white matter lesions, but not in groups without these conditions.
Exposure to bright light during the day may help combat sleep disturbances associated with the evening use of electronic devices emitting blue light, according to a study published online ahead of print June 16 in Sleep Medicine. Following a constant bright light exposure over 6.5 hours, 14 participants read a novel either on a tablet or as a physical book for two hours. Evening concentrations of saliva melatonin were measured repeatedly. Sleepiness was assessed before and after nocturnal sleep. About one week later, experiments were repeated. Participants who had read the novel on a tablet in the first experimental session continued reading the same novel as a physical book, and vice versa. There were no differences in sleep parameters and presleep saliva melatonin levels between the tablet reading and physical book reading conditions.
Treatment immediately after clinically isolated syndrome (CIS) is more beneficial than delayed treatment, according to a study published online ahead of print August 10 in Neurology. Researchers randomized 278 people with CIS to interferon beta-1b or placebo. After two years or a diagnosis of multiple sclerosis (MS), patients receiving placebo could receive treatment. After 11 years, risk of clinically definite MS remained lower in the early-treatment arm, compared with the delayed-treatment arm, with longer time to first relapse and lower overall annualized relapse rate. Twenty-five patients converted to secondary progressive MS. Expanded Disability Status Scale scores remained low and stable, with no difference between treatment arms. The early-treatment group had better Paced Auditory Serial Addition Task-3 total scores. Health resource utilization was low in both groups.
Patients with anemia have increased mortality after stroke, according to a study published online ahead of print August 17 in the Journal of the American Heart Association. Researchers analyzed data from a cohort of 8,013 patients with stroke who were consecutively admitted over 11 years. Anemia was present in 24.5% of the cohort on admission and was associated with increased odds of mortality at most of the time points examined up to one year following stroke. Elevated hemoglobin also was associated with increased mortality. In addition, investigators conducted a systematic review using various databases. When combined with the cohort from the current study, the pooled population had 29,943 patients with stroke. Anemia on admission was associated with an increased risk of mortality in ischemic stroke and hemorrhagic stroke.
Bedside EEG methods may indicate the level of awareness of patients in a vegetative state, according to a study published online ahead of print August 4 in Annals of Neurology. Fourteen patients with severe brain injuries were evaluated with an EEG vibrotactile attention task designed to identify a hierarchy of residual somatosensory and cognitive abilities. Each patient also was assessed with a clinical behavioral scale and two fMRI assessments of covert command following. Six patients produced only sensory responses, with no evidence of cognitive event-related potentials. Furthermore, eight patients demonstrated reliable bottom-up attention-orienting responses. No patient showed evidence of top-down attention. Only patients who followed commands, whether overtly with behavior or covertly with functional neuroimaging, also demonstrated event-related potential evidence of attentional orienting.
The PET tracer [18F]-AV-1451 may help identify the stages of the preclinical and clinical phases of Alzheimer's disease, according to a study published online ahead of print July 25 in JAMA Neurology. In all, 59 participants (64% male; mean age, 74) underwent PET imaging. The [18F]-AV-1451 standardized uptake value ratio (SUVR) in the hippocampus and Alzheimer's disease cortical signature regions distinguished participants with Alzheimer's disease from cognitively normal participants. A SUVR cutoff value of 1.19 from Alzheimer's disease cortical signature regions best distinguished these groups. Amyloid β-positivity was associated with an elevated [18F]-AV-1451 SUVR in Alzheimer's disease cortical signature regions, but not in the hippocampus. Amyloid β-positivity alone was not related to hippocampal volume or Alzheimer's disease signature cortical thickness. An elevated [18F]-AV-1451 SUVR was associated with brain volumetric loss.
Symptom exacerbations after concussion are common among children and may not impede recovery, according to a study published online ahead of print August 1 in JAMA Pediatrics. Eligible participants were between ages 11 and 18 and had sustained a concussion that did not result in an abnormal CT scan or require hospital admission. The mean age of the 63 participants (34.9% girls) was 13.8. Symptom spikes occurred in 31.7% of the sample. An abrupt increase in mental activity from one day to the next increased the risk of a symptom spike. Patients with symptom spikes were initially more symptomatic in the emergency department and throughout the observation period, but did not differ from the group without symptom spikes on cognition or balance 10 days following injury.
The FDA has approved the supplemental Biologics License Application from Ipsen Biopharmaceuticals for Dysport (abobotulinumtoxinA) for injection in the treatment of lower limb spasticity in pediatric patients age 2 and older. This approval is based on a phase III pivotal study of 235 pediatric patients ages 2 to 17 with lower limb spasticity because of cerebral palsy causing dynamic equinus foot deformity. Patients treated with Dysport showed statistically significant improvement in ankle plantar flexor muscle tone. Like all botulinum toxin products, Dysport has a boxed warning stating that the effects of the botulinum toxin may spread from the area of injection to other areas of the body, causing symptoms similar to those of botulism. Ipsen Biopharmaceuticals is headquartered in Basking Ridge, New Jersey.
Lower BMI in late life is associated with greater cortical amyloid burden, according to a study published June 18 in the Journal of Alzheimer's Disease. The study entailed cross-sectional analyses that were completed using baseline data from the Harvard Aging Brain Study, which included 280 cognitively normal adults ages 62 to 90. Assessments included medical histories and physical exams, Pittsburgh compound B (PiB) PET amyloid imaging, and APOE4 genotyping. In the primary analysis, greater PiB retention was associated with lower BMI. In the secondary analyses, APOE4 carrier status and normal BMI, as opposed to overweight or obese BMI, were associated with greater PiB retention. The interaction between BMI and APOE4 also was significant. Future studies should seek to clarify the mechanism of this association, said the researchers.
Sleep-disordered breathing (SDB) and sleep-wake disturbances (SWD) increase the risk of stroke in the general population and affect short- and long-term stroke recovery and outcome, according to a literature review published online ahead of print August 3 in Neurology. Several studies have proven SDB to represent an independent risk factor for stroke. Sleep studies in patients with transient ischemic attack or stroke are recommended in view of the high prevalence of SDB, said the researchers. Treatment of obstructive SDB with continuous positive airway pressure is recommended, given the strength of the evidence that supports the treatment's benefit. Oxygen, biphasic positive airway pressure, and adaptive servoventilation may be considered in patients with central SDB, said the researchers. Experimental studies found that SWD may impair neuroplasticity and functional stroke recovery.
—Kimberly Williams
United States nears 1,400 cases of Zika in pregnant women
The number of new cases of pregnant women with laboratory evidence of Zika infection in the 50 states and the District of Columbia took a big jump during the week ending Aug. 18, 2016, while U.S. territories continued the strong increase that started the previous week, according to the Centers for Disease Control and Prevention.
There were 55 new cases of Zika virus infection among pregnant women in the 50 states and D.C. reported the week ending Aug. 18. The number of new cases had been dropping, with 19 new cases the week of Aug. 11, 31 the week ending Aug. 4, and 46 the week ending July 28.
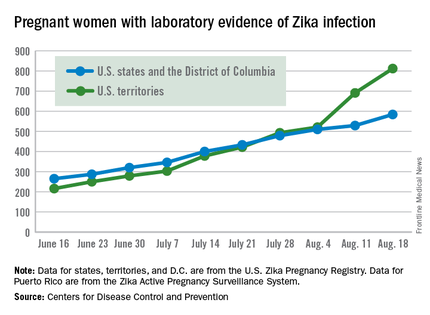
The territories had 121 new cases in the week ending Aug. 18, for a total of 176 new U.S. cases. For the year, there have been 1,396 cases of Zika in pregnant women in the United States: 584 in the states/D.C. and 812 in the territories, the CDC reported on Aug. 25. Among all Americans, there have been 11,528 cases of Zika virus in 2015-2016: 2,517 in the states/D.C. and 9,011 in the territories, of which 8,788 have occurred in Puerto Rico.
There were no new cases of Zika-related poor outcomes reported during the week ending Aug. 18, so the numbers of live-born infants who were born with birth defects remained at 16 in the states/D.C. and 1 in the territories, and pregnancy losses with birth defects held at five in the states/D.C. and one in the territories, the CDC said. State- or territorial-level data are not being reported to protect the privacy of affected women and children.
The figures for states, territories, and D.C. reflect reporting to the U.S. Zika Pregnancy Registry; data for Puerto Rico are reported to the U.S. Zika Active Pregnancy Surveillance System.
Zika virus–related birth defects recorded by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, and termination with evidence of birth defects.
The number of new cases of pregnant women with laboratory evidence of Zika infection in the 50 states and the District of Columbia took a big jump during the week ending Aug. 18, 2016, while U.S. territories continued the strong increase that started the previous week, according to the Centers for Disease Control and Prevention.
There were 55 new cases of Zika virus infection among pregnant women in the 50 states and D.C. reported the week ending Aug. 18. The number of new cases had been dropping, with 19 new cases the week of Aug. 11, 31 the week ending Aug. 4, and 46 the week ending July 28.

The territories had 121 new cases in the week ending Aug. 18, for a total of 176 new U.S. cases. For the year, there have been 1,396 cases of Zika in pregnant women in the United States: 584 in the states/D.C. and 812 in the territories, the CDC reported on Aug. 25. Among all Americans, there have been 11,528 cases of Zika virus in 2015-2016: 2,517 in the states/D.C. and 9,011 in the territories, of which 8,788 have occurred in Puerto Rico.
There were no new cases of Zika-related poor outcomes reported during the week ending Aug. 18, so the numbers of live-born infants who were born with birth defects remained at 16 in the states/D.C. and 1 in the territories, and pregnancy losses with birth defects held at five in the states/D.C. and one in the territories, the CDC said. State- or territorial-level data are not being reported to protect the privacy of affected women and children.
The figures for states, territories, and D.C. reflect reporting to the U.S. Zika Pregnancy Registry; data for Puerto Rico are reported to the U.S. Zika Active Pregnancy Surveillance System.
Zika virus–related birth defects recorded by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, and termination with evidence of birth defects.
The number of new cases of pregnant women with laboratory evidence of Zika infection in the 50 states and the District of Columbia took a big jump during the week ending Aug. 18, 2016, while U.S. territories continued the strong increase that started the previous week, according to the Centers for Disease Control and Prevention.
There were 55 new cases of Zika virus infection among pregnant women in the 50 states and D.C. reported the week ending Aug. 18. The number of new cases had been dropping, with 19 new cases the week of Aug. 11, 31 the week ending Aug. 4, and 46 the week ending July 28.

The territories had 121 new cases in the week ending Aug. 18, for a total of 176 new U.S. cases. For the year, there have been 1,396 cases of Zika in pregnant women in the United States: 584 in the states/D.C. and 812 in the territories, the CDC reported on Aug. 25. Among all Americans, there have been 11,528 cases of Zika virus in 2015-2016: 2,517 in the states/D.C. and 9,011 in the territories, of which 8,788 have occurred in Puerto Rico.
There were no new cases of Zika-related poor outcomes reported during the week ending Aug. 18, so the numbers of live-born infants who were born with birth defects remained at 16 in the states/D.C. and 1 in the territories, and pregnancy losses with birth defects held at five in the states/D.C. and one in the territories, the CDC said. State- or territorial-level data are not being reported to protect the privacy of affected women and children.
The figures for states, territories, and D.C. reflect reporting to the U.S. Zika Pregnancy Registry; data for Puerto Rico are reported to the U.S. Zika Active Pregnancy Surveillance System.
Zika virus–related birth defects recorded by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, and termination with evidence of birth defects.
Calcineurin inhibitor proves effective against lupus nephritis
Patients with highly active lupus nephritis who took the investigational oral calcineurin inhibitor voclosporin plus mycophenolate mofetil and tapered corticosteroids were twice as likely to achieve complete remission by 24 weeks, compared against placebo-treated patients who also received standard of care treatment in a phase IIb study trial reported by Aurinia Pharmaceuticals.
The 24-week complete remission primary endpoint of the AURA-LV(Aurinia Urinary Protein Reduction Active–Lupus With Voclosporin) study – defined as a urine protein/creatinine ratio of 0.5 mg/mg or less as well as normal stable renal function (estimated glomerular filtration rate of 60 mL/min per 1.73 m2 or greater or no confirmed decrease from baseline in eGFR of 20% or greater) – occurred in 32.6% of patients who were randomized to take 23.7 mg of voclosporin twice daily, which was significantly higher than the 19.3% rate observed in the placebo-treated group. The rate was 27.3% in a higher-dose group that received 39.5 mg of voclosporin twice daily.
Serious adverse events occurred at higher rates in both voclosporin arms of the trial than in the placebo arm, but Aurinia said in its statement announcing the results that the nature of the events was consistent with highly active lupus nephritis. A total of 13 deaths occurred, including 2 in the high-dose arm, 10 in the low-dose arm, and 1 in the placebo arm, but the company said that the investigator deemed the deaths as unrelated to voclosporin. Eleven of the deaths occurred in Asia.
Both low- and high-dose voclosporin arms attained a partial response by 24 weeks (50% drop in urine protein per creatinine ratio) in a significantly higher percentage of patients than did the placebo arm (69.7% and 65.9%, respectively, vs. 49.4%).
The Lupus Research Alliance welcomed the results of the study but noted that more needs to be known about the risk-benefit profile of the drug, specifically in reference to the 12 deaths reported in those who took voclosporin. “The magnitude of benefit is quite striking and unprecedented in lupus nephritis, but the number of deaths is a concern that must be taken seriously. We are very hopeful that further analysis of the safety data will confirm that voclosporin can provide a safe and effective treatment,” Margaret G. Dowd, co–chief executive officer of the Lupus Research Alliance, said in a statement.
The trial enrolled and randomized 265 patients diagnosed with highly active lupus nephritis (according to clinical signs and renal biopsy features) across centers in more than 20 countries. Besides being randomized to either active treatment arm or placebo, all patients received mycophenolate mofetil (CellCept) and oral corticosteroids that started at 20-25 mg/daily and then tapered down to 5 mg daily by week 8 and 2.5 mg daily by week 16. All patients also had an initial 500-1,000 mg intravenous dose of steroids.
Aurinia said that the study will continue to 48 weeks, and these data will be available in early 2017.
Patients with highly active lupus nephritis who took the investigational oral calcineurin inhibitor voclosporin plus mycophenolate mofetil and tapered corticosteroids were twice as likely to achieve complete remission by 24 weeks, compared against placebo-treated patients who also received standard of care treatment in a phase IIb study trial reported by Aurinia Pharmaceuticals.
The 24-week complete remission primary endpoint of the AURA-LV(Aurinia Urinary Protein Reduction Active–Lupus With Voclosporin) study – defined as a urine protein/creatinine ratio of 0.5 mg/mg or less as well as normal stable renal function (estimated glomerular filtration rate of 60 mL/min per 1.73 m2 or greater or no confirmed decrease from baseline in eGFR of 20% or greater) – occurred in 32.6% of patients who were randomized to take 23.7 mg of voclosporin twice daily, which was significantly higher than the 19.3% rate observed in the placebo-treated group. The rate was 27.3% in a higher-dose group that received 39.5 mg of voclosporin twice daily.
Serious adverse events occurred at higher rates in both voclosporin arms of the trial than in the placebo arm, but Aurinia said in its statement announcing the results that the nature of the events was consistent with highly active lupus nephritis. A total of 13 deaths occurred, including 2 in the high-dose arm, 10 in the low-dose arm, and 1 in the placebo arm, but the company said that the investigator deemed the deaths as unrelated to voclosporin. Eleven of the deaths occurred in Asia.
Both low- and high-dose voclosporin arms attained a partial response by 24 weeks (50% drop in urine protein per creatinine ratio) in a significantly higher percentage of patients than did the placebo arm (69.7% and 65.9%, respectively, vs. 49.4%).
The Lupus Research Alliance welcomed the results of the study but noted that more needs to be known about the risk-benefit profile of the drug, specifically in reference to the 12 deaths reported in those who took voclosporin. “The magnitude of benefit is quite striking and unprecedented in lupus nephritis, but the number of deaths is a concern that must be taken seriously. We are very hopeful that further analysis of the safety data will confirm that voclosporin can provide a safe and effective treatment,” Margaret G. Dowd, co–chief executive officer of the Lupus Research Alliance, said in a statement.
The trial enrolled and randomized 265 patients diagnosed with highly active lupus nephritis (according to clinical signs and renal biopsy features) across centers in more than 20 countries. Besides being randomized to either active treatment arm or placebo, all patients received mycophenolate mofetil (CellCept) and oral corticosteroids that started at 20-25 mg/daily and then tapered down to 5 mg daily by week 8 and 2.5 mg daily by week 16. All patients also had an initial 500-1,000 mg intravenous dose of steroids.
Aurinia said that the study will continue to 48 weeks, and these data will be available in early 2017.
Patients with highly active lupus nephritis who took the investigational oral calcineurin inhibitor voclosporin plus mycophenolate mofetil and tapered corticosteroids were twice as likely to achieve complete remission by 24 weeks, compared against placebo-treated patients who also received standard of care treatment in a phase IIb study trial reported by Aurinia Pharmaceuticals.
The 24-week complete remission primary endpoint of the AURA-LV(Aurinia Urinary Protein Reduction Active–Lupus With Voclosporin) study – defined as a urine protein/creatinine ratio of 0.5 mg/mg or less as well as normal stable renal function (estimated glomerular filtration rate of 60 mL/min per 1.73 m2 or greater or no confirmed decrease from baseline in eGFR of 20% or greater) – occurred in 32.6% of patients who were randomized to take 23.7 mg of voclosporin twice daily, which was significantly higher than the 19.3% rate observed in the placebo-treated group. The rate was 27.3% in a higher-dose group that received 39.5 mg of voclosporin twice daily.
Serious adverse events occurred at higher rates in both voclosporin arms of the trial than in the placebo arm, but Aurinia said in its statement announcing the results that the nature of the events was consistent with highly active lupus nephritis. A total of 13 deaths occurred, including 2 in the high-dose arm, 10 in the low-dose arm, and 1 in the placebo arm, but the company said that the investigator deemed the deaths as unrelated to voclosporin. Eleven of the deaths occurred in Asia.
Both low- and high-dose voclosporin arms attained a partial response by 24 weeks (50% drop in urine protein per creatinine ratio) in a significantly higher percentage of patients than did the placebo arm (69.7% and 65.9%, respectively, vs. 49.4%).
The Lupus Research Alliance welcomed the results of the study but noted that more needs to be known about the risk-benefit profile of the drug, specifically in reference to the 12 deaths reported in those who took voclosporin. “The magnitude of benefit is quite striking and unprecedented in lupus nephritis, but the number of deaths is a concern that must be taken seriously. We are very hopeful that further analysis of the safety data will confirm that voclosporin can provide a safe and effective treatment,” Margaret G. Dowd, co–chief executive officer of the Lupus Research Alliance, said in a statement.
The trial enrolled and randomized 265 patients diagnosed with highly active lupus nephritis (according to clinical signs and renal biopsy features) across centers in more than 20 countries. Besides being randomized to either active treatment arm or placebo, all patients received mycophenolate mofetil (CellCept) and oral corticosteroids that started at 20-25 mg/daily and then tapered down to 5 mg daily by week 8 and 2.5 mg daily by week 16. All patients also had an initial 500-1,000 mg intravenous dose of steroids.
Aurinia said that the study will continue to 48 weeks, and these data will be available in early 2017.
Changing payments, changing practice
The first article during my tenure as editor of the “Practice Management: The Road Ahead” section of Clinical Gastroenterology and Hepatology published in July 2012 (Clin Gastroenterol Hepatol. 2012;10:692-6) outlined anticipated changes in health care delivery, due in large part to mandates or trends contained in the Patient Protection and Affordable Care Act. A second article was published in 2013 (Health care reform 3.0: The road gets bumpy. Clin Gastroenterol Hepatol. 2013;11:1527-8). In this month’s column, Spencer D. Dorn, MD, MPH, MHA, of the University of North Carolina at Chapel Hill, adds a third update with an article focused on alternative payment models. These new reimbursement models are becoming common and will be part of all of our practice strategies in the years to come. No matter what occurs in the 2016 election, the movement from volume- to value-based payment will continue relentlessly, and practices that do not understand how to respond will struggle. We hope these articles will kick-start conversations in your practice.
Fee-for-service (FFS) reimbursement has been criticized for encouraging quantity over quality, favoring procedures over cognitive services, and fragmenting care.1 The landmark Patient Protection and Affordable Care Act (ACA) and more recent Medicare Access and Children’s Health Insurance Program Reauthorization Act (MACRA) modify Medicare’s FFS and encourage alternative payment models (APMs) that better reward value than volume.
Prior articles in this series have identified the specific trends driving gastroenterology practice strategies and business decisions,2 including an increasing need to demonstrate value, an emphasis on improved population health, an increasing number of practices becoming employees of large integrated delivery networks, reduced FFS reimbursements that are more closely linked to performance metrics, and increasing demands for risk-based contracts.3 In this article, I dive more deeply into these last two trends (declining FFS and the rise of APMs) and consider strategies gastroenterology practices can take in response.
Changes in fee for service
The ACA directed the secretary of Health and Human Services to establish a formal process to review potentially misvalued procedure codes. Compared with the pre-ACA fee schedule, the final 2016 Medicare Physician Fee Schedule includes cuts to professional fees for upper endoscopy, endoscopic retrograde cholangiopancreatography, endoscopic ultrasound, and colonoscopy. At the same time, over the past decade, facility fees paid for procedures performed in hospital outpatient departments have increased. Those to ambulatory surgery centers have gradually increased, although they still remain far below pre-2008 levels. Thus, the full economic impact of fee revaluation on an individual gastroenterology practice depends on whether it collects associated facility and ancillary fees.4
In addition, in the 2016 fee schedule, the Centers for Medicare & Medicaid Services described its intention to remove the value of moderate sedation from all gastrointestinal procedures. This is to prevent paying twice for sedation in procedures that involve anesthesiology professionals (i.e., one payment to the endoscopist as part of the overall procedure fee and a separate payment to the anesthesia professional for sedation they provide and bill for separately). The American Medical Association/Specialty Society Relative Value Scale Update Committee, using survey data from the GI specialty societies and other specialties that perform their own moderate sedation, has submitted recommendations for the value of a new set of moderate sedation Current Procedural Terminology codes to the CMS. The agency is expected to provide the specifics on how it will remove moderate sedation from the GI procedure codes in the 2017 Medicare Physician Fee Schedule Proposed Rule. The more that moderate sedation is valued, the less that endoscopic procedures will be valued. Consequently, gastroenterologists who rely on anesthesiology professionals to sedate their patients will generate less revenue per procedure, unless they rearrange contracts with anesthesia providers. Gastroenterologists who perform moderate sedation will not be impacted, because the sum of the value of the new moderate sedation code plus the underlying endoscopic procedure code will equal the original value of the procedure.
Beyond revaluing services, the CMS outlined its rather ambitious goal “to have 85% of all Medicare fee-for-service (FFS) payments tied to quality or value by 2016, and 90% by 2018.”5 Currently this includes the Physician Quality Reporting System (PQRS), which requires gastroenterologists to report performance on either three or more individual PQRS measures or one PQRS measures group (collection of related individual measures) or face a 2% Medicare payment penalty. It also includes the value-based payment modifier, through which by 2017 all practices with better-than-average quality (linked to PQRS measures) and lower costs will receive bonus payments, whereas those with worse-than-average performance (or who choose not to report) will be penalized.
MACRA changes all of this. Starting in 2019, the meaningful use incentive program, PQRS, and value-based payment modifier will be consolidated into the Merit-Based Incentive Payment System (MIPS). Physicians who elect to remain on an FFS tract will receive a 0-100 composite performance score based on quality (30%), resource use (30%), meaningful use (25%), and clinical practice improvement activities (15%). At the start of a performance period, a composite threshold necessary to achieve incentive payments and avoid penalties will be determined. Throughout the performance period, physicians will receive timely feedback on their performance. At year’s end, those below the threshold will face penalties proportionate to their performance (as much as 4% in 2019 and going up to 9% in 2022), those at threshold will not receive a payment adjustment, and those above threshold will receive bonuses proportionate to their performance (although overall payments will be capped at $500 million).
Alternative payment models
The CMS’s ultimate goal is to move beyond FFS and have “30% of Medicare payments tied to quality or value through APMs by the end of 2016 and 50% of payments by the end of 2018.”5 MACRA supports this ambitious goal: Starting in 2019, providers who “sufficiently” participate in APMs will receive 5% across-the-board bonuses. The three main APMs are bundled payments, accountable care organizations (ACOs), and patient-centered medical homes.
A bundled payment is a single fixed price paid to cover services for a specific episode of care. Depending on how an episode is defined, the bundle may encompass all professional fees, facility fees, and medical device and supply costs for a given service, including postacute care and any complications. If costs are reduced beyond the already discounted price of the bundle and quality metrics are achieved, then participants share the savings. Conversely, if costs exceed the bundled payment amount, then participants lose money. Unlike FFS, bundling incentivizes participants to coordinate care, reduce complications and unnecessary services, and cut purchasing costs.
To date, the CMS has launched three bundling programs. The Acute Care Episode Demonstration Project provided hospitals and clinicians a bundled payment to cover orthopedic and cardiovascular procedure–related episodes of care. This program reduced Medicare costs, primarily because the bundle payment was lower than what the sum of individual payments would have been. Providers were able to cope mainly by reducing their surgical implant costs. Second, more than 6,000 providers are currently participating in Medicare’s Bundled Payments for Care Improvement Program. The results of this program have not yet been released. Third, the CMS recently announced the Comprehensive Care for Joint Replacement Program under which hospitals and physicians in 67 metropolitan areas will be required to participate. Mandatory participation signals the CMS’s strong motivation to shift away from FFS. Beyond Medicare, many commercial insurers offer bundled payment programs, primarily for cardiovascular and orthopedic conditions.6 Although these programs are promising, it is technically challenging to define what is in a bundle, and to adequately risk adjust and mitigate random variation in spending for certain episodes of care. Providers are also challenged to find ways to divide payment among participants, coordinate all care, and accept financial risk.7,8 The American Gastroenterological Association recently published a bundled payment framework for screening and surveillance colonoscopy.9 Bundling other gastroenterology services will be more challenging.
Whereas bundled-care programs focus on a discrete service (e.g., knee replacement or colonoscopy), ACOs are integrated groups of providers who jointly assume responsibility for the cost and quality of all care delivered to a defined population. The ACA requires ACOs to have formal legal, leadership, and management structures; care for at least 5,000 Medicare beneficiaries; fulfill certain patient-centeredness criteria; measure and report quality and cost data; and coordinate care. Different payment models incentivize ACOs to reduce costs and improve quality of care. ACOs operating under a one-sided shared savings model receive FFS payments for each service delivered, along with a bonus for reducing costs below a spending target and meeting quality requirements. There are no potential financial penalties. Alternatively, ACOs operating under a two-sided risk-savings model share a greater proportion of cost savings, in exchange for potential financial penalties if the cost of care exceeds target spending.
To date, Medicare-sponsored ACOs have produced mixed results. In 2014 only 92 of the 322 Medicare Shared Savings ACOs were able to reduce spending below a predetermined benchmark by a predetermined amount (2%-3%) while meeting quality scores, thereby earning a bonus ($341 million in total). Similarly, of the original 32 pioneer ACOs, which by definition are more experienced at managing population health and more willing to take on financial risk, 13 dropped out of the program, and in 2014, only 11 generated enough savings to earn a payout ($82 million in total), whereas 5 incurred financial penalties ($9 million in total) for costs exceeding target thresholds.10 In total, after paying out bonuses, the ACO program cost Medicare a net loss of nearly $3 million, far from the $10-$240 million Medicare had previously projected it would save through the ACO program.11 Clearly, ACOs are not a quick fix for all that ails health care. For many ACOs, the major start-up requirements (time, capital investments, and so forth) needed to manage a population may not be worthwhile.12 Nonetheless, the CMS recently launched the Next Generation ACO model through which 21 participating ACOs will assume higher levels of financial risk (possibly capitated payments) in exchange for greater potential rewards. Similarly, beyond Medicare, there are also many Medicaid-sponsored ACOs and hundreds of commercial payer-sponsored ACOs.13
Finally, practices can qualify for APM status without accepting bundled payments or joining an ACO by qualifying as a patient-centered medical home. One option for gastroenterologists and other specialists is the National Committee for Quality Assurance’s patient-centered specialty practice designation, available to practices that successfully demonstrate their ability to track and coordinate care with primary care providers and other specialists, offer timely appointments and responses to telephone and electronic messages, use evidence-based tools to manage care for specific patient populations, develop patient-centered care plans, and measure and improve performance.14
Consolidation
Health insurers are merging to increase scale (and negotiating power), enhance efficiency (reducing administrative costs makes more room for profits), and diversify their businesses. Recently proposed acquisitions will bring “the big five” health insurers to the “big three.” Likewise, health care systems are rapidly acquiring hospitals and physician groups, so much so that today half of all American health care markets are now considered highly concentrated, and none are considered highly competitive.15 Today only 35% of all physicians are independently employed.16 Physicians employed by health systems trade their complete autonomy to offset declining reimbursement, reduce operating expenses (including health information technology costs), improve work-life balance, and mitigate unknown risks.
Proponents contend that these mergers allow health care systems to better coordinate care, improve care experiences, accommodate new payment models, and assemble the building blocks needed to form ACOs and other integrated care models. Critics argue that locally dominant systems drive volume (by tightening referral relationships and gaining new market share) and increase costs (through enhanced negotiating leverage and by reclassifying newly acquired physician practices as part of the hospital, thereby generating facility fees). It is unclear whether consolidation results in better outcomes or simply increases overall costs.17
Strategic imperatives
What should gastroenterologists do? First, recognize that FFS is not going away anytime soon.18 Most APMs are still largely in their experimentation phase, and it remains unclear which models will work and which will be broadly adopted. Still, it is unrealistic to expect FFS to indefinitely persist as the dominant payment model. For some services FFS may no longer be a payment option (e.g., Medicare’s BCPI [Bundled Payments for Care Improvement]). For others, FFS rates may become so unattractive that APMs seem necessary. Finally, APMs may allow some practices to capture a greater proportion of overall clinical revenue (e.g., academic practices that perform endoscopic procedures within hospital outpatient departments) and to develop new models that meaningfully improve care. Today’s gastroenterology practices must therefore operate on two separate tracks: an FFS track that rewards volume (most practices are optimized for this) and an alternative payment track that rewards value (few practices can accommodate these on their own). The degree and speed with which practices should reorient to the alternative payment track depends on the type of practice and the specific local health care market. But even practices operating in slower-to-evolve markets should start preparing for the APMs, no matter how far off in the distance they may seem. I recommend the following six steps:
1. Integrate. To participate in APMs, preserve referral streams, and maintain negotiating leverage with health plans, independent, community-based practices may need to affiliate or merge with other physician groups, or align with or be acquired by a health care system.19 Academic practices are challenged to define their role within health care systems that are rapidly adding primary care practices, and often community gastroenterology practices, too.
2. Collaborate and communicate. To deliver high-value care to populations of patients, gastroenterologists must closely collaborate and clearly communicate with primary care physicians and other specialists. Collaborative care agreements can help guide these relationships.203. Develop new models of care. Patients with more routine GI and liver-related problems may be served more cost effectively by midlevel providers21 or innovative solutions, such as e-consultations.22 Patients with complex, chronic GI and liver diseases may be best served by multidisciplinary care teams (e.g., gastroenterologists alongside midlevel providers, nurses, care managers, psychologists, and/or pharmacists) who use clinical information systems to identify high-risk patients and to encourage evidence-based decision making, and who support patients to self-manage their own conditions.23 Previously infeasible in a purely FFS world, these models are encouraged by APMs.
4. Care for common, costly conditions. Most gastroenterology practices have built robust colorectal cancer screening programs, sometimes at the expense of cognitive-based services. In today’s more accountable world, practices that can effectively manage common, costly conditions, such as inflammatory bowel disease, functional GI disorders, and advanced liver diseases, will be rewarded better than before and will be more highly sought as partners.
5. Understand and contain costs. The timely, accurate data needed to effectively respond to APMs are challenging to come by.19 Individual clinicians and group practices can roughly gauge their costs of care for Medicare beneficiaries, compared with other practices, using CMS Quality Resource Utilization Reports. Local commercial insurers may be willing to share cost profiles with interested practices. Strategies to reduce costs may include shifting clinically appropriate patients to more cost-effective settings (especially important for academic practices that see the bulk of their patients in costly hospital outpatient departments), standardizing endoscopy supplies and devices, using anesthesia services more selectively, and preferentially prescribing generic drugs, among others.
6. Measure and demonstrate value. Despite the inherent limitations of performance measurement,24 it is imperative that practices measure and report the value of care to their patients, community, and payers so that they are preferred partners and not locked out of insurance or referral networks. Improving patient experiences is intrinsically worthwhile25 and also makes good business sense.26
References
1. Miller, H.D. Creating payment systems to accelerate value-driven health care: Issues and options for policy reform. New York: The Commonwealth Fund, 2007.
2. Allen, J.I. The road ahead. Clin Gastroenterol Hepatol. 2012;10:692–6.
3. Allen, J.I. Health care reform 3.0: The road gets bumpy. Clin Gastroenterol Hepatol. 2013;11:1527-8.
4. Dorn, S.D., Vesy C.J. Medicare’s revaluation of gastrointestinal endoscopic procedures: Implications for academic and community-based practices. Clin Gastroenterol Hepatol. 2016 (in press).
5. Burwell, S.M. Setting value-based payment goals: HHS efforts to improve U.S. health care (N Engl J Med. 2015;372:897–9).
6. The Advisory Board Company. Commercial Bundled Payment Tracker, 2016.
7. Mechanic, R.E. Mandatory Medicare bundled payment – is it ready for prime time? N Engl J Med. 2015;373:1291–3.
8. The National Commission on Physician Payment Reform. Physician Payment Report, 2013.
9. Brill, J.V., Jain, R., Margolis, P.S., et al. A bundled payment framework for colonoscopy performed for colorectal cancer screening or surveillance. Gastroenterology. 2014;146:849–53, e9.
10. Evans, M. Few Medicare ACOs earned bonuses in 2014. Mod Healthc (2015). Available at: www.modernhealthcare.com/article/20150825/NEWS/150829922. Accessed Nov. 14, 2015.
11. Rau, J., Gold, J. Medicare yet to save money through heralded medical payment model. Kaiser Health News. Available at: http://khn.org/news/medicare-yet-to-save-money-through-heralded-medical-payment-model. Accessed Nov. 14, 2015.
12. Goldmsith, J., Kaufman, N. Pioneer ACOs: Anatomy of a victory. Health Affairs Blog, 2015.
13. Tu, T., Muhlestein, D., Kocot, S.L., et al. The impact of accountable care: Origins and future of accountable care organizations. Washington, D.C.: Brookings Institution, 2015.
14. NCQA. Patient-centered specialty practice frequently asked questions.
15. Xu, T., Wu, A.W., Makary, M.A. The potential hazards of hospital consolidation: Implications for quality, access, and price. JAMA. 2015;314:1337–8.
16. The Physician’s Foundation. 2014 survey of America’s physicians. Practice patterns & perspectives: The Physician’s Foundation.
17. Tsai, T.C., Jha, A.K. Hospital consolidation, competition, and quality: Is bigger necessarily better? JAMA. 2014;312:29–30.
18. Ginsburg, P.B. Fee-for-service will remain a feature of major payment reforms, requiring more changes in Medicare physician payment. Health Aff (Millwood). 2012;31:1977–83.
19. Friedberg, M.W., Chen, P.G., White, C., et al. Effects of health care payment models on physician practice in the United States. Santa Monica, Calif.: RAND Corp., 2015.
20. Greenberg, J.O., Barnett, M.L., Spinks, M.A., et al. The “medical neighborhood”: Integrating primary and specialty care for ambulatory patients. JAMA Intern Med. 2014;174:454–7.
21. Dorn, S.D. Mid-level providers in gastroenterology. Am J Gastroenterol. 2010;105:246–51.
22. Wasfy, J.H., Rao, S.K., Kalwani, N., et al. Longer term impact of cardiology e-consults. Am Heart J. 2016;173:86–93.
23. Coleman, K., Austin, B.T., Brach, C., et al. Evidence on the chronic care model in the new millennium. Health Aff (Millwood). 2009;28:75–85.
24. Dorn, S.D. Quality measurement in gastroenterology: confessions of a realist. Clin Gastroenterol Hepatol. 2016;14:648–50.
25. Berwick, D.M. Measuring physicians’ quality and performance: adrift on Lake Wobegon. JAMA. 2009;302:2485-6.
26. Browne, K., Roseman, D., Shaller, D., et al. Analysis & commentary. Measuring patient experience as a strategy for improving primary care. Health Aff (Millwood). 2010;29:921–5.
Dr. Dorn is vice chief, division of gastroenterology and hepatology, associate professor of medicine, health policy & management, University of North Carolina at Chapel Hill. He has received honoraria for consulting and presentations on health reform from AbbVie and Olympus.
The first article during my tenure as editor of the “Practice Management: The Road Ahead” section of Clinical Gastroenterology and Hepatology published in July 2012 (Clin Gastroenterol Hepatol. 2012;10:692-6) outlined anticipated changes in health care delivery, due in large part to mandates or trends contained in the Patient Protection and Affordable Care Act. A second article was published in 2013 (Health care reform 3.0: The road gets bumpy. Clin Gastroenterol Hepatol. 2013;11:1527-8). In this month’s column, Spencer D. Dorn, MD, MPH, MHA, of the University of North Carolina at Chapel Hill, adds a third update with an article focused on alternative payment models. These new reimbursement models are becoming common and will be part of all of our practice strategies in the years to come. No matter what occurs in the 2016 election, the movement from volume- to value-based payment will continue relentlessly, and practices that do not understand how to respond will struggle. We hope these articles will kick-start conversations in your practice.
Fee-for-service (FFS) reimbursement has been criticized for encouraging quantity over quality, favoring procedures over cognitive services, and fragmenting care.1 The landmark Patient Protection and Affordable Care Act (ACA) and more recent Medicare Access and Children’s Health Insurance Program Reauthorization Act (MACRA) modify Medicare’s FFS and encourage alternative payment models (APMs) that better reward value than volume.
Prior articles in this series have identified the specific trends driving gastroenterology practice strategies and business decisions,2 including an increasing need to demonstrate value, an emphasis on improved population health, an increasing number of practices becoming employees of large integrated delivery networks, reduced FFS reimbursements that are more closely linked to performance metrics, and increasing demands for risk-based contracts.3 In this article, I dive more deeply into these last two trends (declining FFS and the rise of APMs) and consider strategies gastroenterology practices can take in response.
Changes in fee for service
The ACA directed the secretary of Health and Human Services to establish a formal process to review potentially misvalued procedure codes. Compared with the pre-ACA fee schedule, the final 2016 Medicare Physician Fee Schedule includes cuts to professional fees for upper endoscopy, endoscopic retrograde cholangiopancreatography, endoscopic ultrasound, and colonoscopy. At the same time, over the past decade, facility fees paid for procedures performed in hospital outpatient departments have increased. Those to ambulatory surgery centers have gradually increased, although they still remain far below pre-2008 levels. Thus, the full economic impact of fee revaluation on an individual gastroenterology practice depends on whether it collects associated facility and ancillary fees.4
In addition, in the 2016 fee schedule, the Centers for Medicare & Medicaid Services described its intention to remove the value of moderate sedation from all gastrointestinal procedures. This is to prevent paying twice for sedation in procedures that involve anesthesiology professionals (i.e., one payment to the endoscopist as part of the overall procedure fee and a separate payment to the anesthesia professional for sedation they provide and bill for separately). The American Medical Association/Specialty Society Relative Value Scale Update Committee, using survey data from the GI specialty societies and other specialties that perform their own moderate sedation, has submitted recommendations for the value of a new set of moderate sedation Current Procedural Terminology codes to the CMS. The agency is expected to provide the specifics on how it will remove moderate sedation from the GI procedure codes in the 2017 Medicare Physician Fee Schedule Proposed Rule. The more that moderate sedation is valued, the less that endoscopic procedures will be valued. Consequently, gastroenterologists who rely on anesthesiology professionals to sedate their patients will generate less revenue per procedure, unless they rearrange contracts with anesthesia providers. Gastroenterologists who perform moderate sedation will not be impacted, because the sum of the value of the new moderate sedation code plus the underlying endoscopic procedure code will equal the original value of the procedure.
Beyond revaluing services, the CMS outlined its rather ambitious goal “to have 85% of all Medicare fee-for-service (FFS) payments tied to quality or value by 2016, and 90% by 2018.”5 Currently this includes the Physician Quality Reporting System (PQRS), which requires gastroenterologists to report performance on either three or more individual PQRS measures or one PQRS measures group (collection of related individual measures) or face a 2% Medicare payment penalty. It also includes the value-based payment modifier, through which by 2017 all practices with better-than-average quality (linked to PQRS measures) and lower costs will receive bonus payments, whereas those with worse-than-average performance (or who choose not to report) will be penalized.
MACRA changes all of this. Starting in 2019, the meaningful use incentive program, PQRS, and value-based payment modifier will be consolidated into the Merit-Based Incentive Payment System (MIPS). Physicians who elect to remain on an FFS tract will receive a 0-100 composite performance score based on quality (30%), resource use (30%), meaningful use (25%), and clinical practice improvement activities (15%). At the start of a performance period, a composite threshold necessary to achieve incentive payments and avoid penalties will be determined. Throughout the performance period, physicians will receive timely feedback on their performance. At year’s end, those below the threshold will face penalties proportionate to their performance (as much as 4% in 2019 and going up to 9% in 2022), those at threshold will not receive a payment adjustment, and those above threshold will receive bonuses proportionate to their performance (although overall payments will be capped at $500 million).
Alternative payment models
The CMS’s ultimate goal is to move beyond FFS and have “30% of Medicare payments tied to quality or value through APMs by the end of 2016 and 50% of payments by the end of 2018.”5 MACRA supports this ambitious goal: Starting in 2019, providers who “sufficiently” participate in APMs will receive 5% across-the-board bonuses. The three main APMs are bundled payments, accountable care organizations (ACOs), and patient-centered medical homes.
A bundled payment is a single fixed price paid to cover services for a specific episode of care. Depending on how an episode is defined, the bundle may encompass all professional fees, facility fees, and medical device and supply costs for a given service, including postacute care and any complications. If costs are reduced beyond the already discounted price of the bundle and quality metrics are achieved, then participants share the savings. Conversely, if costs exceed the bundled payment amount, then participants lose money. Unlike FFS, bundling incentivizes participants to coordinate care, reduce complications and unnecessary services, and cut purchasing costs.
To date, the CMS has launched three bundling programs. The Acute Care Episode Demonstration Project provided hospitals and clinicians a bundled payment to cover orthopedic and cardiovascular procedure–related episodes of care. This program reduced Medicare costs, primarily because the bundle payment was lower than what the sum of individual payments would have been. Providers were able to cope mainly by reducing their surgical implant costs. Second, more than 6,000 providers are currently participating in Medicare’s Bundled Payments for Care Improvement Program. The results of this program have not yet been released. Third, the CMS recently announced the Comprehensive Care for Joint Replacement Program under which hospitals and physicians in 67 metropolitan areas will be required to participate. Mandatory participation signals the CMS’s strong motivation to shift away from FFS. Beyond Medicare, many commercial insurers offer bundled payment programs, primarily for cardiovascular and orthopedic conditions.6 Although these programs are promising, it is technically challenging to define what is in a bundle, and to adequately risk adjust and mitigate random variation in spending for certain episodes of care. Providers are also challenged to find ways to divide payment among participants, coordinate all care, and accept financial risk.7,8 The American Gastroenterological Association recently published a bundled payment framework for screening and surveillance colonoscopy.9 Bundling other gastroenterology services will be more challenging.
Whereas bundled-care programs focus on a discrete service (e.g., knee replacement or colonoscopy), ACOs are integrated groups of providers who jointly assume responsibility for the cost and quality of all care delivered to a defined population. The ACA requires ACOs to have formal legal, leadership, and management structures; care for at least 5,000 Medicare beneficiaries; fulfill certain patient-centeredness criteria; measure and report quality and cost data; and coordinate care. Different payment models incentivize ACOs to reduce costs and improve quality of care. ACOs operating under a one-sided shared savings model receive FFS payments for each service delivered, along with a bonus for reducing costs below a spending target and meeting quality requirements. There are no potential financial penalties. Alternatively, ACOs operating under a two-sided risk-savings model share a greater proportion of cost savings, in exchange for potential financial penalties if the cost of care exceeds target spending.
To date, Medicare-sponsored ACOs have produced mixed results. In 2014 only 92 of the 322 Medicare Shared Savings ACOs were able to reduce spending below a predetermined benchmark by a predetermined amount (2%-3%) while meeting quality scores, thereby earning a bonus ($341 million in total). Similarly, of the original 32 pioneer ACOs, which by definition are more experienced at managing population health and more willing to take on financial risk, 13 dropped out of the program, and in 2014, only 11 generated enough savings to earn a payout ($82 million in total), whereas 5 incurred financial penalties ($9 million in total) for costs exceeding target thresholds.10 In total, after paying out bonuses, the ACO program cost Medicare a net loss of nearly $3 million, far from the $10-$240 million Medicare had previously projected it would save through the ACO program.11 Clearly, ACOs are not a quick fix for all that ails health care. For many ACOs, the major start-up requirements (time, capital investments, and so forth) needed to manage a population may not be worthwhile.12 Nonetheless, the CMS recently launched the Next Generation ACO model through which 21 participating ACOs will assume higher levels of financial risk (possibly capitated payments) in exchange for greater potential rewards. Similarly, beyond Medicare, there are also many Medicaid-sponsored ACOs and hundreds of commercial payer-sponsored ACOs.13
Finally, practices can qualify for APM status without accepting bundled payments or joining an ACO by qualifying as a patient-centered medical home. One option for gastroenterologists and other specialists is the National Committee for Quality Assurance’s patient-centered specialty practice designation, available to practices that successfully demonstrate their ability to track and coordinate care with primary care providers and other specialists, offer timely appointments and responses to telephone and electronic messages, use evidence-based tools to manage care for specific patient populations, develop patient-centered care plans, and measure and improve performance.14
Consolidation
Health insurers are merging to increase scale (and negotiating power), enhance efficiency (reducing administrative costs makes more room for profits), and diversify their businesses. Recently proposed acquisitions will bring “the big five” health insurers to the “big three.” Likewise, health care systems are rapidly acquiring hospitals and physician groups, so much so that today half of all American health care markets are now considered highly concentrated, and none are considered highly competitive.15 Today only 35% of all physicians are independently employed.16 Physicians employed by health systems trade their complete autonomy to offset declining reimbursement, reduce operating expenses (including health information technology costs), improve work-life balance, and mitigate unknown risks.
Proponents contend that these mergers allow health care systems to better coordinate care, improve care experiences, accommodate new payment models, and assemble the building blocks needed to form ACOs and other integrated care models. Critics argue that locally dominant systems drive volume (by tightening referral relationships and gaining new market share) and increase costs (through enhanced negotiating leverage and by reclassifying newly acquired physician practices as part of the hospital, thereby generating facility fees). It is unclear whether consolidation results in better outcomes or simply increases overall costs.17
Strategic imperatives
What should gastroenterologists do? First, recognize that FFS is not going away anytime soon.18 Most APMs are still largely in their experimentation phase, and it remains unclear which models will work and which will be broadly adopted. Still, it is unrealistic to expect FFS to indefinitely persist as the dominant payment model. For some services FFS may no longer be a payment option (e.g., Medicare’s BCPI [Bundled Payments for Care Improvement]). For others, FFS rates may become so unattractive that APMs seem necessary. Finally, APMs may allow some practices to capture a greater proportion of overall clinical revenue (e.g., academic practices that perform endoscopic procedures within hospital outpatient departments) and to develop new models that meaningfully improve care. Today’s gastroenterology practices must therefore operate on two separate tracks: an FFS track that rewards volume (most practices are optimized for this) and an alternative payment track that rewards value (few practices can accommodate these on their own). The degree and speed with which practices should reorient to the alternative payment track depends on the type of practice and the specific local health care market. But even practices operating in slower-to-evolve markets should start preparing for the APMs, no matter how far off in the distance they may seem. I recommend the following six steps:
1. Integrate. To participate in APMs, preserve referral streams, and maintain negotiating leverage with health plans, independent, community-based practices may need to affiliate or merge with other physician groups, or align with or be acquired by a health care system.19 Academic practices are challenged to define their role within health care systems that are rapidly adding primary care practices, and often community gastroenterology practices, too.
2. Collaborate and communicate. To deliver high-value care to populations of patients, gastroenterologists must closely collaborate and clearly communicate with primary care physicians and other specialists. Collaborative care agreements can help guide these relationships.203. Develop new models of care. Patients with more routine GI and liver-related problems may be served more cost effectively by midlevel providers21 or innovative solutions, such as e-consultations.22 Patients with complex, chronic GI and liver diseases may be best served by multidisciplinary care teams (e.g., gastroenterologists alongside midlevel providers, nurses, care managers, psychologists, and/or pharmacists) who use clinical information systems to identify high-risk patients and to encourage evidence-based decision making, and who support patients to self-manage their own conditions.23 Previously infeasible in a purely FFS world, these models are encouraged by APMs.
4. Care for common, costly conditions. Most gastroenterology practices have built robust colorectal cancer screening programs, sometimes at the expense of cognitive-based services. In today’s more accountable world, practices that can effectively manage common, costly conditions, such as inflammatory bowel disease, functional GI disorders, and advanced liver diseases, will be rewarded better than before and will be more highly sought as partners.
5. Understand and contain costs. The timely, accurate data needed to effectively respond to APMs are challenging to come by.19 Individual clinicians and group practices can roughly gauge their costs of care for Medicare beneficiaries, compared with other practices, using CMS Quality Resource Utilization Reports. Local commercial insurers may be willing to share cost profiles with interested practices. Strategies to reduce costs may include shifting clinically appropriate patients to more cost-effective settings (especially important for academic practices that see the bulk of their patients in costly hospital outpatient departments), standardizing endoscopy supplies and devices, using anesthesia services more selectively, and preferentially prescribing generic drugs, among others.
6. Measure and demonstrate value. Despite the inherent limitations of performance measurement,24 it is imperative that practices measure and report the value of care to their patients, community, and payers so that they are preferred partners and not locked out of insurance or referral networks. Improving patient experiences is intrinsically worthwhile25 and also makes good business sense.26
References
1. Miller, H.D. Creating payment systems to accelerate value-driven health care: Issues and options for policy reform. New York: The Commonwealth Fund, 2007.
2. Allen, J.I. The road ahead. Clin Gastroenterol Hepatol. 2012;10:692–6.
3. Allen, J.I. Health care reform 3.0: The road gets bumpy. Clin Gastroenterol Hepatol. 2013;11:1527-8.
4. Dorn, S.D., Vesy C.J. Medicare’s revaluation of gastrointestinal endoscopic procedures: Implications for academic and community-based practices. Clin Gastroenterol Hepatol. 2016 (in press).
5. Burwell, S.M. Setting value-based payment goals: HHS efforts to improve U.S. health care (N Engl J Med. 2015;372:897–9).
6. The Advisory Board Company. Commercial Bundled Payment Tracker, 2016.
7. Mechanic, R.E. Mandatory Medicare bundled payment – is it ready for prime time? N Engl J Med. 2015;373:1291–3.
8. The National Commission on Physician Payment Reform. Physician Payment Report, 2013.
9. Brill, J.V., Jain, R., Margolis, P.S., et al. A bundled payment framework for colonoscopy performed for colorectal cancer screening or surveillance. Gastroenterology. 2014;146:849–53, e9.
10. Evans, M. Few Medicare ACOs earned bonuses in 2014. Mod Healthc (2015). Available at: www.modernhealthcare.com/article/20150825/NEWS/150829922. Accessed Nov. 14, 2015.
11. Rau, J., Gold, J. Medicare yet to save money through heralded medical payment model. Kaiser Health News. Available at: http://khn.org/news/medicare-yet-to-save-money-through-heralded-medical-payment-model. Accessed Nov. 14, 2015.
12. Goldmsith, J., Kaufman, N. Pioneer ACOs: Anatomy of a victory. Health Affairs Blog, 2015.
13. Tu, T., Muhlestein, D., Kocot, S.L., et al. The impact of accountable care: Origins and future of accountable care organizations. Washington, D.C.: Brookings Institution, 2015.
14. NCQA. Patient-centered specialty practice frequently asked questions.
15. Xu, T., Wu, A.W., Makary, M.A. The potential hazards of hospital consolidation: Implications for quality, access, and price. JAMA. 2015;314:1337–8.
16. The Physician’s Foundation. 2014 survey of America’s physicians. Practice patterns & perspectives: The Physician’s Foundation.
17. Tsai, T.C., Jha, A.K. Hospital consolidation, competition, and quality: Is bigger necessarily better? JAMA. 2014;312:29–30.
18. Ginsburg, P.B. Fee-for-service will remain a feature of major payment reforms, requiring more changes in Medicare physician payment. Health Aff (Millwood). 2012;31:1977–83.
19. Friedberg, M.W., Chen, P.G., White, C., et al. Effects of health care payment models on physician practice in the United States. Santa Monica, Calif.: RAND Corp., 2015.
20. Greenberg, J.O., Barnett, M.L., Spinks, M.A., et al. The “medical neighborhood”: Integrating primary and specialty care for ambulatory patients. JAMA Intern Med. 2014;174:454–7.
21. Dorn, S.D. Mid-level providers in gastroenterology. Am J Gastroenterol. 2010;105:246–51.
22. Wasfy, J.H., Rao, S.K., Kalwani, N., et al. Longer term impact of cardiology e-consults. Am Heart J. 2016;173:86–93.
23. Coleman, K., Austin, B.T., Brach, C., et al. Evidence on the chronic care model in the new millennium. Health Aff (Millwood). 2009;28:75–85.
24. Dorn, S.D. Quality measurement in gastroenterology: confessions of a realist. Clin Gastroenterol Hepatol. 2016;14:648–50.
25. Berwick, D.M. Measuring physicians’ quality and performance: adrift on Lake Wobegon. JAMA. 2009;302:2485-6.
26. Browne, K., Roseman, D., Shaller, D., et al. Analysis & commentary. Measuring patient experience as a strategy for improving primary care. Health Aff (Millwood). 2010;29:921–5.
Dr. Dorn is vice chief, division of gastroenterology and hepatology, associate professor of medicine, health policy & management, University of North Carolina at Chapel Hill. He has received honoraria for consulting and presentations on health reform from AbbVie and Olympus.
The first article during my tenure as editor of the “Practice Management: The Road Ahead” section of Clinical Gastroenterology and Hepatology published in July 2012 (Clin Gastroenterol Hepatol. 2012;10:692-6) outlined anticipated changes in health care delivery, due in large part to mandates or trends contained in the Patient Protection and Affordable Care Act. A second article was published in 2013 (Health care reform 3.0: The road gets bumpy. Clin Gastroenterol Hepatol. 2013;11:1527-8). In this month’s column, Spencer D. Dorn, MD, MPH, MHA, of the University of North Carolina at Chapel Hill, adds a third update with an article focused on alternative payment models. These new reimbursement models are becoming common and will be part of all of our practice strategies in the years to come. No matter what occurs in the 2016 election, the movement from volume- to value-based payment will continue relentlessly, and practices that do not understand how to respond will struggle. We hope these articles will kick-start conversations in your practice.
Fee-for-service (FFS) reimbursement has been criticized for encouraging quantity over quality, favoring procedures over cognitive services, and fragmenting care.1 The landmark Patient Protection and Affordable Care Act (ACA) and more recent Medicare Access and Children’s Health Insurance Program Reauthorization Act (MACRA) modify Medicare’s FFS and encourage alternative payment models (APMs) that better reward value than volume.
Prior articles in this series have identified the specific trends driving gastroenterology practice strategies and business decisions,2 including an increasing need to demonstrate value, an emphasis on improved population health, an increasing number of practices becoming employees of large integrated delivery networks, reduced FFS reimbursements that are more closely linked to performance metrics, and increasing demands for risk-based contracts.3 In this article, I dive more deeply into these last two trends (declining FFS and the rise of APMs) and consider strategies gastroenterology practices can take in response.
Changes in fee for service
The ACA directed the secretary of Health and Human Services to establish a formal process to review potentially misvalued procedure codes. Compared with the pre-ACA fee schedule, the final 2016 Medicare Physician Fee Schedule includes cuts to professional fees for upper endoscopy, endoscopic retrograde cholangiopancreatography, endoscopic ultrasound, and colonoscopy. At the same time, over the past decade, facility fees paid for procedures performed in hospital outpatient departments have increased. Those to ambulatory surgery centers have gradually increased, although they still remain far below pre-2008 levels. Thus, the full economic impact of fee revaluation on an individual gastroenterology practice depends on whether it collects associated facility and ancillary fees.4
In addition, in the 2016 fee schedule, the Centers for Medicare & Medicaid Services described its intention to remove the value of moderate sedation from all gastrointestinal procedures. This is to prevent paying twice for sedation in procedures that involve anesthesiology professionals (i.e., one payment to the endoscopist as part of the overall procedure fee and a separate payment to the anesthesia professional for sedation they provide and bill for separately). The American Medical Association/Specialty Society Relative Value Scale Update Committee, using survey data from the GI specialty societies and other specialties that perform their own moderate sedation, has submitted recommendations for the value of a new set of moderate sedation Current Procedural Terminology codes to the CMS. The agency is expected to provide the specifics on how it will remove moderate sedation from the GI procedure codes in the 2017 Medicare Physician Fee Schedule Proposed Rule. The more that moderate sedation is valued, the less that endoscopic procedures will be valued. Consequently, gastroenterologists who rely on anesthesiology professionals to sedate their patients will generate less revenue per procedure, unless they rearrange contracts with anesthesia providers. Gastroenterologists who perform moderate sedation will not be impacted, because the sum of the value of the new moderate sedation code plus the underlying endoscopic procedure code will equal the original value of the procedure.
Beyond revaluing services, the CMS outlined its rather ambitious goal “to have 85% of all Medicare fee-for-service (FFS) payments tied to quality or value by 2016, and 90% by 2018.”5 Currently this includes the Physician Quality Reporting System (PQRS), which requires gastroenterologists to report performance on either three or more individual PQRS measures or one PQRS measures group (collection of related individual measures) or face a 2% Medicare payment penalty. It also includes the value-based payment modifier, through which by 2017 all practices with better-than-average quality (linked to PQRS measures) and lower costs will receive bonus payments, whereas those with worse-than-average performance (or who choose not to report) will be penalized.
MACRA changes all of this. Starting in 2019, the meaningful use incentive program, PQRS, and value-based payment modifier will be consolidated into the Merit-Based Incentive Payment System (MIPS). Physicians who elect to remain on an FFS tract will receive a 0-100 composite performance score based on quality (30%), resource use (30%), meaningful use (25%), and clinical practice improvement activities (15%). At the start of a performance period, a composite threshold necessary to achieve incentive payments and avoid penalties will be determined. Throughout the performance period, physicians will receive timely feedback on their performance. At year’s end, those below the threshold will face penalties proportionate to their performance (as much as 4% in 2019 and going up to 9% in 2022), those at threshold will not receive a payment adjustment, and those above threshold will receive bonuses proportionate to their performance (although overall payments will be capped at $500 million).
Alternative payment models
The CMS’s ultimate goal is to move beyond FFS and have “30% of Medicare payments tied to quality or value through APMs by the end of 2016 and 50% of payments by the end of 2018.”5 MACRA supports this ambitious goal: Starting in 2019, providers who “sufficiently” participate in APMs will receive 5% across-the-board bonuses. The three main APMs are bundled payments, accountable care organizations (ACOs), and patient-centered medical homes.
A bundled payment is a single fixed price paid to cover services for a specific episode of care. Depending on how an episode is defined, the bundle may encompass all professional fees, facility fees, and medical device and supply costs for a given service, including postacute care and any complications. If costs are reduced beyond the already discounted price of the bundle and quality metrics are achieved, then participants share the savings. Conversely, if costs exceed the bundled payment amount, then participants lose money. Unlike FFS, bundling incentivizes participants to coordinate care, reduce complications and unnecessary services, and cut purchasing costs.
To date, the CMS has launched three bundling programs. The Acute Care Episode Demonstration Project provided hospitals and clinicians a bundled payment to cover orthopedic and cardiovascular procedure–related episodes of care. This program reduced Medicare costs, primarily because the bundle payment was lower than what the sum of individual payments would have been. Providers were able to cope mainly by reducing their surgical implant costs. Second, more than 6,000 providers are currently participating in Medicare’s Bundled Payments for Care Improvement Program. The results of this program have not yet been released. Third, the CMS recently announced the Comprehensive Care for Joint Replacement Program under which hospitals and physicians in 67 metropolitan areas will be required to participate. Mandatory participation signals the CMS’s strong motivation to shift away from FFS. Beyond Medicare, many commercial insurers offer bundled payment programs, primarily for cardiovascular and orthopedic conditions.6 Although these programs are promising, it is technically challenging to define what is in a bundle, and to adequately risk adjust and mitigate random variation in spending for certain episodes of care. Providers are also challenged to find ways to divide payment among participants, coordinate all care, and accept financial risk.7,8 The American Gastroenterological Association recently published a bundled payment framework for screening and surveillance colonoscopy.9 Bundling other gastroenterology services will be more challenging.
Whereas bundled-care programs focus on a discrete service (e.g., knee replacement or colonoscopy), ACOs are integrated groups of providers who jointly assume responsibility for the cost and quality of all care delivered to a defined population. The ACA requires ACOs to have formal legal, leadership, and management structures; care for at least 5,000 Medicare beneficiaries; fulfill certain patient-centeredness criteria; measure and report quality and cost data; and coordinate care. Different payment models incentivize ACOs to reduce costs and improve quality of care. ACOs operating under a one-sided shared savings model receive FFS payments for each service delivered, along with a bonus for reducing costs below a spending target and meeting quality requirements. There are no potential financial penalties. Alternatively, ACOs operating under a two-sided risk-savings model share a greater proportion of cost savings, in exchange for potential financial penalties if the cost of care exceeds target spending.
To date, Medicare-sponsored ACOs have produced mixed results. In 2014 only 92 of the 322 Medicare Shared Savings ACOs were able to reduce spending below a predetermined benchmark by a predetermined amount (2%-3%) while meeting quality scores, thereby earning a bonus ($341 million in total). Similarly, of the original 32 pioneer ACOs, which by definition are more experienced at managing population health and more willing to take on financial risk, 13 dropped out of the program, and in 2014, only 11 generated enough savings to earn a payout ($82 million in total), whereas 5 incurred financial penalties ($9 million in total) for costs exceeding target thresholds.10 In total, after paying out bonuses, the ACO program cost Medicare a net loss of nearly $3 million, far from the $10-$240 million Medicare had previously projected it would save through the ACO program.11 Clearly, ACOs are not a quick fix for all that ails health care. For many ACOs, the major start-up requirements (time, capital investments, and so forth) needed to manage a population may not be worthwhile.12 Nonetheless, the CMS recently launched the Next Generation ACO model through which 21 participating ACOs will assume higher levels of financial risk (possibly capitated payments) in exchange for greater potential rewards. Similarly, beyond Medicare, there are also many Medicaid-sponsored ACOs and hundreds of commercial payer-sponsored ACOs.13
Finally, practices can qualify for APM status without accepting bundled payments or joining an ACO by qualifying as a patient-centered medical home. One option for gastroenterologists and other specialists is the National Committee for Quality Assurance’s patient-centered specialty practice designation, available to practices that successfully demonstrate their ability to track and coordinate care with primary care providers and other specialists, offer timely appointments and responses to telephone and electronic messages, use evidence-based tools to manage care for specific patient populations, develop patient-centered care plans, and measure and improve performance.14
Consolidation
Health insurers are merging to increase scale (and negotiating power), enhance efficiency (reducing administrative costs makes more room for profits), and diversify their businesses. Recently proposed acquisitions will bring “the big five” health insurers to the “big three.” Likewise, health care systems are rapidly acquiring hospitals and physician groups, so much so that today half of all American health care markets are now considered highly concentrated, and none are considered highly competitive.15 Today only 35% of all physicians are independently employed.16 Physicians employed by health systems trade their complete autonomy to offset declining reimbursement, reduce operating expenses (including health information technology costs), improve work-life balance, and mitigate unknown risks.
Proponents contend that these mergers allow health care systems to better coordinate care, improve care experiences, accommodate new payment models, and assemble the building blocks needed to form ACOs and other integrated care models. Critics argue that locally dominant systems drive volume (by tightening referral relationships and gaining new market share) and increase costs (through enhanced negotiating leverage and by reclassifying newly acquired physician practices as part of the hospital, thereby generating facility fees). It is unclear whether consolidation results in better outcomes or simply increases overall costs.17
Strategic imperatives
What should gastroenterologists do? First, recognize that FFS is not going away anytime soon.18 Most APMs are still largely in their experimentation phase, and it remains unclear which models will work and which will be broadly adopted. Still, it is unrealistic to expect FFS to indefinitely persist as the dominant payment model. For some services FFS may no longer be a payment option (e.g., Medicare’s BCPI [Bundled Payments for Care Improvement]). For others, FFS rates may become so unattractive that APMs seem necessary. Finally, APMs may allow some practices to capture a greater proportion of overall clinical revenue (e.g., academic practices that perform endoscopic procedures within hospital outpatient departments) and to develop new models that meaningfully improve care. Today’s gastroenterology practices must therefore operate on two separate tracks: an FFS track that rewards volume (most practices are optimized for this) and an alternative payment track that rewards value (few practices can accommodate these on their own). The degree and speed with which practices should reorient to the alternative payment track depends on the type of practice and the specific local health care market. But even practices operating in slower-to-evolve markets should start preparing for the APMs, no matter how far off in the distance they may seem. I recommend the following six steps:
1. Integrate. To participate in APMs, preserve referral streams, and maintain negotiating leverage with health plans, independent, community-based practices may need to affiliate or merge with other physician groups, or align with or be acquired by a health care system.19 Academic practices are challenged to define their role within health care systems that are rapidly adding primary care practices, and often community gastroenterology practices, too.
2. Collaborate and communicate. To deliver high-value care to populations of patients, gastroenterologists must closely collaborate and clearly communicate with primary care physicians and other specialists. Collaborative care agreements can help guide these relationships.203. Develop new models of care. Patients with more routine GI and liver-related problems may be served more cost effectively by midlevel providers21 or innovative solutions, such as e-consultations.22 Patients with complex, chronic GI and liver diseases may be best served by multidisciplinary care teams (e.g., gastroenterologists alongside midlevel providers, nurses, care managers, psychologists, and/or pharmacists) who use clinical information systems to identify high-risk patients and to encourage evidence-based decision making, and who support patients to self-manage their own conditions.23 Previously infeasible in a purely FFS world, these models are encouraged by APMs.
4. Care for common, costly conditions. Most gastroenterology practices have built robust colorectal cancer screening programs, sometimes at the expense of cognitive-based services. In today’s more accountable world, practices that can effectively manage common, costly conditions, such as inflammatory bowel disease, functional GI disorders, and advanced liver diseases, will be rewarded better than before and will be more highly sought as partners.
5. Understand and contain costs. The timely, accurate data needed to effectively respond to APMs are challenging to come by.19 Individual clinicians and group practices can roughly gauge their costs of care for Medicare beneficiaries, compared with other practices, using CMS Quality Resource Utilization Reports. Local commercial insurers may be willing to share cost profiles with interested practices. Strategies to reduce costs may include shifting clinically appropriate patients to more cost-effective settings (especially important for academic practices that see the bulk of their patients in costly hospital outpatient departments), standardizing endoscopy supplies and devices, using anesthesia services more selectively, and preferentially prescribing generic drugs, among others.
6. Measure and demonstrate value. Despite the inherent limitations of performance measurement,24 it is imperative that practices measure and report the value of care to their patients, community, and payers so that they are preferred partners and not locked out of insurance or referral networks. Improving patient experiences is intrinsically worthwhile25 and also makes good business sense.26
References
1. Miller, H.D. Creating payment systems to accelerate value-driven health care: Issues and options for policy reform. New York: The Commonwealth Fund, 2007.
2. Allen, J.I. The road ahead. Clin Gastroenterol Hepatol. 2012;10:692–6.
3. Allen, J.I. Health care reform 3.0: The road gets bumpy. Clin Gastroenterol Hepatol. 2013;11:1527-8.
4. Dorn, S.D., Vesy C.J. Medicare’s revaluation of gastrointestinal endoscopic procedures: Implications for academic and community-based practices. Clin Gastroenterol Hepatol. 2016 (in press).
5. Burwell, S.M. Setting value-based payment goals: HHS efforts to improve U.S. health care (N Engl J Med. 2015;372:897–9).
6. The Advisory Board Company. Commercial Bundled Payment Tracker, 2016.
7. Mechanic, R.E. Mandatory Medicare bundled payment – is it ready for prime time? N Engl J Med. 2015;373:1291–3.
8. The National Commission on Physician Payment Reform. Physician Payment Report, 2013.
9. Brill, J.V., Jain, R., Margolis, P.S., et al. A bundled payment framework for colonoscopy performed for colorectal cancer screening or surveillance. Gastroenterology. 2014;146:849–53, e9.
10. Evans, M. Few Medicare ACOs earned bonuses in 2014. Mod Healthc (2015). Available at: www.modernhealthcare.com/article/20150825/NEWS/150829922. Accessed Nov. 14, 2015.
11. Rau, J., Gold, J. Medicare yet to save money through heralded medical payment model. Kaiser Health News. Available at: http://khn.org/news/medicare-yet-to-save-money-through-heralded-medical-payment-model. Accessed Nov. 14, 2015.
12. Goldmsith, J., Kaufman, N. Pioneer ACOs: Anatomy of a victory. Health Affairs Blog, 2015.
13. Tu, T., Muhlestein, D., Kocot, S.L., et al. The impact of accountable care: Origins and future of accountable care organizations. Washington, D.C.: Brookings Institution, 2015.
14. NCQA. Patient-centered specialty practice frequently asked questions.
15. Xu, T., Wu, A.W., Makary, M.A. The potential hazards of hospital consolidation: Implications for quality, access, and price. JAMA. 2015;314:1337–8.
16. The Physician’s Foundation. 2014 survey of America’s physicians. Practice patterns & perspectives: The Physician’s Foundation.
17. Tsai, T.C., Jha, A.K. Hospital consolidation, competition, and quality: Is bigger necessarily better? JAMA. 2014;312:29–30.
18. Ginsburg, P.B. Fee-for-service will remain a feature of major payment reforms, requiring more changes in Medicare physician payment. Health Aff (Millwood). 2012;31:1977–83.
19. Friedberg, M.W., Chen, P.G., White, C., et al. Effects of health care payment models on physician practice in the United States. Santa Monica, Calif.: RAND Corp., 2015.
20. Greenberg, J.O., Barnett, M.L., Spinks, M.A., et al. The “medical neighborhood”: Integrating primary and specialty care for ambulatory patients. JAMA Intern Med. 2014;174:454–7.
21. Dorn, S.D. Mid-level providers in gastroenterology. Am J Gastroenterol. 2010;105:246–51.
22. Wasfy, J.H., Rao, S.K., Kalwani, N., et al. Longer term impact of cardiology e-consults. Am Heart J. 2016;173:86–93.
23. Coleman, K., Austin, B.T., Brach, C., et al. Evidence on the chronic care model in the new millennium. Health Aff (Millwood). 2009;28:75–85.
24. Dorn, S.D. Quality measurement in gastroenterology: confessions of a realist. Clin Gastroenterol Hepatol. 2016;14:648–50.
25. Berwick, D.M. Measuring physicians’ quality and performance: adrift on Lake Wobegon. JAMA. 2009;302:2485-6.
26. Browne, K., Roseman, D., Shaller, D., et al. Analysis & commentary. Measuring patient experience as a strategy for improving primary care. Health Aff (Millwood). 2010;29:921–5.
Dr. Dorn is vice chief, division of gastroenterology and hepatology, associate professor of medicine, health policy & management, University of North Carolina at Chapel Hill. He has received honoraria for consulting and presentations on health reform from AbbVie and Olympus.
Hepatitis B vaccine response suppressed by maternal antibodies
Maternal antibodies against hepatitis blunt the immune response to the hepatitis B vaccine in newborns, but the booster dose is unaffected by maternal antibodies, a study found.
Previous research also has identified a suppressed response to vaccination due to maternal antibodies with vaccines such as the measles, hepatitis A, mumps and tetanus vaccines.
“Maternal antibodies are a double-edged sword for infants,” wrote X. Chen of the Zhongnan Hospital of Wuhan (China) University, and colleagues (J Viral Hepat. 2016 Jul 29. doi: 10.1111/jvh.12572). “These neutralizing antibodies can protect neonates against most infectious diseases in early life; however, as shown in the study here, these antibodies can also suppress the immune response of infants to vaccines.”
The researchers first assessed transplacental transfer of antibodies by measuring anti–hepatitis B antibodies in 90 mothers and their newborns. The mothers were all positive for anti–hepatitis B antibodies with a median titer of 250. Before the infants had been vaccinated, 97% were positive for anti–hepatitis B antibodies. Among infants whose mothers had titers above 100 IU/L, 100% were positive for antibodies.
Then the researchers measured titers and rate of anti–hepatitis B positivity in 1,055 mothers and their 1,063 infants, aged 7-24 months, after the babies had received doses of the 10 mcg HBV vaccine at ages 0, 1 and 6 months, per the recommended schedule in China. In the United States, infants are recommended to receive the vaccine at birth, at 1-4 months, and then at 6-18 months.
Among the 405 mothers with antibodies of less than 10 IU/L, 89% of their newborns responded sufficiently to the vaccine to be positive for anti–hepatitis B antibodies. Among the 451 mothers with antibodies from 10-499 IU/L, 85% of the infants had positivity, and among the 207 mothers with antibodies of 500 IU/L and higher, 77% of the infants were positive for anti–hepatitis B antibodies.
Titers followed the same inverse pattern. The median titer was 169 in infants whose mothers had low titers, but the median titer was 79 in infants of mothers with high titers. When mothers with titers in the middle range, the infants’ median titer was 141.
Among 162 newborns who tested negative for anti–hepatitis B antibodies after receiving all three doses of the vaccine, 92% showed positivity for the antibodies after receiving a “catch-up” booster dose, without significant interference from maternal antibodies. Of another 11 infants still lacking positivity, 8 assessed 1 month later, achieved it after a second catch-up booster, suggesting “the suppression effects of the maternal anti-HBVs can be overcome.”
The research was funded by the Hongkong Zeshan Foundation, and the authors reported having no disclosures.
Maternal antibodies against hepatitis blunt the immune response to the hepatitis B vaccine in newborns, but the booster dose is unaffected by maternal antibodies, a study found.
Previous research also has identified a suppressed response to vaccination due to maternal antibodies with vaccines such as the measles, hepatitis A, mumps and tetanus vaccines.
“Maternal antibodies are a double-edged sword for infants,” wrote X. Chen of the Zhongnan Hospital of Wuhan (China) University, and colleagues (J Viral Hepat. 2016 Jul 29. doi: 10.1111/jvh.12572). “These neutralizing antibodies can protect neonates against most infectious diseases in early life; however, as shown in the study here, these antibodies can also suppress the immune response of infants to vaccines.”
The researchers first assessed transplacental transfer of antibodies by measuring anti–hepatitis B antibodies in 90 mothers and their newborns. The mothers were all positive for anti–hepatitis B antibodies with a median titer of 250. Before the infants had been vaccinated, 97% were positive for anti–hepatitis B antibodies. Among infants whose mothers had titers above 100 IU/L, 100% were positive for antibodies.
Then the researchers measured titers and rate of anti–hepatitis B positivity in 1,055 mothers and their 1,063 infants, aged 7-24 months, after the babies had received doses of the 10 mcg HBV vaccine at ages 0, 1 and 6 months, per the recommended schedule in China. In the United States, infants are recommended to receive the vaccine at birth, at 1-4 months, and then at 6-18 months.
Among the 405 mothers with antibodies of less than 10 IU/L, 89% of their newborns responded sufficiently to the vaccine to be positive for anti–hepatitis B antibodies. Among the 451 mothers with antibodies from 10-499 IU/L, 85% of the infants had positivity, and among the 207 mothers with antibodies of 500 IU/L and higher, 77% of the infants were positive for anti–hepatitis B antibodies.
Titers followed the same inverse pattern. The median titer was 169 in infants whose mothers had low titers, but the median titer was 79 in infants of mothers with high titers. When mothers with titers in the middle range, the infants’ median titer was 141.
Among 162 newborns who tested negative for anti–hepatitis B antibodies after receiving all three doses of the vaccine, 92% showed positivity for the antibodies after receiving a “catch-up” booster dose, without significant interference from maternal antibodies. Of another 11 infants still lacking positivity, 8 assessed 1 month later, achieved it after a second catch-up booster, suggesting “the suppression effects of the maternal anti-HBVs can be overcome.”
The research was funded by the Hongkong Zeshan Foundation, and the authors reported having no disclosures.
Maternal antibodies against hepatitis blunt the immune response to the hepatitis B vaccine in newborns, but the booster dose is unaffected by maternal antibodies, a study found.
Previous research also has identified a suppressed response to vaccination due to maternal antibodies with vaccines such as the measles, hepatitis A, mumps and tetanus vaccines.
“Maternal antibodies are a double-edged sword for infants,” wrote X. Chen of the Zhongnan Hospital of Wuhan (China) University, and colleagues (J Viral Hepat. 2016 Jul 29. doi: 10.1111/jvh.12572). “These neutralizing antibodies can protect neonates against most infectious diseases in early life; however, as shown in the study here, these antibodies can also suppress the immune response of infants to vaccines.”
The researchers first assessed transplacental transfer of antibodies by measuring anti–hepatitis B antibodies in 90 mothers and their newborns. The mothers were all positive for anti–hepatitis B antibodies with a median titer of 250. Before the infants had been vaccinated, 97% were positive for anti–hepatitis B antibodies. Among infants whose mothers had titers above 100 IU/L, 100% were positive for antibodies.
Then the researchers measured titers and rate of anti–hepatitis B positivity in 1,055 mothers and their 1,063 infants, aged 7-24 months, after the babies had received doses of the 10 mcg HBV vaccine at ages 0, 1 and 6 months, per the recommended schedule in China. In the United States, infants are recommended to receive the vaccine at birth, at 1-4 months, and then at 6-18 months.
Among the 405 mothers with antibodies of less than 10 IU/L, 89% of their newborns responded sufficiently to the vaccine to be positive for anti–hepatitis B antibodies. Among the 451 mothers with antibodies from 10-499 IU/L, 85% of the infants had positivity, and among the 207 mothers with antibodies of 500 IU/L and higher, 77% of the infants were positive for anti–hepatitis B antibodies.
Titers followed the same inverse pattern. The median titer was 169 in infants whose mothers had low titers, but the median titer was 79 in infants of mothers with high titers. When mothers with titers in the middle range, the infants’ median titer was 141.
Among 162 newborns who tested negative for anti–hepatitis B antibodies after receiving all three doses of the vaccine, 92% showed positivity for the antibodies after receiving a “catch-up” booster dose, without significant interference from maternal antibodies. Of another 11 infants still lacking positivity, 8 assessed 1 month later, achieved it after a second catch-up booster, suggesting “the suppression effects of the maternal anti-HBVs can be overcome.”
The research was funded by the Hongkong Zeshan Foundation, and the authors reported having no disclosures.
FROM THE JOURNAL OF VIRAL HEPATITIS
Key clinical point: Maternal immunity to hepatitis B suppresses infant response to vaccination.
Major finding: 89%, 85%, and 77% of newborns tested positive for anti–hepatitis B antibodies after a standard three-dose regimen when born to mothers with low, medium, and high titers, respectively.
Data source: The findings are based on titers and antibody positivity against hepatitis B in 1,055 mothers and 1,063 newborns at multiple centers in China from March 2012 to November 2015 before and after infant immunization against hepatitis B.
Disclosures: The research was funded by the Hongkong Zeshan Foundation, and the authors reported having no disclosures.
Thymidine phosphorylase increases myeloma-induced bone lesions
Myeloma-induced osteolytic bone lesions were reduced by suppressing expression of thymidine phosphorylase (TP), according to Huan Liu of the University of Texas MD Anderson Cancer Center in Houston and associates.
In osteoblast progenitors, methylation of alpha-1/runt-related transcription factor 2 (RUNX2) and osterix was upregulated by TP, resulting in decreased bone formation. TP also upregulated methylation of interferon regulatory factor 8 (IRF8) and enhanced nuclear factor of activated T cells, cytoplasmic 1 protein (NFATc1), which increased bone resorption. Thymidine was catalyzed into thymine and 2-deoxy-d-ribose, which bound to integrins alphavbeta3 and alpha5beta1, activated PI3K/Akt signaling, and increased DNA methyltransferase 3A (DNMT3A) expression, the investigators found.
In an experiment, myeloma was established in severe combined immunodeficient mice, and the mice were injected with ARP-1 cells, which produce high levels of TP. The mice were then treated with 7-deazaxanthine or tipiracil hydrochloride, which reduced ARP-1-induced bone lesions, DNMT3A expression, and 2DDR levels in the serum of tumor-bearing mice.
“This model could facilitate the translation of these inhibitors into human studies of myeloma bone disease. Because TP is often expressed by other malignancies including breast, prostate, and lung cancer, these findings may also have broader implications for the genesis of bone metastasis caused by these and other tumors,” the investigators concluded.
Find the full study in Science Translational Medicine (doi:10.1126/scitranslmed.aad8949)
Myeloma-induced osteolytic bone lesions were reduced by suppressing expression of thymidine phosphorylase (TP), according to Huan Liu of the University of Texas MD Anderson Cancer Center in Houston and associates.
In osteoblast progenitors, methylation of alpha-1/runt-related transcription factor 2 (RUNX2) and osterix was upregulated by TP, resulting in decreased bone formation. TP also upregulated methylation of interferon regulatory factor 8 (IRF8) and enhanced nuclear factor of activated T cells, cytoplasmic 1 protein (NFATc1), which increased bone resorption. Thymidine was catalyzed into thymine and 2-deoxy-d-ribose, which bound to integrins alphavbeta3 and alpha5beta1, activated PI3K/Akt signaling, and increased DNA methyltransferase 3A (DNMT3A) expression, the investigators found.
In an experiment, myeloma was established in severe combined immunodeficient mice, and the mice were injected with ARP-1 cells, which produce high levels of TP. The mice were then treated with 7-deazaxanthine or tipiracil hydrochloride, which reduced ARP-1-induced bone lesions, DNMT3A expression, and 2DDR levels in the serum of tumor-bearing mice.
“This model could facilitate the translation of these inhibitors into human studies of myeloma bone disease. Because TP is often expressed by other malignancies including breast, prostate, and lung cancer, these findings may also have broader implications for the genesis of bone metastasis caused by these and other tumors,” the investigators concluded.
Find the full study in Science Translational Medicine (doi:10.1126/scitranslmed.aad8949)
Myeloma-induced osteolytic bone lesions were reduced by suppressing expression of thymidine phosphorylase (TP), according to Huan Liu of the University of Texas MD Anderson Cancer Center in Houston and associates.
In osteoblast progenitors, methylation of alpha-1/runt-related transcription factor 2 (RUNX2) and osterix was upregulated by TP, resulting in decreased bone formation. TP also upregulated methylation of interferon regulatory factor 8 (IRF8) and enhanced nuclear factor of activated T cells, cytoplasmic 1 protein (NFATc1), which increased bone resorption. Thymidine was catalyzed into thymine and 2-deoxy-d-ribose, which bound to integrins alphavbeta3 and alpha5beta1, activated PI3K/Akt signaling, and increased DNA methyltransferase 3A (DNMT3A) expression, the investigators found.
In an experiment, myeloma was established in severe combined immunodeficient mice, and the mice were injected with ARP-1 cells, which produce high levels of TP. The mice were then treated with 7-deazaxanthine or tipiracil hydrochloride, which reduced ARP-1-induced bone lesions, DNMT3A expression, and 2DDR levels in the serum of tumor-bearing mice.
“This model could facilitate the translation of these inhibitors into human studies of myeloma bone disease. Because TP is often expressed by other malignancies including breast, prostate, and lung cancer, these findings may also have broader implications for the genesis of bone metastasis caused by these and other tumors,” the investigators concluded.
Find the full study in Science Translational Medicine (doi:10.1126/scitranslmed.aad8949)
FROM SCIENCE TRANSLATIONAL MEDICINE