User login
Hyperammonemia May Signal the Presence of Generalized Convulsive Seizures
Elevated blood ammonia levels may help differentiate epileptic generalized convulsive seizures (GCS) from other events, suggests a recent report in Epilepsia. When Rawan Albadareen and associates measured blood ammonia levels in 78 patients with GCS, psychogenic nonepileptic seizures with convulsions (PNES-C), or focal seizures using video–electroencephalography (vEEG) monitoring, they discovered that ammonia levels at or above 80 μmol/L could classify generalized convulsive seizures in 80% of patients with a sensitivity of 53.9% and specificity of 100%. Their findings suggest that transient hyperammonemia may serve as an inexpensive test for the diagnosis of GCS.
Albadareen R, Gronseth G, Landazuri P, et al. Postictal ammonia as a biomarker for electrographic convulsive seizures: a prospective study. Epilepsia. 2016;57(8): 1221-1227.
Elevated blood ammonia levels may help differentiate epileptic generalized convulsive seizures (GCS) from other events, suggests a recent report in Epilepsia. When Rawan Albadareen and associates measured blood ammonia levels in 78 patients with GCS, psychogenic nonepileptic seizures with convulsions (PNES-C), or focal seizures using video–electroencephalography (vEEG) monitoring, they discovered that ammonia levels at or above 80 μmol/L could classify generalized convulsive seizures in 80% of patients with a sensitivity of 53.9% and specificity of 100%. Their findings suggest that transient hyperammonemia may serve as an inexpensive test for the diagnosis of GCS.
Albadareen R, Gronseth G, Landazuri P, et al. Postictal ammonia as a biomarker for electrographic convulsive seizures: a prospective study. Epilepsia. 2016;57(8): 1221-1227.
Elevated blood ammonia levels may help differentiate epileptic generalized convulsive seizures (GCS) from other events, suggests a recent report in Epilepsia. When Rawan Albadareen and associates measured blood ammonia levels in 78 patients with GCS, psychogenic nonepileptic seizures with convulsions (PNES-C), or focal seizures using video–electroencephalography (vEEG) monitoring, they discovered that ammonia levels at or above 80 μmol/L could classify generalized convulsive seizures in 80% of patients with a sensitivity of 53.9% and specificity of 100%. Their findings suggest that transient hyperammonemia may serve as an inexpensive test for the diagnosis of GCS.
Albadareen R, Gronseth G, Landazuri P, et al. Postictal ammonia as a biomarker for electrographic convulsive seizures: a prospective study. Epilepsia. 2016;57(8): 1221-1227.
Finding a Link Between Ictal Fear and Auras
In order to determine if there is an association between ictal fear and other auras and with patients’ gender and age, investigators analyzed 536 participants in the Epilepsy Phenome/Genome Project. Among 36 patients with confirmed ictal fear, the phenomenon was associated with temporal lobe auras, including cephalic, olfactory, and visceral symptoms, as well as déjà vu and derealization. Aphasias were also correlated with ictal fear but researchers found no link between such fear and age or gender.
Chong DJ, Dugan P; The EPGP Investigators. Ictal fear: associations with age, gender, and other experiential phenomena. Epilepsy Behav. 2016;62:153-158.
In order to determine if there is an association between ictal fear and other auras and with patients’ gender and age, investigators analyzed 536 participants in the Epilepsy Phenome/Genome Project. Among 36 patients with confirmed ictal fear, the phenomenon was associated with temporal lobe auras, including cephalic, olfactory, and visceral symptoms, as well as déjà vu and derealization. Aphasias were also correlated with ictal fear but researchers found no link between such fear and age or gender.
Chong DJ, Dugan P; The EPGP Investigators. Ictal fear: associations with age, gender, and other experiential phenomena. Epilepsy Behav. 2016;62:153-158.
In order to determine if there is an association between ictal fear and other auras and with patients’ gender and age, investigators analyzed 536 participants in the Epilepsy Phenome/Genome Project. Among 36 patients with confirmed ictal fear, the phenomenon was associated with temporal lobe auras, including cephalic, olfactory, and visceral symptoms, as well as déjà vu and derealization. Aphasias were also correlated with ictal fear but researchers found no link between such fear and age or gender.
Chong DJ, Dugan P; The EPGP Investigators. Ictal fear: associations with age, gender, and other experiential phenomena. Epilepsy Behav. 2016;62:153-158.
JZP-110 May Have Low Potential for Abuse
DENVER—The abuse potential of the investigational drug JZP-110 may be similar to or lower than that of the Schedule IV stimulant phentermine, according to data presented at the 30th Anniversary Meeting of the Associated Professional Sleep Societies. Results also indicate that the 300-mg therapeutic dose of JZP-110 is well tolerated and entails no new safety concerns.
JZP-110 is a selective dopamine and norepinephrine reuptake inhibitor and wake-promoting agent under evaluation for the treatment of narcolepsy and obstructive sleep apnea. The use of some therapies for narcolepsy is limited by their abuse potential. Lawrence P. Carter, PhD, Senior Director in Clinical Development at Jazz Pharmaceuticals and Assistant Professor of Pharmacology and Toxicology at the University of Arkansas for Medical Sciences in Little Rock, and colleagues sought to evaluate the abuse potential of JZP-110, compared with phentermine as a positive control in a human abuse liability study.
Eligible participants were healthy individuals between ages 18 and 55, had a self-reported history of recreational polydrug use, and had used a stimulant recreationally 10 or more times in the previous five years and once or more in the previous three months. Participants who tolerated phentermine in a qualification phase and who preferred it to placebo were enrolled in the test phase. In the test phase, participants were randomized to one of six double-blind treatment sequences that included single administration of placebo; 300-mg, 600-mg, and 1,200-mg doses of JZP-110; and 45-mg and 90-mg doses of phentermine. The primary end point was peak rating of Liking at the Moment during the first 12 hours on the Visual Analog Scale (VAS). Secondary end points included retrospective VAS ratings at 24 hours after administration for Overall Drug Liking, and how much the participant would like to take the drug again.
In all, 43 participants (mean age, 29.3) were enrolled, 74.4% of whom were African American. Thirty-seven participants completed the study, and two discontinued the study because of adverse events. Liking at the Moment was generally greater after taking phentermine than after taking JZP-110. For all doses of JZP-110, peak Liking at the Moment was significantly greater than for placebo and significantly less than for 90 mg of phentermine. Retrospective evaluation of Overall Drug Liking for JZP-110 at 600 mg and 1,200 mg was not significantly different from placebo and was significantly less than both doses of phentermine. At all doses of JZP-110, participants were significantly less willing to take the drug again, compared with both doses of phentermine.
—Erik Greb
DENVER—The abuse potential of the investigational drug JZP-110 may be similar to or lower than that of the Schedule IV stimulant phentermine, according to data presented at the 30th Anniversary Meeting of the Associated Professional Sleep Societies. Results also indicate that the 300-mg therapeutic dose of JZP-110 is well tolerated and entails no new safety concerns.
JZP-110 is a selective dopamine and norepinephrine reuptake inhibitor and wake-promoting agent under evaluation for the treatment of narcolepsy and obstructive sleep apnea. The use of some therapies for narcolepsy is limited by their abuse potential. Lawrence P. Carter, PhD, Senior Director in Clinical Development at Jazz Pharmaceuticals and Assistant Professor of Pharmacology and Toxicology at the University of Arkansas for Medical Sciences in Little Rock, and colleagues sought to evaluate the abuse potential of JZP-110, compared with phentermine as a positive control in a human abuse liability study.
Eligible participants were healthy individuals between ages 18 and 55, had a self-reported history of recreational polydrug use, and had used a stimulant recreationally 10 or more times in the previous five years and once or more in the previous three months. Participants who tolerated phentermine in a qualification phase and who preferred it to placebo were enrolled in the test phase. In the test phase, participants were randomized to one of six double-blind treatment sequences that included single administration of placebo; 300-mg, 600-mg, and 1,200-mg doses of JZP-110; and 45-mg and 90-mg doses of phentermine. The primary end point was peak rating of Liking at the Moment during the first 12 hours on the Visual Analog Scale (VAS). Secondary end points included retrospective VAS ratings at 24 hours after administration for Overall Drug Liking, and how much the participant would like to take the drug again.
In all, 43 participants (mean age, 29.3) were enrolled, 74.4% of whom were African American. Thirty-seven participants completed the study, and two discontinued the study because of adverse events. Liking at the Moment was generally greater after taking phentermine than after taking JZP-110. For all doses of JZP-110, peak Liking at the Moment was significantly greater than for placebo and significantly less than for 90 mg of phentermine. Retrospective evaluation of Overall Drug Liking for JZP-110 at 600 mg and 1,200 mg was not significantly different from placebo and was significantly less than both doses of phentermine. At all doses of JZP-110, participants were significantly less willing to take the drug again, compared with both doses of phentermine.
—Erik Greb
DENVER—The abuse potential of the investigational drug JZP-110 may be similar to or lower than that of the Schedule IV stimulant phentermine, according to data presented at the 30th Anniversary Meeting of the Associated Professional Sleep Societies. Results also indicate that the 300-mg therapeutic dose of JZP-110 is well tolerated and entails no new safety concerns.
JZP-110 is a selective dopamine and norepinephrine reuptake inhibitor and wake-promoting agent under evaluation for the treatment of narcolepsy and obstructive sleep apnea. The use of some therapies for narcolepsy is limited by their abuse potential. Lawrence P. Carter, PhD, Senior Director in Clinical Development at Jazz Pharmaceuticals and Assistant Professor of Pharmacology and Toxicology at the University of Arkansas for Medical Sciences in Little Rock, and colleagues sought to evaluate the abuse potential of JZP-110, compared with phentermine as a positive control in a human abuse liability study.
Eligible participants were healthy individuals between ages 18 and 55, had a self-reported history of recreational polydrug use, and had used a stimulant recreationally 10 or more times in the previous five years and once or more in the previous three months. Participants who tolerated phentermine in a qualification phase and who preferred it to placebo were enrolled in the test phase. In the test phase, participants were randomized to one of six double-blind treatment sequences that included single administration of placebo; 300-mg, 600-mg, and 1,200-mg doses of JZP-110; and 45-mg and 90-mg doses of phentermine. The primary end point was peak rating of Liking at the Moment during the first 12 hours on the Visual Analog Scale (VAS). Secondary end points included retrospective VAS ratings at 24 hours after administration for Overall Drug Liking, and how much the participant would like to take the drug again.
In all, 43 participants (mean age, 29.3) were enrolled, 74.4% of whom were African American. Thirty-seven participants completed the study, and two discontinued the study because of adverse events. Liking at the Moment was generally greater after taking phentermine than after taking JZP-110. For all doses of JZP-110, peak Liking at the Moment was significantly greater than for placebo and significantly less than for 90 mg of phentermine. Retrospective evaluation of Overall Drug Liking for JZP-110 at 600 mg and 1,200 mg was not significantly different from placebo and was significantly less than both doses of phentermine. At all doses of JZP-110, participants were significantly less willing to take the drug again, compared with both doses of phentermine.
—Erik Greb
Take back a patient who fired you? No way
I refuse to do retreads.
This has nothing to do with my car. If a retread tire gives me the same performance and safety at a lower price, I’m all for it.
Sometimes patients fire me. This is often acrimonious, with them sending me a letter complaining about my competence, bedside manner, personal appearance, staff, office decor, phone system ... whatever. For some reason they weren’t happy with me and instead of just sending a release of records, they decided to let me know in no uncertain terms that they aren’t coming back. We all get these notes.
When I was younger this would really upset me. I took a lot of things personally when I first started out. Now, after almost 20 years on the neurology front lines, it’s just another day. I learned a long time ago that you can’t please everyone or be the doctor they all love. So I fax their chart to wherever they want and move on.
Surprisingly, a few times a year patients will try to come back. Maybe they didn’t like the new doc, or are sorry about their outburst, or can’t find someone else nearby who takes their insurance. So they call and try to make a follow-up. Not surprisingly, they never mention their previous letter. When it’s brought up, they typically claim we misinterpreted it, that they didn’t mean it, or that they’ve decided to forgive me.
This is what I call a retread. A patient who wants to come back after leaving under unpleasant circumstances. I don’t allow it.
To me the doctor-patient relationship is based on trust and objectivity. Once a patient sends an acrimonious letter, it’s very difficult to return to an impartial condition. The fact that they did it once means they may do it again, or think they can get their way with threatening or insulting behavior. These are not things that are good for the connection between us.
So I turn them away. Some just hang up. Others yell, and a few threaten me with legal action. But I don’t back down. Do you want to care for someone who’s done the same to you?
This is a high-stress field. I don’t need the additional distraction of dealing with a toxic medical relationship. If you don’t like me, I have no issue with that. Not everyone does. But once you’ve made that decision, you’re stuck with it.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I refuse to do retreads.
This has nothing to do with my car. If a retread tire gives me the same performance and safety at a lower price, I’m all for it.
Sometimes patients fire me. This is often acrimonious, with them sending me a letter complaining about my competence, bedside manner, personal appearance, staff, office decor, phone system ... whatever. For some reason they weren’t happy with me and instead of just sending a release of records, they decided to let me know in no uncertain terms that they aren’t coming back. We all get these notes.
When I was younger this would really upset me. I took a lot of things personally when I first started out. Now, after almost 20 years on the neurology front lines, it’s just another day. I learned a long time ago that you can’t please everyone or be the doctor they all love. So I fax their chart to wherever they want and move on.
Surprisingly, a few times a year patients will try to come back. Maybe they didn’t like the new doc, or are sorry about their outburst, or can’t find someone else nearby who takes their insurance. So they call and try to make a follow-up. Not surprisingly, they never mention their previous letter. When it’s brought up, they typically claim we misinterpreted it, that they didn’t mean it, or that they’ve decided to forgive me.
This is what I call a retread. A patient who wants to come back after leaving under unpleasant circumstances. I don’t allow it.
To me the doctor-patient relationship is based on trust and objectivity. Once a patient sends an acrimonious letter, it’s very difficult to return to an impartial condition. The fact that they did it once means they may do it again, or think they can get their way with threatening or insulting behavior. These are not things that are good for the connection between us.
So I turn them away. Some just hang up. Others yell, and a few threaten me with legal action. But I don’t back down. Do you want to care for someone who’s done the same to you?
This is a high-stress field. I don’t need the additional distraction of dealing with a toxic medical relationship. If you don’t like me, I have no issue with that. Not everyone does. But once you’ve made that decision, you’re stuck with it.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I refuse to do retreads.
This has nothing to do with my car. If a retread tire gives me the same performance and safety at a lower price, I’m all for it.
Sometimes patients fire me. This is often acrimonious, with them sending me a letter complaining about my competence, bedside manner, personal appearance, staff, office decor, phone system ... whatever. For some reason they weren’t happy with me and instead of just sending a release of records, they decided to let me know in no uncertain terms that they aren’t coming back. We all get these notes.
When I was younger this would really upset me. I took a lot of things personally when I first started out. Now, after almost 20 years on the neurology front lines, it’s just another day. I learned a long time ago that you can’t please everyone or be the doctor they all love. So I fax their chart to wherever they want and move on.
Surprisingly, a few times a year patients will try to come back. Maybe they didn’t like the new doc, or are sorry about their outburst, or can’t find someone else nearby who takes their insurance. So they call and try to make a follow-up. Not surprisingly, they never mention their previous letter. When it’s brought up, they typically claim we misinterpreted it, that they didn’t mean it, or that they’ve decided to forgive me.
This is what I call a retread. A patient who wants to come back after leaving under unpleasant circumstances. I don’t allow it.
To me the doctor-patient relationship is based on trust and objectivity. Once a patient sends an acrimonious letter, it’s very difficult to return to an impartial condition. The fact that they did it once means they may do it again, or think they can get their way with threatening or insulting behavior. These are not things that are good for the connection between us.
So I turn them away. Some just hang up. Others yell, and a few threaten me with legal action. But I don’t back down. Do you want to care for someone who’s done the same to you?
This is a high-stress field. I don’t need the additional distraction of dealing with a toxic medical relationship. If you don’t like me, I have no issue with that. Not everyone does. But once you’ve made that decision, you’re stuck with it.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
IPF Patient Registry will expand
The number of patients enrolled in the Idiopathic Pulmonary Fibrosis–Prospective Outcomes (IPF-PRO) Registry will be increased to 1,500, Boehringer Ingelheim Pharmaceuticals and the Duke Clinical Research Institute have announced.
The organizations plan to accomplish this goal by increasing the number of sites they use to gather IPF patient data, according to a statement; the patients enrolled in the registry will now come from 45 sites instead of 18 sites.
IPF-PRO, which was launched in June 2014, is the first multicenter longitudinal disease state registry in the United States focused specifically on IPF. It was designed for the purpose of studying the progression of IPF and the effectiveness of various treatment approaches for the disease. The registry includes a biorepository that stores blood samples that provide patient genetic material.
“In collecting data from a larger, more diverse group of patients ... this registry will allow us to better assess the impact of the disease over time on clinical and patient-centered outcomes,” said Scott M. Palmer, MD, director of pulmonary research at the Duke Clinical Research Institute, Durham, N.C., in the statement.
More information on the registry is available at clinicaltrials.gov/ct2/show/NCT01915511.
The number of patients enrolled in the Idiopathic Pulmonary Fibrosis–Prospective Outcomes (IPF-PRO) Registry will be increased to 1,500, Boehringer Ingelheim Pharmaceuticals and the Duke Clinical Research Institute have announced.
The organizations plan to accomplish this goal by increasing the number of sites they use to gather IPF patient data, according to a statement; the patients enrolled in the registry will now come from 45 sites instead of 18 sites.
IPF-PRO, which was launched in June 2014, is the first multicenter longitudinal disease state registry in the United States focused specifically on IPF. It was designed for the purpose of studying the progression of IPF and the effectiveness of various treatment approaches for the disease. The registry includes a biorepository that stores blood samples that provide patient genetic material.
“In collecting data from a larger, more diverse group of patients ... this registry will allow us to better assess the impact of the disease over time on clinical and patient-centered outcomes,” said Scott M. Palmer, MD, director of pulmonary research at the Duke Clinical Research Institute, Durham, N.C., in the statement.
More information on the registry is available at clinicaltrials.gov/ct2/show/NCT01915511.
The number of patients enrolled in the Idiopathic Pulmonary Fibrosis–Prospective Outcomes (IPF-PRO) Registry will be increased to 1,500, Boehringer Ingelheim Pharmaceuticals and the Duke Clinical Research Institute have announced.
The organizations plan to accomplish this goal by increasing the number of sites they use to gather IPF patient data, according to a statement; the patients enrolled in the registry will now come from 45 sites instead of 18 sites.
IPF-PRO, which was launched in June 2014, is the first multicenter longitudinal disease state registry in the United States focused specifically on IPF. It was designed for the purpose of studying the progression of IPF and the effectiveness of various treatment approaches for the disease. The registry includes a biorepository that stores blood samples that provide patient genetic material.
“In collecting data from a larger, more diverse group of patients ... this registry will allow us to better assess the impact of the disease over time on clinical and patient-centered outcomes,” said Scott M. Palmer, MD, director of pulmonary research at the Duke Clinical Research Institute, Durham, N.C., in the statement.
More information on the registry is available at clinicaltrials.gov/ct2/show/NCT01915511.
Investigational Drug Improves Maintenance of Wakefulness
DENVER—An investigational drug known as JZP-110 significantly improves the ability to stay awake, compared with placebo, in patients with narcolepsy, according to data presented at the 30th Anniversary Meeting of the Associated Professional Sleep Societies. The large effect sizes associated with the drug confirm the robustness of the results, according to the researchers.
JZP-110 is a selective dopamine and norepinephrine reuptake inhibitor in development as a treatment for excessive sleepiness and impaired wakefulness associated with narcolepsy and obstructive sleep apnea. In phase II trials, the drug improved wakefulness, compared with placebo, on the 40-minute Maintenance of Wakefulness Test (MWT). Studies of other agents, however, have reported sleep latency results using a 20-minute MWT.
Chad Ruoff, MD, Clinical Assistant Professor of Psychiatry and Behavioral Sciences at the Stanford Center for Sleep Sciences and Medicine in California, and colleagues conducted a post hoc analysis of data from two phase II trials to evaluate changes from baseline in mean MWT sleep latency associated with JZP-110, censoring data to include only the first 20 minutes of the 40-minute MWT. In one study, 33 adults with narcolepsy (with or without cataplexy) were randomized to receive two weeks of treatment with placebo or JZP-110. Active treatment was administered at a dose of 150 mg/day during the first week and at a dose of 300 mg/day during the second week. In the second study, 93 adults with narcolepsy (with or without cataplexy) were randomized to receive placebo or JZP-110 for 12 weeks. The dose of drug was 150 mg/day during the first four weeks and 300 mg/day for the remaining eight weeks.
Regardless of whether the data were censored at 20 minutes or at 40 minutes, JZP-110 resulted in a statistically significant increase from baseline in MWT sleep latency, compared with placebo, in both studies. In the first study, mean changes in sleep latency were 12.7 minutes with the drug versus 0.9 minutes with placebo when censored at 40 minutes. The changes were 8.9 minutes with drug and 0.4 minutes with placebo when data were censored at 20 minutes. In the second study, mean changes in MWT were 12.8 minutes with JZP-110 and 2.1 minutes with placebo when data were censored at 40 minutes. Mean changes were 8.9 minutes for drug and 1.1 minutes for placebo when data were censored at 20 minutes.
Effect sizes were large and slightly greater for 20-minute censored data (1.54 in the first study and 1.41 in the second study) than for 40-minute censored data (1.37 in the first study and 1.17 in the second study). Researchers observed two serious adverse events--one case of conversion disorder and one case of acute cholecystitis--that they considered unrelated to the study drug. Few discontinuations resulted from adverse events.
—Erik Greb
DENVER—An investigational drug known as JZP-110 significantly improves the ability to stay awake, compared with placebo, in patients with narcolepsy, according to data presented at the 30th Anniversary Meeting of the Associated Professional Sleep Societies. The large effect sizes associated with the drug confirm the robustness of the results, according to the researchers.
JZP-110 is a selective dopamine and norepinephrine reuptake inhibitor in development as a treatment for excessive sleepiness and impaired wakefulness associated with narcolepsy and obstructive sleep apnea. In phase II trials, the drug improved wakefulness, compared with placebo, on the 40-minute Maintenance of Wakefulness Test (MWT). Studies of other agents, however, have reported sleep latency results using a 20-minute MWT.
Chad Ruoff, MD, Clinical Assistant Professor of Psychiatry and Behavioral Sciences at the Stanford Center for Sleep Sciences and Medicine in California, and colleagues conducted a post hoc analysis of data from two phase II trials to evaluate changes from baseline in mean MWT sleep latency associated with JZP-110, censoring data to include only the first 20 minutes of the 40-minute MWT. In one study, 33 adults with narcolepsy (with or without cataplexy) were randomized to receive two weeks of treatment with placebo or JZP-110. Active treatment was administered at a dose of 150 mg/day during the first week and at a dose of 300 mg/day during the second week. In the second study, 93 adults with narcolepsy (with or without cataplexy) were randomized to receive placebo or JZP-110 for 12 weeks. The dose of drug was 150 mg/day during the first four weeks and 300 mg/day for the remaining eight weeks.
Regardless of whether the data were censored at 20 minutes or at 40 minutes, JZP-110 resulted in a statistically significant increase from baseline in MWT sleep latency, compared with placebo, in both studies. In the first study, mean changes in sleep latency were 12.7 minutes with the drug versus 0.9 minutes with placebo when censored at 40 minutes. The changes were 8.9 minutes with drug and 0.4 minutes with placebo when data were censored at 20 minutes. In the second study, mean changes in MWT were 12.8 minutes with JZP-110 and 2.1 minutes with placebo when data were censored at 40 minutes. Mean changes were 8.9 minutes for drug and 1.1 minutes for placebo when data were censored at 20 minutes.
Effect sizes were large and slightly greater for 20-minute censored data (1.54 in the first study and 1.41 in the second study) than for 40-minute censored data (1.37 in the first study and 1.17 in the second study). Researchers observed two serious adverse events--one case of conversion disorder and one case of acute cholecystitis--that they considered unrelated to the study drug. Few discontinuations resulted from adverse events.
—Erik Greb
DENVER—An investigational drug known as JZP-110 significantly improves the ability to stay awake, compared with placebo, in patients with narcolepsy, according to data presented at the 30th Anniversary Meeting of the Associated Professional Sleep Societies. The large effect sizes associated with the drug confirm the robustness of the results, according to the researchers.
JZP-110 is a selective dopamine and norepinephrine reuptake inhibitor in development as a treatment for excessive sleepiness and impaired wakefulness associated with narcolepsy and obstructive sleep apnea. In phase II trials, the drug improved wakefulness, compared with placebo, on the 40-minute Maintenance of Wakefulness Test (MWT). Studies of other agents, however, have reported sleep latency results using a 20-minute MWT.
Chad Ruoff, MD, Clinical Assistant Professor of Psychiatry and Behavioral Sciences at the Stanford Center for Sleep Sciences and Medicine in California, and colleagues conducted a post hoc analysis of data from two phase II trials to evaluate changes from baseline in mean MWT sleep latency associated with JZP-110, censoring data to include only the first 20 minutes of the 40-minute MWT. In one study, 33 adults with narcolepsy (with or without cataplexy) were randomized to receive two weeks of treatment with placebo or JZP-110. Active treatment was administered at a dose of 150 mg/day during the first week and at a dose of 300 mg/day during the second week. In the second study, 93 adults with narcolepsy (with or without cataplexy) were randomized to receive placebo or JZP-110 for 12 weeks. The dose of drug was 150 mg/day during the first four weeks and 300 mg/day for the remaining eight weeks.
Regardless of whether the data were censored at 20 minutes or at 40 minutes, JZP-110 resulted in a statistically significant increase from baseline in MWT sleep latency, compared with placebo, in both studies. In the first study, mean changes in sleep latency were 12.7 minutes with the drug versus 0.9 minutes with placebo when censored at 40 minutes. The changes were 8.9 minutes with drug and 0.4 minutes with placebo when data were censored at 20 minutes. In the second study, mean changes in MWT were 12.8 minutes with JZP-110 and 2.1 minutes with placebo when data were censored at 40 minutes. Mean changes were 8.9 minutes for drug and 1.1 minutes for placebo when data were censored at 20 minutes.
Effect sizes were large and slightly greater for 20-minute censored data (1.54 in the first study and 1.41 in the second study) than for 40-minute censored data (1.37 in the first study and 1.17 in the second study). Researchers observed two serious adverse events--one case of conversion disorder and one case of acute cholecystitis--that they considered unrelated to the study drug. Few discontinuations resulted from adverse events.
—Erik Greb
Remission of lupus often short-lasting and variable
The time it takes for patients with systemic lupus erythematosus to achieve remission greatly depends on how remission is defined, and such episodes are usually short-lasting, according to an analysis of more than 2,000 patients in the Hopkins Lupus Cohort.
“Our results concerning durability of remission show the relapsing-remitting nature of SLE [systemic lupus erythematosus]. The median duration of remission was only about 3 months for all [four] definitions [used in the study]. This was the time to the next quarterly cohort visit. Even though achieving remission was frequent, durable remission was rare,” wrote the authors, led by Theresa R. Wilhelm, MD, of Johns Hopkins University in Baltimore.
Dr. Wilhelm and her coauthors chose to examine remission in SLE based on four DORIS (Definitions Of Remission In SLE) working group clinical definitions: clinical remission, complete remission, clinical ROT (Remission on Treatment), and complete ROT. To define them, a clinical SLE Disease Activity Index (cSLEDAI) score of 0 and a Physician Global Assessment of less than 0.5 were applicable to all definitions. Zero prednisone was applicable to clinical and complete remission, while clinical ROT and complete ROT allowed for 5 mg or less daily. Immunosuppressive drugs were not allowed for a patient to be deemed in either clinical or complete remission, but were allowed for both ROT definitions. Serologically negative results were deemed permissible for both complete remission and ROT, but not for clinical remission or clinical ROT (Ann Rheum Dis. 2016 Aug 24. doi: 10.1136/annrheumdis-2016-209489).
For a total of 2,307 SLE patients who were enrolled into the Hopkins Lupus Cohort during 1987-2014, the median time for clinical remission to be achieved was 8.7 years, while complete remission took 11.0 years, clinical ROT took 1.8 years, and complete ROT took 3.1 years. These Kaplan-Meier estimates of the distribution of time to remission after entry into the cohort did not include patients who had a gap in their follow-up or dropped out before satisfying the definition of remission.
However, across all four definitions, the median length of remission was only 3 months, as sustained remission proved elusive. Those with high disease activity and greater treatment had longer median times to achieve remission than did those with low disease activity and less treatment.
“Not surprisingly, we found that the level of baseline treatment and baseline disease activity were strongly associated with the time to remission for all definitions,” Dr. Wilhelm and her coauthors noted.
“Testing definitions of remission is of great relevance for clinical practice, as much as for clinical trials,” the authors concluded. “Our future goal is to find out which definitions are most successful in predicting the best possible outcome for our patients.”
The Hopkins Lupus Cohort receives funding from the National Institutes of Health. Dr. Wilhelm and her coauthors did not report any relevant financial disclosures.
The time it takes for patients with systemic lupus erythematosus to achieve remission greatly depends on how remission is defined, and such episodes are usually short-lasting, according to an analysis of more than 2,000 patients in the Hopkins Lupus Cohort.
“Our results concerning durability of remission show the relapsing-remitting nature of SLE [systemic lupus erythematosus]. The median duration of remission was only about 3 months for all [four] definitions [used in the study]. This was the time to the next quarterly cohort visit. Even though achieving remission was frequent, durable remission was rare,” wrote the authors, led by Theresa R. Wilhelm, MD, of Johns Hopkins University in Baltimore.
Dr. Wilhelm and her coauthors chose to examine remission in SLE based on four DORIS (Definitions Of Remission In SLE) working group clinical definitions: clinical remission, complete remission, clinical ROT (Remission on Treatment), and complete ROT. To define them, a clinical SLE Disease Activity Index (cSLEDAI) score of 0 and a Physician Global Assessment of less than 0.5 were applicable to all definitions. Zero prednisone was applicable to clinical and complete remission, while clinical ROT and complete ROT allowed for 5 mg or less daily. Immunosuppressive drugs were not allowed for a patient to be deemed in either clinical or complete remission, but were allowed for both ROT definitions. Serologically negative results were deemed permissible for both complete remission and ROT, but not for clinical remission or clinical ROT (Ann Rheum Dis. 2016 Aug 24. doi: 10.1136/annrheumdis-2016-209489).
For a total of 2,307 SLE patients who were enrolled into the Hopkins Lupus Cohort during 1987-2014, the median time for clinical remission to be achieved was 8.7 years, while complete remission took 11.0 years, clinical ROT took 1.8 years, and complete ROT took 3.1 years. These Kaplan-Meier estimates of the distribution of time to remission after entry into the cohort did not include patients who had a gap in their follow-up or dropped out before satisfying the definition of remission.
However, across all four definitions, the median length of remission was only 3 months, as sustained remission proved elusive. Those with high disease activity and greater treatment had longer median times to achieve remission than did those with low disease activity and less treatment.
“Not surprisingly, we found that the level of baseline treatment and baseline disease activity were strongly associated with the time to remission for all definitions,” Dr. Wilhelm and her coauthors noted.
“Testing definitions of remission is of great relevance for clinical practice, as much as for clinical trials,” the authors concluded. “Our future goal is to find out which definitions are most successful in predicting the best possible outcome for our patients.”
The Hopkins Lupus Cohort receives funding from the National Institutes of Health. Dr. Wilhelm and her coauthors did not report any relevant financial disclosures.
The time it takes for patients with systemic lupus erythematosus to achieve remission greatly depends on how remission is defined, and such episodes are usually short-lasting, according to an analysis of more than 2,000 patients in the Hopkins Lupus Cohort.
“Our results concerning durability of remission show the relapsing-remitting nature of SLE [systemic lupus erythematosus]. The median duration of remission was only about 3 months for all [four] definitions [used in the study]. This was the time to the next quarterly cohort visit. Even though achieving remission was frequent, durable remission was rare,” wrote the authors, led by Theresa R. Wilhelm, MD, of Johns Hopkins University in Baltimore.
Dr. Wilhelm and her coauthors chose to examine remission in SLE based on four DORIS (Definitions Of Remission In SLE) working group clinical definitions: clinical remission, complete remission, clinical ROT (Remission on Treatment), and complete ROT. To define them, a clinical SLE Disease Activity Index (cSLEDAI) score of 0 and a Physician Global Assessment of less than 0.5 were applicable to all definitions. Zero prednisone was applicable to clinical and complete remission, while clinical ROT and complete ROT allowed for 5 mg or less daily. Immunosuppressive drugs were not allowed for a patient to be deemed in either clinical or complete remission, but were allowed for both ROT definitions. Serologically negative results were deemed permissible for both complete remission and ROT, but not for clinical remission or clinical ROT (Ann Rheum Dis. 2016 Aug 24. doi: 10.1136/annrheumdis-2016-209489).
For a total of 2,307 SLE patients who were enrolled into the Hopkins Lupus Cohort during 1987-2014, the median time for clinical remission to be achieved was 8.7 years, while complete remission took 11.0 years, clinical ROT took 1.8 years, and complete ROT took 3.1 years. These Kaplan-Meier estimates of the distribution of time to remission after entry into the cohort did not include patients who had a gap in their follow-up or dropped out before satisfying the definition of remission.
However, across all four definitions, the median length of remission was only 3 months, as sustained remission proved elusive. Those with high disease activity and greater treatment had longer median times to achieve remission than did those with low disease activity and less treatment.
“Not surprisingly, we found that the level of baseline treatment and baseline disease activity were strongly associated with the time to remission for all definitions,” Dr. Wilhelm and her coauthors noted.
“Testing definitions of remission is of great relevance for clinical practice, as much as for clinical trials,” the authors concluded. “Our future goal is to find out which definitions are most successful in predicting the best possible outcome for our patients.”
The Hopkins Lupus Cohort receives funding from the National Institutes of Health. Dr. Wilhelm and her coauthors did not report any relevant financial disclosures.
FROM ANNALS OF THE RHEUMATIC DISEASES
Key clinical point: Sustained remission in systemic lupus erythematosus patients varies considerably according to different definitions and is less likely than previously believed.
Major finding: The median times for remission were 8.7 years (clinical remission), 11.0 years (complete remission), 1.8 years (clinical remission on treatment), and 3.1 years (complete remission on treatment), while median duration of remission was 3 months across all four definitions.
Data source: Prospective clinical cohort study of 2,307 patients enrolled during 1987-2014.
Disclosures: The Hopkins Lupus Cohort receives funding from the National Institutes of Health. The authors reported no relevant financial disclosures.
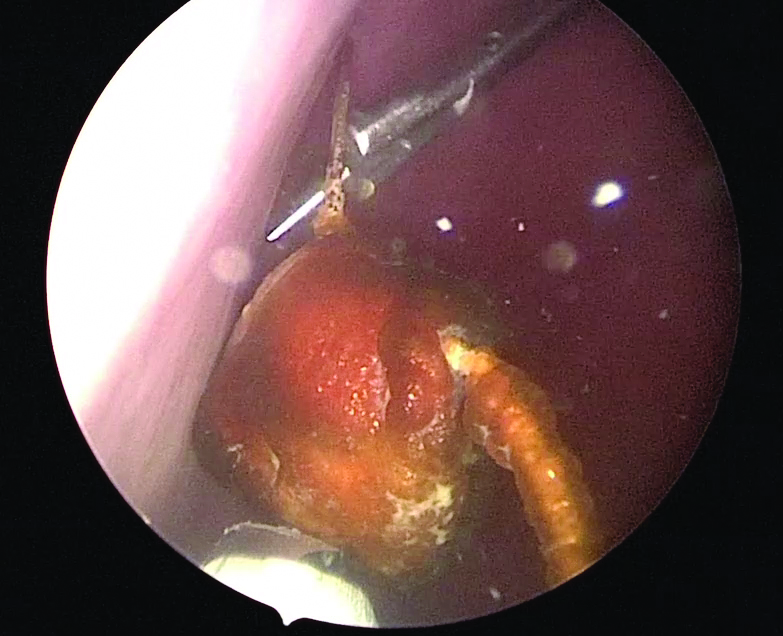
Use of suprapubic Carter-Thomason needle to assist in cystoscopic excision of an intravesical foreign object

For more videos from the Society of Gynecologic Surgeons, click here
Visit the Society of Gynecologic Surgeons online: sgsonline.org
Related articles:
- Uterine artery ligation: Advanced techniques and considerations for the difficult laparoscopic hysterectomy
- Cervical injection of methylene blue for identification of sentinel lymph nodes in cervical cancer
- Misplaced hysteroscopic sterilization micro-insert in the peritoneal cavity: A corpus alienum
- Laparoscopic cystectomy for large, bilateral ovarian dermoids
- Small bowel surgery for the benign gynecologist

For more videos from the Society of Gynecologic Surgeons, click here
Visit the Society of Gynecologic Surgeons online: sgsonline.org
Related articles:
- Uterine artery ligation: Advanced techniques and considerations for the difficult laparoscopic hysterectomy
- Cervical injection of methylene blue for identification of sentinel lymph nodes in cervical cancer
- Misplaced hysteroscopic sterilization micro-insert in the peritoneal cavity: A corpus alienum
- Laparoscopic cystectomy for large, bilateral ovarian dermoids
- Small bowel surgery for the benign gynecologist

For more videos from the Society of Gynecologic Surgeons, click here
Visit the Society of Gynecologic Surgeons online: sgsonline.org
Related articles:
- Uterine artery ligation: Advanced techniques and considerations for the difficult laparoscopic hysterectomy
- Cervical injection of methylene blue for identification of sentinel lymph nodes in cervical cancer
- Misplaced hysteroscopic sterilization micro-insert in the peritoneal cavity: A corpus alienum
- Laparoscopic cystectomy for large, bilateral ovarian dermoids
- Small bowel surgery for the benign gynecologist
CDC reports asymptomatic Zika transmission; FDA begins universal blood testing
Officials at the Centers for Disease Control and Prevention have confirmed a case of Zika virus infection in a nonpregnant Maryland woman who likely contracted the virus through sexual intercourse with her asymptomatic male partner.
“To date, only one other case has been reported in which a man without symptoms might have sexually transmitted Zika virus to his female partner,” Richard B. Brooks, MD, and his colleagues wrote Aug. 26 in the Morbidity and Mortality Weekly Report (doi:10.15585/mmwr.mm6534e2). “However, in that reported case, both the man and the woman had traveled to a country with ongoing Zika virus transmission where they were likely exposed to mosquitoes.”
In the current case, the couple had condomless vaginal sex 10 days and 14 days after his return from the Dominican Republic, along with oral sex on day 14. Two days after the last encounter, the woman began exhibiting symptoms of Zika virus infection, namely, a maculopapular rash and a fever. She sought medical care 3 days later (19 days after her partner returned to the United States). She had no other sexual partners during this time. Meanwhile, the male sex partner reported no symptoms of a Zika virus infection, other than simply being tired from his recent travel.
“The findings in this report indicate that it might be appropriate to consider persons who have condomless sex with partners returning from areas with ongoing Zika virus transmission as exposed to Zika virus, regardless of whether the returning traveler reports symptoms of Zika virus infection,” the researchers wrote.
Transmission of Zika virus through blood transfusions is also a growing concern, particularly if an asymptomatic individual donated blood.
On Aug. 26, the Food and Drug Administration announced recommendations to test all donated blood and blood components across the United States and its territories for the Zika virus, to mitigate the chances of transmitting the virus through transfusions. In February, the FDA first issued guidance recommending that only areas with active Zika virus transmission screen donated blood.
“As new scientific and epidemiological information regarding Zika virus has become available, it’s clear that additional precautionary measures are necessary,” Luciana Borio, MD, FDA’s acting chief scientist, said in a statement. “We are issuing revised guidance for immediate implementation in order to help maintain the safety of the U.S. blood supply.”
In a conference call with reporters, Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, said that while there have not yet been any confirmed cases of such transmission, donors and health care workers must be vigilant. “Given the frequency of travel of individuals within the United States, there is the risk that people without symptoms who are infected with Zika virus could potentially donate blood and thereby transmit Zika virus,” he said.
The CDC also published new numbers on Guillain-Barré syndrome (GBS), which has been on the rise in countries affected by Zika virus.
Individuals who began exhibiting any neurologic symptoms between Jan. 1, 2016, and July 31, 2016, and were suspected of possible GBS total 56. Of those 56 patients, 34 (61%) were found to have evidence of either Zika or another related flavivirus. Ten (18%) of those 56 were confirmed to have Zika virus, and 1 patient who received treatment for GBS died of septic shock. Thirty (88%) of the 34 found to have evidence of flavivirus also reported having an acute illness of some kind before the onset of neurologic symptoms. The figures come from the GBS Passive Surveillance System (MMWR. 2016 Aug 26. doi:10.15585/mmwr.mm6534e1).
“Persons with signs or symptoms consistent with GBS should promptly seek medical attention,” the CDC urged. “Health care providers who evaluate patients with neurologic illnesses should consider GBS and report suspected cases to public health authorities. Residents of and travelers to Puerto Rico are advised to follow existing recommendations for prevention of Zika virus infection.”
Officials at the Centers for Disease Control and Prevention have confirmed a case of Zika virus infection in a nonpregnant Maryland woman who likely contracted the virus through sexual intercourse with her asymptomatic male partner.
“To date, only one other case has been reported in which a man without symptoms might have sexually transmitted Zika virus to his female partner,” Richard B. Brooks, MD, and his colleagues wrote Aug. 26 in the Morbidity and Mortality Weekly Report (doi:10.15585/mmwr.mm6534e2). “However, in that reported case, both the man and the woman had traveled to a country with ongoing Zika virus transmission where they were likely exposed to mosquitoes.”
In the current case, the couple had condomless vaginal sex 10 days and 14 days after his return from the Dominican Republic, along with oral sex on day 14. Two days after the last encounter, the woman began exhibiting symptoms of Zika virus infection, namely, a maculopapular rash and a fever. She sought medical care 3 days later (19 days after her partner returned to the United States). She had no other sexual partners during this time. Meanwhile, the male sex partner reported no symptoms of a Zika virus infection, other than simply being tired from his recent travel.
“The findings in this report indicate that it might be appropriate to consider persons who have condomless sex with partners returning from areas with ongoing Zika virus transmission as exposed to Zika virus, regardless of whether the returning traveler reports symptoms of Zika virus infection,” the researchers wrote.
Transmission of Zika virus through blood transfusions is also a growing concern, particularly if an asymptomatic individual donated blood.
On Aug. 26, the Food and Drug Administration announced recommendations to test all donated blood and blood components across the United States and its territories for the Zika virus, to mitigate the chances of transmitting the virus through transfusions. In February, the FDA first issued guidance recommending that only areas with active Zika virus transmission screen donated blood.
“As new scientific and epidemiological information regarding Zika virus has become available, it’s clear that additional precautionary measures are necessary,” Luciana Borio, MD, FDA’s acting chief scientist, said in a statement. “We are issuing revised guidance for immediate implementation in order to help maintain the safety of the U.S. blood supply.”
In a conference call with reporters, Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, said that while there have not yet been any confirmed cases of such transmission, donors and health care workers must be vigilant. “Given the frequency of travel of individuals within the United States, there is the risk that people without symptoms who are infected with Zika virus could potentially donate blood and thereby transmit Zika virus,” he said.
The CDC also published new numbers on Guillain-Barré syndrome (GBS), which has been on the rise in countries affected by Zika virus.
Individuals who began exhibiting any neurologic symptoms between Jan. 1, 2016, and July 31, 2016, and were suspected of possible GBS total 56. Of those 56 patients, 34 (61%) were found to have evidence of either Zika or another related flavivirus. Ten (18%) of those 56 were confirmed to have Zika virus, and 1 patient who received treatment for GBS died of septic shock. Thirty (88%) of the 34 found to have evidence of flavivirus also reported having an acute illness of some kind before the onset of neurologic symptoms. The figures come from the GBS Passive Surveillance System (MMWR. 2016 Aug 26. doi:10.15585/mmwr.mm6534e1).
“Persons with signs or symptoms consistent with GBS should promptly seek medical attention,” the CDC urged. “Health care providers who evaluate patients with neurologic illnesses should consider GBS and report suspected cases to public health authorities. Residents of and travelers to Puerto Rico are advised to follow existing recommendations for prevention of Zika virus infection.”
Officials at the Centers for Disease Control and Prevention have confirmed a case of Zika virus infection in a nonpregnant Maryland woman who likely contracted the virus through sexual intercourse with her asymptomatic male partner.
“To date, only one other case has been reported in which a man without symptoms might have sexually transmitted Zika virus to his female partner,” Richard B. Brooks, MD, and his colleagues wrote Aug. 26 in the Morbidity and Mortality Weekly Report (doi:10.15585/mmwr.mm6534e2). “However, in that reported case, both the man and the woman had traveled to a country with ongoing Zika virus transmission where they were likely exposed to mosquitoes.”
In the current case, the couple had condomless vaginal sex 10 days and 14 days after his return from the Dominican Republic, along with oral sex on day 14. Two days after the last encounter, the woman began exhibiting symptoms of Zika virus infection, namely, a maculopapular rash and a fever. She sought medical care 3 days later (19 days after her partner returned to the United States). She had no other sexual partners during this time. Meanwhile, the male sex partner reported no symptoms of a Zika virus infection, other than simply being tired from his recent travel.
“The findings in this report indicate that it might be appropriate to consider persons who have condomless sex with partners returning from areas with ongoing Zika virus transmission as exposed to Zika virus, regardless of whether the returning traveler reports symptoms of Zika virus infection,” the researchers wrote.
Transmission of Zika virus through blood transfusions is also a growing concern, particularly if an asymptomatic individual donated blood.
On Aug. 26, the Food and Drug Administration announced recommendations to test all donated blood and blood components across the United States and its territories for the Zika virus, to mitigate the chances of transmitting the virus through transfusions. In February, the FDA first issued guidance recommending that only areas with active Zika virus transmission screen donated blood.
“As new scientific and epidemiological information regarding Zika virus has become available, it’s clear that additional precautionary measures are necessary,” Luciana Borio, MD, FDA’s acting chief scientist, said in a statement. “We are issuing revised guidance for immediate implementation in order to help maintain the safety of the U.S. blood supply.”
In a conference call with reporters, Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, said that while there have not yet been any confirmed cases of such transmission, donors and health care workers must be vigilant. “Given the frequency of travel of individuals within the United States, there is the risk that people without symptoms who are infected with Zika virus could potentially donate blood and thereby transmit Zika virus,” he said.
The CDC also published new numbers on Guillain-Barré syndrome (GBS), which has been on the rise in countries affected by Zika virus.
Individuals who began exhibiting any neurologic symptoms between Jan. 1, 2016, and July 31, 2016, and were suspected of possible GBS total 56. Of those 56 patients, 34 (61%) were found to have evidence of either Zika or another related flavivirus. Ten (18%) of those 56 were confirmed to have Zika virus, and 1 patient who received treatment for GBS died of septic shock. Thirty (88%) of the 34 found to have evidence of flavivirus also reported having an acute illness of some kind before the onset of neurologic symptoms. The figures come from the GBS Passive Surveillance System (MMWR. 2016 Aug 26. doi:10.15585/mmwr.mm6534e1).
“Persons with signs or symptoms consistent with GBS should promptly seek medical attention,” the CDC urged. “Health care providers who evaluate patients with neurologic illnesses should consider GBS and report suspected cases to public health authorities. Residents of and travelers to Puerto Rico are advised to follow existing recommendations for prevention of Zika virus infection.”
Inflammatory bowel disease
For the Spring Postgraduate Course session on inflammatory bowel disease (IBD), we were fortunate to secure four of the best educators on IBD in the American Gastroenterological Association.
William Sandborn, MD, of the University of California, San Diego, led the session with an overview of the immunomodulators and biologics that are employed for the treatment of Crohn’s disease and ulcerative colitis in 2016, including the thiopurines, methotrexate, calcineurin inhibitors, anti–tumor necrosis factor (TNF) agents, anti-integrins, and upcoming therapies such as ustekinumab (anti-interleukins 12 and 23) and tofacitinib (Janus kinase antagonist).
The evidence base for thiopurine monotherapy in IBD is weaker than we had once assumed, and the combination of infliximab and azathioprine remains the gold standard in efficacy with respect to steroid-free remission. Methotrexate remains a reasonable option as an immunomodulator, especially for patients who can’t tolerate thiopurines or who are risk averse. Cyclosporine has a niche indication for the treatment of acute severe colitis, and is at least equivalent to infliximab for that indication.
We have a total of four anti-TNF agents approved for the treatment of moderate to severe Crohn’s disease and ulcerative colitis, including infliximab, adalimumab, certolizumab pegol (Crohn’s only), and golimumab (ulcerative colitis only). The infliximab biosimilar CT-P13 is approved for IBD in several countries. The anti-integrin, vedolizumab, is approved for both moderate to severe ulcerative colitis and Crohn’s disease. Ustekinumab was shown to be more effective than placebo in inducing clinical response and remission in moderate to severe Crohn’s disease patients who were either refractory/intolerant to or naive to anti-TNF therapy. Tofacitinib was recently shown in two trials to be more effective than placebo in inducing clinical remission and mucosal healing in patients with moderate to severe ulcerative colitis.
Maria Abreu, MD, from the University of Miami highlighted the fact that although our current therapies are far more effective than previous ones, they can be problematic, and dose adjustment is often needed. We should tailor our treatment plan based on our assessment of the patient’s risk for intestinal complications or surgery – patients with multiple risk factors such as young age at diagnosis, extensive involvement, deep ulcers, previous surgeries, or penetrating/stricturing behavior at baseline should be treated more aggressively, perhaps with a top-down approach. In general, biologics will be more effective when used in combination with immunomodulators. Monotherapy can be considered in patients with low inflammatory burden, few risk factors for more severe disease, or in those at high risk for complications from combination therapy.
At least one way in which concomitant immunomodulator therapy exerts its beneficial effect is by increasing trough levels of the biologic. One recent study of the relationship between adalimumab levels and endoscopic inflammation suggested that the median adalimumab trough level associated with mucosal healing was 13.5 mcg/mL. Patients with low drug levels and no or low levels of anti-drug antibodies should undergo dose escalation. Those with absent drug levels and high levels of antibodies should switch to a different agent. If the drug level is considered adequate and yet inflammation is still present, consideration should be given to switching to a biologic with a different mechanism of action. In some patients with low levels of antibodies who are on monotherapy, there may be a role for adding an immunomodulator in an attempt to suppress antibody formation. The importance of vedolizumab and ustekinumab as biologics with different mechanisms of action was also highlighted. An exciting class of biologics currently under development is the anti–IL-23 class, including drugs such as risankizumab and MEDI-2070.
David T. Rubin, MD, from the University of Chicago reviewed the various complications associated with IBD and its therapies. Disease-related complications include clinical flares, bowel obstruction, fistula/abscess formation, intestinal cancer, the need for surgery (sometimes more than one), and extraintestinal manifestations. More accurate diagnosis and earlier effective therapies may help to reduce the risk of these complications. Certain infections such as herpes zoster, pneumonia, and Clostridium difficile infection appear to occur more commonly in IBD patients. The TREAT registry showed that the use of corticosteroids is associated with an increased risk of serious infections and mortality, and the use of infliximab is associated with an increased risk of serious infection. C. difficile infection in particular can be problematic, and should be treated aggressively. Thiopurines appear to increase the risk of lymphoma and nonmelanoma skin cancer. Lymphoma risk quickly reverts to baseline after thiopurines are discontinued. Overall, no increased risk of cancer can be demonstrated among patients on anti-TNF therapy, and patients with a history of cancer do not have an increased risk of recurrent or new cancers on anti-TNF therapy. There may be an association between anti-TNF use and melanoma, although this bears further study.
Miguel Regueiro, MD, from the University of Pittsburgh wrapped up the session with a talk on managing postoperative IBD patients. Although the ileal pouch–anal anastomosis (IPAA) broadened access to proctocolectomy, it is associated with several disorders, including pelvic sepsis, pouchitis, Crohn’s disease of the ileoanal pouch, cuffitis, and irritable pouch syndrome. At least 50% of IPAA patients will develop at least one episode of pouchitis. Most patients will respond to ciprofloxacin or metronidazole, but a small percentage will develop chronic pouchitis. Ileocecal resection for the complications of Crohn’s disease generally results in immediate improvements in quality of life, but unfortunately, most patients will develop endoscopic and eventually clinical recurrence. At 6-12 months after surgery, a Rutgeerts score of i2 or higher (at least five aphthous lesions in the neoterminal ileum) is associated with a high risk of clinical recurrence, and this finding can be used as a trigger for escalation of therapy.
Infliximab was associated with a markedly decreased risk of endoscopic recurrence in a small investigator-initiated trial, and subsequent studies have strongly suggested that infliximab and adalimumab reduce the risk of recurrence. Although the primary endpoint of clinical recurrence was not met in the large multicenter PREVENT trial, infliximab was shown to significantly decrease endoscopic recurrence. The POCER study showed that a slightly less proactive approach of watchful waiting in lower-risk patients and making decisions about therapy at the 6-month colonoscopy was a reasonable one. The risk factors one must consider in stratifying postop patients include early age at surgery, short time between diagnosis and surgery, cigarette smoking, penetrating disease behavior, and a history of previous resections.
Dr. Loftus is professor of medicine in the division of gastroenterology and hepatology, Mayo Clinic, Rochester, Minn. He has consulted for and has received research support from Janssen, UCB, Takeda, and AbbVie.
This is a summary provided by the moderator of one of the spring postgraduate course sessions held at DDW 2016.
For the Spring Postgraduate Course session on inflammatory bowel disease (IBD), we were fortunate to secure four of the best educators on IBD in the American Gastroenterological Association.
William Sandborn, MD, of the University of California, San Diego, led the session with an overview of the immunomodulators and biologics that are employed for the treatment of Crohn’s disease and ulcerative colitis in 2016, including the thiopurines, methotrexate, calcineurin inhibitors, anti–tumor necrosis factor (TNF) agents, anti-integrins, and upcoming therapies such as ustekinumab (anti-interleukins 12 and 23) and tofacitinib (Janus kinase antagonist).
The evidence base for thiopurine monotherapy in IBD is weaker than we had once assumed, and the combination of infliximab and azathioprine remains the gold standard in efficacy with respect to steroid-free remission. Methotrexate remains a reasonable option as an immunomodulator, especially for patients who can’t tolerate thiopurines or who are risk averse. Cyclosporine has a niche indication for the treatment of acute severe colitis, and is at least equivalent to infliximab for that indication.
We have a total of four anti-TNF agents approved for the treatment of moderate to severe Crohn’s disease and ulcerative colitis, including infliximab, adalimumab, certolizumab pegol (Crohn’s only), and golimumab (ulcerative colitis only). The infliximab biosimilar CT-P13 is approved for IBD in several countries. The anti-integrin, vedolizumab, is approved for both moderate to severe ulcerative colitis and Crohn’s disease. Ustekinumab was shown to be more effective than placebo in inducing clinical response and remission in moderate to severe Crohn’s disease patients who were either refractory/intolerant to or naive to anti-TNF therapy. Tofacitinib was recently shown in two trials to be more effective than placebo in inducing clinical remission and mucosal healing in patients with moderate to severe ulcerative colitis.
Maria Abreu, MD, from the University of Miami highlighted the fact that although our current therapies are far more effective than previous ones, they can be problematic, and dose adjustment is often needed. We should tailor our treatment plan based on our assessment of the patient’s risk for intestinal complications or surgery – patients with multiple risk factors such as young age at diagnosis, extensive involvement, deep ulcers, previous surgeries, or penetrating/stricturing behavior at baseline should be treated more aggressively, perhaps with a top-down approach. In general, biologics will be more effective when used in combination with immunomodulators. Monotherapy can be considered in patients with low inflammatory burden, few risk factors for more severe disease, or in those at high risk for complications from combination therapy.
At least one way in which concomitant immunomodulator therapy exerts its beneficial effect is by increasing trough levels of the biologic. One recent study of the relationship between adalimumab levels and endoscopic inflammation suggested that the median adalimumab trough level associated with mucosal healing was 13.5 mcg/mL. Patients with low drug levels and no or low levels of anti-drug antibodies should undergo dose escalation. Those with absent drug levels and high levels of antibodies should switch to a different agent. If the drug level is considered adequate and yet inflammation is still present, consideration should be given to switching to a biologic with a different mechanism of action. In some patients with low levels of antibodies who are on monotherapy, there may be a role for adding an immunomodulator in an attempt to suppress antibody formation. The importance of vedolizumab and ustekinumab as biologics with different mechanisms of action was also highlighted. An exciting class of biologics currently under development is the anti–IL-23 class, including drugs such as risankizumab and MEDI-2070.
David T. Rubin, MD, from the University of Chicago reviewed the various complications associated with IBD and its therapies. Disease-related complications include clinical flares, bowel obstruction, fistula/abscess formation, intestinal cancer, the need for surgery (sometimes more than one), and extraintestinal manifestations. More accurate diagnosis and earlier effective therapies may help to reduce the risk of these complications. Certain infections such as herpes zoster, pneumonia, and Clostridium difficile infection appear to occur more commonly in IBD patients. The TREAT registry showed that the use of corticosteroids is associated with an increased risk of serious infections and mortality, and the use of infliximab is associated with an increased risk of serious infection. C. difficile infection in particular can be problematic, and should be treated aggressively. Thiopurines appear to increase the risk of lymphoma and nonmelanoma skin cancer. Lymphoma risk quickly reverts to baseline after thiopurines are discontinued. Overall, no increased risk of cancer can be demonstrated among patients on anti-TNF therapy, and patients with a history of cancer do not have an increased risk of recurrent or new cancers on anti-TNF therapy. There may be an association between anti-TNF use and melanoma, although this bears further study.
Miguel Regueiro, MD, from the University of Pittsburgh wrapped up the session with a talk on managing postoperative IBD patients. Although the ileal pouch–anal anastomosis (IPAA) broadened access to proctocolectomy, it is associated with several disorders, including pelvic sepsis, pouchitis, Crohn’s disease of the ileoanal pouch, cuffitis, and irritable pouch syndrome. At least 50% of IPAA patients will develop at least one episode of pouchitis. Most patients will respond to ciprofloxacin or metronidazole, but a small percentage will develop chronic pouchitis. Ileocecal resection for the complications of Crohn’s disease generally results in immediate improvements in quality of life, but unfortunately, most patients will develop endoscopic and eventually clinical recurrence. At 6-12 months after surgery, a Rutgeerts score of i2 or higher (at least five aphthous lesions in the neoterminal ileum) is associated with a high risk of clinical recurrence, and this finding can be used as a trigger for escalation of therapy.
Infliximab was associated with a markedly decreased risk of endoscopic recurrence in a small investigator-initiated trial, and subsequent studies have strongly suggested that infliximab and adalimumab reduce the risk of recurrence. Although the primary endpoint of clinical recurrence was not met in the large multicenter PREVENT trial, infliximab was shown to significantly decrease endoscopic recurrence. The POCER study showed that a slightly less proactive approach of watchful waiting in lower-risk patients and making decisions about therapy at the 6-month colonoscopy was a reasonable one. The risk factors one must consider in stratifying postop patients include early age at surgery, short time between diagnosis and surgery, cigarette smoking, penetrating disease behavior, and a history of previous resections.
Dr. Loftus is professor of medicine in the division of gastroenterology and hepatology, Mayo Clinic, Rochester, Minn. He has consulted for and has received research support from Janssen, UCB, Takeda, and AbbVie.
This is a summary provided by the moderator of one of the spring postgraduate course sessions held at DDW 2016.
For the Spring Postgraduate Course session on inflammatory bowel disease (IBD), we were fortunate to secure four of the best educators on IBD in the American Gastroenterological Association.
William Sandborn, MD, of the University of California, San Diego, led the session with an overview of the immunomodulators and biologics that are employed for the treatment of Crohn’s disease and ulcerative colitis in 2016, including the thiopurines, methotrexate, calcineurin inhibitors, anti–tumor necrosis factor (TNF) agents, anti-integrins, and upcoming therapies such as ustekinumab (anti-interleukins 12 and 23) and tofacitinib (Janus kinase antagonist).
The evidence base for thiopurine monotherapy in IBD is weaker than we had once assumed, and the combination of infliximab and azathioprine remains the gold standard in efficacy with respect to steroid-free remission. Methotrexate remains a reasonable option as an immunomodulator, especially for patients who can’t tolerate thiopurines or who are risk averse. Cyclosporine has a niche indication for the treatment of acute severe colitis, and is at least equivalent to infliximab for that indication.
We have a total of four anti-TNF agents approved for the treatment of moderate to severe Crohn’s disease and ulcerative colitis, including infliximab, adalimumab, certolizumab pegol (Crohn’s only), and golimumab (ulcerative colitis only). The infliximab biosimilar CT-P13 is approved for IBD in several countries. The anti-integrin, vedolizumab, is approved for both moderate to severe ulcerative colitis and Crohn’s disease. Ustekinumab was shown to be more effective than placebo in inducing clinical response and remission in moderate to severe Crohn’s disease patients who were either refractory/intolerant to or naive to anti-TNF therapy. Tofacitinib was recently shown in two trials to be more effective than placebo in inducing clinical remission and mucosal healing in patients with moderate to severe ulcerative colitis.
Maria Abreu, MD, from the University of Miami highlighted the fact that although our current therapies are far more effective than previous ones, they can be problematic, and dose adjustment is often needed. We should tailor our treatment plan based on our assessment of the patient’s risk for intestinal complications or surgery – patients with multiple risk factors such as young age at diagnosis, extensive involvement, deep ulcers, previous surgeries, or penetrating/stricturing behavior at baseline should be treated more aggressively, perhaps with a top-down approach. In general, biologics will be more effective when used in combination with immunomodulators. Monotherapy can be considered in patients with low inflammatory burden, few risk factors for more severe disease, or in those at high risk for complications from combination therapy.
At least one way in which concomitant immunomodulator therapy exerts its beneficial effect is by increasing trough levels of the biologic. One recent study of the relationship between adalimumab levels and endoscopic inflammation suggested that the median adalimumab trough level associated with mucosal healing was 13.5 mcg/mL. Patients with low drug levels and no or low levels of anti-drug antibodies should undergo dose escalation. Those with absent drug levels and high levels of antibodies should switch to a different agent. If the drug level is considered adequate and yet inflammation is still present, consideration should be given to switching to a biologic with a different mechanism of action. In some patients with low levels of antibodies who are on monotherapy, there may be a role for adding an immunomodulator in an attempt to suppress antibody formation. The importance of vedolizumab and ustekinumab as biologics with different mechanisms of action was also highlighted. An exciting class of biologics currently under development is the anti–IL-23 class, including drugs such as risankizumab and MEDI-2070.
David T. Rubin, MD, from the University of Chicago reviewed the various complications associated with IBD and its therapies. Disease-related complications include clinical flares, bowel obstruction, fistula/abscess formation, intestinal cancer, the need for surgery (sometimes more than one), and extraintestinal manifestations. More accurate diagnosis and earlier effective therapies may help to reduce the risk of these complications. Certain infections such as herpes zoster, pneumonia, and Clostridium difficile infection appear to occur more commonly in IBD patients. The TREAT registry showed that the use of corticosteroids is associated with an increased risk of serious infections and mortality, and the use of infliximab is associated with an increased risk of serious infection. C. difficile infection in particular can be problematic, and should be treated aggressively. Thiopurines appear to increase the risk of lymphoma and nonmelanoma skin cancer. Lymphoma risk quickly reverts to baseline after thiopurines are discontinued. Overall, no increased risk of cancer can be demonstrated among patients on anti-TNF therapy, and patients with a history of cancer do not have an increased risk of recurrent or new cancers on anti-TNF therapy. There may be an association between anti-TNF use and melanoma, although this bears further study.
Miguel Regueiro, MD, from the University of Pittsburgh wrapped up the session with a talk on managing postoperative IBD patients. Although the ileal pouch–anal anastomosis (IPAA) broadened access to proctocolectomy, it is associated with several disorders, including pelvic sepsis, pouchitis, Crohn’s disease of the ileoanal pouch, cuffitis, and irritable pouch syndrome. At least 50% of IPAA patients will develop at least one episode of pouchitis. Most patients will respond to ciprofloxacin or metronidazole, but a small percentage will develop chronic pouchitis. Ileocecal resection for the complications of Crohn’s disease generally results in immediate improvements in quality of life, but unfortunately, most patients will develop endoscopic and eventually clinical recurrence. At 6-12 months after surgery, a Rutgeerts score of i2 or higher (at least five aphthous lesions in the neoterminal ileum) is associated with a high risk of clinical recurrence, and this finding can be used as a trigger for escalation of therapy.
Infliximab was associated with a markedly decreased risk of endoscopic recurrence in a small investigator-initiated trial, and subsequent studies have strongly suggested that infliximab and adalimumab reduce the risk of recurrence. Although the primary endpoint of clinical recurrence was not met in the large multicenter PREVENT trial, infliximab was shown to significantly decrease endoscopic recurrence. The POCER study showed that a slightly less proactive approach of watchful waiting in lower-risk patients and making decisions about therapy at the 6-month colonoscopy was a reasonable one. The risk factors one must consider in stratifying postop patients include early age at surgery, short time between diagnosis and surgery, cigarette smoking, penetrating disease behavior, and a history of previous resections.
Dr. Loftus is professor of medicine in the division of gastroenterology and hepatology, Mayo Clinic, Rochester, Minn. He has consulted for and has received research support from Janssen, UCB, Takeda, and AbbVie.
This is a summary provided by the moderator of one of the spring postgraduate course sessions held at DDW 2016.