User login
Periocular Fillers and Related Anatomy
Rejuvenation of the periocular area is in high demand among patients who want to look and feel their best. Physicians should understand the complicated anatomy surrounding the eyes before attempting to inject this area with facial fillers, both to understand the aging process and to minimize treatment complications.
Basic Oculoplastic and Orbital Anatomy
The injector should understand the anatomy of the periocular muscles, the orbital osteology, and the secretory and lacrimal system, in addition to the fat, ligaments, and vascular anatomy in this area.1
The eyes are surrounded by fat compartments that provide glide planes for the motion of the eyelids and globe. There are 2 upper eyelid fat-pads—nasal and central [preaponeurotic])—in the upper lid, leaving room for the lacrimal gland laterally. There are 3 fat compartments—nasal, central, and lateral—in the lower eyelid. The nasal and central compartments are separated by the inferior oblique muscle, which elevates and extorts the eye. The orbital septum holds the fat-pads in place in the orbit. The brow fat-pad is the retro-orbicularis oculi fat-pad (ROOF). There are fat compartments that lie in the subcutaneous space along the entire forehead and in the temple. The suborbicularis oculi fat-pad (SOOF) lies over the malar eminence. Superficial and deep submuscular fat compartments of the face have been described.2 Deep fat compartments also have been examined on computed tomography.3
Orbital circulation comes from the internal carotid artery and anastomoses with the supply from the external carotid artery to supply the orbit. The first branch off of the carotid artery is the ophthalmic artery, and the first branch off of the ophthalmic artery is the central retinal artery that enters the optic nerve sheath 1 cm behind the globe to supply the retina. The supraorbital and supratrochlear arteries branch off of the ophthalmic artery and supply the forehead. The supraorbital artery runs through the supraorbital notch (foramen in 8%)1 and can usually be palpated with one’s finger. There are 15 to 20 short posterior ciliary arteries leading to the choroid, 2 long posterior ciliary arteries to the iris circle, and 7 anterior ciliary arteries to the extraocular muscles. The superior and inferior venous systems drain into the cavernous sinus.4
The ligaments are important to signs of facial aging because tissue atrophy occurs along them. The main orbital ligaments are the lateral orbital thickening (known as the LOT) that adheres the eyelids to the lateral orbital rim and the orbitomalar ligament (orbicularis retaining ligament), which is a condensation fibrous tissue that attaches the skin to the inferior orbital rim and orbital septum along the arcus marginalis and defines the superior edge of the SOOF.5 The zygomatic ligament not only suspends the zygomaticus major and zygomaticus minor muscles to the malar eminence but there are osseocutaneous attachments that connect the skin over the zygoma’s malar eminence and demarcate the inferior edge of the SOOF.6
Periocular Aging
The skin, fat, muscles, and bones change and rotate with aging, and not all orbits age in the same manner. Older patients with dermatochalasis (excess skin fat and muscle) often undergo rejuvenation with blepharoplasty, a brow-lift, and a midface-lift, but many atrophic changes can be improved with facial fillers.7,8
As adults age, the soft tissue along the ligaments begins to show atrophy, prime signs of aging that are often improved with fillers. Atrophy along the orbitomalar ligament along the infraorbital rim creates a depressed tear trough, which is an early sign of aging. A 3-point grading system reported by Hirmand8 describes the severity of progressive hallowing. There also is atrophy along the zygomatic cutaneous ligament that creates the malar hollow. The SOOF appears to be more prominent when these areas above and below show atrophy, which creates the look of an unwanted bag known as a festoon. Additionally, there is atrophy along the superior orbital notch where the ophthalmic branch of the trigeminal nerve (V1) and the supraorbital artery traverse. Soft-tissue atrophy along the supraorbital notch resembles the peak at the top of the letter A, giving the slang term A-frame deformity.
Periocular fat can atrophy, hypertrophy, herniate forward as the septum weakens, or become ptotic. Some patients develop hypertrophy and herniation of the superior and inferior orbital fat-pads, while others develop unwanted atrophy leaving a hollow superior orbit and loss of support to the levator muscle that contributes to eyelid ptosis. The frontalis fat deflates, leaving veins, arteries, and the hypertrophied corrugators unwantedly visible. Loss of subcutaneous fat in the glabella contributes to the formation of frown lines between the brows (also called number 11’s). The ROOF deflates in some patients adding to brow ptosis. Loss of the facial frame occurs when temple fat atrophies.
Skeletal rotation also occurs. Throughout a patient’s life, the skeleton remodels itself via activity of osteoclasts and osteoblasts. Pessa et al9,10 has described the expansion of the anterior orbital aperture superomedially and inferolaterally as well as maxillary retrusion that results in angular changes of the midface in relation to the orbital rim. Lambros’ algorithm describes the rotational changes of the cranium where the superior orbit protrudes as the maxilla retreats posteriorly.9-11 The equator of the globe does not change its distance from the ROOF of the orbit, presumably because of its suspension in the orbit by the optic nerve after it passes through the optic canal and trochlea via the superior oblique muscle, but the distance of the inferior equator of the globe to the floor of the orbit increases as the floor of the orbit descends.12
Dermal Fillers for Periocular Rejuvenation
Hyaluronic acid (HA) was first pioneered for use in humans in the late 1970s by ophthalmologists for anterior segment surgery.13-15 Biocompatibility for orthopedic and dermal applications was explored in the early 1990s.16
At this time, no dermal filler is approved by the US Food and Drug Administration for use in the periorbital area. Some fillers are approved for subdermal areas extending to the preperiosteal plane and can be used in the midface such as HA fillers (eg, Restylane Lyft [Galderma Laboratories, LP]), Juvéderm Voluma XC [Allergan, Inc]), poly-L-lactic acid (PLLA), and calcium hydroxylapatite (CaHA). No dermal fillers are approved for use in the forehead, glabella, or temples. Their use is becoming increasingly popular but is considered off label. In addition, cannulas are not approved for use in these areas. Cannulas may be beneficial in that they are thought to create less bruising and have less chance of entering a vessel than needles, but some injectors prefer needles because they are stiffer and therefore more precise.
The ideal filler for the tear trough, superior sulcus, ROOF, over the orbitomalar ligament, forehead, and glabella is one that is somewhat moldable but does not migrate, is not hydrophilic, is smooth to inject, and is reversible should there be any complications. No single filler fits this ideal description, but HAs typically are the first choice.
In vitro studies to determine the stiffness (G') and the ability to flow (viscosity) have been performed.17,18 Calcium hydroxylapatite has the most stiffness, while Belotero Balance (Merz Aesthetics) and Juvéderm Ultra XC (Allergan, Inc) are more soft17 (Table). These guidelines are important but may not correlate directly with how the fillers behave in vivo as demonstrated in animal models.18
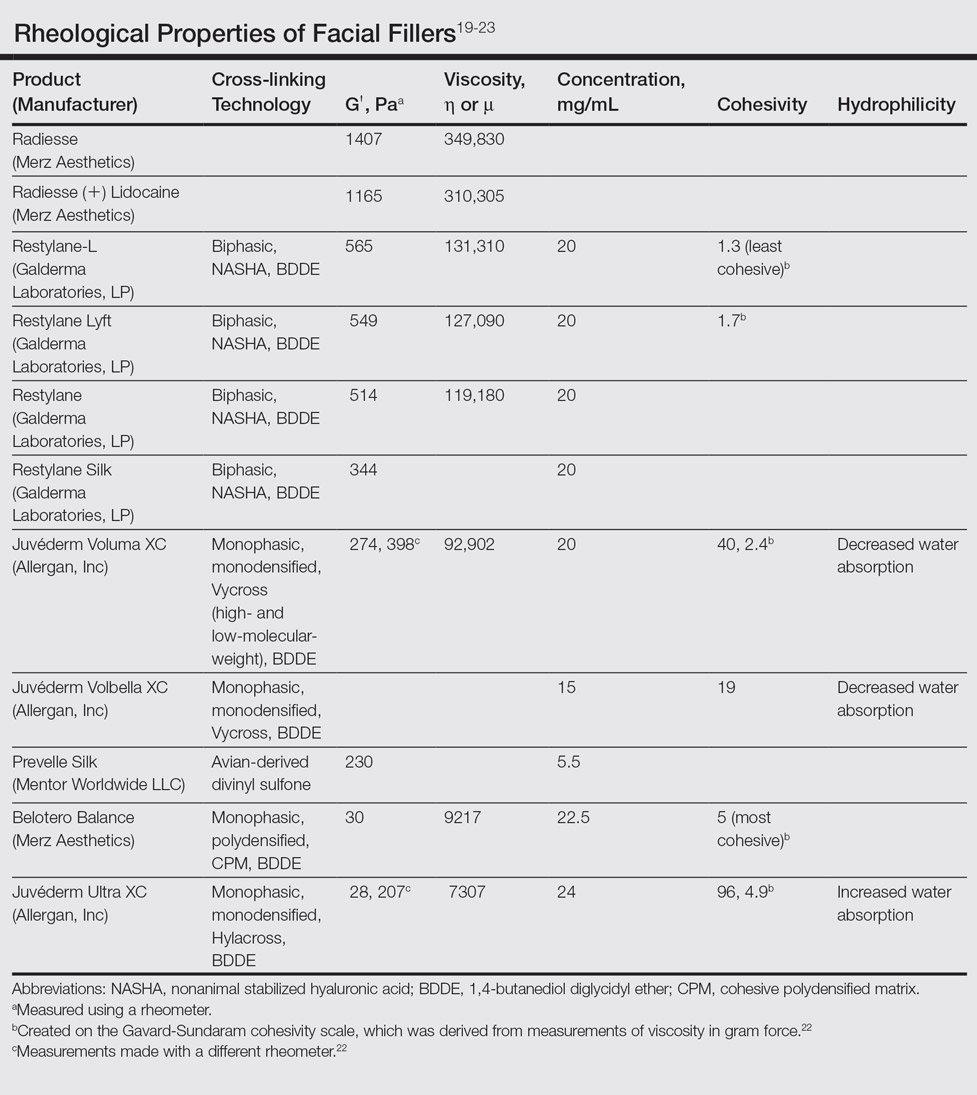
Hyaluronic acid fillers are produced by different technologies to create their cross-link patterns with 1,4-butanediol diglycidyl ether, which determines, to some degree, their behavior in human tissue. Fillers are either monophasic; monodensified; formed by Hylacross (Juvéderm), Vycross (Juvéderm Voluma XC, Juvéderm Volbella XC), or cohesive polydensified matrix technology (Belotero Balance), or biphasic, formed by nonanimal stabilized HA sieving technology (Restylane family). Biopsy has demonstrated that monophasic fillers tend to percolate through and integrate into the tissue, while biphasic fillers dissect tissue to the sides to create a potential space for the filler to reside (Table).24
Periocular Injection Considerations
An experienced injector is one who has developed not only an artistic eye for the face and excellent sense of anatomy but also has a sensitive ability to predict the filler-tissue interaction based on tactile feedback dependent on 3 main qualities: (1) stiffness and viscosity of the filler, (2) gauge of the needle or cannula, and (3) depth of the needle in the tissue. Periocular injections of dermal fillers can be delivered with needles or cannulas, diluted or undiluted. Smaller-gauge needles require more force than larger-gauge needles and cannulas that flow more freely. A needle in the dense dermis will require more force than one placed in the loose subcutaneous space.
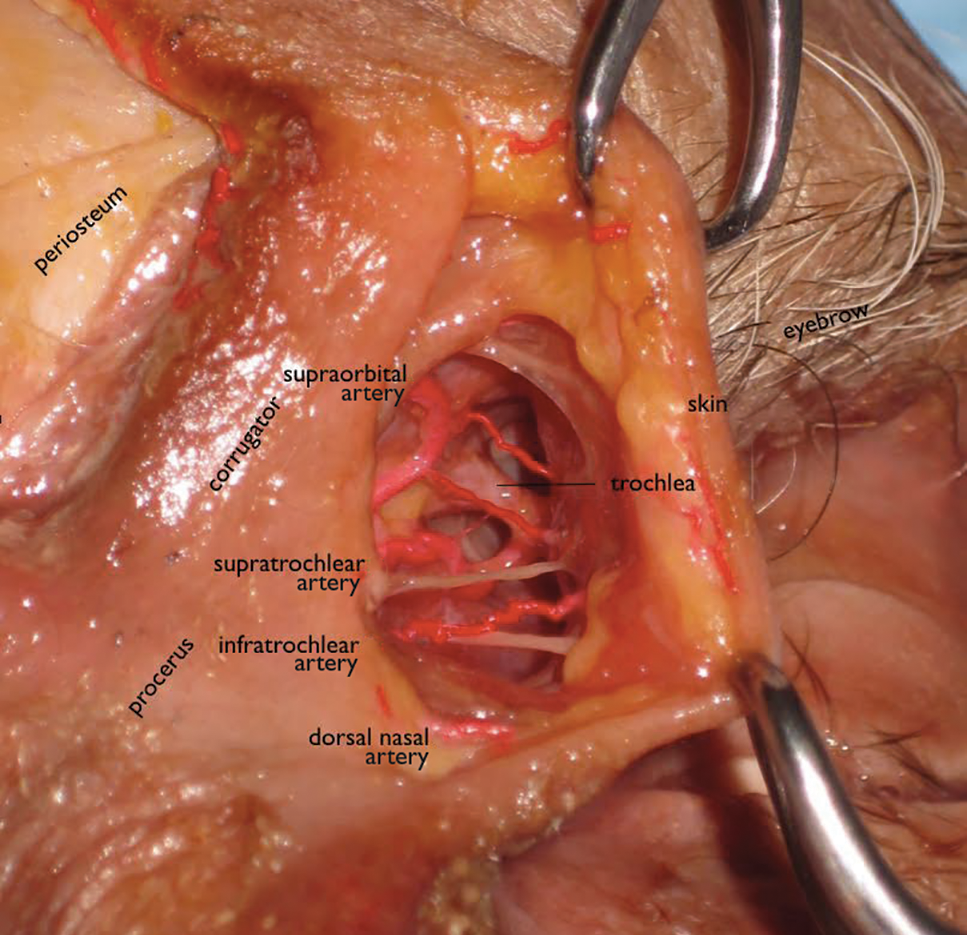
The tear trough is generally preferable to fill with a mid-level G' HA filler that is less apt to migrate. A neutral gaze during the injection is preferred because closing or moving the eyes can distort the position of the inferior orbital fat-pads (Figure 1). A needle or cannula can be used, diluted or undiluted. The tear trough can be filled with the injection directed horizontally or vertically via a fanning technique. If needles are used, the skin should be stretched to view the 3 to 5 vertical veins and then the needle should be advanced beneath them to avoid bruising. Avoidance of hydrophilic fillers in the tear trough is important to avoid edema. The superior sulcus can be filled both anteriorly and posteriorly to the septum, which is a highly advanced injection for experienced injectors because of the proximity to the supratrochlear and supraorbital arteries as well as the superior ophthalmic vein (Figure 2). Sharp creases such as deep lateral periocular rhytides known as crow’s-feet are nicely filled with intradermal HAs with a low G'.
Adding volume to the midface is important because it is the continuum of the lower eyelid. Fillers can be injected into multiple levels in this area: deep (to act as pillars to lift the malar eminence and replace bone that has rotated and soft tissue that has become atrophic or descended) and subcutaneous (to efface soft tissue along the zygomatic cutaneous ligament). Higher G' HA fillers and CaHA often are used in the midface along with PLLA. Facial framing of the temples, lateral cheeks, and preauricular area is often accomplished with PLLA but also can be done with mid to high G' HA fillers or CaHA. A cannula may be used to undermine and break apart the zygomatic cutaneous ligament’s cutaneous attachments prior to delivery of the filler in the subcutaneous plane.26 If not done, filler may track away from the hollow area where the ligament is attached and instead move to adjacent areas that will accentuate the hollow and make it look worse.
The temples and lateral face often are filled with PLLA for framing. Mid or high G' HA fillers and CaHA also are used in the temples both beneath the temporalis muscle and also above the deep temporalis fascia or sometimes in the subcutaneous plane.27
Prevention and Management of Periocular Complications
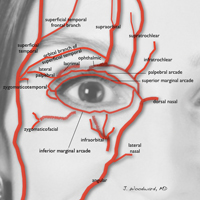
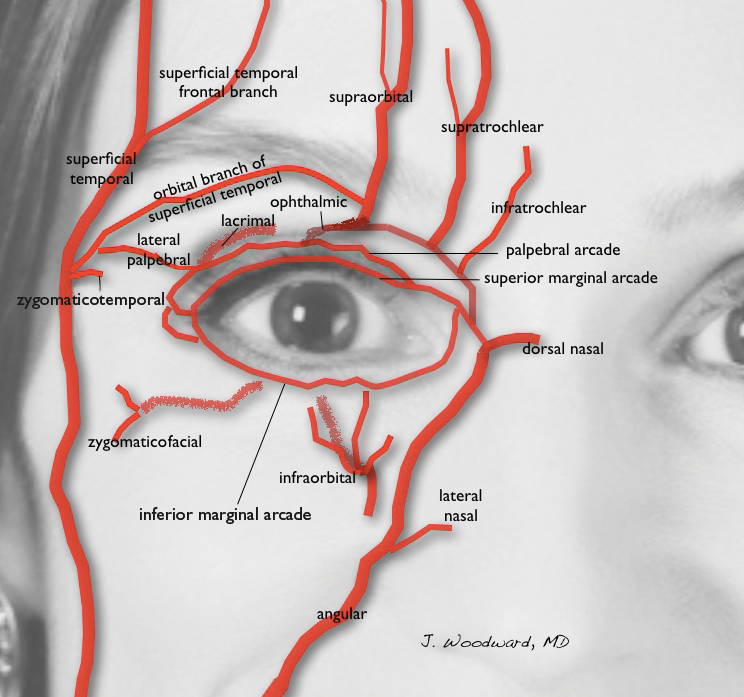
Blindness is the most devastating periocular complication of facial fillers, which is caused by retrograde arterial embolization followed by anterograde flow into the ophthalmic then central retinal arteries. Injectables that have caused blindness include (in descending order of frequency) fat, HA, collagen, paraffin, polymethyl methacrylate, silicone, PLLA, CaHA, polyacrylamide hydrogel, and micronized acellular dermal matrix. Of the 98 cases of blindness from periocular complications from dermal fillers reported in the world literature, the order of affected sites include the glabella (38 cases), nose (25), nasolabial folds (13), superior forehead (12), infraorbital rim (6), temples (1), malar area (1), lip (1), and chin (1). Prevention includes avoidance of danger zone arteries including the supratrochlear, supraorbital, dorsal nasal, angular, infraorbital, zygomaticofacial and zygomaticotemporal arteries.28
Avoiding the average critical volume of 0.84 in any single aliquot dispensed is key to avoid filling of these periocular arteries to the critical bifurcation point that can result in anterograde flow into the eye (Freudenthal Nicolau syndrome). The smallest supratrochlear artery’s volume in this study was 0.04 cc, so aliquots that do not exceed 0.03 cc are ideal.29,30
The injector should always be thinking about the anatomy of the danger zones (eg, infratrochlear and supratrochlear arteries, supraorbital artery, frontal branch of the superficial temporal artery, lacrimal artery, dorsal nasal artery, infraorbital artery, angular artery, zygomaticofacial artery, zygomaticotemporal artery)(Figure 3).
Hyaluronidase can be used off label to hydrolyze unwanted HA. It was first used to aid transcutaneous hydration and was used by ophthalmologists in the 1960s and 1970s to promote the spread of anesthetics by retrobulbar injection.31,32 It can penetrate through soft tissues and blood vessels.33 It is therefore hypothesized that a retrobulbar injection of hyaluronidase could aid in a case of impending blindness34 but has not been successfully accomplished to date. If vision is confirmed to be poor or there is no light perception, a retrobulbar injection of 300 U of hyaluronidase should be given immediately and then repeated in approximately 30 to 45 minutes. The retina begins to show permanent loss of function after being deprived of blood flow for just 97 minutes,35 so there may not be time for an immediate ophthalmology consultation, though such a consultation would be ideal.
Aside from common complications such as bruising and swelling, granulomas and biofilms are well documented in the literature. There are a variety of algorithms to treat such complications, which can happen many weeks after the injection of a dermal filler or years after the injection of a semipermanent filler.36 Postinjection periocular edema can occur years after the initial injection.37,38 Other periocular complications of dermal fillers include nonischemic (eg, bluish hue, filler migration, infection, inflammation, lumps) and ischemic (eg, blindness, necrosis, ophthalmoplegia, ptosis) disturbances.
Conclusion
In summary, periocular injections of facial fillers are useful tools for rejuvenation of the upper face when used with great caution and respect for anatomy.
- Foster J, ed. Orbit, Eyelids, and Lacrimal System. San Francisco, CA: American Academy of Ophthalmology; 2016. 2016-2017 Basic and Clinical Science Course; section 7.
- Rohrich RJ, Pessa JE. The fat compartments of the face: anatomy and clinical implications for cosmetic surgery. Plast Reconstr Surg. 2007;119:2219-2227; discussion 2228-2231.
- Gierloff M, Stöhring C, Buder T, et al. Aging changes of the midfacial fat compartments: a computed tomographic study. Plast Reconst Surg. 2012;129:263-273.
- Zide BM, Jelks GW. Surgical Anatomy of the Orbit: The System of Zones. Philadelphia, PA: Lippincott Williams & Wilkins; 2006.
- Kikkawa DO, Lemke BN, Dortzbach RK. Relations of the superficial musculoaponeurotic system to the orbit and characterization of the oribitomalar ligament. Ophthal Plast Reconstr Surg. 1996;12:77-88.
- Furnas DW. The retaining ligaments of the cheek. Plast Reconstr Surg. 1989;83:11-16.
- Morley AM, Taban M, Malhotra R, et al. Use of hyaluronic acid gel for upper eyelid filling and contouring. Ophthal Plast Reconstr Surg. 2009;25:440-444.
- Hirmand H. Anatomy and nonsurgical correction of the tear trough deformity. Plast Reconstr Surg. 2010;125:699-708.
- Pessa JE, Zadoo VP, Mutimer KL, et al. Relative maxillary retrusion as a natural consequence of aging. Plast Reconstr Surg. 1998;102:205-212.
- Pessa JE, Desvigne LD, Lambros VS, et al. Changes in ocular globe-to-orbital rim position with age: implications for aesthetic blepharoplasty of the lower eyelids. Aesthet Plast Surg. 1999;23:337-345.
- Goldberg RA, Relan A, Hoenig J. Relationship of the eye to the bony orbit, with clinical correlations. Aust N Z J Ophthalmol. 1999;27:398-403.
- Richard MJ, Morris C, Deen BF, et al. Analysis of the anatomic changes of the aging facial skeleton using computer-assisted tomography. Ophthal Plast Reconstr Surg. 2009;25:382-386.
- Miller D, O’Connor P, Williams J. Use of Na-hyaluronate during intraocular lens implantation in rabbits. Ophthalmic Surg. 1977;8:58-61.
- Miller D, Stegmann R. Use of Na-hyaluronate in anterior segment eye surgery. J Am Intraocul Implant Soc. 1980;6:13-15.
- Pape LG, Balazs EA. The use of sodium hyaluronate (Healon) in human anterior segment surgery. Ophthalmology. 1980;87:699-705.
- Larsen NE, Pollak CT, Reiner K, et al. Hylan gel biomaterial: dermal and immunologic compatibility. J Biomed Mater Res. 1993;27:1129-1134.
- Sundaram H, Cassuto D. Biophysical characteristics of hyaluronic acid soft-tissue fillers and their relevance to aesthetic applications. Plast Reconstr Surg. 2013;132(4, suppl 2):5S-21S.
- Hee CK, Shumate GT, Narurkar V, et al. Rheological properties and in vivo performance characteristics of soft tissue fillers. Dermatol Surg. 2015;41(suppl 1):S373-S381.
- Sundaram H, Voigts B, Beer K, et al. Comparison of the rheological properties of viscosity and elasticity in two categories of soft tissue fillers: calcium hydroxylapatite and hyaluronic acid. Dermatol Surg. 2010;36(suppl 3):1859-1865.
- Sundaram H. The new face of fillers: a multi-specialty CME initiative: supplement part II of II. J Drugs Dermatol. 2012;11(suppl 8):S8.
- Stocks D, Sundaram H, Michaels J, et al. Rheological evaluation of the physical properties of hyaluronic acid dermal fillers. J Drugs Dermatol. 2011;10:974-980.
- Goodman GJ, Swift A, Remington BK. Current concepts in the use of Voluma, Volift, and Volbella. Plast Reconstr Surg. 2015;136(suppl 5):139S-148S.
- Sundaram H, Rohrich RJ, Liew S, et al. Cohesivity of hyaluronic acid fillers: development and clinical implications of a novel assay, pilot validation with a five-point grading scale and evaluation of six U.S. Food and Drug Administration–approved fillers. Plast Reconstr Surg. 2015;136:678-686.
- Flynn TC, Sarazin D, Bezzola A, et al. Comparative histology of intradermal implantation of mono and biphasic hyaluronic acid fillers. Dermatol Surg. 2011;37:637-643.
- Woodward JA, Langelier N. Filler enhancement of the superior periocular area [published online Jun 23, 2016]. JAMA Facial Plast Surg. doi:10.1001/jamafacial.2016.0636.
- Cotofana S, Schenck TL, Trevidic P, et al. Midface: clinical anatomy and regional approaches with injectable fillers. Plast Reconstr Surg. 2015;136(suppl 5):219S-234S.
- Buckingham ED, Glasgold R, Kontis T, et al. Volume rejuvenation of the facial upper third. Facial Plast Surg. 2015;31:43-54.
- Beleznay K, Carruthers JD, Humphrey S, et al. Avoiding and treating blindness from fillers: a review of the world literature. Dermatol Surg. 2015;41:1097-1117.
- Coleman SR. Avoidance of arterial occlusion from injection of soft tissue fillers. Aesthet Surg J. 2002;22:555-557.
- Khan T, Colon-Acevedo B, Mettu P, et al. An anatomical analysis of the supratrochlear artery: considerations in facial filler injections and preventing vision loss [published online August 16, 2016]. Aesthet Surg J. pii: sjw132.
- Iserle J, Kumstat Z. Retrobulbar injections of hyaluronidase as a method of increasing safety in cataract surgery [in Czech]. Cesk Oftalmol. 1960;15:126-130.
- Wojtowicz S. Effect of retrobulbar injections of novocaine and lignocaine with adrenalin and hyaluronidase for the immobilization of the eye in electromyography [in Polish]. Klin Oczna. 1964;34:285-296.
- Delorenzi C. Transarterial degradation of hyaluronic acid filler by hyaluronidase. Dermatol Surg. 2014;40:832-841.
- Carruthers J, Fagien S, Dolman P. Retro or peribulbar injections techniques to reverse visual loss after filler injections. 2015;41(suppl 1):S354-S357.
- Hayreh SS, Zimmerman MB, Kimura A, et al. Central retinal artery occlusion. retinal survival time. Exp Eye Res. 2004;78:723-736.
- Woodward J, Khan T, Martin J. Facial filler complications. Facial Plast Surg Clin North Am. 2015;23:447-458.
- Khan TT, Woodward JA. Retained dermal filler in the upper eyelid masquerading as periorbital edema. Dermatol Surg. 2015;41:1182-1184.
- Chang JR, Baharestani S, Salek SS, et al. Delayed superficial migration of retained hyaluronic acid years following periocular injection [published online April 20, 2015]. Ophthal Plast Reconstr Surg. doi:10.1097/IOP.0000000000000434.
Rejuvenation of the periocular area is in high demand among patients who want to look and feel their best. Physicians should understand the complicated anatomy surrounding the eyes before attempting to inject this area with facial fillers, both to understand the aging process and to minimize treatment complications.
Basic Oculoplastic and Orbital Anatomy
The injector should understand the anatomy of the periocular muscles, the orbital osteology, and the secretory and lacrimal system, in addition to the fat, ligaments, and vascular anatomy in this area.1
The eyes are surrounded by fat compartments that provide glide planes for the motion of the eyelids and globe. There are 2 upper eyelid fat-pads—nasal and central [preaponeurotic])—in the upper lid, leaving room for the lacrimal gland laterally. There are 3 fat compartments—nasal, central, and lateral—in the lower eyelid. The nasal and central compartments are separated by the inferior oblique muscle, which elevates and extorts the eye. The orbital septum holds the fat-pads in place in the orbit. The brow fat-pad is the retro-orbicularis oculi fat-pad (ROOF). There are fat compartments that lie in the subcutaneous space along the entire forehead and in the temple. The suborbicularis oculi fat-pad (SOOF) lies over the malar eminence. Superficial and deep submuscular fat compartments of the face have been described.2 Deep fat compartments also have been examined on computed tomography.3
Orbital circulation comes from the internal carotid artery and anastomoses with the supply from the external carotid artery to supply the orbit. The first branch off of the carotid artery is the ophthalmic artery, and the first branch off of the ophthalmic artery is the central retinal artery that enters the optic nerve sheath 1 cm behind the globe to supply the retina. The supraorbital and supratrochlear arteries branch off of the ophthalmic artery and supply the forehead. The supraorbital artery runs through the supraorbital notch (foramen in 8%)1 and can usually be palpated with one’s finger. There are 15 to 20 short posterior ciliary arteries leading to the choroid, 2 long posterior ciliary arteries to the iris circle, and 7 anterior ciliary arteries to the extraocular muscles. The superior and inferior venous systems drain into the cavernous sinus.4
The ligaments are important to signs of facial aging because tissue atrophy occurs along them. The main orbital ligaments are the lateral orbital thickening (known as the LOT) that adheres the eyelids to the lateral orbital rim and the orbitomalar ligament (orbicularis retaining ligament), which is a condensation fibrous tissue that attaches the skin to the inferior orbital rim and orbital septum along the arcus marginalis and defines the superior edge of the SOOF.5 The zygomatic ligament not only suspends the zygomaticus major and zygomaticus minor muscles to the malar eminence but there are osseocutaneous attachments that connect the skin over the zygoma’s malar eminence and demarcate the inferior edge of the SOOF.6
Periocular Aging
The skin, fat, muscles, and bones change and rotate with aging, and not all orbits age in the same manner. Older patients with dermatochalasis (excess skin fat and muscle) often undergo rejuvenation with blepharoplasty, a brow-lift, and a midface-lift, but many atrophic changes can be improved with facial fillers.7,8
As adults age, the soft tissue along the ligaments begins to show atrophy, prime signs of aging that are often improved with fillers. Atrophy along the orbitomalar ligament along the infraorbital rim creates a depressed tear trough, which is an early sign of aging. A 3-point grading system reported by Hirmand8 describes the severity of progressive hallowing. There also is atrophy along the zygomatic cutaneous ligament that creates the malar hollow. The SOOF appears to be more prominent when these areas above and below show atrophy, which creates the look of an unwanted bag known as a festoon. Additionally, there is atrophy along the superior orbital notch where the ophthalmic branch of the trigeminal nerve (V1) and the supraorbital artery traverse. Soft-tissue atrophy along the supraorbital notch resembles the peak at the top of the letter A, giving the slang term A-frame deformity.
Periocular fat can atrophy, hypertrophy, herniate forward as the septum weakens, or become ptotic. Some patients develop hypertrophy and herniation of the superior and inferior orbital fat-pads, while others develop unwanted atrophy leaving a hollow superior orbit and loss of support to the levator muscle that contributes to eyelid ptosis. The frontalis fat deflates, leaving veins, arteries, and the hypertrophied corrugators unwantedly visible. Loss of subcutaneous fat in the glabella contributes to the formation of frown lines between the brows (also called number 11’s). The ROOF deflates in some patients adding to brow ptosis. Loss of the facial frame occurs when temple fat atrophies.
Skeletal rotation also occurs. Throughout a patient’s life, the skeleton remodels itself via activity of osteoclasts and osteoblasts. Pessa et al9,10 has described the expansion of the anterior orbital aperture superomedially and inferolaterally as well as maxillary retrusion that results in angular changes of the midface in relation to the orbital rim. Lambros’ algorithm describes the rotational changes of the cranium where the superior orbit protrudes as the maxilla retreats posteriorly.9-11 The equator of the globe does not change its distance from the ROOF of the orbit, presumably because of its suspension in the orbit by the optic nerve after it passes through the optic canal and trochlea via the superior oblique muscle, but the distance of the inferior equator of the globe to the floor of the orbit increases as the floor of the orbit descends.12
Dermal Fillers for Periocular Rejuvenation
Hyaluronic acid (HA) was first pioneered for use in humans in the late 1970s by ophthalmologists for anterior segment surgery.13-15 Biocompatibility for orthopedic and dermal applications was explored in the early 1990s.16
At this time, no dermal filler is approved by the US Food and Drug Administration for use in the periorbital area. Some fillers are approved for subdermal areas extending to the preperiosteal plane and can be used in the midface such as HA fillers (eg, Restylane Lyft [Galderma Laboratories, LP]), Juvéderm Voluma XC [Allergan, Inc]), poly-L-lactic acid (PLLA), and calcium hydroxylapatite (CaHA). No dermal fillers are approved for use in the forehead, glabella, or temples. Their use is becoming increasingly popular but is considered off label. In addition, cannulas are not approved for use in these areas. Cannulas may be beneficial in that they are thought to create less bruising and have less chance of entering a vessel than needles, but some injectors prefer needles because they are stiffer and therefore more precise.
The ideal filler for the tear trough, superior sulcus, ROOF, over the orbitomalar ligament, forehead, and glabella is one that is somewhat moldable but does not migrate, is not hydrophilic, is smooth to inject, and is reversible should there be any complications. No single filler fits this ideal description, but HAs typically are the first choice.
In vitro studies to determine the stiffness (G') and the ability to flow (viscosity) have been performed.17,18 Calcium hydroxylapatite has the most stiffness, while Belotero Balance (Merz Aesthetics) and Juvéderm Ultra XC (Allergan, Inc) are more soft17 (Table). These guidelines are important but may not correlate directly with how the fillers behave in vivo as demonstrated in animal models.18

Hyaluronic acid fillers are produced by different technologies to create their cross-link patterns with 1,4-butanediol diglycidyl ether, which determines, to some degree, their behavior in human tissue. Fillers are either monophasic; monodensified; formed by Hylacross (Juvéderm), Vycross (Juvéderm Voluma XC, Juvéderm Volbella XC), or cohesive polydensified matrix technology (Belotero Balance), or biphasic, formed by nonanimal stabilized HA sieving technology (Restylane family). Biopsy has demonstrated that monophasic fillers tend to percolate through and integrate into the tissue, while biphasic fillers dissect tissue to the sides to create a potential space for the filler to reside (Table).24
Periocular Injection Considerations
An experienced injector is one who has developed not only an artistic eye for the face and excellent sense of anatomy but also has a sensitive ability to predict the filler-tissue interaction based on tactile feedback dependent on 3 main qualities: (1) stiffness and viscosity of the filler, (2) gauge of the needle or cannula, and (3) depth of the needle in the tissue. Periocular injections of dermal fillers can be delivered with needles or cannulas, diluted or undiluted. Smaller-gauge needles require more force than larger-gauge needles and cannulas that flow more freely. A needle in the dense dermis will require more force than one placed in the loose subcutaneous space.
The tear trough is generally preferable to fill with a mid-level G' HA filler that is less apt to migrate. A neutral gaze during the injection is preferred because closing or moving the eyes can distort the position of the inferior orbital fat-pads (Figure 1). A needle or cannula can be used, diluted or undiluted. The tear trough can be filled with the injection directed horizontally or vertically via a fanning technique. If needles are used, the skin should be stretched to view the 3 to 5 vertical veins and then the needle should be advanced beneath them to avoid bruising. Avoidance of hydrophilic fillers in the tear trough is important to avoid edema. The superior sulcus can be filled both anteriorly and posteriorly to the septum, which is a highly advanced injection for experienced injectors because of the proximity to the supratrochlear and supraorbital arteries as well as the superior ophthalmic vein (Figure 2). Sharp creases such as deep lateral periocular rhytides known as crow’s-feet are nicely filled with intradermal HAs with a low G'.


Adding volume to the midface is important because it is the continuum of the lower eyelid. Fillers can be injected into multiple levels in this area: deep (to act as pillars to lift the malar eminence and replace bone that has rotated and soft tissue that has become atrophic or descended) and subcutaneous (to efface soft tissue along the zygomatic cutaneous ligament). Higher G' HA fillers and CaHA often are used in the midface along with PLLA. Facial framing of the temples, lateral cheeks, and preauricular area is often accomplished with PLLA but also can be done with mid to high G' HA fillers or CaHA. A cannula may be used to undermine and break apart the zygomatic cutaneous ligament’s cutaneous attachments prior to delivery of the filler in the subcutaneous plane.26 If not done, filler may track away from the hollow area where the ligament is attached and instead move to adjacent areas that will accentuate the hollow and make it look worse.
The temples and lateral face often are filled with PLLA for framing. Mid or high G' HA fillers and CaHA also are used in the temples both beneath the temporalis muscle and also above the deep temporalis fascia or sometimes in the subcutaneous plane.27
Prevention and Management of Periocular Complications
Blindness is the most devastating periocular complication of facial fillers, which is caused by retrograde arterial embolization followed by anterograde flow into the ophthalmic then central retinal arteries. Injectables that have caused blindness include (in descending order of frequency) fat, HA, collagen, paraffin, polymethyl methacrylate, silicone, PLLA, CaHA, polyacrylamide hydrogel, and micronized acellular dermal matrix. Of the 98 cases of blindness from periocular complications from dermal fillers reported in the world literature, the order of affected sites include the glabella (38 cases), nose (25), nasolabial folds (13), superior forehead (12), infraorbital rim (6), temples (1), malar area (1), lip (1), and chin (1). Prevention includes avoidance of danger zone arteries including the supratrochlear, supraorbital, dorsal nasal, angular, infraorbital, zygomaticofacial and zygomaticotemporal arteries.28
Avoiding the average critical volume of 0.84 in any single aliquot dispensed is key to avoid filling of these periocular arteries to the critical bifurcation point that can result in anterograde flow into the eye (Freudenthal Nicolau syndrome). The smallest supratrochlear artery’s volume in this study was 0.04 cc, so aliquots that do not exceed 0.03 cc are ideal.29,30
The injector should always be thinking about the anatomy of the danger zones (eg, infratrochlear and supratrochlear arteries, supraorbital artery, frontal branch of the superficial temporal artery, lacrimal artery, dorsal nasal artery, infraorbital artery, angular artery, zygomaticofacial artery, zygomaticotemporal artery)(Figure 3).

Hyaluronidase can be used off label to hydrolyze unwanted HA. It was first used to aid transcutaneous hydration and was used by ophthalmologists in the 1960s and 1970s to promote the spread of anesthetics by retrobulbar injection.31,32 It can penetrate through soft tissues and blood vessels.33 It is therefore hypothesized that a retrobulbar injection of hyaluronidase could aid in a case of impending blindness34 but has not been successfully accomplished to date. If vision is confirmed to be poor or there is no light perception, a retrobulbar injection of 300 U of hyaluronidase should be given immediately and then repeated in approximately 30 to 45 minutes. The retina begins to show permanent loss of function after being deprived of blood flow for just 97 minutes,35 so there may not be time for an immediate ophthalmology consultation, though such a consultation would be ideal.
Aside from common complications such as bruising and swelling, granulomas and biofilms are well documented in the literature. There are a variety of algorithms to treat such complications, which can happen many weeks after the injection of a dermal filler or years after the injection of a semipermanent filler.36 Postinjection periocular edema can occur years after the initial injection.37,38 Other periocular complications of dermal fillers include nonischemic (eg, bluish hue, filler migration, infection, inflammation, lumps) and ischemic (eg, blindness, necrosis, ophthalmoplegia, ptosis) disturbances.
Conclusion
In summary, periocular injections of facial fillers are useful tools for rejuvenation of the upper face when used with great caution and respect for anatomy.
Rejuvenation of the periocular area is in high demand among patients who want to look and feel their best. Physicians should understand the complicated anatomy surrounding the eyes before attempting to inject this area with facial fillers, both to understand the aging process and to minimize treatment complications.
Basic Oculoplastic and Orbital Anatomy
The injector should understand the anatomy of the periocular muscles, the orbital osteology, and the secretory and lacrimal system, in addition to the fat, ligaments, and vascular anatomy in this area.1
The eyes are surrounded by fat compartments that provide glide planes for the motion of the eyelids and globe. There are 2 upper eyelid fat-pads—nasal and central [preaponeurotic])—in the upper lid, leaving room for the lacrimal gland laterally. There are 3 fat compartments—nasal, central, and lateral—in the lower eyelid. The nasal and central compartments are separated by the inferior oblique muscle, which elevates and extorts the eye. The orbital septum holds the fat-pads in place in the orbit. The brow fat-pad is the retro-orbicularis oculi fat-pad (ROOF). There are fat compartments that lie in the subcutaneous space along the entire forehead and in the temple. The suborbicularis oculi fat-pad (SOOF) lies over the malar eminence. Superficial and deep submuscular fat compartments of the face have been described.2 Deep fat compartments also have been examined on computed tomography.3
Orbital circulation comes from the internal carotid artery and anastomoses with the supply from the external carotid artery to supply the orbit. The first branch off of the carotid artery is the ophthalmic artery, and the first branch off of the ophthalmic artery is the central retinal artery that enters the optic nerve sheath 1 cm behind the globe to supply the retina. The supraorbital and supratrochlear arteries branch off of the ophthalmic artery and supply the forehead. The supraorbital artery runs through the supraorbital notch (foramen in 8%)1 and can usually be palpated with one’s finger. There are 15 to 20 short posterior ciliary arteries leading to the choroid, 2 long posterior ciliary arteries to the iris circle, and 7 anterior ciliary arteries to the extraocular muscles. The superior and inferior venous systems drain into the cavernous sinus.4
The ligaments are important to signs of facial aging because tissue atrophy occurs along them. The main orbital ligaments are the lateral orbital thickening (known as the LOT) that adheres the eyelids to the lateral orbital rim and the orbitomalar ligament (orbicularis retaining ligament), which is a condensation fibrous tissue that attaches the skin to the inferior orbital rim and orbital septum along the arcus marginalis and defines the superior edge of the SOOF.5 The zygomatic ligament not only suspends the zygomaticus major and zygomaticus minor muscles to the malar eminence but there are osseocutaneous attachments that connect the skin over the zygoma’s malar eminence and demarcate the inferior edge of the SOOF.6
Periocular Aging
The skin, fat, muscles, and bones change and rotate with aging, and not all orbits age in the same manner. Older patients with dermatochalasis (excess skin fat and muscle) often undergo rejuvenation with blepharoplasty, a brow-lift, and a midface-lift, but many atrophic changes can be improved with facial fillers.7,8
As adults age, the soft tissue along the ligaments begins to show atrophy, prime signs of aging that are often improved with fillers. Atrophy along the orbitomalar ligament along the infraorbital rim creates a depressed tear trough, which is an early sign of aging. A 3-point grading system reported by Hirmand8 describes the severity of progressive hallowing. There also is atrophy along the zygomatic cutaneous ligament that creates the malar hollow. The SOOF appears to be more prominent when these areas above and below show atrophy, which creates the look of an unwanted bag known as a festoon. Additionally, there is atrophy along the superior orbital notch where the ophthalmic branch of the trigeminal nerve (V1) and the supraorbital artery traverse. Soft-tissue atrophy along the supraorbital notch resembles the peak at the top of the letter A, giving the slang term A-frame deformity.
Periocular fat can atrophy, hypertrophy, herniate forward as the septum weakens, or become ptotic. Some patients develop hypertrophy and herniation of the superior and inferior orbital fat-pads, while others develop unwanted atrophy leaving a hollow superior orbit and loss of support to the levator muscle that contributes to eyelid ptosis. The frontalis fat deflates, leaving veins, arteries, and the hypertrophied corrugators unwantedly visible. Loss of subcutaneous fat in the glabella contributes to the formation of frown lines between the brows (also called number 11’s). The ROOF deflates in some patients adding to brow ptosis. Loss of the facial frame occurs when temple fat atrophies.
Skeletal rotation also occurs. Throughout a patient’s life, the skeleton remodels itself via activity of osteoclasts and osteoblasts. Pessa et al9,10 has described the expansion of the anterior orbital aperture superomedially and inferolaterally as well as maxillary retrusion that results in angular changes of the midface in relation to the orbital rim. Lambros’ algorithm describes the rotational changes of the cranium where the superior orbit protrudes as the maxilla retreats posteriorly.9-11 The equator of the globe does not change its distance from the ROOF of the orbit, presumably because of its suspension in the orbit by the optic nerve after it passes through the optic canal and trochlea via the superior oblique muscle, but the distance of the inferior equator of the globe to the floor of the orbit increases as the floor of the orbit descends.12
Dermal Fillers for Periocular Rejuvenation
Hyaluronic acid (HA) was first pioneered for use in humans in the late 1970s by ophthalmologists for anterior segment surgery.13-15 Biocompatibility for orthopedic and dermal applications was explored in the early 1990s.16
At this time, no dermal filler is approved by the US Food and Drug Administration for use in the periorbital area. Some fillers are approved for subdermal areas extending to the preperiosteal plane and can be used in the midface such as HA fillers (eg, Restylane Lyft [Galderma Laboratories, LP]), Juvéderm Voluma XC [Allergan, Inc]), poly-L-lactic acid (PLLA), and calcium hydroxylapatite (CaHA). No dermal fillers are approved for use in the forehead, glabella, or temples. Their use is becoming increasingly popular but is considered off label. In addition, cannulas are not approved for use in these areas. Cannulas may be beneficial in that they are thought to create less bruising and have less chance of entering a vessel than needles, but some injectors prefer needles because they are stiffer and therefore more precise.
The ideal filler for the tear trough, superior sulcus, ROOF, over the orbitomalar ligament, forehead, and glabella is one that is somewhat moldable but does not migrate, is not hydrophilic, is smooth to inject, and is reversible should there be any complications. No single filler fits this ideal description, but HAs typically are the first choice.
In vitro studies to determine the stiffness (G') and the ability to flow (viscosity) have been performed.17,18 Calcium hydroxylapatite has the most stiffness, while Belotero Balance (Merz Aesthetics) and Juvéderm Ultra XC (Allergan, Inc) are more soft17 (Table). These guidelines are important but may not correlate directly with how the fillers behave in vivo as demonstrated in animal models.18

Hyaluronic acid fillers are produced by different technologies to create their cross-link patterns with 1,4-butanediol diglycidyl ether, which determines, to some degree, their behavior in human tissue. Fillers are either monophasic; monodensified; formed by Hylacross (Juvéderm), Vycross (Juvéderm Voluma XC, Juvéderm Volbella XC), or cohesive polydensified matrix technology (Belotero Balance), or biphasic, formed by nonanimal stabilized HA sieving technology (Restylane family). Biopsy has demonstrated that monophasic fillers tend to percolate through and integrate into the tissue, while biphasic fillers dissect tissue to the sides to create a potential space for the filler to reside (Table).24
Periocular Injection Considerations
An experienced injector is one who has developed not only an artistic eye for the face and excellent sense of anatomy but also has a sensitive ability to predict the filler-tissue interaction based on tactile feedback dependent on 3 main qualities: (1) stiffness and viscosity of the filler, (2) gauge of the needle or cannula, and (3) depth of the needle in the tissue. Periocular injections of dermal fillers can be delivered with needles or cannulas, diluted or undiluted. Smaller-gauge needles require more force than larger-gauge needles and cannulas that flow more freely. A needle in the dense dermis will require more force than one placed in the loose subcutaneous space.
The tear trough is generally preferable to fill with a mid-level G' HA filler that is less apt to migrate. A neutral gaze during the injection is preferred because closing or moving the eyes can distort the position of the inferior orbital fat-pads (Figure 1). A needle or cannula can be used, diluted or undiluted. The tear trough can be filled with the injection directed horizontally or vertically via a fanning technique. If needles are used, the skin should be stretched to view the 3 to 5 vertical veins and then the needle should be advanced beneath them to avoid bruising. Avoidance of hydrophilic fillers in the tear trough is important to avoid edema. The superior sulcus can be filled both anteriorly and posteriorly to the septum, which is a highly advanced injection for experienced injectors because of the proximity to the supratrochlear and supraorbital arteries as well as the superior ophthalmic vein (Figure 2). Sharp creases such as deep lateral periocular rhytides known as crow’s-feet are nicely filled with intradermal HAs with a low G'.


Adding volume to the midface is important because it is the continuum of the lower eyelid. Fillers can be injected into multiple levels in this area: deep (to act as pillars to lift the malar eminence and replace bone that has rotated and soft tissue that has become atrophic or descended) and subcutaneous (to efface soft tissue along the zygomatic cutaneous ligament). Higher G' HA fillers and CaHA often are used in the midface along with PLLA. Facial framing of the temples, lateral cheeks, and preauricular area is often accomplished with PLLA but also can be done with mid to high G' HA fillers or CaHA. A cannula may be used to undermine and break apart the zygomatic cutaneous ligament’s cutaneous attachments prior to delivery of the filler in the subcutaneous plane.26 If not done, filler may track away from the hollow area where the ligament is attached and instead move to adjacent areas that will accentuate the hollow and make it look worse.
The temples and lateral face often are filled with PLLA for framing. Mid or high G' HA fillers and CaHA also are used in the temples both beneath the temporalis muscle and also above the deep temporalis fascia or sometimes in the subcutaneous plane.27
Prevention and Management of Periocular Complications
Blindness is the most devastating periocular complication of facial fillers, which is caused by retrograde arterial embolization followed by anterograde flow into the ophthalmic then central retinal arteries. Injectables that have caused blindness include (in descending order of frequency) fat, HA, collagen, paraffin, polymethyl methacrylate, silicone, PLLA, CaHA, polyacrylamide hydrogel, and micronized acellular dermal matrix. Of the 98 cases of blindness from periocular complications from dermal fillers reported in the world literature, the order of affected sites include the glabella (38 cases), nose (25), nasolabial folds (13), superior forehead (12), infraorbital rim (6), temples (1), malar area (1), lip (1), and chin (1). Prevention includes avoidance of danger zone arteries including the supratrochlear, supraorbital, dorsal nasal, angular, infraorbital, zygomaticofacial and zygomaticotemporal arteries.28
Avoiding the average critical volume of 0.84 in any single aliquot dispensed is key to avoid filling of these periocular arteries to the critical bifurcation point that can result in anterograde flow into the eye (Freudenthal Nicolau syndrome). The smallest supratrochlear artery’s volume in this study was 0.04 cc, so aliquots that do not exceed 0.03 cc are ideal.29,30
The injector should always be thinking about the anatomy of the danger zones (eg, infratrochlear and supratrochlear arteries, supraorbital artery, frontal branch of the superficial temporal artery, lacrimal artery, dorsal nasal artery, infraorbital artery, angular artery, zygomaticofacial artery, zygomaticotemporal artery)(Figure 3).

Hyaluronidase can be used off label to hydrolyze unwanted HA. It was first used to aid transcutaneous hydration and was used by ophthalmologists in the 1960s and 1970s to promote the spread of anesthetics by retrobulbar injection.31,32 It can penetrate through soft tissues and blood vessels.33 It is therefore hypothesized that a retrobulbar injection of hyaluronidase could aid in a case of impending blindness34 but has not been successfully accomplished to date. If vision is confirmed to be poor or there is no light perception, a retrobulbar injection of 300 U of hyaluronidase should be given immediately and then repeated in approximately 30 to 45 minutes. The retina begins to show permanent loss of function after being deprived of blood flow for just 97 minutes,35 so there may not be time for an immediate ophthalmology consultation, though such a consultation would be ideal.
Aside from common complications such as bruising and swelling, granulomas and biofilms are well documented in the literature. There are a variety of algorithms to treat such complications, which can happen many weeks after the injection of a dermal filler or years after the injection of a semipermanent filler.36 Postinjection periocular edema can occur years after the initial injection.37,38 Other periocular complications of dermal fillers include nonischemic (eg, bluish hue, filler migration, infection, inflammation, lumps) and ischemic (eg, blindness, necrosis, ophthalmoplegia, ptosis) disturbances.
Conclusion
In summary, periocular injections of facial fillers are useful tools for rejuvenation of the upper face when used with great caution and respect for anatomy.
- Foster J, ed. Orbit, Eyelids, and Lacrimal System. San Francisco, CA: American Academy of Ophthalmology; 2016. 2016-2017 Basic and Clinical Science Course; section 7.
- Rohrich RJ, Pessa JE. The fat compartments of the face: anatomy and clinical implications for cosmetic surgery. Plast Reconstr Surg. 2007;119:2219-2227; discussion 2228-2231.
- Gierloff M, Stöhring C, Buder T, et al. Aging changes of the midfacial fat compartments: a computed tomographic study. Plast Reconst Surg. 2012;129:263-273.
- Zide BM, Jelks GW. Surgical Anatomy of the Orbit: The System of Zones. Philadelphia, PA: Lippincott Williams & Wilkins; 2006.
- Kikkawa DO, Lemke BN, Dortzbach RK. Relations of the superficial musculoaponeurotic system to the orbit and characterization of the oribitomalar ligament. Ophthal Plast Reconstr Surg. 1996;12:77-88.
- Furnas DW. The retaining ligaments of the cheek. Plast Reconstr Surg. 1989;83:11-16.
- Morley AM, Taban M, Malhotra R, et al. Use of hyaluronic acid gel for upper eyelid filling and contouring. Ophthal Plast Reconstr Surg. 2009;25:440-444.
- Hirmand H. Anatomy and nonsurgical correction of the tear trough deformity. Plast Reconstr Surg. 2010;125:699-708.
- Pessa JE, Zadoo VP, Mutimer KL, et al. Relative maxillary retrusion as a natural consequence of aging. Plast Reconstr Surg. 1998;102:205-212.
- Pessa JE, Desvigne LD, Lambros VS, et al. Changes in ocular globe-to-orbital rim position with age: implications for aesthetic blepharoplasty of the lower eyelids. Aesthet Plast Surg. 1999;23:337-345.
- Goldberg RA, Relan A, Hoenig J. Relationship of the eye to the bony orbit, with clinical correlations. Aust N Z J Ophthalmol. 1999;27:398-403.
- Richard MJ, Morris C, Deen BF, et al. Analysis of the anatomic changes of the aging facial skeleton using computer-assisted tomography. Ophthal Plast Reconstr Surg. 2009;25:382-386.
- Miller D, O’Connor P, Williams J. Use of Na-hyaluronate during intraocular lens implantation in rabbits. Ophthalmic Surg. 1977;8:58-61.
- Miller D, Stegmann R. Use of Na-hyaluronate in anterior segment eye surgery. J Am Intraocul Implant Soc. 1980;6:13-15.
- Pape LG, Balazs EA. The use of sodium hyaluronate (Healon) in human anterior segment surgery. Ophthalmology. 1980;87:699-705.
- Larsen NE, Pollak CT, Reiner K, et al. Hylan gel biomaterial: dermal and immunologic compatibility. J Biomed Mater Res. 1993;27:1129-1134.
- Sundaram H, Cassuto D. Biophysical characteristics of hyaluronic acid soft-tissue fillers and their relevance to aesthetic applications. Plast Reconstr Surg. 2013;132(4, suppl 2):5S-21S.
- Hee CK, Shumate GT, Narurkar V, et al. Rheological properties and in vivo performance characteristics of soft tissue fillers. Dermatol Surg. 2015;41(suppl 1):S373-S381.
- Sundaram H, Voigts B, Beer K, et al. Comparison of the rheological properties of viscosity and elasticity in two categories of soft tissue fillers: calcium hydroxylapatite and hyaluronic acid. Dermatol Surg. 2010;36(suppl 3):1859-1865.
- Sundaram H. The new face of fillers: a multi-specialty CME initiative: supplement part II of II. J Drugs Dermatol. 2012;11(suppl 8):S8.
- Stocks D, Sundaram H, Michaels J, et al. Rheological evaluation of the physical properties of hyaluronic acid dermal fillers. J Drugs Dermatol. 2011;10:974-980.
- Goodman GJ, Swift A, Remington BK. Current concepts in the use of Voluma, Volift, and Volbella. Plast Reconstr Surg. 2015;136(suppl 5):139S-148S.
- Sundaram H, Rohrich RJ, Liew S, et al. Cohesivity of hyaluronic acid fillers: development and clinical implications of a novel assay, pilot validation with a five-point grading scale and evaluation of six U.S. Food and Drug Administration–approved fillers. Plast Reconstr Surg. 2015;136:678-686.
- Flynn TC, Sarazin D, Bezzola A, et al. Comparative histology of intradermal implantation of mono and biphasic hyaluronic acid fillers. Dermatol Surg. 2011;37:637-643.
- Woodward JA, Langelier N. Filler enhancement of the superior periocular area [published online Jun 23, 2016]. JAMA Facial Plast Surg. doi:10.1001/jamafacial.2016.0636.
- Cotofana S, Schenck TL, Trevidic P, et al. Midface: clinical anatomy and regional approaches with injectable fillers. Plast Reconstr Surg. 2015;136(suppl 5):219S-234S.
- Buckingham ED, Glasgold R, Kontis T, et al. Volume rejuvenation of the facial upper third. Facial Plast Surg. 2015;31:43-54.
- Beleznay K, Carruthers JD, Humphrey S, et al. Avoiding and treating blindness from fillers: a review of the world literature. Dermatol Surg. 2015;41:1097-1117.
- Coleman SR. Avoidance of arterial occlusion from injection of soft tissue fillers. Aesthet Surg J. 2002;22:555-557.
- Khan T, Colon-Acevedo B, Mettu P, et al. An anatomical analysis of the supratrochlear artery: considerations in facial filler injections and preventing vision loss [published online August 16, 2016]. Aesthet Surg J. pii: sjw132.
- Iserle J, Kumstat Z. Retrobulbar injections of hyaluronidase as a method of increasing safety in cataract surgery [in Czech]. Cesk Oftalmol. 1960;15:126-130.
- Wojtowicz S. Effect of retrobulbar injections of novocaine and lignocaine with adrenalin and hyaluronidase for the immobilization of the eye in electromyography [in Polish]. Klin Oczna. 1964;34:285-296.
- Delorenzi C. Transarterial degradation of hyaluronic acid filler by hyaluronidase. Dermatol Surg. 2014;40:832-841.
- Carruthers J, Fagien S, Dolman P. Retro or peribulbar injections techniques to reverse visual loss after filler injections. 2015;41(suppl 1):S354-S357.
- Hayreh SS, Zimmerman MB, Kimura A, et al. Central retinal artery occlusion. retinal survival time. Exp Eye Res. 2004;78:723-736.
- Woodward J, Khan T, Martin J. Facial filler complications. Facial Plast Surg Clin North Am. 2015;23:447-458.
- Khan TT, Woodward JA. Retained dermal filler in the upper eyelid masquerading as periorbital edema. Dermatol Surg. 2015;41:1182-1184.
- Chang JR, Baharestani S, Salek SS, et al. Delayed superficial migration of retained hyaluronic acid years following periocular injection [published online April 20, 2015]. Ophthal Plast Reconstr Surg. doi:10.1097/IOP.0000000000000434.
- Foster J, ed. Orbit, Eyelids, and Lacrimal System. San Francisco, CA: American Academy of Ophthalmology; 2016. 2016-2017 Basic and Clinical Science Course; section 7.
- Rohrich RJ, Pessa JE. The fat compartments of the face: anatomy and clinical implications for cosmetic surgery. Plast Reconstr Surg. 2007;119:2219-2227; discussion 2228-2231.
- Gierloff M, Stöhring C, Buder T, et al. Aging changes of the midfacial fat compartments: a computed tomographic study. Plast Reconst Surg. 2012;129:263-273.
- Zide BM, Jelks GW. Surgical Anatomy of the Orbit: The System of Zones. Philadelphia, PA: Lippincott Williams & Wilkins; 2006.
- Kikkawa DO, Lemke BN, Dortzbach RK. Relations of the superficial musculoaponeurotic system to the orbit and characterization of the oribitomalar ligament. Ophthal Plast Reconstr Surg. 1996;12:77-88.
- Furnas DW. The retaining ligaments of the cheek. Plast Reconstr Surg. 1989;83:11-16.
- Morley AM, Taban M, Malhotra R, et al. Use of hyaluronic acid gel for upper eyelid filling and contouring. Ophthal Plast Reconstr Surg. 2009;25:440-444.
- Hirmand H. Anatomy and nonsurgical correction of the tear trough deformity. Plast Reconstr Surg. 2010;125:699-708.
- Pessa JE, Zadoo VP, Mutimer KL, et al. Relative maxillary retrusion as a natural consequence of aging. Plast Reconstr Surg. 1998;102:205-212.
- Pessa JE, Desvigne LD, Lambros VS, et al. Changes in ocular globe-to-orbital rim position with age: implications for aesthetic blepharoplasty of the lower eyelids. Aesthet Plast Surg. 1999;23:337-345.
- Goldberg RA, Relan A, Hoenig J. Relationship of the eye to the bony orbit, with clinical correlations. Aust N Z J Ophthalmol. 1999;27:398-403.
- Richard MJ, Morris C, Deen BF, et al. Analysis of the anatomic changes of the aging facial skeleton using computer-assisted tomography. Ophthal Plast Reconstr Surg. 2009;25:382-386.
- Miller D, O’Connor P, Williams J. Use of Na-hyaluronate during intraocular lens implantation in rabbits. Ophthalmic Surg. 1977;8:58-61.
- Miller D, Stegmann R. Use of Na-hyaluronate in anterior segment eye surgery. J Am Intraocul Implant Soc. 1980;6:13-15.
- Pape LG, Balazs EA. The use of sodium hyaluronate (Healon) in human anterior segment surgery. Ophthalmology. 1980;87:699-705.
- Larsen NE, Pollak CT, Reiner K, et al. Hylan gel biomaterial: dermal and immunologic compatibility. J Biomed Mater Res. 1993;27:1129-1134.
- Sundaram H, Cassuto D. Biophysical characteristics of hyaluronic acid soft-tissue fillers and their relevance to aesthetic applications. Plast Reconstr Surg. 2013;132(4, suppl 2):5S-21S.
- Hee CK, Shumate GT, Narurkar V, et al. Rheological properties and in vivo performance characteristics of soft tissue fillers. Dermatol Surg. 2015;41(suppl 1):S373-S381.
- Sundaram H, Voigts B, Beer K, et al. Comparison of the rheological properties of viscosity and elasticity in two categories of soft tissue fillers: calcium hydroxylapatite and hyaluronic acid. Dermatol Surg. 2010;36(suppl 3):1859-1865.
- Sundaram H. The new face of fillers: a multi-specialty CME initiative: supplement part II of II. J Drugs Dermatol. 2012;11(suppl 8):S8.
- Stocks D, Sundaram H, Michaels J, et al. Rheological evaluation of the physical properties of hyaluronic acid dermal fillers. J Drugs Dermatol. 2011;10:974-980.
- Goodman GJ, Swift A, Remington BK. Current concepts in the use of Voluma, Volift, and Volbella. Plast Reconstr Surg. 2015;136(suppl 5):139S-148S.
- Sundaram H, Rohrich RJ, Liew S, et al. Cohesivity of hyaluronic acid fillers: development and clinical implications of a novel assay, pilot validation with a five-point grading scale and evaluation of six U.S. Food and Drug Administration–approved fillers. Plast Reconstr Surg. 2015;136:678-686.
- Flynn TC, Sarazin D, Bezzola A, et al. Comparative histology of intradermal implantation of mono and biphasic hyaluronic acid fillers. Dermatol Surg. 2011;37:637-643.
- Woodward JA, Langelier N. Filler enhancement of the superior periocular area [published online Jun 23, 2016]. JAMA Facial Plast Surg. doi:10.1001/jamafacial.2016.0636.
- Cotofana S, Schenck TL, Trevidic P, et al. Midface: clinical anatomy and regional approaches with injectable fillers. Plast Reconstr Surg. 2015;136(suppl 5):219S-234S.
- Buckingham ED, Glasgold R, Kontis T, et al. Volume rejuvenation of the facial upper third. Facial Plast Surg. 2015;31:43-54.
- Beleznay K, Carruthers JD, Humphrey S, et al. Avoiding and treating blindness from fillers: a review of the world literature. Dermatol Surg. 2015;41:1097-1117.
- Coleman SR. Avoidance of arterial occlusion from injection of soft tissue fillers. Aesthet Surg J. 2002;22:555-557.
- Khan T, Colon-Acevedo B, Mettu P, et al. An anatomical analysis of the supratrochlear artery: considerations in facial filler injections and preventing vision loss [published online August 16, 2016]. Aesthet Surg J. pii: sjw132.
- Iserle J, Kumstat Z. Retrobulbar injections of hyaluronidase as a method of increasing safety in cataract surgery [in Czech]. Cesk Oftalmol. 1960;15:126-130.
- Wojtowicz S. Effect of retrobulbar injections of novocaine and lignocaine with adrenalin and hyaluronidase for the immobilization of the eye in electromyography [in Polish]. Klin Oczna. 1964;34:285-296.
- Delorenzi C. Transarterial degradation of hyaluronic acid filler by hyaluronidase. Dermatol Surg. 2014;40:832-841.
- Carruthers J, Fagien S, Dolman P. Retro or peribulbar injections techniques to reverse visual loss after filler injections. 2015;41(suppl 1):S354-S357.
- Hayreh SS, Zimmerman MB, Kimura A, et al. Central retinal artery occlusion. retinal survival time. Exp Eye Res. 2004;78:723-736.
- Woodward J, Khan T, Martin J. Facial filler complications. Facial Plast Surg Clin North Am. 2015;23:447-458.
- Khan TT, Woodward JA. Retained dermal filler in the upper eyelid masquerading as periorbital edema. Dermatol Surg. 2015;41:1182-1184.
- Chang JR, Baharestani S, Salek SS, et al. Delayed superficial migration of retained hyaluronic acid years following periocular injection [published online April 20, 2015]. Ophthal Plast Reconstr Surg. doi:10.1097/IOP.0000000000000434.
Practice Points
- When performing periocular dermal injections, physicians should understand the complicated anatomy surrounding the eyes and related changes with upper face aging.
- The different rheological properties of facial fillers impact product selection for various areas of the upper face.
- Physicians should be aware of the anatomical danger zones to avoid intravascular embolization.
Letter from the Editor: Value-based reimbursement is here to stay
For years, we advocated to repeal the sustainable growth rate (SGR) payment formula. Congress, by passing the MACRA legislation, eliminated SGR but created a new process that links provider reimbursement to value (quality and cost). Value-based reimbursement is here to stay. We now must help CMS devise reasonable linkages that will truly improve patient care, yet keep us in business. MACRA’s final rule is an improvement over the preliminary rule published earlier this spring. Gastroenterologists that plan to practice (and accept Medicare reimbursement) must educate themselves about both the incentive portion (MIPS) and alternative payment models.
MACRA intends to move independent practices into health systems (whether employed or contracted) that will assume financial and clinical risk. Gastroenterologists must lead clinical service lines for colon cancer prevention and integrated care for patients with IBD or cirrhosis. These care models must demonstrate good outcomes and substantive cost savings.
There are many sources of information about MACRA (see AGA resources at www.gastro.org/MACRA). I have listed four key websites:
https://qpp.cms.gov/docs/QPP_Executive_Summary_of_Final_Rule.pdf
http://healthaffairs.org/healthpolicybriefs/brief_pdfs/healthpolicybrief_156.pdf
https://innovation.cms.gov/initiatives/Transforming-Clinical-Practices/
I hope you will take it to heart that we are moving into a new world of care delivery and payment. We need physician leaders and innovators to make sure this works well for our patients and our profession.
John I. Allen MD, MBA, AGAF
Editor in Chief
For years, we advocated to repeal the sustainable growth rate (SGR) payment formula. Congress, by passing the MACRA legislation, eliminated SGR but created a new process that links provider reimbursement to value (quality and cost). Value-based reimbursement is here to stay. We now must help CMS devise reasonable linkages that will truly improve patient care, yet keep us in business. MACRA’s final rule is an improvement over the preliminary rule published earlier this spring. Gastroenterologists that plan to practice (and accept Medicare reimbursement) must educate themselves about both the incentive portion (MIPS) and alternative payment models.
MACRA intends to move independent practices into health systems (whether employed or contracted) that will assume financial and clinical risk. Gastroenterologists must lead clinical service lines for colon cancer prevention and integrated care for patients with IBD or cirrhosis. These care models must demonstrate good outcomes and substantive cost savings.
There are many sources of information about MACRA (see AGA resources at www.gastro.org/MACRA). I have listed four key websites:
https://qpp.cms.gov/docs/QPP_Executive_Summary_of_Final_Rule.pdf
http://healthaffairs.org/healthpolicybriefs/brief_pdfs/healthpolicybrief_156.pdf
https://innovation.cms.gov/initiatives/Transforming-Clinical-Practices/
I hope you will take it to heart that we are moving into a new world of care delivery and payment. We need physician leaders and innovators to make sure this works well for our patients and our profession.
John I. Allen MD, MBA, AGAF
Editor in Chief
For years, we advocated to repeal the sustainable growth rate (SGR) payment formula. Congress, by passing the MACRA legislation, eliminated SGR but created a new process that links provider reimbursement to value (quality and cost). Value-based reimbursement is here to stay. We now must help CMS devise reasonable linkages that will truly improve patient care, yet keep us in business. MACRA’s final rule is an improvement over the preliminary rule published earlier this spring. Gastroenterologists that plan to practice (and accept Medicare reimbursement) must educate themselves about both the incentive portion (MIPS) and alternative payment models.
MACRA intends to move independent practices into health systems (whether employed or contracted) that will assume financial and clinical risk. Gastroenterologists must lead clinical service lines for colon cancer prevention and integrated care for patients with IBD or cirrhosis. These care models must demonstrate good outcomes and substantive cost savings.
There are many sources of information about MACRA (see AGA resources at www.gastro.org/MACRA). I have listed four key websites:
https://qpp.cms.gov/docs/QPP_Executive_Summary_of_Final_Rule.pdf
http://healthaffairs.org/healthpolicybriefs/brief_pdfs/healthpolicybrief_156.pdf
https://innovation.cms.gov/initiatives/Transforming-Clinical-Practices/
I hope you will take it to heart that we are moving into a new world of care delivery and payment. We need physician leaders and innovators to make sure this works well for our patients and our profession.
John I. Allen MD, MBA, AGAF
Editor in Chief
Asleep Deep Brain Stimulation Placement Offers Advantages in Parkinson’s Disease
PORTLAND, OR—Performing deep brain stimulation (DBS) surgery for Parkinson’s disease using intraoperative CT imaging while the patient is under general anesthesia had clinical advantages and no disadvantages over surgery using microelectrode recording (MER) for lead placement with the patient awake, in a prospective, open-label study of 64 patients. The study was presented at the Fourth World Parkinson Congress.
The change in motor scores following surgery was the same for asleep and awake patients, said study coauthor Shannon Anderson, a physician assistant at Oregon Health & Science University in Portland. “What was surprising to us was that verbal fluency ... the ability to come up with the right word, actually improved in our asleep DBS group, which is a huge complication for patients [and] has a really negative impact on their life.”
Awake surgery with MER for lead targeting has been the preferred method. Surgery under general anesthesia with intraoperative CT (ICT) has been known to have lower morbidity and be more cost effective, but comparative clinical outcomes were previously not known.
Patients with Parkinson’s disease and motor complications (n = 64) were enrolled prospectively at Oregon Health & Science University. Thirty received asleep procedures under general anesthesia with ICT guidance for lead targeting to the globus pallidus pars interna (GPi; n = 21) or to the subthalamic nucleus (STN; n = 9). Thirty-four patients received DBS devices with MER guidance (15 STN; 19 GPi). At baseline, the two groups were similar in age (mean age, 61.1 and 62.7) and off-medication motor subscale scores of the Unified Parkinson’s Disease Rating Scale (mUPDRS; mean, 43.0 and 43.5). The university investigators optimized the DBS parameters at one, two, three, and six months after implantation. The same surgeon performed all the procedures at the same medical center.
Motor improvements were similar between the asleep and awake cohorts. At six months, the ICT (asleep) group experienced a mean improvement in motor abilities of 14.3 on the mUPDRS off medication and on DBS, compared with an improvement of 17.6 for the MER (awake) group.
Greater Fluency With Asleep DBS
Asleep DBS with ICT resulted in improvements in aspects of language, whereas awake patients lost language abilities. The asleep group showed a 0.8-point increase in phonemic fluency and a 1.0-point increase in semantic fluency at six months versus a worsening on both language measures (–3.5 points and –4.7 points, respectively) if DBS was performed via MER on awake patients.
Both cohorts showed significant improvements on the 39-item Parkinson’s Disease Questionnaire at six months. The cohorts did not differ in their degrees of improvement. Similarly, both had improvements on scores of activities of daily living, and both cohorts had a 4 to 4.5 hours per day increase in on time without dyskinesia and a 2.6 to 3.5 hours per day decrease in on time with dyskinesia.
Patients tolerated asleep DBS well, and there were no serious complications.
Surgery while patients are asleep is much shorter. “It is about two hours long, as opposed to four, five, sometimes eight, 10 hours with the awake. There [are fewer] complications, so less risk of hemorrhage or seizures or things like that,” Ms. Anderson said. A separate study found that asleep surgery results in more accurate placement of the electrodes. “All of those things considered, we feel the asleep version is definitely the superior choice between the two,” she said.
Being asleep is much more comfortable for the patient, added study leader Matthew Brodsky, MD, Associate Professor of Neurology at Oregon Health & Science University. “But the biggest advantage is that it is a single pass into the brain as opposed to multiple passes.” The average number of passes using MER is two to three per side of the brain, and in some centers, four or more. “Problems such as speech prosody are related to pokes in the brain, if you will, rather than stimulation,” he said.
Ms. Anderson said MER “is a fantastic research tool, and it gives us a lot of information on the electrophysiology, but really, there is no need for it in the clinical application of DBS.”
Based on the asleep procedure’s accuracy, lower rate of complications, shorter operating room time, and noninferiority in terms of motor outcomes, she said, “Our recommendation is that in more centers, more neurosurgeons be trained in this technique .... We would like to see the clinical field move toward that area and really reserve MER for the research side of things.”
A Barrier for Patients
“If you talk to folks who are considering brain surgery for their Parkinson’s, for some of them, the idea of being awake in the operating room and undergoing this is a barrier that they cannot quite overcome,” Dr. Brodsky said. “So, having this as an option makes it easier for them to sign up for the process.”
Richard Smeyne, PhD, Director of the Jefferson Comprehensive Parkinson’s Center at Thomas Jefferson University in Philadelphia, said that the asleep procedure is the newer one and can target either the GPi or the STN. “The asleep DBS seems to have a little bit better improvement on speech afterwards than the awake DBS, and there could be several causes of this,” he said. “Some might be operative, in that you can make smaller holes, you can get really nice guidance, you do not have to sort of move around as in the awake DBS.”
In addition, CT scanning with the patients asleep in the operating room allows more time in the scanner and greater precision in anatomical placement of the DBS leads.
“If I had to choose, looking at this particular study, it would suggest that the asleep DBS is actually a better overall way to go,” Dr. Smeyne said. However, he had no objection to awake procedures “if the neurosurgeon has a record of good results with it .... But if you have the option ... that becomes an individual choice that you should discuss with the neurosurgeon.”
Some of the work presented in the study was supported by a research grant from Medtronic.
—Daniel M. Keller
PORTLAND, OR—Performing deep brain stimulation (DBS) surgery for Parkinson’s disease using intraoperative CT imaging while the patient is under general anesthesia had clinical advantages and no disadvantages over surgery using microelectrode recording (MER) for lead placement with the patient awake, in a prospective, open-label study of 64 patients. The study was presented at the Fourth World Parkinson Congress.
The change in motor scores following surgery was the same for asleep and awake patients, said study coauthor Shannon Anderson, a physician assistant at Oregon Health & Science University in Portland. “What was surprising to us was that verbal fluency ... the ability to come up with the right word, actually improved in our asleep DBS group, which is a huge complication for patients [and] has a really negative impact on their life.”
Awake surgery with MER for lead targeting has been the preferred method. Surgery under general anesthesia with intraoperative CT (ICT) has been known to have lower morbidity and be more cost effective, but comparative clinical outcomes were previously not known.
Patients with Parkinson’s disease and motor complications (n = 64) were enrolled prospectively at Oregon Health & Science University. Thirty received asleep procedures under general anesthesia with ICT guidance for lead targeting to the globus pallidus pars interna (GPi; n = 21) or to the subthalamic nucleus (STN; n = 9). Thirty-four patients received DBS devices with MER guidance (15 STN; 19 GPi). At baseline, the two groups were similar in age (mean age, 61.1 and 62.7) and off-medication motor subscale scores of the Unified Parkinson’s Disease Rating Scale (mUPDRS; mean, 43.0 and 43.5). The university investigators optimized the DBS parameters at one, two, three, and six months after implantation. The same surgeon performed all the procedures at the same medical center.
Motor improvements were similar between the asleep and awake cohorts. At six months, the ICT (asleep) group experienced a mean improvement in motor abilities of 14.3 on the mUPDRS off medication and on DBS, compared with an improvement of 17.6 for the MER (awake) group.
Greater Fluency With Asleep DBS
Asleep DBS with ICT resulted in improvements in aspects of language, whereas awake patients lost language abilities. The asleep group showed a 0.8-point increase in phonemic fluency and a 1.0-point increase in semantic fluency at six months versus a worsening on both language measures (–3.5 points and –4.7 points, respectively) if DBS was performed via MER on awake patients.
Both cohorts showed significant improvements on the 39-item Parkinson’s Disease Questionnaire at six months. The cohorts did not differ in their degrees of improvement. Similarly, both had improvements on scores of activities of daily living, and both cohorts had a 4 to 4.5 hours per day increase in on time without dyskinesia and a 2.6 to 3.5 hours per day decrease in on time with dyskinesia.
Patients tolerated asleep DBS well, and there were no serious complications.
Surgery while patients are asleep is much shorter. “It is about two hours long, as opposed to four, five, sometimes eight, 10 hours with the awake. There [are fewer] complications, so less risk of hemorrhage or seizures or things like that,” Ms. Anderson said. A separate study found that asleep surgery results in more accurate placement of the electrodes. “All of those things considered, we feel the asleep version is definitely the superior choice between the two,” she said.
Being asleep is much more comfortable for the patient, added study leader Matthew Brodsky, MD, Associate Professor of Neurology at Oregon Health & Science University. “But the biggest advantage is that it is a single pass into the brain as opposed to multiple passes.” The average number of passes using MER is two to three per side of the brain, and in some centers, four or more. “Problems such as speech prosody are related to pokes in the brain, if you will, rather than stimulation,” he said.
Ms. Anderson said MER “is a fantastic research tool, and it gives us a lot of information on the electrophysiology, but really, there is no need for it in the clinical application of DBS.”
Based on the asleep procedure’s accuracy, lower rate of complications, shorter operating room time, and noninferiority in terms of motor outcomes, she said, “Our recommendation is that in more centers, more neurosurgeons be trained in this technique .... We would like to see the clinical field move toward that area and really reserve MER for the research side of things.”
A Barrier for Patients
“If you talk to folks who are considering brain surgery for their Parkinson’s, for some of them, the idea of being awake in the operating room and undergoing this is a barrier that they cannot quite overcome,” Dr. Brodsky said. “So, having this as an option makes it easier for them to sign up for the process.”
Richard Smeyne, PhD, Director of the Jefferson Comprehensive Parkinson’s Center at Thomas Jefferson University in Philadelphia, said that the asleep procedure is the newer one and can target either the GPi or the STN. “The asleep DBS seems to have a little bit better improvement on speech afterwards than the awake DBS, and there could be several causes of this,” he said. “Some might be operative, in that you can make smaller holes, you can get really nice guidance, you do not have to sort of move around as in the awake DBS.”
In addition, CT scanning with the patients asleep in the operating room allows more time in the scanner and greater precision in anatomical placement of the DBS leads.
“If I had to choose, looking at this particular study, it would suggest that the asleep DBS is actually a better overall way to go,” Dr. Smeyne said. However, he had no objection to awake procedures “if the neurosurgeon has a record of good results with it .... But if you have the option ... that becomes an individual choice that you should discuss with the neurosurgeon.”
Some of the work presented in the study was supported by a research grant from Medtronic.
—Daniel M. Keller
PORTLAND, OR—Performing deep brain stimulation (DBS) surgery for Parkinson’s disease using intraoperative CT imaging while the patient is under general anesthesia had clinical advantages and no disadvantages over surgery using microelectrode recording (MER) for lead placement with the patient awake, in a prospective, open-label study of 64 patients. The study was presented at the Fourth World Parkinson Congress.
The change in motor scores following surgery was the same for asleep and awake patients, said study coauthor Shannon Anderson, a physician assistant at Oregon Health & Science University in Portland. “What was surprising to us was that verbal fluency ... the ability to come up with the right word, actually improved in our asleep DBS group, which is a huge complication for patients [and] has a really negative impact on their life.”
Awake surgery with MER for lead targeting has been the preferred method. Surgery under general anesthesia with intraoperative CT (ICT) has been known to have lower morbidity and be more cost effective, but comparative clinical outcomes were previously not known.
Patients with Parkinson’s disease and motor complications (n = 64) were enrolled prospectively at Oregon Health & Science University. Thirty received asleep procedures under general anesthesia with ICT guidance for lead targeting to the globus pallidus pars interna (GPi; n = 21) or to the subthalamic nucleus (STN; n = 9). Thirty-four patients received DBS devices with MER guidance (15 STN; 19 GPi). At baseline, the two groups were similar in age (mean age, 61.1 and 62.7) and off-medication motor subscale scores of the Unified Parkinson’s Disease Rating Scale (mUPDRS; mean, 43.0 and 43.5). The university investigators optimized the DBS parameters at one, two, three, and six months after implantation. The same surgeon performed all the procedures at the same medical center.
Motor improvements were similar between the asleep and awake cohorts. At six months, the ICT (asleep) group experienced a mean improvement in motor abilities of 14.3 on the mUPDRS off medication and on DBS, compared with an improvement of 17.6 for the MER (awake) group.
Greater Fluency With Asleep DBS
Asleep DBS with ICT resulted in improvements in aspects of language, whereas awake patients lost language abilities. The asleep group showed a 0.8-point increase in phonemic fluency and a 1.0-point increase in semantic fluency at six months versus a worsening on both language measures (–3.5 points and –4.7 points, respectively) if DBS was performed via MER on awake patients.
Both cohorts showed significant improvements on the 39-item Parkinson’s Disease Questionnaire at six months. The cohorts did not differ in their degrees of improvement. Similarly, both had improvements on scores of activities of daily living, and both cohorts had a 4 to 4.5 hours per day increase in on time without dyskinesia and a 2.6 to 3.5 hours per day decrease in on time with dyskinesia.
Patients tolerated asleep DBS well, and there were no serious complications.
Surgery while patients are asleep is much shorter. “It is about two hours long, as opposed to four, five, sometimes eight, 10 hours with the awake. There [are fewer] complications, so less risk of hemorrhage or seizures or things like that,” Ms. Anderson said. A separate study found that asleep surgery results in more accurate placement of the electrodes. “All of those things considered, we feel the asleep version is definitely the superior choice between the two,” she said.
Being asleep is much more comfortable for the patient, added study leader Matthew Brodsky, MD, Associate Professor of Neurology at Oregon Health & Science University. “But the biggest advantage is that it is a single pass into the brain as opposed to multiple passes.” The average number of passes using MER is two to three per side of the brain, and in some centers, four or more. “Problems such as speech prosody are related to pokes in the brain, if you will, rather than stimulation,” he said.
Ms. Anderson said MER “is a fantastic research tool, and it gives us a lot of information on the electrophysiology, but really, there is no need for it in the clinical application of DBS.”
Based on the asleep procedure’s accuracy, lower rate of complications, shorter operating room time, and noninferiority in terms of motor outcomes, she said, “Our recommendation is that in more centers, more neurosurgeons be trained in this technique .... We would like to see the clinical field move toward that area and really reserve MER for the research side of things.”
A Barrier for Patients
“If you talk to folks who are considering brain surgery for their Parkinson’s, for some of them, the idea of being awake in the operating room and undergoing this is a barrier that they cannot quite overcome,” Dr. Brodsky said. “So, having this as an option makes it easier for them to sign up for the process.”
Richard Smeyne, PhD, Director of the Jefferson Comprehensive Parkinson’s Center at Thomas Jefferson University in Philadelphia, said that the asleep procedure is the newer one and can target either the GPi or the STN. “The asleep DBS seems to have a little bit better improvement on speech afterwards than the awake DBS, and there could be several causes of this,” he said. “Some might be operative, in that you can make smaller holes, you can get really nice guidance, you do not have to sort of move around as in the awake DBS.”
In addition, CT scanning with the patients asleep in the operating room allows more time in the scanner and greater precision in anatomical placement of the DBS leads.
“If I had to choose, looking at this particular study, it would suggest that the asleep DBS is actually a better overall way to go,” Dr. Smeyne said. However, he had no objection to awake procedures “if the neurosurgeon has a record of good results with it .... But if you have the option ... that becomes an individual choice that you should discuss with the neurosurgeon.”
Some of the work presented in the study was supported by a research grant from Medtronic.
—Daniel M. Keller
What Are New and Potential Therapies for Neuromuscular Disorders?
HILTON HEAD, SC—Biologics, stem cells, and gene therapy may hold promise as treatments for neuromuscular disorders. Ongoing clinical trials are poised to inform treatment strategies and may identify new therapies for amyotrophic lateral sclerosis (ALS), myasthenia gravis, muscular dystrophy, and neuropathy, according to a lecture delivered at the 39th Annual Contemporary Clinical Neurology Symposium.
Recent developments in neuromuscular disorders include the FDA’s approval of a new drug for Duchenne muscular dystrophy and the publication of a randomized trial that found that thymectomy improves clinical outcomes in patients with myasthenia gravis.
ALS
In ALS, many drugs have been studied with little success, said Amanda C. Peltier, MD, Associate Professor of Neurology at Vanderbilt University in Nashville. Riluzole, an approved drug for ALS that may result in a three-month increase in life expectancy or time to mechanical ventilation, targets excitotoxicity. Other drugs targeting excitotoxicity (eg, ceftriaxone and talampanel) have not been found to be effective, however. Agents that target oxidative stress, inflammatory response, protein degradation pathways, mitochondrial dysfunction, and apoptotic pathways have not provided benefit, Dr. Peltier said.
Most trials in ALS use time to mechanical ventilation, time to death, or rate of decline on the ALS Functional Rating Scale as end points. These end points are “very gross measures of how people do,” and researchers are seeking better ways to measure progression in ALS, she said.
Two phase II stem cell studies in ALS are ongoing—the BrainStorm trial in Hassadah, Israel, and the Neuralstem Trial at Emory University and the University of Michigan. Safety data for the Neuralstem Trial that were presented at the 68th Annual Meeting of the American Academy of Neurology (AAN) showed that patients experienced no significant worsening after injections of stem cells in the cervical and lumbar cord. Dose escalation therapy currently is being tested.
Several phase III studies in ALS are under way, including a study of tirasemtiv, an activator of the skeletal muscle troponin complex. “The goal of this [therapy] is to increase muscle force in ALS patients. It is not actually acting at the motor neuron itself,” Dr. Peltier said. In addition, investigators are re-studying creatine, an organic acid involved in ATP formation, at doses of 5 g to 10 g per day. Researchers also are studying masitinib, a biologic targeting inflammation.
Muscular Dystrophies
“There is more gene therapy going on in muscular dystrophy than has ever happened in my lifetime,” Dr. Peltier said. Neuromuscular diseases are caused by genetic mutations in structural proteins that connect the muscle cell membrane to the actin–myosin complex. Dystrophin, one of the largest proteins in the body, is mutated in Duchenne muscular dystrophy and Becker muscular dystrophy.
Some patients with Duchenne muscular dystrophy have a frameshift mutation that results in nonfunctional dystrophin. One treatment strategy entails using an antisense oligonucleotide fragment that binds to messenger RNA to create a more functional protein. “The biggest problem is that those mutations are not the vast majority of mutations in Duchenne,” said Dr. Peltier. “A large proportion of our Duchenne patients are not going to be able to benefit from this technology.”
The FDA on September 19 approved eteplirsen (Exondys 51) injection to treat patients with Duchenne muscular dystrophy who have a confirmed mutation of the dystrophin gene that is amenable to exon 51 skipping. About 13% of people with Duchenne muscular dystrophy have this mutation. The FDA approved eteplirsen under an accelerated pathway that provides patients with access to the drug while the drug developer conducts trials to verify clinical benefit. The FDA said that the approval was based on an increase of dystrophin in skeletal muscle that was observed in some treated patients. Clinical benefit of eteplirsen has not been established.
“The approval of eteplirsen is a positive first step in gene therapy,” Dr. Peltier said. “The FDA is requiring further clinical trials to establish whether clinical improvement will occur, and hopefully these [trials] will be positive.” Gene therapy may have applications for treating other dystrophies and genetic disorders, and the FDA is considering gene therapy for spinal muscular atrophy that is based on increasing messenger RNA transcripts. “Hopefully this is a signal that the FDA is open to gene therapy for these devastating neuromuscular disorders,” she said.
Idebenone, an analog of coenzyme Q10, may reduce loss of respiratory function in patients with Duchenne muscular dystrophy, according to a post hoc analysis of the phase III Duchenne Muscular Dystrophy Long-term Idebenone Study (DELOS). Patients who received idebenone had a lower risk of bronchopulmonary adverse events and a reduced need for systemic antibiotics, compared with patients who received placebo.
Ataluren, a drug intended to make red blood cells less sensitive to premature stop codons, resulted in faster walk times in the Ataluren Confirmatory Trial in Duchenne Muscular Dystrophy (ACT DMD) study. “So far, it seems to be promising,” Dr. Peltier said.
Many patients with Becker or Duchenne muscular dystrophy develop cardiomyopathy. Drugs that typically are used for heart failure and ischemic cardiomyopathy have not been studied in this patient population, however. An ongoing study comparing ACE inhibitors and beta blockers aims to determine the best strategy for treating cardiomyopathy in this population, she said.
In addition, investigators are studying whether follistatin, delivered by an adeno-associated virus, inhibits the degradation of muscle cells in muscular dystrophy and sporadic inclusion body myositis. A recombinant version of histidyl-tRNA synthetase, aTyr1940, is being studied in facioscapulohumeral muscular dystrophy and limb girdle dystrophies.
Among patients with myotonic dystrophy, a small study found that methylphenidate significantly reduces daytime sleepiness, Dr. Peltier said.
Biologics
Several biologics are being studied as treatments for various neuromuscular disorders. In a phase III study in patients with refractory generalized myasthenia gravis, eculizumab, which inhibits complements and the membrane attack complex, did not result in a significant improvement in Myasthenia Gravis–Activities of Daily Living Profile score. Eculizumab did, however, significantly improve a secondary end point, Quantitative Myasthenia Gravis total score.
A phase II trial to evaluate rituximab in myasthenia gravis has completed enrollment. Results may be available in approximately a year. Rituximab also is being studied in juvenile and adult dermatomyositis and chronic inflammatory demyelinating polyneuropathy (CIDP), Dr. Peltier said.
Etanercept and infliximab, treatments for rheumatoid arthritis that act on tumor necrosis factor alpha, are being studied in dermatomyositis and polymyositis. Tocilizumab, a treatment for rheumatoid arthritis that inhibits IL-6 receptors, is being studied in those disorders as well.
Alemtuzumab has been shown to stabilize quantitative muscle testing in sporadic inclusion body myositis and may be helpful in refractory CIDP, Dr. Peltier said.
Myasthenia Gravis
Researchers in August published the results of a randomized trial that found that thymectomy improves clinical outcomes over three years in patients with nonthymomatous myasthenia gravis. Patients who underwent thymectomy and received alternate-day prednisone had a lower time-weighted average Quantitative Myasthenia Gravis score, compared with patients who received alternate-day prednisone alone. Thymectomy had been a mainstay in the treatment of myasthenia gravis, although prior nonrandomized studies had not provided conclusive evidence of its benefit. “The MGTX trial was based on the traditional method of thymectomy requiring sternotomy,” she said. “Further trials comparing the effectiveness of full sternotomy versus newer methods utilizing transcervical approaches or partial sternotomy will most likely be coming.”
A trial of subcutaneous IV immunoglobulin (IVIg) in myasthenia gravis is enrolling patients. Additional data to support the use of IVIg in myasthenia gravis would improve patient outcomes due to the ease of delivering IVIg versus plasma exchange, Dr. Peltier said.
Neuropathy
Investigators have pooled data for patients with neuropathy who have received IVIg. The INSIGHTS Quality Improvement Registry includes data from 585 patients. Researchers have found that patients who fulfill European Federation of Neurological Societies or AAN definite or probable criteria for CIDP or multifocal motor neuropathy respond better to IVIg. This finding “makes sense,” Dr. Peltier said. “If we are more confident of the diagnosis, then it is very likely that IVIg will perform better.”
Among patients with type 2 diabetic neuropathy, exercise significantly improves epidermal nerve fiber density. “Exercise has been interesting, in that it may actually reverse some of the effects” of neuropathy in type 2 diabetes, Dr. Peltier said. “It may be that you will see more exercise studies in the future.”
—Jake Remaly
HILTON HEAD, SC—Biologics, stem cells, and gene therapy may hold promise as treatments for neuromuscular disorders. Ongoing clinical trials are poised to inform treatment strategies and may identify new therapies for amyotrophic lateral sclerosis (ALS), myasthenia gravis, muscular dystrophy, and neuropathy, according to a lecture delivered at the 39th Annual Contemporary Clinical Neurology Symposium.
Recent developments in neuromuscular disorders include the FDA’s approval of a new drug for Duchenne muscular dystrophy and the publication of a randomized trial that found that thymectomy improves clinical outcomes in patients with myasthenia gravis.
ALS
In ALS, many drugs have been studied with little success, said Amanda C. Peltier, MD, Associate Professor of Neurology at Vanderbilt University in Nashville. Riluzole, an approved drug for ALS that may result in a three-month increase in life expectancy or time to mechanical ventilation, targets excitotoxicity. Other drugs targeting excitotoxicity (eg, ceftriaxone and talampanel) have not been found to be effective, however. Agents that target oxidative stress, inflammatory response, protein degradation pathways, mitochondrial dysfunction, and apoptotic pathways have not provided benefit, Dr. Peltier said.
Most trials in ALS use time to mechanical ventilation, time to death, or rate of decline on the ALS Functional Rating Scale as end points. These end points are “very gross measures of how people do,” and researchers are seeking better ways to measure progression in ALS, she said.
Two phase II stem cell studies in ALS are ongoing—the BrainStorm trial in Hassadah, Israel, and the Neuralstem Trial at Emory University and the University of Michigan. Safety data for the Neuralstem Trial that were presented at the 68th Annual Meeting of the American Academy of Neurology (AAN) showed that patients experienced no significant worsening after injections of stem cells in the cervical and lumbar cord. Dose escalation therapy currently is being tested.
Several phase III studies in ALS are under way, including a study of tirasemtiv, an activator of the skeletal muscle troponin complex. “The goal of this [therapy] is to increase muscle force in ALS patients. It is not actually acting at the motor neuron itself,” Dr. Peltier said. In addition, investigators are re-studying creatine, an organic acid involved in ATP formation, at doses of 5 g to 10 g per day. Researchers also are studying masitinib, a biologic targeting inflammation.
Muscular Dystrophies
“There is more gene therapy going on in muscular dystrophy than has ever happened in my lifetime,” Dr. Peltier said. Neuromuscular diseases are caused by genetic mutations in structural proteins that connect the muscle cell membrane to the actin–myosin complex. Dystrophin, one of the largest proteins in the body, is mutated in Duchenne muscular dystrophy and Becker muscular dystrophy.
Some patients with Duchenne muscular dystrophy have a frameshift mutation that results in nonfunctional dystrophin. One treatment strategy entails using an antisense oligonucleotide fragment that binds to messenger RNA to create a more functional protein. “The biggest problem is that those mutations are not the vast majority of mutations in Duchenne,” said Dr. Peltier. “A large proportion of our Duchenne patients are not going to be able to benefit from this technology.”
The FDA on September 19 approved eteplirsen (Exondys 51) injection to treat patients with Duchenne muscular dystrophy who have a confirmed mutation of the dystrophin gene that is amenable to exon 51 skipping. About 13% of people with Duchenne muscular dystrophy have this mutation. The FDA approved eteplirsen under an accelerated pathway that provides patients with access to the drug while the drug developer conducts trials to verify clinical benefit. The FDA said that the approval was based on an increase of dystrophin in skeletal muscle that was observed in some treated patients. Clinical benefit of eteplirsen has not been established.
“The approval of eteplirsen is a positive first step in gene therapy,” Dr. Peltier said. “The FDA is requiring further clinical trials to establish whether clinical improvement will occur, and hopefully these [trials] will be positive.” Gene therapy may have applications for treating other dystrophies and genetic disorders, and the FDA is considering gene therapy for spinal muscular atrophy that is based on increasing messenger RNA transcripts. “Hopefully this is a signal that the FDA is open to gene therapy for these devastating neuromuscular disorders,” she said.
Idebenone, an analog of coenzyme Q10, may reduce loss of respiratory function in patients with Duchenne muscular dystrophy, according to a post hoc analysis of the phase III Duchenne Muscular Dystrophy Long-term Idebenone Study (DELOS). Patients who received idebenone had a lower risk of bronchopulmonary adverse events and a reduced need for systemic antibiotics, compared with patients who received placebo.
Ataluren, a drug intended to make red blood cells less sensitive to premature stop codons, resulted in faster walk times in the Ataluren Confirmatory Trial in Duchenne Muscular Dystrophy (ACT DMD) study. “So far, it seems to be promising,” Dr. Peltier said.
Many patients with Becker or Duchenne muscular dystrophy develop cardiomyopathy. Drugs that typically are used for heart failure and ischemic cardiomyopathy have not been studied in this patient population, however. An ongoing study comparing ACE inhibitors and beta blockers aims to determine the best strategy for treating cardiomyopathy in this population, she said.
In addition, investigators are studying whether follistatin, delivered by an adeno-associated virus, inhibits the degradation of muscle cells in muscular dystrophy and sporadic inclusion body myositis. A recombinant version of histidyl-tRNA synthetase, aTyr1940, is being studied in facioscapulohumeral muscular dystrophy and limb girdle dystrophies.
Among patients with myotonic dystrophy, a small study found that methylphenidate significantly reduces daytime sleepiness, Dr. Peltier said.
Biologics
Several biologics are being studied as treatments for various neuromuscular disorders. In a phase III study in patients with refractory generalized myasthenia gravis, eculizumab, which inhibits complements and the membrane attack complex, did not result in a significant improvement in Myasthenia Gravis–Activities of Daily Living Profile score. Eculizumab did, however, significantly improve a secondary end point, Quantitative Myasthenia Gravis total score.
A phase II trial to evaluate rituximab in myasthenia gravis has completed enrollment. Results may be available in approximately a year. Rituximab also is being studied in juvenile and adult dermatomyositis and chronic inflammatory demyelinating polyneuropathy (CIDP), Dr. Peltier said.
Etanercept and infliximab, treatments for rheumatoid arthritis that act on tumor necrosis factor alpha, are being studied in dermatomyositis and polymyositis. Tocilizumab, a treatment for rheumatoid arthritis that inhibits IL-6 receptors, is being studied in those disorders as well.
Alemtuzumab has been shown to stabilize quantitative muscle testing in sporadic inclusion body myositis and may be helpful in refractory CIDP, Dr. Peltier said.
Myasthenia Gravis
Researchers in August published the results of a randomized trial that found that thymectomy improves clinical outcomes over three years in patients with nonthymomatous myasthenia gravis. Patients who underwent thymectomy and received alternate-day prednisone had a lower time-weighted average Quantitative Myasthenia Gravis score, compared with patients who received alternate-day prednisone alone. Thymectomy had been a mainstay in the treatment of myasthenia gravis, although prior nonrandomized studies had not provided conclusive evidence of its benefit. “The MGTX trial was based on the traditional method of thymectomy requiring sternotomy,” she said. “Further trials comparing the effectiveness of full sternotomy versus newer methods utilizing transcervical approaches or partial sternotomy will most likely be coming.”
A trial of subcutaneous IV immunoglobulin (IVIg) in myasthenia gravis is enrolling patients. Additional data to support the use of IVIg in myasthenia gravis would improve patient outcomes due to the ease of delivering IVIg versus plasma exchange, Dr. Peltier said.
Neuropathy
Investigators have pooled data for patients with neuropathy who have received IVIg. The INSIGHTS Quality Improvement Registry includes data from 585 patients. Researchers have found that patients who fulfill European Federation of Neurological Societies or AAN definite or probable criteria for CIDP or multifocal motor neuropathy respond better to IVIg. This finding “makes sense,” Dr. Peltier said. “If we are more confident of the diagnosis, then it is very likely that IVIg will perform better.”
Among patients with type 2 diabetic neuropathy, exercise significantly improves epidermal nerve fiber density. “Exercise has been interesting, in that it may actually reverse some of the effects” of neuropathy in type 2 diabetes, Dr. Peltier said. “It may be that you will see more exercise studies in the future.”
—Jake Remaly
HILTON HEAD, SC—Biologics, stem cells, and gene therapy may hold promise as treatments for neuromuscular disorders. Ongoing clinical trials are poised to inform treatment strategies and may identify new therapies for amyotrophic lateral sclerosis (ALS), myasthenia gravis, muscular dystrophy, and neuropathy, according to a lecture delivered at the 39th Annual Contemporary Clinical Neurology Symposium.
Recent developments in neuromuscular disorders include the FDA’s approval of a new drug for Duchenne muscular dystrophy and the publication of a randomized trial that found that thymectomy improves clinical outcomes in patients with myasthenia gravis.
ALS
In ALS, many drugs have been studied with little success, said Amanda C. Peltier, MD, Associate Professor of Neurology at Vanderbilt University in Nashville. Riluzole, an approved drug for ALS that may result in a three-month increase in life expectancy or time to mechanical ventilation, targets excitotoxicity. Other drugs targeting excitotoxicity (eg, ceftriaxone and talampanel) have not been found to be effective, however. Agents that target oxidative stress, inflammatory response, protein degradation pathways, mitochondrial dysfunction, and apoptotic pathways have not provided benefit, Dr. Peltier said.
Most trials in ALS use time to mechanical ventilation, time to death, or rate of decline on the ALS Functional Rating Scale as end points. These end points are “very gross measures of how people do,” and researchers are seeking better ways to measure progression in ALS, she said.
Two phase II stem cell studies in ALS are ongoing—the BrainStorm trial in Hassadah, Israel, and the Neuralstem Trial at Emory University and the University of Michigan. Safety data for the Neuralstem Trial that were presented at the 68th Annual Meeting of the American Academy of Neurology (AAN) showed that patients experienced no significant worsening after injections of stem cells in the cervical and lumbar cord. Dose escalation therapy currently is being tested.
Several phase III studies in ALS are under way, including a study of tirasemtiv, an activator of the skeletal muscle troponin complex. “The goal of this [therapy] is to increase muscle force in ALS patients. It is not actually acting at the motor neuron itself,” Dr. Peltier said. In addition, investigators are re-studying creatine, an organic acid involved in ATP formation, at doses of 5 g to 10 g per day. Researchers also are studying masitinib, a biologic targeting inflammation.
Muscular Dystrophies
“There is more gene therapy going on in muscular dystrophy than has ever happened in my lifetime,” Dr. Peltier said. Neuromuscular diseases are caused by genetic mutations in structural proteins that connect the muscle cell membrane to the actin–myosin complex. Dystrophin, one of the largest proteins in the body, is mutated in Duchenne muscular dystrophy and Becker muscular dystrophy.
Some patients with Duchenne muscular dystrophy have a frameshift mutation that results in nonfunctional dystrophin. One treatment strategy entails using an antisense oligonucleotide fragment that binds to messenger RNA to create a more functional protein. “The biggest problem is that those mutations are not the vast majority of mutations in Duchenne,” said Dr. Peltier. “A large proportion of our Duchenne patients are not going to be able to benefit from this technology.”
The FDA on September 19 approved eteplirsen (Exondys 51) injection to treat patients with Duchenne muscular dystrophy who have a confirmed mutation of the dystrophin gene that is amenable to exon 51 skipping. About 13% of people with Duchenne muscular dystrophy have this mutation. The FDA approved eteplirsen under an accelerated pathway that provides patients with access to the drug while the drug developer conducts trials to verify clinical benefit. The FDA said that the approval was based on an increase of dystrophin in skeletal muscle that was observed in some treated patients. Clinical benefit of eteplirsen has not been established.
“The approval of eteplirsen is a positive first step in gene therapy,” Dr. Peltier said. “The FDA is requiring further clinical trials to establish whether clinical improvement will occur, and hopefully these [trials] will be positive.” Gene therapy may have applications for treating other dystrophies and genetic disorders, and the FDA is considering gene therapy for spinal muscular atrophy that is based on increasing messenger RNA transcripts. “Hopefully this is a signal that the FDA is open to gene therapy for these devastating neuromuscular disorders,” she said.
Idebenone, an analog of coenzyme Q10, may reduce loss of respiratory function in patients with Duchenne muscular dystrophy, according to a post hoc analysis of the phase III Duchenne Muscular Dystrophy Long-term Idebenone Study (DELOS). Patients who received idebenone had a lower risk of bronchopulmonary adverse events and a reduced need for systemic antibiotics, compared with patients who received placebo.
Ataluren, a drug intended to make red blood cells less sensitive to premature stop codons, resulted in faster walk times in the Ataluren Confirmatory Trial in Duchenne Muscular Dystrophy (ACT DMD) study. “So far, it seems to be promising,” Dr. Peltier said.
Many patients with Becker or Duchenne muscular dystrophy develop cardiomyopathy. Drugs that typically are used for heart failure and ischemic cardiomyopathy have not been studied in this patient population, however. An ongoing study comparing ACE inhibitors and beta blockers aims to determine the best strategy for treating cardiomyopathy in this population, she said.
In addition, investigators are studying whether follistatin, delivered by an adeno-associated virus, inhibits the degradation of muscle cells in muscular dystrophy and sporadic inclusion body myositis. A recombinant version of histidyl-tRNA synthetase, aTyr1940, is being studied in facioscapulohumeral muscular dystrophy and limb girdle dystrophies.
Among patients with myotonic dystrophy, a small study found that methylphenidate significantly reduces daytime sleepiness, Dr. Peltier said.
Biologics
Several biologics are being studied as treatments for various neuromuscular disorders. In a phase III study in patients with refractory generalized myasthenia gravis, eculizumab, which inhibits complements and the membrane attack complex, did not result in a significant improvement in Myasthenia Gravis–Activities of Daily Living Profile score. Eculizumab did, however, significantly improve a secondary end point, Quantitative Myasthenia Gravis total score.
A phase II trial to evaluate rituximab in myasthenia gravis has completed enrollment. Results may be available in approximately a year. Rituximab also is being studied in juvenile and adult dermatomyositis and chronic inflammatory demyelinating polyneuropathy (CIDP), Dr. Peltier said.
Etanercept and infliximab, treatments for rheumatoid arthritis that act on tumor necrosis factor alpha, are being studied in dermatomyositis and polymyositis. Tocilizumab, a treatment for rheumatoid arthritis that inhibits IL-6 receptors, is being studied in those disorders as well.
Alemtuzumab has been shown to stabilize quantitative muscle testing in sporadic inclusion body myositis and may be helpful in refractory CIDP, Dr. Peltier said.
Myasthenia Gravis
Researchers in August published the results of a randomized trial that found that thymectomy improves clinical outcomes over three years in patients with nonthymomatous myasthenia gravis. Patients who underwent thymectomy and received alternate-day prednisone had a lower time-weighted average Quantitative Myasthenia Gravis score, compared with patients who received alternate-day prednisone alone. Thymectomy had been a mainstay in the treatment of myasthenia gravis, although prior nonrandomized studies had not provided conclusive evidence of its benefit. “The MGTX trial was based on the traditional method of thymectomy requiring sternotomy,” she said. “Further trials comparing the effectiveness of full sternotomy versus newer methods utilizing transcervical approaches or partial sternotomy will most likely be coming.”
A trial of subcutaneous IV immunoglobulin (IVIg) in myasthenia gravis is enrolling patients. Additional data to support the use of IVIg in myasthenia gravis would improve patient outcomes due to the ease of delivering IVIg versus plasma exchange, Dr. Peltier said.
Neuropathy
Investigators have pooled data for patients with neuropathy who have received IVIg. The INSIGHTS Quality Improvement Registry includes data from 585 patients. Researchers have found that patients who fulfill European Federation of Neurological Societies or AAN definite or probable criteria for CIDP or multifocal motor neuropathy respond better to IVIg. This finding “makes sense,” Dr. Peltier said. “If we are more confident of the diagnosis, then it is very likely that IVIg will perform better.”
Among patients with type 2 diabetic neuropathy, exercise significantly improves epidermal nerve fiber density. “Exercise has been interesting, in that it may actually reverse some of the effects” of neuropathy in type 2 diabetes, Dr. Peltier said. “It may be that you will see more exercise studies in the future.”
—Jake Remaly
Current Concepts in Lip Augmentation
Historically, a variety of tools have been used to alter one’s appearance for cultural or religious purposes or to conform to standards of beauty. As a defining feature of the face, the lips provide a unique opportunity for facial aesthetic enhancement. There has been a paradigm shift in medicine favoring preventative health and a desire to slow and even reverse the aging process.1 Acknowledging that product technology, skill sets, and cultural ideals continually evolve, this article highlights perioral anatomy, explains aging of the lower face, and reviews techniques to achieve perioral rejuvenation through volume restoration and muscle control.
Perioral Anatomy
The layers of the lips include the epidermis, subcutaneous tissue, orbicularis oris muscle fibers, and mucosa. The upper lip extends from the base of the nose to the mucosa inferiorly and to the nasolabial folds laterally. The curvilinear lower lip extends from the mucosa to the mandible inferiorly and to the oral commissures laterally.2 Circumferential at the vermilion-cutaneous junction, a raised area of pale skin known as the white roll accentuates the vermilion border and provides an important landmark during lip augmentation.3 At the upper lip, this elevation of the vermilion joins at a V-shaped depression centrally to form the Cupid’s bow. The cutaneous upper lip has 2 raised vertical pillars known as the philtral columns, which are formed from decussating fibers of the orbicularis oris muscle.2 The resultant midline depression is the philtrum. These defining features of the upper lip are to be preserved during augmentation procedures (Figure 1).4
The superior and inferior labial arteries, both branches of the facial artery, supply the upper and lower lip, respectively. The anastomotic arch of the superior labial artery is susceptible to injury from deep injection of the upper lip between the muscle layer and mucosa; therefore, caution must be exercised in this area.5 Injections into the vermilion and lower lip can be safely performed with less concern for vascular compromise. The vermilion derives its red color from the translucency of capillaries in the superficial papillae.2 The capillary plexus at the papillae and rich sensory nerve network render the lip a highly vascular and sensitive structure.
Aging of the Lower Face
Subcutaneous fat atrophy, loss of elasticity, gravitational forces, and remodeling of the skeletal foundation all contribute to aging of the lower face. Starting as early as the third decade of life, intrinsic factors including hormonal changes and genetically determined processes produce alterations in skin quality and structure. Similarly, extrinsic aging through environmental influences, namely exposure to UV radiation and smoking, accelerate the loss of skin integrity.6
The decreased laxity of the skin in combination with repeated contraction of the orbicularis oris muscle results in perioral rhytides.7 For women in particular, vertically oriented perioral rhytides develop above the vermilion; terminal hair follicles, thicker skin, and a greater density of subcutaneous fat are presumptive protective factors for males.8 With time, the cutaneous portion of the upper lip lengthens and there is redistribution of volume with effacement of the upper lip vermilion.9 Additionally, the demarcation of the vermilion becomes blurred secondary to pallor, flattening of the philtral columns, and loss of projection of the Cupid’s bow.10
Downturning of the oral commissures is observed secondary to a combination of gravity, bone resorption, and soft tissue volume loss. Hyperactivity of the depressor anguli oris muscle exacerbates the mesolabial folds, producing marionette lines and a saddened expression.7 With ongoing volume loss and ligament laxity, tissue redistributes near the jaws and chin, giving rise to jowls. Similarly, perioral volume loss and descent of the malar fat-pad deepen the nasolabial folds in the aging midface.6
The main objective of perioral rejuvenation is to reinstate a harmonious refreshed look to the lower face; however, aesthetic analysis should occur within the context of the face as a whole, as the lips should complement the surrounding perioral cosmetic unit and overall skeletal foundation of the face. To accomplish this goal, the dermatologist’s armamentarium contains a broad variety of approaches including restriction of muscle movement, volume restoration, and surface contouring.
Volume Restoration
Treatment Options
In 2015, hyaluronic acid (HA) fillers constituted 80% of all injectable soft-tissue fillers, an 8% increase from 2014.11 Hyaluronic acid has achieved immense popularity as a temporary dermal filler given its biocompatibility, longevity, and reversibility via hyaluronidase.12
Hyaluronic acid is a naturally occurring glycosaminoglycan that comprises the connective tissue matrix. The molecular composition affords HA its hydrophilic property, which augments dermal volume.7 Endogenous HA has a short half-life, and chemical modification by a cross-linking process extends longevity by 6 to 12 months. The various HA fillers are distinguished by method of purification, size of molecules, concentration and degree of cross-linking, and viscosity.7,13,14 These differences dictate overall clinical performance such as flow properties, longevity, and stability. As a general rule, a high-viscosity product is more appropriate for deeper augmentation; fillers with low viscosity are more appropriate for correction of shallow defects.1 Table 1 lists the HA fillers that are currently approved by the US Food and Drug Administration for lip augmentation and/or perioral rhytides in adults 21 years and older.15-17
Randomized controlled trials comparing the efficacy, longevity, and tolerability of different HA products are lacking in the literature and, where present, have strong industry influence.18,19 The advent of assessment scales has provided an objective evaluation of perioral and lip augmentation, facilitating comparisons between products in both clinical research and practice.20
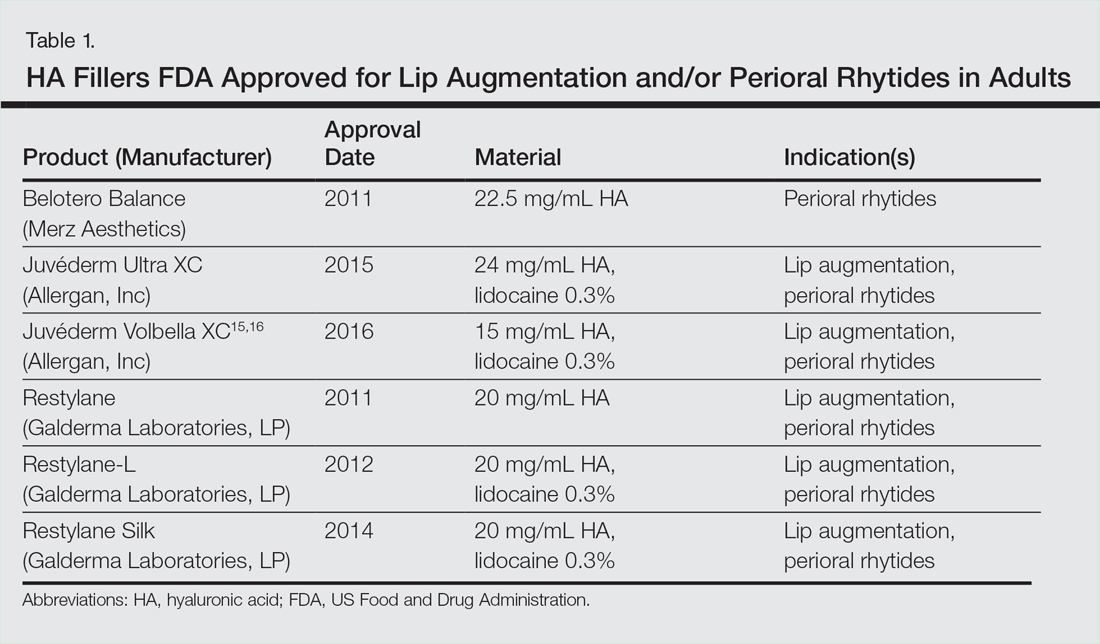
Semipermanent biostimulatory dermal fillers such as calcium hydroxylapatite and poly-L-lactic acid are not recommended for lip augmentation due to an increased incidence of submucosal nodule formation.6,14,21 Likewise, permanent fillers are not recommended given their irreversibility and risk of nodule formation around the lips.14,22 Nonetheless, liquid silicone (purified polydimethylsiloxane) administered via a microdroplet technique (0.01 mL of silicone at a time, no more than 1 cc per lip per session) has been used off label as a permanent filling agent for lip augmentation with limited complications.23 Regardless, trepidations about its use with respect to reported risks continue to limit its application.22
Similarly, surgical lip implants such as expanded polytetrafluoroethylene is an option for a subset of patients desiring permanent enhancement but are less commonly utilized given the side-effect profile, irreversibility, and relatively invasive nature of the procedure.22 Lastly, autologous fat transfer has been used in correction of the nasolabial and mesolabial folds as well as in lip augmentation; however, irregular surface contours and unpredictable longevity secondary to postinjection resorption (20%–90%) has limited its popularity.3,14,21
HA Injection Technique
With respect to HA fillers in the perioral area, numerous approaches have been described.10,22 The techniques in Table 2 provide a foundation for lip rejuvenation.

Several injection techniques exist, including serial puncture, linear threading, cross-hatching, and fanning in a retrograde or anterograde manner.24 A blunt microcannula (27 gauge, 38 mm) may be used in place of sharp needles and offers the benefit of increased patient comfort, reduced edema and ecchymosis, and shortened recovery period.25,26 Gentle massage of the product after injection can assist with an even contour. Lastly, a key determinant of successful outcomes is using an adequate volume of HA filler (1–2 mL for shaping the vermilion border and volumizing the lips).27 Figure 2 highlights a clinical example of HA filler for lip augmentation.
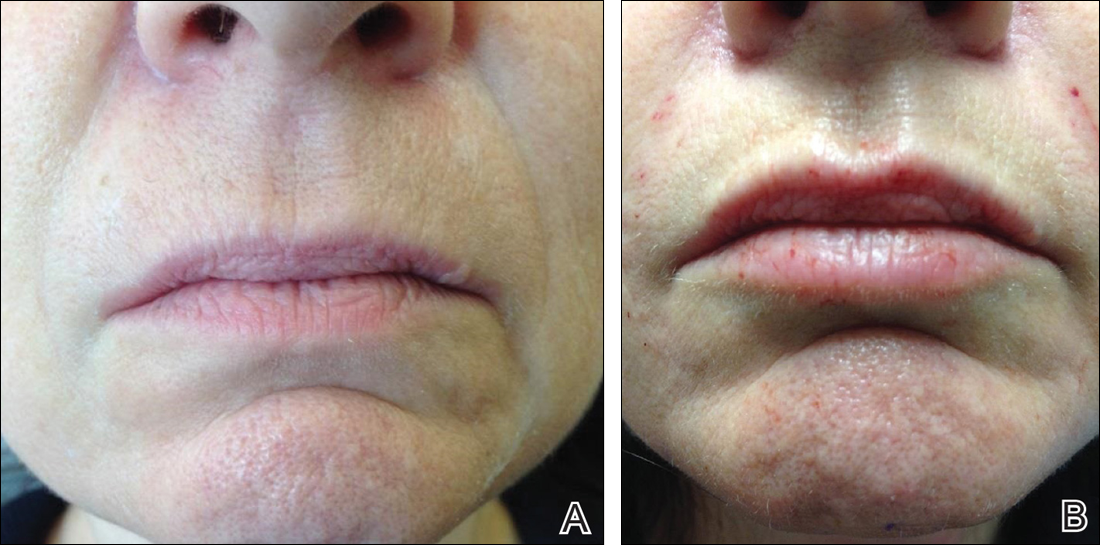
Fortunately, most complications encountered with HA lip augmentation are mild and transient. The most commonly observed side effects include injection-site reactions such as pain, erythema, and edema. Similarly, most adverse effects are related to injection technique. All HA fillers are prone to the Tyndall effect, a consequence of too superficial an injection plane. Patients with history of recurrent herpes simplex virus infections should receive prophylactic antiviral therapy.12
Muscle Control
An emerging concept in rejuvenation of the lower face recognizes not only restoration of volume but also control of muscle movement. Local injection of botulinum toxin type A induces relaxation of hyperfunctional facial muscles through temporary inhibition of neurotransmitter release.6 The potential for paralysis of the oral cavity may limit the application of botulinum toxin type A in that region.7 Nonetheless, the off-label potential of botulinum toxin type A has expanded to include several targets in the lower face. The orbicularis oris muscle is targeted to soften perioral rhytides. Conservative dosing (1–2 U per lip quadrant or approximately 5 U total) and superficial injection is emphasized in this area.27 Similarly, the depressor anguli oris muscle is targeted by injection of 4 U bilaterally to soften the marionette lines. In the chin area, the mentalis muscle can be targeted by injection of 2 U deep into each belly of the muscle to reduce the mental crease and dimpling.28 Combination treatment with dermal filler and neurotoxin demonstrates effects that last longer than either modality alone without additional adverse events.29 With combination therapy, guidelines suggest treating with filler first.27
Conclusion
A greater understanding of the extrinsic and intrinsic factors that contribute to the structural and surface changes of the aging face coupled with a preference for minimally invasive procedures has revolutionized the dermatologist’s approach to perioral rejuvenation. Serving as a focal point of the face, the lips and perioral skin are well poised to benefit from this paradigm shift. A multifaceted approach utilizing dermal fillers and neurotoxins may be most appropriate and has demonstrated optimal outcomes in facial aesthetics.
- Buck DW, Alam M, Kim JYS. Injectable fillers for facial rejuvenation: a review. J Plast Reconstr Aesthet Surg. 2009;62:11-18.
- Guareschi M, Stella E. Lips. In: Goisis M, ed. Injections in Aesthetic Medicine. Milan, Italy: Springer; 2014:125-136.
- Byrne PJ, Hilger PA. Lip augmentation. Facial Plast Surg. 2004;20:31-38.
- Niamtu J. Rejuvenation of the lip and perioral areas. In: Bell WH, Guerroro CA, eds. Distraction Osteogenesis of the Facial Skeleton. Ontario, Canada: BC Decker Inc; 2007:38-48.
- Tansatit T, Apinuntrum P, Phetudom T. A typical pattern of the labial arteries with implication for lip augmentation with injectable fillers. Aesthet Plast Surg. 2014;38:1083-1089.
- Sadick NS, Karcher C, Palmisano L. Cosmetic dermatology of the aging face. Clin Dermatol. 2009;27(suppl):S3-S12.
- Ali MJ, Ende K, Mass CS. Perioral rejuvenation and lip augmentation. Facial Plast Surg Clin N Am. 2007;15:491-500.
- Chien AL, Qi J, Cheng N, et al. Perioral wrinkles are associated with female gender, aging, and smoking: development of a gender-specific photonumeric scale. J Am Acad Dermatol. 2016;74:924-930.
- Iblher N, Stark GB, Penna V. The aging perioral region—do we really know what is happening? J Nutr Health Aging. 2012;16:581-585.
- Sarnoff DS, Gotkin RH. Six steps to the “perfect” lip. J Drugs Dermatol. 2012;11:1081-1088.
- American Society of Plastic Surgeons. 2015 Cosmetic plastic surgery statistics. https://d2wirczt3b6wjm.cloudfront.net/News/Statistics/2015/cosmetic-procedure-trends-2015.pdf. Published February 26, 2015. Accessed October 5, 2016.
- Abduljabbar MH, Basendwh MA. Complications of hyaluronic acid fillers and their managements. J Dermatol Surg. 2016;20:1-7.
- Luebberding S, Alexiades-Armenakas M. Facial volume augmentation in 2014: overview of different filler options. J Drugs Dermatol. 2013;12:1339-1344.
- Huang Attenello N, Mass CS. Injectable fillers: review of material and properties. Facial Plast Surg. 2015;31:29-34.
- Eccleston D, Murphy DK. Juvéderm Volbella in the perioral area: a 12-month perspective, multicenter, open-label study. Clin Cosmet Investig Dermatol. 2012;5:167-172.
- Raspaldo H, Chantrey J, Belhaouari L, et al. Lip and perioral enhancement: a 12-month prospective, randomized, controlled study. J Drugs Dermatol. 2015;14:1444-1452.
- Soft tissue fillers approved by the center for devices and radiological health. US Food and Drug Administration website. http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/CosmeticDevices/WrinkleFillers/ucm227749.htm. Updated July 27, 2015. Accessed October 5, 2016.
- Butterwick K, Marmur E, Narurkar V, et al. HYC-24L demonstrates greater effectiveness with less pain than CPM-22.5 for treatment of perioral lines in a randomized controlled trial. Dermatol Surg. 2015;41:1351-1360.
- San Miguel Moragas J, Reddy RR, Hernández Alfaro F, et al. Systematic review of “filling” procedures for lip augmentation regarding types of material, outcomes and complications. J Craniomaxillofac Surg. 2015;43:883-906.
- Cohen JL, Thomas J, Paradkar D, et al. An interrater and intrarater reliability study of 3 photographic scales for the classification of perioral aesthetic features. Dermatol Surg. 2014;40:663-670.
- Broder KW, Cohen SR. An overview of permanent and semipermanent fillers. Plast Reconstr Surg. 2006;118(3 suppl):7S-14S.
- Sarnoff DS, Saini R, Gotkin RH. Comparison of filling agents for lip augmentation. Aesthet Surg J. 2008;28:556-563.
- Moscona RA, Fodor L. A retrospective study on liquid injectable silicone for lip augmentation: long-term results and patient satisfaction. J Plast Reconstr Aesthet Surg. 2010;63:1694-1698.
- Bertucci V, Lynde CB. Current concepts in the use of small-particle hyaluronic acid. Plast Reconstr Surg. 2015;136(5 suppl):132S-138S.
- Wilson AJ, Taglienti AJ, Chang CS, et al. Current applications of facial volumization with fillers. Plast Reconstr Surg. 2016;137:E872-E889.
- Dewandre L, Caperton C, Fulton J. Filler injections with the blunt-tip microcannula compared to the sharp hypodermic needle. J Drugs Dermatol. 2012;11:1098-1103.
- Carruthers JD, Glogau RG, Blitzer A; Facial Aesthetics Consensus Group Faculty. Advances in facial rejuvenation: botulinum toxin type A, hyaluronic acid dermal fillers, and combination therapies-consensus recommendations. Plast Reconstr Surg. 2008;121(5 suppl):5S-30S.
- Wu DC, Fabi SG, Goldman MP. Neurotoxins: current concepts in cosmetic use on the face and neck-lower face. Plast Reconstr Surg. 2015;136(5 suppl):76S-79S.
- Carruthers A, Carruthers J, Monheit GD, et al. Multicenter, randomized, parallel-group study of the safety and effectiveness of onabotulinumtoxin A and hyaluronic acid dermal fillers (24-mg/mL smooth, cohesive gel) alone and in combination for lower facial rejuvenation. Dermatol Surg. 2010;36:2121-2134.
Historically, a variety of tools have been used to alter one’s appearance for cultural or religious purposes or to conform to standards of beauty. As a defining feature of the face, the lips provide a unique opportunity for facial aesthetic enhancement. There has been a paradigm shift in medicine favoring preventative health and a desire to slow and even reverse the aging process.1 Acknowledging that product technology, skill sets, and cultural ideals continually evolve, this article highlights perioral anatomy, explains aging of the lower face, and reviews techniques to achieve perioral rejuvenation through volume restoration and muscle control.
Perioral Anatomy
The layers of the lips include the epidermis, subcutaneous tissue, orbicularis oris muscle fibers, and mucosa. The upper lip extends from the base of the nose to the mucosa inferiorly and to the nasolabial folds laterally. The curvilinear lower lip extends from the mucosa to the mandible inferiorly and to the oral commissures laterally.2 Circumferential at the vermilion-cutaneous junction, a raised area of pale skin known as the white roll accentuates the vermilion border and provides an important landmark during lip augmentation.3 At the upper lip, this elevation of the vermilion joins at a V-shaped depression centrally to form the Cupid’s bow. The cutaneous upper lip has 2 raised vertical pillars known as the philtral columns, which are formed from decussating fibers of the orbicularis oris muscle.2 The resultant midline depression is the philtrum. These defining features of the upper lip are to be preserved during augmentation procedures (Figure 1).4

The superior and inferior labial arteries, both branches of the facial artery, supply the upper and lower lip, respectively. The anastomotic arch of the superior labial artery is susceptible to injury from deep injection of the upper lip between the muscle layer and mucosa; therefore, caution must be exercised in this area.5 Injections into the vermilion and lower lip can be safely performed with less concern for vascular compromise. The vermilion derives its red color from the translucency of capillaries in the superficial papillae.2 The capillary plexus at the papillae and rich sensory nerve network render the lip a highly vascular and sensitive structure.
Aging of the Lower Face
Subcutaneous fat atrophy, loss of elasticity, gravitational forces, and remodeling of the skeletal foundation all contribute to aging of the lower face. Starting as early as the third decade of life, intrinsic factors including hormonal changes and genetically determined processes produce alterations in skin quality and structure. Similarly, extrinsic aging through environmental influences, namely exposure to UV radiation and smoking, accelerate the loss of skin integrity.6
The decreased laxity of the skin in combination with repeated contraction of the orbicularis oris muscle results in perioral rhytides.7 For women in particular, vertically oriented perioral rhytides develop above the vermilion; terminal hair follicles, thicker skin, and a greater density of subcutaneous fat are presumptive protective factors for males.8 With time, the cutaneous portion of the upper lip lengthens and there is redistribution of volume with effacement of the upper lip vermilion.9 Additionally, the demarcation of the vermilion becomes blurred secondary to pallor, flattening of the philtral columns, and loss of projection of the Cupid’s bow.10
Downturning of the oral commissures is observed secondary to a combination of gravity, bone resorption, and soft tissue volume loss. Hyperactivity of the depressor anguli oris muscle exacerbates the mesolabial folds, producing marionette lines and a saddened expression.7 With ongoing volume loss and ligament laxity, tissue redistributes near the jaws and chin, giving rise to jowls. Similarly, perioral volume loss and descent of the malar fat-pad deepen the nasolabial folds in the aging midface.6
The main objective of perioral rejuvenation is to reinstate a harmonious refreshed look to the lower face; however, aesthetic analysis should occur within the context of the face as a whole, as the lips should complement the surrounding perioral cosmetic unit and overall skeletal foundation of the face. To accomplish this goal, the dermatologist’s armamentarium contains a broad variety of approaches including restriction of muscle movement, volume restoration, and surface contouring.
Volume Restoration
Treatment Options
In 2015, hyaluronic acid (HA) fillers constituted 80% of all injectable soft-tissue fillers, an 8% increase from 2014.11 Hyaluronic acid has achieved immense popularity as a temporary dermal filler given its biocompatibility, longevity, and reversibility via hyaluronidase.12
Hyaluronic acid is a naturally occurring glycosaminoglycan that comprises the connective tissue matrix. The molecular composition affords HA its hydrophilic property, which augments dermal volume.7 Endogenous HA has a short half-life, and chemical modification by a cross-linking process extends longevity by 6 to 12 months. The various HA fillers are distinguished by method of purification, size of molecules, concentration and degree of cross-linking, and viscosity.7,13,14 These differences dictate overall clinical performance such as flow properties, longevity, and stability. As a general rule, a high-viscosity product is more appropriate for deeper augmentation; fillers with low viscosity are more appropriate for correction of shallow defects.1 Table 1 lists the HA fillers that are currently approved by the US Food and Drug Administration for lip augmentation and/or perioral rhytides in adults 21 years and older.15-17
Randomized controlled trials comparing the efficacy, longevity, and tolerability of different HA products are lacking in the literature and, where present, have strong industry influence.18,19 The advent of assessment scales has provided an objective evaluation of perioral and lip augmentation, facilitating comparisons between products in both clinical research and practice.20

Semipermanent biostimulatory dermal fillers such as calcium hydroxylapatite and poly-L-lactic acid are not recommended for lip augmentation due to an increased incidence of submucosal nodule formation.6,14,21 Likewise, permanent fillers are not recommended given their irreversibility and risk of nodule formation around the lips.14,22 Nonetheless, liquid silicone (purified polydimethylsiloxane) administered via a microdroplet technique (0.01 mL of silicone at a time, no more than 1 cc per lip per session) has been used off label as a permanent filling agent for lip augmentation with limited complications.23 Regardless, trepidations about its use with respect to reported risks continue to limit its application.22
Similarly, surgical lip implants such as expanded polytetrafluoroethylene is an option for a subset of patients desiring permanent enhancement but are less commonly utilized given the side-effect profile, irreversibility, and relatively invasive nature of the procedure.22 Lastly, autologous fat transfer has been used in correction of the nasolabial and mesolabial folds as well as in lip augmentation; however, irregular surface contours and unpredictable longevity secondary to postinjection resorption (20%–90%) has limited its popularity.3,14,21
HA Injection Technique
With respect to HA fillers in the perioral area, numerous approaches have been described.10,22 The techniques in Table 2 provide a foundation for lip rejuvenation.

Several injection techniques exist, including serial puncture, linear threading, cross-hatching, and fanning in a retrograde or anterograde manner.24 A blunt microcannula (27 gauge, 38 mm) may be used in place of sharp needles and offers the benefit of increased patient comfort, reduced edema and ecchymosis, and shortened recovery period.25,26 Gentle massage of the product after injection can assist with an even contour. Lastly, a key determinant of successful outcomes is using an adequate volume of HA filler (1–2 mL for shaping the vermilion border and volumizing the lips).27 Figure 2 highlights a clinical example of HA filler for lip augmentation.

Fortunately, most complications encountered with HA lip augmentation are mild and transient. The most commonly observed side effects include injection-site reactions such as pain, erythema, and edema. Similarly, most adverse effects are related to injection technique. All HA fillers are prone to the Tyndall effect, a consequence of too superficial an injection plane. Patients with history of recurrent herpes simplex virus infections should receive prophylactic antiviral therapy.12
Muscle Control
An emerging concept in rejuvenation of the lower face recognizes not only restoration of volume but also control of muscle movement. Local injection of botulinum toxin type A induces relaxation of hyperfunctional facial muscles through temporary inhibition of neurotransmitter release.6 The potential for paralysis of the oral cavity may limit the application of botulinum toxin type A in that region.7 Nonetheless, the off-label potential of botulinum toxin type A has expanded to include several targets in the lower face. The orbicularis oris muscle is targeted to soften perioral rhytides. Conservative dosing (1–2 U per lip quadrant or approximately 5 U total) and superficial injection is emphasized in this area.27 Similarly, the depressor anguli oris muscle is targeted by injection of 4 U bilaterally to soften the marionette lines. In the chin area, the mentalis muscle can be targeted by injection of 2 U deep into each belly of the muscle to reduce the mental crease and dimpling.28 Combination treatment with dermal filler and neurotoxin demonstrates effects that last longer than either modality alone without additional adverse events.29 With combination therapy, guidelines suggest treating with filler first.27
Conclusion
A greater understanding of the extrinsic and intrinsic factors that contribute to the structural and surface changes of the aging face coupled with a preference for minimally invasive procedures has revolutionized the dermatologist’s approach to perioral rejuvenation. Serving as a focal point of the face, the lips and perioral skin are well poised to benefit from this paradigm shift. A multifaceted approach utilizing dermal fillers and neurotoxins may be most appropriate and has demonstrated optimal outcomes in facial aesthetics.
Historically, a variety of tools have been used to alter one’s appearance for cultural or religious purposes or to conform to standards of beauty. As a defining feature of the face, the lips provide a unique opportunity for facial aesthetic enhancement. There has been a paradigm shift in medicine favoring preventative health and a desire to slow and even reverse the aging process.1 Acknowledging that product technology, skill sets, and cultural ideals continually evolve, this article highlights perioral anatomy, explains aging of the lower face, and reviews techniques to achieve perioral rejuvenation through volume restoration and muscle control.
Perioral Anatomy
The layers of the lips include the epidermis, subcutaneous tissue, orbicularis oris muscle fibers, and mucosa. The upper lip extends from the base of the nose to the mucosa inferiorly and to the nasolabial folds laterally. The curvilinear lower lip extends from the mucosa to the mandible inferiorly and to the oral commissures laterally.2 Circumferential at the vermilion-cutaneous junction, a raised area of pale skin known as the white roll accentuates the vermilion border and provides an important landmark during lip augmentation.3 At the upper lip, this elevation of the vermilion joins at a V-shaped depression centrally to form the Cupid’s bow. The cutaneous upper lip has 2 raised vertical pillars known as the philtral columns, which are formed from decussating fibers of the orbicularis oris muscle.2 The resultant midline depression is the philtrum. These defining features of the upper lip are to be preserved during augmentation procedures (Figure 1).4

The superior and inferior labial arteries, both branches of the facial artery, supply the upper and lower lip, respectively. The anastomotic arch of the superior labial artery is susceptible to injury from deep injection of the upper lip between the muscle layer and mucosa; therefore, caution must be exercised in this area.5 Injections into the vermilion and lower lip can be safely performed with less concern for vascular compromise. The vermilion derives its red color from the translucency of capillaries in the superficial papillae.2 The capillary plexus at the papillae and rich sensory nerve network render the lip a highly vascular and sensitive structure.
Aging of the Lower Face
Subcutaneous fat atrophy, loss of elasticity, gravitational forces, and remodeling of the skeletal foundation all contribute to aging of the lower face. Starting as early as the third decade of life, intrinsic factors including hormonal changes and genetically determined processes produce alterations in skin quality and structure. Similarly, extrinsic aging through environmental influences, namely exposure to UV radiation and smoking, accelerate the loss of skin integrity.6
The decreased laxity of the skin in combination with repeated contraction of the orbicularis oris muscle results in perioral rhytides.7 For women in particular, vertically oriented perioral rhytides develop above the vermilion; terminal hair follicles, thicker skin, and a greater density of subcutaneous fat are presumptive protective factors for males.8 With time, the cutaneous portion of the upper lip lengthens and there is redistribution of volume with effacement of the upper lip vermilion.9 Additionally, the demarcation of the vermilion becomes blurred secondary to pallor, flattening of the philtral columns, and loss of projection of the Cupid’s bow.10
Downturning of the oral commissures is observed secondary to a combination of gravity, bone resorption, and soft tissue volume loss. Hyperactivity of the depressor anguli oris muscle exacerbates the mesolabial folds, producing marionette lines and a saddened expression.7 With ongoing volume loss and ligament laxity, tissue redistributes near the jaws and chin, giving rise to jowls. Similarly, perioral volume loss and descent of the malar fat-pad deepen the nasolabial folds in the aging midface.6
The main objective of perioral rejuvenation is to reinstate a harmonious refreshed look to the lower face; however, aesthetic analysis should occur within the context of the face as a whole, as the lips should complement the surrounding perioral cosmetic unit and overall skeletal foundation of the face. To accomplish this goal, the dermatologist’s armamentarium contains a broad variety of approaches including restriction of muscle movement, volume restoration, and surface contouring.
Volume Restoration
Treatment Options
In 2015, hyaluronic acid (HA) fillers constituted 80% of all injectable soft-tissue fillers, an 8% increase from 2014.11 Hyaluronic acid has achieved immense popularity as a temporary dermal filler given its biocompatibility, longevity, and reversibility via hyaluronidase.12
Hyaluronic acid is a naturally occurring glycosaminoglycan that comprises the connective tissue matrix. The molecular composition affords HA its hydrophilic property, which augments dermal volume.7 Endogenous HA has a short half-life, and chemical modification by a cross-linking process extends longevity by 6 to 12 months. The various HA fillers are distinguished by method of purification, size of molecules, concentration and degree of cross-linking, and viscosity.7,13,14 These differences dictate overall clinical performance such as flow properties, longevity, and stability. As a general rule, a high-viscosity product is more appropriate for deeper augmentation; fillers with low viscosity are more appropriate for correction of shallow defects.1 Table 1 lists the HA fillers that are currently approved by the US Food and Drug Administration for lip augmentation and/or perioral rhytides in adults 21 years and older.15-17
Randomized controlled trials comparing the efficacy, longevity, and tolerability of different HA products are lacking in the literature and, where present, have strong industry influence.18,19 The advent of assessment scales has provided an objective evaluation of perioral and lip augmentation, facilitating comparisons between products in both clinical research and practice.20

Semipermanent biostimulatory dermal fillers such as calcium hydroxylapatite and poly-L-lactic acid are not recommended for lip augmentation due to an increased incidence of submucosal nodule formation.6,14,21 Likewise, permanent fillers are not recommended given their irreversibility and risk of nodule formation around the lips.14,22 Nonetheless, liquid silicone (purified polydimethylsiloxane) administered via a microdroplet technique (0.01 mL of silicone at a time, no more than 1 cc per lip per session) has been used off label as a permanent filling agent for lip augmentation with limited complications.23 Regardless, trepidations about its use with respect to reported risks continue to limit its application.22
Similarly, surgical lip implants such as expanded polytetrafluoroethylene is an option for a subset of patients desiring permanent enhancement but are less commonly utilized given the side-effect profile, irreversibility, and relatively invasive nature of the procedure.22 Lastly, autologous fat transfer has been used in correction of the nasolabial and mesolabial folds as well as in lip augmentation; however, irregular surface contours and unpredictable longevity secondary to postinjection resorption (20%–90%) has limited its popularity.3,14,21
HA Injection Technique
With respect to HA fillers in the perioral area, numerous approaches have been described.10,22 The techniques in Table 2 provide a foundation for lip rejuvenation.

Several injection techniques exist, including serial puncture, linear threading, cross-hatching, and fanning in a retrograde or anterograde manner.24 A blunt microcannula (27 gauge, 38 mm) may be used in place of sharp needles and offers the benefit of increased patient comfort, reduced edema and ecchymosis, and shortened recovery period.25,26 Gentle massage of the product after injection can assist with an even contour. Lastly, a key determinant of successful outcomes is using an adequate volume of HA filler (1–2 mL for shaping the vermilion border and volumizing the lips).27 Figure 2 highlights a clinical example of HA filler for lip augmentation.

Fortunately, most complications encountered with HA lip augmentation are mild and transient. The most commonly observed side effects include injection-site reactions such as pain, erythema, and edema. Similarly, most adverse effects are related to injection technique. All HA fillers are prone to the Tyndall effect, a consequence of too superficial an injection plane. Patients with history of recurrent herpes simplex virus infections should receive prophylactic antiviral therapy.12
Muscle Control
An emerging concept in rejuvenation of the lower face recognizes not only restoration of volume but also control of muscle movement. Local injection of botulinum toxin type A induces relaxation of hyperfunctional facial muscles through temporary inhibition of neurotransmitter release.6 The potential for paralysis of the oral cavity may limit the application of botulinum toxin type A in that region.7 Nonetheless, the off-label potential of botulinum toxin type A has expanded to include several targets in the lower face. The orbicularis oris muscle is targeted to soften perioral rhytides. Conservative dosing (1–2 U per lip quadrant or approximately 5 U total) and superficial injection is emphasized in this area.27 Similarly, the depressor anguli oris muscle is targeted by injection of 4 U bilaterally to soften the marionette lines. In the chin area, the mentalis muscle can be targeted by injection of 2 U deep into each belly of the muscle to reduce the mental crease and dimpling.28 Combination treatment with dermal filler and neurotoxin demonstrates effects that last longer than either modality alone without additional adverse events.29 With combination therapy, guidelines suggest treating with filler first.27
Conclusion
A greater understanding of the extrinsic and intrinsic factors that contribute to the structural and surface changes of the aging face coupled with a preference for minimally invasive procedures has revolutionized the dermatologist’s approach to perioral rejuvenation. Serving as a focal point of the face, the lips and perioral skin are well poised to benefit from this paradigm shift. A multifaceted approach utilizing dermal fillers and neurotoxins may be most appropriate and has demonstrated optimal outcomes in facial aesthetics.
- Buck DW, Alam M, Kim JYS. Injectable fillers for facial rejuvenation: a review. J Plast Reconstr Aesthet Surg. 2009;62:11-18.
- Guareschi M, Stella E. Lips. In: Goisis M, ed. Injections in Aesthetic Medicine. Milan, Italy: Springer; 2014:125-136.
- Byrne PJ, Hilger PA. Lip augmentation. Facial Plast Surg. 2004;20:31-38.
- Niamtu J. Rejuvenation of the lip and perioral areas. In: Bell WH, Guerroro CA, eds. Distraction Osteogenesis of the Facial Skeleton. Ontario, Canada: BC Decker Inc; 2007:38-48.
- Tansatit T, Apinuntrum P, Phetudom T. A typical pattern of the labial arteries with implication for lip augmentation with injectable fillers. Aesthet Plast Surg. 2014;38:1083-1089.
- Sadick NS, Karcher C, Palmisano L. Cosmetic dermatology of the aging face. Clin Dermatol. 2009;27(suppl):S3-S12.
- Ali MJ, Ende K, Mass CS. Perioral rejuvenation and lip augmentation. Facial Plast Surg Clin N Am. 2007;15:491-500.
- Chien AL, Qi J, Cheng N, et al. Perioral wrinkles are associated with female gender, aging, and smoking: development of a gender-specific photonumeric scale. J Am Acad Dermatol. 2016;74:924-930.
- Iblher N, Stark GB, Penna V. The aging perioral region—do we really know what is happening? J Nutr Health Aging. 2012;16:581-585.
- Sarnoff DS, Gotkin RH. Six steps to the “perfect” lip. J Drugs Dermatol. 2012;11:1081-1088.
- American Society of Plastic Surgeons. 2015 Cosmetic plastic surgery statistics. https://d2wirczt3b6wjm.cloudfront.net/News/Statistics/2015/cosmetic-procedure-trends-2015.pdf. Published February 26, 2015. Accessed October 5, 2016.
- Abduljabbar MH, Basendwh MA. Complications of hyaluronic acid fillers and their managements. J Dermatol Surg. 2016;20:1-7.
- Luebberding S, Alexiades-Armenakas M. Facial volume augmentation in 2014: overview of different filler options. J Drugs Dermatol. 2013;12:1339-1344.
- Huang Attenello N, Mass CS. Injectable fillers: review of material and properties. Facial Plast Surg. 2015;31:29-34.
- Eccleston D, Murphy DK. Juvéderm Volbella in the perioral area: a 12-month perspective, multicenter, open-label study. Clin Cosmet Investig Dermatol. 2012;5:167-172.
- Raspaldo H, Chantrey J, Belhaouari L, et al. Lip and perioral enhancement: a 12-month prospective, randomized, controlled study. J Drugs Dermatol. 2015;14:1444-1452.
- Soft tissue fillers approved by the center for devices and radiological health. US Food and Drug Administration website. http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/CosmeticDevices/WrinkleFillers/ucm227749.htm. Updated July 27, 2015. Accessed October 5, 2016.
- Butterwick K, Marmur E, Narurkar V, et al. HYC-24L demonstrates greater effectiveness with less pain than CPM-22.5 for treatment of perioral lines in a randomized controlled trial. Dermatol Surg. 2015;41:1351-1360.
- San Miguel Moragas J, Reddy RR, Hernández Alfaro F, et al. Systematic review of “filling” procedures for lip augmentation regarding types of material, outcomes and complications. J Craniomaxillofac Surg. 2015;43:883-906.
- Cohen JL, Thomas J, Paradkar D, et al. An interrater and intrarater reliability study of 3 photographic scales for the classification of perioral aesthetic features. Dermatol Surg. 2014;40:663-670.
- Broder KW, Cohen SR. An overview of permanent and semipermanent fillers. Plast Reconstr Surg. 2006;118(3 suppl):7S-14S.
- Sarnoff DS, Saini R, Gotkin RH. Comparison of filling agents for lip augmentation. Aesthet Surg J. 2008;28:556-563.
- Moscona RA, Fodor L. A retrospective study on liquid injectable silicone for lip augmentation: long-term results and patient satisfaction. J Plast Reconstr Aesthet Surg. 2010;63:1694-1698.
- Bertucci V, Lynde CB. Current concepts in the use of small-particle hyaluronic acid. Plast Reconstr Surg. 2015;136(5 suppl):132S-138S.
- Wilson AJ, Taglienti AJ, Chang CS, et al. Current applications of facial volumization with fillers. Plast Reconstr Surg. 2016;137:E872-E889.
- Dewandre L, Caperton C, Fulton J. Filler injections with the blunt-tip microcannula compared to the sharp hypodermic needle. J Drugs Dermatol. 2012;11:1098-1103.
- Carruthers JD, Glogau RG, Blitzer A; Facial Aesthetics Consensus Group Faculty. Advances in facial rejuvenation: botulinum toxin type A, hyaluronic acid dermal fillers, and combination therapies-consensus recommendations. Plast Reconstr Surg. 2008;121(5 suppl):5S-30S.
- Wu DC, Fabi SG, Goldman MP. Neurotoxins: current concepts in cosmetic use on the face and neck-lower face. Plast Reconstr Surg. 2015;136(5 suppl):76S-79S.
- Carruthers A, Carruthers J, Monheit GD, et al. Multicenter, randomized, parallel-group study of the safety and effectiveness of onabotulinumtoxin A and hyaluronic acid dermal fillers (24-mg/mL smooth, cohesive gel) alone and in combination for lower facial rejuvenation. Dermatol Surg. 2010;36:2121-2134.
- Buck DW, Alam M, Kim JYS. Injectable fillers for facial rejuvenation: a review. J Plast Reconstr Aesthet Surg. 2009;62:11-18.
- Guareschi M, Stella E. Lips. In: Goisis M, ed. Injections in Aesthetic Medicine. Milan, Italy: Springer; 2014:125-136.
- Byrne PJ, Hilger PA. Lip augmentation. Facial Plast Surg. 2004;20:31-38.
- Niamtu J. Rejuvenation of the lip and perioral areas. In: Bell WH, Guerroro CA, eds. Distraction Osteogenesis of the Facial Skeleton. Ontario, Canada: BC Decker Inc; 2007:38-48.
- Tansatit T, Apinuntrum P, Phetudom T. A typical pattern of the labial arteries with implication for lip augmentation with injectable fillers. Aesthet Plast Surg. 2014;38:1083-1089.
- Sadick NS, Karcher C, Palmisano L. Cosmetic dermatology of the aging face. Clin Dermatol. 2009;27(suppl):S3-S12.
- Ali MJ, Ende K, Mass CS. Perioral rejuvenation and lip augmentation. Facial Plast Surg Clin N Am. 2007;15:491-500.
- Chien AL, Qi J, Cheng N, et al. Perioral wrinkles are associated with female gender, aging, and smoking: development of a gender-specific photonumeric scale. J Am Acad Dermatol. 2016;74:924-930.
- Iblher N, Stark GB, Penna V. The aging perioral region—do we really know what is happening? J Nutr Health Aging. 2012;16:581-585.
- Sarnoff DS, Gotkin RH. Six steps to the “perfect” lip. J Drugs Dermatol. 2012;11:1081-1088.
- American Society of Plastic Surgeons. 2015 Cosmetic plastic surgery statistics. https://d2wirczt3b6wjm.cloudfront.net/News/Statistics/2015/cosmetic-procedure-trends-2015.pdf. Published February 26, 2015. Accessed October 5, 2016.
- Abduljabbar MH, Basendwh MA. Complications of hyaluronic acid fillers and their managements. J Dermatol Surg. 2016;20:1-7.
- Luebberding S, Alexiades-Armenakas M. Facial volume augmentation in 2014: overview of different filler options. J Drugs Dermatol. 2013;12:1339-1344.
- Huang Attenello N, Mass CS. Injectable fillers: review of material and properties. Facial Plast Surg. 2015;31:29-34.
- Eccleston D, Murphy DK. Juvéderm Volbella in the perioral area: a 12-month perspective, multicenter, open-label study. Clin Cosmet Investig Dermatol. 2012;5:167-172.
- Raspaldo H, Chantrey J, Belhaouari L, et al. Lip and perioral enhancement: a 12-month prospective, randomized, controlled study. J Drugs Dermatol. 2015;14:1444-1452.
- Soft tissue fillers approved by the center for devices and radiological health. US Food and Drug Administration website. http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/CosmeticDevices/WrinkleFillers/ucm227749.htm. Updated July 27, 2015. Accessed October 5, 2016.
- Butterwick K, Marmur E, Narurkar V, et al. HYC-24L demonstrates greater effectiveness with less pain than CPM-22.5 for treatment of perioral lines in a randomized controlled trial. Dermatol Surg. 2015;41:1351-1360.
- San Miguel Moragas J, Reddy RR, Hernández Alfaro F, et al. Systematic review of “filling” procedures for lip augmentation regarding types of material, outcomes and complications. J Craniomaxillofac Surg. 2015;43:883-906.
- Cohen JL, Thomas J, Paradkar D, et al. An interrater and intrarater reliability study of 3 photographic scales for the classification of perioral aesthetic features. Dermatol Surg. 2014;40:663-670.
- Broder KW, Cohen SR. An overview of permanent and semipermanent fillers. Plast Reconstr Surg. 2006;118(3 suppl):7S-14S.
- Sarnoff DS, Saini R, Gotkin RH. Comparison of filling agents for lip augmentation. Aesthet Surg J. 2008;28:556-563.
- Moscona RA, Fodor L. A retrospective study on liquid injectable silicone for lip augmentation: long-term results and patient satisfaction. J Plast Reconstr Aesthet Surg. 2010;63:1694-1698.
- Bertucci V, Lynde CB. Current concepts in the use of small-particle hyaluronic acid. Plast Reconstr Surg. 2015;136(5 suppl):132S-138S.
- Wilson AJ, Taglienti AJ, Chang CS, et al. Current applications of facial volumization with fillers. Plast Reconstr Surg. 2016;137:E872-E889.
- Dewandre L, Caperton C, Fulton J. Filler injections with the blunt-tip microcannula compared to the sharp hypodermic needle. J Drugs Dermatol. 2012;11:1098-1103.
- Carruthers JD, Glogau RG, Blitzer A; Facial Aesthetics Consensus Group Faculty. Advances in facial rejuvenation: botulinum toxin type A, hyaluronic acid dermal fillers, and combination therapies-consensus recommendations. Plast Reconstr Surg. 2008;121(5 suppl):5S-30S.
- Wu DC, Fabi SG, Goldman MP. Neurotoxins: current concepts in cosmetic use on the face and neck-lower face. Plast Reconstr Surg. 2015;136(5 suppl):76S-79S.
- Carruthers A, Carruthers J, Monheit GD, et al. Multicenter, randomized, parallel-group study of the safety and effectiveness of onabotulinumtoxin A and hyaluronic acid dermal fillers (24-mg/mL smooth, cohesive gel) alone and in combination for lower facial rejuvenation. Dermatol Surg. 2010;36:2121-2134.
Practice Points
- Hyaluronic acid (HA) fillers are approved by the US Food and Drug Administration for lip augmentation and/or treatment of perioral rhytides in adults 21 years and older.
- Most complications encountered with HA lip augmentation are mild and transient and can include injection-site reactions such as pain, erythema, and edema.
- Combination treatment with dermal fillers and neurotoxins (off label) may demonstrate effects that last longer than either modality alone without additional adverse events.
AGA members take Capitol Hill
More than 40 AGA members, representing 24 states, visited Capitol Hill earlier this fall to fight for the science and practice of gastroenterology during AGA’s annual Advocacy Day.
NIH funding
AGA members met with lawmakers and their staffs on Sept. 16 to discuss the success that research and medical breakthroughs have had for their patients and encouraged Congress to support increased funding for NIH. Many members of Congress support increased funding for NIH, but a small group of House members is preventing passage of a bill that would increase funding for the institute by $1.25 billion in fiscal year (FY) 2017. The Senate Appropriations Committee passed a bill in June, on a vote of 29-1, to increase funding for NIH by $2 billion. Advocacy Day attendees urged their congressional offices to support the higher Senate number.
MACRA implementation
Members also discussed the need for congressional oversight to ensure that the Medicare Access & CHIP Reauthorization Act of 2015 (MACRA) law is being implemented as Congress intended. They shared their concerns over the impact this law could have on gastroenterologists, especially those in small or solo practices, and emphasized the need for CMS to provide flexibility to physicians to enable them to comply with the new requirements.
Participants highlighted the recent announcement that CMS will allow physicians more options for reporting in the first year as a positive sign that it is listening to the concerns voiced by Congress and the physician community on the regulatory burdens. AGA members also discussed the barriers that currently exist in qualifying as a specialty-focused alternative payment model (APM) and the need for continued flexibility to ensure that all physicians have the opportunity to participate in more value-based payment models.
Virtual advocacy
In conjunction with the Capitol Hill meetings, all AGA members were invited to participate in a Virtual Advocacy Day campaign. This additional component allowed all members to contact their members of Congress via email to voice their concerns about sustainable NIH funding for FY 2017 and the need for more congressional oversight of MACRA.
It’s not too late for you to show your support for the science and practice of GI. Contact your congressional representatives in support of important issues at www.gastroadvocacy.org/actionalerts.aspx.
More than 40 AGA members, representing 24 states, visited Capitol Hill earlier this fall to fight for the science and practice of gastroenterology during AGA’s annual Advocacy Day.
NIH funding
AGA members met with lawmakers and their staffs on Sept. 16 to discuss the success that research and medical breakthroughs have had for their patients and encouraged Congress to support increased funding for NIH. Many members of Congress support increased funding for NIH, but a small group of House members is preventing passage of a bill that would increase funding for the institute by $1.25 billion in fiscal year (FY) 2017. The Senate Appropriations Committee passed a bill in June, on a vote of 29-1, to increase funding for NIH by $2 billion. Advocacy Day attendees urged their congressional offices to support the higher Senate number.
MACRA implementation
Members also discussed the need for congressional oversight to ensure that the Medicare Access & CHIP Reauthorization Act of 2015 (MACRA) law is being implemented as Congress intended. They shared their concerns over the impact this law could have on gastroenterologists, especially those in small or solo practices, and emphasized the need for CMS to provide flexibility to physicians to enable them to comply with the new requirements.
Participants highlighted the recent announcement that CMS will allow physicians more options for reporting in the first year as a positive sign that it is listening to the concerns voiced by Congress and the physician community on the regulatory burdens. AGA members also discussed the barriers that currently exist in qualifying as a specialty-focused alternative payment model (APM) and the need for continued flexibility to ensure that all physicians have the opportunity to participate in more value-based payment models.
Virtual advocacy
In conjunction with the Capitol Hill meetings, all AGA members were invited to participate in a Virtual Advocacy Day campaign. This additional component allowed all members to contact their members of Congress via email to voice their concerns about sustainable NIH funding for FY 2017 and the need for more congressional oversight of MACRA.
It’s not too late for you to show your support for the science and practice of GI. Contact your congressional representatives in support of important issues at www.gastroadvocacy.org/actionalerts.aspx.
More than 40 AGA members, representing 24 states, visited Capitol Hill earlier this fall to fight for the science and practice of gastroenterology during AGA’s annual Advocacy Day.
NIH funding
AGA members met with lawmakers and their staffs on Sept. 16 to discuss the success that research and medical breakthroughs have had for their patients and encouraged Congress to support increased funding for NIH. Many members of Congress support increased funding for NIH, but a small group of House members is preventing passage of a bill that would increase funding for the institute by $1.25 billion in fiscal year (FY) 2017. The Senate Appropriations Committee passed a bill in June, on a vote of 29-1, to increase funding for NIH by $2 billion. Advocacy Day attendees urged their congressional offices to support the higher Senate number.
MACRA implementation
Members also discussed the need for congressional oversight to ensure that the Medicare Access & CHIP Reauthorization Act of 2015 (MACRA) law is being implemented as Congress intended. They shared their concerns over the impact this law could have on gastroenterologists, especially those in small or solo practices, and emphasized the need for CMS to provide flexibility to physicians to enable them to comply with the new requirements.
Participants highlighted the recent announcement that CMS will allow physicians more options for reporting in the first year as a positive sign that it is listening to the concerns voiced by Congress and the physician community on the regulatory burdens. AGA members also discussed the barriers that currently exist in qualifying as a specialty-focused alternative payment model (APM) and the need for continued flexibility to ensure that all physicians have the opportunity to participate in more value-based payment models.
Virtual advocacy
In conjunction with the Capitol Hill meetings, all AGA members were invited to participate in a Virtual Advocacy Day campaign. This additional component allowed all members to contact their members of Congress via email to voice their concerns about sustainable NIH funding for FY 2017 and the need for more congressional oversight of MACRA.
It’s not too late for you to show your support for the science and practice of GI. Contact your congressional representatives in support of important issues at www.gastroadvocacy.org/actionalerts.aspx.
2017 Membership renewal period now open
AGA is committed to your success in gastroenterology. Make sure to renew your membership at www.gastro.org/renew to maintain your benefits and stay up to date on the latest discoveries in GI through subscriptions to leading publications. These include Gastroenterology, Clinical Gastroenterology and Hepatology, and Cellular and Molecular Gastroenterology and Hepatology, as well as members-only publications covering leading trends in the field.
Renew today and also maintain access to the AGA Community forum, where you can share topics and challenges spanning the GI landscape. You can also sign up for up to 6 of 13 section affiliations to discuss the needs and issues facing GI professionals.
Take advantage of these and other career-enhancing, members-only benefits like valuable patient care resources or research funding and publishing opportunities.
Renew before the Dec. 1 deadline.
While renewing your membership, please take a moment to update your AGA member profile and communications preferences to receive content customized to your needs and interests.
If you have any questions, please contact AGA Member Relations and Constituency Programs at [email protected] or 301-941-2651.
AGA is committed to your success in gastroenterology. Make sure to renew your membership at www.gastro.org/renew to maintain your benefits and stay up to date on the latest discoveries in GI through subscriptions to leading publications. These include Gastroenterology, Clinical Gastroenterology and Hepatology, and Cellular and Molecular Gastroenterology and Hepatology, as well as members-only publications covering leading trends in the field.
Renew today and also maintain access to the AGA Community forum, where you can share topics and challenges spanning the GI landscape. You can also sign up for up to 6 of 13 section affiliations to discuss the needs and issues facing GI professionals.
Take advantage of these and other career-enhancing, members-only benefits like valuable patient care resources or research funding and publishing opportunities.
Renew before the Dec. 1 deadline.
While renewing your membership, please take a moment to update your AGA member profile and communications preferences to receive content customized to your needs and interests.
If you have any questions, please contact AGA Member Relations and Constituency Programs at [email protected] or 301-941-2651.
AGA is committed to your success in gastroenterology. Make sure to renew your membership at www.gastro.org/renew to maintain your benefits and stay up to date on the latest discoveries in GI through subscriptions to leading publications. These include Gastroenterology, Clinical Gastroenterology and Hepatology, and Cellular and Molecular Gastroenterology and Hepatology, as well as members-only publications covering leading trends in the field.
Renew today and also maintain access to the AGA Community forum, where you can share topics and challenges spanning the GI landscape. You can also sign up for up to 6 of 13 section affiliations to discuss the needs and issues facing GI professionals.
Take advantage of these and other career-enhancing, members-only benefits like valuable patient care resources or research funding and publishing opportunities.
Renew before the Dec. 1 deadline.
While renewing your membership, please take a moment to update your AGA member profile and communications preferences to receive content customized to your needs and interests.
If you have any questions, please contact AGA Member Relations and Constituency Programs at [email protected] or 301-941-2651.
Support young researchers through the AGA Research Foundation
The AGA Research Foundation plays an important role in medical research by providing grants to young scientists at a critical time in their careers. In the 2016 research award cycle, the foundation provided more than $2.5 million in research award support to 66 scientists. We funded four Research Scholar Award (RSA) recipients; outside of the RSAs, the foundation provided several other funding vehicles for individuals ranging from high school students to established investigators.
With the support of our donors, we are building a community of researchers whose work serves the greater community and benefits all our patients. Here are just a few of the investigators the AGA Research Foundation is funding.
Massachusetts General Hospital
2016 AGA Research Scholar Award Recipient
“I would like to thank the AGA Research Foundation and its donors for their generous support. This award is a critical step in allowing me to develop an infrastructure to support future research examining additional lifestyle risk factors for IBS.” – He will use this research funding to evaluate the association of early-life risk factors and risk of irritable bowel syndrome (IBS) in prospective multicenter cohorts.
University of Pennsylvania
2016 AGA Microbiome Junior Investigator Research Award
“I am extremely honored to have received this award. This grant is instrumental in my career development as a physician-scientist working in the gut microbiome field. The opportunities and resources this award affords will gear me toward achieving my research goals.” – His research project aims to investigate the role of bacterial urease, which hydrolyzes host-derived urea into ammonia in the colon, in host and gut microbiota amino acid metabolism.
Mt. Saint Mary’s University
2016 AGA Investing in the Future Student Research Fellowship
“I would like to thank the AGA Research Foundation for giving me this once-in-a-lifetime opportunity to conduct valuable research under the mantles of so many well-known scientists in the GI field. This award motivates me to continue to strive for excellence in my professional pursuits.” – Elizabeth notes receiving this grant will enable more students from her university to embark on research activities.
Support talented investigators in gastroenterology and hepatology like Drs. Kyle Staller and Ting-Chin Shen, and student Elizabeth Edouard through the AGA Research Foundation. Make a tax-deductible donation today at www.gastro.org/donateonline.
The AGA Research Foundation plays an important role in medical research by providing grants to young scientists at a critical time in their careers. In the 2016 research award cycle, the foundation provided more than $2.5 million in research award support to 66 scientists. We funded four Research Scholar Award (RSA) recipients; outside of the RSAs, the foundation provided several other funding vehicles for individuals ranging from high school students to established investigators.
With the support of our donors, we are building a community of researchers whose work serves the greater community and benefits all our patients. Here are just a few of the investigators the AGA Research Foundation is funding.
Massachusetts General Hospital
2016 AGA Research Scholar Award Recipient
“I would like to thank the AGA Research Foundation and its donors for their generous support. This award is a critical step in allowing me to develop an infrastructure to support future research examining additional lifestyle risk factors for IBS.” – He will use this research funding to evaluate the association of early-life risk factors and risk of irritable bowel syndrome (IBS) in prospective multicenter cohorts.
University of Pennsylvania
2016 AGA Microbiome Junior Investigator Research Award
“I am extremely honored to have received this award. This grant is instrumental in my career development as a physician-scientist working in the gut microbiome field. The opportunities and resources this award affords will gear me toward achieving my research goals.” – His research project aims to investigate the role of bacterial urease, which hydrolyzes host-derived urea into ammonia in the colon, in host and gut microbiota amino acid metabolism.
Mt. Saint Mary’s University
2016 AGA Investing in the Future Student Research Fellowship
“I would like to thank the AGA Research Foundation for giving me this once-in-a-lifetime opportunity to conduct valuable research under the mantles of so many well-known scientists in the GI field. This award motivates me to continue to strive for excellence in my professional pursuits.” – Elizabeth notes receiving this grant will enable more students from her university to embark on research activities.
Support talented investigators in gastroenterology and hepatology like Drs. Kyle Staller and Ting-Chin Shen, and student Elizabeth Edouard through the AGA Research Foundation. Make a tax-deductible donation today at www.gastro.org/donateonline.
The AGA Research Foundation plays an important role in medical research by providing grants to young scientists at a critical time in their careers. In the 2016 research award cycle, the foundation provided more than $2.5 million in research award support to 66 scientists. We funded four Research Scholar Award (RSA) recipients; outside of the RSAs, the foundation provided several other funding vehicles for individuals ranging from high school students to established investigators.
With the support of our donors, we are building a community of researchers whose work serves the greater community and benefits all our patients. Here are just a few of the investigators the AGA Research Foundation is funding.
Massachusetts General Hospital
2016 AGA Research Scholar Award Recipient
“I would like to thank the AGA Research Foundation and its donors for their generous support. This award is a critical step in allowing me to develop an infrastructure to support future research examining additional lifestyle risk factors for IBS.” – He will use this research funding to evaluate the association of early-life risk factors and risk of irritable bowel syndrome (IBS) in prospective multicenter cohorts.
University of Pennsylvania
2016 AGA Microbiome Junior Investigator Research Award
“I am extremely honored to have received this award. This grant is instrumental in my career development as a physician-scientist working in the gut microbiome field. The opportunities and resources this award affords will gear me toward achieving my research goals.” – His research project aims to investigate the role of bacterial urease, which hydrolyzes host-derived urea into ammonia in the colon, in host and gut microbiota amino acid metabolism.
Mt. Saint Mary’s University
2016 AGA Investing in the Future Student Research Fellowship
“I would like to thank the AGA Research Foundation for giving me this once-in-a-lifetime opportunity to conduct valuable research under the mantles of so many well-known scientists in the GI field. This award motivates me to continue to strive for excellence in my professional pursuits.” – Elizabeth notes receiving this grant will enable more students from her university to embark on research activities.
Support talented investigators in gastroenterology and hepatology like Drs. Kyle Staller and Ting-Chin Shen, and student Elizabeth Edouard through the AGA Research Foundation. Make a tax-deductible donation today at www.gastro.org/donateonline.
Choosing Wisely Initiative Helps Physicians Provide Appropriate Care
HILTON HEAD, SC—Physicians sometimes order unnecessary medical tests and procedures for their patients, which results in wasteful spending and inappropriate care. Following medical associations’ practice recommendations, which have been collected by the Choosing Wisely initiative, can help physicians reduce waste in the health care system and provide appropriate treatment for patients, according to an overview presented at the 39th Annual Contemporary Clinical Neurology Symposium.
Avoiding Unnecessary Treatments and Tests
The American Board of Internal Medicine Foundation created the Choosing Wisely website to encourage dialogue between physicians and patients about the overuse of treatments and tests. An additional goal was to empower patients to make informed treatment decisions. More than 70 societies, including the American Academy of Neurology (AAN) and the American Headache Society, submitted recommendations to advise patients and clinicians about proper healthcare. “You can find a list of all the organizations that contributed on the Choosing Wisely website, and each one was asked to contribute five different topics for Choosing Wisely,” said Peter Donofrio, MD, Professor of Neurology at Vanderbilt University in Nashville.
The AAN recommends that clinicians not perform an EEG for headaches. In addition, the organization recommends that physicians not perform imaging of the carotid arteries for simple syncope without other neurologic symptoms. For patients with migraine, opioids or butalbital treatment should be a last resort. The AAN also recommends that doctors not prescribe interferon-beta or glatiramer acetate for patients with disability resulting from progressive, nonrelapsing forms of multiple sclerosis, because the drugs are ineffective. Finally, it advises doctors not to recommend carotid endarterectomy for asymptomatic carotid stenosis unless the complication rate from surgery is less than 3%.
The American Association of Neuromuscular and Electrodiagnostic Medicine recommends that physicians not perform MRI scans of the brain or spine for patients with peripheral neuropathy without signs of cerebral or spinal cord disease. In addition, the association discourages physicians from performing nerve conduction studies (NCSs) without a needle EMG for radiculopathy assessment. It also recommends that physicians not order or perform four-limb EMG/NCS testing for neck or back pain after trauma.
Treating Acute Low Back Pain and Headache
Other medical associations have made recommendations regarding the assessment and treatment of acute low back pain and headache. The North American Spine Society does not recommend advanced imaging of the spine within the first six weeks in patients with nonspecific acute low back pain in the absence of red flags.
The American Headache Society (AHS) recommends that physicians avoid advising prolonged or frequent use of over-the-counter pain medications for headache. The organization also discourages physicians from prescribing opioid or butalbital-containing medications as first-line treatment for recurrent headache disorders. Furthermore, it does not recommend surgical deactivation of migraine trigger points outside of a clinical trial. In addition, the society advises physicians not to perform CT imaging for headache when an MRI is available, except in emergency settings. The society also recommends that physicians should not perform neuroimaging studies for patients with stable headaches that meet migraine criteria.
When CT Scans Are Unnecessary in Children
The American Academy of Pediatrics (AAP) advises that CT scans and MRI scans are not necessary in a child with simple febrile seizure. The AAP also does not recommend CT scans for the immediate evaluation of minor head injuries; clinical observation and Pediatric Emergency Care Applied Research Network (PECARN) criteria should be used to determine whether imaging is indicated.
Treating Insomnia and Sleep Disorders
The American Academy of Sleep Medicine advises doctors not to prescribe medication for childhood insomnia, which usually arises from parent–child interactions and responds to behavioral intervention. In addition, the academy does no
—Erica Tricarico
Suggested Reading
Callaghan BC, De Lott LB, Kerber KA, et al. Neurology Choosing Wisely recommendations: 74 and growing. Neurol Clin Pract. 2015;5(5):439-447.
Langer-Gould AM, Anderson WE, Armstrong MJ, et al. The American Academy of Neurology’s top five choosing wisely recommendations. Neurology. 2013;81(11):1004-1011.
Loder E, Weisenbaum E, Fishberg B, et al. Choosing wisely in headache medicine: the American Headache Society’s list of five things physicians and patients should question. Headache. 2013;53(10):1651-1659.
HILTON HEAD, SC—Physicians sometimes order unnecessary medical tests and procedures for their patients, which results in wasteful spending and inappropriate care. Following medical associations’ practice recommendations, which have been collected by the Choosing Wisely initiative, can help physicians reduce waste in the health care system and provide appropriate treatment for patients, according to an overview presented at the 39th Annual Contemporary Clinical Neurology Symposium.
Avoiding Unnecessary Treatments and Tests
The American Board of Internal Medicine Foundation created the Choosing Wisely website to encourage dialogue between physicians and patients about the overuse of treatments and tests. An additional goal was to empower patients to make informed treatment decisions. More than 70 societies, including the American Academy of Neurology (AAN) and the American Headache Society, submitted recommendations to advise patients and clinicians about proper healthcare. “You can find a list of all the organizations that contributed on the Choosing Wisely website, and each one was asked to contribute five different topics for Choosing Wisely,” said Peter Donofrio, MD, Professor of Neurology at Vanderbilt University in Nashville.
The AAN recommends that clinicians not perform an EEG for headaches. In addition, the organization recommends that physicians not perform imaging of the carotid arteries for simple syncope without other neurologic symptoms. For patients with migraine, opioids or butalbital treatment should be a last resort. The AAN also recommends that doctors not prescribe interferon-beta or glatiramer acetate for patients with disability resulting from progressive, nonrelapsing forms of multiple sclerosis, because the drugs are ineffective. Finally, it advises doctors not to recommend carotid endarterectomy for asymptomatic carotid stenosis unless the complication rate from surgery is less than 3%.
The American Association of Neuromuscular and Electrodiagnostic Medicine recommends that physicians not perform MRI scans of the brain or spine for patients with peripheral neuropathy without signs of cerebral or spinal cord disease. In addition, the association discourages physicians from performing nerve conduction studies (NCSs) without a needle EMG for radiculopathy assessment. It also recommends that physicians not order or perform four-limb EMG/NCS testing for neck or back pain after trauma.
Treating Acute Low Back Pain and Headache
Other medical associations have made recommendations regarding the assessment and treatment of acute low back pain and headache. The North American Spine Society does not recommend advanced imaging of the spine within the first six weeks in patients with nonspecific acute low back pain in the absence of red flags.
The American Headache Society (AHS) recommends that physicians avoid advising prolonged or frequent use of over-the-counter pain medications for headache. The organization also discourages physicians from prescribing opioid or butalbital-containing medications as first-line treatment for recurrent headache disorders. Furthermore, it does not recommend surgical deactivation of migraine trigger points outside of a clinical trial. In addition, the society advises physicians not to perform CT imaging for headache when an MRI is available, except in emergency settings. The society also recommends that physicians should not perform neuroimaging studies for patients with stable headaches that meet migraine criteria.
When CT Scans Are Unnecessary in Children
The American Academy of Pediatrics (AAP) advises that CT scans and MRI scans are not necessary in a child with simple febrile seizure. The AAP also does not recommend CT scans for the immediate evaluation of minor head injuries; clinical observation and Pediatric Emergency Care Applied Research Network (PECARN) criteria should be used to determine whether imaging is indicated.
Treating Insomnia and Sleep Disorders
The American Academy of Sleep Medicine advises doctors not to prescribe medication for childhood insomnia, which usually arises from parent–child interactions and responds to behavioral intervention. In addition, the academy does no
—Erica Tricarico
Suggested Reading
Callaghan BC, De Lott LB, Kerber KA, et al. Neurology Choosing Wisely recommendations: 74 and growing. Neurol Clin Pract. 2015;5(5):439-447.
Langer-Gould AM, Anderson WE, Armstrong MJ, et al. The American Academy of Neurology’s top five choosing wisely recommendations. Neurology. 2013;81(11):1004-1011.
Loder E, Weisenbaum E, Fishberg B, et al. Choosing wisely in headache medicine: the American Headache Society’s list of five things physicians and patients should question. Headache. 2013;53(10):1651-1659.
HILTON HEAD, SC—Physicians sometimes order unnecessary medical tests and procedures for their patients, which results in wasteful spending and inappropriate care. Following medical associations’ practice recommendations, which have been collected by the Choosing Wisely initiative, can help physicians reduce waste in the health care system and provide appropriate treatment for patients, according to an overview presented at the 39th Annual Contemporary Clinical Neurology Symposium.
Avoiding Unnecessary Treatments and Tests
The American Board of Internal Medicine Foundation created the Choosing Wisely website to encourage dialogue between physicians and patients about the overuse of treatments and tests. An additional goal was to empower patients to make informed treatment decisions. More than 70 societies, including the American Academy of Neurology (AAN) and the American Headache Society, submitted recommendations to advise patients and clinicians about proper healthcare. “You can find a list of all the organizations that contributed on the Choosing Wisely website, and each one was asked to contribute five different topics for Choosing Wisely,” said Peter Donofrio, MD, Professor of Neurology at Vanderbilt University in Nashville.
The AAN recommends that clinicians not perform an EEG for headaches. In addition, the organization recommends that physicians not perform imaging of the carotid arteries for simple syncope without other neurologic symptoms. For patients with migraine, opioids or butalbital treatment should be a last resort. The AAN also recommends that doctors not prescribe interferon-beta or glatiramer acetate for patients with disability resulting from progressive, nonrelapsing forms of multiple sclerosis, because the drugs are ineffective. Finally, it advises doctors not to recommend carotid endarterectomy for asymptomatic carotid stenosis unless the complication rate from surgery is less than 3%.
The American Association of Neuromuscular and Electrodiagnostic Medicine recommends that physicians not perform MRI scans of the brain or spine for patients with peripheral neuropathy without signs of cerebral or spinal cord disease. In addition, the association discourages physicians from performing nerve conduction studies (NCSs) without a needle EMG for radiculopathy assessment. It also recommends that physicians not order or perform four-limb EMG/NCS testing for neck or back pain after trauma.
Treating Acute Low Back Pain and Headache
Other medical associations have made recommendations regarding the assessment and treatment of acute low back pain and headache. The North American Spine Society does not recommend advanced imaging of the spine within the first six weeks in patients with nonspecific acute low back pain in the absence of red flags.
The American Headache Society (AHS) recommends that physicians avoid advising prolonged or frequent use of over-the-counter pain medications for headache. The organization also discourages physicians from prescribing opioid or butalbital-containing medications as first-line treatment for recurrent headache disorders. Furthermore, it does not recommend surgical deactivation of migraine trigger points outside of a clinical trial. In addition, the society advises physicians not to perform CT imaging for headache when an MRI is available, except in emergency settings. The society also recommends that physicians should not perform neuroimaging studies for patients with stable headaches that meet migraine criteria.
When CT Scans Are Unnecessary in Children
The American Academy of Pediatrics (AAP) advises that CT scans and MRI scans are not necessary in a child with simple febrile seizure. The AAP also does not recommend CT scans for the immediate evaluation of minor head injuries; clinical observation and Pediatric Emergency Care Applied Research Network (PECARN) criteria should be used to determine whether imaging is indicated.
Treating Insomnia and Sleep Disorders
The American Academy of Sleep Medicine advises doctors not to prescribe medication for childhood insomnia, which usually arises from parent–child interactions and responds to behavioral intervention. In addition, the academy does no
—Erica Tricarico
Suggested Reading
Callaghan BC, De Lott LB, Kerber KA, et al. Neurology Choosing Wisely recommendations: 74 and growing. Neurol Clin Pract. 2015;5(5):439-447.
Langer-Gould AM, Anderson WE, Armstrong MJ, et al. The American Academy of Neurology’s top five choosing wisely recommendations. Neurology. 2013;81(11):1004-1011.
Loder E, Weisenbaum E, Fishberg B, et al. Choosing wisely in headache medicine: the American Headache Society’s list of five things physicians and patients should question. Headache. 2013;53(10):1651-1659.
Reasons Behind the Ink
Tattoos have been viewed as one of the most exotic forms of art for thousands of years. In ancient times, tattoos were used mainly for therapeutic and status purposes. According to British archeologist Joann Fletcher, the oldest evidence of tattoo use was found on the famous “Iceman,” a 5200-year-old frozen mummy that was discovered more than 20 years ago.1 Tattoos were thought to be a form of therapy used to decrease joint pain. On the other hand, the ancient Egyptians used tattoos as symbols of wealth and high status; surprisingly, only women were tattooed. Fletcher also reported that tattoos were used as a form of therapy during pregnancy in upper-class women.1
Tattoos have served different purposes in the last few centuries, making their way to the United States at the start of the 20th century.2 New York City became the tattoo capital of the country. During this early period, male artists often would tattoo their wives so that they could advertise their work. After the Prohibition era, tattoos became widely used within the US Military, becoming a way to show pride and patriotism.2
Due to the permanent nature of tattoos, we sought to understand the reasons for obtaining this particular genre of body art. The purpose of this study was to provide a greater understanding of the current demographics of individuals who get tattoos, looking at specific trends in age and level of education of those who get tattoos as well as the motivation for tattoo placement. As dermatologists, it is essential to understand this patient population to be able to provide services (ie, tattoo removal) in the safe setting of a physician’s office.
Methods
The study was conducted at a private dermatology clinic in the Chicago (Illinois) metropolitan area with no institutional review board approval. Between January 2011 and December 2012, local patients with at least 1 tattoo were asked, with assumed consent, to fill out an investigator-developed survey containing 18 multiple-choice questions regarding age, educational and family background, and other factors. The race and gender of the respondents as well as the number of patients who declined to complete the survey were not recorded.
Results
A total of 363 patients completed the in-person survey. Responses were tabulated and converted into percentages for comparison (N=363). Data analysis was divided into 3 parameters: education level, health concerns, and motivation for getting a tattoo. Figure 1 shows that 70% of respondents had obtained a college degree or higher.
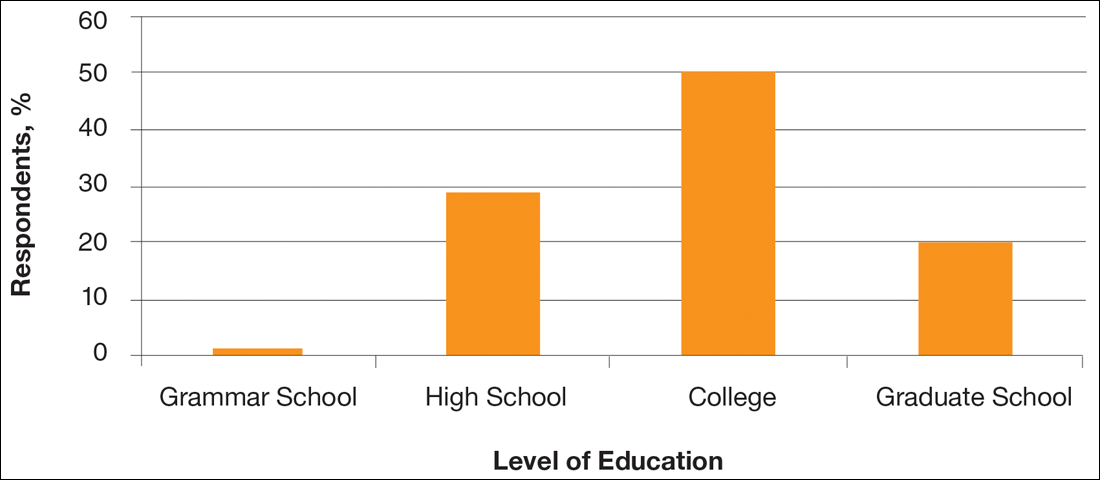
With regard to health concerns associated with tattoos, the majority of respondents (71%) claimed they were not concerned with the health risks (eg, infection with human immunodeficiency virus or hepatitis C virus) associated with getting a tattoo. Also, only 6% of respondents admitted to being under the influence of drugs or alcohol at the time of getting a tattoo. Of 21 respondents who claimed drugs and/or alcohol were part of their tattoo experience, the highest level of education was high school in 7 respondents and 2 got their first tattoo when they were younger than 14 years.
Survey results revealed that the majority of respondents got a tattoo as an act of rememberance (Figure 2). For example, one respondent reported getting a tattoo for religious purposes, while another got a tattoo to celebrate and mark each level of completed education (ie, high school, college, graduate school). However, a high percentage of respondents (26%) got a tattoo for fun.
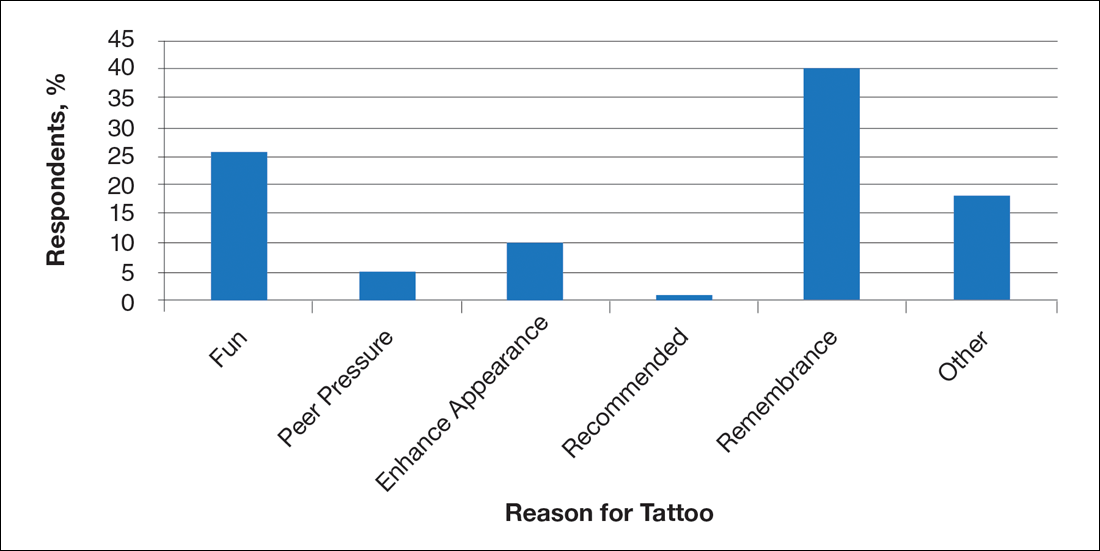
Comment
Although ancient tattoos were used for therapeutic purposes, this study revealed that tattoos are now obtained by individuals with higher levels of education to remember a loved one or purely for enjoyment. The potential health risks associated with getting a tattoo did not deter the respondents in this study. Converse to the popular belief that individuals are under the influence of drugs and/or alcohol when getting a tattoo, our study found that only 6% of respondents were under the influence. A comparable trend was found among US military service members in a similar study.3 The majority of respondents did not regret their tattoos and did not report taking a mind-altering substance. Tattoos serve as a symbol of one’s proud individualism.3 However, a 2001 study found a correlation between greater use of alcohol and marijuana among college students with tattoos and piecings.4 These circumstances may lead patients to seek consultation from a dermatologist for tattoo removal. Therefore, it is important to have a better understanding of this particular patient population to facilitate care in an efficient manner.
Evaluation of the gender and race of survey respondents would be useful in the future. Financial status of respondents also may be explored, as wealth and status were used by the ancient Egyptians to determine who could get a tattoo. A follow-up analysis on removal of tattoos also will be explored in the future.
- Lineberry C. Tattoos: the ancient and mysterious history. Smithsonian. http://www.smithsonianmag.com/history-archaeology/tattoo.html. Published January 1, 2007. Accessed April 29, 2016.
- Bickerstaff L. Tattoos: fad, fashion, or folly? Odyssey. 2005;14:34-36.
- Lande RG, Bahroo BA, Soumoff A. United States military service members and their tattoos: a descriptive study. Mil Med. 2013;178:921-925.
- Forbes GB. College students with tattoos and piercings: motives, family experiences, personality factors, and perception by others. Psychol Rep. 2001;89:774-786.
Tattoos have been viewed as one of the most exotic forms of art for thousands of years. In ancient times, tattoos were used mainly for therapeutic and status purposes. According to British archeologist Joann Fletcher, the oldest evidence of tattoo use was found on the famous “Iceman,” a 5200-year-old frozen mummy that was discovered more than 20 years ago.1 Tattoos were thought to be a form of therapy used to decrease joint pain. On the other hand, the ancient Egyptians used tattoos as symbols of wealth and high status; surprisingly, only women were tattooed. Fletcher also reported that tattoos were used as a form of therapy during pregnancy in upper-class women.1
Tattoos have served different purposes in the last few centuries, making their way to the United States at the start of the 20th century.2 New York City became the tattoo capital of the country. During this early period, male artists often would tattoo their wives so that they could advertise their work. After the Prohibition era, tattoos became widely used within the US Military, becoming a way to show pride and patriotism.2
Due to the permanent nature of tattoos, we sought to understand the reasons for obtaining this particular genre of body art. The purpose of this study was to provide a greater understanding of the current demographics of individuals who get tattoos, looking at specific trends in age and level of education of those who get tattoos as well as the motivation for tattoo placement. As dermatologists, it is essential to understand this patient population to be able to provide services (ie, tattoo removal) in the safe setting of a physician’s office.
Methods
The study was conducted at a private dermatology clinic in the Chicago (Illinois) metropolitan area with no institutional review board approval. Between January 2011 and December 2012, local patients with at least 1 tattoo were asked, with assumed consent, to fill out an investigator-developed survey containing 18 multiple-choice questions regarding age, educational and family background, and other factors. The race and gender of the respondents as well as the number of patients who declined to complete the survey were not recorded.
Results
A total of 363 patients completed the in-person survey. Responses were tabulated and converted into percentages for comparison (N=363). Data analysis was divided into 3 parameters: education level, health concerns, and motivation for getting a tattoo. Figure 1 shows that 70% of respondents had obtained a college degree or higher.

With regard to health concerns associated with tattoos, the majority of respondents (71%) claimed they were not concerned with the health risks (eg, infection with human immunodeficiency virus or hepatitis C virus) associated with getting a tattoo. Also, only 6% of respondents admitted to being under the influence of drugs or alcohol at the time of getting a tattoo. Of 21 respondents who claimed drugs and/or alcohol were part of their tattoo experience, the highest level of education was high school in 7 respondents and 2 got their first tattoo when they were younger than 14 years.
Survey results revealed that the majority of respondents got a tattoo as an act of rememberance (Figure 2). For example, one respondent reported getting a tattoo for religious purposes, while another got a tattoo to celebrate and mark each level of completed education (ie, high school, college, graduate school). However, a high percentage of respondents (26%) got a tattoo for fun.

Comment
Although ancient tattoos were used for therapeutic purposes, this study revealed that tattoos are now obtained by individuals with higher levels of education to remember a loved one or purely for enjoyment. The potential health risks associated with getting a tattoo did not deter the respondents in this study. Converse to the popular belief that individuals are under the influence of drugs and/or alcohol when getting a tattoo, our study found that only 6% of respondents were under the influence. A comparable trend was found among US military service members in a similar study.3 The majority of respondents did not regret their tattoos and did not report taking a mind-altering substance. Tattoos serve as a symbol of one’s proud individualism.3 However, a 2001 study found a correlation between greater use of alcohol and marijuana among college students with tattoos and piecings.4 These circumstances may lead patients to seek consultation from a dermatologist for tattoo removal. Therefore, it is important to have a better understanding of this particular patient population to facilitate care in an efficient manner.
Evaluation of the gender and race of survey respondents would be useful in the future. Financial status of respondents also may be explored, as wealth and status were used by the ancient Egyptians to determine who could get a tattoo. A follow-up analysis on removal of tattoos also will be explored in the future.
Tattoos have been viewed as one of the most exotic forms of art for thousands of years. In ancient times, tattoos were used mainly for therapeutic and status purposes. According to British archeologist Joann Fletcher, the oldest evidence of tattoo use was found on the famous “Iceman,” a 5200-year-old frozen mummy that was discovered more than 20 years ago.1 Tattoos were thought to be a form of therapy used to decrease joint pain. On the other hand, the ancient Egyptians used tattoos as symbols of wealth and high status; surprisingly, only women were tattooed. Fletcher also reported that tattoos were used as a form of therapy during pregnancy in upper-class women.1
Tattoos have served different purposes in the last few centuries, making their way to the United States at the start of the 20th century.2 New York City became the tattoo capital of the country. During this early period, male artists often would tattoo their wives so that they could advertise their work. After the Prohibition era, tattoos became widely used within the US Military, becoming a way to show pride and patriotism.2
Due to the permanent nature of tattoos, we sought to understand the reasons for obtaining this particular genre of body art. The purpose of this study was to provide a greater understanding of the current demographics of individuals who get tattoos, looking at specific trends in age and level of education of those who get tattoos as well as the motivation for tattoo placement. As dermatologists, it is essential to understand this patient population to be able to provide services (ie, tattoo removal) in the safe setting of a physician’s office.
Methods
The study was conducted at a private dermatology clinic in the Chicago (Illinois) metropolitan area with no institutional review board approval. Between January 2011 and December 2012, local patients with at least 1 tattoo were asked, with assumed consent, to fill out an investigator-developed survey containing 18 multiple-choice questions regarding age, educational and family background, and other factors. The race and gender of the respondents as well as the number of patients who declined to complete the survey were not recorded.
Results
A total of 363 patients completed the in-person survey. Responses were tabulated and converted into percentages for comparison (N=363). Data analysis was divided into 3 parameters: education level, health concerns, and motivation for getting a tattoo. Figure 1 shows that 70% of respondents had obtained a college degree or higher.

With regard to health concerns associated with tattoos, the majority of respondents (71%) claimed they were not concerned with the health risks (eg, infection with human immunodeficiency virus or hepatitis C virus) associated with getting a tattoo. Also, only 6% of respondents admitted to being under the influence of drugs or alcohol at the time of getting a tattoo. Of 21 respondents who claimed drugs and/or alcohol were part of their tattoo experience, the highest level of education was high school in 7 respondents and 2 got their first tattoo when they were younger than 14 years.
Survey results revealed that the majority of respondents got a tattoo as an act of rememberance (Figure 2). For example, one respondent reported getting a tattoo for religious purposes, while another got a tattoo to celebrate and mark each level of completed education (ie, high school, college, graduate school). However, a high percentage of respondents (26%) got a tattoo for fun.

Comment
Although ancient tattoos were used for therapeutic purposes, this study revealed that tattoos are now obtained by individuals with higher levels of education to remember a loved one or purely for enjoyment. The potential health risks associated with getting a tattoo did not deter the respondents in this study. Converse to the popular belief that individuals are under the influence of drugs and/or alcohol when getting a tattoo, our study found that only 6% of respondents were under the influence. A comparable trend was found among US military service members in a similar study.3 The majority of respondents did not regret their tattoos and did not report taking a mind-altering substance. Tattoos serve as a symbol of one’s proud individualism.3 However, a 2001 study found a correlation between greater use of alcohol and marijuana among college students with tattoos and piecings.4 These circumstances may lead patients to seek consultation from a dermatologist for tattoo removal. Therefore, it is important to have a better understanding of this particular patient population to facilitate care in an efficient manner.
Evaluation of the gender and race of survey respondents would be useful in the future. Financial status of respondents also may be explored, as wealth and status were used by the ancient Egyptians to determine who could get a tattoo. A follow-up analysis on removal of tattoos also will be explored in the future.
- Lineberry C. Tattoos: the ancient and mysterious history. Smithsonian. http://www.smithsonianmag.com/history-archaeology/tattoo.html. Published January 1, 2007. Accessed April 29, 2016.
- Bickerstaff L. Tattoos: fad, fashion, or folly? Odyssey. 2005;14:34-36.
- Lande RG, Bahroo BA, Soumoff A. United States military service members and their tattoos: a descriptive study. Mil Med. 2013;178:921-925.
- Forbes GB. College students with tattoos and piercings: motives, family experiences, personality factors, and perception by others. Psychol Rep. 2001;89:774-786.
- Lineberry C. Tattoos: the ancient and mysterious history. Smithsonian. http://www.smithsonianmag.com/history-archaeology/tattoo.html. Published January 1, 2007. Accessed April 29, 2016.
- Bickerstaff L. Tattoos: fad, fashion, or folly? Odyssey. 2005;14:34-36.
- Lande RG, Bahroo BA, Soumoff A. United States military service members and their tattoos: a descriptive study. Mil Med. 2013;178:921-925.
- Forbes GB. College students with tattoos and piercings: motives, family experiences, personality factors, and perception by others. Psychol Rep. 2001;89:774-786.
Practice Points
- Individuals who get tattoos often are more educated and well informed than previously thought, more likely leading them to seek removal if desired.
- Our results indicate that tattoos are not regretted as often as previously speculated.