User login
Drug prices, not the health law, top voters’ health priorities for 2017
Until this week, when big increases in insurance premiums were unveiled for next year, the federal health law has not been a major issue in the presidential election. In fact, fixing what ails the Affordable Care Act isn’t even among voters’ top priorities for health issues for next year, according to a new poll.
The monthly October tracking poll from the Kaiser Family Foundation finds that, when voters are asked about what the next president and Congress should do about health care, issues relating to prescription drug prices and out-of-pocket spending far outrank proposals to address the shortcomings of the health law. (Kaiser Health News is an editorially independent project of the foundation.)
By contrast, fewer than a third of all voters favored proposals to repeal requirements in the health law for employers to provide health insurance to their workers or pay a fine; reduce the tax subsidies that help people pay their insurance premiums, and eliminate a tax on high-cost health plans.
Republicans (but not Democrats or independents) still overwhelmingly want to repeal the entire health law, with 60% supporting that action. But Republicans are fractured on why they don’t like the law. Asked what their main reason is for their disapproval, nearly a third (31%) said the law “gives government too big a role in the health care system,” while 27% said “the law is just one of many indications that President Obama took the country in the wrong direction.”
The poll also asked voters about adding a government-sponsored “public option” to health plans available to those purchasing insurance in the health law’s marketplaces. Both President Barack Obama and Democratic nominee Hillary Clinton have called for reconsideration of the idea, which narrowly failed to be included in the original law in 2010.
As with the health law itself, semantics matter in this debate over whether to include a government plan to compete with private plans. Even in describing the same concept, a much larger majority (70%) favored the idea of “creating a public health insurance option to compete with private health insurance plans” than favored “creating a government-administered public health insurance option to compete with private health insurance plans” (53%).
Opinions about a public option are also relatively easily swayed when voters are presented with arguments for and against the idea. For example, 21% of supporters shifted to opposition when told that doctors and hospitals might be paid less under a public option, while 13% shifted from opposition to support when told that having a public plan compete with private plans might help drive down costs.
The survey was conducted between Oct. 12 and Oct. 18 among 1,205 adults, using both land lines and cell phones. The margin of error was plus or minus 3%.
This story was produced by Kaiser Health News, a national health policy news service that is part of the nonpartisan Henry J. Kaiser Family Foundation.
Until this week, when big increases in insurance premiums were unveiled for next year, the federal health law has not been a major issue in the presidential election. In fact, fixing what ails the Affordable Care Act isn’t even among voters’ top priorities for health issues for next year, according to a new poll.
The monthly October tracking poll from the Kaiser Family Foundation finds that, when voters are asked about what the next president and Congress should do about health care, issues relating to prescription drug prices and out-of-pocket spending far outrank proposals to address the shortcomings of the health law. (Kaiser Health News is an editorially independent project of the foundation.)
By contrast, fewer than a third of all voters favored proposals to repeal requirements in the health law for employers to provide health insurance to their workers or pay a fine; reduce the tax subsidies that help people pay their insurance premiums, and eliminate a tax on high-cost health plans.
Republicans (but not Democrats or independents) still overwhelmingly want to repeal the entire health law, with 60% supporting that action. But Republicans are fractured on why they don’t like the law. Asked what their main reason is for their disapproval, nearly a third (31%) said the law “gives government too big a role in the health care system,” while 27% said “the law is just one of many indications that President Obama took the country in the wrong direction.”
The poll also asked voters about adding a government-sponsored “public option” to health plans available to those purchasing insurance in the health law’s marketplaces. Both President Barack Obama and Democratic nominee Hillary Clinton have called for reconsideration of the idea, which narrowly failed to be included in the original law in 2010.
As with the health law itself, semantics matter in this debate over whether to include a government plan to compete with private plans. Even in describing the same concept, a much larger majority (70%) favored the idea of “creating a public health insurance option to compete with private health insurance plans” than favored “creating a government-administered public health insurance option to compete with private health insurance plans” (53%).
Opinions about a public option are also relatively easily swayed when voters are presented with arguments for and against the idea. For example, 21% of supporters shifted to opposition when told that doctors and hospitals might be paid less under a public option, while 13% shifted from opposition to support when told that having a public plan compete with private plans might help drive down costs.
The survey was conducted between Oct. 12 and Oct. 18 among 1,205 adults, using both land lines and cell phones. The margin of error was plus or minus 3%.
This story was produced by Kaiser Health News, a national health policy news service that is part of the nonpartisan Henry J. Kaiser Family Foundation.
Until this week, when big increases in insurance premiums were unveiled for next year, the federal health law has not been a major issue in the presidential election. In fact, fixing what ails the Affordable Care Act isn’t even among voters’ top priorities for health issues for next year, according to a new poll.
The monthly October tracking poll from the Kaiser Family Foundation finds that, when voters are asked about what the next president and Congress should do about health care, issues relating to prescription drug prices and out-of-pocket spending far outrank proposals to address the shortcomings of the health law. (Kaiser Health News is an editorially independent project of the foundation.)
By contrast, fewer than a third of all voters favored proposals to repeal requirements in the health law for employers to provide health insurance to their workers or pay a fine; reduce the tax subsidies that help people pay their insurance premiums, and eliminate a tax on high-cost health plans.
Republicans (but not Democrats or independents) still overwhelmingly want to repeal the entire health law, with 60% supporting that action. But Republicans are fractured on why they don’t like the law. Asked what their main reason is for their disapproval, nearly a third (31%) said the law “gives government too big a role in the health care system,” while 27% said “the law is just one of many indications that President Obama took the country in the wrong direction.”
The poll also asked voters about adding a government-sponsored “public option” to health plans available to those purchasing insurance in the health law’s marketplaces. Both President Barack Obama and Democratic nominee Hillary Clinton have called for reconsideration of the idea, which narrowly failed to be included in the original law in 2010.
As with the health law itself, semantics matter in this debate over whether to include a government plan to compete with private plans. Even in describing the same concept, a much larger majority (70%) favored the idea of “creating a public health insurance option to compete with private health insurance plans” than favored “creating a government-administered public health insurance option to compete with private health insurance plans” (53%).
Opinions about a public option are also relatively easily swayed when voters are presented with arguments for and against the idea. For example, 21% of supporters shifted to opposition when told that doctors and hospitals might be paid less under a public option, while 13% shifted from opposition to support when told that having a public plan compete with private plans might help drive down costs.
The survey was conducted between Oct. 12 and Oct. 18 among 1,205 adults, using both land lines and cell phones. The margin of error was plus or minus 3%.
This story was produced by Kaiser Health News, a national health policy news service that is part of the nonpartisan Henry J. Kaiser Family Foundation.
Lower mortality with left-sided colon tumors
Left-sided primary colon tumors are associated with significantly lower mortality than right-sided tumors, according to a systematic review and meta-analysis published online Oct. 27 in JAMA Oncology.
Previous research has suggested that left- and right-sided colon cancer have different biological, molecular, and clinical features, wrote Fausto Petrelli, MD, from the oncology department at ASST Bergamo Ovest (Italy) and his coauthors.
A meta-analysis of 66 studies involving 1,437,846 patients, in which the cancer side was reported and survival data was available, showed patients with a left-sided primary tumor had a significant 18% lower risk of death (P less than .001), compared with those with right-side tumors. This relationship was independent of other factors including stage, race, adjuvant chemotherapy, and the quality of the study, although studies that only included patients with stage IV disease showed an even greater 27% reduction in death in those with left-side tumors.
“An increasingly large amount of evidence is accumulating showing that colon tumors proximal and distal to the splenic flexure are distinct clinical and biological entities,” the authors wrote. “Apart from having a different embryological origin – proximal colon from midgut and distal colon and rectum from hindgut – the right colon displays peculiar differences in mucosal immunology, probably owing to differences in gut microbiota.”
For example, the proximal colon has been found to have a higher concentration of eosinophils and intraepithelial T cells, which may be due to the fact that immune cells in the distal colorectum have to walk an even finer line between immunogenicity against pathogens and tolerance for normal gut microbiota.
“This observation could also explain the differences in immunological response to tumors developing in the proximal colon characterized by an increased immune activity and, in turn, reflect the specific differences in pathogenesis and outcome.”
Given their findings, the authors argued that side of origin should be included as a prognostic factor for both early and advanced disease, and should also be considered in treatment decision making and in future clinical trials.
No conflicts of interest were declared.
Left-sided primary colon tumors are associated with significantly lower mortality than right-sided tumors, according to a systematic review and meta-analysis published online Oct. 27 in JAMA Oncology.
Previous research has suggested that left- and right-sided colon cancer have different biological, molecular, and clinical features, wrote Fausto Petrelli, MD, from the oncology department at ASST Bergamo Ovest (Italy) and his coauthors.
A meta-analysis of 66 studies involving 1,437,846 patients, in which the cancer side was reported and survival data was available, showed patients with a left-sided primary tumor had a significant 18% lower risk of death (P less than .001), compared with those with right-side tumors. This relationship was independent of other factors including stage, race, adjuvant chemotherapy, and the quality of the study, although studies that only included patients with stage IV disease showed an even greater 27% reduction in death in those with left-side tumors.
“An increasingly large amount of evidence is accumulating showing that colon tumors proximal and distal to the splenic flexure are distinct clinical and biological entities,” the authors wrote. “Apart from having a different embryological origin – proximal colon from midgut and distal colon and rectum from hindgut – the right colon displays peculiar differences in mucosal immunology, probably owing to differences in gut microbiota.”
For example, the proximal colon has been found to have a higher concentration of eosinophils and intraepithelial T cells, which may be due to the fact that immune cells in the distal colorectum have to walk an even finer line between immunogenicity against pathogens and tolerance for normal gut microbiota.
“This observation could also explain the differences in immunological response to tumors developing in the proximal colon characterized by an increased immune activity and, in turn, reflect the specific differences in pathogenesis and outcome.”
Given their findings, the authors argued that side of origin should be included as a prognostic factor for both early and advanced disease, and should also be considered in treatment decision making and in future clinical trials.
No conflicts of interest were declared.
Left-sided primary colon tumors are associated with significantly lower mortality than right-sided tumors, according to a systematic review and meta-analysis published online Oct. 27 in JAMA Oncology.
Previous research has suggested that left- and right-sided colon cancer have different biological, molecular, and clinical features, wrote Fausto Petrelli, MD, from the oncology department at ASST Bergamo Ovest (Italy) and his coauthors.
A meta-analysis of 66 studies involving 1,437,846 patients, in which the cancer side was reported and survival data was available, showed patients with a left-sided primary tumor had a significant 18% lower risk of death (P less than .001), compared with those with right-side tumors. This relationship was independent of other factors including stage, race, adjuvant chemotherapy, and the quality of the study, although studies that only included patients with stage IV disease showed an even greater 27% reduction in death in those with left-side tumors.
“An increasingly large amount of evidence is accumulating showing that colon tumors proximal and distal to the splenic flexure are distinct clinical and biological entities,” the authors wrote. “Apart from having a different embryological origin – proximal colon from midgut and distal colon and rectum from hindgut – the right colon displays peculiar differences in mucosal immunology, probably owing to differences in gut microbiota.”
For example, the proximal colon has been found to have a higher concentration of eosinophils and intraepithelial T cells, which may be due to the fact that immune cells in the distal colorectum have to walk an even finer line between immunogenicity against pathogens and tolerance for normal gut microbiota.
“This observation could also explain the differences in immunological response to tumors developing in the proximal colon characterized by an increased immune activity and, in turn, reflect the specific differences in pathogenesis and outcome.”
Given their findings, the authors argued that side of origin should be included as a prognostic factor for both early and advanced disease, and should also be considered in treatment decision making and in future clinical trials.
No conflicts of interest were declared.
FROM JAMA ONCOLOGY
Key clinical point: Left-sided colon tumors are associated with significantly lower mortality than right-sided tumors.
Major finding: Patients with a left-sided primary tumor had a significant 18% lower risk of death, compared with those with a right-side tumor.
Data source: Systematic review and meta-analysis of 66 studies involving 1,437,846 patients.
Disclosures: No relevant conflicts of interest were declared.
Pediatric OSA improved with oral montelukast
The majority of children with obstructive sleep apnea (OSA) who took oral montelukast showed reductions in their apnea-hypopnea index (AHI) scores, in a randomized, double-blind placebo-controlled study.
Typically, OSA in children is treated by adenotonsillectomy, according to Leila Kheirandish-Gozal, MD, director of clinical sleep research at the University of Chicago, and her colleagues. Prior to this study, only one randomized controlled trial had showed that children with mild OSA “responded favorably” to the leukotriene modifier montelukast (Pediatrics. 2012 Aug 31. doi: 10.1542/peds.2012-0310).
Twenty (71%) of the children who received montelukast had fewer AHI events per hour of total sleep time at the end of the study. The average number of such events for these patients was 4.2 plus or minus 2.8 after taking the drug, compared with 9.2 plus or minus 4.1 at the beginning of the study (P less than .0001). Only two (6.9%) of the patients who took the placebo had lower AHI scores at the end of the study, with the average AHI score for the placebo group having been 8.7 plus or minus 4.9 events per hour of total sleep time. At baseline, the average score for patients in the placebo group was 8.2 plus or minus 5.0 AHI events per hour of total sleep time at baseline.
Another improvement seen by patients who received the drug was a decrease in the number of 3% reductions in arterial oxygen saturation per hour of sleep. At the beginning of the study, these patients had 7.2 plus or minus 3.1 of these events; by the end of the study, the number of these events was down to 2.8 plus or minus 1.8 (P less than .001). No significant decrease in the number of these events was seen among patients in the placebo group.
In this study, “montelukast emerges as favorably reducing the severity of OSA short term in children 2-10 years of age. These findings add to the existing evidence supporting a therapeutic role for anti-inflammatory approaches in the management of this highly prevalent condition in children, and clearly justify future studies targeting the long-term benefits of these approaches in children with OSA,” the researchers wrote.
All patients participated in overnight sleep studies following a referral to one of two sleep clinics by their primary care pediatrician or pediatric otolaryngologist, at the beginning of the study. Children who had been diagnosed with symptomatic snoring and had an AHI score of greater than 2 events per hour of total sleep time, and for whom adenotonsillectomy was contemplated, were included in the study.
Central, obstructive, mixed apneic events were counted and hypopneas were assessed. OSA was defined “as the absence of airflow with continued chest wall and abdominal movement for a duration of at least two breaths,” the investigators said. Hypopneas were defined “as a decrease in oronasal flow greater than 50% on either the thermistor or nasal pressure transducer signal. with a corresponding decrease in arterial oxygen saturation greater than 3% or arousal,” Dr. Kheirandish-Gozal and her coauthors said.
Patients were excluded from the study for a variety of reasons, including having severe OSA requiring early surgical intervention.
Adverse events included headache in two children, one from the experimental group and one from the placebo group, and nausea in two subjects from the placebo group and in one from the montelukast group.
Merck provided tablets used in this study. Dr. Kheirandish-Gozal reported grants from Merck and the National Institutes of Health during the conduct of the study. David Gozal, MD, is supported by the Herbert T. Abelson Chair in Pediatrics at the University of Chicago.
The majority of children with obstructive sleep apnea (OSA) who took oral montelukast showed reductions in their apnea-hypopnea index (AHI) scores, in a randomized, double-blind placebo-controlled study.
Typically, OSA in children is treated by adenotonsillectomy, according to Leila Kheirandish-Gozal, MD, director of clinical sleep research at the University of Chicago, and her colleagues. Prior to this study, only one randomized controlled trial had showed that children with mild OSA “responded favorably” to the leukotriene modifier montelukast (Pediatrics. 2012 Aug 31. doi: 10.1542/peds.2012-0310).
Twenty (71%) of the children who received montelukast had fewer AHI events per hour of total sleep time at the end of the study. The average number of such events for these patients was 4.2 plus or minus 2.8 after taking the drug, compared with 9.2 plus or minus 4.1 at the beginning of the study (P less than .0001). Only two (6.9%) of the patients who took the placebo had lower AHI scores at the end of the study, with the average AHI score for the placebo group having been 8.7 plus or minus 4.9 events per hour of total sleep time. At baseline, the average score for patients in the placebo group was 8.2 plus or minus 5.0 AHI events per hour of total sleep time at baseline.
Another improvement seen by patients who received the drug was a decrease in the number of 3% reductions in arterial oxygen saturation per hour of sleep. At the beginning of the study, these patients had 7.2 plus or minus 3.1 of these events; by the end of the study, the number of these events was down to 2.8 plus or minus 1.8 (P less than .001). No significant decrease in the number of these events was seen among patients in the placebo group.
In this study, “montelukast emerges as favorably reducing the severity of OSA short term in children 2-10 years of age. These findings add to the existing evidence supporting a therapeutic role for anti-inflammatory approaches in the management of this highly prevalent condition in children, and clearly justify future studies targeting the long-term benefits of these approaches in children with OSA,” the researchers wrote.
All patients participated in overnight sleep studies following a referral to one of two sleep clinics by their primary care pediatrician or pediatric otolaryngologist, at the beginning of the study. Children who had been diagnosed with symptomatic snoring and had an AHI score of greater than 2 events per hour of total sleep time, and for whom adenotonsillectomy was contemplated, were included in the study.
Central, obstructive, mixed apneic events were counted and hypopneas were assessed. OSA was defined “as the absence of airflow with continued chest wall and abdominal movement for a duration of at least two breaths,” the investigators said. Hypopneas were defined “as a decrease in oronasal flow greater than 50% on either the thermistor or nasal pressure transducer signal. with a corresponding decrease in arterial oxygen saturation greater than 3% or arousal,” Dr. Kheirandish-Gozal and her coauthors said.
Patients were excluded from the study for a variety of reasons, including having severe OSA requiring early surgical intervention.
Adverse events included headache in two children, one from the experimental group and one from the placebo group, and nausea in two subjects from the placebo group and in one from the montelukast group.
Merck provided tablets used in this study. Dr. Kheirandish-Gozal reported grants from Merck and the National Institutes of Health during the conduct of the study. David Gozal, MD, is supported by the Herbert T. Abelson Chair in Pediatrics at the University of Chicago.
The majority of children with obstructive sleep apnea (OSA) who took oral montelukast showed reductions in their apnea-hypopnea index (AHI) scores, in a randomized, double-blind placebo-controlled study.
Typically, OSA in children is treated by adenotonsillectomy, according to Leila Kheirandish-Gozal, MD, director of clinical sleep research at the University of Chicago, and her colleagues. Prior to this study, only one randomized controlled trial had showed that children with mild OSA “responded favorably” to the leukotriene modifier montelukast (Pediatrics. 2012 Aug 31. doi: 10.1542/peds.2012-0310).
Twenty (71%) of the children who received montelukast had fewer AHI events per hour of total sleep time at the end of the study. The average number of such events for these patients was 4.2 plus or minus 2.8 after taking the drug, compared with 9.2 plus or minus 4.1 at the beginning of the study (P less than .0001). Only two (6.9%) of the patients who took the placebo had lower AHI scores at the end of the study, with the average AHI score for the placebo group having been 8.7 plus or minus 4.9 events per hour of total sleep time. At baseline, the average score for patients in the placebo group was 8.2 plus or minus 5.0 AHI events per hour of total sleep time at baseline.
Another improvement seen by patients who received the drug was a decrease in the number of 3% reductions in arterial oxygen saturation per hour of sleep. At the beginning of the study, these patients had 7.2 plus or minus 3.1 of these events; by the end of the study, the number of these events was down to 2.8 plus or minus 1.8 (P less than .001). No significant decrease in the number of these events was seen among patients in the placebo group.
In this study, “montelukast emerges as favorably reducing the severity of OSA short term in children 2-10 years of age. These findings add to the existing evidence supporting a therapeutic role for anti-inflammatory approaches in the management of this highly prevalent condition in children, and clearly justify future studies targeting the long-term benefits of these approaches in children with OSA,” the researchers wrote.
All patients participated in overnight sleep studies following a referral to one of two sleep clinics by their primary care pediatrician or pediatric otolaryngologist, at the beginning of the study. Children who had been diagnosed with symptomatic snoring and had an AHI score of greater than 2 events per hour of total sleep time, and for whom adenotonsillectomy was contemplated, were included in the study.
Central, obstructive, mixed apneic events were counted and hypopneas were assessed. OSA was defined “as the absence of airflow with continued chest wall and abdominal movement for a duration of at least two breaths,” the investigators said. Hypopneas were defined “as a decrease in oronasal flow greater than 50% on either the thermistor or nasal pressure transducer signal. with a corresponding decrease in arterial oxygen saturation greater than 3% or arousal,” Dr. Kheirandish-Gozal and her coauthors said.
Patients were excluded from the study for a variety of reasons, including having severe OSA requiring early surgical intervention.
Adverse events included headache in two children, one from the experimental group and one from the placebo group, and nausea in two subjects from the placebo group and in one from the montelukast group.
Merck provided tablets used in this study. Dr. Kheirandish-Gozal reported grants from Merck and the National Institutes of Health during the conduct of the study. David Gozal, MD, is supported by the Herbert T. Abelson Chair in Pediatrics at the University of Chicago.
Key clinical point:
Major finding: 71% of patients who took montelukast had a significant reduction in AHI events per hour of total sleep time (P less than .0001).
Data source: A prospective, randomized, double-blind placebo-controlled study of 57 children with obstructive sleep apnea.
Disclosures: Merck provided tablets used in this study. Dr. Kheirandish-Gozal reported grants from Merck and the National Institutes of Health during the conduct of the study. David Gozal, MD, is supported by the Herbert T. Abelson Chair in Pediatrics at the University of Chicago.
Genetic screening for CLL premature, speaker says

Photo courtesy of the
National Institute
of General Medical Science
NEW YORK—Research has shown that family history is a strong risk factor for developing chronic lymphocytic leukemia (CLL).
First-degree relatives have an 8.5-fold risk of getting CLL and an increased risk of other lymphoproliferative disorders, according to a study published in 2009.
However, despite the strong evidence of a genetic contribution, one expert believes it’s premature to bring genetic testing into the clinic for screening in CLL.
“At this time, we do not recommend genetic screening,” said Susan Slager, PhD, of the Mayo Clinic in Rochester, Minnesota.
“There’s no known relationship between the inherited variants and treatment response,” she explained, and the relatively low incidence of CLL argues against active screening in affected families at present.
Dr Slager discussed genetic and non-genetic factors associated with CLL and the clinical implications of these factors at Lymphoma & Myeloma 2016.
Demographic risk factors
Dr Slager noted that age, gender, and race are risk factors for CLL.
Individuals aged 65 to 74 have the highest incidence of CLL, at 28%, while the risk is almost non-existent for those under age 20, she said.
There is a higher incidence of CLL in males than in females, and the reason for this gender disparity is unknown.
There is a higher incidence of CLL in Caucasians than Asians, for both males and females.
“Again, it’s unknown why there’s this variability in incidence in CLL,” Dr Slager said. “Obviously, age, sex, and race—these are things you can’t modify. You’re stuck with them.”
However, several studies have been undertaken to look at some of the potentially modifiable factors associated with CLL.
Beyond demographic factors
The International Lymphoma Epidemiology Consortium, known as InterLymph, was initiated in 2001 to evaluate the association of risk factors in CLL. Study centers are located primarily in North America and Europe, with one in Australia.
In one of the larger InterLymph studies, investigators evaluated risk factors—lifestyle exposure, reproductive history, medical history, occupational exposures, farming exposure, and family history—in 2440 CLL patients and 15,186 controls.
The investigators found that sun exposure and atopy—allergies, asthma, eczema, and hay fever—have a protective effect in CLL, while serological hepatitis C virus (HCV) infections, farming exposure, and family history carry an increased risk of CLL.
This confirmed an earlier study conducted in New South Wales, Australia, that had uncovered an inverse association between sun exposure and non-Hodgkin lymphoma (NHL) risk, which fell significantly with increasing recreational sun exposure.
Medical history
Another earlier study from New South Wales revealed a 20% reduction in the risk of NHL for any specific allergy.
However, the investigators of the large, more recent study observed little to no evidence of reduced risk for asthma and eczema.
The underlying biology for atopy or allergies is a hyper-immune system, Dr Slager explained.
“So if you have a hyper-immune system, then we hypothesize that you have protection against CLL,” she said.
Another medical exposure investigators analyzed that impacts CLL risk is HCV. People infected with HCV have an increased risk of CLL, perhaps due to chronic antigen stimulation or possibly disruption of the T-cell function.
Height is also associated with CLL. CLL risk increases with greater height. The concept is that taller individuals have increased exposure to growth hormones that possibly result in cell proliferation.
Another hypothesis supporting the height association is that people of shorter stature experience more infections, which could result in a stronger immune system. And a stronger immune system perhaps protects against NHL.
Occupational exposures
Investigators consistently observed a 20% increased risk of CLL for people living or working on a farm.
Animal farmers, as opposed to crop farmers, experienced some protection. However, the sample size was too small to be conclusive, with only 29 people across all studies being animal farmers.
Among other occupations evaluated, hairdressers also had an increased risk of CLL, although this too was based on a small sample size.
Family history
One of the strongest risk factors for CLL is family history.
Using population-based registry data from Sweden, investigators found that people with a first-degree relative with CLL have an 8.5-fold risk of CLL.
They also have an elevated risk of other lymphoproliferative disorders, including NHL (1.9-fold risk), Waldenström’s macroglobulinemia (4.0-fold risk), hairy cell leukemia (3.3-fold risk), and follicular lymphoma (1.6-fold risk).
GWAS in CLL
Investigators conducted genome-wide association studies (GWAS) to determine what is driving the familial risk.
Dr Slager described these studies as an agnostic approach that looks across the entire genome to determine which regions are associated with a trait of interest.
Typically, many markers are genotyped—somewhere between half a million to 5 million markers—and each is looked at individually with respect to CLL, she said.
Unrelated cases and controls are included in the studies.
The first GWAS study identifying susceptibility loci for CLL was published in 2008. Subsequently, more studies were published with increasing sample sizes—more cases, more controls, and more genetic variants identified.
In the largest meta-analysis for CLL to date (Slager and Houlston et al, not yet published), investigators analyzed 4400 CLL cases and 13,000 controls.
They identified 9 more inherited variances with CLL, for a total of 43 identified to date.
The genes involved follow an apoptosis pathway, the telomere length pathway, and the B-cell lymphocyte development pathway.
“We have to remember, though, that these are non-causal,” Dr Slager cautioned. “We are just identifying the region in the genome that’s associated with CLL . . . . So now we have to dig deeper in these relationships to understand what’s going on.”
Using the identified CLL single-nucleotide polymorphisms, the investigators computed a polygenic risk score. CLL cases in the highest quintile had 2.7-fold increased risk of CLL.
However, the most common GWAS variants explain only 17% of the genetic heritability of CLL, which suggests that more loci are yet to be identified, Dr Slager clarified.
She went on to say that CLL incidence varies by ethnicity. Caucasians have a very high rate of CLL, while Asians have a very low rate. And African Americans have an incidence rate between those of Caucasians and Asians.
Investigators have hypothesized that the differences in incidence are based on the distinct genetic variants that are associated with the ethnicities.
For example, 4 of the variants with more than 20% frequency in Caucasians are quite rare in Chinese individuals and are also quite uncommon in African Americans, with frequencies less than 10%.
Dr Slager suggested that conducting these kinds of studies in Asians and African Americans will take a large sample size and most likely require an international consortium to bring enough CLL cases together.
Impact on clinical practice
Because of the strong genetic risk, patients with CLL naturally want to know about their offspring and their siblings, Dr Slager has found.
Patients who have monoclonal B-cell lymphocytosis (MBL), which is a precursor to CLL, pose the biggest quandary.
MBL is detected in about 5% of people over age 40. However, it’s detected in about 15% to 18% of people with a first-degree relative with CLL.
“These are individuals who have lymphocytosis,” Dr Slager said. “They come to your clinic and have an elevated blood cell count, flow cytometry. [So] you screen them for MBL, and these individuals who have more than 500 cells per microliter, they are the ones who progress to CLL, at 1% per year.”
Individuals who don’t have the elevated blood counts do have the clonal cells, Dr Slager noted.
“They just don’t have the expansion,” she said. “The progression of these individuals to CLL is still yet to be determined.”
For these reasons, Dr Slager believes “it’s still premature to bring genetic testing into clinical practice.”
Future directions include bringing together the non-environmental issues and the inherited issues to create a model that will accurately predict the risk of CLL. ![]()

Photo courtesy of the
National Institute
of General Medical Science
NEW YORK—Research has shown that family history is a strong risk factor for developing chronic lymphocytic leukemia (CLL).
First-degree relatives have an 8.5-fold risk of getting CLL and an increased risk of other lymphoproliferative disorders, according to a study published in 2009.
However, despite the strong evidence of a genetic contribution, one expert believes it’s premature to bring genetic testing into the clinic for screening in CLL.
“At this time, we do not recommend genetic screening,” said Susan Slager, PhD, of the Mayo Clinic in Rochester, Minnesota.
“There’s no known relationship between the inherited variants and treatment response,” she explained, and the relatively low incidence of CLL argues against active screening in affected families at present.
Dr Slager discussed genetic and non-genetic factors associated with CLL and the clinical implications of these factors at Lymphoma & Myeloma 2016.
Demographic risk factors
Dr Slager noted that age, gender, and race are risk factors for CLL.
Individuals aged 65 to 74 have the highest incidence of CLL, at 28%, while the risk is almost non-existent for those under age 20, she said.
There is a higher incidence of CLL in males than in females, and the reason for this gender disparity is unknown.
There is a higher incidence of CLL in Caucasians than Asians, for both males and females.
“Again, it’s unknown why there’s this variability in incidence in CLL,” Dr Slager said. “Obviously, age, sex, and race—these are things you can’t modify. You’re stuck with them.”
However, several studies have been undertaken to look at some of the potentially modifiable factors associated with CLL.
Beyond demographic factors
The International Lymphoma Epidemiology Consortium, known as InterLymph, was initiated in 2001 to evaluate the association of risk factors in CLL. Study centers are located primarily in North America and Europe, with one in Australia.
In one of the larger InterLymph studies, investigators evaluated risk factors—lifestyle exposure, reproductive history, medical history, occupational exposures, farming exposure, and family history—in 2440 CLL patients and 15,186 controls.
The investigators found that sun exposure and atopy—allergies, asthma, eczema, and hay fever—have a protective effect in CLL, while serological hepatitis C virus (HCV) infections, farming exposure, and family history carry an increased risk of CLL.
This confirmed an earlier study conducted in New South Wales, Australia, that had uncovered an inverse association between sun exposure and non-Hodgkin lymphoma (NHL) risk, which fell significantly with increasing recreational sun exposure.
Medical history
Another earlier study from New South Wales revealed a 20% reduction in the risk of NHL for any specific allergy.
However, the investigators of the large, more recent study observed little to no evidence of reduced risk for asthma and eczema.
The underlying biology for atopy or allergies is a hyper-immune system, Dr Slager explained.
“So if you have a hyper-immune system, then we hypothesize that you have protection against CLL,” she said.
Another medical exposure investigators analyzed that impacts CLL risk is HCV. People infected with HCV have an increased risk of CLL, perhaps due to chronic antigen stimulation or possibly disruption of the T-cell function.
Height is also associated with CLL. CLL risk increases with greater height. The concept is that taller individuals have increased exposure to growth hormones that possibly result in cell proliferation.
Another hypothesis supporting the height association is that people of shorter stature experience more infections, which could result in a stronger immune system. And a stronger immune system perhaps protects against NHL.
Occupational exposures
Investigators consistently observed a 20% increased risk of CLL for people living or working on a farm.
Animal farmers, as opposed to crop farmers, experienced some protection. However, the sample size was too small to be conclusive, with only 29 people across all studies being animal farmers.
Among other occupations evaluated, hairdressers also had an increased risk of CLL, although this too was based on a small sample size.
Family history
One of the strongest risk factors for CLL is family history.
Using population-based registry data from Sweden, investigators found that people with a first-degree relative with CLL have an 8.5-fold risk of CLL.
They also have an elevated risk of other lymphoproliferative disorders, including NHL (1.9-fold risk), Waldenström’s macroglobulinemia (4.0-fold risk), hairy cell leukemia (3.3-fold risk), and follicular lymphoma (1.6-fold risk).
GWAS in CLL
Investigators conducted genome-wide association studies (GWAS) to determine what is driving the familial risk.
Dr Slager described these studies as an agnostic approach that looks across the entire genome to determine which regions are associated with a trait of interest.
Typically, many markers are genotyped—somewhere between half a million to 5 million markers—and each is looked at individually with respect to CLL, she said.
Unrelated cases and controls are included in the studies.
The first GWAS study identifying susceptibility loci for CLL was published in 2008. Subsequently, more studies were published with increasing sample sizes—more cases, more controls, and more genetic variants identified.
In the largest meta-analysis for CLL to date (Slager and Houlston et al, not yet published), investigators analyzed 4400 CLL cases and 13,000 controls.
They identified 9 more inherited variances with CLL, for a total of 43 identified to date.
The genes involved follow an apoptosis pathway, the telomere length pathway, and the B-cell lymphocyte development pathway.
“We have to remember, though, that these are non-causal,” Dr Slager cautioned. “We are just identifying the region in the genome that’s associated with CLL . . . . So now we have to dig deeper in these relationships to understand what’s going on.”
Using the identified CLL single-nucleotide polymorphisms, the investigators computed a polygenic risk score. CLL cases in the highest quintile had 2.7-fold increased risk of CLL.
However, the most common GWAS variants explain only 17% of the genetic heritability of CLL, which suggests that more loci are yet to be identified, Dr Slager clarified.
She went on to say that CLL incidence varies by ethnicity. Caucasians have a very high rate of CLL, while Asians have a very low rate. And African Americans have an incidence rate between those of Caucasians and Asians.
Investigators have hypothesized that the differences in incidence are based on the distinct genetic variants that are associated with the ethnicities.
For example, 4 of the variants with more than 20% frequency in Caucasians are quite rare in Chinese individuals and are also quite uncommon in African Americans, with frequencies less than 10%.
Dr Slager suggested that conducting these kinds of studies in Asians and African Americans will take a large sample size and most likely require an international consortium to bring enough CLL cases together.
Impact on clinical practice
Because of the strong genetic risk, patients with CLL naturally want to know about their offspring and their siblings, Dr Slager has found.
Patients who have monoclonal B-cell lymphocytosis (MBL), which is a precursor to CLL, pose the biggest quandary.
MBL is detected in about 5% of people over age 40. However, it’s detected in about 15% to 18% of people with a first-degree relative with CLL.
“These are individuals who have lymphocytosis,” Dr Slager said. “They come to your clinic and have an elevated blood cell count, flow cytometry. [So] you screen them for MBL, and these individuals who have more than 500 cells per microliter, they are the ones who progress to CLL, at 1% per year.”
Individuals who don’t have the elevated blood counts do have the clonal cells, Dr Slager noted.
“They just don’t have the expansion,” she said. “The progression of these individuals to CLL is still yet to be determined.”
For these reasons, Dr Slager believes “it’s still premature to bring genetic testing into clinical practice.”
Future directions include bringing together the non-environmental issues and the inherited issues to create a model that will accurately predict the risk of CLL. ![]()

Photo courtesy of the
National Institute
of General Medical Science
NEW YORK—Research has shown that family history is a strong risk factor for developing chronic lymphocytic leukemia (CLL).
First-degree relatives have an 8.5-fold risk of getting CLL and an increased risk of other lymphoproliferative disorders, according to a study published in 2009.
However, despite the strong evidence of a genetic contribution, one expert believes it’s premature to bring genetic testing into the clinic for screening in CLL.
“At this time, we do not recommend genetic screening,” said Susan Slager, PhD, of the Mayo Clinic in Rochester, Minnesota.
“There’s no known relationship between the inherited variants and treatment response,” she explained, and the relatively low incidence of CLL argues against active screening in affected families at present.
Dr Slager discussed genetic and non-genetic factors associated with CLL and the clinical implications of these factors at Lymphoma & Myeloma 2016.
Demographic risk factors
Dr Slager noted that age, gender, and race are risk factors for CLL.
Individuals aged 65 to 74 have the highest incidence of CLL, at 28%, while the risk is almost non-existent for those under age 20, she said.
There is a higher incidence of CLL in males than in females, and the reason for this gender disparity is unknown.
There is a higher incidence of CLL in Caucasians than Asians, for both males and females.
“Again, it’s unknown why there’s this variability in incidence in CLL,” Dr Slager said. “Obviously, age, sex, and race—these are things you can’t modify. You’re stuck with them.”
However, several studies have been undertaken to look at some of the potentially modifiable factors associated with CLL.
Beyond demographic factors
The International Lymphoma Epidemiology Consortium, known as InterLymph, was initiated in 2001 to evaluate the association of risk factors in CLL. Study centers are located primarily in North America and Europe, with one in Australia.
In one of the larger InterLymph studies, investigators evaluated risk factors—lifestyle exposure, reproductive history, medical history, occupational exposures, farming exposure, and family history—in 2440 CLL patients and 15,186 controls.
The investigators found that sun exposure and atopy—allergies, asthma, eczema, and hay fever—have a protective effect in CLL, while serological hepatitis C virus (HCV) infections, farming exposure, and family history carry an increased risk of CLL.
This confirmed an earlier study conducted in New South Wales, Australia, that had uncovered an inverse association between sun exposure and non-Hodgkin lymphoma (NHL) risk, which fell significantly with increasing recreational sun exposure.
Medical history
Another earlier study from New South Wales revealed a 20% reduction in the risk of NHL for any specific allergy.
However, the investigators of the large, more recent study observed little to no evidence of reduced risk for asthma and eczema.
The underlying biology for atopy or allergies is a hyper-immune system, Dr Slager explained.
“So if you have a hyper-immune system, then we hypothesize that you have protection against CLL,” she said.
Another medical exposure investigators analyzed that impacts CLL risk is HCV. People infected with HCV have an increased risk of CLL, perhaps due to chronic antigen stimulation or possibly disruption of the T-cell function.
Height is also associated with CLL. CLL risk increases with greater height. The concept is that taller individuals have increased exposure to growth hormones that possibly result in cell proliferation.
Another hypothesis supporting the height association is that people of shorter stature experience more infections, which could result in a stronger immune system. And a stronger immune system perhaps protects against NHL.
Occupational exposures
Investigators consistently observed a 20% increased risk of CLL for people living or working on a farm.
Animal farmers, as opposed to crop farmers, experienced some protection. However, the sample size was too small to be conclusive, with only 29 people across all studies being animal farmers.
Among other occupations evaluated, hairdressers also had an increased risk of CLL, although this too was based on a small sample size.
Family history
One of the strongest risk factors for CLL is family history.
Using population-based registry data from Sweden, investigators found that people with a first-degree relative with CLL have an 8.5-fold risk of CLL.
They also have an elevated risk of other lymphoproliferative disorders, including NHL (1.9-fold risk), Waldenström’s macroglobulinemia (4.0-fold risk), hairy cell leukemia (3.3-fold risk), and follicular lymphoma (1.6-fold risk).
GWAS in CLL
Investigators conducted genome-wide association studies (GWAS) to determine what is driving the familial risk.
Dr Slager described these studies as an agnostic approach that looks across the entire genome to determine which regions are associated with a trait of interest.
Typically, many markers are genotyped—somewhere between half a million to 5 million markers—and each is looked at individually with respect to CLL, she said.
Unrelated cases and controls are included in the studies.
The first GWAS study identifying susceptibility loci for CLL was published in 2008. Subsequently, more studies were published with increasing sample sizes—more cases, more controls, and more genetic variants identified.
In the largest meta-analysis for CLL to date (Slager and Houlston et al, not yet published), investigators analyzed 4400 CLL cases and 13,000 controls.
They identified 9 more inherited variances with CLL, for a total of 43 identified to date.
The genes involved follow an apoptosis pathway, the telomere length pathway, and the B-cell lymphocyte development pathway.
“We have to remember, though, that these are non-causal,” Dr Slager cautioned. “We are just identifying the region in the genome that’s associated with CLL . . . . So now we have to dig deeper in these relationships to understand what’s going on.”
Using the identified CLL single-nucleotide polymorphisms, the investigators computed a polygenic risk score. CLL cases in the highest quintile had 2.7-fold increased risk of CLL.
However, the most common GWAS variants explain only 17% of the genetic heritability of CLL, which suggests that more loci are yet to be identified, Dr Slager clarified.
She went on to say that CLL incidence varies by ethnicity. Caucasians have a very high rate of CLL, while Asians have a very low rate. And African Americans have an incidence rate between those of Caucasians and Asians.
Investigators have hypothesized that the differences in incidence are based on the distinct genetic variants that are associated with the ethnicities.
For example, 4 of the variants with more than 20% frequency in Caucasians are quite rare in Chinese individuals and are also quite uncommon in African Americans, with frequencies less than 10%.
Dr Slager suggested that conducting these kinds of studies in Asians and African Americans will take a large sample size and most likely require an international consortium to bring enough CLL cases together.
Impact on clinical practice
Because of the strong genetic risk, patients with CLL naturally want to know about their offspring and their siblings, Dr Slager has found.
Patients who have monoclonal B-cell lymphocytosis (MBL), which is a precursor to CLL, pose the biggest quandary.
MBL is detected in about 5% of people over age 40. However, it’s detected in about 15% to 18% of people with a first-degree relative with CLL.
“These are individuals who have lymphocytosis,” Dr Slager said. “They come to your clinic and have an elevated blood cell count, flow cytometry. [So] you screen them for MBL, and these individuals who have more than 500 cells per microliter, they are the ones who progress to CLL, at 1% per year.”
Individuals who don’t have the elevated blood counts do have the clonal cells, Dr Slager noted.
“They just don’t have the expansion,” she said. “The progression of these individuals to CLL is still yet to be determined.”
For these reasons, Dr Slager believes “it’s still premature to bring genetic testing into clinical practice.”
Future directions include bringing together the non-environmental issues and the inherited issues to create a model that will accurately predict the risk of CLL. ![]()
Gene-editing method cures thalassemia in mice

Photo by Aaron Logan
A new gene-editing strategy may be able to cure thalassemia, according to preclinical research published in Nature Communications.
The technique—which involves a combination of nanoparticles, synthetic pieces of DNA, and an intravenous injection—was able to alleviate symptoms of thalassemia in mice.
The strategy also decreases the risk of off-target mutations, when compared to other gene-editing techniques, according to researchers.
The new technique involves biocompatible nanoparticles containing peptide nucleic acids (PNAs), which are small, nano-sized, synthetic molecules in which a protein-like backbone is combined with the nucleobases found in DNA and RNA.
PNAs are designed to open up double-stranded DNA and bind near the target site in a highly specific manner. The PNAs fit inside a nanoparticle delivery system that is approved by the US Food and Drug Administration (FDA) and has already been used to treat neurodegenerative diseases in humans.
“We have developed a system that uses FDA-approved nanoparticles to deliver our PNA molecule along with a donor DNA to repair a malfunctioning gene in living mice,” said study author Danith Ly, PhD, of Carnegie Mellon University in Pittsburgh, Pennsylvania. “This has not been achieved with CRISPR.”
Dr Ly and his colleagues designed a PNA to target the malfunctioning gene in beta-thalassemia, the hemoglobin subunit beta (HBB) gene.
The researchers then loaded the nanoparticles with the PNAs, a donor strand of DNA encoding the sequence for a functional HBB gene, and a stem cell factor that enhances gene editing.
When the PNA binds to the target site in the DNA, it forms a PNA-DNA-PNA triplex, leaving a displaced DNA strand. Formation of such a complex enables the donor DNA to bind to the faulty DNA site within the vicinity.
Taken together, this altered helix engages the cell’s own DNA repair pathways to correct the malfunctioning HBB gene.
In addition to testing this system on mouse and human hematopoietic stem cells, the researchers used an intravenous injection to deliver the gene-editing package in mouse models of beta-thalassemia.
The results showed up to 6.9% successful gene-editing in hematopoietic stem cells. The mice showed elevated levels of hemoglobin—into the normal range—for several months after treatment, a reduction in reticulocytosis, and reversal of splenomegaly.
The researchers said this represents a striking increase in efficacy over typical gene-editing methods, which produce a 0.1% success rate.
“The effect may only be 7%, but that’s curative,” Dr Ly said. “In the case of this particular disease model, you don’t need a lot of correction. You don’t need 100% to see the phenotype return to normal.”
In addition, this gene-editing strategy had “extremely low off-target effects,” according to study author Peter Glazer, MD, of Yale University in New Haven, Connecticut.
The overall off-target modification frequency was 0.0032%.
If this strategy proves effective in clinical studies, it could lead to the development of gene therapy for patients with thalassemia, sickle cell disease, and other inherited blood disorders, Dr Glazer said.
“We might get enough cells corrected that individuals are not anemic anymore,” he added. “We could achieve a symptomatic cure.” ![]()

Photo by Aaron Logan
A new gene-editing strategy may be able to cure thalassemia, according to preclinical research published in Nature Communications.
The technique—which involves a combination of nanoparticles, synthetic pieces of DNA, and an intravenous injection—was able to alleviate symptoms of thalassemia in mice.
The strategy also decreases the risk of off-target mutations, when compared to other gene-editing techniques, according to researchers.
The new technique involves biocompatible nanoparticles containing peptide nucleic acids (PNAs), which are small, nano-sized, synthetic molecules in which a protein-like backbone is combined with the nucleobases found in DNA and RNA.
PNAs are designed to open up double-stranded DNA and bind near the target site in a highly specific manner. The PNAs fit inside a nanoparticle delivery system that is approved by the US Food and Drug Administration (FDA) and has already been used to treat neurodegenerative diseases in humans.
“We have developed a system that uses FDA-approved nanoparticles to deliver our PNA molecule along with a donor DNA to repair a malfunctioning gene in living mice,” said study author Danith Ly, PhD, of Carnegie Mellon University in Pittsburgh, Pennsylvania. “This has not been achieved with CRISPR.”
Dr Ly and his colleagues designed a PNA to target the malfunctioning gene in beta-thalassemia, the hemoglobin subunit beta (HBB) gene.
The researchers then loaded the nanoparticles with the PNAs, a donor strand of DNA encoding the sequence for a functional HBB gene, and a stem cell factor that enhances gene editing.
When the PNA binds to the target site in the DNA, it forms a PNA-DNA-PNA triplex, leaving a displaced DNA strand. Formation of such a complex enables the donor DNA to bind to the faulty DNA site within the vicinity.
Taken together, this altered helix engages the cell’s own DNA repair pathways to correct the malfunctioning HBB gene.
In addition to testing this system on mouse and human hematopoietic stem cells, the researchers used an intravenous injection to deliver the gene-editing package in mouse models of beta-thalassemia.
The results showed up to 6.9% successful gene-editing in hematopoietic stem cells. The mice showed elevated levels of hemoglobin—into the normal range—for several months after treatment, a reduction in reticulocytosis, and reversal of splenomegaly.
The researchers said this represents a striking increase in efficacy over typical gene-editing methods, which produce a 0.1% success rate.
“The effect may only be 7%, but that’s curative,” Dr Ly said. “In the case of this particular disease model, you don’t need a lot of correction. You don’t need 100% to see the phenotype return to normal.”
In addition, this gene-editing strategy had “extremely low off-target effects,” according to study author Peter Glazer, MD, of Yale University in New Haven, Connecticut.
The overall off-target modification frequency was 0.0032%.
If this strategy proves effective in clinical studies, it could lead to the development of gene therapy for patients with thalassemia, sickle cell disease, and other inherited blood disorders, Dr Glazer said.
“We might get enough cells corrected that individuals are not anemic anymore,” he added. “We could achieve a symptomatic cure.” ![]()

Photo by Aaron Logan
A new gene-editing strategy may be able to cure thalassemia, according to preclinical research published in Nature Communications.
The technique—which involves a combination of nanoparticles, synthetic pieces of DNA, and an intravenous injection—was able to alleviate symptoms of thalassemia in mice.
The strategy also decreases the risk of off-target mutations, when compared to other gene-editing techniques, according to researchers.
The new technique involves biocompatible nanoparticles containing peptide nucleic acids (PNAs), which are small, nano-sized, synthetic molecules in which a protein-like backbone is combined with the nucleobases found in DNA and RNA.
PNAs are designed to open up double-stranded DNA and bind near the target site in a highly specific manner. The PNAs fit inside a nanoparticle delivery system that is approved by the US Food and Drug Administration (FDA) and has already been used to treat neurodegenerative diseases in humans.
“We have developed a system that uses FDA-approved nanoparticles to deliver our PNA molecule along with a donor DNA to repair a malfunctioning gene in living mice,” said study author Danith Ly, PhD, of Carnegie Mellon University in Pittsburgh, Pennsylvania. “This has not been achieved with CRISPR.”
Dr Ly and his colleagues designed a PNA to target the malfunctioning gene in beta-thalassemia, the hemoglobin subunit beta (HBB) gene.
The researchers then loaded the nanoparticles with the PNAs, a donor strand of DNA encoding the sequence for a functional HBB gene, and a stem cell factor that enhances gene editing.
When the PNA binds to the target site in the DNA, it forms a PNA-DNA-PNA triplex, leaving a displaced DNA strand. Formation of such a complex enables the donor DNA to bind to the faulty DNA site within the vicinity.
Taken together, this altered helix engages the cell’s own DNA repair pathways to correct the malfunctioning HBB gene.
In addition to testing this system on mouse and human hematopoietic stem cells, the researchers used an intravenous injection to deliver the gene-editing package in mouse models of beta-thalassemia.
The results showed up to 6.9% successful gene-editing in hematopoietic stem cells. The mice showed elevated levels of hemoglobin—into the normal range—for several months after treatment, a reduction in reticulocytosis, and reversal of splenomegaly.
The researchers said this represents a striking increase in efficacy over typical gene-editing methods, which produce a 0.1% success rate.
“The effect may only be 7%, but that’s curative,” Dr Ly said. “In the case of this particular disease model, you don’t need a lot of correction. You don’t need 100% to see the phenotype return to normal.”
In addition, this gene-editing strategy had “extremely low off-target effects,” according to study author Peter Glazer, MD, of Yale University in New Haven, Connecticut.
The overall off-target modification frequency was 0.0032%.
If this strategy proves effective in clinical studies, it could lead to the development of gene therapy for patients with thalassemia, sickle cell disease, and other inherited blood disorders, Dr Glazer said.
“We might get enough cells corrected that individuals are not anemic anymore,” he added. “We could achieve a symptomatic cure.” ![]()
FDA approves blood screening assay

Photo by Daniel Gay
The US Food and Drug Administration has approved the blood screening assay cobas® MPX for use on the cobas® 6800 and 8800 Systems.
cobas® MPX is a nucleic acid test designed to screen donated blood and plasma for human immunodeficiency virus (HIV),hepatitis B virus (HBV), and hepatitis C virus (HCV).
The test can detect 5 viral targets—HIV-1 Group M, HIV-1 Group O, HIV-2, HBV, and HCV—in a single sample.
cobas® MPX features a dual-target approach with amplification of separate regions of HIV-1 and dual probes for HCV. It eliminates both the need for discriminatory testing between HIV, HBV, and HCV and the potential for discrepant results.
cobas® MPX is a product of Roche Molecular Diagnostics and can be used on Roche’s cobas® 6800 System or cobas® 8800 System.
These systems are used for routine molecular testing in the areas of donor screening, viral load monitoring, women’s health, and microbiology.
Both systems make it possible for labs to perform up to 3 tests in the same run with no pre-sorting required.
In an 8-hour shift, the cobas® 6800 System can provide 384 results, and the cobas® 8800 System can provide 960 results.
The cobas® 6800 system enables up to 8 hours of walk-away time with minimal user interaction, and the cobas® 8800 enables up to 4 hours of walk-away time. ![]()

Photo by Daniel Gay
The US Food and Drug Administration has approved the blood screening assay cobas® MPX for use on the cobas® 6800 and 8800 Systems.
cobas® MPX is a nucleic acid test designed to screen donated blood and plasma for human immunodeficiency virus (HIV),hepatitis B virus (HBV), and hepatitis C virus (HCV).
The test can detect 5 viral targets—HIV-1 Group M, HIV-1 Group O, HIV-2, HBV, and HCV—in a single sample.
cobas® MPX features a dual-target approach with amplification of separate regions of HIV-1 and dual probes for HCV. It eliminates both the need for discriminatory testing between HIV, HBV, and HCV and the potential for discrepant results.
cobas® MPX is a product of Roche Molecular Diagnostics and can be used on Roche’s cobas® 6800 System or cobas® 8800 System.
These systems are used for routine molecular testing in the areas of donor screening, viral load monitoring, women’s health, and microbiology.
Both systems make it possible for labs to perform up to 3 tests in the same run with no pre-sorting required.
In an 8-hour shift, the cobas® 6800 System can provide 384 results, and the cobas® 8800 System can provide 960 results.
The cobas® 6800 system enables up to 8 hours of walk-away time with minimal user interaction, and the cobas® 8800 enables up to 4 hours of walk-away time. ![]()

Photo by Daniel Gay
The US Food and Drug Administration has approved the blood screening assay cobas® MPX for use on the cobas® 6800 and 8800 Systems.
cobas® MPX is a nucleic acid test designed to screen donated blood and plasma for human immunodeficiency virus (HIV),hepatitis B virus (HBV), and hepatitis C virus (HCV).
The test can detect 5 viral targets—HIV-1 Group M, HIV-1 Group O, HIV-2, HBV, and HCV—in a single sample.
cobas® MPX features a dual-target approach with amplification of separate regions of HIV-1 and dual probes for HCV. It eliminates both the need for discriminatory testing between HIV, HBV, and HCV and the potential for discrepant results.
cobas® MPX is a product of Roche Molecular Diagnostics and can be used on Roche’s cobas® 6800 System or cobas® 8800 System.
These systems are used for routine molecular testing in the areas of donor screening, viral load monitoring, women’s health, and microbiology.
Both systems make it possible for labs to perform up to 3 tests in the same run with no pre-sorting required.
In an 8-hour shift, the cobas® 6800 System can provide 384 results, and the cobas® 8800 System can provide 960 results.
The cobas® 6800 system enables up to 8 hours of walk-away time with minimal user interaction, and the cobas® 8800 enables up to 4 hours of walk-away time. ![]()
EMA recommends orphan status for drug in SCD

and normal red blood cells
Image by Graham Beards
The European Medicines Agency’s (EMA) Committee for Orphan Medicinal Products (COMP) has issued a positive opinion recommending that LJPC-401 receive orphan designation as a treatment for patients with sickle cell disease (SCD).
LJPC-401 is a formulation of synthetic human hepcidin.
La Jolla Pharmaceutical Company is developing LJPC-401 for the treatment of iron overload, which can occur in SCD and other diseases.
LJPC-401 already has orphan designation in the European Union as a treatment for patients with beta-thalassemia intermedia and major.
La Jolla recently reported positive results from a phase 1 study of LJPC-401 in patients at risk of iron overload suffering from hereditary hemochromatosis, thalassemia, or SCD. Fifteen patients received LJPC-401 at escalating dose levels ranging from 1 mg to 20 mg.
The researchers observed a dose-dependent, statistically significant reduction in serum iron (P=0.008 for dose response; not adjusted for multiple comparisons).
At the 20 mg dose level, LJPC-401 reduced serum iron by an average of 58.1% from baseline to hour 8 (P=0.001; not adjusted for potential regression to the mean effect), and serum iron had not returned to baseline through day 7 (21.2% reduction from baseline to the end of day 7).
The researchers also said LJPC-401 was well tolerated, with no dose-limiting toxicities. Injection-site reactions were the most commonly reported adverse event. These were all mild or moderate in severity, self-limiting, and fully resolved.
Now, La Jolla is working to initiate a pivotal study of LJPC-401. This will be a randomized, controlled, multicenter study in beta-thalassemia patients suffering from iron overload. La Jolla plans to initiate this study in mid-2017.
About orphan designation
The EMA’s COMP adopts an opinion on the granting of orphan drug designation, and that opinion is submitted to the European Commission for a final decision.
Orphan designation provides regulatory and financial incentives for companies to develop and market therapies that treat life-threatening or chronically debilitating conditions affecting no more than 5 in 10,000 people in the European Union, and where no satisfactory treatment is available.
Orphan designation provides a 10-year period of marketing exclusivity if the drug receives regulatory approval. The designation also provides incentives for companies seeking protocol assistance from the EMA during the product development phase and direct access to the centralized authorization procedure. ![]()

and normal red blood cells
Image by Graham Beards
The European Medicines Agency’s (EMA) Committee for Orphan Medicinal Products (COMP) has issued a positive opinion recommending that LJPC-401 receive orphan designation as a treatment for patients with sickle cell disease (SCD).
LJPC-401 is a formulation of synthetic human hepcidin.
La Jolla Pharmaceutical Company is developing LJPC-401 for the treatment of iron overload, which can occur in SCD and other diseases.
LJPC-401 already has orphan designation in the European Union as a treatment for patients with beta-thalassemia intermedia and major.
La Jolla recently reported positive results from a phase 1 study of LJPC-401 in patients at risk of iron overload suffering from hereditary hemochromatosis, thalassemia, or SCD. Fifteen patients received LJPC-401 at escalating dose levels ranging from 1 mg to 20 mg.
The researchers observed a dose-dependent, statistically significant reduction in serum iron (P=0.008 for dose response; not adjusted for multiple comparisons).
At the 20 mg dose level, LJPC-401 reduced serum iron by an average of 58.1% from baseline to hour 8 (P=0.001; not adjusted for potential regression to the mean effect), and serum iron had not returned to baseline through day 7 (21.2% reduction from baseline to the end of day 7).
The researchers also said LJPC-401 was well tolerated, with no dose-limiting toxicities. Injection-site reactions were the most commonly reported adverse event. These were all mild or moderate in severity, self-limiting, and fully resolved.
Now, La Jolla is working to initiate a pivotal study of LJPC-401. This will be a randomized, controlled, multicenter study in beta-thalassemia patients suffering from iron overload. La Jolla plans to initiate this study in mid-2017.
About orphan designation
The EMA’s COMP adopts an opinion on the granting of orphan drug designation, and that opinion is submitted to the European Commission for a final decision.
Orphan designation provides regulatory and financial incentives for companies to develop and market therapies that treat life-threatening or chronically debilitating conditions affecting no more than 5 in 10,000 people in the European Union, and where no satisfactory treatment is available.
Orphan designation provides a 10-year period of marketing exclusivity if the drug receives regulatory approval. The designation also provides incentives for companies seeking protocol assistance from the EMA during the product development phase and direct access to the centralized authorization procedure. ![]()

and normal red blood cells
Image by Graham Beards
The European Medicines Agency’s (EMA) Committee for Orphan Medicinal Products (COMP) has issued a positive opinion recommending that LJPC-401 receive orphan designation as a treatment for patients with sickle cell disease (SCD).
LJPC-401 is a formulation of synthetic human hepcidin.
La Jolla Pharmaceutical Company is developing LJPC-401 for the treatment of iron overload, which can occur in SCD and other diseases.
LJPC-401 already has orphan designation in the European Union as a treatment for patients with beta-thalassemia intermedia and major.
La Jolla recently reported positive results from a phase 1 study of LJPC-401 in patients at risk of iron overload suffering from hereditary hemochromatosis, thalassemia, or SCD. Fifteen patients received LJPC-401 at escalating dose levels ranging from 1 mg to 20 mg.
The researchers observed a dose-dependent, statistically significant reduction in serum iron (P=0.008 for dose response; not adjusted for multiple comparisons).
At the 20 mg dose level, LJPC-401 reduced serum iron by an average of 58.1% from baseline to hour 8 (P=0.001; not adjusted for potential regression to the mean effect), and serum iron had not returned to baseline through day 7 (21.2% reduction from baseline to the end of day 7).
The researchers also said LJPC-401 was well tolerated, with no dose-limiting toxicities. Injection-site reactions were the most commonly reported adverse event. These were all mild or moderate in severity, self-limiting, and fully resolved.
Now, La Jolla is working to initiate a pivotal study of LJPC-401. This will be a randomized, controlled, multicenter study in beta-thalassemia patients suffering from iron overload. La Jolla plans to initiate this study in mid-2017.
About orphan designation
The EMA’s COMP adopts an opinion on the granting of orphan drug designation, and that opinion is submitted to the European Commission for a final decision.
Orphan designation provides regulatory and financial incentives for companies to develop and market therapies that treat life-threatening or chronically debilitating conditions affecting no more than 5 in 10,000 people in the European Union, and where no satisfactory treatment is available.
Orphan designation provides a 10-year period of marketing exclusivity if the drug receives regulatory approval. The designation also provides incentives for companies seeking protocol assistance from the EMA during the product development phase and direct access to the centralized authorization procedure. ![]()
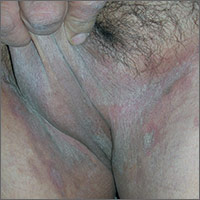
Itching in groin
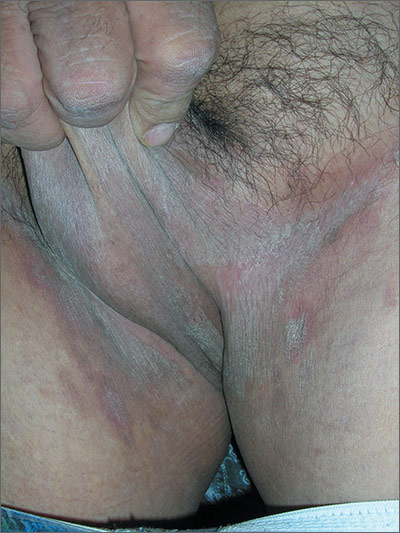
The FP suspected tinea cruris and asked the patient if he had athlete’s foot. The patient stated that his feet were fine, but the FP asked to examine them anyway and found signs of tinea pedis between the toes (especially in the interspace between toes 4 and 5). While this supported the diagnosis of tinea cruris, the FP decided to confirm his diagnosis with a potassium hydroxide (KOH) preparation. (See video on how to perform a KOH preparation here.) The KOH preparation showed branching hyphae with septate and visible nuclei, confirming the tinea infection.
Tinea cruris is an intensely pruritic superficial fungal infection of the groin and adjacent skin that is more common in men than women and rarely affects children. It is most commonly caused by the dermatophytes Trichophyton rubrum, Epidermophyton floccosum, Trichophyton mentagrophytes, and Trichophyton verrucosum. T rubrum is the most common organism and can be spread by fomites, such as contaminated towels. Autoinoculation can occur from fungus on the feet or hands. Risk factors include obesity and diabetes.
Most topical antifungals can be used to treat tinea cruris, except for nystatin, which only works for Candida. The fungicidal allylamines (naftifine and terbinafine) and butenafine (allylamine derivative) are more convenient, as they allow for a shorter duration of treatment compared with fungistatic azoles (clotrimazole, econazole, ketoconazole, oxiconazole, miconazole, and sulconazole). Topical azoles should be continued for 4 weeks and topical allylamines for 2 weeks or until clinical cure. Fluconazole 150 mg once weekly for 2 to 4 weeks can effectively treat tinea cruris when topical agents are failing. If there are multiple sites infected with fungus, such as the groin and feet, it helps to treat all active areas of infection simultaneously to prevent reinfection of the groin from other body sites.
For this patient, the FP suggested that he buy over-the-counter topical terbinafine to treat the groin and feet twice daily for a minimum of 2 weeks until the fungal infection was no longer visible and symptomatic. At a follow-up visit 4 weeks later, the tinea had resolved and the patient was very happy with the results.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Usatine R, Smith M. Tinea cruris. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:795-798.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com

The FP suspected tinea cruris and asked the patient if he had athlete’s foot. The patient stated that his feet were fine, but the FP asked to examine them anyway and found signs of tinea pedis between the toes (especially in the interspace between toes 4 and 5). While this supported the diagnosis of tinea cruris, the FP decided to confirm his diagnosis with a potassium hydroxide (KOH) preparation. (See video on how to perform a KOH preparation here.) The KOH preparation showed branching hyphae with septate and visible nuclei, confirming the tinea infection.
Tinea cruris is an intensely pruritic superficial fungal infection of the groin and adjacent skin that is more common in men than women and rarely affects children. It is most commonly caused by the dermatophytes Trichophyton rubrum, Epidermophyton floccosum, Trichophyton mentagrophytes, and Trichophyton verrucosum. T rubrum is the most common organism and can be spread by fomites, such as contaminated towels. Autoinoculation can occur from fungus on the feet or hands. Risk factors include obesity and diabetes.
Most topical antifungals can be used to treat tinea cruris, except for nystatin, which only works for Candida. The fungicidal allylamines (naftifine and terbinafine) and butenafine (allylamine derivative) are more convenient, as they allow for a shorter duration of treatment compared with fungistatic azoles (clotrimazole, econazole, ketoconazole, oxiconazole, miconazole, and sulconazole). Topical azoles should be continued for 4 weeks and topical allylamines for 2 weeks or until clinical cure. Fluconazole 150 mg once weekly for 2 to 4 weeks can effectively treat tinea cruris when topical agents are failing. If there are multiple sites infected with fungus, such as the groin and feet, it helps to treat all active areas of infection simultaneously to prevent reinfection of the groin from other body sites.
For this patient, the FP suggested that he buy over-the-counter topical terbinafine to treat the groin and feet twice daily for a minimum of 2 weeks until the fungal infection was no longer visible and symptomatic. At a follow-up visit 4 weeks later, the tinea had resolved and the patient was very happy with the results.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Usatine R, Smith M. Tinea cruris. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:795-798.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com

The FP suspected tinea cruris and asked the patient if he had athlete’s foot. The patient stated that his feet were fine, but the FP asked to examine them anyway and found signs of tinea pedis between the toes (especially in the interspace between toes 4 and 5). While this supported the diagnosis of tinea cruris, the FP decided to confirm his diagnosis with a potassium hydroxide (KOH) preparation. (See video on how to perform a KOH preparation here.) The KOH preparation showed branching hyphae with septate and visible nuclei, confirming the tinea infection.
Tinea cruris is an intensely pruritic superficial fungal infection of the groin and adjacent skin that is more common in men than women and rarely affects children. It is most commonly caused by the dermatophytes Trichophyton rubrum, Epidermophyton floccosum, Trichophyton mentagrophytes, and Trichophyton verrucosum. T rubrum is the most common organism and can be spread by fomites, such as contaminated towels. Autoinoculation can occur from fungus on the feet or hands. Risk factors include obesity and diabetes.
Most topical antifungals can be used to treat tinea cruris, except for nystatin, which only works for Candida. The fungicidal allylamines (naftifine and terbinafine) and butenafine (allylamine derivative) are more convenient, as they allow for a shorter duration of treatment compared with fungistatic azoles (clotrimazole, econazole, ketoconazole, oxiconazole, miconazole, and sulconazole). Topical azoles should be continued for 4 weeks and topical allylamines for 2 weeks or until clinical cure. Fluconazole 150 mg once weekly for 2 to 4 weeks can effectively treat tinea cruris when topical agents are failing. If there are multiple sites infected with fungus, such as the groin and feet, it helps to treat all active areas of infection simultaneously to prevent reinfection of the groin from other body sites.
For this patient, the FP suggested that he buy over-the-counter topical terbinafine to treat the groin and feet twice daily for a minimum of 2 weeks until the fungal infection was no longer visible and symptomatic. At a follow-up visit 4 weeks later, the tinea had resolved and the patient was very happy with the results.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Usatine R, Smith M. Tinea cruris. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:795-798.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
Diabetes, stroke linked to recurrent C. difficile
LAS VEGAS – Diabetes and stroke are risk factors for recurrent Clostridium difficile infection (CDI), with stroke patients at about 10 times the risk of recurrence.
The underlying cause for the association is a mystery, but one-sided paralysis is one possibility. “A lot of stroke patients may be hemiplegic, and they may be bedridden, so that may be a risk factor by itself. It’s something that may need to be studied in the future,” Alan Putrus, MD, a gastroenterology fellow at St. John Providence Hospital, Detroit, said in an interview.
CDI recurrence rates range from 5% to 47%, depending on the institution. Although risk factors of initial CDI have been well defined, few studies have looked at risk factors associated with recurrence.
In order to get at the question, the researchers conducted a study of 108 initial CDIs and 113 recurrences at two urban and one suburban hospital. Patients who experienced recurrence were matched 1:1 to age- and gender-matched controls with no recurrent CDI.
CDI recurrence rates were 16.5% and 15.9% in the two urban hospitals, and 14.9% in the suburban hospital.
Logistic regression revealed risk factors associated with CDI recurrence, including diabetes (odds ratio, 1.91; 95% confidence interval, 1.05-3.47; P = .04), stroke (OR, 9.73; 95% CI, 1.15-82.35; P = .04), exposure to proton pump inhibitors in the past 3 months (OR, 1.82; 95% CI, 1.03-3.23; P = .04), and admission to an intensive care unit in the past 3 months (OR, 1.95; 95% CI, 1.0-3.83; P = .04).
The results suggest that diabetes and especially stroke may be important risk factors for CDI recurrence, and their presence should prompt physicians to alter patient care accordingly, according to Dr. Putrus.
He stressed the importance of antibiotic stewardship. “Once you find a certain bug or pathogen, try to deescalate the antibiotics as soon as you can. If a patient is diabetic, controlling their blood sugar may also help,” Dr. Putrus said.
Finally, physicians should consider whether proton pump inhibitors are really necessary. Some patients start on PPIs but remain on the drugs long after symptoms have abated. “A lot of patients just have some discomfort in their abdomen, and they never stop taking it. They keep refilling it. So that’s a problem,” said Dr. Putrus.
Dr. Putrus has declared no conflicts of interest.
LAS VEGAS – Diabetes and stroke are risk factors for recurrent Clostridium difficile infection (CDI), with stroke patients at about 10 times the risk of recurrence.
The underlying cause for the association is a mystery, but one-sided paralysis is one possibility. “A lot of stroke patients may be hemiplegic, and they may be bedridden, so that may be a risk factor by itself. It’s something that may need to be studied in the future,” Alan Putrus, MD, a gastroenterology fellow at St. John Providence Hospital, Detroit, said in an interview.
CDI recurrence rates range from 5% to 47%, depending on the institution. Although risk factors of initial CDI have been well defined, few studies have looked at risk factors associated with recurrence.
In order to get at the question, the researchers conducted a study of 108 initial CDIs and 113 recurrences at two urban and one suburban hospital. Patients who experienced recurrence were matched 1:1 to age- and gender-matched controls with no recurrent CDI.
CDI recurrence rates were 16.5% and 15.9% in the two urban hospitals, and 14.9% in the suburban hospital.
Logistic regression revealed risk factors associated with CDI recurrence, including diabetes (odds ratio, 1.91; 95% confidence interval, 1.05-3.47; P = .04), stroke (OR, 9.73; 95% CI, 1.15-82.35; P = .04), exposure to proton pump inhibitors in the past 3 months (OR, 1.82; 95% CI, 1.03-3.23; P = .04), and admission to an intensive care unit in the past 3 months (OR, 1.95; 95% CI, 1.0-3.83; P = .04).
The results suggest that diabetes and especially stroke may be important risk factors for CDI recurrence, and their presence should prompt physicians to alter patient care accordingly, according to Dr. Putrus.
He stressed the importance of antibiotic stewardship. “Once you find a certain bug or pathogen, try to deescalate the antibiotics as soon as you can. If a patient is diabetic, controlling their blood sugar may also help,” Dr. Putrus said.
Finally, physicians should consider whether proton pump inhibitors are really necessary. Some patients start on PPIs but remain on the drugs long after symptoms have abated. “A lot of patients just have some discomfort in their abdomen, and they never stop taking it. They keep refilling it. So that’s a problem,” said Dr. Putrus.
Dr. Putrus has declared no conflicts of interest.
LAS VEGAS – Diabetes and stroke are risk factors for recurrent Clostridium difficile infection (CDI), with stroke patients at about 10 times the risk of recurrence.
The underlying cause for the association is a mystery, but one-sided paralysis is one possibility. “A lot of stroke patients may be hemiplegic, and they may be bedridden, so that may be a risk factor by itself. It’s something that may need to be studied in the future,” Alan Putrus, MD, a gastroenterology fellow at St. John Providence Hospital, Detroit, said in an interview.
CDI recurrence rates range from 5% to 47%, depending on the institution. Although risk factors of initial CDI have been well defined, few studies have looked at risk factors associated with recurrence.
In order to get at the question, the researchers conducted a study of 108 initial CDIs and 113 recurrences at two urban and one suburban hospital. Patients who experienced recurrence were matched 1:1 to age- and gender-matched controls with no recurrent CDI.
CDI recurrence rates were 16.5% and 15.9% in the two urban hospitals, and 14.9% in the suburban hospital.
Logistic regression revealed risk factors associated with CDI recurrence, including diabetes (odds ratio, 1.91; 95% confidence interval, 1.05-3.47; P = .04), stroke (OR, 9.73; 95% CI, 1.15-82.35; P = .04), exposure to proton pump inhibitors in the past 3 months (OR, 1.82; 95% CI, 1.03-3.23; P = .04), and admission to an intensive care unit in the past 3 months (OR, 1.95; 95% CI, 1.0-3.83; P = .04).
The results suggest that diabetes and especially stroke may be important risk factors for CDI recurrence, and their presence should prompt physicians to alter patient care accordingly, according to Dr. Putrus.
He stressed the importance of antibiotic stewardship. “Once you find a certain bug or pathogen, try to deescalate the antibiotics as soon as you can. If a patient is diabetic, controlling their blood sugar may also help,” Dr. Putrus said.
Finally, physicians should consider whether proton pump inhibitors are really necessary. Some patients start on PPIs but remain on the drugs long after symptoms have abated. “A lot of patients just have some discomfort in their abdomen, and they never stop taking it. They keep refilling it. So that’s a problem,” said Dr. Putrus.
Dr. Putrus has declared no conflicts of interest.
AT ACG 2016
Key clinical point:
Major finding: Diabetes, stroke, and a history of PPI use were all associated with higher risks of recurrence.
Data source: Case-control, retrospective study.
Disclosures: Dr. Putrus has declared no conflicts of interest.
Oxygen therapy no advantage in stable COPD with moderate desaturation
Longterm supplemental oxygen had no benefit on multiple outcome measures in patients with stable chronic obstructive pulmonary disease (COPD) and resting or exercise-induced moderate desaturation, Robert Wise, MD, and his colleagues in The Long-Term Oxygen Treatment Trial (LOTT) Research Group reported.
Recommendations that supplemental oxygen be administered to patients with severe desaturation – an oxyhemoglobin saturation of less than 89% on pulse oximetry (SpO2) – date to two trials performed in the 1970s. Since that time, subsequent studies have been performed in patients with COPD and mild-to-moderate daytime hypoxemia, but the studies were underpowered to assess mortality and the impact of oxygen therapy on hospitalization, exercise performance, and quality of life were unclear.
Dr. Wise, professor of medicine and director of research, in the division of pulmonary and critical care medicine, Johns Hopkins University, Baltimore, and his fellow LOTT researchers examined whether longterm treatment with supplemental oxygen would extend life and avoid hospitalization among patients who had stable COPD with moderate resting desaturation – defined as an SpO2 of 89% to 93% – and patients who had stable COPD with moderate exercise-induced desaturation during the 6-minute walk test – defined as an SpO2 of at least 80% for at least 5 minutes and less than 90% for 10 seconds or more.
The 738 study participants, about 75% of whom were men, were randomly assigned at one of 42 centers either to receive (368) or not to receive (370) longterm supplemental oxygen. In the supplemental oxygen group, patients with resting desaturation were prescribed 24-hour oxygen, and those with desaturation only during exercise were prescribed oxygen during exercise and sleep (N Engl J Med. 2016;375:1617-27. DOI: 10.1056/NEJMoa1604344).
The groups were balanced for oxygen-desaturation type: 60 (16%) and 73 (20%) had oxygen desaturation only at rest, 171 (46%) and 148 (40%) had oxygen desaturation only upon exercise, and 139 (38%) and 147 (40%) had oxygen desaturation at rest and upon exercise. Patients were followed for 1 to 6 years.
Supplemental oxygen, regardless of prescription type or adherence, failed to benefit patients overall or any subgroup of patients with stable COPD and moderate desaturation. The results were similar for all groups based on measures of time to death or first hospitalization (hazard ratio, 0.94; 95% confidence interval [CI], 0.79 to 1.12; P = .52), hospitalization for a COPD-related hospitalizations (rate ratio, 0.99; 95% CI, 0.83 to 1.17), non–COPD-related hospitalizations (rate ratio, 1.03; 95% CI, 0.90 to 1.18), the rate of all hospitalizations (rate ratio, 1.01; 95% CI, 0.91 to 1.13), and the rate of all COPD exacerbations (rate ratio, 1.08; 95% CI, 0.98 to 1.19). Additionally, patients who did and did not receive oxygen treatment did not differ based on changes on measures of quality of life, depression, anxiety, or functional status.
Oxygen treatment also was not without risk. Among the 51 adverse events attributed to the use of supplemental oxygen were 23 reports of tripping over equipment, including two cases that necessitated hospitalization. There were five patients who reported six cases of fires or burns, including one who had to be hospitalized.
The researchers acknowledged that some patients may not have enrolled in the trial because they were too ill or felt that oxygen was beneficial. “Highly symptomatic patients who declined enrollment might have had a different response to oxygen than what we observed in the enrolled patients,” they noted.
Additionally, uniform devices weren’t used for oxygen delivery, so the amount of oxygen delivered may have varied, and the study did not evaluate the immediate effects of oxygen on symptoms or exercise performance. Nocturnal oxygen saturation was not measured, and “some patients with COPD and severe nocturnal desaturation might benefit from nocturnal oxygen supplementation,” they pointed out. Moreover, “patients’ self-reported adherence may have been an overestimate of their actual oxygen use,” they added, noting, however, that there was good agreement with use “as measured by means of serial meter readings on the concentrator.”
Based on the results, the authors concluded, “the consistency of the null findings strengthens the overall conclusion that long-term supplemental oxygen in patients with stable COPD and resting or exercise-induced moderate desaturation has no benefit with regard to the multiple outcomes measured.”
LOTT was funded by the National Heart, Lung, and Blood Institute and the Centers for Medicare and Medicaid Services. LOTT researchers reported relationships with a wide variety of drug companies.
[email protected]
On Twitter @maryjodales
This landmark study is the largest to date to provide quality evidence about clinically relevant outcomes of longterm oxygen therapy for COPD patients with moderate hypoxemia, a prescribing decision that is relatively costly and potentially places a burden on patients. For patients with moderate hypoxemia, longterm oxygen therapy consistently did not affect outcomes, and the results were not modified by the type of oxygen prescription, desaturation profile, oxygen use, sex, smoking status, or lung function.
Given the available current data, longterm oxygen therapy should be prescribed to prolong survival among patients with COPD who have more than 3 weeks of severe resting hypoxemia (PaO2 of no more than 55 mm Hg or SaO2 of less than 88%) while they are breathing ambient air. Oxygen therapy might still be appropriate in selected patients with moderate exertional hypoxemia and intractable breathlessness despite appropriate evidence-based treatment.
Ambient air or oxygen can be used to evaluate the potential benefit. Oxygen therapy can be discontinued if the patient perceives no benefit within a day or two. Selected patients who benefit should be prescribed oxygen, and I think that this treatment that should be covered by insurance payers. However, longterm oxygen therapy should not be routinely prescribed in patients with mild or moderate hypoxemia at rest or during exercise.
Magnus Ekström, MD, PhD , is with the Division of Respiratory Medicine and Allergology, Lund (Sweden) University, and the department of medicine, Blekinge Hospital, Karlskrona, Sweden. He had no relevant financial disclosures and made these remarks in an editorial that accompanied the published study ( N Engl J. Med. 2016;375: 1683-4 ).
This landmark study is the largest to date to provide quality evidence about clinically relevant outcomes of longterm oxygen therapy for COPD patients with moderate hypoxemia, a prescribing decision that is relatively costly and potentially places a burden on patients. For patients with moderate hypoxemia, longterm oxygen therapy consistently did not affect outcomes, and the results were not modified by the type of oxygen prescription, desaturation profile, oxygen use, sex, smoking status, or lung function.
Given the available current data, longterm oxygen therapy should be prescribed to prolong survival among patients with COPD who have more than 3 weeks of severe resting hypoxemia (PaO2 of no more than 55 mm Hg or SaO2 of less than 88%) while they are breathing ambient air. Oxygen therapy might still be appropriate in selected patients with moderate exertional hypoxemia and intractable breathlessness despite appropriate evidence-based treatment.
Ambient air or oxygen can be used to evaluate the potential benefit. Oxygen therapy can be discontinued if the patient perceives no benefit within a day or two. Selected patients who benefit should be prescribed oxygen, and I think that this treatment that should be covered by insurance payers. However, longterm oxygen therapy should not be routinely prescribed in patients with mild or moderate hypoxemia at rest or during exercise.
Magnus Ekström, MD, PhD , is with the Division of Respiratory Medicine and Allergology, Lund (Sweden) University, and the department of medicine, Blekinge Hospital, Karlskrona, Sweden. He had no relevant financial disclosures and made these remarks in an editorial that accompanied the published study ( N Engl J. Med. 2016;375: 1683-4 ).
This landmark study is the largest to date to provide quality evidence about clinically relevant outcomes of longterm oxygen therapy for COPD patients with moderate hypoxemia, a prescribing decision that is relatively costly and potentially places a burden on patients. For patients with moderate hypoxemia, longterm oxygen therapy consistently did not affect outcomes, and the results were not modified by the type of oxygen prescription, desaturation profile, oxygen use, sex, smoking status, or lung function.
Given the available current data, longterm oxygen therapy should be prescribed to prolong survival among patients with COPD who have more than 3 weeks of severe resting hypoxemia (PaO2 of no more than 55 mm Hg or SaO2 of less than 88%) while they are breathing ambient air. Oxygen therapy might still be appropriate in selected patients with moderate exertional hypoxemia and intractable breathlessness despite appropriate evidence-based treatment.
Ambient air or oxygen can be used to evaluate the potential benefit. Oxygen therapy can be discontinued if the patient perceives no benefit within a day or two. Selected patients who benefit should be prescribed oxygen, and I think that this treatment that should be covered by insurance payers. However, longterm oxygen therapy should not be routinely prescribed in patients with mild or moderate hypoxemia at rest or during exercise.
Magnus Ekström, MD, PhD , is with the Division of Respiratory Medicine and Allergology, Lund (Sweden) University, and the department of medicine, Blekinge Hospital, Karlskrona, Sweden. He had no relevant financial disclosures and made these remarks in an editorial that accompanied the published study ( N Engl J. Med. 2016;375: 1683-4 ).
Longterm supplemental oxygen had no benefit on multiple outcome measures in patients with stable chronic obstructive pulmonary disease (COPD) and resting or exercise-induced moderate desaturation, Robert Wise, MD, and his colleagues in The Long-Term Oxygen Treatment Trial (LOTT) Research Group reported.
Recommendations that supplemental oxygen be administered to patients with severe desaturation – an oxyhemoglobin saturation of less than 89% on pulse oximetry (SpO2) – date to two trials performed in the 1970s. Since that time, subsequent studies have been performed in patients with COPD and mild-to-moderate daytime hypoxemia, but the studies were underpowered to assess mortality and the impact of oxygen therapy on hospitalization, exercise performance, and quality of life were unclear.
Dr. Wise, professor of medicine and director of research, in the division of pulmonary and critical care medicine, Johns Hopkins University, Baltimore, and his fellow LOTT researchers examined whether longterm treatment with supplemental oxygen would extend life and avoid hospitalization among patients who had stable COPD with moderate resting desaturation – defined as an SpO2 of 89% to 93% – and patients who had stable COPD with moderate exercise-induced desaturation during the 6-minute walk test – defined as an SpO2 of at least 80% for at least 5 minutes and less than 90% for 10 seconds or more.
The 738 study participants, about 75% of whom were men, were randomly assigned at one of 42 centers either to receive (368) or not to receive (370) longterm supplemental oxygen. In the supplemental oxygen group, patients with resting desaturation were prescribed 24-hour oxygen, and those with desaturation only during exercise were prescribed oxygen during exercise and sleep (N Engl J Med. 2016;375:1617-27. DOI: 10.1056/NEJMoa1604344).
The groups were balanced for oxygen-desaturation type: 60 (16%) and 73 (20%) had oxygen desaturation only at rest, 171 (46%) and 148 (40%) had oxygen desaturation only upon exercise, and 139 (38%) and 147 (40%) had oxygen desaturation at rest and upon exercise. Patients were followed for 1 to 6 years.
Supplemental oxygen, regardless of prescription type or adherence, failed to benefit patients overall or any subgroup of patients with stable COPD and moderate desaturation. The results were similar for all groups based on measures of time to death or first hospitalization (hazard ratio, 0.94; 95% confidence interval [CI], 0.79 to 1.12; P = .52), hospitalization for a COPD-related hospitalizations (rate ratio, 0.99; 95% CI, 0.83 to 1.17), non–COPD-related hospitalizations (rate ratio, 1.03; 95% CI, 0.90 to 1.18), the rate of all hospitalizations (rate ratio, 1.01; 95% CI, 0.91 to 1.13), and the rate of all COPD exacerbations (rate ratio, 1.08; 95% CI, 0.98 to 1.19). Additionally, patients who did and did not receive oxygen treatment did not differ based on changes on measures of quality of life, depression, anxiety, or functional status.
Oxygen treatment also was not without risk. Among the 51 adverse events attributed to the use of supplemental oxygen were 23 reports of tripping over equipment, including two cases that necessitated hospitalization. There were five patients who reported six cases of fires or burns, including one who had to be hospitalized.
The researchers acknowledged that some patients may not have enrolled in the trial because they were too ill or felt that oxygen was beneficial. “Highly symptomatic patients who declined enrollment might have had a different response to oxygen than what we observed in the enrolled patients,” they noted.
Additionally, uniform devices weren’t used for oxygen delivery, so the amount of oxygen delivered may have varied, and the study did not evaluate the immediate effects of oxygen on symptoms or exercise performance. Nocturnal oxygen saturation was not measured, and “some patients with COPD and severe nocturnal desaturation might benefit from nocturnal oxygen supplementation,” they pointed out. Moreover, “patients’ self-reported adherence may have been an overestimate of their actual oxygen use,” they added, noting, however, that there was good agreement with use “as measured by means of serial meter readings on the concentrator.”
Based on the results, the authors concluded, “the consistency of the null findings strengthens the overall conclusion that long-term supplemental oxygen in patients with stable COPD and resting or exercise-induced moderate desaturation has no benefit with regard to the multiple outcomes measured.”
LOTT was funded by the National Heart, Lung, and Blood Institute and the Centers for Medicare and Medicaid Services. LOTT researchers reported relationships with a wide variety of drug companies.
[email protected]
On Twitter @maryjodales
Longterm supplemental oxygen had no benefit on multiple outcome measures in patients with stable chronic obstructive pulmonary disease (COPD) and resting or exercise-induced moderate desaturation, Robert Wise, MD, and his colleagues in The Long-Term Oxygen Treatment Trial (LOTT) Research Group reported.
Recommendations that supplemental oxygen be administered to patients with severe desaturation – an oxyhemoglobin saturation of less than 89% on pulse oximetry (SpO2) – date to two trials performed in the 1970s. Since that time, subsequent studies have been performed in patients with COPD and mild-to-moderate daytime hypoxemia, but the studies were underpowered to assess mortality and the impact of oxygen therapy on hospitalization, exercise performance, and quality of life were unclear.
Dr. Wise, professor of medicine and director of research, in the division of pulmonary and critical care medicine, Johns Hopkins University, Baltimore, and his fellow LOTT researchers examined whether longterm treatment with supplemental oxygen would extend life and avoid hospitalization among patients who had stable COPD with moderate resting desaturation – defined as an SpO2 of 89% to 93% – and patients who had stable COPD with moderate exercise-induced desaturation during the 6-minute walk test – defined as an SpO2 of at least 80% for at least 5 minutes and less than 90% for 10 seconds or more.
The 738 study participants, about 75% of whom were men, were randomly assigned at one of 42 centers either to receive (368) or not to receive (370) longterm supplemental oxygen. In the supplemental oxygen group, patients with resting desaturation were prescribed 24-hour oxygen, and those with desaturation only during exercise were prescribed oxygen during exercise and sleep (N Engl J Med. 2016;375:1617-27. DOI: 10.1056/NEJMoa1604344).
The groups were balanced for oxygen-desaturation type: 60 (16%) and 73 (20%) had oxygen desaturation only at rest, 171 (46%) and 148 (40%) had oxygen desaturation only upon exercise, and 139 (38%) and 147 (40%) had oxygen desaturation at rest and upon exercise. Patients were followed for 1 to 6 years.
Supplemental oxygen, regardless of prescription type or adherence, failed to benefit patients overall or any subgroup of patients with stable COPD and moderate desaturation. The results were similar for all groups based on measures of time to death or first hospitalization (hazard ratio, 0.94; 95% confidence interval [CI], 0.79 to 1.12; P = .52), hospitalization for a COPD-related hospitalizations (rate ratio, 0.99; 95% CI, 0.83 to 1.17), non–COPD-related hospitalizations (rate ratio, 1.03; 95% CI, 0.90 to 1.18), the rate of all hospitalizations (rate ratio, 1.01; 95% CI, 0.91 to 1.13), and the rate of all COPD exacerbations (rate ratio, 1.08; 95% CI, 0.98 to 1.19). Additionally, patients who did and did not receive oxygen treatment did not differ based on changes on measures of quality of life, depression, anxiety, or functional status.
Oxygen treatment also was not without risk. Among the 51 adverse events attributed to the use of supplemental oxygen were 23 reports of tripping over equipment, including two cases that necessitated hospitalization. There were five patients who reported six cases of fires or burns, including one who had to be hospitalized.
The researchers acknowledged that some patients may not have enrolled in the trial because they were too ill or felt that oxygen was beneficial. “Highly symptomatic patients who declined enrollment might have had a different response to oxygen than what we observed in the enrolled patients,” they noted.
Additionally, uniform devices weren’t used for oxygen delivery, so the amount of oxygen delivered may have varied, and the study did not evaluate the immediate effects of oxygen on symptoms or exercise performance. Nocturnal oxygen saturation was not measured, and “some patients with COPD and severe nocturnal desaturation might benefit from nocturnal oxygen supplementation,” they pointed out. Moreover, “patients’ self-reported adherence may have been an overestimate of their actual oxygen use,” they added, noting, however, that there was good agreement with use “as measured by means of serial meter readings on the concentrator.”
Based on the results, the authors concluded, “the consistency of the null findings strengthens the overall conclusion that long-term supplemental oxygen in patients with stable COPD and resting or exercise-induced moderate desaturation has no benefit with regard to the multiple outcomes measured.”
LOTT was funded by the National Heart, Lung, and Blood Institute and the Centers for Medicare and Medicaid Services. LOTT researchers reported relationships with a wide variety of drug companies.
[email protected]
On Twitter @maryjodales
FROM NEJM
Key clinical point:
Major finding: The results were similar for all groups based on measures of time to death or first hospitalization (hazard ratio, 0.94; 95% confidence interval [CI], 0.79 to 1.12; P = .52).
Data source: 738 study participants were randomly assigned to receive (368) or not to receive (370) longterm supplemental oxygen in The Long-Term Oxygen Treatment Trial (LOTT).
Disclosures: LOTT was funded by the National Heart, Lung, and Blood Institute and the Centers for Medicare and Medicaid Services. LOTT researchers reported relationships with a wide variety of drug companies.