User login
Professional time
As I write this article, the snow is piling up outside. While Cleveland’s west side citizens are raking up the last of fallen leaves, its east siders will dig out of 2 feet of snow. The lake effect is affecting us. The snow plow trucks vainly clear a path only for it to disappear in minutes. There seems to be no end to the torrents of white flakes that are each unique and tiny, but in aggregate uniform and overwhelming.
A blizzard of patients awaits my return from the annual meeting of the American Society of Hematology in San Diego. Like snowflakes, they are each unique, but in aggregate can be overwhelming. Plowing through a clinic, we go from patient to patient knowing that we will eventually see them all, then return to our offices or home to finish the labor of charting.
For some physicians, this is a daily reality. Whether patients in the clinic, or cases in the queue, some hematologists revisit the storm every day. Most, however, are engaged in an academic practice where at least some respite from direct patient care is offered. Whether teaching medical students, analyzing data, participating in administrative meetings, or writing manuscripts, most of us do something more beyond the clinic. We do this during our “protected time.”
But what are we protected from? Patients and their concerns? Really, this is what we want to be protected from?
“Protected” is the wrong word. The time we spend pursuing academics is really “professional” time. Some centers call it administrative time, but this also falls short. Time allotted to nonclinical activities keeps us fresh, sharpens our intellect, and ultimately helps our patients. Professional time helps prevent burnout by making us more present when we are in clinic. Professional time allows for scientific inquiry to advance treatments, and encourages continuing education to remain at the cutting edge of technology. Professional time, though, competes with patient time and that tension can drive disengagement.
Patients, and their problems, do not operate according to half-day clinic schedules. When there exists any professional time, patient time is always interfering. The interference becomes more acute as academic success increases and the allotted professional time seems inadequate. Hematologists then start to blame patients for interfering with their careers. A pernicious disdain for patient care may develop because it interrupts the academic motivations that drive many physicians once they get a taste of success. Manifestations of this attitude include dread of inpatient service, negotiations to reduce clinic time for research, and refusal to see or sometimes even talk to patients when not assigned to clinic. The more successful the academic hematologist becomes, the less he or she wants to be troubled with patients without whom professional success could not have been achieved.
The professional and patient time balance is as important to recognize as work and life balance, as one tension directly impacts the other. When nature sends a snowstorm, a warm home allows survival, but if one never ventures from home, the beauty and grandeur of nature is lost. True satisfaction comes from a balance of the two and no one person knows how best to accomplish it. I believe we can learn to manage our professional and patient time better by exchanging ideas and best practices. Please email me at [email protected] with your ideas and we will post as many as we can on the Hematology News website for all to learn from.
Dr. Kalaycio is Editor in Chief of Hematology News. Dr. Kalaycio chairs the department of hematologic oncology and blood disorders at Cleveland Clinic Taussig Cancer Institute. Contact him at [email protected].
As I write this article, the snow is piling up outside. While Cleveland’s west side citizens are raking up the last of fallen leaves, its east siders will dig out of 2 feet of snow. The lake effect is affecting us. The snow plow trucks vainly clear a path only for it to disappear in minutes. There seems to be no end to the torrents of white flakes that are each unique and tiny, but in aggregate uniform and overwhelming.
A blizzard of patients awaits my return from the annual meeting of the American Society of Hematology in San Diego. Like snowflakes, they are each unique, but in aggregate can be overwhelming. Plowing through a clinic, we go from patient to patient knowing that we will eventually see them all, then return to our offices or home to finish the labor of charting.
For some physicians, this is a daily reality. Whether patients in the clinic, or cases in the queue, some hematologists revisit the storm every day. Most, however, are engaged in an academic practice where at least some respite from direct patient care is offered. Whether teaching medical students, analyzing data, participating in administrative meetings, or writing manuscripts, most of us do something more beyond the clinic. We do this during our “protected time.”
But what are we protected from? Patients and their concerns? Really, this is what we want to be protected from?
“Protected” is the wrong word. The time we spend pursuing academics is really “professional” time. Some centers call it administrative time, but this also falls short. Time allotted to nonclinical activities keeps us fresh, sharpens our intellect, and ultimately helps our patients. Professional time helps prevent burnout by making us more present when we are in clinic. Professional time allows for scientific inquiry to advance treatments, and encourages continuing education to remain at the cutting edge of technology. Professional time, though, competes with patient time and that tension can drive disengagement.
Patients, and their problems, do not operate according to half-day clinic schedules. When there exists any professional time, patient time is always interfering. The interference becomes more acute as academic success increases and the allotted professional time seems inadequate. Hematologists then start to blame patients for interfering with their careers. A pernicious disdain for patient care may develop because it interrupts the academic motivations that drive many physicians once they get a taste of success. Manifestations of this attitude include dread of inpatient service, negotiations to reduce clinic time for research, and refusal to see or sometimes even talk to patients when not assigned to clinic. The more successful the academic hematologist becomes, the less he or she wants to be troubled with patients without whom professional success could not have been achieved.
The professional and patient time balance is as important to recognize as work and life balance, as one tension directly impacts the other. When nature sends a snowstorm, a warm home allows survival, but if one never ventures from home, the beauty and grandeur of nature is lost. True satisfaction comes from a balance of the two and no one person knows how best to accomplish it. I believe we can learn to manage our professional and patient time better by exchanging ideas and best practices. Please email me at [email protected] with your ideas and we will post as many as we can on the Hematology News website for all to learn from.
Dr. Kalaycio is Editor in Chief of Hematology News. Dr. Kalaycio chairs the department of hematologic oncology and blood disorders at Cleveland Clinic Taussig Cancer Institute. Contact him at [email protected].
As I write this article, the snow is piling up outside. While Cleveland’s west side citizens are raking up the last of fallen leaves, its east siders will dig out of 2 feet of snow. The lake effect is affecting us. The snow plow trucks vainly clear a path only for it to disappear in minutes. There seems to be no end to the torrents of white flakes that are each unique and tiny, but in aggregate uniform and overwhelming.
A blizzard of patients awaits my return from the annual meeting of the American Society of Hematology in San Diego. Like snowflakes, they are each unique, but in aggregate can be overwhelming. Plowing through a clinic, we go from patient to patient knowing that we will eventually see them all, then return to our offices or home to finish the labor of charting.
For some physicians, this is a daily reality. Whether patients in the clinic, or cases in the queue, some hematologists revisit the storm every day. Most, however, are engaged in an academic practice where at least some respite from direct patient care is offered. Whether teaching medical students, analyzing data, participating in administrative meetings, or writing manuscripts, most of us do something more beyond the clinic. We do this during our “protected time.”
But what are we protected from? Patients and their concerns? Really, this is what we want to be protected from?
“Protected” is the wrong word. The time we spend pursuing academics is really “professional” time. Some centers call it administrative time, but this also falls short. Time allotted to nonclinical activities keeps us fresh, sharpens our intellect, and ultimately helps our patients. Professional time helps prevent burnout by making us more present when we are in clinic. Professional time allows for scientific inquiry to advance treatments, and encourages continuing education to remain at the cutting edge of technology. Professional time, though, competes with patient time and that tension can drive disengagement.
Patients, and their problems, do not operate according to half-day clinic schedules. When there exists any professional time, patient time is always interfering. The interference becomes more acute as academic success increases and the allotted professional time seems inadequate. Hematologists then start to blame patients for interfering with their careers. A pernicious disdain for patient care may develop because it interrupts the academic motivations that drive many physicians once they get a taste of success. Manifestations of this attitude include dread of inpatient service, negotiations to reduce clinic time for research, and refusal to see or sometimes even talk to patients when not assigned to clinic. The more successful the academic hematologist becomes, the less he or she wants to be troubled with patients without whom professional success could not have been achieved.
The professional and patient time balance is as important to recognize as work and life balance, as one tension directly impacts the other. When nature sends a snowstorm, a warm home allows survival, but if one never ventures from home, the beauty and grandeur of nature is lost. True satisfaction comes from a balance of the two and no one person knows how best to accomplish it. I believe we can learn to manage our professional and patient time better by exchanging ideas and best practices. Please email me at [email protected] with your ideas and we will post as many as we can on the Hematology News website for all to learn from.
Dr. Kalaycio is Editor in Chief of Hematology News. Dr. Kalaycio chairs the department of hematologic oncology and blood disorders at Cleveland Clinic Taussig Cancer Institute. Contact him at [email protected].
Cutaneous Adnexal Carcinoma With Apocrine Differentiation
Differentiation between a primary adnexal carcinoma and a metastatic carcinoma to the skin is a challenging yet critical task for dermatologists and pathologists. Carcinomas that have metastasized to the skin are a sign of widespread systemic involvement and poor prognosis, while primary adnexal carcinomas tend to progress with an indolent clinical course. Although many patients with cutaneous metastases from an internal primary neoplasm can expect a median survival of no more than 12 months,1 patients with primary adnexal carcinomas are reported to have a 5-year survival rate of 95.5% for localized disease and 85% with spread to regional lymph nodes.2 We report a case of multiple cutaneous neoplasms of unknown primary origin in a 71-year-old man and describe our approach to identification of the possible primary site as well as management of the disease.
Case Report
A 71-year-old man initially presented to his primary physician for evaluation of a mass on the left side of the neck of 3 months' duration. On physical examination, a firm 2.5×3.0-cm nodule was noted at the anterior border of the trapezius muscle. Palpation of the thyroid revealed an additional right-sided nodule. The submandibular and parotid glands were unremarkable to palpation. The patient was referred to general surgery for biopsy, which revealed an infiltrating, moderately differentiated adenocarcinoma with extensive lymphatic permeation. Immunohistochemical staining for cytokeratin (CK) 7 was positive, while CK20 and thyroid transcription factor 1 were negative. A positron emission tomography/computed tomography (CT) fusion scan demonstrated 3 areas of enhanced uptake: one in the right side of the thyroid, a second corresponding to the mass on the left side of the neck at the level of the trapezius muscle, and a third in the left masseter muscle. Surgical excision with negative margins with possible chemotherapy was recommended; however, the patient declined treatment and was lost to follow-up until 2 years later when he presented to his primary physician with an additional lesion on his scalp.
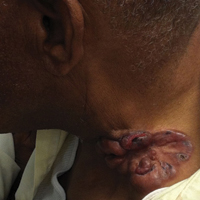
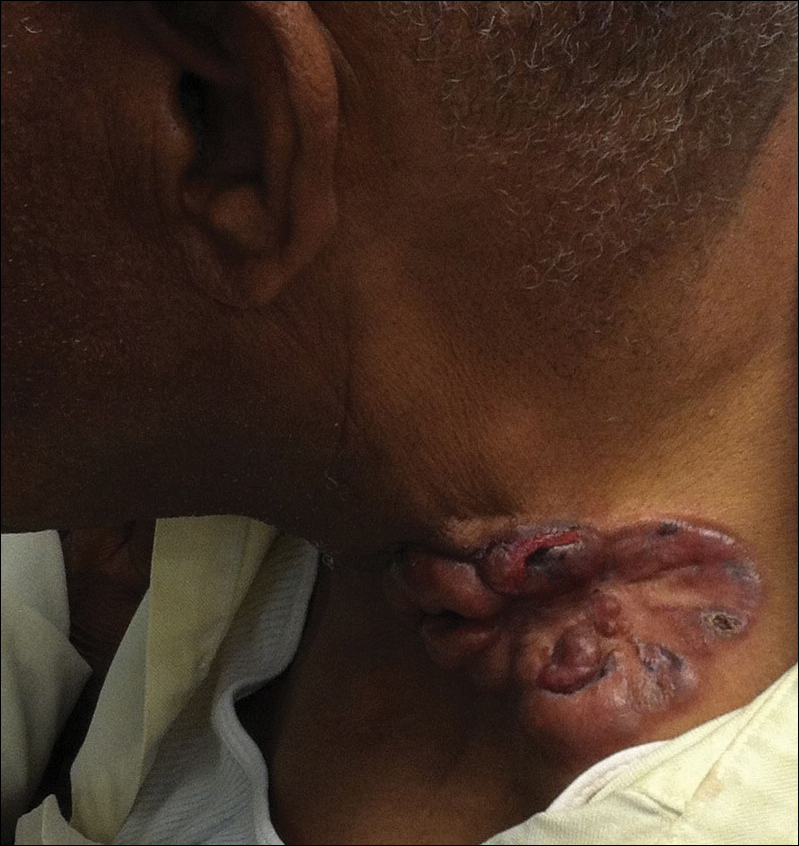
Four years after the biopsy, the patient presented to the dermatology department with additional tumor nodules including a 4-cm, annular, indurated, focally eroded plaque on the left side of the lateral neck (Figure 1); 3 separate 1-cm nodules on the right side of the lateral neck; and an ulcerated, crusted, 10×8-cm plaque on the posterior aspect of the scalp. Despite the extensive lesions, the patient remained in good health and reported no recent weight loss or signs or symptoms of systemic involvement. The posterior scalp lesion, which developed 2 years after the initial appearance of the mass on the neck and was thought to represent a possible metastasis of the tumor, was biopsied and showed diffuse infiltration of the dermis by poorly differentiated tumor cells with vacuolated cytoplasm arranged in nests and cords and sometimes in a single-file arrangement (Figure 2). A CT scan demonstrated pretracheal lymphadenopathy as well as small intraparenchymal and subpleural pulmonary nodules throughout both lung fields.
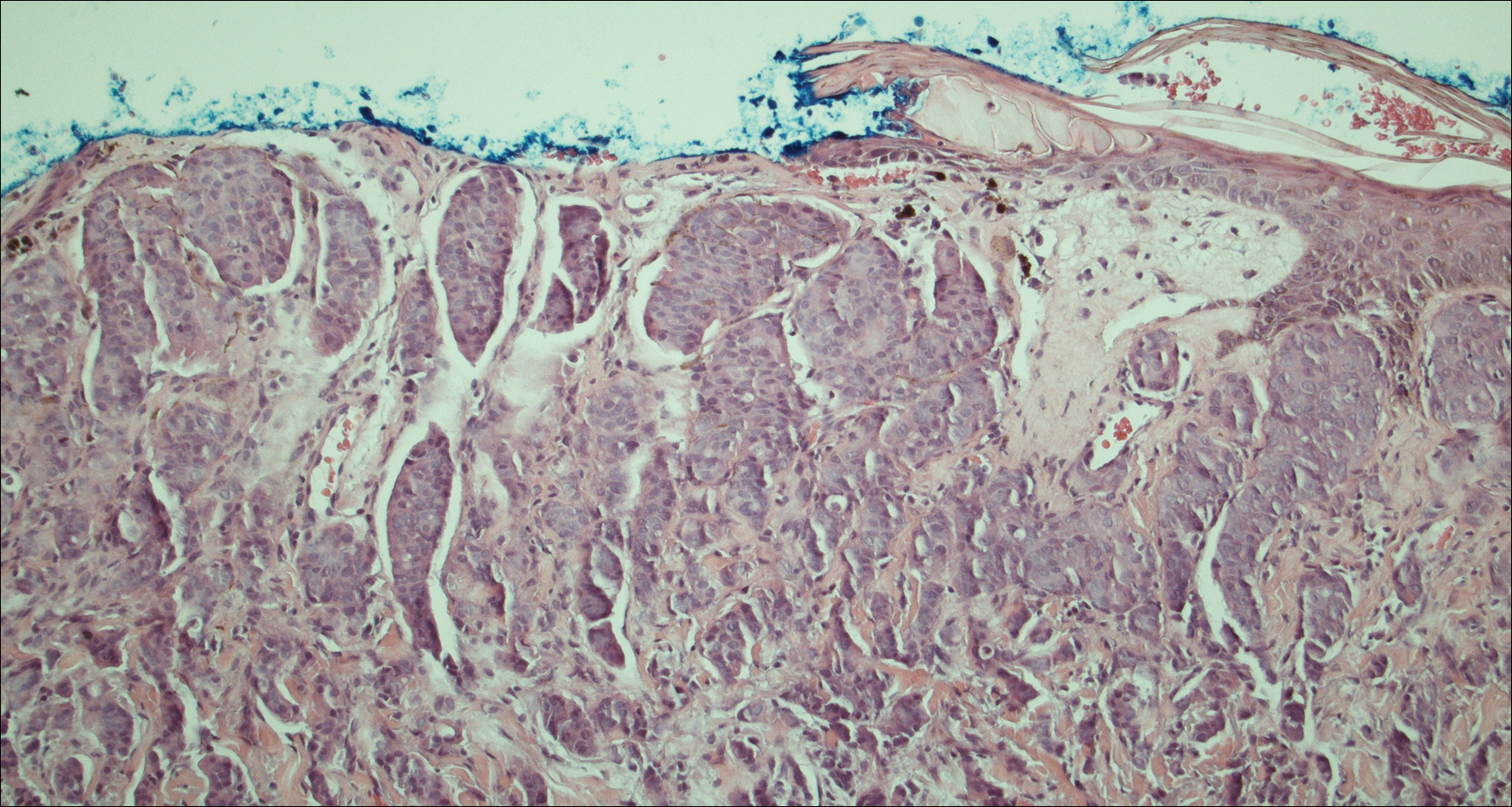
Another scalp biopsy was taken. Tumor cells were negative on mucicarmine staining. Additional immunohistochemical staining, including a periodic acid-Schiff stain with diastase digestion for epithelial mucin revealed minimal luminal positivity. Immunostaining was positive for CK7, carcinoembryonic antigen, CD15, estrogen receptor, progesterone receptor, gross cystic disease fluid protein 15 (GCDFP-15), and mammaglobin, and negative for CK20, podoplanin, thyroid transcription factor 1, S-100 protein, p63, and prostate specific antigen. ERBB2 (formerly HER2/neu) staining was negative according to fluorescence in situ hybridization analysis. Tumor cells showed a Ki-67 nuclear proliferation index of greater than 50%, indicating progression to aggressive carcinoma.
Based on the histological and immunochemical studies, the differential diagnosis included primary cutaneous apocrine carcinoma versus breast carcinoma; however, the prolonged clinical progression of these lesions favored a primary cutaneous adnexal tumor over a metastatic adenocarcinoma. Nevertheless, despite the initially indolent growth of the lesions over the first 5 years, the Ki-67 proliferation index and presence of widespread metastases on the posterior scalp indicated progression to an aggressive carcinoma. Chemotherapy was recommended as the treatment of choice. At his most recent follow-up visit 4 months later, the patient chose to begin treatment with tamoxifen and refused other treatment options.
Comment
The distinction between primary adnexal and metastatic adenocarcinomas of the skin is challenging both clinically and histologically. Some pathologists have argued that metastatic breast carcinomas and primary cutaneous apocrine carcinomas are essentially indistinguishable.3 Patients with cutaneous metastases, which occur in approximately 5.3% of all malignancies,4 typically can expect survival of no more than 12 months from the time of detection.1 In contrast, primary apocrine carcinomas of the skin, though much less common, carry a remarkably better prognosis, with 5-year relative survival rates of 95.5% and 85.5% reported for patients with localized disease and spread to regional lymph nodes, respectively.2
Fewer than 100 cases of primary cutaneous adnexal (apocrine) carcinomas have been reported overall, with the earliest known report dating back to 1944.5 According to the literature, primary apocrine carcinomas were diagnosed at a median age of 66 years and were slightly more common in females than males.2,6 Apocrine carcinomas were seen most frequently on the head, neck, and trunk,2 generally presenting in the form of asymptomatic nodules or plaques of 2 to 3 cm in size, with gradual progression occurring over months to years.6 Approximately 40% of patients have been reported with positive regional lymph nodes at diagnosis. Treatment of apocrine carcinoma typically has involved local excision with clear margins with or without lymph node dissection. Chemotherapy and radiation therapy have shown no proven benefit.7
Currently, there is no standardized approach to evaluating patients with possible cutaneous metastasis versus primary cutaneous adnexal carcinomas. Imaging studies such as mammography and abdominal CT typically reveal an internal primary cancer in one-third of patients. However, additional studies such as gastrointestinal radiography, chest and pelvic CT, barium enema, and intravenous pyelogram have shown to be of limited value.8 Although specificity and sensitivity of immunohistochemistry is limited, a number of immunomarkers, including CK7 and CK20, are routinely studied to narrow the differential diagnosis of a cutaneous neoplasm of unclear origin. Urothelial, gastric, colorectal, and pancreatic carcinomas generally are positive for CK20; CK7-positive adenocarcinomas include salivary, non-small cell lung, breast, ovarian, pancreatic, endometrial, and transitional cell adenocarcinomas. Carcinomas negative for both CK7 and CK20 include colorectal, hepatocellular, renal cell, prostate, and squamous cell carcinoma of the lung.
The presence of positive staining for estrogen and progesterone receptors as well as GCDFP-15 and mammaglobin raised the possibility of primary breast adenocarcinoma in our patient, but given that these markers can be positive in primary cutaneous adnexal tumors, immunohistochemistry results were not able to provide a definitive primary site. The overall staining pattern was nearly identical to 26 cases of primary cutaneous cribriform apocrine carcinoma, which was found to be positive for CK7 and carcinoembryonic antigen, and negative for CK20 and S-100. The only difference was in GCDFP-15 staining, which was positive in our case and negative in the cases of cribriform apocrine carcinoma.9 Histologic features favoring a primary apocrine origin include normal apocrine glands in the vicinity, glandular structures with decapitation secretion high in the dermis, and intracytoplasmic iron granules.10 Additionally, positive estrogen receptor staining appears to be much more common in apocrine carcinomas (5/10) than in eccrine carcinomas (1/7).11
A number of other markers have been investigated for possible diagnostic utility for distinction between primary adnexal carcinomas and metastatic adenocarcinomas. The nuclear transcription factor p63, which plays a role in keratinocyte differentiation, is preferentially expressed in a number of primary adnexal carcinomas and is purported to be the most sensitive marker overall, with a sensitivity of 78% to 91%.12-14 However, p63 has shown incomplete specificity for primary adnexal neoplasms, having been reported as positive in 11% to 22% of adenocarcinomas metastatic to skin.15-18 Nestin and CK15, which are expressed in hair follicle progenitor cells, also are potential specific markers for some primary adnexal lesions, specifically eccrine carcinoma, porocarcinoma, hidradenocarcinoma, and microcystic adnexal carcinoma; however, in one report, none of the apocrine carcinomas were positive for p63, cytokeratin 15, or D2-40.19 Thus, while markers for some primary adnexal neoplasms are emerging, specific tests at the immunohistochemical level for the apocrine carcinoma subgroup are still lacking.
Conclusion
In summary, a conclusive distinction between primary cutaneous apocrine carcinoma and metastatic adenocarcinoma to the skin remains challenging. Although new markers provide more specificity and sensitivity for neoplasms of eccrine origin, these markers do not appear to differentiate between primary apocrine carcinoma and metastatic breast carcinoma. In this case, as in other recent reports, diagnosis remained dependent on the clinical course of the patient. Although considerable progress has been made regarding immunohistochemical analysis of these cases, additional markers, especially ones more specific for primary skin cancers with apocrine differentiation, are still needed.
- Nashan D, Müller ML, Braun-Falco M, et al. Cutaneous metastases of visceral tumours: a review. J Cancer Res Clin Oncol. 2009;135:1-14.
- Blake PW, Bradford PT, Devesa SS, et al. Cutaneous appendageal carcinoma incidence and survival patterns in the United States: a population-based study. Arch Dermatol. 2010;146:625-632.
- Fernandez-Flores A. The elusive differential diagnosis of cutaneous apocrine adenocarcinoma vs. metastasis: the current role of clinical correlation. Acta Dermatovenerol Alp Pannonica Adriat. 2009;18:141-142.
- Lookingbill DP, Spangler N, Sexton FM. Skin involvement as the presenting sign of internal carcinoma. A retrospective study of 7316 cancer patients. J Am Acad Dermatol. 1990;22:19-26.
- Horn RC. Malignant papillary cystadenoma of sweat glands with metastases to the regional lymph nodes. Surgery. 1944;16:348-355.
- Pucevich B, Catinchi-Jaime S, Ho J, et al. Invasive primary ductal apocrine adenocarcinoma of axilla: a case report with immunohistochemical profiling and a review of literature. Dermatol Online J. 2008;14:5.
- Vasilakaki T, Skafida E, Moustou E, et al. Primary cutaneous apocrine carcinoma of sweat glands: a rare case report [published online December 17, 2011]. Case Rep Oncol. 2011;4:597-601.
- Hainsworth JD, Greco FA. Treatment of patients with cancer of an unknown primary site. N Engl J Med. 1993;329:257-263.
- Rutten A, Kutzner H, Mentzel T, et al. Primary cutaneous cribriform apocrine carcinoma: a clinicopathologic and immunohistochemical study of 26 cases of an under-recognized cutaneous adnexal neoplasm. J Am Acad Dermatol. 2009;61:644-651.
- Elder DE, Elenitsas R, Johnson BL Jr, et al, eds. Lever's Histopathology of the Skin. 10th ed. Philadelphia, PA: Lippincott, Williams, and Wilkins; 2009.
- Le LP, Dias-Santagata D, Pawlak AC, et al. Apocrine-eccrine carcinomas: molecular and immunohistochemical analyses. PLoS One. 2012;7:e47290.
- Levrero M, De Laurenzi V, Costanzo A, et al. The p53/p63/p73 family of transcription factors: overlapping and distinct functions. J Cell Sci. 2000;113:1661-1670.
- Pellegrini G, Dellambra E, Golisano O, et al. p63 identifies keratinocyte stem cells. Proc Natl Acad Sci U S A. 2001;98:3156-3161.
- Reis-Filho JS, Torio B, Albergaria A, et al. p63 expression in normal skin and usual cutaneous carcinomas. J Cutan Pathol. 2002;29:517-523.
- Sariya D, Ruth K, Adams-McDonnell R, et al. Clinicopathologic correlation of cutaneous metastases: experience from a cancer center. Arch Dermatol. 2007;143:613-620.
- Liang H, Wu H, Giorgadze TA, et al. Podoplanin is a highly sensitive and specific marker to distinguish primary skin adnexal carcinomas from adenocarcinomas metastatic to skin. Am J Surg Pathol. 2007;31:304-310.
- Kanitakis J, Chouvet B. Expression of p63 in cutaneous metastases. Am J Clin Pathol. 2007;128:753-758.
- Qureshi HS, Ormsby AH, Lee MW, et al. The diagnostic utility of p63, CK5/6, CK 7, and CK 20 in distinguishing primary cutaneous adnexal neoplasms from metastatic carcinomas. J Cutan Pathol. 2004;31:145-152.
- Mahalingam M, Nguyen LP, Richards JE, et al. The diagnostic utility of immunohistochemistry in distinguishing primary skin adnexal carcinomas from metastatic adenocarcinoma to skin: an immunohistochemical reappraisal using cytokeratin 15, nestin, p63, D2-40, and calretinin. Mod Pathol. 2010;23:713-719.
Differentiation between a primary adnexal carcinoma and a metastatic carcinoma to the skin is a challenging yet critical task for dermatologists and pathologists. Carcinomas that have metastasized to the skin are a sign of widespread systemic involvement and poor prognosis, while primary adnexal carcinomas tend to progress with an indolent clinical course. Although many patients with cutaneous metastases from an internal primary neoplasm can expect a median survival of no more than 12 months,1 patients with primary adnexal carcinomas are reported to have a 5-year survival rate of 95.5% for localized disease and 85% with spread to regional lymph nodes.2 We report a case of multiple cutaneous neoplasms of unknown primary origin in a 71-year-old man and describe our approach to identification of the possible primary site as well as management of the disease.
Case Report
A 71-year-old man initially presented to his primary physician for evaluation of a mass on the left side of the neck of 3 months' duration. On physical examination, a firm 2.5×3.0-cm nodule was noted at the anterior border of the trapezius muscle. Palpation of the thyroid revealed an additional right-sided nodule. The submandibular and parotid glands were unremarkable to palpation. The patient was referred to general surgery for biopsy, which revealed an infiltrating, moderately differentiated adenocarcinoma with extensive lymphatic permeation. Immunohistochemical staining for cytokeratin (CK) 7 was positive, while CK20 and thyroid transcription factor 1 were negative. A positron emission tomography/computed tomography (CT) fusion scan demonstrated 3 areas of enhanced uptake: one in the right side of the thyroid, a second corresponding to the mass on the left side of the neck at the level of the trapezius muscle, and a third in the left masseter muscle. Surgical excision with negative margins with possible chemotherapy was recommended; however, the patient declined treatment and was lost to follow-up until 2 years later when he presented to his primary physician with an additional lesion on his scalp.
Four years after the biopsy, the patient presented to the dermatology department with additional tumor nodules including a 4-cm, annular, indurated, focally eroded plaque on the left side of the lateral neck (Figure 1); 3 separate 1-cm nodules on the right side of the lateral neck; and an ulcerated, crusted, 10×8-cm plaque on the posterior aspect of the scalp. Despite the extensive lesions, the patient remained in good health and reported no recent weight loss or signs or symptoms of systemic involvement. The posterior scalp lesion, which developed 2 years after the initial appearance of the mass on the neck and was thought to represent a possible metastasis of the tumor, was biopsied and showed diffuse infiltration of the dermis by poorly differentiated tumor cells with vacuolated cytoplasm arranged in nests and cords and sometimes in a single-file arrangement (Figure 2). A CT scan demonstrated pretracheal lymphadenopathy as well as small intraparenchymal and subpleural pulmonary nodules throughout both lung fields.


Another scalp biopsy was taken. Tumor cells were negative on mucicarmine staining. Additional immunohistochemical staining, including a periodic acid-Schiff stain with diastase digestion for epithelial mucin revealed minimal luminal positivity. Immunostaining was positive for CK7, carcinoembryonic antigen, CD15, estrogen receptor, progesterone receptor, gross cystic disease fluid protein 15 (GCDFP-15), and mammaglobin, and negative for CK20, podoplanin, thyroid transcription factor 1, S-100 protein, p63, and prostate specific antigen. ERBB2 (formerly HER2/neu) staining was negative according to fluorescence in situ hybridization analysis. Tumor cells showed a Ki-67 nuclear proliferation index of greater than 50%, indicating progression to aggressive carcinoma.
Based on the histological and immunochemical studies, the differential diagnosis included primary cutaneous apocrine carcinoma versus breast carcinoma; however, the prolonged clinical progression of these lesions favored a primary cutaneous adnexal tumor over a metastatic adenocarcinoma. Nevertheless, despite the initially indolent growth of the lesions over the first 5 years, the Ki-67 proliferation index and presence of widespread metastases on the posterior scalp indicated progression to an aggressive carcinoma. Chemotherapy was recommended as the treatment of choice. At his most recent follow-up visit 4 months later, the patient chose to begin treatment with tamoxifen and refused other treatment options.
Comment
The distinction between primary adnexal and metastatic adenocarcinomas of the skin is challenging both clinically and histologically. Some pathologists have argued that metastatic breast carcinomas and primary cutaneous apocrine carcinomas are essentially indistinguishable.3 Patients with cutaneous metastases, which occur in approximately 5.3% of all malignancies,4 typically can expect survival of no more than 12 months from the time of detection.1 In contrast, primary apocrine carcinomas of the skin, though much less common, carry a remarkably better prognosis, with 5-year relative survival rates of 95.5% and 85.5% reported for patients with localized disease and spread to regional lymph nodes, respectively.2
Fewer than 100 cases of primary cutaneous adnexal (apocrine) carcinomas have been reported overall, with the earliest known report dating back to 1944.5 According to the literature, primary apocrine carcinomas were diagnosed at a median age of 66 years and were slightly more common in females than males.2,6 Apocrine carcinomas were seen most frequently on the head, neck, and trunk,2 generally presenting in the form of asymptomatic nodules or plaques of 2 to 3 cm in size, with gradual progression occurring over months to years.6 Approximately 40% of patients have been reported with positive regional lymph nodes at diagnosis. Treatment of apocrine carcinoma typically has involved local excision with clear margins with or without lymph node dissection. Chemotherapy and radiation therapy have shown no proven benefit.7
Currently, there is no standardized approach to evaluating patients with possible cutaneous metastasis versus primary cutaneous adnexal carcinomas. Imaging studies such as mammography and abdominal CT typically reveal an internal primary cancer in one-third of patients. However, additional studies such as gastrointestinal radiography, chest and pelvic CT, barium enema, and intravenous pyelogram have shown to be of limited value.8 Although specificity and sensitivity of immunohistochemistry is limited, a number of immunomarkers, including CK7 and CK20, are routinely studied to narrow the differential diagnosis of a cutaneous neoplasm of unclear origin. Urothelial, gastric, colorectal, and pancreatic carcinomas generally are positive for CK20; CK7-positive adenocarcinomas include salivary, non-small cell lung, breast, ovarian, pancreatic, endometrial, and transitional cell adenocarcinomas. Carcinomas negative for both CK7 and CK20 include colorectal, hepatocellular, renal cell, prostate, and squamous cell carcinoma of the lung.
The presence of positive staining for estrogen and progesterone receptors as well as GCDFP-15 and mammaglobin raised the possibility of primary breast adenocarcinoma in our patient, but given that these markers can be positive in primary cutaneous adnexal tumors, immunohistochemistry results were not able to provide a definitive primary site. The overall staining pattern was nearly identical to 26 cases of primary cutaneous cribriform apocrine carcinoma, which was found to be positive for CK7 and carcinoembryonic antigen, and negative for CK20 and S-100. The only difference was in GCDFP-15 staining, which was positive in our case and negative in the cases of cribriform apocrine carcinoma.9 Histologic features favoring a primary apocrine origin include normal apocrine glands in the vicinity, glandular structures with decapitation secretion high in the dermis, and intracytoplasmic iron granules.10 Additionally, positive estrogen receptor staining appears to be much more common in apocrine carcinomas (5/10) than in eccrine carcinomas (1/7).11
A number of other markers have been investigated for possible diagnostic utility for distinction between primary adnexal carcinomas and metastatic adenocarcinomas. The nuclear transcription factor p63, which plays a role in keratinocyte differentiation, is preferentially expressed in a number of primary adnexal carcinomas and is purported to be the most sensitive marker overall, with a sensitivity of 78% to 91%.12-14 However, p63 has shown incomplete specificity for primary adnexal neoplasms, having been reported as positive in 11% to 22% of adenocarcinomas metastatic to skin.15-18 Nestin and CK15, which are expressed in hair follicle progenitor cells, also are potential specific markers for some primary adnexal lesions, specifically eccrine carcinoma, porocarcinoma, hidradenocarcinoma, and microcystic adnexal carcinoma; however, in one report, none of the apocrine carcinomas were positive for p63, cytokeratin 15, or D2-40.19 Thus, while markers for some primary adnexal neoplasms are emerging, specific tests at the immunohistochemical level for the apocrine carcinoma subgroup are still lacking.
Conclusion
In summary, a conclusive distinction between primary cutaneous apocrine carcinoma and metastatic adenocarcinoma to the skin remains challenging. Although new markers provide more specificity and sensitivity for neoplasms of eccrine origin, these markers do not appear to differentiate between primary apocrine carcinoma and metastatic breast carcinoma. In this case, as in other recent reports, diagnosis remained dependent on the clinical course of the patient. Although considerable progress has been made regarding immunohistochemical analysis of these cases, additional markers, especially ones more specific for primary skin cancers with apocrine differentiation, are still needed.
Differentiation between a primary adnexal carcinoma and a metastatic carcinoma to the skin is a challenging yet critical task for dermatologists and pathologists. Carcinomas that have metastasized to the skin are a sign of widespread systemic involvement and poor prognosis, while primary adnexal carcinomas tend to progress with an indolent clinical course. Although many patients with cutaneous metastases from an internal primary neoplasm can expect a median survival of no more than 12 months,1 patients with primary adnexal carcinomas are reported to have a 5-year survival rate of 95.5% for localized disease and 85% with spread to regional lymph nodes.2 We report a case of multiple cutaneous neoplasms of unknown primary origin in a 71-year-old man and describe our approach to identification of the possible primary site as well as management of the disease.
Case Report
A 71-year-old man initially presented to his primary physician for evaluation of a mass on the left side of the neck of 3 months' duration. On physical examination, a firm 2.5×3.0-cm nodule was noted at the anterior border of the trapezius muscle. Palpation of the thyroid revealed an additional right-sided nodule. The submandibular and parotid glands were unremarkable to palpation. The patient was referred to general surgery for biopsy, which revealed an infiltrating, moderately differentiated adenocarcinoma with extensive lymphatic permeation. Immunohistochemical staining for cytokeratin (CK) 7 was positive, while CK20 and thyroid transcription factor 1 were negative. A positron emission tomography/computed tomography (CT) fusion scan demonstrated 3 areas of enhanced uptake: one in the right side of the thyroid, a second corresponding to the mass on the left side of the neck at the level of the trapezius muscle, and a third in the left masseter muscle. Surgical excision with negative margins with possible chemotherapy was recommended; however, the patient declined treatment and was lost to follow-up until 2 years later when he presented to his primary physician with an additional lesion on his scalp.
Four years after the biopsy, the patient presented to the dermatology department with additional tumor nodules including a 4-cm, annular, indurated, focally eroded plaque on the left side of the lateral neck (Figure 1); 3 separate 1-cm nodules on the right side of the lateral neck; and an ulcerated, crusted, 10×8-cm plaque on the posterior aspect of the scalp. Despite the extensive lesions, the patient remained in good health and reported no recent weight loss or signs or symptoms of systemic involvement. The posterior scalp lesion, which developed 2 years after the initial appearance of the mass on the neck and was thought to represent a possible metastasis of the tumor, was biopsied and showed diffuse infiltration of the dermis by poorly differentiated tumor cells with vacuolated cytoplasm arranged in nests and cords and sometimes in a single-file arrangement (Figure 2). A CT scan demonstrated pretracheal lymphadenopathy as well as small intraparenchymal and subpleural pulmonary nodules throughout both lung fields.


Another scalp biopsy was taken. Tumor cells were negative on mucicarmine staining. Additional immunohistochemical staining, including a periodic acid-Schiff stain with diastase digestion for epithelial mucin revealed minimal luminal positivity. Immunostaining was positive for CK7, carcinoembryonic antigen, CD15, estrogen receptor, progesterone receptor, gross cystic disease fluid protein 15 (GCDFP-15), and mammaglobin, and negative for CK20, podoplanin, thyroid transcription factor 1, S-100 protein, p63, and prostate specific antigen. ERBB2 (formerly HER2/neu) staining was negative according to fluorescence in situ hybridization analysis. Tumor cells showed a Ki-67 nuclear proliferation index of greater than 50%, indicating progression to aggressive carcinoma.
Based on the histological and immunochemical studies, the differential diagnosis included primary cutaneous apocrine carcinoma versus breast carcinoma; however, the prolonged clinical progression of these lesions favored a primary cutaneous adnexal tumor over a metastatic adenocarcinoma. Nevertheless, despite the initially indolent growth of the lesions over the first 5 years, the Ki-67 proliferation index and presence of widespread metastases on the posterior scalp indicated progression to an aggressive carcinoma. Chemotherapy was recommended as the treatment of choice. At his most recent follow-up visit 4 months later, the patient chose to begin treatment with tamoxifen and refused other treatment options.
Comment
The distinction between primary adnexal and metastatic adenocarcinomas of the skin is challenging both clinically and histologically. Some pathologists have argued that metastatic breast carcinomas and primary cutaneous apocrine carcinomas are essentially indistinguishable.3 Patients with cutaneous metastases, which occur in approximately 5.3% of all malignancies,4 typically can expect survival of no more than 12 months from the time of detection.1 In contrast, primary apocrine carcinomas of the skin, though much less common, carry a remarkably better prognosis, with 5-year relative survival rates of 95.5% and 85.5% reported for patients with localized disease and spread to regional lymph nodes, respectively.2
Fewer than 100 cases of primary cutaneous adnexal (apocrine) carcinomas have been reported overall, with the earliest known report dating back to 1944.5 According to the literature, primary apocrine carcinomas were diagnosed at a median age of 66 years and were slightly more common in females than males.2,6 Apocrine carcinomas were seen most frequently on the head, neck, and trunk,2 generally presenting in the form of asymptomatic nodules or plaques of 2 to 3 cm in size, with gradual progression occurring over months to years.6 Approximately 40% of patients have been reported with positive regional lymph nodes at diagnosis. Treatment of apocrine carcinoma typically has involved local excision with clear margins with or without lymph node dissection. Chemotherapy and radiation therapy have shown no proven benefit.7
Currently, there is no standardized approach to evaluating patients with possible cutaneous metastasis versus primary cutaneous adnexal carcinomas. Imaging studies such as mammography and abdominal CT typically reveal an internal primary cancer in one-third of patients. However, additional studies such as gastrointestinal radiography, chest and pelvic CT, barium enema, and intravenous pyelogram have shown to be of limited value.8 Although specificity and sensitivity of immunohistochemistry is limited, a number of immunomarkers, including CK7 and CK20, are routinely studied to narrow the differential diagnosis of a cutaneous neoplasm of unclear origin. Urothelial, gastric, colorectal, and pancreatic carcinomas generally are positive for CK20; CK7-positive adenocarcinomas include salivary, non-small cell lung, breast, ovarian, pancreatic, endometrial, and transitional cell adenocarcinomas. Carcinomas negative for both CK7 and CK20 include colorectal, hepatocellular, renal cell, prostate, and squamous cell carcinoma of the lung.
The presence of positive staining for estrogen and progesterone receptors as well as GCDFP-15 and mammaglobin raised the possibility of primary breast adenocarcinoma in our patient, but given that these markers can be positive in primary cutaneous adnexal tumors, immunohistochemistry results were not able to provide a definitive primary site. The overall staining pattern was nearly identical to 26 cases of primary cutaneous cribriform apocrine carcinoma, which was found to be positive for CK7 and carcinoembryonic antigen, and negative for CK20 and S-100. The only difference was in GCDFP-15 staining, which was positive in our case and negative in the cases of cribriform apocrine carcinoma.9 Histologic features favoring a primary apocrine origin include normal apocrine glands in the vicinity, glandular structures with decapitation secretion high in the dermis, and intracytoplasmic iron granules.10 Additionally, positive estrogen receptor staining appears to be much more common in apocrine carcinomas (5/10) than in eccrine carcinomas (1/7).11
A number of other markers have been investigated for possible diagnostic utility for distinction between primary adnexal carcinomas and metastatic adenocarcinomas. The nuclear transcription factor p63, which plays a role in keratinocyte differentiation, is preferentially expressed in a number of primary adnexal carcinomas and is purported to be the most sensitive marker overall, with a sensitivity of 78% to 91%.12-14 However, p63 has shown incomplete specificity for primary adnexal neoplasms, having been reported as positive in 11% to 22% of adenocarcinomas metastatic to skin.15-18 Nestin and CK15, which are expressed in hair follicle progenitor cells, also are potential specific markers for some primary adnexal lesions, specifically eccrine carcinoma, porocarcinoma, hidradenocarcinoma, and microcystic adnexal carcinoma; however, in one report, none of the apocrine carcinomas were positive for p63, cytokeratin 15, or D2-40.19 Thus, while markers for some primary adnexal neoplasms are emerging, specific tests at the immunohistochemical level for the apocrine carcinoma subgroup are still lacking.
Conclusion
In summary, a conclusive distinction between primary cutaneous apocrine carcinoma and metastatic adenocarcinoma to the skin remains challenging. Although new markers provide more specificity and sensitivity for neoplasms of eccrine origin, these markers do not appear to differentiate between primary apocrine carcinoma and metastatic breast carcinoma. In this case, as in other recent reports, diagnosis remained dependent on the clinical course of the patient. Although considerable progress has been made regarding immunohistochemical analysis of these cases, additional markers, especially ones more specific for primary skin cancers with apocrine differentiation, are still needed.
- Nashan D, Müller ML, Braun-Falco M, et al. Cutaneous metastases of visceral tumours: a review. J Cancer Res Clin Oncol. 2009;135:1-14.
- Blake PW, Bradford PT, Devesa SS, et al. Cutaneous appendageal carcinoma incidence and survival patterns in the United States: a population-based study. Arch Dermatol. 2010;146:625-632.
- Fernandez-Flores A. The elusive differential diagnosis of cutaneous apocrine adenocarcinoma vs. metastasis: the current role of clinical correlation. Acta Dermatovenerol Alp Pannonica Adriat. 2009;18:141-142.
- Lookingbill DP, Spangler N, Sexton FM. Skin involvement as the presenting sign of internal carcinoma. A retrospective study of 7316 cancer patients. J Am Acad Dermatol. 1990;22:19-26.
- Horn RC. Malignant papillary cystadenoma of sweat glands with metastases to the regional lymph nodes. Surgery. 1944;16:348-355.
- Pucevich B, Catinchi-Jaime S, Ho J, et al. Invasive primary ductal apocrine adenocarcinoma of axilla: a case report with immunohistochemical profiling and a review of literature. Dermatol Online J. 2008;14:5.
- Vasilakaki T, Skafida E, Moustou E, et al. Primary cutaneous apocrine carcinoma of sweat glands: a rare case report [published online December 17, 2011]. Case Rep Oncol. 2011;4:597-601.
- Hainsworth JD, Greco FA. Treatment of patients with cancer of an unknown primary site. N Engl J Med. 1993;329:257-263.
- Rutten A, Kutzner H, Mentzel T, et al. Primary cutaneous cribriform apocrine carcinoma: a clinicopathologic and immunohistochemical study of 26 cases of an under-recognized cutaneous adnexal neoplasm. J Am Acad Dermatol. 2009;61:644-651.
- Elder DE, Elenitsas R, Johnson BL Jr, et al, eds. Lever's Histopathology of the Skin. 10th ed. Philadelphia, PA: Lippincott, Williams, and Wilkins; 2009.
- Le LP, Dias-Santagata D, Pawlak AC, et al. Apocrine-eccrine carcinomas: molecular and immunohistochemical analyses. PLoS One. 2012;7:e47290.
- Levrero M, De Laurenzi V, Costanzo A, et al. The p53/p63/p73 family of transcription factors: overlapping and distinct functions. J Cell Sci. 2000;113:1661-1670.
- Pellegrini G, Dellambra E, Golisano O, et al. p63 identifies keratinocyte stem cells. Proc Natl Acad Sci U S A. 2001;98:3156-3161.
- Reis-Filho JS, Torio B, Albergaria A, et al. p63 expression in normal skin and usual cutaneous carcinomas. J Cutan Pathol. 2002;29:517-523.
- Sariya D, Ruth K, Adams-McDonnell R, et al. Clinicopathologic correlation of cutaneous metastases: experience from a cancer center. Arch Dermatol. 2007;143:613-620.
- Liang H, Wu H, Giorgadze TA, et al. Podoplanin is a highly sensitive and specific marker to distinguish primary skin adnexal carcinomas from adenocarcinomas metastatic to skin. Am J Surg Pathol. 2007;31:304-310.
- Kanitakis J, Chouvet B. Expression of p63 in cutaneous metastases. Am J Clin Pathol. 2007;128:753-758.
- Qureshi HS, Ormsby AH, Lee MW, et al. The diagnostic utility of p63, CK5/6, CK 7, and CK 20 in distinguishing primary cutaneous adnexal neoplasms from metastatic carcinomas. J Cutan Pathol. 2004;31:145-152.
- Mahalingam M, Nguyen LP, Richards JE, et al. The diagnostic utility of immunohistochemistry in distinguishing primary skin adnexal carcinomas from metastatic adenocarcinoma to skin: an immunohistochemical reappraisal using cytokeratin 15, nestin, p63, D2-40, and calretinin. Mod Pathol. 2010;23:713-719.
- Nashan D, Müller ML, Braun-Falco M, et al. Cutaneous metastases of visceral tumours: a review. J Cancer Res Clin Oncol. 2009;135:1-14.
- Blake PW, Bradford PT, Devesa SS, et al. Cutaneous appendageal carcinoma incidence and survival patterns in the United States: a population-based study. Arch Dermatol. 2010;146:625-632.
- Fernandez-Flores A. The elusive differential diagnosis of cutaneous apocrine adenocarcinoma vs. metastasis: the current role of clinical correlation. Acta Dermatovenerol Alp Pannonica Adriat. 2009;18:141-142.
- Lookingbill DP, Spangler N, Sexton FM. Skin involvement as the presenting sign of internal carcinoma. A retrospective study of 7316 cancer patients. J Am Acad Dermatol. 1990;22:19-26.
- Horn RC. Malignant papillary cystadenoma of sweat glands with metastases to the regional lymph nodes. Surgery. 1944;16:348-355.
- Pucevich B, Catinchi-Jaime S, Ho J, et al. Invasive primary ductal apocrine adenocarcinoma of axilla: a case report with immunohistochemical profiling and a review of literature. Dermatol Online J. 2008;14:5.
- Vasilakaki T, Skafida E, Moustou E, et al. Primary cutaneous apocrine carcinoma of sweat glands: a rare case report [published online December 17, 2011]. Case Rep Oncol. 2011;4:597-601.
- Hainsworth JD, Greco FA. Treatment of patients with cancer of an unknown primary site. N Engl J Med. 1993;329:257-263.
- Rutten A, Kutzner H, Mentzel T, et al. Primary cutaneous cribriform apocrine carcinoma: a clinicopathologic and immunohistochemical study of 26 cases of an under-recognized cutaneous adnexal neoplasm. J Am Acad Dermatol. 2009;61:644-651.
- Elder DE, Elenitsas R, Johnson BL Jr, et al, eds. Lever's Histopathology of the Skin. 10th ed. Philadelphia, PA: Lippincott, Williams, and Wilkins; 2009.
- Le LP, Dias-Santagata D, Pawlak AC, et al. Apocrine-eccrine carcinomas: molecular and immunohistochemical analyses. PLoS One. 2012;7:e47290.
- Levrero M, De Laurenzi V, Costanzo A, et al. The p53/p63/p73 family of transcription factors: overlapping and distinct functions. J Cell Sci. 2000;113:1661-1670.
- Pellegrini G, Dellambra E, Golisano O, et al. p63 identifies keratinocyte stem cells. Proc Natl Acad Sci U S A. 2001;98:3156-3161.
- Reis-Filho JS, Torio B, Albergaria A, et al. p63 expression in normal skin and usual cutaneous carcinomas. J Cutan Pathol. 2002;29:517-523.
- Sariya D, Ruth K, Adams-McDonnell R, et al. Clinicopathologic correlation of cutaneous metastases: experience from a cancer center. Arch Dermatol. 2007;143:613-620.
- Liang H, Wu H, Giorgadze TA, et al. Podoplanin is a highly sensitive and specific marker to distinguish primary skin adnexal carcinomas from adenocarcinomas metastatic to skin. Am J Surg Pathol. 2007;31:304-310.
- Kanitakis J, Chouvet B. Expression of p63 in cutaneous metastases. Am J Clin Pathol. 2007;128:753-758.
- Qureshi HS, Ormsby AH, Lee MW, et al. The diagnostic utility of p63, CK5/6, CK 7, and CK 20 in distinguishing primary cutaneous adnexal neoplasms from metastatic carcinomas. J Cutan Pathol. 2004;31:145-152.
- Mahalingam M, Nguyen LP, Richards JE, et al. The diagnostic utility of immunohistochemistry in distinguishing primary skin adnexal carcinomas from metastatic adenocarcinoma to skin: an immunohistochemical reappraisal using cytokeratin 15, nestin, p63, D2-40, and calretinin. Mod Pathol. 2010;23:713-719.
Practice Points
- Despite advances in immunohistochemical analysis, differentiating between primary apocrine carcinoma and metastatic breast carcinoma remains largely dependent on the clinical course of the patient.
- Treatment of apocrine carcinoma typically involves local excision with clear margins with or without lymph node dissection.
Choline and prevention of prevalent mental illnesses
Advocating on behalf of the power of prevention in psychiatry has been my life’s work. I ran a world-class community mental health center with a strong wellness component; have taught, researched, written, and spoken extensively about the importance of prevention; and have incorporated preventive ideas into my current clinical practice.
I would like to think that I have been one of the forces that helped start a new movement called “positive psychiatry,” the idea that mental health must encompass more than the reduction or elimination of psychiatric illness. In the new book edited by American Psychiatric Association Past-President Dilip V. Jeste, MD, and Barton W. Palmer, PhD, called “Positive Psychiatry” (Arlington, Va.: American Psychiatric Association Publishing, 2015), I contributed a chapter on the psychosocial factors tied to positive outcomes. In addition, I am part of a group of psychiatrists and researchers affiliated with the World Psychiatric Association who are starting an interest group focusing on positive psychiatry.
Recently, because of the prevalence of neurobehavioral disorder associated with prenatal alcohol exposure (ND-PAE) (the American Psychiatric Association’s DSM-5 version of fetal alcohol spectrum disorders) in my community, I have begun to tout this problem as a major public health issue. When we formulated the Institute of Medicine’s 2009 Preventing Mental, Emotional, and Behavioral Disorders Among Young People: Progress and Possibilities report, we did not include the problem of fetal alcohol exposure – and this was an unfortunate oversight.
However, this area of interest had not yet fully developed, and nearly 8 years later, there have been some confluent developments regarding potential prevention of this problem. They both involve choline.
First, we know that when women drink while pregnant, the alcohol they consume rids their bodies of choline, a nutrient the fetus needs for proper cell construction, neurogenesis, and neurodevelopment. Accordingly, several scientists are exploring using choline both pre- and postnatally to see if the defects on ND-PAE can be ameliorated or prevented. All of the research in this area is new, but it looks very promising.
Recently, I had the good fortune to present an idea during the Andrea Delgado Memorial Lecture at the Black Psychiatrists of America transcultural conference in the Bahamas. I also spoke at a mini-plenary at the 32nd Annual Rosalynn Carter Mental Health Policy Symposium in Atlanta. The core of the presentations were not too deep (to paraphrase a line Morgan Freeman used on Jack Nicholson in the movie “The Bucket List” – ‘I have seen bathtubs that are deeper’), but I think it explicated an essential idea. Jessie Aujla, a 4th-year medical student, and I explored the content of choline in the 25 top prenatal vitamins and found none of them contained the 450-mg daily recommended dose of choline advised by the Institute of Medicine in 1998. In fact, only two contain 50 mg; six others contain less than 30 mg; and the other 17 have no choline whatsoever (this study is in press at the Journal of Family Medicine and Prevention). So we are advocating that the prenatal vitamin manufacturers increase the choline content of their prenatal vitamins, because although women may be getting some choline from their food diets, we found one large study illustrating that 90% of pregnant women are choline deficient.
The other area of interest regarding choline as a preventive agent for mental illness is work published by researchers at the University of Colorado Denver. This research group is proposing that choline may prevent the development of autism, attention-deficit/hyperactivity disorder, and schizophrenia by an epigenetic mechanism involving a nicotinic acetylcholine receptor. This makes perfectly good sense clinically among those of us who are treating patients with ND-PAE. Some of us are starting to think of ND-PAE as a choline deficiency disorder and see symptoms that are extremely similar to autism, ADHD, and schizophrenia in such patients. Many patients with ND-PAE are misdiagnosed with these disorders. Accordingly, there appears to be some common ground between ideas aimed at preventing fetal alcohol exposure and those aimed at preventing autism, ADHD, and schizophrenia – specifically, ensuring that pregnant women get an adequate supply of choline.
There is certainly a great need to do more research to nail down these two potential preventive actions. But until that research is done, it seems to me that the least we can do is to advocate for a position that the manufacturers of prenatal vitamins at least include the daily recommended dose of choline (450 mg/day) pregnant women need per the findings of the Institute of Medicine’s Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and its Panel on Folate, Other B Vitamins, and Choline, published in 1998.
Dr. Bell is a staff psychiatrist at Jackson Park Hospital Family Medicine Clinic in Chicago; clinical psychiatrist emeritus, department of psychiatry, at the University of Illinois at Chicago; former president/CEO of Community Mental Health Council; and former director of the Institute for Juvenile Research (birthplace of child psychiatry), also in Chicago.
Advocating on behalf of the power of prevention in psychiatry has been my life’s work. I ran a world-class community mental health center with a strong wellness component; have taught, researched, written, and spoken extensively about the importance of prevention; and have incorporated preventive ideas into my current clinical practice.
I would like to think that I have been one of the forces that helped start a new movement called “positive psychiatry,” the idea that mental health must encompass more than the reduction or elimination of psychiatric illness. In the new book edited by American Psychiatric Association Past-President Dilip V. Jeste, MD, and Barton W. Palmer, PhD, called “Positive Psychiatry” (Arlington, Va.: American Psychiatric Association Publishing, 2015), I contributed a chapter on the psychosocial factors tied to positive outcomes. In addition, I am part of a group of psychiatrists and researchers affiliated with the World Psychiatric Association who are starting an interest group focusing on positive psychiatry.
Recently, because of the prevalence of neurobehavioral disorder associated with prenatal alcohol exposure (ND-PAE) (the American Psychiatric Association’s DSM-5 version of fetal alcohol spectrum disorders) in my community, I have begun to tout this problem as a major public health issue. When we formulated the Institute of Medicine’s 2009 Preventing Mental, Emotional, and Behavioral Disorders Among Young People: Progress and Possibilities report, we did not include the problem of fetal alcohol exposure – and this was an unfortunate oversight.
However, this area of interest had not yet fully developed, and nearly 8 years later, there have been some confluent developments regarding potential prevention of this problem. They both involve choline.
First, we know that when women drink while pregnant, the alcohol they consume rids their bodies of choline, a nutrient the fetus needs for proper cell construction, neurogenesis, and neurodevelopment. Accordingly, several scientists are exploring using choline both pre- and postnatally to see if the defects on ND-PAE can be ameliorated or prevented. All of the research in this area is new, but it looks very promising.
Recently, I had the good fortune to present an idea during the Andrea Delgado Memorial Lecture at the Black Psychiatrists of America transcultural conference in the Bahamas. I also spoke at a mini-plenary at the 32nd Annual Rosalynn Carter Mental Health Policy Symposium in Atlanta. The core of the presentations were not too deep (to paraphrase a line Morgan Freeman used on Jack Nicholson in the movie “The Bucket List” – ‘I have seen bathtubs that are deeper’), but I think it explicated an essential idea. Jessie Aujla, a 4th-year medical student, and I explored the content of choline in the 25 top prenatal vitamins and found none of them contained the 450-mg daily recommended dose of choline advised by the Institute of Medicine in 1998. In fact, only two contain 50 mg; six others contain less than 30 mg; and the other 17 have no choline whatsoever (this study is in press at the Journal of Family Medicine and Prevention). So we are advocating that the prenatal vitamin manufacturers increase the choline content of their prenatal vitamins, because although women may be getting some choline from their food diets, we found one large study illustrating that 90% of pregnant women are choline deficient.
The other area of interest regarding choline as a preventive agent for mental illness is work published by researchers at the University of Colorado Denver. This research group is proposing that choline may prevent the development of autism, attention-deficit/hyperactivity disorder, and schizophrenia by an epigenetic mechanism involving a nicotinic acetylcholine receptor. This makes perfectly good sense clinically among those of us who are treating patients with ND-PAE. Some of us are starting to think of ND-PAE as a choline deficiency disorder and see symptoms that are extremely similar to autism, ADHD, and schizophrenia in such patients. Many patients with ND-PAE are misdiagnosed with these disorders. Accordingly, there appears to be some common ground between ideas aimed at preventing fetal alcohol exposure and those aimed at preventing autism, ADHD, and schizophrenia – specifically, ensuring that pregnant women get an adequate supply of choline.
There is certainly a great need to do more research to nail down these two potential preventive actions. But until that research is done, it seems to me that the least we can do is to advocate for a position that the manufacturers of prenatal vitamins at least include the daily recommended dose of choline (450 mg/day) pregnant women need per the findings of the Institute of Medicine’s Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and its Panel on Folate, Other B Vitamins, and Choline, published in 1998.
Dr. Bell is a staff psychiatrist at Jackson Park Hospital Family Medicine Clinic in Chicago; clinical psychiatrist emeritus, department of psychiatry, at the University of Illinois at Chicago; former president/CEO of Community Mental Health Council; and former director of the Institute for Juvenile Research (birthplace of child psychiatry), also in Chicago.
Advocating on behalf of the power of prevention in psychiatry has been my life’s work. I ran a world-class community mental health center with a strong wellness component; have taught, researched, written, and spoken extensively about the importance of prevention; and have incorporated preventive ideas into my current clinical practice.
I would like to think that I have been one of the forces that helped start a new movement called “positive psychiatry,” the idea that mental health must encompass more than the reduction or elimination of psychiatric illness. In the new book edited by American Psychiatric Association Past-President Dilip V. Jeste, MD, and Barton W. Palmer, PhD, called “Positive Psychiatry” (Arlington, Va.: American Psychiatric Association Publishing, 2015), I contributed a chapter on the psychosocial factors tied to positive outcomes. In addition, I am part of a group of psychiatrists and researchers affiliated with the World Psychiatric Association who are starting an interest group focusing on positive psychiatry.
Recently, because of the prevalence of neurobehavioral disorder associated with prenatal alcohol exposure (ND-PAE) (the American Psychiatric Association’s DSM-5 version of fetal alcohol spectrum disorders) in my community, I have begun to tout this problem as a major public health issue. When we formulated the Institute of Medicine’s 2009 Preventing Mental, Emotional, and Behavioral Disorders Among Young People: Progress and Possibilities report, we did not include the problem of fetal alcohol exposure – and this was an unfortunate oversight.
However, this area of interest had not yet fully developed, and nearly 8 years later, there have been some confluent developments regarding potential prevention of this problem. They both involve choline.
First, we know that when women drink while pregnant, the alcohol they consume rids their bodies of choline, a nutrient the fetus needs for proper cell construction, neurogenesis, and neurodevelopment. Accordingly, several scientists are exploring using choline both pre- and postnatally to see if the defects on ND-PAE can be ameliorated or prevented. All of the research in this area is new, but it looks very promising.
Recently, I had the good fortune to present an idea during the Andrea Delgado Memorial Lecture at the Black Psychiatrists of America transcultural conference in the Bahamas. I also spoke at a mini-plenary at the 32nd Annual Rosalynn Carter Mental Health Policy Symposium in Atlanta. The core of the presentations were not too deep (to paraphrase a line Morgan Freeman used on Jack Nicholson in the movie “The Bucket List” – ‘I have seen bathtubs that are deeper’), but I think it explicated an essential idea. Jessie Aujla, a 4th-year medical student, and I explored the content of choline in the 25 top prenatal vitamins and found none of them contained the 450-mg daily recommended dose of choline advised by the Institute of Medicine in 1998. In fact, only two contain 50 mg; six others contain less than 30 mg; and the other 17 have no choline whatsoever (this study is in press at the Journal of Family Medicine and Prevention). So we are advocating that the prenatal vitamin manufacturers increase the choline content of their prenatal vitamins, because although women may be getting some choline from their food diets, we found one large study illustrating that 90% of pregnant women are choline deficient.
The other area of interest regarding choline as a preventive agent for mental illness is work published by researchers at the University of Colorado Denver. This research group is proposing that choline may prevent the development of autism, attention-deficit/hyperactivity disorder, and schizophrenia by an epigenetic mechanism involving a nicotinic acetylcholine receptor. This makes perfectly good sense clinically among those of us who are treating patients with ND-PAE. Some of us are starting to think of ND-PAE as a choline deficiency disorder and see symptoms that are extremely similar to autism, ADHD, and schizophrenia in such patients. Many patients with ND-PAE are misdiagnosed with these disorders. Accordingly, there appears to be some common ground between ideas aimed at preventing fetal alcohol exposure and those aimed at preventing autism, ADHD, and schizophrenia – specifically, ensuring that pregnant women get an adequate supply of choline.
There is certainly a great need to do more research to nail down these two potential preventive actions. But until that research is done, it seems to me that the least we can do is to advocate for a position that the manufacturers of prenatal vitamins at least include the daily recommended dose of choline (450 mg/day) pregnant women need per the findings of the Institute of Medicine’s Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and its Panel on Folate, Other B Vitamins, and Choline, published in 1998.
Dr. Bell is a staff psychiatrist at Jackson Park Hospital Family Medicine Clinic in Chicago; clinical psychiatrist emeritus, department of psychiatry, at the University of Illinois at Chicago; former president/CEO of Community Mental Health Council; and former director of the Institute for Juvenile Research (birthplace of child psychiatry), also in Chicago.
Secukinumab tames severe scalp psoriasis
VIENNA – Secukinumab proved highly effective specifically for the treatment of moderate to severe scalp psoriasis in a phase IIIb clinical trial, Mark G. Lebwohl, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
The scalp is one of the areas most commonly affected by psoriasis, yet few treatment trials have focused on patients with primarily moderate to severe scalp psoriasis. This phase IIIb study was designed to do just that. The 102 participants had psoriasis over a mean of 60% of their scalp for at least 6 months at baseline despite various forms of therapy; 40% had 70% or greater scalp involvement. The study population’s mean baseline Psoriasis Scalp Severity Index score was 34 out of a possible 72, noted Dr. Lebwohl, professor and chairman of the department of dermatology at the Icahn School of Medicine at Mount Sinai, New York.
“The mean involved body surface area was only 11.2%, and the PASI was 8.4. That is below the entry score required for most biologic studies, yet scalp involvement was substantial,” he observed.
Participants in the double-blind trial were randomized to either subcutaneous secukinumab (Cosentyx) at 300 mg on the approved treatment schedule for psoriasis or to placebo, with the primary endpoint being at least a 90% improvement in Psoriasis Area and Severity Index scores (PASI 90 response) at 12 weeks.
“The results were striking. Quite stunning,” Dr. Lebwohl said.
A PASI 90 response was achieved in 53% of secukinumab-treated patients, compared with 2% of controls. Already by week 3 a significant difference was apparent between the two study arms: At that early point, 12% of the secukinumab group, but none of the controls, had a PASI 90 response.
The secondary endpoint was change in the Investigator’s Global Assessment of scalp disease. At baseline, roughly 80% of patients had an IGA of 3 out of a possible 4 and the rest were at 4. At 3 weeks, 26% of the secukinumab group had a score of 0 or 1, signifying a clear or almost clear scalp, compared with 6% of controls. At 12 weeks, 57% of patients on secukinumab had an IGA of 0 or 1, as did 6% of those on placebo.
Side effects of secukinumab in the 12-week study were minimal. There were no serious adverse events. One case of candidiasis occurred in each study arm. Both responded readily to standard therapy.
Secukinumab is a fully human monoclonal antibody that inhibits interleukin-17A. It’s approved for treatment of moderate-to-severe psoriasis, psoriatic arthritis, and ankylosing spondylitis.
This phase IIIb clinical trial was sponsored by Novartis. Dr. Lebwohl reported that his department receives research funding from Novartis and roughly a dozen other pharmaceutical companies.
VIENNA – Secukinumab proved highly effective specifically for the treatment of moderate to severe scalp psoriasis in a phase IIIb clinical trial, Mark G. Lebwohl, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
The scalp is one of the areas most commonly affected by psoriasis, yet few treatment trials have focused on patients with primarily moderate to severe scalp psoriasis. This phase IIIb study was designed to do just that. The 102 participants had psoriasis over a mean of 60% of their scalp for at least 6 months at baseline despite various forms of therapy; 40% had 70% or greater scalp involvement. The study population’s mean baseline Psoriasis Scalp Severity Index score was 34 out of a possible 72, noted Dr. Lebwohl, professor and chairman of the department of dermatology at the Icahn School of Medicine at Mount Sinai, New York.
“The mean involved body surface area was only 11.2%, and the PASI was 8.4. That is below the entry score required for most biologic studies, yet scalp involvement was substantial,” he observed.
Participants in the double-blind trial were randomized to either subcutaneous secukinumab (Cosentyx) at 300 mg on the approved treatment schedule for psoriasis or to placebo, with the primary endpoint being at least a 90% improvement in Psoriasis Area and Severity Index scores (PASI 90 response) at 12 weeks.
“The results were striking. Quite stunning,” Dr. Lebwohl said.
A PASI 90 response was achieved in 53% of secukinumab-treated patients, compared with 2% of controls. Already by week 3 a significant difference was apparent between the two study arms: At that early point, 12% of the secukinumab group, but none of the controls, had a PASI 90 response.
The secondary endpoint was change in the Investigator’s Global Assessment of scalp disease. At baseline, roughly 80% of patients had an IGA of 3 out of a possible 4 and the rest were at 4. At 3 weeks, 26% of the secukinumab group had a score of 0 or 1, signifying a clear or almost clear scalp, compared with 6% of controls. At 12 weeks, 57% of patients on secukinumab had an IGA of 0 or 1, as did 6% of those on placebo.
Side effects of secukinumab in the 12-week study were minimal. There were no serious adverse events. One case of candidiasis occurred in each study arm. Both responded readily to standard therapy.
Secukinumab is a fully human monoclonal antibody that inhibits interleukin-17A. It’s approved for treatment of moderate-to-severe psoriasis, psoriatic arthritis, and ankylosing spondylitis.
This phase IIIb clinical trial was sponsored by Novartis. Dr. Lebwohl reported that his department receives research funding from Novartis and roughly a dozen other pharmaceutical companies.
VIENNA – Secukinumab proved highly effective specifically for the treatment of moderate to severe scalp psoriasis in a phase IIIb clinical trial, Mark G. Lebwohl, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
The scalp is one of the areas most commonly affected by psoriasis, yet few treatment trials have focused on patients with primarily moderate to severe scalp psoriasis. This phase IIIb study was designed to do just that. The 102 participants had psoriasis over a mean of 60% of their scalp for at least 6 months at baseline despite various forms of therapy; 40% had 70% or greater scalp involvement. The study population’s mean baseline Psoriasis Scalp Severity Index score was 34 out of a possible 72, noted Dr. Lebwohl, professor and chairman of the department of dermatology at the Icahn School of Medicine at Mount Sinai, New York.
“The mean involved body surface area was only 11.2%, and the PASI was 8.4. That is below the entry score required for most biologic studies, yet scalp involvement was substantial,” he observed.
Participants in the double-blind trial were randomized to either subcutaneous secukinumab (Cosentyx) at 300 mg on the approved treatment schedule for psoriasis or to placebo, with the primary endpoint being at least a 90% improvement in Psoriasis Area and Severity Index scores (PASI 90 response) at 12 weeks.
“The results were striking. Quite stunning,” Dr. Lebwohl said.
A PASI 90 response was achieved in 53% of secukinumab-treated patients, compared with 2% of controls. Already by week 3 a significant difference was apparent between the two study arms: At that early point, 12% of the secukinumab group, but none of the controls, had a PASI 90 response.
The secondary endpoint was change in the Investigator’s Global Assessment of scalp disease. At baseline, roughly 80% of patients had an IGA of 3 out of a possible 4 and the rest were at 4. At 3 weeks, 26% of the secukinumab group had a score of 0 or 1, signifying a clear or almost clear scalp, compared with 6% of controls. At 12 weeks, 57% of patients on secukinumab had an IGA of 0 or 1, as did 6% of those on placebo.
Side effects of secukinumab in the 12-week study were minimal. There were no serious adverse events. One case of candidiasis occurred in each study arm. Both responded readily to standard therapy.
Secukinumab is a fully human monoclonal antibody that inhibits interleukin-17A. It’s approved for treatment of moderate-to-severe psoriasis, psoriatic arthritis, and ankylosing spondylitis.
This phase IIIb clinical trial was sponsored by Novartis. Dr. Lebwohl reported that his department receives research funding from Novartis and roughly a dozen other pharmaceutical companies.
AT THE EADV CONGRESS
Key clinical point:
Major finding: 53% of patients with chronic moderate to severe scalp psoriasis experienced at least a 90% improvement after 12 weeks on secukinumab, compared with 2% of controls.
Data source: This prospective, double-blind, phase IIIb clinical trial randomized 102 patients with moderate to severe scalp psoriasis to secukinumab or placebo.
Disclosures: The study was sponsored by Novartis. The presenter reported that his academic department receives research funding from Novartis and roughly a dozen other pharmaceutical companies.
Teledermatology in Tijuana, Mexico
The Health Frontiers in Tijuana (HFiT) clinic is a binational partnership between the University of California, San Diego School of Medicine (San Diego, California); the Universidad Autónoma de Baja California School of Medicine (Tijuana, Mexico); and Desayunador Salesiano Padre Chava, a community grassroots organization in Tijuana, Mexico. Health Frontiers in Tijuana provides accessible quality health care for the underserved in Tijuana's Zona Norte.1 This article is a narrative meant to share my clinical experience as a dermatology resident who worked with HFiT to establish teledermatology services at this clinic.
Teledermatology in Tijuana
The patient population served by the HFiT clinic includes substance users, sex workers, the homeless, deportees, indigent patients, and recently Haitian immigrants.1 We established teledermatology services under the faculty leadership of Casey Carlos, MD, who was awarded a SkinCare for Developing Countries grant from the American Academy of Dermatology in April 2015 to address the need for teledermatology support for the clinic.2
Over the last 2 years, we have worked closely with 2 medical students from the University of California, San Diego--Nicole Herrick, BS, and Nicole DeMartinis, BA--to apply for the grant and create a system whereby volunteer residents and faculty consultants at the University of California, San Diego, can provide teledermatology services on a weekly basis to support the HFiT staff as they see patients with dermatologic conditions. Initially, we purchased touch screen tablets to use the Africa Teledermatology Project (africa.telederm.org) web-based program. The clinic was already functioning with electronic medical records with volunteers who carried tablets and scribed for the providers as they saw patients. We felt this method would be a great way to incorporate teledermatology into the clinic, and it functioned moderately well for several weeks but was very labor intensive on our part, as we frequently had to travel to Tijuana to retrain rotating clinic volunteers on how to use the program. Often, the Internet connection was slow, which made pulling up the Africa Teledermatology Project website difficult, and photographs also would take too long to upload in the middle of a busy clinic.
We are now exploring how to use a more simple email format to send the teledermatology consultations while still being compliant with the Health Insurance Portability and Accountability Act. We currently use secure university email accounts. Although we are still working out the details, this email-based method seems to work well. It has been a simple solution to accommodate a slow Internet connection and many rotating volunteers without requiring additional training. The email format also allows the photographs to be saved in draft messages, even if the Internet connection times out.
Once the teledermatology consultation is sent, the medical students and I review them and then get an attending physician's input on our proposed working diagnosis and plan. We work to have this process complete within several days to return the answered consultation to the requesting provider.
Final Thoughts
The HFiT providers have shared a lot of positive verbal feedback about this project. One frequent comment is how helpful it is to have access to a dermatologist for challenging cases. We also have heard many times that this project has inspired medical students and volunteers to expand their knowledge of dermatology. We are continuing to form new collaborative relationships with physicians in Tijuana. We will soon have the ability to train primary care providers at HFiT on performing simple skin biopsies and managing basic dermatologic conditions. Through our support of these providers, we are creating a sustainable partnership that is mutually beneficial to the patients in Tijuana as well as the medical students and residents in the United States. It is highly rewarding to all those involved with this project, and I am excited to see what challenges this next year will bring as we welcome many new patients from Haiti into the HFiT patient population.
- About Health Frontiers in Tijuana. University of California, San Diego School of Medicine website. https://meded.ucsd.edu/index.cfm/groups/hfit/about/. Accessed November 29, 2016.
- SkinCare for developing countries. American Academy of Dermatology website. https://www.aad.org/members/awards/skincare-for-developing-countries#undefined. Accessed November 29, 2016.
The Health Frontiers in Tijuana (HFiT) clinic is a binational partnership between the University of California, San Diego School of Medicine (San Diego, California); the Universidad Autónoma de Baja California School of Medicine (Tijuana, Mexico); and Desayunador Salesiano Padre Chava, a community grassroots organization in Tijuana, Mexico. Health Frontiers in Tijuana provides accessible quality health care for the underserved in Tijuana's Zona Norte.1 This article is a narrative meant to share my clinical experience as a dermatology resident who worked with HFiT to establish teledermatology services at this clinic.
Teledermatology in Tijuana
The patient population served by the HFiT clinic includes substance users, sex workers, the homeless, deportees, indigent patients, and recently Haitian immigrants.1 We established teledermatology services under the faculty leadership of Casey Carlos, MD, who was awarded a SkinCare for Developing Countries grant from the American Academy of Dermatology in April 2015 to address the need for teledermatology support for the clinic.2
Over the last 2 years, we have worked closely with 2 medical students from the University of California, San Diego--Nicole Herrick, BS, and Nicole DeMartinis, BA--to apply for the grant and create a system whereby volunteer residents and faculty consultants at the University of California, San Diego, can provide teledermatology services on a weekly basis to support the HFiT staff as they see patients with dermatologic conditions. Initially, we purchased touch screen tablets to use the Africa Teledermatology Project (africa.telederm.org) web-based program. The clinic was already functioning with electronic medical records with volunteers who carried tablets and scribed for the providers as they saw patients. We felt this method would be a great way to incorporate teledermatology into the clinic, and it functioned moderately well for several weeks but was very labor intensive on our part, as we frequently had to travel to Tijuana to retrain rotating clinic volunteers on how to use the program. Often, the Internet connection was slow, which made pulling up the Africa Teledermatology Project website difficult, and photographs also would take too long to upload in the middle of a busy clinic.
We are now exploring how to use a more simple email format to send the teledermatology consultations while still being compliant with the Health Insurance Portability and Accountability Act. We currently use secure university email accounts. Although we are still working out the details, this email-based method seems to work well. It has been a simple solution to accommodate a slow Internet connection and many rotating volunteers without requiring additional training. The email format also allows the photographs to be saved in draft messages, even if the Internet connection times out.
Once the teledermatology consultation is sent, the medical students and I review them and then get an attending physician's input on our proposed working diagnosis and plan. We work to have this process complete within several days to return the answered consultation to the requesting provider.
Final Thoughts
The HFiT providers have shared a lot of positive verbal feedback about this project. One frequent comment is how helpful it is to have access to a dermatologist for challenging cases. We also have heard many times that this project has inspired medical students and volunteers to expand their knowledge of dermatology. We are continuing to form new collaborative relationships with physicians in Tijuana. We will soon have the ability to train primary care providers at HFiT on performing simple skin biopsies and managing basic dermatologic conditions. Through our support of these providers, we are creating a sustainable partnership that is mutually beneficial to the patients in Tijuana as well as the medical students and residents in the United States. It is highly rewarding to all those involved with this project, and I am excited to see what challenges this next year will bring as we welcome many new patients from Haiti into the HFiT patient population.
The Health Frontiers in Tijuana (HFiT) clinic is a binational partnership between the University of California, San Diego School of Medicine (San Diego, California); the Universidad Autónoma de Baja California School of Medicine (Tijuana, Mexico); and Desayunador Salesiano Padre Chava, a community grassroots organization in Tijuana, Mexico. Health Frontiers in Tijuana provides accessible quality health care for the underserved in Tijuana's Zona Norte.1 This article is a narrative meant to share my clinical experience as a dermatology resident who worked with HFiT to establish teledermatology services at this clinic.
Teledermatology in Tijuana
The patient population served by the HFiT clinic includes substance users, sex workers, the homeless, deportees, indigent patients, and recently Haitian immigrants.1 We established teledermatology services under the faculty leadership of Casey Carlos, MD, who was awarded a SkinCare for Developing Countries grant from the American Academy of Dermatology in April 2015 to address the need for teledermatology support for the clinic.2
Over the last 2 years, we have worked closely with 2 medical students from the University of California, San Diego--Nicole Herrick, BS, and Nicole DeMartinis, BA--to apply for the grant and create a system whereby volunteer residents and faculty consultants at the University of California, San Diego, can provide teledermatology services on a weekly basis to support the HFiT staff as they see patients with dermatologic conditions. Initially, we purchased touch screen tablets to use the Africa Teledermatology Project (africa.telederm.org) web-based program. The clinic was already functioning with electronic medical records with volunteers who carried tablets and scribed for the providers as they saw patients. We felt this method would be a great way to incorporate teledermatology into the clinic, and it functioned moderately well for several weeks but was very labor intensive on our part, as we frequently had to travel to Tijuana to retrain rotating clinic volunteers on how to use the program. Often, the Internet connection was slow, which made pulling up the Africa Teledermatology Project website difficult, and photographs also would take too long to upload in the middle of a busy clinic.
We are now exploring how to use a more simple email format to send the teledermatology consultations while still being compliant with the Health Insurance Portability and Accountability Act. We currently use secure university email accounts. Although we are still working out the details, this email-based method seems to work well. It has been a simple solution to accommodate a slow Internet connection and many rotating volunteers without requiring additional training. The email format also allows the photographs to be saved in draft messages, even if the Internet connection times out.
Once the teledermatology consultation is sent, the medical students and I review them and then get an attending physician's input on our proposed working diagnosis and plan. We work to have this process complete within several days to return the answered consultation to the requesting provider.
Final Thoughts
The HFiT providers have shared a lot of positive verbal feedback about this project. One frequent comment is how helpful it is to have access to a dermatologist for challenging cases. We also have heard many times that this project has inspired medical students and volunteers to expand their knowledge of dermatology. We are continuing to form new collaborative relationships with physicians in Tijuana. We will soon have the ability to train primary care providers at HFiT on performing simple skin biopsies and managing basic dermatologic conditions. Through our support of these providers, we are creating a sustainable partnership that is mutually beneficial to the patients in Tijuana as well as the medical students and residents in the United States. It is highly rewarding to all those involved with this project, and I am excited to see what challenges this next year will bring as we welcome many new patients from Haiti into the HFiT patient population.
- About Health Frontiers in Tijuana. University of California, San Diego School of Medicine website. https://meded.ucsd.edu/index.cfm/groups/hfit/about/. Accessed November 29, 2016.
- SkinCare for developing countries. American Academy of Dermatology website. https://www.aad.org/members/awards/skincare-for-developing-countries#undefined. Accessed November 29, 2016.
- About Health Frontiers in Tijuana. University of California, San Diego School of Medicine website. https://meded.ucsd.edu/index.cfm/groups/hfit/about/. Accessed November 29, 2016.
- SkinCare for developing countries. American Academy of Dermatology website. https://www.aad.org/members/awards/skincare-for-developing-countries#undefined. Accessed November 29, 2016.
Ixekizumab proves highly effective for palmoplantar, scalp psoriasis
VIENNA – Ixekizumab proved markedly more effective than etanercept for treatment of palmoplantar psoriasis in a head-to-head contest in the landmark phase III UNCOVER-3 trial, Alan Menter, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
Significant improvement in palmoplantar disease was seen as early as week 2 in patients randomized to ixekizumab (Taltz), a humanized monoclonal antibody directed against interleukin-17A. Moreover, the early improvement was maintained out to week 60 with administration of 80 mg of ixekizumab by subcutaneous injection every 4 weeks in the open-label extension phase of UNCOVER-3. This pivotal trial, including 1,346 patients with moderate to severe psoriasis, helped win regulatory approval for ixekizumab for treatment of chronic plaque psoriasis.
The primary results of UNCOVER-3 have been published. At week 60, at least 80% of patients on maintenance therapy with ixekizumab had a PASI 75 response and at least 73% were rated clear or almost clear (N Engl J Med. 2016 Jul 28;375[4]:345-56).
Dr. Menter and Dr. Reich presented new subgroup analyses focused specifically on palmoplantar and scalp psoriasis because these two expressions of the disease are very important in clinical practice, albeit for different reasons.
Scalp psoriasis is extremely common in patients with plaque psoriasis. In fact, nearly 91% of subjects in UNCOVER-3 had scalp involvement.
“That’s a higher percentage than we’re accustomed to seeing in daily practice. It suggests scalp psoriasis may be more common than previously thought in patients with moderate or severe psoriasis,” said Dr. Reich, professor of dermatology at Georg-August-University in Gottingen, Germany, and a partner at the Dermatologikum Hamburg.
At week 60, more than 77% of patients on ixekizumab achieved a Psoriasis Scalp Severity Index 100 response (PSSI 100), meaning they had complete resolution of their scalp psoriasis. More than 80% achieved a PSSI 90 response indicative of complete or near complete resolution of their scalp involvement, the dermatologist reported.
“I often tell my patients that it’s because of palmoplantar psoriasis that I have so many white hairs. It’s certainly a disease that none of us cope with well topically, phototherapy-wise, PUVA-wise, or with systemic therapy. All of the studies done to date with our systemic therapies show significantly lower effect on palmoplantar psoriasis than for psoriasis at other sites. When I did the REVEAL study for Humira [adalimumab], we published a week 16 PASI 75 rate of 71%. When we did the palmoplantar psoriasis cohort, it was less than 40%,” recalled Dr. Menter, who is chair of dermatology at Baylor University Medical Center, Dallas.
“Even though palmoplantar disease affects less than 5% of the body surface area, the quality of life impact for patients with significant palmoplantar pustular or plaque psoriasis is very significant,” Dr. Menter continued. “We’ve worked with our hand surgeons and our foot surgeons to show that the impairment equals that seen in patients with severe rheumatoid arthritis or osteoarthritis of the hands and feet. So it is a huge issue.”
He reported on the 115 UNCOVER-3 participants with palmoplantar involvement. Within 2 weeks after the first 80-mg dose of ixekizumab, recipients had a 60% improvement in their Palmoplantar Psoriasis Area and Severity Index (PPSI) scores.
“It was very dramatic. These are figures that we haven’t seen with methotrexate, with retinoids, or with TNF-alpha blockers,” according to Dr. Menter.
At week 12 in UNCOVER-3, patients randomized to ixekizumab at 80 mg every 2 weeks showed an 85% improvement from baseline in PPSI scores. Those on ixekizumab at 80 mg every 4 weeks had a 78% improvement from baseline, while patients on etanercept at 50 mg twice weekly showed a 52% improvement.
At 60 weeks, PPSI 100 response rates – that is, clear palms and soles – were 60%-70% in the various ixekizumab-treated groups.
“To me, the big issue now is what about palmoplantar pustulosis, a totally different disease, and a disease with equally serious issues for our patients. I’m looking forward to studies in that population. I sincerely hope these new agents such as ixekizumab will have a significant role to play,” he said.
Dr. Menter and Dr. Reich reported receiving research support from and serving as consultants to Eli Lilly, which sponsored the UNCOVER-3 trial and markets ixekizumab, as well as numerous other pharmaceutical companies.
VIENNA – Ixekizumab proved markedly more effective than etanercept for treatment of palmoplantar psoriasis in a head-to-head contest in the landmark phase III UNCOVER-3 trial, Alan Menter, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
Significant improvement in palmoplantar disease was seen as early as week 2 in patients randomized to ixekizumab (Taltz), a humanized monoclonal antibody directed against interleukin-17A. Moreover, the early improvement was maintained out to week 60 with administration of 80 mg of ixekizumab by subcutaneous injection every 4 weeks in the open-label extension phase of UNCOVER-3. This pivotal trial, including 1,346 patients with moderate to severe psoriasis, helped win regulatory approval for ixekizumab for treatment of chronic plaque psoriasis.
The primary results of UNCOVER-3 have been published. At week 60, at least 80% of patients on maintenance therapy with ixekizumab had a PASI 75 response and at least 73% were rated clear or almost clear (N Engl J Med. 2016 Jul 28;375[4]:345-56).
Dr. Menter and Dr. Reich presented new subgroup analyses focused specifically on palmoplantar and scalp psoriasis because these two expressions of the disease are very important in clinical practice, albeit for different reasons.
Scalp psoriasis is extremely common in patients with plaque psoriasis. In fact, nearly 91% of subjects in UNCOVER-3 had scalp involvement.
“That’s a higher percentage than we’re accustomed to seeing in daily practice. It suggests scalp psoriasis may be more common than previously thought in patients with moderate or severe psoriasis,” said Dr. Reich, professor of dermatology at Georg-August-University in Gottingen, Germany, and a partner at the Dermatologikum Hamburg.
At week 60, more than 77% of patients on ixekizumab achieved a Psoriasis Scalp Severity Index 100 response (PSSI 100), meaning they had complete resolution of their scalp psoriasis. More than 80% achieved a PSSI 90 response indicative of complete or near complete resolution of their scalp involvement, the dermatologist reported.
“I often tell my patients that it’s because of palmoplantar psoriasis that I have so many white hairs. It’s certainly a disease that none of us cope with well topically, phototherapy-wise, PUVA-wise, or with systemic therapy. All of the studies done to date with our systemic therapies show significantly lower effect on palmoplantar psoriasis than for psoriasis at other sites. When I did the REVEAL study for Humira [adalimumab], we published a week 16 PASI 75 rate of 71%. When we did the palmoplantar psoriasis cohort, it was less than 40%,” recalled Dr. Menter, who is chair of dermatology at Baylor University Medical Center, Dallas.
“Even though palmoplantar disease affects less than 5% of the body surface area, the quality of life impact for patients with significant palmoplantar pustular or plaque psoriasis is very significant,” Dr. Menter continued. “We’ve worked with our hand surgeons and our foot surgeons to show that the impairment equals that seen in patients with severe rheumatoid arthritis or osteoarthritis of the hands and feet. So it is a huge issue.”
He reported on the 115 UNCOVER-3 participants with palmoplantar involvement. Within 2 weeks after the first 80-mg dose of ixekizumab, recipients had a 60% improvement in their Palmoplantar Psoriasis Area and Severity Index (PPSI) scores.
“It was very dramatic. These are figures that we haven’t seen with methotrexate, with retinoids, or with TNF-alpha blockers,” according to Dr. Menter.
At week 12 in UNCOVER-3, patients randomized to ixekizumab at 80 mg every 2 weeks showed an 85% improvement from baseline in PPSI scores. Those on ixekizumab at 80 mg every 4 weeks had a 78% improvement from baseline, while patients on etanercept at 50 mg twice weekly showed a 52% improvement.
At 60 weeks, PPSI 100 response rates – that is, clear palms and soles – were 60%-70% in the various ixekizumab-treated groups.
“To me, the big issue now is what about palmoplantar pustulosis, a totally different disease, and a disease with equally serious issues for our patients. I’m looking forward to studies in that population. I sincerely hope these new agents such as ixekizumab will have a significant role to play,” he said.
Dr. Menter and Dr. Reich reported receiving research support from and serving as consultants to Eli Lilly, which sponsored the UNCOVER-3 trial and markets ixekizumab, as well as numerous other pharmaceutical companies.
VIENNA – Ixekizumab proved markedly more effective than etanercept for treatment of palmoplantar psoriasis in a head-to-head contest in the landmark phase III UNCOVER-3 trial, Alan Menter, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
Significant improvement in palmoplantar disease was seen as early as week 2 in patients randomized to ixekizumab (Taltz), a humanized monoclonal antibody directed against interleukin-17A. Moreover, the early improvement was maintained out to week 60 with administration of 80 mg of ixekizumab by subcutaneous injection every 4 weeks in the open-label extension phase of UNCOVER-3. This pivotal trial, including 1,346 patients with moderate to severe psoriasis, helped win regulatory approval for ixekizumab for treatment of chronic plaque psoriasis.
The primary results of UNCOVER-3 have been published. At week 60, at least 80% of patients on maintenance therapy with ixekizumab had a PASI 75 response and at least 73% were rated clear or almost clear (N Engl J Med. 2016 Jul 28;375[4]:345-56).
Dr. Menter and Dr. Reich presented new subgroup analyses focused specifically on palmoplantar and scalp psoriasis because these two expressions of the disease are very important in clinical practice, albeit for different reasons.
Scalp psoriasis is extremely common in patients with plaque psoriasis. In fact, nearly 91% of subjects in UNCOVER-3 had scalp involvement.
“That’s a higher percentage than we’re accustomed to seeing in daily practice. It suggests scalp psoriasis may be more common than previously thought in patients with moderate or severe psoriasis,” said Dr. Reich, professor of dermatology at Georg-August-University in Gottingen, Germany, and a partner at the Dermatologikum Hamburg.
At week 60, more than 77% of patients on ixekizumab achieved a Psoriasis Scalp Severity Index 100 response (PSSI 100), meaning they had complete resolution of their scalp psoriasis. More than 80% achieved a PSSI 90 response indicative of complete or near complete resolution of their scalp involvement, the dermatologist reported.
“I often tell my patients that it’s because of palmoplantar psoriasis that I have so many white hairs. It’s certainly a disease that none of us cope with well topically, phototherapy-wise, PUVA-wise, or with systemic therapy. All of the studies done to date with our systemic therapies show significantly lower effect on palmoplantar psoriasis than for psoriasis at other sites. When I did the REVEAL study for Humira [adalimumab], we published a week 16 PASI 75 rate of 71%. When we did the palmoplantar psoriasis cohort, it was less than 40%,” recalled Dr. Menter, who is chair of dermatology at Baylor University Medical Center, Dallas.
“Even though palmoplantar disease affects less than 5% of the body surface area, the quality of life impact for patients with significant palmoplantar pustular or plaque psoriasis is very significant,” Dr. Menter continued. “We’ve worked with our hand surgeons and our foot surgeons to show that the impairment equals that seen in patients with severe rheumatoid arthritis or osteoarthritis of the hands and feet. So it is a huge issue.”
He reported on the 115 UNCOVER-3 participants with palmoplantar involvement. Within 2 weeks after the first 80-mg dose of ixekizumab, recipients had a 60% improvement in their Palmoplantar Psoriasis Area and Severity Index (PPSI) scores.
“It was very dramatic. These are figures that we haven’t seen with methotrexate, with retinoids, or with TNF-alpha blockers,” according to Dr. Menter.
At week 12 in UNCOVER-3, patients randomized to ixekizumab at 80 mg every 2 weeks showed an 85% improvement from baseline in PPSI scores. Those on ixekizumab at 80 mg every 4 weeks had a 78% improvement from baseline, while patients on etanercept at 50 mg twice weekly showed a 52% improvement.
At 60 weeks, PPSI 100 response rates – that is, clear palms and soles – were 60%-70% in the various ixekizumab-treated groups.
“To me, the big issue now is what about palmoplantar pustulosis, a totally different disease, and a disease with equally serious issues for our patients. I’m looking forward to studies in that population. I sincerely hope these new agents such as ixekizumab will have a significant role to play,” he said.
Dr. Menter and Dr. Reich reported receiving research support from and serving as consultants to Eli Lilly, which sponsored the UNCOVER-3 trial and markets ixekizumab, as well as numerous other pharmaceutical companies.
EXPERT ANALYSIS FROM THE EADV CONGRESS
David Henry's JCSO podcast, December 2016
In the December podcast for The Journal of Community and Supportive Oncology, Dr David Henry discusses a round-up and review by Linda Bosserman, an Editor on the Journal, of the 2016 oncology landscape – from new therapy approvals, to practice pathways and value-based care, and the implementation of MACRA. Also included are Original Reports on patients’ retrospective assessment of palliative chemotherapy for lung or gastrointestinal cancers, social support needs among patients with advanced breast cancer, and quality of life after surgery for pleural malignant mesothelioma. As always, we focus on cutting edge care for the cancer patient, with two features, one by our regular contributor, Jane de Lartigue, who brings us up to date on new therapies for pancreatic cancer, and a second, practice-oriented article that reports on adopting a team approach for co-managing the clinical and palliative components in caring for our patients. Among the regular offerings, we have Case Reports on paraneoplastic Isaacs syndrome that led to diagnosis of small-cell lung cancer and unicentric Castleman disease that was disguised as a pancreatic neoplasm, and a Community Translations report on the approval of pembrolizumab as the first immune checkpoint inhibitor to receive approval for head and neck cancer.
Listen to the podcast below.
In the December podcast for The Journal of Community and Supportive Oncology, Dr David Henry discusses a round-up and review by Linda Bosserman, an Editor on the Journal, of the 2016 oncology landscape – from new therapy approvals, to practice pathways and value-based care, and the implementation of MACRA. Also included are Original Reports on patients’ retrospective assessment of palliative chemotherapy for lung or gastrointestinal cancers, social support needs among patients with advanced breast cancer, and quality of life after surgery for pleural malignant mesothelioma. As always, we focus on cutting edge care for the cancer patient, with two features, one by our regular contributor, Jane de Lartigue, who brings us up to date on new therapies for pancreatic cancer, and a second, practice-oriented article that reports on adopting a team approach for co-managing the clinical and palliative components in caring for our patients. Among the regular offerings, we have Case Reports on paraneoplastic Isaacs syndrome that led to diagnosis of small-cell lung cancer and unicentric Castleman disease that was disguised as a pancreatic neoplasm, and a Community Translations report on the approval of pembrolizumab as the first immune checkpoint inhibitor to receive approval for head and neck cancer.
Listen to the podcast below.
In the December podcast for The Journal of Community and Supportive Oncology, Dr David Henry discusses a round-up and review by Linda Bosserman, an Editor on the Journal, of the 2016 oncology landscape – from new therapy approvals, to practice pathways and value-based care, and the implementation of MACRA. Also included are Original Reports on patients’ retrospective assessment of palliative chemotherapy for lung or gastrointestinal cancers, social support needs among patients with advanced breast cancer, and quality of life after surgery for pleural malignant mesothelioma. As always, we focus on cutting edge care for the cancer patient, with two features, one by our regular contributor, Jane de Lartigue, who brings us up to date on new therapies for pancreatic cancer, and a second, practice-oriented article that reports on adopting a team approach for co-managing the clinical and palliative components in caring for our patients. Among the regular offerings, we have Case Reports on paraneoplastic Isaacs syndrome that led to diagnosis of small-cell lung cancer and unicentric Castleman disease that was disguised as a pancreatic neoplasm, and a Community Translations report on the approval of pembrolizumab as the first immune checkpoint inhibitor to receive approval for head and neck cancer.
Listen to the podcast below.
Intermittent fasting fights ALL, not AML, in mice

Photo by Steve Berger
Intermittent fasting inhibits the development and progression of acute lymphoblastic leukemia (ALL), according to preclinical research published in Nature Medicine.
Fasting had an inhibitory effect in mouse models of T-cell and B-cell ALL but not acute myeloid leukemia (AML).
“This study using mouse models indicates that the effects of fasting on blood cancers are type-dependent and provides a platform for identifying new targets for leukemia treatments,” said study author Chengcheng “Alec” Zhang, PhD, of UT Southwestern Medical Center in Dallas, Texas.
“We also identified a mechanism responsible for the differing response to the fasting treatment.”
For this study, Dr Zhang and his colleagues created mouse models of acute leukemia—N-Myc B-ALL, activated Notch1 T-ALL, MLL-AF9 AML, and AML driven by the AML1-Eto9a oncogene—and tested the effects of various dietary restriction plans.
The team used green or yellow florescent proteins to mark and trace the leukemia cells so they could determine if the cells’ levels rose or fell in response to the fasting treatment.
“Strikingly, we found that, in models of ALL, a regimen consisting of 6 cycles of 1 day of fasting followed by 1 day of feeding completely inhibited cancer development,” Dr Zhang said.
At the end of 7 weeks, fasted mice with B-ALL had virtually no detectible cancerous cells—an average of 0.48%—compared to an average of 67.68% of cells found to be cancerous in the test areas of the non-fasted B-ALL mice.
Dr Zhang noted that, compared to B-ALL mice that ate normally, the mice on alternate-day fasting had dramatic reductions in the percentage of ALL cells in the bone marrow and spleen, as well as reduced numbers of white blood cells.
In addition, the spleens and lymph nodes in the fasted mice with B-ALL were similar in size to those of normal mice.
“Although initially cancerous, the few fluorescent cells that remained in the fasted mice after 7 weeks appeared to behave like normal cells,” Dr Zhang said. “Mice in the [B-ALL] model group that ate normally died within 59 days, while 75% of the fasted mice survived more than 120 days without signs of leukemia.”
Dr Zhang and his colleagues said they observed similar results in the T-ALL model but not the AML models. There was no decrease in leukemia cells among fasted mice with AML. And fasting actually shortened survival time in these mice.
Identifying the mechanism
Fasting is known to reduce the level of leptin, a cell signaling molecule created by fat tissue. In addition, previous studies have shown weakened activity by leptin receptors in humans with ALL. For those reasons, the researchers studied both leptin levels and leptin receptors in the mouse models.
The team found that mice with ALL showed reduced leptin receptor activity that increased with intermittent fasting.
“We found that fasting decreased the levels of leptin circulating in the bloodstream as well as decreased the leptin levels in the bone marrow,” Dr Zhang said. “These effects became more pronounced with repeated cycles of fasting. After fasting, the rate at which the leptin levels recovered seemed to correspond to the rate at which the cancerous ALL cells were cleared from the blood.”
The researchers also found that AML was associated with higher levels of leptin receptors that were unaffected by fasting, which could help explain why the fasting treatment was ineffective against this type of leukemia.
It also suggests a mechanism—the leptin receptor pathway—by which fasting exerts its effects in ALL, Dr Zhang said.
“It will be important to determine whether ALL cells can become resistant to the effects of fasting,” he noted. “It also will be interesting to investigate whether we can find alternative ways that mimic fasting to block ALL development.”
Given that this study did not involve drug treatment, researchers are discussing with clinicians whether the tested regimen might be able to move forward quickly to clinical trials. ![]()

Photo by Steve Berger
Intermittent fasting inhibits the development and progression of acute lymphoblastic leukemia (ALL), according to preclinical research published in Nature Medicine.
Fasting had an inhibitory effect in mouse models of T-cell and B-cell ALL but not acute myeloid leukemia (AML).
“This study using mouse models indicates that the effects of fasting on blood cancers are type-dependent and provides a platform for identifying new targets for leukemia treatments,” said study author Chengcheng “Alec” Zhang, PhD, of UT Southwestern Medical Center in Dallas, Texas.
“We also identified a mechanism responsible for the differing response to the fasting treatment.”
For this study, Dr Zhang and his colleagues created mouse models of acute leukemia—N-Myc B-ALL, activated Notch1 T-ALL, MLL-AF9 AML, and AML driven by the AML1-Eto9a oncogene—and tested the effects of various dietary restriction plans.
The team used green or yellow florescent proteins to mark and trace the leukemia cells so they could determine if the cells’ levels rose or fell in response to the fasting treatment.
“Strikingly, we found that, in models of ALL, a regimen consisting of 6 cycles of 1 day of fasting followed by 1 day of feeding completely inhibited cancer development,” Dr Zhang said.
At the end of 7 weeks, fasted mice with B-ALL had virtually no detectible cancerous cells—an average of 0.48%—compared to an average of 67.68% of cells found to be cancerous in the test areas of the non-fasted B-ALL mice.
Dr Zhang noted that, compared to B-ALL mice that ate normally, the mice on alternate-day fasting had dramatic reductions in the percentage of ALL cells in the bone marrow and spleen, as well as reduced numbers of white blood cells.
In addition, the spleens and lymph nodes in the fasted mice with B-ALL were similar in size to those of normal mice.
“Although initially cancerous, the few fluorescent cells that remained in the fasted mice after 7 weeks appeared to behave like normal cells,” Dr Zhang said. “Mice in the [B-ALL] model group that ate normally died within 59 days, while 75% of the fasted mice survived more than 120 days without signs of leukemia.”
Dr Zhang and his colleagues said they observed similar results in the T-ALL model but not the AML models. There was no decrease in leukemia cells among fasted mice with AML. And fasting actually shortened survival time in these mice.
Identifying the mechanism
Fasting is known to reduce the level of leptin, a cell signaling molecule created by fat tissue. In addition, previous studies have shown weakened activity by leptin receptors in humans with ALL. For those reasons, the researchers studied both leptin levels and leptin receptors in the mouse models.
The team found that mice with ALL showed reduced leptin receptor activity that increased with intermittent fasting.
“We found that fasting decreased the levels of leptin circulating in the bloodstream as well as decreased the leptin levels in the bone marrow,” Dr Zhang said. “These effects became more pronounced with repeated cycles of fasting. After fasting, the rate at which the leptin levels recovered seemed to correspond to the rate at which the cancerous ALL cells were cleared from the blood.”
The researchers also found that AML was associated with higher levels of leptin receptors that were unaffected by fasting, which could help explain why the fasting treatment was ineffective against this type of leukemia.
It also suggests a mechanism—the leptin receptor pathway—by which fasting exerts its effects in ALL, Dr Zhang said.
“It will be important to determine whether ALL cells can become resistant to the effects of fasting,” he noted. “It also will be interesting to investigate whether we can find alternative ways that mimic fasting to block ALL development.”
Given that this study did not involve drug treatment, researchers are discussing with clinicians whether the tested regimen might be able to move forward quickly to clinical trials. ![]()

Photo by Steve Berger
Intermittent fasting inhibits the development and progression of acute lymphoblastic leukemia (ALL), according to preclinical research published in Nature Medicine.
Fasting had an inhibitory effect in mouse models of T-cell and B-cell ALL but not acute myeloid leukemia (AML).
“This study using mouse models indicates that the effects of fasting on blood cancers are type-dependent and provides a platform for identifying new targets for leukemia treatments,” said study author Chengcheng “Alec” Zhang, PhD, of UT Southwestern Medical Center in Dallas, Texas.
“We also identified a mechanism responsible for the differing response to the fasting treatment.”
For this study, Dr Zhang and his colleagues created mouse models of acute leukemia—N-Myc B-ALL, activated Notch1 T-ALL, MLL-AF9 AML, and AML driven by the AML1-Eto9a oncogene—and tested the effects of various dietary restriction plans.
The team used green or yellow florescent proteins to mark and trace the leukemia cells so they could determine if the cells’ levels rose or fell in response to the fasting treatment.
“Strikingly, we found that, in models of ALL, a regimen consisting of 6 cycles of 1 day of fasting followed by 1 day of feeding completely inhibited cancer development,” Dr Zhang said.
At the end of 7 weeks, fasted mice with B-ALL had virtually no detectible cancerous cells—an average of 0.48%—compared to an average of 67.68% of cells found to be cancerous in the test areas of the non-fasted B-ALL mice.
Dr Zhang noted that, compared to B-ALL mice that ate normally, the mice on alternate-day fasting had dramatic reductions in the percentage of ALL cells in the bone marrow and spleen, as well as reduced numbers of white blood cells.
In addition, the spleens and lymph nodes in the fasted mice with B-ALL were similar in size to those of normal mice.
“Although initially cancerous, the few fluorescent cells that remained in the fasted mice after 7 weeks appeared to behave like normal cells,” Dr Zhang said. “Mice in the [B-ALL] model group that ate normally died within 59 days, while 75% of the fasted mice survived more than 120 days without signs of leukemia.”
Dr Zhang and his colleagues said they observed similar results in the T-ALL model but not the AML models. There was no decrease in leukemia cells among fasted mice with AML. And fasting actually shortened survival time in these mice.
Identifying the mechanism
Fasting is known to reduce the level of leptin, a cell signaling molecule created by fat tissue. In addition, previous studies have shown weakened activity by leptin receptors in humans with ALL. For those reasons, the researchers studied both leptin levels and leptin receptors in the mouse models.
The team found that mice with ALL showed reduced leptin receptor activity that increased with intermittent fasting.
“We found that fasting decreased the levels of leptin circulating in the bloodstream as well as decreased the leptin levels in the bone marrow,” Dr Zhang said. “These effects became more pronounced with repeated cycles of fasting. After fasting, the rate at which the leptin levels recovered seemed to correspond to the rate at which the cancerous ALL cells were cleared from the blood.”
The researchers also found that AML was associated with higher levels of leptin receptors that were unaffected by fasting, which could help explain why the fasting treatment was ineffective against this type of leukemia.
It also suggests a mechanism—the leptin receptor pathway—by which fasting exerts its effects in ALL, Dr Zhang said.
“It will be important to determine whether ALL cells can become resistant to the effects of fasting,” he noted. “It also will be interesting to investigate whether we can find alternative ways that mimic fasting to block ALL development.”
Given that this study did not involve drug treatment, researchers are discussing with clinicians whether the tested regimen might be able to move forward quickly to clinical trials. ![]()
JCAR017 gets PRIME access, breakthrough designation

The chimeric antigen receptor (CAR) T-cell therapy JCAR017 has received breakthrough therapy designation from the US Food and Drug Administration (FDA) and access to the European Medicines Agency’s (EMA) Priority Medicines (PRIME) program.
JCAR017 has gained access to the PRIME program as a treatment for relapsed/refractory diffuse large B-cell lymphoma (DLBCL).
The breakthrough designation is for JCAR017 in the treatment of patients with relapsed/refractory, aggressive, large B-cell non-Hodgkin lymphoma, including DLBCL not otherwise specified (de novo or transformed from indolent lymphoma), primary mediastinal B-cell lymphoma, and grade 3B follicular lymphoma.
JCAR017 uses a defined CD4:CD8 cell composition and 4-1BB as the costimulatory domain. The product is being developed by Juno Therapeutics, Inc. and Celgene Corporation.
The breakthrough therapy designation and PRIME eligibility for JCAR017 were granted by the FDA and EMA, respectively, on the basis of early clinical results with JCAR017 in relapsed/refractory DLBCL.
Results from a phase 1 trial of JCAR017 in relapsed/refractory DLBCL and mantle cell lymphoma were recently presented at the 2016 ASH Annual Meeting (abstract 4192).
About the PRIME program
The goal of the EMA’s PRIME program is to accelerate the development of therapies that target unmet medical needs.
The program provides enhanced EMA support and increased interaction to developers, in order to optimize development plans and speed regulatory evaluations to potentially bring these therapies to patients more quickly.
To be accepted for PRIME, a therapy must demonstrate the potential to benefit patients with unmet medical need through early clinical or nonclinical data.
About breakthrough designation
The FDA’s breakthrough therapy designation is intended to expedite the development and review of new treatments for serious or life-threatening conditions.
Breakthrough designation entitles the company developing a therapy to more intensive FDA guidance on an efficient and accelerated development program, as well as eligibility for other actions to expedite FDA review, such as a rolling submission and priority review.
To earn breakthrough designation, a treatment must show encouraging early clinical results demonstrating substantial improvement over available therapies with regard to a clinically significant endpoint, or it must fulfill an unmet need. ![]()

The chimeric antigen receptor (CAR) T-cell therapy JCAR017 has received breakthrough therapy designation from the US Food and Drug Administration (FDA) and access to the European Medicines Agency’s (EMA) Priority Medicines (PRIME) program.
JCAR017 has gained access to the PRIME program as a treatment for relapsed/refractory diffuse large B-cell lymphoma (DLBCL).
The breakthrough designation is for JCAR017 in the treatment of patients with relapsed/refractory, aggressive, large B-cell non-Hodgkin lymphoma, including DLBCL not otherwise specified (de novo or transformed from indolent lymphoma), primary mediastinal B-cell lymphoma, and grade 3B follicular lymphoma.
JCAR017 uses a defined CD4:CD8 cell composition and 4-1BB as the costimulatory domain. The product is being developed by Juno Therapeutics, Inc. and Celgene Corporation.
The breakthrough therapy designation and PRIME eligibility for JCAR017 were granted by the FDA and EMA, respectively, on the basis of early clinical results with JCAR017 in relapsed/refractory DLBCL.
Results from a phase 1 trial of JCAR017 in relapsed/refractory DLBCL and mantle cell lymphoma were recently presented at the 2016 ASH Annual Meeting (abstract 4192).
About the PRIME program
The goal of the EMA’s PRIME program is to accelerate the development of therapies that target unmet medical needs.
The program provides enhanced EMA support and increased interaction to developers, in order to optimize development plans and speed regulatory evaluations to potentially bring these therapies to patients more quickly.
To be accepted for PRIME, a therapy must demonstrate the potential to benefit patients with unmet medical need through early clinical or nonclinical data.
About breakthrough designation
The FDA’s breakthrough therapy designation is intended to expedite the development and review of new treatments for serious or life-threatening conditions.
Breakthrough designation entitles the company developing a therapy to more intensive FDA guidance on an efficient and accelerated development program, as well as eligibility for other actions to expedite FDA review, such as a rolling submission and priority review.
To earn breakthrough designation, a treatment must show encouraging early clinical results demonstrating substantial improvement over available therapies with regard to a clinically significant endpoint, or it must fulfill an unmet need. ![]()

The chimeric antigen receptor (CAR) T-cell therapy JCAR017 has received breakthrough therapy designation from the US Food and Drug Administration (FDA) and access to the European Medicines Agency’s (EMA) Priority Medicines (PRIME) program.
JCAR017 has gained access to the PRIME program as a treatment for relapsed/refractory diffuse large B-cell lymphoma (DLBCL).
The breakthrough designation is for JCAR017 in the treatment of patients with relapsed/refractory, aggressive, large B-cell non-Hodgkin lymphoma, including DLBCL not otherwise specified (de novo or transformed from indolent lymphoma), primary mediastinal B-cell lymphoma, and grade 3B follicular lymphoma.
JCAR017 uses a defined CD4:CD8 cell composition and 4-1BB as the costimulatory domain. The product is being developed by Juno Therapeutics, Inc. and Celgene Corporation.
The breakthrough therapy designation and PRIME eligibility for JCAR017 were granted by the FDA and EMA, respectively, on the basis of early clinical results with JCAR017 in relapsed/refractory DLBCL.
Results from a phase 1 trial of JCAR017 in relapsed/refractory DLBCL and mantle cell lymphoma were recently presented at the 2016 ASH Annual Meeting (abstract 4192).
About the PRIME program
The goal of the EMA’s PRIME program is to accelerate the development of therapies that target unmet medical needs.
The program provides enhanced EMA support and increased interaction to developers, in order to optimize development plans and speed regulatory evaluations to potentially bring these therapies to patients more quickly.
To be accepted for PRIME, a therapy must demonstrate the potential to benefit patients with unmet medical need through early clinical or nonclinical data.
About breakthrough designation
The FDA’s breakthrough therapy designation is intended to expedite the development and review of new treatments for serious or life-threatening conditions.
Breakthrough designation entitles the company developing a therapy to more intensive FDA guidance on an efficient and accelerated development program, as well as eligibility for other actions to expedite FDA review, such as a rolling submission and priority review.
To earn breakthrough designation, a treatment must show encouraging early clinical results demonstrating substantial improvement over available therapies with regard to a clinically significant endpoint, or it must fulfill an unmet need. ![]()
Why some patients relapse: The case for consolidation therapy in Hodgkin lymphoma

In this editorial, Andreas Engert, MD, makes the case for consolidation therapy in advanced Hodgkin lymphoma.
Dr Engert is a professor of internal medicine, hematology, and oncology at University Hospital of Cologne in Germany. He has received research funding and consultancy fees from Takeda/Millennium Pharmaceuticals and Affimed as well as research funding from Bristol-Myers Squibb.
Historically, Hodgkin lymphoma has been viewed as a cancer with generally favorable outcomes. However, it’s clear that there is an unmet need for patients with advanced stage disease.
Physicians treat newly diagnosed patients with a curative intent, but up to 30% fail to respond to initial therapy or relapse, depending on the treatment regimen used, stage of disease, and risk factors.1-3 Additionally, toxicity from frontline treatment has the potential to impact patients throughout their lives.
In line with the current standard of care, the majority of patients who fail frontline therapy will receive high-dose chemotherapy followed by an autologous stem cell transplant (ASCT).
This path of treatment, similar to frontline regimens, can be effective in eradicating the disease, but approximately half of those who undergo an ASCT subsequently relapse. Outcomes are generally poor for patients whose disease returns post-ASCT, especially if the relapse occurs within the first year.4
Consolidation therapy, used to kill remaining cancer cells after ASCT, may offer a new treatment option to address this problem. Unlike longer-term maintenance therapy, consolidation typically lasts for a short period of time—normally months instead of years—and involves intense treatment to eradicate any remaining disease.
The evidence for consolidation therapy in Hodgkin lymphoma
To understand the rationale for consolidation therapy, first consider why some patients with Hodgkin lymphoma relapse following ASCT. A small number of cancer cells, undetectable using traditional diagnostics, may remain following ASCT. This is known as minimal residual disease, and it may indicate the potential for the cancer to return.
The goal of consolidation therapy is to eliminate minimal residual disease before it progresses and causes a relapse. Unsurprisingly, timing plays a crucial role in the likelihood of achieving that goal.
In order to allow for the best chance for optimal patient outcomes, consolidation treatment should be initiated shortly after ASCT, before regrowth of cancer cells can occur. Tolerability is paramount, though, and timing must be carefully weighed by the treating physician.
Physicians and researchers learned about the impact and use of consolidation therapy from its success in other blood cancers like chronic myeloid leukemia.5,6
To prove the concept of consolidation treatment in Hodgkin lymphoma, a controlled clinical trial was conducted. The AETHERA study evaluated the use of brentuximab vedotin as consolidation therapy in patients with advanced Hodgkin lymphoma who were at increased risk of relapse or progression following ASCT.7
AETHERA was the first completed phase 3 study to explore consolidation treatment immediately following ASCT as a way of extending the effect of transplant in patients with Hodgkin lymphoma.
The results made a strong argument in favor of consolidation therapy, as patients who received brentuximab vedotin plus best supportive care after ASCT lived significantly longer without their disease worsening versus those on the placebo regimen. The safety profile of brentuximab vedotin in the AETHERA trial was generally consistent with the existing prescribing information.
Based on these data, consolidation therapy with brentuximab vedotin has been approved in several countries as a treatment option for patients with Hodgkin lymphoma who are at increased risk for relapse or progression following ASCT.
An important next step: Treating the right patients at the right time
Translating clinical evidence into real-world practice, physicians must look at which patients are most likely to benefit from consolidation therapy following ASCT—namely, those who are at increased risk of relapse. The effort to identify clear risk factors for relapse is still in progress.
Researchers across the world are currently studying patient characteristics and outcomes to determine a definitive set of risk factors that can better illustrate which patients should receive consolidation treatment.
Examples of factors under consideration include the stage of disease at diagnosis, tumor size, time to relapse, and response to previous treatment.8 Experts generally agree, however, that increased risk is cumulative and that it is not clear that any one risk factor is more important than others.
As researchers work to answer outstanding questions about consolidation therapy, there are a number of actions that the Hodgkin lymphoma community can take to help bring the right treatment options to patients.
Existing guidelines need to be evaluated and, if appropriate, adapted to give physicians across the globe the information that will allow them to provide the best care for patients at increased risk of relapse following ASCT.
Hematologists and oncologists then have the responsibility to stay informed of revisions to guidelines and to practically apply the latest research of consolidation therapy into their clinical practices.
The possibility now exists to potentially cure some Hodgkin lymphoma patients within a group that has traditionally experienced poor outcomes. As a result, a new treatment paradigm in this setting is emerging—one that may help solve the challenge of post-ASCT relapse in Hodgkin lymphoma.
Additional information on the use of consolidation therapy in Hodgkin lymphoma is available in the paper, Consolidation Therapy After ASCT in Hodgkin Lymphoma: Why and Who to Treat? ![]()
1 Diehl, V, Franklin, J, Pfreundschuh, M, et al. Standard and Increased-Dose BEACOPP Chemotherapy Compared with COPP-ABVD for Advanced Hodgkin’s Disease. N Engl J Med 2003;348:2386-95.
2 Duggan, D, Petroni, G, Johnson, J, et al. Randomized Comparison of ABVD and MOPP/ABV Hybrid for the Treatment of Advanced Hodgkin’s Disease: Report of an Intergroup Trial. J Clin Oncol 2003;21:607-614.
3 Federico, M, Luminari, S, Iannitto, E, et al. ABVD Compared With BEACOPP Compared With CEC for the Initial Treatment of Patients With Advanced Hodgkin’s Lymphoma: Results From the HD2000 Gruppo Italiano per lo Studio dei Linfomi Trial. J Clin Oncol 2009;27:805-811.
4 Arai S, Fanale M, deVos S, et al. Defining a Hodgkin lymphoma population for novel therapeutics after relapse from autologous hematopoietic cell transplant. Leuk Lymphoma. 2013;54:2531–2533.
5 Zonder, J and Schiffer, C. Update on practical aspects of the treatment of chronic myeloid leukemia with imatinib mesylate. Curr Hematol Malig Rep 2006;1:141.
6 Giralt SA, Arora M, Goldman JM, et al. Impact of imatinib therapy on the use of allogeneic haematopoietic progenitor cell transplantation for the treatment of chronic myeloid leukaemia. Br J Haematol 2007;137(5):461-467.
7 Moskowitz CH, Nadamanee A, Masszi T, et al; for the AETHERA Study Group. Brentuximab vedotin as consolidation therapy after autologous stem-cell transplantation in patients with Hodgkin’s lymphoma at risk of relapse or progression (AETHERA): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;385:1853-1862.
8 Bröckelmann PJ, Müller H, Casasnovas O, et al. Risk factors and a prognostic score for progression free survival after treatment with autologous stem cell transplantation (ASCT) in patients with relapsed or refractory Hodgkin lymphoma (rrHL). Poster presented at: 57th American Society of Hematology (ASH) Annual Meeting & Exposition; December 5-8, 2015; Orlando, FL.

In this editorial, Andreas Engert, MD, makes the case for consolidation therapy in advanced Hodgkin lymphoma.
Dr Engert is a professor of internal medicine, hematology, and oncology at University Hospital of Cologne in Germany. He has received research funding and consultancy fees from Takeda/Millennium Pharmaceuticals and Affimed as well as research funding from Bristol-Myers Squibb.
Historically, Hodgkin lymphoma has been viewed as a cancer with generally favorable outcomes. However, it’s clear that there is an unmet need for patients with advanced stage disease.
Physicians treat newly diagnosed patients with a curative intent, but up to 30% fail to respond to initial therapy or relapse, depending on the treatment regimen used, stage of disease, and risk factors.1-3 Additionally, toxicity from frontline treatment has the potential to impact patients throughout their lives.
In line with the current standard of care, the majority of patients who fail frontline therapy will receive high-dose chemotherapy followed by an autologous stem cell transplant (ASCT).
This path of treatment, similar to frontline regimens, can be effective in eradicating the disease, but approximately half of those who undergo an ASCT subsequently relapse. Outcomes are generally poor for patients whose disease returns post-ASCT, especially if the relapse occurs within the first year.4
Consolidation therapy, used to kill remaining cancer cells after ASCT, may offer a new treatment option to address this problem. Unlike longer-term maintenance therapy, consolidation typically lasts for a short period of time—normally months instead of years—and involves intense treatment to eradicate any remaining disease.
The evidence for consolidation therapy in Hodgkin lymphoma
To understand the rationale for consolidation therapy, first consider why some patients with Hodgkin lymphoma relapse following ASCT. A small number of cancer cells, undetectable using traditional diagnostics, may remain following ASCT. This is known as minimal residual disease, and it may indicate the potential for the cancer to return.
The goal of consolidation therapy is to eliminate minimal residual disease before it progresses and causes a relapse. Unsurprisingly, timing plays a crucial role in the likelihood of achieving that goal.
In order to allow for the best chance for optimal patient outcomes, consolidation treatment should be initiated shortly after ASCT, before regrowth of cancer cells can occur. Tolerability is paramount, though, and timing must be carefully weighed by the treating physician.
Physicians and researchers learned about the impact and use of consolidation therapy from its success in other blood cancers like chronic myeloid leukemia.5,6
To prove the concept of consolidation treatment in Hodgkin lymphoma, a controlled clinical trial was conducted. The AETHERA study evaluated the use of brentuximab vedotin as consolidation therapy in patients with advanced Hodgkin lymphoma who were at increased risk of relapse or progression following ASCT.7
AETHERA was the first completed phase 3 study to explore consolidation treatment immediately following ASCT as a way of extending the effect of transplant in patients with Hodgkin lymphoma.
The results made a strong argument in favor of consolidation therapy, as patients who received brentuximab vedotin plus best supportive care after ASCT lived significantly longer without their disease worsening versus those on the placebo regimen. The safety profile of brentuximab vedotin in the AETHERA trial was generally consistent with the existing prescribing information.
Based on these data, consolidation therapy with brentuximab vedotin has been approved in several countries as a treatment option for patients with Hodgkin lymphoma who are at increased risk for relapse or progression following ASCT.
An important next step: Treating the right patients at the right time
Translating clinical evidence into real-world practice, physicians must look at which patients are most likely to benefit from consolidation therapy following ASCT—namely, those who are at increased risk of relapse. The effort to identify clear risk factors for relapse is still in progress.
Researchers across the world are currently studying patient characteristics and outcomes to determine a definitive set of risk factors that can better illustrate which patients should receive consolidation treatment.
Examples of factors under consideration include the stage of disease at diagnosis, tumor size, time to relapse, and response to previous treatment.8 Experts generally agree, however, that increased risk is cumulative and that it is not clear that any one risk factor is more important than others.
As researchers work to answer outstanding questions about consolidation therapy, there are a number of actions that the Hodgkin lymphoma community can take to help bring the right treatment options to patients.
Existing guidelines need to be evaluated and, if appropriate, adapted to give physicians across the globe the information that will allow them to provide the best care for patients at increased risk of relapse following ASCT.
Hematologists and oncologists then have the responsibility to stay informed of revisions to guidelines and to practically apply the latest research of consolidation therapy into their clinical practices.
The possibility now exists to potentially cure some Hodgkin lymphoma patients within a group that has traditionally experienced poor outcomes. As a result, a new treatment paradigm in this setting is emerging—one that may help solve the challenge of post-ASCT relapse in Hodgkin lymphoma.
Additional information on the use of consolidation therapy in Hodgkin lymphoma is available in the paper, Consolidation Therapy After ASCT in Hodgkin Lymphoma: Why and Who to Treat? ![]()
1 Diehl, V, Franklin, J, Pfreundschuh, M, et al. Standard and Increased-Dose BEACOPP Chemotherapy Compared with COPP-ABVD for Advanced Hodgkin’s Disease. N Engl J Med 2003;348:2386-95.
2 Duggan, D, Petroni, G, Johnson, J, et al. Randomized Comparison of ABVD and MOPP/ABV Hybrid for the Treatment of Advanced Hodgkin’s Disease: Report of an Intergroup Trial. J Clin Oncol 2003;21:607-614.
3 Federico, M, Luminari, S, Iannitto, E, et al. ABVD Compared With BEACOPP Compared With CEC for the Initial Treatment of Patients With Advanced Hodgkin’s Lymphoma: Results From the HD2000 Gruppo Italiano per lo Studio dei Linfomi Trial. J Clin Oncol 2009;27:805-811.
4 Arai S, Fanale M, deVos S, et al. Defining a Hodgkin lymphoma population for novel therapeutics after relapse from autologous hematopoietic cell transplant. Leuk Lymphoma. 2013;54:2531–2533.
5 Zonder, J and Schiffer, C. Update on practical aspects of the treatment of chronic myeloid leukemia with imatinib mesylate. Curr Hematol Malig Rep 2006;1:141.
6 Giralt SA, Arora M, Goldman JM, et al. Impact of imatinib therapy on the use of allogeneic haematopoietic progenitor cell transplantation for the treatment of chronic myeloid leukaemia. Br J Haematol 2007;137(5):461-467.
7 Moskowitz CH, Nadamanee A, Masszi T, et al; for the AETHERA Study Group. Brentuximab vedotin as consolidation therapy after autologous stem-cell transplantation in patients with Hodgkin’s lymphoma at risk of relapse or progression (AETHERA): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;385:1853-1862.
8 Bröckelmann PJ, Müller H, Casasnovas O, et al. Risk factors and a prognostic score for progression free survival after treatment with autologous stem cell transplantation (ASCT) in patients with relapsed or refractory Hodgkin lymphoma (rrHL). Poster presented at: 57th American Society of Hematology (ASH) Annual Meeting & Exposition; December 5-8, 2015; Orlando, FL.

In this editorial, Andreas Engert, MD, makes the case for consolidation therapy in advanced Hodgkin lymphoma.
Dr Engert is a professor of internal medicine, hematology, and oncology at University Hospital of Cologne in Germany. He has received research funding and consultancy fees from Takeda/Millennium Pharmaceuticals and Affimed as well as research funding from Bristol-Myers Squibb.
Historically, Hodgkin lymphoma has been viewed as a cancer with generally favorable outcomes. However, it’s clear that there is an unmet need for patients with advanced stage disease.
Physicians treat newly diagnosed patients with a curative intent, but up to 30% fail to respond to initial therapy or relapse, depending on the treatment regimen used, stage of disease, and risk factors.1-3 Additionally, toxicity from frontline treatment has the potential to impact patients throughout their lives.
In line with the current standard of care, the majority of patients who fail frontline therapy will receive high-dose chemotherapy followed by an autologous stem cell transplant (ASCT).
This path of treatment, similar to frontline regimens, can be effective in eradicating the disease, but approximately half of those who undergo an ASCT subsequently relapse. Outcomes are generally poor for patients whose disease returns post-ASCT, especially if the relapse occurs within the first year.4
Consolidation therapy, used to kill remaining cancer cells after ASCT, may offer a new treatment option to address this problem. Unlike longer-term maintenance therapy, consolidation typically lasts for a short period of time—normally months instead of years—and involves intense treatment to eradicate any remaining disease.
The evidence for consolidation therapy in Hodgkin lymphoma
To understand the rationale for consolidation therapy, first consider why some patients with Hodgkin lymphoma relapse following ASCT. A small number of cancer cells, undetectable using traditional diagnostics, may remain following ASCT. This is known as minimal residual disease, and it may indicate the potential for the cancer to return.
The goal of consolidation therapy is to eliminate minimal residual disease before it progresses and causes a relapse. Unsurprisingly, timing plays a crucial role in the likelihood of achieving that goal.
In order to allow for the best chance for optimal patient outcomes, consolidation treatment should be initiated shortly after ASCT, before regrowth of cancer cells can occur. Tolerability is paramount, though, and timing must be carefully weighed by the treating physician.
Physicians and researchers learned about the impact and use of consolidation therapy from its success in other blood cancers like chronic myeloid leukemia.5,6
To prove the concept of consolidation treatment in Hodgkin lymphoma, a controlled clinical trial was conducted. The AETHERA study evaluated the use of brentuximab vedotin as consolidation therapy in patients with advanced Hodgkin lymphoma who were at increased risk of relapse or progression following ASCT.7
AETHERA was the first completed phase 3 study to explore consolidation treatment immediately following ASCT as a way of extending the effect of transplant in patients with Hodgkin lymphoma.
The results made a strong argument in favor of consolidation therapy, as patients who received brentuximab vedotin plus best supportive care after ASCT lived significantly longer without their disease worsening versus those on the placebo regimen. The safety profile of brentuximab vedotin in the AETHERA trial was generally consistent with the existing prescribing information.
Based on these data, consolidation therapy with brentuximab vedotin has been approved in several countries as a treatment option for patients with Hodgkin lymphoma who are at increased risk for relapse or progression following ASCT.
An important next step: Treating the right patients at the right time
Translating clinical evidence into real-world practice, physicians must look at which patients are most likely to benefit from consolidation therapy following ASCT—namely, those who are at increased risk of relapse. The effort to identify clear risk factors for relapse is still in progress.
Researchers across the world are currently studying patient characteristics and outcomes to determine a definitive set of risk factors that can better illustrate which patients should receive consolidation treatment.
Examples of factors under consideration include the stage of disease at diagnosis, tumor size, time to relapse, and response to previous treatment.8 Experts generally agree, however, that increased risk is cumulative and that it is not clear that any one risk factor is more important than others.
As researchers work to answer outstanding questions about consolidation therapy, there are a number of actions that the Hodgkin lymphoma community can take to help bring the right treatment options to patients.
Existing guidelines need to be evaluated and, if appropriate, adapted to give physicians across the globe the information that will allow them to provide the best care for patients at increased risk of relapse following ASCT.
Hematologists and oncologists then have the responsibility to stay informed of revisions to guidelines and to practically apply the latest research of consolidation therapy into their clinical practices.
The possibility now exists to potentially cure some Hodgkin lymphoma patients within a group that has traditionally experienced poor outcomes. As a result, a new treatment paradigm in this setting is emerging—one that may help solve the challenge of post-ASCT relapse in Hodgkin lymphoma.
Additional information on the use of consolidation therapy in Hodgkin lymphoma is available in the paper, Consolidation Therapy After ASCT in Hodgkin Lymphoma: Why and Who to Treat? ![]()
1 Diehl, V, Franklin, J, Pfreundschuh, M, et al. Standard and Increased-Dose BEACOPP Chemotherapy Compared with COPP-ABVD for Advanced Hodgkin’s Disease. N Engl J Med 2003;348:2386-95.
2 Duggan, D, Petroni, G, Johnson, J, et al. Randomized Comparison of ABVD and MOPP/ABV Hybrid for the Treatment of Advanced Hodgkin’s Disease: Report of an Intergroup Trial. J Clin Oncol 2003;21:607-614.
3 Federico, M, Luminari, S, Iannitto, E, et al. ABVD Compared With BEACOPP Compared With CEC for the Initial Treatment of Patients With Advanced Hodgkin’s Lymphoma: Results From the HD2000 Gruppo Italiano per lo Studio dei Linfomi Trial. J Clin Oncol 2009;27:805-811.
4 Arai S, Fanale M, deVos S, et al. Defining a Hodgkin lymphoma population for novel therapeutics after relapse from autologous hematopoietic cell transplant. Leuk Lymphoma. 2013;54:2531–2533.
5 Zonder, J and Schiffer, C. Update on practical aspects of the treatment of chronic myeloid leukemia with imatinib mesylate. Curr Hematol Malig Rep 2006;1:141.
6 Giralt SA, Arora M, Goldman JM, et al. Impact of imatinib therapy on the use of allogeneic haematopoietic progenitor cell transplantation for the treatment of chronic myeloid leukaemia. Br J Haematol 2007;137(5):461-467.
7 Moskowitz CH, Nadamanee A, Masszi T, et al; for the AETHERA Study Group. Brentuximab vedotin as consolidation therapy after autologous stem-cell transplantation in patients with Hodgkin’s lymphoma at risk of relapse or progression (AETHERA): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;385:1853-1862.
8 Bröckelmann PJ, Müller H, Casasnovas O, et al. Risk factors and a prognostic score for progression free survival after treatment with autologous stem cell transplantation (ASCT) in patients with relapsed or refractory Hodgkin lymphoma (rrHL). Poster presented at: 57th American Society of Hematology (ASH) Annual Meeting & Exposition; December 5-8, 2015; Orlando, FL.