User login
Trial supports early treatment of lower-risk ET

Photo courtesy of CDC
SAN DIEGO—Results from the ARETA trial suggest patients with essential thrombocythemia (ET) can benefit from early treatment even if they are not considered high-risk.
In this phase 3 trial, non-high-risk patients were less likely to experience ET-related cardiovascular events or disease progression if they received extended-release anagrelide rather than placebo.
Patients who received extended-release anagrelide were also less likely to become high-risk over time.
And extended-release anagrelide had a safety profile consistent with conventional anagrelide formulations, according to investigator Heinz Gisslinger, MD, of the Medical University of Vienna in Austria.
Dr Gisslinger reported final results of the ARETA trial at the 2016 ASH Annual Meeting (abstract 476).
The trial was sponsored by AOP Orphan Pharmaceuticals AG, the company developing the extended-release formulation of anagrelide, known as anagrelide retard (AR).
Dr Gisslinger noted that the goals of developing AR are to achieve lower peak plasma concentration of anagrelide, reduce the frequency and intensity of peak concentration related to adverse events (AEs), allow for an easier dosing scheme (once daily vs 2 to 3 times daily), and improve patient compliance.
He also pointed out that results of the phase 3 TEAM-ET trial suggest AR is non-inferior to the standard formulation of anagrelide (Thromboreductin, also a product of AOP Orphan Pharmaceuticals).
So with the ARETA trial, Dr Gisslinger and his colleagues set out to determine if AR would be beneficial as an early intervention in patients with non-high-risk ET.
Patients
The trial enrolled 146 patients who had platelet counts below 1000 G/L and met at least 1 of the following criteria:
- Age 40 to 60 years
- ET duration of more than 3 years
- Any risk factor for thrombotic complications (JAK2 mutation, protein C and/or S deficiency, antithrombin III deficiency, factor V Leiden or prothrombin mutation, or cardiovascular risk factors).
Seventy-seven patients were randomized to AR, and 69 were randomized to placebo. In both treatment arms, 100% of patients were Caucasian, and about 74% were female.
The mean age was 40.9 (range, 20-60) in the AR arm and 45.2 (range, 19-59) in the placebo arm. The median disease duration was 75.0 days (range, 1-2502) and 78.0 days (range, 1-2195), respectively. The mean platelet count was 748.6 G/L and 745.3 G/L, respectively.
A majority of patients in both arms had JAK2 mutations (62.7% in the AR arm and 63.8% in the placebo arm). Fewer had CALR mutations (22.7% and 13.6%, respectively) and MPL mutations (16.7% and 12.5%).
Treatment
Patients were stratified by JAK2 status and randomized to receive AR at 2 to 8 mg/day or placebo.
The dosing of AR started at 1 tablet (2 mg) per day during week 1 and was titrated up according to platelet response to 2 tablets in week 2. Dosing was further increased or decreased according to platelet response in weeks 3 and 4.
The maximum dose was 4 tablets (8 mg) per day. After week 4, the maximum dose to achieve optimal platelet counts (<450 G/L) was maintained, and patients continued with weekly visits through week 6.
After that, patients had visits every 3 months in both the main phase of this study and the extension phase. The main phase lasted 1 year, and the extension phase lasted up to 3 years.
Sixty patients (77.9%) in the AR arm and 52 (75.4%) in the placebo arm completed the main phase of the study.
Fifty-seven patients in the AR arm entered the extension phase, and 44 (57.1%) completed it. Thirty-four patients in the placebo arm entered the extension phase, and 21 (30.4%) completed it.
Efficacy
The primary endpoint was time to ET-related cardiovascular events (as confirmed by a blinded expert panel) or disease progression/worsening (platelet increase >1000 G/L).
The 1-year event-free rate (patients who did not meet criteria for the primary endpoint) was 87% in the AR arm and 69% in the placebo arm (hazard ratio=0.356, P=0.0008).
According to the expert panel, there were 12 ET-related events in 11 patients in the AR arm, as well as 17 such events in 14 patients in the placebo arm. This included major and minor arterial, venous, and bleeding events.
In total, there were 13 patients who had ET-related events or met platelet criteria in the AR arm (13 events) and 26 patients who had ET-related events or met platelet criteria in the placebo arm (30 events).
Nine patients in the AR arm (11.7%) and 18 in the placebo arm (26.1%) changed to high-risk status at some point during the trial (odds ratio=2.67, P=0.033).
Safety
The overall incidence of AEs was 88.3% in the AR arm and 69.6% in the placebo arm. The incidence of treatment-related AEs was 76.6% and 27.5%, respectively.
The incidence of treatment-related serious AEs was 1.3% and 0%, respectively. And the incidence of treatment-related AEs leading to withdrawal was 9.1% and 7.2%, respectively.
Treatment-related AEs occurring in more than 10% of patients in either arm (the AR and placebo arms, respectively) included headache (41.6% and 15.9%), dizziness (35.1% and 14.5%), palpitations (28.6% and 1.4%), and tachycardia (10.4% and 1.4%).
In closing, Dr Gisslinger noted that the primary endpoint of this study was met, and AR allowed for platelet count normalization and delayed progression to high-risk status.
Furthermore, the safety profile of AR is consistent with conventional anagrelide formulations, but AR allows for a more convenient dosing schedule.
Dr Gisslinger concluded, “[T]hese data from the ARETA study support an early treatment concept for all ET patients where platelet count or symptom reduction is a goal and those patients who can be attributed as intermediate-risk patients.” ![]()

Photo courtesy of CDC
SAN DIEGO—Results from the ARETA trial suggest patients with essential thrombocythemia (ET) can benefit from early treatment even if they are not considered high-risk.
In this phase 3 trial, non-high-risk patients were less likely to experience ET-related cardiovascular events or disease progression if they received extended-release anagrelide rather than placebo.
Patients who received extended-release anagrelide were also less likely to become high-risk over time.
And extended-release anagrelide had a safety profile consistent with conventional anagrelide formulations, according to investigator Heinz Gisslinger, MD, of the Medical University of Vienna in Austria.
Dr Gisslinger reported final results of the ARETA trial at the 2016 ASH Annual Meeting (abstract 476).
The trial was sponsored by AOP Orphan Pharmaceuticals AG, the company developing the extended-release formulation of anagrelide, known as anagrelide retard (AR).
Dr Gisslinger noted that the goals of developing AR are to achieve lower peak plasma concentration of anagrelide, reduce the frequency and intensity of peak concentration related to adverse events (AEs), allow for an easier dosing scheme (once daily vs 2 to 3 times daily), and improve patient compliance.
He also pointed out that results of the phase 3 TEAM-ET trial suggest AR is non-inferior to the standard formulation of anagrelide (Thromboreductin, also a product of AOP Orphan Pharmaceuticals).
So with the ARETA trial, Dr Gisslinger and his colleagues set out to determine if AR would be beneficial as an early intervention in patients with non-high-risk ET.
Patients
The trial enrolled 146 patients who had platelet counts below 1000 G/L and met at least 1 of the following criteria:
- Age 40 to 60 years
- ET duration of more than 3 years
- Any risk factor for thrombotic complications (JAK2 mutation, protein C and/or S deficiency, antithrombin III deficiency, factor V Leiden or prothrombin mutation, or cardiovascular risk factors).
Seventy-seven patients were randomized to AR, and 69 were randomized to placebo. In both treatment arms, 100% of patients were Caucasian, and about 74% were female.
The mean age was 40.9 (range, 20-60) in the AR arm and 45.2 (range, 19-59) in the placebo arm. The median disease duration was 75.0 days (range, 1-2502) and 78.0 days (range, 1-2195), respectively. The mean platelet count was 748.6 G/L and 745.3 G/L, respectively.
A majority of patients in both arms had JAK2 mutations (62.7% in the AR arm and 63.8% in the placebo arm). Fewer had CALR mutations (22.7% and 13.6%, respectively) and MPL mutations (16.7% and 12.5%).
Treatment
Patients were stratified by JAK2 status and randomized to receive AR at 2 to 8 mg/day or placebo.
The dosing of AR started at 1 tablet (2 mg) per day during week 1 and was titrated up according to platelet response to 2 tablets in week 2. Dosing was further increased or decreased according to platelet response in weeks 3 and 4.
The maximum dose was 4 tablets (8 mg) per day. After week 4, the maximum dose to achieve optimal platelet counts (<450 G/L) was maintained, and patients continued with weekly visits through week 6.
After that, patients had visits every 3 months in both the main phase of this study and the extension phase. The main phase lasted 1 year, and the extension phase lasted up to 3 years.
Sixty patients (77.9%) in the AR arm and 52 (75.4%) in the placebo arm completed the main phase of the study.
Fifty-seven patients in the AR arm entered the extension phase, and 44 (57.1%) completed it. Thirty-four patients in the placebo arm entered the extension phase, and 21 (30.4%) completed it.
Efficacy
The primary endpoint was time to ET-related cardiovascular events (as confirmed by a blinded expert panel) or disease progression/worsening (platelet increase >1000 G/L).
The 1-year event-free rate (patients who did not meet criteria for the primary endpoint) was 87% in the AR arm and 69% in the placebo arm (hazard ratio=0.356, P=0.0008).
According to the expert panel, there were 12 ET-related events in 11 patients in the AR arm, as well as 17 such events in 14 patients in the placebo arm. This included major and minor arterial, venous, and bleeding events.
In total, there were 13 patients who had ET-related events or met platelet criteria in the AR arm (13 events) and 26 patients who had ET-related events or met platelet criteria in the placebo arm (30 events).
Nine patients in the AR arm (11.7%) and 18 in the placebo arm (26.1%) changed to high-risk status at some point during the trial (odds ratio=2.67, P=0.033).
Safety
The overall incidence of AEs was 88.3% in the AR arm and 69.6% in the placebo arm. The incidence of treatment-related AEs was 76.6% and 27.5%, respectively.
The incidence of treatment-related serious AEs was 1.3% and 0%, respectively. And the incidence of treatment-related AEs leading to withdrawal was 9.1% and 7.2%, respectively.
Treatment-related AEs occurring in more than 10% of patients in either arm (the AR and placebo arms, respectively) included headache (41.6% and 15.9%), dizziness (35.1% and 14.5%), palpitations (28.6% and 1.4%), and tachycardia (10.4% and 1.4%).
In closing, Dr Gisslinger noted that the primary endpoint of this study was met, and AR allowed for platelet count normalization and delayed progression to high-risk status.
Furthermore, the safety profile of AR is consistent with conventional anagrelide formulations, but AR allows for a more convenient dosing schedule.
Dr Gisslinger concluded, “[T]hese data from the ARETA study support an early treatment concept for all ET patients where platelet count or symptom reduction is a goal and those patients who can be attributed as intermediate-risk patients.” ![]()

Photo courtesy of CDC
SAN DIEGO—Results from the ARETA trial suggest patients with essential thrombocythemia (ET) can benefit from early treatment even if they are not considered high-risk.
In this phase 3 trial, non-high-risk patients were less likely to experience ET-related cardiovascular events or disease progression if they received extended-release anagrelide rather than placebo.
Patients who received extended-release anagrelide were also less likely to become high-risk over time.
And extended-release anagrelide had a safety profile consistent with conventional anagrelide formulations, according to investigator Heinz Gisslinger, MD, of the Medical University of Vienna in Austria.
Dr Gisslinger reported final results of the ARETA trial at the 2016 ASH Annual Meeting (abstract 476).
The trial was sponsored by AOP Orphan Pharmaceuticals AG, the company developing the extended-release formulation of anagrelide, known as anagrelide retard (AR).
Dr Gisslinger noted that the goals of developing AR are to achieve lower peak plasma concentration of anagrelide, reduce the frequency and intensity of peak concentration related to adverse events (AEs), allow for an easier dosing scheme (once daily vs 2 to 3 times daily), and improve patient compliance.
He also pointed out that results of the phase 3 TEAM-ET trial suggest AR is non-inferior to the standard formulation of anagrelide (Thromboreductin, also a product of AOP Orphan Pharmaceuticals).
So with the ARETA trial, Dr Gisslinger and his colleagues set out to determine if AR would be beneficial as an early intervention in patients with non-high-risk ET.
Patients
The trial enrolled 146 patients who had platelet counts below 1000 G/L and met at least 1 of the following criteria:
- Age 40 to 60 years
- ET duration of more than 3 years
- Any risk factor for thrombotic complications (JAK2 mutation, protein C and/or S deficiency, antithrombin III deficiency, factor V Leiden or prothrombin mutation, or cardiovascular risk factors).
Seventy-seven patients were randomized to AR, and 69 were randomized to placebo. In both treatment arms, 100% of patients were Caucasian, and about 74% were female.
The mean age was 40.9 (range, 20-60) in the AR arm and 45.2 (range, 19-59) in the placebo arm. The median disease duration was 75.0 days (range, 1-2502) and 78.0 days (range, 1-2195), respectively. The mean platelet count was 748.6 G/L and 745.3 G/L, respectively.
A majority of patients in both arms had JAK2 mutations (62.7% in the AR arm and 63.8% in the placebo arm). Fewer had CALR mutations (22.7% and 13.6%, respectively) and MPL mutations (16.7% and 12.5%).
Treatment
Patients were stratified by JAK2 status and randomized to receive AR at 2 to 8 mg/day or placebo.
The dosing of AR started at 1 tablet (2 mg) per day during week 1 and was titrated up according to platelet response to 2 tablets in week 2. Dosing was further increased or decreased according to platelet response in weeks 3 and 4.
The maximum dose was 4 tablets (8 mg) per day. After week 4, the maximum dose to achieve optimal platelet counts (<450 G/L) was maintained, and patients continued with weekly visits through week 6.
After that, patients had visits every 3 months in both the main phase of this study and the extension phase. The main phase lasted 1 year, and the extension phase lasted up to 3 years.
Sixty patients (77.9%) in the AR arm and 52 (75.4%) in the placebo arm completed the main phase of the study.
Fifty-seven patients in the AR arm entered the extension phase, and 44 (57.1%) completed it. Thirty-four patients in the placebo arm entered the extension phase, and 21 (30.4%) completed it.
Efficacy
The primary endpoint was time to ET-related cardiovascular events (as confirmed by a blinded expert panel) or disease progression/worsening (platelet increase >1000 G/L).
The 1-year event-free rate (patients who did not meet criteria for the primary endpoint) was 87% in the AR arm and 69% in the placebo arm (hazard ratio=0.356, P=0.0008).
According to the expert panel, there were 12 ET-related events in 11 patients in the AR arm, as well as 17 such events in 14 patients in the placebo arm. This included major and minor arterial, venous, and bleeding events.
In total, there were 13 patients who had ET-related events or met platelet criteria in the AR arm (13 events) and 26 patients who had ET-related events or met platelet criteria in the placebo arm (30 events).
Nine patients in the AR arm (11.7%) and 18 in the placebo arm (26.1%) changed to high-risk status at some point during the trial (odds ratio=2.67, P=0.033).
Safety
The overall incidence of AEs was 88.3% in the AR arm and 69.6% in the placebo arm. The incidence of treatment-related AEs was 76.6% and 27.5%, respectively.
The incidence of treatment-related serious AEs was 1.3% and 0%, respectively. And the incidence of treatment-related AEs leading to withdrawal was 9.1% and 7.2%, respectively.
Treatment-related AEs occurring in more than 10% of patients in either arm (the AR and placebo arms, respectively) included headache (41.6% and 15.9%), dizziness (35.1% and 14.5%), palpitations (28.6% and 1.4%), and tachycardia (10.4% and 1.4%).
In closing, Dr Gisslinger noted that the primary endpoint of this study was met, and AR allowed for platelet count normalization and delayed progression to high-risk status.
Furthermore, the safety profile of AR is consistent with conventional anagrelide formulations, but AR allows for a more convenient dosing schedule.
Dr Gisslinger concluded, “[T]hese data from the ARETA study support an early treatment concept for all ET patients where platelet count or symptom reduction is a goal and those patients who can be attributed as intermediate-risk patients.” ![]()
Agios stops developing drug for PK deficiency

Agios Pharmaceuticals, Inc. is no longer developing one of its pyruvate kinase-R (PKR) activators, AG-519, for the treatment of pyruvate kinase (PK) deficiency.
The company withdrew its investigational new drug application for AG-519 following a verbal notification of a clinical hold from the US Food and Drug Administration (FDA).
The hold resulted from an adverse event—cholestatic hepatitis—observed in a phase 1 trial of healthy volunteers.
“[W]e received feedback from the FDA that AG-519 no longer has an appropriate risk-benefit ratio to move forward in clinical development and was placed on clinical hold due to that case of cholestatic hepatitis,” said David Schenkein, MD, chief executive officer at Agios.
“We made the decision to withdraw the IND [investigational new drug application] and discontinue development of AG-519 and advance AG-348, our first-in-class and lead pyruvate kinase activator into pivotal development. We share the FDA’s commitment to patient safety and believe this is the right decision to ultimately help people with PK deficiency.”
About AG-519
Agios has described AG-519 as a potent, highly selective, and orally bioavailable PKR activator devoid of the aromatase inhibitory effects that were observed with the company’s other PKR activator, AG-348.
AG-519 was evaluated in a phase 1 study of healthy volunteers in the UK. The goal of this study was to assess the drug’s safety, tolerability, pharmacokinetics, pharmacodynamics, and bioavailability.
A case of drug-induced cholestatic hepatitis occurred in the bioavailability portion of the study. This volunteer continues to be monitored and is showing improvement, according to Dr Schenkein.
Agios said other adverse events observed in this trial were largely mild or moderate (grade 1/2). The most common of these was headache.
The company did note a case of grade 2 thrombocytopenia that resolved spontaneously within 7 days after the last dose of AG-519.
Results from this trial were presented at the 2015 ASH Annual Meeting (abstract 1264).
AG-519 was also under investigation in a palatability study of volunteers in the US. The goal of this study was to develop a formulation of the drug for potential future development.
In total, 98 volunteers have received AG-519. No volunteers or patients are currently receiving the drug.
About AG-348
Agios’s decision to stop developing AG-519 does not affect the company’s ongoing phase 2 study (DRIVE PK) of AG-348, an activator of both wild-type and mutated PKR enzymes.
“AG-348 and AG-519 are different molecules with different structures,” Dr Schenkein noted.
Agios is advancing AG-348 into development as the first potential disease-modifying treatment for PK deficiency.
Results from a pair of phase 1 studies of AG-348 were presented at the 2014 ASH Annual Meeting (abstract 4007).
About PK deficiency
PK deficiency is a rare inherited disease that presents as hemolytic anemia. The inherited mutations in PKR enzymes cause a deficit in cellular energy within the red blood cell, as evidenced by lower PK enzyme activity, a decline in adenosine triphosphate levels, and a build-up of upstream metabolites, including 2,3-DPG.
The current standard of care for PK deficiency is supportive care, including blood transfusions, splenectomy, chelation therapy to address iron overload, and/or interventions for other treatment- and disease-related morbidities.
There is, at present, no approved therapy to treat the underlying cause of PK deficiency. ![]()

Agios Pharmaceuticals, Inc. is no longer developing one of its pyruvate kinase-R (PKR) activators, AG-519, for the treatment of pyruvate kinase (PK) deficiency.
The company withdrew its investigational new drug application for AG-519 following a verbal notification of a clinical hold from the US Food and Drug Administration (FDA).
The hold resulted from an adverse event—cholestatic hepatitis—observed in a phase 1 trial of healthy volunteers.
“[W]e received feedback from the FDA that AG-519 no longer has an appropriate risk-benefit ratio to move forward in clinical development and was placed on clinical hold due to that case of cholestatic hepatitis,” said David Schenkein, MD, chief executive officer at Agios.
“We made the decision to withdraw the IND [investigational new drug application] and discontinue development of AG-519 and advance AG-348, our first-in-class and lead pyruvate kinase activator into pivotal development. We share the FDA’s commitment to patient safety and believe this is the right decision to ultimately help people with PK deficiency.”
About AG-519
Agios has described AG-519 as a potent, highly selective, and orally bioavailable PKR activator devoid of the aromatase inhibitory effects that were observed with the company’s other PKR activator, AG-348.
AG-519 was evaluated in a phase 1 study of healthy volunteers in the UK. The goal of this study was to assess the drug’s safety, tolerability, pharmacokinetics, pharmacodynamics, and bioavailability.
A case of drug-induced cholestatic hepatitis occurred in the bioavailability portion of the study. This volunteer continues to be monitored and is showing improvement, according to Dr Schenkein.
Agios said other adverse events observed in this trial were largely mild or moderate (grade 1/2). The most common of these was headache.
The company did note a case of grade 2 thrombocytopenia that resolved spontaneously within 7 days after the last dose of AG-519.
Results from this trial were presented at the 2015 ASH Annual Meeting (abstract 1264).
AG-519 was also under investigation in a palatability study of volunteers in the US. The goal of this study was to develop a formulation of the drug for potential future development.
In total, 98 volunteers have received AG-519. No volunteers or patients are currently receiving the drug.
About AG-348
Agios’s decision to stop developing AG-519 does not affect the company’s ongoing phase 2 study (DRIVE PK) of AG-348, an activator of both wild-type and mutated PKR enzymes.
“AG-348 and AG-519 are different molecules with different structures,” Dr Schenkein noted.
Agios is advancing AG-348 into development as the first potential disease-modifying treatment for PK deficiency.
Results from a pair of phase 1 studies of AG-348 were presented at the 2014 ASH Annual Meeting (abstract 4007).
About PK deficiency
PK deficiency is a rare inherited disease that presents as hemolytic anemia. The inherited mutations in PKR enzymes cause a deficit in cellular energy within the red blood cell, as evidenced by lower PK enzyme activity, a decline in adenosine triphosphate levels, and a build-up of upstream metabolites, including 2,3-DPG.
The current standard of care for PK deficiency is supportive care, including blood transfusions, splenectomy, chelation therapy to address iron overload, and/or interventions for other treatment- and disease-related morbidities.
There is, at present, no approved therapy to treat the underlying cause of PK deficiency. ![]()

Agios Pharmaceuticals, Inc. is no longer developing one of its pyruvate kinase-R (PKR) activators, AG-519, for the treatment of pyruvate kinase (PK) deficiency.
The company withdrew its investigational new drug application for AG-519 following a verbal notification of a clinical hold from the US Food and Drug Administration (FDA).
The hold resulted from an adverse event—cholestatic hepatitis—observed in a phase 1 trial of healthy volunteers.
“[W]e received feedback from the FDA that AG-519 no longer has an appropriate risk-benefit ratio to move forward in clinical development and was placed on clinical hold due to that case of cholestatic hepatitis,” said David Schenkein, MD, chief executive officer at Agios.
“We made the decision to withdraw the IND [investigational new drug application] and discontinue development of AG-519 and advance AG-348, our first-in-class and lead pyruvate kinase activator into pivotal development. We share the FDA’s commitment to patient safety and believe this is the right decision to ultimately help people with PK deficiency.”
About AG-519
Agios has described AG-519 as a potent, highly selective, and orally bioavailable PKR activator devoid of the aromatase inhibitory effects that were observed with the company’s other PKR activator, AG-348.
AG-519 was evaluated in a phase 1 study of healthy volunteers in the UK. The goal of this study was to assess the drug’s safety, tolerability, pharmacokinetics, pharmacodynamics, and bioavailability.
A case of drug-induced cholestatic hepatitis occurred in the bioavailability portion of the study. This volunteer continues to be monitored and is showing improvement, according to Dr Schenkein.
Agios said other adverse events observed in this trial were largely mild or moderate (grade 1/2). The most common of these was headache.
The company did note a case of grade 2 thrombocytopenia that resolved spontaneously within 7 days after the last dose of AG-519.
Results from this trial were presented at the 2015 ASH Annual Meeting (abstract 1264).
AG-519 was also under investigation in a palatability study of volunteers in the US. The goal of this study was to develop a formulation of the drug for potential future development.
In total, 98 volunteers have received AG-519. No volunteers or patients are currently receiving the drug.
About AG-348
Agios’s decision to stop developing AG-519 does not affect the company’s ongoing phase 2 study (DRIVE PK) of AG-348, an activator of both wild-type and mutated PKR enzymes.
“AG-348 and AG-519 are different molecules with different structures,” Dr Schenkein noted.
Agios is advancing AG-348 into development as the first potential disease-modifying treatment for PK deficiency.
Results from a pair of phase 1 studies of AG-348 were presented at the 2014 ASH Annual Meeting (abstract 4007).
About PK deficiency
PK deficiency is a rare inherited disease that presents as hemolytic anemia. The inherited mutations in PKR enzymes cause a deficit in cellular energy within the red blood cell, as evidenced by lower PK enzyme activity, a decline in adenosine triphosphate levels, and a build-up of upstream metabolites, including 2,3-DPG.
The current standard of care for PK deficiency is supportive care, including blood transfusions, splenectomy, chelation therapy to address iron overload, and/or interventions for other treatment- and disease-related morbidities.
There is, at present, no approved therapy to treat the underlying cause of PK deficiency. ![]()
Hospitalized patients may fare better with female doctors

Photo courtesy of CDC
New research suggests that hospitalized patients on Medicare may fare better when treated by female internists.
Researchers analyzed data on more than 1.5 million hospitalizations of Medicare beneficiaries and found that patients treated by female physicians had lower rates of 30-day mortality and hospital readmission than those treated by male physicians.
The results were published in JAMA Internal Medicine alongside a related editorial.
“There’s a lot of evidence out there that male and female physicians practice medicine differently,” noted study author Ashish K. Jha, MD, of the Harvard T. H. Chan School of Public Health in Boston, Massachusetts.
“Female physicians are more likely to adhere to clinical practice guidelines. They’re more likely to practice evidence-based medicine. And while that data has been out there, we don’t really know to what extent that actually matters for patient outcomes.”
So with this study, Dr Jha and his colleagues set out to determine if differences in practice patterns translate into differences in patient outcomes.
The researchers analyzed data on 1,583,028 hospitalizations to assess 30-day mortality rates and 1,540,797 hospitalizations to assess readmissions. The hospitalizations occurred from January 1, 2011, to December 31, 2014.
In the 30-day mortality analysis, the patients’ mean age was 80.2 years, 621,412 patients were male, and 961,616 were female.
In the hospital readmission analysis, the mean patient age was 80.1 years, 602,115 patients were male, and 938,682 were female.
Physician characteristics
During the study period, 58,344 internists treated at least 1 hospitalized Medicare beneficiary. Among those physicians, 18,751 were women (32.1%).
Female physicians tended to be younger than males, with mean ages of 42.8 and 47.8, respectively. Females were also more likely than males to have had osteopathic training—8.4% and 7.0%, respectively.
Females were more likely than males to work in large hospitals (41.9% vs 35.7%), nonprofit hospitals (78.2% vs 75.6%), major teaching hospitals (29.0% vs 21.1%), and hospitals located in the Northeast (26.8% vs 22.7%).
Female physicians tended to treat fewer patients than males—131.9 and 180.5 hospitalizations per year, respectively.
Patient characteristics were largely similar between male and female physicians. However, female physicians treated a higher proportion of female patients than male physicians did—62.1% and 60.2%, respectively.
Results
An adjusted analysis showed that patients treated by female physicians had lower 30-day mortality rates than those treated by males—11.07% and 11.49%, respectively (risk difference, –0.43%; 95% confidence interval, –0.57% to –0.28%; P<0.001; number needed to treat to prevent 1 death, 233).
An adjusted analysis for 30-day hospital readmission rates showed a lower rate for patients treated by females than those treated by males—15.02% and 15.57%, respectively (risk difference, –0.55%; 95% confidence interval, –0.71% to –0.39%; P<0.001; number needed to treat to prevent 1 readmission, 182).
These analyses were adjusted for patient characteristics, hospital-fixed effects, and physician characteristics.
The researchers noted that patients treated by female physicians had lower 30-day mortality and readmission rates regardless of their medical condition or the severity of their illness.
“Across a wide range of conditions, we see a very consistent pattern—that patients who are treated by female physicians had modest but consistently better outcomes than patients treated by male physicians,” Dr Jha said.
“That was true across conditions. It was also true across severity of illness. In fact, among the patients who were the sickest, that’s where we saw some of the largest gaps between female and male physicians.”
The researchers also adjusted their analyses for patients’ length of stay, use of care, discharge location, patient volume, and physicians’ years of practice. But this did not affect the results.
Dr Jha and his colleagues said the results of this study suggest differences in practice patterns between male and female physicians may have important clinical implications for patients. And understanding why these differences exist may provide valuable insights into improving the quality of patient care. ![]()

Photo courtesy of CDC
New research suggests that hospitalized patients on Medicare may fare better when treated by female internists.
Researchers analyzed data on more than 1.5 million hospitalizations of Medicare beneficiaries and found that patients treated by female physicians had lower rates of 30-day mortality and hospital readmission than those treated by male physicians.
The results were published in JAMA Internal Medicine alongside a related editorial.
“There’s a lot of evidence out there that male and female physicians practice medicine differently,” noted study author Ashish K. Jha, MD, of the Harvard T. H. Chan School of Public Health in Boston, Massachusetts.
“Female physicians are more likely to adhere to clinical practice guidelines. They’re more likely to practice evidence-based medicine. And while that data has been out there, we don’t really know to what extent that actually matters for patient outcomes.”
So with this study, Dr Jha and his colleagues set out to determine if differences in practice patterns translate into differences in patient outcomes.
The researchers analyzed data on 1,583,028 hospitalizations to assess 30-day mortality rates and 1,540,797 hospitalizations to assess readmissions. The hospitalizations occurred from January 1, 2011, to December 31, 2014.
In the 30-day mortality analysis, the patients’ mean age was 80.2 years, 621,412 patients were male, and 961,616 were female.
In the hospital readmission analysis, the mean patient age was 80.1 years, 602,115 patients were male, and 938,682 were female.
Physician characteristics
During the study period, 58,344 internists treated at least 1 hospitalized Medicare beneficiary. Among those physicians, 18,751 were women (32.1%).
Female physicians tended to be younger than males, with mean ages of 42.8 and 47.8, respectively. Females were also more likely than males to have had osteopathic training—8.4% and 7.0%, respectively.
Females were more likely than males to work in large hospitals (41.9% vs 35.7%), nonprofit hospitals (78.2% vs 75.6%), major teaching hospitals (29.0% vs 21.1%), and hospitals located in the Northeast (26.8% vs 22.7%).
Female physicians tended to treat fewer patients than males—131.9 and 180.5 hospitalizations per year, respectively.
Patient characteristics were largely similar between male and female physicians. However, female physicians treated a higher proportion of female patients than male physicians did—62.1% and 60.2%, respectively.
Results
An adjusted analysis showed that patients treated by female physicians had lower 30-day mortality rates than those treated by males—11.07% and 11.49%, respectively (risk difference, –0.43%; 95% confidence interval, –0.57% to –0.28%; P<0.001; number needed to treat to prevent 1 death, 233).
An adjusted analysis for 30-day hospital readmission rates showed a lower rate for patients treated by females than those treated by males—15.02% and 15.57%, respectively (risk difference, –0.55%; 95% confidence interval, –0.71% to –0.39%; P<0.001; number needed to treat to prevent 1 readmission, 182).
These analyses were adjusted for patient characteristics, hospital-fixed effects, and physician characteristics.
The researchers noted that patients treated by female physicians had lower 30-day mortality and readmission rates regardless of their medical condition or the severity of their illness.
“Across a wide range of conditions, we see a very consistent pattern—that patients who are treated by female physicians had modest but consistently better outcomes than patients treated by male physicians,” Dr Jha said.
“That was true across conditions. It was also true across severity of illness. In fact, among the patients who were the sickest, that’s where we saw some of the largest gaps between female and male physicians.”
The researchers also adjusted their analyses for patients’ length of stay, use of care, discharge location, patient volume, and physicians’ years of practice. But this did not affect the results.
Dr Jha and his colleagues said the results of this study suggest differences in practice patterns between male and female physicians may have important clinical implications for patients. And understanding why these differences exist may provide valuable insights into improving the quality of patient care. ![]()

Photo courtesy of CDC
New research suggests that hospitalized patients on Medicare may fare better when treated by female internists.
Researchers analyzed data on more than 1.5 million hospitalizations of Medicare beneficiaries and found that patients treated by female physicians had lower rates of 30-day mortality and hospital readmission than those treated by male physicians.
The results were published in JAMA Internal Medicine alongside a related editorial.
“There’s a lot of evidence out there that male and female physicians practice medicine differently,” noted study author Ashish K. Jha, MD, of the Harvard T. H. Chan School of Public Health in Boston, Massachusetts.
“Female physicians are more likely to adhere to clinical practice guidelines. They’re more likely to practice evidence-based medicine. And while that data has been out there, we don’t really know to what extent that actually matters for patient outcomes.”
So with this study, Dr Jha and his colleagues set out to determine if differences in practice patterns translate into differences in patient outcomes.
The researchers analyzed data on 1,583,028 hospitalizations to assess 30-day mortality rates and 1,540,797 hospitalizations to assess readmissions. The hospitalizations occurred from January 1, 2011, to December 31, 2014.
In the 30-day mortality analysis, the patients’ mean age was 80.2 years, 621,412 patients were male, and 961,616 were female.
In the hospital readmission analysis, the mean patient age was 80.1 years, 602,115 patients were male, and 938,682 were female.
Physician characteristics
During the study period, 58,344 internists treated at least 1 hospitalized Medicare beneficiary. Among those physicians, 18,751 were women (32.1%).
Female physicians tended to be younger than males, with mean ages of 42.8 and 47.8, respectively. Females were also more likely than males to have had osteopathic training—8.4% and 7.0%, respectively.
Females were more likely than males to work in large hospitals (41.9% vs 35.7%), nonprofit hospitals (78.2% vs 75.6%), major teaching hospitals (29.0% vs 21.1%), and hospitals located in the Northeast (26.8% vs 22.7%).
Female physicians tended to treat fewer patients than males—131.9 and 180.5 hospitalizations per year, respectively.
Patient characteristics were largely similar between male and female physicians. However, female physicians treated a higher proportion of female patients than male physicians did—62.1% and 60.2%, respectively.
Results
An adjusted analysis showed that patients treated by female physicians had lower 30-day mortality rates than those treated by males—11.07% and 11.49%, respectively (risk difference, –0.43%; 95% confidence interval, –0.57% to –0.28%; P<0.001; number needed to treat to prevent 1 death, 233).
An adjusted analysis for 30-day hospital readmission rates showed a lower rate for patients treated by females than those treated by males—15.02% and 15.57%, respectively (risk difference, –0.55%; 95% confidence interval, –0.71% to –0.39%; P<0.001; number needed to treat to prevent 1 readmission, 182).
These analyses were adjusted for patient characteristics, hospital-fixed effects, and physician characteristics.
The researchers noted that patients treated by female physicians had lower 30-day mortality and readmission rates regardless of their medical condition or the severity of their illness.
“Across a wide range of conditions, we see a very consistent pattern—that patients who are treated by female physicians had modest but consistently better outcomes than patients treated by male physicians,” Dr Jha said.
“That was true across conditions. It was also true across severity of illness. In fact, among the patients who were the sickest, that’s where we saw some of the largest gaps between female and male physicians.”
The researchers also adjusted their analyses for patients’ length of stay, use of care, discharge location, patient volume, and physicians’ years of practice. But this did not affect the results.
Dr Jha and his colleagues said the results of this study suggest differences in practice patterns between male and female physicians may have important clinical implications for patients. And understanding why these differences exist may provide valuable insights into improving the quality of patient care. ![]()
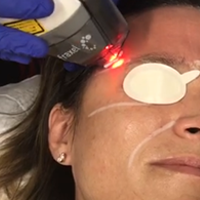
Facial Rejuvenation With Fractional Laser Resurfacing



Sneak Peek: The Hospital Leader blog
To my next patient:
I often avoid putting my politics on my sleeve, as I don’t want that to get in the way of our relationship. I want you to know that I treat you as a fellow human being, no matter your race, gender, sexual orientation. With the election results, what will change about how I treat you at the bedside? Nothing.
I may know about your criminal past. I see that tattoo underneath your gown. I hear your profanity-filled screed because you won’t get that MRI today. I know you don’t follow the treatment plan, that you are here illegally or that you are a refugee from another country.
I will still care for you no matter what. It’s one of the blessed things we instill in each other in medicine.
I saw someone like you recently: 28 years old, working hard, with two jobs, neither of which provided insurance. She was doing well, without health problems, but then she became fatigued and swollen. She came to the ER after weeks of suffering with what turned out to be failing kidneys. Lupus. She required expensive medications that would aim to reverse her kidney disease. She left the hospital not knowing what would happen next, as there was no way she could afford the treatment. The fates of medicine handed her an unexpected illness, and we had no good way to reassure her of what would come next. I am sorry that more patients without insurance will arrive, instead of the steady decline I had been used to the past few years.
You also remind me of another patient I saw last week. She was sweet in the face, smiling despite her travails, and wore the skimpy gown with pride. She had some fluid just outside her lung that shouldn’t be there: a pleural effusion. We discussed the different possible diagnoses. She had cancer in the past, surgically treated and presumably cured. Was this the cancer back? Was it an infection, easily treated? We couldn’t tell by the exam or the x-ray.
On Tuesday, we took the fluid out. The results trickled in slowly, and initial tests suggested it was benign. We allowed a smile, but final tests were pending. What will turn up? When the final results return? Can we dance in the room with joy? Or will we hold hands, bear the cross, shed a tear, but then lift our heads up and know we will fight for another day, and another day, and not stop fighting until the cancer upon us is gone?
Read the full post at www.hospitalleader.org.
Also on The Hospital Leader blog ...
Post: An open letter to hospital executives about their hospitalist programs
By Leslie Flores, MHA, SFHM
Post: What’s under the hood? A quick look at hospital expenses
By Brad Flansbaum, DO, MPH, MHM
Post: A quick lesson on bundled payments
By John Nelson, MD, MHM
Post: The ABIM Has new plans for MOC and wants your opinion. Give it to ’em!
By Burke Kealey, MD, SFHM
To my next patient:
I often avoid putting my politics on my sleeve, as I don’t want that to get in the way of our relationship. I want you to know that I treat you as a fellow human being, no matter your race, gender, sexual orientation. With the election results, what will change about how I treat you at the bedside? Nothing.
I may know about your criminal past. I see that tattoo underneath your gown. I hear your profanity-filled screed because you won’t get that MRI today. I know you don’t follow the treatment plan, that you are here illegally or that you are a refugee from another country.
I will still care for you no matter what. It’s one of the blessed things we instill in each other in medicine.
I saw someone like you recently: 28 years old, working hard, with two jobs, neither of which provided insurance. She was doing well, without health problems, but then she became fatigued and swollen. She came to the ER after weeks of suffering with what turned out to be failing kidneys. Lupus. She required expensive medications that would aim to reverse her kidney disease. She left the hospital not knowing what would happen next, as there was no way she could afford the treatment. The fates of medicine handed her an unexpected illness, and we had no good way to reassure her of what would come next. I am sorry that more patients without insurance will arrive, instead of the steady decline I had been used to the past few years.
You also remind me of another patient I saw last week. She was sweet in the face, smiling despite her travails, and wore the skimpy gown with pride. She had some fluid just outside her lung that shouldn’t be there: a pleural effusion. We discussed the different possible diagnoses. She had cancer in the past, surgically treated and presumably cured. Was this the cancer back? Was it an infection, easily treated? We couldn’t tell by the exam or the x-ray.
On Tuesday, we took the fluid out. The results trickled in slowly, and initial tests suggested it was benign. We allowed a smile, but final tests were pending. What will turn up? When the final results return? Can we dance in the room with joy? Or will we hold hands, bear the cross, shed a tear, but then lift our heads up and know we will fight for another day, and another day, and not stop fighting until the cancer upon us is gone?
Read the full post at www.hospitalleader.org.
Also on The Hospital Leader blog ...
Post: An open letter to hospital executives about their hospitalist programs
By Leslie Flores, MHA, SFHM
Post: What’s under the hood? A quick look at hospital expenses
By Brad Flansbaum, DO, MPH, MHM
Post: A quick lesson on bundled payments
By John Nelson, MD, MHM
Post: The ABIM Has new plans for MOC and wants your opinion. Give it to ’em!
By Burke Kealey, MD, SFHM
To my next patient:
I often avoid putting my politics on my sleeve, as I don’t want that to get in the way of our relationship. I want you to know that I treat you as a fellow human being, no matter your race, gender, sexual orientation. With the election results, what will change about how I treat you at the bedside? Nothing.
I may know about your criminal past. I see that tattoo underneath your gown. I hear your profanity-filled screed because you won’t get that MRI today. I know you don’t follow the treatment plan, that you are here illegally or that you are a refugee from another country.
I will still care for you no matter what. It’s one of the blessed things we instill in each other in medicine.
I saw someone like you recently: 28 years old, working hard, with two jobs, neither of which provided insurance. She was doing well, without health problems, but then she became fatigued and swollen. She came to the ER after weeks of suffering with what turned out to be failing kidneys. Lupus. She required expensive medications that would aim to reverse her kidney disease. She left the hospital not knowing what would happen next, as there was no way she could afford the treatment. The fates of medicine handed her an unexpected illness, and we had no good way to reassure her of what would come next. I am sorry that more patients without insurance will arrive, instead of the steady decline I had been used to the past few years.
You also remind me of another patient I saw last week. She was sweet in the face, smiling despite her travails, and wore the skimpy gown with pride. She had some fluid just outside her lung that shouldn’t be there: a pleural effusion. We discussed the different possible diagnoses. She had cancer in the past, surgically treated and presumably cured. Was this the cancer back? Was it an infection, easily treated? We couldn’t tell by the exam or the x-ray.
On Tuesday, we took the fluid out. The results trickled in slowly, and initial tests suggested it was benign. We allowed a smile, but final tests were pending. What will turn up? When the final results return? Can we dance in the room with joy? Or will we hold hands, bear the cross, shed a tear, but then lift our heads up and know we will fight for another day, and another day, and not stop fighting until the cancer upon us is gone?
Read the full post at www.hospitalleader.org.
Also on The Hospital Leader blog ...
Post: An open letter to hospital executives about their hospitalist programs
By Leslie Flores, MHA, SFHM
Post: What’s under the hood? A quick look at hospital expenses
By Brad Flansbaum, DO, MPH, MHM
Post: A quick lesson on bundled payments
By John Nelson, MD, MHM
Post: The ABIM Has new plans for MOC and wants your opinion. Give it to ’em!
By Burke Kealey, MD, SFHM
SHM member spotlight
Editor’s note: Each month, SHM puts the spotlight on some of our most active members who are making substantial contributions to hospital medicine.
Visit www.hospitalmedicine.org/getinvolved for more information on how you can help SHM improve the care of hospitalized patients.
Dr. Venkataraman Palabindala, FHM, is a hospitalist at the University of Mississippi Medical Center in Jackson. Dr. Palabindala is an active member of SHM’s IT Committee and has been instrumental in growing the Gulf States Chapter.
Answer: I was exploring my options during my second year of residency at Greater Baltimore Medical Center as to what my final career path should be. I always loved inpatient medicine, mostly critical care, so I was thinking of completing a pulmonary critical-care fellowship. Completing a hospitalist rotation changed everything about how I saw my future and led me to specialize in hospital medicine.
Once I learned about SHM and the wealth of activities and opportunities membership offered from a few of my attendings, I applied to be part of the Leadership Committee. I attended every meeting and kept my committee work as a top priority. At the time, with little experience in hospital medicine, I knew I might not have as much to contribute as the rest, but my goal was to learn as much as I could. Never once did I feel that my voice was any more or less valuable than those of the rest of the committee members; our committee work was truly a collaborative effort.
As my career in hospital medicine has evolved, so have my contributions to SHM’s committees; I now am a proud member of the IT Committee. We’re currently working on a white paper about hospitalists’ attitudes toward electronic health record (EHR) systems and look forward to sharing more about that next month.
In addition, throughout my time with SHM, I have become a Fellow in Hospital Medicine, attended two “Hill Days” to learn about the policies, and made a concerted effort to be present at as many meetings as possible, especially SHM’s annual meetings. The networking, coupled with the workshops and lectures, is unparalleled. I have missed only one annual meeting, and I feel like I missed a Thanksgiving dinner with my family!
Q: Can you tell us about your role in the revitalization of the Gulf States Chapter and the Chapter Development Program?
A: During my time as a member of the SHM Leadership Committee, I quickly realized that hospitalists in small cities like Dothan, Ala., were not as exposed to networking and education activities as were those in big cities. To unite hospitalists in that area of the country, I founded the Wiregrass Chapter; obtaining 20 signatures to start it was an uphill task. After Dan Dressler, MD, [in Atlanta] and I gave a talk about updates in hospital medicine, the Wiregrass Chapter was awarded the Silver Chapter Award [after its first year in inception], and everything changed. The buzz around the chapter helped it continue to grow.
After I moved to Jackson, I applied for a pilot funding project to start a Jackson Chapter, as I realized the Gulf States Chapter was a bit far away. I thought a local chapter would bring all hospitalists in this area together. However, I received a call from Lisa Chester, our chapter liaison at SHM, about being a part of the Gulf States Chapter and serving as a catalyst to revitalize the chapter.
I was thrilled to work with Randy Roth, MD, and Steven Deitelzweig, MD; both are hospitalist leaders in this area. The Chapter Development Program surely helped us to create new goals and develop a realistic timeline. It kept us on track to achieve what we originally set out to do. By creating coupons to encourage membership and arranging more local meetings using this fund, we have been able to experience even more success. We are now recognizing that residents are very excited about SHM meetings and are identifying young leaders to be part of the hospital medicine movement.
Q: How has your participation in HMX – and, more broadly, engagement with SHM – helped you improve your practice?
A: HMX [connect.hospitalmedicine.org] is a great platform for asking questions and exchanging ideas. Being active on HMX has helped me learn important information about performance metrics, observation unit models, EHRs, coding and billing questions, and sometimes even ethical questions.
Although I still have mentors helping me, I know if I post a question on HMX, that I will get many ideas from hospitalists across the nation. I also make it a point to encourage friends every month to download the HMX app on their phones and present it as a valuable resource to my students and residents. As hospitalists, this is our forum with experts available all the time.
To encourage others to use the platform and make myself and fellow committee members accessible to other members, we actively take turns assuming responsibility for maintaining the momentum on HMX by finding intriguing topics of discussion.
Q: As we ring in 2017 after a year of many changes for HM and the health care system in general, what do you see as the biggest HM opportunities this year?
A: We know physician retention and burnout are some of the biggest challenges in hospital medicine. Given the pace at which we are growing as a specialty, I would like to see more time dedicated to addressing and attempting to alleviate these specific issues.
Also, now that hospitalists have left their stamp on inpatient medicine, specialties like critical care, nephrology, cardiology, and ob.gyn. are moving toward this model. We need to do everything we can to integrate them into our pool, move forward together, and learn from each other.
Lastly, mentorship is of paramount importance as we head into the future. We must encourage young hospitalists to mentor students and residents and recruit them to be part of SHM when they return home.
Brett Radler is SHM’s communications specialist.
Editor’s note: Each month, SHM puts the spotlight on some of our most active members who are making substantial contributions to hospital medicine.
Visit www.hospitalmedicine.org/getinvolved for more information on how you can help SHM improve the care of hospitalized patients.
Dr. Venkataraman Palabindala, FHM, is a hospitalist at the University of Mississippi Medical Center in Jackson. Dr. Palabindala is an active member of SHM’s IT Committee and has been instrumental in growing the Gulf States Chapter.
Answer: I was exploring my options during my second year of residency at Greater Baltimore Medical Center as to what my final career path should be. I always loved inpatient medicine, mostly critical care, so I was thinking of completing a pulmonary critical-care fellowship. Completing a hospitalist rotation changed everything about how I saw my future and led me to specialize in hospital medicine.
Once I learned about SHM and the wealth of activities and opportunities membership offered from a few of my attendings, I applied to be part of the Leadership Committee. I attended every meeting and kept my committee work as a top priority. At the time, with little experience in hospital medicine, I knew I might not have as much to contribute as the rest, but my goal was to learn as much as I could. Never once did I feel that my voice was any more or less valuable than those of the rest of the committee members; our committee work was truly a collaborative effort.
As my career in hospital medicine has evolved, so have my contributions to SHM’s committees; I now am a proud member of the IT Committee. We’re currently working on a white paper about hospitalists’ attitudes toward electronic health record (EHR) systems and look forward to sharing more about that next month.
In addition, throughout my time with SHM, I have become a Fellow in Hospital Medicine, attended two “Hill Days” to learn about the policies, and made a concerted effort to be present at as many meetings as possible, especially SHM’s annual meetings. The networking, coupled with the workshops and lectures, is unparalleled. I have missed only one annual meeting, and I feel like I missed a Thanksgiving dinner with my family!
Q: Can you tell us about your role in the revitalization of the Gulf States Chapter and the Chapter Development Program?
A: During my time as a member of the SHM Leadership Committee, I quickly realized that hospitalists in small cities like Dothan, Ala., were not as exposed to networking and education activities as were those in big cities. To unite hospitalists in that area of the country, I founded the Wiregrass Chapter; obtaining 20 signatures to start it was an uphill task. After Dan Dressler, MD, [in Atlanta] and I gave a talk about updates in hospital medicine, the Wiregrass Chapter was awarded the Silver Chapter Award [after its first year in inception], and everything changed. The buzz around the chapter helped it continue to grow.
After I moved to Jackson, I applied for a pilot funding project to start a Jackson Chapter, as I realized the Gulf States Chapter was a bit far away. I thought a local chapter would bring all hospitalists in this area together. However, I received a call from Lisa Chester, our chapter liaison at SHM, about being a part of the Gulf States Chapter and serving as a catalyst to revitalize the chapter.
I was thrilled to work with Randy Roth, MD, and Steven Deitelzweig, MD; both are hospitalist leaders in this area. The Chapter Development Program surely helped us to create new goals and develop a realistic timeline. It kept us on track to achieve what we originally set out to do. By creating coupons to encourage membership and arranging more local meetings using this fund, we have been able to experience even more success. We are now recognizing that residents are very excited about SHM meetings and are identifying young leaders to be part of the hospital medicine movement.
Q: How has your participation in HMX – and, more broadly, engagement with SHM – helped you improve your practice?
A: HMX [connect.hospitalmedicine.org] is a great platform for asking questions and exchanging ideas. Being active on HMX has helped me learn important information about performance metrics, observation unit models, EHRs, coding and billing questions, and sometimes even ethical questions.
Although I still have mentors helping me, I know if I post a question on HMX, that I will get many ideas from hospitalists across the nation. I also make it a point to encourage friends every month to download the HMX app on their phones and present it as a valuable resource to my students and residents. As hospitalists, this is our forum with experts available all the time.
To encourage others to use the platform and make myself and fellow committee members accessible to other members, we actively take turns assuming responsibility for maintaining the momentum on HMX by finding intriguing topics of discussion.
Q: As we ring in 2017 after a year of many changes for HM and the health care system in general, what do you see as the biggest HM opportunities this year?
A: We know physician retention and burnout are some of the biggest challenges in hospital medicine. Given the pace at which we are growing as a specialty, I would like to see more time dedicated to addressing and attempting to alleviate these specific issues.
Also, now that hospitalists have left their stamp on inpatient medicine, specialties like critical care, nephrology, cardiology, and ob.gyn. are moving toward this model. We need to do everything we can to integrate them into our pool, move forward together, and learn from each other.
Lastly, mentorship is of paramount importance as we head into the future. We must encourage young hospitalists to mentor students and residents and recruit them to be part of SHM when they return home.
Brett Radler is SHM’s communications specialist.
Editor’s note: Each month, SHM puts the spotlight on some of our most active members who are making substantial contributions to hospital medicine.
Visit www.hospitalmedicine.org/getinvolved for more information on how you can help SHM improve the care of hospitalized patients.
Dr. Venkataraman Palabindala, FHM, is a hospitalist at the University of Mississippi Medical Center in Jackson. Dr. Palabindala is an active member of SHM’s IT Committee and has been instrumental in growing the Gulf States Chapter.
Answer: I was exploring my options during my second year of residency at Greater Baltimore Medical Center as to what my final career path should be. I always loved inpatient medicine, mostly critical care, so I was thinking of completing a pulmonary critical-care fellowship. Completing a hospitalist rotation changed everything about how I saw my future and led me to specialize in hospital medicine.
Once I learned about SHM and the wealth of activities and opportunities membership offered from a few of my attendings, I applied to be part of the Leadership Committee. I attended every meeting and kept my committee work as a top priority. At the time, with little experience in hospital medicine, I knew I might not have as much to contribute as the rest, but my goal was to learn as much as I could. Never once did I feel that my voice was any more or less valuable than those of the rest of the committee members; our committee work was truly a collaborative effort.
As my career in hospital medicine has evolved, so have my contributions to SHM’s committees; I now am a proud member of the IT Committee. We’re currently working on a white paper about hospitalists’ attitudes toward electronic health record (EHR) systems and look forward to sharing more about that next month.
In addition, throughout my time with SHM, I have become a Fellow in Hospital Medicine, attended two “Hill Days” to learn about the policies, and made a concerted effort to be present at as many meetings as possible, especially SHM’s annual meetings. The networking, coupled with the workshops and lectures, is unparalleled. I have missed only one annual meeting, and I feel like I missed a Thanksgiving dinner with my family!
Q: Can you tell us about your role in the revitalization of the Gulf States Chapter and the Chapter Development Program?
A: During my time as a member of the SHM Leadership Committee, I quickly realized that hospitalists in small cities like Dothan, Ala., were not as exposed to networking and education activities as were those in big cities. To unite hospitalists in that area of the country, I founded the Wiregrass Chapter; obtaining 20 signatures to start it was an uphill task. After Dan Dressler, MD, [in Atlanta] and I gave a talk about updates in hospital medicine, the Wiregrass Chapter was awarded the Silver Chapter Award [after its first year in inception], and everything changed. The buzz around the chapter helped it continue to grow.
After I moved to Jackson, I applied for a pilot funding project to start a Jackson Chapter, as I realized the Gulf States Chapter was a bit far away. I thought a local chapter would bring all hospitalists in this area together. However, I received a call from Lisa Chester, our chapter liaison at SHM, about being a part of the Gulf States Chapter and serving as a catalyst to revitalize the chapter.
I was thrilled to work with Randy Roth, MD, and Steven Deitelzweig, MD; both are hospitalist leaders in this area. The Chapter Development Program surely helped us to create new goals and develop a realistic timeline. It kept us on track to achieve what we originally set out to do. By creating coupons to encourage membership and arranging more local meetings using this fund, we have been able to experience even more success. We are now recognizing that residents are very excited about SHM meetings and are identifying young leaders to be part of the hospital medicine movement.
Q: How has your participation in HMX – and, more broadly, engagement with SHM – helped you improve your practice?
A: HMX [connect.hospitalmedicine.org] is a great platform for asking questions and exchanging ideas. Being active on HMX has helped me learn important information about performance metrics, observation unit models, EHRs, coding and billing questions, and sometimes even ethical questions.
Although I still have mentors helping me, I know if I post a question on HMX, that I will get many ideas from hospitalists across the nation. I also make it a point to encourage friends every month to download the HMX app on their phones and present it as a valuable resource to my students and residents. As hospitalists, this is our forum with experts available all the time.
To encourage others to use the platform and make myself and fellow committee members accessible to other members, we actively take turns assuming responsibility for maintaining the momentum on HMX by finding intriguing topics of discussion.
Q: As we ring in 2017 after a year of many changes for HM and the health care system in general, what do you see as the biggest HM opportunities this year?
A: We know physician retention and burnout are some of the biggest challenges in hospital medicine. Given the pace at which we are growing as a specialty, I would like to see more time dedicated to addressing and attempting to alleviate these specific issues.
Also, now that hospitalists have left their stamp on inpatient medicine, specialties like critical care, nephrology, cardiology, and ob.gyn. are moving toward this model. We need to do everything we can to integrate them into our pool, move forward together, and learn from each other.
Lastly, mentorship is of paramount importance as we head into the future. We must encourage young hospitalists to mentor students and residents and recruit them to be part of SHM when they return home.
Brett Radler is SHM’s communications specialist.
Add-on fenfluramine reduces seizure frequency in Dravet syndrome
HOUSTON – Low-dose fenfluramine was found to reduce seizures significantly among a small cohort with Dravet syndrome without the appearance of valvular abnormalities or pulmonary hypertension, according to a prospective study presented at a poster session of the annual meeting of the American Epilepsy Society.
Six of nine Dravet syndrome (DS) patients (66%) had at least a 50% reduction in major motor seizure frequency for at least 90% of the period during which they took fenfluramine. Five of the nine DS patients (56%) experienced a reduction in major motor seizure frequency of 75% or more for at least 60% of the median 1.9 years they were on fenfluramine.
According to lead author An-Sofie Schoonjans, MD, and her collaborators, the results suggest that “low-dose fenfluramine provides significant improvement in seizure frequency while being generally well tolerated in DS patients.”
The study criteria included patients aged 6 months to 50 years who had a DS diagnosis; enrollees ranged in age from 1.2 to 29.8 years when starting fenfluramine. Though criteria allowed enrollment of patients with and without a mutation in the SCN1A gene, all participants did have a de novo mutation of the SCN1A gene, according to Dr. Schoonjans of the department of pediatric neurology at Antwerp (Belgium) University Hospital and her colleagues. They wrote that mutations in the gene, which encodes the alpha subunit of type 1 voltage-gated sodium channels, are found in about 80% of DS patients.
During the 3-month run-in period that began the study, patients had a median seizure frequency of 15 seizures per month. Patients remained on their baseline antiepilepsy regimen during the run-in period and throughout the study, with fenfluramine used as add-on therapy. At baseline, all patients were taking valproic acid and at least one other antiepileptic medication; three patients were taking four medications and one was taking five medications. Three patients also had vagal nerve stimulators with stable settings.
Throughout the study period, patients or their caregivers kept a seizure diary, recording major motor seizures. Those keeping the diary were instructed to record all tonic-clonic, tonic, atonic, and myoclonic seizures lasting more than 30 seconds.
Three months after beginning treatment, the study population’s median seizure frequency fell to 2.0 per month (–84%). Frequency fell further during the first year, to 1.0 per month (–79%; a smaller percent reduction because data were not available for this time period for the patient with the highest seizure frequency). For the total treatment period, the median seizure frequency was 1.9 per month (–76%). The reduction in seizure frequency was statistically significant at all time points (P less than .05; compared with baseline).
Fenfluramine was generally well-tolerated. Five patients experienced somnolence, and four had loss of appetite.
To track cardiovascular safety, all patients had echocardiographs at baseline and every 3 months during the first year of treatment. Echocardiographs were performed every 6 months during the second year, and annually thereafter. One patient had systolic dysfunction characterized by a reduced ejection fraction (53%) and fractional shortening (26%), findings of “no clinical significance,” according to Dr. Schoonjans and her colleagues.
Fenfluramine was part of an oral weight loss drug combination, along with phentermine. The combo, known as “fen-phen,” was associated with increased rates of pulmonary hypertension and valve disease, particularly aortic valve thickening and regurgitation. It was withdrawn from the market in 1997. Though pulmonary hypertension would frequently resolve after discontinuing fen-phen, not all patients with valvulopathy experienced resolution, and case reports of patients with the aortic valve thickening typically seen with fenfluramine are still surfacing many years after the drug’s discontinuation (e.g., Tex Heart Inst J. 2011;38[5]:581-3).
Fenfluramine was typically given at doses up to 60 mg when used with phentermine for weight loss. The dosing for Dravet syndrome patients in this study was much lower and weight based, ranging from 0.1 to 1.0 mg/kg per day, with a maximum permitted dose of 20 mg/day.
Fenfluramine is a serotonin releaser, and serotonin is known to modulate the action of voltage-gated sodium channels. However, the exact mechanism by which the drug reduces seizure frequency is not known. Clinical trials are underway in the United States for both DS and Lennox Gastaut epilepsy, and fenfluramine has been granted orphan drug status in the United States and Europe, according to an announcement from Zogenix, the drug’s manufacturer.
The study was funded by Zogenix, which holds a Royal Decree to dispense the drug under the study conditions in Belgium, where the study took place. Zogenix also funded writing and editorial assistance for the poster presentation.
[email protected]
On Twitter @karioakes
HOUSTON – Low-dose fenfluramine was found to reduce seizures significantly among a small cohort with Dravet syndrome without the appearance of valvular abnormalities or pulmonary hypertension, according to a prospective study presented at a poster session of the annual meeting of the American Epilepsy Society.
Six of nine Dravet syndrome (DS) patients (66%) had at least a 50% reduction in major motor seizure frequency for at least 90% of the period during which they took fenfluramine. Five of the nine DS patients (56%) experienced a reduction in major motor seizure frequency of 75% or more for at least 60% of the median 1.9 years they were on fenfluramine.
According to lead author An-Sofie Schoonjans, MD, and her collaborators, the results suggest that “low-dose fenfluramine provides significant improvement in seizure frequency while being generally well tolerated in DS patients.”
The study criteria included patients aged 6 months to 50 years who had a DS diagnosis; enrollees ranged in age from 1.2 to 29.8 years when starting fenfluramine. Though criteria allowed enrollment of patients with and without a mutation in the SCN1A gene, all participants did have a de novo mutation of the SCN1A gene, according to Dr. Schoonjans of the department of pediatric neurology at Antwerp (Belgium) University Hospital and her colleagues. They wrote that mutations in the gene, which encodes the alpha subunit of type 1 voltage-gated sodium channels, are found in about 80% of DS patients.
During the 3-month run-in period that began the study, patients had a median seizure frequency of 15 seizures per month. Patients remained on their baseline antiepilepsy regimen during the run-in period and throughout the study, with fenfluramine used as add-on therapy. At baseline, all patients were taking valproic acid and at least one other antiepileptic medication; three patients were taking four medications and one was taking five medications. Three patients also had vagal nerve stimulators with stable settings.
Throughout the study period, patients or their caregivers kept a seizure diary, recording major motor seizures. Those keeping the diary were instructed to record all tonic-clonic, tonic, atonic, and myoclonic seizures lasting more than 30 seconds.
Three months after beginning treatment, the study population’s median seizure frequency fell to 2.0 per month (–84%). Frequency fell further during the first year, to 1.0 per month (–79%; a smaller percent reduction because data were not available for this time period for the patient with the highest seizure frequency). For the total treatment period, the median seizure frequency was 1.9 per month (–76%). The reduction in seizure frequency was statistically significant at all time points (P less than .05; compared with baseline).
Fenfluramine was generally well-tolerated. Five patients experienced somnolence, and four had loss of appetite.
To track cardiovascular safety, all patients had echocardiographs at baseline and every 3 months during the first year of treatment. Echocardiographs were performed every 6 months during the second year, and annually thereafter. One patient had systolic dysfunction characterized by a reduced ejection fraction (53%) and fractional shortening (26%), findings of “no clinical significance,” according to Dr. Schoonjans and her colleagues.
Fenfluramine was part of an oral weight loss drug combination, along with phentermine. The combo, known as “fen-phen,” was associated with increased rates of pulmonary hypertension and valve disease, particularly aortic valve thickening and regurgitation. It was withdrawn from the market in 1997. Though pulmonary hypertension would frequently resolve after discontinuing fen-phen, not all patients with valvulopathy experienced resolution, and case reports of patients with the aortic valve thickening typically seen with fenfluramine are still surfacing many years after the drug’s discontinuation (e.g., Tex Heart Inst J. 2011;38[5]:581-3).
Fenfluramine was typically given at doses up to 60 mg when used with phentermine for weight loss. The dosing for Dravet syndrome patients in this study was much lower and weight based, ranging from 0.1 to 1.0 mg/kg per day, with a maximum permitted dose of 20 mg/day.
Fenfluramine is a serotonin releaser, and serotonin is known to modulate the action of voltage-gated sodium channels. However, the exact mechanism by which the drug reduces seizure frequency is not known. Clinical trials are underway in the United States for both DS and Lennox Gastaut epilepsy, and fenfluramine has been granted orphan drug status in the United States and Europe, according to an announcement from Zogenix, the drug’s manufacturer.
The study was funded by Zogenix, which holds a Royal Decree to dispense the drug under the study conditions in Belgium, where the study took place. Zogenix also funded writing and editorial assistance for the poster presentation.
[email protected]
On Twitter @karioakes
HOUSTON – Low-dose fenfluramine was found to reduce seizures significantly among a small cohort with Dravet syndrome without the appearance of valvular abnormalities or pulmonary hypertension, according to a prospective study presented at a poster session of the annual meeting of the American Epilepsy Society.
Six of nine Dravet syndrome (DS) patients (66%) had at least a 50% reduction in major motor seizure frequency for at least 90% of the period during which they took fenfluramine. Five of the nine DS patients (56%) experienced a reduction in major motor seizure frequency of 75% or more for at least 60% of the median 1.9 years they were on fenfluramine.
According to lead author An-Sofie Schoonjans, MD, and her collaborators, the results suggest that “low-dose fenfluramine provides significant improvement in seizure frequency while being generally well tolerated in DS patients.”
The study criteria included patients aged 6 months to 50 years who had a DS diagnosis; enrollees ranged in age from 1.2 to 29.8 years when starting fenfluramine. Though criteria allowed enrollment of patients with and without a mutation in the SCN1A gene, all participants did have a de novo mutation of the SCN1A gene, according to Dr. Schoonjans of the department of pediatric neurology at Antwerp (Belgium) University Hospital and her colleagues. They wrote that mutations in the gene, which encodes the alpha subunit of type 1 voltage-gated sodium channels, are found in about 80% of DS patients.
During the 3-month run-in period that began the study, patients had a median seizure frequency of 15 seizures per month. Patients remained on their baseline antiepilepsy regimen during the run-in period and throughout the study, with fenfluramine used as add-on therapy. At baseline, all patients were taking valproic acid and at least one other antiepileptic medication; three patients were taking four medications and one was taking five medications. Three patients also had vagal nerve stimulators with stable settings.
Throughout the study period, patients or their caregivers kept a seizure diary, recording major motor seizures. Those keeping the diary were instructed to record all tonic-clonic, tonic, atonic, and myoclonic seizures lasting more than 30 seconds.
Three months after beginning treatment, the study population’s median seizure frequency fell to 2.0 per month (–84%). Frequency fell further during the first year, to 1.0 per month (–79%; a smaller percent reduction because data were not available for this time period for the patient with the highest seizure frequency). For the total treatment period, the median seizure frequency was 1.9 per month (–76%). The reduction in seizure frequency was statistically significant at all time points (P less than .05; compared with baseline).
Fenfluramine was generally well-tolerated. Five patients experienced somnolence, and four had loss of appetite.
To track cardiovascular safety, all patients had echocardiographs at baseline and every 3 months during the first year of treatment. Echocardiographs were performed every 6 months during the second year, and annually thereafter. One patient had systolic dysfunction characterized by a reduced ejection fraction (53%) and fractional shortening (26%), findings of “no clinical significance,” according to Dr. Schoonjans and her colleagues.
Fenfluramine was part of an oral weight loss drug combination, along with phentermine. The combo, known as “fen-phen,” was associated with increased rates of pulmonary hypertension and valve disease, particularly aortic valve thickening and regurgitation. It was withdrawn from the market in 1997. Though pulmonary hypertension would frequently resolve after discontinuing fen-phen, not all patients with valvulopathy experienced resolution, and case reports of patients with the aortic valve thickening typically seen with fenfluramine are still surfacing many years after the drug’s discontinuation (e.g., Tex Heart Inst J. 2011;38[5]:581-3).
Fenfluramine was typically given at doses up to 60 mg when used with phentermine for weight loss. The dosing for Dravet syndrome patients in this study was much lower and weight based, ranging from 0.1 to 1.0 mg/kg per day, with a maximum permitted dose of 20 mg/day.
Fenfluramine is a serotonin releaser, and serotonin is known to modulate the action of voltage-gated sodium channels. However, the exact mechanism by which the drug reduces seizure frequency is not known. Clinical trials are underway in the United States for both DS and Lennox Gastaut epilepsy, and fenfluramine has been granted orphan drug status in the United States and Europe, according to an announcement from Zogenix, the drug’s manufacturer.
The study was funded by Zogenix, which holds a Royal Decree to dispense the drug under the study conditions in Belgium, where the study took place. Zogenix also funded writing and editorial assistance for the poster presentation.
[email protected]
On Twitter @karioakes
AT AES 2016
Key clinical point:
Major finding: Six of nine patients (66%) had a reduction in seizure frequency of at least 50% for at least 90% of the time they were taking fenfluramine.
Data source: Prospective cohort study of nine patients with Dravet syndrome who took fenfluramine as add-on therapy for a median 1.9 years.
Disclosures: The study was funded by Zogenix, which funded editorial and writing support for the poster presentation.
FDA eases mental health warnings in smoking cessation drugs’ labels
Labels on two smoking cessation treatments will offer less severe warnings for mental health risk potentials in people with no history of psychiatric disorders, the Food and Drug Administration has announced.
Varenicline (Chantix) will no longer include a boxed warning for serious mental health side effects. The label for bupropion (Zyban) will still include a boxed warning, but language describing the potential for serious psychiatric adverse events will no longer appear within it. Updates will also be made to both labels to describe side effects on mood, behavior, or thinking.
In addition, varenicline’s label will reflect trial data showing its superior efficacy, compared with oral bupropion or nicotine patch. Although a patient medication guide will still be included with each prescription, the risk evaluation and mitigation strategy that prompted the guide will no longer be in place.
Earlier this year, two FDA advisory committees voted in favor of updating varenicline’s label, based on data from a randomized, controlled trial of more than 8,000 smokers, half of whom had a history of psychiatric disorders.
The trial showed no clinically significant difference in risk of adverse events across the smoking cessation treatments varenicline, bupropion, nicotine patch, or placebo study arms, although the risk was higher in the psychiatric cohorts in each.
Overall, 2% of those without a history of mental illness experienced neuropsychiatric adverse events, compared with between 5% and 7% of those with such a history.
The trial was cosponsored by Pfizer, maker of Chantix, and GlaxoSmithKline, maker of Zyban.
The FDA approved varenicline for smoking cessation in 2006 and approved bupropion, which also is indicated to treat depression and seasonal affective disorder, in 1997. After numerous postmarketing reports of increased incidents of psychiatric disorders occurring in smokers who used either drug, the agency added the boxed warning to each in 2009.
FDA officials advised clinicians to guard against changes in mental health status in smokers using these therapies. However, “the results of the trial confirm that the benefits of stopping smoking outweigh the risks of these medicines,” they noted.
On Twitter @whitneymcknight
Labels on two smoking cessation treatments will offer less severe warnings for mental health risk potentials in people with no history of psychiatric disorders, the Food and Drug Administration has announced.
Varenicline (Chantix) will no longer include a boxed warning for serious mental health side effects. The label for bupropion (Zyban) will still include a boxed warning, but language describing the potential for serious psychiatric adverse events will no longer appear within it. Updates will also be made to both labels to describe side effects on mood, behavior, or thinking.
In addition, varenicline’s label will reflect trial data showing its superior efficacy, compared with oral bupropion or nicotine patch. Although a patient medication guide will still be included with each prescription, the risk evaluation and mitigation strategy that prompted the guide will no longer be in place.
Earlier this year, two FDA advisory committees voted in favor of updating varenicline’s label, based on data from a randomized, controlled trial of more than 8,000 smokers, half of whom had a history of psychiatric disorders.
The trial showed no clinically significant difference in risk of adverse events across the smoking cessation treatments varenicline, bupropion, nicotine patch, or placebo study arms, although the risk was higher in the psychiatric cohorts in each.
Overall, 2% of those without a history of mental illness experienced neuropsychiatric adverse events, compared with between 5% and 7% of those with such a history.
The trial was cosponsored by Pfizer, maker of Chantix, and GlaxoSmithKline, maker of Zyban.
The FDA approved varenicline for smoking cessation in 2006 and approved bupropion, which also is indicated to treat depression and seasonal affective disorder, in 1997. After numerous postmarketing reports of increased incidents of psychiatric disorders occurring in smokers who used either drug, the agency added the boxed warning to each in 2009.
FDA officials advised clinicians to guard against changes in mental health status in smokers using these therapies. However, “the results of the trial confirm that the benefits of stopping smoking outweigh the risks of these medicines,” they noted.
On Twitter @whitneymcknight
Labels on two smoking cessation treatments will offer less severe warnings for mental health risk potentials in people with no history of psychiatric disorders, the Food and Drug Administration has announced.
Varenicline (Chantix) will no longer include a boxed warning for serious mental health side effects. The label for bupropion (Zyban) will still include a boxed warning, but language describing the potential for serious psychiatric adverse events will no longer appear within it. Updates will also be made to both labels to describe side effects on mood, behavior, or thinking.
In addition, varenicline’s label will reflect trial data showing its superior efficacy, compared with oral bupropion or nicotine patch. Although a patient medication guide will still be included with each prescription, the risk evaluation and mitigation strategy that prompted the guide will no longer be in place.
Earlier this year, two FDA advisory committees voted in favor of updating varenicline’s label, based on data from a randomized, controlled trial of more than 8,000 smokers, half of whom had a history of psychiatric disorders.
The trial showed no clinically significant difference in risk of adverse events across the smoking cessation treatments varenicline, bupropion, nicotine patch, or placebo study arms, although the risk was higher in the psychiatric cohorts in each.
Overall, 2% of those without a history of mental illness experienced neuropsychiatric adverse events, compared with between 5% and 7% of those with such a history.
The trial was cosponsored by Pfizer, maker of Chantix, and GlaxoSmithKline, maker of Zyban.
The FDA approved varenicline for smoking cessation in 2006 and approved bupropion, which also is indicated to treat depression and seasonal affective disorder, in 1997. After numerous postmarketing reports of increased incidents of psychiatric disorders occurring in smokers who used either drug, the agency added the boxed warning to each in 2009.
FDA officials advised clinicians to guard against changes in mental health status in smokers using these therapies. However, “the results of the trial confirm that the benefits of stopping smoking outweigh the risks of these medicines,” they noted.
On Twitter @whitneymcknight
Severe postoperative pain following thoracotomy predicts persistent pain months later
Patients who suffer from severe pain in the days immediately following an open thoracotomy are significantly more likely to still be experiencing pain from the procedure 6 months later, according to a study published in the Journal of Clinical Anesthesia.
“A recognized cause of persistent postsurgical pain is poorly controlled immediate postoperative pain,” wrote the authors, led by Gopinath Niraj, MD, of the University Hospitals of Leicester (England) NHS Trust. “Open thoracotomy can induce significant pain during the immediate postoperative period. Patients undergoing thoracotomy also have one of the greatest incidences of chronic postoperative pain and disability among all the surgical procedures.”
Dr. Niraj and his coinvestigators conducted an audit on 504 patients who underwent open thoracotomy at a single center between May 2010 and April 2012. The audit consisted of a questionnaire composed of 15 questions, which asked yes/no questions about the existence of and location of postoperative pain, and numerical questions regarding the severity of pain. Scores of 7 or higher on a 10-point scale indicated “severe pain,” according to the investigators (J Clin Anesth. 2017;36:174-7). Subjects were evaluated at 72 hours and at 6 months after the operation.
Of the 504 patients, there were 364 survivors, of which 306 received questionnaires. Of those 306, 133 (43%) reported at least five incidents of severe pain within 72 hours of undergoing the operation. Within this group, 109 (82%) reported feeling some amount of persistent pain 6 months later. Chronic post-thoracotomy pain was considered severe in 10% of those subjects, while 24% reported it as moderate and 48% said it was mild.
A total of 289 of the 306 subjects (95%) received an epidural analgesic in the 72 hours after thoracotomy. In terms of satisfaction with pain management, patients were overall positive; 36.3% rated it “excellent,” 43.8% called it “good,” while only 15.8% said it was “fair” and 3.8% said it was “poor.”
“Our audit has some limitations,” the authors noted. “The retrospective project relied on patient self-report and recall.”
Dr. Niraj and his coauthors did not report any financial conflicts. No funding sources for this study were disclosed.
Patients who suffer from severe pain in the days immediately following an open thoracotomy are significantly more likely to still be experiencing pain from the procedure 6 months later, according to a study published in the Journal of Clinical Anesthesia.
“A recognized cause of persistent postsurgical pain is poorly controlled immediate postoperative pain,” wrote the authors, led by Gopinath Niraj, MD, of the University Hospitals of Leicester (England) NHS Trust. “Open thoracotomy can induce significant pain during the immediate postoperative period. Patients undergoing thoracotomy also have one of the greatest incidences of chronic postoperative pain and disability among all the surgical procedures.”
Dr. Niraj and his coinvestigators conducted an audit on 504 patients who underwent open thoracotomy at a single center between May 2010 and April 2012. The audit consisted of a questionnaire composed of 15 questions, which asked yes/no questions about the existence of and location of postoperative pain, and numerical questions regarding the severity of pain. Scores of 7 or higher on a 10-point scale indicated “severe pain,” according to the investigators (J Clin Anesth. 2017;36:174-7). Subjects were evaluated at 72 hours and at 6 months after the operation.
Of the 504 patients, there were 364 survivors, of which 306 received questionnaires. Of those 306, 133 (43%) reported at least five incidents of severe pain within 72 hours of undergoing the operation. Within this group, 109 (82%) reported feeling some amount of persistent pain 6 months later. Chronic post-thoracotomy pain was considered severe in 10% of those subjects, while 24% reported it as moderate and 48% said it was mild.
A total of 289 of the 306 subjects (95%) received an epidural analgesic in the 72 hours after thoracotomy. In terms of satisfaction with pain management, patients were overall positive; 36.3% rated it “excellent,” 43.8% called it “good,” while only 15.8% said it was “fair” and 3.8% said it was “poor.”
“Our audit has some limitations,” the authors noted. “The retrospective project relied on patient self-report and recall.”
Dr. Niraj and his coauthors did not report any financial conflicts. No funding sources for this study were disclosed.
Patients who suffer from severe pain in the days immediately following an open thoracotomy are significantly more likely to still be experiencing pain from the procedure 6 months later, according to a study published in the Journal of Clinical Anesthesia.
“A recognized cause of persistent postsurgical pain is poorly controlled immediate postoperative pain,” wrote the authors, led by Gopinath Niraj, MD, of the University Hospitals of Leicester (England) NHS Trust. “Open thoracotomy can induce significant pain during the immediate postoperative period. Patients undergoing thoracotomy also have one of the greatest incidences of chronic postoperative pain and disability among all the surgical procedures.”
Dr. Niraj and his coinvestigators conducted an audit on 504 patients who underwent open thoracotomy at a single center between May 2010 and April 2012. The audit consisted of a questionnaire composed of 15 questions, which asked yes/no questions about the existence of and location of postoperative pain, and numerical questions regarding the severity of pain. Scores of 7 or higher on a 10-point scale indicated “severe pain,” according to the investigators (J Clin Anesth. 2017;36:174-7). Subjects were evaluated at 72 hours and at 6 months after the operation.
Of the 504 patients, there were 364 survivors, of which 306 received questionnaires. Of those 306, 133 (43%) reported at least five incidents of severe pain within 72 hours of undergoing the operation. Within this group, 109 (82%) reported feeling some amount of persistent pain 6 months later. Chronic post-thoracotomy pain was considered severe in 10% of those subjects, while 24% reported it as moderate and 48% said it was mild.
A total of 289 of the 306 subjects (95%) received an epidural analgesic in the 72 hours after thoracotomy. In terms of satisfaction with pain management, patients were overall positive; 36.3% rated it “excellent,” 43.8% called it “good,” while only 15.8% said it was “fair” and 3.8% said it was “poor.”
“Our audit has some limitations,” the authors noted. “The retrospective project relied on patient self-report and recall.”
Dr. Niraj and his coauthors did not report any financial conflicts. No funding sources for this study were disclosed.
FROM THE JOURNAL OF CLINICAL ANESTHESIA
Key clinical point:
Major finding: 133 of 306 patients were in severe pain 72 hours after thoracotomy; of these, 109 (82%) still had pain 6 months later.
Data source: Retrospective, single-center study of 504 thoracotomy patients between May 2010 and April 2012.
Disclosures: Authors reported no financial disclosures nor funding source.
CMS nixes Part B drug payment demonstration
A controversial demonstration project that would have tested new methods to pay for the drugs administered in medical offices has been canceled by the Centers for Medicare & Medicaid Services.
The agency received considerable backlash from physicians, Congress, and others when the demonstration project was announced in March 2016.
The agency said it received “a great deal of support from some” for the proposed demonstration. However, “a number of stakeholders expressed strong concerns about the model. While CMS was working to address these concerns, the complexity of the issues and the limited time available led to the decision not to finalize the rule at this time.”
The demonstration project was designed to test new methods to “improve how Medicare Part B pays for prescription drugs and supports physicians and other clinicians in delivering high quality care,” according to a fact sheet published in March.
Under the project, medical practices would have been divided into two groups. A control group would continue to be paid for Part B drugs at the current rate of 106% of average sales price (ASP), while the other would have been paid at 102.5% of ASP plus a flat fee of $16.80 per drug payment. Starting in January 2017, each group would have been further subdivided with a portion of each being subjected to value-based purchasing tools.
One key criticism of the demonstration project centered on the proposed randomization of practices, which was based on primary care service areas (clusters of zip codes with similar Part B medical care patterns). That randomization scheme could have caused different payment levels – and patient out-of-pocket spending – for geographically close areas. Further, participation in the demonstration project would have been mandatory, with no mechanism to opt out.
“This is a model for how Washington should, but often doesn’t, work,” American Medical Association President Andrew W. Gurman, MD, said in a statement. “We are grateful that CMS came to the right decision after listening to stakeholders.”
An analysis of the proposed demonstration project by Avalere found that specialists would likely see a decrease in their drug payments under the proposal, while primary care doctors would likely see an increase, and that 7 of the 10 drugs most affected by this proposal were drugs used to treat cancer.
A controversial demonstration project that would have tested new methods to pay for the drugs administered in medical offices has been canceled by the Centers for Medicare & Medicaid Services.
The agency received considerable backlash from physicians, Congress, and others when the demonstration project was announced in March 2016.
The agency said it received “a great deal of support from some” for the proposed demonstration. However, “a number of stakeholders expressed strong concerns about the model. While CMS was working to address these concerns, the complexity of the issues and the limited time available led to the decision not to finalize the rule at this time.”
The demonstration project was designed to test new methods to “improve how Medicare Part B pays for prescription drugs and supports physicians and other clinicians in delivering high quality care,” according to a fact sheet published in March.
Under the project, medical practices would have been divided into two groups. A control group would continue to be paid for Part B drugs at the current rate of 106% of average sales price (ASP), while the other would have been paid at 102.5% of ASP plus a flat fee of $16.80 per drug payment. Starting in January 2017, each group would have been further subdivided with a portion of each being subjected to value-based purchasing tools.
One key criticism of the demonstration project centered on the proposed randomization of practices, which was based on primary care service areas (clusters of zip codes with similar Part B medical care patterns). That randomization scheme could have caused different payment levels – and patient out-of-pocket spending – for geographically close areas. Further, participation in the demonstration project would have been mandatory, with no mechanism to opt out.
“This is a model for how Washington should, but often doesn’t, work,” American Medical Association President Andrew W. Gurman, MD, said in a statement. “We are grateful that CMS came to the right decision after listening to stakeholders.”
An analysis of the proposed demonstration project by Avalere found that specialists would likely see a decrease in their drug payments under the proposal, while primary care doctors would likely see an increase, and that 7 of the 10 drugs most affected by this proposal were drugs used to treat cancer.
A controversial demonstration project that would have tested new methods to pay for the drugs administered in medical offices has been canceled by the Centers for Medicare & Medicaid Services.
The agency received considerable backlash from physicians, Congress, and others when the demonstration project was announced in March 2016.
The agency said it received “a great deal of support from some” for the proposed demonstration. However, “a number of stakeholders expressed strong concerns about the model. While CMS was working to address these concerns, the complexity of the issues and the limited time available led to the decision not to finalize the rule at this time.”
The demonstration project was designed to test new methods to “improve how Medicare Part B pays for prescription drugs and supports physicians and other clinicians in delivering high quality care,” according to a fact sheet published in March.
Under the project, medical practices would have been divided into two groups. A control group would continue to be paid for Part B drugs at the current rate of 106% of average sales price (ASP), while the other would have been paid at 102.5% of ASP plus a flat fee of $16.80 per drug payment. Starting in January 2017, each group would have been further subdivided with a portion of each being subjected to value-based purchasing tools.
One key criticism of the demonstration project centered on the proposed randomization of practices, which was based on primary care service areas (clusters of zip codes with similar Part B medical care patterns). That randomization scheme could have caused different payment levels – and patient out-of-pocket spending – for geographically close areas. Further, participation in the demonstration project would have been mandatory, with no mechanism to opt out.
“This is a model for how Washington should, but often doesn’t, work,” American Medical Association President Andrew W. Gurman, MD, said in a statement. “We are grateful that CMS came to the right decision after listening to stakeholders.”
An analysis of the proposed demonstration project by Avalere found that specialists would likely see a decrease in their drug payments under the proposal, while primary care doctors would likely see an increase, and that 7 of the 10 drugs most affected by this proposal were drugs used to treat cancer.