User login
Lymphoma patients report high levels of distress

Photo courtesy of ESMO
SINGAPORE—Cancer survivors in Malaysia may have impaired health-related quality of life (HRQOL) and high levels of psychological distress 1 year after diagnosis, according to research presented at the ESMO Asia 2016 Congress.*
Of all the patients studied, those with lymphoma had the lowest global health score (according to the EORTC QLQ C30 questionnaire) and the highest levels of psychological distress (anxiety and depression).
“We urgently need new ways of supporting cancer survivors and addressing wider aspects of wellbeing,” said study investigator Shridevi Subramaniam, of the Clinical Research Centre, Ministry of Health Malaysia, in Kuala Lumpur.
“Instead of just focusing on clinical outcome, doctors must focus equally on quality of life for cancer patients, especially psychologically, financially, and socially.”
Patient population
Subramaniam and her colleagues assessed HRQOL in 1376 cancer patients who had survived 12 months from diagnosis. The patients’ mean age was 52, and 64% were female.
Patients had breast cancer (n=403), lymphomas (n=349), colorectal cancer (n=160), mouth cancer (n=108), and female reproductive cancer (n=91).
Forty-one percent of patients had late-stage cancer, 30% had early stage, and 29% had hematologic cancer. Twenty-eight percent of patients had comorbidities.
Thirty-five percent of patients underwent surgery, 73% received chemotherapy, 43% received radiotherapy, and 13% received hormonal therapy.
Forty-six percent of patients were treated at public hospitals, 48% were treated at academic hospitals, and 6% were treated at private hospitals.
Nearly three-quarters of patients (73%) had no insurance, 20% had private insurance, 5% had insurance via their employers, and 4% had other insurance. Forty-one percent of patients had low income status, 30% middle income, and 20% high income.
Overall HRQOL
The patients reported their HRQOL using the EORTC QLQ C30, Hospital Anxiety and Depression Scale, and EQ-5D questionnaire.
For the entire patient cohort, EORTC QLQ-Q30 scores were as follows:
- Mean global health score—53.0
- Mean physical function score—72.6
- Mean emotional function score—63.0
- Mean fatigue score—32.3
- Mean pain score—26.5
The patients’ mean generic HRQOL index score (according to EQ-5D) was 0.7. And a majority of patients reported anxiety (83.5%, n=949) and depression (79.1%, n=899).
HRQOL by cancer type
Anxiety and depression was most common among patients with lymphoma. These patients also had the lowest global health score. Subramaniam said these findings might be explained by side effects from aggressive treatment.
Mean global health scores were 33.3 for lymphoma patients, 59.4 for female breast cancer patients, 59.6 for colorectal cancer patients, 59.8 for patients with mouth cancer, and 67.8 for patients with female reproductive cancers.
The generic HRQOL index scores were 0.69 (colorectal), 0.73 (lymphoma, breast, and mouth), and 0.80 (reproductive).
The proportion of patients with anxiety was 94% (lymphoma), 87.4% (colorectal), 80.5% (breast), 72.6% (mouth), and 51.7% (reproductive).
The proportion of patients with depression was 86.7% (lymphoma), 80.9% (colorectal), 75.8% (mouth), 74.4% (breast), and 50.5% (reproductive).
Predictors of HRQOL
Subramaniam and her colleagues found several significant predictors of HRQOL.
Older patients had decreased physical function and global health, as well as increased pain and fatigue. Married patients had increased fatigue. And patients without comorbidities had increased physical and emotional function as well as decreased fatigue.
Compared with those treated at private hospitals, patients treated at academic hospitals had decreased physical and emotional functions and increased fatigue. Subramaniam said this could be due to long wait times at academic hospitals, which lead to worsening conditions and more pain and discomfort.
Compared to patients with high income status, those from low income groups had increased global health and physical and emotional functions, as well as decreased pain and fatigue. Subramaniam said this could be due to higher expectations among patients with higher incomes.
Compared to patients with early stage cancers, patients with hematologic and late stage cancers had decreased global health and physical function, as well as increased pain and fatigue. Subramaniam said this could be attributed to side effects of treatment.
She added that treatment side effects might also explain why patients who did not receive chemotherapy had higher global health scores.
Patients who didn’t receive radiotherapy were twice as likely as those who did to report psychological distress. And Subramaniam attributed this to a loss of hope among patients who were not treated.
Patients treated in public and academic hospitals were less likely to be psychologically distressed than those treated in private centers. Subramaniam said this could be related to financial distress.
In closing, Subramaniam said this study indicates that cancer survivors in Malaysia have impaired HRQOL, and many experience psychological distress. Therefore, clinicians should focus on “supporting patients throughout their whole cancer ‘journey,’ especially in their lives after treatment.” ![]()
*Abstract 496O_PR—“Health-related quality of life and psychological distress among cancer survivors in a middle-income Asian country.” (Information in the abstract differs from the presentation.)

Photo courtesy of ESMO
SINGAPORE—Cancer survivors in Malaysia may have impaired health-related quality of life (HRQOL) and high levels of psychological distress 1 year after diagnosis, according to research presented at the ESMO Asia 2016 Congress.*
Of all the patients studied, those with lymphoma had the lowest global health score (according to the EORTC QLQ C30 questionnaire) and the highest levels of psychological distress (anxiety and depression).
“We urgently need new ways of supporting cancer survivors and addressing wider aspects of wellbeing,” said study investigator Shridevi Subramaniam, of the Clinical Research Centre, Ministry of Health Malaysia, in Kuala Lumpur.
“Instead of just focusing on clinical outcome, doctors must focus equally on quality of life for cancer patients, especially psychologically, financially, and socially.”
Patient population
Subramaniam and her colleagues assessed HRQOL in 1376 cancer patients who had survived 12 months from diagnosis. The patients’ mean age was 52, and 64% were female.
Patients had breast cancer (n=403), lymphomas (n=349), colorectal cancer (n=160), mouth cancer (n=108), and female reproductive cancer (n=91).
Forty-one percent of patients had late-stage cancer, 30% had early stage, and 29% had hematologic cancer. Twenty-eight percent of patients had comorbidities.
Thirty-five percent of patients underwent surgery, 73% received chemotherapy, 43% received radiotherapy, and 13% received hormonal therapy.
Forty-six percent of patients were treated at public hospitals, 48% were treated at academic hospitals, and 6% were treated at private hospitals.
Nearly three-quarters of patients (73%) had no insurance, 20% had private insurance, 5% had insurance via their employers, and 4% had other insurance. Forty-one percent of patients had low income status, 30% middle income, and 20% high income.
Overall HRQOL
The patients reported their HRQOL using the EORTC QLQ C30, Hospital Anxiety and Depression Scale, and EQ-5D questionnaire.
For the entire patient cohort, EORTC QLQ-Q30 scores were as follows:
- Mean global health score—53.0
- Mean physical function score—72.6
- Mean emotional function score—63.0
- Mean fatigue score—32.3
- Mean pain score—26.5
The patients’ mean generic HRQOL index score (according to EQ-5D) was 0.7. And a majority of patients reported anxiety (83.5%, n=949) and depression (79.1%, n=899).
HRQOL by cancer type
Anxiety and depression was most common among patients with lymphoma. These patients also had the lowest global health score. Subramaniam said these findings might be explained by side effects from aggressive treatment.
Mean global health scores were 33.3 for lymphoma patients, 59.4 for female breast cancer patients, 59.6 for colorectal cancer patients, 59.8 for patients with mouth cancer, and 67.8 for patients with female reproductive cancers.
The generic HRQOL index scores were 0.69 (colorectal), 0.73 (lymphoma, breast, and mouth), and 0.80 (reproductive).
The proportion of patients with anxiety was 94% (lymphoma), 87.4% (colorectal), 80.5% (breast), 72.6% (mouth), and 51.7% (reproductive).
The proportion of patients with depression was 86.7% (lymphoma), 80.9% (colorectal), 75.8% (mouth), 74.4% (breast), and 50.5% (reproductive).
Predictors of HRQOL
Subramaniam and her colleagues found several significant predictors of HRQOL.
Older patients had decreased physical function and global health, as well as increased pain and fatigue. Married patients had increased fatigue. And patients without comorbidities had increased physical and emotional function as well as decreased fatigue.
Compared with those treated at private hospitals, patients treated at academic hospitals had decreased physical and emotional functions and increased fatigue. Subramaniam said this could be due to long wait times at academic hospitals, which lead to worsening conditions and more pain and discomfort.
Compared to patients with high income status, those from low income groups had increased global health and physical and emotional functions, as well as decreased pain and fatigue. Subramaniam said this could be due to higher expectations among patients with higher incomes.
Compared to patients with early stage cancers, patients with hematologic and late stage cancers had decreased global health and physical function, as well as increased pain and fatigue. Subramaniam said this could be attributed to side effects of treatment.
She added that treatment side effects might also explain why patients who did not receive chemotherapy had higher global health scores.
Patients who didn’t receive radiotherapy were twice as likely as those who did to report psychological distress. And Subramaniam attributed this to a loss of hope among patients who were not treated.
Patients treated in public and academic hospitals were less likely to be psychologically distressed than those treated in private centers. Subramaniam said this could be related to financial distress.
In closing, Subramaniam said this study indicates that cancer survivors in Malaysia have impaired HRQOL, and many experience psychological distress. Therefore, clinicians should focus on “supporting patients throughout their whole cancer ‘journey,’ especially in their lives after treatment.” ![]()
*Abstract 496O_PR—“Health-related quality of life and psychological distress among cancer survivors in a middle-income Asian country.” (Information in the abstract differs from the presentation.)

Photo courtesy of ESMO
SINGAPORE—Cancer survivors in Malaysia may have impaired health-related quality of life (HRQOL) and high levels of psychological distress 1 year after diagnosis, according to research presented at the ESMO Asia 2016 Congress.*
Of all the patients studied, those with lymphoma had the lowest global health score (according to the EORTC QLQ C30 questionnaire) and the highest levels of psychological distress (anxiety and depression).
“We urgently need new ways of supporting cancer survivors and addressing wider aspects of wellbeing,” said study investigator Shridevi Subramaniam, of the Clinical Research Centre, Ministry of Health Malaysia, in Kuala Lumpur.
“Instead of just focusing on clinical outcome, doctors must focus equally on quality of life for cancer patients, especially psychologically, financially, and socially.”
Patient population
Subramaniam and her colleagues assessed HRQOL in 1376 cancer patients who had survived 12 months from diagnosis. The patients’ mean age was 52, and 64% were female.
Patients had breast cancer (n=403), lymphomas (n=349), colorectal cancer (n=160), mouth cancer (n=108), and female reproductive cancer (n=91).
Forty-one percent of patients had late-stage cancer, 30% had early stage, and 29% had hematologic cancer. Twenty-eight percent of patients had comorbidities.
Thirty-five percent of patients underwent surgery, 73% received chemotherapy, 43% received radiotherapy, and 13% received hormonal therapy.
Forty-six percent of patients were treated at public hospitals, 48% were treated at academic hospitals, and 6% were treated at private hospitals.
Nearly three-quarters of patients (73%) had no insurance, 20% had private insurance, 5% had insurance via their employers, and 4% had other insurance. Forty-one percent of patients had low income status, 30% middle income, and 20% high income.
Overall HRQOL
The patients reported their HRQOL using the EORTC QLQ C30, Hospital Anxiety and Depression Scale, and EQ-5D questionnaire.
For the entire patient cohort, EORTC QLQ-Q30 scores were as follows:
- Mean global health score—53.0
- Mean physical function score—72.6
- Mean emotional function score—63.0
- Mean fatigue score—32.3
- Mean pain score—26.5
The patients’ mean generic HRQOL index score (according to EQ-5D) was 0.7. And a majority of patients reported anxiety (83.5%, n=949) and depression (79.1%, n=899).
HRQOL by cancer type
Anxiety and depression was most common among patients with lymphoma. These patients also had the lowest global health score. Subramaniam said these findings might be explained by side effects from aggressive treatment.
Mean global health scores were 33.3 for lymphoma patients, 59.4 for female breast cancer patients, 59.6 for colorectal cancer patients, 59.8 for patients with mouth cancer, and 67.8 for patients with female reproductive cancers.
The generic HRQOL index scores were 0.69 (colorectal), 0.73 (lymphoma, breast, and mouth), and 0.80 (reproductive).
The proportion of patients with anxiety was 94% (lymphoma), 87.4% (colorectal), 80.5% (breast), 72.6% (mouth), and 51.7% (reproductive).
The proportion of patients with depression was 86.7% (lymphoma), 80.9% (colorectal), 75.8% (mouth), 74.4% (breast), and 50.5% (reproductive).
Predictors of HRQOL
Subramaniam and her colleagues found several significant predictors of HRQOL.
Older patients had decreased physical function and global health, as well as increased pain and fatigue. Married patients had increased fatigue. And patients without comorbidities had increased physical and emotional function as well as decreased fatigue.
Compared with those treated at private hospitals, patients treated at academic hospitals had decreased physical and emotional functions and increased fatigue. Subramaniam said this could be due to long wait times at academic hospitals, which lead to worsening conditions and more pain and discomfort.
Compared to patients with high income status, those from low income groups had increased global health and physical and emotional functions, as well as decreased pain and fatigue. Subramaniam said this could be due to higher expectations among patients with higher incomes.
Compared to patients with early stage cancers, patients with hematologic and late stage cancers had decreased global health and physical function, as well as increased pain and fatigue. Subramaniam said this could be attributed to side effects of treatment.
She added that treatment side effects might also explain why patients who did not receive chemotherapy had higher global health scores.
Patients who didn’t receive radiotherapy were twice as likely as those who did to report psychological distress. And Subramaniam attributed this to a loss of hope among patients who were not treated.
Patients treated in public and academic hospitals were less likely to be psychologically distressed than those treated in private centers. Subramaniam said this could be related to financial distress.
In closing, Subramaniam said this study indicates that cancer survivors in Malaysia have impaired HRQOL, and many experience psychological distress. Therefore, clinicians should focus on “supporting patients throughout their whole cancer ‘journey,’ especially in their lives after treatment.” ![]()
*Abstract 496O_PR—“Health-related quality of life and psychological distress among cancer survivors in a middle-income Asian country.” (Information in the abstract differs from the presentation.)
Sleep Disorders
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Bariatric surgery or total joint replacement: which first?
NEW ORLEANS – Performing bariatric surgery prior to total knee or hip replacement instead of vice versa resulted in significantly shorter orthopedic surgical operating time and length of stay in an observational study, Emanuel E. Nearing II, MD, reported at Obesity Week 2016.
“We propose that strong consideration be given to bariatric surgery as a means of weight loss and BMI [body mass index] reduction in patients with obesity prior to total joint replacement,” he said at the meeting presented by the Obesity Society of America and the American Society for Metabolic and Bariatric Surgery.
“A common complaint of patients presenting with obesity is that their osteoarthritis has limited their mobility and that their weight gain is secondary to that reduced mobility. They believe that a new joint will help them regain their mobility and then lose weight. Interestingly, this does not appear to be the case. In fact, the majority of patients in our study actually gained weight following joint replacement. Given that, these patients need to be weight-optimized prior to total joint replacement. Bariatric surgery is a durable way to facilitate this,” he continued.
Dr. Nearing presented a retrospective observational study of 102 patients who underwent either laparoscopic Roux-en-Y gastric bypass or laparoscopic sleeve gastrectomy plus a total knee or hip replacement in the Gundersen system. Sixty-six patients had their bariatric surgery first, by a mean of 4.3 years, while the other 36 had arthroplasty a mean of 4.9 years before their bariatric surgery. The two groups were similar in terms of demographics and baseline comorbid conditions.
Patients who had their total joint replacement first had a mean preoperative BMI of 43.7 kg/m2 and a mean pre–bariatric surgery BMI of 46.3 kg/m2. The patients who had bariatric surgery first had a preoperative BMI of 49.6 kg/m2 and a mean pre–orthopedic surgery BMI of 37.6 kgm2. One year after joint replacement surgery, patients who had that operation first had a mean BMI of 43.9 kg/m2, compared with 37.8 kg/m2 for those who waited until after they underwent bariatric surgery.
Mean operative time for total joint replacement when it was the first operation was 113.5 minutes and substantially less at 71 minutes when it was done after bariatric surgery. Mean hospital length of stay for total joint replacement when it followed bariatric surgery was 2.9 days, a full day less than when joint replacement came first.
Rates of complications including skin or soft tissue infection, venous thromboembolism, hematoma, need for transfusion, and periprosthetic infection at 30 and 90 days didn’t differ between the two groups. Neither did the need for late reinterventions.
Dr. Nearing noted that a working group of the American Association of Hip and Knee Surgeons has conducted a review of the orthopedic surgery literature and concluded that all patients with a BMI of 30 kg/m2 or more undergoing total knee or hip arthroplasty are at increased risk for perioperative respiratory complications, thromboembolic events, delayed wound healing, infection, and need for joint revision surgery (J Arthroplasty. 2013 May;28[5]:714-21).
He observed that a retrospective study such as his cannot shed light on the optimal time interval for total joint replacement following bariatric surgery. That key question is being addressed by the ongoing prospective SWIFT (Surgical Weight-Loss to Improve Functional Status Trajectories Following Total Knee Arthroplasty) trial. The study hypothesis is that bariatric surgery prior to the knee replacement surgery will reduce risk and improve long-term outcomes and physical function.
Several audience member commented that, based upon their experience, they would have anticipated that complication rates would have been significantly lower in total joint replacement patients when that operation followed bariatric surgery.
“We were surprised, too,” Dr. Nearing replied. “I think the explanation is that at Gundersen we have three bariatric surgeons and only a handful of orthopedic surgeons, and we use protocols and pathways. We just routinely do our operations the same way each and every time.”
John M. Morton, MD, a former American Society for Metabolic and Bariatric Surgery president, commented that the Gundersen study findings sound a call for more cross-specialty collaboration in steering obese patients with severe knee or hip osteoarthritis to bariatric surgery first in order to maximize the results of the joint replacement surgery.
“I think we’re all seeing weight loss as another form of prehabilitation for other specialties. Our orthopedic colleagues are kind of like us – surgeons – so this seems to be a great place for us to partner with them,” said Dr. Morton, chief of bariatric and minimally invasive surgery at Stanford (Calif.) University.
Dr. Nearing reported having no financial interests relevant to his study.
NEW ORLEANS – Performing bariatric surgery prior to total knee or hip replacement instead of vice versa resulted in significantly shorter orthopedic surgical operating time and length of stay in an observational study, Emanuel E. Nearing II, MD, reported at Obesity Week 2016.
“We propose that strong consideration be given to bariatric surgery as a means of weight loss and BMI [body mass index] reduction in patients with obesity prior to total joint replacement,” he said at the meeting presented by the Obesity Society of America and the American Society for Metabolic and Bariatric Surgery.
“A common complaint of patients presenting with obesity is that their osteoarthritis has limited their mobility and that their weight gain is secondary to that reduced mobility. They believe that a new joint will help them regain their mobility and then lose weight. Interestingly, this does not appear to be the case. In fact, the majority of patients in our study actually gained weight following joint replacement. Given that, these patients need to be weight-optimized prior to total joint replacement. Bariatric surgery is a durable way to facilitate this,” he continued.
Dr. Nearing presented a retrospective observational study of 102 patients who underwent either laparoscopic Roux-en-Y gastric bypass or laparoscopic sleeve gastrectomy plus a total knee or hip replacement in the Gundersen system. Sixty-six patients had their bariatric surgery first, by a mean of 4.3 years, while the other 36 had arthroplasty a mean of 4.9 years before their bariatric surgery. The two groups were similar in terms of demographics and baseline comorbid conditions.
Patients who had their total joint replacement first had a mean preoperative BMI of 43.7 kg/m2 and a mean pre–bariatric surgery BMI of 46.3 kg/m2. The patients who had bariatric surgery first had a preoperative BMI of 49.6 kg/m2 and a mean pre–orthopedic surgery BMI of 37.6 kgm2. One year after joint replacement surgery, patients who had that operation first had a mean BMI of 43.9 kg/m2, compared with 37.8 kg/m2 for those who waited until after they underwent bariatric surgery.
Mean operative time for total joint replacement when it was the first operation was 113.5 minutes and substantially less at 71 minutes when it was done after bariatric surgery. Mean hospital length of stay for total joint replacement when it followed bariatric surgery was 2.9 days, a full day less than when joint replacement came first.
Rates of complications including skin or soft tissue infection, venous thromboembolism, hematoma, need for transfusion, and periprosthetic infection at 30 and 90 days didn’t differ between the two groups. Neither did the need for late reinterventions.
Dr. Nearing noted that a working group of the American Association of Hip and Knee Surgeons has conducted a review of the orthopedic surgery literature and concluded that all patients with a BMI of 30 kg/m2 or more undergoing total knee or hip arthroplasty are at increased risk for perioperative respiratory complications, thromboembolic events, delayed wound healing, infection, and need for joint revision surgery (J Arthroplasty. 2013 May;28[5]:714-21).
He observed that a retrospective study such as his cannot shed light on the optimal time interval for total joint replacement following bariatric surgery. That key question is being addressed by the ongoing prospective SWIFT (Surgical Weight-Loss to Improve Functional Status Trajectories Following Total Knee Arthroplasty) trial. The study hypothesis is that bariatric surgery prior to the knee replacement surgery will reduce risk and improve long-term outcomes and physical function.
Several audience member commented that, based upon their experience, they would have anticipated that complication rates would have been significantly lower in total joint replacement patients when that operation followed bariatric surgery.
“We were surprised, too,” Dr. Nearing replied. “I think the explanation is that at Gundersen we have three bariatric surgeons and only a handful of orthopedic surgeons, and we use protocols and pathways. We just routinely do our operations the same way each and every time.”
John M. Morton, MD, a former American Society for Metabolic and Bariatric Surgery president, commented that the Gundersen study findings sound a call for more cross-specialty collaboration in steering obese patients with severe knee or hip osteoarthritis to bariatric surgery first in order to maximize the results of the joint replacement surgery.
“I think we’re all seeing weight loss as another form of prehabilitation for other specialties. Our orthopedic colleagues are kind of like us – surgeons – so this seems to be a great place for us to partner with them,” said Dr. Morton, chief of bariatric and minimally invasive surgery at Stanford (Calif.) University.
Dr. Nearing reported having no financial interests relevant to his study.
NEW ORLEANS – Performing bariatric surgery prior to total knee or hip replacement instead of vice versa resulted in significantly shorter orthopedic surgical operating time and length of stay in an observational study, Emanuel E. Nearing II, MD, reported at Obesity Week 2016.
“We propose that strong consideration be given to bariatric surgery as a means of weight loss and BMI [body mass index] reduction in patients with obesity prior to total joint replacement,” he said at the meeting presented by the Obesity Society of America and the American Society for Metabolic and Bariatric Surgery.
“A common complaint of patients presenting with obesity is that their osteoarthritis has limited their mobility and that their weight gain is secondary to that reduced mobility. They believe that a new joint will help them regain their mobility and then lose weight. Interestingly, this does not appear to be the case. In fact, the majority of patients in our study actually gained weight following joint replacement. Given that, these patients need to be weight-optimized prior to total joint replacement. Bariatric surgery is a durable way to facilitate this,” he continued.
Dr. Nearing presented a retrospective observational study of 102 patients who underwent either laparoscopic Roux-en-Y gastric bypass or laparoscopic sleeve gastrectomy plus a total knee or hip replacement in the Gundersen system. Sixty-six patients had their bariatric surgery first, by a mean of 4.3 years, while the other 36 had arthroplasty a mean of 4.9 years before their bariatric surgery. The two groups were similar in terms of demographics and baseline comorbid conditions.
Patients who had their total joint replacement first had a mean preoperative BMI of 43.7 kg/m2 and a mean pre–bariatric surgery BMI of 46.3 kg/m2. The patients who had bariatric surgery first had a preoperative BMI of 49.6 kg/m2 and a mean pre–orthopedic surgery BMI of 37.6 kgm2. One year after joint replacement surgery, patients who had that operation first had a mean BMI of 43.9 kg/m2, compared with 37.8 kg/m2 for those who waited until after they underwent bariatric surgery.
Mean operative time for total joint replacement when it was the first operation was 113.5 minutes and substantially less at 71 minutes when it was done after bariatric surgery. Mean hospital length of stay for total joint replacement when it followed bariatric surgery was 2.9 days, a full day less than when joint replacement came first.
Rates of complications including skin or soft tissue infection, venous thromboembolism, hematoma, need for transfusion, and periprosthetic infection at 30 and 90 days didn’t differ between the two groups. Neither did the need for late reinterventions.
Dr. Nearing noted that a working group of the American Association of Hip and Knee Surgeons has conducted a review of the orthopedic surgery literature and concluded that all patients with a BMI of 30 kg/m2 or more undergoing total knee or hip arthroplasty are at increased risk for perioperative respiratory complications, thromboembolic events, delayed wound healing, infection, and need for joint revision surgery (J Arthroplasty. 2013 May;28[5]:714-21).
He observed that a retrospective study such as his cannot shed light on the optimal time interval for total joint replacement following bariatric surgery. That key question is being addressed by the ongoing prospective SWIFT (Surgical Weight-Loss to Improve Functional Status Trajectories Following Total Knee Arthroplasty) trial. The study hypothesis is that bariatric surgery prior to the knee replacement surgery will reduce risk and improve long-term outcomes and physical function.
Several audience member commented that, based upon their experience, they would have anticipated that complication rates would have been significantly lower in total joint replacement patients when that operation followed bariatric surgery.
“We were surprised, too,” Dr. Nearing replied. “I think the explanation is that at Gundersen we have three bariatric surgeons and only a handful of orthopedic surgeons, and we use protocols and pathways. We just routinely do our operations the same way each and every time.”
John M. Morton, MD, a former American Society for Metabolic and Bariatric Surgery president, commented that the Gundersen study findings sound a call for more cross-specialty collaboration in steering obese patients with severe knee or hip osteoarthritis to bariatric surgery first in order to maximize the results of the joint replacement surgery.
“I think we’re all seeing weight loss as another form of prehabilitation for other specialties. Our orthopedic colleagues are kind of like us – surgeons – so this seems to be a great place for us to partner with them,” said Dr. Morton, chief of bariatric and minimally invasive surgery at Stanford (Calif.) University.
Dr. Nearing reported having no financial interests relevant to his study.
AT OBESITY WEEK 2016
Key clinical point:
Major finding: When total joint replacement in obese patients was performed after bariatric surgery, mean hospital length of stay was a full day less than when the orthopedic surgery preceded the bariatric surgery.
Data source: This retrospective observational study included 102 obese patients who underwent bariatric surgery and total knee or hip replacement.
Disclosures: The study presenter reported having no financial conflicts of interest.
ADHD versus anxiety? An approach for pediatricians
This month’s column comes directly by request from a pediatric colleague. She asked about a common diagnostic dilemma for pediatricians that involves what at least on the surface appears like disruptive or oppositional behavior at the home, school, or both, but is complicated by the possibility that the primary engine of this behavior is anxiety. This is an important challenge to try and get right because the treatment plan will take different paths, depending on the final call that is made.
Case summary
Devin is a 6-year-old boy who comes in with his parents for concerns about his behavior. His parents note that he has always been “high strung” but not disruptive or aggressive. When he was younger, Devin was quite sensitive to sounds, textures, and tactile sensations, but this has improved on its own. Thunderstorms continue to bother him quite a bit, though, and he often will ask his parents repeated questions when it is cloudy about the possibility of a thunderstorm. With some extra teacher and parent support, Devin made the transition to kindergarten fairly well. Now, however, he is struggling in a larger 1st grade class. His teacher states that he often seems distracted, fidgety, and easily frustrated, causing him to “shut down” and refuse to do his work. This past week, during a more challenging assignment, he crawled under his desk and would not come out. The teacher is now recommending an evaluation for attention-deficit/hyperactivity disorder (ADHD).
Discussion
1. Are there other times in the child’s life when clearly he is very anxious? The presence of developmentally elevated levels of anxiety in areas outside the particular situations in question can provide a clue that anxiety is contributing to what otherwise might be seen as more oppositional behavior. In this case, the high levels of anxiety about thunderstorms show that anxiety is present in the child and could be playing a role in his disruptive behavior at school.
2. When he’s not focusing on the task at hand, what is he thinking about? Nonanxious children with or without ADHD can frequently daydream and go “off task,” but the content of those thoughts frequently involves anticipation for more preferred activities, reminisces of positive events from the past, or attention to other stimuli in the environment (for example, the bird in a tree outside). More anxious children, by contrast, may have more worried and ruminating thoughts about poor performance, possible bad events that might happen in the future, or “what if?” kinds of concerns.
3. Is there a family history of anxiety? While one should not over-rely on family history, the presence of one or more family members with clinically significant anxiety does raise the possibility of anxiety in the identified patient. Research indicates that the heritability of anxiety is about 50%,1,2 but that a significant amount of the transmission of anxiety from parent to child comes from environmental mechanisms.3
4. Is there a consistent trigger to his outbursts? For anxious children, meltdowns are frequently provoked by situations in which a child feels uncomfortable, overstimulated, or overwhelmed, and the outburst is a reflection of those intense feelings that are difficult to manage. An outburst like that above, which occurs when a child is pushed to finish difficult work, might be a good example of one that is triggered by anxiety.
5. What does the rating scale show? A broad-based rating scale that assesses multiple domains of symptoms can be a big help for diagnostic dilemmas such as this one. Our clinic uses the Child Behavior Checklist4 which has subscales for both anxiety and attention problems. Evidence of a spike in either of those domains, or both, really can help guide our thinking.
Of course, it is very possible that the answer to the ADHD versus anxiety question is that both are present. This is a common conclusion when it comes to mental health assessment, and it is different from the traditional “this or that” thinking present in more classic differential diagnosis decision making. Research indicates that the ADHD and anxiety disorders frequently co-occur.5 When that happens, concurrent evidence-based psychotherapy for anxiety in conjunction with multimodal treatment for ADHD has been recommended as a first step.6
Case follow-up
Based on all the information, the pediatrician judges that Devin’s disruptive behavior is in large part being driven by his level of anxiety. She makes a referral to a child psychologist to begin evidence-based psychotherapy and recommends that the school consider some modifications and accommodations that may help his behavior at school. At a follow-up appointment, Devin’s difficulties have improved, and there is little evidence of ADHD now that the anxiety has been fully addressed.
References
1. Genes Brain Behav. 2005;4(8):466-81.
2. J Am Acad Child Adolesc Psychiatry. 2010;49(3):248-55.
3. Am J Psychiatry. 2015;172(7):630-7.
4. Manual for the ASEBA School-Age Forms & Profiles (Burlington, Vt.: University of Vermont, Research Center for Children, Youth, and Families, 2001).
5. J Anxiety Disord. 1997;11(4):377-94.
6. J Abnorm Child Psychol. 2000;28(6):527-41.
Dr. Rettew is a child and adolescent psychiatrist and assistant professor of psychiatry and pediatrics at the University of Vermont Larner College of Medicine, Burlington. Follow him on Twitter @PediPsych. Email him at [email protected].
This month’s column comes directly by request from a pediatric colleague. She asked about a common diagnostic dilemma for pediatricians that involves what at least on the surface appears like disruptive or oppositional behavior at the home, school, or both, but is complicated by the possibility that the primary engine of this behavior is anxiety. This is an important challenge to try and get right because the treatment plan will take different paths, depending on the final call that is made.
Case summary
Devin is a 6-year-old boy who comes in with his parents for concerns about his behavior. His parents note that he has always been “high strung” but not disruptive or aggressive. When he was younger, Devin was quite sensitive to sounds, textures, and tactile sensations, but this has improved on its own. Thunderstorms continue to bother him quite a bit, though, and he often will ask his parents repeated questions when it is cloudy about the possibility of a thunderstorm. With some extra teacher and parent support, Devin made the transition to kindergarten fairly well. Now, however, he is struggling in a larger 1st grade class. His teacher states that he often seems distracted, fidgety, and easily frustrated, causing him to “shut down” and refuse to do his work. This past week, during a more challenging assignment, he crawled under his desk and would not come out. The teacher is now recommending an evaluation for attention-deficit/hyperactivity disorder (ADHD).
Discussion
1. Are there other times in the child’s life when clearly he is very anxious? The presence of developmentally elevated levels of anxiety in areas outside the particular situations in question can provide a clue that anxiety is contributing to what otherwise might be seen as more oppositional behavior. In this case, the high levels of anxiety about thunderstorms show that anxiety is present in the child and could be playing a role in his disruptive behavior at school.
2. When he’s not focusing on the task at hand, what is he thinking about? Nonanxious children with or without ADHD can frequently daydream and go “off task,” but the content of those thoughts frequently involves anticipation for more preferred activities, reminisces of positive events from the past, or attention to other stimuli in the environment (for example, the bird in a tree outside). More anxious children, by contrast, may have more worried and ruminating thoughts about poor performance, possible bad events that might happen in the future, or “what if?” kinds of concerns.
3. Is there a family history of anxiety? While one should not over-rely on family history, the presence of one or more family members with clinically significant anxiety does raise the possibility of anxiety in the identified patient. Research indicates that the heritability of anxiety is about 50%,1,2 but that a significant amount of the transmission of anxiety from parent to child comes from environmental mechanisms.3
4. Is there a consistent trigger to his outbursts? For anxious children, meltdowns are frequently provoked by situations in which a child feels uncomfortable, overstimulated, or overwhelmed, and the outburst is a reflection of those intense feelings that are difficult to manage. An outburst like that above, which occurs when a child is pushed to finish difficult work, might be a good example of one that is triggered by anxiety.
5. What does the rating scale show? A broad-based rating scale that assesses multiple domains of symptoms can be a big help for diagnostic dilemmas such as this one. Our clinic uses the Child Behavior Checklist4 which has subscales for both anxiety and attention problems. Evidence of a spike in either of those domains, or both, really can help guide our thinking.
Of course, it is very possible that the answer to the ADHD versus anxiety question is that both are present. This is a common conclusion when it comes to mental health assessment, and it is different from the traditional “this or that” thinking present in more classic differential diagnosis decision making. Research indicates that the ADHD and anxiety disorders frequently co-occur.5 When that happens, concurrent evidence-based psychotherapy for anxiety in conjunction with multimodal treatment for ADHD has been recommended as a first step.6
Case follow-up
Based on all the information, the pediatrician judges that Devin’s disruptive behavior is in large part being driven by his level of anxiety. She makes a referral to a child psychologist to begin evidence-based psychotherapy and recommends that the school consider some modifications and accommodations that may help his behavior at school. At a follow-up appointment, Devin’s difficulties have improved, and there is little evidence of ADHD now that the anxiety has been fully addressed.
References
1. Genes Brain Behav. 2005;4(8):466-81.
2. J Am Acad Child Adolesc Psychiatry. 2010;49(3):248-55.
3. Am J Psychiatry. 2015;172(7):630-7.
4. Manual for the ASEBA School-Age Forms & Profiles (Burlington, Vt.: University of Vermont, Research Center for Children, Youth, and Families, 2001).
5. J Anxiety Disord. 1997;11(4):377-94.
6. J Abnorm Child Psychol. 2000;28(6):527-41.
Dr. Rettew is a child and adolescent psychiatrist and assistant professor of psychiatry and pediatrics at the University of Vermont Larner College of Medicine, Burlington. Follow him on Twitter @PediPsych. Email him at [email protected].
This month’s column comes directly by request from a pediatric colleague. She asked about a common diagnostic dilemma for pediatricians that involves what at least on the surface appears like disruptive or oppositional behavior at the home, school, or both, but is complicated by the possibility that the primary engine of this behavior is anxiety. This is an important challenge to try and get right because the treatment plan will take different paths, depending on the final call that is made.
Case summary
Devin is a 6-year-old boy who comes in with his parents for concerns about his behavior. His parents note that he has always been “high strung” but not disruptive or aggressive. When he was younger, Devin was quite sensitive to sounds, textures, and tactile sensations, but this has improved on its own. Thunderstorms continue to bother him quite a bit, though, and he often will ask his parents repeated questions when it is cloudy about the possibility of a thunderstorm. With some extra teacher and parent support, Devin made the transition to kindergarten fairly well. Now, however, he is struggling in a larger 1st grade class. His teacher states that he often seems distracted, fidgety, and easily frustrated, causing him to “shut down” and refuse to do his work. This past week, during a more challenging assignment, he crawled under his desk and would not come out. The teacher is now recommending an evaluation for attention-deficit/hyperactivity disorder (ADHD).
Discussion
1. Are there other times in the child’s life when clearly he is very anxious? The presence of developmentally elevated levels of anxiety in areas outside the particular situations in question can provide a clue that anxiety is contributing to what otherwise might be seen as more oppositional behavior. In this case, the high levels of anxiety about thunderstorms show that anxiety is present in the child and could be playing a role in his disruptive behavior at school.
2. When he’s not focusing on the task at hand, what is he thinking about? Nonanxious children with or without ADHD can frequently daydream and go “off task,” but the content of those thoughts frequently involves anticipation for more preferred activities, reminisces of positive events from the past, or attention to other stimuli in the environment (for example, the bird in a tree outside). More anxious children, by contrast, may have more worried and ruminating thoughts about poor performance, possible bad events that might happen in the future, or “what if?” kinds of concerns.
3. Is there a family history of anxiety? While one should not over-rely on family history, the presence of one or more family members with clinically significant anxiety does raise the possibility of anxiety in the identified patient. Research indicates that the heritability of anxiety is about 50%,1,2 but that a significant amount of the transmission of anxiety from parent to child comes from environmental mechanisms.3
4. Is there a consistent trigger to his outbursts? For anxious children, meltdowns are frequently provoked by situations in which a child feels uncomfortable, overstimulated, or overwhelmed, and the outburst is a reflection of those intense feelings that are difficult to manage. An outburst like that above, which occurs when a child is pushed to finish difficult work, might be a good example of one that is triggered by anxiety.
5. What does the rating scale show? A broad-based rating scale that assesses multiple domains of symptoms can be a big help for diagnostic dilemmas such as this one. Our clinic uses the Child Behavior Checklist4 which has subscales for both anxiety and attention problems. Evidence of a spike in either of those domains, or both, really can help guide our thinking.
Of course, it is very possible that the answer to the ADHD versus anxiety question is that both are present. This is a common conclusion when it comes to mental health assessment, and it is different from the traditional “this or that” thinking present in more classic differential diagnosis decision making. Research indicates that the ADHD and anxiety disorders frequently co-occur.5 When that happens, concurrent evidence-based psychotherapy for anxiety in conjunction with multimodal treatment for ADHD has been recommended as a first step.6
Case follow-up
Based on all the information, the pediatrician judges that Devin’s disruptive behavior is in large part being driven by his level of anxiety. She makes a referral to a child psychologist to begin evidence-based psychotherapy and recommends that the school consider some modifications and accommodations that may help his behavior at school. At a follow-up appointment, Devin’s difficulties have improved, and there is little evidence of ADHD now that the anxiety has been fully addressed.
References
1. Genes Brain Behav. 2005;4(8):466-81.
2. J Am Acad Child Adolesc Psychiatry. 2010;49(3):248-55.
3. Am J Psychiatry. 2015;172(7):630-7.
4. Manual for the ASEBA School-Age Forms & Profiles (Burlington, Vt.: University of Vermont, Research Center for Children, Youth, and Families, 2001).
5. J Anxiety Disord. 1997;11(4):377-94.
6. J Abnorm Child Psychol. 2000;28(6):527-41.
Dr. Rettew is a child and adolescent psychiatrist and assistant professor of psychiatry and pediatrics at the University of Vermont Larner College of Medicine, Burlington. Follow him on Twitter @PediPsych. Email him at [email protected].
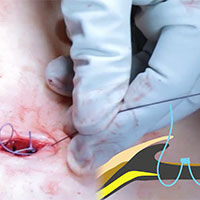
Tips and tricks for open laparoscopy

Visit the Society of Gynecologic Surgeons online: sgsonline.org
More videos from SGS:
- Complete colpectomy & colpocleisis: Model for simulation
- Natural orifice sacral colpopexy
- Alternative options for visualizing ureteral patency during intraoperative cystoscopy
- Use of suprapubic Carter-Thomason needle to assist in cystoscopic excision of an intravesical foreign object
- Uterine artery ligation: Advanced techniques and considerations for the difficult laparoscopic hysterectomy
- Cervical injection of methylene blue for identification of sentinel lymph nodes in cervical cancer
- Misplaced hysteroscopic sterilization micro-insert in the peritoneal cavity: A corpus alienum
- Laparoscopic cystectomy for large, bilateral ovarian dermoids
- Small bowel surgery for the benign gynecologist

Visit the Society of Gynecologic Surgeons online: sgsonline.org
More videos from SGS:
- Complete colpectomy & colpocleisis: Model for simulation
- Natural orifice sacral colpopexy
- Alternative options for visualizing ureteral patency during intraoperative cystoscopy
- Use of suprapubic Carter-Thomason needle to assist in cystoscopic excision of an intravesical foreign object
- Uterine artery ligation: Advanced techniques and considerations for the difficult laparoscopic hysterectomy
- Cervical injection of methylene blue for identification of sentinel lymph nodes in cervical cancer
- Misplaced hysteroscopic sterilization micro-insert in the peritoneal cavity: A corpus alienum
- Laparoscopic cystectomy for large, bilateral ovarian dermoids
- Small bowel surgery for the benign gynecologist

Visit the Society of Gynecologic Surgeons online: sgsonline.org
More videos from SGS:
- Complete colpectomy & colpocleisis: Model for simulation
- Natural orifice sacral colpopexy
- Alternative options for visualizing ureteral patency during intraoperative cystoscopy
- Use of suprapubic Carter-Thomason needle to assist in cystoscopic excision of an intravesical foreign object
- Uterine artery ligation: Advanced techniques and considerations for the difficult laparoscopic hysterectomy
- Cervical injection of methylene blue for identification of sentinel lymph nodes in cervical cancer
- Misplaced hysteroscopic sterilization micro-insert in the peritoneal cavity: A corpus alienum
- Laparoscopic cystectomy for large, bilateral ovarian dermoids
- Small bowel surgery for the benign gynecologist
This video is brought to you by
Ladostigil advances to phase III after hints of slowing brain atrophy in MCI patients
SAN DIEGO – Ladostigil, a drug that combines molecular characteristics of rivastigmine and rasagiline, was associated with some modest memory improvement and – perhaps more interestingly – a signal of brain volume preservation in a phase IIb study of patients with mild cognitive impairment.
Both whole brain and hippocampal volume after 3 years were about 1% higher in the trial’s active group than in the placebo group.
The volumetric data are potentially of more interest than the cognitive results, which didn’t meet the study’s primary endpoint of delaying or preventing conversion from mild cognitive impairment (MCI) to Alzheimer’s disease, Lon Schneider, MD, said at the Clinical Trials on Alzheimer’s Disease conference.
“The whole brain and hippocampal volume started to separate after 1 year and maximized at 2 years,” said Dr. Schneider of the University of Southern California, Los Angeles. “With hippocampal volume, we lose the significance of the effect by year 3, but when we continue on to the end there is a nominal significance in whole brain volume favoring the drug.”
He said the trial was plagued by a 35% dropout rate, probably because of perceived ineffectiveness of the drug. “Those numbers got in the way of looking at this outcome, particularly with 12 drops between years 2 and 3.”
Nevertheless, the drug will continue along its developmental pathway. The Israeli company developing it, Avraham Pharma, is seeking a larger pharmaceutical company with which to partner, according to its website.
Ladostigil pieces together molecular structures from both the monoamine oxidase-B inhibitor rasagiline and the anticholinesterase inhibitor rivastigmine (Exelon).
Avraham suggests that ladostigil could modify Alzheimer’s by neuroprotection, immune modulation, and reducing oxidative stress that leads the release of proinflammatory cytokines and microglial activation.
In 2012, the drug failed a phase II trial focusing on cognition in patients with Alzheimer’s. Afterward, Avraham lowered the dose from 80 mg to 20 mg and launched the current trial, with a refocus on MCI conversion.
It comprised 210 patients with MCI. The mean age was 71 years; mean Mini-Mental State Examination score was 28. About one-third were carriers of the high-risk apolipoprotein E4 allele. Patients were randomized to placebo or 20 mg ladostigil daily for 36 months. Brain MRI was performed at baseline and every 6 months throughout the trial.
The study failed to meet its primary endpoint of MCI conversion to Alzheimer’s. Fourteen patients in the active group and 20 in the placebo group progressed to Alzheimer’s. In year 3, there was a separation of curves with 4 vs. 10 patients progressing to Alzheimer’s, but overall the endpoint was not statistically significant (P = .16).
The two groups began to separate in the decline of brain volume around 1 year into the trial. For hippocampal volume, the difference was statistically significant only at 24 months. By 36 months, volume in the active and placebo groups overlapped slightly with a P value of .31.
The changes in whole-brain volume were somewhat more pronounced. The declining slopes in whole-brain volume began to separate at 12 months. At both 24 and 36 months, the differences were statistically significant (P = .033 and .036, respectively), with curves continuing to diverge at the study’s end.
Adverse events occurred in about 80% of patients (82% with active treatment vs. 79% with placebo); about 25% of both arms experienced serious adverse events.
Common adverse events were atrial fibrillation (8% vs. 3%), chest pain (5% vs. 3%), and benign prostatic hyperplasia (6% vs. 3%). Other events included dizziness, arthralgia, depression, and confusion.
None of the 35% who dropped out of the trial did so for an adverse event, Dr. Schneider noted.
The Neuropsychological Test Battery (NTB) and the Rey Auditory Verbal Learning Test (RAVLT) were secondary measures of behavior and cognition. Both showed positive trends for ladostigil, but neither was statistically significant.
Dr. Schneider has disclosed financial relationships with numerous pharmaceutical companies, but has no financial ties with Avraham.
[email protected]
On Twitter @alz_gal
SAN DIEGO – Ladostigil, a drug that combines molecular characteristics of rivastigmine and rasagiline, was associated with some modest memory improvement and – perhaps more interestingly – a signal of brain volume preservation in a phase IIb study of patients with mild cognitive impairment.
Both whole brain and hippocampal volume after 3 years were about 1% higher in the trial’s active group than in the placebo group.
The volumetric data are potentially of more interest than the cognitive results, which didn’t meet the study’s primary endpoint of delaying or preventing conversion from mild cognitive impairment (MCI) to Alzheimer’s disease, Lon Schneider, MD, said at the Clinical Trials on Alzheimer’s Disease conference.
“The whole brain and hippocampal volume started to separate after 1 year and maximized at 2 years,” said Dr. Schneider of the University of Southern California, Los Angeles. “With hippocampal volume, we lose the significance of the effect by year 3, but when we continue on to the end there is a nominal significance in whole brain volume favoring the drug.”
He said the trial was plagued by a 35% dropout rate, probably because of perceived ineffectiveness of the drug. “Those numbers got in the way of looking at this outcome, particularly with 12 drops between years 2 and 3.”
Nevertheless, the drug will continue along its developmental pathway. The Israeli company developing it, Avraham Pharma, is seeking a larger pharmaceutical company with which to partner, according to its website.
Ladostigil pieces together molecular structures from both the monoamine oxidase-B inhibitor rasagiline and the anticholinesterase inhibitor rivastigmine (Exelon).
Avraham suggests that ladostigil could modify Alzheimer’s by neuroprotection, immune modulation, and reducing oxidative stress that leads the release of proinflammatory cytokines and microglial activation.
In 2012, the drug failed a phase II trial focusing on cognition in patients with Alzheimer’s. Afterward, Avraham lowered the dose from 80 mg to 20 mg and launched the current trial, with a refocus on MCI conversion.
It comprised 210 patients with MCI. The mean age was 71 years; mean Mini-Mental State Examination score was 28. About one-third were carriers of the high-risk apolipoprotein E4 allele. Patients were randomized to placebo or 20 mg ladostigil daily for 36 months. Brain MRI was performed at baseline and every 6 months throughout the trial.
The study failed to meet its primary endpoint of MCI conversion to Alzheimer’s. Fourteen patients in the active group and 20 in the placebo group progressed to Alzheimer’s. In year 3, there was a separation of curves with 4 vs. 10 patients progressing to Alzheimer’s, but overall the endpoint was not statistically significant (P = .16).
The two groups began to separate in the decline of brain volume around 1 year into the trial. For hippocampal volume, the difference was statistically significant only at 24 months. By 36 months, volume in the active and placebo groups overlapped slightly with a P value of .31.
The changes in whole-brain volume were somewhat more pronounced. The declining slopes in whole-brain volume began to separate at 12 months. At both 24 and 36 months, the differences were statistically significant (P = .033 and .036, respectively), with curves continuing to diverge at the study’s end.
Adverse events occurred in about 80% of patients (82% with active treatment vs. 79% with placebo); about 25% of both arms experienced serious adverse events.
Common adverse events were atrial fibrillation (8% vs. 3%), chest pain (5% vs. 3%), and benign prostatic hyperplasia (6% vs. 3%). Other events included dizziness, arthralgia, depression, and confusion.
None of the 35% who dropped out of the trial did so for an adverse event, Dr. Schneider noted.
The Neuropsychological Test Battery (NTB) and the Rey Auditory Verbal Learning Test (RAVLT) were secondary measures of behavior and cognition. Both showed positive trends for ladostigil, but neither was statistically significant.
Dr. Schneider has disclosed financial relationships with numerous pharmaceutical companies, but has no financial ties with Avraham.
[email protected]
On Twitter @alz_gal
SAN DIEGO – Ladostigil, a drug that combines molecular characteristics of rivastigmine and rasagiline, was associated with some modest memory improvement and – perhaps more interestingly – a signal of brain volume preservation in a phase IIb study of patients with mild cognitive impairment.
Both whole brain and hippocampal volume after 3 years were about 1% higher in the trial’s active group than in the placebo group.
The volumetric data are potentially of more interest than the cognitive results, which didn’t meet the study’s primary endpoint of delaying or preventing conversion from mild cognitive impairment (MCI) to Alzheimer’s disease, Lon Schneider, MD, said at the Clinical Trials on Alzheimer’s Disease conference.
“The whole brain and hippocampal volume started to separate after 1 year and maximized at 2 years,” said Dr. Schneider of the University of Southern California, Los Angeles. “With hippocampal volume, we lose the significance of the effect by year 3, but when we continue on to the end there is a nominal significance in whole brain volume favoring the drug.”
He said the trial was plagued by a 35% dropout rate, probably because of perceived ineffectiveness of the drug. “Those numbers got in the way of looking at this outcome, particularly with 12 drops between years 2 and 3.”
Nevertheless, the drug will continue along its developmental pathway. The Israeli company developing it, Avraham Pharma, is seeking a larger pharmaceutical company with which to partner, according to its website.
Ladostigil pieces together molecular structures from both the monoamine oxidase-B inhibitor rasagiline and the anticholinesterase inhibitor rivastigmine (Exelon).
Avraham suggests that ladostigil could modify Alzheimer’s by neuroprotection, immune modulation, and reducing oxidative stress that leads the release of proinflammatory cytokines and microglial activation.
In 2012, the drug failed a phase II trial focusing on cognition in patients with Alzheimer’s. Afterward, Avraham lowered the dose from 80 mg to 20 mg and launched the current trial, with a refocus on MCI conversion.
It comprised 210 patients with MCI. The mean age was 71 years; mean Mini-Mental State Examination score was 28. About one-third were carriers of the high-risk apolipoprotein E4 allele. Patients were randomized to placebo or 20 mg ladostigil daily for 36 months. Brain MRI was performed at baseline and every 6 months throughout the trial.
The study failed to meet its primary endpoint of MCI conversion to Alzheimer’s. Fourteen patients in the active group and 20 in the placebo group progressed to Alzheimer’s. In year 3, there was a separation of curves with 4 vs. 10 patients progressing to Alzheimer’s, but overall the endpoint was not statistically significant (P = .16).
The two groups began to separate in the decline of brain volume around 1 year into the trial. For hippocampal volume, the difference was statistically significant only at 24 months. By 36 months, volume in the active and placebo groups overlapped slightly with a P value of .31.
The changes in whole-brain volume were somewhat more pronounced. The declining slopes in whole-brain volume began to separate at 12 months. At both 24 and 36 months, the differences were statistically significant (P = .033 and .036, respectively), with curves continuing to diverge at the study’s end.
Adverse events occurred in about 80% of patients (82% with active treatment vs. 79% with placebo); about 25% of both arms experienced serious adverse events.
Common adverse events were atrial fibrillation (8% vs. 3%), chest pain (5% vs. 3%), and benign prostatic hyperplasia (6% vs. 3%). Other events included dizziness, arthralgia, depression, and confusion.
None of the 35% who dropped out of the trial did so for an adverse event, Dr. Schneider noted.
The Neuropsychological Test Battery (NTB) and the Rey Auditory Verbal Learning Test (RAVLT) were secondary measures of behavior and cognition. Both showed positive trends for ladostigil, but neither was statistically significant.
Dr. Schneider has disclosed financial relationships with numerous pharmaceutical companies, but has no financial ties with Avraham.
[email protected]
On Twitter @alz_gal
AT CTAD
Key clinical point:
Major finding: After 3 years, whole brain and hippocampal volume were slightly better in the ladostigil group, compared with placebo.
Data source: A phase IIb trial that randomized 210 patients to placebo or 20 mg ladostigil for 36 months.
Disclosures: Dr. Schneider disclosed financial ties with numerous pharmaceutical companies, but has no financial ties with Avraham, which sponsored the study.
50 years in pediatrics: ‘You have to get involved’
A few years before the launch of Pediatric News in 1967, Dr. Henry Kempe and his colleagues at the University of Colorado, Denver, published a groundbreaking report on child abuse, “The Battered Child Syndrome,” in JAMA.
It had a major impact on Calvin C.J. Sia, MD, a now-retired pediatrician in Hawaii, not only because it was pioneering work and one of the milestones of 20th century pediatrics, but also because Dr. Kempe was a close friend and mentor of Dr. Sia, going back to when Dr. Sia was a resident in the 1950s.
Dr. Kempe “alerted me to the battered child syndrome and taught me the importance of looking at the whole child within the context of the family, and he repeatedly reminded me of the importance of preventive care. He taught me how to make preventive change, with the emphasis on the first 3 years” of life, Dr. Sia said in an interview from his home in Honolulu.
To celebrate the 50th anniversary of Pediatric News, it seemed appropriate to turn to Dr. Sia. His practice, launched in 1958, not only spanned our 50 years, but also illustrated one of the major themes in pediatrics over the past half-century. With the old scourges of infectious disease, malnutrition, and infant mortality largely brought under control, pediatrics turned to the broader struggles of childhood, including learning, poverty, and abusive parenting.
Dr. Sia has been president of the Hawaii Medical Association; president of the Hawaii chapter of the American Academy of Pediatrics; chief of staff at the Kauikeolani Children’s Hospital, Honolulu (now the Kapi‘olani Medical Center for Women and Children); chair of the American Medical Association Pediatric Delegation; and founder of the AMA’s Section Council on Pediatrics.
Positions like these eventually led him to contacts with legislators willing to listen and fund child abuse prevention, immunization programs, and other initiatives to help children. It often meant lobbying politicians for money.
“It doesn’t occur overnight; it takes chance and dedication,” said Dr. Sia, whose name, as one of its earliest champions, is now nearly synonymous with the concept of the medical home – an ideal of cradling children in a physician, family, and social services safety net of coordinated care.
Dr. Sia’s career spanned all of the developments that Pediatric News has covered over its 50 years, including the increasing recognition of autism, the earlier survival of premature infants, and phenylketonuria and other screenings at birth. He remembers all of them, and took action on many at the state and federal level.
Taking a step took courage, even when he wasn’t quite sure what to do. “I recognized autism back in the 60s. Children looked normal, were born normal, but then had trouble. I didn’t know what to do with them. That’s how I got involved with learning disabilities. I didn’t know anything, but I got involved,” he said.
With the advice of Robert Cooke, MD, another mentor and an eventual founder of the Head Start program, Dr. Sia cofounded Honolulu’s Variety School for Learning Disabilities in 1967. It’s still in operation; Don Ho and other local “variety hour” entertainers helped with early fundraising.
Dr. Sia also helped launch Healthy Start, one of the nation’s first home visit programs for at-risk kids, and an amendment to the federal Education of the Handicapped Act to extend aid to children with family and developmental challenges.
He convinced Sen. Daniel Inouye, another personal friend, to introduce the Emergency Medical Services for Children Act in the 1980s, which funded states to develop emergency medical services for children. “It was really important because kids need special instruments, IVs, and medications. I had to lobby. It was under Reaganomics,” Dr. Sia said.
At 89 years of age and recovering from a recent heart attack and open heart bypass, he is still trying to help children. Dr. Sia, who was born in Beijing, has been promoting the medical home concept in Asia. Meanwhile, he said he is worried about the fragmentation of health care in the United States, and the likely cutback in federal and state spending on kids.
Even so, “I believe in the future. I see in the generation coming up much more awareness of the whole child. I think they are going to do a much better job than I did,” but they need to learn “how to work the system.” Young physicians also must be mentored to believe in themselves, and take up the torch, he said.
A few years before the launch of Pediatric News in 1967, Dr. Henry Kempe and his colleagues at the University of Colorado, Denver, published a groundbreaking report on child abuse, “The Battered Child Syndrome,” in JAMA.
It had a major impact on Calvin C.J. Sia, MD, a now-retired pediatrician in Hawaii, not only because it was pioneering work and one of the milestones of 20th century pediatrics, but also because Dr. Kempe was a close friend and mentor of Dr. Sia, going back to when Dr. Sia was a resident in the 1950s.
Dr. Kempe “alerted me to the battered child syndrome and taught me the importance of looking at the whole child within the context of the family, and he repeatedly reminded me of the importance of preventive care. He taught me how to make preventive change, with the emphasis on the first 3 years” of life, Dr. Sia said in an interview from his home in Honolulu.
To celebrate the 50th anniversary of Pediatric News, it seemed appropriate to turn to Dr. Sia. His practice, launched in 1958, not only spanned our 50 years, but also illustrated one of the major themes in pediatrics over the past half-century. With the old scourges of infectious disease, malnutrition, and infant mortality largely brought under control, pediatrics turned to the broader struggles of childhood, including learning, poverty, and abusive parenting.
Dr. Sia has been president of the Hawaii Medical Association; president of the Hawaii chapter of the American Academy of Pediatrics; chief of staff at the Kauikeolani Children’s Hospital, Honolulu (now the Kapi‘olani Medical Center for Women and Children); chair of the American Medical Association Pediatric Delegation; and founder of the AMA’s Section Council on Pediatrics.
Positions like these eventually led him to contacts with legislators willing to listen and fund child abuse prevention, immunization programs, and other initiatives to help children. It often meant lobbying politicians for money.
“It doesn’t occur overnight; it takes chance and dedication,” said Dr. Sia, whose name, as one of its earliest champions, is now nearly synonymous with the concept of the medical home – an ideal of cradling children in a physician, family, and social services safety net of coordinated care.
Dr. Sia’s career spanned all of the developments that Pediatric News has covered over its 50 years, including the increasing recognition of autism, the earlier survival of premature infants, and phenylketonuria and other screenings at birth. He remembers all of them, and took action on many at the state and federal level.
Taking a step took courage, even when he wasn’t quite sure what to do. “I recognized autism back in the 60s. Children looked normal, were born normal, but then had trouble. I didn’t know what to do with them. That’s how I got involved with learning disabilities. I didn’t know anything, but I got involved,” he said.
With the advice of Robert Cooke, MD, another mentor and an eventual founder of the Head Start program, Dr. Sia cofounded Honolulu’s Variety School for Learning Disabilities in 1967. It’s still in operation; Don Ho and other local “variety hour” entertainers helped with early fundraising.
Dr. Sia also helped launch Healthy Start, one of the nation’s first home visit programs for at-risk kids, and an amendment to the federal Education of the Handicapped Act to extend aid to children with family and developmental challenges.
He convinced Sen. Daniel Inouye, another personal friend, to introduce the Emergency Medical Services for Children Act in the 1980s, which funded states to develop emergency medical services for children. “It was really important because kids need special instruments, IVs, and medications. I had to lobby. It was under Reaganomics,” Dr. Sia said.
At 89 years of age and recovering from a recent heart attack and open heart bypass, he is still trying to help children. Dr. Sia, who was born in Beijing, has been promoting the medical home concept in Asia. Meanwhile, he said he is worried about the fragmentation of health care in the United States, and the likely cutback in federal and state spending on kids.
Even so, “I believe in the future. I see in the generation coming up much more awareness of the whole child. I think they are going to do a much better job than I did,” but they need to learn “how to work the system.” Young physicians also must be mentored to believe in themselves, and take up the torch, he said.
A few years before the launch of Pediatric News in 1967, Dr. Henry Kempe and his colleagues at the University of Colorado, Denver, published a groundbreaking report on child abuse, “The Battered Child Syndrome,” in JAMA.
It had a major impact on Calvin C.J. Sia, MD, a now-retired pediatrician in Hawaii, not only because it was pioneering work and one of the milestones of 20th century pediatrics, but also because Dr. Kempe was a close friend and mentor of Dr. Sia, going back to when Dr. Sia was a resident in the 1950s.
Dr. Kempe “alerted me to the battered child syndrome and taught me the importance of looking at the whole child within the context of the family, and he repeatedly reminded me of the importance of preventive care. He taught me how to make preventive change, with the emphasis on the first 3 years” of life, Dr. Sia said in an interview from his home in Honolulu.
To celebrate the 50th anniversary of Pediatric News, it seemed appropriate to turn to Dr. Sia. His practice, launched in 1958, not only spanned our 50 years, but also illustrated one of the major themes in pediatrics over the past half-century. With the old scourges of infectious disease, malnutrition, and infant mortality largely brought under control, pediatrics turned to the broader struggles of childhood, including learning, poverty, and abusive parenting.
Dr. Sia has been president of the Hawaii Medical Association; president of the Hawaii chapter of the American Academy of Pediatrics; chief of staff at the Kauikeolani Children’s Hospital, Honolulu (now the Kapi‘olani Medical Center for Women and Children); chair of the American Medical Association Pediatric Delegation; and founder of the AMA’s Section Council on Pediatrics.
Positions like these eventually led him to contacts with legislators willing to listen and fund child abuse prevention, immunization programs, and other initiatives to help children. It often meant lobbying politicians for money.
“It doesn’t occur overnight; it takes chance and dedication,” said Dr. Sia, whose name, as one of its earliest champions, is now nearly synonymous with the concept of the medical home – an ideal of cradling children in a physician, family, and social services safety net of coordinated care.
Dr. Sia’s career spanned all of the developments that Pediatric News has covered over its 50 years, including the increasing recognition of autism, the earlier survival of premature infants, and phenylketonuria and other screenings at birth. He remembers all of them, and took action on many at the state and federal level.
Taking a step took courage, even when he wasn’t quite sure what to do. “I recognized autism back in the 60s. Children looked normal, were born normal, but then had trouble. I didn’t know what to do with them. That’s how I got involved with learning disabilities. I didn’t know anything, but I got involved,” he said.
With the advice of Robert Cooke, MD, another mentor and an eventual founder of the Head Start program, Dr. Sia cofounded Honolulu’s Variety School for Learning Disabilities in 1967. It’s still in operation; Don Ho and other local “variety hour” entertainers helped with early fundraising.
Dr. Sia also helped launch Healthy Start, one of the nation’s first home visit programs for at-risk kids, and an amendment to the federal Education of the Handicapped Act to extend aid to children with family and developmental challenges.
He convinced Sen. Daniel Inouye, another personal friend, to introduce the Emergency Medical Services for Children Act in the 1980s, which funded states to develop emergency medical services for children. “It was really important because kids need special instruments, IVs, and medications. I had to lobby. It was under Reaganomics,” Dr. Sia said.
At 89 years of age and recovering from a recent heart attack and open heart bypass, he is still trying to help children. Dr. Sia, who was born in Beijing, has been promoting the medical home concept in Asia. Meanwhile, he said he is worried about the fragmentation of health care in the United States, and the likely cutback in federal and state spending on kids.
Even so, “I believe in the future. I see in the generation coming up much more awareness of the whole child. I think they are going to do a much better job than I did,” but they need to learn “how to work the system.” Young physicians also must be mentored to believe in themselves, and take up the torch, he said.
Intensifying vedolizumab could counter loss of response in IBD
ORLANDO – In a study of 644 people with moderate to severe inflammatory bowel disease treated with vedolizumab (Entyvio, Takeda Pharmaceuticals), 346 achieved remission or a significant response. This 54% response rate includes 192 people with Crohn’s disease and 154 others with ulcerative colitis.
However, after a median of 143 days, some of the initial responders experienced a loss of response to vedolizumab therapy.
“In our real-world study of vedolizumab use in patients with IBD predominantly refractory to anti-TNF [tumor necrosis factor] therapy, loss of response was observed in about 40% of patients at 12 months,” said Eugenia Shmidt, MD, a gastroenterology fellow at the Icahn School of Medicine at Mount Sinai hospital in New York City.
To counter the loss of response, Dr. Shmidt and her colleagues shortened the dosing interval of vedolizumab to either 4 or 6 weeks in a subgroup of 36 patients who lost response. They also shortened the dosing interval to try to attempt an initial response in a subgroup of 47 people who did not respond to initial induction therapy in the first place. “Dose intensification led to a successful recapture of response in 32% of patients who had initial response and in 19% of patients who did not have an initial response,” Dr. Shmidt said at the Advances in Inflammatory Bowel Diseases meeting.
“We find it interesting that there was greater success in recapturing significant response in patients who responded to vedolizumab initially compared to those who did not initially respond,” she said when asked if she found any of her findings surprising. “This emphasizes a likely need for different management strategies for initial vedolizumab responders and nonresponders, as well as early recognition of the need for dose optimization,” she added.
The study included adults with mild to moderate inflammatory bowel disease, based on clinical factors or confirmed through endoscopy. There were no significant differences in severity of disease between patients who were able to recapture response versus those were not. The mean age among the 374 participants with Crohn’s disease was 39 years; similarly, the mean age in the group of 270 with ulcerative colitis was 41 years. Mean duration of disease was 15 years and 9 years in the two groups, respectively.
To identify risk factors associated with loss of response to vedolizumab, Dr. Shmidt and her colleagues performed univariable and multivariable Cox proportional hazard analyses. They found concomitant use of an immunomodulator for Crohn’s disease was protective against loss of response (hazard ratio, 0.44). In the ulcerative colitis group, a baseline serum albumin level below a normal value achieved lower vedolizumab response rates; a value below 3.2 g/dL was an independent predictor of cumulative loss of response over time (HR, 2.39).
Inflammatory bowel disease patients treated at higher volume centers, which were defined as enrolling at least 100 patients in the study, had higher rates of loss of response to vedolizumab (HR, 1.92 on multivariable analysis).
The multicenter VICTORY consortium coinvestigators in this study are affiliated with Mayo Clinic in Rochester, Minn.; Cleveland Clinic Foundation in Ohio; University of California, San Diego; New York University in New York City; Montefiore Medical Center/Albert Einstein College of Medicine in the Bronx, N.Y.; and Dartmouth-Hitchcock Medical Center in Lebanon, N.H.
Future studies are warranted to evaluate the pharmacodynamics and pharmacokinetics of vedolizumab, the authors noted, as well as to determine an optimal dosing strategy among people with high drug clearance.
The study was funded in part by Takeda Pharmaceuticals. Dr. Eugenia Shmidt did not have any relevant disclosures. The meeting was sponsored by the Crohn’s & Colitis Foundation of America.
ORLANDO – In a study of 644 people with moderate to severe inflammatory bowel disease treated with vedolizumab (Entyvio, Takeda Pharmaceuticals), 346 achieved remission or a significant response. This 54% response rate includes 192 people with Crohn’s disease and 154 others with ulcerative colitis.
However, after a median of 143 days, some of the initial responders experienced a loss of response to vedolizumab therapy.
“In our real-world study of vedolizumab use in patients with IBD predominantly refractory to anti-TNF [tumor necrosis factor] therapy, loss of response was observed in about 40% of patients at 12 months,” said Eugenia Shmidt, MD, a gastroenterology fellow at the Icahn School of Medicine at Mount Sinai hospital in New York City.
To counter the loss of response, Dr. Shmidt and her colleagues shortened the dosing interval of vedolizumab to either 4 or 6 weeks in a subgroup of 36 patients who lost response. They also shortened the dosing interval to try to attempt an initial response in a subgroup of 47 people who did not respond to initial induction therapy in the first place. “Dose intensification led to a successful recapture of response in 32% of patients who had initial response and in 19% of patients who did not have an initial response,” Dr. Shmidt said at the Advances in Inflammatory Bowel Diseases meeting.
“We find it interesting that there was greater success in recapturing significant response in patients who responded to vedolizumab initially compared to those who did not initially respond,” she said when asked if she found any of her findings surprising. “This emphasizes a likely need for different management strategies for initial vedolizumab responders and nonresponders, as well as early recognition of the need for dose optimization,” she added.
The study included adults with mild to moderate inflammatory bowel disease, based on clinical factors or confirmed through endoscopy. There were no significant differences in severity of disease between patients who were able to recapture response versus those were not. The mean age among the 374 participants with Crohn’s disease was 39 years; similarly, the mean age in the group of 270 with ulcerative colitis was 41 years. Mean duration of disease was 15 years and 9 years in the two groups, respectively.
To identify risk factors associated with loss of response to vedolizumab, Dr. Shmidt and her colleagues performed univariable and multivariable Cox proportional hazard analyses. They found concomitant use of an immunomodulator for Crohn’s disease was protective against loss of response (hazard ratio, 0.44). In the ulcerative colitis group, a baseline serum albumin level below a normal value achieved lower vedolizumab response rates; a value below 3.2 g/dL was an independent predictor of cumulative loss of response over time (HR, 2.39).
Inflammatory bowel disease patients treated at higher volume centers, which were defined as enrolling at least 100 patients in the study, had higher rates of loss of response to vedolizumab (HR, 1.92 on multivariable analysis).
The multicenter VICTORY consortium coinvestigators in this study are affiliated with Mayo Clinic in Rochester, Minn.; Cleveland Clinic Foundation in Ohio; University of California, San Diego; New York University in New York City; Montefiore Medical Center/Albert Einstein College of Medicine in the Bronx, N.Y.; and Dartmouth-Hitchcock Medical Center in Lebanon, N.H.
Future studies are warranted to evaluate the pharmacodynamics and pharmacokinetics of vedolizumab, the authors noted, as well as to determine an optimal dosing strategy among people with high drug clearance.
The study was funded in part by Takeda Pharmaceuticals. Dr. Eugenia Shmidt did not have any relevant disclosures. The meeting was sponsored by the Crohn’s & Colitis Foundation of America.
ORLANDO – In a study of 644 people with moderate to severe inflammatory bowel disease treated with vedolizumab (Entyvio, Takeda Pharmaceuticals), 346 achieved remission or a significant response. This 54% response rate includes 192 people with Crohn’s disease and 154 others with ulcerative colitis.
However, after a median of 143 days, some of the initial responders experienced a loss of response to vedolizumab therapy.
“In our real-world study of vedolizumab use in patients with IBD predominantly refractory to anti-TNF [tumor necrosis factor] therapy, loss of response was observed in about 40% of patients at 12 months,” said Eugenia Shmidt, MD, a gastroenterology fellow at the Icahn School of Medicine at Mount Sinai hospital in New York City.
To counter the loss of response, Dr. Shmidt and her colleagues shortened the dosing interval of vedolizumab to either 4 or 6 weeks in a subgroup of 36 patients who lost response. They also shortened the dosing interval to try to attempt an initial response in a subgroup of 47 people who did not respond to initial induction therapy in the first place. “Dose intensification led to a successful recapture of response in 32% of patients who had initial response and in 19% of patients who did not have an initial response,” Dr. Shmidt said at the Advances in Inflammatory Bowel Diseases meeting.
“We find it interesting that there was greater success in recapturing significant response in patients who responded to vedolizumab initially compared to those who did not initially respond,” she said when asked if she found any of her findings surprising. “This emphasizes a likely need for different management strategies for initial vedolizumab responders and nonresponders, as well as early recognition of the need for dose optimization,” she added.
The study included adults with mild to moderate inflammatory bowel disease, based on clinical factors or confirmed through endoscopy. There were no significant differences in severity of disease between patients who were able to recapture response versus those were not. The mean age among the 374 participants with Crohn’s disease was 39 years; similarly, the mean age in the group of 270 with ulcerative colitis was 41 years. Mean duration of disease was 15 years and 9 years in the two groups, respectively.
To identify risk factors associated with loss of response to vedolizumab, Dr. Shmidt and her colleagues performed univariable and multivariable Cox proportional hazard analyses. They found concomitant use of an immunomodulator for Crohn’s disease was protective against loss of response (hazard ratio, 0.44). In the ulcerative colitis group, a baseline serum albumin level below a normal value achieved lower vedolizumab response rates; a value below 3.2 g/dL was an independent predictor of cumulative loss of response over time (HR, 2.39).
Inflammatory bowel disease patients treated at higher volume centers, which were defined as enrolling at least 100 patients in the study, had higher rates of loss of response to vedolizumab (HR, 1.92 on multivariable analysis).
The multicenter VICTORY consortium coinvestigators in this study are affiliated with Mayo Clinic in Rochester, Minn.; Cleveland Clinic Foundation in Ohio; University of California, San Diego; New York University in New York City; Montefiore Medical Center/Albert Einstein College of Medicine in the Bronx, N.Y.; and Dartmouth-Hitchcock Medical Center in Lebanon, N.H.
Future studies are warranted to evaluate the pharmacodynamics and pharmacokinetics of vedolizumab, the authors noted, as well as to determine an optimal dosing strategy among people with high drug clearance.
The study was funded in part by Takeda Pharmaceuticals. Dr. Eugenia Shmidt did not have any relevant disclosures. The meeting was sponsored by the Crohn’s & Colitis Foundation of America.
Key clinical point: Some patients with moderate to severe inflammatory bowel disease experience loss of response to vedolizumab over time.
Major finding: At a median 143 days, 41% of patients with Crohn’s disease and 42% of those with ulcerative colitis who initially had a strong response or experienced remission with vedolizumab experienced subsequent loss of response.
Data source: Study of 644 patients with moderate to severe Crohn’s disease or ulcerative colitis followed for 12 months.
Disclosures: The study was funded in part by Takeda Pharmaceuticals. Dr. Eugenia Shmidt did not have any relevant disclosures.
Immediate postpartum LARC requires cross-disciplinary cooperation in the hospital
Hospitals that aim to offer women long-acting reversible contraception immediately after giving birth require up-front coordination across departments, including early recruitment of nonclinical staff, researchers have found.
One of the advantages to offering LARC postpartum in the hospital, instead of an outpatient clinic, is that patients are not required to present for repeat visits. The American College of Obstetricians and Gynecologists has called the immediate postpartum period an optimal time for LARC placement.
In an effort to fill in this knowledge gap, a team of investigators led by Lisa G. Hofler, MD, MPH, of Emory University in Atlanta, sought to identify barriers to implementation and characteristics of successful efforts among hospitals developing postpartum LARC programs.
Dr. Hofler’s team interviewed clinicians and staff members, including pharmacists and billing employees, at 10 Georgia hospitals starting in March 2015, about a year after the state approved a separate Medicaid-reimbursement protocol for immediate postpartum LARC. Of the hospitals in the study, nine were attempting to launch programs during the study period, and four had active programs by the study endpoint (Obstet Gynecol 2017;129:3–9).
Dr. Hofler and her colleagues found – through interviews conducted separately with 32 employees in clinical or administrative roles – that the hospitals that had succeeded had engaged multidisciplinary teams early in the process.
“We found that implementing an immediate postpartum LARC program in the hospital is initially more complicated than people think, and involves the participation of departments that people might overlook,” Dr. Hofler said in an interview. “It’s about engaging a pharmacy person, a billing person, or a health records expert in addition to the usual nursing and physician staff that one engages when you have some sort of clinical practice change.”
Barriers to successful programs included staff lack of knowledge about LARC, financial concerns, and competing priorities within hospitals, the team found.
“Several participants had little previous exposure to LARC, and clinicians did not always easily appreciate the differences between providing LARC in the inpatient and outpatient settings,” Dr. Hofler and her colleagues reported in their study.
“Early involvement of the necessary members of the implementation team leads to better communication and understanding of the project,” the researchers concluded, noting that implementation cannot move forward without “financial reassurance early in the process.”
Teams should include representation from direct clinical care personnel, pharmacy, or finance and billing, they reported, though the specific team members may vary by hospital.
“Consistent communication and team planning with clear roles and responsibilities are key to navigating the complex and interconnected steps” in launching a program, they wrote.
Though Dr. Hofler stressed that the report was not meant to substitute for formal guidance, it maps the steps needed, and the departments involved, at each stage of the implementation process, from exploration of a program to its eventual launch and maintenance.
The study was supported by a grant from the Society of Family Planning Research Fund. Two of the coauthors disclosed research funding or other relationships with LARC manufacturers.
Hospitals that aim to offer women long-acting reversible contraception immediately after giving birth require up-front coordination across departments, including early recruitment of nonclinical staff, researchers have found.
One of the advantages to offering LARC postpartum in the hospital, instead of an outpatient clinic, is that patients are not required to present for repeat visits. The American College of Obstetricians and Gynecologists has called the immediate postpartum period an optimal time for LARC placement.
In an effort to fill in this knowledge gap, a team of investigators led by Lisa G. Hofler, MD, MPH, of Emory University in Atlanta, sought to identify barriers to implementation and characteristics of successful efforts among hospitals developing postpartum LARC programs.
Dr. Hofler’s team interviewed clinicians and staff members, including pharmacists and billing employees, at 10 Georgia hospitals starting in March 2015, about a year after the state approved a separate Medicaid-reimbursement protocol for immediate postpartum LARC. Of the hospitals in the study, nine were attempting to launch programs during the study period, and four had active programs by the study endpoint (Obstet Gynecol 2017;129:3–9).
Dr. Hofler and her colleagues found – through interviews conducted separately with 32 employees in clinical or administrative roles – that the hospitals that had succeeded had engaged multidisciplinary teams early in the process.
“We found that implementing an immediate postpartum LARC program in the hospital is initially more complicated than people think, and involves the participation of departments that people might overlook,” Dr. Hofler said in an interview. “It’s about engaging a pharmacy person, a billing person, or a health records expert in addition to the usual nursing and physician staff that one engages when you have some sort of clinical practice change.”
Barriers to successful programs included staff lack of knowledge about LARC, financial concerns, and competing priorities within hospitals, the team found.
“Several participants had little previous exposure to LARC, and clinicians did not always easily appreciate the differences between providing LARC in the inpatient and outpatient settings,” Dr. Hofler and her colleagues reported in their study.
“Early involvement of the necessary members of the implementation team leads to better communication and understanding of the project,” the researchers concluded, noting that implementation cannot move forward without “financial reassurance early in the process.”
Teams should include representation from direct clinical care personnel, pharmacy, or finance and billing, they reported, though the specific team members may vary by hospital.
“Consistent communication and team planning with clear roles and responsibilities are key to navigating the complex and interconnected steps” in launching a program, they wrote.
Though Dr. Hofler stressed that the report was not meant to substitute for formal guidance, it maps the steps needed, and the departments involved, at each stage of the implementation process, from exploration of a program to its eventual launch and maintenance.
The study was supported by a grant from the Society of Family Planning Research Fund. Two of the coauthors disclosed research funding or other relationships with LARC manufacturers.
Hospitals that aim to offer women long-acting reversible contraception immediately after giving birth require up-front coordination across departments, including early recruitment of nonclinical staff, researchers have found.
One of the advantages to offering LARC postpartum in the hospital, instead of an outpatient clinic, is that patients are not required to present for repeat visits. The American College of Obstetricians and Gynecologists has called the immediate postpartum period an optimal time for LARC placement.
In an effort to fill in this knowledge gap, a team of investigators led by Lisa G. Hofler, MD, MPH, of Emory University in Atlanta, sought to identify barriers to implementation and characteristics of successful efforts among hospitals developing postpartum LARC programs.
Dr. Hofler’s team interviewed clinicians and staff members, including pharmacists and billing employees, at 10 Georgia hospitals starting in March 2015, about a year after the state approved a separate Medicaid-reimbursement protocol for immediate postpartum LARC. Of the hospitals in the study, nine were attempting to launch programs during the study period, and four had active programs by the study endpoint (Obstet Gynecol 2017;129:3–9).
Dr. Hofler and her colleagues found – through interviews conducted separately with 32 employees in clinical or administrative roles – that the hospitals that had succeeded had engaged multidisciplinary teams early in the process.
“We found that implementing an immediate postpartum LARC program in the hospital is initially more complicated than people think, and involves the participation of departments that people might overlook,” Dr. Hofler said in an interview. “It’s about engaging a pharmacy person, a billing person, or a health records expert in addition to the usual nursing and physician staff that one engages when you have some sort of clinical practice change.”
Barriers to successful programs included staff lack of knowledge about LARC, financial concerns, and competing priorities within hospitals, the team found.
“Several participants had little previous exposure to LARC, and clinicians did not always easily appreciate the differences between providing LARC in the inpatient and outpatient settings,” Dr. Hofler and her colleagues reported in their study.
“Early involvement of the necessary members of the implementation team leads to better communication and understanding of the project,” the researchers concluded, noting that implementation cannot move forward without “financial reassurance early in the process.”
Teams should include representation from direct clinical care personnel, pharmacy, or finance and billing, they reported, though the specific team members may vary by hospital.
“Consistent communication and team planning with clear roles and responsibilities are key to navigating the complex and interconnected steps” in launching a program, they wrote.
Though Dr. Hofler stressed that the report was not meant to substitute for formal guidance, it maps the steps needed, and the departments involved, at each stage of the implementation process, from exploration of a program to its eventual launch and maintenance.
The study was supported by a grant from the Society of Family Planning Research Fund. Two of the coauthors disclosed research funding or other relationships with LARC manufacturers.
FROM OBSTETRICS & GYNECOLOGY
Key clinical point: Success in establishing an immediate postpartum LARC program involves team-building across hospital disciplines.
Major finding: Factors associated with success included early coordination among financial, administrative, pharmacy, and clinical personnel.
Data source: A qualitative analysis of interviews with 32 employees (clinical and nonclinical) from 10 hospitals in Georgia.
Disclosures: Two authors disclosed relationships with LARC manufacturers.
Ketamine emerging as top treatment for cocaine dependence
VIENNA – The prospect on the horizon of two new effective therapies for chronic cocaine dependence – sustained-release dextroamphetamine and subanesthetic ketamine infusions – was among the top developments of the year in addiction medicine, Wim van den Brink, MD, PhD, said at the annual congress of the European College of Neuropsychopharmacology.
Other highlights on his list included:
• Studies establishing that comorbid attention-deficit/hyperactivity disorder (ADHD) and substance use disorder now can be treated effectively with either extended-release mixed amphetamine salts or high-dose methylphenidate.
• Release of a puzzling array of conflicting studies on the use of high-dose baclofen for treatment of alcohol dependence. It’s tough to reconcile this mishmash of polar opposite results. And that dictates it’s time to declare a moratorium on the use of this therapy in clinical practice, which in many places is now widespread, said Dr. van den Brink, professor of psychiatry and addiction at the University of Amsterdam and director of the Amsterdam Institute for Addiction Research.
“It’s too strange that we have such conflicting evidence out there. Too many people are prescribing crazy-high doses of baclofen with no strong supporting evidence,” Dr. van den Brink said.
Cocaine dependence
Dr. van den Brink was a coinvestigator in a Dutch multicenter randomized, double-blind, placebo-controlled trial of multitreatment-refractory comorbid cocaine dependence in 73 heroin-dependent patients in heroin-assisted treatment. Patients assigned to 60 mg/day of sustained-release dextroamphetamine, in addition to the background methadone and diacetylmorphine all participants were on for their heroin dependence, had significantly fewer days of cocaine use in the 12-week study: a mean of 44.9 days, compared with 60.6 days in placebo-treated controls. Adverse events were transient and well tolerated (Lancet. 2016 May 28;387[10034]:2226-34).
“A lot of medications have been tried for treatment of cocaine dependence, but actually none of them has been shown to be effective with the exception of substitution treatment with stimulants. Ours is one of the most successful trials. These patients were using cocaine an average of 24 days per month along with a lot of other drugs, despite being in heroin treatment for 4 years,” Dr. van den Brink said. “Patients were very willing to take the sustained-release dextroamphetamine. In the last 4 weeks, 84% of them used at least 80% of their medication. And they were blinded to what they were using.
“We saw good effect sizes: 0.6-0.7 for self-report measures and 0.31 for negative urine samples. So this is a very promising approach. But it also means that, like with tobacco dependence or alcohol dependence, we have to start thinking about substitution therapy in stimulant-dependent patients,” he said.
Dr. van den Brink said subanesthetic ketamine as a novel treatment for cocaine dependence is not yet ready for prime time use in clinical practice, because it’s just not practical to bring patients in for a roughly hour-long intravenous infusion on a daily basis, as was done in a highly impressive proof-of-concept study. But new formulations of ketamine are under development that should better lend themselves to use in clinical practice.
In the proof-of-concept study, investigators at the New York State Psychiatric Institute brought into the laboratory cocaine-dependent volunteers not seeking treatment or abstinence and administered 52-minute infusions of ketamine at 0.41 or 71 mg/kg or lorazepam at 2 mg (Biol Psychiatry. 2014 Jul 1;76[1]:40-6). Lorazepam had absolutely no effect on motivation to change, but ketamine was a different story.
“As soon as you give a low dose of ketamine, you see a wonderful effect on motivation to change and on craving ratings in assessments at 24 hours post infusion. This looks like another promising way of treating cocaine dependence,” he said.
Doxazosin for alcoholism
Investigators at the National Institute on Alcohol Abuse and Alcoholism and several U.S. universities hypothesized that the norepinephrine system could be an important treatment target in alcohol dependence. They conducted a double-blind, placebo-controlled randomized trial in which alcohol-dependent patients seeking outpatient treatment were assigned to the alpha1-adrenergic blocker doxazosin (Cardura) titrated to a maximum of 16 mg/day or placebo. They found doxazosin significantly reduced drinks per week and the number of heavy drinking days per week, but only in the subgroup of patients with a strong family history of alcoholism. In patients without such a family history, doxazosin paradoxically increased drinking (Addict Biol. 2016 Jul;21[4]:904-14).
One of the reasons adult ADHD is greatly underrecognized is that it tends to occur in combination with flashier substance use disorders. “Addiction is very comorbid with all kinds of disorders, but especially with externalizing childhood disorders like conduct disorder and ADHD,” Dr. van den Brink said.
It was shown half-a-decade ago that normal doses of methylphenidate have no effect on ADHD symptoms or substance use in comorbid adults. Then Swedish investigators reported that treating criminal offenders with high-dose methylphenidate – roughly three times greater than standard dosing – was effective in reducing both ADHD symptoms and comorbid substance use in criminal offenders. Those findings prompted investigators at the New York State Psychiatric Institute and the University of Minnesota to examine whether prescribing extended-release mixed amphetamine salts in adults with comorbid cocaine use disorder and ADHD would achieve improvement in both conditions. Indeed, it did, Dr. van den Brink said.
One hundred twenty-six affected patients were randomized to 60 or 80 mg/day of extended-release mixed amphetamine salts or placebo for 13 weeks coupled with weekly individual cognitive-behavioral therapy for all in this double-blind, three-arm clinical trial.
“They showed a number-needed-to-treat of about 2.5 in order to achieve a significant reduction in cocaine use and a very nice reduction in ADHD symptoms with a number-needed-to-treat of 3,” Dr. van den Brink said.
The rate of continuous cocaine abstinence in the last 3 weeks of the trial was 30% in the 80-mg group and 17.5% with 60 mg of extended-release mixed amphetamine salts, compared with just 7% with placebo (JAMA Psychiatry. 2015 Jun;72[6]:593-602).
Interpreting baclofen studies
The first high-quality multicenter, randomized, placebo-controlled, double-blind clinical trial, conducted in Germany, showed baclofen (Lioresal) at a mean dose of 180 mg/day was effective in maintaining alcohol abstinence (Eur Neuropsychopharmacol. 2015 Aug;25[8]:1167-77).
“They got wonderful results, with a number-needed-to-treat of 2.3. That is something we’re not used to seeing in the treatment of alcoholism. But there was no dose-response effect, which is a little unusual,” the psychiatrist observed.
Then a multicenter group of Dutch investigators, including Dr. van den Brink, carried out what they believed would be a confirmatory randomized, double-blind, placebo-controlled trial. However, it showed no difference between high- or low-dose baclofen and placebo in time to relapse (Eur Neuropsychopharmacol. 2016 Dec;26[12]:1950-9).
Little further light was shed by the two large French randomized, placebo-controlled clinical trials presented at the 2016 World Congress for Alcohol and Alcoholism in Berlin. One, the BACLOVILLE trial, included 320 patients treated in 60 family practice clinics; it showed strongly positive results for high-dose baclofen. In contrast, the 316-patient ALPADIR study proved negative. These conflicting results were particularly disappointing because France has been at the forefront of using high-dose baclofen to treat alcoholism, Dr. van den Brink said.
“Maybe some 100,000 people have been treated with high-dose baclofen for alcoholism in France,” he said. “What is the conclusion from all these baclofen studies? You can interpret them in many ways. Maybe there are two positive trials and two negative trials, or maybe there are two positive trials and two failed trials. The debate is not closed, even after four randomized trials.”
Dr. van den Brink reported receiving research funding from and/or serving as a consultant to more than half a dozen pharmaceutical companies.
VIENNA – The prospect on the horizon of two new effective therapies for chronic cocaine dependence – sustained-release dextroamphetamine and subanesthetic ketamine infusions – was among the top developments of the year in addiction medicine, Wim van den Brink, MD, PhD, said at the annual congress of the European College of Neuropsychopharmacology.
Other highlights on his list included:
• Studies establishing that comorbid attention-deficit/hyperactivity disorder (ADHD) and substance use disorder now can be treated effectively with either extended-release mixed amphetamine salts or high-dose methylphenidate.
• Release of a puzzling array of conflicting studies on the use of high-dose baclofen for treatment of alcohol dependence. It’s tough to reconcile this mishmash of polar opposite results. And that dictates it’s time to declare a moratorium on the use of this therapy in clinical practice, which in many places is now widespread, said Dr. van den Brink, professor of psychiatry and addiction at the University of Amsterdam and director of the Amsterdam Institute for Addiction Research.
“It’s too strange that we have such conflicting evidence out there. Too many people are prescribing crazy-high doses of baclofen with no strong supporting evidence,” Dr. van den Brink said.
Cocaine dependence
Dr. van den Brink was a coinvestigator in a Dutch multicenter randomized, double-blind, placebo-controlled trial of multitreatment-refractory comorbid cocaine dependence in 73 heroin-dependent patients in heroin-assisted treatment. Patients assigned to 60 mg/day of sustained-release dextroamphetamine, in addition to the background methadone and diacetylmorphine all participants were on for their heroin dependence, had significantly fewer days of cocaine use in the 12-week study: a mean of 44.9 days, compared with 60.6 days in placebo-treated controls. Adverse events were transient and well tolerated (Lancet. 2016 May 28;387[10034]:2226-34).
“A lot of medications have been tried for treatment of cocaine dependence, but actually none of them has been shown to be effective with the exception of substitution treatment with stimulants. Ours is one of the most successful trials. These patients were using cocaine an average of 24 days per month along with a lot of other drugs, despite being in heroin treatment for 4 years,” Dr. van den Brink said. “Patients were very willing to take the sustained-release dextroamphetamine. In the last 4 weeks, 84% of them used at least 80% of their medication. And they were blinded to what they were using.
“We saw good effect sizes: 0.6-0.7 for self-report measures and 0.31 for negative urine samples. So this is a very promising approach. But it also means that, like with tobacco dependence or alcohol dependence, we have to start thinking about substitution therapy in stimulant-dependent patients,” he said.
Dr. van den Brink said subanesthetic ketamine as a novel treatment for cocaine dependence is not yet ready for prime time use in clinical practice, because it’s just not practical to bring patients in for a roughly hour-long intravenous infusion on a daily basis, as was done in a highly impressive proof-of-concept study. But new formulations of ketamine are under development that should better lend themselves to use in clinical practice.
In the proof-of-concept study, investigators at the New York State Psychiatric Institute brought into the laboratory cocaine-dependent volunteers not seeking treatment or abstinence and administered 52-minute infusions of ketamine at 0.41 or 71 mg/kg or lorazepam at 2 mg (Biol Psychiatry. 2014 Jul 1;76[1]:40-6). Lorazepam had absolutely no effect on motivation to change, but ketamine was a different story.
“As soon as you give a low dose of ketamine, you see a wonderful effect on motivation to change and on craving ratings in assessments at 24 hours post infusion. This looks like another promising way of treating cocaine dependence,” he said.
Doxazosin for alcoholism
Investigators at the National Institute on Alcohol Abuse and Alcoholism and several U.S. universities hypothesized that the norepinephrine system could be an important treatment target in alcohol dependence. They conducted a double-blind, placebo-controlled randomized trial in which alcohol-dependent patients seeking outpatient treatment were assigned to the alpha1-adrenergic blocker doxazosin (Cardura) titrated to a maximum of 16 mg/day or placebo. They found doxazosin significantly reduced drinks per week and the number of heavy drinking days per week, but only in the subgroup of patients with a strong family history of alcoholism. In patients without such a family history, doxazosin paradoxically increased drinking (Addict Biol. 2016 Jul;21[4]:904-14).
One of the reasons adult ADHD is greatly underrecognized is that it tends to occur in combination with flashier substance use disorders. “Addiction is very comorbid with all kinds of disorders, but especially with externalizing childhood disorders like conduct disorder and ADHD,” Dr. van den Brink said.
It was shown half-a-decade ago that normal doses of methylphenidate have no effect on ADHD symptoms or substance use in comorbid adults. Then Swedish investigators reported that treating criminal offenders with high-dose methylphenidate – roughly three times greater than standard dosing – was effective in reducing both ADHD symptoms and comorbid substance use in criminal offenders. Those findings prompted investigators at the New York State Psychiatric Institute and the University of Minnesota to examine whether prescribing extended-release mixed amphetamine salts in adults with comorbid cocaine use disorder and ADHD would achieve improvement in both conditions. Indeed, it did, Dr. van den Brink said.
One hundred twenty-six affected patients were randomized to 60 or 80 mg/day of extended-release mixed amphetamine salts or placebo for 13 weeks coupled with weekly individual cognitive-behavioral therapy for all in this double-blind, three-arm clinical trial.
“They showed a number-needed-to-treat of about 2.5 in order to achieve a significant reduction in cocaine use and a very nice reduction in ADHD symptoms with a number-needed-to-treat of 3,” Dr. van den Brink said.
The rate of continuous cocaine abstinence in the last 3 weeks of the trial was 30% in the 80-mg group and 17.5% with 60 mg of extended-release mixed amphetamine salts, compared with just 7% with placebo (JAMA Psychiatry. 2015 Jun;72[6]:593-602).
Interpreting baclofen studies
The first high-quality multicenter, randomized, placebo-controlled, double-blind clinical trial, conducted in Germany, showed baclofen (Lioresal) at a mean dose of 180 mg/day was effective in maintaining alcohol abstinence (Eur Neuropsychopharmacol. 2015 Aug;25[8]:1167-77).
“They got wonderful results, with a number-needed-to-treat of 2.3. That is something we’re not used to seeing in the treatment of alcoholism. But there was no dose-response effect, which is a little unusual,” the psychiatrist observed.
Then a multicenter group of Dutch investigators, including Dr. van den Brink, carried out what they believed would be a confirmatory randomized, double-blind, placebo-controlled trial. However, it showed no difference between high- or low-dose baclofen and placebo in time to relapse (Eur Neuropsychopharmacol. 2016 Dec;26[12]:1950-9).
Little further light was shed by the two large French randomized, placebo-controlled clinical trials presented at the 2016 World Congress for Alcohol and Alcoholism in Berlin. One, the BACLOVILLE trial, included 320 patients treated in 60 family practice clinics; it showed strongly positive results for high-dose baclofen. In contrast, the 316-patient ALPADIR study proved negative. These conflicting results were particularly disappointing because France has been at the forefront of using high-dose baclofen to treat alcoholism, Dr. van den Brink said.
“Maybe some 100,000 people have been treated with high-dose baclofen for alcoholism in France,” he said. “What is the conclusion from all these baclofen studies? You can interpret them in many ways. Maybe there are two positive trials and two negative trials, or maybe there are two positive trials and two failed trials. The debate is not closed, even after four randomized trials.”
Dr. van den Brink reported receiving research funding from and/or serving as a consultant to more than half a dozen pharmaceutical companies.
VIENNA – The prospect on the horizon of two new effective therapies for chronic cocaine dependence – sustained-release dextroamphetamine and subanesthetic ketamine infusions – was among the top developments of the year in addiction medicine, Wim van den Brink, MD, PhD, said at the annual congress of the European College of Neuropsychopharmacology.
Other highlights on his list included:
• Studies establishing that comorbid attention-deficit/hyperactivity disorder (ADHD) and substance use disorder now can be treated effectively with either extended-release mixed amphetamine salts or high-dose methylphenidate.
• Release of a puzzling array of conflicting studies on the use of high-dose baclofen for treatment of alcohol dependence. It’s tough to reconcile this mishmash of polar opposite results. And that dictates it’s time to declare a moratorium on the use of this therapy in clinical practice, which in many places is now widespread, said Dr. van den Brink, professor of psychiatry and addiction at the University of Amsterdam and director of the Amsterdam Institute for Addiction Research.
“It’s too strange that we have such conflicting evidence out there. Too many people are prescribing crazy-high doses of baclofen with no strong supporting evidence,” Dr. van den Brink said.
Cocaine dependence
Dr. van den Brink was a coinvestigator in a Dutch multicenter randomized, double-blind, placebo-controlled trial of multitreatment-refractory comorbid cocaine dependence in 73 heroin-dependent patients in heroin-assisted treatment. Patients assigned to 60 mg/day of sustained-release dextroamphetamine, in addition to the background methadone and diacetylmorphine all participants were on for their heroin dependence, had significantly fewer days of cocaine use in the 12-week study: a mean of 44.9 days, compared with 60.6 days in placebo-treated controls. Adverse events were transient and well tolerated (Lancet. 2016 May 28;387[10034]:2226-34).
“A lot of medications have been tried for treatment of cocaine dependence, but actually none of them has been shown to be effective with the exception of substitution treatment with stimulants. Ours is one of the most successful trials. These patients were using cocaine an average of 24 days per month along with a lot of other drugs, despite being in heroin treatment for 4 years,” Dr. van den Brink said. “Patients were very willing to take the sustained-release dextroamphetamine. In the last 4 weeks, 84% of them used at least 80% of their medication. And they were blinded to what they were using.
“We saw good effect sizes: 0.6-0.7 for self-report measures and 0.31 for negative urine samples. So this is a very promising approach. But it also means that, like with tobacco dependence or alcohol dependence, we have to start thinking about substitution therapy in stimulant-dependent patients,” he said.
Dr. van den Brink said subanesthetic ketamine as a novel treatment for cocaine dependence is not yet ready for prime time use in clinical practice, because it’s just not practical to bring patients in for a roughly hour-long intravenous infusion on a daily basis, as was done in a highly impressive proof-of-concept study. But new formulations of ketamine are under development that should better lend themselves to use in clinical practice.
In the proof-of-concept study, investigators at the New York State Psychiatric Institute brought into the laboratory cocaine-dependent volunteers not seeking treatment or abstinence and administered 52-minute infusions of ketamine at 0.41 or 71 mg/kg or lorazepam at 2 mg (Biol Psychiatry. 2014 Jul 1;76[1]:40-6). Lorazepam had absolutely no effect on motivation to change, but ketamine was a different story.
“As soon as you give a low dose of ketamine, you see a wonderful effect on motivation to change and on craving ratings in assessments at 24 hours post infusion. This looks like another promising way of treating cocaine dependence,” he said.
Doxazosin for alcoholism
Investigators at the National Institute on Alcohol Abuse and Alcoholism and several U.S. universities hypothesized that the norepinephrine system could be an important treatment target in alcohol dependence. They conducted a double-blind, placebo-controlled randomized trial in which alcohol-dependent patients seeking outpatient treatment were assigned to the alpha1-adrenergic blocker doxazosin (Cardura) titrated to a maximum of 16 mg/day or placebo. They found doxazosin significantly reduced drinks per week and the number of heavy drinking days per week, but only in the subgroup of patients with a strong family history of alcoholism. In patients without such a family history, doxazosin paradoxically increased drinking (Addict Biol. 2016 Jul;21[4]:904-14).
One of the reasons adult ADHD is greatly underrecognized is that it tends to occur in combination with flashier substance use disorders. “Addiction is very comorbid with all kinds of disorders, but especially with externalizing childhood disorders like conduct disorder and ADHD,” Dr. van den Brink said.
It was shown half-a-decade ago that normal doses of methylphenidate have no effect on ADHD symptoms or substance use in comorbid adults. Then Swedish investigators reported that treating criminal offenders with high-dose methylphenidate – roughly three times greater than standard dosing – was effective in reducing both ADHD symptoms and comorbid substance use in criminal offenders. Those findings prompted investigators at the New York State Psychiatric Institute and the University of Minnesota to examine whether prescribing extended-release mixed amphetamine salts in adults with comorbid cocaine use disorder and ADHD would achieve improvement in both conditions. Indeed, it did, Dr. van den Brink said.
One hundred twenty-six affected patients were randomized to 60 or 80 mg/day of extended-release mixed amphetamine salts or placebo for 13 weeks coupled with weekly individual cognitive-behavioral therapy for all in this double-blind, three-arm clinical trial.
“They showed a number-needed-to-treat of about 2.5 in order to achieve a significant reduction in cocaine use and a very nice reduction in ADHD symptoms with a number-needed-to-treat of 3,” Dr. van den Brink said.
The rate of continuous cocaine abstinence in the last 3 weeks of the trial was 30% in the 80-mg group and 17.5% with 60 mg of extended-release mixed amphetamine salts, compared with just 7% with placebo (JAMA Psychiatry. 2015 Jun;72[6]:593-602).
Interpreting baclofen studies
The first high-quality multicenter, randomized, placebo-controlled, double-blind clinical trial, conducted in Germany, showed baclofen (Lioresal) at a mean dose of 180 mg/day was effective in maintaining alcohol abstinence (Eur Neuropsychopharmacol. 2015 Aug;25[8]:1167-77).
“They got wonderful results, with a number-needed-to-treat of 2.3. That is something we’re not used to seeing in the treatment of alcoholism. But there was no dose-response effect, which is a little unusual,” the psychiatrist observed.
Then a multicenter group of Dutch investigators, including Dr. van den Brink, carried out what they believed would be a confirmatory randomized, double-blind, placebo-controlled trial. However, it showed no difference between high- or low-dose baclofen and placebo in time to relapse (Eur Neuropsychopharmacol. 2016 Dec;26[12]:1950-9).
Little further light was shed by the two large French randomized, placebo-controlled clinical trials presented at the 2016 World Congress for Alcohol and Alcoholism in Berlin. One, the BACLOVILLE trial, included 320 patients treated in 60 family practice clinics; it showed strongly positive results for high-dose baclofen. In contrast, the 316-patient ALPADIR study proved negative. These conflicting results were particularly disappointing because France has been at the forefront of using high-dose baclofen to treat alcoholism, Dr. van den Brink said.
“Maybe some 100,000 people have been treated with high-dose baclofen for alcoholism in France,” he said. “What is the conclusion from all these baclofen studies? You can interpret them in many ways. Maybe there are two positive trials and two negative trials, or maybe there are two positive trials and two failed trials. The debate is not closed, even after four randomized trials.”
Dr. van den Brink reported receiving research funding from and/or serving as a consultant to more than half a dozen pharmaceutical companies.
EXPERT ANALYSIS FROM THE ECNP CONGRESS