User login
Meningococcal conjugate vaccination may be associated with increased risk of Bell’s palsy
A postlicensure safety study of a meningococcal conjugate vaccine in Southern California has shown that the vaccine may be associated with an increase in the risk of Bell’s palsy, but only if the vaccine is taken concomitantly with another vaccine.
Researchers set out to evaluate the safety of one quadrivalent meningococcal conjugate vaccine, MenACWY-CRM. Two MenACWY vaccines are currently licensed in the United States; MenACWY-D is the other. The vaccines underwent studies on the road to approval, but researchers saw an absence of data about how the vaccine was faring in routine clinical use.
Researchers looked through the electronic health records of the study population for “26 prespecified events of interest (EOIs) under investigation, including neurologic, rheumatologic, hematologic, endocrine, renal, pediatric, and pediatric infectious disease EOIs. Occurrence of incident episodes of these EOIs was identified during a 1-year observation period after the index vaccination for each individual.”
They identified 4,240 EOIs, but dismissed 3,000 of them as probable preexisting conditions. With what was left, some of the EOIs did not occur at all (such as Guillain-Barré syndrome, myasthenia gravis, or systemic lupus erythematosus). Of transverse myelitis and autoimmune hemolytic anemia, among others, there was only 1 case.
Seizure, iridocyclitis, Hashimoto’s disease, and anaphylaxis initially showed statistically significant risk incidence, but were all ruled out (of the hypothesis of possible cause by vaccination) by further review from a physician investigator.
But in the case of Bell’s palsy, the independent case review committee did not rule out the possibility that the MenACWY-CRM vaccine increased the risk incidence of the condition.
However, the increased risk was present only for subjects who received a concomitant vaccine along with the MenACWY-CRM, such as Tdap, influenza, or human papillomavirus vaccine. “Stratified analyses demonstrated an increased risk for Bell’s palsy in subjects receiving concomitant vaccines (risk incidence, 5.0; 95% confidence interval, 1.4-17.8), and no increased risk for those without concomitant vaccine (RI, 1.1; 95% CI, 0.2-5.5),” Dr. Tseng and his coauthors wrote. All eight cases of Bell’s palsy resolved completely.
They concluded, “we observed a temporal association between occurrence of Bell’s palsy and receipt of MenACWY-CRM concomitantly with other vaccines. The association needs further investigation because it could be due to chance, concomitant vaccination, or underlying medical history predisposing to Bell’s palsy.”
Dr. Tseng and numerous coauthors reported receiving research support from Novartis Vaccines, the sponsor of the study. Three coauthors were employees of Novartis at the time of the study.
A postlicensure safety study of a meningococcal conjugate vaccine in Southern California has shown that the vaccine may be associated with an increase in the risk of Bell’s palsy, but only if the vaccine is taken concomitantly with another vaccine.
Researchers set out to evaluate the safety of one quadrivalent meningococcal conjugate vaccine, MenACWY-CRM. Two MenACWY vaccines are currently licensed in the United States; MenACWY-D is the other. The vaccines underwent studies on the road to approval, but researchers saw an absence of data about how the vaccine was faring in routine clinical use.
Researchers looked through the electronic health records of the study population for “26 prespecified events of interest (EOIs) under investigation, including neurologic, rheumatologic, hematologic, endocrine, renal, pediatric, and pediatric infectious disease EOIs. Occurrence of incident episodes of these EOIs was identified during a 1-year observation period after the index vaccination for each individual.”
They identified 4,240 EOIs, but dismissed 3,000 of them as probable preexisting conditions. With what was left, some of the EOIs did not occur at all (such as Guillain-Barré syndrome, myasthenia gravis, or systemic lupus erythematosus). Of transverse myelitis and autoimmune hemolytic anemia, among others, there was only 1 case.
Seizure, iridocyclitis, Hashimoto’s disease, and anaphylaxis initially showed statistically significant risk incidence, but were all ruled out (of the hypothesis of possible cause by vaccination) by further review from a physician investigator.
But in the case of Bell’s palsy, the independent case review committee did not rule out the possibility that the MenACWY-CRM vaccine increased the risk incidence of the condition.
However, the increased risk was present only for subjects who received a concomitant vaccine along with the MenACWY-CRM, such as Tdap, influenza, or human papillomavirus vaccine. “Stratified analyses demonstrated an increased risk for Bell’s palsy in subjects receiving concomitant vaccines (risk incidence, 5.0; 95% confidence interval, 1.4-17.8), and no increased risk for those without concomitant vaccine (RI, 1.1; 95% CI, 0.2-5.5),” Dr. Tseng and his coauthors wrote. All eight cases of Bell’s palsy resolved completely.
They concluded, “we observed a temporal association between occurrence of Bell’s palsy and receipt of MenACWY-CRM concomitantly with other vaccines. The association needs further investigation because it could be due to chance, concomitant vaccination, or underlying medical history predisposing to Bell’s palsy.”
Dr. Tseng and numerous coauthors reported receiving research support from Novartis Vaccines, the sponsor of the study. Three coauthors were employees of Novartis at the time of the study.
A postlicensure safety study of a meningococcal conjugate vaccine in Southern California has shown that the vaccine may be associated with an increase in the risk of Bell’s palsy, but only if the vaccine is taken concomitantly with another vaccine.
Researchers set out to evaluate the safety of one quadrivalent meningococcal conjugate vaccine, MenACWY-CRM. Two MenACWY vaccines are currently licensed in the United States; MenACWY-D is the other. The vaccines underwent studies on the road to approval, but researchers saw an absence of data about how the vaccine was faring in routine clinical use.
Researchers looked through the electronic health records of the study population for “26 prespecified events of interest (EOIs) under investigation, including neurologic, rheumatologic, hematologic, endocrine, renal, pediatric, and pediatric infectious disease EOIs. Occurrence of incident episodes of these EOIs was identified during a 1-year observation period after the index vaccination for each individual.”
They identified 4,240 EOIs, but dismissed 3,000 of them as probable preexisting conditions. With what was left, some of the EOIs did not occur at all (such as Guillain-Barré syndrome, myasthenia gravis, or systemic lupus erythematosus). Of transverse myelitis and autoimmune hemolytic anemia, among others, there was only 1 case.
Seizure, iridocyclitis, Hashimoto’s disease, and anaphylaxis initially showed statistically significant risk incidence, but were all ruled out (of the hypothesis of possible cause by vaccination) by further review from a physician investigator.
But in the case of Bell’s palsy, the independent case review committee did not rule out the possibility that the MenACWY-CRM vaccine increased the risk incidence of the condition.
However, the increased risk was present only for subjects who received a concomitant vaccine along with the MenACWY-CRM, such as Tdap, influenza, or human papillomavirus vaccine. “Stratified analyses demonstrated an increased risk for Bell’s palsy in subjects receiving concomitant vaccines (risk incidence, 5.0; 95% confidence interval, 1.4-17.8), and no increased risk for those without concomitant vaccine (RI, 1.1; 95% CI, 0.2-5.5),” Dr. Tseng and his coauthors wrote. All eight cases of Bell’s palsy resolved completely.
They concluded, “we observed a temporal association between occurrence of Bell’s palsy and receipt of MenACWY-CRM concomitantly with other vaccines. The association needs further investigation because it could be due to chance, concomitant vaccination, or underlying medical history predisposing to Bell’s palsy.”
Dr. Tseng and numerous coauthors reported receiving research support from Novartis Vaccines, the sponsor of the study. Three coauthors were employees of Novartis at the time of the study.
Chronic Cough
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Combo produces high response rate in CLL trial

Results of a phase 2 trial suggest a 2-drug combination may be effective in patients with chronic lymphocytic leukemia (CLL), particularly those with high-risk disease.
The combination consists of ublituximab (TG-1101), a glycoengineered anti-CD20 monoclonal antibody, and the oral BTK inhibitor ibrutinib.
Six months after starting treatment, the overall response rate was 88% among all evaluable patients and 95% among those with high-risk CLL.
Researchers said the long-term clinical benefit of the combination will be defined by an ongoing phase 3 trial.
The team reported results from the phase 2 trial in the British Journal of Haematology. The study was sponsored by TG Therapeutics, Inc., the company developing ublituximab.
The trial included 45 patients. Their median age was 71 (range, 39-86), about half were female, and the median ECOG performance score was 1.
Nearly half of patients (47%, n=21) had high-risk CLL. Twelve patients had del 17p, 12 had del 11q, 5 patients had both, and 2 had a TP53 mutation.
The patients had a median of 2 (range, 1-7) prior treatments, including purine analogues (n=22), bendamustine (n=21), idelalisib (n=2), a spleen-tyrosine kinase inhibitor (n=2), and the BTK inhibitor CC-292 (n=1).
Treatment
For this study, patients received ibrutinib at 420 mg once daily and 2 different doses of ublituximab. The study had a dose-confirmation safety run-in period that was followed by an open enrollment into phase 2.
The dose-confirmation safety assessment enrolled 6 patients in each of 2 cohorts. Patients in cohort 1 received ublituximab at 600 mg on days 1, 8, and 15 of cycle 1. If there was ≤1 dose-limiting toxicity (DLT) in this cohort, the dose escalation would proceed to cohort 2.
In cohort 2, patients’ ublituximab dose increased to 900 mg on days 1, 8, and 15 of cycle 1. If ≤ 1 DLT was reported in this cohort, the dose was considered safe for phase 2.
There were no DLTs observed in either cohort. So subsequent patients were enrolled into the open phase 2 part of the study, in which they received ublituximab at 900 mg on days 1, 8, and 15 of cycle 1, as well as on day 1 of cycles 2 to 6.
Patients had response assessments at cycles 3 and 6. After that, they continued on ibrutinib monotherapy off study.
Safety
All 45 patients were evaluable for safety. The most common adverse events (AEs) were infusion-related reactions (IRRs, 53%), diarrhea (40%), fatigue (33%), cough (27%), rash (27%), and nausea (24%).
Grade 3/4 AEs included anemia (11%), neutropenia (11%), IRRs (7%), thrombocytopenia (7%), diarrhea (4%), and arthralgia (2%).
All rash and grade 3/4 diarrhea events were attributed to ibrutinib, and all IRRs were related to ublituximab. Twenty-one patients (47%) had dose interruptions due to IRRs, and 1 patient had a dose reduction to 600 mg.
Four patients had ublituximab-related dose interruptions—2 due to neutropenia and 2 because of elevated aspartate aminotransferase.
Two patients had ibrutinib-related dose reductions (for diarrhea and dizziness). Ten patients had ibrutinib-related dose interruptions—3 due to rash, 2 due to neutropenia, and 1 each because of anemia, thrombocytopenia, nausea, hypercalcemia, and dehydration.
Efficacy
Forty-one patients were evaluable for efficacy. Two patients were lost to follow-up, and 2 discontinued due to AEs. One of the AEs, diarrhea, was considered related to ibrutinib. The other patient discontinued due to pneumonia and pleural effusion, which were not attributed to study treatment.
At 6 months, the overall response rate was 88% among evaluable patients and 95% among high-risk patients. The median time to response was 8 weeks.
Two patients had a complete response, 34 had a partial response, and 3 had stable disease.
Both complete responders and 1 of the partial responders achieved minimal residual disease negativity. All 3 of these patients had high-risk disease.
“[T]he addition of ublituximab to ibrutinib not only produced high response rates but also allowed patients to achieve deeper responses, with complete responses and minimal residual disease negativity seen, which is rare with ibrutinib alone,” said study author Jeff Sharman, MD, of Willamette Valley Cancer Institute in Eugene, Oregon.
“We look forward to exploring how the increased depth of response may affect the sequence of treatments given to patients.” ![]()

Results of a phase 2 trial suggest a 2-drug combination may be effective in patients with chronic lymphocytic leukemia (CLL), particularly those with high-risk disease.
The combination consists of ublituximab (TG-1101), a glycoengineered anti-CD20 monoclonal antibody, and the oral BTK inhibitor ibrutinib.
Six months after starting treatment, the overall response rate was 88% among all evaluable patients and 95% among those with high-risk CLL.
Researchers said the long-term clinical benefit of the combination will be defined by an ongoing phase 3 trial.
The team reported results from the phase 2 trial in the British Journal of Haematology. The study was sponsored by TG Therapeutics, Inc., the company developing ublituximab.
The trial included 45 patients. Their median age was 71 (range, 39-86), about half were female, and the median ECOG performance score was 1.
Nearly half of patients (47%, n=21) had high-risk CLL. Twelve patients had del 17p, 12 had del 11q, 5 patients had both, and 2 had a TP53 mutation.
The patients had a median of 2 (range, 1-7) prior treatments, including purine analogues (n=22), bendamustine (n=21), idelalisib (n=2), a spleen-tyrosine kinase inhibitor (n=2), and the BTK inhibitor CC-292 (n=1).
Treatment
For this study, patients received ibrutinib at 420 mg once daily and 2 different doses of ublituximab. The study had a dose-confirmation safety run-in period that was followed by an open enrollment into phase 2.
The dose-confirmation safety assessment enrolled 6 patients in each of 2 cohorts. Patients in cohort 1 received ublituximab at 600 mg on days 1, 8, and 15 of cycle 1. If there was ≤1 dose-limiting toxicity (DLT) in this cohort, the dose escalation would proceed to cohort 2.
In cohort 2, patients’ ublituximab dose increased to 900 mg on days 1, 8, and 15 of cycle 1. If ≤ 1 DLT was reported in this cohort, the dose was considered safe for phase 2.
There were no DLTs observed in either cohort. So subsequent patients were enrolled into the open phase 2 part of the study, in which they received ublituximab at 900 mg on days 1, 8, and 15 of cycle 1, as well as on day 1 of cycles 2 to 6.
Patients had response assessments at cycles 3 and 6. After that, they continued on ibrutinib monotherapy off study.
Safety
All 45 patients were evaluable for safety. The most common adverse events (AEs) were infusion-related reactions (IRRs, 53%), diarrhea (40%), fatigue (33%), cough (27%), rash (27%), and nausea (24%).
Grade 3/4 AEs included anemia (11%), neutropenia (11%), IRRs (7%), thrombocytopenia (7%), diarrhea (4%), and arthralgia (2%).
All rash and grade 3/4 diarrhea events were attributed to ibrutinib, and all IRRs were related to ublituximab. Twenty-one patients (47%) had dose interruptions due to IRRs, and 1 patient had a dose reduction to 600 mg.
Four patients had ublituximab-related dose interruptions—2 due to neutropenia and 2 because of elevated aspartate aminotransferase.
Two patients had ibrutinib-related dose reductions (for diarrhea and dizziness). Ten patients had ibrutinib-related dose interruptions—3 due to rash, 2 due to neutropenia, and 1 each because of anemia, thrombocytopenia, nausea, hypercalcemia, and dehydration.
Efficacy
Forty-one patients were evaluable for efficacy. Two patients were lost to follow-up, and 2 discontinued due to AEs. One of the AEs, diarrhea, was considered related to ibrutinib. The other patient discontinued due to pneumonia and pleural effusion, which were not attributed to study treatment.
At 6 months, the overall response rate was 88% among evaluable patients and 95% among high-risk patients. The median time to response was 8 weeks.
Two patients had a complete response, 34 had a partial response, and 3 had stable disease.
Both complete responders and 1 of the partial responders achieved minimal residual disease negativity. All 3 of these patients had high-risk disease.
“[T]he addition of ublituximab to ibrutinib not only produced high response rates but also allowed patients to achieve deeper responses, with complete responses and minimal residual disease negativity seen, which is rare with ibrutinib alone,” said study author Jeff Sharman, MD, of Willamette Valley Cancer Institute in Eugene, Oregon.
“We look forward to exploring how the increased depth of response may affect the sequence of treatments given to patients.” ![]()

Results of a phase 2 trial suggest a 2-drug combination may be effective in patients with chronic lymphocytic leukemia (CLL), particularly those with high-risk disease.
The combination consists of ublituximab (TG-1101), a glycoengineered anti-CD20 monoclonal antibody, and the oral BTK inhibitor ibrutinib.
Six months after starting treatment, the overall response rate was 88% among all evaluable patients and 95% among those with high-risk CLL.
Researchers said the long-term clinical benefit of the combination will be defined by an ongoing phase 3 trial.
The team reported results from the phase 2 trial in the British Journal of Haematology. The study was sponsored by TG Therapeutics, Inc., the company developing ublituximab.
The trial included 45 patients. Their median age was 71 (range, 39-86), about half were female, and the median ECOG performance score was 1.
Nearly half of patients (47%, n=21) had high-risk CLL. Twelve patients had del 17p, 12 had del 11q, 5 patients had both, and 2 had a TP53 mutation.
The patients had a median of 2 (range, 1-7) prior treatments, including purine analogues (n=22), bendamustine (n=21), idelalisib (n=2), a spleen-tyrosine kinase inhibitor (n=2), and the BTK inhibitor CC-292 (n=1).
Treatment
For this study, patients received ibrutinib at 420 mg once daily and 2 different doses of ublituximab. The study had a dose-confirmation safety run-in period that was followed by an open enrollment into phase 2.
The dose-confirmation safety assessment enrolled 6 patients in each of 2 cohorts. Patients in cohort 1 received ublituximab at 600 mg on days 1, 8, and 15 of cycle 1. If there was ≤1 dose-limiting toxicity (DLT) in this cohort, the dose escalation would proceed to cohort 2.
In cohort 2, patients’ ublituximab dose increased to 900 mg on days 1, 8, and 15 of cycle 1. If ≤ 1 DLT was reported in this cohort, the dose was considered safe for phase 2.
There were no DLTs observed in either cohort. So subsequent patients were enrolled into the open phase 2 part of the study, in which they received ublituximab at 900 mg on days 1, 8, and 15 of cycle 1, as well as on day 1 of cycles 2 to 6.
Patients had response assessments at cycles 3 and 6. After that, they continued on ibrutinib monotherapy off study.
Safety
All 45 patients were evaluable for safety. The most common adverse events (AEs) were infusion-related reactions (IRRs, 53%), diarrhea (40%), fatigue (33%), cough (27%), rash (27%), and nausea (24%).
Grade 3/4 AEs included anemia (11%), neutropenia (11%), IRRs (7%), thrombocytopenia (7%), diarrhea (4%), and arthralgia (2%).
All rash and grade 3/4 diarrhea events were attributed to ibrutinib, and all IRRs were related to ublituximab. Twenty-one patients (47%) had dose interruptions due to IRRs, and 1 patient had a dose reduction to 600 mg.
Four patients had ublituximab-related dose interruptions—2 due to neutropenia and 2 because of elevated aspartate aminotransferase.
Two patients had ibrutinib-related dose reductions (for diarrhea and dizziness). Ten patients had ibrutinib-related dose interruptions—3 due to rash, 2 due to neutropenia, and 1 each because of anemia, thrombocytopenia, nausea, hypercalcemia, and dehydration.
Efficacy
Forty-one patients were evaluable for efficacy. Two patients were lost to follow-up, and 2 discontinued due to AEs. One of the AEs, diarrhea, was considered related to ibrutinib. The other patient discontinued due to pneumonia and pleural effusion, which were not attributed to study treatment.
At 6 months, the overall response rate was 88% among evaluable patients and 95% among high-risk patients. The median time to response was 8 weeks.
Two patients had a complete response, 34 had a partial response, and 3 had stable disease.
Both complete responders and 1 of the partial responders achieved minimal residual disease negativity. All 3 of these patients had high-risk disease.
“[T]he addition of ublituximab to ibrutinib not only produced high response rates but also allowed patients to achieve deeper responses, with complete responses and minimal residual disease negativity seen, which is rare with ibrutinib alone,” said study author Jeff Sharman, MD, of Willamette Valley Cancer Institute in Eugene, Oregon.
“We look forward to exploring how the increased depth of response may affect the sequence of treatments given to patients.” ![]()
Memory Skills Classes to Address Cognitive Concerns in Older Veterans With a History of Posttraumatic Stress Disorder
The Geriatric Research Education and Clinical Center (GRECC) Memory Disorders Clinic at the VA Puget Sound Health Care System (VAPSHCS) in Seattle, Washington, receives referrals from primary and specialty care. About a decade ago, this clinic began to see an influx of Vietnam-era veterans who presented with a variety of symptoms: not remembering where they were going when driving, forgetting why they went into another room, not remembering what their spouse told them, and feeling “out of it.” These symptoms were not associated with the loss of independence, but they were cause for concern. Family members and care providers typically corroborated the symptom description and perception of decline. Yet during workups, these veterans showed no primary medical causes for cognitive impairments and on neuropsychological evaluation demonstrated essentially normal cognition.
Memory Disorders Clinic staff largely were at a loss to know how to care for these patients. The simple reassurance, “You do not have dementia now,” seemed unsatisfactory given the patients’ ongoing concerns and the established risk factors for neurodegenerative disease.1,2 One theme emerged when talking with these veterans and their families: They all had a diagnosis of or history of treatment for posttraumatic stress disorder (PTSD).
To help these veterans, the VAPSHCS GRECC sought to address their key areas of concern related to memory. With input from veterans and their families, a quality improvement project was developed with the following goals: (1) to educate veterans and their families about PTSD and cognitive changes; (2) to build and field test a psychoeducational class to teach memory skills in this population; and (3) to inform VA staff about PTSD and cognitive change. In this article, the authors focus on how the first 2 goals were addressed and present preliminary results related to quality improvement.
Memory Skills Classes
Several strategies might promote memory skills, including printed materials for self-directed learning, individual sessions, interactive technologies, or groups. Given the patients’ reports about concentration problems, asking them to work through structured materials independently seemed unproductive. Individual clinical evaluations and cognitive interventions likely would not meet the demand or be cost-effective. Groups have long been used to treat PTSD, and Norrie and colleagues reported that at-risk adults benefited from a group psychoeducation program targeting healthy brain aging.3 At the same time, the Memory Disorders Clinic sought to distinguish itself from PTSD groups, because these groups tend to focus on treating active PTSD.
A better fit for this offering was the description of the sessions as classes. Although the focus was on promoting memory skills among those capable of learning them, the authors were mindful that some veterans might truly have prodromal dementia or acute PTSD symptoms that would require clinical management. The classes were not intended to address all these issues, and there was a plan to refer participants either before or during the class if warranted.
There was no formal evaluation of memory prior to starting the class. These classes were not developed as a research intervention and were exempt from institutional review board (IRB) approval requirements, according to prescreening by the VAPSHCS IRB and a memo from the GRECC director.
Core Components of Memory Skills
It may not be evident at first glance that PTSD or a history of PTSD influences memory. The symptom criteria for PTSD (involving reexperiencing, hyperarousal, and avoidance) might be described as “too much remembering” rather than forgetting. Yet problems with attention and concentration often occur in the setting of intrusive memories and alterations in reactivity. Research has found that older adults with PTSD have deficits of memory, especially new learning.
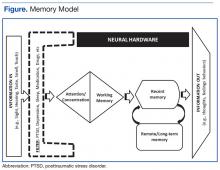
To appreciate these effects, it was important for participants in the memory skills classes to have some understanding of how memory works. The authors developed the Memory Model (Figure) as a visual aid and reference point to discuss the stages of new learning and how different aspects of brain activity are required for new learning and for memory to occur. This straightforward model is based on cognitive science and presented in layman’s terms. An important part of this model is the “filter” stage, which controls the information and stimuli that are available to the brain. Posttraumatic stress disorder involves involuntary emotional responses and efforts to avoid them and selects and colors the information that is processed in some situations (eg, avoidance of situations associated with trauma or dissociation of extreme memories). At other times, such as when a powerful stimulus is presented (eg, a helicopter flying close overhead), the filter may try to block out all inputs in order to preserve safety. The Memory Model also served as a visual aid during class discussions of normal cognitive aging.
Class sessions incorporated specific, measurable, attainable, realistic, and timely (SMART) goals, regular exercises based on mindfulness-based stress reduction approaches, and principles of behavioral activation.5 The SMART goals structure the sessions and permit customization of learning for participants. Class leaders record a goal for each participant and use these throughout the sessions to build rapport, develop communication, and teach memory skills.
Mindfulness-based stress reduction is an evidence-based treatment used in PTSD.6 It provides a counterpoint to the more didactic memory skills and is a method that even those with objective memory impairments can practice and apply successfully. Being in the current moment and emotional regulation are important skills to teach veterans as they learn to exert
Organization
Class sessions occurred weekly for 1 hour for a total of 8 sessions. The weekly class topics included introduction to memory; mood disorders, cognition, and cognitive disorders; barriers to effective memory: assessing readiness for change; developing a routine and becoming organized; attention and concentration; memory improvement (strategies internal and external aids); and reassessing goals.
Over the 3 years of classes reported in this article, the class sizes varied from 4 to 12 participants based on veteran interest, retention, and room size. The classes were structured so that important content areas were covered but with enough elasticity so leaders and veterans would develop a rapport and explore in greater depth the topics that resonated most for the attendees. Group participation was strongly encouraged. Veterans were expressly informed that the class was not for treatment of PTSD and that evidence-based therapies were encouraged to address PTSD especially if their symptoms flared up when compared with previous levels. The attendees also understood that they did not receive formal cognitive or memory testing but were encouraged to pursue testing if they showed significant deficits.
Preliminary Findings
From spring 2012 until spring 2015, 69 veterans agreed to participate and attended at least 1 memory skills class. Eighty-seven percent of participants (n = 60) attended 4 or more classes. The mean age (SD) was 67.3 years (4.2). All the participants were men, and the race/ethnic distribution was similar to that of the aging veteran population and very close to racial demographics for Washington state: 80% white, 14% African American, 2% Asian/Pacific Islander, 2% Native American, and 2% unknown.
Attendees were asked, but not required, to complete questionnaires before the classes began and again at completion. These questionnaires included self-assessments of cognitive strategies and compensatory methods used; an assessment of concern regarding cognition, life satisfaction, and community integration; the PTSD CheckList-Civilian Version (PCL-C); and the Geriatric Depression Scale (GDS).7,8 The questionnaire also included open response questions to providefeedback on what attendees liked about the classes and recommendations for improvements. The majority of comments for improvement focused on attendees’ desire for longer sessions and repeat offerings. Five veterans did not complete the full set of questionnaires at the beginning of the classes, and 7 did not complete the questionnaires at completion (the 2 subsets did not perfectly overlap).
At the start of the class, on average, veteran participants were experiencing mild depression and moderate symptoms of PTSD as measured by the GDS (n = 54) and the PCL-C (n = 56), respectively. Preliminary comparisons of ratings pre- and post-classes, using simple paired t tests, indicated a reduction in symptoms of depression on the GDS, improved sense of mastery over their memory symptoms, as well as improved quality of life ratings (all P < .01, no corrections). There was no evidence for a significant reduction in PTSD symptoms or report of elimination of cognitive difficulties. With the small sample and modest effects, the clinical significance of these scores cannot be determined. The authors are planning more detailed analyses on a larger set of participants, including measures of health care utilization before and after the class.
Future Directions
1. Chopra MP, Zhang H, Pless Kaiser A, et al. PTSD is a chronic, fluctuating disorder affecting the mental quality of life in older adults. Am J Geriatr Psychiatry. 2014;22(1):86-97.
2. Donovan NJ, Amariglio RE, Zoller AS, et al. Subjective cognitive concerns and neuropsychiatric predictors of progression to the early clinical stages of Alzheimer disease. Am J Geriatr Psychiatry. 2014;22(12):1642-1651.
3. Norrie LM, Diamond K, Hickie IB, Rogers NL, Fearns S, Naismith SL. Can older “at risk” adults benefit from psychoeducation targeting healthy brain aging? Int Psychogeriatr. 2011;23(3):413-424.
4. Hopko DR, Robertson SMC, Lejuez CW. Behavioral activation for anxiety disorders. Behav Anal Today. 2006;7(2):212-232.
5. Schuitevoerder S, Rosen JW, Twamley EW, et al. A meta-analysis of cognitive functioning in older adults with PTSD. J Anxiety Disord. 2013;27(6):550-558.
6. Polusny MA, Erbes CR, Thuras P, et al. Mindfulness-based stress reduction for posttraumatic stress disorder among veterans: a randomized clinical trial. JAMA. 2015;314(5):456-465.
7. Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982-1983;17(1):37-49.
8. Mental Illness Research, Education and Clinical Center. PTSD CheckList-Civilian Version (PCL-C). http://www.mirecc.va.gov/docs/visn6/3_PTSD _CheckList_and_Scoring.pdf Published December 2013. Accessed November 3, 2016.
9. Scott JC, Matt GE, Wrocklage KM, et al. A quantitative meta-analysis of neurocognitive functioning in posttraumatic stress disorder. Psychol Bull. 2015;141(1):105-140.
10. Wrocklage KM, Schweinsburg BC, Krystal JH, et al. Neuropsychological functioning in veterans with posttraumatic stress disorder: associations with performance validity, comorbidities, and functional outcomes. J Int Neuropsychol Soc. 2016;22(4):399-411.
11. Cook JM, O’Donnell C. Assessment and psychological treatment of posttraumatic stress disorder in older adults. J Geriatr Psychiatry Neurol. 2005;18(2):61-71.
12. Mota N, Tsai J, Kirwin PD, et al. Late-life exacerbation of PTSD symptoms in US veterans: results from the National Health and Resilience in Veterans Study. J Clin Psychiatry. 2016;77(3):348-354.
13. Yaffe K, Vittinghoff E, Lindquist K, et al. Posttraumatic stress disorder and risk of dementia among US veterans. Arch Gen Psychiatry. 2010;67(6):608-613.
The Geriatric Research Education and Clinical Center (GRECC) Memory Disorders Clinic at the VA Puget Sound Health Care System (VAPSHCS) in Seattle, Washington, receives referrals from primary and specialty care. About a decade ago, this clinic began to see an influx of Vietnam-era veterans who presented with a variety of symptoms: not remembering where they were going when driving, forgetting why they went into another room, not remembering what their spouse told them, and feeling “out of it.” These symptoms were not associated with the loss of independence, but they were cause for concern. Family members and care providers typically corroborated the symptom description and perception of decline. Yet during workups, these veterans showed no primary medical causes for cognitive impairments and on neuropsychological evaluation demonstrated essentially normal cognition.
Memory Disorders Clinic staff largely were at a loss to know how to care for these patients. The simple reassurance, “You do not have dementia now,” seemed unsatisfactory given the patients’ ongoing concerns and the established risk factors for neurodegenerative disease.1,2 One theme emerged when talking with these veterans and their families: They all had a diagnosis of or history of treatment for posttraumatic stress disorder (PTSD).
To help these veterans, the VAPSHCS GRECC sought to address their key areas of concern related to memory. With input from veterans and their families, a quality improvement project was developed with the following goals: (1) to educate veterans and their families about PTSD and cognitive changes; (2) to build and field test a psychoeducational class to teach memory skills in this population; and (3) to inform VA staff about PTSD and cognitive change. In this article, the authors focus on how the first 2 goals were addressed and present preliminary results related to quality improvement.
Memory Skills Classes
Several strategies might promote memory skills, including printed materials for self-directed learning, individual sessions, interactive technologies, or groups. Given the patients’ reports about concentration problems, asking them to work through structured materials independently seemed unproductive. Individual clinical evaluations and cognitive interventions likely would not meet the demand or be cost-effective. Groups have long been used to treat PTSD, and Norrie and colleagues reported that at-risk adults benefited from a group psychoeducation program targeting healthy brain aging.3 At the same time, the Memory Disorders Clinic sought to distinguish itself from PTSD groups, because these groups tend to focus on treating active PTSD.
A better fit for this offering was the description of the sessions as classes. Although the focus was on promoting memory skills among those capable of learning them, the authors were mindful that some veterans might truly have prodromal dementia or acute PTSD symptoms that would require clinical management. The classes were not intended to address all these issues, and there was a plan to refer participants either before or during the class if warranted.
There was no formal evaluation of memory prior to starting the class. These classes were not developed as a research intervention and were exempt from institutional review board (IRB) approval requirements, according to prescreening by the VAPSHCS IRB and a memo from the GRECC director.
Core Components of Memory Skills
It may not be evident at first glance that PTSD or a history of PTSD influences memory. The symptom criteria for PTSD (involving reexperiencing, hyperarousal, and avoidance) might be described as “too much remembering” rather than forgetting. Yet problems with attention and concentration often occur in the setting of intrusive memories and alterations in reactivity. Research has found that older adults with PTSD have deficits of memory, especially new learning.
To appreciate these effects, it was important for participants in the memory skills classes to have some understanding of how memory works. The authors developed the Memory Model (Figure) as a visual aid and reference point to discuss the stages of new learning and how different aspects of brain activity are required for new learning and for memory to occur. This straightforward model is based on cognitive science and presented in layman’s terms. An important part of this model is the “filter” stage, which controls the information and stimuli that are available to the brain. Posttraumatic stress disorder involves involuntary emotional responses and efforts to avoid them and selects and colors the information that is processed in some situations (eg, avoidance of situations associated with trauma or dissociation of extreme memories). At other times, such as when a powerful stimulus is presented (eg, a helicopter flying close overhead), the filter may try to block out all inputs in order to preserve safety. The Memory Model also served as a visual aid during class discussions of normal cognitive aging.
Class sessions incorporated specific, measurable, attainable, realistic, and timely (SMART) goals, regular exercises based on mindfulness-based stress reduction approaches, and principles of behavioral activation.5 The SMART goals structure the sessions and permit customization of learning for participants. Class leaders record a goal for each participant and use these throughout the sessions to build rapport, develop communication, and teach memory skills.
Mindfulness-based stress reduction is an evidence-based treatment used in PTSD.6 It provides a counterpoint to the more didactic memory skills and is a method that even those with objective memory impairments can practice and apply successfully. Being in the current moment and emotional regulation are important skills to teach veterans as they learn to exert
Organization
Class sessions occurred weekly for 1 hour for a total of 8 sessions. The weekly class topics included introduction to memory; mood disorders, cognition, and cognitive disorders; barriers to effective memory: assessing readiness for change; developing a routine and becoming organized; attention and concentration; memory improvement (strategies internal and external aids); and reassessing goals.
Over the 3 years of classes reported in this article, the class sizes varied from 4 to 12 participants based on veteran interest, retention, and room size. The classes were structured so that important content areas were covered but with enough elasticity so leaders and veterans would develop a rapport and explore in greater depth the topics that resonated most for the attendees. Group participation was strongly encouraged. Veterans were expressly informed that the class was not for treatment of PTSD and that evidence-based therapies were encouraged to address PTSD especially if their symptoms flared up when compared with previous levels. The attendees also understood that they did not receive formal cognitive or memory testing but were encouraged to pursue testing if they showed significant deficits.
Preliminary Findings
From spring 2012 until spring 2015, 69 veterans agreed to participate and attended at least 1 memory skills class. Eighty-seven percent of participants (n = 60) attended 4 or more classes. The mean age (SD) was 67.3 years (4.2). All the participants were men, and the race/ethnic distribution was similar to that of the aging veteran population and very close to racial demographics for Washington state: 80% white, 14% African American, 2% Asian/Pacific Islander, 2% Native American, and 2% unknown.
Attendees were asked, but not required, to complete questionnaires before the classes began and again at completion. These questionnaires included self-assessments of cognitive strategies and compensatory methods used; an assessment of concern regarding cognition, life satisfaction, and community integration; the PTSD CheckList-Civilian Version (PCL-C); and the Geriatric Depression Scale (GDS).7,8 The questionnaire also included open response questions to providefeedback on what attendees liked about the classes and recommendations for improvements. The majority of comments for improvement focused on attendees’ desire for longer sessions and repeat offerings. Five veterans did not complete the full set of questionnaires at the beginning of the classes, and 7 did not complete the questionnaires at completion (the 2 subsets did not perfectly overlap).
At the start of the class, on average, veteran participants were experiencing mild depression and moderate symptoms of PTSD as measured by the GDS (n = 54) and the PCL-C (n = 56), respectively. Preliminary comparisons of ratings pre- and post-classes, using simple paired t tests, indicated a reduction in symptoms of depression on the GDS, improved sense of mastery over their memory symptoms, as well as improved quality of life ratings (all P < .01, no corrections). There was no evidence for a significant reduction in PTSD symptoms or report of elimination of cognitive difficulties. With the small sample and modest effects, the clinical significance of these scores cannot be determined. The authors are planning more detailed analyses on a larger set of participants, including measures of health care utilization before and after the class.
Future Directions
The Geriatric Research Education and Clinical Center (GRECC) Memory Disorders Clinic at the VA Puget Sound Health Care System (VAPSHCS) in Seattle, Washington, receives referrals from primary and specialty care. About a decade ago, this clinic began to see an influx of Vietnam-era veterans who presented with a variety of symptoms: not remembering where they were going when driving, forgetting why they went into another room, not remembering what their spouse told them, and feeling “out of it.” These symptoms were not associated with the loss of independence, but they were cause for concern. Family members and care providers typically corroborated the symptom description and perception of decline. Yet during workups, these veterans showed no primary medical causes for cognitive impairments and on neuropsychological evaluation demonstrated essentially normal cognition.
Memory Disorders Clinic staff largely were at a loss to know how to care for these patients. The simple reassurance, “You do not have dementia now,” seemed unsatisfactory given the patients’ ongoing concerns and the established risk factors for neurodegenerative disease.1,2 One theme emerged when talking with these veterans and their families: They all had a diagnosis of or history of treatment for posttraumatic stress disorder (PTSD).
To help these veterans, the VAPSHCS GRECC sought to address their key areas of concern related to memory. With input from veterans and their families, a quality improvement project was developed with the following goals: (1) to educate veterans and their families about PTSD and cognitive changes; (2) to build and field test a psychoeducational class to teach memory skills in this population; and (3) to inform VA staff about PTSD and cognitive change. In this article, the authors focus on how the first 2 goals were addressed and present preliminary results related to quality improvement.
Memory Skills Classes
Several strategies might promote memory skills, including printed materials for self-directed learning, individual sessions, interactive technologies, or groups. Given the patients’ reports about concentration problems, asking them to work through structured materials independently seemed unproductive. Individual clinical evaluations and cognitive interventions likely would not meet the demand or be cost-effective. Groups have long been used to treat PTSD, and Norrie and colleagues reported that at-risk adults benefited from a group psychoeducation program targeting healthy brain aging.3 At the same time, the Memory Disorders Clinic sought to distinguish itself from PTSD groups, because these groups tend to focus on treating active PTSD.
A better fit for this offering was the description of the sessions as classes. Although the focus was on promoting memory skills among those capable of learning them, the authors were mindful that some veterans might truly have prodromal dementia or acute PTSD symptoms that would require clinical management. The classes were not intended to address all these issues, and there was a plan to refer participants either before or during the class if warranted.
There was no formal evaluation of memory prior to starting the class. These classes were not developed as a research intervention and were exempt from institutional review board (IRB) approval requirements, according to prescreening by the VAPSHCS IRB and a memo from the GRECC director.
Core Components of Memory Skills
It may not be evident at first glance that PTSD or a history of PTSD influences memory. The symptom criteria for PTSD (involving reexperiencing, hyperarousal, and avoidance) might be described as “too much remembering” rather than forgetting. Yet problems with attention and concentration often occur in the setting of intrusive memories and alterations in reactivity. Research has found that older adults with PTSD have deficits of memory, especially new learning.
To appreciate these effects, it was important for participants in the memory skills classes to have some understanding of how memory works. The authors developed the Memory Model (Figure) as a visual aid and reference point to discuss the stages of new learning and how different aspects of brain activity are required for new learning and for memory to occur. This straightforward model is based on cognitive science and presented in layman’s terms. An important part of this model is the “filter” stage, which controls the information and stimuli that are available to the brain. Posttraumatic stress disorder involves involuntary emotional responses and efforts to avoid them and selects and colors the information that is processed in some situations (eg, avoidance of situations associated with trauma or dissociation of extreme memories). At other times, such as when a powerful stimulus is presented (eg, a helicopter flying close overhead), the filter may try to block out all inputs in order to preserve safety. The Memory Model also served as a visual aid during class discussions of normal cognitive aging.
Class sessions incorporated specific, measurable, attainable, realistic, and timely (SMART) goals, regular exercises based on mindfulness-based stress reduction approaches, and principles of behavioral activation.5 The SMART goals structure the sessions and permit customization of learning for participants. Class leaders record a goal for each participant and use these throughout the sessions to build rapport, develop communication, and teach memory skills.
Mindfulness-based stress reduction is an evidence-based treatment used in PTSD.6 It provides a counterpoint to the more didactic memory skills and is a method that even those with objective memory impairments can practice and apply successfully. Being in the current moment and emotional regulation are important skills to teach veterans as they learn to exert
Organization
Class sessions occurred weekly for 1 hour for a total of 8 sessions. The weekly class topics included introduction to memory; mood disorders, cognition, and cognitive disorders; barriers to effective memory: assessing readiness for change; developing a routine and becoming organized; attention and concentration; memory improvement (strategies internal and external aids); and reassessing goals.
Over the 3 years of classes reported in this article, the class sizes varied from 4 to 12 participants based on veteran interest, retention, and room size. The classes were structured so that important content areas were covered but with enough elasticity so leaders and veterans would develop a rapport and explore in greater depth the topics that resonated most for the attendees. Group participation was strongly encouraged. Veterans were expressly informed that the class was not for treatment of PTSD and that evidence-based therapies were encouraged to address PTSD especially if their symptoms flared up when compared with previous levels. The attendees also understood that they did not receive formal cognitive or memory testing but were encouraged to pursue testing if they showed significant deficits.
Preliminary Findings
From spring 2012 until spring 2015, 69 veterans agreed to participate and attended at least 1 memory skills class. Eighty-seven percent of participants (n = 60) attended 4 or more classes. The mean age (SD) was 67.3 years (4.2). All the participants were men, and the race/ethnic distribution was similar to that of the aging veteran population and very close to racial demographics for Washington state: 80% white, 14% African American, 2% Asian/Pacific Islander, 2% Native American, and 2% unknown.
Attendees were asked, but not required, to complete questionnaires before the classes began and again at completion. These questionnaires included self-assessments of cognitive strategies and compensatory methods used; an assessment of concern regarding cognition, life satisfaction, and community integration; the PTSD CheckList-Civilian Version (PCL-C); and the Geriatric Depression Scale (GDS).7,8 The questionnaire also included open response questions to providefeedback on what attendees liked about the classes and recommendations for improvements. The majority of comments for improvement focused on attendees’ desire for longer sessions and repeat offerings. Five veterans did not complete the full set of questionnaires at the beginning of the classes, and 7 did not complete the questionnaires at completion (the 2 subsets did not perfectly overlap).
At the start of the class, on average, veteran participants were experiencing mild depression and moderate symptoms of PTSD as measured by the GDS (n = 54) and the PCL-C (n = 56), respectively. Preliminary comparisons of ratings pre- and post-classes, using simple paired t tests, indicated a reduction in symptoms of depression on the GDS, improved sense of mastery over their memory symptoms, as well as improved quality of life ratings (all P < .01, no corrections). There was no evidence for a significant reduction in PTSD symptoms or report of elimination of cognitive difficulties. With the small sample and modest effects, the clinical significance of these scores cannot be determined. The authors are planning more detailed analyses on a larger set of participants, including measures of health care utilization before and after the class.
Future Directions
1. Chopra MP, Zhang H, Pless Kaiser A, et al. PTSD is a chronic, fluctuating disorder affecting the mental quality of life in older adults. Am J Geriatr Psychiatry. 2014;22(1):86-97.
2. Donovan NJ, Amariglio RE, Zoller AS, et al. Subjective cognitive concerns and neuropsychiatric predictors of progression to the early clinical stages of Alzheimer disease. Am J Geriatr Psychiatry. 2014;22(12):1642-1651.
3. Norrie LM, Diamond K, Hickie IB, Rogers NL, Fearns S, Naismith SL. Can older “at risk” adults benefit from psychoeducation targeting healthy brain aging? Int Psychogeriatr. 2011;23(3):413-424.
4. Hopko DR, Robertson SMC, Lejuez CW. Behavioral activation for anxiety disorders. Behav Anal Today. 2006;7(2):212-232.
5. Schuitevoerder S, Rosen JW, Twamley EW, et al. A meta-analysis of cognitive functioning in older adults with PTSD. J Anxiety Disord. 2013;27(6):550-558.
6. Polusny MA, Erbes CR, Thuras P, et al. Mindfulness-based stress reduction for posttraumatic stress disorder among veterans: a randomized clinical trial. JAMA. 2015;314(5):456-465.
7. Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982-1983;17(1):37-49.
8. Mental Illness Research, Education and Clinical Center. PTSD CheckList-Civilian Version (PCL-C). http://www.mirecc.va.gov/docs/visn6/3_PTSD _CheckList_and_Scoring.pdf Published December 2013. Accessed November 3, 2016.
9. Scott JC, Matt GE, Wrocklage KM, et al. A quantitative meta-analysis of neurocognitive functioning in posttraumatic stress disorder. Psychol Bull. 2015;141(1):105-140.
10. Wrocklage KM, Schweinsburg BC, Krystal JH, et al. Neuropsychological functioning in veterans with posttraumatic stress disorder: associations with performance validity, comorbidities, and functional outcomes. J Int Neuropsychol Soc. 2016;22(4):399-411.
11. Cook JM, O’Donnell C. Assessment and psychological treatment of posttraumatic stress disorder in older adults. J Geriatr Psychiatry Neurol. 2005;18(2):61-71.
12. Mota N, Tsai J, Kirwin PD, et al. Late-life exacerbation of PTSD symptoms in US veterans: results from the National Health and Resilience in Veterans Study. J Clin Psychiatry. 2016;77(3):348-354.
13. Yaffe K, Vittinghoff E, Lindquist K, et al. Posttraumatic stress disorder and risk of dementia among US veterans. Arch Gen Psychiatry. 2010;67(6):608-613.
1. Chopra MP, Zhang H, Pless Kaiser A, et al. PTSD is a chronic, fluctuating disorder affecting the mental quality of life in older adults. Am J Geriatr Psychiatry. 2014;22(1):86-97.
2. Donovan NJ, Amariglio RE, Zoller AS, et al. Subjective cognitive concerns and neuropsychiatric predictors of progression to the early clinical stages of Alzheimer disease. Am J Geriatr Psychiatry. 2014;22(12):1642-1651.
3. Norrie LM, Diamond K, Hickie IB, Rogers NL, Fearns S, Naismith SL. Can older “at risk” adults benefit from psychoeducation targeting healthy brain aging? Int Psychogeriatr. 2011;23(3):413-424.
4. Hopko DR, Robertson SMC, Lejuez CW. Behavioral activation for anxiety disorders. Behav Anal Today. 2006;7(2):212-232.
5. Schuitevoerder S, Rosen JW, Twamley EW, et al. A meta-analysis of cognitive functioning in older adults with PTSD. J Anxiety Disord. 2013;27(6):550-558.
6. Polusny MA, Erbes CR, Thuras P, et al. Mindfulness-based stress reduction for posttraumatic stress disorder among veterans: a randomized clinical trial. JAMA. 2015;314(5):456-465.
7. Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982-1983;17(1):37-49.
8. Mental Illness Research, Education and Clinical Center. PTSD CheckList-Civilian Version (PCL-C). http://www.mirecc.va.gov/docs/visn6/3_PTSD _CheckList_and_Scoring.pdf Published December 2013. Accessed November 3, 2016.
9. Scott JC, Matt GE, Wrocklage KM, et al. A quantitative meta-analysis of neurocognitive functioning in posttraumatic stress disorder. Psychol Bull. 2015;141(1):105-140.
10. Wrocklage KM, Schweinsburg BC, Krystal JH, et al. Neuropsychological functioning in veterans with posttraumatic stress disorder: associations with performance validity, comorbidities, and functional outcomes. J Int Neuropsychol Soc. 2016;22(4):399-411.
11. Cook JM, O’Donnell C. Assessment and psychological treatment of posttraumatic stress disorder in older adults. J Geriatr Psychiatry Neurol. 2005;18(2):61-71.
12. Mota N, Tsai J, Kirwin PD, et al. Late-life exacerbation of PTSD symptoms in US veterans: results from the National Health and Resilience in Veterans Study. J Clin Psychiatry. 2016;77(3):348-354.
13. Yaffe K, Vittinghoff E, Lindquist K, et al. Posttraumatic stress disorder and risk of dementia among US veterans. Arch Gen Psychiatry. 2010;67(6):608-613.
MM patients with t(11;14) benefit from venetoclax

SAN DIEGO—Venetoclax, the oral BCL-2 inhibitor approved by the US Food and Drug Administration to treat chronic lymphocytic leukemia (CLL) patients with 17p deletion, is also showing activity in multiple myeloma (MM) patients, particularly those with t(11;14).
Final results of a phase 1 study showed venetoclax to be safe as monotherapy in relapsed or refractory MM, producing a response rate of 40% in patients with the translocation and 21% overall.
Preliminary results of the study were presented at the 2015 ASH Annual Meeting, and final results were presented at the 2016 ASH Annual Meeting.
“So I think we have a drug that potentially can change the outcome of a lot of patients with myeloma,” Shaji Kumar, MD, of the Mayo Clinic in Rochester, Minnesota, said during the presentation of the findings at ASH (abstract 488*).
“[It] also opens the possibility of being combined with a variety of other therapeutics that we have in this disease today.”
Venetoclax induces cell death in MM cell lines, particularly those positive for t(11;14). The translocation correlates with higher ratios of BCL-2 to MCL-1 and BCL-2 to MCL-2L1 (BCL-XL) mRNA. BCL-2 and MCL-1 promote survival of MM cells.
Study design and enrollment
The phase 1, open-label, multicenter study was designed to determine the best tolerated dose of venetoclax.
Secondary and exploratory objectives included overall response rate (ORR), time to progression, duration of response, and predictive biomarkers.
Patients had to have previously treated MM with measurable disease, ECOG status of 0 or 1, and adequate organ function.
They were excluded if they had an active infection, a history of significant renal, neurologic, psychiatric, endocrine, immunologic, cardiovascular, or hepatic disease within 6 months of study entry, or a history of other active malignancies within 3 years of study entry.
The study called for a 2-week lead-in period of venetoclax with weekly dose escalation. Four different dose cohorts were evaluated—300 mg, 600 mg, 900 mg, and 1200 mg.
Thirty patients were enrolled during the lead-in period, and 36 additional patients enrolled at the maximum evaluated dose of 1200 mg in the safety expansion cohort, for a total of 66 patients.
Patients were treated on a 21-day cycle with daily venetoclax. They could also receive dexamethasone to continue on the study if they progressed while receiving the monotherapy.
Patient characteristics
Patient characteristics were “similar to what you would see in relapsed/refractory multiple myloma,” Dr Kumar said.
Median age was 63 (range, 31–79), and most (62%) were ISS stage II/III.
“I want to draw your attention to two features here,” Dr Kumar said.
“Thirty patients, or 46% of the patients, had 11;14 translocation, and that reflects the interest in this drug for this particular class of patients.”
Twelve patients (18%) had 17p deletion, 32 (48%) had 13q deletion, and 27 (41%) were hyperdiploid.
“What is most striking in this cohort of patients,” Dr Kumar added, “is the fact that the median number of prior lines of therapy was 5, with some as high as 15 prior lines of therapy.”
Seventy percent were refractory to bortezomib, 77% refractory to lenalidomide, and 61% refractory to both. Fifty-two patients (79%) were refractory to their last prior therapy.
Patient disposition
At the time of data cutoff on August 19, 2016, 11 patients (17%) were still active on the study.
The median time on study was 3.3 months (range, 0.2–27), median time on venetoclax monotherapy was 2.5 months (range, 0.2–25), and median time on venetoclax plus dexamethasone was 1.4 months (range, 1–13). Seventeen patients received the combination after disease progression.
Fifty-five patients (83%) discontinued treatment, 41 (62%) because of disease progression, 5 (8%) because of adverse events, 2 (3%) withdrew consent, 1 (2%) was lost to follow-up, and 6 (9%) for unspecified reasons.
The 5 adverse events leading to withdrawal included renal failure (n=2), worsening pulmonary disorder (n=1), paralyzing sciatica (n=1), and shortness of breath and pain (n=1).
“Eight patients died on study,” Dr Kumar said, “none thought to be related to the drug.”
Adverse events
The toxicity profile was primarily hematologic and gastrointestinal.
All patients experienced an adverse event of any grade, and 45 (68%) had a grade 3 or 4 event.
“I wanted to highlight that the majority of the gastrointestinal and non-hematologic toxicity we saw were grades 1 and 2,” Dr Kumar pointed out, “and could be managed symptomatically or with dose modifications.”
Grade 3-4 hematologic adverse events included thrombocytopenia (26%), neutropenia (21%), anemia (14%), leukopenia (14%), and lymphopenia (15%).
Grade 3-4 non-hematologic adverse events included nausea (3%), diarrhea (3%), fatigue (5%), back pain (8%), and vomiting (3%).
Serious adverse events occurring in 2% or more of patients included pneumonia (8%), sepsis (5%), pain, pyrexia, cough, and hypotension (3% each).
Two patients had dose-limiting toxicities of abdominal pain and nausea at the 600 mg dose.
No events of tumor lysis syndrome (TLS) were reported. Dr Kumar explained that this may have been the case because patients thought to be at high risk for TLS were mandated to be in the hospital and observed for early tumor lysis in the initial part of the study.
Response
The ORR was 21% in all patients, including a stringent complete response (sCR) of 3% and a CR of 4%.
“But what was really striking was the response rate that we observed in the 30 patients with translocation 11;14,” Dr Kumar said. “The overall response rate was 40%, with 14% of the patients having complete response or better [stringent CR] and 13% of the patients with very good partial response.”
The 36 patients without t(11;14) had a 6% ORR, 3% sCR, and 3% very good partial response.
“If you look at the response rates based on the type of therapy they were coming off or the drugs they were refractory to, the response rate is very similar across all these patient subgroups, irrespective of what groups of drugs they were refractory to,” he added.
Time to progression for all patients was about 2.5 months. For patients with the translocation, it was about 6.6 months.
“Responses were fairly durable among those who had a response,” Dr Kumar said, “considering these are patients with a median of 5 prior lines of therapy.”
Duration of response for patients with t(11;14) was close to 10 months.
Biomarker analysis
The underlying biology for the response was the BCL-2 to BCL-2L1 ratio, as the investigators had observed in the cell lines.
So they analyzed the BCL-2 gene expression ratio in 24 of the 30 patients with t(11;14).
The investigators used droplet digital PCR performed on CD138-selected bone marrow mononuclear cells collected at baseline.
Nine patients had a high ratio, and their ORR was 88%. Fifteen patients had a low ratio, and their ORR was 20%.
Median time to progression for patients with a high ratio was about 12 months. For those with a low ratio, it was about 9 months.
Median change in M protein for patients with t(11;14) was –53%, compared to +11% in the patients without the translocation.
The investigators recommend additional studies with venetoclax in MM, including those with alternative combination therapies.
Venetoclax is being developed by AbbVie, in partnership with Genentech and Roche. This study was sponsored by AbbVie. ![]()
*Data in the abstract differ from the presentation.

SAN DIEGO—Venetoclax, the oral BCL-2 inhibitor approved by the US Food and Drug Administration to treat chronic lymphocytic leukemia (CLL) patients with 17p deletion, is also showing activity in multiple myeloma (MM) patients, particularly those with t(11;14).
Final results of a phase 1 study showed venetoclax to be safe as monotherapy in relapsed or refractory MM, producing a response rate of 40% in patients with the translocation and 21% overall.
Preliminary results of the study were presented at the 2015 ASH Annual Meeting, and final results were presented at the 2016 ASH Annual Meeting.
“So I think we have a drug that potentially can change the outcome of a lot of patients with myeloma,” Shaji Kumar, MD, of the Mayo Clinic in Rochester, Minnesota, said during the presentation of the findings at ASH (abstract 488*).
“[It] also opens the possibility of being combined with a variety of other therapeutics that we have in this disease today.”
Venetoclax induces cell death in MM cell lines, particularly those positive for t(11;14). The translocation correlates with higher ratios of BCL-2 to MCL-1 and BCL-2 to MCL-2L1 (BCL-XL) mRNA. BCL-2 and MCL-1 promote survival of MM cells.
Study design and enrollment
The phase 1, open-label, multicenter study was designed to determine the best tolerated dose of venetoclax.
Secondary and exploratory objectives included overall response rate (ORR), time to progression, duration of response, and predictive biomarkers.
Patients had to have previously treated MM with measurable disease, ECOG status of 0 or 1, and adequate organ function.
They were excluded if they had an active infection, a history of significant renal, neurologic, psychiatric, endocrine, immunologic, cardiovascular, or hepatic disease within 6 months of study entry, or a history of other active malignancies within 3 years of study entry.
The study called for a 2-week lead-in period of venetoclax with weekly dose escalation. Four different dose cohorts were evaluated—300 mg, 600 mg, 900 mg, and 1200 mg.
Thirty patients were enrolled during the lead-in period, and 36 additional patients enrolled at the maximum evaluated dose of 1200 mg in the safety expansion cohort, for a total of 66 patients.
Patients were treated on a 21-day cycle with daily venetoclax. They could also receive dexamethasone to continue on the study if they progressed while receiving the monotherapy.
Patient characteristics
Patient characteristics were “similar to what you would see in relapsed/refractory multiple myloma,” Dr Kumar said.
Median age was 63 (range, 31–79), and most (62%) were ISS stage II/III.
“I want to draw your attention to two features here,” Dr Kumar said.
“Thirty patients, or 46% of the patients, had 11;14 translocation, and that reflects the interest in this drug for this particular class of patients.”
Twelve patients (18%) had 17p deletion, 32 (48%) had 13q deletion, and 27 (41%) were hyperdiploid.
“What is most striking in this cohort of patients,” Dr Kumar added, “is the fact that the median number of prior lines of therapy was 5, with some as high as 15 prior lines of therapy.”
Seventy percent were refractory to bortezomib, 77% refractory to lenalidomide, and 61% refractory to both. Fifty-two patients (79%) were refractory to their last prior therapy.
Patient disposition
At the time of data cutoff on August 19, 2016, 11 patients (17%) were still active on the study.
The median time on study was 3.3 months (range, 0.2–27), median time on venetoclax monotherapy was 2.5 months (range, 0.2–25), and median time on venetoclax plus dexamethasone was 1.4 months (range, 1–13). Seventeen patients received the combination after disease progression.
Fifty-five patients (83%) discontinued treatment, 41 (62%) because of disease progression, 5 (8%) because of adverse events, 2 (3%) withdrew consent, 1 (2%) was lost to follow-up, and 6 (9%) for unspecified reasons.
The 5 adverse events leading to withdrawal included renal failure (n=2), worsening pulmonary disorder (n=1), paralyzing sciatica (n=1), and shortness of breath and pain (n=1).
“Eight patients died on study,” Dr Kumar said, “none thought to be related to the drug.”
Adverse events
The toxicity profile was primarily hematologic and gastrointestinal.
All patients experienced an adverse event of any grade, and 45 (68%) had a grade 3 or 4 event.
“I wanted to highlight that the majority of the gastrointestinal and non-hematologic toxicity we saw were grades 1 and 2,” Dr Kumar pointed out, “and could be managed symptomatically or with dose modifications.”
Grade 3-4 hematologic adverse events included thrombocytopenia (26%), neutropenia (21%), anemia (14%), leukopenia (14%), and lymphopenia (15%).
Grade 3-4 non-hematologic adverse events included nausea (3%), diarrhea (3%), fatigue (5%), back pain (8%), and vomiting (3%).
Serious adverse events occurring in 2% or more of patients included pneumonia (8%), sepsis (5%), pain, pyrexia, cough, and hypotension (3% each).
Two patients had dose-limiting toxicities of abdominal pain and nausea at the 600 mg dose.
No events of tumor lysis syndrome (TLS) were reported. Dr Kumar explained that this may have been the case because patients thought to be at high risk for TLS were mandated to be in the hospital and observed for early tumor lysis in the initial part of the study.
Response
The ORR was 21% in all patients, including a stringent complete response (sCR) of 3% and a CR of 4%.
“But what was really striking was the response rate that we observed in the 30 patients with translocation 11;14,” Dr Kumar said. “The overall response rate was 40%, with 14% of the patients having complete response or better [stringent CR] and 13% of the patients with very good partial response.”
The 36 patients without t(11;14) had a 6% ORR, 3% sCR, and 3% very good partial response.
“If you look at the response rates based on the type of therapy they were coming off or the drugs they were refractory to, the response rate is very similar across all these patient subgroups, irrespective of what groups of drugs they were refractory to,” he added.
Time to progression for all patients was about 2.5 months. For patients with the translocation, it was about 6.6 months.
“Responses were fairly durable among those who had a response,” Dr Kumar said, “considering these are patients with a median of 5 prior lines of therapy.”
Duration of response for patients with t(11;14) was close to 10 months.
Biomarker analysis
The underlying biology for the response was the BCL-2 to BCL-2L1 ratio, as the investigators had observed in the cell lines.
So they analyzed the BCL-2 gene expression ratio in 24 of the 30 patients with t(11;14).
The investigators used droplet digital PCR performed on CD138-selected bone marrow mononuclear cells collected at baseline.
Nine patients had a high ratio, and their ORR was 88%. Fifteen patients had a low ratio, and their ORR was 20%.
Median time to progression for patients with a high ratio was about 12 months. For those with a low ratio, it was about 9 months.
Median change in M protein for patients with t(11;14) was –53%, compared to +11% in the patients without the translocation.
The investigators recommend additional studies with venetoclax in MM, including those with alternative combination therapies.
Venetoclax is being developed by AbbVie, in partnership with Genentech and Roche. This study was sponsored by AbbVie. ![]()
*Data in the abstract differ from the presentation.

SAN DIEGO—Venetoclax, the oral BCL-2 inhibitor approved by the US Food and Drug Administration to treat chronic lymphocytic leukemia (CLL) patients with 17p deletion, is also showing activity in multiple myeloma (MM) patients, particularly those with t(11;14).
Final results of a phase 1 study showed venetoclax to be safe as monotherapy in relapsed or refractory MM, producing a response rate of 40% in patients with the translocation and 21% overall.
Preliminary results of the study were presented at the 2015 ASH Annual Meeting, and final results were presented at the 2016 ASH Annual Meeting.
“So I think we have a drug that potentially can change the outcome of a lot of patients with myeloma,” Shaji Kumar, MD, of the Mayo Clinic in Rochester, Minnesota, said during the presentation of the findings at ASH (abstract 488*).
“[It] also opens the possibility of being combined with a variety of other therapeutics that we have in this disease today.”
Venetoclax induces cell death in MM cell lines, particularly those positive for t(11;14). The translocation correlates with higher ratios of BCL-2 to MCL-1 and BCL-2 to MCL-2L1 (BCL-XL) mRNA. BCL-2 and MCL-1 promote survival of MM cells.
Study design and enrollment
The phase 1, open-label, multicenter study was designed to determine the best tolerated dose of venetoclax.
Secondary and exploratory objectives included overall response rate (ORR), time to progression, duration of response, and predictive biomarkers.
Patients had to have previously treated MM with measurable disease, ECOG status of 0 or 1, and adequate organ function.
They were excluded if they had an active infection, a history of significant renal, neurologic, psychiatric, endocrine, immunologic, cardiovascular, or hepatic disease within 6 months of study entry, or a history of other active malignancies within 3 years of study entry.
The study called for a 2-week lead-in period of venetoclax with weekly dose escalation. Four different dose cohorts were evaluated—300 mg, 600 mg, 900 mg, and 1200 mg.
Thirty patients were enrolled during the lead-in period, and 36 additional patients enrolled at the maximum evaluated dose of 1200 mg in the safety expansion cohort, for a total of 66 patients.
Patients were treated on a 21-day cycle with daily venetoclax. They could also receive dexamethasone to continue on the study if they progressed while receiving the monotherapy.
Patient characteristics
Patient characteristics were “similar to what you would see in relapsed/refractory multiple myloma,” Dr Kumar said.
Median age was 63 (range, 31–79), and most (62%) were ISS stage II/III.
“I want to draw your attention to two features here,” Dr Kumar said.
“Thirty patients, or 46% of the patients, had 11;14 translocation, and that reflects the interest in this drug for this particular class of patients.”
Twelve patients (18%) had 17p deletion, 32 (48%) had 13q deletion, and 27 (41%) were hyperdiploid.
“What is most striking in this cohort of patients,” Dr Kumar added, “is the fact that the median number of prior lines of therapy was 5, with some as high as 15 prior lines of therapy.”
Seventy percent were refractory to bortezomib, 77% refractory to lenalidomide, and 61% refractory to both. Fifty-two patients (79%) were refractory to their last prior therapy.
Patient disposition
At the time of data cutoff on August 19, 2016, 11 patients (17%) were still active on the study.
The median time on study was 3.3 months (range, 0.2–27), median time on venetoclax monotherapy was 2.5 months (range, 0.2–25), and median time on venetoclax plus dexamethasone was 1.4 months (range, 1–13). Seventeen patients received the combination after disease progression.
Fifty-five patients (83%) discontinued treatment, 41 (62%) because of disease progression, 5 (8%) because of adverse events, 2 (3%) withdrew consent, 1 (2%) was lost to follow-up, and 6 (9%) for unspecified reasons.
The 5 adverse events leading to withdrawal included renal failure (n=2), worsening pulmonary disorder (n=1), paralyzing sciatica (n=1), and shortness of breath and pain (n=1).
“Eight patients died on study,” Dr Kumar said, “none thought to be related to the drug.”
Adverse events
The toxicity profile was primarily hematologic and gastrointestinal.
All patients experienced an adverse event of any grade, and 45 (68%) had a grade 3 or 4 event.
“I wanted to highlight that the majority of the gastrointestinal and non-hematologic toxicity we saw were grades 1 and 2,” Dr Kumar pointed out, “and could be managed symptomatically or with dose modifications.”
Grade 3-4 hematologic adverse events included thrombocytopenia (26%), neutropenia (21%), anemia (14%), leukopenia (14%), and lymphopenia (15%).
Grade 3-4 non-hematologic adverse events included nausea (3%), diarrhea (3%), fatigue (5%), back pain (8%), and vomiting (3%).
Serious adverse events occurring in 2% or more of patients included pneumonia (8%), sepsis (5%), pain, pyrexia, cough, and hypotension (3% each).
Two patients had dose-limiting toxicities of abdominal pain and nausea at the 600 mg dose.
No events of tumor lysis syndrome (TLS) were reported. Dr Kumar explained that this may have been the case because patients thought to be at high risk for TLS were mandated to be in the hospital and observed for early tumor lysis in the initial part of the study.
Response
The ORR was 21% in all patients, including a stringent complete response (sCR) of 3% and a CR of 4%.
“But what was really striking was the response rate that we observed in the 30 patients with translocation 11;14,” Dr Kumar said. “The overall response rate was 40%, with 14% of the patients having complete response or better [stringent CR] and 13% of the patients with very good partial response.”
The 36 patients without t(11;14) had a 6% ORR, 3% sCR, and 3% very good partial response.
“If you look at the response rates based on the type of therapy they were coming off or the drugs they were refractory to, the response rate is very similar across all these patient subgroups, irrespective of what groups of drugs they were refractory to,” he added.
Time to progression for all patients was about 2.5 months. For patients with the translocation, it was about 6.6 months.
“Responses were fairly durable among those who had a response,” Dr Kumar said, “considering these are patients with a median of 5 prior lines of therapy.”
Duration of response for patients with t(11;14) was close to 10 months.
Biomarker analysis
The underlying biology for the response was the BCL-2 to BCL-2L1 ratio, as the investigators had observed in the cell lines.
So they analyzed the BCL-2 gene expression ratio in 24 of the 30 patients with t(11;14).
The investigators used droplet digital PCR performed on CD138-selected bone marrow mononuclear cells collected at baseline.
Nine patients had a high ratio, and their ORR was 88%. Fifteen patients had a low ratio, and their ORR was 20%.
Median time to progression for patients with a high ratio was about 12 months. For those with a low ratio, it was about 9 months.
Median change in M protein for patients with t(11;14) was –53%, compared to +11% in the patients without the translocation.
The investigators recommend additional studies with venetoclax in MM, including those with alternative combination therapies.
Venetoclax is being developed by AbbVie, in partnership with Genentech and Roche. This study was sponsored by AbbVie. ![]()
*Data in the abstract differ from the presentation.
Explaining lack of response to malaria vaccines

Image by Peter H. Seeberger
Researchers say they have uncovered one potential reason why it has been difficult to generate protective immunity against the early liver stage of malaria infection in regions where the incidence of malaria is high.
Their research, conducted in mice and published in Cell Reports, suggests that exposure to the blood stage of malaria infection inhibits the formation of the protective immune cells (and their antibodies) that can prevent liver-stage infection.
“The blood stage of malaria infection has a very profound impact on the liver stage immune response, and that impact had never been dissected and visualized at this level,” said study author Marion Pepper, PhD, of the University of Washington School of Medicine in Seattle.
“These studies really suggest that you need a vaccine that is protective against both stages of infection to effectively prevent malaria.”
To track how the blood stage of malaria infection overpowers the liver-stage immune response, Dr Pepper and her colleagues infected 2 groups of mice with different forms of malaria parasites.
One group of mice was infected with Plasmodium yoelii wild-type sporozoites, which complete the pre-erythrocytic stage of infection and establish a blood-stage infection.
The other group was infected with a genetically attenuated Plasmodium yoelii parasite that arrests late in liver stage development and does not cause blood-stage infection.
Six days after infection, the researchers found the levels of antibodies were significantly lower in the mice with the blood stage infection than in mice that only had the parasite targeted to the liver.
To understand this discrepancy, the team tracked the differentiation of Plasmodium liver stage-specific B cells. B cells can differentiate into antibody-secreting early effector cells or long-lived memory cells, both of which contribute to protection against malaria.
The researchers discovered that, 14 days after infection, the B cells in the blood-stage-infected mice never went through the necessary changes to make rapidly responsive memory cells.
However, in the mice that received the liver-stage attenuated version of the parasite, the B cells were still able to differentiate and create the necessary antibodies and memory cells for an effective immune response.
“This work really highlights the importance of looking at antigen-specific B cells,” Dr Pepper said. “These data also suggest that if you’re getting a vaccine while you have an ongoing blood-stage infection, there is a chance that the vaccine will not generate good memory cells because the blood stage disrupts all the processes that are involved in making that immunological memory.”
Dr Pepper and her colleagues are now looking into the possibility of treatment to solve this problem.
The team found that when they treated the second stage of the infection with the anti-malarial drug atovaquone, the B cells were able to create the optimally responsive memory cells.
For now, the researchers hope their work can be used to answer immediate questions about the efficacy of malaria vaccines in regions that are most significantly affected by the disease.
“Malaria has evolved with us throughout human existence and therefore has some potent immune evasion strategies,” Dr Pepper said. “We really tried to tease apart some of the factors that could be driving the loss of protective immunity during natural infection and with current vaccine strategies in areas of high malaria transmission.”
“Our next step is to compare malaria-specific B cells after vaccination or natural infection in humans so we can translate these findings and start to determine how to solve this problem.” ![]()

Image by Peter H. Seeberger
Researchers say they have uncovered one potential reason why it has been difficult to generate protective immunity against the early liver stage of malaria infection in regions where the incidence of malaria is high.
Their research, conducted in mice and published in Cell Reports, suggests that exposure to the blood stage of malaria infection inhibits the formation of the protective immune cells (and their antibodies) that can prevent liver-stage infection.
“The blood stage of malaria infection has a very profound impact on the liver stage immune response, and that impact had never been dissected and visualized at this level,” said study author Marion Pepper, PhD, of the University of Washington School of Medicine in Seattle.
“These studies really suggest that you need a vaccine that is protective against both stages of infection to effectively prevent malaria.”
To track how the blood stage of malaria infection overpowers the liver-stage immune response, Dr Pepper and her colleagues infected 2 groups of mice with different forms of malaria parasites.
One group of mice was infected with Plasmodium yoelii wild-type sporozoites, which complete the pre-erythrocytic stage of infection and establish a blood-stage infection.
The other group was infected with a genetically attenuated Plasmodium yoelii parasite that arrests late in liver stage development and does not cause blood-stage infection.
Six days after infection, the researchers found the levels of antibodies were significantly lower in the mice with the blood stage infection than in mice that only had the parasite targeted to the liver.
To understand this discrepancy, the team tracked the differentiation of Plasmodium liver stage-specific B cells. B cells can differentiate into antibody-secreting early effector cells or long-lived memory cells, both of which contribute to protection against malaria.
The researchers discovered that, 14 days after infection, the B cells in the blood-stage-infected mice never went through the necessary changes to make rapidly responsive memory cells.
However, in the mice that received the liver-stage attenuated version of the parasite, the B cells were still able to differentiate and create the necessary antibodies and memory cells for an effective immune response.
“This work really highlights the importance of looking at antigen-specific B cells,” Dr Pepper said. “These data also suggest that if you’re getting a vaccine while you have an ongoing blood-stage infection, there is a chance that the vaccine will not generate good memory cells because the blood stage disrupts all the processes that are involved in making that immunological memory.”
Dr Pepper and her colleagues are now looking into the possibility of treatment to solve this problem.
The team found that when they treated the second stage of the infection with the anti-malarial drug atovaquone, the B cells were able to create the optimally responsive memory cells.
For now, the researchers hope their work can be used to answer immediate questions about the efficacy of malaria vaccines in regions that are most significantly affected by the disease.
“Malaria has evolved with us throughout human existence and therefore has some potent immune evasion strategies,” Dr Pepper said. “We really tried to tease apart some of the factors that could be driving the loss of protective immunity during natural infection and with current vaccine strategies in areas of high malaria transmission.”
“Our next step is to compare malaria-specific B cells after vaccination or natural infection in humans so we can translate these findings and start to determine how to solve this problem.” ![]()

Image by Peter H. Seeberger
Researchers say they have uncovered one potential reason why it has been difficult to generate protective immunity against the early liver stage of malaria infection in regions where the incidence of malaria is high.
Their research, conducted in mice and published in Cell Reports, suggests that exposure to the blood stage of malaria infection inhibits the formation of the protective immune cells (and their antibodies) that can prevent liver-stage infection.
“The blood stage of malaria infection has a very profound impact on the liver stage immune response, and that impact had never been dissected and visualized at this level,” said study author Marion Pepper, PhD, of the University of Washington School of Medicine in Seattle.
“These studies really suggest that you need a vaccine that is protective against both stages of infection to effectively prevent malaria.”
To track how the blood stage of malaria infection overpowers the liver-stage immune response, Dr Pepper and her colleagues infected 2 groups of mice with different forms of malaria parasites.
One group of mice was infected with Plasmodium yoelii wild-type sporozoites, which complete the pre-erythrocytic stage of infection and establish a blood-stage infection.
The other group was infected with a genetically attenuated Plasmodium yoelii parasite that arrests late in liver stage development and does not cause blood-stage infection.
Six days after infection, the researchers found the levels of antibodies were significantly lower in the mice with the blood stage infection than in mice that only had the parasite targeted to the liver.
To understand this discrepancy, the team tracked the differentiation of Plasmodium liver stage-specific B cells. B cells can differentiate into antibody-secreting early effector cells or long-lived memory cells, both of which contribute to protection against malaria.
The researchers discovered that, 14 days after infection, the B cells in the blood-stage-infected mice never went through the necessary changes to make rapidly responsive memory cells.
However, in the mice that received the liver-stage attenuated version of the parasite, the B cells were still able to differentiate and create the necessary antibodies and memory cells for an effective immune response.
“This work really highlights the importance of looking at antigen-specific B cells,” Dr Pepper said. “These data also suggest that if you’re getting a vaccine while you have an ongoing blood-stage infection, there is a chance that the vaccine will not generate good memory cells because the blood stage disrupts all the processes that are involved in making that immunological memory.”
Dr Pepper and her colleagues are now looking into the possibility of treatment to solve this problem.
The team found that when they treated the second stage of the infection with the anti-malarial drug atovaquone, the B cells were able to create the optimally responsive memory cells.
For now, the researchers hope their work can be used to answer immediate questions about the efficacy of malaria vaccines in regions that are most significantly affected by the disease.
“Malaria has evolved with us throughout human existence and therefore has some potent immune evasion strategies,” Dr Pepper said. “We really tried to tease apart some of the factors that could be driving the loss of protective immunity during natural infection and with current vaccine strategies in areas of high malaria transmission.”
“Our next step is to compare malaria-specific B cells after vaccination or natural infection in humans so we can translate these findings and start to determine how to solve this problem.” ![]()
Bendamustine approved for new indication in Japan

Bendamustine hydrochloride (TREAKISYM®) has been approved in Japan as first-line treatment for patients with low-grade B-cell non-Hodgkin lymphoma (NHL) and mantle cell lymphoma (MCL).
The drug will now be available for adjunctive use with rituximab in these patients.
Bendamustine hydrochloride is already approved in Japan as monotherapy for relapsed or refractory low-grade B-cell NHL and MCL, as well as chronic lymphocytic leukemia.
Bendamustine hydrochloride is the subject of a licensing agreement concluded between Eisai Co., Ltd and SymBio Pharmaceuticals Limited. Under the licensing agreement, Eisai has been marketing the product since December 2010.
Bendamustine hydrochloride is available at doses of 25 mg and 100 mg for intravenous infusion. The recommended dosage and administration is as follows:
- For low-grade B-cell NHL and MCL
- As first-line treatment
When used adjunctively with rituximab, the usual adult dose of bendamustine hydrochloride is 90 mg/m2 body surface area infused intravenously over 60 minutes on days 1 and 2 of repeated 28-day cycles.
- For relapsed or refractory disease
The usual adult dose of bendamustine hydrochloride is 120 mg/m2 body surface area infused intravenously over 60 minutes on days 1 and 2 of repeated 21-day cycles.
- As first-line treatment
- For chronic lymphocytic leukemia
- The usual adult dose of bendamustine hydrochloride is 100 mg/m2 body surface area infused intravenously over 60 minutes on days 1 and 2 of repeated 28-day cycles.
All of the aforementioned doses may be reduced appropriately according to the patient’s condition. ![]()

Bendamustine hydrochloride (TREAKISYM®) has been approved in Japan as first-line treatment for patients with low-grade B-cell non-Hodgkin lymphoma (NHL) and mantle cell lymphoma (MCL).
The drug will now be available for adjunctive use with rituximab in these patients.
Bendamustine hydrochloride is already approved in Japan as monotherapy for relapsed or refractory low-grade B-cell NHL and MCL, as well as chronic lymphocytic leukemia.
Bendamustine hydrochloride is the subject of a licensing agreement concluded between Eisai Co., Ltd and SymBio Pharmaceuticals Limited. Under the licensing agreement, Eisai has been marketing the product since December 2010.
Bendamustine hydrochloride is available at doses of 25 mg and 100 mg for intravenous infusion. The recommended dosage and administration is as follows:
- For low-grade B-cell NHL and MCL
- As first-line treatment
When used adjunctively with rituximab, the usual adult dose of bendamustine hydrochloride is 90 mg/m2 body surface area infused intravenously over 60 minutes on days 1 and 2 of repeated 28-day cycles.
- For relapsed or refractory disease
The usual adult dose of bendamustine hydrochloride is 120 mg/m2 body surface area infused intravenously over 60 minutes on days 1 and 2 of repeated 21-day cycles.
- As first-line treatment
- For chronic lymphocytic leukemia
- The usual adult dose of bendamustine hydrochloride is 100 mg/m2 body surface area infused intravenously over 60 minutes on days 1 and 2 of repeated 28-day cycles.
All of the aforementioned doses may be reduced appropriately according to the patient’s condition. ![]()

Bendamustine hydrochloride (TREAKISYM®) has been approved in Japan as first-line treatment for patients with low-grade B-cell non-Hodgkin lymphoma (NHL) and mantle cell lymphoma (MCL).
The drug will now be available for adjunctive use with rituximab in these patients.
Bendamustine hydrochloride is already approved in Japan as monotherapy for relapsed or refractory low-grade B-cell NHL and MCL, as well as chronic lymphocytic leukemia.
Bendamustine hydrochloride is the subject of a licensing agreement concluded between Eisai Co., Ltd and SymBio Pharmaceuticals Limited. Under the licensing agreement, Eisai has been marketing the product since December 2010.
Bendamustine hydrochloride is available at doses of 25 mg and 100 mg for intravenous infusion. The recommended dosage and administration is as follows:
- For low-grade B-cell NHL and MCL
- As first-line treatment
When used adjunctively with rituximab, the usual adult dose of bendamustine hydrochloride is 90 mg/m2 body surface area infused intravenously over 60 minutes on days 1 and 2 of repeated 28-day cycles.
- For relapsed or refractory disease
The usual adult dose of bendamustine hydrochloride is 120 mg/m2 body surface area infused intravenously over 60 minutes on days 1 and 2 of repeated 21-day cycles.
- As first-line treatment
- For chronic lymphocytic leukemia
- The usual adult dose of bendamustine hydrochloride is 100 mg/m2 body surface area infused intravenously over 60 minutes on days 1 and 2 of repeated 28-day cycles.
All of the aforementioned doses may be reduced appropriately according to the patient’s condition. ![]()
KTE-C19 feasible in most young, high-risk ALL patients, study suggests

Photo by Bill Branson
SAN DIEGO—Trial results suggest treatment with the chimeric antigen receptor (CAR) T-cell therapy KTE-C19 is feasible for most young patients with high-risk B-cell acute lymphoblastic leukemia (ALL).
Nearly all ALL patients in this trial were able to receive their assigned dose of KTE-C19 after a preparative chemotherapy regimen.
The complete response (CR) rate in these patients was 62%, and the rate of severe cytokine release syndrome (CRS) was low.
Daniel W. Lee III, MD, of the University of Virginia in Charlottesville, presented these results at the 2016 ASH Annual Meeting (abstract 218).
Dr Lee noted that CAR T cells have shown promise in early studies, but morbidity related to high-grade CRS and/or neurotoxicity could limit wide applicability of this treatment in patients with high disease burden. Among those who achieve CR to CD19 CAR T-cell therapy, nearly half of patients relapse in the first year.
At ASH, Dr Lee reported results of a non-randomized clinical trial of KTE-C19, a CD19 CAR T-cell therapy under development by Kite Pharmaceuticals. The company did not sponsor this study, although investigators reported relationships with Kite and other companies. The trial was sponsored by the National Cancer Institute.
The trial included 53 children and young adults with relapsed/refractory ALL (n=51) or diffuse large B-cell lymphoma (n=2). The patients’ median age was 13 (range, 4-30), and most were male (n=41).
Of the ALL patients, 11 had primary refractory disease, 5 had Ph-positive ALL, 3 had Down syndrome, 6 had central nervous system (CNS) disease (2 with CNS3, 4 with CNS2), and 2 had MLL-rearranged ALL. The median ALL disease burden was 27%.
The first 21 patients received a low-dose fludarabine/cyclophosphamide preparative regimen, and the subsequent 32 patients received an alternative intensified preparative regimen in an attempt to mitigate severe CRS risk and improve response.
Possible intensive preparative regimens included higher-dose fludarabine/cyclophosphamide, fludarabine/high-dose cytarabine/G-CSF, and ifosfamide/etoposide.
All 53 patients had peripheral blood cells collected, and 52 were infused with CAR T cells. One patient did not receive an infusion due to progressive fungal pneumonia, and 2 patients received less than their assigned dose.
Therefore, Dr Lee said KTE-C19 was feasible in 94% of patients.
Efficacy
The median follow-up was 18.7 months.
Dr Lee said KTE-C19 “produced robust responses in very high-risk ALL patients.” He noted, however, that the CR rate was lower among patients with high disease burden.
The CR rate among the ALL patients was 62%. Of the 31 patients who achieved a CR, 28 had a minimal residual disease (MRD)-negative remission.
The rate of MRD-negative CR was 100% among the 11 patients with primary refractory ALL, 100% among the 6 patients with CNS disease, 60% among the 5 patients with Ph+ ALL, and 67% among the 3 with Down syndrome. Neither of the 2 patients with MLL-rearranged ALL responded.
“Attempts to increase response rate by modifying the preparative regimen have not yet been successful,” Dr Lee pointed out.
However, he noted superior response and overall survival rates among patients who received a fludarabine/cyclophosphamide preparative regimen.
“Median overall survival in all enrolled patients is 13.3 months with fludarabine/cyclophosphamide prep versus 5.5 months with other regimens,” he said.
The overall survival rate for the ALL patients was 28%, and the median overall survival was 11.2 months.
For patients who achieved an MRD-negative remission, the leukemia-free survival (LFS) rate was 56%. The median LFS was not reached.
Dr Lee noted that hematopoietic stem cell transplant (HSCT) after KTE-C19 correlated with decreased relapse rates and led to superior LFS.
Of the 28 patients who achieved MRD-negative CR, 21 went on to HSCT after KTE-C19. The median time to HSCT after CAR T-cell dose was 54 days. (Ten of the 28 patients had HSCT before receiving KTE-C19.)
Nineteen (91%) of the patients who proceeded to HSCT after KTE-C19 did not relapse, compared to 1 (14%) of the patients who did not have a post-CAR T transplant.
The median LFS was 4.9 months among the MRD responders who did not proceed to HSCT and undefined among MRD responders with a transplant after KTE-C19.
The probability of survival was 65% beginning at 18 months among patients who underwent HSCT and 14% beginning at 9.8 months among patients without a post-KTE-C19 transplant.
CD19 escape remains a challenge, Dr Lee said. The risk may be diminished, but not eradicated, with HSCT.
Toxicity
“There was a low rate of CRS, which was successfully managed with a grade-driven algorithm,” Dr Lee noted.
Five patients (10%) had grade 3 CRS, and 2 (4%) had grade 4 CRS.
Other grade 3/4 adverse events that were considered at least possibly related to therapy included fever (38% grade 3), febrile neutropenia (23% grade
3), hypotension (9% grade 3, 4% grade 4), LV systolic dysfunction (9% grade 3), prolonged QTc (2% grade 3), dysphasia (2% grade 3), cardiac arrest (2% grade 4), multi-organ failure (2% grade 3), hypoxia (2% grade 3, 2% grade 4), and pulmonary embolism (2% grade 3).
“There were no severe or permanent neurologic toxicities,” Dr Lee said. “Intensive neuropsychological testing in 13 patients revealed no consistent treatment-related neurocognitive decline, and several patients improved following therapy.”
In all, there were 46 cases of neurotoxicity, including visual hallucination (8 grade 1, 17%), headache (1 grade 3 [2%], 3 grade 2 [6%]), confusion (2 grade 1, 4%),
dysphasia (1 grade 3, 2%), delirium (1 grade 3, 2%), seizure (1 grade 2, 1 grade 1 [2% each]), ataxia (1 grade 2, 2%), tremor (1 grade 2, 2%), dysesthesia (1 grade 2, 2%), and dysarthria (1 grade 1, 2%). ![]()

Photo by Bill Branson
SAN DIEGO—Trial results suggest treatment with the chimeric antigen receptor (CAR) T-cell therapy KTE-C19 is feasible for most young patients with high-risk B-cell acute lymphoblastic leukemia (ALL).
Nearly all ALL patients in this trial were able to receive their assigned dose of KTE-C19 after a preparative chemotherapy regimen.
The complete response (CR) rate in these patients was 62%, and the rate of severe cytokine release syndrome (CRS) was low.
Daniel W. Lee III, MD, of the University of Virginia in Charlottesville, presented these results at the 2016 ASH Annual Meeting (abstract 218).
Dr Lee noted that CAR T cells have shown promise in early studies, but morbidity related to high-grade CRS and/or neurotoxicity could limit wide applicability of this treatment in patients with high disease burden. Among those who achieve CR to CD19 CAR T-cell therapy, nearly half of patients relapse in the first year.
At ASH, Dr Lee reported results of a non-randomized clinical trial of KTE-C19, a CD19 CAR T-cell therapy under development by Kite Pharmaceuticals. The company did not sponsor this study, although investigators reported relationships with Kite and other companies. The trial was sponsored by the National Cancer Institute.
The trial included 53 children and young adults with relapsed/refractory ALL (n=51) or diffuse large B-cell lymphoma (n=2). The patients’ median age was 13 (range, 4-30), and most were male (n=41).
Of the ALL patients, 11 had primary refractory disease, 5 had Ph-positive ALL, 3 had Down syndrome, 6 had central nervous system (CNS) disease (2 with CNS3, 4 with CNS2), and 2 had MLL-rearranged ALL. The median ALL disease burden was 27%.
The first 21 patients received a low-dose fludarabine/cyclophosphamide preparative regimen, and the subsequent 32 patients received an alternative intensified preparative regimen in an attempt to mitigate severe CRS risk and improve response.
Possible intensive preparative regimens included higher-dose fludarabine/cyclophosphamide, fludarabine/high-dose cytarabine/G-CSF, and ifosfamide/etoposide.
All 53 patients had peripheral blood cells collected, and 52 were infused with CAR T cells. One patient did not receive an infusion due to progressive fungal pneumonia, and 2 patients received less than their assigned dose.
Therefore, Dr Lee said KTE-C19 was feasible in 94% of patients.
Efficacy
The median follow-up was 18.7 months.
Dr Lee said KTE-C19 “produced robust responses in very high-risk ALL patients.” He noted, however, that the CR rate was lower among patients with high disease burden.
The CR rate among the ALL patients was 62%. Of the 31 patients who achieved a CR, 28 had a minimal residual disease (MRD)-negative remission.
The rate of MRD-negative CR was 100% among the 11 patients with primary refractory ALL, 100% among the 6 patients with CNS disease, 60% among the 5 patients with Ph+ ALL, and 67% among the 3 with Down syndrome. Neither of the 2 patients with MLL-rearranged ALL responded.
“Attempts to increase response rate by modifying the preparative regimen have not yet been successful,” Dr Lee pointed out.
However, he noted superior response and overall survival rates among patients who received a fludarabine/cyclophosphamide preparative regimen.
“Median overall survival in all enrolled patients is 13.3 months with fludarabine/cyclophosphamide prep versus 5.5 months with other regimens,” he said.
The overall survival rate for the ALL patients was 28%, and the median overall survival was 11.2 months.
For patients who achieved an MRD-negative remission, the leukemia-free survival (LFS) rate was 56%. The median LFS was not reached.
Dr Lee noted that hematopoietic stem cell transplant (HSCT) after KTE-C19 correlated with decreased relapse rates and led to superior LFS.
Of the 28 patients who achieved MRD-negative CR, 21 went on to HSCT after KTE-C19. The median time to HSCT after CAR T-cell dose was 54 days. (Ten of the 28 patients had HSCT before receiving KTE-C19.)
Nineteen (91%) of the patients who proceeded to HSCT after KTE-C19 did not relapse, compared to 1 (14%) of the patients who did not have a post-CAR T transplant.
The median LFS was 4.9 months among the MRD responders who did not proceed to HSCT and undefined among MRD responders with a transplant after KTE-C19.
The probability of survival was 65% beginning at 18 months among patients who underwent HSCT and 14% beginning at 9.8 months among patients without a post-KTE-C19 transplant.
CD19 escape remains a challenge, Dr Lee said. The risk may be diminished, but not eradicated, with HSCT.
Toxicity
“There was a low rate of CRS, which was successfully managed with a grade-driven algorithm,” Dr Lee noted.
Five patients (10%) had grade 3 CRS, and 2 (4%) had grade 4 CRS.
Other grade 3/4 adverse events that were considered at least possibly related to therapy included fever (38% grade 3), febrile neutropenia (23% grade
3), hypotension (9% grade 3, 4% grade 4), LV systolic dysfunction (9% grade 3), prolonged QTc (2% grade 3), dysphasia (2% grade 3), cardiac arrest (2% grade 4), multi-organ failure (2% grade 3), hypoxia (2% grade 3, 2% grade 4), and pulmonary embolism (2% grade 3).
“There were no severe or permanent neurologic toxicities,” Dr Lee said. “Intensive neuropsychological testing in 13 patients revealed no consistent treatment-related neurocognitive decline, and several patients improved following therapy.”
In all, there were 46 cases of neurotoxicity, including visual hallucination (8 grade 1, 17%), headache (1 grade 3 [2%], 3 grade 2 [6%]), confusion (2 grade 1, 4%),
dysphasia (1 grade 3, 2%), delirium (1 grade 3, 2%), seizure (1 grade 2, 1 grade 1 [2% each]), ataxia (1 grade 2, 2%), tremor (1 grade 2, 2%), dysesthesia (1 grade 2, 2%), and dysarthria (1 grade 1, 2%). ![]()

Photo by Bill Branson
SAN DIEGO—Trial results suggest treatment with the chimeric antigen receptor (CAR) T-cell therapy KTE-C19 is feasible for most young patients with high-risk B-cell acute lymphoblastic leukemia (ALL).
Nearly all ALL patients in this trial were able to receive their assigned dose of KTE-C19 after a preparative chemotherapy regimen.
The complete response (CR) rate in these patients was 62%, and the rate of severe cytokine release syndrome (CRS) was low.
Daniel W. Lee III, MD, of the University of Virginia in Charlottesville, presented these results at the 2016 ASH Annual Meeting (abstract 218).
Dr Lee noted that CAR T cells have shown promise in early studies, but morbidity related to high-grade CRS and/or neurotoxicity could limit wide applicability of this treatment in patients with high disease burden. Among those who achieve CR to CD19 CAR T-cell therapy, nearly half of patients relapse in the first year.
At ASH, Dr Lee reported results of a non-randomized clinical trial of KTE-C19, a CD19 CAR T-cell therapy under development by Kite Pharmaceuticals. The company did not sponsor this study, although investigators reported relationships with Kite and other companies. The trial was sponsored by the National Cancer Institute.
The trial included 53 children and young adults with relapsed/refractory ALL (n=51) or diffuse large B-cell lymphoma (n=2). The patients’ median age was 13 (range, 4-30), and most were male (n=41).
Of the ALL patients, 11 had primary refractory disease, 5 had Ph-positive ALL, 3 had Down syndrome, 6 had central nervous system (CNS) disease (2 with CNS3, 4 with CNS2), and 2 had MLL-rearranged ALL. The median ALL disease burden was 27%.
The first 21 patients received a low-dose fludarabine/cyclophosphamide preparative regimen, and the subsequent 32 patients received an alternative intensified preparative regimen in an attempt to mitigate severe CRS risk and improve response.
Possible intensive preparative regimens included higher-dose fludarabine/cyclophosphamide, fludarabine/high-dose cytarabine/G-CSF, and ifosfamide/etoposide.
All 53 patients had peripheral blood cells collected, and 52 were infused with CAR T cells. One patient did not receive an infusion due to progressive fungal pneumonia, and 2 patients received less than their assigned dose.
Therefore, Dr Lee said KTE-C19 was feasible in 94% of patients.
Efficacy
The median follow-up was 18.7 months.
Dr Lee said KTE-C19 “produced robust responses in very high-risk ALL patients.” He noted, however, that the CR rate was lower among patients with high disease burden.
The CR rate among the ALL patients was 62%. Of the 31 patients who achieved a CR, 28 had a minimal residual disease (MRD)-negative remission.
The rate of MRD-negative CR was 100% among the 11 patients with primary refractory ALL, 100% among the 6 patients with CNS disease, 60% among the 5 patients with Ph+ ALL, and 67% among the 3 with Down syndrome. Neither of the 2 patients with MLL-rearranged ALL responded.
“Attempts to increase response rate by modifying the preparative regimen have not yet been successful,” Dr Lee pointed out.
However, he noted superior response and overall survival rates among patients who received a fludarabine/cyclophosphamide preparative regimen.
“Median overall survival in all enrolled patients is 13.3 months with fludarabine/cyclophosphamide prep versus 5.5 months with other regimens,” he said.
The overall survival rate for the ALL patients was 28%, and the median overall survival was 11.2 months.
For patients who achieved an MRD-negative remission, the leukemia-free survival (LFS) rate was 56%. The median LFS was not reached.
Dr Lee noted that hematopoietic stem cell transplant (HSCT) after KTE-C19 correlated with decreased relapse rates and led to superior LFS.
Of the 28 patients who achieved MRD-negative CR, 21 went on to HSCT after KTE-C19. The median time to HSCT after CAR T-cell dose was 54 days. (Ten of the 28 patients had HSCT before receiving KTE-C19.)
Nineteen (91%) of the patients who proceeded to HSCT after KTE-C19 did not relapse, compared to 1 (14%) of the patients who did not have a post-CAR T transplant.
The median LFS was 4.9 months among the MRD responders who did not proceed to HSCT and undefined among MRD responders with a transplant after KTE-C19.
The probability of survival was 65% beginning at 18 months among patients who underwent HSCT and 14% beginning at 9.8 months among patients without a post-KTE-C19 transplant.
CD19 escape remains a challenge, Dr Lee said. The risk may be diminished, but not eradicated, with HSCT.
Toxicity
“There was a low rate of CRS, which was successfully managed with a grade-driven algorithm,” Dr Lee noted.
Five patients (10%) had grade 3 CRS, and 2 (4%) had grade 4 CRS.
Other grade 3/4 adverse events that were considered at least possibly related to therapy included fever (38% grade 3), febrile neutropenia (23% grade
3), hypotension (9% grade 3, 4% grade 4), LV systolic dysfunction (9% grade 3), prolonged QTc (2% grade 3), dysphasia (2% grade 3), cardiac arrest (2% grade 4), multi-organ failure (2% grade 3), hypoxia (2% grade 3, 2% grade 4), and pulmonary embolism (2% grade 3).
“There were no severe or permanent neurologic toxicities,” Dr Lee said. “Intensive neuropsychological testing in 13 patients revealed no consistent treatment-related neurocognitive decline, and several patients improved following therapy.”
In all, there were 46 cases of neurotoxicity, including visual hallucination (8 grade 1, 17%), headache (1 grade 3 [2%], 3 grade 2 [6%]), confusion (2 grade 1, 4%),
dysphasia (1 grade 3, 2%), delirium (1 grade 3, 2%), seizure (1 grade 2, 1 grade 1 [2% each]), ataxia (1 grade 2, 2%), tremor (1 grade 2, 2%), dysesthesia (1 grade 2, 2%), and dysarthria (1 grade 1, 2%). ![]()
It’s working! (No it’s not! Yes it is!)
A man walks into a doctor’s office, snapping his fingers.
“Why are you snapping your fingers?” asks the doctor.
“To keep the elephants away,” says the man, still snapping his fingers.
“That’s ridiculous!” says the doctor. “There are no elephants within 3,000 miles of here!”
“You see,” says the man, still snapping. “It’s working!”
Even saying that sounds strange. Don’t we physicians apply the evidence-based fruits of science? What does the patient’s mind have to do with that?
Yesterday we saw Emma, who spent 5 years in Austria. On her back was perfectly circular purpura.
“Who does your cupping?” I asked her.
“My acupuncturist,” said Emma. “He does cupping too.”
“What’s it for?”
“Aches and pains, stress, that sort of thing.”
“Does it help?”
“It seems to,” said Emma. Sometimes, anyway.”
Later I asked my student what she thought Emma meant. “What did Emma see or feel to make her conclude that cupping was working, at least sometimes? Did she feel better Tuesday than Monday? What if she felt worse again Wednesday? Would that mean the treatment wasn’t working anymore? That it works some days and not others?”
If you think this line of analysis applies only to exotic forms of alternative medicine, consider how often we could ask the same questions about the medically approved treatments we prescribe every day.
Acne
• Henrietta, for whom I’d prescribed clindamycin in the morning and tretinoin at night. Her verdict? “I stopped the clindamycin because it didn’t work. But I love the tretinoin—it works great!”
Since she was putting both creams on exactly the same area, what did Henrietta observe to lead her to this paradoxical conclusion?
• Janet has two pimples, yet she’s decided that minocycline doesn’t work. Her evidence? “I still get breakouts around my period.”
Eczema
• “Amcinonide worked amazingly but clobetasol didn’t work at all!”
• “I stopped the betamethasone. Calendula works better.”
• And of course: “Sure the cream helped, but the rash came back!”
Patients say things like this all day long. From a medical standpoint, active ingredients work better than inert vehicles. In theory, class 1 steroids are more effective than class 3 steroids.
Perhaps, but many of my patients don’t agree. Maybe their eczema has gone into remission, in which case anything will work. Even if so, there is no way I can prove that to them. So I usually don’t try.
Psoriasis
“Your psoriasis looks better.”
“No, it’s worse.”
“Why? It covers a lot less skin than it used to.”
“But now it’s coming in new places.”
One could go on. With my students, I often do. If they learn nothing else, I try to convey the essential difference between a person and a toaster. Which is this:
If you know how to fix a toaster, the toaster does not have to agree with you.
A person is another matter. Patients have minds to go with their parts. They pick up knowledge from places doctors have never been and make inferences doctors would never make. Then they act on this knowledge and those inferences by saying things like: “The morning cream didn’t work but the night cream did, so I stopped the morning cream.”
I therefore advise students to ask patients two questions first thing:
• What treatments are the patients actually using? Assuming that they are doing what the chart says you asked them to do can jam your foot so deep in your mouth that you’ll never get it out.
• How do the patients themselves think they’re doing? One man with a couple of pimples or scaly spots is thrilled. Another with the same pimples or spots is miserable. It’s helpful to find out which he is before making suggestions. (See foot in mouth, above.)
Emma, by the way, was unhappy that she couldn’t find a practitioner of craniosacral therapy (look it up) as proficient as the one she had in Austria.
I asked her how she judged proficiency but won’t bother you with her answer. I just referred her to a physician who practices both Eastern and Western medicine.
That worked for her.
Dr. Rockoff practices dermatology in Brookline, Mass, and is a longtime contributor to Dermatology News. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years. His new book “Act Like a Doctor, Think Like a Patient” is now available at amazon.com and barnesandnoble.com. This is his second book. Write to him at [email protected] .
A man walks into a doctor’s office, snapping his fingers.
“Why are you snapping your fingers?” asks the doctor.
“To keep the elephants away,” says the man, still snapping his fingers.
“That’s ridiculous!” says the doctor. “There are no elephants within 3,000 miles of here!”
“You see,” says the man, still snapping. “It’s working!”
Even saying that sounds strange. Don’t we physicians apply the evidence-based fruits of science? What does the patient’s mind have to do with that?
Yesterday we saw Emma, who spent 5 years in Austria. On her back was perfectly circular purpura.
“Who does your cupping?” I asked her.
“My acupuncturist,” said Emma. “He does cupping too.”
“What’s it for?”
“Aches and pains, stress, that sort of thing.”
“Does it help?”
“It seems to,” said Emma. Sometimes, anyway.”
Later I asked my student what she thought Emma meant. “What did Emma see or feel to make her conclude that cupping was working, at least sometimes? Did she feel better Tuesday than Monday? What if she felt worse again Wednesday? Would that mean the treatment wasn’t working anymore? That it works some days and not others?”
If you think this line of analysis applies only to exotic forms of alternative medicine, consider how often we could ask the same questions about the medically approved treatments we prescribe every day.
Acne
• Henrietta, for whom I’d prescribed clindamycin in the morning and tretinoin at night. Her verdict? “I stopped the clindamycin because it didn’t work. But I love the tretinoin—it works great!”
Since she was putting both creams on exactly the same area, what did Henrietta observe to lead her to this paradoxical conclusion?
• Janet has two pimples, yet she’s decided that minocycline doesn’t work. Her evidence? “I still get breakouts around my period.”
Eczema
• “Amcinonide worked amazingly but clobetasol didn’t work at all!”
• “I stopped the betamethasone. Calendula works better.”
• And of course: “Sure the cream helped, but the rash came back!”
Patients say things like this all day long. From a medical standpoint, active ingredients work better than inert vehicles. In theory, class 1 steroids are more effective than class 3 steroids.
Perhaps, but many of my patients don’t agree. Maybe their eczema has gone into remission, in which case anything will work. Even if so, there is no way I can prove that to them. So I usually don’t try.
Psoriasis
“Your psoriasis looks better.”
“No, it’s worse.”
“Why? It covers a lot less skin than it used to.”
“But now it’s coming in new places.”
One could go on. With my students, I often do. If they learn nothing else, I try to convey the essential difference between a person and a toaster. Which is this:
If you know how to fix a toaster, the toaster does not have to agree with you.
A person is another matter. Patients have minds to go with their parts. They pick up knowledge from places doctors have never been and make inferences doctors would never make. Then they act on this knowledge and those inferences by saying things like: “The morning cream didn’t work but the night cream did, so I stopped the morning cream.”
I therefore advise students to ask patients two questions first thing:
• What treatments are the patients actually using? Assuming that they are doing what the chart says you asked them to do can jam your foot so deep in your mouth that you’ll never get it out.
• How do the patients themselves think they’re doing? One man with a couple of pimples or scaly spots is thrilled. Another with the same pimples or spots is miserable. It’s helpful to find out which he is before making suggestions. (See foot in mouth, above.)
Emma, by the way, was unhappy that she couldn’t find a practitioner of craniosacral therapy (look it up) as proficient as the one she had in Austria.
I asked her how she judged proficiency but won’t bother you with her answer. I just referred her to a physician who practices both Eastern and Western medicine.
That worked for her.
Dr. Rockoff practices dermatology in Brookline, Mass, and is a longtime contributor to Dermatology News. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years. His new book “Act Like a Doctor, Think Like a Patient” is now available at amazon.com and barnesandnoble.com. This is his second book. Write to him at [email protected] .
A man walks into a doctor’s office, snapping his fingers.
“Why are you snapping your fingers?” asks the doctor.
“To keep the elephants away,” says the man, still snapping his fingers.
“That’s ridiculous!” says the doctor. “There are no elephants within 3,000 miles of here!”
“You see,” says the man, still snapping. “It’s working!”
Even saying that sounds strange. Don’t we physicians apply the evidence-based fruits of science? What does the patient’s mind have to do with that?
Yesterday we saw Emma, who spent 5 years in Austria. On her back was perfectly circular purpura.
“Who does your cupping?” I asked her.
“My acupuncturist,” said Emma. “He does cupping too.”
“What’s it for?”
“Aches and pains, stress, that sort of thing.”
“Does it help?”
“It seems to,” said Emma. Sometimes, anyway.”
Later I asked my student what she thought Emma meant. “What did Emma see or feel to make her conclude that cupping was working, at least sometimes? Did she feel better Tuesday than Monday? What if she felt worse again Wednesday? Would that mean the treatment wasn’t working anymore? That it works some days and not others?”
If you think this line of analysis applies only to exotic forms of alternative medicine, consider how often we could ask the same questions about the medically approved treatments we prescribe every day.
Acne
• Henrietta, for whom I’d prescribed clindamycin in the morning and tretinoin at night. Her verdict? “I stopped the clindamycin because it didn’t work. But I love the tretinoin—it works great!”
Since she was putting both creams on exactly the same area, what did Henrietta observe to lead her to this paradoxical conclusion?
• Janet has two pimples, yet she’s decided that minocycline doesn’t work. Her evidence? “I still get breakouts around my period.”
Eczema
• “Amcinonide worked amazingly but clobetasol didn’t work at all!”
• “I stopped the betamethasone. Calendula works better.”
• And of course: “Sure the cream helped, but the rash came back!”
Patients say things like this all day long. From a medical standpoint, active ingredients work better than inert vehicles. In theory, class 1 steroids are more effective than class 3 steroids.
Perhaps, but many of my patients don’t agree. Maybe their eczema has gone into remission, in which case anything will work. Even if so, there is no way I can prove that to them. So I usually don’t try.
Psoriasis
“Your psoriasis looks better.”
“No, it’s worse.”
“Why? It covers a lot less skin than it used to.”
“But now it’s coming in new places.”
One could go on. With my students, I often do. If they learn nothing else, I try to convey the essential difference between a person and a toaster. Which is this:
If you know how to fix a toaster, the toaster does not have to agree with you.
A person is another matter. Patients have minds to go with their parts. They pick up knowledge from places doctors have never been and make inferences doctors would never make. Then they act on this knowledge and those inferences by saying things like: “The morning cream didn’t work but the night cream did, so I stopped the morning cream.”
I therefore advise students to ask patients two questions first thing:
• What treatments are the patients actually using? Assuming that they are doing what the chart says you asked them to do can jam your foot so deep in your mouth that you’ll never get it out.
• How do the patients themselves think they’re doing? One man with a couple of pimples or scaly spots is thrilled. Another with the same pimples or spots is miserable. It’s helpful to find out which he is before making suggestions. (See foot in mouth, above.)
Emma, by the way, was unhappy that she couldn’t find a practitioner of craniosacral therapy (look it up) as proficient as the one she had in Austria.
I asked her how she judged proficiency but won’t bother you with her answer. I just referred her to a physician who practices both Eastern and Western medicine.
That worked for her.
Dr. Rockoff practices dermatology in Brookline, Mass, and is a longtime contributor to Dermatology News. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years. His new book “Act Like a Doctor, Think Like a Patient” is now available at amazon.com and barnesandnoble.com. This is his second book. Write to him at [email protected] .
FOUND IN TRANSLATION Minimal nomenclature and maximum sensitivity complicate MRD measures
In hematologic malignancies, there is a deep and direct connection between each individual patient, that patient’s symptoms, the visible cells that cause the disease, and the direct measurements and assessments of those cells. The totality of these factors helps to determine the diagnosis and treatment plan. As a butterfly needle often is sufficient for obtaining a diagnostic tumor biopsy, it is not surprising that these same diagnostic techniques are now standardly being used to monitor disease response.
The techniques differ in their limits of detection, however. With sequencing depths able to reliably detect variant allele frequencies of less than 10%, even when patients’ overt leukemia may no longer be detectable, clinicians may be left to ponder what to do with persistent “preleukemic” or “rising clones.”1-3
These patients, now more appropriately stratified for risk of recurrence, are in desperate need of better care algorithms. Standard MRD assessment by flow cytometric analysis is able to detect less than 1 x 10-4 cells. While it can be applied to most patients, its sensitivity will likely be surpassed by new and emerging genomic assays. Real time quantitative polymerase chain reaction (RT-qPCR) and next generation sequencing (NGS) require a leukemia-specific abnormality but have the potential for far greater sensitivity with deeper sequencing techniques.
Long-term follow up data in acute promyelocytic leukemia (APL) provides the illustrative example where morphologic remission is not durable in the setting of a persistent PML-RARa transcript and therapeutic goals for PCR negativity irrespective of morphology are standard. Pathologic fusion proteins are ideal for marker-driven therapy, but are found in only about 50% of patients, mainly those with APL and Philadelphia chromosome-positive leukemias.
With driver mutations identified in the majority of patients, we can be hopeful that NGS negativity may be a useful clinical endpoint. In work presented at ASH 2016 by Bartlomiej M Getta, MBBS, of Memorial Sloan Kettering Cancer Center, New York, and his colleagues, patients with concordant MRD positivity by flow cytometry and NGS had inferior outcomes, even after allogeneic transplant, compared to patients with MRD positivity on one assay but not both.5 Nonetheless, caution should be taken in early adoption of NGS as a independent marker of MRD status for two main reasons: 1) False positives and lack of standardization make current interpretation difficult. 2) The presence of “preleukemic” clones remains enigmatic – and no matter the nomenclature used, can a DNMT3A or IDH-mutant clone really be deemed “clonal hematopoiesis of indeterminate potential” when a patient has already had clonal transformation?
Conversely, not all patients reported in the work by Klco2 and Getta ultimately relapse. Thus, while it would be preferred to clear all mutant clones, as a therapeutic goal this likely would subject many patients to unnecessary toxicity. One half of the patients reported by Getta were disease free at a year with concordant flow and NGS positive MRD while patients with NGS positivity alone had outcomes equivalent to those of MRD-negative patients, highlighting that certain persistent clones in NGS-only, MRD-positive patients might be amenable to immunotherapy, either with checkpoint inhibitors or allogeneic transplant. Insight into which clones remain quiescent and which are more sinister will require more investigation, but there does seem to be an additive role to NGS-positivity, whereby all MRD is not created equal and the precision and clinical utility of MRD status will likely take on nuanced nomenclature.
References
1. Jan, M. et al. Clonal evolution of preleukemic hematopoietic stem cells precedes human acute myeloid leukemia. Science Translational Medicine 4, 149ra118, doi: 10.1126/scitranslmed.3004315 (2012).
2. Klco, J. M. et al. Association Between Mutation Clearance After Induction Therapy and Outcomes in Acute Myeloid Leukemia. JAMA 2015;314:811-22. doi: 10.1001/jama.2015.9643.
3. Wong, T. N. et al. Rapid expansion of preexisting nonleukemic hematopoietic clones frequently follows induction therapy for de novo AML. Blood 2016;127:893-7. doi: 10.1182/blood-2015-10-677021 (2016).
4. Lane, A. A. et al. Results from Ongoing Phase II Trial of SL-401 As Consolidation Therapy in Patients with Acute Myeloid Leukemia (AML) in Remission with High Relapse Risk Including Minimal Residual Disease (MRD), Abstract 215, ASH 2016.
5. Getta, B. M. et al. Multicolor Flow Cytometry and Multi-Gene Next Generation Sequencing Are Complimentary and Highly Predictive for Relapse in Acute Myeloid Leukemia Following Allogeneic Hematopoietic Stem Cell Transplant, Abstract 834, ASH 2016.
Dr. Viny is with the Memorial Sloan-Kettering Cancer Center, New York, where he is a clinical instructor, on the staff of the leukemia service, and a clinical researcher in The Ross Levine Lab. Contact Dr. Viny at [email protected].
In hematologic malignancies, there is a deep and direct connection between each individual patient, that patient’s symptoms, the visible cells that cause the disease, and the direct measurements and assessments of those cells. The totality of these factors helps to determine the diagnosis and treatment plan. As a butterfly needle often is sufficient for obtaining a diagnostic tumor biopsy, it is not surprising that these same diagnostic techniques are now standardly being used to monitor disease response.
The techniques differ in their limits of detection, however. With sequencing depths able to reliably detect variant allele frequencies of less than 10%, even when patients’ overt leukemia may no longer be detectable, clinicians may be left to ponder what to do with persistent “preleukemic” or “rising clones.”1-3
These patients, now more appropriately stratified for risk of recurrence, are in desperate need of better care algorithms. Standard MRD assessment by flow cytometric analysis is able to detect less than 1 x 10-4 cells. While it can be applied to most patients, its sensitivity will likely be surpassed by new and emerging genomic assays. Real time quantitative polymerase chain reaction (RT-qPCR) and next generation sequencing (NGS) require a leukemia-specific abnormality but have the potential for far greater sensitivity with deeper sequencing techniques.
Long-term follow up data in acute promyelocytic leukemia (APL) provides the illustrative example where morphologic remission is not durable in the setting of a persistent PML-RARa transcript and therapeutic goals for PCR negativity irrespective of morphology are standard. Pathologic fusion proteins are ideal for marker-driven therapy, but are found in only about 50% of patients, mainly those with APL and Philadelphia chromosome-positive leukemias.
With driver mutations identified in the majority of patients, we can be hopeful that NGS negativity may be a useful clinical endpoint. In work presented at ASH 2016 by Bartlomiej M Getta, MBBS, of Memorial Sloan Kettering Cancer Center, New York, and his colleagues, patients with concordant MRD positivity by flow cytometry and NGS had inferior outcomes, even after allogeneic transplant, compared to patients with MRD positivity on one assay but not both.5 Nonetheless, caution should be taken in early adoption of NGS as a independent marker of MRD status for two main reasons: 1) False positives and lack of standardization make current interpretation difficult. 2) The presence of “preleukemic” clones remains enigmatic – and no matter the nomenclature used, can a DNMT3A or IDH-mutant clone really be deemed “clonal hematopoiesis of indeterminate potential” when a patient has already had clonal transformation?
Conversely, not all patients reported in the work by Klco2 and Getta ultimately relapse. Thus, while it would be preferred to clear all mutant clones, as a therapeutic goal this likely would subject many patients to unnecessary toxicity. One half of the patients reported by Getta were disease free at a year with concordant flow and NGS positive MRD while patients with NGS positivity alone had outcomes equivalent to those of MRD-negative patients, highlighting that certain persistent clones in NGS-only, MRD-positive patients might be amenable to immunotherapy, either with checkpoint inhibitors or allogeneic transplant. Insight into which clones remain quiescent and which are more sinister will require more investigation, but there does seem to be an additive role to NGS-positivity, whereby all MRD is not created equal and the precision and clinical utility of MRD status will likely take on nuanced nomenclature.
References
1. Jan, M. et al. Clonal evolution of preleukemic hematopoietic stem cells precedes human acute myeloid leukemia. Science Translational Medicine 4, 149ra118, doi: 10.1126/scitranslmed.3004315 (2012).
2. Klco, J. M. et al. Association Between Mutation Clearance After Induction Therapy and Outcomes in Acute Myeloid Leukemia. JAMA 2015;314:811-22. doi: 10.1001/jama.2015.9643.
3. Wong, T. N. et al. Rapid expansion of preexisting nonleukemic hematopoietic clones frequently follows induction therapy for de novo AML. Blood 2016;127:893-7. doi: 10.1182/blood-2015-10-677021 (2016).
4. Lane, A. A. et al. Results from Ongoing Phase II Trial of SL-401 As Consolidation Therapy in Patients with Acute Myeloid Leukemia (AML) in Remission with High Relapse Risk Including Minimal Residual Disease (MRD), Abstract 215, ASH 2016.
5. Getta, B. M. et al. Multicolor Flow Cytometry and Multi-Gene Next Generation Sequencing Are Complimentary and Highly Predictive for Relapse in Acute Myeloid Leukemia Following Allogeneic Hematopoietic Stem Cell Transplant, Abstract 834, ASH 2016.
Dr. Viny is with the Memorial Sloan-Kettering Cancer Center, New York, where he is a clinical instructor, on the staff of the leukemia service, and a clinical researcher in The Ross Levine Lab. Contact Dr. Viny at [email protected].
In hematologic malignancies, there is a deep and direct connection between each individual patient, that patient’s symptoms, the visible cells that cause the disease, and the direct measurements and assessments of those cells. The totality of these factors helps to determine the diagnosis and treatment plan. As a butterfly needle often is sufficient for obtaining a diagnostic tumor biopsy, it is not surprising that these same diagnostic techniques are now standardly being used to monitor disease response.
The techniques differ in their limits of detection, however. With sequencing depths able to reliably detect variant allele frequencies of less than 10%, even when patients’ overt leukemia may no longer be detectable, clinicians may be left to ponder what to do with persistent “preleukemic” or “rising clones.”1-3
These patients, now more appropriately stratified for risk of recurrence, are in desperate need of better care algorithms. Standard MRD assessment by flow cytometric analysis is able to detect less than 1 x 10-4 cells. While it can be applied to most patients, its sensitivity will likely be surpassed by new and emerging genomic assays. Real time quantitative polymerase chain reaction (RT-qPCR) and next generation sequencing (NGS) require a leukemia-specific abnormality but have the potential for far greater sensitivity with deeper sequencing techniques.
Long-term follow up data in acute promyelocytic leukemia (APL) provides the illustrative example where morphologic remission is not durable in the setting of a persistent PML-RARa transcript and therapeutic goals for PCR negativity irrespective of morphology are standard. Pathologic fusion proteins are ideal for marker-driven therapy, but are found in only about 50% of patients, mainly those with APL and Philadelphia chromosome-positive leukemias.
With driver mutations identified in the majority of patients, we can be hopeful that NGS negativity may be a useful clinical endpoint. In work presented at ASH 2016 by Bartlomiej M Getta, MBBS, of Memorial Sloan Kettering Cancer Center, New York, and his colleagues, patients with concordant MRD positivity by flow cytometry and NGS had inferior outcomes, even after allogeneic transplant, compared to patients with MRD positivity on one assay but not both.5 Nonetheless, caution should be taken in early adoption of NGS as a independent marker of MRD status for two main reasons: 1) False positives and lack of standardization make current interpretation difficult. 2) The presence of “preleukemic” clones remains enigmatic – and no matter the nomenclature used, can a DNMT3A or IDH-mutant clone really be deemed “clonal hematopoiesis of indeterminate potential” when a patient has already had clonal transformation?
Conversely, not all patients reported in the work by Klco2 and Getta ultimately relapse. Thus, while it would be preferred to clear all mutant clones, as a therapeutic goal this likely would subject many patients to unnecessary toxicity. One half of the patients reported by Getta were disease free at a year with concordant flow and NGS positive MRD while patients with NGS positivity alone had outcomes equivalent to those of MRD-negative patients, highlighting that certain persistent clones in NGS-only, MRD-positive patients might be amenable to immunotherapy, either with checkpoint inhibitors or allogeneic transplant. Insight into which clones remain quiescent and which are more sinister will require more investigation, but there does seem to be an additive role to NGS-positivity, whereby all MRD is not created equal and the precision and clinical utility of MRD status will likely take on nuanced nomenclature.
References
1. Jan, M. et al. Clonal evolution of preleukemic hematopoietic stem cells precedes human acute myeloid leukemia. Science Translational Medicine 4, 149ra118, doi: 10.1126/scitranslmed.3004315 (2012).
2. Klco, J. M. et al. Association Between Mutation Clearance After Induction Therapy and Outcomes in Acute Myeloid Leukemia. JAMA 2015;314:811-22. doi: 10.1001/jama.2015.9643.
3. Wong, T. N. et al. Rapid expansion of preexisting nonleukemic hematopoietic clones frequently follows induction therapy for de novo AML. Blood 2016;127:893-7. doi: 10.1182/blood-2015-10-677021 (2016).
4. Lane, A. A. et al. Results from Ongoing Phase II Trial of SL-401 As Consolidation Therapy in Patients with Acute Myeloid Leukemia (AML) in Remission with High Relapse Risk Including Minimal Residual Disease (MRD), Abstract 215, ASH 2016.
5. Getta, B. M. et al. Multicolor Flow Cytometry and Multi-Gene Next Generation Sequencing Are Complimentary and Highly Predictive for Relapse in Acute Myeloid Leukemia Following Allogeneic Hematopoietic Stem Cell Transplant, Abstract 834, ASH 2016.
Dr. Viny is with the Memorial Sloan-Kettering Cancer Center, New York, where he is a clinical instructor, on the staff of the leukemia service, and a clinical researcher in The Ross Levine Lab. Contact Dr. Viny at [email protected].