User login
Why kids with cancer have a higher risk of treatment-related toxicity
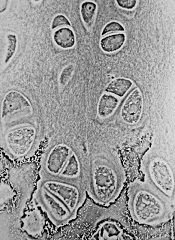
Preclinical research appears to explain why certain tissues in very young children are more sensitive to collateral damage from cancer treatment than tissues in older individuals.
Researchers found evidence to suggest that, early in life, cells in the brain, heart, and kidney are primed for apoptosis.
On the other hand, cells in the healthy adult brain, heart, and kidneys are apoptosis-refractory.
Kristopher A. Sarosiek, PhD, of the Harvard T.H. Chan School of Public Health in Boston, Massachusetts, and his colleagues reported these findings in Cancer Cell.
The researchers used BH3 profiling to measure the relative dominance of pro-survival or pro-death signals inside cells.
A cancer cell in which apoptotic signals are dominant is said to be “highly primed” for self-destruction and therefore easily killed by therapy, while a cell with low priming is more resistant to death or damage.
Dr Sarosiek and his colleagues measured the priming of cells in tissues from adult mice and young mice.
In the adult mice, cells of the hematopoietic lineage from the periphery, thymus, spleen, and bone marrow were the most primed for apoptosis. Cells from the large intestine, small intestine, lungs, and liver were relatively unprimed. And cells in brain, heart, and kidney tissues were far less primed.
However, in embryonic and very young mice, cells in the brain, heart, and kidney were extremely primed for apoptosis.
The researchers found that, in the adult brains, hearts, and kidneys, the molecular machinery needed to perform apoptosis was nearly completely absent.
In contrast, this machinery was abundant in the brains, hearts, and kidneys of young mice. As a result, brain, heart, and kidney cells were much more vulnerable to cell death when exposed to chemotherapy or radiation.
After determining in mouse models that certain cells grew more resistant to treatment toxicity with age, the researchers tested human cells. The team obtained fresh samples of tissue that had been removed from brains of children and adults to prevent intractable epileptic seizures.
As in the mice, the youngest human brain cells were more highly primed with apoptotic machinery and vulnerable to chemotherapy and radiation damage.
The researchers said there was a period of higher heterogeneity in apoptotic priming among patients between 2 and 6 years of age. After that, the brain transitions to full apoptotic resistance.
The team also found that, in young tissues, expression of the apoptotic protein machinery is driven by c-Myc. This transcription factor drives an apoptotically primed state by directly activating transcription of the pro-apoptotic genes Bax, Bim, and Bid.
“[This research] has uncovered some opportunities to selectively block apoptosis in our healthy tissues and prevent toxicity from radiation or chemotherapy while still maintaining sensitivity within cancer cells,” Dr Sarosiek said. “We are actively pursuing the identification of new medicines that can be used exactly for this purpose.” ![]()

Preclinical research appears to explain why certain tissues in very young children are more sensitive to collateral damage from cancer treatment than tissues in older individuals.
Researchers found evidence to suggest that, early in life, cells in the brain, heart, and kidney are primed for apoptosis.
On the other hand, cells in the healthy adult brain, heart, and kidneys are apoptosis-refractory.
Kristopher A. Sarosiek, PhD, of the Harvard T.H. Chan School of Public Health in Boston, Massachusetts, and his colleagues reported these findings in Cancer Cell.
The researchers used BH3 profiling to measure the relative dominance of pro-survival or pro-death signals inside cells.
A cancer cell in which apoptotic signals are dominant is said to be “highly primed” for self-destruction and therefore easily killed by therapy, while a cell with low priming is more resistant to death or damage.
Dr Sarosiek and his colleagues measured the priming of cells in tissues from adult mice and young mice.
In the adult mice, cells of the hematopoietic lineage from the periphery, thymus, spleen, and bone marrow were the most primed for apoptosis. Cells from the large intestine, small intestine, lungs, and liver were relatively unprimed. And cells in brain, heart, and kidney tissues were far less primed.
However, in embryonic and very young mice, cells in the brain, heart, and kidney were extremely primed for apoptosis.
The researchers found that, in the adult brains, hearts, and kidneys, the molecular machinery needed to perform apoptosis was nearly completely absent.
In contrast, this machinery was abundant in the brains, hearts, and kidneys of young mice. As a result, brain, heart, and kidney cells were much more vulnerable to cell death when exposed to chemotherapy or radiation.
After determining in mouse models that certain cells grew more resistant to treatment toxicity with age, the researchers tested human cells. The team obtained fresh samples of tissue that had been removed from brains of children and adults to prevent intractable epileptic seizures.
As in the mice, the youngest human brain cells were more highly primed with apoptotic machinery and vulnerable to chemotherapy and radiation damage.
The researchers said there was a period of higher heterogeneity in apoptotic priming among patients between 2 and 6 years of age. After that, the brain transitions to full apoptotic resistance.
The team also found that, in young tissues, expression of the apoptotic protein machinery is driven by c-Myc. This transcription factor drives an apoptotically primed state by directly activating transcription of the pro-apoptotic genes Bax, Bim, and Bid.
“[This research] has uncovered some opportunities to selectively block apoptosis in our healthy tissues and prevent toxicity from radiation or chemotherapy while still maintaining sensitivity within cancer cells,” Dr Sarosiek said. “We are actively pursuing the identification of new medicines that can be used exactly for this purpose.” ![]()

Preclinical research appears to explain why certain tissues in very young children are more sensitive to collateral damage from cancer treatment than tissues in older individuals.
Researchers found evidence to suggest that, early in life, cells in the brain, heart, and kidney are primed for apoptosis.
On the other hand, cells in the healthy adult brain, heart, and kidneys are apoptosis-refractory.
Kristopher A. Sarosiek, PhD, of the Harvard T.H. Chan School of Public Health in Boston, Massachusetts, and his colleagues reported these findings in Cancer Cell.
The researchers used BH3 profiling to measure the relative dominance of pro-survival or pro-death signals inside cells.
A cancer cell in which apoptotic signals are dominant is said to be “highly primed” for self-destruction and therefore easily killed by therapy, while a cell with low priming is more resistant to death or damage.
Dr Sarosiek and his colleagues measured the priming of cells in tissues from adult mice and young mice.
In the adult mice, cells of the hematopoietic lineage from the periphery, thymus, spleen, and bone marrow were the most primed for apoptosis. Cells from the large intestine, small intestine, lungs, and liver were relatively unprimed. And cells in brain, heart, and kidney tissues were far less primed.
However, in embryonic and very young mice, cells in the brain, heart, and kidney were extremely primed for apoptosis.
The researchers found that, in the adult brains, hearts, and kidneys, the molecular machinery needed to perform apoptosis was nearly completely absent.
In contrast, this machinery was abundant in the brains, hearts, and kidneys of young mice. As a result, brain, heart, and kidney cells were much more vulnerable to cell death when exposed to chemotherapy or radiation.
After determining in mouse models that certain cells grew more resistant to treatment toxicity with age, the researchers tested human cells. The team obtained fresh samples of tissue that had been removed from brains of children and adults to prevent intractable epileptic seizures.
As in the mice, the youngest human brain cells were more highly primed with apoptotic machinery and vulnerable to chemotherapy and radiation damage.
The researchers said there was a period of higher heterogeneity in apoptotic priming among patients between 2 and 6 years of age. After that, the brain transitions to full apoptotic resistance.
The team also found that, in young tissues, expression of the apoptotic protein machinery is driven by c-Myc. This transcription factor drives an apoptotically primed state by directly activating transcription of the pro-apoptotic genes Bax, Bim, and Bid.
“[This research] has uncovered some opportunities to selectively block apoptosis in our healthy tissues and prevent toxicity from radiation or chemotherapy while still maintaining sensitivity within cancer cells,” Dr Sarosiek said. “We are actively pursuing the identification of new medicines that can be used exactly for this purpose.” ![]()
Smoking Cessation
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Recovery From TBI and Better Sleep Go Hand in Hand
After a traumatic brain injury (TBI), people also experience major sleep problems, including changes in their sleep–wake cycle. A new study published online ahead of print December 21, 2016, in Neurology showed that recovering from these two conditions occurs in parallel.
“These results suggest that monitoring a person’s sleep–wake cycle may be a useful tool for assessing their recovery after TBI,” said study author Nadia Gosselin, PhD, an Assistant Professor in the Department of Psychology at the University of Montréal in Québec. “We found that when someone sustained a brain injury and had not recovered a certain level of consciousness to keep them awake and aware of their surroundings, they were not able to generate a good sleep–wake cycle. But as they recovered, their quality of sleep improved.”
The study involved 30 people, ages 17 to 58, who had been hospitalized for moderate to severe TBI. Most of the patients were in a coma when they were admitted to the hospital, and all initially received care in an ICU. The injuries were caused by motor vehicle accidents for 20 people, falls for seven people, recreational or sports activities for two people and a blow to the head for one person. They were hospitalized for an average of 45 days, with monitoring for the study beginning an average of 21 days into the patient’s stay.
Each person was monitored daily for an average of 11 days for level of consciousness and thinking abilities using the Rancho Los Amigos scale, which ranges from 1 to 8. Each person also wore an activity monitor on the wrist so researchers could measure their sleep.
Researchers found that consciousness and thinking abilities improved hand in hand with measures of quality of sleep, showing a linear relationship.
One measure, the daytime activity ratio, reflects the percentage of activity that occurs during the day. Immediately after the injury, activity occurs throughout the day and night. The study showed that participants reached an acceptable sleep–wake cycle, with a daytime activity ratio of at least 80%, at the same point when they emerged from a minimally conscious state.
The participants still had inadequate sleep–wake cycles, at a score of 5 on the Rancho Los Amigos scale, where people are confused and give inappropriate responses to stimuli, but are able to follow simple commands. Sleep–wake cycles reached adequate levels at the same time that people reached a score of 6 on the Rancho Los Amigos scale, which is when people can give appropriate responses while still depending on outside input for direction. At that level, they can remember relearned tasks, but cannot remember new tasks.
The results were the same when researchers adjusted for the amount of time that had passed since the injury and the amount of medications they had received while they were in the ICU.
“It is possible that there are common underlying brain mechanisms involved in both recovery from TBI and improvement in sleep,” said Dr. Gosselin. “Still, more study needs to be done, and future research may want to examine how hospital lighting and noise also affect quality of sleep for those with TBI.”
Suggested Reading
Duclos C, Dumont M, Arbour C, et al. Parallel recovery of consciousness and sleep in acute traumatic brain injury. Neurology. 2016 Dec 21 [Epub ahead of print].
Soddu A, Bassetti CL. A good sleep for a fresh mind in patients with acute tramatic brain injury. Neurology. 2016 Dec 21 [Epub ahead of print].
After a traumatic brain injury (TBI), people also experience major sleep problems, including changes in their sleep–wake cycle. A new study published online ahead of print December 21, 2016, in Neurology showed that recovering from these two conditions occurs in parallel.
“These results suggest that monitoring a person’s sleep–wake cycle may be a useful tool for assessing their recovery after TBI,” said study author Nadia Gosselin, PhD, an Assistant Professor in the Department of Psychology at the University of Montréal in Québec. “We found that when someone sustained a brain injury and had not recovered a certain level of consciousness to keep them awake and aware of their surroundings, they were not able to generate a good sleep–wake cycle. But as they recovered, their quality of sleep improved.”
The study involved 30 people, ages 17 to 58, who had been hospitalized for moderate to severe TBI. Most of the patients were in a coma when they were admitted to the hospital, and all initially received care in an ICU. The injuries were caused by motor vehicle accidents for 20 people, falls for seven people, recreational or sports activities for two people and a blow to the head for one person. They were hospitalized for an average of 45 days, with monitoring for the study beginning an average of 21 days into the patient’s stay.
Each person was monitored daily for an average of 11 days for level of consciousness and thinking abilities using the Rancho Los Amigos scale, which ranges from 1 to 8. Each person also wore an activity monitor on the wrist so researchers could measure their sleep.
Researchers found that consciousness and thinking abilities improved hand in hand with measures of quality of sleep, showing a linear relationship.
One measure, the daytime activity ratio, reflects the percentage of activity that occurs during the day. Immediately after the injury, activity occurs throughout the day and night. The study showed that participants reached an acceptable sleep–wake cycle, with a daytime activity ratio of at least 80%, at the same point when they emerged from a minimally conscious state.
The participants still had inadequate sleep–wake cycles, at a score of 5 on the Rancho Los Amigos scale, where people are confused and give inappropriate responses to stimuli, but are able to follow simple commands. Sleep–wake cycles reached adequate levels at the same time that people reached a score of 6 on the Rancho Los Amigos scale, which is when people can give appropriate responses while still depending on outside input for direction. At that level, they can remember relearned tasks, but cannot remember new tasks.
The results were the same when researchers adjusted for the amount of time that had passed since the injury and the amount of medications they had received while they were in the ICU.
“It is possible that there are common underlying brain mechanisms involved in both recovery from TBI and improvement in sleep,” said Dr. Gosselin. “Still, more study needs to be done, and future research may want to examine how hospital lighting and noise also affect quality of sleep for those with TBI.”
Suggested Reading
Duclos C, Dumont M, Arbour C, et al. Parallel recovery of consciousness and sleep in acute traumatic brain injury. Neurology. 2016 Dec 21 [Epub ahead of print].
Soddu A, Bassetti CL. A good sleep for a fresh mind in patients with acute tramatic brain injury. Neurology. 2016 Dec 21 [Epub ahead of print].
After a traumatic brain injury (TBI), people also experience major sleep problems, including changes in their sleep–wake cycle. A new study published online ahead of print December 21, 2016, in Neurology showed that recovering from these two conditions occurs in parallel.
“These results suggest that monitoring a person’s sleep–wake cycle may be a useful tool for assessing their recovery after TBI,” said study author Nadia Gosselin, PhD, an Assistant Professor in the Department of Psychology at the University of Montréal in Québec. “We found that when someone sustained a brain injury and had not recovered a certain level of consciousness to keep them awake and aware of their surroundings, they were not able to generate a good sleep–wake cycle. But as they recovered, their quality of sleep improved.”
The study involved 30 people, ages 17 to 58, who had been hospitalized for moderate to severe TBI. Most of the patients were in a coma when they were admitted to the hospital, and all initially received care in an ICU. The injuries were caused by motor vehicle accidents for 20 people, falls for seven people, recreational or sports activities for two people and a blow to the head for one person. They were hospitalized for an average of 45 days, with monitoring for the study beginning an average of 21 days into the patient’s stay.
Each person was monitored daily for an average of 11 days for level of consciousness and thinking abilities using the Rancho Los Amigos scale, which ranges from 1 to 8. Each person also wore an activity monitor on the wrist so researchers could measure their sleep.
Researchers found that consciousness and thinking abilities improved hand in hand with measures of quality of sleep, showing a linear relationship.
One measure, the daytime activity ratio, reflects the percentage of activity that occurs during the day. Immediately after the injury, activity occurs throughout the day and night. The study showed that participants reached an acceptable sleep–wake cycle, with a daytime activity ratio of at least 80%, at the same point when they emerged from a minimally conscious state.
The participants still had inadequate sleep–wake cycles, at a score of 5 on the Rancho Los Amigos scale, where people are confused and give inappropriate responses to stimuli, but are able to follow simple commands. Sleep–wake cycles reached adequate levels at the same time that people reached a score of 6 on the Rancho Los Amigos scale, which is when people can give appropriate responses while still depending on outside input for direction. At that level, they can remember relearned tasks, but cannot remember new tasks.
The results were the same when researchers adjusted for the amount of time that had passed since the injury and the amount of medications they had received while they were in the ICU.
“It is possible that there are common underlying brain mechanisms involved in both recovery from TBI and improvement in sleep,” said Dr. Gosselin. “Still, more study needs to be done, and future research may want to examine how hospital lighting and noise also affect quality of sleep for those with TBI.”
Suggested Reading
Duclos C, Dumont M, Arbour C, et al. Parallel recovery of consciousness and sleep in acute traumatic brain injury. Neurology. 2016 Dec 21 [Epub ahead of print].
Soddu A, Bassetti CL. A good sleep for a fresh mind in patients with acute tramatic brain injury. Neurology. 2016 Dec 21 [Epub ahead of print].
Tips for Living With Muscular Dystrophy
Click here to download the PDF.
Click here to download the PDF.
Click here to download the PDF.
Cardiovascular Comorbidity Is Common Among Adults With Epilepsy
HOUSTON—Adults with epilepsy report cardiovascular disease more often than adults without epilepsy, according to research presented at the 70th Annual Meeting of the American Epilepsy Society.
In the 2013 US National Health Interview Survey, women with epilepsy reported significantly more hypertension, stroke, and angina pectoris than women without epilepsy. Men with epilepsy reported significantly more stroke than men without epilepsy, said Matthew Zack, MD, MPH, an epidemiologist in the Division of Population Health in the National Center for Chronic Disease Prevention and Health Promotion at the CDC, and colleagues.
Recommending that patients with epilepsy practice healthier behaviors to reduce cardiovascular risk (eg, quitting cigarette smoking, increasing aerobic activity, and eating a healthy diet) “will reduce the burden from these outcomes,” the researchers said. Among patients with cardiovascular disease, adherence to treatments and self-management also will reduce risk, they said.
Cardiovascular diseases are among the most common potentially preventable comorbidities. To compare how often adults with and without epilepsy in the general United States population report common cardiovascular diseases, Dr. Zack and his research colleagues analyzed data from the US National Health Interview Survey, a cross-sectional survey of the civilian noninstitutionalized population.
Participants were age 18 or older and answered questions about epilepsy and cardiovascular disease. In all, 587 adults reported ever having been told by a health professional that they had a seizure disorder or epilepsy, and 33,946 adults did not report a history of epilepsy. Participants also reported whether they had been told by a health professional that they had hypertension, coronary heart disease, angina pectoris, heart attack, other heart condition, or stroke. The investigators adjusted results for age, race/ethnicity, marital status, educational attainment, the ratio of family income to the poverty level, and geographic region.
Compared with people without epilepsy, people with epilepsy reported significantly more hypertension (36.4% vs 30.2%), angina pectoris (3.9% vs 2.0%), heart attack (5.2% vs 3.3%), other heart condition (11.8% vs 7.4%), and stroke (12.2% vs 2.6%). Women with epilepsy reported significantly more hypertension (36.4% vs 29.6%), angina pectoris (3.9% vs 1.7%), and stroke (14.1% vs 2.6%) than women without epilepsy. Men with epilepsy reported significantly more stroke (10.1% vs 2.7%) than men without epilepsy.
People with epilepsy may report more cardiovascular disease than people without epilepsy because of behavioral risk factors, genetic predisposition, seizure-related damage to the heart, or medication effects, the researchers said. The sex-specific differences require further study. The investigators noted that the study’s reliance on self-report increases the likelihood of misclassification of epilepsy status and cardiovascular disease outcomes. In addition, researchers do not know whether cardiovascular outcomes occurred before or after the onset of epilepsy.
—Jake Remaly
Suggested Reading
Cui W, Zack MM, Kobau R, Helmers SL. Health behaviors among people with epilepsy—results from the 2010 National Health Interview Survey. Epilepsy Behav. 2015;44:121-126.
Kadima NT, Kobau R, Zack MM, Helmers S. Comorbidity in adults with epilepsy—United States, 2010. MMWR Morb Mortal Wkly Rep. 2013;62(43):849-853.
HOUSTON—Adults with epilepsy report cardiovascular disease more often than adults without epilepsy, according to research presented at the 70th Annual Meeting of the American Epilepsy Society.
In the 2013 US National Health Interview Survey, women with epilepsy reported significantly more hypertension, stroke, and angina pectoris than women without epilepsy. Men with epilepsy reported significantly more stroke than men without epilepsy, said Matthew Zack, MD, MPH, an epidemiologist in the Division of Population Health in the National Center for Chronic Disease Prevention and Health Promotion at the CDC, and colleagues.
Recommending that patients with epilepsy practice healthier behaviors to reduce cardiovascular risk (eg, quitting cigarette smoking, increasing aerobic activity, and eating a healthy diet) “will reduce the burden from these outcomes,” the researchers said. Among patients with cardiovascular disease, adherence to treatments and self-management also will reduce risk, they said.
Cardiovascular diseases are among the most common potentially preventable comorbidities. To compare how often adults with and without epilepsy in the general United States population report common cardiovascular diseases, Dr. Zack and his research colleagues analyzed data from the US National Health Interview Survey, a cross-sectional survey of the civilian noninstitutionalized population.
Participants were age 18 or older and answered questions about epilepsy and cardiovascular disease. In all, 587 adults reported ever having been told by a health professional that they had a seizure disorder or epilepsy, and 33,946 adults did not report a history of epilepsy. Participants also reported whether they had been told by a health professional that they had hypertension, coronary heart disease, angina pectoris, heart attack, other heart condition, or stroke. The investigators adjusted results for age, race/ethnicity, marital status, educational attainment, the ratio of family income to the poverty level, and geographic region.
Compared with people without epilepsy, people with epilepsy reported significantly more hypertension (36.4% vs 30.2%), angina pectoris (3.9% vs 2.0%), heart attack (5.2% vs 3.3%), other heart condition (11.8% vs 7.4%), and stroke (12.2% vs 2.6%). Women with epilepsy reported significantly more hypertension (36.4% vs 29.6%), angina pectoris (3.9% vs 1.7%), and stroke (14.1% vs 2.6%) than women without epilepsy. Men with epilepsy reported significantly more stroke (10.1% vs 2.7%) than men without epilepsy.
People with epilepsy may report more cardiovascular disease than people without epilepsy because of behavioral risk factors, genetic predisposition, seizure-related damage to the heart, or medication effects, the researchers said. The sex-specific differences require further study. The investigators noted that the study’s reliance on self-report increases the likelihood of misclassification of epilepsy status and cardiovascular disease outcomes. In addition, researchers do not know whether cardiovascular outcomes occurred before or after the onset of epilepsy.
—Jake Remaly
Suggested Reading
Cui W, Zack MM, Kobau R, Helmers SL. Health behaviors among people with epilepsy—results from the 2010 National Health Interview Survey. Epilepsy Behav. 2015;44:121-126.
Kadima NT, Kobau R, Zack MM, Helmers S. Comorbidity in adults with epilepsy—United States, 2010. MMWR Morb Mortal Wkly Rep. 2013;62(43):849-853.
HOUSTON—Adults with epilepsy report cardiovascular disease more often than adults without epilepsy, according to research presented at the 70th Annual Meeting of the American Epilepsy Society.
In the 2013 US National Health Interview Survey, women with epilepsy reported significantly more hypertension, stroke, and angina pectoris than women without epilepsy. Men with epilepsy reported significantly more stroke than men without epilepsy, said Matthew Zack, MD, MPH, an epidemiologist in the Division of Population Health in the National Center for Chronic Disease Prevention and Health Promotion at the CDC, and colleagues.
Recommending that patients with epilepsy practice healthier behaviors to reduce cardiovascular risk (eg, quitting cigarette smoking, increasing aerobic activity, and eating a healthy diet) “will reduce the burden from these outcomes,” the researchers said. Among patients with cardiovascular disease, adherence to treatments and self-management also will reduce risk, they said.
Cardiovascular diseases are among the most common potentially preventable comorbidities. To compare how often adults with and without epilepsy in the general United States population report common cardiovascular diseases, Dr. Zack and his research colleagues analyzed data from the US National Health Interview Survey, a cross-sectional survey of the civilian noninstitutionalized population.
Participants were age 18 or older and answered questions about epilepsy and cardiovascular disease. In all, 587 adults reported ever having been told by a health professional that they had a seizure disorder or epilepsy, and 33,946 adults did not report a history of epilepsy. Participants also reported whether they had been told by a health professional that they had hypertension, coronary heart disease, angina pectoris, heart attack, other heart condition, or stroke. The investigators adjusted results for age, race/ethnicity, marital status, educational attainment, the ratio of family income to the poverty level, and geographic region.
Compared with people without epilepsy, people with epilepsy reported significantly more hypertension (36.4% vs 30.2%), angina pectoris (3.9% vs 2.0%), heart attack (5.2% vs 3.3%), other heart condition (11.8% vs 7.4%), and stroke (12.2% vs 2.6%). Women with epilepsy reported significantly more hypertension (36.4% vs 29.6%), angina pectoris (3.9% vs 1.7%), and stroke (14.1% vs 2.6%) than women without epilepsy. Men with epilepsy reported significantly more stroke (10.1% vs 2.7%) than men without epilepsy.
People with epilepsy may report more cardiovascular disease than people without epilepsy because of behavioral risk factors, genetic predisposition, seizure-related damage to the heart, or medication effects, the researchers said. The sex-specific differences require further study. The investigators noted that the study’s reliance on self-report increases the likelihood of misclassification of epilepsy status and cardiovascular disease outcomes. In addition, researchers do not know whether cardiovascular outcomes occurred before or after the onset of epilepsy.
—Jake Remaly
Suggested Reading
Cui W, Zack MM, Kobau R, Helmers SL. Health behaviors among people with epilepsy—results from the 2010 National Health Interview Survey. Epilepsy Behav. 2015;44:121-126.
Kadima NT, Kobau R, Zack MM, Helmers S. Comorbidity in adults with epilepsy—United States, 2010. MMWR Morb Mortal Wkly Rep. 2013;62(43):849-853.
Treatment Advances in Parkinson’s Disease and Other Movement Disorders
LAS VEGAS—An oral extended-release agent composed of carbidopa–levodopa microbeads that was shown, in clinical studies, to improve symptoms in patients with early or advanced Parkinson’s disease is one of several promising new therapies for this neurodegenerative disorder, according to a presentation at the American Academy of Neurology’s Fall 2016 Conference.
In addition to extended-release carbidopa–levodopa (Rytary), these therapies include drugs recently approved by the FDA for orthostatic hypotension (droxidopa) and psychosis (pimavanserin) in Parkinson’s disease and investigational agents such as an inhaled levodopa (CVT-301) and a leukemia drug (nilotinib) currently in clinical trials for use in patients with Parkinson’s disease. “These are things that patients are going to be talking to you about and that you all can use in taking care of patients with Parkinson’s disease,” said Jeff A. Kraakevik, MD, Associate Professor of Neurology at Oregon Health & Science University in Portland.
Expanding the Armamentarium
Dr. Kraakevik presented data on two new deep brain stimulation systems—Vercise from Boston Scientific, which has not yet been approved by the FDA, and Infinity from St. Jude Medical, which has been FDA approved—and a third surgical device from Medtronic, which is not new, but now has new FDA labeling regarding broader MRI compatibility for limited body scans. He also reported on a randomized trial of focused ultrasound for essential tremor that has led to FDA approval and touched on a clinical trial of a specific gene therapy for Huntington’s disease that is now enrolling patients. However, the bulk of his presentation focused on pharmacologic therapy.
Extended-Release Carbidopa–Levodopa (Rytary)
Citing a 2016 review of cumulative data by Dhall and Kreitzman that included five randomized controlled trials, Dr. Kraakevik indicated that Rytary is a superior formulation of carbidopa–levodopa, compared with a prior controlled-release formulation—largely owing to factors related to gut absorption, such as the slow rate at which the extended-release component of the latter formulation goes through the stomach. “Parkinson’s patients are often constipated, so there is a variable gut transport, as well as a protein competing with the carbidopa–levodopa as it [gets absorbed],” he said. “All those things led to this being a fairly unpredictable [process]. If you talk to patients about it, they usually are rather unhappy with the controlled-release formulation, especially as the disease progresses.”
The extended-release formulation of Rytary can entail what essentially amounts to a dosing-related trial period for some patients started on it; but, in Dr. Kraakevik’s experience, patients who stick it out usually are glad that they did. For one, the combination of immediate-release and sustained-release beads in each capsule makes the formulation much more predictable than controlled release. “The patients I’ve been able to get through the transition period have really, really liked it,” he said. “We tell people we are going to have … maybe at least a couple of weeks to a month of trying to figure out what the best dose of Rytary is.”
He also commented on the high cost of the drug. “The company has a support desk that patients can call; but, even with that, every time I’ve had to do a dose adjustment and change the pill I’ve had to do another prior authorization, which then sets it back,” said Dr. Kraakevik. “That also has been a barrier for some of my patients to get started on Rytary. That being said, especially for later-onset … motor fluctuations, it is a very good formulation.”
Droxidopa (Northera)
In terms of treating orthostatic hypotension in Parkinson’s disease, prior agents include midodrine, fludrocortisone, and pyridostigmine. They all work relatively well, according to Dr. Kraakevik; however, if one looks at the studies on midodrine, for example, the data are somewhat equivocal. “It is nice that we have this additional therapeutic in our armamentarium,” he said of droxidopa (Northera). “It is a precursor to norepinephrine, so it has a different mechanism of action than [any] of the medications we have to treat orthostasis.”
Symptom and symptom-impact composite scores from the trial that resulted in FDA approval for droxidopa show a statistically significant difference versus placebo. In his practice, Dr. Kraakevik talks to his patients a lot more about whether they are fainting or feeling lightheaded and a lot less about listing all their orthostatic blood pressure readings over the previous several weeks. Most of his patients have been seeing primary care physicians who, for years, have been telling them that their blood pressure should not exceed 140/90 and that, if it does, they should call their doctor and, if it is more than 180 or 185, they should go to the emergency room.
“I get a lot of phone calls because these medications are going to make these people have blood pressures that are going to be above those ranges,” said Dr. Kraakevik. “It is probably going to be up there for only about five, 10 minutes, and then it is going to come back down. So that is why I do not tell people to focus on the numbers, but more on symptoms. You can see [in the aforementioned trial] that for dizziness/lightheadedness, visual disturbances … [droxidopa] has a nice effect. Looking at symptoms [such as] when they are standing for a long time, standing for a short time, all these things—except for walking for a long time—symptoms were significantly reduced with use of droxidopa.”
Pimavanserin (Nuplazid)
Pimavanserin (Nuplazid) has recently been FDA approved for treatment of hallucinations in Parkinson’s disease. It is a 5-HT2A inverse agonist. Pimavanserin blocks the serotonergic receptor and reduces the stimulation effect, causing a decrease in the ability to create hallucinations to the striatal limit. In a 2014 trial, pimavanserin was shown to significantly decrease hallucinations over 43 days. “It is an effective medication … not much in the way of side effects at this point,” said Dr. Kraakevik. “It does have that QT prolongation possibility, so you need to be a little careful in patients who have some cardiac abnormalities or rhythm abnormalities when you use this medication. But otherwise it is relatively well tolerated.”
Therapeutics on the Horizon
Medications in the pipeline include an inhaled carbidopa–levodopa agent (CVT-301). In a randomized controlled trial of 86 subjects, investigators found it to be effective in rapidly reversing “off” symptoms, Dr. Kraakevik reported. “Most of the patients who were in this trial had reversal of their symptoms within about 10 minutes,” he said, adding that CVT-301 should be in phase III studies soon and coming up for approval within the next two years.
Nilotinib was approved by the FDA in 2010 as a treatment for adult patients with a form of chronic myeloid leukemia. Given its mechanism of action as a tyrosine kinase inhibitor, researchers hypothesize that it could be applicable to Parkinson’s disease. In a phase I study involving 12 subjects, investigators found that all of the patients who received nilotinib had a reversible decrease in their Unified Parkinson’s Disease Rating Scale score while also scoring “much better on their Mini-Mental State Examination,” according to Dr. Kraakevik.
“It reversed when the medication was taken away,” he emphasized. “This led to a lot of press and a lot of questions in my clinic, where we are asked … why is not every [therapy] able to do this? And my response to patients is, ‘Right now we have 12 people in an open-label study. We need more data.’”
Such data could come from a double-blind, placebo-controlled clinical trial currently in the planning stages, “but there have allegedly been some disagreements between the key entities about the best way to proceed, and this has slowed the process significantly.”
—Fred Balzac
Suggested Reading
Cummings J, Isaacson S, Mills R, et al. Pimavanserin for patients with Parkinson’s disease psychosis: a randomised, placebo-controlled phase 3 trial. Lancet. 2014;383(9916):533-540.
Dhall R, Kreitzman DL. Advances in levodopa therapy for Parkinson disease: review of RYTARY (carbidopa and levodopa) clinical efficacy and safety. Neurology. 2016;86(14 suppl 1):S13-S24.
Fox SH. Pimavanserin as treatment for Parkinson’s disease psychosis. Lancet. 2014;383(9916):494-496.
Kaufmann H, Freeman R, Biaggioni I, et al for the NOH301 Investigators. Droxidopa for neurogenic orthostatic hypotension: a randomized, placebo-controlled, phase 3 trial. Neurology. 2014;83(4):328-335.
LeWitt PA, Hauser RA, Grosset DG, et al. A randomized trial of inhaled levodopa (CVT-301) for motor fluctuations in Parkinson’s disease. Mov Disord. 2016;31(9):1356-1365.
Pagan F, Hebron M, Valadez EH, et al. Nilotinib effects in Parkinson’s disease and dementia with Lewy bodies. J Parkinsons Dis. 2016;6(3):503-517.
LAS VEGAS—An oral extended-release agent composed of carbidopa–levodopa microbeads that was shown, in clinical studies, to improve symptoms in patients with early or advanced Parkinson’s disease is one of several promising new therapies for this neurodegenerative disorder, according to a presentation at the American Academy of Neurology’s Fall 2016 Conference.
In addition to extended-release carbidopa–levodopa (Rytary), these therapies include drugs recently approved by the FDA for orthostatic hypotension (droxidopa) and psychosis (pimavanserin) in Parkinson’s disease and investigational agents such as an inhaled levodopa (CVT-301) and a leukemia drug (nilotinib) currently in clinical trials for use in patients with Parkinson’s disease. “These are things that patients are going to be talking to you about and that you all can use in taking care of patients with Parkinson’s disease,” said Jeff A. Kraakevik, MD, Associate Professor of Neurology at Oregon Health & Science University in Portland.
Expanding the Armamentarium
Dr. Kraakevik presented data on two new deep brain stimulation systems—Vercise from Boston Scientific, which has not yet been approved by the FDA, and Infinity from St. Jude Medical, which has been FDA approved—and a third surgical device from Medtronic, which is not new, but now has new FDA labeling regarding broader MRI compatibility for limited body scans. He also reported on a randomized trial of focused ultrasound for essential tremor that has led to FDA approval and touched on a clinical trial of a specific gene therapy for Huntington’s disease that is now enrolling patients. However, the bulk of his presentation focused on pharmacologic therapy.
Extended-Release Carbidopa–Levodopa (Rytary)
Citing a 2016 review of cumulative data by Dhall and Kreitzman that included five randomized controlled trials, Dr. Kraakevik indicated that Rytary is a superior formulation of carbidopa–levodopa, compared with a prior controlled-release formulation—largely owing to factors related to gut absorption, such as the slow rate at which the extended-release component of the latter formulation goes through the stomach. “Parkinson’s patients are often constipated, so there is a variable gut transport, as well as a protein competing with the carbidopa–levodopa as it [gets absorbed],” he said. “All those things led to this being a fairly unpredictable [process]. If you talk to patients about it, they usually are rather unhappy with the controlled-release formulation, especially as the disease progresses.”
The extended-release formulation of Rytary can entail what essentially amounts to a dosing-related trial period for some patients started on it; but, in Dr. Kraakevik’s experience, patients who stick it out usually are glad that they did. For one, the combination of immediate-release and sustained-release beads in each capsule makes the formulation much more predictable than controlled release. “The patients I’ve been able to get through the transition period have really, really liked it,” he said. “We tell people we are going to have … maybe at least a couple of weeks to a month of trying to figure out what the best dose of Rytary is.”
He also commented on the high cost of the drug. “The company has a support desk that patients can call; but, even with that, every time I’ve had to do a dose adjustment and change the pill I’ve had to do another prior authorization, which then sets it back,” said Dr. Kraakevik. “That also has been a barrier for some of my patients to get started on Rytary. That being said, especially for later-onset … motor fluctuations, it is a very good formulation.”
Droxidopa (Northera)
In terms of treating orthostatic hypotension in Parkinson’s disease, prior agents include midodrine, fludrocortisone, and pyridostigmine. They all work relatively well, according to Dr. Kraakevik; however, if one looks at the studies on midodrine, for example, the data are somewhat equivocal. “It is nice that we have this additional therapeutic in our armamentarium,” he said of droxidopa (Northera). “It is a precursor to norepinephrine, so it has a different mechanism of action than [any] of the medications we have to treat orthostasis.”
Symptom and symptom-impact composite scores from the trial that resulted in FDA approval for droxidopa show a statistically significant difference versus placebo. In his practice, Dr. Kraakevik talks to his patients a lot more about whether they are fainting or feeling lightheaded and a lot less about listing all their orthostatic blood pressure readings over the previous several weeks. Most of his patients have been seeing primary care physicians who, for years, have been telling them that their blood pressure should not exceed 140/90 and that, if it does, they should call their doctor and, if it is more than 180 or 185, they should go to the emergency room.
“I get a lot of phone calls because these medications are going to make these people have blood pressures that are going to be above those ranges,” said Dr. Kraakevik. “It is probably going to be up there for only about five, 10 minutes, and then it is going to come back down. So that is why I do not tell people to focus on the numbers, but more on symptoms. You can see [in the aforementioned trial] that for dizziness/lightheadedness, visual disturbances … [droxidopa] has a nice effect. Looking at symptoms [such as] when they are standing for a long time, standing for a short time, all these things—except for walking for a long time—symptoms were significantly reduced with use of droxidopa.”
Pimavanserin (Nuplazid)
Pimavanserin (Nuplazid) has recently been FDA approved for treatment of hallucinations in Parkinson’s disease. It is a 5-HT2A inverse agonist. Pimavanserin blocks the serotonergic receptor and reduces the stimulation effect, causing a decrease in the ability to create hallucinations to the striatal limit. In a 2014 trial, pimavanserin was shown to significantly decrease hallucinations over 43 days. “It is an effective medication … not much in the way of side effects at this point,” said Dr. Kraakevik. “It does have that QT prolongation possibility, so you need to be a little careful in patients who have some cardiac abnormalities or rhythm abnormalities when you use this medication. But otherwise it is relatively well tolerated.”
Therapeutics on the Horizon
Medications in the pipeline include an inhaled carbidopa–levodopa agent (CVT-301). In a randomized controlled trial of 86 subjects, investigators found it to be effective in rapidly reversing “off” symptoms, Dr. Kraakevik reported. “Most of the patients who were in this trial had reversal of their symptoms within about 10 minutes,” he said, adding that CVT-301 should be in phase III studies soon and coming up for approval within the next two years.
Nilotinib was approved by the FDA in 2010 as a treatment for adult patients with a form of chronic myeloid leukemia. Given its mechanism of action as a tyrosine kinase inhibitor, researchers hypothesize that it could be applicable to Parkinson’s disease. In a phase I study involving 12 subjects, investigators found that all of the patients who received nilotinib had a reversible decrease in their Unified Parkinson’s Disease Rating Scale score while also scoring “much better on their Mini-Mental State Examination,” according to Dr. Kraakevik.
“It reversed when the medication was taken away,” he emphasized. “This led to a lot of press and a lot of questions in my clinic, where we are asked … why is not every [therapy] able to do this? And my response to patients is, ‘Right now we have 12 people in an open-label study. We need more data.’”
Such data could come from a double-blind, placebo-controlled clinical trial currently in the planning stages, “but there have allegedly been some disagreements between the key entities about the best way to proceed, and this has slowed the process significantly.”
—Fred Balzac
Suggested Reading
Cummings J, Isaacson S, Mills R, et al. Pimavanserin for patients with Parkinson’s disease psychosis: a randomised, placebo-controlled phase 3 trial. Lancet. 2014;383(9916):533-540.
Dhall R, Kreitzman DL. Advances in levodopa therapy for Parkinson disease: review of RYTARY (carbidopa and levodopa) clinical efficacy and safety. Neurology. 2016;86(14 suppl 1):S13-S24.
Fox SH. Pimavanserin as treatment for Parkinson’s disease psychosis. Lancet. 2014;383(9916):494-496.
Kaufmann H, Freeman R, Biaggioni I, et al for the NOH301 Investigators. Droxidopa for neurogenic orthostatic hypotension: a randomized, placebo-controlled, phase 3 trial. Neurology. 2014;83(4):328-335.
LeWitt PA, Hauser RA, Grosset DG, et al. A randomized trial of inhaled levodopa (CVT-301) for motor fluctuations in Parkinson’s disease. Mov Disord. 2016;31(9):1356-1365.
Pagan F, Hebron M, Valadez EH, et al. Nilotinib effects in Parkinson’s disease and dementia with Lewy bodies. J Parkinsons Dis. 2016;6(3):503-517.
LAS VEGAS—An oral extended-release agent composed of carbidopa–levodopa microbeads that was shown, in clinical studies, to improve symptoms in patients with early or advanced Parkinson’s disease is one of several promising new therapies for this neurodegenerative disorder, according to a presentation at the American Academy of Neurology’s Fall 2016 Conference.
In addition to extended-release carbidopa–levodopa (Rytary), these therapies include drugs recently approved by the FDA for orthostatic hypotension (droxidopa) and psychosis (pimavanserin) in Parkinson’s disease and investigational agents such as an inhaled levodopa (CVT-301) and a leukemia drug (nilotinib) currently in clinical trials for use in patients with Parkinson’s disease. “These are things that patients are going to be talking to you about and that you all can use in taking care of patients with Parkinson’s disease,” said Jeff A. Kraakevik, MD, Associate Professor of Neurology at Oregon Health & Science University in Portland.
Expanding the Armamentarium
Dr. Kraakevik presented data on two new deep brain stimulation systems—Vercise from Boston Scientific, which has not yet been approved by the FDA, and Infinity from St. Jude Medical, which has been FDA approved—and a third surgical device from Medtronic, which is not new, but now has new FDA labeling regarding broader MRI compatibility for limited body scans. He also reported on a randomized trial of focused ultrasound for essential tremor that has led to FDA approval and touched on a clinical trial of a specific gene therapy for Huntington’s disease that is now enrolling patients. However, the bulk of his presentation focused on pharmacologic therapy.
Extended-Release Carbidopa–Levodopa (Rytary)
Citing a 2016 review of cumulative data by Dhall and Kreitzman that included five randomized controlled trials, Dr. Kraakevik indicated that Rytary is a superior formulation of carbidopa–levodopa, compared with a prior controlled-release formulation—largely owing to factors related to gut absorption, such as the slow rate at which the extended-release component of the latter formulation goes through the stomach. “Parkinson’s patients are often constipated, so there is a variable gut transport, as well as a protein competing with the carbidopa–levodopa as it [gets absorbed],” he said. “All those things led to this being a fairly unpredictable [process]. If you talk to patients about it, they usually are rather unhappy with the controlled-release formulation, especially as the disease progresses.”
The extended-release formulation of Rytary can entail what essentially amounts to a dosing-related trial period for some patients started on it; but, in Dr. Kraakevik’s experience, patients who stick it out usually are glad that they did. For one, the combination of immediate-release and sustained-release beads in each capsule makes the formulation much more predictable than controlled release. “The patients I’ve been able to get through the transition period have really, really liked it,” he said. “We tell people we are going to have … maybe at least a couple of weeks to a month of trying to figure out what the best dose of Rytary is.”
He also commented on the high cost of the drug. “The company has a support desk that patients can call; but, even with that, every time I’ve had to do a dose adjustment and change the pill I’ve had to do another prior authorization, which then sets it back,” said Dr. Kraakevik. “That also has been a barrier for some of my patients to get started on Rytary. That being said, especially for later-onset … motor fluctuations, it is a very good formulation.”
Droxidopa (Northera)
In terms of treating orthostatic hypotension in Parkinson’s disease, prior agents include midodrine, fludrocortisone, and pyridostigmine. They all work relatively well, according to Dr. Kraakevik; however, if one looks at the studies on midodrine, for example, the data are somewhat equivocal. “It is nice that we have this additional therapeutic in our armamentarium,” he said of droxidopa (Northera). “It is a precursor to norepinephrine, so it has a different mechanism of action than [any] of the medications we have to treat orthostasis.”
Symptom and symptom-impact composite scores from the trial that resulted in FDA approval for droxidopa show a statistically significant difference versus placebo. In his practice, Dr. Kraakevik talks to his patients a lot more about whether they are fainting or feeling lightheaded and a lot less about listing all their orthostatic blood pressure readings over the previous several weeks. Most of his patients have been seeing primary care physicians who, for years, have been telling them that their blood pressure should not exceed 140/90 and that, if it does, they should call their doctor and, if it is more than 180 or 185, they should go to the emergency room.
“I get a lot of phone calls because these medications are going to make these people have blood pressures that are going to be above those ranges,” said Dr. Kraakevik. “It is probably going to be up there for only about five, 10 minutes, and then it is going to come back down. So that is why I do not tell people to focus on the numbers, but more on symptoms. You can see [in the aforementioned trial] that for dizziness/lightheadedness, visual disturbances … [droxidopa] has a nice effect. Looking at symptoms [such as] when they are standing for a long time, standing for a short time, all these things—except for walking for a long time—symptoms were significantly reduced with use of droxidopa.”
Pimavanserin (Nuplazid)
Pimavanserin (Nuplazid) has recently been FDA approved for treatment of hallucinations in Parkinson’s disease. It is a 5-HT2A inverse agonist. Pimavanserin blocks the serotonergic receptor and reduces the stimulation effect, causing a decrease in the ability to create hallucinations to the striatal limit. In a 2014 trial, pimavanserin was shown to significantly decrease hallucinations over 43 days. “It is an effective medication … not much in the way of side effects at this point,” said Dr. Kraakevik. “It does have that QT prolongation possibility, so you need to be a little careful in patients who have some cardiac abnormalities or rhythm abnormalities when you use this medication. But otherwise it is relatively well tolerated.”
Therapeutics on the Horizon
Medications in the pipeline include an inhaled carbidopa–levodopa agent (CVT-301). In a randomized controlled trial of 86 subjects, investigators found it to be effective in rapidly reversing “off” symptoms, Dr. Kraakevik reported. “Most of the patients who were in this trial had reversal of their symptoms within about 10 minutes,” he said, adding that CVT-301 should be in phase III studies soon and coming up for approval within the next two years.
Nilotinib was approved by the FDA in 2010 as a treatment for adult patients with a form of chronic myeloid leukemia. Given its mechanism of action as a tyrosine kinase inhibitor, researchers hypothesize that it could be applicable to Parkinson’s disease. In a phase I study involving 12 subjects, investigators found that all of the patients who received nilotinib had a reversible decrease in their Unified Parkinson’s Disease Rating Scale score while also scoring “much better on their Mini-Mental State Examination,” according to Dr. Kraakevik.
“It reversed when the medication was taken away,” he emphasized. “This led to a lot of press and a lot of questions in my clinic, where we are asked … why is not every [therapy] able to do this? And my response to patients is, ‘Right now we have 12 people in an open-label study. We need more data.’”
Such data could come from a double-blind, placebo-controlled clinical trial currently in the planning stages, “but there have allegedly been some disagreements between the key entities about the best way to proceed, and this has slowed the process significantly.”
—Fred Balzac
Suggested Reading
Cummings J, Isaacson S, Mills R, et al. Pimavanserin for patients with Parkinson’s disease psychosis: a randomised, placebo-controlled phase 3 trial. Lancet. 2014;383(9916):533-540.
Dhall R, Kreitzman DL. Advances in levodopa therapy for Parkinson disease: review of RYTARY (carbidopa and levodopa) clinical efficacy and safety. Neurology. 2016;86(14 suppl 1):S13-S24.
Fox SH. Pimavanserin as treatment for Parkinson’s disease psychosis. Lancet. 2014;383(9916):494-496.
Kaufmann H, Freeman R, Biaggioni I, et al for the NOH301 Investigators. Droxidopa for neurogenic orthostatic hypotension: a randomized, placebo-controlled, phase 3 trial. Neurology. 2014;83(4):328-335.
LeWitt PA, Hauser RA, Grosset DG, et al. A randomized trial of inhaled levodopa (CVT-301) for motor fluctuations in Parkinson’s disease. Mov Disord. 2016;31(9):1356-1365.
Pagan F, Hebron M, Valadez EH, et al. Nilotinib effects in Parkinson’s disease and dementia with Lewy bodies. J Parkinsons Dis. 2016;6(3):503-517.
Age and Stroke Volume May Predict Poststroke Epilepsy
HOUSTON—Younger age and greater stroke volume are risk factors for the development of poststroke epilepsy, according to research presented at the 70th Annual Meeting of the American Epilepsy Society.
Beate Diehl, MD, PhD, neurologist and clinical neurophysiologist at University College London, and colleagues examined information from the Predicting Language Outcome and Recovery After Stroke (PLORAS) database to ascertain the frequency of poststroke epilepsy and compare lesion characteristics between people with and without poststroke epilepsy. The database includes T1-weighted whole brain MRIs acquired with a 3-T scanner.
The investigators identified 369 patients with left-hemisphere strokes, and 42 of them (11.4%) had poststroke epilepsy. Of 81 patients with right-hemisphere strokes, nine (11.1%) had poststroke epilepsy. Gender, handedness, and stroke etiology were similar between patients with and without poststroke epilepsy.
Patients with poststroke epilepsy were significantly younger than those without poststroke epilepsy, however (44 vs 56). In addition, patients with poststroke epilepsy had significantly larger lesions than patients without poststroke epilepsy (148 cm3 vs 73 cm3). Large lesions are more likely to damage deep white matter, said Dr. Diehl.
The most consistent lesion sites among all patients with poststroke epilepsy included the basal ganglia (ie, globus pallidus and caudate nucleus) and most nuclei of the thalamus (ie, anterior and ventral nuclei and posterior regions, including pulvinar). Damage to these regions occurred in 27 (64%) of patients with left-hemisphere stroke and poststroke epilepsy. Furthermore, 55 (17%) patients with left-hemisphere stroke without poststroke epilepsy also had damage in the same regions. Damage to these regions thus was associated with poststroke epilepsy in 27 of 82 (33%) patients.
“Many physicians treating stroke patients do not realize that falls, episodes of confusion, and loss of consciousness may be signs of poststroke epilepsy,” said Dr. Diehl. “Poststroke epileptic seizures can negatively affect stroke recovery and rehabilitation.”
—Erik Greb
HOUSTON—Younger age and greater stroke volume are risk factors for the development of poststroke epilepsy, according to research presented at the 70th Annual Meeting of the American Epilepsy Society.
Beate Diehl, MD, PhD, neurologist and clinical neurophysiologist at University College London, and colleagues examined information from the Predicting Language Outcome and Recovery After Stroke (PLORAS) database to ascertain the frequency of poststroke epilepsy and compare lesion characteristics between people with and without poststroke epilepsy. The database includes T1-weighted whole brain MRIs acquired with a 3-T scanner.
The investigators identified 369 patients with left-hemisphere strokes, and 42 of them (11.4%) had poststroke epilepsy. Of 81 patients with right-hemisphere strokes, nine (11.1%) had poststroke epilepsy. Gender, handedness, and stroke etiology were similar between patients with and without poststroke epilepsy.
Patients with poststroke epilepsy were significantly younger than those without poststroke epilepsy, however (44 vs 56). In addition, patients with poststroke epilepsy had significantly larger lesions than patients without poststroke epilepsy (148 cm3 vs 73 cm3). Large lesions are more likely to damage deep white matter, said Dr. Diehl.
The most consistent lesion sites among all patients with poststroke epilepsy included the basal ganglia (ie, globus pallidus and caudate nucleus) and most nuclei of the thalamus (ie, anterior and ventral nuclei and posterior regions, including pulvinar). Damage to these regions occurred in 27 (64%) of patients with left-hemisphere stroke and poststroke epilepsy. Furthermore, 55 (17%) patients with left-hemisphere stroke without poststroke epilepsy also had damage in the same regions. Damage to these regions thus was associated with poststroke epilepsy in 27 of 82 (33%) patients.
“Many physicians treating stroke patients do not realize that falls, episodes of confusion, and loss of consciousness may be signs of poststroke epilepsy,” said Dr. Diehl. “Poststroke epileptic seizures can negatively affect stroke recovery and rehabilitation.”
—Erik Greb
HOUSTON—Younger age and greater stroke volume are risk factors for the development of poststroke epilepsy, according to research presented at the 70th Annual Meeting of the American Epilepsy Society.
Beate Diehl, MD, PhD, neurologist and clinical neurophysiologist at University College London, and colleagues examined information from the Predicting Language Outcome and Recovery After Stroke (PLORAS) database to ascertain the frequency of poststroke epilepsy and compare lesion characteristics between people with and without poststroke epilepsy. The database includes T1-weighted whole brain MRIs acquired with a 3-T scanner.
The investigators identified 369 patients with left-hemisphere strokes, and 42 of them (11.4%) had poststroke epilepsy. Of 81 patients with right-hemisphere strokes, nine (11.1%) had poststroke epilepsy. Gender, handedness, and stroke etiology were similar between patients with and without poststroke epilepsy.
Patients with poststroke epilepsy were significantly younger than those without poststroke epilepsy, however (44 vs 56). In addition, patients with poststroke epilepsy had significantly larger lesions than patients without poststroke epilepsy (148 cm3 vs 73 cm3). Large lesions are more likely to damage deep white matter, said Dr. Diehl.
The most consistent lesion sites among all patients with poststroke epilepsy included the basal ganglia (ie, globus pallidus and caudate nucleus) and most nuclei of the thalamus (ie, anterior and ventral nuclei and posterior regions, including pulvinar). Damage to these regions occurred in 27 (64%) of patients with left-hemisphere stroke and poststroke epilepsy. Furthermore, 55 (17%) patients with left-hemisphere stroke without poststroke epilepsy also had damage in the same regions. Damage to these regions thus was associated with poststroke epilepsy in 27 of 82 (33%) patients.
“Many physicians treating stroke patients do not realize that falls, episodes of confusion, and loss of consciousness may be signs of poststroke epilepsy,” said Dr. Diehl. “Poststroke epileptic seizures can negatively affect stroke recovery and rehabilitation.”
—Erik Greb
Epilepsy Armamentarium Continues to Grow
LAS VEGAS—Several newer antiepileptic drugs (AEDs) are, along with new surgical options and nontraditional interventions such as medical marijuana, among a growing number of epilepsy treatments offering the potential for increased efficacy and improved patient care. However, subpopulations such as pregnant women pose special challenges for treatment selection, and the large number of patients who remain refractory to any epilepsy therapy represents perhaps the greatest challenge of all. Of the estimated three million people in the United States who have epilepsy, approximately one-third have uncontrolled seizures because no available treatment works for them, said Tracey A. Milligan, MD, at the American Academy of Neurology’s Fall 2016 Conference. Dr. Milligan is Vice Chair for Education in the Department of Neurology at Brigham and Women’s Hospital and Assistant Professor of Neurology at Harvard Medical School in Boston.
Illustrating the challenges of trying to achieve more widespread seizure control, Dr. Milligan cited a study of new-onset epilepsy by Brodie and colleagues, who found that 37% of patients had their seizures initially controlled, 22% had them eventually controlled, 16% had fluctuating control, and 25% had seizures that were refractory to treatment. This breakdown resonated with Dr. Milligan in terms of what she sees in her own practice—starting with the patients whose seizures are initially controlled. “They start a medication, they do well, they go on to live very full, active lives,” she said. “For the other 22%, we need to spend a little more time adjusting medications. Maybe they are adjusting lifestyle factors, but they do get to full control as well.”
Patients in the refractory group should be referred to epilepsy treatment centers, where surgical options can be considered. It is patients with fluctuating seizure control whom Dr. Milligan finds particularly interesting. “This is our sort of ‘relapsing-remitting’ epilepsy,” she said. “You start an agent, you think you have the seizures under control, and the drug works for a little while. Then the patient starts having seizures again…. These patients also need to be referred to an epilepsy treatment center to … be worked up and think about surgery if it is drug-resistant epilepsy.”
Questions for AED Selection
Clinical decisions such as the choice of an AED should be informed by the latest guidelines. Citing a 2015 report on unprovoked first seizures in adults, Dr. Milligan said that after one unprovoked seizure, there is a 21% to 45% risk of another seizure in two years. In her view, this new guideline raises more questions than it provides answers—like the new International League Against Epilepsy definition of epilepsy, in which one unprovoked seizure (rather than the previously required two) can be enough to support a diagnosis of epilepsy.
“How do we know which [patients] to diagnose with epilepsy after a single seizure?” she asked. “How do we know which patients are at highest risk of having a second seizure and we’re going to start with an AED?.... At this point we do not have an agreed-upon and reliable formula where we can plug in the demographics and the factors of the seizure and calculate the risk.”
In lieu of such information, clinicians need to ask several questions when selecting an AED, including which epilepsy syndrome the patient has, which medicines work best with it, and which patient factors apply. Dr. Milligan discussed four newer AEDS that are clinically available—clobazam, perampanel, eslicarbazepine, and brivaracetam. “These, for the most part, have been in trials involving patients with refractory seizures and all have a similar efficacy as add-on therapy.”
Onfi (Clobazam)
A benzodiazepine that offers fewer tolerance/sedation issues than others in its class, clobazam has been used since the 1970s as an anxiolytic/anticonvulsant. In 2011, Onfi (clobazam) was approved by the FDA for adjunctive treatment of seizures associated with Lennox-Gastaut syndrome in patients age 2 and older. Adverse effects include dizziness, somnolence, restlessness, and aggression. “It is a nice option when you are thinking about a benzodiazepine, because it does not have the same sort of tolerance/dependence [issues] as the other benzodiazepines do with epilepsy,” said Dr. Milligan.
Fycompa (Perampanel)
Fycompa (perampanel) is a noncompetitive AMPA-receptor inhibitor with a long half-life (70 to 100 hours). It is indicated for adjunctive treatment of focal epilepsy and primary generalized tonic-clonic seizures in patients age 12 and older. Side effects include dizziness, somnolence, and rash. The drug’s labeling includes a black-box warning for an occurrence Dr. Milligan described as unusual but important to be aware of: “Very interesting conversations you have with your patients when you prescribe this—where you have to say, ‘You may develop homicidal ideation on this medication. If you start to feel like killing somebody, please let me know right away.’”
Aptiom (Eslicarbazepine)
Approved by the FDA in 2013 for partial epilepsy (monotherapy or adjunctive treatment), Aptiom (eslicarbazepine) is a sodium-channel blocker whose longer half-life (20 hours) may help with peak effects and reduce hyponatremia and rash. It has few drug interactions and can be used with carbamazepine, though not with oxcarbazepine. Although the list of its most common side effects is long—dizziness, somnolence, nausea, headache, diplopia, vomiting, fatigue, vertigo, ataxia, blurred vision, and tremor—it “probably has fewer adverse effects associated with it [overall] than other AEDs do.”
Briviact (Brivaracetam)
The newest agent, Briviact (brivaracetam), was approved in February 2016 as add-on therapy for refractory partial seizures in patients age 16 and older. Clinical trial results showed adverse effects such as somnolence, headache, dizziness, and fatigue to be mild and similar to what control patients experienced on placebo, but with a higher incidence of psychiatric/cognitive problems versus placebo. It is 20 times more potent at the SV2A receptor than levetiracetam, a related agent. “Unlike levetiracetam, it does [affect] oral contraceptives at a higher dose,” said Dr. Milligan, adding that she is inclined to use it after she has “tried tried agents that have been around longer”—at least until more data are in.
Marijuana, Pregnancy, and Depression
Dr. Milligan devoted the rest of her lecture to pregnancy, depression, and “the thing that everybody asks us about”—marijuana. She cited an open-label interventional trial by Devinsky and colleagues that found that cannabidiol might reduce seizure frequency and have an adequate safety profile in children and young adults with highly treatment-resistant epilepsy. “We do not have many randomized placebo-controlled trials at this point,” she said. “[Cannabidiol] probably works well for some syndromes but it does have adverse effects.”
Regarding pregnancy, a study by MacDonald and colleagues that examined complications in women giving birth showed a 10-times higher risk of death in women with epilepsy, along with a greater rate of every complication the investigators assessed. Other risks are associated with AED therapy. “We know that there are risks of seizures during pregnancy, and these are important to control,” said Dr. Milligan. “There are also risks that come along with taking certain AEDs—neural tube defects, small for gestational age, cleft lip and palate, and autism.”
One drug associated with cleft lip/palate and small size for gestational age is topiramate (FDA pregnancy category D). “Being a baby with small for gestational age carries an increased risk of obesity, cardiovascular disease, diabetes, and cognition [problems]—so it is important for us to be aware of [this fact] when we use topiramate,” said Dr. Milligan, who also cited findings from the North American Pregnancy Registry showing lamotrigine and levetiracetam to be the safest among AEDs assessed.
Depression is present in more than 33% of patients with epilepsy and entails a three- to four-times greater risk of suicide. Sudden death is 27 times more likely in patients with epilepsy than in the general population. “Seizures kill—this is something people are not aware of,” Dr. Milligan emphasized. “Depression is very prevalent, but there is also this high risk of death that goes along with epilepsy that is not just sudden unexplained death in epilepsy [SUDEP] but accidental death, assault….
“Mortality risk and years of potential life lost from epilepsy are second only to stroke. We need to think about education. For example, most patients who are found dead after a presumed seizure or from SUDEP are in a prone position. Maybe we should have for those refractory patients [something] like the sudden infant death syndrome campaign for babies to sleep on their backs, and not prone. It’s something to think about.”
—Fred Balzac
Suggested Reading
Brodie MJ, Barry SJ, Bamagous GA, et al. Patterns of treatment response in newly diagnosed epilepsy. Neurology. 2012;78(20):1548-1554.
Devinsky O, Marsh E, Friedman D, et al. Cannabidiol in patients with treatment-resistant epilepsy: an open-label interventional trial. Lancet Neurol. 2016;15(3):270-278.
Devinsky O, Spruill T, Thurman D, Friedman D. Recognizing and preventing epilepsy-related mortality: a call for action. Neurology. 2016;86(8):779-786.
Hernandez-Diaz S. Evidence accumulates on the association between topiramate use early in pregnancy and the risk of oral clefts. Pharmacoepidemiol Drug Saf. 2014;23(10):1026-1028.
Krumholz A, Shinnar S, French J, et al. Evidence-based guideline: management of an unprovoked first seizure in adults: Report of the Guideline Development Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology. 2015;85(17):1526-1527.
Kwan P, Arzimanoglou A, Berg AT, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2010;51(6):1069-1077.
MacDonald SC, Bateman BT, McElrath TF, et al. Mortality and morbidity during delivery hospitalization among pregnant women with epilepsy in the United States. JAMA Neurol. 2015;72(9):981-988.
Martin RC, Faught E, Richman J, et al. Psychiatric and neurologic risk factors for incident cases of new-onset epilepsy in older adults: data from U.S. Medicare beneficiaries. Epilepsia. 2014;55(7):1120-1127.
Pennell PB. Use of antiepileptic drugs during pregnancy: evolving concepts. Neurotherapeutics. 2016;13(4):811-820.
Vajda FJ, O’Brien TJ, Lander CM, et al. The teratogenicity of the newer antiepileptic drugs - an update. Acta Neurol Scand. 2014;130(4):234-238.
LAS VEGAS—Several newer antiepileptic drugs (AEDs) are, along with new surgical options and nontraditional interventions such as medical marijuana, among a growing number of epilepsy treatments offering the potential for increased efficacy and improved patient care. However, subpopulations such as pregnant women pose special challenges for treatment selection, and the large number of patients who remain refractory to any epilepsy therapy represents perhaps the greatest challenge of all. Of the estimated three million people in the United States who have epilepsy, approximately one-third have uncontrolled seizures because no available treatment works for them, said Tracey A. Milligan, MD, at the American Academy of Neurology’s Fall 2016 Conference. Dr. Milligan is Vice Chair for Education in the Department of Neurology at Brigham and Women’s Hospital and Assistant Professor of Neurology at Harvard Medical School in Boston.
Illustrating the challenges of trying to achieve more widespread seizure control, Dr. Milligan cited a study of new-onset epilepsy by Brodie and colleagues, who found that 37% of patients had their seizures initially controlled, 22% had them eventually controlled, 16% had fluctuating control, and 25% had seizures that were refractory to treatment. This breakdown resonated with Dr. Milligan in terms of what she sees in her own practice—starting with the patients whose seizures are initially controlled. “They start a medication, they do well, they go on to live very full, active lives,” she said. “For the other 22%, we need to spend a little more time adjusting medications. Maybe they are adjusting lifestyle factors, but they do get to full control as well.”
Patients in the refractory group should be referred to epilepsy treatment centers, where surgical options can be considered. It is patients with fluctuating seizure control whom Dr. Milligan finds particularly interesting. “This is our sort of ‘relapsing-remitting’ epilepsy,” she said. “You start an agent, you think you have the seizures under control, and the drug works for a little while. Then the patient starts having seizures again…. These patients also need to be referred to an epilepsy treatment center to … be worked up and think about surgery if it is drug-resistant epilepsy.”
Questions for AED Selection
Clinical decisions such as the choice of an AED should be informed by the latest guidelines. Citing a 2015 report on unprovoked first seizures in adults, Dr. Milligan said that after one unprovoked seizure, there is a 21% to 45% risk of another seizure in two years. In her view, this new guideline raises more questions than it provides answers—like the new International League Against Epilepsy definition of epilepsy, in which one unprovoked seizure (rather than the previously required two) can be enough to support a diagnosis of epilepsy.
“How do we know which [patients] to diagnose with epilepsy after a single seizure?” she asked. “How do we know which patients are at highest risk of having a second seizure and we’re going to start with an AED?.... At this point we do not have an agreed-upon and reliable formula where we can plug in the demographics and the factors of the seizure and calculate the risk.”
In lieu of such information, clinicians need to ask several questions when selecting an AED, including which epilepsy syndrome the patient has, which medicines work best with it, and which patient factors apply. Dr. Milligan discussed four newer AEDS that are clinically available—clobazam, perampanel, eslicarbazepine, and brivaracetam. “These, for the most part, have been in trials involving patients with refractory seizures and all have a similar efficacy as add-on therapy.”
Onfi (Clobazam)
A benzodiazepine that offers fewer tolerance/sedation issues than others in its class, clobazam has been used since the 1970s as an anxiolytic/anticonvulsant. In 2011, Onfi (clobazam) was approved by the FDA for adjunctive treatment of seizures associated with Lennox-Gastaut syndrome in patients age 2 and older. Adverse effects include dizziness, somnolence, restlessness, and aggression. “It is a nice option when you are thinking about a benzodiazepine, because it does not have the same sort of tolerance/dependence [issues] as the other benzodiazepines do with epilepsy,” said Dr. Milligan.
Fycompa (Perampanel)
Fycompa (perampanel) is a noncompetitive AMPA-receptor inhibitor with a long half-life (70 to 100 hours). It is indicated for adjunctive treatment of focal epilepsy and primary generalized tonic-clonic seizures in patients age 12 and older. Side effects include dizziness, somnolence, and rash. The drug’s labeling includes a black-box warning for an occurrence Dr. Milligan described as unusual but important to be aware of: “Very interesting conversations you have with your patients when you prescribe this—where you have to say, ‘You may develop homicidal ideation on this medication. If you start to feel like killing somebody, please let me know right away.’”
Aptiom (Eslicarbazepine)
Approved by the FDA in 2013 for partial epilepsy (monotherapy or adjunctive treatment), Aptiom (eslicarbazepine) is a sodium-channel blocker whose longer half-life (20 hours) may help with peak effects and reduce hyponatremia and rash. It has few drug interactions and can be used with carbamazepine, though not with oxcarbazepine. Although the list of its most common side effects is long—dizziness, somnolence, nausea, headache, diplopia, vomiting, fatigue, vertigo, ataxia, blurred vision, and tremor—it “probably has fewer adverse effects associated with it [overall] than other AEDs do.”
Briviact (Brivaracetam)
The newest agent, Briviact (brivaracetam), was approved in February 2016 as add-on therapy for refractory partial seizures in patients age 16 and older. Clinical trial results showed adverse effects such as somnolence, headache, dizziness, and fatigue to be mild and similar to what control patients experienced on placebo, but with a higher incidence of psychiatric/cognitive problems versus placebo. It is 20 times more potent at the SV2A receptor than levetiracetam, a related agent. “Unlike levetiracetam, it does [affect] oral contraceptives at a higher dose,” said Dr. Milligan, adding that she is inclined to use it after she has “tried tried agents that have been around longer”—at least until more data are in.
Marijuana, Pregnancy, and Depression
Dr. Milligan devoted the rest of her lecture to pregnancy, depression, and “the thing that everybody asks us about”—marijuana. She cited an open-label interventional trial by Devinsky and colleagues that found that cannabidiol might reduce seizure frequency and have an adequate safety profile in children and young adults with highly treatment-resistant epilepsy. “We do not have many randomized placebo-controlled trials at this point,” she said. “[Cannabidiol] probably works well for some syndromes but it does have adverse effects.”
Regarding pregnancy, a study by MacDonald and colleagues that examined complications in women giving birth showed a 10-times higher risk of death in women with epilepsy, along with a greater rate of every complication the investigators assessed. Other risks are associated with AED therapy. “We know that there are risks of seizures during pregnancy, and these are important to control,” said Dr. Milligan. “There are also risks that come along with taking certain AEDs—neural tube defects, small for gestational age, cleft lip and palate, and autism.”
One drug associated with cleft lip/palate and small size for gestational age is topiramate (FDA pregnancy category D). “Being a baby with small for gestational age carries an increased risk of obesity, cardiovascular disease, diabetes, and cognition [problems]—so it is important for us to be aware of [this fact] when we use topiramate,” said Dr. Milligan, who also cited findings from the North American Pregnancy Registry showing lamotrigine and levetiracetam to be the safest among AEDs assessed.
Depression is present in more than 33% of patients with epilepsy and entails a three- to four-times greater risk of suicide. Sudden death is 27 times more likely in patients with epilepsy than in the general population. “Seizures kill—this is something people are not aware of,” Dr. Milligan emphasized. “Depression is very prevalent, but there is also this high risk of death that goes along with epilepsy that is not just sudden unexplained death in epilepsy [SUDEP] but accidental death, assault….
“Mortality risk and years of potential life lost from epilepsy are second only to stroke. We need to think about education. For example, most patients who are found dead after a presumed seizure or from SUDEP are in a prone position. Maybe we should have for those refractory patients [something] like the sudden infant death syndrome campaign for babies to sleep on their backs, and not prone. It’s something to think about.”
—Fred Balzac
Suggested Reading
Brodie MJ, Barry SJ, Bamagous GA, et al. Patterns of treatment response in newly diagnosed epilepsy. Neurology. 2012;78(20):1548-1554.
Devinsky O, Marsh E, Friedman D, et al. Cannabidiol in patients with treatment-resistant epilepsy: an open-label interventional trial. Lancet Neurol. 2016;15(3):270-278.
Devinsky O, Spruill T, Thurman D, Friedman D. Recognizing and preventing epilepsy-related mortality: a call for action. Neurology. 2016;86(8):779-786.
Hernandez-Diaz S. Evidence accumulates on the association between topiramate use early in pregnancy and the risk of oral clefts. Pharmacoepidemiol Drug Saf. 2014;23(10):1026-1028.
Krumholz A, Shinnar S, French J, et al. Evidence-based guideline: management of an unprovoked first seizure in adults: Report of the Guideline Development Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology. 2015;85(17):1526-1527.
Kwan P, Arzimanoglou A, Berg AT, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2010;51(6):1069-1077.
MacDonald SC, Bateman BT, McElrath TF, et al. Mortality and morbidity during delivery hospitalization among pregnant women with epilepsy in the United States. JAMA Neurol. 2015;72(9):981-988.
Martin RC, Faught E, Richman J, et al. Psychiatric and neurologic risk factors for incident cases of new-onset epilepsy in older adults: data from U.S. Medicare beneficiaries. Epilepsia. 2014;55(7):1120-1127.
Pennell PB. Use of antiepileptic drugs during pregnancy: evolving concepts. Neurotherapeutics. 2016;13(4):811-820.
Vajda FJ, O’Brien TJ, Lander CM, et al. The teratogenicity of the newer antiepileptic drugs - an update. Acta Neurol Scand. 2014;130(4):234-238.
LAS VEGAS—Several newer antiepileptic drugs (AEDs) are, along with new surgical options and nontraditional interventions such as medical marijuana, among a growing number of epilepsy treatments offering the potential for increased efficacy and improved patient care. However, subpopulations such as pregnant women pose special challenges for treatment selection, and the large number of patients who remain refractory to any epilepsy therapy represents perhaps the greatest challenge of all. Of the estimated three million people in the United States who have epilepsy, approximately one-third have uncontrolled seizures because no available treatment works for them, said Tracey A. Milligan, MD, at the American Academy of Neurology’s Fall 2016 Conference. Dr. Milligan is Vice Chair for Education in the Department of Neurology at Brigham and Women’s Hospital and Assistant Professor of Neurology at Harvard Medical School in Boston.
Illustrating the challenges of trying to achieve more widespread seizure control, Dr. Milligan cited a study of new-onset epilepsy by Brodie and colleagues, who found that 37% of patients had their seizures initially controlled, 22% had them eventually controlled, 16% had fluctuating control, and 25% had seizures that were refractory to treatment. This breakdown resonated with Dr. Milligan in terms of what she sees in her own practice—starting with the patients whose seizures are initially controlled. “They start a medication, they do well, they go on to live very full, active lives,” she said. “For the other 22%, we need to spend a little more time adjusting medications. Maybe they are adjusting lifestyle factors, but they do get to full control as well.”
Patients in the refractory group should be referred to epilepsy treatment centers, where surgical options can be considered. It is patients with fluctuating seizure control whom Dr. Milligan finds particularly interesting. “This is our sort of ‘relapsing-remitting’ epilepsy,” she said. “You start an agent, you think you have the seizures under control, and the drug works for a little while. Then the patient starts having seizures again…. These patients also need to be referred to an epilepsy treatment center to … be worked up and think about surgery if it is drug-resistant epilepsy.”
Questions for AED Selection
Clinical decisions such as the choice of an AED should be informed by the latest guidelines. Citing a 2015 report on unprovoked first seizures in adults, Dr. Milligan said that after one unprovoked seizure, there is a 21% to 45% risk of another seizure in two years. In her view, this new guideline raises more questions than it provides answers—like the new International League Against Epilepsy definition of epilepsy, in which one unprovoked seizure (rather than the previously required two) can be enough to support a diagnosis of epilepsy.
“How do we know which [patients] to diagnose with epilepsy after a single seizure?” she asked. “How do we know which patients are at highest risk of having a second seizure and we’re going to start with an AED?.... At this point we do not have an agreed-upon and reliable formula where we can plug in the demographics and the factors of the seizure and calculate the risk.”
In lieu of such information, clinicians need to ask several questions when selecting an AED, including which epilepsy syndrome the patient has, which medicines work best with it, and which patient factors apply. Dr. Milligan discussed four newer AEDS that are clinically available—clobazam, perampanel, eslicarbazepine, and brivaracetam. “These, for the most part, have been in trials involving patients with refractory seizures and all have a similar efficacy as add-on therapy.”
Onfi (Clobazam)
A benzodiazepine that offers fewer tolerance/sedation issues than others in its class, clobazam has been used since the 1970s as an anxiolytic/anticonvulsant. In 2011, Onfi (clobazam) was approved by the FDA for adjunctive treatment of seizures associated with Lennox-Gastaut syndrome in patients age 2 and older. Adverse effects include dizziness, somnolence, restlessness, and aggression. “It is a nice option when you are thinking about a benzodiazepine, because it does not have the same sort of tolerance/dependence [issues] as the other benzodiazepines do with epilepsy,” said Dr. Milligan.
Fycompa (Perampanel)
Fycompa (perampanel) is a noncompetitive AMPA-receptor inhibitor with a long half-life (70 to 100 hours). It is indicated for adjunctive treatment of focal epilepsy and primary generalized tonic-clonic seizures in patients age 12 and older. Side effects include dizziness, somnolence, and rash. The drug’s labeling includes a black-box warning for an occurrence Dr. Milligan described as unusual but important to be aware of: “Very interesting conversations you have with your patients when you prescribe this—where you have to say, ‘You may develop homicidal ideation on this medication. If you start to feel like killing somebody, please let me know right away.’”
Aptiom (Eslicarbazepine)
Approved by the FDA in 2013 for partial epilepsy (monotherapy or adjunctive treatment), Aptiom (eslicarbazepine) is a sodium-channel blocker whose longer half-life (20 hours) may help with peak effects and reduce hyponatremia and rash. It has few drug interactions and can be used with carbamazepine, though not with oxcarbazepine. Although the list of its most common side effects is long—dizziness, somnolence, nausea, headache, diplopia, vomiting, fatigue, vertigo, ataxia, blurred vision, and tremor—it “probably has fewer adverse effects associated with it [overall] than other AEDs do.”
Briviact (Brivaracetam)
The newest agent, Briviact (brivaracetam), was approved in February 2016 as add-on therapy for refractory partial seizures in patients age 16 and older. Clinical trial results showed adverse effects such as somnolence, headache, dizziness, and fatigue to be mild and similar to what control patients experienced on placebo, but with a higher incidence of psychiatric/cognitive problems versus placebo. It is 20 times more potent at the SV2A receptor than levetiracetam, a related agent. “Unlike levetiracetam, it does [affect] oral contraceptives at a higher dose,” said Dr. Milligan, adding that she is inclined to use it after she has “tried tried agents that have been around longer”—at least until more data are in.
Marijuana, Pregnancy, and Depression
Dr. Milligan devoted the rest of her lecture to pregnancy, depression, and “the thing that everybody asks us about”—marijuana. She cited an open-label interventional trial by Devinsky and colleagues that found that cannabidiol might reduce seizure frequency and have an adequate safety profile in children and young adults with highly treatment-resistant epilepsy. “We do not have many randomized placebo-controlled trials at this point,” she said. “[Cannabidiol] probably works well for some syndromes but it does have adverse effects.”
Regarding pregnancy, a study by MacDonald and colleagues that examined complications in women giving birth showed a 10-times higher risk of death in women with epilepsy, along with a greater rate of every complication the investigators assessed. Other risks are associated with AED therapy. “We know that there are risks of seizures during pregnancy, and these are important to control,” said Dr. Milligan. “There are also risks that come along with taking certain AEDs—neural tube defects, small for gestational age, cleft lip and palate, and autism.”
One drug associated with cleft lip/palate and small size for gestational age is topiramate (FDA pregnancy category D). “Being a baby with small for gestational age carries an increased risk of obesity, cardiovascular disease, diabetes, and cognition [problems]—so it is important for us to be aware of [this fact] when we use topiramate,” said Dr. Milligan, who also cited findings from the North American Pregnancy Registry showing lamotrigine and levetiracetam to be the safest among AEDs assessed.
Depression is present in more than 33% of patients with epilepsy and entails a three- to four-times greater risk of suicide. Sudden death is 27 times more likely in patients with epilepsy than in the general population. “Seizures kill—this is something people are not aware of,” Dr. Milligan emphasized. “Depression is very prevalent, but there is also this high risk of death that goes along with epilepsy that is not just sudden unexplained death in epilepsy [SUDEP] but accidental death, assault….
“Mortality risk and years of potential life lost from epilepsy are second only to stroke. We need to think about education. For example, most patients who are found dead after a presumed seizure or from SUDEP are in a prone position. Maybe we should have for those refractory patients [something] like the sudden infant death syndrome campaign for babies to sleep on their backs, and not prone. It’s something to think about.”
—Fred Balzac
Suggested Reading
Brodie MJ, Barry SJ, Bamagous GA, et al. Patterns of treatment response in newly diagnosed epilepsy. Neurology. 2012;78(20):1548-1554.
Devinsky O, Marsh E, Friedman D, et al. Cannabidiol in patients with treatment-resistant epilepsy: an open-label interventional trial. Lancet Neurol. 2016;15(3):270-278.
Devinsky O, Spruill T, Thurman D, Friedman D. Recognizing and preventing epilepsy-related mortality: a call for action. Neurology. 2016;86(8):779-786.
Hernandez-Diaz S. Evidence accumulates on the association between topiramate use early in pregnancy and the risk of oral clefts. Pharmacoepidemiol Drug Saf. 2014;23(10):1026-1028.
Krumholz A, Shinnar S, French J, et al. Evidence-based guideline: management of an unprovoked first seizure in adults: Report of the Guideline Development Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology. 2015;85(17):1526-1527.
Kwan P, Arzimanoglou A, Berg AT, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2010;51(6):1069-1077.
MacDonald SC, Bateman BT, McElrath TF, et al. Mortality and morbidity during delivery hospitalization among pregnant women with epilepsy in the United States. JAMA Neurol. 2015;72(9):981-988.
Martin RC, Faught E, Richman J, et al. Psychiatric and neurologic risk factors for incident cases of new-onset epilepsy in older adults: data from U.S. Medicare beneficiaries. Epilepsia. 2014;55(7):1120-1127.
Pennell PB. Use of antiepileptic drugs during pregnancy: evolving concepts. Neurotherapeutics. 2016;13(4):811-820.
Vajda FJ, O’Brien TJ, Lander CM, et al. The teratogenicity of the newer antiepileptic drugs - an update. Acta Neurol Scand. 2014;130(4):234-238.
Combined Erythropoietin and Hypothermia May Provide Neuroprotection in Neonates With Hypoxic-Ischemic Encephalopathy
VANCOUVER—Erythropoietin (EPO) plus therapeutic hypothermia may reduce brain injury and improve one-year motor outcomes in neonates with hypoxic-ischemic encephalopathy (HIE), according to phase II trial results presented at the 45th Annual Meeting of the Child Neurology Society.
A large number of in vitro and in vivo studies have shown that EPO has neuroprotective effects after neonatal HIE. “Acutely, it reduces inflammation and apoptosis and improves cell survival. In the long term, it enhances brain repair through a number of mechanisms,” said Yvonne Wu, MD, MPH, Professor of Neurology and Pediatrics at the University of California, San Francisco.
Hypothermia reduces the risk of death and moderate to severe disability, including cerebral palsy; however, about 40% of infants who are cooled still have adverse outcomes, said Dr. Wu. Studies suggest that multiple doses of EPO provide optimal neuroprotection. In addition, studies in animals have shown that EPO can be neuroprotective even when given up to seven days after the hypoxic-ischemic insult.
To study the effect of EPO and hypothermia combined in neonates with moderate to severe HIE, Dr. Wu and colleagues conducted a randomized, double-blind, placebo-controlled multicenter trial. They evaluated safety, feasibility, and biomarkers of brain injury.
Researchers randomized 50 patients at seven sites to receive either 1,000 U/kg of EPO plus hypothermia or placebo plus hypothermia. Twenty-four babies were randomized to receive EPO and 26 were randomized to receive placebo. Babies received the study drug on five days during the first week of age. Investigators performed MRI on days 4 through 7. They assessed patients’ outcomes at six months and 12 months.
Babies included in the study met standard cooling criteria, including evidence of perinatal distress (eg, Apgar score of less than 5 at 10 minutes). Investigators excluded babies with a genetic disorder, congenital malformation, birth weight less than 1,800 g, microcephaly, or no indwelling line, as well as babies for whom withdrawal of care was considered or who were unlikely to be followed up at 12 months.
To assess neurodevelopmental outcomes at 12 months, researchers administered the Warner Initial Developmental Evaluation (WIDEA), a parental questionnaire that assesses four domains of infant development. In addition, they rated motor function using the Alberta Infant Motor Scale (AIMS). At 12 months, the EPOgroup had a significantly higher AIMS score and a trend toward improvement on the WIDEA score, compared with the placebo group.
Out of 24 infants receiving EPO, 23 had MRI and received three or more doses of treatment. About half of the babies received four to five doses of EPO prior to the MRI.
Researchers scored eight regions of the brain based on the extent of abnormal signal intensity. They found that the babies in the EPO-treated group had a lower median global injury score than those in the placebo group (2 vs 11). In addition, the number of patients with moderate or severe brain MRI abnormalities was lower in the EPO group (one out of 24) versus the placebo group (11 out of 26).
Researchers also noted that EPO appeared to protect the subcortical region of the brain; fewer babies in the EPO-treated group had injury to the deep gray nuclei, compared with babies who received placebo. Two babies died before hospital discharge in the EPO plus hypothermia group, and five died in the hypothermia-alone group. One baby in each group was lost to follow-up at 12 months. No adverse events were considered related to EPO treatment.
“These are small numbers, but our findings raise the possibility that EPO is really doing what we see in animals, which is reducing injury and enhancing repair, so that outcomes are better than expected hypoxic-ischemic encephalopathy,” said Dr. Wu.
—Erica Tricarico
Suggested Reading
Rogers EE, Bonifacio Sl, Glass HC, et al. Erythropoieten and hypothermia for hypoxic-ischemic encephalopathy. Pediatr Neurol. 2014 Aug 24 [Epub ahead of print].
Wu YW, Bauer LA, Ballard RA, et al. Erythropoietin for neuroprotection in neonatal encephalopathy: safety and pharmacokinetics. Pediatrics. 2012 Sep 24 [Epub ahead of print].
Wu YW, Mathur AM, Chang T, et al. High-dose erythropoietin and hypothermia for hypoxic-ischemic encephalopathy: a phase II trial. Pediatrics. 2016;137(6).
VANCOUVER—Erythropoietin (EPO) plus therapeutic hypothermia may reduce brain injury and improve one-year motor outcomes in neonates with hypoxic-ischemic encephalopathy (HIE), according to phase II trial results presented at the 45th Annual Meeting of the Child Neurology Society.
A large number of in vitro and in vivo studies have shown that EPO has neuroprotective effects after neonatal HIE. “Acutely, it reduces inflammation and apoptosis and improves cell survival. In the long term, it enhances brain repair through a number of mechanisms,” said Yvonne Wu, MD, MPH, Professor of Neurology and Pediatrics at the University of California, San Francisco.
Hypothermia reduces the risk of death and moderate to severe disability, including cerebral palsy; however, about 40% of infants who are cooled still have adverse outcomes, said Dr. Wu. Studies suggest that multiple doses of EPO provide optimal neuroprotection. In addition, studies in animals have shown that EPO can be neuroprotective even when given up to seven days after the hypoxic-ischemic insult.
To study the effect of EPO and hypothermia combined in neonates with moderate to severe HIE, Dr. Wu and colleagues conducted a randomized, double-blind, placebo-controlled multicenter trial. They evaluated safety, feasibility, and biomarkers of brain injury.
Researchers randomized 50 patients at seven sites to receive either 1,000 U/kg of EPO plus hypothermia or placebo plus hypothermia. Twenty-four babies were randomized to receive EPO and 26 were randomized to receive placebo. Babies received the study drug on five days during the first week of age. Investigators performed MRI on days 4 through 7. They assessed patients’ outcomes at six months and 12 months.
Babies included in the study met standard cooling criteria, including evidence of perinatal distress (eg, Apgar score of less than 5 at 10 minutes). Investigators excluded babies with a genetic disorder, congenital malformation, birth weight less than 1,800 g, microcephaly, or no indwelling line, as well as babies for whom withdrawal of care was considered or who were unlikely to be followed up at 12 months.
To assess neurodevelopmental outcomes at 12 months, researchers administered the Warner Initial Developmental Evaluation (WIDEA), a parental questionnaire that assesses four domains of infant development. In addition, they rated motor function using the Alberta Infant Motor Scale (AIMS). At 12 months, the EPOgroup had a significantly higher AIMS score and a trend toward improvement on the WIDEA score, compared with the placebo group.
Out of 24 infants receiving EPO, 23 had MRI and received three or more doses of treatment. About half of the babies received four to five doses of EPO prior to the MRI.
Researchers scored eight regions of the brain based on the extent of abnormal signal intensity. They found that the babies in the EPO-treated group had a lower median global injury score than those in the placebo group (2 vs 11). In addition, the number of patients with moderate or severe brain MRI abnormalities was lower in the EPO group (one out of 24) versus the placebo group (11 out of 26).
Researchers also noted that EPO appeared to protect the subcortical region of the brain; fewer babies in the EPO-treated group had injury to the deep gray nuclei, compared with babies who received placebo. Two babies died before hospital discharge in the EPO plus hypothermia group, and five died in the hypothermia-alone group. One baby in each group was lost to follow-up at 12 months. No adverse events were considered related to EPO treatment.
“These are small numbers, but our findings raise the possibility that EPO is really doing what we see in animals, which is reducing injury and enhancing repair, so that outcomes are better than expected hypoxic-ischemic encephalopathy,” said Dr. Wu.
—Erica Tricarico
Suggested Reading
Rogers EE, Bonifacio Sl, Glass HC, et al. Erythropoieten and hypothermia for hypoxic-ischemic encephalopathy. Pediatr Neurol. 2014 Aug 24 [Epub ahead of print].
Wu YW, Bauer LA, Ballard RA, et al. Erythropoietin for neuroprotection in neonatal encephalopathy: safety and pharmacokinetics. Pediatrics. 2012 Sep 24 [Epub ahead of print].
Wu YW, Mathur AM, Chang T, et al. High-dose erythropoietin and hypothermia for hypoxic-ischemic encephalopathy: a phase II trial. Pediatrics. 2016;137(6).
VANCOUVER—Erythropoietin (EPO) plus therapeutic hypothermia may reduce brain injury and improve one-year motor outcomes in neonates with hypoxic-ischemic encephalopathy (HIE), according to phase II trial results presented at the 45th Annual Meeting of the Child Neurology Society.
A large number of in vitro and in vivo studies have shown that EPO has neuroprotective effects after neonatal HIE. “Acutely, it reduces inflammation and apoptosis and improves cell survival. In the long term, it enhances brain repair through a number of mechanisms,” said Yvonne Wu, MD, MPH, Professor of Neurology and Pediatrics at the University of California, San Francisco.
Hypothermia reduces the risk of death and moderate to severe disability, including cerebral palsy; however, about 40% of infants who are cooled still have adverse outcomes, said Dr. Wu. Studies suggest that multiple doses of EPO provide optimal neuroprotection. In addition, studies in animals have shown that EPO can be neuroprotective even when given up to seven days after the hypoxic-ischemic insult.
To study the effect of EPO and hypothermia combined in neonates with moderate to severe HIE, Dr. Wu and colleagues conducted a randomized, double-blind, placebo-controlled multicenter trial. They evaluated safety, feasibility, and biomarkers of brain injury.
Researchers randomized 50 patients at seven sites to receive either 1,000 U/kg of EPO plus hypothermia or placebo plus hypothermia. Twenty-four babies were randomized to receive EPO and 26 were randomized to receive placebo. Babies received the study drug on five days during the first week of age. Investigators performed MRI on days 4 through 7. They assessed patients’ outcomes at six months and 12 months.
Babies included in the study met standard cooling criteria, including evidence of perinatal distress (eg, Apgar score of less than 5 at 10 minutes). Investigators excluded babies with a genetic disorder, congenital malformation, birth weight less than 1,800 g, microcephaly, or no indwelling line, as well as babies for whom withdrawal of care was considered or who were unlikely to be followed up at 12 months.
To assess neurodevelopmental outcomes at 12 months, researchers administered the Warner Initial Developmental Evaluation (WIDEA), a parental questionnaire that assesses four domains of infant development. In addition, they rated motor function using the Alberta Infant Motor Scale (AIMS). At 12 months, the EPOgroup had a significantly higher AIMS score and a trend toward improvement on the WIDEA score, compared with the placebo group.
Out of 24 infants receiving EPO, 23 had MRI and received three or more doses of treatment. About half of the babies received four to five doses of EPO prior to the MRI.
Researchers scored eight regions of the brain based on the extent of abnormal signal intensity. They found that the babies in the EPO-treated group had a lower median global injury score than those in the placebo group (2 vs 11). In addition, the number of patients with moderate or severe brain MRI abnormalities was lower in the EPO group (one out of 24) versus the placebo group (11 out of 26).
Researchers also noted that EPO appeared to protect the subcortical region of the brain; fewer babies in the EPO-treated group had injury to the deep gray nuclei, compared with babies who received placebo. Two babies died before hospital discharge in the EPO plus hypothermia group, and five died in the hypothermia-alone group. One baby in each group was lost to follow-up at 12 months. No adverse events were considered related to EPO treatment.
“These are small numbers, but our findings raise the possibility that EPO is really doing what we see in animals, which is reducing injury and enhancing repair, so that outcomes are better than expected hypoxic-ischemic encephalopathy,” said Dr. Wu.
—Erica Tricarico
Suggested Reading
Rogers EE, Bonifacio Sl, Glass HC, et al. Erythropoieten and hypothermia for hypoxic-ischemic encephalopathy. Pediatr Neurol. 2014 Aug 24 [Epub ahead of print].
Wu YW, Bauer LA, Ballard RA, et al. Erythropoietin for neuroprotection in neonatal encephalopathy: safety and pharmacokinetics. Pediatrics. 2012 Sep 24 [Epub ahead of print].
Wu YW, Mathur AM, Chang T, et al. High-dose erythropoietin and hypothermia for hypoxic-ischemic encephalopathy: a phase II trial. Pediatrics. 2016;137(6).
Diabetes, ischemic heart disease, pain lead health spending
Diabetes, ischemic heart disease, and back and neck pain are the top three conditions accounting for the highest spending on personal health care in the United States, according to a report published online Dec. 27 in JAMA.
In addition, spending on pharmaceuticals – particularly diabetes therapies, antihypertensive drugs, and medications for hyperlipidemia – drove much of the massive increase in health care spending during the past 2 decades, said Joseph L. Dieleman, PhD, of the Institute for Health Metrics and Evaluation, University of Washington, Seattle, and his associates.
Recent increases in health care spending are well documented, but less is known about what is spent for individual conditions, in different health care settings, and in various patient age groups. To assess health care spending across these categories, the investigators collected and analyzed data for 1996 through 2013 from nationally representative surveys of households, nationally representative surveys of medical facilities, insurance claims, government budgets, and other official records.
They grouped the data into six type-of-care categories: inpatient care, ambulatory care, emergency department care, nursing facility care, dental care, and prescribed pharmaceuticals. “Spending on the six types of personal health care was then disaggregated across 155 mutually exclusive and collectively exhaustive conditions and 38 age and sex groups,” with each sex being divided into 5-year age groups, the researchers noted.
Based on these data, the investigators came to the following conclusions:
• Twenty conditions accounted for approximately 58% of personal health care spending, which totaled an estimated $1.2 trillion in 2013.
• More resources were spent on diabetes than any other condition in 2013, at an estimated $101.4 billion. Prescribed medications accounted for nearly 60% of diabetes costs.
• The second-highest amount of health care spending was for ischemic heart disease, which accounted for $88.1 billion in 2013. Most such spending occurred in inpatient settings.
• Low-back and neck pain, comprising the third-highest level of spending, cost an estimated $87.6 billion. Approximately 60% of this spending occurred in ambulatory settings.
• Among all 155 conditions, spending for diabetes and low-back and neck pain increased the most during the 18-year study period.
• Among all six types of care, spending on pharmaceuticals and emergency care increased the most during the study period.
It is important to note that for the purposes of this study, cancer was disaggregated into 29 separate conditions, and none of them placed in the top 20 for health care spending, Dr. Dieleman and his associates noted (JAMA. 2016;316[24]:2627-46. doi: 10.1001/jama.2016.16885).
When spending was categorized by patient age groups, working-age adults accounted for the greatest amount spent in 2013, estimated at $1,070.1 billion. But that was followed closely by patients aged 65 and older, who accounted for an estimated $796.5 billion, much of which was spent on care in nursing facilities. The smallest amount of health care spending was in children over age 1 and adolescents, who accounted for an estimated $233.5 billion.
Among the other study findings:
• Spending on pharmaceutical treatment of two conditions, hypertension and hyperlipidemia, increased at more than double the rate of total health care spending. It totaled an estimated $135.7 billion in 2013.
• Other top-20 conditions included falls, depression, skin disorders such as acne and eczema, sense disorders such as vision correction and hearing loss, dental care, urinary disorders, and lower respiratory tract infection.
This work was supported by the National Institute on Aging and the Vitality Institute. Dr. Dieleman and his associates reported having no relevant financial disclosures.
Dieleman et al. have “followed” the health care money, and the trail could ultimately lead to the United States changing how it spends a staggering, almost unimaginable amount – roughly $3.2 trillion in 2015 – on health care.
At the very least, their data indicate that the United States should pay more attention to managing physical pain, controlling the costs of pharmaceuticals, and promoting lifestyle interventions that prevent or ameliorate obesity and other factors contributing to diabetes and heart disease.
Ezekiel J. Emanuel, MD, is provost of the department of medical ethics and health policy at the Perelman School of Medicine and in the department of health care management at The Wharton School, University of Pennsylvania, Philadelphia. He reported receiving speaking fees from numerous industry sources. Dr. Emanuel made these remarks in an editorial comment accompanying Dr. Dieleman’s report (JAMA 2016;316:2604-6. doi: 10.1001/jama.2016.16739).
Dieleman et al. have “followed” the health care money, and the trail could ultimately lead to the United States changing how it spends a staggering, almost unimaginable amount – roughly $3.2 trillion in 2015 – on health care.
At the very least, their data indicate that the United States should pay more attention to managing physical pain, controlling the costs of pharmaceuticals, and promoting lifestyle interventions that prevent or ameliorate obesity and other factors contributing to diabetes and heart disease.
Ezekiel J. Emanuel, MD, is provost of the department of medical ethics and health policy at the Perelman School of Medicine and in the department of health care management at The Wharton School, University of Pennsylvania, Philadelphia. He reported receiving speaking fees from numerous industry sources. Dr. Emanuel made these remarks in an editorial comment accompanying Dr. Dieleman’s report (JAMA 2016;316:2604-6. doi: 10.1001/jama.2016.16739).
Dieleman et al. have “followed” the health care money, and the trail could ultimately lead to the United States changing how it spends a staggering, almost unimaginable amount – roughly $3.2 trillion in 2015 – on health care.
At the very least, their data indicate that the United States should pay more attention to managing physical pain, controlling the costs of pharmaceuticals, and promoting lifestyle interventions that prevent or ameliorate obesity and other factors contributing to diabetes and heart disease.
Ezekiel J. Emanuel, MD, is provost of the department of medical ethics and health policy at the Perelman School of Medicine and in the department of health care management at The Wharton School, University of Pennsylvania, Philadelphia. He reported receiving speaking fees from numerous industry sources. Dr. Emanuel made these remarks in an editorial comment accompanying Dr. Dieleman’s report (JAMA 2016;316:2604-6. doi: 10.1001/jama.2016.16739).
Diabetes, ischemic heart disease, and back and neck pain are the top three conditions accounting for the highest spending on personal health care in the United States, according to a report published online Dec. 27 in JAMA.
In addition, spending on pharmaceuticals – particularly diabetes therapies, antihypertensive drugs, and medications for hyperlipidemia – drove much of the massive increase in health care spending during the past 2 decades, said Joseph L. Dieleman, PhD, of the Institute for Health Metrics and Evaluation, University of Washington, Seattle, and his associates.
Recent increases in health care spending are well documented, but less is known about what is spent for individual conditions, in different health care settings, and in various patient age groups. To assess health care spending across these categories, the investigators collected and analyzed data for 1996 through 2013 from nationally representative surveys of households, nationally representative surveys of medical facilities, insurance claims, government budgets, and other official records.
They grouped the data into six type-of-care categories: inpatient care, ambulatory care, emergency department care, nursing facility care, dental care, and prescribed pharmaceuticals. “Spending on the six types of personal health care was then disaggregated across 155 mutually exclusive and collectively exhaustive conditions and 38 age and sex groups,” with each sex being divided into 5-year age groups, the researchers noted.
Based on these data, the investigators came to the following conclusions:
• Twenty conditions accounted for approximately 58% of personal health care spending, which totaled an estimated $1.2 trillion in 2013.
• More resources were spent on diabetes than any other condition in 2013, at an estimated $101.4 billion. Prescribed medications accounted for nearly 60% of diabetes costs.
• The second-highest amount of health care spending was for ischemic heart disease, which accounted for $88.1 billion in 2013. Most such spending occurred in inpatient settings.
• Low-back and neck pain, comprising the third-highest level of spending, cost an estimated $87.6 billion. Approximately 60% of this spending occurred in ambulatory settings.
• Among all 155 conditions, spending for diabetes and low-back and neck pain increased the most during the 18-year study period.
• Among all six types of care, spending on pharmaceuticals and emergency care increased the most during the study period.
It is important to note that for the purposes of this study, cancer was disaggregated into 29 separate conditions, and none of them placed in the top 20 for health care spending, Dr. Dieleman and his associates noted (JAMA. 2016;316[24]:2627-46. doi: 10.1001/jama.2016.16885).
When spending was categorized by patient age groups, working-age adults accounted for the greatest amount spent in 2013, estimated at $1,070.1 billion. But that was followed closely by patients aged 65 and older, who accounted for an estimated $796.5 billion, much of which was spent on care in nursing facilities. The smallest amount of health care spending was in children over age 1 and adolescents, who accounted for an estimated $233.5 billion.
Among the other study findings:
• Spending on pharmaceutical treatment of two conditions, hypertension and hyperlipidemia, increased at more than double the rate of total health care spending. It totaled an estimated $135.7 billion in 2013.
• Other top-20 conditions included falls, depression, skin disorders such as acne and eczema, sense disorders such as vision correction and hearing loss, dental care, urinary disorders, and lower respiratory tract infection.
This work was supported by the National Institute on Aging and the Vitality Institute. Dr. Dieleman and his associates reported having no relevant financial disclosures.
Diabetes, ischemic heart disease, and back and neck pain are the top three conditions accounting for the highest spending on personal health care in the United States, according to a report published online Dec. 27 in JAMA.
In addition, spending on pharmaceuticals – particularly diabetes therapies, antihypertensive drugs, and medications for hyperlipidemia – drove much of the massive increase in health care spending during the past 2 decades, said Joseph L. Dieleman, PhD, of the Institute for Health Metrics and Evaluation, University of Washington, Seattle, and his associates.
Recent increases in health care spending are well documented, but less is known about what is spent for individual conditions, in different health care settings, and in various patient age groups. To assess health care spending across these categories, the investigators collected and analyzed data for 1996 through 2013 from nationally representative surveys of households, nationally representative surveys of medical facilities, insurance claims, government budgets, and other official records.
They grouped the data into six type-of-care categories: inpatient care, ambulatory care, emergency department care, nursing facility care, dental care, and prescribed pharmaceuticals. “Spending on the six types of personal health care was then disaggregated across 155 mutually exclusive and collectively exhaustive conditions and 38 age and sex groups,” with each sex being divided into 5-year age groups, the researchers noted.
Based on these data, the investigators came to the following conclusions:
• Twenty conditions accounted for approximately 58% of personal health care spending, which totaled an estimated $1.2 trillion in 2013.
• More resources were spent on diabetes than any other condition in 2013, at an estimated $101.4 billion. Prescribed medications accounted for nearly 60% of diabetes costs.
• The second-highest amount of health care spending was for ischemic heart disease, which accounted for $88.1 billion in 2013. Most such spending occurred in inpatient settings.
• Low-back and neck pain, comprising the third-highest level of spending, cost an estimated $87.6 billion. Approximately 60% of this spending occurred in ambulatory settings.
• Among all 155 conditions, spending for diabetes and low-back and neck pain increased the most during the 18-year study period.
• Among all six types of care, spending on pharmaceuticals and emergency care increased the most during the study period.
It is important to note that for the purposes of this study, cancer was disaggregated into 29 separate conditions, and none of them placed in the top 20 for health care spending, Dr. Dieleman and his associates noted (JAMA. 2016;316[24]:2627-46. doi: 10.1001/jama.2016.16885).
When spending was categorized by patient age groups, working-age adults accounted for the greatest amount spent in 2013, estimated at $1,070.1 billion. But that was followed closely by patients aged 65 and older, who accounted for an estimated $796.5 billion, much of which was spent on care in nursing facilities. The smallest amount of health care spending was in children over age 1 and adolescents, who accounted for an estimated $233.5 billion.
Among the other study findings:
• Spending on pharmaceutical treatment of two conditions, hypertension and hyperlipidemia, increased at more than double the rate of total health care spending. It totaled an estimated $135.7 billion in 2013.
• Other top-20 conditions included falls, depression, skin disorders such as acne and eczema, sense disorders such as vision correction and hearing loss, dental care, urinary disorders, and lower respiratory tract infection.
This work was supported by the National Institute on Aging and the Vitality Institute. Dr. Dieleman and his associates reported having no relevant financial disclosures.
FROM JAMA
Key clinical point:
Major finding: More resources were spent on diabetes than any other condition in 2013, at an estimated $101.4 billion.
Data source: A comprehensive estimate of U.S. spending on personal health care, based on information collected from nationally representative surveys of households and medical facilities, government budgets, insurance claims, and official records from 1996 through 2013.
Disclosures: The National Institute on Aging and the Vitality Institute supported the work. Dr. Dieleman and his associates reported having no relevant financial disclosures.
















