User login
CDC: More preterm births after fertility treatments
Infants conceived through assisted reproductive technology (ART) represented less than 2% of all children born in the United States in 2014, but they experienced disproportionately large shares of adverse perinatal outcomes, according to the Centers for Disease Control and Prevention.
Of the 4.02 million children born in the United States in 2014, a total of 65,296 (1.6%) were conceived with ART procedures. ART-conceived children, however, represented 5.5% of all low-birth-weight infants (less than 2,500 g) and 5.6% of all very-low-birth-weight infants (less than 1,500 g). ART-conceived children also represented 4.7% of all preterm births (less than 37 weeks) in 2014 and 5.0% of all very preterm (less than 32 weeks) infants nationally, the CDC reported (MMWR Surveill Summ. 2017;66[SS-6]:1-24).
These poor outcomes are related, at least in part, to the effect of infertility treatment on multiple births. The percentage of triplets or higher-order infants among ART-conceived infants has decreased since 2000, but “the percentage of twin infants has remained persistently high,” the CDC noted. Multiple-birth infants represent 39.4% of all ART infants but only 3.5% of all infants, and ART-conceived multiple-birth infants represent 18.3% of all multiple-birth infants, the CDC said.
The multiple-birth effect varied considerably by state, however. In Hawaii, ART-conceived multiple-birth infants accounted for 37.3% of all multiple births in 2014, while Alaska and West Virginia both had rates of 5.5%. Poor outcomes also varied by state: In Massachusetts, ART infants represented 14.2% of all low-birth-weight infants, compared with 1.2% in West Virginia. Massachusetts had the highest rate of ART preterm infants to all infants, 13.4%, and Puerto Rico had the lowest, 1.2%, according to the CDC report, which was based on data from the National ART Surveillance System.
Puerto Rico’s rate of 364 ART procedures per 1 million women aged 15-44 years was the lowest in the country, and the highest rate belonged to Massachusetts at 6,726 per 1 million women aged 15-44 years.
Infants conceived through assisted reproductive technology (ART) represented less than 2% of all children born in the United States in 2014, but they experienced disproportionately large shares of adverse perinatal outcomes, according to the Centers for Disease Control and Prevention.
Of the 4.02 million children born in the United States in 2014, a total of 65,296 (1.6%) were conceived with ART procedures. ART-conceived children, however, represented 5.5% of all low-birth-weight infants (less than 2,500 g) and 5.6% of all very-low-birth-weight infants (less than 1,500 g). ART-conceived children also represented 4.7% of all preterm births (less than 37 weeks) in 2014 and 5.0% of all very preterm (less than 32 weeks) infants nationally, the CDC reported (MMWR Surveill Summ. 2017;66[SS-6]:1-24).
These poor outcomes are related, at least in part, to the effect of infertility treatment on multiple births. The percentage of triplets or higher-order infants among ART-conceived infants has decreased since 2000, but “the percentage of twin infants has remained persistently high,” the CDC noted. Multiple-birth infants represent 39.4% of all ART infants but only 3.5% of all infants, and ART-conceived multiple-birth infants represent 18.3% of all multiple-birth infants, the CDC said.
The multiple-birth effect varied considerably by state, however. In Hawaii, ART-conceived multiple-birth infants accounted for 37.3% of all multiple births in 2014, while Alaska and West Virginia both had rates of 5.5%. Poor outcomes also varied by state: In Massachusetts, ART infants represented 14.2% of all low-birth-weight infants, compared with 1.2% in West Virginia. Massachusetts had the highest rate of ART preterm infants to all infants, 13.4%, and Puerto Rico had the lowest, 1.2%, according to the CDC report, which was based on data from the National ART Surveillance System.
Puerto Rico’s rate of 364 ART procedures per 1 million women aged 15-44 years was the lowest in the country, and the highest rate belonged to Massachusetts at 6,726 per 1 million women aged 15-44 years.
Infants conceived through assisted reproductive technology (ART) represented less than 2% of all children born in the United States in 2014, but they experienced disproportionately large shares of adverse perinatal outcomes, according to the Centers for Disease Control and Prevention.
Of the 4.02 million children born in the United States in 2014, a total of 65,296 (1.6%) were conceived with ART procedures. ART-conceived children, however, represented 5.5% of all low-birth-weight infants (less than 2,500 g) and 5.6% of all very-low-birth-weight infants (less than 1,500 g). ART-conceived children also represented 4.7% of all preterm births (less than 37 weeks) in 2014 and 5.0% of all very preterm (less than 32 weeks) infants nationally, the CDC reported (MMWR Surveill Summ. 2017;66[SS-6]:1-24).
These poor outcomes are related, at least in part, to the effect of infertility treatment on multiple births. The percentage of triplets or higher-order infants among ART-conceived infants has decreased since 2000, but “the percentage of twin infants has remained persistently high,” the CDC noted. Multiple-birth infants represent 39.4% of all ART infants but only 3.5% of all infants, and ART-conceived multiple-birth infants represent 18.3% of all multiple-birth infants, the CDC said.
The multiple-birth effect varied considerably by state, however. In Hawaii, ART-conceived multiple-birth infants accounted for 37.3% of all multiple births in 2014, while Alaska and West Virginia both had rates of 5.5%. Poor outcomes also varied by state: In Massachusetts, ART infants represented 14.2% of all low-birth-weight infants, compared with 1.2% in West Virginia. Massachusetts had the highest rate of ART preterm infants to all infants, 13.4%, and Puerto Rico had the lowest, 1.2%, according to the CDC report, which was based on data from the National ART Surveillance System.
Puerto Rico’s rate of 364 ART procedures per 1 million women aged 15-44 years was the lowest in the country, and the highest rate belonged to Massachusetts at 6,726 per 1 million women aged 15-44 years.
FROM MMWR SURVEILLANCE SUMMARIES
Steroid use in adolescents
Anabolic androgenic steroid (AAS) use in athletes is not a new topic. In fact, many teens can relate a story involving a famous athlete and use of a performance-enhancing drug and the consequences associated with it. Although published data do not support a significant increase in use of performance-enhancing drugs among adolescents,1 more recent studies show that anabolic steroids are being found in nonprescription supplements, and their use among adolescents may be substantially underestimated.
Supplements are not regulated by the Food and Drug Administration, so many of these products are easily found on the Internet and sold in local stores.2,3 Some of these are marketed for increasing muscle mass, strength, and performance, which is appealing to the young athlete. Much of the marketing of these products minimize the side effects associated with their use and, therefore, most users are unaware of their harmful effects.
Approximately 5%-11% of teen athletes use AAS to improve physique and performance.3 Given its promotion for improved physique, many nonathletes also are turning to AAS for weight loss in both males and females. Side effects of steroid use are extrapolated from the data of therapeutic use, but most adolescents using steroids are not using under medical supervision. The dosing for building muscle mass uses a pyramid type dosing over a 6- to 12-week period and can be up to forty times the therapeutic dosing; therefore, the side effects can be greater than reported.4,5 Common side effects of AAS are hypogonadism, gynecomastia, decreased sperm count and infertility, acne, and aggressiveness. Liver tumors and psychosis have been reported, and increased depressed mood have been identified with discontinuation of use.4,6 Studies suggest that these side effects are reversible with discontinuation, but more studies are needed.4,6
Given that many of the side effects are not identifiable on physical exam, pediatricians must be proactive in questioning adolescents about their knowledge and use of AAS. Testing for steroid use is difficult and not very sensitive. Urine test for carbon isotopes 13/12 is the most common test, but if not done within hours of ingestion it will not be detected. T/E test (testosterone/epitestosterone glucuronide) is another test, but also limited by the timing of the test.6 Home screening also is available but the American Academy of Pediatrics 2014 guidelines warns against parents using these given the high false positive rate and risk of confrontation.7
Home and school screening may function more to deter athletes from using steroids to avoid consequences of being caught more than actually identifying use. If an athlete is suspected of using steroids, a test should be done. If negative, it should be repeated another time, as repeated testing is more likely to identify use. Studies have shown a correlation between steroid use and use of other illicit drugs so further screening should be done to identify if other drugs are being used.8
Widespread screening has not been shown to be cost effective and, therefore, should not be encouraged. Educating the patient on the risk of use and potential side effects along with healthy alternatives that improve performance and physique is much more effective.9 Being observant to signs and symptoms of AAS use helps to initiate conversation on the risk of using anabolic steroids.
References
1. Arch Pediatr Adolesc Med. 1997 Dec;151(12):1197-206.
2. Subst Use Misuse. 2012 Feb; 47(3):329-41.
3. Am J Clin Nutr. 2000 Feb;71(2):399-400.
4. J Sports Sci Med. 2006 Jun 1;5(2):182-93.
5. Br J Sports Med. 2006 Jul; 40(Suppl 1): i21-24.
6. J Athl Train. 1994 Mar; 29(1):60-4.
7. Pediatrics. 2014;133:e1798-1807.
8. Pediatrics. 1995 Jul;96(1 Pt 1):23-8.
9. Pediatrics. 1997 Jun;99(6):904-8.
Dr. Pearce is a pediatrician in Frankfort, Ill. She said she had no relevant financial disclosures. Email her at [email protected].
Anabolic androgenic steroid (AAS) use in athletes is not a new topic. In fact, many teens can relate a story involving a famous athlete and use of a performance-enhancing drug and the consequences associated with it. Although published data do not support a significant increase in use of performance-enhancing drugs among adolescents,1 more recent studies show that anabolic steroids are being found in nonprescription supplements, and their use among adolescents may be substantially underestimated.
Supplements are not regulated by the Food and Drug Administration, so many of these products are easily found on the Internet and sold in local stores.2,3 Some of these are marketed for increasing muscle mass, strength, and performance, which is appealing to the young athlete. Much of the marketing of these products minimize the side effects associated with their use and, therefore, most users are unaware of their harmful effects.
Approximately 5%-11% of teen athletes use AAS to improve physique and performance.3 Given its promotion for improved physique, many nonathletes also are turning to AAS for weight loss in both males and females. Side effects of steroid use are extrapolated from the data of therapeutic use, but most adolescents using steroids are not using under medical supervision. The dosing for building muscle mass uses a pyramid type dosing over a 6- to 12-week period and can be up to forty times the therapeutic dosing; therefore, the side effects can be greater than reported.4,5 Common side effects of AAS are hypogonadism, gynecomastia, decreased sperm count and infertility, acne, and aggressiveness. Liver tumors and psychosis have been reported, and increased depressed mood have been identified with discontinuation of use.4,6 Studies suggest that these side effects are reversible with discontinuation, but more studies are needed.4,6
Given that many of the side effects are not identifiable on physical exam, pediatricians must be proactive in questioning adolescents about their knowledge and use of AAS. Testing for steroid use is difficult and not very sensitive. Urine test for carbon isotopes 13/12 is the most common test, but if not done within hours of ingestion it will not be detected. T/E test (testosterone/epitestosterone glucuronide) is another test, but also limited by the timing of the test.6 Home screening also is available but the American Academy of Pediatrics 2014 guidelines warns against parents using these given the high false positive rate and risk of confrontation.7
Home and school screening may function more to deter athletes from using steroids to avoid consequences of being caught more than actually identifying use. If an athlete is suspected of using steroids, a test should be done. If negative, it should be repeated another time, as repeated testing is more likely to identify use. Studies have shown a correlation between steroid use and use of other illicit drugs so further screening should be done to identify if other drugs are being used.8
Widespread screening has not been shown to be cost effective and, therefore, should not be encouraged. Educating the patient on the risk of use and potential side effects along with healthy alternatives that improve performance and physique is much more effective.9 Being observant to signs and symptoms of AAS use helps to initiate conversation on the risk of using anabolic steroids.
References
1. Arch Pediatr Adolesc Med. 1997 Dec;151(12):1197-206.
2. Subst Use Misuse. 2012 Feb; 47(3):329-41.
3. Am J Clin Nutr. 2000 Feb;71(2):399-400.
4. J Sports Sci Med. 2006 Jun 1;5(2):182-93.
5. Br J Sports Med. 2006 Jul; 40(Suppl 1): i21-24.
6. J Athl Train. 1994 Mar; 29(1):60-4.
7. Pediatrics. 2014;133:e1798-1807.
8. Pediatrics. 1995 Jul;96(1 Pt 1):23-8.
9. Pediatrics. 1997 Jun;99(6):904-8.
Dr. Pearce is a pediatrician in Frankfort, Ill. She said she had no relevant financial disclosures. Email her at [email protected].
Anabolic androgenic steroid (AAS) use in athletes is not a new topic. In fact, many teens can relate a story involving a famous athlete and use of a performance-enhancing drug and the consequences associated with it. Although published data do not support a significant increase in use of performance-enhancing drugs among adolescents,1 more recent studies show that anabolic steroids are being found in nonprescription supplements, and their use among adolescents may be substantially underestimated.
Supplements are not regulated by the Food and Drug Administration, so many of these products are easily found on the Internet and sold in local stores.2,3 Some of these are marketed for increasing muscle mass, strength, and performance, which is appealing to the young athlete. Much of the marketing of these products minimize the side effects associated with their use and, therefore, most users are unaware of their harmful effects.
Approximately 5%-11% of teen athletes use AAS to improve physique and performance.3 Given its promotion for improved physique, many nonathletes also are turning to AAS for weight loss in both males and females. Side effects of steroid use are extrapolated from the data of therapeutic use, but most adolescents using steroids are not using under medical supervision. The dosing for building muscle mass uses a pyramid type dosing over a 6- to 12-week period and can be up to forty times the therapeutic dosing; therefore, the side effects can be greater than reported.4,5 Common side effects of AAS are hypogonadism, gynecomastia, decreased sperm count and infertility, acne, and aggressiveness. Liver tumors and psychosis have been reported, and increased depressed mood have been identified with discontinuation of use.4,6 Studies suggest that these side effects are reversible with discontinuation, but more studies are needed.4,6
Given that many of the side effects are not identifiable on physical exam, pediatricians must be proactive in questioning adolescents about their knowledge and use of AAS. Testing for steroid use is difficult and not very sensitive. Urine test for carbon isotopes 13/12 is the most common test, but if not done within hours of ingestion it will not be detected. T/E test (testosterone/epitestosterone glucuronide) is another test, but also limited by the timing of the test.6 Home screening also is available but the American Academy of Pediatrics 2014 guidelines warns against parents using these given the high false positive rate and risk of confrontation.7
Home and school screening may function more to deter athletes from using steroids to avoid consequences of being caught more than actually identifying use. If an athlete is suspected of using steroids, a test should be done. If negative, it should be repeated another time, as repeated testing is more likely to identify use. Studies have shown a correlation between steroid use and use of other illicit drugs so further screening should be done to identify if other drugs are being used.8
Widespread screening has not been shown to be cost effective and, therefore, should not be encouraged. Educating the patient on the risk of use and potential side effects along with healthy alternatives that improve performance and physique is much more effective.9 Being observant to signs and symptoms of AAS use helps to initiate conversation on the risk of using anabolic steroids.
References
1. Arch Pediatr Adolesc Med. 1997 Dec;151(12):1197-206.
2. Subst Use Misuse. 2012 Feb; 47(3):329-41.
3. Am J Clin Nutr. 2000 Feb;71(2):399-400.
4. J Sports Sci Med. 2006 Jun 1;5(2):182-93.
5. Br J Sports Med. 2006 Jul; 40(Suppl 1): i21-24.
6. J Athl Train. 1994 Mar; 29(1):60-4.
7. Pediatrics. 2014;133:e1798-1807.
8. Pediatrics. 1995 Jul;96(1 Pt 1):23-8.
9. Pediatrics. 1997 Jun;99(6):904-8.
Dr. Pearce is a pediatrician in Frankfort, Ill. She said she had no relevant financial disclosures. Email her at [email protected].
Sirolimus shows promise for pediatric refractory IBD treatment
Sirolimus may be an effective rescue treatment option for children with refractory inflammatory bowel disease, according to Dr. Mohamed Mutalib and his associates.
In a retrospective analysis of 11 ulcerative colitis (UC) and 3 Crohn’s disease patients treated with sirolimus, 5 of the UC patients and all of the Crohn’s disease patients achieved clinical remission. An additional 2 UC patients achieved clinical response. The remaining 4 UC patients did not respond to sirolimus treatment.
Mucosal healing was achieved in 5 of 11 UC patients and 2 of 3 Crohn’s patients. Clinical response to treatment occurred at least 2 weeks after treatment was started. The only significant side effect reported was minor gastrointestinal distress.
“Our data provide compelling evidence that sirolimus is effective as rescue therapy in a subgroup of children with severe [inflammatory bowel disease] refractory to conventional therapies by inducing both clinical remission and mucosal healing. However, randomized placebo-controlled studies are warranted to extend our encouraging initial findings,” the investigators concluded.
Find the full study in the Journal of Crohn’s and Colitis (doi: 10.1016/j.crohns.2014.08.014).
Sirolimus may be an effective rescue treatment option for children with refractory inflammatory bowel disease, according to Dr. Mohamed Mutalib and his associates.
In a retrospective analysis of 11 ulcerative colitis (UC) and 3 Crohn’s disease patients treated with sirolimus, 5 of the UC patients and all of the Crohn’s disease patients achieved clinical remission. An additional 2 UC patients achieved clinical response. The remaining 4 UC patients did not respond to sirolimus treatment.
Mucosal healing was achieved in 5 of 11 UC patients and 2 of 3 Crohn’s patients. Clinical response to treatment occurred at least 2 weeks after treatment was started. The only significant side effect reported was minor gastrointestinal distress.
“Our data provide compelling evidence that sirolimus is effective as rescue therapy in a subgroup of children with severe [inflammatory bowel disease] refractory to conventional therapies by inducing both clinical remission and mucosal healing. However, randomized placebo-controlled studies are warranted to extend our encouraging initial findings,” the investigators concluded.
Find the full study in the Journal of Crohn’s and Colitis (doi: 10.1016/j.crohns.2014.08.014).
Sirolimus may be an effective rescue treatment option for children with refractory inflammatory bowel disease, according to Dr. Mohamed Mutalib and his associates.
In a retrospective analysis of 11 ulcerative colitis (UC) and 3 Crohn’s disease patients treated with sirolimus, 5 of the UC patients and all of the Crohn’s disease patients achieved clinical remission. An additional 2 UC patients achieved clinical response. The remaining 4 UC patients did not respond to sirolimus treatment.
Mucosal healing was achieved in 5 of 11 UC patients and 2 of 3 Crohn’s patients. Clinical response to treatment occurred at least 2 weeks after treatment was started. The only significant side effect reported was minor gastrointestinal distress.
“Our data provide compelling evidence that sirolimus is effective as rescue therapy in a subgroup of children with severe [inflammatory bowel disease] refractory to conventional therapies by inducing both clinical remission and mucosal healing. However, randomized placebo-controlled studies are warranted to extend our encouraging initial findings,” the investigators concluded.
Find the full study in the Journal of Crohn’s and Colitis (doi: 10.1016/j.crohns.2014.08.014).
FROM THE JOURNAL OF CROHN'S AND COLITIS
Magnetic implant may offer new drug delivery method
A tiny magnetic implant could provide a new method of drug delivery, according to research published in Advanced Functional Materials.
The device is a silicone sponge with magnetic carbonyl iron particles wrapped in a round polymer layer. It measures 6 mm in diameter.
A drug is injected into the device, which is surgically implanted in the area being treated.
Passing a magnet over the implant activates the device by deforming the sponge and triggering the release of the drug into surrounding tissue through a tiny opening.
“Drug implants can be safe and effective for treating many conditions, and magnetically controlled implants are particularly interesting because you can adjust the dose after implantation by using different magnet strengths,” said study author Ali Shademani, a PhD student at the University of British Columbia (UBC) in Vancouver, British Columbia, Canada.
“This device lets you release the actual dose that the patient needs when they need it, and it’s sufficiently easy to use that patients could administer their own medication one day without having to go to a hospital,” added John K. Jackson, also of UBC.
The researchers tested the device on animal tissue in the lab using the prostate cancer drug docetaxel. The device was able to deliver the drug on demand even after repeated use.
The drug also produced an effect on cancer cells comparable to that of freshly administered docetaxel, suggesting that drugs stored in the device stay effective.
The researchers are now working on refining the device and narrowing down the conditions for its use.
“This could one day be used for administering painkillers, hormones, chemotherapy drugs, and other treatments for a wide range of health conditions,” said Mu Chiao, PhD, of UBC. “In the next few years, we hope to be able to test it for long-term use and for viability in living models.” ![]()
A tiny magnetic implant could provide a new method of drug delivery, according to research published in Advanced Functional Materials.
The device is a silicone sponge with magnetic carbonyl iron particles wrapped in a round polymer layer. It measures 6 mm in diameter.
A drug is injected into the device, which is surgically implanted in the area being treated.
Passing a magnet over the implant activates the device by deforming the sponge and triggering the release of the drug into surrounding tissue through a tiny opening.
“Drug implants can be safe and effective for treating many conditions, and magnetically controlled implants are particularly interesting because you can adjust the dose after implantation by using different magnet strengths,” said study author Ali Shademani, a PhD student at the University of British Columbia (UBC) in Vancouver, British Columbia, Canada.
“This device lets you release the actual dose that the patient needs when they need it, and it’s sufficiently easy to use that patients could administer their own medication one day without having to go to a hospital,” added John K. Jackson, also of UBC.
The researchers tested the device on animal tissue in the lab using the prostate cancer drug docetaxel. The device was able to deliver the drug on demand even after repeated use.
The drug also produced an effect on cancer cells comparable to that of freshly administered docetaxel, suggesting that drugs stored in the device stay effective.
The researchers are now working on refining the device and narrowing down the conditions for its use.
“This could one day be used for administering painkillers, hormones, chemotherapy drugs, and other treatments for a wide range of health conditions,” said Mu Chiao, PhD, of UBC. “In the next few years, we hope to be able to test it for long-term use and for viability in living models.” ![]()
A tiny magnetic implant could provide a new method of drug delivery, according to research published in Advanced Functional Materials.
The device is a silicone sponge with magnetic carbonyl iron particles wrapped in a round polymer layer. It measures 6 mm in diameter.
A drug is injected into the device, which is surgically implanted in the area being treated.
Passing a magnet over the implant activates the device by deforming the sponge and triggering the release of the drug into surrounding tissue through a tiny opening.
“Drug implants can be safe and effective for treating many conditions, and magnetically controlled implants are particularly interesting because you can adjust the dose after implantation by using different magnet strengths,” said study author Ali Shademani, a PhD student at the University of British Columbia (UBC) in Vancouver, British Columbia, Canada.
“This device lets you release the actual dose that the patient needs when they need it, and it’s sufficiently easy to use that patients could administer their own medication one day without having to go to a hospital,” added John K. Jackson, also of UBC.
The researchers tested the device on animal tissue in the lab using the prostate cancer drug docetaxel. The device was able to deliver the drug on demand even after repeated use.
The drug also produced an effect on cancer cells comparable to that of freshly administered docetaxel, suggesting that drugs stored in the device stay effective.
The researchers are now working on refining the device and narrowing down the conditions for its use.
“This could one day be used for administering painkillers, hormones, chemotherapy drugs, and other treatments for a wide range of health conditions,” said Mu Chiao, PhD, of UBC. “In the next few years, we hope to be able to test it for long-term use and for viability in living models.” ![]()
Styrene exposure linked to myeloid leukemia, HL
A new study links styrene—a chemical used in the manufacture of plastics, rubber, and resins—to certain cancers.
The research showed that, contrary to previous suggestions, employees who have worked with styrene do not have an increased incidence of esophageal, pancreatic, lung, kidney, or bladder cancer.
On the other hand, they may have an increased risk of nasal and paranasal cancer, as well as myeloid leukemia and Hodgkin lymphoma (HL).
The research was published in Epidemiology.
“It is important to know for present and former workers exposed to styrene that they are unlikely to have become ill by doing their job if they have developed cancer of the esophagus, pancreas, lungs, kidneys, bladder, or a wide range of other types of cancer,” said study author Henrik A. Kolstad, MD, PhD, of Aarhus University in Denmark.
“This is also new and important knowledge in the USA, where styrene was added to the list of carcinogenic substances in 2011.”
In relation to the cancers for which the study shows a possible increased risk, Dr Kolstad emphasized that additional research is needed to determine if styrene is the actual cause of the employees’ disease.
For the current study, Dr Kolstad and his colleagues analyzed data on 72,292 employees who worked for 1 of 443 small and medium-sized companies in Denmark that used styrene for the production of wind turbines, pleasure boats, and other products from 1964 to 2007.
There were 8961 incident cases of cancer in this cohort from 1968 to 2012. The standardized incidence rate ratio (SIR) for all cancers was 1.04. When the researchers included a 10-year lag period, the SIR for all cancers was still 1.04.
As for hematologic malignancies, the researchers said they observed increased rate ratios associated with increased duration of employment for HL and myeloid leukemia.
For HL, the SIRs were 1.21 with no lag and 1.22 with a 10-year lag. For myeloid leukemia, the SIRs were 1.06 and 1.13, respectively.
The SIRs for non-Hodgkin lymphoma were 0.97 with no lag and 0.94 with a 10-year lag. The SIRs for multiple myeloma were 0.79 and 0.77, respectively.
For cancers of lymphatic and hematopoietic tissue, the SIRs were 0.97 with no lag and 0.96 with a 10-year lag. For lymphatic leukemia, the SIR was 0.96 for both time points.
The SIRs for monocytic leukemia were 0.77 with no lag and 0.56 with a 10-year lag. The SIRs for other and unspecified leukemias were 1.05 and 1.26, respectively.
The researchers noted that workers first employed in the 1960s had a higher risk of HL than workers first employed in subsequent years.
The SIRs were 2.12 for those first employed in 1964-1969, 0.82 for 1970-1979, 1.07 for 1980-1989, 1.52 for 1990-1999, and 1.10 for those first employed in 2000-2007.
There were no such associations for other cancer sites. ![]()
A new study links styrene—a chemical used in the manufacture of plastics, rubber, and resins—to certain cancers.
The research showed that, contrary to previous suggestions, employees who have worked with styrene do not have an increased incidence of esophageal, pancreatic, lung, kidney, or bladder cancer.
On the other hand, they may have an increased risk of nasal and paranasal cancer, as well as myeloid leukemia and Hodgkin lymphoma (HL).
The research was published in Epidemiology.
“It is important to know for present and former workers exposed to styrene that they are unlikely to have become ill by doing their job if they have developed cancer of the esophagus, pancreas, lungs, kidneys, bladder, or a wide range of other types of cancer,” said study author Henrik A. Kolstad, MD, PhD, of Aarhus University in Denmark.
“This is also new and important knowledge in the USA, where styrene was added to the list of carcinogenic substances in 2011.”
In relation to the cancers for which the study shows a possible increased risk, Dr Kolstad emphasized that additional research is needed to determine if styrene is the actual cause of the employees’ disease.
For the current study, Dr Kolstad and his colleagues analyzed data on 72,292 employees who worked for 1 of 443 small and medium-sized companies in Denmark that used styrene for the production of wind turbines, pleasure boats, and other products from 1964 to 2007.
There were 8961 incident cases of cancer in this cohort from 1968 to 2012. The standardized incidence rate ratio (SIR) for all cancers was 1.04. When the researchers included a 10-year lag period, the SIR for all cancers was still 1.04.
As for hematologic malignancies, the researchers said they observed increased rate ratios associated with increased duration of employment for HL and myeloid leukemia.
For HL, the SIRs were 1.21 with no lag and 1.22 with a 10-year lag. For myeloid leukemia, the SIRs were 1.06 and 1.13, respectively.
The SIRs for non-Hodgkin lymphoma were 0.97 with no lag and 0.94 with a 10-year lag. The SIRs for multiple myeloma were 0.79 and 0.77, respectively.
For cancers of lymphatic and hematopoietic tissue, the SIRs were 0.97 with no lag and 0.96 with a 10-year lag. For lymphatic leukemia, the SIR was 0.96 for both time points.
The SIRs for monocytic leukemia were 0.77 with no lag and 0.56 with a 10-year lag. The SIRs for other and unspecified leukemias were 1.05 and 1.26, respectively.
The researchers noted that workers first employed in the 1960s had a higher risk of HL than workers first employed in subsequent years.
The SIRs were 2.12 for those first employed in 1964-1969, 0.82 for 1970-1979, 1.07 for 1980-1989, 1.52 for 1990-1999, and 1.10 for those first employed in 2000-2007.
There were no such associations for other cancer sites. ![]()
A new study links styrene—a chemical used in the manufacture of plastics, rubber, and resins—to certain cancers.
The research showed that, contrary to previous suggestions, employees who have worked with styrene do not have an increased incidence of esophageal, pancreatic, lung, kidney, or bladder cancer.
On the other hand, they may have an increased risk of nasal and paranasal cancer, as well as myeloid leukemia and Hodgkin lymphoma (HL).
The research was published in Epidemiology.
“It is important to know for present and former workers exposed to styrene that they are unlikely to have become ill by doing their job if they have developed cancer of the esophagus, pancreas, lungs, kidneys, bladder, or a wide range of other types of cancer,” said study author Henrik A. Kolstad, MD, PhD, of Aarhus University in Denmark.
“This is also new and important knowledge in the USA, where styrene was added to the list of carcinogenic substances in 2011.”
In relation to the cancers for which the study shows a possible increased risk, Dr Kolstad emphasized that additional research is needed to determine if styrene is the actual cause of the employees’ disease.
For the current study, Dr Kolstad and his colleagues analyzed data on 72,292 employees who worked for 1 of 443 small and medium-sized companies in Denmark that used styrene for the production of wind turbines, pleasure boats, and other products from 1964 to 2007.
There were 8961 incident cases of cancer in this cohort from 1968 to 2012. The standardized incidence rate ratio (SIR) for all cancers was 1.04. When the researchers included a 10-year lag period, the SIR for all cancers was still 1.04.
As for hematologic malignancies, the researchers said they observed increased rate ratios associated with increased duration of employment for HL and myeloid leukemia.
For HL, the SIRs were 1.21 with no lag and 1.22 with a 10-year lag. For myeloid leukemia, the SIRs were 1.06 and 1.13, respectively.
The SIRs for non-Hodgkin lymphoma were 0.97 with no lag and 0.94 with a 10-year lag. The SIRs for multiple myeloma were 0.79 and 0.77, respectively.
For cancers of lymphatic and hematopoietic tissue, the SIRs were 0.97 with no lag and 0.96 with a 10-year lag. For lymphatic leukemia, the SIR was 0.96 for both time points.
The SIRs for monocytic leukemia were 0.77 with no lag and 0.56 with a 10-year lag. The SIRs for other and unspecified leukemias were 1.05 and 1.26, respectively.
The researchers noted that workers first employed in the 1960s had a higher risk of HL than workers first employed in subsequent years.
The SIRs were 2.12 for those first employed in 1964-1969, 0.82 for 1970-1979, 1.07 for 1980-1989, 1.52 for 1990-1999, and 1.10 for those first employed in 2000-2007.
There were no such associations for other cancer sites. ![]()
Vaccination approach prevents malaria
A novel vaccination approach can offer 100% protection against malaria, according to research published in Nature.
The approach involved direct venous inoculation of aseptic, purified, cryopreserved, non-irradiated Plasmodium falciparum sporozoites (PfSPZ) in healthy adults taking chloroquine for antimalarial chemoprophylaxis (PfSPZ-CVac).
At the optimal dose and schedule, PfSPZ-CVac prevented infection in 9 of 9 volunteers, and this protection lasted 10 weeks after the last dose.
In addition, researchers said PfSPZ-CVac was well-tolerated.
PfSPZ was provided by Sanaria. This research was supported by federal funding and the Bill and Melinda Gates Foundation.
Treatment and challenge
The researchers tested PfSPZ-CVac in healthy adults, ages 18 to 45, who were malaria-naive. They received different doses of PfSPZ at different intervals and different doses/schedules of chloroquine.
In the first part of the study, test subjects received 3 doses of PfSPZ at 3.2 × 103 (n=9), 1.28 × 104 (n=9), or 5.12 × 104 (n=9), and controls (n=13) received 3 doses of normal saline.
Doses were given at 28-day intervals, and controlled human malaria infection (CHMI) was performed at 8 to 10 weeks after immunization.
In the second part of the study, test subjects received 3 doses of PfSPZ at 5.12 × 104 at 14-day intervals (n=9) or 5-day intervals (n=9), and controls (n=6) received 3 doses of normal saline.
CHMI was performed at 10 weeks post-immunization.
Most subjects received chloroquine at a base loading dose of 10 mg kg-1 or 620 mg two days before the first dose of PfSPZ, whichever dose was less, followed by weekly doses of 5 mg kg-1 or 310 mg through 5 days after the last dose of PfSPZ. Subjects who were immunized on days 0, 5, and 10 received chloroquine on days 0, 5, 10, and 15.
Results
All controls, who received normal saline plus chloroquine, developed parasitemia.
However, 3 doses of PfSPZ at 5.12 × 104, given at 28-day intervals, prevented infection in 9 of 9 subjects (100%).
The 3.2 × 103 dose of PfSPZ (also given at 28-day intervals) protected 3 of 9 subjects (33%), and the 1.28 × 104 dose protected 6 of 9 subjects (67%).
Three doses of PfSPZ at 5.12 × 104, given at 5-day intervals, protected 5 of 8 subjects (63%). And 3 doses of PfSPZ at 5.12 × 104, given at 14-day intervals, protected 6 of 9 subjects (67%).
“By vaccinating with a live, fully active pathogen, it seems clear that we were able to set off a very strong immune response,” said study author Benjamin Mordmüller, of the University of Tübingen in Germany.
“Additionally, all the data we have so far indicate that what we have here is relatively stable, long-lasting protection.”
“That protection was probably caused by specific T lymphocytes and antibody responses to the parasites in the liver,” added Peter Kremsner, also of the University of Tübingen.
The researchers said there were no serious adverse events, and the frequencies of grade 1-3 events were similar in subjects who received PfSPZ-CVac and controls.
Chloroquine was considered well-tolerated, although 2 subjects discontinued the trial after the loading dose because they experienced nausea and vomiting. ![]()
A novel vaccination approach can offer 100% protection against malaria, according to research published in Nature.
The approach involved direct venous inoculation of aseptic, purified, cryopreserved, non-irradiated Plasmodium falciparum sporozoites (PfSPZ) in healthy adults taking chloroquine for antimalarial chemoprophylaxis (PfSPZ-CVac).
At the optimal dose and schedule, PfSPZ-CVac prevented infection in 9 of 9 volunteers, and this protection lasted 10 weeks after the last dose.
In addition, researchers said PfSPZ-CVac was well-tolerated.
PfSPZ was provided by Sanaria. This research was supported by federal funding and the Bill and Melinda Gates Foundation.
Treatment and challenge
The researchers tested PfSPZ-CVac in healthy adults, ages 18 to 45, who were malaria-naive. They received different doses of PfSPZ at different intervals and different doses/schedules of chloroquine.
In the first part of the study, test subjects received 3 doses of PfSPZ at 3.2 × 103 (n=9), 1.28 × 104 (n=9), or 5.12 × 104 (n=9), and controls (n=13) received 3 doses of normal saline.
Doses were given at 28-day intervals, and controlled human malaria infection (CHMI) was performed at 8 to 10 weeks after immunization.
In the second part of the study, test subjects received 3 doses of PfSPZ at 5.12 × 104 at 14-day intervals (n=9) or 5-day intervals (n=9), and controls (n=6) received 3 doses of normal saline.
CHMI was performed at 10 weeks post-immunization.
Most subjects received chloroquine at a base loading dose of 10 mg kg-1 or 620 mg two days before the first dose of PfSPZ, whichever dose was less, followed by weekly doses of 5 mg kg-1 or 310 mg through 5 days after the last dose of PfSPZ. Subjects who were immunized on days 0, 5, and 10 received chloroquine on days 0, 5, 10, and 15.
Results
All controls, who received normal saline plus chloroquine, developed parasitemia.
However, 3 doses of PfSPZ at 5.12 × 104, given at 28-day intervals, prevented infection in 9 of 9 subjects (100%).
The 3.2 × 103 dose of PfSPZ (also given at 28-day intervals) protected 3 of 9 subjects (33%), and the 1.28 × 104 dose protected 6 of 9 subjects (67%).
Three doses of PfSPZ at 5.12 × 104, given at 5-day intervals, protected 5 of 8 subjects (63%). And 3 doses of PfSPZ at 5.12 × 104, given at 14-day intervals, protected 6 of 9 subjects (67%).
“By vaccinating with a live, fully active pathogen, it seems clear that we were able to set off a very strong immune response,” said study author Benjamin Mordmüller, of the University of Tübingen in Germany.
“Additionally, all the data we have so far indicate that what we have here is relatively stable, long-lasting protection.”
“That protection was probably caused by specific T lymphocytes and antibody responses to the parasites in the liver,” added Peter Kremsner, also of the University of Tübingen.
The researchers said there were no serious adverse events, and the frequencies of grade 1-3 events were similar in subjects who received PfSPZ-CVac and controls.
Chloroquine was considered well-tolerated, although 2 subjects discontinued the trial after the loading dose because they experienced nausea and vomiting. ![]()
A novel vaccination approach can offer 100% protection against malaria, according to research published in Nature.
The approach involved direct venous inoculation of aseptic, purified, cryopreserved, non-irradiated Plasmodium falciparum sporozoites (PfSPZ) in healthy adults taking chloroquine for antimalarial chemoprophylaxis (PfSPZ-CVac).
At the optimal dose and schedule, PfSPZ-CVac prevented infection in 9 of 9 volunteers, and this protection lasted 10 weeks after the last dose.
In addition, researchers said PfSPZ-CVac was well-tolerated.
PfSPZ was provided by Sanaria. This research was supported by federal funding and the Bill and Melinda Gates Foundation.
Treatment and challenge
The researchers tested PfSPZ-CVac in healthy adults, ages 18 to 45, who were malaria-naive. They received different doses of PfSPZ at different intervals and different doses/schedules of chloroquine.
In the first part of the study, test subjects received 3 doses of PfSPZ at 3.2 × 103 (n=9), 1.28 × 104 (n=9), or 5.12 × 104 (n=9), and controls (n=13) received 3 doses of normal saline.
Doses were given at 28-day intervals, and controlled human malaria infection (CHMI) was performed at 8 to 10 weeks after immunization.
In the second part of the study, test subjects received 3 doses of PfSPZ at 5.12 × 104 at 14-day intervals (n=9) or 5-day intervals (n=9), and controls (n=6) received 3 doses of normal saline.
CHMI was performed at 10 weeks post-immunization.
Most subjects received chloroquine at a base loading dose of 10 mg kg-1 or 620 mg two days before the first dose of PfSPZ, whichever dose was less, followed by weekly doses of 5 mg kg-1 or 310 mg through 5 days after the last dose of PfSPZ. Subjects who were immunized on days 0, 5, and 10 received chloroquine on days 0, 5, 10, and 15.
Results
All controls, who received normal saline plus chloroquine, developed parasitemia.
However, 3 doses of PfSPZ at 5.12 × 104, given at 28-day intervals, prevented infection in 9 of 9 subjects (100%).
The 3.2 × 103 dose of PfSPZ (also given at 28-day intervals) protected 3 of 9 subjects (33%), and the 1.28 × 104 dose protected 6 of 9 subjects (67%).
Three doses of PfSPZ at 5.12 × 104, given at 5-day intervals, protected 5 of 8 subjects (63%). And 3 doses of PfSPZ at 5.12 × 104, given at 14-day intervals, protected 6 of 9 subjects (67%).
“By vaccinating with a live, fully active pathogen, it seems clear that we were able to set off a very strong immune response,” said study author Benjamin Mordmüller, of the University of Tübingen in Germany.
“Additionally, all the data we have so far indicate that what we have here is relatively stable, long-lasting protection.”
“That protection was probably caused by specific T lymphocytes and antibody responses to the parasites in the liver,” added Peter Kremsner, also of the University of Tübingen.
The researchers said there were no serious adverse events, and the frequencies of grade 1-3 events were similar in subjects who received PfSPZ-CVac and controls.
Chloroquine was considered well-tolerated, although 2 subjects discontinued the trial after the loading dose because they experienced nausea and vomiting. ![]()
Eating disorders in transgender youth
The field of transgender health is growing. What began as a lone German physician in 1918 defying the norms of treating gender identity as a disease now has burgeoned into a field that includes 1,079 PubMed articles,two medical guidelines1,2, and a multitude of books. As we learn more about the complexity of gender and gender identity, we also are discovering potential problems that occur when providing care to our transgender patients. One is eating disorders.
A systematic review by Jones et al. showed only a handful of studies on eating disorders in transgender individuals, most of them restricted to case studies.3 In some situations, the issue of gender identity arises during treatment for an eating disorder, as the individual realizes that body dissatisfaction is due to the gender identity instead of a fear of gaining weight. In other cases, a transgender person in the process of transitioning to the affirmed gender develops an eating disorder.
There are two larger quantitative studies on eating disorders among transgender individuals. One study of 289,024 college students reveals that transgender students, compared to cisgender students, are almost five times as likely to report an eating disorder and two times as likely to use unhealthy compensatory methods (e.g., vomiting) for weight control.4 Another study of almost 2,500 teenagers shows that transgender individuals are almost three times as likely to restrict their eating, almost nine times as likely to take diet pills, and seven times as likely to take laxatives.5
The most commonly suggested reason for the possible elevated risk for eating disorders among transgender individuals is that many of them are trying to achieve the unrealistic standards of the ideal masculine or feminine body type. Another explanation is that eating disorders among transgender individuals are maladaptive coping mechanisms to stress from antitrans stigma and discrimination.4 However, these explanations are not mutually exclusive and could simultaneously drive disordered eating among transgender individuals.
To further appreciate the relationship between these two conditions, one must understand their similarities and differences. The Diagnostic Statistical Manual of Mental Disorders V characterizes eating disorders as “a persistent disturbance of eating or eating-related behavior that results in the altered consumption or absorption of food and ... significantly impairs physical health or psychosocial functioning.”6 Anorexia nervosa and bulimia nervosa are driven by fear of gaining weight or by a self-esteem unduly influenced by weight or appearance.6
Gender dysphoria, in comparison, is the distress caused by the incongruence between one’s gender identity and one’s anatomy, along with the desire to have the characteristics of one’s affirmed gender identity. This condition also could severely alter physical and psychosocial functioning,7 partly because of the distress from the incongruence, and partly because of the stress from antitrans stigma and discrimination, as an individual attempts to match the body with the gender identity8 (e.g., wearing clothing to match the gender identity).
The higher risk of developing an eating disorder among transgender individuals makes sense. Dissatisfaction with one’s body characterizes both conditions. The high standards on what is masculine or feminine affects everyone, especially transgender individuals who may feel that they’re “far behind” when they begin to transition to their affirmed gender. In addition, both involve identity. Those who have anorexia nervosa also incorporate this into their own identity.9 This is why treating an eating disorder can be very difficult.
Finally, individuals afflicted by an eating disorder or gender dysphoria engage in certain behaviors to achieve their desired appearance. However, this is where the similarities end. One major distinction between an eating disorder and gender dysphoria is the treatment approach. The goal in treating an eating disorder is to discourage the disordered behavior and encourage healthier eating habits and a more positive body image. Affirming the identity of someone with an eating disorder can be deadly, as it will encourage more disordered eating.10 In contrast, affirming the identity of someone with gender dysphoria through social transition, cross-sex hormones, and/or surgical reassignment is life-saving and therapeutic.11
There is little guidance on how to treat the these disorders simultaneously. What complicates treating both conditions at the same time is that when an eating disorder is accompanied by another mental health disorder (e.g., substance use), one condition over the other is prioritized.12 There is no guidance on whether the eating disorder or gender dysphoria should take priority over the other, or if it is possible to treat both conditions at the same time.
Strandjord et al. suggest a hierarchal approach, in which life-threatening issues (such as suicide or electrolyte disturbances) take priority.13 In addition, if the patient is malnourished, weight restoration should be the initial focus. A patient who is severely malnourished may not have the cognitive capacity nor the physiological ability to manage comorbidities such as anxiety or depression,12 much less have the capacity to process something as complex as gender and gender identity, nor understand the steps necessary for a successful transition to the affirmed gender. However, this does not mean providers should wait to successfully manage an eating disorder before addressing gender dysphoria. Studies have suggested that gender-affirming medical therapies (e.g., cross sex hormones) can be therapeutic for both gender dysphoria and eating disorder symptoms.14 Finally, because of the two ways a transgender patient with an eating disorder can present, I recommend screening for eating disorders in transgender individuals and inquiring about gender identity among those with an eating disorder. Doing so may save a life.
References
1. J Clin Endocrinol Metab. 2009 Sep;94(9):3132-54.
2. Adv Urol. 2012;2012:581712.
3. Int Rev Psychiatry. 2016;28(1):81-94.
4. J Adolesc Health. 2015 Aug;57(2):144-9.
5. J Adolesc Health. 2016. doi: 10.1016/j.jadohealth.2016.08.027.
6. Feeding and Eating Disorders. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. (Washington: American Psychiatric Association, 2013).
7. Gender Dysphoria. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. (Washington: American Psychiatric Association, 2013).
8. Psychol Bull. 2003 Sep;129(5):674-97.
9. Int J Law Psychiatry. 2003 Sep-Oct;26(5):533-48.
10. Arch Gen Psychiatry. 2011 Jul;68(7):724-31.
11. Clin Endocrinol (Oxf). 2010 Feb;72(2):214-31.
12. CNS drugs. 2006;20(8):655-63.
13. Int J Eat Disord. 2015 Nov;48(7):942-5.
14. Eat Disord. 2012;20(4):300-11.
The field of transgender health is growing. What began as a lone German physician in 1918 defying the norms of treating gender identity as a disease now has burgeoned into a field that includes 1,079 PubMed articles,two medical guidelines1,2, and a multitude of books. As we learn more about the complexity of gender and gender identity, we also are discovering potential problems that occur when providing care to our transgender patients. One is eating disorders.
A systematic review by Jones et al. showed only a handful of studies on eating disorders in transgender individuals, most of them restricted to case studies.3 In some situations, the issue of gender identity arises during treatment for an eating disorder, as the individual realizes that body dissatisfaction is due to the gender identity instead of a fear of gaining weight. In other cases, a transgender person in the process of transitioning to the affirmed gender develops an eating disorder.
There are two larger quantitative studies on eating disorders among transgender individuals. One study of 289,024 college students reveals that transgender students, compared to cisgender students, are almost five times as likely to report an eating disorder and two times as likely to use unhealthy compensatory methods (e.g., vomiting) for weight control.4 Another study of almost 2,500 teenagers shows that transgender individuals are almost three times as likely to restrict their eating, almost nine times as likely to take diet pills, and seven times as likely to take laxatives.5
The most commonly suggested reason for the possible elevated risk for eating disorders among transgender individuals is that many of them are trying to achieve the unrealistic standards of the ideal masculine or feminine body type. Another explanation is that eating disorders among transgender individuals are maladaptive coping mechanisms to stress from antitrans stigma and discrimination.4 However, these explanations are not mutually exclusive and could simultaneously drive disordered eating among transgender individuals.
To further appreciate the relationship between these two conditions, one must understand their similarities and differences. The Diagnostic Statistical Manual of Mental Disorders V characterizes eating disorders as “a persistent disturbance of eating or eating-related behavior that results in the altered consumption or absorption of food and ... significantly impairs physical health or psychosocial functioning.”6 Anorexia nervosa and bulimia nervosa are driven by fear of gaining weight or by a self-esteem unduly influenced by weight or appearance.6
Gender dysphoria, in comparison, is the distress caused by the incongruence between one’s gender identity and one’s anatomy, along with the desire to have the characteristics of one’s affirmed gender identity. This condition also could severely alter physical and psychosocial functioning,7 partly because of the distress from the incongruence, and partly because of the stress from antitrans stigma and discrimination, as an individual attempts to match the body with the gender identity8 (e.g., wearing clothing to match the gender identity).
The higher risk of developing an eating disorder among transgender individuals makes sense. Dissatisfaction with one’s body characterizes both conditions. The high standards on what is masculine or feminine affects everyone, especially transgender individuals who may feel that they’re “far behind” when they begin to transition to their affirmed gender. In addition, both involve identity. Those who have anorexia nervosa also incorporate this into their own identity.9 This is why treating an eating disorder can be very difficult.
Finally, individuals afflicted by an eating disorder or gender dysphoria engage in certain behaviors to achieve their desired appearance. However, this is where the similarities end. One major distinction between an eating disorder and gender dysphoria is the treatment approach. The goal in treating an eating disorder is to discourage the disordered behavior and encourage healthier eating habits and a more positive body image. Affirming the identity of someone with an eating disorder can be deadly, as it will encourage more disordered eating.10 In contrast, affirming the identity of someone with gender dysphoria through social transition, cross-sex hormones, and/or surgical reassignment is life-saving and therapeutic.11
There is little guidance on how to treat the these disorders simultaneously. What complicates treating both conditions at the same time is that when an eating disorder is accompanied by another mental health disorder (e.g., substance use), one condition over the other is prioritized.12 There is no guidance on whether the eating disorder or gender dysphoria should take priority over the other, or if it is possible to treat both conditions at the same time.
Strandjord et al. suggest a hierarchal approach, in which life-threatening issues (such as suicide or electrolyte disturbances) take priority.13 In addition, if the patient is malnourished, weight restoration should be the initial focus. A patient who is severely malnourished may not have the cognitive capacity nor the physiological ability to manage comorbidities such as anxiety or depression,12 much less have the capacity to process something as complex as gender and gender identity, nor understand the steps necessary for a successful transition to the affirmed gender. However, this does not mean providers should wait to successfully manage an eating disorder before addressing gender dysphoria. Studies have suggested that gender-affirming medical therapies (e.g., cross sex hormones) can be therapeutic for both gender dysphoria and eating disorder symptoms.14 Finally, because of the two ways a transgender patient with an eating disorder can present, I recommend screening for eating disorders in transgender individuals and inquiring about gender identity among those with an eating disorder. Doing so may save a life.
References
1. J Clin Endocrinol Metab. 2009 Sep;94(9):3132-54.
2. Adv Urol. 2012;2012:581712.
3. Int Rev Psychiatry. 2016;28(1):81-94.
4. J Adolesc Health. 2015 Aug;57(2):144-9.
5. J Adolesc Health. 2016. doi: 10.1016/j.jadohealth.2016.08.027.
6. Feeding and Eating Disorders. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. (Washington: American Psychiatric Association, 2013).
7. Gender Dysphoria. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. (Washington: American Psychiatric Association, 2013).
8. Psychol Bull. 2003 Sep;129(5):674-97.
9. Int J Law Psychiatry. 2003 Sep-Oct;26(5):533-48.
10. Arch Gen Psychiatry. 2011 Jul;68(7):724-31.
11. Clin Endocrinol (Oxf). 2010 Feb;72(2):214-31.
12. CNS drugs. 2006;20(8):655-63.
13. Int J Eat Disord. 2015 Nov;48(7):942-5.
14. Eat Disord. 2012;20(4):300-11.
The field of transgender health is growing. What began as a lone German physician in 1918 defying the norms of treating gender identity as a disease now has burgeoned into a field that includes 1,079 PubMed articles,two medical guidelines1,2, and a multitude of books. As we learn more about the complexity of gender and gender identity, we also are discovering potential problems that occur when providing care to our transgender patients. One is eating disorders.
A systematic review by Jones et al. showed only a handful of studies on eating disorders in transgender individuals, most of them restricted to case studies.3 In some situations, the issue of gender identity arises during treatment for an eating disorder, as the individual realizes that body dissatisfaction is due to the gender identity instead of a fear of gaining weight. In other cases, a transgender person in the process of transitioning to the affirmed gender develops an eating disorder.
There are two larger quantitative studies on eating disorders among transgender individuals. One study of 289,024 college students reveals that transgender students, compared to cisgender students, are almost five times as likely to report an eating disorder and two times as likely to use unhealthy compensatory methods (e.g., vomiting) for weight control.4 Another study of almost 2,500 teenagers shows that transgender individuals are almost three times as likely to restrict their eating, almost nine times as likely to take diet pills, and seven times as likely to take laxatives.5
The most commonly suggested reason for the possible elevated risk for eating disorders among transgender individuals is that many of them are trying to achieve the unrealistic standards of the ideal masculine or feminine body type. Another explanation is that eating disorders among transgender individuals are maladaptive coping mechanisms to stress from antitrans stigma and discrimination.4 However, these explanations are not mutually exclusive and could simultaneously drive disordered eating among transgender individuals.
To further appreciate the relationship between these two conditions, one must understand their similarities and differences. The Diagnostic Statistical Manual of Mental Disorders V characterizes eating disorders as “a persistent disturbance of eating or eating-related behavior that results in the altered consumption or absorption of food and ... significantly impairs physical health or psychosocial functioning.”6 Anorexia nervosa and bulimia nervosa are driven by fear of gaining weight or by a self-esteem unduly influenced by weight or appearance.6
Gender dysphoria, in comparison, is the distress caused by the incongruence between one’s gender identity and one’s anatomy, along with the desire to have the characteristics of one’s affirmed gender identity. This condition also could severely alter physical and psychosocial functioning,7 partly because of the distress from the incongruence, and partly because of the stress from antitrans stigma and discrimination, as an individual attempts to match the body with the gender identity8 (e.g., wearing clothing to match the gender identity).
The higher risk of developing an eating disorder among transgender individuals makes sense. Dissatisfaction with one’s body characterizes both conditions. The high standards on what is masculine or feminine affects everyone, especially transgender individuals who may feel that they’re “far behind” when they begin to transition to their affirmed gender. In addition, both involve identity. Those who have anorexia nervosa also incorporate this into their own identity.9 This is why treating an eating disorder can be very difficult.
Finally, individuals afflicted by an eating disorder or gender dysphoria engage in certain behaviors to achieve their desired appearance. However, this is where the similarities end. One major distinction between an eating disorder and gender dysphoria is the treatment approach. The goal in treating an eating disorder is to discourage the disordered behavior and encourage healthier eating habits and a more positive body image. Affirming the identity of someone with an eating disorder can be deadly, as it will encourage more disordered eating.10 In contrast, affirming the identity of someone with gender dysphoria through social transition, cross-sex hormones, and/or surgical reassignment is life-saving and therapeutic.11
There is little guidance on how to treat the these disorders simultaneously. What complicates treating both conditions at the same time is that when an eating disorder is accompanied by another mental health disorder (e.g., substance use), one condition over the other is prioritized.12 There is no guidance on whether the eating disorder or gender dysphoria should take priority over the other, or if it is possible to treat both conditions at the same time.
Strandjord et al. suggest a hierarchal approach, in which life-threatening issues (such as suicide or electrolyte disturbances) take priority.13 In addition, if the patient is malnourished, weight restoration should be the initial focus. A patient who is severely malnourished may not have the cognitive capacity nor the physiological ability to manage comorbidities such as anxiety or depression,12 much less have the capacity to process something as complex as gender and gender identity, nor understand the steps necessary for a successful transition to the affirmed gender. However, this does not mean providers should wait to successfully manage an eating disorder before addressing gender dysphoria. Studies have suggested that gender-affirming medical therapies (e.g., cross sex hormones) can be therapeutic for both gender dysphoria and eating disorder symptoms.14 Finally, because of the two ways a transgender patient with an eating disorder can present, I recommend screening for eating disorders in transgender individuals and inquiring about gender identity among those with an eating disorder. Doing so may save a life.
References
1. J Clin Endocrinol Metab. 2009 Sep;94(9):3132-54.
2. Adv Urol. 2012;2012:581712.
3. Int Rev Psychiatry. 2016;28(1):81-94.
4. J Adolesc Health. 2015 Aug;57(2):144-9.
5. J Adolesc Health. 2016. doi: 10.1016/j.jadohealth.2016.08.027.
6. Feeding and Eating Disorders. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. (Washington: American Psychiatric Association, 2013).
7. Gender Dysphoria. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. (Washington: American Psychiatric Association, 2013).
8. Psychol Bull. 2003 Sep;129(5):674-97.
9. Int J Law Psychiatry. 2003 Sep-Oct;26(5):533-48.
10. Arch Gen Psychiatry. 2011 Jul;68(7):724-31.
11. Clin Endocrinol (Oxf). 2010 Feb;72(2):214-31.
12. CNS drugs. 2006;20(8):655-63.
13. Int J Eat Disord. 2015 Nov;48(7):942-5.
14. Eat Disord. 2012;20(4):300-11.
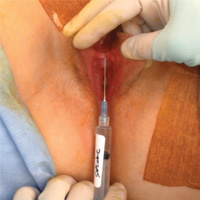
Novel classification of labial anatomy and evaluation in the treatment of labial agglutination

References
Pardo J, Sola V, Ricci P, Guillof E. Laser labioplasty of labia minora. Int J Gynaecol Obstet. 2006;93(1):38–43.
Chang P, Salisbury MA, Narsete T, Buckspan R, Derrick D, Ersek RA. Vaginal labiaplasty: defense of the simple "clip and snip" and a new classification system. Aesthetic Plast Surg. 2013;37(5): p. 887–891.
Malone DG, Clark TB, Wei N. Ultrasound-guided percutaneous injection, hydrodissection, and fenestration for carpel tunnel syndrome description of a new technique. J Appl Res. 2010;10(3):116–123.
Visit the Society of Gynecologic Surgeons online: sgsonline.org
More videos from SGS:
- Strategies for prophylactic oophoropexy
- Tips and tricks for open laparoscopy
- Complete colpectomy & colpocleisis: Model for simulation
- Natural orifice sacral colpopexy
- Alternative options for visualizing ureteral patency during intraoperative cystoscopy
- Use of suprapubic Carter-Thomason needle to assist in cystoscopic excision of an intravesical foreign object
- Uterine artery ligation: Advanced techniques and considerations for the difficult laparoscopic hysterectomy
- Cervical injection of methylene blue for identification of sentinel lymph nodes in cervical cancer
- Misplaced hysteroscopic sterilization micro-insert in the peritoneal cavity: A corpus alienum
- Laparoscopic cystectomy for large, bilateral ovarian dermoids
- Small bowel surgery for the benign gynecologist

References
Pardo J, Sola V, Ricci P, Guillof E. Laser labioplasty of labia minora. Int J Gynaecol Obstet. 2006;93(1):38–43.
Chang P, Salisbury MA, Narsete T, Buckspan R, Derrick D, Ersek RA. Vaginal labiaplasty: defense of the simple "clip and snip" and a new classification system. Aesthetic Plast Surg. 2013;37(5): p. 887–891.
Malone DG, Clark TB, Wei N. Ultrasound-guided percutaneous injection, hydrodissection, and fenestration for carpel tunnel syndrome description of a new technique. J Appl Res. 2010;10(3):116–123.
Visit the Society of Gynecologic Surgeons online: sgsonline.org
More videos from SGS:
- Strategies for prophylactic oophoropexy
- Tips and tricks for open laparoscopy
- Complete colpectomy & colpocleisis: Model for simulation
- Natural orifice sacral colpopexy
- Alternative options for visualizing ureteral patency during intraoperative cystoscopy
- Use of suprapubic Carter-Thomason needle to assist in cystoscopic excision of an intravesical foreign object
- Uterine artery ligation: Advanced techniques and considerations for the difficult laparoscopic hysterectomy
- Cervical injection of methylene blue for identification of sentinel lymph nodes in cervical cancer
- Misplaced hysteroscopic sterilization micro-insert in the peritoneal cavity: A corpus alienum
- Laparoscopic cystectomy for large, bilateral ovarian dermoids
- Small bowel surgery for the benign gynecologist

References
Pardo J, Sola V, Ricci P, Guillof E. Laser labioplasty of labia minora. Int J Gynaecol Obstet. 2006;93(1):38–43.
Chang P, Salisbury MA, Narsete T, Buckspan R, Derrick D, Ersek RA. Vaginal labiaplasty: defense of the simple "clip and snip" and a new classification system. Aesthetic Plast Surg. 2013;37(5): p. 887–891.
Malone DG, Clark TB, Wei N. Ultrasound-guided percutaneous injection, hydrodissection, and fenestration for carpel tunnel syndrome description of a new technique. J Appl Res. 2010;10(3):116–123.
Visit the Society of Gynecologic Surgeons online: sgsonline.org
More videos from SGS:
- Strategies for prophylactic oophoropexy
- Tips and tricks for open laparoscopy
- Complete colpectomy & colpocleisis: Model for simulation
- Natural orifice sacral colpopexy
- Alternative options for visualizing ureteral patency during intraoperative cystoscopy
- Use of suprapubic Carter-Thomason needle to assist in cystoscopic excision of an intravesical foreign object
- Uterine artery ligation: Advanced techniques and considerations for the difficult laparoscopic hysterectomy
- Cervical injection of methylene blue for identification of sentinel lymph nodes in cervical cancer
- Misplaced hysteroscopic sterilization micro-insert in the peritoneal cavity: A corpus alienum
- Laparoscopic cystectomy for large, bilateral ovarian dermoids
- Small bowel surgery for the benign gynecologist
This video is brought to you by
Not enough time? Time to rethink
Raising children is a lot like drinking out of a fire hose. Feeding, cleaning, dressing, transporting, teaching, entertaining, protecting, comforting, and managing one child is demanding, but is increased exponentially by multiple children, a spouse, and a job.
In our dataset of more than 74,900 parents of 0- to 3-year-olds completing a routine previsit questionnaire about the “best” and “hardest” parts of parenting their child, the most frequent spontaneous comment for the hardest part was “time-life balance.” The goal of asking these questions is to broaden the agenda for the pediatric visit to address stresses that are highly relevant to the child’s life in the family, and their current well-being and future outcome. The hardest part also rather succinctly captures the stress I hear every day from parents coming to me not only for health supervision, but especially for child behavior problems.
Setting limits on work to free up more time is not possible for everyone. Many people are grateful to have a job at all or need multiple jobs to make ends meet. They may not be in a position to negotiate for fewer tasks, hours, or roles. But others more fortunate may have fallen into a habit of taking on extra duties, taking work home, or simply not examining where they might set limits to preserve time for themselves and their family.
Working parents may need to prepare themselves for the onslaught when they get home. If the returning parent retreats into TV, the computer, or the bedroom, it makes the children feel angry and rejected. The parent who has been managing the household for the preceding hour(s) feels resentful, unappreciated, and often exhausted. I sometimes suggest that the returning parent pause 15 minutes to take a walk before picking children up at day care or go to the gym before coming home to be ready to engage, accept, and be present for whatever happens when they open the door.
Eliciting the “hardest part” can insert a pause for some much-needed problem-solving. Pointing out to parents the value to their child of working on their own time-life balance often gives them needed permission to make changes.
Balancing time for some parents may include setting some privacy for “alone time.” Individual desire to be alone varies, but trouble getting it is universal, especially with young children who don’t even respect a closed bathroom door! Given a young child’s need for contact about every 3-5 minutes, parents need to revise their expectations, wait until after bedtime, get some help, learn to do “token” relaxation, or all of these.
Parents often feel guilty for not attending more to their child, but then feel irritable about getting behind on other chores. It can be useful to cite the fact that mothers at home full time typically spend only 20 minutes of exclusive playtime with their child. I regularly prescribe 15 minutes of “special time” daily to break this irritability cycle for both the parent and child. Getting a babysitter does not mean that the parent has to leave the house and the undone laundry. I often suggest to resource-strapped families that they pay an 8-year-old neighbor to play with their kids for an hour several times per week. While not expecting to leave the child alone with such a “sitter,” one could relax in the tub, read a magazine, or make an uninterrupted phone call to a friend with such help.
The same parents feeling the pinch of too little time often are lacking in social support, a major buffer of stress. Sometimes, the solutions overlap. For example, trading play dates with another family by taking all their kids on a regular basis and vice versa requires no money exchange. Several kids playing together are often easier to care for than one’s own with their usual sibling struggles or boredom. And sharing of this kind can build lasting friendships and social support for the adults. Another often forgotten source of adult rest coupled with social support is religious services that offer “Sunday School.” The service has built-in cues to meditation, the kids make new friends protected by accepting teachers, and the social hour builds social support for the parents.
But we can’t really insert more hours in the day, right? Actually, one of the most valuable suggestions may be for parents to keep a diary of their activities for a few days. The average American in 2015 clocked 147 minutes watching TV, 103 minutes in front of a computer, 151 minutes on smart phones, and 43 minutes with a tablet. These time wasters may not only not feel satisfying or even relaxing, but even prompt anxiety or envy, and certainly take away from sleep, exercise, and intimacy. The American Academy of Pediatrics recently provided a Media Calculator and Family Media Plan intended to help families consider these choices for their child’s media life within all the other required activities of a day (including sleep), but adults could benefit from the same approach to making decisions about how they budget their time.
By mapping out actual time spent, parents can then reevaluate and choose differently. A useful question we might ask frazzled parents is “What fills your tank?” to help them come up with a list of activities that (used to be) regenerative to put on the new schedule. Most people blurt out “go on a cruise” (not practical) when “token” activities can suffice and be immediately possible. Coach them to be creative! Instead of a cruise, take a walk around the block; instead of going to a spa, request a back rub at bedtime; instead of a movie, watch a YouTube clip. When allowing oneself to be fully present to such “tokens,” they can have immense value. The practice of mindfulness (for which many training apps are available) can heighten awareness of each moment and expand the sense of time. Meditation and yoga training both are proven to provide benefits for relaxation and well-being that can be fit into anyone’s day.
While this column is intended to help with pediatric practice, I’ll bet you thought I was talking about you! With the pace of current health care practice and emphasis on “productivity,” many pediatricians are struggling with balancing time for themselves and their families as well. All the ideas just discussed also apply to you, but maybe, just maybe, you have the resources to insist on limits on work you haven’t seized. Cherishing the years when you have children in your life is for you, too, not just your patients. Remember, “The days are long, but the years are short.”
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS. She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to Frontline Medical News. Email her at [email protected].
Raising children is a lot like drinking out of a fire hose. Feeding, cleaning, dressing, transporting, teaching, entertaining, protecting, comforting, and managing one child is demanding, but is increased exponentially by multiple children, a spouse, and a job.
In our dataset of more than 74,900 parents of 0- to 3-year-olds completing a routine previsit questionnaire about the “best” and “hardest” parts of parenting their child, the most frequent spontaneous comment for the hardest part was “time-life balance.” The goal of asking these questions is to broaden the agenda for the pediatric visit to address stresses that are highly relevant to the child’s life in the family, and their current well-being and future outcome. The hardest part also rather succinctly captures the stress I hear every day from parents coming to me not only for health supervision, but especially for child behavior problems.
Setting limits on work to free up more time is not possible for everyone. Many people are grateful to have a job at all or need multiple jobs to make ends meet. They may not be in a position to negotiate for fewer tasks, hours, or roles. But others more fortunate may have fallen into a habit of taking on extra duties, taking work home, or simply not examining where they might set limits to preserve time for themselves and their family.
Working parents may need to prepare themselves for the onslaught when they get home. If the returning parent retreats into TV, the computer, or the bedroom, it makes the children feel angry and rejected. The parent who has been managing the household for the preceding hour(s) feels resentful, unappreciated, and often exhausted. I sometimes suggest that the returning parent pause 15 minutes to take a walk before picking children up at day care or go to the gym before coming home to be ready to engage, accept, and be present for whatever happens when they open the door.
Eliciting the “hardest part” can insert a pause for some much-needed problem-solving. Pointing out to parents the value to their child of working on their own time-life balance often gives them needed permission to make changes.
Balancing time for some parents may include setting some privacy for “alone time.” Individual desire to be alone varies, but trouble getting it is universal, especially with young children who don’t even respect a closed bathroom door! Given a young child’s need for contact about every 3-5 minutes, parents need to revise their expectations, wait until after bedtime, get some help, learn to do “token” relaxation, or all of these.
Parents often feel guilty for not attending more to their child, but then feel irritable about getting behind on other chores. It can be useful to cite the fact that mothers at home full time typically spend only 20 minutes of exclusive playtime with their child. I regularly prescribe 15 minutes of “special time” daily to break this irritability cycle for both the parent and child. Getting a babysitter does not mean that the parent has to leave the house and the undone laundry. I often suggest to resource-strapped families that they pay an 8-year-old neighbor to play with their kids for an hour several times per week. While not expecting to leave the child alone with such a “sitter,” one could relax in the tub, read a magazine, or make an uninterrupted phone call to a friend with such help.
The same parents feeling the pinch of too little time often are lacking in social support, a major buffer of stress. Sometimes, the solutions overlap. For example, trading play dates with another family by taking all their kids on a regular basis and vice versa requires no money exchange. Several kids playing together are often easier to care for than one’s own with their usual sibling struggles or boredom. And sharing of this kind can build lasting friendships and social support for the adults. Another often forgotten source of adult rest coupled with social support is religious services that offer “Sunday School.” The service has built-in cues to meditation, the kids make new friends protected by accepting teachers, and the social hour builds social support for the parents.
But we can’t really insert more hours in the day, right? Actually, one of the most valuable suggestions may be for parents to keep a diary of their activities for a few days. The average American in 2015 clocked 147 minutes watching TV, 103 minutes in front of a computer, 151 minutes on smart phones, and 43 minutes with a tablet. These time wasters may not only not feel satisfying or even relaxing, but even prompt anxiety or envy, and certainly take away from sleep, exercise, and intimacy. The American Academy of Pediatrics recently provided a Media Calculator and Family Media Plan intended to help families consider these choices for their child’s media life within all the other required activities of a day (including sleep), but adults could benefit from the same approach to making decisions about how they budget their time.
By mapping out actual time spent, parents can then reevaluate and choose differently. A useful question we might ask frazzled parents is “What fills your tank?” to help them come up with a list of activities that (used to be) regenerative to put on the new schedule. Most people blurt out “go on a cruise” (not practical) when “token” activities can suffice and be immediately possible. Coach them to be creative! Instead of a cruise, take a walk around the block; instead of going to a spa, request a back rub at bedtime; instead of a movie, watch a YouTube clip. When allowing oneself to be fully present to such “tokens,” they can have immense value. The practice of mindfulness (for which many training apps are available) can heighten awareness of each moment and expand the sense of time. Meditation and yoga training both are proven to provide benefits for relaxation and well-being that can be fit into anyone’s day.
While this column is intended to help with pediatric practice, I’ll bet you thought I was talking about you! With the pace of current health care practice and emphasis on “productivity,” many pediatricians are struggling with balancing time for themselves and their families as well. All the ideas just discussed also apply to you, but maybe, just maybe, you have the resources to insist on limits on work you haven’t seized. Cherishing the years when you have children in your life is for you, too, not just your patients. Remember, “The days are long, but the years are short.”
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS. She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to Frontline Medical News. Email her at [email protected].
Raising children is a lot like drinking out of a fire hose. Feeding, cleaning, dressing, transporting, teaching, entertaining, protecting, comforting, and managing one child is demanding, but is increased exponentially by multiple children, a spouse, and a job.
In our dataset of more than 74,900 parents of 0- to 3-year-olds completing a routine previsit questionnaire about the “best” and “hardest” parts of parenting their child, the most frequent spontaneous comment for the hardest part was “time-life balance.” The goal of asking these questions is to broaden the agenda for the pediatric visit to address stresses that are highly relevant to the child’s life in the family, and their current well-being and future outcome. The hardest part also rather succinctly captures the stress I hear every day from parents coming to me not only for health supervision, but especially for child behavior problems.
Setting limits on work to free up more time is not possible for everyone. Many people are grateful to have a job at all or need multiple jobs to make ends meet. They may not be in a position to negotiate for fewer tasks, hours, or roles. But others more fortunate may have fallen into a habit of taking on extra duties, taking work home, or simply not examining where they might set limits to preserve time for themselves and their family.
Working parents may need to prepare themselves for the onslaught when they get home. If the returning parent retreats into TV, the computer, or the bedroom, it makes the children feel angry and rejected. The parent who has been managing the household for the preceding hour(s) feels resentful, unappreciated, and often exhausted. I sometimes suggest that the returning parent pause 15 minutes to take a walk before picking children up at day care or go to the gym before coming home to be ready to engage, accept, and be present for whatever happens when they open the door.
Eliciting the “hardest part” can insert a pause for some much-needed problem-solving. Pointing out to parents the value to their child of working on their own time-life balance often gives them needed permission to make changes.
Balancing time for some parents may include setting some privacy for “alone time.” Individual desire to be alone varies, but trouble getting it is universal, especially with young children who don’t even respect a closed bathroom door! Given a young child’s need for contact about every 3-5 minutes, parents need to revise their expectations, wait until after bedtime, get some help, learn to do “token” relaxation, or all of these.
Parents often feel guilty for not attending more to their child, but then feel irritable about getting behind on other chores. It can be useful to cite the fact that mothers at home full time typically spend only 20 minutes of exclusive playtime with their child. I regularly prescribe 15 minutes of “special time” daily to break this irritability cycle for both the parent and child. Getting a babysitter does not mean that the parent has to leave the house and the undone laundry. I often suggest to resource-strapped families that they pay an 8-year-old neighbor to play with their kids for an hour several times per week. While not expecting to leave the child alone with such a “sitter,” one could relax in the tub, read a magazine, or make an uninterrupted phone call to a friend with such help.
The same parents feeling the pinch of too little time often are lacking in social support, a major buffer of stress. Sometimes, the solutions overlap. For example, trading play dates with another family by taking all their kids on a regular basis and vice versa requires no money exchange. Several kids playing together are often easier to care for than one’s own with their usual sibling struggles or boredom. And sharing of this kind can build lasting friendships and social support for the adults. Another often forgotten source of adult rest coupled with social support is religious services that offer “Sunday School.” The service has built-in cues to meditation, the kids make new friends protected by accepting teachers, and the social hour builds social support for the parents.
But we can’t really insert more hours in the day, right? Actually, one of the most valuable suggestions may be for parents to keep a diary of their activities for a few days. The average American in 2015 clocked 147 minutes watching TV, 103 minutes in front of a computer, 151 minutes on smart phones, and 43 minutes with a tablet. These time wasters may not only not feel satisfying or even relaxing, but even prompt anxiety or envy, and certainly take away from sleep, exercise, and intimacy. The American Academy of Pediatrics recently provided a Media Calculator and Family Media Plan intended to help families consider these choices for their child’s media life within all the other required activities of a day (including sleep), but adults could benefit from the same approach to making decisions about how they budget their time.
By mapping out actual time spent, parents can then reevaluate and choose differently. A useful question we might ask frazzled parents is “What fills your tank?” to help them come up with a list of activities that (used to be) regenerative to put on the new schedule. Most people blurt out “go on a cruise” (not practical) when “token” activities can suffice and be immediately possible. Coach them to be creative! Instead of a cruise, take a walk around the block; instead of going to a spa, request a back rub at bedtime; instead of a movie, watch a YouTube clip. When allowing oneself to be fully present to such “tokens,” they can have immense value. The practice of mindfulness (for which many training apps are available) can heighten awareness of each moment and expand the sense of time. Meditation and yoga training both are proven to provide benefits for relaxation and well-being that can be fit into anyone’s day.
While this column is intended to help with pediatric practice, I’ll bet you thought I was talking about you! With the pace of current health care practice and emphasis on “productivity,” many pediatricians are struggling with balancing time for themselves and their families as well. All the ideas just discussed also apply to you, but maybe, just maybe, you have the resources to insist on limits on work you haven’t seized. Cherishing the years when you have children in your life is for you, too, not just your patients. Remember, “The days are long, but the years are short.”
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS. She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to Frontline Medical News. Email her at [email protected].
Brodalumab approved for psoriasis with REMS required
The Food and Drug Administration has approved brodalumab, an interleukin-17 receptor A-antagonist, for treating adults with moderate to severe plaque psoriasis, with a Risk Evaluation and Mitigation Strategy (REMS) that addresses the “observed risk of suicidal ideation and behavior” in clinical trials, the agency announced on Feb. 16.
In the three pivotal phase III studies of 4,373 adults with moderate to severe plaque psoriasis who were candidates for systemic therapy or phototherapy, 83%-86% of those treated with brodalumab achieved Psoriasis Area and Severity Index (PASI 75) scores at 12 weeks of treatment, compared with 3%-8% of those on placebo. In addition, 37%-44% of those on brodalumab achieved PASI 100 scores, compared with 1% or fewer of those on placebo. In the psoriasis clinical trials, suicidal ideation and behavior, which included four completed suicides, occurred in patients treated with brodalumab, according to the prescribing information
In the statement, the FDA points out that “a causal association between treatment with Siliq and increased risk of suicidal ideation and behavior has not been established,” but that “suicidal ideation and behavior, including completed suicides, have occurred in patients treated with Siliq during clinical trials. Siliq users with a history of suicidality or depression had an increased incidence of suicidal ideation and behavior, compared to users without this history.”
At a meeting in July 2016, the FDA’s Dermatologic and Ophthalmic Drugs Advisory Committee voted 18-0 in favor of approving brodalumab, with the majority (14) recommending risk management options beyond labeling to address these concerns.
Elements of the REMS include requirements for prescribers, pharmacy certification, and a medication guide for patients with information about the risks of suicidal ideation and behavior. Prescribers are required to counsel patients about this risk, and patients are required to sign a “Patient-Prescriber Agreement Form and be made aware of the need to seek medical attention should they experience new or worsening suicidal thoughts or behavior, feelings of depression, anxiety or other mood changes,” the FDA said. The prescribing information also includes a boxed warning about suicidal ideation and behavior.
The most common adverse events associated with brodalumab, the FDA statement noted, include arthralgia, headache, fatigue, diarrhea, oropharyngeal pain, nausea, myalgia, injection site reactions, neutropenia, and fungal infections. The recommended dose of brodalumab is 210 mg, administered subcutaneously, at weeks 0, 1, and 2, followed by 210 mg every 2 weeks.
The Food and Drug Administration has approved brodalumab, an interleukin-17 receptor A-antagonist, for treating adults with moderate to severe plaque psoriasis, with a Risk Evaluation and Mitigation Strategy (REMS) that addresses the “observed risk of suicidal ideation and behavior” in clinical trials, the agency announced on Feb. 16.
In the three pivotal phase III studies of 4,373 adults with moderate to severe plaque psoriasis who were candidates for systemic therapy or phototherapy, 83%-86% of those treated with brodalumab achieved Psoriasis Area and Severity Index (PASI 75) scores at 12 weeks of treatment, compared with 3%-8% of those on placebo. In addition, 37%-44% of those on brodalumab achieved PASI 100 scores, compared with 1% or fewer of those on placebo. In the psoriasis clinical trials, suicidal ideation and behavior, which included four completed suicides, occurred in patients treated with brodalumab, according to the prescribing information
In the statement, the FDA points out that “a causal association between treatment with Siliq and increased risk of suicidal ideation and behavior has not been established,” but that “suicidal ideation and behavior, including completed suicides, have occurred in patients treated with Siliq during clinical trials. Siliq users with a history of suicidality or depression had an increased incidence of suicidal ideation and behavior, compared to users without this history.”
At a meeting in July 2016, the FDA’s Dermatologic and Ophthalmic Drugs Advisory Committee voted 18-0 in favor of approving brodalumab, with the majority (14) recommending risk management options beyond labeling to address these concerns.
Elements of the REMS include requirements for prescribers, pharmacy certification, and a medication guide for patients with information about the risks of suicidal ideation and behavior. Prescribers are required to counsel patients about this risk, and patients are required to sign a “Patient-Prescriber Agreement Form and be made aware of the need to seek medical attention should they experience new or worsening suicidal thoughts or behavior, feelings of depression, anxiety or other mood changes,” the FDA said. The prescribing information also includes a boxed warning about suicidal ideation and behavior.
The most common adverse events associated with brodalumab, the FDA statement noted, include arthralgia, headache, fatigue, diarrhea, oropharyngeal pain, nausea, myalgia, injection site reactions, neutropenia, and fungal infections. The recommended dose of brodalumab is 210 mg, administered subcutaneously, at weeks 0, 1, and 2, followed by 210 mg every 2 weeks.
The Food and Drug Administration has approved brodalumab, an interleukin-17 receptor A-antagonist, for treating adults with moderate to severe plaque psoriasis, with a Risk Evaluation and Mitigation Strategy (REMS) that addresses the “observed risk of suicidal ideation and behavior” in clinical trials, the agency announced on Feb. 16.
In the three pivotal phase III studies of 4,373 adults with moderate to severe plaque psoriasis who were candidates for systemic therapy or phototherapy, 83%-86% of those treated with brodalumab achieved Psoriasis Area and Severity Index (PASI 75) scores at 12 weeks of treatment, compared with 3%-8% of those on placebo. In addition, 37%-44% of those on brodalumab achieved PASI 100 scores, compared with 1% or fewer of those on placebo. In the psoriasis clinical trials, suicidal ideation and behavior, which included four completed suicides, occurred in patients treated with brodalumab, according to the prescribing information
In the statement, the FDA points out that “a causal association between treatment with Siliq and increased risk of suicidal ideation and behavior has not been established,” but that “suicidal ideation and behavior, including completed suicides, have occurred in patients treated with Siliq during clinical trials. Siliq users with a history of suicidality or depression had an increased incidence of suicidal ideation and behavior, compared to users without this history.”
At a meeting in July 2016, the FDA’s Dermatologic and Ophthalmic Drugs Advisory Committee voted 18-0 in favor of approving brodalumab, with the majority (14) recommending risk management options beyond labeling to address these concerns.
Elements of the REMS include requirements for prescribers, pharmacy certification, and a medication guide for patients with information about the risks of suicidal ideation and behavior. Prescribers are required to counsel patients about this risk, and patients are required to sign a “Patient-Prescriber Agreement Form and be made aware of the need to seek medical attention should they experience new or worsening suicidal thoughts or behavior, feelings of depression, anxiety or other mood changes,” the FDA said. The prescribing information also includes a boxed warning about suicidal ideation and behavior.
The most common adverse events associated with brodalumab, the FDA statement noted, include arthralgia, headache, fatigue, diarrhea, oropharyngeal pain, nausea, myalgia, injection site reactions, neutropenia, and fungal infections. The recommended dose of brodalumab is 210 mg, administered subcutaneously, at weeks 0, 1, and 2, followed by 210 mg every 2 weeks.