User login
Vitamin D reduces respiratory infection risk
Administering doses of a vitamin D supplement to patients can significantly mitigate their risk of developing acute respiratory tract infections, according to a recent study published by the BMJ.
“[Existing] epidemiological and in vitro data have prompted numerous randomized controlled trials to determine whether vitamin D supplementation can decrease the risk of acute respiratory tract infection,” wrote the authors of the study, led by Adrian R. Martineau, PhD, of Queen Mary University of London. “A total of five aggregate data meta-analyses incorporating data from up to 15 primary trials have been conducted to date [but] all but one of these aggregate data meta-analyses reported statistically significant heterogeneity of effect between primary trials.”
A total of 532 studies were reviewed by a panel, of which 25 studies were ultimately selected for inclusion in this analysis. The studies included were of varying lengths in terms of trial periods and involved a total of 11,321 subjects ranging from 0 to 95 years of age. Of these, 10,933 (96.6%) subjects experienced at least one acute respiratory tract infection.
No significant benefit was found in subjects who had already experienced an infection, yielding an odds ratio of 0.98 (95% CI, 0.80-1.20; P = .83). Analysis performed to quantify the risk of infection with or without Vitamin D showed that taking Vitamin D supplements significantly decreased infection risk, with an OR of 0.88 (95% CI, 0.81-0.96; P less than .001) after adjusting for age, sex, and the duration of the trial.
Results also demonstrated that bolus doses of Vitamin D did not offer any beneficial value to subjects. Those who received daily or weekly doses without bolus had a better OR, compared with those who did receive at least one bolus dose: 0.81 (95% CI, 0.72-0.91) versus 0.97 (95% CI, 0.86-1.10), respectively (P = .05). Individuals whose baseline 25-hydroxyvitamin D levels were lower than 25 nanomols per liter experienced a greater benefit than those whose levels were above 25: OR = 0.30 (95% CI, 0.17-0.53) and OR = 0.75 (95% CI, 0.60-0.95), respectively (P = .006).
“Our study reports a major new indication for vitamin D supplementation: the prevention of acute respiratory tract infection,” Dr. Martineau and his coauthors concluded, adding that a potential application for these findings would be “the introduction of public health measures such as food fortification to improve vitamin D status, particularly in settings where profound vitamin D deficiency is common.”
The study was funded by a grant from the National Institute of Health Research. Dr. Martineau and his coauthors did not report any relevant financial disclosures.
While the work undertaken by Dr. Martineau et al. is commendable, the results themselves are ultimately underwhelming. The study’s results are too heterogeneous and offer too slight a reduction in overall risk to justify a complete overhaul of clinical procedure and prescribing protocols. These findings should not change clinical practice in any significant way, and there are other groups of individuals, such as those with low serum concentrations of vitamin D, that were omitted from this analysis altogether.
Mark J. Bolland is an associate professor of medicine at the University of Auckland (New Zealand). Alison Avenell is a professor at the University of Aberdeen (Scotland).
While the work undertaken by Dr. Martineau et al. is commendable, the results themselves are ultimately underwhelming. The study’s results are too heterogeneous and offer too slight a reduction in overall risk to justify a complete overhaul of clinical procedure and prescribing protocols. These findings should not change clinical practice in any significant way, and there are other groups of individuals, such as those with low serum concentrations of vitamin D, that were omitted from this analysis altogether.
Mark J. Bolland is an associate professor of medicine at the University of Auckland (New Zealand). Alison Avenell is a professor at the University of Aberdeen (Scotland).
While the work undertaken by Dr. Martineau et al. is commendable, the results themselves are ultimately underwhelming. The study’s results are too heterogeneous and offer too slight a reduction in overall risk to justify a complete overhaul of clinical procedure and prescribing protocols. These findings should not change clinical practice in any significant way, and there are other groups of individuals, such as those with low serum concentrations of vitamin D, that were omitted from this analysis altogether.
Mark J. Bolland is an associate professor of medicine at the University of Auckland (New Zealand). Alison Avenell is a professor at the University of Aberdeen (Scotland).
Administering doses of a vitamin D supplement to patients can significantly mitigate their risk of developing acute respiratory tract infections, according to a recent study published by the BMJ.
“[Existing] epidemiological and in vitro data have prompted numerous randomized controlled trials to determine whether vitamin D supplementation can decrease the risk of acute respiratory tract infection,” wrote the authors of the study, led by Adrian R. Martineau, PhD, of Queen Mary University of London. “A total of five aggregate data meta-analyses incorporating data from up to 15 primary trials have been conducted to date [but] all but one of these aggregate data meta-analyses reported statistically significant heterogeneity of effect between primary trials.”
A total of 532 studies were reviewed by a panel, of which 25 studies were ultimately selected for inclusion in this analysis. The studies included were of varying lengths in terms of trial periods and involved a total of 11,321 subjects ranging from 0 to 95 years of age. Of these, 10,933 (96.6%) subjects experienced at least one acute respiratory tract infection.
No significant benefit was found in subjects who had already experienced an infection, yielding an odds ratio of 0.98 (95% CI, 0.80-1.20; P = .83). Analysis performed to quantify the risk of infection with or without Vitamin D showed that taking Vitamin D supplements significantly decreased infection risk, with an OR of 0.88 (95% CI, 0.81-0.96; P less than .001) after adjusting for age, sex, and the duration of the trial.
Results also demonstrated that bolus doses of Vitamin D did not offer any beneficial value to subjects. Those who received daily or weekly doses without bolus had a better OR, compared with those who did receive at least one bolus dose: 0.81 (95% CI, 0.72-0.91) versus 0.97 (95% CI, 0.86-1.10), respectively (P = .05). Individuals whose baseline 25-hydroxyvitamin D levels were lower than 25 nanomols per liter experienced a greater benefit than those whose levels were above 25: OR = 0.30 (95% CI, 0.17-0.53) and OR = 0.75 (95% CI, 0.60-0.95), respectively (P = .006).
“Our study reports a major new indication for vitamin D supplementation: the prevention of acute respiratory tract infection,” Dr. Martineau and his coauthors concluded, adding that a potential application for these findings would be “the introduction of public health measures such as food fortification to improve vitamin D status, particularly in settings where profound vitamin D deficiency is common.”
The study was funded by a grant from the National Institute of Health Research. Dr. Martineau and his coauthors did not report any relevant financial disclosures.
Administering doses of a vitamin D supplement to patients can significantly mitigate their risk of developing acute respiratory tract infections, according to a recent study published by the BMJ.
“[Existing] epidemiological and in vitro data have prompted numerous randomized controlled trials to determine whether vitamin D supplementation can decrease the risk of acute respiratory tract infection,” wrote the authors of the study, led by Adrian R. Martineau, PhD, of Queen Mary University of London. “A total of five aggregate data meta-analyses incorporating data from up to 15 primary trials have been conducted to date [but] all but one of these aggregate data meta-analyses reported statistically significant heterogeneity of effect between primary trials.”
A total of 532 studies were reviewed by a panel, of which 25 studies were ultimately selected for inclusion in this analysis. The studies included were of varying lengths in terms of trial periods and involved a total of 11,321 subjects ranging from 0 to 95 years of age. Of these, 10,933 (96.6%) subjects experienced at least one acute respiratory tract infection.
No significant benefit was found in subjects who had already experienced an infection, yielding an odds ratio of 0.98 (95% CI, 0.80-1.20; P = .83). Analysis performed to quantify the risk of infection with or without Vitamin D showed that taking Vitamin D supplements significantly decreased infection risk, with an OR of 0.88 (95% CI, 0.81-0.96; P less than .001) after adjusting for age, sex, and the duration of the trial.
Results also demonstrated that bolus doses of Vitamin D did not offer any beneficial value to subjects. Those who received daily or weekly doses without bolus had a better OR, compared with those who did receive at least one bolus dose: 0.81 (95% CI, 0.72-0.91) versus 0.97 (95% CI, 0.86-1.10), respectively (P = .05). Individuals whose baseline 25-hydroxyvitamin D levels were lower than 25 nanomols per liter experienced a greater benefit than those whose levels were above 25: OR = 0.30 (95% CI, 0.17-0.53) and OR = 0.75 (95% CI, 0.60-0.95), respectively (P = .006).
“Our study reports a major new indication for vitamin D supplementation: the prevention of acute respiratory tract infection,” Dr. Martineau and his coauthors concluded, adding that a potential application for these findings would be “the introduction of public health measures such as food fortification to improve vitamin D status, particularly in settings where profound vitamin D deficiency is common.”
The study was funded by a grant from the National Institute of Health Research. Dr. Martineau and his coauthors did not report any relevant financial disclosures.
Key clinical point:
Major finding: For those receiving vitamin D, the odds ratio of reducing respiratory infection risk was 0.88 (P less than .001).
Data source: Systematic review and meta-analysis of data on 11,321 subjects taken from 25 different trials.
Disclosures: Funded by the National Institute for Health Research; authors reported no relevant financial disclosures.
Methotrexate for RA: A 'fascinating drug’
SNOWMASS, COLO. – “When I started working with methotrexate in 1982, I never would have predicted that methotrexate would become the standard of care in treating rheumatoid arthritis. There’s just no way,” Michael E. Weinblatt, MD, recalled at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
“Even now, 35 years later, we continue to learn more about this fascinating drug,” added Dr. Weinblatt, professor of medicine at Harvard Medical School, Boston.
He highlighted recent developments in this ongoing story and presented some tricks of the trade gained in 35 years of up-close experience with the drug.
Enhancing effectiveness of biologics
One of the hottest topics in methotrexate research is the drug’s ability to enhance the effectiveness of many, but not all, biologic agents. All of the anti–tumor necrosis factor (anti-TNF) biologics as well as rituximab (Rituxan) are demonstrably more effective when used in combination with methotrexate. Dr. Weinblatt considers the widespread underutilization of this combination strategy scandalous.
“This is an incredibly important point: All of you use biologics and all of you use methotrexate, but I’ve been depressed by the fact that up to 30%-40% of patients – no matter which data set you look at – are on monotherapy biological therapy,” he said.
He cited data from the ongoing BRASS Registry (Brigham and Women’s Hospital Rheumatoid Arthritis Sequential Registry) to underscore his point that good things happen when biologics and methotrexate are used together. Of 1,395 BRASS Registry participants prospectively followed since 2003, the proportion on biologic therapy has climbed steadily from 41% at the outset to 68% in 2016. Remarkably, 82% of patients on biologic therapy remain on their first biologic agent. Fewer than 4% have switched biologics more than twice. That’s very unlike the experiences reported elsewhere.
“I think one of the reasons we have such positive data is that we have a high percentage of patients staying on their background methotrexate,” said Dr. Weinblatt, codirector of clinical rheumatology and associate director of the Center for Arthritis and Joint Diseases at Brigham and Women’s Hospital, Boston.
He noted that Dutch investigators reported at the 2016 annual meeting of the American College of Rheumatology that among 1,230 consecutive rheumatoid arthritis patients started on etanercept (Enbrel) or adalimumab (Humira), 28% in the etanercept group were on concomitant methotrexate, as were 64% of those who started on adalimumab. The patients spent a median of 1.3-1.6 years and a maximum of 9.2-9.3 years on their biologic agent. Patients on adalimumab monotherapy were 2.61-fold more likely to drop out than were those on dual therapy with methotrexate. Patients on etanercept monotherapy were 1.2-fold more likely to drop out, a difference that didn’t achieve statistical significance.
Although the investigators did not study the mechanism of prolonged on-treatment survival, they speculated that it probably involved methotrexate’s documented ability to prevent formation of anti-adalimumab antibodies. In contrast, patients on etanercept don’t develop blocking antibodies, Dr. Weinblatt observed.
The randomized, double-blind CONCERTO trial conducted in 395 methotrexate- and biologic-naive RA patients demonstrated that methotrexate reduces the immunogenicity of adalimumab in dose-dependent fashion. Participants were randomized to open-label adalimumab at 40 mg every 2 weeks plus weekly double-blind methotrexate at 2.5, 5, 10, or 20 mg. Clinical outcomes at 26 weeks as reflected in 28-joint count disease activity score and the Clinical Disease Activity Index were significantly better in patients on 10 or 20 mg/week of methotrexate than in those on 2.5 or 5 mg/week. Serum adalimumab levels were higher in patients on the two higher doses of methotrexate as well (Ann Rheum Dis. 2015 Jun;74[6]:1037-44).
“It ends up that if you don’t use methotrexate or you use a very low dose you increase the risk of developing antibodies against adalimumab and decrease the efficacy of the drug. So in clinical practice, if you’re going to be working with dose titration of methotrexate and your patient is on adalimumab, there’s a threshold below which you probably shouldn’t go. In this study, doses of 10 mg/week or more induced a greater clinical response,” he said.
With infliximab (Remicade), based upon 20-year-old studies, the threshold is 7.5 mg of methotrexate per week.
“With etanercept, we don’t know what the threshold is. You don’t develop blocking anti-drug antibodies with etanercept, but we do know that methotrexate enhances the efficacy of etanercept, and it doesn’t do it by changing the biologic’s pharmacokinetics and there’s no increase in methotrexate blood levels,” the rheumatologist continued.
Unlike the anti-TNF biologics and rituximab, the efficacy of the Janus kinase inhibitors tofacitinib (Xeljanz) and baricitinib is not enhanced when the drugs are used in combination with methotrexate, studies indicate.
The efficacy of certolizumab pegol (Cimzia) wasn’t affected by methotrexate dose category in a prespecified pooled subgroup analysis of the phase III RAPID 1 and RAPID 2 clinical trials. In the 1,273 certolizumab-treated patients, the week-24 treatment response was similar regardless of whether patients were on methotrexate at 10 mg/week or less, 10-15 mg/week, or more than 15 mg/week. The investigators concluded that to minimize treatment-emergent adverse events, physicians can tailor background methotrexate dosing based upon individual patient tolerance without affecting certolizumab’s efficacy (Arthritis Care Res [Hoboken]. 2016 Mar;68[3]:299-307).
An important aspect of this analysis was that among the 325 subjects randomized to placebo rather than certolizumab, the treatment response at week 24 was significantly better in those on more than 15 mg/week of methotrexate than with lower doses of the drug.
“Most patients on methotrexate need more than 15 mg/week. So it astonishes me that such a high percentage of patients enrolled in clinical trials around the world are on, like, 14 mg/week. I mean, most patients need somewhere between 15 and 25 mg/week for a response, although over time you might be able to decrease that dose,” Dr. Weinblatt said.
Side effects of methotrexate
“The biggest issue with methotrexate is the tolerability problem, since serious adverse events are incredibly rare with this molecule,” he said.
Hepatotoxicity is a concern, but Dr. Weinblatt emphasized that elevated liver function tests do not equal cirrhosis.
“Historically, during the first 6 months on methotrexate 20%-25% of patients increase their transaminases in every clinical trial where that’s been looked at. Over time, the liver compensates for the drug. But 5%-6% of patients experience repeated moderate elevations more than 1.5 times the upper limit of normal,” he said.
Key risk factors for methotrexate-related hepatotoxicity were identified in a national observational cohort study of 659 military veterans over age 65 when they started methotrexate for rheumatic diseases. The investigators found a 6% incidence of moderately elevated liver enzymes during a mean follow-up period of 7 months. Obesity was associated with a 1.9-fold increased risk, a serum total cholesterol greater than 240 mg/dL conferred a 5.8-fold elevated risk, and abnormal liver function tests at baseline were associated with a 3.2-fold increased risk (Arthritis Care Res [Hoboken]. 2014 Aug;66[8]:1159-66).
“No surprise: It’s patients who weigh more who are at increased risk for methotrexate-related transaminase increases. I actually think the biggest factor with regard to methotrexate liver disease is the patient’s [body mass index]. Patients in North America aren’t getting any slimmer, so you need to look at this with your patients. If you have a morbidly obese patient on methotrexate whose transaminases suddenly start going up, that’s the patient who’s at greatest risk for methotrexate hepatotoxicity,” he cautioned.
The 3.2-fold increased risk of repeated elevated transaminases associated with abnormal baseline liver function tests in the Veterans Affairs study should be a red flag for rheumatologists.
“I personally think patients shouldn’t start on methotrexate if they have elevated transaminases. They ought to be normal at the start. There are too many other good options now to treat our patients,” Dr. Weinblatt said.
He reported receiving research grants from half a dozen companies and serving as a consultant to more than two dozen.
SNOWMASS, COLO. – “When I started working with methotrexate in 1982, I never would have predicted that methotrexate would become the standard of care in treating rheumatoid arthritis. There’s just no way,” Michael E. Weinblatt, MD, recalled at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
“Even now, 35 years later, we continue to learn more about this fascinating drug,” added Dr. Weinblatt, professor of medicine at Harvard Medical School, Boston.
He highlighted recent developments in this ongoing story and presented some tricks of the trade gained in 35 years of up-close experience with the drug.
Enhancing effectiveness of biologics
One of the hottest topics in methotrexate research is the drug’s ability to enhance the effectiveness of many, but not all, biologic agents. All of the anti–tumor necrosis factor (anti-TNF) biologics as well as rituximab (Rituxan) are demonstrably more effective when used in combination with methotrexate. Dr. Weinblatt considers the widespread underutilization of this combination strategy scandalous.
“This is an incredibly important point: All of you use biologics and all of you use methotrexate, but I’ve been depressed by the fact that up to 30%-40% of patients – no matter which data set you look at – are on monotherapy biological therapy,” he said.
He cited data from the ongoing BRASS Registry (Brigham and Women’s Hospital Rheumatoid Arthritis Sequential Registry) to underscore his point that good things happen when biologics and methotrexate are used together. Of 1,395 BRASS Registry participants prospectively followed since 2003, the proportion on biologic therapy has climbed steadily from 41% at the outset to 68% in 2016. Remarkably, 82% of patients on biologic therapy remain on their first biologic agent. Fewer than 4% have switched biologics more than twice. That’s very unlike the experiences reported elsewhere.
“I think one of the reasons we have such positive data is that we have a high percentage of patients staying on their background methotrexate,” said Dr. Weinblatt, codirector of clinical rheumatology and associate director of the Center for Arthritis and Joint Diseases at Brigham and Women’s Hospital, Boston.
He noted that Dutch investigators reported at the 2016 annual meeting of the American College of Rheumatology that among 1,230 consecutive rheumatoid arthritis patients started on etanercept (Enbrel) or adalimumab (Humira), 28% in the etanercept group were on concomitant methotrexate, as were 64% of those who started on adalimumab. The patients spent a median of 1.3-1.6 years and a maximum of 9.2-9.3 years on their biologic agent. Patients on adalimumab monotherapy were 2.61-fold more likely to drop out than were those on dual therapy with methotrexate. Patients on etanercept monotherapy were 1.2-fold more likely to drop out, a difference that didn’t achieve statistical significance.
Although the investigators did not study the mechanism of prolonged on-treatment survival, they speculated that it probably involved methotrexate’s documented ability to prevent formation of anti-adalimumab antibodies. In contrast, patients on etanercept don’t develop blocking antibodies, Dr. Weinblatt observed.
The randomized, double-blind CONCERTO trial conducted in 395 methotrexate- and biologic-naive RA patients demonstrated that methotrexate reduces the immunogenicity of adalimumab in dose-dependent fashion. Participants were randomized to open-label adalimumab at 40 mg every 2 weeks plus weekly double-blind methotrexate at 2.5, 5, 10, or 20 mg. Clinical outcomes at 26 weeks as reflected in 28-joint count disease activity score and the Clinical Disease Activity Index were significantly better in patients on 10 or 20 mg/week of methotrexate than in those on 2.5 or 5 mg/week. Serum adalimumab levels were higher in patients on the two higher doses of methotrexate as well (Ann Rheum Dis. 2015 Jun;74[6]:1037-44).
“It ends up that if you don’t use methotrexate or you use a very low dose you increase the risk of developing antibodies against adalimumab and decrease the efficacy of the drug. So in clinical practice, if you’re going to be working with dose titration of methotrexate and your patient is on adalimumab, there’s a threshold below which you probably shouldn’t go. In this study, doses of 10 mg/week or more induced a greater clinical response,” he said.
With infliximab (Remicade), based upon 20-year-old studies, the threshold is 7.5 mg of methotrexate per week.
“With etanercept, we don’t know what the threshold is. You don’t develop blocking anti-drug antibodies with etanercept, but we do know that methotrexate enhances the efficacy of etanercept, and it doesn’t do it by changing the biologic’s pharmacokinetics and there’s no increase in methotrexate blood levels,” the rheumatologist continued.
Unlike the anti-TNF biologics and rituximab, the efficacy of the Janus kinase inhibitors tofacitinib (Xeljanz) and baricitinib is not enhanced when the drugs are used in combination with methotrexate, studies indicate.
The efficacy of certolizumab pegol (Cimzia) wasn’t affected by methotrexate dose category in a prespecified pooled subgroup analysis of the phase III RAPID 1 and RAPID 2 clinical trials. In the 1,273 certolizumab-treated patients, the week-24 treatment response was similar regardless of whether patients were on methotrexate at 10 mg/week or less, 10-15 mg/week, or more than 15 mg/week. The investigators concluded that to minimize treatment-emergent adverse events, physicians can tailor background methotrexate dosing based upon individual patient tolerance without affecting certolizumab’s efficacy (Arthritis Care Res [Hoboken]. 2016 Mar;68[3]:299-307).
An important aspect of this analysis was that among the 325 subjects randomized to placebo rather than certolizumab, the treatment response at week 24 was significantly better in those on more than 15 mg/week of methotrexate than with lower doses of the drug.
“Most patients on methotrexate need more than 15 mg/week. So it astonishes me that such a high percentage of patients enrolled in clinical trials around the world are on, like, 14 mg/week. I mean, most patients need somewhere between 15 and 25 mg/week for a response, although over time you might be able to decrease that dose,” Dr. Weinblatt said.
Side effects of methotrexate
“The biggest issue with methotrexate is the tolerability problem, since serious adverse events are incredibly rare with this molecule,” he said.
Hepatotoxicity is a concern, but Dr. Weinblatt emphasized that elevated liver function tests do not equal cirrhosis.
“Historically, during the first 6 months on methotrexate 20%-25% of patients increase their transaminases in every clinical trial where that’s been looked at. Over time, the liver compensates for the drug. But 5%-6% of patients experience repeated moderate elevations more than 1.5 times the upper limit of normal,” he said.
Key risk factors for methotrexate-related hepatotoxicity were identified in a national observational cohort study of 659 military veterans over age 65 when they started methotrexate for rheumatic diseases. The investigators found a 6% incidence of moderately elevated liver enzymes during a mean follow-up period of 7 months. Obesity was associated with a 1.9-fold increased risk, a serum total cholesterol greater than 240 mg/dL conferred a 5.8-fold elevated risk, and abnormal liver function tests at baseline were associated with a 3.2-fold increased risk (Arthritis Care Res [Hoboken]. 2014 Aug;66[8]:1159-66).
“No surprise: It’s patients who weigh more who are at increased risk for methotrexate-related transaminase increases. I actually think the biggest factor with regard to methotrexate liver disease is the patient’s [body mass index]. Patients in North America aren’t getting any slimmer, so you need to look at this with your patients. If you have a morbidly obese patient on methotrexate whose transaminases suddenly start going up, that’s the patient who’s at greatest risk for methotrexate hepatotoxicity,” he cautioned.
The 3.2-fold increased risk of repeated elevated transaminases associated with abnormal baseline liver function tests in the Veterans Affairs study should be a red flag for rheumatologists.
“I personally think patients shouldn’t start on methotrexate if they have elevated transaminases. They ought to be normal at the start. There are too many other good options now to treat our patients,” Dr. Weinblatt said.
He reported receiving research grants from half a dozen companies and serving as a consultant to more than two dozen.
SNOWMASS, COLO. – “When I started working with methotrexate in 1982, I never would have predicted that methotrexate would become the standard of care in treating rheumatoid arthritis. There’s just no way,” Michael E. Weinblatt, MD, recalled at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
“Even now, 35 years later, we continue to learn more about this fascinating drug,” added Dr. Weinblatt, professor of medicine at Harvard Medical School, Boston.
He highlighted recent developments in this ongoing story and presented some tricks of the trade gained in 35 years of up-close experience with the drug.
Enhancing effectiveness of biologics
One of the hottest topics in methotrexate research is the drug’s ability to enhance the effectiveness of many, but not all, biologic agents. All of the anti–tumor necrosis factor (anti-TNF) biologics as well as rituximab (Rituxan) are demonstrably more effective when used in combination with methotrexate. Dr. Weinblatt considers the widespread underutilization of this combination strategy scandalous.
“This is an incredibly important point: All of you use biologics and all of you use methotrexate, but I’ve been depressed by the fact that up to 30%-40% of patients – no matter which data set you look at – are on monotherapy biological therapy,” he said.
He cited data from the ongoing BRASS Registry (Brigham and Women’s Hospital Rheumatoid Arthritis Sequential Registry) to underscore his point that good things happen when biologics and methotrexate are used together. Of 1,395 BRASS Registry participants prospectively followed since 2003, the proportion on biologic therapy has climbed steadily from 41% at the outset to 68% in 2016. Remarkably, 82% of patients on biologic therapy remain on their first biologic agent. Fewer than 4% have switched biologics more than twice. That’s very unlike the experiences reported elsewhere.
“I think one of the reasons we have such positive data is that we have a high percentage of patients staying on their background methotrexate,” said Dr. Weinblatt, codirector of clinical rheumatology and associate director of the Center for Arthritis and Joint Diseases at Brigham and Women’s Hospital, Boston.
He noted that Dutch investigators reported at the 2016 annual meeting of the American College of Rheumatology that among 1,230 consecutive rheumatoid arthritis patients started on etanercept (Enbrel) or adalimumab (Humira), 28% in the etanercept group were on concomitant methotrexate, as were 64% of those who started on adalimumab. The patients spent a median of 1.3-1.6 years and a maximum of 9.2-9.3 years on their biologic agent. Patients on adalimumab monotherapy were 2.61-fold more likely to drop out than were those on dual therapy with methotrexate. Patients on etanercept monotherapy were 1.2-fold more likely to drop out, a difference that didn’t achieve statistical significance.
Although the investigators did not study the mechanism of prolonged on-treatment survival, they speculated that it probably involved methotrexate’s documented ability to prevent formation of anti-adalimumab antibodies. In contrast, patients on etanercept don’t develop blocking antibodies, Dr. Weinblatt observed.
The randomized, double-blind CONCERTO trial conducted in 395 methotrexate- and biologic-naive RA patients demonstrated that methotrexate reduces the immunogenicity of adalimumab in dose-dependent fashion. Participants were randomized to open-label adalimumab at 40 mg every 2 weeks plus weekly double-blind methotrexate at 2.5, 5, 10, or 20 mg. Clinical outcomes at 26 weeks as reflected in 28-joint count disease activity score and the Clinical Disease Activity Index were significantly better in patients on 10 or 20 mg/week of methotrexate than in those on 2.5 or 5 mg/week. Serum adalimumab levels were higher in patients on the two higher doses of methotrexate as well (Ann Rheum Dis. 2015 Jun;74[6]:1037-44).
“It ends up that if you don’t use methotrexate or you use a very low dose you increase the risk of developing antibodies against adalimumab and decrease the efficacy of the drug. So in clinical practice, if you’re going to be working with dose titration of methotrexate and your patient is on adalimumab, there’s a threshold below which you probably shouldn’t go. In this study, doses of 10 mg/week or more induced a greater clinical response,” he said.
With infliximab (Remicade), based upon 20-year-old studies, the threshold is 7.5 mg of methotrexate per week.
“With etanercept, we don’t know what the threshold is. You don’t develop blocking anti-drug antibodies with etanercept, but we do know that methotrexate enhances the efficacy of etanercept, and it doesn’t do it by changing the biologic’s pharmacokinetics and there’s no increase in methotrexate blood levels,” the rheumatologist continued.
Unlike the anti-TNF biologics and rituximab, the efficacy of the Janus kinase inhibitors tofacitinib (Xeljanz) and baricitinib is not enhanced when the drugs are used in combination with methotrexate, studies indicate.
The efficacy of certolizumab pegol (Cimzia) wasn’t affected by methotrexate dose category in a prespecified pooled subgroup analysis of the phase III RAPID 1 and RAPID 2 clinical trials. In the 1,273 certolizumab-treated patients, the week-24 treatment response was similar regardless of whether patients were on methotrexate at 10 mg/week or less, 10-15 mg/week, or more than 15 mg/week. The investigators concluded that to minimize treatment-emergent adverse events, physicians can tailor background methotrexate dosing based upon individual patient tolerance without affecting certolizumab’s efficacy (Arthritis Care Res [Hoboken]. 2016 Mar;68[3]:299-307).
An important aspect of this analysis was that among the 325 subjects randomized to placebo rather than certolizumab, the treatment response at week 24 was significantly better in those on more than 15 mg/week of methotrexate than with lower doses of the drug.
“Most patients on methotrexate need more than 15 mg/week. So it astonishes me that such a high percentage of patients enrolled in clinical trials around the world are on, like, 14 mg/week. I mean, most patients need somewhere between 15 and 25 mg/week for a response, although over time you might be able to decrease that dose,” Dr. Weinblatt said.
Side effects of methotrexate
“The biggest issue with methotrexate is the tolerability problem, since serious adverse events are incredibly rare with this molecule,” he said.
Hepatotoxicity is a concern, but Dr. Weinblatt emphasized that elevated liver function tests do not equal cirrhosis.
“Historically, during the first 6 months on methotrexate 20%-25% of patients increase their transaminases in every clinical trial where that’s been looked at. Over time, the liver compensates for the drug. But 5%-6% of patients experience repeated moderate elevations more than 1.5 times the upper limit of normal,” he said.
Key risk factors for methotrexate-related hepatotoxicity were identified in a national observational cohort study of 659 military veterans over age 65 when they started methotrexate for rheumatic diseases. The investigators found a 6% incidence of moderately elevated liver enzymes during a mean follow-up period of 7 months. Obesity was associated with a 1.9-fold increased risk, a serum total cholesterol greater than 240 mg/dL conferred a 5.8-fold elevated risk, and abnormal liver function tests at baseline were associated with a 3.2-fold increased risk (Arthritis Care Res [Hoboken]. 2014 Aug;66[8]:1159-66).
“No surprise: It’s patients who weigh more who are at increased risk for methotrexate-related transaminase increases. I actually think the biggest factor with regard to methotrexate liver disease is the patient’s [body mass index]. Patients in North America aren’t getting any slimmer, so you need to look at this with your patients. If you have a morbidly obese patient on methotrexate whose transaminases suddenly start going up, that’s the patient who’s at greatest risk for methotrexate hepatotoxicity,” he cautioned.
The 3.2-fold increased risk of repeated elevated transaminases associated with abnormal baseline liver function tests in the Veterans Affairs study should be a red flag for rheumatologists.
“I personally think patients shouldn’t start on methotrexate if they have elevated transaminases. They ought to be normal at the start. There are too many other good options now to treat our patients,” Dr. Weinblatt said.
He reported receiving research grants from half a dozen companies and serving as a consultant to more than two dozen.
EXPERT ANALYSIS FROM THE WINTER RHEUMATOLOGY SYMPOSIUM
March is Colorectal Cancer Awareness Month
Each year, AGA participates in a series of activities in support of Colorectal Cancer Awareness Month – and 2017 is no exception. March provides us with an important platform to help remind patients of the necessity of getting screened. Here are a few easy ways to join us in raising awareness:
- In-person: Take time this month to talk to your patients about their personal history and encourage timely screening. Visit www.gastro.org/CRC for materials you can provide to your patients to help them understand risk factors and screening options.
- On your practice website: When patients visit your website, make sure there is a prominent CRC screening reminder. You can link to AGA’s patient materials or use our awareness videos (also available via the above link) to help spread the word.
- On Facebook: AGA will be running a campaign throughout March to remind patients over 50 to get screened. Make sure to like us (facebook.com/AmerGastroAssn) to see our CRC posts, which you can share with your family and friends. If your practice has a Facebook page, the page can share all of our CRC awareness materials, as well.
- On Twitter: Tweeting is a great way to raise awareness among the public. Follow @AmerGastroAssn (twitter.com/AmerGastroAssn) for information on Twitter chats you can take part in to help raise awareness.
With your support, we can improve the public’s understanding of this deadly cancer and continue to increase screening rates. Stay tuned to AGA eDigest and AGA’s website (gastro.org) for timely CRC Awareness Month updates, and join CRC-related discussions with other AGA members on the AGA Community (community.gastro.org).
Each year, AGA participates in a series of activities in support of Colorectal Cancer Awareness Month – and 2017 is no exception. March provides us with an important platform to help remind patients of the necessity of getting screened. Here are a few easy ways to join us in raising awareness:
- In-person: Take time this month to talk to your patients about their personal history and encourage timely screening. Visit www.gastro.org/CRC for materials you can provide to your patients to help them understand risk factors and screening options.
- On your practice website: When patients visit your website, make sure there is a prominent CRC screening reminder. You can link to AGA’s patient materials or use our awareness videos (also available via the above link) to help spread the word.
- On Facebook: AGA will be running a campaign throughout March to remind patients over 50 to get screened. Make sure to like us (facebook.com/AmerGastroAssn) to see our CRC posts, which you can share with your family and friends. If your practice has a Facebook page, the page can share all of our CRC awareness materials, as well.
- On Twitter: Tweeting is a great way to raise awareness among the public. Follow @AmerGastroAssn (twitter.com/AmerGastroAssn) for information on Twitter chats you can take part in to help raise awareness.
With your support, we can improve the public’s understanding of this deadly cancer and continue to increase screening rates. Stay tuned to AGA eDigest and AGA’s website (gastro.org) for timely CRC Awareness Month updates, and join CRC-related discussions with other AGA members on the AGA Community (community.gastro.org).
Each year, AGA participates in a series of activities in support of Colorectal Cancer Awareness Month – and 2017 is no exception. March provides us with an important platform to help remind patients of the necessity of getting screened. Here are a few easy ways to join us in raising awareness:
- In-person: Take time this month to talk to your patients about their personal history and encourage timely screening. Visit www.gastro.org/CRC for materials you can provide to your patients to help them understand risk factors and screening options.
- On your practice website: When patients visit your website, make sure there is a prominent CRC screening reminder. You can link to AGA’s patient materials or use our awareness videos (also available via the above link) to help spread the word.
- On Facebook: AGA will be running a campaign throughout March to remind patients over 50 to get screened. Make sure to like us (facebook.com/AmerGastroAssn) to see our CRC posts, which you can share with your family and friends. If your practice has a Facebook page, the page can share all of our CRC awareness materials, as well.
- On Twitter: Tweeting is a great way to raise awareness among the public. Follow @AmerGastroAssn (twitter.com/AmerGastroAssn) for information on Twitter chats you can take part in to help raise awareness.
With your support, we can improve the public’s understanding of this deadly cancer and continue to increase screening rates. Stay tuned to AGA eDigest and AGA’s website (gastro.org) for timely CRC Awareness Month updates, and join CRC-related discussions with other AGA members on the AGA Community (community.gastro.org).
CGM safe and effective without additional blood glucose testing
according to the authors of a noninferiority trial published in the April issue of Diabetes Care.
Before December 2016, continuous glucose monitoring (CGM) was approved by the Food and Drug Administration for use only as an adjunct to standard home blood glucose monitoring (BGM), which was required to confirm the continuous glucose monitoring reading before making a decision on insulin dosing.
In this randomized, noninferiority trial, 217 participants with type 1 diabetes who used an insulin pump were randomized either to CGM only (142 individuals) or to CGM with additional BGM before an insulin bolus was administered (75 individuals).
The primary outcome of the 26-week trial was time in the range of 70-180 mg/dL, according to findings from the CGM, and the study found that mean time in this range was the same for the two groups: 65%.
There were no severe hypoglycemic events in the CGM-only group, and only one in the CGM plus BGM group. There were also no significant changes from baseline to 26 weeks in other metrics of glucose control for mean glucose, hyperglycemia, hypoglycemia, and glycemic variability, and no significant differences between the two groups. The results were also similar for subgroups of age, duration of disease, education, use of CGM before study enrollment, baseline hemoglobin A1c, and baseline time in range.
“In addition to randomization and multiple center participation, the strengths of this study include a high degree of participant retention, CGM use, and treatment group adherence,” the authors wrote. “Notably, there was good separation between the treatment groups in the number of BGM tests per day, particularly when recognizing that two of the BGM measurements per day were required for CGM calibration and that, according to the protocol, the calibrations were performed at times such that they would not influence insulin bolusing.”
The authors said the results were likely to be equally applicable to individuals who use multiple daily insulin injections rather than an insulin pump, as the accuracy of the CGM sensor was just as relevant to this group.
They stressed that one major limitation of the trial was that it only included adults with well-controlled type 1 diabetes who were likely to adhere to the study protocol, and it excluded those with less well controlled disease.
Given this, they called for future studies to examine the safety of CGM alone in young people and adults who might be less compliant with their diabetes control, such as those with higher HbA1c levels, who test their blood glucose fewer than four times a day, and who are hypoglycemia-unaware.
“The application of this trial’s results to clinical practice can benefit people with T1D by reducing their burden of multiple daily fingersticks when using CGM and can enhance the cost-effectiveness of CGM therapy by reducing the number of daily BGM test strips,” they wrote. “Furthermore, the demonstration that insulin dosing based on CGM alone is safe has applicability to assessing risk involved with artificial pancreas systems that automate insulin delivery based on CGM sensor glucose measurements.”
The study was funded by the Leona M. and Harry B. Helmsley Charitable Trust, and Dexcom provided the CGM systems used in the trial. Authors declared speakers fees, consultancies, board positions and other funding from a range of pharmaceutical companies, including Dexcom. One author declared stock in Dexcom.
according to the authors of a noninferiority trial published in the April issue of Diabetes Care.
Before December 2016, continuous glucose monitoring (CGM) was approved by the Food and Drug Administration for use only as an adjunct to standard home blood glucose monitoring (BGM), which was required to confirm the continuous glucose monitoring reading before making a decision on insulin dosing.
In this randomized, noninferiority trial, 217 participants with type 1 diabetes who used an insulin pump were randomized either to CGM only (142 individuals) or to CGM with additional BGM before an insulin bolus was administered (75 individuals).
The primary outcome of the 26-week trial was time in the range of 70-180 mg/dL, according to findings from the CGM, and the study found that mean time in this range was the same for the two groups: 65%.
There were no severe hypoglycemic events in the CGM-only group, and only one in the CGM plus BGM group. There were also no significant changes from baseline to 26 weeks in other metrics of glucose control for mean glucose, hyperglycemia, hypoglycemia, and glycemic variability, and no significant differences between the two groups. The results were also similar for subgroups of age, duration of disease, education, use of CGM before study enrollment, baseline hemoglobin A1c, and baseline time in range.
“In addition to randomization and multiple center participation, the strengths of this study include a high degree of participant retention, CGM use, and treatment group adherence,” the authors wrote. “Notably, there was good separation between the treatment groups in the number of BGM tests per day, particularly when recognizing that two of the BGM measurements per day were required for CGM calibration and that, according to the protocol, the calibrations were performed at times such that they would not influence insulin bolusing.”
The authors said the results were likely to be equally applicable to individuals who use multiple daily insulin injections rather than an insulin pump, as the accuracy of the CGM sensor was just as relevant to this group.
They stressed that one major limitation of the trial was that it only included adults with well-controlled type 1 diabetes who were likely to adhere to the study protocol, and it excluded those with less well controlled disease.
Given this, they called for future studies to examine the safety of CGM alone in young people and adults who might be less compliant with their diabetes control, such as those with higher HbA1c levels, who test their blood glucose fewer than four times a day, and who are hypoglycemia-unaware.
“The application of this trial’s results to clinical practice can benefit people with T1D by reducing their burden of multiple daily fingersticks when using CGM and can enhance the cost-effectiveness of CGM therapy by reducing the number of daily BGM test strips,” they wrote. “Furthermore, the demonstration that insulin dosing based on CGM alone is safe has applicability to assessing risk involved with artificial pancreas systems that automate insulin delivery based on CGM sensor glucose measurements.”
The study was funded by the Leona M. and Harry B. Helmsley Charitable Trust, and Dexcom provided the CGM systems used in the trial. Authors declared speakers fees, consultancies, board positions and other funding from a range of pharmaceutical companies, including Dexcom. One author declared stock in Dexcom.
according to the authors of a noninferiority trial published in the April issue of Diabetes Care.
Before December 2016, continuous glucose monitoring (CGM) was approved by the Food and Drug Administration for use only as an adjunct to standard home blood glucose monitoring (BGM), which was required to confirm the continuous glucose monitoring reading before making a decision on insulin dosing.
In this randomized, noninferiority trial, 217 participants with type 1 diabetes who used an insulin pump were randomized either to CGM only (142 individuals) or to CGM with additional BGM before an insulin bolus was administered (75 individuals).
The primary outcome of the 26-week trial was time in the range of 70-180 mg/dL, according to findings from the CGM, and the study found that mean time in this range was the same for the two groups: 65%.
There were no severe hypoglycemic events in the CGM-only group, and only one in the CGM plus BGM group. There were also no significant changes from baseline to 26 weeks in other metrics of glucose control for mean glucose, hyperglycemia, hypoglycemia, and glycemic variability, and no significant differences between the two groups. The results were also similar for subgroups of age, duration of disease, education, use of CGM before study enrollment, baseline hemoglobin A1c, and baseline time in range.
“In addition to randomization and multiple center participation, the strengths of this study include a high degree of participant retention, CGM use, and treatment group adherence,” the authors wrote. “Notably, there was good separation between the treatment groups in the number of BGM tests per day, particularly when recognizing that two of the BGM measurements per day were required for CGM calibration and that, according to the protocol, the calibrations were performed at times such that they would not influence insulin bolusing.”
The authors said the results were likely to be equally applicable to individuals who use multiple daily insulin injections rather than an insulin pump, as the accuracy of the CGM sensor was just as relevant to this group.
They stressed that one major limitation of the trial was that it only included adults with well-controlled type 1 diabetes who were likely to adhere to the study protocol, and it excluded those with less well controlled disease.
Given this, they called for future studies to examine the safety of CGM alone in young people and adults who might be less compliant with their diabetes control, such as those with higher HbA1c levels, who test their blood glucose fewer than four times a day, and who are hypoglycemia-unaware.
“The application of this trial’s results to clinical practice can benefit people with T1D by reducing their burden of multiple daily fingersticks when using CGM and can enhance the cost-effectiveness of CGM therapy by reducing the number of daily BGM test strips,” they wrote. “Furthermore, the demonstration that insulin dosing based on CGM alone is safe has applicability to assessing risk involved with artificial pancreas systems that automate insulin delivery based on CGM sensor glucose measurements.”
The study was funded by the Leona M. and Harry B. Helmsley Charitable Trust, and Dexcom provided the CGM systems used in the trial. Authors declared speakers fees, consultancies, board positions and other funding from a range of pharmaceutical companies, including Dexcom. One author declared stock in Dexcom.
Key clinical point: Continuous glucose monitoring alone is safe and effective without the addition of confirmatory blood glucose monitoring.
Major finding: Mean time spent with blood glucose in the 70-180 mg/dL range was the same for individuals with continuous glucose monitoring and those with additional confirmatory blood glucose testing.
Data source: A randomized noninferiority trial in 217 adults with well-controlled type 1 diabetes.
Disclosures: The study was funded by the Leona M. and Harry B. Helmsley Charitable Trust. Dexcom provided the continuous glucose monitoring systems used in the trial. Authors declared speaking fees, consultancies, board positions and other funding from a range of pharmaceutical companies, including Dexcom. One author declared stock ownership in Dexcom.
Drug produces high response rates in AITL

Photo by Larry Young
SAN FRANCISCO—Treatment with 5-azacitidine (5-AZA) can produce a high response rate in patients with relapsed/refractory angioimmunoblastic T-cell lymphoma (AITL), according to a small study.
The overall response rate (ORR) among AITL patients was 75%, and the complete response (CR) rate was 42%.
However, this study also included patients with other types of peripheral T-cell lymphoma (PTCL), and most of these patients did not respond to 5-AZA.
Richard Delarue, MD, of Necker University Hospital in Paris, France, presented these results at the 9th Annual T-cell Lymphoma Forum.
Results were also presented at the 2016 ASH Annual Meeting (abstract 4164). Dr Delarue reported receiving honoraria from Celgene.
Patients
The study included 19 patients with relapsed/refractory PTCL. Twelve patients had AITL, 3 had adult T-cell leukemia/lymphoma (ATLL), 2 had PTCL not otherwise specified, 1 had enteropathy-associated T-cell lymphoma, and 1 had transformed mycosis fungoides.
At diagnosis, the median age was 71 (range, 39-85) for AITL patients and 59 (range, 32-83) for the other PTCL patients. Seventy-five percent of AITL patients had an IPI score of 3 to 5 and a PIT score of 3 to 4. Eighty-six percent of the other PTCL patients had an IPI score of 3 to 5, and 57% had a PIT score of 3 to 4.
At the time of 5-AZA treatment, all patients had stage III/IV disease. The AITL patients had received a median of 2 (range, 0-6) prior lines of therapy, and the other PTCL patients had received a median of 3 (range, 0-7).
Two patients did not receive chemotherapy before 5-AZA because of the presence of associated chronic myelomonocytic leukemia (CMML) that required treatment first.
Ninety-two percent of AITL patients had TET2 mutations (n=11), 33% had DNMT3A mutations (n=4), and 0% had IDH2 mutations. One of the non-AITL patients had a TET2 mutation.
Treatment
Patients received a subcutaneous injection of 5-AZA at 75 mg/m² for 7 consecutive days every 28 days until progression or unacceptable toxicity. Six patients also received 4 to 8 infusions of rituximab because of EBV-DNA positivity.
The patients received a median of 3 cycles of 5-AZA. At the time of analysis, 4 patients were still receiving therapy.
The median follow-up was 84 days (range, 19 to 1236).
Toxicity
“Hematological toxicity was as expected with 5-azacitidine,” Dr Delarue said.
However, 2 patients had “unusual” adverse reactions. One patient had grade 2 polyneuropathy, which was considered related to a paraneoplastic syndrome.
The other patient had grade 3 diarrhea related to colitis of unknown origin, and this led to treatment interruption.
There were no treatment-related deaths.
Efficacy
Dr Delarue noted that the ORR was significantly higher in AITL patients than in patients with the other PTCL subtypes (P=0.0198).
The ORR was 53% in the entire cohort (10/19), 75% (9/12) among AITL patients, and 14% among patients with other PTCLs (1/7).
“The only patient with a response in the ‘other PTCL’ group was a patient with HTLV1-associated ATLL . . . , but he relapsed a couple of weeks after the second cycle,” Dr Delarue explained.
Among the AITL patients, the CR rate was 42% (5/12), the partial response rate was 33% (4/12), and the rate of stable disease was 25% (3/12).
Six AITL patients eventually progressed—after 2, 2, 3, 4, 4, and 20 cycles of therapy, respectively.
Two AITL patients are off therapy but remain in CR after 9 and 10 months (5 and 6 cycles of treatment), respectively.
The median progression-free survival for AITL patients was 16 months, and the median overall survival was 17 months.
Dr DeLarue noted that 4 of the AITL patients had CMML, 1 had non-CMML myelodysplastic syndrome, 3 had monocytosis without CMML, and 4 had normal monocyte counts.
He also said that, at present, it’s not possible to correlate the results observed in the AITL patients with their mutational status.
However, he and his colleagues are planning a prospective study of 5-AZA in patients with relapsed/refractory AITL and T follicular helper cell PTCL not otherwise specified. 5-AZA will be compared to investigator’s choice in this study.
Dr DeLarue said this trial will provide an opportunity to use the new oral formulation of 5-AZA (CC-486). And he and his colleagues welcome collaborators. ![]()

Photo by Larry Young
SAN FRANCISCO—Treatment with 5-azacitidine (5-AZA) can produce a high response rate in patients with relapsed/refractory angioimmunoblastic T-cell lymphoma (AITL), according to a small study.
The overall response rate (ORR) among AITL patients was 75%, and the complete response (CR) rate was 42%.
However, this study also included patients with other types of peripheral T-cell lymphoma (PTCL), and most of these patients did not respond to 5-AZA.
Richard Delarue, MD, of Necker University Hospital in Paris, France, presented these results at the 9th Annual T-cell Lymphoma Forum.
Results were also presented at the 2016 ASH Annual Meeting (abstract 4164). Dr Delarue reported receiving honoraria from Celgene.
Patients
The study included 19 patients with relapsed/refractory PTCL. Twelve patients had AITL, 3 had adult T-cell leukemia/lymphoma (ATLL), 2 had PTCL not otherwise specified, 1 had enteropathy-associated T-cell lymphoma, and 1 had transformed mycosis fungoides.
At diagnosis, the median age was 71 (range, 39-85) for AITL patients and 59 (range, 32-83) for the other PTCL patients. Seventy-five percent of AITL patients had an IPI score of 3 to 5 and a PIT score of 3 to 4. Eighty-six percent of the other PTCL patients had an IPI score of 3 to 5, and 57% had a PIT score of 3 to 4.
At the time of 5-AZA treatment, all patients had stage III/IV disease. The AITL patients had received a median of 2 (range, 0-6) prior lines of therapy, and the other PTCL patients had received a median of 3 (range, 0-7).
Two patients did not receive chemotherapy before 5-AZA because of the presence of associated chronic myelomonocytic leukemia (CMML) that required treatment first.
Ninety-two percent of AITL patients had TET2 mutations (n=11), 33% had DNMT3A mutations (n=4), and 0% had IDH2 mutations. One of the non-AITL patients had a TET2 mutation.
Treatment
Patients received a subcutaneous injection of 5-AZA at 75 mg/m² for 7 consecutive days every 28 days until progression or unacceptable toxicity. Six patients also received 4 to 8 infusions of rituximab because of EBV-DNA positivity.
The patients received a median of 3 cycles of 5-AZA. At the time of analysis, 4 patients were still receiving therapy.
The median follow-up was 84 days (range, 19 to 1236).
Toxicity
“Hematological toxicity was as expected with 5-azacitidine,” Dr Delarue said.
However, 2 patients had “unusual” adverse reactions. One patient had grade 2 polyneuropathy, which was considered related to a paraneoplastic syndrome.
The other patient had grade 3 diarrhea related to colitis of unknown origin, and this led to treatment interruption.
There were no treatment-related deaths.
Efficacy
Dr Delarue noted that the ORR was significantly higher in AITL patients than in patients with the other PTCL subtypes (P=0.0198).
The ORR was 53% in the entire cohort (10/19), 75% (9/12) among AITL patients, and 14% among patients with other PTCLs (1/7).
“The only patient with a response in the ‘other PTCL’ group was a patient with HTLV1-associated ATLL . . . , but he relapsed a couple of weeks after the second cycle,” Dr Delarue explained.
Among the AITL patients, the CR rate was 42% (5/12), the partial response rate was 33% (4/12), and the rate of stable disease was 25% (3/12).
Six AITL patients eventually progressed—after 2, 2, 3, 4, 4, and 20 cycles of therapy, respectively.
Two AITL patients are off therapy but remain in CR after 9 and 10 months (5 and 6 cycles of treatment), respectively.
The median progression-free survival for AITL patients was 16 months, and the median overall survival was 17 months.
Dr DeLarue noted that 4 of the AITL patients had CMML, 1 had non-CMML myelodysplastic syndrome, 3 had monocytosis without CMML, and 4 had normal monocyte counts.
He also said that, at present, it’s not possible to correlate the results observed in the AITL patients with their mutational status.
However, he and his colleagues are planning a prospective study of 5-AZA in patients with relapsed/refractory AITL and T follicular helper cell PTCL not otherwise specified. 5-AZA will be compared to investigator’s choice in this study.
Dr DeLarue said this trial will provide an opportunity to use the new oral formulation of 5-AZA (CC-486). And he and his colleagues welcome collaborators. ![]()

Photo by Larry Young
SAN FRANCISCO—Treatment with 5-azacitidine (5-AZA) can produce a high response rate in patients with relapsed/refractory angioimmunoblastic T-cell lymphoma (AITL), according to a small study.
The overall response rate (ORR) among AITL patients was 75%, and the complete response (CR) rate was 42%.
However, this study also included patients with other types of peripheral T-cell lymphoma (PTCL), and most of these patients did not respond to 5-AZA.
Richard Delarue, MD, of Necker University Hospital in Paris, France, presented these results at the 9th Annual T-cell Lymphoma Forum.
Results were also presented at the 2016 ASH Annual Meeting (abstract 4164). Dr Delarue reported receiving honoraria from Celgene.
Patients
The study included 19 patients with relapsed/refractory PTCL. Twelve patients had AITL, 3 had adult T-cell leukemia/lymphoma (ATLL), 2 had PTCL not otherwise specified, 1 had enteropathy-associated T-cell lymphoma, and 1 had transformed mycosis fungoides.
At diagnosis, the median age was 71 (range, 39-85) for AITL patients and 59 (range, 32-83) for the other PTCL patients. Seventy-five percent of AITL patients had an IPI score of 3 to 5 and a PIT score of 3 to 4. Eighty-six percent of the other PTCL patients had an IPI score of 3 to 5, and 57% had a PIT score of 3 to 4.
At the time of 5-AZA treatment, all patients had stage III/IV disease. The AITL patients had received a median of 2 (range, 0-6) prior lines of therapy, and the other PTCL patients had received a median of 3 (range, 0-7).
Two patients did not receive chemotherapy before 5-AZA because of the presence of associated chronic myelomonocytic leukemia (CMML) that required treatment first.
Ninety-two percent of AITL patients had TET2 mutations (n=11), 33% had DNMT3A mutations (n=4), and 0% had IDH2 mutations. One of the non-AITL patients had a TET2 mutation.
Treatment
Patients received a subcutaneous injection of 5-AZA at 75 mg/m² for 7 consecutive days every 28 days until progression or unacceptable toxicity. Six patients also received 4 to 8 infusions of rituximab because of EBV-DNA positivity.
The patients received a median of 3 cycles of 5-AZA. At the time of analysis, 4 patients were still receiving therapy.
The median follow-up was 84 days (range, 19 to 1236).
Toxicity
“Hematological toxicity was as expected with 5-azacitidine,” Dr Delarue said.
However, 2 patients had “unusual” adverse reactions. One patient had grade 2 polyneuropathy, which was considered related to a paraneoplastic syndrome.
The other patient had grade 3 diarrhea related to colitis of unknown origin, and this led to treatment interruption.
There were no treatment-related deaths.
Efficacy
Dr Delarue noted that the ORR was significantly higher in AITL patients than in patients with the other PTCL subtypes (P=0.0198).
The ORR was 53% in the entire cohort (10/19), 75% (9/12) among AITL patients, and 14% among patients with other PTCLs (1/7).
“The only patient with a response in the ‘other PTCL’ group was a patient with HTLV1-associated ATLL . . . , but he relapsed a couple of weeks after the second cycle,” Dr Delarue explained.
Among the AITL patients, the CR rate was 42% (5/12), the partial response rate was 33% (4/12), and the rate of stable disease was 25% (3/12).
Six AITL patients eventually progressed—after 2, 2, 3, 4, 4, and 20 cycles of therapy, respectively.
Two AITL patients are off therapy but remain in CR after 9 and 10 months (5 and 6 cycles of treatment), respectively.
The median progression-free survival for AITL patients was 16 months, and the median overall survival was 17 months.
Dr DeLarue noted that 4 of the AITL patients had CMML, 1 had non-CMML myelodysplastic syndrome, 3 had monocytosis without CMML, and 4 had normal monocyte counts.
He also said that, at present, it’s not possible to correlate the results observed in the AITL patients with their mutational status.
However, he and his colleagues are planning a prospective study of 5-AZA in patients with relapsed/refractory AITL and T follicular helper cell PTCL not otherwise specified. 5-AZA will be compared to investigator’s choice in this study.
Dr DeLarue said this trial will provide an opportunity to use the new oral formulation of 5-AZA (CC-486). And he and his colleagues welcome collaborators. ![]()
Mindfulness in the Workplace
Mindfulness practices, such as meditation, yoga, tai chi, and qigong, are on the rise in the workplace, as more studies show how they can improve health and reduce the costs of stress.
Researchers who analyzed data on 85,004 respondents to the National Health Interview Survey say that their findings are “encouraging”; about 1 in 7 workers report engaging in some form of mindfulness-based activity. From 2002 to 2012, the number of yoga practitioners nearly doubled from 6% to 11%. From 2002 to 2007, the number of people who began meditating increased from 8% to 9.9%.
Related: Mindfulness to Reduce Stress
The rise in activity was seen across different groups of workers. In 2002, 2.2% of farm workers reported engaging in at least 1 of the practices studied, as did 18.2% of white-collar workers in 2007. Mindfulness found a foothold among 9% to 12% of the unemployed as well.
Still the trend is mostly seen among white-collar workers, attributable mainly to differences in household income and education level, the researchers say. After they controlled for those 2 factors, blue-collar workers were still less likely to engage in meditation or yoga, and farm workers were less likely to engage in any of the 4 practices.
Related: Preventing Burnout With Cognitive Empathy
But the lack of engagement among blue-collar and farm workers can’t be explained by sociodemographic factors alone, the researchers say. White-collar workers may have more time, access, and opportunity to practice mindfulness and might have different beliefs about the value of these practices.
Lack of engagement could be ameliorated in part by developing interventions to target those occupational groups, the researchers suggest. They found no studies that focused on blue-collar or farm workers—the low prevalence of mindfulness practices in those groups indicates a “pressing need” for interventions, even though those workplace settings may present “unique implementation challenges.”
Mindfulness practices, such as meditation, yoga, tai chi, and qigong, are on the rise in the workplace, as more studies show how they can improve health and reduce the costs of stress.
Researchers who analyzed data on 85,004 respondents to the National Health Interview Survey say that their findings are “encouraging”; about 1 in 7 workers report engaging in some form of mindfulness-based activity. From 2002 to 2012, the number of yoga practitioners nearly doubled from 6% to 11%. From 2002 to 2007, the number of people who began meditating increased from 8% to 9.9%.
Related: Mindfulness to Reduce Stress
The rise in activity was seen across different groups of workers. In 2002, 2.2% of farm workers reported engaging in at least 1 of the practices studied, as did 18.2% of white-collar workers in 2007. Mindfulness found a foothold among 9% to 12% of the unemployed as well.
Still the trend is mostly seen among white-collar workers, attributable mainly to differences in household income and education level, the researchers say. After they controlled for those 2 factors, blue-collar workers were still less likely to engage in meditation or yoga, and farm workers were less likely to engage in any of the 4 practices.
Related: Preventing Burnout With Cognitive Empathy
But the lack of engagement among blue-collar and farm workers can’t be explained by sociodemographic factors alone, the researchers say. White-collar workers may have more time, access, and opportunity to practice mindfulness and might have different beliefs about the value of these practices.
Lack of engagement could be ameliorated in part by developing interventions to target those occupational groups, the researchers suggest. They found no studies that focused on blue-collar or farm workers—the low prevalence of mindfulness practices in those groups indicates a “pressing need” for interventions, even though those workplace settings may present “unique implementation challenges.”
Mindfulness practices, such as meditation, yoga, tai chi, and qigong, are on the rise in the workplace, as more studies show how they can improve health and reduce the costs of stress.
Researchers who analyzed data on 85,004 respondents to the National Health Interview Survey say that their findings are “encouraging”; about 1 in 7 workers report engaging in some form of mindfulness-based activity. From 2002 to 2012, the number of yoga practitioners nearly doubled from 6% to 11%. From 2002 to 2007, the number of people who began meditating increased from 8% to 9.9%.
Related: Mindfulness to Reduce Stress
The rise in activity was seen across different groups of workers. In 2002, 2.2% of farm workers reported engaging in at least 1 of the practices studied, as did 18.2% of white-collar workers in 2007. Mindfulness found a foothold among 9% to 12% of the unemployed as well.
Still the trend is mostly seen among white-collar workers, attributable mainly to differences in household income and education level, the researchers say. After they controlled for those 2 factors, blue-collar workers were still less likely to engage in meditation or yoga, and farm workers were less likely to engage in any of the 4 practices.
Related: Preventing Burnout With Cognitive Empathy
But the lack of engagement among blue-collar and farm workers can’t be explained by sociodemographic factors alone, the researchers say. White-collar workers may have more time, access, and opportunity to practice mindfulness and might have different beliefs about the value of these practices.
Lack of engagement could be ameliorated in part by developing interventions to target those occupational groups, the researchers suggest. They found no studies that focused on blue-collar or farm workers—the low prevalence of mindfulness practices in those groups indicates a “pressing need” for interventions, even though those workplace settings may present “unique implementation challenges.”
Blurred vision
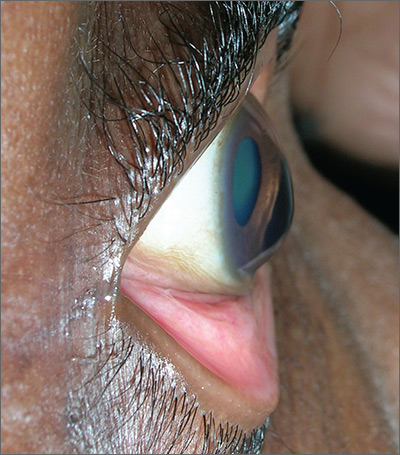
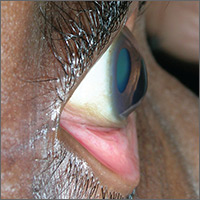
The FP advised the patient that she had keratoconus, a condition in which the cornea bulges out in the middle (like a cone). Keratoconus, which can adversely affect the health of the eye, is one of several eye findings related to atopic dermatitis. Others include recurrent conjunctivitis, cataracts, and periorbital darkening.
The patient in this case was referred to her ophthalmologist for further evaluation and the FP advised her to avoid rubbing her eyes. In some severe cases, keratoconus treatment requires corneal transplantation.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Usatine R, Finklea L. Atopic dermatitis. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:584-590.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com

The FP advised the patient that she had keratoconus, a condition in which the cornea bulges out in the middle (like a cone). Keratoconus, which can adversely affect the health of the eye, is one of several eye findings related to atopic dermatitis. Others include recurrent conjunctivitis, cataracts, and periorbital darkening.
The patient in this case was referred to her ophthalmologist for further evaluation and the FP advised her to avoid rubbing her eyes. In some severe cases, keratoconus treatment requires corneal transplantation.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Usatine R, Finklea L. Atopic dermatitis. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:584-590.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com

The FP advised the patient that she had keratoconus, a condition in which the cornea bulges out in the middle (like a cone). Keratoconus, which can adversely affect the health of the eye, is one of several eye findings related to atopic dermatitis. Others include recurrent conjunctivitis, cataracts, and periorbital darkening.
The patient in this case was referred to her ophthalmologist for further evaluation and the FP advised her to avoid rubbing her eyes. In some severe cases, keratoconus treatment requires corneal transplantation.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Usatine R, Finklea L. Atopic dermatitis. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:584-590.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
Breakfast and weight loss
Eating breakfast is sometimes promulgated as a component of an effective weight loss strategy. Correlational studies have suggested that breakfast consumption is associated with lower body weight. As the thinking goes, breakfast promotes morning satiety, thus suppressing caloric intake later in the day. Skipping breakfast, however, results in increased caloric intake later in the day.
But most people are breakfast eaters. Data exist suggesting that the adverse effects of skipping breakfast may occur only in habitual breakfast eaters. In other words, it may be harmful only if you suddenly change your habits.
So, what should we be telling our “breakfast-skippers” about breakfast and weight loss?
Gabrielle LeCheminant and her colleagues conducted a randomized trial of habitual breakfast skippers to evaluate the effects of eating breakfast versus not on energy, macronutrient consumption, and physical activity over 1 month (Appetite. 2017 May 1. doi: 10.1016/j.appet.2016.12.041).
Subjects were required to eat within 90 minutes of waking up and finish eating by 8:30 a.m. No eating or snack restrictions were imposed after breakfast. Subjects were 18-55 years of age, ate breakfast (at least 2 days/week), slept at least 6 hours per night, and woke up consistently before 8:00 am. Biometric and food diaries were completed.
Breakfast-skippers randomized to eat breakfast consumed more calories (266) per day and weighed more (0.7 kg) at 1 month. No changes were observed in caloric compensation with subsequent meals nor in self-reported hunger or satiety. No additional physical activity was observed with the addition of breakfast.
Weight gain was minimal, and the time frame of the study was short. Even so, I think the take-home message from this is: Don’t tell habitual breakfast skippers to start eating breakfast with the goal of losing weight. It appears that the opposite may be true.
Dr. Ebbert is a professor of medicine and general internist at the Mayo Clinic in Rochester, Minn., and a diplomate of the American Board of Addiction Medicine. The opinions expressed are those of the author and do not necessarily represent the views and opinions of the Mayo Clinic. The opinions expressed in this article should not be used to diagnose or treat any medical condition, nor should they be used as a substitute for medical advice from a qualified, board-certified practicing clinician. Dr. Ebbert has no relevant financial disclosures about this article.
Eating breakfast is sometimes promulgated as a component of an effective weight loss strategy. Correlational studies have suggested that breakfast consumption is associated with lower body weight. As the thinking goes, breakfast promotes morning satiety, thus suppressing caloric intake later in the day. Skipping breakfast, however, results in increased caloric intake later in the day.
But most people are breakfast eaters. Data exist suggesting that the adverse effects of skipping breakfast may occur only in habitual breakfast eaters. In other words, it may be harmful only if you suddenly change your habits.
So, what should we be telling our “breakfast-skippers” about breakfast and weight loss?
Gabrielle LeCheminant and her colleagues conducted a randomized trial of habitual breakfast skippers to evaluate the effects of eating breakfast versus not on energy, macronutrient consumption, and physical activity over 1 month (Appetite. 2017 May 1. doi: 10.1016/j.appet.2016.12.041).
Subjects were required to eat within 90 minutes of waking up and finish eating by 8:30 a.m. No eating or snack restrictions were imposed after breakfast. Subjects were 18-55 years of age, ate breakfast (at least 2 days/week), slept at least 6 hours per night, and woke up consistently before 8:00 am. Biometric and food diaries were completed.
Breakfast-skippers randomized to eat breakfast consumed more calories (266) per day and weighed more (0.7 kg) at 1 month. No changes were observed in caloric compensation with subsequent meals nor in self-reported hunger or satiety. No additional physical activity was observed with the addition of breakfast.
Weight gain was minimal, and the time frame of the study was short. Even so, I think the take-home message from this is: Don’t tell habitual breakfast skippers to start eating breakfast with the goal of losing weight. It appears that the opposite may be true.
Dr. Ebbert is a professor of medicine and general internist at the Mayo Clinic in Rochester, Minn., and a diplomate of the American Board of Addiction Medicine. The opinions expressed are those of the author and do not necessarily represent the views and opinions of the Mayo Clinic. The opinions expressed in this article should not be used to diagnose or treat any medical condition, nor should they be used as a substitute for medical advice from a qualified, board-certified practicing clinician. Dr. Ebbert has no relevant financial disclosures about this article.
Eating breakfast is sometimes promulgated as a component of an effective weight loss strategy. Correlational studies have suggested that breakfast consumption is associated with lower body weight. As the thinking goes, breakfast promotes morning satiety, thus suppressing caloric intake later in the day. Skipping breakfast, however, results in increased caloric intake later in the day.
But most people are breakfast eaters. Data exist suggesting that the adverse effects of skipping breakfast may occur only in habitual breakfast eaters. In other words, it may be harmful only if you suddenly change your habits.
So, what should we be telling our “breakfast-skippers” about breakfast and weight loss?
Gabrielle LeCheminant and her colleagues conducted a randomized trial of habitual breakfast skippers to evaluate the effects of eating breakfast versus not on energy, macronutrient consumption, and physical activity over 1 month (Appetite. 2017 May 1. doi: 10.1016/j.appet.2016.12.041).
Subjects were required to eat within 90 minutes of waking up and finish eating by 8:30 a.m. No eating or snack restrictions were imposed after breakfast. Subjects were 18-55 years of age, ate breakfast (at least 2 days/week), slept at least 6 hours per night, and woke up consistently before 8:00 am. Biometric and food diaries were completed.
Breakfast-skippers randomized to eat breakfast consumed more calories (266) per day and weighed more (0.7 kg) at 1 month. No changes were observed in caloric compensation with subsequent meals nor in self-reported hunger or satiety. No additional physical activity was observed with the addition of breakfast.
Weight gain was minimal, and the time frame of the study was short. Even so, I think the take-home message from this is: Don’t tell habitual breakfast skippers to start eating breakfast with the goal of losing weight. It appears that the opposite may be true.
Dr. Ebbert is a professor of medicine and general internist at the Mayo Clinic in Rochester, Minn., and a diplomate of the American Board of Addiction Medicine. The opinions expressed are those of the author and do not necessarily represent the views and opinions of the Mayo Clinic. The opinions expressed in this article should not be used to diagnose or treat any medical condition, nor should they be used as a substitute for medical advice from a qualified, board-certified practicing clinician. Dr. Ebbert has no relevant financial disclosures about this article.
RA-BEAM trial shows that baricitinib improves RA symptoms, slows joint damage
Oral baricitinib, when added to standard rheumatoid arthritis treatment, improved symptoms and slowed the progression of joint damage, compared with both placebo and adalimumab, according to a report published online Feb. 15 in the New England Journal of Medicine.
The JAK inhibitor’s beneficial effects were noted as early as the first week of treatment and persisted throughout 1 year of follow-up in an international manufacturer-sponsored phase III trial (RA-BEAM) involving 1,305 adults with moderate to severe active rheumatoid arthritis (RA). The study participants had not responded to methotrexate and had never been treated with biologics; the majority had previously received at least two conventional synthetic disease-modifying antirheumatic drugs (DMARDs), said Peter C. Taylor, MD, PhD, of the Nuffield Department of Orthopedics, Rheumatology, and Musculoskeletal Sciences and the Kennedy Institute of Rheumatology at the University of Oxford (England), and his associates.
The primary efficacy endpoint – the proportion of patients at week 12 who showed a response of 20% or more according to American College of Rheumatology criteria (ACR20) – was 70% for baricitinib, compared with 40% for placebo. Baricitinib also was superior to placebo regarding all the major secondary efficacy endpoints of the study, including scores on the Health Assessment Questionnaire–Disability Index; results on the Disease Activity Score for 28 joints using high-sensitivity C-reactive protein (DAS28-CRP); remission according to the Simplified Disease Activity Index criteria; and the patients’ daily diary reports on duration and severity of morning joint stiffness, worst level of tiredness, and worst level of pain.
In addition, baricitinib was “considered to be significantly superior to adalimumab” according to the ACR20 (61% for adalimumab) and the DAS28-CRP responses at week 12. Both baricitinib and adalimumab were associated with significant reductions in the radiographic progression of structural joint damage, compared with placebo, at week 24 and week 52, the investigators reported (N Engl J Med. 2017;376:652-62).
Regarding safety outcomes, adverse events including infections were more frequent with baricitinib and adalimumab than with placebo, as were decreases in neutrophil counts, increases in aminotransferase and creatinine levels, and increases in low-density lipoprotein cholesterol levels. At 1 year, three patients in the baricitinib group and two in the adalimumab group discontinued treatment because of liver abnormalities.
Baricitinib is currently under consideration for approval by the U.S. Food and Drug Administration and was approved for marketing in the European Union on Feb. 13.
The RA-BEAM trial was supported by Eli Lilly and Incyte. Dr. Taylor reported receiving personal fees from Eli Lilly, and he and his associates reported ties to numerous other industry sources.
Oral baricitinib, when added to standard rheumatoid arthritis treatment, improved symptoms and slowed the progression of joint damage, compared with both placebo and adalimumab, according to a report published online Feb. 15 in the New England Journal of Medicine.
The JAK inhibitor’s beneficial effects were noted as early as the first week of treatment and persisted throughout 1 year of follow-up in an international manufacturer-sponsored phase III trial (RA-BEAM) involving 1,305 adults with moderate to severe active rheumatoid arthritis (RA). The study participants had not responded to methotrexate and had never been treated with biologics; the majority had previously received at least two conventional synthetic disease-modifying antirheumatic drugs (DMARDs), said Peter C. Taylor, MD, PhD, of the Nuffield Department of Orthopedics, Rheumatology, and Musculoskeletal Sciences and the Kennedy Institute of Rheumatology at the University of Oxford (England), and his associates.
The primary efficacy endpoint – the proportion of patients at week 12 who showed a response of 20% or more according to American College of Rheumatology criteria (ACR20) – was 70% for baricitinib, compared with 40% for placebo. Baricitinib also was superior to placebo regarding all the major secondary efficacy endpoints of the study, including scores on the Health Assessment Questionnaire–Disability Index; results on the Disease Activity Score for 28 joints using high-sensitivity C-reactive protein (DAS28-CRP); remission according to the Simplified Disease Activity Index criteria; and the patients’ daily diary reports on duration and severity of morning joint stiffness, worst level of tiredness, and worst level of pain.
In addition, baricitinib was “considered to be significantly superior to adalimumab” according to the ACR20 (61% for adalimumab) and the DAS28-CRP responses at week 12. Both baricitinib and adalimumab were associated with significant reductions in the radiographic progression of structural joint damage, compared with placebo, at week 24 and week 52, the investigators reported (N Engl J Med. 2017;376:652-62).
Regarding safety outcomes, adverse events including infections were more frequent with baricitinib and adalimumab than with placebo, as were decreases in neutrophil counts, increases in aminotransferase and creatinine levels, and increases in low-density lipoprotein cholesterol levels. At 1 year, three patients in the baricitinib group and two in the adalimumab group discontinued treatment because of liver abnormalities.
Baricitinib is currently under consideration for approval by the U.S. Food and Drug Administration and was approved for marketing in the European Union on Feb. 13.
The RA-BEAM trial was supported by Eli Lilly and Incyte. Dr. Taylor reported receiving personal fees from Eli Lilly, and he and his associates reported ties to numerous other industry sources.
Oral baricitinib, when added to standard rheumatoid arthritis treatment, improved symptoms and slowed the progression of joint damage, compared with both placebo and adalimumab, according to a report published online Feb. 15 in the New England Journal of Medicine.
The JAK inhibitor’s beneficial effects were noted as early as the first week of treatment and persisted throughout 1 year of follow-up in an international manufacturer-sponsored phase III trial (RA-BEAM) involving 1,305 adults with moderate to severe active rheumatoid arthritis (RA). The study participants had not responded to methotrexate and had never been treated with biologics; the majority had previously received at least two conventional synthetic disease-modifying antirheumatic drugs (DMARDs), said Peter C. Taylor, MD, PhD, of the Nuffield Department of Orthopedics, Rheumatology, and Musculoskeletal Sciences and the Kennedy Institute of Rheumatology at the University of Oxford (England), and his associates.
The primary efficacy endpoint – the proportion of patients at week 12 who showed a response of 20% or more according to American College of Rheumatology criteria (ACR20) – was 70% for baricitinib, compared with 40% for placebo. Baricitinib also was superior to placebo regarding all the major secondary efficacy endpoints of the study, including scores on the Health Assessment Questionnaire–Disability Index; results on the Disease Activity Score for 28 joints using high-sensitivity C-reactive protein (DAS28-CRP); remission according to the Simplified Disease Activity Index criteria; and the patients’ daily diary reports on duration and severity of morning joint stiffness, worst level of tiredness, and worst level of pain.
In addition, baricitinib was “considered to be significantly superior to adalimumab” according to the ACR20 (61% for adalimumab) and the DAS28-CRP responses at week 12. Both baricitinib and adalimumab were associated with significant reductions in the radiographic progression of structural joint damage, compared with placebo, at week 24 and week 52, the investigators reported (N Engl J Med. 2017;376:652-62).
Regarding safety outcomes, adverse events including infections were more frequent with baricitinib and adalimumab than with placebo, as were decreases in neutrophil counts, increases in aminotransferase and creatinine levels, and increases in low-density lipoprotein cholesterol levels. At 1 year, three patients in the baricitinib group and two in the adalimumab group discontinued treatment because of liver abnormalities.
Baricitinib is currently under consideration for approval by the U.S. Food and Drug Administration and was approved for marketing in the European Union on Feb. 13.
The RA-BEAM trial was supported by Eli Lilly and Incyte. Dr. Taylor reported receiving personal fees from Eli Lilly, and he and his associates reported ties to numerous other industry sources.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point:
Major finding: The primary efficacy end point – the proportion of patients at week 12 who showed an ACR20 response – was 70% for baricitinib, compared with 40% for placebo.
Data source: A manufacturer-sponsored, international, randomized, double-blind phase III clinical trial involving 1,305 adults with moderate to severe active RA.
Disclosures: This study was supported by Eli Lilly and Incyte. Dr. Taylor reported receiving personal fees from Eli Lilly, and he and his associates reported ties to numerous other industry sources.
More periviable infants survive without neurodevelopmental impairment
Among periviable infants born at 11 tertiary care centers in 2000 through 2011, the rate of survival without neurodevelopmental impairment increased a small but significant 4%, according to a report published online Feb. 16 in the New England Journal of Medicine.
The rate of survival with neurodevelopmental impairment also increased, although to a lesser extent (1%).
“These findings are important for guiding counseling and decision making with respect to periviable birth. Prognosis continues to be guarded; in the most recent epoch [time period in our study], mortality was 64%, and 43% of surviving infants had neurodevelopmental impairment,” they noted.
The investigators defined such impairment as moderate or severe cerebral palsy, Gross Motor Function Classification System level of at least 2 on a scale of 1-5, profound hearing loss requiring amplification in both ears, profound visual impairment in both eyes, or cognitive impairment such as a Mental Developmental Index score of less than 70 or a Cognitive Composite score of less than 85.
To examine time trends in the outcomes of periviable infants, Dr. Younge of Duke University, Durham, N.C., and her associates analyzed data from the network’s registry of births at 11 academic tertiary care centers nationwide. They focused on 4,274 infants who were born during 3 epochs – 2000-2003, 2004-2007, and 2008-2011 – and were evaluated for motor function, sensory impairment, and cognitive delay at a corrected age of 18-22 months.
The percentage of infants who survived without neurodevelopmental impairment increased over time, from 16% during the first epoch to 20% during the third epoch. However, the percentage who survived with neurodevelopmental impairment also increased, from 15% during the first epoch to 16% during the third epoch (New Engl. J. Med. 2017 Feb 16. doi: 10.1056/NEJMoa1605566).
The rates of active treatment of these periviable infants didn’t change significantly over time. Overall, 22% of infants born at 22 weeks, 71% of those born at 23 weeks, and 95% of those born at 24 weeks received active treatment at birth. Therefore, the overall decrease in mortality and the 4% improvement in neurodevelopmental outcomes wasn’t attributable to greater use of active treatment for periviable infants over time, said Dr. Younge and her associates.
Despite these small but significant improvements in outcomes, “the incidence of death, neurodevelopmental impairment, and other adverse outcomes remains high in this population,” they noted.
This study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Institutes of Health, the National Center for Research Resources, and the National Center for Advancing Translational Sciences for the Neonatal Research Network’s Generic Database and Follow-up Studies. Dr. Younge reported having no relevant financial disclosures; two of her associates reported ties to Pediatrix Medical Group and rEVO Biologics.
The study by Younge et al. was limited in that it only included infants born in 11 academic tertiary care medical centers.
This study population represents only 4%-5% of periviable infants born in the United States, so the findings are not generalizable.
Prakesh S. Shah, MD, is in the department of pediatrics and the Institute of Health Policy, Management, and Evaluation at Mount Sinai Hospital, Toronto, and the University of Toronto. He reported having no relevant financial disclosures. Dr. Shah made these remarks in an editorial accompanying Dr. Younge’s report (N Engl J Med. 2017 Feb 16. doi: 10.1056/NEJMe1616539).
The study by Younge et al. was limited in that it only included infants born in 11 academic tertiary care medical centers.
This study population represents only 4%-5% of periviable infants born in the United States, so the findings are not generalizable.
Prakesh S. Shah, MD, is in the department of pediatrics and the Institute of Health Policy, Management, and Evaluation at Mount Sinai Hospital, Toronto, and the University of Toronto. He reported having no relevant financial disclosures. Dr. Shah made these remarks in an editorial accompanying Dr. Younge’s report (N Engl J Med. 2017 Feb 16. doi: 10.1056/NEJMe1616539).
The study by Younge et al. was limited in that it only included infants born in 11 academic tertiary care medical centers.
This study population represents only 4%-5% of periviable infants born in the United States, so the findings are not generalizable.
Prakesh S. Shah, MD, is in the department of pediatrics and the Institute of Health Policy, Management, and Evaluation at Mount Sinai Hospital, Toronto, and the University of Toronto. He reported having no relevant financial disclosures. Dr. Shah made these remarks in an editorial accompanying Dr. Younge’s report (N Engl J Med. 2017 Feb 16. doi: 10.1056/NEJMe1616539).
Among periviable infants born at 11 tertiary care centers in 2000 through 2011, the rate of survival without neurodevelopmental impairment increased a small but significant 4%, according to a report published online Feb. 16 in the New England Journal of Medicine.
The rate of survival with neurodevelopmental impairment also increased, although to a lesser extent (1%).
“These findings are important for guiding counseling and decision making with respect to periviable birth. Prognosis continues to be guarded; in the most recent epoch [time period in our study], mortality was 64%, and 43% of surviving infants had neurodevelopmental impairment,” they noted.
The investigators defined such impairment as moderate or severe cerebral palsy, Gross Motor Function Classification System level of at least 2 on a scale of 1-5, profound hearing loss requiring amplification in both ears, profound visual impairment in both eyes, or cognitive impairment such as a Mental Developmental Index score of less than 70 or a Cognitive Composite score of less than 85.
To examine time trends in the outcomes of periviable infants, Dr. Younge of Duke University, Durham, N.C., and her associates analyzed data from the network’s registry of births at 11 academic tertiary care centers nationwide. They focused on 4,274 infants who were born during 3 epochs – 2000-2003, 2004-2007, and 2008-2011 – and were evaluated for motor function, sensory impairment, and cognitive delay at a corrected age of 18-22 months.
The percentage of infants who survived without neurodevelopmental impairment increased over time, from 16% during the first epoch to 20% during the third epoch. However, the percentage who survived with neurodevelopmental impairment also increased, from 15% during the first epoch to 16% during the third epoch (New Engl. J. Med. 2017 Feb 16. doi: 10.1056/NEJMoa1605566).
The rates of active treatment of these periviable infants didn’t change significantly over time. Overall, 22% of infants born at 22 weeks, 71% of those born at 23 weeks, and 95% of those born at 24 weeks received active treatment at birth. Therefore, the overall decrease in mortality and the 4% improvement in neurodevelopmental outcomes wasn’t attributable to greater use of active treatment for periviable infants over time, said Dr. Younge and her associates.
Despite these small but significant improvements in outcomes, “the incidence of death, neurodevelopmental impairment, and other adverse outcomes remains high in this population,” they noted.
This study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Institutes of Health, the National Center for Research Resources, and the National Center for Advancing Translational Sciences for the Neonatal Research Network’s Generic Database and Follow-up Studies. Dr. Younge reported having no relevant financial disclosures; two of her associates reported ties to Pediatrix Medical Group and rEVO Biologics.
Among periviable infants born at 11 tertiary care centers in 2000 through 2011, the rate of survival without neurodevelopmental impairment increased a small but significant 4%, according to a report published online Feb. 16 in the New England Journal of Medicine.
The rate of survival with neurodevelopmental impairment also increased, although to a lesser extent (1%).
“These findings are important for guiding counseling and decision making with respect to periviable birth. Prognosis continues to be guarded; in the most recent epoch [time period in our study], mortality was 64%, and 43% of surviving infants had neurodevelopmental impairment,” they noted.
The investigators defined such impairment as moderate or severe cerebral palsy, Gross Motor Function Classification System level of at least 2 on a scale of 1-5, profound hearing loss requiring amplification in both ears, profound visual impairment in both eyes, or cognitive impairment such as a Mental Developmental Index score of less than 70 or a Cognitive Composite score of less than 85.
To examine time trends in the outcomes of periviable infants, Dr. Younge of Duke University, Durham, N.C., and her associates analyzed data from the network’s registry of births at 11 academic tertiary care centers nationwide. They focused on 4,274 infants who were born during 3 epochs – 2000-2003, 2004-2007, and 2008-2011 – and were evaluated for motor function, sensory impairment, and cognitive delay at a corrected age of 18-22 months.
The percentage of infants who survived without neurodevelopmental impairment increased over time, from 16% during the first epoch to 20% during the third epoch. However, the percentage who survived with neurodevelopmental impairment also increased, from 15% during the first epoch to 16% during the third epoch (New Engl. J. Med. 2017 Feb 16. doi: 10.1056/NEJMoa1605566).
The rates of active treatment of these periviable infants didn’t change significantly over time. Overall, 22% of infants born at 22 weeks, 71% of those born at 23 weeks, and 95% of those born at 24 weeks received active treatment at birth. Therefore, the overall decrease in mortality and the 4% improvement in neurodevelopmental outcomes wasn’t attributable to greater use of active treatment for periviable infants over time, said Dr. Younge and her associates.
Despite these small but significant improvements in outcomes, “the incidence of death, neurodevelopmental impairment, and other adverse outcomes remains high in this population,” they noted.
This study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Institutes of Health, the National Center for Research Resources, and the National Center for Advancing Translational Sciences for the Neonatal Research Network’s Generic Database and Follow-up Studies. Dr. Younge reported having no relevant financial disclosures; two of her associates reported ties to Pediatrix Medical Group and rEVO Biologics.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point:
Major finding: The percentage of infants who survived without neurodevelopmental impairment increased over time, from 16% to 20%, as did the percentage who survived with neurodevelopmental impairment, from 15% to 16%.
Data source: A cohort study involving 4,274 infants in an NIH registry born at 22-24 weeks’ gestation and evaluated for neurodevelopmental impairment at a corrected age of 18-22 months.
Disclosures: This study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Institutes of Health, the National Center for Research Resources, and the National Center for Advancing Translational Sciences for the Neonatal Research Network’s Generic Database and Follow-up Studies. Dr. Younge reported having no relevant financial disclosures; two of her associates reported ties to Pediatrix Medical Group and rEVO Biologics.