User login
Bleeding esophageal varices: Who should receive a shunt?
A transjugular intrahepatic portosystemic shunt (TIPS) has been shown in randomized controlled trials to be effective for:
- Secondary prevention of variceal bleeding
- Controlling refractory ascites in patients with liver cirrhosis.
In addition, findings from retrospective case series have suggested that it helps in cases of:
- Acute variceal bleeding refractory to endoscopic therapy
- Gastropathy due to portal hypertension
- Bleeding gastric varices
- Refractory hepatic hydrothorax
- Hepatorenal syndrome
- Budd-Chiari syndrome
- Veno-occlusive disease
- Hepatopulmonary syndrome.
Here, we discuss the indications for a TIPS in cirrhotic patients with esophageal variceal bleeding.
CIRRHOSIS CAN LEAD TO PORTAL HYPERTENSION, BLEEDING
Cirrhosis of the liver alters the hepatic architecture. Development of regenerating nodules and deposition of connective tissue between these nodules increase the resistance to portal blood flow, which can lead to portal hypertension.1
Esophageal variceal bleeding is a complication of portal hypertension and a major cause of death in patients with liver cirrhosis. Combined treatment with vasoactive drugs, prophylactic antibiotics, and endoscopic band ligation is the standard of care for patients with acute bleeding. However, this treatment fails in about 10% to 15% of these patients. A TIPS creates a connection between the portal and hepatic veins, resulting in portal decompression and homeostasis.2
PRE-TIPS EVALUATION
Patients being considered for a TIPS should be medically assessed before the procedure. The workup should include the following:
- Routine blood tests, including blood type and screen (indirect Coombs test), complete blood cell count, basic metabolic panel, liver function tests, prothrombin time, and partial thromboplastin time
- Doppler ultrasonography of the liver to ensure that the portal and hepatic veins are patent
- Echocardiography to assess pulmonary arterial pressure and right-side heart function
- The hepatic venous pressure gradient, which is measured at the time of TIPS placement, reflects the degree of portal hypertension. A hepatic vein is catheterized, and the right atrial pressure or the free hepatic venous pressure is subtracted from the wedged hepatic venous pressure. The gradient is normally 1 to 5 mm Hg. A gradient greater than 5 mm Hg indicates portal hypertension, and esophageal varices may start to bleed when the gradient is greater than 12 mm Hg. The goal of TIPS placement is to reduce the gradient to less than 12 mm Hg, or at least by 50%.
Heart failure is a contraindication
Pulmonary hypertension may follow TIPS placement because the shunt increases venous return to the heart. Additionally, systemic vascular resistance decreases in patients who have a shunt. This further worsens the hyperdynamic circulatory state already present in patients with cirrhosis. Cardiac output increases in response to these changes. When the heart’s ability to handle this “volume overload” is exceeded, pulmonary venous pressures rise, with increasing ventilation-perfusion mismatch, hypoxia, and pulmonary vasoconstriction; pulmonary edema may ensue.
Congestive heart failure, severe tricuspid regurgitation, and severe pulmonary hypertension (mean pulmonary pressures > 45 mm Hg) are therefore considered absolute contraindications to TIPS placement.3,4 This is why echocardiography is recommended to assess pulmonary pressure along with the size and function of the right side of the heart before proceeding with TIPS insertion.
Other considerations
TIPS insertion is not recommended in patients with active hepatic encephalopathy, which should be adequately controlled before insertion of a TIPS. This can be achieved with lactulose and rifaximin. Lactulose is a laxative; the recommended target is 3 to 4 bowel movements daily. Rifaximin is a poorly absorbed antibiotic that has a wide spectrum of coverage, affecting gram-negative and gram-positive aerobes and anaerobes. It wipes out the gut bacteria and so decreases the production of ammonia by the gut.
Paracentesis is recommended before TIPS placement if a large volume of ascites is present. Draining the fluid allows the liver to drop down and makes it easier to access the portal vein from the hepatic vein.
WHEN TO CONSIDER A TIPS IN ESOPHAGEAL VARICEAL BLEEDING
Acute bleeding refractory to endoscopic therapy
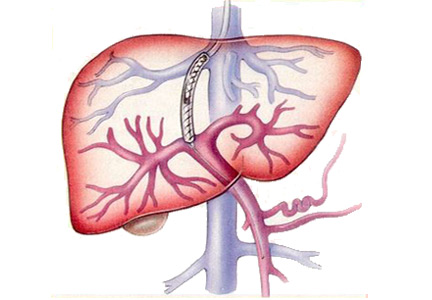
A TIPS remains the only choice to control acute variceal bleeding refractory to medical and endoscopic therapy (Figure 1), with a success rate of 90% to 100%.5 The urgency of TIPS placement is an independent predictor of early mortality.
Esophageal variceal rebleeding
Once varices bleed, the risk of rebleeding is higher than 50%, and rebleeding is associated with a high mortality rate. TIPS should be considered if nonselective beta-blockers and surveillance with upper endoscopy and banding fail to prevent rebleeding, with many studies showing a TIPS to be superior to pharmacologic and endoscopic therapies.6
A meta-analysis in 1999 by Papatheodoridis et al6 found that variceal rebleeding was significantly more frequent with endoscopic therapies, at 47% vs 19% with a TIPS, but the incidence of hepatic encephalopathy was higher with TIPS (34% vs 19%; P < .001), and there was no difference in mortality rates.
Hepatic encephalopathy occurs in 15% to 25% of patients after TIPS procedures. Risk factors include advanced age, poor renal function, and a history of hepatic encephalopathy. Hepatic encephalopathy can be managed with lactulose or rifaximin, or both (see above). Narcotics, antihistamines, and benzodiazepines should be avoided. In rare cases (5%) when hepatic encephalopathy is refractory to medical therapy, liver transplant should be considered.
A surgical distal splenorenal shunt is another option for patients with refractory or recurrent variceal bleeding. In a large randomized controlled trial,7 140 cirrhotic patients with recurrent variceal bleeding were randomized to receive either a distal splenorenal shunt or a TIPS. At a mean follow-up of 48 months, there was no difference in the rates of rebleeding between the two groups (5.5% with a surgical shunt vs 10.5% with a TIPS, P = .29) or in hepatic encephalopathy (50% in both groups). Survival rates were comparable between the two groups at 2 years (81% with a surgical shunt vs 88% with a TIPS) and 5 years (62% vs 61%).
Early use of TIPS after first variceal bleeding
In a 2010 randomized controlled trial,8 63 patients with cirrhosis (Child-Pugh class B or C) and acute variceal bleeding who had received standard medical and endoscopic therapy were randomized to receive either a TIPS within 72 hours of admission or long-term conservative treatment with nonselective beta-blockers and endoscopic band ligation. The 1-year actuarial probability of remaining free of rebleeding or failure to control bleeding was 50% in the conservative treatment group vs 97% in the early-TIPS group (P < .001). The 1-year actuarial survival rate was 61% in the conservative treatment group vs 86% in the early-TIPS group (P < .001).
The authors8 concluded that early use of TIPS in patients with cirrhosis and Child-Pugh scores of 7 to 13 who were hospitalized for acute variceal bleeding was associated with significant reductions in rates of treatment failure and mortality.
- Brenner D, Rippe RA. Pathogenesis of hepatic fibrosis. In: Yamada T, Alpers DH, Laine L, Kaplowitz N, Owyang C, Powell DW, editors. Textbook of Gastroenterology. 4th edition. Philadelphia, PA: Lippincott Williams & Wilkins; 2003.
- Bhogal HK, Sanyal AJ. Using transjugular intrahepatic portosystemic shunts for complications of cirrhosis. Clin Gastroenterol Hepatol 2011; 9:936–946.
- Garcia-Tsao G, Sanyal AJ, Grace ND, Carey WD; Practice Guidelines Committee of American Association for Study of Liver Diseases; Practice Parameters Committee of American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Am J Gastroenterol 2007; 102:2086–2102.
- Azoulay D, Castaing D, Dennison A, Martino W, Eyraud D, Bismuth H. Transjugular intrahepatic portosystemic shunt worsens the hyperdynamic circulatory state of the cirrhotic patient: preliminary report of a prospective study. Hepatology 1994; 19:129–132.
- Rodríguez-Laiz JM, Bañares R, Echenagusia A, et al. Effects of transjugular intrahepatic portasystemic shunt (TIPS) on splanchnic and systemic hemodynamics, and hepatic function in patients with portal hypertension. Preliminary results. Dig Dis Sci 1995; 40:2121–2127.
- Papatheodoridis GV, Goulis J, Leandro G, Patch D, Burroughs AK. Transjugular intrahepatic portosystemic shunt compared with endoscopic treatment for prevention of variceal rebleeding: a meta-analysis. Hepatology 1999; 30:612–622.
- Henderson JM, Boyer TD, Kutner MH, et al; DIVERT Study Group. Distal splenorenal shunt versus transjugular intrahepatic portal systemic shunt for variceal bleeding: a randomized trial. Gastroenterology 2006; 130:1643–1651.
- García-Pagán JC, Caca K, Bureau C, et al; Early TIPS (Transjugular Intrahepatic Portosystemic Shunt) Cooperative Study Group. Early use of TIPS in patients with cirrhosis and variceal bleeding. N Engl J Med 2010; 362:2370–2379.
A transjugular intrahepatic portosystemic shunt (TIPS) has been shown in randomized controlled trials to be effective for:
- Secondary prevention of variceal bleeding
- Controlling refractory ascites in patients with liver cirrhosis.
In addition, findings from retrospective case series have suggested that it helps in cases of:
- Acute variceal bleeding refractory to endoscopic therapy
- Gastropathy due to portal hypertension
- Bleeding gastric varices
- Refractory hepatic hydrothorax
- Hepatorenal syndrome
- Budd-Chiari syndrome
- Veno-occlusive disease
- Hepatopulmonary syndrome.
Here, we discuss the indications for a TIPS in cirrhotic patients with esophageal variceal bleeding.
CIRRHOSIS CAN LEAD TO PORTAL HYPERTENSION, BLEEDING
Cirrhosis of the liver alters the hepatic architecture. Development of regenerating nodules and deposition of connective tissue between these nodules increase the resistance to portal blood flow, which can lead to portal hypertension.1
Esophageal variceal bleeding is a complication of portal hypertension and a major cause of death in patients with liver cirrhosis. Combined treatment with vasoactive drugs, prophylactic antibiotics, and endoscopic band ligation is the standard of care for patients with acute bleeding. However, this treatment fails in about 10% to 15% of these patients. A TIPS creates a connection between the portal and hepatic veins, resulting in portal decompression and homeostasis.2
PRE-TIPS EVALUATION
Patients being considered for a TIPS should be medically assessed before the procedure. The workup should include the following:
- Routine blood tests, including blood type and screen (indirect Coombs test), complete blood cell count, basic metabolic panel, liver function tests, prothrombin time, and partial thromboplastin time
- Doppler ultrasonography of the liver to ensure that the portal and hepatic veins are patent
- Echocardiography to assess pulmonary arterial pressure and right-side heart function
- The hepatic venous pressure gradient, which is measured at the time of TIPS placement, reflects the degree of portal hypertension. A hepatic vein is catheterized, and the right atrial pressure or the free hepatic venous pressure is subtracted from the wedged hepatic venous pressure. The gradient is normally 1 to 5 mm Hg. A gradient greater than 5 mm Hg indicates portal hypertension, and esophageal varices may start to bleed when the gradient is greater than 12 mm Hg. The goal of TIPS placement is to reduce the gradient to less than 12 mm Hg, or at least by 50%.
Heart failure is a contraindication
Pulmonary hypertension may follow TIPS placement because the shunt increases venous return to the heart. Additionally, systemic vascular resistance decreases in patients who have a shunt. This further worsens the hyperdynamic circulatory state already present in patients with cirrhosis. Cardiac output increases in response to these changes. When the heart’s ability to handle this “volume overload” is exceeded, pulmonary venous pressures rise, with increasing ventilation-perfusion mismatch, hypoxia, and pulmonary vasoconstriction; pulmonary edema may ensue.
Congestive heart failure, severe tricuspid regurgitation, and severe pulmonary hypertension (mean pulmonary pressures > 45 mm Hg) are therefore considered absolute contraindications to TIPS placement.3,4 This is why echocardiography is recommended to assess pulmonary pressure along with the size and function of the right side of the heart before proceeding with TIPS insertion.
Other considerations
TIPS insertion is not recommended in patients with active hepatic encephalopathy, which should be adequately controlled before insertion of a TIPS. This can be achieved with lactulose and rifaximin. Lactulose is a laxative; the recommended target is 3 to 4 bowel movements daily. Rifaximin is a poorly absorbed antibiotic that has a wide spectrum of coverage, affecting gram-negative and gram-positive aerobes and anaerobes. It wipes out the gut bacteria and so decreases the production of ammonia by the gut.
Paracentesis is recommended before TIPS placement if a large volume of ascites is present. Draining the fluid allows the liver to drop down and makes it easier to access the portal vein from the hepatic vein.
WHEN TO CONSIDER A TIPS IN ESOPHAGEAL VARICEAL BLEEDING
Acute bleeding refractory to endoscopic therapy
A TIPS remains the only choice to control acute variceal bleeding refractory to medical and endoscopic therapy (Figure 1), with a success rate of 90% to 100%.5 The urgency of TIPS placement is an independent predictor of early mortality.
Esophageal variceal rebleeding
Once varices bleed, the risk of rebleeding is higher than 50%, and rebleeding is associated with a high mortality rate. TIPS should be considered if nonselective beta-blockers and surveillance with upper endoscopy and banding fail to prevent rebleeding, with many studies showing a TIPS to be superior to pharmacologic and endoscopic therapies.6
A meta-analysis in 1999 by Papatheodoridis et al6 found that variceal rebleeding was significantly more frequent with endoscopic therapies, at 47% vs 19% with a TIPS, but the incidence of hepatic encephalopathy was higher with TIPS (34% vs 19%; P < .001), and there was no difference in mortality rates.
Hepatic encephalopathy occurs in 15% to 25% of patients after TIPS procedures. Risk factors include advanced age, poor renal function, and a history of hepatic encephalopathy. Hepatic encephalopathy can be managed with lactulose or rifaximin, or both (see above). Narcotics, antihistamines, and benzodiazepines should be avoided. In rare cases (5%) when hepatic encephalopathy is refractory to medical therapy, liver transplant should be considered.
A surgical distal splenorenal shunt is another option for patients with refractory or recurrent variceal bleeding. In a large randomized controlled trial,7 140 cirrhotic patients with recurrent variceal bleeding were randomized to receive either a distal splenorenal shunt or a TIPS. At a mean follow-up of 48 months, there was no difference in the rates of rebleeding between the two groups (5.5% with a surgical shunt vs 10.5% with a TIPS, P = .29) or in hepatic encephalopathy (50% in both groups). Survival rates were comparable between the two groups at 2 years (81% with a surgical shunt vs 88% with a TIPS) and 5 years (62% vs 61%).
Early use of TIPS after first variceal bleeding
In a 2010 randomized controlled trial,8 63 patients with cirrhosis (Child-Pugh class B or C) and acute variceal bleeding who had received standard medical and endoscopic therapy were randomized to receive either a TIPS within 72 hours of admission or long-term conservative treatment with nonselective beta-blockers and endoscopic band ligation. The 1-year actuarial probability of remaining free of rebleeding or failure to control bleeding was 50% in the conservative treatment group vs 97% in the early-TIPS group (P < .001). The 1-year actuarial survival rate was 61% in the conservative treatment group vs 86% in the early-TIPS group (P < .001).
The authors8 concluded that early use of TIPS in patients with cirrhosis and Child-Pugh scores of 7 to 13 who were hospitalized for acute variceal bleeding was associated with significant reductions in rates of treatment failure and mortality.
A transjugular intrahepatic portosystemic shunt (TIPS) has been shown in randomized controlled trials to be effective for:
- Secondary prevention of variceal bleeding
- Controlling refractory ascites in patients with liver cirrhosis.
In addition, findings from retrospective case series have suggested that it helps in cases of:
- Acute variceal bleeding refractory to endoscopic therapy
- Gastropathy due to portal hypertension
- Bleeding gastric varices
- Refractory hepatic hydrothorax
- Hepatorenal syndrome
- Budd-Chiari syndrome
- Veno-occlusive disease
- Hepatopulmonary syndrome.
Here, we discuss the indications for a TIPS in cirrhotic patients with esophageal variceal bleeding.
CIRRHOSIS CAN LEAD TO PORTAL HYPERTENSION, BLEEDING
Cirrhosis of the liver alters the hepatic architecture. Development of regenerating nodules and deposition of connective tissue between these nodules increase the resistance to portal blood flow, which can lead to portal hypertension.1
Esophageal variceal bleeding is a complication of portal hypertension and a major cause of death in patients with liver cirrhosis. Combined treatment with vasoactive drugs, prophylactic antibiotics, and endoscopic band ligation is the standard of care for patients with acute bleeding. However, this treatment fails in about 10% to 15% of these patients. A TIPS creates a connection between the portal and hepatic veins, resulting in portal decompression and homeostasis.2
PRE-TIPS EVALUATION
Patients being considered for a TIPS should be medically assessed before the procedure. The workup should include the following:
- Routine blood tests, including blood type and screen (indirect Coombs test), complete blood cell count, basic metabolic panel, liver function tests, prothrombin time, and partial thromboplastin time
- Doppler ultrasonography of the liver to ensure that the portal and hepatic veins are patent
- Echocardiography to assess pulmonary arterial pressure and right-side heart function
- The hepatic venous pressure gradient, which is measured at the time of TIPS placement, reflects the degree of portal hypertension. A hepatic vein is catheterized, and the right atrial pressure or the free hepatic venous pressure is subtracted from the wedged hepatic venous pressure. The gradient is normally 1 to 5 mm Hg. A gradient greater than 5 mm Hg indicates portal hypertension, and esophageal varices may start to bleed when the gradient is greater than 12 mm Hg. The goal of TIPS placement is to reduce the gradient to less than 12 mm Hg, or at least by 50%.
Heart failure is a contraindication
Pulmonary hypertension may follow TIPS placement because the shunt increases venous return to the heart. Additionally, systemic vascular resistance decreases in patients who have a shunt. This further worsens the hyperdynamic circulatory state already present in patients with cirrhosis. Cardiac output increases in response to these changes. When the heart’s ability to handle this “volume overload” is exceeded, pulmonary venous pressures rise, with increasing ventilation-perfusion mismatch, hypoxia, and pulmonary vasoconstriction; pulmonary edema may ensue.
Congestive heart failure, severe tricuspid regurgitation, and severe pulmonary hypertension (mean pulmonary pressures > 45 mm Hg) are therefore considered absolute contraindications to TIPS placement.3,4 This is why echocardiography is recommended to assess pulmonary pressure along with the size and function of the right side of the heart before proceeding with TIPS insertion.
Other considerations
TIPS insertion is not recommended in patients with active hepatic encephalopathy, which should be adequately controlled before insertion of a TIPS. This can be achieved with lactulose and rifaximin. Lactulose is a laxative; the recommended target is 3 to 4 bowel movements daily. Rifaximin is a poorly absorbed antibiotic that has a wide spectrum of coverage, affecting gram-negative and gram-positive aerobes and anaerobes. It wipes out the gut bacteria and so decreases the production of ammonia by the gut.
Paracentesis is recommended before TIPS placement if a large volume of ascites is present. Draining the fluid allows the liver to drop down and makes it easier to access the portal vein from the hepatic vein.
WHEN TO CONSIDER A TIPS IN ESOPHAGEAL VARICEAL BLEEDING
Acute bleeding refractory to endoscopic therapy
A TIPS remains the only choice to control acute variceal bleeding refractory to medical and endoscopic therapy (Figure 1), with a success rate of 90% to 100%.5 The urgency of TIPS placement is an independent predictor of early mortality.
Esophageal variceal rebleeding
Once varices bleed, the risk of rebleeding is higher than 50%, and rebleeding is associated with a high mortality rate. TIPS should be considered if nonselective beta-blockers and surveillance with upper endoscopy and banding fail to prevent rebleeding, with many studies showing a TIPS to be superior to pharmacologic and endoscopic therapies.6
A meta-analysis in 1999 by Papatheodoridis et al6 found that variceal rebleeding was significantly more frequent with endoscopic therapies, at 47% vs 19% with a TIPS, but the incidence of hepatic encephalopathy was higher with TIPS (34% vs 19%; P < .001), and there was no difference in mortality rates.
Hepatic encephalopathy occurs in 15% to 25% of patients after TIPS procedures. Risk factors include advanced age, poor renal function, and a history of hepatic encephalopathy. Hepatic encephalopathy can be managed with lactulose or rifaximin, or both (see above). Narcotics, antihistamines, and benzodiazepines should be avoided. In rare cases (5%) when hepatic encephalopathy is refractory to medical therapy, liver transplant should be considered.
A surgical distal splenorenal shunt is another option for patients with refractory or recurrent variceal bleeding. In a large randomized controlled trial,7 140 cirrhotic patients with recurrent variceal bleeding were randomized to receive either a distal splenorenal shunt or a TIPS. At a mean follow-up of 48 months, there was no difference in the rates of rebleeding between the two groups (5.5% with a surgical shunt vs 10.5% with a TIPS, P = .29) or in hepatic encephalopathy (50% in both groups). Survival rates were comparable between the two groups at 2 years (81% with a surgical shunt vs 88% with a TIPS) and 5 years (62% vs 61%).
Early use of TIPS after first variceal bleeding
In a 2010 randomized controlled trial,8 63 patients with cirrhosis (Child-Pugh class B or C) and acute variceal bleeding who had received standard medical and endoscopic therapy were randomized to receive either a TIPS within 72 hours of admission or long-term conservative treatment with nonselective beta-blockers and endoscopic band ligation. The 1-year actuarial probability of remaining free of rebleeding or failure to control bleeding was 50% in the conservative treatment group vs 97% in the early-TIPS group (P < .001). The 1-year actuarial survival rate was 61% in the conservative treatment group vs 86% in the early-TIPS group (P < .001).
The authors8 concluded that early use of TIPS in patients with cirrhosis and Child-Pugh scores of 7 to 13 who were hospitalized for acute variceal bleeding was associated with significant reductions in rates of treatment failure and mortality.
- Brenner D, Rippe RA. Pathogenesis of hepatic fibrosis. In: Yamada T, Alpers DH, Laine L, Kaplowitz N, Owyang C, Powell DW, editors. Textbook of Gastroenterology. 4th edition. Philadelphia, PA: Lippincott Williams & Wilkins; 2003.
- Bhogal HK, Sanyal AJ. Using transjugular intrahepatic portosystemic shunts for complications of cirrhosis. Clin Gastroenterol Hepatol 2011; 9:936–946.
- Garcia-Tsao G, Sanyal AJ, Grace ND, Carey WD; Practice Guidelines Committee of American Association for Study of Liver Diseases; Practice Parameters Committee of American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Am J Gastroenterol 2007; 102:2086–2102.
- Azoulay D, Castaing D, Dennison A, Martino W, Eyraud D, Bismuth H. Transjugular intrahepatic portosystemic shunt worsens the hyperdynamic circulatory state of the cirrhotic patient: preliminary report of a prospective study. Hepatology 1994; 19:129–132.
- Rodríguez-Laiz JM, Bañares R, Echenagusia A, et al. Effects of transjugular intrahepatic portasystemic shunt (TIPS) on splanchnic and systemic hemodynamics, and hepatic function in patients with portal hypertension. Preliminary results. Dig Dis Sci 1995; 40:2121–2127.
- Papatheodoridis GV, Goulis J, Leandro G, Patch D, Burroughs AK. Transjugular intrahepatic portosystemic shunt compared with endoscopic treatment for prevention of variceal rebleeding: a meta-analysis. Hepatology 1999; 30:612–622.
- Henderson JM, Boyer TD, Kutner MH, et al; DIVERT Study Group. Distal splenorenal shunt versus transjugular intrahepatic portal systemic shunt for variceal bleeding: a randomized trial. Gastroenterology 2006; 130:1643–1651.
- García-Pagán JC, Caca K, Bureau C, et al; Early TIPS (Transjugular Intrahepatic Portosystemic Shunt) Cooperative Study Group. Early use of TIPS in patients with cirrhosis and variceal bleeding. N Engl J Med 2010; 362:2370–2379.
- Brenner D, Rippe RA. Pathogenesis of hepatic fibrosis. In: Yamada T, Alpers DH, Laine L, Kaplowitz N, Owyang C, Powell DW, editors. Textbook of Gastroenterology. 4th edition. Philadelphia, PA: Lippincott Williams & Wilkins; 2003.
- Bhogal HK, Sanyal AJ. Using transjugular intrahepatic portosystemic shunts for complications of cirrhosis. Clin Gastroenterol Hepatol 2011; 9:936–946.
- Garcia-Tsao G, Sanyal AJ, Grace ND, Carey WD; Practice Guidelines Committee of American Association for Study of Liver Diseases; Practice Parameters Committee of American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Am J Gastroenterol 2007; 102:2086–2102.
- Azoulay D, Castaing D, Dennison A, Martino W, Eyraud D, Bismuth H. Transjugular intrahepatic portosystemic shunt worsens the hyperdynamic circulatory state of the cirrhotic patient: preliminary report of a prospective study. Hepatology 1994; 19:129–132.
- Rodríguez-Laiz JM, Bañares R, Echenagusia A, et al. Effects of transjugular intrahepatic portasystemic shunt (TIPS) on splanchnic and systemic hemodynamics, and hepatic function in patients with portal hypertension. Preliminary results. Dig Dis Sci 1995; 40:2121–2127.
- Papatheodoridis GV, Goulis J, Leandro G, Patch D, Burroughs AK. Transjugular intrahepatic portosystemic shunt compared with endoscopic treatment for prevention of variceal rebleeding: a meta-analysis. Hepatology 1999; 30:612–622.
- Henderson JM, Boyer TD, Kutner MH, et al; DIVERT Study Group. Distal splenorenal shunt versus transjugular intrahepatic portal systemic shunt for variceal bleeding: a randomized trial. Gastroenterology 2006; 130:1643–1651.
- García-Pagán JC, Caca K, Bureau C, et al; Early TIPS (Transjugular Intrahepatic Portosystemic Shunt) Cooperative Study Group. Early use of TIPS in patients with cirrhosis and variceal bleeding. N Engl J Med 2010; 362:2370–2379.
Minimizing use of antipsychotics
Iodine deficiency: Clinical implications
A 65-year-old woman is found to have a goiter. She is clinically euthyroid. She is a strict vegan and only uses noniodized Himalayan salt for cooking. Her thyroid gland is diffusely enlarged with no nodules. The estimated weight of the thyroid gland is 50 g (normal 10–20 g) based on ultrasonography. Her thyroid-stimulating hormone (TSH) level is 2.95 mU/L (reference range 0.5–5 mU/L), and her free thyroxine level is 0.8 ng/dL (0.7–1.8 ng/dL). Testing for TSH receptor antibody is negative. Her 24-hour urine iodine is undetectable (urine iodine concentration < 10 μg/L with urine volume 3,175 mL). What may be the cause of her goiter?
Iodine is an essential element needed for the production of thyroid hormone, which controls metabolism and plays a major role in fetal neurodevelopment. Its ionized form is called iodide. Iodine deficiency results in impairment of thyroid hormone synthesis and may lead to several undesirable consequences. Physicians should be aware of the risks iodine deficiency poses, especially during pregnancy, and should be familiar with approaches to testing and current indications for iodine supplementation.
SOURCES OF IODINE AND SALT IODIZATION
The major environmental source of iodine is the ocean. Elemental iodine in the ocean volatilizes into the atmosphere and returns to the soil by rain. The effects of glaciation, flooding, and leaching into soil have resulted in the variable geographic distribution of iodine. Mountainous areas (eg, the Alps, Andes, Himalayas) and areas with frequent flooding typically have iodine-deficient soil due to slow iodine cycling.1 Seafood is a good source of iodine because marine plants and animals concentrate iodine from seawater. The iodine content of other foods varies widely, depending on the source and any additives.
In the United States, the major sources of dietary iodine are dairy products (due to livestock iodine supplements and use of iodophors for cleaning milk udders) and iodized salt.1,2 Seafood contains a higher amount of iodine by weight than dairy products but is consumed far less than dairy.3,4 Further, the iodine content of milk can range from 88 to 168 μg per 250 mL (about 1 cup), depending on the product manufacturer. Also, iodine content is often omitted from the food label. Even if it is reported, the package labeling may not accurately predict the iodine content.5
Less common sources of iodine are radiographic contrast, bread with iodate dough conditioners, red food coloring (erythrosine), and drugs such as amiodarone.1
Using iodized salt is an effective and stable way to ensure adequate iodine intake. In the United States, only table salt is iodized, and the salt typically used in processed food has only minimal iodine content.6 Nearly 70% of the salt we ingest is from processed food. Table salt provides only 15% of dietary salt intake, and only 70% of consumers choose iodized salt for home cooking.7
IODINE REQUIREMENTS
Daily requirements of iodine suggested by the World Health Organization (WHO) and by the US Institute of Medicine are in the range of 90 to 150 µg/day.8,9 The iodine requirement is higher in pregnancy (220–250 µg/day) because of increased maternal thyroid hormone production required to maintain euthyroidism and increased renal iodine clearance, and it is even higher in lactating women (250–290 µg/day).
IODINE STATUS IN POPULATIONS
Since the establishment of universal salt iodization programs under the influence of the WHO and the International Council for Control of Iodine Deficiency Disorders (ICCIDD) in 1990, global iodine status has continued to improve. Yet only 70% of households worldwide currently have access to adequately iodized salt, because many countries lack a national program for iodine supplementation. The population of the United States was historically iodine-deficient, but since the introduction of salt iodization in the 1920s, the iodine status in the United States has been considered adequate.1
The WHO defines iodine status for a population by the median spot urinary iodine concentration. Because a urinary iodine concentration of 100 μg/L represents an iodine intake of about 150 μg/day, the WHO uses a median urinary iodine concentration of 100 to 199 μg/L to define adequate iodine intake for a nonpregnant population.9
The National Health and Nutrition Examination Survey (NHANES) found that the median urinary iodine concentration decreased by more than 50% from the 1970s to the 1990s, indicating declining iodine status in the US population.2 Of particular concern, the percentage of women of childbearing age with moderate iodine deficiency increased from 4% to 15% over this period.2 Still, the NHANES survey in 2009–2010 indicated that the overall US population is still iodine-sufficient (median urinary iodine concentration 144 μg/L).10 The decline in the US iodine status may be due to reduction of iodine content in dairy products, increased use of noniodized salt by the food industry, and recommendations to avoid salt for blood pressure control.
Although US iodine status has been considered generally adequate, iodine intake varies greatly across the population. Vegans tend to have iodine-deficient diets, while kelp consumers may have excessive iodine intake.11 Individuals with lactose intolerance are at risk of iodine deficiency, given that dairy products are a major source of iodine in the United States. Physicians should be aware of these risk factors for iodine deficiency.
PREGNANCY AND LACTATION
It is crucial to maintain euthyroidism during pregnancy. In early gestation, maternal thyroid hormone production increases 50% due to an increase in thyroid-binding globulin and stimulation by human chorionic gonadotropin. The glomerular filtration rate increases by 30% to 50% during pregnancy, thus increasing renal iodine clearance. Fetal thyroid hormone production increases during the second half of pregnancy, further contributing to increased maternal iodine requirements because iodine readily crosses the placenta.12
Women with sufficient iodine intake before and during pregnancy generally have adequate intrathyroidal iodine storage and can adapt to the increased demand for thyroid hormone throughout gestation. But in the setting of even mild iodine deficiency, total body iodine stores decline gradually from the first to third trimester of pregnancy.13
The fetal thyroid gland does not begin to concentrate iodine until 10 to 12 weeks of gestation and is not controlled by TSH until the full development of the pituitary-portal vascular system at 20 weeks of gestation.12 Therefore, the fetus relies on maternal thyroid hormone during this critical stage of neurodevelopment. Thyroid hormone is essential for oligodendrocyte differentiation and myelin distribution14 as well as fetal neuronal proliferation and migration in the first and second trimesters. Iodine deficiency leading to maternal hypothyroidism can result in irreversible fetal brain damage.
Because of the greater requirement during pregnancy, the WHO recommends using a median urinary iodine concentration of 150 to 249 μg/L to define a population that has no iodine deficiency.9 The NHANES data from 2007 to 2010 showed that pregnant US women were mildly iodine-deficient (median urinary iodine concentration 135 μg/L),10 and the National Children’s Study of 501 pregnant US women during the third trimester in 2009 to 2010 showed they had adequate iodine intake (median urinary iodine concentration 167 μg/L). Interestingly, pregnant non-Hispanic blacks were the only ethnic group with a median urinary iodine concentration less than 150 μg/L, suggesting that race or ethnicity is a predictor of iodine status in pregnant women.10
Iodine requirements during lactation
During lactation, thyroid hormone production and renal iodine clearance return to the prepregnancy state. However, a significant amount of iodine is excreted into breast milk at a concentration 20 to 50 times greater than that in plasma.15 It is recommended that lactating women continue high iodine intake to ensure sufficient iodine in breast milk to build reserves in the newborn’s thyroid gland.
The iodine requirement during lactation is 225 to 350 μg/day.16 Breast milk containing 100 to 200 μg/L of iodine appears to provide adequate iodine to meet Institute of Medicine recommendations for infants.17 The amount of iodine excreted into breast milk depends on maternal iodine intake. In the setting of iodine sufficiency, the iodine content of breast milk is 150 to 180 μg/L, but it is much lower (9–32 μg/L) in women from iodine-deficient areas, eg, the “goiter belt,” which included the Great Lakes, the Appalachians, and northwestern states. While iodized salt has virtually eliminated the goiter belt, the risk of iodine deficiency remains for people who avoid iodized salt and dairy.15
To ensure adequate iodine intake, the American Thyroid Association recommends that women receive iodine supplementation daily during pregnancy and lactation.11 However, the iodine content of prenatal multivitamins is currently not mandated in the United States. Only half of marketed prenatal vitamins in the United States contain iodine, in the form of either potassium iodide or kelp. Though most iodine-containing products claim to contain at least 150 μg of iodine per daily dose, when measured, the actual iodine content varied between 33 and 610 μg.18
CONSEQUENCES OF IODINE DEFICIENCY
Goiter
Goiter in iodine-deficient areas is considered to be an adaptation to chronic iodine deficiency. Low iodine intake leads to reduced thyroid hormone production, which in turn stimulates TSH secretion from the pituitary. TSH increases iodine uptake by the thyroid, stimulates thyroid growth, and leads to goiter development.
Initially, goiter is characterized by diffuse thyroid enlargement, but over time it may become nodular from progressive accumulation of new thyroid follicles. Goiter in children from iodine-deficient areas is diffusely enlarged, whereas in older adults it tends to be multinodular.
Iodine deficiency and chronic TSH stimulation may play a role in TSH receptor-activating mutations of thyroid follicles. These “gain-of-function” mutations are more common in the glands of patients with goiter in areas of iodine deficiency but are relatively rare in areas of iodine sufficiency.19 Toxic multinodular goiter may eventually develop, and hyperthyroidism may occur if iodine deficiency is not severe.
Goiter generally does not cause obstructive symptoms, since the thyroid usually grows outward. However, a very large goiter may descend to the thoracic inlet and compress the trachea and esophagus. The obstructive effect of a large goiter can be demonstrated by having a patient raise the arms adjacent to the face (the Pemberton maneuver). Signs suggesting obstruction are engorged neck veins, facial plethora, increased dyspnea, and stridor during the maneuver. Computed tomography of the neck and upper thorax may provide information on the degree of tracheal compression.20
Hypothyroidism
A normal or low triiodothyronine (T3), a low serum thyroxine (T4), and a variably elevated TSH are features of thyroid function tests in iodine deficiency.11,21,22 As long as daily iodine intake exceeds 50 μg/day, the absolute uptake of iodine by the thyroid gland usually remains adequate to maintain euthyroidism. Below 50 μg/day, iodine storage in the thyroid becomes depleted, leading to hypothyroidism.1
The clinical manifestations of hypothyroidism from iodine deficiency are similar to those of hypothyroidism from other causes. Because of thyroid hormone’s role in neural and somatic development, the manifestations of hypothyroidism differ among age groups (Table 1).
Cretinism
Before the development of fetal thyroid tissue in the 10th to 12th week of gestation, the fetus is dependent on maternal thyroid hormone, which crosses the placenta to support general and neural development. Iodine deficiency leading to maternal hypothyroidism (in early gestation) or inadequate fetal thyroid hormone production (in late gestation) may result in various degrees of mental retardation or lower than expected IQ.
Severe iodine deficiency during gestation typically results in cretinism, characterized by severe mental retardation accompanied by other neurologic or physical defects. Cretinism is divided into two subtypes according to clinical manifestations (neurologic and myxomatous cretinism; Table 2), which may reflect the different timing of intrauterine insult to the developing fetal nervous system and whether the iodine deficiency continues into the postnatal period. Both types can be prevented by adequate maternal iodine intake before and during pregnancy.23,24
Although mild gestational iodine deficiency does not result in cretinism, it nevertheless has an adverse impact on fetal neurodevelopment and subsequent functioning. Children of mothers with mild gestational iodine deficiency were found to have reductions in spelling, grammar, and English literacy performance despite growing up in iodine-replete environments.25
Impaired cognitive development
Reduction in IQ has been noted in affected youth from regions of severe and mild iodine deficiency. A meta-analysis of studies relating iodine deficiency to cognitive development suggested that chronic moderate to severe iodine deficiency reduced expected average IQ by about 13.5 points.26
The effects of mild iodine deficiency during childhood are more difficult to quantify. The results of one study suggested that mild iodine deficiency was associated with subtle neurodevelopmental deficits and that iodine supplementation might improve cognitive function in mildly iodine-deficient children.27
In a 2009 randomized, placebo-controlled study in New Zealand, 184 children ages 10 to 13 with mild iodine deficiency (median urinary iodine concentration of 63 μg/L) received iodine supplementation (150 μg/day) or placebo for 28 weeks. Iodine supplementation increased the median urinary iodine concentration to 145 μg/L and significantly improved perceptual reasoning measures and overall cognitive score compared with placebo.28
These findings suggest that correcting mild iodine deficiency in children could improve certain components of cognition. More research is needed to understand the effects of mild iodine deficiency and iodine supplementation on cognitive function.
ASSESSING IODINE STATUS
The diagnosis of iodine deficiency is based on clinical and laboratory assessments. Clinical manifestations compatible with iodine deficiency and careful history-taking focused on the patient’s dietary iodine intake and geographic data are keys to the diagnosis.
Four main methods are used to assess iodine status at a population level: urinary iodine, serum thyroglobulin, serum TSH, and thyroid size. Urinary iodine is a sensitive marker for recent iodine intake (within days); thyroglobulin represents iodine nutrition over a period of months and thyroid size over a period of years.1
Urinary iodine
Most dietary iodine is excreted into the urine within 24 hours of ingestion, and the 24-hour urinary iodine is considered a reference standard for the measurement of individual daily iodine intake. However, the process of collection is cumbersome, and the 24-hour urinary iodine can vary from day to day in the same person, depending on the amount of iodine ingested.
A study in healthy women from an iodine-sufficient area suggested that 10 repeated 24-hour urine collections estimated the person’s iodine status at a precision of 20% because of variable daily iodine intake.29 Therefore, when necessary, several 24-hour urine iodine determinations should be performed.
A single, random, spot urinary iodine is expressed as the urinary iodine concentration and is affected by the amount of iodine and fluids the individual ingests in a day, thus resulting in high variation both within an individual person and between individuals. Expressing the urinary iodine concentration as the ratio of urine iodine to creatinine is useful in correcting for the influence of fluid intake. The ratio of urine iodine to creatinine can be used to estimate 24-hour urine iodine with the following formula: urine iodine (μg/L)/creatinine (g/L)× age- and sex-specific estimated 24-hour creatinine excretion (g/day). Another clinical use of the spot urine iodine is to screen for exposure to a large amount of iodine from a source such as radiographic contrast.30
Although individual urine iodine excretion and urine volume can vary from day to day, this variation tends to even out in a large number of samples. In study populations of at least 500, the median value of the spot urinary iodine concentration is considered a reliable measure of iodine intake in that population.30 The spot urine iodine test is convenient, making it the test of choice to study iodine status in a large cohort. The WHO recommends using the median value of the spot urine iodine to evaluate the iodine status of a population.9
Thyroglobulin
Thyroglobulin is a thyroid-specific protein involved in the synthesis of thyroid hormone. Small amounts can be detected in the blood of healthy people. In the absence of thyroid damage, the amount of serum thyroglobulin depends on thyroid cell mass and TSH stimulation. The serum level is elevated in iodine deficiency as a result of chronic TSH stimulation and thyroid hyperplasia. Thus, thyroglobulin can serve as a marker of iodine deficiency.
Serum thyroglobulin assays have been adapted for use on dried whole-blood spots, which require only a few drops of whole blood collected on filter paper and left to air-dry. The results of the dried whole-blood assay correlate closely with those of the serum assay.31 An established international dried whole-blood thyroglobulin reference range for iodine-sufficient school-age children is 4 to 40 μg/L.32 A median level of less than 13 μg/L in school-age children indicates iodine sufficiency in the population.33
Thyroid-stimulating hormone
Iodine deficiency lowers serum T4, which in turn leads to increased serum TSH. Therefore, iodine-deficient populations generally have higher TSH than iodine-sufficient groups. However, the TSH values in older children and adults with iodine deficiency are not significantly different from values of those with adequate iodine intake. Therefore, TSH is not a practical marker of iodine deficiency in the general population.
In contrast, TSH in newborns is a reasonable indicator of population iodine status. The newborn thyroid has limited iodine stores compared with that of an adult and hence a much higher iodine turnover rate. TSH from the cord blood is markedly elevated in newborns of mothers with moderate to severe iodine deficiency.34 A high prevalence of newborns with elevated TSH should therefore reflect iodine deficiency in the area where the mothers of the newborns live.
TSH is now routinely checked in newborns to screen for congenital hypothyroidism. TSH is typically checked 2 to 5 days after delivery to avoid confusion with transient physiologic TSH elevation, which occurs within a few hours after birth and decreases rapidly in 24 hours. The WHO has proposed that a more than 3% prevalence of newborns with TSH values higher than 5 mU/L from blood samples collected 3 to 4 days after birth indicates iodine deficiency in a population.1 This threshold appears to correlate well with the iodine status of the population defined by the WHO’s median urinary iodine concentration.35,36
But several other factors can influence the measurement of newborn TSH, such as prematurity, time of blood collection, maternal or newborn exposure to iodine-containing antiseptics, and the TSH assay methodology. These potential confounding factors limit the role of neonatal TSH as a reliable monitoring tool for iodine deficiency.35,37
Thyroid size
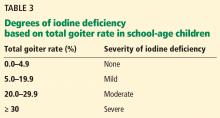
The size of the thyroid gland varies inversely with iodine intake. Thyroid size can be assessed by either palpation or ultrasonography, with the latter being more sensitive. The goiter rate in school-age children can be used to determine the severity of iodine deficiency in the population (Table 3). A goiter rate of 5% or more in school-age children suggests the presence of iodine deficiency in the community.
Although thyroid size is easy to estimate by palpation, it has low sensitivity and specificity to detect iodine deficiency and high interobserver variation. Thyroid ultrasonography provides a more precise measurement of thyroid gland volume. Zimmermann et al38 provided reference data on thyroid volume stratified by age, sex, and body surface area of school-age children in iodine-sufficient areas.38 Results of ultrasonography in a population is then compared with these reference data. The higher the percentage of the population with thyroid volume exceeding the 97th percentile of the reference range, the more severe the iodine deficiency. However, the WHO does not specify how to grade the degree of iodine deficiency based on the thyroid volume obtained with ultrasonography. Follow-up studies showed no significant correlation between urinary iodine concentration and thyroid size.39,40
Thyroid size decreases slowly after iodine repletion. Therefore, the goiter rate may remain high for several years after iodine supplementation begins.1,9
TREATMENT AND PREVENTION
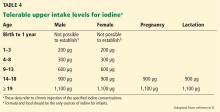
Treatment of iodine deficiency should be instituted at the levels recommended by the Institute of Medicine and the WHO. The tolerable upper intake levels for iodine are outlined in Table 4. In a nonpregnant adult, 150 μg/day is sufficient for normal thyroid function. Iodine intake should be higher for pregnant and lactating women (250 μg/day according to the WHO recommendation).
Iodine supplementation is easily achieved by using iodized salt or an iodine-containing daily multivitamin.
In patients with overt hypothyroidism from iodine deficiency, we recommend initiating levothyroxine treatment along with iodine supplementation to restore euthyroidism, with consideration of possible interruption in 6 to 12 months when the urine iodine has normalized and goiter size has decreased. Thyroid function should be reassessed 4 to 6 weeks after discontinuation of levothyroxine.
At the population level, iodine deficiency can usually be prevented by iodization of food products or the water supply. In developing countries where salt iodization is not practical, iodine deficiency has been eradicated by adding iodine drops to well water or by injecting people with iodized oil.
TAKE-HOME POINTS
Iodine is essential for thyroid hormone synthesis. It can be obtained by eating iodine-containing foods or by using iodized salt. The WHO classifies iodine deficiency based on the median urinary iodine concentration. Iodine nutrition at the community level is best assessed by measurements of urinary iodine, thyroglobulin, serum TSH, and thyroid size.
Adequate iodine intake during pregnancy is important for fetal development. Iodine deficiency is associated with goiter and hypothyroidism. Severe iodine deficiency during pregnancy is associated with cretinism.
There is evidence that mild to moderate iodine deficiency can cause impaired cognitive development and that correcting the iodine deficiency can significantly improve cognitive function.
CASE FOLLOW-UP
This patient had been following a strict vegan diet with very little intake of iodized salt. Her dietary history and the presence of goiter suggested iodine deficiency. She was instructed to take an iodine supplement 150 μg/day to meet her daily requirement. After 2 months of iodine supplementation, her urine iodine concentration had increased to 58 μg/L. She remained biochemically euthyroid.
- Zimmermann MB. Iodine deficiency. Endocr Rev 2009; 30:376–408.
- Pearce EN, Andersson M, Zimmermann MB. Global iodine nutrition: where do we stand in 2013? Thyroid 2013; 23:523–528.
- Huang SW. Seafood and iodine: an analysis of a medical myth. Allergy Asthma Proc 2005; 26:468–469.
- US Census Bureau. The 2012 Statistical abstract. Health and nutrition. www.census.gov/prod/2011pubs/12statab/health.pdf. Accessed December 1, 2016.
- Pearce EN, Pino S, He X, Bazrafshan HR, Lee SL, Braverman LE. Sources of dietary iodine: bread, cows’ milk, and infant formula in the Boston area. J Clin Endocrinol Metab 2004; 89:3421–3424.
- Salt Institute. Production and Industry. www.saltinstitute.org/salt-101/production-industry. Accessed September 20, 2016.
- Dunn JT. Guarding our nation's thyroid health. J Clin Endocrinol Metab 2002; 87:486–488.
- Institute of Medicine (US) Panel on Micronutrients. Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Washington, DC: National Academy Press; 2001.
- World Health Organization (WHO). Assessment of iodine deficiency disorders and monitoring their elimination: a guide for programme managers. www.who.int/nutrition/publications/micronutrients/iodine_deficiency/9789241595827/en. Accessed December 1, 2016.
- Caldwell KL, Pan Y, Mortensen ME, Makhmudov A, Merrill L, Moye J. Iodine status in pregnant women in the National Children's Study and in US women (15–44 years), National Health and Nutrition Examination Survey 2005–2010. Thyroid 2013; 23:927–937.
- Public Health Committee of the American Thyroid Association; Becker DV, Braverman LE, Delange F, et al. Iodine supplementation for pregnancy and lactation—United States and Canada: recommendations of the American Thyroid Association. Thyroid 2006; 16:949–951.
- Leung AM, Pearce EN, Braverman LE. Iodine nutrition in pregnancy and lactation. Endocrinol Metab Clin North Am 2011; 40:765–777.
- Pearce EN. Iodine in pregnancy: is salt iodization enough? J Clin Endocrinol Metab 2008; 93:2466–2468.
- Younes-Rapozo V, Berendonk J, Savignon T, Manhaes AC, Barradas PC. Thyroid hormone deficiency changes the distribution of oligodendrocyte/myelin markers during oligodendroglial differentiation in vitro. Int J Dev Neurosci 2006; 24:445–453.
- Azizi F, Smyth P. Breastfeeding and maternal and infant iodine nutrition. Clin Endocrinol (Oxf) 2009; 70:803–809.
- Delange F. Iodine requirements during pregnancy, lactation and the neonatal period and indicators of optimal iodine nutrition. Public Health Nutr 2007; 10:1571–1583.
- Semba RD, Delange F. Iodine in human milk: perspectives for infant health. Nutr Rev 2001; 59:269–278.
- Leung AM, Pearce EN, Braverman LE. Iodine content of prenatal multivitamins in the United States. N Engl J Med 2009; 360:939–940.
- Tonacchera M, Agretti P, Chiovato L, et al. Activating thyrotropin receptor mutations are present in nonadenomatous hyperfunctioning nodules of toxic or autonomous multinodular goiter. J Clin Endocrinol Metab 2000; 85:2270–2274.
- Medeiros-Neto G, Camargo RY, Tomimori EK. Approach to and treatment of goiters. Med Clin North Am 2012; 96:351–368.
- Heidemann P, Stubbe P. Serum 3,5,3'-triiodothyronine, thyroxine, and thyrotropin in hypothyroid infants with congenital goiter and the response to iodine. J Clin Endocrinol Metab 1978; 47:189–192.
- Patel YC, Pharoah PO, Hornabrook RW, Hetzel BS. Serum triiodothyronine, thyroxine and thyroid-stimulating hormone in endemic goiter: a comparison of goitrous and nongoitrous subjects in New Guinea. J Clin Endocrinol Metab 1973; 37:783–789.
- Boyages SC, Halpern JP. Endemic cretinism: toward a unifying hypothesis. Thyroid 1993; 3:59–69.
- Chen ZP, Hetzel BS. Cretinism revisited. Best Pract Res Clin Endocrinol Metab 2010; 24:39–50.
- Hynes KL, Otahal P, Hay I, Burgess JR. Mild iodine deficiency during pregnancy is associated with reduced educational outcomes in the offspring: 9-year follow-up of the gestational iodine cohort. J Clin Endocrinol Metab 2013; 98:1954–1962.
- Bleichrodt N, Born MP. A metaanalysis of research on iodine and its relationship to cognitive development. In: Stanbury JB, ed. The Damaged Brain of Iodine Deficiency. 1st ed. New York, NY: Cognizant Communication; 1994:195–200.
- Melse-Boonstra A, Jaiswal N. Iodine deficiency in pregnancy, infancy and childhood and its consequences for brain development. Best Pract Res Clin Endocrinol Metab 2010; 24:29–38.
- Gordon RC, Rose MC, Skeaff SA, Gray AR, Morgan KM, Ruffman T. Iodine supplementation improves cognition in mildly iodine-deficient children. Am J Clin Nutr 2009; 90:1264–1271.
- Konig F, Andersson M, Hotz K, Aeberli I, Zimmermann MB. Ten repeat collections for urinary iodine from spot samples or 24-hour samples are needed to reliably estimate individual iodine status in women. J Nutr 2011; 141:2049–2054.
- Vejbjerg P, Knudsen N, Perrild H, et al. Estimation of iodine intake from various urinary iodine measurements in population studies. Thyroid 2009; 19:1281–1286.
- Zimmermann MB, Hess SY, Adou P, Toresanni T, Wegmuller R, Hurrell RF. Thyroid size and goiter prevalence after introduction of iodized salt: a 5-y prospective study in schoolchildren in Cote d’Ivoire. Am J Clin Nutr 2003; 77:663–667.
- Zimmermann MB, de Benoist B, Corigliano S, et al. Assessment of iodine status using dried blood spot thyroglobulin: development of reference material and establishment of an international reference range in iodine-sufficient children. J Clin Endocrinol Metab 2006; 91:4881–4887.
- Zimmermann MB, Aeberli I, Andersson M, et al. Thyroglobulin is a sensitive measure of both deficient and excess iodine intakes in children and indicates no adverse effects on thyroid function in the UIC range of 100-299 mug/L: a UNICEF/ICCIDD study group report. J Clin Endocrinol Metab 2013; 98:1271–1280.
- Thilly CH, Delange F, Lagasse R, et al. Fetal hypothyroidism and maternal thyroid status in severe endemic goiter. J Clin Endocrinol Metab 1978; 47:354–360.
- Sullivan KM, May W, Nordenberg D, Houston R, Maberly GF. Use of thyroid stimulating hormone testing in newborns to identify iodine deficiency. J Nutr 1997; 127:55–58.
- Delange F. Neonatal thyroid screening as a monitoring tool for the control of iodine deficiency. Acta Paediatr Suppl 1999; 88:21–24.
- Li M, Eastman CJ. Neonatal TSH screening: is it a sensitive and reliable tool for monitoring iodine status in populations? Best Pract Res Clin Endocrinol Metab 2010; 24:63–75.
- Zimmermann MB, Moretti D, Chaouki N, Torresani T. Development of a dried whole-blood spot thyroglobulin assay and its evaluation as an indicator of thyroid status in goitrous children receiving iodized salt. Am J Clin Nutr 2003; 77:1453–1458.
- Brahmbhatt S, Brahmbhatt RM, Boyages SC. Thyroid ultrasound is the best prevalence indicator for assessment of iodine deficiency disorders: a study in rural/tribal schoolchildren from Gujarat (Western India). Eur J Endocrinol 2000; 143:37–46.
- Moradi M, Hashemipour M, Akbari S, Kor Z, Mirbod SA, Kooshanmehr MR. Ultrasonographic evaluation of the thyroid gland volume among 8-15-year-old children in Isfahan, Iran. Adv Biomed Res 2014; 3:9.
A 65-year-old woman is found to have a goiter. She is clinically euthyroid. She is a strict vegan and only uses noniodized Himalayan salt for cooking. Her thyroid gland is diffusely enlarged with no nodules. The estimated weight of the thyroid gland is 50 g (normal 10–20 g) based on ultrasonography. Her thyroid-stimulating hormone (TSH) level is 2.95 mU/L (reference range 0.5–5 mU/L), and her free thyroxine level is 0.8 ng/dL (0.7–1.8 ng/dL). Testing for TSH receptor antibody is negative. Her 24-hour urine iodine is undetectable (urine iodine concentration < 10 μg/L with urine volume 3,175 mL). What may be the cause of her goiter?
Iodine is an essential element needed for the production of thyroid hormone, which controls metabolism and plays a major role in fetal neurodevelopment. Its ionized form is called iodide. Iodine deficiency results in impairment of thyroid hormone synthesis and may lead to several undesirable consequences. Physicians should be aware of the risks iodine deficiency poses, especially during pregnancy, and should be familiar with approaches to testing and current indications for iodine supplementation.
SOURCES OF IODINE AND SALT IODIZATION
The major environmental source of iodine is the ocean. Elemental iodine in the ocean volatilizes into the atmosphere and returns to the soil by rain. The effects of glaciation, flooding, and leaching into soil have resulted in the variable geographic distribution of iodine. Mountainous areas (eg, the Alps, Andes, Himalayas) and areas with frequent flooding typically have iodine-deficient soil due to slow iodine cycling.1 Seafood is a good source of iodine because marine plants and animals concentrate iodine from seawater. The iodine content of other foods varies widely, depending on the source and any additives.
In the United States, the major sources of dietary iodine are dairy products (due to livestock iodine supplements and use of iodophors for cleaning milk udders) and iodized salt.1,2 Seafood contains a higher amount of iodine by weight than dairy products but is consumed far less than dairy.3,4 Further, the iodine content of milk can range from 88 to 168 μg per 250 mL (about 1 cup), depending on the product manufacturer. Also, iodine content is often omitted from the food label. Even if it is reported, the package labeling may not accurately predict the iodine content.5
Less common sources of iodine are radiographic contrast, bread with iodate dough conditioners, red food coloring (erythrosine), and drugs such as amiodarone.1
Using iodized salt is an effective and stable way to ensure adequate iodine intake. In the United States, only table salt is iodized, and the salt typically used in processed food has only minimal iodine content.6 Nearly 70% of the salt we ingest is from processed food. Table salt provides only 15% of dietary salt intake, and only 70% of consumers choose iodized salt for home cooking.7
IODINE REQUIREMENTS
Daily requirements of iodine suggested by the World Health Organization (WHO) and by the US Institute of Medicine are in the range of 90 to 150 µg/day.8,9 The iodine requirement is higher in pregnancy (220–250 µg/day) because of increased maternal thyroid hormone production required to maintain euthyroidism and increased renal iodine clearance, and it is even higher in lactating women (250–290 µg/day).
IODINE STATUS IN POPULATIONS
Since the establishment of universal salt iodization programs under the influence of the WHO and the International Council for Control of Iodine Deficiency Disorders (ICCIDD) in 1990, global iodine status has continued to improve. Yet only 70% of households worldwide currently have access to adequately iodized salt, because many countries lack a national program for iodine supplementation. The population of the United States was historically iodine-deficient, but since the introduction of salt iodization in the 1920s, the iodine status in the United States has been considered adequate.1
The WHO defines iodine status for a population by the median spot urinary iodine concentration. Because a urinary iodine concentration of 100 μg/L represents an iodine intake of about 150 μg/day, the WHO uses a median urinary iodine concentration of 100 to 199 μg/L to define adequate iodine intake for a nonpregnant population.9
The National Health and Nutrition Examination Survey (NHANES) found that the median urinary iodine concentration decreased by more than 50% from the 1970s to the 1990s, indicating declining iodine status in the US population.2 Of particular concern, the percentage of women of childbearing age with moderate iodine deficiency increased from 4% to 15% over this period.2 Still, the NHANES survey in 2009–2010 indicated that the overall US population is still iodine-sufficient (median urinary iodine concentration 144 μg/L).10 The decline in the US iodine status may be due to reduction of iodine content in dairy products, increased use of noniodized salt by the food industry, and recommendations to avoid salt for blood pressure control.
Although US iodine status has been considered generally adequate, iodine intake varies greatly across the population. Vegans tend to have iodine-deficient diets, while kelp consumers may have excessive iodine intake.11 Individuals with lactose intolerance are at risk of iodine deficiency, given that dairy products are a major source of iodine in the United States. Physicians should be aware of these risk factors for iodine deficiency.
PREGNANCY AND LACTATION
It is crucial to maintain euthyroidism during pregnancy. In early gestation, maternal thyroid hormone production increases 50% due to an increase in thyroid-binding globulin and stimulation by human chorionic gonadotropin. The glomerular filtration rate increases by 30% to 50% during pregnancy, thus increasing renal iodine clearance. Fetal thyroid hormone production increases during the second half of pregnancy, further contributing to increased maternal iodine requirements because iodine readily crosses the placenta.12
Women with sufficient iodine intake before and during pregnancy generally have adequate intrathyroidal iodine storage and can adapt to the increased demand for thyroid hormone throughout gestation. But in the setting of even mild iodine deficiency, total body iodine stores decline gradually from the first to third trimester of pregnancy.13
The fetal thyroid gland does not begin to concentrate iodine until 10 to 12 weeks of gestation and is not controlled by TSH until the full development of the pituitary-portal vascular system at 20 weeks of gestation.12 Therefore, the fetus relies on maternal thyroid hormone during this critical stage of neurodevelopment. Thyroid hormone is essential for oligodendrocyte differentiation and myelin distribution14 as well as fetal neuronal proliferation and migration in the first and second trimesters. Iodine deficiency leading to maternal hypothyroidism can result in irreversible fetal brain damage.
Because of the greater requirement during pregnancy, the WHO recommends using a median urinary iodine concentration of 150 to 249 μg/L to define a population that has no iodine deficiency.9 The NHANES data from 2007 to 2010 showed that pregnant US women were mildly iodine-deficient (median urinary iodine concentration 135 μg/L),10 and the National Children’s Study of 501 pregnant US women during the third trimester in 2009 to 2010 showed they had adequate iodine intake (median urinary iodine concentration 167 μg/L). Interestingly, pregnant non-Hispanic blacks were the only ethnic group with a median urinary iodine concentration less than 150 μg/L, suggesting that race or ethnicity is a predictor of iodine status in pregnant women.10
Iodine requirements during lactation
During lactation, thyroid hormone production and renal iodine clearance return to the prepregnancy state. However, a significant amount of iodine is excreted into breast milk at a concentration 20 to 50 times greater than that in plasma.15 It is recommended that lactating women continue high iodine intake to ensure sufficient iodine in breast milk to build reserves in the newborn’s thyroid gland.
The iodine requirement during lactation is 225 to 350 μg/day.16 Breast milk containing 100 to 200 μg/L of iodine appears to provide adequate iodine to meet Institute of Medicine recommendations for infants.17 The amount of iodine excreted into breast milk depends on maternal iodine intake. In the setting of iodine sufficiency, the iodine content of breast milk is 150 to 180 μg/L, but it is much lower (9–32 μg/L) in women from iodine-deficient areas, eg, the “goiter belt,” which included the Great Lakes, the Appalachians, and northwestern states. While iodized salt has virtually eliminated the goiter belt, the risk of iodine deficiency remains for people who avoid iodized salt and dairy.15
To ensure adequate iodine intake, the American Thyroid Association recommends that women receive iodine supplementation daily during pregnancy and lactation.11 However, the iodine content of prenatal multivitamins is currently not mandated in the United States. Only half of marketed prenatal vitamins in the United States contain iodine, in the form of either potassium iodide or kelp. Though most iodine-containing products claim to contain at least 150 μg of iodine per daily dose, when measured, the actual iodine content varied between 33 and 610 μg.18
CONSEQUENCES OF IODINE DEFICIENCY
Goiter
Goiter in iodine-deficient areas is considered to be an adaptation to chronic iodine deficiency. Low iodine intake leads to reduced thyroid hormone production, which in turn stimulates TSH secretion from the pituitary. TSH increases iodine uptake by the thyroid, stimulates thyroid growth, and leads to goiter development.
Initially, goiter is characterized by diffuse thyroid enlargement, but over time it may become nodular from progressive accumulation of new thyroid follicles. Goiter in children from iodine-deficient areas is diffusely enlarged, whereas in older adults it tends to be multinodular.
Iodine deficiency and chronic TSH stimulation may play a role in TSH receptor-activating mutations of thyroid follicles. These “gain-of-function” mutations are more common in the glands of patients with goiter in areas of iodine deficiency but are relatively rare in areas of iodine sufficiency.19 Toxic multinodular goiter may eventually develop, and hyperthyroidism may occur if iodine deficiency is not severe.
Goiter generally does not cause obstructive symptoms, since the thyroid usually grows outward. However, a very large goiter may descend to the thoracic inlet and compress the trachea and esophagus. The obstructive effect of a large goiter can be demonstrated by having a patient raise the arms adjacent to the face (the Pemberton maneuver). Signs suggesting obstruction are engorged neck veins, facial plethora, increased dyspnea, and stridor during the maneuver. Computed tomography of the neck and upper thorax may provide information on the degree of tracheal compression.20
Hypothyroidism
A normal or low triiodothyronine (T3), a low serum thyroxine (T4), and a variably elevated TSH are features of thyroid function tests in iodine deficiency.11,21,22 As long as daily iodine intake exceeds 50 μg/day, the absolute uptake of iodine by the thyroid gland usually remains adequate to maintain euthyroidism. Below 50 μg/day, iodine storage in the thyroid becomes depleted, leading to hypothyroidism.1
The clinical manifestations of hypothyroidism from iodine deficiency are similar to those of hypothyroidism from other causes. Because of thyroid hormone’s role in neural and somatic development, the manifestations of hypothyroidism differ among age groups (Table 1).
Cretinism
Before the development of fetal thyroid tissue in the 10th to 12th week of gestation, the fetus is dependent on maternal thyroid hormone, which crosses the placenta to support general and neural development. Iodine deficiency leading to maternal hypothyroidism (in early gestation) or inadequate fetal thyroid hormone production (in late gestation) may result in various degrees of mental retardation or lower than expected IQ.
Severe iodine deficiency during gestation typically results in cretinism, characterized by severe mental retardation accompanied by other neurologic or physical defects. Cretinism is divided into two subtypes according to clinical manifestations (neurologic and myxomatous cretinism; Table 2), which may reflect the different timing of intrauterine insult to the developing fetal nervous system and whether the iodine deficiency continues into the postnatal period. Both types can be prevented by adequate maternal iodine intake before and during pregnancy.23,24
Although mild gestational iodine deficiency does not result in cretinism, it nevertheless has an adverse impact on fetal neurodevelopment and subsequent functioning. Children of mothers with mild gestational iodine deficiency were found to have reductions in spelling, grammar, and English literacy performance despite growing up in iodine-replete environments.25
Impaired cognitive development
Reduction in IQ has been noted in affected youth from regions of severe and mild iodine deficiency. A meta-analysis of studies relating iodine deficiency to cognitive development suggested that chronic moderate to severe iodine deficiency reduced expected average IQ by about 13.5 points.26
The effects of mild iodine deficiency during childhood are more difficult to quantify. The results of one study suggested that mild iodine deficiency was associated with subtle neurodevelopmental deficits and that iodine supplementation might improve cognitive function in mildly iodine-deficient children.27
In a 2009 randomized, placebo-controlled study in New Zealand, 184 children ages 10 to 13 with mild iodine deficiency (median urinary iodine concentration of 63 μg/L) received iodine supplementation (150 μg/day) or placebo for 28 weeks. Iodine supplementation increased the median urinary iodine concentration to 145 μg/L and significantly improved perceptual reasoning measures and overall cognitive score compared with placebo.28
These findings suggest that correcting mild iodine deficiency in children could improve certain components of cognition. More research is needed to understand the effects of mild iodine deficiency and iodine supplementation on cognitive function.
ASSESSING IODINE STATUS
The diagnosis of iodine deficiency is based on clinical and laboratory assessments. Clinical manifestations compatible with iodine deficiency and careful history-taking focused on the patient’s dietary iodine intake and geographic data are keys to the diagnosis.
Four main methods are used to assess iodine status at a population level: urinary iodine, serum thyroglobulin, serum TSH, and thyroid size. Urinary iodine is a sensitive marker for recent iodine intake (within days); thyroglobulin represents iodine nutrition over a period of months and thyroid size over a period of years.1
Urinary iodine
Most dietary iodine is excreted into the urine within 24 hours of ingestion, and the 24-hour urinary iodine is considered a reference standard for the measurement of individual daily iodine intake. However, the process of collection is cumbersome, and the 24-hour urinary iodine can vary from day to day in the same person, depending on the amount of iodine ingested.
A study in healthy women from an iodine-sufficient area suggested that 10 repeated 24-hour urine collections estimated the person’s iodine status at a precision of 20% because of variable daily iodine intake.29 Therefore, when necessary, several 24-hour urine iodine determinations should be performed.
A single, random, spot urinary iodine is expressed as the urinary iodine concentration and is affected by the amount of iodine and fluids the individual ingests in a day, thus resulting in high variation both within an individual person and between individuals. Expressing the urinary iodine concentration as the ratio of urine iodine to creatinine is useful in correcting for the influence of fluid intake. The ratio of urine iodine to creatinine can be used to estimate 24-hour urine iodine with the following formula: urine iodine (μg/L)/creatinine (g/L)× age- and sex-specific estimated 24-hour creatinine excretion (g/day). Another clinical use of the spot urine iodine is to screen for exposure to a large amount of iodine from a source such as radiographic contrast.30
Although individual urine iodine excretion and urine volume can vary from day to day, this variation tends to even out in a large number of samples. In study populations of at least 500, the median value of the spot urinary iodine concentration is considered a reliable measure of iodine intake in that population.30 The spot urine iodine test is convenient, making it the test of choice to study iodine status in a large cohort. The WHO recommends using the median value of the spot urine iodine to evaluate the iodine status of a population.9
Thyroglobulin
Thyroglobulin is a thyroid-specific protein involved in the synthesis of thyroid hormone. Small amounts can be detected in the blood of healthy people. In the absence of thyroid damage, the amount of serum thyroglobulin depends on thyroid cell mass and TSH stimulation. The serum level is elevated in iodine deficiency as a result of chronic TSH stimulation and thyroid hyperplasia. Thus, thyroglobulin can serve as a marker of iodine deficiency.
Serum thyroglobulin assays have been adapted for use on dried whole-blood spots, which require only a few drops of whole blood collected on filter paper and left to air-dry. The results of the dried whole-blood assay correlate closely with those of the serum assay.31 An established international dried whole-blood thyroglobulin reference range for iodine-sufficient school-age children is 4 to 40 μg/L.32 A median level of less than 13 μg/L in school-age children indicates iodine sufficiency in the population.33
Thyroid-stimulating hormone
Iodine deficiency lowers serum T4, which in turn leads to increased serum TSH. Therefore, iodine-deficient populations generally have higher TSH than iodine-sufficient groups. However, the TSH values in older children and adults with iodine deficiency are not significantly different from values of those with adequate iodine intake. Therefore, TSH is not a practical marker of iodine deficiency in the general population.
In contrast, TSH in newborns is a reasonable indicator of population iodine status. The newborn thyroid has limited iodine stores compared with that of an adult and hence a much higher iodine turnover rate. TSH from the cord blood is markedly elevated in newborns of mothers with moderate to severe iodine deficiency.34 A high prevalence of newborns with elevated TSH should therefore reflect iodine deficiency in the area where the mothers of the newborns live.
TSH is now routinely checked in newborns to screen for congenital hypothyroidism. TSH is typically checked 2 to 5 days after delivery to avoid confusion with transient physiologic TSH elevation, which occurs within a few hours after birth and decreases rapidly in 24 hours. The WHO has proposed that a more than 3% prevalence of newborns with TSH values higher than 5 mU/L from blood samples collected 3 to 4 days after birth indicates iodine deficiency in a population.1 This threshold appears to correlate well with the iodine status of the population defined by the WHO’s median urinary iodine concentration.35,36
But several other factors can influence the measurement of newborn TSH, such as prematurity, time of blood collection, maternal or newborn exposure to iodine-containing antiseptics, and the TSH assay methodology. These potential confounding factors limit the role of neonatal TSH as a reliable monitoring tool for iodine deficiency.35,37
Thyroid size
The size of the thyroid gland varies inversely with iodine intake. Thyroid size can be assessed by either palpation or ultrasonography, with the latter being more sensitive. The goiter rate in school-age children can be used to determine the severity of iodine deficiency in the population (Table 3). A goiter rate of 5% or more in school-age children suggests the presence of iodine deficiency in the community.
Although thyroid size is easy to estimate by palpation, it has low sensitivity and specificity to detect iodine deficiency and high interobserver variation. Thyroid ultrasonography provides a more precise measurement of thyroid gland volume. Zimmermann et al38 provided reference data on thyroid volume stratified by age, sex, and body surface area of school-age children in iodine-sufficient areas.38 Results of ultrasonography in a population is then compared with these reference data. The higher the percentage of the population with thyroid volume exceeding the 97th percentile of the reference range, the more severe the iodine deficiency. However, the WHO does not specify how to grade the degree of iodine deficiency based on the thyroid volume obtained with ultrasonography. Follow-up studies showed no significant correlation between urinary iodine concentration and thyroid size.39,40
Thyroid size decreases slowly after iodine repletion. Therefore, the goiter rate may remain high for several years after iodine supplementation begins.1,9
TREATMENT AND PREVENTION
Treatment of iodine deficiency should be instituted at the levels recommended by the Institute of Medicine and the WHO. The tolerable upper intake levels for iodine are outlined in Table 4. In a nonpregnant adult, 150 μg/day is sufficient for normal thyroid function. Iodine intake should be higher for pregnant and lactating women (250 μg/day according to the WHO recommendation).
Iodine supplementation is easily achieved by using iodized salt or an iodine-containing daily multivitamin.
In patients with overt hypothyroidism from iodine deficiency, we recommend initiating levothyroxine treatment along with iodine supplementation to restore euthyroidism, with consideration of possible interruption in 6 to 12 months when the urine iodine has normalized and goiter size has decreased. Thyroid function should be reassessed 4 to 6 weeks after discontinuation of levothyroxine.
At the population level, iodine deficiency can usually be prevented by iodization of food products or the water supply. In developing countries where salt iodization is not practical, iodine deficiency has been eradicated by adding iodine drops to well water or by injecting people with iodized oil.
TAKE-HOME POINTS
Iodine is essential for thyroid hormone synthesis. It can be obtained by eating iodine-containing foods or by using iodized salt. The WHO classifies iodine deficiency based on the median urinary iodine concentration. Iodine nutrition at the community level is best assessed by measurements of urinary iodine, thyroglobulin, serum TSH, and thyroid size.
Adequate iodine intake during pregnancy is important for fetal development. Iodine deficiency is associated with goiter and hypothyroidism. Severe iodine deficiency during pregnancy is associated with cretinism.
There is evidence that mild to moderate iodine deficiency can cause impaired cognitive development and that correcting the iodine deficiency can significantly improve cognitive function.
CASE FOLLOW-UP
This patient had been following a strict vegan diet with very little intake of iodized salt. Her dietary history and the presence of goiter suggested iodine deficiency. She was instructed to take an iodine supplement 150 μg/day to meet her daily requirement. After 2 months of iodine supplementation, her urine iodine concentration had increased to 58 μg/L. She remained biochemically euthyroid.
A 65-year-old woman is found to have a goiter. She is clinically euthyroid. She is a strict vegan and only uses noniodized Himalayan salt for cooking. Her thyroid gland is diffusely enlarged with no nodules. The estimated weight of the thyroid gland is 50 g (normal 10–20 g) based on ultrasonography. Her thyroid-stimulating hormone (TSH) level is 2.95 mU/L (reference range 0.5–5 mU/L), and her free thyroxine level is 0.8 ng/dL (0.7–1.8 ng/dL). Testing for TSH receptor antibody is negative. Her 24-hour urine iodine is undetectable (urine iodine concentration < 10 μg/L with urine volume 3,175 mL). What may be the cause of her goiter?
Iodine is an essential element needed for the production of thyroid hormone, which controls metabolism and plays a major role in fetal neurodevelopment. Its ionized form is called iodide. Iodine deficiency results in impairment of thyroid hormone synthesis and may lead to several undesirable consequences. Physicians should be aware of the risks iodine deficiency poses, especially during pregnancy, and should be familiar with approaches to testing and current indications for iodine supplementation.
SOURCES OF IODINE AND SALT IODIZATION
The major environmental source of iodine is the ocean. Elemental iodine in the ocean volatilizes into the atmosphere and returns to the soil by rain. The effects of glaciation, flooding, and leaching into soil have resulted in the variable geographic distribution of iodine. Mountainous areas (eg, the Alps, Andes, Himalayas) and areas with frequent flooding typically have iodine-deficient soil due to slow iodine cycling.1 Seafood is a good source of iodine because marine plants and animals concentrate iodine from seawater. The iodine content of other foods varies widely, depending on the source and any additives.
In the United States, the major sources of dietary iodine are dairy products (due to livestock iodine supplements and use of iodophors for cleaning milk udders) and iodized salt.1,2 Seafood contains a higher amount of iodine by weight than dairy products but is consumed far less than dairy.3,4 Further, the iodine content of milk can range from 88 to 168 μg per 250 mL (about 1 cup), depending on the product manufacturer. Also, iodine content is often omitted from the food label. Even if it is reported, the package labeling may not accurately predict the iodine content.5
Less common sources of iodine are radiographic contrast, bread with iodate dough conditioners, red food coloring (erythrosine), and drugs such as amiodarone.1
Using iodized salt is an effective and stable way to ensure adequate iodine intake. In the United States, only table salt is iodized, and the salt typically used in processed food has only minimal iodine content.6 Nearly 70% of the salt we ingest is from processed food. Table salt provides only 15% of dietary salt intake, and only 70% of consumers choose iodized salt for home cooking.7
IODINE REQUIREMENTS
Daily requirements of iodine suggested by the World Health Organization (WHO) and by the US Institute of Medicine are in the range of 90 to 150 µg/day.8,9 The iodine requirement is higher in pregnancy (220–250 µg/day) because of increased maternal thyroid hormone production required to maintain euthyroidism and increased renal iodine clearance, and it is even higher in lactating women (250–290 µg/day).
IODINE STATUS IN POPULATIONS
Since the establishment of universal salt iodization programs under the influence of the WHO and the International Council for Control of Iodine Deficiency Disorders (ICCIDD) in 1990, global iodine status has continued to improve. Yet only 70% of households worldwide currently have access to adequately iodized salt, because many countries lack a national program for iodine supplementation. The population of the United States was historically iodine-deficient, but since the introduction of salt iodization in the 1920s, the iodine status in the United States has been considered adequate.1
The WHO defines iodine status for a population by the median spot urinary iodine concentration. Because a urinary iodine concentration of 100 μg/L represents an iodine intake of about 150 μg/day, the WHO uses a median urinary iodine concentration of 100 to 199 μg/L to define adequate iodine intake for a nonpregnant population.9
The National Health and Nutrition Examination Survey (NHANES) found that the median urinary iodine concentration decreased by more than 50% from the 1970s to the 1990s, indicating declining iodine status in the US population.2 Of particular concern, the percentage of women of childbearing age with moderate iodine deficiency increased from 4% to 15% over this period.2 Still, the NHANES survey in 2009–2010 indicated that the overall US population is still iodine-sufficient (median urinary iodine concentration 144 μg/L).10 The decline in the US iodine status may be due to reduction of iodine content in dairy products, increased use of noniodized salt by the food industry, and recommendations to avoid salt for blood pressure control.
Although US iodine status has been considered generally adequate, iodine intake varies greatly across the population. Vegans tend to have iodine-deficient diets, while kelp consumers may have excessive iodine intake.11 Individuals with lactose intolerance are at risk of iodine deficiency, given that dairy products are a major source of iodine in the United States. Physicians should be aware of these risk factors for iodine deficiency.
PREGNANCY AND LACTATION
It is crucial to maintain euthyroidism during pregnancy. In early gestation, maternal thyroid hormone production increases 50% due to an increase in thyroid-binding globulin and stimulation by human chorionic gonadotropin. The glomerular filtration rate increases by 30% to 50% during pregnancy, thus increasing renal iodine clearance. Fetal thyroid hormone production increases during the second half of pregnancy, further contributing to increased maternal iodine requirements because iodine readily crosses the placenta.12
Women with sufficient iodine intake before and during pregnancy generally have adequate intrathyroidal iodine storage and can adapt to the increased demand for thyroid hormone throughout gestation. But in the setting of even mild iodine deficiency, total body iodine stores decline gradually from the first to third trimester of pregnancy.13
The fetal thyroid gland does not begin to concentrate iodine until 10 to 12 weeks of gestation and is not controlled by TSH until the full development of the pituitary-portal vascular system at 20 weeks of gestation.12 Therefore, the fetus relies on maternal thyroid hormone during this critical stage of neurodevelopment. Thyroid hormone is essential for oligodendrocyte differentiation and myelin distribution14 as well as fetal neuronal proliferation and migration in the first and second trimesters. Iodine deficiency leading to maternal hypothyroidism can result in irreversible fetal brain damage.
Because of the greater requirement during pregnancy, the WHO recommends using a median urinary iodine concentration of 150 to 249 μg/L to define a population that has no iodine deficiency.9 The NHANES data from 2007 to 2010 showed that pregnant US women were mildly iodine-deficient (median urinary iodine concentration 135 μg/L),10 and the National Children’s Study of 501 pregnant US women during the third trimester in 2009 to 2010 showed they had adequate iodine intake (median urinary iodine concentration 167 μg/L). Interestingly, pregnant non-Hispanic blacks were the only ethnic group with a median urinary iodine concentration less than 150 μg/L, suggesting that race or ethnicity is a predictor of iodine status in pregnant women.10
Iodine requirements during lactation
During lactation, thyroid hormone production and renal iodine clearance return to the prepregnancy state. However, a significant amount of iodine is excreted into breast milk at a concentration 20 to 50 times greater than that in plasma.15 It is recommended that lactating women continue high iodine intake to ensure sufficient iodine in breast milk to build reserves in the newborn’s thyroid gland.
The iodine requirement during lactation is 225 to 350 μg/day.16 Breast milk containing 100 to 200 μg/L of iodine appears to provide adequate iodine to meet Institute of Medicine recommendations for infants.17 The amount of iodine excreted into breast milk depends on maternal iodine intake. In the setting of iodine sufficiency, the iodine content of breast milk is 150 to 180 μg/L, but it is much lower (9–32 μg/L) in women from iodine-deficient areas, eg, the “goiter belt,” which included the Great Lakes, the Appalachians, and northwestern states. While iodized salt has virtually eliminated the goiter belt, the risk of iodine deficiency remains for people who avoid iodized salt and dairy.15
To ensure adequate iodine intake, the American Thyroid Association recommends that women receive iodine supplementation daily during pregnancy and lactation.11 However, the iodine content of prenatal multivitamins is currently not mandated in the United States. Only half of marketed prenatal vitamins in the United States contain iodine, in the form of either potassium iodide or kelp. Though most iodine-containing products claim to contain at least 150 μg of iodine per daily dose, when measured, the actual iodine content varied between 33 and 610 μg.18
CONSEQUENCES OF IODINE DEFICIENCY
Goiter
Goiter in iodine-deficient areas is considered to be an adaptation to chronic iodine deficiency. Low iodine intake leads to reduced thyroid hormone production, which in turn stimulates TSH secretion from the pituitary. TSH increases iodine uptake by the thyroid, stimulates thyroid growth, and leads to goiter development.
Initially, goiter is characterized by diffuse thyroid enlargement, but over time it may become nodular from progressive accumulation of new thyroid follicles. Goiter in children from iodine-deficient areas is diffusely enlarged, whereas in older adults it tends to be multinodular.
Iodine deficiency and chronic TSH stimulation may play a role in TSH receptor-activating mutations of thyroid follicles. These “gain-of-function” mutations are more common in the glands of patients with goiter in areas of iodine deficiency but are relatively rare in areas of iodine sufficiency.19 Toxic multinodular goiter may eventually develop, and hyperthyroidism may occur if iodine deficiency is not severe.
Goiter generally does not cause obstructive symptoms, since the thyroid usually grows outward. However, a very large goiter may descend to the thoracic inlet and compress the trachea and esophagus. The obstructive effect of a large goiter can be demonstrated by having a patient raise the arms adjacent to the face (the Pemberton maneuver). Signs suggesting obstruction are engorged neck veins, facial plethora, increased dyspnea, and stridor during the maneuver. Computed tomography of the neck and upper thorax may provide information on the degree of tracheal compression.20
Hypothyroidism
A normal or low triiodothyronine (T3), a low serum thyroxine (T4), and a variably elevated TSH are features of thyroid function tests in iodine deficiency.11,21,22 As long as daily iodine intake exceeds 50 μg/day, the absolute uptake of iodine by the thyroid gland usually remains adequate to maintain euthyroidism. Below 50 μg/day, iodine storage in the thyroid becomes depleted, leading to hypothyroidism.1
The clinical manifestations of hypothyroidism from iodine deficiency are similar to those of hypothyroidism from other causes. Because of thyroid hormone’s role in neural and somatic development, the manifestations of hypothyroidism differ among age groups (Table 1).
Cretinism
Before the development of fetal thyroid tissue in the 10th to 12th week of gestation, the fetus is dependent on maternal thyroid hormone, which crosses the placenta to support general and neural development. Iodine deficiency leading to maternal hypothyroidism (in early gestation) or inadequate fetal thyroid hormone production (in late gestation) may result in various degrees of mental retardation or lower than expected IQ.
Severe iodine deficiency during gestation typically results in cretinism, characterized by severe mental retardation accompanied by other neurologic or physical defects. Cretinism is divided into two subtypes according to clinical manifestations (neurologic and myxomatous cretinism; Table 2), which may reflect the different timing of intrauterine insult to the developing fetal nervous system and whether the iodine deficiency continues into the postnatal period. Both types can be prevented by adequate maternal iodine intake before and during pregnancy.23,24
Although mild gestational iodine deficiency does not result in cretinism, it nevertheless has an adverse impact on fetal neurodevelopment and subsequent functioning. Children of mothers with mild gestational iodine deficiency were found to have reductions in spelling, grammar, and English literacy performance despite growing up in iodine-replete environments.25
Impaired cognitive development
Reduction in IQ has been noted in affected youth from regions of severe and mild iodine deficiency. A meta-analysis of studies relating iodine deficiency to cognitive development suggested that chronic moderate to severe iodine deficiency reduced expected average IQ by about 13.5 points.26
The effects of mild iodine deficiency during childhood are more difficult to quantify. The results of one study suggested that mild iodine deficiency was associated with subtle neurodevelopmental deficits and that iodine supplementation might improve cognitive function in mildly iodine-deficient children.27
In a 2009 randomized, placebo-controlled study in New Zealand, 184 children ages 10 to 13 with mild iodine deficiency (median urinary iodine concentration of 63 μg/L) received iodine supplementation (150 μg/day) or placebo for 28 weeks. Iodine supplementation increased the median urinary iodine concentration to 145 μg/L and significantly improved perceptual reasoning measures and overall cognitive score compared with placebo.28
These findings suggest that correcting mild iodine deficiency in children could improve certain components of cognition. More research is needed to understand the effects of mild iodine deficiency and iodine supplementation on cognitive function.
ASSESSING IODINE STATUS
The diagnosis of iodine deficiency is based on clinical and laboratory assessments. Clinical manifestations compatible with iodine deficiency and careful history-taking focused on the patient’s dietary iodine intake and geographic data are keys to the diagnosis.
Four main methods are used to assess iodine status at a population level: urinary iodine, serum thyroglobulin, serum TSH, and thyroid size. Urinary iodine is a sensitive marker for recent iodine intake (within days); thyroglobulin represents iodine nutrition over a period of months and thyroid size over a period of years.1
Urinary iodine
Most dietary iodine is excreted into the urine within 24 hours of ingestion, and the 24-hour urinary iodine is considered a reference standard for the measurement of individual daily iodine intake. However, the process of collection is cumbersome, and the 24-hour urinary iodine can vary from day to day in the same person, depending on the amount of iodine ingested.
A study in healthy women from an iodine-sufficient area suggested that 10 repeated 24-hour urine collections estimated the person’s iodine status at a precision of 20% because of variable daily iodine intake.29 Therefore, when necessary, several 24-hour urine iodine determinations should be performed.
A single, random, spot urinary iodine is expressed as the urinary iodine concentration and is affected by the amount of iodine and fluids the individual ingests in a day, thus resulting in high variation both within an individual person and between individuals. Expressing the urinary iodine concentration as the ratio of urine iodine to creatinine is useful in correcting for the influence of fluid intake. The ratio of urine iodine to creatinine can be used to estimate 24-hour urine iodine with the following formula: urine iodine (μg/L)/creatinine (g/L)× age- and sex-specific estimated 24-hour creatinine excretion (g/day). Another clinical use of the spot urine iodine is to screen for exposure to a large amount of iodine from a source such as radiographic contrast.30
Although individual urine iodine excretion and urine volume can vary from day to day, this variation tends to even out in a large number of samples. In study populations of at least 500, the median value of the spot urinary iodine concentration is considered a reliable measure of iodine intake in that population.30 The spot urine iodine test is convenient, making it the test of choice to study iodine status in a large cohort. The WHO recommends using the median value of the spot urine iodine to evaluate the iodine status of a population.9
Thyroglobulin
Thyroglobulin is a thyroid-specific protein involved in the synthesis of thyroid hormone. Small amounts can be detected in the blood of healthy people. In the absence of thyroid damage, the amount of serum thyroglobulin depends on thyroid cell mass and TSH stimulation. The serum level is elevated in iodine deficiency as a result of chronic TSH stimulation and thyroid hyperplasia. Thus, thyroglobulin can serve as a marker of iodine deficiency.
Serum thyroglobulin assays have been adapted for use on dried whole-blood spots, which require only a few drops of whole blood collected on filter paper and left to air-dry. The results of the dried whole-blood assay correlate closely with those of the serum assay.31 An established international dried whole-blood thyroglobulin reference range for iodine-sufficient school-age children is 4 to 40 μg/L.32 A median level of less than 13 μg/L in school-age children indicates iodine sufficiency in the population.33
Thyroid-stimulating hormone
Iodine deficiency lowers serum T4, which in turn leads to increased serum TSH. Therefore, iodine-deficient populations generally have higher TSH than iodine-sufficient groups. However, the TSH values in older children and adults with iodine deficiency are not significantly different from values of those with adequate iodine intake. Therefore, TSH is not a practical marker of iodine deficiency in the general population.
In contrast, TSH in newborns is a reasonable indicator of population iodine status. The newborn thyroid has limited iodine stores compared with that of an adult and hence a much higher iodine turnover rate. TSH from the cord blood is markedly elevated in newborns of mothers with moderate to severe iodine deficiency.34 A high prevalence of newborns with elevated TSH should therefore reflect iodine deficiency in the area where the mothers of the newborns live.
TSH is now routinely checked in newborns to screen for congenital hypothyroidism. TSH is typically checked 2 to 5 days after delivery to avoid confusion with transient physiologic TSH elevation, which occurs within a few hours after birth and decreases rapidly in 24 hours. The WHO has proposed that a more than 3% prevalence of newborns with TSH values higher than 5 mU/L from blood samples collected 3 to 4 days after birth indicates iodine deficiency in a population.1 This threshold appears to correlate well with the iodine status of the population defined by the WHO’s median urinary iodine concentration.35,36
But several other factors can influence the measurement of newborn TSH, such as prematurity, time of blood collection, maternal or newborn exposure to iodine-containing antiseptics, and the TSH assay methodology. These potential confounding factors limit the role of neonatal TSH as a reliable monitoring tool for iodine deficiency.35,37
Thyroid size
The size of the thyroid gland varies inversely with iodine intake. Thyroid size can be assessed by either palpation or ultrasonography, with the latter being more sensitive. The goiter rate in school-age children can be used to determine the severity of iodine deficiency in the population (Table 3). A goiter rate of 5% or more in school-age children suggests the presence of iodine deficiency in the community.
Although thyroid size is easy to estimate by palpation, it has low sensitivity and specificity to detect iodine deficiency and high interobserver variation. Thyroid ultrasonography provides a more precise measurement of thyroid gland volume. Zimmermann et al38 provided reference data on thyroid volume stratified by age, sex, and body surface area of school-age children in iodine-sufficient areas.38 Results of ultrasonography in a population is then compared with these reference data. The higher the percentage of the population with thyroid volume exceeding the 97th percentile of the reference range, the more severe the iodine deficiency. However, the WHO does not specify how to grade the degree of iodine deficiency based on the thyroid volume obtained with ultrasonography. Follow-up studies showed no significant correlation between urinary iodine concentration and thyroid size.39,40
Thyroid size decreases slowly after iodine repletion. Therefore, the goiter rate may remain high for several years after iodine supplementation begins.1,9
TREATMENT AND PREVENTION
Treatment of iodine deficiency should be instituted at the levels recommended by the Institute of Medicine and the WHO. The tolerable upper intake levels for iodine are outlined in Table 4. In a nonpregnant adult, 150 μg/day is sufficient for normal thyroid function. Iodine intake should be higher for pregnant and lactating women (250 μg/day according to the WHO recommendation).
Iodine supplementation is easily achieved by using iodized salt or an iodine-containing daily multivitamin.
In patients with overt hypothyroidism from iodine deficiency, we recommend initiating levothyroxine treatment along with iodine supplementation to restore euthyroidism, with consideration of possible interruption in 6 to 12 months when the urine iodine has normalized and goiter size has decreased. Thyroid function should be reassessed 4 to 6 weeks after discontinuation of levothyroxine.
At the population level, iodine deficiency can usually be prevented by iodization of food products or the water supply. In developing countries where salt iodization is not practical, iodine deficiency has been eradicated by adding iodine drops to well water or by injecting people with iodized oil.
TAKE-HOME POINTS
Iodine is essential for thyroid hormone synthesis. It can be obtained by eating iodine-containing foods or by using iodized salt. The WHO classifies iodine deficiency based on the median urinary iodine concentration. Iodine nutrition at the community level is best assessed by measurements of urinary iodine, thyroglobulin, serum TSH, and thyroid size.
Adequate iodine intake during pregnancy is important for fetal development. Iodine deficiency is associated with goiter and hypothyroidism. Severe iodine deficiency during pregnancy is associated with cretinism.
There is evidence that mild to moderate iodine deficiency can cause impaired cognitive development and that correcting the iodine deficiency can significantly improve cognitive function.
CASE FOLLOW-UP
This patient had been following a strict vegan diet with very little intake of iodized salt. Her dietary history and the presence of goiter suggested iodine deficiency. She was instructed to take an iodine supplement 150 μg/day to meet her daily requirement. After 2 months of iodine supplementation, her urine iodine concentration had increased to 58 μg/L. She remained biochemically euthyroid.
- Zimmermann MB. Iodine deficiency. Endocr Rev 2009; 30:376–408.
- Pearce EN, Andersson M, Zimmermann MB. Global iodine nutrition: where do we stand in 2013? Thyroid 2013; 23:523–528.
- Huang SW. Seafood and iodine: an analysis of a medical myth. Allergy Asthma Proc 2005; 26:468–469.
- US Census Bureau. The 2012 Statistical abstract. Health and nutrition. www.census.gov/prod/2011pubs/12statab/health.pdf. Accessed December 1, 2016.
- Pearce EN, Pino S, He X, Bazrafshan HR, Lee SL, Braverman LE. Sources of dietary iodine: bread, cows’ milk, and infant formula in the Boston area. J Clin Endocrinol Metab 2004; 89:3421–3424.
- Salt Institute. Production and Industry. www.saltinstitute.org/salt-101/production-industry. Accessed September 20, 2016.
- Dunn JT. Guarding our nation's thyroid health. J Clin Endocrinol Metab 2002; 87:486–488.
- Institute of Medicine (US) Panel on Micronutrients. Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Washington, DC: National Academy Press; 2001.
- World Health Organization (WHO). Assessment of iodine deficiency disorders and monitoring their elimination: a guide for programme managers. www.who.int/nutrition/publications/micronutrients/iodine_deficiency/9789241595827/en. Accessed December 1, 2016.
- Caldwell KL, Pan Y, Mortensen ME, Makhmudov A, Merrill L, Moye J. Iodine status in pregnant women in the National Children's Study and in US women (15–44 years), National Health and Nutrition Examination Survey 2005–2010. Thyroid 2013; 23:927–937.
- Public Health Committee of the American Thyroid Association; Becker DV, Braverman LE, Delange F, et al. Iodine supplementation for pregnancy and lactation—United States and Canada: recommendations of the American Thyroid Association. Thyroid 2006; 16:949–951.
- Leung AM, Pearce EN, Braverman LE. Iodine nutrition in pregnancy and lactation. Endocrinol Metab Clin North Am 2011; 40:765–777.
- Pearce EN. Iodine in pregnancy: is salt iodization enough? J Clin Endocrinol Metab 2008; 93:2466–2468.
- Younes-Rapozo V, Berendonk J, Savignon T, Manhaes AC, Barradas PC. Thyroid hormone deficiency changes the distribution of oligodendrocyte/myelin markers during oligodendroglial differentiation in vitro. Int J Dev Neurosci 2006; 24:445–453.
- Azizi F, Smyth P. Breastfeeding and maternal and infant iodine nutrition. Clin Endocrinol (Oxf) 2009; 70:803–809.
- Delange F. Iodine requirements during pregnancy, lactation and the neonatal period and indicators of optimal iodine nutrition. Public Health Nutr 2007; 10:1571–1583.
- Semba RD, Delange F. Iodine in human milk: perspectives for infant health. Nutr Rev 2001; 59:269–278.
- Leung AM, Pearce EN, Braverman LE. Iodine content of prenatal multivitamins in the United States. N Engl J Med 2009; 360:939–940.
- Tonacchera M, Agretti P, Chiovato L, et al. Activating thyrotropin receptor mutations are present in nonadenomatous hyperfunctioning nodules of toxic or autonomous multinodular goiter. J Clin Endocrinol Metab 2000; 85:2270–2274.
- Medeiros-Neto G, Camargo RY, Tomimori EK. Approach to and treatment of goiters. Med Clin North Am 2012; 96:351–368.
- Heidemann P, Stubbe P. Serum 3,5,3'-triiodothyronine, thyroxine, and thyrotropin in hypothyroid infants with congenital goiter and the response to iodine. J Clin Endocrinol Metab 1978; 47:189–192.
- Patel YC, Pharoah PO, Hornabrook RW, Hetzel BS. Serum triiodothyronine, thyroxine and thyroid-stimulating hormone in endemic goiter: a comparison of goitrous and nongoitrous subjects in New Guinea. J Clin Endocrinol Metab 1973; 37:783–789.
- Boyages SC, Halpern JP. Endemic cretinism: toward a unifying hypothesis. Thyroid 1993; 3:59–69.
- Chen ZP, Hetzel BS. Cretinism revisited. Best Pract Res Clin Endocrinol Metab 2010; 24:39–50.
- Hynes KL, Otahal P, Hay I, Burgess JR. Mild iodine deficiency during pregnancy is associated with reduced educational outcomes in the offspring: 9-year follow-up of the gestational iodine cohort. J Clin Endocrinol Metab 2013; 98:1954–1962.
- Bleichrodt N, Born MP. A metaanalysis of research on iodine and its relationship to cognitive development. In: Stanbury JB, ed. The Damaged Brain of Iodine Deficiency. 1st ed. New York, NY: Cognizant Communication; 1994:195–200.
- Melse-Boonstra A, Jaiswal N. Iodine deficiency in pregnancy, infancy and childhood and its consequences for brain development. Best Pract Res Clin Endocrinol Metab 2010; 24:29–38.
- Gordon RC, Rose MC, Skeaff SA, Gray AR, Morgan KM, Ruffman T. Iodine supplementation improves cognition in mildly iodine-deficient children. Am J Clin Nutr 2009; 90:1264–1271.
- Konig F, Andersson M, Hotz K, Aeberli I, Zimmermann MB. Ten repeat collections for urinary iodine from spot samples or 24-hour samples are needed to reliably estimate individual iodine status in women. J Nutr 2011; 141:2049–2054.
- Vejbjerg P, Knudsen N, Perrild H, et al. Estimation of iodine intake from various urinary iodine measurements in population studies. Thyroid 2009; 19:1281–1286.
- Zimmermann MB, Hess SY, Adou P, Toresanni T, Wegmuller R, Hurrell RF. Thyroid size and goiter prevalence after introduction of iodized salt: a 5-y prospective study in schoolchildren in Cote d’Ivoire. Am J Clin Nutr 2003; 77:663–667.
- Zimmermann MB, de Benoist B, Corigliano S, et al. Assessment of iodine status using dried blood spot thyroglobulin: development of reference material and establishment of an international reference range in iodine-sufficient children. J Clin Endocrinol Metab 2006; 91:4881–4887.
- Zimmermann MB, Aeberli I, Andersson M, et al. Thyroglobulin is a sensitive measure of both deficient and excess iodine intakes in children and indicates no adverse effects on thyroid function in the UIC range of 100-299 mug/L: a UNICEF/ICCIDD study group report. J Clin Endocrinol Metab 2013; 98:1271–1280.
- Thilly CH, Delange F, Lagasse R, et al. Fetal hypothyroidism and maternal thyroid status in severe endemic goiter. J Clin Endocrinol Metab 1978; 47:354–360.
- Sullivan KM, May W, Nordenberg D, Houston R, Maberly GF. Use of thyroid stimulating hormone testing in newborns to identify iodine deficiency. J Nutr 1997; 127:55–58.
- Delange F. Neonatal thyroid screening as a monitoring tool for the control of iodine deficiency. Acta Paediatr Suppl 1999; 88:21–24.
- Li M, Eastman CJ. Neonatal TSH screening: is it a sensitive and reliable tool for monitoring iodine status in populations? Best Pract Res Clin Endocrinol Metab 2010; 24:63–75.
- Zimmermann MB, Moretti D, Chaouki N, Torresani T. Development of a dried whole-blood spot thyroglobulin assay and its evaluation as an indicator of thyroid status in goitrous children receiving iodized salt. Am J Clin Nutr 2003; 77:1453–1458.
- Brahmbhatt S, Brahmbhatt RM, Boyages SC. Thyroid ultrasound is the best prevalence indicator for assessment of iodine deficiency disorders: a study in rural/tribal schoolchildren from Gujarat (Western India). Eur J Endocrinol 2000; 143:37–46.
- Moradi M, Hashemipour M, Akbari S, Kor Z, Mirbod SA, Kooshanmehr MR. Ultrasonographic evaluation of the thyroid gland volume among 8-15-year-old children in Isfahan, Iran. Adv Biomed Res 2014; 3:9.
- Zimmermann MB. Iodine deficiency. Endocr Rev 2009; 30:376–408.
- Pearce EN, Andersson M, Zimmermann MB. Global iodine nutrition: where do we stand in 2013? Thyroid 2013; 23:523–528.
- Huang SW. Seafood and iodine: an analysis of a medical myth. Allergy Asthma Proc 2005; 26:468–469.
- US Census Bureau. The 2012 Statistical abstract. Health and nutrition. www.census.gov/prod/2011pubs/12statab/health.pdf. Accessed December 1, 2016.
- Pearce EN, Pino S, He X, Bazrafshan HR, Lee SL, Braverman LE. Sources of dietary iodine: bread, cows’ milk, and infant formula in the Boston area. J Clin Endocrinol Metab 2004; 89:3421–3424.
- Salt Institute. Production and Industry. www.saltinstitute.org/salt-101/production-industry. Accessed September 20, 2016.
- Dunn JT. Guarding our nation's thyroid health. J Clin Endocrinol Metab 2002; 87:486–488.
- Institute of Medicine (US) Panel on Micronutrients. Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Washington, DC: National Academy Press; 2001.
- World Health Organization (WHO). Assessment of iodine deficiency disorders and monitoring their elimination: a guide for programme managers. www.who.int/nutrition/publications/micronutrients/iodine_deficiency/9789241595827/en. Accessed December 1, 2016.
- Caldwell KL, Pan Y, Mortensen ME, Makhmudov A, Merrill L, Moye J. Iodine status in pregnant women in the National Children's Study and in US women (15–44 years), National Health and Nutrition Examination Survey 2005–2010. Thyroid 2013; 23:927–937.
- Public Health Committee of the American Thyroid Association; Becker DV, Braverman LE, Delange F, et al. Iodine supplementation for pregnancy and lactation—United States and Canada: recommendations of the American Thyroid Association. Thyroid 2006; 16:949–951.
- Leung AM, Pearce EN, Braverman LE. Iodine nutrition in pregnancy and lactation. Endocrinol Metab Clin North Am 2011; 40:765–777.
- Pearce EN. Iodine in pregnancy: is salt iodization enough? J Clin Endocrinol Metab 2008; 93:2466–2468.
- Younes-Rapozo V, Berendonk J, Savignon T, Manhaes AC, Barradas PC. Thyroid hormone deficiency changes the distribution of oligodendrocyte/myelin markers during oligodendroglial differentiation in vitro. Int J Dev Neurosci 2006; 24:445–453.
- Azizi F, Smyth P. Breastfeeding and maternal and infant iodine nutrition. Clin Endocrinol (Oxf) 2009; 70:803–809.
- Delange F. Iodine requirements during pregnancy, lactation and the neonatal period and indicators of optimal iodine nutrition. Public Health Nutr 2007; 10:1571–1583.
- Semba RD, Delange F. Iodine in human milk: perspectives for infant health. Nutr Rev 2001; 59:269–278.
- Leung AM, Pearce EN, Braverman LE. Iodine content of prenatal multivitamins in the United States. N Engl J Med 2009; 360:939–940.
- Tonacchera M, Agretti P, Chiovato L, et al. Activating thyrotropin receptor mutations are present in nonadenomatous hyperfunctioning nodules of toxic or autonomous multinodular goiter. J Clin Endocrinol Metab 2000; 85:2270–2274.
- Medeiros-Neto G, Camargo RY, Tomimori EK. Approach to and treatment of goiters. Med Clin North Am 2012; 96:351–368.
- Heidemann P, Stubbe P. Serum 3,5,3'-triiodothyronine, thyroxine, and thyrotropin in hypothyroid infants with congenital goiter and the response to iodine. J Clin Endocrinol Metab 1978; 47:189–192.
- Patel YC, Pharoah PO, Hornabrook RW, Hetzel BS. Serum triiodothyronine, thyroxine and thyroid-stimulating hormone in endemic goiter: a comparison of goitrous and nongoitrous subjects in New Guinea. J Clin Endocrinol Metab 1973; 37:783–789.
- Boyages SC, Halpern JP. Endemic cretinism: toward a unifying hypothesis. Thyroid 1993; 3:59–69.
- Chen ZP, Hetzel BS. Cretinism revisited. Best Pract Res Clin Endocrinol Metab 2010; 24:39–50.
- Hynes KL, Otahal P, Hay I, Burgess JR. Mild iodine deficiency during pregnancy is associated with reduced educational outcomes in the offspring: 9-year follow-up of the gestational iodine cohort. J Clin Endocrinol Metab 2013; 98:1954–1962.
- Bleichrodt N, Born MP. A metaanalysis of research on iodine and its relationship to cognitive development. In: Stanbury JB, ed. The Damaged Brain of Iodine Deficiency. 1st ed. New York, NY: Cognizant Communication; 1994:195–200.
- Melse-Boonstra A, Jaiswal N. Iodine deficiency in pregnancy, infancy and childhood and its consequences for brain development. Best Pract Res Clin Endocrinol Metab 2010; 24:29–38.
- Gordon RC, Rose MC, Skeaff SA, Gray AR, Morgan KM, Ruffman T. Iodine supplementation improves cognition in mildly iodine-deficient children. Am J Clin Nutr 2009; 90:1264–1271.
- Konig F, Andersson M, Hotz K, Aeberli I, Zimmermann MB. Ten repeat collections for urinary iodine from spot samples or 24-hour samples are needed to reliably estimate individual iodine status in women. J Nutr 2011; 141:2049–2054.
- Vejbjerg P, Knudsen N, Perrild H, et al. Estimation of iodine intake from various urinary iodine measurements in population studies. Thyroid 2009; 19:1281–1286.
- Zimmermann MB, Hess SY, Adou P, Toresanni T, Wegmuller R, Hurrell RF. Thyroid size and goiter prevalence after introduction of iodized salt: a 5-y prospective study in schoolchildren in Cote d’Ivoire. Am J Clin Nutr 2003; 77:663–667.
- Zimmermann MB, de Benoist B, Corigliano S, et al. Assessment of iodine status using dried blood spot thyroglobulin: development of reference material and establishment of an international reference range in iodine-sufficient children. J Clin Endocrinol Metab 2006; 91:4881–4887.
- Zimmermann MB, Aeberli I, Andersson M, et al. Thyroglobulin is a sensitive measure of both deficient and excess iodine intakes in children and indicates no adverse effects on thyroid function in the UIC range of 100-299 mug/L: a UNICEF/ICCIDD study group report. J Clin Endocrinol Metab 2013; 98:1271–1280.
- Thilly CH, Delange F, Lagasse R, et al. Fetal hypothyroidism and maternal thyroid status in severe endemic goiter. J Clin Endocrinol Metab 1978; 47:354–360.
- Sullivan KM, May W, Nordenberg D, Houston R, Maberly GF. Use of thyroid stimulating hormone testing in newborns to identify iodine deficiency. J Nutr 1997; 127:55–58.
- Delange F. Neonatal thyroid screening as a monitoring tool for the control of iodine deficiency. Acta Paediatr Suppl 1999; 88:21–24.
- Li M, Eastman CJ. Neonatal TSH screening: is it a sensitive and reliable tool for monitoring iodine status in populations? Best Pract Res Clin Endocrinol Metab 2010; 24:63–75.
- Zimmermann MB, Moretti D, Chaouki N, Torresani T. Development of a dried whole-blood spot thyroglobulin assay and its evaluation as an indicator of thyroid status in goitrous children receiving iodized salt. Am J Clin Nutr 2003; 77:1453–1458.
- Brahmbhatt S, Brahmbhatt RM, Boyages SC. Thyroid ultrasound is the best prevalence indicator for assessment of iodine deficiency disorders: a study in rural/tribal schoolchildren from Gujarat (Western India). Eur J Endocrinol 2000; 143:37–46.
- Moradi M, Hashemipour M, Akbari S, Kor Z, Mirbod SA, Kooshanmehr MR. Ultrasonographic evaluation of the thyroid gland volume among 8-15-year-old children in Isfahan, Iran. Adv Biomed Res 2014; 3:9.
KEY POINTS
- Adequate iodine intake during pregnancy is critical for normal fetal development.
- Major sources of dietary iodine in the United States are dairy products and iodized salt.
- The daily iodine requirement for nonpregnant adults is 150 µg, and for pregnant women it is 220 to 250 μg. Pregnant and lactating women should take a daily iodine supplement to ensure adequate iodine intake.
- Assessing the risk of iodine deficiency from clinical signs and from the history is key to diagnosing iodine deficiency. Individual urine iodine concentrations may vary from day to day. Repeated samples can be used to confirm iodine deficiency.
Vulvovaginitis: Find the cause to treat it
Although vulvovaginitis has several possible causes, the typical presenting symptoms are similar regardless of the cause: itching, burning, and vaginal discharge. Physical examination often reveals atrophy, redness, excoriations, and fissures in the vulvovaginal and perianal areas. Determining the cause is key to successful treatment.
This article reviews the diagnosis and treatment of many common and less common infectious and noninfectious causes of vulvovaginitis, the use of special tests, and the management of persistent cases.
DIAGNOSIS CAN BE CHALLENGING
Vulvar and vaginal symptoms are most commonly caused by local infections, but other causes must be also be considered, including several noninfectious ones (Table 1). Challenges in diagnosing vulvovaginitis are many and include distinguishing contact from allergic dermatitis, recognizing vaginal atrophy, and recognizing a parasitic infection. Determining whether a patient has an infectious process is important so that antibiotics can be used only when truly needed.
Foreign bodies in the vagina should also be considered, especially in children,1 as should sexual abuse. A 15-year retrospective review of prepubertal girls presenting with recurrent vaginal discharge found that sexual abuse might have been involved in about 5% of cases.2
Systemic diseases, such as eczema and psoriasis, may also present with gynecologic symptoms.
Heavy vaginal discharge may also be normal. This situation is a diagnosis of exclusion but is important to recognize in order to allay the patient’s anxiety and avoid unnecessary treatment.
SIMPLE OFFICE-BASED ASSESSMENT
A thorough history and physical examination are always warranted.
Simple tests of vaginal secretions can often determine the diagnosis (Table 2). Vaginal secretions should be analyzed in the following order:
Testing the pH. The pH can help determine likely diagnoses and streamline further testing (Figure 1).
Saline microscopy. Some of the vaginal discharge sample should be diluted with 1 or 2 drops of normal saline and examined under a microscope, first at × 10 magnification, then at × 40. The sample should be searched for epithelial cells, blood cells, “clue” cells (ie, epithelial cells with borders studded or obscured by bacteria), and motile trichomonads.
10% KOH whiff test and microscopy. To a second vaginal sample, a small amount of 10% potassium hydroxide should be added, and the examiner should sniff it. An amine or fishy odor is a sign of bacterial vaginosis.
If pH paper, KOH, and a microscope are unavailable, other point-of-care tests can be used for specific conditions as discussed below.
INFECTIOUS CAUSES
Infectious causes of vulvovaginitis include bacterial vaginosis, candidiasis, trichomoniasis, and herpes simplex virus (HSV) infection.
BACTERIAL VAGINOSIS
Bacterial vaginosis is the most common vaginal disorder worldwide. It has been linked to preterm delivery, intra-amniotic infection, endometritis, postabortion infection, and vaginal cuff cellulitis after hysterectomy.3 It may also be a risk factor for human immunodeficiency virus (HIV) infection.4
The condition reflects a microbial imbalance in the vaginal ecosystem, characterized by depletion of the dominant hydrogen peroxide-producing lactobacilli and overgrowth of anaerobic and facultative aerobic organisms such as Gardnerella vaginalis, Mycoplasma hominis, Atopobium vaginae, and Prevotella and Mobiluncus species.
Diagnosis of bacterial vaginosis
The Amsel criteria consist of the following:
- pH greater than 4.5
- Positive whiff test
- Homogeneous discharge
- Clue cells.
Three of the four criteria must be present for a diagnosis of bacterial vaginosis. This method is inexpensive and provides immediate results in the clinic.
The Nugent score, based on seeing certain bacteria from a vaginal swab on Gram stain microscopy, is the diagnostic standard for research.5
DNA tests. Affirm VPIII (BD Diagnostics, Sparks, MD) is a nonamplified nucleic acid probe hybridization test that detects Trichomonas vaginalis, Candida albicans, and G vaginalis. Although it is more expensive than testing for the Amsel criteria, it is commonly used in private offices because it is simple to use, gives rapid results, and does not require a microscope.6 Insurance pays for it when the test is indicated, but we know of a patient who received a bill for approximately $500 when the insurance company thought the test was not indicated.
In a study of 109 patients with symptoms of vulvovaginitis, the Affirm VPIII was found comparable to saline microscopy when tested on residual vaginal samples. Compared with Gram stain using Nugent scoring, the test has a sensitivity of 87.7% to 95.2% and a specificity of 81% to 99.1% for bacterial vaginosis.7
In 323 symptomatic women, a polymerase chain reaction (PCR) assay for bacterial vaginosis was 96.9% sensitive and 92.6% specific for bacterial vaginosis, and Affirm VPIII was 90.1% sensitive and 67.6% specific, compared with a reference standard incorporating Nugent Gram-stain scores and Amsel criteria.8 The test is commercially available.
Management of bacterial vaginosis
Initial treatment. Bacterial vaginosis can be treated with oral or topical metronidazole, oral tinidazole, or oral or topical clindamycin.9 All options offer equivalent efficacy as initial treatments, so the choice may be based on cost and preferred route of administration.
Treatment for recurrent disease. Women who have 3 or more episodes in 12 months should receive initial treatment each time as described above and should then be offered additional suppressive therapy with 0.75% metronidazole intravaginal gel 2 times a week for 4 months. A side effect of therapy is vulvovaginal candidiasis, which should be treated as needed.
In a multicenter study, Sobel et al10 randomized patients who had recurrent bacterial vaginosis to twice-weekly metronidazole gel or placebo for 16 weeks after their initial treatment. During the 28 weeks of follow-up, recurrences occurred in 51% of treated women vs 75% of those on placebo.
Another option for chronic therapy is oral metronidazole and boric acid vaginal suppositories.
Reichman et al11 treated women with oral metronidazole or tinidazole 500 mg twice a day for 7 days, followed by vaginal boric acid 600 mg daily for 21 days. This was followed by twice-weekly vaginal metronidazole gel for 16 weeks. At follow-up, the cure rate was 92% at 7 weeks, dropping to 88% at 12 weeks and 50% at 36 weeks.
Patients with recurrent bacterial vaginosis despite therapy should be referred to a vulvovaginal or infectious disease specialist.
VULVOVAGINAL CANDIDIASIS
Vulvovaginal candidiasis is the second most common cause of vaginitis.
Diagnosis can be clinical
Vulvovaginal candidiasis can be clinically diagnosed on the basis of cottage cheese-like clumpy discharge; external dysuria (a burning sensation when urine comes in contact with the vulva); and vulvar itching, pain, swelling, and redness. Edema, fissures, and excoriations may be seen on examination of the vulva. (Figure 3).
Saline microscopy (Figure 2) with the addition of 10% KOH may reveal the characteristic fungal elements, but its sensitivity is only 50%.
Fungal culture remains the gold standard for diagnosis and is needed to determine the sensitivity of specific strains of Candida to therapy.12
DNA tests can also be helpful. In a study of patients with symptomatic vaginitis, Affirm VPIII detected Candida in 11% of samples, whereas microscopy detected it in only 7%.13 Another study7 found that Affirm VPIII produced comparable results whether the sample was collected from residual vaginal discharge found on the speculum or was collected in the traditional way (by swabbing).
Cartwright et al8 compared the performance of a multiplexed, real-time PCR assay and Affirm VPIII in 102 patients. PCR was much more sensitive (97.7% vs 58.1%) but less specific (93.2% vs 100%), with culture serving as the gold standard.
Management of candidiasis
Uncomplicated cases can be managed with prescription or over-the-counter topical or oral antifungal medications for 1 to 7 days, depending on the medication.9 However, most of the common antifungals may not be effective against non-albicans Candida.
In immunosuppressed patients and diabetic patients, if symptoms do not improve with regular treatment, a vaginal sample should be cultured for C albicans. If the culture is positive, the patient should be treated with fluconazole 150 mg orally every 3 days for 3 doses.14
Patients with recurrent episodes (3 or more in 12 months) should follow initial treatment with maintenance therapy of weekly fluconazole 150 mg orally for 6 months.15
Non-albicans Candida may be azole-resistant, and fungal culture and sensitivity should be obtained. Sobel et al13 documented successful treatment of non-albicans Candida using boric acid and flucytosine. Phillips16 documented successful use of compounded amphotericin B in a 50-mg vaginal suppository for 14 days. Therefore, in patients who have Candida species other than C albicans, treatment should be one of the following:
- Vaginal boric acid 600 mg daily for 14 to 21 days
- Flucytosine in 15.5% vaginal cream, intravaginally administered as 5 g for 14 days
- Amphotericin B 50 mg vaginal suppositories for 14 days.
Boric acid is readily available, but flucytosine vaginal cream and amphotericin B vaginal suppositories must usually be compounded by a pharmacist.
Of note: All that itches is not yeast. Patients with persistent itching despite treatment should be referred to a specialist to search for another cause.
TRICHOMONIASIS
The incidence of T vaginalis infection is higher than that of Neisseria gonorrhoeae and Chlamydia trachomatis combined, with an estimated 7.4 million new cases occurring in the year 2000 in the United States.17 Infection increases the sexual transmission of HIV.18–20 It is often asymptomatic and so is likely underdiagnosed.
Diagnosis of trichomoniasis
Vaginal pH may be normal or elevated (> 4.5).
Direct microscopy. Observation by saline microscopy of motile trichomonads with their characteristic jerky movements is 100% specific but only 50% sensitive. Sensitivity is reduced by delaying microscopy on the sample by as little as 10 minutes.21
The incidental finding of T vaginalis on a conventional Papanicolaou (Pap) smear has poor sensitivity and specificity, and patients diagnosed with T vaginalis by conventional Pap smear should have a second test performed. The liquid-based Pap test is more accurate for microscopic diagnosis, and its results can be used to determine if treatment is needed (sensitivity 60%–90%; specificity 98%–100%).22,23
Culture. Amplification of T vaginalis in liquid culture usually provides results within 3 days.24 It is more sensitive than microscopy but less sensitive than a nucleic acid amplification test: compared with a nucleic acid amplification test, culture is 44% to 75% sensitive for detecting T vaginalis and 100% specific.19 Culture is the preferred test for resistant strains.
Non–culture-based or nucleic acid tests do not require viable organisms, so they allow for a wider range of specimen storage temperatures and time intervals between collection and processing. This quality limits them for testing treatment success; if performed too early, they may detect nonviable organisms. A 2-week interval is recommended between the end of treatment and retesting.25
Nonamplified tests such as Affirm VPIII and the Osom Trichomonas Test (Sekisui Diagnostics, Lexington, MA) are 40% to 95% sensitive, depending on the test and reference standard used, and 92% to 100% specific.26,27
Nucleic acid amplification tests are usually not performed as point-of-care tests. They are more expensive and require special equipment with trained personnel. Sensitivities range from 76% to 100%, making these tests more suitable for screening and testing of asymptomatic women, in whom the concentration of organisms may be lower.
Treatment of trichomoniasis
Treatment is a single 2-g oral dose of metronidazole or tinidazole.9
If initial treatment is ineffective, an additional regimen can be either of the following:
- Oral metronidazole 500 mg twice a day for 7 days
- Oral metronidazole or tinidazole, 2 g daily for 5 days.
Patients allergic to nitroimidazoles should be referred for desensitization.
If these treatments are unsuccessful, the patient should be referred to an infectious disease specialist or gynecologist who specializes in vulvovaginal disorders. Treatment failure is uncommon and is usually related to noncompliance, reinfection, or metronidazole resistance.28 The US Centers for Disease Control and Prevention offers testing for resistance by request.
Reportedly successful regimens for refractory trichomoniasis include 14 days of either:
- Oral tinidazole 500 mg 4 times daily plus vaginal tinidazole 500 mg twice daily29
- Oral tinidazole 1 g 3 times daily plus compounded 5% intravaginal paromomycin 5 g nightly.30
HERPES SIMPLEX VIRUS INFECTION
HSV (HSV-1 and HSV-2) causes lifelong infection. About 50 million people in the United States are infected with HSV-2, the most common cause of recurrent infections.31 Owing to changes in sexual practices, an increasing number of young people are acquiring anogenital HSV-1 infection.32,33
Diagnosis of herpes
Diagnosis may be difficult because the painful vesicular or ulcerative lesions (Figure 4) may not be visible at the time of presentation. Diagnosis is based on specific virologic and serologic tests. Nonspecific tests (eg, Tzanck smear, direct immunofluorescence) are neither sensitive nor specific and should not be relied on for diagnosis.34 HSV culture or HSV-PCR testing of a lesion is preferred. The sensitivity of viral culture can be low and is dependent on the stage of healing of a lesion and obtaining an adequate sample.
Accurate type-specific HSV serologic assays are based on HSV-specific glycoprotein G1 (HSV-1) and glycoprotein G2 (HSV-2). Unless a patient’s serologic status has already been determined, serologic testing should be done concurrently with HSV culture or PCR testing. Serologic testing enables classification of an infection as primary, nonprimary, or recurrent. For example, a patient with a positive HSV culture and negative serology most likely has primary HSV infection, and serologic study should be repeated after 6 to 8 weeks to assess for seroconversion.
Immunoglobulin M (IgM) testing for HSV-1 or HSV-2 is not diagnostic or type-specific and may be positive during recurrent genital or oral episodes of herpes.35
Treatment of herpes
In general, antiviral medications (eg, acyclovir, valacyclovir, famciclovir) are effective for managing HSV.12 Episodic or continuous suppression therapy may be needed for patients experiencing more than four outbreaks in 12 months. Patients who do not respond to treatment should be referred to an infectious disease specialist and undergo a viral culture with sensitivities.
NONINFECTIOUS CAUSES
Desquamative inflammatory vaginitis
Desquamative inflammatory vaginitis is a chronic vaginal disorder of unknown cause. It is a diagnosis of exclusion, and some patients may have a superimposed bacterial infection. It occurs mostly in perimenopausal woman and is often associated with low estrogen levels.
Diagnosis. Patients may report copious green-yellow mucoid discharge, vulvar or vaginal pain, and dyspareunia. On examination, the vulva may be erythematous, friable, and tender to the touch. The vagina may have ecchymoses, be diffusely erythematous, and have linear lesions. Mucoid or purulent discharge may be seen.
The vaginal pH is greater than 4.5.
Saline microscopy shows increased parabasal cells and leukorrhea (Figure 5).
Diagnosis is based on all of the following:
- At least 1 symptom (ie, vaginal discharge, dyspareunia, pruritus, pain, irritation, or burning)
- Vaginal inflammation on examination
- pH higher than 4.5
- Presence of parabasal cells and leukorrhea on microscopy (a ratio of leukocytes to vaginal epithelial cells > 1:1).36
Treatment involves use of 2% intravaginal clindamycin or 10% intravaginal compounded hydrocortisone cream for 4 to 6 weeks. Patients who are not cured with single-agent therapy may benefit from compounded clindamycin and hydrocortisone, with estrogen added to the formulation for hypoestrogenic patients.
Atrophic vaginitis
Atrophic vaginitis is often seen in menopausal or hypoestrogenic women. Presenting symptoms include vulvar or vaginal pain and dyspareunia.
Diagnosis. On physical examination, the vulva appears pale and atrophic, with narrowing of the introitus. Vaginal examination may reveal a pale mucosa that lacks elasticity and rugation. The examination should be performed with caution, as the vagina may bleed easily.
The vaginal pH is usually elevated.
Saline microscopy may show parabasal cells and a paucity of epithelial cells. (Figure 6).
The Vaginal Maturation Index is an indicator of the maturity of the epithelial cell types being exfoliated; these normally include parabasal (immature) cells, intermediate, and superficial (mature) cells. A predominance of immature cells indicates a hypoestrogenic state.
Infection should be considered and treated as needed.
Treatment. Patients with no contraindication may benefit from systemic hormone therapy or topical estrogen, or both.
Contact dermatitis
Contact dermatitis is classified into two types:
Irritant dermatitis, caused by the destructive action of contactants, eg, urine, feces, topical agents, feminine wipes
Allergic dermatitis, also contactant-induced, but immunologically mediated.
If a diagnosis cannot be made from the patient history and physical examination, biopsy should be performed.
Treatment of contact dermatitis involves removing the irritant, hydrating the skin with sitz baths, and using an emollient (eg, petroleum jelly) and midpotent topical steroids until resolution. Some patients benefit from topical immunosuppressive agents (eg, tacrolimus). Patients with severe symptoms may be treated with a tapering course of oral steroids for 5 to 7 days. Recalcitrant cases should be referred to a specialist.
Lichen planus
Lichen sclerosus is a benign, chronic, progressive dermatologic condition characterized by marked inflammation, epithelial thinning, and distinctive dermal changes accompanied by pruritus and pain (Figures 7 and 8).
Treatment. High-potency topical steroids are the mainstay of therapy for lichen disease. Although these are not infectious processes, superimposed infections (mostly bacterial and fungal) may also be present and should be treated.
- Van Eyk N, Allen L, Giesbrecht E, et al. Pediatric vulvovaginal disorders: a diagnostic approach and review of the literature. J Obstet Gynaecol Can 2009; 31:850–862.
- McGreal S, Wood P. Recurrent vaginal discharge in children. J Pediatr Adolesc Gynecol 2013; 26:205–208.
- Livengood CH. Bacterial vaginosis: an overview for 2009. Rev Obstet Gynecol 2009; 2:28–37.
- Atashili J, Poole C, Ndumbe PM, Adimora AA, Smith JS. Bacterial vaginosis and HIV acquisition: a meta-analysis of published studies. AIDS 2008; 22:1493–1501.
- Money D. The laboratory diagnosis of bacterial vaginosis. Can J Infect Dis Med Microbiol 2005; 16:77–79.
- Lowe NK, Neal JL, Ryan-Wenger NA, et al. Accuracy of the clinical diagnosis of vaginitis compared with a DNA probe laboratory standard. Obstet Gynecol 2009; 113:89–95.
- Mulhem E, Boyanton BL Jr, Robinson-Dunn B, Ebert C, Dzebo R. Performance of the Affirm VP-III using residual vaginal discharge collected from the speculum to characterize vaginitis in symptomatic women. J Low Genit Tract Dis 2014; 18:344–346.
- Cartwright CP, Lembke BD, Ramachandran K, et al. Comparison of nucleic acid amplification assays with BD Affirm VPIII for diagnosis of vaginitis in symptomatic women. J Clin Microbiol 2013; 51:3694–3699.
- Workowski KA, Bolan GA; Centers for Disease Control and Prevention (CDC). Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 2015; 64:1–137.
- Sobel JD, Ferris D, Schwebke J, et al. Suppressive antibacterial therapy with 0.75% metronidazole vaginal gel to prevent recurrent bacterial vaginosis. Am J Obstet Gynecol 2006; 194:1283–1289.
- Reichman O, Akins R, Sobel JD. Boric acid addition to suppressive antimicrobial therapy for recurrent bacterial vaginosis. Sex Transm Dis 2009; 36:732–734.
- Carr PL, Felsenstein D, Friedman RH. Evaluation and management of vaginitis. J Gen Intern Med 1998; 13:335–346.
- Sobel JD, Chaim W, Nagappan V, Leaman D. Treatment of vaginitis caused by Candida glabrata: use of topical boric acid and flucytosine. Am J Obstet Gynecol 2003; 189:1297–1300.
- Sobel JD, Kapernick PS, Zervos M, et al. Treatment of complicated Candida vaginitis: comparison of single and sequential doses of fluconazole. Am J Obstet Gynecol 2001; 185:363–369.
- Sobel JD, Wiesenfeld HC, Martens M, et al. Maintenance fluconazole therapy for recurrent vulvovaginal candidiasis. N Engl J Med 2004; 351:876–883.
- Phillips AJ. Treatment of non-albicans Candida vaginitis with amphotericin B vaginal suppositories. Am J Obstet Gynecol 2005; 192:2009–2013.
- Weinstock H, Berman S, Cates W Jr. Sexually transmitted diseases among American youth: incidence and prevalence estimates, 2000. Perspect Sex Reprod Health 2004; 36:6–10.
- Gatski M, Martin DH, Clark RA, Harville E, Schmidt N, Kissinger P. Co-occurrence of Trichomonas vaginalis and bacterial vaginosis among HIV-positive women. Sex Transm Dis 2011; 38:163–166.
- Hobbs MM, Seña AC. Modern diagnosis of Trichomonas vaginalis infection. Sex Transm Infect 2013; 89:434–438.
- McClelland RS, Sangare L, Hassan WM, et al. Infection with Trichomonas vaginalis increases the risk of HIV-1 acquisition. J Infect Dis 2007; 195:698–702.
- Kingston MA, Bansal D, Carlin EM. ‘Shelf life’ of Trichomonas vaginalis. Int J STD AIDS 2003; 14:28–29.
- Aslan DL, Gulbahce HE, Stelow EB, et al. The diagnosis of Trichomonas vaginalis in liquid-based Pap tests: correlation with PCR. Diagn Cytopathol 2005; 32:341–344.
- Lara-Torre E, Pinkerton JS. Accuracy of detection of Trichomonas vaginalis organisms on a liquid-based papanicolaou smear. Am J Obstet Gynecol 2003; 188:354–356.
- Hobbs MM, Lapple DM, Lawing LF, et al. Methods for detection of Trichomonas vaginalis in the male partners of infected women: implications for control of trichomoniasis. J Clin Microbiol 2006; 44:3994–3999.
- Van Der Pol B, Williams JA, Orr DP, Batteiger BE, Fortenberry JD. Prevalence, incidence, natural history, and response to treatment of Trichomonas vaginalis infection among adolescent women. J Infect Dis 2005; 192:2039–2044.
- Campbell L, Woods V, Lloyd T, Elsayed S, Church DL. Evaluation of the OSOM Trichomonas rapid test versus wet preparation examination for detection of Trichomonas vaginalis vaginitis in specimens from women with a low prevalence of infection. J Clin Microbiol 2008; 46:3467–3469.
- Chapin K, Andrea S. APTIMA Trichomonas vaginalis, a transcription-mediated amplification assay for detection of Trichomonas vaginalis in urogenital specimens. Expert Rev Mol Diagn 2011; 11:679–688.
- Krashin JW, Koumans EH, Bradshaw-Sydnor AC, et al. Trichomonas vaginalis prevalence, incidence, risk factors and antibiotic-resistance in an adolescent population. Sex Transm Dis 2010; 37:440–444.
- Sobel JD, Nyirjesy P, Brown W. Tinidazole therapy for metronidazole-resistant vaginal trichomoniasis. Clin Infect Dis 2001; 33:1341–1346.
- Nyirjesy P, Gilbert J, Mulcahy LJ. Resistant trichomoniasis: successful treatment with combination therapy. Sex Transm Dis 2011; 38:962–963.
- Bradley H, Markowitz LE, Gibson T, McQuillan GM. Seroprevalence of herpes simplex virus types 1 and 2—United States, 1999–2010. J Infect Dis 2014; 209:325–333.
- Roberts CM, Pfister JR, Spear SJ. Increasing proportion of herpes simplex virus type 1 as a cause of genital herpes infection in college students. Sex Transm Dis 2003; 30:797–800.
- Ryder N, Jin F, McNulty AM, Grulich AE, Donovan B. Increasing role of herpes simplex virus type 1 in first-episode anogenital herpes in heterosexual women and younger men who have sex with men, 1992-2006. Sex Transm Infect 2009; 85:416–419.
- Caviness AC, Oelze LL, Saz UE, Greer JM, Demmler-Harrison GJ. Direct immunofluorescence assay compared to cell culture for the diagnosis of mucocutaneous herpes simplex virus infections in children. J Clin Virol 2010; 49:58–60.
- Morrow R, Friedrich D. Performance of a novel test for IgM and IgG antibodies in subjects with culture-documented genital herpes simplex virus-1 or -2 infection. Clin Microbiol Infect 2006; 12:463–469.
- Bradford J, Fischer G. Desquamative inflammatory vaginitis: differential diagnosis and alternate diagnostic criteria. J Low Genit Tract Dis 2010; 14:306–310.
Although vulvovaginitis has several possible causes, the typical presenting symptoms are similar regardless of the cause: itching, burning, and vaginal discharge. Physical examination often reveals atrophy, redness, excoriations, and fissures in the vulvovaginal and perianal areas. Determining the cause is key to successful treatment.
This article reviews the diagnosis and treatment of many common and less common infectious and noninfectious causes of vulvovaginitis, the use of special tests, and the management of persistent cases.
DIAGNOSIS CAN BE CHALLENGING
Vulvar and vaginal symptoms are most commonly caused by local infections, but other causes must be also be considered, including several noninfectious ones (Table 1). Challenges in diagnosing vulvovaginitis are many and include distinguishing contact from allergic dermatitis, recognizing vaginal atrophy, and recognizing a parasitic infection. Determining whether a patient has an infectious process is important so that antibiotics can be used only when truly needed.
Foreign bodies in the vagina should also be considered, especially in children,1 as should sexual abuse. A 15-year retrospective review of prepubertal girls presenting with recurrent vaginal discharge found that sexual abuse might have been involved in about 5% of cases.2
Systemic diseases, such as eczema and psoriasis, may also present with gynecologic symptoms.
Heavy vaginal discharge may also be normal. This situation is a diagnosis of exclusion but is important to recognize in order to allay the patient’s anxiety and avoid unnecessary treatment.
SIMPLE OFFICE-BASED ASSESSMENT
A thorough history and physical examination are always warranted.
Simple tests of vaginal secretions can often determine the diagnosis (Table 2). Vaginal secretions should be analyzed in the following order:
Testing the pH. The pH can help determine likely diagnoses and streamline further testing (Figure 1).
Saline microscopy. Some of the vaginal discharge sample should be diluted with 1 or 2 drops of normal saline and examined under a microscope, first at × 10 magnification, then at × 40. The sample should be searched for epithelial cells, blood cells, “clue” cells (ie, epithelial cells with borders studded or obscured by bacteria), and motile trichomonads.
10% KOH whiff test and microscopy. To a second vaginal sample, a small amount of 10% potassium hydroxide should be added, and the examiner should sniff it. An amine or fishy odor is a sign of bacterial vaginosis.
If pH paper, KOH, and a microscope are unavailable, other point-of-care tests can be used for specific conditions as discussed below.
INFECTIOUS CAUSES
Infectious causes of vulvovaginitis include bacterial vaginosis, candidiasis, trichomoniasis, and herpes simplex virus (HSV) infection.
BACTERIAL VAGINOSIS
Bacterial vaginosis is the most common vaginal disorder worldwide. It has been linked to preterm delivery, intra-amniotic infection, endometritis, postabortion infection, and vaginal cuff cellulitis after hysterectomy.3 It may also be a risk factor for human immunodeficiency virus (HIV) infection.4
The condition reflects a microbial imbalance in the vaginal ecosystem, characterized by depletion of the dominant hydrogen peroxide-producing lactobacilli and overgrowth of anaerobic and facultative aerobic organisms such as Gardnerella vaginalis, Mycoplasma hominis, Atopobium vaginae, and Prevotella and Mobiluncus species.
Diagnosis of bacterial vaginosis
The Amsel criteria consist of the following:
- pH greater than 4.5
- Positive whiff test
- Homogeneous discharge
- Clue cells.
Three of the four criteria must be present for a diagnosis of bacterial vaginosis. This method is inexpensive and provides immediate results in the clinic.
The Nugent score, based on seeing certain bacteria from a vaginal swab on Gram stain microscopy, is the diagnostic standard for research.5
DNA tests. Affirm VPIII (BD Diagnostics, Sparks, MD) is a nonamplified nucleic acid probe hybridization test that detects Trichomonas vaginalis, Candida albicans, and G vaginalis. Although it is more expensive than testing for the Amsel criteria, it is commonly used in private offices because it is simple to use, gives rapid results, and does not require a microscope.6 Insurance pays for it when the test is indicated, but we know of a patient who received a bill for approximately $500 when the insurance company thought the test was not indicated.
In a study of 109 patients with symptoms of vulvovaginitis, the Affirm VPIII was found comparable to saline microscopy when tested on residual vaginal samples. Compared with Gram stain using Nugent scoring, the test has a sensitivity of 87.7% to 95.2% and a specificity of 81% to 99.1% for bacterial vaginosis.7
In 323 symptomatic women, a polymerase chain reaction (PCR) assay for bacterial vaginosis was 96.9% sensitive and 92.6% specific for bacterial vaginosis, and Affirm VPIII was 90.1% sensitive and 67.6% specific, compared with a reference standard incorporating Nugent Gram-stain scores and Amsel criteria.8 The test is commercially available.
Management of bacterial vaginosis
Initial treatment. Bacterial vaginosis can be treated with oral or topical metronidazole, oral tinidazole, or oral or topical clindamycin.9 All options offer equivalent efficacy as initial treatments, so the choice may be based on cost and preferred route of administration.
Treatment for recurrent disease. Women who have 3 or more episodes in 12 months should receive initial treatment each time as described above and should then be offered additional suppressive therapy with 0.75% metronidazole intravaginal gel 2 times a week for 4 months. A side effect of therapy is vulvovaginal candidiasis, which should be treated as needed.
In a multicenter study, Sobel et al10 randomized patients who had recurrent bacterial vaginosis to twice-weekly metronidazole gel or placebo for 16 weeks after their initial treatment. During the 28 weeks of follow-up, recurrences occurred in 51% of treated women vs 75% of those on placebo.
Another option for chronic therapy is oral metronidazole and boric acid vaginal suppositories.
Reichman et al11 treated women with oral metronidazole or tinidazole 500 mg twice a day for 7 days, followed by vaginal boric acid 600 mg daily for 21 days. This was followed by twice-weekly vaginal metronidazole gel for 16 weeks. At follow-up, the cure rate was 92% at 7 weeks, dropping to 88% at 12 weeks and 50% at 36 weeks.
Patients with recurrent bacterial vaginosis despite therapy should be referred to a vulvovaginal or infectious disease specialist.
VULVOVAGINAL CANDIDIASIS
Vulvovaginal candidiasis is the second most common cause of vaginitis.
Diagnosis can be clinical
Vulvovaginal candidiasis can be clinically diagnosed on the basis of cottage cheese-like clumpy discharge; external dysuria (a burning sensation when urine comes in contact with the vulva); and vulvar itching, pain, swelling, and redness. Edema, fissures, and excoriations may be seen on examination of the vulva. (Figure 3).
Saline microscopy (Figure 2) with the addition of 10% KOH may reveal the characteristic fungal elements, but its sensitivity is only 50%.
Fungal culture remains the gold standard for diagnosis and is needed to determine the sensitivity of specific strains of Candida to therapy.12
DNA tests can also be helpful. In a study of patients with symptomatic vaginitis, Affirm VPIII detected Candida in 11% of samples, whereas microscopy detected it in only 7%.13 Another study7 found that Affirm VPIII produced comparable results whether the sample was collected from residual vaginal discharge found on the speculum or was collected in the traditional way (by swabbing).
Cartwright et al8 compared the performance of a multiplexed, real-time PCR assay and Affirm VPIII in 102 patients. PCR was much more sensitive (97.7% vs 58.1%) but less specific (93.2% vs 100%), with culture serving as the gold standard.
Management of candidiasis
Uncomplicated cases can be managed with prescription or over-the-counter topical or oral antifungal medications for 1 to 7 days, depending on the medication.9 However, most of the common antifungals may not be effective against non-albicans Candida.
In immunosuppressed patients and diabetic patients, if symptoms do not improve with regular treatment, a vaginal sample should be cultured for C albicans. If the culture is positive, the patient should be treated with fluconazole 150 mg orally every 3 days for 3 doses.14
Patients with recurrent episodes (3 or more in 12 months) should follow initial treatment with maintenance therapy of weekly fluconazole 150 mg orally for 6 months.15
Non-albicans Candida may be azole-resistant, and fungal culture and sensitivity should be obtained. Sobel et al13 documented successful treatment of non-albicans Candida using boric acid and flucytosine. Phillips16 documented successful use of compounded amphotericin B in a 50-mg vaginal suppository for 14 days. Therefore, in patients who have Candida species other than C albicans, treatment should be one of the following:
- Vaginal boric acid 600 mg daily for 14 to 21 days
- Flucytosine in 15.5% vaginal cream, intravaginally administered as 5 g for 14 days
- Amphotericin B 50 mg vaginal suppositories for 14 days.
Boric acid is readily available, but flucytosine vaginal cream and amphotericin B vaginal suppositories must usually be compounded by a pharmacist.
Of note: All that itches is not yeast. Patients with persistent itching despite treatment should be referred to a specialist to search for another cause.
TRICHOMONIASIS
The incidence of T vaginalis infection is higher than that of Neisseria gonorrhoeae and Chlamydia trachomatis combined, with an estimated 7.4 million new cases occurring in the year 2000 in the United States.17 Infection increases the sexual transmission of HIV.18–20 It is often asymptomatic and so is likely underdiagnosed.
Diagnosis of trichomoniasis
Vaginal pH may be normal or elevated (> 4.5).
Direct microscopy. Observation by saline microscopy of motile trichomonads with their characteristic jerky movements is 100% specific but only 50% sensitive. Sensitivity is reduced by delaying microscopy on the sample by as little as 10 minutes.21
The incidental finding of T vaginalis on a conventional Papanicolaou (Pap) smear has poor sensitivity and specificity, and patients diagnosed with T vaginalis by conventional Pap smear should have a second test performed. The liquid-based Pap test is more accurate for microscopic diagnosis, and its results can be used to determine if treatment is needed (sensitivity 60%–90%; specificity 98%–100%).22,23
Culture. Amplification of T vaginalis in liquid culture usually provides results within 3 days.24 It is more sensitive than microscopy but less sensitive than a nucleic acid amplification test: compared with a nucleic acid amplification test, culture is 44% to 75% sensitive for detecting T vaginalis and 100% specific.19 Culture is the preferred test for resistant strains.
Non–culture-based or nucleic acid tests do not require viable organisms, so they allow for a wider range of specimen storage temperatures and time intervals between collection and processing. This quality limits them for testing treatment success; if performed too early, they may detect nonviable organisms. A 2-week interval is recommended between the end of treatment and retesting.25
Nonamplified tests such as Affirm VPIII and the Osom Trichomonas Test (Sekisui Diagnostics, Lexington, MA) are 40% to 95% sensitive, depending on the test and reference standard used, and 92% to 100% specific.26,27
Nucleic acid amplification tests are usually not performed as point-of-care tests. They are more expensive and require special equipment with trained personnel. Sensitivities range from 76% to 100%, making these tests more suitable for screening and testing of asymptomatic women, in whom the concentration of organisms may be lower.
Treatment of trichomoniasis
Treatment is a single 2-g oral dose of metronidazole or tinidazole.9
If initial treatment is ineffective, an additional regimen can be either of the following:
- Oral metronidazole 500 mg twice a day for 7 days
- Oral metronidazole or tinidazole, 2 g daily for 5 days.
Patients allergic to nitroimidazoles should be referred for desensitization.
If these treatments are unsuccessful, the patient should be referred to an infectious disease specialist or gynecologist who specializes in vulvovaginal disorders. Treatment failure is uncommon and is usually related to noncompliance, reinfection, or metronidazole resistance.28 The US Centers for Disease Control and Prevention offers testing for resistance by request.
Reportedly successful regimens for refractory trichomoniasis include 14 days of either:
- Oral tinidazole 500 mg 4 times daily plus vaginal tinidazole 500 mg twice daily29
- Oral tinidazole 1 g 3 times daily plus compounded 5% intravaginal paromomycin 5 g nightly.30
HERPES SIMPLEX VIRUS INFECTION
HSV (HSV-1 and HSV-2) causes lifelong infection. About 50 million people in the United States are infected with HSV-2, the most common cause of recurrent infections.31 Owing to changes in sexual practices, an increasing number of young people are acquiring anogenital HSV-1 infection.32,33
Diagnosis of herpes
Diagnosis may be difficult because the painful vesicular or ulcerative lesions (Figure 4) may not be visible at the time of presentation. Diagnosis is based on specific virologic and serologic tests. Nonspecific tests (eg, Tzanck smear, direct immunofluorescence) are neither sensitive nor specific and should not be relied on for diagnosis.34 HSV culture or HSV-PCR testing of a lesion is preferred. The sensitivity of viral culture can be low and is dependent on the stage of healing of a lesion and obtaining an adequate sample.
Accurate type-specific HSV serologic assays are based on HSV-specific glycoprotein G1 (HSV-1) and glycoprotein G2 (HSV-2). Unless a patient’s serologic status has already been determined, serologic testing should be done concurrently with HSV culture or PCR testing. Serologic testing enables classification of an infection as primary, nonprimary, or recurrent. For example, a patient with a positive HSV culture and negative serology most likely has primary HSV infection, and serologic study should be repeated after 6 to 8 weeks to assess for seroconversion.
Immunoglobulin M (IgM) testing for HSV-1 or HSV-2 is not diagnostic or type-specific and may be positive during recurrent genital or oral episodes of herpes.35
Treatment of herpes
In general, antiviral medications (eg, acyclovir, valacyclovir, famciclovir) are effective for managing HSV.12 Episodic or continuous suppression therapy may be needed for patients experiencing more than four outbreaks in 12 months. Patients who do not respond to treatment should be referred to an infectious disease specialist and undergo a viral culture with sensitivities.
NONINFECTIOUS CAUSES
Desquamative inflammatory vaginitis
Desquamative inflammatory vaginitis is a chronic vaginal disorder of unknown cause. It is a diagnosis of exclusion, and some patients may have a superimposed bacterial infection. It occurs mostly in perimenopausal woman and is often associated with low estrogen levels.
Diagnosis. Patients may report copious green-yellow mucoid discharge, vulvar or vaginal pain, and dyspareunia. On examination, the vulva may be erythematous, friable, and tender to the touch. The vagina may have ecchymoses, be diffusely erythematous, and have linear lesions. Mucoid or purulent discharge may be seen.
The vaginal pH is greater than 4.5.
Saline microscopy shows increased parabasal cells and leukorrhea (Figure 5).
Diagnosis is based on all of the following:
- At least 1 symptom (ie, vaginal discharge, dyspareunia, pruritus, pain, irritation, or burning)
- Vaginal inflammation on examination
- pH higher than 4.5
- Presence of parabasal cells and leukorrhea on microscopy (a ratio of leukocytes to vaginal epithelial cells > 1:1).36
Treatment involves use of 2% intravaginal clindamycin or 10% intravaginal compounded hydrocortisone cream for 4 to 6 weeks. Patients who are not cured with single-agent therapy may benefit from compounded clindamycin and hydrocortisone, with estrogen added to the formulation for hypoestrogenic patients.
Atrophic vaginitis
Atrophic vaginitis is often seen in menopausal or hypoestrogenic women. Presenting symptoms include vulvar or vaginal pain and dyspareunia.
Diagnosis. On physical examination, the vulva appears pale and atrophic, with narrowing of the introitus. Vaginal examination may reveal a pale mucosa that lacks elasticity and rugation. The examination should be performed with caution, as the vagina may bleed easily.
The vaginal pH is usually elevated.
Saline microscopy may show parabasal cells and a paucity of epithelial cells. (Figure 6).
The Vaginal Maturation Index is an indicator of the maturity of the epithelial cell types being exfoliated; these normally include parabasal (immature) cells, intermediate, and superficial (mature) cells. A predominance of immature cells indicates a hypoestrogenic state.
Infection should be considered and treated as needed.
Treatment. Patients with no contraindication may benefit from systemic hormone therapy or topical estrogen, or both.
Contact dermatitis
Contact dermatitis is classified into two types:
Irritant dermatitis, caused by the destructive action of contactants, eg, urine, feces, topical agents, feminine wipes
Allergic dermatitis, also contactant-induced, but immunologically mediated.
If a diagnosis cannot be made from the patient history and physical examination, biopsy should be performed.
Treatment of contact dermatitis involves removing the irritant, hydrating the skin with sitz baths, and using an emollient (eg, petroleum jelly) and midpotent topical steroids until resolution. Some patients benefit from topical immunosuppressive agents (eg, tacrolimus). Patients with severe symptoms may be treated with a tapering course of oral steroids for 5 to 7 days. Recalcitrant cases should be referred to a specialist.
Lichen planus
Lichen sclerosus is a benign, chronic, progressive dermatologic condition characterized by marked inflammation, epithelial thinning, and distinctive dermal changes accompanied by pruritus and pain (Figures 7 and 8).
Treatment. High-potency topical steroids are the mainstay of therapy for lichen disease. Although these are not infectious processes, superimposed infections (mostly bacterial and fungal) may also be present and should be treated.
Although vulvovaginitis has several possible causes, the typical presenting symptoms are similar regardless of the cause: itching, burning, and vaginal discharge. Physical examination often reveals atrophy, redness, excoriations, and fissures in the vulvovaginal and perianal areas. Determining the cause is key to successful treatment.
This article reviews the diagnosis and treatment of many common and less common infectious and noninfectious causes of vulvovaginitis, the use of special tests, and the management of persistent cases.
DIAGNOSIS CAN BE CHALLENGING
Vulvar and vaginal symptoms are most commonly caused by local infections, but other causes must be also be considered, including several noninfectious ones (Table 1). Challenges in diagnosing vulvovaginitis are many and include distinguishing contact from allergic dermatitis, recognizing vaginal atrophy, and recognizing a parasitic infection. Determining whether a patient has an infectious process is important so that antibiotics can be used only when truly needed.
Foreign bodies in the vagina should also be considered, especially in children,1 as should sexual abuse. A 15-year retrospective review of prepubertal girls presenting with recurrent vaginal discharge found that sexual abuse might have been involved in about 5% of cases.2
Systemic diseases, such as eczema and psoriasis, may also present with gynecologic symptoms.
Heavy vaginal discharge may also be normal. This situation is a diagnosis of exclusion but is important to recognize in order to allay the patient’s anxiety and avoid unnecessary treatment.
SIMPLE OFFICE-BASED ASSESSMENT
A thorough history and physical examination are always warranted.
Simple tests of vaginal secretions can often determine the diagnosis (Table 2). Vaginal secretions should be analyzed in the following order:
Testing the pH. The pH can help determine likely diagnoses and streamline further testing (Figure 1).
Saline microscopy. Some of the vaginal discharge sample should be diluted with 1 or 2 drops of normal saline and examined under a microscope, first at × 10 magnification, then at × 40. The sample should be searched for epithelial cells, blood cells, “clue” cells (ie, epithelial cells with borders studded or obscured by bacteria), and motile trichomonads.
10% KOH whiff test and microscopy. To a second vaginal sample, a small amount of 10% potassium hydroxide should be added, and the examiner should sniff it. An amine or fishy odor is a sign of bacterial vaginosis.
If pH paper, KOH, and a microscope are unavailable, other point-of-care tests can be used for specific conditions as discussed below.
INFECTIOUS CAUSES
Infectious causes of vulvovaginitis include bacterial vaginosis, candidiasis, trichomoniasis, and herpes simplex virus (HSV) infection.
BACTERIAL VAGINOSIS
Bacterial vaginosis is the most common vaginal disorder worldwide. It has been linked to preterm delivery, intra-amniotic infection, endometritis, postabortion infection, and vaginal cuff cellulitis after hysterectomy.3 It may also be a risk factor for human immunodeficiency virus (HIV) infection.4
The condition reflects a microbial imbalance in the vaginal ecosystem, characterized by depletion of the dominant hydrogen peroxide-producing lactobacilli and overgrowth of anaerobic and facultative aerobic organisms such as Gardnerella vaginalis, Mycoplasma hominis, Atopobium vaginae, and Prevotella and Mobiluncus species.
Diagnosis of bacterial vaginosis
The Amsel criteria consist of the following:
- pH greater than 4.5
- Positive whiff test
- Homogeneous discharge
- Clue cells.
Three of the four criteria must be present for a diagnosis of bacterial vaginosis. This method is inexpensive and provides immediate results in the clinic.
The Nugent score, based on seeing certain bacteria from a vaginal swab on Gram stain microscopy, is the diagnostic standard for research.5
DNA tests. Affirm VPIII (BD Diagnostics, Sparks, MD) is a nonamplified nucleic acid probe hybridization test that detects Trichomonas vaginalis, Candida albicans, and G vaginalis. Although it is more expensive than testing for the Amsel criteria, it is commonly used in private offices because it is simple to use, gives rapid results, and does not require a microscope.6 Insurance pays for it when the test is indicated, but we know of a patient who received a bill for approximately $500 when the insurance company thought the test was not indicated.
In a study of 109 patients with symptoms of vulvovaginitis, the Affirm VPIII was found comparable to saline microscopy when tested on residual vaginal samples. Compared with Gram stain using Nugent scoring, the test has a sensitivity of 87.7% to 95.2% and a specificity of 81% to 99.1% for bacterial vaginosis.7
In 323 symptomatic women, a polymerase chain reaction (PCR) assay for bacterial vaginosis was 96.9% sensitive and 92.6% specific for bacterial vaginosis, and Affirm VPIII was 90.1% sensitive and 67.6% specific, compared with a reference standard incorporating Nugent Gram-stain scores and Amsel criteria.8 The test is commercially available.
Management of bacterial vaginosis
Initial treatment. Bacterial vaginosis can be treated with oral or topical metronidazole, oral tinidazole, or oral or topical clindamycin.9 All options offer equivalent efficacy as initial treatments, so the choice may be based on cost and preferred route of administration.
Treatment for recurrent disease. Women who have 3 or more episodes in 12 months should receive initial treatment each time as described above and should then be offered additional suppressive therapy with 0.75% metronidazole intravaginal gel 2 times a week for 4 months. A side effect of therapy is vulvovaginal candidiasis, which should be treated as needed.
In a multicenter study, Sobel et al10 randomized patients who had recurrent bacterial vaginosis to twice-weekly metronidazole gel or placebo for 16 weeks after their initial treatment. During the 28 weeks of follow-up, recurrences occurred in 51% of treated women vs 75% of those on placebo.
Another option for chronic therapy is oral metronidazole and boric acid vaginal suppositories.
Reichman et al11 treated women with oral metronidazole or tinidazole 500 mg twice a day for 7 days, followed by vaginal boric acid 600 mg daily for 21 days. This was followed by twice-weekly vaginal metronidazole gel for 16 weeks. At follow-up, the cure rate was 92% at 7 weeks, dropping to 88% at 12 weeks and 50% at 36 weeks.
Patients with recurrent bacterial vaginosis despite therapy should be referred to a vulvovaginal or infectious disease specialist.
VULVOVAGINAL CANDIDIASIS
Vulvovaginal candidiasis is the second most common cause of vaginitis.
Diagnosis can be clinical
Vulvovaginal candidiasis can be clinically diagnosed on the basis of cottage cheese-like clumpy discharge; external dysuria (a burning sensation when urine comes in contact with the vulva); and vulvar itching, pain, swelling, and redness. Edema, fissures, and excoriations may be seen on examination of the vulva. (Figure 3).
Saline microscopy (Figure 2) with the addition of 10% KOH may reveal the characteristic fungal elements, but its sensitivity is only 50%.
Fungal culture remains the gold standard for diagnosis and is needed to determine the sensitivity of specific strains of Candida to therapy.12
DNA tests can also be helpful. In a study of patients with symptomatic vaginitis, Affirm VPIII detected Candida in 11% of samples, whereas microscopy detected it in only 7%.13 Another study7 found that Affirm VPIII produced comparable results whether the sample was collected from residual vaginal discharge found on the speculum or was collected in the traditional way (by swabbing).
Cartwright et al8 compared the performance of a multiplexed, real-time PCR assay and Affirm VPIII in 102 patients. PCR was much more sensitive (97.7% vs 58.1%) but less specific (93.2% vs 100%), with culture serving as the gold standard.
Management of candidiasis
Uncomplicated cases can be managed with prescription or over-the-counter topical or oral antifungal medications for 1 to 7 days, depending on the medication.9 However, most of the common antifungals may not be effective against non-albicans Candida.
In immunosuppressed patients and diabetic patients, if symptoms do not improve with regular treatment, a vaginal sample should be cultured for C albicans. If the culture is positive, the patient should be treated with fluconazole 150 mg orally every 3 days for 3 doses.14
Patients with recurrent episodes (3 or more in 12 months) should follow initial treatment with maintenance therapy of weekly fluconazole 150 mg orally for 6 months.15
Non-albicans Candida may be azole-resistant, and fungal culture and sensitivity should be obtained. Sobel et al13 documented successful treatment of non-albicans Candida using boric acid and flucytosine. Phillips16 documented successful use of compounded amphotericin B in a 50-mg vaginal suppository for 14 days. Therefore, in patients who have Candida species other than C albicans, treatment should be one of the following:
- Vaginal boric acid 600 mg daily for 14 to 21 days
- Flucytosine in 15.5% vaginal cream, intravaginally administered as 5 g for 14 days
- Amphotericin B 50 mg vaginal suppositories for 14 days.
Boric acid is readily available, but flucytosine vaginal cream and amphotericin B vaginal suppositories must usually be compounded by a pharmacist.
Of note: All that itches is not yeast. Patients with persistent itching despite treatment should be referred to a specialist to search for another cause.
TRICHOMONIASIS
The incidence of T vaginalis infection is higher than that of Neisseria gonorrhoeae and Chlamydia trachomatis combined, with an estimated 7.4 million new cases occurring in the year 2000 in the United States.17 Infection increases the sexual transmission of HIV.18–20 It is often asymptomatic and so is likely underdiagnosed.
Diagnosis of trichomoniasis
Vaginal pH may be normal or elevated (> 4.5).
Direct microscopy. Observation by saline microscopy of motile trichomonads with their characteristic jerky movements is 100% specific but only 50% sensitive. Sensitivity is reduced by delaying microscopy on the sample by as little as 10 minutes.21
The incidental finding of T vaginalis on a conventional Papanicolaou (Pap) smear has poor sensitivity and specificity, and patients diagnosed with T vaginalis by conventional Pap smear should have a second test performed. The liquid-based Pap test is more accurate for microscopic diagnosis, and its results can be used to determine if treatment is needed (sensitivity 60%–90%; specificity 98%–100%).22,23
Culture. Amplification of T vaginalis in liquid culture usually provides results within 3 days.24 It is more sensitive than microscopy but less sensitive than a nucleic acid amplification test: compared with a nucleic acid amplification test, culture is 44% to 75% sensitive for detecting T vaginalis and 100% specific.19 Culture is the preferred test for resistant strains.
Non–culture-based or nucleic acid tests do not require viable organisms, so they allow for a wider range of specimen storage temperatures and time intervals between collection and processing. This quality limits them for testing treatment success; if performed too early, they may detect nonviable organisms. A 2-week interval is recommended between the end of treatment and retesting.25
Nonamplified tests such as Affirm VPIII and the Osom Trichomonas Test (Sekisui Diagnostics, Lexington, MA) are 40% to 95% sensitive, depending on the test and reference standard used, and 92% to 100% specific.26,27
Nucleic acid amplification tests are usually not performed as point-of-care tests. They are more expensive and require special equipment with trained personnel. Sensitivities range from 76% to 100%, making these tests more suitable for screening and testing of asymptomatic women, in whom the concentration of organisms may be lower.
Treatment of trichomoniasis
Treatment is a single 2-g oral dose of metronidazole or tinidazole.9
If initial treatment is ineffective, an additional regimen can be either of the following:
- Oral metronidazole 500 mg twice a day for 7 days
- Oral metronidazole or tinidazole, 2 g daily for 5 days.
Patients allergic to nitroimidazoles should be referred for desensitization.
If these treatments are unsuccessful, the patient should be referred to an infectious disease specialist or gynecologist who specializes in vulvovaginal disorders. Treatment failure is uncommon and is usually related to noncompliance, reinfection, or metronidazole resistance.28 The US Centers for Disease Control and Prevention offers testing for resistance by request.
Reportedly successful regimens for refractory trichomoniasis include 14 days of either:
- Oral tinidazole 500 mg 4 times daily plus vaginal tinidazole 500 mg twice daily29
- Oral tinidazole 1 g 3 times daily plus compounded 5% intravaginal paromomycin 5 g nightly.30
HERPES SIMPLEX VIRUS INFECTION
HSV (HSV-1 and HSV-2) causes lifelong infection. About 50 million people in the United States are infected with HSV-2, the most common cause of recurrent infections.31 Owing to changes in sexual practices, an increasing number of young people are acquiring anogenital HSV-1 infection.32,33
Diagnosis of herpes
Diagnosis may be difficult because the painful vesicular or ulcerative lesions (Figure 4) may not be visible at the time of presentation. Diagnosis is based on specific virologic and serologic tests. Nonspecific tests (eg, Tzanck smear, direct immunofluorescence) are neither sensitive nor specific and should not be relied on for diagnosis.34 HSV culture or HSV-PCR testing of a lesion is preferred. The sensitivity of viral culture can be low and is dependent on the stage of healing of a lesion and obtaining an adequate sample.
Accurate type-specific HSV serologic assays are based on HSV-specific glycoprotein G1 (HSV-1) and glycoprotein G2 (HSV-2). Unless a patient’s serologic status has already been determined, serologic testing should be done concurrently with HSV culture or PCR testing. Serologic testing enables classification of an infection as primary, nonprimary, or recurrent. For example, a patient with a positive HSV culture and negative serology most likely has primary HSV infection, and serologic study should be repeated after 6 to 8 weeks to assess for seroconversion.
Immunoglobulin M (IgM) testing for HSV-1 or HSV-2 is not diagnostic or type-specific and may be positive during recurrent genital or oral episodes of herpes.35
Treatment of herpes
In general, antiviral medications (eg, acyclovir, valacyclovir, famciclovir) are effective for managing HSV.12 Episodic or continuous suppression therapy may be needed for patients experiencing more than four outbreaks in 12 months. Patients who do not respond to treatment should be referred to an infectious disease specialist and undergo a viral culture with sensitivities.
NONINFECTIOUS CAUSES
Desquamative inflammatory vaginitis
Desquamative inflammatory vaginitis is a chronic vaginal disorder of unknown cause. It is a diagnosis of exclusion, and some patients may have a superimposed bacterial infection. It occurs mostly in perimenopausal woman and is often associated with low estrogen levels.
Diagnosis. Patients may report copious green-yellow mucoid discharge, vulvar or vaginal pain, and dyspareunia. On examination, the vulva may be erythematous, friable, and tender to the touch. The vagina may have ecchymoses, be diffusely erythematous, and have linear lesions. Mucoid or purulent discharge may be seen.
The vaginal pH is greater than 4.5.
Saline microscopy shows increased parabasal cells and leukorrhea (Figure 5).
Diagnosis is based on all of the following:
- At least 1 symptom (ie, vaginal discharge, dyspareunia, pruritus, pain, irritation, or burning)
- Vaginal inflammation on examination
- pH higher than 4.5
- Presence of parabasal cells and leukorrhea on microscopy (a ratio of leukocytes to vaginal epithelial cells > 1:1).36
Treatment involves use of 2% intravaginal clindamycin or 10% intravaginal compounded hydrocortisone cream for 4 to 6 weeks. Patients who are not cured with single-agent therapy may benefit from compounded clindamycin and hydrocortisone, with estrogen added to the formulation for hypoestrogenic patients.
Atrophic vaginitis
Atrophic vaginitis is often seen in menopausal or hypoestrogenic women. Presenting symptoms include vulvar or vaginal pain and dyspareunia.
Diagnosis. On physical examination, the vulva appears pale and atrophic, with narrowing of the introitus. Vaginal examination may reveal a pale mucosa that lacks elasticity and rugation. The examination should be performed with caution, as the vagina may bleed easily.
The vaginal pH is usually elevated.
Saline microscopy may show parabasal cells and a paucity of epithelial cells. (Figure 6).
The Vaginal Maturation Index is an indicator of the maturity of the epithelial cell types being exfoliated; these normally include parabasal (immature) cells, intermediate, and superficial (mature) cells. A predominance of immature cells indicates a hypoestrogenic state.
Infection should be considered and treated as needed.
Treatment. Patients with no contraindication may benefit from systemic hormone therapy or topical estrogen, or both.
Contact dermatitis
Contact dermatitis is classified into two types:
Irritant dermatitis, caused by the destructive action of contactants, eg, urine, feces, topical agents, feminine wipes
Allergic dermatitis, also contactant-induced, but immunologically mediated.
If a diagnosis cannot be made from the patient history and physical examination, biopsy should be performed.
Treatment of contact dermatitis involves removing the irritant, hydrating the skin with sitz baths, and using an emollient (eg, petroleum jelly) and midpotent topical steroids until resolution. Some patients benefit from topical immunosuppressive agents (eg, tacrolimus). Patients with severe symptoms may be treated with a tapering course of oral steroids for 5 to 7 days. Recalcitrant cases should be referred to a specialist.
Lichen planus
Lichen sclerosus is a benign, chronic, progressive dermatologic condition characterized by marked inflammation, epithelial thinning, and distinctive dermal changes accompanied by pruritus and pain (Figures 7 and 8).
Treatment. High-potency topical steroids are the mainstay of therapy for lichen disease. Although these are not infectious processes, superimposed infections (mostly bacterial and fungal) may also be present and should be treated.
- Van Eyk N, Allen L, Giesbrecht E, et al. Pediatric vulvovaginal disorders: a diagnostic approach and review of the literature. J Obstet Gynaecol Can 2009; 31:850–862.
- McGreal S, Wood P. Recurrent vaginal discharge in children. J Pediatr Adolesc Gynecol 2013; 26:205–208.
- Livengood CH. Bacterial vaginosis: an overview for 2009. Rev Obstet Gynecol 2009; 2:28–37.
- Atashili J, Poole C, Ndumbe PM, Adimora AA, Smith JS. Bacterial vaginosis and HIV acquisition: a meta-analysis of published studies. AIDS 2008; 22:1493–1501.
- Money D. The laboratory diagnosis of bacterial vaginosis. Can J Infect Dis Med Microbiol 2005; 16:77–79.
- Lowe NK, Neal JL, Ryan-Wenger NA, et al. Accuracy of the clinical diagnosis of vaginitis compared with a DNA probe laboratory standard. Obstet Gynecol 2009; 113:89–95.
- Mulhem E, Boyanton BL Jr, Robinson-Dunn B, Ebert C, Dzebo R. Performance of the Affirm VP-III using residual vaginal discharge collected from the speculum to characterize vaginitis in symptomatic women. J Low Genit Tract Dis 2014; 18:344–346.
- Cartwright CP, Lembke BD, Ramachandran K, et al. Comparison of nucleic acid amplification assays with BD Affirm VPIII for diagnosis of vaginitis in symptomatic women. J Clin Microbiol 2013; 51:3694–3699.
- Workowski KA, Bolan GA; Centers for Disease Control and Prevention (CDC). Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 2015; 64:1–137.
- Sobel JD, Ferris D, Schwebke J, et al. Suppressive antibacterial therapy with 0.75% metronidazole vaginal gel to prevent recurrent bacterial vaginosis. Am J Obstet Gynecol 2006; 194:1283–1289.
- Reichman O, Akins R, Sobel JD. Boric acid addition to suppressive antimicrobial therapy for recurrent bacterial vaginosis. Sex Transm Dis 2009; 36:732–734.
- Carr PL, Felsenstein D, Friedman RH. Evaluation and management of vaginitis. J Gen Intern Med 1998; 13:335–346.
- Sobel JD, Chaim W, Nagappan V, Leaman D. Treatment of vaginitis caused by Candida glabrata: use of topical boric acid and flucytosine. Am J Obstet Gynecol 2003; 189:1297–1300.
- Sobel JD, Kapernick PS, Zervos M, et al. Treatment of complicated Candida vaginitis: comparison of single and sequential doses of fluconazole. Am J Obstet Gynecol 2001; 185:363–369.
- Sobel JD, Wiesenfeld HC, Martens M, et al. Maintenance fluconazole therapy for recurrent vulvovaginal candidiasis. N Engl J Med 2004; 351:876–883.
- Phillips AJ. Treatment of non-albicans Candida vaginitis with amphotericin B vaginal suppositories. Am J Obstet Gynecol 2005; 192:2009–2013.
- Weinstock H, Berman S, Cates W Jr. Sexually transmitted diseases among American youth: incidence and prevalence estimates, 2000. Perspect Sex Reprod Health 2004; 36:6–10.
- Gatski M, Martin DH, Clark RA, Harville E, Schmidt N, Kissinger P. Co-occurrence of Trichomonas vaginalis and bacterial vaginosis among HIV-positive women. Sex Transm Dis 2011; 38:163–166.
- Hobbs MM, Seña AC. Modern diagnosis of Trichomonas vaginalis infection. Sex Transm Infect 2013; 89:434–438.
- McClelland RS, Sangare L, Hassan WM, et al. Infection with Trichomonas vaginalis increases the risk of HIV-1 acquisition. J Infect Dis 2007; 195:698–702.
- Kingston MA, Bansal D, Carlin EM. ‘Shelf life’ of Trichomonas vaginalis. Int J STD AIDS 2003; 14:28–29.
- Aslan DL, Gulbahce HE, Stelow EB, et al. The diagnosis of Trichomonas vaginalis in liquid-based Pap tests: correlation with PCR. Diagn Cytopathol 2005; 32:341–344.
- Lara-Torre E, Pinkerton JS. Accuracy of detection of Trichomonas vaginalis organisms on a liquid-based papanicolaou smear. Am J Obstet Gynecol 2003; 188:354–356.
- Hobbs MM, Lapple DM, Lawing LF, et al. Methods for detection of Trichomonas vaginalis in the male partners of infected women: implications for control of trichomoniasis. J Clin Microbiol 2006; 44:3994–3999.
- Van Der Pol B, Williams JA, Orr DP, Batteiger BE, Fortenberry JD. Prevalence, incidence, natural history, and response to treatment of Trichomonas vaginalis infection among adolescent women. J Infect Dis 2005; 192:2039–2044.
- Campbell L, Woods V, Lloyd T, Elsayed S, Church DL. Evaluation of the OSOM Trichomonas rapid test versus wet preparation examination for detection of Trichomonas vaginalis vaginitis in specimens from women with a low prevalence of infection. J Clin Microbiol 2008; 46:3467–3469.
- Chapin K, Andrea S. APTIMA Trichomonas vaginalis, a transcription-mediated amplification assay for detection of Trichomonas vaginalis in urogenital specimens. Expert Rev Mol Diagn 2011; 11:679–688.
- Krashin JW, Koumans EH, Bradshaw-Sydnor AC, et al. Trichomonas vaginalis prevalence, incidence, risk factors and antibiotic-resistance in an adolescent population. Sex Transm Dis 2010; 37:440–444.
- Sobel JD, Nyirjesy P, Brown W. Tinidazole therapy for metronidazole-resistant vaginal trichomoniasis. Clin Infect Dis 2001; 33:1341–1346.
- Nyirjesy P, Gilbert J, Mulcahy LJ. Resistant trichomoniasis: successful treatment with combination therapy. Sex Transm Dis 2011; 38:962–963.
- Bradley H, Markowitz LE, Gibson T, McQuillan GM. Seroprevalence of herpes simplex virus types 1 and 2—United States, 1999–2010. J Infect Dis 2014; 209:325–333.
- Roberts CM, Pfister JR, Spear SJ. Increasing proportion of herpes simplex virus type 1 as a cause of genital herpes infection in college students. Sex Transm Dis 2003; 30:797–800.
- Ryder N, Jin F, McNulty AM, Grulich AE, Donovan B. Increasing role of herpes simplex virus type 1 in first-episode anogenital herpes in heterosexual women and younger men who have sex with men, 1992-2006. Sex Transm Infect 2009; 85:416–419.
- Caviness AC, Oelze LL, Saz UE, Greer JM, Demmler-Harrison GJ. Direct immunofluorescence assay compared to cell culture for the diagnosis of mucocutaneous herpes simplex virus infections in children. J Clin Virol 2010; 49:58–60.
- Morrow R, Friedrich D. Performance of a novel test for IgM and IgG antibodies in subjects with culture-documented genital herpes simplex virus-1 or -2 infection. Clin Microbiol Infect 2006; 12:463–469.
- Bradford J, Fischer G. Desquamative inflammatory vaginitis: differential diagnosis and alternate diagnostic criteria. J Low Genit Tract Dis 2010; 14:306–310.
- Van Eyk N, Allen L, Giesbrecht E, et al. Pediatric vulvovaginal disorders: a diagnostic approach and review of the literature. J Obstet Gynaecol Can 2009; 31:850–862.
- McGreal S, Wood P. Recurrent vaginal discharge in children. J Pediatr Adolesc Gynecol 2013; 26:205–208.
- Livengood CH. Bacterial vaginosis: an overview for 2009. Rev Obstet Gynecol 2009; 2:28–37.
- Atashili J, Poole C, Ndumbe PM, Adimora AA, Smith JS. Bacterial vaginosis and HIV acquisition: a meta-analysis of published studies. AIDS 2008; 22:1493–1501.
- Money D. The laboratory diagnosis of bacterial vaginosis. Can J Infect Dis Med Microbiol 2005; 16:77–79.
- Lowe NK, Neal JL, Ryan-Wenger NA, et al. Accuracy of the clinical diagnosis of vaginitis compared with a DNA probe laboratory standard. Obstet Gynecol 2009; 113:89–95.
- Mulhem E, Boyanton BL Jr, Robinson-Dunn B, Ebert C, Dzebo R. Performance of the Affirm VP-III using residual vaginal discharge collected from the speculum to characterize vaginitis in symptomatic women. J Low Genit Tract Dis 2014; 18:344–346.
- Cartwright CP, Lembke BD, Ramachandran K, et al. Comparison of nucleic acid amplification assays with BD Affirm VPIII for diagnosis of vaginitis in symptomatic women. J Clin Microbiol 2013; 51:3694–3699.
- Workowski KA, Bolan GA; Centers for Disease Control and Prevention (CDC). Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 2015; 64:1–137.
- Sobel JD, Ferris D, Schwebke J, et al. Suppressive antibacterial therapy with 0.75% metronidazole vaginal gel to prevent recurrent bacterial vaginosis. Am J Obstet Gynecol 2006; 194:1283–1289.
- Reichman O, Akins R, Sobel JD. Boric acid addition to suppressive antimicrobial therapy for recurrent bacterial vaginosis. Sex Transm Dis 2009; 36:732–734.
- Carr PL, Felsenstein D, Friedman RH. Evaluation and management of vaginitis. J Gen Intern Med 1998; 13:335–346.
- Sobel JD, Chaim W, Nagappan V, Leaman D. Treatment of vaginitis caused by Candida glabrata: use of topical boric acid and flucytosine. Am J Obstet Gynecol 2003; 189:1297–1300.
- Sobel JD, Kapernick PS, Zervos M, et al. Treatment of complicated Candida vaginitis: comparison of single and sequential doses of fluconazole. Am J Obstet Gynecol 2001; 185:363–369.
- Sobel JD, Wiesenfeld HC, Martens M, et al. Maintenance fluconazole therapy for recurrent vulvovaginal candidiasis. N Engl J Med 2004; 351:876–883.
- Phillips AJ. Treatment of non-albicans Candida vaginitis with amphotericin B vaginal suppositories. Am J Obstet Gynecol 2005; 192:2009–2013.
- Weinstock H, Berman S, Cates W Jr. Sexually transmitted diseases among American youth: incidence and prevalence estimates, 2000. Perspect Sex Reprod Health 2004; 36:6–10.
- Gatski M, Martin DH, Clark RA, Harville E, Schmidt N, Kissinger P. Co-occurrence of Trichomonas vaginalis and bacterial vaginosis among HIV-positive women. Sex Transm Dis 2011; 38:163–166.
- Hobbs MM, Seña AC. Modern diagnosis of Trichomonas vaginalis infection. Sex Transm Infect 2013; 89:434–438.
- McClelland RS, Sangare L, Hassan WM, et al. Infection with Trichomonas vaginalis increases the risk of HIV-1 acquisition. J Infect Dis 2007; 195:698–702.
- Kingston MA, Bansal D, Carlin EM. ‘Shelf life’ of Trichomonas vaginalis. Int J STD AIDS 2003; 14:28–29.
- Aslan DL, Gulbahce HE, Stelow EB, et al. The diagnosis of Trichomonas vaginalis in liquid-based Pap tests: correlation with PCR. Diagn Cytopathol 2005; 32:341–344.
- Lara-Torre E, Pinkerton JS. Accuracy of detection of Trichomonas vaginalis organisms on a liquid-based papanicolaou smear. Am J Obstet Gynecol 2003; 188:354–356.
- Hobbs MM, Lapple DM, Lawing LF, et al. Methods for detection of Trichomonas vaginalis in the male partners of infected women: implications for control of trichomoniasis. J Clin Microbiol 2006; 44:3994–3999.
- Van Der Pol B, Williams JA, Orr DP, Batteiger BE, Fortenberry JD. Prevalence, incidence, natural history, and response to treatment of Trichomonas vaginalis infection among adolescent women. J Infect Dis 2005; 192:2039–2044.
- Campbell L, Woods V, Lloyd T, Elsayed S, Church DL. Evaluation of the OSOM Trichomonas rapid test versus wet preparation examination for detection of Trichomonas vaginalis vaginitis in specimens from women with a low prevalence of infection. J Clin Microbiol 2008; 46:3467–3469.
- Chapin K, Andrea S. APTIMA Trichomonas vaginalis, a transcription-mediated amplification assay for detection of Trichomonas vaginalis in urogenital specimens. Expert Rev Mol Diagn 2011; 11:679–688.
- Krashin JW, Koumans EH, Bradshaw-Sydnor AC, et al. Trichomonas vaginalis prevalence, incidence, risk factors and antibiotic-resistance in an adolescent population. Sex Transm Dis 2010; 37:440–444.
- Sobel JD, Nyirjesy P, Brown W. Tinidazole therapy for metronidazole-resistant vaginal trichomoniasis. Clin Infect Dis 2001; 33:1341–1346.
- Nyirjesy P, Gilbert J, Mulcahy LJ. Resistant trichomoniasis: successful treatment with combination therapy. Sex Transm Dis 2011; 38:962–963.
- Bradley H, Markowitz LE, Gibson T, McQuillan GM. Seroprevalence of herpes simplex virus types 1 and 2—United States, 1999–2010. J Infect Dis 2014; 209:325–333.
- Roberts CM, Pfister JR, Spear SJ. Increasing proportion of herpes simplex virus type 1 as a cause of genital herpes infection in college students. Sex Transm Dis 2003; 30:797–800.
- Ryder N, Jin F, McNulty AM, Grulich AE, Donovan B. Increasing role of herpes simplex virus type 1 in first-episode anogenital herpes in heterosexual women and younger men who have sex with men, 1992-2006. Sex Transm Infect 2009; 85:416–419.
- Caviness AC, Oelze LL, Saz UE, Greer JM, Demmler-Harrison GJ. Direct immunofluorescence assay compared to cell culture for the diagnosis of mucocutaneous herpes simplex virus infections in children. J Clin Virol 2010; 49:58–60.
- Morrow R, Friedrich D. Performance of a novel test for IgM and IgG antibodies in subjects with culture-documented genital herpes simplex virus-1 or -2 infection. Clin Microbiol Infect 2006; 12:463–469.
- Bradford J, Fischer G. Desquamative inflammatory vaginitis: differential diagnosis and alternate diagnostic criteria. J Low Genit Tract Dis 2010; 14:306–310.
KEY POINTS
- Typical presenting symptoms of vulvovaginitis are itching, burning, and abnormal discharge.
- Evaluating vaginal secretions with simple office-based tools is often sufficient for diagnosis, although DNA testing is also available.
- Depending on the cause, vulvovaginitis is generally treated with a course of oral or topical antibiotics, antiviral or antifungal drugs, anti-inflammatory agents, or hormonal therapy.
- Cases that do not resolve may require maintenance therapy. Patients who have persistent or unusual symptoms should be referred to a specialist.
Channeling the flow of medical information
New, changing technologies are being incorporated into every aspect of medical care and education, and the impact cannot be overstated. While the ultimate qualitative impact (beneficial, intrusive, efficient, obstructive, or neutral) of specific individual implementations remains to be seen, there is no doubt that the faces of patient care and the financial management of healthcare delivery have been forever altered.
The amount of information now literally at our fingertips is overwhelming. Some of my patients bring the latest from www.clinicaltrials.gov to their appointments. One patient recently brought downloaded online testimony from patients who were being given an oil supplement to treat disorders including gout, neuropathy, carpal tunnel, sinusitis, headache, and postop hip and knee pain and wanted to know why I hadn’t suggested it for her. Accessing the information pipeline is like drinking from a firehose, and there is no perfect valve that can adjust the flow to every thirst.
Lest we think that only patients use sites of variable reliability in getting their online medical information, in a recent survey by Kantar Media, when 508 physicians were asked which of 49 sites they looked at most often, Wikipedia came in at number 3 (UpToDate was number 1 and Cleveland Clinic Journal of Medicine was number 25). But when asked to rate the 49 sites for “quality clinical content,” responders listed Wikipedia as 47 (UpToDate was still number 1 and CCJM was number 6).
We healthcare providers can assess the accuracy and quality of clinical content. But it is not always easy, especially when trying to access, digest, and utilize information within the real-time constraints of an office visit or inpatient consultation. The now almost universal use of electronic medical records (EMRs) in major health systems provides access to true point-of-care information to assist in clinical decision-making, but how we filter and channel this information and apply it to the patient sitting in the exam room is not always straightforward. It is naïve to think that one source can fit all of our information needs.
A “smart” EMR can reflexively direct me to diagnosis-linked clinical guidelines or care paths. But without knowing the specifics of how the guidelines were written, I can’t know if they are ideally applicable to the patient in front of me. Guidelines based solely on clinical trial data may not be ideal for a given patient due to constraints of the clinical trial design and the tested clinical populations. There are times in areas outside my clinical field that I seek clinically nuanced expertise in interpreting clinical trials rather than the actual clinical trial data. Other times, when approaching problems within my own expertise, I want to see the raw data to reach a conclusion on its applicability and likely magnitude of effect in a specific patient. Using a trusted source of predigested, summarized data (as opposed to the raw data), knowing whether an author has a relationship with a specific company, and knowing that the clinical trials of a specific therapy are not intrinsically bad or good—all of these contribute to contextual decisions that lead to my clinical recommendations. I always want to know the nature of authors’ commercial relationships, as well as the track record of my information sources.
It is in this context that the paper by Andrews et al in this issue of the Journal addresses in a very practical way some of the nuances in using several popular point-of-care information resources. This is the second of 2 papers that these authors have contributed to the CCJM in an effort to inform and direct us how to quench our thirst for information without getting bloated.
New, changing technologies are being incorporated into every aspect of medical care and education, and the impact cannot be overstated. While the ultimate qualitative impact (beneficial, intrusive, efficient, obstructive, or neutral) of specific individual implementations remains to be seen, there is no doubt that the faces of patient care and the financial management of healthcare delivery have been forever altered.
The amount of information now literally at our fingertips is overwhelming. Some of my patients bring the latest from www.clinicaltrials.gov to their appointments. One patient recently brought downloaded online testimony from patients who were being given an oil supplement to treat disorders including gout, neuropathy, carpal tunnel, sinusitis, headache, and postop hip and knee pain and wanted to know why I hadn’t suggested it for her. Accessing the information pipeline is like drinking from a firehose, and there is no perfect valve that can adjust the flow to every thirst.
Lest we think that only patients use sites of variable reliability in getting their online medical information, in a recent survey by Kantar Media, when 508 physicians were asked which of 49 sites they looked at most often, Wikipedia came in at number 3 (UpToDate was number 1 and Cleveland Clinic Journal of Medicine was number 25). But when asked to rate the 49 sites for “quality clinical content,” responders listed Wikipedia as 47 (UpToDate was still number 1 and CCJM was number 6).
We healthcare providers can assess the accuracy and quality of clinical content. But it is not always easy, especially when trying to access, digest, and utilize information within the real-time constraints of an office visit or inpatient consultation. The now almost universal use of electronic medical records (EMRs) in major health systems provides access to true point-of-care information to assist in clinical decision-making, but how we filter and channel this information and apply it to the patient sitting in the exam room is not always straightforward. It is naïve to think that one source can fit all of our information needs.
A “smart” EMR can reflexively direct me to diagnosis-linked clinical guidelines or care paths. But without knowing the specifics of how the guidelines were written, I can’t know if they are ideally applicable to the patient in front of me. Guidelines based solely on clinical trial data may not be ideal for a given patient due to constraints of the clinical trial design and the tested clinical populations. There are times in areas outside my clinical field that I seek clinically nuanced expertise in interpreting clinical trials rather than the actual clinical trial data. Other times, when approaching problems within my own expertise, I want to see the raw data to reach a conclusion on its applicability and likely magnitude of effect in a specific patient. Using a trusted source of predigested, summarized data (as opposed to the raw data), knowing whether an author has a relationship with a specific company, and knowing that the clinical trials of a specific therapy are not intrinsically bad or good—all of these contribute to contextual decisions that lead to my clinical recommendations. I always want to know the nature of authors’ commercial relationships, as well as the track record of my information sources.
It is in this context that the paper by Andrews et al in this issue of the Journal addresses in a very practical way some of the nuances in using several popular point-of-care information resources. This is the second of 2 papers that these authors have contributed to the CCJM in an effort to inform and direct us how to quench our thirst for information without getting bloated.
New, changing technologies are being incorporated into every aspect of medical care and education, and the impact cannot be overstated. While the ultimate qualitative impact (beneficial, intrusive, efficient, obstructive, or neutral) of specific individual implementations remains to be seen, there is no doubt that the faces of patient care and the financial management of healthcare delivery have been forever altered.
The amount of information now literally at our fingertips is overwhelming. Some of my patients bring the latest from www.clinicaltrials.gov to their appointments. One patient recently brought downloaded online testimony from patients who were being given an oil supplement to treat disorders including gout, neuropathy, carpal tunnel, sinusitis, headache, and postop hip and knee pain and wanted to know why I hadn’t suggested it for her. Accessing the information pipeline is like drinking from a firehose, and there is no perfect valve that can adjust the flow to every thirst.
Lest we think that only patients use sites of variable reliability in getting their online medical information, in a recent survey by Kantar Media, when 508 physicians were asked which of 49 sites they looked at most often, Wikipedia came in at number 3 (UpToDate was number 1 and Cleveland Clinic Journal of Medicine was number 25). But when asked to rate the 49 sites for “quality clinical content,” responders listed Wikipedia as 47 (UpToDate was still number 1 and CCJM was number 6).
We healthcare providers can assess the accuracy and quality of clinical content. But it is not always easy, especially when trying to access, digest, and utilize information within the real-time constraints of an office visit or inpatient consultation. The now almost universal use of electronic medical records (EMRs) in major health systems provides access to true point-of-care information to assist in clinical decision-making, but how we filter and channel this information and apply it to the patient sitting in the exam room is not always straightforward. It is naïve to think that one source can fit all of our information needs.
A “smart” EMR can reflexively direct me to diagnosis-linked clinical guidelines or care paths. But without knowing the specifics of how the guidelines were written, I can’t know if they are ideally applicable to the patient in front of me. Guidelines based solely on clinical trial data may not be ideal for a given patient due to constraints of the clinical trial design and the tested clinical populations. There are times in areas outside my clinical field that I seek clinically nuanced expertise in interpreting clinical trials rather than the actual clinical trial data. Other times, when approaching problems within my own expertise, I want to see the raw data to reach a conclusion on its applicability and likely magnitude of effect in a specific patient. Using a trusted source of predigested, summarized data (as opposed to the raw data), knowing whether an author has a relationship with a specific company, and knowing that the clinical trials of a specific therapy are not intrinsically bad or good—all of these contribute to contextual decisions that lead to my clinical recommendations. I always want to know the nature of authors’ commercial relationships, as well as the track record of my information sources.
It is in this context that the paper by Andrews et al in this issue of the Journal addresses in a very practical way some of the nuances in using several popular point-of-care information resources. This is the second of 2 papers that these authors have contributed to the CCJM in an effort to inform and direct us how to quench our thirst for information without getting bloated.
Caring for international patients
To the Editor: We read with great interest the article by Drs. Cawcutt and Wilson on caring for international patients.1 They provide an overview of the challenges of delivering medical care for these patients (eg, cultural differences) and the likely benefits from such interactions (eg, gaining cultural knowledge). Having practiced medicine in 3 different continents and experienced working in various medical centers caring for international patients, we would like to offer a slightly different viewpoint.
First, gaining cultural knowledge should be regarded as a prerequisite for healthcare workers involved in the care of international patients, rather than the expected benefit and consequence of such encounters. Healthcare workers with some knowledge of an international patient’s culture are best able to serve that patient.2 Indeed, unless knowledge of cultural differences is obtained before such interactions, there is a significant risk of stereotyping by amplifying the sense of “otherness,” which is unfortunately too often mistaken for cultural sensitivity. The perception of the stereotypes and prejudices during the second stage of cultural adaptation (ie, irritation, hostility) often stems from the host’s lack of cultural knowledge. Table 1 of their article clearly reflects such risk: the authors have tried to exemplify the concepts they discussed through a number of real-life scenarios. But indeed some of those cases (eg, the man from Saudi Arabia) could be interpreted more as examples of stereotyping than cultural sensitivity.
Second, the authors do not mention requests by family members of international patients for nondisclosure of serious medical diagnoses, one we have frequently received from family members from different cultural backgrounds. These requests represent another challenge of caring for these patients as they counter our obligation for full disclosure and the patients’ right to know in order to be able to make informed decisions regarding their medical care.3
- Cawcutt KA, Wilson JW. Benefits and challenges of caring for international patients. Cleve Clin J Med 2016; 83:794–800.
- Martin DR. Challenges and opportunities in the care of international patients: clinical and health services issues for academic medical centers. Acad Med 2006;81:189–192.
- American Medical Association Code of Ethics. https://www.ama-assn.org/sites/default/files/media-browser/code-of-medical-ethics-chapter-2.pdf. Accessed November 28, 2016.
To the Editor: We read with great interest the article by Drs. Cawcutt and Wilson on caring for international patients.1 They provide an overview of the challenges of delivering medical care for these patients (eg, cultural differences) and the likely benefits from such interactions (eg, gaining cultural knowledge). Having practiced medicine in 3 different continents and experienced working in various medical centers caring for international patients, we would like to offer a slightly different viewpoint.
First, gaining cultural knowledge should be regarded as a prerequisite for healthcare workers involved in the care of international patients, rather than the expected benefit and consequence of such encounters. Healthcare workers with some knowledge of an international patient’s culture are best able to serve that patient.2 Indeed, unless knowledge of cultural differences is obtained before such interactions, there is a significant risk of stereotyping by amplifying the sense of “otherness,” which is unfortunately too often mistaken for cultural sensitivity. The perception of the stereotypes and prejudices during the second stage of cultural adaptation (ie, irritation, hostility) often stems from the host’s lack of cultural knowledge. Table 1 of their article clearly reflects such risk: the authors have tried to exemplify the concepts they discussed through a number of real-life scenarios. But indeed some of those cases (eg, the man from Saudi Arabia) could be interpreted more as examples of stereotyping than cultural sensitivity.
Second, the authors do not mention requests by family members of international patients for nondisclosure of serious medical diagnoses, one we have frequently received from family members from different cultural backgrounds. These requests represent another challenge of caring for these patients as they counter our obligation for full disclosure and the patients’ right to know in order to be able to make informed decisions regarding their medical care.3
To the Editor: We read with great interest the article by Drs. Cawcutt and Wilson on caring for international patients.1 They provide an overview of the challenges of delivering medical care for these patients (eg, cultural differences) and the likely benefits from such interactions (eg, gaining cultural knowledge). Having practiced medicine in 3 different continents and experienced working in various medical centers caring for international patients, we would like to offer a slightly different viewpoint.
First, gaining cultural knowledge should be regarded as a prerequisite for healthcare workers involved in the care of international patients, rather than the expected benefit and consequence of such encounters. Healthcare workers with some knowledge of an international patient’s culture are best able to serve that patient.2 Indeed, unless knowledge of cultural differences is obtained before such interactions, there is a significant risk of stereotyping by amplifying the sense of “otherness,” which is unfortunately too often mistaken for cultural sensitivity. The perception of the stereotypes and prejudices during the second stage of cultural adaptation (ie, irritation, hostility) often stems from the host’s lack of cultural knowledge. Table 1 of their article clearly reflects such risk: the authors have tried to exemplify the concepts they discussed through a number of real-life scenarios. But indeed some of those cases (eg, the man from Saudi Arabia) could be interpreted more as examples of stereotyping than cultural sensitivity.
Second, the authors do not mention requests by family members of international patients for nondisclosure of serious medical diagnoses, one we have frequently received from family members from different cultural backgrounds. These requests represent another challenge of caring for these patients as they counter our obligation for full disclosure and the patients’ right to know in order to be able to make informed decisions regarding their medical care.3
- Cawcutt KA, Wilson JW. Benefits and challenges of caring for international patients. Cleve Clin J Med 2016; 83:794–800.
- Martin DR. Challenges and opportunities in the care of international patients: clinical and health services issues for academic medical centers. Acad Med 2006;81:189–192.
- American Medical Association Code of Ethics. https://www.ama-assn.org/sites/default/files/media-browser/code-of-medical-ethics-chapter-2.pdf. Accessed November 28, 2016.
- Cawcutt KA, Wilson JW. Benefits and challenges of caring for international patients. Cleve Clin J Med 2016; 83:794–800.
- Martin DR. Challenges and opportunities in the care of international patients: clinical and health services issues for academic medical centers. Acad Med 2006;81:189–192.
- American Medical Association Code of Ethics. https://www.ama-assn.org/sites/default/files/media-browser/code-of-medical-ethics-chapter-2.pdf. Accessed November 28, 2016.
In reply: Caring for international patients
In Reply: We appreciate the comments, and we fully agree about the dangers of blurring sensitivity and stereotyping in medicine. We also recognize that health providers working around the world have distinct backgrounds and unique perspectives, which serve to enrich the discussion.
We agree that gaining cultural knowledge should be a prerequisite for healthcare workers. However, healthcare providers may not uniformly have the opportunity, time, or resources for this training. Additionally, providers working in large group practices including referral and academic medical centers often do not have control over scheduling of patient appointments. Therefore, rather than prohibiting the evaluations of international patients, we advocate for the utilization of a few guiding and common principles to optimize a mutually beneficial patient care experience. Despite inherent inadequacies and potential prejudices, healthcare providers do learn through patient encounters. Within this learning environment, mistakes will be made, but there are also opportunities for further self-improvement.
We agree there is a fine line between sensitivity and stereotyping, along with common misunderstandings regarding patient labeling. Identifying the geographic homeland of a patient could be misconstrued as intent to stereotype patients. However, numerous infectious diseases and many noncommunicable syndromes are disproportionately represented within select countries. Thus, we feel the identification of a patient’s homeland along with ethnicity, age, gender, and pertinent socioeconomic details can be done respectfully and remain an important collective part of the active medical history and serve to optimize care for each patient. Within medical education, we often find ourselves generalizing patient presentations and symptom profiles.
Yet we must recognize that the generalized concepts cannot apply to everyone. Medicine remains a profession of humility—both in our willingness to consider additional diagnoses and in our openness to care for patients of different backgrounds. With this humility, we hope to avoid the pitfalls of patient stereotyping, misjudgments, and misunderstandings.
Finally, the nondisclosure of serious medical diagnoses at the request of family members can be a tricky issue. It can be most difficult to balance unique wishes of a family with the ethics of accurate patient communication and compliance with legal statutes and medical center policies. We advocate a team approach with family members of international patients as a way to avoid breaches in medical ethics or breaks in mutual family trust.
In Reply: We appreciate the comments, and we fully agree about the dangers of blurring sensitivity and stereotyping in medicine. We also recognize that health providers working around the world have distinct backgrounds and unique perspectives, which serve to enrich the discussion.
We agree that gaining cultural knowledge should be a prerequisite for healthcare workers. However, healthcare providers may not uniformly have the opportunity, time, or resources for this training. Additionally, providers working in large group practices including referral and academic medical centers often do not have control over scheduling of patient appointments. Therefore, rather than prohibiting the evaluations of international patients, we advocate for the utilization of a few guiding and common principles to optimize a mutually beneficial patient care experience. Despite inherent inadequacies and potential prejudices, healthcare providers do learn through patient encounters. Within this learning environment, mistakes will be made, but there are also opportunities for further self-improvement.
We agree there is a fine line between sensitivity and stereotyping, along with common misunderstandings regarding patient labeling. Identifying the geographic homeland of a patient could be misconstrued as intent to stereotype patients. However, numerous infectious diseases and many noncommunicable syndromes are disproportionately represented within select countries. Thus, we feel the identification of a patient’s homeland along with ethnicity, age, gender, and pertinent socioeconomic details can be done respectfully and remain an important collective part of the active medical history and serve to optimize care for each patient. Within medical education, we often find ourselves generalizing patient presentations and symptom profiles.
Yet we must recognize that the generalized concepts cannot apply to everyone. Medicine remains a profession of humility—both in our willingness to consider additional diagnoses and in our openness to care for patients of different backgrounds. With this humility, we hope to avoid the pitfalls of patient stereotyping, misjudgments, and misunderstandings.
Finally, the nondisclosure of serious medical diagnoses at the request of family members can be a tricky issue. It can be most difficult to balance unique wishes of a family with the ethics of accurate patient communication and compliance with legal statutes and medical center policies. We advocate a team approach with family members of international patients as a way to avoid breaches in medical ethics or breaks in mutual family trust.
In Reply: We appreciate the comments, and we fully agree about the dangers of blurring sensitivity and stereotyping in medicine. We also recognize that health providers working around the world have distinct backgrounds and unique perspectives, which serve to enrich the discussion.
We agree that gaining cultural knowledge should be a prerequisite for healthcare workers. However, healthcare providers may not uniformly have the opportunity, time, or resources for this training. Additionally, providers working in large group practices including referral and academic medical centers often do not have control over scheduling of patient appointments. Therefore, rather than prohibiting the evaluations of international patients, we advocate for the utilization of a few guiding and common principles to optimize a mutually beneficial patient care experience. Despite inherent inadequacies and potential prejudices, healthcare providers do learn through patient encounters. Within this learning environment, mistakes will be made, but there are also opportunities for further self-improvement.
We agree there is a fine line between sensitivity and stereotyping, along with common misunderstandings regarding patient labeling. Identifying the geographic homeland of a patient could be misconstrued as intent to stereotype patients. However, numerous infectious diseases and many noncommunicable syndromes are disproportionately represented within select countries. Thus, we feel the identification of a patient’s homeland along with ethnicity, age, gender, and pertinent socioeconomic details can be done respectfully and remain an important collective part of the active medical history and serve to optimize care for each patient. Within medical education, we often find ourselves generalizing patient presentations and symptom profiles.
Yet we must recognize that the generalized concepts cannot apply to everyone. Medicine remains a profession of humility—both in our willingness to consider additional diagnoses and in our openness to care for patients of different backgrounds. With this humility, we hope to avoid the pitfalls of patient stereotyping, misjudgments, and misunderstandings.
Finally, the nondisclosure of serious medical diagnoses at the request of family members can be a tricky issue. It can be most difficult to balance unique wishes of a family with the ethics of accurate patient communication and compliance with legal statutes and medical center policies. We advocate a team approach with family members of international patients as a way to avoid breaches in medical ethics or breaks in mutual family trust.
Acid-base disturbances
To the Editor: In their article “A patient with altered mental status and an acid-base disturbance,”1 Drs. Shylaja Mani and Gregory W. Rutecki state that 5-oxoproline or pyroglutamic acidosis is associated with an elevated osmol gap. This is not the case. The cited reference by Tan et al2 describes a patient who most likely had ketoacidosis, perhaps complicated by isopropyl alcohol ingestion.
Those disorders can certainly generate an osmol gap. Although pyroglutamic acidosis was mentioned in the differential diagnosis of that case, that condition was never documented. The accumulation of 5-oxoproline or pyroglutamic acid should not elevate the serum osmolality or generate an osmol gap.
- Mani S, Rutecki GW. A patient with altered mental status and an acid-base disturbance. Cleve Clin J Med 2017; 84:27–34.
- Tan EM, Kalimullah E, Sohail MR, Ramar K. Diagnostic challenge in a patient with severe anion gap metabolic acidosis. Case Rep Crit Care 2015; 2015:272914.
To the Editor: In their article “A patient with altered mental status and an acid-base disturbance,”1 Drs. Shylaja Mani and Gregory W. Rutecki state that 5-oxoproline or pyroglutamic acidosis is associated with an elevated osmol gap. This is not the case. The cited reference by Tan et al2 describes a patient who most likely had ketoacidosis, perhaps complicated by isopropyl alcohol ingestion.
Those disorders can certainly generate an osmol gap. Although pyroglutamic acidosis was mentioned in the differential diagnosis of that case, that condition was never documented. The accumulation of 5-oxoproline or pyroglutamic acid should not elevate the serum osmolality or generate an osmol gap.
To the Editor: In their article “A patient with altered mental status and an acid-base disturbance,”1 Drs. Shylaja Mani and Gregory W. Rutecki state that 5-oxoproline or pyroglutamic acidosis is associated with an elevated osmol gap. This is not the case. The cited reference by Tan et al2 describes a patient who most likely had ketoacidosis, perhaps complicated by isopropyl alcohol ingestion.
Those disorders can certainly generate an osmol gap. Although pyroglutamic acidosis was mentioned in the differential diagnosis of that case, that condition was never documented. The accumulation of 5-oxoproline or pyroglutamic acid should not elevate the serum osmolality or generate an osmol gap.
- Mani S, Rutecki GW. A patient with altered mental status and an acid-base disturbance. Cleve Clin J Med 2017; 84:27–34.
- Tan EM, Kalimullah E, Sohail MR, Ramar K. Diagnostic challenge in a patient with severe anion gap metabolic acidosis. Case Rep Crit Care 2015; 2015:272914.
- Mani S, Rutecki GW. A patient with altered mental status and an acid-base disturbance. Cleve Clin J Med 2017; 84:27–34.
- Tan EM, Kalimullah E, Sohail MR, Ramar K. Diagnostic challenge in a patient with severe anion gap metabolic acidosis. Case Rep Crit Care 2015; 2015:272914.
In reply: Acid-base disturbances
In Reply: We thank Dr. Emmett for his insightful comment. He is correct that in the case reported by Tan et al the elevated osmol gap was not a direct result of the patient’s presumed acetaminophen ingestion but more likely another unidentified toxic ingestion. The online version of our article has been modified accordingly (also see page 214 of this issue).
In Reply: We thank Dr. Emmett for his insightful comment. He is correct that in the case reported by Tan et al the elevated osmol gap was not a direct result of the patient’s presumed acetaminophen ingestion but more likely another unidentified toxic ingestion. The online version of our article has been modified accordingly (also see page 214 of this issue).
In Reply: We thank Dr. Emmett for his insightful comment. He is correct that in the case reported by Tan et al the elevated osmol gap was not a direct result of the patient’s presumed acetaminophen ingestion but more likely another unidentified toxic ingestion. The online version of our article has been modified accordingly (also see page 214 of this issue).
Correction: Altered mental status and an acid-base disturbance
In the article “A patient with altered mental status and an acid-base disturbance” (Mani S, Rutecki GW, Cleve Clin J Med 2017; 84:27–34), 2 errors occurred in Table 2. The corrected table appears with corrections shown in red:
In addition, two sentences in the text regarding the osmol gap should be revised as follows:
On page 31, the last 3 lines should read as follows: “When the anion gap metabolic acidosis is multifactorial, as it was suspected to be in a case reported by Tan et al,23 the osmol gap may be elevated as a consequence of additional toxic ingestions, as it was in the reported patient.”
And on page 33, the last sentence should read as follows: “As reflected in the revisions to MUD PILES and in the newer GOLD MARK acronym, the osmol gap has become more valuable in differential diagnosis of metabolic acidosis with an elevated anion gap consequent to an expanding array of toxic ingestions (methanol, propylene glycol, ethylene glycol, and diethylene glycol), which may accompany pyroglutamic acid-oxoproline.”
In the article “A patient with altered mental status and an acid-base disturbance” (Mani S, Rutecki GW, Cleve Clin J Med 2017; 84:27–34), 2 errors occurred in Table 2. The corrected table appears with corrections shown in red:
In addition, two sentences in the text regarding the osmol gap should be revised as follows:
On page 31, the last 3 lines should read as follows: “When the anion gap metabolic acidosis is multifactorial, as it was suspected to be in a case reported by Tan et al,23 the osmol gap may be elevated as a consequence of additional toxic ingestions, as it was in the reported patient.”
And on page 33, the last sentence should read as follows: “As reflected in the revisions to MUD PILES and in the newer GOLD MARK acronym, the osmol gap has become more valuable in differential diagnosis of metabolic acidosis with an elevated anion gap consequent to an expanding array of toxic ingestions (methanol, propylene glycol, ethylene glycol, and diethylene glycol), which may accompany pyroglutamic acid-oxoproline.”
In the article “A patient with altered mental status and an acid-base disturbance” (Mani S, Rutecki GW, Cleve Clin J Med 2017; 84:27–34), 2 errors occurred in Table 2. The corrected table appears with corrections shown in red:
In addition, two sentences in the text regarding the osmol gap should be revised as follows:
On page 31, the last 3 lines should read as follows: “When the anion gap metabolic acidosis is multifactorial, as it was suspected to be in a case reported by Tan et al,23 the osmol gap may be elevated as a consequence of additional toxic ingestions, as it was in the reported patient.”
And on page 33, the last sentence should read as follows: “As reflected in the revisions to MUD PILES and in the newer GOLD MARK acronym, the osmol gap has become more valuable in differential diagnosis of metabolic acidosis with an elevated anion gap consequent to an expanding array of toxic ingestions (methanol, propylene glycol, ethylene glycol, and diethylene glycol), which may accompany pyroglutamic acid-oxoproline.”