User login
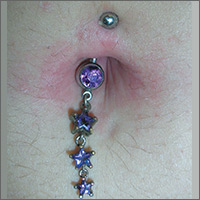
Rash around belly button
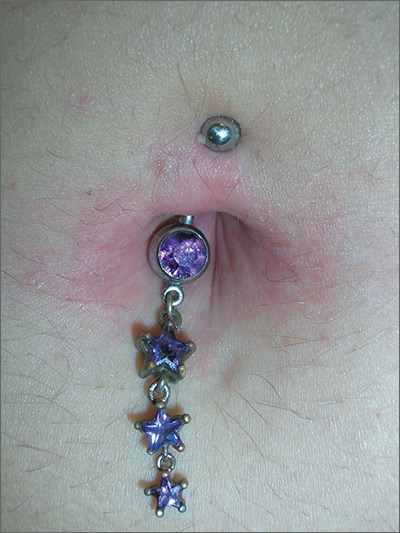
The family physician (FP) recognized that the patient’s new belly ring was the cause of this case of contact dermatitis. The FP suspected that the belly ring contained nickel—a common culprit in cases of allergic contact dermatitis (ACD).
ACD is a delayed-type hypersensitivity reaction that occurs when skin proteins form an antigen complex in reaction to a foreign substance. Upon reexposure of the epidermis to the antigen, the sensitized T cells initiate an inflammatory cascade, leading to the skin changes seen in ACD.
The FP told the patient that patch testing could be used to confirm her allergy, but it required wearing patches on her back for 3 days and involved 3 office visits to complete the testing. He also asked her if she wanted to have her jewelry tested for nickel content.
The patient removed the belly button jewelry and the FP used a nickel testing kit, which showed the jewelry was, in fact, positive for nickel. The patient asked if she could still wear the jewelry if she got medication to treat the allergy, but the FP explained that it was unlikely that the rash would go away completely with a topical cream if the jewelry remained in place. The FP prescribed 0.1% triamcinolone cream to be applied twice daily to the area. He also suggested that she look for a jewelry replacement that was nickel-free.
At a one-month follow-up visit, the patient acknowledged that the FP had been right: While she was able to wear the jewelry for another 2 weeks with decreased erythema and pruritus while using the triamcinolone cream, the rash never went away. The patient switched to a nickel-free belly button ring and her rash cleared completely.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Usatine R. Contact dermatitis. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:591-596.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com

The family physician (FP) recognized that the patient’s new belly ring was the cause of this case of contact dermatitis. The FP suspected that the belly ring contained nickel—a common culprit in cases of allergic contact dermatitis (ACD).
ACD is a delayed-type hypersensitivity reaction that occurs when skin proteins form an antigen complex in reaction to a foreign substance. Upon reexposure of the epidermis to the antigen, the sensitized T cells initiate an inflammatory cascade, leading to the skin changes seen in ACD.
The FP told the patient that patch testing could be used to confirm her allergy, but it required wearing patches on her back for 3 days and involved 3 office visits to complete the testing. He also asked her if she wanted to have her jewelry tested for nickel content.
The patient removed the belly button jewelry and the FP used a nickel testing kit, which showed the jewelry was, in fact, positive for nickel. The patient asked if she could still wear the jewelry if she got medication to treat the allergy, but the FP explained that it was unlikely that the rash would go away completely with a topical cream if the jewelry remained in place. The FP prescribed 0.1% triamcinolone cream to be applied twice daily to the area. He also suggested that she look for a jewelry replacement that was nickel-free.
At a one-month follow-up visit, the patient acknowledged that the FP had been right: While she was able to wear the jewelry for another 2 weeks with decreased erythema and pruritus while using the triamcinolone cream, the rash never went away. The patient switched to a nickel-free belly button ring and her rash cleared completely.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Usatine R. Contact dermatitis. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:591-596.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com

The family physician (FP) recognized that the patient’s new belly ring was the cause of this case of contact dermatitis. The FP suspected that the belly ring contained nickel—a common culprit in cases of allergic contact dermatitis (ACD).
ACD is a delayed-type hypersensitivity reaction that occurs when skin proteins form an antigen complex in reaction to a foreign substance. Upon reexposure of the epidermis to the antigen, the sensitized T cells initiate an inflammatory cascade, leading to the skin changes seen in ACD.
The FP told the patient that patch testing could be used to confirm her allergy, but it required wearing patches on her back for 3 days and involved 3 office visits to complete the testing. He also asked her if she wanted to have her jewelry tested for nickel content.
The patient removed the belly button jewelry and the FP used a nickel testing kit, which showed the jewelry was, in fact, positive for nickel. The patient asked if she could still wear the jewelry if she got medication to treat the allergy, but the FP explained that it was unlikely that the rash would go away completely with a topical cream if the jewelry remained in place. The FP prescribed 0.1% triamcinolone cream to be applied twice daily to the area. He also suggested that she look for a jewelry replacement that was nickel-free.
At a one-month follow-up visit, the patient acknowledged that the FP had been right: While she was able to wear the jewelry for another 2 weeks with decreased erythema and pruritus while using the triamcinolone cream, the rash never went away. The patient switched to a nickel-free belly button ring and her rash cleared completely.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Usatine R. Contact dermatitis. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:591-596.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
APA task force urges caution in off-label use of ketamine for mood disorders
A new consensus statement on the use of ketamine as an off-label treatment for mood disorders provides psychiatrists some guidance for patient selection and evaluation, as well as dosing and duration of therapy. The statement was issued against the backdrop of newer studies showing ketamine’s rapid, though not durable, efficacy in treating depression and anxiety disorders.
From start to end, however, the statement urges caution and marks out areas for further study, citing a paucity of data on longer-term efficacy and safety and a literature largely made up of smaller studies.
Dr. Sanacora, professor of psychiatry at Yale University, New Haven, Conn., and his coauthors made clear that “this report is not intended to serve as a standard, guideline, clinical policy, or absolute requirement.” Rather, the document identifies the current state of the field and identifies factors to consider in determining the appropriateness of ketamine therapy.
When a clinician is considering giving ketamine to a patient, a preprocedural evaluation is recommended, both to assess whether ketamine is likely to benefit the patient and to evaluate potential risks. Recommended components include a comprehensive psychiatric diagnostic assessment, paying particular attention to substance use and psychosis history, if any; assessment of baseline symptom severity; history of prior antidepressant treatment; review of systems to assess risk factors; physical examination and laboratory screening that follows accepted guidelines for the anticipated level of sedation, with the recommended addition of a baseline urine toxicology screen; and records review and family corroboration with a particular eye to past substance use.
Finally, the informed consent process should ensure that the patient is aware not only of potential treatment risks, but also of the relatively limited data on the off-label use of ketamine for mood disorders. Thorough written documentation is recommended.
Regarding the appropriate amount of experience and training a clinician should have before administering ketamine, Dr. Sanacora and his coauthors noted that the subanesthetic doses of ketamine that are used to treat mood disorders have been found generally very safe in the limited studies that have been done. Typical peak plasma concentrations will not induce general anesthesia. However, systolic and diastolic blood pressure and heart rate elevations are not uncommon. The statement cites a study that found “blood pressure levels exceeding 180/100 mm Hg or heart rates exceeding 110 beats per minute in approximately 30% of the patients treated.”
Accordingly, the statement recommends that clinicians administering ketamine have Advanced Cardiac Life Support certification and be able to administer Drug Enforcement Administration Schedule III medications. Individual organizations, according to the statement, should follow accepted standards of practice for granting privileges to administer ketamine. A statement developed by the American Society of Anesthesiologists on granting privileges for conscious sedation to nonanesthesia professionals may be used for guidance.
The most-studied dose of ketamine in the treatment of mood disorders is 0.5 mg/kg, administered intravenously over 40 minutes. Though other routes, and lower doses, have been studied, Dr. Sanacora and his colleagues wrote that they consider that “insufficient benefit was provided in those studies to allow any meaningful analysis of any specific dose or route of treatment compared with the standard dose.”
The statement includes a strong recommendation to develop a site-specific–standard operating procedure for the administration of ketamine that incorporates the preprocedural evaluation. In addition, the procedure should assess baseline vital signs, include criteria for acceptable vital signs, and incorporate a “time-out” procedure before initiating ketamine treatment.
Also, standard operating procedures should include continued assessment of respiratory, cardiovascular, and mental status throughout the infusion. Stopping criteria should be clearly defined, as should a plan aimed at managing any cardiovascular or behavioral problems. Discharge procedures should include documentation of vital signs and mental status, ensuring that an adult caregiver can take the patient home, and a thorough review of discharge instructions.
The statement’s section on follow-up and assessments noted: “The existing data surrounding the benefits of repeated infusions of ketamine remain limited.” However, some studies have shown that twice-weekly infusions were as efficacious as infusions given three times a week. In terms of longer-term treatment, the data are sparse, wrote Dr. Sanacora and his coauthors. Some clinics, they said, are giving ketamine two or three times weekly for 2 or 3 weeks, then tapering or adjusting the regimen based on clinical response, but high-quality studies are lacking.” The scarcity of this information is one of the major drawbacks to be considered before initiating ketamine therapy for patients with mood disorders and should be discussed with the patient before beginning treatment,” they wrote.
The known risks of cognitive impairment and cystitis with more chronic high-frequency ketamine use, taken together with the significant potential for abuse, mean that ketamine should be discontinued if more than once-weekly dosing is required after 2 months of treatment. “The goal remains to eventually taper and discontinue treatment until more long-term safety data can be collected,” wrote Dr. Sanacora and his coauthors.
The statement, while acknowledging the hope and excitement that currently surround the use of ketamine for mood disorders, calls for further research that would include a patient registry and coordinated data collection to facilitate answering the many important questions remaining about ketamine’s efficacy and safety.
The report was neither endorsed nor promulgated as policy of the APA. Dr. Sanacora reported multiple relationships with pharmaceutical companies, as did all of his coauthors.
[email protected]
On Twitter @karioakes
A clear need exists for better treatments for the up to one-third of patients with major depression who do not respond to currently available therapies. Ketamine, which can produce rapid antidepressant effects, holds promise for this population and is increasingly being used off-label.
However, clinical guidance is lacking, and the small studies done to date leave many knowledge gaps. Though ketamine is known to be an N-methyl-D-aspartate (NMDA) glutamate receptor antagonist, rodent studies show that its action, or perhaps that of its metabolites, on another glutamate pathway may primarily underlie its antidepressant effects. Also, researchers are trialling adjunctive agents such as clonidine to help mute the undesirable psychomimetic and cardiorespiratory effects of ketamine. As the science rapidly develops, more targeted rapid treatments may become available, an evolution that could fundamentally change many aspects of the practice of psychiatry.
Uncertainty created by the current limitations in knowledge permeates the consensus statement and appropriately so. The authors provide clinical guidance based on what data are available but call for caution and transparency when treating mood disorder patients with ketamine. Gaps in data can be ameliorated by the formation of a patient registry for coordinated data collection and safety monitoring.
Charles F. Zorumski, MD, and Charles R. Conway, MD, are professors of psychiatry at Washington University in St. Louis, where Dr. Zorumski also holds an appointment in the department of neuroscience. Dr. Zorumski reported serving on the scientific advisory board of Sage Therapeutics, which also has funded research conducted by both authors. Dr. Conway reported serving as an unpaid consultant to LivaNova. These remarks were drawn from invited commentary accompanying the consensus statement.
A clear need exists for better treatments for the up to one-third of patients with major depression who do not respond to currently available therapies. Ketamine, which can produce rapid antidepressant effects, holds promise for this population and is increasingly being used off-label.
However, clinical guidance is lacking, and the small studies done to date leave many knowledge gaps. Though ketamine is known to be an N-methyl-D-aspartate (NMDA) glutamate receptor antagonist, rodent studies show that its action, or perhaps that of its metabolites, on another glutamate pathway may primarily underlie its antidepressant effects. Also, researchers are trialling adjunctive agents such as clonidine to help mute the undesirable psychomimetic and cardiorespiratory effects of ketamine. As the science rapidly develops, more targeted rapid treatments may become available, an evolution that could fundamentally change many aspects of the practice of psychiatry.
Uncertainty created by the current limitations in knowledge permeates the consensus statement and appropriately so. The authors provide clinical guidance based on what data are available but call for caution and transparency when treating mood disorder patients with ketamine. Gaps in data can be ameliorated by the formation of a patient registry for coordinated data collection and safety monitoring.
Charles F. Zorumski, MD, and Charles R. Conway, MD, are professors of psychiatry at Washington University in St. Louis, where Dr. Zorumski also holds an appointment in the department of neuroscience. Dr. Zorumski reported serving on the scientific advisory board of Sage Therapeutics, which also has funded research conducted by both authors. Dr. Conway reported serving as an unpaid consultant to LivaNova. These remarks were drawn from invited commentary accompanying the consensus statement.
A clear need exists for better treatments for the up to one-third of patients with major depression who do not respond to currently available therapies. Ketamine, which can produce rapid antidepressant effects, holds promise for this population and is increasingly being used off-label.
However, clinical guidance is lacking, and the small studies done to date leave many knowledge gaps. Though ketamine is known to be an N-methyl-D-aspartate (NMDA) glutamate receptor antagonist, rodent studies show that its action, or perhaps that of its metabolites, on another glutamate pathway may primarily underlie its antidepressant effects. Also, researchers are trialling adjunctive agents such as clonidine to help mute the undesirable psychomimetic and cardiorespiratory effects of ketamine. As the science rapidly develops, more targeted rapid treatments may become available, an evolution that could fundamentally change many aspects of the practice of psychiatry.
Uncertainty created by the current limitations in knowledge permeates the consensus statement and appropriately so. The authors provide clinical guidance based on what data are available but call for caution and transparency when treating mood disorder patients with ketamine. Gaps in data can be ameliorated by the formation of a patient registry for coordinated data collection and safety monitoring.
Charles F. Zorumski, MD, and Charles R. Conway, MD, are professors of psychiatry at Washington University in St. Louis, where Dr. Zorumski also holds an appointment in the department of neuroscience. Dr. Zorumski reported serving on the scientific advisory board of Sage Therapeutics, which also has funded research conducted by both authors. Dr. Conway reported serving as an unpaid consultant to LivaNova. These remarks were drawn from invited commentary accompanying the consensus statement.
A new consensus statement on the use of ketamine as an off-label treatment for mood disorders provides psychiatrists some guidance for patient selection and evaluation, as well as dosing and duration of therapy. The statement was issued against the backdrop of newer studies showing ketamine’s rapid, though not durable, efficacy in treating depression and anxiety disorders.
From start to end, however, the statement urges caution and marks out areas for further study, citing a paucity of data on longer-term efficacy and safety and a literature largely made up of smaller studies.
Dr. Sanacora, professor of psychiatry at Yale University, New Haven, Conn., and his coauthors made clear that “this report is not intended to serve as a standard, guideline, clinical policy, or absolute requirement.” Rather, the document identifies the current state of the field and identifies factors to consider in determining the appropriateness of ketamine therapy.
When a clinician is considering giving ketamine to a patient, a preprocedural evaluation is recommended, both to assess whether ketamine is likely to benefit the patient and to evaluate potential risks. Recommended components include a comprehensive psychiatric diagnostic assessment, paying particular attention to substance use and psychosis history, if any; assessment of baseline symptom severity; history of prior antidepressant treatment; review of systems to assess risk factors; physical examination and laboratory screening that follows accepted guidelines for the anticipated level of sedation, with the recommended addition of a baseline urine toxicology screen; and records review and family corroboration with a particular eye to past substance use.
Finally, the informed consent process should ensure that the patient is aware not only of potential treatment risks, but also of the relatively limited data on the off-label use of ketamine for mood disorders. Thorough written documentation is recommended.
Regarding the appropriate amount of experience and training a clinician should have before administering ketamine, Dr. Sanacora and his coauthors noted that the subanesthetic doses of ketamine that are used to treat mood disorders have been found generally very safe in the limited studies that have been done. Typical peak plasma concentrations will not induce general anesthesia. However, systolic and diastolic blood pressure and heart rate elevations are not uncommon. The statement cites a study that found “blood pressure levels exceeding 180/100 mm Hg or heart rates exceeding 110 beats per minute in approximately 30% of the patients treated.”
Accordingly, the statement recommends that clinicians administering ketamine have Advanced Cardiac Life Support certification and be able to administer Drug Enforcement Administration Schedule III medications. Individual organizations, according to the statement, should follow accepted standards of practice for granting privileges to administer ketamine. A statement developed by the American Society of Anesthesiologists on granting privileges for conscious sedation to nonanesthesia professionals may be used for guidance.
The most-studied dose of ketamine in the treatment of mood disorders is 0.5 mg/kg, administered intravenously over 40 minutes. Though other routes, and lower doses, have been studied, Dr. Sanacora and his colleagues wrote that they consider that “insufficient benefit was provided in those studies to allow any meaningful analysis of any specific dose or route of treatment compared with the standard dose.”
The statement includes a strong recommendation to develop a site-specific–standard operating procedure for the administration of ketamine that incorporates the preprocedural evaluation. In addition, the procedure should assess baseline vital signs, include criteria for acceptable vital signs, and incorporate a “time-out” procedure before initiating ketamine treatment.
Also, standard operating procedures should include continued assessment of respiratory, cardiovascular, and mental status throughout the infusion. Stopping criteria should be clearly defined, as should a plan aimed at managing any cardiovascular or behavioral problems. Discharge procedures should include documentation of vital signs and mental status, ensuring that an adult caregiver can take the patient home, and a thorough review of discharge instructions.
The statement’s section on follow-up and assessments noted: “The existing data surrounding the benefits of repeated infusions of ketamine remain limited.” However, some studies have shown that twice-weekly infusions were as efficacious as infusions given three times a week. In terms of longer-term treatment, the data are sparse, wrote Dr. Sanacora and his coauthors. Some clinics, they said, are giving ketamine two or three times weekly for 2 or 3 weeks, then tapering or adjusting the regimen based on clinical response, but high-quality studies are lacking.” The scarcity of this information is one of the major drawbacks to be considered before initiating ketamine therapy for patients with mood disorders and should be discussed with the patient before beginning treatment,” they wrote.
The known risks of cognitive impairment and cystitis with more chronic high-frequency ketamine use, taken together with the significant potential for abuse, mean that ketamine should be discontinued if more than once-weekly dosing is required after 2 months of treatment. “The goal remains to eventually taper and discontinue treatment until more long-term safety data can be collected,” wrote Dr. Sanacora and his coauthors.
The statement, while acknowledging the hope and excitement that currently surround the use of ketamine for mood disorders, calls for further research that would include a patient registry and coordinated data collection to facilitate answering the many important questions remaining about ketamine’s efficacy and safety.
The report was neither endorsed nor promulgated as policy of the APA. Dr. Sanacora reported multiple relationships with pharmaceutical companies, as did all of his coauthors.
[email protected]
On Twitter @karioakes
A new consensus statement on the use of ketamine as an off-label treatment for mood disorders provides psychiatrists some guidance for patient selection and evaluation, as well as dosing and duration of therapy. The statement was issued against the backdrop of newer studies showing ketamine’s rapid, though not durable, efficacy in treating depression and anxiety disorders.
From start to end, however, the statement urges caution and marks out areas for further study, citing a paucity of data on longer-term efficacy and safety and a literature largely made up of smaller studies.
Dr. Sanacora, professor of psychiatry at Yale University, New Haven, Conn., and his coauthors made clear that “this report is not intended to serve as a standard, guideline, clinical policy, or absolute requirement.” Rather, the document identifies the current state of the field and identifies factors to consider in determining the appropriateness of ketamine therapy.
When a clinician is considering giving ketamine to a patient, a preprocedural evaluation is recommended, both to assess whether ketamine is likely to benefit the patient and to evaluate potential risks. Recommended components include a comprehensive psychiatric diagnostic assessment, paying particular attention to substance use and psychosis history, if any; assessment of baseline symptom severity; history of prior antidepressant treatment; review of systems to assess risk factors; physical examination and laboratory screening that follows accepted guidelines for the anticipated level of sedation, with the recommended addition of a baseline urine toxicology screen; and records review and family corroboration with a particular eye to past substance use.
Finally, the informed consent process should ensure that the patient is aware not only of potential treatment risks, but also of the relatively limited data on the off-label use of ketamine for mood disorders. Thorough written documentation is recommended.
Regarding the appropriate amount of experience and training a clinician should have before administering ketamine, Dr. Sanacora and his coauthors noted that the subanesthetic doses of ketamine that are used to treat mood disorders have been found generally very safe in the limited studies that have been done. Typical peak plasma concentrations will not induce general anesthesia. However, systolic and diastolic blood pressure and heart rate elevations are not uncommon. The statement cites a study that found “blood pressure levels exceeding 180/100 mm Hg or heart rates exceeding 110 beats per minute in approximately 30% of the patients treated.”
Accordingly, the statement recommends that clinicians administering ketamine have Advanced Cardiac Life Support certification and be able to administer Drug Enforcement Administration Schedule III medications. Individual organizations, according to the statement, should follow accepted standards of practice for granting privileges to administer ketamine. A statement developed by the American Society of Anesthesiologists on granting privileges for conscious sedation to nonanesthesia professionals may be used for guidance.
The most-studied dose of ketamine in the treatment of mood disorders is 0.5 mg/kg, administered intravenously over 40 minutes. Though other routes, and lower doses, have been studied, Dr. Sanacora and his colleagues wrote that they consider that “insufficient benefit was provided in those studies to allow any meaningful analysis of any specific dose or route of treatment compared with the standard dose.”
The statement includes a strong recommendation to develop a site-specific–standard operating procedure for the administration of ketamine that incorporates the preprocedural evaluation. In addition, the procedure should assess baseline vital signs, include criteria for acceptable vital signs, and incorporate a “time-out” procedure before initiating ketamine treatment.
Also, standard operating procedures should include continued assessment of respiratory, cardiovascular, and mental status throughout the infusion. Stopping criteria should be clearly defined, as should a plan aimed at managing any cardiovascular or behavioral problems. Discharge procedures should include documentation of vital signs and mental status, ensuring that an adult caregiver can take the patient home, and a thorough review of discharge instructions.
The statement’s section on follow-up and assessments noted: “The existing data surrounding the benefits of repeated infusions of ketamine remain limited.” However, some studies have shown that twice-weekly infusions were as efficacious as infusions given three times a week. In terms of longer-term treatment, the data are sparse, wrote Dr. Sanacora and his coauthors. Some clinics, they said, are giving ketamine two or three times weekly for 2 or 3 weeks, then tapering or adjusting the regimen based on clinical response, but high-quality studies are lacking.” The scarcity of this information is one of the major drawbacks to be considered before initiating ketamine therapy for patients with mood disorders and should be discussed with the patient before beginning treatment,” they wrote.
The known risks of cognitive impairment and cystitis with more chronic high-frequency ketamine use, taken together with the significant potential for abuse, mean that ketamine should be discontinued if more than once-weekly dosing is required after 2 months of treatment. “The goal remains to eventually taper and discontinue treatment until more long-term safety data can be collected,” wrote Dr. Sanacora and his coauthors.
The statement, while acknowledging the hope and excitement that currently surround the use of ketamine for mood disorders, calls for further research that would include a patient registry and coordinated data collection to facilitate answering the many important questions remaining about ketamine’s efficacy and safety.
The report was neither endorsed nor promulgated as policy of the APA. Dr. Sanacora reported multiple relationships with pharmaceutical companies, as did all of his coauthors.
[email protected]
On Twitter @karioakes
FROM JAMA PSYCHIATRY
CLL boost in PFS came at high cost in side effects
Add-on idelalisib boosted the progression-free survival (PFS) of patients with relapsed and refractory chronic lymphocytic leukemia by over 9 months in a double-blind, placebo-controlled trial of 416 participants. But that improvement came at the price of a 4% absolute increase in treatment-emergent adverse events leading to death.
At a median follow-up of 14 months, the median PFS was 20.8 months in the 207 patients in the idelalisib group and 11.1 months in the 209 patients in the placebo group. However, 11% of patients in the idelalisib group and 7% of those in the placebo group had treatment-related adverse events that resulted in death.
Overall, 68% of those in the idelalisib group, and 44% in the placebo group, had serious adverse events that included febrile neutropenia, pneumonia, and pyrexia. Six deaths resulted from infections in the idelalisib group, and three deaths resulted from infections in the placebo group.
All 416 participants in the international, multicenter study had measurable lymphadenopathy by CT or MRI and progression of chronic lymphocytic leukemia within 36 months of their last therapy. Patients were stratified by high-risk genetic mutations (IGHV, del[17p], or TP53) and refractory versus relapsed disease. Participants were randomly assigned to receive daily idelalisib or placebo, plus a maximum of six cycles of bendamustine and rituximab.
The primary endpoint of PFS was assessed by an independent review committee in the intention-to-treat population.
A 150 mg dose of idelalisib or placebo was given twice daily until disease progressed or drug-related toxicity was intolerable. Bendamustine was given intravenously at 70 mg/m2 on days 1 and 2 for six 28-day cycles. Rituximab was given at 375 mg/m2 on day 1 of cycle 1 and at 500 mg/m2 on day 1 of cycles 2–6.
The trial is ongoing, the researchers reported.
The trial is funded by Gilead Sciences, the maker of idelalisib (Zydelig). Dr. Zelenetz disclosed consulting with multiple drug companies, including Gilead.
[email protected]
On Twitter @maryjodales
Add-on idelalisib boosted the progression-free survival (PFS) of patients with relapsed and refractory chronic lymphocytic leukemia by over 9 months in a double-blind, placebo-controlled trial of 416 participants. But that improvement came at the price of a 4% absolute increase in treatment-emergent adverse events leading to death.
At a median follow-up of 14 months, the median PFS was 20.8 months in the 207 patients in the idelalisib group and 11.1 months in the 209 patients in the placebo group. However, 11% of patients in the idelalisib group and 7% of those in the placebo group had treatment-related adverse events that resulted in death.
Overall, 68% of those in the idelalisib group, and 44% in the placebo group, had serious adverse events that included febrile neutropenia, pneumonia, and pyrexia. Six deaths resulted from infections in the idelalisib group, and three deaths resulted from infections in the placebo group.
All 416 participants in the international, multicenter study had measurable lymphadenopathy by CT or MRI and progression of chronic lymphocytic leukemia within 36 months of their last therapy. Patients were stratified by high-risk genetic mutations (IGHV, del[17p], or TP53) and refractory versus relapsed disease. Participants were randomly assigned to receive daily idelalisib or placebo, plus a maximum of six cycles of bendamustine and rituximab.
The primary endpoint of PFS was assessed by an independent review committee in the intention-to-treat population.
A 150 mg dose of idelalisib or placebo was given twice daily until disease progressed or drug-related toxicity was intolerable. Bendamustine was given intravenously at 70 mg/m2 on days 1 and 2 for six 28-day cycles. Rituximab was given at 375 mg/m2 on day 1 of cycle 1 and at 500 mg/m2 on day 1 of cycles 2–6.
The trial is ongoing, the researchers reported.
The trial is funded by Gilead Sciences, the maker of idelalisib (Zydelig). Dr. Zelenetz disclosed consulting with multiple drug companies, including Gilead.
[email protected]
On Twitter @maryjodales
Add-on idelalisib boosted the progression-free survival (PFS) of patients with relapsed and refractory chronic lymphocytic leukemia by over 9 months in a double-blind, placebo-controlled trial of 416 participants. But that improvement came at the price of a 4% absolute increase in treatment-emergent adverse events leading to death.
At a median follow-up of 14 months, the median PFS was 20.8 months in the 207 patients in the idelalisib group and 11.1 months in the 209 patients in the placebo group. However, 11% of patients in the idelalisib group and 7% of those in the placebo group had treatment-related adverse events that resulted in death.
Overall, 68% of those in the idelalisib group, and 44% in the placebo group, had serious adverse events that included febrile neutropenia, pneumonia, and pyrexia. Six deaths resulted from infections in the idelalisib group, and three deaths resulted from infections in the placebo group.
All 416 participants in the international, multicenter study had measurable lymphadenopathy by CT or MRI and progression of chronic lymphocytic leukemia within 36 months of their last therapy. Patients were stratified by high-risk genetic mutations (IGHV, del[17p], or TP53) and refractory versus relapsed disease. Participants were randomly assigned to receive daily idelalisib or placebo, plus a maximum of six cycles of bendamustine and rituximab.
The primary endpoint of PFS was assessed by an independent review committee in the intention-to-treat population.
A 150 mg dose of idelalisib or placebo was given twice daily until disease progressed or drug-related toxicity was intolerable. Bendamustine was given intravenously at 70 mg/m2 on days 1 and 2 for six 28-day cycles. Rituximab was given at 375 mg/m2 on day 1 of cycle 1 and at 500 mg/m2 on day 1 of cycles 2–6.
The trial is ongoing, the researchers reported.
The trial is funded by Gilead Sciences, the maker of idelalisib (Zydelig). Dr. Zelenetz disclosed consulting with multiple drug companies, including Gilead.
[email protected]
On Twitter @maryjodales
Key clinical point:
Major finding: Add-on idelalisib boosted the progression-free survival of patients with relapsed and refractory chronic lymphocytic leukemia by over 9 months.
Data source: A double-blind, placebo-controlled trial of 416 participants.
Disclosures: Gilead Sciences, the maker of idelalisib (Zydelig), sponsored the study. Dr. Zelenetz disclosed consulting with multiple drug companies, including Gilead.
In CML, imatinib benefits persist at 10 years
In patients with chronic myeloid leukemia (CML), the safety and benefits of imatinib persisted over the course of 10 years in a follow-up study reported online March 9 in the New England Journal of Medicine.
The initial results of the phase III International Randomized Study of Interferon and ST1571 (IRIS) trial “fundamentally changed CML treatment and led to marked improvements in prognosis for patients” when imatinib was shown more effective than interferon plus cytarabine among newly diagnosed patients in the chronic phase of the disease, said Andreas Hochhaus, MD, of the Klinik für Innere Medizin II, Universitätsklinikum Jena (Germany), and his associates. The researchers are now reporting the final follow-up results after a median of 10.9 years for the 553 patients who had been randomly assigned to receive daily oral imatinib in the IRIS trial.
The high rate of crossover to the imatinib group among IRIS study participants precluded a direct comparison of overall survival between the two study groups at 10 years. However, the estimated overall 10-year survival in the imatinib group alone was 83.3%. “A total of 260 patients (47.0%) were alive and still receiving study treatment at 10 years, 96 patients (17.4%) were alive and not receiving study treatment, 86 known deaths (15.6% of patients) had occurred, and 11 patients (20.1%) had unknown survival status,” Dr. Hochhaus and his associates reported (N Engl J Med. 2017 Mar 9. doi: 10.1056/NEJMoa1609324).
Approximately 9% of those in the imatinib group had a serious adverse event during follow-up, including 4 patients (0.7%) in whom the event was considered to be related to the drug. Most occurred during the first year of treatment and declined over time. No new safety signals were observed after the 5-year follow-up.
“These results highlight the safety and efficacy of imatinib therapy, with a clear improvement over the outcomes that were expected n patients who received a diagnosis of CML before the introduction of tyrosine kinase inhibitor therapy, when interferon alfa and hematopoietic stem-cell transplantation were the standard therapies,” the investigators said.
Second-generation tyrosine kinase inhibitors have been developed since the IRIS trial was begun, and “it remains to be seen whether they will have similarly favorable long-term safety. Given the long-term safety and efficacy results with imatinib and the increasing availability of generic imatinib, comparative analyses evaluating the available tyrosine kinase inhibitors for first-line therapy are likely to be forthcoming,” they noted.
In patients with chronic myeloid leukemia (CML), the safety and benefits of imatinib persisted over the course of 10 years in a follow-up study reported online March 9 in the New England Journal of Medicine.
The initial results of the phase III International Randomized Study of Interferon and ST1571 (IRIS) trial “fundamentally changed CML treatment and led to marked improvements in prognosis for patients” when imatinib was shown more effective than interferon plus cytarabine among newly diagnosed patients in the chronic phase of the disease, said Andreas Hochhaus, MD, of the Klinik für Innere Medizin II, Universitätsklinikum Jena (Germany), and his associates. The researchers are now reporting the final follow-up results after a median of 10.9 years for the 553 patients who had been randomly assigned to receive daily oral imatinib in the IRIS trial.
The high rate of crossover to the imatinib group among IRIS study participants precluded a direct comparison of overall survival between the two study groups at 10 years. However, the estimated overall 10-year survival in the imatinib group alone was 83.3%. “A total of 260 patients (47.0%) were alive and still receiving study treatment at 10 years, 96 patients (17.4%) were alive and not receiving study treatment, 86 known deaths (15.6% of patients) had occurred, and 11 patients (20.1%) had unknown survival status,” Dr. Hochhaus and his associates reported (N Engl J Med. 2017 Mar 9. doi: 10.1056/NEJMoa1609324).
Approximately 9% of those in the imatinib group had a serious adverse event during follow-up, including 4 patients (0.7%) in whom the event was considered to be related to the drug. Most occurred during the first year of treatment and declined over time. No new safety signals were observed after the 5-year follow-up.
“These results highlight the safety and efficacy of imatinib therapy, with a clear improvement over the outcomes that were expected n patients who received a diagnosis of CML before the introduction of tyrosine kinase inhibitor therapy, when interferon alfa and hematopoietic stem-cell transplantation were the standard therapies,” the investigators said.
Second-generation tyrosine kinase inhibitors have been developed since the IRIS trial was begun, and “it remains to be seen whether they will have similarly favorable long-term safety. Given the long-term safety and efficacy results with imatinib and the increasing availability of generic imatinib, comparative analyses evaluating the available tyrosine kinase inhibitors for first-line therapy are likely to be forthcoming,” they noted.
In patients with chronic myeloid leukemia (CML), the safety and benefits of imatinib persisted over the course of 10 years in a follow-up study reported online March 9 in the New England Journal of Medicine.
The initial results of the phase III International Randomized Study of Interferon and ST1571 (IRIS) trial “fundamentally changed CML treatment and led to marked improvements in prognosis for patients” when imatinib was shown more effective than interferon plus cytarabine among newly diagnosed patients in the chronic phase of the disease, said Andreas Hochhaus, MD, of the Klinik für Innere Medizin II, Universitätsklinikum Jena (Germany), and his associates. The researchers are now reporting the final follow-up results after a median of 10.9 years for the 553 patients who had been randomly assigned to receive daily oral imatinib in the IRIS trial.
The high rate of crossover to the imatinib group among IRIS study participants precluded a direct comparison of overall survival between the two study groups at 10 years. However, the estimated overall 10-year survival in the imatinib group alone was 83.3%. “A total of 260 patients (47.0%) were alive and still receiving study treatment at 10 years, 96 patients (17.4%) were alive and not receiving study treatment, 86 known deaths (15.6% of patients) had occurred, and 11 patients (20.1%) had unknown survival status,” Dr. Hochhaus and his associates reported (N Engl J Med. 2017 Mar 9. doi: 10.1056/NEJMoa1609324).
Approximately 9% of those in the imatinib group had a serious adverse event during follow-up, including 4 patients (0.7%) in whom the event was considered to be related to the drug. Most occurred during the first year of treatment and declined over time. No new safety signals were observed after the 5-year follow-up.
“These results highlight the safety and efficacy of imatinib therapy, with a clear improvement over the outcomes that were expected n patients who received a diagnosis of CML before the introduction of tyrosine kinase inhibitor therapy, when interferon alfa and hematopoietic stem-cell transplantation were the standard therapies,” the investigators said.
Second-generation tyrosine kinase inhibitors have been developed since the IRIS trial was begun, and “it remains to be seen whether they will have similarly favorable long-term safety. Given the long-term safety and efficacy results with imatinib and the increasing availability of generic imatinib, comparative analyses evaluating the available tyrosine kinase inhibitors for first-line therapy are likely to be forthcoming,” they noted.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Imatinib’s benefits in chronic myeloid leukemia and its safety profile persisted over the long term in a 10-year follow-up study.
Major finding: The estimated overall 10-year survival was 83.3%, and no new safety signals were observed after 5-year follow-up.
Data source: Extended follow-up of an open-label international randomized trial involving 553 adults with CML.
Disclosures: This study was funded by Novartis. Dr. Hochhaus reported ties to Novartis and other drug companies.
Did posthysterectomy hemorrhage cause woman’s brain damage?
Did posthysterectomy hemorrhage cause woman’s brain damage?
A 42-year-old woman underwent elective subtotal hysterectomy to treat a large uterine fibroid. In the recovery unit, she had extremely low blood pressure and tachycardia and lost consciousness for 15 minutes. She was given a blood transfusion, but was not returned to the operating room for emergency exploratory laparotomy until 3 hours later. Surgery revealed a hemorrhage from the left uterine artery requiring ligation.
PATIENT’S CLAIM:
The hemorrhage caused hypoxia resulting in an anoxic brain injury with memory and concentration difficulties. The gynecologist and hospital staff were negligent in delaying emergency treatment.
DEFENDANTS’ DEFENSE:
Postoperative bleeding is a known complication of hysterectomy. The patient was at increased risk of bleeding because of the numerous blood vessels feeding the fibroid. A morphine reaction is believed to be the cause of her becoming unconscious. The patient had not sustained an anoxic or hypoxic brain damage because she was alert and oriented immediately after the damage allegedly occurred.
VERDICT:
The Illinois jury deadlocked. The parties entered into a settlement agreement for a confidential sum before a mistrial was declared.
Related article:
7 Myomectomy myths debunked
Choriocarcinoma diagnosis missed
A 25-year-old woman had a miscarriage. A follow-up test to measure the patient’s human chorionic gonadotropin (hCG) hormone level test was not performed. The patient died from choriocarcinoma.
ESTATE’S CLAIM:
The ObGyn did not follow the standard of care: the patient’s hCG level should have been tested after the miscarriage. It is a well-known fact that an hCG level that does not return to zero after a miscarriage is cause for concern, especially from choriocarcinoma.
PHYSICIAN’S DEFENSE:
There was nothing that could have been done to save the woman’s life; choriocarcinoma is a quickly spreading cancer.
VERDICT:
A $1,800,000 Massachusetts settlement was reached.
Related article:
Manual vacuum aspiration: A safe and effective treatment for early miscarriage
Bowel perforation during hysterectomy: $860,000 verdict
A 58-year-old woman underwent laparoscopic hysterectomy and was discharged the next day although she had not urinated or defecated. After eating solid food that evening, she experienced immediate vomiting, nausea, and abdominal pain. When she saw her ObGyn the next morning, he immediately hospitalized her. During the next 8 days in the hospital, she was unable to pass gas, was febrile, and was given antibiotics. On postoperative day 11, a general surgeon transferred her to the intensive care unit due to shortness of breath and tachycardia. During exploratory abdominal surgery, several abscesses and a 1-cm injury to the rectosigmoid colon were discovered necessitating a colostomy. The patient underwent 5 additional abdominal washout procedures and was hospitalized for 40 days. Colostomy reversal surgery occurred 8 months later.
PATIENT’S CLAIM:
The ObGyn never provided an explanation of what went wrong; the patient was told the hysterectomy was accomplished without incident. An expert colorectal surgeon stated that the perforation likely occurred within 24 hours of surgery and was probably caused by the electromechanical device used during surgery.
DEFENDANTS’ DEFENSE:
The perforation was caused by a sudden rupture of a diverticulum 10 days after hysterectomy. The injury was treated in a timely manner.
VERDICT:
An $860,000 Virginia verdict was returned.
Related article:
How to avoid intestinal and urinary tract injuries during gynecologic laparoscopy
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Did posthysterectomy hemorrhage cause woman’s brain damage?
A 42-year-old woman underwent elective subtotal hysterectomy to treat a large uterine fibroid. In the recovery unit, she had extremely low blood pressure and tachycardia and lost consciousness for 15 minutes. She was given a blood transfusion, but was not returned to the operating room for emergency exploratory laparotomy until 3 hours later. Surgery revealed a hemorrhage from the left uterine artery requiring ligation.
PATIENT’S CLAIM:
The hemorrhage caused hypoxia resulting in an anoxic brain injury with memory and concentration difficulties. The gynecologist and hospital staff were negligent in delaying emergency treatment.
DEFENDANTS’ DEFENSE:
Postoperative bleeding is a known complication of hysterectomy. The patient was at increased risk of bleeding because of the numerous blood vessels feeding the fibroid. A morphine reaction is believed to be the cause of her becoming unconscious. The patient had not sustained an anoxic or hypoxic brain damage because she was alert and oriented immediately after the damage allegedly occurred.
VERDICT:
The Illinois jury deadlocked. The parties entered into a settlement agreement for a confidential sum before a mistrial was declared.
Related article:
7 Myomectomy myths debunked
Choriocarcinoma diagnosis missed
A 25-year-old woman had a miscarriage. A follow-up test to measure the patient’s human chorionic gonadotropin (hCG) hormone level test was not performed. The patient died from choriocarcinoma.
ESTATE’S CLAIM:
The ObGyn did not follow the standard of care: the patient’s hCG level should have been tested after the miscarriage. It is a well-known fact that an hCG level that does not return to zero after a miscarriage is cause for concern, especially from choriocarcinoma.
PHYSICIAN’S DEFENSE:
There was nothing that could have been done to save the woman’s life; choriocarcinoma is a quickly spreading cancer.
VERDICT:
A $1,800,000 Massachusetts settlement was reached.
Related article:
Manual vacuum aspiration: A safe and effective treatment for early miscarriage
Bowel perforation during hysterectomy: $860,000 verdict
A 58-year-old woman underwent laparoscopic hysterectomy and was discharged the next day although she had not urinated or defecated. After eating solid food that evening, she experienced immediate vomiting, nausea, and abdominal pain. When she saw her ObGyn the next morning, he immediately hospitalized her. During the next 8 days in the hospital, she was unable to pass gas, was febrile, and was given antibiotics. On postoperative day 11, a general surgeon transferred her to the intensive care unit due to shortness of breath and tachycardia. During exploratory abdominal surgery, several abscesses and a 1-cm injury to the rectosigmoid colon were discovered necessitating a colostomy. The patient underwent 5 additional abdominal washout procedures and was hospitalized for 40 days. Colostomy reversal surgery occurred 8 months later.
PATIENT’S CLAIM:
The ObGyn never provided an explanation of what went wrong; the patient was told the hysterectomy was accomplished without incident. An expert colorectal surgeon stated that the perforation likely occurred within 24 hours of surgery and was probably caused by the electromechanical device used during surgery.
DEFENDANTS’ DEFENSE:
The perforation was caused by a sudden rupture of a diverticulum 10 days after hysterectomy. The injury was treated in a timely manner.
VERDICT:
An $860,000 Virginia verdict was returned.
Related article:
How to avoid intestinal and urinary tract injuries during gynecologic laparoscopy
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Did posthysterectomy hemorrhage cause woman’s brain damage?
A 42-year-old woman underwent elective subtotal hysterectomy to treat a large uterine fibroid. In the recovery unit, she had extremely low blood pressure and tachycardia and lost consciousness for 15 minutes. She was given a blood transfusion, but was not returned to the operating room for emergency exploratory laparotomy until 3 hours later. Surgery revealed a hemorrhage from the left uterine artery requiring ligation.
PATIENT’S CLAIM:
The hemorrhage caused hypoxia resulting in an anoxic brain injury with memory and concentration difficulties. The gynecologist and hospital staff were negligent in delaying emergency treatment.
DEFENDANTS’ DEFENSE:
Postoperative bleeding is a known complication of hysterectomy. The patient was at increased risk of bleeding because of the numerous blood vessels feeding the fibroid. A morphine reaction is believed to be the cause of her becoming unconscious. The patient had not sustained an anoxic or hypoxic brain damage because she was alert and oriented immediately after the damage allegedly occurred.
VERDICT:
The Illinois jury deadlocked. The parties entered into a settlement agreement for a confidential sum before a mistrial was declared.
Related article:
7 Myomectomy myths debunked
Choriocarcinoma diagnosis missed
A 25-year-old woman had a miscarriage. A follow-up test to measure the patient’s human chorionic gonadotropin (hCG) hormone level test was not performed. The patient died from choriocarcinoma.
ESTATE’S CLAIM:
The ObGyn did not follow the standard of care: the patient’s hCG level should have been tested after the miscarriage. It is a well-known fact that an hCG level that does not return to zero after a miscarriage is cause for concern, especially from choriocarcinoma.
PHYSICIAN’S DEFENSE:
There was nothing that could have been done to save the woman’s life; choriocarcinoma is a quickly spreading cancer.
VERDICT:
A $1,800,000 Massachusetts settlement was reached.
Related article:
Manual vacuum aspiration: A safe and effective treatment for early miscarriage
Bowel perforation during hysterectomy: $860,000 verdict
A 58-year-old woman underwent laparoscopic hysterectomy and was discharged the next day although she had not urinated or defecated. After eating solid food that evening, she experienced immediate vomiting, nausea, and abdominal pain. When she saw her ObGyn the next morning, he immediately hospitalized her. During the next 8 days in the hospital, she was unable to pass gas, was febrile, and was given antibiotics. On postoperative day 11, a general surgeon transferred her to the intensive care unit due to shortness of breath and tachycardia. During exploratory abdominal surgery, several abscesses and a 1-cm injury to the rectosigmoid colon were discovered necessitating a colostomy. The patient underwent 5 additional abdominal washout procedures and was hospitalized for 40 days. Colostomy reversal surgery occurred 8 months later.
PATIENT’S CLAIM:
The ObGyn never provided an explanation of what went wrong; the patient was told the hysterectomy was accomplished without incident. An expert colorectal surgeon stated that the perforation likely occurred within 24 hours of surgery and was probably caused by the electromechanical device used during surgery.
DEFENDANTS’ DEFENSE:
The perforation was caused by a sudden rupture of a diverticulum 10 days after hysterectomy. The injury was treated in a timely manner.
VERDICT:
An $860,000 Virginia verdict was returned.
Related article:
How to avoid intestinal and urinary tract injuries during gynecologic laparoscopy
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Two boys, a dog, and our electronic health records
“Speak clearly, if you speak at all; carve every word before you let it fall.” – Oliver Wendell Holmes Sr.
One of our favorite stories is that of two boys talking to one another with a dog sitting nearby. One boy says to the other, “I taught my dog how to whistle.” Skeptically, the other boy responds, “Really? I don’t hear him whistling.” The first boys then replies, “I said I taught him. I didn’t say he learned!”
We spend a lot of time as physicians going over information with our patients, yet, according to the best data available, they retain only a small portion of what we tell them. Medication adherence rates for chronic disease range from 30% to 70%, showing that many doses of important medications are missed. Patients often don’t even remember the last instructions we give them as they are walking out of the office. This raises questions about both the way we explain information and how we can use the tools at our disposal to enhance the communication so vital to patient outcomes.
Obviously, we need to consider our words carefully and focus on teaching, not just speaking. What sets teaching apart from speaking is consideration of the learner. The better we understand our patients’ perspectives, the better the knowledge transfer will be. A simple way to address this may be better eye contact.
We have all heard the expression “the eyes are a window to the soul.” Yet, we now have computers that acts as a virtual shades, covering that window and drawing our gaze away from our patients. These shades can blind us to important clues, impeding communication and leading to misunderstanding, missed opportunity, and even patient harm. This is why some practices have chosen to use scribes to handle documentation, freeing up physicians’ eyes and addressing another obstacle to communication: time.
One of the most cited complaints from physicians is lack of time. There is an ever-growing demand on us to see more patients, manage more data, and “check off more boxes” to meet bureaucratic requirements. It should come as no surprise that these impede good patient care. We are thankful that attempts to modernize payment models are recognizing this problem. For example, the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA) helps to blaze the trail by focusing on care quality, practice improvement, and patient satisfaction for incentive payments. While these are early steps, they certainly point to a future more concerned with value than with volume.
As we move toward that future, we need to acknowledge that information technology can be both the problem and the answer. The current state of health IT is far from perfect. The tools we use have been designed, seemingly, around financial performance or developed to meet government requirements. It appears that neither physicians nor patients were consulted to ensure their usability or utility. Step No. 1 was getting EHRs out there. Steps 2-10 will be making them useful to clinicians, patients, and health care systems. Part of that utility will come in their ability to enhance communication.
Take patient portals, for example. The “meaningful use program” set as a requirement the ability for patients to “view, download, or transmit” their health information through electronic means. EHR vendors complied with this request but seem to have missed the intent of the measure. Patients accessing the information often are confronted with a morass of technical jargon and unfamiliar medical terms, which may even be offensive. For example, we recently spoke to a parent of a teenager with moderate intellectual disabilities. A hold-out ICD-9 code on the teen’s chart translated to her portal as “318.0 – Imbecile.” Her mother was appropriately upset, and she decided to leave the practice.
As we begin to understand technology’s advantages – and learn its pitfalls – we believe EHR vendors must enhance their offerings while engaging both providers and patients in the process of improvement. We also believe physicians need to leverage the entire care team to realize the software’s full potential. This approach may present new challenges in communication, but it also presents new opportunities. We hope that this collaborative approach will allow physicians to have more time to spend connecting with patients, leading to enhanced understanding and satisfaction.
Our knowledge of human health and disease is growing more sophisticated and so is the challenge of imparting that knowledge to patients. It is critical to find ways to do so that are relevant and understandable and give patients the tools they need to reinforce and remember what we say. This is one of the promises that we are just beginning to see fulfilled by modern EHR technology. Unlike the boy who was trying to teach his dog to whistle, our words have deep impact, and our roles as educators have never been more important.
This article was updated 3/24/17.
Dr. Notte is a family physician and clinical informaticist for Abington (Pa.) Memorial Hospital. He is a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records. Dr. Skolnik is associate director of the family medicine residency program at Abington Memorial Hospital and professor of family and community medicine at Temple University in Philadelphia.
“Speak clearly, if you speak at all; carve every word before you let it fall.” – Oliver Wendell Holmes Sr.
One of our favorite stories is that of two boys talking to one another with a dog sitting nearby. One boy says to the other, “I taught my dog how to whistle.” Skeptically, the other boy responds, “Really? I don’t hear him whistling.” The first boys then replies, “I said I taught him. I didn’t say he learned!”
We spend a lot of time as physicians going over information with our patients, yet, according to the best data available, they retain only a small portion of what we tell them. Medication adherence rates for chronic disease range from 30% to 70%, showing that many doses of important medications are missed. Patients often don’t even remember the last instructions we give them as they are walking out of the office. This raises questions about both the way we explain information and how we can use the tools at our disposal to enhance the communication so vital to patient outcomes.
Obviously, we need to consider our words carefully and focus on teaching, not just speaking. What sets teaching apart from speaking is consideration of the learner. The better we understand our patients’ perspectives, the better the knowledge transfer will be. A simple way to address this may be better eye contact.
We have all heard the expression “the eyes are a window to the soul.” Yet, we now have computers that acts as a virtual shades, covering that window and drawing our gaze away from our patients. These shades can blind us to important clues, impeding communication and leading to misunderstanding, missed opportunity, and even patient harm. This is why some practices have chosen to use scribes to handle documentation, freeing up physicians’ eyes and addressing another obstacle to communication: time.
One of the most cited complaints from physicians is lack of time. There is an ever-growing demand on us to see more patients, manage more data, and “check off more boxes” to meet bureaucratic requirements. It should come as no surprise that these impede good patient care. We are thankful that attempts to modernize payment models are recognizing this problem. For example, the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA) helps to blaze the trail by focusing on care quality, practice improvement, and patient satisfaction for incentive payments. While these are early steps, they certainly point to a future more concerned with value than with volume.
As we move toward that future, we need to acknowledge that information technology can be both the problem and the answer. The current state of health IT is far from perfect. The tools we use have been designed, seemingly, around financial performance or developed to meet government requirements. It appears that neither physicians nor patients were consulted to ensure their usability or utility. Step No. 1 was getting EHRs out there. Steps 2-10 will be making them useful to clinicians, patients, and health care systems. Part of that utility will come in their ability to enhance communication.
Take patient portals, for example. The “meaningful use program” set as a requirement the ability for patients to “view, download, or transmit” their health information through electronic means. EHR vendors complied with this request but seem to have missed the intent of the measure. Patients accessing the information often are confronted with a morass of technical jargon and unfamiliar medical terms, which may even be offensive. For example, we recently spoke to a parent of a teenager with moderate intellectual disabilities. A hold-out ICD-9 code on the teen’s chart translated to her portal as “318.0 – Imbecile.” Her mother was appropriately upset, and she decided to leave the practice.
As we begin to understand technology’s advantages – and learn its pitfalls – we believe EHR vendors must enhance their offerings while engaging both providers and patients in the process of improvement. We also believe physicians need to leverage the entire care team to realize the software’s full potential. This approach may present new challenges in communication, but it also presents new opportunities. We hope that this collaborative approach will allow physicians to have more time to spend connecting with patients, leading to enhanced understanding and satisfaction.
Our knowledge of human health and disease is growing more sophisticated and so is the challenge of imparting that knowledge to patients. It is critical to find ways to do so that are relevant and understandable and give patients the tools they need to reinforce and remember what we say. This is one of the promises that we are just beginning to see fulfilled by modern EHR technology. Unlike the boy who was trying to teach his dog to whistle, our words have deep impact, and our roles as educators have never been more important.
This article was updated 3/24/17.
Dr. Notte is a family physician and clinical informaticist for Abington (Pa.) Memorial Hospital. He is a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records. Dr. Skolnik is associate director of the family medicine residency program at Abington Memorial Hospital and professor of family and community medicine at Temple University in Philadelphia.
“Speak clearly, if you speak at all; carve every word before you let it fall.” – Oliver Wendell Holmes Sr.
One of our favorite stories is that of two boys talking to one another with a dog sitting nearby. One boy says to the other, “I taught my dog how to whistle.” Skeptically, the other boy responds, “Really? I don’t hear him whistling.” The first boys then replies, “I said I taught him. I didn’t say he learned!”
We spend a lot of time as physicians going over information with our patients, yet, according to the best data available, they retain only a small portion of what we tell them. Medication adherence rates for chronic disease range from 30% to 70%, showing that many doses of important medications are missed. Patients often don’t even remember the last instructions we give them as they are walking out of the office. This raises questions about both the way we explain information and how we can use the tools at our disposal to enhance the communication so vital to patient outcomes.
Obviously, we need to consider our words carefully and focus on teaching, not just speaking. What sets teaching apart from speaking is consideration of the learner. The better we understand our patients’ perspectives, the better the knowledge transfer will be. A simple way to address this may be better eye contact.
We have all heard the expression “the eyes are a window to the soul.” Yet, we now have computers that acts as a virtual shades, covering that window and drawing our gaze away from our patients. These shades can blind us to important clues, impeding communication and leading to misunderstanding, missed opportunity, and even patient harm. This is why some practices have chosen to use scribes to handle documentation, freeing up physicians’ eyes and addressing another obstacle to communication: time.
One of the most cited complaints from physicians is lack of time. There is an ever-growing demand on us to see more patients, manage more data, and “check off more boxes” to meet bureaucratic requirements. It should come as no surprise that these impede good patient care. We are thankful that attempts to modernize payment models are recognizing this problem. For example, the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA) helps to blaze the trail by focusing on care quality, practice improvement, and patient satisfaction for incentive payments. While these are early steps, they certainly point to a future more concerned with value than with volume.
As we move toward that future, we need to acknowledge that information technology can be both the problem and the answer. The current state of health IT is far from perfect. The tools we use have been designed, seemingly, around financial performance or developed to meet government requirements. It appears that neither physicians nor patients were consulted to ensure their usability or utility. Step No. 1 was getting EHRs out there. Steps 2-10 will be making them useful to clinicians, patients, and health care systems. Part of that utility will come in their ability to enhance communication.
Take patient portals, for example. The “meaningful use program” set as a requirement the ability for patients to “view, download, or transmit” their health information through electronic means. EHR vendors complied with this request but seem to have missed the intent of the measure. Patients accessing the information often are confronted with a morass of technical jargon and unfamiliar medical terms, which may even be offensive. For example, we recently spoke to a parent of a teenager with moderate intellectual disabilities. A hold-out ICD-9 code on the teen’s chart translated to her portal as “318.0 – Imbecile.” Her mother was appropriately upset, and she decided to leave the practice.
As we begin to understand technology’s advantages – and learn its pitfalls – we believe EHR vendors must enhance their offerings while engaging both providers and patients in the process of improvement. We also believe physicians need to leverage the entire care team to realize the software’s full potential. This approach may present new challenges in communication, but it also presents new opportunities. We hope that this collaborative approach will allow physicians to have more time to spend connecting with patients, leading to enhanced understanding and satisfaction.
Our knowledge of human health and disease is growing more sophisticated and so is the challenge of imparting that knowledge to patients. It is critical to find ways to do so that are relevant and understandable and give patients the tools they need to reinforce and remember what we say. This is one of the promises that we are just beginning to see fulfilled by modern EHR technology. Unlike the boy who was trying to teach his dog to whistle, our words have deep impact, and our roles as educators have never been more important.
This article was updated 3/24/17.
Dr. Notte is a family physician and clinical informaticist for Abington (Pa.) Memorial Hospital. He is a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records. Dr. Skolnik is associate director of the family medicine residency program at Abington Memorial Hospital and professor of family and community medicine at Temple University in Philadelphia.
Social media may be isolating young adults
Among young adults, those who most frequently use social media are more likely to feel socially isolated than are less frequent users, according to results of a survey of almost 1,800 respondents.
Adults aged 19-32 years who were in the highest quartile of social media use – 58 or more times a week – had an adjusted odds ratio of 3.4 for perceived social isolation, compared with those in the lowest quartile, who reported using social media less than 9 times a week, said Brian A. Primack, MD, PhD, of the University of Pittsburgh and his associates (Am J Prev Med. 2017 Mar 6. doi: 10.1016/j.amepre.2017.01.010).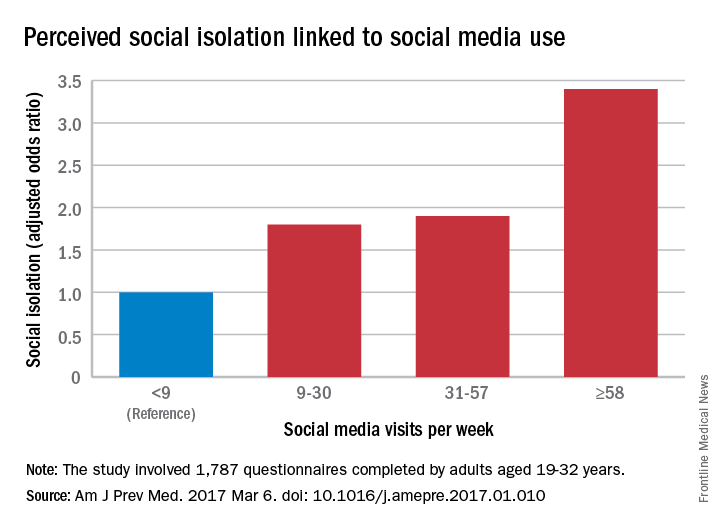
“We are inherently social creatures, but modern life tends to compartmentalize us instead of bringing us together. While it may seem that social media presents opportunities to fill that social void, I think this study suggests that it may not be the solution people were hoping for,” Dr. Primack said in a separate written statement.
The cross-sectional survey was conducted in October and November 2014, and the 11 social media platforms included were Facebook, Twitter, Google+, YouTube, LinkedIn, Instagram, Pinterest, Tumblr, Vine, Snapchat, and Reddit.
Among young adults, those who most frequently use social media are more likely to feel socially isolated than are less frequent users, according to results of a survey of almost 1,800 respondents.
Adults aged 19-32 years who were in the highest quartile of social media use – 58 or more times a week – had an adjusted odds ratio of 3.4 for perceived social isolation, compared with those in the lowest quartile, who reported using social media less than 9 times a week, said Brian A. Primack, MD, PhD, of the University of Pittsburgh and his associates (Am J Prev Med. 2017 Mar 6. doi: 10.1016/j.amepre.2017.01.010).
“We are inherently social creatures, but modern life tends to compartmentalize us instead of bringing us together. While it may seem that social media presents opportunities to fill that social void, I think this study suggests that it may not be the solution people were hoping for,” Dr. Primack said in a separate written statement.
The cross-sectional survey was conducted in October and November 2014, and the 11 social media platforms included were Facebook, Twitter, Google+, YouTube, LinkedIn, Instagram, Pinterest, Tumblr, Vine, Snapchat, and Reddit.
Among young adults, those who most frequently use social media are more likely to feel socially isolated than are less frequent users, according to results of a survey of almost 1,800 respondents.
Adults aged 19-32 years who were in the highest quartile of social media use – 58 or more times a week – had an adjusted odds ratio of 3.4 for perceived social isolation, compared with those in the lowest quartile, who reported using social media less than 9 times a week, said Brian A. Primack, MD, PhD, of the University of Pittsburgh and his associates (Am J Prev Med. 2017 Mar 6. doi: 10.1016/j.amepre.2017.01.010).
“We are inherently social creatures, but modern life tends to compartmentalize us instead of bringing us together. While it may seem that social media presents opportunities to fill that social void, I think this study suggests that it may not be the solution people were hoping for,” Dr. Primack said in a separate written statement.
The cross-sectional survey was conducted in October and November 2014, and the 11 social media platforms included were Facebook, Twitter, Google+, YouTube, LinkedIn, Instagram, Pinterest, Tumblr, Vine, Snapchat, and Reddit.
FROM THE AMERICAN JOURNAL OF PREVENTIVE MEDICINE
FDA approves first dedicated bifurcation device to treat coronary bifurcation lesions
Tryton Medical announced on March 6 that the Food and Drug Administration has approved Tryton Side Branch Stent for the treatment of coronary bifurcation lesions involving large side branches, becoming the first dedicated bifurcation device to receive regulatory approval in the U.S.
In a post hoc analysis of a randomized clinical trial, treatment with the Tryton Side Branch Stent in the intended population of patients with large side branches (stent greater than 2.5mm) reduced the need for additional bailout stenting (0.7% vs. 5.6%, P = .02). It led to significantly lower side branch percent diameter stenosis at a 9-month follow-up (30.4% vs. 40.6%, P = .004) when compared with provisional stenting. The analysis also showed comparable major adverse cardiovascular events and myocardial infarction rates when compared with provisional stenting at 3 years.
“Treatment of complex lesions at the site of a bifurcation has historically been inconsistent, with results varying depending on the procedure and the experience of the interventionist,” said Aaron Kaplan, MD, Professor of Medicine at Dartmouth Hitchcock Medical Center, Lebanon, N.H., and Chief Medical Officer of Tryton Medical, in a press release. “A predictable bifurcation solution helps alleviate some of the stress in these procedures by limiting variability and reducing the need for bailout stenting. This important FDA decision could have a profound impact on treatment protocols and guidelines for significant bifurcation lesions in the years ahead.”
There have been no randomized studies to compare the results of percutaneous coronary interventions (PCI) with coronary artery bypass grafting in a bifurcation-only patient population. But this new device should benefit results from treatment using PCI.
Coronary artery disease is the leading cause of death in the U.S. in both men and women, and often results in bifurcation. Provisional stenting of the main branch is the current standard of care, but in many cases the side branch is not stented, leaving it vulnerable to complications like occlusion requiring bailout stenting.
Read more on Tryton Side Branch Stent on Tryton’s website.
Tryton Medical announced on March 6 that the Food and Drug Administration has approved Tryton Side Branch Stent for the treatment of coronary bifurcation lesions involving large side branches, becoming the first dedicated bifurcation device to receive regulatory approval in the U.S.
In a post hoc analysis of a randomized clinical trial, treatment with the Tryton Side Branch Stent in the intended population of patients with large side branches (stent greater than 2.5mm) reduced the need for additional bailout stenting (0.7% vs. 5.6%, P = .02). It led to significantly lower side branch percent diameter stenosis at a 9-month follow-up (30.4% vs. 40.6%, P = .004) when compared with provisional stenting. The analysis also showed comparable major adverse cardiovascular events and myocardial infarction rates when compared with provisional stenting at 3 years.
“Treatment of complex lesions at the site of a bifurcation has historically been inconsistent, with results varying depending on the procedure and the experience of the interventionist,” said Aaron Kaplan, MD, Professor of Medicine at Dartmouth Hitchcock Medical Center, Lebanon, N.H., and Chief Medical Officer of Tryton Medical, in a press release. “A predictable bifurcation solution helps alleviate some of the stress in these procedures by limiting variability and reducing the need for bailout stenting. This important FDA decision could have a profound impact on treatment protocols and guidelines for significant bifurcation lesions in the years ahead.”
There have been no randomized studies to compare the results of percutaneous coronary interventions (PCI) with coronary artery bypass grafting in a bifurcation-only patient population. But this new device should benefit results from treatment using PCI.
Coronary artery disease is the leading cause of death in the U.S. in both men and women, and often results in bifurcation. Provisional stenting of the main branch is the current standard of care, but in many cases the side branch is not stented, leaving it vulnerable to complications like occlusion requiring bailout stenting.
Read more on Tryton Side Branch Stent on Tryton’s website.
Tryton Medical announced on March 6 that the Food and Drug Administration has approved Tryton Side Branch Stent for the treatment of coronary bifurcation lesions involving large side branches, becoming the first dedicated bifurcation device to receive regulatory approval in the U.S.
In a post hoc analysis of a randomized clinical trial, treatment with the Tryton Side Branch Stent in the intended population of patients with large side branches (stent greater than 2.5mm) reduced the need for additional bailout stenting (0.7% vs. 5.6%, P = .02). It led to significantly lower side branch percent diameter stenosis at a 9-month follow-up (30.4% vs. 40.6%, P = .004) when compared with provisional stenting. The analysis also showed comparable major adverse cardiovascular events and myocardial infarction rates when compared with provisional stenting at 3 years.
“Treatment of complex lesions at the site of a bifurcation has historically been inconsistent, with results varying depending on the procedure and the experience of the interventionist,” said Aaron Kaplan, MD, Professor of Medicine at Dartmouth Hitchcock Medical Center, Lebanon, N.H., and Chief Medical Officer of Tryton Medical, in a press release. “A predictable bifurcation solution helps alleviate some of the stress in these procedures by limiting variability and reducing the need for bailout stenting. This important FDA decision could have a profound impact on treatment protocols and guidelines for significant bifurcation lesions in the years ahead.”
There have been no randomized studies to compare the results of percutaneous coronary interventions (PCI) with coronary artery bypass grafting in a bifurcation-only patient population. But this new device should benefit results from treatment using PCI.
Coronary artery disease is the leading cause of death in the U.S. in both men and women, and often results in bifurcation. Provisional stenting of the main branch is the current standard of care, but in many cases the side branch is not stented, leaving it vulnerable to complications like occlusion requiring bailout stenting.
Read more on Tryton Side Branch Stent on Tryton’s website.
Malpractice bill would impose $250,000 damages cap
New legislation approved by the House Judiciary Committee could mean legal relief for health providers in the form of capped damages and a tighter time frame for lawsuits.
The House Judiciary Committee passed the Protecting Access to Care Act of 2017 (H.R. 1215) on Feb. 28 by a vote of 18-17. The bill, modeled after California’s Medical Injury Compensation Reform Act (MICRA), would limit noneconomic damages in medical malpractice cases to $250,000, restrict contingency fees charged by attorneys, and enforce a 3-year statute of limitations for liability lawsuits from the date of alleged injury. The bill also includes a “fair share” rule in which defendants are liable only for the damages in direct proportion to their percentage of responsibility.
The bill is the first significant medical professional liability reform legislation to be approved by the committee since 2011, said Brian K. Atchinson, president and CEO of PIAA, a national trade association for medical liability insurers.
“Unlike previous federal bills, the bill is focused solely on health care professionals and entities, includes detailed flexibility for states for all its reforms, and is linked with the expenditure of federal dollars to address states’ rights concerns,” Mr. Atchinson said in a statement. “H.R. 1215 will help ensure fair and timely compensation to injured patients, improve access to patient care, and promote affordable and accessible medical liability insurance coverage.”
The proposed statute would apply to any patient who receives medical care provided via a federal program, such as Medicare or Medicaid, or via a subsidy or tax benefit, such as coverage purchased under the Affordable Care Act or a future* replacement. Medical care paid by employer health plans would fall under the legislation’s umbrella since insurance premiums receive federal tax exemptions. The bill would not preempt state medical malpractice laws that impose damage caps, whether higher or lower than $250,000, nor would the legislation affect the availability of economic damages, according to bill language.
As part of the H.R. 1215, courts could limit how much attorneys receive from a patient’s ultimate award. Specifically, courts would have the power to restrict payments from a plaintiff’s damage recovery to an attorney who claims a financial stake in the outcome by virtue of a contingent fee.
“The Protecting Access to Care Act will help keep the rising costs of health care from being passed along to the American people,” Rep. Goodlatte said in a statement. “The Congressional Budget Office estimates that the reforms contained in the bill would lower health care costs by tens of billions of dollars.”
Public Citizen, a consumer rights group, criticized the legislation as misleading to consumers and harmful to patients.
“Proposals to shield providers from liability are nothing but a giveaway to industry,” Lisa Gilbert, director of Public Citizen’s Congress Watch, said in a statement. “Members supporting this bill would further harm those who are suffering from doctors’ mistakes and abandon the GOP’s supposedly unwavering commitment to state’s rights.”
“There are so many moving parts to this bill, I think the likelihood of its being passed as is is low,” said Dr. Segal, founder of Medical Justice, a company that works to deter frivolous medical malpractice lawsuits. “The biggest challenge will be whether the Republicans have to get eight Democratic senators to join the bill. To make it more palatable, something will need to give. Such provisions on tort reform are likely to be the first items offered for sacrifice.”
[email protected]
On Twitter @legal_med
New legislation approved by the House Judiciary Committee could mean legal relief for health providers in the form of capped damages and a tighter time frame for lawsuits.
The House Judiciary Committee passed the Protecting Access to Care Act of 2017 (H.R. 1215) on Feb. 28 by a vote of 18-17. The bill, modeled after California’s Medical Injury Compensation Reform Act (MICRA), would limit noneconomic damages in medical malpractice cases to $250,000, restrict contingency fees charged by attorneys, and enforce a 3-year statute of limitations for liability lawsuits from the date of alleged injury. The bill also includes a “fair share” rule in which defendants are liable only for the damages in direct proportion to their percentage of responsibility.
The bill is the first significant medical professional liability reform legislation to be approved by the committee since 2011, said Brian K. Atchinson, president and CEO of PIAA, a national trade association for medical liability insurers.
“Unlike previous federal bills, the bill is focused solely on health care professionals and entities, includes detailed flexibility for states for all its reforms, and is linked with the expenditure of federal dollars to address states’ rights concerns,” Mr. Atchinson said in a statement. “H.R. 1215 will help ensure fair and timely compensation to injured patients, improve access to patient care, and promote affordable and accessible medical liability insurance coverage.”
The proposed statute would apply to any patient who receives medical care provided via a federal program, such as Medicare or Medicaid, or via a subsidy or tax benefit, such as coverage purchased under the Affordable Care Act or a future* replacement. Medical care paid by employer health plans would fall under the legislation’s umbrella since insurance premiums receive federal tax exemptions. The bill would not preempt state medical malpractice laws that impose damage caps, whether higher or lower than $250,000, nor would the legislation affect the availability of economic damages, according to bill language.
As part of the H.R. 1215, courts could limit how much attorneys receive from a patient’s ultimate award. Specifically, courts would have the power to restrict payments from a plaintiff’s damage recovery to an attorney who claims a financial stake in the outcome by virtue of a contingent fee.
“The Protecting Access to Care Act will help keep the rising costs of health care from being passed along to the American people,” Rep. Goodlatte said in a statement. “The Congressional Budget Office estimates that the reforms contained in the bill would lower health care costs by tens of billions of dollars.”
Public Citizen, a consumer rights group, criticized the legislation as misleading to consumers and harmful to patients.
“Proposals to shield providers from liability are nothing but a giveaway to industry,” Lisa Gilbert, director of Public Citizen’s Congress Watch, said in a statement. “Members supporting this bill would further harm those who are suffering from doctors’ mistakes and abandon the GOP’s supposedly unwavering commitment to state’s rights.”
“There are so many moving parts to this bill, I think the likelihood of its being passed as is is low,” said Dr. Segal, founder of Medical Justice, a company that works to deter frivolous medical malpractice lawsuits. “The biggest challenge will be whether the Republicans have to get eight Democratic senators to join the bill. To make it more palatable, something will need to give. Such provisions on tort reform are likely to be the first items offered for sacrifice.”
[email protected]
On Twitter @legal_med
New legislation approved by the House Judiciary Committee could mean legal relief for health providers in the form of capped damages and a tighter time frame for lawsuits.
The House Judiciary Committee passed the Protecting Access to Care Act of 2017 (H.R. 1215) on Feb. 28 by a vote of 18-17. The bill, modeled after California’s Medical Injury Compensation Reform Act (MICRA), would limit noneconomic damages in medical malpractice cases to $250,000, restrict contingency fees charged by attorneys, and enforce a 3-year statute of limitations for liability lawsuits from the date of alleged injury. The bill also includes a “fair share” rule in which defendants are liable only for the damages in direct proportion to their percentage of responsibility.
The bill is the first significant medical professional liability reform legislation to be approved by the committee since 2011, said Brian K. Atchinson, president and CEO of PIAA, a national trade association for medical liability insurers.
“Unlike previous federal bills, the bill is focused solely on health care professionals and entities, includes detailed flexibility for states for all its reforms, and is linked with the expenditure of federal dollars to address states’ rights concerns,” Mr. Atchinson said in a statement. “H.R. 1215 will help ensure fair and timely compensation to injured patients, improve access to patient care, and promote affordable and accessible medical liability insurance coverage.”
The proposed statute would apply to any patient who receives medical care provided via a federal program, such as Medicare or Medicaid, or via a subsidy or tax benefit, such as coverage purchased under the Affordable Care Act or a future* replacement. Medical care paid by employer health plans would fall under the legislation’s umbrella since insurance premiums receive federal tax exemptions. The bill would not preempt state medical malpractice laws that impose damage caps, whether higher or lower than $250,000, nor would the legislation affect the availability of economic damages, according to bill language.
As part of the H.R. 1215, courts could limit how much attorneys receive from a patient’s ultimate award. Specifically, courts would have the power to restrict payments from a plaintiff’s damage recovery to an attorney who claims a financial stake in the outcome by virtue of a contingent fee.
“The Protecting Access to Care Act will help keep the rising costs of health care from being passed along to the American people,” Rep. Goodlatte said in a statement. “The Congressional Budget Office estimates that the reforms contained in the bill would lower health care costs by tens of billions of dollars.”
Public Citizen, a consumer rights group, criticized the legislation as misleading to consumers and harmful to patients.
“Proposals to shield providers from liability are nothing but a giveaway to industry,” Lisa Gilbert, director of Public Citizen’s Congress Watch, said in a statement. “Members supporting this bill would further harm those who are suffering from doctors’ mistakes and abandon the GOP’s supposedly unwavering commitment to state’s rights.”
“There are so many moving parts to this bill, I think the likelihood of its being passed as is is low,” said Dr. Segal, founder of Medical Justice, a company that works to deter frivolous medical malpractice lawsuits. “The biggest challenge will be whether the Republicans have to get eight Democratic senators to join the bill. To make it more palatable, something will need to give. Such provisions on tort reform are likely to be the first items offered for sacrifice.”
[email protected]
On Twitter @legal_med
TAVR can be performed safely within 30 days of PCI
WASHINGTON – The risk of adverse events from a transcatheter aortic valve replacement (TAVR) does not appear to be significantly increased in those who have undergone a recent percutaneous intervention (PCI), according to a matched retrospective analysis.
“PCI prior to TAVR in patients with severe aortic stenosis and significant coronary artery disease appears to be feasible and safe,” reported Ashwat S. Dhillon, MD, a cardiology fellow at the University of Southern California, Los Angeles, at CRT 2017 sponsored by the Cardiovascular Research Institute at Washington Hospital Center.
The conclusion that PCI can be performed safely prior to TAVR was drawn from a series of 286 patients treated with TAVR over a nearly 5-year period. Within this group, 29 patients underwent PCI for CAD within 30 days prior to TAVR. They were matched in a 1:1 fashion based on age, sex, history of prior myocardial infarction, and left ventricular ejection fraction to patients undergoing PCI without subsequent TAVR.
The primary endpoint of the analysis was a composite of major in-hospital adverse cardiovascular events (MACE) that included MI and stroke. In addition, the two groups were compared for mortality and readmission rates 30 days after TAVR.
Most of the patients (69%) were male, and the mean age was 77 years. About 20% had a prior MI, roughly 30% had a prior coronary artery bypass graft procedure, and approximately 30% had a prior PCI. There were numerical differences in the rates of hypertension, chronic kidney disease, and diabetes when the two groups were compared, but none were statistically significant.
The procedural details of the PCI were also similar, according to Dr. Dhillon. Although there was a significantly greater proportion of patients treated for lesions in the left circumflex artery in the group that did not undergo TAVR (31.03% vs. 3.45%; P = .02), there were no significant differences in procedures performed in other arteries. There were also no significant differences in the average number of stents and the average total stent length for those who underwent TAVR relative to those who did not.
The rate of in-hospital MI was 14% in both groups. No patient in either group had a stroke. At 30 days, mortality was 3% in each group. Although 30-day readmissions were higher in the group that underwent both PCI and TAVR than those who underwent PCI alone (10% vs. 0%), the difference did not reach significance.
Data evaluating the safety of performing PCI and TAVR procedures in close proximity is needed because “a significant proportion of patients with severe aortic stenosis have coexisting and significant CAD,” Dr. Dhillon explained. Although he suggested that a larger pool of data is needed to confirm the preliminary findings of this study, he suggested that these data are reassuring.
WASHINGTON – The risk of adverse events from a transcatheter aortic valve replacement (TAVR) does not appear to be significantly increased in those who have undergone a recent percutaneous intervention (PCI), according to a matched retrospective analysis.
“PCI prior to TAVR in patients with severe aortic stenosis and significant coronary artery disease appears to be feasible and safe,” reported Ashwat S. Dhillon, MD, a cardiology fellow at the University of Southern California, Los Angeles, at CRT 2017 sponsored by the Cardiovascular Research Institute at Washington Hospital Center.
The conclusion that PCI can be performed safely prior to TAVR was drawn from a series of 286 patients treated with TAVR over a nearly 5-year period. Within this group, 29 patients underwent PCI for CAD within 30 days prior to TAVR. They were matched in a 1:1 fashion based on age, sex, history of prior myocardial infarction, and left ventricular ejection fraction to patients undergoing PCI without subsequent TAVR.
The primary endpoint of the analysis was a composite of major in-hospital adverse cardiovascular events (MACE) that included MI and stroke. In addition, the two groups were compared for mortality and readmission rates 30 days after TAVR.
Most of the patients (69%) were male, and the mean age was 77 years. About 20% had a prior MI, roughly 30% had a prior coronary artery bypass graft procedure, and approximately 30% had a prior PCI. There were numerical differences in the rates of hypertension, chronic kidney disease, and diabetes when the two groups were compared, but none were statistically significant.
The procedural details of the PCI were also similar, according to Dr. Dhillon. Although there was a significantly greater proportion of patients treated for lesions in the left circumflex artery in the group that did not undergo TAVR (31.03% vs. 3.45%; P = .02), there were no significant differences in procedures performed in other arteries. There were also no significant differences in the average number of stents and the average total stent length for those who underwent TAVR relative to those who did not.
The rate of in-hospital MI was 14% in both groups. No patient in either group had a stroke. At 30 days, mortality was 3% in each group. Although 30-day readmissions were higher in the group that underwent both PCI and TAVR than those who underwent PCI alone (10% vs. 0%), the difference did not reach significance.
Data evaluating the safety of performing PCI and TAVR procedures in close proximity is needed because “a significant proportion of patients with severe aortic stenosis have coexisting and significant CAD,” Dr. Dhillon explained. Although he suggested that a larger pool of data is needed to confirm the preliminary findings of this study, he suggested that these data are reassuring.
WASHINGTON – The risk of adverse events from a transcatheter aortic valve replacement (TAVR) does not appear to be significantly increased in those who have undergone a recent percutaneous intervention (PCI), according to a matched retrospective analysis.
“PCI prior to TAVR in patients with severe aortic stenosis and significant coronary artery disease appears to be feasible and safe,” reported Ashwat S. Dhillon, MD, a cardiology fellow at the University of Southern California, Los Angeles, at CRT 2017 sponsored by the Cardiovascular Research Institute at Washington Hospital Center.
The conclusion that PCI can be performed safely prior to TAVR was drawn from a series of 286 patients treated with TAVR over a nearly 5-year period. Within this group, 29 patients underwent PCI for CAD within 30 days prior to TAVR. They were matched in a 1:1 fashion based on age, sex, history of prior myocardial infarction, and left ventricular ejection fraction to patients undergoing PCI without subsequent TAVR.
The primary endpoint of the analysis was a composite of major in-hospital adverse cardiovascular events (MACE) that included MI and stroke. In addition, the two groups were compared for mortality and readmission rates 30 days after TAVR.
Most of the patients (69%) were male, and the mean age was 77 years. About 20% had a prior MI, roughly 30% had a prior coronary artery bypass graft procedure, and approximately 30% had a prior PCI. There were numerical differences in the rates of hypertension, chronic kidney disease, and diabetes when the two groups were compared, but none were statistically significant.
The procedural details of the PCI were also similar, according to Dr. Dhillon. Although there was a significantly greater proportion of patients treated for lesions in the left circumflex artery in the group that did not undergo TAVR (31.03% vs. 3.45%; P = .02), there were no significant differences in procedures performed in other arteries. There were also no significant differences in the average number of stents and the average total stent length for those who underwent TAVR relative to those who did not.
The rate of in-hospital MI was 14% in both groups. No patient in either group had a stroke. At 30 days, mortality was 3% in each group. Although 30-day readmissions were higher in the group that underwent both PCI and TAVR than those who underwent PCI alone (10% vs. 0%), the difference did not reach significance.
Data evaluating the safety of performing PCI and TAVR procedures in close proximity is needed because “a significant proportion of patients with severe aortic stenosis have coexisting and significant CAD,” Dr. Dhillon explained. Although he suggested that a larger pool of data is needed to confirm the preliminary findings of this study, he suggested that these data are reassuring.
AT CRT 2017
Key clinical point: Adverse outcomes from transcatheter aortic valve replacement are not increased in patients who had a recent percutaneous intervention.
Major finding: Rates of in-hospital MI (14%), stroke (0%), and 30-day mortality (3%) were exactly the same for those with or without prior PCI.
Data source: A nonrandomized, retrospective, matched analysis.
Disclosures: Dr. Dhillon reported no financial relationships to disclose.