User login
Dose Titration May Reduce Risk of ARIA Associated With Aducanumab
BOSTON—Compared with fixed dosing, dose titration appears to reduce the risk of amyloid-related imaging abnormalities (ARIA) associated with aducanumab, according to research presented at the 69th Annual Meeting of the American Academy of Neurology. The drug also may reduce the burden of amyloid plaques and slow cognitive decline.
Aducanumab is a human anti-amyloid beta monoclonal antibody under investigation for early Alzheimer’s disease. In an interim analysis of the PRIME study, dose- and APOE ε4-dependent ARIA of the edema type was the main safety and tolerability finding. Ahmed Enayetallah, MD, PhD, of Biogen, and colleagues investigated whether dose titration would reduce the incidence of ARIA, compared with fixed dosing.
In a double-blind, placebo-controlled study, the researchers randomized participants with prodromal or mild Alzheimer’s disease 3:1 to fixed doses of aducanumab or placebo every four weeks for 52 weeks, stratified by APOE ε4 status. After the enrollment of this cohort, the investigators added a cohort of APOE ε4 carriers who received titrated aducanumab or placebo. Patients assigned to aducanumab received two 1-mg/kg doses, four 3-mg/kg doses, five 6-mg/kg doses, and 10-mg/kg doses thereafter.
The trial’s primary end points were safety and tolerability. Exploratory efficacy end points included amyloid beta reduction by PET at one year, Clinical Dementia Rating–Sum of Boxes (CDR–SB), and Mini-Mental State Examination (MMSE).
A total of 196 patients were dosed in the study, and the titration cohort included 31 participants. The treatment groups were well balanced. Compared with placebo, titrated aducanumab was associated with significant decreases in brain amyloid beta at 12 months. The adjusted mean change from baseline in PET standard uptake value ratio was –0.171 versus 0.014, respectively. The difference was observable at week 26 and was maintained to week 54.
Titrated aducanumab also was associated with a slowing of clinical decline on CDR–SB and MMSE. Results in the titration cohort were generally consistent with those reported in fixed-dose cohorts. The incidence of ARIA was lower with titrated dosing versus higher fixed dosing of aducanumab in APOE ε4 carriers. The researchers did not find any new safety signals in the titration cohort during the placebo-controlled period.
Biogen funded the study.
Suggested Reading
Sevigny J, Chiao P, Bussière T, et al. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature. 2016;537(7618):50-56.
BOSTON—Compared with fixed dosing, dose titration appears to reduce the risk of amyloid-related imaging abnormalities (ARIA) associated with aducanumab, according to research presented at the 69th Annual Meeting of the American Academy of Neurology. The drug also may reduce the burden of amyloid plaques and slow cognitive decline.
Aducanumab is a human anti-amyloid beta monoclonal antibody under investigation for early Alzheimer’s disease. In an interim analysis of the PRIME study, dose- and APOE ε4-dependent ARIA of the edema type was the main safety and tolerability finding. Ahmed Enayetallah, MD, PhD, of Biogen, and colleagues investigated whether dose titration would reduce the incidence of ARIA, compared with fixed dosing.
In a double-blind, placebo-controlled study, the researchers randomized participants with prodromal or mild Alzheimer’s disease 3:1 to fixed doses of aducanumab or placebo every four weeks for 52 weeks, stratified by APOE ε4 status. After the enrollment of this cohort, the investigators added a cohort of APOE ε4 carriers who received titrated aducanumab or placebo. Patients assigned to aducanumab received two 1-mg/kg doses, four 3-mg/kg doses, five 6-mg/kg doses, and 10-mg/kg doses thereafter.
The trial’s primary end points were safety and tolerability. Exploratory efficacy end points included amyloid beta reduction by PET at one year, Clinical Dementia Rating–Sum of Boxes (CDR–SB), and Mini-Mental State Examination (MMSE).
A total of 196 patients were dosed in the study, and the titration cohort included 31 participants. The treatment groups were well balanced. Compared with placebo, titrated aducanumab was associated with significant decreases in brain amyloid beta at 12 months. The adjusted mean change from baseline in PET standard uptake value ratio was –0.171 versus 0.014, respectively. The difference was observable at week 26 and was maintained to week 54.
Titrated aducanumab also was associated with a slowing of clinical decline on CDR–SB and MMSE. Results in the titration cohort were generally consistent with those reported in fixed-dose cohorts. The incidence of ARIA was lower with titrated dosing versus higher fixed dosing of aducanumab in APOE ε4 carriers. The researchers did not find any new safety signals in the titration cohort during the placebo-controlled period.
Biogen funded the study.
Suggested Reading
Sevigny J, Chiao P, Bussière T, et al. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature. 2016;537(7618):50-56.
BOSTON—Compared with fixed dosing, dose titration appears to reduce the risk of amyloid-related imaging abnormalities (ARIA) associated with aducanumab, according to research presented at the 69th Annual Meeting of the American Academy of Neurology. The drug also may reduce the burden of amyloid plaques and slow cognitive decline.
Aducanumab is a human anti-amyloid beta monoclonal antibody under investigation for early Alzheimer’s disease. In an interim analysis of the PRIME study, dose- and APOE ε4-dependent ARIA of the edema type was the main safety and tolerability finding. Ahmed Enayetallah, MD, PhD, of Biogen, and colleagues investigated whether dose titration would reduce the incidence of ARIA, compared with fixed dosing.
In a double-blind, placebo-controlled study, the researchers randomized participants with prodromal or mild Alzheimer’s disease 3:1 to fixed doses of aducanumab or placebo every four weeks for 52 weeks, stratified by APOE ε4 status. After the enrollment of this cohort, the investigators added a cohort of APOE ε4 carriers who received titrated aducanumab or placebo. Patients assigned to aducanumab received two 1-mg/kg doses, four 3-mg/kg doses, five 6-mg/kg doses, and 10-mg/kg doses thereafter.
The trial’s primary end points were safety and tolerability. Exploratory efficacy end points included amyloid beta reduction by PET at one year, Clinical Dementia Rating–Sum of Boxes (CDR–SB), and Mini-Mental State Examination (MMSE).
A total of 196 patients were dosed in the study, and the titration cohort included 31 participants. The treatment groups were well balanced. Compared with placebo, titrated aducanumab was associated with significant decreases in brain amyloid beta at 12 months. The adjusted mean change from baseline in PET standard uptake value ratio was –0.171 versus 0.014, respectively. The difference was observable at week 26 and was maintained to week 54.
Titrated aducanumab also was associated with a slowing of clinical decline on CDR–SB and MMSE. Results in the titration cohort were generally consistent with those reported in fixed-dose cohorts. The incidence of ARIA was lower with titrated dosing versus higher fixed dosing of aducanumab in APOE ε4 carriers. The researchers did not find any new safety signals in the titration cohort during the placebo-controlled period.
Biogen funded the study.
Suggested Reading
Sevigny J, Chiao P, Bussière T, et al. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature. 2016;537(7618):50-56.
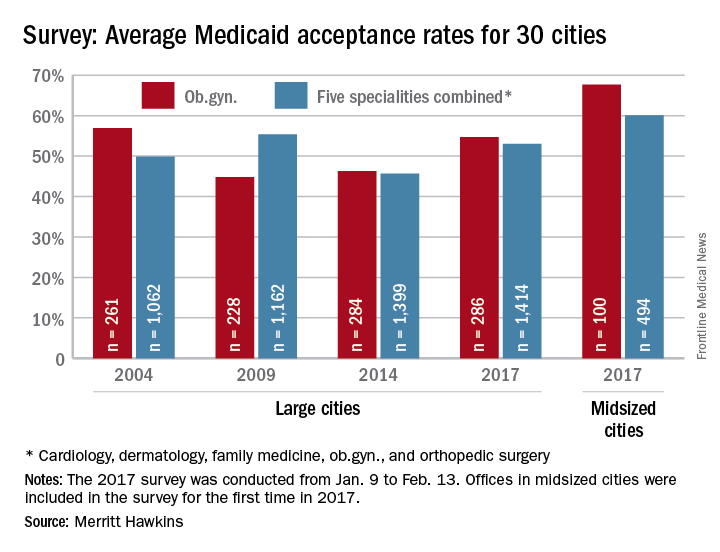
Just over half of ob.gyns. accept Medicaid
Medicaid acceptance was nearly 55% among ob.gyns. in the 2017 edition of an ongoing survey conducted in 15 large cities by physician recruitment firm Merritt Hawkins.
It was up from almost 46% in the previous survey, conducted in 2014, but lower than the average of 68% for ob.gyns. in 15 midsized cities that were included for the first time in 2017, the company reported.
There were two large cities with Medicaid acceptance rates of 100% – Boston and Minneapolis (up from 40% in 2014) – along with four midsized cities – Billings, Mt.; Fargo, N.D.; Fort Smith, Ark.; and Yakima, Wash. The lowest rate among the large cities was in Dallas (15%), with the midsized basement occupied by Lafayette, La., at 10%, Merritt Hawkins said.
Investigators called 286 randomly selected ob.gyns. in the large cities and 100 ob.gyns. in the midsized cities in January and February. It was the fourth such survey the company has conducted since 2004.
The survey also included four other specialties – cardiology, dermatology, family medicine, and orthopedic surgery. The Medicaid acceptance rate for all 1,414 physicians in all five specialties in the 15 large cities was 53%, and the average rate for all specialties in the midsized cities was 60% for the 494 offices surveyed, the company said.
Cardiology had the highest rates by specialty and dermatology the lowest in both the large and midsized cities. For all five specialties combined, Fargo (100%) and Minneapolis (97%) had the highest acceptance rates, and Dallas (17%) and Lafayette (11%) had the lowest, the report showed.
Medicaid acceptance was nearly 55% among ob.gyns. in the 2017 edition of an ongoing survey conducted in 15 large cities by physician recruitment firm Merritt Hawkins.
It was up from almost 46% in the previous survey, conducted in 2014, but lower than the average of 68% for ob.gyns. in 15 midsized cities that were included for the first time in 2017, the company reported.
There were two large cities with Medicaid acceptance rates of 100% – Boston and Minneapolis (up from 40% in 2014) – along with four midsized cities – Billings, Mt.; Fargo, N.D.; Fort Smith, Ark.; and Yakima, Wash. The lowest rate among the large cities was in Dallas (15%), with the midsized basement occupied by Lafayette, La., at 10%, Merritt Hawkins said.
Investigators called 286 randomly selected ob.gyns. in the large cities and 100 ob.gyns. in the midsized cities in January and February. It was the fourth such survey the company has conducted since 2004.
The survey also included four other specialties – cardiology, dermatology, family medicine, and orthopedic surgery. The Medicaid acceptance rate for all 1,414 physicians in all five specialties in the 15 large cities was 53%, and the average rate for all specialties in the midsized cities was 60% for the 494 offices surveyed, the company said.
Cardiology had the highest rates by specialty and dermatology the lowest in both the large and midsized cities. For all five specialties combined, Fargo (100%) and Minneapolis (97%) had the highest acceptance rates, and Dallas (17%) and Lafayette (11%) had the lowest, the report showed.
Medicaid acceptance was nearly 55% among ob.gyns. in the 2017 edition of an ongoing survey conducted in 15 large cities by physician recruitment firm Merritt Hawkins.
It was up from almost 46% in the previous survey, conducted in 2014, but lower than the average of 68% for ob.gyns. in 15 midsized cities that were included for the first time in 2017, the company reported.
There were two large cities with Medicaid acceptance rates of 100% – Boston and Minneapolis (up from 40% in 2014) – along with four midsized cities – Billings, Mt.; Fargo, N.D.; Fort Smith, Ark.; and Yakima, Wash. The lowest rate among the large cities was in Dallas (15%), with the midsized basement occupied by Lafayette, La., at 10%, Merritt Hawkins said.
Investigators called 286 randomly selected ob.gyns. in the large cities and 100 ob.gyns. in the midsized cities in January and February. It was the fourth such survey the company has conducted since 2004.
The survey also included four other specialties – cardiology, dermatology, family medicine, and orthopedic surgery. The Medicaid acceptance rate for all 1,414 physicians in all five specialties in the 15 large cities was 53%, and the average rate for all specialties in the midsized cities was 60% for the 494 offices surveyed, the company said.
Cardiology had the highest rates by specialty and dermatology the lowest in both the large and midsized cities. For all five specialties combined, Fargo (100%) and Minneapolis (97%) had the highest acceptance rates, and Dallas (17%) and Lafayette (11%) had the lowest, the report showed.
Bezlotoxumab for prevention of recurrent Clostridium difficile infection
Clinical question: Does administration of monoclonal antibodies to C. difficile toxins A and B, in addition to standard-of-care antibiotics, prevent recurrent infection?
Background: Currently, no therapy has been approved to prevent recurrent C. difficile infection. A new approach to the prevention of recurrent C. difficile infection is the administration of monoclonal antibodies against C. difficile toxins (in addition to antibiotic therapy) as a form of passive immunity. Actoxumab and bezlotoxumab are fully human monoclonal antibodies that bind and neutralize C. difficile toxins A and B, respectively. In humans, the level of circulating antibodies against toxin A or toxin B has been correlated with protection against primary and recurrent C. difficile infection.
Study design: Two (MODIFY [MK-6072 and MK-3415A in Participants Receiving Antibiotic Therapy for Clostridium Difficile Infection] I and MODIFY II) double-blind, randomized, placebo-controlled, phase III trials.
Setting: 322 sites (~68% inpatient) in 30 countries from Nov. 1, 2011, through May 22, 2015.
Synopsis: Trials pooled data from 2,174 adults who were receiving oral standard-of-care antibiotics for primary or recurrent C. difficile infections. Participants received an infusion of either bezlotoxumab, actoxumab plus bezlotoxumab, or placebo for MODIFY II; actoxumab alone was also given in MODIFY I. The primary endpoint was recurrent infection within 12 weeks.
The rate of recurrent C. difficile infection was significantly lower with bezlotoxumab alone than with placebo (MODIFY I: 17% vs. 28%; 95% CI, −15.9 to −4.3; P less than .001; MODIFY II: 16% vs. 26%; 95% CI, −15.5 to −4.3; P less than .001) and was significantly lower with actoxumab plus bezlotoxumab than with placebo (MODIFY I: 16% vs. 28%; 95% CI, −17.4 to −5.9; P less than .001; MODIFY II: 15% vs. 26%; 95% CI, −16.4 to −5.1; P less than .001).
The serious adverse events were similar with most groups, the exception being actoxumab alone. Given the higher rate of recurrent infection and deaths in the actoxumab group from interim analysis, the enrollment was discontinued in MODIFY I.
Investigators did admit that safety assessments were limited because of the relatively small number of patients who received bezlotoxumab, making it difficult to detect potentially serious but low-frequency toxic effects.
Bottom line: In patients receiving oral standard-of-care antibiotics for primary or recurrent C. difficile infection, a single intravenous infusion of bezlotoxumab was associated with a significantly lower rate of recurrent infection than placebo and had a safety profile similar to that of placebo.
Citation: Wilcox MH, Gerding DN, Poxton IR, et al. “Bezlotoxumab for prevention of recurrent Clostridium difficile infection.” N Engl J Med. 2017 Jan 26;376(4):305-17.
Dr. White is an instructor in the Division of Hospital Medicine, Loyola University Chicago.
Clinical question: Does administration of monoclonal antibodies to C. difficile toxins A and B, in addition to standard-of-care antibiotics, prevent recurrent infection?
Background: Currently, no therapy has been approved to prevent recurrent C. difficile infection. A new approach to the prevention of recurrent C. difficile infection is the administration of monoclonal antibodies against C. difficile toxins (in addition to antibiotic therapy) as a form of passive immunity. Actoxumab and bezlotoxumab are fully human monoclonal antibodies that bind and neutralize C. difficile toxins A and B, respectively. In humans, the level of circulating antibodies against toxin A or toxin B has been correlated with protection against primary and recurrent C. difficile infection.
Study design: Two (MODIFY [MK-6072 and MK-3415A in Participants Receiving Antibiotic Therapy for Clostridium Difficile Infection] I and MODIFY II) double-blind, randomized, placebo-controlled, phase III trials.
Setting: 322 sites (~68% inpatient) in 30 countries from Nov. 1, 2011, through May 22, 2015.
Synopsis: Trials pooled data from 2,174 adults who were receiving oral standard-of-care antibiotics for primary or recurrent C. difficile infections. Participants received an infusion of either bezlotoxumab, actoxumab plus bezlotoxumab, or placebo for MODIFY II; actoxumab alone was also given in MODIFY I. The primary endpoint was recurrent infection within 12 weeks.
The rate of recurrent C. difficile infection was significantly lower with bezlotoxumab alone than with placebo (MODIFY I: 17% vs. 28%; 95% CI, −15.9 to −4.3; P less than .001; MODIFY II: 16% vs. 26%; 95% CI, −15.5 to −4.3; P less than .001) and was significantly lower with actoxumab plus bezlotoxumab than with placebo (MODIFY I: 16% vs. 28%; 95% CI, −17.4 to −5.9; P less than .001; MODIFY II: 15% vs. 26%; 95% CI, −16.4 to −5.1; P less than .001).
The serious adverse events were similar with most groups, the exception being actoxumab alone. Given the higher rate of recurrent infection and deaths in the actoxumab group from interim analysis, the enrollment was discontinued in MODIFY I.
Investigators did admit that safety assessments were limited because of the relatively small number of patients who received bezlotoxumab, making it difficult to detect potentially serious but low-frequency toxic effects.
Bottom line: In patients receiving oral standard-of-care antibiotics for primary or recurrent C. difficile infection, a single intravenous infusion of bezlotoxumab was associated with a significantly lower rate of recurrent infection than placebo and had a safety profile similar to that of placebo.
Citation: Wilcox MH, Gerding DN, Poxton IR, et al. “Bezlotoxumab for prevention of recurrent Clostridium difficile infection.” N Engl J Med. 2017 Jan 26;376(4):305-17.
Dr. White is an instructor in the Division of Hospital Medicine, Loyola University Chicago.
Clinical question: Does administration of monoclonal antibodies to C. difficile toxins A and B, in addition to standard-of-care antibiotics, prevent recurrent infection?
Background: Currently, no therapy has been approved to prevent recurrent C. difficile infection. A new approach to the prevention of recurrent C. difficile infection is the administration of monoclonal antibodies against C. difficile toxins (in addition to antibiotic therapy) as a form of passive immunity. Actoxumab and bezlotoxumab are fully human monoclonal antibodies that bind and neutralize C. difficile toxins A and B, respectively. In humans, the level of circulating antibodies against toxin A or toxin B has been correlated with protection against primary and recurrent C. difficile infection.
Study design: Two (MODIFY [MK-6072 and MK-3415A in Participants Receiving Antibiotic Therapy for Clostridium Difficile Infection] I and MODIFY II) double-blind, randomized, placebo-controlled, phase III trials.
Setting: 322 sites (~68% inpatient) in 30 countries from Nov. 1, 2011, through May 22, 2015.
Synopsis: Trials pooled data from 2,174 adults who were receiving oral standard-of-care antibiotics for primary or recurrent C. difficile infections. Participants received an infusion of either bezlotoxumab, actoxumab plus bezlotoxumab, or placebo for MODIFY II; actoxumab alone was also given in MODIFY I. The primary endpoint was recurrent infection within 12 weeks.
The rate of recurrent C. difficile infection was significantly lower with bezlotoxumab alone than with placebo (MODIFY I: 17% vs. 28%; 95% CI, −15.9 to −4.3; P less than .001; MODIFY II: 16% vs. 26%; 95% CI, −15.5 to −4.3; P less than .001) and was significantly lower with actoxumab plus bezlotoxumab than with placebo (MODIFY I: 16% vs. 28%; 95% CI, −17.4 to −5.9; P less than .001; MODIFY II: 15% vs. 26%; 95% CI, −16.4 to −5.1; P less than .001).
The serious adverse events were similar with most groups, the exception being actoxumab alone. Given the higher rate of recurrent infection and deaths in the actoxumab group from interim analysis, the enrollment was discontinued in MODIFY I.
Investigators did admit that safety assessments were limited because of the relatively small number of patients who received bezlotoxumab, making it difficult to detect potentially serious but low-frequency toxic effects.
Bottom line: In patients receiving oral standard-of-care antibiotics for primary or recurrent C. difficile infection, a single intravenous infusion of bezlotoxumab was associated with a significantly lower rate of recurrent infection than placebo and had a safety profile similar to that of placebo.
Citation: Wilcox MH, Gerding DN, Poxton IR, et al. “Bezlotoxumab for prevention of recurrent Clostridium difficile infection.” N Engl J Med. 2017 Jan 26;376(4):305-17.
Dr. White is an instructor in the Division of Hospital Medicine, Loyola University Chicago.
HHS Secretary Price promises reduced health IT burden for physicians
WASHINGTON – Reducing IT burden for doctors and fostering interoperability are two top tech priorities for Health and Human Services Secretary Tom Price, MD.
“We simply have to do a better job of reducing the burden of health IT on physicians and all health care providers,” Dr. Price said April 27 at Health Datapalooza, an annual conference on health data transparency. “The promise of big data and health information technology is so great and absolutely remarkable but we must not, we cannot continue to get this wrong.”
“Now we are seeing physicians leaving the practice of medicine when they are 60 or 55,” he said. “Many of my colleagues, my personal friends who have been practicing, right now they are looking for the exit doors. They are trying to figure out how to get out of practicing medicine and I think it is incredibly important for us as a society to step back and ask, why?”
A significant factor is the shift to electronic health records, which has caused doctors to spend much more time looking at screens and feeling more like data entry clerks than health care providers, he said.
“I know that we have lost more than one physician to retirement because of the kinds of burdens that have been placed on a lot of them and that simply ought to be unacceptable to us,” he said. “You think of the intellectual capital that has been lost by this nation because of the kinds of burdens that clinicians have seen.”
That said, “data is absolutely crucial,” Dr. Price said. “Don’t misunderstand me. It is absolutely critical that we have all the data that we can and that we use it in an evidence-based manner so that we can provide better care and better quality of care to patients.”
He challenged the health IT professionals at the meeting to make their products more user friendly.
“We will work on reducing the burdens at the federal level, but we also need clinicians and IT folks on the ground to help make certain that technology implementation is done in a way that it enhances usability and increases efficiency,” Dr. Price said.
He also called for true interoperability, a common goal that has persisted since electronic health records were mandated under the HITECH Act but remains an elusive target.
“This has always been the goal and it just seems so simple,” he said. “Somehow something has happened between the idea of interoperability and now that has made it so much more challenging.”
He placed that fault on current federal regulation around interoperability and pledged to create an environment that reduces regulatory roadblocks and allows the technology sector to innovate and foster the free flow of data.
“From my perspective it seems that what we ought to be doing is deciding the rules of the road,” Dr. Price said. “We are going to drive on the right side. We are going to stop at the red light. This is the language we are going to do. This is what a triangular sign looks like, as opposed to stipulating every single dot... all the way down the line.”
WASHINGTON – Reducing IT burden for doctors and fostering interoperability are two top tech priorities for Health and Human Services Secretary Tom Price, MD.
“We simply have to do a better job of reducing the burden of health IT on physicians and all health care providers,” Dr. Price said April 27 at Health Datapalooza, an annual conference on health data transparency. “The promise of big data and health information technology is so great and absolutely remarkable but we must not, we cannot continue to get this wrong.”
“Now we are seeing physicians leaving the practice of medicine when they are 60 or 55,” he said. “Many of my colleagues, my personal friends who have been practicing, right now they are looking for the exit doors. They are trying to figure out how to get out of practicing medicine and I think it is incredibly important for us as a society to step back and ask, why?”
A significant factor is the shift to electronic health records, which has caused doctors to spend much more time looking at screens and feeling more like data entry clerks than health care providers, he said.
“I know that we have lost more than one physician to retirement because of the kinds of burdens that have been placed on a lot of them and that simply ought to be unacceptable to us,” he said. “You think of the intellectual capital that has been lost by this nation because of the kinds of burdens that clinicians have seen.”
That said, “data is absolutely crucial,” Dr. Price said. “Don’t misunderstand me. It is absolutely critical that we have all the data that we can and that we use it in an evidence-based manner so that we can provide better care and better quality of care to patients.”
He challenged the health IT professionals at the meeting to make their products more user friendly.
“We will work on reducing the burdens at the federal level, but we also need clinicians and IT folks on the ground to help make certain that technology implementation is done in a way that it enhances usability and increases efficiency,” Dr. Price said.
He also called for true interoperability, a common goal that has persisted since electronic health records were mandated under the HITECH Act but remains an elusive target.
“This has always been the goal and it just seems so simple,” he said. “Somehow something has happened between the idea of interoperability and now that has made it so much more challenging.”
He placed that fault on current federal regulation around interoperability and pledged to create an environment that reduces regulatory roadblocks and allows the technology sector to innovate and foster the free flow of data.
“From my perspective it seems that what we ought to be doing is deciding the rules of the road,” Dr. Price said. “We are going to drive on the right side. We are going to stop at the red light. This is the language we are going to do. This is what a triangular sign looks like, as opposed to stipulating every single dot... all the way down the line.”
WASHINGTON – Reducing IT burden for doctors and fostering interoperability are two top tech priorities for Health and Human Services Secretary Tom Price, MD.
“We simply have to do a better job of reducing the burden of health IT on physicians and all health care providers,” Dr. Price said April 27 at Health Datapalooza, an annual conference on health data transparency. “The promise of big data and health information technology is so great and absolutely remarkable but we must not, we cannot continue to get this wrong.”
“Now we are seeing physicians leaving the practice of medicine when they are 60 or 55,” he said. “Many of my colleagues, my personal friends who have been practicing, right now they are looking for the exit doors. They are trying to figure out how to get out of practicing medicine and I think it is incredibly important for us as a society to step back and ask, why?”
A significant factor is the shift to electronic health records, which has caused doctors to spend much more time looking at screens and feeling more like data entry clerks than health care providers, he said.
“I know that we have lost more than one physician to retirement because of the kinds of burdens that have been placed on a lot of them and that simply ought to be unacceptable to us,” he said. “You think of the intellectual capital that has been lost by this nation because of the kinds of burdens that clinicians have seen.”
That said, “data is absolutely crucial,” Dr. Price said. “Don’t misunderstand me. It is absolutely critical that we have all the data that we can and that we use it in an evidence-based manner so that we can provide better care and better quality of care to patients.”
He challenged the health IT professionals at the meeting to make their products more user friendly.
“We will work on reducing the burdens at the federal level, but we also need clinicians and IT folks on the ground to help make certain that technology implementation is done in a way that it enhances usability and increases efficiency,” Dr. Price said.
He also called for true interoperability, a common goal that has persisted since electronic health records were mandated under the HITECH Act but remains an elusive target.
“This has always been the goal and it just seems so simple,” he said. “Somehow something has happened between the idea of interoperability and now that has made it so much more challenging.”
He placed that fault on current federal regulation around interoperability and pledged to create an environment that reduces regulatory roadblocks and allows the technology sector to innovate and foster the free flow of data.
“From my perspective it seems that what we ought to be doing is deciding the rules of the road,” Dr. Price said. “We are going to drive on the right side. We are going to stop at the red light. This is the language we are going to do. This is what a triangular sign looks like, as opposed to stipulating every single dot... all the way down the line.”
AT HEALTH DATAPALOOZA 2017
Hospitalists: Leading health care innovation
As I begin my year as SHM president, I continue to be energized by the opportunity to be part of an organization that has such a positive impact on our nation’s health care system. From the beginning of my medical career to now, never have I witnessed a health care movement quite like hospital medicine.
Even when I first arrived in Southern California as a pulmonary/critical-care physician in 1987, there were groups of physicians who had taken financial risk on populations of managed-care patients and were paid using an “alternative payment model” called capitation. One of the innovations they had utilized since the early ’80s to successfully manage their risk – and their patients’ – was to have dedicated inpatient physicians caring for their hospitalized patients 24/7, while most of their primary care partners managed the group’s patients in the outpatient setting.
This year will see a continued reshaping of our delivery system, driven by emerging federal policy like the Medicare Access and CHIP Reauthorization Act (MACRA). All of this policy is designed to create a health care system that delivers high-quality care in a much more cost effective way. Many of these policies will result in groups of providers being pushed away from fee-for-service payment toward alternative payment models that involve higher levels of risk and opportunity. If we, as providers, are going to be successful in managing our “at risk” populations, we are going to have to be as innovative as our managed care forefathers. If we are not, we, as a society, are not going to be able to afford to deliver high-quality care to our nations sickest citizens.
At the center of much of this innovation will be hospitalists. After all, by its very nature, our model is a delivery system reform. The drive to deliver more-efficient quality care is in the very DNA of our specialty.
As decisions are made, they will have a significant impact on our patients and our careers. It will continue to be a priority for SHM to make sure that the voice of hospital medicine is heard loud and clear. We will continue to ask our members to ensure that the hospital medicine community has a prominent place in these conversations. Those who step up in this effort will lead us as we insist on having a prominent seat at the table and as new models of care emerge and new incentives are created for the provider community. We will continue to strive to make sure that our patients get the care they deserve and that we continue to help build a sustainable health care delivery system.
This year, you will also see a focused effort to strengthen SHM’s system of state and local chapters. The vitality of these local organizations is important to our efforts to effectively serve our members by engaging them with their colleagues at the local level. In our attempts to further connect our members with others who share similar interests and focuses, we will be rolling out a new structure of special interest groups. These local chapters and these interest groups will fuel new ideas that will continue to improve our specialty and the effectiveness of the society to speak for hospital medicine with a strong voice.
Of course, SHM will continue to be the only organization that was created to represent our nation’s hospitalists and will be totally committed to providing our members with clinical and administrative education, dedicated publications, leadership training, research opportunities, and advocacy. I look forward to serving you and helping you get the most from your SHM experience. Together, we will continue to move the hospital medicine movement forward, shaping our health care system and improving patient care.
Dr. Greeno is the incoming president of the Society of Hospital Medicine and senior adviser for medical affairs at TeamHealth.
As I begin my year as SHM president, I continue to be energized by the opportunity to be part of an organization that has such a positive impact on our nation’s health care system. From the beginning of my medical career to now, never have I witnessed a health care movement quite like hospital medicine.
Even when I first arrived in Southern California as a pulmonary/critical-care physician in 1987, there were groups of physicians who had taken financial risk on populations of managed-care patients and were paid using an “alternative payment model” called capitation. One of the innovations they had utilized since the early ’80s to successfully manage their risk – and their patients’ – was to have dedicated inpatient physicians caring for their hospitalized patients 24/7, while most of their primary care partners managed the group’s patients in the outpatient setting.
This year will see a continued reshaping of our delivery system, driven by emerging federal policy like the Medicare Access and CHIP Reauthorization Act (MACRA). All of this policy is designed to create a health care system that delivers high-quality care in a much more cost effective way. Many of these policies will result in groups of providers being pushed away from fee-for-service payment toward alternative payment models that involve higher levels of risk and opportunity. If we, as providers, are going to be successful in managing our “at risk” populations, we are going to have to be as innovative as our managed care forefathers. If we are not, we, as a society, are not going to be able to afford to deliver high-quality care to our nations sickest citizens.
At the center of much of this innovation will be hospitalists. After all, by its very nature, our model is a delivery system reform. The drive to deliver more-efficient quality care is in the very DNA of our specialty.
As decisions are made, they will have a significant impact on our patients and our careers. It will continue to be a priority for SHM to make sure that the voice of hospital medicine is heard loud and clear. We will continue to ask our members to ensure that the hospital medicine community has a prominent place in these conversations. Those who step up in this effort will lead us as we insist on having a prominent seat at the table and as new models of care emerge and new incentives are created for the provider community. We will continue to strive to make sure that our patients get the care they deserve and that we continue to help build a sustainable health care delivery system.
This year, you will also see a focused effort to strengthen SHM’s system of state and local chapters. The vitality of these local organizations is important to our efforts to effectively serve our members by engaging them with their colleagues at the local level. In our attempts to further connect our members with others who share similar interests and focuses, we will be rolling out a new structure of special interest groups. These local chapters and these interest groups will fuel new ideas that will continue to improve our specialty and the effectiveness of the society to speak for hospital medicine with a strong voice.
Of course, SHM will continue to be the only organization that was created to represent our nation’s hospitalists and will be totally committed to providing our members with clinical and administrative education, dedicated publications, leadership training, research opportunities, and advocacy. I look forward to serving you and helping you get the most from your SHM experience. Together, we will continue to move the hospital medicine movement forward, shaping our health care system and improving patient care.
Dr. Greeno is the incoming president of the Society of Hospital Medicine and senior adviser for medical affairs at TeamHealth.
As I begin my year as SHM president, I continue to be energized by the opportunity to be part of an organization that has such a positive impact on our nation’s health care system. From the beginning of my medical career to now, never have I witnessed a health care movement quite like hospital medicine.
Even when I first arrived in Southern California as a pulmonary/critical-care physician in 1987, there were groups of physicians who had taken financial risk on populations of managed-care patients and were paid using an “alternative payment model” called capitation. One of the innovations they had utilized since the early ’80s to successfully manage their risk – and their patients’ – was to have dedicated inpatient physicians caring for their hospitalized patients 24/7, while most of their primary care partners managed the group’s patients in the outpatient setting.
This year will see a continued reshaping of our delivery system, driven by emerging federal policy like the Medicare Access and CHIP Reauthorization Act (MACRA). All of this policy is designed to create a health care system that delivers high-quality care in a much more cost effective way. Many of these policies will result in groups of providers being pushed away from fee-for-service payment toward alternative payment models that involve higher levels of risk and opportunity. If we, as providers, are going to be successful in managing our “at risk” populations, we are going to have to be as innovative as our managed care forefathers. If we are not, we, as a society, are not going to be able to afford to deliver high-quality care to our nations sickest citizens.
At the center of much of this innovation will be hospitalists. After all, by its very nature, our model is a delivery system reform. The drive to deliver more-efficient quality care is in the very DNA of our specialty.
As decisions are made, they will have a significant impact on our patients and our careers. It will continue to be a priority for SHM to make sure that the voice of hospital medicine is heard loud and clear. We will continue to ask our members to ensure that the hospital medicine community has a prominent place in these conversations. Those who step up in this effort will lead us as we insist on having a prominent seat at the table and as new models of care emerge and new incentives are created for the provider community. We will continue to strive to make sure that our patients get the care they deserve and that we continue to help build a sustainable health care delivery system.
This year, you will also see a focused effort to strengthen SHM’s system of state and local chapters. The vitality of these local organizations is important to our efforts to effectively serve our members by engaging them with their colleagues at the local level. In our attempts to further connect our members with others who share similar interests and focuses, we will be rolling out a new structure of special interest groups. These local chapters and these interest groups will fuel new ideas that will continue to improve our specialty and the effectiveness of the society to speak for hospital medicine with a strong voice.
Of course, SHM will continue to be the only organization that was created to represent our nation’s hospitalists and will be totally committed to providing our members with clinical and administrative education, dedicated publications, leadership training, research opportunities, and advocacy. I look forward to serving you and helping you get the most from your SHM experience. Together, we will continue to move the hospital medicine movement forward, shaping our health care system and improving patient care.
Dr. Greeno is the incoming president of the Society of Hospital Medicine and senior adviser for medical affairs at TeamHealth.
Study Reveals Tumor Blood Vessels Cluster in Beltlike Zones
Digital pathology has made it possible to measure microscopic objects, such as blood vessels in tumor tissue and then visualize them in density maps, showing hotspot regions. But for many applications in histopathology, there is no clear-cut definition of the hotspots, say researchers from Heidelberg University in Germany. Thus, most tumor models “implicitly assume” that blood vessels are equally abundant in different parts of a tumor. But the researchers’ new computational approach to mapping angiogenesis in colorectal cancer (CRC) could change that assumption.
Related: In Rare Case Colorectal Cancer Causes Thrombus
Their method analyzes blood vessels based on spatial statistics, identifying all hotspot areas that are unlikely to occur by chance. The researchers found that in nearly all cases, the blood vessels grouped in a distinctive beltlike pattern. In 33 of 34 untreated colorectal tumor samples, the blood vessels were aggregated at the interface of tumor tissue to the intestinal wall. The researchers found similar “hypervascularized” zones at the boundaries of liver tissue in 100% of the samples of CRC liver metastases. Ultimately, they describe a new model of tumor vascularization: a highly vascularized zone about 1.5-mm wide close to the intestinal lumen in CRC primary tumors and a highly vascularized zone approximately 1-mm wide close to the invasion front in CRC liver metastases.
Related: Colorectal Screening: Available but Underused
Their model has immediate and far-reaching implications, the researchers say. For instance, because vascular patterns determine how chemotherapeutic drugs are distributed in tumor tissue, it is likely that these drugs reach the luminal side of CRC tumors much easier than they reach the the basolateral side. Their new information also could be used in timing surgery, since the tumor parts of the deep invasion front may be less sensitive to chemotherapy. The researchers suggest that using their model could help optimize treatment in any number of ways: explaining early symptoms like gastrointestinal bleeding, the architecture of CRC, metastasis, and opening new pathways for investigation.
Source:
Kather JN, Zöllner FG, Schad LR. PLoS One. 2017;12(3):e0171378.
doi: 10.1371/journal.pone.0171378.
Digital pathology has made it possible to measure microscopic objects, such as blood vessels in tumor tissue and then visualize them in density maps, showing hotspot regions. But for many applications in histopathology, there is no clear-cut definition of the hotspots, say researchers from Heidelberg University in Germany. Thus, most tumor models “implicitly assume” that blood vessels are equally abundant in different parts of a tumor. But the researchers’ new computational approach to mapping angiogenesis in colorectal cancer (CRC) could change that assumption.
Related: In Rare Case Colorectal Cancer Causes Thrombus
Their method analyzes blood vessels based on spatial statistics, identifying all hotspot areas that are unlikely to occur by chance. The researchers found that in nearly all cases, the blood vessels grouped in a distinctive beltlike pattern. In 33 of 34 untreated colorectal tumor samples, the blood vessels were aggregated at the interface of tumor tissue to the intestinal wall. The researchers found similar “hypervascularized” zones at the boundaries of liver tissue in 100% of the samples of CRC liver metastases. Ultimately, they describe a new model of tumor vascularization: a highly vascularized zone about 1.5-mm wide close to the intestinal lumen in CRC primary tumors and a highly vascularized zone approximately 1-mm wide close to the invasion front in CRC liver metastases.
Related: Colorectal Screening: Available but Underused
Their model has immediate and far-reaching implications, the researchers say. For instance, because vascular patterns determine how chemotherapeutic drugs are distributed in tumor tissue, it is likely that these drugs reach the luminal side of CRC tumors much easier than they reach the the basolateral side. Their new information also could be used in timing surgery, since the tumor parts of the deep invasion front may be less sensitive to chemotherapy. The researchers suggest that using their model could help optimize treatment in any number of ways: explaining early symptoms like gastrointestinal bleeding, the architecture of CRC, metastasis, and opening new pathways for investigation.
Source:
Kather JN, Zöllner FG, Schad LR. PLoS One. 2017;12(3):e0171378.
doi: 10.1371/journal.pone.0171378.
Digital pathology has made it possible to measure microscopic objects, such as blood vessels in tumor tissue and then visualize them in density maps, showing hotspot regions. But for many applications in histopathology, there is no clear-cut definition of the hotspots, say researchers from Heidelberg University in Germany. Thus, most tumor models “implicitly assume” that blood vessels are equally abundant in different parts of a tumor. But the researchers’ new computational approach to mapping angiogenesis in colorectal cancer (CRC) could change that assumption.
Related: In Rare Case Colorectal Cancer Causes Thrombus
Their method analyzes blood vessels based on spatial statistics, identifying all hotspot areas that are unlikely to occur by chance. The researchers found that in nearly all cases, the blood vessels grouped in a distinctive beltlike pattern. In 33 of 34 untreated colorectal tumor samples, the blood vessels were aggregated at the interface of tumor tissue to the intestinal wall. The researchers found similar “hypervascularized” zones at the boundaries of liver tissue in 100% of the samples of CRC liver metastases. Ultimately, they describe a new model of tumor vascularization: a highly vascularized zone about 1.5-mm wide close to the intestinal lumen in CRC primary tumors and a highly vascularized zone approximately 1-mm wide close to the invasion front in CRC liver metastases.
Related: Colorectal Screening: Available but Underused
Their model has immediate and far-reaching implications, the researchers say. For instance, because vascular patterns determine how chemotherapeutic drugs are distributed in tumor tissue, it is likely that these drugs reach the luminal side of CRC tumors much easier than they reach the the basolateral side. Their new information also could be used in timing surgery, since the tumor parts of the deep invasion front may be less sensitive to chemotherapy. The researchers suggest that using their model could help optimize treatment in any number of ways: explaining early symptoms like gastrointestinal bleeding, the architecture of CRC, metastasis, and opening new pathways for investigation.
Source:
Kather JN, Zöllner FG, Schad LR. PLoS One. 2017;12(3):e0171378.
doi: 10.1371/journal.pone.0171378.
Fertility treatments linked to risk of pediatric cancers
Children born to mothers who underwent fertility treatments have an increased risk of developing pediatric neoplasms, according to research published in the American Journal of Obstetrics & Gynecology.
The study showed an increased risk of malignancies and benign tumors among children conceived after fertility treatments.
However, the risk of leukemias and lymphomas among these children was not significantly different from the risk among children who were conceived spontaneously.
The study was a population-based cohort analysis of babies born between 1991 and 2013 at Soroka University Medical Center in Beer-Sheva, Israel, with follow-up to age 18.
Of the 242,187 newborn infants in the study, 237,863 (98.3%) were conceived spontaneously, 2603 (1.1%) were conceived after in vitro fertilization (IVF), and 1721 (0.7%) were conceived after ovulation induction (OI) treatments.
During a median follow-up of 10.55 years, there were 1498 neoplasms reported, including 1074 benign tumors and 429 malignancies.
The rate of neoplasms per 10,000 children was 61.85 for the entire study cohort, 111.41 for the IVF group, 110.40 for the OI group, and 60.96 for the spontaneous conception group (P<0.001 for the comparison between spontaneous conception and both types of fertility treatments).
The rate of benign tumors per 10,000 children was 44.35 for the entire study cohort, 84.51 for the IVF group, 69.73 for the OI group, and 43.72 for the spontaneous conception group (P=0.002).
The rate of malignancies per 10,000 children was 17.71 for the entire study cohort, 26.89 for the IVF group, 40.67 for the OI group, and 17.44 for the spontaneous conception group (P=0.038).
The rate of leukemia per 10,000 children was 3.72 for the entire study cohort (n=90 leukemia cases total), 0 for the IVF group (n=0), 5.81 for the OI group (n=1), and 3.74 for the spontaneous conception group (n=89, P=0.56).
The rate of lymphoma per 10,000 children was 2.27 for the entire study cohort (n=55), 7.68 for the IVF group (n=2), 0 for the OI group (n=0), and 2.23 for the spontaneous conception group (n=53, P=0.15).
The researchers said the association between fertility treatments and total pediatric neoplasms or total malignancies remained significant in analyses controlled for confounders such as gestational diabetes mellitus, hypertensive disorders, preterm birth, and maternal age.
For any fertility treatment, the adjusted hazard ratio (aHR) for all neoplasms was 1.97, and the aHR for all malignancies was 1.96.
For IVF, the aHR was 2.48 for all neoplasms and 1.89 for all malignancies. For OI, the aHR was 1.51 for all neoplasms and 2.03 for all malignancies.
“The research concludes that the association between IVF and total pediatric neoplasms and malignancies is significant,” said study author Eyal Sheiner, MD, PhD, of Ben-Gurion University of the Negev in Beer-Sheva, Israel.
“With increasing numbers of offspring conceived after fertility treatments, it is important to follow up on their health.” ![]()
Children born to mothers who underwent fertility treatments have an increased risk of developing pediatric neoplasms, according to research published in the American Journal of Obstetrics & Gynecology.
The study showed an increased risk of malignancies and benign tumors among children conceived after fertility treatments.
However, the risk of leukemias and lymphomas among these children was not significantly different from the risk among children who were conceived spontaneously.
The study was a population-based cohort analysis of babies born between 1991 and 2013 at Soroka University Medical Center in Beer-Sheva, Israel, with follow-up to age 18.
Of the 242,187 newborn infants in the study, 237,863 (98.3%) were conceived spontaneously, 2603 (1.1%) were conceived after in vitro fertilization (IVF), and 1721 (0.7%) were conceived after ovulation induction (OI) treatments.
During a median follow-up of 10.55 years, there were 1498 neoplasms reported, including 1074 benign tumors and 429 malignancies.
The rate of neoplasms per 10,000 children was 61.85 for the entire study cohort, 111.41 for the IVF group, 110.40 for the OI group, and 60.96 for the spontaneous conception group (P<0.001 for the comparison between spontaneous conception and both types of fertility treatments).
The rate of benign tumors per 10,000 children was 44.35 for the entire study cohort, 84.51 for the IVF group, 69.73 for the OI group, and 43.72 for the spontaneous conception group (P=0.002).
The rate of malignancies per 10,000 children was 17.71 for the entire study cohort, 26.89 for the IVF group, 40.67 for the OI group, and 17.44 for the spontaneous conception group (P=0.038).
The rate of leukemia per 10,000 children was 3.72 for the entire study cohort (n=90 leukemia cases total), 0 for the IVF group (n=0), 5.81 for the OI group (n=1), and 3.74 for the spontaneous conception group (n=89, P=0.56).
The rate of lymphoma per 10,000 children was 2.27 for the entire study cohort (n=55), 7.68 for the IVF group (n=2), 0 for the OI group (n=0), and 2.23 for the spontaneous conception group (n=53, P=0.15).
The researchers said the association between fertility treatments and total pediatric neoplasms or total malignancies remained significant in analyses controlled for confounders such as gestational diabetes mellitus, hypertensive disorders, preterm birth, and maternal age.
For any fertility treatment, the adjusted hazard ratio (aHR) for all neoplasms was 1.97, and the aHR for all malignancies was 1.96.
For IVF, the aHR was 2.48 for all neoplasms and 1.89 for all malignancies. For OI, the aHR was 1.51 for all neoplasms and 2.03 for all malignancies.
“The research concludes that the association between IVF and total pediatric neoplasms and malignancies is significant,” said study author Eyal Sheiner, MD, PhD, of Ben-Gurion University of the Negev in Beer-Sheva, Israel.
“With increasing numbers of offspring conceived after fertility treatments, it is important to follow up on their health.” ![]()
Children born to mothers who underwent fertility treatments have an increased risk of developing pediatric neoplasms, according to research published in the American Journal of Obstetrics & Gynecology.
The study showed an increased risk of malignancies and benign tumors among children conceived after fertility treatments.
However, the risk of leukemias and lymphomas among these children was not significantly different from the risk among children who were conceived spontaneously.
The study was a population-based cohort analysis of babies born between 1991 and 2013 at Soroka University Medical Center in Beer-Sheva, Israel, with follow-up to age 18.
Of the 242,187 newborn infants in the study, 237,863 (98.3%) were conceived spontaneously, 2603 (1.1%) were conceived after in vitro fertilization (IVF), and 1721 (0.7%) were conceived after ovulation induction (OI) treatments.
During a median follow-up of 10.55 years, there were 1498 neoplasms reported, including 1074 benign tumors and 429 malignancies.
The rate of neoplasms per 10,000 children was 61.85 for the entire study cohort, 111.41 for the IVF group, 110.40 for the OI group, and 60.96 for the spontaneous conception group (P<0.001 for the comparison between spontaneous conception and both types of fertility treatments).
The rate of benign tumors per 10,000 children was 44.35 for the entire study cohort, 84.51 for the IVF group, 69.73 for the OI group, and 43.72 for the spontaneous conception group (P=0.002).
The rate of malignancies per 10,000 children was 17.71 for the entire study cohort, 26.89 for the IVF group, 40.67 for the OI group, and 17.44 for the spontaneous conception group (P=0.038).
The rate of leukemia per 10,000 children was 3.72 for the entire study cohort (n=90 leukemia cases total), 0 for the IVF group (n=0), 5.81 for the OI group (n=1), and 3.74 for the spontaneous conception group (n=89, P=0.56).
The rate of lymphoma per 10,000 children was 2.27 for the entire study cohort (n=55), 7.68 for the IVF group (n=2), 0 for the OI group (n=0), and 2.23 for the spontaneous conception group (n=53, P=0.15).
The researchers said the association between fertility treatments and total pediatric neoplasms or total malignancies remained significant in analyses controlled for confounders such as gestational diabetes mellitus, hypertensive disorders, preterm birth, and maternal age.
For any fertility treatment, the adjusted hazard ratio (aHR) for all neoplasms was 1.97, and the aHR for all malignancies was 1.96.
For IVF, the aHR was 2.48 for all neoplasms and 1.89 for all malignancies. For OI, the aHR was 1.51 for all neoplasms and 2.03 for all malignancies.
“The research concludes that the association between IVF and total pediatric neoplasms and malignancies is significant,” said study author Eyal Sheiner, MD, PhD, of Ben-Gurion University of the Negev in Beer-Sheva, Israel.
“With increasing numbers of offspring conceived after fertility treatments, it is important to follow up on their health.” ![]()
TXA lowers risk of death from post-partum hemorrhage
Treatment with tranexamic acid (TXA) should become the frontline response to major bleeding after childbirth, according to researchers.
Results of a global study showed that TXA can reduce death due to bleeding in women with post-partum hemorrhage, particularly when the drug is given early.
TXA reduced deaths from bleeding by 19% overall and by 31% when patients received TXA within 3 hours of giving birth.
In addition, there was no significant difference in adverse events for women who received TXA and those who received placebo.
Researchers reported these results in The Lancet.
Funds to support the drug and placebo costs in the run-in phase of the trial were provided by Pfizer. The trial was also funded by the London School of Hygiene & Tropical Medicine, UK Department of Health, Wellcome Trust, and Bill & Melinda Gates Foundation.
The trial included 20,060 mothers, age 16 and older, who were treated at 193 hospitals in 21 countries.
All of the women had a clinical diagnosis of post-partum hemorrhage—defined as a blood loss of more than 500 ml within 24 hours of giving birth—after a vaginal birth or caesarean section.
The women were randomized to receive either 1 g of intravenous TXA (n=10,051) or matching placebo (n=10,009) in addition to standard care. The patients could receive a second dose of TXA or placebo if their bleeding continued after 30 minutes or stopped and restarted within 24 hours of the first dose.
The patients and their caregivers were blinded to randomization.
Results
In the final analysis, there were 10,036 women in the TXA arm and 9985 in the placebo arm.
TXA significantly reduced the risk of death from bleeding. The incidence of death from bleeding was 1.5% (n=155) among women who received TXA and 1.9% (n=191) in the placebo group. The risk ratio (RR) was 0.81 (P=0.045).
The incidence of death due to bleeding was 1.2% (n=89) among women who received TXA within 3 hours of giving birth and 1.7% (n=127) among women who received placebo within the same time period. The RR was 0.69 (P=0.008).
There was no significant difference between the treatment groups for other causes of death, and there was no significant difference in the use of hysterectomy.
Likewise, there was no significant difference between the treatment groups when it came to the composite endpoint of death from all causes or hysterectomy within 42 days of giving birth. This endpoint occurred in 5.3% (n=534) of patients in the TXA group and 5.6% (n=546) of those in the placebo group. The RR was 0.97 (P=0.65).
There was no significant difference between the treatment groups in the use of blood products. Fifty-four percent of patients in each group received blood transfusions. Among women who received transfusions, there was no significant difference in the mean number of units received.
On the other hand, there was a significant reduction in laparotomy to control bleeding for patients who received TXA (0.8%, n=82) compared to placebo (1.3%, n=127), with an RR of 0.64 (P=0.002).
There was no significant difference between the treatment groups (TXA and placebo, respectively) with regard to thromboembolic events (0.3% vs 0.3%), renal failure (1.3% vs 1.2%), cardiac failure (1.1% vs 1.2%), respiratory failure (1.1% vs 1.2%), hepatic failure (0.3% vs 0.3%), sepsis (1.8% vs 1.9%), or seizure (0.3% vs 0.4%).
And there was no significant difference between the treatment groups in quality of life measures, such as pain/discomfort, anxiety/depression, and mobility.
“We now have important evidence that the early use of tranexamic acid can save women’s lives and ensure more children grow up with a mother,” said study author Haleema Shakur, of the London School of Hygiene & Tropical Medicine in the UK.
“[TXA is] safe, affordable, and easy to administer, and we hope that doctors will use it as early as possible following the onset of severe bleeding after childbirth.”
Current World Health Organization guidelines recommend the use of TXA in post-partum hemorrhage as a treatment option if uterotonics fail to control the bleeding or if the bleeding is thought to be due to trauma. ![]()
Treatment with tranexamic acid (TXA) should become the frontline response to major bleeding after childbirth, according to researchers.
Results of a global study showed that TXA can reduce death due to bleeding in women with post-partum hemorrhage, particularly when the drug is given early.
TXA reduced deaths from bleeding by 19% overall and by 31% when patients received TXA within 3 hours of giving birth.
In addition, there was no significant difference in adverse events for women who received TXA and those who received placebo.
Researchers reported these results in The Lancet.
Funds to support the drug and placebo costs in the run-in phase of the trial were provided by Pfizer. The trial was also funded by the London School of Hygiene & Tropical Medicine, UK Department of Health, Wellcome Trust, and Bill & Melinda Gates Foundation.
The trial included 20,060 mothers, age 16 and older, who were treated at 193 hospitals in 21 countries.
All of the women had a clinical diagnosis of post-partum hemorrhage—defined as a blood loss of more than 500 ml within 24 hours of giving birth—after a vaginal birth or caesarean section.
The women were randomized to receive either 1 g of intravenous TXA (n=10,051) or matching placebo (n=10,009) in addition to standard care. The patients could receive a second dose of TXA or placebo if their bleeding continued after 30 minutes or stopped and restarted within 24 hours of the first dose.
The patients and their caregivers were blinded to randomization.
Results
In the final analysis, there were 10,036 women in the TXA arm and 9985 in the placebo arm.
TXA significantly reduced the risk of death from bleeding. The incidence of death from bleeding was 1.5% (n=155) among women who received TXA and 1.9% (n=191) in the placebo group. The risk ratio (RR) was 0.81 (P=0.045).
The incidence of death due to bleeding was 1.2% (n=89) among women who received TXA within 3 hours of giving birth and 1.7% (n=127) among women who received placebo within the same time period. The RR was 0.69 (P=0.008).
There was no significant difference between the treatment groups for other causes of death, and there was no significant difference in the use of hysterectomy.
Likewise, there was no significant difference between the treatment groups when it came to the composite endpoint of death from all causes or hysterectomy within 42 days of giving birth. This endpoint occurred in 5.3% (n=534) of patients in the TXA group and 5.6% (n=546) of those in the placebo group. The RR was 0.97 (P=0.65).
There was no significant difference between the treatment groups in the use of blood products. Fifty-four percent of patients in each group received blood transfusions. Among women who received transfusions, there was no significant difference in the mean number of units received.
On the other hand, there was a significant reduction in laparotomy to control bleeding for patients who received TXA (0.8%, n=82) compared to placebo (1.3%, n=127), with an RR of 0.64 (P=0.002).
There was no significant difference between the treatment groups (TXA and placebo, respectively) with regard to thromboembolic events (0.3% vs 0.3%), renal failure (1.3% vs 1.2%), cardiac failure (1.1% vs 1.2%), respiratory failure (1.1% vs 1.2%), hepatic failure (0.3% vs 0.3%), sepsis (1.8% vs 1.9%), or seizure (0.3% vs 0.4%).
And there was no significant difference between the treatment groups in quality of life measures, such as pain/discomfort, anxiety/depression, and mobility.
“We now have important evidence that the early use of tranexamic acid can save women’s lives and ensure more children grow up with a mother,” said study author Haleema Shakur, of the London School of Hygiene & Tropical Medicine in the UK.
“[TXA is] safe, affordable, and easy to administer, and we hope that doctors will use it as early as possible following the onset of severe bleeding after childbirth.”
Current World Health Organization guidelines recommend the use of TXA in post-partum hemorrhage as a treatment option if uterotonics fail to control the bleeding or if the bleeding is thought to be due to trauma. ![]()
Treatment with tranexamic acid (TXA) should become the frontline response to major bleeding after childbirth, according to researchers.
Results of a global study showed that TXA can reduce death due to bleeding in women with post-partum hemorrhage, particularly when the drug is given early.
TXA reduced deaths from bleeding by 19% overall and by 31% when patients received TXA within 3 hours of giving birth.
In addition, there was no significant difference in adverse events for women who received TXA and those who received placebo.
Researchers reported these results in The Lancet.
Funds to support the drug and placebo costs in the run-in phase of the trial were provided by Pfizer. The trial was also funded by the London School of Hygiene & Tropical Medicine, UK Department of Health, Wellcome Trust, and Bill & Melinda Gates Foundation.
The trial included 20,060 mothers, age 16 and older, who were treated at 193 hospitals in 21 countries.
All of the women had a clinical diagnosis of post-partum hemorrhage—defined as a blood loss of more than 500 ml within 24 hours of giving birth—after a vaginal birth or caesarean section.
The women were randomized to receive either 1 g of intravenous TXA (n=10,051) or matching placebo (n=10,009) in addition to standard care. The patients could receive a second dose of TXA or placebo if their bleeding continued after 30 minutes or stopped and restarted within 24 hours of the first dose.
The patients and their caregivers were blinded to randomization.
Results
In the final analysis, there were 10,036 women in the TXA arm and 9985 in the placebo arm.
TXA significantly reduced the risk of death from bleeding. The incidence of death from bleeding was 1.5% (n=155) among women who received TXA and 1.9% (n=191) in the placebo group. The risk ratio (RR) was 0.81 (P=0.045).
The incidence of death due to bleeding was 1.2% (n=89) among women who received TXA within 3 hours of giving birth and 1.7% (n=127) among women who received placebo within the same time period. The RR was 0.69 (P=0.008).
There was no significant difference between the treatment groups for other causes of death, and there was no significant difference in the use of hysterectomy.
Likewise, there was no significant difference between the treatment groups when it came to the composite endpoint of death from all causes or hysterectomy within 42 days of giving birth. This endpoint occurred in 5.3% (n=534) of patients in the TXA group and 5.6% (n=546) of those in the placebo group. The RR was 0.97 (P=0.65).
There was no significant difference between the treatment groups in the use of blood products. Fifty-four percent of patients in each group received blood transfusions. Among women who received transfusions, there was no significant difference in the mean number of units received.
On the other hand, there was a significant reduction in laparotomy to control bleeding for patients who received TXA (0.8%, n=82) compared to placebo (1.3%, n=127), with an RR of 0.64 (P=0.002).
There was no significant difference between the treatment groups (TXA and placebo, respectively) with regard to thromboembolic events (0.3% vs 0.3%), renal failure (1.3% vs 1.2%), cardiac failure (1.1% vs 1.2%), respiratory failure (1.1% vs 1.2%), hepatic failure (0.3% vs 0.3%), sepsis (1.8% vs 1.9%), or seizure (0.3% vs 0.4%).
And there was no significant difference between the treatment groups in quality of life measures, such as pain/discomfort, anxiety/depression, and mobility.
“We now have important evidence that the early use of tranexamic acid can save women’s lives and ensure more children grow up with a mother,” said study author Haleema Shakur, of the London School of Hygiene & Tropical Medicine in the UK.
“[TXA is] safe, affordable, and easy to administer, and we hope that doctors will use it as early as possible following the onset of severe bleeding after childbirth.”
Current World Health Organization guidelines recommend the use of TXA in post-partum hemorrhage as a treatment option if uterotonics fail to control the bleeding or if the bleeding is thought to be due to trauma. ![]()
Program allows select Europeans access to belinostat
A managed access program is making the histone deacetylase inhibitor belinostat (Beleodaq®) available to patients in Europe who have relapsed or refractory peripheral T-cell lymphoma (PTCL).
The program allows physicians to request belinostat for individual PTCL patients who have no alternative treatment options.
This enables patients to use belinostat ahead of a potential European approval. There are currently no approved treatments for PTCL in Europe.
The program will provide access to belinostat for patients in the UK, Germany, France, Spain, Italy, Denmark, Sweden, Norway, Finland, Belgium, The Netherlands, Luxembourg, and Austria.
The managed access program was made possible via an agreement between Onxeo, the company developing belinostat, and Clinigen Group plc., a company focused on providing access to medicines.
Healthcare professionals can obtain details about the belinostat managed access program by calling a Clinigen representative at +44 (0) 1283 44 347 or emailing [email protected]. ![]()
A managed access program is making the histone deacetylase inhibitor belinostat (Beleodaq®) available to patients in Europe who have relapsed or refractory peripheral T-cell lymphoma (PTCL).
The program allows physicians to request belinostat for individual PTCL patients who have no alternative treatment options.
This enables patients to use belinostat ahead of a potential European approval. There are currently no approved treatments for PTCL in Europe.
The program will provide access to belinostat for patients in the UK, Germany, France, Spain, Italy, Denmark, Sweden, Norway, Finland, Belgium, The Netherlands, Luxembourg, and Austria.
The managed access program was made possible via an agreement between Onxeo, the company developing belinostat, and Clinigen Group plc., a company focused on providing access to medicines.
Healthcare professionals can obtain details about the belinostat managed access program by calling a Clinigen representative at +44 (0) 1283 44 347 or emailing [email protected]. ![]()
A managed access program is making the histone deacetylase inhibitor belinostat (Beleodaq®) available to patients in Europe who have relapsed or refractory peripheral T-cell lymphoma (PTCL).
The program allows physicians to request belinostat for individual PTCL patients who have no alternative treatment options.
This enables patients to use belinostat ahead of a potential European approval. There are currently no approved treatments for PTCL in Europe.
The program will provide access to belinostat for patients in the UK, Germany, France, Spain, Italy, Denmark, Sweden, Norway, Finland, Belgium, The Netherlands, Luxembourg, and Austria.
The managed access program was made possible via an agreement between Onxeo, the company developing belinostat, and Clinigen Group plc., a company focused on providing access to medicines.
Healthcare professionals can obtain details about the belinostat managed access program by calling a Clinigen representative at +44 (0) 1283 44 347 or emailing [email protected]. ![]()
DLA tablets cure drug-resistant malaria
Researchers have reported that tablets made from dried leaves of the Artemisia annua plant were able to cure malaria in 18 critically ill patients in a clinic in Africa.
When standard malaria medications failed to help the patients, the attending physician at the clinic acted under the compassionate use doctrine and prescribed the unapproved therapy, known as dried-leaf Artemisia (DLA).
Within 5 days, all 18 patients had fully recovered, with lab tests showing no parasites in their blood.
Details on this small trial were published in Phytomedicine.
The trial included 18 patients in the North Kivu province of the Democratic Republic of Congo who showed symptoms of malaria and were originally treated with artemisinin-based combination therapy (ACT).
The patients, who ranged in age from 14 months to 60 years, did not respond to ACT and lapsed into severe malaria.
Seven of the patients had anemia, 6 had convulsions, and 4 had vomiting and diarrhea. One patient, a 5-year-old child, became comatose.
All of the patients were then treated with intravenous artesunate, but they showed no improvement.
As a last resort, doctors turned to DLA. After 5 days of treatment with DLA tablets, all 18 patients fully recovered. Tests showed they had no parasites remaining in their blood.
The researchers said the DLA tablets were well-tolerated, as there were no side effects observed.
“These 18 patients were dying,” said study author Pamela Weathers, PhD, of Worcester Polytechnic Institute in Massachusetts.
“So to see 100% recover, even the child who had lapsed into a coma, was just amazing. It’s a small study, but the results are powerful.”
Dr Weathers noted that more than 100 other drug-resistant patients have been successfully treated with DLA tablets.
She said the superior performance of DLA in comparison to ACT, as well as DLA’s ability to kill drug-resistant parasites and avoid the resistance trap itself, is likely due to the synergistic effects of a complex array of phytochemicals contained in the plant’s leaves.
Several of these phytochemicals are known to have antimalarial properties, and others may act both to enhance the absorption of artemisinin into the bloodstream and bolster its effectiveness against malaria.
In effect, the dried leaves constitute a robust natural combination therapy, one whose benefits surpass those of ACT and other combination drugs.
“We have done a lot of work to understand the biochemistry of these compounds, which include a number of flavonoids and terpenes, so we can better understand the role they play in the pharmacological activity of the dried leaves,” Dr Weathers said.
“The more we learn, the more excited we become about the potential for DLA to be the medication of choice for combating malaria worldwide. Artemisia annua is known to be efficacious against a range of other diseases, including other tropical maladies and certain cancers. So, in our lab, we are already at work investigating the effectiveness of DLA with other diseases.”
Another advantage of DLA over conventional malaria treatments is its low cost and the relative simplicity of its manufacture, Dr Weathers said. When compared to ACT, producing DLA tablets can be accomplished with simpler equipment and a modest amount of training.
Growing Artemisia annua and producing and testing the tablets, Dr Weathers noted, are ideal local business ventures that can provide jobs in impoverished areas and greatly expand access to antimalarial therapy.
In fact, she has already established a supply chain in Africa that includes growing and harvesting high-producing cultivars in East Africa, along with Good Manufacturing Practice processing operations in Uganda where the leaves are dried, pulverized, and homogenized; the powder is compacted into tablets; and the tablets are tested to verify their dosage.
This supply chain helped produce the tablets used to treat the 18 patients in the Democratic Republic of Congo. ![]()
Researchers have reported that tablets made from dried leaves of the Artemisia annua plant were able to cure malaria in 18 critically ill patients in a clinic in Africa.
When standard malaria medications failed to help the patients, the attending physician at the clinic acted under the compassionate use doctrine and prescribed the unapproved therapy, known as dried-leaf Artemisia (DLA).
Within 5 days, all 18 patients had fully recovered, with lab tests showing no parasites in their blood.
Details on this small trial were published in Phytomedicine.
The trial included 18 patients in the North Kivu province of the Democratic Republic of Congo who showed symptoms of malaria and were originally treated with artemisinin-based combination therapy (ACT).
The patients, who ranged in age from 14 months to 60 years, did not respond to ACT and lapsed into severe malaria.
Seven of the patients had anemia, 6 had convulsions, and 4 had vomiting and diarrhea. One patient, a 5-year-old child, became comatose.
All of the patients were then treated with intravenous artesunate, but they showed no improvement.
As a last resort, doctors turned to DLA. After 5 days of treatment with DLA tablets, all 18 patients fully recovered. Tests showed they had no parasites remaining in their blood.
The researchers said the DLA tablets were well-tolerated, as there were no side effects observed.
“These 18 patients were dying,” said study author Pamela Weathers, PhD, of Worcester Polytechnic Institute in Massachusetts.
“So to see 100% recover, even the child who had lapsed into a coma, was just amazing. It’s a small study, but the results are powerful.”
Dr Weathers noted that more than 100 other drug-resistant patients have been successfully treated with DLA tablets.
She said the superior performance of DLA in comparison to ACT, as well as DLA’s ability to kill drug-resistant parasites and avoid the resistance trap itself, is likely due to the synergistic effects of a complex array of phytochemicals contained in the plant’s leaves.
Several of these phytochemicals are known to have antimalarial properties, and others may act both to enhance the absorption of artemisinin into the bloodstream and bolster its effectiveness against malaria.
In effect, the dried leaves constitute a robust natural combination therapy, one whose benefits surpass those of ACT and other combination drugs.
“We have done a lot of work to understand the biochemistry of these compounds, which include a number of flavonoids and terpenes, so we can better understand the role they play in the pharmacological activity of the dried leaves,” Dr Weathers said.
“The more we learn, the more excited we become about the potential for DLA to be the medication of choice for combating malaria worldwide. Artemisia annua is known to be efficacious against a range of other diseases, including other tropical maladies and certain cancers. So, in our lab, we are already at work investigating the effectiveness of DLA with other diseases.”
Another advantage of DLA over conventional malaria treatments is its low cost and the relative simplicity of its manufacture, Dr Weathers said. When compared to ACT, producing DLA tablets can be accomplished with simpler equipment and a modest amount of training.
Growing Artemisia annua and producing and testing the tablets, Dr Weathers noted, are ideal local business ventures that can provide jobs in impoverished areas and greatly expand access to antimalarial therapy.
In fact, she has already established a supply chain in Africa that includes growing and harvesting high-producing cultivars in East Africa, along with Good Manufacturing Practice processing operations in Uganda where the leaves are dried, pulverized, and homogenized; the powder is compacted into tablets; and the tablets are tested to verify their dosage.
This supply chain helped produce the tablets used to treat the 18 patients in the Democratic Republic of Congo. ![]()
Researchers have reported that tablets made from dried leaves of the Artemisia annua plant were able to cure malaria in 18 critically ill patients in a clinic in Africa.
When standard malaria medications failed to help the patients, the attending physician at the clinic acted under the compassionate use doctrine and prescribed the unapproved therapy, known as dried-leaf Artemisia (DLA).
Within 5 days, all 18 patients had fully recovered, with lab tests showing no parasites in their blood.
Details on this small trial were published in Phytomedicine.
The trial included 18 patients in the North Kivu province of the Democratic Republic of Congo who showed symptoms of malaria and were originally treated with artemisinin-based combination therapy (ACT).
The patients, who ranged in age from 14 months to 60 years, did not respond to ACT and lapsed into severe malaria.
Seven of the patients had anemia, 6 had convulsions, and 4 had vomiting and diarrhea. One patient, a 5-year-old child, became comatose.
All of the patients were then treated with intravenous artesunate, but they showed no improvement.
As a last resort, doctors turned to DLA. After 5 days of treatment with DLA tablets, all 18 patients fully recovered. Tests showed they had no parasites remaining in their blood.
The researchers said the DLA tablets were well-tolerated, as there were no side effects observed.
“These 18 patients were dying,” said study author Pamela Weathers, PhD, of Worcester Polytechnic Institute in Massachusetts.
“So to see 100% recover, even the child who had lapsed into a coma, was just amazing. It’s a small study, but the results are powerful.”
Dr Weathers noted that more than 100 other drug-resistant patients have been successfully treated with DLA tablets.
She said the superior performance of DLA in comparison to ACT, as well as DLA’s ability to kill drug-resistant parasites and avoid the resistance trap itself, is likely due to the synergistic effects of a complex array of phytochemicals contained in the plant’s leaves.
Several of these phytochemicals are known to have antimalarial properties, and others may act both to enhance the absorption of artemisinin into the bloodstream and bolster its effectiveness against malaria.
In effect, the dried leaves constitute a robust natural combination therapy, one whose benefits surpass those of ACT and other combination drugs.
“We have done a lot of work to understand the biochemistry of these compounds, which include a number of flavonoids and terpenes, so we can better understand the role they play in the pharmacological activity of the dried leaves,” Dr Weathers said.
“The more we learn, the more excited we become about the potential for DLA to be the medication of choice for combating malaria worldwide. Artemisia annua is known to be efficacious against a range of other diseases, including other tropical maladies and certain cancers. So, in our lab, we are already at work investigating the effectiveness of DLA with other diseases.”
Another advantage of DLA over conventional malaria treatments is its low cost and the relative simplicity of its manufacture, Dr Weathers said. When compared to ACT, producing DLA tablets can be accomplished with simpler equipment and a modest amount of training.
Growing Artemisia annua and producing and testing the tablets, Dr Weathers noted, are ideal local business ventures that can provide jobs in impoverished areas and greatly expand access to antimalarial therapy.
In fact, she has already established a supply chain in Africa that includes growing and harvesting high-producing cultivars in East Africa, along with Good Manufacturing Practice processing operations in Uganda where the leaves are dried, pulverized, and homogenized; the powder is compacted into tablets; and the tablets are tested to verify their dosage.
This supply chain helped produce the tablets used to treat the 18 patients in the Democratic Republic of Congo. ![]()