User login
USPSTF recommendations: A 2017 roundup
Since the last Practice Alert update on the United States Preventive Services Task Force in May of 2016,1 the Task Force has released 19 recommendations on 13 topics that include: the use of aspirin and statins for the prevention of cardiovascular disease (CVD); support for breastfeeding; use of folic acid during pregnancy; and screening for syphilis, latent tuberculosis (TB), herpes, chronic obstructive pulmonary disease (COPD), colorectal cancer (CRC), obstructive sleep apnea (OSA), celiac disease, and skin cancer. The Task Force also released a draft recommendation regarding prostate cancer screening in asymptomatic men (see “A change for prostate cancer screening?”) and addressed screening pelvic examinations in asymptomatic women, the subject of this month’s audiocast. (To listen, go to: http://bit.ly/2nIVoD5.)
Recommendations to implement
Recommendations from the past year that family physicians should put into practice are detailed below and in TABLE 1.2-8

CRC: Screen all individuals ages 50 to 75, but 76 to 85 selectively. The Task Force reaffirmed its 2008 finding that screening for CRC in adults ages 50 to 75 years is substantially beneficial.2 In contrast to the previous recommendation, however, the new one does not state which screening tests are preferred. The tests considered were 3 stool tests (fecal immunochemical test [FIT], FIT-tumor DNA testing [FIT-DNA], and guaiac-based fecal occult blood test [gFOBT]), as well as 3 direct visualization tests (colonoscopy, sigmoidoscopy, and CT colonoscopy). The Task Force assessed various testing frequencies of each test and some test combinations. While the Task Force does not recommend any one screening strategy, there are still significant unknowns about FIT-DNA and CT colonoscopy. The American Academy of Family Physicians does not recommend using these 2 tests for screening purposes at this time.9
CRC screening for adults ages 76 to 85 was given a “C” recommendation, which means the value of the service to the population overall is small, but that certain individuals may benefit from it. The Task Force advises selectively offering a “C” service to individuals based on professional judgment and patient preferences. Regarding CRC screening in individuals 76 years or older, the ones most likely to benefit are those who have never been screened and those without significant comorbidities that could limit life expectancy. All “C” recommendations from the past year are listed in TABLE 2.2-4

CVD prevention: When aspirin or a statin is indicated. The Task Force released 2 recommendations for the prevention of CVD this past year. One pertained to the use of low-dose aspirin3 (which also helps to prevent CRC), and the other addressed the use of low- to moderate-dose statins.4 Each recommendation is fairly complicated and nuanced in terms of age and risk for CVD. A decision to use low-dose aspirin must also consider the risk of bleeding.
To calculate a patient’s risk for CVD, the Task Force recommends using the risk calculator developed by the American College of Cardiology and the American Heart Association (http://www.cvriskcalculator.com/).
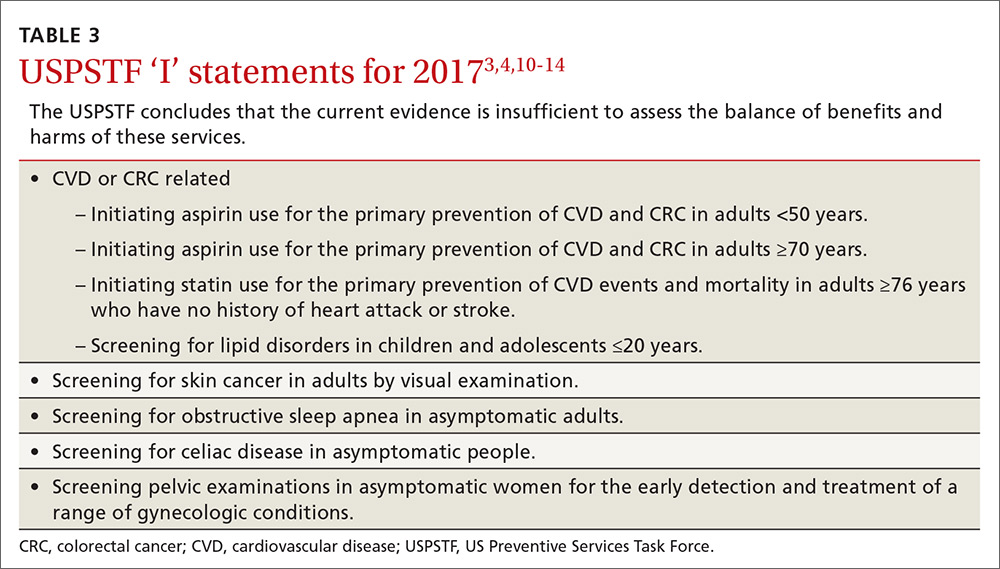
Adults for whom low-dose aspirin and low- to moderate-dose statins are recommended are described in TABLE 1.2-8 Patients for whom individual decision making is advised, rather than a generalized recommendation, are reviewed in TABLE 2.2-4 There is insufficient evidence to make a recommendation for the use of aspirin before age 50 or at age 70 and older,3 and for the use of statins in adults age 76 and older who do not have a history of CVD4 (TABLE 33,4,10-14). The use of low-dose aspirin and low-to-moderate dose statins have been the subject of JFP audiocasts in May 2016 and January 2017. (See http://bit.ly/2oiun8d and http://bit.ly/2oqkohR.)
2 pregnancy-related recommendations. To prevent neural tube defects in newborns, the Task Force now recommends daily folic acid, 0.4 to 0.8 mg (400 to 800 mcg), for all women who are planning on or are capable of becoming pregnant.5 This is an update of a 2009 recommendation that was worded slightly differently, recommending the supplement for all women of childbearing age.
A new recommendation on breastfeeding recognizes its benefits for both mother and baby and finds that interventions to encourage breastfeeding increase the prevalence of this practice and its duration.6 Interventions—provided to individuals or groups by professionals or peers or through formal education—include promoting the benefits of breastfeeding, giving practical advice and direct support on how to breastfeed, and offering psychological support.
Latent TB: Advantages of newer testing method. The recommendation on screening for latent tuberculosis (TB) is an update from the one made in 1996.7 At that time, screening for latent infection was performed using a tuberculin skin test (TST). Now a TST or interferon-gamma release assay (IGRA) can be used. Testing with IGRA may be the best option for those who have received a bacille Calmette–Guérin vaccination (because it can cause a false-positive TST) or for those who are not likely to return to have their TST read.
Those at high risk for latent TB include people who were born or have resided in countries with a high TB prevalence, those who have lived in a correctional institution or homeless shelter, and anyone in a high-risk group based on local epidemiology of the disease. (Read more on TB in this month’s Case Report.) Others at high risk are those who are immune suppressed because of infection or medications, and those who work in health care or correctional facilities. Screening of these groups is usually conducted as part of occupational health or is considered part of routine health care.
Syphilis: Screen high-risk individuals in 2 steps. The recommendation on syphilis screening basically reaffirms the one from 2004.8 Those at high risk for syphilis include men who have sex with men (who now account for 90% of new cases), those who are HIV positive, and those who engage in commercial sex work. Other racial and demographic groups can be at high risk depending on the local epidemiology of the disease. In a separate recommendation, the Task Force advises screening all pregnant women for syphilis.
Screening for syphilis infection involves 2 steps: first, a nontreponemal test (Venereal Disease Research Laboratory [VDRL] or rapid plasma reagin [RPR] test); second, a confirmatory treponemal antibody detection test (fluorescent treponemal antibody absorption [FTA-ABS] or Treponema pallidum particle agglutination [TPPA] test). Treatment for syphilis remains benzathine penicillin with the number of injections depending on the stage of infection. The Centers for Disease Control and Prevention is the best source for current recommendations for treatment of all sexually transmitted infections.15
Screening tests to avoid
TABLE 416,17 lists screening tests the Task Force recommends against. While chronic obstructive pulmonary disease afflicts 14% of US adults ages 40 to 79 years and is the third leading cause of death in the country, the Task Force found that early detection in asymptomatic adults does not affect the course of the illness and is of no benefit.16

Genital herpes, also prevalent, infects an estimated one out of 6 individuals, ages 14 to 49. It causes little mortality, except in neonates, but those infected can have recurrent flares and suffer psychological harms from stigmatization. Most genital herpes is caused by herpes simplex virus-2, and there is a serological test to detect it. However, the Task Force recommends against using the test to screen asymptomatic adults and adolescents, including those who are pregnant. This recommendation is based on the test’s high false-positive rate, which can cause emotional harm, and on the lack of evidence that detection through screening improves outcomes.17
The evidence is lacking for these practices
The Task Force is one of only a few organizations that will not make a recommendation if evidence is lacking on benefits and harms. In addition to the ‘I’ statements regarding CVD and CRC mentioned earlier, the Task Force found insufficient evidence to recommend screening for lipid disorders in individuals ages 20 years or younger,10 performing a visual skin exam as a screening tool for skin cancer,11 screening for celiac disease,12 performing a periodic pelvic examination in asymptomatic women,13 and screening for obstructive sleep apnea using screening questionnaires14 (TABLE 33,4,10-14).
SIDEBAR
A change for prostate cancer screening?The USPSTF recently issued new draft recommendations regarding prostate cancer screening in asymptomatic men (available at: https://screeningforprostatecancer.org/).
The draft now divides men into 2 age groups, stating that the decision to screen for prostate cancer using a prostate specific antigen (PSA) test should be individualized for men ages 55 to 69 years (a C recommendation, meaning that there is at least moderate certainty that the net benefit is small), and that men ages 70 and older (lowered from age 75 in the previous 2012 recommendation1) should not be screened (a D recommendation, meaning that there is moderate or high certainty that the service has no net benefit or that the harms outweigh the benefits).
The USPSTF believes that clinicians should explain to men ages 55 to 69 years that screening offers a small potential benefit of reducing the chance of dying from prostate cancer, but also comes with potential harms, including false-positive results requiring additional testing/procedures, overdiagnosis and overtreatment, and treatment complications such as incontinence and impotence. In this way, each man has the chance to incorporate his values and preferences into the decision.
For men ages 70 and older, the potential benefits simply do not outweigh the potential harms, according to the USPSTF.
1. USPSTF. Final recommendation statement. Prostate cancer: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/prostate-cancer-screening#Pod1. Accessed April 11, 2017.
1. Campos-Outcalt D. Eight USPSTF recommendations FPs need to know about. J Fam Pract. 2016;65:338-341.
2. USPSTF. Colorectal cancer: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/colorectal-cancer-screening2. Accessed March 22, 2017.
3. USPSTF. Aspirin use to prevent cardiovascular disease and colorectal cancer: preventive medications. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/aspirin-to-prevent-cardiovascular-disease-and-cancer. Accessed March 22, 2017.
4. USPSTF. Statin use for the prevention of cardiovascular disease in adults: preventive medication. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/statin-use-in-adults-preventive-medication1. Accessed March 22, 2017.
5. USPSTF. Folic acid for the prevention of neural tube defects: preventive medication. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/folic-acid-for-the-prevention-of-neural-tube-defects-preventive-medication. Accessed March 22, 2017.
6. USPSTF. Breastfeeding: primary care interventions. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/breastfeeding-primary-care-interventions. Accessed March 22, 2017.
7. USPSTF. Latent tuberculosis infection: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/latent-tuberculosis-infection-screening. Accessed March 22, 2017.
8. USPSTF. Syphilis infection in nonpregnant adults and adolescents: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/syphilis-infection-in-nonpregnant-adults-and-adolescents. Accessed March 22, 2017.
9. AAFP. Colorectal cancer screening, adults. Available at: http://www.aafp.org/patient-care/clinical-recommendations/all/colorectal-cancer.html. Accessed March 22, 2017.
10. USPSTF. Lipid disorders in children and adolescents: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/lipid-disorders-in-children-screening1. Accessed March 22, 2017.
11. USPSTF. Skin cancer: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/skin-cancer-screening2. Accessed March 22, 2017.
12. USPSTF. Celiac disease: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/celiac-disease-screening. Accessed March 22, 2017.
13. USPSTF. Gynecological conditions: periodic screening with the pelvic examination. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/gynecological-conditions-screening-with-the-pelvic-examination. Accessed March 22, 2017.
14. USPSTF. Obstructive sleep apnea in adults: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/obstructive-sleep-apnea-in-adults-screening. Accessed March 22, 2017.
15. CDC. 2015 sexually transmitted diseases treatment guidelines. Available at: https://www.cdc.gov/std/tg2015/default.htm. Accessed March 22, 2017.
16. USPSTF. Chronic obstructive pulmonary disease: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/chronic-obstructive-pulmonary-disease-screening. Accessed March 22, 2017.
17. USPSTF. Genital herpes infection: serologic screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/genital-herpes-screening1. Accessed March 22, 2017.
Since the last Practice Alert update on the United States Preventive Services Task Force in May of 2016,1 the Task Force has released 19 recommendations on 13 topics that include: the use of aspirin and statins for the prevention of cardiovascular disease (CVD); support for breastfeeding; use of folic acid during pregnancy; and screening for syphilis, latent tuberculosis (TB), herpes, chronic obstructive pulmonary disease (COPD), colorectal cancer (CRC), obstructive sleep apnea (OSA), celiac disease, and skin cancer. The Task Force also released a draft recommendation regarding prostate cancer screening in asymptomatic men (see “A change for prostate cancer screening?”) and addressed screening pelvic examinations in asymptomatic women, the subject of this month’s audiocast. (To listen, go to: http://bit.ly/2nIVoD5.)
Recommendations to implement
Recommendations from the past year that family physicians should put into practice are detailed below and in TABLE 1.2-8

CRC: Screen all individuals ages 50 to 75, but 76 to 85 selectively. The Task Force reaffirmed its 2008 finding that screening for CRC in adults ages 50 to 75 years is substantially beneficial.2 In contrast to the previous recommendation, however, the new one does not state which screening tests are preferred. The tests considered were 3 stool tests (fecal immunochemical test [FIT], FIT-tumor DNA testing [FIT-DNA], and guaiac-based fecal occult blood test [gFOBT]), as well as 3 direct visualization tests (colonoscopy, sigmoidoscopy, and CT colonoscopy). The Task Force assessed various testing frequencies of each test and some test combinations. While the Task Force does not recommend any one screening strategy, there are still significant unknowns about FIT-DNA and CT colonoscopy. The American Academy of Family Physicians does not recommend using these 2 tests for screening purposes at this time.9
CRC screening for adults ages 76 to 85 was given a “C” recommendation, which means the value of the service to the population overall is small, but that certain individuals may benefit from it. The Task Force advises selectively offering a “C” service to individuals based on professional judgment and patient preferences. Regarding CRC screening in individuals 76 years or older, the ones most likely to benefit are those who have never been screened and those without significant comorbidities that could limit life expectancy. All “C” recommendations from the past year are listed in TABLE 2.2-4

CVD prevention: When aspirin or a statin is indicated. The Task Force released 2 recommendations for the prevention of CVD this past year. One pertained to the use of low-dose aspirin3 (which also helps to prevent CRC), and the other addressed the use of low- to moderate-dose statins.4 Each recommendation is fairly complicated and nuanced in terms of age and risk for CVD. A decision to use low-dose aspirin must also consider the risk of bleeding.
To calculate a patient’s risk for CVD, the Task Force recommends using the risk calculator developed by the American College of Cardiology and the American Heart Association (http://www.cvriskcalculator.com/).
Adults for whom low-dose aspirin and low- to moderate-dose statins are recommended are described in TABLE 1.2-8 Patients for whom individual decision making is advised, rather than a generalized recommendation, are reviewed in TABLE 2.2-4 There is insufficient evidence to make a recommendation for the use of aspirin before age 50 or at age 70 and older,3 and for the use of statins in adults age 76 and older who do not have a history of CVD4 (TABLE 33,4,10-14). The use of low-dose aspirin and low-to-moderate dose statins have been the subject of JFP audiocasts in May 2016 and January 2017. (See http://bit.ly/2oiun8d and http://bit.ly/2oqkohR.)

2 pregnancy-related recommendations. To prevent neural tube defects in newborns, the Task Force now recommends daily folic acid, 0.4 to 0.8 mg (400 to 800 mcg), for all women who are planning on or are capable of becoming pregnant.5 This is an update of a 2009 recommendation that was worded slightly differently, recommending the supplement for all women of childbearing age.
A new recommendation on breastfeeding recognizes its benefits for both mother and baby and finds that interventions to encourage breastfeeding increase the prevalence of this practice and its duration.6 Interventions—provided to individuals or groups by professionals or peers or through formal education—include promoting the benefits of breastfeeding, giving practical advice and direct support on how to breastfeed, and offering psychological support.
Latent TB: Advantages of newer testing method. The recommendation on screening for latent tuberculosis (TB) is an update from the one made in 1996.7 At that time, screening for latent infection was performed using a tuberculin skin test (TST). Now a TST or interferon-gamma release assay (IGRA) can be used. Testing with IGRA may be the best option for those who have received a bacille Calmette–Guérin vaccination (because it can cause a false-positive TST) or for those who are not likely to return to have their TST read.
Those at high risk for latent TB include people who were born or have resided in countries with a high TB prevalence, those who have lived in a correctional institution or homeless shelter, and anyone in a high-risk group based on local epidemiology of the disease. (Read more on TB in this month’s Case Report.) Others at high risk are those who are immune suppressed because of infection or medications, and those who work in health care or correctional facilities. Screening of these groups is usually conducted as part of occupational health or is considered part of routine health care.
Syphilis: Screen high-risk individuals in 2 steps. The recommendation on syphilis screening basically reaffirms the one from 2004.8 Those at high risk for syphilis include men who have sex with men (who now account for 90% of new cases), those who are HIV positive, and those who engage in commercial sex work. Other racial and demographic groups can be at high risk depending on the local epidemiology of the disease. In a separate recommendation, the Task Force advises screening all pregnant women for syphilis.
Screening for syphilis infection involves 2 steps: first, a nontreponemal test (Venereal Disease Research Laboratory [VDRL] or rapid plasma reagin [RPR] test); second, a confirmatory treponemal antibody detection test (fluorescent treponemal antibody absorption [FTA-ABS] or Treponema pallidum particle agglutination [TPPA] test). Treatment for syphilis remains benzathine penicillin with the number of injections depending on the stage of infection. The Centers for Disease Control and Prevention is the best source for current recommendations for treatment of all sexually transmitted infections.15
Screening tests to avoid
TABLE 416,17 lists screening tests the Task Force recommends against. While chronic obstructive pulmonary disease afflicts 14% of US adults ages 40 to 79 years and is the third leading cause of death in the country, the Task Force found that early detection in asymptomatic adults does not affect the course of the illness and is of no benefit.16

Genital herpes, also prevalent, infects an estimated one out of 6 individuals, ages 14 to 49. It causes little mortality, except in neonates, but those infected can have recurrent flares and suffer psychological harms from stigmatization. Most genital herpes is caused by herpes simplex virus-2, and there is a serological test to detect it. However, the Task Force recommends against using the test to screen asymptomatic adults and adolescents, including those who are pregnant. This recommendation is based on the test’s high false-positive rate, which can cause emotional harm, and on the lack of evidence that detection through screening improves outcomes.17
The evidence is lacking for these practices
The Task Force is one of only a few organizations that will not make a recommendation if evidence is lacking on benefits and harms. In addition to the ‘I’ statements regarding CVD and CRC mentioned earlier, the Task Force found insufficient evidence to recommend screening for lipid disorders in individuals ages 20 years or younger,10 performing a visual skin exam as a screening tool for skin cancer,11 screening for celiac disease,12 performing a periodic pelvic examination in asymptomatic women,13 and screening for obstructive sleep apnea using screening questionnaires14 (TABLE 33,4,10-14).
SIDEBAR
A change for prostate cancer screening?The USPSTF recently issued new draft recommendations regarding prostate cancer screening in asymptomatic men (available at: https://screeningforprostatecancer.org/).
The draft now divides men into 2 age groups, stating that the decision to screen for prostate cancer using a prostate specific antigen (PSA) test should be individualized for men ages 55 to 69 years (a C recommendation, meaning that there is at least moderate certainty that the net benefit is small), and that men ages 70 and older (lowered from age 75 in the previous 2012 recommendation1) should not be screened (a D recommendation, meaning that there is moderate or high certainty that the service has no net benefit or that the harms outweigh the benefits).
The USPSTF believes that clinicians should explain to men ages 55 to 69 years that screening offers a small potential benefit of reducing the chance of dying from prostate cancer, but also comes with potential harms, including false-positive results requiring additional testing/procedures, overdiagnosis and overtreatment, and treatment complications such as incontinence and impotence. In this way, each man has the chance to incorporate his values and preferences into the decision.
For men ages 70 and older, the potential benefits simply do not outweigh the potential harms, according to the USPSTF.
1. USPSTF. Final recommendation statement. Prostate cancer: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/prostate-cancer-screening#Pod1. Accessed April 11, 2017.
Since the last Practice Alert update on the United States Preventive Services Task Force in May of 2016,1 the Task Force has released 19 recommendations on 13 topics that include: the use of aspirin and statins for the prevention of cardiovascular disease (CVD); support for breastfeeding; use of folic acid during pregnancy; and screening for syphilis, latent tuberculosis (TB), herpes, chronic obstructive pulmonary disease (COPD), colorectal cancer (CRC), obstructive sleep apnea (OSA), celiac disease, and skin cancer. The Task Force also released a draft recommendation regarding prostate cancer screening in asymptomatic men (see “A change for prostate cancer screening?”) and addressed screening pelvic examinations in asymptomatic women, the subject of this month’s audiocast. (To listen, go to: http://bit.ly/2nIVoD5.)
Recommendations to implement
Recommendations from the past year that family physicians should put into practice are detailed below and in TABLE 1.2-8

CRC: Screen all individuals ages 50 to 75, but 76 to 85 selectively. The Task Force reaffirmed its 2008 finding that screening for CRC in adults ages 50 to 75 years is substantially beneficial.2 In contrast to the previous recommendation, however, the new one does not state which screening tests are preferred. The tests considered were 3 stool tests (fecal immunochemical test [FIT], FIT-tumor DNA testing [FIT-DNA], and guaiac-based fecal occult blood test [gFOBT]), as well as 3 direct visualization tests (colonoscopy, sigmoidoscopy, and CT colonoscopy). The Task Force assessed various testing frequencies of each test and some test combinations. While the Task Force does not recommend any one screening strategy, there are still significant unknowns about FIT-DNA and CT colonoscopy. The American Academy of Family Physicians does not recommend using these 2 tests for screening purposes at this time.9
CRC screening for adults ages 76 to 85 was given a “C” recommendation, which means the value of the service to the population overall is small, but that certain individuals may benefit from it. The Task Force advises selectively offering a “C” service to individuals based on professional judgment and patient preferences. Regarding CRC screening in individuals 76 years or older, the ones most likely to benefit are those who have never been screened and those without significant comorbidities that could limit life expectancy. All “C” recommendations from the past year are listed in TABLE 2.2-4

CVD prevention: When aspirin or a statin is indicated. The Task Force released 2 recommendations for the prevention of CVD this past year. One pertained to the use of low-dose aspirin3 (which also helps to prevent CRC), and the other addressed the use of low- to moderate-dose statins.4 Each recommendation is fairly complicated and nuanced in terms of age and risk for CVD. A decision to use low-dose aspirin must also consider the risk of bleeding.
To calculate a patient’s risk for CVD, the Task Force recommends using the risk calculator developed by the American College of Cardiology and the American Heart Association (http://www.cvriskcalculator.com/).
Adults for whom low-dose aspirin and low- to moderate-dose statins are recommended are described in TABLE 1.2-8 Patients for whom individual decision making is advised, rather than a generalized recommendation, are reviewed in TABLE 2.2-4 There is insufficient evidence to make a recommendation for the use of aspirin before age 50 or at age 70 and older,3 and for the use of statins in adults age 76 and older who do not have a history of CVD4 (TABLE 33,4,10-14). The use of low-dose aspirin and low-to-moderate dose statins have been the subject of JFP audiocasts in May 2016 and January 2017. (See http://bit.ly/2oiun8d and http://bit.ly/2oqkohR.)

2 pregnancy-related recommendations. To prevent neural tube defects in newborns, the Task Force now recommends daily folic acid, 0.4 to 0.8 mg (400 to 800 mcg), for all women who are planning on or are capable of becoming pregnant.5 This is an update of a 2009 recommendation that was worded slightly differently, recommending the supplement for all women of childbearing age.
A new recommendation on breastfeeding recognizes its benefits for both mother and baby and finds that interventions to encourage breastfeeding increase the prevalence of this practice and its duration.6 Interventions—provided to individuals or groups by professionals or peers or through formal education—include promoting the benefits of breastfeeding, giving practical advice and direct support on how to breastfeed, and offering psychological support.
Latent TB: Advantages of newer testing method. The recommendation on screening for latent tuberculosis (TB) is an update from the one made in 1996.7 At that time, screening for latent infection was performed using a tuberculin skin test (TST). Now a TST or interferon-gamma release assay (IGRA) can be used. Testing with IGRA may be the best option for those who have received a bacille Calmette–Guérin vaccination (because it can cause a false-positive TST) or for those who are not likely to return to have their TST read.
Those at high risk for latent TB include people who were born or have resided in countries with a high TB prevalence, those who have lived in a correctional institution or homeless shelter, and anyone in a high-risk group based on local epidemiology of the disease. (Read more on TB in this month’s Case Report.) Others at high risk are those who are immune suppressed because of infection or medications, and those who work in health care or correctional facilities. Screening of these groups is usually conducted as part of occupational health or is considered part of routine health care.
Syphilis: Screen high-risk individuals in 2 steps. The recommendation on syphilis screening basically reaffirms the one from 2004.8 Those at high risk for syphilis include men who have sex with men (who now account for 90% of new cases), those who are HIV positive, and those who engage in commercial sex work. Other racial and demographic groups can be at high risk depending on the local epidemiology of the disease. In a separate recommendation, the Task Force advises screening all pregnant women for syphilis.
Screening for syphilis infection involves 2 steps: first, a nontreponemal test (Venereal Disease Research Laboratory [VDRL] or rapid plasma reagin [RPR] test); second, a confirmatory treponemal antibody detection test (fluorescent treponemal antibody absorption [FTA-ABS] or Treponema pallidum particle agglutination [TPPA] test). Treatment for syphilis remains benzathine penicillin with the number of injections depending on the stage of infection. The Centers for Disease Control and Prevention is the best source for current recommendations for treatment of all sexually transmitted infections.15
Screening tests to avoid
TABLE 416,17 lists screening tests the Task Force recommends against. While chronic obstructive pulmonary disease afflicts 14% of US adults ages 40 to 79 years and is the third leading cause of death in the country, the Task Force found that early detection in asymptomatic adults does not affect the course of the illness and is of no benefit.16

Genital herpes, also prevalent, infects an estimated one out of 6 individuals, ages 14 to 49. It causes little mortality, except in neonates, but those infected can have recurrent flares and suffer psychological harms from stigmatization. Most genital herpes is caused by herpes simplex virus-2, and there is a serological test to detect it. However, the Task Force recommends against using the test to screen asymptomatic adults and adolescents, including those who are pregnant. This recommendation is based on the test’s high false-positive rate, which can cause emotional harm, and on the lack of evidence that detection through screening improves outcomes.17
The evidence is lacking for these practices
The Task Force is one of only a few organizations that will not make a recommendation if evidence is lacking on benefits and harms. In addition to the ‘I’ statements regarding CVD and CRC mentioned earlier, the Task Force found insufficient evidence to recommend screening for lipid disorders in individuals ages 20 years or younger,10 performing a visual skin exam as a screening tool for skin cancer,11 screening for celiac disease,12 performing a periodic pelvic examination in asymptomatic women,13 and screening for obstructive sleep apnea using screening questionnaires14 (TABLE 33,4,10-14).
SIDEBAR
A change for prostate cancer screening?The USPSTF recently issued new draft recommendations regarding prostate cancer screening in asymptomatic men (available at: https://screeningforprostatecancer.org/).
The draft now divides men into 2 age groups, stating that the decision to screen for prostate cancer using a prostate specific antigen (PSA) test should be individualized for men ages 55 to 69 years (a C recommendation, meaning that there is at least moderate certainty that the net benefit is small), and that men ages 70 and older (lowered from age 75 in the previous 2012 recommendation1) should not be screened (a D recommendation, meaning that there is moderate or high certainty that the service has no net benefit or that the harms outweigh the benefits).
The USPSTF believes that clinicians should explain to men ages 55 to 69 years that screening offers a small potential benefit of reducing the chance of dying from prostate cancer, but also comes with potential harms, including false-positive results requiring additional testing/procedures, overdiagnosis and overtreatment, and treatment complications such as incontinence and impotence. In this way, each man has the chance to incorporate his values and preferences into the decision.
For men ages 70 and older, the potential benefits simply do not outweigh the potential harms, according to the USPSTF.
1. USPSTF. Final recommendation statement. Prostate cancer: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/prostate-cancer-screening#Pod1. Accessed April 11, 2017.
1. Campos-Outcalt D. Eight USPSTF recommendations FPs need to know about. J Fam Pract. 2016;65:338-341.
2. USPSTF. Colorectal cancer: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/colorectal-cancer-screening2. Accessed March 22, 2017.
3. USPSTF. Aspirin use to prevent cardiovascular disease and colorectal cancer: preventive medications. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/aspirin-to-prevent-cardiovascular-disease-and-cancer. Accessed March 22, 2017.
4. USPSTF. Statin use for the prevention of cardiovascular disease in adults: preventive medication. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/statin-use-in-adults-preventive-medication1. Accessed March 22, 2017.
5. USPSTF. Folic acid for the prevention of neural tube defects: preventive medication. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/folic-acid-for-the-prevention-of-neural-tube-defects-preventive-medication. Accessed March 22, 2017.
6. USPSTF. Breastfeeding: primary care interventions. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/breastfeeding-primary-care-interventions. Accessed March 22, 2017.
7. USPSTF. Latent tuberculosis infection: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/latent-tuberculosis-infection-screening. Accessed March 22, 2017.
8. USPSTF. Syphilis infection in nonpregnant adults and adolescents: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/syphilis-infection-in-nonpregnant-adults-and-adolescents. Accessed March 22, 2017.
9. AAFP. Colorectal cancer screening, adults. Available at: http://www.aafp.org/patient-care/clinical-recommendations/all/colorectal-cancer.html. Accessed March 22, 2017.
10. USPSTF. Lipid disorders in children and adolescents: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/lipid-disorders-in-children-screening1. Accessed March 22, 2017.
11. USPSTF. Skin cancer: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/skin-cancer-screening2. Accessed March 22, 2017.
12. USPSTF. Celiac disease: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/celiac-disease-screening. Accessed March 22, 2017.
13. USPSTF. Gynecological conditions: periodic screening with the pelvic examination. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/gynecological-conditions-screening-with-the-pelvic-examination. Accessed March 22, 2017.
14. USPSTF. Obstructive sleep apnea in adults: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/obstructive-sleep-apnea-in-adults-screening. Accessed March 22, 2017.
15. CDC. 2015 sexually transmitted diseases treatment guidelines. Available at: https://www.cdc.gov/std/tg2015/default.htm. Accessed March 22, 2017.
16. USPSTF. Chronic obstructive pulmonary disease: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/chronic-obstructive-pulmonary-disease-screening. Accessed March 22, 2017.
17. USPSTF. Genital herpes infection: serologic screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/genital-herpes-screening1. Accessed March 22, 2017.
1. Campos-Outcalt D. Eight USPSTF recommendations FPs need to know about. J Fam Pract. 2016;65:338-341.
2. USPSTF. Colorectal cancer: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/colorectal-cancer-screening2. Accessed March 22, 2017.
3. USPSTF. Aspirin use to prevent cardiovascular disease and colorectal cancer: preventive medications. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/aspirin-to-prevent-cardiovascular-disease-and-cancer. Accessed March 22, 2017.
4. USPSTF. Statin use for the prevention of cardiovascular disease in adults: preventive medication. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/statin-use-in-adults-preventive-medication1. Accessed March 22, 2017.
5. USPSTF. Folic acid for the prevention of neural tube defects: preventive medication. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/folic-acid-for-the-prevention-of-neural-tube-defects-preventive-medication. Accessed March 22, 2017.
6. USPSTF. Breastfeeding: primary care interventions. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/breastfeeding-primary-care-interventions. Accessed March 22, 2017.
7. USPSTF. Latent tuberculosis infection: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/latent-tuberculosis-infection-screening. Accessed March 22, 2017.
8. USPSTF. Syphilis infection in nonpregnant adults and adolescents: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/syphilis-infection-in-nonpregnant-adults-and-adolescents. Accessed March 22, 2017.
9. AAFP. Colorectal cancer screening, adults. Available at: http://www.aafp.org/patient-care/clinical-recommendations/all/colorectal-cancer.html. Accessed March 22, 2017.
10. USPSTF. Lipid disorders in children and adolescents: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/lipid-disorders-in-children-screening1. Accessed March 22, 2017.
11. USPSTF. Skin cancer: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/skin-cancer-screening2. Accessed March 22, 2017.
12. USPSTF. Celiac disease: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/celiac-disease-screening. Accessed March 22, 2017.
13. USPSTF. Gynecological conditions: periodic screening with the pelvic examination. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/gynecological-conditions-screening-with-the-pelvic-examination. Accessed March 22, 2017.
14. USPSTF. Obstructive sleep apnea in adults: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/obstructive-sleep-apnea-in-adults-screening. Accessed March 22, 2017.
15. CDC. 2015 sexually transmitted diseases treatment guidelines. Available at: https://www.cdc.gov/std/tg2015/default.htm. Accessed March 22, 2017.
16. USPSTF. Chronic obstructive pulmonary disease: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/chronic-obstructive-pulmonary-disease-screening. Accessed March 22, 2017.
17. USPSTF. Genital herpes infection: serologic screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/genital-herpes-screening1. Accessed March 22, 2017.
Management of bow legs in children: A primary care protocol
ABSTRACT
Objective To reduce unnecessary orthopedic referrals by developing a protocol for managing physiologic bow legs in the primary care environment through the use of a noninvasive technique that simultaneously tracks normal varus progression and screens for potential pathologic bowing requiring an orthopedic referral.
Methods Retrospective study of 155 patients with physiologic genu varum and 10 with infantile Blount’s disease. We used fingerbreadth measurements to document progression or resolution of bow legs. Final diagnoses were made by one orthopedic surgeon using clinical and radiographic evidence. We divided genu varum patients into 3 groups: patients presenting with bow legs before 18 months of age (MOA), patients presenting between 18 and 23 MOA, and patients presenting at 24 MOA or older for analyses relevant to the development of the follow-up protocol.
Results Physiologic genu varum patients walked earlier than average infants (10 months vs 12-15 months; P<.001). Physiologic genu varum patients presenting before 18 MOA demonstrated initial signs of correction between 18 and 24 MOA and resolution by 30 MOA. Physiologic genu varum patients presenting between 18 and 23 MOA demonstrated initial signs of correction between 24 MOA and 30 MOA and resolution by 36 MOA.
Conclusion Primary care physicians can manage most children presenting with bow legs. Management focuses on following the progression or resolution of varus with regular follow-up. For patients presenting with bow legs, we recommend a follow-up protocol using mainly well-child checkups and a simple clinical assessment to monitor varus progression and screen for pathologic bowing.
Bow legs in young children can be a concern for parents.1,2 By far, the most common reason for bow legs is physiologic genu varum,3-5 a nonprogressive stage of normal development in young children that generally resolves spontaneously without treatment.1,6-11 Normally developing children undergo a varus phase between birth and 18 to 24 months of age (MOA), at which time there is usually a transition in alignment from varus to straight to valgus (knock knees), which will correct to straight or mild valgus throughout adolescence.1,6,7,9,10,12-17
The most common form of pathologic bow legs is Blount’s disease, also known as tibia vara, which must be differentiated from physiologic genu varum.8-10,15,18-24 The progressive varus deformity of Blount’s disease usually requires orthopedic intervention.1,10,23-26 Early diagnosis may spare patients complex interventions, improve prognosis, and limit complications that include gait abnormalities,4,8,10,27 knee joint instability,4,24,27 osteoarthritis,9,20,27 meniscal tears,27 and degenerative joint disease.19,20,27
Although variables such as walking age, race, weight, and gender have been suggested as risk factors for Blount’s disease, they have not been useful in differentiating between Blount’s pathology and physiologic genu varum.1,4,5,7,10,20,28 In the primary care setting, distinguishing physiologic from pathologic forms of bow legs is possible with a thorough history and physical exam and with radiographs, as warranted.1,2,15 More than 40% of genu varum/genu valgum cases referred for orthopedic consultation turn out to be the physiologic form,2 suggesting a need for guidelines in the primary care setting to help direct referral and follow-up. The purpose of this study was to provide recommendations to family physicians for evaluating and managing children with bow legs.
Materials and methods
This study, approved by the Internal Review Board of Akron Children’s Hospital, is a retrospective review of children seen by a single pediatric orthopedic surgeon (DSW) from 1970 to 2012. Four-hundred twenty-four children were received for evaluation of bow legs. Excluded from our final analysis were 220 subjects seen only once for this specific referral and 39 subjects diagnosed with a condition other than genu varum or Blount’s disease (ie, rickets, skeletal dysplasia, sequelae of trauma, or infection). Ten subjects with Blount’s disease and 155 subjects with physiologic genu varum were included in the final data analysis.
In addition to noting the age at which a patient walked independently, at each visit we documented age and the fingerbreadth (varus) distance between the medial femoral condyles with the child’s ankles held together. Parents reported age of independent walking for just 3 children with Blount’s disease and for 134 children with physiologic genu varum. Study variables for the genu varum data analysis were age of walking, age at presentation, age at varus correction, age at varus resolution, time between presentation and varus correction, and time between presentation and varus resolution. Varus correction is defined as any decrease in varus angulation since presentation. Varus resolution is defined as varus correction to less than or equal to half of the varus angulation at presentation. For inclusion in the age-at-resolution analysis, a child must have been evaluated at regular follow-up visits (all rechecks within 8 months).
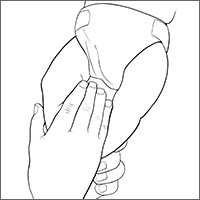
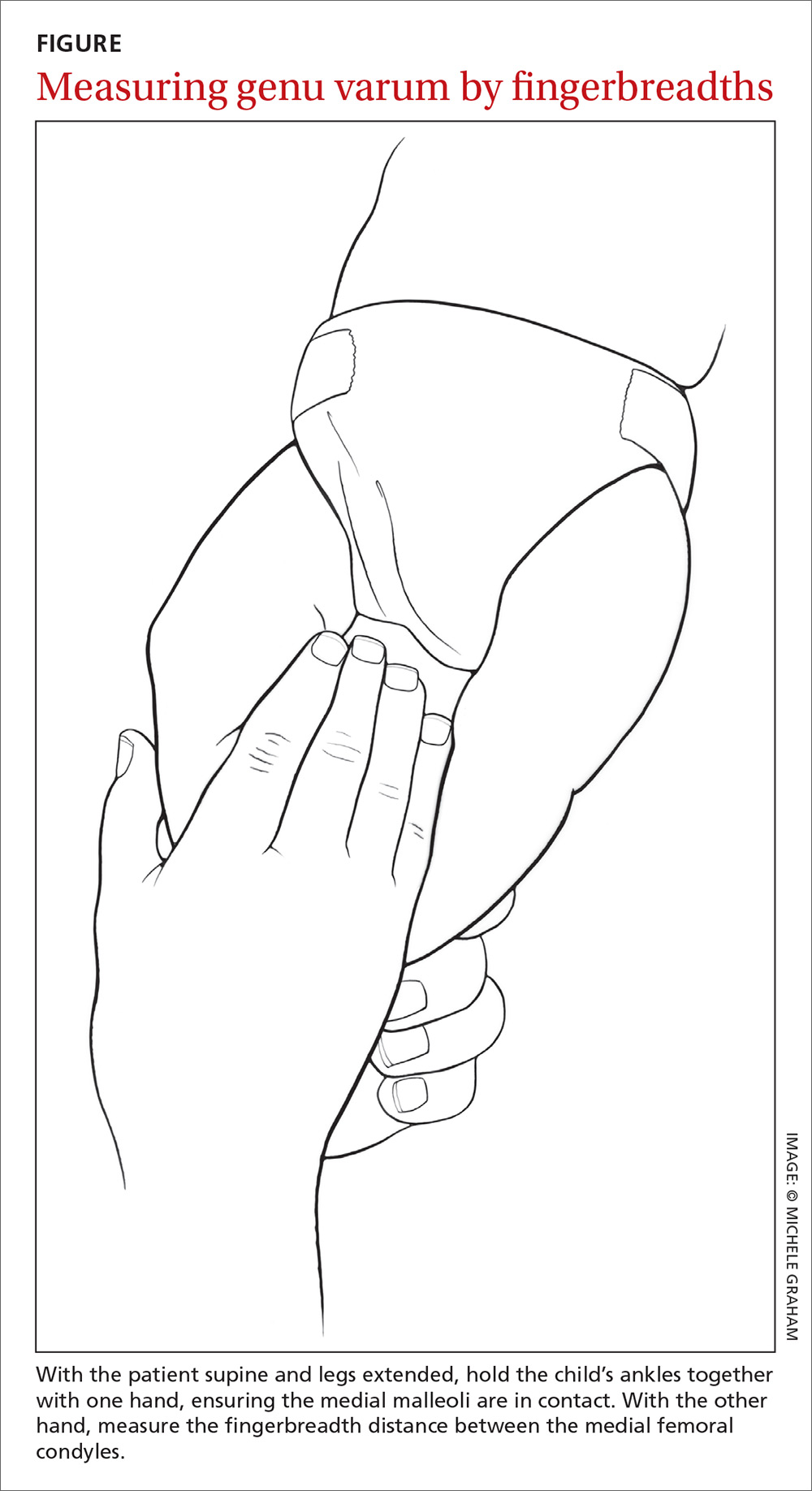
To measure varus distance, we used the fingerbreadth method described by Weiner in a study of 600 cases (FIGURE).6 This simple technique, which requires no special equipment, accurately detected differences in varus angulation and tracked the normal pattern of lower limb angular development. The patient should be supine on the examination table with legs extended. With one hand, the examiner holds the child’s ankles together, ensuring the medial malleoli are in contact. With the other hand, the examiner measures the fingerbreadth distance between the medial femoral condyles. Alternatively, a ruler may be used to measure the distance. This latter method may be especially useful in practices where the patient is likely to see more than one provider for well child care.
We divided the genu varum subject group into 3 subgroups by age at presentation: 103 subjects were younger than 18 months; 47 were 18 to 23 months; and 5 were 24 months or older. We used the data analysis toolkit in Microsoft Excel 2013 to perform a statistical analysis of study variables. We assumed the genu varum population is a normally distributed population. We used a 95% confidence level (α=0.05) for all calculations of confidence intervals (CIs), student t-tests, and tolerance intervals. Based on the data analysis results, we developed a series of follow-up and referral guidelines for practitioners.
Results
The mean walking age for those diagnosed with physiologic genu varum was 10 months (95% CI, 9.8-10.4), which is significantly younger than the 12 months of age (at the earliest) typical of toddlers in general (P<.001). There was no significant difference between the walking age of male and female children diagnosed with genu varum (P=.37).
Of the children presenting with the primary complaint of bow legs, 6% subsequently developed Blount’s disease. These patients presented at a mean age of 20.9 months and were diagnosed at a mean age of 23.9 months. Following the Blount’s disease diagnosis, we initiated therapy in all cases (3 surgical, 7 bracing).
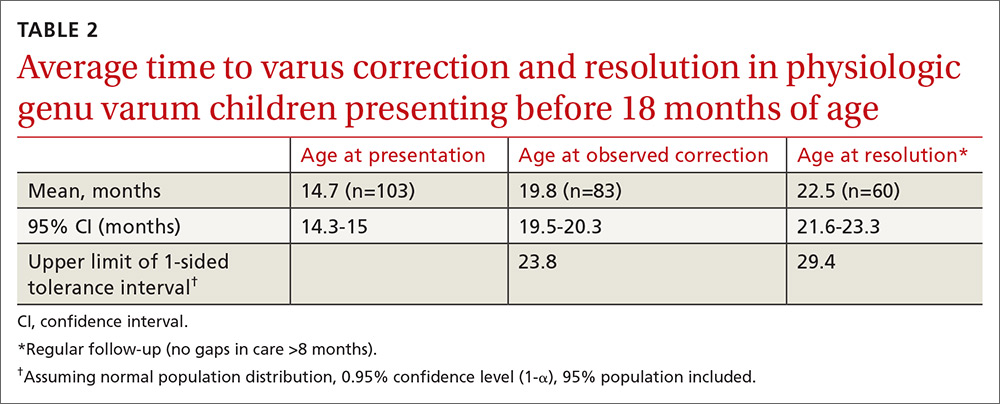
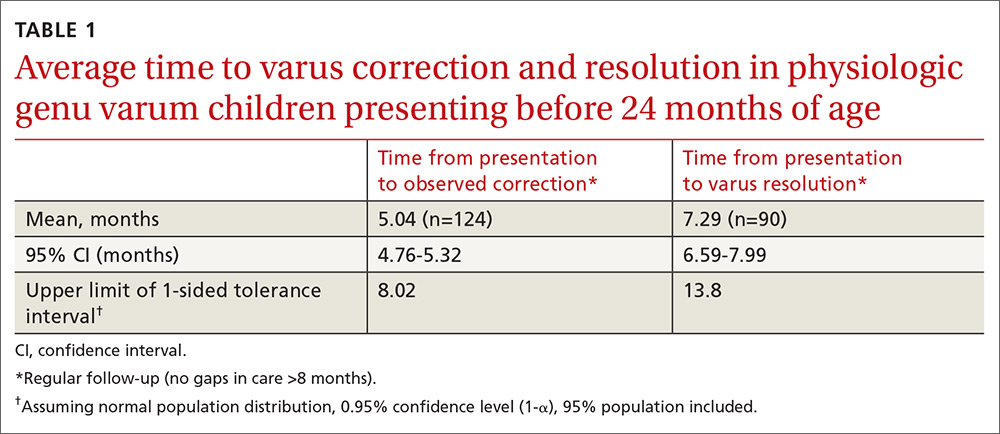
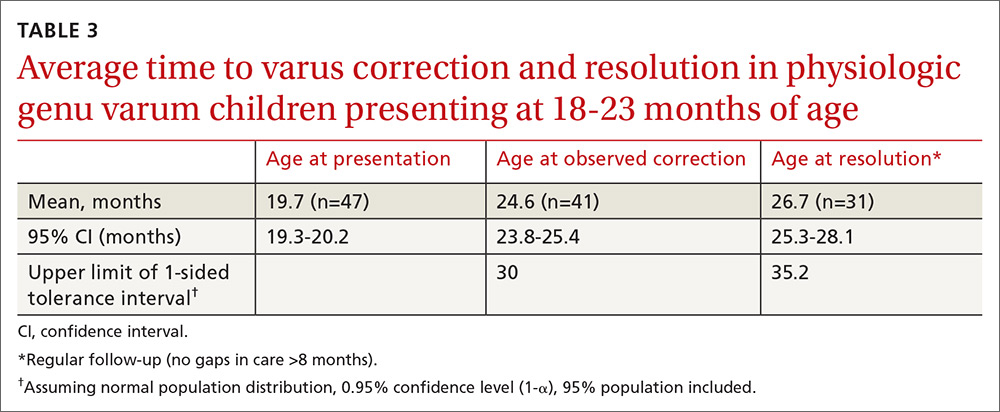
Physiologic genu varum patients presented at a mean age of 16.4 months, with only 3.23% presenting at older than 23 months. On average, physiologic genu varum patients presenting before 24 months of age showed measurable varus correction 5 months after presentation and achieved varus resolution 7.3 months after presentation (TABLE 1). Assuming the patient population is normally distributed, we can be 95% confident that 95% of physiologic genu varum patients presenting before 18 months of age will show measurable varus correction by 24 months and will resolve without intervention by 30 months (TABLE 2). Patients presenting between 18 and 23 months of age should show measurable varus correction by 30 months and resolution by 36 months (TABLE 3).
Discussion
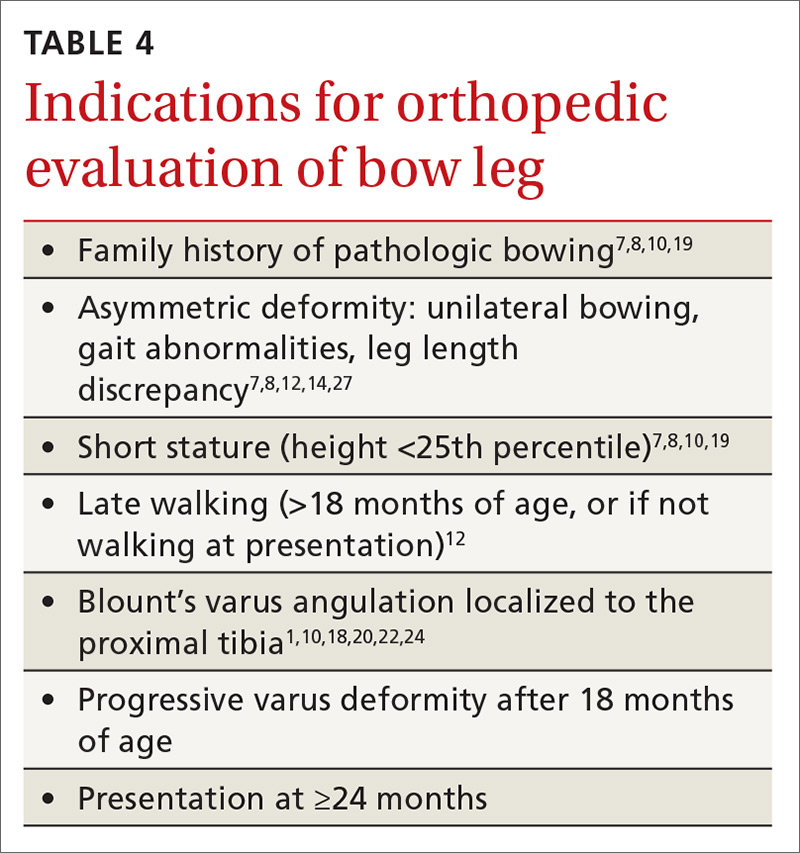
Primary care physicians have the ability to differentiate physiologic genu varum from pathologic forms of bow legs with a thorough history, physical exam, and radiographic examination, if necessary1,2,13 (TABLE 41,7,8,10,12,14,18-20,22,24,27). Several approaches to differentiating Blount’s disease and physiologic genu varum have been described in the literature.1,4,7,8,10,14,22,23
The average age at which children begin to walk independently is between 13 and 15 months.5,18,29-31 Recently, it has been suggested that the range be expanded to include 12 months of age.30 The association between early walking (at 10-11 months)12,20,22 and Blount’s disease is generally accepted in the orthopedic literature.1,4,7,10,19-22 However, some authors have suggested early walking also contributes to genu varum.1,5,8,10,18,28 The mean age of independent walking for children with physiologic genu varum suggested in the literature (10 months) was confirmed in our study and found to be significantly younger than the average for toddlers generally.1,22 Early walking is clearly associated with both physiologic genu varum and Blount’s disease, but no direct causation has been identified in either case. An alternative means of differentiating these entities is needed.
Radiographic examination of the knee is essential to the diagnosis of Blount’s disease as well as other, less common causes of pathologic bow legs (skeletal dysplasia, rickets, traumatic growth plate insults, infections, neoplasms).1,8,14,19 The common radiologic classification of staging for Blount’s disease is the Langenskiöld staging system, which involves identification of characteristic radiographic changes at the tibial physis.5,8,14,15,18,22,24
Sequential measurement of genu varum is most useful in differentiating between physiologic and pathologic processes. Physiologic genu varum, an exaggeration of the normal developmental pattern, characteristically resolves and evolves into physiologic genu valgum by 3 years of age.1,6-11 The pathophysiology of Blount’s disease is believed to be related to biomechanical overloading of the posteromedial proximal tibia during gait with the knee in a varus orientation. Excess loading on the proximal medial physis contributes to varus progression.4,10,14,20,25,27 Patients with Blount’s disease progress with varus and concomitant internal tibial torsion associated with growth plate irregularities and eventually exhibit premature closure.1,10,14,18,20,23,24,26 In the months prior to Blount’s disease diagnosis, increasing varus has been reported.4,7,10,19 Varus progression that differs from the expected pattern indicates possible pathologic bow legs and should prompt radiologic evaluation and, often, an orthopedic referral.3,4,7-9,12,13,21
In our study, only 3% of children with physiologic genu varum presented at 24 months of age or older, compared with 20% of Blount’s disease patients. We recommend considering orthopedic referral for any patient presenting with bow legs at 24 months of age or older. Additionally, consider orthopedic referral for any patient whose varus has not begun to correct within 8 months or has not resolved within 14 months of presentation, as more than 95% of patients with physiologic genu varum are expected to meet these milestones (TABLE 1). And do not hesitate to refer patients at any stage of follow-up if you suspect pathology or if parents are anxious.
If no sign of pathology is immediately identified, we recommend the following course of action:
- Record a reference fingerbreadth or ruler measurement at the initial presentation.
- Re-examine the knee varus at the next regular well-child visit (TABLE 5).
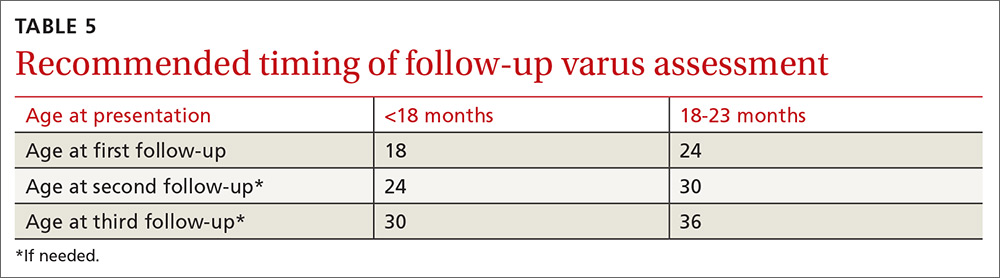
Re-examining the patient prior to the next well-child visit is unnecessary, as some degree of bowing is typical until age 18 to 24 months.1,6,7,9,12,13,17 Recommend orthopedic referral for any patient with varus that has progressed since initial presentation. Without signs of pathology, repeat varus assessment at the next well-child visit. This schedule minimizes the need for additional physician appointments by integrating follow-up into the typical well-child visits at 18, 24, 30, and 36 months of age.32 The 6-month follow-up interval was a feature of our study and is recommended in the related literature.12
- Consider orthopedic referral for patients whose varus has not corrected by the second follow-up appointment, as more than 95% of patients should have measurable varus correction at this visit. Most patients will have exhibited varus resolution by this time and will not require additional follow-up. For patients with observable correction who do not yet meet the criteria for resolution, we recommend a third, final follow-up appointment in another 6 months.
- Refer any patient whose varus has not resolved by the third follow-up appointment, as more than 95% of genu varum cases should have resolved by this time. This finding is echoed in the literature; any varus beyond 36 months of age is considered abnormal and suggestive of pathology.5,7,8,13,14 If evidence of Blount’s or skeletal dysplasia is identified, orthopedic management will likely consist of bracing (orthotics) or surgical management.
CORRESPONDENCE
Dennis S. Weiner, MD, Department of Orthopedic Surgery, Akron Children’s Hospital, 300 Locust Street, Suite 250, Akron, OH, 44302; [email protected].
ACKNOWLEDGEMENTS
The authors thank Meadow Newton, BS, assistant research coordinator, Akron Children’s Hospital, for her editing and technical assistance and Richard Steiner, PhD, The University of Akron, for his statistical review.
1. Weiner DS. Pediatric orthopedics for primary care physicians. 2nd ed. Jones K, ed. Cambridge, United Kingdom: Cambridge University Press; 2004.
2. Carli A, Saran N, Kruijt J, et al. Physiological referrals for paediatric musculoskeletal complaints: a costly problem that needs to be addressed. Paediatr Child Health. 2012;17:e93-e97.
3. Fabry G. Clinical practice. Static, axial, and rotational deformities of the lower extremities in children. Eur J Pediatr. 2010;169:529-534.
4. Davids JR, Blackhurst DW, Allen Jr BL. Clinical evaluation of bowed legs in children. J Pediatr Orthop B. 2000;9:278-284.
5. Bateson EM. The relationship between Blount’s disease and bow legs. Br J Radiol. 1968;41:107-114.
6. Weiner DS. The natural history of “bow legs” and “knock knees” in childhood. Orthopedics. 1981;4:156-160.
7. Greene WB. Genu varum and genu valgum in children: differential diagnosis and guidelines for evaluation. Compr Ther. 1996;22:22-29.
8. Do TT. Clinical and radiographic evaluation of bowlegs. Curr Opin Pediatr. 2001;13:42-46.
9. Bleck EE. Developmental orthopaedics. III: Toddlers. Dev Med Child Neurol. 1982;24:533-555.
10. Brooks WC, Gross RH. Genu Varum in Children: Diagnosis and Treatment. J Am Acad Orthop Surg. 1995;3:326-335.
11. Greenberg LA, Swartz AA. Genu varum and genu valgum. Another look. Am J Dis Child. 1971;121:219-221.
12. Scherl SA. Common lower extremity problems in children. Pediatr Rev. 2004;25:52-62.
13. Wall EJ. Practical primary pediatric orthopedics. Nurs Clin North Am. 2000;35:95-113.
14. Cheema JI, Grissom LE, Harcke HT. Radiographic characteristics of lower-extremity bowing in children. Radiographics. 2003;23:871-880.
15. McCarthy JJ, Betz RR, Kim A, et al. Early radiographic differentiation of infantile tibia vara from physiologic bowing using the femoral-tibial ratio. J Pediatr Orthop. 2001;21:545-548.
16. Salenius P, Vankka E. The development of the tibiofemoral angle in children. J Bone Joint Surg Am. 1975;57:259-261.
17. Engel GM, Staheli LT. The natural history of torsion and other factors influencing gait in childhood. A study of the angle of gait, tibial torsion, knee angle, hip rotation, and development of the arch in normal children. Clin Orthop Relat Res. 1974;99:12-17.
18. Golding J, Bateson E, McNeil-Smith G. Infantile tibia vara. In: The Growth Plate and Its Disorders. Rang M, ed. Baltimore, MD: Williams and Wilkins; 1969:109-119.
19. Greene WB. Infantile tibia vara. J Bone Joint Surg Am. 1993;75:130-143.
20. Golding J, McNeil-Smith JDG. Observations on the etiology of tibia vara. J Bone Joint Surg Br. 1963;45-B:320-325.
21. Eggert P, Viemann M. Physiological bowlegs or infantile Blount’s disease. Some new aspects on an old problem. Pediatr Radiol. 1996;26:349-352.
22. Levine AM, Drennan JC. Physiological bowing and tibia vara. The metaphyseal-diaphyseal angle in the measurement of bowleg deformities. J Bone Joint Surg Am. 1982;64:1158-1163.
23. Kessel L. Annotations on the etiology and treatment of tibia vara. J Bone Joint Surg Br. 1970;52:93-99.
24. Blount WP. Tibia vara: osteochondrosis deformans tibiae. J Bone Joint Surg Am. 1937;19:1-29.
25. Davids JR, Blackhurst DW, Allen BL Jr. Radiographic evaluation of bowed legs in children. J Pediatr Orthop. 2001;21:257-263.
26. Cook SD, Lavernia CJ, Burke SW, et al. A biomechanical analysis of the etiology of tibia vara. J Pediatr Orthop. 1983;3:449-454.
27. Birch JG. Blount disease. J Am Acad Orthop Surg. 2013;21:408-418.
28. Bateson EM. Non-rachitic bow leg and knock-knee deformities in young Jamaican children. Br J Radiol. 1966;39:92-101.
29. Grantham-McGregor SM, Back EH. Gross motor development in Jamaican infants. Dev Med Child Neurol. 1971;13:79-87.
30. Størvold GV, Aarethun K, Bratberg GH. Age for onset of walking and prewalking strategies. Early Hum Dev. 2013;89:655-659.
31. Garrett M, McElroy AM, Staines A. Locomotor milestones and babywalkers: cross sectional study. BMJ. 2002;324:1494.
32. Simon GR, Baker C, Barden GA 3rd, et al; Committee on Practice and Ambulatory Medicine, Curry ES, Dunca PM, Hagan JF Jr, et al; Bright Futures Periodicity Schedule Workgroup. 2014 recommendations for pediatric preventive health care. Pediatrics. 2014;133:568-570.
ABSTRACT
Objective To reduce unnecessary orthopedic referrals by developing a protocol for managing physiologic bow legs in the primary care environment through the use of a noninvasive technique that simultaneously tracks normal varus progression and screens for potential pathologic bowing requiring an orthopedic referral.
Methods Retrospective study of 155 patients with physiologic genu varum and 10 with infantile Blount’s disease. We used fingerbreadth measurements to document progression or resolution of bow legs. Final diagnoses were made by one orthopedic surgeon using clinical and radiographic evidence. We divided genu varum patients into 3 groups: patients presenting with bow legs before 18 months of age (MOA), patients presenting between 18 and 23 MOA, and patients presenting at 24 MOA or older for analyses relevant to the development of the follow-up protocol.
Results Physiologic genu varum patients walked earlier than average infants (10 months vs 12-15 months; P<.001). Physiologic genu varum patients presenting before 18 MOA demonstrated initial signs of correction between 18 and 24 MOA and resolution by 30 MOA. Physiologic genu varum patients presenting between 18 and 23 MOA demonstrated initial signs of correction between 24 MOA and 30 MOA and resolution by 36 MOA.
Conclusion Primary care physicians can manage most children presenting with bow legs. Management focuses on following the progression or resolution of varus with regular follow-up. For patients presenting with bow legs, we recommend a follow-up protocol using mainly well-child checkups and a simple clinical assessment to monitor varus progression and screen for pathologic bowing.
Bow legs in young children can be a concern for parents.1,2 By far, the most common reason for bow legs is physiologic genu varum,3-5 a nonprogressive stage of normal development in young children that generally resolves spontaneously without treatment.1,6-11 Normally developing children undergo a varus phase between birth and 18 to 24 months of age (MOA), at which time there is usually a transition in alignment from varus to straight to valgus (knock knees), which will correct to straight or mild valgus throughout adolescence.1,6,7,9,10,12-17
The most common form of pathologic bow legs is Blount’s disease, also known as tibia vara, which must be differentiated from physiologic genu varum.8-10,15,18-24 The progressive varus deformity of Blount’s disease usually requires orthopedic intervention.1,10,23-26 Early diagnosis may spare patients complex interventions, improve prognosis, and limit complications that include gait abnormalities,4,8,10,27 knee joint instability,4,24,27 osteoarthritis,9,20,27 meniscal tears,27 and degenerative joint disease.19,20,27
Although variables such as walking age, race, weight, and gender have been suggested as risk factors for Blount’s disease, they have not been useful in differentiating between Blount’s pathology and physiologic genu varum.1,4,5,7,10,20,28 In the primary care setting, distinguishing physiologic from pathologic forms of bow legs is possible with a thorough history and physical exam and with radiographs, as warranted.1,2,15 More than 40% of genu varum/genu valgum cases referred for orthopedic consultation turn out to be the physiologic form,2 suggesting a need for guidelines in the primary care setting to help direct referral and follow-up. The purpose of this study was to provide recommendations to family physicians for evaluating and managing children with bow legs.
Materials and methods
This study, approved by the Internal Review Board of Akron Children’s Hospital, is a retrospective review of children seen by a single pediatric orthopedic surgeon (DSW) from 1970 to 2012. Four-hundred twenty-four children were received for evaluation of bow legs. Excluded from our final analysis were 220 subjects seen only once for this specific referral and 39 subjects diagnosed with a condition other than genu varum or Blount’s disease (ie, rickets, skeletal dysplasia, sequelae of trauma, or infection). Ten subjects with Blount’s disease and 155 subjects with physiologic genu varum were included in the final data analysis.
In addition to noting the age at which a patient walked independently, at each visit we documented age and the fingerbreadth (varus) distance between the medial femoral condyles with the child’s ankles held together. Parents reported age of independent walking for just 3 children with Blount’s disease and for 134 children with physiologic genu varum. Study variables for the genu varum data analysis were age of walking, age at presentation, age at varus correction, age at varus resolution, time between presentation and varus correction, and time between presentation and varus resolution. Varus correction is defined as any decrease in varus angulation since presentation. Varus resolution is defined as varus correction to less than or equal to half of the varus angulation at presentation. For inclusion in the age-at-resolution analysis, a child must have been evaluated at regular follow-up visits (all rechecks within 8 months).
To measure varus distance, we used the fingerbreadth method described by Weiner in a study of 600 cases (FIGURE).6 This simple technique, which requires no special equipment, accurately detected differences in varus angulation and tracked the normal pattern of lower limb angular development. The patient should be supine on the examination table with legs extended. With one hand, the examiner holds the child’s ankles together, ensuring the medial malleoli are in contact. With the other hand, the examiner measures the fingerbreadth distance between the medial femoral condyles. Alternatively, a ruler may be used to measure the distance. This latter method may be especially useful in practices where the patient is likely to see more than one provider for well child care.

We divided the genu varum subject group into 3 subgroups by age at presentation: 103 subjects were younger than 18 months; 47 were 18 to 23 months; and 5 were 24 months or older. We used the data analysis toolkit in Microsoft Excel 2013 to perform a statistical analysis of study variables. We assumed the genu varum population is a normally distributed population. We used a 95% confidence level (α=0.05) for all calculations of confidence intervals (CIs), student t-tests, and tolerance intervals. Based on the data analysis results, we developed a series of follow-up and referral guidelines for practitioners.
Results
The mean walking age for those diagnosed with physiologic genu varum was 10 months (95% CI, 9.8-10.4), which is significantly younger than the 12 months of age (at the earliest) typical of toddlers in general (P<.001). There was no significant difference between the walking age of male and female children diagnosed with genu varum (P=.37).

Of the children presenting with the primary complaint of bow legs, 6% subsequently developed Blount’s disease. These patients presented at a mean age of 20.9 months and were diagnosed at a mean age of 23.9 months. Following the Blount’s disease diagnosis, we initiated therapy in all cases (3 surgical, 7 bracing).

Physiologic genu varum patients presented at a mean age of 16.4 months, with only 3.23% presenting at older than 23 months. On average, physiologic genu varum patients presenting before 24 months of age showed measurable varus correction 5 months after presentation and achieved varus resolution 7.3 months after presentation (TABLE 1). Assuming the patient population is normally distributed, we can be 95% confident that 95% of physiologic genu varum patients presenting before 18 months of age will show measurable varus correction by 24 months and will resolve without intervention by 30 months (TABLE 2). Patients presenting between 18 and 23 months of age should show measurable varus correction by 30 months and resolution by 36 months (TABLE 3).

Discussion
Primary care physicians have the ability to differentiate physiologic genu varum from pathologic forms of bow legs with a thorough history, physical exam, and radiographic examination, if necessary1,2,13 (TABLE 41,7,8,10,12,14,18-20,22,24,27). Several approaches to differentiating Blount’s disease and physiologic genu varum have been described in the literature.1,4,7,8,10,14,22,23

The average age at which children begin to walk independently is between 13 and 15 months.5,18,29-31 Recently, it has been suggested that the range be expanded to include 12 months of age.30 The association between early walking (at 10-11 months)12,20,22 and Blount’s disease is generally accepted in the orthopedic literature.1,4,7,10,19-22 However, some authors have suggested early walking also contributes to genu varum.1,5,8,10,18,28 The mean age of independent walking for children with physiologic genu varum suggested in the literature (10 months) was confirmed in our study and found to be significantly younger than the average for toddlers generally.1,22 Early walking is clearly associated with both physiologic genu varum and Blount’s disease, but no direct causation has been identified in either case. An alternative means of differentiating these entities is needed.
Radiographic examination of the knee is essential to the diagnosis of Blount’s disease as well as other, less common causes of pathologic bow legs (skeletal dysplasia, rickets, traumatic growth plate insults, infections, neoplasms).1,8,14,19 The common radiologic classification of staging for Blount’s disease is the Langenskiöld staging system, which involves identification of characteristic radiographic changes at the tibial physis.5,8,14,15,18,22,24
Sequential measurement of genu varum is most useful in differentiating between physiologic and pathologic processes. Physiologic genu varum, an exaggeration of the normal developmental pattern, characteristically resolves and evolves into physiologic genu valgum by 3 years of age.1,6-11 The pathophysiology of Blount’s disease is believed to be related to biomechanical overloading of the posteromedial proximal tibia during gait with the knee in a varus orientation. Excess loading on the proximal medial physis contributes to varus progression.4,10,14,20,25,27 Patients with Blount’s disease progress with varus and concomitant internal tibial torsion associated with growth plate irregularities and eventually exhibit premature closure.1,10,14,18,20,23,24,26 In the months prior to Blount’s disease diagnosis, increasing varus has been reported.4,7,10,19 Varus progression that differs from the expected pattern indicates possible pathologic bow legs and should prompt radiologic evaluation and, often, an orthopedic referral.3,4,7-9,12,13,21
In our study, only 3% of children with physiologic genu varum presented at 24 months of age or older, compared with 20% of Blount’s disease patients. We recommend considering orthopedic referral for any patient presenting with bow legs at 24 months of age or older. Additionally, consider orthopedic referral for any patient whose varus has not begun to correct within 8 months or has not resolved within 14 months of presentation, as more than 95% of patients with physiologic genu varum are expected to meet these milestones (TABLE 1). And do not hesitate to refer patients at any stage of follow-up if you suspect pathology or if parents are anxious.
If no sign of pathology is immediately identified, we recommend the following course of action:
- Record a reference fingerbreadth or ruler measurement at the initial presentation.
- Re-examine the knee varus at the next regular well-child visit (TABLE 5).

Re-examining the patient prior to the next well-child visit is unnecessary, as some degree of bowing is typical until age 18 to 24 months.1,6,7,9,12,13,17 Recommend orthopedic referral for any patient with varus that has progressed since initial presentation. Without signs of pathology, repeat varus assessment at the next well-child visit. This schedule minimizes the need for additional physician appointments by integrating follow-up into the typical well-child visits at 18, 24, 30, and 36 months of age.32 The 6-month follow-up interval was a feature of our study and is recommended in the related literature.12
- Consider orthopedic referral for patients whose varus has not corrected by the second follow-up appointment, as more than 95% of patients should have measurable varus correction at this visit. Most patients will have exhibited varus resolution by this time and will not require additional follow-up. For patients with observable correction who do not yet meet the criteria for resolution, we recommend a third, final follow-up appointment in another 6 months.
- Refer any patient whose varus has not resolved by the third follow-up appointment, as more than 95% of genu varum cases should have resolved by this time. This finding is echoed in the literature; any varus beyond 36 months of age is considered abnormal and suggestive of pathology.5,7,8,13,14 If evidence of Blount’s or skeletal dysplasia is identified, orthopedic management will likely consist of bracing (orthotics) or surgical management.
CORRESPONDENCE
Dennis S. Weiner, MD, Department of Orthopedic Surgery, Akron Children’s Hospital, 300 Locust Street, Suite 250, Akron, OH, 44302; [email protected].
ACKNOWLEDGEMENTS
The authors thank Meadow Newton, BS, assistant research coordinator, Akron Children’s Hospital, for her editing and technical assistance and Richard Steiner, PhD, The University of Akron, for his statistical review.
ABSTRACT
Objective To reduce unnecessary orthopedic referrals by developing a protocol for managing physiologic bow legs in the primary care environment through the use of a noninvasive technique that simultaneously tracks normal varus progression and screens for potential pathologic bowing requiring an orthopedic referral.
Methods Retrospective study of 155 patients with physiologic genu varum and 10 with infantile Blount’s disease. We used fingerbreadth measurements to document progression or resolution of bow legs. Final diagnoses were made by one orthopedic surgeon using clinical and radiographic evidence. We divided genu varum patients into 3 groups: patients presenting with bow legs before 18 months of age (MOA), patients presenting between 18 and 23 MOA, and patients presenting at 24 MOA or older for analyses relevant to the development of the follow-up protocol.
Results Physiologic genu varum patients walked earlier than average infants (10 months vs 12-15 months; P<.001). Physiologic genu varum patients presenting before 18 MOA demonstrated initial signs of correction between 18 and 24 MOA and resolution by 30 MOA. Physiologic genu varum patients presenting between 18 and 23 MOA demonstrated initial signs of correction between 24 MOA and 30 MOA and resolution by 36 MOA.
Conclusion Primary care physicians can manage most children presenting with bow legs. Management focuses on following the progression or resolution of varus with regular follow-up. For patients presenting with bow legs, we recommend a follow-up protocol using mainly well-child checkups and a simple clinical assessment to monitor varus progression and screen for pathologic bowing.
Bow legs in young children can be a concern for parents.1,2 By far, the most common reason for bow legs is physiologic genu varum,3-5 a nonprogressive stage of normal development in young children that generally resolves spontaneously without treatment.1,6-11 Normally developing children undergo a varus phase between birth and 18 to 24 months of age (MOA), at which time there is usually a transition in alignment from varus to straight to valgus (knock knees), which will correct to straight or mild valgus throughout adolescence.1,6,7,9,10,12-17
The most common form of pathologic bow legs is Blount’s disease, also known as tibia vara, which must be differentiated from physiologic genu varum.8-10,15,18-24 The progressive varus deformity of Blount’s disease usually requires orthopedic intervention.1,10,23-26 Early diagnosis may spare patients complex interventions, improve prognosis, and limit complications that include gait abnormalities,4,8,10,27 knee joint instability,4,24,27 osteoarthritis,9,20,27 meniscal tears,27 and degenerative joint disease.19,20,27
Although variables such as walking age, race, weight, and gender have been suggested as risk factors for Blount’s disease, they have not been useful in differentiating between Blount’s pathology and physiologic genu varum.1,4,5,7,10,20,28 In the primary care setting, distinguishing physiologic from pathologic forms of bow legs is possible with a thorough history and physical exam and with radiographs, as warranted.1,2,15 More than 40% of genu varum/genu valgum cases referred for orthopedic consultation turn out to be the physiologic form,2 suggesting a need for guidelines in the primary care setting to help direct referral and follow-up. The purpose of this study was to provide recommendations to family physicians for evaluating and managing children with bow legs.
Materials and methods
This study, approved by the Internal Review Board of Akron Children’s Hospital, is a retrospective review of children seen by a single pediatric orthopedic surgeon (DSW) from 1970 to 2012. Four-hundred twenty-four children were received for evaluation of bow legs. Excluded from our final analysis were 220 subjects seen only once for this specific referral and 39 subjects diagnosed with a condition other than genu varum or Blount’s disease (ie, rickets, skeletal dysplasia, sequelae of trauma, or infection). Ten subjects with Blount’s disease and 155 subjects with physiologic genu varum were included in the final data analysis.
In addition to noting the age at which a patient walked independently, at each visit we documented age and the fingerbreadth (varus) distance between the medial femoral condyles with the child’s ankles held together. Parents reported age of independent walking for just 3 children with Blount’s disease and for 134 children with physiologic genu varum. Study variables for the genu varum data analysis were age of walking, age at presentation, age at varus correction, age at varus resolution, time between presentation and varus correction, and time between presentation and varus resolution. Varus correction is defined as any decrease in varus angulation since presentation. Varus resolution is defined as varus correction to less than or equal to half of the varus angulation at presentation. For inclusion in the age-at-resolution analysis, a child must have been evaluated at regular follow-up visits (all rechecks within 8 months).
To measure varus distance, we used the fingerbreadth method described by Weiner in a study of 600 cases (FIGURE).6 This simple technique, which requires no special equipment, accurately detected differences in varus angulation and tracked the normal pattern of lower limb angular development. The patient should be supine on the examination table with legs extended. With one hand, the examiner holds the child’s ankles together, ensuring the medial malleoli are in contact. With the other hand, the examiner measures the fingerbreadth distance between the medial femoral condyles. Alternatively, a ruler may be used to measure the distance. This latter method may be especially useful in practices where the patient is likely to see more than one provider for well child care.

We divided the genu varum subject group into 3 subgroups by age at presentation: 103 subjects were younger than 18 months; 47 were 18 to 23 months; and 5 were 24 months or older. We used the data analysis toolkit in Microsoft Excel 2013 to perform a statistical analysis of study variables. We assumed the genu varum population is a normally distributed population. We used a 95% confidence level (α=0.05) for all calculations of confidence intervals (CIs), student t-tests, and tolerance intervals. Based on the data analysis results, we developed a series of follow-up and referral guidelines for practitioners.
Results
The mean walking age for those diagnosed with physiologic genu varum was 10 months (95% CI, 9.8-10.4), which is significantly younger than the 12 months of age (at the earliest) typical of toddlers in general (P<.001). There was no significant difference between the walking age of male and female children diagnosed with genu varum (P=.37).

Of the children presenting with the primary complaint of bow legs, 6% subsequently developed Blount’s disease. These patients presented at a mean age of 20.9 months and were diagnosed at a mean age of 23.9 months. Following the Blount’s disease diagnosis, we initiated therapy in all cases (3 surgical, 7 bracing).

Physiologic genu varum patients presented at a mean age of 16.4 months, with only 3.23% presenting at older than 23 months. On average, physiologic genu varum patients presenting before 24 months of age showed measurable varus correction 5 months after presentation and achieved varus resolution 7.3 months after presentation (TABLE 1). Assuming the patient population is normally distributed, we can be 95% confident that 95% of physiologic genu varum patients presenting before 18 months of age will show measurable varus correction by 24 months and will resolve without intervention by 30 months (TABLE 2). Patients presenting between 18 and 23 months of age should show measurable varus correction by 30 months and resolution by 36 months (TABLE 3).

Discussion
Primary care physicians have the ability to differentiate physiologic genu varum from pathologic forms of bow legs with a thorough history, physical exam, and radiographic examination, if necessary1,2,13 (TABLE 41,7,8,10,12,14,18-20,22,24,27). Several approaches to differentiating Blount’s disease and physiologic genu varum have been described in the literature.1,4,7,8,10,14,22,23

The average age at which children begin to walk independently is between 13 and 15 months.5,18,29-31 Recently, it has been suggested that the range be expanded to include 12 months of age.30 The association between early walking (at 10-11 months)12,20,22 and Blount’s disease is generally accepted in the orthopedic literature.1,4,7,10,19-22 However, some authors have suggested early walking also contributes to genu varum.1,5,8,10,18,28 The mean age of independent walking for children with physiologic genu varum suggested in the literature (10 months) was confirmed in our study and found to be significantly younger than the average for toddlers generally.1,22 Early walking is clearly associated with both physiologic genu varum and Blount’s disease, but no direct causation has been identified in either case. An alternative means of differentiating these entities is needed.
Radiographic examination of the knee is essential to the diagnosis of Blount’s disease as well as other, less common causes of pathologic bow legs (skeletal dysplasia, rickets, traumatic growth plate insults, infections, neoplasms).1,8,14,19 The common radiologic classification of staging for Blount’s disease is the Langenskiöld staging system, which involves identification of characteristic radiographic changes at the tibial physis.5,8,14,15,18,22,24
Sequential measurement of genu varum is most useful in differentiating between physiologic and pathologic processes. Physiologic genu varum, an exaggeration of the normal developmental pattern, characteristically resolves and evolves into physiologic genu valgum by 3 years of age.1,6-11 The pathophysiology of Blount’s disease is believed to be related to biomechanical overloading of the posteromedial proximal tibia during gait with the knee in a varus orientation. Excess loading on the proximal medial physis contributes to varus progression.4,10,14,20,25,27 Patients with Blount’s disease progress with varus and concomitant internal tibial torsion associated with growth plate irregularities and eventually exhibit premature closure.1,10,14,18,20,23,24,26 In the months prior to Blount’s disease diagnosis, increasing varus has been reported.4,7,10,19 Varus progression that differs from the expected pattern indicates possible pathologic bow legs and should prompt radiologic evaluation and, often, an orthopedic referral.3,4,7-9,12,13,21
In our study, only 3% of children with physiologic genu varum presented at 24 months of age or older, compared with 20% of Blount’s disease patients. We recommend considering orthopedic referral for any patient presenting with bow legs at 24 months of age or older. Additionally, consider orthopedic referral for any patient whose varus has not begun to correct within 8 months or has not resolved within 14 months of presentation, as more than 95% of patients with physiologic genu varum are expected to meet these milestones (TABLE 1). And do not hesitate to refer patients at any stage of follow-up if you suspect pathology or if parents are anxious.
If no sign of pathology is immediately identified, we recommend the following course of action:
- Record a reference fingerbreadth or ruler measurement at the initial presentation.
- Re-examine the knee varus at the next regular well-child visit (TABLE 5).

Re-examining the patient prior to the next well-child visit is unnecessary, as some degree of bowing is typical until age 18 to 24 months.1,6,7,9,12,13,17 Recommend orthopedic referral for any patient with varus that has progressed since initial presentation. Without signs of pathology, repeat varus assessment at the next well-child visit. This schedule minimizes the need for additional physician appointments by integrating follow-up into the typical well-child visits at 18, 24, 30, and 36 months of age.32 The 6-month follow-up interval was a feature of our study and is recommended in the related literature.12
- Consider orthopedic referral for patients whose varus has not corrected by the second follow-up appointment, as more than 95% of patients should have measurable varus correction at this visit. Most patients will have exhibited varus resolution by this time and will not require additional follow-up. For patients with observable correction who do not yet meet the criteria for resolution, we recommend a third, final follow-up appointment in another 6 months.
- Refer any patient whose varus has not resolved by the third follow-up appointment, as more than 95% of genu varum cases should have resolved by this time. This finding is echoed in the literature; any varus beyond 36 months of age is considered abnormal and suggestive of pathology.5,7,8,13,14 If evidence of Blount’s or skeletal dysplasia is identified, orthopedic management will likely consist of bracing (orthotics) or surgical management.
CORRESPONDENCE
Dennis S. Weiner, MD, Department of Orthopedic Surgery, Akron Children’s Hospital, 300 Locust Street, Suite 250, Akron, OH, 44302; [email protected].
ACKNOWLEDGEMENTS
The authors thank Meadow Newton, BS, assistant research coordinator, Akron Children’s Hospital, for her editing and technical assistance and Richard Steiner, PhD, The University of Akron, for his statistical review.
1. Weiner DS. Pediatric orthopedics for primary care physicians. 2nd ed. Jones K, ed. Cambridge, United Kingdom: Cambridge University Press; 2004.
2. Carli A, Saran N, Kruijt J, et al. Physiological referrals for paediatric musculoskeletal complaints: a costly problem that needs to be addressed. Paediatr Child Health. 2012;17:e93-e97.
3. Fabry G. Clinical practice. Static, axial, and rotational deformities of the lower extremities in children. Eur J Pediatr. 2010;169:529-534.
4. Davids JR, Blackhurst DW, Allen Jr BL. Clinical evaluation of bowed legs in children. J Pediatr Orthop B. 2000;9:278-284.
5. Bateson EM. The relationship between Blount’s disease and bow legs. Br J Radiol. 1968;41:107-114.
6. Weiner DS. The natural history of “bow legs” and “knock knees” in childhood. Orthopedics. 1981;4:156-160.
7. Greene WB. Genu varum and genu valgum in children: differential diagnosis and guidelines for evaluation. Compr Ther. 1996;22:22-29.
8. Do TT. Clinical and radiographic evaluation of bowlegs. Curr Opin Pediatr. 2001;13:42-46.
9. Bleck EE. Developmental orthopaedics. III: Toddlers. Dev Med Child Neurol. 1982;24:533-555.
10. Brooks WC, Gross RH. Genu Varum in Children: Diagnosis and Treatment. J Am Acad Orthop Surg. 1995;3:326-335.
11. Greenberg LA, Swartz AA. Genu varum and genu valgum. Another look. Am J Dis Child. 1971;121:219-221.
12. Scherl SA. Common lower extremity problems in children. Pediatr Rev. 2004;25:52-62.
13. Wall EJ. Practical primary pediatric orthopedics. Nurs Clin North Am. 2000;35:95-113.
14. Cheema JI, Grissom LE, Harcke HT. Radiographic characteristics of lower-extremity bowing in children. Radiographics. 2003;23:871-880.
15. McCarthy JJ, Betz RR, Kim A, et al. Early radiographic differentiation of infantile tibia vara from physiologic bowing using the femoral-tibial ratio. J Pediatr Orthop. 2001;21:545-548.
16. Salenius P, Vankka E. The development of the tibiofemoral angle in children. J Bone Joint Surg Am. 1975;57:259-261.
17. Engel GM, Staheli LT. The natural history of torsion and other factors influencing gait in childhood. A study of the angle of gait, tibial torsion, knee angle, hip rotation, and development of the arch in normal children. Clin Orthop Relat Res. 1974;99:12-17.
18. Golding J, Bateson E, McNeil-Smith G. Infantile tibia vara. In: The Growth Plate and Its Disorders. Rang M, ed. Baltimore, MD: Williams and Wilkins; 1969:109-119.
19. Greene WB. Infantile tibia vara. J Bone Joint Surg Am. 1993;75:130-143.
20. Golding J, McNeil-Smith JDG. Observations on the etiology of tibia vara. J Bone Joint Surg Br. 1963;45-B:320-325.
21. Eggert P, Viemann M. Physiological bowlegs or infantile Blount’s disease. Some new aspects on an old problem. Pediatr Radiol. 1996;26:349-352.
22. Levine AM, Drennan JC. Physiological bowing and tibia vara. The metaphyseal-diaphyseal angle in the measurement of bowleg deformities. J Bone Joint Surg Am. 1982;64:1158-1163.
23. Kessel L. Annotations on the etiology and treatment of tibia vara. J Bone Joint Surg Br. 1970;52:93-99.
24. Blount WP. Tibia vara: osteochondrosis deformans tibiae. J Bone Joint Surg Am. 1937;19:1-29.
25. Davids JR, Blackhurst DW, Allen BL Jr. Radiographic evaluation of bowed legs in children. J Pediatr Orthop. 2001;21:257-263.
26. Cook SD, Lavernia CJ, Burke SW, et al. A biomechanical analysis of the etiology of tibia vara. J Pediatr Orthop. 1983;3:449-454.
27. Birch JG. Blount disease. J Am Acad Orthop Surg. 2013;21:408-418.
28. Bateson EM. Non-rachitic bow leg and knock-knee deformities in young Jamaican children. Br J Radiol. 1966;39:92-101.
29. Grantham-McGregor SM, Back EH. Gross motor development in Jamaican infants. Dev Med Child Neurol. 1971;13:79-87.
30. Størvold GV, Aarethun K, Bratberg GH. Age for onset of walking and prewalking strategies. Early Hum Dev. 2013;89:655-659.
31. Garrett M, McElroy AM, Staines A. Locomotor milestones and babywalkers: cross sectional study. BMJ. 2002;324:1494.
32. Simon GR, Baker C, Barden GA 3rd, et al; Committee on Practice and Ambulatory Medicine, Curry ES, Dunca PM, Hagan JF Jr, et al; Bright Futures Periodicity Schedule Workgroup. 2014 recommendations for pediatric preventive health care. Pediatrics. 2014;133:568-570.
1. Weiner DS. Pediatric orthopedics for primary care physicians. 2nd ed. Jones K, ed. Cambridge, United Kingdom: Cambridge University Press; 2004.
2. Carli A, Saran N, Kruijt J, et al. Physiological referrals for paediatric musculoskeletal complaints: a costly problem that needs to be addressed. Paediatr Child Health. 2012;17:e93-e97.
3. Fabry G. Clinical practice. Static, axial, and rotational deformities of the lower extremities in children. Eur J Pediatr. 2010;169:529-534.
4. Davids JR, Blackhurst DW, Allen Jr BL. Clinical evaluation of bowed legs in children. J Pediatr Orthop B. 2000;9:278-284.
5. Bateson EM. The relationship between Blount’s disease and bow legs. Br J Radiol. 1968;41:107-114.
6. Weiner DS. The natural history of “bow legs” and “knock knees” in childhood. Orthopedics. 1981;4:156-160.
7. Greene WB. Genu varum and genu valgum in children: differential diagnosis and guidelines for evaluation. Compr Ther. 1996;22:22-29.
8. Do TT. Clinical and radiographic evaluation of bowlegs. Curr Opin Pediatr. 2001;13:42-46.
9. Bleck EE. Developmental orthopaedics. III: Toddlers. Dev Med Child Neurol. 1982;24:533-555.
10. Brooks WC, Gross RH. Genu Varum in Children: Diagnosis and Treatment. J Am Acad Orthop Surg. 1995;3:326-335.
11. Greenberg LA, Swartz AA. Genu varum and genu valgum. Another look. Am J Dis Child. 1971;121:219-221.
12. Scherl SA. Common lower extremity problems in children. Pediatr Rev. 2004;25:52-62.
13. Wall EJ. Practical primary pediatric orthopedics. Nurs Clin North Am. 2000;35:95-113.
14. Cheema JI, Grissom LE, Harcke HT. Radiographic characteristics of lower-extremity bowing in children. Radiographics. 2003;23:871-880.
15. McCarthy JJ, Betz RR, Kim A, et al. Early radiographic differentiation of infantile tibia vara from physiologic bowing using the femoral-tibial ratio. J Pediatr Orthop. 2001;21:545-548.
16. Salenius P, Vankka E. The development of the tibiofemoral angle in children. J Bone Joint Surg Am. 1975;57:259-261.
17. Engel GM, Staheli LT. The natural history of torsion and other factors influencing gait in childhood. A study of the angle of gait, tibial torsion, knee angle, hip rotation, and development of the arch in normal children. Clin Orthop Relat Res. 1974;99:12-17.
18. Golding J, Bateson E, McNeil-Smith G. Infantile tibia vara. In: The Growth Plate and Its Disorders. Rang M, ed. Baltimore, MD: Williams and Wilkins; 1969:109-119.
19. Greene WB. Infantile tibia vara. J Bone Joint Surg Am. 1993;75:130-143.
20. Golding J, McNeil-Smith JDG. Observations on the etiology of tibia vara. J Bone Joint Surg Br. 1963;45-B:320-325.
21. Eggert P, Viemann M. Physiological bowlegs or infantile Blount’s disease. Some new aspects on an old problem. Pediatr Radiol. 1996;26:349-352.
22. Levine AM, Drennan JC. Physiological bowing and tibia vara. The metaphyseal-diaphyseal angle in the measurement of bowleg deformities. J Bone Joint Surg Am. 1982;64:1158-1163.
23. Kessel L. Annotations on the etiology and treatment of tibia vara. J Bone Joint Surg Br. 1970;52:93-99.
24. Blount WP. Tibia vara: osteochondrosis deformans tibiae. J Bone Joint Surg Am. 1937;19:1-29.
25. Davids JR, Blackhurst DW, Allen BL Jr. Radiographic evaluation of bowed legs in children. J Pediatr Orthop. 2001;21:257-263.
26. Cook SD, Lavernia CJ, Burke SW, et al. A biomechanical analysis of the etiology of tibia vara. J Pediatr Orthop. 1983;3:449-454.
27. Birch JG. Blount disease. J Am Acad Orthop Surg. 2013;21:408-418.
28. Bateson EM. Non-rachitic bow leg and knock-knee deformities in young Jamaican children. Br J Radiol. 1966;39:92-101.
29. Grantham-McGregor SM, Back EH. Gross motor development in Jamaican infants. Dev Med Child Neurol. 1971;13:79-87.
30. Størvold GV, Aarethun K, Bratberg GH. Age for onset of walking and prewalking strategies. Early Hum Dev. 2013;89:655-659.
31. Garrett M, McElroy AM, Staines A. Locomotor milestones and babywalkers: cross sectional study. BMJ. 2002;324:1494.
32. Simon GR, Baker C, Barden GA 3rd, et al; Committee on Practice and Ambulatory Medicine, Curry ES, Dunca PM, Hagan JF Jr, et al; Bright Futures Periodicity Schedule Workgroup. 2014 recommendations for pediatric preventive health care. Pediatrics. 2014;133:568-570.
Assessment steps and treatment tips for ankle arthritis
CASE › A 57-year-old man had been experiencing intermittent pain in his left ankle for the past 2.5 years. About 6 weeks before coming to our clinic, his symptoms became significantly worse after playing a pickup game of basketball. At the clinic visit, he reported no other recent injury or trauma to the leg. However, 15 years earlier he had fractured his left ankle and was treated conservatively with a short period in a cast followed by a course of physical therapy. After completing the physical therapy, he noted significant improvement, although he continued to have minor episodes of pain. He felt no instability or mechanical locking but did note a decreased ability to move the ankle. And it felt much stiffer than his right ankle.
Examination of his left ankle revealed tenderness over the anterior aspect at the tibiotalar joint. He also exhibited decreased dorsiflexion and was unable to perform a toe raise. There was no tenderness over the major ligaments, and results of anterior drawer and talar tilt tests were normal. X-rays revealed tibiotalar joint arthritis (FIGURE).
How would you proceed if this were your patient?
Arthritis of the tibiotalar joint, which has an estimated prevalence of approximately 1%, occurs much less frequently than arthritis of the knee or hip joints.1 This low prevalence is primarily due to the ankle joint’s unique biomechanics and the features of the cartilage within the joint, including its thickness.2
Specifically, the hip and knee joints have greater degrees of freedom than the tibiotalar articulation, which is significantly constrained. The bony congruity between the talus, tibia, and fibula provides inherent stability to the ankle joint, thus protecting against primary osteoarthritis (OA).
Additionally, the large number of ligamentous structures and overall strength of the ligaments provide significant supplemental stability to the ankle joint articulation. Articular cartilage within the ankle joint is thicker than that of the knee and hip (1-1.7 mm). This cartilage also tends to retain its tensile strength with age, unlike cartilage in the hip; the ankle is therefore more resistant to age-related degeneration.3
Metabolic factors also protect against arthritis. Chondrocytes in the ankle are less responsive to inflammatory mediators, including interleukin-1 (IL-1), and therefore produce fewer matrix metalloproteinases.1,2,4 There are also fewer IL-1 receptors on ankle chondrocytes.
The role of trauma in ankle OA. Given the ankle joint’s inherent stability, the most common cause of ankle OA is trauma,4 mainly ankle fracture and, less commonly, ligamentous injury.5,6 Other rarer causes of ankle arthritis include primary OA, crystalline arthropathy, inflammatory disease, septic arthritis, neuroarthropathy, hemochromatosis, and ochronosis.
The ankle’s characteristics that protect it against primary OA may facilitate the pathogenesis of post-traumatic OA through 2 main mechanisms. First, direct trauma to the chondral surfaces can hasten the onset of progressive degeneration. Second, articular incongruity from a fracture can lead to insidious deterioration. The stiffer cartilage layer may be less adaptable to malalignment, and incongruity may cause secondary instability and chronic overloading. Ultimately, the joint breaks down with associated cartilage wear.6,7
The importance of the normal ankle’s congruity and stability became clear in the landmark study by Ramsey and colleagues,8 showing that the contact area between the talus and the tibia decreases as talar displacement increases laterally. This innate stability explains why the contact area of the ankle joint can bear loads similar to those of the hip and knee, yet does not experience primary OA nearly as often.
A stepwise diagnostic appraisal
Ask these questions. Since most ankle pain results from trauma, ask about any recent or remote injury to the affected ankle. Knowing the type of injury that occurred and the exact treatment, if received, may shed light on the relationship between the injury and current symptoms. Acute traumatic events can cause fractures or injury to various soft-tissue structures traversing the ankle joint. Ankle ligament sprains or tendon strains may result after abnormal rotation of the foot. Alternatively, chronic overuse injuries may lead to tendinopathy in any of the tendons that control motion throughout the foot and ankle or degenerative changes within the tibiotalar joint. Knowing the exact location of pain may also help identify the pathology (TABLE 1).
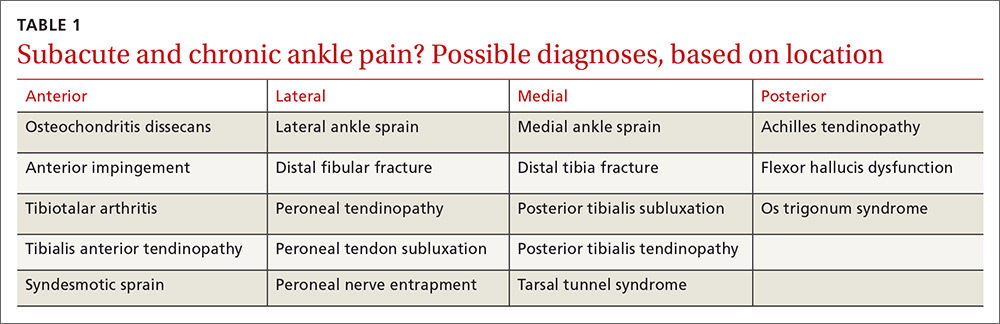
The patient in our case had not suffered a recent injury, so it was important to learn as much as possible about his prior fracture. Was the injury treated conservatively or surgically? If management was conservative, the type and duration of treatment could offer clues to the mechanism underlying symptoms. If a patient has undergone surgery, knowledge of the exact procedure could suggest specific problems. For example, surgical fixation would likely indicate there was ankle instability, thus altering the normal biomechanics in the injured tibiotalar joint.
Other key questions to ask. Most patients with ankle pain also complain of limitations in their usual activities. Ask about the duration and type of pain and other symptoms. Also ask about the position of the foot and ankle when the pain is at its greatest, which will provide insight into likely areas of pathology. For example, if pain arises when the patient navigates uneven ground, subtalar pathology is highly likely. If the patient complains of pain while walking down stairs, suspect injury to the posterior (plantar flexed) ankle; pain while walking up stairs more likely indicates anterior (dorsiflexed) pathology.
Finally, ask about nonorthopedic medical problems and all medications being taken. Systemic conditions, too, can lead to ankle pain—eg, inflammatory arthropathies, infections, and crystalline arthropathy.
Physical examination. Observe the patient’s gait to assess any functional or range-of-motion limitations or abnormal loading throughout the foot and ankle.9 With the patient standing, evaluate any malalignment from the foot through the knees and to the hips. Evaluate the skin for any lesions, wounds, or evidence of trauma or surgery. Next, with the patient seated, examine carefully for neuropathy or vascular abnormalities. Evaluate the ankle’s range of motion and assess for any mechanical locking, clicking, or crepitus. Palpate all bony and ligamentous landmarks to reveal areas of tenderness or swelling. Perform anterior drawer and varus tilt tests to determine overall ligamentous stability of the ankle, and compare your findings with test results of the opposite, uninjured ankle.
Diagnostic imaging. Order weight-bearing radiographs of the foot and ankle. Including the foot allows you to identify additional potential concerns such as malalignment, deformity, or adjacent joint arthritis. Look particularly for joint space narrowing, malalignment, post-traumatic changes, or implanted hardware. Advanced imaging studies—computerized tomography, magnetic resonance imaging, bone scan—are reserved for cases that necessitate ruling out alternative diagnoses, or for preoperative evaluation by an orthopedic surgeon.
Management: Make use of multiple modalities
Conservative management options for ankle OA are limited, and high-quality evidence of efficacy is lacking. Surgical alternatives, however, are invasive and yield modest outcomes. Therefore, unless specific indications for surgery are present, exhaust conservative options (TABLE 2) before considering referral.
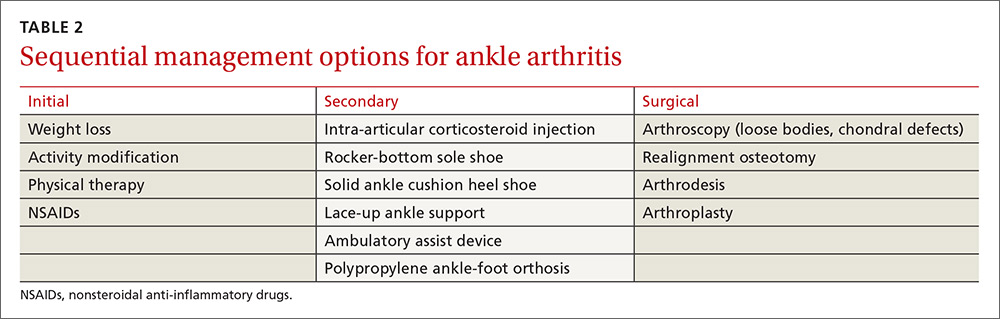
Weight loss is important for those who are overweight—as with knee OA management—to decrease the reactive forces within the ankle joint and to decrease pain. Weight loss will also enhance the outcomes of other treatment modalities and improve overall health.10,11
Activity modification is usually required, even though this may make weight loss more difficult. Avoiding vigorous activities, restricting work-related movements that place high-impact stress on the ankle, and decreasing overall walking time often reduce the severity of symptoms and improve functioning in other activities. Use of assistive-devices, such as a cane, can decrease the weight-bearing load on the affected joint.10,11
Physical therapy has not been shown to alleviate pain in ankle arthritis, although stretching, joint mobilization, and gait training may help prevent further progression of arthritis and improve function.11 The strength of dorsiflexion and plantar-flexion muscles is often decreased in individuals with ankle arthritis. Strengthening exercises may be indicated in individuals exhibiting deficits.
Prescriptive conservative management. Begin with a combination, as needed, of anti-inflammatory medications, orthotic devices, and footwear modifications.
Nonsteroidal anti-inflammatory agents are generally safe, but long-term use requires monitoring. Intra-articular steroid injections have some supporting evidence of effectiveness, but any benefit is short-lived.12 Glucosamine and chondroitin, although unlikely to cause harm, are not supported by the evidence for use in ankle arthritis. Intra-articular viscosupplementation is controversial, and evidence is limited regarding its efficacy.1
Adding a rocker-bottom sole and a solid ankle cushion heel to a shoe helps decrease heel strike impact in individuals with decreased ankle motion, and they aid in the transition from the heel strike to the push-off during level walking.11 If the arthritic joint is unstable, a lace-up ankle support may help with proprioception and stability. A polypropylene ankle-foot orthosis, custom leather ankle corset, or a double-upright brace with a patellar-tendon-bearing support are options to restrict ankle motion and decrease weight-bearing forces.10,11
Immobilization is not recommended except for short-term use during an arthritic flare. Limiting ankle motion reduces pain, but the downside tradeoff is acquired stiffness and weakness that accompanies prolonged periods of immobilization. A controlled ankle motion walking boot or walking plaster cast are both reasonable options for the short term.
Consider surgical referral for specific indications such as osteophytes, loose bodies, and chondral defects, which may be treated with arthroscopy. Patients with large areas of exposed chondral bone or rapid onset of degeneration have poorer outcomes with conservative management and should also be referred to a surgeon earlier. Otherwise, consider surgical referral only after a full trial of conservative management.11
Surgical options vary in scope and effectiveness and include osteotomy, arthrodesis, and arthroplasty. Osteotomies can be performed in early OA to correct bony alignment deformities. Arthrodesis in neutral dorsiflexion with roughly 5 degrees of external rotation is reserved for end-stage ankle OA to allow for near normal gait and pain relief. Total ankle arthroplasty is an emerging option for severe ankle OA, resulting in improved pain relief, gait, and patient satisfaction, but potentially has a higher reoperation rate when compared with arthrodesis.1,2
CASE › We prescribed short-term immobilization with a controlled ankle motion boot and administered an intra-articular corticosteroid injection. At the patient’s follow-up visit 6 weeks later, he reported only moderate improvement in pain. We then advised physical therapy at a specialty ankle rehabilitation program to focus on mobilization, strengthening, and gait training. Nearly one year after his initial visit to our clinic, he is doing well. He understands, however, that the nature of his ankle arthrosis may necessitate surgical intervention in the future.
CORRESPONDENCE
Adam Bitterman, DO, Department of Orthopedic Surgery, Hofstra Northwell School of Medicine at Huntington Hospital, 155 East Main Street, Huntington, NY 11743; [email protected].
1. Valderrabano V, Horisberger M, Russell I, et al. Etiology of ankle osteoarthritis. Clin Orthop Relat Res. 2009;467:1800-1806.
2. Huch K, Kuettner KE, Dieppe P. Osteoarthritis in ankle and knee joints. Semin Arthritis Rheum. 1997;26:667-674.
3. Kempson GE. Age-related changes in the tensile properties of human articular cartilage: a comparative study between the femoral head of the hip joint and the talus of the ankle joint. Biochim Biophys Acta. 1991;1075:223-230.
4. Saltzman CL, Salamon ML, Blanchard GM, et al. Epidemiology of ankle arthritis: Report of a consecutive series of 639 patients from a tertiary orthopaedic center. Iowa Orthop J. 2005;25:44-46.
5. Brown T, Johnston R, Saltzman C, et al. Posttraumatic osteoarthritis: a first estimate of incidence, prevalence, and burden of disease. J Orthop Trauma. 2006;20:739-744.
6. Barg A, Pagenstert G, Hügle T, et al. Ankle osteoarthritis etiology, diagnostics and classification. Foot Ankle Clin. 2013;18:411-426.
7. Schenker M, Mauck R, Ahn J, et al. Pathogenesis and prevention of posttraumatic osteoarthritis after intra-articular fracture. J Am Acad Orthop Surg. 2014;22:20-28.
8. Ramsey PL, Hamilton W. Changes in tibiotalar area of contact caused by lateral talar shift. J Bone Joint Surg Am. 1976;58:356-357.
9. Hayes BJ, Gonzalez T, Smith JT, et al. Ankle arthritis: you can’t always replace it. J Am Acad Orthop Surg. 2016;24:e29-e38.
10. Thomas R, Daniels T. Current concepts review ankle arthritis. J Bone Joint Surg Am. 2003;85A:923-936.
11. Martin RL, Stewart GW, Conti SF. Posttraumatic ankle arthritis: an update on conservative and surgical management. J Orthop Sports Phys Ther. 2007:37:253-259.
12. Pekarek B, Osher L, Buck S, et al. Intra-articular corticosteroid injections: a critical literature review with up-to-date findings. Foot. 2011;21:66-70.
13. Abate M, Schiavone C, Salini V. Hyaluronic acid in ankle arthritis: why evidence of efficacy is still lacking? Clin Exp Rheumatol. 2012;30:277-281.
14. Witteveen AG, Hofstad CJ, Kerkhoffs GM. Hyaluronic acid and other conservative treatment options for osteoarthritis of the ankle. Cochrane Database Syst Rev. 2015;(10):CD010643.
15. Rao S, Ellis SJ, Deland JT, et al. Nonmedicinal therapy in the management of ankle arthritis. Curr Opin Rheumatol. 2010;22:223-228.
CASE › A 57-year-old man had been experiencing intermittent pain in his left ankle for the past 2.5 years. About 6 weeks before coming to our clinic, his symptoms became significantly worse after playing a pickup game of basketball. At the clinic visit, he reported no other recent injury or trauma to the leg. However, 15 years earlier he had fractured his left ankle and was treated conservatively with a short period in a cast followed by a course of physical therapy. After completing the physical therapy, he noted significant improvement, although he continued to have minor episodes of pain. He felt no instability or mechanical locking but did note a decreased ability to move the ankle. And it felt much stiffer than his right ankle.
Examination of his left ankle revealed tenderness over the anterior aspect at the tibiotalar joint. He also exhibited decreased dorsiflexion and was unable to perform a toe raise. There was no tenderness over the major ligaments, and results of anterior drawer and talar tilt tests were normal. X-rays revealed tibiotalar joint arthritis (FIGURE).

How would you proceed if this were your patient?
Arthritis of the tibiotalar joint, which has an estimated prevalence of approximately 1%, occurs much less frequently than arthritis of the knee or hip joints.1 This low prevalence is primarily due to the ankle joint’s unique biomechanics and the features of the cartilage within the joint, including its thickness.2
Specifically, the hip and knee joints have greater degrees of freedom than the tibiotalar articulation, which is significantly constrained. The bony congruity between the talus, tibia, and fibula provides inherent stability to the ankle joint, thus protecting against primary osteoarthritis (OA).
Additionally, the large number of ligamentous structures and overall strength of the ligaments provide significant supplemental stability to the ankle joint articulation. Articular cartilage within the ankle joint is thicker than that of the knee and hip (1-1.7 mm). This cartilage also tends to retain its tensile strength with age, unlike cartilage in the hip; the ankle is therefore more resistant to age-related degeneration.3
Metabolic factors also protect against arthritis. Chondrocytes in the ankle are less responsive to inflammatory mediators, including interleukin-1 (IL-1), and therefore produce fewer matrix metalloproteinases.1,2,4 There are also fewer IL-1 receptors on ankle chondrocytes.
The role of trauma in ankle OA. Given the ankle joint’s inherent stability, the most common cause of ankle OA is trauma,4 mainly ankle fracture and, less commonly, ligamentous injury.5,6 Other rarer causes of ankle arthritis include primary OA, crystalline arthropathy, inflammatory disease, septic arthritis, neuroarthropathy, hemochromatosis, and ochronosis.
The ankle’s characteristics that protect it against primary OA may facilitate the pathogenesis of post-traumatic OA through 2 main mechanisms. First, direct trauma to the chondral surfaces can hasten the onset of progressive degeneration. Second, articular incongruity from a fracture can lead to insidious deterioration. The stiffer cartilage layer may be less adaptable to malalignment, and incongruity may cause secondary instability and chronic overloading. Ultimately, the joint breaks down with associated cartilage wear.6,7
The importance of the normal ankle’s congruity and stability became clear in the landmark study by Ramsey and colleagues,8 showing that the contact area between the talus and the tibia decreases as talar displacement increases laterally. This innate stability explains why the contact area of the ankle joint can bear loads similar to those of the hip and knee, yet does not experience primary OA nearly as often.
A stepwise diagnostic appraisal
Ask these questions. Since most ankle pain results from trauma, ask about any recent or remote injury to the affected ankle. Knowing the type of injury that occurred and the exact treatment, if received, may shed light on the relationship between the injury and current symptoms. Acute traumatic events can cause fractures or injury to various soft-tissue structures traversing the ankle joint. Ankle ligament sprains or tendon strains may result after abnormal rotation of the foot. Alternatively, chronic overuse injuries may lead to tendinopathy in any of the tendons that control motion throughout the foot and ankle or degenerative changes within the tibiotalar joint. Knowing the exact location of pain may also help identify the pathology (TABLE 1).

The patient in our case had not suffered a recent injury, so it was important to learn as much as possible about his prior fracture. Was the injury treated conservatively or surgically? If management was conservative, the type and duration of treatment could offer clues to the mechanism underlying symptoms. If a patient has undergone surgery, knowledge of the exact procedure could suggest specific problems. For example, surgical fixation would likely indicate there was ankle instability, thus altering the normal biomechanics in the injured tibiotalar joint.
Other key questions to ask. Most patients with ankle pain also complain of limitations in their usual activities. Ask about the duration and type of pain and other symptoms. Also ask about the position of the foot and ankle when the pain is at its greatest, which will provide insight into likely areas of pathology. For example, if pain arises when the patient navigates uneven ground, subtalar pathology is highly likely. If the patient complains of pain while walking down stairs, suspect injury to the posterior (plantar flexed) ankle; pain while walking up stairs more likely indicates anterior (dorsiflexed) pathology.
Finally, ask about nonorthopedic medical problems and all medications being taken. Systemic conditions, too, can lead to ankle pain—eg, inflammatory arthropathies, infections, and crystalline arthropathy.
Physical examination. Observe the patient’s gait to assess any functional or range-of-motion limitations or abnormal loading throughout the foot and ankle.9 With the patient standing, evaluate any malalignment from the foot through the knees and to the hips. Evaluate the skin for any lesions, wounds, or evidence of trauma or surgery. Next, with the patient seated, examine carefully for neuropathy or vascular abnormalities. Evaluate the ankle’s range of motion and assess for any mechanical locking, clicking, or crepitus. Palpate all bony and ligamentous landmarks to reveal areas of tenderness or swelling. Perform anterior drawer and varus tilt tests to determine overall ligamentous stability of the ankle, and compare your findings with test results of the opposite, uninjured ankle.
Diagnostic imaging. Order weight-bearing radiographs of the foot and ankle. Including the foot allows you to identify additional potential concerns such as malalignment, deformity, or adjacent joint arthritis. Look particularly for joint space narrowing, malalignment, post-traumatic changes, or implanted hardware. Advanced imaging studies—computerized tomography, magnetic resonance imaging, bone scan—are reserved for cases that necessitate ruling out alternative diagnoses, or for preoperative evaluation by an orthopedic surgeon.
Management: Make use of multiple modalities
Conservative management options for ankle OA are limited, and high-quality evidence of efficacy is lacking. Surgical alternatives, however, are invasive and yield modest outcomes. Therefore, unless specific indications for surgery are present, exhaust conservative options (TABLE 2) before considering referral.

Weight loss is important for those who are overweight—as with knee OA management—to decrease the reactive forces within the ankle joint and to decrease pain. Weight loss will also enhance the outcomes of other treatment modalities and improve overall health.10,11
Activity modification is usually required, even though this may make weight loss more difficult. Avoiding vigorous activities, restricting work-related movements that place high-impact stress on the ankle, and decreasing overall walking time often reduce the severity of symptoms and improve functioning in other activities. Use of assistive-devices, such as a cane, can decrease the weight-bearing load on the affected joint.10,11
Physical therapy has not been shown to alleviate pain in ankle arthritis, although stretching, joint mobilization, and gait training may help prevent further progression of arthritis and improve function.11 The strength of dorsiflexion and plantar-flexion muscles is often decreased in individuals with ankle arthritis. Strengthening exercises may be indicated in individuals exhibiting deficits.
Prescriptive conservative management. Begin with a combination, as needed, of anti-inflammatory medications, orthotic devices, and footwear modifications.
Nonsteroidal anti-inflammatory agents are generally safe, but long-term use requires monitoring. Intra-articular steroid injections have some supporting evidence of effectiveness, but any benefit is short-lived.12 Glucosamine and chondroitin, although unlikely to cause harm, are not supported by the evidence for use in ankle arthritis. Intra-articular viscosupplementation is controversial, and evidence is limited regarding its efficacy.1
Adding a rocker-bottom sole and a solid ankle cushion heel to a shoe helps decrease heel strike impact in individuals with decreased ankle motion, and they aid in the transition from the heel strike to the push-off during level walking.11 If the arthritic joint is unstable, a lace-up ankle support may help with proprioception and stability. A polypropylene ankle-foot orthosis, custom leather ankle corset, or a double-upright brace with a patellar-tendon-bearing support are options to restrict ankle motion and decrease weight-bearing forces.10,11
Immobilization is not recommended except for short-term use during an arthritic flare. Limiting ankle motion reduces pain, but the downside tradeoff is acquired stiffness and weakness that accompanies prolonged periods of immobilization. A controlled ankle motion walking boot or walking plaster cast are both reasonable options for the short term.
Consider surgical referral for specific indications such as osteophytes, loose bodies, and chondral defects, which may be treated with arthroscopy. Patients with large areas of exposed chondral bone or rapid onset of degeneration have poorer outcomes with conservative management and should also be referred to a surgeon earlier. Otherwise, consider surgical referral only after a full trial of conservative management.11
Surgical options vary in scope and effectiveness and include osteotomy, arthrodesis, and arthroplasty. Osteotomies can be performed in early OA to correct bony alignment deformities. Arthrodesis in neutral dorsiflexion with roughly 5 degrees of external rotation is reserved for end-stage ankle OA to allow for near normal gait and pain relief. Total ankle arthroplasty is an emerging option for severe ankle OA, resulting in improved pain relief, gait, and patient satisfaction, but potentially has a higher reoperation rate when compared with arthrodesis.1,2
CASE › We prescribed short-term immobilization with a controlled ankle motion boot and administered an intra-articular corticosteroid injection. At the patient’s follow-up visit 6 weeks later, he reported only moderate improvement in pain. We then advised physical therapy at a specialty ankle rehabilitation program to focus on mobilization, strengthening, and gait training. Nearly one year after his initial visit to our clinic, he is doing well. He understands, however, that the nature of his ankle arthrosis may necessitate surgical intervention in the future.
CORRESPONDENCE
Adam Bitterman, DO, Department of Orthopedic Surgery, Hofstra Northwell School of Medicine at Huntington Hospital, 155 East Main Street, Huntington, NY 11743; [email protected].
CASE › A 57-year-old man had been experiencing intermittent pain in his left ankle for the past 2.5 years. About 6 weeks before coming to our clinic, his symptoms became significantly worse after playing a pickup game of basketball. At the clinic visit, he reported no other recent injury or trauma to the leg. However, 15 years earlier he had fractured his left ankle and was treated conservatively with a short period in a cast followed by a course of physical therapy. After completing the physical therapy, he noted significant improvement, although he continued to have minor episodes of pain. He felt no instability or mechanical locking but did note a decreased ability to move the ankle. And it felt much stiffer than his right ankle.
Examination of his left ankle revealed tenderness over the anterior aspect at the tibiotalar joint. He also exhibited decreased dorsiflexion and was unable to perform a toe raise. There was no tenderness over the major ligaments, and results of anterior drawer and talar tilt tests were normal. X-rays revealed tibiotalar joint arthritis (FIGURE).

How would you proceed if this were your patient?
Arthritis of the tibiotalar joint, which has an estimated prevalence of approximately 1%, occurs much less frequently than arthritis of the knee or hip joints.1 This low prevalence is primarily due to the ankle joint’s unique biomechanics and the features of the cartilage within the joint, including its thickness.2
Specifically, the hip and knee joints have greater degrees of freedom than the tibiotalar articulation, which is significantly constrained. The bony congruity between the talus, tibia, and fibula provides inherent stability to the ankle joint, thus protecting against primary osteoarthritis (OA).
Additionally, the large number of ligamentous structures and overall strength of the ligaments provide significant supplemental stability to the ankle joint articulation. Articular cartilage within the ankle joint is thicker than that of the knee and hip (1-1.7 mm). This cartilage also tends to retain its tensile strength with age, unlike cartilage in the hip; the ankle is therefore more resistant to age-related degeneration.3
Metabolic factors also protect against arthritis. Chondrocytes in the ankle are less responsive to inflammatory mediators, including interleukin-1 (IL-1), and therefore produce fewer matrix metalloproteinases.1,2,4 There are also fewer IL-1 receptors on ankle chondrocytes.
The role of trauma in ankle OA. Given the ankle joint’s inherent stability, the most common cause of ankle OA is trauma,4 mainly ankle fracture and, less commonly, ligamentous injury.5,6 Other rarer causes of ankle arthritis include primary OA, crystalline arthropathy, inflammatory disease, septic arthritis, neuroarthropathy, hemochromatosis, and ochronosis.
The ankle’s characteristics that protect it against primary OA may facilitate the pathogenesis of post-traumatic OA through 2 main mechanisms. First, direct trauma to the chondral surfaces can hasten the onset of progressive degeneration. Second, articular incongruity from a fracture can lead to insidious deterioration. The stiffer cartilage layer may be less adaptable to malalignment, and incongruity may cause secondary instability and chronic overloading. Ultimately, the joint breaks down with associated cartilage wear.6,7
The importance of the normal ankle’s congruity and stability became clear in the landmark study by Ramsey and colleagues,8 showing that the contact area between the talus and the tibia decreases as talar displacement increases laterally. This innate stability explains why the contact area of the ankle joint can bear loads similar to those of the hip and knee, yet does not experience primary OA nearly as often.
A stepwise diagnostic appraisal
Ask these questions. Since most ankle pain results from trauma, ask about any recent or remote injury to the affected ankle. Knowing the type of injury that occurred and the exact treatment, if received, may shed light on the relationship between the injury and current symptoms. Acute traumatic events can cause fractures or injury to various soft-tissue structures traversing the ankle joint. Ankle ligament sprains or tendon strains may result after abnormal rotation of the foot. Alternatively, chronic overuse injuries may lead to tendinopathy in any of the tendons that control motion throughout the foot and ankle or degenerative changes within the tibiotalar joint. Knowing the exact location of pain may also help identify the pathology (TABLE 1).

The patient in our case had not suffered a recent injury, so it was important to learn as much as possible about his prior fracture. Was the injury treated conservatively or surgically? If management was conservative, the type and duration of treatment could offer clues to the mechanism underlying symptoms. If a patient has undergone surgery, knowledge of the exact procedure could suggest specific problems. For example, surgical fixation would likely indicate there was ankle instability, thus altering the normal biomechanics in the injured tibiotalar joint.
Other key questions to ask. Most patients with ankle pain also complain of limitations in their usual activities. Ask about the duration and type of pain and other symptoms. Also ask about the position of the foot and ankle when the pain is at its greatest, which will provide insight into likely areas of pathology. For example, if pain arises when the patient navigates uneven ground, subtalar pathology is highly likely. If the patient complains of pain while walking down stairs, suspect injury to the posterior (plantar flexed) ankle; pain while walking up stairs more likely indicates anterior (dorsiflexed) pathology.
Finally, ask about nonorthopedic medical problems and all medications being taken. Systemic conditions, too, can lead to ankle pain—eg, inflammatory arthropathies, infections, and crystalline arthropathy.
Physical examination. Observe the patient’s gait to assess any functional or range-of-motion limitations or abnormal loading throughout the foot and ankle.9 With the patient standing, evaluate any malalignment from the foot through the knees and to the hips. Evaluate the skin for any lesions, wounds, or evidence of trauma or surgery. Next, with the patient seated, examine carefully for neuropathy or vascular abnormalities. Evaluate the ankle’s range of motion and assess for any mechanical locking, clicking, or crepitus. Palpate all bony and ligamentous landmarks to reveal areas of tenderness or swelling. Perform anterior drawer and varus tilt tests to determine overall ligamentous stability of the ankle, and compare your findings with test results of the opposite, uninjured ankle.
Diagnostic imaging. Order weight-bearing radiographs of the foot and ankle. Including the foot allows you to identify additional potential concerns such as malalignment, deformity, or adjacent joint arthritis. Look particularly for joint space narrowing, malalignment, post-traumatic changes, or implanted hardware. Advanced imaging studies—computerized tomography, magnetic resonance imaging, bone scan—are reserved for cases that necessitate ruling out alternative diagnoses, or for preoperative evaluation by an orthopedic surgeon.
Management: Make use of multiple modalities
Conservative management options for ankle OA are limited, and high-quality evidence of efficacy is lacking. Surgical alternatives, however, are invasive and yield modest outcomes. Therefore, unless specific indications for surgery are present, exhaust conservative options (TABLE 2) before considering referral.

Weight loss is important for those who are overweight—as with knee OA management—to decrease the reactive forces within the ankle joint and to decrease pain. Weight loss will also enhance the outcomes of other treatment modalities and improve overall health.10,11
Activity modification is usually required, even though this may make weight loss more difficult. Avoiding vigorous activities, restricting work-related movements that place high-impact stress on the ankle, and decreasing overall walking time often reduce the severity of symptoms and improve functioning in other activities. Use of assistive-devices, such as a cane, can decrease the weight-bearing load on the affected joint.10,11
Physical therapy has not been shown to alleviate pain in ankle arthritis, although stretching, joint mobilization, and gait training may help prevent further progression of arthritis and improve function.11 The strength of dorsiflexion and plantar-flexion muscles is often decreased in individuals with ankle arthritis. Strengthening exercises may be indicated in individuals exhibiting deficits.
Prescriptive conservative management. Begin with a combination, as needed, of anti-inflammatory medications, orthotic devices, and footwear modifications.
Nonsteroidal anti-inflammatory agents are generally safe, but long-term use requires monitoring. Intra-articular steroid injections have some supporting evidence of effectiveness, but any benefit is short-lived.12 Glucosamine and chondroitin, although unlikely to cause harm, are not supported by the evidence for use in ankle arthritis. Intra-articular viscosupplementation is controversial, and evidence is limited regarding its efficacy.1
Adding a rocker-bottom sole and a solid ankle cushion heel to a shoe helps decrease heel strike impact in individuals with decreased ankle motion, and they aid in the transition from the heel strike to the push-off during level walking.11 If the arthritic joint is unstable, a lace-up ankle support may help with proprioception and stability. A polypropylene ankle-foot orthosis, custom leather ankle corset, or a double-upright brace with a patellar-tendon-bearing support are options to restrict ankle motion and decrease weight-bearing forces.10,11
Immobilization is not recommended except for short-term use during an arthritic flare. Limiting ankle motion reduces pain, but the downside tradeoff is acquired stiffness and weakness that accompanies prolonged periods of immobilization. A controlled ankle motion walking boot or walking plaster cast are both reasonable options for the short term.
Consider surgical referral for specific indications such as osteophytes, loose bodies, and chondral defects, which may be treated with arthroscopy. Patients with large areas of exposed chondral bone or rapid onset of degeneration have poorer outcomes with conservative management and should also be referred to a surgeon earlier. Otherwise, consider surgical referral only after a full trial of conservative management.11
Surgical options vary in scope and effectiveness and include osteotomy, arthrodesis, and arthroplasty. Osteotomies can be performed in early OA to correct bony alignment deformities. Arthrodesis in neutral dorsiflexion with roughly 5 degrees of external rotation is reserved for end-stage ankle OA to allow for near normal gait and pain relief. Total ankle arthroplasty is an emerging option for severe ankle OA, resulting in improved pain relief, gait, and patient satisfaction, but potentially has a higher reoperation rate when compared with arthrodesis.1,2
CASE › We prescribed short-term immobilization with a controlled ankle motion boot and administered an intra-articular corticosteroid injection. At the patient’s follow-up visit 6 weeks later, he reported only moderate improvement in pain. We then advised physical therapy at a specialty ankle rehabilitation program to focus on mobilization, strengthening, and gait training. Nearly one year after his initial visit to our clinic, he is doing well. He understands, however, that the nature of his ankle arthrosis may necessitate surgical intervention in the future.
CORRESPONDENCE
Adam Bitterman, DO, Department of Orthopedic Surgery, Hofstra Northwell School of Medicine at Huntington Hospital, 155 East Main Street, Huntington, NY 11743; [email protected].
1. Valderrabano V, Horisberger M, Russell I, et al. Etiology of ankle osteoarthritis. Clin Orthop Relat Res. 2009;467:1800-1806.
2. Huch K, Kuettner KE, Dieppe P. Osteoarthritis in ankle and knee joints. Semin Arthritis Rheum. 1997;26:667-674.
3. Kempson GE. Age-related changes in the tensile properties of human articular cartilage: a comparative study between the femoral head of the hip joint and the talus of the ankle joint. Biochim Biophys Acta. 1991;1075:223-230.
4. Saltzman CL, Salamon ML, Blanchard GM, et al. Epidemiology of ankle arthritis: Report of a consecutive series of 639 patients from a tertiary orthopaedic center. Iowa Orthop J. 2005;25:44-46.
5. Brown T, Johnston R, Saltzman C, et al. Posttraumatic osteoarthritis: a first estimate of incidence, prevalence, and burden of disease. J Orthop Trauma. 2006;20:739-744.
6. Barg A, Pagenstert G, Hügle T, et al. Ankle osteoarthritis etiology, diagnostics and classification. Foot Ankle Clin. 2013;18:411-426.
7. Schenker M, Mauck R, Ahn J, et al. Pathogenesis and prevention of posttraumatic osteoarthritis after intra-articular fracture. J Am Acad Orthop Surg. 2014;22:20-28.
8. Ramsey PL, Hamilton W. Changes in tibiotalar area of contact caused by lateral talar shift. J Bone Joint Surg Am. 1976;58:356-357.
9. Hayes BJ, Gonzalez T, Smith JT, et al. Ankle arthritis: you can’t always replace it. J Am Acad Orthop Surg. 2016;24:e29-e38.
10. Thomas R, Daniels T. Current concepts review ankle arthritis. J Bone Joint Surg Am. 2003;85A:923-936.
11. Martin RL, Stewart GW, Conti SF. Posttraumatic ankle arthritis: an update on conservative and surgical management. J Orthop Sports Phys Ther. 2007:37:253-259.
12. Pekarek B, Osher L, Buck S, et al. Intra-articular corticosteroid injections: a critical literature review with up-to-date findings. Foot. 2011;21:66-70.
13. Abate M, Schiavone C, Salini V. Hyaluronic acid in ankle arthritis: why evidence of efficacy is still lacking? Clin Exp Rheumatol. 2012;30:277-281.
14. Witteveen AG, Hofstad CJ, Kerkhoffs GM. Hyaluronic acid and other conservative treatment options for osteoarthritis of the ankle. Cochrane Database Syst Rev. 2015;(10):CD010643.
15. Rao S, Ellis SJ, Deland JT, et al. Nonmedicinal therapy in the management of ankle arthritis. Curr Opin Rheumatol. 2010;22:223-228.
1. Valderrabano V, Horisberger M, Russell I, et al. Etiology of ankle osteoarthritis. Clin Orthop Relat Res. 2009;467:1800-1806.
2. Huch K, Kuettner KE, Dieppe P. Osteoarthritis in ankle and knee joints. Semin Arthritis Rheum. 1997;26:667-674.
3. Kempson GE. Age-related changes in the tensile properties of human articular cartilage: a comparative study between the femoral head of the hip joint and the talus of the ankle joint. Biochim Biophys Acta. 1991;1075:223-230.
4. Saltzman CL, Salamon ML, Blanchard GM, et al. Epidemiology of ankle arthritis: Report of a consecutive series of 639 patients from a tertiary orthopaedic center. Iowa Orthop J. 2005;25:44-46.
5. Brown T, Johnston R, Saltzman C, et al. Posttraumatic osteoarthritis: a first estimate of incidence, prevalence, and burden of disease. J Orthop Trauma. 2006;20:739-744.
6. Barg A, Pagenstert G, Hügle T, et al. Ankle osteoarthritis etiology, diagnostics and classification. Foot Ankle Clin. 2013;18:411-426.
7. Schenker M, Mauck R, Ahn J, et al. Pathogenesis and prevention of posttraumatic osteoarthritis after intra-articular fracture. J Am Acad Orthop Surg. 2014;22:20-28.
8. Ramsey PL, Hamilton W. Changes in tibiotalar area of contact caused by lateral talar shift. J Bone Joint Surg Am. 1976;58:356-357.
9. Hayes BJ, Gonzalez T, Smith JT, et al. Ankle arthritis: you can’t always replace it. J Am Acad Orthop Surg. 2016;24:e29-e38.
10. Thomas R, Daniels T. Current concepts review ankle arthritis. J Bone Joint Surg Am. 2003;85A:923-936.
11. Martin RL, Stewart GW, Conti SF. Posttraumatic ankle arthritis: an update on conservative and surgical management. J Orthop Sports Phys Ther. 2007:37:253-259.
12. Pekarek B, Osher L, Buck S, et al. Intra-articular corticosteroid injections: a critical literature review with up-to-date findings. Foot. 2011;21:66-70.
13. Abate M, Schiavone C, Salini V. Hyaluronic acid in ankle arthritis: why evidence of efficacy is still lacking? Clin Exp Rheumatol. 2012;30:277-281.
14. Witteveen AG, Hofstad CJ, Kerkhoffs GM. Hyaluronic acid and other conservative treatment options for osteoarthritis of the ankle. Cochrane Database Syst Rev. 2015;(10):CD010643.
15. Rao S, Ellis SJ, Deland JT, et al. Nonmedicinal therapy in the management of ankle arthritis. Curr Opin Rheumatol. 2010;22:223-228.
PRACTICE RECOMMENDATIONS
› Always ask that the foot be included in ankle x-rays to aid in identifying malalignment, deformity, or joint arthritis. C
› Use anti-inflammatory medications, orthotic devices, and footwear modifications, as needed, for ankle osteoarthritis. C
› Avoid ankle immobilization except, perhaps, during arthritic flare. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
A stepwise approach to pediatric asthma
Pediatric asthma is the most commonly encountered childhood chronic disease, occurring in approximately 13.5% of children.1 Due to the interplay between patient, family physician (FP), and the environment, asthma often proves challenging to control. Although national guidelines for the treatment of asthma have not changed since 2007, significant research continues to examine the optimal means of preventing, controlling, and treating asthma in children. This review summarizes the evidence to date so that you can maximize your care for these young patients.
A stepwise approach to asthma control
The 2007 National Heart, Lung, and Blood Institute (NHLBI) guidelines provide a common pathway for the management of asthma (FIGURE).2 These guidelines emphasize stepwise treatment, based on symptom severity, which maximizes quality of life while minimizing morbidity. Treatment is escalated after careful assessment of frequency of daytime symptoms, frequency of nighttime symptoms, forced expiratory volume in one second (FEV1), and the number of exacerbations requiring systemic steroids over the past year. It’s also appropriate to de-escalate care when symptoms are controlled to minimize adverse effects.
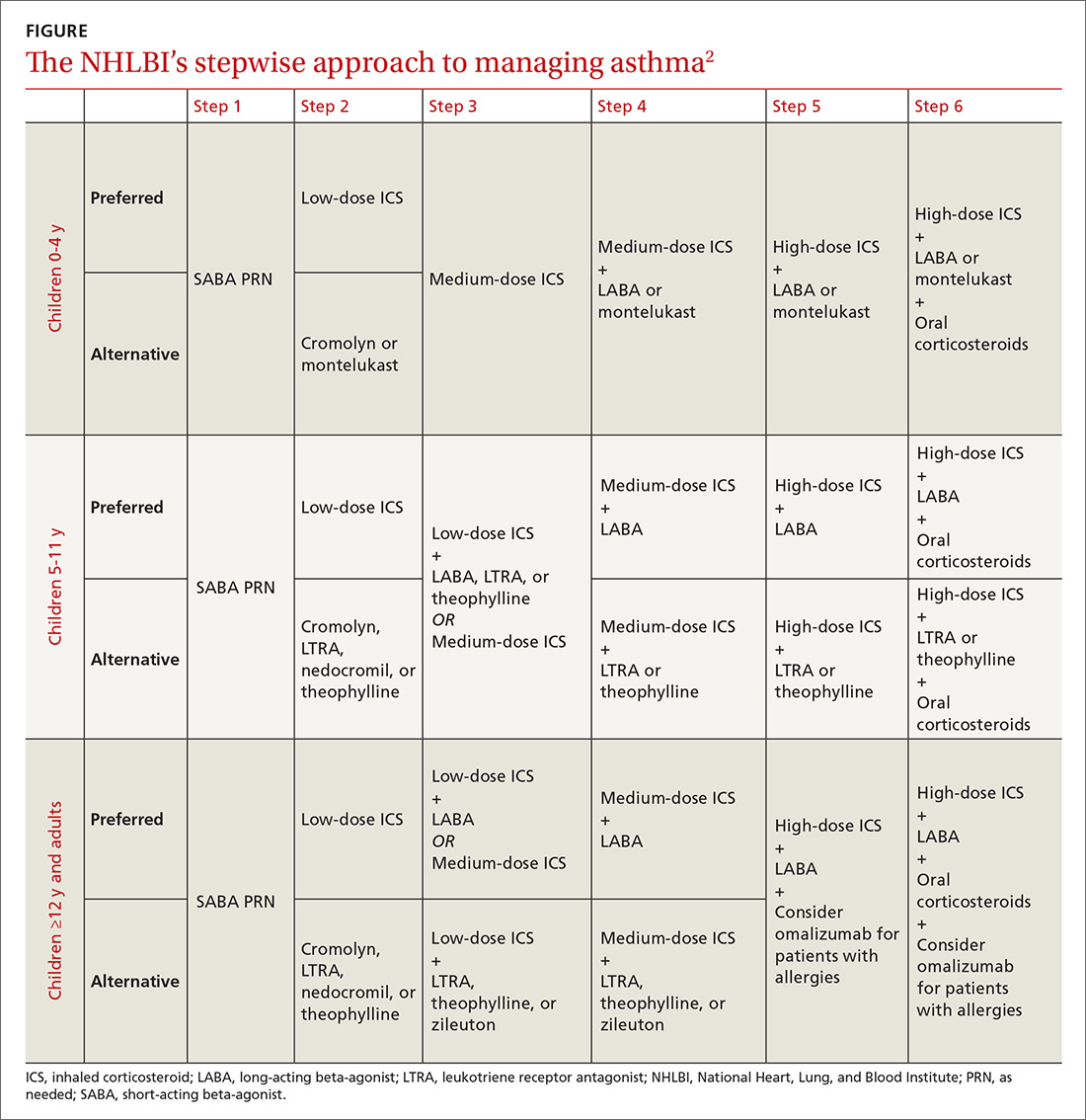
The 2017 Global Initiative for Asthma (GINA) guidelines recommend a similar stepwise approach, with generally the same progression of control medications as the NHLBI guidelines. One variation is that GINA guidelines recommend considering inhaled corticosteroids (ICS) for all asthma patients—even those with intermittent symptoms.3
The Asthma Control Test and Asthma Control Questionnaire are also available for assessment of efficacy of asthma control and can help guide FPs with the decision of when to escalate control medications. (They are available at: http://bit.ly/2o3CzGX and http://bit.ly/2p1LvAc, respectively.)
A systematic review found that both tools were effective in determining if a patient was well controlled vs not well controlled.4 Similarly, a 2012 study found that using the Asthma Control Test score was useful for assessing control and directing changes to treatment.5 In addition, using these tools consistently in a primary care setting increased the frequency of assessment without negatively impacting patient flow through a clinic.6
Short-acting beta-agonists: A mainstay for intermittent asthma
Inhaled short-acting beta-agonists (SABAs) are the mainstay of treatment for intermittent asthma as well as asthma exacerbations. Short-acting beta-agonists are effective for symptomatic relief and preventing exacerbations prior to known exposures because they dilate smooth muscle in bronchioles and relieve bronchospasm.
Albuterol, the most commonly used SABA, is a mixture of the active R-enantiomer and inactive L-enantiomer. Levalbuterol, which consists of only the active R-enantiomer, is also available and is theoretically more effective with fewer adverse effects. Studies examining the difference in efficacy between albuterol and levalbuterol, however, have been mixed, and current guidelines do not recommend one over the other.7
Metered-dose inhalers vs nebulizers
SABAs are typically prescribed in metered-dose inhalers (MDIs), dry powder inhalers, or nebulizers. A meta-analysis comparing the efficacy of nebulizers to MDIs with spacers in both the outpatient and emergency department (ED) settings indicated that MDIs work at least as well as nebulizers and may also reduce length of ED stay.8 This trend appears consistent even with children younger than 24 months.9
If you are prescribing an MDI, be sure to routinely prescribe spacers to help ensure the medication is properly administered. Various types of spacers are available; some consist of an extension of the mouthpiece, while others serve as a chamber with a one-way valve to help improve the ability of the child to inhale the medication. Spacers that do not have antistatic coating should be gently washed with water and detergent.
Masks are also available for younger children and should be properly sized. The same spacer can be used for multiple medications, although they should be administered one at a time. Generally, albuterol should be administered prior to other medications to maximize distribution of subsequent inhaled medications.
Start low with inhaled corticosteroids
Treatment of persistent asthma consists of regular use of inhaled corticosteroids (ICSs; first line) with SABAs, as needed, for exacerbations. Guidelines recommend starting at a low dose and increasing the dose based on symptom control. Patients must consistently use ICSs for one to 2 weeks prior to obtaining full effect of the medication, and parents should be counseled to set appropriate expectations.2,3 ICSs are dispensed as MDIs, dry powder inhalers, and nebulizers; spacers should be considered.
Studies have shown a slight decrease in height for children on ICSs, with long-term studies indicating a persistent 1- to 2-cm decrease in adult height.10,11
Patient isn’t well controlled? Time for a long-acting beta-agonist
For patients not well controlled on low-dose ICSs, the dose can be increased or a long-acting beta-agonist (LABA) or leukotriene receptor antagonist (LTRA) can be added. A recent meta-analysis examining children not well controlled with ICSs, found that the addition of LABAs resulted in improved FEV1 and nighttime symptoms, and reduced the requirement for rescue inhalers when compared to increasing the ICS dose alone.12 In addition, there was no difference in adverse events between the 2 agents, although patients taking ICSs and LABAs had greater growth in height than those with an increased dose of ICS.12 Adding a LABA, however, did not decrease the need for systemic steroids and also did not reduce the number of exacerbations requiring hospitalizations.12
Another recent study demonstrated that the combination of ICSs and LABAs was noninferior to ICSs alone in preventing hospitalizations, intubations, and deaths.13 There are limited data on whether patients already on LABAs and ICSs should be continued on dual medications. Additionally, there is no clear method describing how to de-escalate therapy for those patients who are well controlled on ICSs and LABAs. A reasonable approach is to reduce doses of both medications and discontinue the LABA if tolerated.14,15 The US Food and Drug Administration has issued a black box warning that LABAs should not be used as a single controller medication because patients may be at increased risk of asthma-related deaths.16,17
What role for leukotriene receptor antagonists?
According to NHLBI guidelines, LTRAs can be considered as an alternative to ICSs when starting a control medication for mild persistent asthma.2 A recent meta-analysis, however, showed that there were increased rates of hospitalizations with LTRAs alone when compared to ICSs.18 The NHLBI guidelines also suggest that LTRAs can be used as adjunctive medication for those patients not well controlled on ICSs rather than increasing the ICS dose or adding a LABA.2
A 2011 clinical trial found no difference in quality of life measures between LTRAs and LABAs as adjunctive therapy at 2 months, but LABAs were more effective when patients were reassessed in 2 years.19 Similarly, the same study also found that adding LTRAs to low-dose ICSs rather than increasing the ICS dose was equivalent in the short term but not at 2 years.19
2 other adjunctive therapy options: Xanthines, cromolyn
Similar to LTRAs, xanthines can be considered as adjunctive therapy for children older than 5 years who are not well controlled on a low-dose ICS. Although xanthines decrease asthma symptoms when compared to placebo alone, they are not more effective than ICSs alone and should be considered only as adjunctive therapy.2,20 There have been few studies comparing xanthines to other adjunctive medications.21
Cromolyn is another adjunctive medication cited in the NHLBI guidelines for escalation of therapy.2 Although the medication has few adverse effects, its use is generally limited in the United States because data supporting its efficacy are lacking.
Omalizumab for allergy-related asthma exacerbations
Omalizumab, an anti-IgE antibody injected every 2 to 4 weeks, is available for children older than 6 years with moderate to severe asthma that is not responsive to ICSs and LABAs.22 The medication is effective in reducing allergy-related asthma exacerbations and hospitalizations, but data comparing it to other adjunctive medications are limited.22 Due to their significant systemic effects, the role of oral steroids as control medications is reserved for patients with severe asthma who are refractory to other medications. Children should be placed on oral steroids for the least amount of time required to achieve symptom control.
Acute exacerbation treatment: What to consider
Although there is no agreed-upon definition for an acute asthma exacerbation, the American Thoracic Society defines it as "an event characterized by a change from the patient’s previous status."23 All patients should be given an asthma action plan that clearly delineates the escalation of therapy in the event of an exacerbation, although only half of all patients report experiencing one.24 Symptom-based plans may prevent more acute care visits when compared to plans that use peak-flow measurements, although children on peak-flow plans may have fewer symptomatic days.25
Start with short-acting beta-agonists
The SABAs serve as the initial treatment of choice for management of asthma exacerbations. In young children (0-3 years), SABAs delivered by MDI with a spacer were more effective in reducing admission rates (11.3% vs 21.7%) when compared to SABAs delivered by nebulizers, resulting in a number needed to treat to prevent one admission of 10.26
In older children (3-18 years), SABAs delivered via spacer reduced ED length of stay, but did not significantly affect hospitalization rates. Additionally, SABAs administered with anticholinergics such as ipratropium bromide were more effective than SABAs alone in reducing admissions (16.9% vs 23.2%), particularly in older children with moderate to severe asthma, while also minimizing adverse effects.8,26
Corticosteroids: A mainstay in the ED
In addition to albuterol administration, corticosteroids remain the mainstay of ED management for asthma exacerbations. Administration of systemic steroids has been shown to reduce hospitalizations in children under 6 years, although, paradoxically, studies examining outpatient administration have demonstrated an increase in hospitalizations when compared to placebo.27
Dexamethasone and prednisone are the 2 most commonly used systemic steroids, and studies haven't indicated superiority of either.28,29 There is no difference in efficacy between oral and intravenous steroids.30 A recent clinical trial found a 2-day course of dexamethasone (0.6 mg/kg) had similar efficacy with fewer adverse effects when compared to a 5-day course of prednisone (1-2 mg/kg/day).28
Patient isn’t responding? Try IV magnesium
For patients who don't respond to corticosteroids and albuterol treatments, IV magnesium sulfate (usual dose, 25-75 mg/kg/d; maximum dose, 2000 mg/d) has been shown to improve respiratory function, but not necessarily decrease admission rates.31 Inhaled magnesium sulfate hasn't been shown to be more effective than IV administration and isn'trecommended.32
Evidence doesn’t support use of heliox
Heliox, which consists of 80% helium, is theorized to be effective in the treatment of asthma by increasing laminar flow and increasing the delivery of medications to the alveoli.32 Overall, the evidence does not support the use of heliox, which is typically restricted to patients with severe asthma exacerbations.33
Is it worth considering noninvasive positive pressure ventilation?
If patients don't improve with medical treatment, noninvasive positive pressure ventilation can be considered. A recent meta-analysis suggests that there is no definitive benefit or harm from this treatment, although several studies have indicated a decrease in symptom severity.34
Intubation should be considered for hypoxemia unresponsive to medications, or in cases of exhaustion, worsening mental status, or respiratory acidosis unresponsive to medication. Ventilation should allow for a permissive respiratory acidosis (pH, 7.2), while maintaining adequate oxygenation.35
Reducing the burden of asthma
Due to the complex task of reducing triggers and providing effective controller medications, working with parents and children is integral to improving the quality of life for patients with asthma. Although there is an obvious genetic predisposition, family physicians can help reduce the risk of developing asthma by encouraging healthy behaviors at home before the child is born. In the prenatal period, this includes avoiding tobacco-smoke exposure, lessening maternal obesity, decreasing maternal antibiotic and acetaminophen use, and curtailing stress.
Evidence suggests that after birth, breastfeeding and reducing childhood obesity can help lower the risk of asthma.36 Atopic disease, in general, can be reduced by breastfeeding until at least 4 months, as well as encouraging a varied diet that does not restrict potential allergens during pregnancy or lactation, and introducing foods (including potential allergens) after the age of 4 months.
The risk of atopic disease can also be lowered by lessening potential triggers at home. These include restricting exposure to cats (but not dogs), reducing home mold by decreasing humidity and ensuring adequate ventilation, avoiding volatile organic compounds, such as chlorine, and curtailing exposure to vehicle emissions. Although often marketed to be effective in reducing allergies, dust-mite covers and soy-based formulas don't prevent or minimize allergies and are often costly.37,38 In addition, there is no evidence that vaccinations areassociated with allergies.39
CORRESPONDENCE
Douglas M. Maurer, 9040A Jackson Ave, Joint-Base Lewis-McChord, WA 98431; [email protected].
1. US Department of Health and Human Services. Centers for Disease Control and Prevention. Asthma and Schools. Available at: www.cdc.gov/healthyschools/asthma/index.htm. Updated June 17, 2015. Accessed September 28, 2016.
2. National Asthma Education and Prevention Program. Expert Panel Report 3: guidelines for the diagnosis and management of asthma. Bethesda, Md: National Heart, Lung, and Blood Institute; 2007. Report No.:07-4051.
3. Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention, 2017. Available at: www.ginasthma.org. Accessed April 11, 2017.
4. Jia CE, Zhang HP, Lv Y, et al. The asthma control test and asthma control questionnaire for assessing asthma control: systematic review and meta-analysis. J Allergy Clin Immunol. 2013;131:695-703.
5. Ko FW, Hui DS, Leung TS, et al. Evaluation of the asthma control test: a reliable determinant of disease stability and a predictor of future exacerbations. Respirology. 2012;17: 370-378.
6. Sudhanthar S, Thakur K, Sigal Y, et al. Improving asthma severity and control screening in a primary care pediatric practice. BMJ Qual Improv Rep. 2016;5
7. Wilkinson M, Bulloch B, Garcia-Filion P, et al. Efficacy of racemic albuterol versus levalbuterol used as a continuous nebulization for the treatment of acute asthma exacerbations: a randomized, double-blind, clinical trial. J Asthma. 2011;48:188-193.
8. Cates CJ, Crilly JA, Rowe BH. Holding chambers (spacers) versus nebulisers for beta-agonist treatment of acute asthma. Cochrane Database Syst Rev. 2006;19.
9. Delgado A, Chou KJ, Silver EJ, et al. Nebulizers vs metered-dose inhalers with spacers for bronchodilator therapy to treat wheezing in children aged 2 to 24 months in a pediatric emergency department. Arch Pediatr Adolesc Med. 2003;157:76-80.
10. Kelly HW, Sternberg AL, Lescher R, et al. Effect of inhaled glucocorticoids in childhood on adult height. N Engl J Med. 2012;367:904-912.
11. Loke YK, Blanco P, Thavarajh M, et al. Impact of inhaled corticosteroids on growth in children with asthma: systematic review and meta-analysis. PLoS ONE. 2015;10:e0133428.
12. Chauhan BF, Chartrand C, Ni Chroinin M, et al. Addition of long-acting beta2-agonists to inhaled corticosteroids for chronic asthma in children. Cochrane Database Syst Rev. 2015;24:CD007949.
13. Stempel DA, Szefler SJ, Pedersen S, et al; VESTRI Investigators. Safety of adding salmeterol to fluticasone propionate in children with asthma. N Engl J Med. 2016;375:840-849.
14. Blair MM. PL Detail-Document, Safety of long-acting beta-agonists in asthma. Pharmacist’s Letter/Prescriber’s Letter. November 2012.
15. Kew KM, Beggs S, Ahmad S. Stopping long-acting beta2-agonists (LABA) for children with asthma well controlled on LABA and inhaled corticosteroids. Cochrane Database Syst Rev. 2015;5:CD011316.
16. Nelson HS, Weiss ST, Bleecker ER, et al; SMART Study Group. The Salmeterol Multicenter Asthma Research Trial: a comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterol. Chest. 2006;129:15-26.
17. US Food and Drug Administration. FDA Drug Safety Communication: new safety requirements for long-acting inhaled asthma medications called long-acting beta-agonists (LABAs). Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm200776.htm. Accessed December 23, 2016.
18. Chauhan BF, Ducharme FM. Anti-leukotriene agents compared to inhaled corticosteroids in the management of recurrent and/or chronic asthma in adults and children. Cochrane Database Syst Rev. 2012;5:CD002314.
19. Price D, Musgrave SD, Shepstone L, et al. Leukotriene antagonists as first-line or add-on asthma-controller therapy. N Engl J Med. 2011;364:1695-1707.
20. Seddon P, Bara A, Ducharme FM, et al. Oral xanthines as maintenance treatment for asthma in children. Cochrane Database Syst Rev. 2006;1:CD002885.
21. van der Mark LB, Lyklema PHE, Geskus RB, et al. A systematic review with attempted network meta-analysis of asthma therapy recommended for five to eighteen year olds in GINA steps three and four. BMC Pulm Med. 2012;12:63.
22. Normansell R, Walker S, Milan SJ. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev. 2014;1:CD003559.
23. Reddel HK, Taylor DR, Bateman ED, et al. An official American Thoracic Society/European Respiratory Society Statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med. 2009;180:59-99.
24. Simon AE, Akinbami LJ. Asthma action plan receipt among children with asthma 2-17 years of age, United States, 2002-2013. J Pediatr. 2016;171:283-289.
25. Bhogal SK, Zemek R, Ducharme FM. Written action plans for asthma in children. Cochrane Database Syst Rev. 2006;3: CD005306.
26. Pollock M, Sinha IP, Hartling L, et al. Inhaled short-acting bronchodilators for managing emergency childhood asthma: an overview of reviews. Allergy. 2017;72:183-200.
27. Castro-Rodriguez JA, Beckhaus AA, Forno E. Efficacy of oral corticosteroids in the treatment of acute wheezing episodes in asthmatic preschoolers: systematic review with meta-analysis. Pediatr Pulmonol. 2016;51:868-876.
28. Keeney GE, Gray MP, Morrison AK, et al. Dexamethasone for acute asthma exacerbations in children: a meta-analysis. Pediatrics. 2014;133:493-499.
29. Normansell R, Kew KM, Mansour G. Different oral corticosteroid regimens for acute asthma. Cochrane Database Syst Rev. 2016;5:CD011801.
30. Rowe BH, Keller JL, Oxman AD. Effectiveness of steroid therapy in acute exacerbations of asthma: a meta-analysis. Am J Emerg Med. 1992;10:301-310.
31. Rowe BH, Bretzlaff J, Bourdon C, et al. Magnesium sulfate for treating exacerbations of acute asthma in the emergency department. Cochrane Database Syst Rev. 2000;1:CD001490.
32. Powell C, Dwan K, Milan SJ, et al. Inhaled magnesium sulfate in the treatment of acute asthma. Cochrane Database Syst Rev. 2012;12:CD003898.
33. Rodrigo GJ, Pollack CV, Rodrigo C, et al. Heliox for non-intubated acute asthma patients. Cochrane Database Syst Rev. 2006;4:CD002884.
34. Korang SK, Feinberg J, Wetterslev J, et al. Non-invasive positive pressure ventilation for acute asthma in children. Cochrane Database System Rev. 2016;9:CD012067.
35. Kline-Krammes S, Patel NH, Robinson S. Childhood asthma: a guide for pediatric emergency medicine providers. Emerg Med Clin North Am. 2013;31:705-732.
36. Castro-Rodriguez JA, Forno E, Rodriguez-Martinez CE, et al. Risk and protective factors for childhood asthma: what is the evidence? J Allergy Clin Immunol Pract. 2016;4:1111-1122.
37. Gøtzsche P, Johansen HK. House dust mite control measures for asthma. Cochrane Database Syst Rev. 2008;2:1465-1858.
38. Osborn DA, Sinn JKH. Soy formula for prevention of allergy and food intolerance in infants. Cochrane Database of Syst Rev. 2006;4:1465-1858.
39. Schäfer T, Bauer CP, Beyer K, et al. S3-Guideline on allergy prevention: 2014 update: Guideline of the German Society for Allergology and Clinical Immunology (DGAKI) and the German society for Pediatric and Adolescent Medicine (DGKJ). Allegro J Int. 2014;23:186-199.
Pediatric asthma is the most commonly encountered childhood chronic disease, occurring in approximately 13.5% of children.1 Due to the interplay between patient, family physician (FP), and the environment, asthma often proves challenging to control. Although national guidelines for the treatment of asthma have not changed since 2007, significant research continues to examine the optimal means of preventing, controlling, and treating asthma in children. This review summarizes the evidence to date so that you can maximize your care for these young patients.
A stepwise approach to asthma control
The 2007 National Heart, Lung, and Blood Institute (NHLBI) guidelines provide a common pathway for the management of asthma (FIGURE).2 These guidelines emphasize stepwise treatment, based on symptom severity, which maximizes quality of life while minimizing morbidity. Treatment is escalated after careful assessment of frequency of daytime symptoms, frequency of nighttime symptoms, forced expiratory volume in one second (FEV1), and the number of exacerbations requiring systemic steroids over the past year. It’s also appropriate to de-escalate care when symptoms are controlled to minimize adverse effects.

The 2017 Global Initiative for Asthma (GINA) guidelines recommend a similar stepwise approach, with generally the same progression of control medications as the NHLBI guidelines. One variation is that GINA guidelines recommend considering inhaled corticosteroids (ICS) for all asthma patients—even those with intermittent symptoms.3
The Asthma Control Test and Asthma Control Questionnaire are also available for assessment of efficacy of asthma control and can help guide FPs with the decision of when to escalate control medications. (They are available at: http://bit.ly/2o3CzGX and http://bit.ly/2p1LvAc, respectively.)
A systematic review found that both tools were effective in determining if a patient was well controlled vs not well controlled.4 Similarly, a 2012 study found that using the Asthma Control Test score was useful for assessing control and directing changes to treatment.5 In addition, using these tools consistently in a primary care setting increased the frequency of assessment without negatively impacting patient flow through a clinic.6
Short-acting beta-agonists: A mainstay for intermittent asthma
Inhaled short-acting beta-agonists (SABAs) are the mainstay of treatment for intermittent asthma as well as asthma exacerbations. Short-acting beta-agonists are effective for symptomatic relief and preventing exacerbations prior to known exposures because they dilate smooth muscle in bronchioles and relieve bronchospasm.
Albuterol, the most commonly used SABA, is a mixture of the active R-enantiomer and inactive L-enantiomer. Levalbuterol, which consists of only the active R-enantiomer, is also available and is theoretically more effective with fewer adverse effects. Studies examining the difference in efficacy between albuterol and levalbuterol, however, have been mixed, and current guidelines do not recommend one over the other.7
Metered-dose inhalers vs nebulizers
SABAs are typically prescribed in metered-dose inhalers (MDIs), dry powder inhalers, or nebulizers. A meta-analysis comparing the efficacy of nebulizers to MDIs with spacers in both the outpatient and emergency department (ED) settings indicated that MDIs work at least as well as nebulizers and may also reduce length of ED stay.8 This trend appears consistent even with children younger than 24 months.9
If you are prescribing an MDI, be sure to routinely prescribe spacers to help ensure the medication is properly administered. Various types of spacers are available; some consist of an extension of the mouthpiece, while others serve as a chamber with a one-way valve to help improve the ability of the child to inhale the medication. Spacers that do not have antistatic coating should be gently washed with water and detergent.
Masks are also available for younger children and should be properly sized. The same spacer can be used for multiple medications, although they should be administered one at a time. Generally, albuterol should be administered prior to other medications to maximize distribution of subsequent inhaled medications.
Start low with inhaled corticosteroids
Treatment of persistent asthma consists of regular use of inhaled corticosteroids (ICSs; first line) with SABAs, as needed, for exacerbations. Guidelines recommend starting at a low dose and increasing the dose based on symptom control. Patients must consistently use ICSs for one to 2 weeks prior to obtaining full effect of the medication, and parents should be counseled to set appropriate expectations.2,3 ICSs are dispensed as MDIs, dry powder inhalers, and nebulizers; spacers should be considered.
Studies have shown a slight decrease in height for children on ICSs, with long-term studies indicating a persistent 1- to 2-cm decrease in adult height.10,11
Patient isn’t well controlled? Time for a long-acting beta-agonist
For patients not well controlled on low-dose ICSs, the dose can be increased or a long-acting beta-agonist (LABA) or leukotriene receptor antagonist (LTRA) can be added. A recent meta-analysis examining children not well controlled with ICSs, found that the addition of LABAs resulted in improved FEV1 and nighttime symptoms, and reduced the requirement for rescue inhalers when compared to increasing the ICS dose alone.12 In addition, there was no difference in adverse events between the 2 agents, although patients taking ICSs and LABAs had greater growth in height than those with an increased dose of ICS.12 Adding a LABA, however, did not decrease the need for systemic steroids and also did not reduce the number of exacerbations requiring hospitalizations.12
Another recent study demonstrated that the combination of ICSs and LABAs was noninferior to ICSs alone in preventing hospitalizations, intubations, and deaths.13 There are limited data on whether patients already on LABAs and ICSs should be continued on dual medications. Additionally, there is no clear method describing how to de-escalate therapy for those patients who are well controlled on ICSs and LABAs. A reasonable approach is to reduce doses of both medications and discontinue the LABA if tolerated.14,15 The US Food and Drug Administration has issued a black box warning that LABAs should not be used as a single controller medication because patients may be at increased risk of asthma-related deaths.16,17
What role for leukotriene receptor antagonists?
According to NHLBI guidelines, LTRAs can be considered as an alternative to ICSs when starting a control medication for mild persistent asthma.2 A recent meta-analysis, however, showed that there were increased rates of hospitalizations with LTRAs alone when compared to ICSs.18 The NHLBI guidelines also suggest that LTRAs can be used as adjunctive medication for those patients not well controlled on ICSs rather than increasing the ICS dose or adding a LABA.2
A 2011 clinical trial found no difference in quality of life measures between LTRAs and LABAs as adjunctive therapy at 2 months, but LABAs were more effective when patients were reassessed in 2 years.19 Similarly, the same study also found that adding LTRAs to low-dose ICSs rather than increasing the ICS dose was equivalent in the short term but not at 2 years.19
2 other adjunctive therapy options: Xanthines, cromolyn
Similar to LTRAs, xanthines can be considered as adjunctive therapy for children older than 5 years who are not well controlled on a low-dose ICS. Although xanthines decrease asthma symptoms when compared to placebo alone, they are not more effective than ICSs alone and should be considered only as adjunctive therapy.2,20 There have been few studies comparing xanthines to other adjunctive medications.21
Cromolyn is another adjunctive medication cited in the NHLBI guidelines for escalation of therapy.2 Although the medication has few adverse effects, its use is generally limited in the United States because data supporting its efficacy are lacking.
Omalizumab for allergy-related asthma exacerbations
Omalizumab, an anti-IgE antibody injected every 2 to 4 weeks, is available for children older than 6 years with moderate to severe asthma that is not responsive to ICSs and LABAs.22 The medication is effective in reducing allergy-related asthma exacerbations and hospitalizations, but data comparing it to other adjunctive medications are limited.22 Due to their significant systemic effects, the role of oral steroids as control medications is reserved for patients with severe asthma who are refractory to other medications. Children should be placed on oral steroids for the least amount of time required to achieve symptom control.
Acute exacerbation treatment: What to consider
Although there is no agreed-upon definition for an acute asthma exacerbation, the American Thoracic Society defines it as "an event characterized by a change from the patient’s previous status."23 All patients should be given an asthma action plan that clearly delineates the escalation of therapy in the event of an exacerbation, although only half of all patients report experiencing one.24 Symptom-based plans may prevent more acute care visits when compared to plans that use peak-flow measurements, although children on peak-flow plans may have fewer symptomatic days.25
Start with short-acting beta-agonists
The SABAs serve as the initial treatment of choice for management of asthma exacerbations. In young children (0-3 years), SABAs delivered by MDI with a spacer were more effective in reducing admission rates (11.3% vs 21.7%) when compared to SABAs delivered by nebulizers, resulting in a number needed to treat to prevent one admission of 10.26
In older children (3-18 years), SABAs delivered via spacer reduced ED length of stay, but did not significantly affect hospitalization rates. Additionally, SABAs administered with anticholinergics such as ipratropium bromide were more effective than SABAs alone in reducing admissions (16.9% vs 23.2%), particularly in older children with moderate to severe asthma, while also minimizing adverse effects.8,26
Corticosteroids: A mainstay in the ED
In addition to albuterol administration, corticosteroids remain the mainstay of ED management for asthma exacerbations. Administration of systemic steroids has been shown to reduce hospitalizations in children under 6 years, although, paradoxically, studies examining outpatient administration have demonstrated an increase in hospitalizations when compared to placebo.27
Dexamethasone and prednisone are the 2 most commonly used systemic steroids, and studies haven't indicated superiority of either.28,29 There is no difference in efficacy between oral and intravenous steroids.30 A recent clinical trial found a 2-day course of dexamethasone (0.6 mg/kg) had similar efficacy with fewer adverse effects when compared to a 5-day course of prednisone (1-2 mg/kg/day).28
Patient isn’t responding? Try IV magnesium
For patients who don't respond to corticosteroids and albuterol treatments, IV magnesium sulfate (usual dose, 25-75 mg/kg/d; maximum dose, 2000 mg/d) has been shown to improve respiratory function, but not necessarily decrease admission rates.31 Inhaled magnesium sulfate hasn't been shown to be more effective than IV administration and isn'trecommended.32
Evidence doesn’t support use of heliox
Heliox, which consists of 80% helium, is theorized to be effective in the treatment of asthma by increasing laminar flow and increasing the delivery of medications to the alveoli.32 Overall, the evidence does not support the use of heliox, which is typically restricted to patients with severe asthma exacerbations.33
Is it worth considering noninvasive positive pressure ventilation?
If patients don't improve with medical treatment, noninvasive positive pressure ventilation can be considered. A recent meta-analysis suggests that there is no definitive benefit or harm from this treatment, although several studies have indicated a decrease in symptom severity.34
Intubation should be considered for hypoxemia unresponsive to medications, or in cases of exhaustion, worsening mental status, or respiratory acidosis unresponsive to medication. Ventilation should allow for a permissive respiratory acidosis (pH, 7.2), while maintaining adequate oxygenation.35
Reducing the burden of asthma
Due to the complex task of reducing triggers and providing effective controller medications, working with parents and children is integral to improving the quality of life for patients with asthma. Although there is an obvious genetic predisposition, family physicians can help reduce the risk of developing asthma by encouraging healthy behaviors at home before the child is born. In the prenatal period, this includes avoiding tobacco-smoke exposure, lessening maternal obesity, decreasing maternal antibiotic and acetaminophen use, and curtailing stress.
Evidence suggests that after birth, breastfeeding and reducing childhood obesity can help lower the risk of asthma.36 Atopic disease, in general, can be reduced by breastfeeding until at least 4 months, as well as encouraging a varied diet that does not restrict potential allergens during pregnancy or lactation, and introducing foods (including potential allergens) after the age of 4 months.
The risk of atopic disease can also be lowered by lessening potential triggers at home. These include restricting exposure to cats (but not dogs), reducing home mold by decreasing humidity and ensuring adequate ventilation, avoiding volatile organic compounds, such as chlorine, and curtailing exposure to vehicle emissions. Although often marketed to be effective in reducing allergies, dust-mite covers and soy-based formulas don't prevent or minimize allergies and are often costly.37,38 In addition, there is no evidence that vaccinations areassociated with allergies.39
CORRESPONDENCE
Douglas M. Maurer, 9040A Jackson Ave, Joint-Base Lewis-McChord, WA 98431; [email protected].
Pediatric asthma is the most commonly encountered childhood chronic disease, occurring in approximately 13.5% of children.1 Due to the interplay between patient, family physician (FP), and the environment, asthma often proves challenging to control. Although national guidelines for the treatment of asthma have not changed since 2007, significant research continues to examine the optimal means of preventing, controlling, and treating asthma in children. This review summarizes the evidence to date so that you can maximize your care for these young patients.
A stepwise approach to asthma control
The 2007 National Heart, Lung, and Blood Institute (NHLBI) guidelines provide a common pathway for the management of asthma (FIGURE).2 These guidelines emphasize stepwise treatment, based on symptom severity, which maximizes quality of life while minimizing morbidity. Treatment is escalated after careful assessment of frequency of daytime symptoms, frequency of nighttime symptoms, forced expiratory volume in one second (FEV1), and the number of exacerbations requiring systemic steroids over the past year. It’s also appropriate to de-escalate care when symptoms are controlled to minimize adverse effects.

The 2017 Global Initiative for Asthma (GINA) guidelines recommend a similar stepwise approach, with generally the same progression of control medications as the NHLBI guidelines. One variation is that GINA guidelines recommend considering inhaled corticosteroids (ICS) for all asthma patients—even those with intermittent symptoms.3
The Asthma Control Test and Asthma Control Questionnaire are also available for assessment of efficacy of asthma control and can help guide FPs with the decision of when to escalate control medications. (They are available at: http://bit.ly/2o3CzGX and http://bit.ly/2p1LvAc, respectively.)
A systematic review found that both tools were effective in determining if a patient was well controlled vs not well controlled.4 Similarly, a 2012 study found that using the Asthma Control Test score was useful for assessing control and directing changes to treatment.5 In addition, using these tools consistently in a primary care setting increased the frequency of assessment without negatively impacting patient flow through a clinic.6
Short-acting beta-agonists: A mainstay for intermittent asthma
Inhaled short-acting beta-agonists (SABAs) are the mainstay of treatment for intermittent asthma as well as asthma exacerbations. Short-acting beta-agonists are effective for symptomatic relief and preventing exacerbations prior to known exposures because they dilate smooth muscle in bronchioles and relieve bronchospasm.
Albuterol, the most commonly used SABA, is a mixture of the active R-enantiomer and inactive L-enantiomer. Levalbuterol, which consists of only the active R-enantiomer, is also available and is theoretically more effective with fewer adverse effects. Studies examining the difference in efficacy between albuterol and levalbuterol, however, have been mixed, and current guidelines do not recommend one over the other.7
Metered-dose inhalers vs nebulizers
SABAs are typically prescribed in metered-dose inhalers (MDIs), dry powder inhalers, or nebulizers. A meta-analysis comparing the efficacy of nebulizers to MDIs with spacers in both the outpatient and emergency department (ED) settings indicated that MDIs work at least as well as nebulizers and may also reduce length of ED stay.8 This trend appears consistent even with children younger than 24 months.9
If you are prescribing an MDI, be sure to routinely prescribe spacers to help ensure the medication is properly administered. Various types of spacers are available; some consist of an extension of the mouthpiece, while others serve as a chamber with a one-way valve to help improve the ability of the child to inhale the medication. Spacers that do not have antistatic coating should be gently washed with water and detergent.
Masks are also available for younger children and should be properly sized. The same spacer can be used for multiple medications, although they should be administered one at a time. Generally, albuterol should be administered prior to other medications to maximize distribution of subsequent inhaled medications.
Start low with inhaled corticosteroids
Treatment of persistent asthma consists of regular use of inhaled corticosteroids (ICSs; first line) with SABAs, as needed, for exacerbations. Guidelines recommend starting at a low dose and increasing the dose based on symptom control. Patients must consistently use ICSs for one to 2 weeks prior to obtaining full effect of the medication, and parents should be counseled to set appropriate expectations.2,3 ICSs are dispensed as MDIs, dry powder inhalers, and nebulizers; spacers should be considered.
Studies have shown a slight decrease in height for children on ICSs, with long-term studies indicating a persistent 1- to 2-cm decrease in adult height.10,11
Patient isn’t well controlled? Time for a long-acting beta-agonist
For patients not well controlled on low-dose ICSs, the dose can be increased or a long-acting beta-agonist (LABA) or leukotriene receptor antagonist (LTRA) can be added. A recent meta-analysis examining children not well controlled with ICSs, found that the addition of LABAs resulted in improved FEV1 and nighttime symptoms, and reduced the requirement for rescue inhalers when compared to increasing the ICS dose alone.12 In addition, there was no difference in adverse events between the 2 agents, although patients taking ICSs and LABAs had greater growth in height than those with an increased dose of ICS.12 Adding a LABA, however, did not decrease the need for systemic steroids and also did not reduce the number of exacerbations requiring hospitalizations.12
Another recent study demonstrated that the combination of ICSs and LABAs was noninferior to ICSs alone in preventing hospitalizations, intubations, and deaths.13 There are limited data on whether patients already on LABAs and ICSs should be continued on dual medications. Additionally, there is no clear method describing how to de-escalate therapy for those patients who are well controlled on ICSs and LABAs. A reasonable approach is to reduce doses of both medications and discontinue the LABA if tolerated.14,15 The US Food and Drug Administration has issued a black box warning that LABAs should not be used as a single controller medication because patients may be at increased risk of asthma-related deaths.16,17
What role for leukotriene receptor antagonists?
According to NHLBI guidelines, LTRAs can be considered as an alternative to ICSs when starting a control medication for mild persistent asthma.2 A recent meta-analysis, however, showed that there were increased rates of hospitalizations with LTRAs alone when compared to ICSs.18 The NHLBI guidelines also suggest that LTRAs can be used as adjunctive medication for those patients not well controlled on ICSs rather than increasing the ICS dose or adding a LABA.2
A 2011 clinical trial found no difference in quality of life measures between LTRAs and LABAs as adjunctive therapy at 2 months, but LABAs were more effective when patients were reassessed in 2 years.19 Similarly, the same study also found that adding LTRAs to low-dose ICSs rather than increasing the ICS dose was equivalent in the short term but not at 2 years.19
2 other adjunctive therapy options: Xanthines, cromolyn
Similar to LTRAs, xanthines can be considered as adjunctive therapy for children older than 5 years who are not well controlled on a low-dose ICS. Although xanthines decrease asthma symptoms when compared to placebo alone, they are not more effective than ICSs alone and should be considered only as adjunctive therapy.2,20 There have been few studies comparing xanthines to other adjunctive medications.21
Cromolyn is another adjunctive medication cited in the NHLBI guidelines for escalation of therapy.2 Although the medication has few adverse effects, its use is generally limited in the United States because data supporting its efficacy are lacking.
Omalizumab for allergy-related asthma exacerbations
Omalizumab, an anti-IgE antibody injected every 2 to 4 weeks, is available for children older than 6 years with moderate to severe asthma that is not responsive to ICSs and LABAs.22 The medication is effective in reducing allergy-related asthma exacerbations and hospitalizations, but data comparing it to other adjunctive medications are limited.22 Due to their significant systemic effects, the role of oral steroids as control medications is reserved for patients with severe asthma who are refractory to other medications. Children should be placed on oral steroids for the least amount of time required to achieve symptom control.
Acute exacerbation treatment: What to consider
Although there is no agreed-upon definition for an acute asthma exacerbation, the American Thoracic Society defines it as "an event characterized by a change from the patient’s previous status."23 All patients should be given an asthma action plan that clearly delineates the escalation of therapy in the event of an exacerbation, although only half of all patients report experiencing one.24 Symptom-based plans may prevent more acute care visits when compared to plans that use peak-flow measurements, although children on peak-flow plans may have fewer symptomatic days.25
Start with short-acting beta-agonists
The SABAs serve as the initial treatment of choice for management of asthma exacerbations. In young children (0-3 years), SABAs delivered by MDI with a spacer were more effective in reducing admission rates (11.3% vs 21.7%) when compared to SABAs delivered by nebulizers, resulting in a number needed to treat to prevent one admission of 10.26
In older children (3-18 years), SABAs delivered via spacer reduced ED length of stay, but did not significantly affect hospitalization rates. Additionally, SABAs administered with anticholinergics such as ipratropium bromide were more effective than SABAs alone in reducing admissions (16.9% vs 23.2%), particularly in older children with moderate to severe asthma, while also minimizing adverse effects.8,26
Corticosteroids: A mainstay in the ED
In addition to albuterol administration, corticosteroids remain the mainstay of ED management for asthma exacerbations. Administration of systemic steroids has been shown to reduce hospitalizations in children under 6 years, although, paradoxically, studies examining outpatient administration have demonstrated an increase in hospitalizations when compared to placebo.27
Dexamethasone and prednisone are the 2 most commonly used systemic steroids, and studies haven't indicated superiority of either.28,29 There is no difference in efficacy between oral and intravenous steroids.30 A recent clinical trial found a 2-day course of dexamethasone (0.6 mg/kg) had similar efficacy with fewer adverse effects when compared to a 5-day course of prednisone (1-2 mg/kg/day).28
Patient isn’t responding? Try IV magnesium
For patients who don't respond to corticosteroids and albuterol treatments, IV magnesium sulfate (usual dose, 25-75 mg/kg/d; maximum dose, 2000 mg/d) has been shown to improve respiratory function, but not necessarily decrease admission rates.31 Inhaled magnesium sulfate hasn't been shown to be more effective than IV administration and isn'trecommended.32
Evidence doesn’t support use of heliox
Heliox, which consists of 80% helium, is theorized to be effective in the treatment of asthma by increasing laminar flow and increasing the delivery of medications to the alveoli.32 Overall, the evidence does not support the use of heliox, which is typically restricted to patients with severe asthma exacerbations.33
Is it worth considering noninvasive positive pressure ventilation?
If patients don't improve with medical treatment, noninvasive positive pressure ventilation can be considered. A recent meta-analysis suggests that there is no definitive benefit or harm from this treatment, although several studies have indicated a decrease in symptom severity.34
Intubation should be considered for hypoxemia unresponsive to medications, or in cases of exhaustion, worsening mental status, or respiratory acidosis unresponsive to medication. Ventilation should allow for a permissive respiratory acidosis (pH, 7.2), while maintaining adequate oxygenation.35
Reducing the burden of asthma
Due to the complex task of reducing triggers and providing effective controller medications, working with parents and children is integral to improving the quality of life for patients with asthma. Although there is an obvious genetic predisposition, family physicians can help reduce the risk of developing asthma by encouraging healthy behaviors at home before the child is born. In the prenatal period, this includes avoiding tobacco-smoke exposure, lessening maternal obesity, decreasing maternal antibiotic and acetaminophen use, and curtailing stress.
Evidence suggests that after birth, breastfeeding and reducing childhood obesity can help lower the risk of asthma.36 Atopic disease, in general, can be reduced by breastfeeding until at least 4 months, as well as encouraging a varied diet that does not restrict potential allergens during pregnancy or lactation, and introducing foods (including potential allergens) after the age of 4 months.
The risk of atopic disease can also be lowered by lessening potential triggers at home. These include restricting exposure to cats (but not dogs), reducing home mold by decreasing humidity and ensuring adequate ventilation, avoiding volatile organic compounds, such as chlorine, and curtailing exposure to vehicle emissions. Although often marketed to be effective in reducing allergies, dust-mite covers and soy-based formulas don't prevent or minimize allergies and are often costly.37,38 In addition, there is no evidence that vaccinations areassociated with allergies.39
CORRESPONDENCE
Douglas M. Maurer, 9040A Jackson Ave, Joint-Base Lewis-McChord, WA 98431; [email protected].
1. US Department of Health and Human Services. Centers for Disease Control and Prevention. Asthma and Schools. Available at: www.cdc.gov/healthyschools/asthma/index.htm. Updated June 17, 2015. Accessed September 28, 2016.
2. National Asthma Education and Prevention Program. Expert Panel Report 3: guidelines for the diagnosis and management of asthma. Bethesda, Md: National Heart, Lung, and Blood Institute; 2007. Report No.:07-4051.
3. Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention, 2017. Available at: www.ginasthma.org. Accessed April 11, 2017.
4. Jia CE, Zhang HP, Lv Y, et al. The asthma control test and asthma control questionnaire for assessing asthma control: systematic review and meta-analysis. J Allergy Clin Immunol. 2013;131:695-703.
5. Ko FW, Hui DS, Leung TS, et al. Evaluation of the asthma control test: a reliable determinant of disease stability and a predictor of future exacerbations. Respirology. 2012;17: 370-378.
6. Sudhanthar S, Thakur K, Sigal Y, et al. Improving asthma severity and control screening in a primary care pediatric practice. BMJ Qual Improv Rep. 2016;5
7. Wilkinson M, Bulloch B, Garcia-Filion P, et al. Efficacy of racemic albuterol versus levalbuterol used as a continuous nebulization for the treatment of acute asthma exacerbations: a randomized, double-blind, clinical trial. J Asthma. 2011;48:188-193.
8. Cates CJ, Crilly JA, Rowe BH. Holding chambers (spacers) versus nebulisers for beta-agonist treatment of acute asthma. Cochrane Database Syst Rev. 2006;19.
9. Delgado A, Chou KJ, Silver EJ, et al. Nebulizers vs metered-dose inhalers with spacers for bronchodilator therapy to treat wheezing in children aged 2 to 24 months in a pediatric emergency department. Arch Pediatr Adolesc Med. 2003;157:76-80.
10. Kelly HW, Sternberg AL, Lescher R, et al. Effect of inhaled glucocorticoids in childhood on adult height. N Engl J Med. 2012;367:904-912.
11. Loke YK, Blanco P, Thavarajh M, et al. Impact of inhaled corticosteroids on growth in children with asthma: systematic review and meta-analysis. PLoS ONE. 2015;10:e0133428.
12. Chauhan BF, Chartrand C, Ni Chroinin M, et al. Addition of long-acting beta2-agonists to inhaled corticosteroids for chronic asthma in children. Cochrane Database Syst Rev. 2015;24:CD007949.
13. Stempel DA, Szefler SJ, Pedersen S, et al; VESTRI Investigators. Safety of adding salmeterol to fluticasone propionate in children with asthma. N Engl J Med. 2016;375:840-849.
14. Blair MM. PL Detail-Document, Safety of long-acting beta-agonists in asthma. Pharmacist’s Letter/Prescriber’s Letter. November 2012.
15. Kew KM, Beggs S, Ahmad S. Stopping long-acting beta2-agonists (LABA) for children with asthma well controlled on LABA and inhaled corticosteroids. Cochrane Database Syst Rev. 2015;5:CD011316.
16. Nelson HS, Weiss ST, Bleecker ER, et al; SMART Study Group. The Salmeterol Multicenter Asthma Research Trial: a comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterol. Chest. 2006;129:15-26.
17. US Food and Drug Administration. FDA Drug Safety Communication: new safety requirements for long-acting inhaled asthma medications called long-acting beta-agonists (LABAs). Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm200776.htm. Accessed December 23, 2016.
18. Chauhan BF, Ducharme FM. Anti-leukotriene agents compared to inhaled corticosteroids in the management of recurrent and/or chronic asthma in adults and children. Cochrane Database Syst Rev. 2012;5:CD002314.
19. Price D, Musgrave SD, Shepstone L, et al. Leukotriene antagonists as first-line or add-on asthma-controller therapy. N Engl J Med. 2011;364:1695-1707.
20. Seddon P, Bara A, Ducharme FM, et al. Oral xanthines as maintenance treatment for asthma in children. Cochrane Database Syst Rev. 2006;1:CD002885.
21. van der Mark LB, Lyklema PHE, Geskus RB, et al. A systematic review with attempted network meta-analysis of asthma therapy recommended for five to eighteen year olds in GINA steps three and four. BMC Pulm Med. 2012;12:63.
22. Normansell R, Walker S, Milan SJ. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev. 2014;1:CD003559.
23. Reddel HK, Taylor DR, Bateman ED, et al. An official American Thoracic Society/European Respiratory Society Statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med. 2009;180:59-99.
24. Simon AE, Akinbami LJ. Asthma action plan receipt among children with asthma 2-17 years of age, United States, 2002-2013. J Pediatr. 2016;171:283-289.
25. Bhogal SK, Zemek R, Ducharme FM. Written action plans for asthma in children. Cochrane Database Syst Rev. 2006;3: CD005306.
26. Pollock M, Sinha IP, Hartling L, et al. Inhaled short-acting bronchodilators for managing emergency childhood asthma: an overview of reviews. Allergy. 2017;72:183-200.
27. Castro-Rodriguez JA, Beckhaus AA, Forno E. Efficacy of oral corticosteroids in the treatment of acute wheezing episodes in asthmatic preschoolers: systematic review with meta-analysis. Pediatr Pulmonol. 2016;51:868-876.
28. Keeney GE, Gray MP, Morrison AK, et al. Dexamethasone for acute asthma exacerbations in children: a meta-analysis. Pediatrics. 2014;133:493-499.
29. Normansell R, Kew KM, Mansour G. Different oral corticosteroid regimens for acute asthma. Cochrane Database Syst Rev. 2016;5:CD011801.
30. Rowe BH, Keller JL, Oxman AD. Effectiveness of steroid therapy in acute exacerbations of asthma: a meta-analysis. Am J Emerg Med. 1992;10:301-310.
31. Rowe BH, Bretzlaff J, Bourdon C, et al. Magnesium sulfate for treating exacerbations of acute asthma in the emergency department. Cochrane Database Syst Rev. 2000;1:CD001490.
32. Powell C, Dwan K, Milan SJ, et al. Inhaled magnesium sulfate in the treatment of acute asthma. Cochrane Database Syst Rev. 2012;12:CD003898.
33. Rodrigo GJ, Pollack CV, Rodrigo C, et al. Heliox for non-intubated acute asthma patients. Cochrane Database Syst Rev. 2006;4:CD002884.
34. Korang SK, Feinberg J, Wetterslev J, et al. Non-invasive positive pressure ventilation for acute asthma in children. Cochrane Database System Rev. 2016;9:CD012067.
35. Kline-Krammes S, Patel NH, Robinson S. Childhood asthma: a guide for pediatric emergency medicine providers. Emerg Med Clin North Am. 2013;31:705-732.
36. Castro-Rodriguez JA, Forno E, Rodriguez-Martinez CE, et al. Risk and protective factors for childhood asthma: what is the evidence? J Allergy Clin Immunol Pract. 2016;4:1111-1122.
37. Gøtzsche P, Johansen HK. House dust mite control measures for asthma. Cochrane Database Syst Rev. 2008;2:1465-1858.
38. Osborn DA, Sinn JKH. Soy formula for prevention of allergy and food intolerance in infants. Cochrane Database of Syst Rev. 2006;4:1465-1858.
39. Schäfer T, Bauer CP, Beyer K, et al. S3-Guideline on allergy prevention: 2014 update: Guideline of the German Society for Allergology and Clinical Immunology (DGAKI) and the German society for Pediatric and Adolescent Medicine (DGKJ). Allegro J Int. 2014;23:186-199.
1. US Department of Health and Human Services. Centers for Disease Control and Prevention. Asthma and Schools. Available at: www.cdc.gov/healthyschools/asthma/index.htm. Updated June 17, 2015. Accessed September 28, 2016.
2. National Asthma Education and Prevention Program. Expert Panel Report 3: guidelines for the diagnosis and management of asthma. Bethesda, Md: National Heart, Lung, and Blood Institute; 2007. Report No.:07-4051.
3. Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention, 2017. Available at: www.ginasthma.org. Accessed April 11, 2017.
4. Jia CE, Zhang HP, Lv Y, et al. The asthma control test and asthma control questionnaire for assessing asthma control: systematic review and meta-analysis. J Allergy Clin Immunol. 2013;131:695-703.
5. Ko FW, Hui DS, Leung TS, et al. Evaluation of the asthma control test: a reliable determinant of disease stability and a predictor of future exacerbations. Respirology. 2012;17: 370-378.
6. Sudhanthar S, Thakur K, Sigal Y, et al. Improving asthma severity and control screening in a primary care pediatric practice. BMJ Qual Improv Rep. 2016;5
7. Wilkinson M, Bulloch B, Garcia-Filion P, et al. Efficacy of racemic albuterol versus levalbuterol used as a continuous nebulization for the treatment of acute asthma exacerbations: a randomized, double-blind, clinical trial. J Asthma. 2011;48:188-193.
8. Cates CJ, Crilly JA, Rowe BH. Holding chambers (spacers) versus nebulisers for beta-agonist treatment of acute asthma. Cochrane Database Syst Rev. 2006;19.
9. Delgado A, Chou KJ, Silver EJ, et al. Nebulizers vs metered-dose inhalers with spacers for bronchodilator therapy to treat wheezing in children aged 2 to 24 months in a pediatric emergency department. Arch Pediatr Adolesc Med. 2003;157:76-80.
10. Kelly HW, Sternberg AL, Lescher R, et al. Effect of inhaled glucocorticoids in childhood on adult height. N Engl J Med. 2012;367:904-912.
11. Loke YK, Blanco P, Thavarajh M, et al. Impact of inhaled corticosteroids on growth in children with asthma: systematic review and meta-analysis. PLoS ONE. 2015;10:e0133428.
12. Chauhan BF, Chartrand C, Ni Chroinin M, et al. Addition of long-acting beta2-agonists to inhaled corticosteroids for chronic asthma in children. Cochrane Database Syst Rev. 2015;24:CD007949.
13. Stempel DA, Szefler SJ, Pedersen S, et al; VESTRI Investigators. Safety of adding salmeterol to fluticasone propionate in children with asthma. N Engl J Med. 2016;375:840-849.
14. Blair MM. PL Detail-Document, Safety of long-acting beta-agonists in asthma. Pharmacist’s Letter/Prescriber’s Letter. November 2012.
15. Kew KM, Beggs S, Ahmad S. Stopping long-acting beta2-agonists (LABA) for children with asthma well controlled on LABA and inhaled corticosteroids. Cochrane Database Syst Rev. 2015;5:CD011316.
16. Nelson HS, Weiss ST, Bleecker ER, et al; SMART Study Group. The Salmeterol Multicenter Asthma Research Trial: a comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterol. Chest. 2006;129:15-26.
17. US Food and Drug Administration. FDA Drug Safety Communication: new safety requirements for long-acting inhaled asthma medications called long-acting beta-agonists (LABAs). Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm200776.htm. Accessed December 23, 2016.
18. Chauhan BF, Ducharme FM. Anti-leukotriene agents compared to inhaled corticosteroids in the management of recurrent and/or chronic asthma in adults and children. Cochrane Database Syst Rev. 2012;5:CD002314.
19. Price D, Musgrave SD, Shepstone L, et al. Leukotriene antagonists as first-line or add-on asthma-controller therapy. N Engl J Med. 2011;364:1695-1707.
20. Seddon P, Bara A, Ducharme FM, et al. Oral xanthines as maintenance treatment for asthma in children. Cochrane Database Syst Rev. 2006;1:CD002885.
21. van der Mark LB, Lyklema PHE, Geskus RB, et al. A systematic review with attempted network meta-analysis of asthma therapy recommended for five to eighteen year olds in GINA steps three and four. BMC Pulm Med. 2012;12:63.
22. Normansell R, Walker S, Milan SJ. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev. 2014;1:CD003559.
23. Reddel HK, Taylor DR, Bateman ED, et al. An official American Thoracic Society/European Respiratory Society Statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med. 2009;180:59-99.
24. Simon AE, Akinbami LJ. Asthma action plan receipt among children with asthma 2-17 years of age, United States, 2002-2013. J Pediatr. 2016;171:283-289.
25. Bhogal SK, Zemek R, Ducharme FM. Written action plans for asthma in children. Cochrane Database Syst Rev. 2006;3: CD005306.
26. Pollock M, Sinha IP, Hartling L, et al. Inhaled short-acting bronchodilators for managing emergency childhood asthma: an overview of reviews. Allergy. 2017;72:183-200.
27. Castro-Rodriguez JA, Beckhaus AA, Forno E. Efficacy of oral corticosteroids in the treatment of acute wheezing episodes in asthmatic preschoolers: systematic review with meta-analysis. Pediatr Pulmonol. 2016;51:868-876.
28. Keeney GE, Gray MP, Morrison AK, et al. Dexamethasone for acute asthma exacerbations in children: a meta-analysis. Pediatrics. 2014;133:493-499.
29. Normansell R, Kew KM, Mansour G. Different oral corticosteroid regimens for acute asthma. Cochrane Database Syst Rev. 2016;5:CD011801.
30. Rowe BH, Keller JL, Oxman AD. Effectiveness of steroid therapy in acute exacerbations of asthma: a meta-analysis. Am J Emerg Med. 1992;10:301-310.
31. Rowe BH, Bretzlaff J, Bourdon C, et al. Magnesium sulfate for treating exacerbations of acute asthma in the emergency department. Cochrane Database Syst Rev. 2000;1:CD001490.
32. Powell C, Dwan K, Milan SJ, et al. Inhaled magnesium sulfate in the treatment of acute asthma. Cochrane Database Syst Rev. 2012;12:CD003898.
33. Rodrigo GJ, Pollack CV, Rodrigo C, et al. Heliox for non-intubated acute asthma patients. Cochrane Database Syst Rev. 2006;4:CD002884.
34. Korang SK, Feinberg J, Wetterslev J, et al. Non-invasive positive pressure ventilation for acute asthma in children. Cochrane Database System Rev. 2016;9:CD012067.
35. Kline-Krammes S, Patel NH, Robinson S. Childhood asthma: a guide for pediatric emergency medicine providers. Emerg Med Clin North Am. 2013;31:705-732.
36. Castro-Rodriguez JA, Forno E, Rodriguez-Martinez CE, et al. Risk and protective factors for childhood asthma: what is the evidence? J Allergy Clin Immunol Pract. 2016;4:1111-1122.
37. Gøtzsche P, Johansen HK. House dust mite control measures for asthma. Cochrane Database Syst Rev. 2008;2:1465-1858.
38. Osborn DA, Sinn JKH. Soy formula for prevention of allergy and food intolerance in infants. Cochrane Database of Syst Rev. 2006;4:1465-1858.
39. Schäfer T, Bauer CP, Beyer K, et al. S3-Guideline on allergy prevention: 2014 update: Guideline of the German Society for Allergology and Clinical Immunology (DGAKI) and the German society for Pediatric and Adolescent Medicine (DGKJ). Allegro J Int. 2014;23:186-199.
PRACTICE RECOMMENDATIONS
› Reassure parents that metered-dose inhalers are as effective as nebulizers for asthma exacerbations. A
› Use a 2-day course of systemic steroids for asthma exacerbations rather than extended regimens. B
› Develop an asthma action plan for every patient with asthma to decrease acute care visits. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
These agents do double duty by reducing CV risk in diabetes
In this issue of JFP, Skolnik et al discuss ways we can assist patients with type 2 diabetes mellitus (T2DM) in lowering their cardiovascular (CV) risk. It is well established that the main predictors of the development of CV disease in patients with T2DM are blood pressure (BP) and lipid levels. Many randomized controlled trials (RCTs) have demonstrated the benefit of lowering BP and lipid levels on reducing CV disease in these patients.
The problem has been that other than a modest CV benefit from metformin, no glucose-lowering drug has been shown to have a significant effect on CV outcomes—until recently. Now there is solid evidence from RCTs that treatment with one of 3 newer agents—empagliflozin (a sodium-glucose cotransporter [SGLT]-2 inhibitor), liraglutide, and semaglutide (both glucagon-like peptide [GLP]-1 receptor agonists)—is associated with reductions in CV morbidity and mortality for patients with T2DM who have established, or are at high risk for, CV disease. (Of note: Semaglutide is not yet on the market. Its manufacturer submitted a New Drug Application late last year.)
For empagliflozin, an RCT involving more than 7000 patients calculated that the number needed to treat (NNT) over a 3-year period to prevent one CV event was 63 and the NNT to prevent one death from any cause was 38.1 For liraglutide, a double-blind trial involving over 9000 patients reported the NNT to prevent one CV event in 3 years was 53, and the NNT to prevent one death from any cause was 71.2 The RCT for semaglutide involved more than 3000 patients and reported the NNT to prevent one major CVD event was 43, but there was no significant difference in CV mortality between the semaglutide and placebo groups in that clinical trial.3
1. Zinman B, Wanner C, Lachin JM, et al, for the EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
2. Marso SP, Daniels GH, Brown-Frandsen K, et al, for the LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
3. Marso SP, Bain SC, Consoli A, et al, for the SUSTAIN-6 Investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834-1844.
4. Ebell MH, Siwek J, Weiss BD, et al. Simplifying the language of evidence to improve patient care: Strength of recommendation taxonomy (SORT): a patient-centered approach to grading evidence in medical literature. J Fam Pract. 2004;53:111-120.
In this issue of JFP, Skolnik et al discuss ways we can assist patients with type 2 diabetes mellitus (T2DM) in lowering their cardiovascular (CV) risk. It is well established that the main predictors of the development of CV disease in patients with T2DM are blood pressure (BP) and lipid levels. Many randomized controlled trials (RCTs) have demonstrated the benefit of lowering BP and lipid levels on reducing CV disease in these patients.
The problem has been that other than a modest CV benefit from metformin, no glucose-lowering drug has been shown to have a significant effect on CV outcomes—until recently. Now there is solid evidence from RCTs that treatment with one of 3 newer agents—empagliflozin (a sodium-glucose cotransporter [SGLT]-2 inhibitor), liraglutide, and semaglutide (both glucagon-like peptide [GLP]-1 receptor agonists)—is associated with reductions in CV morbidity and mortality for patients with T2DM who have established, or are at high risk for, CV disease. (Of note: Semaglutide is not yet on the market. Its manufacturer submitted a New Drug Application late last year.)
For empagliflozin, an RCT involving more than 7000 patients calculated that the number needed to treat (NNT) over a 3-year period to prevent one CV event was 63 and the NNT to prevent one death from any cause was 38.1 For liraglutide, a double-blind trial involving over 9000 patients reported the NNT to prevent one CV event in 3 years was 53, and the NNT to prevent one death from any cause was 71.2 The RCT for semaglutide involved more than 3000 patients and reported the NNT to prevent one major CVD event was 43, but there was no significant difference in CV mortality between the semaglutide and placebo groups in that clinical trial.3
In this issue of JFP, Skolnik et al discuss ways we can assist patients with type 2 diabetes mellitus (T2DM) in lowering their cardiovascular (CV) risk. It is well established that the main predictors of the development of CV disease in patients with T2DM are blood pressure (BP) and lipid levels. Many randomized controlled trials (RCTs) have demonstrated the benefit of lowering BP and lipid levels on reducing CV disease in these patients.
The problem has been that other than a modest CV benefit from metformin, no glucose-lowering drug has been shown to have a significant effect on CV outcomes—until recently. Now there is solid evidence from RCTs that treatment with one of 3 newer agents—empagliflozin (a sodium-glucose cotransporter [SGLT]-2 inhibitor), liraglutide, and semaglutide (both glucagon-like peptide [GLP]-1 receptor agonists)—is associated with reductions in CV morbidity and mortality for patients with T2DM who have established, or are at high risk for, CV disease. (Of note: Semaglutide is not yet on the market. Its manufacturer submitted a New Drug Application late last year.)
For empagliflozin, an RCT involving more than 7000 patients calculated that the number needed to treat (NNT) over a 3-year period to prevent one CV event was 63 and the NNT to prevent one death from any cause was 38.1 For liraglutide, a double-blind trial involving over 9000 patients reported the NNT to prevent one CV event in 3 years was 53, and the NNT to prevent one death from any cause was 71.2 The RCT for semaglutide involved more than 3000 patients and reported the NNT to prevent one major CVD event was 43, but there was no significant difference in CV mortality between the semaglutide and placebo groups in that clinical trial.3
1. Zinman B, Wanner C, Lachin JM, et al, for the EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
2. Marso SP, Daniels GH, Brown-Frandsen K, et al, for the LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
3. Marso SP, Bain SC, Consoli A, et al, for the SUSTAIN-6 Investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834-1844.
4. Ebell MH, Siwek J, Weiss BD, et al. Simplifying the language of evidence to improve patient care: Strength of recommendation taxonomy (SORT): a patient-centered approach to grading evidence in medical literature. J Fam Pract. 2004;53:111-120.
1. Zinman B, Wanner C, Lachin JM, et al, for the EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
2. Marso SP, Daniels GH, Brown-Frandsen K, et al, for the LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
3. Marso SP, Bain SC, Consoli A, et al, for the SUSTAIN-6 Investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834-1844.
4. Ebell MH, Siwek J, Weiss BD, et al. Simplifying the language of evidence to improve patient care: Strength of recommendation taxonomy (SORT): a patient-centered approach to grading evidence in medical literature. J Fam Pract. 2004;53:111-120.
USPSTF changes stance on routine pelvic exams
AGA Guideline: Transient elastography in liver fibrosis, most used and most accurate
Vibration-controlled transient elastography (VCTE) can accurately diagnose cirrhosis in most patients with chronic liver disease, particularly those with chronic hepatitis B or C, states a new guideline from the AGA Institute, published in the May issue of Gastroenterology (doi: 10.1053/j.gastro.2017.03.017).
However, magnetic resonance elastography (MRE) is somewhat more accurate for detecting cirrhosis in nonalcoholic fatty liver disease, wrote Joseph K. Lim, MD, AGAF, of Yale University in New Haven, Conn., with his associates from the Clinical Guidelines Committee of the AGA. VCTE is convenient but performs unevenly in some liver conditions and is especially unreliable in patients with acute hepatitis, alcohol abuse, food intake within 2-3 hours, congestive heart failure, or extrahepatic cholestasis, the guideline notes. Yet, VCTE remains the most common imaging tool for diagnosing fibrosis in the United States, and the guideline addresses “focused, clinically relevant questions” to guide its use.
When possible, clinicians should use VCTE instead of noninvasive serum tests for cirrhosis in patients with chronic hepatitis C, the guideline asserts. In pooled analyses of 62 studies, VCTE detected about 89% of cirrhosis cases (95% confidence interval, 84%-92%), Fibrosis-4 test (FIB-4) detected 87% (95% CI, 74%-94%), and aspartate aminotransferase to platelet ratio index (APRI) detected 77% (95% CI, 73%-81%). The specificity of VCTE (91%) also equaled or exceeded that of FIB-4 (91%) or APRI (78%), the guideline noted.
For chronic hepatitis C, MRE had “poorer specificity with higher false-positive rates, suggesting poorer diagnostic performance,” compared with VCTE. Lower cost and lower point-of-care availability make VCTE “an attractive solution compared to MRE,” the guideline adds. It conditionally recommends VCTE cutoffs of 12.5 kPa for cirrhosis and 9.5 kPa for advanced (F3-F4) liver fibrosis after patients have a sustained virologic response to therapy. The 9.5-kPa cutoff would misclassify only 1% of low-risk patients and 7% of high-risk patients, but noncirrhotic patients (less than 9.5 kPa) may reasonably choose to continue specialty care if they prioritize avoiding “the small risk” of hepatocellular carcinoma over the “inconvenience and risks of continued laboratory and fibrosis testing.”
For chronic hepatitis B, the guideline conditionally recommends VCTE with an 11.0-kPa cutoff over APRI or FIB-4. In a pooled analysis of 28 studies, VCTE detected cirrhosis with a sensitivity of 86% and a specificity of 85%, compared with 66% and 74%, respectively, for APRI, and 87% and 65%, respectively, for FIB-4. However, the overall diagnostic performance of VCTE resembled that of the serum tests, and clinicians should interpret VCTE in the context of other clinical cirrhosis data, the guideline states.
Among 17 studies of VCTE cutoffs in hepatitis B, an 11.0-kPa threshold diagnosed cirrhosis with a sensitivity of 81% and a specificity of 83%. This cutoff would miss cirrhosis in less than 1% of low-risk patients and about 5% of high-risk patients and would yield false positives in 10%-15% of patients. Thus, its cutoff minimizes false negatives, reflecting “a judgment that the harm of missing cirrhosis is greater than the harm of over diagnosis,” the authors write.
For chronic alcoholic liver disease, the AGA conditionally recommends VCTE with a cirrhosis cutoff of 12.5 kPa. In pooled analyses, this value had a sensitivity of 95% and a specificity of 71%. For suspected compensated cirrhosis, the guideline conditionally suggests a 19.5-kPa cutoff when assessing the need for esophagogastroduodenoscopy (EGD) to identify high-risk esophageal varices. Patients who fall below this cutoff can reasonably pursue screening endoscopy if they are concerned about the small risk of acute variceal hemorrhage, the guideline adds.
The guideline also conditionally recommends a 17-kPa cutoff to detect clinically significant portal hypertension in patients with suspected chronic liver disease who are undergoing elective nonhepatic surgeries. This cutoff will miss about 0.1% of very low-risk patients, 0.8% of low-risk patients, and 7% of high-risk patients. Because the failure to detect portal hypertension contributes to operative morbidity and mortality, higher-risk patients might “reasonably” pursue screening endoscopy even if their kPa is below the cutoff, the guideline states.
The guideline made no recommendation about VCTE versus APRI or FIB-4 in adults with nonalcoholic fatty liver disease (NAFLD), citing “unacceptable bias” in 12 studies that excluded obese patients, used per-protocol rather than intention-to-diagnose analyses, and ignored “unsuccessful or inadequate” liver stiffness measurements, which are relatively common in NAFLD, the guideline notes. It conditionally recommends MRE over VCTE in high-risk adults with NAFLD, including those who are older, diabetic, or obese (especially with central adiposity) or who have alanine levels more than twice the upper limit of normal. However, it cites insufficient evidence to extend this recommendation to low-risk patients who only have imaging evidence of fatty liver.
Overall, the guideline focuses on “routine clinical management issues, and [does] not address comparisons with proprietary serum fibrosis assays, other emerging imaging-based fibrosis assessment techniques, or combinations of more than one noninvasive fibrosis test,” the authors note. They also limited VCTE cutoffs to single thresholds that prioritized sensitivity over specificity. “Additional studies are needed to further define the role of VCTE, MRE, and emerging diagnostic studies in the assessment of liver fibrosis, for which a significant unmet medical need remains, particularly in conditions such as NAFLD/[nonalcoholic steatohepatitis],” they add. “In particular, defining the implications for serial liver stiffness measurements over time on management decisions is of great interest.”
Dr. Muir has served as a consultant for AbbVie, Bristol-Myers Squibb, Gilead, and Merck. Dr. Lim has served as a consultant for Bristol Myers-Squibb, Gilead, Merck, and Boehringer Ingelheim. Dr. Flamm has served as a consultant or received research support from Gilead, Bristol-Myers Squibb, AbbVie, Salix Pharmaceuticals, and Intercept Pharmaceuticals. Dr. Dieterich has presented lectures for Gilead and Merck products. The rest of the authors disclosed no conflicts related to the content of this guideline.
Vibration-controlled transient elastography (VCTE) can accurately diagnose cirrhosis in most patients with chronic liver disease, particularly those with chronic hepatitis B or C, states a new guideline from the AGA Institute, published in the May issue of Gastroenterology (doi: 10.1053/j.gastro.2017.03.017).
However, magnetic resonance elastography (MRE) is somewhat more accurate for detecting cirrhosis in nonalcoholic fatty liver disease, wrote Joseph K. Lim, MD, AGAF, of Yale University in New Haven, Conn., with his associates from the Clinical Guidelines Committee of the AGA. VCTE is convenient but performs unevenly in some liver conditions and is especially unreliable in patients with acute hepatitis, alcohol abuse, food intake within 2-3 hours, congestive heart failure, or extrahepatic cholestasis, the guideline notes. Yet, VCTE remains the most common imaging tool for diagnosing fibrosis in the United States, and the guideline addresses “focused, clinically relevant questions” to guide its use.
When possible, clinicians should use VCTE instead of noninvasive serum tests for cirrhosis in patients with chronic hepatitis C, the guideline asserts. In pooled analyses of 62 studies, VCTE detected about 89% of cirrhosis cases (95% confidence interval, 84%-92%), Fibrosis-4 test (FIB-4) detected 87% (95% CI, 74%-94%), and aspartate aminotransferase to platelet ratio index (APRI) detected 77% (95% CI, 73%-81%). The specificity of VCTE (91%) also equaled or exceeded that of FIB-4 (91%) or APRI (78%), the guideline noted.
For chronic hepatitis C, MRE had “poorer specificity with higher false-positive rates, suggesting poorer diagnostic performance,” compared with VCTE. Lower cost and lower point-of-care availability make VCTE “an attractive solution compared to MRE,” the guideline adds. It conditionally recommends VCTE cutoffs of 12.5 kPa for cirrhosis and 9.5 kPa for advanced (F3-F4) liver fibrosis after patients have a sustained virologic response to therapy. The 9.5-kPa cutoff would misclassify only 1% of low-risk patients and 7% of high-risk patients, but noncirrhotic patients (less than 9.5 kPa) may reasonably choose to continue specialty care if they prioritize avoiding “the small risk” of hepatocellular carcinoma over the “inconvenience and risks of continued laboratory and fibrosis testing.”
For chronic hepatitis B, the guideline conditionally recommends VCTE with an 11.0-kPa cutoff over APRI or FIB-4. In a pooled analysis of 28 studies, VCTE detected cirrhosis with a sensitivity of 86% and a specificity of 85%, compared with 66% and 74%, respectively, for APRI, and 87% and 65%, respectively, for FIB-4. However, the overall diagnostic performance of VCTE resembled that of the serum tests, and clinicians should interpret VCTE in the context of other clinical cirrhosis data, the guideline states.
Among 17 studies of VCTE cutoffs in hepatitis B, an 11.0-kPa threshold diagnosed cirrhosis with a sensitivity of 81% and a specificity of 83%. This cutoff would miss cirrhosis in less than 1% of low-risk patients and about 5% of high-risk patients and would yield false positives in 10%-15% of patients. Thus, its cutoff minimizes false negatives, reflecting “a judgment that the harm of missing cirrhosis is greater than the harm of over diagnosis,” the authors write.
For chronic alcoholic liver disease, the AGA conditionally recommends VCTE with a cirrhosis cutoff of 12.5 kPa. In pooled analyses, this value had a sensitivity of 95% and a specificity of 71%. For suspected compensated cirrhosis, the guideline conditionally suggests a 19.5-kPa cutoff when assessing the need for esophagogastroduodenoscopy (EGD) to identify high-risk esophageal varices. Patients who fall below this cutoff can reasonably pursue screening endoscopy if they are concerned about the small risk of acute variceal hemorrhage, the guideline adds.
The guideline also conditionally recommends a 17-kPa cutoff to detect clinically significant portal hypertension in patients with suspected chronic liver disease who are undergoing elective nonhepatic surgeries. This cutoff will miss about 0.1% of very low-risk patients, 0.8% of low-risk patients, and 7% of high-risk patients. Because the failure to detect portal hypertension contributes to operative morbidity and mortality, higher-risk patients might “reasonably” pursue screening endoscopy even if their kPa is below the cutoff, the guideline states.
The guideline made no recommendation about VCTE versus APRI or FIB-4 in adults with nonalcoholic fatty liver disease (NAFLD), citing “unacceptable bias” in 12 studies that excluded obese patients, used per-protocol rather than intention-to-diagnose analyses, and ignored “unsuccessful or inadequate” liver stiffness measurements, which are relatively common in NAFLD, the guideline notes. It conditionally recommends MRE over VCTE in high-risk adults with NAFLD, including those who are older, diabetic, or obese (especially with central adiposity) or who have alanine levels more than twice the upper limit of normal. However, it cites insufficient evidence to extend this recommendation to low-risk patients who only have imaging evidence of fatty liver.
Overall, the guideline focuses on “routine clinical management issues, and [does] not address comparisons with proprietary serum fibrosis assays, other emerging imaging-based fibrosis assessment techniques, or combinations of more than one noninvasive fibrosis test,” the authors note. They also limited VCTE cutoffs to single thresholds that prioritized sensitivity over specificity. “Additional studies are needed to further define the role of VCTE, MRE, and emerging diagnostic studies in the assessment of liver fibrosis, for which a significant unmet medical need remains, particularly in conditions such as NAFLD/[nonalcoholic steatohepatitis],” they add. “In particular, defining the implications for serial liver stiffness measurements over time on management decisions is of great interest.”
Dr. Muir has served as a consultant for AbbVie, Bristol-Myers Squibb, Gilead, and Merck. Dr. Lim has served as a consultant for Bristol Myers-Squibb, Gilead, Merck, and Boehringer Ingelheim. Dr. Flamm has served as a consultant or received research support from Gilead, Bristol-Myers Squibb, AbbVie, Salix Pharmaceuticals, and Intercept Pharmaceuticals. Dr. Dieterich has presented lectures for Gilead and Merck products. The rest of the authors disclosed no conflicts related to the content of this guideline.
Vibration-controlled transient elastography (VCTE) can accurately diagnose cirrhosis in most patients with chronic liver disease, particularly those with chronic hepatitis B or C, states a new guideline from the AGA Institute, published in the May issue of Gastroenterology (doi: 10.1053/j.gastro.2017.03.017).
However, magnetic resonance elastography (MRE) is somewhat more accurate for detecting cirrhosis in nonalcoholic fatty liver disease, wrote Joseph K. Lim, MD, AGAF, of Yale University in New Haven, Conn., with his associates from the Clinical Guidelines Committee of the AGA. VCTE is convenient but performs unevenly in some liver conditions and is especially unreliable in patients with acute hepatitis, alcohol abuse, food intake within 2-3 hours, congestive heart failure, or extrahepatic cholestasis, the guideline notes. Yet, VCTE remains the most common imaging tool for diagnosing fibrosis in the United States, and the guideline addresses “focused, clinically relevant questions” to guide its use.
When possible, clinicians should use VCTE instead of noninvasive serum tests for cirrhosis in patients with chronic hepatitis C, the guideline asserts. In pooled analyses of 62 studies, VCTE detected about 89% of cirrhosis cases (95% confidence interval, 84%-92%), Fibrosis-4 test (FIB-4) detected 87% (95% CI, 74%-94%), and aspartate aminotransferase to platelet ratio index (APRI) detected 77% (95% CI, 73%-81%). The specificity of VCTE (91%) also equaled or exceeded that of FIB-4 (91%) or APRI (78%), the guideline noted.
For chronic hepatitis C, MRE had “poorer specificity with higher false-positive rates, suggesting poorer diagnostic performance,” compared with VCTE. Lower cost and lower point-of-care availability make VCTE “an attractive solution compared to MRE,” the guideline adds. It conditionally recommends VCTE cutoffs of 12.5 kPa for cirrhosis and 9.5 kPa for advanced (F3-F4) liver fibrosis after patients have a sustained virologic response to therapy. The 9.5-kPa cutoff would misclassify only 1% of low-risk patients and 7% of high-risk patients, but noncirrhotic patients (less than 9.5 kPa) may reasonably choose to continue specialty care if they prioritize avoiding “the small risk” of hepatocellular carcinoma over the “inconvenience and risks of continued laboratory and fibrosis testing.”
For chronic hepatitis B, the guideline conditionally recommends VCTE with an 11.0-kPa cutoff over APRI or FIB-4. In a pooled analysis of 28 studies, VCTE detected cirrhosis with a sensitivity of 86% and a specificity of 85%, compared with 66% and 74%, respectively, for APRI, and 87% and 65%, respectively, for FIB-4. However, the overall diagnostic performance of VCTE resembled that of the serum tests, and clinicians should interpret VCTE in the context of other clinical cirrhosis data, the guideline states.
Among 17 studies of VCTE cutoffs in hepatitis B, an 11.0-kPa threshold diagnosed cirrhosis with a sensitivity of 81% and a specificity of 83%. This cutoff would miss cirrhosis in less than 1% of low-risk patients and about 5% of high-risk patients and would yield false positives in 10%-15% of patients. Thus, its cutoff minimizes false negatives, reflecting “a judgment that the harm of missing cirrhosis is greater than the harm of over diagnosis,” the authors write.
For chronic alcoholic liver disease, the AGA conditionally recommends VCTE with a cirrhosis cutoff of 12.5 kPa. In pooled analyses, this value had a sensitivity of 95% and a specificity of 71%. For suspected compensated cirrhosis, the guideline conditionally suggests a 19.5-kPa cutoff when assessing the need for esophagogastroduodenoscopy (EGD) to identify high-risk esophageal varices. Patients who fall below this cutoff can reasonably pursue screening endoscopy if they are concerned about the small risk of acute variceal hemorrhage, the guideline adds.
The guideline also conditionally recommends a 17-kPa cutoff to detect clinically significant portal hypertension in patients with suspected chronic liver disease who are undergoing elective nonhepatic surgeries. This cutoff will miss about 0.1% of very low-risk patients, 0.8% of low-risk patients, and 7% of high-risk patients. Because the failure to detect portal hypertension contributes to operative morbidity and mortality, higher-risk patients might “reasonably” pursue screening endoscopy even if their kPa is below the cutoff, the guideline states.
The guideline made no recommendation about VCTE versus APRI or FIB-4 in adults with nonalcoholic fatty liver disease (NAFLD), citing “unacceptable bias” in 12 studies that excluded obese patients, used per-protocol rather than intention-to-diagnose analyses, and ignored “unsuccessful or inadequate” liver stiffness measurements, which are relatively common in NAFLD, the guideline notes. It conditionally recommends MRE over VCTE in high-risk adults with NAFLD, including those who are older, diabetic, or obese (especially with central adiposity) or who have alanine levels more than twice the upper limit of normal. However, it cites insufficient evidence to extend this recommendation to low-risk patients who only have imaging evidence of fatty liver.
Overall, the guideline focuses on “routine clinical management issues, and [does] not address comparisons with proprietary serum fibrosis assays, other emerging imaging-based fibrosis assessment techniques, or combinations of more than one noninvasive fibrosis test,” the authors note. They also limited VCTE cutoffs to single thresholds that prioritized sensitivity over specificity. “Additional studies are needed to further define the role of VCTE, MRE, and emerging diagnostic studies in the assessment of liver fibrosis, for which a significant unmet medical need remains, particularly in conditions such as NAFLD/[nonalcoholic steatohepatitis],” they add. “In particular, defining the implications for serial liver stiffness measurements over time on management decisions is of great interest.”
Dr. Muir has served as a consultant for AbbVie, Bristol-Myers Squibb, Gilead, and Merck. Dr. Lim has served as a consultant for Bristol Myers-Squibb, Gilead, Merck, and Boehringer Ingelheim. Dr. Flamm has served as a consultant or received research support from Gilead, Bristol-Myers Squibb, AbbVie, Salix Pharmaceuticals, and Intercept Pharmaceuticals. Dr. Dieterich has presented lectures for Gilead and Merck products. The rest of the authors disclosed no conflicts related to the content of this guideline.
FROM GASTROENTEROLOGY
AGA Clinical Practice Update: Expert review recommendations on post-SVR hepatitis C care
The AGA Institute issued a clinical practice update for managing hepatitis C virus–infected patients who achieve a sustained virologic response after antiviral therapy, who still require ongoing care for their liver disease. The expert review appears in the May issue of Gastroenterology (doi: 10.1053/j.gastro.2017.03.018).
Even though direct-acting antiviral regimens have produced remarkably high sustained virologic response (SVR) rates and it appears that fewer than 1% of patients relapse, and even though liver fibrosis and cirrhosis may regress with this therapy, continued surveillance and even intervention may be needed “to reduce complications arising from liver damage that has already accrued by the time SVR was attained,” said Ira M. Jacobson, MD, AGAF, chair of the department of medicine, Mount Sinai Beth Israel Medical Center, New York, and his associates.
Dr. Jacobson and his associates at the AGA Institute reviewed the current literature and expert opinion to formulate 11 best-practice recommendations for managing this patient population. Among their recommendations:
SVR should be confirmed by hepatitis C virus RNA testing at 12 weeks after completion of an all-oral direct-acting antiviral regimen, and routine confirmation after 48 weeks is also “prudent.” Further testing for later virologic relapse is not supported by the available evidence. However, further periodic testing is advised for patients at risk for reinfection, such as those who continue to use IV drugs.
All patients with stage 3 or higher liver fibrosis or cirrhosis before achieving SVR should continue to be monitored by liver imaging (with or without serum alpha fetoprotein testing) twice a year “for an indefinite duration.” At present, there is no evidence of a finite point beyond which the risk of hepatocellular carcinoma is reduced to the level of people who don’t have a history of liver disease. And there have been documented cases of hepatocellular carcinoma developing more than 5 years after attaining SVR.
Regardless of SVR status, all patients with liver cirrhosis should undergo endoscopic screening for esophagogastric varices. If no varices or only small varices are detected, repeat endoscopy should be done 2-3 years after achieving SVR. If no varices are identified then, “cessation of further endoscopic screening may be considered on an individual patient basis if there are no risk factors for progressive cirrhosis.”
Noninvasive assessment of fibrosis, such as liver elastography, may be considered on an individual basis after SVR is attained, to assess whether fibrosis has progressed or regressed or to guide clinical management.
All patients who achieve SVR must be counseled regarding factors that could further injure the liver and contribute to the progression of fibrosis, hepatic decompensation, or the development of hepatocellular carcinoma. These include alcohol consumption, fatty liver, diabetes, and potential toxins. If serum liver enzyme levels rise, all patients should be evaluated for possible liver injury.
The AGA Institute issued a clinical practice update for managing hepatitis C virus–infected patients who achieve a sustained virologic response after antiviral therapy, who still require ongoing care for their liver disease. The expert review appears in the May issue of Gastroenterology (doi: 10.1053/j.gastro.2017.03.018).
Even though direct-acting antiviral regimens have produced remarkably high sustained virologic response (SVR) rates and it appears that fewer than 1% of patients relapse, and even though liver fibrosis and cirrhosis may regress with this therapy, continued surveillance and even intervention may be needed “to reduce complications arising from liver damage that has already accrued by the time SVR was attained,” said Ira M. Jacobson, MD, AGAF, chair of the department of medicine, Mount Sinai Beth Israel Medical Center, New York, and his associates.
Dr. Jacobson and his associates at the AGA Institute reviewed the current literature and expert opinion to formulate 11 best-practice recommendations for managing this patient population. Among their recommendations:
SVR should be confirmed by hepatitis C virus RNA testing at 12 weeks after completion of an all-oral direct-acting antiviral regimen, and routine confirmation after 48 weeks is also “prudent.” Further testing for later virologic relapse is not supported by the available evidence. However, further periodic testing is advised for patients at risk for reinfection, such as those who continue to use IV drugs.
All patients with stage 3 or higher liver fibrosis or cirrhosis before achieving SVR should continue to be monitored by liver imaging (with or without serum alpha fetoprotein testing) twice a year “for an indefinite duration.” At present, there is no evidence of a finite point beyond which the risk of hepatocellular carcinoma is reduced to the level of people who don’t have a history of liver disease. And there have been documented cases of hepatocellular carcinoma developing more than 5 years after attaining SVR.
Regardless of SVR status, all patients with liver cirrhosis should undergo endoscopic screening for esophagogastric varices. If no varices or only small varices are detected, repeat endoscopy should be done 2-3 years after achieving SVR. If no varices are identified then, “cessation of further endoscopic screening may be considered on an individual patient basis if there are no risk factors for progressive cirrhosis.”
Noninvasive assessment of fibrosis, such as liver elastography, may be considered on an individual basis after SVR is attained, to assess whether fibrosis has progressed or regressed or to guide clinical management.
All patients who achieve SVR must be counseled regarding factors that could further injure the liver and contribute to the progression of fibrosis, hepatic decompensation, or the development of hepatocellular carcinoma. These include alcohol consumption, fatty liver, diabetes, and potential toxins. If serum liver enzyme levels rise, all patients should be evaluated for possible liver injury.
The AGA Institute issued a clinical practice update for managing hepatitis C virus–infected patients who achieve a sustained virologic response after antiviral therapy, who still require ongoing care for their liver disease. The expert review appears in the May issue of Gastroenterology (doi: 10.1053/j.gastro.2017.03.018).
Even though direct-acting antiviral regimens have produced remarkably high sustained virologic response (SVR) rates and it appears that fewer than 1% of patients relapse, and even though liver fibrosis and cirrhosis may regress with this therapy, continued surveillance and even intervention may be needed “to reduce complications arising from liver damage that has already accrued by the time SVR was attained,” said Ira M. Jacobson, MD, AGAF, chair of the department of medicine, Mount Sinai Beth Israel Medical Center, New York, and his associates.
Dr. Jacobson and his associates at the AGA Institute reviewed the current literature and expert opinion to formulate 11 best-practice recommendations for managing this patient population. Among their recommendations:
SVR should be confirmed by hepatitis C virus RNA testing at 12 weeks after completion of an all-oral direct-acting antiviral regimen, and routine confirmation after 48 weeks is also “prudent.” Further testing for later virologic relapse is not supported by the available evidence. However, further periodic testing is advised for patients at risk for reinfection, such as those who continue to use IV drugs.
All patients with stage 3 or higher liver fibrosis or cirrhosis before achieving SVR should continue to be monitored by liver imaging (with or without serum alpha fetoprotein testing) twice a year “for an indefinite duration.” At present, there is no evidence of a finite point beyond which the risk of hepatocellular carcinoma is reduced to the level of people who don’t have a history of liver disease. And there have been documented cases of hepatocellular carcinoma developing more than 5 years after attaining SVR.
Regardless of SVR status, all patients with liver cirrhosis should undergo endoscopic screening for esophagogastric varices. If no varices or only small varices are detected, repeat endoscopy should be done 2-3 years after achieving SVR. If no varices are identified then, “cessation of further endoscopic screening may be considered on an individual patient basis if there are no risk factors for progressive cirrhosis.”
Noninvasive assessment of fibrosis, such as liver elastography, may be considered on an individual basis after SVR is attained, to assess whether fibrosis has progressed or regressed or to guide clinical management.
All patients who achieve SVR must be counseled regarding factors that could further injure the liver and contribute to the progression of fibrosis, hepatic decompensation, or the development of hepatocellular carcinoma. These include alcohol consumption, fatty liver, diabetes, and potential toxins. If serum liver enzyme levels rise, all patients should be evaluated for possible liver injury.
Key clinical point: The AGA Institute issued a clinical practice update for managing HCV patients who achieve a sustained virologic response after antiviral therapy, who still require ongoing care for their liver disease.
Major finding: SVR should be confirmed by HCV RNA testing at 12 weeks after completion of an all-oral direct-acting antiviral regimen, and routine confirmation after 48 weeks is also “prudent.”
Data source: A review of the literature and of expert opinion to compile 11 best-practice recommendations for managing post-SVR HCV care.
Disclosures: This work was supported by the AGA Institute. Dr. Jacobson reported ties to AbbVie, Bristol-Myers Squibb, Gilead, Intercept, Janssen, Merck, and Trek; one of his associates reported ties to those groups and to Target PharmaSolutions.
Thrombosis in Pregnancy
INTRODUCTION
Venous thromboembolism (VTE), comprising deep vein thrombosis (DVT) and pulmonary embolism (PE), is a leading nonobstetric cause of maternal death in the United States and in developed countries.1,2 During pregnancy, the risk for VTE increases four- to six-fold, and although the risk is present throughout pregnancy, the mother is at highest risk immediately postpartum.3–5
VTE risk is increased due to physiologic and anatomic changes that occur in pregnancy. These changes include hypercoagulability, progesterone-induced venous stasis, decreased venous outflow, compression of the inferior vena cava and pelvic veins by the expanding uterus, and decreased mobility. The hypercoagulability of pregnancy is due to increased levels of coagulation factors I (fibrinogen), VII, VIII, and X, and von Willebrand factor; decreased free protein S, a natural anticoagulant; acquired resistance to activated protein C; and decreased fibrinolysis due to increased levels of plasminogen activator inhibitor-1 and -2.6,7 These changes confer increased hemostasis to the mother for delivery but also place her at higher risk for thrombosis.
A review of the literature found that more than 70% of pregnancy-associated DVTs are located in the ileofemoral region, as compared with approximately 9% in non-pregnant patients.8 The proximal location is associated with a higher risk for post-thrombotic syndrome and embolization as compared with calf DVTs.9 Proximal postnatal thrombosis, smoking, and older age are independent predictors of the development of post-thrombotic syndrome.10
RISK FACTORS
Clinical risk factors that increase the risk for VTE during pregnancy include a prior history of estrogen-related or unprovoked VTE, being a carrier of severe inherited thrombophilia (homozygotes for factor V Leiden or factor II G20210A variants, double heterozygotes, or persons with antithrombin, protein C, or protein S deficiencies), and the presence of antiphospholipid (aPL) antibodies.11 Women with systemic lupus erythematosus, diabetes, sickle cell disease, and heart disease also have a high risk for VTE during pregnancy.12 Other risk factors predisposing to thrombosis include black ethnicity, smoking, operative procedures, conception after assisted reproductive techniques, high body mass index, antepartum immobilization, severe preeclampsia, advanced age and parity, and a family history of VTE.13 A prospective cohort study of 1,297,037 pregnancies and related puerperium identified the following risk factors for thrombosis: hospitalization, infection, hyperemesis, multiple pregnancies, preeclampsia, obesity, cesarean section, major postpartum hemorrhage, intrauterine growth restriction, and fetal death.14 Risk factors identified in an Agency for Healthcare Research and Quality study include: age 35 or older, black ethnicity, lupus, sickle cell disease, heart disease, postpartum infection, and transfusion.15 The combination of more than one risk factor increases the risk for VTE. All these factors have to be considered when deciding on prophylactic or therapeutic anticoagulation therapy in pregnancy. In addition, the risks of anticoagulation, including bruising, bleeding, and other side effects (eg, reduced bone mineral density with therapeutic-dose unfractionated heparin), allergic reactions, and rarely thrombocytopenia, must be considered.
EVALUATION AND DIAGNOSIS
CASE PRESENTATION I
A 31-year-old woman G1P0 at 10 weeks’ gestation with no personal or family history of thrombosis presents with acute onset of shortness of breath and left-sided chest pain that awoke her the morning of presentation. Her vital signs are significant for a heart rate of 106 beats/min, respiration rate of 22 breaths/min, blood pressure of 105/76 mm Hg, and pulse oximetry of 98% on room air. The patient denies previous exposure to oral contraceptives. She does not smoke. She reports that she had noticed left calf pain and swelling, which worsened with walking after a 4-hour drive 2 days prior.
What is the approach to diagnosis of thromboembolism in pregnant patients?
DEEP VEIN THROMBOSIS
Although a clinical diagnosis of DVT in pregnancy is unreliable, a history and physical examination are necessary to exclude other diagnoses and to assess the likelihood of thrombosis. Unfortunately, studies of the accuracy of history and physical examination for detecting DVT and PE have not included pregnant patients. In most pregnant patients with clinically suspected DVT, the diagnosis is not confirmed. Other causes of leg pain and swelling are not uncommon during pregnancy and include cellulitis, ruptured Baker’s cyst, or muscular pain.
A cross-sectional study described the derivation of the LEFt clinical decision rule, which relies on 3 variables in pregnant women with suspected DVT: left leg presentation (L), ≥ 2 cm calf circumference difference (E for edema), and first trimester presentation (Ft). If none of these variables is present, the negative predictive value is 100%.16 A validation study suggested that a negative LEFt rule accurately identifies pregnant women in whom the risk for confirmed DVT appears to be very low. The rule should not be used as an individual test for excluding DVT during pregnancy, but could be applied in a diagnostic approach in association with D-dimer measurement and compression ultrasonography (CUS); however, it has not been prospectively validated for safety and efficacy.17 In a study of 149 consecutive pregnant women with suspected DVT, a whole-blood agglutination D-dimer had a sensitivity of 100% and specificity of 60%.18 A 2006 systematic review found only 4 diagnostic studies of VTE in pregnancy in the literature. One of these studies showed that a combination of a negative CUS and normal D-dimer can accurately exclude DVT.19
Serial CUS is necessary for pregnant women with a high clinical suspicion of DVT but a negative initial investigation. In a study of 221 pregnant women in whom DVT was clinically suspected, 16 women (7.2%) were diagnosed with DVT by initial CUS, and none were diagnosed with DVT onserial testing.20 During follow-up (≥ 3 months), 6 of the 205 women with normal serial CUS results presented with symptoms of DVT, PE, or both, and 1 of them was diagnosed with DVT and PE. The sensitivity of serial CUS with Doppler imaging was 94.1% (95% confidence interval [CI] 69.2% to 99.7%), and the negative predictive value was 99.5% (95% CI 96.9% to 100%).20 All ultrasounds undertaken for investigation of pregnancy-associated DVT should include imaging of the iliac veins if there is a high index of suspicion and the CUS is negative for femoral DVT. Serial CUS with Doppler imaging of the iliac vein performed over a 7-day period excludes DVT in symptomatic pregnant women.20 Repeat CUS may be done 2 to 4 days and 6 to 8 days after the initial scan.
Ileofemoral vein thrombosis accounts for approximately 90% of proximal thromboses in pregnancy, occurring most often in the left lower extremity.20 The incidence of isolated iliac vein thrombosis in pregnancy is low, but when it does occur, delay in diagnosis can lead to significant morbidity. Therefore, for women with suspected isolated iliac vein thrombosis in whom CUS is negative or nondiagnostic, magnetic resonance direct thrombus imaging (MRDTI) should be performed.21 Patients with iliac vein thrombosis may present with unexplained inguinal, pelvic, or abdominal pain, which may be accompanied by back pain, and they usually present with swelling of the entire leg. MRDTI does not require gadolinium contrast and its accuracy appears to be similar to that of venography for iliac vein thrombi in the nonpregnant population.21 Exposure to gadolinium during pregnancy is associated with an increased risk for rheumatologic, inflammatory, or infiltrative skin conditions and stillbirth or neonatal death.22
Ovarian vein thrombosis is a rare but serious diagnosis. It occurs mostly in the postpartum period, mainly after cesarean delivery, and usually affects the right ovarian vein. The diagnosis is confirmed by ultrasound, computed tomography (CT), or magnetic resonance imaging.23
PULMONARY EMBOLISM
PE is more difficult to diagnose than DVT, particularly because clinical signs of PE are unreliable in the pregnant patient. The mortality rate of untreated PE is high, ranging from 18% to 38%, and approximately one-third of patients with untreated thromboembolic disease develop recurrent embolism.24 Studies have reported a PE prevalence between 1.4% and 4.2% in pregnant women with suspected clinical diagnosis of PE.25
The clinical presentation of PE and associated laboratory testing results may be subtler in pregnant than in nonpregnant patients. Arterial blood gases (ABG) may show hypoxemia or hypocapnia. The ABG in pregnancy has a sensitivity of 76.9%, specificity of 20.2%, and negative and positive predictive values of 80% and 11.5% for PE, respectively.26 The alveolar-arterial oxygen gradient is a poor screening test for PE during pregnancy and postpartum. A retrospective chart review of 17 pregnant women with documented PE showed that 58% had normal alveolar-arterial gradients.27 Therefore, in a pregnant woman with a history suspicious for PE, objective imaging studies should be performed even if the patient has normal ABG.
The 2011 guidelines from the American Thoracic Society (ATS) and the Society of Thoracic Radiology (STR) recommend against using D-dimer to diagnose PE in pregnancy.28 In addition, lower extremity CUS should only be performed as the first diagnostic imaging procedure if the patient has signs or symptoms of DVT. Instead, the ATS/STR guidelines recommend a plain radiograph of the chest as the first imaging test. If the chest radiograph is normal, a ventilation/perfusion scan (V/Q) scan is preferred over CT pulmonary angiography (CTPA). Diagnostic accuracy of the V/Q scan may be superior to CTPA in pregnancy, and it is preferable because of the lower prevalence of indeterminate V/Q scan in pregnant women.29 Moreover, there is lower radiation exposure to the maternal breast and lung tissue with a V/Q scan than with CTPA. CTPA confers lower fetal radiation doses than V/Q scans (0.03–0.66 mGy versus 0.32–0.74 mGy, respectively) but higher total body maternal radiation (4–16 mSv versus 1–2.5 mSv).30 A quantitative approach to lung scan interpretation, based on the distribution histogram of V/Q ratios, may be helpful in categorizing patients with suspected PE.28 A study of 121 suspected episodes of PE in 120 pregnant women showed that 104 women with normal or nondiagnostic scans did not develop subsequent episodes of VTE during a mean follow-up period of 20 months.31
If the baseline chest radiograph is abnormal in a pregnant woman with clinical suspicion of PE, a CTPA should be performed. As noted, fetal radiation doses for CTPA examinations in which the fetus is not directly imaged are minimal. If CTPA is recommended for the diagnosis of PE, the patient should be informed that radiation to the breast may increase her baseline risk for breast cancer. The ATS guidelines state that “given the lack of evidence documenting clear superiority of any one diagnostic test, the values and preferences of a patient and her physician likely will and should determine the final choice and sequence of tests performed.”28
CASE I CONTINUED
Upon presentation to the emergency department, the circumference of the patient’s left leg is not significantly greater than that of her right leg, and her leg pain has resolved. Bilateral CUS is negative for proximal or distal DVT. Chest radiograph shows an opacification of her left lower lobe. CTPA shows bilateral segmental and subsegmental lower lobe pulmonary emboli.
How does risk for VTE change throughout pregnancy?
Women are at increased risk for VTE throughout the entire pregnancy, starting from conception, but mainly during the postpartum period. A Danish historical controlled cohort study of 819,751 pregnant women (ages 15–49 years) over a 10-year period identified 727 women with VTE. The absolute risk for VTE per 10,000 pregnancy-years increased from 4.1 (95% CI 3.2 to 5.2) during weeks 1 to 11 to 59.0 (95% CI 46.1 to 76.4) in week 40 and decreased in the postpartum period from 60 (95% CI 47.2 to 76.4) during the first week after birth to 2.1 during weeks 9–12 after birth (95% CI 1.1 to 4.2).32 This study showed that the risk of VTE increases throughout pregnancy and reaches its maximum during the peripartum period and is not significantly increased after 6 weeks post-delivery. In a retrospective cross-over cohort study of 1,687,930 women in California who delivered their first newborn, an elevated risk of VTE persisted until at least 12 weeks after delivery. However, the absolute increase in risk after 6 weeks postpartum was low.33
CASE 1 CONCLUSION
The patient is started on anticoagulation therapy and carefully monitored during the remainder of the pregnancy and postpartum period. Anticoagulation is discontinued 6 weeks after delivery.
TREATMENT
ANTICOAGULATION THERAPY
The treatment of VTE can be lifesaving. In a study comparing 35 patients with PE randomly assigned to treatment with anticoagulants versus no treatment, 5 of 19 patients in the untreated group died from PE and an additional 5 had nonfatal recurrences, as compared with none in the treated group.24 Unfractionated heparin (UFH) and low-molecular-weight heparin (LMWH) are both safe and effective anticoagulants during pregnancy as neither crosses the placenta. In a review of 186 reports of fetal and infant outcomes following anticoagulant therapy during pregnancy in 1325 pregnancies, outcomes in UFH-treated patients were similar to those in the normal population after excluding pregnancies with comorbid conditions independently associated with adverse outcomes.34 A 2005 systematic review of LMWH for prophylaxis and treatment of VTE during pregnancy included 64 studies of 277 pregnancies. There were no maternal deaths, live births resulted from 94.7% of the pregnancies, VTE or arterial thrombosis occurred in 0.86%, and significant bleeding occurred in 1.98%.35
The standard UFH regimen is an initial bolus of 5000 units subcutaneously and 17,500 units every 12 hours, with dose adjustment made based on a mid-interval activated partial thromboplastin time (aPTT).36 Although still controversial, it has been suggested that the anti-Xa assay with a mid-dosing interval target of 0.3 to 0.7 U/mL is a more reliable measure of therapeutic UFH activity than the aPTT, as the aPTT response is suppressed due to a pregnancy-related increase in factor VIII. LMWH is dosed based on weight; regimens are enoxaparin 1 mg/kg subcutaneously twice daily or 1.5 mg/kg subcutaneously once daily, and dalteparin 100 units/kg every 12 hours or 150 units/kg daily.
A 2017 Cochrane review of the effect of LMWH compared with UFH for the treatment of VTE in the nonpregnant setting included 23 studies with 9587 patients. Thrombotic complications (odds ratio [OR] 0.70 [CI 0.57 to 0.85]) and major hemorrhage (OR 0.58 [CI 0.40 to 0.83]) were lower in patients receiving LMWH, with a trend toward lower mortality.37 In addition, the incidence of bleeding complications in patients treated with subcutaneous LMWH versus intravenous heparin was compared in a 2012 systematic review of 27 randomized controlled trials with a total of 28,637 patients. In patients treated with LMWH, there was a nonstatistically significant lower incidence of major bleeding events (OR 0.79 [95% CI 0.60 to 1.04]) and a statistically significant reduction in bleeding risk (OR 0.68 [95% CI 0.47 to 1.00]) compared to patients treated with UFH.38 Additionally, a trial comparing the use of standard UFH versus LMWH found a significantly lower incidence of thrombocytopenia in patients treated with LMWH.39,40 Overall, LMWH is more effective at decreasing both thrombotic and bleeding complications, and the risk for osteoporosis is lower with LMWH. Based on these results, the American College of Chest Physicians (ACCP) recommends LMWH as the first-line treatment for VTE in pregnancy.41
In specific clinical situations, such as patients with renal dysfunction with creatinine clearance (CrCl) less than 30 mL/min, UFH is indicated. In a study of 103 pregnancies in 93 women given anti-coagulation during pregnancy, 89.3% received UFH. There were no maternal deaths, and fetal demise occurred in 8 pregnancies (7.8%) at a median of 14 weeks’ gestation. There were 2 episodes of PE (1.9%) and 2 major bleeding events requiring transfusion (1.9%).42 UFH costs much less than LMWH, and therefore UFH remains an important, inexpensive, and efficacious anticoagulant option for pregnant women who require anticoagulation and cannot afford LMWH.43
Due to the physiologic changes associated with pregnancy, LMWH and UFH dosages may need to be adjusted. An observational study of 20 pregnant women with acute VTE found no recurrent VTE or major bleeding after treatment with dalteparin. Dalteparin doses approximately 10% to 20% higher than those recommended in nonpregnant women were required to reach therapeutic target anti-Xa activity.44
Caution Regarding Oral Anticoagulants
Due to its teratogenicity, warfarin is not a first-line anticoagulation option. It is strictly contraindicated during the first trimester during organogenesis, and its use during pregnancy is restricted to women with mechanical heart valves. Warfarin crosses the placenta and has been associated with nasal hypoplasia, stippled epiphyses, and growth restriction, particularly between 6 to 9 weeks’ gestation. Every effort should be made to substitute UFH or LMWH for warfarin between 6 and 12 weeks of gestation. The bridging process should begin as early in the gestational age as possible due to the long half-life of warfarin.45 When used later in gestation, warfarin has been associated with fetal hemorrhage and central nervous system abnormalities. Other complications from use during the second and third trimesters include microcephaly, blindness, deafness, and fetal growth restriction.46,47 Its use also increases the risk for abortion and fetal death in utero.48–50
The direct oral anticoagulants (DOACs) are not approved for use in pregnancy. Although there are limited anecdotal reports of DOAC use in pregnancy,51 there is preclinical evidence of placental transfer with the DOACs rivaroxaban and apixaban (direct Xa inhibitors) and the oral thrombin inhibitor dabigatran, thus increasing the risk to the fetus.52–54 Edoxaban, another direct Xa inhibitor, should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. It should be discontinued in nursing mothers.55
THROMBOLYSIS
Fetal as well as maternal survival is dependent on adequate maternal perfusion and oxygenation. The risk of death from PE is significant, with a cross-sectional study of 58 patients with acute, massive PE showing a 55% mortality rate.56 Thus, pregnancy is not an absolute contraindication to mechanical or systemic (recombinant tissue plasminogen activator or streptokinase) thrombolysis in an unstable patient at high risk for death.57–59 There are no major studies of this approach, although a small review of 13 cases using systemic thrombolysis showed no increased risk of maternal mortality.58 Thrombolysis should be considered for appropriate indications in pregnant patients as it would be in nonpregnant patients. However, caution is required when drawing conclusions regarding maternal and fetal safety, given the lack of controlled clinical trials including pregnant women.
SURGICAL PULMONARY EMBOLECTOMY
Surgical pulmonary embolectomy is an important therapeutic and potentially life-saving option in women presenting with massive PE in the immediate postpartum period. Because of the risk of massive uterine bleeding immediately postpartum, thrombolytic therapy should not be used.60
INFERIOR VENA CAVA FILTER
Placement of an inferior vena cava (IVC) filter is indicated in patients who have an acute VTE with absolute contraindications for anticoagulation. In addition, it can be considered in patients with extensive ileofemoral venous thrombosis within 2 weeks prior to expected delivery.61 In a systematic review of 44 studies of IVC filters placed in pregnant patients, the IVC filter complication rate was 8.87% and the failure-to-retrieve rate was 11.25%.62 The complication rate is similar to that found in the nonpregnant population. Thus, IVC filters may be used when appropriately indicated and should be removed as soon as clinically feasible.
RECURRENT THROMBOSIS AND THROMBOPHILIAS
CASE PRESENTATION 2
A 34-year-old pregnant woman G1P0 at 38 weeks’ gestation presents with a painful, swollen left calf that is associated with difficulty on walking; the circumference of the left calf is 2 cm greater than that of the right. She has no shortness of breath or chest pain. She has a prior history of distal right lower extremity DVT while on combined oral contraceptives. Her mother also has a history of DVT while bedbound during a prolonged hospitalization at an older age. CUS is negative, and the patient is discharged home. However, 24 hours later she returns to the hospital with worsening swelling and pain in her left leg. Magnetic resonance venography demonstrates a large left external iliac and common iliac DVT. She is admitted and is started on UFH, and a retrievable IVC filter is placed in anticipation of delivery.
What is the risk for VTE recurrence during pregnancy?
A personal and family history of VTE should be obtained when evaluating pregnant patients. A retrospective study of 109 women with prior history of VTE showed recurrence rates per patient-year of 10.9% during pregnancy and 3.7% in the nonpregnant period; the relative risk of recurrent VTE during pregnancy was 3.5 (95% CI 1.6 to 7.8).63 Two large European retrospective cohort studies of VTE in pregnancy showed that the recurrence rate of VTE in women with a history of thrombosis is around 6% during pregnancy, equally distributed among trimesters. The highest incidence of recurrence was in the postpartum period, ranging from 8.3% to 10%.64 The recurrence risk during pregnancy in women with a history of a single episode of VTE was 2.4% antepartum (95% CI 0.2% to 6.9%).65 These risks may be lower in women without thrombophilia or with a temporary risk factor associated with their previous thromboembolic event.65 Recurrence risk is higher if the previous VTE was estrogen-related, either due to pregnancy or through hormonal contraception (10%), than if the previous VTE was non-estrogen-related (2.7%).64,66
The timing of the case patient’s presentation is consistent with reports of increased risk of VTE during the peripartum period. Her prior history of estrogen-related DVT is concerning for a risk of recurrence, particularly during pregnancy. A retrospective cohort study of 1104 women with previous VTE, 88 of whom became pregnant without receiving thromboprophylaxis, showed that the overall rate of VTE recurrence was 5.8% (95% CI 3.0% to 10.6%) and 8.3% (95% CI 4.5% to 14.6%) during pregnancy and postpartum, respectively. The risk of VTE recurrence was absent if the first VTE was related to a transient risk factor other than pregnancy, postpartum period, or hormonal contraception.67 However, the recurrence rate of VTE in women with prior unprovoked VTE and/or thrombophilia has been reported as 5.9% (95% CI 1.2% to 16.2%).65 The presence of an underlying hypercoagulable state can increase the recurrence risk by 25% to 50%, depending on the disorder.68 A retrospective cohort study of 270 pregnancies in 105 carriers of factor V Leiden, identified because of a symptomatic relative with the factor V Leiden mutation, found a VTE risk (mostly in the postpartum period) of 6.4% for heterozygous women, 16.7% for homozygous women, 20% for double heterozygous women, and 1.2% for noncarriers.69
Should the patient be screened for a thrombophilia disorder?
Half of all index thromboses in patients with thrombophilia occur in association with an additional risk factor. In women of child-bearing age, pregnancy, the postpartum period, and the use of combined hormonal contraception are all risk factors for VTE. A 2010 guideline from the British hematology community recommended testing for thrombophilia in women with prior VTE secondary to a minor provoking factor before or during pregnancy, but not testing women with unprovoked VTE (who would receive prophylaxis regardless) or those with VTE secondary to a major provoking factor (who would not require prophylaxis).70 Indications to screen for aPL antibodies include: women with (1) 3 unexplained recurrent first-trimester pregnancy losses or 1 second or third trimester fetal loss of morphologically normal fetuses; (2) severe preeclampsia; (3) intrauterine growth restriction; or (4) premature labor (< 34 weeks’ gestation).71,72
CASE 2 CONCLUSION
The patient is subsequently screened for inherited thrombophilia disorders and is found to be heterozygous for factor V Leiden.
CASE PRESENTATION 3
A 25-year-old woman is diagnosed with antiphospholipid syndrome (APS) during her second pregnancy when she experiences fetal loss during her second trimester. Pathologic examination of the placenta reveals infarcts. Laboratory evaluation reveals positive high-titer anticardiolipin and anti-beta-2 glycoprotein 1 antibodies (IgG isotype) and lupus anticoagulant on 2 separate occasions 12 weeks apart. In a subsequent pregnancy, she is started on prophylactic LMWH and daily low-dose aspirin (81 mg). At 36 weeks’ gestation, she presents with a blood pressure of 210/104 mm Hg and a platelet count of 94,000 cells/µL. She is diagnosed with preeclampsia and hemolysis, elevated liver enzymes, and low platelets (HELLP) syndrome and is induced for early delivery. About 2 weeks after vaginal delivery, she notices shortness of breath and chest pain. A CTPA demonstrates a right lower lobe lobar defect consistent with a PE. Her anticoagulation is increased to therapeutic dosage LMWH.
To what extent does thrombophilia increase the risk for VTE in pregnancy?
Approximately 50% of pregnancy-related VTEs are associated with inherited thrombophilia. A systematic review of 79 studies, in which 9 studies (n = 2526 patients) assessed the risk of VTE associated with inherited thrombophilia in pregnancy, revealed that the odds ratio for individuals with thrombophilia to develop VTE ranged from 0.74 to 34.40.73 Although women with thrombophilia have an increased relative risk of developing VTE in pregnancy, the absolute risk of VTE remains low (Table 1).41,73,74
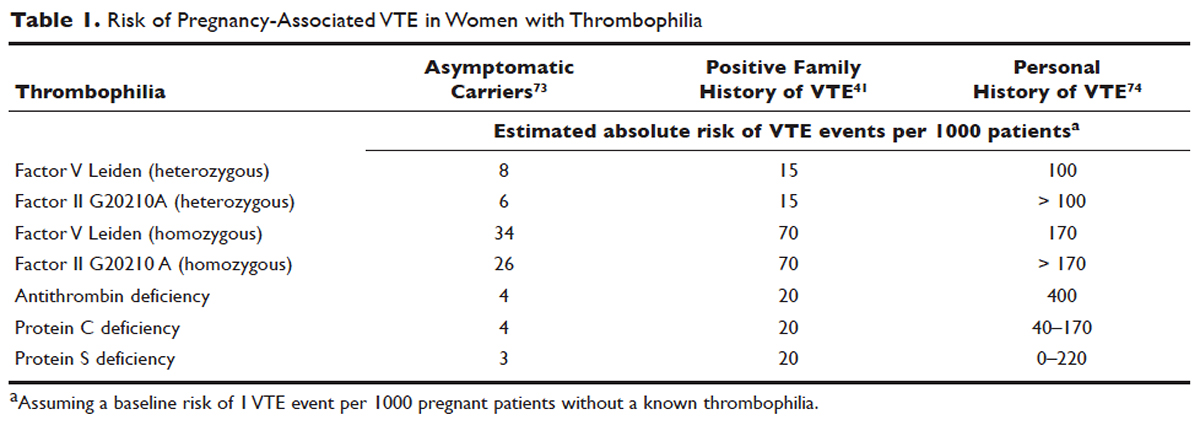
How is APS managed in pregnant patients?
Women with history of recurrent early pregnancy loss (< 10 weeks’ gestation) related to the presence of aPL antibodies are managed with low-dose aspirin and prophylactic-dose UFH or LMWH. This treatment increases the rate of subsequent successful pregnancy outcomes and reduces the risk for thrombosis. A 2010 systematic review and meta-analysis of UFH plus low-dose aspirin compared with low-dose aspirin alone in patients with APS and recurrent pregnancy loss included 5 trials and 334 patients. Patients receiving dual therapy had higher rates of live births (74.3%; relative risk [RR] 1.30 [CI 1.04 to 1.63]) compared to the aspirin-only group (55.8%).75 A 2009 randomized controlled trial compared low-dose aspirin to low-dose aspirin plus LMWH in women with recurrent pregnancy loss and either aPL antibodies, antinuclear antibody, or inherited thrombophilia. The study was stopped early after 4 years and found no difference in rates of live births between the groups (77.8% versus 79.1%).76 However, a randomized case-control trial of women with aPL antibodies and recurrent miscarriage found a 72% live birth rate in 47 women randomly assigned to low-dose aspirin and LMWH.77 A 2012 guideline from the American College of Chest Physicians (ACCP) recommends that women with aPL antibodies with a history of 3 or more pregnancy losses receive low-dose aspirin plus prophylactic-dose LMWH or UFH.78 A 2014 systematic review and meta-analysis showed that the combination of low-dose aspirin and UFH resulted in a higher live-birth rate than aspirin alone in 803 women with APS (RR 1.54 [95% CI 1.25 to 1.89]).79 Further large randomized controlled trials are needed to confirm optimal management of recurrent miscarriage and aPL antibodies.
The addition of prednisone to aspirin, heparin, or both has shown no benefits in pregnant women with aPL antibodies. Indeed, prolonged use of steroids may cause serious pregnancy complications, such as prematurity and hypertension.80–83 Intravenous infusions of immunoglobulin (IVIG) have not been shown to be superior to heparin and aspirin. This finding was confirmed in a multicenter clinical trial that tested the effects of IVIG compared with LMWH plus low-dose aspirin for the treatment of women with aPL antibodies and recurrent miscarriage. The rate of live-birth was 72.5% in the group treated with heparin plus low-dose aspirin compared with 39.5% in the IVIG group.84
Preeclampsia and HELLP syndrome complicated the case patient’s pregnancy even though she was being treated with prophylactic-dose LMWH and low-dose aspirin, the current standard of care for pregnant women with APS (UFH can be used as well). It is important to note that complications may still occur despite standard treatment. Indeed, PE is more common in the postpartum than in the antepartum period. Prompt diagnosis is paramount to initiate the appropriate treatment; in this case the dose of LMWH was increased from prophylactic to therapeutic dose. However, additional therapeutic modalities are necessary to improve outcomes. A randomized controlled trial comparing standard of care with or without hydroxychloroquine is under way to address this issue.
PROPHYLAXIS
CASE PRESENTATION 4
A 34-year-old woman G1P0 at 6 weeks’ gestation with a past medical history of a proximal lower extremity DVT while on oral contraception is treated with warfarin anticoagulation for 6 months. Her obstetrician consults the hematologist to advise regarding antithrombotic management during this pregnancy.
What is the approach to prophylaxis in women at high risk for pregnancy-associated VTE?
All women at high risk for pregnancy-associated VTE should be counseled about the signs and symptoms of DVT or PE during preconception and pregnancy and have a plan developed should these symptoms arise. The ACCP guidelines on antithrombotic therapy outline recommendations ranging from clinical vigilance to prophylactic and intermediate-dose anticoagulation, depending on the risk for VTE recurrence, based on the personal and family history of VTE and type of thrombophilia (Table 2).78 These recommendations range from grade 2B to 2C.
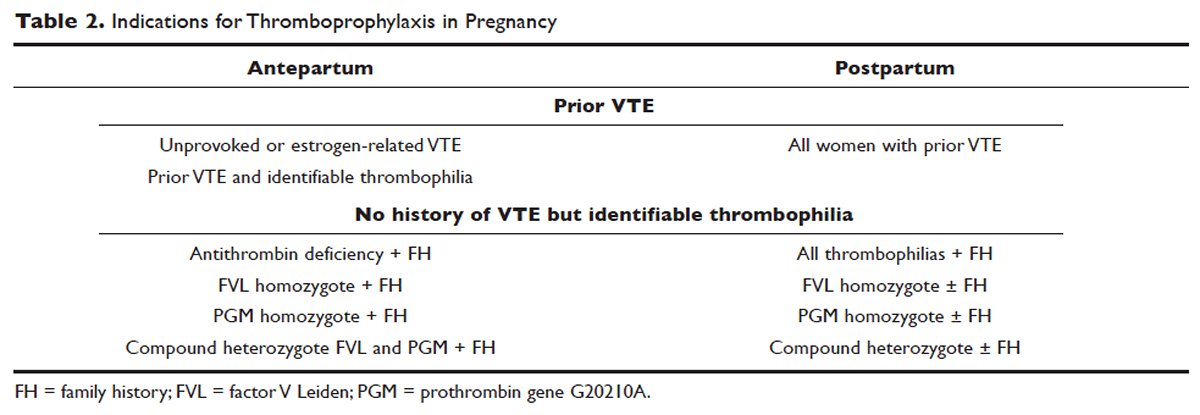
For women with a history of estrogen-related VTE, single unprovoked VTE, or recurrent unprovoked VTE not on chronic anticoagulation, antepartum and postpartum pharmacologic thromboprophylaxis with either prophylactic or intermediate-dose LMWH is recommended (grade 2C). In patients with prior history of provoked VTE (non-estrogen related), antepartum clinical vigilance and postpartum pharmacologic thromboprophylaxis is recommended (grade 2C, 2B).
In asymptomatic pregnant women who are homozygote carriers for factor V Leiden or prothrombin G20210A variants and have a positive family history of thrombosis, antepartum and postpartum pharmacologic thromboprophylaxis is recommended (grade 2B). In asymptomatic homozygote carriers of factor V Leiden or prothrombin G20210A variants with no family history of thrombosis and women with all other thrombophilias with a positive family history of thrombosis, postpartum pharmacologic thromboprophylaxis is indicated (grade 2B and 2C, respectively). For women with confirmed APS and clinical criteria of obstetric APS with recurrent pregnancy loss, antepartum thromboprophylaxis with LMWH and low-dose aspirin is recommended (grade 1B). For pregnant women with all other thrombophilias with no personal or family history of thrombosis, clinical vigilance is suggested (grade 2 C).78
As an alternative to LMWH, vitamin K antagonists (VKA) such as warfarin can be used for postpartum thromboprophylaxis; in patients with protein C or S deficiency, due to the risk of warfarin-induced skin necrosis, a rapid-onset anticoagulant must be concomitantly administered. Warfarin and LMWH are safe anticoagulants during lactation, but there are no clinical data on the effects of the DOACs on infants during lactation. Data from animal studies indicate that DOACs are secreted into breast milk.85
What risks are associated with anticoagulant therapy in pregnancy?
VKAs cross the placenta and can cause teratogenicity, pregnancy loss, fetal bleeding, and neurodevelopmental deficits. Therefore, discontinuation of VKAs prior to the sixth week of gestation is necessary to avoid warfarin embryopathy. DOACs have been shown to readily cross the placenta but with unknown human reproductive risks. Fondaparinux, a synthetic pentasaccharide, crosses the placenta in small quantities. Though there are reports of the successful use of fondaparinux in pregnancy, there is limited reported experience of its use in the first trimester.86
The risk for bleeding with anticoagulation is notably acceptable. In a case-control study of 88 pregnant women receiving therapeutic-dose anticoagulation, the risk of postpartum hemorrhage (PPH) after vaginal delivery was 30% in those who received LMWH anticoagulation versus 18% in those who did not (OR 1.9 [95% CI 1.1 to 3.5]).87 However, the risk for severe PPH (≥ 500 mL) was similar (5.6% versus 5.0%; OR 1.1 [95% CI 0.4 to 3.6]). The risk for PPH after cesarean section was 12% in LMWH users versus 4% in LMWH non-users (OR 2.9 [95% CI 0.5 to 19.4]). The risk for PPH associated with delivery within 24 hours after the last dose of LMWH was 1.2 times higher (95% CI 0.4 to 3.6) compared to a longer interval. Therefore, therapeutic LMWH increases the risk for blood loss after vaginal delivery, but not the risk for severe PPH. The risk for PPH is influenced by the interval between the last dose of LMWH and delivery. Of note in this study, per the institution’s protocol, the anticoagulation was stopped with signs of labor or determination of need for delivery. The risk for blood loss may be mitigated in more planned delivery scenarios.87
CASE 4 CONTINUED
The patient is placed on prophylactic-dose LMWH with good tolerance and delivers at 39 weeks' gestation via caesarian section due to nonprogression of labor. Postpartum she is restarted on prophylactic-dose anticoagulation with LMWH. Two weeks after discharge from the hospital, she presents with right calf pain and mild shortness of breath. On physical exam, her leg circumferences are equal. A D-dimer assay is 3375 ng/mL (normal 0–229). CUS of the right leg shows a complete occlusive DVT of the mid-distal superficial femoral and popliteal veins and partially occlusive acute DVT of the right posterior tibial and peroneal veins. CTPA reveals a right lower lobe PE. Because she had developed VTE despite prophylactic LMWH, her anticoagulation is changed to therapeutic dose. She is treated with anticoagulation with LMWH for a total of 3 months, after which a repeat CUS shows no residual thrombosis.
What is the recommended dosing of heparin and LMWH during pregnancy?
A prospective study of 14 pregnant women receiving UFH prophylaxis found that a prophylactic dose of 5000 units twice a day was inadequate to achieve prophylactic heparin levels in any patient in the second or third trimester.88 Similar to treatment dosage, there is no consensus evidence for prophylactic dosing, and dosage recommendations are based on expert opinion. In a retrospective study of 25 pregnant women on intermediate-dose UFH, the mean UFH dose required to achieve a target anti-factor Xa level of 0.1 to 0.3 units/mL was 236.9 units/kg/day.89 However, the use of anti-factor Xa levels for monitoring is controversial as there is no data to support a difference in outcomes with its use in prophylactic or therapeutic dosing.
The timing of the previous VTE history is important when deciding on the anticoagulant dose in pregnancy. In pregnant women with a VTE that occurred within the previous 4 to 6 weeks, full-dose anticoagulation with LMWH should be considered; an intermediate dose (three-fourths of a therapeutic dose) may be used if the thrombotic episode occurred more than 6 weeks earlier but still within a year. Prophylactic dosing may be sufficient if the episode occurred more than a year earlier.90 A clinical trial (High-Low) is under way to explore the optimal dose of LMWH in pregnant women with prior history of VTE who are not on chronic anticoagulation therapy.91
How is anticoagulation therapy managed in the peripartum period?
Neuraxial anesthesia during active labor while on anticoagulation increases the risk for central nervous system bleeding. Therefore, if spontaneous labor occurs in women on therapeutic dose anticoagulation, neuraxial anesthesia cannot be used. However, in the event of elective induction of labor or caesarean section, neuroaxial anesthesia may be performed 12 hours after the administration of the last prophylactic dose of LMWH or 24 hours after the last therapeutic dose of LMWH. Intravenous UFH should be stopped for 6 hours before induction of labor with a confirmed normal aPTT before placement of neuraxial anesthesia. There is no contraindication for using neuraxial anesthesia during subcutaneous standard UFH at total doses of 10,000 units daily. The risk of spinal hematoma with larger daily subcutaneous doses is unclear; therefore, a documented normal aPTT must be obtained before placement of neuroaxial anesthesia.
Postpartum, reinitiation of prophylactic-dose LMWH should be delayed for at least 12 hours after the removal of an epidural catheter. Therapeutic-dose LMWH should be administered no earlier than 24 hours after neuraxial anesthesia, providing that proper hemostasis is achieved. In the absence of persistent bleeding, if no regional anesthesia was used, LMWH may be resumed 12 hours after delivery.92 Anticoagulation with either LMWH or warfarin is recommended for at least 6 to 12 weeks postpartum.33
COUNSELING
Patients should be advised to manage controllable risk factors, including avoiding prolonged immobilization, avoiding excessive weight gain in pregnancy, and stopping smoking. Periods of immobilization tend to cause reduced blood flow (stasis), which predisposes to thrombosis. In a systematic review of records of all patients with confirmed PE after arrival at Charles de Gaulle airport in Paris during a 13-year period, women had a higher risk of PE after a long-distance flight than men, with an estimated incidence of 0.61 per million passengers versus 0.20, respectively; the incidence reached 7.24 and 2.35 cases, respectively, in passengers traveling more than 10,000 kilometers.93,94
The risk of air travel-related thrombosis in pregnant women is estimated to be between 0.03% and 0.1%. Physicians must decide on an individual basis how to prevent travel-related thrombosis in their pregnant patients. In most passengers, prevention can be limited to encouraging exercise, avoidance of long sleeping periods, and not using a window seat. Women at high risk for VTE, such as women with a prior history of VTE who are not on anticoagulation or women with known asymptomatic thrombophilia or other risk factors for thrombosis such as obesity, may benefit from a short period (1–3 days) of LMWH starting 2 hours before a long-distance flight.95
Activation of the coagulation system has been demonstrated in cigarette smokers.96 Heavy smoking was found to be a significant risk factor for VTE in a cross-sectional analysis of 2404 men and women.97 An increased risk for thrombosis during pregnancy is seen in cigarette smokers15,98 and is enhanced with the concomitant use of illicit drugs.99 Other obstetric complications associated with smoking and illicit drug use during pregnancy include preterm labor, spontaneous abortion, perinatal death, low birth weight, and abruption placenta. The efficacy of nicotine replacement therapy in pregnancy is uncertain.100 Recommendations are to advise patients to stop smoking, obtain psychosocial counseling, and utilize adjunctive therapies, which have been shown to have some effect on abstinence rates.101
CONCLUSION
Women are at increased risk for VTE during pregnancy and the postpartum period. Awareness of risk factors and the signs and symptoms of VTE is paramount. Prompt diagnosis and treatment is mandatory to decrease complications of VTE. LMWH is the mainstay treatment of VTE in pregnancy, as it does not cross the placenta. Both LMWH and warfarin are safe during lactation. Close communication among the patient, obstetrician, hematologist, anesthesiologist, and neonatologist is crucial to optimize the care of these patients.
- Knight M, Nour M, Tuffnell D, et al, eds. on behalf of MBRRACE-UK. Saving lives, improving mothers’ care—surveillance of maternal deaths in the UK 2012-14 and lessons learned to inform maternity care from the UK and Ireland confidential enquiries into maternal deaths and morbidity 2009-14. Oxford: National Perinatal Epidemiology Unit, University of Oxford; 2016: 69–75.
- Berg CJ, Callaghan WM, Syverson C, Henderson Z. Pregnancy-related mortality in the United States, 1998 to 2005. Obstet Gynecol 2010;116:1302–9.
- Salonen Ros H, Lichtenstein P, Bellocco R, et al. Increased risks of circulatory diseases in late pregnancy and puerperium. Epidemiology 2001;12:456–60.
- Heit JA, Kobbervig CE, James AH, et al. Trends in the incidence of venous thromboembolism during pregnancy or postpartum: a 30-year population-based study. Ann Intern Med 2005;143:697–706.
- Greer IA. Thrombosis in pregnancy: updates in diagnosis and management. Hematology Am Soc Hematol Educ Program 2012;2012:203–7.
- Hellgren M. Hemostasis during normal pregnancy and puerperium. Semin Thromb Hemost 2003;29:125–30.
- Brenner B. Haemostatic changes in pregnancy. Thromb Res 2004;114:409–14.
- Chan W-S, Spencer FA, Ginsberg JS. Anatomic distribution of deep vein thrombosis in pregnancy. CMAJ 2010;182:657–60.
- Greer IA. Prevention and management of venous thromboembolism in pregnancy. Clin Chest Med 2003;24:123–37.
- Wik HS, Jacobsen AF, Sandvik L, Sandset PM. Prevalence and predictors for post-thrombotic syndrome 3 to 16 years after pregnancy-related venous thrombosis: a population-based, cross-sectional, case-control study. J Thromb Haemost 2012;10:840–7.
- Pomp ER, Lenselink AM, Rosendaal FR, Doggen CJM. Pregnancy, the postpartum period and prothrombotic defects: risk of venous thrombosis in the MEGA study. J Thromb Haemost 2008;6:632–7.
- Marik PE, Plante LA. Venous thromboembolic disease and pregnancy. N Engl J Med 2008;359:2025–33.
- Jacobsen AF, Skjeldestad FE, Sandset PM. Ante- and postnatal risk factors of venous thrombosis: a hospital-based case-control study. J Thromb Haemost 2008;6:905–12.
- Virkus RA, Løkkegaard E, Lidegaard Ø, et al. Risk factors for venous thromboembolism in 1.3 million pregnancies: a nationwide prospective cohort. PloS One 2014;9:e96495.
- James AH, Jamison MG, Brancazio LR, Myers ER. Venous thromboembolism during pregnancy and the postpartum period: incidence, risk factors, and mortality. Am J Obstet Gynecol 2006;194:1311–5.
- Chan W-S, Lee A, Spencer FA, et al. Predicting deep venous thrombosis in pregnancy: out in “LEFt” field? Ann Intern Med 2009;151:85–92.
- Righini M, Jobic C, Boehlen F, et al. Predicting deep venous thrombosis in pregnancy: external validation of the LEFT clinical prediction rule. Haematologica 2013;98:545–8.
- Chan W-S, Chunilal S, Lee A, et al. A red blood cell agglutination D-dimer test to exclude deep venous thrombosis in pregnancy. Ann Intern Med 2007;147:165–70.
- Nijkeuter M, Ginsberg JS, Huisman MV. Diagnosis of deep vein thrombosis and pulmonary embolism in pregnancy: a systematic review. J Thromb Haemost 2006;4:496–500.
- Chan W-S, Spencer FA, Lee AY, et al. Safety of withholding anticoagulation in pregnant women with suspected deep vein thrombosis following negative serial compression ultrasound and iliac vein imaging. CMAJ 2013;185:E194–200.
- Dronkers CE, Srámek A, Huisman MV, Klok FA. Accurate diagnosis of iliac vein thrombosis in pregnancy with magnetic resonance direct thrombus imaging (MRDTI). BMJ Case Rep 2016;2016. pii: bcr2016218091.
- Ray JG, Vermeulen MJ, Bharatha A, et al. Association between MRI exposure during pregnancy and fetal and childhood outcomes. JAMA 2016;316:952–61.
- Royal College of Obstretricians and Gynaecologists. Thromboembolic disease in pregnancy and the puerperium: acute management. Green-top Guideline No. 37b. London: RCOG; 2015.
- Barritt DW, Jordan SC. Anticoagulant drugs in the treatment of pulmonary embolism. A controlled trial. Lancet 1960;1(7138):1309–12.
- Bourjeily G, Khalil H, Raker C, et al. Outcomes of negative multidetector computed tomography with pulmonary angiography in pregnant women suspected of pulmonary embolism. Lung 2012;190:105–11.
- Deutsch AB, Twitty P, Downes K, Parsons MT. Assessment of the alveolar-arterial oxygen gradient as a screening test for pulmonary embolism in pregnancy. Am J Obstet Gynecol 2010;203:373.e1–4.
- Powrie RO, Larson L, Rosene-Montella K, et al. Alveolar-arterial oxygen gradient in acute pulmonary embolism in pregnancy. Am J Obstet Gynecol 1998;178:394–6.
- Leung AN, Bull TM, Jaeschke R, et al. American Thoracic Society documents: an official American Thoracic Society/Society of Thoracic Radiology clinical practice guideline--evaluation of suspected pulmonary embolism in pregnancy. Radiology 2012;262:635–46.
- Cahill AG, Stout MJ, Macones GA, Bhalla S. Diagnosing pulmonary embolism in pregnancy using computed-tomographic angiography or ventilation-perfusion. Obstet Gynecol 2009;114:124–9.
- Bourjeily G, Paidas M, Khalil H, et al. Pulmonary embolism in pregnancy. Lancet 2010;375(9713):500–12.
- Chan WS, Ray JG, Murray S, et al. Suspected pulmonary embolism in pregnancy: clinical presentation, results of lung scanning, and subsequent maternal and pediatric outcomes. Arch Intern Med 2002;162:1170–5.
- Virkus RA, Løkkegaard ECL, Bergholt T, et al. Venous thromboembolism in pregnant and puerperal women in Denmark 1995-2005. A national cohort study. Thromb Haemost 2011;106:304–9.
- Kamel H, Navi BB, Sriram N, et al. Risk of a thrombotic event after the 6-week postpartum period. N Engl J Med 2014;370:1307–15.
- Ginsberg JS, Hirsh J, Turner DC, et al. Risks to the fetus of anticoagulant therapy during pregnancy. Thromb Haemost 1989;61:197–203.
- Greer IA, Nelson-Piercy C. Low-molecular-weight heparins for thromboprophylaxis and treatment of venous thromboembolism in pregnancy: a systematic review of safety and efficacy. Blood 2005;106:401–7.
- Prandoni P, Carnovali M, Marchiori A, Galilei Investigators. Subcutaneous adjusted-dose unfractionated heparin vs fixed-dose low-molecular-weight heparin in the initial treatment of venous thromboembolism. Arch Intern Med 2004;164:1077–83.
- Robertson L, Jones LE. Fixed dose subcutaneous low molecular weight heparins versus adjusted dose unfractionated heparin for venous thromboembolism. Cochrane Database Syst Rev 2017;(9):eb9;2:CD001100.
- Costantino G, Ceriani E, Rusconi AM, et al. Bleeding risk during treatment of acute thrombotic events with subcutaneous LMWH compared to intravenous unfractionated heparin; a systematic review. PloS One 2012;7:e44553.
- Warkentin TE, Levine MN, Hirsh J, et al. Heparin-induced thrombocytopenia in patients treated with low-molecular-weight heparin or unfractionated heparin. N Engl J Med 1995;332:1330–5.
- Junqueira DRG, Perini E, Penholati RRM, Carvalho MG. Unfractionated heparin versus low molecular weight heparin for avoiding heparin-induced thrombocytopenia in postoperative patients. Cochrane Database Syst Rev 2012;(9):CD007557.
- Bates SM, Greer IA, Middeldorp S, et al. VTE, thrombophilia, antithrombotic therapy, and pregnancy. Chest 2012;141:e691S–e736S.
- Clark NP, Delate T, Witt DM, et al. A descriptive evaluation of unfractionated heparin use during pregnancy. J Thromb Thrombolysis 2009;27:267–73.
- Clark NP, Delate T, Cleary SJ, Witt DM. Analysis of unfractionated heparin dose requirements to target therapeutic anti-Xa intensity during pregnancy. Thromb Res 2010;125:402–5.
- Jacobsen AF, Qvigstad E, Sandset PM. Low molecular weight heparin (dalteparin) for the treatment of venous thromboembolism in pregnancy. BJOG 2003;110:139–44.
- Walfisch A, Koren G. The “warfarin window” in pregnancy: the importance of half-life. J Obstet Gynaecol Can 2010;32:988–9.
- Bates SM, Ginsberg JS. Anticoagulants in pregnancy: fetal effects. Baillières Clin Obstet Gynaecol 1997;11:479–88.
- Stevenson RE, Burton OM, Ferlauto GJ, Taylor HA. Hazards of oral anticoagulants during pregnancy. JAMA 1980;243:1549–51.
- Ginsberg JS, Hirsh J. Anticoagulants during pregnancy. Annu Rev Med 1989;40:79–86.
- Wong V, Cheng CH, Chan KC. Fetal and neonatal outcome of exposure to anticoagulants during pregnancy. Am J Med Genet 1993;45:17–21.
- Blickstein D, Blickstein I. The risk of fetal loss associated with Warfarin anticoagulation. Int J Gynaecol Obstet 2002;78:221–5.
- Burnett AE, Mahan CE, Vazquez SR, et al. Guidance for the practical management of the direct oral anticoagulants (DOACs) in VTE treatment. J Thromb Thrombolysis 2016;41:206–32.
- Bapat P, Pinto LSR, Lubetsky A, et al. Examining the transplacental passage of apixaban using the dually perfused human placenta. J Thromb Haemost 2016;14:1436–41.
- Bapat P, Pinto LSR, Lubetsky A, et al. Rivaroxaban transfer across the dually perfused isolated human placental cotyledon. Am J Obstet Gynecol 2015;213:710.e1–6.
- Bapat P, Kedar R, Lubetsky A, et al. Transfer of dabigatran and dabigatran etexilate mesylate across the dually perfused human placenta. Obstet Gynecol 2014;123:1256–61.
- Savaysa [package insert]. Parsippany (NJ): Daiichi Sankyo, Inc; 2015.
- Filipecki S, Tomkowski W, Hajduk B, et al. [Outcome of patients with clinically acute massive pulmonary embolism]. Pneumonol Alergol Pol 1994;62:132–7.
- Holden EL, Ranu H, Sheth A, et al. Thrombolysis for massive pulmonary embolism in pregnancy--a report of three cases and follow up over a two year period. Thromb Res 2011;127:58–9.
- te Raa GD, Ribbert LS, Snijder RJ, Biesma DH. Treatment options in massive pulmonary embolism during pregnancy; a case-report and review of literature. Thromb Res 2009;124:1–5.
- Leonhardt G, Gaul C, Nietsch HH, et al. Thrombolytic therapy in pregnancy. J Thromb Thrombolysis 2006;21:271–6.
- Colombier S, Niclauss L. Successful surgical pulmonary embolectomy for massive perinatal embolism after emergency cesarean section. Ann Vasc Surg 2015;29:1452.e1–4.
- British Committee for Standards in Haematology Writing Group, Baglin TP, Brush J, Streiff M. Guidelines on use of vena cava filters. Br J Haematol 2006;134:590–5.
- Harris SA, Velineni R, Davies AH. Inferior vena cava filters in pregnancy: a systematic review. J Vasc Interv Radiol 2016;27:354–360.
- Pabinger I, Grafenhofer H, Kyrle PA, et al. Temporary increase in the risk for recurrence during pregnancy in women with a history of venous thromboembolism. Blood 2002;100:1060–2.
- Pabinger I, Grafenhofer H, Kaider A, et al. Risk of pregnancy-associated recurrent venous thromboembolism in women with a history of venous thrombosis. J Thromb Haemost 2005;3:949–54.
- Brill-Edwards P, Ginsberg JS, Gent M, et al. Safety of withholding heparin in pregnant women with a history of venous thromboembolism. Recurrence of Clot in This Pregnancy Study Group. N Engl J Med 2000;343:1439–44.
- De Stefano V, Martinelli I, Rossi E, et al. The risk of recurrent venous thromboembolism in pregnancy and puerperium without antithrombotic prophylaxis. Br J Haematol 2006;135:386–91.
- De Stefano V, Martinelli I, Rossi E, et al. The risk of recurrent venous thromboembolism in pregnancy and puerperium without antithrombotic prophylaxis. Br J Haematol 2006;135:386–91.
- Lim W, Eikelboom JW, Ginsberg JS. Inherited thrombophilia and pregnancy associated venous thromboembolism. BMJ 2007;334:1318–21.
- Tormene D, Simioni P, Prandoni P, et al. Factor V Leiden mutation and the risk of venous thromboembolism in pregnant women. Haematologica 2001;86:1305–9.
- Baglin T, Gray E, Greaves M, et al. Clinical guidelines for testing for heritable thrombophilia. Br J Haematol 2010;149:209–20.
- Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006;4:295–306.
- Pengo V, Tripodi A, Reber G, et al. Update of the guidelines for lupus anticoagulant detection. J Thromb Haemost 2009;7:1737–40.
- Robertson L, Wu O, Langhorne P, et al. Thrombophilia in pregnancy: a systematic review. Br J Haematol 2006;132:171–96.
- American College of Obstetricians and Gynecologists Women’s Health Care Physicians. ACOG Practice Bulletin No. 138: Inherited thrombophilias in pregnancy. Obstet Gynecol 2013;122:706–17.
- Mak A, Cheung MW, Cheak AA, Ho RC. Combination of heparin and aspirin is superior to aspirin alone in enhancing live births in patients with recurrent pregnancy loss and positive anti-phospholipid antibodies: a meta-analysis of randomized controlled trials and meta-regression. Rheumatology (Oxf) 2010;49:281–8.
- Laskin CA, Spitzer KA, Clark CA, et al. Low molecular weight heparin and aspirin for recurrent pregnancy loss: results from the randomized, controlled HepASA Trial. J Rheumatol 2009;36:279–87.
- Farquharson RG, Quenby S, Greaves M. Antiphospholipid syndrome in pregnancy: a randomized, controlled trial of treatment. Obstet Gynecol 2002;100:408–13.
- Bates SM, Jaeschke R, Stevens SM, et al. Diagnosis of DVT: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141(2 Suppl):e351S–418S.
- Lubbe WF, Butler WS, Palmer SJ, Liggins GC. Fetal survival after prednisone suppression of maternal lupus-anticoagulant. Lancet 1983;1(8338):1361–3.
- Lockshin MD, Druzin ML, Qamar T. Prednisone does not prevent recurrent fetal death in women with antiphospholipid antibody. Am J Obstet Gynecol 1989;160:439–43.
- Silver RK, MacGregor SN, Sholl JS, et al. Comparative trial of prednisone plus aspirin versus aspirin alone in the treatment of anticardiolipin antibody-positive obstetric patients. Am J Obstet Gynecol 1993;169:1411–7.
- Cowchock FS, Reece EA, Balaban D, et al. Repeated fetal losses associated with antiphospholipid antibodies: a collaborative randomized trial comparing prednisone with low-dose heparin treatment. Am J Obstet Gynecol 1992;166:1318–23.
- Laskin CA, Bombardier C, Hannah ME, et al. Prednisone and aspirin in women with autoantibodies and unexplained recurrent fetal loss. N Engl J Med 1997;337:148–53.
- Dendrinos S, Sakkas E, Makrakis E. Low-molecular-weight heparin versus intravenous immunoglobulin for recurrent abortion associated with antiphospholipid antibody syndrome. Int J Gynaecol Obstet 2009;104:223–5.
- Cohen H, Arachchillage DRJ, Beyer-Westendorf J, et al. Direct oral anticoagulants and women. Semin Thromb Hemost 2016;42:789–97.
- Bates SM, Middeldorp S, Rodger M, et al. Guidance for the treatment and prevention of obstetric-associated venous thromboembolism. J Thromb Thrombolysis 2016;41:92–128.
- Knol HM, Schultinge L, Veeger NJ, et al. The risk of postpartum hemorrhage in women using high dose of low-molecular-weight heparins during pregnancy. Thromb Res 2012;130:334–8.
- Barbour LA, Smith JM, Marlar RA. Heparin levels to guide thromboembolism prophylaxis during pregnancy. Am J Obstet Gynecol 1995;173:1869–73.
- Bergqvist A, Bergqvist D, Lindhagen A, Mätzsch T. Late symptoms after pregnancy-related deep vein thrombosis. Br J Obstet Gynaecol 1990;97:338–41.
- Rodger M. Evidence base for the management of venous thromboembolism in pregnancy. Hematology Am Soc Hematol Educ Program. 2010;2010:173–80.
- Bleker SM, Buchmüller A, Chauleur C, et al. Low-molecular-weight heparin to prevent recurrent venous thromboembolism in pregnancy: Rationale and design of the Highlow study, a randomised trial of two doses. Thromb Res 2016;144:62–8.
- Horlocker TT, Wedel DJ, Rowlingson JC, et al. Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy: American Society of Regional Anesthesia and Pain Medicine evidence-based guidelines (third edition). Reg Anesth Pain Med 2010;35:64–101.
- Lapostolle F, Surget V, Borron SW, et al. Severe pulmonary embolism associated with air travel. N Engl J Med 2001;345:779–83.
- Lapostolle F, Le Toumelin P, Chassery C, et al. Gender as a risk factor for pulmonary embolism after air travel. Thromb Haemost 2009;102:1165–8.
- Cannegieter SC, Rosendaal FR. Pregnancy and travel-related thromboembolism. Thromb Res 2013;131 Suppl 1:S55–58.
- Miller GJ, Bauer KA, Cooper JA, Rosenberg RD. Activation of the coagulant pathway in cigarette smokers. Thromb Haemost 1998;79:549–53.
- Golomb BA, Chan VT, Denenberg JO, et al. Risk marker associations with venous thrombotic events: a cross-sectional analysis. BMJ Open 2014;4:e003208.
- Lindqvist P, Dahlbäck B, Marŝál K. Thrombotic risk during pregnancy: a population study. Obstet Gynecol 1999;94:595–9.
- Black M, Bhattacharya S, Fairley T, et al. Outcomes of pregnancy in women using illegal drugs and in women who smoke cigarettes. Acta Obstet Gynecol Scand 2013;92:47–52.
- Mendelsohn C, Gould GS, Oncken C. Management of smoking in pregnant women. Aust Fam Physician 2014;43:46–51.
- Chamberlain C, O’Mara-Eves A, Oliver S, et al. Psychosocial interventions for supporting women to stop smoking in pregnancy. Cochrane Database Syst Rev 2013;10:CD001055.
INTRODUCTION
Venous thromboembolism (VTE), comprising deep vein thrombosis (DVT) and pulmonary embolism (PE), is a leading nonobstetric cause of maternal death in the United States and in developed countries.1,2 During pregnancy, the risk for VTE increases four- to six-fold, and although the risk is present throughout pregnancy, the mother is at highest risk immediately postpartum.3–5
VTE risk is increased due to physiologic and anatomic changes that occur in pregnancy. These changes include hypercoagulability, progesterone-induced venous stasis, decreased venous outflow, compression of the inferior vena cava and pelvic veins by the expanding uterus, and decreased mobility. The hypercoagulability of pregnancy is due to increased levels of coagulation factors I (fibrinogen), VII, VIII, and X, and von Willebrand factor; decreased free protein S, a natural anticoagulant; acquired resistance to activated protein C; and decreased fibrinolysis due to increased levels of plasminogen activator inhibitor-1 and -2.6,7 These changes confer increased hemostasis to the mother for delivery but also place her at higher risk for thrombosis.
A review of the literature found that more than 70% of pregnancy-associated DVTs are located in the ileofemoral region, as compared with approximately 9% in non-pregnant patients.8 The proximal location is associated with a higher risk for post-thrombotic syndrome and embolization as compared with calf DVTs.9 Proximal postnatal thrombosis, smoking, and older age are independent predictors of the development of post-thrombotic syndrome.10
RISK FACTORS
Clinical risk factors that increase the risk for VTE during pregnancy include a prior history of estrogen-related or unprovoked VTE, being a carrier of severe inherited thrombophilia (homozygotes for factor V Leiden or factor II G20210A variants, double heterozygotes, or persons with antithrombin, protein C, or protein S deficiencies), and the presence of antiphospholipid (aPL) antibodies.11 Women with systemic lupus erythematosus, diabetes, sickle cell disease, and heart disease also have a high risk for VTE during pregnancy.12 Other risk factors predisposing to thrombosis include black ethnicity, smoking, operative procedures, conception after assisted reproductive techniques, high body mass index, antepartum immobilization, severe preeclampsia, advanced age and parity, and a family history of VTE.13 A prospective cohort study of 1,297,037 pregnancies and related puerperium identified the following risk factors for thrombosis: hospitalization, infection, hyperemesis, multiple pregnancies, preeclampsia, obesity, cesarean section, major postpartum hemorrhage, intrauterine growth restriction, and fetal death.14 Risk factors identified in an Agency for Healthcare Research and Quality study include: age 35 or older, black ethnicity, lupus, sickle cell disease, heart disease, postpartum infection, and transfusion.15 The combination of more than one risk factor increases the risk for VTE. All these factors have to be considered when deciding on prophylactic or therapeutic anticoagulation therapy in pregnancy. In addition, the risks of anticoagulation, including bruising, bleeding, and other side effects (eg, reduced bone mineral density with therapeutic-dose unfractionated heparin), allergic reactions, and rarely thrombocytopenia, must be considered.
EVALUATION AND DIAGNOSIS
CASE PRESENTATION I
A 31-year-old woman G1P0 at 10 weeks’ gestation with no personal or family history of thrombosis presents with acute onset of shortness of breath and left-sided chest pain that awoke her the morning of presentation. Her vital signs are significant for a heart rate of 106 beats/min, respiration rate of 22 breaths/min, blood pressure of 105/76 mm Hg, and pulse oximetry of 98% on room air. The patient denies previous exposure to oral contraceptives. She does not smoke. She reports that she had noticed left calf pain and swelling, which worsened with walking after a 4-hour drive 2 days prior.
What is the approach to diagnosis of thromboembolism in pregnant patients?
DEEP VEIN THROMBOSIS
Although a clinical diagnosis of DVT in pregnancy is unreliable, a history and physical examination are necessary to exclude other diagnoses and to assess the likelihood of thrombosis. Unfortunately, studies of the accuracy of history and physical examination for detecting DVT and PE have not included pregnant patients. In most pregnant patients with clinically suspected DVT, the diagnosis is not confirmed. Other causes of leg pain and swelling are not uncommon during pregnancy and include cellulitis, ruptured Baker’s cyst, or muscular pain.
A cross-sectional study described the derivation of the LEFt clinical decision rule, which relies on 3 variables in pregnant women with suspected DVT: left leg presentation (L), ≥ 2 cm calf circumference difference (E for edema), and first trimester presentation (Ft). If none of these variables is present, the negative predictive value is 100%.16 A validation study suggested that a negative LEFt rule accurately identifies pregnant women in whom the risk for confirmed DVT appears to be very low. The rule should not be used as an individual test for excluding DVT during pregnancy, but could be applied in a diagnostic approach in association with D-dimer measurement and compression ultrasonography (CUS); however, it has not been prospectively validated for safety and efficacy.17 In a study of 149 consecutive pregnant women with suspected DVT, a whole-blood agglutination D-dimer had a sensitivity of 100% and specificity of 60%.18 A 2006 systematic review found only 4 diagnostic studies of VTE in pregnancy in the literature. One of these studies showed that a combination of a negative CUS and normal D-dimer can accurately exclude DVT.19
Serial CUS is necessary for pregnant women with a high clinical suspicion of DVT but a negative initial investigation. In a study of 221 pregnant women in whom DVT was clinically suspected, 16 women (7.2%) were diagnosed with DVT by initial CUS, and none were diagnosed with DVT onserial testing.20 During follow-up (≥ 3 months), 6 of the 205 women with normal serial CUS results presented with symptoms of DVT, PE, or both, and 1 of them was diagnosed with DVT and PE. The sensitivity of serial CUS with Doppler imaging was 94.1% (95% confidence interval [CI] 69.2% to 99.7%), and the negative predictive value was 99.5% (95% CI 96.9% to 100%).20 All ultrasounds undertaken for investigation of pregnancy-associated DVT should include imaging of the iliac veins if there is a high index of suspicion and the CUS is negative for femoral DVT. Serial CUS with Doppler imaging of the iliac vein performed over a 7-day period excludes DVT in symptomatic pregnant women.20 Repeat CUS may be done 2 to 4 days and 6 to 8 days after the initial scan.
Ileofemoral vein thrombosis accounts for approximately 90% of proximal thromboses in pregnancy, occurring most often in the left lower extremity.20 The incidence of isolated iliac vein thrombosis in pregnancy is low, but when it does occur, delay in diagnosis can lead to significant morbidity. Therefore, for women with suspected isolated iliac vein thrombosis in whom CUS is negative or nondiagnostic, magnetic resonance direct thrombus imaging (MRDTI) should be performed.21 Patients with iliac vein thrombosis may present with unexplained inguinal, pelvic, or abdominal pain, which may be accompanied by back pain, and they usually present with swelling of the entire leg. MRDTI does not require gadolinium contrast and its accuracy appears to be similar to that of venography for iliac vein thrombi in the nonpregnant population.21 Exposure to gadolinium during pregnancy is associated with an increased risk for rheumatologic, inflammatory, or infiltrative skin conditions and stillbirth or neonatal death.22
Ovarian vein thrombosis is a rare but serious diagnosis. It occurs mostly in the postpartum period, mainly after cesarean delivery, and usually affects the right ovarian vein. The diagnosis is confirmed by ultrasound, computed tomography (CT), or magnetic resonance imaging.23
PULMONARY EMBOLISM
PE is more difficult to diagnose than DVT, particularly because clinical signs of PE are unreliable in the pregnant patient. The mortality rate of untreated PE is high, ranging from 18% to 38%, and approximately one-third of patients with untreated thromboembolic disease develop recurrent embolism.24 Studies have reported a PE prevalence between 1.4% and 4.2% in pregnant women with suspected clinical diagnosis of PE.25
The clinical presentation of PE and associated laboratory testing results may be subtler in pregnant than in nonpregnant patients. Arterial blood gases (ABG) may show hypoxemia or hypocapnia. The ABG in pregnancy has a sensitivity of 76.9%, specificity of 20.2%, and negative and positive predictive values of 80% and 11.5% for PE, respectively.26 The alveolar-arterial oxygen gradient is a poor screening test for PE during pregnancy and postpartum. A retrospective chart review of 17 pregnant women with documented PE showed that 58% had normal alveolar-arterial gradients.27 Therefore, in a pregnant woman with a history suspicious for PE, objective imaging studies should be performed even if the patient has normal ABG.
The 2011 guidelines from the American Thoracic Society (ATS) and the Society of Thoracic Radiology (STR) recommend against using D-dimer to diagnose PE in pregnancy.28 In addition, lower extremity CUS should only be performed as the first diagnostic imaging procedure if the patient has signs or symptoms of DVT. Instead, the ATS/STR guidelines recommend a plain radiograph of the chest as the first imaging test. If the chest radiograph is normal, a ventilation/perfusion scan (V/Q) scan is preferred over CT pulmonary angiography (CTPA). Diagnostic accuracy of the V/Q scan may be superior to CTPA in pregnancy, and it is preferable because of the lower prevalence of indeterminate V/Q scan in pregnant women.29 Moreover, there is lower radiation exposure to the maternal breast and lung tissue with a V/Q scan than with CTPA. CTPA confers lower fetal radiation doses than V/Q scans (0.03–0.66 mGy versus 0.32–0.74 mGy, respectively) but higher total body maternal radiation (4–16 mSv versus 1–2.5 mSv).30 A quantitative approach to lung scan interpretation, based on the distribution histogram of V/Q ratios, may be helpful in categorizing patients with suspected PE.28 A study of 121 suspected episodes of PE in 120 pregnant women showed that 104 women with normal or nondiagnostic scans did not develop subsequent episodes of VTE during a mean follow-up period of 20 months.31
If the baseline chest radiograph is abnormal in a pregnant woman with clinical suspicion of PE, a CTPA should be performed. As noted, fetal radiation doses for CTPA examinations in which the fetus is not directly imaged are minimal. If CTPA is recommended for the diagnosis of PE, the patient should be informed that radiation to the breast may increase her baseline risk for breast cancer. The ATS guidelines state that “given the lack of evidence documenting clear superiority of any one diagnostic test, the values and preferences of a patient and her physician likely will and should determine the final choice and sequence of tests performed.”28
CASE I CONTINUED
Upon presentation to the emergency department, the circumference of the patient’s left leg is not significantly greater than that of her right leg, and her leg pain has resolved. Bilateral CUS is negative for proximal or distal DVT. Chest radiograph shows an opacification of her left lower lobe. CTPA shows bilateral segmental and subsegmental lower lobe pulmonary emboli.
How does risk for VTE change throughout pregnancy?
Women are at increased risk for VTE throughout the entire pregnancy, starting from conception, but mainly during the postpartum period. A Danish historical controlled cohort study of 819,751 pregnant women (ages 15–49 years) over a 10-year period identified 727 women with VTE. The absolute risk for VTE per 10,000 pregnancy-years increased from 4.1 (95% CI 3.2 to 5.2) during weeks 1 to 11 to 59.0 (95% CI 46.1 to 76.4) in week 40 and decreased in the postpartum period from 60 (95% CI 47.2 to 76.4) during the first week after birth to 2.1 during weeks 9–12 after birth (95% CI 1.1 to 4.2).32 This study showed that the risk of VTE increases throughout pregnancy and reaches its maximum during the peripartum period and is not significantly increased after 6 weeks post-delivery. In a retrospective cross-over cohort study of 1,687,930 women in California who delivered their first newborn, an elevated risk of VTE persisted until at least 12 weeks after delivery. However, the absolute increase in risk after 6 weeks postpartum was low.33
CASE 1 CONCLUSION
The patient is started on anticoagulation therapy and carefully monitored during the remainder of the pregnancy and postpartum period. Anticoagulation is discontinued 6 weeks after delivery.
TREATMENT
ANTICOAGULATION THERAPY
The treatment of VTE can be lifesaving. In a study comparing 35 patients with PE randomly assigned to treatment with anticoagulants versus no treatment, 5 of 19 patients in the untreated group died from PE and an additional 5 had nonfatal recurrences, as compared with none in the treated group.24 Unfractionated heparin (UFH) and low-molecular-weight heparin (LMWH) are both safe and effective anticoagulants during pregnancy as neither crosses the placenta. In a review of 186 reports of fetal and infant outcomes following anticoagulant therapy during pregnancy in 1325 pregnancies, outcomes in UFH-treated patients were similar to those in the normal population after excluding pregnancies with comorbid conditions independently associated with adverse outcomes.34 A 2005 systematic review of LMWH for prophylaxis and treatment of VTE during pregnancy included 64 studies of 277 pregnancies. There were no maternal deaths, live births resulted from 94.7% of the pregnancies, VTE or arterial thrombosis occurred in 0.86%, and significant bleeding occurred in 1.98%.35
The standard UFH regimen is an initial bolus of 5000 units subcutaneously and 17,500 units every 12 hours, with dose adjustment made based on a mid-interval activated partial thromboplastin time (aPTT).36 Although still controversial, it has been suggested that the anti-Xa assay with a mid-dosing interval target of 0.3 to 0.7 U/mL is a more reliable measure of therapeutic UFH activity than the aPTT, as the aPTT response is suppressed due to a pregnancy-related increase in factor VIII. LMWH is dosed based on weight; regimens are enoxaparin 1 mg/kg subcutaneously twice daily or 1.5 mg/kg subcutaneously once daily, and dalteparin 100 units/kg every 12 hours or 150 units/kg daily.
A 2017 Cochrane review of the effect of LMWH compared with UFH for the treatment of VTE in the nonpregnant setting included 23 studies with 9587 patients. Thrombotic complications (odds ratio [OR] 0.70 [CI 0.57 to 0.85]) and major hemorrhage (OR 0.58 [CI 0.40 to 0.83]) were lower in patients receiving LMWH, with a trend toward lower mortality.37 In addition, the incidence of bleeding complications in patients treated with subcutaneous LMWH versus intravenous heparin was compared in a 2012 systematic review of 27 randomized controlled trials with a total of 28,637 patients. In patients treated with LMWH, there was a nonstatistically significant lower incidence of major bleeding events (OR 0.79 [95% CI 0.60 to 1.04]) and a statistically significant reduction in bleeding risk (OR 0.68 [95% CI 0.47 to 1.00]) compared to patients treated with UFH.38 Additionally, a trial comparing the use of standard UFH versus LMWH found a significantly lower incidence of thrombocytopenia in patients treated with LMWH.39,40 Overall, LMWH is more effective at decreasing both thrombotic and bleeding complications, and the risk for osteoporosis is lower with LMWH. Based on these results, the American College of Chest Physicians (ACCP) recommends LMWH as the first-line treatment for VTE in pregnancy.41
In specific clinical situations, such as patients with renal dysfunction with creatinine clearance (CrCl) less than 30 mL/min, UFH is indicated. In a study of 103 pregnancies in 93 women given anti-coagulation during pregnancy, 89.3% received UFH. There were no maternal deaths, and fetal demise occurred in 8 pregnancies (7.8%) at a median of 14 weeks’ gestation. There were 2 episodes of PE (1.9%) and 2 major bleeding events requiring transfusion (1.9%).42 UFH costs much less than LMWH, and therefore UFH remains an important, inexpensive, and efficacious anticoagulant option for pregnant women who require anticoagulation and cannot afford LMWH.43
Due to the physiologic changes associated with pregnancy, LMWH and UFH dosages may need to be adjusted. An observational study of 20 pregnant women with acute VTE found no recurrent VTE or major bleeding after treatment with dalteparin. Dalteparin doses approximately 10% to 20% higher than those recommended in nonpregnant women were required to reach therapeutic target anti-Xa activity.44
Caution Regarding Oral Anticoagulants
Due to its teratogenicity, warfarin is not a first-line anticoagulation option. It is strictly contraindicated during the first trimester during organogenesis, and its use during pregnancy is restricted to women with mechanical heart valves. Warfarin crosses the placenta and has been associated with nasal hypoplasia, stippled epiphyses, and growth restriction, particularly between 6 to 9 weeks’ gestation. Every effort should be made to substitute UFH or LMWH for warfarin between 6 and 12 weeks of gestation. The bridging process should begin as early in the gestational age as possible due to the long half-life of warfarin.45 When used later in gestation, warfarin has been associated with fetal hemorrhage and central nervous system abnormalities. Other complications from use during the second and third trimesters include microcephaly, blindness, deafness, and fetal growth restriction.46,47 Its use also increases the risk for abortion and fetal death in utero.48–50
The direct oral anticoagulants (DOACs) are not approved for use in pregnancy. Although there are limited anecdotal reports of DOAC use in pregnancy,51 there is preclinical evidence of placental transfer with the DOACs rivaroxaban and apixaban (direct Xa inhibitors) and the oral thrombin inhibitor dabigatran, thus increasing the risk to the fetus.52–54 Edoxaban, another direct Xa inhibitor, should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. It should be discontinued in nursing mothers.55
THROMBOLYSIS
Fetal as well as maternal survival is dependent on adequate maternal perfusion and oxygenation. The risk of death from PE is significant, with a cross-sectional study of 58 patients with acute, massive PE showing a 55% mortality rate.56 Thus, pregnancy is not an absolute contraindication to mechanical or systemic (recombinant tissue plasminogen activator or streptokinase) thrombolysis in an unstable patient at high risk for death.57–59 There are no major studies of this approach, although a small review of 13 cases using systemic thrombolysis showed no increased risk of maternal mortality.58 Thrombolysis should be considered for appropriate indications in pregnant patients as it would be in nonpregnant patients. However, caution is required when drawing conclusions regarding maternal and fetal safety, given the lack of controlled clinical trials including pregnant women.
SURGICAL PULMONARY EMBOLECTOMY
Surgical pulmonary embolectomy is an important therapeutic and potentially life-saving option in women presenting with massive PE in the immediate postpartum period. Because of the risk of massive uterine bleeding immediately postpartum, thrombolytic therapy should not be used.60
INFERIOR VENA CAVA FILTER
Placement of an inferior vena cava (IVC) filter is indicated in patients who have an acute VTE with absolute contraindications for anticoagulation. In addition, it can be considered in patients with extensive ileofemoral venous thrombosis within 2 weeks prior to expected delivery.61 In a systematic review of 44 studies of IVC filters placed in pregnant patients, the IVC filter complication rate was 8.87% and the failure-to-retrieve rate was 11.25%.62 The complication rate is similar to that found in the nonpregnant population. Thus, IVC filters may be used when appropriately indicated and should be removed as soon as clinically feasible.
RECURRENT THROMBOSIS AND THROMBOPHILIAS
CASE PRESENTATION 2
A 34-year-old pregnant woman G1P0 at 38 weeks’ gestation presents with a painful, swollen left calf that is associated with difficulty on walking; the circumference of the left calf is 2 cm greater than that of the right. She has no shortness of breath or chest pain. She has a prior history of distal right lower extremity DVT while on combined oral contraceptives. Her mother also has a history of DVT while bedbound during a prolonged hospitalization at an older age. CUS is negative, and the patient is discharged home. However, 24 hours later she returns to the hospital with worsening swelling and pain in her left leg. Magnetic resonance venography demonstrates a large left external iliac and common iliac DVT. She is admitted and is started on UFH, and a retrievable IVC filter is placed in anticipation of delivery.
What is the risk for VTE recurrence during pregnancy?
A personal and family history of VTE should be obtained when evaluating pregnant patients. A retrospective study of 109 women with prior history of VTE showed recurrence rates per patient-year of 10.9% during pregnancy and 3.7% in the nonpregnant period; the relative risk of recurrent VTE during pregnancy was 3.5 (95% CI 1.6 to 7.8).63 Two large European retrospective cohort studies of VTE in pregnancy showed that the recurrence rate of VTE in women with a history of thrombosis is around 6% during pregnancy, equally distributed among trimesters. The highest incidence of recurrence was in the postpartum period, ranging from 8.3% to 10%.64 The recurrence risk during pregnancy in women with a history of a single episode of VTE was 2.4% antepartum (95% CI 0.2% to 6.9%).65 These risks may be lower in women without thrombophilia or with a temporary risk factor associated with their previous thromboembolic event.65 Recurrence risk is higher if the previous VTE was estrogen-related, either due to pregnancy or through hormonal contraception (10%), than if the previous VTE was non-estrogen-related (2.7%).64,66
The timing of the case patient’s presentation is consistent with reports of increased risk of VTE during the peripartum period. Her prior history of estrogen-related DVT is concerning for a risk of recurrence, particularly during pregnancy. A retrospective cohort study of 1104 women with previous VTE, 88 of whom became pregnant without receiving thromboprophylaxis, showed that the overall rate of VTE recurrence was 5.8% (95% CI 3.0% to 10.6%) and 8.3% (95% CI 4.5% to 14.6%) during pregnancy and postpartum, respectively. The risk of VTE recurrence was absent if the first VTE was related to a transient risk factor other than pregnancy, postpartum period, or hormonal contraception.67 However, the recurrence rate of VTE in women with prior unprovoked VTE and/or thrombophilia has been reported as 5.9% (95% CI 1.2% to 16.2%).65 The presence of an underlying hypercoagulable state can increase the recurrence risk by 25% to 50%, depending on the disorder.68 A retrospective cohort study of 270 pregnancies in 105 carriers of factor V Leiden, identified because of a symptomatic relative with the factor V Leiden mutation, found a VTE risk (mostly in the postpartum period) of 6.4% for heterozygous women, 16.7% for homozygous women, 20% for double heterozygous women, and 1.2% for noncarriers.69
Should the patient be screened for a thrombophilia disorder?
Half of all index thromboses in patients with thrombophilia occur in association with an additional risk factor. In women of child-bearing age, pregnancy, the postpartum period, and the use of combined hormonal contraception are all risk factors for VTE. A 2010 guideline from the British hematology community recommended testing for thrombophilia in women with prior VTE secondary to a minor provoking factor before or during pregnancy, but not testing women with unprovoked VTE (who would receive prophylaxis regardless) or those with VTE secondary to a major provoking factor (who would not require prophylaxis).70 Indications to screen for aPL antibodies include: women with (1) 3 unexplained recurrent first-trimester pregnancy losses or 1 second or third trimester fetal loss of morphologically normal fetuses; (2) severe preeclampsia; (3) intrauterine growth restriction; or (4) premature labor (< 34 weeks’ gestation).71,72
CASE 2 CONCLUSION
The patient is subsequently screened for inherited thrombophilia disorders and is found to be heterozygous for factor V Leiden.
CASE PRESENTATION 3
A 25-year-old woman is diagnosed with antiphospholipid syndrome (APS) during her second pregnancy when she experiences fetal loss during her second trimester. Pathologic examination of the placenta reveals infarcts. Laboratory evaluation reveals positive high-titer anticardiolipin and anti-beta-2 glycoprotein 1 antibodies (IgG isotype) and lupus anticoagulant on 2 separate occasions 12 weeks apart. In a subsequent pregnancy, she is started on prophylactic LMWH and daily low-dose aspirin (81 mg). At 36 weeks’ gestation, she presents with a blood pressure of 210/104 mm Hg and a platelet count of 94,000 cells/µL. She is diagnosed with preeclampsia and hemolysis, elevated liver enzymes, and low platelets (HELLP) syndrome and is induced for early delivery. About 2 weeks after vaginal delivery, she notices shortness of breath and chest pain. A CTPA demonstrates a right lower lobe lobar defect consistent with a PE. Her anticoagulation is increased to therapeutic dosage LMWH.
To what extent does thrombophilia increase the risk for VTE in pregnancy?
Approximately 50% of pregnancy-related VTEs are associated with inherited thrombophilia. A systematic review of 79 studies, in which 9 studies (n = 2526 patients) assessed the risk of VTE associated with inherited thrombophilia in pregnancy, revealed that the odds ratio for individuals with thrombophilia to develop VTE ranged from 0.74 to 34.40.73 Although women with thrombophilia have an increased relative risk of developing VTE in pregnancy, the absolute risk of VTE remains low (Table 1).41,73,74

How is APS managed in pregnant patients?
Women with history of recurrent early pregnancy loss (< 10 weeks’ gestation) related to the presence of aPL antibodies are managed with low-dose aspirin and prophylactic-dose UFH or LMWH. This treatment increases the rate of subsequent successful pregnancy outcomes and reduces the risk for thrombosis. A 2010 systematic review and meta-analysis of UFH plus low-dose aspirin compared with low-dose aspirin alone in patients with APS and recurrent pregnancy loss included 5 trials and 334 patients. Patients receiving dual therapy had higher rates of live births (74.3%; relative risk [RR] 1.30 [CI 1.04 to 1.63]) compared to the aspirin-only group (55.8%).75 A 2009 randomized controlled trial compared low-dose aspirin to low-dose aspirin plus LMWH in women with recurrent pregnancy loss and either aPL antibodies, antinuclear antibody, or inherited thrombophilia. The study was stopped early after 4 years and found no difference in rates of live births between the groups (77.8% versus 79.1%).76 However, a randomized case-control trial of women with aPL antibodies and recurrent miscarriage found a 72% live birth rate in 47 women randomly assigned to low-dose aspirin and LMWH.77 A 2012 guideline from the American College of Chest Physicians (ACCP) recommends that women with aPL antibodies with a history of 3 or more pregnancy losses receive low-dose aspirin plus prophylactic-dose LMWH or UFH.78 A 2014 systematic review and meta-analysis showed that the combination of low-dose aspirin and UFH resulted in a higher live-birth rate than aspirin alone in 803 women with APS (RR 1.54 [95% CI 1.25 to 1.89]).79 Further large randomized controlled trials are needed to confirm optimal management of recurrent miscarriage and aPL antibodies.
The addition of prednisone to aspirin, heparin, or both has shown no benefits in pregnant women with aPL antibodies. Indeed, prolonged use of steroids may cause serious pregnancy complications, such as prematurity and hypertension.80–83 Intravenous infusions of immunoglobulin (IVIG) have not been shown to be superior to heparin and aspirin. This finding was confirmed in a multicenter clinical trial that tested the effects of IVIG compared with LMWH plus low-dose aspirin for the treatment of women with aPL antibodies and recurrent miscarriage. The rate of live-birth was 72.5% in the group treated with heparin plus low-dose aspirin compared with 39.5% in the IVIG group.84
Preeclampsia and HELLP syndrome complicated the case patient’s pregnancy even though she was being treated with prophylactic-dose LMWH and low-dose aspirin, the current standard of care for pregnant women with APS (UFH can be used as well). It is important to note that complications may still occur despite standard treatment. Indeed, PE is more common in the postpartum than in the antepartum period. Prompt diagnosis is paramount to initiate the appropriate treatment; in this case the dose of LMWH was increased from prophylactic to therapeutic dose. However, additional therapeutic modalities are necessary to improve outcomes. A randomized controlled trial comparing standard of care with or without hydroxychloroquine is under way to address this issue.
PROPHYLAXIS
CASE PRESENTATION 4
A 34-year-old woman G1P0 at 6 weeks’ gestation with a past medical history of a proximal lower extremity DVT while on oral contraception is treated with warfarin anticoagulation for 6 months. Her obstetrician consults the hematologist to advise regarding antithrombotic management during this pregnancy.
What is the approach to prophylaxis in women at high risk for pregnancy-associated VTE?
All women at high risk for pregnancy-associated VTE should be counseled about the signs and symptoms of DVT or PE during preconception and pregnancy and have a plan developed should these symptoms arise. The ACCP guidelines on antithrombotic therapy outline recommendations ranging from clinical vigilance to prophylactic and intermediate-dose anticoagulation, depending on the risk for VTE recurrence, based on the personal and family history of VTE and type of thrombophilia (Table 2).78 These recommendations range from grade 2B to 2C.

For women with a history of estrogen-related VTE, single unprovoked VTE, or recurrent unprovoked VTE not on chronic anticoagulation, antepartum and postpartum pharmacologic thromboprophylaxis with either prophylactic or intermediate-dose LMWH is recommended (grade 2C). In patients with prior history of provoked VTE (non-estrogen related), antepartum clinical vigilance and postpartum pharmacologic thromboprophylaxis is recommended (grade 2C, 2B).
In asymptomatic pregnant women who are homozygote carriers for factor V Leiden or prothrombin G20210A variants and have a positive family history of thrombosis, antepartum and postpartum pharmacologic thromboprophylaxis is recommended (grade 2B). In asymptomatic homozygote carriers of factor V Leiden or prothrombin G20210A variants with no family history of thrombosis and women with all other thrombophilias with a positive family history of thrombosis, postpartum pharmacologic thromboprophylaxis is indicated (grade 2B and 2C, respectively). For women with confirmed APS and clinical criteria of obstetric APS with recurrent pregnancy loss, antepartum thromboprophylaxis with LMWH and low-dose aspirin is recommended (grade 1B). For pregnant women with all other thrombophilias with no personal or family history of thrombosis, clinical vigilance is suggested (grade 2 C).78
As an alternative to LMWH, vitamin K antagonists (VKA) such as warfarin can be used for postpartum thromboprophylaxis; in patients with protein C or S deficiency, due to the risk of warfarin-induced skin necrosis, a rapid-onset anticoagulant must be concomitantly administered. Warfarin and LMWH are safe anticoagulants during lactation, but there are no clinical data on the effects of the DOACs on infants during lactation. Data from animal studies indicate that DOACs are secreted into breast milk.85
What risks are associated with anticoagulant therapy in pregnancy?
VKAs cross the placenta and can cause teratogenicity, pregnancy loss, fetal bleeding, and neurodevelopmental deficits. Therefore, discontinuation of VKAs prior to the sixth week of gestation is necessary to avoid warfarin embryopathy. DOACs have been shown to readily cross the placenta but with unknown human reproductive risks. Fondaparinux, a synthetic pentasaccharide, crosses the placenta in small quantities. Though there are reports of the successful use of fondaparinux in pregnancy, there is limited reported experience of its use in the first trimester.86
The risk for bleeding with anticoagulation is notably acceptable. In a case-control study of 88 pregnant women receiving therapeutic-dose anticoagulation, the risk of postpartum hemorrhage (PPH) after vaginal delivery was 30% in those who received LMWH anticoagulation versus 18% in those who did not (OR 1.9 [95% CI 1.1 to 3.5]).87 However, the risk for severe PPH (≥ 500 mL) was similar (5.6% versus 5.0%; OR 1.1 [95% CI 0.4 to 3.6]). The risk for PPH after cesarean section was 12% in LMWH users versus 4% in LMWH non-users (OR 2.9 [95% CI 0.5 to 19.4]). The risk for PPH associated with delivery within 24 hours after the last dose of LMWH was 1.2 times higher (95% CI 0.4 to 3.6) compared to a longer interval. Therefore, therapeutic LMWH increases the risk for blood loss after vaginal delivery, but not the risk for severe PPH. The risk for PPH is influenced by the interval between the last dose of LMWH and delivery. Of note in this study, per the institution’s protocol, the anticoagulation was stopped with signs of labor or determination of need for delivery. The risk for blood loss may be mitigated in more planned delivery scenarios.87
CASE 4 CONTINUED
The patient is placed on prophylactic-dose LMWH with good tolerance and delivers at 39 weeks' gestation via caesarian section due to nonprogression of labor. Postpartum she is restarted on prophylactic-dose anticoagulation with LMWH. Two weeks after discharge from the hospital, she presents with right calf pain and mild shortness of breath. On physical exam, her leg circumferences are equal. A D-dimer assay is 3375 ng/mL (normal 0–229). CUS of the right leg shows a complete occlusive DVT of the mid-distal superficial femoral and popliteal veins and partially occlusive acute DVT of the right posterior tibial and peroneal veins. CTPA reveals a right lower lobe PE. Because she had developed VTE despite prophylactic LMWH, her anticoagulation is changed to therapeutic dose. She is treated with anticoagulation with LMWH for a total of 3 months, after which a repeat CUS shows no residual thrombosis.
What is the recommended dosing of heparin and LMWH during pregnancy?
A prospective study of 14 pregnant women receiving UFH prophylaxis found that a prophylactic dose of 5000 units twice a day was inadequate to achieve prophylactic heparin levels in any patient in the second or third trimester.88 Similar to treatment dosage, there is no consensus evidence for prophylactic dosing, and dosage recommendations are based on expert opinion. In a retrospective study of 25 pregnant women on intermediate-dose UFH, the mean UFH dose required to achieve a target anti-factor Xa level of 0.1 to 0.3 units/mL was 236.9 units/kg/day.89 However, the use of anti-factor Xa levels for monitoring is controversial as there is no data to support a difference in outcomes with its use in prophylactic or therapeutic dosing.
The timing of the previous VTE history is important when deciding on the anticoagulant dose in pregnancy. In pregnant women with a VTE that occurred within the previous 4 to 6 weeks, full-dose anticoagulation with LMWH should be considered; an intermediate dose (three-fourths of a therapeutic dose) may be used if the thrombotic episode occurred more than 6 weeks earlier but still within a year. Prophylactic dosing may be sufficient if the episode occurred more than a year earlier.90 A clinical trial (High-Low) is under way to explore the optimal dose of LMWH in pregnant women with prior history of VTE who are not on chronic anticoagulation therapy.91
How is anticoagulation therapy managed in the peripartum period?
Neuraxial anesthesia during active labor while on anticoagulation increases the risk for central nervous system bleeding. Therefore, if spontaneous labor occurs in women on therapeutic dose anticoagulation, neuraxial anesthesia cannot be used. However, in the event of elective induction of labor or caesarean section, neuroaxial anesthesia may be performed 12 hours after the administration of the last prophylactic dose of LMWH or 24 hours after the last therapeutic dose of LMWH. Intravenous UFH should be stopped for 6 hours before induction of labor with a confirmed normal aPTT before placement of neuraxial anesthesia. There is no contraindication for using neuraxial anesthesia during subcutaneous standard UFH at total doses of 10,000 units daily. The risk of spinal hematoma with larger daily subcutaneous doses is unclear; therefore, a documented normal aPTT must be obtained before placement of neuroaxial anesthesia.
Postpartum, reinitiation of prophylactic-dose LMWH should be delayed for at least 12 hours after the removal of an epidural catheter. Therapeutic-dose LMWH should be administered no earlier than 24 hours after neuraxial anesthesia, providing that proper hemostasis is achieved. In the absence of persistent bleeding, if no regional anesthesia was used, LMWH may be resumed 12 hours after delivery.92 Anticoagulation with either LMWH or warfarin is recommended for at least 6 to 12 weeks postpartum.33
COUNSELING
Patients should be advised to manage controllable risk factors, including avoiding prolonged immobilization, avoiding excessive weight gain in pregnancy, and stopping smoking. Periods of immobilization tend to cause reduced blood flow (stasis), which predisposes to thrombosis. In a systematic review of records of all patients with confirmed PE after arrival at Charles de Gaulle airport in Paris during a 13-year period, women had a higher risk of PE after a long-distance flight than men, with an estimated incidence of 0.61 per million passengers versus 0.20, respectively; the incidence reached 7.24 and 2.35 cases, respectively, in passengers traveling more than 10,000 kilometers.93,94
The risk of air travel-related thrombosis in pregnant women is estimated to be between 0.03% and 0.1%. Physicians must decide on an individual basis how to prevent travel-related thrombosis in their pregnant patients. In most passengers, prevention can be limited to encouraging exercise, avoidance of long sleeping periods, and not using a window seat. Women at high risk for VTE, such as women with a prior history of VTE who are not on anticoagulation or women with known asymptomatic thrombophilia or other risk factors for thrombosis such as obesity, may benefit from a short period (1–3 days) of LMWH starting 2 hours before a long-distance flight.95
Activation of the coagulation system has been demonstrated in cigarette smokers.96 Heavy smoking was found to be a significant risk factor for VTE in a cross-sectional analysis of 2404 men and women.97 An increased risk for thrombosis during pregnancy is seen in cigarette smokers15,98 and is enhanced with the concomitant use of illicit drugs.99 Other obstetric complications associated with smoking and illicit drug use during pregnancy include preterm labor, spontaneous abortion, perinatal death, low birth weight, and abruption placenta. The efficacy of nicotine replacement therapy in pregnancy is uncertain.100 Recommendations are to advise patients to stop smoking, obtain psychosocial counseling, and utilize adjunctive therapies, which have been shown to have some effect on abstinence rates.101
CONCLUSION
Women are at increased risk for VTE during pregnancy and the postpartum period. Awareness of risk factors and the signs and symptoms of VTE is paramount. Prompt diagnosis and treatment is mandatory to decrease complications of VTE. LMWH is the mainstay treatment of VTE in pregnancy, as it does not cross the placenta. Both LMWH and warfarin are safe during lactation. Close communication among the patient, obstetrician, hematologist, anesthesiologist, and neonatologist is crucial to optimize the care of these patients.
INTRODUCTION
Venous thromboembolism (VTE), comprising deep vein thrombosis (DVT) and pulmonary embolism (PE), is a leading nonobstetric cause of maternal death in the United States and in developed countries.1,2 During pregnancy, the risk for VTE increases four- to six-fold, and although the risk is present throughout pregnancy, the mother is at highest risk immediately postpartum.3–5
VTE risk is increased due to physiologic and anatomic changes that occur in pregnancy. These changes include hypercoagulability, progesterone-induced venous stasis, decreased venous outflow, compression of the inferior vena cava and pelvic veins by the expanding uterus, and decreased mobility. The hypercoagulability of pregnancy is due to increased levels of coagulation factors I (fibrinogen), VII, VIII, and X, and von Willebrand factor; decreased free protein S, a natural anticoagulant; acquired resistance to activated protein C; and decreased fibrinolysis due to increased levels of plasminogen activator inhibitor-1 and -2.6,7 These changes confer increased hemostasis to the mother for delivery but also place her at higher risk for thrombosis.
A review of the literature found that more than 70% of pregnancy-associated DVTs are located in the ileofemoral region, as compared with approximately 9% in non-pregnant patients.8 The proximal location is associated with a higher risk for post-thrombotic syndrome and embolization as compared with calf DVTs.9 Proximal postnatal thrombosis, smoking, and older age are independent predictors of the development of post-thrombotic syndrome.10
RISK FACTORS
Clinical risk factors that increase the risk for VTE during pregnancy include a prior history of estrogen-related or unprovoked VTE, being a carrier of severe inherited thrombophilia (homozygotes for factor V Leiden or factor II G20210A variants, double heterozygotes, or persons with antithrombin, protein C, or protein S deficiencies), and the presence of antiphospholipid (aPL) antibodies.11 Women with systemic lupus erythematosus, diabetes, sickle cell disease, and heart disease also have a high risk for VTE during pregnancy.12 Other risk factors predisposing to thrombosis include black ethnicity, smoking, operative procedures, conception after assisted reproductive techniques, high body mass index, antepartum immobilization, severe preeclampsia, advanced age and parity, and a family history of VTE.13 A prospective cohort study of 1,297,037 pregnancies and related puerperium identified the following risk factors for thrombosis: hospitalization, infection, hyperemesis, multiple pregnancies, preeclampsia, obesity, cesarean section, major postpartum hemorrhage, intrauterine growth restriction, and fetal death.14 Risk factors identified in an Agency for Healthcare Research and Quality study include: age 35 or older, black ethnicity, lupus, sickle cell disease, heart disease, postpartum infection, and transfusion.15 The combination of more than one risk factor increases the risk for VTE. All these factors have to be considered when deciding on prophylactic or therapeutic anticoagulation therapy in pregnancy. In addition, the risks of anticoagulation, including bruising, bleeding, and other side effects (eg, reduced bone mineral density with therapeutic-dose unfractionated heparin), allergic reactions, and rarely thrombocytopenia, must be considered.
EVALUATION AND DIAGNOSIS
CASE PRESENTATION I
A 31-year-old woman G1P0 at 10 weeks’ gestation with no personal or family history of thrombosis presents with acute onset of shortness of breath and left-sided chest pain that awoke her the morning of presentation. Her vital signs are significant for a heart rate of 106 beats/min, respiration rate of 22 breaths/min, blood pressure of 105/76 mm Hg, and pulse oximetry of 98% on room air. The patient denies previous exposure to oral contraceptives. She does not smoke. She reports that she had noticed left calf pain and swelling, which worsened with walking after a 4-hour drive 2 days prior.
What is the approach to diagnosis of thromboembolism in pregnant patients?
DEEP VEIN THROMBOSIS
Although a clinical diagnosis of DVT in pregnancy is unreliable, a history and physical examination are necessary to exclude other diagnoses and to assess the likelihood of thrombosis. Unfortunately, studies of the accuracy of history and physical examination for detecting DVT and PE have not included pregnant patients. In most pregnant patients with clinically suspected DVT, the diagnosis is not confirmed. Other causes of leg pain and swelling are not uncommon during pregnancy and include cellulitis, ruptured Baker’s cyst, or muscular pain.
A cross-sectional study described the derivation of the LEFt clinical decision rule, which relies on 3 variables in pregnant women with suspected DVT: left leg presentation (L), ≥ 2 cm calf circumference difference (E for edema), and first trimester presentation (Ft). If none of these variables is present, the negative predictive value is 100%.16 A validation study suggested that a negative LEFt rule accurately identifies pregnant women in whom the risk for confirmed DVT appears to be very low. The rule should not be used as an individual test for excluding DVT during pregnancy, but could be applied in a diagnostic approach in association with D-dimer measurement and compression ultrasonography (CUS); however, it has not been prospectively validated for safety and efficacy.17 In a study of 149 consecutive pregnant women with suspected DVT, a whole-blood agglutination D-dimer had a sensitivity of 100% and specificity of 60%.18 A 2006 systematic review found only 4 diagnostic studies of VTE in pregnancy in the literature. One of these studies showed that a combination of a negative CUS and normal D-dimer can accurately exclude DVT.19
Serial CUS is necessary for pregnant women with a high clinical suspicion of DVT but a negative initial investigation. In a study of 221 pregnant women in whom DVT was clinically suspected, 16 women (7.2%) were diagnosed with DVT by initial CUS, and none were diagnosed with DVT onserial testing.20 During follow-up (≥ 3 months), 6 of the 205 women with normal serial CUS results presented with symptoms of DVT, PE, or both, and 1 of them was diagnosed with DVT and PE. The sensitivity of serial CUS with Doppler imaging was 94.1% (95% confidence interval [CI] 69.2% to 99.7%), and the negative predictive value was 99.5% (95% CI 96.9% to 100%).20 All ultrasounds undertaken for investigation of pregnancy-associated DVT should include imaging of the iliac veins if there is a high index of suspicion and the CUS is negative for femoral DVT. Serial CUS with Doppler imaging of the iliac vein performed over a 7-day period excludes DVT in symptomatic pregnant women.20 Repeat CUS may be done 2 to 4 days and 6 to 8 days after the initial scan.
Ileofemoral vein thrombosis accounts for approximately 90% of proximal thromboses in pregnancy, occurring most often in the left lower extremity.20 The incidence of isolated iliac vein thrombosis in pregnancy is low, but when it does occur, delay in diagnosis can lead to significant morbidity. Therefore, for women with suspected isolated iliac vein thrombosis in whom CUS is negative or nondiagnostic, magnetic resonance direct thrombus imaging (MRDTI) should be performed.21 Patients with iliac vein thrombosis may present with unexplained inguinal, pelvic, or abdominal pain, which may be accompanied by back pain, and they usually present with swelling of the entire leg. MRDTI does not require gadolinium contrast and its accuracy appears to be similar to that of venography for iliac vein thrombi in the nonpregnant population.21 Exposure to gadolinium during pregnancy is associated with an increased risk for rheumatologic, inflammatory, or infiltrative skin conditions and stillbirth or neonatal death.22
Ovarian vein thrombosis is a rare but serious diagnosis. It occurs mostly in the postpartum period, mainly after cesarean delivery, and usually affects the right ovarian vein. The diagnosis is confirmed by ultrasound, computed tomography (CT), or magnetic resonance imaging.23
PULMONARY EMBOLISM
PE is more difficult to diagnose than DVT, particularly because clinical signs of PE are unreliable in the pregnant patient. The mortality rate of untreated PE is high, ranging from 18% to 38%, and approximately one-third of patients with untreated thromboembolic disease develop recurrent embolism.24 Studies have reported a PE prevalence between 1.4% and 4.2% in pregnant women with suspected clinical diagnosis of PE.25
The clinical presentation of PE and associated laboratory testing results may be subtler in pregnant than in nonpregnant patients. Arterial blood gases (ABG) may show hypoxemia or hypocapnia. The ABG in pregnancy has a sensitivity of 76.9%, specificity of 20.2%, and negative and positive predictive values of 80% and 11.5% for PE, respectively.26 The alveolar-arterial oxygen gradient is a poor screening test for PE during pregnancy and postpartum. A retrospective chart review of 17 pregnant women with documented PE showed that 58% had normal alveolar-arterial gradients.27 Therefore, in a pregnant woman with a history suspicious for PE, objective imaging studies should be performed even if the patient has normal ABG.
The 2011 guidelines from the American Thoracic Society (ATS) and the Society of Thoracic Radiology (STR) recommend against using D-dimer to diagnose PE in pregnancy.28 In addition, lower extremity CUS should only be performed as the first diagnostic imaging procedure if the patient has signs or symptoms of DVT. Instead, the ATS/STR guidelines recommend a plain radiograph of the chest as the first imaging test. If the chest radiograph is normal, a ventilation/perfusion scan (V/Q) scan is preferred over CT pulmonary angiography (CTPA). Diagnostic accuracy of the V/Q scan may be superior to CTPA in pregnancy, and it is preferable because of the lower prevalence of indeterminate V/Q scan in pregnant women.29 Moreover, there is lower radiation exposure to the maternal breast and lung tissue with a V/Q scan than with CTPA. CTPA confers lower fetal radiation doses than V/Q scans (0.03–0.66 mGy versus 0.32–0.74 mGy, respectively) but higher total body maternal radiation (4–16 mSv versus 1–2.5 mSv).30 A quantitative approach to lung scan interpretation, based on the distribution histogram of V/Q ratios, may be helpful in categorizing patients with suspected PE.28 A study of 121 suspected episodes of PE in 120 pregnant women showed that 104 women with normal or nondiagnostic scans did not develop subsequent episodes of VTE during a mean follow-up period of 20 months.31
If the baseline chest radiograph is abnormal in a pregnant woman with clinical suspicion of PE, a CTPA should be performed. As noted, fetal radiation doses for CTPA examinations in which the fetus is not directly imaged are minimal. If CTPA is recommended for the diagnosis of PE, the patient should be informed that radiation to the breast may increase her baseline risk for breast cancer. The ATS guidelines state that “given the lack of evidence documenting clear superiority of any one diagnostic test, the values and preferences of a patient and her physician likely will and should determine the final choice and sequence of tests performed.”28
CASE I CONTINUED
Upon presentation to the emergency department, the circumference of the patient’s left leg is not significantly greater than that of her right leg, and her leg pain has resolved. Bilateral CUS is negative for proximal or distal DVT. Chest radiograph shows an opacification of her left lower lobe. CTPA shows bilateral segmental and subsegmental lower lobe pulmonary emboli.
How does risk for VTE change throughout pregnancy?
Women are at increased risk for VTE throughout the entire pregnancy, starting from conception, but mainly during the postpartum period. A Danish historical controlled cohort study of 819,751 pregnant women (ages 15–49 years) over a 10-year period identified 727 women with VTE. The absolute risk for VTE per 10,000 pregnancy-years increased from 4.1 (95% CI 3.2 to 5.2) during weeks 1 to 11 to 59.0 (95% CI 46.1 to 76.4) in week 40 and decreased in the postpartum period from 60 (95% CI 47.2 to 76.4) during the first week after birth to 2.1 during weeks 9–12 after birth (95% CI 1.1 to 4.2).32 This study showed that the risk of VTE increases throughout pregnancy and reaches its maximum during the peripartum period and is not significantly increased after 6 weeks post-delivery. In a retrospective cross-over cohort study of 1,687,930 women in California who delivered their first newborn, an elevated risk of VTE persisted until at least 12 weeks after delivery. However, the absolute increase in risk after 6 weeks postpartum was low.33
CASE 1 CONCLUSION
The patient is started on anticoagulation therapy and carefully monitored during the remainder of the pregnancy and postpartum period. Anticoagulation is discontinued 6 weeks after delivery.
TREATMENT
ANTICOAGULATION THERAPY
The treatment of VTE can be lifesaving. In a study comparing 35 patients with PE randomly assigned to treatment with anticoagulants versus no treatment, 5 of 19 patients in the untreated group died from PE and an additional 5 had nonfatal recurrences, as compared with none in the treated group.24 Unfractionated heparin (UFH) and low-molecular-weight heparin (LMWH) are both safe and effective anticoagulants during pregnancy as neither crosses the placenta. In a review of 186 reports of fetal and infant outcomes following anticoagulant therapy during pregnancy in 1325 pregnancies, outcomes in UFH-treated patients were similar to those in the normal population after excluding pregnancies with comorbid conditions independently associated with adverse outcomes.34 A 2005 systematic review of LMWH for prophylaxis and treatment of VTE during pregnancy included 64 studies of 277 pregnancies. There were no maternal deaths, live births resulted from 94.7% of the pregnancies, VTE or arterial thrombosis occurred in 0.86%, and significant bleeding occurred in 1.98%.35
The standard UFH regimen is an initial bolus of 5000 units subcutaneously and 17,500 units every 12 hours, with dose adjustment made based on a mid-interval activated partial thromboplastin time (aPTT).36 Although still controversial, it has been suggested that the anti-Xa assay with a mid-dosing interval target of 0.3 to 0.7 U/mL is a more reliable measure of therapeutic UFH activity than the aPTT, as the aPTT response is suppressed due to a pregnancy-related increase in factor VIII. LMWH is dosed based on weight; regimens are enoxaparin 1 mg/kg subcutaneously twice daily or 1.5 mg/kg subcutaneously once daily, and dalteparin 100 units/kg every 12 hours or 150 units/kg daily.
A 2017 Cochrane review of the effect of LMWH compared with UFH for the treatment of VTE in the nonpregnant setting included 23 studies with 9587 patients. Thrombotic complications (odds ratio [OR] 0.70 [CI 0.57 to 0.85]) and major hemorrhage (OR 0.58 [CI 0.40 to 0.83]) were lower in patients receiving LMWH, with a trend toward lower mortality.37 In addition, the incidence of bleeding complications in patients treated with subcutaneous LMWH versus intravenous heparin was compared in a 2012 systematic review of 27 randomized controlled trials with a total of 28,637 patients. In patients treated with LMWH, there was a nonstatistically significant lower incidence of major bleeding events (OR 0.79 [95% CI 0.60 to 1.04]) and a statistically significant reduction in bleeding risk (OR 0.68 [95% CI 0.47 to 1.00]) compared to patients treated with UFH.38 Additionally, a trial comparing the use of standard UFH versus LMWH found a significantly lower incidence of thrombocytopenia in patients treated with LMWH.39,40 Overall, LMWH is more effective at decreasing both thrombotic and bleeding complications, and the risk for osteoporosis is lower with LMWH. Based on these results, the American College of Chest Physicians (ACCP) recommends LMWH as the first-line treatment for VTE in pregnancy.41
In specific clinical situations, such as patients with renal dysfunction with creatinine clearance (CrCl) less than 30 mL/min, UFH is indicated. In a study of 103 pregnancies in 93 women given anti-coagulation during pregnancy, 89.3% received UFH. There were no maternal deaths, and fetal demise occurred in 8 pregnancies (7.8%) at a median of 14 weeks’ gestation. There were 2 episodes of PE (1.9%) and 2 major bleeding events requiring transfusion (1.9%).42 UFH costs much less than LMWH, and therefore UFH remains an important, inexpensive, and efficacious anticoagulant option for pregnant women who require anticoagulation and cannot afford LMWH.43
Due to the physiologic changes associated with pregnancy, LMWH and UFH dosages may need to be adjusted. An observational study of 20 pregnant women with acute VTE found no recurrent VTE or major bleeding after treatment with dalteparin. Dalteparin doses approximately 10% to 20% higher than those recommended in nonpregnant women were required to reach therapeutic target anti-Xa activity.44
Caution Regarding Oral Anticoagulants
Due to its teratogenicity, warfarin is not a first-line anticoagulation option. It is strictly contraindicated during the first trimester during organogenesis, and its use during pregnancy is restricted to women with mechanical heart valves. Warfarin crosses the placenta and has been associated with nasal hypoplasia, stippled epiphyses, and growth restriction, particularly between 6 to 9 weeks’ gestation. Every effort should be made to substitute UFH or LMWH for warfarin between 6 and 12 weeks of gestation. The bridging process should begin as early in the gestational age as possible due to the long half-life of warfarin.45 When used later in gestation, warfarin has been associated with fetal hemorrhage and central nervous system abnormalities. Other complications from use during the second and third trimesters include microcephaly, blindness, deafness, and fetal growth restriction.46,47 Its use also increases the risk for abortion and fetal death in utero.48–50
The direct oral anticoagulants (DOACs) are not approved for use in pregnancy. Although there are limited anecdotal reports of DOAC use in pregnancy,51 there is preclinical evidence of placental transfer with the DOACs rivaroxaban and apixaban (direct Xa inhibitors) and the oral thrombin inhibitor dabigatran, thus increasing the risk to the fetus.52–54 Edoxaban, another direct Xa inhibitor, should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. It should be discontinued in nursing mothers.55
THROMBOLYSIS
Fetal as well as maternal survival is dependent on adequate maternal perfusion and oxygenation. The risk of death from PE is significant, with a cross-sectional study of 58 patients with acute, massive PE showing a 55% mortality rate.56 Thus, pregnancy is not an absolute contraindication to mechanical or systemic (recombinant tissue plasminogen activator or streptokinase) thrombolysis in an unstable patient at high risk for death.57–59 There are no major studies of this approach, although a small review of 13 cases using systemic thrombolysis showed no increased risk of maternal mortality.58 Thrombolysis should be considered for appropriate indications in pregnant patients as it would be in nonpregnant patients. However, caution is required when drawing conclusions regarding maternal and fetal safety, given the lack of controlled clinical trials including pregnant women.
SURGICAL PULMONARY EMBOLECTOMY
Surgical pulmonary embolectomy is an important therapeutic and potentially life-saving option in women presenting with massive PE in the immediate postpartum period. Because of the risk of massive uterine bleeding immediately postpartum, thrombolytic therapy should not be used.60
INFERIOR VENA CAVA FILTER
Placement of an inferior vena cava (IVC) filter is indicated in patients who have an acute VTE with absolute contraindications for anticoagulation. In addition, it can be considered in patients with extensive ileofemoral venous thrombosis within 2 weeks prior to expected delivery.61 In a systematic review of 44 studies of IVC filters placed in pregnant patients, the IVC filter complication rate was 8.87% and the failure-to-retrieve rate was 11.25%.62 The complication rate is similar to that found in the nonpregnant population. Thus, IVC filters may be used when appropriately indicated and should be removed as soon as clinically feasible.
RECURRENT THROMBOSIS AND THROMBOPHILIAS
CASE PRESENTATION 2
A 34-year-old pregnant woman G1P0 at 38 weeks’ gestation presents with a painful, swollen left calf that is associated with difficulty on walking; the circumference of the left calf is 2 cm greater than that of the right. She has no shortness of breath or chest pain. She has a prior history of distal right lower extremity DVT while on combined oral contraceptives. Her mother also has a history of DVT while bedbound during a prolonged hospitalization at an older age. CUS is negative, and the patient is discharged home. However, 24 hours later she returns to the hospital with worsening swelling and pain in her left leg. Magnetic resonance venography demonstrates a large left external iliac and common iliac DVT. She is admitted and is started on UFH, and a retrievable IVC filter is placed in anticipation of delivery.
What is the risk for VTE recurrence during pregnancy?
A personal and family history of VTE should be obtained when evaluating pregnant patients. A retrospective study of 109 women with prior history of VTE showed recurrence rates per patient-year of 10.9% during pregnancy and 3.7% in the nonpregnant period; the relative risk of recurrent VTE during pregnancy was 3.5 (95% CI 1.6 to 7.8).63 Two large European retrospective cohort studies of VTE in pregnancy showed that the recurrence rate of VTE in women with a history of thrombosis is around 6% during pregnancy, equally distributed among trimesters. The highest incidence of recurrence was in the postpartum period, ranging from 8.3% to 10%.64 The recurrence risk during pregnancy in women with a history of a single episode of VTE was 2.4% antepartum (95% CI 0.2% to 6.9%).65 These risks may be lower in women without thrombophilia or with a temporary risk factor associated with their previous thromboembolic event.65 Recurrence risk is higher if the previous VTE was estrogen-related, either due to pregnancy or through hormonal contraception (10%), than if the previous VTE was non-estrogen-related (2.7%).64,66
The timing of the case patient’s presentation is consistent with reports of increased risk of VTE during the peripartum period. Her prior history of estrogen-related DVT is concerning for a risk of recurrence, particularly during pregnancy. A retrospective cohort study of 1104 women with previous VTE, 88 of whom became pregnant without receiving thromboprophylaxis, showed that the overall rate of VTE recurrence was 5.8% (95% CI 3.0% to 10.6%) and 8.3% (95% CI 4.5% to 14.6%) during pregnancy and postpartum, respectively. The risk of VTE recurrence was absent if the first VTE was related to a transient risk factor other than pregnancy, postpartum period, or hormonal contraception.67 However, the recurrence rate of VTE in women with prior unprovoked VTE and/or thrombophilia has been reported as 5.9% (95% CI 1.2% to 16.2%).65 The presence of an underlying hypercoagulable state can increase the recurrence risk by 25% to 50%, depending on the disorder.68 A retrospective cohort study of 270 pregnancies in 105 carriers of factor V Leiden, identified because of a symptomatic relative with the factor V Leiden mutation, found a VTE risk (mostly in the postpartum period) of 6.4% for heterozygous women, 16.7% for homozygous women, 20% for double heterozygous women, and 1.2% for noncarriers.69
Should the patient be screened for a thrombophilia disorder?
Half of all index thromboses in patients with thrombophilia occur in association with an additional risk factor. In women of child-bearing age, pregnancy, the postpartum period, and the use of combined hormonal contraception are all risk factors for VTE. A 2010 guideline from the British hematology community recommended testing for thrombophilia in women with prior VTE secondary to a minor provoking factor before or during pregnancy, but not testing women with unprovoked VTE (who would receive prophylaxis regardless) or those with VTE secondary to a major provoking factor (who would not require prophylaxis).70 Indications to screen for aPL antibodies include: women with (1) 3 unexplained recurrent first-trimester pregnancy losses or 1 second or third trimester fetal loss of morphologically normal fetuses; (2) severe preeclampsia; (3) intrauterine growth restriction; or (4) premature labor (< 34 weeks’ gestation).71,72
CASE 2 CONCLUSION
The patient is subsequently screened for inherited thrombophilia disorders and is found to be heterozygous for factor V Leiden.
CASE PRESENTATION 3
A 25-year-old woman is diagnosed with antiphospholipid syndrome (APS) during her second pregnancy when she experiences fetal loss during her second trimester. Pathologic examination of the placenta reveals infarcts. Laboratory evaluation reveals positive high-titer anticardiolipin and anti-beta-2 glycoprotein 1 antibodies (IgG isotype) and lupus anticoagulant on 2 separate occasions 12 weeks apart. In a subsequent pregnancy, she is started on prophylactic LMWH and daily low-dose aspirin (81 mg). At 36 weeks’ gestation, she presents with a blood pressure of 210/104 mm Hg and a platelet count of 94,000 cells/µL. She is diagnosed with preeclampsia and hemolysis, elevated liver enzymes, and low platelets (HELLP) syndrome and is induced for early delivery. About 2 weeks after vaginal delivery, she notices shortness of breath and chest pain. A CTPA demonstrates a right lower lobe lobar defect consistent with a PE. Her anticoagulation is increased to therapeutic dosage LMWH.
To what extent does thrombophilia increase the risk for VTE in pregnancy?
Approximately 50% of pregnancy-related VTEs are associated with inherited thrombophilia. A systematic review of 79 studies, in which 9 studies (n = 2526 patients) assessed the risk of VTE associated with inherited thrombophilia in pregnancy, revealed that the odds ratio for individuals with thrombophilia to develop VTE ranged from 0.74 to 34.40.73 Although women with thrombophilia have an increased relative risk of developing VTE in pregnancy, the absolute risk of VTE remains low (Table 1).41,73,74

How is APS managed in pregnant patients?
Women with history of recurrent early pregnancy loss (< 10 weeks’ gestation) related to the presence of aPL antibodies are managed with low-dose aspirin and prophylactic-dose UFH or LMWH. This treatment increases the rate of subsequent successful pregnancy outcomes and reduces the risk for thrombosis. A 2010 systematic review and meta-analysis of UFH plus low-dose aspirin compared with low-dose aspirin alone in patients with APS and recurrent pregnancy loss included 5 trials and 334 patients. Patients receiving dual therapy had higher rates of live births (74.3%; relative risk [RR] 1.30 [CI 1.04 to 1.63]) compared to the aspirin-only group (55.8%).75 A 2009 randomized controlled trial compared low-dose aspirin to low-dose aspirin plus LMWH in women with recurrent pregnancy loss and either aPL antibodies, antinuclear antibody, or inherited thrombophilia. The study was stopped early after 4 years and found no difference in rates of live births between the groups (77.8% versus 79.1%).76 However, a randomized case-control trial of women with aPL antibodies and recurrent miscarriage found a 72% live birth rate in 47 women randomly assigned to low-dose aspirin and LMWH.77 A 2012 guideline from the American College of Chest Physicians (ACCP) recommends that women with aPL antibodies with a history of 3 or more pregnancy losses receive low-dose aspirin plus prophylactic-dose LMWH or UFH.78 A 2014 systematic review and meta-analysis showed that the combination of low-dose aspirin and UFH resulted in a higher live-birth rate than aspirin alone in 803 women with APS (RR 1.54 [95% CI 1.25 to 1.89]).79 Further large randomized controlled trials are needed to confirm optimal management of recurrent miscarriage and aPL antibodies.
The addition of prednisone to aspirin, heparin, or both has shown no benefits in pregnant women with aPL antibodies. Indeed, prolonged use of steroids may cause serious pregnancy complications, such as prematurity and hypertension.80–83 Intravenous infusions of immunoglobulin (IVIG) have not been shown to be superior to heparin and aspirin. This finding was confirmed in a multicenter clinical trial that tested the effects of IVIG compared with LMWH plus low-dose aspirin for the treatment of women with aPL antibodies and recurrent miscarriage. The rate of live-birth was 72.5% in the group treated with heparin plus low-dose aspirin compared with 39.5% in the IVIG group.84
Preeclampsia and HELLP syndrome complicated the case patient’s pregnancy even though she was being treated with prophylactic-dose LMWH and low-dose aspirin, the current standard of care for pregnant women with APS (UFH can be used as well). It is important to note that complications may still occur despite standard treatment. Indeed, PE is more common in the postpartum than in the antepartum period. Prompt diagnosis is paramount to initiate the appropriate treatment; in this case the dose of LMWH was increased from prophylactic to therapeutic dose. However, additional therapeutic modalities are necessary to improve outcomes. A randomized controlled trial comparing standard of care with or without hydroxychloroquine is under way to address this issue.
PROPHYLAXIS
CASE PRESENTATION 4
A 34-year-old woman G1P0 at 6 weeks’ gestation with a past medical history of a proximal lower extremity DVT while on oral contraception is treated with warfarin anticoagulation for 6 months. Her obstetrician consults the hematologist to advise regarding antithrombotic management during this pregnancy.
What is the approach to prophylaxis in women at high risk for pregnancy-associated VTE?
All women at high risk for pregnancy-associated VTE should be counseled about the signs and symptoms of DVT or PE during preconception and pregnancy and have a plan developed should these symptoms arise. The ACCP guidelines on antithrombotic therapy outline recommendations ranging from clinical vigilance to prophylactic and intermediate-dose anticoagulation, depending on the risk for VTE recurrence, based on the personal and family history of VTE and type of thrombophilia (Table 2).78 These recommendations range from grade 2B to 2C.

For women with a history of estrogen-related VTE, single unprovoked VTE, or recurrent unprovoked VTE not on chronic anticoagulation, antepartum and postpartum pharmacologic thromboprophylaxis with either prophylactic or intermediate-dose LMWH is recommended (grade 2C). In patients with prior history of provoked VTE (non-estrogen related), antepartum clinical vigilance and postpartum pharmacologic thromboprophylaxis is recommended (grade 2C, 2B).
In asymptomatic pregnant women who are homozygote carriers for factor V Leiden or prothrombin G20210A variants and have a positive family history of thrombosis, antepartum and postpartum pharmacologic thromboprophylaxis is recommended (grade 2B). In asymptomatic homozygote carriers of factor V Leiden or prothrombin G20210A variants with no family history of thrombosis and women with all other thrombophilias with a positive family history of thrombosis, postpartum pharmacologic thromboprophylaxis is indicated (grade 2B and 2C, respectively). For women with confirmed APS and clinical criteria of obstetric APS with recurrent pregnancy loss, antepartum thromboprophylaxis with LMWH and low-dose aspirin is recommended (grade 1B). For pregnant women with all other thrombophilias with no personal or family history of thrombosis, clinical vigilance is suggested (grade 2 C).78
As an alternative to LMWH, vitamin K antagonists (VKA) such as warfarin can be used for postpartum thromboprophylaxis; in patients with protein C or S deficiency, due to the risk of warfarin-induced skin necrosis, a rapid-onset anticoagulant must be concomitantly administered. Warfarin and LMWH are safe anticoagulants during lactation, but there are no clinical data on the effects of the DOACs on infants during lactation. Data from animal studies indicate that DOACs are secreted into breast milk.85
What risks are associated with anticoagulant therapy in pregnancy?
VKAs cross the placenta and can cause teratogenicity, pregnancy loss, fetal bleeding, and neurodevelopmental deficits. Therefore, discontinuation of VKAs prior to the sixth week of gestation is necessary to avoid warfarin embryopathy. DOACs have been shown to readily cross the placenta but with unknown human reproductive risks. Fondaparinux, a synthetic pentasaccharide, crosses the placenta in small quantities. Though there are reports of the successful use of fondaparinux in pregnancy, there is limited reported experience of its use in the first trimester.86
The risk for bleeding with anticoagulation is notably acceptable. In a case-control study of 88 pregnant women receiving therapeutic-dose anticoagulation, the risk of postpartum hemorrhage (PPH) after vaginal delivery was 30% in those who received LMWH anticoagulation versus 18% in those who did not (OR 1.9 [95% CI 1.1 to 3.5]).87 However, the risk for severe PPH (≥ 500 mL) was similar (5.6% versus 5.0%; OR 1.1 [95% CI 0.4 to 3.6]). The risk for PPH after cesarean section was 12% in LMWH users versus 4% in LMWH non-users (OR 2.9 [95% CI 0.5 to 19.4]). The risk for PPH associated with delivery within 24 hours after the last dose of LMWH was 1.2 times higher (95% CI 0.4 to 3.6) compared to a longer interval. Therefore, therapeutic LMWH increases the risk for blood loss after vaginal delivery, but not the risk for severe PPH. The risk for PPH is influenced by the interval between the last dose of LMWH and delivery. Of note in this study, per the institution’s protocol, the anticoagulation was stopped with signs of labor or determination of need for delivery. The risk for blood loss may be mitigated in more planned delivery scenarios.87
CASE 4 CONTINUED
The patient is placed on prophylactic-dose LMWH with good tolerance and delivers at 39 weeks' gestation via caesarian section due to nonprogression of labor. Postpartum she is restarted on prophylactic-dose anticoagulation with LMWH. Two weeks after discharge from the hospital, she presents with right calf pain and mild shortness of breath. On physical exam, her leg circumferences are equal. A D-dimer assay is 3375 ng/mL (normal 0–229). CUS of the right leg shows a complete occlusive DVT of the mid-distal superficial femoral and popliteal veins and partially occlusive acute DVT of the right posterior tibial and peroneal veins. CTPA reveals a right lower lobe PE. Because she had developed VTE despite prophylactic LMWH, her anticoagulation is changed to therapeutic dose. She is treated with anticoagulation with LMWH for a total of 3 months, after which a repeat CUS shows no residual thrombosis.
What is the recommended dosing of heparin and LMWH during pregnancy?
A prospective study of 14 pregnant women receiving UFH prophylaxis found that a prophylactic dose of 5000 units twice a day was inadequate to achieve prophylactic heparin levels in any patient in the second or third trimester.88 Similar to treatment dosage, there is no consensus evidence for prophylactic dosing, and dosage recommendations are based on expert opinion. In a retrospective study of 25 pregnant women on intermediate-dose UFH, the mean UFH dose required to achieve a target anti-factor Xa level of 0.1 to 0.3 units/mL was 236.9 units/kg/day.89 However, the use of anti-factor Xa levels for monitoring is controversial as there is no data to support a difference in outcomes with its use in prophylactic or therapeutic dosing.
The timing of the previous VTE history is important when deciding on the anticoagulant dose in pregnancy. In pregnant women with a VTE that occurred within the previous 4 to 6 weeks, full-dose anticoagulation with LMWH should be considered; an intermediate dose (three-fourths of a therapeutic dose) may be used if the thrombotic episode occurred more than 6 weeks earlier but still within a year. Prophylactic dosing may be sufficient if the episode occurred more than a year earlier.90 A clinical trial (High-Low) is under way to explore the optimal dose of LMWH in pregnant women with prior history of VTE who are not on chronic anticoagulation therapy.91
How is anticoagulation therapy managed in the peripartum period?
Neuraxial anesthesia during active labor while on anticoagulation increases the risk for central nervous system bleeding. Therefore, if spontaneous labor occurs in women on therapeutic dose anticoagulation, neuraxial anesthesia cannot be used. However, in the event of elective induction of labor or caesarean section, neuroaxial anesthesia may be performed 12 hours after the administration of the last prophylactic dose of LMWH or 24 hours after the last therapeutic dose of LMWH. Intravenous UFH should be stopped for 6 hours before induction of labor with a confirmed normal aPTT before placement of neuraxial anesthesia. There is no contraindication for using neuraxial anesthesia during subcutaneous standard UFH at total doses of 10,000 units daily. The risk of spinal hematoma with larger daily subcutaneous doses is unclear; therefore, a documented normal aPTT must be obtained before placement of neuroaxial anesthesia.
Postpartum, reinitiation of prophylactic-dose LMWH should be delayed for at least 12 hours after the removal of an epidural catheter. Therapeutic-dose LMWH should be administered no earlier than 24 hours after neuraxial anesthesia, providing that proper hemostasis is achieved. In the absence of persistent bleeding, if no regional anesthesia was used, LMWH may be resumed 12 hours after delivery.92 Anticoagulation with either LMWH or warfarin is recommended for at least 6 to 12 weeks postpartum.33
COUNSELING
Patients should be advised to manage controllable risk factors, including avoiding prolonged immobilization, avoiding excessive weight gain in pregnancy, and stopping smoking. Periods of immobilization tend to cause reduced blood flow (stasis), which predisposes to thrombosis. In a systematic review of records of all patients with confirmed PE after arrival at Charles de Gaulle airport in Paris during a 13-year period, women had a higher risk of PE after a long-distance flight than men, with an estimated incidence of 0.61 per million passengers versus 0.20, respectively; the incidence reached 7.24 and 2.35 cases, respectively, in passengers traveling more than 10,000 kilometers.93,94
The risk of air travel-related thrombosis in pregnant women is estimated to be between 0.03% and 0.1%. Physicians must decide on an individual basis how to prevent travel-related thrombosis in their pregnant patients. In most passengers, prevention can be limited to encouraging exercise, avoidance of long sleeping periods, and not using a window seat. Women at high risk for VTE, such as women with a prior history of VTE who are not on anticoagulation or women with known asymptomatic thrombophilia or other risk factors for thrombosis such as obesity, may benefit from a short period (1–3 days) of LMWH starting 2 hours before a long-distance flight.95
Activation of the coagulation system has been demonstrated in cigarette smokers.96 Heavy smoking was found to be a significant risk factor for VTE in a cross-sectional analysis of 2404 men and women.97 An increased risk for thrombosis during pregnancy is seen in cigarette smokers15,98 and is enhanced with the concomitant use of illicit drugs.99 Other obstetric complications associated with smoking and illicit drug use during pregnancy include preterm labor, spontaneous abortion, perinatal death, low birth weight, and abruption placenta. The efficacy of nicotine replacement therapy in pregnancy is uncertain.100 Recommendations are to advise patients to stop smoking, obtain psychosocial counseling, and utilize adjunctive therapies, which have been shown to have some effect on abstinence rates.101
CONCLUSION
Women are at increased risk for VTE during pregnancy and the postpartum period. Awareness of risk factors and the signs and symptoms of VTE is paramount. Prompt diagnosis and treatment is mandatory to decrease complications of VTE. LMWH is the mainstay treatment of VTE in pregnancy, as it does not cross the placenta. Both LMWH and warfarin are safe during lactation. Close communication among the patient, obstetrician, hematologist, anesthesiologist, and neonatologist is crucial to optimize the care of these patients.
- Knight M, Nour M, Tuffnell D, et al, eds. on behalf of MBRRACE-UK. Saving lives, improving mothers’ care—surveillance of maternal deaths in the UK 2012-14 and lessons learned to inform maternity care from the UK and Ireland confidential enquiries into maternal deaths and morbidity 2009-14. Oxford: National Perinatal Epidemiology Unit, University of Oxford; 2016: 69–75.
- Berg CJ, Callaghan WM, Syverson C, Henderson Z. Pregnancy-related mortality in the United States, 1998 to 2005. Obstet Gynecol 2010;116:1302–9.
- Salonen Ros H, Lichtenstein P, Bellocco R, et al. Increased risks of circulatory diseases in late pregnancy and puerperium. Epidemiology 2001;12:456–60.
- Heit JA, Kobbervig CE, James AH, et al. Trends in the incidence of venous thromboembolism during pregnancy or postpartum: a 30-year population-based study. Ann Intern Med 2005;143:697–706.
- Greer IA. Thrombosis in pregnancy: updates in diagnosis and management. Hematology Am Soc Hematol Educ Program 2012;2012:203–7.
- Hellgren M. Hemostasis during normal pregnancy and puerperium. Semin Thromb Hemost 2003;29:125–30.
- Brenner B. Haemostatic changes in pregnancy. Thromb Res 2004;114:409–14.
- Chan W-S, Spencer FA, Ginsberg JS. Anatomic distribution of deep vein thrombosis in pregnancy. CMAJ 2010;182:657–60.
- Greer IA. Prevention and management of venous thromboembolism in pregnancy. Clin Chest Med 2003;24:123–37.
- Wik HS, Jacobsen AF, Sandvik L, Sandset PM. Prevalence and predictors for post-thrombotic syndrome 3 to 16 years after pregnancy-related venous thrombosis: a population-based, cross-sectional, case-control study. J Thromb Haemost 2012;10:840–7.
- Pomp ER, Lenselink AM, Rosendaal FR, Doggen CJM. Pregnancy, the postpartum period and prothrombotic defects: risk of venous thrombosis in the MEGA study. J Thromb Haemost 2008;6:632–7.
- Marik PE, Plante LA. Venous thromboembolic disease and pregnancy. N Engl J Med 2008;359:2025–33.
- Jacobsen AF, Skjeldestad FE, Sandset PM. Ante- and postnatal risk factors of venous thrombosis: a hospital-based case-control study. J Thromb Haemost 2008;6:905–12.
- Virkus RA, Løkkegaard E, Lidegaard Ø, et al. Risk factors for venous thromboembolism in 1.3 million pregnancies: a nationwide prospective cohort. PloS One 2014;9:e96495.
- James AH, Jamison MG, Brancazio LR, Myers ER. Venous thromboembolism during pregnancy and the postpartum period: incidence, risk factors, and mortality. Am J Obstet Gynecol 2006;194:1311–5.
- Chan W-S, Lee A, Spencer FA, et al. Predicting deep venous thrombosis in pregnancy: out in “LEFt” field? Ann Intern Med 2009;151:85–92.
- Righini M, Jobic C, Boehlen F, et al. Predicting deep venous thrombosis in pregnancy: external validation of the LEFT clinical prediction rule. Haematologica 2013;98:545–8.
- Chan W-S, Chunilal S, Lee A, et al. A red blood cell agglutination D-dimer test to exclude deep venous thrombosis in pregnancy. Ann Intern Med 2007;147:165–70.
- Nijkeuter M, Ginsberg JS, Huisman MV. Diagnosis of deep vein thrombosis and pulmonary embolism in pregnancy: a systematic review. J Thromb Haemost 2006;4:496–500.
- Chan W-S, Spencer FA, Lee AY, et al. Safety of withholding anticoagulation in pregnant women with suspected deep vein thrombosis following negative serial compression ultrasound and iliac vein imaging. CMAJ 2013;185:E194–200.
- Dronkers CE, Srámek A, Huisman MV, Klok FA. Accurate diagnosis of iliac vein thrombosis in pregnancy with magnetic resonance direct thrombus imaging (MRDTI). BMJ Case Rep 2016;2016. pii: bcr2016218091.
- Ray JG, Vermeulen MJ, Bharatha A, et al. Association between MRI exposure during pregnancy and fetal and childhood outcomes. JAMA 2016;316:952–61.
- Royal College of Obstretricians and Gynaecologists. Thromboembolic disease in pregnancy and the puerperium: acute management. Green-top Guideline No. 37b. London: RCOG; 2015.
- Barritt DW, Jordan SC. Anticoagulant drugs in the treatment of pulmonary embolism. A controlled trial. Lancet 1960;1(7138):1309–12.
- Bourjeily G, Khalil H, Raker C, et al. Outcomes of negative multidetector computed tomography with pulmonary angiography in pregnant women suspected of pulmonary embolism. Lung 2012;190:105–11.
- Deutsch AB, Twitty P, Downes K, Parsons MT. Assessment of the alveolar-arterial oxygen gradient as a screening test for pulmonary embolism in pregnancy. Am J Obstet Gynecol 2010;203:373.e1–4.
- Powrie RO, Larson L, Rosene-Montella K, et al. Alveolar-arterial oxygen gradient in acute pulmonary embolism in pregnancy. Am J Obstet Gynecol 1998;178:394–6.
- Leung AN, Bull TM, Jaeschke R, et al. American Thoracic Society documents: an official American Thoracic Society/Society of Thoracic Radiology clinical practice guideline--evaluation of suspected pulmonary embolism in pregnancy. Radiology 2012;262:635–46.
- Cahill AG, Stout MJ, Macones GA, Bhalla S. Diagnosing pulmonary embolism in pregnancy using computed-tomographic angiography or ventilation-perfusion. Obstet Gynecol 2009;114:124–9.
- Bourjeily G, Paidas M, Khalil H, et al. Pulmonary embolism in pregnancy. Lancet 2010;375(9713):500–12.
- Chan WS, Ray JG, Murray S, et al. Suspected pulmonary embolism in pregnancy: clinical presentation, results of lung scanning, and subsequent maternal and pediatric outcomes. Arch Intern Med 2002;162:1170–5.
- Virkus RA, Løkkegaard ECL, Bergholt T, et al. Venous thromboembolism in pregnant and puerperal women in Denmark 1995-2005. A national cohort study. Thromb Haemost 2011;106:304–9.
- Kamel H, Navi BB, Sriram N, et al. Risk of a thrombotic event after the 6-week postpartum period. N Engl J Med 2014;370:1307–15.
- Ginsberg JS, Hirsh J, Turner DC, et al. Risks to the fetus of anticoagulant therapy during pregnancy. Thromb Haemost 1989;61:197–203.
- Greer IA, Nelson-Piercy C. Low-molecular-weight heparins for thromboprophylaxis and treatment of venous thromboembolism in pregnancy: a systematic review of safety and efficacy. Blood 2005;106:401–7.
- Prandoni P, Carnovali M, Marchiori A, Galilei Investigators. Subcutaneous adjusted-dose unfractionated heparin vs fixed-dose low-molecular-weight heparin in the initial treatment of venous thromboembolism. Arch Intern Med 2004;164:1077–83.
- Robertson L, Jones LE. Fixed dose subcutaneous low molecular weight heparins versus adjusted dose unfractionated heparin for venous thromboembolism. Cochrane Database Syst Rev 2017;(9):eb9;2:CD001100.
- Costantino G, Ceriani E, Rusconi AM, et al. Bleeding risk during treatment of acute thrombotic events with subcutaneous LMWH compared to intravenous unfractionated heparin; a systematic review. PloS One 2012;7:e44553.
- Warkentin TE, Levine MN, Hirsh J, et al. Heparin-induced thrombocytopenia in patients treated with low-molecular-weight heparin or unfractionated heparin. N Engl J Med 1995;332:1330–5.
- Junqueira DRG, Perini E, Penholati RRM, Carvalho MG. Unfractionated heparin versus low molecular weight heparin for avoiding heparin-induced thrombocytopenia in postoperative patients. Cochrane Database Syst Rev 2012;(9):CD007557.
- Bates SM, Greer IA, Middeldorp S, et al. VTE, thrombophilia, antithrombotic therapy, and pregnancy. Chest 2012;141:e691S–e736S.
- Clark NP, Delate T, Witt DM, et al. A descriptive evaluation of unfractionated heparin use during pregnancy. J Thromb Thrombolysis 2009;27:267–73.
- Clark NP, Delate T, Cleary SJ, Witt DM. Analysis of unfractionated heparin dose requirements to target therapeutic anti-Xa intensity during pregnancy. Thromb Res 2010;125:402–5.
- Jacobsen AF, Qvigstad E, Sandset PM. Low molecular weight heparin (dalteparin) for the treatment of venous thromboembolism in pregnancy. BJOG 2003;110:139–44.
- Walfisch A, Koren G. The “warfarin window” in pregnancy: the importance of half-life. J Obstet Gynaecol Can 2010;32:988–9.
- Bates SM, Ginsberg JS. Anticoagulants in pregnancy: fetal effects. Baillières Clin Obstet Gynaecol 1997;11:479–88.
- Stevenson RE, Burton OM, Ferlauto GJ, Taylor HA. Hazards of oral anticoagulants during pregnancy. JAMA 1980;243:1549–51.
- Ginsberg JS, Hirsh J. Anticoagulants during pregnancy. Annu Rev Med 1989;40:79–86.
- Wong V, Cheng CH, Chan KC. Fetal and neonatal outcome of exposure to anticoagulants during pregnancy. Am J Med Genet 1993;45:17–21.
- Blickstein D, Blickstein I. The risk of fetal loss associated with Warfarin anticoagulation. Int J Gynaecol Obstet 2002;78:221–5.
- Burnett AE, Mahan CE, Vazquez SR, et al. Guidance for the practical management of the direct oral anticoagulants (DOACs) in VTE treatment. J Thromb Thrombolysis 2016;41:206–32.
- Bapat P, Pinto LSR, Lubetsky A, et al. Examining the transplacental passage of apixaban using the dually perfused human placenta. J Thromb Haemost 2016;14:1436–41.
- Bapat P, Pinto LSR, Lubetsky A, et al. Rivaroxaban transfer across the dually perfused isolated human placental cotyledon. Am J Obstet Gynecol 2015;213:710.e1–6.
- Bapat P, Kedar R, Lubetsky A, et al. Transfer of dabigatran and dabigatran etexilate mesylate across the dually perfused human placenta. Obstet Gynecol 2014;123:1256–61.
- Savaysa [package insert]. Parsippany (NJ): Daiichi Sankyo, Inc; 2015.
- Filipecki S, Tomkowski W, Hajduk B, et al. [Outcome of patients with clinically acute massive pulmonary embolism]. Pneumonol Alergol Pol 1994;62:132–7.
- Holden EL, Ranu H, Sheth A, et al. Thrombolysis for massive pulmonary embolism in pregnancy--a report of three cases and follow up over a two year period. Thromb Res 2011;127:58–9.
- te Raa GD, Ribbert LS, Snijder RJ, Biesma DH. Treatment options in massive pulmonary embolism during pregnancy; a case-report and review of literature. Thromb Res 2009;124:1–5.
- Leonhardt G, Gaul C, Nietsch HH, et al. Thrombolytic therapy in pregnancy. J Thromb Thrombolysis 2006;21:271–6.
- Colombier S, Niclauss L. Successful surgical pulmonary embolectomy for massive perinatal embolism after emergency cesarean section. Ann Vasc Surg 2015;29:1452.e1–4.
- British Committee for Standards in Haematology Writing Group, Baglin TP, Brush J, Streiff M. Guidelines on use of vena cava filters. Br J Haematol 2006;134:590–5.
- Harris SA, Velineni R, Davies AH. Inferior vena cava filters in pregnancy: a systematic review. J Vasc Interv Radiol 2016;27:354–360.
- Pabinger I, Grafenhofer H, Kyrle PA, et al. Temporary increase in the risk for recurrence during pregnancy in women with a history of venous thromboembolism. Blood 2002;100:1060–2.
- Pabinger I, Grafenhofer H, Kaider A, et al. Risk of pregnancy-associated recurrent venous thromboembolism in women with a history of venous thrombosis. J Thromb Haemost 2005;3:949–54.
- Brill-Edwards P, Ginsberg JS, Gent M, et al. Safety of withholding heparin in pregnant women with a history of venous thromboembolism. Recurrence of Clot in This Pregnancy Study Group. N Engl J Med 2000;343:1439–44.
- De Stefano V, Martinelli I, Rossi E, et al. The risk of recurrent venous thromboembolism in pregnancy and puerperium without antithrombotic prophylaxis. Br J Haematol 2006;135:386–91.
- De Stefano V, Martinelli I, Rossi E, et al. The risk of recurrent venous thromboembolism in pregnancy and puerperium without antithrombotic prophylaxis. Br J Haematol 2006;135:386–91.
- Lim W, Eikelboom JW, Ginsberg JS. Inherited thrombophilia and pregnancy associated venous thromboembolism. BMJ 2007;334:1318–21.
- Tormene D, Simioni P, Prandoni P, et al. Factor V Leiden mutation and the risk of venous thromboembolism in pregnant women. Haematologica 2001;86:1305–9.
- Baglin T, Gray E, Greaves M, et al. Clinical guidelines for testing for heritable thrombophilia. Br J Haematol 2010;149:209–20.
- Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006;4:295–306.
- Pengo V, Tripodi A, Reber G, et al. Update of the guidelines for lupus anticoagulant detection. J Thromb Haemost 2009;7:1737–40.
- Robertson L, Wu O, Langhorne P, et al. Thrombophilia in pregnancy: a systematic review. Br J Haematol 2006;132:171–96.
- American College of Obstetricians and Gynecologists Women’s Health Care Physicians. ACOG Practice Bulletin No. 138: Inherited thrombophilias in pregnancy. Obstet Gynecol 2013;122:706–17.
- Mak A, Cheung MW, Cheak AA, Ho RC. Combination of heparin and aspirin is superior to aspirin alone in enhancing live births in patients with recurrent pregnancy loss and positive anti-phospholipid antibodies: a meta-analysis of randomized controlled trials and meta-regression. Rheumatology (Oxf) 2010;49:281–8.
- Laskin CA, Spitzer KA, Clark CA, et al. Low molecular weight heparin and aspirin for recurrent pregnancy loss: results from the randomized, controlled HepASA Trial. J Rheumatol 2009;36:279–87.
- Farquharson RG, Quenby S, Greaves M. Antiphospholipid syndrome in pregnancy: a randomized, controlled trial of treatment. Obstet Gynecol 2002;100:408–13.
- Bates SM, Jaeschke R, Stevens SM, et al. Diagnosis of DVT: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141(2 Suppl):e351S–418S.
- Lubbe WF, Butler WS, Palmer SJ, Liggins GC. Fetal survival after prednisone suppression of maternal lupus-anticoagulant. Lancet 1983;1(8338):1361–3.
- Lockshin MD, Druzin ML, Qamar T. Prednisone does not prevent recurrent fetal death in women with antiphospholipid antibody. Am J Obstet Gynecol 1989;160:439–43.
- Silver RK, MacGregor SN, Sholl JS, et al. Comparative trial of prednisone plus aspirin versus aspirin alone in the treatment of anticardiolipin antibody-positive obstetric patients. Am J Obstet Gynecol 1993;169:1411–7.
- Cowchock FS, Reece EA, Balaban D, et al. Repeated fetal losses associated with antiphospholipid antibodies: a collaborative randomized trial comparing prednisone with low-dose heparin treatment. Am J Obstet Gynecol 1992;166:1318–23.
- Laskin CA, Bombardier C, Hannah ME, et al. Prednisone and aspirin in women with autoantibodies and unexplained recurrent fetal loss. N Engl J Med 1997;337:148–53.
- Dendrinos S, Sakkas E, Makrakis E. Low-molecular-weight heparin versus intravenous immunoglobulin for recurrent abortion associated with antiphospholipid antibody syndrome. Int J Gynaecol Obstet 2009;104:223–5.
- Cohen H, Arachchillage DRJ, Beyer-Westendorf J, et al. Direct oral anticoagulants and women. Semin Thromb Hemost 2016;42:789–97.
- Bates SM, Middeldorp S, Rodger M, et al. Guidance for the treatment and prevention of obstetric-associated venous thromboembolism. J Thromb Thrombolysis 2016;41:92–128.
- Knol HM, Schultinge L, Veeger NJ, et al. The risk of postpartum hemorrhage in women using high dose of low-molecular-weight heparins during pregnancy. Thromb Res 2012;130:334–8.
- Barbour LA, Smith JM, Marlar RA. Heparin levels to guide thromboembolism prophylaxis during pregnancy. Am J Obstet Gynecol 1995;173:1869–73.
- Bergqvist A, Bergqvist D, Lindhagen A, Mätzsch T. Late symptoms after pregnancy-related deep vein thrombosis. Br J Obstet Gynaecol 1990;97:338–41.
- Rodger M. Evidence base for the management of venous thromboembolism in pregnancy. Hematology Am Soc Hematol Educ Program. 2010;2010:173–80.
- Bleker SM, Buchmüller A, Chauleur C, et al. Low-molecular-weight heparin to prevent recurrent venous thromboembolism in pregnancy: Rationale and design of the Highlow study, a randomised trial of two doses. Thromb Res 2016;144:62–8.
- Horlocker TT, Wedel DJ, Rowlingson JC, et al. Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy: American Society of Regional Anesthesia and Pain Medicine evidence-based guidelines (third edition). Reg Anesth Pain Med 2010;35:64–101.
- Lapostolle F, Surget V, Borron SW, et al. Severe pulmonary embolism associated with air travel. N Engl J Med 2001;345:779–83.
- Lapostolle F, Le Toumelin P, Chassery C, et al. Gender as a risk factor for pulmonary embolism after air travel. Thromb Haemost 2009;102:1165–8.
- Cannegieter SC, Rosendaal FR. Pregnancy and travel-related thromboembolism. Thromb Res 2013;131 Suppl 1:S55–58.
- Miller GJ, Bauer KA, Cooper JA, Rosenberg RD. Activation of the coagulant pathway in cigarette smokers. Thromb Haemost 1998;79:549–53.
- Golomb BA, Chan VT, Denenberg JO, et al. Risk marker associations with venous thrombotic events: a cross-sectional analysis. BMJ Open 2014;4:e003208.
- Lindqvist P, Dahlbäck B, Marŝál K. Thrombotic risk during pregnancy: a population study. Obstet Gynecol 1999;94:595–9.
- Black M, Bhattacharya S, Fairley T, et al. Outcomes of pregnancy in women using illegal drugs and in women who smoke cigarettes. Acta Obstet Gynecol Scand 2013;92:47–52.
- Mendelsohn C, Gould GS, Oncken C. Management of smoking in pregnant women. Aust Fam Physician 2014;43:46–51.
- Chamberlain C, O’Mara-Eves A, Oliver S, et al. Psychosocial interventions for supporting women to stop smoking in pregnancy. Cochrane Database Syst Rev 2013;10:CD001055.
- Knight M, Nour M, Tuffnell D, et al, eds. on behalf of MBRRACE-UK. Saving lives, improving mothers’ care—surveillance of maternal deaths in the UK 2012-14 and lessons learned to inform maternity care from the UK and Ireland confidential enquiries into maternal deaths and morbidity 2009-14. Oxford: National Perinatal Epidemiology Unit, University of Oxford; 2016: 69–75.
- Berg CJ, Callaghan WM, Syverson C, Henderson Z. Pregnancy-related mortality in the United States, 1998 to 2005. Obstet Gynecol 2010;116:1302–9.
- Salonen Ros H, Lichtenstein P, Bellocco R, et al. Increased risks of circulatory diseases in late pregnancy and puerperium. Epidemiology 2001;12:456–60.
- Heit JA, Kobbervig CE, James AH, et al. Trends in the incidence of venous thromboembolism during pregnancy or postpartum: a 30-year population-based study. Ann Intern Med 2005;143:697–706.
- Greer IA. Thrombosis in pregnancy: updates in diagnosis and management. Hematology Am Soc Hematol Educ Program 2012;2012:203–7.
- Hellgren M. Hemostasis during normal pregnancy and puerperium. Semin Thromb Hemost 2003;29:125–30.
- Brenner B. Haemostatic changes in pregnancy. Thromb Res 2004;114:409–14.
- Chan W-S, Spencer FA, Ginsberg JS. Anatomic distribution of deep vein thrombosis in pregnancy. CMAJ 2010;182:657–60.
- Greer IA. Prevention and management of venous thromboembolism in pregnancy. Clin Chest Med 2003;24:123–37.
- Wik HS, Jacobsen AF, Sandvik L, Sandset PM. Prevalence and predictors for post-thrombotic syndrome 3 to 16 years after pregnancy-related venous thrombosis: a population-based, cross-sectional, case-control study. J Thromb Haemost 2012;10:840–7.
- Pomp ER, Lenselink AM, Rosendaal FR, Doggen CJM. Pregnancy, the postpartum period and prothrombotic defects: risk of venous thrombosis in the MEGA study. J Thromb Haemost 2008;6:632–7.
- Marik PE, Plante LA. Venous thromboembolic disease and pregnancy. N Engl J Med 2008;359:2025–33.
- Jacobsen AF, Skjeldestad FE, Sandset PM. Ante- and postnatal risk factors of venous thrombosis: a hospital-based case-control study. J Thromb Haemost 2008;6:905–12.
- Virkus RA, Løkkegaard E, Lidegaard Ø, et al. Risk factors for venous thromboembolism in 1.3 million pregnancies: a nationwide prospective cohort. PloS One 2014;9:e96495.
- James AH, Jamison MG, Brancazio LR, Myers ER. Venous thromboembolism during pregnancy and the postpartum period: incidence, risk factors, and mortality. Am J Obstet Gynecol 2006;194:1311–5.
- Chan W-S, Lee A, Spencer FA, et al. Predicting deep venous thrombosis in pregnancy: out in “LEFt” field? Ann Intern Med 2009;151:85–92.
- Righini M, Jobic C, Boehlen F, et al. Predicting deep venous thrombosis in pregnancy: external validation of the LEFT clinical prediction rule. Haematologica 2013;98:545–8.
- Chan W-S, Chunilal S, Lee A, et al. A red blood cell agglutination D-dimer test to exclude deep venous thrombosis in pregnancy. Ann Intern Med 2007;147:165–70.
- Nijkeuter M, Ginsberg JS, Huisman MV. Diagnosis of deep vein thrombosis and pulmonary embolism in pregnancy: a systematic review. J Thromb Haemost 2006;4:496–500.
- Chan W-S, Spencer FA, Lee AY, et al. Safety of withholding anticoagulation in pregnant women with suspected deep vein thrombosis following negative serial compression ultrasound and iliac vein imaging. CMAJ 2013;185:E194–200.
- Dronkers CE, Srámek A, Huisman MV, Klok FA. Accurate diagnosis of iliac vein thrombosis in pregnancy with magnetic resonance direct thrombus imaging (MRDTI). BMJ Case Rep 2016;2016. pii: bcr2016218091.
- Ray JG, Vermeulen MJ, Bharatha A, et al. Association between MRI exposure during pregnancy and fetal and childhood outcomes. JAMA 2016;316:952–61.
- Royal College of Obstretricians and Gynaecologists. Thromboembolic disease in pregnancy and the puerperium: acute management. Green-top Guideline No. 37b. London: RCOG; 2015.
- Barritt DW, Jordan SC. Anticoagulant drugs in the treatment of pulmonary embolism. A controlled trial. Lancet 1960;1(7138):1309–12.
- Bourjeily G, Khalil H, Raker C, et al. Outcomes of negative multidetector computed tomography with pulmonary angiography in pregnant women suspected of pulmonary embolism. Lung 2012;190:105–11.
- Deutsch AB, Twitty P, Downes K, Parsons MT. Assessment of the alveolar-arterial oxygen gradient as a screening test for pulmonary embolism in pregnancy. Am J Obstet Gynecol 2010;203:373.e1–4.
- Powrie RO, Larson L, Rosene-Montella K, et al. Alveolar-arterial oxygen gradient in acute pulmonary embolism in pregnancy. Am J Obstet Gynecol 1998;178:394–6.
- Leung AN, Bull TM, Jaeschke R, et al. American Thoracic Society documents: an official American Thoracic Society/Society of Thoracic Radiology clinical practice guideline--evaluation of suspected pulmonary embolism in pregnancy. Radiology 2012;262:635–46.
- Cahill AG, Stout MJ, Macones GA, Bhalla S. Diagnosing pulmonary embolism in pregnancy using computed-tomographic angiography or ventilation-perfusion. Obstet Gynecol 2009;114:124–9.
- Bourjeily G, Paidas M, Khalil H, et al. Pulmonary embolism in pregnancy. Lancet 2010;375(9713):500–12.
- Chan WS, Ray JG, Murray S, et al. Suspected pulmonary embolism in pregnancy: clinical presentation, results of lung scanning, and subsequent maternal and pediatric outcomes. Arch Intern Med 2002;162:1170–5.
- Virkus RA, Løkkegaard ECL, Bergholt T, et al. Venous thromboembolism in pregnant and puerperal women in Denmark 1995-2005. A national cohort study. Thromb Haemost 2011;106:304–9.
- Kamel H, Navi BB, Sriram N, et al. Risk of a thrombotic event after the 6-week postpartum period. N Engl J Med 2014;370:1307–15.
- Ginsberg JS, Hirsh J, Turner DC, et al. Risks to the fetus of anticoagulant therapy during pregnancy. Thromb Haemost 1989;61:197–203.
- Greer IA, Nelson-Piercy C. Low-molecular-weight heparins for thromboprophylaxis and treatment of venous thromboembolism in pregnancy: a systematic review of safety and efficacy. Blood 2005;106:401–7.
- Prandoni P, Carnovali M, Marchiori A, Galilei Investigators. Subcutaneous adjusted-dose unfractionated heparin vs fixed-dose low-molecular-weight heparin in the initial treatment of venous thromboembolism. Arch Intern Med 2004;164:1077–83.
- Robertson L, Jones LE. Fixed dose subcutaneous low molecular weight heparins versus adjusted dose unfractionated heparin for venous thromboembolism. Cochrane Database Syst Rev 2017;(9):eb9;2:CD001100.
- Costantino G, Ceriani E, Rusconi AM, et al. Bleeding risk during treatment of acute thrombotic events with subcutaneous LMWH compared to intravenous unfractionated heparin; a systematic review. PloS One 2012;7:e44553.
- Warkentin TE, Levine MN, Hirsh J, et al. Heparin-induced thrombocytopenia in patients treated with low-molecular-weight heparin or unfractionated heparin. N Engl J Med 1995;332:1330–5.
- Junqueira DRG, Perini E, Penholati RRM, Carvalho MG. Unfractionated heparin versus low molecular weight heparin for avoiding heparin-induced thrombocytopenia in postoperative patients. Cochrane Database Syst Rev 2012;(9):CD007557.
- Bates SM, Greer IA, Middeldorp S, et al. VTE, thrombophilia, antithrombotic therapy, and pregnancy. Chest 2012;141:e691S–e736S.
- Clark NP, Delate T, Witt DM, et al. A descriptive evaluation of unfractionated heparin use during pregnancy. J Thromb Thrombolysis 2009;27:267–73.
- Clark NP, Delate T, Cleary SJ, Witt DM. Analysis of unfractionated heparin dose requirements to target therapeutic anti-Xa intensity during pregnancy. Thromb Res 2010;125:402–5.
- Jacobsen AF, Qvigstad E, Sandset PM. Low molecular weight heparin (dalteparin) for the treatment of venous thromboembolism in pregnancy. BJOG 2003;110:139–44.
- Walfisch A, Koren G. The “warfarin window” in pregnancy: the importance of half-life. J Obstet Gynaecol Can 2010;32:988–9.
- Bates SM, Ginsberg JS. Anticoagulants in pregnancy: fetal effects. Baillières Clin Obstet Gynaecol 1997;11:479–88.
- Stevenson RE, Burton OM, Ferlauto GJ, Taylor HA. Hazards of oral anticoagulants during pregnancy. JAMA 1980;243:1549–51.
- Ginsberg JS, Hirsh J. Anticoagulants during pregnancy. Annu Rev Med 1989;40:79–86.
- Wong V, Cheng CH, Chan KC. Fetal and neonatal outcome of exposure to anticoagulants during pregnancy. Am J Med Genet 1993;45:17–21.
- Blickstein D, Blickstein I. The risk of fetal loss associated with Warfarin anticoagulation. Int J Gynaecol Obstet 2002;78:221–5.
- Burnett AE, Mahan CE, Vazquez SR, et al. Guidance for the practical management of the direct oral anticoagulants (DOACs) in VTE treatment. J Thromb Thrombolysis 2016;41:206–32.
- Bapat P, Pinto LSR, Lubetsky A, et al. Examining the transplacental passage of apixaban using the dually perfused human placenta. J Thromb Haemost 2016;14:1436–41.
- Bapat P, Pinto LSR, Lubetsky A, et al. Rivaroxaban transfer across the dually perfused isolated human placental cotyledon. Am J Obstet Gynecol 2015;213:710.e1–6.
- Bapat P, Kedar R, Lubetsky A, et al. Transfer of dabigatran and dabigatran etexilate mesylate across the dually perfused human placenta. Obstet Gynecol 2014;123:1256–61.
- Savaysa [package insert]. Parsippany (NJ): Daiichi Sankyo, Inc; 2015.
- Filipecki S, Tomkowski W, Hajduk B, et al. [Outcome of patients with clinically acute massive pulmonary embolism]. Pneumonol Alergol Pol 1994;62:132–7.
- Holden EL, Ranu H, Sheth A, et al. Thrombolysis for massive pulmonary embolism in pregnancy--a report of three cases and follow up over a two year period. Thromb Res 2011;127:58–9.
- te Raa GD, Ribbert LS, Snijder RJ, Biesma DH. Treatment options in massive pulmonary embolism during pregnancy; a case-report and review of literature. Thromb Res 2009;124:1–5.
- Leonhardt G, Gaul C, Nietsch HH, et al. Thrombolytic therapy in pregnancy. J Thromb Thrombolysis 2006;21:271–6.
- Colombier S, Niclauss L. Successful surgical pulmonary embolectomy for massive perinatal embolism after emergency cesarean section. Ann Vasc Surg 2015;29:1452.e1–4.
- British Committee for Standards in Haematology Writing Group, Baglin TP, Brush J, Streiff M. Guidelines on use of vena cava filters. Br J Haematol 2006;134:590–5.
- Harris SA, Velineni R, Davies AH. Inferior vena cava filters in pregnancy: a systematic review. J Vasc Interv Radiol 2016;27:354–360.
- Pabinger I, Grafenhofer H, Kyrle PA, et al. Temporary increase in the risk for recurrence during pregnancy in women with a history of venous thromboembolism. Blood 2002;100:1060–2.
- Pabinger I, Grafenhofer H, Kaider A, et al. Risk of pregnancy-associated recurrent venous thromboembolism in women with a history of venous thrombosis. J Thromb Haemost 2005;3:949–54.
- Brill-Edwards P, Ginsberg JS, Gent M, et al. Safety of withholding heparin in pregnant women with a history of venous thromboembolism. Recurrence of Clot in This Pregnancy Study Group. N Engl J Med 2000;343:1439–44.
- De Stefano V, Martinelli I, Rossi E, et al. The risk of recurrent venous thromboembolism in pregnancy and puerperium without antithrombotic prophylaxis. Br J Haematol 2006;135:386–91.
- De Stefano V, Martinelli I, Rossi E, et al. The risk of recurrent venous thromboembolism in pregnancy and puerperium without antithrombotic prophylaxis. Br J Haematol 2006;135:386–91.
- Lim W, Eikelboom JW, Ginsberg JS. Inherited thrombophilia and pregnancy associated venous thromboembolism. BMJ 2007;334:1318–21.
- Tormene D, Simioni P, Prandoni P, et al. Factor V Leiden mutation and the risk of venous thromboembolism in pregnant women. Haematologica 2001;86:1305–9.
- Baglin T, Gray E, Greaves M, et al. Clinical guidelines for testing for heritable thrombophilia. Br J Haematol 2010;149:209–20.
- Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006;4:295–306.
- Pengo V, Tripodi A, Reber G, et al. Update of the guidelines for lupus anticoagulant detection. J Thromb Haemost 2009;7:1737–40.
- Robertson L, Wu O, Langhorne P, et al. Thrombophilia in pregnancy: a systematic review. Br J Haematol 2006;132:171–96.
- American College of Obstetricians and Gynecologists Women’s Health Care Physicians. ACOG Practice Bulletin No. 138: Inherited thrombophilias in pregnancy. Obstet Gynecol 2013;122:706–17.
- Mak A, Cheung MW, Cheak AA, Ho RC. Combination of heparin and aspirin is superior to aspirin alone in enhancing live births in patients with recurrent pregnancy loss and positive anti-phospholipid antibodies: a meta-analysis of randomized controlled trials and meta-regression. Rheumatology (Oxf) 2010;49:281–8.
- Laskin CA, Spitzer KA, Clark CA, et al. Low molecular weight heparin and aspirin for recurrent pregnancy loss: results from the randomized, controlled HepASA Trial. J Rheumatol 2009;36:279–87.
- Farquharson RG, Quenby S, Greaves M. Antiphospholipid syndrome in pregnancy: a randomized, controlled trial of treatment. Obstet Gynecol 2002;100:408–13.
- Bates SM, Jaeschke R, Stevens SM, et al. Diagnosis of DVT: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141(2 Suppl):e351S–418S.
- Lubbe WF, Butler WS, Palmer SJ, Liggins GC. Fetal survival after prednisone suppression of maternal lupus-anticoagulant. Lancet 1983;1(8338):1361–3.
- Lockshin MD, Druzin ML, Qamar T. Prednisone does not prevent recurrent fetal death in women with antiphospholipid antibody. Am J Obstet Gynecol 1989;160:439–43.
- Silver RK, MacGregor SN, Sholl JS, et al. Comparative trial of prednisone plus aspirin versus aspirin alone in the treatment of anticardiolipin antibody-positive obstetric patients. Am J Obstet Gynecol 1993;169:1411–7.
- Cowchock FS, Reece EA, Balaban D, et al. Repeated fetal losses associated with antiphospholipid antibodies: a collaborative randomized trial comparing prednisone with low-dose heparin treatment. Am J Obstet Gynecol 1992;166:1318–23.
- Laskin CA, Bombardier C, Hannah ME, et al. Prednisone and aspirin in women with autoantibodies and unexplained recurrent fetal loss. N Engl J Med 1997;337:148–53.
- Dendrinos S, Sakkas E, Makrakis E. Low-molecular-weight heparin versus intravenous immunoglobulin for recurrent abortion associated with antiphospholipid antibody syndrome. Int J Gynaecol Obstet 2009;104:223–5.
- Cohen H, Arachchillage DRJ, Beyer-Westendorf J, et al. Direct oral anticoagulants and women. Semin Thromb Hemost 2016;42:789–97.
- Bates SM, Middeldorp S, Rodger M, et al. Guidance for the treatment and prevention of obstetric-associated venous thromboembolism. J Thromb Thrombolysis 2016;41:92–128.
- Knol HM, Schultinge L, Veeger NJ, et al. The risk of postpartum hemorrhage in women using high dose of low-molecular-weight heparins during pregnancy. Thromb Res 2012;130:334–8.
- Barbour LA, Smith JM, Marlar RA. Heparin levels to guide thromboembolism prophylaxis during pregnancy. Am J Obstet Gynecol 1995;173:1869–73.
- Bergqvist A, Bergqvist D, Lindhagen A, Mätzsch T. Late symptoms after pregnancy-related deep vein thrombosis. Br J Obstet Gynaecol 1990;97:338–41.
- Rodger M. Evidence base for the management of venous thromboembolism in pregnancy. Hematology Am Soc Hematol Educ Program. 2010;2010:173–80.
- Bleker SM, Buchmüller A, Chauleur C, et al. Low-molecular-weight heparin to prevent recurrent venous thromboembolism in pregnancy: Rationale and design of the Highlow study, a randomised trial of two doses. Thromb Res 2016;144:62–8.
- Horlocker TT, Wedel DJ, Rowlingson JC, et al. Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy: American Society of Regional Anesthesia and Pain Medicine evidence-based guidelines (third edition). Reg Anesth Pain Med 2010;35:64–101.
- Lapostolle F, Surget V, Borron SW, et al. Severe pulmonary embolism associated with air travel. N Engl J Med 2001;345:779–83.
- Lapostolle F, Le Toumelin P, Chassery C, et al. Gender as a risk factor for pulmonary embolism after air travel. Thromb Haemost 2009;102:1165–8.
- Cannegieter SC, Rosendaal FR. Pregnancy and travel-related thromboembolism. Thromb Res 2013;131 Suppl 1:S55–58.
- Miller GJ, Bauer KA, Cooper JA, Rosenberg RD. Activation of the coagulant pathway in cigarette smokers. Thromb Haemost 1998;79:549–53.
- Golomb BA, Chan VT, Denenberg JO, et al. Risk marker associations with venous thrombotic events: a cross-sectional analysis. BMJ Open 2014;4:e003208.
- Lindqvist P, Dahlbäck B, Marŝál K. Thrombotic risk during pregnancy: a population study. Obstet Gynecol 1999;94:595–9.
- Black M, Bhattacharya S, Fairley T, et al. Outcomes of pregnancy in women using illegal drugs and in women who smoke cigarettes. Acta Obstet Gynecol Scand 2013;92:47–52.
- Mendelsohn C, Gould GS, Oncken C. Management of smoking in pregnant women. Aust Fam Physician 2014;43:46–51.
- Chamberlain C, O’Mara-Eves A, Oliver S, et al. Psychosocial interventions for supporting women to stop smoking in pregnancy. Cochrane Database Syst Rev 2013;10:CD001055.
Locally Advanced Pancreatic Cancer
INTRODUCTION
Pancreatic cancer is one of the most rapidly rising causes of mortality in the United States. In 2016, the number of deaths from pancreatic cancer exceeded those from breast cancer, making it the third leading cause of cancer-related death in the United States.1 It is projected that by 2020 pancreatic cancer will overtake colorectal malignancies to become the second most common cause of cancer death in this country.1,2 The term pancreatic cancer encompasses both exocrine and endocrine tumors. However, since 80% of pancreatic cancers are classified as pancreatic ductal adenocarcinoma (PDA), when speaking about pancreatic cancer most clinicians and scientists are referring to PDA.
Even with advances in chemotherapy and radiotherapy over the past decade, the only curative option for PDA is surgical resection. Unfortunately, only 20% of patients are appropriate surgical candidates at the time of diagnosis.3 Considering the lack of screening options and the ambiguity of symptomatology, roughly 4 out 5 patients with PDA are diagnosed as having locally advanced or metastatic disease that is initially not amenable to surgery.
Locally advanced pancreatic adenocarcinoma presents unique challenges in management and treatment. Treatment options include multi-agent chemotherapy, chemoradiation, or radiotherapy. Some patients can be successfully down-staged with these therapies and be deemed surgical candidates. Other challenges include selecting the appropriate sequence of therapies and stratifying therapies based on comorbidities. In this article, we review the epidemiology, biology, and diagnostic approach to PDA and focus on current treatment strategies for locally advanced pancreatic cancer (LAPC).
EPIDEMIOLOGY
In 2012, GLOBOCAN estimated that PDA caused 331,000 deaths per year, accounting for 4% of all worldwide mortality.4,5 Despite high incidence rates internationally, PDA is a disease of Western and industrialized nations. In the Unites States, PDA is a malignancy of middle to late adulthood, with a sharp upsurge in incidence after age 50 years.6 More than one third of new cases are diagnosed in patients older than 70 years, and more than half of patients diagnosed are older than 60 years of age.2 The incidence of pancreatic cancer is fairly equal among men and women, with a slightly higher rate for the male sex. It has an incidence preference for African-Americans by 4.8 cases per 100,000 persons nationally.7
Risk factors for the development of exocrine pancreatic cancer include hereditary disposition, underlying medical conditions, and environmental factors. One of the most significant environmental risk factors for the development of PDA is smoking,8 which is associated with up to 25% of all cases.9 Smoking cessation leads to a rapid reduction in risk for pancreatic cancer, with the risk among former smokers approaching that for never smokers less than 10 years after quitting.9 Other environmental factors that contribute to the development of pancreatic cancer include increased body mass index, a high-salt and high-saturated fat diet, heavy alcohol intake, and increased utilization of nonsteroidal inflammatory drugs.10–13
There is a strong association between new-onset diabetes and increased risk for developing PDA.14,15 Data also suggest that diabetes may be a risk factor and/or a consequence of tissue destruction that arises during the development or progression of PDA.16,17 Interestingly, ABO blood grouping is another underlying medical disposition that confers an altered risk profile. Studies have shown that patients with blood group O were less likely than those with type A, B, or AB to develop pancreatic cancer.18
Genetic predisposition syndromes can elevate an individual patient’s risk for developing PDA. Genetic syndromes and gene alterations that increase the risk for PDA include BRCA1/2, Peutz-Jeghers syndrome, and Lynch syndrome risk.19–21 Up to 10% to 15% of PDA cases may be due to an inherited familial cancer.22 Having a first-degree relative with PDA increases the odds of developing PDA 1.76-fold compared to those without a family history.23 The exact biologic and molecular mechanisms of familial pancreatic cancer are unclear. It is estimated that about 10% of patients with familial pancreatic cancer (FPC) carry BRCA2 mutations.24 Individuals at risk for FPC should undergo genetic screening for the presence of the most frequently inherited pancreatic cancer susceptibility genetic defects: BRCA2, PALB2, and ATM germline mutations.25 Carriers of BRCA2, who are also at increased risk for developing breast, ovarian, and prostate cancer, should be monitored closely. Of all hereditary conditions, hereditary pancreatitis confers the highest risk for developing PDA, with an approximate risk elevation of 40% to 50%.26,27 Although several genetic predisposition syndromes have been identified, most cases of pancreatic adenocarcinoma are thought to be sporadic.
CANCER BIOLOGY AND PATHOLOGY
The pathologic predecessor of PDA is pancreatic intraepithelial neoplasia (PIN). With further dysplastic changes that result from increasing genetic alterations, these precancerous lesions progress from low- to high-grade and finally to adenocarcinoma. More than 90% of all PINs across all grades have oncogenic KRAS mutations.28 Additionally, inactivating mutations in the tumor suppressor genes SMAD4, p53, and CDKN2A are found with increasing frequency in higher grade PINs. The frequency and presence of mutations in both oncogenes and tumor suppressor genes in precursor neoplasias mirror the genetic mutations noted in advanced PDA.29 Among all mutations, KRAS is the most common and most functionally important for pancreatic cancer cell survival. KRAS mutations not only have profound effects on downstream mediators of tumor growth and metastasis, but they are implicated in reprograming of cellular metabolism.30,31
Pancreatic adenocarcinoma has a unique microenvironment that makes it a difficult target for current therapeutic modalities. First, it is one of the most stroma-rich malignancies. The dense stroma surrounding pancreatic tumor cells leads to increased tumor pressures and alterations in tumor vascular perfusion.32 It also serves as a barrier that prevents chemotherapeutic drugs from reaching the tumor cells. Thus, clinical trials are under way to investigate agents such has hyaluronidase, which may degrade components of the extracellular matrix that supports thestromal environment. Additionally, there is data to suggest that the microenvironment of PDA downregulates immune monitoring, leading to further tumor growth.27,33 The molecular, cellular, and immunologic complexity of PDA may contribute to its resistance to traditional therapeutics.
EVALUATION AND DIAGNOSIS
CASE PRESENTATION
A 61-year-old man with a history of type 2 diabetes mellitus and chronic tobacco use presents to the emergency department (ED) with a 4-month history of progressively worsening abdominal discomfort and fatigue. He has also noticed darkening of his urine and slight yellow discoloration of his eyes. His weight measured 5 months ago in his primary care physician’s office was 91 kg (200 lb, BMI 29.5) and in the ED is 75 kg (165 lb, BMI 24.4). He has noticed bulky, malodorous, oily stools for about 2 months. Preliminary laboratory studies reveal elevated levels of total bilirubin (2.7 mg/dL) and alkaline phosphatase (204 IU/L). Transabdominal ultrasound (US) is obtained and reveals a 3-cm pancreatic mass with biliary tract dilation.
Does this patient have pancreatic cancer?
CLINICAL SIGNS AND SYMPTOMS
Establishing the diagnosis of pancreatic cancer in a patient who presents with a high index of suspicion is critical. Patients with pancreatic cancer usually present after a period of nonspecific and vague symptoms, which typically are experienced as abdominal discomfort, weight loss, and weakness. It is estimated that approximately 25% of patients may complain of vague abdominal pain up to 6 months prior to diagnosis. Up to 15% of patients may seek medical attention more than 6 months prior to establishing a diagnosis of PDA.34 The most common symptoms associated with pancreatic cancer in order of decreasing reported frequency are weight loss, anorexia, abdominal/epigastric pain, dark-colored urine, jaundice, nausea, back pain, and diarrhea with associated steatorrhea.35 Upwards of 15% of patients present with painless jaundice, a term that is often associated with pancreatic cancer.36 On exam these patients may have scleral icterus, sublingual jaundice, epigastric pain on palpation, weight loss, hepatomegaly, lymphadenopathy and a nontender, distended, palpable gallbladder (also known as Courvoisier sign).34 Abdominal signs and symptoms arise from tumor growth into surrounding vessels, tissues, and ducts within the abdominal cavity. Compression of the common bile duct accounts for the development of jaundice. Tumor growth around the stomach and duodenum can lead to delayed gastric emptying and subsequently nausea and vomiting. Constriction of the pancreatic duct leads to pancreatic insufficiency, precipitation of weight loss, and steatorrhea. Pancreatic insufficiency can worsen abdominal pain, and lead to increased weight loss and flatulence.
Less common symptoms include pain, erythema, and edema involving the lower extremities, which may be reflective of migratory thrombophlebitis (commonly known as Trousseau syndrome). Thromboembolic disease, including pulmonary embolism, portal vein, and deep vein thromboses are frequently encountered complications of pancreatic cancer. The incidence of thromboembolic events in patients with PDA has been reported to be as high as 54%.37 Of all signs encountered, weight loss is the most common and most profound. Patients with advanced PDA have severe degrees of cachexia. Some patients present with as much as a 5 kg/m2 decrease in their BMI from pre-illness baseline BMI, and lose another 3 to 4 kg/m2 through disease progression.38 At the time of diagnosis, many patients have already undergone significant weight loss, which can have substantial implications on treatment planning and clinical outcomes.
What other studies can be done to assist in making the diagnosis?
LABORATORY ABNORMALITIES AND TUMOR MARKERS
Elevations in alkaline phosphatase, γ-glutamyltransferase (GGT), serum aspartate aminotransferase (AST), serum alanine aminotransferase (ALT), and direct fractions of bilirubin are common in patients with PDA. Patients will usually have an obstructive pattern on their liver panel, with predominant elevations in direct bilirubin, alkaline phosphatase, and GGT, as compared with AST and ALT. Other baseline laboratory studies, including a complete blood count and basic metabolic panel, should be obtained because patients commonly have thrombocytosis, anemia, and electrolyte abnormalities due to the tumor itself and pancreatic insufficiency (Table 1).
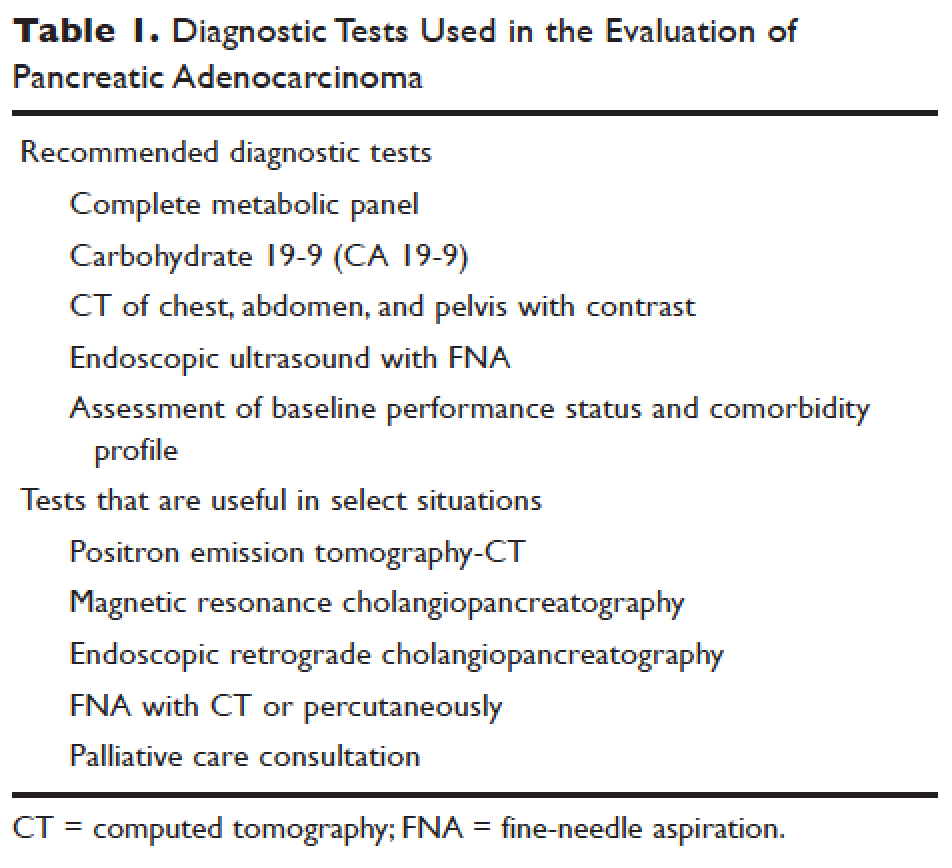
Measurement of glycated hemoglobin (HBA1C) is an emerging and important diagnostic test in the diagnosis of pancreatic cancer. Recently, data has emerged to suggest that new-onset diabetes is present in about 50% of patients diagnosed with pancreatic cancer.39 The temporal relationship of pancreatic cancer and diabetes is supported by evidence showing that patients who undergo resection commonly have resolution of their diabetes.17 This study suggested that hyperglycemia, elevated HBA1C, and symptoms of diabetes in patients older than 50 years may identify patients who have early pancreatic cancer. The entity of pancreatic cancer–associated diabetes needs to be better defined and the algorithmic approach to evaluation and diagnosis, utilizing signs, symptoms, and laboratory values associated with diabetes, needs to be clearly established.
The only serum marker for PDA is carbohydrate antigen 19-9 (CA 19-9), also known as sialylated Lewis antigen or cancer-associated antigen. It was first identified in pancreatic cancer patients in 1981.40,41 The sensitivity and specificity of CA 19-9 ranges from 70% to approximately 90%.42,43 Hereditary predispositions and comorbid disease cross-reactivity contribute to the diminished sensitivity and specificity of CA 19-9. In about 5% to 10% of the population, CA 19-9 is not expressed (Lewis antigen A and B negative). Additionally, since CA 19-9 is expressed in the cells that line the biliary tree, diseases that lead to pancreatic or liver inflammation may falsely elevate CA 19-9.44 As a result, CA 19-9 is not an ideal screening test. However, data has shown that CA 19-9 may have prognostic value postoperatively and serve as a marker for therapeutic response.45,46
Is biopsy needed for this patient and if so, what is the most appropriate technique?
ENDOSCOPIC ULTRASOUND
Generally, diagnosis with tissue is not necessary for patients who clearly have resectable disease and will proceed directly to surgery for management. Nevertheless, it is still commonly obtained in this group of patients. However, in patients with LAPC or with features suggestive of LAPC, such as tumor approximation to critical vessels such as the superior mesenteric artery (SMA) or celiac axis, biopsy is necessary. These patients will receive neoadjuvant therapy, and biopsy is important in establishing a diagnosis. The ideal way to obtain a biopsy is through fine-needle aspiration (FNA) or biopsy (FNB) utilizing endoscopic ultrasound (EUS). Percutaneous and computed tomography (CT)–guided FNB can also be used to obtain a biopsy for diagnosis. In comparison to percutaneous and CT-guided FNB, EUS-FNA/FNB has low rates of complications, a decreased rate of peritoneal seeding, and is cost effective.47,48
CASE CONTINUED
Abdominal CT obtained following abdominal ultrasound reveals a 3.5-cm mass in the head of the pancreas in close approximation to the SMA and celiac axis.
Does the patient have borderline resectable or unresectable disease?
IMAGING
Abdominal ultrasound is a reasonable, inexpensive, and safe alternative to abdominal CT as it does not utilize ionizing radiation. It is particularly useful in patients who present with jaundice or have concern for biliary obstruction based on laboratory evaluation. It is particularly sensitive for detecting tumors greater than 3 cm in size.49,50 In patients whose abdominal ultrasound is unrevealing and whose index of suspicion remains high for PDA, abdominal CT should be the next imaging modality.
Abdominal CT obtained utilizing a pancreatic protocol is ideal for detection and staging of pancreatic tumors. By implementing a triple-phase protocol with arterial, late arterial, and venous phases, tumors, which have a density different from that of the pancreatic parenchyma, are accentuated. Abdominal CT is also able to provide critical information about tumor resectability.51 By revealing the degree of tumor encasement around the aorta, level of destruction of the superior mesenteric vein, or degree of involvement of the SMA or celiac vessels, abdominal CT determines if a patient should be deemed resectable, borderline resectable, or unresectable (Table 2).52,53 Resectability is based on thorough imaging evaluation, expert opinion of a multidisciplinary team, and guidelines proposed by American Hepatopancreaticobiliary Association, Society of Surgical Oncology, Society for Surgery of the Alimentary Tract, and the NCCN.54
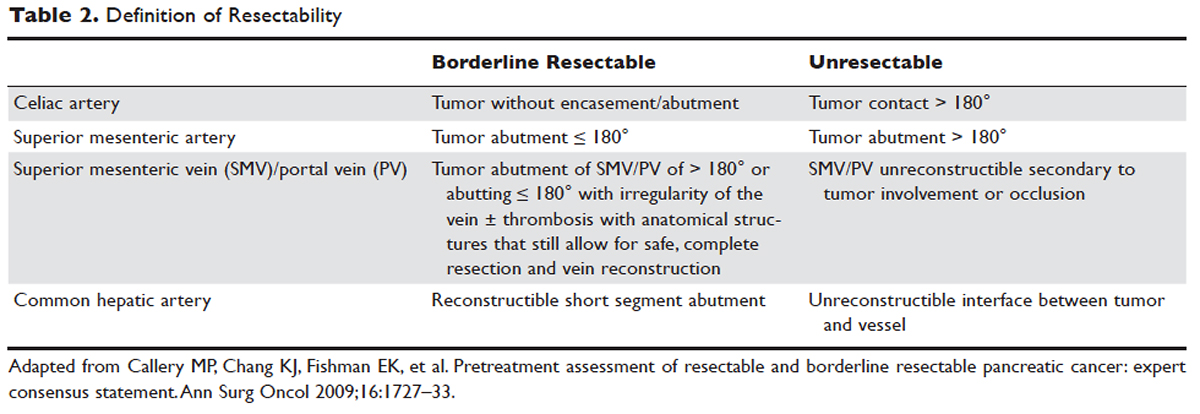
Other imaging modalities have a less clearly established role in the diagnostic approach to PDA. In patients who have contraindications to obtaining CT, magnetic resonance imaging can be utilized as a secondary imaging modality.55 The role of positron emission tomography 18F-fluorodeoxyglucose (PET-FDG) is not clearly defined among clinicians, nor reflected in consensus guidelines by the National Comprehensive Cancer Network (NCCN). In clinical practice, it is still often combined with CT to detect metastatic disease, particularly in high-risk patients such has those with LAPC. The role of PET-CT in staging and its impact on clinical outcomes has not been fully established.
Endoscopic retrograde cholangiopancreatography (ERCP) and magnetic resonance cholangiopancreatography (MRCP) can also assist in the diagnosis and management of PDA. In patients with obstructive jaundice, both MRCP and ERCP visualize obstructions and dilations within the biliary tree, with the latter having the ability to intervene. ERCP allows for the collection of tissue to aid in diagnosis, and has the ability to relieve biliary obstruction via stenting.56
TREATMENT
CASE CONTINUED
After an abdominal CT is obtained, the patient is referred to an outpatient oncologist because of concern for pancreatic adenocarcinoma. After consultation, the patient is advised to obtain EUS with biopsy and to return immediately afterwards for further treatment planning. The pathology report following EUS confirms that the mass is a poorly differentiated PDA. The patient’s case is discussed at a multidisciplinary meeting with radiation, surgical, and medical oncology. The abdominal CT and PET-CT scan are thoroughly reviewed. After imaging review, the multidisciplinary team concludes that the tumor is in contact with the SMA at 120° and with the common hepatic artery without extension in the celiac axis and without evidence of metastasis.
What is the appropriate management of borderline resectable pancreatic cancer?
BORDERLINE RESECTABLE CANCER
Patients who have nonmetastatic disease and are deemed resectable and without contraindications to surgery or high-risk features, as defined by NCCN guidelines, should proceed directly to surgery. A large body of evidence suggests that complete surgical resection with negative margins is a significant predictor of survival and currently provides the only option for cure.57–59 Despite the curative intent of surgery, the rate of recurrence remains high in patients who undergo surgical resection. Even in patients with negative resection margins (R0 resection), the 5-year survival is 20% to 30%, with a median survival ranging from 12 to 25 months, suggesting the presence of regional and distant occult disease at the time of diagnosis.60–62
Additionally, in half the patients who undergo surgical resection with resultant positive microscopic (R1 resection) or gross (R2 resection) margins, the median survival is no greater than 12 months. In this subset of patients, clinical outcomes are similar to outcomes in patients with locally advanced and metastatic pancreatic cancer, suggesting that upfront surgery and adjuvant therapy may not be the ideal therapeutic option. This raises 2 important points: first, resectability should be assessed carefully in all patients with LAPC, and second, for those patients who are deemed borderline resectable, neoadjuvant therapy should be considered.63 Borderline resectability is defined as tumor abutment ≤ 180° of the celiac artery, and tumor abutment of the superior mesenteric vein /portal vein of > 180° or abutting ≤ 180° with irregularity of the vein with or without thrombosis with anatomical structures that still allows for safe and complete resection and vein reconstruction (Table 2).
Neoadjuvant Therapy
The goal of neoadjuvant therapy is to minimize the negative impact of upfront surgery in patients who have a high likelihood of having microscopic or grossly positive margins. Research has suggested that neoadjuvant therapy may improve resectability, decrease the rate of recurrence, and improve overall survival.64–66
There is no clear consensus on the ideal management of patients with borderline resectable disease. However, expert guidelines are in agreement that upfront surgery in patients with LAPC is not appropriate, as most patients will not be able to achieve an R0 resection.67 As staging and management of patients with LAPC is difficult, expertise of a multidisciplinary team can be helpful.68
Several studies and the NCCN guidelines support the use of neoadjuvant therapy in patients deemed borderline resectable.69,70 Treatment of borderline resectable disease is similar to unresectable LAPC and generally involves 2 chemotherapy treatment backbones: FOLFIRINOX (folinic acid [leucovorin], fluorouracil [5-FU], irinotecan, and oxaliplatin) or gemcitabine-based therapy.
Phase 1 to 2/3 clinical trials conducted by Conroy et al from 2005 to 2011, including the landmark ACCORD-11 trial, established the safety and role of FOLFIRINOX in metastatic pancreatic cancer and also demonstrated an improved overall survival with the use of this therapy in these patients.71,72 These findings led to interest in FOLFIRINOX as a neoadjuvant therapy for patients with LAPC. Since then, multiple prospective and retrospective studies have shown that 54% to 100% of patients with borderline resectable LAPC who were treated with FOLFIRINOX were down-staged significantly enough to undergo resection. Of those patients, more than 90% had a R0 resection following surgery (Table 3).73–79
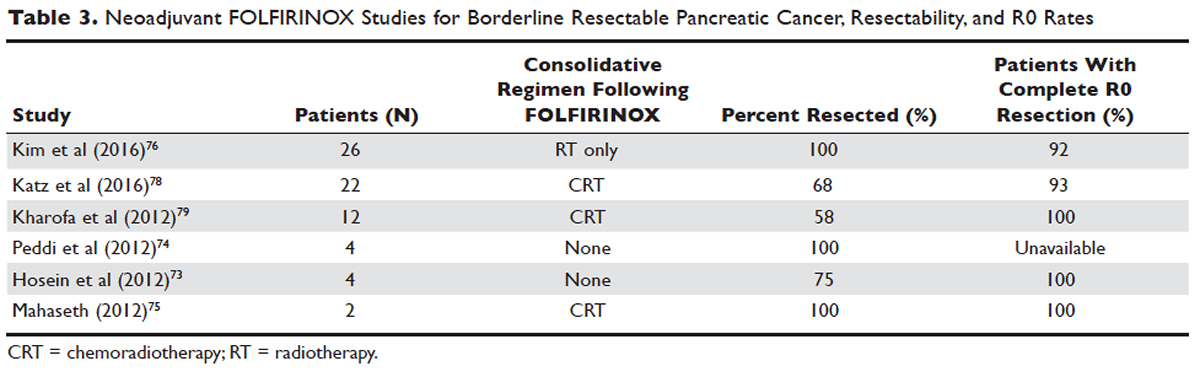
Data over the past 7 years suggests that neoadjuvant FOLFIRINOX improves overall survival and resectability in patients with borderline disease. However, treatment with FOLFIRINOX is not without limitations. FOLFIRINOX is associated with higher rates of febrile neutropenia, thrombocytopenia, diarrhea, and sensory neuropathy as compared with gemcitabine-based therapy.72 Other less commonly observed toxicities associated with FOLFIRINOX include mucositis, hand-foot syndrome, pulmonary toxicity, and alopecia. Dose-attenuated FOLFIRINOX-based regimens, including those that exclude the bolus fluorouracil dose and augment upfront filgrastim, have demonstrated improved safety and comparable efficacy as compared to standard FOLFIRINOX.80
Gemcitabine has been the fundamental treatment backbone for PDA since the results of the phase 3 CONKO-001 trial were published.81 Gemcitabine is a pyrimidine antimetabolite and potent inhibitor of DNA polymerase and ribonucleotide reductase.82 In recent years, multiple combination therapies with gemcitabine have been investigated, including regimens with nab-paclitaxel, oxaliplatin, or docetaxel. Resection rates and negative margin outcomes have been shown to be comparable to patients who received FOLFIRINOX in the neoadjuvant setting with borderline locally advanced disease.83–85 In addition to having a more tolerable side effect profile in comparison to fluorouracil-based regimens, gemcitabine is considered to be a potent radiosensitizer.86 For this reason, studies have also investigated the role of radiotherapy in conjunction with gemcitabine, revealing negative margin resection rates above 80% in patients with borderline resectable disease.87,88
Because very few studies directly comparing FOLFIRINOX with gemcitabine-based combination regimens have been completed, there is no clear consensus on the preferred treatment regimen, in both borderline and unresectable LAPC. Decisions to treat are influenced predominantly by comorbidities, adverse effect profiles, and performance status of patients, as FOLFIRINOX is the more toxic of the 2 treatment backbones. Therefore, FOLFIRINOX has mostly been utilized in patients with relatively good functional status (Eastern Cooperative Oncology Group [ECOG] performance status 0 to 1).89 In elderly patients and those with poor functional status, ECOG 2 to 4, gemcitabine as a single agent is a reasonable alternative in the neoadjuvant setting of borderline resectable disease.
The exact role of radiation therapy in addition to induction chemotherapy in borderline resectable pancreatic cancer has not been clearly established because of the lack of prospective studies in this area. Multiple large retrospective series have identified high rates of conversion to margin-negative resection with neoadjuvant chemoradiation alone.90 Based on available data, it is reasonable for patients with borderline resectable disease to proceed with any of the following treatment options: chemotherapy, chemoradiation, or induction chemotherapy followed by chemoradiation (Figure). Chemotherapy and chemoradiation are generally more appropriate with patients with high CA 19-9 levels or those at an elevated risk of having positive margins or occult metastatic disease.91 Obtaining negative margin resections is the predominant goal of neoadjuvant radiotherapy.89 Many studies have identified margin status to be one of the most significant prognostic factors in PDA.57,59,92,93 Additionally, several studies have highlighted that radiotherapy in the neoadjuvant setting could improve negative margin resection rates, local control, and clinical outcomes in patients with borderline resectable locally advanced disease.94–97 A common multimodal regimen utilized in the neoadjuvant setting combines capecitabine, an oral prodrug that is converted to fluorouracil, with radiation therapy. This combination has also been shown to improve resectability rates and long-term clinical outcomes in patients with borderline resectable disease.98 Additionally, neoadjuvant radiation therapy can potentially downstage patients with unresectable disease at the time of diagnosis to become surgical candidates.99 Despite the paucity of data, interval scans utilizing CT following neoadjuvant therapy should be obtained 2 to 4 months after completion of therapy to determine therapeutic response, evaluate for disease progression, and, most important, reassess surgical stage/resectability. It is clinically acceptable to proceed to resection with radiographically stable disease post-neoadjuvant therapy.

Many patients classified as borderline resectable are able to proceed with surgery following neoadjuvant therapy. Unfortunately, specific data on adjuvant therapy following neoadjuvant chemotherapy or chemoradiotherapy and surgical resection in borderline resectable patients is scarce. Clinical practice guidelines are extrapolated from studies where upfront resection in clearly resectable patients was followed by adjuvant therapy. Based on these data, approximately 6 months of perioperative chemotherapy with or without chemoradiotherapy is a reasonable consideration. Nevertheless, about 80% of patients at the time of diagnosis are deemed to be unresectable, and a smaller number do not proceed to surgery despite an initial classification as borderline resectable. Of the 80% of patients with advanced disease, about half are metastatic at presentation and the remaining 30% to 40% are defined as having unresectable LAPC.100
CASE CONTINUED
The patient is deemed borderline resectable. He receives neoadjuvant therapy with gemcitabine and nab-paclitaxel. Two months after therapy, interval imaging with abdominal CT does not show improvement in tumor size and there is now evidence that the tumor has invaded the celiac axis and is abutting more than 180° of the SMA. The patient presents to the oncologist to discuss further management. He has lost about 15 lb since his last evaluation, is capable of self-care, but is unable to carry on with any work activities.
What is the appropriate management of unresectable nonmetastatic LAPC?
UNRESECTABLE LOCALLY ADVANCED CANCER
As in the case of borderline resectable disease, there are many treatment options for patients with unresectable LAPC. Timing, optimal chemotherapy regimen, and the addition of regularly and hypofractionated radiotherapy are issues currently under investigation. However, there are some general considerations and principles that are followed as reflected in the NCCN guidelines and recent studies. The primary therapeutic aims in patients with unresectable locally advanced disease are to increase survival and improve palliation.
The elderly comprise a large percentage of the patients diagnosed with unresectable locally advanced disease. Pharmacokinetics and toxicity profiles are altered in the elderly population.101,102 Therefore, it is important to assess functional status and comorbidities as these are critical factors in determining treatment regimens, similar to patients with borderline resectable disease. Currently, the most common first-line therapies in advanced pancreatic cancer are gemcitabine alone, gemcitabine and nab-paclitaxel, FOLFIRINOX, gemcitabine/capecitabine, and gemcitabine/oxaliplatin.103 The overall treatment approach to unresectable locally advanced pancreatic adenocarcinoma closely mirrors that of patients with borderline resectable disease and metastatic disease. Much of the data supporting treatment regimens in unresectable LAPC is extrapolated from clinical trials looking at advanced or metastatic pancreatic cancer.
Consensus opinions domestically and from Europe recommend that patients with locally advanced unresectable disease undergo upfront chemotherapy (Figure).104 This is based on the premise that initial chemotherapy may destroy occult metastatic cells and increase the efficacy of consolidative chemotherapy, particularly with radiation in the future. Upfront chemoradiotherapy has only been investigated in a small series of trials in which no clear survival benefit was observed and has the added consequence of treatment-related toxicity.105 However, data is limited in this regard, with variations in treatment protocols and cohort compositions contributing to the inconclusive findings.
Despite advances in immunotherapy, targeted therapies, and gene sequencing, initial chemotherapy for unresectable disease is still either gemcitabine-based combination therapy or FOLFIRINOX. Across numerous studies, patients with unresectable LAPC receiving FOLFIRINOX have a median progression-free survival of 3 to 20 months and a median overall survival of 10 to 32.7 months.106 As with borderline resectable patients, FOLFIRINOX (Table 4) is generally reserved for unresectable patients with good functional status (ECOG 0–1 or Karnofsky Performance Status 90–100) and those at low risk for developing grade 3 or 4 systemic toxicities.103 For these reasons it has generally not been frequently combined with other chemotherapeutic agents. However, FOLFIRINOX has been combined with radiation therapy in the consolidative neoadjuvant setting after induction chemotherapy. There have also been studies where traditional FOLFIRONIX was modified to improve tolerability, as evidenced by Gunturu et al’s study, which dose-reduced both fluorouracil and irinotecan by 25%, without compromising efficacy and simultaneously increasing tolerability.107 Additionally, FOLFIRINOX requires infusional administration of the fluorouracil component, which may not be practical in certain patients. In that subset, capecitabine can be substituted. Among radiosensitizers during neoadjuvant therapy for unresectable LAPC, capecitabine has been shown to be as efficacious and less toxic than even gemcitabine.108
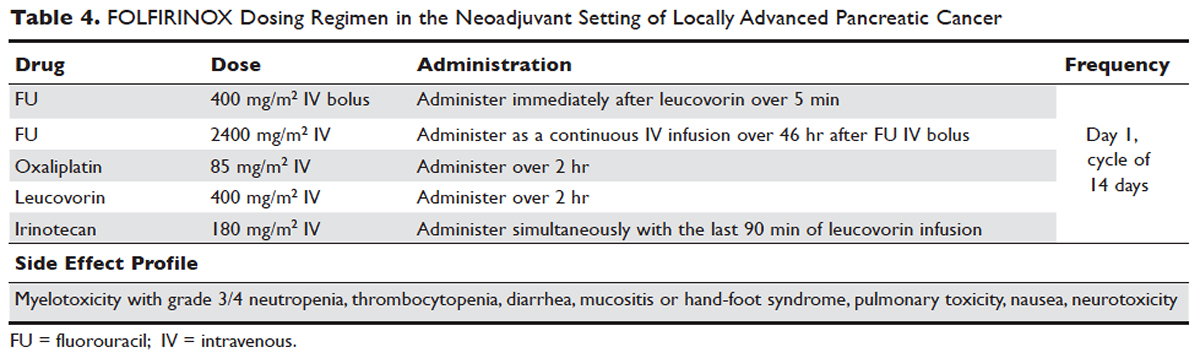
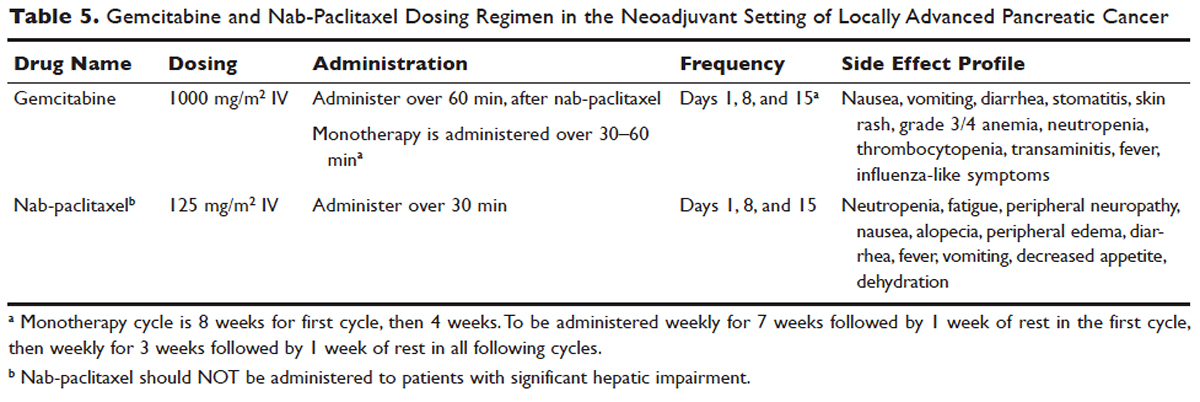
No head-to-head studies investigating FOLFIRINOX versus nab-paclitaxel and gemcitabine in patients with locally advanced disease have been published, but clinical trials are under way. Other combination therapies have been looked at through small retrospective or prospective studies, but no robust, large-scale clinical trials have been completed. For this reason, NCCN guidelines recommend enrollment of patients with LAPC into active clinical trials.
What is the role of radiation therapy in unresectable LAPC?
Despite the reported advantages of neoadjuvant radiation in patients with potentially resectable disease, there is significant debate regarding the timing and role of neoadjuvant radiation in patients with unresectable disease. Numerous comprehensive analyses and studiest indicate that chemoradiotherapy leads to significantly better overall survival compared to no therapy or radiation therapy alone in LAPC.68,110,111 However, conflicting data support the use of upfront chemoradiotherapy in unresectable LAPC when compared to chemotherapy alone. Unfortunately, most prospective studies investigating the role of radiotherapy were performed following administration of single-agent gemcitabine, which is no longer considered standard of care for patients with LAPC. In spite of this, ECOG 4201 identified a statistically significant improvement in median overall survival following the addition of gemcitabine-based radiotherapy. Huguet et al in his review pointed out that upfront chemoradiotherapy was not superior to chemotherapy only and was associated with increased treatment toxicity.105 Additionally, a recent phase 3 study looking at chemoradiotherapy versus chemotherapy alone in patients treated with gemcitabine found no difference in overall survival.112 This can potentially be attributed to the fact that about 30% of patients with LAPC develop metastatic disease in the early phases of treatment due to poor control of local and systemically occult disease.113 Given the propensity for high rates of occult metastatic disease in LAPC, treatment paradigms and consensus guidelines recommend multi-agent systemic chemotherapy followed by chemoradiotherapy in select patients.
Based on current studies and until further clinical investigations are completed, consensus opinion indicates that the most appropriate approach in unresectable LAPC is to begin with induction chemotherapy (with either gemcitabine plus nab-paclitaxel, FOLFIRINOX, capecitabine, or other treatment combinations), followed by chemoradiation in the absence of disease progression when the first repeat imaging evaluation is completed (Figure). One important caveat regarding reimaging with CT in the neoadjuvant setting is that radiologic response may not correlate with pathologic response.114 PET-CT may have a role in predicting response to neoadjuvant therapy. Overall, induction chemotherapy followed by consolidative chemoradiation may confer numerous benefits: it removes the unnecessary burden and toxicity associated with radiotherapy in the nearly one third of patients who have pervasive disease progression during initial treatment; it allows testing and increases the chances of tolerating full-dose systemic chemotherapy; and it raises the likelihood of converting patients who do not progress to metastasis during the initial phase of treatment from unresectable to resectable status.103,115 Despite the lack of strong conclusive data, the general agreement is that neoadjuvant chemoradiotherapy converts about one third of borderline and unresectable LAPC to an R0 resection.95,103 There are very specific rationales for the addition of radiotherapy in LAPC, and these functions need to be better defined through further clinical trials.
PALLIATIVE CARE
CASE CONTINUED
The patient is unable to tolerate his first round of second-line therapy with modified FOLFIRINOX. His overall treatment plan is transitioned to palliation. He continues to have pain, despite increasing doses of narcotics.
What is the next step for patients in whom second-line therapy fails and who have intractable pain while on high-dose narcotics?
A subset of patients with unresectable LAPC may not be amenable to chemotherapy with or without radiation due to significant comorbidities or because they present with or progress to ECOG scores 3 or 4. The goal in these patients should be palliation. Pain is one of the most predominant and difficult to manage symptoms in progressive LAPC. Opioid-based medications are the primary treatment for pain in LAPC. However, patients sometimes become refractory to opioid medications. In this group of patients, it is reasonable to consider palliative radiation as an alternative method for pain control.116
An alternative to palliative radiation in the setting of progressive pain in PDA is celiac plexus block or neurolysis. By injecting an anesthetic or alcohol into the celiac plexus, neural signaling pathways involved in the propagation of pain are inhibited without leading to significant nerve destruction. Additionally, chemical splanchnicectomy allows for reduced opioid medication use and associated side effects.117
In general patients with LAPC have profound weight loss prior to and during treatment. This has significant implications prognostically and on treatment options. The underlying etiology is multifactorial, but one of the primary driving factors is pancreatic insufficiency. An estimated 65% of pancreatic cancer patients have fat malabsorption, and 50% have protein malabsorption, leading to steatorrhea and weight loss.118 Patients diagnosed with pancreatic cancer should be given enzyme replacement with formulations that include lipase, amylase, and protease. A minimum dose of enzyme replacement should include 40,000 to 50,000 U of lipase during meals and 25,000 U during snack intake. If maldigestion, symptoms, or nutritional endpoints (BMI, albumin, prealbumin, cholesterol) do not improve, the pancreatic enzyme dose should be escalated and a proton-pump inhibitor (PPI) added. In patients with pancreatic insufficiency, PPIs have been shown to improve fat absorption.119 Enzyme replacement therapy has been shown to prevent weight loss in patients with unresectable pancreatic cancer.120
As most patients with LAPC go on to develop progressive disease, palliative care becomes an integral aspect of the therapeutic paradigm. Palliation in LAPC has a significant role in determining quality of life and ensuring patient’s goals of care have been meet. Studies have suggested that pancreatic cancer is second only to lung cancer in terms of the number of emergency department visits in the later stages of disease.120 Additionally, aggressive care in the setting of incurable diseases such as LAPC has been associated with poor quality of life.121 More recently it has been shown that involvement of palliative care in patients with advanced pancreatic is associated with less aggressive care near death.122 Therefore, the incorporation of palliative or supportive care teams in the treatment of patients with progressive LAPC can improve quality of life and alleviate suffering associated with increasing symptom burden.
CONCLUSION
LAPC is a difficult disease for both provider and patient. There is a paucity of robust clinical trials in the neoadjuvant setting for LAPC. Current research is complicated by varying consensus definitions of resectability and the varying treatment configurations across studies. The optimal type, timing, and sequence of treatment and whether to add radiation therapy in LAPC have not been clearly defined. However, based on the available studies and consensus guidelines, patients who are deemed to have LAPC should have neoadjuvant therapy. FOLFIRINOX or gemcitabine with nab-paclitaxel should be considered first-line treatments. Patients with LAPC who respond to chemotherapy or are ineligible for multi-drug chemotherapy may benefit from chemoradiotherapy. In patients with unresectable disease, chemoradiotherapy has been shown to enhance survival as compared to best supportive care or radiation alone. For borderline resectable disease, it is reasonable to treat patients with either chemoradiotherapy, chemotherapy alone, or chemotherapy followed by chemoradiotherapy.
Considering the invasive nature of LAPC and the controversy around neoadjuvant treatment protocols, enrollment of patients with LAPC into clinical trials is important and will help determine the optimal treatment regimen for future patients.
- Network PCA. Pancreatic cancer facts 2016. 2016. https://www.pancan.org/wp-content/uploads/2016/02/2016-GAA-PC-Facts.pdf. Accessed April 24, 2017.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7–30.
- Konstantinidis IT, Warshaw AL, Allen JN, et al. Pancreatic ductal adenocarcinoma: is there a survival difference for R1 resections versus locally advanced unresectable tumors? What is a “true” R0 resection? Ann Surg 2013;257:731–6.
- Ilic M, Ilic I. Epidemiology of pancreatic cancer. World J Gastroenterol 2016;22:9694–705.
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69–90.
- Zhang J, Dhakal I, Ning B, Kesteloot H. Patterns and trends of pancreatic cancer mortality rates in Arkansas, 1969-2002: a comparison with the US population. Eur J Cancer Prev 2008;17:18–27.
- National Cancer Institute. SEER cancer statistics review, 1975-2013. http://seer.cancer.gov/csr/1975_2013/. Accessed April 24, 2017.
- Lowenfels AB, Maisonneuve P. Epidemiology and risk factors for pancreatic cancer. Best Pract Res Clin Gastroenterol 2006;20:197–209.
- Fuchs CS, Colditz GA, Stampfer MJ, et al. A prospective study of cigarette smoking and the risk of pancreatic cancer. Arch Intern Med 1996;156:2255–60.
- Lucenteforte E, La Vecchia C, Silverman D, et al. Alcohol consumption and pancreatic cancer: a pooled analysis in the International Pancreatic Cancer Case-Control Consortium (PanC4). Ann Oncol 2012;23:374–82.
- Schernhammer ES, Kang JH, Chan AT, et al. A prospective study of aspirin use and the risk of pancreatic cancer in women. J Natl Cancer Inst 2004;96:22–28.
- Michaud DS, Giovannucci E, Willett WC, et al. Physical activity, obesity, height, and the risk of pancreatic cancer. JAMA 2001;286:921–9.
- Nothlings U, Wilkens LR, Murphy SP, et al. Meat and fat intake as risk factors for pancreatic cancer: the multiethnic cohort study. J Natl Cancer Inst 2005;97:1458–65.
- Chari ST, Leibson CL, Rabe KG, et al. Probability of pancreatic cancer following diabetes: a population-based study. Gastroenterology 2005;129:504–11.
- Batabyal P, Vander Hoorn S, Christophi C, Nikfarjam M. Association of diabetes mellitus and pancreatic adenocarcinoma: a meta-analysis of 88 studies. Ann Surg Oncol 2014;21:2453–62.
- Chari ST, Leibson CL, Rabe KG, et al. Pancreatic cancer-associated diabetes mellitus: prevalence and temporal association with diagnosis of cancer. Gastroenterology 2008;134:95–101.
- Pannala R, Basu A, Petersen GM, Chari ST. New-onset diabetes: a potential clue to the early diagnosis of pancreatic cancer. Lancet Oncol 2009;10:88–95.
- Wolpin BM, Chan AT, Hartge P, et al. ABO blood group and the risk of pancreatic cancer. J Natl Cancer Inst 2009;101:424–31.
- Iqbal J, Ragone A, Lubinski J, et al. The incidence of pancreatic cancer in BRCA1 and BRCA2 mutation carriers. Br J Cancer 2012;107:2005–9.
- Giardiello FM, Brensinger JD, Tersmette AC, et al. Very high risk of cancer in familial Peutz-Jeghers syndrome. Gastroenterology 2000;119:1447–53.
- Kastrinos F, Mukherjee B, Tayob N, et al. Risk of pancreatic cancer in families with Lynch syndrome. JAMA 2009;302:1790–5.
- Brand RE, Lynch HT. Hereditary pancreatic adenocarcinoma. A clinical perspective. Med Clin North Am 2000;84:665–75.
- Jacobs EJ, Chanock SJ, Fuchs CS, et al. Family history of cancer and risk of pancreatic cancer: a pooled analysis from the Pancreatic Cancer Cohort Consortium (PanScan). Int J Cancer 2010;127:1421–8.
- Rustgi AK. Familial pancreatic cancer: genetic advances. Genes Dev 2014;28:1–7.
- Reznik R, Hendifar AE, Tuli R. Genetic determinants and potential therapeutic targets for pancreatic adenocarcinoma. Front Physiol 2014;5:87.
- Lowenfels AB, Maisonneuve P, DiMagno EP, et al. Hereditary pancreatitis and the risk of pancreatic cancer. International Hereditary Pancreatitis Study Group. J Natl Cancer Inst 1997;89:442–6.
- Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med 2014;371:2140–1.
- Kanda M, Matthaei H, Wu J, et al. Presence of somatic mutations in most early-stage pancreatic intraepithelial neoplasia. Gastroenterology 2012;142:730–3.
- Feldmann G, Maitra A. Molecular genetics of pancreatic ductal adenocarcinomas and recent implications for translational efforts. J Mol Diagn 2008;10:111–22.
- Eser S, Schnieke A, Schneider G, Saur D. Oncogenic KRAS signalling in pancreatic cancer. Br J Cancer 2014;111:817–22.
- Ying H, Kimmelman AC, Lyssiotis CA, et al. Oncogenic KRAS maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell 2012;149:656–70.
- Provenzano PP, Cuevas C, Chang AE, et al. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell 2012;21:418–29.
- Vonderheide RH, Bayne LJ. Inflammatory networks and immune surveillance of pancreatic carcinoma. Curr Opin Immunol 2013;25:200–5.
- DiMagno EP. Pancreatic cancer: Clinical presentation, pitfalls and early clues. Ann Oncol 1999;10(suppl 4):S140–S142.
- Porta M, Fabregat X, Malats N, et al. Exocrine pancreatic cancer: symptoms at presentation and their relation to tumour site and stage. Clin Transl Oncol 2005;7:189–97.
- Gullo L, Tomassetti P, Migliori M, et al. Do early symptoms of pancreatic cancer exist that can allow an earlier diagnosis? Pancreas 2001;22:210–3.
- Khorana AA, Fine RL. Pancreatic cancer and thromboembolic disease. Lancet Oncol 2004;5(11):655-663.
- Wigmore SJ, Plester CE, Richardson RA, Fearon KC. Changes in nutritional status associated with unresectable pancreatic cancer. Br J Cancer 1997;75:106–9.
- Aggarwal G, Rabe KG, Petersen GM, Chari ST. New-onset diabetes in pancreatic cancer: a study in the primary care setting. Pancreatology 2012;12:156–61.
- Koprowski H, Herlyn M, Steplewski Z, Sears HF. Specific antigen in serum of patients with colon carcinoma. Science 1981;212:53–5.
- Bond-Smith G, Banga N, Hammond TM, Imber CJ. Pancreatic adenocarcinoma. BMJ 2012;344:e2476.
- Cwik G, Wallner G, Skoczylas T, et al. Cancer antigens 19-9 and 125 in the differential diagnosis of pancreatic mass lesions. Arch Surg 2006;141:968–73.
- van den Bosch RP, van Eijck CH, Mulder PG, Jeekel J. Serum CA19-9 determination in the management of pancreatic cancer. Hepatogastroenterology 1996;43:710–3.
- Lamerz R. Role of tumour markers, cytogenetics. Ann Oncol 1999;10 Suppl 4:145–9.
- Hess V, Glimelius B, Grawe P, et al. CA 19-9 tumour-marker response to chemotherapy in patients with advanced pancreatic cancer enrolled in a randomised controlled trial. Lancet Oncol 2008;9:132–8.
- Montgomery RC, Hoffman JP, Riley LB, et al. Prediction of recurrence and survival by post-resection CA 19-9 values in patients with adenocarcinoma of the pancreas. Ann Surg Oncol 1997;4:551–6.
- Zamboni GA, D’Onofrio M, Idili A, et al. Ultrasound-guided percutaneous fine-needle aspiration of 545 focal pancreatic lesions. AJR Am J Roentgenol 2009;193:1691–5.
- Micames C, Jowell PS, White R, et al. Lower frequency of peritoneal carcinomatosis in patients with pancreatic cancer diagnosed by EUS-guided FNA vs. percutaneous FNA. Gastrointest Endosc 2003;58:690–5.
- Brambs HJ, Claussen CD. Pancreatic and ampullary carcinoma. Ultrasound, computed tomography, magnetic resonance imaging and angiography. Endoscopy 1993;25:58–68.
- Karlson BM, Ekbom A, Lindgren PG, et al. Abdominal US for diagnosis of pancreatic tumor: prospective cohort analysis. Radiology 1999;213:107–11.
- Imbriaco M, Megibow AJ, Camera L, et al. Dual-phase versus single-phase helical CT to detect and assess resectability of pancreatic carcinoma. AJR Am J Roentgenol 2002;178:1473–9.
- Schrag D. Optimizing treatment for locally advanced pancreas cancer: progress but no precision. JAMA 2016;315:1837–8.
- House MG, Yeo CJ, Cameron JL, et al. Predicting resectability of periampullary cancer with three-dimensional computed tomography. J Gastrointest Surg 2004;8:280–8.
- Callery MP, Chang KJ, Fishman EK, et al. Pretreatment assessment of resectable and borderline resectable pancreatic cancer: expert consensus statement. Ann Surg Oncol 2009;16:1727–33.
- Horton KM, Fishman EK. Adenocarcinoma of the pancreas: CT imaging. Radiol Clin North Am 2002;40:1263–72.
- Ross WA, Wasan SM, Evans DB, et al. Combined EUS with FNA and ERCP for the evaluation of patients with obstructive jaundice from presumed pancreatic malignancy. Gastrointest Endosc 2008;68:461–6.
- Hartwig W, Hackert T, Hinz U, et al. Pancreatic cancer surgery in the new millennium: better prediction of outcome. Ann Surg 2011;254:311–9.
- Jamieson NB, Chan NI, Foulis AK, et al. The prognostic influence of resection margin clearance following pancreaticoduodenectomy for pancreatic ductal adenocarcinoma. J Gastrointest Surg 2013;17:511–21.
- Ethun CG, Kooby DA. The importance of surgical margins in pancreatic cancer. J Surg Oncol 2016;113:283–8.
- Fischer R, Breidert M, Keck T, et al. Early recurrence of pancreatic cancer after resection and during adjuvant chemotherapy. Saudi J Gastroenterol 2012;18:118–21.
- Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med 2004;350:1200–10.
- Neoptolemos JP, Palmer DH, Ghaneh P, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet 2017;389(10073):1011–24.
- Cardenes HR, Chiorean EG, Dewitt J, et al. Locally advanced pancreatic cancer: current therapeutic approach. Oncologist 2006;11:612–23.
- Yeung RS, Weese JL, Hoffman JP, et al. Neoadjuvant chemoradiation in pancreatic and duodenal carcinoma. A Phase II Study. Cancer 1993;72:2124–33.
- Spitz FR, Abbruzzese JL, Lee JE, et al. Preoperative and postoperative chemoradiation strategies in patients treated with pancreaticoduodenectomy for adenocarcinoma of the pancreas. J Clin Oncol 1997;15:928–37.
- McClaine RJ, Lowy AM, Sussman JJ, et al. Neoadjuvant therapy may lead to successful surgical resection and improved survival in patients with borderline resectable pancreatic cancer. HPB (Oxford) 2010;12:73–9.
- Balaban EP, Mangu PB, Khorana AA, et al. Locally advanced, unresectable pancreatic cCancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2016;34:2654–68.
- Shaib WL, Ip A, Cardona K, et al. Contemporary management of borderline resectable and locally advanced unresectable pancreatic cancer. Oncologist 2016;21:178–87.
- Chun YS, Milestone BN, Watson JC, et al. Defining venous involvement in borderline resectable pancreatic cancer. Ann Surg Oncol 2010;17:2832–8.
- Evans DB, Erickson BA, Ritch P. Borderline resectable pancreatic cancer: definitions and the importance of multimodality therapy. Ann Surg Oncol 2010;17:2803–5.
- Conroy T, Paillot B, Francois E, et al. Irinotecan plus oxaliplatin and leucovorin-modulated fluorouracil in advanced pancreatic cancer--a Groupe Tumeurs Digestives of the Federation Nationale des Centres de Lutte Contre le Cancer study. J Clin Oncol 2005;23:1228–36.
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817–25.
- Hosein PJ, Macintyre J, Kawamura C, et al. A retrospective study of neoadjuvant FOLFIRINOX in unresectable or borderline-resectable locally advanced pancreatic adenocarcinoma. BMC Cancer 2012;12:199.
- Peddi PF, Lubner S, McWilliams R, et al. Multi-institutional experience with FOLFIRINOX in pancreatic adenocarcinoma. JOP 2012;13:497–501.
- Mahaseth H, Kauh JS, Brutcher E, et al. Safety and efficacy of modified FOLFIRINOX in pancreatic cancer: A retrospective experience. J Clin Oncol 2012;30 (suppl; abstr e14614).
- Kim SS, Nakakura EK, Wang ZJ, et al. Preoperative FOLFIRINOX for borderline resectable pancreatic cancer: Is radiation necessary in the modern era of chemotherapy? J Surg Oncol 2016;114:587–96.
- Conroy T, Gavoille C, Samalin E, et al. The role of the FOLFIRINOX regimen for advanced pancreatic cancer. Curr Oncol Rep 2013;15:182–9.
- Katz MH, Shi Q, Ahmad SA, et al. Preoperative modified FOLFIRINOX treatment followed by capecitabine-based chemoradiation for borderline resectable pancreatic cancer: Alliance for Clinical Trials in Oncology Trial A021101. JAMA Surg 2016;151:e161137.
- Kharofa J, Kelly TR, Ritch PS, et al. 5-FU/leucovorin, irinotecan, oxaliplatin (FOLFIRINOX) induction followed by chemoXRT in borderline resectable pancreatic cancer. J Clin Oncol 2012;30 (suppl; abstr e14613).
- Blazer M, Wu C, Goldberg RM, et al. Neoadjuvant modified (m) FOLFIRINOX for locally advanced unresectable (LAPC) and borderline resectable (BRPC) adenocarcinoma of the pancreas. Ann Surg Oncol 2015;22:1153–9.
- Oettle H, Post S, Neuhaus P, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA 2007;297:267–77.
- Plunkett W, Huang P, Xu YZ, et al. Gemcitabine: metabolism, mechanisms of action, and self-potentiation. Semin Oncol 1995;22(4 Suppl 11):3–10.
- Sahora K, Kuehrer I, Eisenhut A, et al. NeoGemOx: Gemcitabine and oxaliplatin as neoadjuvant treatment for locally advanced, nonmetastasized pancreatic cancer. Surgery 2011;149(3):311–20.
- Lee JL, Kim SC, Kim JH, et al. Prospective efficacy and safety study of neoadjuvant gemcitabine with capecitabine combination chemotherapy for borderline-resectable or unresectable locally advanced pancreatic adenocarcinoma. Surgery 2012;152:851–62.
- Leone F, Gatti M, Massucco P, et al. Induction gemcitabine and oxaliplatin therapy followed by a twice-weekly infusion of gemcitabine and concurrent external-beam radiation for neoadjuvant treatment of locally advanced pancreatic cancer: a single institutional experience. Cancer 2013;119:277–84.
- Lawrence TS, Eisbruch A, Shewach DS. Gemcitabine-mediated radiosensitization. Semin Oncol 1997;24(2 Suppl 7):S7–24-S27–28.
- Kang CM, Chung YE, Park JY, et al. Potential contribution of preoperative neoadjuvant concurrent chemoradiation therapy on margin-negative resection in borderline resectable pancreatic cancer. J Gastrointest Surg 2012;16:509–17.
- Chuong MD, Hayman TJ, Patel MR, et al. Comparison of 1-, 2-, and 3-dimensional tumor response assessment after neoadjuvant GTX-RT in borderline-resectable pancreatic cancer. Gastrointest Cancer Res 2011;4:128–34.
- Loehrer AP, Kinnier CV, Ferrone CR. Treatment of locally advanced pancreatic ductal adenocarcinoma. Adv Surg 2016;50:115–28.
- Katz MH, Wang H, Balachandran A, et al. Effect of neoadjuvant chemoradiation and surgical technique on recurrence of localized pancreatic cancer. J Gastrointest Surg 2012;16:68–78.
- Franke AJ, Rosati LM, Pawlik TM, et al. The role of radiation therapy in pancreatic ductal adenocarcinoma in the neoadjuvant and adjuvant settings. Semin Oncol 2015;42:144–62.
- Butturini G, Stocken DD, Wente MN, et al. Influence of resection margins and treatment on survival in patients with pancreatic cancer: meta-analysis of randomized controlled trials. Arch Surg 2008;143:75–83.
- Paniccia A, Hosokawa P, Henderson W, et al. Characteristics of 10-year survivors of pancreatic ductal adenocarcinoma. JAMA Surg 2015;150:701–10.
- Massucco P, Capussotti L, Magnino A, et al. Pancreatic resections after chemoradiotherapy for locally advanced ductal adenocarcinoma: analysis of perioperative outcome and survival. Ann Surg Oncol 2006;13:1201–8.
- Patel M, Hoffe S, Malafa M, et al. Neoadjuvant GTX chemotherapy and IMRT-based chemoradiation for borderline resectable pancreatic cancer. J Surg Oncol 2011;104:155–161.
- Landry J, Catalano PJ, Staley C, et al. Randomized phase II study of gemcitabine plus radiotherapy versus gemcitabine, 5-fluorouracil, and cisplatin followed by radiotherapy and 5-fluorouracil for patients with locally advanced, potentially resectable pancreatic adenocarcinoma. J Surg Oncol 2010;101:587–92.
- Lamb R, Ozsvari B, Lisanti CL, et al. Antibiotics that target mitochondria effectively eradicate cancer stem cells, across multiple tumor types: treating cancer like an infectious disease. Oncotarget 2015;6:4569–84.
- Stokes JB, Nolan NJ, Stelow EB, et al. Preoperative capecitabine and concurrent radiation for borderline resectable pancreatic cancer. Ann Surg Oncol 2011;18:619–27.
- White R, Lee C, Anscher M, et al. Preoperative chemoradiation for patients with locally advanced adenocarcinoma of the pancreas. Ann Surg Oncol 1999;6:38–45.
- Martin RC 2nd. Management of locally advanced pancreatic cancer. Surg Clin North Am 2016;96:1371–89.
- Higuera O, Ghanem I, Nasimi R, et al. Management of pancreatic cancer in the elderly. World J Gastroenterol 2016;22:764–75.
- Hurria A, Lichtman SM. Clinical pharmacology of cancer therapies in older adults. Br J Cancer 2008;98:517–22.
- Spadi R, Brusa F, Ponzetti A, et al. Current therapeutic strategies for advanced pancreatic cancer: A review for clinicians. World J Clin Oncol 2016;7:27–43.
- Seufferlein T, Bachet JB, Van Cutsem E, Rougier P, Group EGW. Pancreatic adenocarcinoma: ESMO-ESDO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2012;23 Suppl 7:vii33–40.
- Huguet F, Girard N, Guerche CS, et al. Chemoradiotherapy in the management of locally advanced pancreatic carcinoma: a qualitative systematic review. J Clin Oncol 2009;27:2269–77.
- Suker M, Beumer BR, Sadot E, et al. FOLFIRINOX for locally advanced pancreatic cancer: a systematic review and patient-level meta-analysis. Lancet Oncol 2016;17:801–10.
- Gunturu KS, Yao X, Cong X, et al. FOLFIRINOX for locally advanced and metastatic pancreatic cancer: single institution retrospective review of efficacy and toxicity. Med Oncol 2013;30:361.
- Mukherjee S, Hurt CN, Bridgewater J, et al. Gemcitabine-based or capecitabine-based chemoradiotherapy for locally advanced pancreatic cancer (SCALOP): a multicentre, randomised, phase 2 trial. Lancet Oncol 2013;14:317–26.
- Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013;369:1691–1703.
- Krzyzanowska MK, Weeks JC, Earle CC. Treatment of locally advanced pancreatic cancer in the real world: population-based practices and effectiveness. J Clin Oncol 2003;21:3409–14.
- Sultana A, Tudur Smith C, Cunningham D, et al. Meta-analyses of chemotherapy for locally advanced and metastatic pancreatic cancer: results of secondary end points analyses. Br J Cancer 2008;99:6–13.
- Hammel P, Huguet F, van Laethem JL, et al. Effect of chemoradiotherapy vs chemotherapy on survival in patients with locally advanced pancreatic cancer controlled after 4 months of gemcitabine with or without erlotinib: the LAP07 randomized clinical trial. JAMA 2016;315:1844–53.
- Huguet F, Andre T, Hammel P, et al. Impact of chemoradiotherapy after disease control with chemotherapy in locally advanced pancreatic adenocarcinoma in GERCOR phase II and III studies. J Clin Oncol 2007;25:326–31.
- Dholakia AS, Hacker-Prietz A, Wild AT, et al. Resection of borderline resectable pancreatic cancer after neoadjuvant chemoradiation does not depend on improved radiographic appearance of tumor-vessel relationships. J Radiat Oncol 2013;2:413–25.
- Heinemann V, Haas M, Boeck S. Neoadjuvant treatment of borderline resectable and non-resectable pancreatic cancer. Ann Oncol 2013;24:2484–92.
- Morganti AG, Trodella L, Valentini V, et al. Pain relief with short-term irradiation in locally advanced carcinoma of the pancreas. J Palliat Care 2003;19:258–62.
- Arcidiacono PG, Calori G, Carrara S, et al. Celiac plexus block for pancreatic cancer pain in adults. Cochrane Database Syst Rev 2011(3):CD007519.
- Pezzilli R, Andriulli A, Bassi C, et al. Exocrine pancreatic insufficiency in adults: a shared position statement of the Italian Association for the Study of the Pancreas. World J Gastroenterol 2013;19:7930–46.
- Dominguez-Munoz JE. Pancreatic exocrine insufficiency: diagnosis and treatment. J Gastroenterol Hepatol 2011;26 Suppl 2:12–16.
- Bruno MJ, Haverkort EB, Tijssen GP, et al. Placebo controlled trial of enteric coated pancreatin microsphere treatment in patients with unresectable cancer of the pancreatic head region. Gut 1998;42:92–6.
- Wright AA, Keating NL, Balboni TA, et al. Place of death: correlations with quality of life of patients with cancer and predictors of bereaved caregivers’ mental health. J Clin Oncol 2010;28:4457–64.
- Jang RW, Krzyzanowska MK, Zimmermann C, et al. Palliative care and the aggressiveness of end-of-life care in patients with advanced pancreatic cancer. J Natl Cancer Inst 2015;107(3). pii: dju424.
INTRODUCTION
Pancreatic cancer is one of the most rapidly rising causes of mortality in the United States. In 2016, the number of deaths from pancreatic cancer exceeded those from breast cancer, making it the third leading cause of cancer-related death in the United States.1 It is projected that by 2020 pancreatic cancer will overtake colorectal malignancies to become the second most common cause of cancer death in this country.1,2 The term pancreatic cancer encompasses both exocrine and endocrine tumors. However, since 80% of pancreatic cancers are classified as pancreatic ductal adenocarcinoma (PDA), when speaking about pancreatic cancer most clinicians and scientists are referring to PDA.
Even with advances in chemotherapy and radiotherapy over the past decade, the only curative option for PDA is surgical resection. Unfortunately, only 20% of patients are appropriate surgical candidates at the time of diagnosis.3 Considering the lack of screening options and the ambiguity of symptomatology, roughly 4 out 5 patients with PDA are diagnosed as having locally advanced or metastatic disease that is initially not amenable to surgery.
Locally advanced pancreatic adenocarcinoma presents unique challenges in management and treatment. Treatment options include multi-agent chemotherapy, chemoradiation, or radiotherapy. Some patients can be successfully down-staged with these therapies and be deemed surgical candidates. Other challenges include selecting the appropriate sequence of therapies and stratifying therapies based on comorbidities. In this article, we review the epidemiology, biology, and diagnostic approach to PDA and focus on current treatment strategies for locally advanced pancreatic cancer (LAPC).
EPIDEMIOLOGY
In 2012, GLOBOCAN estimated that PDA caused 331,000 deaths per year, accounting for 4% of all worldwide mortality.4,5 Despite high incidence rates internationally, PDA is a disease of Western and industrialized nations. In the Unites States, PDA is a malignancy of middle to late adulthood, with a sharp upsurge in incidence after age 50 years.6 More than one third of new cases are diagnosed in patients older than 70 years, and more than half of patients diagnosed are older than 60 years of age.2 The incidence of pancreatic cancer is fairly equal among men and women, with a slightly higher rate for the male sex. It has an incidence preference for African-Americans by 4.8 cases per 100,000 persons nationally.7
Risk factors for the development of exocrine pancreatic cancer include hereditary disposition, underlying medical conditions, and environmental factors. One of the most significant environmental risk factors for the development of PDA is smoking,8 which is associated with up to 25% of all cases.9 Smoking cessation leads to a rapid reduction in risk for pancreatic cancer, with the risk among former smokers approaching that for never smokers less than 10 years after quitting.9 Other environmental factors that contribute to the development of pancreatic cancer include increased body mass index, a high-salt and high-saturated fat diet, heavy alcohol intake, and increased utilization of nonsteroidal inflammatory drugs.10–13
There is a strong association between new-onset diabetes and increased risk for developing PDA.14,15 Data also suggest that diabetes may be a risk factor and/or a consequence of tissue destruction that arises during the development or progression of PDA.16,17 Interestingly, ABO blood grouping is another underlying medical disposition that confers an altered risk profile. Studies have shown that patients with blood group O were less likely than those with type A, B, or AB to develop pancreatic cancer.18
Genetic predisposition syndromes can elevate an individual patient’s risk for developing PDA. Genetic syndromes and gene alterations that increase the risk for PDA include BRCA1/2, Peutz-Jeghers syndrome, and Lynch syndrome risk.19–21 Up to 10% to 15% of PDA cases may be due to an inherited familial cancer.22 Having a first-degree relative with PDA increases the odds of developing PDA 1.76-fold compared to those without a family history.23 The exact biologic and molecular mechanisms of familial pancreatic cancer are unclear. It is estimated that about 10% of patients with familial pancreatic cancer (FPC) carry BRCA2 mutations.24 Individuals at risk for FPC should undergo genetic screening for the presence of the most frequently inherited pancreatic cancer susceptibility genetic defects: BRCA2, PALB2, and ATM germline mutations.25 Carriers of BRCA2, who are also at increased risk for developing breast, ovarian, and prostate cancer, should be monitored closely. Of all hereditary conditions, hereditary pancreatitis confers the highest risk for developing PDA, with an approximate risk elevation of 40% to 50%.26,27 Although several genetic predisposition syndromes have been identified, most cases of pancreatic adenocarcinoma are thought to be sporadic.
CANCER BIOLOGY AND PATHOLOGY
The pathologic predecessor of PDA is pancreatic intraepithelial neoplasia (PIN). With further dysplastic changes that result from increasing genetic alterations, these precancerous lesions progress from low- to high-grade and finally to adenocarcinoma. More than 90% of all PINs across all grades have oncogenic KRAS mutations.28 Additionally, inactivating mutations in the tumor suppressor genes SMAD4, p53, and CDKN2A are found with increasing frequency in higher grade PINs. The frequency and presence of mutations in both oncogenes and tumor suppressor genes in precursor neoplasias mirror the genetic mutations noted in advanced PDA.29 Among all mutations, KRAS is the most common and most functionally important for pancreatic cancer cell survival. KRAS mutations not only have profound effects on downstream mediators of tumor growth and metastasis, but they are implicated in reprograming of cellular metabolism.30,31
Pancreatic adenocarcinoma has a unique microenvironment that makes it a difficult target for current therapeutic modalities. First, it is one of the most stroma-rich malignancies. The dense stroma surrounding pancreatic tumor cells leads to increased tumor pressures and alterations in tumor vascular perfusion.32 It also serves as a barrier that prevents chemotherapeutic drugs from reaching the tumor cells. Thus, clinical trials are under way to investigate agents such has hyaluronidase, which may degrade components of the extracellular matrix that supports thestromal environment. Additionally, there is data to suggest that the microenvironment of PDA downregulates immune monitoring, leading to further tumor growth.27,33 The molecular, cellular, and immunologic complexity of PDA may contribute to its resistance to traditional therapeutics.
EVALUATION AND DIAGNOSIS
CASE PRESENTATION
A 61-year-old man with a history of type 2 diabetes mellitus and chronic tobacco use presents to the emergency department (ED) with a 4-month history of progressively worsening abdominal discomfort and fatigue. He has also noticed darkening of his urine and slight yellow discoloration of his eyes. His weight measured 5 months ago in his primary care physician’s office was 91 kg (200 lb, BMI 29.5) and in the ED is 75 kg (165 lb, BMI 24.4). He has noticed bulky, malodorous, oily stools for about 2 months. Preliminary laboratory studies reveal elevated levels of total bilirubin (2.7 mg/dL) and alkaline phosphatase (204 IU/L). Transabdominal ultrasound (US) is obtained and reveals a 3-cm pancreatic mass with biliary tract dilation.
Does this patient have pancreatic cancer?
CLINICAL SIGNS AND SYMPTOMS
Establishing the diagnosis of pancreatic cancer in a patient who presents with a high index of suspicion is critical. Patients with pancreatic cancer usually present after a period of nonspecific and vague symptoms, which typically are experienced as abdominal discomfort, weight loss, and weakness. It is estimated that approximately 25% of patients may complain of vague abdominal pain up to 6 months prior to diagnosis. Up to 15% of patients may seek medical attention more than 6 months prior to establishing a diagnosis of PDA.34 The most common symptoms associated with pancreatic cancer in order of decreasing reported frequency are weight loss, anorexia, abdominal/epigastric pain, dark-colored urine, jaundice, nausea, back pain, and diarrhea with associated steatorrhea.35 Upwards of 15% of patients present with painless jaundice, a term that is often associated with pancreatic cancer.36 On exam these patients may have scleral icterus, sublingual jaundice, epigastric pain on palpation, weight loss, hepatomegaly, lymphadenopathy and a nontender, distended, palpable gallbladder (also known as Courvoisier sign).34 Abdominal signs and symptoms arise from tumor growth into surrounding vessels, tissues, and ducts within the abdominal cavity. Compression of the common bile duct accounts for the development of jaundice. Tumor growth around the stomach and duodenum can lead to delayed gastric emptying and subsequently nausea and vomiting. Constriction of the pancreatic duct leads to pancreatic insufficiency, precipitation of weight loss, and steatorrhea. Pancreatic insufficiency can worsen abdominal pain, and lead to increased weight loss and flatulence.
Less common symptoms include pain, erythema, and edema involving the lower extremities, which may be reflective of migratory thrombophlebitis (commonly known as Trousseau syndrome). Thromboembolic disease, including pulmonary embolism, portal vein, and deep vein thromboses are frequently encountered complications of pancreatic cancer. The incidence of thromboembolic events in patients with PDA has been reported to be as high as 54%.37 Of all signs encountered, weight loss is the most common and most profound. Patients with advanced PDA have severe degrees of cachexia. Some patients present with as much as a 5 kg/m2 decrease in their BMI from pre-illness baseline BMI, and lose another 3 to 4 kg/m2 through disease progression.38 At the time of diagnosis, many patients have already undergone significant weight loss, which can have substantial implications on treatment planning and clinical outcomes.
What other studies can be done to assist in making the diagnosis?
LABORATORY ABNORMALITIES AND TUMOR MARKERS
Elevations in alkaline phosphatase, γ-glutamyltransferase (GGT), serum aspartate aminotransferase (AST), serum alanine aminotransferase (ALT), and direct fractions of bilirubin are common in patients with PDA. Patients will usually have an obstructive pattern on their liver panel, with predominant elevations in direct bilirubin, alkaline phosphatase, and GGT, as compared with AST and ALT. Other baseline laboratory studies, including a complete blood count and basic metabolic panel, should be obtained because patients commonly have thrombocytosis, anemia, and electrolyte abnormalities due to the tumor itself and pancreatic insufficiency (Table 1).

Measurement of glycated hemoglobin (HBA1C) is an emerging and important diagnostic test in the diagnosis of pancreatic cancer. Recently, data has emerged to suggest that new-onset diabetes is present in about 50% of patients diagnosed with pancreatic cancer.39 The temporal relationship of pancreatic cancer and diabetes is supported by evidence showing that patients who undergo resection commonly have resolution of their diabetes.17 This study suggested that hyperglycemia, elevated HBA1C, and symptoms of diabetes in patients older than 50 years may identify patients who have early pancreatic cancer. The entity of pancreatic cancer–associated diabetes needs to be better defined and the algorithmic approach to evaluation and diagnosis, utilizing signs, symptoms, and laboratory values associated with diabetes, needs to be clearly established.
The only serum marker for PDA is carbohydrate antigen 19-9 (CA 19-9), also known as sialylated Lewis antigen or cancer-associated antigen. It was first identified in pancreatic cancer patients in 1981.40,41 The sensitivity and specificity of CA 19-9 ranges from 70% to approximately 90%.42,43 Hereditary predispositions and comorbid disease cross-reactivity contribute to the diminished sensitivity and specificity of CA 19-9. In about 5% to 10% of the population, CA 19-9 is not expressed (Lewis antigen A and B negative). Additionally, since CA 19-9 is expressed in the cells that line the biliary tree, diseases that lead to pancreatic or liver inflammation may falsely elevate CA 19-9.44 As a result, CA 19-9 is not an ideal screening test. However, data has shown that CA 19-9 may have prognostic value postoperatively and serve as a marker for therapeutic response.45,46
Is biopsy needed for this patient and if so, what is the most appropriate technique?
ENDOSCOPIC ULTRASOUND
Generally, diagnosis with tissue is not necessary for patients who clearly have resectable disease and will proceed directly to surgery for management. Nevertheless, it is still commonly obtained in this group of patients. However, in patients with LAPC or with features suggestive of LAPC, such as tumor approximation to critical vessels such as the superior mesenteric artery (SMA) or celiac axis, biopsy is necessary. These patients will receive neoadjuvant therapy, and biopsy is important in establishing a diagnosis. The ideal way to obtain a biopsy is through fine-needle aspiration (FNA) or biopsy (FNB) utilizing endoscopic ultrasound (EUS). Percutaneous and computed tomography (CT)–guided FNB can also be used to obtain a biopsy for diagnosis. In comparison to percutaneous and CT-guided FNB, EUS-FNA/FNB has low rates of complications, a decreased rate of peritoneal seeding, and is cost effective.47,48
CASE CONTINUED
Abdominal CT obtained following abdominal ultrasound reveals a 3.5-cm mass in the head of the pancreas in close approximation to the SMA and celiac axis.
Does the patient have borderline resectable or unresectable disease?
IMAGING
Abdominal ultrasound is a reasonable, inexpensive, and safe alternative to abdominal CT as it does not utilize ionizing radiation. It is particularly useful in patients who present with jaundice or have concern for biliary obstruction based on laboratory evaluation. It is particularly sensitive for detecting tumors greater than 3 cm in size.49,50 In patients whose abdominal ultrasound is unrevealing and whose index of suspicion remains high for PDA, abdominal CT should be the next imaging modality.
Abdominal CT obtained utilizing a pancreatic protocol is ideal for detection and staging of pancreatic tumors. By implementing a triple-phase protocol with arterial, late arterial, and venous phases, tumors, which have a density different from that of the pancreatic parenchyma, are accentuated. Abdominal CT is also able to provide critical information about tumor resectability.51 By revealing the degree of tumor encasement around the aorta, level of destruction of the superior mesenteric vein, or degree of involvement of the SMA or celiac vessels, abdominal CT determines if a patient should be deemed resectable, borderline resectable, or unresectable (Table 2).52,53 Resectability is based on thorough imaging evaluation, expert opinion of a multidisciplinary team, and guidelines proposed by American Hepatopancreaticobiliary Association, Society of Surgical Oncology, Society for Surgery of the Alimentary Tract, and the NCCN.54

Other imaging modalities have a less clearly established role in the diagnostic approach to PDA. In patients who have contraindications to obtaining CT, magnetic resonance imaging can be utilized as a secondary imaging modality.55 The role of positron emission tomography 18F-fluorodeoxyglucose (PET-FDG) is not clearly defined among clinicians, nor reflected in consensus guidelines by the National Comprehensive Cancer Network (NCCN). In clinical practice, it is still often combined with CT to detect metastatic disease, particularly in high-risk patients such has those with LAPC. The role of PET-CT in staging and its impact on clinical outcomes has not been fully established.
Endoscopic retrograde cholangiopancreatography (ERCP) and magnetic resonance cholangiopancreatography (MRCP) can also assist in the diagnosis and management of PDA. In patients with obstructive jaundice, both MRCP and ERCP visualize obstructions and dilations within the biliary tree, with the latter having the ability to intervene. ERCP allows for the collection of tissue to aid in diagnosis, and has the ability to relieve biliary obstruction via stenting.56
TREATMENT
CASE CONTINUED
After an abdominal CT is obtained, the patient is referred to an outpatient oncologist because of concern for pancreatic adenocarcinoma. After consultation, the patient is advised to obtain EUS with biopsy and to return immediately afterwards for further treatment planning. The pathology report following EUS confirms that the mass is a poorly differentiated PDA. The patient’s case is discussed at a multidisciplinary meeting with radiation, surgical, and medical oncology. The abdominal CT and PET-CT scan are thoroughly reviewed. After imaging review, the multidisciplinary team concludes that the tumor is in contact with the SMA at 120° and with the common hepatic artery without extension in the celiac axis and without evidence of metastasis.
What is the appropriate management of borderline resectable pancreatic cancer?
BORDERLINE RESECTABLE CANCER
Patients who have nonmetastatic disease and are deemed resectable and without contraindications to surgery or high-risk features, as defined by NCCN guidelines, should proceed directly to surgery. A large body of evidence suggests that complete surgical resection with negative margins is a significant predictor of survival and currently provides the only option for cure.57–59 Despite the curative intent of surgery, the rate of recurrence remains high in patients who undergo surgical resection. Even in patients with negative resection margins (R0 resection), the 5-year survival is 20% to 30%, with a median survival ranging from 12 to 25 months, suggesting the presence of regional and distant occult disease at the time of diagnosis.60–62
Additionally, in half the patients who undergo surgical resection with resultant positive microscopic (R1 resection) or gross (R2 resection) margins, the median survival is no greater than 12 months. In this subset of patients, clinical outcomes are similar to outcomes in patients with locally advanced and metastatic pancreatic cancer, suggesting that upfront surgery and adjuvant therapy may not be the ideal therapeutic option. This raises 2 important points: first, resectability should be assessed carefully in all patients with LAPC, and second, for those patients who are deemed borderline resectable, neoadjuvant therapy should be considered.63 Borderline resectability is defined as tumor abutment ≤ 180° of the celiac artery, and tumor abutment of the superior mesenteric vein /portal vein of > 180° or abutting ≤ 180° with irregularity of the vein with or without thrombosis with anatomical structures that still allows for safe and complete resection and vein reconstruction (Table 2).
Neoadjuvant Therapy
The goal of neoadjuvant therapy is to minimize the negative impact of upfront surgery in patients who have a high likelihood of having microscopic or grossly positive margins. Research has suggested that neoadjuvant therapy may improve resectability, decrease the rate of recurrence, and improve overall survival.64–66
There is no clear consensus on the ideal management of patients with borderline resectable disease. However, expert guidelines are in agreement that upfront surgery in patients with LAPC is not appropriate, as most patients will not be able to achieve an R0 resection.67 As staging and management of patients with LAPC is difficult, expertise of a multidisciplinary team can be helpful.68
Several studies and the NCCN guidelines support the use of neoadjuvant therapy in patients deemed borderline resectable.69,70 Treatment of borderline resectable disease is similar to unresectable LAPC and generally involves 2 chemotherapy treatment backbones: FOLFIRINOX (folinic acid [leucovorin], fluorouracil [5-FU], irinotecan, and oxaliplatin) or gemcitabine-based therapy.
Phase 1 to 2/3 clinical trials conducted by Conroy et al from 2005 to 2011, including the landmark ACCORD-11 trial, established the safety and role of FOLFIRINOX in metastatic pancreatic cancer and also demonstrated an improved overall survival with the use of this therapy in these patients.71,72 These findings led to interest in FOLFIRINOX as a neoadjuvant therapy for patients with LAPC. Since then, multiple prospective and retrospective studies have shown that 54% to 100% of patients with borderline resectable LAPC who were treated with FOLFIRINOX were down-staged significantly enough to undergo resection. Of those patients, more than 90% had a R0 resection following surgery (Table 3).73–79

Data over the past 7 years suggests that neoadjuvant FOLFIRINOX improves overall survival and resectability in patients with borderline disease. However, treatment with FOLFIRINOX is not without limitations. FOLFIRINOX is associated with higher rates of febrile neutropenia, thrombocytopenia, diarrhea, and sensory neuropathy as compared with gemcitabine-based therapy.72 Other less commonly observed toxicities associated with FOLFIRINOX include mucositis, hand-foot syndrome, pulmonary toxicity, and alopecia. Dose-attenuated FOLFIRINOX-based regimens, including those that exclude the bolus fluorouracil dose and augment upfront filgrastim, have demonstrated improved safety and comparable efficacy as compared to standard FOLFIRINOX.80
Gemcitabine has been the fundamental treatment backbone for PDA since the results of the phase 3 CONKO-001 trial were published.81 Gemcitabine is a pyrimidine antimetabolite and potent inhibitor of DNA polymerase and ribonucleotide reductase.82 In recent years, multiple combination therapies with gemcitabine have been investigated, including regimens with nab-paclitaxel, oxaliplatin, or docetaxel. Resection rates and negative margin outcomes have been shown to be comparable to patients who received FOLFIRINOX in the neoadjuvant setting with borderline locally advanced disease.83–85 In addition to having a more tolerable side effect profile in comparison to fluorouracil-based regimens, gemcitabine is considered to be a potent radiosensitizer.86 For this reason, studies have also investigated the role of radiotherapy in conjunction with gemcitabine, revealing negative margin resection rates above 80% in patients with borderline resectable disease.87,88
Because very few studies directly comparing FOLFIRINOX with gemcitabine-based combination regimens have been completed, there is no clear consensus on the preferred treatment regimen, in both borderline and unresectable LAPC. Decisions to treat are influenced predominantly by comorbidities, adverse effect profiles, and performance status of patients, as FOLFIRINOX is the more toxic of the 2 treatment backbones. Therefore, FOLFIRINOX has mostly been utilized in patients with relatively good functional status (Eastern Cooperative Oncology Group [ECOG] performance status 0 to 1).89 In elderly patients and those with poor functional status, ECOG 2 to 4, gemcitabine as a single agent is a reasonable alternative in the neoadjuvant setting of borderline resectable disease.
The exact role of radiation therapy in addition to induction chemotherapy in borderline resectable pancreatic cancer has not been clearly established because of the lack of prospective studies in this area. Multiple large retrospective series have identified high rates of conversion to margin-negative resection with neoadjuvant chemoradiation alone.90 Based on available data, it is reasonable for patients with borderline resectable disease to proceed with any of the following treatment options: chemotherapy, chemoradiation, or induction chemotherapy followed by chemoradiation (Figure). Chemotherapy and chemoradiation are generally more appropriate with patients with high CA 19-9 levels or those at an elevated risk of having positive margins or occult metastatic disease.91 Obtaining negative margin resections is the predominant goal of neoadjuvant radiotherapy.89 Many studies have identified margin status to be one of the most significant prognostic factors in PDA.57,59,92,93 Additionally, several studies have highlighted that radiotherapy in the neoadjuvant setting could improve negative margin resection rates, local control, and clinical outcomes in patients with borderline resectable locally advanced disease.94–97 A common multimodal regimen utilized in the neoadjuvant setting combines capecitabine, an oral prodrug that is converted to fluorouracil, with radiation therapy. This combination has also been shown to improve resectability rates and long-term clinical outcomes in patients with borderline resectable disease.98 Additionally, neoadjuvant radiation therapy can potentially downstage patients with unresectable disease at the time of diagnosis to become surgical candidates.99 Despite the paucity of data, interval scans utilizing CT following neoadjuvant therapy should be obtained 2 to 4 months after completion of therapy to determine therapeutic response, evaluate for disease progression, and, most important, reassess surgical stage/resectability. It is clinically acceptable to proceed to resection with radiographically stable disease post-neoadjuvant therapy.

Many patients classified as borderline resectable are able to proceed with surgery following neoadjuvant therapy. Unfortunately, specific data on adjuvant therapy following neoadjuvant chemotherapy or chemoradiotherapy and surgical resection in borderline resectable patients is scarce. Clinical practice guidelines are extrapolated from studies where upfront resection in clearly resectable patients was followed by adjuvant therapy. Based on these data, approximately 6 months of perioperative chemotherapy with or without chemoradiotherapy is a reasonable consideration. Nevertheless, about 80% of patients at the time of diagnosis are deemed to be unresectable, and a smaller number do not proceed to surgery despite an initial classification as borderline resectable. Of the 80% of patients with advanced disease, about half are metastatic at presentation and the remaining 30% to 40% are defined as having unresectable LAPC.100
CASE CONTINUED
The patient is deemed borderline resectable. He receives neoadjuvant therapy with gemcitabine and nab-paclitaxel. Two months after therapy, interval imaging with abdominal CT does not show improvement in tumor size and there is now evidence that the tumor has invaded the celiac axis and is abutting more than 180° of the SMA. The patient presents to the oncologist to discuss further management. He has lost about 15 lb since his last evaluation, is capable of self-care, but is unable to carry on with any work activities.
What is the appropriate management of unresectable nonmetastatic LAPC?
UNRESECTABLE LOCALLY ADVANCED CANCER
As in the case of borderline resectable disease, there are many treatment options for patients with unresectable LAPC. Timing, optimal chemotherapy regimen, and the addition of regularly and hypofractionated radiotherapy are issues currently under investigation. However, there are some general considerations and principles that are followed as reflected in the NCCN guidelines and recent studies. The primary therapeutic aims in patients with unresectable locally advanced disease are to increase survival and improve palliation.
The elderly comprise a large percentage of the patients diagnosed with unresectable locally advanced disease. Pharmacokinetics and toxicity profiles are altered in the elderly population.101,102 Therefore, it is important to assess functional status and comorbidities as these are critical factors in determining treatment regimens, similar to patients with borderline resectable disease. Currently, the most common first-line therapies in advanced pancreatic cancer are gemcitabine alone, gemcitabine and nab-paclitaxel, FOLFIRINOX, gemcitabine/capecitabine, and gemcitabine/oxaliplatin.103 The overall treatment approach to unresectable locally advanced pancreatic adenocarcinoma closely mirrors that of patients with borderline resectable disease and metastatic disease. Much of the data supporting treatment regimens in unresectable LAPC is extrapolated from clinical trials looking at advanced or metastatic pancreatic cancer.
Consensus opinions domestically and from Europe recommend that patients with locally advanced unresectable disease undergo upfront chemotherapy (Figure).104 This is based on the premise that initial chemotherapy may destroy occult metastatic cells and increase the efficacy of consolidative chemotherapy, particularly with radiation in the future. Upfront chemoradiotherapy has only been investigated in a small series of trials in which no clear survival benefit was observed and has the added consequence of treatment-related toxicity.105 However, data is limited in this regard, with variations in treatment protocols and cohort compositions contributing to the inconclusive findings.
Despite advances in immunotherapy, targeted therapies, and gene sequencing, initial chemotherapy for unresectable disease is still either gemcitabine-based combination therapy or FOLFIRINOX. Across numerous studies, patients with unresectable LAPC receiving FOLFIRINOX have a median progression-free survival of 3 to 20 months and a median overall survival of 10 to 32.7 months.106 As with borderline resectable patients, FOLFIRINOX (Table 4) is generally reserved for unresectable patients with good functional status (ECOG 0–1 or Karnofsky Performance Status 90–100) and those at low risk for developing grade 3 or 4 systemic toxicities.103 For these reasons it has generally not been frequently combined with other chemotherapeutic agents. However, FOLFIRINOX has been combined with radiation therapy in the consolidative neoadjuvant setting after induction chemotherapy. There have also been studies where traditional FOLFIRONIX was modified to improve tolerability, as evidenced by Gunturu et al’s study, which dose-reduced both fluorouracil and irinotecan by 25%, without compromising efficacy and simultaneously increasing tolerability.107 Additionally, FOLFIRINOX requires infusional administration of the fluorouracil component, which may not be practical in certain patients. In that subset, capecitabine can be substituted. Among radiosensitizers during neoadjuvant therapy for unresectable LAPC, capecitabine has been shown to be as efficacious and less toxic than even gemcitabine.108


No head-to-head studies investigating FOLFIRINOX versus nab-paclitaxel and gemcitabine in patients with locally advanced disease have been published, but clinical trials are under way. Other combination therapies have been looked at through small retrospective or prospective studies, but no robust, large-scale clinical trials have been completed. For this reason, NCCN guidelines recommend enrollment of patients with LAPC into active clinical trials.
What is the role of radiation therapy in unresectable LAPC?
Despite the reported advantages of neoadjuvant radiation in patients with potentially resectable disease, there is significant debate regarding the timing and role of neoadjuvant radiation in patients with unresectable disease. Numerous comprehensive analyses and studiest indicate that chemoradiotherapy leads to significantly better overall survival compared to no therapy or radiation therapy alone in LAPC.68,110,111 However, conflicting data support the use of upfront chemoradiotherapy in unresectable LAPC when compared to chemotherapy alone. Unfortunately, most prospective studies investigating the role of radiotherapy were performed following administration of single-agent gemcitabine, which is no longer considered standard of care for patients with LAPC. In spite of this, ECOG 4201 identified a statistically significant improvement in median overall survival following the addition of gemcitabine-based radiotherapy. Huguet et al in his review pointed out that upfront chemoradiotherapy was not superior to chemotherapy only and was associated with increased treatment toxicity.105 Additionally, a recent phase 3 study looking at chemoradiotherapy versus chemotherapy alone in patients treated with gemcitabine found no difference in overall survival.112 This can potentially be attributed to the fact that about 30% of patients with LAPC develop metastatic disease in the early phases of treatment due to poor control of local and systemically occult disease.113 Given the propensity for high rates of occult metastatic disease in LAPC, treatment paradigms and consensus guidelines recommend multi-agent systemic chemotherapy followed by chemoradiotherapy in select patients.
Based on current studies and until further clinical investigations are completed, consensus opinion indicates that the most appropriate approach in unresectable LAPC is to begin with induction chemotherapy (with either gemcitabine plus nab-paclitaxel, FOLFIRINOX, capecitabine, or other treatment combinations), followed by chemoradiation in the absence of disease progression when the first repeat imaging evaluation is completed (Figure). One important caveat regarding reimaging with CT in the neoadjuvant setting is that radiologic response may not correlate with pathologic response.114 PET-CT may have a role in predicting response to neoadjuvant therapy. Overall, induction chemotherapy followed by consolidative chemoradiation may confer numerous benefits: it removes the unnecessary burden and toxicity associated with radiotherapy in the nearly one third of patients who have pervasive disease progression during initial treatment; it allows testing and increases the chances of tolerating full-dose systemic chemotherapy; and it raises the likelihood of converting patients who do not progress to metastasis during the initial phase of treatment from unresectable to resectable status.103,115 Despite the lack of strong conclusive data, the general agreement is that neoadjuvant chemoradiotherapy converts about one third of borderline and unresectable LAPC to an R0 resection.95,103 There are very specific rationales for the addition of radiotherapy in LAPC, and these functions need to be better defined through further clinical trials.
PALLIATIVE CARE
CASE CONTINUED
The patient is unable to tolerate his first round of second-line therapy with modified FOLFIRINOX. His overall treatment plan is transitioned to palliation. He continues to have pain, despite increasing doses of narcotics.
What is the next step for patients in whom second-line therapy fails and who have intractable pain while on high-dose narcotics?
A subset of patients with unresectable LAPC may not be amenable to chemotherapy with or without radiation due to significant comorbidities or because they present with or progress to ECOG scores 3 or 4. The goal in these patients should be palliation. Pain is one of the most predominant and difficult to manage symptoms in progressive LAPC. Opioid-based medications are the primary treatment for pain in LAPC. However, patients sometimes become refractory to opioid medications. In this group of patients, it is reasonable to consider palliative radiation as an alternative method for pain control.116
An alternative to palliative radiation in the setting of progressive pain in PDA is celiac plexus block or neurolysis. By injecting an anesthetic or alcohol into the celiac plexus, neural signaling pathways involved in the propagation of pain are inhibited without leading to significant nerve destruction. Additionally, chemical splanchnicectomy allows for reduced opioid medication use and associated side effects.117
In general patients with LAPC have profound weight loss prior to and during treatment. This has significant implications prognostically and on treatment options. The underlying etiology is multifactorial, but one of the primary driving factors is pancreatic insufficiency. An estimated 65% of pancreatic cancer patients have fat malabsorption, and 50% have protein malabsorption, leading to steatorrhea and weight loss.118 Patients diagnosed with pancreatic cancer should be given enzyme replacement with formulations that include lipase, amylase, and protease. A minimum dose of enzyme replacement should include 40,000 to 50,000 U of lipase during meals and 25,000 U during snack intake. If maldigestion, symptoms, or nutritional endpoints (BMI, albumin, prealbumin, cholesterol) do not improve, the pancreatic enzyme dose should be escalated and a proton-pump inhibitor (PPI) added. In patients with pancreatic insufficiency, PPIs have been shown to improve fat absorption.119 Enzyme replacement therapy has been shown to prevent weight loss in patients with unresectable pancreatic cancer.120
As most patients with LAPC go on to develop progressive disease, palliative care becomes an integral aspect of the therapeutic paradigm. Palliation in LAPC has a significant role in determining quality of life and ensuring patient’s goals of care have been meet. Studies have suggested that pancreatic cancer is second only to lung cancer in terms of the number of emergency department visits in the later stages of disease.120 Additionally, aggressive care in the setting of incurable diseases such as LAPC has been associated with poor quality of life.121 More recently it has been shown that involvement of palliative care in patients with advanced pancreatic is associated with less aggressive care near death.122 Therefore, the incorporation of palliative or supportive care teams in the treatment of patients with progressive LAPC can improve quality of life and alleviate suffering associated with increasing symptom burden.
CONCLUSION
LAPC is a difficult disease for both provider and patient. There is a paucity of robust clinical trials in the neoadjuvant setting for LAPC. Current research is complicated by varying consensus definitions of resectability and the varying treatment configurations across studies. The optimal type, timing, and sequence of treatment and whether to add radiation therapy in LAPC have not been clearly defined. However, based on the available studies and consensus guidelines, patients who are deemed to have LAPC should have neoadjuvant therapy. FOLFIRINOX or gemcitabine with nab-paclitaxel should be considered first-line treatments. Patients with LAPC who respond to chemotherapy or are ineligible for multi-drug chemotherapy may benefit from chemoradiotherapy. In patients with unresectable disease, chemoradiotherapy has been shown to enhance survival as compared to best supportive care or radiation alone. For borderline resectable disease, it is reasonable to treat patients with either chemoradiotherapy, chemotherapy alone, or chemotherapy followed by chemoradiotherapy.
Considering the invasive nature of LAPC and the controversy around neoadjuvant treatment protocols, enrollment of patients with LAPC into clinical trials is important and will help determine the optimal treatment regimen for future patients.
INTRODUCTION
Pancreatic cancer is one of the most rapidly rising causes of mortality in the United States. In 2016, the number of deaths from pancreatic cancer exceeded those from breast cancer, making it the third leading cause of cancer-related death in the United States.1 It is projected that by 2020 pancreatic cancer will overtake colorectal malignancies to become the second most common cause of cancer death in this country.1,2 The term pancreatic cancer encompasses both exocrine and endocrine tumors. However, since 80% of pancreatic cancers are classified as pancreatic ductal adenocarcinoma (PDA), when speaking about pancreatic cancer most clinicians and scientists are referring to PDA.
Even with advances in chemotherapy and radiotherapy over the past decade, the only curative option for PDA is surgical resection. Unfortunately, only 20% of patients are appropriate surgical candidates at the time of diagnosis.3 Considering the lack of screening options and the ambiguity of symptomatology, roughly 4 out 5 patients with PDA are diagnosed as having locally advanced or metastatic disease that is initially not amenable to surgery.
Locally advanced pancreatic adenocarcinoma presents unique challenges in management and treatment. Treatment options include multi-agent chemotherapy, chemoradiation, or radiotherapy. Some patients can be successfully down-staged with these therapies and be deemed surgical candidates. Other challenges include selecting the appropriate sequence of therapies and stratifying therapies based on comorbidities. In this article, we review the epidemiology, biology, and diagnostic approach to PDA and focus on current treatment strategies for locally advanced pancreatic cancer (LAPC).
EPIDEMIOLOGY
In 2012, GLOBOCAN estimated that PDA caused 331,000 deaths per year, accounting for 4% of all worldwide mortality.4,5 Despite high incidence rates internationally, PDA is a disease of Western and industrialized nations. In the Unites States, PDA is a malignancy of middle to late adulthood, with a sharp upsurge in incidence after age 50 years.6 More than one third of new cases are diagnosed in patients older than 70 years, and more than half of patients diagnosed are older than 60 years of age.2 The incidence of pancreatic cancer is fairly equal among men and women, with a slightly higher rate for the male sex. It has an incidence preference for African-Americans by 4.8 cases per 100,000 persons nationally.7
Risk factors for the development of exocrine pancreatic cancer include hereditary disposition, underlying medical conditions, and environmental factors. One of the most significant environmental risk factors for the development of PDA is smoking,8 which is associated with up to 25% of all cases.9 Smoking cessation leads to a rapid reduction in risk for pancreatic cancer, with the risk among former smokers approaching that for never smokers less than 10 years after quitting.9 Other environmental factors that contribute to the development of pancreatic cancer include increased body mass index, a high-salt and high-saturated fat diet, heavy alcohol intake, and increased utilization of nonsteroidal inflammatory drugs.10–13
There is a strong association between new-onset diabetes and increased risk for developing PDA.14,15 Data also suggest that diabetes may be a risk factor and/or a consequence of tissue destruction that arises during the development or progression of PDA.16,17 Interestingly, ABO blood grouping is another underlying medical disposition that confers an altered risk profile. Studies have shown that patients with blood group O were less likely than those with type A, B, or AB to develop pancreatic cancer.18
Genetic predisposition syndromes can elevate an individual patient’s risk for developing PDA. Genetic syndromes and gene alterations that increase the risk for PDA include BRCA1/2, Peutz-Jeghers syndrome, and Lynch syndrome risk.19–21 Up to 10% to 15% of PDA cases may be due to an inherited familial cancer.22 Having a first-degree relative with PDA increases the odds of developing PDA 1.76-fold compared to those without a family history.23 The exact biologic and molecular mechanisms of familial pancreatic cancer are unclear. It is estimated that about 10% of patients with familial pancreatic cancer (FPC) carry BRCA2 mutations.24 Individuals at risk for FPC should undergo genetic screening for the presence of the most frequently inherited pancreatic cancer susceptibility genetic defects: BRCA2, PALB2, and ATM germline mutations.25 Carriers of BRCA2, who are also at increased risk for developing breast, ovarian, and prostate cancer, should be monitored closely. Of all hereditary conditions, hereditary pancreatitis confers the highest risk for developing PDA, with an approximate risk elevation of 40% to 50%.26,27 Although several genetic predisposition syndromes have been identified, most cases of pancreatic adenocarcinoma are thought to be sporadic.
CANCER BIOLOGY AND PATHOLOGY
The pathologic predecessor of PDA is pancreatic intraepithelial neoplasia (PIN). With further dysplastic changes that result from increasing genetic alterations, these precancerous lesions progress from low- to high-grade and finally to adenocarcinoma. More than 90% of all PINs across all grades have oncogenic KRAS mutations.28 Additionally, inactivating mutations in the tumor suppressor genes SMAD4, p53, and CDKN2A are found with increasing frequency in higher grade PINs. The frequency and presence of mutations in both oncogenes and tumor suppressor genes in precursor neoplasias mirror the genetic mutations noted in advanced PDA.29 Among all mutations, KRAS is the most common and most functionally important for pancreatic cancer cell survival. KRAS mutations not only have profound effects on downstream mediators of tumor growth and metastasis, but they are implicated in reprograming of cellular metabolism.30,31
Pancreatic adenocarcinoma has a unique microenvironment that makes it a difficult target for current therapeutic modalities. First, it is one of the most stroma-rich malignancies. The dense stroma surrounding pancreatic tumor cells leads to increased tumor pressures and alterations in tumor vascular perfusion.32 It also serves as a barrier that prevents chemotherapeutic drugs from reaching the tumor cells. Thus, clinical trials are under way to investigate agents such has hyaluronidase, which may degrade components of the extracellular matrix that supports thestromal environment. Additionally, there is data to suggest that the microenvironment of PDA downregulates immune monitoring, leading to further tumor growth.27,33 The molecular, cellular, and immunologic complexity of PDA may contribute to its resistance to traditional therapeutics.
EVALUATION AND DIAGNOSIS
CASE PRESENTATION
A 61-year-old man with a history of type 2 diabetes mellitus and chronic tobacco use presents to the emergency department (ED) with a 4-month history of progressively worsening abdominal discomfort and fatigue. He has also noticed darkening of his urine and slight yellow discoloration of his eyes. His weight measured 5 months ago in his primary care physician’s office was 91 kg (200 lb, BMI 29.5) and in the ED is 75 kg (165 lb, BMI 24.4). He has noticed bulky, malodorous, oily stools for about 2 months. Preliminary laboratory studies reveal elevated levels of total bilirubin (2.7 mg/dL) and alkaline phosphatase (204 IU/L). Transabdominal ultrasound (US) is obtained and reveals a 3-cm pancreatic mass with biliary tract dilation.
Does this patient have pancreatic cancer?
CLINICAL SIGNS AND SYMPTOMS
Establishing the diagnosis of pancreatic cancer in a patient who presents with a high index of suspicion is critical. Patients with pancreatic cancer usually present after a period of nonspecific and vague symptoms, which typically are experienced as abdominal discomfort, weight loss, and weakness. It is estimated that approximately 25% of patients may complain of vague abdominal pain up to 6 months prior to diagnosis. Up to 15% of patients may seek medical attention more than 6 months prior to establishing a diagnosis of PDA.34 The most common symptoms associated with pancreatic cancer in order of decreasing reported frequency are weight loss, anorexia, abdominal/epigastric pain, dark-colored urine, jaundice, nausea, back pain, and diarrhea with associated steatorrhea.35 Upwards of 15% of patients present with painless jaundice, a term that is often associated with pancreatic cancer.36 On exam these patients may have scleral icterus, sublingual jaundice, epigastric pain on palpation, weight loss, hepatomegaly, lymphadenopathy and a nontender, distended, palpable gallbladder (also known as Courvoisier sign).34 Abdominal signs and symptoms arise from tumor growth into surrounding vessels, tissues, and ducts within the abdominal cavity. Compression of the common bile duct accounts for the development of jaundice. Tumor growth around the stomach and duodenum can lead to delayed gastric emptying and subsequently nausea and vomiting. Constriction of the pancreatic duct leads to pancreatic insufficiency, precipitation of weight loss, and steatorrhea. Pancreatic insufficiency can worsen abdominal pain, and lead to increased weight loss and flatulence.
Less common symptoms include pain, erythema, and edema involving the lower extremities, which may be reflective of migratory thrombophlebitis (commonly known as Trousseau syndrome). Thromboembolic disease, including pulmonary embolism, portal vein, and deep vein thromboses are frequently encountered complications of pancreatic cancer. The incidence of thromboembolic events in patients with PDA has been reported to be as high as 54%.37 Of all signs encountered, weight loss is the most common and most profound. Patients with advanced PDA have severe degrees of cachexia. Some patients present with as much as a 5 kg/m2 decrease in their BMI from pre-illness baseline BMI, and lose another 3 to 4 kg/m2 through disease progression.38 At the time of diagnosis, many patients have already undergone significant weight loss, which can have substantial implications on treatment planning and clinical outcomes.
What other studies can be done to assist in making the diagnosis?
LABORATORY ABNORMALITIES AND TUMOR MARKERS
Elevations in alkaline phosphatase, γ-glutamyltransferase (GGT), serum aspartate aminotransferase (AST), serum alanine aminotransferase (ALT), and direct fractions of bilirubin are common in patients with PDA. Patients will usually have an obstructive pattern on their liver panel, with predominant elevations in direct bilirubin, alkaline phosphatase, and GGT, as compared with AST and ALT. Other baseline laboratory studies, including a complete blood count and basic metabolic panel, should be obtained because patients commonly have thrombocytosis, anemia, and electrolyte abnormalities due to the tumor itself and pancreatic insufficiency (Table 1).

Measurement of glycated hemoglobin (HBA1C) is an emerging and important diagnostic test in the diagnosis of pancreatic cancer. Recently, data has emerged to suggest that new-onset diabetes is present in about 50% of patients diagnosed with pancreatic cancer.39 The temporal relationship of pancreatic cancer and diabetes is supported by evidence showing that patients who undergo resection commonly have resolution of their diabetes.17 This study suggested that hyperglycemia, elevated HBA1C, and symptoms of diabetes in patients older than 50 years may identify patients who have early pancreatic cancer. The entity of pancreatic cancer–associated diabetes needs to be better defined and the algorithmic approach to evaluation and diagnosis, utilizing signs, symptoms, and laboratory values associated with diabetes, needs to be clearly established.
The only serum marker for PDA is carbohydrate antigen 19-9 (CA 19-9), also known as sialylated Lewis antigen or cancer-associated antigen. It was first identified in pancreatic cancer patients in 1981.40,41 The sensitivity and specificity of CA 19-9 ranges from 70% to approximately 90%.42,43 Hereditary predispositions and comorbid disease cross-reactivity contribute to the diminished sensitivity and specificity of CA 19-9. In about 5% to 10% of the population, CA 19-9 is not expressed (Lewis antigen A and B negative). Additionally, since CA 19-9 is expressed in the cells that line the biliary tree, diseases that lead to pancreatic or liver inflammation may falsely elevate CA 19-9.44 As a result, CA 19-9 is not an ideal screening test. However, data has shown that CA 19-9 may have prognostic value postoperatively and serve as a marker for therapeutic response.45,46
Is biopsy needed for this patient and if so, what is the most appropriate technique?
ENDOSCOPIC ULTRASOUND
Generally, diagnosis with tissue is not necessary for patients who clearly have resectable disease and will proceed directly to surgery for management. Nevertheless, it is still commonly obtained in this group of patients. However, in patients with LAPC or with features suggestive of LAPC, such as tumor approximation to critical vessels such as the superior mesenteric artery (SMA) or celiac axis, biopsy is necessary. These patients will receive neoadjuvant therapy, and biopsy is important in establishing a diagnosis. The ideal way to obtain a biopsy is through fine-needle aspiration (FNA) or biopsy (FNB) utilizing endoscopic ultrasound (EUS). Percutaneous and computed tomography (CT)–guided FNB can also be used to obtain a biopsy for diagnosis. In comparison to percutaneous and CT-guided FNB, EUS-FNA/FNB has low rates of complications, a decreased rate of peritoneal seeding, and is cost effective.47,48
CASE CONTINUED
Abdominal CT obtained following abdominal ultrasound reveals a 3.5-cm mass in the head of the pancreas in close approximation to the SMA and celiac axis.
Does the patient have borderline resectable or unresectable disease?
IMAGING
Abdominal ultrasound is a reasonable, inexpensive, and safe alternative to abdominal CT as it does not utilize ionizing radiation. It is particularly useful in patients who present with jaundice or have concern for biliary obstruction based on laboratory evaluation. It is particularly sensitive for detecting tumors greater than 3 cm in size.49,50 In patients whose abdominal ultrasound is unrevealing and whose index of suspicion remains high for PDA, abdominal CT should be the next imaging modality.
Abdominal CT obtained utilizing a pancreatic protocol is ideal for detection and staging of pancreatic tumors. By implementing a triple-phase protocol with arterial, late arterial, and venous phases, tumors, which have a density different from that of the pancreatic parenchyma, are accentuated. Abdominal CT is also able to provide critical information about tumor resectability.51 By revealing the degree of tumor encasement around the aorta, level of destruction of the superior mesenteric vein, or degree of involvement of the SMA or celiac vessels, abdominal CT determines if a patient should be deemed resectable, borderline resectable, or unresectable (Table 2).52,53 Resectability is based on thorough imaging evaluation, expert opinion of a multidisciplinary team, and guidelines proposed by American Hepatopancreaticobiliary Association, Society of Surgical Oncology, Society for Surgery of the Alimentary Tract, and the NCCN.54

Other imaging modalities have a less clearly established role in the diagnostic approach to PDA. In patients who have contraindications to obtaining CT, magnetic resonance imaging can be utilized as a secondary imaging modality.55 The role of positron emission tomography 18F-fluorodeoxyglucose (PET-FDG) is not clearly defined among clinicians, nor reflected in consensus guidelines by the National Comprehensive Cancer Network (NCCN). In clinical practice, it is still often combined with CT to detect metastatic disease, particularly in high-risk patients such has those with LAPC. The role of PET-CT in staging and its impact on clinical outcomes has not been fully established.
Endoscopic retrograde cholangiopancreatography (ERCP) and magnetic resonance cholangiopancreatography (MRCP) can also assist in the diagnosis and management of PDA. In patients with obstructive jaundice, both MRCP and ERCP visualize obstructions and dilations within the biliary tree, with the latter having the ability to intervene. ERCP allows for the collection of tissue to aid in diagnosis, and has the ability to relieve biliary obstruction via stenting.56
TREATMENT
CASE CONTINUED
After an abdominal CT is obtained, the patient is referred to an outpatient oncologist because of concern for pancreatic adenocarcinoma. After consultation, the patient is advised to obtain EUS with biopsy and to return immediately afterwards for further treatment planning. The pathology report following EUS confirms that the mass is a poorly differentiated PDA. The patient’s case is discussed at a multidisciplinary meeting with radiation, surgical, and medical oncology. The abdominal CT and PET-CT scan are thoroughly reviewed. After imaging review, the multidisciplinary team concludes that the tumor is in contact with the SMA at 120° and with the common hepatic artery without extension in the celiac axis and without evidence of metastasis.
What is the appropriate management of borderline resectable pancreatic cancer?
BORDERLINE RESECTABLE CANCER
Patients who have nonmetastatic disease and are deemed resectable and without contraindications to surgery or high-risk features, as defined by NCCN guidelines, should proceed directly to surgery. A large body of evidence suggests that complete surgical resection with negative margins is a significant predictor of survival and currently provides the only option for cure.57–59 Despite the curative intent of surgery, the rate of recurrence remains high in patients who undergo surgical resection. Even in patients with negative resection margins (R0 resection), the 5-year survival is 20% to 30%, with a median survival ranging from 12 to 25 months, suggesting the presence of regional and distant occult disease at the time of diagnosis.60–62
Additionally, in half the patients who undergo surgical resection with resultant positive microscopic (R1 resection) or gross (R2 resection) margins, the median survival is no greater than 12 months. In this subset of patients, clinical outcomes are similar to outcomes in patients with locally advanced and metastatic pancreatic cancer, suggesting that upfront surgery and adjuvant therapy may not be the ideal therapeutic option. This raises 2 important points: first, resectability should be assessed carefully in all patients with LAPC, and second, for those patients who are deemed borderline resectable, neoadjuvant therapy should be considered.63 Borderline resectability is defined as tumor abutment ≤ 180° of the celiac artery, and tumor abutment of the superior mesenteric vein /portal vein of > 180° or abutting ≤ 180° with irregularity of the vein with or without thrombosis with anatomical structures that still allows for safe and complete resection and vein reconstruction (Table 2).
Neoadjuvant Therapy
The goal of neoadjuvant therapy is to minimize the negative impact of upfront surgery in patients who have a high likelihood of having microscopic or grossly positive margins. Research has suggested that neoadjuvant therapy may improve resectability, decrease the rate of recurrence, and improve overall survival.64–66
There is no clear consensus on the ideal management of patients with borderline resectable disease. However, expert guidelines are in agreement that upfront surgery in patients with LAPC is not appropriate, as most patients will not be able to achieve an R0 resection.67 As staging and management of patients with LAPC is difficult, expertise of a multidisciplinary team can be helpful.68
Several studies and the NCCN guidelines support the use of neoadjuvant therapy in patients deemed borderline resectable.69,70 Treatment of borderline resectable disease is similar to unresectable LAPC and generally involves 2 chemotherapy treatment backbones: FOLFIRINOX (folinic acid [leucovorin], fluorouracil [5-FU], irinotecan, and oxaliplatin) or gemcitabine-based therapy.
Phase 1 to 2/3 clinical trials conducted by Conroy et al from 2005 to 2011, including the landmark ACCORD-11 trial, established the safety and role of FOLFIRINOX in metastatic pancreatic cancer and also demonstrated an improved overall survival with the use of this therapy in these patients.71,72 These findings led to interest in FOLFIRINOX as a neoadjuvant therapy for patients with LAPC. Since then, multiple prospective and retrospective studies have shown that 54% to 100% of patients with borderline resectable LAPC who were treated with FOLFIRINOX were down-staged significantly enough to undergo resection. Of those patients, more than 90% had a R0 resection following surgery (Table 3).73–79

Data over the past 7 years suggests that neoadjuvant FOLFIRINOX improves overall survival and resectability in patients with borderline disease. However, treatment with FOLFIRINOX is not without limitations. FOLFIRINOX is associated with higher rates of febrile neutropenia, thrombocytopenia, diarrhea, and sensory neuropathy as compared with gemcitabine-based therapy.72 Other less commonly observed toxicities associated with FOLFIRINOX include mucositis, hand-foot syndrome, pulmonary toxicity, and alopecia. Dose-attenuated FOLFIRINOX-based regimens, including those that exclude the bolus fluorouracil dose and augment upfront filgrastim, have demonstrated improved safety and comparable efficacy as compared to standard FOLFIRINOX.80
Gemcitabine has been the fundamental treatment backbone for PDA since the results of the phase 3 CONKO-001 trial were published.81 Gemcitabine is a pyrimidine antimetabolite and potent inhibitor of DNA polymerase and ribonucleotide reductase.82 In recent years, multiple combination therapies with gemcitabine have been investigated, including regimens with nab-paclitaxel, oxaliplatin, or docetaxel. Resection rates and negative margin outcomes have been shown to be comparable to patients who received FOLFIRINOX in the neoadjuvant setting with borderline locally advanced disease.83–85 In addition to having a more tolerable side effect profile in comparison to fluorouracil-based regimens, gemcitabine is considered to be a potent radiosensitizer.86 For this reason, studies have also investigated the role of radiotherapy in conjunction with gemcitabine, revealing negative margin resection rates above 80% in patients with borderline resectable disease.87,88
Because very few studies directly comparing FOLFIRINOX with gemcitabine-based combination regimens have been completed, there is no clear consensus on the preferred treatment regimen, in both borderline and unresectable LAPC. Decisions to treat are influenced predominantly by comorbidities, adverse effect profiles, and performance status of patients, as FOLFIRINOX is the more toxic of the 2 treatment backbones. Therefore, FOLFIRINOX has mostly been utilized in patients with relatively good functional status (Eastern Cooperative Oncology Group [ECOG] performance status 0 to 1).89 In elderly patients and those with poor functional status, ECOG 2 to 4, gemcitabine as a single agent is a reasonable alternative in the neoadjuvant setting of borderline resectable disease.
The exact role of radiation therapy in addition to induction chemotherapy in borderline resectable pancreatic cancer has not been clearly established because of the lack of prospective studies in this area. Multiple large retrospective series have identified high rates of conversion to margin-negative resection with neoadjuvant chemoradiation alone.90 Based on available data, it is reasonable for patients with borderline resectable disease to proceed with any of the following treatment options: chemotherapy, chemoradiation, or induction chemotherapy followed by chemoradiation (Figure). Chemotherapy and chemoradiation are generally more appropriate with patients with high CA 19-9 levels or those at an elevated risk of having positive margins or occult metastatic disease.91 Obtaining negative margin resections is the predominant goal of neoadjuvant radiotherapy.89 Many studies have identified margin status to be one of the most significant prognostic factors in PDA.57,59,92,93 Additionally, several studies have highlighted that radiotherapy in the neoadjuvant setting could improve negative margin resection rates, local control, and clinical outcomes in patients with borderline resectable locally advanced disease.94–97 A common multimodal regimen utilized in the neoadjuvant setting combines capecitabine, an oral prodrug that is converted to fluorouracil, with radiation therapy. This combination has also been shown to improve resectability rates and long-term clinical outcomes in patients with borderline resectable disease.98 Additionally, neoadjuvant radiation therapy can potentially downstage patients with unresectable disease at the time of diagnosis to become surgical candidates.99 Despite the paucity of data, interval scans utilizing CT following neoadjuvant therapy should be obtained 2 to 4 months after completion of therapy to determine therapeutic response, evaluate for disease progression, and, most important, reassess surgical stage/resectability. It is clinically acceptable to proceed to resection with radiographically stable disease post-neoadjuvant therapy.

Many patients classified as borderline resectable are able to proceed with surgery following neoadjuvant therapy. Unfortunately, specific data on adjuvant therapy following neoadjuvant chemotherapy or chemoradiotherapy and surgical resection in borderline resectable patients is scarce. Clinical practice guidelines are extrapolated from studies where upfront resection in clearly resectable patients was followed by adjuvant therapy. Based on these data, approximately 6 months of perioperative chemotherapy with or without chemoradiotherapy is a reasonable consideration. Nevertheless, about 80% of patients at the time of diagnosis are deemed to be unresectable, and a smaller number do not proceed to surgery despite an initial classification as borderline resectable. Of the 80% of patients with advanced disease, about half are metastatic at presentation and the remaining 30% to 40% are defined as having unresectable LAPC.100
CASE CONTINUED
The patient is deemed borderline resectable. He receives neoadjuvant therapy with gemcitabine and nab-paclitaxel. Two months after therapy, interval imaging with abdominal CT does not show improvement in tumor size and there is now evidence that the tumor has invaded the celiac axis and is abutting more than 180° of the SMA. The patient presents to the oncologist to discuss further management. He has lost about 15 lb since his last evaluation, is capable of self-care, but is unable to carry on with any work activities.
What is the appropriate management of unresectable nonmetastatic LAPC?
UNRESECTABLE LOCALLY ADVANCED CANCER
As in the case of borderline resectable disease, there are many treatment options for patients with unresectable LAPC. Timing, optimal chemotherapy regimen, and the addition of regularly and hypofractionated radiotherapy are issues currently under investigation. However, there are some general considerations and principles that are followed as reflected in the NCCN guidelines and recent studies. The primary therapeutic aims in patients with unresectable locally advanced disease are to increase survival and improve palliation.
The elderly comprise a large percentage of the patients diagnosed with unresectable locally advanced disease. Pharmacokinetics and toxicity profiles are altered in the elderly population.101,102 Therefore, it is important to assess functional status and comorbidities as these are critical factors in determining treatment regimens, similar to patients with borderline resectable disease. Currently, the most common first-line therapies in advanced pancreatic cancer are gemcitabine alone, gemcitabine and nab-paclitaxel, FOLFIRINOX, gemcitabine/capecitabine, and gemcitabine/oxaliplatin.103 The overall treatment approach to unresectable locally advanced pancreatic adenocarcinoma closely mirrors that of patients with borderline resectable disease and metastatic disease. Much of the data supporting treatment regimens in unresectable LAPC is extrapolated from clinical trials looking at advanced or metastatic pancreatic cancer.
Consensus opinions domestically and from Europe recommend that patients with locally advanced unresectable disease undergo upfront chemotherapy (Figure).104 This is based on the premise that initial chemotherapy may destroy occult metastatic cells and increase the efficacy of consolidative chemotherapy, particularly with radiation in the future. Upfront chemoradiotherapy has only been investigated in a small series of trials in which no clear survival benefit was observed and has the added consequence of treatment-related toxicity.105 However, data is limited in this regard, with variations in treatment protocols and cohort compositions contributing to the inconclusive findings.
Despite advances in immunotherapy, targeted therapies, and gene sequencing, initial chemotherapy for unresectable disease is still either gemcitabine-based combination therapy or FOLFIRINOX. Across numerous studies, patients with unresectable LAPC receiving FOLFIRINOX have a median progression-free survival of 3 to 20 months and a median overall survival of 10 to 32.7 months.106 As with borderline resectable patients, FOLFIRINOX (Table 4) is generally reserved for unresectable patients with good functional status (ECOG 0–1 or Karnofsky Performance Status 90–100) and those at low risk for developing grade 3 or 4 systemic toxicities.103 For these reasons it has generally not been frequently combined with other chemotherapeutic agents. However, FOLFIRINOX has been combined with radiation therapy in the consolidative neoadjuvant setting after induction chemotherapy. There have also been studies where traditional FOLFIRONIX was modified to improve tolerability, as evidenced by Gunturu et al’s study, which dose-reduced both fluorouracil and irinotecan by 25%, without compromising efficacy and simultaneously increasing tolerability.107 Additionally, FOLFIRINOX requires infusional administration of the fluorouracil component, which may not be practical in certain patients. In that subset, capecitabine can be substituted. Among radiosensitizers during neoadjuvant therapy for unresectable LAPC, capecitabine has been shown to be as efficacious and less toxic than even gemcitabine.108


No head-to-head studies investigating FOLFIRINOX versus nab-paclitaxel and gemcitabine in patients with locally advanced disease have been published, but clinical trials are under way. Other combination therapies have been looked at through small retrospective or prospective studies, but no robust, large-scale clinical trials have been completed. For this reason, NCCN guidelines recommend enrollment of patients with LAPC into active clinical trials.
What is the role of radiation therapy in unresectable LAPC?
Despite the reported advantages of neoadjuvant radiation in patients with potentially resectable disease, there is significant debate regarding the timing and role of neoadjuvant radiation in patients with unresectable disease. Numerous comprehensive analyses and studiest indicate that chemoradiotherapy leads to significantly better overall survival compared to no therapy or radiation therapy alone in LAPC.68,110,111 However, conflicting data support the use of upfront chemoradiotherapy in unresectable LAPC when compared to chemotherapy alone. Unfortunately, most prospective studies investigating the role of radiotherapy were performed following administration of single-agent gemcitabine, which is no longer considered standard of care for patients with LAPC. In spite of this, ECOG 4201 identified a statistically significant improvement in median overall survival following the addition of gemcitabine-based radiotherapy. Huguet et al in his review pointed out that upfront chemoradiotherapy was not superior to chemotherapy only and was associated with increased treatment toxicity.105 Additionally, a recent phase 3 study looking at chemoradiotherapy versus chemotherapy alone in patients treated with gemcitabine found no difference in overall survival.112 This can potentially be attributed to the fact that about 30% of patients with LAPC develop metastatic disease in the early phases of treatment due to poor control of local and systemically occult disease.113 Given the propensity for high rates of occult metastatic disease in LAPC, treatment paradigms and consensus guidelines recommend multi-agent systemic chemotherapy followed by chemoradiotherapy in select patients.
Based on current studies and until further clinical investigations are completed, consensus opinion indicates that the most appropriate approach in unresectable LAPC is to begin with induction chemotherapy (with either gemcitabine plus nab-paclitaxel, FOLFIRINOX, capecitabine, or other treatment combinations), followed by chemoradiation in the absence of disease progression when the first repeat imaging evaluation is completed (Figure). One important caveat regarding reimaging with CT in the neoadjuvant setting is that radiologic response may not correlate with pathologic response.114 PET-CT may have a role in predicting response to neoadjuvant therapy. Overall, induction chemotherapy followed by consolidative chemoradiation may confer numerous benefits: it removes the unnecessary burden and toxicity associated with radiotherapy in the nearly one third of patients who have pervasive disease progression during initial treatment; it allows testing and increases the chances of tolerating full-dose systemic chemotherapy; and it raises the likelihood of converting patients who do not progress to metastasis during the initial phase of treatment from unresectable to resectable status.103,115 Despite the lack of strong conclusive data, the general agreement is that neoadjuvant chemoradiotherapy converts about one third of borderline and unresectable LAPC to an R0 resection.95,103 There are very specific rationales for the addition of radiotherapy in LAPC, and these functions need to be better defined through further clinical trials.
PALLIATIVE CARE
CASE CONTINUED
The patient is unable to tolerate his first round of second-line therapy with modified FOLFIRINOX. His overall treatment plan is transitioned to palliation. He continues to have pain, despite increasing doses of narcotics.
What is the next step for patients in whom second-line therapy fails and who have intractable pain while on high-dose narcotics?
A subset of patients with unresectable LAPC may not be amenable to chemotherapy with or without radiation due to significant comorbidities or because they present with or progress to ECOG scores 3 or 4. The goal in these patients should be palliation. Pain is one of the most predominant and difficult to manage symptoms in progressive LAPC. Opioid-based medications are the primary treatment for pain in LAPC. However, patients sometimes become refractory to opioid medications. In this group of patients, it is reasonable to consider palliative radiation as an alternative method for pain control.116
An alternative to palliative radiation in the setting of progressive pain in PDA is celiac plexus block or neurolysis. By injecting an anesthetic or alcohol into the celiac plexus, neural signaling pathways involved in the propagation of pain are inhibited without leading to significant nerve destruction. Additionally, chemical splanchnicectomy allows for reduced opioid medication use and associated side effects.117
In general patients with LAPC have profound weight loss prior to and during treatment. This has significant implications prognostically and on treatment options. The underlying etiology is multifactorial, but one of the primary driving factors is pancreatic insufficiency. An estimated 65% of pancreatic cancer patients have fat malabsorption, and 50% have protein malabsorption, leading to steatorrhea and weight loss.118 Patients diagnosed with pancreatic cancer should be given enzyme replacement with formulations that include lipase, amylase, and protease. A minimum dose of enzyme replacement should include 40,000 to 50,000 U of lipase during meals and 25,000 U during snack intake. If maldigestion, symptoms, or nutritional endpoints (BMI, albumin, prealbumin, cholesterol) do not improve, the pancreatic enzyme dose should be escalated and a proton-pump inhibitor (PPI) added. In patients with pancreatic insufficiency, PPIs have been shown to improve fat absorption.119 Enzyme replacement therapy has been shown to prevent weight loss in patients with unresectable pancreatic cancer.120
As most patients with LAPC go on to develop progressive disease, palliative care becomes an integral aspect of the therapeutic paradigm. Palliation in LAPC has a significant role in determining quality of life and ensuring patient’s goals of care have been meet. Studies have suggested that pancreatic cancer is second only to lung cancer in terms of the number of emergency department visits in the later stages of disease.120 Additionally, aggressive care in the setting of incurable diseases such as LAPC has been associated with poor quality of life.121 More recently it has been shown that involvement of palliative care in patients with advanced pancreatic is associated with less aggressive care near death.122 Therefore, the incorporation of palliative or supportive care teams in the treatment of patients with progressive LAPC can improve quality of life and alleviate suffering associated with increasing symptom burden.
CONCLUSION
LAPC is a difficult disease for both provider and patient. There is a paucity of robust clinical trials in the neoadjuvant setting for LAPC. Current research is complicated by varying consensus definitions of resectability and the varying treatment configurations across studies. The optimal type, timing, and sequence of treatment and whether to add radiation therapy in LAPC have not been clearly defined. However, based on the available studies and consensus guidelines, patients who are deemed to have LAPC should have neoadjuvant therapy. FOLFIRINOX or gemcitabine with nab-paclitaxel should be considered first-line treatments. Patients with LAPC who respond to chemotherapy or are ineligible for multi-drug chemotherapy may benefit from chemoradiotherapy. In patients with unresectable disease, chemoradiotherapy has been shown to enhance survival as compared to best supportive care or radiation alone. For borderline resectable disease, it is reasonable to treat patients with either chemoradiotherapy, chemotherapy alone, or chemotherapy followed by chemoradiotherapy.
Considering the invasive nature of LAPC and the controversy around neoadjuvant treatment protocols, enrollment of patients with LAPC into clinical trials is important and will help determine the optimal treatment regimen for future patients.
- Network PCA. Pancreatic cancer facts 2016. 2016. https://www.pancan.org/wp-content/uploads/2016/02/2016-GAA-PC-Facts.pdf. Accessed April 24, 2017.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7–30.
- Konstantinidis IT, Warshaw AL, Allen JN, et al. Pancreatic ductal adenocarcinoma: is there a survival difference for R1 resections versus locally advanced unresectable tumors? What is a “true” R0 resection? Ann Surg 2013;257:731–6.
- Ilic M, Ilic I. Epidemiology of pancreatic cancer. World J Gastroenterol 2016;22:9694–705.
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69–90.
- Zhang J, Dhakal I, Ning B, Kesteloot H. Patterns and trends of pancreatic cancer mortality rates in Arkansas, 1969-2002: a comparison with the US population. Eur J Cancer Prev 2008;17:18–27.
- National Cancer Institute. SEER cancer statistics review, 1975-2013. http://seer.cancer.gov/csr/1975_2013/. Accessed April 24, 2017.
- Lowenfels AB, Maisonneuve P. Epidemiology and risk factors for pancreatic cancer. Best Pract Res Clin Gastroenterol 2006;20:197–209.
- Fuchs CS, Colditz GA, Stampfer MJ, et al. A prospective study of cigarette smoking and the risk of pancreatic cancer. Arch Intern Med 1996;156:2255–60.
- Lucenteforte E, La Vecchia C, Silverman D, et al. Alcohol consumption and pancreatic cancer: a pooled analysis in the International Pancreatic Cancer Case-Control Consortium (PanC4). Ann Oncol 2012;23:374–82.
- Schernhammer ES, Kang JH, Chan AT, et al. A prospective study of aspirin use and the risk of pancreatic cancer in women. J Natl Cancer Inst 2004;96:22–28.
- Michaud DS, Giovannucci E, Willett WC, et al. Physical activity, obesity, height, and the risk of pancreatic cancer. JAMA 2001;286:921–9.
- Nothlings U, Wilkens LR, Murphy SP, et al. Meat and fat intake as risk factors for pancreatic cancer: the multiethnic cohort study. J Natl Cancer Inst 2005;97:1458–65.
- Chari ST, Leibson CL, Rabe KG, et al. Probability of pancreatic cancer following diabetes: a population-based study. Gastroenterology 2005;129:504–11.
- Batabyal P, Vander Hoorn S, Christophi C, Nikfarjam M. Association of diabetes mellitus and pancreatic adenocarcinoma: a meta-analysis of 88 studies. Ann Surg Oncol 2014;21:2453–62.
- Chari ST, Leibson CL, Rabe KG, et al. Pancreatic cancer-associated diabetes mellitus: prevalence and temporal association with diagnosis of cancer. Gastroenterology 2008;134:95–101.
- Pannala R, Basu A, Petersen GM, Chari ST. New-onset diabetes: a potential clue to the early diagnosis of pancreatic cancer. Lancet Oncol 2009;10:88–95.
- Wolpin BM, Chan AT, Hartge P, et al. ABO blood group and the risk of pancreatic cancer. J Natl Cancer Inst 2009;101:424–31.
- Iqbal J, Ragone A, Lubinski J, et al. The incidence of pancreatic cancer in BRCA1 and BRCA2 mutation carriers. Br J Cancer 2012;107:2005–9.
- Giardiello FM, Brensinger JD, Tersmette AC, et al. Very high risk of cancer in familial Peutz-Jeghers syndrome. Gastroenterology 2000;119:1447–53.
- Kastrinos F, Mukherjee B, Tayob N, et al. Risk of pancreatic cancer in families with Lynch syndrome. JAMA 2009;302:1790–5.
- Brand RE, Lynch HT. Hereditary pancreatic adenocarcinoma. A clinical perspective. Med Clin North Am 2000;84:665–75.
- Jacobs EJ, Chanock SJ, Fuchs CS, et al. Family history of cancer and risk of pancreatic cancer: a pooled analysis from the Pancreatic Cancer Cohort Consortium (PanScan). Int J Cancer 2010;127:1421–8.
- Rustgi AK. Familial pancreatic cancer: genetic advances. Genes Dev 2014;28:1–7.
- Reznik R, Hendifar AE, Tuli R. Genetic determinants and potential therapeutic targets for pancreatic adenocarcinoma. Front Physiol 2014;5:87.
- Lowenfels AB, Maisonneuve P, DiMagno EP, et al. Hereditary pancreatitis and the risk of pancreatic cancer. International Hereditary Pancreatitis Study Group. J Natl Cancer Inst 1997;89:442–6.
- Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med 2014;371:2140–1.
- Kanda M, Matthaei H, Wu J, et al. Presence of somatic mutations in most early-stage pancreatic intraepithelial neoplasia. Gastroenterology 2012;142:730–3.
- Feldmann G, Maitra A. Molecular genetics of pancreatic ductal adenocarcinomas and recent implications for translational efforts. J Mol Diagn 2008;10:111–22.
- Eser S, Schnieke A, Schneider G, Saur D. Oncogenic KRAS signalling in pancreatic cancer. Br J Cancer 2014;111:817–22.
- Ying H, Kimmelman AC, Lyssiotis CA, et al. Oncogenic KRAS maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell 2012;149:656–70.
- Provenzano PP, Cuevas C, Chang AE, et al. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell 2012;21:418–29.
- Vonderheide RH, Bayne LJ. Inflammatory networks and immune surveillance of pancreatic carcinoma. Curr Opin Immunol 2013;25:200–5.
- DiMagno EP. Pancreatic cancer: Clinical presentation, pitfalls and early clues. Ann Oncol 1999;10(suppl 4):S140–S142.
- Porta M, Fabregat X, Malats N, et al. Exocrine pancreatic cancer: symptoms at presentation and their relation to tumour site and stage. Clin Transl Oncol 2005;7:189–97.
- Gullo L, Tomassetti P, Migliori M, et al. Do early symptoms of pancreatic cancer exist that can allow an earlier diagnosis? Pancreas 2001;22:210–3.
- Khorana AA, Fine RL. Pancreatic cancer and thromboembolic disease. Lancet Oncol 2004;5(11):655-663.
- Wigmore SJ, Plester CE, Richardson RA, Fearon KC. Changes in nutritional status associated with unresectable pancreatic cancer. Br J Cancer 1997;75:106–9.
- Aggarwal G, Rabe KG, Petersen GM, Chari ST. New-onset diabetes in pancreatic cancer: a study in the primary care setting. Pancreatology 2012;12:156–61.
- Koprowski H, Herlyn M, Steplewski Z, Sears HF. Specific antigen in serum of patients with colon carcinoma. Science 1981;212:53–5.
- Bond-Smith G, Banga N, Hammond TM, Imber CJ. Pancreatic adenocarcinoma. BMJ 2012;344:e2476.
- Cwik G, Wallner G, Skoczylas T, et al. Cancer antigens 19-9 and 125 in the differential diagnosis of pancreatic mass lesions. Arch Surg 2006;141:968–73.
- van den Bosch RP, van Eijck CH, Mulder PG, Jeekel J. Serum CA19-9 determination in the management of pancreatic cancer. Hepatogastroenterology 1996;43:710–3.
- Lamerz R. Role of tumour markers, cytogenetics. Ann Oncol 1999;10 Suppl 4:145–9.
- Hess V, Glimelius B, Grawe P, et al. CA 19-9 tumour-marker response to chemotherapy in patients with advanced pancreatic cancer enrolled in a randomised controlled trial. Lancet Oncol 2008;9:132–8.
- Montgomery RC, Hoffman JP, Riley LB, et al. Prediction of recurrence and survival by post-resection CA 19-9 values in patients with adenocarcinoma of the pancreas. Ann Surg Oncol 1997;4:551–6.
- Zamboni GA, D’Onofrio M, Idili A, et al. Ultrasound-guided percutaneous fine-needle aspiration of 545 focal pancreatic lesions. AJR Am J Roentgenol 2009;193:1691–5.
- Micames C, Jowell PS, White R, et al. Lower frequency of peritoneal carcinomatosis in patients with pancreatic cancer diagnosed by EUS-guided FNA vs. percutaneous FNA. Gastrointest Endosc 2003;58:690–5.
- Brambs HJ, Claussen CD. Pancreatic and ampullary carcinoma. Ultrasound, computed tomography, magnetic resonance imaging and angiography. Endoscopy 1993;25:58–68.
- Karlson BM, Ekbom A, Lindgren PG, et al. Abdominal US for diagnosis of pancreatic tumor: prospective cohort analysis. Radiology 1999;213:107–11.
- Imbriaco M, Megibow AJ, Camera L, et al. Dual-phase versus single-phase helical CT to detect and assess resectability of pancreatic carcinoma. AJR Am J Roentgenol 2002;178:1473–9.
- Schrag D. Optimizing treatment for locally advanced pancreas cancer: progress but no precision. JAMA 2016;315:1837–8.
- House MG, Yeo CJ, Cameron JL, et al. Predicting resectability of periampullary cancer with three-dimensional computed tomography. J Gastrointest Surg 2004;8:280–8.
- Callery MP, Chang KJ, Fishman EK, et al. Pretreatment assessment of resectable and borderline resectable pancreatic cancer: expert consensus statement. Ann Surg Oncol 2009;16:1727–33.
- Horton KM, Fishman EK. Adenocarcinoma of the pancreas: CT imaging. Radiol Clin North Am 2002;40:1263–72.
- Ross WA, Wasan SM, Evans DB, et al. Combined EUS with FNA and ERCP for the evaluation of patients with obstructive jaundice from presumed pancreatic malignancy. Gastrointest Endosc 2008;68:461–6.
- Hartwig W, Hackert T, Hinz U, et al. Pancreatic cancer surgery in the new millennium: better prediction of outcome. Ann Surg 2011;254:311–9.
- Jamieson NB, Chan NI, Foulis AK, et al. The prognostic influence of resection margin clearance following pancreaticoduodenectomy for pancreatic ductal adenocarcinoma. J Gastrointest Surg 2013;17:511–21.
- Ethun CG, Kooby DA. The importance of surgical margins in pancreatic cancer. J Surg Oncol 2016;113:283–8.
- Fischer R, Breidert M, Keck T, et al. Early recurrence of pancreatic cancer after resection and during adjuvant chemotherapy. Saudi J Gastroenterol 2012;18:118–21.
- Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med 2004;350:1200–10.
- Neoptolemos JP, Palmer DH, Ghaneh P, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet 2017;389(10073):1011–24.
- Cardenes HR, Chiorean EG, Dewitt J, et al. Locally advanced pancreatic cancer: current therapeutic approach. Oncologist 2006;11:612–23.
- Yeung RS, Weese JL, Hoffman JP, et al. Neoadjuvant chemoradiation in pancreatic and duodenal carcinoma. A Phase II Study. Cancer 1993;72:2124–33.
- Spitz FR, Abbruzzese JL, Lee JE, et al. Preoperative and postoperative chemoradiation strategies in patients treated with pancreaticoduodenectomy for adenocarcinoma of the pancreas. J Clin Oncol 1997;15:928–37.
- McClaine RJ, Lowy AM, Sussman JJ, et al. Neoadjuvant therapy may lead to successful surgical resection and improved survival in patients with borderline resectable pancreatic cancer. HPB (Oxford) 2010;12:73–9.
- Balaban EP, Mangu PB, Khorana AA, et al. Locally advanced, unresectable pancreatic cCancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2016;34:2654–68.
- Shaib WL, Ip A, Cardona K, et al. Contemporary management of borderline resectable and locally advanced unresectable pancreatic cancer. Oncologist 2016;21:178–87.
- Chun YS, Milestone BN, Watson JC, et al. Defining venous involvement in borderline resectable pancreatic cancer. Ann Surg Oncol 2010;17:2832–8.
- Evans DB, Erickson BA, Ritch P. Borderline resectable pancreatic cancer: definitions and the importance of multimodality therapy. Ann Surg Oncol 2010;17:2803–5.
- Conroy T, Paillot B, Francois E, et al. Irinotecan plus oxaliplatin and leucovorin-modulated fluorouracil in advanced pancreatic cancer--a Groupe Tumeurs Digestives of the Federation Nationale des Centres de Lutte Contre le Cancer study. J Clin Oncol 2005;23:1228–36.
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817–25.
- Hosein PJ, Macintyre J, Kawamura C, et al. A retrospective study of neoadjuvant FOLFIRINOX in unresectable or borderline-resectable locally advanced pancreatic adenocarcinoma. BMC Cancer 2012;12:199.
- Peddi PF, Lubner S, McWilliams R, et al. Multi-institutional experience with FOLFIRINOX in pancreatic adenocarcinoma. JOP 2012;13:497–501.
- Mahaseth H, Kauh JS, Brutcher E, et al. Safety and efficacy of modified FOLFIRINOX in pancreatic cancer: A retrospective experience. J Clin Oncol 2012;30 (suppl; abstr e14614).
- Kim SS, Nakakura EK, Wang ZJ, et al. Preoperative FOLFIRINOX for borderline resectable pancreatic cancer: Is radiation necessary in the modern era of chemotherapy? J Surg Oncol 2016;114:587–96.
- Conroy T, Gavoille C, Samalin E, et al. The role of the FOLFIRINOX regimen for advanced pancreatic cancer. Curr Oncol Rep 2013;15:182–9.
- Katz MH, Shi Q, Ahmad SA, et al. Preoperative modified FOLFIRINOX treatment followed by capecitabine-based chemoradiation for borderline resectable pancreatic cancer: Alliance for Clinical Trials in Oncology Trial A021101. JAMA Surg 2016;151:e161137.
- Kharofa J, Kelly TR, Ritch PS, et al. 5-FU/leucovorin, irinotecan, oxaliplatin (FOLFIRINOX) induction followed by chemoXRT in borderline resectable pancreatic cancer. J Clin Oncol 2012;30 (suppl; abstr e14613).
- Blazer M, Wu C, Goldberg RM, et al. Neoadjuvant modified (m) FOLFIRINOX for locally advanced unresectable (LAPC) and borderline resectable (BRPC) adenocarcinoma of the pancreas. Ann Surg Oncol 2015;22:1153–9.
- Oettle H, Post S, Neuhaus P, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA 2007;297:267–77.
- Plunkett W, Huang P, Xu YZ, et al. Gemcitabine: metabolism, mechanisms of action, and self-potentiation. Semin Oncol 1995;22(4 Suppl 11):3–10.
- Sahora K, Kuehrer I, Eisenhut A, et al. NeoGemOx: Gemcitabine and oxaliplatin as neoadjuvant treatment for locally advanced, nonmetastasized pancreatic cancer. Surgery 2011;149(3):311–20.
- Lee JL, Kim SC, Kim JH, et al. Prospective efficacy and safety study of neoadjuvant gemcitabine with capecitabine combination chemotherapy for borderline-resectable or unresectable locally advanced pancreatic adenocarcinoma. Surgery 2012;152:851–62.
- Leone F, Gatti M, Massucco P, et al. Induction gemcitabine and oxaliplatin therapy followed by a twice-weekly infusion of gemcitabine and concurrent external-beam radiation for neoadjuvant treatment of locally advanced pancreatic cancer: a single institutional experience. Cancer 2013;119:277–84.
- Lawrence TS, Eisbruch A, Shewach DS. Gemcitabine-mediated radiosensitization. Semin Oncol 1997;24(2 Suppl 7):S7–24-S27–28.
- Kang CM, Chung YE, Park JY, et al. Potential contribution of preoperative neoadjuvant concurrent chemoradiation therapy on margin-negative resection in borderline resectable pancreatic cancer. J Gastrointest Surg 2012;16:509–17.
- Chuong MD, Hayman TJ, Patel MR, et al. Comparison of 1-, 2-, and 3-dimensional tumor response assessment after neoadjuvant GTX-RT in borderline-resectable pancreatic cancer. Gastrointest Cancer Res 2011;4:128–34.
- Loehrer AP, Kinnier CV, Ferrone CR. Treatment of locally advanced pancreatic ductal adenocarcinoma. Adv Surg 2016;50:115–28.
- Katz MH, Wang H, Balachandran A, et al. Effect of neoadjuvant chemoradiation and surgical technique on recurrence of localized pancreatic cancer. J Gastrointest Surg 2012;16:68–78.
- Franke AJ, Rosati LM, Pawlik TM, et al. The role of radiation therapy in pancreatic ductal adenocarcinoma in the neoadjuvant and adjuvant settings. Semin Oncol 2015;42:144–62.
- Butturini G, Stocken DD, Wente MN, et al. Influence of resection margins and treatment on survival in patients with pancreatic cancer: meta-analysis of randomized controlled trials. Arch Surg 2008;143:75–83.
- Paniccia A, Hosokawa P, Henderson W, et al. Characteristics of 10-year survivors of pancreatic ductal adenocarcinoma. JAMA Surg 2015;150:701–10.
- Massucco P, Capussotti L, Magnino A, et al. Pancreatic resections after chemoradiotherapy for locally advanced ductal adenocarcinoma: analysis of perioperative outcome and survival. Ann Surg Oncol 2006;13:1201–8.
- Patel M, Hoffe S, Malafa M, et al. Neoadjuvant GTX chemotherapy and IMRT-based chemoradiation for borderline resectable pancreatic cancer. J Surg Oncol 2011;104:155–161.
- Landry J, Catalano PJ, Staley C, et al. Randomized phase II study of gemcitabine plus radiotherapy versus gemcitabine, 5-fluorouracil, and cisplatin followed by radiotherapy and 5-fluorouracil for patients with locally advanced, potentially resectable pancreatic adenocarcinoma. J Surg Oncol 2010;101:587–92.
- Lamb R, Ozsvari B, Lisanti CL, et al. Antibiotics that target mitochondria effectively eradicate cancer stem cells, across multiple tumor types: treating cancer like an infectious disease. Oncotarget 2015;6:4569–84.
- Stokes JB, Nolan NJ, Stelow EB, et al. Preoperative capecitabine and concurrent radiation for borderline resectable pancreatic cancer. Ann Surg Oncol 2011;18:619–27.
- White R, Lee C, Anscher M, et al. Preoperative chemoradiation for patients with locally advanced adenocarcinoma of the pancreas. Ann Surg Oncol 1999;6:38–45.
- Martin RC 2nd. Management of locally advanced pancreatic cancer. Surg Clin North Am 2016;96:1371–89.
- Higuera O, Ghanem I, Nasimi R, et al. Management of pancreatic cancer in the elderly. World J Gastroenterol 2016;22:764–75.
- Hurria A, Lichtman SM. Clinical pharmacology of cancer therapies in older adults. Br J Cancer 2008;98:517–22.
- Spadi R, Brusa F, Ponzetti A, et al. Current therapeutic strategies for advanced pancreatic cancer: A review for clinicians. World J Clin Oncol 2016;7:27–43.
- Seufferlein T, Bachet JB, Van Cutsem E, Rougier P, Group EGW. Pancreatic adenocarcinoma: ESMO-ESDO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2012;23 Suppl 7:vii33–40.
- Huguet F, Girard N, Guerche CS, et al. Chemoradiotherapy in the management of locally advanced pancreatic carcinoma: a qualitative systematic review. J Clin Oncol 2009;27:2269–77.
- Suker M, Beumer BR, Sadot E, et al. FOLFIRINOX for locally advanced pancreatic cancer: a systematic review and patient-level meta-analysis. Lancet Oncol 2016;17:801–10.
- Gunturu KS, Yao X, Cong X, et al. FOLFIRINOX for locally advanced and metastatic pancreatic cancer: single institution retrospective review of efficacy and toxicity. Med Oncol 2013;30:361.
- Mukherjee S, Hurt CN, Bridgewater J, et al. Gemcitabine-based or capecitabine-based chemoradiotherapy for locally advanced pancreatic cancer (SCALOP): a multicentre, randomised, phase 2 trial. Lancet Oncol 2013;14:317–26.
- Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013;369:1691–1703.
- Krzyzanowska MK, Weeks JC, Earle CC. Treatment of locally advanced pancreatic cancer in the real world: population-based practices and effectiveness. J Clin Oncol 2003;21:3409–14.
- Sultana A, Tudur Smith C, Cunningham D, et al. Meta-analyses of chemotherapy for locally advanced and metastatic pancreatic cancer: results of secondary end points analyses. Br J Cancer 2008;99:6–13.
- Hammel P, Huguet F, van Laethem JL, et al. Effect of chemoradiotherapy vs chemotherapy on survival in patients with locally advanced pancreatic cancer controlled after 4 months of gemcitabine with or without erlotinib: the LAP07 randomized clinical trial. JAMA 2016;315:1844–53.
- Huguet F, Andre T, Hammel P, et al. Impact of chemoradiotherapy after disease control with chemotherapy in locally advanced pancreatic adenocarcinoma in GERCOR phase II and III studies. J Clin Oncol 2007;25:326–31.
- Dholakia AS, Hacker-Prietz A, Wild AT, et al. Resection of borderline resectable pancreatic cancer after neoadjuvant chemoradiation does not depend on improved radiographic appearance of tumor-vessel relationships. J Radiat Oncol 2013;2:413–25.
- Heinemann V, Haas M, Boeck S. Neoadjuvant treatment of borderline resectable and non-resectable pancreatic cancer. Ann Oncol 2013;24:2484–92.
- Morganti AG, Trodella L, Valentini V, et al. Pain relief with short-term irradiation in locally advanced carcinoma of the pancreas. J Palliat Care 2003;19:258–62.
- Arcidiacono PG, Calori G, Carrara S, et al. Celiac plexus block for pancreatic cancer pain in adults. Cochrane Database Syst Rev 2011(3):CD007519.
- Pezzilli R, Andriulli A, Bassi C, et al. Exocrine pancreatic insufficiency in adults: a shared position statement of the Italian Association for the Study of the Pancreas. World J Gastroenterol 2013;19:7930–46.
- Dominguez-Munoz JE. Pancreatic exocrine insufficiency: diagnosis and treatment. J Gastroenterol Hepatol 2011;26 Suppl 2:12–16.
- Bruno MJ, Haverkort EB, Tijssen GP, et al. Placebo controlled trial of enteric coated pancreatin microsphere treatment in patients with unresectable cancer of the pancreatic head region. Gut 1998;42:92–6.
- Wright AA, Keating NL, Balboni TA, et al. Place of death: correlations with quality of life of patients with cancer and predictors of bereaved caregivers’ mental health. J Clin Oncol 2010;28:4457–64.
- Jang RW, Krzyzanowska MK, Zimmermann C, et al. Palliative care and the aggressiveness of end-of-life care in patients with advanced pancreatic cancer. J Natl Cancer Inst 2015;107(3). pii: dju424.
- Network PCA. Pancreatic cancer facts 2016. 2016. https://www.pancan.org/wp-content/uploads/2016/02/2016-GAA-PC-Facts.pdf. Accessed April 24, 2017.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7–30.
- Konstantinidis IT, Warshaw AL, Allen JN, et al. Pancreatic ductal adenocarcinoma: is there a survival difference for R1 resections versus locally advanced unresectable tumors? What is a “true” R0 resection? Ann Surg 2013;257:731–6.
- Ilic M, Ilic I. Epidemiology of pancreatic cancer. World J Gastroenterol 2016;22:9694–705.
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69–90.
- Zhang J, Dhakal I, Ning B, Kesteloot H. Patterns and trends of pancreatic cancer mortality rates in Arkansas, 1969-2002: a comparison with the US population. Eur J Cancer Prev 2008;17:18–27.
- National Cancer Institute. SEER cancer statistics review, 1975-2013. http://seer.cancer.gov/csr/1975_2013/. Accessed April 24, 2017.
- Lowenfels AB, Maisonneuve P. Epidemiology and risk factors for pancreatic cancer. Best Pract Res Clin Gastroenterol 2006;20:197–209.
- Fuchs CS, Colditz GA, Stampfer MJ, et al. A prospective study of cigarette smoking and the risk of pancreatic cancer. Arch Intern Med 1996;156:2255–60.
- Lucenteforte E, La Vecchia C, Silverman D, et al. Alcohol consumption and pancreatic cancer: a pooled analysis in the International Pancreatic Cancer Case-Control Consortium (PanC4). Ann Oncol 2012;23:374–82.
- Schernhammer ES, Kang JH, Chan AT, et al. A prospective study of aspirin use and the risk of pancreatic cancer in women. J Natl Cancer Inst 2004;96:22–28.
- Michaud DS, Giovannucci E, Willett WC, et al. Physical activity, obesity, height, and the risk of pancreatic cancer. JAMA 2001;286:921–9.
- Nothlings U, Wilkens LR, Murphy SP, et al. Meat and fat intake as risk factors for pancreatic cancer: the multiethnic cohort study. J Natl Cancer Inst 2005;97:1458–65.
- Chari ST, Leibson CL, Rabe KG, et al. Probability of pancreatic cancer following diabetes: a population-based study. Gastroenterology 2005;129:504–11.
- Batabyal P, Vander Hoorn S, Christophi C, Nikfarjam M. Association of diabetes mellitus and pancreatic adenocarcinoma: a meta-analysis of 88 studies. Ann Surg Oncol 2014;21:2453–62.
- Chari ST, Leibson CL, Rabe KG, et al. Pancreatic cancer-associated diabetes mellitus: prevalence and temporal association with diagnosis of cancer. Gastroenterology 2008;134:95–101.
- Pannala R, Basu A, Petersen GM, Chari ST. New-onset diabetes: a potential clue to the early diagnosis of pancreatic cancer. Lancet Oncol 2009;10:88–95.
- Wolpin BM, Chan AT, Hartge P, et al. ABO blood group and the risk of pancreatic cancer. J Natl Cancer Inst 2009;101:424–31.
- Iqbal J, Ragone A, Lubinski J, et al. The incidence of pancreatic cancer in BRCA1 and BRCA2 mutation carriers. Br J Cancer 2012;107:2005–9.
- Giardiello FM, Brensinger JD, Tersmette AC, et al. Very high risk of cancer in familial Peutz-Jeghers syndrome. Gastroenterology 2000;119:1447–53.
- Kastrinos F, Mukherjee B, Tayob N, et al. Risk of pancreatic cancer in families with Lynch syndrome. JAMA 2009;302:1790–5.
- Brand RE, Lynch HT. Hereditary pancreatic adenocarcinoma. A clinical perspective. Med Clin North Am 2000;84:665–75.
- Jacobs EJ, Chanock SJ, Fuchs CS, et al. Family history of cancer and risk of pancreatic cancer: a pooled analysis from the Pancreatic Cancer Cohort Consortium (PanScan). Int J Cancer 2010;127:1421–8.
- Rustgi AK. Familial pancreatic cancer: genetic advances. Genes Dev 2014;28:1–7.
- Reznik R, Hendifar AE, Tuli R. Genetic determinants and potential therapeutic targets for pancreatic adenocarcinoma. Front Physiol 2014;5:87.
- Lowenfels AB, Maisonneuve P, DiMagno EP, et al. Hereditary pancreatitis and the risk of pancreatic cancer. International Hereditary Pancreatitis Study Group. J Natl Cancer Inst 1997;89:442–6.
- Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med 2014;371:2140–1.
- Kanda M, Matthaei H, Wu J, et al. Presence of somatic mutations in most early-stage pancreatic intraepithelial neoplasia. Gastroenterology 2012;142:730–3.
- Feldmann G, Maitra A. Molecular genetics of pancreatic ductal adenocarcinomas and recent implications for translational efforts. J Mol Diagn 2008;10:111–22.
- Eser S, Schnieke A, Schneider G, Saur D. Oncogenic KRAS signalling in pancreatic cancer. Br J Cancer 2014;111:817–22.
- Ying H, Kimmelman AC, Lyssiotis CA, et al. Oncogenic KRAS maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell 2012;149:656–70.
- Provenzano PP, Cuevas C, Chang AE, et al. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell 2012;21:418–29.
- Vonderheide RH, Bayne LJ. Inflammatory networks and immune surveillance of pancreatic carcinoma. Curr Opin Immunol 2013;25:200–5.
- DiMagno EP. Pancreatic cancer: Clinical presentation, pitfalls and early clues. Ann Oncol 1999;10(suppl 4):S140–S142.
- Porta M, Fabregat X, Malats N, et al. Exocrine pancreatic cancer: symptoms at presentation and their relation to tumour site and stage. Clin Transl Oncol 2005;7:189–97.
- Gullo L, Tomassetti P, Migliori M, et al. Do early symptoms of pancreatic cancer exist that can allow an earlier diagnosis? Pancreas 2001;22:210–3.
- Khorana AA, Fine RL. Pancreatic cancer and thromboembolic disease. Lancet Oncol 2004;5(11):655-663.
- Wigmore SJ, Plester CE, Richardson RA, Fearon KC. Changes in nutritional status associated with unresectable pancreatic cancer. Br J Cancer 1997;75:106–9.
- Aggarwal G, Rabe KG, Petersen GM, Chari ST. New-onset diabetes in pancreatic cancer: a study in the primary care setting. Pancreatology 2012;12:156–61.
- Koprowski H, Herlyn M, Steplewski Z, Sears HF. Specific antigen in serum of patients with colon carcinoma. Science 1981;212:53–5.
- Bond-Smith G, Banga N, Hammond TM, Imber CJ. Pancreatic adenocarcinoma. BMJ 2012;344:e2476.
- Cwik G, Wallner G, Skoczylas T, et al. Cancer antigens 19-9 and 125 in the differential diagnosis of pancreatic mass lesions. Arch Surg 2006;141:968–73.
- van den Bosch RP, van Eijck CH, Mulder PG, Jeekel J. Serum CA19-9 determination in the management of pancreatic cancer. Hepatogastroenterology 1996;43:710–3.
- Lamerz R. Role of tumour markers, cytogenetics. Ann Oncol 1999;10 Suppl 4:145–9.
- Hess V, Glimelius B, Grawe P, et al. CA 19-9 tumour-marker response to chemotherapy in patients with advanced pancreatic cancer enrolled in a randomised controlled trial. Lancet Oncol 2008;9:132–8.
- Montgomery RC, Hoffman JP, Riley LB, et al. Prediction of recurrence and survival by post-resection CA 19-9 values in patients with adenocarcinoma of the pancreas. Ann Surg Oncol 1997;4:551–6.
- Zamboni GA, D’Onofrio M, Idili A, et al. Ultrasound-guided percutaneous fine-needle aspiration of 545 focal pancreatic lesions. AJR Am J Roentgenol 2009;193:1691–5.
- Micames C, Jowell PS, White R, et al. Lower frequency of peritoneal carcinomatosis in patients with pancreatic cancer diagnosed by EUS-guided FNA vs. percutaneous FNA. Gastrointest Endosc 2003;58:690–5.
- Brambs HJ, Claussen CD. Pancreatic and ampullary carcinoma. Ultrasound, computed tomography, magnetic resonance imaging and angiography. Endoscopy 1993;25:58–68.
- Karlson BM, Ekbom A, Lindgren PG, et al. Abdominal US for diagnosis of pancreatic tumor: prospective cohort analysis. Radiology 1999;213:107–11.
- Imbriaco M, Megibow AJ, Camera L, et al. Dual-phase versus single-phase helical CT to detect and assess resectability of pancreatic carcinoma. AJR Am J Roentgenol 2002;178:1473–9.
- Schrag D. Optimizing treatment for locally advanced pancreas cancer: progress but no precision. JAMA 2016;315:1837–8.
- House MG, Yeo CJ, Cameron JL, et al. Predicting resectability of periampullary cancer with three-dimensional computed tomography. J Gastrointest Surg 2004;8:280–8.
- Callery MP, Chang KJ, Fishman EK, et al. Pretreatment assessment of resectable and borderline resectable pancreatic cancer: expert consensus statement. Ann Surg Oncol 2009;16:1727–33.
- Horton KM, Fishman EK. Adenocarcinoma of the pancreas: CT imaging. Radiol Clin North Am 2002;40:1263–72.
- Ross WA, Wasan SM, Evans DB, et al. Combined EUS with FNA and ERCP for the evaluation of patients with obstructive jaundice from presumed pancreatic malignancy. Gastrointest Endosc 2008;68:461–6.
- Hartwig W, Hackert T, Hinz U, et al. Pancreatic cancer surgery in the new millennium: better prediction of outcome. Ann Surg 2011;254:311–9.
- Jamieson NB, Chan NI, Foulis AK, et al. The prognostic influence of resection margin clearance following pancreaticoduodenectomy for pancreatic ductal adenocarcinoma. J Gastrointest Surg 2013;17:511–21.
- Ethun CG, Kooby DA. The importance of surgical margins in pancreatic cancer. J Surg Oncol 2016;113:283–8.
- Fischer R, Breidert M, Keck T, et al. Early recurrence of pancreatic cancer after resection and during adjuvant chemotherapy. Saudi J Gastroenterol 2012;18:118–21.
- Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med 2004;350:1200–10.
- Neoptolemos JP, Palmer DH, Ghaneh P, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet 2017;389(10073):1011–24.
- Cardenes HR, Chiorean EG, Dewitt J, et al. Locally advanced pancreatic cancer: current therapeutic approach. Oncologist 2006;11:612–23.
- Yeung RS, Weese JL, Hoffman JP, et al. Neoadjuvant chemoradiation in pancreatic and duodenal carcinoma. A Phase II Study. Cancer 1993;72:2124–33.
- Spitz FR, Abbruzzese JL, Lee JE, et al. Preoperative and postoperative chemoradiation strategies in patients treated with pancreaticoduodenectomy for adenocarcinoma of the pancreas. J Clin Oncol 1997;15:928–37.
- McClaine RJ, Lowy AM, Sussman JJ, et al. Neoadjuvant therapy may lead to successful surgical resection and improved survival in patients with borderline resectable pancreatic cancer. HPB (Oxford) 2010;12:73–9.
- Balaban EP, Mangu PB, Khorana AA, et al. Locally advanced, unresectable pancreatic cCancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2016;34:2654–68.
- Shaib WL, Ip A, Cardona K, et al. Contemporary management of borderline resectable and locally advanced unresectable pancreatic cancer. Oncologist 2016;21:178–87.
- Chun YS, Milestone BN, Watson JC, et al. Defining venous involvement in borderline resectable pancreatic cancer. Ann Surg Oncol 2010;17:2832–8.
- Evans DB, Erickson BA, Ritch P. Borderline resectable pancreatic cancer: definitions and the importance of multimodality therapy. Ann Surg Oncol 2010;17:2803–5.
- Conroy T, Paillot B, Francois E, et al. Irinotecan plus oxaliplatin and leucovorin-modulated fluorouracil in advanced pancreatic cancer--a Groupe Tumeurs Digestives of the Federation Nationale des Centres de Lutte Contre le Cancer study. J Clin Oncol 2005;23:1228–36.
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817–25.
- Hosein PJ, Macintyre J, Kawamura C, et al. A retrospective study of neoadjuvant FOLFIRINOX in unresectable or borderline-resectable locally advanced pancreatic adenocarcinoma. BMC Cancer 2012;12:199.
- Peddi PF, Lubner S, McWilliams R, et al. Multi-institutional experience with FOLFIRINOX in pancreatic adenocarcinoma. JOP 2012;13:497–501.
- Mahaseth H, Kauh JS, Brutcher E, et al. Safety and efficacy of modified FOLFIRINOX in pancreatic cancer: A retrospective experience. J Clin Oncol 2012;30 (suppl; abstr e14614).
- Kim SS, Nakakura EK, Wang ZJ, et al. Preoperative FOLFIRINOX for borderline resectable pancreatic cancer: Is radiation necessary in the modern era of chemotherapy? J Surg Oncol 2016;114:587–96.
- Conroy T, Gavoille C, Samalin E, et al. The role of the FOLFIRINOX regimen for advanced pancreatic cancer. Curr Oncol Rep 2013;15:182–9.
- Katz MH, Shi Q, Ahmad SA, et al. Preoperative modified FOLFIRINOX treatment followed by capecitabine-based chemoradiation for borderline resectable pancreatic cancer: Alliance for Clinical Trials in Oncology Trial A021101. JAMA Surg 2016;151:e161137.
- Kharofa J, Kelly TR, Ritch PS, et al. 5-FU/leucovorin, irinotecan, oxaliplatin (FOLFIRINOX) induction followed by chemoXRT in borderline resectable pancreatic cancer. J Clin Oncol 2012;30 (suppl; abstr e14613).
- Blazer M, Wu C, Goldberg RM, et al. Neoadjuvant modified (m) FOLFIRINOX for locally advanced unresectable (LAPC) and borderline resectable (BRPC) adenocarcinoma of the pancreas. Ann Surg Oncol 2015;22:1153–9.
- Oettle H, Post S, Neuhaus P, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA 2007;297:267–77.
- Plunkett W, Huang P, Xu YZ, et al. Gemcitabine: metabolism, mechanisms of action, and self-potentiation. Semin Oncol 1995;22(4 Suppl 11):3–10.
- Sahora K, Kuehrer I, Eisenhut A, et al. NeoGemOx: Gemcitabine and oxaliplatin as neoadjuvant treatment for locally advanced, nonmetastasized pancreatic cancer. Surgery 2011;149(3):311–20.
- Lee JL, Kim SC, Kim JH, et al. Prospective efficacy and safety study of neoadjuvant gemcitabine with capecitabine combination chemotherapy for borderline-resectable or unresectable locally advanced pancreatic adenocarcinoma. Surgery 2012;152:851–62.
- Leone F, Gatti M, Massucco P, et al. Induction gemcitabine and oxaliplatin therapy followed by a twice-weekly infusion of gemcitabine and concurrent external-beam radiation for neoadjuvant treatment of locally advanced pancreatic cancer: a single institutional experience. Cancer 2013;119:277–84.
- Lawrence TS, Eisbruch A, Shewach DS. Gemcitabine-mediated radiosensitization. Semin Oncol 1997;24(2 Suppl 7):S7–24-S27–28.
- Kang CM, Chung YE, Park JY, et al. Potential contribution of preoperative neoadjuvant concurrent chemoradiation therapy on margin-negative resection in borderline resectable pancreatic cancer. J Gastrointest Surg 2012;16:509–17.
- Chuong MD, Hayman TJ, Patel MR, et al. Comparison of 1-, 2-, and 3-dimensional tumor response assessment after neoadjuvant GTX-RT in borderline-resectable pancreatic cancer. Gastrointest Cancer Res 2011;4:128–34.
- Loehrer AP, Kinnier CV, Ferrone CR. Treatment of locally advanced pancreatic ductal adenocarcinoma. Adv Surg 2016;50:115–28.
- Katz MH, Wang H, Balachandran A, et al. Effect of neoadjuvant chemoradiation and surgical technique on recurrence of localized pancreatic cancer. J Gastrointest Surg 2012;16:68–78.
- Franke AJ, Rosati LM, Pawlik TM, et al. The role of radiation therapy in pancreatic ductal adenocarcinoma in the neoadjuvant and adjuvant settings. Semin Oncol 2015;42:144–62.
- Butturini G, Stocken DD, Wente MN, et al. Influence of resection margins and treatment on survival in patients with pancreatic cancer: meta-analysis of randomized controlled trials. Arch Surg 2008;143:75–83.
- Paniccia A, Hosokawa P, Henderson W, et al. Characteristics of 10-year survivors of pancreatic ductal adenocarcinoma. JAMA Surg 2015;150:701–10.
- Massucco P, Capussotti L, Magnino A, et al. Pancreatic resections after chemoradiotherapy for locally advanced ductal adenocarcinoma: analysis of perioperative outcome and survival. Ann Surg Oncol 2006;13:1201–8.
- Patel M, Hoffe S, Malafa M, et al. Neoadjuvant GTX chemotherapy and IMRT-based chemoradiation for borderline resectable pancreatic cancer. J Surg Oncol 2011;104:155–161.
- Landry J, Catalano PJ, Staley C, et al. Randomized phase II study of gemcitabine plus radiotherapy versus gemcitabine, 5-fluorouracil, and cisplatin followed by radiotherapy and 5-fluorouracil for patients with locally advanced, potentially resectable pancreatic adenocarcinoma. J Surg Oncol 2010;101:587–92.
- Lamb R, Ozsvari B, Lisanti CL, et al. Antibiotics that target mitochondria effectively eradicate cancer stem cells, across multiple tumor types: treating cancer like an infectious disease. Oncotarget 2015;6:4569–84.
- Stokes JB, Nolan NJ, Stelow EB, et al. Preoperative capecitabine and concurrent radiation for borderline resectable pancreatic cancer. Ann Surg Oncol 2011;18:619–27.
- White R, Lee C, Anscher M, et al. Preoperative chemoradiation for patients with locally advanced adenocarcinoma of the pancreas. Ann Surg Oncol 1999;6:38–45.
- Martin RC 2nd. Management of locally advanced pancreatic cancer. Surg Clin North Am 2016;96:1371–89.
- Higuera O, Ghanem I, Nasimi R, et al. Management of pancreatic cancer in the elderly. World J Gastroenterol 2016;22:764–75.
- Hurria A, Lichtman SM. Clinical pharmacology of cancer therapies in older adults. Br J Cancer 2008;98:517–22.
- Spadi R, Brusa F, Ponzetti A, et al. Current therapeutic strategies for advanced pancreatic cancer: A review for clinicians. World J Clin Oncol 2016;7:27–43.
- Seufferlein T, Bachet JB, Van Cutsem E, Rougier P, Group EGW. Pancreatic adenocarcinoma: ESMO-ESDO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2012;23 Suppl 7:vii33–40.
- Huguet F, Girard N, Guerche CS, et al. Chemoradiotherapy in the management of locally advanced pancreatic carcinoma: a qualitative systematic review. J Clin Oncol 2009;27:2269–77.
- Suker M, Beumer BR, Sadot E, et al. FOLFIRINOX for locally advanced pancreatic cancer: a systematic review and patient-level meta-analysis. Lancet Oncol 2016;17:801–10.
- Gunturu KS, Yao X, Cong X, et al. FOLFIRINOX for locally advanced and metastatic pancreatic cancer: single institution retrospective review of efficacy and toxicity. Med Oncol 2013;30:361.
- Mukherjee S, Hurt CN, Bridgewater J, et al. Gemcitabine-based or capecitabine-based chemoradiotherapy for locally advanced pancreatic cancer (SCALOP): a multicentre, randomised, phase 2 trial. Lancet Oncol 2013;14:317–26.
- Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013;369:1691–1703.
- Krzyzanowska MK, Weeks JC, Earle CC. Treatment of locally advanced pancreatic cancer in the real world: population-based practices and effectiveness. J Clin Oncol 2003;21:3409–14.
- Sultana A, Tudur Smith C, Cunningham D, et al. Meta-analyses of chemotherapy for locally advanced and metastatic pancreatic cancer: results of secondary end points analyses. Br J Cancer 2008;99:6–13.
- Hammel P, Huguet F, van Laethem JL, et al. Effect of chemoradiotherapy vs chemotherapy on survival in patients with locally advanced pancreatic cancer controlled after 4 months of gemcitabine with or without erlotinib: the LAP07 randomized clinical trial. JAMA 2016;315:1844–53.
- Huguet F, Andre T, Hammel P, et al. Impact of chemoradiotherapy after disease control with chemotherapy in locally advanced pancreatic adenocarcinoma in GERCOR phase II and III studies. J Clin Oncol 2007;25:326–31.
- Dholakia AS, Hacker-Prietz A, Wild AT, et al. Resection of borderline resectable pancreatic cancer after neoadjuvant chemoradiation does not depend on improved radiographic appearance of tumor-vessel relationships. J Radiat Oncol 2013;2:413–25.
- Heinemann V, Haas M, Boeck S. Neoadjuvant treatment of borderline resectable and non-resectable pancreatic cancer. Ann Oncol 2013;24:2484–92.
- Morganti AG, Trodella L, Valentini V, et al. Pain relief with short-term irradiation in locally advanced carcinoma of the pancreas. J Palliat Care 2003;19:258–62.
- Arcidiacono PG, Calori G, Carrara S, et al. Celiac plexus block for pancreatic cancer pain in adults. Cochrane Database Syst Rev 2011(3):CD007519.
- Pezzilli R, Andriulli A, Bassi C, et al. Exocrine pancreatic insufficiency in adults: a shared position statement of the Italian Association for the Study of the Pancreas. World J Gastroenterol 2013;19:7930–46.
- Dominguez-Munoz JE. Pancreatic exocrine insufficiency: diagnosis and treatment. J Gastroenterol Hepatol 2011;26 Suppl 2:12–16.
- Bruno MJ, Haverkort EB, Tijssen GP, et al. Placebo controlled trial of enteric coated pancreatin microsphere treatment in patients with unresectable cancer of the pancreatic head region. Gut 1998;42:92–6.
- Wright AA, Keating NL, Balboni TA, et al. Place of death: correlations with quality of life of patients with cancer and predictors of bereaved caregivers’ mental health. J Clin Oncol 2010;28:4457–64.
- Jang RW, Krzyzanowska MK, Zimmermann C, et al. Palliative care and the aggressiveness of end-of-life care in patients with advanced pancreatic cancer. J Natl Cancer Inst 2015;107(3). pii: dju424.