User login
Ostomy Innovation Grabs ‘Shark Tank’ Win
The “Shark Tank” winning innovation at the American Gastroenterological Association (AGA) Tech Summit in Chicago this April has “life-altering” potential for ostomy patients, according to one of the judges, and eliminates the need for constant pouch wear.
The innovation is called Twistomy and it is designed to replace current ostomy-pouch systems that can cause leaks, odor, skin irritation, embarrassment, and social and emotional distress. The AGA Committee for GI Innovation and Technology (CGIT) organizes the annual Tech Summit.
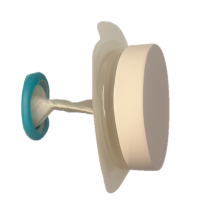
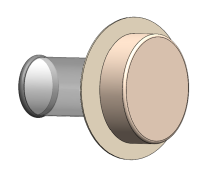
Twistomy’s winning design includes a flexible ring and sleeve, which are inserted into the stoma and secured on the outside with a set of rings that make up the housing unit attached to a standard wafer. The housing unit twists the sleeve closed, allowing the user to control fecal output. For evacuation, the user attaches a pouch, untwists the sleeve, evacuates cleanly and effectively, and then discards the pouch.
Twistomy cofounders Devon Horton, BS, senior bioengineer, and Lily Williams, BS, biomedical researcher and engineer, both work for the department of surgery at University of Colorado, Denver.
Horton said in an interview that when he was approached with the idea to create a better ostomy solution for a senior-year capstone project he was intrigued because the traditional ostomy system “has not changed in more than 70 years. It was crazy that no one had done anything to change that.”
The Twistomy team also won the Grand Prize this spring at the Emerging Medical Innovation Valuation Competition at the Design of Medical Devices Conference held at the University of Minnesota, Minneapolis.
Witnessing the Struggle as a CNA
Horton also works as a certified nursing assistant at an inpatient unit at University of Colorado Hospital and the ostomy patients he sees there every shift help drive his passion to find a better solution.
He hears the emotional stories of people who manage their ostomy daily.
“Many express feelings of depression and anxiety, feeling isolated with their severe inability to go out and do things because of the fear of the noise the stoma makes, or the crinkling of the plastic bag in a yoga class,” he said. “We want to help them regain that control of quality of life.”
They also hope to cut down on the ostomy management time. “Initial user testing [for Twistomy] was less than 75 seconds to insert and assemble,” he said. “I did an interview with a patient yesterday who said they probably spend an hour a day managing their ostomy,” including cleaning and replacing.
Horton and Williams have a patent on the device and currently use three-dimensional printing for the prototypes.
Williams said they are now conducting consumer discovery studies through the National Science Foundation and are interviewing 30 stakeholders — “anyone who has a relationship with an ostomy,” whether a colorectal surgeon, a gastrointestinal nurse, ostomy patients, or insurers.
Those interviews will help in refining the device so they can start consulting with manufacturers and work toward approval as a Class II medical device from the US Food and Drug Administration (FDA), Williams said.
Saving Healthcare Costs
Another potential benefit for Twistomy is its ability to cut healthcare costs, Horton said. Traditional ostomies are prone to leakage, which can lead to peristomal skin complications.
He pointed to a National Institutes of Health analysis that found that on average peristomal skin complications caused upwards of $80,000 more per ostomy patient in increased healthcare costs over a 3-month period than for those without the complications.
“With Twistomy, we are reducing leakage most likely to zero,” Horton said. “We set out to say if we could reduce [infections] by half or a little less than half, we can cut out those tens of thousands of dollars that insurance companies and payers are spending.”
Permanent and Temporary Ostomy Markets
He pointed out that not all ostomies are permanent ostomies, adding that the reversal rate “is about 65%.” Often those reversal surgeries cannot take place until peristomal skin complications have been healed.
“We’re not only hoping to market to the permanent stoma patients, but the patients with temporary stomas as well,” he said.
The team estimates it will need $4 million–$6 million in funding for manufacturing and consultation costs as well as costs involved in seeking FDA approval.
Horton and Williams project the housing unit cost will be $399 based on known out-of-pocket expenses for patients with ostomy care products and the unit would be replaced annually. Disposable elements would be an additional cost.
Assuming insurance acceptance of the product, he said, “With about an 80/20 insurance coverage, typical for many patients, it would be about $100 in out-of-pocket expenses per month to use our device, which is around the lower end of what a lot of patients are spending out of pocket.”
One of the Tech Summit judges, Somaya Albhaisi, MD, a gastroenterology/hepatology fellow at University of Southern California, Los Angeles, said in an interview that the Shark Tank results were unanimous among the five judges and Twistomy also took the fan favorite vote.
She said the teams were judged on quality of pitch, potential clinical impact, and feasibility of business plan. Teams got 5-7 minutes to pitch and answered questions afterward.
“Deep Understanding” of Patient Need
“They combined smart engineering with deep understanding of patient need, which is restoring control, dignity, and quality of life for ostomy users while also reducing healthcare costs. It is rare to see a solution this scalable and impactful. It was a deeply empathetic solution overall.” She noted that nearly 1 million people in the United States currently use an ostomy.
Ostomy users’ quality of life is compromised, and they often have mental health challenges, Albhaisi said. This innovation appears to offer easy use, more dignity and control.
The other four Shark Tank finalists were:
- AI Lumen, which developed a retroview camera system, which attaches to the colonoscope and enhances imaging to detect hidden polyps that may evade conventional endoscopes.
- Amplified Sciences, which developed an ultrasensitive diagnostic platform that detects biomarker activities in minute volumes of fluid from pancreatic cystic lesions, helping to stratify patients into low risk or potential malignancy, reducing unneeded surgeries, costs, and comorbidities.
- KITE Endoscopic Innovations, which designed the Dynaflex TruCut needle to offer a simpler endoscopic ultrasound (EUS)–guided biopsy procedure with fewer needle passes, deeper insights into tumor pathology, and more tissue for geonomic analysis.
- MicroSteer, which designed a device to facilitate semiautomated endoscopic submucosal dissection (ESD) by decoupling the dissecting knife from the endoscope, enhancing safety and effectiveness during the procedure.
The Twistomy Team “Surprised Everyone”
The competitors’ scores were “very close,” one of the judges, Kevin Berliner, said in an interview. “The Twistomy team surprised everyone — the judges and the crowd — with their succinct, informative, and impactful pitch. That presentation disparity was the tiebreaker for me,” said Berliner, who works for Medtronic, a sponsor of the competition, in Chicago.
He said Horton and Williams were the youngest presenters and had the earliest stage pitch they judged, but they “outpresented other competitors in clarity, simplification, and storytelling.”
Also impressive was their description of their “commercially viable path to success” and their plan for the challenges ahead, he said.
Those challenges to get Twistomy to market center “on the ongoing changing climate we have with research funds lately,” Horton said. “We’re giving it an estimate of 3-5 years.”
Horton, Williams, Albhaisi, and Berliner reported no relevant financial relationships.
The “Shark Tank” winning innovation at the American Gastroenterological Association (AGA) Tech Summit in Chicago this April has “life-altering” potential for ostomy patients, according to one of the judges, and eliminates the need for constant pouch wear.
The innovation is called Twistomy and it is designed to replace current ostomy-pouch systems that can cause leaks, odor, skin irritation, embarrassment, and social and emotional distress. The AGA Committee for GI Innovation and Technology (CGIT) organizes the annual Tech Summit.
Twistomy’s winning design includes a flexible ring and sleeve, which are inserted into the stoma and secured on the outside with a set of rings that make up the housing unit attached to a standard wafer. The housing unit twists the sleeve closed, allowing the user to control fecal output. For evacuation, the user attaches a pouch, untwists the sleeve, evacuates cleanly and effectively, and then discards the pouch.
Twistomy cofounders Devon Horton, BS, senior bioengineer, and Lily Williams, BS, biomedical researcher and engineer, both work for the department of surgery at University of Colorado, Denver.
Horton said in an interview that when he was approached with the idea to create a better ostomy solution for a senior-year capstone project he was intrigued because the traditional ostomy system “has not changed in more than 70 years. It was crazy that no one had done anything to change that.”
The Twistomy team also won the Grand Prize this spring at the Emerging Medical Innovation Valuation Competition at the Design of Medical Devices Conference held at the University of Minnesota, Minneapolis.
Witnessing the Struggle as a CNA
Horton also works as a certified nursing assistant at an inpatient unit at University of Colorado Hospital and the ostomy patients he sees there every shift help drive his passion to find a better solution.
He hears the emotional stories of people who manage their ostomy daily.
“Many express feelings of depression and anxiety, feeling isolated with their severe inability to go out and do things because of the fear of the noise the stoma makes, or the crinkling of the plastic bag in a yoga class,” he said. “We want to help them regain that control of quality of life.”
They also hope to cut down on the ostomy management time. “Initial user testing [for Twistomy] was less than 75 seconds to insert and assemble,” he said. “I did an interview with a patient yesterday who said they probably spend an hour a day managing their ostomy,” including cleaning and replacing.
Horton and Williams have a patent on the device and currently use three-dimensional printing for the prototypes.
Williams said they are now conducting consumer discovery studies through the National Science Foundation and are interviewing 30 stakeholders — “anyone who has a relationship with an ostomy,” whether a colorectal surgeon, a gastrointestinal nurse, ostomy patients, or insurers.
Those interviews will help in refining the device so they can start consulting with manufacturers and work toward approval as a Class II medical device from the US Food and Drug Administration (FDA), Williams said.
Saving Healthcare Costs
Another potential benefit for Twistomy is its ability to cut healthcare costs, Horton said. Traditional ostomies are prone to leakage, which can lead to peristomal skin complications.
He pointed to a National Institutes of Health analysis that found that on average peristomal skin complications caused upwards of $80,000 more per ostomy patient in increased healthcare costs over a 3-month period than for those without the complications.
“With Twistomy, we are reducing leakage most likely to zero,” Horton said. “We set out to say if we could reduce [infections] by half or a little less than half, we can cut out those tens of thousands of dollars that insurance companies and payers are spending.”
Permanent and Temporary Ostomy Markets
He pointed out that not all ostomies are permanent ostomies, adding that the reversal rate “is about 65%.” Often those reversal surgeries cannot take place until peristomal skin complications have been healed.
“We’re not only hoping to market to the permanent stoma patients, but the patients with temporary stomas as well,” he said.
The team estimates it will need $4 million–$6 million in funding for manufacturing and consultation costs as well as costs involved in seeking FDA approval.
Horton and Williams project the housing unit cost will be $399 based on known out-of-pocket expenses for patients with ostomy care products and the unit would be replaced annually. Disposable elements would be an additional cost.
Assuming insurance acceptance of the product, he said, “With about an 80/20 insurance coverage, typical for many patients, it would be about $100 in out-of-pocket expenses per month to use our device, which is around the lower end of what a lot of patients are spending out of pocket.”
One of the Tech Summit judges, Somaya Albhaisi, MD, a gastroenterology/hepatology fellow at University of Southern California, Los Angeles, said in an interview that the Shark Tank results were unanimous among the five judges and Twistomy also took the fan favorite vote.
She said the teams were judged on quality of pitch, potential clinical impact, and feasibility of business plan. Teams got 5-7 minutes to pitch and answered questions afterward.
“Deep Understanding” of Patient Need
“They combined smart engineering with deep understanding of patient need, which is restoring control, dignity, and quality of life for ostomy users while also reducing healthcare costs. It is rare to see a solution this scalable and impactful. It was a deeply empathetic solution overall.” She noted that nearly 1 million people in the United States currently use an ostomy.
Ostomy users’ quality of life is compromised, and they often have mental health challenges, Albhaisi said. This innovation appears to offer easy use, more dignity and control.
The other four Shark Tank finalists were:
- AI Lumen, which developed a retroview camera system, which attaches to the colonoscope and enhances imaging to detect hidden polyps that may evade conventional endoscopes.
- Amplified Sciences, which developed an ultrasensitive diagnostic platform that detects biomarker activities in minute volumes of fluid from pancreatic cystic lesions, helping to stratify patients into low risk or potential malignancy, reducing unneeded surgeries, costs, and comorbidities.
- KITE Endoscopic Innovations, which designed the Dynaflex TruCut needle to offer a simpler endoscopic ultrasound (EUS)–guided biopsy procedure with fewer needle passes, deeper insights into tumor pathology, and more tissue for geonomic analysis.
- MicroSteer, which designed a device to facilitate semiautomated endoscopic submucosal dissection (ESD) by decoupling the dissecting knife from the endoscope, enhancing safety and effectiveness during the procedure.
The Twistomy Team “Surprised Everyone”
The competitors’ scores were “very close,” one of the judges, Kevin Berliner, said in an interview. “The Twistomy team surprised everyone — the judges and the crowd — with their succinct, informative, and impactful pitch. That presentation disparity was the tiebreaker for me,” said Berliner, who works for Medtronic, a sponsor of the competition, in Chicago.
He said Horton and Williams were the youngest presenters and had the earliest stage pitch they judged, but they “outpresented other competitors in clarity, simplification, and storytelling.”
Also impressive was their description of their “commercially viable path to success” and their plan for the challenges ahead, he said.
Those challenges to get Twistomy to market center “on the ongoing changing climate we have with research funds lately,” Horton said. “We’re giving it an estimate of 3-5 years.”
Horton, Williams, Albhaisi, and Berliner reported no relevant financial relationships.
The “Shark Tank” winning innovation at the American Gastroenterological Association (AGA) Tech Summit in Chicago this April has “life-altering” potential for ostomy patients, according to one of the judges, and eliminates the need for constant pouch wear.
The innovation is called Twistomy and it is designed to replace current ostomy-pouch systems that can cause leaks, odor, skin irritation, embarrassment, and social and emotional distress. The AGA Committee for GI Innovation and Technology (CGIT) organizes the annual Tech Summit.
Twistomy’s winning design includes a flexible ring and sleeve, which are inserted into the stoma and secured on the outside with a set of rings that make up the housing unit attached to a standard wafer. The housing unit twists the sleeve closed, allowing the user to control fecal output. For evacuation, the user attaches a pouch, untwists the sleeve, evacuates cleanly and effectively, and then discards the pouch.
Twistomy cofounders Devon Horton, BS, senior bioengineer, and Lily Williams, BS, biomedical researcher and engineer, both work for the department of surgery at University of Colorado, Denver.
Horton said in an interview that when he was approached with the idea to create a better ostomy solution for a senior-year capstone project he was intrigued because the traditional ostomy system “has not changed in more than 70 years. It was crazy that no one had done anything to change that.”
The Twistomy team also won the Grand Prize this spring at the Emerging Medical Innovation Valuation Competition at the Design of Medical Devices Conference held at the University of Minnesota, Minneapolis.
Witnessing the Struggle as a CNA
Horton also works as a certified nursing assistant at an inpatient unit at University of Colorado Hospital and the ostomy patients he sees there every shift help drive his passion to find a better solution.
He hears the emotional stories of people who manage their ostomy daily.
“Many express feelings of depression and anxiety, feeling isolated with their severe inability to go out and do things because of the fear of the noise the stoma makes, or the crinkling of the plastic bag in a yoga class,” he said. “We want to help them regain that control of quality of life.”
They also hope to cut down on the ostomy management time. “Initial user testing [for Twistomy] was less than 75 seconds to insert and assemble,” he said. “I did an interview with a patient yesterday who said they probably spend an hour a day managing their ostomy,” including cleaning and replacing.
Horton and Williams have a patent on the device and currently use three-dimensional printing for the prototypes.
Williams said they are now conducting consumer discovery studies through the National Science Foundation and are interviewing 30 stakeholders — “anyone who has a relationship with an ostomy,” whether a colorectal surgeon, a gastrointestinal nurse, ostomy patients, or insurers.
Those interviews will help in refining the device so they can start consulting with manufacturers and work toward approval as a Class II medical device from the US Food and Drug Administration (FDA), Williams said.
Saving Healthcare Costs
Another potential benefit for Twistomy is its ability to cut healthcare costs, Horton said. Traditional ostomies are prone to leakage, which can lead to peristomal skin complications.
He pointed to a National Institutes of Health analysis that found that on average peristomal skin complications caused upwards of $80,000 more per ostomy patient in increased healthcare costs over a 3-month period than for those without the complications.
“With Twistomy, we are reducing leakage most likely to zero,” Horton said. “We set out to say if we could reduce [infections] by half or a little less than half, we can cut out those tens of thousands of dollars that insurance companies and payers are spending.”
Permanent and Temporary Ostomy Markets
He pointed out that not all ostomies are permanent ostomies, adding that the reversal rate “is about 65%.” Often those reversal surgeries cannot take place until peristomal skin complications have been healed.
“We’re not only hoping to market to the permanent stoma patients, but the patients with temporary stomas as well,” he said.
The team estimates it will need $4 million–$6 million in funding for manufacturing and consultation costs as well as costs involved in seeking FDA approval.
Horton and Williams project the housing unit cost will be $399 based on known out-of-pocket expenses for patients with ostomy care products and the unit would be replaced annually. Disposable elements would be an additional cost.
Assuming insurance acceptance of the product, he said, “With about an 80/20 insurance coverage, typical for many patients, it would be about $100 in out-of-pocket expenses per month to use our device, which is around the lower end of what a lot of patients are spending out of pocket.”
One of the Tech Summit judges, Somaya Albhaisi, MD, a gastroenterology/hepatology fellow at University of Southern California, Los Angeles, said in an interview that the Shark Tank results were unanimous among the five judges and Twistomy also took the fan favorite vote.
She said the teams were judged on quality of pitch, potential clinical impact, and feasibility of business plan. Teams got 5-7 minutes to pitch and answered questions afterward.
“Deep Understanding” of Patient Need
“They combined smart engineering with deep understanding of patient need, which is restoring control, dignity, and quality of life for ostomy users while also reducing healthcare costs. It is rare to see a solution this scalable and impactful. It was a deeply empathetic solution overall.” She noted that nearly 1 million people in the United States currently use an ostomy.
Ostomy users’ quality of life is compromised, and they often have mental health challenges, Albhaisi said. This innovation appears to offer easy use, more dignity and control.
The other four Shark Tank finalists were:
- AI Lumen, which developed a retroview camera system, which attaches to the colonoscope and enhances imaging to detect hidden polyps that may evade conventional endoscopes.
- Amplified Sciences, which developed an ultrasensitive diagnostic platform that detects biomarker activities in minute volumes of fluid from pancreatic cystic lesions, helping to stratify patients into low risk or potential malignancy, reducing unneeded surgeries, costs, and comorbidities.
- KITE Endoscopic Innovations, which designed the Dynaflex TruCut needle to offer a simpler endoscopic ultrasound (EUS)–guided biopsy procedure with fewer needle passes, deeper insights into tumor pathology, and more tissue for geonomic analysis.
- MicroSteer, which designed a device to facilitate semiautomated endoscopic submucosal dissection (ESD) by decoupling the dissecting knife from the endoscope, enhancing safety and effectiveness during the procedure.
The Twistomy Team “Surprised Everyone”
The competitors’ scores were “very close,” one of the judges, Kevin Berliner, said in an interview. “The Twistomy team surprised everyone — the judges and the crowd — with their succinct, informative, and impactful pitch. That presentation disparity was the tiebreaker for me,” said Berliner, who works for Medtronic, a sponsor of the competition, in Chicago.
He said Horton and Williams were the youngest presenters and had the earliest stage pitch they judged, but they “outpresented other competitors in clarity, simplification, and storytelling.”
Also impressive was their description of their “commercially viable path to success” and their plan for the challenges ahead, he said.
Those challenges to get Twistomy to market center “on the ongoing changing climate we have with research funds lately,” Horton said. “We’re giving it an estimate of 3-5 years.”
Horton, Williams, Albhaisi, and Berliner reported no relevant financial relationships.
Advice, Support for Entrepreneurs at AGA Tech 2024
CHICAGO — Have a great tech idea to improve gastroenterology? Start-up companies have the potential to transform the practice of medicine, and to make founders a nice pot of money, but it is a difficult road. At the 2024 AGA Tech Summit, held at the Chicago headquarters of MATTER, a global healthcare startup incubator, investors and gastroenterologists discussed some of the key challenges and opportunities for GI startups.
The road is daunting, and founders must be dedicated to their companies but also maintain life balance. “It is very easy, following your passion, for your life to get out of check. I don’t know what the divorce rate is for entrepreneurs, but I personally was a victim of that. The culture that we built was addictive and it became all encompassing, and at the same time [I neglected] my home life,” Scott Fraser, managing director of the consulting company Fraser Healthcare, said during a “Scars and Stripes” panel at the summit.
For those willing to navigate those waters, there is help. Investors are prepared to provide seed money for companies with good ideas and a strong market. AGA itself has stepped into the investment field with its GI Opportunity Fund, which it launched in 2022 through a partnership with Varia Ventures. The fund’s capital comes from AGA members, with a minimum investment of $25,000. To date, AGA has made investments in six companies, at around $100,000 per company. “It’s not a large amount that we’re investing. We’re a lead investor that signals to other venture capital companies that this is a viable company,” Tom Serena, CEO of AGA, said in an interview.
The fund grew out of AGA’s commitment to boosting early-stage companies in the gastroenterology space. AGA has always supported GI device and tech companies through its Center for GI Innovation and Technology, which sponsored the AGA Tech Summit. The center now provides resources and advice for GI innovators and startups. The AGA Tech Summit has created a gathering place for entrepreneurs and innovators to share their experiences and learn from one another. “But what we were missing was the last mile, which is getting funding to the companies,” said Mr. Serena. The summit itself has been modified to increase the venture capital presence. “That’s the networking we’re trying to [create] here. Venture capitalists are well acquainted with these companies, but we feel that AGA can bring clinical due diligence, and the startups want to be exposed to venture capital,” said Mr. Serena.
During the “Learn from VC Strategists” panel, investors shared advice for entrepreneurs. The emphasis throughout was on marketable ideas that can fundamentally change healthcare practice, though inventions may not have the whiz-bang appeal of some new technologies of years past.
“We’re particularly focused on clinical models that actually work. There were a lot of companies for many years that were doing things that had minimal impact, or very incremental impact. Maybe they were helping identify certain patients, but they weren’t actually engaging those patients. We’re now looking very end-to-end and trying to make sure that it’s not just a good idea, but one that you can actually roll out, engage patients, and see the [return on investment] in that patient data,” said Kelsey Maguire, managing director of the Blue Venture Fund, which is a collaborative effort across Blue Cross Blue Shield companies.
Part of the reason for that shift is that healthcare has evolved in a way that has put more pressure on physicians, according to Barbara H. Jung, MD, AGAF, past president of AGA, who was present for the session. “I think that there’s huge burnout among gastroenterologists, [partly because] some of the systems have been optimized to get the most out of each specialist. I think we just have to get back to making work more enjoyable. [It could be less] fighting with the insurance companies, it could be that you spend less time typing after hours. It could be that it helps the team work more seamlessly, or it could be something that helps the patient prepare, so they have everything ready when they see the doctors. It’s thinking about how healthcare is delivered, and really in a patient and physician-centric way,” Dr. Jung said in an interview.
Anna Haghgooie, managing director of Valtruis, noted that, historically, new technology has been rewarded by the healthcare system. “It’s part of why we find ourselves where we are as an industry: There was nobody in the marketplace that was incented to roll out a cost-reducing technology, and those weren’t necessarily considered grand slams. But [I think] we’re at a tipping point on cost, and as a country will start purchasing in pretty meaningfully different ways, which opens up a lot of opportunities for those practical solutions to be grand slams. Everything that we look at has a component of virtual care, leveraging technology, whether it’s AI or just better workflow tools, better data and intelligence to make business decisions,” said Ms. Haghgooie. She did note that Valtruis does not work much with medical devices.
Specifically in the GI space, one panelist called for a shift away from novel colonoscopy technology. “I don’t know how many more bells and whistles we can ask for colonoscopy, which we’re very dependent on. Not that it’s not important, but I don’t think that’s where the real innovation is going to come. When you think about the cognitive side of the GI business: New diagnostics, things that are predictive of disease states, things that monitor disease, things that help you to know what people’s disease courses will be. I think as more and more interventions are done by endoscopists, you need more tools,” said Thomas Shehab, MD, managing partner at Arboretum Ventures.
Finally, AI has become a central component to investment decisions. Ms. Haghgooie said that Valtruis is focused on the infrastructure surrounding AI, such as the data that it requires to make or help guide decisions. That data can vary widely in quality, is difficult to index, exists in various silos, and is subject to a number of regulatory constraints on how to move or aggregate it. “So, a lot of what we’re focused on are the systems and tools that can enable the next gen application of AI. That’s one piece of the puzzle. The other is, I’d say that every company that we’ve either invested in or are looking at investing in, we ask the question: How are you planning to incorporate and leverage this next gen technology to drive your marginal cost-to-deliver down? In many cases you have to do that through business model redesign, because there is no fee-for-service code to get paid for leveraging AI to reduce your costs. You’ve got to have different payment structures in order to get the benefit of leveraging those types of technologies. When we’re sourcing and looking at deals, we’re looking at both of those angles,” she said.
CHICAGO — Have a great tech idea to improve gastroenterology? Start-up companies have the potential to transform the practice of medicine, and to make founders a nice pot of money, but it is a difficult road. At the 2024 AGA Tech Summit, held at the Chicago headquarters of MATTER, a global healthcare startup incubator, investors and gastroenterologists discussed some of the key challenges and opportunities for GI startups.
The road is daunting, and founders must be dedicated to their companies but also maintain life balance. “It is very easy, following your passion, for your life to get out of check. I don’t know what the divorce rate is for entrepreneurs, but I personally was a victim of that. The culture that we built was addictive and it became all encompassing, and at the same time [I neglected] my home life,” Scott Fraser, managing director of the consulting company Fraser Healthcare, said during a “Scars and Stripes” panel at the summit.
For those willing to navigate those waters, there is help. Investors are prepared to provide seed money for companies with good ideas and a strong market. AGA itself has stepped into the investment field with its GI Opportunity Fund, which it launched in 2022 through a partnership with Varia Ventures. The fund’s capital comes from AGA members, with a minimum investment of $25,000. To date, AGA has made investments in six companies, at around $100,000 per company. “It’s not a large amount that we’re investing. We’re a lead investor that signals to other venture capital companies that this is a viable company,” Tom Serena, CEO of AGA, said in an interview.
The fund grew out of AGA’s commitment to boosting early-stage companies in the gastroenterology space. AGA has always supported GI device and tech companies through its Center for GI Innovation and Technology, which sponsored the AGA Tech Summit. The center now provides resources and advice for GI innovators and startups. The AGA Tech Summit has created a gathering place for entrepreneurs and innovators to share their experiences and learn from one another. “But what we were missing was the last mile, which is getting funding to the companies,” said Mr. Serena. The summit itself has been modified to increase the venture capital presence. “That’s the networking we’re trying to [create] here. Venture capitalists are well acquainted with these companies, but we feel that AGA can bring clinical due diligence, and the startups want to be exposed to venture capital,” said Mr. Serena.
During the “Learn from VC Strategists” panel, investors shared advice for entrepreneurs. The emphasis throughout was on marketable ideas that can fundamentally change healthcare practice, though inventions may not have the whiz-bang appeal of some new technologies of years past.
“We’re particularly focused on clinical models that actually work. There were a lot of companies for many years that were doing things that had minimal impact, or very incremental impact. Maybe they were helping identify certain patients, but they weren’t actually engaging those patients. We’re now looking very end-to-end and trying to make sure that it’s not just a good idea, but one that you can actually roll out, engage patients, and see the [return on investment] in that patient data,” said Kelsey Maguire, managing director of the Blue Venture Fund, which is a collaborative effort across Blue Cross Blue Shield companies.
Part of the reason for that shift is that healthcare has evolved in a way that has put more pressure on physicians, according to Barbara H. Jung, MD, AGAF, past president of AGA, who was present for the session. “I think that there’s huge burnout among gastroenterologists, [partly because] some of the systems have been optimized to get the most out of each specialist. I think we just have to get back to making work more enjoyable. [It could be less] fighting with the insurance companies, it could be that you spend less time typing after hours. It could be that it helps the team work more seamlessly, or it could be something that helps the patient prepare, so they have everything ready when they see the doctors. It’s thinking about how healthcare is delivered, and really in a patient and physician-centric way,” Dr. Jung said in an interview.
Anna Haghgooie, managing director of Valtruis, noted that, historically, new technology has been rewarded by the healthcare system. “It’s part of why we find ourselves where we are as an industry: There was nobody in the marketplace that was incented to roll out a cost-reducing technology, and those weren’t necessarily considered grand slams. But [I think] we’re at a tipping point on cost, and as a country will start purchasing in pretty meaningfully different ways, which opens up a lot of opportunities for those practical solutions to be grand slams. Everything that we look at has a component of virtual care, leveraging technology, whether it’s AI or just better workflow tools, better data and intelligence to make business decisions,” said Ms. Haghgooie. She did note that Valtruis does not work much with medical devices.
Specifically in the GI space, one panelist called for a shift away from novel colonoscopy technology. “I don’t know how many more bells and whistles we can ask for colonoscopy, which we’re very dependent on. Not that it’s not important, but I don’t think that’s where the real innovation is going to come. When you think about the cognitive side of the GI business: New diagnostics, things that are predictive of disease states, things that monitor disease, things that help you to know what people’s disease courses will be. I think as more and more interventions are done by endoscopists, you need more tools,” said Thomas Shehab, MD, managing partner at Arboretum Ventures.
Finally, AI has become a central component to investment decisions. Ms. Haghgooie said that Valtruis is focused on the infrastructure surrounding AI, such as the data that it requires to make or help guide decisions. That data can vary widely in quality, is difficult to index, exists in various silos, and is subject to a number of regulatory constraints on how to move or aggregate it. “So, a lot of what we’re focused on are the systems and tools that can enable the next gen application of AI. That’s one piece of the puzzle. The other is, I’d say that every company that we’ve either invested in or are looking at investing in, we ask the question: How are you planning to incorporate and leverage this next gen technology to drive your marginal cost-to-deliver down? In many cases you have to do that through business model redesign, because there is no fee-for-service code to get paid for leveraging AI to reduce your costs. You’ve got to have different payment structures in order to get the benefit of leveraging those types of technologies. When we’re sourcing and looking at deals, we’re looking at both of those angles,” she said.
CHICAGO — Have a great tech idea to improve gastroenterology? Start-up companies have the potential to transform the practice of medicine, and to make founders a nice pot of money, but it is a difficult road. At the 2024 AGA Tech Summit, held at the Chicago headquarters of MATTER, a global healthcare startup incubator, investors and gastroenterologists discussed some of the key challenges and opportunities for GI startups.
The road is daunting, and founders must be dedicated to their companies but also maintain life balance. “It is very easy, following your passion, for your life to get out of check. I don’t know what the divorce rate is for entrepreneurs, but I personally was a victim of that. The culture that we built was addictive and it became all encompassing, and at the same time [I neglected] my home life,” Scott Fraser, managing director of the consulting company Fraser Healthcare, said during a “Scars and Stripes” panel at the summit.
For those willing to navigate those waters, there is help. Investors are prepared to provide seed money for companies with good ideas and a strong market. AGA itself has stepped into the investment field with its GI Opportunity Fund, which it launched in 2022 through a partnership with Varia Ventures. The fund’s capital comes from AGA members, with a minimum investment of $25,000. To date, AGA has made investments in six companies, at around $100,000 per company. “It’s not a large amount that we’re investing. We’re a lead investor that signals to other venture capital companies that this is a viable company,” Tom Serena, CEO of AGA, said in an interview.
The fund grew out of AGA’s commitment to boosting early-stage companies in the gastroenterology space. AGA has always supported GI device and tech companies through its Center for GI Innovation and Technology, which sponsored the AGA Tech Summit. The center now provides resources and advice for GI innovators and startups. The AGA Tech Summit has created a gathering place for entrepreneurs and innovators to share their experiences and learn from one another. “But what we were missing was the last mile, which is getting funding to the companies,” said Mr. Serena. The summit itself has been modified to increase the venture capital presence. “That’s the networking we’re trying to [create] here. Venture capitalists are well acquainted with these companies, but we feel that AGA can bring clinical due diligence, and the startups want to be exposed to venture capital,” said Mr. Serena.
During the “Learn from VC Strategists” panel, investors shared advice for entrepreneurs. The emphasis throughout was on marketable ideas that can fundamentally change healthcare practice, though inventions may not have the whiz-bang appeal of some new technologies of years past.
“We’re particularly focused on clinical models that actually work. There were a lot of companies for many years that were doing things that had minimal impact, or very incremental impact. Maybe they were helping identify certain patients, but they weren’t actually engaging those patients. We’re now looking very end-to-end and trying to make sure that it’s not just a good idea, but one that you can actually roll out, engage patients, and see the [return on investment] in that patient data,” said Kelsey Maguire, managing director of the Blue Venture Fund, which is a collaborative effort across Blue Cross Blue Shield companies.
Part of the reason for that shift is that healthcare has evolved in a way that has put more pressure on physicians, according to Barbara H. Jung, MD, AGAF, past president of AGA, who was present for the session. “I think that there’s huge burnout among gastroenterologists, [partly because] some of the systems have been optimized to get the most out of each specialist. I think we just have to get back to making work more enjoyable. [It could be less] fighting with the insurance companies, it could be that you spend less time typing after hours. It could be that it helps the team work more seamlessly, or it could be something that helps the patient prepare, so they have everything ready when they see the doctors. It’s thinking about how healthcare is delivered, and really in a patient and physician-centric way,” Dr. Jung said in an interview.
Anna Haghgooie, managing director of Valtruis, noted that, historically, new technology has been rewarded by the healthcare system. “It’s part of why we find ourselves where we are as an industry: There was nobody in the marketplace that was incented to roll out a cost-reducing technology, and those weren’t necessarily considered grand slams. But [I think] we’re at a tipping point on cost, and as a country will start purchasing in pretty meaningfully different ways, which opens up a lot of opportunities for those practical solutions to be grand slams. Everything that we look at has a component of virtual care, leveraging technology, whether it’s AI or just better workflow tools, better data and intelligence to make business decisions,” said Ms. Haghgooie. She did note that Valtruis does not work much with medical devices.
Specifically in the GI space, one panelist called for a shift away from novel colonoscopy technology. “I don’t know how many more bells and whistles we can ask for colonoscopy, which we’re very dependent on. Not that it’s not important, but I don’t think that’s where the real innovation is going to come. When you think about the cognitive side of the GI business: New diagnostics, things that are predictive of disease states, things that monitor disease, things that help you to know what people’s disease courses will be. I think as more and more interventions are done by endoscopists, you need more tools,” said Thomas Shehab, MD, managing partner at Arboretum Ventures.
Finally, AI has become a central component to investment decisions. Ms. Haghgooie said that Valtruis is focused on the infrastructure surrounding AI, such as the data that it requires to make or help guide decisions. That data can vary widely in quality, is difficult to index, exists in various silos, and is subject to a number of regulatory constraints on how to move or aggregate it. “So, a lot of what we’re focused on are the systems and tools that can enable the next gen application of AI. That’s one piece of the puzzle. The other is, I’d say that every company that we’ve either invested in or are looking at investing in, we ask the question: How are you planning to incorporate and leverage this next gen technology to drive your marginal cost-to-deliver down? In many cases you have to do that through business model redesign, because there is no fee-for-service code to get paid for leveraging AI to reduce your costs. You’ve got to have different payment structures in order to get the benefit of leveraging those types of technologies. When we’re sourcing and looking at deals, we’re looking at both of those angles,” she said.
FROM THE 2024 AGA TECH SUMMIT
AI Wins AGA’s Shark Tank Competition
CHICAGO — At the 2024 AGA Tech Summit, held April 11-12 at the Chicago headquarters of MATTER, a global healthcare startup incubator, five companies made their pitch to be the winner of the Shark Tank competition that recognizes an outstanding tech start up in the gastroenterology field.
After the companies’ rapid-fire pitches and Q&A sessions, four judges convened to determine a winner and returned to make an announcement.
The winner was Arithmedics, which uses AI technology to automate billing codes. Founder Venthan Elango, PhD, has worked as a software engineer at Google, Urban Engines, and Georgia Tech, and his wife of 17 years is Renumathy Dhanasekaran, MD, PhD, a gastroenterologist and assistant professor of medicine at Stanford (California) University.
Their marriage has brought a unique perspective, according to Dr. Elango. “There isn’t a single day that goes by when she talks to me about the inefficiencies in healthcare, and then I say, ‘this can be easily solved with a software solution,’ ” he said.
When they decided to try a start-up, the two initiated conversations with healthcare providers to identify a key unmet need. “The common recurring theme was that medical billing was a problem, because of [insufficient] institutional knowledge, staff shortage, and inconsistencies with the payers,” said Dr. Elango. During their presentation, the two noted that about 80% of claims include at least one coding error, and this leads to an estimated $125 billion in annual losses.
Generative AI presented a solution. “Automating the medical billing code [determination] from a clinical record became 10 times easier than what it was before. So I thought, I can build a product that actually brings in augmented analytics and generative AI and do something that is tremendously useful to physicians,” he said.
The future goal is to make life easier for healthcare providers, according to Dr. Dhanasekaran. “As physicians, we went into medicine to talk with patients, but a lot of us are just typing away when patients are sitting in the room, because there are all of these requirements for documentation to get the billing so that we can get paid at the end of the day,” she said.
Arithmedics aims to initially target small-group medical practices that are tech savvy. They will analyze a year’s worth of claims for errors and resubmit claims for the past 3 months and split any additional revenue that ensues. They plan to expand to revenue cycle management companies and hospital systems. On the technology side, they will expand to data intelligence and integrate with electronic health records, and ultimately plan to charge 1%-2% of revenue.
The other Shark Tank finalists were:
- Aspero Medical: Balloon overtube that maximizes frictional properties to improve mucosal wall traction and anchoring consistency. (Voted ‘fan favorite’ by AGA Tech Summit attendees)
- Aurora Medical Technologies: Minimally invasive, guided, tissue-anchoring suturing system for complex endoscopic procedures.
- Ergami Endoscopy: Flexible overtube capable of automatic insertion and fixation in the colon, which could potentially eliminate sedation and prevent endoscopic injuries to the physician.
- Lazurite: Wireless surgical camera that eliminates the need for light or video cables, avoiding the associated fire, trip, and contamination hazards.
The judges were swayed by Arithmedics’ practical solution to a widespread problem. “There is for sure a need in terms of inaccurate billing and billing codes that are wrong. There’s lost revenue for physicians around that. So I think we were really focused from a judging standpoint on the fact that their solution was filling truly an unmet need,” said judge Andrea Vossler, a managing director of Varia Ventures, which has partnered with AGA to launch and manage the GI Opportunity Fund, an AGA-member venture fund.
“We were really focused on how to assist physicians in terms of supporting their practices, and really changing what you’re doing. I think AI has the ability to do that, so we liked that about the company,” she added.
The company is an example of how AI is poised to alter healthcare, according to Ms. Vossler. “I think it’s massive. I think we’re at the very beginning of its impact on healthcare,” she said.
Another judge had a similar view. “They won because there is a screaming need to fix billing. So, it’s well known that lots of money is indeed lost in billing practices, which are stressful for office personnel and stressful for physicians. They can fulfill a long-standing need, and we thought that that was the success story,” said Christopher Gostout, MD, emeritus professor of medicine at Mayo Clinic in Rochester, Minnesota.
Dr. Gostout offered advice for gastroenterologists and other physicians interested in starting tech companies. It’s imperative to be a realist, he said. “Is there a real market for it, or [is it just] a niche market? Does your device have legs — can it expand and can evolve into other [spin-off] products? These are things you need to think about because one-offs or single-trick ponies are pretty hard to move along now,” said Dr. Gostout.
He recommended that entrepreneurs apply for Small Business Innovation Research (SBIR) grants. “I think it’s a great opportunity to bring in money and get the ball rolling.”
Finally, he advised entrepreneurs to be thoughtful about their advisory groups. Founders may be tempted to find the highest profile names they can to give the business gravitas, but those big names may not have the best knowledge base to understand the problems that the technology is meant to address. “I’ve seen businesses fail because they went for marquee names that really were not helpful, and they didn’t do their due diligence in seeking out really useful value. You don’t need a lot of advisers, just a couple of really good ones,” said Dr. Gostout.
The summit was sponsored by the AGA Center for GI Innovation and Technology.
Dr. Gostout has founded and advises AdaptivEndo and Lean Medical. He is a consultant to Boston Scientific. Dr. Dhanasekaran has no financial disclosures. Ms. Vossler is an employee of Varia Ventures, which is an investment partner to AGA. Dr. Elango is an employee of Arithmedics.
CHICAGO — At the 2024 AGA Tech Summit, held April 11-12 at the Chicago headquarters of MATTER, a global healthcare startup incubator, five companies made their pitch to be the winner of the Shark Tank competition that recognizes an outstanding tech start up in the gastroenterology field.
After the companies’ rapid-fire pitches and Q&A sessions, four judges convened to determine a winner and returned to make an announcement.
The winner was Arithmedics, which uses AI technology to automate billing codes. Founder Venthan Elango, PhD, has worked as a software engineer at Google, Urban Engines, and Georgia Tech, and his wife of 17 years is Renumathy Dhanasekaran, MD, PhD, a gastroenterologist and assistant professor of medicine at Stanford (California) University.
Their marriage has brought a unique perspective, according to Dr. Elango. “There isn’t a single day that goes by when she talks to me about the inefficiencies in healthcare, and then I say, ‘this can be easily solved with a software solution,’ ” he said.
When they decided to try a start-up, the two initiated conversations with healthcare providers to identify a key unmet need. “The common recurring theme was that medical billing was a problem, because of [insufficient] institutional knowledge, staff shortage, and inconsistencies with the payers,” said Dr. Elango. During their presentation, the two noted that about 80% of claims include at least one coding error, and this leads to an estimated $125 billion in annual losses.
Generative AI presented a solution. “Automating the medical billing code [determination] from a clinical record became 10 times easier than what it was before. So I thought, I can build a product that actually brings in augmented analytics and generative AI and do something that is tremendously useful to physicians,” he said.
The future goal is to make life easier for healthcare providers, according to Dr. Dhanasekaran. “As physicians, we went into medicine to talk with patients, but a lot of us are just typing away when patients are sitting in the room, because there are all of these requirements for documentation to get the billing so that we can get paid at the end of the day,” she said.
Arithmedics aims to initially target small-group medical practices that are tech savvy. They will analyze a year’s worth of claims for errors and resubmit claims for the past 3 months and split any additional revenue that ensues. They plan to expand to revenue cycle management companies and hospital systems. On the technology side, they will expand to data intelligence and integrate with electronic health records, and ultimately plan to charge 1%-2% of revenue.
The other Shark Tank finalists were:
- Aspero Medical: Balloon overtube that maximizes frictional properties to improve mucosal wall traction and anchoring consistency. (Voted ‘fan favorite’ by AGA Tech Summit attendees)
- Aurora Medical Technologies: Minimally invasive, guided, tissue-anchoring suturing system for complex endoscopic procedures.
- Ergami Endoscopy: Flexible overtube capable of automatic insertion and fixation in the colon, which could potentially eliminate sedation and prevent endoscopic injuries to the physician.
- Lazurite: Wireless surgical camera that eliminates the need for light or video cables, avoiding the associated fire, trip, and contamination hazards.
The judges were swayed by Arithmedics’ practical solution to a widespread problem. “There is for sure a need in terms of inaccurate billing and billing codes that are wrong. There’s lost revenue for physicians around that. So I think we were really focused from a judging standpoint on the fact that their solution was filling truly an unmet need,” said judge Andrea Vossler, a managing director of Varia Ventures, which has partnered with AGA to launch and manage the GI Opportunity Fund, an AGA-member venture fund.
“We were really focused on how to assist physicians in terms of supporting their practices, and really changing what you’re doing. I think AI has the ability to do that, so we liked that about the company,” she added.
The company is an example of how AI is poised to alter healthcare, according to Ms. Vossler. “I think it’s massive. I think we’re at the very beginning of its impact on healthcare,” she said.
Another judge had a similar view. “They won because there is a screaming need to fix billing. So, it’s well known that lots of money is indeed lost in billing practices, which are stressful for office personnel and stressful for physicians. They can fulfill a long-standing need, and we thought that that was the success story,” said Christopher Gostout, MD, emeritus professor of medicine at Mayo Clinic in Rochester, Minnesota.
Dr. Gostout offered advice for gastroenterologists and other physicians interested in starting tech companies. It’s imperative to be a realist, he said. “Is there a real market for it, or [is it just] a niche market? Does your device have legs — can it expand and can evolve into other [spin-off] products? These are things you need to think about because one-offs or single-trick ponies are pretty hard to move along now,” said Dr. Gostout.
He recommended that entrepreneurs apply for Small Business Innovation Research (SBIR) grants. “I think it’s a great opportunity to bring in money and get the ball rolling.”
Finally, he advised entrepreneurs to be thoughtful about their advisory groups. Founders may be tempted to find the highest profile names they can to give the business gravitas, but those big names may not have the best knowledge base to understand the problems that the technology is meant to address. “I’ve seen businesses fail because they went for marquee names that really were not helpful, and they didn’t do their due diligence in seeking out really useful value. You don’t need a lot of advisers, just a couple of really good ones,” said Dr. Gostout.
The summit was sponsored by the AGA Center for GI Innovation and Technology.
Dr. Gostout has founded and advises AdaptivEndo and Lean Medical. He is a consultant to Boston Scientific. Dr. Dhanasekaran has no financial disclosures. Ms. Vossler is an employee of Varia Ventures, which is an investment partner to AGA. Dr. Elango is an employee of Arithmedics.
CHICAGO — At the 2024 AGA Tech Summit, held April 11-12 at the Chicago headquarters of MATTER, a global healthcare startup incubator, five companies made their pitch to be the winner of the Shark Tank competition that recognizes an outstanding tech start up in the gastroenterology field.
After the companies’ rapid-fire pitches and Q&A sessions, four judges convened to determine a winner and returned to make an announcement.
The winner was Arithmedics, which uses AI technology to automate billing codes. Founder Venthan Elango, PhD, has worked as a software engineer at Google, Urban Engines, and Georgia Tech, and his wife of 17 years is Renumathy Dhanasekaran, MD, PhD, a gastroenterologist and assistant professor of medicine at Stanford (California) University.
Their marriage has brought a unique perspective, according to Dr. Elango. “There isn’t a single day that goes by when she talks to me about the inefficiencies in healthcare, and then I say, ‘this can be easily solved with a software solution,’ ” he said.
When they decided to try a start-up, the two initiated conversations with healthcare providers to identify a key unmet need. “The common recurring theme was that medical billing was a problem, because of [insufficient] institutional knowledge, staff shortage, and inconsistencies with the payers,” said Dr. Elango. During their presentation, the two noted that about 80% of claims include at least one coding error, and this leads to an estimated $125 billion in annual losses.
Generative AI presented a solution. “Automating the medical billing code [determination] from a clinical record became 10 times easier than what it was before. So I thought, I can build a product that actually brings in augmented analytics and generative AI and do something that is tremendously useful to physicians,” he said.
The future goal is to make life easier for healthcare providers, according to Dr. Dhanasekaran. “As physicians, we went into medicine to talk with patients, but a lot of us are just typing away when patients are sitting in the room, because there are all of these requirements for documentation to get the billing so that we can get paid at the end of the day,” she said.
Arithmedics aims to initially target small-group medical practices that are tech savvy. They will analyze a year’s worth of claims for errors and resubmit claims for the past 3 months and split any additional revenue that ensues. They plan to expand to revenue cycle management companies and hospital systems. On the technology side, they will expand to data intelligence and integrate with electronic health records, and ultimately plan to charge 1%-2% of revenue.
The other Shark Tank finalists were:
- Aspero Medical: Balloon overtube that maximizes frictional properties to improve mucosal wall traction and anchoring consistency. (Voted ‘fan favorite’ by AGA Tech Summit attendees)
- Aurora Medical Technologies: Minimally invasive, guided, tissue-anchoring suturing system for complex endoscopic procedures.
- Ergami Endoscopy: Flexible overtube capable of automatic insertion and fixation in the colon, which could potentially eliminate sedation and prevent endoscopic injuries to the physician.
- Lazurite: Wireless surgical camera that eliminates the need for light or video cables, avoiding the associated fire, trip, and contamination hazards.
The judges were swayed by Arithmedics’ practical solution to a widespread problem. “There is for sure a need in terms of inaccurate billing and billing codes that are wrong. There’s lost revenue for physicians around that. So I think we were really focused from a judging standpoint on the fact that their solution was filling truly an unmet need,” said judge Andrea Vossler, a managing director of Varia Ventures, which has partnered with AGA to launch and manage the GI Opportunity Fund, an AGA-member venture fund.
“We were really focused on how to assist physicians in terms of supporting their practices, and really changing what you’re doing. I think AI has the ability to do that, so we liked that about the company,” she added.
The company is an example of how AI is poised to alter healthcare, according to Ms. Vossler. “I think it’s massive. I think we’re at the very beginning of its impact on healthcare,” she said.
Another judge had a similar view. “They won because there is a screaming need to fix billing. So, it’s well known that lots of money is indeed lost in billing practices, which are stressful for office personnel and stressful for physicians. They can fulfill a long-standing need, and we thought that that was the success story,” said Christopher Gostout, MD, emeritus professor of medicine at Mayo Clinic in Rochester, Minnesota.
Dr. Gostout offered advice for gastroenterologists and other physicians interested in starting tech companies. It’s imperative to be a realist, he said. “Is there a real market for it, or [is it just] a niche market? Does your device have legs — can it expand and can evolve into other [spin-off] products? These are things you need to think about because one-offs or single-trick ponies are pretty hard to move along now,” said Dr. Gostout.
He recommended that entrepreneurs apply for Small Business Innovation Research (SBIR) grants. “I think it’s a great opportunity to bring in money and get the ball rolling.”
Finally, he advised entrepreneurs to be thoughtful about their advisory groups. Founders may be tempted to find the highest profile names they can to give the business gravitas, but those big names may not have the best knowledge base to understand the problems that the technology is meant to address. “I’ve seen businesses fail because they went for marquee names that really were not helpful, and they didn’t do their due diligence in seeking out really useful value. You don’t need a lot of advisers, just a couple of really good ones,” said Dr. Gostout.
The summit was sponsored by the AGA Center for GI Innovation and Technology.
Dr. Gostout has founded and advises AdaptivEndo and Lean Medical. He is a consultant to Boston Scientific. Dr. Dhanasekaran has no financial disclosures. Ms. Vossler is an employee of Varia Ventures, which is an investment partner to AGA. Dr. Elango is an employee of Arithmedics.
FROM THE 2024 AGA TECH SUMMIT
AGA Tech Summit Focuses on Accelerating Innovation
The AGA Tech Summit is building on the success of past summits and moving in a new direction. The reimagined summit will accelerate innovation by bringing together MedTech startups, innovators, investors and leaders in the field.
“It’s a new world out there. The Tech Summit now reflects the new direction AGA is taking in innovation,” said Lawrence R. Kosinski, MD, AGA at-large councilor for development and growth. “We want to help GI innovators successfully navigate the innovation lifecycle from start to finish and bring new technologies to market.”
The Tech Summit will take place April 11-12 in Chicago at MATTER, located at the Merchandise Mart. MATTER supports healthcare startups at all stages of growth and brings together industry executives, entrepreneurs, and investors to accelerate innovation, advance care and improve lives.
Highlights of the Tech Summit include:
- Keynote addresses from leaders in the field of GI innovation.
- Panel discussions with VC strategists.
- The Shark Tank Pitch Competition featuring emerging GI technologies.
- Multiple opportunities to network innovators, investors and leaders in the field.
- One-on-one consultations with VCs.
.
The AGA Tech Summit is building on the success of past summits and moving in a new direction. The reimagined summit will accelerate innovation by bringing together MedTech startups, innovators, investors and leaders in the field.
“It’s a new world out there. The Tech Summit now reflects the new direction AGA is taking in innovation,” said Lawrence R. Kosinski, MD, AGA at-large councilor for development and growth. “We want to help GI innovators successfully navigate the innovation lifecycle from start to finish and bring new technologies to market.”
The Tech Summit will take place April 11-12 in Chicago at MATTER, located at the Merchandise Mart. MATTER supports healthcare startups at all stages of growth and brings together industry executives, entrepreneurs, and investors to accelerate innovation, advance care and improve lives.
Highlights of the Tech Summit include:
- Keynote addresses from leaders in the field of GI innovation.
- Panel discussions with VC strategists.
- The Shark Tank Pitch Competition featuring emerging GI technologies.
- Multiple opportunities to network innovators, investors and leaders in the field.
- One-on-one consultations with VCs.
.
The AGA Tech Summit is building on the success of past summits and moving in a new direction. The reimagined summit will accelerate innovation by bringing together MedTech startups, innovators, investors and leaders in the field.
“It’s a new world out there. The Tech Summit now reflects the new direction AGA is taking in innovation,” said Lawrence R. Kosinski, MD, AGA at-large councilor for development and growth. “We want to help GI innovators successfully navigate the innovation lifecycle from start to finish and bring new technologies to market.”
The Tech Summit will take place April 11-12 in Chicago at MATTER, located at the Merchandise Mart. MATTER supports healthcare startups at all stages of growth and brings together industry executives, entrepreneurs, and investors to accelerate innovation, advance care and improve lives.
Highlights of the Tech Summit include:
- Keynote addresses from leaders in the field of GI innovation.
- Panel discussions with VC strategists.
- The Shark Tank Pitch Competition featuring emerging GI technologies.
- Multiple opportunities to network innovators, investors and leaders in the field.
- One-on-one consultations with VCs.
.
Noninvasive esophageal cancer screening approaches may reach more at-risk patients
A rise in esophageal adenocarcinoma (EAC) cases and deaths showcases a need for noninvasive screening methods that can be performed by nonendoscopists, such as nurses or technicians, according to a presentation at the 2022 AGA Tech Summit that reviewed the new approaches. AGA’s annual innovation summit is sponsored by the AGA Center for GI Innovation and Technology.
Mortality rates are high, because the cancer is usually found after obstructive symptoms. Screening for Barrett’s esophagus (BE) and associated dysplasia could lead to earlier diagnosis and better prognoses, but endoscopic screening is costly and invasive, and few at-risk patients take advantage of it.
Some new approaches have the potential to screen more patients and detect earlier stages of disease, according to Prasad Iyer, MD, director of the esophageal interest group in the division of gastroenterology and hepatology at the Mayo Clinic in Rochester, Minn.
The estimated rise in EAC ranges from 400% to 600% between 1975 and 2000. The 5-year survival of EAC hovers at around 20%. “Not only is the incidence increasing, but the mortality associated with the disease is also increasing at a similar pace,” said Dr. Iyer during his presentation.
The only known precursor to EAC is BE, which has made the condition a focal point in screening. “If we can screen those with risk factors, we can identify those with prevalent Barrett’s. We then can put those with known Barrett’s into surveillance to detect cancer or high-grade or low-grade dysplasia. And then we when we find dysplasia or early cancer, we can intervene hopefully endoscopically to prevent or treat this progression from Barrett’s to adenocarcinoma,” said Dr. Iyer.
Endoscopic treatment of dysplasia achieves similar long-term survival outcomes to esophagectomy,Dr. Iyer said. Clinical studies have shown that radiofrequency ablation of high-grade and low-grade dysplasia reduces progression to cancer.
Low screening rates miss at-risk patients
Unfortunately, only 10%-12% of esophageal cancers are detected during surveillance, partly because many with BE are unaware of the condition and therefore don’t enter surveillance. “Two-thirds of the patients with Barrett’s are not under surveillance, so it’s not surprising that most esophageal cancers, unfortunately, are still being diagnosed after the onset of obstructive symptoms,” said Dr. Iyer.
A key issue is that sedated endoscopy is the only available screening tool, and it is expensive and invasive. “Only 10% of those who should get evaluated for the presence of Barrett’s are currently getting evaluated,” said Dr. Iyer.
Those issues have led to a movement to develop noninvasive methods for screening that could be performed by nonendoscopists, such as nurses or technicians. Dr. Iyer noted the importance of sensitivity and specificity of any test, but access to the test and participation are often overlooked factors.
“We hope that, by developing a nonendoscopic, minimally invasive test, we can increase access by allowing nonphysicians to perform this test. By keeping the costs low, we make this strategy cost effective, and hopefully get buy in for reimbursement from payers,” said Dr. Iyer.
New screening methods on horizon
He reviewed several noninvasive screening methodologies in development.
Unsedated transnasal endoscopy has been used successfully to diagnose BE, but the technique has not gained much traction in the United States.
Some devices collect esophageal cells, and then test them for various biomarkers. These include EsophaCap, CytoSponge, and the ESOCHEK Balloon. The procedure requires the patient to swallow a device, which is attached to a string or cord. After a few minutes, the device expands into a sphere or balloon, and the operator pulls it out through the esophagus, collecting 3-4 million esophageal cells in the process.
Biomarker analysis of the cells can include the protein trefoil factor 3 and methylated DNA markers. Case-control studies have shown this approach can achieve sensitivities of 76%-94%, and specificities of 62%-92%. “At least in case-control studies, this technology has been shown in thousands of patients now to be well tolerated, very safe, with a low risk of detachment, and can be done by a nurse in an office setting in less than 10 minutes,” said Dr. Iyer.
Earlier detection of Barrett’s
He summarized a randomized, controlled trial, published in 2020 in The Lancet, which tested this approach in patients who had taken proton pump inhibitors for at least 6 months. It compared 6,983 patients screened using the CytoSponge/TFF3 with 6,531 usual-care patients who only underwent screening if their physicians recommended it.
In the screening group, 140 patients were diagnosed with Barrett’s Esophagus, compared with 13 in the usual-care group. There were nine cases of dysplastic Barrett’s and five cases of stage I EAC in the screening group, versus no dysplastic Barrett’s and three advanced stage EAC cases in the usual care group. “You can see how we can shift the spectrum of patients with Barrett’s if we go for early detection,” said Dr. Iyer.
Another noninvasive strategy relies on sensors to detect exhaled volatile organic compounds. After a patient breathes into the detector for about 5 minutes, an artificial neural network distinguishes molecular patterns indicative of the presence or absence of BE. The technique had just moderate sensitivity and specificity, “But this is very noninvasive and even less invasive than [sponge or balloon]-based technology,” said Dr. Iyer.
Other efforts are underway to identify plasma biomarkers for screening. Dr. Iyer and colleagues have developed methylated DNA markers for EAC and squamous cell cancer. So far, they have achieved sensitivity and specificity just above 80%. “Not where we would want it to be, but certainly not terrible,” said Dr. Iyer, adding that they are performing a larger prospective study.
He described a potential screening program that could draw from electronic medical records or even apps to identify patients with risk above a defined threshold who would then be tested with minimally invasive techniques. Those with positive results would go on to confirmatory endoscopy. His group found that such a strategy would be cost effective even if reflux was not used as a qualifying criterion for screening.
Answering audience questions after the talk, Dr. Iyer was asked if noninvasive methods would directly compete with endoscopy, or if some patients would be better candidates for one or the other.
“That’s something we need to think through. It’s going to be very difficult for us to say every patient at risk should get an endoscopy. I just don’t think that strategy is probably practical or cost effective. On the other hand, I think an all-of-the-above strategy is probably just fine. It’s like elections. You have to be very local, your message has to be cost effective, available, and have adequate patient as well as provider buy-in,” he said.
Dr. Iyer has received research funding from Exact Sciences, Pentax Medical, and Cernostics. He has consulted for Exact Sciences, Pentax Medical, Medtronic, Ambu, Cernostics, CDx Diagnostics, and Symple Surgical. The 2022 AGA Tech Summit was supported by independent grants from Castle Biosciences, Medtronic, Boston Scientific, Exact Sciences, Olympus, 3-D Matrix, Apollo Endosurgery, Motus GI Holdings, STERIS Endoscopy, Cook Medical, FUJIFILM Healthcare Americas, and Virgo.
This article was updated 5/10/22.
A rise in esophageal adenocarcinoma (EAC) cases and deaths showcases a need for noninvasive screening methods that can be performed by nonendoscopists, such as nurses or technicians, according to a presentation at the 2022 AGA Tech Summit that reviewed the new approaches. AGA’s annual innovation summit is sponsored by the AGA Center for GI Innovation and Technology.
Mortality rates are high, because the cancer is usually found after obstructive symptoms. Screening for Barrett’s esophagus (BE) and associated dysplasia could lead to earlier diagnosis and better prognoses, but endoscopic screening is costly and invasive, and few at-risk patients take advantage of it.
Some new approaches have the potential to screen more patients and detect earlier stages of disease, according to Prasad Iyer, MD, director of the esophageal interest group in the division of gastroenterology and hepatology at the Mayo Clinic in Rochester, Minn.
The estimated rise in EAC ranges from 400% to 600% between 1975 and 2000. The 5-year survival of EAC hovers at around 20%. “Not only is the incidence increasing, but the mortality associated with the disease is also increasing at a similar pace,” said Dr. Iyer during his presentation.
The only known precursor to EAC is BE, which has made the condition a focal point in screening. “If we can screen those with risk factors, we can identify those with prevalent Barrett’s. We then can put those with known Barrett’s into surveillance to detect cancer or high-grade or low-grade dysplasia. And then we when we find dysplasia or early cancer, we can intervene hopefully endoscopically to prevent or treat this progression from Barrett’s to adenocarcinoma,” said Dr. Iyer.
Endoscopic treatment of dysplasia achieves similar long-term survival outcomes to esophagectomy,Dr. Iyer said. Clinical studies have shown that radiofrequency ablation of high-grade and low-grade dysplasia reduces progression to cancer.
Low screening rates miss at-risk patients
Unfortunately, only 10%-12% of esophageal cancers are detected during surveillance, partly because many with BE are unaware of the condition and therefore don’t enter surveillance. “Two-thirds of the patients with Barrett’s are not under surveillance, so it’s not surprising that most esophageal cancers, unfortunately, are still being diagnosed after the onset of obstructive symptoms,” said Dr. Iyer.
A key issue is that sedated endoscopy is the only available screening tool, and it is expensive and invasive. “Only 10% of those who should get evaluated for the presence of Barrett’s are currently getting evaluated,” said Dr. Iyer.
Those issues have led to a movement to develop noninvasive methods for screening that could be performed by nonendoscopists, such as nurses or technicians. Dr. Iyer noted the importance of sensitivity and specificity of any test, but access to the test and participation are often overlooked factors.
“We hope that, by developing a nonendoscopic, minimally invasive test, we can increase access by allowing nonphysicians to perform this test. By keeping the costs low, we make this strategy cost effective, and hopefully get buy in for reimbursement from payers,” said Dr. Iyer.
New screening methods on horizon
He reviewed several noninvasive screening methodologies in development.
Unsedated transnasal endoscopy has been used successfully to diagnose BE, but the technique has not gained much traction in the United States.
Some devices collect esophageal cells, and then test them for various biomarkers. These include EsophaCap, CytoSponge, and the ESOCHEK Balloon. The procedure requires the patient to swallow a device, which is attached to a string or cord. After a few minutes, the device expands into a sphere or balloon, and the operator pulls it out through the esophagus, collecting 3-4 million esophageal cells in the process.
Biomarker analysis of the cells can include the protein trefoil factor 3 and methylated DNA markers. Case-control studies have shown this approach can achieve sensitivities of 76%-94%, and specificities of 62%-92%. “At least in case-control studies, this technology has been shown in thousands of patients now to be well tolerated, very safe, with a low risk of detachment, and can be done by a nurse in an office setting in less than 10 minutes,” said Dr. Iyer.
Earlier detection of Barrett’s
He summarized a randomized, controlled trial, published in 2020 in The Lancet, which tested this approach in patients who had taken proton pump inhibitors for at least 6 months. It compared 6,983 patients screened using the CytoSponge/TFF3 with 6,531 usual-care patients who only underwent screening if their physicians recommended it.
In the screening group, 140 patients were diagnosed with Barrett’s Esophagus, compared with 13 in the usual-care group. There were nine cases of dysplastic Barrett’s and five cases of stage I EAC in the screening group, versus no dysplastic Barrett’s and three advanced stage EAC cases in the usual care group. “You can see how we can shift the spectrum of patients with Barrett’s if we go for early detection,” said Dr. Iyer.
Another noninvasive strategy relies on sensors to detect exhaled volatile organic compounds. After a patient breathes into the detector for about 5 minutes, an artificial neural network distinguishes molecular patterns indicative of the presence or absence of BE. The technique had just moderate sensitivity and specificity, “But this is very noninvasive and even less invasive than [sponge or balloon]-based technology,” said Dr. Iyer.
Other efforts are underway to identify plasma biomarkers for screening. Dr. Iyer and colleagues have developed methylated DNA markers for EAC and squamous cell cancer. So far, they have achieved sensitivity and specificity just above 80%. “Not where we would want it to be, but certainly not terrible,” said Dr. Iyer, adding that they are performing a larger prospective study.
He described a potential screening program that could draw from electronic medical records or even apps to identify patients with risk above a defined threshold who would then be tested with minimally invasive techniques. Those with positive results would go on to confirmatory endoscopy. His group found that such a strategy would be cost effective even if reflux was not used as a qualifying criterion for screening.
Answering audience questions after the talk, Dr. Iyer was asked if noninvasive methods would directly compete with endoscopy, or if some patients would be better candidates for one or the other.
“That’s something we need to think through. It’s going to be very difficult for us to say every patient at risk should get an endoscopy. I just don’t think that strategy is probably practical or cost effective. On the other hand, I think an all-of-the-above strategy is probably just fine. It’s like elections. You have to be very local, your message has to be cost effective, available, and have adequate patient as well as provider buy-in,” he said.
Dr. Iyer has received research funding from Exact Sciences, Pentax Medical, and Cernostics. He has consulted for Exact Sciences, Pentax Medical, Medtronic, Ambu, Cernostics, CDx Diagnostics, and Symple Surgical. The 2022 AGA Tech Summit was supported by independent grants from Castle Biosciences, Medtronic, Boston Scientific, Exact Sciences, Olympus, 3-D Matrix, Apollo Endosurgery, Motus GI Holdings, STERIS Endoscopy, Cook Medical, FUJIFILM Healthcare Americas, and Virgo.
This article was updated 5/10/22.
A rise in esophageal adenocarcinoma (EAC) cases and deaths showcases a need for noninvasive screening methods that can be performed by nonendoscopists, such as nurses or technicians, according to a presentation at the 2022 AGA Tech Summit that reviewed the new approaches. AGA’s annual innovation summit is sponsored by the AGA Center for GI Innovation and Technology.
Mortality rates are high, because the cancer is usually found after obstructive symptoms. Screening for Barrett’s esophagus (BE) and associated dysplasia could lead to earlier diagnosis and better prognoses, but endoscopic screening is costly and invasive, and few at-risk patients take advantage of it.
Some new approaches have the potential to screen more patients and detect earlier stages of disease, according to Prasad Iyer, MD, director of the esophageal interest group in the division of gastroenterology and hepatology at the Mayo Clinic in Rochester, Minn.
The estimated rise in EAC ranges from 400% to 600% between 1975 and 2000. The 5-year survival of EAC hovers at around 20%. “Not only is the incidence increasing, but the mortality associated with the disease is also increasing at a similar pace,” said Dr. Iyer during his presentation.
The only known precursor to EAC is BE, which has made the condition a focal point in screening. “If we can screen those with risk factors, we can identify those with prevalent Barrett’s. We then can put those with known Barrett’s into surveillance to detect cancer or high-grade or low-grade dysplasia. And then we when we find dysplasia or early cancer, we can intervene hopefully endoscopically to prevent or treat this progression from Barrett’s to adenocarcinoma,” said Dr. Iyer.
Endoscopic treatment of dysplasia achieves similar long-term survival outcomes to esophagectomy,Dr. Iyer said. Clinical studies have shown that radiofrequency ablation of high-grade and low-grade dysplasia reduces progression to cancer.
Low screening rates miss at-risk patients
Unfortunately, only 10%-12% of esophageal cancers are detected during surveillance, partly because many with BE are unaware of the condition and therefore don’t enter surveillance. “Two-thirds of the patients with Barrett’s are not under surveillance, so it’s not surprising that most esophageal cancers, unfortunately, are still being diagnosed after the onset of obstructive symptoms,” said Dr. Iyer.
A key issue is that sedated endoscopy is the only available screening tool, and it is expensive and invasive. “Only 10% of those who should get evaluated for the presence of Barrett’s are currently getting evaluated,” said Dr. Iyer.
Those issues have led to a movement to develop noninvasive methods for screening that could be performed by nonendoscopists, such as nurses or technicians. Dr. Iyer noted the importance of sensitivity and specificity of any test, but access to the test and participation are often overlooked factors.
“We hope that, by developing a nonendoscopic, minimally invasive test, we can increase access by allowing nonphysicians to perform this test. By keeping the costs low, we make this strategy cost effective, and hopefully get buy in for reimbursement from payers,” said Dr. Iyer.
New screening methods on horizon
He reviewed several noninvasive screening methodologies in development.
Unsedated transnasal endoscopy has been used successfully to diagnose BE, but the technique has not gained much traction in the United States.
Some devices collect esophageal cells, and then test them for various biomarkers. These include EsophaCap, CytoSponge, and the ESOCHEK Balloon. The procedure requires the patient to swallow a device, which is attached to a string or cord. After a few minutes, the device expands into a sphere or balloon, and the operator pulls it out through the esophagus, collecting 3-4 million esophageal cells in the process.
Biomarker analysis of the cells can include the protein trefoil factor 3 and methylated DNA markers. Case-control studies have shown this approach can achieve sensitivities of 76%-94%, and specificities of 62%-92%. “At least in case-control studies, this technology has been shown in thousands of patients now to be well tolerated, very safe, with a low risk of detachment, and can be done by a nurse in an office setting in less than 10 minutes,” said Dr. Iyer.
Earlier detection of Barrett’s
He summarized a randomized, controlled trial, published in 2020 in The Lancet, which tested this approach in patients who had taken proton pump inhibitors for at least 6 months. It compared 6,983 patients screened using the CytoSponge/TFF3 with 6,531 usual-care patients who only underwent screening if their physicians recommended it.
In the screening group, 140 patients were diagnosed with Barrett’s Esophagus, compared with 13 in the usual-care group. There were nine cases of dysplastic Barrett’s and five cases of stage I EAC in the screening group, versus no dysplastic Barrett’s and three advanced stage EAC cases in the usual care group. “You can see how we can shift the spectrum of patients with Barrett’s if we go for early detection,” said Dr. Iyer.
Another noninvasive strategy relies on sensors to detect exhaled volatile organic compounds. After a patient breathes into the detector for about 5 minutes, an artificial neural network distinguishes molecular patterns indicative of the presence or absence of BE. The technique had just moderate sensitivity and specificity, “But this is very noninvasive and even less invasive than [sponge or balloon]-based technology,” said Dr. Iyer.
Other efforts are underway to identify plasma biomarkers for screening. Dr. Iyer and colleagues have developed methylated DNA markers for EAC and squamous cell cancer. So far, they have achieved sensitivity and specificity just above 80%. “Not where we would want it to be, but certainly not terrible,” said Dr. Iyer, adding that they are performing a larger prospective study.
He described a potential screening program that could draw from electronic medical records or even apps to identify patients with risk above a defined threshold who would then be tested with minimally invasive techniques. Those with positive results would go on to confirmatory endoscopy. His group found that such a strategy would be cost effective even if reflux was not used as a qualifying criterion for screening.
Answering audience questions after the talk, Dr. Iyer was asked if noninvasive methods would directly compete with endoscopy, or if some patients would be better candidates for one or the other.
“That’s something we need to think through. It’s going to be very difficult for us to say every patient at risk should get an endoscopy. I just don’t think that strategy is probably practical or cost effective. On the other hand, I think an all-of-the-above strategy is probably just fine. It’s like elections. You have to be very local, your message has to be cost effective, available, and have adequate patient as well as provider buy-in,” he said.
Dr. Iyer has received research funding from Exact Sciences, Pentax Medical, and Cernostics. He has consulted for Exact Sciences, Pentax Medical, Medtronic, Ambu, Cernostics, CDx Diagnostics, and Symple Surgical. The 2022 AGA Tech Summit was supported by independent grants from Castle Biosciences, Medtronic, Boston Scientific, Exact Sciences, Olympus, 3-D Matrix, Apollo Endosurgery, Motus GI Holdings, STERIS Endoscopy, Cook Medical, FUJIFILM Healthcare Americas, and Virgo.
This article was updated 5/10/22.
FROM THE 2022 AGA TECH SUMMIT
Endoscopic ultrasound survives the sharks at AGA Tech Summit
After a 3-year, pandemic-induced hiatus, the American Gastroenterological Association’s Tech Summit returned to a live meeting in San Francisco. As usual, the highlight of the 2-day event, which is sponsored by the AGA Center for GI Innovation and Technology, was the Shark Tank, where selected companies presented lightning-round overviews of their technology and business plans. A panel of sharks and the audience voted for their favorite.
The contestants presented technologies such as a cell phone app to improve gut health (Agora Health), a polypectomy suite (IzoMed), an implantable weight-loss device (Lean Medical), a device to alleviate gastric obstruction in pancreatic cancer (Myka Labs), a pill designed to map out the gastrointestinal system to aid in diagnosis (Rock West Medical Devices), and an endoscopic ultrasound device (EndoSound).
Six finalists were selected from 20 submissions, and EndoSound was the winner. According to Raman Muthusamy, MD, medical director of endoscopy at UCLA Health and professor of clinical medicine at the University of California, Los Angeles, and past chair of the AGA Center for GI Innovation and Technology, the quality of presentations and the sophistication of the companies have increased year after year. “This was really the very best,” said Dr. Muthusamy.
Both the judges and the audience chose EndoSound. Endoscopic ultrasound (EUS) focuses on diagnosis and treatment of chest and abdomen disorders, particularly the pancreas. The EndoSound device attaches to an upper endoscope and converts it to a fully therapeutic endoscope that can perform all standard EUS procedures. Moreover, it does not use an elevator, which has been linked to infection risk.
Most clinical facilities lack EUS capability: 97% of ambulatory surgical centers and 80% of hospitals. EUS systems have hardly changed since the late 20th century, and they cost about $450,000. The projected cost of the EndoSound device is closer to $50,000.
“Just like colonoscopies and upper endoscopies, most endoscopic ultrasounds ought to be done in surgical centers. The idea that they can do them efficiently, and at lower cost and greater convenience to their patients and themselves, seems to me the way everything is going, and the way this procedure ought to go as well. The only obstacle to that has been the cost of the equipment. If we can take away that obstacle, then people who are already doing procedures in hospitals where it’s not convenient and not efficient, will be able to do the procedures in surgical centers,” said Stephen Steinberg, MD, founder and President of EndoSound.
“It’s a radical redesign. You’ve cut cost and you’ve cut space. And it’s something that could be put on at a moment’s notice. Rather than referring the patient for [ultrasound], it could allow you to do it on the spot, and perhaps save a second trip for a patient. It allows flexibility in terms of site of service,” said Dr. Muthusamy.
Dr. Muthusamy called it a “godsend” for low-resource institutions in the United States or abroad who have the expertise, but not the equipment, to perform EUS. “There’s no question that more EUS procedures could be done than are currently being done because of issues of availability, and this device takes a significant step to alleviate that.”
The Food and Drug Administration has granted a breakthrough device designation to EndoSound, which allows the company to forgo human clinical trials to support the application. “We’re hoping and expecting to have our application in the beginning of the fourth quarter, and with a little bit of luck to be approved by the end of the year. That’s our goal,” said Dr. Steinberg.
The technology started out as a challenge that Dr. Steinberg set for himself. His career overlapped with some of the earliest innovators of therapeutic endoscopy. “They were the stars. I wasn’t, but I was there,” said Dr. Steinberg. In his practice, Dr. Steinberg was doing procedures that included endoscopic ultrasound.
By the new millennium, EUS had gained a lot of interest, but there was a problem. “It was expensive, and it could only be done in hospitals. I started wondering if we couldn’t get it into a different environment by having a simpler solution,” said Dr. Steinberg.
But success didn’t come quickly. “I started drawing on the back of napkins to see if there wasn’t some solution,” said Dr. Steinberg. It wasn’t until a serendipitous meeting occurred that the concept took shape. Dr. Steinberg’s wife was the CEO and provost of Oregon Health Sciences University, Portland, as well as head of the technology transfer program. Dr. Steinberg’s practice, however, was in Florida so he commuted to Oregon every weekend.
One day, she told him about a presentation by Scott Corbett, MD. “My wife said: ‘Hey, they’re doing ultrasound. Why don’t you come and sit in [on the meeting] because I don’t know anything about it.’ [Dr.] Corbett was working with Sonivate, a point-of-care ultrasound company that was developing an ultrasound that could be placed over the end of the finger, to be used in battlefield triage. I thought, well, if you could put it on a finger, why couldn’t you put it on a scope? So, Scott and I got to talking, and went through a couple of iterations that didn’t work, and then finally came up with one that seemed like it was suitable.”
The device has been tested in five animal models with 20 EUS physicians who concluded that the images were equivalent to legacy devices and that they could be adopted quickly. The company also presented results from a human study that demonstrated noninferiority to the latest EUS system from Pentax.
Dr. Steinberg is an employee and stockholder of Sonivate. Dr. Muthusamy has no relevant financial disclosures. The 2022 AGA Tech Summit was supported by independent grants from Castle Biosciences, Medtronic, Boston Scientific, Exact Sciences, Olympus, 3-D Matrix, Apollo Endosurgery, Motus GI Holdings, STERIS Endoscopy, Cook Medical, FUJIFILM Healthcare Americas, and Virgo.
This article was updated 5/10/22.
*Correction, 5/17/22: An earlier version of this article stated that Geneoscopy was a finalist in the competition. It was not. Also, EndoSound should have been listed as a finalist in this paragraph.
After a 3-year, pandemic-induced hiatus, the American Gastroenterological Association’s Tech Summit returned to a live meeting in San Francisco. As usual, the highlight of the 2-day event, which is sponsored by the AGA Center for GI Innovation and Technology, was the Shark Tank, where selected companies presented lightning-round overviews of their technology and business plans. A panel of sharks and the audience voted for their favorite.
The contestants presented technologies such as a cell phone app to improve gut health (Agora Health), a polypectomy suite (IzoMed), an implantable weight-loss device (Lean Medical), a device to alleviate gastric obstruction in pancreatic cancer (Myka Labs), a pill designed to map out the gastrointestinal system to aid in diagnosis (Rock West Medical Devices), and an endoscopic ultrasound device (EndoSound).
Six finalists were selected from 20 submissions, and EndoSound was the winner. According to Raman Muthusamy, MD, medical director of endoscopy at UCLA Health and professor of clinical medicine at the University of California, Los Angeles, and past chair of the AGA Center for GI Innovation and Technology, the quality of presentations and the sophistication of the companies have increased year after year. “This was really the very best,” said Dr. Muthusamy.
Both the judges and the audience chose EndoSound. Endoscopic ultrasound (EUS) focuses on diagnosis and treatment of chest and abdomen disorders, particularly the pancreas. The EndoSound device attaches to an upper endoscope and converts it to a fully therapeutic endoscope that can perform all standard EUS procedures. Moreover, it does not use an elevator, which has been linked to infection risk.
Most clinical facilities lack EUS capability: 97% of ambulatory surgical centers and 80% of hospitals. EUS systems have hardly changed since the late 20th century, and they cost about $450,000. The projected cost of the EndoSound device is closer to $50,000.
“Just like colonoscopies and upper endoscopies, most endoscopic ultrasounds ought to be done in surgical centers. The idea that they can do them efficiently, and at lower cost and greater convenience to their patients and themselves, seems to me the way everything is going, and the way this procedure ought to go as well. The only obstacle to that has been the cost of the equipment. If we can take away that obstacle, then people who are already doing procedures in hospitals where it’s not convenient and not efficient, will be able to do the procedures in surgical centers,” said Stephen Steinberg, MD, founder and President of EndoSound.
“It’s a radical redesign. You’ve cut cost and you’ve cut space. And it’s something that could be put on at a moment’s notice. Rather than referring the patient for [ultrasound], it could allow you to do it on the spot, and perhaps save a second trip for a patient. It allows flexibility in terms of site of service,” said Dr. Muthusamy.
Dr. Muthusamy called it a “godsend” for low-resource institutions in the United States or abroad who have the expertise, but not the equipment, to perform EUS. “There’s no question that more EUS procedures could be done than are currently being done because of issues of availability, and this device takes a significant step to alleviate that.”
The Food and Drug Administration has granted a breakthrough device designation to EndoSound, which allows the company to forgo human clinical trials to support the application. “We’re hoping and expecting to have our application in the beginning of the fourth quarter, and with a little bit of luck to be approved by the end of the year. That’s our goal,” said Dr. Steinberg.
The technology started out as a challenge that Dr. Steinberg set for himself. His career overlapped with some of the earliest innovators of therapeutic endoscopy. “They were the stars. I wasn’t, but I was there,” said Dr. Steinberg. In his practice, Dr. Steinberg was doing procedures that included endoscopic ultrasound.
By the new millennium, EUS had gained a lot of interest, but there was a problem. “It was expensive, and it could only be done in hospitals. I started wondering if we couldn’t get it into a different environment by having a simpler solution,” said Dr. Steinberg.
But success didn’t come quickly. “I started drawing on the back of napkins to see if there wasn’t some solution,” said Dr. Steinberg. It wasn’t until a serendipitous meeting occurred that the concept took shape. Dr. Steinberg’s wife was the CEO and provost of Oregon Health Sciences University, Portland, as well as head of the technology transfer program. Dr. Steinberg’s practice, however, was in Florida so he commuted to Oregon every weekend.
One day, she told him about a presentation by Scott Corbett, MD. “My wife said: ‘Hey, they’re doing ultrasound. Why don’t you come and sit in [on the meeting] because I don’t know anything about it.’ [Dr.] Corbett was working with Sonivate, a point-of-care ultrasound company that was developing an ultrasound that could be placed over the end of the finger, to be used in battlefield triage. I thought, well, if you could put it on a finger, why couldn’t you put it on a scope? So, Scott and I got to talking, and went through a couple of iterations that didn’t work, and then finally came up with one that seemed like it was suitable.”
The device has been tested in five animal models with 20 EUS physicians who concluded that the images were equivalent to legacy devices and that they could be adopted quickly. The company also presented results from a human study that demonstrated noninferiority to the latest EUS system from Pentax.
Dr. Steinberg is an employee and stockholder of Sonivate. Dr. Muthusamy has no relevant financial disclosures. The 2022 AGA Tech Summit was supported by independent grants from Castle Biosciences, Medtronic, Boston Scientific, Exact Sciences, Olympus, 3-D Matrix, Apollo Endosurgery, Motus GI Holdings, STERIS Endoscopy, Cook Medical, FUJIFILM Healthcare Americas, and Virgo.
This article was updated 5/10/22.
*Correction, 5/17/22: An earlier version of this article stated that Geneoscopy was a finalist in the competition. It was not. Also, EndoSound should have been listed as a finalist in this paragraph.
After a 3-year, pandemic-induced hiatus, the American Gastroenterological Association’s Tech Summit returned to a live meeting in San Francisco. As usual, the highlight of the 2-day event, which is sponsored by the AGA Center for GI Innovation and Technology, was the Shark Tank, where selected companies presented lightning-round overviews of their technology and business plans. A panel of sharks and the audience voted for their favorite.
The contestants presented technologies such as a cell phone app to improve gut health (Agora Health), a polypectomy suite (IzoMed), an implantable weight-loss device (Lean Medical), a device to alleviate gastric obstruction in pancreatic cancer (Myka Labs), a pill designed to map out the gastrointestinal system to aid in diagnosis (Rock West Medical Devices), and an endoscopic ultrasound device (EndoSound).
Six finalists were selected from 20 submissions, and EndoSound was the winner. According to Raman Muthusamy, MD, medical director of endoscopy at UCLA Health and professor of clinical medicine at the University of California, Los Angeles, and past chair of the AGA Center for GI Innovation and Technology, the quality of presentations and the sophistication of the companies have increased year after year. “This was really the very best,” said Dr. Muthusamy.
Both the judges and the audience chose EndoSound. Endoscopic ultrasound (EUS) focuses on diagnosis and treatment of chest and abdomen disorders, particularly the pancreas. The EndoSound device attaches to an upper endoscope and converts it to a fully therapeutic endoscope that can perform all standard EUS procedures. Moreover, it does not use an elevator, which has been linked to infection risk.
Most clinical facilities lack EUS capability: 97% of ambulatory surgical centers and 80% of hospitals. EUS systems have hardly changed since the late 20th century, and they cost about $450,000. The projected cost of the EndoSound device is closer to $50,000.
“Just like colonoscopies and upper endoscopies, most endoscopic ultrasounds ought to be done in surgical centers. The idea that they can do them efficiently, and at lower cost and greater convenience to their patients and themselves, seems to me the way everything is going, and the way this procedure ought to go as well. The only obstacle to that has been the cost of the equipment. If we can take away that obstacle, then people who are already doing procedures in hospitals where it’s not convenient and not efficient, will be able to do the procedures in surgical centers,” said Stephen Steinberg, MD, founder and President of EndoSound.
“It’s a radical redesign. You’ve cut cost and you’ve cut space. And it’s something that could be put on at a moment’s notice. Rather than referring the patient for [ultrasound], it could allow you to do it on the spot, and perhaps save a second trip for a patient. It allows flexibility in terms of site of service,” said Dr. Muthusamy.
Dr. Muthusamy called it a “godsend” for low-resource institutions in the United States or abroad who have the expertise, but not the equipment, to perform EUS. “There’s no question that more EUS procedures could be done than are currently being done because of issues of availability, and this device takes a significant step to alleviate that.”
The Food and Drug Administration has granted a breakthrough device designation to EndoSound, which allows the company to forgo human clinical trials to support the application. “We’re hoping and expecting to have our application in the beginning of the fourth quarter, and with a little bit of luck to be approved by the end of the year. That’s our goal,” said Dr. Steinberg.
The technology started out as a challenge that Dr. Steinberg set for himself. His career overlapped with some of the earliest innovators of therapeutic endoscopy. “They were the stars. I wasn’t, but I was there,” said Dr. Steinberg. In his practice, Dr. Steinberg was doing procedures that included endoscopic ultrasound.
By the new millennium, EUS had gained a lot of interest, but there was a problem. “It was expensive, and it could only be done in hospitals. I started wondering if we couldn’t get it into a different environment by having a simpler solution,” said Dr. Steinberg.
But success didn’t come quickly. “I started drawing on the back of napkins to see if there wasn’t some solution,” said Dr. Steinberg. It wasn’t until a serendipitous meeting occurred that the concept took shape. Dr. Steinberg’s wife was the CEO and provost of Oregon Health Sciences University, Portland, as well as head of the technology transfer program. Dr. Steinberg’s practice, however, was in Florida so he commuted to Oregon every weekend.
One day, she told him about a presentation by Scott Corbett, MD. “My wife said: ‘Hey, they’re doing ultrasound. Why don’t you come and sit in [on the meeting] because I don’t know anything about it.’ [Dr.] Corbett was working with Sonivate, a point-of-care ultrasound company that was developing an ultrasound that could be placed over the end of the finger, to be used in battlefield triage. I thought, well, if you could put it on a finger, why couldn’t you put it on a scope? So, Scott and I got to talking, and went through a couple of iterations that didn’t work, and then finally came up with one that seemed like it was suitable.”
The device has been tested in five animal models with 20 EUS physicians who concluded that the images were equivalent to legacy devices and that they could be adopted quickly. The company also presented results from a human study that demonstrated noninferiority to the latest EUS system from Pentax.
Dr. Steinberg is an employee and stockholder of Sonivate. Dr. Muthusamy has no relevant financial disclosures. The 2022 AGA Tech Summit was supported by independent grants from Castle Biosciences, Medtronic, Boston Scientific, Exact Sciences, Olympus, 3-D Matrix, Apollo Endosurgery, Motus GI Holdings, STERIS Endoscopy, Cook Medical, FUJIFILM Healthcare Americas, and Virgo.
This article was updated 5/10/22.
*Correction, 5/17/22: An earlier version of this article stated that Geneoscopy was a finalist in the competition. It was not. Also, EndoSound should have been listed as a finalist in this paragraph.
FROM 2022 AGA TECH SUMMIT
Endoscopic obesity treatments offer alternatives to surgery
SAN FRANCISCO – Endoscopic treatments for obesity are under-utilized but represent an opportunity for gastroenterologists to help address the metabolic epidemic that affects up to 40% of people in the United States, according to a presentation reviewing these techniques.
Lifestyle modification is the first intervention, but results in just a 5% average weight loss, according to Allison Schulman, MD, MPH, who discussed these options at the 2022 AGA Tech Summit sponsored by the AGA Center for GI Innovation and Technology. Although surgical interventions induce more weight loss and greater improvement of metabolic outcomes, they come with significant risks and many patients are reluctant to pursue them, she added. In fact, fewer than 1% of obese individuals who qualify for bariatric surgery ultimately undergo it.
Dr. Schulman emphasized another option: Endoscopic bariatric therapies fill this void in between those two extremes, as they are clearly less invasive” said Dr. Schulman, who is an assistant professor of gastroenterology and hepatology at the University of Michigan, Ann Arbor. “They may appeal to those who do not qualify or do not want bariatric surgery. They also could bridge a critical gap in the treatment of obesity, as they reach patients earlier, at BMIs [body mass indexes] where they may not be surgical candidates. Furthermore, these therapies are oftentimes repeatable and commonly can be used in combination [with other weight loss approaches].”
Endoscopic therapies for obesity include devices that occupy space in the stomach, such as intragastric balloons, gastric remodeling procedures like endoscopic sleeve gastroplasty (ESG), and aspiration therapy.
Potential candidates for noninvasive approaches include patients with a BMI over 30 kg/m2 who have not lost sufficient weight through nonsurgical methods or those who do not want to undergo surgery or require a bridge therapy to surgery.
Fluid-filled balloons can be placed and filled to an appropriate volume. One network meta-analysis found that fluid-filled balloons were more likely to lead to weight loss, but also more likely to be removed due to intolerance. She also noted that the Elipse balloon (Allurion Technologies) is designed to be swallowed and thus avoid procedures entirely; it is currently under review by Food and Drug Administration.
Although balloons are linked to 7%-10% weight loss in some studies and reviews, Dr. Schulman said, “we know … that the majority of these lead to much more weight loss in clinical practice, oftentimes closer to 13%-%15.”
One review found that balloons also lead to improvement in obesity-related comorbidities, compared with conventional nonsurgical approaches, and this benefit extends past 1 year. A study of 21 patients with nonalcoholic steatohepatitis (NASH) treated with intragastric balloons found that 90% had an improvement in nonalcoholic fatty liver disease activity score, with a median drop of 3 points, and 80% had a drop of at least 2 points. Of these patients, 50% also had an improvement in fibrosis determined by magnetic resonance elastography.
Balloon therapy should be highly individualized, according to Dr. Schulman.
Dr. Schulman also described ESG, which uses sutures to remodel the stomach and reduce volume by up to 70%. She outlined studies and reviews, such as those from Sharaiha and colleagues and Hedjoudje and colleagues, showing that ESG leads to significant and sustained weight loss. The procedure was also quite safe, with one large, single-center study showing that both fever and significant blood loss each occurred in less than 1% of patients (Gastrointest Endosc. 2019 Jun;89[6]:1132-8), while the systematic review and meta-analysis from Hedjoudje and colleagues found an adverse event frequency of 2.2%.
In a matched control study, laparoscopic sleeve gastrectomy led to more weight loss, but ESG had fewer adverse events (5.2% versus 16.9%; P < .01) and had a greater effect on gastroesophageal reflux disease.
ESG can be effective when repeated, while surgical revisions are associated with much higher morbidity, according to Dr. Schulman.
During her presentation, Dr. Schulman mentioned the AspireAssist device developed by Aspire Bariatrics, which is similar to a percutaneous endoscopic gastrostomy (PEG) tube. It leads to the removal of about 30% of calories consumed during a meal, with patients instructed to aspirate 20-30 minutes after a meal, two to three times a day. It gained Food and Drug Administration approval on the strength of the PATHWAY study, which showed significant weight loss.
“But perhaps more impressive is the overall patient satisfaction and willingness to recommend this device to others,” said Dr. Schulman.
Another approach she described is the transpyloric shuttle (TPS), which leads to faster filling times and delayed gastric emptying, though it must be removed endoscopically at 12 months.
Dr. Schulman also discussed endoscopic bariatric and metabolic therapy. This approach is currently a primary therapy for obesity, and is in development for the treatment of diabetes and non-alcoholic fatty liver disease. The approach is predicated on the idea that obesity is a disorder of energy homeostasis, and that enteric neurons in the small bowel are key players, possibly through reduced production of as yet unknown signaling molecules, leading to insulin resistance. It’s also known that diets high in fat and sugar alter the duodenum, which causes changes in nutrient signaling to the brain.
“It’s thought that this leads to duodenal endocrine hyperactivity and ultimately metabolic disease,” said Dr. Schulman.
Finally, she described small-bowel therapies like endobarrier sleeves, duodenal mucosal resurfacing, and an incisionless anastomosis system designed to improve glycemic control by altering the gut through noninvasive means.
Dr. Schulman has consulted for Apollo Endosurgery, Boston Scientific, Olympus, and MicroTech, and has received research support from GI Dynamics.
SAN FRANCISCO – Endoscopic treatments for obesity are under-utilized but represent an opportunity for gastroenterologists to help address the metabolic epidemic that affects up to 40% of people in the United States, according to a presentation reviewing these techniques.
Lifestyle modification is the first intervention, but results in just a 5% average weight loss, according to Allison Schulman, MD, MPH, who discussed these options at the 2022 AGA Tech Summit sponsored by the AGA Center for GI Innovation and Technology. Although surgical interventions induce more weight loss and greater improvement of metabolic outcomes, they come with significant risks and many patients are reluctant to pursue them, she added. In fact, fewer than 1% of obese individuals who qualify for bariatric surgery ultimately undergo it.
Dr. Schulman emphasized another option: Endoscopic bariatric therapies fill this void in between those two extremes, as they are clearly less invasive” said Dr. Schulman, who is an assistant professor of gastroenterology and hepatology at the University of Michigan, Ann Arbor. “They may appeal to those who do not qualify or do not want bariatric surgery. They also could bridge a critical gap in the treatment of obesity, as they reach patients earlier, at BMIs [body mass indexes] where they may not be surgical candidates. Furthermore, these therapies are oftentimes repeatable and commonly can be used in combination [with other weight loss approaches].”
Endoscopic therapies for obesity include devices that occupy space in the stomach, such as intragastric balloons, gastric remodeling procedures like endoscopic sleeve gastroplasty (ESG), and aspiration therapy.
Potential candidates for noninvasive approaches include patients with a BMI over 30 kg/m2 who have not lost sufficient weight through nonsurgical methods or those who do not want to undergo surgery or require a bridge therapy to surgery.
Fluid-filled balloons can be placed and filled to an appropriate volume. One network meta-analysis found that fluid-filled balloons were more likely to lead to weight loss, but also more likely to be removed due to intolerance. She also noted that the Elipse balloon (Allurion Technologies) is designed to be swallowed and thus avoid procedures entirely; it is currently under review by Food and Drug Administration.
Although balloons are linked to 7%-10% weight loss in some studies and reviews, Dr. Schulman said, “we know … that the majority of these lead to much more weight loss in clinical practice, oftentimes closer to 13%-%15.”
One review found that balloons also lead to improvement in obesity-related comorbidities, compared with conventional nonsurgical approaches, and this benefit extends past 1 year. A study of 21 patients with nonalcoholic steatohepatitis (NASH) treated with intragastric balloons found that 90% had an improvement in nonalcoholic fatty liver disease activity score, with a median drop of 3 points, and 80% had a drop of at least 2 points. Of these patients, 50% also had an improvement in fibrosis determined by magnetic resonance elastography.
Balloon therapy should be highly individualized, according to Dr. Schulman.
Dr. Schulman also described ESG, which uses sutures to remodel the stomach and reduce volume by up to 70%. She outlined studies and reviews, such as those from Sharaiha and colleagues and Hedjoudje and colleagues, showing that ESG leads to significant and sustained weight loss. The procedure was also quite safe, with one large, single-center study showing that both fever and significant blood loss each occurred in less than 1% of patients (Gastrointest Endosc. 2019 Jun;89[6]:1132-8), while the systematic review and meta-analysis from Hedjoudje and colleagues found an adverse event frequency of 2.2%.
In a matched control study, laparoscopic sleeve gastrectomy led to more weight loss, but ESG had fewer adverse events (5.2% versus 16.9%; P < .01) and had a greater effect on gastroesophageal reflux disease.
ESG can be effective when repeated, while surgical revisions are associated with much higher morbidity, according to Dr. Schulman.
During her presentation, Dr. Schulman mentioned the AspireAssist device developed by Aspire Bariatrics, which is similar to a percutaneous endoscopic gastrostomy (PEG) tube. It leads to the removal of about 30% of calories consumed during a meal, with patients instructed to aspirate 20-30 minutes after a meal, two to three times a day. It gained Food and Drug Administration approval on the strength of the PATHWAY study, which showed significant weight loss.
“But perhaps more impressive is the overall patient satisfaction and willingness to recommend this device to others,” said Dr. Schulman.
Another approach she described is the transpyloric shuttle (TPS), which leads to faster filling times and delayed gastric emptying, though it must be removed endoscopically at 12 months.
Dr. Schulman also discussed endoscopic bariatric and metabolic therapy. This approach is currently a primary therapy for obesity, and is in development for the treatment of diabetes and non-alcoholic fatty liver disease. The approach is predicated on the idea that obesity is a disorder of energy homeostasis, and that enteric neurons in the small bowel are key players, possibly through reduced production of as yet unknown signaling molecules, leading to insulin resistance. It’s also known that diets high in fat and sugar alter the duodenum, which causes changes in nutrient signaling to the brain.
“It’s thought that this leads to duodenal endocrine hyperactivity and ultimately metabolic disease,” said Dr. Schulman.
Finally, she described small-bowel therapies like endobarrier sleeves, duodenal mucosal resurfacing, and an incisionless anastomosis system designed to improve glycemic control by altering the gut through noninvasive means.
Dr. Schulman has consulted for Apollo Endosurgery, Boston Scientific, Olympus, and MicroTech, and has received research support from GI Dynamics.
SAN FRANCISCO – Endoscopic treatments for obesity are under-utilized but represent an opportunity for gastroenterologists to help address the metabolic epidemic that affects up to 40% of people in the United States, according to a presentation reviewing these techniques.
Lifestyle modification is the first intervention, but results in just a 5% average weight loss, according to Allison Schulman, MD, MPH, who discussed these options at the 2022 AGA Tech Summit sponsored by the AGA Center for GI Innovation and Technology. Although surgical interventions induce more weight loss and greater improvement of metabolic outcomes, they come with significant risks and many patients are reluctant to pursue them, she added. In fact, fewer than 1% of obese individuals who qualify for bariatric surgery ultimately undergo it.
Dr. Schulman emphasized another option: Endoscopic bariatric therapies fill this void in between those two extremes, as they are clearly less invasive” said Dr. Schulman, who is an assistant professor of gastroenterology and hepatology at the University of Michigan, Ann Arbor. “They may appeal to those who do not qualify or do not want bariatric surgery. They also could bridge a critical gap in the treatment of obesity, as they reach patients earlier, at BMIs [body mass indexes] where they may not be surgical candidates. Furthermore, these therapies are oftentimes repeatable and commonly can be used in combination [with other weight loss approaches].”
Endoscopic therapies for obesity include devices that occupy space in the stomach, such as intragastric balloons, gastric remodeling procedures like endoscopic sleeve gastroplasty (ESG), and aspiration therapy.
Potential candidates for noninvasive approaches include patients with a BMI over 30 kg/m2 who have not lost sufficient weight through nonsurgical methods or those who do not want to undergo surgery or require a bridge therapy to surgery.
Fluid-filled balloons can be placed and filled to an appropriate volume. One network meta-analysis found that fluid-filled balloons were more likely to lead to weight loss, but also more likely to be removed due to intolerance. She also noted that the Elipse balloon (Allurion Technologies) is designed to be swallowed and thus avoid procedures entirely; it is currently under review by Food and Drug Administration.
Although balloons are linked to 7%-10% weight loss in some studies and reviews, Dr. Schulman said, “we know … that the majority of these lead to much more weight loss in clinical practice, oftentimes closer to 13%-%15.”
One review found that balloons also lead to improvement in obesity-related comorbidities, compared with conventional nonsurgical approaches, and this benefit extends past 1 year. A study of 21 patients with nonalcoholic steatohepatitis (NASH) treated with intragastric balloons found that 90% had an improvement in nonalcoholic fatty liver disease activity score, with a median drop of 3 points, and 80% had a drop of at least 2 points. Of these patients, 50% also had an improvement in fibrosis determined by magnetic resonance elastography.
Balloon therapy should be highly individualized, according to Dr. Schulman.
Dr. Schulman also described ESG, which uses sutures to remodel the stomach and reduce volume by up to 70%. She outlined studies and reviews, such as those from Sharaiha and colleagues and Hedjoudje and colleagues, showing that ESG leads to significant and sustained weight loss. The procedure was also quite safe, with one large, single-center study showing that both fever and significant blood loss each occurred in less than 1% of patients (Gastrointest Endosc. 2019 Jun;89[6]:1132-8), while the systematic review and meta-analysis from Hedjoudje and colleagues found an adverse event frequency of 2.2%.
In a matched control study, laparoscopic sleeve gastrectomy led to more weight loss, but ESG had fewer adverse events (5.2% versus 16.9%; P < .01) and had a greater effect on gastroesophageal reflux disease.
ESG can be effective when repeated, while surgical revisions are associated with much higher morbidity, according to Dr. Schulman.
During her presentation, Dr. Schulman mentioned the AspireAssist device developed by Aspire Bariatrics, which is similar to a percutaneous endoscopic gastrostomy (PEG) tube. It leads to the removal of about 30% of calories consumed during a meal, with patients instructed to aspirate 20-30 minutes after a meal, two to three times a day. It gained Food and Drug Administration approval on the strength of the PATHWAY study, which showed significant weight loss.
“But perhaps more impressive is the overall patient satisfaction and willingness to recommend this device to others,” said Dr. Schulman.
Another approach she described is the transpyloric shuttle (TPS), which leads to faster filling times and delayed gastric emptying, though it must be removed endoscopically at 12 months.
Dr. Schulman also discussed endoscopic bariatric and metabolic therapy. This approach is currently a primary therapy for obesity, and is in development for the treatment of diabetes and non-alcoholic fatty liver disease. The approach is predicated on the idea that obesity is a disorder of energy homeostasis, and that enteric neurons in the small bowel are key players, possibly through reduced production of as yet unknown signaling molecules, leading to insulin resistance. It’s also known that diets high in fat and sugar alter the duodenum, which causes changes in nutrient signaling to the brain.
“It’s thought that this leads to duodenal endocrine hyperactivity and ultimately metabolic disease,” said Dr. Schulman.
Finally, she described small-bowel therapies like endobarrier sleeves, duodenal mucosal resurfacing, and an incisionless anastomosis system designed to improve glycemic control by altering the gut through noninvasive means.
Dr. Schulman has consulted for Apollo Endosurgery, Boston Scientific, Olympus, and MicroTech, and has received research support from GI Dynamics.
AT 2022 AGA TECH SUMMIT
Weighing the pros and cons of disposable duodenoscopes
Disposable duodenoscopes have one irrefutable advantage over their reusable counterparts: They definitively solve the problem of scope-related multidrug-resistant organism (MDRO) infections. Yet they also come with trade-offs, such as increased cost and medical waste, which has triggered pushback from skeptical endoscopists. How endoscopists weigh their differing concerns will ultimately determine the uptake of these devices going forward, according to Andrew S. Ross, MD, medical director for strategic growth at Virginia Mason Medical Center, Seattle.
“What would you pay to not have to deal with the scope infection issue at all?” Dr. Ross asked during a virtual presentation at the 2021 AGA Tech Summit sponsored by the AGA Center for GI Innovation and Technology. “I think that x-factor is going to depend [on] who you’re talking to and how much they really believe in [duodenoscope-related infection] as an issue.”
Dr. Ross explained that some endoscopists doubt the clinical relevance of duodenoscope-related MDRO infections, possibly because of a lack of direct experience.
“There still is a prevailing sentiment among some endoscopists that duodenoscope infection is really not a problem,” Dr. Ross said. “Or [they may say,]: ‘We haven’t had that issue here in our medical center, so therefore it is not a problem.’ ”
In fact, the exact magnitude of the problem remains unknown.
“In the end, we have an unquantifiable risk to patients wherever [reusable duodenoscopes] are used,” Dr. Ross said.
Just how common are scope-related MDRO infections?
According to V. Raman Muthusamy, MD, AGAF, immediate former chair of the AGA Center for GI Innovation and Technology, and director of endoscopy at the University of California, Los Angeles Health System, scope-related MDRO infections are “relatively uncommon,” but they do occur.
MDRO infections are generally linked with contaminated endoscopes, but duodenoscopes are the most common culprit because they pose a unique risk.
“Traditionally, when outbreaks have occurred [with nonduodenoscopes], it has usually been due to a breach in the reprocessing protocol,” Dr. Muthusamy said in an interview. “But with duodenoscopes, we’ve found that that does not appear to be necessary, and that in many cases there are no identified breaches, and yet there are still outbreaks.”
Dr. Muthusamy, the first endoscopist to test a disposable duodenoscope in a human patient, noted that it’s challenging to definitively prove infection from a reusable scope. Citing an Executive Summary from the Food and Drug Administration, he said, “We know it’s happened 300-400 times over the past decade or so,” with infection rates peaking in 2014-2016 and steadily declining since then.
Approximately 5% of reprocessed duodenoscopes harbor pathogenic bacteria, according to Dr. Muthusamy, but the rate of infection is significantly lower.
“[The use of a contaminated duodenoscope] doesn’t mean a patient will actually get sick ... but it does mean the potential exists, obviously,” he said. “It just shows that these devices are hard to clean and a fraction of people have the potential of becoming ill. It’s our goal to improve on those numbers, and really try to eliminate the risk of this problem, as best we can.”
Infection isn’t the only concern
There are several potential ways to tackle the issue of scope-related infections, Dr. Ross said during his presentation, including designing devices that are easier to clean and optimizing the cleaning process; however, the only definitive solution is to eliminate cleaning altogether.
This is where disposable duodenoscopes come in.
At present, there are two such FDA-approved devices, the Ascope Duodeno from Ambu and the Exalt Model D from Boston Scientific, both of which Dr. Ross characterized as being “in their infancy.”
Studies testing the Exalt Model D suggest that performance compares favorably with reusable duodenoscopes.
“The scope works in a benchtop model, it works in a lab, and it seems to be functional in expert hands,” Dr. Ross said. “With inexperienced users, we also see that this device works, albeit with a rate of crossover that may approach up to 10%. So, a functional, disposable scope has been produced.”
Despite availability, several pain points may slow adoption, Dr. Ross said, including reluctance to use new technology, skepticism about the clinical impact of scope-related infections, environmental concerns of increased medical waste, and increased cost.
On this latter topic, Dr. Ross pointed out that the true cost of a reusable scope goes beyond the purchase or lease price to include repair costs, reprocessing costs, and, potentially, the cost of litigation from scope-related infection.
“If you have an outbreak in your medical center, you can rest assured that you will have some litigation exposure,” Dr. Ross said.
Fitting disposable duodenoscopes into routine practice
Currently, both FDA-approved disposable duodenoscopes are covered by outpatient pass-through reimbursement for Medicare, and in October, both will be covered on an inpatient basis, according to Dr. Ross.
“I think the big question regarding pass-through reimbursement is what happens when the codes get revalued,” he said. “How long will the additional reimbursement stay in place?”
For now, Dr. Ross suggested that endoscopists reach for disposable duodenoscopes in unique scenarios, such as weekend or night procedures, to avoid calling in a scope-reprocessing technician; or in operating room cases when the scope enters a sterile field. Disposable scopes should also be considered for patients with known MDROs, he added, and conversely, for patients who are immunocompromised or critically ill and “can least afford a scope-related infection.”
Ultimately, the role of disposable duodenoscopes may be decided by the patients themselves, Dr. Ross concluded.
“Certainly, patients know about this – they may come in and demand the use of a single-use scope in certain situations,” Dr. Ross said. “We have to remember when we’re bringing any new technology into the marketplace that while it’s important to understand the input and perspectives of multiple stakeholders, the single-most important stakeholder at the end of the day are our patients.”
Dr. Ross disclosed a relationship with Boston Scientific. Dr. Muthusamy disclosed a relationship with Boston Scientific and Medivators.
Disposable duodenoscopes have one irrefutable advantage over their reusable counterparts: They definitively solve the problem of scope-related multidrug-resistant organism (MDRO) infections. Yet they also come with trade-offs, such as increased cost and medical waste, which has triggered pushback from skeptical endoscopists. How endoscopists weigh their differing concerns will ultimately determine the uptake of these devices going forward, according to Andrew S. Ross, MD, medical director for strategic growth at Virginia Mason Medical Center, Seattle.
“What would you pay to not have to deal with the scope infection issue at all?” Dr. Ross asked during a virtual presentation at the 2021 AGA Tech Summit sponsored by the AGA Center for GI Innovation and Technology. “I think that x-factor is going to depend [on] who you’re talking to and how much they really believe in [duodenoscope-related infection] as an issue.”
Dr. Ross explained that some endoscopists doubt the clinical relevance of duodenoscope-related MDRO infections, possibly because of a lack of direct experience.
“There still is a prevailing sentiment among some endoscopists that duodenoscope infection is really not a problem,” Dr. Ross said. “Or [they may say,]: ‘We haven’t had that issue here in our medical center, so therefore it is not a problem.’ ”
In fact, the exact magnitude of the problem remains unknown.
“In the end, we have an unquantifiable risk to patients wherever [reusable duodenoscopes] are used,” Dr. Ross said.
Just how common are scope-related MDRO infections?
According to V. Raman Muthusamy, MD, AGAF, immediate former chair of the AGA Center for GI Innovation and Technology, and director of endoscopy at the University of California, Los Angeles Health System, scope-related MDRO infections are “relatively uncommon,” but they do occur.
MDRO infections are generally linked with contaminated endoscopes, but duodenoscopes are the most common culprit because they pose a unique risk.
“Traditionally, when outbreaks have occurred [with nonduodenoscopes], it has usually been due to a breach in the reprocessing protocol,” Dr. Muthusamy said in an interview. “But with duodenoscopes, we’ve found that that does not appear to be necessary, and that in many cases there are no identified breaches, and yet there are still outbreaks.”
Dr. Muthusamy, the first endoscopist to test a disposable duodenoscope in a human patient, noted that it’s challenging to definitively prove infection from a reusable scope. Citing an Executive Summary from the Food and Drug Administration, he said, “We know it’s happened 300-400 times over the past decade or so,” with infection rates peaking in 2014-2016 and steadily declining since then.
Approximately 5% of reprocessed duodenoscopes harbor pathogenic bacteria, according to Dr. Muthusamy, but the rate of infection is significantly lower.
“[The use of a contaminated duodenoscope] doesn’t mean a patient will actually get sick ... but it does mean the potential exists, obviously,” he said. “It just shows that these devices are hard to clean and a fraction of people have the potential of becoming ill. It’s our goal to improve on those numbers, and really try to eliminate the risk of this problem, as best we can.”
Infection isn’t the only concern
There are several potential ways to tackle the issue of scope-related infections, Dr. Ross said during his presentation, including designing devices that are easier to clean and optimizing the cleaning process; however, the only definitive solution is to eliminate cleaning altogether.
This is where disposable duodenoscopes come in.
At present, there are two such FDA-approved devices, the Ascope Duodeno from Ambu and the Exalt Model D from Boston Scientific, both of which Dr. Ross characterized as being “in their infancy.”
Studies testing the Exalt Model D suggest that performance compares favorably with reusable duodenoscopes.
“The scope works in a benchtop model, it works in a lab, and it seems to be functional in expert hands,” Dr. Ross said. “With inexperienced users, we also see that this device works, albeit with a rate of crossover that may approach up to 10%. So, a functional, disposable scope has been produced.”
Despite availability, several pain points may slow adoption, Dr. Ross said, including reluctance to use new technology, skepticism about the clinical impact of scope-related infections, environmental concerns of increased medical waste, and increased cost.
On this latter topic, Dr. Ross pointed out that the true cost of a reusable scope goes beyond the purchase or lease price to include repair costs, reprocessing costs, and, potentially, the cost of litigation from scope-related infection.
“If you have an outbreak in your medical center, you can rest assured that you will have some litigation exposure,” Dr. Ross said.
Fitting disposable duodenoscopes into routine practice
Currently, both FDA-approved disposable duodenoscopes are covered by outpatient pass-through reimbursement for Medicare, and in October, both will be covered on an inpatient basis, according to Dr. Ross.
“I think the big question regarding pass-through reimbursement is what happens when the codes get revalued,” he said. “How long will the additional reimbursement stay in place?”
For now, Dr. Ross suggested that endoscopists reach for disposable duodenoscopes in unique scenarios, such as weekend or night procedures, to avoid calling in a scope-reprocessing technician; or in operating room cases when the scope enters a sterile field. Disposable scopes should also be considered for patients with known MDROs, he added, and conversely, for patients who are immunocompromised or critically ill and “can least afford a scope-related infection.”
Ultimately, the role of disposable duodenoscopes may be decided by the patients themselves, Dr. Ross concluded.
“Certainly, patients know about this – they may come in and demand the use of a single-use scope in certain situations,” Dr. Ross said. “We have to remember when we’re bringing any new technology into the marketplace that while it’s important to understand the input and perspectives of multiple stakeholders, the single-most important stakeholder at the end of the day are our patients.”
Dr. Ross disclosed a relationship with Boston Scientific. Dr. Muthusamy disclosed a relationship with Boston Scientific and Medivators.
Disposable duodenoscopes have one irrefutable advantage over their reusable counterparts: They definitively solve the problem of scope-related multidrug-resistant organism (MDRO) infections. Yet they also come with trade-offs, such as increased cost and medical waste, which has triggered pushback from skeptical endoscopists. How endoscopists weigh their differing concerns will ultimately determine the uptake of these devices going forward, according to Andrew S. Ross, MD, medical director for strategic growth at Virginia Mason Medical Center, Seattle.
“What would you pay to not have to deal with the scope infection issue at all?” Dr. Ross asked during a virtual presentation at the 2021 AGA Tech Summit sponsored by the AGA Center for GI Innovation and Technology. “I think that x-factor is going to depend [on] who you’re talking to and how much they really believe in [duodenoscope-related infection] as an issue.”
Dr. Ross explained that some endoscopists doubt the clinical relevance of duodenoscope-related MDRO infections, possibly because of a lack of direct experience.
“There still is a prevailing sentiment among some endoscopists that duodenoscope infection is really not a problem,” Dr. Ross said. “Or [they may say,]: ‘We haven’t had that issue here in our medical center, so therefore it is not a problem.’ ”
In fact, the exact magnitude of the problem remains unknown.
“In the end, we have an unquantifiable risk to patients wherever [reusable duodenoscopes] are used,” Dr. Ross said.
Just how common are scope-related MDRO infections?
According to V. Raman Muthusamy, MD, AGAF, immediate former chair of the AGA Center for GI Innovation and Technology, and director of endoscopy at the University of California, Los Angeles Health System, scope-related MDRO infections are “relatively uncommon,” but they do occur.
MDRO infections are generally linked with contaminated endoscopes, but duodenoscopes are the most common culprit because they pose a unique risk.
“Traditionally, when outbreaks have occurred [with nonduodenoscopes], it has usually been due to a breach in the reprocessing protocol,” Dr. Muthusamy said in an interview. “But with duodenoscopes, we’ve found that that does not appear to be necessary, and that in many cases there are no identified breaches, and yet there are still outbreaks.”
Dr. Muthusamy, the first endoscopist to test a disposable duodenoscope in a human patient, noted that it’s challenging to definitively prove infection from a reusable scope. Citing an Executive Summary from the Food and Drug Administration, he said, “We know it’s happened 300-400 times over the past decade or so,” with infection rates peaking in 2014-2016 and steadily declining since then.
Approximately 5% of reprocessed duodenoscopes harbor pathogenic bacteria, according to Dr. Muthusamy, but the rate of infection is significantly lower.
“[The use of a contaminated duodenoscope] doesn’t mean a patient will actually get sick ... but it does mean the potential exists, obviously,” he said. “It just shows that these devices are hard to clean and a fraction of people have the potential of becoming ill. It’s our goal to improve on those numbers, and really try to eliminate the risk of this problem, as best we can.”
Infection isn’t the only concern
There are several potential ways to tackle the issue of scope-related infections, Dr. Ross said during his presentation, including designing devices that are easier to clean and optimizing the cleaning process; however, the only definitive solution is to eliminate cleaning altogether.
This is where disposable duodenoscopes come in.
At present, there are two such FDA-approved devices, the Ascope Duodeno from Ambu and the Exalt Model D from Boston Scientific, both of which Dr. Ross characterized as being “in their infancy.”
Studies testing the Exalt Model D suggest that performance compares favorably with reusable duodenoscopes.
“The scope works in a benchtop model, it works in a lab, and it seems to be functional in expert hands,” Dr. Ross said. “With inexperienced users, we also see that this device works, albeit with a rate of crossover that may approach up to 10%. So, a functional, disposable scope has been produced.”
Despite availability, several pain points may slow adoption, Dr. Ross said, including reluctance to use new technology, skepticism about the clinical impact of scope-related infections, environmental concerns of increased medical waste, and increased cost.
On this latter topic, Dr. Ross pointed out that the true cost of a reusable scope goes beyond the purchase or lease price to include repair costs, reprocessing costs, and, potentially, the cost of litigation from scope-related infection.
“If you have an outbreak in your medical center, you can rest assured that you will have some litigation exposure,” Dr. Ross said.
Fitting disposable duodenoscopes into routine practice
Currently, both FDA-approved disposable duodenoscopes are covered by outpatient pass-through reimbursement for Medicare, and in October, both will be covered on an inpatient basis, according to Dr. Ross.
“I think the big question regarding pass-through reimbursement is what happens when the codes get revalued,” he said. “How long will the additional reimbursement stay in place?”
For now, Dr. Ross suggested that endoscopists reach for disposable duodenoscopes in unique scenarios, such as weekend or night procedures, to avoid calling in a scope-reprocessing technician; or in operating room cases when the scope enters a sterile field. Disposable scopes should also be considered for patients with known MDROs, he added, and conversely, for patients who are immunocompromised or critically ill and “can least afford a scope-related infection.”
Ultimately, the role of disposable duodenoscopes may be decided by the patients themselves, Dr. Ross concluded.
“Certainly, patients know about this – they may come in and demand the use of a single-use scope in certain situations,” Dr. Ross said. “We have to remember when we’re bringing any new technology into the marketplace that while it’s important to understand the input and perspectives of multiple stakeholders, the single-most important stakeholder at the end of the day are our patients.”
Dr. Ross disclosed a relationship with Boston Scientific. Dr. Muthusamy disclosed a relationship with Boston Scientific and Medivators.
FROM THE 2021 AGA TECH SUMMIT
Admit or send home for GI bleeding? AI may help you decide
GI Genius recently became the first Food and Drug Administration–approved device to use artificial intelligence (AI) for endoscopy. Soon, similar technology may give gastroenterologists an edge before they even walk into the procedure room.
AI can provide highly accurate risk scores for patients with suspected upper GI bleeding, and make a recommendation for discharge or hospitalization, according to Dennis Shung, MD, MHS, a clinical instructor at Yale University, New Haven, Conn. And this could provide extensive benefit.
“Acute gastrointestinal bleeding is the most common gastrointestinal diagnosis requiring hospitalization. It costs around $19.2 billion per year,” Dr. Shung said, citing a study from Gastroenterology. He made these remarks during a virtual presentation at the 2021 AGA Tech Summit sponsored by the AGA Center for GI Innovation and Technology.
Emergency department visits for upper GI bleeding increased 17% from 2006 to 2014, Dr. Shung added, suggesting a rising trend.
The trouble with using risk scores
A variety of conventional risk scores are presently available to help manage these patients. Generally, they use a composite outcome of hemostatic intervention, transfusion, or death to determine which patients should be hospitalized (high risk) and which patients can go home (low risk). Although these models can offer high sensitivity, they remain underutilized.
“[Clinical risk scores] are cumbersome, it’s difficult to calculate them, [and] you may not remember to do that in your busy workflow,” Dr. Shung said.
He pointed out that low implementation may also stem from poorly defined clinical responsibilities.
“[Observing] providers caring for patients with GI bleeding showed that there was a culture of not taking ownership,” he said. “Emergency department physicians thought that it was the gastroenterologists who needed to [perform risk scoring]. Gastroenterologists thought it was the ED [physicians’ responsibility].”
To overcome these pitfalls, Dr. Shung and colleagues are developing AI that automates risk analysis for upper GI bleeding by integrating the process into the clinical workflow. Like GI Genius, their strategy relies upon machine learning, which is a type of AI that can improve automatically without being explicitly programmed.
Their most recent study (Sci Rep. 2021 Apr 23;11[1]:8827) involved a machine learning model that could predict transfusion in patients admitted for acute GI bleeding. The model was developed and internally validated in a cohort of 2,524 patients, then shown to outperform conventional regression-based models when externally validated in 1,526 patients similarly admitted at large urban hospitals.
Google Maps for GI bleeding
“The future, as I envision it, is a Google Maps for GI bleeding,” Dr. Shung said, referring to how the popular web-mapping product analyzes real-time data, such as weather and traffic patterns, to provide the best route and an estimated time of arrival. “With the electronic health record, we have the ability to personalize care by basically using data obtained during the clinical encounter to generate risk assessment in real time.”
In other words, machine learning software reads a patient’s electronic health record, runs relevant data through an algorithm, and produces both a risk score and a clinical recommendation. In the case of suspected upper GI bleeding, the clinician is advised to either discharge for outpatient endoscopy or hospitalize for inpatient evaluation.
Because the quality and consistency of data in EHRs can vary, the most advanced form of machine learning – deep learning – is needed to make this a clinical reality. Deep learning converts simpler concepts into complex ones. In this scenario, that would mean deciding which clinical data are relevant and which are just noise. Taking this a step further, deep learning can actually “draw conclusions” from what’s missing.
“There are huge challenges in [irregular data] that need to be overcome,” Dr. Shung said in an interview. “But I see it as an opportunity. When you see things that are irregularly sampled, when you see things are missing – they mean something. They mean that a human has decided that that is not the way we should do things because this patient doesn’t need it. And I think there is a lot of value in learning how to model those things.”
The road to clinical implementation
With further research and validation, deep learning models for gastroenterology are likely to play a role in clinical decision-making, according to Dr. Shung. But to reach the clinic floor, developers will need to outsmart some more fundamental obstacles. “The main thing that’s really barring [AI risk modeling] from being used is the reimbursement issue,” he said, referring to uncertainty in how payers will cover associated costs.
In an interview, Sushovan Guha, MD, PhD, moderator of the virtual session and codirector of the center for interventional gastroenterology at UTHealth (iGUT) in Houston, pointed out another financial unknown: liability.
“What happens if there is an error?” he asked. “It’s done by the computers, but who is at fault?”
In addition to these challenges, some clinicians may need to be persuaded before they are willing to trust an algorithm with a patient’s life.
“We have to have community physicians convinced about the importance of using these tools to further improve their clinical practice,” Dr. Guha said. To this end, he added, “It’s time for us to accept and adapt, and make our decision-making process much more efficient.”
The investigators disclosed no relevant conflicts of interest.
GI Genius recently became the first Food and Drug Administration–approved device to use artificial intelligence (AI) for endoscopy. Soon, similar technology may give gastroenterologists an edge before they even walk into the procedure room.
AI can provide highly accurate risk scores for patients with suspected upper GI bleeding, and make a recommendation for discharge or hospitalization, according to Dennis Shung, MD, MHS, a clinical instructor at Yale University, New Haven, Conn. And this could provide extensive benefit.
“Acute gastrointestinal bleeding is the most common gastrointestinal diagnosis requiring hospitalization. It costs around $19.2 billion per year,” Dr. Shung said, citing a study from Gastroenterology. He made these remarks during a virtual presentation at the 2021 AGA Tech Summit sponsored by the AGA Center for GI Innovation and Technology.
Emergency department visits for upper GI bleeding increased 17% from 2006 to 2014, Dr. Shung added, suggesting a rising trend.
The trouble with using risk scores
A variety of conventional risk scores are presently available to help manage these patients. Generally, they use a composite outcome of hemostatic intervention, transfusion, or death to determine which patients should be hospitalized (high risk) and which patients can go home (low risk). Although these models can offer high sensitivity, they remain underutilized.
“[Clinical risk scores] are cumbersome, it’s difficult to calculate them, [and] you may not remember to do that in your busy workflow,” Dr. Shung said.
He pointed out that low implementation may also stem from poorly defined clinical responsibilities.
“[Observing] providers caring for patients with GI bleeding showed that there was a culture of not taking ownership,” he said. “Emergency department physicians thought that it was the gastroenterologists who needed to [perform risk scoring]. Gastroenterologists thought it was the ED [physicians’ responsibility].”
To overcome these pitfalls, Dr. Shung and colleagues are developing AI that automates risk analysis for upper GI bleeding by integrating the process into the clinical workflow. Like GI Genius, their strategy relies upon machine learning, which is a type of AI that can improve automatically without being explicitly programmed.
Their most recent study (Sci Rep. 2021 Apr 23;11[1]:8827) involved a machine learning model that could predict transfusion in patients admitted for acute GI bleeding. The model was developed and internally validated in a cohort of 2,524 patients, then shown to outperform conventional regression-based models when externally validated in 1,526 patients similarly admitted at large urban hospitals.
Google Maps for GI bleeding
“The future, as I envision it, is a Google Maps for GI bleeding,” Dr. Shung said, referring to how the popular web-mapping product analyzes real-time data, such as weather and traffic patterns, to provide the best route and an estimated time of arrival. “With the electronic health record, we have the ability to personalize care by basically using data obtained during the clinical encounter to generate risk assessment in real time.”
In other words, machine learning software reads a patient’s electronic health record, runs relevant data through an algorithm, and produces both a risk score and a clinical recommendation. In the case of suspected upper GI bleeding, the clinician is advised to either discharge for outpatient endoscopy or hospitalize for inpatient evaluation.
Because the quality and consistency of data in EHRs can vary, the most advanced form of machine learning – deep learning – is needed to make this a clinical reality. Deep learning converts simpler concepts into complex ones. In this scenario, that would mean deciding which clinical data are relevant and which are just noise. Taking this a step further, deep learning can actually “draw conclusions” from what’s missing.
“There are huge challenges in [irregular data] that need to be overcome,” Dr. Shung said in an interview. “But I see it as an opportunity. When you see things that are irregularly sampled, when you see things are missing – they mean something. They mean that a human has decided that that is not the way we should do things because this patient doesn’t need it. And I think there is a lot of value in learning how to model those things.”
The road to clinical implementation
With further research and validation, deep learning models for gastroenterology are likely to play a role in clinical decision-making, according to Dr. Shung. But to reach the clinic floor, developers will need to outsmart some more fundamental obstacles. “The main thing that’s really barring [AI risk modeling] from being used is the reimbursement issue,” he said, referring to uncertainty in how payers will cover associated costs.
In an interview, Sushovan Guha, MD, PhD, moderator of the virtual session and codirector of the center for interventional gastroenterology at UTHealth (iGUT) in Houston, pointed out another financial unknown: liability.
“What happens if there is an error?” he asked. “It’s done by the computers, but who is at fault?”
In addition to these challenges, some clinicians may need to be persuaded before they are willing to trust an algorithm with a patient’s life.
“We have to have community physicians convinced about the importance of using these tools to further improve their clinical practice,” Dr. Guha said. To this end, he added, “It’s time for us to accept and adapt, and make our decision-making process much more efficient.”
The investigators disclosed no relevant conflicts of interest.
GI Genius recently became the first Food and Drug Administration–approved device to use artificial intelligence (AI) for endoscopy. Soon, similar technology may give gastroenterologists an edge before they even walk into the procedure room.
AI can provide highly accurate risk scores for patients with suspected upper GI bleeding, and make a recommendation for discharge or hospitalization, according to Dennis Shung, MD, MHS, a clinical instructor at Yale University, New Haven, Conn. And this could provide extensive benefit.
“Acute gastrointestinal bleeding is the most common gastrointestinal diagnosis requiring hospitalization. It costs around $19.2 billion per year,” Dr. Shung said, citing a study from Gastroenterology. He made these remarks during a virtual presentation at the 2021 AGA Tech Summit sponsored by the AGA Center for GI Innovation and Technology.
Emergency department visits for upper GI bleeding increased 17% from 2006 to 2014, Dr. Shung added, suggesting a rising trend.
The trouble with using risk scores
A variety of conventional risk scores are presently available to help manage these patients. Generally, they use a composite outcome of hemostatic intervention, transfusion, or death to determine which patients should be hospitalized (high risk) and which patients can go home (low risk). Although these models can offer high sensitivity, they remain underutilized.
“[Clinical risk scores] are cumbersome, it’s difficult to calculate them, [and] you may not remember to do that in your busy workflow,” Dr. Shung said.
He pointed out that low implementation may also stem from poorly defined clinical responsibilities.
“[Observing] providers caring for patients with GI bleeding showed that there was a culture of not taking ownership,” he said. “Emergency department physicians thought that it was the gastroenterologists who needed to [perform risk scoring]. Gastroenterologists thought it was the ED [physicians’ responsibility].”
To overcome these pitfalls, Dr. Shung and colleagues are developing AI that automates risk analysis for upper GI bleeding by integrating the process into the clinical workflow. Like GI Genius, their strategy relies upon machine learning, which is a type of AI that can improve automatically without being explicitly programmed.
Their most recent study (Sci Rep. 2021 Apr 23;11[1]:8827) involved a machine learning model that could predict transfusion in patients admitted for acute GI bleeding. The model was developed and internally validated in a cohort of 2,524 patients, then shown to outperform conventional regression-based models when externally validated in 1,526 patients similarly admitted at large urban hospitals.
Google Maps for GI bleeding
“The future, as I envision it, is a Google Maps for GI bleeding,” Dr. Shung said, referring to how the popular web-mapping product analyzes real-time data, such as weather and traffic patterns, to provide the best route and an estimated time of arrival. “With the electronic health record, we have the ability to personalize care by basically using data obtained during the clinical encounter to generate risk assessment in real time.”
In other words, machine learning software reads a patient’s electronic health record, runs relevant data through an algorithm, and produces both a risk score and a clinical recommendation. In the case of suspected upper GI bleeding, the clinician is advised to either discharge for outpatient endoscopy or hospitalize for inpatient evaluation.
Because the quality and consistency of data in EHRs can vary, the most advanced form of machine learning – deep learning – is needed to make this a clinical reality. Deep learning converts simpler concepts into complex ones. In this scenario, that would mean deciding which clinical data are relevant and which are just noise. Taking this a step further, deep learning can actually “draw conclusions” from what’s missing.
“There are huge challenges in [irregular data] that need to be overcome,” Dr. Shung said in an interview. “But I see it as an opportunity. When you see things that are irregularly sampled, when you see things are missing – they mean something. They mean that a human has decided that that is not the way we should do things because this patient doesn’t need it. And I think there is a lot of value in learning how to model those things.”
The road to clinical implementation
With further research and validation, deep learning models for gastroenterology are likely to play a role in clinical decision-making, according to Dr. Shung. But to reach the clinic floor, developers will need to outsmart some more fundamental obstacles. “The main thing that’s really barring [AI risk modeling] from being used is the reimbursement issue,” he said, referring to uncertainty in how payers will cover associated costs.
In an interview, Sushovan Guha, MD, PhD, moderator of the virtual session and codirector of the center for interventional gastroenterology at UTHealth (iGUT) in Houston, pointed out another financial unknown: liability.
“What happens if there is an error?” he asked. “It’s done by the computers, but who is at fault?”
In addition to these challenges, some clinicians may need to be persuaded before they are willing to trust an algorithm with a patient’s life.
“We have to have community physicians convinced about the importance of using these tools to further improve their clinical practice,” Dr. Guha said. To this end, he added, “It’s time for us to accept and adapt, and make our decision-making process much more efficient.”
The investigators disclosed no relevant conflicts of interest.
FROM 2021 AGA TECH SUMMIT
AGA Shark Tank 2021: A simple design survives
William of Ockham would have been proud because, at this year’s American Gastroenterological Association’s Shark Tank pitch competition, one product clearly demonstrated Ockham’s razor – that sometimes the simplest solution is best – and came away as the winner at the 2021 AGA Tech Summit sponsored by the AGA Center for GI Innovation and Technology.
Out of five innovative products, ranging from an educational app to a high-tech anorectal sensor, all aimed at improving outcomes in patients with gastrointestinal disorders, the winner was ... drumroll please ...
A needle.
That’s it. A needle. But not like any other needle.
Winner: Toufic Kachaamy, MD, FASGE, AGAF – An EUS-guided access needle
This EUS-guided access needle, invented by Dr. Kachaamy, enterprise clinical leader at Cancer Treatment Centers of America, Phoenix, is a simple device that overcomes a longstanding challenge presented by endoscopic retrograde cholangiopancreatography (ERCP): biliary access.
Many “ERCPs are considered difficult, and sometimes fail, depending on the center and the endoscopist,” Dr. Kachaamy said during a virtual presentation. “Most failures are due to failed initial access to the bile duct.”
Indeed, one study cited a failure rate in ductal cannulation of 5%-15% even among experienced hands.
Failure can have several consequences, Dr. Kachaamy noted, including increased complications, higher cost, delayed care, longer hospitalization, and greater likelihood of patient transfer.
He went on to explain why biliary access can be so challenging and how this EUS-guided access needle helps address these issues.
“[The] two main limitations [during endoscopic ultrasound–guided biliary access] are directing the wire into the narrowed areas and the wire shearing as we are manipulating the wire to get it to where we want it,” Dr. Kachaamy said. “[This EUS-guided access needle] is a 19-22 gauge, rotatable needle with a smooth, side exit for the wire to allow wire manipulation and direction without shearing.”
Dr. Kachaamy highlighted the simple design, which will keep the production cost below $300 per unit, and suggested that failed ERCPs are just the first potential indication of many. Future uses may include gallbladder access, peri-GI collection, gastrojejunostomy, and others.
In an interview, Dr. Kachaamy reacted to the win, which follows 2 years of collaborative development with Cancer Treatment Centers of America.
“For people who are innovators, there’s nothing that feels more rewarding than their ideas being recognized as adding something to the field and potentially helping people and patients,” Dr. Kachaamy said. “So [this is] very, very, very exciting. Very rewarding. Pride would probably be the best way I’d describe it.”
Dr. Kachaamy anticipates that this EUS-guided access needle will be commercially available within 1-2 years, pending regulatory approval. In the meantime, he and his colleagues are seeking a strategic partner.
A shark speaks
V. Raman Muthusamy, MD, AGAF, immediate past chair of the AGA Center for GI Innovation and Technology and director of endoscopy at UCLA Health System, moderated the Shark Tank session, calling it “the highlight” of the AGA Tech Summit.
Dr. Muthusamy and four other “sharks,” including a gastroenterologist, venture capitalist, regulatory device reviewer, and entrepreneur, scored the pitches using three equally weighted categories: the quality of the pitch, the level of innovation and impact on the field, and the quality of the business plan and overall feasibility.
“We saw a full spectrum [of innovations],” Dr. Muthusamy said. “I think it was an enjoyable session.”
Behind closed doors, the sharks narrowed the field to two top contenders. Ultimately, however, there could be only one winner: Dr. Kachaamy. Their decision aligned with a “Fan Favorite” audience poll.
“A lot of [Dr. Kachaamy’s win] had to do with the potential applications and commonality of the problem,” Dr. Muthusamy said in an interview. He highlighted how the EUS-guided access needle allows for an immediate response to ERCP failure without the need for a second procedure.
Dr. Muthusamy also noted that several product designs previously failed to achieve what the EUS-guided access needle has the potential to do.
“I think the feeling was that this seemed to be a way that may address some of the limitations and challenges that we’ve had with earlier [attempts at solving this problem],” Dr. Muthusamy said.
For innovators who didn’t make the cut this year, or those with products still in development, Dr. Muthusamy suggested applying next year.
“We encourage our colleagues and members of the AGA to continue to apply to this program,” Dr. Muthusamy said.
Other fish in the sea
Four other innovators entered the AGA Shark Tank this year. Here are snippets of their pitches:
Hans Gregersen, MD, PhD, MPH – Fecobionics
“Fecobionics is a simulated electronic stool with the consistency and shape of normal stool,” Dr. Gregersen said.
The balloon device, which contains multiple sensors, provides “real-time, quantitative, and mechanistic insights by simulating defecation.”
“It ... is inserted into the rectum,” Dr. Gregersen said. “It measures multiple pressures; it has gyroscopes that measure orientation; we can compute the bending of the device; and we can calculate the shape of the device.”
According to Dr. Gregersen, Fecobionics has “diagnostic potential for patients with fecal incontinence and for subtyping patients with constipation.” He highlighted fewer false-positives than current technology, alongside greater efficiency and lower cost.
Dr. Gregersen is a research professor at California Medical Innovations Institute, San Diego.
Mary J. Pattison, RN – Trans-Abdominal Gastric Surgical System (TAGSS)
TAGSS is a trans-abdominal gastric access device that “represents a novel and exciting means to address multiple gastrointestinal conditions that are without a standardized approach,” Ms. Pattison said. “Placed as simply as a [percutaneous endoscopic gastrostomy tube], TAGSS offers disruptive technology to address [gastroesophageal reflux disease], fundoplication, achalasia, gastroparesis, gastric tumors, and even obesity in a safe, efficient, and cost effective manner. TAGSS offers the first true hybrid approach for endoscopic/laparoscopic collaboration.”
Ms. Pattison is a nurse clinician and endoscopy assistant at WestGlen GI Consultants, Weston, Mo.
Pankaj Rajvanshi, MD, FAASLD – Healthswim App
“At this time, most patient education is provided by Dr. Google,” Dr. Rajvanshi said, “and we want to change that. We have built a platform which allows you, the physician, to create custom, curated, credible content that can be delivered seamlessly to your patients on an ongoing basis.”
Through the Healthswim app, patients subscribe to their providers, allowing access physician-approved content. Subscribers also receive provider updates through their social media feeds.
Dr. Rajvanshi is a gastroenterologist at Swedish Medical Center, Seattle.
Ali S. Karakurum, MD, FACP, FACG – A Device for Removal of Esophageal Food Impactions
“I would like to propose a device which consists of a clear overtube, a collapsible plastic cylindrical basket secured to the distal end of the overtube ... and a snare wire attached to the distal end of the basket which is controlled by the snare handle externally,” Dr. Karakurum said. “The device is ... gradually advanced over the scope for the basket to encompass the food bolus under direct visualization. Once the food bolus is within the basket, the wire loop at the end of the basket is closed via the external handle, securing the food bolus in the basket for safe removal.”
Dr. Karakurum is a gastroenterologist at Advanced Gastroenterology & Endoscopy, Port Jefferson, N.Y.
This article was updated 5/14/21.
William of Ockham would have been proud because, at this year’s American Gastroenterological Association’s Shark Tank pitch competition, one product clearly demonstrated Ockham’s razor – that sometimes the simplest solution is best – and came away as the winner at the 2021 AGA Tech Summit sponsored by the AGA Center for GI Innovation and Technology.
Out of five innovative products, ranging from an educational app to a high-tech anorectal sensor, all aimed at improving outcomes in patients with gastrointestinal disorders, the winner was ... drumroll please ...
A needle.
That’s it. A needle. But not like any other needle.
Winner: Toufic Kachaamy, MD, FASGE, AGAF – An EUS-guided access needle
This EUS-guided access needle, invented by Dr. Kachaamy, enterprise clinical leader at Cancer Treatment Centers of America, Phoenix, is a simple device that overcomes a longstanding challenge presented by endoscopic retrograde cholangiopancreatography (ERCP): biliary access.
Many “ERCPs are considered difficult, and sometimes fail, depending on the center and the endoscopist,” Dr. Kachaamy said during a virtual presentation. “Most failures are due to failed initial access to the bile duct.”
Indeed, one study cited a failure rate in ductal cannulation of 5%-15% even among experienced hands.
Failure can have several consequences, Dr. Kachaamy noted, including increased complications, higher cost, delayed care, longer hospitalization, and greater likelihood of patient transfer.
He went on to explain why biliary access can be so challenging and how this EUS-guided access needle helps address these issues.
“[The] two main limitations [during endoscopic ultrasound–guided biliary access] are directing the wire into the narrowed areas and the wire shearing as we are manipulating the wire to get it to where we want it,” Dr. Kachaamy said. “[This EUS-guided access needle] is a 19-22 gauge, rotatable needle with a smooth, side exit for the wire to allow wire manipulation and direction without shearing.”
Dr. Kachaamy highlighted the simple design, which will keep the production cost below $300 per unit, and suggested that failed ERCPs are just the first potential indication of many. Future uses may include gallbladder access, peri-GI collection, gastrojejunostomy, and others.
In an interview, Dr. Kachaamy reacted to the win, which follows 2 years of collaborative development with Cancer Treatment Centers of America.
“For people who are innovators, there’s nothing that feels more rewarding than their ideas being recognized as adding something to the field and potentially helping people and patients,” Dr. Kachaamy said. “So [this is] very, very, very exciting. Very rewarding. Pride would probably be the best way I’d describe it.”
Dr. Kachaamy anticipates that this EUS-guided access needle will be commercially available within 1-2 years, pending regulatory approval. In the meantime, he and his colleagues are seeking a strategic partner.
A shark speaks
V. Raman Muthusamy, MD, AGAF, immediate past chair of the AGA Center for GI Innovation and Technology and director of endoscopy at UCLA Health System, moderated the Shark Tank session, calling it “the highlight” of the AGA Tech Summit.
Dr. Muthusamy and four other “sharks,” including a gastroenterologist, venture capitalist, regulatory device reviewer, and entrepreneur, scored the pitches using three equally weighted categories: the quality of the pitch, the level of innovation and impact on the field, and the quality of the business plan and overall feasibility.
“We saw a full spectrum [of innovations],” Dr. Muthusamy said. “I think it was an enjoyable session.”
Behind closed doors, the sharks narrowed the field to two top contenders. Ultimately, however, there could be only one winner: Dr. Kachaamy. Their decision aligned with a “Fan Favorite” audience poll.
“A lot of [Dr. Kachaamy’s win] had to do with the potential applications and commonality of the problem,” Dr. Muthusamy said in an interview. He highlighted how the EUS-guided access needle allows for an immediate response to ERCP failure without the need for a second procedure.
Dr. Muthusamy also noted that several product designs previously failed to achieve what the EUS-guided access needle has the potential to do.
“I think the feeling was that this seemed to be a way that may address some of the limitations and challenges that we’ve had with earlier [attempts at solving this problem],” Dr. Muthusamy said.
For innovators who didn’t make the cut this year, or those with products still in development, Dr. Muthusamy suggested applying next year.
“We encourage our colleagues and members of the AGA to continue to apply to this program,” Dr. Muthusamy said.
Other fish in the sea
Four other innovators entered the AGA Shark Tank this year. Here are snippets of their pitches:
Hans Gregersen, MD, PhD, MPH – Fecobionics
“Fecobionics is a simulated electronic stool with the consistency and shape of normal stool,” Dr. Gregersen said.
The balloon device, which contains multiple sensors, provides “real-time, quantitative, and mechanistic insights by simulating defecation.”
“It ... is inserted into the rectum,” Dr. Gregersen said. “It measures multiple pressures; it has gyroscopes that measure orientation; we can compute the bending of the device; and we can calculate the shape of the device.”
According to Dr. Gregersen, Fecobionics has “diagnostic potential for patients with fecal incontinence and for subtyping patients with constipation.” He highlighted fewer false-positives than current technology, alongside greater efficiency and lower cost.
Dr. Gregersen is a research professor at California Medical Innovations Institute, San Diego.
Mary J. Pattison, RN – Trans-Abdominal Gastric Surgical System (TAGSS)
TAGSS is a trans-abdominal gastric access device that “represents a novel and exciting means to address multiple gastrointestinal conditions that are without a standardized approach,” Ms. Pattison said. “Placed as simply as a [percutaneous endoscopic gastrostomy tube], TAGSS offers disruptive technology to address [gastroesophageal reflux disease], fundoplication, achalasia, gastroparesis, gastric tumors, and even obesity in a safe, efficient, and cost effective manner. TAGSS offers the first true hybrid approach for endoscopic/laparoscopic collaboration.”
Ms. Pattison is a nurse clinician and endoscopy assistant at WestGlen GI Consultants, Weston, Mo.
Pankaj Rajvanshi, MD, FAASLD – Healthswim App
“At this time, most patient education is provided by Dr. Google,” Dr. Rajvanshi said, “and we want to change that. We have built a platform which allows you, the physician, to create custom, curated, credible content that can be delivered seamlessly to your patients on an ongoing basis.”
Through the Healthswim app, patients subscribe to their providers, allowing access physician-approved content. Subscribers also receive provider updates through their social media feeds.
Dr. Rajvanshi is a gastroenterologist at Swedish Medical Center, Seattle.
Ali S. Karakurum, MD, FACP, FACG – A Device for Removal of Esophageal Food Impactions
“I would like to propose a device which consists of a clear overtube, a collapsible plastic cylindrical basket secured to the distal end of the overtube ... and a snare wire attached to the distal end of the basket which is controlled by the snare handle externally,” Dr. Karakurum said. “The device is ... gradually advanced over the scope for the basket to encompass the food bolus under direct visualization. Once the food bolus is within the basket, the wire loop at the end of the basket is closed via the external handle, securing the food bolus in the basket for safe removal.”
Dr. Karakurum is a gastroenterologist at Advanced Gastroenterology & Endoscopy, Port Jefferson, N.Y.
This article was updated 5/14/21.
William of Ockham would have been proud because, at this year’s American Gastroenterological Association’s Shark Tank pitch competition, one product clearly demonstrated Ockham’s razor – that sometimes the simplest solution is best – and came away as the winner at the 2021 AGA Tech Summit sponsored by the AGA Center for GI Innovation and Technology.
Out of five innovative products, ranging from an educational app to a high-tech anorectal sensor, all aimed at improving outcomes in patients with gastrointestinal disorders, the winner was ... drumroll please ...
A needle.
That’s it. A needle. But not like any other needle.
Winner: Toufic Kachaamy, MD, FASGE, AGAF – An EUS-guided access needle
This EUS-guided access needle, invented by Dr. Kachaamy, enterprise clinical leader at Cancer Treatment Centers of America, Phoenix, is a simple device that overcomes a longstanding challenge presented by endoscopic retrograde cholangiopancreatography (ERCP): biliary access.
Many “ERCPs are considered difficult, and sometimes fail, depending on the center and the endoscopist,” Dr. Kachaamy said during a virtual presentation. “Most failures are due to failed initial access to the bile duct.”
Indeed, one study cited a failure rate in ductal cannulation of 5%-15% even among experienced hands.
Failure can have several consequences, Dr. Kachaamy noted, including increased complications, higher cost, delayed care, longer hospitalization, and greater likelihood of patient transfer.
He went on to explain why biliary access can be so challenging and how this EUS-guided access needle helps address these issues.
“[The] two main limitations [during endoscopic ultrasound–guided biliary access] are directing the wire into the narrowed areas and the wire shearing as we are manipulating the wire to get it to where we want it,” Dr. Kachaamy said. “[This EUS-guided access needle] is a 19-22 gauge, rotatable needle with a smooth, side exit for the wire to allow wire manipulation and direction without shearing.”
Dr. Kachaamy highlighted the simple design, which will keep the production cost below $300 per unit, and suggested that failed ERCPs are just the first potential indication of many. Future uses may include gallbladder access, peri-GI collection, gastrojejunostomy, and others.
In an interview, Dr. Kachaamy reacted to the win, which follows 2 years of collaborative development with Cancer Treatment Centers of America.
“For people who are innovators, there’s nothing that feels more rewarding than their ideas being recognized as adding something to the field and potentially helping people and patients,” Dr. Kachaamy said. “So [this is] very, very, very exciting. Very rewarding. Pride would probably be the best way I’d describe it.”
Dr. Kachaamy anticipates that this EUS-guided access needle will be commercially available within 1-2 years, pending regulatory approval. In the meantime, he and his colleagues are seeking a strategic partner.
A shark speaks
V. Raman Muthusamy, MD, AGAF, immediate past chair of the AGA Center for GI Innovation and Technology and director of endoscopy at UCLA Health System, moderated the Shark Tank session, calling it “the highlight” of the AGA Tech Summit.
Dr. Muthusamy and four other “sharks,” including a gastroenterologist, venture capitalist, regulatory device reviewer, and entrepreneur, scored the pitches using three equally weighted categories: the quality of the pitch, the level of innovation and impact on the field, and the quality of the business plan and overall feasibility.
“We saw a full spectrum [of innovations],” Dr. Muthusamy said. “I think it was an enjoyable session.”
Behind closed doors, the sharks narrowed the field to two top contenders. Ultimately, however, there could be only one winner: Dr. Kachaamy. Their decision aligned with a “Fan Favorite” audience poll.
“A lot of [Dr. Kachaamy’s win] had to do with the potential applications and commonality of the problem,” Dr. Muthusamy said in an interview. He highlighted how the EUS-guided access needle allows for an immediate response to ERCP failure without the need for a second procedure.
Dr. Muthusamy also noted that several product designs previously failed to achieve what the EUS-guided access needle has the potential to do.
“I think the feeling was that this seemed to be a way that may address some of the limitations and challenges that we’ve had with earlier [attempts at solving this problem],” Dr. Muthusamy said.
For innovators who didn’t make the cut this year, or those with products still in development, Dr. Muthusamy suggested applying next year.
“We encourage our colleagues and members of the AGA to continue to apply to this program,” Dr. Muthusamy said.
Other fish in the sea
Four other innovators entered the AGA Shark Tank this year. Here are snippets of their pitches:
Hans Gregersen, MD, PhD, MPH – Fecobionics
“Fecobionics is a simulated electronic stool with the consistency and shape of normal stool,” Dr. Gregersen said.
The balloon device, which contains multiple sensors, provides “real-time, quantitative, and mechanistic insights by simulating defecation.”
“It ... is inserted into the rectum,” Dr. Gregersen said. “It measures multiple pressures; it has gyroscopes that measure orientation; we can compute the bending of the device; and we can calculate the shape of the device.”
According to Dr. Gregersen, Fecobionics has “diagnostic potential for patients with fecal incontinence and for subtyping patients with constipation.” He highlighted fewer false-positives than current technology, alongside greater efficiency and lower cost.
Dr. Gregersen is a research professor at California Medical Innovations Institute, San Diego.
Mary J. Pattison, RN – Trans-Abdominal Gastric Surgical System (TAGSS)
TAGSS is a trans-abdominal gastric access device that “represents a novel and exciting means to address multiple gastrointestinal conditions that are without a standardized approach,” Ms. Pattison said. “Placed as simply as a [percutaneous endoscopic gastrostomy tube], TAGSS offers disruptive technology to address [gastroesophageal reflux disease], fundoplication, achalasia, gastroparesis, gastric tumors, and even obesity in a safe, efficient, and cost effective manner. TAGSS offers the first true hybrid approach for endoscopic/laparoscopic collaboration.”
Ms. Pattison is a nurse clinician and endoscopy assistant at WestGlen GI Consultants, Weston, Mo.
Pankaj Rajvanshi, MD, FAASLD – Healthswim App
“At this time, most patient education is provided by Dr. Google,” Dr. Rajvanshi said, “and we want to change that. We have built a platform which allows you, the physician, to create custom, curated, credible content that can be delivered seamlessly to your patients on an ongoing basis.”
Through the Healthswim app, patients subscribe to their providers, allowing access physician-approved content. Subscribers also receive provider updates through their social media feeds.
Dr. Rajvanshi is a gastroenterologist at Swedish Medical Center, Seattle.
Ali S. Karakurum, MD, FACP, FACG – A Device for Removal of Esophageal Food Impactions
“I would like to propose a device which consists of a clear overtube, a collapsible plastic cylindrical basket secured to the distal end of the overtube ... and a snare wire attached to the distal end of the basket which is controlled by the snare handle externally,” Dr. Karakurum said. “The device is ... gradually advanced over the scope for the basket to encompass the food bolus under direct visualization. Once the food bolus is within the basket, the wire loop at the end of the basket is closed via the external handle, securing the food bolus in the basket for safe removal.”
Dr. Karakurum is a gastroenterologist at Advanced Gastroenterology & Endoscopy, Port Jefferson, N.Y.
This article was updated 5/14/21.
FROM THE 2021 AGA TECH SUMMIT MEETING