User login
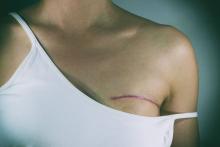
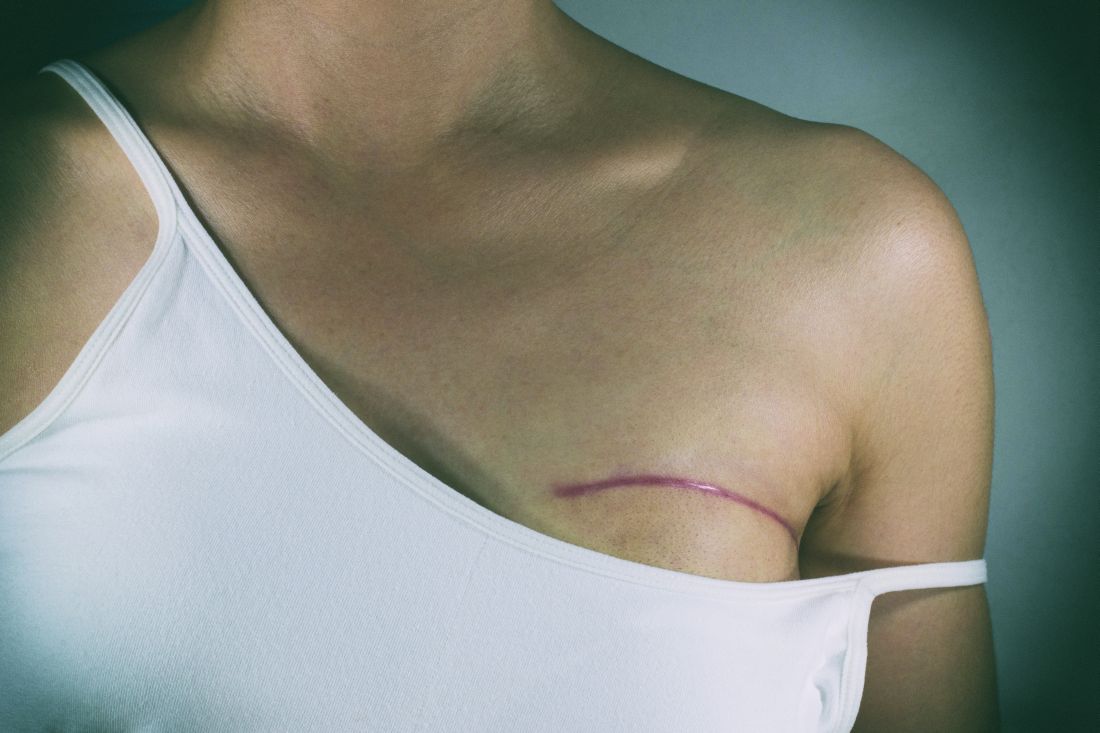
Don’t rely on MRI findings after DCIS needle biopsy
LAS VEGAS – MRI results after percutaneous biopsy for ductal carcinoma in situ (DCIS) are often unreliable, overestimating the extent of disease and leading to over treatment, investigators from the Mayo Clinic in Phoenix concluded after reviewing 54 cases.
Of the 54 women, 7 (13%) had mastectomies driven by postbiopsy MRI findings that were not indicated on final pathology, Barbara Pockaj, MD, senior investigator and surgical oncologist at the Mayo Clinic in Phoenix, reported at the annual meeting of the American Society of Breast Surgeons.
Dr. Pockaj and the Mayo radiologists had a hunch that there was a problem and were uncomfortable interpreting MRI findings after needle biopsy, but there was nothing in the literature about it. The goal of the study was to quantify the problem and “bring this issue to light,” she said.
“I feel better, now that we have this data in hand, as I counsel patients,” who are sometimes so alarmed by MRI findings after needle biopsy that they opt for mastectomies. Now, Dr. Pockaj can tell them with certainty that MRI “may overestimate the extent of disease, so we have to take MRI findings with a grain of salt,” she said.
“Surgeons should be aware that, in patients with a postbiopsy MRI, tumor size may be different than anticipated” and may adversely “affect surgical decision-making and potentially cosmetic results. Percutaneous biopsy significantly limits the ability to accurately interpret the extent of DCIS on preoperative breast MRI by overestimating extent of disease,” the investigators concluded.
Postbiopsy MRIs were performed in 38 of the 54 women (70%), and 14 women (26%) had bigger surgeries because of their MRI results.
Three women had larger lumpectomies, and 11 had mastectomies. “Three really needed it, and one maybe half way,” but the remaining seven turned out on pathology to have only needed lumpectomies, Dr. Pockaj said.
Mean lesion size on preoperative MRI was 3.6 cm while mean lesion size on pathologic specimen was 1.6 cm. Postbiopsy MRI did not significantly correlate with the actual size of the tumor specimen (r = 0.028; P = 0.921).
If the core needle biopsy shows DCIS, in many cases, “I think you can go right to surgery,” Dr. Pockaj said. MRI might still be indicted for very large lesions, suspicions of invasive disease, or very dense breasts, but, even so, the results need to be put into context with the postbiopsy changes, she said.
Women in the study were a mean of 59 years old. Most had high- or intermediate-grade DCIS. Women upgraded to invasive disease at surgery were excluded from the analysis.
There was no industry funding for the work, and the investigators reported having no relevant financial disclosures.
LAS VEGAS – MRI results after percutaneous biopsy for ductal carcinoma in situ (DCIS) are often unreliable, overestimating the extent of disease and leading to over treatment, investigators from the Mayo Clinic in Phoenix concluded after reviewing 54 cases.
Of the 54 women, 7 (13%) had mastectomies driven by postbiopsy MRI findings that were not indicated on final pathology, Barbara Pockaj, MD, senior investigator and surgical oncologist at the Mayo Clinic in Phoenix, reported at the annual meeting of the American Society of Breast Surgeons.
Dr. Pockaj and the Mayo radiologists had a hunch that there was a problem and were uncomfortable interpreting MRI findings after needle biopsy, but there was nothing in the literature about it. The goal of the study was to quantify the problem and “bring this issue to light,” she said.
“I feel better, now that we have this data in hand, as I counsel patients,” who are sometimes so alarmed by MRI findings after needle biopsy that they opt for mastectomies. Now, Dr. Pockaj can tell them with certainty that MRI “may overestimate the extent of disease, so we have to take MRI findings with a grain of salt,” she said.
“Surgeons should be aware that, in patients with a postbiopsy MRI, tumor size may be different than anticipated” and may adversely “affect surgical decision-making and potentially cosmetic results. Percutaneous biopsy significantly limits the ability to accurately interpret the extent of DCIS on preoperative breast MRI by overestimating extent of disease,” the investigators concluded.
Postbiopsy MRIs were performed in 38 of the 54 women (70%), and 14 women (26%) had bigger surgeries because of their MRI results.
Three women had larger lumpectomies, and 11 had mastectomies. “Three really needed it, and one maybe half way,” but the remaining seven turned out on pathology to have only needed lumpectomies, Dr. Pockaj said.
Mean lesion size on preoperative MRI was 3.6 cm while mean lesion size on pathologic specimen was 1.6 cm. Postbiopsy MRI did not significantly correlate with the actual size of the tumor specimen (r = 0.028; P = 0.921).
If the core needle biopsy shows DCIS, in many cases, “I think you can go right to surgery,” Dr. Pockaj said. MRI might still be indicted for very large lesions, suspicions of invasive disease, or very dense breasts, but, even so, the results need to be put into context with the postbiopsy changes, she said.
Women in the study were a mean of 59 years old. Most had high- or intermediate-grade DCIS. Women upgraded to invasive disease at surgery were excluded from the analysis.
There was no industry funding for the work, and the investigators reported having no relevant financial disclosures.
LAS VEGAS – MRI results after percutaneous biopsy for ductal carcinoma in situ (DCIS) are often unreliable, overestimating the extent of disease and leading to over treatment, investigators from the Mayo Clinic in Phoenix concluded after reviewing 54 cases.
Of the 54 women, 7 (13%) had mastectomies driven by postbiopsy MRI findings that were not indicated on final pathology, Barbara Pockaj, MD, senior investigator and surgical oncologist at the Mayo Clinic in Phoenix, reported at the annual meeting of the American Society of Breast Surgeons.
Dr. Pockaj and the Mayo radiologists had a hunch that there was a problem and were uncomfortable interpreting MRI findings after needle biopsy, but there was nothing in the literature about it. The goal of the study was to quantify the problem and “bring this issue to light,” she said.
“I feel better, now that we have this data in hand, as I counsel patients,” who are sometimes so alarmed by MRI findings after needle biopsy that they opt for mastectomies. Now, Dr. Pockaj can tell them with certainty that MRI “may overestimate the extent of disease, so we have to take MRI findings with a grain of salt,” she said.
“Surgeons should be aware that, in patients with a postbiopsy MRI, tumor size may be different than anticipated” and may adversely “affect surgical decision-making and potentially cosmetic results. Percutaneous biopsy significantly limits the ability to accurately interpret the extent of DCIS on preoperative breast MRI by overestimating extent of disease,” the investigators concluded.
Postbiopsy MRIs were performed in 38 of the 54 women (70%), and 14 women (26%) had bigger surgeries because of their MRI results.
Three women had larger lumpectomies, and 11 had mastectomies. “Three really needed it, and one maybe half way,” but the remaining seven turned out on pathology to have only needed lumpectomies, Dr. Pockaj said.
Mean lesion size on preoperative MRI was 3.6 cm while mean lesion size on pathologic specimen was 1.6 cm. Postbiopsy MRI did not significantly correlate with the actual size of the tumor specimen (r = 0.028; P = 0.921).
If the core needle biopsy shows DCIS, in many cases, “I think you can go right to surgery,” Dr. Pockaj said. MRI might still be indicted for very large lesions, suspicions of invasive disease, or very dense breasts, but, even so, the results need to be put into context with the postbiopsy changes, she said.
Women in the study were a mean of 59 years old. Most had high- or intermediate-grade DCIS. Women upgraded to invasive disease at surgery were excluded from the analysis.
There was no industry funding for the work, and the investigators reported having no relevant financial disclosures.
At ASBS 2017
Key clinical point:
Major finding: Of 54 women, 7 (13%) had mastectomies driven by postbiopsy MRI findings that were not indicated on final pathology.
Data source: Single-center review of 54 DCIS cases.
Disclosures: There was no industry funding for the work, and the investigators reported having no relevant financial disclosures.
Lumpectomy plus reconstruction outperforms mastectomy plus reconstruction
LAS VEGAS – Lumpectomy plus oncoplastic surgery has a total cost that is comparable to lumpectomy alone and that is less than mastectomy plus reconstructive surgery, according to an analysis of nearly 40,000 women.
While the complication rate was slightly higher than lumpectomy alone, the difference disappeared in the last year of data, possibly because surgical techniques had improved. The study looked at MarketScan Commercial Claims and Encounters Database data from 2000 to 2011 and confirms findings from an earlier study that looked at data from the MD Anderson Cancer Center (Ann Surg Oncol. 2016 Oct;23[10]:3190-8).
Lumpectomy plus oncoplastic surgery is increasingly being performed, but it still only represented about 2% of surgeries in women with invasive epithelial breast cancer, although this percentage had grown to 8.4% by 2011 and is likely higher now, according to Dr. Hwang, an associate professor of breast surgical oncology and surgical oncology at the University of Texas MD Anderson Cancer Center, Houston.
Candidates for lumpectomy plus oncoplastic surgery include those with large volume disease, multifocal/centric disease, poorly located tumors, or macromastia. “If oncoplastic reconstruction was not available, these patients would oftentimes be undergoing total mastectomy with reconstruction,” Dr. Hwang said.
The study included records from 39,518 women who underwent breast surgery with or without reconstruction. It excluded patients who received postmastectomy radiation or neoadjuvant chemotherapy.
A total of 40% underwent lumpectomy plus whole breast irradiation (BCT), 2% received BCT plus oncoplastic reconstruction (BCT+R), 30% had total mastectomy (TM), and 29% had total mastectomy plus reconstruction (TM+R).
After adjusting for age, race, comorbidity, chemotherapy, axillary surgery, and nodal positivity, the complication rate was lowest in the TM group (25%; relative risk compared with BCT+R, 0.71; 95% confidence interval, 0.64-0.78; P less than .001), followed by BCT (29%; RR, 0.80; 95% CI, 0.72-0.88; P less than .001), BCT+R (37%), and TM+R (54%; RR, 1.49; 95% CI, 1.35-1.64; P less than .001).
The rate of complications in BCT+R fell over time, so that, by 2011, it was only slightly higher than those of BCT alone (31.5% vs 29.4%). That trend is “probably just additional experience with the technique,” Dr. Hwang said.
Total costs for TM+R was $89,187, compared with $48,767 for TM, $66,217 for BCT, and $69,781 for BCT+R. The cost difference between BCT and TM+R was about $23,000.
The findings are encouraging, said Judy Boughey, MD, professor of surgery at the Mayo Clinic, Rochester, Minn., who moderated the session. However, she called for caution in interpreting the results because the population of interest made up just 2% of the overall sample. “I think you’re going to have the widest variability of confidence intervals around that group,” Dr. Boughey said. “It’s eye-opening, but I would just view it with caution.”
Dr. Hwang and Dr. Boughey reported having no financial disclosures.
LAS VEGAS – Lumpectomy plus oncoplastic surgery has a total cost that is comparable to lumpectomy alone and that is less than mastectomy plus reconstructive surgery, according to an analysis of nearly 40,000 women.
While the complication rate was slightly higher than lumpectomy alone, the difference disappeared in the last year of data, possibly because surgical techniques had improved. The study looked at MarketScan Commercial Claims and Encounters Database data from 2000 to 2011 and confirms findings from an earlier study that looked at data from the MD Anderson Cancer Center (Ann Surg Oncol. 2016 Oct;23[10]:3190-8).
Lumpectomy plus oncoplastic surgery is increasingly being performed, but it still only represented about 2% of surgeries in women with invasive epithelial breast cancer, although this percentage had grown to 8.4% by 2011 and is likely higher now, according to Dr. Hwang, an associate professor of breast surgical oncology and surgical oncology at the University of Texas MD Anderson Cancer Center, Houston.
Candidates for lumpectomy plus oncoplastic surgery include those with large volume disease, multifocal/centric disease, poorly located tumors, or macromastia. “If oncoplastic reconstruction was not available, these patients would oftentimes be undergoing total mastectomy with reconstruction,” Dr. Hwang said.
The study included records from 39,518 women who underwent breast surgery with or without reconstruction. It excluded patients who received postmastectomy radiation or neoadjuvant chemotherapy.
A total of 40% underwent lumpectomy plus whole breast irradiation (BCT), 2% received BCT plus oncoplastic reconstruction (BCT+R), 30% had total mastectomy (TM), and 29% had total mastectomy plus reconstruction (TM+R).
After adjusting for age, race, comorbidity, chemotherapy, axillary surgery, and nodal positivity, the complication rate was lowest in the TM group (25%; relative risk compared with BCT+R, 0.71; 95% confidence interval, 0.64-0.78; P less than .001), followed by BCT (29%; RR, 0.80; 95% CI, 0.72-0.88; P less than .001), BCT+R (37%), and TM+R (54%; RR, 1.49; 95% CI, 1.35-1.64; P less than .001).
The rate of complications in BCT+R fell over time, so that, by 2011, it was only slightly higher than those of BCT alone (31.5% vs 29.4%). That trend is “probably just additional experience with the technique,” Dr. Hwang said.
Total costs for TM+R was $89,187, compared with $48,767 for TM, $66,217 for BCT, and $69,781 for BCT+R. The cost difference between BCT and TM+R was about $23,000.
The findings are encouraging, said Judy Boughey, MD, professor of surgery at the Mayo Clinic, Rochester, Minn., who moderated the session. However, she called for caution in interpreting the results because the population of interest made up just 2% of the overall sample. “I think you’re going to have the widest variability of confidence intervals around that group,” Dr. Boughey said. “It’s eye-opening, but I would just view it with caution.”
Dr. Hwang and Dr. Boughey reported having no financial disclosures.
LAS VEGAS – Lumpectomy plus oncoplastic surgery has a total cost that is comparable to lumpectomy alone and that is less than mastectomy plus reconstructive surgery, according to an analysis of nearly 40,000 women.
While the complication rate was slightly higher than lumpectomy alone, the difference disappeared in the last year of data, possibly because surgical techniques had improved. The study looked at MarketScan Commercial Claims and Encounters Database data from 2000 to 2011 and confirms findings from an earlier study that looked at data from the MD Anderson Cancer Center (Ann Surg Oncol. 2016 Oct;23[10]:3190-8).
Lumpectomy plus oncoplastic surgery is increasingly being performed, but it still only represented about 2% of surgeries in women with invasive epithelial breast cancer, although this percentage had grown to 8.4% by 2011 and is likely higher now, according to Dr. Hwang, an associate professor of breast surgical oncology and surgical oncology at the University of Texas MD Anderson Cancer Center, Houston.
Candidates for lumpectomy plus oncoplastic surgery include those with large volume disease, multifocal/centric disease, poorly located tumors, or macromastia. “If oncoplastic reconstruction was not available, these patients would oftentimes be undergoing total mastectomy with reconstruction,” Dr. Hwang said.
The study included records from 39,518 women who underwent breast surgery with or without reconstruction. It excluded patients who received postmastectomy radiation or neoadjuvant chemotherapy.
A total of 40% underwent lumpectomy plus whole breast irradiation (BCT), 2% received BCT plus oncoplastic reconstruction (BCT+R), 30% had total mastectomy (TM), and 29% had total mastectomy plus reconstruction (TM+R).
After adjusting for age, race, comorbidity, chemotherapy, axillary surgery, and nodal positivity, the complication rate was lowest in the TM group (25%; relative risk compared with BCT+R, 0.71; 95% confidence interval, 0.64-0.78; P less than .001), followed by BCT (29%; RR, 0.80; 95% CI, 0.72-0.88; P less than .001), BCT+R (37%), and TM+R (54%; RR, 1.49; 95% CI, 1.35-1.64; P less than .001).
The rate of complications in BCT+R fell over time, so that, by 2011, it was only slightly higher than those of BCT alone (31.5% vs 29.4%). That trend is “probably just additional experience with the technique,” Dr. Hwang said.
Total costs for TM+R was $89,187, compared with $48,767 for TM, $66,217 for BCT, and $69,781 for BCT+R. The cost difference between BCT and TM+R was about $23,000.
The findings are encouraging, said Judy Boughey, MD, professor of surgery at the Mayo Clinic, Rochester, Minn., who moderated the session. However, she called for caution in interpreting the results because the population of interest made up just 2% of the overall sample. “I think you’re going to have the widest variability of confidence intervals around that group,” Dr. Boughey said. “It’s eye-opening, but I would just view it with caution.”
Dr. Hwang and Dr. Boughey reported having no financial disclosures.
At ASBS 2017
Key clinical point:
Major finding: Mastectomy with reconstruction had a 49% higher complication rate and $20,000 higher cost, compared with lumpectomy plus reconstruction.
Data source: Nationwide retrospective analysis of 39,518 women.
Disclosures: Dr. Hwang and Dr. Boughey reported having no financial disclosures.
Schizophrenia patients’ mortality is 14.5 years shorter
SAN DIEGO – A new systematic review and meta-analysis, the first of its kind, suggests that most people with schizophrenia – especially men – do not survive past their 60s.
According to findings released at the 2017 International Congress on Schizophrenia Research, schizophrenia patients appear to die a weighted average of 14.5 years earlier than the rest of the population (95% confidence interval, 11.2-17.8).
The weighted average life expectancy for schizophrenia patients was 64.7 years (95% CI, 61.1-71.3) – 59.9 years for men (95% CI, 55.5-64.3) and 67.6 years for women (95% CI, 63.1-72.1).
“There were no indications that this is improving over time,” lead author Carsten Hjorthøj, PhD, senior researcher at the University of Copenhagen, said in an interview.
The researchers launched their paper in order to provide a wider perspective on lives lost in schizophrenia.
Previous studies had used measures like the standardized mortality ratio, which compared schizophrenia patients with a matched group from the general population, Dr. Hjorthøj said. “Saying that people with schizophrenia are, for instance, 2.5 times more likely to die at any given age may be a little difficult to understand. Identifying the actual number of years they die earlier than the rest of the population is easily understood and will probably also make the issue more obvious to policymakers.”
The researchers, whose findings were previously published in Lancet Psychiatry (2017 Apr;4[4]:295-301), examined 11 studies, mostly from Europe (n = 5) and North America (n = 3) with single studies from Africa, Asia, and Australia. (The lowest life expectancies were reported in Asia and Africa.)
One study was published in 1991, and the rest from 2001-2016. Together, the studies tracked 302,691 patients with schizophrenia.
What explains the gap between the life expectancies in men and women? “We did not investigate the reasons for this,” Dr. Hjorthøj said, “but men typically have worse adherence to treatment than women, and men typically also have poorer health-seeking behavior than women even in the general population. Given that schizophrenia is associated with a multitude of adverse somatic outcomes, this could then be aggravated by this difference in health-seeking behavior.”
In addition, he said, “It could also be the case that use of alcohol, tobacco, and illicit substances is even more skewed in schizophrenia than in the general population. Finally, it could also be attributed in part to the fact that men are more likely than women to die from suicide.”
Dr. Hjorthøj said that the findings emphasize the importance of treating physical symptoms in people with schizophrenia.
“I am aware of several cases where physical complaints were not taken seriously because they were raised by people who were known to suffer from delusions,” he said. “But, I do believe that most psychiatrists are aware of this, and the problem is perhaps more that the system in general is underfunded and that somatic and psychiatric hospitals are not good enough at communicating with each other.”
The researchers report no funding and no disclosures.
SAN DIEGO – A new systematic review and meta-analysis, the first of its kind, suggests that most people with schizophrenia – especially men – do not survive past their 60s.
According to findings released at the 2017 International Congress on Schizophrenia Research, schizophrenia patients appear to die a weighted average of 14.5 years earlier than the rest of the population (95% confidence interval, 11.2-17.8).
The weighted average life expectancy for schizophrenia patients was 64.7 years (95% CI, 61.1-71.3) – 59.9 years for men (95% CI, 55.5-64.3) and 67.6 years for women (95% CI, 63.1-72.1).
“There were no indications that this is improving over time,” lead author Carsten Hjorthøj, PhD, senior researcher at the University of Copenhagen, said in an interview.
The researchers launched their paper in order to provide a wider perspective on lives lost in schizophrenia.
Previous studies had used measures like the standardized mortality ratio, which compared schizophrenia patients with a matched group from the general population, Dr. Hjorthøj said. “Saying that people with schizophrenia are, for instance, 2.5 times more likely to die at any given age may be a little difficult to understand. Identifying the actual number of years they die earlier than the rest of the population is easily understood and will probably also make the issue more obvious to policymakers.”
The researchers, whose findings were previously published in Lancet Psychiatry (2017 Apr;4[4]:295-301), examined 11 studies, mostly from Europe (n = 5) and North America (n = 3) with single studies from Africa, Asia, and Australia. (The lowest life expectancies were reported in Asia and Africa.)
One study was published in 1991, and the rest from 2001-2016. Together, the studies tracked 302,691 patients with schizophrenia.
What explains the gap between the life expectancies in men and women? “We did not investigate the reasons for this,” Dr. Hjorthøj said, “but men typically have worse adherence to treatment than women, and men typically also have poorer health-seeking behavior than women even in the general population. Given that schizophrenia is associated with a multitude of adverse somatic outcomes, this could then be aggravated by this difference in health-seeking behavior.”
In addition, he said, “It could also be the case that use of alcohol, tobacco, and illicit substances is even more skewed in schizophrenia than in the general population. Finally, it could also be attributed in part to the fact that men are more likely than women to die from suicide.”
Dr. Hjorthøj said that the findings emphasize the importance of treating physical symptoms in people with schizophrenia.
“I am aware of several cases where physical complaints were not taken seriously because they were raised by people who were known to suffer from delusions,” he said. “But, I do believe that most psychiatrists are aware of this, and the problem is perhaps more that the system in general is underfunded and that somatic and psychiatric hospitals are not good enough at communicating with each other.”
The researchers report no funding and no disclosures.
SAN DIEGO – A new systematic review and meta-analysis, the first of its kind, suggests that most people with schizophrenia – especially men – do not survive past their 60s.
According to findings released at the 2017 International Congress on Schizophrenia Research, schizophrenia patients appear to die a weighted average of 14.5 years earlier than the rest of the population (95% confidence interval, 11.2-17.8).
The weighted average life expectancy for schizophrenia patients was 64.7 years (95% CI, 61.1-71.3) – 59.9 years for men (95% CI, 55.5-64.3) and 67.6 years for women (95% CI, 63.1-72.1).
“There were no indications that this is improving over time,” lead author Carsten Hjorthøj, PhD, senior researcher at the University of Copenhagen, said in an interview.
The researchers launched their paper in order to provide a wider perspective on lives lost in schizophrenia.
Previous studies had used measures like the standardized mortality ratio, which compared schizophrenia patients with a matched group from the general population, Dr. Hjorthøj said. “Saying that people with schizophrenia are, for instance, 2.5 times more likely to die at any given age may be a little difficult to understand. Identifying the actual number of years they die earlier than the rest of the population is easily understood and will probably also make the issue more obvious to policymakers.”
The researchers, whose findings were previously published in Lancet Psychiatry (2017 Apr;4[4]:295-301), examined 11 studies, mostly from Europe (n = 5) and North America (n = 3) with single studies from Africa, Asia, and Australia. (The lowest life expectancies were reported in Asia and Africa.)
One study was published in 1991, and the rest from 2001-2016. Together, the studies tracked 302,691 patients with schizophrenia.
What explains the gap between the life expectancies in men and women? “We did not investigate the reasons for this,” Dr. Hjorthøj said, “but men typically have worse adherence to treatment than women, and men typically also have poorer health-seeking behavior than women even in the general population. Given that schizophrenia is associated with a multitude of adverse somatic outcomes, this could then be aggravated by this difference in health-seeking behavior.”
In addition, he said, “It could also be the case that use of alcohol, tobacco, and illicit substances is even more skewed in schizophrenia than in the general population. Finally, it could also be attributed in part to the fact that men are more likely than women to die from suicide.”
Dr. Hjorthøj said that the findings emphasize the importance of treating physical symptoms in people with schizophrenia.
“I am aware of several cases where physical complaints were not taken seriously because they were raised by people who were known to suffer from delusions,” he said. “But, I do believe that most psychiatrists are aware of this, and the problem is perhaps more that the system in general is underfunded and that somatic and psychiatric hospitals are not good enough at communicating with each other.”
The researchers report no funding and no disclosures.
At the ICSR Biennial Meeting
Key clinical point:
Major finding: Schizophrenia patients die a weighted average of 14.5 years earlier than those in the general population (95% CI, 11.2-17.8), and their weighted average life expectancy is 64.7 years (95% CI, 61-71.3) – 59.9 for men (95% CI, 55.5-64.3) and 67.6 for women (95% CI, 63.1-72.1).
Data source: Review and meta-analysis of 11 studies of 302,691 schizophrenia patients.
Disclosures: No specific funding was reported, and the authors reported no disclosures.
Study finds predictors of progression to Sjögren’s syndrome
Elevated serum gamma globulin or low complement levels in patients with symptoms of Sjögren’s syndrome (SS) may predict their likelihood of progression to the autoimmune disease, according to findings from a longitudinal cohort study.
“Identification of serological variables that predict the development of Sjögren’s syndrome may contribute to earlier diagnosis and treatment of the disease and, ultimately, better long-term outcome,” Caroline H. Shiboski, DDS, PhD, chair of the department of orofacial sciences at the University of California, San Francisco, and her coauthors wrote.
The researchers described the results of a follow-up visit 2 years after baseline for 771 of 3,310 participants in the Sjögren’s International Collaborative Clinical Alliance (SICCA) registry.
Approximately half of the participants did not meet either the 2012 American College of Rheumatology criteria or the 2016 ACR-European League Against Rheumatism criteria for Sjögren’s syndrome at baseline. At follow-up, 8% met the ACR criteria, and 9% met the ACR-EULAR criteria (Arthritis Care Res. 2017 April 24. doi: 10.1002/acr.23264).
For the other patients, those who did not progress according to either criteria, the researchers “observed remarkable stability over 2-3 years for both individual phenotypic features of SS and SS status, a finding consistent with smaller prospective studies with longer durations of follow-up.”
The participants with hypergammaglobulinemia at study entry, defined as immunoglobulin G greater than 1,445 mg/dL, were four times more likely to progress to full disease after 2 years (P = .006), while those with hypocomplementemia were six times more likely to progress (P = .004) than were those without. Hypocomplementemia was defined as C3 less than 90 mg/dL and/or C4 less than 16 mg/dL.
“However, the highest percentage of progression from not meeting the SS criteria at baseline to meeting them at the 2- to 3-year follow-up was among those who reported receiving immunomodulating/suppressive medications at both time points, both when using the ACR criteria (18% progressors) and the ACR-EULAR criteria (12% progressors),” the authors wrote.
In general, those with confirmed diagnosis at study entry showed reasonable stability in both individual phenotypic features and disease status.
Overall, 93% of participants who met the ACR criteria for SS at baseline still met those criteria at the 2- to 3-year follow-up, as did 89% of participants who met the ACR-EULAR criteria at baseline.
A majority of the participants who reverted from SS classification at baseline to failure to meet the criteria at follow-up had a change in labial salivary gland biopsy results. This occurred in 60% who met ACR criteria at baseline and 75% who met ACR-EULAR criteria at baseline.
More than 85% of participants in the study reported dry mouth or dry eyes, but participants with SS were much more likely to have positive anti-SSA, positive rheumatoid factor, antinuclear antibody titer of 1:320 or greater, hypergammaglobulinemia, and focal lymphocytic sialadenitis with a focus score of 1 or more focus per 4 mm2.
The researchers also reported a very low incidence (0.5%) of lymphoma among the participants available for follow-up.
The study was supported by the National Institutes of Health, the Jerome L. Greene Foundation, and the Rosalind Russell/Ephraim P. Engleman Rheumatology Research Center. Six authors declared consultancies, honoraria, and grants from pharmaceutical companies.
Elevated serum gamma globulin or low complement levels in patients with symptoms of Sjögren’s syndrome (SS) may predict their likelihood of progression to the autoimmune disease, according to findings from a longitudinal cohort study.
“Identification of serological variables that predict the development of Sjögren’s syndrome may contribute to earlier diagnosis and treatment of the disease and, ultimately, better long-term outcome,” Caroline H. Shiboski, DDS, PhD, chair of the department of orofacial sciences at the University of California, San Francisco, and her coauthors wrote.
The researchers described the results of a follow-up visit 2 years after baseline for 771 of 3,310 participants in the Sjögren’s International Collaborative Clinical Alliance (SICCA) registry.
Approximately half of the participants did not meet either the 2012 American College of Rheumatology criteria or the 2016 ACR-European League Against Rheumatism criteria for Sjögren’s syndrome at baseline. At follow-up, 8% met the ACR criteria, and 9% met the ACR-EULAR criteria (Arthritis Care Res. 2017 April 24. doi: 10.1002/acr.23264).
For the other patients, those who did not progress according to either criteria, the researchers “observed remarkable stability over 2-3 years for both individual phenotypic features of SS and SS status, a finding consistent with smaller prospective studies with longer durations of follow-up.”
The participants with hypergammaglobulinemia at study entry, defined as immunoglobulin G greater than 1,445 mg/dL, were four times more likely to progress to full disease after 2 years (P = .006), while those with hypocomplementemia were six times more likely to progress (P = .004) than were those without. Hypocomplementemia was defined as C3 less than 90 mg/dL and/or C4 less than 16 mg/dL.
“However, the highest percentage of progression from not meeting the SS criteria at baseline to meeting them at the 2- to 3-year follow-up was among those who reported receiving immunomodulating/suppressive medications at both time points, both when using the ACR criteria (18% progressors) and the ACR-EULAR criteria (12% progressors),” the authors wrote.
In general, those with confirmed diagnosis at study entry showed reasonable stability in both individual phenotypic features and disease status.
Overall, 93% of participants who met the ACR criteria for SS at baseline still met those criteria at the 2- to 3-year follow-up, as did 89% of participants who met the ACR-EULAR criteria at baseline.
A majority of the participants who reverted from SS classification at baseline to failure to meet the criteria at follow-up had a change in labial salivary gland biopsy results. This occurred in 60% who met ACR criteria at baseline and 75% who met ACR-EULAR criteria at baseline.
More than 85% of participants in the study reported dry mouth or dry eyes, but participants with SS were much more likely to have positive anti-SSA, positive rheumatoid factor, antinuclear antibody titer of 1:320 or greater, hypergammaglobulinemia, and focal lymphocytic sialadenitis with a focus score of 1 or more focus per 4 mm2.
The researchers also reported a very low incidence (0.5%) of lymphoma among the participants available for follow-up.
The study was supported by the National Institutes of Health, the Jerome L. Greene Foundation, and the Rosalind Russell/Ephraim P. Engleman Rheumatology Research Center. Six authors declared consultancies, honoraria, and grants from pharmaceutical companies.
Elevated serum gamma globulin or low complement levels in patients with symptoms of Sjögren’s syndrome (SS) may predict their likelihood of progression to the autoimmune disease, according to findings from a longitudinal cohort study.
“Identification of serological variables that predict the development of Sjögren’s syndrome may contribute to earlier diagnosis and treatment of the disease and, ultimately, better long-term outcome,” Caroline H. Shiboski, DDS, PhD, chair of the department of orofacial sciences at the University of California, San Francisco, and her coauthors wrote.
The researchers described the results of a follow-up visit 2 years after baseline for 771 of 3,310 participants in the Sjögren’s International Collaborative Clinical Alliance (SICCA) registry.
Approximately half of the participants did not meet either the 2012 American College of Rheumatology criteria or the 2016 ACR-European League Against Rheumatism criteria for Sjögren’s syndrome at baseline. At follow-up, 8% met the ACR criteria, and 9% met the ACR-EULAR criteria (Arthritis Care Res. 2017 April 24. doi: 10.1002/acr.23264).
For the other patients, those who did not progress according to either criteria, the researchers “observed remarkable stability over 2-3 years for both individual phenotypic features of SS and SS status, a finding consistent with smaller prospective studies with longer durations of follow-up.”
The participants with hypergammaglobulinemia at study entry, defined as immunoglobulin G greater than 1,445 mg/dL, were four times more likely to progress to full disease after 2 years (P = .006), while those with hypocomplementemia were six times more likely to progress (P = .004) than were those without. Hypocomplementemia was defined as C3 less than 90 mg/dL and/or C4 less than 16 mg/dL.
“However, the highest percentage of progression from not meeting the SS criteria at baseline to meeting them at the 2- to 3-year follow-up was among those who reported receiving immunomodulating/suppressive medications at both time points, both when using the ACR criteria (18% progressors) and the ACR-EULAR criteria (12% progressors),” the authors wrote.
In general, those with confirmed diagnosis at study entry showed reasonable stability in both individual phenotypic features and disease status.
Overall, 93% of participants who met the ACR criteria for SS at baseline still met those criteria at the 2- to 3-year follow-up, as did 89% of participants who met the ACR-EULAR criteria at baseline.
A majority of the participants who reverted from SS classification at baseline to failure to meet the criteria at follow-up had a change in labial salivary gland biopsy results. This occurred in 60% who met ACR criteria at baseline and 75% who met ACR-EULAR criteria at baseline.
More than 85% of participants in the study reported dry mouth or dry eyes, but participants with SS were much more likely to have positive anti-SSA, positive rheumatoid factor, antinuclear antibody titer of 1:320 or greater, hypergammaglobulinemia, and focal lymphocytic sialadenitis with a focus score of 1 or more focus per 4 mm2.
The researchers also reported a very low incidence (0.5%) of lymphoma among the participants available for follow-up.
The study was supported by the National Institutes of Health, the Jerome L. Greene Foundation, and the Rosalind Russell/Ephraim P. Engleman Rheumatology Research Center. Six authors declared consultancies, honoraria, and grants from pharmaceutical companies.
Key clinical point:
Major finding: Patients with symptoms of Sjögren’s syndrome and hypergammaglobulinemia are four times more likely to progress to full disease than are those without.
Data source: A longitudinal cohort study of 3,310 participants in the Sjögren’s International Collaborative Clinical Alliance (SICCA) registry.
Disclosures: The study was supported by the National Institutes of Health, the Jerome L. Greene Foundation, and the Rosalind Russell/Ephraim P. Engleman Rheumatology Research Center. Six authors declared consultancies, honoraria, and grants from pharmaceutical companies.
Vitamin D3 supplementation beat placebo in atopic dermatitis trial
PORTLAND – Short-term daily oral supplementation with 5000 IU of vitamin D3 was safe and significantly outperformed placebo for lessening the signs and symptoms of atopic dermatitis (AD), according to the results of a randomized, double-blind trial of 65 patients with moderate to severe disease.
At week 12, average improvements on the Scoring Atopic Dermatitis (SCORAD) index (standard deviation, 11.1 points) were 21.2 points in the vitamin D3 group and 13.9 points (standard deviation, 12.3 points) in the placebo group (P = .02), Karen Sanchez-Armendariz, MD, reported at the annual meeting of the Society for Investigative Dermatology. “Vitamin D3 should be considered a valuable adjuvant in the treatment of AD, especially in patients with relapses,” she said.
Past studies have reported an inverse relationship between 25(OH)D (vitamin D) serum levels and the severity of AD, noted Dr. Sanchez-Armendariz, who is with the National Autonomous University of Mexico in Mexico City. This finding makes sense, as vitamin D3 induces the topical production of antimicrobial peptides, which have direct antimicrobial activity and induce a host response characterized by cytokine release, chemotaxis, inflammation, angiogenesis, and re-epithelialization, she added.
Consequently, 25(OH)D deficiency has been posited as a culprit in the pathophysiology of AD. However, scientific and endocrine societies have published differing recommendations about safe and optimal doses for vitamin D3 supplementation, while experts have noted that 5000 IU per day is needed to achieve an increase of 10 ng 25(OH)D per mL serum (J Am Board Fam Med. 2014 Jul-Aug;27[4]:495-509).
For the study, therefore, Dr. Sanchez-Armendariz and her associates evaluated this dose as adjuvant therapy in patients with moderate to severe AD despite topical hydrocortisone aceponate, soap substitute, and emollient. Patients continued this usual care and received either a cellulose capsule placebo or 5000 IU oral vitamin D3 per day. Based on previously recommended cutoff values, the researchers considered serum 25(OH)D levels sufficient if they exceeded 30 ng per mL, insufficient if they were between 20 and 30 ng per mL, and deficient if they were less than 20 ng per mL (J Bone Miner Res. 2011 Mar;26[3]:455-7).
At baseline, the placebo and intervention groups resembled each other in terms of baseline levels of 25(OH)D, calcium, and phosphorus, pruritus, and scores on SCORAD and the six-area Total Body Severity Assessment. A total of 59% of patients had insufficient serum 25(OH)D, and 39% were deficient.
Patients in both groups improved on the Total Body Severity Assessment, but the extent of improvement did not statistically differ between groups, Dr. Sanchez-Armendariz reported. Supplementation did significantly increase serum 25(OH)D levels, which at week 12 averaged 58.3 ng per mL (SD, 18.5 ng per mL), compared with 22.2 (SD, 7.6 ng per mL) with placebo (P less than .001).
Interestingly, at week 12, all patients in the vitamin D3 group achieved serum 25(OH)D levels of at least 30 ng per mL, as did 41% of patients in the placebo arm. Patients in both groups who achieved this “sufficient” serum 25(OH)D level averaged a nearly 21-point improvement on the SCORAD (SD, 11.1 points), compared with 7.5 points (SD, 9.9 points) among patients who remained deficient, and 17.4 points (SD, 12.7 points) among patients who remained insufficient (P = .004 for difference among groups; P = .02 for difference between sufficient and insufficient groups).
An analysis of variance confirmed this finding, showing that, regardless of treatment group, patients whose final serum 25(OH)D level was at least 20 ng per mL scored an average of 20 points on the SCORAD, compared with 38 points for patients whose 25(OH)D level remained below 20 ng per mL. Patients whose final 25(OH)D level exceeded 20 ng per mL had fivefold greater odds of having a SCORAD below 25 than did patients with lower serum 25(OH)D levels (odds ratio, 5.1; 95% confidence interval, 1.2-22.6; P = .03).
The investigators identified no adverse effects of vitamin D3 supplementation, Dr. Sanchez-Armendariz said. “Attaining 25(OH)D levels above 20 ng per mL in addition to baseline therapy reduced the SCORAD of patients with moderate to severe atopic dermatitis,” she added. Clinicians should consider vitamin D3 deficiency and supplementation in patients with relapsing or refractory AD, she emphasized.
Dr. Sanchez-Armendariz reported no external funding sources and no conflicts of interest.
PORTLAND – Short-term daily oral supplementation with 5000 IU of vitamin D3 was safe and significantly outperformed placebo for lessening the signs and symptoms of atopic dermatitis (AD), according to the results of a randomized, double-blind trial of 65 patients with moderate to severe disease.
At week 12, average improvements on the Scoring Atopic Dermatitis (SCORAD) index (standard deviation, 11.1 points) were 21.2 points in the vitamin D3 group and 13.9 points (standard deviation, 12.3 points) in the placebo group (P = .02), Karen Sanchez-Armendariz, MD, reported at the annual meeting of the Society for Investigative Dermatology. “Vitamin D3 should be considered a valuable adjuvant in the treatment of AD, especially in patients with relapses,” she said.
Past studies have reported an inverse relationship between 25(OH)D (vitamin D) serum levels and the severity of AD, noted Dr. Sanchez-Armendariz, who is with the National Autonomous University of Mexico in Mexico City. This finding makes sense, as vitamin D3 induces the topical production of antimicrobial peptides, which have direct antimicrobial activity and induce a host response characterized by cytokine release, chemotaxis, inflammation, angiogenesis, and re-epithelialization, she added.
Consequently, 25(OH)D deficiency has been posited as a culprit in the pathophysiology of AD. However, scientific and endocrine societies have published differing recommendations about safe and optimal doses for vitamin D3 supplementation, while experts have noted that 5000 IU per day is needed to achieve an increase of 10 ng 25(OH)D per mL serum (J Am Board Fam Med. 2014 Jul-Aug;27[4]:495-509).
For the study, therefore, Dr. Sanchez-Armendariz and her associates evaluated this dose as adjuvant therapy in patients with moderate to severe AD despite topical hydrocortisone aceponate, soap substitute, and emollient. Patients continued this usual care and received either a cellulose capsule placebo or 5000 IU oral vitamin D3 per day. Based on previously recommended cutoff values, the researchers considered serum 25(OH)D levels sufficient if they exceeded 30 ng per mL, insufficient if they were between 20 and 30 ng per mL, and deficient if they were less than 20 ng per mL (J Bone Miner Res. 2011 Mar;26[3]:455-7).
At baseline, the placebo and intervention groups resembled each other in terms of baseline levels of 25(OH)D, calcium, and phosphorus, pruritus, and scores on SCORAD and the six-area Total Body Severity Assessment. A total of 59% of patients had insufficient serum 25(OH)D, and 39% were deficient.
Patients in both groups improved on the Total Body Severity Assessment, but the extent of improvement did not statistically differ between groups, Dr. Sanchez-Armendariz reported. Supplementation did significantly increase serum 25(OH)D levels, which at week 12 averaged 58.3 ng per mL (SD, 18.5 ng per mL), compared with 22.2 (SD, 7.6 ng per mL) with placebo (P less than .001).
Interestingly, at week 12, all patients in the vitamin D3 group achieved serum 25(OH)D levels of at least 30 ng per mL, as did 41% of patients in the placebo arm. Patients in both groups who achieved this “sufficient” serum 25(OH)D level averaged a nearly 21-point improvement on the SCORAD (SD, 11.1 points), compared with 7.5 points (SD, 9.9 points) among patients who remained deficient, and 17.4 points (SD, 12.7 points) among patients who remained insufficient (P = .004 for difference among groups; P = .02 for difference between sufficient and insufficient groups).
An analysis of variance confirmed this finding, showing that, regardless of treatment group, patients whose final serum 25(OH)D level was at least 20 ng per mL scored an average of 20 points on the SCORAD, compared with 38 points for patients whose 25(OH)D level remained below 20 ng per mL. Patients whose final 25(OH)D level exceeded 20 ng per mL had fivefold greater odds of having a SCORAD below 25 than did patients with lower serum 25(OH)D levels (odds ratio, 5.1; 95% confidence interval, 1.2-22.6; P = .03).
The investigators identified no adverse effects of vitamin D3 supplementation, Dr. Sanchez-Armendariz said. “Attaining 25(OH)D levels above 20 ng per mL in addition to baseline therapy reduced the SCORAD of patients with moderate to severe atopic dermatitis,” she added. Clinicians should consider vitamin D3 deficiency and supplementation in patients with relapsing or refractory AD, she emphasized.
Dr. Sanchez-Armendariz reported no external funding sources and no conflicts of interest.
PORTLAND – Short-term daily oral supplementation with 5000 IU of vitamin D3 was safe and significantly outperformed placebo for lessening the signs and symptoms of atopic dermatitis (AD), according to the results of a randomized, double-blind trial of 65 patients with moderate to severe disease.
At week 12, average improvements on the Scoring Atopic Dermatitis (SCORAD) index (standard deviation, 11.1 points) were 21.2 points in the vitamin D3 group and 13.9 points (standard deviation, 12.3 points) in the placebo group (P = .02), Karen Sanchez-Armendariz, MD, reported at the annual meeting of the Society for Investigative Dermatology. “Vitamin D3 should be considered a valuable adjuvant in the treatment of AD, especially in patients with relapses,” she said.
Past studies have reported an inverse relationship between 25(OH)D (vitamin D) serum levels and the severity of AD, noted Dr. Sanchez-Armendariz, who is with the National Autonomous University of Mexico in Mexico City. This finding makes sense, as vitamin D3 induces the topical production of antimicrobial peptides, which have direct antimicrobial activity and induce a host response characterized by cytokine release, chemotaxis, inflammation, angiogenesis, and re-epithelialization, she added.
Consequently, 25(OH)D deficiency has been posited as a culprit in the pathophysiology of AD. However, scientific and endocrine societies have published differing recommendations about safe and optimal doses for vitamin D3 supplementation, while experts have noted that 5000 IU per day is needed to achieve an increase of 10 ng 25(OH)D per mL serum (J Am Board Fam Med. 2014 Jul-Aug;27[4]:495-509).
For the study, therefore, Dr. Sanchez-Armendariz and her associates evaluated this dose as adjuvant therapy in patients with moderate to severe AD despite topical hydrocortisone aceponate, soap substitute, and emollient. Patients continued this usual care and received either a cellulose capsule placebo or 5000 IU oral vitamin D3 per day. Based on previously recommended cutoff values, the researchers considered serum 25(OH)D levels sufficient if they exceeded 30 ng per mL, insufficient if they were between 20 and 30 ng per mL, and deficient if they were less than 20 ng per mL (J Bone Miner Res. 2011 Mar;26[3]:455-7).
At baseline, the placebo and intervention groups resembled each other in terms of baseline levels of 25(OH)D, calcium, and phosphorus, pruritus, and scores on SCORAD and the six-area Total Body Severity Assessment. A total of 59% of patients had insufficient serum 25(OH)D, and 39% were deficient.
Patients in both groups improved on the Total Body Severity Assessment, but the extent of improvement did not statistically differ between groups, Dr. Sanchez-Armendariz reported. Supplementation did significantly increase serum 25(OH)D levels, which at week 12 averaged 58.3 ng per mL (SD, 18.5 ng per mL), compared with 22.2 (SD, 7.6 ng per mL) with placebo (P less than .001).
Interestingly, at week 12, all patients in the vitamin D3 group achieved serum 25(OH)D levels of at least 30 ng per mL, as did 41% of patients in the placebo arm. Patients in both groups who achieved this “sufficient” serum 25(OH)D level averaged a nearly 21-point improvement on the SCORAD (SD, 11.1 points), compared with 7.5 points (SD, 9.9 points) among patients who remained deficient, and 17.4 points (SD, 12.7 points) among patients who remained insufficient (P = .004 for difference among groups; P = .02 for difference between sufficient and insufficient groups).
An analysis of variance confirmed this finding, showing that, regardless of treatment group, patients whose final serum 25(OH)D level was at least 20 ng per mL scored an average of 20 points on the SCORAD, compared with 38 points for patients whose 25(OH)D level remained below 20 ng per mL. Patients whose final 25(OH)D level exceeded 20 ng per mL had fivefold greater odds of having a SCORAD below 25 than did patients with lower serum 25(OH)D levels (odds ratio, 5.1; 95% confidence interval, 1.2-22.6; P = .03).
The investigators identified no adverse effects of vitamin D3 supplementation, Dr. Sanchez-Armendariz said. “Attaining 25(OH)D levels above 20 ng per mL in addition to baseline therapy reduced the SCORAD of patients with moderate to severe atopic dermatitis,” she added. Clinicians should consider vitamin D3 deficiency and supplementation in patients with relapsing or refractory AD, she emphasized.
Dr. Sanchez-Armendariz reported no external funding sources and no conflicts of interest.
Key clinical point:
Major finding: At week 12, average improvements on the SCORAD index were 21.2 points with vitamin D3 and 13.9 points (SD, 12.3 points) with placebo (P = .02).
Data source: A double-blind, placebo-controlled, randomized trial of 65 patients with moderate to severe atopic dermatitis despite usual therapy with topical steroids, soap substitute, and topical emollient.
Disclosures: Dr. Sanchez-Armendariz did not acknowledge external funding sources. She reported having no conflicts of interest.
EC expands approval for daratumumab in MM
The European Commission (EC) has expanded the approved use of the anti-CD38 antibody daratumumab (Darzalex®).
Daratumumab is now approved for use in combination with lenalidomide and dexamethasone or bortezomib and dexamethasone for the treatment of adults with multiple myeloma (MM) who have received at least 1 prior therapy.
The EC previously granted conditional approval for daratumumab as monotherapy for the treatment of adults with relapsed or refractory MM whose prior treatment included a proteasome inhibitor and an immunomodulatory agent and who demonstrated disease progression on their last therapy.
Now, the EC has granted daratumumab full approval for this indication. The conditional approval was contingent upon Janssen, the company developing daratumumab, providing additional data from the phase 3 POLLUX and CASTOR trials.
As these data have been provided, the EC said the specific obligations associated with the conditional approval have been fulfilled, allowing the switch from conditional to full approval.
The EC’s decision to grant daratumumab approval in combination with lenalidomide and dexamethasone or bortezomib and dexamethasone was also based on data from the POLLUX and CASTOR trials.
In the POLLUX trial, researchers compared treatment with lenalidomide and dexamethasone to treatment with daratumumab, lenalidomide, and dexamethasone in patients with relapsed or refractory MM.
Patients who received daratumumab in combination had a significantly higher response rate and longer progression-free survival than patients who received the 2-drug combination.
However, treatment with daratumumab was associated with infusion-related reactions and a higher incidence of neutropenia.
Results from this trial were published in NEJM in October 2016.
In the CASTOR trial, researchers compared treatment with bortezomib and dexamethasone to treatment with daratumumab, bortezomib, and dexamethasone in patients with previously treated MM.
Patients who received the 3-drug combination had a higher response rate, longer progression-free survival, and a higher incidence of grade 3/4 adverse events than those who received the 2-drug combination.
Results from this trial were published in NEJM in August 2016. ![]()
The European Commission (EC) has expanded the approved use of the anti-CD38 antibody daratumumab (Darzalex®).
Daratumumab is now approved for use in combination with lenalidomide and dexamethasone or bortezomib and dexamethasone for the treatment of adults with multiple myeloma (MM) who have received at least 1 prior therapy.
The EC previously granted conditional approval for daratumumab as monotherapy for the treatment of adults with relapsed or refractory MM whose prior treatment included a proteasome inhibitor and an immunomodulatory agent and who demonstrated disease progression on their last therapy.
Now, the EC has granted daratumumab full approval for this indication. The conditional approval was contingent upon Janssen, the company developing daratumumab, providing additional data from the phase 3 POLLUX and CASTOR trials.
As these data have been provided, the EC said the specific obligations associated with the conditional approval have been fulfilled, allowing the switch from conditional to full approval.
The EC’s decision to grant daratumumab approval in combination with lenalidomide and dexamethasone or bortezomib and dexamethasone was also based on data from the POLLUX and CASTOR trials.
In the POLLUX trial, researchers compared treatment with lenalidomide and dexamethasone to treatment with daratumumab, lenalidomide, and dexamethasone in patients with relapsed or refractory MM.
Patients who received daratumumab in combination had a significantly higher response rate and longer progression-free survival than patients who received the 2-drug combination.
However, treatment with daratumumab was associated with infusion-related reactions and a higher incidence of neutropenia.
Results from this trial were published in NEJM in October 2016.
In the CASTOR trial, researchers compared treatment with bortezomib and dexamethasone to treatment with daratumumab, bortezomib, and dexamethasone in patients with previously treated MM.
Patients who received the 3-drug combination had a higher response rate, longer progression-free survival, and a higher incidence of grade 3/4 adverse events than those who received the 2-drug combination.
Results from this trial were published in NEJM in August 2016. ![]()
The European Commission (EC) has expanded the approved use of the anti-CD38 antibody daratumumab (Darzalex®).
Daratumumab is now approved for use in combination with lenalidomide and dexamethasone or bortezomib and dexamethasone for the treatment of adults with multiple myeloma (MM) who have received at least 1 prior therapy.
The EC previously granted conditional approval for daratumumab as monotherapy for the treatment of adults with relapsed or refractory MM whose prior treatment included a proteasome inhibitor and an immunomodulatory agent and who demonstrated disease progression on their last therapy.
Now, the EC has granted daratumumab full approval for this indication. The conditional approval was contingent upon Janssen, the company developing daratumumab, providing additional data from the phase 3 POLLUX and CASTOR trials.
As these data have been provided, the EC said the specific obligations associated with the conditional approval have been fulfilled, allowing the switch from conditional to full approval.
The EC’s decision to grant daratumumab approval in combination with lenalidomide and dexamethasone or bortezomib and dexamethasone was also based on data from the POLLUX and CASTOR trials.
In the POLLUX trial, researchers compared treatment with lenalidomide and dexamethasone to treatment with daratumumab, lenalidomide, and dexamethasone in patients with relapsed or refractory MM.
Patients who received daratumumab in combination had a significantly higher response rate and longer progression-free survival than patients who received the 2-drug combination.
However, treatment with daratumumab was associated with infusion-related reactions and a higher incidence of neutropenia.
Results from this trial were published in NEJM in October 2016.
In the CASTOR trial, researchers compared treatment with bortezomib and dexamethasone to treatment with daratumumab, bortezomib, and dexamethasone in patients with previously treated MM.
Patients who received the 3-drug combination had a higher response rate, longer progression-free survival, and a higher incidence of grade 3/4 adverse events than those who received the 2-drug combination.
Results from this trial were published in NEJM in August 2016. ![]()
Cannabidiol cuts drop seizure frequency in Lennox-Gastaut syndrome
BOSTON – Cannabidiol add-on treatment significantly reduces the frequency of drop seizures in patients with Lennox-Gastaut syndrome, according to findings from the randomized, double-blind, placebo-controlled GWPCARE4 and GWPCARE3 trials.
In GWPCARE4 – the first controlled trial of cannabidiol in Lennox-Gastaut syndrome (LGS) – 171 patients were randomized to receive either placebo or cannabidiol oral solution at a dose of 20 mg/kg per day for 14 weeks, including 2 weeks of titration and 12 weeks of maintenance therapy. The drop seizure frequency decreased by a median of 44% in the cannabidiol group vs. 22% in the placebo group. The difference between the groups was statistically significant and was apparent within the first 4 weeks of the maintenance period, Jacqueline French, MD, director of translational research and clinical trials in epilepsy at New York University Langone Medical Center reported at the annual meeting of the American Academy of Neurology.
Study participants had a mean age of 15 years, and 34% were aged 18 years or older. The median drop seizure frequency was 74 per month at baseline.
“That is a very high seizure burden,” Dr. French said, noting that the median total drop and non-drop seizure frequency was in the mid to high 100s. “So you can see that these children were very afflicted by seizures.”
Interestingly, there was a “very substantial drop” in non-drop seizures, as well, she said.
“And that’s really hard to demonstrate, so that’s impressive to me, at least,” she added.
Patients had taken a median of six antiepileptic drugs (AEDs) in their past and were taking a median of three AEDs in addition to the cannabidiol throughout the study period.
Adverse events were common, occurring in 86% of cannabidiol patients and 69% of placebo patients. The most common were diarrhea, somnolence, pyrexia, decreased appetite, and vomiting, but most were mild or moderate. Treatment-related serious adverse events were reported in nine cannabidiol patients and one placebo patient. All patients who completed the trial entered an open-label extension study, Dr. French said.
GWPCARE4 was followed by GWPCARE3, which compared both 20 mg/kg per day and 10 mg/kg per day doses of cannabidiol with placebo.
In that study, 225 patients with a mean age of 16 years (30% were aged 18 years or older) were randomized, and the median drop in seizure frequency was 42% with 20 mg/kg of cannabidiol and 37% with 10 mg/kg of cannabidiol versus 17% with placebo, Anup D. Patel, MD, reported at the meeting.
Approximately 40% and 36% of patients in the 20 mg/kg and 10 mg/kg groups, respectively, achieved the secondary outcome of a 50% or greater drop in seizure frequency, compared with 15% of placebo patients.
“Overall, 5 patients in the 20 mg/kg per day arm, 3 in the 10 [mg/kg per day arm], and 1 in the placebo arm achieved drop seizure freedom during the maintenance period of our study,” said Dr. Patel, a pediatric neurologist at Nationwide Children’s Hospital, Columbus, Ohio.
The median monthly drop seizure frequency at baseline was 80-87 in the three groups, and patients had previously failed a median of six to seven AEDs. They took a median of three AEDs during the study.
Adverse events occurred in 94% of patients in the 20-mg/kg cannabidiol group, 84% of those in the 10-mg/kg group, and 72% of placebo patients, and most were mild or moderate. The most common were somnolence and decreased appetite.
Treatment-related serious adverse events were reported in 5, 2, and 0 patients in the groups, respectively. Some elevations in transaminases were seen (in 11 patients in the 20-mg/kg dose group, and 2 in the 10-mg/kg dose group), but no patients met criteria for drug-induced liver injury with concurrent elevated bilirubin, and all cases resolved, Dr. Patel said.
Similar findings were noted in GWPCARE4, and both Dr. Patel and Dr. French noted that most patients with elevated transaminases were also taking valproic acid.
No deaths occurred in either GWPCARE4 or GWPCARE3.
“We feel both doses... produce significantly greater reductions in drop seizures as compared to placebo,” Dr. Patel said.
In an interview, he noted that the two cannabidiol doses were not compared directly to determine if the differences in response rates and adverse events were statistically significant, but said that the rates appeared comparable, and that the adverse event rates were high in all of the groups, reflecting a very sick patient population.
Of the 212 patients who completed GWPCARE3, 99% entered an open-label extension study.
Eligible patients in both trials were children and adults aged 2-55 years with a clinical diagnosis of LGS, at least two drop seizures (including tonic, atonic, and tonic-clonic seizures) each week at baseline, and documented failures on at least one AED.
Patients and caregivers in both trials were more likely to report improvement in overall condition among treated vs. placebo patients, as measured using the Subject/Caregiver Global Impression of Change scale.
The findings provide class 1 evidence that cannabidiol as add-on therapy in LGS is efficacious for reducing seizure frequency, and is generally well tolerated, Dr. French and Dr. Patel said.
Dr. French noted that enthusiasm for the trials was high, with many study sites reporting waiting lists for patient enrollment.
Some of the authors in each study have consulted for, conducted studies funded by, or received honoraria from GW Pharmaceuticals, which funded the GWPCARE4 and GWPCARE3 trials and manufacturers the oral cannabidiol solution. Several authors in each study were employees of GW Pharmaceuticals.
[email protected]
BOSTON – Cannabidiol add-on treatment significantly reduces the frequency of drop seizures in patients with Lennox-Gastaut syndrome, according to findings from the randomized, double-blind, placebo-controlled GWPCARE4 and GWPCARE3 trials.
In GWPCARE4 – the first controlled trial of cannabidiol in Lennox-Gastaut syndrome (LGS) – 171 patients were randomized to receive either placebo or cannabidiol oral solution at a dose of 20 mg/kg per day for 14 weeks, including 2 weeks of titration and 12 weeks of maintenance therapy. The drop seizure frequency decreased by a median of 44% in the cannabidiol group vs. 22% in the placebo group. The difference between the groups was statistically significant and was apparent within the first 4 weeks of the maintenance period, Jacqueline French, MD, director of translational research and clinical trials in epilepsy at New York University Langone Medical Center reported at the annual meeting of the American Academy of Neurology.
Study participants had a mean age of 15 years, and 34% were aged 18 years or older. The median drop seizure frequency was 74 per month at baseline.
“That is a very high seizure burden,” Dr. French said, noting that the median total drop and non-drop seizure frequency was in the mid to high 100s. “So you can see that these children were very afflicted by seizures.”
Interestingly, there was a “very substantial drop” in non-drop seizures, as well, she said.
“And that’s really hard to demonstrate, so that’s impressive to me, at least,” she added.
Patients had taken a median of six antiepileptic drugs (AEDs) in their past and were taking a median of three AEDs in addition to the cannabidiol throughout the study period.
Adverse events were common, occurring in 86% of cannabidiol patients and 69% of placebo patients. The most common were diarrhea, somnolence, pyrexia, decreased appetite, and vomiting, but most were mild or moderate. Treatment-related serious adverse events were reported in nine cannabidiol patients and one placebo patient. All patients who completed the trial entered an open-label extension study, Dr. French said.
GWPCARE4 was followed by GWPCARE3, which compared both 20 mg/kg per day and 10 mg/kg per day doses of cannabidiol with placebo.
In that study, 225 patients with a mean age of 16 years (30% were aged 18 years or older) were randomized, and the median drop in seizure frequency was 42% with 20 mg/kg of cannabidiol and 37% with 10 mg/kg of cannabidiol versus 17% with placebo, Anup D. Patel, MD, reported at the meeting.
Approximately 40% and 36% of patients in the 20 mg/kg and 10 mg/kg groups, respectively, achieved the secondary outcome of a 50% or greater drop in seizure frequency, compared with 15% of placebo patients.
“Overall, 5 patients in the 20 mg/kg per day arm, 3 in the 10 [mg/kg per day arm], and 1 in the placebo arm achieved drop seizure freedom during the maintenance period of our study,” said Dr. Patel, a pediatric neurologist at Nationwide Children’s Hospital, Columbus, Ohio.
The median monthly drop seizure frequency at baseline was 80-87 in the three groups, and patients had previously failed a median of six to seven AEDs. They took a median of three AEDs during the study.
Adverse events occurred in 94% of patients in the 20-mg/kg cannabidiol group, 84% of those in the 10-mg/kg group, and 72% of placebo patients, and most were mild or moderate. The most common were somnolence and decreased appetite.
Treatment-related serious adverse events were reported in 5, 2, and 0 patients in the groups, respectively. Some elevations in transaminases were seen (in 11 patients in the 20-mg/kg dose group, and 2 in the 10-mg/kg dose group), but no patients met criteria for drug-induced liver injury with concurrent elevated bilirubin, and all cases resolved, Dr. Patel said.
Similar findings were noted in GWPCARE4, and both Dr. Patel and Dr. French noted that most patients with elevated transaminases were also taking valproic acid.
No deaths occurred in either GWPCARE4 or GWPCARE3.
“We feel both doses... produce significantly greater reductions in drop seizures as compared to placebo,” Dr. Patel said.
In an interview, he noted that the two cannabidiol doses were not compared directly to determine if the differences in response rates and adverse events were statistically significant, but said that the rates appeared comparable, and that the adverse event rates were high in all of the groups, reflecting a very sick patient population.
Of the 212 patients who completed GWPCARE3, 99% entered an open-label extension study.
Eligible patients in both trials were children and adults aged 2-55 years with a clinical diagnosis of LGS, at least two drop seizures (including tonic, atonic, and tonic-clonic seizures) each week at baseline, and documented failures on at least one AED.
Patients and caregivers in both trials were more likely to report improvement in overall condition among treated vs. placebo patients, as measured using the Subject/Caregiver Global Impression of Change scale.
The findings provide class 1 evidence that cannabidiol as add-on therapy in LGS is efficacious for reducing seizure frequency, and is generally well tolerated, Dr. French and Dr. Patel said.
Dr. French noted that enthusiasm for the trials was high, with many study sites reporting waiting lists for patient enrollment.
Some of the authors in each study have consulted for, conducted studies funded by, or received honoraria from GW Pharmaceuticals, which funded the GWPCARE4 and GWPCARE3 trials and manufacturers the oral cannabidiol solution. Several authors in each study were employees of GW Pharmaceuticals.
[email protected]
BOSTON – Cannabidiol add-on treatment significantly reduces the frequency of drop seizures in patients with Lennox-Gastaut syndrome, according to findings from the randomized, double-blind, placebo-controlled GWPCARE4 and GWPCARE3 trials.
In GWPCARE4 – the first controlled trial of cannabidiol in Lennox-Gastaut syndrome (LGS) – 171 patients were randomized to receive either placebo or cannabidiol oral solution at a dose of 20 mg/kg per day for 14 weeks, including 2 weeks of titration and 12 weeks of maintenance therapy. The drop seizure frequency decreased by a median of 44% in the cannabidiol group vs. 22% in the placebo group. The difference between the groups was statistically significant and was apparent within the first 4 weeks of the maintenance period, Jacqueline French, MD, director of translational research and clinical trials in epilepsy at New York University Langone Medical Center reported at the annual meeting of the American Academy of Neurology.
Study participants had a mean age of 15 years, and 34% were aged 18 years or older. The median drop seizure frequency was 74 per month at baseline.
“That is a very high seizure burden,” Dr. French said, noting that the median total drop and non-drop seizure frequency was in the mid to high 100s. “So you can see that these children were very afflicted by seizures.”
Interestingly, there was a “very substantial drop” in non-drop seizures, as well, she said.
“And that’s really hard to demonstrate, so that’s impressive to me, at least,” she added.
Patients had taken a median of six antiepileptic drugs (AEDs) in their past and were taking a median of three AEDs in addition to the cannabidiol throughout the study period.
Adverse events were common, occurring in 86% of cannabidiol patients and 69% of placebo patients. The most common were diarrhea, somnolence, pyrexia, decreased appetite, and vomiting, but most were mild or moderate. Treatment-related serious adverse events were reported in nine cannabidiol patients and one placebo patient. All patients who completed the trial entered an open-label extension study, Dr. French said.
GWPCARE4 was followed by GWPCARE3, which compared both 20 mg/kg per day and 10 mg/kg per day doses of cannabidiol with placebo.
In that study, 225 patients with a mean age of 16 years (30% were aged 18 years or older) were randomized, and the median drop in seizure frequency was 42% with 20 mg/kg of cannabidiol and 37% with 10 mg/kg of cannabidiol versus 17% with placebo, Anup D. Patel, MD, reported at the meeting.
Approximately 40% and 36% of patients in the 20 mg/kg and 10 mg/kg groups, respectively, achieved the secondary outcome of a 50% or greater drop in seizure frequency, compared with 15% of placebo patients.
“Overall, 5 patients in the 20 mg/kg per day arm, 3 in the 10 [mg/kg per day arm], and 1 in the placebo arm achieved drop seizure freedom during the maintenance period of our study,” said Dr. Patel, a pediatric neurologist at Nationwide Children’s Hospital, Columbus, Ohio.
The median monthly drop seizure frequency at baseline was 80-87 in the three groups, and patients had previously failed a median of six to seven AEDs. They took a median of three AEDs during the study.
Adverse events occurred in 94% of patients in the 20-mg/kg cannabidiol group, 84% of those in the 10-mg/kg group, and 72% of placebo patients, and most were mild or moderate. The most common were somnolence and decreased appetite.
Treatment-related serious adverse events were reported in 5, 2, and 0 patients in the groups, respectively. Some elevations in transaminases were seen (in 11 patients in the 20-mg/kg dose group, and 2 in the 10-mg/kg dose group), but no patients met criteria for drug-induced liver injury with concurrent elevated bilirubin, and all cases resolved, Dr. Patel said.
Similar findings were noted in GWPCARE4, and both Dr. Patel and Dr. French noted that most patients with elevated transaminases were also taking valproic acid.
No deaths occurred in either GWPCARE4 or GWPCARE3.
“We feel both doses... produce significantly greater reductions in drop seizures as compared to placebo,” Dr. Patel said.
In an interview, he noted that the two cannabidiol doses were not compared directly to determine if the differences in response rates and adverse events were statistically significant, but said that the rates appeared comparable, and that the adverse event rates were high in all of the groups, reflecting a very sick patient population.
Of the 212 patients who completed GWPCARE3, 99% entered an open-label extension study.
Eligible patients in both trials were children and adults aged 2-55 years with a clinical diagnosis of LGS, at least two drop seizures (including tonic, atonic, and tonic-clonic seizures) each week at baseline, and documented failures on at least one AED.
Patients and caregivers in both trials were more likely to report improvement in overall condition among treated vs. placebo patients, as measured using the Subject/Caregiver Global Impression of Change scale.
The findings provide class 1 evidence that cannabidiol as add-on therapy in LGS is efficacious for reducing seizure frequency, and is generally well tolerated, Dr. French and Dr. Patel said.
Dr. French noted that enthusiasm for the trials was high, with many study sites reporting waiting lists for patient enrollment.
Some of the authors in each study have consulted for, conducted studies funded by, or received honoraria from GW Pharmaceuticals, which funded the GWPCARE4 and GWPCARE3 trials and manufacturers the oral cannabidiol solution. Several authors in each study were employees of GW Pharmaceuticals.
[email protected]
Key clinical point:
Major finding: The drop seizure frequency decreased by a median of 42%-44% in the 20 mg/kg per day cannabidiol groups vs. 17%-22% in the placebo groups.
Data source: The randomized, placebo-controlled GWPCARE4 and GWPCARE3 trials, including 171 and 225 patients, respectively.
Disclosures: Some of the authors in each study have consulted for, conducted studies funded by, or received honoraria from GW Pharmaceuticals, which funded the GWPCARE4 and GWPCARE3 trials and manufactures the oral cannabidiol solution. Several authors in each study were employees of GW Pharmaceuticals.
FDA approves brigatinib for second-line advanced ALK-positive NSCLC
The Food and Drug Administration has granted accelerated approval to brigatinib for the treatment of patients with metastatic anaplastic lymphoma kinase (ALK)–positive non–small cell lung cancer (NSCLC) who have progressed on or are intolerant to crizotinib.
Approval was based on a meaningful and durable overall response rate in a two-arm, open-label, multicenter trial of 222 patients with locally advanced or metastatic ALK-positive NSCLC who had progressed on crizotinib. Patients were randomized to brigatinib orally either 90 mg once daily (112 patients) or 180 mg once daily following a 7-day lead-in at 90 mg once daily (110 patients).
The median duration of response was 13.8 months in both arms.
The most common adverse reactions in 219 patients who received at least one dose were nausea, diarrhea, fatigue, cough, and headache. The most common serious adverse reactions were pneumonia and interstitial lung disease/pneumonitis. The rate of fatal adverse reactions was 3.7%; they were pneumonia in two patients and sudden death, dyspnea, respiratory failure, pulmonary embolism, bacterial meningitis, and urosepsis in one patient each. Visual disturbances also occurred in patients receiving brigatinib. The FDA cautions that patients receiving brigatinib should be monitored for new or worsening respiratory symptoms; hypertension; bradycardia; visual symptoms; and elevations in amylase, lipase, blood glucose, and creatine phosphokinase.
The recommended dosing of brigatinib, marketed as Alunbrig by Takeda Pharmaceutical, is 90 mg orally once daily for the first 7 days then, if tolerated, increase to 180 mg orally once daily.
Full prescribing information is available here.
The Food and Drug Administration has granted accelerated approval to brigatinib for the treatment of patients with metastatic anaplastic lymphoma kinase (ALK)–positive non–small cell lung cancer (NSCLC) who have progressed on or are intolerant to crizotinib.
Approval was based on a meaningful and durable overall response rate in a two-arm, open-label, multicenter trial of 222 patients with locally advanced or metastatic ALK-positive NSCLC who had progressed on crizotinib. Patients were randomized to brigatinib orally either 90 mg once daily (112 patients) or 180 mg once daily following a 7-day lead-in at 90 mg once daily (110 patients).
The median duration of response was 13.8 months in both arms.
The most common adverse reactions in 219 patients who received at least one dose were nausea, diarrhea, fatigue, cough, and headache. The most common serious adverse reactions were pneumonia and interstitial lung disease/pneumonitis. The rate of fatal adverse reactions was 3.7%; they were pneumonia in two patients and sudden death, dyspnea, respiratory failure, pulmonary embolism, bacterial meningitis, and urosepsis in one patient each. Visual disturbances also occurred in patients receiving brigatinib. The FDA cautions that patients receiving brigatinib should be monitored for new or worsening respiratory symptoms; hypertension; bradycardia; visual symptoms; and elevations in amylase, lipase, blood glucose, and creatine phosphokinase.
The recommended dosing of brigatinib, marketed as Alunbrig by Takeda Pharmaceutical, is 90 mg orally once daily for the first 7 days then, if tolerated, increase to 180 mg orally once daily.
Full prescribing information is available here.
The Food and Drug Administration has granted accelerated approval to brigatinib for the treatment of patients with metastatic anaplastic lymphoma kinase (ALK)–positive non–small cell lung cancer (NSCLC) who have progressed on or are intolerant to crizotinib.
Approval was based on a meaningful and durable overall response rate in a two-arm, open-label, multicenter trial of 222 patients with locally advanced or metastatic ALK-positive NSCLC who had progressed on crizotinib. Patients were randomized to brigatinib orally either 90 mg once daily (112 patients) or 180 mg once daily following a 7-day lead-in at 90 mg once daily (110 patients).
The median duration of response was 13.8 months in both arms.
The most common adverse reactions in 219 patients who received at least one dose were nausea, diarrhea, fatigue, cough, and headache. The most common serious adverse reactions were pneumonia and interstitial lung disease/pneumonitis. The rate of fatal adverse reactions was 3.7%; they were pneumonia in two patients and sudden death, dyspnea, respiratory failure, pulmonary embolism, bacterial meningitis, and urosepsis in one patient each. Visual disturbances also occurred in patients receiving brigatinib. The FDA cautions that patients receiving brigatinib should be monitored for new or worsening respiratory symptoms; hypertension; bradycardia; visual symptoms; and elevations in amylase, lipase, blood glucose, and creatine phosphokinase.
The recommended dosing of brigatinib, marketed as Alunbrig by Takeda Pharmaceutical, is 90 mg orally once daily for the first 7 days then, if tolerated, increase to 180 mg orally once daily.
Full prescribing information is available here.
Increased Length of Stay for Invasive Monitoring Does Not Reduce Hospital’s Bottom Line
Length of stay (LOS) may not be the best metric to use when trying to determine the cost effectiveness of invasive monitoring with subdural grid implantation or stereoelectroencephalography, suggests a recent analysis of 76 patients over a 2 year period. Researchers found that as LOS increased a hospital’s profit and contribution margins also increased, while patients maintained a low rate of complications. Investigators concluded that increased LOS does not necessarily result in lower financial gain for institutions.
Chan AY, Kharrat S. Lundeen K, et al. Length of stay for patients undergoing invasive electrode monitoring with stereoelectroencephalography and subdural grids correlates positively with increased institutional profitability [published online April 20, 2017]. Epilespia. doi:10.1111/epi.13737
Length of stay (LOS) may not be the best metric to use when trying to determine the cost effectiveness of invasive monitoring with subdural grid implantation or stereoelectroencephalography, suggests a recent analysis of 76 patients over a 2 year period. Researchers found that as LOS increased a hospital’s profit and contribution margins also increased, while patients maintained a low rate of complications. Investigators concluded that increased LOS does not necessarily result in lower financial gain for institutions.
Chan AY, Kharrat S. Lundeen K, et al. Length of stay for patients undergoing invasive electrode monitoring with stereoelectroencephalography and subdural grids correlates positively with increased institutional profitability [published online April 20, 2017]. Epilespia. doi:10.1111/epi.13737
Length of stay (LOS) may not be the best metric to use when trying to determine the cost effectiveness of invasive monitoring with subdural grid implantation or stereoelectroencephalography, suggests a recent analysis of 76 patients over a 2 year period. Researchers found that as LOS increased a hospital’s profit and contribution margins also increased, while patients maintained a low rate of complications. Investigators concluded that increased LOS does not necessarily result in lower financial gain for institutions.
Chan AY, Kharrat S. Lundeen K, et al. Length of stay for patients undergoing invasive electrode monitoring with stereoelectroencephalography and subdural grids correlates positively with increased institutional profitability [published online April 20, 2017]. Epilespia. doi:10.1111/epi.13737
Uniform Approach Needed for Death Certification in SUDEP
Death certificates fail to consistently report sudden unexplained death in epilepsy (SUDEP), making it difficult for researchers to study the phenomenon, according to a recent survey sent to medical examiners (MEs). Among 847 responses on 11 case vignettes, MEs did not use the ICD-10 seizure code in 3% to 62% of cases, depending on the specific vignette. Several factors may be responsible for the shortfall, including the complicated nature of SUDEP and uncertain circumstances involved in any individual death.
Atherton DS, Davis GG, Wright C, Devinsky O, Hesdorffer D. A Survey of medical examiner death certification of vignettes on death in epilepsy: gaps in identifying SUDEP [published online April 21, 2017]. Epilepsy Res. doi: http://dx.doi.org/10.1016/j.eplepsyres.2017.04.013.
Death certificates fail to consistently report sudden unexplained death in epilepsy (SUDEP), making it difficult for researchers to study the phenomenon, according to a recent survey sent to medical examiners (MEs). Among 847 responses on 11 case vignettes, MEs did not use the ICD-10 seizure code in 3% to 62% of cases, depending on the specific vignette. Several factors may be responsible for the shortfall, including the complicated nature of SUDEP and uncertain circumstances involved in any individual death.
Atherton DS, Davis GG, Wright C, Devinsky O, Hesdorffer D. A Survey of medical examiner death certification of vignettes on death in epilepsy: gaps in identifying SUDEP [published online April 21, 2017]. Epilepsy Res. doi: http://dx.doi.org/10.1016/j.eplepsyres.2017.04.013.
Death certificates fail to consistently report sudden unexplained death in epilepsy (SUDEP), making it difficult for researchers to study the phenomenon, according to a recent survey sent to medical examiners (MEs). Among 847 responses on 11 case vignettes, MEs did not use the ICD-10 seizure code in 3% to 62% of cases, depending on the specific vignette. Several factors may be responsible for the shortfall, including the complicated nature of SUDEP and uncertain circumstances involved in any individual death.
Atherton DS, Davis GG, Wright C, Devinsky O, Hesdorffer D. A Survey of medical examiner death certification of vignettes on death in epilepsy: gaps in identifying SUDEP [published online April 21, 2017]. Epilepsy Res. doi: http://dx.doi.org/10.1016/j.eplepsyres.2017.04.013.