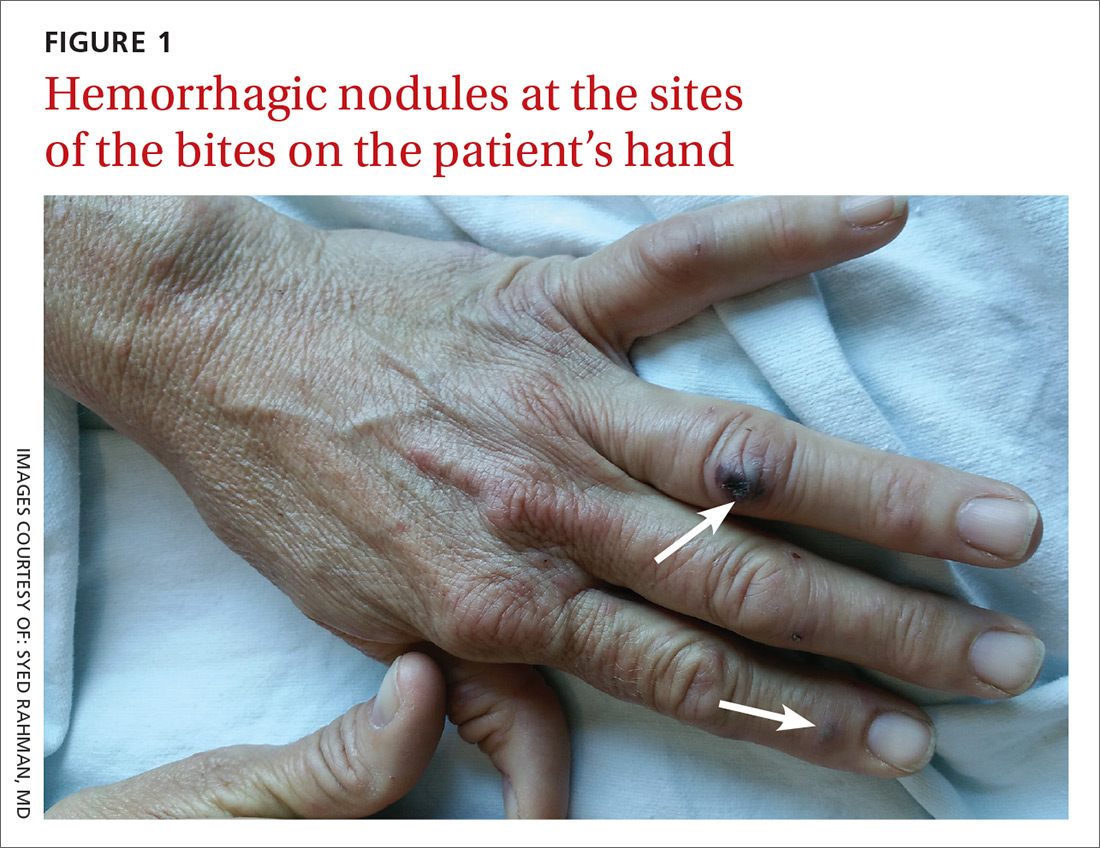
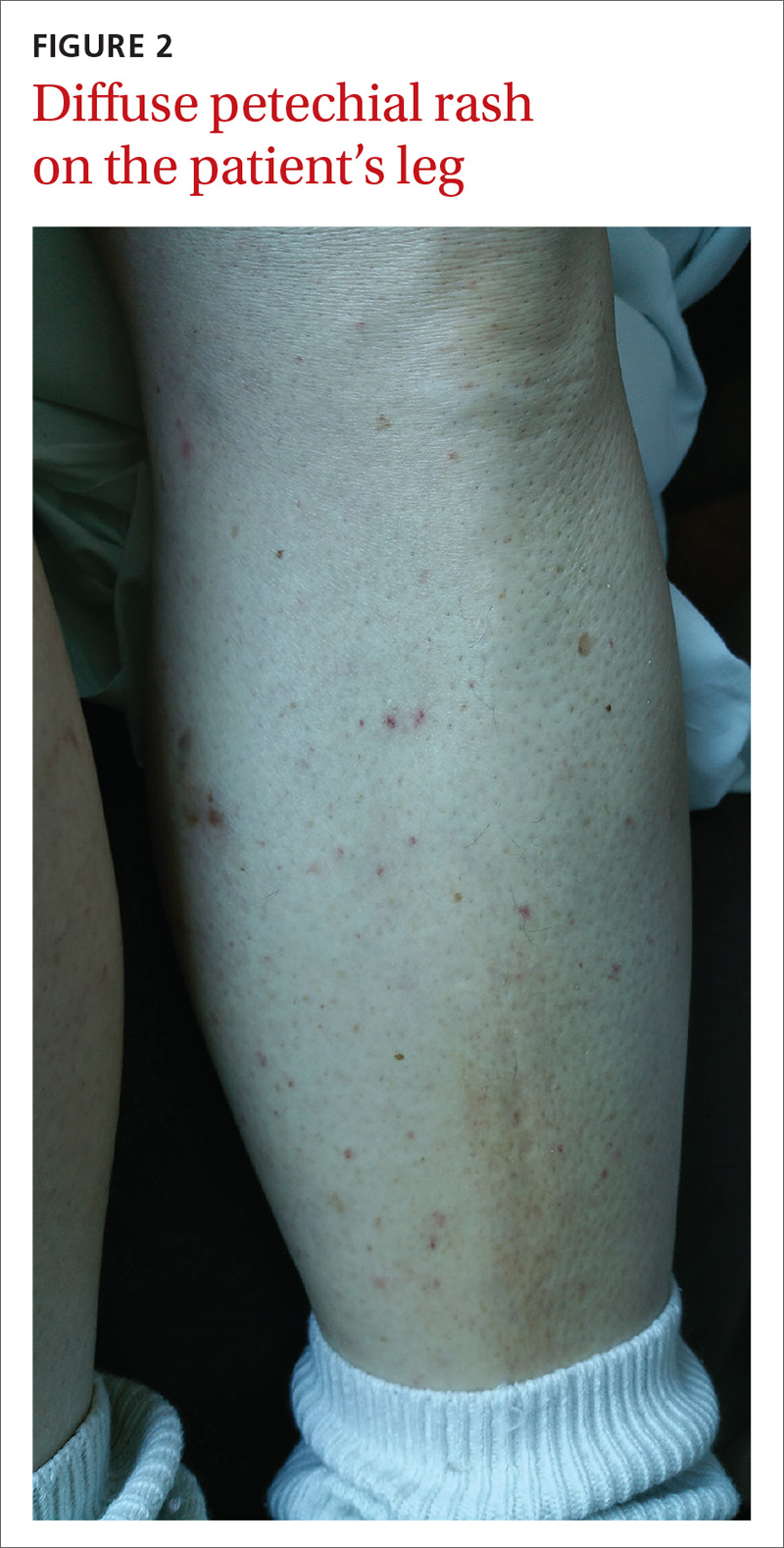
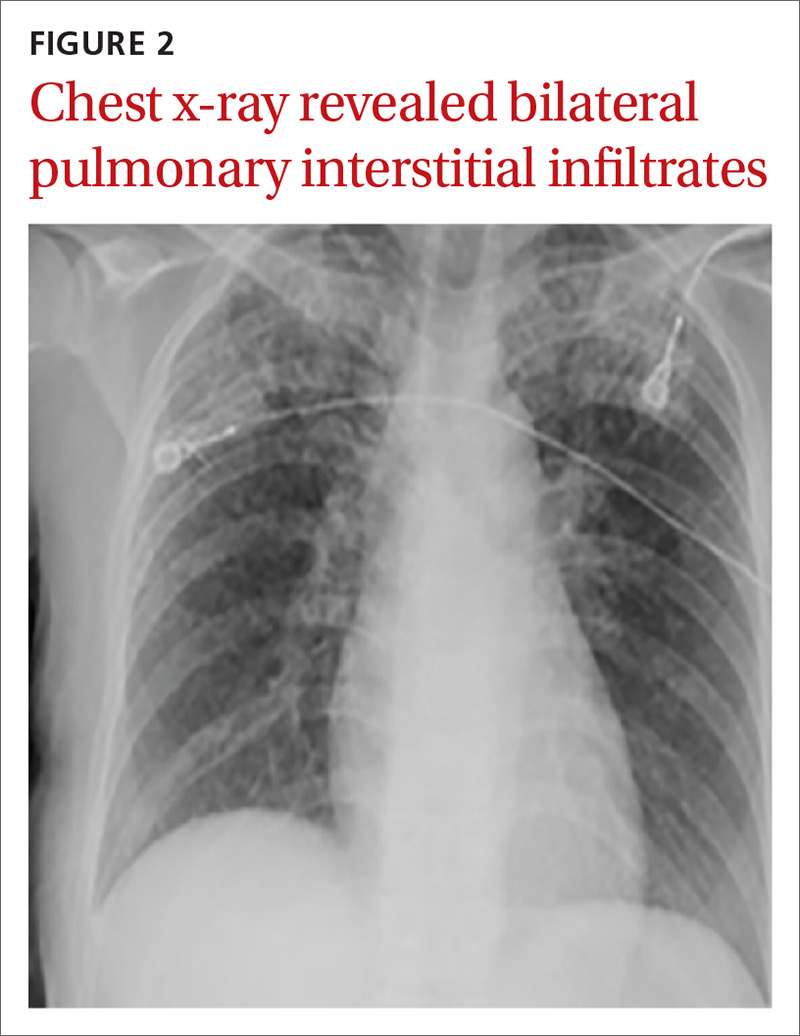
User login
Opioid abuse
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Reducing CV risk in diabetes: An ADA update
More than 29 million Americans have diabetes, and each year another 1.7 million are given the diagnosis.1 Prediabetes is even more common; over one-third of US adults ages 20 years and older, and more than half of those who are ages 65 and older, have attained this precursor status, representing another 86 million Americans.1
Because the evidence base for the management of diabetes is rapidly expanding, the American Diabetes Association’s (ADA) Professional Practice Committee updates its Standards of Medical Care in Diabetes annually to incorporate new evidence into its recommendations. The 2017 Standards of Care are available at: professional.diabetes.org/jfp.2
Atherosclerotic cardiovascular disease (ASCVD) is the leading cause of morbidity and mortality for people with diabetes, and is the largest contributor to the direct and indirect costs of the disease.2 As a result, all patients with diabetes should have cardiovascular (CV) risk factors, including dyslipidemia, hypertension, smoking, a family history of premature coronary disease, and the presence of albuminuria, assessed at least annually.2 Numerous studies have demonstrated the efficacy of controlling individual CV risk factors in preventing or slowing ASCVD in people with diabetes. Even larger benefits, including reduced ASCVD morbidity and mortality, can be achieved when multiple risk factors are addressed simultaneously.3
To hone your management of CV risks in patients with diabetes, we’ve put together this Q&A pointing out the elements of the ADA’s 2017 Standards of Care that are most relevant to the management of patients at risk for, or with established, ASCVD.
Screening
Since ASCVD so commonly co-occurs with diabetes, should I routinely screen asymptomatic patients with diabetes for heart disease?
No. The current evidence suggests that outcomes are NOT improved by screening people before they develop symptoms of ASCVD,4 and widespread ASCVD screening has not been shown to be cost-effective. Cardiac testing should be reserved for those with typical or atypical symptoms or those with an abnormal resting electrocardiogram (EKG).
Lifestyle modification
What are the benefits of lifestyle interventions?
The benefits include not only lost pounds, but improved mobility, physical and sexual functioning, and health-related quality of life. Recommend that all overweight patients with diabetes take advantage of intensive lifestyle interventions focusing on weight loss through decreased caloric intake and increased physical activity as per the Look AHEAD (Action for Health in Diabetes) trial.5 Although the intensive lifestyle intervention in the Look AHEAD trial did not decrease CV outcomes over 10 years of follow-up, it did improve control of CV risk factors and led to people in the intervention group taking fewer glucose-, blood pressure (BP)-, and lipid-lowering medications than those in the standard care group.
There is no one diet that is recommended for all people with diabetes. Weight reduction often requires intensive intervention. In order for weight loss diets to be sustainable, they must include patient preferences.
People with diabetes should be encouraged to receive individualized medical nutrition therapy (MNT), preferably from a registered dietitian who is well versed in nutritional management for diabetes. Such MNT is associated with a 0.5% to 2% decrease in A1c levels for people with type 2 diabetes.6-9 Specific healthy diets include the Mediterranean, Dietary Approaches to Stop Hypertension (DASH), and plant-based diets.
A new lifestyle recommendation in this year’s ADA Standards is that periods of prolonged sitting should be interrupted every 30 minutes with a period of physical activity. This appears to have glycemic benefits.2
Hypertension/BP management
When should I initiate hypertension treatment in patients with diabetes?
Nonpharmacologic therapy is reasonable in people with diabetes and mildly elevated BP (>120/80 mm Hg). If systolic blood pressure (SBP) is confirmed to be >140 mm Hg and/or diastolic blood pressure (DBP) is confirmed to be >90 mm Hg, the ADA recommends initiating pharmacologic therapy along with nonpharmacologic strategies. For patients with confirmed office-based BP >160/100 mm Hg, the ADA advises initiating lifestyle modifications as well as 2 pharmacologic medications (or a single pill combination of agents).2
What is the recommended BP target for patients with diabetes and hypertension?
These patients should be treated with a combination of measures, including lifestyle modification and pharmacologic therapy, to a target BP of <140/90 mm Hg. Randomized controlled trials (RCTs) have shown benefits with this target in terms of a reduction in the incidence of coronary heart disease (CHD) events, stroke, and diabetic kidney disease.10,11
A 2012 meta-analysis of randomized trials involving adults with type 2 diabetes mellitus (T2DM) and comparing intensive BP targets (≤130 mm Hg SBP and ≤80 mm Hg DBP) with standard targets (≤140-160 mm Hg SBP and ≤85-100 mm Hg DBP) found no significant reduction in mortality or nonfatal MIs associated with more intense BP control. There was a statistically significant 35% relative risk (RR) reduction in stroke with intensive targets, but lower BP was also associated with an increased risk of hypotension and syncope.12
The 2010 Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial,13 which randomized 5518 patients with T2DM at high risk for ASCVD to either a target SBP of <120 mm Hg or 130 to 140 mm Hg, found that the patients with the lower SBP target did not benefit in the primary end point (a composite of nonfatal MI, nonfatal stroke, and CV death), but did benefit from nominally significant lower rates of total stroke and nonfatal stroke.
Based on these data, the ADA Standards of Care suggest that, “more intensive BP control may be reasonable in certain motivated, ACCORD-like patients (40-79 years of age with prior evidence of CVD or multiple CV risk factors) who have been educated about the added treatment burden, side effects, and costs of more intensive BP control and for patients who prefer to lower their risk of stroke beyond what can be achieved with usual care.”
Another major study, the 2015 Systolic Blood Pressure Intervention Trial (SPRINT) trial,14 demonstrated that treating patients with hypertension to a target SBP <120 mm Hg compared to the usual target of <140 mm Hg resulted in a 25% lower RR of the primary outcome (a composite of MI, other acute coronary syndromes, stroke, heart failure, or death from CV causes) and about a 25% reduction in all-cause mortality; however, people with diabetes were not included in the trial, so the applicability of the results to decisions about BP management in patients with diabetes is not known.
A 2015 systematic review and meta-analysis of over 100,000 participants looked at SBP lowering in adults with T2DM and found that each 10-mm Hg reduction in SBP was associated with a significantly lower risk of morbidity, CV events, CHD, stroke, albuminuria, and retinopathy.10 When trials were stratified by mean baseline SBP (<140 mm Hg or ≥140 mm Hg), RRs for outcomes other than stroke, retinopathy, and renal failure were lower in studies with greater baseline SBP.
The latest ADA Standards of Care recommend that a lower BP target of 130/80 mm Hg may be appropriate for patients at high risk of CVD if this target can be achieved without undue treatment burden. A DBP of <80 mm Hg may also be appropriate in certain patients including those with a long life expectancy, CKD, elevated urinary albumin excretion, and those with evidence of CVD or associated risk factors.15 Of note, treating older adults with diabetes to an SBP target of <130 mm Hg has not been shown to improve cardiovascular outcomes,16 and treating to a diastolic target of <70 mm Hg has been associated with a greater risk of mortality.17
What are the current recommended treatment options?
Treatment for hypertension in adults with diabetes without albuminuria should include any of the classes of medications demonstrated to reduce CV events in patients with diabetes, such as:
- angiotensin-converting enzyme (ACE) inhibitors,
- angiotensin receptor blockers (ARBs),
- thiazide-like diuretics, and
- dihydropyridine calcium channel blockers.
These recommendations are based on evidence suggesting the lack of superiority of ACE inhibitors and ARBs over other classes of antihypertensive agents for the prevention of CV outcomes in all patients with diabetes.18 However, in people with diabetes at high risk for ASCVD and/or with albuminuria, ACE inhibitors and ARBs do reduce ASCVD outcomes and the progression of kidney disease.19-24 Thus, ACE inhibitors and ARBs continue to be recommended as first-line medications for the treatment of hypertension in patients with diabetes and urine albumin/creatinine ratios ≥30 mg/g, as these medications are associated with a reduction in the rate of kidney disease progression.
The use of both an ACE inhibitor and an ARB in combination is not recommended.25,26 For patients treated with ACE inhibitors, ARBs, or diuretics, serum creatinine/estimated glomerular filtration rate (eGFR) and serum potassium levels should be monitored.
What are the recommended lifestyle modifications for patients with diabetes and hypertension?
Regular exercise and healthy eating are recommended for all people with diabetes to optimize glycemic control and lose weight (if they are overweight or obese). For patients with hypertension, the DASH diet (available at: https://www.nhlbi.nih.gov/health/health-topics/topics/dash/) is effective at lowering BP. The DASH diet emphasizes reducing sodium intake, increasing potassium intake, limiting alcohol intake, and increasing physical activity. Specifically, sodium intake should be restricted to <2300 mg/d and patients should consume approximately 8 to 10 servings of fruits and vegetables per day and 2 to 3 servings of low-fat dairy per day. Alcohol should be limited to 2 drinks per day for men and one drink per day for women.
Most adults with diabetes should perform 150 minutes per week of moderate to vigorous exercise, spread over at least 3 days/week. In addition, it is recommended that resistance exercises be performed at least 2 to 3 days/week. Prolonged inactivity is detrimental to health and should be interrupted with activity every 30 minutes.27
Finally, as a part of lifestyle management for all patients with diabetes, smoking cessation is important, as is attention to stress, depression, and anxiety.
Is there an advantage to nighttime dosing of antihypertensive medications?
Yes. Growing evidence suggests that there is an ASCVD benefit to avoiding nocturnal BP dipping. A 2011 RCT of 448 participants with T2DM and hypertension showed a decrease in CV events and mortality during 5.4 years of follow-up if at least one antihypertensive medication was taken at bedtime.28 As a result of this and other evidence,29 consider administering one or more antihypertensive medications at bedtime, although this is not a formal recommendation in the ADA Standards of Care.
Are there any additional issues to be aware of when treating patients with diabetes and hypertension?
Yes. Sometimes patients who have had diabetes for many years have significant orthostatic hypotension secondary to autonomic neuropathy. Postural changes in BP and pulse may require adjustment of BP targets. Home BP self-monitoring and 24-hour ambulatory BP monitoring may indicate white-coat or masked hypertension.
Lipid management
What is the current evidence for lipid treatment in diabetes?
Lipid abnormalities are common in people with diabetes and contribute to the overall high risk of ASCVD in these patients. Subgroup analyses of patients in large trials with diabetes30 and trials involving patients with diabetes31 have shown significant improvements in primary and secondary prevention of ASCVD with statin use. A 2008 meta-analysis of 18,686 people with diabetes showed a 9% reduction in all-cause mortality and a 13% reduction in vascular mortality for each 39-mg/dL reduction in low-density lipoprotein (LDL) cholesterol.32 Absolute reductions in mortality are greatest in those with highest risk, but the benefits of statin therapy are clear for low- and moderate-risk individuals with diabetes, too.33,34 As a result, statins are the medications of choice for lipid lowering and CV risk reduction and should be used in addition to lifestyle management.
Who should get a statin, and how do I choose the optimum dosage?
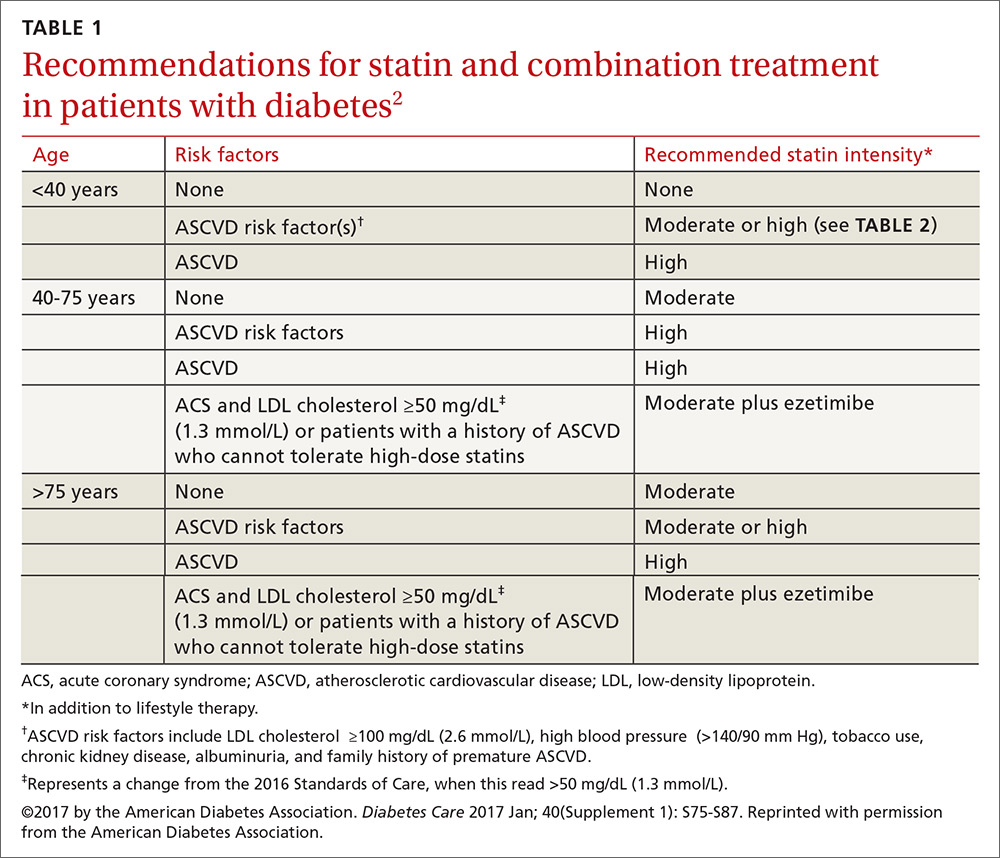
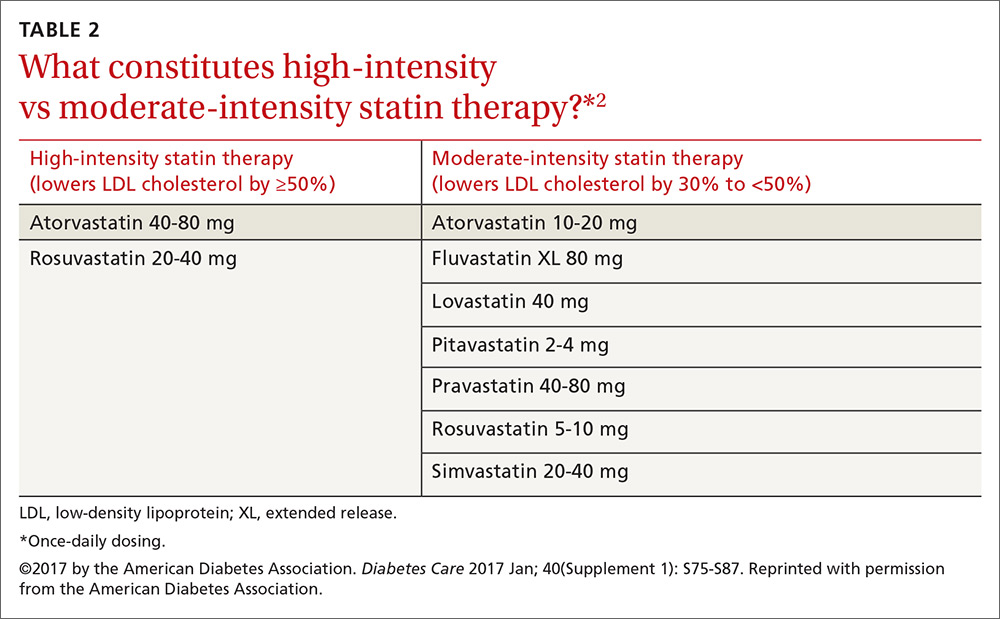
Patients ages 40 to 75 years with diabetes but without additional ASCVD risk factors should receive a moderate-intensity statin, according to the ADA (see TABLES 12 and 22). For those with additional CV risk factors, a high-intensity statin should be considered. The American College of Cardiology/American Heart Association ASCVD risk calculator (available at: http://www.cvriskcalculator.com/) may be useful for some patients, but generally, risk is already known to be high for most patients with diabetes. For patients of all ages with diabetes and established ASCVD, high-intensity statin therapy should be added to lifestyle modifications.35-37
For patients with diabetes who are <40 years with additional ASCVD risk factors, few clinical trial data exist; nevertheless, consider a moderate- or high-intensity statin and lifestyle therapy. Similarly, for patients >75 years who have diabetes and no additional ASCVD risk factors, consider a moderate-intensity statin and lifestyle modifications. For older adults with additional ASCVD risk factors, consider high-intensity statin therapy.35-37
Statins and cognition. It should be noted that published data have not demonstrated an adverse effect of statins on cognition.38 Statins, however, have been linked to an increased risk of developing diabetes,39,40 although the absolute increase in risk is small, and much smaller than the benefit derived from preventing the development of coronary disease.
Should total cholesterol and LDL levels be used as targets with statin treatment?
No. Statin doses have primarily been tested against placebo in clinical trials, rather than testing to specific target LDL levels, suggesting that the initiation and intensification of statin therapy be based on a patient’s risk profile.35 When maximally tolerated doses of statins do not lower LDL cholesterol by more than 30% from the patient’s baseline, there is currently no good evidence that combination therapy would be helpful, so regular monitoring of lipid levels has limited value. A lipid profile that includes levels of total cholesterol, LDL cholesterol, high-density lipoprotein (HDL) cholesterol, and triglycerides should be obtained at initial medical evaluation, at diagnosis of diabetes, and every 5 years thereafter or before the initiation of statin therapy. Ongoing testing may be appropriate in individual circumstances and to monitor for adherence to, or efficacy of, therapy.
What should I do for my patients who can’t tolerate statins?
Try a lower dose or a different statin before eliminating the class. Research has shown that even small doses (eg, rosuvastatin 5 mg) have some benefit.41
How do combination treatments figure into the current treatment of lipids in patients with diabetes?
It depends on the agent and the patient’s profile.
Fenofibrate. The ADA does not recommend automatically adding fenofibrate to statin therapy because the combination is associated with increased risks for abnormal transaminase levels, myositis, and rhabdomyolysis. In the ACCORD trial, the combination of fenofibrate and simvastatin did not reduce the rate of fatal CV events, nonfatal MIs, or nonfatal strokes compared with simvastatin alone.42
That said, a subgroup analysis suggested a benefit for men with both a triglyceride level ≥204 mg/dL (2.3 mmol/L) and an HDL cholesterol level ≤34 mg/dL (0.9 mmol/L).42 For this reason, the combination of a statin and fenofibrate may be considered for men who meet these laboratory parameters. In addition, consider medical therapy for triglyceride levels ≥500 mg/dL to reduce the risk of pancreatitis.
Ezetimibe. Recommendations regarding ezetimibe are based on the IMPROVE-IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial), a 2015 RCT including over 18,000 patients that compared treatment with ezetimibe and simvastatin to simvastatin alone.43 Individuals in the trial were ≥50 years of age and had experienced an ACS within the preceding 10 days. In those with diabetes, the combination of moderate-intensity simvastatin (40 mg) and ezetimibe (10 mg) significantly reduced major adverse CV events with an absolute risk reduction of 5% (40% vs 45%) and an RR reduction of 14% over moderate-intensity simvastatin (40 mg) alone.
Based on these results, patients with diabetes and a recent ACS should be considered for combination therapy with ezetimibe and a moderate-intensity statin. The combination should also be considered in patients with diabetes and a history of ASCVD who cannot tolerate high-intensity statins.43
Niacin. The ADA currently does not recommend niacin in combination with a statin because of lack of efficacy on major ASCVD outcomes, possible increased risk of ischemic stroke, and adverse effects.44
What are the recommendations for the use of PCSK-9 inhibitors?
Proprotein convertase subtilisin/kexin type 9 (PCSK-9) inhibitors (ie, evolucumab and alirocumab) may be considered as adjunctive therapy to statins for patients with diabetes at high risk for ASCVD events who require additional lowering of LDL cholesterol. They may also be considered for those in whom high-intensity statin therapy is indicated, but not tolerated.
Antiplatelet agents
Who should take aspirin for primary prevention of CVD?
Both women and men ages ≥50 years who have diabetes and at least one additional CV risk factor (family history of premature ASCVD, hypertension, tobacco use, dyslipidemia, or albuminuria) should consider taking daily aspirin therapy (75-162 mg/d) if they do not have an excessive bleeding risk.45,46 The most common dose in the United States is 81 mg. This recommendation is supported by a 2010 consensus statement of the American Diabetes Association, American Heart Association, and the American College of Cardiology.47
Should patients with diabetes and heart disease receive antiplatelet therapy?
Yes. The evidence is clear that people with known diabetes and ASCVD benefit from aspirin therapy, according to the 2017 Standards of Care. Clopidogrel 75 mg/d is an appropriate alternative for patients who are allergic to aspirin. Dual antiplatelet therapy (a P2Y12 receptor antagonist and aspirin) should be used for as long as one year after an ACS and may have benefits beyond this period.48
Established heart disease
Are there specific recommendations for patients with diabetes and CHD?
According to the ADA Standards, there is good evidence that both aspirin and statin therapy are beneficial for patients with known ASCVD, and that high-intensity statin therapy should be used. In addition, consider ACE inhibitors to reduce the future risk of CV events. In patients with a prior MI, continue beta-blocker therapy for at least 2 years post event.49
Which medications should I avoid, or approach with caution, in patients with congestive heart failure (CHF)?
Thiazolidinediones, dipeptidyl peptidase 4 (DPP-4) inhibitors, and metformin all require careful attention. This is especially important to know when you consider that almost half of all patients with T2DM will develop heart failure.50
Thiazolidinediones. The 2017 Standards of Care state that patients with diabetes and symptomatic congestive heart failure should not receive thiazolidinediones, as they can worsen heart failure status via fluid retention. As such, they are contraindicated in patients with class III and IV heart failure.51
DPP-4 inhibitors. The studies on DPP-4 inhibitors and heart failure have had mixed results. The 2013 SAVOR-TIMI (Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus–Thrombolysis in Myocardial Infarction) 53 trial52 showed that patients treated with saxagliptin were more likely to be hospitalized for heart failure than those taking placebo (3.5% vs 2.8%, respectively). However, the 2015 EXAMINE (Examination of Cardiovascular Outcomes with Alogliptin vs Standard of Care)53 trial and the 2015 TECOS (Trial Evaluating Cardiovascular Outcomes with Sitagliptin)54 trial evaluated heart failure and mortality outcomes in patients with alogliptin and sitagliptin, respectively, compared to placebo, and did not show a relationship to heart failure.
Metformin may be used in people who have T2DM and stable CHF if their eGFR remains >30 mL/min; it should be withheld from patients with unstable heart failure and those who are hospitalized with CHF.
Are there antihyperglycemic medications that reduce CV morbidity and mortality in those with established ASCVD?
Yes. This year’s ADA Standards indicate that certain glucose-lowering medications—specifically empagliflozin (a sodium–glucose cotransporter [SGLT]-2 inhibitor) and liraglutide (a glucagon-like peptide [GLP]-1 receptor agonist)—have been shown to be beneficial for those with established CVD. According to the 2017 Standards of Care, “In patients with longstanding suboptimally controlled T2DM and established ASCVD, empagliflozin or liraglutide should be considered, as they have been shown to reduce CV and all-cause mortality when added to standard care.”2 The studies that provide support for their use are summarized below. Ongoing studies are investigating the CV effects of other agents in these drug classes.
Empagliflozin. The 2015 EMPA-REG OUTCOME (Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients) study55 was a randomized double-blind study of empagliflozin vs placebo and usual care in patients with diabetes and established CVD. Over a median follow-up of 3.1 years, treatment with empagliflozin reduced the aggregate outcome of MI, stroke, and CV death by 14%, reduced CV deaths by 38%, and decreased deaths from any cause by 32%. In December 2016, the FDA announced a new indication for empagliflozin: to reduce the risk of CV death in adult patients with T2DM and CVD.56
Liraglutide. The LEADER (Liraglutide Effect and Action in Diabetes Evaluation of Cardiovascular Outcome Results: A Long Term Evaluation) trial57 was a double-blind randomized trial of liraglutide vs placebo added to usual care in patients with T2DM at high risk for CVD or with existing CVD. More than 80% of the participants had existing CVD including a history of prior MI, cerebrovascular disease, or peripheral vascular disease. After a median follow-up of 3.8 years, the group taking liraglutide demonstrated a 13% reduction in the composite outcome of MI, stroke, or CV death, a 22% reduction in CV death, and a 15% reduction in death from any cause, compared with placebo.57
CORRESPONDENCE
Neil Skolnik, MD, Abington-Jefferson Health, 500 Old York Rd, Ste 108, Jenkintown, PA 19046; [email protected].
The authors thank Sarah Bradley, director, professional engagement & collaboration at the American Diabetes Association, for her editorial and organizational assistance in the preparation of this manuscript.
1. Centers for Disease Control and Prevention. National Center for Chronic Disease Prevention and Health Promotion. National diabetes statistics report, 2014. Estimates of diabetes and its burden in the United States. Available at: http://templatelab.com/national-diabetes-report-2014/. Accessed April 7, 2017.
2. American Diabetes Association. Standards of Medical Care in Diabetes—2017. Available at: http://professional.diabetes.org/sites/professional.diabetes.org/files/media/dc_40_s1_final.pdf. Accessed April 7, 2017.
3. Gaede P, Lund-Andersen H, Parving HH, et al. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358:580-591.
4. Bax JJ, Young LH, Frye RL, et al; American Diabetes Association. Screening for coronary artery disease in patients with diabetes. Diabetes Care. 2007;30:2729-2736.
5. The Look AHEAD Research Group. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369:145-154.
6. UK Prospective Diabetes Study (UKDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKDS 34). Lancet. 1998;352:854-865.
7. Ziemer DC, Berkowitz KJ, Panayioto RM, et al. A simple meal plan emphasizing healthy food choices is as effective as an exchange-based meal plan for urban African Americans with type 2 diabetes. Diabetes Care. 2003;26:1719-1724.
8. Wolf AM, Conaway RM, Crowther JQ, et al; Improving Control with Activity and Nutrition (ICAN) Study. Translating lifestyle intervention to practice in obese patients with type 2 diabetes: Improving Control with Activity and Nutrition (ICAN) study. Diabetes Care. 2004;27:1570-1576.
9. Coppell KJ, Kataoka M, Williams SM, et al. Nutritional intervention in patients with type 2 diabetes who are hyperglycaemic despite optimised drug treatment-Lifestyle Over and Above Drugs in Diabetes (LOADD) study: randomised controlled trial. BMJ. 2010;341:c3337.
10. Emdin CA, Rahimi K, Neal B, et al. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2015;313:603-615.
11. Arguedas JA, Leiva V, Wright JM. Blood pressure targets for hypertension in people with diabetes mellitus. Cochrane Database Syst Rev. 2013;10:CD008277.
12. McBrien K, Rabi DM, Campbell N, et al. Intensive and standard blood pressure targets in patients with type 2 diabetes mellitus: systematic review and meta-analysis. Arch Intern Med. 2012;172:1296-1303.
13. ACCORD Study Group, Cushman WC, Evans GW, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575-1585.
14. SPRINT Research Group, Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
15. Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet. 1998;351:1755-1762.
16. Kirkman MS, Briscoe VJ, Clark N, et al. Diabetes in older adults. Diabetes Care. 2012;35:2650-2664.
17. Anderson RJ, Bahn GD, Moritz TE, et al; VADT Study Group. Blood pressure and cardiovascular disease risk in the Veterans Affairs Diabetes Trial. Diabetes Care. 2011;34:34-38.
18. Bangalore S, Fakheri R, Toklu B, et al. Diabetes mellitus as a compelling indication for use of renin angiotensin system blockers: systematic review and meta-analysis of randomized trials. BMJ. 2016;352:i438.
19. Heart Outcomes Prevention Evaluation Study Investigators. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Lancet. 2000;355:253-259.
20. Granger CB, McMurray JJ, Yusuf S, et al; CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting enzyme inhibitors: the CHARM-Alternative trial. Lancet. 2003;362:772-776.
21. McMurray JJ, Ostergren J, Swedberg K, et al; CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. Lancet. 2003;362:767-771.
22. Pfeffer MA, Swedberg K, Granger CB, et al; CHARM Investigators and Committees. Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-Overall programme. Lancet. 2003;362:759-766.
23. Brenner BM, Cooper ME, de Zeeuw D, et al; RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861-869.
24. Palmer SC, Mavridis D, Navarese E, et al. Comparative efficacy and safety of blood pressure-lowering agents in adults with diabetes and kidney disease: a network meta-analysis. Lancet. 2015;385:2047-2056.
25. The ONTARGET Investigators. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547-1559.
26. Fried LF, Emanuele N, Zhang JH, et al; VA NEPHRON-D Investigators. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med. 2013;369:1892-1903.
27. Colberg SR, Sigal RJ, Yardley JE, et al. Physical activity/exercise and diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2016;39:2065-2079.
28. Hermida RC, Ayala DE, Mojón A, et al. Influence of time of day of blood pressure-lowering treatment on cardiovascular risk in hypertensive patients with type 2 diabetes. Diabetes Care. 2011;34:1270-1276.
29. Zhao P, Xu P, Wan C, et al. Evening versus morning dosing regimen drug therapy for hypertension. Cochrane Database Syst Rev. 2011;10:CD004184.
30. Py̆orälä K, Pedersen TR, Kjekshus J, et al. Cholesterol lowering with simvastatin improves prognosis of diabetic patients with coronary heart disease. A subgroup analysis of the Scandinavian Simvastatin Survival Study (4S). Diabetes Care. 1997;20:614-620.
31. Knopp RH, d’Emden M, Smilde JG, et al. Efficacy and safety of atorvastatin in the prevention of cardiovascular end points in subjects with type 2 diabetes: the Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in Non-Insulin-Dependent Diabetes Mellitus (ASPEN). Diabetes Care. 2006;29:1478-1485.
32. Cholesterol Treatment Trialists’ (CTT) Collaborators, Kearney PM, Blackwell L, Collins R, et al. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet. 2008;371:117-125.
33. Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013:CD004816.
34. Carter AA, Gomes T, Camacho X, et al. Risk of incident diabetes among patients treated with statins: population based study. BMJ. 2013;346:f2610.
35. Hayward RA, Hofer TP, Vijan S. Narrative review: lack of evidence for recommended low-density lipoprotein treatment targets: a solvable problem. Ann Intern Med. 2006;145:520-530.
36. Cannon CP, Braunwald E, McCabe CH, et al; Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495-1504.
37. de Lemos JA, Blazing MA, Wiviott SD, et al. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA. 2004;292:1307-1316.
38. Richardson K, Schoen M, French B, et al. Statins and cognitive function: a systematic review. Ann Intern Med. 2013;159:688-697.
39. Rajpathak SN, Kumbhani DJ, Crandall J, et al. Statin therapy and risk of developing type 2 diabetes: a meta-analysis. Diabetes Care. 2009;32:1924-1929.
40. Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet. 2010;375:735-742.
41. Meek C, Wierzbicki AS, Jewkes C, et al. Daily and intermittent rosuvastatin 5 mg therapy in statin intolerant patients: an observational study. Curr Med Res Opin. 2012;28:371-378.
42. ACCORD Study Group, Ginsberg HN, Bam MB, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563-1574.
43. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-2397.
44. AIM-HIGH Investigators, Boden WE, Probstfield JL, Anderson T, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255-2267.
45. Antithrombotic Trialists’ (ATT) Collaboration, Baigent C, Blackwell L, Collins R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373:1849-1860.
46. Perk J, De Backer G, Gohlke H, et al; European Association for Cardiovascular Prevention & Rehabilitation (EACPR); ESC Committee for Practice Guidelines (CPG). European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Eur Heart J. 2012;33:1635-1701.
47. Pignone M, Alberts MJ, Colwell JA, et al. Aspirin for primary prevention of cardiovascular events in people with diabetes. A position statement of the American Diabetes Association, a scientific statement of the American Heart Association, and an expert consensus document of the American College of Cardiology Foundation. Diabetes Care. 2010;33:1395-1402.
48. Vandvik PO, Lincoff AM, Gore JM, et al; American College of Chest Physicians. Primary and secondary prevention of cardiovascular disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl):e637S-e668S.
49. Kezerashvilli A, Marzo K, De Leon J. Beta blocker use after acute myocardial infarction in the patient with normal systolic function: when is it “ok” to discontinue? Curr Cardiol Rev. 2012;8:77-84.
50. Kannel WB, Hjortland M, Castelli WP. Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol. 1974;34:29-34.
51. Pioglitazone Package Insert. Available at: http://medlibrary.org/lib/rx/meds/pioglitazone-3/. Accessed April 10, 2017.
52. Scirica BM, Bhatt DL, Braunwald E, et al; SAVOR-TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317-1326.
53. Zannad F, Cannon CP, Cushman WC, et al; EXAMINE Investigators. Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double-blind trial. Lancet. 2015;385:2067-2076.
54. Green JB, Bethel MA, Armstrong PW, et al; TECOS Study Group. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373:232-242.
55. Zinman B, Wanner C, Lachin JM, et al, for the EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
56. FDA approves Jardiance to reduce cardiovascular death in adults with type 2 diabetes. FDA News Release, December 2, 2016. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm531517.htm. Accessed February 9, 2017.
57. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
More than 29 million Americans have diabetes, and each year another 1.7 million are given the diagnosis.1 Prediabetes is even more common; over one-third of US adults ages 20 years and older, and more than half of those who are ages 65 and older, have attained this precursor status, representing another 86 million Americans.1
Because the evidence base for the management of diabetes is rapidly expanding, the American Diabetes Association’s (ADA) Professional Practice Committee updates its Standards of Medical Care in Diabetes annually to incorporate new evidence into its recommendations. The 2017 Standards of Care are available at: professional.diabetes.org/jfp.2
Atherosclerotic cardiovascular disease (ASCVD) is the leading cause of morbidity and mortality for people with diabetes, and is the largest contributor to the direct and indirect costs of the disease.2 As a result, all patients with diabetes should have cardiovascular (CV) risk factors, including dyslipidemia, hypertension, smoking, a family history of premature coronary disease, and the presence of albuminuria, assessed at least annually.2 Numerous studies have demonstrated the efficacy of controlling individual CV risk factors in preventing or slowing ASCVD in people with diabetes. Even larger benefits, including reduced ASCVD morbidity and mortality, can be achieved when multiple risk factors are addressed simultaneously.3
To hone your management of CV risks in patients with diabetes, we’ve put together this Q&A pointing out the elements of the ADA’s 2017 Standards of Care that are most relevant to the management of patients at risk for, or with established, ASCVD.
Screening
Since ASCVD so commonly co-occurs with diabetes, should I routinely screen asymptomatic patients with diabetes for heart disease?
No. The current evidence suggests that outcomes are NOT improved by screening people before they develop symptoms of ASCVD,4 and widespread ASCVD screening has not been shown to be cost-effective. Cardiac testing should be reserved for those with typical or atypical symptoms or those with an abnormal resting electrocardiogram (EKG).
Lifestyle modification
What are the benefits of lifestyle interventions?
The benefits include not only lost pounds, but improved mobility, physical and sexual functioning, and health-related quality of life. Recommend that all overweight patients with diabetes take advantage of intensive lifestyle interventions focusing on weight loss through decreased caloric intake and increased physical activity as per the Look AHEAD (Action for Health in Diabetes) trial.5 Although the intensive lifestyle intervention in the Look AHEAD trial did not decrease CV outcomes over 10 years of follow-up, it did improve control of CV risk factors and led to people in the intervention group taking fewer glucose-, blood pressure (BP)-, and lipid-lowering medications than those in the standard care group.
There is no one diet that is recommended for all people with diabetes. Weight reduction often requires intensive intervention. In order for weight loss diets to be sustainable, they must include patient preferences.
People with diabetes should be encouraged to receive individualized medical nutrition therapy (MNT), preferably from a registered dietitian who is well versed in nutritional management for diabetes. Such MNT is associated with a 0.5% to 2% decrease in A1c levels for people with type 2 diabetes.6-9 Specific healthy diets include the Mediterranean, Dietary Approaches to Stop Hypertension (DASH), and plant-based diets.
A new lifestyle recommendation in this year’s ADA Standards is that periods of prolonged sitting should be interrupted every 30 minutes with a period of physical activity. This appears to have glycemic benefits.2
Hypertension/BP management
When should I initiate hypertension treatment in patients with diabetes?
Nonpharmacologic therapy is reasonable in people with diabetes and mildly elevated BP (>120/80 mm Hg). If systolic blood pressure (SBP) is confirmed to be >140 mm Hg and/or diastolic blood pressure (DBP) is confirmed to be >90 mm Hg, the ADA recommends initiating pharmacologic therapy along with nonpharmacologic strategies. For patients with confirmed office-based BP >160/100 mm Hg, the ADA advises initiating lifestyle modifications as well as 2 pharmacologic medications (or a single pill combination of agents).2
What is the recommended BP target for patients with diabetes and hypertension?
These patients should be treated with a combination of measures, including lifestyle modification and pharmacologic therapy, to a target BP of <140/90 mm Hg. Randomized controlled trials (RCTs) have shown benefits with this target in terms of a reduction in the incidence of coronary heart disease (CHD) events, stroke, and diabetic kidney disease.10,11
A 2012 meta-analysis of randomized trials involving adults with type 2 diabetes mellitus (T2DM) and comparing intensive BP targets (≤130 mm Hg SBP and ≤80 mm Hg DBP) with standard targets (≤140-160 mm Hg SBP and ≤85-100 mm Hg DBP) found no significant reduction in mortality or nonfatal MIs associated with more intense BP control. There was a statistically significant 35% relative risk (RR) reduction in stroke with intensive targets, but lower BP was also associated with an increased risk of hypotension and syncope.12
The 2010 Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial,13 which randomized 5518 patients with T2DM at high risk for ASCVD to either a target SBP of <120 mm Hg or 130 to 140 mm Hg, found that the patients with the lower SBP target did not benefit in the primary end point (a composite of nonfatal MI, nonfatal stroke, and CV death), but did benefit from nominally significant lower rates of total stroke and nonfatal stroke.
Based on these data, the ADA Standards of Care suggest that, “more intensive BP control may be reasonable in certain motivated, ACCORD-like patients (40-79 years of age with prior evidence of CVD or multiple CV risk factors) who have been educated about the added treatment burden, side effects, and costs of more intensive BP control and for patients who prefer to lower their risk of stroke beyond what can be achieved with usual care.”
Another major study, the 2015 Systolic Blood Pressure Intervention Trial (SPRINT) trial,14 demonstrated that treating patients with hypertension to a target SBP <120 mm Hg compared to the usual target of <140 mm Hg resulted in a 25% lower RR of the primary outcome (a composite of MI, other acute coronary syndromes, stroke, heart failure, or death from CV causes) and about a 25% reduction in all-cause mortality; however, people with diabetes were not included in the trial, so the applicability of the results to decisions about BP management in patients with diabetes is not known.
A 2015 systematic review and meta-analysis of over 100,000 participants looked at SBP lowering in adults with T2DM and found that each 10-mm Hg reduction in SBP was associated with a significantly lower risk of morbidity, CV events, CHD, stroke, albuminuria, and retinopathy.10 When trials were stratified by mean baseline SBP (<140 mm Hg or ≥140 mm Hg), RRs for outcomes other than stroke, retinopathy, and renal failure were lower in studies with greater baseline SBP.
The latest ADA Standards of Care recommend that a lower BP target of 130/80 mm Hg may be appropriate for patients at high risk of CVD if this target can be achieved without undue treatment burden. A DBP of <80 mm Hg may also be appropriate in certain patients including those with a long life expectancy, CKD, elevated urinary albumin excretion, and those with evidence of CVD or associated risk factors.15 Of note, treating older adults with diabetes to an SBP target of <130 mm Hg has not been shown to improve cardiovascular outcomes,16 and treating to a diastolic target of <70 mm Hg has been associated with a greater risk of mortality.17
What are the current recommended treatment options?
Treatment for hypertension in adults with diabetes without albuminuria should include any of the classes of medications demonstrated to reduce CV events in patients with diabetes, such as:
- angiotensin-converting enzyme (ACE) inhibitors,
- angiotensin receptor blockers (ARBs),
- thiazide-like diuretics, and
- dihydropyridine calcium channel blockers.
These recommendations are based on evidence suggesting the lack of superiority of ACE inhibitors and ARBs over other classes of antihypertensive agents for the prevention of CV outcomes in all patients with diabetes.18 However, in people with diabetes at high risk for ASCVD and/or with albuminuria, ACE inhibitors and ARBs do reduce ASCVD outcomes and the progression of kidney disease.19-24 Thus, ACE inhibitors and ARBs continue to be recommended as first-line medications for the treatment of hypertension in patients with diabetes and urine albumin/creatinine ratios ≥30 mg/g, as these medications are associated with a reduction in the rate of kidney disease progression.
The use of both an ACE inhibitor and an ARB in combination is not recommended.25,26 For patients treated with ACE inhibitors, ARBs, or diuretics, serum creatinine/estimated glomerular filtration rate (eGFR) and serum potassium levels should be monitored.
What are the recommended lifestyle modifications for patients with diabetes and hypertension?
Regular exercise and healthy eating are recommended for all people with diabetes to optimize glycemic control and lose weight (if they are overweight or obese). For patients with hypertension, the DASH diet (available at: https://www.nhlbi.nih.gov/health/health-topics/topics/dash/) is effective at lowering BP. The DASH diet emphasizes reducing sodium intake, increasing potassium intake, limiting alcohol intake, and increasing physical activity. Specifically, sodium intake should be restricted to <2300 mg/d and patients should consume approximately 8 to 10 servings of fruits and vegetables per day and 2 to 3 servings of low-fat dairy per day. Alcohol should be limited to 2 drinks per day for men and one drink per day for women.
Most adults with diabetes should perform 150 minutes per week of moderate to vigorous exercise, spread over at least 3 days/week. In addition, it is recommended that resistance exercises be performed at least 2 to 3 days/week. Prolonged inactivity is detrimental to health and should be interrupted with activity every 30 minutes.27
Finally, as a part of lifestyle management for all patients with diabetes, smoking cessation is important, as is attention to stress, depression, and anxiety.
Is there an advantage to nighttime dosing of antihypertensive medications?
Yes. Growing evidence suggests that there is an ASCVD benefit to avoiding nocturnal BP dipping. A 2011 RCT of 448 participants with T2DM and hypertension showed a decrease in CV events and mortality during 5.4 years of follow-up if at least one antihypertensive medication was taken at bedtime.28 As a result of this and other evidence,29 consider administering one or more antihypertensive medications at bedtime, although this is not a formal recommendation in the ADA Standards of Care.
Are there any additional issues to be aware of when treating patients with diabetes and hypertension?
Yes. Sometimes patients who have had diabetes for many years have significant orthostatic hypotension secondary to autonomic neuropathy. Postural changes in BP and pulse may require adjustment of BP targets. Home BP self-monitoring and 24-hour ambulatory BP monitoring may indicate white-coat or masked hypertension.
Lipid management
What is the current evidence for lipid treatment in diabetes?
Lipid abnormalities are common in people with diabetes and contribute to the overall high risk of ASCVD in these patients. Subgroup analyses of patients in large trials with diabetes30 and trials involving patients with diabetes31 have shown significant improvements in primary and secondary prevention of ASCVD with statin use. A 2008 meta-analysis of 18,686 people with diabetes showed a 9% reduction in all-cause mortality and a 13% reduction in vascular mortality for each 39-mg/dL reduction in low-density lipoprotein (LDL) cholesterol.32 Absolute reductions in mortality are greatest in those with highest risk, but the benefits of statin therapy are clear for low- and moderate-risk individuals with diabetes, too.33,34 As a result, statins are the medications of choice for lipid lowering and CV risk reduction and should be used in addition to lifestyle management.
Who should get a statin, and how do I choose the optimum dosage?
Patients ages 40 to 75 years with diabetes but without additional ASCVD risk factors should receive a moderate-intensity statin, according to the ADA (see TABLES 12 and 22). For those with additional CV risk factors, a high-intensity statin should be considered. The American College of Cardiology/American Heart Association ASCVD risk calculator (available at: http://www.cvriskcalculator.com/) may be useful for some patients, but generally, risk is already known to be high for most patients with diabetes. For patients of all ages with diabetes and established ASCVD, high-intensity statin therapy should be added to lifestyle modifications.35-37
For patients with diabetes who are <40 years with additional ASCVD risk factors, few clinical trial data exist; nevertheless, consider a moderate- or high-intensity statin and lifestyle therapy. Similarly, for patients >75 years who have diabetes and no additional ASCVD risk factors, consider a moderate-intensity statin and lifestyle modifications. For older adults with additional ASCVD risk factors, consider high-intensity statin therapy.35-37
Statins and cognition. It should be noted that published data have not demonstrated an adverse effect of statins on cognition.38 Statins, however, have been linked to an increased risk of developing diabetes,39,40 although the absolute increase in risk is small, and much smaller than the benefit derived from preventing the development of coronary disease.
Should total cholesterol and LDL levels be used as targets with statin treatment?
No. Statin doses have primarily been tested against placebo in clinical trials, rather than testing to specific target LDL levels, suggesting that the initiation and intensification of statin therapy be based on a patient’s risk profile.35 When maximally tolerated doses of statins do not lower LDL cholesterol by more than 30% from the patient’s baseline, there is currently no good evidence that combination therapy would be helpful, so regular monitoring of lipid levels has limited value. A lipid profile that includes levels of total cholesterol, LDL cholesterol, high-density lipoprotein (HDL) cholesterol, and triglycerides should be obtained at initial medical evaluation, at diagnosis of diabetes, and every 5 years thereafter or before the initiation of statin therapy. Ongoing testing may be appropriate in individual circumstances and to monitor for adherence to, or efficacy of, therapy.
What should I do for my patients who can’t tolerate statins?
Try a lower dose or a different statin before eliminating the class. Research has shown that even small doses (eg, rosuvastatin 5 mg) have some benefit.41
How do combination treatments figure into the current treatment of lipids in patients with diabetes?
It depends on the agent and the patient’s profile.
Fenofibrate. The ADA does not recommend automatically adding fenofibrate to statin therapy because the combination is associated with increased risks for abnormal transaminase levels, myositis, and rhabdomyolysis. In the ACCORD trial, the combination of fenofibrate and simvastatin did not reduce the rate of fatal CV events, nonfatal MIs, or nonfatal strokes compared with simvastatin alone.42
That said, a subgroup analysis suggested a benefit for men with both a triglyceride level ≥204 mg/dL (2.3 mmol/L) and an HDL cholesterol level ≤34 mg/dL (0.9 mmol/L).42 For this reason, the combination of a statin and fenofibrate may be considered for men who meet these laboratory parameters. In addition, consider medical therapy for triglyceride levels ≥500 mg/dL to reduce the risk of pancreatitis.
Ezetimibe. Recommendations regarding ezetimibe are based on the IMPROVE-IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial), a 2015 RCT including over 18,000 patients that compared treatment with ezetimibe and simvastatin to simvastatin alone.43 Individuals in the trial were ≥50 years of age and had experienced an ACS within the preceding 10 days. In those with diabetes, the combination of moderate-intensity simvastatin (40 mg) and ezetimibe (10 mg) significantly reduced major adverse CV events with an absolute risk reduction of 5% (40% vs 45%) and an RR reduction of 14% over moderate-intensity simvastatin (40 mg) alone.
Based on these results, patients with diabetes and a recent ACS should be considered for combination therapy with ezetimibe and a moderate-intensity statin. The combination should also be considered in patients with diabetes and a history of ASCVD who cannot tolerate high-intensity statins.43
Niacin. The ADA currently does not recommend niacin in combination with a statin because of lack of efficacy on major ASCVD outcomes, possible increased risk of ischemic stroke, and adverse effects.44
What are the recommendations for the use of PCSK-9 inhibitors?
Proprotein convertase subtilisin/kexin type 9 (PCSK-9) inhibitors (ie, evolucumab and alirocumab) may be considered as adjunctive therapy to statins for patients with diabetes at high risk for ASCVD events who require additional lowering of LDL cholesterol. They may also be considered for those in whom high-intensity statin therapy is indicated, but not tolerated.
Antiplatelet agents
Who should take aspirin for primary prevention of CVD?
Both women and men ages ≥50 years who have diabetes and at least one additional CV risk factor (family history of premature ASCVD, hypertension, tobacco use, dyslipidemia, or albuminuria) should consider taking daily aspirin therapy (75-162 mg/d) if they do not have an excessive bleeding risk.45,46 The most common dose in the United States is 81 mg. This recommendation is supported by a 2010 consensus statement of the American Diabetes Association, American Heart Association, and the American College of Cardiology.47
Should patients with diabetes and heart disease receive antiplatelet therapy?
Yes. The evidence is clear that people with known diabetes and ASCVD benefit from aspirin therapy, according to the 2017 Standards of Care. Clopidogrel 75 mg/d is an appropriate alternative for patients who are allergic to aspirin. Dual antiplatelet therapy (a P2Y12 receptor antagonist and aspirin) should be used for as long as one year after an ACS and may have benefits beyond this period.48
Established heart disease
Are there specific recommendations for patients with diabetes and CHD?
According to the ADA Standards, there is good evidence that both aspirin and statin therapy are beneficial for patients with known ASCVD, and that high-intensity statin therapy should be used. In addition, consider ACE inhibitors to reduce the future risk of CV events. In patients with a prior MI, continue beta-blocker therapy for at least 2 years post event.49
Which medications should I avoid, or approach with caution, in patients with congestive heart failure (CHF)?
Thiazolidinediones, dipeptidyl peptidase 4 (DPP-4) inhibitors, and metformin all require careful attention. This is especially important to know when you consider that almost half of all patients with T2DM will develop heart failure.50
Thiazolidinediones. The 2017 Standards of Care state that patients with diabetes and symptomatic congestive heart failure should not receive thiazolidinediones, as they can worsen heart failure status via fluid retention. As such, they are contraindicated in patients with class III and IV heart failure.51
DPP-4 inhibitors. The studies on DPP-4 inhibitors and heart failure have had mixed results. The 2013 SAVOR-TIMI (Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus–Thrombolysis in Myocardial Infarction) 53 trial52 showed that patients treated with saxagliptin were more likely to be hospitalized for heart failure than those taking placebo (3.5% vs 2.8%, respectively). However, the 2015 EXAMINE (Examination of Cardiovascular Outcomes with Alogliptin vs Standard of Care)53 trial and the 2015 TECOS (Trial Evaluating Cardiovascular Outcomes with Sitagliptin)54 trial evaluated heart failure and mortality outcomes in patients with alogliptin and sitagliptin, respectively, compared to placebo, and did not show a relationship to heart failure.
Metformin may be used in people who have T2DM and stable CHF if their eGFR remains >30 mL/min; it should be withheld from patients with unstable heart failure and those who are hospitalized with CHF.
Are there antihyperglycemic medications that reduce CV morbidity and mortality in those with established ASCVD?
Yes. This year’s ADA Standards indicate that certain glucose-lowering medications—specifically empagliflozin (a sodium–glucose cotransporter [SGLT]-2 inhibitor) and liraglutide (a glucagon-like peptide [GLP]-1 receptor agonist)—have been shown to be beneficial for those with established CVD. According to the 2017 Standards of Care, “In patients with longstanding suboptimally controlled T2DM and established ASCVD, empagliflozin or liraglutide should be considered, as they have been shown to reduce CV and all-cause mortality when added to standard care.”2 The studies that provide support for their use are summarized below. Ongoing studies are investigating the CV effects of other agents in these drug classes.
Empagliflozin. The 2015 EMPA-REG OUTCOME (Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients) study55 was a randomized double-blind study of empagliflozin vs placebo and usual care in patients with diabetes and established CVD. Over a median follow-up of 3.1 years, treatment with empagliflozin reduced the aggregate outcome of MI, stroke, and CV death by 14%, reduced CV deaths by 38%, and decreased deaths from any cause by 32%. In December 2016, the FDA announced a new indication for empagliflozin: to reduce the risk of CV death in adult patients with T2DM and CVD.56
Liraglutide. The LEADER (Liraglutide Effect and Action in Diabetes Evaluation of Cardiovascular Outcome Results: A Long Term Evaluation) trial57 was a double-blind randomized trial of liraglutide vs placebo added to usual care in patients with T2DM at high risk for CVD or with existing CVD. More than 80% of the participants had existing CVD including a history of prior MI, cerebrovascular disease, or peripheral vascular disease. After a median follow-up of 3.8 years, the group taking liraglutide demonstrated a 13% reduction in the composite outcome of MI, stroke, or CV death, a 22% reduction in CV death, and a 15% reduction in death from any cause, compared with placebo.57
CORRESPONDENCE
Neil Skolnik, MD, Abington-Jefferson Health, 500 Old York Rd, Ste 108, Jenkintown, PA 19046; [email protected].
The authors thank Sarah Bradley, director, professional engagement & collaboration at the American Diabetes Association, for her editorial and organizational assistance in the preparation of this manuscript.
More than 29 million Americans have diabetes, and each year another 1.7 million are given the diagnosis.1 Prediabetes is even more common; over one-third of US adults ages 20 years and older, and more than half of those who are ages 65 and older, have attained this precursor status, representing another 86 million Americans.1
Because the evidence base for the management of diabetes is rapidly expanding, the American Diabetes Association’s (ADA) Professional Practice Committee updates its Standards of Medical Care in Diabetes annually to incorporate new evidence into its recommendations. The 2017 Standards of Care are available at: professional.diabetes.org/jfp.2
Atherosclerotic cardiovascular disease (ASCVD) is the leading cause of morbidity and mortality for people with diabetes, and is the largest contributor to the direct and indirect costs of the disease.2 As a result, all patients with diabetes should have cardiovascular (CV) risk factors, including dyslipidemia, hypertension, smoking, a family history of premature coronary disease, and the presence of albuminuria, assessed at least annually.2 Numerous studies have demonstrated the efficacy of controlling individual CV risk factors in preventing or slowing ASCVD in people with diabetes. Even larger benefits, including reduced ASCVD morbidity and mortality, can be achieved when multiple risk factors are addressed simultaneously.3
To hone your management of CV risks in patients with diabetes, we’ve put together this Q&A pointing out the elements of the ADA’s 2017 Standards of Care that are most relevant to the management of patients at risk for, or with established, ASCVD.
Screening
Since ASCVD so commonly co-occurs with diabetes, should I routinely screen asymptomatic patients with diabetes for heart disease?
No. The current evidence suggests that outcomes are NOT improved by screening people before they develop symptoms of ASCVD,4 and widespread ASCVD screening has not been shown to be cost-effective. Cardiac testing should be reserved for those with typical or atypical symptoms or those with an abnormal resting electrocardiogram (EKG).
Lifestyle modification
What are the benefits of lifestyle interventions?
The benefits include not only lost pounds, but improved mobility, physical and sexual functioning, and health-related quality of life. Recommend that all overweight patients with diabetes take advantage of intensive lifestyle interventions focusing on weight loss through decreased caloric intake and increased physical activity as per the Look AHEAD (Action for Health in Diabetes) trial.5 Although the intensive lifestyle intervention in the Look AHEAD trial did not decrease CV outcomes over 10 years of follow-up, it did improve control of CV risk factors and led to people in the intervention group taking fewer glucose-, blood pressure (BP)-, and lipid-lowering medications than those in the standard care group.
There is no one diet that is recommended for all people with diabetes. Weight reduction often requires intensive intervention. In order for weight loss diets to be sustainable, they must include patient preferences.
People with diabetes should be encouraged to receive individualized medical nutrition therapy (MNT), preferably from a registered dietitian who is well versed in nutritional management for diabetes. Such MNT is associated with a 0.5% to 2% decrease in A1c levels for people with type 2 diabetes.6-9 Specific healthy diets include the Mediterranean, Dietary Approaches to Stop Hypertension (DASH), and plant-based diets.
A new lifestyle recommendation in this year’s ADA Standards is that periods of prolonged sitting should be interrupted every 30 minutes with a period of physical activity. This appears to have glycemic benefits.2
Hypertension/BP management
When should I initiate hypertension treatment in patients with diabetes?
Nonpharmacologic therapy is reasonable in people with diabetes and mildly elevated BP (>120/80 mm Hg). If systolic blood pressure (SBP) is confirmed to be >140 mm Hg and/or diastolic blood pressure (DBP) is confirmed to be >90 mm Hg, the ADA recommends initiating pharmacologic therapy along with nonpharmacologic strategies. For patients with confirmed office-based BP >160/100 mm Hg, the ADA advises initiating lifestyle modifications as well as 2 pharmacologic medications (or a single pill combination of agents).2
What is the recommended BP target for patients with diabetes and hypertension?
These patients should be treated with a combination of measures, including lifestyle modification and pharmacologic therapy, to a target BP of <140/90 mm Hg. Randomized controlled trials (RCTs) have shown benefits with this target in terms of a reduction in the incidence of coronary heart disease (CHD) events, stroke, and diabetic kidney disease.10,11
A 2012 meta-analysis of randomized trials involving adults with type 2 diabetes mellitus (T2DM) and comparing intensive BP targets (≤130 mm Hg SBP and ≤80 mm Hg DBP) with standard targets (≤140-160 mm Hg SBP and ≤85-100 mm Hg DBP) found no significant reduction in mortality or nonfatal MIs associated with more intense BP control. There was a statistically significant 35% relative risk (RR) reduction in stroke with intensive targets, but lower BP was also associated with an increased risk of hypotension and syncope.12
The 2010 Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial,13 which randomized 5518 patients with T2DM at high risk for ASCVD to either a target SBP of <120 mm Hg or 130 to 140 mm Hg, found that the patients with the lower SBP target did not benefit in the primary end point (a composite of nonfatal MI, nonfatal stroke, and CV death), but did benefit from nominally significant lower rates of total stroke and nonfatal stroke.
Based on these data, the ADA Standards of Care suggest that, “more intensive BP control may be reasonable in certain motivated, ACCORD-like patients (40-79 years of age with prior evidence of CVD or multiple CV risk factors) who have been educated about the added treatment burden, side effects, and costs of more intensive BP control and for patients who prefer to lower their risk of stroke beyond what can be achieved with usual care.”
Another major study, the 2015 Systolic Blood Pressure Intervention Trial (SPRINT) trial,14 demonstrated that treating patients with hypertension to a target SBP <120 mm Hg compared to the usual target of <140 mm Hg resulted in a 25% lower RR of the primary outcome (a composite of MI, other acute coronary syndromes, stroke, heart failure, or death from CV causes) and about a 25% reduction in all-cause mortality; however, people with diabetes were not included in the trial, so the applicability of the results to decisions about BP management in patients with diabetes is not known.
A 2015 systematic review and meta-analysis of over 100,000 participants looked at SBP lowering in adults with T2DM and found that each 10-mm Hg reduction in SBP was associated with a significantly lower risk of morbidity, CV events, CHD, stroke, albuminuria, and retinopathy.10 When trials were stratified by mean baseline SBP (<140 mm Hg or ≥140 mm Hg), RRs for outcomes other than stroke, retinopathy, and renal failure were lower in studies with greater baseline SBP.
The latest ADA Standards of Care recommend that a lower BP target of 130/80 mm Hg may be appropriate for patients at high risk of CVD if this target can be achieved without undue treatment burden. A DBP of <80 mm Hg may also be appropriate in certain patients including those with a long life expectancy, CKD, elevated urinary albumin excretion, and those with evidence of CVD or associated risk factors.15 Of note, treating older adults with diabetes to an SBP target of <130 mm Hg has not been shown to improve cardiovascular outcomes,16 and treating to a diastolic target of <70 mm Hg has been associated with a greater risk of mortality.17
What are the current recommended treatment options?
Treatment for hypertension in adults with diabetes without albuminuria should include any of the classes of medications demonstrated to reduce CV events in patients with diabetes, such as:
- angiotensin-converting enzyme (ACE) inhibitors,
- angiotensin receptor blockers (ARBs),
- thiazide-like diuretics, and
- dihydropyridine calcium channel blockers.
These recommendations are based on evidence suggesting the lack of superiority of ACE inhibitors and ARBs over other classes of antihypertensive agents for the prevention of CV outcomes in all patients with diabetes.18 However, in people with diabetes at high risk for ASCVD and/or with albuminuria, ACE inhibitors and ARBs do reduce ASCVD outcomes and the progression of kidney disease.19-24 Thus, ACE inhibitors and ARBs continue to be recommended as first-line medications for the treatment of hypertension in patients with diabetes and urine albumin/creatinine ratios ≥30 mg/g, as these medications are associated with a reduction in the rate of kidney disease progression.
The use of both an ACE inhibitor and an ARB in combination is not recommended.25,26 For patients treated with ACE inhibitors, ARBs, or diuretics, serum creatinine/estimated glomerular filtration rate (eGFR) and serum potassium levels should be monitored.
What are the recommended lifestyle modifications for patients with diabetes and hypertension?
Regular exercise and healthy eating are recommended for all people with diabetes to optimize glycemic control and lose weight (if they are overweight or obese). For patients with hypertension, the DASH diet (available at: https://www.nhlbi.nih.gov/health/health-topics/topics/dash/) is effective at lowering BP. The DASH diet emphasizes reducing sodium intake, increasing potassium intake, limiting alcohol intake, and increasing physical activity. Specifically, sodium intake should be restricted to <2300 mg/d and patients should consume approximately 8 to 10 servings of fruits and vegetables per day and 2 to 3 servings of low-fat dairy per day. Alcohol should be limited to 2 drinks per day for men and one drink per day for women.
Most adults with diabetes should perform 150 minutes per week of moderate to vigorous exercise, spread over at least 3 days/week. In addition, it is recommended that resistance exercises be performed at least 2 to 3 days/week. Prolonged inactivity is detrimental to health and should be interrupted with activity every 30 minutes.27
Finally, as a part of lifestyle management for all patients with diabetes, smoking cessation is important, as is attention to stress, depression, and anxiety.
Is there an advantage to nighttime dosing of antihypertensive medications?
Yes. Growing evidence suggests that there is an ASCVD benefit to avoiding nocturnal BP dipping. A 2011 RCT of 448 participants with T2DM and hypertension showed a decrease in CV events and mortality during 5.4 years of follow-up if at least one antihypertensive medication was taken at bedtime.28 As a result of this and other evidence,29 consider administering one or more antihypertensive medications at bedtime, although this is not a formal recommendation in the ADA Standards of Care.
Are there any additional issues to be aware of when treating patients with diabetes and hypertension?
Yes. Sometimes patients who have had diabetes for many years have significant orthostatic hypotension secondary to autonomic neuropathy. Postural changes in BP and pulse may require adjustment of BP targets. Home BP self-monitoring and 24-hour ambulatory BP monitoring may indicate white-coat or masked hypertension.
Lipid management
What is the current evidence for lipid treatment in diabetes?
Lipid abnormalities are common in people with diabetes and contribute to the overall high risk of ASCVD in these patients. Subgroup analyses of patients in large trials with diabetes30 and trials involving patients with diabetes31 have shown significant improvements in primary and secondary prevention of ASCVD with statin use. A 2008 meta-analysis of 18,686 people with diabetes showed a 9% reduction in all-cause mortality and a 13% reduction in vascular mortality for each 39-mg/dL reduction in low-density lipoprotein (LDL) cholesterol.32 Absolute reductions in mortality are greatest in those with highest risk, but the benefits of statin therapy are clear for low- and moderate-risk individuals with diabetes, too.33,34 As a result, statins are the medications of choice for lipid lowering and CV risk reduction and should be used in addition to lifestyle management.
Who should get a statin, and how do I choose the optimum dosage?
Patients ages 40 to 75 years with diabetes but without additional ASCVD risk factors should receive a moderate-intensity statin, according to the ADA (see TABLES 12 and 22). For those with additional CV risk factors, a high-intensity statin should be considered. The American College of Cardiology/American Heart Association ASCVD risk calculator (available at: http://www.cvriskcalculator.com/) may be useful for some patients, but generally, risk is already known to be high for most patients with diabetes. For patients of all ages with diabetes and established ASCVD, high-intensity statin therapy should be added to lifestyle modifications.35-37
For patients with diabetes who are <40 years with additional ASCVD risk factors, few clinical trial data exist; nevertheless, consider a moderate- or high-intensity statin and lifestyle therapy. Similarly, for patients >75 years who have diabetes and no additional ASCVD risk factors, consider a moderate-intensity statin and lifestyle modifications. For older adults with additional ASCVD risk factors, consider high-intensity statin therapy.35-37
Statins and cognition. It should be noted that published data have not demonstrated an adverse effect of statins on cognition.38 Statins, however, have been linked to an increased risk of developing diabetes,39,40 although the absolute increase in risk is small, and much smaller than the benefit derived from preventing the development of coronary disease.
Should total cholesterol and LDL levels be used as targets with statin treatment?
No. Statin doses have primarily been tested against placebo in clinical trials, rather than testing to specific target LDL levels, suggesting that the initiation and intensification of statin therapy be based on a patient’s risk profile.35 When maximally tolerated doses of statins do not lower LDL cholesterol by more than 30% from the patient’s baseline, there is currently no good evidence that combination therapy would be helpful, so regular monitoring of lipid levels has limited value. A lipid profile that includes levels of total cholesterol, LDL cholesterol, high-density lipoprotein (HDL) cholesterol, and triglycerides should be obtained at initial medical evaluation, at diagnosis of diabetes, and every 5 years thereafter or before the initiation of statin therapy. Ongoing testing may be appropriate in individual circumstances and to monitor for adherence to, or efficacy of, therapy.
What should I do for my patients who can’t tolerate statins?
Try a lower dose or a different statin before eliminating the class. Research has shown that even small doses (eg, rosuvastatin 5 mg) have some benefit.41
How do combination treatments figure into the current treatment of lipids in patients with diabetes?
It depends on the agent and the patient’s profile.
Fenofibrate. The ADA does not recommend automatically adding fenofibrate to statin therapy because the combination is associated with increased risks for abnormal transaminase levels, myositis, and rhabdomyolysis. In the ACCORD trial, the combination of fenofibrate and simvastatin did not reduce the rate of fatal CV events, nonfatal MIs, or nonfatal strokes compared with simvastatin alone.42
That said, a subgroup analysis suggested a benefit for men with both a triglyceride level ≥204 mg/dL (2.3 mmol/L) and an HDL cholesterol level ≤34 mg/dL (0.9 mmol/L).42 For this reason, the combination of a statin and fenofibrate may be considered for men who meet these laboratory parameters. In addition, consider medical therapy for triglyceride levels ≥500 mg/dL to reduce the risk of pancreatitis.
Ezetimibe. Recommendations regarding ezetimibe are based on the IMPROVE-IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial), a 2015 RCT including over 18,000 patients that compared treatment with ezetimibe and simvastatin to simvastatin alone.43 Individuals in the trial were ≥50 years of age and had experienced an ACS within the preceding 10 days. In those with diabetes, the combination of moderate-intensity simvastatin (40 mg) and ezetimibe (10 mg) significantly reduced major adverse CV events with an absolute risk reduction of 5% (40% vs 45%) and an RR reduction of 14% over moderate-intensity simvastatin (40 mg) alone.
Based on these results, patients with diabetes and a recent ACS should be considered for combination therapy with ezetimibe and a moderate-intensity statin. The combination should also be considered in patients with diabetes and a history of ASCVD who cannot tolerate high-intensity statins.43
Niacin. The ADA currently does not recommend niacin in combination with a statin because of lack of efficacy on major ASCVD outcomes, possible increased risk of ischemic stroke, and adverse effects.44
What are the recommendations for the use of PCSK-9 inhibitors?
Proprotein convertase subtilisin/kexin type 9 (PCSK-9) inhibitors (ie, evolucumab and alirocumab) may be considered as adjunctive therapy to statins for patients with diabetes at high risk for ASCVD events who require additional lowering of LDL cholesterol. They may also be considered for those in whom high-intensity statin therapy is indicated, but not tolerated.
Antiplatelet agents
Who should take aspirin for primary prevention of CVD?
Both women and men ages ≥50 years who have diabetes and at least one additional CV risk factor (family history of premature ASCVD, hypertension, tobacco use, dyslipidemia, or albuminuria) should consider taking daily aspirin therapy (75-162 mg/d) if they do not have an excessive bleeding risk.45,46 The most common dose in the United States is 81 mg. This recommendation is supported by a 2010 consensus statement of the American Diabetes Association, American Heart Association, and the American College of Cardiology.47
Should patients with diabetes and heart disease receive antiplatelet therapy?
Yes. The evidence is clear that people with known diabetes and ASCVD benefit from aspirin therapy, according to the 2017 Standards of Care. Clopidogrel 75 mg/d is an appropriate alternative for patients who are allergic to aspirin. Dual antiplatelet therapy (a P2Y12 receptor antagonist and aspirin) should be used for as long as one year after an ACS and may have benefits beyond this period.48
Established heart disease
Are there specific recommendations for patients with diabetes and CHD?
According to the ADA Standards, there is good evidence that both aspirin and statin therapy are beneficial for patients with known ASCVD, and that high-intensity statin therapy should be used. In addition, consider ACE inhibitors to reduce the future risk of CV events. In patients with a prior MI, continue beta-blocker therapy for at least 2 years post event.49
Which medications should I avoid, or approach with caution, in patients with congestive heart failure (CHF)?
Thiazolidinediones, dipeptidyl peptidase 4 (DPP-4) inhibitors, and metformin all require careful attention. This is especially important to know when you consider that almost half of all patients with T2DM will develop heart failure.50
Thiazolidinediones. The 2017 Standards of Care state that patients with diabetes and symptomatic congestive heart failure should not receive thiazolidinediones, as they can worsen heart failure status via fluid retention. As such, they are contraindicated in patients with class III and IV heart failure.51
DPP-4 inhibitors. The studies on DPP-4 inhibitors and heart failure have had mixed results. The 2013 SAVOR-TIMI (Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus–Thrombolysis in Myocardial Infarction) 53 trial52 showed that patients treated with saxagliptin were more likely to be hospitalized for heart failure than those taking placebo (3.5% vs 2.8%, respectively). However, the 2015 EXAMINE (Examination of Cardiovascular Outcomes with Alogliptin vs Standard of Care)53 trial and the 2015 TECOS (Trial Evaluating Cardiovascular Outcomes with Sitagliptin)54 trial evaluated heart failure and mortality outcomes in patients with alogliptin and sitagliptin, respectively, compared to placebo, and did not show a relationship to heart failure.
Metformin may be used in people who have T2DM and stable CHF if their eGFR remains >30 mL/min; it should be withheld from patients with unstable heart failure and those who are hospitalized with CHF.
Are there antihyperglycemic medications that reduce CV morbidity and mortality in those with established ASCVD?
Yes. This year’s ADA Standards indicate that certain glucose-lowering medications—specifically empagliflozin (a sodium–glucose cotransporter [SGLT]-2 inhibitor) and liraglutide (a glucagon-like peptide [GLP]-1 receptor agonist)—have been shown to be beneficial for those with established CVD. According to the 2017 Standards of Care, “In patients with longstanding suboptimally controlled T2DM and established ASCVD, empagliflozin or liraglutide should be considered, as they have been shown to reduce CV and all-cause mortality when added to standard care.”2 The studies that provide support for their use are summarized below. Ongoing studies are investigating the CV effects of other agents in these drug classes.
Empagliflozin. The 2015 EMPA-REG OUTCOME (Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients) study55 was a randomized double-blind study of empagliflozin vs placebo and usual care in patients with diabetes and established CVD. Over a median follow-up of 3.1 years, treatment with empagliflozin reduced the aggregate outcome of MI, stroke, and CV death by 14%, reduced CV deaths by 38%, and decreased deaths from any cause by 32%. In December 2016, the FDA announced a new indication for empagliflozin: to reduce the risk of CV death in adult patients with T2DM and CVD.56
Liraglutide. The LEADER (Liraglutide Effect and Action in Diabetes Evaluation of Cardiovascular Outcome Results: A Long Term Evaluation) trial57 was a double-blind randomized trial of liraglutide vs placebo added to usual care in patients with T2DM at high risk for CVD or with existing CVD. More than 80% of the participants had existing CVD including a history of prior MI, cerebrovascular disease, or peripheral vascular disease. After a median follow-up of 3.8 years, the group taking liraglutide demonstrated a 13% reduction in the composite outcome of MI, stroke, or CV death, a 22% reduction in CV death, and a 15% reduction in death from any cause, compared with placebo.57
CORRESPONDENCE
Neil Skolnik, MD, Abington-Jefferson Health, 500 Old York Rd, Ste 108, Jenkintown, PA 19046; [email protected].
The authors thank Sarah Bradley, director, professional engagement & collaboration at the American Diabetes Association, for her editorial and organizational assistance in the preparation of this manuscript.
1. Centers for Disease Control and Prevention. National Center for Chronic Disease Prevention and Health Promotion. National diabetes statistics report, 2014. Estimates of diabetes and its burden in the United States. Available at: http://templatelab.com/national-diabetes-report-2014/. Accessed April 7, 2017.
2. American Diabetes Association. Standards of Medical Care in Diabetes—2017. Available at: http://professional.diabetes.org/sites/professional.diabetes.org/files/media/dc_40_s1_final.pdf. Accessed April 7, 2017.
3. Gaede P, Lund-Andersen H, Parving HH, et al. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358:580-591.
4. Bax JJ, Young LH, Frye RL, et al; American Diabetes Association. Screening for coronary artery disease in patients with diabetes. Diabetes Care. 2007;30:2729-2736.
5. The Look AHEAD Research Group. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369:145-154.
6. UK Prospective Diabetes Study (UKDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKDS 34). Lancet. 1998;352:854-865.
7. Ziemer DC, Berkowitz KJ, Panayioto RM, et al. A simple meal plan emphasizing healthy food choices is as effective as an exchange-based meal plan for urban African Americans with type 2 diabetes. Diabetes Care. 2003;26:1719-1724.
8. Wolf AM, Conaway RM, Crowther JQ, et al; Improving Control with Activity and Nutrition (ICAN) Study. Translating lifestyle intervention to practice in obese patients with type 2 diabetes: Improving Control with Activity and Nutrition (ICAN) study. Diabetes Care. 2004;27:1570-1576.
9. Coppell KJ, Kataoka M, Williams SM, et al. Nutritional intervention in patients with type 2 diabetes who are hyperglycaemic despite optimised drug treatment-Lifestyle Over and Above Drugs in Diabetes (LOADD) study: randomised controlled trial. BMJ. 2010;341:c3337.
10. Emdin CA, Rahimi K, Neal B, et al. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2015;313:603-615.
11. Arguedas JA, Leiva V, Wright JM. Blood pressure targets for hypertension in people with diabetes mellitus. Cochrane Database Syst Rev. 2013;10:CD008277.
12. McBrien K, Rabi DM, Campbell N, et al. Intensive and standard blood pressure targets in patients with type 2 diabetes mellitus: systematic review and meta-analysis. Arch Intern Med. 2012;172:1296-1303.
13. ACCORD Study Group, Cushman WC, Evans GW, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575-1585.
14. SPRINT Research Group, Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
15. Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet. 1998;351:1755-1762.
16. Kirkman MS, Briscoe VJ, Clark N, et al. Diabetes in older adults. Diabetes Care. 2012;35:2650-2664.
17. Anderson RJ, Bahn GD, Moritz TE, et al; VADT Study Group. Blood pressure and cardiovascular disease risk in the Veterans Affairs Diabetes Trial. Diabetes Care. 2011;34:34-38.
18. Bangalore S, Fakheri R, Toklu B, et al. Diabetes mellitus as a compelling indication for use of renin angiotensin system blockers: systematic review and meta-analysis of randomized trials. BMJ. 2016;352:i438.
19. Heart Outcomes Prevention Evaluation Study Investigators. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Lancet. 2000;355:253-259.
20. Granger CB, McMurray JJ, Yusuf S, et al; CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting enzyme inhibitors: the CHARM-Alternative trial. Lancet. 2003;362:772-776.
21. McMurray JJ, Ostergren J, Swedberg K, et al; CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. Lancet. 2003;362:767-771.
22. Pfeffer MA, Swedberg K, Granger CB, et al; CHARM Investigators and Committees. Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-Overall programme. Lancet. 2003;362:759-766.
23. Brenner BM, Cooper ME, de Zeeuw D, et al; RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861-869.
24. Palmer SC, Mavridis D, Navarese E, et al. Comparative efficacy and safety of blood pressure-lowering agents in adults with diabetes and kidney disease: a network meta-analysis. Lancet. 2015;385:2047-2056.
25. The ONTARGET Investigators. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547-1559.
26. Fried LF, Emanuele N, Zhang JH, et al; VA NEPHRON-D Investigators. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med. 2013;369:1892-1903.
27. Colberg SR, Sigal RJ, Yardley JE, et al. Physical activity/exercise and diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2016;39:2065-2079.
28. Hermida RC, Ayala DE, Mojón A, et al. Influence of time of day of blood pressure-lowering treatment on cardiovascular risk in hypertensive patients with type 2 diabetes. Diabetes Care. 2011;34:1270-1276.
29. Zhao P, Xu P, Wan C, et al. Evening versus morning dosing regimen drug therapy for hypertension. Cochrane Database Syst Rev. 2011;10:CD004184.
30. Py̆orälä K, Pedersen TR, Kjekshus J, et al. Cholesterol lowering with simvastatin improves prognosis of diabetic patients with coronary heart disease. A subgroup analysis of the Scandinavian Simvastatin Survival Study (4S). Diabetes Care. 1997;20:614-620.
31. Knopp RH, d’Emden M, Smilde JG, et al. Efficacy and safety of atorvastatin in the prevention of cardiovascular end points in subjects with type 2 diabetes: the Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in Non-Insulin-Dependent Diabetes Mellitus (ASPEN). Diabetes Care. 2006;29:1478-1485.
32. Cholesterol Treatment Trialists’ (CTT) Collaborators, Kearney PM, Blackwell L, Collins R, et al. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet. 2008;371:117-125.
33. Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013:CD004816.
34. Carter AA, Gomes T, Camacho X, et al. Risk of incident diabetes among patients treated with statins: population based study. BMJ. 2013;346:f2610.
35. Hayward RA, Hofer TP, Vijan S. Narrative review: lack of evidence for recommended low-density lipoprotein treatment targets: a solvable problem. Ann Intern Med. 2006;145:520-530.
36. Cannon CP, Braunwald E, McCabe CH, et al; Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495-1504.
37. de Lemos JA, Blazing MA, Wiviott SD, et al. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA. 2004;292:1307-1316.
38. Richardson K, Schoen M, French B, et al. Statins and cognitive function: a systematic review. Ann Intern Med. 2013;159:688-697.
39. Rajpathak SN, Kumbhani DJ, Crandall J, et al. Statin therapy and risk of developing type 2 diabetes: a meta-analysis. Diabetes Care. 2009;32:1924-1929.
40. Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet. 2010;375:735-742.
41. Meek C, Wierzbicki AS, Jewkes C, et al. Daily and intermittent rosuvastatin 5 mg therapy in statin intolerant patients: an observational study. Curr Med Res Opin. 2012;28:371-378.
42. ACCORD Study Group, Ginsberg HN, Bam MB, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563-1574.
43. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-2397.
44. AIM-HIGH Investigators, Boden WE, Probstfield JL, Anderson T, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255-2267.
45. Antithrombotic Trialists’ (ATT) Collaboration, Baigent C, Blackwell L, Collins R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373:1849-1860.
46. Perk J, De Backer G, Gohlke H, et al; European Association for Cardiovascular Prevention & Rehabilitation (EACPR); ESC Committee for Practice Guidelines (CPG). European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Eur Heart J. 2012;33:1635-1701.
47. Pignone M, Alberts MJ, Colwell JA, et al. Aspirin for primary prevention of cardiovascular events in people with diabetes. A position statement of the American Diabetes Association, a scientific statement of the American Heart Association, and an expert consensus document of the American College of Cardiology Foundation. Diabetes Care. 2010;33:1395-1402.
48. Vandvik PO, Lincoff AM, Gore JM, et al; American College of Chest Physicians. Primary and secondary prevention of cardiovascular disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl):e637S-e668S.
49. Kezerashvilli A, Marzo K, De Leon J. Beta blocker use after acute myocardial infarction in the patient with normal systolic function: when is it “ok” to discontinue? Curr Cardiol Rev. 2012;8:77-84.
50. Kannel WB, Hjortland M, Castelli WP. Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol. 1974;34:29-34.
51. Pioglitazone Package Insert. Available at: http://medlibrary.org/lib/rx/meds/pioglitazone-3/. Accessed April 10, 2017.
52. Scirica BM, Bhatt DL, Braunwald E, et al; SAVOR-TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317-1326.
53. Zannad F, Cannon CP, Cushman WC, et al; EXAMINE Investigators. Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double-blind trial. Lancet. 2015;385:2067-2076.
54. Green JB, Bethel MA, Armstrong PW, et al; TECOS Study Group. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373:232-242.
55. Zinman B, Wanner C, Lachin JM, et al, for the EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
56. FDA approves Jardiance to reduce cardiovascular death in adults with type 2 diabetes. FDA News Release, December 2, 2016. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm531517.htm. Accessed February 9, 2017.
57. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
1. Centers for Disease Control and Prevention. National Center for Chronic Disease Prevention and Health Promotion. National diabetes statistics report, 2014. Estimates of diabetes and its burden in the United States. Available at: http://templatelab.com/national-diabetes-report-2014/. Accessed April 7, 2017.
2. American Diabetes Association. Standards of Medical Care in Diabetes—2017. Available at: http://professional.diabetes.org/sites/professional.diabetes.org/files/media/dc_40_s1_final.pdf. Accessed April 7, 2017.
3. Gaede P, Lund-Andersen H, Parving HH, et al. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358:580-591.
4. Bax JJ, Young LH, Frye RL, et al; American Diabetes Association. Screening for coronary artery disease in patients with diabetes. Diabetes Care. 2007;30:2729-2736.
5. The Look AHEAD Research Group. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369:145-154.
6. UK Prospective Diabetes Study (UKDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKDS 34). Lancet. 1998;352:854-865.
7. Ziemer DC, Berkowitz KJ, Panayioto RM, et al. A simple meal plan emphasizing healthy food choices is as effective as an exchange-based meal plan for urban African Americans with type 2 diabetes. Diabetes Care. 2003;26:1719-1724.
8. Wolf AM, Conaway RM, Crowther JQ, et al; Improving Control with Activity and Nutrition (ICAN) Study. Translating lifestyle intervention to practice in obese patients with type 2 diabetes: Improving Control with Activity and Nutrition (ICAN) study. Diabetes Care. 2004;27:1570-1576.
9. Coppell KJ, Kataoka M, Williams SM, et al. Nutritional intervention in patients with type 2 diabetes who are hyperglycaemic despite optimised drug treatment-Lifestyle Over and Above Drugs in Diabetes (LOADD) study: randomised controlled trial. BMJ. 2010;341:c3337.
10. Emdin CA, Rahimi K, Neal B, et al. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2015;313:603-615.
11. Arguedas JA, Leiva V, Wright JM. Blood pressure targets for hypertension in people with diabetes mellitus. Cochrane Database Syst Rev. 2013;10:CD008277.
12. McBrien K, Rabi DM, Campbell N, et al. Intensive and standard blood pressure targets in patients with type 2 diabetes mellitus: systematic review and meta-analysis. Arch Intern Med. 2012;172:1296-1303.
13. ACCORD Study Group, Cushman WC, Evans GW, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575-1585.
14. SPRINT Research Group, Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
15. Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet. 1998;351:1755-1762.
16. Kirkman MS, Briscoe VJ, Clark N, et al. Diabetes in older adults. Diabetes Care. 2012;35:2650-2664.
17. Anderson RJ, Bahn GD, Moritz TE, et al; VADT Study Group. Blood pressure and cardiovascular disease risk in the Veterans Affairs Diabetes Trial. Diabetes Care. 2011;34:34-38.
18. Bangalore S, Fakheri R, Toklu B, et al. Diabetes mellitus as a compelling indication for use of renin angiotensin system blockers: systematic review and meta-analysis of randomized trials. BMJ. 2016;352:i438.
19. Heart Outcomes Prevention Evaluation Study Investigators. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Lancet. 2000;355:253-259.
20. Granger CB, McMurray JJ, Yusuf S, et al; CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting enzyme inhibitors: the CHARM-Alternative trial. Lancet. 2003;362:772-776.
21. McMurray JJ, Ostergren J, Swedberg K, et al; CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. Lancet. 2003;362:767-771.
22. Pfeffer MA, Swedberg K, Granger CB, et al; CHARM Investigators and Committees. Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-Overall programme. Lancet. 2003;362:759-766.
23. Brenner BM, Cooper ME, de Zeeuw D, et al; RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861-869.
24. Palmer SC, Mavridis D, Navarese E, et al. Comparative efficacy and safety of blood pressure-lowering agents in adults with diabetes and kidney disease: a network meta-analysis. Lancet. 2015;385:2047-2056.
25. The ONTARGET Investigators. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547-1559.
26. Fried LF, Emanuele N, Zhang JH, et al; VA NEPHRON-D Investigators. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med. 2013;369:1892-1903.
27. Colberg SR, Sigal RJ, Yardley JE, et al. Physical activity/exercise and diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2016;39:2065-2079.
28. Hermida RC, Ayala DE, Mojón A, et al. Influence of time of day of blood pressure-lowering treatment on cardiovascular risk in hypertensive patients with type 2 diabetes. Diabetes Care. 2011;34:1270-1276.
29. Zhao P, Xu P, Wan C, et al. Evening versus morning dosing regimen drug therapy for hypertension. Cochrane Database Syst Rev. 2011;10:CD004184.
30. Py̆orälä K, Pedersen TR, Kjekshus J, et al. Cholesterol lowering with simvastatin improves prognosis of diabetic patients with coronary heart disease. A subgroup analysis of the Scandinavian Simvastatin Survival Study (4S). Diabetes Care. 1997;20:614-620.
31. Knopp RH, d’Emden M, Smilde JG, et al. Efficacy and safety of atorvastatin in the prevention of cardiovascular end points in subjects with type 2 diabetes: the Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in Non-Insulin-Dependent Diabetes Mellitus (ASPEN). Diabetes Care. 2006;29:1478-1485.
32. Cholesterol Treatment Trialists’ (CTT) Collaborators, Kearney PM, Blackwell L, Collins R, et al. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet. 2008;371:117-125.
33. Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013:CD004816.
34. Carter AA, Gomes T, Camacho X, et al. Risk of incident diabetes among patients treated with statins: population based study. BMJ. 2013;346:f2610.
35. Hayward RA, Hofer TP, Vijan S. Narrative review: lack of evidence for recommended low-density lipoprotein treatment targets: a solvable problem. Ann Intern Med. 2006;145:520-530.
36. Cannon CP, Braunwald E, McCabe CH, et al; Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495-1504.
37. de Lemos JA, Blazing MA, Wiviott SD, et al. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA. 2004;292:1307-1316.
38. Richardson K, Schoen M, French B, et al. Statins and cognitive function: a systematic review. Ann Intern Med. 2013;159:688-697.
39. Rajpathak SN, Kumbhani DJ, Crandall J, et al. Statin therapy and risk of developing type 2 diabetes: a meta-analysis. Diabetes Care. 2009;32:1924-1929.
40. Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet. 2010;375:735-742.
41. Meek C, Wierzbicki AS, Jewkes C, et al. Daily and intermittent rosuvastatin 5 mg therapy in statin intolerant patients: an observational study. Curr Med Res Opin. 2012;28:371-378.
42. ACCORD Study Group, Ginsberg HN, Bam MB, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563-1574.
43. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-2397.
44. AIM-HIGH Investigators, Boden WE, Probstfield JL, Anderson T, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255-2267.
45. Antithrombotic Trialists’ (ATT) Collaboration, Baigent C, Blackwell L, Collins R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373:1849-1860.
46. Perk J, De Backer G, Gohlke H, et al; European Association for Cardiovascular Prevention & Rehabilitation (EACPR); ESC Committee for Practice Guidelines (CPG). European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Eur Heart J. 2012;33:1635-1701.
47. Pignone M, Alberts MJ, Colwell JA, et al. Aspirin for primary prevention of cardiovascular events in people with diabetes. A position statement of the American Diabetes Association, a scientific statement of the American Heart Association, and an expert consensus document of the American College of Cardiology Foundation. Diabetes Care. 2010;33:1395-1402.
48. Vandvik PO, Lincoff AM, Gore JM, et al; American College of Chest Physicians. Primary and secondary prevention of cardiovascular disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl):e637S-e668S.
49. Kezerashvilli A, Marzo K, De Leon J. Beta blocker use after acute myocardial infarction in the patient with normal systolic function: when is it “ok” to discontinue? Curr Cardiol Rev. 2012;8:77-84.
50. Kannel WB, Hjortland M, Castelli WP. Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol. 1974;34:29-34.
51. Pioglitazone Package Insert. Available at: http://medlibrary.org/lib/rx/meds/pioglitazone-3/. Accessed April 10, 2017.
52. Scirica BM, Bhatt DL, Braunwald E, et al; SAVOR-TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317-1326.
53. Zannad F, Cannon CP, Cushman WC, et al; EXAMINE Investigators. Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double-blind trial. Lancet. 2015;385:2067-2076.
54. Green JB, Bethel MA, Armstrong PW, et al; TECOS Study Group. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373:232-242.
55. Zinman B, Wanner C, Lachin JM, et al, for the EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
56. FDA approves Jardiance to reduce cardiovascular death in adults with type 2 diabetes. FDA News Release, December 2, 2016. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm531517.htm. Accessed February 9, 2017.
57. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
FDA approves midostaurin to treat FLT3+ AML, advanced SM
The US Food and Drug Administration (FDA) has granted approval for the oral, multi-targeted kinase inhibitor midostaurin (Rydapt).
The drug is now approved for the treatment of adults with advanced systemic mastocytosis (SM), including aggressive SM (ASM), SM with associated hematologic neoplasm (SM-AHN), and mast cell leukemia (MCL).
Midostaurin is also approved for use in combination with standard cytarabine and daunorubicin induction, followed by cytarabine consolidation, in adults with newly diagnosed acute myeloid leukemia (AML) who are FLT3 mutation-positive, as detected by an FDA-approved test.
The FDA approved a companion diagnostic, the LeukoStrat CDx FLT3 Mutation Assay, for use with midostaurin to test AML patients for the FLT3 mutation.
Midostaurin is a product of Novartis. The companion diagnostic was developed by Novartis and Invivoscribe Technologies, Inc.
Midostaurin in AML
The FDA’s approval of midostaurin in AML is based on results from the phase 3 RATIFY trial, which were presented at the 2015 ASH Annual Meeting.
In RATIFY, researchers compared midostaurin plus standard chemotherapy to placebo plus standard chemotherapy in 717 adults younger than age 60 who had FLT3-mutated AML.
Patients in the midostaurin arm experienced a statistically significant improvement in overall survival, with a 23% reduction in risk of death compared to the placebo arm (hazard ratio=0.77, P=0.016).
The median event-free survival was significantly longer in the midostaurin arm than the placebo arm—8.2 months and 3.0 months, respectively (hazard ratio=0.78, P=0.004).
The most frequent adverse events (AEs) in the midostaurin arm (occurring in at least 20% of patients) were febrile neutropenia, nausea, vomiting, mucositis, headache, musculoskeletal pain, petechiae, device-related infection, epistaxis, hyperglycemia, and upper respiratory tract infections.
The most frequent grade 3/4 AEs (occurring in at least 10% of patients) were febrile neutropenia, device-related infection, and mucositis. Nine percent of patients in the midostaurin arm stopped treatment due to AEs, as did 6% in the placebo arm.
Midostaurin in advanced SM
The FDA’s approval of midostaurin in advanced SM was based on results from a pair of phase 2, single-arm studies, hereafter referred to as Study 2 and Study 3.
Data from Study 2 were published in NEJM in June 2016, and data from Study 3 were presented at the 2010 ASH Annual Meeting.
Study 2 included 116 patients, 115 of whom were evaluable for response.
The overall response rate (ORR) was 17% in the entire cohort, 31% among patients with ASM, 11% among patients with SM-AHN, and 19% among patients with MCL. The complete response rates were 2%, 6%, 0%, and 5%, respectively.
Study 3 included 26 patients with advanced SM. In 3 of the patients, the subtype of SM was unconfirmed.
Among the 17 patients with SM-AHN, there were 10 response (ORR=59%), including 1 partial response and 9 major responses. In the 6 patients with MCL, there were 2 responses (ORR=33%), which included 1 partial response and 1 major response.
In both studies combined, there were 142 adults with ASM, SM-AHN, or MCL.
The most frequent AEs (excluding laboratory abnormalities) that occurred in at least 20% of these patients were nausea, vomiting, diarrhea, edema, musculoskeletal pain, abdominal pain, fatigue, upper respiratory tract infection, constipation, pyrexia, headache, and dyspnea.
The most frequent grade 3 or higher AEs (excluding laboratory abnormalities) that occurred in at least 5% of patients were fatigue, sepsis, gastrointestinal hemorrhage, pneumonia, diarrhea, febrile neutropenia, edema, dyspnea, nausea, vomiting, abdominal pain, and renal insufficiency.
Serious AEs occurred in 68% of patients, most commonly infections and gastrointestinal disorders. Twenty-one percent of patients discontinued treatment due to AEs, the most frequent of which were infection, nausea or vomiting, QT prolongation, and gastrointestinal hemorrhage. ![]()
The US Food and Drug Administration (FDA) has granted approval for the oral, multi-targeted kinase inhibitor midostaurin (Rydapt).
The drug is now approved for the treatment of adults with advanced systemic mastocytosis (SM), including aggressive SM (ASM), SM with associated hematologic neoplasm (SM-AHN), and mast cell leukemia (MCL).
Midostaurin is also approved for use in combination with standard cytarabine and daunorubicin induction, followed by cytarabine consolidation, in adults with newly diagnosed acute myeloid leukemia (AML) who are FLT3 mutation-positive, as detected by an FDA-approved test.
The FDA approved a companion diagnostic, the LeukoStrat CDx FLT3 Mutation Assay, for use with midostaurin to test AML patients for the FLT3 mutation.
Midostaurin is a product of Novartis. The companion diagnostic was developed by Novartis and Invivoscribe Technologies, Inc.
Midostaurin in AML
The FDA’s approval of midostaurin in AML is based on results from the phase 3 RATIFY trial, which were presented at the 2015 ASH Annual Meeting.
In RATIFY, researchers compared midostaurin plus standard chemotherapy to placebo plus standard chemotherapy in 717 adults younger than age 60 who had FLT3-mutated AML.
Patients in the midostaurin arm experienced a statistically significant improvement in overall survival, with a 23% reduction in risk of death compared to the placebo arm (hazard ratio=0.77, P=0.016).
The median event-free survival was significantly longer in the midostaurin arm than the placebo arm—8.2 months and 3.0 months, respectively (hazard ratio=0.78, P=0.004).
The most frequent adverse events (AEs) in the midostaurin arm (occurring in at least 20% of patients) were febrile neutropenia, nausea, vomiting, mucositis, headache, musculoskeletal pain, petechiae, device-related infection, epistaxis, hyperglycemia, and upper respiratory tract infections.
The most frequent grade 3/4 AEs (occurring in at least 10% of patients) were febrile neutropenia, device-related infection, and mucositis. Nine percent of patients in the midostaurin arm stopped treatment due to AEs, as did 6% in the placebo arm.
Midostaurin in advanced SM
The FDA’s approval of midostaurin in advanced SM was based on results from a pair of phase 2, single-arm studies, hereafter referred to as Study 2 and Study 3.
Data from Study 2 were published in NEJM in June 2016, and data from Study 3 were presented at the 2010 ASH Annual Meeting.
Study 2 included 116 patients, 115 of whom were evaluable for response.
The overall response rate (ORR) was 17% in the entire cohort, 31% among patients with ASM, 11% among patients with SM-AHN, and 19% among patients with MCL. The complete response rates were 2%, 6%, 0%, and 5%, respectively.
Study 3 included 26 patients with advanced SM. In 3 of the patients, the subtype of SM was unconfirmed.
Among the 17 patients with SM-AHN, there were 10 response (ORR=59%), including 1 partial response and 9 major responses. In the 6 patients with MCL, there were 2 responses (ORR=33%), which included 1 partial response and 1 major response.
In both studies combined, there were 142 adults with ASM, SM-AHN, or MCL.
The most frequent AEs (excluding laboratory abnormalities) that occurred in at least 20% of these patients were nausea, vomiting, diarrhea, edema, musculoskeletal pain, abdominal pain, fatigue, upper respiratory tract infection, constipation, pyrexia, headache, and dyspnea.
The most frequent grade 3 or higher AEs (excluding laboratory abnormalities) that occurred in at least 5% of patients were fatigue, sepsis, gastrointestinal hemorrhage, pneumonia, diarrhea, febrile neutropenia, edema, dyspnea, nausea, vomiting, abdominal pain, and renal insufficiency.
Serious AEs occurred in 68% of patients, most commonly infections and gastrointestinal disorders. Twenty-one percent of patients discontinued treatment due to AEs, the most frequent of which were infection, nausea or vomiting, QT prolongation, and gastrointestinal hemorrhage. ![]()
The US Food and Drug Administration (FDA) has granted approval for the oral, multi-targeted kinase inhibitor midostaurin (Rydapt).
The drug is now approved for the treatment of adults with advanced systemic mastocytosis (SM), including aggressive SM (ASM), SM with associated hematologic neoplasm (SM-AHN), and mast cell leukemia (MCL).
Midostaurin is also approved for use in combination with standard cytarabine and daunorubicin induction, followed by cytarabine consolidation, in adults with newly diagnosed acute myeloid leukemia (AML) who are FLT3 mutation-positive, as detected by an FDA-approved test.
The FDA approved a companion diagnostic, the LeukoStrat CDx FLT3 Mutation Assay, for use with midostaurin to test AML patients for the FLT3 mutation.
Midostaurin is a product of Novartis. The companion diagnostic was developed by Novartis and Invivoscribe Technologies, Inc.
Midostaurin in AML
The FDA’s approval of midostaurin in AML is based on results from the phase 3 RATIFY trial, which were presented at the 2015 ASH Annual Meeting.
In RATIFY, researchers compared midostaurin plus standard chemotherapy to placebo plus standard chemotherapy in 717 adults younger than age 60 who had FLT3-mutated AML.
Patients in the midostaurin arm experienced a statistically significant improvement in overall survival, with a 23% reduction in risk of death compared to the placebo arm (hazard ratio=0.77, P=0.016).
The median event-free survival was significantly longer in the midostaurin arm than the placebo arm—8.2 months and 3.0 months, respectively (hazard ratio=0.78, P=0.004).
The most frequent adverse events (AEs) in the midostaurin arm (occurring in at least 20% of patients) were febrile neutropenia, nausea, vomiting, mucositis, headache, musculoskeletal pain, petechiae, device-related infection, epistaxis, hyperglycemia, and upper respiratory tract infections.
The most frequent grade 3/4 AEs (occurring in at least 10% of patients) were febrile neutropenia, device-related infection, and mucositis. Nine percent of patients in the midostaurin arm stopped treatment due to AEs, as did 6% in the placebo arm.
Midostaurin in advanced SM
The FDA’s approval of midostaurin in advanced SM was based on results from a pair of phase 2, single-arm studies, hereafter referred to as Study 2 and Study 3.
Data from Study 2 were published in NEJM in June 2016, and data from Study 3 were presented at the 2010 ASH Annual Meeting.
Study 2 included 116 patients, 115 of whom were evaluable for response.
The overall response rate (ORR) was 17% in the entire cohort, 31% among patients with ASM, 11% among patients with SM-AHN, and 19% among patients with MCL. The complete response rates were 2%, 6%, 0%, and 5%, respectively.
Study 3 included 26 patients with advanced SM. In 3 of the patients, the subtype of SM was unconfirmed.
Among the 17 patients with SM-AHN, there were 10 response (ORR=59%), including 1 partial response and 9 major responses. In the 6 patients with MCL, there were 2 responses (ORR=33%), which included 1 partial response and 1 major response.
In both studies combined, there were 142 adults with ASM, SM-AHN, or MCL.
The most frequent AEs (excluding laboratory abnormalities) that occurred in at least 20% of these patients were nausea, vomiting, diarrhea, edema, musculoskeletal pain, abdominal pain, fatigue, upper respiratory tract infection, constipation, pyrexia, headache, and dyspnea.
The most frequent grade 3 or higher AEs (excluding laboratory abnormalities) that occurred in at least 5% of patients were fatigue, sepsis, gastrointestinal hemorrhage, pneumonia, diarrhea, febrile neutropenia, edema, dyspnea, nausea, vomiting, abdominal pain, and renal insufficiency.
Serious AEs occurred in 68% of patients, most commonly infections and gastrointestinal disorders. Twenty-one percent of patients discontinued treatment due to AEs, the most frequent of which were infection, nausea or vomiting, QT prolongation, and gastrointestinal hemorrhage. ![]()
FDA approves test to detect FLT3 mutations
The US Food and Drug Administration (FDA) has approved use of the LeukoStrat® CDx FLT3 Mutation Assay as a companion diagnostic test.
The LeukoStrat® CDx FLT3 Mutation Assay is a signal ratio assay that identifies both internal tandem duplication and tyrosine kinase domain mutations.
The assay is the first FDA-approved companion diagnostic for acute myeloid leukemia (AML).
It is approved for use in patients newly diagnosed with AML to determine if they have FLT3 mutations and are therefore eligible to receive treatment with midostaurin (Rydapt).
The FDA granted approval for the LeukoStrat® CDx FLT3 Mutation Assay and midostaurin simultaneously.
The assay was developed by Invivoscribe Technologies, Inc. and Novartis. Midostaurin is a product of Novartis.
Under the current labeling, FLT3 mutation testing with the LeukoStrat® CDx FLT3 Mutation Assay is exclusively performed by The Laboratory for Personalized Molecular Medicine, a subsidiary of Invivoscribe Technologies, Inc.
Under terms of a previously announced agreement with Thermo Fisher, Invivoscribe will also seek FDA approval of the LeukoStrat® CDx FLT3 Mutation Assay that will allow the sale of kits to other laboratories. ![]()
The US Food and Drug Administration (FDA) has approved use of the LeukoStrat® CDx FLT3 Mutation Assay as a companion diagnostic test.
The LeukoStrat® CDx FLT3 Mutation Assay is a signal ratio assay that identifies both internal tandem duplication and tyrosine kinase domain mutations.
The assay is the first FDA-approved companion diagnostic for acute myeloid leukemia (AML).
It is approved for use in patients newly diagnosed with AML to determine if they have FLT3 mutations and are therefore eligible to receive treatment with midostaurin (Rydapt).
The FDA granted approval for the LeukoStrat® CDx FLT3 Mutation Assay and midostaurin simultaneously.
The assay was developed by Invivoscribe Technologies, Inc. and Novartis. Midostaurin is a product of Novartis.
Under the current labeling, FLT3 mutation testing with the LeukoStrat® CDx FLT3 Mutation Assay is exclusively performed by The Laboratory for Personalized Molecular Medicine, a subsidiary of Invivoscribe Technologies, Inc.
Under terms of a previously announced agreement with Thermo Fisher, Invivoscribe will also seek FDA approval of the LeukoStrat® CDx FLT3 Mutation Assay that will allow the sale of kits to other laboratories. ![]()
The US Food and Drug Administration (FDA) has approved use of the LeukoStrat® CDx FLT3 Mutation Assay as a companion diagnostic test.
The LeukoStrat® CDx FLT3 Mutation Assay is a signal ratio assay that identifies both internal tandem duplication and tyrosine kinase domain mutations.
The assay is the first FDA-approved companion diagnostic for acute myeloid leukemia (AML).
It is approved for use in patients newly diagnosed with AML to determine if they have FLT3 mutations and are therefore eligible to receive treatment with midostaurin (Rydapt).
The FDA granted approval for the LeukoStrat® CDx FLT3 Mutation Assay and midostaurin simultaneously.
The assay was developed by Invivoscribe Technologies, Inc. and Novartis. Midostaurin is a product of Novartis.
Under the current labeling, FLT3 mutation testing with the LeukoStrat® CDx FLT3 Mutation Assay is exclusively performed by The Laboratory for Personalized Molecular Medicine, a subsidiary of Invivoscribe Technologies, Inc.
Under terms of a previously announced agreement with Thermo Fisher, Invivoscribe will also seek FDA approval of the LeukoStrat® CDx FLT3 Mutation Assay that will allow the sale of kits to other laboratories. ![]()
Consider melatonin for migraine prevention
ILLUSTRATIVE CASE
A 32-year-old woman comes to your office for help with her recurrent migraines, which she’s had since her early 20s. She is otherwise healthy and active. She is frustrated over the frequency of her migraines and the debilitation they cause. She has tried prophylactic medications in the past, but stopped taking them because of the adverse effects. What do you recommend for treatment?
Daily preventive medication can be helpful for chronic migraine sufferers whose headaches have a significant impact on their lives and who have a goal of reducing headache frequency or severity, disability, and/or avoiding acute headache medication escalation.2 An estimated 38% of patients with migraines are appropriate candidates for prophylactic therapy, but only 3% to 13% are taking preventive medications.3
Evidence-based guidelines from the American Academy of Neurology and the American Headache Society state that antiepileptic drugs (divalproex sodium, sodium valproate, topiramate) and many beta-blockers (metoprolol, propranolol, timolol) are effective and should be recommended for migraine prevention (level A recommendation; based on ≥2 class I trials).2 Medications such as antidepressants (amitriptyline, venlafaxine) and other beta-blockers (atenolol, nadolol) are probably effective and can be considered (level B recommendation; based on one class I trial or 2 class II trials).2 However, adverse effects, such as somnolence, are listed as frequent with amitriptyline and occasional to frequent with topiramate.4
Researchers have investigated melatonin before. But a 2010 double-blind, crossover, randomized controlled trial (RCT) of 46 patients with 2 to 7 migraine attacks per month found no significant difference in reduction of headache frequency with extended-release melatonin 2 mg taken one hour before bed compared to placebo over an 8-week period.5
[polldaddy:9724288]
STUDY SUMMARY
Melatonin tops amitriptyline in >50% improvement in headache frequency
This RCT conducted in Brazil compared the effectiveness of melatonin to amitriptyline and placebo for migraine prevention in 196 adults (ages 18-65 years) with chronic migraines.1 Eligible patients had a history of at least 3 migraine attacks or 4 migraine headache days per month. Patients were randomized to take identically-appearing melatonin 3 mg, amitriptyline 25 mg, or placebo nightly. The investigators appear to have concealed allocation adequately, and used double-blinding.
The primary outcome was the number of headache days per month, comparing baseline with the 4 weeks of treatment. Secondary endpoints included reduction in migraine intensity, duration, number of analgesics used, and percentage of patients with more than 50% reduction in migraine headache days.
Compared to placebo, headache days per month were reduced in both the melatonin group (6.2 days vs 4.6 days, respectively; mean difference [MD], -1.6; 95% confidence interval [CI], -2.4 to -0.9) and the amitriptyline group (6.2 days vs 5 days, respectively; MD, -1.1; 95% CI, -1.5 to -0.7) at 12 weeks, based on intention-to-treat analysis. Mean headache intensity (0-10 pain scale) was also lower at 12 weeks in the melatonin group (4.8 vs 3.6; MD, -1.2; 95% CI, -1.6 to -0.8) and in the amitriptyline group (4.8 vs 3.5; MD, -1.3; 95% CI, -1.7 to -0.9), when compared to placebo.
Headache duration (hours/month) at 12 weeks was reduced in both groups (amitriptyline MD, -4.4 hours; 95% CI, -5.1 to -3.9; melatonin MD, -4.8 hours; 95% CI, -5.7 to -3.9), as was the number of analgesics used (amitriptyline MD, -1; 95% CI, -1.5 to -0.5; melatonin MD, -1; 95% CI, -1.4 to -0.6) when compared to placebo. There was no significant difference between the melatonin and amitriptyline groups for these outcomes.
Patients taking melatonin were more likely to have a >50% improvement in headache frequency compared to amitriptyline (54% vs 39%; number needed to treat [NNT]=7; P<.05); melatonin worked much better than placebo (54% vs 20%; NNT=3; P<.01).
Adverse events were reported more often in the amitriptyline group than in the melatonin group (46 vs 16; P<.03) with daytime sleepiness being the most frequent complaint (41% of patients in the amitriptyline group vs 18% of the melatonin group; number needed to harm [NNH]=5). There was no significant difference in adverse events between melatonin and placebo (16 vs 17; P=not significant). Melatonin resulted in weight loss (mean, -0.14 kg), whereas those taking amitriptyline gained weight (+0.97 kg; P<.01).
WHAT’S NEW
An effective migraine prevention alternative with minimal adverse effects
Melatonin is an accessible and affordable option for preventing migraine headaches in chronic sufferers. The 3-mg dosing reduces headache frequency—both in terms of the number of migraine headache days per month and in terms of the percentage of patients with a >50% reduction in headache events—as well as headache intensity, with minimal adverse effects.
CAVEATS
Product consistency, missing study data
This trial used 3-mg dosing, so it is not clear if other doses are also effective. In addition, because melatonin is available over-the-counter, the quality/actual doses may be less well regulated, and thus, there may be a lack of consistency between brands. Unlike clinical practice, neither the amitriptyline nor the melatonin dose was titrated according to patient response or adverse effects. As a result, we are not sure of the actual lowest effective dose, or if greater effect (with continued minimal adverse effects) could be achieved with higher doses.
Lastly, 69% to 75% of patients in the treatment groups completed the 16-week trial, but the authors of the study reported using 3 different analytic techniques to estimate missing data. The primary outcome included 178 of 196 randomized patients (90.8%). For the primary endpoint, the authors treated all missing data as non-headache days. It is unclear how these missing data would affect the outcome, although an analysis like this would tend towards a null effect.
CHALLENGES TO IMPLEMENTATION
Challenges are negligible
There are really no challenges to implementing this practice changer; melatonin is readily available over-the-counter and it is affordable.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Gonçalves AL, Martini Ferreira A, Ribeiro RT, et al. Randomised clinical trial comparing melatonin 3 mg, amitriptyline 25 mg and placebo for migraine prevention. J Neurol Neurosurg Psychiatry. 2016;87:1127-1132.
2. Silberstein SD, Holland S, Freitag F, et al. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1337-1345.
3. Lipton RB, Bigal ME, Diamond M, et al; The American Migraine Prevalence and Prevention Advisory Group. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68:343-349.
4. Silberstein SD. Practice parameter: evidence-based guidelines for migraine headache (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2000;55:754-762.
5. Alstadhaug KB, Odeh F, Salvesen R, et al. Prophylaxis of migraine with melatonin: a randomized controlled trial. Neurology. 2010;75:1527-1532.
ILLUSTRATIVE CASE
A 32-year-old woman comes to your office for help with her recurrent migraines, which she’s had since her early 20s. She is otherwise healthy and active. She is frustrated over the frequency of her migraines and the debilitation they cause. She has tried prophylactic medications in the past, but stopped taking them because of the adverse effects. What do you recommend for treatment?
Daily preventive medication can be helpful for chronic migraine sufferers whose headaches have a significant impact on their lives and who have a goal of reducing headache frequency or severity, disability, and/or avoiding acute headache medication escalation.2 An estimated 38% of patients with migraines are appropriate candidates for prophylactic therapy, but only 3% to 13% are taking preventive medications.3
Evidence-based guidelines from the American Academy of Neurology and the American Headache Society state that antiepileptic drugs (divalproex sodium, sodium valproate, topiramate) and many beta-blockers (metoprolol, propranolol, timolol) are effective and should be recommended for migraine prevention (level A recommendation; based on ≥2 class I trials).2 Medications such as antidepressants (amitriptyline, venlafaxine) and other beta-blockers (atenolol, nadolol) are probably effective and can be considered (level B recommendation; based on one class I trial or 2 class II trials).2 However, adverse effects, such as somnolence, are listed as frequent with amitriptyline and occasional to frequent with topiramate.4
Researchers have investigated melatonin before. But a 2010 double-blind, crossover, randomized controlled trial (RCT) of 46 patients with 2 to 7 migraine attacks per month found no significant difference in reduction of headache frequency with extended-release melatonin 2 mg taken one hour before bed compared to placebo over an 8-week period.5
[polldaddy:9724288]
STUDY SUMMARY
Melatonin tops amitriptyline in >50% improvement in headache frequency
This RCT conducted in Brazil compared the effectiveness of melatonin to amitriptyline and placebo for migraine prevention in 196 adults (ages 18-65 years) with chronic migraines.1 Eligible patients had a history of at least 3 migraine attacks or 4 migraine headache days per month. Patients were randomized to take identically-appearing melatonin 3 mg, amitriptyline 25 mg, or placebo nightly. The investigators appear to have concealed allocation adequately, and used double-blinding.
The primary outcome was the number of headache days per month, comparing baseline with the 4 weeks of treatment. Secondary endpoints included reduction in migraine intensity, duration, number of analgesics used, and percentage of patients with more than 50% reduction in migraine headache days.
Compared to placebo, headache days per month were reduced in both the melatonin group (6.2 days vs 4.6 days, respectively; mean difference [MD], -1.6; 95% confidence interval [CI], -2.4 to -0.9) and the amitriptyline group (6.2 days vs 5 days, respectively; MD, -1.1; 95% CI, -1.5 to -0.7) at 12 weeks, based on intention-to-treat analysis. Mean headache intensity (0-10 pain scale) was also lower at 12 weeks in the melatonin group (4.8 vs 3.6; MD, -1.2; 95% CI, -1.6 to -0.8) and in the amitriptyline group (4.8 vs 3.5; MD, -1.3; 95% CI, -1.7 to -0.9), when compared to placebo.
Headache duration (hours/month) at 12 weeks was reduced in both groups (amitriptyline MD, -4.4 hours; 95% CI, -5.1 to -3.9; melatonin MD, -4.8 hours; 95% CI, -5.7 to -3.9), as was the number of analgesics used (amitriptyline MD, -1; 95% CI, -1.5 to -0.5; melatonin MD, -1; 95% CI, -1.4 to -0.6) when compared to placebo. There was no significant difference between the melatonin and amitriptyline groups for these outcomes.
Patients taking melatonin were more likely to have a >50% improvement in headache frequency compared to amitriptyline (54% vs 39%; number needed to treat [NNT]=7; P<.05); melatonin worked much better than placebo (54% vs 20%; NNT=3; P<.01).
Adverse events were reported more often in the amitriptyline group than in the melatonin group (46 vs 16; P<.03) with daytime sleepiness being the most frequent complaint (41% of patients in the amitriptyline group vs 18% of the melatonin group; number needed to harm [NNH]=5). There was no significant difference in adverse events between melatonin and placebo (16 vs 17; P=not significant). Melatonin resulted in weight loss (mean, -0.14 kg), whereas those taking amitriptyline gained weight (+0.97 kg; P<.01).
WHAT’S NEW
An effective migraine prevention alternative with minimal adverse effects
Melatonin is an accessible and affordable option for preventing migraine headaches in chronic sufferers. The 3-mg dosing reduces headache frequency—both in terms of the number of migraine headache days per month and in terms of the percentage of patients with a >50% reduction in headache events—as well as headache intensity, with minimal adverse effects.
CAVEATS
Product consistency, missing study data
This trial used 3-mg dosing, so it is not clear if other doses are also effective. In addition, because melatonin is available over-the-counter, the quality/actual doses may be less well regulated, and thus, there may be a lack of consistency between brands. Unlike clinical practice, neither the amitriptyline nor the melatonin dose was titrated according to patient response or adverse effects. As a result, we are not sure of the actual lowest effective dose, or if greater effect (with continued minimal adverse effects) could be achieved with higher doses.
Lastly, 69% to 75% of patients in the treatment groups completed the 16-week trial, but the authors of the study reported using 3 different analytic techniques to estimate missing data. The primary outcome included 178 of 196 randomized patients (90.8%). For the primary endpoint, the authors treated all missing data as non-headache days. It is unclear how these missing data would affect the outcome, although an analysis like this would tend towards a null effect.
CHALLENGES TO IMPLEMENTATION
Challenges are negligible
There are really no challenges to implementing this practice changer; melatonin is readily available over-the-counter and it is affordable.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 32-year-old woman comes to your office for help with her recurrent migraines, which she’s had since her early 20s. She is otherwise healthy and active. She is frustrated over the frequency of her migraines and the debilitation they cause. She has tried prophylactic medications in the past, but stopped taking them because of the adverse effects. What do you recommend for treatment?
Daily preventive medication can be helpful for chronic migraine sufferers whose headaches have a significant impact on their lives and who have a goal of reducing headache frequency or severity, disability, and/or avoiding acute headache medication escalation.2 An estimated 38% of patients with migraines are appropriate candidates for prophylactic therapy, but only 3% to 13% are taking preventive medications.3
Evidence-based guidelines from the American Academy of Neurology and the American Headache Society state that antiepileptic drugs (divalproex sodium, sodium valproate, topiramate) and many beta-blockers (metoprolol, propranolol, timolol) are effective and should be recommended for migraine prevention (level A recommendation; based on ≥2 class I trials).2 Medications such as antidepressants (amitriptyline, venlafaxine) and other beta-blockers (atenolol, nadolol) are probably effective and can be considered (level B recommendation; based on one class I trial or 2 class II trials).2 However, adverse effects, such as somnolence, are listed as frequent with amitriptyline and occasional to frequent with topiramate.4
Researchers have investigated melatonin before. But a 2010 double-blind, crossover, randomized controlled trial (RCT) of 46 patients with 2 to 7 migraine attacks per month found no significant difference in reduction of headache frequency with extended-release melatonin 2 mg taken one hour before bed compared to placebo over an 8-week period.5
[polldaddy:9724288]
STUDY SUMMARY
Melatonin tops amitriptyline in >50% improvement in headache frequency
This RCT conducted in Brazil compared the effectiveness of melatonin to amitriptyline and placebo for migraine prevention in 196 adults (ages 18-65 years) with chronic migraines.1 Eligible patients had a history of at least 3 migraine attacks or 4 migraine headache days per month. Patients were randomized to take identically-appearing melatonin 3 mg, amitriptyline 25 mg, or placebo nightly. The investigators appear to have concealed allocation adequately, and used double-blinding.
The primary outcome was the number of headache days per month, comparing baseline with the 4 weeks of treatment. Secondary endpoints included reduction in migraine intensity, duration, number of analgesics used, and percentage of patients with more than 50% reduction in migraine headache days.
Compared to placebo, headache days per month were reduced in both the melatonin group (6.2 days vs 4.6 days, respectively; mean difference [MD], -1.6; 95% confidence interval [CI], -2.4 to -0.9) and the amitriptyline group (6.2 days vs 5 days, respectively; MD, -1.1; 95% CI, -1.5 to -0.7) at 12 weeks, based on intention-to-treat analysis. Mean headache intensity (0-10 pain scale) was also lower at 12 weeks in the melatonin group (4.8 vs 3.6; MD, -1.2; 95% CI, -1.6 to -0.8) and in the amitriptyline group (4.8 vs 3.5; MD, -1.3; 95% CI, -1.7 to -0.9), when compared to placebo.
Headache duration (hours/month) at 12 weeks was reduced in both groups (amitriptyline MD, -4.4 hours; 95% CI, -5.1 to -3.9; melatonin MD, -4.8 hours; 95% CI, -5.7 to -3.9), as was the number of analgesics used (amitriptyline MD, -1; 95% CI, -1.5 to -0.5; melatonin MD, -1; 95% CI, -1.4 to -0.6) when compared to placebo. There was no significant difference between the melatonin and amitriptyline groups for these outcomes.
Patients taking melatonin were more likely to have a >50% improvement in headache frequency compared to amitriptyline (54% vs 39%; number needed to treat [NNT]=7; P<.05); melatonin worked much better than placebo (54% vs 20%; NNT=3; P<.01).
Adverse events were reported more often in the amitriptyline group than in the melatonin group (46 vs 16; P<.03) with daytime sleepiness being the most frequent complaint (41% of patients in the amitriptyline group vs 18% of the melatonin group; number needed to harm [NNH]=5). There was no significant difference in adverse events between melatonin and placebo (16 vs 17; P=not significant). Melatonin resulted in weight loss (mean, -0.14 kg), whereas those taking amitriptyline gained weight (+0.97 kg; P<.01).
WHAT’S NEW
An effective migraine prevention alternative with minimal adverse effects
Melatonin is an accessible and affordable option for preventing migraine headaches in chronic sufferers. The 3-mg dosing reduces headache frequency—both in terms of the number of migraine headache days per month and in terms of the percentage of patients with a >50% reduction in headache events—as well as headache intensity, with minimal adverse effects.
CAVEATS
Product consistency, missing study data
This trial used 3-mg dosing, so it is not clear if other doses are also effective. In addition, because melatonin is available over-the-counter, the quality/actual doses may be less well regulated, and thus, there may be a lack of consistency between brands. Unlike clinical practice, neither the amitriptyline nor the melatonin dose was titrated according to patient response or adverse effects. As a result, we are not sure of the actual lowest effective dose, or if greater effect (with continued minimal adverse effects) could be achieved with higher doses.
Lastly, 69% to 75% of patients in the treatment groups completed the 16-week trial, but the authors of the study reported using 3 different analytic techniques to estimate missing data. The primary outcome included 178 of 196 randomized patients (90.8%). For the primary endpoint, the authors treated all missing data as non-headache days. It is unclear how these missing data would affect the outcome, although an analysis like this would tend towards a null effect.
CHALLENGES TO IMPLEMENTATION
Challenges are negligible
There are really no challenges to implementing this practice changer; melatonin is readily available over-the-counter and it is affordable.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Gonçalves AL, Martini Ferreira A, Ribeiro RT, et al. Randomised clinical trial comparing melatonin 3 mg, amitriptyline 25 mg and placebo for migraine prevention. J Neurol Neurosurg Psychiatry. 2016;87:1127-1132.
2. Silberstein SD, Holland S, Freitag F, et al. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1337-1345.
3. Lipton RB, Bigal ME, Diamond M, et al; The American Migraine Prevalence and Prevention Advisory Group. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68:343-349.
4. Silberstein SD. Practice parameter: evidence-based guidelines for migraine headache (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2000;55:754-762.
5. Alstadhaug KB, Odeh F, Salvesen R, et al. Prophylaxis of migraine with melatonin: a randomized controlled trial. Neurology. 2010;75:1527-1532.
1. Gonçalves AL, Martini Ferreira A, Ribeiro RT, et al. Randomised clinical trial comparing melatonin 3 mg, amitriptyline 25 mg and placebo for migraine prevention. J Neurol Neurosurg Psychiatry. 2016;87:1127-1132.
2. Silberstein SD, Holland S, Freitag F, et al. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1337-1345.
3. Lipton RB, Bigal ME, Diamond M, et al; The American Migraine Prevalence and Prevention Advisory Group. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68:343-349.
4. Silberstein SD. Practice parameter: evidence-based guidelines for migraine headache (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2000;55:754-762.
5. Alstadhaug KB, Odeh F, Salvesen R, et al. Prophylaxis of migraine with melatonin: a randomized controlled trial. Neurology. 2010;75:1527-1532.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
PRACTICE CHANGER
Recommend nightly melatonin 3 mg to your patients with chronic migraines, as it appears to be as effective as amitriptyline in reducing headaches and causes fewer adverse effects.
STRENGTH OF RECOMMENDATION
B: Based on a single, good quality randomized controlled trial.
Gonçalves AL, Martini Ferreira A, Ribeiro RT, et al. Randomised clinical trial comparing melatonin 3 mg, amitriptyline 25 mg and placebo for migraine prevention. J Neurol Neurosurg Psychiatry. 2016;87:1127-1132.1
Green fingernail
A 34-year-old woman came to our clinic because she was concerned about her thumbnail, which had turned green. Although her finger didn’t hurt, she was bothered by its appearance. Several months earlier, the woman had sought care at a different clinic because the same nail had become brittle and come loose from the nail bed, which was spongy. The physician advised her that she had onychomycosis and prescribed ciclopirox lacquer, but it didn’t help.
Over the next 3 weeks, she noticed a faint green hue developing at the tip of the nail, which expanded and intensified in color (FIGURE). The patient was a mother who worked at home, washed dishes by hand daily, and bathed her children. Her past medical history was significant for type 1 diabetes mellitus and Hashimoto’s thyroiditis. She had no other symptoms.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Green nail syndrome caused by Pseudomonas aeruginosa
This patient had green nail syndrome (GNS), an infection of the nail bed caused by Pseudomonas aeruginosa. These bacteria produce pyocyanin, a blue-green pigment that discolors the nail.1 GNS often occurs in patients with prior nail problems, such as onychomycosis, onycholysis, trauma, chronic paronychia, or psoriasis.
Nail disease disrupts the integumentary barrier and allows a portal of entry for bacteria. Scanning electron microscopy of patients with GNS has shown that fungal infections create tunnel-like structures in the nail keratin, and P aeruginosa grows in these spaces.2 Nails with prior nail disease that are chronically exposed to moisture are at greatest risk of developing GNS,3,4 and it is typical for only one nail to be involved.5Pseudomonas is the most common bacterial infection of the nails, but is not well known because it is rarely reported and patients often don’t seek care.6
In our patient’s case, her prior onychomycosis helped to create a favorable environment for the growth of the bacteria. Onycholysis—characterized by separation of the nail plate from the nail bed—was also present in our patient, based on her description of a “spongy” nail bed and loose nail, allowing moisture and bacteria to infiltrate the space. Onycholysis is associated with hypothyroidism, which the patient also had.7 The frequent soaking of her hands during dishwashing and bathing her children helped to provide the moist environment in which Pseudomonas thrives.
As was the case in this patient, GNS is often painless, or may be accompanied by mild tenderness of the nail. Patients may seek treatment primarily for cosmetic reasons.
GNS can be diagnosed by clinical observation and characteristic pigmentation along with an appropriate patient history.4 Culture of the nail bed may be helpful if bacterial resistance or co-infection with fungal organisms is suspected.
Changes in nail color can be a sign of many conditions
Nail discoloration, or chromonychia, can present in a variety of colors. Nail findings may represent an isolated disease or provide an important clinical clue to other systemic diseases.8 The specific shade of discoloration helps to differentiate the underlying pathology.
Yellow nail syndrome. As the name implies, this syndrome typically causes yellow discoloration of the nail (although yellow-green is also possible). Yellow nail syndrome is believed to be due to microvascular permeability, which also accounts for its associated clinical triad: hypoalbuminemia, pleural effusion, and lymphedema. Yellow nail syndrome may be seen in patients with bronchiectasis, internal malignancies, immunodeficiency, and rheumatoid arthritis.8
Nail bed hematoma. Among the most common causes of nail discoloration, these lesions typically appear as reddish to reddish-black, depending on the age of the bleed, and will often have streaks at the distal margin of the lesion.9 Risk factors for hemorrhage include blood thinners and clotting disorders. Subungual hemorrhages that do not grow out with the nail, or that recur in the same place, may require biopsy.9
Subungual melanoma causes black-brown discoloration of the nails, and may form a longitudinal band in the nail.9 Longitudinal melanonychia is a common variant in African American individuals.10 Features that increase the likelihood of melanoma include a family history of melanoma, a sudden change in the appearance of the lesion, band width greater than 3 mm, pigment changes extending into the cuticle (known as Hutchinson’s sign), and nail plate disruption.
Dermoscopy, the technique of using surface microscopy to examine the skin, may be helpful in distinguishing nail lesions. (See a video on how to perform dermoscopy here: http://bit.ly/2pyJ3xN.)
Nonmelanocytic lesions tend to have homogeneously distributed pigment, while melanocytic lesions contain granules of pigment in cellular inclusions. Any suspicion of melanoma warrants a punch biopsy.11
Medication-induced effects. Minocycline may cause bluish nail discoloration similar to that produced by infection with P aeruginosa, but it is rare for only a single nail to be involved. In addition, pigmentation changes are often present elsewhere on the body, including the sclerae, teeth, and pinna.
Another medication known to color the nails blue is colloidal silver, which is still sold as a dietary supplement or homeopathic remedy to treat a wide range of ailments.6 (Of note: In 1999, the Food and Drug Administration issued a final rule saying that colloidal silver isn’t safe or effective for treating any disease or condition.12)
Glomus tumor. Another cause of blue nails is glomus tumors, relatively uncommon perivascular neoplasms that are typically found in the subungual region. These tumors are generally accompanied by localized tenderness, cold sensitivity, and paroxysms of excruciating pain that are disproportional to the size of the tumor.
Imaging studies may aid in the diagnosis, in addition to pathologic confirmation. Magnetic resonance imaging is the most sensitive imaging modality; if a glomus tumor is present, it most often appears as a well-circumscribed T2 hyperintense lesion.13
Exogenous pigmentation. Nails may become discolored due to exposure to various toxins or chemicals. Frequent culprits include eosin, methylene blue, henna, hair dye, and tobacco.9
Antibiotics and measures to keep the nail dry will help resolve infection
When chronic nail wetness is a contributing factor, treatment begins with measures to keep the nails dry. In addition, either topical or systemic antibiotics may be used to eradicate the infection. Topical applications with agents such as nadifloxacin have been shown to be effective in several case reports,3 but large-scale controlled trials are lacking. Fluoroquinolones are regarded as first-line systemic treatment.5 Briefly soaking the nail in a diluted sodium hypochlorite (bleach) solution also helps to suppress bacterial growth. Nail extraction may be required in refractory cases.
For our patient, we prescribed ciprofloxacin 500 mg twice a day for 10 days, plus bleach soaks (one part bleach to 4 parts water) twice a day. We recommended that our patient wear gloves for household tasks that involved immersing her hands in water, and drying her finger with a hair dryer after bathing.
CORRESPONDENCE
David Gish, MD, University of Virginia Health System, 1215 Lee St. Charlottesville, VA 22908; [email protected].
1. Greene SL, Su WP, Muller SA. Pseudomonas aeruginosa infections of the skin. Am Fam Physician. 1984;29:193-200.
2. de Almeida HL Jr, Duquia RP, de Castro LA, et al. Scanning electron microscopy of the green nail. Int J Dermatol. 2010;49:962-963.
3. Hengge UR, Bardeli V. Images in clinical medicine. Green nails. N Engl J Med. 2009;360:1125.
4. Chiriac A, Brzezinski P, Foia L, et al. Chloronychia: green nail syndrome caused by Pseudomonas aeruginosa in elderly persons. Clin Interv Aging. 2015;10:265-267.
5. Müller S, Ebnöther M, Itin P. Green nail syndrome (Pseudomonas aeruginosa nail infection): Two cases successfully treated with topical nadifloxacin, an acne medication. Case Rep Dermatol. 2014;6:180-184.
6. Raam R, DeClerck B, Jhun P, et al. That’s some weird nail polish you got there! Ann Emerg Med. 2015;66:585-588.
7. Gregoriou S, Argyriou G, Larios G, et al. Nail disorders and systemic disease: what the nails tell us. J Fam Pract. 2008;57:509-514.
8. Fawcett RS, Linford S, Stulberg DL. Nail abnormalities: clues to systemic disease. Am Fam Physician. 2004;69:1417-1424.
9. Braun RP, Baran R, Le Gal FA, et al. Diagnosis and management of nail pigmentations. J Am Acad Dermatol. 2007;56:835-847.
10. Buka R, Friedman KA, Phelps RG, et al. Childhood longitudinal melanonychia: case reports and review of the literature. Mt Sinai J Med. 2001;68:331-335.
11. Tully AS, Trayes KP, Studdiford JS. Evaluation of nail abnormalities. Am Fam Physician. 2012;85:779-787.
12. US Food and Drug Administration. Over-the-counter drug products containing colloidal silver ingredients or silver salts. 1999. Available at: https://www.fda.gov/ohrms/dockets/98fr/081799a.txt. Accessed April 11, 2017.
13. Glazebrook KN, Laundre BJ, Schiefer TK, et al. Imaging features of glomus tumors. Skeletal Radiol. 2011;40:855-862.
A 34-year-old woman came to our clinic because she was concerned about her thumbnail, which had turned green. Although her finger didn’t hurt, she was bothered by its appearance. Several months earlier, the woman had sought care at a different clinic because the same nail had become brittle and come loose from the nail bed, which was spongy. The physician advised her that she had onychomycosis and prescribed ciclopirox lacquer, but it didn’t help.
Over the next 3 weeks, she noticed a faint green hue developing at the tip of the nail, which expanded and intensified in color (FIGURE). The patient was a mother who worked at home, washed dishes by hand daily, and bathed her children. Her past medical history was significant for type 1 diabetes mellitus and Hashimoto’s thyroiditis. She had no other symptoms.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Green nail syndrome caused by Pseudomonas aeruginosa
This patient had green nail syndrome (GNS), an infection of the nail bed caused by Pseudomonas aeruginosa. These bacteria produce pyocyanin, a blue-green pigment that discolors the nail.1 GNS often occurs in patients with prior nail problems, such as onychomycosis, onycholysis, trauma, chronic paronychia, or psoriasis.
Nail disease disrupts the integumentary barrier and allows a portal of entry for bacteria. Scanning electron microscopy of patients with GNS has shown that fungal infections create tunnel-like structures in the nail keratin, and P aeruginosa grows in these spaces.2 Nails with prior nail disease that are chronically exposed to moisture are at greatest risk of developing GNS,3,4 and it is typical for only one nail to be involved.5Pseudomonas is the most common bacterial infection of the nails, but is not well known because it is rarely reported and patients often don’t seek care.6
In our patient’s case, her prior onychomycosis helped to create a favorable environment for the growth of the bacteria. Onycholysis—characterized by separation of the nail plate from the nail bed—was also present in our patient, based on her description of a “spongy” nail bed and loose nail, allowing moisture and bacteria to infiltrate the space. Onycholysis is associated with hypothyroidism, which the patient also had.7 The frequent soaking of her hands during dishwashing and bathing her children helped to provide the moist environment in which Pseudomonas thrives.
As was the case in this patient, GNS is often painless, or may be accompanied by mild tenderness of the nail. Patients may seek treatment primarily for cosmetic reasons.
GNS can be diagnosed by clinical observation and characteristic pigmentation along with an appropriate patient history.4 Culture of the nail bed may be helpful if bacterial resistance or co-infection with fungal organisms is suspected.
Changes in nail color can be a sign of many conditions
Nail discoloration, or chromonychia, can present in a variety of colors. Nail findings may represent an isolated disease or provide an important clinical clue to other systemic diseases.8 The specific shade of discoloration helps to differentiate the underlying pathology.
Yellow nail syndrome. As the name implies, this syndrome typically causes yellow discoloration of the nail (although yellow-green is also possible). Yellow nail syndrome is believed to be due to microvascular permeability, which also accounts for its associated clinical triad: hypoalbuminemia, pleural effusion, and lymphedema. Yellow nail syndrome may be seen in patients with bronchiectasis, internal malignancies, immunodeficiency, and rheumatoid arthritis.8
Nail bed hematoma. Among the most common causes of nail discoloration, these lesions typically appear as reddish to reddish-black, depending on the age of the bleed, and will often have streaks at the distal margin of the lesion.9 Risk factors for hemorrhage include blood thinners and clotting disorders. Subungual hemorrhages that do not grow out with the nail, or that recur in the same place, may require biopsy.9
Subungual melanoma causes black-brown discoloration of the nails, and may form a longitudinal band in the nail.9 Longitudinal melanonychia is a common variant in African American individuals.10 Features that increase the likelihood of melanoma include a family history of melanoma, a sudden change in the appearance of the lesion, band width greater than 3 mm, pigment changes extending into the cuticle (known as Hutchinson’s sign), and nail plate disruption.
Dermoscopy, the technique of using surface microscopy to examine the skin, may be helpful in distinguishing nail lesions. (See a video on how to perform dermoscopy here: http://bit.ly/2pyJ3xN.)
Nonmelanocytic lesions tend to have homogeneously distributed pigment, while melanocytic lesions contain granules of pigment in cellular inclusions. Any suspicion of melanoma warrants a punch biopsy.11
Medication-induced effects. Minocycline may cause bluish nail discoloration similar to that produced by infection with P aeruginosa, but it is rare for only a single nail to be involved. In addition, pigmentation changes are often present elsewhere on the body, including the sclerae, teeth, and pinna.
Another medication known to color the nails blue is colloidal silver, which is still sold as a dietary supplement or homeopathic remedy to treat a wide range of ailments.6 (Of note: In 1999, the Food and Drug Administration issued a final rule saying that colloidal silver isn’t safe or effective for treating any disease or condition.12)
Glomus tumor. Another cause of blue nails is glomus tumors, relatively uncommon perivascular neoplasms that are typically found in the subungual region. These tumors are generally accompanied by localized tenderness, cold sensitivity, and paroxysms of excruciating pain that are disproportional to the size of the tumor.
Imaging studies may aid in the diagnosis, in addition to pathologic confirmation. Magnetic resonance imaging is the most sensitive imaging modality; if a glomus tumor is present, it most often appears as a well-circumscribed T2 hyperintense lesion.13
Exogenous pigmentation. Nails may become discolored due to exposure to various toxins or chemicals. Frequent culprits include eosin, methylene blue, henna, hair dye, and tobacco.9
Antibiotics and measures to keep the nail dry will help resolve infection
When chronic nail wetness is a contributing factor, treatment begins with measures to keep the nails dry. In addition, either topical or systemic antibiotics may be used to eradicate the infection. Topical applications with agents such as nadifloxacin have been shown to be effective in several case reports,3 but large-scale controlled trials are lacking. Fluoroquinolones are regarded as first-line systemic treatment.5 Briefly soaking the nail in a diluted sodium hypochlorite (bleach) solution also helps to suppress bacterial growth. Nail extraction may be required in refractory cases.
For our patient, we prescribed ciprofloxacin 500 mg twice a day for 10 days, plus bleach soaks (one part bleach to 4 parts water) twice a day. We recommended that our patient wear gloves for household tasks that involved immersing her hands in water, and drying her finger with a hair dryer after bathing.
CORRESPONDENCE
David Gish, MD, University of Virginia Health System, 1215 Lee St. Charlottesville, VA 22908; [email protected].
A 34-year-old woman came to our clinic because she was concerned about her thumbnail, which had turned green. Although her finger didn’t hurt, she was bothered by its appearance. Several months earlier, the woman had sought care at a different clinic because the same nail had become brittle and come loose from the nail bed, which was spongy. The physician advised her that she had onychomycosis and prescribed ciclopirox lacquer, but it didn’t help.
Over the next 3 weeks, she noticed a faint green hue developing at the tip of the nail, which expanded and intensified in color (FIGURE). The patient was a mother who worked at home, washed dishes by hand daily, and bathed her children. Her past medical history was significant for type 1 diabetes mellitus and Hashimoto’s thyroiditis. She had no other symptoms.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Green nail syndrome caused by Pseudomonas aeruginosa
This patient had green nail syndrome (GNS), an infection of the nail bed caused by Pseudomonas aeruginosa. These bacteria produce pyocyanin, a blue-green pigment that discolors the nail.1 GNS often occurs in patients with prior nail problems, such as onychomycosis, onycholysis, trauma, chronic paronychia, or psoriasis.
Nail disease disrupts the integumentary barrier and allows a portal of entry for bacteria. Scanning electron microscopy of patients with GNS has shown that fungal infections create tunnel-like structures in the nail keratin, and P aeruginosa grows in these spaces.2 Nails with prior nail disease that are chronically exposed to moisture are at greatest risk of developing GNS,3,4 and it is typical for only one nail to be involved.5Pseudomonas is the most common bacterial infection of the nails, but is not well known because it is rarely reported and patients often don’t seek care.6
In our patient’s case, her prior onychomycosis helped to create a favorable environment for the growth of the bacteria. Onycholysis—characterized by separation of the nail plate from the nail bed—was also present in our patient, based on her description of a “spongy” nail bed and loose nail, allowing moisture and bacteria to infiltrate the space. Onycholysis is associated with hypothyroidism, which the patient also had.7 The frequent soaking of her hands during dishwashing and bathing her children helped to provide the moist environment in which Pseudomonas thrives.
As was the case in this patient, GNS is often painless, or may be accompanied by mild tenderness of the nail. Patients may seek treatment primarily for cosmetic reasons.
GNS can be diagnosed by clinical observation and characteristic pigmentation along with an appropriate patient history.4 Culture of the nail bed may be helpful if bacterial resistance or co-infection with fungal organisms is suspected.
Changes in nail color can be a sign of many conditions
Nail discoloration, or chromonychia, can present in a variety of colors. Nail findings may represent an isolated disease or provide an important clinical clue to other systemic diseases.8 The specific shade of discoloration helps to differentiate the underlying pathology.
Yellow nail syndrome. As the name implies, this syndrome typically causes yellow discoloration of the nail (although yellow-green is also possible). Yellow nail syndrome is believed to be due to microvascular permeability, which also accounts for its associated clinical triad: hypoalbuminemia, pleural effusion, and lymphedema. Yellow nail syndrome may be seen in patients with bronchiectasis, internal malignancies, immunodeficiency, and rheumatoid arthritis.8
Nail bed hematoma. Among the most common causes of nail discoloration, these lesions typically appear as reddish to reddish-black, depending on the age of the bleed, and will often have streaks at the distal margin of the lesion.9 Risk factors for hemorrhage include blood thinners and clotting disorders. Subungual hemorrhages that do not grow out with the nail, or that recur in the same place, may require biopsy.9
Subungual melanoma causes black-brown discoloration of the nails, and may form a longitudinal band in the nail.9 Longitudinal melanonychia is a common variant in African American individuals.10 Features that increase the likelihood of melanoma include a family history of melanoma, a sudden change in the appearance of the lesion, band width greater than 3 mm, pigment changes extending into the cuticle (known as Hutchinson’s sign), and nail plate disruption.
Dermoscopy, the technique of using surface microscopy to examine the skin, may be helpful in distinguishing nail lesions. (See a video on how to perform dermoscopy here: http://bit.ly/2pyJ3xN.)
Nonmelanocytic lesions tend to have homogeneously distributed pigment, while melanocytic lesions contain granules of pigment in cellular inclusions. Any suspicion of melanoma warrants a punch biopsy.11
Medication-induced effects. Minocycline may cause bluish nail discoloration similar to that produced by infection with P aeruginosa, but it is rare for only a single nail to be involved. In addition, pigmentation changes are often present elsewhere on the body, including the sclerae, teeth, and pinna.
Another medication known to color the nails blue is colloidal silver, which is still sold as a dietary supplement or homeopathic remedy to treat a wide range of ailments.6 (Of note: In 1999, the Food and Drug Administration issued a final rule saying that colloidal silver isn’t safe or effective for treating any disease or condition.12)
Glomus tumor. Another cause of blue nails is glomus tumors, relatively uncommon perivascular neoplasms that are typically found in the subungual region. These tumors are generally accompanied by localized tenderness, cold sensitivity, and paroxysms of excruciating pain that are disproportional to the size of the tumor.
Imaging studies may aid in the diagnosis, in addition to pathologic confirmation. Magnetic resonance imaging is the most sensitive imaging modality; if a glomus tumor is present, it most often appears as a well-circumscribed T2 hyperintense lesion.13
Exogenous pigmentation. Nails may become discolored due to exposure to various toxins or chemicals. Frequent culprits include eosin, methylene blue, henna, hair dye, and tobacco.9
Antibiotics and measures to keep the nail dry will help resolve infection
When chronic nail wetness is a contributing factor, treatment begins with measures to keep the nails dry. In addition, either topical or systemic antibiotics may be used to eradicate the infection. Topical applications with agents such as nadifloxacin have been shown to be effective in several case reports,3 but large-scale controlled trials are lacking. Fluoroquinolones are regarded as first-line systemic treatment.5 Briefly soaking the nail in a diluted sodium hypochlorite (bleach) solution also helps to suppress bacterial growth. Nail extraction may be required in refractory cases.
For our patient, we prescribed ciprofloxacin 500 mg twice a day for 10 days, plus bleach soaks (one part bleach to 4 parts water) twice a day. We recommended that our patient wear gloves for household tasks that involved immersing her hands in water, and drying her finger with a hair dryer after bathing.
CORRESPONDENCE
David Gish, MD, University of Virginia Health System, 1215 Lee St. Charlottesville, VA 22908; [email protected].
1. Greene SL, Su WP, Muller SA. Pseudomonas aeruginosa infections of the skin. Am Fam Physician. 1984;29:193-200.
2. de Almeida HL Jr, Duquia RP, de Castro LA, et al. Scanning electron microscopy of the green nail. Int J Dermatol. 2010;49:962-963.
3. Hengge UR, Bardeli V. Images in clinical medicine. Green nails. N Engl J Med. 2009;360:1125.
4. Chiriac A, Brzezinski P, Foia L, et al. Chloronychia: green nail syndrome caused by Pseudomonas aeruginosa in elderly persons. Clin Interv Aging. 2015;10:265-267.
5. Müller S, Ebnöther M, Itin P. Green nail syndrome (Pseudomonas aeruginosa nail infection): Two cases successfully treated with topical nadifloxacin, an acne medication. Case Rep Dermatol. 2014;6:180-184.
6. Raam R, DeClerck B, Jhun P, et al. That’s some weird nail polish you got there! Ann Emerg Med. 2015;66:585-588.
7. Gregoriou S, Argyriou G, Larios G, et al. Nail disorders and systemic disease: what the nails tell us. J Fam Pract. 2008;57:509-514.
8. Fawcett RS, Linford S, Stulberg DL. Nail abnormalities: clues to systemic disease. Am Fam Physician. 2004;69:1417-1424.
9. Braun RP, Baran R, Le Gal FA, et al. Diagnosis and management of nail pigmentations. J Am Acad Dermatol. 2007;56:835-847.
10. Buka R, Friedman KA, Phelps RG, et al. Childhood longitudinal melanonychia: case reports and review of the literature. Mt Sinai J Med. 2001;68:331-335.
11. Tully AS, Trayes KP, Studdiford JS. Evaluation of nail abnormalities. Am Fam Physician. 2012;85:779-787.
12. US Food and Drug Administration. Over-the-counter drug products containing colloidal silver ingredients or silver salts. 1999. Available at: https://www.fda.gov/ohrms/dockets/98fr/081799a.txt. Accessed April 11, 2017.
13. Glazebrook KN, Laundre BJ, Schiefer TK, et al. Imaging features of glomus tumors. Skeletal Radiol. 2011;40:855-862.
1. Greene SL, Su WP, Muller SA. Pseudomonas aeruginosa infections of the skin. Am Fam Physician. 1984;29:193-200.
2. de Almeida HL Jr, Duquia RP, de Castro LA, et al. Scanning electron microscopy of the green nail. Int J Dermatol. 2010;49:962-963.
3. Hengge UR, Bardeli V. Images in clinical medicine. Green nails. N Engl J Med. 2009;360:1125.
4. Chiriac A, Brzezinski P, Foia L, et al. Chloronychia: green nail syndrome caused by Pseudomonas aeruginosa in elderly persons. Clin Interv Aging. 2015;10:265-267.
5. Müller S, Ebnöther M, Itin P. Green nail syndrome (Pseudomonas aeruginosa nail infection): Two cases successfully treated with topical nadifloxacin, an acne medication. Case Rep Dermatol. 2014;6:180-184.
6. Raam R, DeClerck B, Jhun P, et al. That’s some weird nail polish you got there! Ann Emerg Med. 2015;66:585-588.
7. Gregoriou S, Argyriou G, Larios G, et al. Nail disorders and systemic disease: what the nails tell us. J Fam Pract. 2008;57:509-514.
8. Fawcett RS, Linford S, Stulberg DL. Nail abnormalities: clues to systemic disease. Am Fam Physician. 2004;69:1417-1424.
9. Braun RP, Baran R, Le Gal FA, et al. Diagnosis and management of nail pigmentations. J Am Acad Dermatol. 2007;56:835-847.
10. Buka R, Friedman KA, Phelps RG, et al. Childhood longitudinal melanonychia: case reports and review of the literature. Mt Sinai J Med. 2001;68:331-335.
11. Tully AS, Trayes KP, Studdiford JS. Evaluation of nail abnormalities. Am Fam Physician. 2012;85:779-787.
12. US Food and Drug Administration. Over-the-counter drug products containing colloidal silver ingredients or silver salts. 1999. Available at: https://www.fda.gov/ohrms/dockets/98fr/081799a.txt. Accessed April 11, 2017.
13. Glazebrook KN, Laundre BJ, Schiefer TK, et al. Imaging features of glomus tumors. Skeletal Radiol. 2011;40:855-862.
Fever, petechiae, and joint pain
A 59-year-old woman presented to our emergency department with a rash, severe acute pain in her left hip and lower back, and dyspnea on exertion. She denied having a headache and her mental status was at baseline. The woman reported exposure to rats and snakes one week prior to presentation, and mentioned getting bitten by a rat multiple times on the back of both of her hands while feeding it to her son’s pet snake. The patient had a history of a left hip replacement, with a revision and bone graft 5 years earlier.
The patient had a fever of 103° F during the physical examination. She had erythematous papules and central hemorrhagic eschars at the sites of the bites (FIGURE 1). She also had nonblanching petechiae on both of her lower legs (FIGURE 2) and on the dorsal and palmar aspects of her hands.
The patient’s lab work showed mild normocytic anemia with a hemoglobin level of 11.4 g/dL (normal, 12-16 g/dL) and a platelet count of 129,000/mcL (normal, 130,000-400,000/mcL). Her white blood cell count, chemistries, brain natriuretic peptide test, and chest x-ray were normal.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Rat bite fever
Based on the patient’s symptoms, history, and lab work, we concluded that this was a case of rat bite fever. RBF is a zoonotic systemic illness caused by infection from either the gram-negative bacillus Streptobacillus moniliformis, commonly found in the United States, or the gram-negative rod Spirillum minus, commonly seen in Asia. Anyone with exposure to rats is at risk for RBF, especially pet shop employees, lab workers, and people living in areas with rat infestations.1
The rash associated with RBF can be petechial, purpuric, or maculopapular, but the presence of hemorrhagic nodules and ulcers at the site of the bite is especially indicative of the illness. The rash often involves the hands and feet, including the palms and soles.
To make the diagnosis of RBF, a careful history and a high index of suspicion are important. Fever and rigor are often the first symptoms to appear, beginning 3 to 10 days after the bite. Three to 4 days after the onset of fever, up to 75% of patients will develop a rash.2 Joint and muscle aches are also common, as is a migrating pattern of arthritis.2,3
Rule out other infections related to animal exposure
The differential diagnosis for RBF includes other animal-related infections, such as those from snake bites, leptospirosis, rabies, and pasteurellosis.
Symptoms associated with snake bite injuries appear rapidly after the bite and vary with the type of snake toxin. Hemotoxic symptoms may include intense pain, edema, petechiae, and ecchymosis from coagulopathy. Neurotoxic symptoms may include ptosis, weakness, and paresthesias. All snake bites should be treated with supportive care, and antivenin is indicated when symptoms or history indicate a bite from a venomous snake. Venomous snakes are rarely intentionally kept as pets.2
Leptospirosis is a zoonotic bacterial infection that may be spread through the urine of rats, dogs, or other mammals. Symptoms may be mild and limited to conjunctivitis, vomiting, and fever; life-threatening symptoms include hemorrhage and kidney failure. A petechial rash is not typical.4 Beta-lactam antibiotics are the treatment of choice.
Rabies is a viral infection that occurs after exposure to infected animals (most commonly raccoons, bats, skunks, and foxes). Symptoms include fever and mental status changes that can lead to death; rash is not a typical symptom. Exposed patients should receive post-exposure prophylaxis with immune globulin or a rabies vaccine.5
Pasteurellosis may also cause hemorrhagic nodules at the site of the bite or scratch, but bites are typically caused by larger animals such as dogs and livestock. Other symptoms include fever, sepsis, and osteomyelitis. Treatment includes amoxicillin-clavulanate or a fluoroquinolone-clindamycin combination.6
In cases of high suspicion, special culture tubes may be needed
Blood cultures and cerebrospinal fluid cultures are often falsely negative. Special culture tubes without polyanethol sulfonate preservative, which inhibits the growth of S moniliformis, may be required in cases of high suspicion. S moniliformis polymerase chain reaction may be available in some specialized labs.7,8
Treatment options include 7 to 10 days of antibiotic therapy with oral penicillin 500 mg 4 times daily, amoxicillin-clavulanate 875/125 mg twice daily, or oral doxycycline 100 mg every 12 hours.9
RBF may be fatal if not treated.3 Complications may include bacteremia, septicemia, meningitis, and endocarditis.
Our patient received empiric intravenous ceftriaxone 1 g every 24 hours and her fever and joint pain resolved within 48 hours. On Day 3 she was discharged home to complete a 10-day course of oral amoxicillin-clavulanate 875/125 mg. Her primary care physician reported that the rash resolved and the patient made a full recovery.
CORRESPONDENCE
Kate Rowland, MD, MS, Rush-Copley Family Medicine Residency, 2020 Ogden Ave. Suite 325, Aurora, IL 60504; [email protected].
1. Centers for Disease Control and Prevention. Rat-bite fever (RBF). Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/rat-bite-fever/index.html. Accessed December 1, 2015.
2. Elliott SP. Rat bite fever and Streptobacillus moniliformis. Clin Microbiol Rev. 2007;20:13-22.
3. Juckett G, Hancox JG. Venomous snakebites in the United States: management review and update. Am Fam Physician. 2002;65:1367-1374.
4. Rabinowitz PM, Gordon Z, Odofin L. Pet-related infections. Am Fam Physician. 2007;76:1314-1322.
5. Fishbein DB, Robinson LE. Rabies. N Engl J Med. 1993;329:1632-1638.
6. Wilson BA, Ho M. Pasteurella multocida: from zoonosis to cellular microbiology. Clin Microbiol Rev. 2013;26:631-655.
7. Eng J. Effect of sodium polyanethol sulfonate in blood cultures. J Clin Microbiol. 1975;1:119-123.
8. Nakagomi D, Deguchi N, Yagasaki A, et al. Rat-bite fever identified by polymerase chain reaction detection of Streptobacillus moniliformis DNA. J Dermatol. 2008;35:667-670.
9. Bush LM, Perez MT. Rat-bite fever. In: The Merck Manual of Diagnosis and Therapy. Whitehouse Station, NJ: Merck Sharp & Dohme Corp.; 2011.
A 59-year-old woman presented to our emergency department with a rash, severe acute pain in her left hip and lower back, and dyspnea on exertion. She denied having a headache and her mental status was at baseline. The woman reported exposure to rats and snakes one week prior to presentation, and mentioned getting bitten by a rat multiple times on the back of both of her hands while feeding it to her son’s pet snake. The patient had a history of a left hip replacement, with a revision and bone graft 5 years earlier.
The patient had a fever of 103° F during the physical examination. She had erythematous papules and central hemorrhagic eschars at the sites of the bites (FIGURE 1). She also had nonblanching petechiae on both of her lower legs (FIGURE 2) and on the dorsal and palmar aspects of her hands.
The patient’s lab work showed mild normocytic anemia with a hemoglobin level of 11.4 g/dL (normal, 12-16 g/dL) and a platelet count of 129,000/mcL (normal, 130,000-400,000/mcL). Her white blood cell count, chemistries, brain natriuretic peptide test, and chest x-ray were normal.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Rat bite fever
Based on the patient’s symptoms, history, and lab work, we concluded that this was a case of rat bite fever. RBF is a zoonotic systemic illness caused by infection from either the gram-negative bacillus Streptobacillus moniliformis, commonly found in the United States, or the gram-negative rod Spirillum minus, commonly seen in Asia. Anyone with exposure to rats is at risk for RBF, especially pet shop employees, lab workers, and people living in areas with rat infestations.1
The rash associated with RBF can be petechial, purpuric, or maculopapular, but the presence of hemorrhagic nodules and ulcers at the site of the bite is especially indicative of the illness. The rash often involves the hands and feet, including the palms and soles.
To make the diagnosis of RBF, a careful history and a high index of suspicion are important. Fever and rigor are often the first symptoms to appear, beginning 3 to 10 days after the bite. Three to 4 days after the onset of fever, up to 75% of patients will develop a rash.2 Joint and muscle aches are also common, as is a migrating pattern of arthritis.2,3
Rule out other infections related to animal exposure
The differential diagnosis for RBF includes other animal-related infections, such as those from snake bites, leptospirosis, rabies, and pasteurellosis.
Symptoms associated with snake bite injuries appear rapidly after the bite and vary with the type of snake toxin. Hemotoxic symptoms may include intense pain, edema, petechiae, and ecchymosis from coagulopathy. Neurotoxic symptoms may include ptosis, weakness, and paresthesias. All snake bites should be treated with supportive care, and antivenin is indicated when symptoms or history indicate a bite from a venomous snake. Venomous snakes are rarely intentionally kept as pets.2
Leptospirosis is a zoonotic bacterial infection that may be spread through the urine of rats, dogs, or other mammals. Symptoms may be mild and limited to conjunctivitis, vomiting, and fever; life-threatening symptoms include hemorrhage and kidney failure. A petechial rash is not typical.4 Beta-lactam antibiotics are the treatment of choice.
Rabies is a viral infection that occurs after exposure to infected animals (most commonly raccoons, bats, skunks, and foxes). Symptoms include fever and mental status changes that can lead to death; rash is not a typical symptom. Exposed patients should receive post-exposure prophylaxis with immune globulin or a rabies vaccine.5
Pasteurellosis may also cause hemorrhagic nodules at the site of the bite or scratch, but bites are typically caused by larger animals such as dogs and livestock. Other symptoms include fever, sepsis, and osteomyelitis. Treatment includes amoxicillin-clavulanate or a fluoroquinolone-clindamycin combination.6
In cases of high suspicion, special culture tubes may be needed
Blood cultures and cerebrospinal fluid cultures are often falsely negative. Special culture tubes without polyanethol sulfonate preservative, which inhibits the growth of S moniliformis, may be required in cases of high suspicion. S moniliformis polymerase chain reaction may be available in some specialized labs.7,8
Treatment options include 7 to 10 days of antibiotic therapy with oral penicillin 500 mg 4 times daily, amoxicillin-clavulanate 875/125 mg twice daily, or oral doxycycline 100 mg every 12 hours.9
RBF may be fatal if not treated.3 Complications may include bacteremia, septicemia, meningitis, and endocarditis.
Our patient received empiric intravenous ceftriaxone 1 g every 24 hours and her fever and joint pain resolved within 48 hours. On Day 3 she was discharged home to complete a 10-day course of oral amoxicillin-clavulanate 875/125 mg. Her primary care physician reported that the rash resolved and the patient made a full recovery.
CORRESPONDENCE
Kate Rowland, MD, MS, Rush-Copley Family Medicine Residency, 2020 Ogden Ave. Suite 325, Aurora, IL 60504; [email protected].
A 59-year-old woman presented to our emergency department with a rash, severe acute pain in her left hip and lower back, and dyspnea on exertion. She denied having a headache and her mental status was at baseline. The woman reported exposure to rats and snakes one week prior to presentation, and mentioned getting bitten by a rat multiple times on the back of both of her hands while feeding it to her son’s pet snake. The patient had a history of a left hip replacement, with a revision and bone graft 5 years earlier.
The patient had a fever of 103° F during the physical examination. She had erythematous papules and central hemorrhagic eschars at the sites of the bites (FIGURE 1). She also had nonblanching petechiae on both of her lower legs (FIGURE 2) and on the dorsal and palmar aspects of her hands.
The patient’s lab work showed mild normocytic anemia with a hemoglobin level of 11.4 g/dL (normal, 12-16 g/dL) and a platelet count of 129,000/mcL (normal, 130,000-400,000/mcL). Her white blood cell count, chemistries, brain natriuretic peptide test, and chest x-ray were normal.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Rat bite fever
Based on the patient’s symptoms, history, and lab work, we concluded that this was a case of rat bite fever. RBF is a zoonotic systemic illness caused by infection from either the gram-negative bacillus Streptobacillus moniliformis, commonly found in the United States, or the gram-negative rod Spirillum minus, commonly seen in Asia. Anyone with exposure to rats is at risk for RBF, especially pet shop employees, lab workers, and people living in areas with rat infestations.1
The rash associated with RBF can be petechial, purpuric, or maculopapular, but the presence of hemorrhagic nodules and ulcers at the site of the bite is especially indicative of the illness. The rash often involves the hands and feet, including the palms and soles.
To make the diagnosis of RBF, a careful history and a high index of suspicion are important. Fever and rigor are often the first symptoms to appear, beginning 3 to 10 days after the bite. Three to 4 days after the onset of fever, up to 75% of patients will develop a rash.2 Joint and muscle aches are also common, as is a migrating pattern of arthritis.2,3
Rule out other infections related to animal exposure
The differential diagnosis for RBF includes other animal-related infections, such as those from snake bites, leptospirosis, rabies, and pasteurellosis.
Symptoms associated with snake bite injuries appear rapidly after the bite and vary with the type of snake toxin. Hemotoxic symptoms may include intense pain, edema, petechiae, and ecchymosis from coagulopathy. Neurotoxic symptoms may include ptosis, weakness, and paresthesias. All snake bites should be treated with supportive care, and antivenin is indicated when symptoms or history indicate a bite from a venomous snake. Venomous snakes are rarely intentionally kept as pets.2
Leptospirosis is a zoonotic bacterial infection that may be spread through the urine of rats, dogs, or other mammals. Symptoms may be mild and limited to conjunctivitis, vomiting, and fever; life-threatening symptoms include hemorrhage and kidney failure. A petechial rash is not typical.4 Beta-lactam antibiotics are the treatment of choice.
Rabies is a viral infection that occurs after exposure to infected animals (most commonly raccoons, bats, skunks, and foxes). Symptoms include fever and mental status changes that can lead to death; rash is not a typical symptom. Exposed patients should receive post-exposure prophylaxis with immune globulin or a rabies vaccine.5
Pasteurellosis may also cause hemorrhagic nodules at the site of the bite or scratch, but bites are typically caused by larger animals such as dogs and livestock. Other symptoms include fever, sepsis, and osteomyelitis. Treatment includes amoxicillin-clavulanate or a fluoroquinolone-clindamycin combination.6
In cases of high suspicion, special culture tubes may be needed
Blood cultures and cerebrospinal fluid cultures are often falsely negative. Special culture tubes without polyanethol sulfonate preservative, which inhibits the growth of S moniliformis, may be required in cases of high suspicion. S moniliformis polymerase chain reaction may be available in some specialized labs.7,8
Treatment options include 7 to 10 days of antibiotic therapy with oral penicillin 500 mg 4 times daily, amoxicillin-clavulanate 875/125 mg twice daily, or oral doxycycline 100 mg every 12 hours.9
RBF may be fatal if not treated.3 Complications may include bacteremia, septicemia, meningitis, and endocarditis.
Our patient received empiric intravenous ceftriaxone 1 g every 24 hours and her fever and joint pain resolved within 48 hours. On Day 3 she was discharged home to complete a 10-day course of oral amoxicillin-clavulanate 875/125 mg. Her primary care physician reported that the rash resolved and the patient made a full recovery.
CORRESPONDENCE
Kate Rowland, MD, MS, Rush-Copley Family Medicine Residency, 2020 Ogden Ave. Suite 325, Aurora, IL 60504; [email protected].
1. Centers for Disease Control and Prevention. Rat-bite fever (RBF). Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/rat-bite-fever/index.html. Accessed December 1, 2015.
2. Elliott SP. Rat bite fever and Streptobacillus moniliformis. Clin Microbiol Rev. 2007;20:13-22.
3. Juckett G, Hancox JG. Venomous snakebites in the United States: management review and update. Am Fam Physician. 2002;65:1367-1374.
4. Rabinowitz PM, Gordon Z, Odofin L. Pet-related infections. Am Fam Physician. 2007;76:1314-1322.
5. Fishbein DB, Robinson LE. Rabies. N Engl J Med. 1993;329:1632-1638.
6. Wilson BA, Ho M. Pasteurella multocida: from zoonosis to cellular microbiology. Clin Microbiol Rev. 2013;26:631-655.
7. Eng J. Effect of sodium polyanethol sulfonate in blood cultures. J Clin Microbiol. 1975;1:119-123.
8. Nakagomi D, Deguchi N, Yagasaki A, et al. Rat-bite fever identified by polymerase chain reaction detection of Streptobacillus moniliformis DNA. J Dermatol. 2008;35:667-670.
9. Bush LM, Perez MT. Rat-bite fever. In: The Merck Manual of Diagnosis and Therapy. Whitehouse Station, NJ: Merck Sharp & Dohme Corp.; 2011.
1. Centers for Disease Control and Prevention. Rat-bite fever (RBF). Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/rat-bite-fever/index.html. Accessed December 1, 2015.
2. Elliott SP. Rat bite fever and Streptobacillus moniliformis. Clin Microbiol Rev. 2007;20:13-22.
3. Juckett G, Hancox JG. Venomous snakebites in the United States: management review and update. Am Fam Physician. 2002;65:1367-1374.
4. Rabinowitz PM, Gordon Z, Odofin L. Pet-related infections. Am Fam Physician. 2007;76:1314-1322.
5. Fishbein DB, Robinson LE. Rabies. N Engl J Med. 1993;329:1632-1638.
6. Wilson BA, Ho M. Pasteurella multocida: from zoonosis to cellular microbiology. Clin Microbiol Rev. 2013;26:631-655.
7. Eng J. Effect of sodium polyanethol sulfonate in blood cultures. J Clin Microbiol. 1975;1:119-123.
8. Nakagomi D, Deguchi N, Yagasaki A, et al. Rat-bite fever identified by polymerase chain reaction detection of Streptobacillus moniliformis DNA. J Dermatol. 2008;35:667-670.
9. Bush LM, Perez MT. Rat-bite fever. In: The Merck Manual of Diagnosis and Therapy. Whitehouse Station, NJ: Merck Sharp & Dohme Corp.; 2011.
Severe headache • neck pain • intermittent cough • Dx?
THE CASE
A 32-year-old Chinese woman sought care from our family medicine clinic because she had a headache, neck pain, and an intermittent cough that had produced white sputum for 7 days. She described the headache as severe and pressure-like, and said that it had progressively worsened over the previous 3 weeks, coinciding with her first trip outside of China to the United States. The patient indicated that she also had occasional vomiting, dizziness, a low-grade fever, chills, night sweats, and increasing fatigue.
Prior to this visit, the patient had gone to the emergency department (ED) twice in one week, but was told that she had a migraine headache and a viral syndrome and was sent home. She was also told to make a follow-up appointment at our family medicine outpatient clinic.
Besides the symptoms that brought her to our clinic, the only other notable element of the patient’s history was a “neck mass” resection in China 8 years earlier. (The diagnosis of the neck mass was unknown.)
Concerned about her presenting signs and symptoms, we sent the patient to the ED, where she was admitted for further evaluation and treatment of possible meningitis. In the ED, she had a temperature of 101.5° F; her other vital signs were normal. A physical exam revealed mild neck stiffness.
THE DIAGNOSIS
A chest computed tomography (CT) scan demonstrated extensive confluent nodular infiltrates in the lung apices bilaterally with the largest confluent nodule measuring 6 cm (FIGURE 1). A chest x-ray demonstrated extensive bilateral pulmonary interstitial infiltrates that were most pronounced in the upper lung fields (FIGURE 2).
Lumbar puncture results revealed lymphocytic pleocytosis with elevated protein and low glucose levels (TABLE). Based on these results, the family medicine team suspected that our patient had tuberculous meningitis (TBM).
The team consulted with Infectious Diseases for management of TBM, and they placed our patient in a negative pressure room on airborne isolation. In addition, she was started on rifampin 450 mg/d, pyrazinamide 1000 mg/d, ethambutol 800 mg/d, and isoniazid (INH) 800 mg/d, as well as pyridoxine and intravenous dexamethasone.
DISCUSSION
TBM accounts for approximately 1% of all cases of TB and 5% of extrapulmonary diseases in immunocompetent individuals.1 In 2015, there were approximately 10.4 million cases of TB worldwide, and 6 countries accounted for 60% of the global total: India, Indonesia, China, Nigeria, Pakistan, and South Africa.2 TBM is typically a subacute disease with symptoms that can persist for weeks before diagnosis.3 An early diagnosis is critical, as the mortality rate remains relatively high (as high as nearly 70% in underdeveloped and developed countries) despite effective treatment regimens.3 (For updated recommendations on TB screening, see this month’s Practice Alert.)
Most health care facilities use AFB smears to determine when patients with suspected TB should be isolated. However, AFB smears are positive in only 60% of TB cases.4 One study indicated that nucleic acid amplification by PCR can improve sensitivity from 60% to 87% and specificity from 98% to 100%.5
The presentation of TBM varies by phase of disease:
- The prodromal phase typically lasts for 2 to 3 weeks. It is characterized by an insidious onset of malaise, headache, low-grade fever, irritability, and personality changes.
- The meningitis phase is characterized by pronounced neurologic features such as meningismus, protracted headache, confusion, myelopathy, and sensory deficits, as well as vomiting, lethargy, and urinary retention.
- During the paralytic phase, patients experience profound confusion, followed by stupor, coma, seizures, progressive paraplegia, and often, hemiparesis.1,3,6
Treatment should be given for a total of 9 to 12 months
Initiate treatment for TB based on a strong clinical suspicion for the disease. Treatment of TBM consists of an intensive phase with 4 anti-TB drugs for 2 months (typically INH 800 mg/d, rifampin 450 mg/d, pyrazinamide 1000 mg/d, and ethambutol 800 mg/d) and a continuation phase with 2 drugs (INH and rifampin) for 7 to 10 additional months, resulting in a total treatment duration of 9 to 12 months.
Our patient was discharged from the hospital after 2 weeks on an anti-TB medication regimen of INH, rifampin, and pyrazinamide, along with pyridoxine and a tapering dose of dexamethasone. After the initial 2 months of intensive phase therapy, she was switched to INH 300 mg/d and rifampin 450 mg/d for the continuation phase. The patient followed up at our family medicine outpatient clinic with slow improvement of her muscle weakness before returning to China once she was placed on the continuation phase drugs.
THE TAKEAWAY
Suspect TB in high-risk patients traveling from endemic areas. Our patient, a Chinese woman visiting Brooklyn, New York, should’ve been considered high risk for TB even without her travel history from China because Brooklyn has a high rate of TB, as well. (In 2015, Sunset Park, Brooklyn had 18.2 cases of TB per 100,000 people, which was more than double the citywide rate.7)
TBM is a subacute disease with an often subtle presentation. Once you suspect TBM, isolate the patient, obtain appropriate cultures and smears, and start anti-TB drugs and adjunctive corticosteroids immediately, while the results of studies for AFB are still pending. Prompt diagnosis and treatment can save a patient’s life.
1. Garcia-Monco JC. Central nervous system tuberculosis. Neurol Clin. 1999;17:737-759.
2. World Health Organization. Global tuberculosis report, 2016. Available at: http://apps.who.int/iris/bitstream/10665/250441/1/9789241565394-eng.pdf?ua=1. Accessed March 29, 2017.
3. Marx GE, Chan ED. Tuberculous meningitis: diagnosis and treatment overview. Tuberc Res Treat. 2011;2011:798764.
4. Siddiqui AH, Perl TM, Conlon M, et al. Preventing nosocomial transmission of pulmonary tuberculosis: when may isolation be discontinued for patients with suspected tuberculosis? Infect Control Hosp Epidemiol. 2002;23:141-144.
5. Tang YW, Meng S, Li H, et al. PCR enhances acid-fast bacillus stain-based rapid detection of Mycobacterium tuberculosis. J Clin Microbiol. 2004;42:1849-1850.
6. Long R, Gardam M. Tumour necrosis factor-alpha inhibitors and the reactivation of latent tuberculosis infection. CMAJ. 2003;168:1153-1156.
7. New York City Department of Health and Mental Hygiene. Tuberculosis in New York City, 2015. New York City Bureau of Tuberculosis Control Annual Summary. Available at: http://www1.nyc.gov/assets/doh/downloads/pdf/tb/tb2015.pdf. Accessed April 7, 2017.
THE CASE
A 32-year-old Chinese woman sought care from our family medicine clinic because she had a headache, neck pain, and an intermittent cough that had produced white sputum for 7 days. She described the headache as severe and pressure-like, and said that it had progressively worsened over the previous 3 weeks, coinciding with her first trip outside of China to the United States. The patient indicated that she also had occasional vomiting, dizziness, a low-grade fever, chills, night sweats, and increasing fatigue.
Prior to this visit, the patient had gone to the emergency department (ED) twice in one week, but was told that she had a migraine headache and a viral syndrome and was sent home. She was also told to make a follow-up appointment at our family medicine outpatient clinic.
Besides the symptoms that brought her to our clinic, the only other notable element of the patient’s history was a “neck mass” resection in China 8 years earlier. (The diagnosis of the neck mass was unknown.)
Concerned about her presenting signs and symptoms, we sent the patient to the ED, where she was admitted for further evaluation and treatment of possible meningitis. In the ED, she had a temperature of 101.5° F; her other vital signs were normal. A physical exam revealed mild neck stiffness.
THE DIAGNOSIS
A chest computed tomography (CT) scan demonstrated extensive confluent nodular infiltrates in the lung apices bilaterally with the largest confluent nodule measuring 6 cm (FIGURE 1). A chest x-ray demonstrated extensive bilateral pulmonary interstitial infiltrates that were most pronounced in the upper lung fields (FIGURE 2).
Lumbar puncture results revealed lymphocytic pleocytosis with elevated protein and low glucose levels (TABLE). Based on these results, the family medicine team suspected that our patient had tuberculous meningitis (TBM).
The team consulted with Infectious Diseases for management of TBM, and they placed our patient in a negative pressure room on airborne isolation. In addition, she was started on rifampin 450 mg/d, pyrazinamide 1000 mg/d, ethambutol 800 mg/d, and isoniazid (INH) 800 mg/d, as well as pyridoxine and intravenous dexamethasone.
DISCUSSION
TBM accounts for approximately 1% of all cases of TB and 5% of extrapulmonary diseases in immunocompetent individuals.1 In 2015, there were approximately 10.4 million cases of TB worldwide, and 6 countries accounted for 60% of the global total: India, Indonesia, China, Nigeria, Pakistan, and South Africa.2 TBM is typically a subacute disease with symptoms that can persist for weeks before diagnosis.3 An early diagnosis is critical, as the mortality rate remains relatively high (as high as nearly 70% in underdeveloped and developed countries) despite effective treatment regimens.3 (For updated recommendations on TB screening, see this month’s Practice Alert.)
Most health care facilities use AFB smears to determine when patients with suspected TB should be isolated. However, AFB smears are positive in only 60% of TB cases.4 One study indicated that nucleic acid amplification by PCR can improve sensitivity from 60% to 87% and specificity from 98% to 100%.5
The presentation of TBM varies by phase of disease:
- The prodromal phase typically lasts for 2 to 3 weeks. It is characterized by an insidious onset of malaise, headache, low-grade fever, irritability, and personality changes.
- The meningitis phase is characterized by pronounced neurologic features such as meningismus, protracted headache, confusion, myelopathy, and sensory deficits, as well as vomiting, lethargy, and urinary retention.
- During the paralytic phase, patients experience profound confusion, followed by stupor, coma, seizures, progressive paraplegia, and often, hemiparesis.1,3,6
Treatment should be given for a total of 9 to 12 months
Initiate treatment for TB based on a strong clinical suspicion for the disease. Treatment of TBM consists of an intensive phase with 4 anti-TB drugs for 2 months (typically INH 800 mg/d, rifampin 450 mg/d, pyrazinamide 1000 mg/d, and ethambutol 800 mg/d) and a continuation phase with 2 drugs (INH and rifampin) for 7 to 10 additional months, resulting in a total treatment duration of 9 to 12 months.
Our patient was discharged from the hospital after 2 weeks on an anti-TB medication regimen of INH, rifampin, and pyrazinamide, along with pyridoxine and a tapering dose of dexamethasone. After the initial 2 months of intensive phase therapy, she was switched to INH 300 mg/d and rifampin 450 mg/d for the continuation phase. The patient followed up at our family medicine outpatient clinic with slow improvement of her muscle weakness before returning to China once she was placed on the continuation phase drugs.
THE TAKEAWAY
Suspect TB in high-risk patients traveling from endemic areas. Our patient, a Chinese woman visiting Brooklyn, New York, should’ve been considered high risk for TB even without her travel history from China because Brooklyn has a high rate of TB, as well. (In 2015, Sunset Park, Brooklyn had 18.2 cases of TB per 100,000 people, which was more than double the citywide rate.7)
TBM is a subacute disease with an often subtle presentation. Once you suspect TBM, isolate the patient, obtain appropriate cultures and smears, and start anti-TB drugs and adjunctive corticosteroids immediately, while the results of studies for AFB are still pending. Prompt diagnosis and treatment can save a patient’s life.
THE CASE
A 32-year-old Chinese woman sought care from our family medicine clinic because she had a headache, neck pain, and an intermittent cough that had produced white sputum for 7 days. She described the headache as severe and pressure-like, and said that it had progressively worsened over the previous 3 weeks, coinciding with her first trip outside of China to the United States. The patient indicated that she also had occasional vomiting, dizziness, a low-grade fever, chills, night sweats, and increasing fatigue.
Prior to this visit, the patient had gone to the emergency department (ED) twice in one week, but was told that she had a migraine headache and a viral syndrome and was sent home. She was also told to make a follow-up appointment at our family medicine outpatient clinic.
Besides the symptoms that brought her to our clinic, the only other notable element of the patient’s history was a “neck mass” resection in China 8 years earlier. (The diagnosis of the neck mass was unknown.)
Concerned about her presenting signs and symptoms, we sent the patient to the ED, where she was admitted for further evaluation and treatment of possible meningitis. In the ED, she had a temperature of 101.5° F; her other vital signs were normal. A physical exam revealed mild neck stiffness.
THE DIAGNOSIS
A chest computed tomography (CT) scan demonstrated extensive confluent nodular infiltrates in the lung apices bilaterally with the largest confluent nodule measuring 6 cm (FIGURE 1). A chest x-ray demonstrated extensive bilateral pulmonary interstitial infiltrates that were most pronounced in the upper lung fields (FIGURE 2).
Lumbar puncture results revealed lymphocytic pleocytosis with elevated protein and low glucose levels (TABLE). Based on these results, the family medicine team suspected that our patient had tuberculous meningitis (TBM).
The team consulted with Infectious Diseases for management of TBM, and they placed our patient in a negative pressure room on airborne isolation. In addition, she was started on rifampin 450 mg/d, pyrazinamide 1000 mg/d, ethambutol 800 mg/d, and isoniazid (INH) 800 mg/d, as well as pyridoxine and intravenous dexamethasone.
DISCUSSION
TBM accounts for approximately 1% of all cases of TB and 5% of extrapulmonary diseases in immunocompetent individuals.1 In 2015, there were approximately 10.4 million cases of TB worldwide, and 6 countries accounted for 60% of the global total: India, Indonesia, China, Nigeria, Pakistan, and South Africa.2 TBM is typically a subacute disease with symptoms that can persist for weeks before diagnosis.3 An early diagnosis is critical, as the mortality rate remains relatively high (as high as nearly 70% in underdeveloped and developed countries) despite effective treatment regimens.3 (For updated recommendations on TB screening, see this month’s Practice Alert.)
Most health care facilities use AFB smears to determine when patients with suspected TB should be isolated. However, AFB smears are positive in only 60% of TB cases.4 One study indicated that nucleic acid amplification by PCR can improve sensitivity from 60% to 87% and specificity from 98% to 100%.5
The presentation of TBM varies by phase of disease:
- The prodromal phase typically lasts for 2 to 3 weeks. It is characterized by an insidious onset of malaise, headache, low-grade fever, irritability, and personality changes.
- The meningitis phase is characterized by pronounced neurologic features such as meningismus, protracted headache, confusion, myelopathy, and sensory deficits, as well as vomiting, lethargy, and urinary retention.
- During the paralytic phase, patients experience profound confusion, followed by stupor, coma, seizures, progressive paraplegia, and often, hemiparesis.1,3,6
Treatment should be given for a total of 9 to 12 months
Initiate treatment for TB based on a strong clinical suspicion for the disease. Treatment of TBM consists of an intensive phase with 4 anti-TB drugs for 2 months (typically INH 800 mg/d, rifampin 450 mg/d, pyrazinamide 1000 mg/d, and ethambutol 800 mg/d) and a continuation phase with 2 drugs (INH and rifampin) for 7 to 10 additional months, resulting in a total treatment duration of 9 to 12 months.
Our patient was discharged from the hospital after 2 weeks on an anti-TB medication regimen of INH, rifampin, and pyrazinamide, along with pyridoxine and a tapering dose of dexamethasone. After the initial 2 months of intensive phase therapy, she was switched to INH 300 mg/d and rifampin 450 mg/d for the continuation phase. The patient followed up at our family medicine outpatient clinic with slow improvement of her muscle weakness before returning to China once she was placed on the continuation phase drugs.
THE TAKEAWAY
Suspect TB in high-risk patients traveling from endemic areas. Our patient, a Chinese woman visiting Brooklyn, New York, should’ve been considered high risk for TB even without her travel history from China because Brooklyn has a high rate of TB, as well. (In 2015, Sunset Park, Brooklyn had 18.2 cases of TB per 100,000 people, which was more than double the citywide rate.7)
TBM is a subacute disease with an often subtle presentation. Once you suspect TBM, isolate the patient, obtain appropriate cultures and smears, and start anti-TB drugs and adjunctive corticosteroids immediately, while the results of studies for AFB are still pending. Prompt diagnosis and treatment can save a patient’s life.
1. Garcia-Monco JC. Central nervous system tuberculosis. Neurol Clin. 1999;17:737-759.
2. World Health Organization. Global tuberculosis report, 2016. Available at: http://apps.who.int/iris/bitstream/10665/250441/1/9789241565394-eng.pdf?ua=1. Accessed March 29, 2017.
3. Marx GE, Chan ED. Tuberculous meningitis: diagnosis and treatment overview. Tuberc Res Treat. 2011;2011:798764.
4. Siddiqui AH, Perl TM, Conlon M, et al. Preventing nosocomial transmission of pulmonary tuberculosis: when may isolation be discontinued for patients with suspected tuberculosis? Infect Control Hosp Epidemiol. 2002;23:141-144.
5. Tang YW, Meng S, Li H, et al. PCR enhances acid-fast bacillus stain-based rapid detection of Mycobacterium tuberculosis. J Clin Microbiol. 2004;42:1849-1850.
6. Long R, Gardam M. Tumour necrosis factor-alpha inhibitors and the reactivation of latent tuberculosis infection. CMAJ. 2003;168:1153-1156.
7. New York City Department of Health and Mental Hygiene. Tuberculosis in New York City, 2015. New York City Bureau of Tuberculosis Control Annual Summary. Available at: http://www1.nyc.gov/assets/doh/downloads/pdf/tb/tb2015.pdf. Accessed April 7, 2017.
1. Garcia-Monco JC. Central nervous system tuberculosis. Neurol Clin. 1999;17:737-759.
2. World Health Organization. Global tuberculosis report, 2016. Available at: http://apps.who.int/iris/bitstream/10665/250441/1/9789241565394-eng.pdf?ua=1. Accessed March 29, 2017.
3. Marx GE, Chan ED. Tuberculous meningitis: diagnosis and treatment overview. Tuberc Res Treat. 2011;2011:798764.
4. Siddiqui AH, Perl TM, Conlon M, et al. Preventing nosocomial transmission of pulmonary tuberculosis: when may isolation be discontinued for patients with suspected tuberculosis? Infect Control Hosp Epidemiol. 2002;23:141-144.
5. Tang YW, Meng S, Li H, et al. PCR enhances acid-fast bacillus stain-based rapid detection of Mycobacterium tuberculosis. J Clin Microbiol. 2004;42:1849-1850.
6. Long R, Gardam M. Tumour necrosis factor-alpha inhibitors and the reactivation of latent tuberculosis infection. CMAJ. 2003;168:1153-1156.
7. New York City Department of Health and Mental Hygiene. Tuberculosis in New York City, 2015. New York City Bureau of Tuberculosis Control Annual Summary. Available at: http://www1.nyc.gov/assets/doh/downloads/pdf/tb/tb2015.pdf. Accessed April 7, 2017.
USPSTF recommendations: A 2017 roundup
Since the last Practice Alert update on the United States Preventive Services Task Force in May of 2016,1 the Task Force has released 19 recommendations on 13 topics that include: the use of aspirin and statins for the prevention of cardiovascular disease (CVD); support for breastfeeding; use of folic acid during pregnancy; and screening for syphilis, latent tuberculosis (TB), herpes, chronic obstructive pulmonary disease (COPD), colorectal cancer (CRC), obstructive sleep apnea (OSA), celiac disease, and skin cancer. The Task Force also released a draft recommendation regarding prostate cancer screening in asymptomatic men (see “A change for prostate cancer screening?”) and addressed screening pelvic examinations in asymptomatic women, the subject of this month’s audiocast. (To listen, go to: http://bit.ly/2nIVoD5.)
Recommendations to implement
Recommendations from the past year that family physicians should put into practice are detailed below and in TABLE 1.2-8
CRC: Screen all individuals ages 50 to 75, but 76 to 85 selectively. The Task Force reaffirmed its 2008 finding that screening for CRC in adults ages 50 to 75 years is substantially beneficial.2 In contrast to the previous recommendation, however, the new one does not state which screening tests are preferred. The tests considered were 3 stool tests (fecal immunochemical test [FIT], FIT-tumor DNA testing [FIT-DNA], and guaiac-based fecal occult blood test [gFOBT]), as well as 3 direct visualization tests (colonoscopy, sigmoidoscopy, and CT colonoscopy). The Task Force assessed various testing frequencies of each test and some test combinations. While the Task Force does not recommend any one screening strategy, there are still significant unknowns about FIT-DNA and CT colonoscopy. The American Academy of Family Physicians does not recommend using these 2 tests for screening purposes at this time.9
CRC screening for adults ages 76 to 85 was given a “C” recommendation, which means the value of the service to the population overall is small, but that certain individuals may benefit from it. The Task Force advises selectively offering a “C” service to individuals based on professional judgment and patient preferences. Regarding CRC screening in individuals 76 years or older, the ones most likely to benefit are those who have never been screened and those without significant comorbidities that could limit life expectancy. All “C” recommendations from the past year are listed in TABLE 2.2-4
CVD prevention: When aspirin or a statin is indicated. The Task Force released 2 recommendations for the prevention of CVD this past year. One pertained to the use of low-dose aspirin3 (which also helps to prevent CRC), and the other addressed the use of low- to moderate-dose statins.4 Each recommendation is fairly complicated and nuanced in terms of age and risk for CVD. A decision to use low-dose aspirin must also consider the risk of bleeding.
To calculate a patient’s risk for CVD, the Task Force recommends using the risk calculator developed by the American College of Cardiology and the American Heart Association (http://www.cvriskcalculator.com/).
Adults for whom low-dose aspirin and low- to moderate-dose statins are recommended are described in TABLE 1.2-8 Patients for whom individual decision making is advised, rather than a generalized recommendation, are reviewed in TABLE 2.2-4 There is insufficient evidence to make a recommendation for the use of aspirin before age 50 or at age 70 and older,3 and for the use of statins in adults age 76 and older who do not have a history of CVD4 (TABLE 33,4,10-14). The use of low-dose aspirin and low-to-moderate dose statins have been the subject of JFP audiocasts in May 2016 and January 2017. (See http://bit.ly/2oiun8d and http://bit.ly/2oqkohR.)
2 pregnancy-related recommendations. To prevent neural tube defects in newborns, the Task Force now recommends daily folic acid, 0.4 to 0.8 mg (400 to 800 mcg), for all women who are planning on or are capable of becoming pregnant.5 This is an update of a 2009 recommendation that was worded slightly differently, recommending the supplement for all women of childbearing age.
A new recommendation on breastfeeding recognizes its benefits for both mother and baby and finds that interventions to encourage breastfeeding increase the prevalence of this practice and its duration.6 Interventions—provided to individuals or groups by professionals or peers or through formal education—include promoting the benefits of breastfeeding, giving practical advice and direct support on how to breastfeed, and offering psychological support.
Latent TB: Advantages of newer testing method. The recommendation on screening for latent tuberculosis (TB) is an update from the one made in 1996.7 At that time, screening for latent infection was performed using a tuberculin skin test (TST). Now a TST or interferon-gamma release assay (IGRA) can be used. Testing with IGRA may be the best option for those who have received a bacille Calmette–Guérin vaccination (because it can cause a false-positive TST) or for those who are not likely to return to have their TST read.
Those at high risk for latent TB include people who were born or have resided in countries with a high TB prevalence, those who have lived in a correctional institution or homeless shelter, and anyone in a high-risk group based on local epidemiology of the disease. (Read more on TB in this month’s Case Report.) Others at high risk are those who are immune suppressed because of infection or medications, and those who work in health care or correctional facilities. Screening of these groups is usually conducted as part of occupational health or is considered part of routine health care.
Syphilis: Screen high-risk individuals in 2 steps. The recommendation on syphilis screening basically reaffirms the one from 2004.8 Those at high risk for syphilis include men who have sex with men (who now account for 90% of new cases), those who are HIV positive, and those who engage in commercial sex work. Other racial and demographic groups can be at high risk depending on the local epidemiology of the disease. In a separate recommendation, the Task Force advises screening all pregnant women for syphilis.
Screening for syphilis infection involves 2 steps: first, a nontreponemal test (Venereal Disease Research Laboratory [VDRL] or rapid plasma reagin [RPR] test); second, a confirmatory treponemal antibody detection test (fluorescent treponemal antibody absorption [FTA-ABS] or Treponema pallidum particle agglutination [TPPA] test). Treatment for syphilis remains benzathine penicillin with the number of injections depending on the stage of infection. The Centers for Disease Control and Prevention is the best source for current recommendations for treatment of all sexually transmitted infections.15
Screening tests to avoid
TABLE 416,17 lists screening tests the Task Force recommends against. While chronic obstructive pulmonary disease afflicts 14% of US adults ages 40 to 79 years and is the third leading cause of death in the country, the Task Force found that early detection in asymptomatic adults does not affect the course of the illness and is of no benefit.16
Genital herpes, also prevalent, infects an estimated one out of 6 individuals, ages 14 to 49. It causes little mortality, except in neonates, but those infected can have recurrent flares and suffer psychological harms from stigmatization. Most genital herpes is caused by herpes simplex virus-2, and there is a serological test to detect it. However, the Task Force recommends against using the test to screen asymptomatic adults and adolescents, including those who are pregnant. This recommendation is based on the test’s high false-positive rate, which can cause emotional harm, and on the lack of evidence that detection through screening improves outcomes.17
The evidence is lacking for these practices
The Task Force is one of only a few organizations that will not make a recommendation if evidence is lacking on benefits and harms. In addition to the ‘I’ statements regarding CVD and CRC mentioned earlier, the Task Force found insufficient evidence to recommend screening for lipid disorders in individuals ages 20 years or younger,10 performing a visual skin exam as a screening tool for skin cancer,11 screening for celiac disease,12 performing a periodic pelvic examination in asymptomatic women,13 and screening for obstructive sleep apnea using screening questionnaires14 (TABLE 33,4,10-14).
SIDEBAR
A change for prostate cancer screening?The USPSTF recently issued new draft recommendations regarding prostate cancer screening in asymptomatic men (available at: https://screeningforprostatecancer.org/).
The draft now divides men into 2 age groups, stating that the decision to screen for prostate cancer using a prostate specific antigen (PSA) test should be individualized for men ages 55 to 69 years (a C recommendation, meaning that there is at least moderate certainty that the net benefit is small), and that men ages 70 and older (lowered from age 75 in the previous 2012 recommendation1) should not be screened (a D recommendation, meaning that there is moderate or high certainty that the service has no net benefit or that the harms outweigh the benefits).
The USPSTF believes that clinicians should explain to men ages 55 to 69 years that screening offers a small potential benefit of reducing the chance of dying from prostate cancer, but also comes with potential harms, including false-positive results requiring additional testing/procedures, overdiagnosis and overtreatment, and treatment complications such as incontinence and impotence. In this way, each man has the chance to incorporate his values and preferences into the decision.
For men ages 70 and older, the potential benefits simply do not outweigh the potential harms, according to the USPSTF.
1. USPSTF. Final recommendation statement. Prostate cancer: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/prostate-cancer-screening#Pod1. Accessed April 11, 2017.
1. Campos-Outcalt D. Eight USPSTF recommendations FPs need to know about. J Fam Pract. 2016;65:338-341.
2. USPSTF. Colorectal cancer: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/colorectal-cancer-screening2. Accessed March 22, 2017.
3. USPSTF. Aspirin use to prevent cardiovascular disease and colorectal cancer: preventive medications. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/aspirin-to-prevent-cardiovascular-disease-and-cancer. Accessed March 22, 2017.
4. USPSTF. Statin use for the prevention of cardiovascular disease in adults: preventive medication. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/statin-use-in-adults-preventive-medication1. Accessed March 22, 2017.
5. USPSTF. Folic acid for the prevention of neural tube defects: preventive medication. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/folic-acid-for-the-prevention-of-neural-tube-defects-preventive-medication. Accessed March 22, 2017.
6. USPSTF. Breastfeeding: primary care interventions. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/breastfeeding-primary-care-interventions. Accessed March 22, 2017.
7. USPSTF. Latent tuberculosis infection: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/latent-tuberculosis-infection-screening. Accessed March 22, 2017.
8. USPSTF. Syphilis infection in nonpregnant adults and adolescents: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/syphilis-infection-in-nonpregnant-adults-and-adolescents. Accessed March 22, 2017.
9. AAFP. Colorectal cancer screening, adults. Available at: http://www.aafp.org/patient-care/clinical-recommendations/all/colorectal-cancer.html. Accessed March 22, 2017.
10. USPSTF. Lipid disorders in children and adolescents: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/lipid-disorders-in-children-screening1. Accessed March 22, 2017.
11. USPSTF. Skin cancer: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/skin-cancer-screening2. Accessed March 22, 2017.
12. USPSTF. Celiac disease: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/celiac-disease-screening. Accessed March 22, 2017.
13. USPSTF. Gynecological conditions: periodic screening with the pelvic examination. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/gynecological-conditions-screening-with-the-pelvic-examination. Accessed March 22, 2017.
14. USPSTF. Obstructive sleep apnea in adults: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/obstructive-sleep-apnea-in-adults-screening. Accessed March 22, 2017.
15. CDC. 2015 sexually transmitted diseases treatment guidelines. Available at: https://www.cdc.gov/std/tg2015/default.htm. Accessed March 22, 2017.
16. USPSTF. Chronic obstructive pulmonary disease: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/chronic-obstructive-pulmonary-disease-screening. Accessed March 22, 2017.
17. USPSTF. Genital herpes infection: serologic screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/genital-herpes-screening1. Accessed March 22, 2017.
Since the last Practice Alert update on the United States Preventive Services Task Force in May of 2016,1 the Task Force has released 19 recommendations on 13 topics that include: the use of aspirin and statins for the prevention of cardiovascular disease (CVD); support for breastfeeding; use of folic acid during pregnancy; and screening for syphilis, latent tuberculosis (TB), herpes, chronic obstructive pulmonary disease (COPD), colorectal cancer (CRC), obstructive sleep apnea (OSA), celiac disease, and skin cancer. The Task Force also released a draft recommendation regarding prostate cancer screening in asymptomatic men (see “A change for prostate cancer screening?”) and addressed screening pelvic examinations in asymptomatic women, the subject of this month’s audiocast. (To listen, go to: http://bit.ly/2nIVoD5.)
Recommendations to implement
Recommendations from the past year that family physicians should put into practice are detailed below and in TABLE 1.2-8
CRC: Screen all individuals ages 50 to 75, but 76 to 85 selectively. The Task Force reaffirmed its 2008 finding that screening for CRC in adults ages 50 to 75 years is substantially beneficial.2 In contrast to the previous recommendation, however, the new one does not state which screening tests are preferred. The tests considered were 3 stool tests (fecal immunochemical test [FIT], FIT-tumor DNA testing [FIT-DNA], and guaiac-based fecal occult blood test [gFOBT]), as well as 3 direct visualization tests (colonoscopy, sigmoidoscopy, and CT colonoscopy). The Task Force assessed various testing frequencies of each test and some test combinations. While the Task Force does not recommend any one screening strategy, there are still significant unknowns about FIT-DNA and CT colonoscopy. The American Academy of Family Physicians does not recommend using these 2 tests for screening purposes at this time.9
CRC screening for adults ages 76 to 85 was given a “C” recommendation, which means the value of the service to the population overall is small, but that certain individuals may benefit from it. The Task Force advises selectively offering a “C” service to individuals based on professional judgment and patient preferences. Regarding CRC screening in individuals 76 years or older, the ones most likely to benefit are those who have never been screened and those without significant comorbidities that could limit life expectancy. All “C” recommendations from the past year are listed in TABLE 2.2-4
CVD prevention: When aspirin or a statin is indicated. The Task Force released 2 recommendations for the prevention of CVD this past year. One pertained to the use of low-dose aspirin3 (which also helps to prevent CRC), and the other addressed the use of low- to moderate-dose statins.4 Each recommendation is fairly complicated and nuanced in terms of age and risk for CVD. A decision to use low-dose aspirin must also consider the risk of bleeding.
To calculate a patient’s risk for CVD, the Task Force recommends using the risk calculator developed by the American College of Cardiology and the American Heart Association (http://www.cvriskcalculator.com/).
Adults for whom low-dose aspirin and low- to moderate-dose statins are recommended are described in TABLE 1.2-8 Patients for whom individual decision making is advised, rather than a generalized recommendation, are reviewed in TABLE 2.2-4 There is insufficient evidence to make a recommendation for the use of aspirin before age 50 or at age 70 and older,3 and for the use of statins in adults age 76 and older who do not have a history of CVD4 (TABLE 33,4,10-14). The use of low-dose aspirin and low-to-moderate dose statins have been the subject of JFP audiocasts in May 2016 and January 2017. (See http://bit.ly/2oiun8d and http://bit.ly/2oqkohR.)
2 pregnancy-related recommendations. To prevent neural tube defects in newborns, the Task Force now recommends daily folic acid, 0.4 to 0.8 mg (400 to 800 mcg), for all women who are planning on or are capable of becoming pregnant.5 This is an update of a 2009 recommendation that was worded slightly differently, recommending the supplement for all women of childbearing age.
A new recommendation on breastfeeding recognizes its benefits for both mother and baby and finds that interventions to encourage breastfeeding increase the prevalence of this practice and its duration.6 Interventions—provided to individuals or groups by professionals or peers or through formal education—include promoting the benefits of breastfeeding, giving practical advice and direct support on how to breastfeed, and offering psychological support.
Latent TB: Advantages of newer testing method. The recommendation on screening for latent tuberculosis (TB) is an update from the one made in 1996.7 At that time, screening for latent infection was performed using a tuberculin skin test (TST). Now a TST or interferon-gamma release assay (IGRA) can be used. Testing with IGRA may be the best option for those who have received a bacille Calmette–Guérin vaccination (because it can cause a false-positive TST) or for those who are not likely to return to have their TST read.
Those at high risk for latent TB include people who were born or have resided in countries with a high TB prevalence, those who have lived in a correctional institution or homeless shelter, and anyone in a high-risk group based on local epidemiology of the disease. (Read more on TB in this month’s Case Report.) Others at high risk are those who are immune suppressed because of infection or medications, and those who work in health care or correctional facilities. Screening of these groups is usually conducted as part of occupational health or is considered part of routine health care.
Syphilis: Screen high-risk individuals in 2 steps. The recommendation on syphilis screening basically reaffirms the one from 2004.8 Those at high risk for syphilis include men who have sex with men (who now account for 90% of new cases), those who are HIV positive, and those who engage in commercial sex work. Other racial and demographic groups can be at high risk depending on the local epidemiology of the disease. In a separate recommendation, the Task Force advises screening all pregnant women for syphilis.
Screening for syphilis infection involves 2 steps: first, a nontreponemal test (Venereal Disease Research Laboratory [VDRL] or rapid plasma reagin [RPR] test); second, a confirmatory treponemal antibody detection test (fluorescent treponemal antibody absorption [FTA-ABS] or Treponema pallidum particle agglutination [TPPA] test). Treatment for syphilis remains benzathine penicillin with the number of injections depending on the stage of infection. The Centers for Disease Control and Prevention is the best source for current recommendations for treatment of all sexually transmitted infections.15
Screening tests to avoid
TABLE 416,17 lists screening tests the Task Force recommends against. While chronic obstructive pulmonary disease afflicts 14% of US adults ages 40 to 79 years and is the third leading cause of death in the country, the Task Force found that early detection in asymptomatic adults does not affect the course of the illness and is of no benefit.16
Genital herpes, also prevalent, infects an estimated one out of 6 individuals, ages 14 to 49. It causes little mortality, except in neonates, but those infected can have recurrent flares and suffer psychological harms from stigmatization. Most genital herpes is caused by herpes simplex virus-2, and there is a serological test to detect it. However, the Task Force recommends against using the test to screen asymptomatic adults and adolescents, including those who are pregnant. This recommendation is based on the test’s high false-positive rate, which can cause emotional harm, and on the lack of evidence that detection through screening improves outcomes.17
The evidence is lacking for these practices
The Task Force is one of only a few organizations that will not make a recommendation if evidence is lacking on benefits and harms. In addition to the ‘I’ statements regarding CVD and CRC mentioned earlier, the Task Force found insufficient evidence to recommend screening for lipid disorders in individuals ages 20 years or younger,10 performing a visual skin exam as a screening tool for skin cancer,11 screening for celiac disease,12 performing a periodic pelvic examination in asymptomatic women,13 and screening for obstructive sleep apnea using screening questionnaires14 (TABLE 33,4,10-14).
SIDEBAR
A change for prostate cancer screening?The USPSTF recently issued new draft recommendations regarding prostate cancer screening in asymptomatic men (available at: https://screeningforprostatecancer.org/).
The draft now divides men into 2 age groups, stating that the decision to screen for prostate cancer using a prostate specific antigen (PSA) test should be individualized for men ages 55 to 69 years (a C recommendation, meaning that there is at least moderate certainty that the net benefit is small), and that men ages 70 and older (lowered from age 75 in the previous 2012 recommendation1) should not be screened (a D recommendation, meaning that there is moderate or high certainty that the service has no net benefit or that the harms outweigh the benefits).
The USPSTF believes that clinicians should explain to men ages 55 to 69 years that screening offers a small potential benefit of reducing the chance of dying from prostate cancer, but also comes with potential harms, including false-positive results requiring additional testing/procedures, overdiagnosis and overtreatment, and treatment complications such as incontinence and impotence. In this way, each man has the chance to incorporate his values and preferences into the decision.
For men ages 70 and older, the potential benefits simply do not outweigh the potential harms, according to the USPSTF.
1. USPSTF. Final recommendation statement. Prostate cancer: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/prostate-cancer-screening#Pod1. Accessed April 11, 2017.
Since the last Practice Alert update on the United States Preventive Services Task Force in May of 2016,1 the Task Force has released 19 recommendations on 13 topics that include: the use of aspirin and statins for the prevention of cardiovascular disease (CVD); support for breastfeeding; use of folic acid during pregnancy; and screening for syphilis, latent tuberculosis (TB), herpes, chronic obstructive pulmonary disease (COPD), colorectal cancer (CRC), obstructive sleep apnea (OSA), celiac disease, and skin cancer. The Task Force also released a draft recommendation regarding prostate cancer screening in asymptomatic men (see “A change for prostate cancer screening?”) and addressed screening pelvic examinations in asymptomatic women, the subject of this month’s audiocast. (To listen, go to: http://bit.ly/2nIVoD5.)
Recommendations to implement
Recommendations from the past year that family physicians should put into practice are detailed below and in TABLE 1.2-8
CRC: Screen all individuals ages 50 to 75, but 76 to 85 selectively. The Task Force reaffirmed its 2008 finding that screening for CRC in adults ages 50 to 75 years is substantially beneficial.2 In contrast to the previous recommendation, however, the new one does not state which screening tests are preferred. The tests considered were 3 stool tests (fecal immunochemical test [FIT], FIT-tumor DNA testing [FIT-DNA], and guaiac-based fecal occult blood test [gFOBT]), as well as 3 direct visualization tests (colonoscopy, sigmoidoscopy, and CT colonoscopy). The Task Force assessed various testing frequencies of each test and some test combinations. While the Task Force does not recommend any one screening strategy, there are still significant unknowns about FIT-DNA and CT colonoscopy. The American Academy of Family Physicians does not recommend using these 2 tests for screening purposes at this time.9
CRC screening for adults ages 76 to 85 was given a “C” recommendation, which means the value of the service to the population overall is small, but that certain individuals may benefit from it. The Task Force advises selectively offering a “C” service to individuals based on professional judgment and patient preferences. Regarding CRC screening in individuals 76 years or older, the ones most likely to benefit are those who have never been screened and those without significant comorbidities that could limit life expectancy. All “C” recommendations from the past year are listed in TABLE 2.2-4
CVD prevention: When aspirin or a statin is indicated. The Task Force released 2 recommendations for the prevention of CVD this past year. One pertained to the use of low-dose aspirin3 (which also helps to prevent CRC), and the other addressed the use of low- to moderate-dose statins.4 Each recommendation is fairly complicated and nuanced in terms of age and risk for CVD. A decision to use low-dose aspirin must also consider the risk of bleeding.
To calculate a patient’s risk for CVD, the Task Force recommends using the risk calculator developed by the American College of Cardiology and the American Heart Association (http://www.cvriskcalculator.com/).
Adults for whom low-dose aspirin and low- to moderate-dose statins are recommended are described in TABLE 1.2-8 Patients for whom individual decision making is advised, rather than a generalized recommendation, are reviewed in TABLE 2.2-4 There is insufficient evidence to make a recommendation for the use of aspirin before age 50 or at age 70 and older,3 and for the use of statins in adults age 76 and older who do not have a history of CVD4 (TABLE 33,4,10-14). The use of low-dose aspirin and low-to-moderate dose statins have been the subject of JFP audiocasts in May 2016 and January 2017. (See http://bit.ly/2oiun8d and http://bit.ly/2oqkohR.)
2 pregnancy-related recommendations. To prevent neural tube defects in newborns, the Task Force now recommends daily folic acid, 0.4 to 0.8 mg (400 to 800 mcg), for all women who are planning on or are capable of becoming pregnant.5 This is an update of a 2009 recommendation that was worded slightly differently, recommending the supplement for all women of childbearing age.
A new recommendation on breastfeeding recognizes its benefits for both mother and baby and finds that interventions to encourage breastfeeding increase the prevalence of this practice and its duration.6 Interventions—provided to individuals or groups by professionals or peers or through formal education—include promoting the benefits of breastfeeding, giving practical advice and direct support on how to breastfeed, and offering psychological support.
Latent TB: Advantages of newer testing method. The recommendation on screening for latent tuberculosis (TB) is an update from the one made in 1996.7 At that time, screening for latent infection was performed using a tuberculin skin test (TST). Now a TST or interferon-gamma release assay (IGRA) can be used. Testing with IGRA may be the best option for those who have received a bacille Calmette–Guérin vaccination (because it can cause a false-positive TST) or for those who are not likely to return to have their TST read.
Those at high risk for latent TB include people who were born or have resided in countries with a high TB prevalence, those who have lived in a correctional institution or homeless shelter, and anyone in a high-risk group based on local epidemiology of the disease. (Read more on TB in this month’s Case Report.) Others at high risk are those who are immune suppressed because of infection or medications, and those who work in health care or correctional facilities. Screening of these groups is usually conducted as part of occupational health or is considered part of routine health care.
Syphilis: Screen high-risk individuals in 2 steps. The recommendation on syphilis screening basically reaffirms the one from 2004.8 Those at high risk for syphilis include men who have sex with men (who now account for 90% of new cases), those who are HIV positive, and those who engage in commercial sex work. Other racial and demographic groups can be at high risk depending on the local epidemiology of the disease. In a separate recommendation, the Task Force advises screening all pregnant women for syphilis.
Screening for syphilis infection involves 2 steps: first, a nontreponemal test (Venereal Disease Research Laboratory [VDRL] or rapid plasma reagin [RPR] test); second, a confirmatory treponemal antibody detection test (fluorescent treponemal antibody absorption [FTA-ABS] or Treponema pallidum particle agglutination [TPPA] test). Treatment for syphilis remains benzathine penicillin with the number of injections depending on the stage of infection. The Centers for Disease Control and Prevention is the best source for current recommendations for treatment of all sexually transmitted infections.15
Screening tests to avoid
TABLE 416,17 lists screening tests the Task Force recommends against. While chronic obstructive pulmonary disease afflicts 14% of US adults ages 40 to 79 years and is the third leading cause of death in the country, the Task Force found that early detection in asymptomatic adults does not affect the course of the illness and is of no benefit.16
Genital herpes, also prevalent, infects an estimated one out of 6 individuals, ages 14 to 49. It causes little mortality, except in neonates, but those infected can have recurrent flares and suffer psychological harms from stigmatization. Most genital herpes is caused by herpes simplex virus-2, and there is a serological test to detect it. However, the Task Force recommends against using the test to screen asymptomatic adults and adolescents, including those who are pregnant. This recommendation is based on the test’s high false-positive rate, which can cause emotional harm, and on the lack of evidence that detection through screening improves outcomes.17
The evidence is lacking for these practices
The Task Force is one of only a few organizations that will not make a recommendation if evidence is lacking on benefits and harms. In addition to the ‘I’ statements regarding CVD and CRC mentioned earlier, the Task Force found insufficient evidence to recommend screening for lipid disorders in individuals ages 20 years or younger,10 performing a visual skin exam as a screening tool for skin cancer,11 screening for celiac disease,12 performing a periodic pelvic examination in asymptomatic women,13 and screening for obstructive sleep apnea using screening questionnaires14 (TABLE 33,4,10-14).
SIDEBAR
A change for prostate cancer screening?The USPSTF recently issued new draft recommendations regarding prostate cancer screening in asymptomatic men (available at: https://screeningforprostatecancer.org/).
The draft now divides men into 2 age groups, stating that the decision to screen for prostate cancer using a prostate specific antigen (PSA) test should be individualized for men ages 55 to 69 years (a C recommendation, meaning that there is at least moderate certainty that the net benefit is small), and that men ages 70 and older (lowered from age 75 in the previous 2012 recommendation1) should not be screened (a D recommendation, meaning that there is moderate or high certainty that the service has no net benefit or that the harms outweigh the benefits).
The USPSTF believes that clinicians should explain to men ages 55 to 69 years that screening offers a small potential benefit of reducing the chance of dying from prostate cancer, but also comes with potential harms, including false-positive results requiring additional testing/procedures, overdiagnosis and overtreatment, and treatment complications such as incontinence and impotence. In this way, each man has the chance to incorporate his values and preferences into the decision.
For men ages 70 and older, the potential benefits simply do not outweigh the potential harms, according to the USPSTF.
1. USPSTF. Final recommendation statement. Prostate cancer: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/prostate-cancer-screening#Pod1. Accessed April 11, 2017.
1. Campos-Outcalt D. Eight USPSTF recommendations FPs need to know about. J Fam Pract. 2016;65:338-341.
2. USPSTF. Colorectal cancer: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/colorectal-cancer-screening2. Accessed March 22, 2017.
3. USPSTF. Aspirin use to prevent cardiovascular disease and colorectal cancer: preventive medications. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/aspirin-to-prevent-cardiovascular-disease-and-cancer. Accessed March 22, 2017.
4. USPSTF. Statin use for the prevention of cardiovascular disease in adults: preventive medication. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/statin-use-in-adults-preventive-medication1. Accessed March 22, 2017.
5. USPSTF. Folic acid for the prevention of neural tube defects: preventive medication. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/folic-acid-for-the-prevention-of-neural-tube-defects-preventive-medication. Accessed March 22, 2017.
6. USPSTF. Breastfeeding: primary care interventions. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/breastfeeding-primary-care-interventions. Accessed March 22, 2017.
7. USPSTF. Latent tuberculosis infection: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/latent-tuberculosis-infection-screening. Accessed March 22, 2017.
8. USPSTF. Syphilis infection in nonpregnant adults and adolescents: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/syphilis-infection-in-nonpregnant-adults-and-adolescents. Accessed March 22, 2017.
9. AAFP. Colorectal cancer screening, adults. Available at: http://www.aafp.org/patient-care/clinical-recommendations/all/colorectal-cancer.html. Accessed March 22, 2017.
10. USPSTF. Lipid disorders in children and adolescents: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/lipid-disorders-in-children-screening1. Accessed March 22, 2017.
11. USPSTF. Skin cancer: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/skin-cancer-screening2. Accessed March 22, 2017.
12. USPSTF. Celiac disease: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/celiac-disease-screening. Accessed March 22, 2017.
13. USPSTF. Gynecological conditions: periodic screening with the pelvic examination. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/gynecological-conditions-screening-with-the-pelvic-examination. Accessed March 22, 2017.
14. USPSTF. Obstructive sleep apnea in adults: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/obstructive-sleep-apnea-in-adults-screening. Accessed March 22, 2017.
15. CDC. 2015 sexually transmitted diseases treatment guidelines. Available at: https://www.cdc.gov/std/tg2015/default.htm. Accessed March 22, 2017.
16. USPSTF. Chronic obstructive pulmonary disease: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/chronic-obstructive-pulmonary-disease-screening. Accessed March 22, 2017.
17. USPSTF. Genital herpes infection: serologic screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/genital-herpes-screening1. Accessed March 22, 2017.
1. Campos-Outcalt D. Eight USPSTF recommendations FPs need to know about. J Fam Pract. 2016;65:338-341.
2. USPSTF. Colorectal cancer: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/colorectal-cancer-screening2. Accessed March 22, 2017.
3. USPSTF. Aspirin use to prevent cardiovascular disease and colorectal cancer: preventive medications. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/aspirin-to-prevent-cardiovascular-disease-and-cancer. Accessed March 22, 2017.
4. USPSTF. Statin use for the prevention of cardiovascular disease in adults: preventive medication. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/statin-use-in-adults-preventive-medication1. Accessed March 22, 2017.
5. USPSTF. Folic acid for the prevention of neural tube defects: preventive medication. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/folic-acid-for-the-prevention-of-neural-tube-defects-preventive-medication. Accessed March 22, 2017.
6. USPSTF. Breastfeeding: primary care interventions. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/breastfeeding-primary-care-interventions. Accessed March 22, 2017.
7. USPSTF. Latent tuberculosis infection: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/latent-tuberculosis-infection-screening. Accessed March 22, 2017.
8. USPSTF. Syphilis infection in nonpregnant adults and adolescents: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/syphilis-infection-in-nonpregnant-adults-and-adolescents. Accessed March 22, 2017.
9. AAFP. Colorectal cancer screening, adults. Available at: http://www.aafp.org/patient-care/clinical-recommendations/all/colorectal-cancer.html. Accessed March 22, 2017.
10. USPSTF. Lipid disorders in children and adolescents: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/lipid-disorders-in-children-screening1. Accessed March 22, 2017.
11. USPSTF. Skin cancer: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/skin-cancer-screening2. Accessed March 22, 2017.
12. USPSTF. Celiac disease: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/celiac-disease-screening. Accessed March 22, 2017.
13. USPSTF. Gynecological conditions: periodic screening with the pelvic examination. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/gynecological-conditions-screening-with-the-pelvic-examination. Accessed March 22, 2017.
14. USPSTF. Obstructive sleep apnea in adults: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/obstructive-sleep-apnea-in-adults-screening. Accessed March 22, 2017.
15. CDC. 2015 sexually transmitted diseases treatment guidelines. Available at: https://www.cdc.gov/std/tg2015/default.htm. Accessed March 22, 2017.
16. USPSTF. Chronic obstructive pulmonary disease: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/chronic-obstructive-pulmonary-disease-screening. Accessed March 22, 2017.
17. USPSTF. Genital herpes infection: serologic screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/genital-herpes-screening1. Accessed March 22, 2017.
Management of bow legs in children: A primary care protocol
ABSTRACT
Objective To reduce unnecessary orthopedic referrals by developing a protocol for managing physiologic bow legs in the primary care environment through the use of a noninvasive technique that simultaneously tracks normal varus progression and screens for potential pathologic bowing requiring an orthopedic referral.
Methods Retrospective study of 155 patients with physiologic genu varum and 10 with infantile Blount’s disease. We used fingerbreadth measurements to document progression or resolution of bow legs. Final diagnoses were made by one orthopedic surgeon using clinical and radiographic evidence. We divided genu varum patients into 3 groups: patients presenting with bow legs before 18 months of age (MOA), patients presenting between 18 and 23 MOA, and patients presenting at 24 MOA or older for analyses relevant to the development of the follow-up protocol.
Results Physiologic genu varum patients walked earlier than average infants (10 months vs 12-15 months; P<.001). Physiologic genu varum patients presenting before 18 MOA demonstrated initial signs of correction between 18 and 24 MOA and resolution by 30 MOA. Physiologic genu varum patients presenting between 18 and 23 MOA demonstrated initial signs of correction between 24 MOA and 30 MOA and resolution by 36 MOA.
Conclusion Primary care physicians can manage most children presenting with bow legs. Management focuses on following the progression or resolution of varus with regular follow-up. For patients presenting with bow legs, we recommend a follow-up protocol using mainly well-child checkups and a simple clinical assessment to monitor varus progression and screen for pathologic bowing.
Bow legs in young children can be a concern for parents.1,2 By far, the most common reason for bow legs is physiologic genu varum,3-5 a nonprogressive stage of normal development in young children that generally resolves spontaneously without treatment.1,6-11 Normally developing children undergo a varus phase between birth and 18 to 24 months of age (MOA), at which time there is usually a transition in alignment from varus to straight to valgus (knock knees), which will correct to straight or mild valgus throughout adolescence.1,6,7,9,10,12-17
The most common form of pathologic bow legs is Blount’s disease, also known as tibia vara, which must be differentiated from physiologic genu varum.8-10,15,18-24 The progressive varus deformity of Blount’s disease usually requires orthopedic intervention.1,10,23-26 Early diagnosis may spare patients complex interventions, improve prognosis, and limit complications that include gait abnormalities,4,8,10,27 knee joint instability,4,24,27 osteoarthritis,9,20,27 meniscal tears,27 and degenerative joint disease.19,20,27
Although variables such as walking age, race, weight, and gender have been suggested as risk factors for Blount’s disease, they have not been useful in differentiating between Blount’s pathology and physiologic genu varum.1,4,5,7,10,20,28 In the primary care setting, distinguishing physiologic from pathologic forms of bow legs is possible with a thorough history and physical exam and with radiographs, as warranted.1,2,15 More than 40% of genu varum/genu valgum cases referred for orthopedic consultation turn out to be the physiologic form,2 suggesting a need for guidelines in the primary care setting to help direct referral and follow-up. The purpose of this study was to provide recommendations to family physicians for evaluating and managing children with bow legs.
Materials and methods
This study, approved by the Internal Review Board of Akron Children’s Hospital, is a retrospective review of children seen by a single pediatric orthopedic surgeon (DSW) from 1970 to 2012. Four-hundred twenty-four children were received for evaluation of bow legs. Excluded from our final analysis were 220 subjects seen only once for this specific referral and 39 subjects diagnosed with a condition other than genu varum or Blount’s disease (ie, rickets, skeletal dysplasia, sequelae of trauma, or infection). Ten subjects with Blount’s disease and 155 subjects with physiologic genu varum were included in the final data analysis.
In addition to noting the age at which a patient walked independently, at each visit we documented age and the fingerbreadth (varus) distance between the medial femoral condyles with the child’s ankles held together. Parents reported age of independent walking for just 3 children with Blount’s disease and for 134 children with physiologic genu varum. Study variables for the genu varum data analysis were age of walking, age at presentation, age at varus correction, age at varus resolution, time between presentation and varus correction, and time between presentation and varus resolution. Varus correction is defined as any decrease in varus angulation since presentation. Varus resolution is defined as varus correction to less than or equal to half of the varus angulation at presentation. For inclusion in the age-at-resolution analysis, a child must have been evaluated at regular follow-up visits (all rechecks within 8 months).
To measure varus distance, we used the fingerbreadth method described by Weiner in a study of 600 cases (FIGURE).6 This simple technique, which requires no special equipment, accurately detected differences in varus angulation and tracked the normal pattern of lower limb angular development. The patient should be supine on the examination table with legs extended. With one hand, the examiner holds the child’s ankles together, ensuring the medial malleoli are in contact. With the other hand, the examiner measures the fingerbreadth distance between the medial femoral condyles. Alternatively, a ruler may be used to measure the distance. This latter method may be especially useful in practices where the patient is likely to see more than one provider for well child care.
We divided the genu varum subject group into 3 subgroups by age at presentation: 103 subjects were younger than 18 months; 47 were 18 to 23 months; and 5 were 24 months or older. We used the data analysis toolkit in Microsoft Excel 2013 to perform a statistical analysis of study variables. We assumed the genu varum population is a normally distributed population. We used a 95% confidence level (α=0.05) for all calculations of confidence intervals (CIs), student t-tests, and tolerance intervals. Based on the data analysis results, we developed a series of follow-up and referral guidelines for practitioners.
Results
The mean walking age for those diagnosed with physiologic genu varum was 10 months (95% CI, 9.8-10.4), which is significantly younger than the 12 months of age (at the earliest) typical of toddlers in general (P<.001). There was no significant difference between the walking age of male and female children diagnosed with genu varum (P=.37).
Of the children presenting with the primary complaint of bow legs, 6% subsequently developed Blount’s disease. These patients presented at a mean age of 20.9 months and were diagnosed at a mean age of 23.9 months. Following the Blount’s disease diagnosis, we initiated therapy in all cases (3 surgical, 7 bracing).
Physiologic genu varum patients presented at a mean age of 16.4 months, with only 3.23% presenting at older than 23 months. On average, physiologic genu varum patients presenting before 24 months of age showed measurable varus correction 5 months after presentation and achieved varus resolution 7.3 months after presentation (TABLE 1). Assuming the patient population is normally distributed, we can be 95% confident that 95% of physiologic genu varum patients presenting before 18 months of age will show measurable varus correction by 24 months and will resolve without intervention by 30 months (TABLE 2). Patients presenting between 18 and 23 months of age should show measurable varus correction by 30 months and resolution by 36 months (TABLE 3).
Discussion
Primary care physicians have the ability to differentiate physiologic genu varum from pathologic forms of bow legs with a thorough history, physical exam, and radiographic examination, if necessary1,2,13 (TABLE 41,7,8,10,12,14,18-20,22,24,27). Several approaches to differentiating Blount’s disease and physiologic genu varum have been described in the literature.1,4,7,8,10,14,22,23
The average age at which children begin to walk independently is between 13 and 15 months.5,18,29-31 Recently, it has been suggested that the range be expanded to include 12 months of age.30 The association between early walking (at 10-11 months)12,20,22 and Blount’s disease is generally accepted in the orthopedic literature.1,4,7,10,19-22 However, some authors have suggested early walking also contributes to genu varum.1,5,8,10,18,28 The mean age of independent walking for children with physiologic genu varum suggested in the literature (10 months) was confirmed in our study and found to be significantly younger than the average for toddlers generally.1,22 Early walking is clearly associated with both physiologic genu varum and Blount’s disease, but no direct causation has been identified in either case. An alternative means of differentiating these entities is needed.
Radiographic examination of the knee is essential to the diagnosis of Blount’s disease as well as other, less common causes of pathologic bow legs (skeletal dysplasia, rickets, traumatic growth plate insults, infections, neoplasms).1,8,14,19 The common radiologic classification of staging for Blount’s disease is the Langenskiöld staging system, which involves identification of characteristic radiographic changes at the tibial physis.5,8,14,15,18,22,24
Sequential measurement of genu varum is most useful in differentiating between physiologic and pathologic processes. Physiologic genu varum, an exaggeration of the normal developmental pattern, characteristically resolves and evolves into physiologic genu valgum by 3 years of age.1,6-11 The pathophysiology of Blount’s disease is believed to be related to biomechanical overloading of the posteromedial proximal tibia during gait with the knee in a varus orientation. Excess loading on the proximal medial physis contributes to varus progression.4,10,14,20,25,27 Patients with Blount’s disease progress with varus and concomitant internal tibial torsion associated with growth plate irregularities and eventually exhibit premature closure.1,10,14,18,20,23,24,26 In the months prior to Blount’s disease diagnosis, increasing varus has been reported.4,7,10,19 Varus progression that differs from the expected pattern indicates possible pathologic bow legs and should prompt radiologic evaluation and, often, an orthopedic referral.3,4,7-9,12,13,21
In our study, only 3% of children with physiologic genu varum presented at 24 months of age or older, compared with 20% of Blount’s disease patients. We recommend considering orthopedic referral for any patient presenting with bow legs at 24 months of age or older. Additionally, consider orthopedic referral for any patient whose varus has not begun to correct within 8 months or has not resolved within 14 months of presentation, as more than 95% of patients with physiologic genu varum are expected to meet these milestones (TABLE 1). And do not hesitate to refer patients at any stage of follow-up if you suspect pathology or if parents are anxious.
If no sign of pathology is immediately identified, we recommend the following course of action:
- Record a reference fingerbreadth or ruler measurement at the initial presentation.
- Re-examine the knee varus at the next regular well-child visit (TABLE 5).
Re-examining the patient prior to the next well-child visit is unnecessary, as some degree of bowing is typical until age 18 to 24 months.1,6,7,9,12,13,17 Recommend orthopedic referral for any patient with varus that has progressed since initial presentation. Without signs of pathology, repeat varus assessment at the next well-child visit. This schedule minimizes the need for additional physician appointments by integrating follow-up into the typical well-child visits at 18, 24, 30, and 36 months of age.32 The 6-month follow-up interval was a feature of our study and is recommended in the related literature.12
- Consider orthopedic referral for patients whose varus has not corrected by the second follow-up appointment, as more than 95% of patients should have measurable varus correction at this visit. Most patients will have exhibited varus resolution by this time and will not require additional follow-up. For patients with observable correction who do not yet meet the criteria for resolution, we recommend a third, final follow-up appointment in another 6 months.
- Refer any patient whose varus has not resolved by the third follow-up appointment, as more than 95% of genu varum cases should have resolved by this time. This finding is echoed in the literature; any varus beyond 36 months of age is considered abnormal and suggestive of pathology.5,7,8,13,14 If evidence of Blount’s or skeletal dysplasia is identified, orthopedic management will likely consist of bracing (orthotics) or surgical management.
CORRESPONDENCE
Dennis S. Weiner, MD, Department of Orthopedic Surgery, Akron Children’s Hospital, 300 Locust Street, Suite 250, Akron, OH, 44302; [email protected].
ACKNOWLEDGEMENTS
The authors thank Meadow Newton, BS, assistant research coordinator, Akron Children’s Hospital, for her editing and technical assistance and Richard Steiner, PhD, The University of Akron, for his statistical review.
1. Weiner DS. Pediatric orthopedics for primary care physicians. 2nd ed. Jones K, ed. Cambridge, United Kingdom: Cambridge University Press; 2004.
2. Carli A, Saran N, Kruijt J, et al. Physiological referrals for paediatric musculoskeletal complaints: a costly problem that needs to be addressed. Paediatr Child Health. 2012;17:e93-e97.
3. Fabry G. Clinical practice. Static, axial, and rotational deformities of the lower extremities in children. Eur J Pediatr. 2010;169:529-534.
4. Davids JR, Blackhurst DW, Allen Jr BL. Clinical evaluation of bowed legs in children. J Pediatr Orthop B. 2000;9:278-284.
5. Bateson EM. The relationship between Blount’s disease and bow legs. Br J Radiol. 1968;41:107-114.
6. Weiner DS. The natural history of “bow legs” and “knock knees” in childhood. Orthopedics. 1981;4:156-160.
7. Greene WB. Genu varum and genu valgum in children: differential diagnosis and guidelines for evaluation. Compr Ther. 1996;22:22-29.
8. Do TT. Clinical and radiographic evaluation of bowlegs. Curr Opin Pediatr. 2001;13:42-46.
9. Bleck EE. Developmental orthopaedics. III: Toddlers. Dev Med Child Neurol. 1982;24:533-555.
10. Brooks WC, Gross RH. Genu Varum in Children: Diagnosis and Treatment. J Am Acad Orthop Surg. 1995;3:326-335.
11. Greenberg LA, Swartz AA. Genu varum and genu valgum. Another look. Am J Dis Child. 1971;121:219-221.
12. Scherl SA. Common lower extremity problems in children. Pediatr Rev. 2004;25:52-62.
13. Wall EJ. Practical primary pediatric orthopedics. Nurs Clin North Am. 2000;35:95-113.
14. Cheema JI, Grissom LE, Harcke HT. Radiographic characteristics of lower-extremity bowing in children. Radiographics. 2003;23:871-880.
15. McCarthy JJ, Betz RR, Kim A, et al. Early radiographic differentiation of infantile tibia vara from physiologic bowing using the femoral-tibial ratio. J Pediatr Orthop. 2001;21:545-548.
16. Salenius P, Vankka E. The development of the tibiofemoral angle in children. J Bone Joint Surg Am. 1975;57:259-261.
17. Engel GM, Staheli LT. The natural history of torsion and other factors influencing gait in childhood. A study of the angle of gait, tibial torsion, knee angle, hip rotation, and development of the arch in normal children. Clin Orthop Relat Res. 1974;99:12-17.
18. Golding J, Bateson E, McNeil-Smith G. Infantile tibia vara. In: The Growth Plate and Its Disorders. Rang M, ed. Baltimore, MD: Williams and Wilkins; 1969:109-119.
19. Greene WB. Infantile tibia vara. J Bone Joint Surg Am. 1993;75:130-143.
20. Golding J, McNeil-Smith JDG. Observations on the etiology of tibia vara. J Bone Joint Surg Br. 1963;45-B:320-325.
21. Eggert P, Viemann M. Physiological bowlegs or infantile Blount’s disease. Some new aspects on an old problem. Pediatr Radiol. 1996;26:349-352.
22. Levine AM, Drennan JC. Physiological bowing and tibia vara. The metaphyseal-diaphyseal angle in the measurement of bowleg deformities. J Bone Joint Surg Am. 1982;64:1158-1163.
23. Kessel L. Annotations on the etiology and treatment of tibia vara. J Bone Joint Surg Br. 1970;52:93-99.
24. Blount WP. Tibia vara: osteochondrosis deformans tibiae. J Bone Joint Surg Am. 1937;19:1-29.
25. Davids JR, Blackhurst DW, Allen BL Jr. Radiographic evaluation of bowed legs in children. J Pediatr Orthop. 2001;21:257-263.
26. Cook SD, Lavernia CJ, Burke SW, et al. A biomechanical analysis of the etiology of tibia vara. J Pediatr Orthop. 1983;3:449-454.
27. Birch JG. Blount disease. J Am Acad Orthop Surg. 2013;21:408-418.
28. Bateson EM. Non-rachitic bow leg and knock-knee deformities in young Jamaican children. Br J Radiol. 1966;39:92-101.
29. Grantham-McGregor SM, Back EH. Gross motor development in Jamaican infants. Dev Med Child Neurol. 1971;13:79-87.
30. Størvold GV, Aarethun K, Bratberg GH. Age for onset of walking and prewalking strategies. Early Hum Dev. 2013;89:655-659.
31. Garrett M, McElroy AM, Staines A. Locomotor milestones and babywalkers: cross sectional study. BMJ. 2002;324:1494.
32. Simon GR, Baker C, Barden GA 3rd, et al; Committee on Practice and Ambulatory Medicine, Curry ES, Dunca PM, Hagan JF Jr, et al; Bright Futures Periodicity Schedule Workgroup. 2014 recommendations for pediatric preventive health care. Pediatrics. 2014;133:568-570.
ABSTRACT
Objective To reduce unnecessary orthopedic referrals by developing a protocol for managing physiologic bow legs in the primary care environment through the use of a noninvasive technique that simultaneously tracks normal varus progression and screens for potential pathologic bowing requiring an orthopedic referral.
Methods Retrospective study of 155 patients with physiologic genu varum and 10 with infantile Blount’s disease. We used fingerbreadth measurements to document progression or resolution of bow legs. Final diagnoses were made by one orthopedic surgeon using clinical and radiographic evidence. We divided genu varum patients into 3 groups: patients presenting with bow legs before 18 months of age (MOA), patients presenting between 18 and 23 MOA, and patients presenting at 24 MOA or older for analyses relevant to the development of the follow-up protocol.
Results Physiologic genu varum patients walked earlier than average infants (10 months vs 12-15 months; P<.001). Physiologic genu varum patients presenting before 18 MOA demonstrated initial signs of correction between 18 and 24 MOA and resolution by 30 MOA. Physiologic genu varum patients presenting between 18 and 23 MOA demonstrated initial signs of correction between 24 MOA and 30 MOA and resolution by 36 MOA.
Conclusion Primary care physicians can manage most children presenting with bow legs. Management focuses on following the progression or resolution of varus with regular follow-up. For patients presenting with bow legs, we recommend a follow-up protocol using mainly well-child checkups and a simple clinical assessment to monitor varus progression and screen for pathologic bowing.
Bow legs in young children can be a concern for parents.1,2 By far, the most common reason for bow legs is physiologic genu varum,3-5 a nonprogressive stage of normal development in young children that generally resolves spontaneously without treatment.1,6-11 Normally developing children undergo a varus phase between birth and 18 to 24 months of age (MOA), at which time there is usually a transition in alignment from varus to straight to valgus (knock knees), which will correct to straight or mild valgus throughout adolescence.1,6,7,9,10,12-17
The most common form of pathologic bow legs is Blount’s disease, also known as tibia vara, which must be differentiated from physiologic genu varum.8-10,15,18-24 The progressive varus deformity of Blount’s disease usually requires orthopedic intervention.1,10,23-26 Early diagnosis may spare patients complex interventions, improve prognosis, and limit complications that include gait abnormalities,4,8,10,27 knee joint instability,4,24,27 osteoarthritis,9,20,27 meniscal tears,27 and degenerative joint disease.19,20,27
Although variables such as walking age, race, weight, and gender have been suggested as risk factors for Blount’s disease, they have not been useful in differentiating between Blount’s pathology and physiologic genu varum.1,4,5,7,10,20,28 In the primary care setting, distinguishing physiologic from pathologic forms of bow legs is possible with a thorough history and physical exam and with radiographs, as warranted.1,2,15 More than 40% of genu varum/genu valgum cases referred for orthopedic consultation turn out to be the physiologic form,2 suggesting a need for guidelines in the primary care setting to help direct referral and follow-up. The purpose of this study was to provide recommendations to family physicians for evaluating and managing children with bow legs.
Materials and methods
This study, approved by the Internal Review Board of Akron Children’s Hospital, is a retrospective review of children seen by a single pediatric orthopedic surgeon (DSW) from 1970 to 2012. Four-hundred twenty-four children were received for evaluation of bow legs. Excluded from our final analysis were 220 subjects seen only once for this specific referral and 39 subjects diagnosed with a condition other than genu varum or Blount’s disease (ie, rickets, skeletal dysplasia, sequelae of trauma, or infection). Ten subjects with Blount’s disease and 155 subjects with physiologic genu varum were included in the final data analysis.
In addition to noting the age at which a patient walked independently, at each visit we documented age and the fingerbreadth (varus) distance between the medial femoral condyles with the child’s ankles held together. Parents reported age of independent walking for just 3 children with Blount’s disease and for 134 children with physiologic genu varum. Study variables for the genu varum data analysis were age of walking, age at presentation, age at varus correction, age at varus resolution, time between presentation and varus correction, and time between presentation and varus resolution. Varus correction is defined as any decrease in varus angulation since presentation. Varus resolution is defined as varus correction to less than or equal to half of the varus angulation at presentation. For inclusion in the age-at-resolution analysis, a child must have been evaluated at regular follow-up visits (all rechecks within 8 months).
To measure varus distance, we used the fingerbreadth method described by Weiner in a study of 600 cases (FIGURE).6 This simple technique, which requires no special equipment, accurately detected differences in varus angulation and tracked the normal pattern of lower limb angular development. The patient should be supine on the examination table with legs extended. With one hand, the examiner holds the child’s ankles together, ensuring the medial malleoli are in contact. With the other hand, the examiner measures the fingerbreadth distance between the medial femoral condyles. Alternatively, a ruler may be used to measure the distance. This latter method may be especially useful in practices where the patient is likely to see more than one provider for well child care.
We divided the genu varum subject group into 3 subgroups by age at presentation: 103 subjects were younger than 18 months; 47 were 18 to 23 months; and 5 were 24 months or older. We used the data analysis toolkit in Microsoft Excel 2013 to perform a statistical analysis of study variables. We assumed the genu varum population is a normally distributed population. We used a 95% confidence level (α=0.05) for all calculations of confidence intervals (CIs), student t-tests, and tolerance intervals. Based on the data analysis results, we developed a series of follow-up and referral guidelines for practitioners.
Results
The mean walking age for those diagnosed with physiologic genu varum was 10 months (95% CI, 9.8-10.4), which is significantly younger than the 12 months of age (at the earliest) typical of toddlers in general (P<.001). There was no significant difference between the walking age of male and female children diagnosed with genu varum (P=.37).
Of the children presenting with the primary complaint of bow legs, 6% subsequently developed Blount’s disease. These patients presented at a mean age of 20.9 months and were diagnosed at a mean age of 23.9 months. Following the Blount’s disease diagnosis, we initiated therapy in all cases (3 surgical, 7 bracing).
Physiologic genu varum patients presented at a mean age of 16.4 months, with only 3.23% presenting at older than 23 months. On average, physiologic genu varum patients presenting before 24 months of age showed measurable varus correction 5 months after presentation and achieved varus resolution 7.3 months after presentation (TABLE 1). Assuming the patient population is normally distributed, we can be 95% confident that 95% of physiologic genu varum patients presenting before 18 months of age will show measurable varus correction by 24 months and will resolve without intervention by 30 months (TABLE 2). Patients presenting between 18 and 23 months of age should show measurable varus correction by 30 months and resolution by 36 months (TABLE 3).
Discussion
Primary care physicians have the ability to differentiate physiologic genu varum from pathologic forms of bow legs with a thorough history, physical exam, and radiographic examination, if necessary1,2,13 (TABLE 41,7,8,10,12,14,18-20,22,24,27). Several approaches to differentiating Blount’s disease and physiologic genu varum have been described in the literature.1,4,7,8,10,14,22,23
The average age at which children begin to walk independently is between 13 and 15 months.5,18,29-31 Recently, it has been suggested that the range be expanded to include 12 months of age.30 The association between early walking (at 10-11 months)12,20,22 and Blount’s disease is generally accepted in the orthopedic literature.1,4,7,10,19-22 However, some authors have suggested early walking also contributes to genu varum.1,5,8,10,18,28 The mean age of independent walking for children with physiologic genu varum suggested in the literature (10 months) was confirmed in our study and found to be significantly younger than the average for toddlers generally.1,22 Early walking is clearly associated with both physiologic genu varum and Blount’s disease, but no direct causation has been identified in either case. An alternative means of differentiating these entities is needed.
Radiographic examination of the knee is essential to the diagnosis of Blount’s disease as well as other, less common causes of pathologic bow legs (skeletal dysplasia, rickets, traumatic growth plate insults, infections, neoplasms).1,8,14,19 The common radiologic classification of staging for Blount’s disease is the Langenskiöld staging system, which involves identification of characteristic radiographic changes at the tibial physis.5,8,14,15,18,22,24
Sequential measurement of genu varum is most useful in differentiating between physiologic and pathologic processes. Physiologic genu varum, an exaggeration of the normal developmental pattern, characteristically resolves and evolves into physiologic genu valgum by 3 years of age.1,6-11 The pathophysiology of Blount’s disease is believed to be related to biomechanical overloading of the posteromedial proximal tibia during gait with the knee in a varus orientation. Excess loading on the proximal medial physis contributes to varus progression.4,10,14,20,25,27 Patients with Blount’s disease progress with varus and concomitant internal tibial torsion associated with growth plate irregularities and eventually exhibit premature closure.1,10,14,18,20,23,24,26 In the months prior to Blount’s disease diagnosis, increasing varus has been reported.4,7,10,19 Varus progression that differs from the expected pattern indicates possible pathologic bow legs and should prompt radiologic evaluation and, often, an orthopedic referral.3,4,7-9,12,13,21
In our study, only 3% of children with physiologic genu varum presented at 24 months of age or older, compared with 20% of Blount’s disease patients. We recommend considering orthopedic referral for any patient presenting with bow legs at 24 months of age or older. Additionally, consider orthopedic referral for any patient whose varus has not begun to correct within 8 months or has not resolved within 14 months of presentation, as more than 95% of patients with physiologic genu varum are expected to meet these milestones (TABLE 1). And do not hesitate to refer patients at any stage of follow-up if you suspect pathology or if parents are anxious.
If no sign of pathology is immediately identified, we recommend the following course of action:
- Record a reference fingerbreadth or ruler measurement at the initial presentation.
- Re-examine the knee varus at the next regular well-child visit (TABLE 5).
Re-examining the patient prior to the next well-child visit is unnecessary, as some degree of bowing is typical until age 18 to 24 months.1,6,7,9,12,13,17 Recommend orthopedic referral for any patient with varus that has progressed since initial presentation. Without signs of pathology, repeat varus assessment at the next well-child visit. This schedule minimizes the need for additional physician appointments by integrating follow-up into the typical well-child visits at 18, 24, 30, and 36 months of age.32 The 6-month follow-up interval was a feature of our study and is recommended in the related literature.12
- Consider orthopedic referral for patients whose varus has not corrected by the second follow-up appointment, as more than 95% of patients should have measurable varus correction at this visit. Most patients will have exhibited varus resolution by this time and will not require additional follow-up. For patients with observable correction who do not yet meet the criteria for resolution, we recommend a third, final follow-up appointment in another 6 months.
- Refer any patient whose varus has not resolved by the third follow-up appointment, as more than 95% of genu varum cases should have resolved by this time. This finding is echoed in the literature; any varus beyond 36 months of age is considered abnormal and suggestive of pathology.5,7,8,13,14 If evidence of Blount’s or skeletal dysplasia is identified, orthopedic management will likely consist of bracing (orthotics) or surgical management.
CORRESPONDENCE
Dennis S. Weiner, MD, Department of Orthopedic Surgery, Akron Children’s Hospital, 300 Locust Street, Suite 250, Akron, OH, 44302; [email protected].
ACKNOWLEDGEMENTS
The authors thank Meadow Newton, BS, assistant research coordinator, Akron Children’s Hospital, for her editing and technical assistance and Richard Steiner, PhD, The University of Akron, for his statistical review.
ABSTRACT
Objective To reduce unnecessary orthopedic referrals by developing a protocol for managing physiologic bow legs in the primary care environment through the use of a noninvasive technique that simultaneously tracks normal varus progression and screens for potential pathologic bowing requiring an orthopedic referral.
Methods Retrospective study of 155 patients with physiologic genu varum and 10 with infantile Blount’s disease. We used fingerbreadth measurements to document progression or resolution of bow legs. Final diagnoses were made by one orthopedic surgeon using clinical and radiographic evidence. We divided genu varum patients into 3 groups: patients presenting with bow legs before 18 months of age (MOA), patients presenting between 18 and 23 MOA, and patients presenting at 24 MOA or older for analyses relevant to the development of the follow-up protocol.
Results Physiologic genu varum patients walked earlier than average infants (10 months vs 12-15 months; P<.001). Physiologic genu varum patients presenting before 18 MOA demonstrated initial signs of correction between 18 and 24 MOA and resolution by 30 MOA. Physiologic genu varum patients presenting between 18 and 23 MOA demonstrated initial signs of correction between 24 MOA and 30 MOA and resolution by 36 MOA.
Conclusion Primary care physicians can manage most children presenting with bow legs. Management focuses on following the progression or resolution of varus with regular follow-up. For patients presenting with bow legs, we recommend a follow-up protocol using mainly well-child checkups and a simple clinical assessment to monitor varus progression and screen for pathologic bowing.
Bow legs in young children can be a concern for parents.1,2 By far, the most common reason for bow legs is physiologic genu varum,3-5 a nonprogressive stage of normal development in young children that generally resolves spontaneously without treatment.1,6-11 Normally developing children undergo a varus phase between birth and 18 to 24 months of age (MOA), at which time there is usually a transition in alignment from varus to straight to valgus (knock knees), which will correct to straight or mild valgus throughout adolescence.1,6,7,9,10,12-17
The most common form of pathologic bow legs is Blount’s disease, also known as tibia vara, which must be differentiated from physiologic genu varum.8-10,15,18-24 The progressive varus deformity of Blount’s disease usually requires orthopedic intervention.1,10,23-26 Early diagnosis may spare patients complex interventions, improve prognosis, and limit complications that include gait abnormalities,4,8,10,27 knee joint instability,4,24,27 osteoarthritis,9,20,27 meniscal tears,27 and degenerative joint disease.19,20,27
Although variables such as walking age, race, weight, and gender have been suggested as risk factors for Blount’s disease, they have not been useful in differentiating between Blount’s pathology and physiologic genu varum.1,4,5,7,10,20,28 In the primary care setting, distinguishing physiologic from pathologic forms of bow legs is possible with a thorough history and physical exam and with radiographs, as warranted.1,2,15 More than 40% of genu varum/genu valgum cases referred for orthopedic consultation turn out to be the physiologic form,2 suggesting a need for guidelines in the primary care setting to help direct referral and follow-up. The purpose of this study was to provide recommendations to family physicians for evaluating and managing children with bow legs.
Materials and methods
This study, approved by the Internal Review Board of Akron Children’s Hospital, is a retrospective review of children seen by a single pediatric orthopedic surgeon (DSW) from 1970 to 2012. Four-hundred twenty-four children were received for evaluation of bow legs. Excluded from our final analysis were 220 subjects seen only once for this specific referral and 39 subjects diagnosed with a condition other than genu varum or Blount’s disease (ie, rickets, skeletal dysplasia, sequelae of trauma, or infection). Ten subjects with Blount’s disease and 155 subjects with physiologic genu varum were included in the final data analysis.
In addition to noting the age at which a patient walked independently, at each visit we documented age and the fingerbreadth (varus) distance between the medial femoral condyles with the child’s ankles held together. Parents reported age of independent walking for just 3 children with Blount’s disease and for 134 children with physiologic genu varum. Study variables for the genu varum data analysis were age of walking, age at presentation, age at varus correction, age at varus resolution, time between presentation and varus correction, and time between presentation and varus resolution. Varus correction is defined as any decrease in varus angulation since presentation. Varus resolution is defined as varus correction to less than or equal to half of the varus angulation at presentation. For inclusion in the age-at-resolution analysis, a child must have been evaluated at regular follow-up visits (all rechecks within 8 months).
To measure varus distance, we used the fingerbreadth method described by Weiner in a study of 600 cases (FIGURE).6 This simple technique, which requires no special equipment, accurately detected differences in varus angulation and tracked the normal pattern of lower limb angular development. The patient should be supine on the examination table with legs extended. With one hand, the examiner holds the child’s ankles together, ensuring the medial malleoli are in contact. With the other hand, the examiner measures the fingerbreadth distance between the medial femoral condyles. Alternatively, a ruler may be used to measure the distance. This latter method may be especially useful in practices where the patient is likely to see more than one provider for well child care.
We divided the genu varum subject group into 3 subgroups by age at presentation: 103 subjects were younger than 18 months; 47 were 18 to 23 months; and 5 were 24 months or older. We used the data analysis toolkit in Microsoft Excel 2013 to perform a statistical analysis of study variables. We assumed the genu varum population is a normally distributed population. We used a 95% confidence level (α=0.05) for all calculations of confidence intervals (CIs), student t-tests, and tolerance intervals. Based on the data analysis results, we developed a series of follow-up and referral guidelines for practitioners.
Results
The mean walking age for those diagnosed with physiologic genu varum was 10 months (95% CI, 9.8-10.4), which is significantly younger than the 12 months of age (at the earliest) typical of toddlers in general (P<.001). There was no significant difference between the walking age of male and female children diagnosed with genu varum (P=.37).
Of the children presenting with the primary complaint of bow legs, 6% subsequently developed Blount’s disease. These patients presented at a mean age of 20.9 months and were diagnosed at a mean age of 23.9 months. Following the Blount’s disease diagnosis, we initiated therapy in all cases (3 surgical, 7 bracing).
Physiologic genu varum patients presented at a mean age of 16.4 months, with only 3.23% presenting at older than 23 months. On average, physiologic genu varum patients presenting before 24 months of age showed measurable varus correction 5 months after presentation and achieved varus resolution 7.3 months after presentation (TABLE 1). Assuming the patient population is normally distributed, we can be 95% confident that 95% of physiologic genu varum patients presenting before 18 months of age will show measurable varus correction by 24 months and will resolve without intervention by 30 months (TABLE 2). Patients presenting between 18 and 23 months of age should show measurable varus correction by 30 months and resolution by 36 months (TABLE 3).
Discussion
Primary care physicians have the ability to differentiate physiologic genu varum from pathologic forms of bow legs with a thorough history, physical exam, and radiographic examination, if necessary1,2,13 (TABLE 41,7,8,10,12,14,18-20,22,24,27). Several approaches to differentiating Blount’s disease and physiologic genu varum have been described in the literature.1,4,7,8,10,14,22,23
The average age at which children begin to walk independently is between 13 and 15 months.5,18,29-31 Recently, it has been suggested that the range be expanded to include 12 months of age.30 The association between early walking (at 10-11 months)12,20,22 and Blount’s disease is generally accepted in the orthopedic literature.1,4,7,10,19-22 However, some authors have suggested early walking also contributes to genu varum.1,5,8,10,18,28 The mean age of independent walking for children with physiologic genu varum suggested in the literature (10 months) was confirmed in our study and found to be significantly younger than the average for toddlers generally.1,22 Early walking is clearly associated with both physiologic genu varum and Blount’s disease, but no direct causation has been identified in either case. An alternative means of differentiating these entities is needed.
Radiographic examination of the knee is essential to the diagnosis of Blount’s disease as well as other, less common causes of pathologic bow legs (skeletal dysplasia, rickets, traumatic growth plate insults, infections, neoplasms).1,8,14,19 The common radiologic classification of staging for Blount’s disease is the Langenskiöld staging system, which involves identification of characteristic radiographic changes at the tibial physis.5,8,14,15,18,22,24
Sequential measurement of genu varum is most useful in differentiating between physiologic and pathologic processes. Physiologic genu varum, an exaggeration of the normal developmental pattern, characteristically resolves and evolves into physiologic genu valgum by 3 years of age.1,6-11 The pathophysiology of Blount’s disease is believed to be related to biomechanical overloading of the posteromedial proximal tibia during gait with the knee in a varus orientation. Excess loading on the proximal medial physis contributes to varus progression.4,10,14,20,25,27 Patients with Blount’s disease progress with varus and concomitant internal tibial torsion associated with growth plate irregularities and eventually exhibit premature closure.1,10,14,18,20,23,24,26 In the months prior to Blount’s disease diagnosis, increasing varus has been reported.4,7,10,19 Varus progression that differs from the expected pattern indicates possible pathologic bow legs and should prompt radiologic evaluation and, often, an orthopedic referral.3,4,7-9,12,13,21
In our study, only 3% of children with physiologic genu varum presented at 24 months of age or older, compared with 20% of Blount’s disease patients. We recommend considering orthopedic referral for any patient presenting with bow legs at 24 months of age or older. Additionally, consider orthopedic referral for any patient whose varus has not begun to correct within 8 months or has not resolved within 14 months of presentation, as more than 95% of patients with physiologic genu varum are expected to meet these milestones (TABLE 1). And do not hesitate to refer patients at any stage of follow-up if you suspect pathology or if parents are anxious.
If no sign of pathology is immediately identified, we recommend the following course of action:
- Record a reference fingerbreadth or ruler measurement at the initial presentation.
- Re-examine the knee varus at the next regular well-child visit (TABLE 5).
Re-examining the patient prior to the next well-child visit is unnecessary, as some degree of bowing is typical until age 18 to 24 months.1,6,7,9,12,13,17 Recommend orthopedic referral for any patient with varus that has progressed since initial presentation. Without signs of pathology, repeat varus assessment at the next well-child visit. This schedule minimizes the need for additional physician appointments by integrating follow-up into the typical well-child visits at 18, 24, 30, and 36 months of age.32 The 6-month follow-up interval was a feature of our study and is recommended in the related literature.12
- Consider orthopedic referral for patients whose varus has not corrected by the second follow-up appointment, as more than 95% of patients should have measurable varus correction at this visit. Most patients will have exhibited varus resolution by this time and will not require additional follow-up. For patients with observable correction who do not yet meet the criteria for resolution, we recommend a third, final follow-up appointment in another 6 months.
- Refer any patient whose varus has not resolved by the third follow-up appointment, as more than 95% of genu varum cases should have resolved by this time. This finding is echoed in the literature; any varus beyond 36 months of age is considered abnormal and suggestive of pathology.5,7,8,13,14 If evidence of Blount’s or skeletal dysplasia is identified, orthopedic management will likely consist of bracing (orthotics) or surgical management.
CORRESPONDENCE
Dennis S. Weiner, MD, Department of Orthopedic Surgery, Akron Children’s Hospital, 300 Locust Street, Suite 250, Akron, OH, 44302; [email protected].
ACKNOWLEDGEMENTS
The authors thank Meadow Newton, BS, assistant research coordinator, Akron Children’s Hospital, for her editing and technical assistance and Richard Steiner, PhD, The University of Akron, for his statistical review.
1. Weiner DS. Pediatric orthopedics for primary care physicians. 2nd ed. Jones K, ed. Cambridge, United Kingdom: Cambridge University Press; 2004.
2. Carli A, Saran N, Kruijt J, et al. Physiological referrals for paediatric musculoskeletal complaints: a costly problem that needs to be addressed. Paediatr Child Health. 2012;17:e93-e97.
3. Fabry G. Clinical practice. Static, axial, and rotational deformities of the lower extremities in children. Eur J Pediatr. 2010;169:529-534.
4. Davids JR, Blackhurst DW, Allen Jr BL. Clinical evaluation of bowed legs in children. J Pediatr Orthop B. 2000;9:278-284.
5. Bateson EM. The relationship between Blount’s disease and bow legs. Br J Radiol. 1968;41:107-114.
6. Weiner DS. The natural history of “bow legs” and “knock knees” in childhood. Orthopedics. 1981;4:156-160.
7. Greene WB. Genu varum and genu valgum in children: differential diagnosis and guidelines for evaluation. Compr Ther. 1996;22:22-29.
8. Do TT. Clinical and radiographic evaluation of bowlegs. Curr Opin Pediatr. 2001;13:42-46.
9. Bleck EE. Developmental orthopaedics. III: Toddlers. Dev Med Child Neurol. 1982;24:533-555.
10. Brooks WC, Gross RH. Genu Varum in Children: Diagnosis and Treatment. J Am Acad Orthop Surg. 1995;3:326-335.
11. Greenberg LA, Swartz AA. Genu varum and genu valgum. Another look. Am J Dis Child. 1971;121:219-221.
12. Scherl SA. Common lower extremity problems in children. Pediatr Rev. 2004;25:52-62.
13. Wall EJ. Practical primary pediatric orthopedics. Nurs Clin North Am. 2000;35:95-113.
14. Cheema JI, Grissom LE, Harcke HT. Radiographic characteristics of lower-extremity bowing in children. Radiographics. 2003;23:871-880.
15. McCarthy JJ, Betz RR, Kim A, et al. Early radiographic differentiation of infantile tibia vara from physiologic bowing using the femoral-tibial ratio. J Pediatr Orthop. 2001;21:545-548.
16. Salenius P, Vankka E. The development of the tibiofemoral angle in children. J Bone Joint Surg Am. 1975;57:259-261.
17. Engel GM, Staheli LT. The natural history of torsion and other factors influencing gait in childhood. A study of the angle of gait, tibial torsion, knee angle, hip rotation, and development of the arch in normal children. Clin Orthop Relat Res. 1974;99:12-17.
18. Golding J, Bateson E, McNeil-Smith G. Infantile tibia vara. In: The Growth Plate and Its Disorders. Rang M, ed. Baltimore, MD: Williams and Wilkins; 1969:109-119.
19. Greene WB. Infantile tibia vara. J Bone Joint Surg Am. 1993;75:130-143.
20. Golding J, McNeil-Smith JDG. Observations on the etiology of tibia vara. J Bone Joint Surg Br. 1963;45-B:320-325.
21. Eggert P, Viemann M. Physiological bowlegs or infantile Blount’s disease. Some new aspects on an old problem. Pediatr Radiol. 1996;26:349-352.
22. Levine AM, Drennan JC. Physiological bowing and tibia vara. The metaphyseal-diaphyseal angle in the measurement of bowleg deformities. J Bone Joint Surg Am. 1982;64:1158-1163.
23. Kessel L. Annotations on the etiology and treatment of tibia vara. J Bone Joint Surg Br. 1970;52:93-99.
24. Blount WP. Tibia vara: osteochondrosis deformans tibiae. J Bone Joint Surg Am. 1937;19:1-29.
25. Davids JR, Blackhurst DW, Allen BL Jr. Radiographic evaluation of bowed legs in children. J Pediatr Orthop. 2001;21:257-263.
26. Cook SD, Lavernia CJ, Burke SW, et al. A biomechanical analysis of the etiology of tibia vara. J Pediatr Orthop. 1983;3:449-454.
27. Birch JG. Blount disease. J Am Acad Orthop Surg. 2013;21:408-418.
28. Bateson EM. Non-rachitic bow leg and knock-knee deformities in young Jamaican children. Br J Radiol. 1966;39:92-101.
29. Grantham-McGregor SM, Back EH. Gross motor development in Jamaican infants. Dev Med Child Neurol. 1971;13:79-87.
30. Størvold GV, Aarethun K, Bratberg GH. Age for onset of walking and prewalking strategies. Early Hum Dev. 2013;89:655-659.
31. Garrett M, McElroy AM, Staines A. Locomotor milestones and babywalkers: cross sectional study. BMJ. 2002;324:1494.
32. Simon GR, Baker C, Barden GA 3rd, et al; Committee on Practice and Ambulatory Medicine, Curry ES, Dunca PM, Hagan JF Jr, et al; Bright Futures Periodicity Schedule Workgroup. 2014 recommendations for pediatric preventive health care. Pediatrics. 2014;133:568-570.
1. Weiner DS. Pediatric orthopedics for primary care physicians. 2nd ed. Jones K, ed. Cambridge, United Kingdom: Cambridge University Press; 2004.
2. Carli A, Saran N, Kruijt J, et al. Physiological referrals for paediatric musculoskeletal complaints: a costly problem that needs to be addressed. Paediatr Child Health. 2012;17:e93-e97.
3. Fabry G. Clinical practice. Static, axial, and rotational deformities of the lower extremities in children. Eur J Pediatr. 2010;169:529-534.
4. Davids JR, Blackhurst DW, Allen Jr BL. Clinical evaluation of bowed legs in children. J Pediatr Orthop B. 2000;9:278-284.
5. Bateson EM. The relationship between Blount’s disease and bow legs. Br J Radiol. 1968;41:107-114.
6. Weiner DS. The natural history of “bow legs” and “knock knees” in childhood. Orthopedics. 1981;4:156-160.
7. Greene WB. Genu varum and genu valgum in children: differential diagnosis and guidelines for evaluation. Compr Ther. 1996;22:22-29.
8. Do TT. Clinical and radiographic evaluation of bowlegs. Curr Opin Pediatr. 2001;13:42-46.
9. Bleck EE. Developmental orthopaedics. III: Toddlers. Dev Med Child Neurol. 1982;24:533-555.
10. Brooks WC, Gross RH. Genu Varum in Children: Diagnosis and Treatment. J Am Acad Orthop Surg. 1995;3:326-335.
11. Greenberg LA, Swartz AA. Genu varum and genu valgum. Another look. Am J Dis Child. 1971;121:219-221.
12. Scherl SA. Common lower extremity problems in children. Pediatr Rev. 2004;25:52-62.
13. Wall EJ. Practical primary pediatric orthopedics. Nurs Clin North Am. 2000;35:95-113.
14. Cheema JI, Grissom LE, Harcke HT. Radiographic characteristics of lower-extremity bowing in children. Radiographics. 2003;23:871-880.
15. McCarthy JJ, Betz RR, Kim A, et al. Early radiographic differentiation of infantile tibia vara from physiologic bowing using the femoral-tibial ratio. J Pediatr Orthop. 2001;21:545-548.
16. Salenius P, Vankka E. The development of the tibiofemoral angle in children. J Bone Joint Surg Am. 1975;57:259-261.
17. Engel GM, Staheli LT. The natural history of torsion and other factors influencing gait in childhood. A study of the angle of gait, tibial torsion, knee angle, hip rotation, and development of the arch in normal children. Clin Orthop Relat Res. 1974;99:12-17.
18. Golding J, Bateson E, McNeil-Smith G. Infantile tibia vara. In: The Growth Plate and Its Disorders. Rang M, ed. Baltimore, MD: Williams and Wilkins; 1969:109-119.
19. Greene WB. Infantile tibia vara. J Bone Joint Surg Am. 1993;75:130-143.
20. Golding J, McNeil-Smith JDG. Observations on the etiology of tibia vara. J Bone Joint Surg Br. 1963;45-B:320-325.
21. Eggert P, Viemann M. Physiological bowlegs or infantile Blount’s disease. Some new aspects on an old problem. Pediatr Radiol. 1996;26:349-352.
22. Levine AM, Drennan JC. Physiological bowing and tibia vara. The metaphyseal-diaphyseal angle in the measurement of bowleg deformities. J Bone Joint Surg Am. 1982;64:1158-1163.
23. Kessel L. Annotations on the etiology and treatment of tibia vara. J Bone Joint Surg Br. 1970;52:93-99.
24. Blount WP. Tibia vara: osteochondrosis deformans tibiae. J Bone Joint Surg Am. 1937;19:1-29.
25. Davids JR, Blackhurst DW, Allen BL Jr. Radiographic evaluation of bowed legs in children. J Pediatr Orthop. 2001;21:257-263.
26. Cook SD, Lavernia CJ, Burke SW, et al. A biomechanical analysis of the etiology of tibia vara. J Pediatr Orthop. 1983;3:449-454.
27. Birch JG. Blount disease. J Am Acad Orthop Surg. 2013;21:408-418.
28. Bateson EM. Non-rachitic bow leg and knock-knee deformities in young Jamaican children. Br J Radiol. 1966;39:92-101.
29. Grantham-McGregor SM, Back EH. Gross motor development in Jamaican infants. Dev Med Child Neurol. 1971;13:79-87.
30. Størvold GV, Aarethun K, Bratberg GH. Age for onset of walking and prewalking strategies. Early Hum Dev. 2013;89:655-659.
31. Garrett M, McElroy AM, Staines A. Locomotor milestones and babywalkers: cross sectional study. BMJ. 2002;324:1494.
32. Simon GR, Baker C, Barden GA 3rd, et al; Committee on Practice and Ambulatory Medicine, Curry ES, Dunca PM, Hagan JF Jr, et al; Bright Futures Periodicity Schedule Workgroup. 2014 recommendations for pediatric preventive health care. Pediatrics. 2014;133:568-570.