User login
Bromocriptine shows efficacy, safety for peripartum cardiomyopathy
PARIS – Two regimens of bromocriptine treatment administered with anticoagulation and standard heart failure management were safe, and often effective, for normalizing ejection fraction levels in women diagnosed with peripartum cardiomyopathy in a study with 63 women and designed to be the pivotal trial for this management strategy.
“Bromocriptine therapy applied for 1 week seems sufficient to promote healing from PPCM [peripartum cardiomyopathy] in most patients, although critically ill patients may profit from prolonged [8 week] treatment,” Denise Hilfiker-Kleiner, PhD, said at a meeting of the Heart Failure Association of the ESC.
Women who are suspected of having PPCM but with less compromised ventricular output, with an ejection fraction of 40%-45%, should be closely followed with repeated clinical examinations for 6 months and echocardiography examinations at 3 and 6 months to see if their cardiac output worsens enough to justify initiating a bromocriptine regimen, she advised. “We don’t recommend that every woman with an postpartum ejection fraction of 45% needs to immediately stop lactation [with bromocriptine treatment], but she should be frequently seen by a cardiologist to see whether she recovers or deteriorates further.”
The PPCM (Effect of Bromocriptine on Left Ventricular Function in Women With Peripartum Cardiomyopathy) trial enrolled 63 women with PPCM and severely depressed left ventricular ejection fraction at 12 German centers. Randomization placed 32 women into a group assigned to received 1 week of bromocriptine treatment, with 26 completing the study, and 31 in a group treated with bromocriptine for 8 weeks, with all 31 completing the study. The patients averaged 34 years of age. All patients also received standard heart failure treatment.
The study’s primary endpoint, the change in left ventricular ejection fraction from baseline to 6-month follow-up, was similar in the two treatment groups, with the 1-week regimen leading to an average 21% improvement in ejection fraction and the 8-week regimen averaging a 24% gain in pump function. Among a subgroup of 37 women who entered the study with a left ventricular ejection fraction of less than 30%, slightly more than 60% achieved full heart function by 6-month follow-up with an ejection fraction of 50% or greater, and an additional 35% had partial recovery, with a follow-up ejection fraction of 35%-49%, Dr. Hilfiker-Kleiner reported.
No women in the study developed a thrombotic complication, a potential danger of the bromocriptine intervention. All participants received antithrombotic prophylaxis with either warfarin or subcutaneous heparin. Although the bromocriptine strategy has already been adopted for routine treatment of PPCM in Germany and in many other parts of the world, its uptake in the United States has lagged, largely because of concerns about thrombotic complications, noted Dr. Sliwa, professor of medicine and director of the Hatter Institute for Cardiovascular Research in Africa at the University of Cape Town, South Africa.
Among all 57 women available for a follow-up echocardiography assessment 6 months after the start of treatment, roughly 60% had a left ventricular ejection fraction of 50% or greater, and more than 20% achieved an ejection fraction of 35%-49%. The remaining roughly 18% of women either did not have a 6-month follow-up or failed to reach at least a 35% ejection fraction at 6 months.
PPCM can be a diagnostic challenge, said Dr. Hilfiker-Kleiner, but it is relatively common, with an average worldwide incidence of about 1 case for every 1,000 deliveries. The incidence may be even higher with many cases going undetected, often because the clinical signs of PPCM, including fatigue, difficulty sleeping, edema, and dyspnea, can be dismissed as the results of recent pregnancy or caring for a newborn baby. Certain racial or ethnic groups appear to have an increased incidence of the disease, including African and Hispanic women, likely because of genetic factors, said Dr. Sliwa. Clinical factors that boost risk include pre-eclampsia, smoking, obesity, older age, and multiparity, but not diabetes.
Testing for N-terminal-pro B-type natriuretic peptide levels appears to be a good screen for women who have developed PPCM, with a level of at least 500 pg/mL high enough to warrant further assessment, Dr. Sliwa said. She recommended running an NT-proBNP test on any recent postpartum women with a clinical or demographic risk factor or suggestive clinical presentation, but she also stressed that PPCM can occur in younger, totally healthy, and athletic women who appear to have a normal delivery.
A significant concern about bromocriptine treatment is that it precludes breastfeeding, a reason not to use the drug in women with an ejection fraction of 40% or greater, especially in settings where access to safe baby formula is a challenge.
The PPCM trial enrolled 63 women at 12 German centers.
The trial received no commercial funding. Dr. Hilfiker-Kleiner and Dr. Sliwa had no disclosures.
[email protected]
On Twitter @mitchelzoler
This is a very important trial that was extremely difficult to conduct, and the results are exciting. It represents an effective bedside to bench to bedside sequence of research. The problem of peripartum cardiomyopathy was recognized in clinical practice, understood through basic research that led to a potential treatment, and the treatment is now confirmed through clinical testing. The results provide a reason for hope for the women who develop this disease.
These trial results are important for all mothers, for all women, and for anyone born from a woman.
Mariell Jessup, MD, is a heart failure specialist and chief scientific officer of the Leducq organization in Boston. She had no disclosures. She made these comments as designated discussant for the report by Dr. Hilfiker-Kleiner.
This is a very important trial that was extremely difficult to conduct, and the results are exciting. It represents an effective bedside to bench to bedside sequence of research. The problem of peripartum cardiomyopathy was recognized in clinical practice, understood through basic research that led to a potential treatment, and the treatment is now confirmed through clinical testing. The results provide a reason for hope for the women who develop this disease.
These trial results are important for all mothers, for all women, and for anyone born from a woman.
Mariell Jessup, MD, is a heart failure specialist and chief scientific officer of the Leducq organization in Boston. She had no disclosures. She made these comments as designated discussant for the report by Dr. Hilfiker-Kleiner.
This is a very important trial that was extremely difficult to conduct, and the results are exciting. It represents an effective bedside to bench to bedside sequence of research. The problem of peripartum cardiomyopathy was recognized in clinical practice, understood through basic research that led to a potential treatment, and the treatment is now confirmed through clinical testing. The results provide a reason for hope for the women who develop this disease.
These trial results are important for all mothers, for all women, and for anyone born from a woman.
Mariell Jessup, MD, is a heart failure specialist and chief scientific officer of the Leducq organization in Boston. She had no disclosures. She made these comments as designated discussant for the report by Dr. Hilfiker-Kleiner.
PARIS – Two regimens of bromocriptine treatment administered with anticoagulation and standard heart failure management were safe, and often effective, for normalizing ejection fraction levels in women diagnosed with peripartum cardiomyopathy in a study with 63 women and designed to be the pivotal trial for this management strategy.
“Bromocriptine therapy applied for 1 week seems sufficient to promote healing from PPCM [peripartum cardiomyopathy] in most patients, although critically ill patients may profit from prolonged [8 week] treatment,” Denise Hilfiker-Kleiner, PhD, said at a meeting of the Heart Failure Association of the ESC.
Women who are suspected of having PPCM but with less compromised ventricular output, with an ejection fraction of 40%-45%, should be closely followed with repeated clinical examinations for 6 months and echocardiography examinations at 3 and 6 months to see if their cardiac output worsens enough to justify initiating a bromocriptine regimen, she advised. “We don’t recommend that every woman with an postpartum ejection fraction of 45% needs to immediately stop lactation [with bromocriptine treatment], but she should be frequently seen by a cardiologist to see whether she recovers or deteriorates further.”
The PPCM (Effect of Bromocriptine on Left Ventricular Function in Women With Peripartum Cardiomyopathy) trial enrolled 63 women with PPCM and severely depressed left ventricular ejection fraction at 12 German centers. Randomization placed 32 women into a group assigned to received 1 week of bromocriptine treatment, with 26 completing the study, and 31 in a group treated with bromocriptine for 8 weeks, with all 31 completing the study. The patients averaged 34 years of age. All patients also received standard heart failure treatment.
The study’s primary endpoint, the change in left ventricular ejection fraction from baseline to 6-month follow-up, was similar in the two treatment groups, with the 1-week regimen leading to an average 21% improvement in ejection fraction and the 8-week regimen averaging a 24% gain in pump function. Among a subgroup of 37 women who entered the study with a left ventricular ejection fraction of less than 30%, slightly more than 60% achieved full heart function by 6-month follow-up with an ejection fraction of 50% or greater, and an additional 35% had partial recovery, with a follow-up ejection fraction of 35%-49%, Dr. Hilfiker-Kleiner reported.
No women in the study developed a thrombotic complication, a potential danger of the bromocriptine intervention. All participants received antithrombotic prophylaxis with either warfarin or subcutaneous heparin. Although the bromocriptine strategy has already been adopted for routine treatment of PPCM in Germany and in many other parts of the world, its uptake in the United States has lagged, largely because of concerns about thrombotic complications, noted Dr. Sliwa, professor of medicine and director of the Hatter Institute for Cardiovascular Research in Africa at the University of Cape Town, South Africa.
Among all 57 women available for a follow-up echocardiography assessment 6 months after the start of treatment, roughly 60% had a left ventricular ejection fraction of 50% or greater, and more than 20% achieved an ejection fraction of 35%-49%. The remaining roughly 18% of women either did not have a 6-month follow-up or failed to reach at least a 35% ejection fraction at 6 months.
PPCM can be a diagnostic challenge, said Dr. Hilfiker-Kleiner, but it is relatively common, with an average worldwide incidence of about 1 case for every 1,000 deliveries. The incidence may be even higher with many cases going undetected, often because the clinical signs of PPCM, including fatigue, difficulty sleeping, edema, and dyspnea, can be dismissed as the results of recent pregnancy or caring for a newborn baby. Certain racial or ethnic groups appear to have an increased incidence of the disease, including African and Hispanic women, likely because of genetic factors, said Dr. Sliwa. Clinical factors that boost risk include pre-eclampsia, smoking, obesity, older age, and multiparity, but not diabetes.
Testing for N-terminal-pro B-type natriuretic peptide levels appears to be a good screen for women who have developed PPCM, with a level of at least 500 pg/mL high enough to warrant further assessment, Dr. Sliwa said. She recommended running an NT-proBNP test on any recent postpartum women with a clinical or demographic risk factor or suggestive clinical presentation, but she also stressed that PPCM can occur in younger, totally healthy, and athletic women who appear to have a normal delivery.
A significant concern about bromocriptine treatment is that it precludes breastfeeding, a reason not to use the drug in women with an ejection fraction of 40% or greater, especially in settings where access to safe baby formula is a challenge.
The PPCM trial enrolled 63 women at 12 German centers.
The trial received no commercial funding. Dr. Hilfiker-Kleiner and Dr. Sliwa had no disclosures.
[email protected]
On Twitter @mitchelzoler
PARIS – Two regimens of bromocriptine treatment administered with anticoagulation and standard heart failure management were safe, and often effective, for normalizing ejection fraction levels in women diagnosed with peripartum cardiomyopathy in a study with 63 women and designed to be the pivotal trial for this management strategy.
“Bromocriptine therapy applied for 1 week seems sufficient to promote healing from PPCM [peripartum cardiomyopathy] in most patients, although critically ill patients may profit from prolonged [8 week] treatment,” Denise Hilfiker-Kleiner, PhD, said at a meeting of the Heart Failure Association of the ESC.
Women who are suspected of having PPCM but with less compromised ventricular output, with an ejection fraction of 40%-45%, should be closely followed with repeated clinical examinations for 6 months and echocardiography examinations at 3 and 6 months to see if their cardiac output worsens enough to justify initiating a bromocriptine regimen, she advised. “We don’t recommend that every woman with an postpartum ejection fraction of 45% needs to immediately stop lactation [with bromocriptine treatment], but she should be frequently seen by a cardiologist to see whether she recovers or deteriorates further.”
The PPCM (Effect of Bromocriptine on Left Ventricular Function in Women With Peripartum Cardiomyopathy) trial enrolled 63 women with PPCM and severely depressed left ventricular ejection fraction at 12 German centers. Randomization placed 32 women into a group assigned to received 1 week of bromocriptine treatment, with 26 completing the study, and 31 in a group treated with bromocriptine for 8 weeks, with all 31 completing the study. The patients averaged 34 years of age. All patients also received standard heart failure treatment.
The study’s primary endpoint, the change in left ventricular ejection fraction from baseline to 6-month follow-up, was similar in the two treatment groups, with the 1-week regimen leading to an average 21% improvement in ejection fraction and the 8-week regimen averaging a 24% gain in pump function. Among a subgroup of 37 women who entered the study with a left ventricular ejection fraction of less than 30%, slightly more than 60% achieved full heart function by 6-month follow-up with an ejection fraction of 50% or greater, and an additional 35% had partial recovery, with a follow-up ejection fraction of 35%-49%, Dr. Hilfiker-Kleiner reported.
No women in the study developed a thrombotic complication, a potential danger of the bromocriptine intervention. All participants received antithrombotic prophylaxis with either warfarin or subcutaneous heparin. Although the bromocriptine strategy has already been adopted for routine treatment of PPCM in Germany and in many other parts of the world, its uptake in the United States has lagged, largely because of concerns about thrombotic complications, noted Dr. Sliwa, professor of medicine and director of the Hatter Institute for Cardiovascular Research in Africa at the University of Cape Town, South Africa.
Among all 57 women available for a follow-up echocardiography assessment 6 months after the start of treatment, roughly 60% had a left ventricular ejection fraction of 50% or greater, and more than 20% achieved an ejection fraction of 35%-49%. The remaining roughly 18% of women either did not have a 6-month follow-up or failed to reach at least a 35% ejection fraction at 6 months.
PPCM can be a diagnostic challenge, said Dr. Hilfiker-Kleiner, but it is relatively common, with an average worldwide incidence of about 1 case for every 1,000 deliveries. The incidence may be even higher with many cases going undetected, often because the clinical signs of PPCM, including fatigue, difficulty sleeping, edema, and dyspnea, can be dismissed as the results of recent pregnancy or caring for a newborn baby. Certain racial or ethnic groups appear to have an increased incidence of the disease, including African and Hispanic women, likely because of genetic factors, said Dr. Sliwa. Clinical factors that boost risk include pre-eclampsia, smoking, obesity, older age, and multiparity, but not diabetes.
Testing for N-terminal-pro B-type natriuretic peptide levels appears to be a good screen for women who have developed PPCM, with a level of at least 500 pg/mL high enough to warrant further assessment, Dr. Sliwa said. She recommended running an NT-proBNP test on any recent postpartum women with a clinical or demographic risk factor or suggestive clinical presentation, but she also stressed that PPCM can occur in younger, totally healthy, and athletic women who appear to have a normal delivery.
A significant concern about bromocriptine treatment is that it precludes breastfeeding, a reason not to use the drug in women with an ejection fraction of 40% or greater, especially in settings where access to safe baby formula is a challenge.
The PPCM trial enrolled 63 women at 12 German centers.
The trial received no commercial funding. Dr. Hilfiker-Kleiner and Dr. Sliwa had no disclosures.
[email protected]
On Twitter @mitchelzoler
AT HEART FAILURE 2017
Key clinical point:
Major finding: At 6-month follow-up, more than 80% of patients had full or partial restoration of their left ventricular function.
Data source: The PPCM trial, which enrolled 63 women at 12 German centers.
Disclosures: The trial received no commercial funding. Dr. Hilfiker-Kleiner and Dr. Sliwa had no disclosures.
Hospitalists get hands-on training at POC ultrasound pre-course
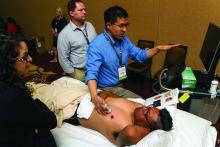
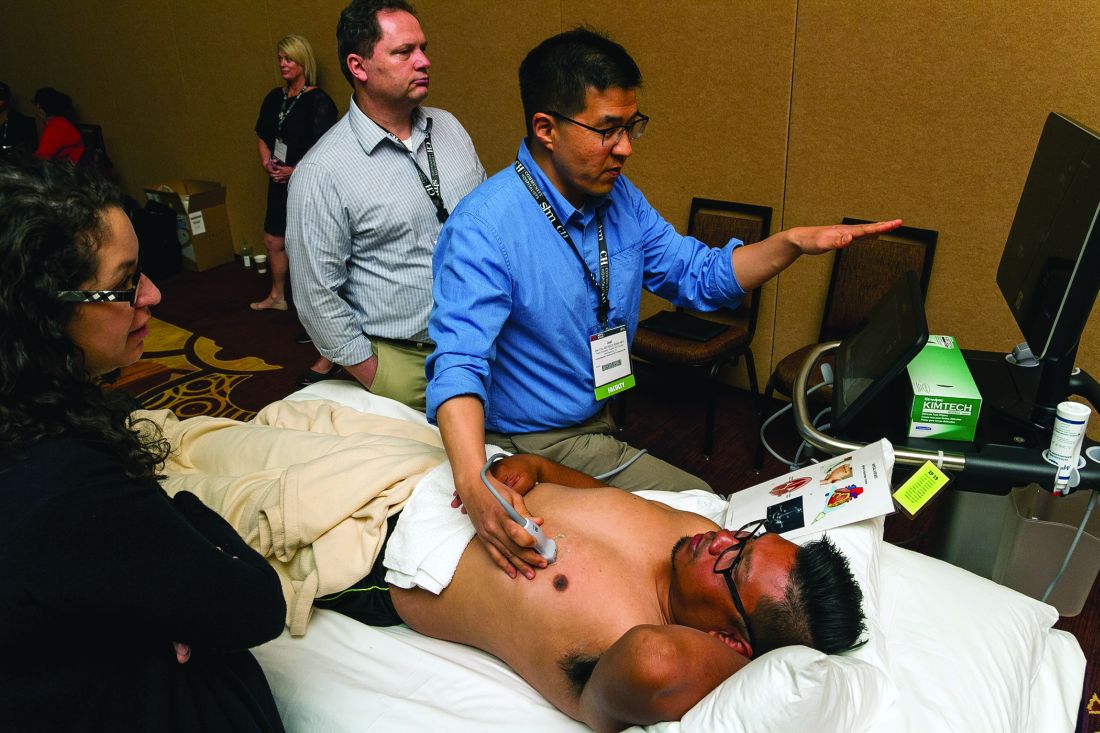
Hospitalists participated in a double-header of hands-on point-of-care ultrasound training here on Monday, looking to gain an edge in expertise in a role that’s becoming more and more common.
Nearly 100 hospitalists and other health care professionals heard talks on the fundamental principles of ultrasound and cardiac, lung and vascular, and abdominal ultrasound. The highlights of the sessions were two 80-minute hands-on segments using the probes.
“This course has grown and grown – this is the largest we’ve ever done,” said pre-course director Nilam Soni, MD, MS, FHM, associate professor of medicine at the University of Texas Health Science Center San Antonio.
A morning and afternoon session were held, each attended by 48 registrants. Because of high demand, the society added 12 spots to each session – and there was still a wait list, said Ricardo Franco-Sadud, MD, the other director of the course and associate professor of medicine at the Medical College of Wisconsin, Milwaukee.
“The idea is to give you the most amount of time with the probe in their hand,” Dr. Franco said.
In one of the hands-on sessions, Adam Merando, MD, a hospitalist and associate program director of the internal medicine residency program at Saint Louis University, slid and rocked the probe on the stomach of a volunteer as the picture came into view.
“Now we’re getting an image,” his bedside instructor, Brandon Boesch, DO, a hospitalist at Highland Hospital in Oakland, Calif., told him. Dr. Merando had found the liver.
He eventually found the main target, the inferior vena cava, and assessed its diameter in relation to the breathing of the “patient.” This information is used to gauge how responsive acute circulatory failure patients are to fluid therapy.
At one point, with another learner, the image shifted.
“You see how it feels like your hand is not moving, but the image is changing?” Dr. Boesch said. “That’s part of the fine motor skill.”
Kirk Spencer, MD, professor of medicine and a cardiologist at the University of Chicago and perennial participant in the course, said it’s a great way for hospitalists who were hesitant about learning ultrasound to get over the hump.
Benji Mathews, MD, assistant professor of medicine at the University of Minnesota, Minneapolis, another bedside instructor, said the enthusiasm about the course is well founded.
“This is one of the few technologies that brings you back to the bedside.”
Hospitalists participated in a double-header of hands-on point-of-care ultrasound training here on Monday, looking to gain an edge in expertise in a role that’s becoming more and more common.
Nearly 100 hospitalists and other health care professionals heard talks on the fundamental principles of ultrasound and cardiac, lung and vascular, and abdominal ultrasound. The highlights of the sessions were two 80-minute hands-on segments using the probes.
“This course has grown and grown – this is the largest we’ve ever done,” said pre-course director Nilam Soni, MD, MS, FHM, associate professor of medicine at the University of Texas Health Science Center San Antonio.
A morning and afternoon session were held, each attended by 48 registrants. Because of high demand, the society added 12 spots to each session – and there was still a wait list, said Ricardo Franco-Sadud, MD, the other director of the course and associate professor of medicine at the Medical College of Wisconsin, Milwaukee.
“The idea is to give you the most amount of time with the probe in their hand,” Dr. Franco said.
In one of the hands-on sessions, Adam Merando, MD, a hospitalist and associate program director of the internal medicine residency program at Saint Louis University, slid and rocked the probe on the stomach of a volunteer as the picture came into view.
“Now we’re getting an image,” his bedside instructor, Brandon Boesch, DO, a hospitalist at Highland Hospital in Oakland, Calif., told him. Dr. Merando had found the liver.
He eventually found the main target, the inferior vena cava, and assessed its diameter in relation to the breathing of the “patient.” This information is used to gauge how responsive acute circulatory failure patients are to fluid therapy.
At one point, with another learner, the image shifted.
“You see how it feels like your hand is not moving, but the image is changing?” Dr. Boesch said. “That’s part of the fine motor skill.”
Kirk Spencer, MD, professor of medicine and a cardiologist at the University of Chicago and perennial participant in the course, said it’s a great way for hospitalists who were hesitant about learning ultrasound to get over the hump.
Benji Mathews, MD, assistant professor of medicine at the University of Minnesota, Minneapolis, another bedside instructor, said the enthusiasm about the course is well founded.
“This is one of the few technologies that brings you back to the bedside.”
Hospitalists participated in a double-header of hands-on point-of-care ultrasound training here on Monday, looking to gain an edge in expertise in a role that’s becoming more and more common.
Nearly 100 hospitalists and other health care professionals heard talks on the fundamental principles of ultrasound and cardiac, lung and vascular, and abdominal ultrasound. The highlights of the sessions were two 80-minute hands-on segments using the probes.
“This course has grown and grown – this is the largest we’ve ever done,” said pre-course director Nilam Soni, MD, MS, FHM, associate professor of medicine at the University of Texas Health Science Center San Antonio.
A morning and afternoon session were held, each attended by 48 registrants. Because of high demand, the society added 12 spots to each session – and there was still a wait list, said Ricardo Franco-Sadud, MD, the other director of the course and associate professor of medicine at the Medical College of Wisconsin, Milwaukee.
“The idea is to give you the most amount of time with the probe in their hand,” Dr. Franco said.
In one of the hands-on sessions, Adam Merando, MD, a hospitalist and associate program director of the internal medicine residency program at Saint Louis University, slid and rocked the probe on the stomach of a volunteer as the picture came into view.
“Now we’re getting an image,” his bedside instructor, Brandon Boesch, DO, a hospitalist at Highland Hospital in Oakland, Calif., told him. Dr. Merando had found the liver.
He eventually found the main target, the inferior vena cava, and assessed its diameter in relation to the breathing of the “patient.” This information is used to gauge how responsive acute circulatory failure patients are to fluid therapy.
At one point, with another learner, the image shifted.
“You see how it feels like your hand is not moving, but the image is changing?” Dr. Boesch said. “That’s part of the fine motor skill.”
Kirk Spencer, MD, professor of medicine and a cardiologist at the University of Chicago and perennial participant in the course, said it’s a great way for hospitalists who were hesitant about learning ultrasound to get over the hump.
Benji Mathews, MD, assistant professor of medicine at the University of Minnesota, Minneapolis, another bedside instructor, said the enthusiasm about the course is well founded.
“This is one of the few technologies that brings you back to the bedside.”
What’s Eating You? Cheyletiella Mites
Identifying Characteristics and Disease Transmission
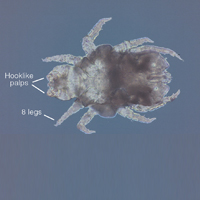
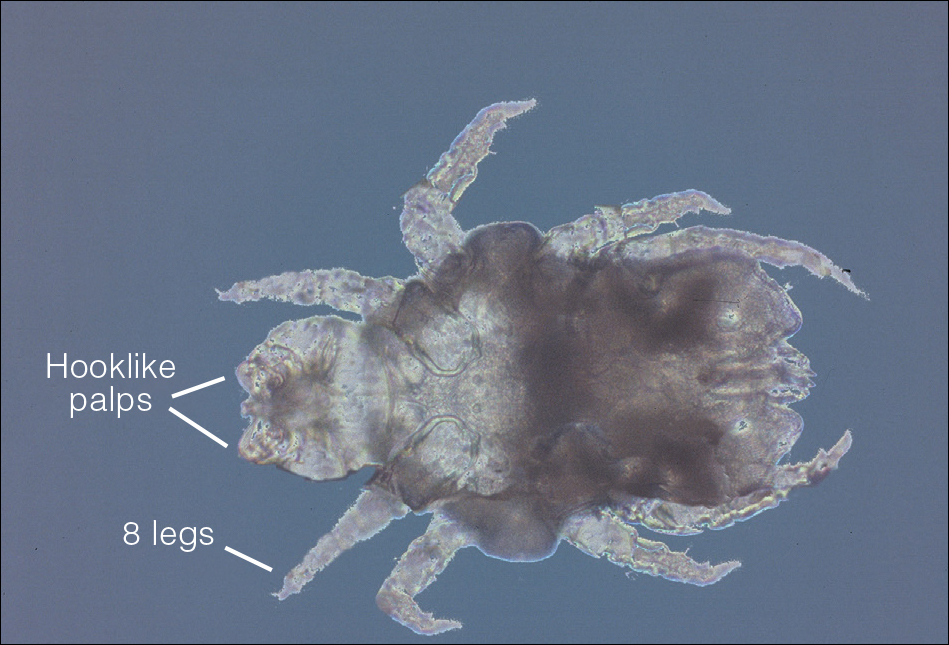
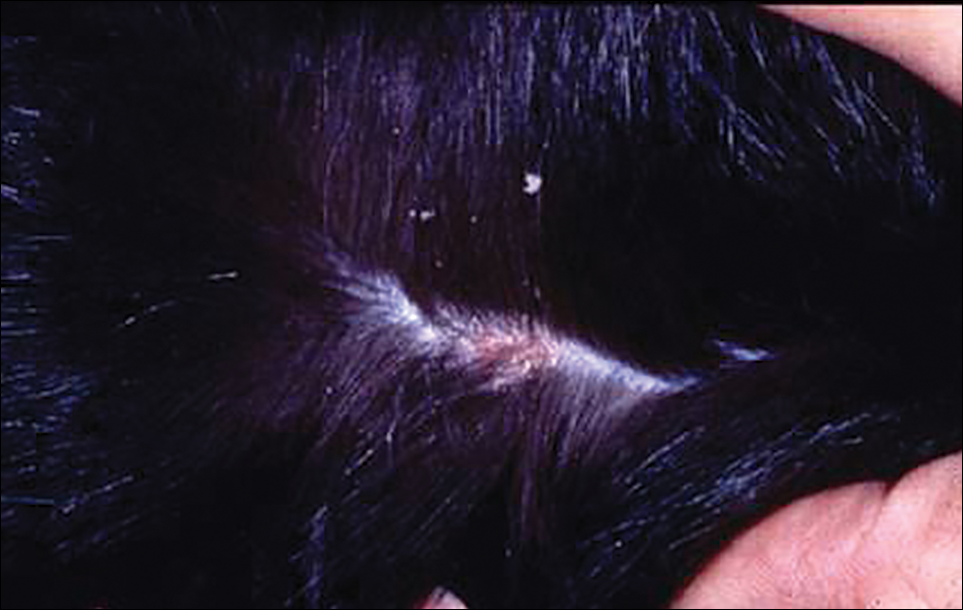
Cheyletiella are nonburrowing mites characterized by hooklike anterior palps (Figure 1) that have a worldwide distribution. Human dermatitis is the result of contact with an affected animal and may present as papular or bullous lesions. Cheyletiella blakei affects cats, Cheyletiella parasitovorax is found on rabbits, and Cheyletiella yasguri is found on dogs. The mites live in the outer layer of the epidermis of the host animal and feed on surface debris and tissue fluids.1 They complete an entire 35-day life cycle on a single animal host. The larval, nymph, and adult male mites die within 48 hours of separation from a host. The female mite and possibly the eggs can live up to 10 days off the host, which makes environmental decontamination a critical part of pest control.2 In animals, the mite often produces a subtle dermatitis sometimes called walking dandruff (Figure 2).3 Affected animals also can be asymptomatic, and up to 50% of rabbits in commercial colonies may harbor Cheyletiella or other mites.4
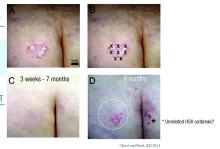
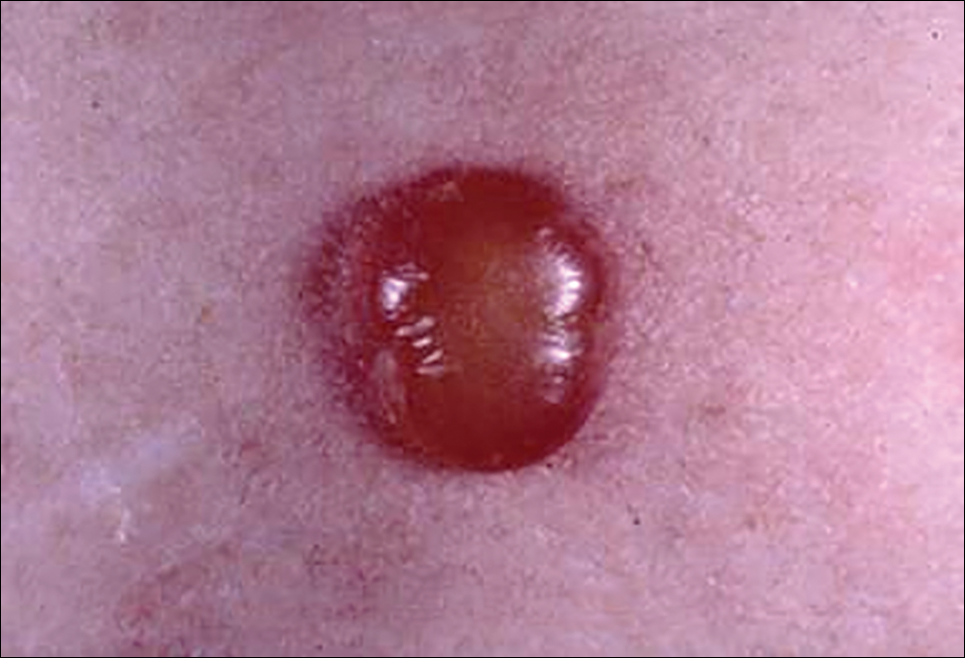
The typical human patient with Cheyletiella-associated dermatitis is a female 40 years or younger who presents with grouped pruritic papules.5 Although papules usually are grouped on exposed areas, they also may be widespread.6,7 Bullous eruptions caused by Cheyletiella mites may mimic those found in immunobullous diseases (Figure 3).8 Children may experience widespread dermatitis after taking a nap where a dog has slept.9 Pet owners, farmers, and veterinarians frequently present with zoonotic mite-induced dermatitis.10 Arthralgia and peripheral eosinophilia caused by Cheyletiella infestation also has been reported.11
Management of Affected Pets
In a case of human infestation resulting from an affected pet, the implicated pet should be evaluated by a qualified veterinarian. Various diagnostic techniques for animals have been used, including adhesive tape preparations.12 A rapid knockdown insecticidal spray marketed for use on animals has been used to facilitate collection of mites, but some pets may be susceptible to toxicity from insecticides. The scaly area should be carefully brushed with a toothbrush or fine-tooth comb, and all scales, crust, and hair collected should be placed in a resealable plastic storage bag. When alcohol is added to the bag, most contents will sink, but the mites tend to float. Vacuum cleaners fitted with in-line filters also have been used to collect mites. The filter samples can be treated with hot potassium hydroxide, then floated in a concentrated sugar solution to collect the ectoparasites.13 Often, a straightforward approach using a #10 blade to provide a skin scraping from the animal in question is effective.14
Various treatment modalities may be employed by the veterinarian, including dips or shampoos, as well as fipronil.15,16 A single application of fipronil 10% has been shown to be highly effective in the elimination of mites after a single application in cats.17 Oral ivermectin and topical amitraz also have been used.18,19 A veterinarian should treat the animals, as some are more susceptible to toxicity from topical or systemic agents.
Treatment in Humans
Cheyletiella infestations in humans usually are self-limited and resolve within a few weeks after treatment of the source animal. Symptomatic treatment with antipruritic medications and topical steroids may be of use while awaiting resolution. Identification and treatment of the vector is key to eliminating the infestation and preventing recurrence.
- Angarano DW, Parish LC. Comparative dermatology: parasitic disorders. Clin Dermatol. 1994;12:543-550.
- Kunkle GA, Miller WH Jr. Cheyletiella infestation in humans. Arch Dermatol. 1980;116:1345.
- Rivers JK, Martin J, Pukay B. Walking dandruff and Cheyletiella dermatitis. J Am Acad Dermatol. 1986;15:1130-1133.
- Flatt RE, Wiemers J. A survey of fur mites in domestic rabbits. Lab Animal Sci. 1976;26:758-761.
- Lee BW. Cheyletiella dermatitis: a report of fourteen cases. Cutis. 1991;47:111-114.
- Cohen SR. Cheyletiella dermatitis. A mite infestation of rabbit, cat, dog and man. Arch Dermatol. 1980;116:435-437.
- Bradrup F, Andersen KE, Kristensen S. Infection in man and dog with the mite, Cheyletiella yasguri Smiley [in German]. Hautarzt. 1979;30:497-500.
- Cvancara JL, Elston DM. Bullous eruption in a patient with systemic lupus erythematosus: mite dermatitis caused by Cheyletiella blakei. J Am Acad Dermatol. 1997;37:265-267.
- Shelley ED, Shelley WB, Pula JF, et al. The diagnostic challenge of nonburrowing mite bites. Cheyletiella yasguri. JAMA. 1984;251:2690-2691.
- Beck W. Farm animals as disease vectors of parasitic epizoonoses and zoophilic dermatophytes and their importance in dermatology [in German]. Hautartz. 1999;50:621-628.
- Dobrosavljevic DD, Popovic ND, Radovanovic SS. Systemic manifestations of Cheyletiella infestation in man. Int J Dermatol. 2007;46:397-399.
- Ottenschot TR, Gil D. Cheyletiellosis in long-haired cats. Tijdschr Diergeneeskd. 1978;103:1104-1108.
- Klayman E, Schillhorn van Veen TW. Diagnosis of ectoparasitism. Mod Vet Pract. 1981;62:767-771.
- Milley C, Dryden M, Rosenkrantz W, et al. Comparison of parasitic mite retrieval methods in a population of community cats [published online Jun 3, 2016]. J Feline Med Surg. pii:1098612X16650717.
- McKeever PJ, Allen SK. Dermatitis associated with Cheyletiella infestation in cats. J Am Vet Med Assoc. 1979;174:718-720.
- Chadwick AJ. Use of a 0.25 per cent fipronil pump spray formulation to treat canine cheyletiellosis. J Small Anim Pract. 1997;38:261-262.
- Scarampella F, Pollmeier M, Visser M, et al. Efficacy of fipronil in the treatment of feline cheyletiellosis. Vet Parasitol. 2005;129:333-339.
- Folz SD, Kakuk TJ, Henke CL, et al. Clinical evaluation of amitraz for treatment of canine scabies. Mod Vet Pract. 1984;65:597-600.
- Dourmishev AL, Dourmishev LA, Schwartz RA. Ivermectin: pharmacology and application in dermatology. Int J Dermatol. 2005;44:981-988.
Identifying Characteristics and Disease Transmission
Cheyletiella are nonburrowing mites characterized by hooklike anterior palps (Figure 1) that have a worldwide distribution. Human dermatitis is the result of contact with an affected animal and may present as papular or bullous lesions. Cheyletiella blakei affects cats, Cheyletiella parasitovorax is found on rabbits, and Cheyletiella yasguri is found on dogs. The mites live in the outer layer of the epidermis of the host animal and feed on surface debris and tissue fluids.1 They complete an entire 35-day life cycle on a single animal host. The larval, nymph, and adult male mites die within 48 hours of separation from a host. The female mite and possibly the eggs can live up to 10 days off the host, which makes environmental decontamination a critical part of pest control.2 In animals, the mite often produces a subtle dermatitis sometimes called walking dandruff (Figure 2).3 Affected animals also can be asymptomatic, and up to 50% of rabbits in commercial colonies may harbor Cheyletiella or other mites.4


The typical human patient with Cheyletiella-associated dermatitis is a female 40 years or younger who presents with grouped pruritic papules.5 Although papules usually are grouped on exposed areas, they also may be widespread.6,7 Bullous eruptions caused by Cheyletiella mites may mimic those found in immunobullous diseases (Figure 3).8 Children may experience widespread dermatitis after taking a nap where a dog has slept.9 Pet owners, farmers, and veterinarians frequently present with zoonotic mite-induced dermatitis.10 Arthralgia and peripheral eosinophilia caused by Cheyletiella infestation also has been reported.11

Management of Affected Pets
In a case of human infestation resulting from an affected pet, the implicated pet should be evaluated by a qualified veterinarian. Various diagnostic techniques for animals have been used, including adhesive tape preparations.12 A rapid knockdown insecticidal spray marketed for use on animals has been used to facilitate collection of mites, but some pets may be susceptible to toxicity from insecticides. The scaly area should be carefully brushed with a toothbrush or fine-tooth comb, and all scales, crust, and hair collected should be placed in a resealable plastic storage bag. When alcohol is added to the bag, most contents will sink, but the mites tend to float. Vacuum cleaners fitted with in-line filters also have been used to collect mites. The filter samples can be treated with hot potassium hydroxide, then floated in a concentrated sugar solution to collect the ectoparasites.13 Often, a straightforward approach using a #10 blade to provide a skin scraping from the animal in question is effective.14
Various treatment modalities may be employed by the veterinarian, including dips or shampoos, as well as fipronil.15,16 A single application of fipronil 10% has been shown to be highly effective in the elimination of mites after a single application in cats.17 Oral ivermectin and topical amitraz also have been used.18,19 A veterinarian should treat the animals, as some are more susceptible to toxicity from topical or systemic agents.
Treatment in Humans
Cheyletiella infestations in humans usually are self-limited and resolve within a few weeks after treatment of the source animal. Symptomatic treatment with antipruritic medications and topical steroids may be of use while awaiting resolution. Identification and treatment of the vector is key to eliminating the infestation and preventing recurrence.
Identifying Characteristics and Disease Transmission
Cheyletiella are nonburrowing mites characterized by hooklike anterior palps (Figure 1) that have a worldwide distribution. Human dermatitis is the result of contact with an affected animal and may present as papular or bullous lesions. Cheyletiella blakei affects cats, Cheyletiella parasitovorax is found on rabbits, and Cheyletiella yasguri is found on dogs. The mites live in the outer layer of the epidermis of the host animal and feed on surface debris and tissue fluids.1 They complete an entire 35-day life cycle on a single animal host. The larval, nymph, and adult male mites die within 48 hours of separation from a host. The female mite and possibly the eggs can live up to 10 days off the host, which makes environmental decontamination a critical part of pest control.2 In animals, the mite often produces a subtle dermatitis sometimes called walking dandruff (Figure 2).3 Affected animals also can be asymptomatic, and up to 50% of rabbits in commercial colonies may harbor Cheyletiella or other mites.4


The typical human patient with Cheyletiella-associated dermatitis is a female 40 years or younger who presents with grouped pruritic papules.5 Although papules usually are grouped on exposed areas, they also may be widespread.6,7 Bullous eruptions caused by Cheyletiella mites may mimic those found in immunobullous diseases (Figure 3).8 Children may experience widespread dermatitis after taking a nap where a dog has slept.9 Pet owners, farmers, and veterinarians frequently present with zoonotic mite-induced dermatitis.10 Arthralgia and peripheral eosinophilia caused by Cheyletiella infestation also has been reported.11

Management of Affected Pets
In a case of human infestation resulting from an affected pet, the implicated pet should be evaluated by a qualified veterinarian. Various diagnostic techniques for animals have been used, including adhesive tape preparations.12 A rapid knockdown insecticidal spray marketed for use on animals has been used to facilitate collection of mites, but some pets may be susceptible to toxicity from insecticides. The scaly area should be carefully brushed with a toothbrush or fine-tooth comb, and all scales, crust, and hair collected should be placed in a resealable plastic storage bag. When alcohol is added to the bag, most contents will sink, but the mites tend to float. Vacuum cleaners fitted with in-line filters also have been used to collect mites. The filter samples can be treated with hot potassium hydroxide, then floated in a concentrated sugar solution to collect the ectoparasites.13 Often, a straightforward approach using a #10 blade to provide a skin scraping from the animal in question is effective.14
Various treatment modalities may be employed by the veterinarian, including dips or shampoos, as well as fipronil.15,16 A single application of fipronil 10% has been shown to be highly effective in the elimination of mites after a single application in cats.17 Oral ivermectin and topical amitraz also have been used.18,19 A veterinarian should treat the animals, as some are more susceptible to toxicity from topical or systemic agents.
Treatment in Humans
Cheyletiella infestations in humans usually are self-limited and resolve within a few weeks after treatment of the source animal. Symptomatic treatment with antipruritic medications and topical steroids may be of use while awaiting resolution. Identification and treatment of the vector is key to eliminating the infestation and preventing recurrence.
- Angarano DW, Parish LC. Comparative dermatology: parasitic disorders. Clin Dermatol. 1994;12:543-550.
- Kunkle GA, Miller WH Jr. Cheyletiella infestation in humans. Arch Dermatol. 1980;116:1345.
- Rivers JK, Martin J, Pukay B. Walking dandruff and Cheyletiella dermatitis. J Am Acad Dermatol. 1986;15:1130-1133.
- Flatt RE, Wiemers J. A survey of fur mites in domestic rabbits. Lab Animal Sci. 1976;26:758-761.
- Lee BW. Cheyletiella dermatitis: a report of fourteen cases. Cutis. 1991;47:111-114.
- Cohen SR. Cheyletiella dermatitis. A mite infestation of rabbit, cat, dog and man. Arch Dermatol. 1980;116:435-437.
- Bradrup F, Andersen KE, Kristensen S. Infection in man and dog with the mite, Cheyletiella yasguri Smiley [in German]. Hautarzt. 1979;30:497-500.
- Cvancara JL, Elston DM. Bullous eruption in a patient with systemic lupus erythematosus: mite dermatitis caused by Cheyletiella blakei. J Am Acad Dermatol. 1997;37:265-267.
- Shelley ED, Shelley WB, Pula JF, et al. The diagnostic challenge of nonburrowing mite bites. Cheyletiella yasguri. JAMA. 1984;251:2690-2691.
- Beck W. Farm animals as disease vectors of parasitic epizoonoses and zoophilic dermatophytes and their importance in dermatology [in German]. Hautartz. 1999;50:621-628.
- Dobrosavljevic DD, Popovic ND, Radovanovic SS. Systemic manifestations of Cheyletiella infestation in man. Int J Dermatol. 2007;46:397-399.
- Ottenschot TR, Gil D. Cheyletiellosis in long-haired cats. Tijdschr Diergeneeskd. 1978;103:1104-1108.
- Klayman E, Schillhorn van Veen TW. Diagnosis of ectoparasitism. Mod Vet Pract. 1981;62:767-771.
- Milley C, Dryden M, Rosenkrantz W, et al. Comparison of parasitic mite retrieval methods in a population of community cats [published online Jun 3, 2016]. J Feline Med Surg. pii:1098612X16650717.
- McKeever PJ, Allen SK. Dermatitis associated with Cheyletiella infestation in cats. J Am Vet Med Assoc. 1979;174:718-720.
- Chadwick AJ. Use of a 0.25 per cent fipronil pump spray formulation to treat canine cheyletiellosis. J Small Anim Pract. 1997;38:261-262.
- Scarampella F, Pollmeier M, Visser M, et al. Efficacy of fipronil in the treatment of feline cheyletiellosis. Vet Parasitol. 2005;129:333-339.
- Folz SD, Kakuk TJ, Henke CL, et al. Clinical evaluation of amitraz for treatment of canine scabies. Mod Vet Pract. 1984;65:597-600.
- Dourmishev AL, Dourmishev LA, Schwartz RA. Ivermectin: pharmacology and application in dermatology. Int J Dermatol. 2005;44:981-988.
- Angarano DW, Parish LC. Comparative dermatology: parasitic disorders. Clin Dermatol. 1994;12:543-550.
- Kunkle GA, Miller WH Jr. Cheyletiella infestation in humans. Arch Dermatol. 1980;116:1345.
- Rivers JK, Martin J, Pukay B. Walking dandruff and Cheyletiella dermatitis. J Am Acad Dermatol. 1986;15:1130-1133.
- Flatt RE, Wiemers J. A survey of fur mites in domestic rabbits. Lab Animal Sci. 1976;26:758-761.
- Lee BW. Cheyletiella dermatitis: a report of fourteen cases. Cutis. 1991;47:111-114.
- Cohen SR. Cheyletiella dermatitis. A mite infestation of rabbit, cat, dog and man. Arch Dermatol. 1980;116:435-437.
- Bradrup F, Andersen KE, Kristensen S. Infection in man and dog with the mite, Cheyletiella yasguri Smiley [in German]. Hautarzt. 1979;30:497-500.
- Cvancara JL, Elston DM. Bullous eruption in a patient with systemic lupus erythematosus: mite dermatitis caused by Cheyletiella blakei. J Am Acad Dermatol. 1997;37:265-267.
- Shelley ED, Shelley WB, Pula JF, et al. The diagnostic challenge of nonburrowing mite bites. Cheyletiella yasguri. JAMA. 1984;251:2690-2691.
- Beck W. Farm animals as disease vectors of parasitic epizoonoses and zoophilic dermatophytes and their importance in dermatology [in German]. Hautartz. 1999;50:621-628.
- Dobrosavljevic DD, Popovic ND, Radovanovic SS. Systemic manifestations of Cheyletiella infestation in man. Int J Dermatol. 2007;46:397-399.
- Ottenschot TR, Gil D. Cheyletiellosis in long-haired cats. Tijdschr Diergeneeskd. 1978;103:1104-1108.
- Klayman E, Schillhorn van Veen TW. Diagnosis of ectoparasitism. Mod Vet Pract. 1981;62:767-771.
- Milley C, Dryden M, Rosenkrantz W, et al. Comparison of parasitic mite retrieval methods in a population of community cats [published online Jun 3, 2016]. J Feline Med Surg. pii:1098612X16650717.
- McKeever PJ, Allen SK. Dermatitis associated with Cheyletiella infestation in cats. J Am Vet Med Assoc. 1979;174:718-720.
- Chadwick AJ. Use of a 0.25 per cent fipronil pump spray formulation to treat canine cheyletiellosis. J Small Anim Pract. 1997;38:261-262.
- Scarampella F, Pollmeier M, Visser M, et al. Efficacy of fipronil in the treatment of feline cheyletiellosis. Vet Parasitol. 2005;129:333-339.
- Folz SD, Kakuk TJ, Henke CL, et al. Clinical evaluation of amitraz for treatment of canine scabies. Mod Vet Pract. 1984;65:597-600.
- Dourmishev AL, Dourmishev LA, Schwartz RA. Ivermectin: pharmacology and application in dermatology. Int J Dermatol. 2005;44:981-988.
Practice Points
- Cheyletiella mites can cause a range of cutaneous and systemic symptoms in affected individuals.
- Diagnosis can be difficult and requires a high level of suspicion, with inquiries directed at animal exposures.
- Identification of the animal vector and treatment by a knowledgeable veterinarian is necessary to prevent recurrence in humans.
Living With Psoriasis: How the Disease Impacts the Daily Activities of Patients
Psoriasis impacts the ability to perform activities, causes embarrassment and social discrimination, and leads to a severe emotional impact in both adult and pediatric patients, according to a public meeting hosted by the US Food and Drug Administration (FDA) to hear patient perspectives. A common source of distress in daily life among psoriasis patients was the lack of understanding of the disease in the general population with wrongful concerns that psoriasis is infectious or contagious.
Approximately 70 psoriasis patients or patient representatives attended the meeting in person and others attended through a live webcast. The impact of psoriasis on daily life was underscored throughout the meeting. Daily activities impacted by psoriasis included physical limitations such as an inability to participate in sports among children due to cracking of the hands and feet, or the impracticability of managing a household or going to work among adults. The inconsistency and unpredictability of the condition led patients to be viewed as unreliable. One participant explained, “If you join a team you can play this week but you can’t play next week.”
Patients and their loved ones often experienced embarrassment and social discrimination. A caregiver stated, “Specifically to a child, psoriasis means something different. It means hiding. It means feeling ashamed and it means being ashamed, and it means thinking twice before being yourself. No child should have to think twice before learning to express themselves.” Social isolation and bullying also were prominent in children, mostly because an uniformed parent or classmate did not understand the disease process.
These effects on the daily life of psoriasis patients often led to a severe emotional impact and social isolation. At a young age, psoriasis can have a devastating social and emotional toll. One caregiver shared that his/her child admitted to having thoughts of suicide. The FDA asked how many participants missed days from work and school because of the emotional toll of their psoriasis symptoms and the majority of participants raised their hands. Several participants also indicated that they had sought treatment for depression and anxiety. Many adult patients also noted that they reconsidered having children because of the destructive effects psoriasis has had on multiple generations of family members.
Dermatologists may use these patient insights to monitor the psychological impact of psoriasis on patients and refer them to a psychiatrist or psychologist when needed.
The psoriasis public meeting in March 2016 was the FDA’s 18th patient-focused drug development meeting. The FDA sought this information to have a greater understanding of the burden of psoriasis on patients and the treatments currently used to treat psoriasis and its symptoms. This information will help guide the FDA as they consider future drug approvals.
Psoriasis impacts the ability to perform activities, causes embarrassment and social discrimination, and leads to a severe emotional impact in both adult and pediatric patients, according to a public meeting hosted by the US Food and Drug Administration (FDA) to hear patient perspectives. A common source of distress in daily life among psoriasis patients was the lack of understanding of the disease in the general population with wrongful concerns that psoriasis is infectious or contagious.
Approximately 70 psoriasis patients or patient representatives attended the meeting in person and others attended through a live webcast. The impact of psoriasis on daily life was underscored throughout the meeting. Daily activities impacted by psoriasis included physical limitations such as an inability to participate in sports among children due to cracking of the hands and feet, or the impracticability of managing a household or going to work among adults. The inconsistency and unpredictability of the condition led patients to be viewed as unreliable. One participant explained, “If you join a team you can play this week but you can’t play next week.”
Patients and their loved ones often experienced embarrassment and social discrimination. A caregiver stated, “Specifically to a child, psoriasis means something different. It means hiding. It means feeling ashamed and it means being ashamed, and it means thinking twice before being yourself. No child should have to think twice before learning to express themselves.” Social isolation and bullying also were prominent in children, mostly because an uniformed parent or classmate did not understand the disease process.
These effects on the daily life of psoriasis patients often led to a severe emotional impact and social isolation. At a young age, psoriasis can have a devastating social and emotional toll. One caregiver shared that his/her child admitted to having thoughts of suicide. The FDA asked how many participants missed days from work and school because of the emotional toll of their psoriasis symptoms and the majority of participants raised their hands. Several participants also indicated that they had sought treatment for depression and anxiety. Many adult patients also noted that they reconsidered having children because of the destructive effects psoriasis has had on multiple generations of family members.
Dermatologists may use these patient insights to monitor the psychological impact of psoriasis on patients and refer them to a psychiatrist or psychologist when needed.
The psoriasis public meeting in March 2016 was the FDA’s 18th patient-focused drug development meeting. The FDA sought this information to have a greater understanding of the burden of psoriasis on patients and the treatments currently used to treat psoriasis and its symptoms. This information will help guide the FDA as they consider future drug approvals.
Psoriasis impacts the ability to perform activities, causes embarrassment and social discrimination, and leads to a severe emotional impact in both adult and pediatric patients, according to a public meeting hosted by the US Food and Drug Administration (FDA) to hear patient perspectives. A common source of distress in daily life among psoriasis patients was the lack of understanding of the disease in the general population with wrongful concerns that psoriasis is infectious or contagious.
Approximately 70 psoriasis patients or patient representatives attended the meeting in person and others attended through a live webcast. The impact of psoriasis on daily life was underscored throughout the meeting. Daily activities impacted by psoriasis included physical limitations such as an inability to participate in sports among children due to cracking of the hands and feet, or the impracticability of managing a household or going to work among adults. The inconsistency and unpredictability of the condition led patients to be viewed as unreliable. One participant explained, “If you join a team you can play this week but you can’t play next week.”
Patients and their loved ones often experienced embarrassment and social discrimination. A caregiver stated, “Specifically to a child, psoriasis means something different. It means hiding. It means feeling ashamed and it means being ashamed, and it means thinking twice before being yourself. No child should have to think twice before learning to express themselves.” Social isolation and bullying also were prominent in children, mostly because an uniformed parent or classmate did not understand the disease process.
These effects on the daily life of psoriasis patients often led to a severe emotional impact and social isolation. At a young age, psoriasis can have a devastating social and emotional toll. One caregiver shared that his/her child admitted to having thoughts of suicide. The FDA asked how many participants missed days from work and school because of the emotional toll of their psoriasis symptoms and the majority of participants raised their hands. Several participants also indicated that they had sought treatment for depression and anxiety. Many adult patients also noted that they reconsidered having children because of the destructive effects psoriasis has had on multiple generations of family members.
Dermatologists may use these patient insights to monitor the psychological impact of psoriasis on patients and refer them to a psychiatrist or psychologist when needed.
The psoriasis public meeting in March 2016 was the FDA’s 18th patient-focused drug development meeting. The FDA sought this information to have a greater understanding of the burden of psoriasis on patients and the treatments currently used to treat psoriasis and its symptoms. This information will help guide the FDA as they consider future drug approvals.
Filling the gap: Hospitalists & palliative care
Most Americans diagnosed with serious illness will be hospitalized in their last months. During these hospitalizations, hospitalists direct their care.
For seriously ill patients, consultation with palliative care specialists has been shown to promote patient- and family-centered care, ensuring that care is consistent with patients’ goals, values, and preferences. Yet, many hospitalized patients lack access to palliative care consultation, and specialists have identified key domains of primary palliative care that can be delivered by nonspecialists.
To fill this gap, SHM’s Center for Quality Improvement partnered with The Hastings Center, a world-renowned bioethics research institution, to develop a resource room focused on hospitalists’ role in providing high-quality communication about prognosis and goals of care. The resource room presents a Prognosis and Goals of Care Communication Pathway, which highlights key processes and maps them onto the daily workflows of hospitalist physicians.
The care pathway is grounded in palliative care communication research and the consensus guidance of The Hastings Center Guidelines for Decisions on Life-Sustaining Treatment and Care Near the End of Life. It was informed by a national stakeholder meeting of hospitalists, other hospital clinicians, patient and family advocates, bioethicists, social scientists, and other experts, who identified professional values of hospital medicine aligned with communication as part of good care for seriously ill patients.
A collaborative interdisciplinary work group convened by SHM and including hospitalists, palliative medicine physicians, a bioethicist, and a palliative nursing specialist constructed the care pathway in terms of key processes occurring at admission, during hospitalization, and in discharge planning to support primary palliative care integration into normal workflow. The resource room also includes skills-building tools and resources for individual hospitals, teams, and institutions.
The work group will present a workshop on the care pathway at Hospital Medicine 2017: “Demystifying Difficult Decisions: Strategies and Skills to Equip Hospitalists for High-Quality Goals of Care Conversations with Seriously Ill Patients and Their Families.” For more information on the resource room, visit www.hospitalmedicine.org/EOL.
Dr. Anderson is associate professor in residence in the division of hospital medicine at the University of California, San Francisco. She also serves as attending physician in the Palliative Care Program and codirector of the School of Nursing Interprofessional Palliative Care Training Program at UCSF.
Most Americans diagnosed with serious illness will be hospitalized in their last months. During these hospitalizations, hospitalists direct their care.
For seriously ill patients, consultation with palliative care specialists has been shown to promote patient- and family-centered care, ensuring that care is consistent with patients’ goals, values, and preferences. Yet, many hospitalized patients lack access to palliative care consultation, and specialists have identified key domains of primary palliative care that can be delivered by nonspecialists.
To fill this gap, SHM’s Center for Quality Improvement partnered with The Hastings Center, a world-renowned bioethics research institution, to develop a resource room focused on hospitalists’ role in providing high-quality communication about prognosis and goals of care. The resource room presents a Prognosis and Goals of Care Communication Pathway, which highlights key processes and maps them onto the daily workflows of hospitalist physicians.
The care pathway is grounded in palliative care communication research and the consensus guidance of The Hastings Center Guidelines for Decisions on Life-Sustaining Treatment and Care Near the End of Life. It was informed by a national stakeholder meeting of hospitalists, other hospital clinicians, patient and family advocates, bioethicists, social scientists, and other experts, who identified professional values of hospital medicine aligned with communication as part of good care for seriously ill patients.
A collaborative interdisciplinary work group convened by SHM and including hospitalists, palliative medicine physicians, a bioethicist, and a palliative nursing specialist constructed the care pathway in terms of key processes occurring at admission, during hospitalization, and in discharge planning to support primary palliative care integration into normal workflow. The resource room also includes skills-building tools and resources for individual hospitals, teams, and institutions.
The work group will present a workshop on the care pathway at Hospital Medicine 2017: “Demystifying Difficult Decisions: Strategies and Skills to Equip Hospitalists for High-Quality Goals of Care Conversations with Seriously Ill Patients and Their Families.” For more information on the resource room, visit www.hospitalmedicine.org/EOL.
Dr. Anderson is associate professor in residence in the division of hospital medicine at the University of California, San Francisco. She also serves as attending physician in the Palliative Care Program and codirector of the School of Nursing Interprofessional Palliative Care Training Program at UCSF.
Most Americans diagnosed with serious illness will be hospitalized in their last months. During these hospitalizations, hospitalists direct their care.
For seriously ill patients, consultation with palliative care specialists has been shown to promote patient- and family-centered care, ensuring that care is consistent with patients’ goals, values, and preferences. Yet, many hospitalized patients lack access to palliative care consultation, and specialists have identified key domains of primary palliative care that can be delivered by nonspecialists.
To fill this gap, SHM’s Center for Quality Improvement partnered with The Hastings Center, a world-renowned bioethics research institution, to develop a resource room focused on hospitalists’ role in providing high-quality communication about prognosis and goals of care. The resource room presents a Prognosis and Goals of Care Communication Pathway, which highlights key processes and maps them onto the daily workflows of hospitalist physicians.
The care pathway is grounded in palliative care communication research and the consensus guidance of The Hastings Center Guidelines for Decisions on Life-Sustaining Treatment and Care Near the End of Life. It was informed by a national stakeholder meeting of hospitalists, other hospital clinicians, patient and family advocates, bioethicists, social scientists, and other experts, who identified professional values of hospital medicine aligned with communication as part of good care for seriously ill patients.
A collaborative interdisciplinary work group convened by SHM and including hospitalists, palliative medicine physicians, a bioethicist, and a palliative nursing specialist constructed the care pathway in terms of key processes occurring at admission, during hospitalization, and in discharge planning to support primary palliative care integration into normal workflow. The resource room also includes skills-building tools and resources for individual hospitals, teams, and institutions.
The work group will present a workshop on the care pathway at Hospital Medicine 2017: “Demystifying Difficult Decisions: Strategies and Skills to Equip Hospitalists for High-Quality Goals of Care Conversations with Seriously Ill Patients and Their Families.” For more information on the resource room, visit www.hospitalmedicine.org/EOL.
Dr. Anderson is associate professor in residence in the division of hospital medicine at the University of California, San Francisco. She also serves as attending physician in the Palliative Care Program and codirector of the School of Nursing Interprofessional Palliative Care Training Program at UCSF.
Some data support botulinum toxin for psoriasis and rosacea
ORLANDO – Botulinum toxin may have a place in treating psoriasis and rosacea.
There is not a huge body of literature supporting the use of neuromodulators for these conditions, but a smattering of case reports have shown positive results and some clinicians are exploring their off label use, Erin Gilbert, MD, said at the annual meeting of the American Academy of Dermatology.
Her own interest was originally piqued when she began working with Nicole Ward, PhD, director of the morphology core of the Skin Diseases Research Center in the department of dermatology at Case Western Reserve University, Cleveland, who developed a transgenic mouse model of psoriasis. Dr. Ward discovered that transecting the thoracic-level cutaneous nerves at their entry site into back skin resulted in rapid and significant changes in the psoriatic phenotype (J Invest Dermatol. 2011 Jul;131[7]:1530–8). These included decreases of up to 40% in various immune cell populations and a 30% improvement in acanthosis relative to sham surgery sites on the same animals.
This gave rise to a new thought, Dr. Gilbert said. Could chemical denervation produce similar improvements?
Using the same mouse model, she and Dr. Ward evaluated the effect of injecting botulinum neurotoxin A (BoNT-A) 9 units/kg diluted in 1 ml saline at one site, and saline control at another site (J Invest Dermatol. 2012 Jul;132[7]:1927–30). The mice were euthanized at 2 and 6 weeks after treatment. The results were similar to those of the surgical denervation: At 6 weeks, a 25% reduction in acanthosis was observed relative to the control site, with decreases in immune cells and inflammatory markers.
BoNT-A inhibits the release of neurotransmitters by cleaving the SPAP25 protein, an inhibitor of acetylcholine, at the neuromuscular junction. This is the root of the toxin’s ability to relax muscle spasm and decrease hyperhidrosis. The investigators also suggested that BoNT-A inhibits nerve-derived release of calcitonin gene-related peptide and substance P – important peptides in pain and itch sensation.
Dr. Gilbert and Dr. Ward also published a case report in which abobotulinumtoxinA was used off label to treat a recalcitrant psoriatic plaque in a 75-year-old woman (J Drugs Dermatol. 2014;13[11]:1407-8).
“This patient had psoriatic plaques concentrated on her trunk, arms, buttocks, and legs. She had been using strong topical corticosteroids for quite a long time with incomplete relief. I asked her to withdraw from all steroids for 3 months and then treated one lesion.”
The treated plaque was on the patient’s buttock. Dr. Gilbert injected a total of 30 units of abobotulinumtoxinA intradermally at eight points, about 1 cm apart. Within 3 weeks, there was complete remission of that plaque, sustained for 7 months. During this time, new lesions formed on other areas of her body. At 8 months, the treated plaque returned in the same place.
“Some of my patients had been completely recalcitrant to other therapies, and, following off label injection with neuromodulators, they have had life-changing results. In my experience, the key to consistently successful treatment is using adequate doses of toxin.”
This practice is supported by case reports in 2012 and 2015 (J Drugs Dermatol. 2012;11[12]:e76-e79; Dermatology 2015;230:299-301). Some investigators seem to think that, along with the anti-inflammatory and neurotransmitter effects, the toxin alters vascular tone.
Dr. Gilbert acknowledged that these treatments are expensive and cannot, in the case of psoriasis, be used in disseminated disease. However, she said that, for many patients, the relief is so profound and the benefit so long-lasting, that the expense is worth it. An argument in favor of this approach is that, where effective, BoNT-A could be used as a steroid-sparing agent and one that might reduce the need for systemic therapies.
“I will tell you that, sometimes, we get only partial relief and still need adjunctive therapies. Ultimately, neuromodulators may be especially useful for psoriatic plaques that are of cosmetic concern, such as those in the scalp or on the face. Limitations to their use include cost, the need for further studies, and safety concerns, such as muscle weakness.”
Dr. Gilbert had no relevant financial disclosures.
[email protected]
On Twitter @alz_gal
ORLANDO – Botulinum toxin may have a place in treating psoriasis and rosacea.
There is not a huge body of literature supporting the use of neuromodulators for these conditions, but a smattering of case reports have shown positive results and some clinicians are exploring their off label use, Erin Gilbert, MD, said at the annual meeting of the American Academy of Dermatology.
Her own interest was originally piqued when she began working with Nicole Ward, PhD, director of the morphology core of the Skin Diseases Research Center in the department of dermatology at Case Western Reserve University, Cleveland, who developed a transgenic mouse model of psoriasis. Dr. Ward discovered that transecting the thoracic-level cutaneous nerves at their entry site into back skin resulted in rapid and significant changes in the psoriatic phenotype (J Invest Dermatol. 2011 Jul;131[7]:1530–8). These included decreases of up to 40% in various immune cell populations and a 30% improvement in acanthosis relative to sham surgery sites on the same animals.
This gave rise to a new thought, Dr. Gilbert said. Could chemical denervation produce similar improvements?
Using the same mouse model, she and Dr. Ward evaluated the effect of injecting botulinum neurotoxin A (BoNT-A) 9 units/kg diluted in 1 ml saline at one site, and saline control at another site (J Invest Dermatol. 2012 Jul;132[7]:1927–30). The mice were euthanized at 2 and 6 weeks after treatment. The results were similar to those of the surgical denervation: At 6 weeks, a 25% reduction in acanthosis was observed relative to the control site, with decreases in immune cells and inflammatory markers.
BoNT-A inhibits the release of neurotransmitters by cleaving the SPAP25 protein, an inhibitor of acetylcholine, at the neuromuscular junction. This is the root of the toxin’s ability to relax muscle spasm and decrease hyperhidrosis. The investigators also suggested that BoNT-A inhibits nerve-derived release of calcitonin gene-related peptide and substance P – important peptides in pain and itch sensation.
Dr. Gilbert and Dr. Ward also published a case report in which abobotulinumtoxinA was used off label to treat a recalcitrant psoriatic plaque in a 75-year-old woman (J Drugs Dermatol. 2014;13[11]:1407-8).
“This patient had psoriatic plaques concentrated on her trunk, arms, buttocks, and legs. She had been using strong topical corticosteroids for quite a long time with incomplete relief. I asked her to withdraw from all steroids for 3 months and then treated one lesion.”
The treated plaque was on the patient’s buttock. Dr. Gilbert injected a total of 30 units of abobotulinumtoxinA intradermally at eight points, about 1 cm apart. Within 3 weeks, there was complete remission of that plaque, sustained for 7 months. During this time, new lesions formed on other areas of her body. At 8 months, the treated plaque returned in the same place.
“Some of my patients had been completely recalcitrant to other therapies, and, following off label injection with neuromodulators, they have had life-changing results. In my experience, the key to consistently successful treatment is using adequate doses of toxin.”
This practice is supported by case reports in 2012 and 2015 (J Drugs Dermatol. 2012;11[12]:e76-e79; Dermatology 2015;230:299-301). Some investigators seem to think that, along with the anti-inflammatory and neurotransmitter effects, the toxin alters vascular tone.
Dr. Gilbert acknowledged that these treatments are expensive and cannot, in the case of psoriasis, be used in disseminated disease. However, she said that, for many patients, the relief is so profound and the benefit so long-lasting, that the expense is worth it. An argument in favor of this approach is that, where effective, BoNT-A could be used as a steroid-sparing agent and one that might reduce the need for systemic therapies.
“I will tell you that, sometimes, we get only partial relief and still need adjunctive therapies. Ultimately, neuromodulators may be especially useful for psoriatic plaques that are of cosmetic concern, such as those in the scalp or on the face. Limitations to their use include cost, the need for further studies, and safety concerns, such as muscle weakness.”
Dr. Gilbert had no relevant financial disclosures.
[email protected]
On Twitter @alz_gal
ORLANDO – Botulinum toxin may have a place in treating psoriasis and rosacea.
There is not a huge body of literature supporting the use of neuromodulators for these conditions, but a smattering of case reports have shown positive results and some clinicians are exploring their off label use, Erin Gilbert, MD, said at the annual meeting of the American Academy of Dermatology.
Her own interest was originally piqued when she began working with Nicole Ward, PhD, director of the morphology core of the Skin Diseases Research Center in the department of dermatology at Case Western Reserve University, Cleveland, who developed a transgenic mouse model of psoriasis. Dr. Ward discovered that transecting the thoracic-level cutaneous nerves at their entry site into back skin resulted in rapid and significant changes in the psoriatic phenotype (J Invest Dermatol. 2011 Jul;131[7]:1530–8). These included decreases of up to 40% in various immune cell populations and a 30% improvement in acanthosis relative to sham surgery sites on the same animals.
This gave rise to a new thought, Dr. Gilbert said. Could chemical denervation produce similar improvements?
Using the same mouse model, she and Dr. Ward evaluated the effect of injecting botulinum neurotoxin A (BoNT-A) 9 units/kg diluted in 1 ml saline at one site, and saline control at another site (J Invest Dermatol. 2012 Jul;132[7]:1927–30). The mice were euthanized at 2 and 6 weeks after treatment. The results were similar to those of the surgical denervation: At 6 weeks, a 25% reduction in acanthosis was observed relative to the control site, with decreases in immune cells and inflammatory markers.
BoNT-A inhibits the release of neurotransmitters by cleaving the SPAP25 protein, an inhibitor of acetylcholine, at the neuromuscular junction. This is the root of the toxin’s ability to relax muscle spasm and decrease hyperhidrosis. The investigators also suggested that BoNT-A inhibits nerve-derived release of calcitonin gene-related peptide and substance P – important peptides in pain and itch sensation.
Dr. Gilbert and Dr. Ward also published a case report in which abobotulinumtoxinA was used off label to treat a recalcitrant psoriatic plaque in a 75-year-old woman (J Drugs Dermatol. 2014;13[11]:1407-8).
“This patient had psoriatic plaques concentrated on her trunk, arms, buttocks, and legs. She had been using strong topical corticosteroids for quite a long time with incomplete relief. I asked her to withdraw from all steroids for 3 months and then treated one lesion.”
The treated plaque was on the patient’s buttock. Dr. Gilbert injected a total of 30 units of abobotulinumtoxinA intradermally at eight points, about 1 cm apart. Within 3 weeks, there was complete remission of that plaque, sustained for 7 months. During this time, new lesions formed on other areas of her body. At 8 months, the treated plaque returned in the same place.
“Some of my patients had been completely recalcitrant to other therapies, and, following off label injection with neuromodulators, they have had life-changing results. In my experience, the key to consistently successful treatment is using adequate doses of toxin.”
This practice is supported by case reports in 2012 and 2015 (J Drugs Dermatol. 2012;11[12]:e76-e79; Dermatology 2015;230:299-301). Some investigators seem to think that, along with the anti-inflammatory and neurotransmitter effects, the toxin alters vascular tone.
Dr. Gilbert acknowledged that these treatments are expensive and cannot, in the case of psoriasis, be used in disseminated disease. However, she said that, for many patients, the relief is so profound and the benefit so long-lasting, that the expense is worth it. An argument in favor of this approach is that, where effective, BoNT-A could be used as a steroid-sparing agent and one that might reduce the need for systemic therapies.
“I will tell you that, sometimes, we get only partial relief and still need adjunctive therapies. Ultimately, neuromodulators may be especially useful for psoriatic plaques that are of cosmetic concern, such as those in the scalp or on the face. Limitations to their use include cost, the need for further studies, and safety concerns, such as muscle weakness.”
Dr. Gilbert had no relevant financial disclosures.
[email protected]
On Twitter @alz_gal
EXPERT ANALYSIS FROM AAD 17
Noncardiovascular comorbidities spike in acute heart failure
PARIS – Patients hospitalized for heart failure increasingly present with a growing number of noncardiovascular comorbidities, according to registry data from more than 300 U.S. hospitals.
During the decade of 2005-2014, the percentage of patients hospitalized for heart failure diagnosed with three or more noncardiovascular comorbidities (NCCs) jumped from abut 17% of these patients in 2005 to about 28% in 2015, Abhinav Sharma, MD, said at a meeting held by the Heart Failure Association of the ESC. This increase occurred as the percentages of hospitalized heart failure patients with none or one NCC showed clear decreases.
U.S. patients hospitalized for heart failure “appear to now be sicker and more medically complex. Probably, a large number of the noncardiovascular comorbidities are not being recognized when the focus is on treating the patient’s heart failure,” he said in an interview. “If we can identify the noncardiovascular comorbidities and target appropriate treatment, it may potentially decrease the risk of readmissions.”
He included five NCCs in his analysis: chronic obstructive pulmonary disease, anemia, diabetes, chronic kidney disease, and obesity.
His analysis showed that a higher rate of readmissions, as well as increased mortality both in hospital and during the 30 days following discharge, are outcomes that all connect with increased numbers of NCCs. Patients with three or more NCCs at the time of their heart failure admission were about 50% more likely to die in hospital, about 65% more likely to die during the 30 days following admission, about 35% more likely to be readmitted, and about half as likely to be discharged home following hospitalization, when compared with patients with no NCC in multivariate analyses that adjusted for demographic and other clinical variables. Patients with three or more NCCs were also about 67% more likely to have an index hospitalization of at least 4 days, compared with patients with no NCC.
Dr. Sharma speculated that the increased prevalence of multiple NCCs in acute heart failure patients may result, in part, from secular trends in the rates of diabetes and obesity and the noncardiovascular comorbidities associated with these conditions. All five of the NCCs included in his analysis showed increased prevalence rates from 2005 to 2014 in the patients he studied. The biggest jump occurred in the prevalence of chronic obstructive pulmonary disease, which rose from about 27% in 2005 to about 35% in 2014.
His study used data collected in the Get With the Guidelines–Heart Failure Registry, which began in 2005, and included just under 208,000 total patients. He acknowledged that it is hard to know how representative these patients are of the entire population of U.S. patients hospitalized for heart failure during the study period, because the patients he studied came from a self-selected group of more than 300 hospitals that opted to participate in the registry. “We need to see if this can be extrapolated to all U.S. hospitals,” Dr. Sharma said.
[email protected]
On Twitter @mitchelzoler
PARIS – Patients hospitalized for heart failure increasingly present with a growing number of noncardiovascular comorbidities, according to registry data from more than 300 U.S. hospitals.
During the decade of 2005-2014, the percentage of patients hospitalized for heart failure diagnosed with three or more noncardiovascular comorbidities (NCCs) jumped from abut 17% of these patients in 2005 to about 28% in 2015, Abhinav Sharma, MD, said at a meeting held by the Heart Failure Association of the ESC. This increase occurred as the percentages of hospitalized heart failure patients with none or one NCC showed clear decreases.
U.S. patients hospitalized for heart failure “appear to now be sicker and more medically complex. Probably, a large number of the noncardiovascular comorbidities are not being recognized when the focus is on treating the patient’s heart failure,” he said in an interview. “If we can identify the noncardiovascular comorbidities and target appropriate treatment, it may potentially decrease the risk of readmissions.”
He included five NCCs in his analysis: chronic obstructive pulmonary disease, anemia, diabetes, chronic kidney disease, and obesity.
His analysis showed that a higher rate of readmissions, as well as increased mortality both in hospital and during the 30 days following discharge, are outcomes that all connect with increased numbers of NCCs. Patients with three or more NCCs at the time of their heart failure admission were about 50% more likely to die in hospital, about 65% more likely to die during the 30 days following admission, about 35% more likely to be readmitted, and about half as likely to be discharged home following hospitalization, when compared with patients with no NCC in multivariate analyses that adjusted for demographic and other clinical variables. Patients with three or more NCCs were also about 67% more likely to have an index hospitalization of at least 4 days, compared with patients with no NCC.
Dr. Sharma speculated that the increased prevalence of multiple NCCs in acute heart failure patients may result, in part, from secular trends in the rates of diabetes and obesity and the noncardiovascular comorbidities associated with these conditions. All five of the NCCs included in his analysis showed increased prevalence rates from 2005 to 2014 in the patients he studied. The biggest jump occurred in the prevalence of chronic obstructive pulmonary disease, which rose from about 27% in 2005 to about 35% in 2014.
His study used data collected in the Get With the Guidelines–Heart Failure Registry, which began in 2005, and included just under 208,000 total patients. He acknowledged that it is hard to know how representative these patients are of the entire population of U.S. patients hospitalized for heart failure during the study period, because the patients he studied came from a self-selected group of more than 300 hospitals that opted to participate in the registry. “We need to see if this can be extrapolated to all U.S. hospitals,” Dr. Sharma said.
[email protected]
On Twitter @mitchelzoler
PARIS – Patients hospitalized for heart failure increasingly present with a growing number of noncardiovascular comorbidities, according to registry data from more than 300 U.S. hospitals.
During the decade of 2005-2014, the percentage of patients hospitalized for heart failure diagnosed with three or more noncardiovascular comorbidities (NCCs) jumped from abut 17% of these patients in 2005 to about 28% in 2015, Abhinav Sharma, MD, said at a meeting held by the Heart Failure Association of the ESC. This increase occurred as the percentages of hospitalized heart failure patients with none or one NCC showed clear decreases.
U.S. patients hospitalized for heart failure “appear to now be sicker and more medically complex. Probably, a large number of the noncardiovascular comorbidities are not being recognized when the focus is on treating the patient’s heart failure,” he said in an interview. “If we can identify the noncardiovascular comorbidities and target appropriate treatment, it may potentially decrease the risk of readmissions.”
He included five NCCs in his analysis: chronic obstructive pulmonary disease, anemia, diabetes, chronic kidney disease, and obesity.
His analysis showed that a higher rate of readmissions, as well as increased mortality both in hospital and during the 30 days following discharge, are outcomes that all connect with increased numbers of NCCs. Patients with three or more NCCs at the time of their heart failure admission were about 50% more likely to die in hospital, about 65% more likely to die during the 30 days following admission, about 35% more likely to be readmitted, and about half as likely to be discharged home following hospitalization, when compared with patients with no NCC in multivariate analyses that adjusted for demographic and other clinical variables. Patients with three or more NCCs were also about 67% more likely to have an index hospitalization of at least 4 days, compared with patients with no NCC.
Dr. Sharma speculated that the increased prevalence of multiple NCCs in acute heart failure patients may result, in part, from secular trends in the rates of diabetes and obesity and the noncardiovascular comorbidities associated with these conditions. All five of the NCCs included in his analysis showed increased prevalence rates from 2005 to 2014 in the patients he studied. The biggest jump occurred in the prevalence of chronic obstructive pulmonary disease, which rose from about 27% in 2005 to about 35% in 2014.
His study used data collected in the Get With the Guidelines–Heart Failure Registry, which began in 2005, and included just under 208,000 total patients. He acknowledged that it is hard to know how representative these patients are of the entire population of U.S. patients hospitalized for heart failure during the study period, because the patients he studied came from a self-selected group of more than 300 hospitals that opted to participate in the registry. “We need to see if this can be extrapolated to all U.S. hospitals,” Dr. Sharma said.
[email protected]
On Twitter @mitchelzoler
AT HEART FAILURE 2017
Key clinical point:
Major finding: The prevalence of three or more noncardiovascular comorbidities jumped from about 17% in 2005 to about 28% in 2014.
Data source: Review of 207,984 U.S. hospitalized heart failure patients included in the Get With the Guidelines–Heart Failure Registry during 2005-2014.
Disclosures: Dr. Sharma has received research support from Roche Diagnostics and Takeda.
Radioembolization could be alternative to sorafenib in advanced liver cancer trial
AMSTERDAM – While there was no overall or progression-free survival benefit, results of the randomized, controlled phase III SARAH trial showed that the use of selective internal radioactive therapy (SIRT) was better tolerated than sorafenib for the treatment of patients with advanced liver cancer.
There was also early evidence that SIRT, using the radiopharmaceutical yttrium-90 (SIR-Spheres microspheres), reduced radiologic progression in the liver and improved tumor responses to a greater extent that did sorafenib.
“I feel [the results] will open a new door in the treatment of HCC [hepatocellular carcinoma],” study investigator Valérie Vilgrain, MD, of Hôpital Beaujon, Paris, said at the International Liver Congress, sponsored by the European Association for the Study of the Liver.
The standard treatment for advanced HCC at present is sorafenib, which is based on the SHARP trial findings that showed an overall survival of around 10.7 months, Dr. Vilgrain observed.
Transarterial chemoembolization (TACE) is the reference treatment for intermediate HCC, she said, and of the 467 patients included in the SARAH trial, just over 40% had failed to respond to two rounds of this therapy and had inoperable or advanced disease.
Dr. Frank Tacke, EASL vice secretary and professor of medicine in the department of gastroenterology, metabolic diseases, and intensive care medicine at University Hospital Aachen, Germany, said during a press briefing that the trial was “the first phase III, randomized controlled trial putting this intervention in perspective with current therapy.”
Dr. Tacke, who was not involved in the trial, added: “The overall survival is the same but there are so many patient-relevant parameters that are actually better in the one treatment [SIRT].”
The SARAH trial was conduced at 25 centers in France and there were 237 patients with a life expectancy of at least 3 months who were randomized to treatment with SIRT and 222 to treatment with sorafenib 800 mg daily.
The mean age of all randomized patients was 65 years, 90% were male, and 90% had cirrhosis. Cirrhosis was caused by alcoholic liver disease in 65%, hepatitis C virus infection in about one-quarter, and nonalcoholic steatohepatitis in roughly one-fifth of cases.
Comparing SIRT with sorafenib in all patients who were randomized, the median overall survival was 8.0 months versus 9.9 months (P = .018) and the median progression-free survival was a respective 4.1 months versus 3.7 months (P = .76). No differences in survival were observed when only patients who actually received the treatments were compared.
While there was no difference in radiologic progression seen overall – one of the secondary endpoints – when progression in the liver as the first site of progression was considered, there was a significant difference favoring the use of SIRT over sorafenib (P = .014), Dr. Vilgrain reported.
In addition, 26 (19%) of SIRT versus 23 (11.6%) of sorafenib-treated patients showed an objective response at 6 months, so there was “a strong signal” that SIRT could have an eventual edge over sorafenib but perhaps effects take longer to materialize (P = .042).
Fewer patients receiving SIRT than sorafenib experienced treatment-related adverse events (76.5% vs. 95%), with a median number of adverse events per patient of 5 and 10, respectively.
Adverse events of note that occurred less frequently in the SIRT than sorafenib arm were fatigue (20% vs. 41%), diarrhea (3% vs. 30%), abdominal pain (6% vs. 14%), hand-foot reaction (1% vs. 12%), infection (3% vs. 9%), weight loss (0% vs. 6%), and hypertension (0% vs. 5%).
Quality of life was significantly better in the SIRT group than in the sorafenib group over time, Dr. Vilgrain said. Quality of life was measured via the Global Health Status subscore of the EORTC QLQ-C30 scale.
Dr. Vilgrain noted during a press briefing that patients in the sorafenib group were able to start oral treatment 1 or 2 days after randomization, whereas there was a delay of 1-2 weeks before the patients randomized to SIRT could receive their treatment and just over one-quarter did not get SIRT as per the protocol, compared to 7% of those randomized to sorafenib.
Whether the lag time in patients getting their treatment might have affected the survival outcomes seen with the treatment is not known. Future studies look at reducing the time between randomization and SIRT, she said.
Dr. Vilgrain noted in an interview that SIRT was given as a single injection after an initial test injection. While it is possible to retreat patients, the study design was such that patients had to wait 6 months before they could be retreated and in theory a second treatment should not be needed.
The study was sponsored Assistance Publique – Hôpitaux de Paris (supported by grants from Sirtex Medical Limited. Dr. Vilgrain disclosed acting as a study investigator and receiving speaker fees from Guerbet and Sirtex, and speaker fees from SuperSonic Imagine and Toshiba.
AMSTERDAM – While there was no overall or progression-free survival benefit, results of the randomized, controlled phase III SARAH trial showed that the use of selective internal radioactive therapy (SIRT) was better tolerated than sorafenib for the treatment of patients with advanced liver cancer.
There was also early evidence that SIRT, using the radiopharmaceutical yttrium-90 (SIR-Spheres microspheres), reduced radiologic progression in the liver and improved tumor responses to a greater extent that did sorafenib.
“I feel [the results] will open a new door in the treatment of HCC [hepatocellular carcinoma],” study investigator Valérie Vilgrain, MD, of Hôpital Beaujon, Paris, said at the International Liver Congress, sponsored by the European Association for the Study of the Liver.
The standard treatment for advanced HCC at present is sorafenib, which is based on the SHARP trial findings that showed an overall survival of around 10.7 months, Dr. Vilgrain observed.
Transarterial chemoembolization (TACE) is the reference treatment for intermediate HCC, she said, and of the 467 patients included in the SARAH trial, just over 40% had failed to respond to two rounds of this therapy and had inoperable or advanced disease.
Dr. Frank Tacke, EASL vice secretary and professor of medicine in the department of gastroenterology, metabolic diseases, and intensive care medicine at University Hospital Aachen, Germany, said during a press briefing that the trial was “the first phase III, randomized controlled trial putting this intervention in perspective with current therapy.”
Dr. Tacke, who was not involved in the trial, added: “The overall survival is the same but there are so many patient-relevant parameters that are actually better in the one treatment [SIRT].”
The SARAH trial was conduced at 25 centers in France and there were 237 patients with a life expectancy of at least 3 months who were randomized to treatment with SIRT and 222 to treatment with sorafenib 800 mg daily.
The mean age of all randomized patients was 65 years, 90% were male, and 90% had cirrhosis. Cirrhosis was caused by alcoholic liver disease in 65%, hepatitis C virus infection in about one-quarter, and nonalcoholic steatohepatitis in roughly one-fifth of cases.
Comparing SIRT with sorafenib in all patients who were randomized, the median overall survival was 8.0 months versus 9.9 months (P = .018) and the median progression-free survival was a respective 4.1 months versus 3.7 months (P = .76). No differences in survival were observed when only patients who actually received the treatments were compared.
While there was no difference in radiologic progression seen overall – one of the secondary endpoints – when progression in the liver as the first site of progression was considered, there was a significant difference favoring the use of SIRT over sorafenib (P = .014), Dr. Vilgrain reported.
In addition, 26 (19%) of SIRT versus 23 (11.6%) of sorafenib-treated patients showed an objective response at 6 months, so there was “a strong signal” that SIRT could have an eventual edge over sorafenib but perhaps effects take longer to materialize (P = .042).
Fewer patients receiving SIRT than sorafenib experienced treatment-related adverse events (76.5% vs. 95%), with a median number of adverse events per patient of 5 and 10, respectively.
Adverse events of note that occurred less frequently in the SIRT than sorafenib arm were fatigue (20% vs. 41%), diarrhea (3% vs. 30%), abdominal pain (6% vs. 14%), hand-foot reaction (1% vs. 12%), infection (3% vs. 9%), weight loss (0% vs. 6%), and hypertension (0% vs. 5%).
Quality of life was significantly better in the SIRT group than in the sorafenib group over time, Dr. Vilgrain said. Quality of life was measured via the Global Health Status subscore of the EORTC QLQ-C30 scale.
Dr. Vilgrain noted during a press briefing that patients in the sorafenib group were able to start oral treatment 1 or 2 days after randomization, whereas there was a delay of 1-2 weeks before the patients randomized to SIRT could receive their treatment and just over one-quarter did not get SIRT as per the protocol, compared to 7% of those randomized to sorafenib.
Whether the lag time in patients getting their treatment might have affected the survival outcomes seen with the treatment is not known. Future studies look at reducing the time between randomization and SIRT, she said.
Dr. Vilgrain noted in an interview that SIRT was given as a single injection after an initial test injection. While it is possible to retreat patients, the study design was such that patients had to wait 6 months before they could be retreated and in theory a second treatment should not be needed.
The study was sponsored Assistance Publique – Hôpitaux de Paris (supported by grants from Sirtex Medical Limited. Dr. Vilgrain disclosed acting as a study investigator and receiving speaker fees from Guerbet and Sirtex, and speaker fees from SuperSonic Imagine and Toshiba.
AMSTERDAM – While there was no overall or progression-free survival benefit, results of the randomized, controlled phase III SARAH trial showed that the use of selective internal radioactive therapy (SIRT) was better tolerated than sorafenib for the treatment of patients with advanced liver cancer.
There was also early evidence that SIRT, using the radiopharmaceutical yttrium-90 (SIR-Spheres microspheres), reduced radiologic progression in the liver and improved tumor responses to a greater extent that did sorafenib.
“I feel [the results] will open a new door in the treatment of HCC [hepatocellular carcinoma],” study investigator Valérie Vilgrain, MD, of Hôpital Beaujon, Paris, said at the International Liver Congress, sponsored by the European Association for the Study of the Liver.
The standard treatment for advanced HCC at present is sorafenib, which is based on the SHARP trial findings that showed an overall survival of around 10.7 months, Dr. Vilgrain observed.
Transarterial chemoembolization (TACE) is the reference treatment for intermediate HCC, she said, and of the 467 patients included in the SARAH trial, just over 40% had failed to respond to two rounds of this therapy and had inoperable or advanced disease.
Dr. Frank Tacke, EASL vice secretary and professor of medicine in the department of gastroenterology, metabolic diseases, and intensive care medicine at University Hospital Aachen, Germany, said during a press briefing that the trial was “the first phase III, randomized controlled trial putting this intervention in perspective with current therapy.”
Dr. Tacke, who was not involved in the trial, added: “The overall survival is the same but there are so many patient-relevant parameters that are actually better in the one treatment [SIRT].”
The SARAH trial was conduced at 25 centers in France and there were 237 patients with a life expectancy of at least 3 months who were randomized to treatment with SIRT and 222 to treatment with sorafenib 800 mg daily.
The mean age of all randomized patients was 65 years, 90% were male, and 90% had cirrhosis. Cirrhosis was caused by alcoholic liver disease in 65%, hepatitis C virus infection in about one-quarter, and nonalcoholic steatohepatitis in roughly one-fifth of cases.
Comparing SIRT with sorafenib in all patients who were randomized, the median overall survival was 8.0 months versus 9.9 months (P = .018) and the median progression-free survival was a respective 4.1 months versus 3.7 months (P = .76). No differences in survival were observed when only patients who actually received the treatments were compared.
While there was no difference in radiologic progression seen overall – one of the secondary endpoints – when progression in the liver as the first site of progression was considered, there was a significant difference favoring the use of SIRT over sorafenib (P = .014), Dr. Vilgrain reported.
In addition, 26 (19%) of SIRT versus 23 (11.6%) of sorafenib-treated patients showed an objective response at 6 months, so there was “a strong signal” that SIRT could have an eventual edge over sorafenib but perhaps effects take longer to materialize (P = .042).
Fewer patients receiving SIRT than sorafenib experienced treatment-related adverse events (76.5% vs. 95%), with a median number of adverse events per patient of 5 and 10, respectively.
Adverse events of note that occurred less frequently in the SIRT than sorafenib arm were fatigue (20% vs. 41%), diarrhea (3% vs. 30%), abdominal pain (6% vs. 14%), hand-foot reaction (1% vs. 12%), infection (3% vs. 9%), weight loss (0% vs. 6%), and hypertension (0% vs. 5%).
Quality of life was significantly better in the SIRT group than in the sorafenib group over time, Dr. Vilgrain said. Quality of life was measured via the Global Health Status subscore of the EORTC QLQ-C30 scale.
Dr. Vilgrain noted during a press briefing that patients in the sorafenib group were able to start oral treatment 1 or 2 days after randomization, whereas there was a delay of 1-2 weeks before the patients randomized to SIRT could receive their treatment and just over one-quarter did not get SIRT as per the protocol, compared to 7% of those randomized to sorafenib.
Whether the lag time in patients getting their treatment might have affected the survival outcomes seen with the treatment is not known. Future studies look at reducing the time between randomization and SIRT, she said.
Dr. Vilgrain noted in an interview that SIRT was given as a single injection after an initial test injection. While it is possible to retreat patients, the study design was such that patients had to wait 6 months before they could be retreated and in theory a second treatment should not be needed.
The study was sponsored Assistance Publique – Hôpitaux de Paris (supported by grants from Sirtex Medical Limited. Dr. Vilgrain disclosed acting as a study investigator and receiving speaker fees from Guerbet and Sirtex, and speaker fees from SuperSonic Imagine and Toshiba.
AT ILC 2017
Key clinical point: Selective internal radiotherapy (SIRT) was found to be better tolerated than standard therapy but did not meet the primary endpoint of improved overall survival.
Major finding: Comparing SIRT with sorafenib in all patients who received it, the median overall survival was 8.0 months versus 9.9 months (P = .018).
Data source: SARAH, a prospective, randomized, open-label, multicenter, controlled phase III trial of radioembolization versus sorafenib for the treatment of 459 patients with advanced or inoperable hepatocellular carcinoma.
Disclosures: The study was sponsored Assistance Publique – Hôpitaux de Paris (supported by grants from Sirtex Medical Limited. Dr. Vilgrain disclosed acting as a study investigator and receiving speaker fees from Guerbet and Sirtex, and speaker fees from SuperSonic Imagine and Toshiba.
DCIS tool IDs axillary node biopsy candidates
LAS VEGAS – A new tool identifies patients with ductal carcinoma in situ (DCIS) who are at high risk for being upstaged as a result of the pathology report. The screen could encourage patients to undergo axillary nodal staging during the core needle biopsy (CNB), thus avoiding a second procedure.
“The risk factors have been well described, but they haven’t helped give individual risk or individual percentages. Our goal was to try to individualize that risk so that we could counsel patients,” said lead researcher James Jakub, MD, chair of the division of breast endocrine and metabolic surgery at Mayo Clinic Rochester, at the annual meeting of the American Society of Breast Surgeons.
The researchers found that grade on CNB, mass lesion on imaging, multifocal/centric disease, and linear dimension combined to predict the likelihood of being upstaged to invasive disease (C statistic, 0.71; 95% confidence interval, 0.66-0.77).
They then combined those characteristics to create a nomogram and tested it against the validation set. In that group, 11% of patients were upstaged to invasive disease. The nomogram performed almost identically in the external validation set (C statistic, 0.71; 95% CI, 0.63-0.79). The model predicted 56 upstages, and 46 occurred.
This wasn’t the first attempt to create a nomogram to predict upstaging in DCIS patients, but others were not tested against external datasets. “That’s a weakness with other studies. Unless it’s validated externally, you don’t really know if you can apply it to your population. That was a very large plus: having colleagues who were willing to collaborate to validate it,” said Dr. Jakub.
The team is working on posting the nomogram online for widespread use.
It remains to be seen whether the availability of the nomogram will, in fact, change patients’ decision-making and result in more axillary nodal biopsies during CNB procedures in high risk patients.
“We haven’t looked into that. It will be interesting to see how it influences [sentinel lymph node] biopsy rate at our institution, and we’d love to hear from institutions who apply it. My sense is that patients who are at high risk are going to be interested in doing that. I think it will definitely change their management,” said Dr. Jakub.
LAS VEGAS – A new tool identifies patients with ductal carcinoma in situ (DCIS) who are at high risk for being upstaged as a result of the pathology report. The screen could encourage patients to undergo axillary nodal staging during the core needle biopsy (CNB), thus avoiding a second procedure.
“The risk factors have been well described, but they haven’t helped give individual risk or individual percentages. Our goal was to try to individualize that risk so that we could counsel patients,” said lead researcher James Jakub, MD, chair of the division of breast endocrine and metabolic surgery at Mayo Clinic Rochester, at the annual meeting of the American Society of Breast Surgeons.
The researchers found that grade on CNB, mass lesion on imaging, multifocal/centric disease, and linear dimension combined to predict the likelihood of being upstaged to invasive disease (C statistic, 0.71; 95% confidence interval, 0.66-0.77).
They then combined those characteristics to create a nomogram and tested it against the validation set. In that group, 11% of patients were upstaged to invasive disease. The nomogram performed almost identically in the external validation set (C statistic, 0.71; 95% CI, 0.63-0.79). The model predicted 56 upstages, and 46 occurred.
This wasn’t the first attempt to create a nomogram to predict upstaging in DCIS patients, but others were not tested against external datasets. “That’s a weakness with other studies. Unless it’s validated externally, you don’t really know if you can apply it to your population. That was a very large plus: having colleagues who were willing to collaborate to validate it,” said Dr. Jakub.
The team is working on posting the nomogram online for widespread use.
It remains to be seen whether the availability of the nomogram will, in fact, change patients’ decision-making and result in more axillary nodal biopsies during CNB procedures in high risk patients.
“We haven’t looked into that. It will be interesting to see how it influences [sentinel lymph node] biopsy rate at our institution, and we’d love to hear from institutions who apply it. My sense is that patients who are at high risk are going to be interested in doing that. I think it will definitely change their management,” said Dr. Jakub.
LAS VEGAS – A new tool identifies patients with ductal carcinoma in situ (DCIS) who are at high risk for being upstaged as a result of the pathology report. The screen could encourage patients to undergo axillary nodal staging during the core needle biopsy (CNB), thus avoiding a second procedure.
“The risk factors have been well described, but they haven’t helped give individual risk or individual percentages. Our goal was to try to individualize that risk so that we could counsel patients,” said lead researcher James Jakub, MD, chair of the division of breast endocrine and metabolic surgery at Mayo Clinic Rochester, at the annual meeting of the American Society of Breast Surgeons.
The researchers found that grade on CNB, mass lesion on imaging, multifocal/centric disease, and linear dimension combined to predict the likelihood of being upstaged to invasive disease (C statistic, 0.71; 95% confidence interval, 0.66-0.77).
They then combined those characteristics to create a nomogram and tested it against the validation set. In that group, 11% of patients were upstaged to invasive disease. The nomogram performed almost identically in the external validation set (C statistic, 0.71; 95% CI, 0.63-0.79). The model predicted 56 upstages, and 46 occurred.
This wasn’t the first attempt to create a nomogram to predict upstaging in DCIS patients, but others were not tested against external datasets. “That’s a weakness with other studies. Unless it’s validated externally, you don’t really know if you can apply it to your population. That was a very large plus: having colleagues who were willing to collaborate to validate it,” said Dr. Jakub.
The team is working on posting the nomogram online for widespread use.
It remains to be seen whether the availability of the nomogram will, in fact, change patients’ decision-making and result in more axillary nodal biopsies during CNB procedures in high risk patients.
“We haven’t looked into that. It will be interesting to see how it influences [sentinel lymph node] biopsy rate at our institution, and we’d love to hear from institutions who apply it. My sense is that patients who are at high risk are going to be interested in doing that. I think it will definitely change their management,” said Dr. Jakub.
AT ASBS 2017
Key clinical point: High risk patients who choose axillary biopsy could avoid a second procedure.
Major finding: The 4-item nomogram predicted upstaging with a C statistic of 0.71.
Data source: A retrospective sample (n = 827) and validation study (n = 579).
Disclosures: The source of funding was not disclosed. Dr. Jakub reported having no financial disclosures.
Transradial catheterizations boost operators’ radiation exposure
WASHINGTON – Coronary catheterization procedures done via transradial access produce significantly better patient outcomes, compared with transfemoral approaches, but transradial also leads to substantially higher radiation exposure to the operator, according to an analysis of 650 procedures.
When operators performed transradial coronary catheterizations they received on average nearly twice the radiation dose to their chest as they did when they performed transfemoral procedures, in a randomized trial that included 14 interventional cardiologists, Alessandro Sciahbasi, MD, reported at the annual meeting of the American College of Cardiology.
Radiation exposures to the operators’ wrists and eyes showed no significant differences between the two catheterization routes. The difference was limited to thorax exposure.
“These data worry me,” commented Sunil V. Rao, MD, a long-time advocate for increased transradial catheterizations in U.S. practice. “We are pretty obsessive about radiation detection in our catheterization laboratory,” said Dr. Rao, an interventional cardiologist at Duke University in Durham, N.C.
The RAD-MATRIX substudy focused on the radiation exposures received by 14 participating operators who wore radiation dosimeters on their wrist, chest, and near their eyes during each procedure. All operators performed both transradial and transfemoral catheterizations with the choice dictated by the randomization protocol. The transradial procedures underwent further randomization to access through either the patient’s left or right arm. The full analysis included 398 procedures performed by transfemoral access, and 252 with transradial access with 131 via the left forearm and 121 via the right forearm.
The analysis showed that, when the operators performed transradial catheterizations, they received an average, normalized, effective dose to their thorax of 2.3 mcSv per procedure and 1.2 mcSv when performing transfemoral catheterization, a statistically significant difference. The exposures from left and from right transradial access did not differ significantly. Concurrently with Dr. Sciahbasi’s report at the meeting the results also appeared in an online article (J Am Coll Card. 2017 Mar 18. doi: 10.1016/j.jacc.2017.03.018).
The analysis also showed a 10-fold variability in exposure level among the 14 participating operators. Dr. Sciahbasi hypothesized that this reflected substantive differences in the degree of radiation protection used by each operator and suggested this implied that some interventionalists were not protecting themselves from exposure as thoroughly as possible.
RAD-MATRIX received partial funding from The Medicines Company and Terumo. Dr. Sciahbasi had no disclosures. Dr. Rao has been a consultant to Boehringer Ingelheim, Cardinal Health, Corindus, Cardiovascular Systems, and Medtronic.
[email protected]
On Twitter @mitchelzoler
The findings from RAD-MATRIX are alarming. Although radiation exposure is generally acknowledged as an occupational hazard of interventional cardiology, it is rarely taken seriously. It is common to see individuals performing procedures without wearing leaded eyewear, or casually strolling into catheterization laboratories without wearing appropriate aprons. The topic is often approached jocularly with comments about childbearing with little thought given to cancer risk. However, the radiation-induced health risks to adults are nontrivial and outweigh by an order of magnitude any risk for hereditary disease.
Although the findings of RAD-MATRIX are unlikely to impede the swelling tide of the transradial approach, the implications are very important. They should prompt radial and femoral operators to heed radiation safety principles. In addition, we need rigorously collected registry data concerning the safety of percutaneous coronary interventions for operators and staff.
Neil S. Kleiman, MD , is professor of cardiology at Houston Methodist. Norman J. Kleiman, PhD , is director of the Eye Radiation on Environmental Research Laboratory at Columbia University in New York. They had no disclosures. These comments are from an editorial they wrote to accompany the published RAD-MATRIX results (J Am Coll Card. 2017 Mar 18. doi: 10.1016/j.jacc.2017.03.016 ).
The findings from RAD-MATRIX are alarming. Although radiation exposure is generally acknowledged as an occupational hazard of interventional cardiology, it is rarely taken seriously. It is common to see individuals performing procedures without wearing leaded eyewear, or casually strolling into catheterization laboratories without wearing appropriate aprons. The topic is often approached jocularly with comments about childbearing with little thought given to cancer risk. However, the radiation-induced health risks to adults are nontrivial and outweigh by an order of magnitude any risk for hereditary disease.
Although the findings of RAD-MATRIX are unlikely to impede the swelling tide of the transradial approach, the implications are very important. They should prompt radial and femoral operators to heed radiation safety principles. In addition, we need rigorously collected registry data concerning the safety of percutaneous coronary interventions for operators and staff.
Neil S. Kleiman, MD , is professor of cardiology at Houston Methodist. Norman J. Kleiman, PhD , is director of the Eye Radiation on Environmental Research Laboratory at Columbia University in New York. They had no disclosures. These comments are from an editorial they wrote to accompany the published RAD-MATRIX results (J Am Coll Card. 2017 Mar 18. doi: 10.1016/j.jacc.2017.03.016 ).
The findings from RAD-MATRIX are alarming. Although radiation exposure is generally acknowledged as an occupational hazard of interventional cardiology, it is rarely taken seriously. It is common to see individuals performing procedures without wearing leaded eyewear, or casually strolling into catheterization laboratories without wearing appropriate aprons. The topic is often approached jocularly with comments about childbearing with little thought given to cancer risk. However, the radiation-induced health risks to adults are nontrivial and outweigh by an order of magnitude any risk for hereditary disease.
Although the findings of RAD-MATRIX are unlikely to impede the swelling tide of the transradial approach, the implications are very important. They should prompt radial and femoral operators to heed radiation safety principles. In addition, we need rigorously collected registry data concerning the safety of percutaneous coronary interventions for operators and staff.
Neil S. Kleiman, MD , is professor of cardiology at Houston Methodist. Norman J. Kleiman, PhD , is director of the Eye Radiation on Environmental Research Laboratory at Columbia University in New York. They had no disclosures. These comments are from an editorial they wrote to accompany the published RAD-MATRIX results (J Am Coll Card. 2017 Mar 18. doi: 10.1016/j.jacc.2017.03.016 ).
WASHINGTON – Coronary catheterization procedures done via transradial access produce significantly better patient outcomes, compared with transfemoral approaches, but transradial also leads to substantially higher radiation exposure to the operator, according to an analysis of 650 procedures.
When operators performed transradial coronary catheterizations they received on average nearly twice the radiation dose to their chest as they did when they performed transfemoral procedures, in a randomized trial that included 14 interventional cardiologists, Alessandro Sciahbasi, MD, reported at the annual meeting of the American College of Cardiology.
Radiation exposures to the operators’ wrists and eyes showed no significant differences between the two catheterization routes. The difference was limited to thorax exposure.
“These data worry me,” commented Sunil V. Rao, MD, a long-time advocate for increased transradial catheterizations in U.S. practice. “We are pretty obsessive about radiation detection in our catheterization laboratory,” said Dr. Rao, an interventional cardiologist at Duke University in Durham, N.C.
The RAD-MATRIX substudy focused on the radiation exposures received by 14 participating operators who wore radiation dosimeters on their wrist, chest, and near their eyes during each procedure. All operators performed both transradial and transfemoral catheterizations with the choice dictated by the randomization protocol. The transradial procedures underwent further randomization to access through either the patient’s left or right arm. The full analysis included 398 procedures performed by transfemoral access, and 252 with transradial access with 131 via the left forearm and 121 via the right forearm.
The analysis showed that, when the operators performed transradial catheterizations, they received an average, normalized, effective dose to their thorax of 2.3 mcSv per procedure and 1.2 mcSv when performing transfemoral catheterization, a statistically significant difference. The exposures from left and from right transradial access did not differ significantly. Concurrently with Dr. Sciahbasi’s report at the meeting the results also appeared in an online article (J Am Coll Card. 2017 Mar 18. doi: 10.1016/j.jacc.2017.03.018).
The analysis also showed a 10-fold variability in exposure level among the 14 participating operators. Dr. Sciahbasi hypothesized that this reflected substantive differences in the degree of radiation protection used by each operator and suggested this implied that some interventionalists were not protecting themselves from exposure as thoroughly as possible.
RAD-MATRIX received partial funding from The Medicines Company and Terumo. Dr. Sciahbasi had no disclosures. Dr. Rao has been a consultant to Boehringer Ingelheim, Cardinal Health, Corindus, Cardiovascular Systems, and Medtronic.
[email protected]
On Twitter @mitchelzoler
WASHINGTON – Coronary catheterization procedures done via transradial access produce significantly better patient outcomes, compared with transfemoral approaches, but transradial also leads to substantially higher radiation exposure to the operator, according to an analysis of 650 procedures.
When operators performed transradial coronary catheterizations they received on average nearly twice the radiation dose to their chest as they did when they performed transfemoral procedures, in a randomized trial that included 14 interventional cardiologists, Alessandro Sciahbasi, MD, reported at the annual meeting of the American College of Cardiology.
Radiation exposures to the operators’ wrists and eyes showed no significant differences between the two catheterization routes. The difference was limited to thorax exposure.
“These data worry me,” commented Sunil V. Rao, MD, a long-time advocate for increased transradial catheterizations in U.S. practice. “We are pretty obsessive about radiation detection in our catheterization laboratory,” said Dr. Rao, an interventional cardiologist at Duke University in Durham, N.C.
The RAD-MATRIX substudy focused on the radiation exposures received by 14 participating operators who wore radiation dosimeters on their wrist, chest, and near their eyes during each procedure. All operators performed both transradial and transfemoral catheterizations with the choice dictated by the randomization protocol. The transradial procedures underwent further randomization to access through either the patient’s left or right arm. The full analysis included 398 procedures performed by transfemoral access, and 252 with transradial access with 131 via the left forearm and 121 via the right forearm.
The analysis showed that, when the operators performed transradial catheterizations, they received an average, normalized, effective dose to their thorax of 2.3 mcSv per procedure and 1.2 mcSv when performing transfemoral catheterization, a statistically significant difference. The exposures from left and from right transradial access did not differ significantly. Concurrently with Dr. Sciahbasi’s report at the meeting the results also appeared in an online article (J Am Coll Card. 2017 Mar 18. doi: 10.1016/j.jacc.2017.03.018).
The analysis also showed a 10-fold variability in exposure level among the 14 participating operators. Dr. Sciahbasi hypothesized that this reflected substantive differences in the degree of radiation protection used by each operator and suggested this implied that some interventionalists were not protecting themselves from exposure as thoroughly as possible.
RAD-MATRIX received partial funding from The Medicines Company and Terumo. Dr. Sciahbasi had no disclosures. Dr. Rao has been a consultant to Boehringer Ingelheim, Cardinal Health, Corindus, Cardiovascular Systems, and Medtronic.
[email protected]
On Twitter @mitchelzoler
AT ACC 17
Key clinical point:
Major finding: Transradial catheterizations delivered an average effective thorax radiation dose of 2.3 mcSv, compared with 1.2 mcSv from transfemoral catheterizations.
Data source: RAD-MATRIX, a randomized study with 14 operators who performed 650 total procedures.
Disclosures: RAD-MATRIX received partial funding from The Medicines Company and Terumo. Dr. Sciahbasi had no disclosures.