User login
Biceps Tenodesis: An Evolution of Treatment
Take-Home Points
- The LHB tendon has been shown to be a significant pain generator in the shoulder.
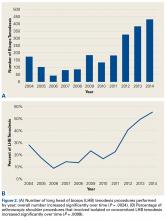
- At our institution, the number of LHB tenodeses significantly increased from 2004 to 2014.
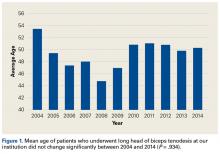
- The age of patients who underwent a LHB tenodesis did not change significantly over the study period.
- Furthermore, the percentage of shoulder procedures that involved a LHB tenodesis significantly increased over the study period.
- Biceps tenodesis has become a more common procedure to treat shoulder pathology.
Although the exact function of the long head of the biceps (LHB) tendon is not completely understood, it is accepted that the LHB tendon can be a significant source of pain within the shoulder.1-4 Patients with symptoms related to biceps pathology often present with anterior shoulder pain that worsens with flexion and supination of the affected elbow and wrist.5 Although the sensitivity and specificity of physical examination maneuvers have been called into question, special tests have been developed to aid in the diagnosis of tendonitis of the LHB. These tests include the Speed, Yergason, bear hug, and uppercut tests as well as the O’Brien test (cross-body adduction).6,7 Recent studies have found LHB pathology in 45% of patients who undergo rotator cuff repair and in 63% of patients with a subscapularis tear.8,9
Pathology of the LHB tendon, including superior labrum anterior to posterior (SLAP) tears, can be treated in many ways.5,10,11 Options include SLAP repair, biceps tenodesis, débridement, and biceps tenotomy.11,12 Results of SLAP repairs have been less than optimal, but biceps tenodesis has been effective, and avoids the issue of cramping as can be seen with biceps tenotomy and débridement.10,12,13 Surgical methods for biceps tenodesis include open subpectoral and all-arthroscopic.11,12 Both methods have had good, reliable outcomes, but the all-arthroscopic technique is relatively new.11,12,14We conducted a study to determine LHB tenodesis trends, including patient age at time of surgery. We used surgical data from fellowship-trained sports or shoulder/elbow orthopedic surgeons at a busy subspecialty-based shoulder orthopedic practice. We hypothesized that the rate of LHB tenodesis would increase significantly over time and that there would be no significant change in the age of patients who underwent LHB tenodesis.
Methods
Our Institutional Review Board exempted this study. To determine the number of LHB tenodesis procedures performed at our institution, overall and in comparison with other common arthroscopic shoulder procedures, we queried the surgical database of 4 fellowship-trained orthopedic surgeons (shoulder/elbow, Drs. Nicholson and Cole; sports, Drs. Romeo and Verma) for the period January 1, 2004 to December 31, 2014. We used Current Procedural Terminology (CPT) code 23430 to determine the number of LHB tenodesis cases, as the surgeons primarily perform an open subpectoral biceps tenodesis. Patient age at time of surgery and the date of surgery were recorded. All patients who underwent LHB tenodesis between January 1, 2004 and December 31, 2014 were included. Number of procedures performed each year by each surgeon was recorded, as were concomitant procedures performed at the same time as the LHB tenodesis. To get the denominator (and reference point) for the number of arthroscopic shoulder surgeries performed by these 4 surgeons during the study period, and thereby determine the rate of LHB tenodesis, we selected the most common shoulder arthroscopy CPT codes used in our practice: 23430, 29806, 29807, 29822, 29823, 29825, 29826, and 29827. For a patient who underwent multiple procedures on the same day (multiple CPT codes entered on the same day), only one code was counted for that day. If 23430 was among the codes, it was included, and the case was placed in the numerator; if 23430 was not among the codes, the case was placed in the denominator.
The Arthroscopy Association of North America provides descriptions for the CPT codes: 23430 (tenodesis of long tendon of biceps), 29806 (arthroscopy, shoulder, surgical; capsulorrhaphy), 29807 (arthroscopy, shoulder, surgical; repair of SLAP lesion), 29822 (arthroscopy, shoulder, surgical; débridement, limited), 29823 (arthroscopy, shoulder, surgical; débridement, extensive), 29825 (arthroscopy, shoulder, surgical; with lysis and resection of adhesions, with or without manipulation), 29826 (arthroscopy, shoulder, surgical; decompression of subacromial space with partial acromioplasty, with or without coracoacromial release), and 29827 (arthroscopy, shoulder, surgical; with rotator cuff repair).
For analysis, we divided the data into total number of arthroscopic shoulder procedures performed by each surgeon each year and number of LHB tenodesis procedures performed by each surgeon each year. Total number of patients who had an arthroscopic procedure was used to create a denominator, and number of LHB tenodesis procedures showed the percentage of arthroscopic shoulder surgery patients who underwent LHB tenodesis. (All patients who undergo biceps tenodesis also have, at the least, diagnostic shoulder arthroscopy with or without tenotomy; if the tendon is ruptured, tenotomy is unnecessary.)
Descriptive statistics were calculated as means (SDs) for continuous variables and as frequencies with percentages for categorical variables. Linear regression analysis was used to determine whether the number of LHB tenodesis procedures changed during the study period and whether patient age changed over time. Significance was set at P < .05.
Results
Of the 7640 patients who underwent arthroscopic shoulder procedures between 2004 and 2014, 2125 had LHB tenodesis (CPT code 23430).
Discussion
Tenodesis has become a common treatment option for several pathologic shoulder conditions involving the LHB tendon.5 We set out to determine trends in LHB tenodesis at a subspecialty-focused shoulder orthopedic practice and hypothesized that the rate of LHB tenodesis would increase significantly over time and that there would be no significant change in the age of patients who underwent LHB tenodesis. Our hypotheses were confirmed: The number of LHB tenodesis cases increased significantly without a significant change in patient age.
Treatment options for LHB pathology and SLAP tears include simple tenotomy, débridement, open biceps tenodesis, and arthroscopic tenodesis.11,12,15
Recent evidence has called into question the results of SLAP repairs and suggested biceps tenodesis may be a better treatment option for SLAP tears.10,13,21 Studies have found excellent outcomes with open subpectoral biceps tenodesis in the treatment of SLAP tears, and others have found better restoration of pitchers’ thoracic rotation with open subpectoral biceps tenodesis than with SLAP repair.13,14 Similarly, comparison studies have largely favored biceps tenodesis over SLAP repair, particularly in patients older than 35 years to 40 years.22 Given these results, it is not surprising that, querying the American Board of Orthopaedic Surgeons (ABOS) part II database for isolated SLAP lesions treated between 2002 and 2011, Patterson and colleagues23 found the percentage of SLAP repairs decreased from 69.3% to 44.8% (P < .0001), whereas the percentage of biceps tenodesis procedures increased from 1.9% to 18.8% (P < .0001), indicating the realization of improved outcomes with LHB tenodesis in the treatment of SLAP tears. On the other hand, in the ABOS part II database for the period 2003 to 2008, Weber and colleagues24 found that, despite a decrease in the percentage of SLAP repairs, total number of SLAP repairs increased from 9.4% to 10.1% (P = .0163). According to our study results, the number of SLAP repairs is decreasing over time, whereas the number of LHB tenodesis procedures is continuing to rise. The practice patterns seen in our study correlate with those in previous studies of the treatment of SLAP tears: good results in tenodesis groups and poor results in SLAP repair groups.10,13Werner and colleagues25 recently used the large PearlDiver database, which includes information from both private payers and Medicare, to determine overall LHB tenodesis trends in the United States for the period 2008 to 2011. Over those years, the incidence of LHB tenodesis increased 1.7-fold, and the rate of arthroscopic LHB tenodesis increased significantly more than the rate of open LHB tenodesis. These results are similar to ours in that the number of LHB tenodesis cases increased significantly over time. However, as the overwhelming majority of patients in our practice undergo open biceps tenodesis, the faster rate of growth in the arthroscopic cohort relative to the open cohort cannot be assessed. Additional randomized studies comparing biceps tenodesis, both open and arthroscopic, with SLAP repair are needed to properly determine the superiority of LHB tenodesis over SLAP repair.
One strength of this database study was the number of patients: more than 7000, 2125 of whom underwent biceps tenodesis performed by 1 of 4 fellowship-trained orthopedic surgeons. There were several study limitations. First, because the original diagnoses were not recorded, it was unclear exactly which pathologies were treated with tenodesis, limiting our ability to make recommendations regarding treatment trends for specific pathologies. Similarly, we did not assess outcome variables, which would have allowed us to draw conclusions about the effectiveness of the biceps tenodesis procedures. Furthermore, some procedures may have been coded incorrectly, and therefore some patients may have been erroneously included or excluded. In addition, using data from only one institution may have introduced bias into our conclusions, though the results are consistent with national trends. Finally, there was some variability among the 4 surgeons in the number of LHB tenodesis procedures performed, and this variability may have confounded results, though these surgeons treat biceps pathology in similar ways.
Am J Orthop. 2017;46(4):E219-E223. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Denard PJ, Dai X, Hanypsiak BT, Burkhart SS. Anatomy of the biceps tendon: implications for restoring physiological length–tension relation during biceps tenodesis with interference screw fixation. Arthroscopy. 2012;28(10):1352-1358.
2. Ejnisman B, Monteiro GC, Andreoli CV, de Castro Pochini A. Disorder of the long head of the biceps tendon. Br J Sports Med. 2010;44(5):347-354.
3. Mellano CR, Shin JJ, Yanke AB, Verma NN. Disorders of the long head of the biceps tendon. Instr Course Lect. 2015;64:567-576.
4. Szabo I, Boileau P, Walch G. The proximal biceps as a pain generator and results of tenotomy. Sports Med Arthrosc Rev. 2008;16(3):180-186.
5. Harwin SF, Birns ME, Mbabuike JJ, Porter DA, Galano GJ. Arthroscopic tenodesis of the long head of the biceps. Orthopedics. 2014;37(11):743-747.
6. Holtby R, Razmjou H. Accuracy of the Speed’s and Yergason’s tests in detecting biceps pathology and SLAP lesions: comparison with arthroscopic findings. Arthroscopy. 2004;20(3):231-236.
7. Ben Kibler W, Sciascia AD, Hester P, Dome D, Jacobs C. Clinical utility of traditional and new tests in the diagnosis of biceps tendon injuries and superior labrum anterior and posterior lesions in the shoulder. Am J Sports Med. 2009;37(9):1840-1847.
8. Lafosse L, Reiland Y, Baier GP, Toussaint B, Jost B. Anterior and posterior instability of the long head of the biceps tendon in rotator cuff tears: a new classification based on arthroscopic observations. Arthroscopy. 2007;23(1):73-80.
9. Adams CR, Schoolfield JD, Burkhart SS. The results of arthroscopic subscapularis tendon repairs. Arthroscopy. 2008;24(12):1381-1389.
10. Provencher MT, McCormick F, Dewing C, McIntire S, Solomon D. A prospective analysis of 179 type 2 superior labrum anterior and posterior repairs: outcomes and factors associated with success and failure. Am J Sports Med. 2013;41(4):880-886.
11. Gombera MM, Kahlenberg CA, Nair R, Saltzman MD, Terry MA. All-arthroscopic suprapectoral versus open subpectoral tenodesis of the long head of the biceps brachii. Am J Sports Med. 2015;43(5):1077-1083.
12. Delle Rose G, Borroni M, Silvestro A, et al. The long head of biceps as a source of pain in active population: tenotomy or tenodesis? A comparison of 2 case series with isolated lesions. Musculoskelet Surg. 2012;96(suppl 1):S47-S52.
13. Chalmers PN, Trombley R, Cip J, et al. Postoperative restoration of upper extremity motion and neuromuscular control during the overhand pitch: evaluation of tenodesis and repair for superior labral anterior-posterior tears. Am J Sports Med. 2014;42(12):2825-2836.
14. Gupta AK, Chalmers PN, Klosterman EL, et al. Subpectoral biceps tenodesis for bicipital tendonitis with SLAP tear. Orthopedics. 2015;38(1):e48-e53.
15. Ge H, Zhang Q, Sun Y, Li J, Sun L, Cheng B. Tenotomy or tenodesis for the long head of biceps lesions in shoulders: a systematic review and meta-analysis. PLoS One. 2015;10(3):e0121286.
16. Kaback LA, Gowda AL, Paller D, Green A, Blaine T. Long head biceps tenodesis with a knotless cinch suture anchor: a biomechanical analysis. Arthroscopy. 2015;31(5):831-835.
17. Kany J, Guinand R, Amaravathi RS, Alassaf I. The keyhole technique for arthroscopic tenodesis of the long head of the biceps tendon. In vivo prospective study with a radio-opaque marker. Orthop Traumatol Surg Res. 2015;101(1):31-34.
18. Mazzocca AD, Cote MP, Arciero CL, Romeo AA, Arciero RA. Clinical outcomes after subpectoral biceps tenodesis with an interference screw. Am J Sports Med. 2008;36(10):1922-1929.
19. Provencher MT, LeClere LE, Romeo AA. Subpectoral biceps tenodesis. Sports Med Arthrosc Rev. 2008;16(3):170-176.
20. Erickson BJ, Jain A, Abrams GD, et al. SLAP lesions: trends in treatment. Arthroscopy. 2016;32(6):976-981.
21. Erickson J, Lavery K, Monica J, Gatt C, Dhawan A. Surgical treatment of symptomatic superior labrum anterior-posterior tears in patients older than 40 years: a systematic review. Am J Sports Med. 2015;43(5):1274-1282.
22. Denard PJ, Ladermann A, Parsley BK, Burkhart SS. Arthroscopic biceps tenodesis compared with repair of isolated type II SLAP lesions in patients older than 35 years. Orthopedics. 2014;37(3):e292-e297.
23. Patterson BM, Creighton RA, Spang JT, Roberson JR, Kamath GV. Surgical trends in the treatment of superior labrum anterior and posterior lesions of the shoulder: analysis of data from the American Board of Orthopaedic Surgery certification examination database. Am J Sports Med. 2014;42(8):1904-1910.
24. Weber SC, Martin DF, Seiler JG 3rd, Harrast JJ. Superior labrum anterior and posterior lesions of the shoulder: incidence rates, complications, and outcomes as reported by American Board of Orthopedic Surgery. Part II candidates. Am J Sports Med. 2012;40(7):1538-1543.
25. Werner BC, Brockmeier SF, Gwathmey FW. Trends in long head biceps tenodesis. Am J Sports Med. 2015;43(3):570-578.
Take-Home Points
- The LHB tendon has been shown to be a significant pain generator in the shoulder.
- At our institution, the number of LHB tenodeses significantly increased from 2004 to 2014.
- The age of patients who underwent a LHB tenodesis did not change significantly over the study period.
- Furthermore, the percentage of shoulder procedures that involved a LHB tenodesis significantly increased over the study period.
- Biceps tenodesis has become a more common procedure to treat shoulder pathology.
Although the exact function of the long head of the biceps (LHB) tendon is not completely understood, it is accepted that the LHB tendon can be a significant source of pain within the shoulder.1-4 Patients with symptoms related to biceps pathology often present with anterior shoulder pain that worsens with flexion and supination of the affected elbow and wrist.5 Although the sensitivity and specificity of physical examination maneuvers have been called into question, special tests have been developed to aid in the diagnosis of tendonitis of the LHB. These tests include the Speed, Yergason, bear hug, and uppercut tests as well as the O’Brien test (cross-body adduction).6,7 Recent studies have found LHB pathology in 45% of patients who undergo rotator cuff repair and in 63% of patients with a subscapularis tear.8,9
Pathology of the LHB tendon, including superior labrum anterior to posterior (SLAP) tears, can be treated in many ways.5,10,11 Options include SLAP repair, biceps tenodesis, débridement, and biceps tenotomy.11,12 Results of SLAP repairs have been less than optimal, but biceps tenodesis has been effective, and avoids the issue of cramping as can be seen with biceps tenotomy and débridement.10,12,13 Surgical methods for biceps tenodesis include open subpectoral and all-arthroscopic.11,12 Both methods have had good, reliable outcomes, but the all-arthroscopic technique is relatively new.11,12,14We conducted a study to determine LHB tenodesis trends, including patient age at time of surgery. We used surgical data from fellowship-trained sports or shoulder/elbow orthopedic surgeons at a busy subspecialty-based shoulder orthopedic practice. We hypothesized that the rate of LHB tenodesis would increase significantly over time and that there would be no significant change in the age of patients who underwent LHB tenodesis.
Methods
Our Institutional Review Board exempted this study. To determine the number of LHB tenodesis procedures performed at our institution, overall and in comparison with other common arthroscopic shoulder procedures, we queried the surgical database of 4 fellowship-trained orthopedic surgeons (shoulder/elbow, Drs. Nicholson and Cole; sports, Drs. Romeo and Verma) for the period January 1, 2004 to December 31, 2014. We used Current Procedural Terminology (CPT) code 23430 to determine the number of LHB tenodesis cases, as the surgeons primarily perform an open subpectoral biceps tenodesis. Patient age at time of surgery and the date of surgery were recorded. All patients who underwent LHB tenodesis between January 1, 2004 and December 31, 2014 were included. Number of procedures performed each year by each surgeon was recorded, as were concomitant procedures performed at the same time as the LHB tenodesis. To get the denominator (and reference point) for the number of arthroscopic shoulder surgeries performed by these 4 surgeons during the study period, and thereby determine the rate of LHB tenodesis, we selected the most common shoulder arthroscopy CPT codes used in our practice: 23430, 29806, 29807, 29822, 29823, 29825, 29826, and 29827. For a patient who underwent multiple procedures on the same day (multiple CPT codes entered on the same day), only one code was counted for that day. If 23430 was among the codes, it was included, and the case was placed in the numerator; if 23430 was not among the codes, the case was placed in the denominator.
The Arthroscopy Association of North America provides descriptions for the CPT codes: 23430 (tenodesis of long tendon of biceps), 29806 (arthroscopy, shoulder, surgical; capsulorrhaphy), 29807 (arthroscopy, shoulder, surgical; repair of SLAP lesion), 29822 (arthroscopy, shoulder, surgical; débridement, limited), 29823 (arthroscopy, shoulder, surgical; débridement, extensive), 29825 (arthroscopy, shoulder, surgical; with lysis and resection of adhesions, with or without manipulation), 29826 (arthroscopy, shoulder, surgical; decompression of subacromial space with partial acromioplasty, with or without coracoacromial release), and 29827 (arthroscopy, shoulder, surgical; with rotator cuff repair).
For analysis, we divided the data into total number of arthroscopic shoulder procedures performed by each surgeon each year and number of LHB tenodesis procedures performed by each surgeon each year. Total number of patients who had an arthroscopic procedure was used to create a denominator, and number of LHB tenodesis procedures showed the percentage of arthroscopic shoulder surgery patients who underwent LHB tenodesis. (All patients who undergo biceps tenodesis also have, at the least, diagnostic shoulder arthroscopy with or without tenotomy; if the tendon is ruptured, tenotomy is unnecessary.)
Descriptive statistics were calculated as means (SDs) for continuous variables and as frequencies with percentages for categorical variables. Linear regression analysis was used to determine whether the number of LHB tenodesis procedures changed during the study period and whether patient age changed over time. Significance was set at P < .05.
Results
Of the 7640 patients who underwent arthroscopic shoulder procedures between 2004 and 2014, 2125 had LHB tenodesis (CPT code 23430).
Discussion
Tenodesis has become a common treatment option for several pathologic shoulder conditions involving the LHB tendon.5 We set out to determine trends in LHB tenodesis at a subspecialty-focused shoulder orthopedic practice and hypothesized that the rate of LHB tenodesis would increase significantly over time and that there would be no significant change in the age of patients who underwent LHB tenodesis. Our hypotheses were confirmed: The number of LHB tenodesis cases increased significantly without a significant change in patient age.
Treatment options for LHB pathology and SLAP tears include simple tenotomy, débridement, open biceps tenodesis, and arthroscopic tenodesis.11,12,15
Recent evidence has called into question the results of SLAP repairs and suggested biceps tenodesis may be a better treatment option for SLAP tears.10,13,21 Studies have found excellent outcomes with open subpectoral biceps tenodesis in the treatment of SLAP tears, and others have found better restoration of pitchers’ thoracic rotation with open subpectoral biceps tenodesis than with SLAP repair.13,14 Similarly, comparison studies have largely favored biceps tenodesis over SLAP repair, particularly in patients older than 35 years to 40 years.22 Given these results, it is not surprising that, querying the American Board of Orthopaedic Surgeons (ABOS) part II database for isolated SLAP lesions treated between 2002 and 2011, Patterson and colleagues23 found the percentage of SLAP repairs decreased from 69.3% to 44.8% (P < .0001), whereas the percentage of biceps tenodesis procedures increased from 1.9% to 18.8% (P < .0001), indicating the realization of improved outcomes with LHB tenodesis in the treatment of SLAP tears. On the other hand, in the ABOS part II database for the period 2003 to 2008, Weber and colleagues24 found that, despite a decrease in the percentage of SLAP repairs, total number of SLAP repairs increased from 9.4% to 10.1% (P = .0163). According to our study results, the number of SLAP repairs is decreasing over time, whereas the number of LHB tenodesis procedures is continuing to rise. The practice patterns seen in our study correlate with those in previous studies of the treatment of SLAP tears: good results in tenodesis groups and poor results in SLAP repair groups.10,13Werner and colleagues25 recently used the large PearlDiver database, which includes information from both private payers and Medicare, to determine overall LHB tenodesis trends in the United States for the period 2008 to 2011. Over those years, the incidence of LHB tenodesis increased 1.7-fold, and the rate of arthroscopic LHB tenodesis increased significantly more than the rate of open LHB tenodesis. These results are similar to ours in that the number of LHB tenodesis cases increased significantly over time. However, as the overwhelming majority of patients in our practice undergo open biceps tenodesis, the faster rate of growth in the arthroscopic cohort relative to the open cohort cannot be assessed. Additional randomized studies comparing biceps tenodesis, both open and arthroscopic, with SLAP repair are needed to properly determine the superiority of LHB tenodesis over SLAP repair.
One strength of this database study was the number of patients: more than 7000, 2125 of whom underwent biceps tenodesis performed by 1 of 4 fellowship-trained orthopedic surgeons. There were several study limitations. First, because the original diagnoses were not recorded, it was unclear exactly which pathologies were treated with tenodesis, limiting our ability to make recommendations regarding treatment trends for specific pathologies. Similarly, we did not assess outcome variables, which would have allowed us to draw conclusions about the effectiveness of the biceps tenodesis procedures. Furthermore, some procedures may have been coded incorrectly, and therefore some patients may have been erroneously included or excluded. In addition, using data from only one institution may have introduced bias into our conclusions, though the results are consistent with national trends. Finally, there was some variability among the 4 surgeons in the number of LHB tenodesis procedures performed, and this variability may have confounded results, though these surgeons treat biceps pathology in similar ways.
Am J Orthop. 2017;46(4):E219-E223. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- The LHB tendon has been shown to be a significant pain generator in the shoulder.
- At our institution, the number of LHB tenodeses significantly increased from 2004 to 2014.
- The age of patients who underwent a LHB tenodesis did not change significantly over the study period.
- Furthermore, the percentage of shoulder procedures that involved a LHB tenodesis significantly increased over the study period.
- Biceps tenodesis has become a more common procedure to treat shoulder pathology.
Although the exact function of the long head of the biceps (LHB) tendon is not completely understood, it is accepted that the LHB tendon can be a significant source of pain within the shoulder.1-4 Patients with symptoms related to biceps pathology often present with anterior shoulder pain that worsens with flexion and supination of the affected elbow and wrist.5 Although the sensitivity and specificity of physical examination maneuvers have been called into question, special tests have been developed to aid in the diagnosis of tendonitis of the LHB. These tests include the Speed, Yergason, bear hug, and uppercut tests as well as the O’Brien test (cross-body adduction).6,7 Recent studies have found LHB pathology in 45% of patients who undergo rotator cuff repair and in 63% of patients with a subscapularis tear.8,9
Pathology of the LHB tendon, including superior labrum anterior to posterior (SLAP) tears, can be treated in many ways.5,10,11 Options include SLAP repair, biceps tenodesis, débridement, and biceps tenotomy.11,12 Results of SLAP repairs have been less than optimal, but biceps tenodesis has been effective, and avoids the issue of cramping as can be seen with biceps tenotomy and débridement.10,12,13 Surgical methods for biceps tenodesis include open subpectoral and all-arthroscopic.11,12 Both methods have had good, reliable outcomes, but the all-arthroscopic technique is relatively new.11,12,14We conducted a study to determine LHB tenodesis trends, including patient age at time of surgery. We used surgical data from fellowship-trained sports or shoulder/elbow orthopedic surgeons at a busy subspecialty-based shoulder orthopedic practice. We hypothesized that the rate of LHB tenodesis would increase significantly over time and that there would be no significant change in the age of patients who underwent LHB tenodesis.
Methods
Our Institutional Review Board exempted this study. To determine the number of LHB tenodesis procedures performed at our institution, overall and in comparison with other common arthroscopic shoulder procedures, we queried the surgical database of 4 fellowship-trained orthopedic surgeons (shoulder/elbow, Drs. Nicholson and Cole; sports, Drs. Romeo and Verma) for the period January 1, 2004 to December 31, 2014. We used Current Procedural Terminology (CPT) code 23430 to determine the number of LHB tenodesis cases, as the surgeons primarily perform an open subpectoral biceps tenodesis. Patient age at time of surgery and the date of surgery were recorded. All patients who underwent LHB tenodesis between January 1, 2004 and December 31, 2014 were included. Number of procedures performed each year by each surgeon was recorded, as were concomitant procedures performed at the same time as the LHB tenodesis. To get the denominator (and reference point) for the number of arthroscopic shoulder surgeries performed by these 4 surgeons during the study period, and thereby determine the rate of LHB tenodesis, we selected the most common shoulder arthroscopy CPT codes used in our practice: 23430, 29806, 29807, 29822, 29823, 29825, 29826, and 29827. For a patient who underwent multiple procedures on the same day (multiple CPT codes entered on the same day), only one code was counted for that day. If 23430 was among the codes, it was included, and the case was placed in the numerator; if 23430 was not among the codes, the case was placed in the denominator.
The Arthroscopy Association of North America provides descriptions for the CPT codes: 23430 (tenodesis of long tendon of biceps), 29806 (arthroscopy, shoulder, surgical; capsulorrhaphy), 29807 (arthroscopy, shoulder, surgical; repair of SLAP lesion), 29822 (arthroscopy, shoulder, surgical; débridement, limited), 29823 (arthroscopy, shoulder, surgical; débridement, extensive), 29825 (arthroscopy, shoulder, surgical; with lysis and resection of adhesions, with or without manipulation), 29826 (arthroscopy, shoulder, surgical; decompression of subacromial space with partial acromioplasty, with or without coracoacromial release), and 29827 (arthroscopy, shoulder, surgical; with rotator cuff repair).
For analysis, we divided the data into total number of arthroscopic shoulder procedures performed by each surgeon each year and number of LHB tenodesis procedures performed by each surgeon each year. Total number of patients who had an arthroscopic procedure was used to create a denominator, and number of LHB tenodesis procedures showed the percentage of arthroscopic shoulder surgery patients who underwent LHB tenodesis. (All patients who undergo biceps tenodesis also have, at the least, diagnostic shoulder arthroscopy with or without tenotomy; if the tendon is ruptured, tenotomy is unnecessary.)
Descriptive statistics were calculated as means (SDs) for continuous variables and as frequencies with percentages for categorical variables. Linear regression analysis was used to determine whether the number of LHB tenodesis procedures changed during the study period and whether patient age changed over time. Significance was set at P < .05.
Results
Of the 7640 patients who underwent arthroscopic shoulder procedures between 2004 and 2014, 2125 had LHB tenodesis (CPT code 23430).
Discussion
Tenodesis has become a common treatment option for several pathologic shoulder conditions involving the LHB tendon.5 We set out to determine trends in LHB tenodesis at a subspecialty-focused shoulder orthopedic practice and hypothesized that the rate of LHB tenodesis would increase significantly over time and that there would be no significant change in the age of patients who underwent LHB tenodesis. Our hypotheses were confirmed: The number of LHB tenodesis cases increased significantly without a significant change in patient age.
Treatment options for LHB pathology and SLAP tears include simple tenotomy, débridement, open biceps tenodesis, and arthroscopic tenodesis.11,12,15
Recent evidence has called into question the results of SLAP repairs and suggested biceps tenodesis may be a better treatment option for SLAP tears.10,13,21 Studies have found excellent outcomes with open subpectoral biceps tenodesis in the treatment of SLAP tears, and others have found better restoration of pitchers’ thoracic rotation with open subpectoral biceps tenodesis than with SLAP repair.13,14 Similarly, comparison studies have largely favored biceps tenodesis over SLAP repair, particularly in patients older than 35 years to 40 years.22 Given these results, it is not surprising that, querying the American Board of Orthopaedic Surgeons (ABOS) part II database for isolated SLAP lesions treated between 2002 and 2011, Patterson and colleagues23 found the percentage of SLAP repairs decreased from 69.3% to 44.8% (P < .0001), whereas the percentage of biceps tenodesis procedures increased from 1.9% to 18.8% (P < .0001), indicating the realization of improved outcomes with LHB tenodesis in the treatment of SLAP tears. On the other hand, in the ABOS part II database for the period 2003 to 2008, Weber and colleagues24 found that, despite a decrease in the percentage of SLAP repairs, total number of SLAP repairs increased from 9.4% to 10.1% (P = .0163). According to our study results, the number of SLAP repairs is decreasing over time, whereas the number of LHB tenodesis procedures is continuing to rise. The practice patterns seen in our study correlate with those in previous studies of the treatment of SLAP tears: good results in tenodesis groups and poor results in SLAP repair groups.10,13Werner and colleagues25 recently used the large PearlDiver database, which includes information from both private payers and Medicare, to determine overall LHB tenodesis trends in the United States for the period 2008 to 2011. Over those years, the incidence of LHB tenodesis increased 1.7-fold, and the rate of arthroscopic LHB tenodesis increased significantly more than the rate of open LHB tenodesis. These results are similar to ours in that the number of LHB tenodesis cases increased significantly over time. However, as the overwhelming majority of patients in our practice undergo open biceps tenodesis, the faster rate of growth in the arthroscopic cohort relative to the open cohort cannot be assessed. Additional randomized studies comparing biceps tenodesis, both open and arthroscopic, with SLAP repair are needed to properly determine the superiority of LHB tenodesis over SLAP repair.
One strength of this database study was the number of patients: more than 7000, 2125 of whom underwent biceps tenodesis performed by 1 of 4 fellowship-trained orthopedic surgeons. There were several study limitations. First, because the original diagnoses were not recorded, it was unclear exactly which pathologies were treated with tenodesis, limiting our ability to make recommendations regarding treatment trends for specific pathologies. Similarly, we did not assess outcome variables, which would have allowed us to draw conclusions about the effectiveness of the biceps tenodesis procedures. Furthermore, some procedures may have been coded incorrectly, and therefore some patients may have been erroneously included or excluded. In addition, using data from only one institution may have introduced bias into our conclusions, though the results are consistent with national trends. Finally, there was some variability among the 4 surgeons in the number of LHB tenodesis procedures performed, and this variability may have confounded results, though these surgeons treat biceps pathology in similar ways.
Am J Orthop. 2017;46(4):E219-E223. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Denard PJ, Dai X, Hanypsiak BT, Burkhart SS. Anatomy of the biceps tendon: implications for restoring physiological length–tension relation during biceps tenodesis with interference screw fixation. Arthroscopy. 2012;28(10):1352-1358.
2. Ejnisman B, Monteiro GC, Andreoli CV, de Castro Pochini A. Disorder of the long head of the biceps tendon. Br J Sports Med. 2010;44(5):347-354.
3. Mellano CR, Shin JJ, Yanke AB, Verma NN. Disorders of the long head of the biceps tendon. Instr Course Lect. 2015;64:567-576.
4. Szabo I, Boileau P, Walch G. The proximal biceps as a pain generator and results of tenotomy. Sports Med Arthrosc Rev. 2008;16(3):180-186.
5. Harwin SF, Birns ME, Mbabuike JJ, Porter DA, Galano GJ. Arthroscopic tenodesis of the long head of the biceps. Orthopedics. 2014;37(11):743-747.
6. Holtby R, Razmjou H. Accuracy of the Speed’s and Yergason’s tests in detecting biceps pathology and SLAP lesions: comparison with arthroscopic findings. Arthroscopy. 2004;20(3):231-236.
7. Ben Kibler W, Sciascia AD, Hester P, Dome D, Jacobs C. Clinical utility of traditional and new tests in the diagnosis of biceps tendon injuries and superior labrum anterior and posterior lesions in the shoulder. Am J Sports Med. 2009;37(9):1840-1847.
8. Lafosse L, Reiland Y, Baier GP, Toussaint B, Jost B. Anterior and posterior instability of the long head of the biceps tendon in rotator cuff tears: a new classification based on arthroscopic observations. Arthroscopy. 2007;23(1):73-80.
9. Adams CR, Schoolfield JD, Burkhart SS. The results of arthroscopic subscapularis tendon repairs. Arthroscopy. 2008;24(12):1381-1389.
10. Provencher MT, McCormick F, Dewing C, McIntire S, Solomon D. A prospective analysis of 179 type 2 superior labrum anterior and posterior repairs: outcomes and factors associated with success and failure. Am J Sports Med. 2013;41(4):880-886.
11. Gombera MM, Kahlenberg CA, Nair R, Saltzman MD, Terry MA. All-arthroscopic suprapectoral versus open subpectoral tenodesis of the long head of the biceps brachii. Am J Sports Med. 2015;43(5):1077-1083.
12. Delle Rose G, Borroni M, Silvestro A, et al. The long head of biceps as a source of pain in active population: tenotomy or tenodesis? A comparison of 2 case series with isolated lesions. Musculoskelet Surg. 2012;96(suppl 1):S47-S52.
13. Chalmers PN, Trombley R, Cip J, et al. Postoperative restoration of upper extremity motion and neuromuscular control during the overhand pitch: evaluation of tenodesis and repair for superior labral anterior-posterior tears. Am J Sports Med. 2014;42(12):2825-2836.
14. Gupta AK, Chalmers PN, Klosterman EL, et al. Subpectoral biceps tenodesis for bicipital tendonitis with SLAP tear. Orthopedics. 2015;38(1):e48-e53.
15. Ge H, Zhang Q, Sun Y, Li J, Sun L, Cheng B. Tenotomy or tenodesis for the long head of biceps lesions in shoulders: a systematic review and meta-analysis. PLoS One. 2015;10(3):e0121286.
16. Kaback LA, Gowda AL, Paller D, Green A, Blaine T. Long head biceps tenodesis with a knotless cinch suture anchor: a biomechanical analysis. Arthroscopy. 2015;31(5):831-835.
17. Kany J, Guinand R, Amaravathi RS, Alassaf I. The keyhole technique for arthroscopic tenodesis of the long head of the biceps tendon. In vivo prospective study with a radio-opaque marker. Orthop Traumatol Surg Res. 2015;101(1):31-34.
18. Mazzocca AD, Cote MP, Arciero CL, Romeo AA, Arciero RA. Clinical outcomes after subpectoral biceps tenodesis with an interference screw. Am J Sports Med. 2008;36(10):1922-1929.
19. Provencher MT, LeClere LE, Romeo AA. Subpectoral biceps tenodesis. Sports Med Arthrosc Rev. 2008;16(3):170-176.
20. Erickson BJ, Jain A, Abrams GD, et al. SLAP lesions: trends in treatment. Arthroscopy. 2016;32(6):976-981.
21. Erickson J, Lavery K, Monica J, Gatt C, Dhawan A. Surgical treatment of symptomatic superior labrum anterior-posterior tears in patients older than 40 years: a systematic review. Am J Sports Med. 2015;43(5):1274-1282.
22. Denard PJ, Ladermann A, Parsley BK, Burkhart SS. Arthroscopic biceps tenodesis compared with repair of isolated type II SLAP lesions in patients older than 35 years. Orthopedics. 2014;37(3):e292-e297.
23. Patterson BM, Creighton RA, Spang JT, Roberson JR, Kamath GV. Surgical trends in the treatment of superior labrum anterior and posterior lesions of the shoulder: analysis of data from the American Board of Orthopaedic Surgery certification examination database. Am J Sports Med. 2014;42(8):1904-1910.
24. Weber SC, Martin DF, Seiler JG 3rd, Harrast JJ. Superior labrum anterior and posterior lesions of the shoulder: incidence rates, complications, and outcomes as reported by American Board of Orthopedic Surgery. Part II candidates. Am J Sports Med. 2012;40(7):1538-1543.
25. Werner BC, Brockmeier SF, Gwathmey FW. Trends in long head biceps tenodesis. Am J Sports Med. 2015;43(3):570-578.
1. Denard PJ, Dai X, Hanypsiak BT, Burkhart SS. Anatomy of the biceps tendon: implications for restoring physiological length–tension relation during biceps tenodesis with interference screw fixation. Arthroscopy. 2012;28(10):1352-1358.
2. Ejnisman B, Monteiro GC, Andreoli CV, de Castro Pochini A. Disorder of the long head of the biceps tendon. Br J Sports Med. 2010;44(5):347-354.
3. Mellano CR, Shin JJ, Yanke AB, Verma NN. Disorders of the long head of the biceps tendon. Instr Course Lect. 2015;64:567-576.
4. Szabo I, Boileau P, Walch G. The proximal biceps as a pain generator and results of tenotomy. Sports Med Arthrosc Rev. 2008;16(3):180-186.
5. Harwin SF, Birns ME, Mbabuike JJ, Porter DA, Galano GJ. Arthroscopic tenodesis of the long head of the biceps. Orthopedics. 2014;37(11):743-747.
6. Holtby R, Razmjou H. Accuracy of the Speed’s and Yergason’s tests in detecting biceps pathology and SLAP lesions: comparison with arthroscopic findings. Arthroscopy. 2004;20(3):231-236.
7. Ben Kibler W, Sciascia AD, Hester P, Dome D, Jacobs C. Clinical utility of traditional and new tests in the diagnosis of biceps tendon injuries and superior labrum anterior and posterior lesions in the shoulder. Am J Sports Med. 2009;37(9):1840-1847.
8. Lafosse L, Reiland Y, Baier GP, Toussaint B, Jost B. Anterior and posterior instability of the long head of the biceps tendon in rotator cuff tears: a new classification based on arthroscopic observations. Arthroscopy. 2007;23(1):73-80.
9. Adams CR, Schoolfield JD, Burkhart SS. The results of arthroscopic subscapularis tendon repairs. Arthroscopy. 2008;24(12):1381-1389.
10. Provencher MT, McCormick F, Dewing C, McIntire S, Solomon D. A prospective analysis of 179 type 2 superior labrum anterior and posterior repairs: outcomes and factors associated with success and failure. Am J Sports Med. 2013;41(4):880-886.
11. Gombera MM, Kahlenberg CA, Nair R, Saltzman MD, Terry MA. All-arthroscopic suprapectoral versus open subpectoral tenodesis of the long head of the biceps brachii. Am J Sports Med. 2015;43(5):1077-1083.
12. Delle Rose G, Borroni M, Silvestro A, et al. The long head of biceps as a source of pain in active population: tenotomy or tenodesis? A comparison of 2 case series with isolated lesions. Musculoskelet Surg. 2012;96(suppl 1):S47-S52.
13. Chalmers PN, Trombley R, Cip J, et al. Postoperative restoration of upper extremity motion and neuromuscular control during the overhand pitch: evaluation of tenodesis and repair for superior labral anterior-posterior tears. Am J Sports Med. 2014;42(12):2825-2836.
14. Gupta AK, Chalmers PN, Klosterman EL, et al. Subpectoral biceps tenodesis for bicipital tendonitis with SLAP tear. Orthopedics. 2015;38(1):e48-e53.
15. Ge H, Zhang Q, Sun Y, Li J, Sun L, Cheng B. Tenotomy or tenodesis for the long head of biceps lesions in shoulders: a systematic review and meta-analysis. PLoS One. 2015;10(3):e0121286.
16. Kaback LA, Gowda AL, Paller D, Green A, Blaine T. Long head biceps tenodesis with a knotless cinch suture anchor: a biomechanical analysis. Arthroscopy. 2015;31(5):831-835.
17. Kany J, Guinand R, Amaravathi RS, Alassaf I. The keyhole technique for arthroscopic tenodesis of the long head of the biceps tendon. In vivo prospective study with a radio-opaque marker. Orthop Traumatol Surg Res. 2015;101(1):31-34.
18. Mazzocca AD, Cote MP, Arciero CL, Romeo AA, Arciero RA. Clinical outcomes after subpectoral biceps tenodesis with an interference screw. Am J Sports Med. 2008;36(10):1922-1929.
19. Provencher MT, LeClere LE, Romeo AA. Subpectoral biceps tenodesis. Sports Med Arthrosc Rev. 2008;16(3):170-176.
20. Erickson BJ, Jain A, Abrams GD, et al. SLAP lesions: trends in treatment. Arthroscopy. 2016;32(6):976-981.
21. Erickson J, Lavery K, Monica J, Gatt C, Dhawan A. Surgical treatment of symptomatic superior labrum anterior-posterior tears in patients older than 40 years: a systematic review. Am J Sports Med. 2015;43(5):1274-1282.
22. Denard PJ, Ladermann A, Parsley BK, Burkhart SS. Arthroscopic biceps tenodesis compared with repair of isolated type II SLAP lesions in patients older than 35 years. Orthopedics. 2014;37(3):e292-e297.
23. Patterson BM, Creighton RA, Spang JT, Roberson JR, Kamath GV. Surgical trends in the treatment of superior labrum anterior and posterior lesions of the shoulder: analysis of data from the American Board of Orthopaedic Surgery certification examination database. Am J Sports Med. 2014;42(8):1904-1910.
24. Weber SC, Martin DF, Seiler JG 3rd, Harrast JJ. Superior labrum anterior and posterior lesions of the shoulder: incidence rates, complications, and outcomes as reported by American Board of Orthopedic Surgery. Part II candidates. Am J Sports Med. 2012;40(7):1538-1543.
25. Werner BC, Brockmeier SF, Gwathmey FW. Trends in long head biceps tenodesis. Am J Sports Med. 2015;43(3):570-578.
No survival benefit with adjuvant girentuximab in high-risk RCC
The monoclonal antibody girentuximab does not appear to have a clinical benefit in patients with high-risk clear cell renal cell carcinoma (ccRCC).
Adjuvant therapy with girentuximab failed to improve either disease-free or overall survival, compared with placebo, in a cohort of patients who had undergone full surgical resection, according to phase 3 results of the ARISER trial.
In a subset of patients with CAIX scores of 200 or greater, however, there was disease-free survival benefit although it did not reach significance, reported lead author Karim Chamie, MD, of the David Geffen School of Medicine at UCLA, and his colleagues (JAMA Oncol. 2017;3[7]:913-20).
The drug was well tolerated, with an excellent safety profile, and there were no reported drug-related serious adverse events.
“Our finding that girentuximab was more effective in the subgroup of patients younger than 65 years is consistent with these observations and, while requiring prospective confirmation, suggests that an insufficient activation of [antibody-dependent cellular cytotoxicity] could explain the failure of its efficacy,” wrote Dr. Chamie and his colleagues.
The authors also point out that “virtually all trials of adjuvant therapy in high-risk RCC have failed to show a treatment benefit,” and thus illustrates the difficulty in identifying successful adjuvant therapies.
“The success of future adjuvant trials will require use of inclusion criteria sufficiently broad to meet accrual goals while limiting inclusion to patients who are most likely to benefit from therapy,” they write.
Girentuximab is a chimeric monoclonal antibody that binds carbonic anhydrase IX, a cell surface glycoprotein that is ubiquitously expressed in ccRCC. Phase 2 studies showed the agent to be safe and active in this setting, and served as the basis for conducting the current phase 3 ARISER trial (Adjuvant Rencarex Immunotherapy Phase 3 Trial to Study Efficacy in Nonmetastatic RCC).
The randomized, double-blind, placebo-controlled trial was conducted at 142 academic medical centers in 15 countries in North and South America and Europe and included a cohort of 864 patients who had undergone partial or radical nephrectomy for ccRCC and fell into one of the following high-risk groups: pT3/pT4Nx/N0M0 or pTanyN+M0 or pT1b/pT2Nx/N0M0 with nuclear grade 3 or greater.
The cohort was randomly assigned to either a single loading dose of girentuximab, 50 mg (week 1), followed by weekly intravenous infusions of girentuximab, 20 mg (weeks 2-24), or placebo, stratified by risk group and region.
The overall disease-free survival was comparable for the two groups: at 5 years it was 53.9% and 51.6% for the girentuximab and placebo groups, respectively, while the median disease-free survival was 71.4 months (interquartile range, 3 months to not reached) for patients who received girentuximab and was not reached for those receiving placebo (P = .74).
Median overall survival was not reached for either of the two groups, and there was no difference in overall survival between arms (hazard ratio, 0.99; 95% confidence interval, 0.74-1.32).
Adverse events were reported in 185 patients (21.6%), and were comparable between groups, and serious events occurred in 72 patients (8.4%), and were also comparable between the two arms.
The study was funded by Wilex, AG. Dr. Chamie receives research support from and serves as a consultant for UroGen Pharma, receives grant support from Phase One Foundation and Stop Cancer, and is a consultant for Cold Genesys and a scientific advisory board member of Altor. Several coauthors reported relationships with industry.
The monoclonal antibody girentuximab does not appear to have a clinical benefit in patients with high-risk clear cell renal cell carcinoma (ccRCC).
Adjuvant therapy with girentuximab failed to improve either disease-free or overall survival, compared with placebo, in a cohort of patients who had undergone full surgical resection, according to phase 3 results of the ARISER trial.
In a subset of patients with CAIX scores of 200 or greater, however, there was disease-free survival benefit although it did not reach significance, reported lead author Karim Chamie, MD, of the David Geffen School of Medicine at UCLA, and his colleagues (JAMA Oncol. 2017;3[7]:913-20).
The drug was well tolerated, with an excellent safety profile, and there were no reported drug-related serious adverse events.
“Our finding that girentuximab was more effective in the subgroup of patients younger than 65 years is consistent with these observations and, while requiring prospective confirmation, suggests that an insufficient activation of [antibody-dependent cellular cytotoxicity] could explain the failure of its efficacy,” wrote Dr. Chamie and his colleagues.
The authors also point out that “virtually all trials of adjuvant therapy in high-risk RCC have failed to show a treatment benefit,” and thus illustrates the difficulty in identifying successful adjuvant therapies.
“The success of future adjuvant trials will require use of inclusion criteria sufficiently broad to meet accrual goals while limiting inclusion to patients who are most likely to benefit from therapy,” they write.
Girentuximab is a chimeric monoclonal antibody that binds carbonic anhydrase IX, a cell surface glycoprotein that is ubiquitously expressed in ccRCC. Phase 2 studies showed the agent to be safe and active in this setting, and served as the basis for conducting the current phase 3 ARISER trial (Adjuvant Rencarex Immunotherapy Phase 3 Trial to Study Efficacy in Nonmetastatic RCC).
The randomized, double-blind, placebo-controlled trial was conducted at 142 academic medical centers in 15 countries in North and South America and Europe and included a cohort of 864 patients who had undergone partial or radical nephrectomy for ccRCC and fell into one of the following high-risk groups: pT3/pT4Nx/N0M0 or pTanyN+M0 or pT1b/pT2Nx/N0M0 with nuclear grade 3 or greater.
The cohort was randomly assigned to either a single loading dose of girentuximab, 50 mg (week 1), followed by weekly intravenous infusions of girentuximab, 20 mg (weeks 2-24), or placebo, stratified by risk group and region.
The overall disease-free survival was comparable for the two groups: at 5 years it was 53.9% and 51.6% for the girentuximab and placebo groups, respectively, while the median disease-free survival was 71.4 months (interquartile range, 3 months to not reached) for patients who received girentuximab and was not reached for those receiving placebo (P = .74).
Median overall survival was not reached for either of the two groups, and there was no difference in overall survival between arms (hazard ratio, 0.99; 95% confidence interval, 0.74-1.32).
Adverse events were reported in 185 patients (21.6%), and were comparable between groups, and serious events occurred in 72 patients (8.4%), and were also comparable between the two arms.
The study was funded by Wilex, AG. Dr. Chamie receives research support from and serves as a consultant for UroGen Pharma, receives grant support from Phase One Foundation and Stop Cancer, and is a consultant for Cold Genesys and a scientific advisory board member of Altor. Several coauthors reported relationships with industry.
The monoclonal antibody girentuximab does not appear to have a clinical benefit in patients with high-risk clear cell renal cell carcinoma (ccRCC).
Adjuvant therapy with girentuximab failed to improve either disease-free or overall survival, compared with placebo, in a cohort of patients who had undergone full surgical resection, according to phase 3 results of the ARISER trial.
In a subset of patients with CAIX scores of 200 or greater, however, there was disease-free survival benefit although it did not reach significance, reported lead author Karim Chamie, MD, of the David Geffen School of Medicine at UCLA, and his colleagues (JAMA Oncol. 2017;3[7]:913-20).
The drug was well tolerated, with an excellent safety profile, and there were no reported drug-related serious adverse events.
“Our finding that girentuximab was more effective in the subgroup of patients younger than 65 years is consistent with these observations and, while requiring prospective confirmation, suggests that an insufficient activation of [antibody-dependent cellular cytotoxicity] could explain the failure of its efficacy,” wrote Dr. Chamie and his colleagues.
The authors also point out that “virtually all trials of adjuvant therapy in high-risk RCC have failed to show a treatment benefit,” and thus illustrates the difficulty in identifying successful adjuvant therapies.
“The success of future adjuvant trials will require use of inclusion criteria sufficiently broad to meet accrual goals while limiting inclusion to patients who are most likely to benefit from therapy,” they write.
Girentuximab is a chimeric monoclonal antibody that binds carbonic anhydrase IX, a cell surface glycoprotein that is ubiquitously expressed in ccRCC. Phase 2 studies showed the agent to be safe and active in this setting, and served as the basis for conducting the current phase 3 ARISER trial (Adjuvant Rencarex Immunotherapy Phase 3 Trial to Study Efficacy in Nonmetastatic RCC).
The randomized, double-blind, placebo-controlled trial was conducted at 142 academic medical centers in 15 countries in North and South America and Europe and included a cohort of 864 patients who had undergone partial or radical nephrectomy for ccRCC and fell into one of the following high-risk groups: pT3/pT4Nx/N0M0 or pTanyN+M0 or pT1b/pT2Nx/N0M0 with nuclear grade 3 or greater.
The cohort was randomly assigned to either a single loading dose of girentuximab, 50 mg (week 1), followed by weekly intravenous infusions of girentuximab, 20 mg (weeks 2-24), or placebo, stratified by risk group and region.
The overall disease-free survival was comparable for the two groups: at 5 years it was 53.9% and 51.6% for the girentuximab and placebo groups, respectively, while the median disease-free survival was 71.4 months (interquartile range, 3 months to not reached) for patients who received girentuximab and was not reached for those receiving placebo (P = .74).
Median overall survival was not reached for either of the two groups, and there was no difference in overall survival between arms (hazard ratio, 0.99; 95% confidence interval, 0.74-1.32).
Adverse events were reported in 185 patients (21.6%), and were comparable between groups, and serious events occurred in 72 patients (8.4%), and were also comparable between the two arms.
The study was funded by Wilex, AG. Dr. Chamie receives research support from and serves as a consultant for UroGen Pharma, receives grant support from Phase One Foundation and Stop Cancer, and is a consultant for Cold Genesys and a scientific advisory board member of Altor. Several coauthors reported relationships with industry.
FROM JAMA ONCOLOGY
Key clinical point: Adjuvant girentuximab did not confer a survival benefit in patients with high-risk clear cell renal cell carcinoma.
Major finding: Five-year disease-free survival was 53.9% and 51.6% for the girentuximab and placebo groups, respectively.
Data source: A phase 3 placebo controlled multicenter clinical trial that included 864 patients with high-risk clear cell renal cell carcinoma.
Disclosures: The study was funded by Wilex, AG. Dr. Chamie receives research support from and serves as a consultant for UroGen Pharma, receives grant support from Phase One Foundation and Stop Cancer, and is a consultant for Cold Genesys and a scientific advisory board member of Altor. Several coauthors reported relationships with industry.
New tool predicts antimicrobial resistance in sepsis
Use of a clinical decision tree predicted antibiotic resistance in sepsis patients infected with gram-negative bacteria, based on data from 1,618 patients.
Increasing rates of bacterial resistance have “contributed to the unwarranted empiric administration of broad-spectrum antibiotics, further promoting resistance emergence across microbial species,” said M. Cristina Vazquez Guillamet, MD, of the University of New Mexico, Albuquerque, and her colleagues (Clin Infect Dis. cix612. 2017 Jul 10. doi: 10.1093/cid/cix612).
The researchers identified adults with sepsis or septic shock caused by bloodstream infections who were treated at a single center between 2008 and 2015. They developed clinical decision trees using the CHAID algorithm (Chi squared Automatic Interaction Detection) to analyze risk factors for resistance associated with three antibiotics: piperacillin-tazobactam (PT), cefepime (CE), and meropenem (ME).
Overall, resistance rates to PT, CE, and ME were 29%, 22%, and 9%, respectively, and 6.6% of the isolates were resistant to all three antibiotics.
Factors associated with increased resistance risk included residence in a nursing home, transfer from an outside hospital, and prior antibiotics use. Resistance to ME was associated with infection with Pseudomonas or Acinetobacter spp, the researchers noted, and resistance to PT was associated with central nervous system and central venous catheter infections.
Clinical decision trees were able to separate patients at low risk for resistance to PT and CE, as well as those with a risk greater than 30% of resistance to PT, CE, or ME. “We also found good overall agreement between the accuracies of the [multivariable logistic regression] models and the decision tree analyses for predicting antibiotic resistance,” the researchers said.
The findings were limited by several factors, including the use of data from a single center and incomplete reporting of previous antibiotic exposure, the researchers noted. However, the results “provide a framework for how empiric antibiotics can be tailored according to decision tree patient clusters,” they said.
Combining user-friendly clinical decision trees and multivariable logistic regression models may offer the best opportunities for hospitals to derive local models to help with antimicrobial prescription.
The researchers had no financial conflicts to disclose.
Use of a clinical decision tree predicted antibiotic resistance in sepsis patients infected with gram-negative bacteria, based on data from 1,618 patients.
Increasing rates of bacterial resistance have “contributed to the unwarranted empiric administration of broad-spectrum antibiotics, further promoting resistance emergence across microbial species,” said M. Cristina Vazquez Guillamet, MD, of the University of New Mexico, Albuquerque, and her colleagues (Clin Infect Dis. cix612. 2017 Jul 10. doi: 10.1093/cid/cix612).
The researchers identified adults with sepsis or septic shock caused by bloodstream infections who were treated at a single center between 2008 and 2015. They developed clinical decision trees using the CHAID algorithm (Chi squared Automatic Interaction Detection) to analyze risk factors for resistance associated with three antibiotics: piperacillin-tazobactam (PT), cefepime (CE), and meropenem (ME).
Overall, resistance rates to PT, CE, and ME were 29%, 22%, and 9%, respectively, and 6.6% of the isolates were resistant to all three antibiotics.
Factors associated with increased resistance risk included residence in a nursing home, transfer from an outside hospital, and prior antibiotics use. Resistance to ME was associated with infection with Pseudomonas or Acinetobacter spp, the researchers noted, and resistance to PT was associated with central nervous system and central venous catheter infections.
Clinical decision trees were able to separate patients at low risk for resistance to PT and CE, as well as those with a risk greater than 30% of resistance to PT, CE, or ME. “We also found good overall agreement between the accuracies of the [multivariable logistic regression] models and the decision tree analyses for predicting antibiotic resistance,” the researchers said.
The findings were limited by several factors, including the use of data from a single center and incomplete reporting of previous antibiotic exposure, the researchers noted. However, the results “provide a framework for how empiric antibiotics can be tailored according to decision tree patient clusters,” they said.
Combining user-friendly clinical decision trees and multivariable logistic regression models may offer the best opportunities for hospitals to derive local models to help with antimicrobial prescription.
The researchers had no financial conflicts to disclose.
Use of a clinical decision tree predicted antibiotic resistance in sepsis patients infected with gram-negative bacteria, based on data from 1,618 patients.
Increasing rates of bacterial resistance have “contributed to the unwarranted empiric administration of broad-spectrum antibiotics, further promoting resistance emergence across microbial species,” said M. Cristina Vazquez Guillamet, MD, of the University of New Mexico, Albuquerque, and her colleagues (Clin Infect Dis. cix612. 2017 Jul 10. doi: 10.1093/cid/cix612).
The researchers identified adults with sepsis or septic shock caused by bloodstream infections who were treated at a single center between 2008 and 2015. They developed clinical decision trees using the CHAID algorithm (Chi squared Automatic Interaction Detection) to analyze risk factors for resistance associated with three antibiotics: piperacillin-tazobactam (PT), cefepime (CE), and meropenem (ME).
Overall, resistance rates to PT, CE, and ME were 29%, 22%, and 9%, respectively, and 6.6% of the isolates were resistant to all three antibiotics.
Factors associated with increased resistance risk included residence in a nursing home, transfer from an outside hospital, and prior antibiotics use. Resistance to ME was associated with infection with Pseudomonas or Acinetobacter spp, the researchers noted, and resistance to PT was associated with central nervous system and central venous catheter infections.
Clinical decision trees were able to separate patients at low risk for resistance to PT and CE, as well as those with a risk greater than 30% of resistance to PT, CE, or ME. “We also found good overall agreement between the accuracies of the [multivariable logistic regression] models and the decision tree analyses for predicting antibiotic resistance,” the researchers said.
The findings were limited by several factors, including the use of data from a single center and incomplete reporting of previous antibiotic exposure, the researchers noted. However, the results “provide a framework for how empiric antibiotics can be tailored according to decision tree patient clusters,” they said.
Combining user-friendly clinical decision trees and multivariable logistic regression models may offer the best opportunities for hospitals to derive local models to help with antimicrobial prescription.
The researchers had no financial conflicts to disclose.
FROM CLINICAL INFECTIOUS DISEASES
Key clinical point:
Major finding: The model found prevalence rates for resistance to piperacillin-tazobactam, cefepime, and meropenem of 28.6%, 21.8%, and 8.5%, respectively.
Data source: A review of 1,618 adults with sepsis.
Disclosures: The researchers had no financial conflicts to disclose.
VA Commits to Improving Health Care Provider Efficiency
The VA is working to implement Government Accountability Office (GAO) recommendations on improving efficiency and reporting of health care providers, according to Carolyn Clancy, MD, deputy under secretary for organizational excellence at the VHA. Dr. Clancy told members of the House Committee on Veterans’ Affairs that the “VA concurred with GAO’s recommendations and is already working to complete them.”
In July 2017, the GAO issued the report “Improvements Needed in Data and Monitoring of Clinical Productivity and Efficiency.” The report found that “VA’s productivity metrics and efficiency models may not provide complete and accurate information on provider productivity and VAMC efficiency.” Based on its findings, the GAO recommended that the “VA develop a policy requiring VAMCs to monitor and improve clinical inefficiency through a standard process, such as establishing performance standards based on VA’s efficiency models, and develop remediation plans for addressing clinical inefficiencies.” The GAO also made 4 specific recommendations:
- Expand existing productivity metrics to track the productivity of all providers of care to veterans, including contract physicians and some advanced practice providers;
- Ensure the accuracy of underlying staffing and workload data by, training all providers on coding clinical procedures;
- Create a policy for all VAMCs to monitor and improve clinical efficiency by establishing performance standards based on VA’s efficiency models and developing a remediation plan for addressing clinical inefficiency; and
- Establish an ongoing process to systematically review VAMCs and ensure that VAMCs and VISNs are implementing those plans and addressing low clinical productivity and inefficiency.
In her testimony, Dr. Clancy took pains to reassure the House committee that she agreed with the GAO recommendations. “VA appreciates our colleagues at GAO’s efforts and the efforts of others to improve clinical efficiency and productivity,” she told the panel. “Mr. Chairman, I am proud of the health care our employees provide to our nation’s veterans. Together with Congress, I look forward to making sure that VA will be a good steward of taxpayer dollars while providing this care in a productive and efficient manner.”
Dr. Clancy explained to the Committee that the VA will expand the use of some of its measures, such as the Specialty Productivity-Access Report and Quadrant (SPARQ) tool. In addition, Dr. Clancy pledged that the VA would take up training in clinical coding for health care providers as well as an effort to improve the efficiency of specialty providers. “We have also undertaken a comprehensive education and communication plan about the specialty physician productivity and staffing standards,” she told the committee. “Our specialty physicians are committed to demonstrating and improving specialty productivity and access.”
In addition, Dr. Clancy insisted that plans to improve clinical efficiency must be developed at each VAMC and that remediation plans would be tracked at both the facility and VISN. The central office will “review the progress VAMCs are making on the remediation plans for addressing low clinical productivity twice a year with the VISN,” she said. The expected completion date for this will be March 2018.
The VA is working to implement Government Accountability Office (GAO) recommendations on improving efficiency and reporting of health care providers, according to Carolyn Clancy, MD, deputy under secretary for organizational excellence at the VHA. Dr. Clancy told members of the House Committee on Veterans’ Affairs that the “VA concurred with GAO’s recommendations and is already working to complete them.”
In July 2017, the GAO issued the report “Improvements Needed in Data and Monitoring of Clinical Productivity and Efficiency.” The report found that “VA’s productivity metrics and efficiency models may not provide complete and accurate information on provider productivity and VAMC efficiency.” Based on its findings, the GAO recommended that the “VA develop a policy requiring VAMCs to monitor and improve clinical inefficiency through a standard process, such as establishing performance standards based on VA’s efficiency models, and develop remediation plans for addressing clinical inefficiencies.” The GAO also made 4 specific recommendations:
- Expand existing productivity metrics to track the productivity of all providers of care to veterans, including contract physicians and some advanced practice providers;
- Ensure the accuracy of underlying staffing and workload data by, training all providers on coding clinical procedures;
- Create a policy for all VAMCs to monitor and improve clinical efficiency by establishing performance standards based on VA’s efficiency models and developing a remediation plan for addressing clinical inefficiency; and
- Establish an ongoing process to systematically review VAMCs and ensure that VAMCs and VISNs are implementing those plans and addressing low clinical productivity and inefficiency.
In her testimony, Dr. Clancy took pains to reassure the House committee that she agreed with the GAO recommendations. “VA appreciates our colleagues at GAO’s efforts and the efforts of others to improve clinical efficiency and productivity,” she told the panel. “Mr. Chairman, I am proud of the health care our employees provide to our nation’s veterans. Together with Congress, I look forward to making sure that VA will be a good steward of taxpayer dollars while providing this care in a productive and efficient manner.”
Dr. Clancy explained to the Committee that the VA will expand the use of some of its measures, such as the Specialty Productivity-Access Report and Quadrant (SPARQ) tool. In addition, Dr. Clancy pledged that the VA would take up training in clinical coding for health care providers as well as an effort to improve the efficiency of specialty providers. “We have also undertaken a comprehensive education and communication plan about the specialty physician productivity and staffing standards,” she told the committee. “Our specialty physicians are committed to demonstrating and improving specialty productivity and access.”
In addition, Dr. Clancy insisted that plans to improve clinical efficiency must be developed at each VAMC and that remediation plans would be tracked at both the facility and VISN. The central office will “review the progress VAMCs are making on the remediation plans for addressing low clinical productivity twice a year with the VISN,” she said. The expected completion date for this will be March 2018.
The VA is working to implement Government Accountability Office (GAO) recommendations on improving efficiency and reporting of health care providers, according to Carolyn Clancy, MD, deputy under secretary for organizational excellence at the VHA. Dr. Clancy told members of the House Committee on Veterans’ Affairs that the “VA concurred with GAO’s recommendations and is already working to complete them.”
In July 2017, the GAO issued the report “Improvements Needed in Data and Monitoring of Clinical Productivity and Efficiency.” The report found that “VA’s productivity metrics and efficiency models may not provide complete and accurate information on provider productivity and VAMC efficiency.” Based on its findings, the GAO recommended that the “VA develop a policy requiring VAMCs to monitor and improve clinical inefficiency through a standard process, such as establishing performance standards based on VA’s efficiency models, and develop remediation plans for addressing clinical inefficiencies.” The GAO also made 4 specific recommendations:
- Expand existing productivity metrics to track the productivity of all providers of care to veterans, including contract physicians and some advanced practice providers;
- Ensure the accuracy of underlying staffing and workload data by, training all providers on coding clinical procedures;
- Create a policy for all VAMCs to monitor and improve clinical efficiency by establishing performance standards based on VA’s efficiency models and developing a remediation plan for addressing clinical inefficiency; and
- Establish an ongoing process to systematically review VAMCs and ensure that VAMCs and VISNs are implementing those plans and addressing low clinical productivity and inefficiency.
In her testimony, Dr. Clancy took pains to reassure the House committee that she agreed with the GAO recommendations. “VA appreciates our colleagues at GAO’s efforts and the efforts of others to improve clinical efficiency and productivity,” she told the panel. “Mr. Chairman, I am proud of the health care our employees provide to our nation’s veterans. Together with Congress, I look forward to making sure that VA will be a good steward of taxpayer dollars while providing this care in a productive and efficient manner.”
Dr. Clancy explained to the Committee that the VA will expand the use of some of its measures, such as the Specialty Productivity-Access Report and Quadrant (SPARQ) tool. In addition, Dr. Clancy pledged that the VA would take up training in clinical coding for health care providers as well as an effort to improve the efficiency of specialty providers. “We have also undertaken a comprehensive education and communication plan about the specialty physician productivity and staffing standards,” she told the committee. “Our specialty physicians are committed to demonstrating and improving specialty productivity and access.”
In addition, Dr. Clancy insisted that plans to improve clinical efficiency must be developed at each VAMC and that remediation plans would be tracked at both the facility and VISN. The central office will “review the progress VAMCs are making on the remediation plans for addressing low clinical productivity twice a year with the VISN,” she said. The expected completion date for this will be March 2018.
An Action Plan for Better COPD Care
A “detailed, patient-centered roadmap” for addressing the third leading cause of death in the U.S.—chronic obstructive pulmonary disease (COPD)—will provide a “cohesive tool” for health professionals, according to the National Heart, Lung, and Blood Institute (NHLBI). Together with federal and non-federal partners, NHLBI released the first-ever COPD National Action Plan in May at the American Thoracic Society International Conference in Washington, DC.
The plan was developed from comments shared at a “COPD Town Hall” by patients and their families, health care providers, academics, and industry representatives. It takes a unified approach identifying the specific work doctors, educators, researchers, federal agencies, patients, advocates, and the biomedical industry can do to make a difference, according to official at NHLBI.
An estimated 16 million Americans have COPD—and millions more may have it and not know. However COPD often is preventable and highly treatable, early diagnosis can lead to better outcomes. With that as the goal, the plan’s developers aim to:
- Empower patients, families, and caregivers to recognize and reduce the burden of COPD
- Equip health care professionals to provide comprehensive care to people with COPD
- Collect, analyze, report, and disseminate COPD data
- Increase and sustain COPD research
- Turn COPD recommendations into research and public health care actions
Involving patients and families has been “invaluable,” said James Kiley, PhD, director of NHLBI’s division of Lung Diseases. “The different perspectives brought by those who live these issues every day contributed to making this a clear, coordinated way forward for all stakeholders.”
A “detailed, patient-centered roadmap” for addressing the third leading cause of death in the U.S.—chronic obstructive pulmonary disease (COPD)—will provide a “cohesive tool” for health professionals, according to the National Heart, Lung, and Blood Institute (NHLBI). Together with federal and non-federal partners, NHLBI released the first-ever COPD National Action Plan in May at the American Thoracic Society International Conference in Washington, DC.
The plan was developed from comments shared at a “COPD Town Hall” by patients and their families, health care providers, academics, and industry representatives. It takes a unified approach identifying the specific work doctors, educators, researchers, federal agencies, patients, advocates, and the biomedical industry can do to make a difference, according to official at NHLBI.
An estimated 16 million Americans have COPD—and millions more may have it and not know. However COPD often is preventable and highly treatable, early diagnosis can lead to better outcomes. With that as the goal, the plan’s developers aim to:
- Empower patients, families, and caregivers to recognize and reduce the burden of COPD
- Equip health care professionals to provide comprehensive care to people with COPD
- Collect, analyze, report, and disseminate COPD data
- Increase and sustain COPD research
- Turn COPD recommendations into research and public health care actions
Involving patients and families has been “invaluable,” said James Kiley, PhD, director of NHLBI’s division of Lung Diseases. “The different perspectives brought by those who live these issues every day contributed to making this a clear, coordinated way forward for all stakeholders.”
A “detailed, patient-centered roadmap” for addressing the third leading cause of death in the U.S.—chronic obstructive pulmonary disease (COPD)—will provide a “cohesive tool” for health professionals, according to the National Heart, Lung, and Blood Institute (NHLBI). Together with federal and non-federal partners, NHLBI released the first-ever COPD National Action Plan in May at the American Thoracic Society International Conference in Washington, DC.
The plan was developed from comments shared at a “COPD Town Hall” by patients and their families, health care providers, academics, and industry representatives. It takes a unified approach identifying the specific work doctors, educators, researchers, federal agencies, patients, advocates, and the biomedical industry can do to make a difference, according to official at NHLBI.
An estimated 16 million Americans have COPD—and millions more may have it and not know. However COPD often is preventable and highly treatable, early diagnosis can lead to better outcomes. With that as the goal, the plan’s developers aim to:
- Empower patients, families, and caregivers to recognize and reduce the burden of COPD
- Equip health care professionals to provide comprehensive care to people with COPD
- Collect, analyze, report, and disseminate COPD data
- Increase and sustain COPD research
- Turn COPD recommendations into research and public health care actions
Involving patients and families has been “invaluable,” said James Kiley, PhD, director of NHLBI’s division of Lung Diseases. “The different perspectives brought by those who live these issues every day contributed to making this a clear, coordinated way forward for all stakeholders.”
CAR T-cell therapy ‘highly effective’ in high-risk CLL
A chimeric antigen receptor (CAR) T-cell therapy is “highly effective” in high-risk patients with chronic lymphocytic leukemia (CLL), according to researchers.
The CD19 CAR T-cell therapy, JCAR014, produced an overall response rate of 71% and a complete response (CR) rate of 17% in a phase 1/2 trial of patients with relapsed/refractory CLL.
Eighty-three percent of patients experienced cytokine release syndrome (CRS), and 33% developed neurotoxicity. One patient died of CRS/neurotoxicity.
Researchers reported these results in The Journal of Clinical Oncology.
The study was supported by Juno Therapeutics, Life Science Discovery Fund, the Bezos Family, the University of British Columbia Clinical Investigator Program, and grants from the National Cancer Institute and National Institute of Diabetes and Digestive and Kidney Diseases.
The trial included 24 patients with relapsed or refractory CLL. The patients’ median age was 61 (range, 40-73), and they had received a median of 5 prior therapies (range, 3-9). Nineteen patients had progressed while on ibrutinib, and 3 could not tolerate the drug.
“It was not known whether CAR T cells could be used to treat these high-risk CLL patients,” said study author Cameron Turtle, MBBS, PhD, of Fred Hutchinson Cancer Research Center in Seattle, Washington.
“Our study shows that CD19 CAR T cells are a highly promising treatment for CLL patients who have failed ibrutinib.”
Treatment and safety
All 24 patients received lymphodepletion prior to JCAR014. Lymphodepleting regimens consisted of cyclophosphamide, fludarabine, or both.
Patients received JCAR014 at 1 of 3 dose levels—2 x 105, 2 x 106, or 2 x 107 CAR T cells/kg.
Twenty patients (83%) developed CRS—18 with grade 1/2, 1 with grade 4, and 1 with grade 5 CRS.
Eight patients (33%) developed neurotoxicity, all of whom also had CRS. Five patients had grade 3 neurotoxicity, and 1 had grade 5 (same patient with grade 5 CRS).
Initial response
The overall response rate, according to IWCLL criteria, was 71%. Seventeen patients responded, 4 with CRs and 13 with partial responses (PRs).
One of the patients who achieved a CR had received a second dose of JCAR014, without lymphodepletion, 14 days after the first dose.
One of the patients with a PR initially had stable disease (SD) at the 4-week assessment but was in PR 8 weeks later.
Twenty-one patients had a bone marrow evaluation 4 weeks after treatment with JCAR014. Seventeen of these patients (81%) had no residual disease according to high-resolution flow cytometry.
The researchers also performed IGH sequencing of bone marrow in 12 patients with no residual disease by flow cytometry. Seven of these patients (58%) had no detectable malignant IGH sequences 4 weeks after receiving JCAR014.
Second dose
Six patients who had persistent disease or who relapsed after receiving JCAR014 received a second cycle of lymphodepletion and JCAR014 at the same dose (n=1) or a 10-fold higher dose (n=5).
Four of these patients developed CRS (2 grade 3 or higher), and 1 developed reversible neurotoxicity (grade 3).
Two patients achieved a CR and had no residual disease by flow cytometry or IGH sequencing.
Survival
Responders had significantly superior progression-free survival (PFS) and overall survival (OS) compared to non-responders.
The median PFS was 9.8 months in patients who achieved a CR, was not reached in those with a PR, and was 1.1 months in patients with progressive disease (PD) or SD (P=0.0068 for CR/PR vs SD/PD).
The median OS was not reached in patients who achieved a CR or a PR, but it was 11.2 months in patients with SD or PD (P=0.0011 for CR/PR vs SD/PD).
The researchers also found that IGH sequencing revealed patients with durable PFS.
The median PFS was not reached in patients with no malignant IGH sequences, but it was 8.5 months in IGH-positive patients (P=0.253). The median OS was not reached in either group (P=0.25). ![]()
A chimeric antigen receptor (CAR) T-cell therapy is “highly effective” in high-risk patients with chronic lymphocytic leukemia (CLL), according to researchers.
The CD19 CAR T-cell therapy, JCAR014, produced an overall response rate of 71% and a complete response (CR) rate of 17% in a phase 1/2 trial of patients with relapsed/refractory CLL.
Eighty-three percent of patients experienced cytokine release syndrome (CRS), and 33% developed neurotoxicity. One patient died of CRS/neurotoxicity.
Researchers reported these results in The Journal of Clinical Oncology.
The study was supported by Juno Therapeutics, Life Science Discovery Fund, the Bezos Family, the University of British Columbia Clinical Investigator Program, and grants from the National Cancer Institute and National Institute of Diabetes and Digestive and Kidney Diseases.
The trial included 24 patients with relapsed or refractory CLL. The patients’ median age was 61 (range, 40-73), and they had received a median of 5 prior therapies (range, 3-9). Nineteen patients had progressed while on ibrutinib, and 3 could not tolerate the drug.
“It was not known whether CAR T cells could be used to treat these high-risk CLL patients,” said study author Cameron Turtle, MBBS, PhD, of Fred Hutchinson Cancer Research Center in Seattle, Washington.
“Our study shows that CD19 CAR T cells are a highly promising treatment for CLL patients who have failed ibrutinib.”
Treatment and safety
All 24 patients received lymphodepletion prior to JCAR014. Lymphodepleting regimens consisted of cyclophosphamide, fludarabine, or both.
Patients received JCAR014 at 1 of 3 dose levels—2 x 105, 2 x 106, or 2 x 107 CAR T cells/kg.
Twenty patients (83%) developed CRS—18 with grade 1/2, 1 with grade 4, and 1 with grade 5 CRS.
Eight patients (33%) developed neurotoxicity, all of whom also had CRS. Five patients had grade 3 neurotoxicity, and 1 had grade 5 (same patient with grade 5 CRS).
Initial response
The overall response rate, according to IWCLL criteria, was 71%. Seventeen patients responded, 4 with CRs and 13 with partial responses (PRs).
One of the patients who achieved a CR had received a second dose of JCAR014, without lymphodepletion, 14 days after the first dose.
One of the patients with a PR initially had stable disease (SD) at the 4-week assessment but was in PR 8 weeks later.
Twenty-one patients had a bone marrow evaluation 4 weeks after treatment with JCAR014. Seventeen of these patients (81%) had no residual disease according to high-resolution flow cytometry.
The researchers also performed IGH sequencing of bone marrow in 12 patients with no residual disease by flow cytometry. Seven of these patients (58%) had no detectable malignant IGH sequences 4 weeks after receiving JCAR014.
Second dose
Six patients who had persistent disease or who relapsed after receiving JCAR014 received a second cycle of lymphodepletion and JCAR014 at the same dose (n=1) or a 10-fold higher dose (n=5).
Four of these patients developed CRS (2 grade 3 or higher), and 1 developed reversible neurotoxicity (grade 3).
Two patients achieved a CR and had no residual disease by flow cytometry or IGH sequencing.
Survival
Responders had significantly superior progression-free survival (PFS) and overall survival (OS) compared to non-responders.
The median PFS was 9.8 months in patients who achieved a CR, was not reached in those with a PR, and was 1.1 months in patients with progressive disease (PD) or SD (P=0.0068 for CR/PR vs SD/PD).
The median OS was not reached in patients who achieved a CR or a PR, but it was 11.2 months in patients with SD or PD (P=0.0011 for CR/PR vs SD/PD).
The researchers also found that IGH sequencing revealed patients with durable PFS.
The median PFS was not reached in patients with no malignant IGH sequences, but it was 8.5 months in IGH-positive patients (P=0.253). The median OS was not reached in either group (P=0.25). ![]()
A chimeric antigen receptor (CAR) T-cell therapy is “highly effective” in high-risk patients with chronic lymphocytic leukemia (CLL), according to researchers.
The CD19 CAR T-cell therapy, JCAR014, produced an overall response rate of 71% and a complete response (CR) rate of 17% in a phase 1/2 trial of patients with relapsed/refractory CLL.
Eighty-three percent of patients experienced cytokine release syndrome (CRS), and 33% developed neurotoxicity. One patient died of CRS/neurotoxicity.
Researchers reported these results in The Journal of Clinical Oncology.
The study was supported by Juno Therapeutics, Life Science Discovery Fund, the Bezos Family, the University of British Columbia Clinical Investigator Program, and grants from the National Cancer Institute and National Institute of Diabetes and Digestive and Kidney Diseases.
The trial included 24 patients with relapsed or refractory CLL. The patients’ median age was 61 (range, 40-73), and they had received a median of 5 prior therapies (range, 3-9). Nineteen patients had progressed while on ibrutinib, and 3 could not tolerate the drug.
“It was not known whether CAR T cells could be used to treat these high-risk CLL patients,” said study author Cameron Turtle, MBBS, PhD, of Fred Hutchinson Cancer Research Center in Seattle, Washington.
“Our study shows that CD19 CAR T cells are a highly promising treatment for CLL patients who have failed ibrutinib.”
Treatment and safety
All 24 patients received lymphodepletion prior to JCAR014. Lymphodepleting regimens consisted of cyclophosphamide, fludarabine, or both.
Patients received JCAR014 at 1 of 3 dose levels—2 x 105, 2 x 106, or 2 x 107 CAR T cells/kg.
Twenty patients (83%) developed CRS—18 with grade 1/2, 1 with grade 4, and 1 with grade 5 CRS.
Eight patients (33%) developed neurotoxicity, all of whom also had CRS. Five patients had grade 3 neurotoxicity, and 1 had grade 5 (same patient with grade 5 CRS).
Initial response
The overall response rate, according to IWCLL criteria, was 71%. Seventeen patients responded, 4 with CRs and 13 with partial responses (PRs).
One of the patients who achieved a CR had received a second dose of JCAR014, without lymphodepletion, 14 days after the first dose.
One of the patients with a PR initially had stable disease (SD) at the 4-week assessment but was in PR 8 weeks later.
Twenty-one patients had a bone marrow evaluation 4 weeks after treatment with JCAR014. Seventeen of these patients (81%) had no residual disease according to high-resolution flow cytometry.
The researchers also performed IGH sequencing of bone marrow in 12 patients with no residual disease by flow cytometry. Seven of these patients (58%) had no detectable malignant IGH sequences 4 weeks after receiving JCAR014.
Second dose
Six patients who had persistent disease or who relapsed after receiving JCAR014 received a second cycle of lymphodepletion and JCAR014 at the same dose (n=1) or a 10-fold higher dose (n=5).
Four of these patients developed CRS (2 grade 3 or higher), and 1 developed reversible neurotoxicity (grade 3).
Two patients achieved a CR and had no residual disease by flow cytometry or IGH sequencing.
Survival
Responders had significantly superior progression-free survival (PFS) and overall survival (OS) compared to non-responders.
The median PFS was 9.8 months in patients who achieved a CR, was not reached in those with a PR, and was 1.1 months in patients with progressive disease (PD) or SD (P=0.0068 for CR/PR vs SD/PD).
The median OS was not reached in patients who achieved a CR or a PR, but it was 11.2 months in patients with SD or PD (P=0.0011 for CR/PR vs SD/PD).
The researchers also found that IGH sequencing revealed patients with durable PFS.
The median PFS was not reached in patients with no malignant IGH sequences, but it was 8.5 months in IGH-positive patients (P=0.253). The median OS was not reached in either group (P=0.25). ![]()
Death is most frequent major adverse outcome after VTE
BERLIN—Data from the GARFIELD-VTE registry showed that, in the first 6 months after a patient was diagnosed with venous thromboembolism (VTE), death was the most frequent major adverse outcome.
More than half of the deaths were related to cancer, with small percentages of patients dying from VTE complications and bleeding events.
GARFIELD-VTE is a prospective registry designed to provide insight into the management of VTE in everyday clinical practice.
Six-month results from the registry were presented in a poster at the International Society on Thrombosis and Haemostasis (ISTH) 2017 Congress (PB 1196).
The GARFIELD-VTE registry has enrolled more than 10,000 patients with acute VTE—including deep vein thrombosis (DVT) and pulmonary embolism (PE)—from across 415 sites in 28 countries.
Alexander G. G. Turpie, MD, of McMaster University in Hamilton, Ontario, Canada, and his colleagues presented data on 10,315 patients with at least 6 months of follow-up.
Baseline characteristics
The patients’ median age was 60.2 (range, 46.2-71.7), and 49.9% were female. Most patients were white (69.3%), 19.6% were Asian, 4.4% were black, 0.6% were multiracial, 4.3% were classified as “other,” and 1.9% were of unknown race.
Six percent of patients had a family history of VTE (first-degree relatives), 15.1% had a prior episode of DVT and/or PE themselves, and 37.5% had at least 1 provoking factor for VTE.
Most patients (61.8%) had DVT alone, but 38.3% had PE with or without DVT.
The registry included patients with active cancer (9.1%), a history of cancer (10.7%), thrombophilia (2.9%), chronic immobilization (5.6%), heart failure (3.2%), and renal insufficiency (3.5%).
Outcomes
Over 6 months of follow-up, the following events were reported:
- Any bleeding—622 total bleeds or 13.6 per 100 person-years
- All-cause mortality—460 total deaths or 9.7 per 100 person-years
- Recurrent VTE—169 events or 3.6 per 100 person-years
- Major bleeding—106 events or 2.2 per 100 person-years
- Myocardial infarction—42 events or 0.9 per 100 person-years
- Stroke/transient ischemic attack—38 events or 0.8 per 100 person-years.
Nearly 5% of patients died (4.5%, n=460). More than half (54.3%, n=250) of these deaths were cancer-related.
Other causes of death included:
- Cardiac death—7.0% (n=32)
- PE—3.5% (n=16)
- Bleeding—3.3% (n=15)
- VTE complications—1.3% (n=6)
- Stroke—1.1% (n=5)
- Other cause—17.8% (n=82)
- Unknown cause—11.7% (n=54).
Additional data from the GARFIELD-VTE registry were presented at ISTH 2017 as posters (PB 460 and PB 1188) and in an oral presentation (ASY 35.4). The next set of data from the registry is slated to be presented at the 2017 ASH Annual Meeting.
GARFIELD-VTE is supported by an unrestricted educational grant from Bayer AG. For further information on the registry, visit http://www.garfieldregistry.org. ![]()
BERLIN—Data from the GARFIELD-VTE registry showed that, in the first 6 months after a patient was diagnosed with venous thromboembolism (VTE), death was the most frequent major adverse outcome.
More than half of the deaths were related to cancer, with small percentages of patients dying from VTE complications and bleeding events.
GARFIELD-VTE is a prospective registry designed to provide insight into the management of VTE in everyday clinical practice.
Six-month results from the registry were presented in a poster at the International Society on Thrombosis and Haemostasis (ISTH) 2017 Congress (PB 1196).
The GARFIELD-VTE registry has enrolled more than 10,000 patients with acute VTE—including deep vein thrombosis (DVT) and pulmonary embolism (PE)—from across 415 sites in 28 countries.
Alexander G. G. Turpie, MD, of McMaster University in Hamilton, Ontario, Canada, and his colleagues presented data on 10,315 patients with at least 6 months of follow-up.
Baseline characteristics
The patients’ median age was 60.2 (range, 46.2-71.7), and 49.9% were female. Most patients were white (69.3%), 19.6% were Asian, 4.4% were black, 0.6% were multiracial, 4.3% were classified as “other,” and 1.9% were of unknown race.
Six percent of patients had a family history of VTE (first-degree relatives), 15.1% had a prior episode of DVT and/or PE themselves, and 37.5% had at least 1 provoking factor for VTE.
Most patients (61.8%) had DVT alone, but 38.3% had PE with or without DVT.
The registry included patients with active cancer (9.1%), a history of cancer (10.7%), thrombophilia (2.9%), chronic immobilization (5.6%), heart failure (3.2%), and renal insufficiency (3.5%).
Outcomes
Over 6 months of follow-up, the following events were reported:
- Any bleeding—622 total bleeds or 13.6 per 100 person-years
- All-cause mortality—460 total deaths or 9.7 per 100 person-years
- Recurrent VTE—169 events or 3.6 per 100 person-years
- Major bleeding—106 events or 2.2 per 100 person-years
- Myocardial infarction—42 events or 0.9 per 100 person-years
- Stroke/transient ischemic attack—38 events or 0.8 per 100 person-years.
Nearly 5% of patients died (4.5%, n=460). More than half (54.3%, n=250) of these deaths were cancer-related.
Other causes of death included:
- Cardiac death—7.0% (n=32)
- PE—3.5% (n=16)
- Bleeding—3.3% (n=15)
- VTE complications—1.3% (n=6)
- Stroke—1.1% (n=5)
- Other cause—17.8% (n=82)
- Unknown cause—11.7% (n=54).
Additional data from the GARFIELD-VTE registry were presented at ISTH 2017 as posters (PB 460 and PB 1188) and in an oral presentation (ASY 35.4). The next set of data from the registry is slated to be presented at the 2017 ASH Annual Meeting.
GARFIELD-VTE is supported by an unrestricted educational grant from Bayer AG. For further information on the registry, visit http://www.garfieldregistry.org. ![]()
BERLIN—Data from the GARFIELD-VTE registry showed that, in the first 6 months after a patient was diagnosed with venous thromboembolism (VTE), death was the most frequent major adverse outcome.
More than half of the deaths were related to cancer, with small percentages of patients dying from VTE complications and bleeding events.
GARFIELD-VTE is a prospective registry designed to provide insight into the management of VTE in everyday clinical practice.
Six-month results from the registry were presented in a poster at the International Society on Thrombosis and Haemostasis (ISTH) 2017 Congress (PB 1196).
The GARFIELD-VTE registry has enrolled more than 10,000 patients with acute VTE—including deep vein thrombosis (DVT) and pulmonary embolism (PE)—from across 415 sites in 28 countries.
Alexander G. G. Turpie, MD, of McMaster University in Hamilton, Ontario, Canada, and his colleagues presented data on 10,315 patients with at least 6 months of follow-up.
Baseline characteristics
The patients’ median age was 60.2 (range, 46.2-71.7), and 49.9% were female. Most patients were white (69.3%), 19.6% were Asian, 4.4% were black, 0.6% were multiracial, 4.3% were classified as “other,” and 1.9% were of unknown race.
Six percent of patients had a family history of VTE (first-degree relatives), 15.1% had a prior episode of DVT and/or PE themselves, and 37.5% had at least 1 provoking factor for VTE.
Most patients (61.8%) had DVT alone, but 38.3% had PE with or without DVT.
The registry included patients with active cancer (9.1%), a history of cancer (10.7%), thrombophilia (2.9%), chronic immobilization (5.6%), heart failure (3.2%), and renal insufficiency (3.5%).
Outcomes
Over 6 months of follow-up, the following events were reported:
- Any bleeding—622 total bleeds or 13.6 per 100 person-years
- All-cause mortality—460 total deaths or 9.7 per 100 person-years
- Recurrent VTE—169 events or 3.6 per 100 person-years
- Major bleeding—106 events or 2.2 per 100 person-years
- Myocardial infarction—42 events or 0.9 per 100 person-years
- Stroke/transient ischemic attack—38 events or 0.8 per 100 person-years.
Nearly 5% of patients died (4.5%, n=460). More than half (54.3%, n=250) of these deaths were cancer-related.
Other causes of death included:
- Cardiac death—7.0% (n=32)
- PE—3.5% (n=16)
- Bleeding—3.3% (n=15)
- VTE complications—1.3% (n=6)
- Stroke—1.1% (n=5)
- Other cause—17.8% (n=82)
- Unknown cause—11.7% (n=54).
Additional data from the GARFIELD-VTE registry were presented at ISTH 2017 as posters (PB 460 and PB 1188) and in an oral presentation (ASY 35.4). The next set of data from the registry is slated to be presented at the 2017 ASH Annual Meeting.
GARFIELD-VTE is supported by an unrestricted educational grant from Bayer AG. For further information on the registry, visit http://www.garfieldregistry.org. ![]()
Combo may be option for elderly patients with untreated AML
MADRID—The combination of venetoclax and low-dose cytarabine (VEN+LDAC) appears to be a feasible treatment option for elderly patients with untreated acute myeloid leukemia (AML) who are ineligible for intensive chemotherapy.
In a phase 1/2 study of such patients, VEN+LDAC was considered well-tolerated, conferring moderate myelosuppression and largely low-grade non-hematologic toxicities.
In addition, the combination produced “rapid and durable” responses, and early death rates were low, according to Andrew H. Wei, MBBS, PhD, of Monash University in Melbourne, Victoria, Australia.
However, nearly three-quarters of patients ultimately discontinued the treatment, many due to disease progression.
Dr Wei presented these results at the 22nd Congress of the European Hematology Association (EHA) as abstract S473. AbbVie and Genentech, the companies developing and marketing venetoclax, provided financial support for this study.
“Expression of pro-survival proteins is an established mechanism of chemoresistance in diverse cancers,” Dr Wei noted. “BCL-2 is 1 of 5 pro-survival molecules which functions to sequester pro-apoptotic molecules and tip the balance in favor of cell survival.”
“Venetoclax is a potent and specific inhibitor of BCL-2 which releases these pro-apoptotic molecules, tipping the balance in favor of cell death. Cytotoxic drugs are well-known to increase the burden of BH3-only proteins, and so it was surmised that the combination of chemotherapy, such as cytarabine, with venetoclax could augment the clinical response.”
Patients
Dr Wei presented data on 61 AML patients treated with VEN+LDAC. He noted that this was a poor-risk population, with nearly half of patients over the age of 75 at baseline.
The patients’ median age was 74 (range, 66-87), and 64% were male. Nearly half of patients had an ECOG performance status of 1 (49%), 30% had a status of 0, and 21% had a status of 2.
Forty-four percent of patients had secondary AML, and 28% had prior treatment with a hypomethylating agent (HMA). Sixty-one percent of patients had intermediate-risk cytogenetics, and 31% had poor-risk cytogenetics.
Treatment
The patients received oral venetoclax at 600 mg daily on days 1 to 28 and subcutaneous cytarabine at 20 mg/m2 daily on days 1 to 10 of each 28-day cycle.
In the first cycle, the dose of venetoclax was ramped up gradually—no dose on day 1, 50 mg on day 2, 100 mg on day 3, 200 mg on day 4, 400 mg on day 5, and 600 mg thereafter.
Patients received prophylaxis for tumor lysis syndrome prior to starting cycle 1, and they were hospitalized to enable observation.
The median time on study treatment was 6 months (range, <1 to 19 months). Seventy-two percent of patients discontinued treatment.
Reasons for discontinuation included:
- Progressive disease without death—26%
- Progressive disease with death—10%
- Adverse event (AE) related to progression—10%
- AE not related to progression—8%
- Withdrawn consent—8%
- Other reasons—18%.
Safety
The most common AEs of any grade (occurring in at least 30% of patients) were nausea (74%), hypokalemia (46%), diarrhea (46%), fatigue (44%), decreased appetite (41%), constipation (34%), hypomagnesemia (34%), vomiting (31%), thrombocytopenia (44%), febrile neutropenia (38%), and neutropenia (33%).
Grade 3/4 hematologic AEs (occurring in at least 10% of patients) included thrombocytopenia (44%), febrile neutropenia (36%), neutropenia (33%), and anemia (28%).
Grade 3/4 non-hematologic AEs (occurring in at least 10% of patients) included hypokalemia (16%), hypophosphatemia (13%), and hypertension (12%).
Response and survival
The overall response rate was 65%, with 25% of patients achieving a complete response (CR), 38% having a CR with incomplete blood count recovery (CRi), and 2% experiencing a partial response.
Dr Wei noted that VEN+LDAC was active across subgroups.
The CR/CRi rate was 76% among patients with intermediate-risk cytogenetics and 47% among patients with poor-risk cytogenetics.
The CR/CRi rate was 70% among patients older than 75, 52% among patients with secondary AML, 66% among patients with no prior HMA exposure, and 53% in patients with prior HMA exposure.
“Although responses were slightly lower in patients with poor cytogenetic risk, prior HMA exposure, and secondary AML . . ., these responses are far in excess of what we would expect with [LDAC] alone,” Dr Wei said.
“Furthermore, the median time to response was very rapid, and this is extremely important to get patients into remission and avoid the medium-term consequences of active AML.”
The median time to response was 1 month (range, <1 to 9 months).
The 30-day death rate was 3%, the 60-day death rate was 15%, and the median overall survival was approximately 12 months.
Based on these results, AbbVie has initiated a phase 3 trial comparing VEN+LDAC to LDAC alone in elderly patients with untreated AML who are ineligible for intensive chemotherapy. ![]()
MADRID—The combination of venetoclax and low-dose cytarabine (VEN+LDAC) appears to be a feasible treatment option for elderly patients with untreated acute myeloid leukemia (AML) who are ineligible for intensive chemotherapy.
In a phase 1/2 study of such patients, VEN+LDAC was considered well-tolerated, conferring moderate myelosuppression and largely low-grade non-hematologic toxicities.
In addition, the combination produced “rapid and durable” responses, and early death rates were low, according to Andrew H. Wei, MBBS, PhD, of Monash University in Melbourne, Victoria, Australia.
However, nearly three-quarters of patients ultimately discontinued the treatment, many due to disease progression.
Dr Wei presented these results at the 22nd Congress of the European Hematology Association (EHA) as abstract S473. AbbVie and Genentech, the companies developing and marketing venetoclax, provided financial support for this study.
“Expression of pro-survival proteins is an established mechanism of chemoresistance in diverse cancers,” Dr Wei noted. “BCL-2 is 1 of 5 pro-survival molecules which functions to sequester pro-apoptotic molecules and tip the balance in favor of cell survival.”
“Venetoclax is a potent and specific inhibitor of BCL-2 which releases these pro-apoptotic molecules, tipping the balance in favor of cell death. Cytotoxic drugs are well-known to increase the burden of BH3-only proteins, and so it was surmised that the combination of chemotherapy, such as cytarabine, with venetoclax could augment the clinical response.”
Patients
Dr Wei presented data on 61 AML patients treated with VEN+LDAC. He noted that this was a poor-risk population, with nearly half of patients over the age of 75 at baseline.
The patients’ median age was 74 (range, 66-87), and 64% were male. Nearly half of patients had an ECOG performance status of 1 (49%), 30% had a status of 0, and 21% had a status of 2.
Forty-four percent of patients had secondary AML, and 28% had prior treatment with a hypomethylating agent (HMA). Sixty-one percent of patients had intermediate-risk cytogenetics, and 31% had poor-risk cytogenetics.
Treatment
The patients received oral venetoclax at 600 mg daily on days 1 to 28 and subcutaneous cytarabine at 20 mg/m2 daily on days 1 to 10 of each 28-day cycle.
In the first cycle, the dose of venetoclax was ramped up gradually—no dose on day 1, 50 mg on day 2, 100 mg on day 3, 200 mg on day 4, 400 mg on day 5, and 600 mg thereafter.
Patients received prophylaxis for tumor lysis syndrome prior to starting cycle 1, and they were hospitalized to enable observation.
The median time on study treatment was 6 months (range, <1 to 19 months). Seventy-two percent of patients discontinued treatment.
Reasons for discontinuation included:
- Progressive disease without death—26%
- Progressive disease with death—10%
- Adverse event (AE) related to progression—10%
- AE not related to progression—8%
- Withdrawn consent—8%
- Other reasons—18%.
Safety
The most common AEs of any grade (occurring in at least 30% of patients) were nausea (74%), hypokalemia (46%), diarrhea (46%), fatigue (44%), decreased appetite (41%), constipation (34%), hypomagnesemia (34%), vomiting (31%), thrombocytopenia (44%), febrile neutropenia (38%), and neutropenia (33%).
Grade 3/4 hematologic AEs (occurring in at least 10% of patients) included thrombocytopenia (44%), febrile neutropenia (36%), neutropenia (33%), and anemia (28%).
Grade 3/4 non-hematologic AEs (occurring in at least 10% of patients) included hypokalemia (16%), hypophosphatemia (13%), and hypertension (12%).
Response and survival
The overall response rate was 65%, with 25% of patients achieving a complete response (CR), 38% having a CR with incomplete blood count recovery (CRi), and 2% experiencing a partial response.
Dr Wei noted that VEN+LDAC was active across subgroups.
The CR/CRi rate was 76% among patients with intermediate-risk cytogenetics and 47% among patients with poor-risk cytogenetics.
The CR/CRi rate was 70% among patients older than 75, 52% among patients with secondary AML, 66% among patients with no prior HMA exposure, and 53% in patients with prior HMA exposure.
“Although responses were slightly lower in patients with poor cytogenetic risk, prior HMA exposure, and secondary AML . . ., these responses are far in excess of what we would expect with [LDAC] alone,” Dr Wei said.
“Furthermore, the median time to response was very rapid, and this is extremely important to get patients into remission and avoid the medium-term consequences of active AML.”
The median time to response was 1 month (range, <1 to 9 months).
The 30-day death rate was 3%, the 60-day death rate was 15%, and the median overall survival was approximately 12 months.
Based on these results, AbbVie has initiated a phase 3 trial comparing VEN+LDAC to LDAC alone in elderly patients with untreated AML who are ineligible for intensive chemotherapy. ![]()
MADRID—The combination of venetoclax and low-dose cytarabine (VEN+LDAC) appears to be a feasible treatment option for elderly patients with untreated acute myeloid leukemia (AML) who are ineligible for intensive chemotherapy.
In a phase 1/2 study of such patients, VEN+LDAC was considered well-tolerated, conferring moderate myelosuppression and largely low-grade non-hematologic toxicities.
In addition, the combination produced “rapid and durable” responses, and early death rates were low, according to Andrew H. Wei, MBBS, PhD, of Monash University in Melbourne, Victoria, Australia.
However, nearly three-quarters of patients ultimately discontinued the treatment, many due to disease progression.
Dr Wei presented these results at the 22nd Congress of the European Hematology Association (EHA) as abstract S473. AbbVie and Genentech, the companies developing and marketing venetoclax, provided financial support for this study.
“Expression of pro-survival proteins is an established mechanism of chemoresistance in diverse cancers,” Dr Wei noted. “BCL-2 is 1 of 5 pro-survival molecules which functions to sequester pro-apoptotic molecules and tip the balance in favor of cell survival.”
“Venetoclax is a potent and specific inhibitor of BCL-2 which releases these pro-apoptotic molecules, tipping the balance in favor of cell death. Cytotoxic drugs are well-known to increase the burden of BH3-only proteins, and so it was surmised that the combination of chemotherapy, such as cytarabine, with venetoclax could augment the clinical response.”
Patients
Dr Wei presented data on 61 AML patients treated with VEN+LDAC. He noted that this was a poor-risk population, with nearly half of patients over the age of 75 at baseline.
The patients’ median age was 74 (range, 66-87), and 64% were male. Nearly half of patients had an ECOG performance status of 1 (49%), 30% had a status of 0, and 21% had a status of 2.
Forty-four percent of patients had secondary AML, and 28% had prior treatment with a hypomethylating agent (HMA). Sixty-one percent of patients had intermediate-risk cytogenetics, and 31% had poor-risk cytogenetics.
Treatment
The patients received oral venetoclax at 600 mg daily on days 1 to 28 and subcutaneous cytarabine at 20 mg/m2 daily on days 1 to 10 of each 28-day cycle.
In the first cycle, the dose of venetoclax was ramped up gradually—no dose on day 1, 50 mg on day 2, 100 mg on day 3, 200 mg on day 4, 400 mg on day 5, and 600 mg thereafter.
Patients received prophylaxis for tumor lysis syndrome prior to starting cycle 1, and they were hospitalized to enable observation.
The median time on study treatment was 6 months (range, <1 to 19 months). Seventy-two percent of patients discontinued treatment.
Reasons for discontinuation included:
- Progressive disease without death—26%
- Progressive disease with death—10%
- Adverse event (AE) related to progression—10%
- AE not related to progression—8%
- Withdrawn consent—8%
- Other reasons—18%.
Safety
The most common AEs of any grade (occurring in at least 30% of patients) were nausea (74%), hypokalemia (46%), diarrhea (46%), fatigue (44%), decreased appetite (41%), constipation (34%), hypomagnesemia (34%), vomiting (31%), thrombocytopenia (44%), febrile neutropenia (38%), and neutropenia (33%).
Grade 3/4 hematologic AEs (occurring in at least 10% of patients) included thrombocytopenia (44%), febrile neutropenia (36%), neutropenia (33%), and anemia (28%).
Grade 3/4 non-hematologic AEs (occurring in at least 10% of patients) included hypokalemia (16%), hypophosphatemia (13%), and hypertension (12%).
Response and survival
The overall response rate was 65%, with 25% of patients achieving a complete response (CR), 38% having a CR with incomplete blood count recovery (CRi), and 2% experiencing a partial response.
Dr Wei noted that VEN+LDAC was active across subgroups.
The CR/CRi rate was 76% among patients with intermediate-risk cytogenetics and 47% among patients with poor-risk cytogenetics.
The CR/CRi rate was 70% among patients older than 75, 52% among patients with secondary AML, 66% among patients with no prior HMA exposure, and 53% in patients with prior HMA exposure.
“Although responses were slightly lower in patients with poor cytogenetic risk, prior HMA exposure, and secondary AML . . ., these responses are far in excess of what we would expect with [LDAC] alone,” Dr Wei said.
“Furthermore, the median time to response was very rapid, and this is extremely important to get patients into remission and avoid the medium-term consequences of active AML.”
The median time to response was 1 month (range, <1 to 9 months).
The 30-day death rate was 3%, the 60-day death rate was 15%, and the median overall survival was approximately 12 months.
Based on these results, AbbVie has initiated a phase 3 trial comparing VEN+LDAC to LDAC alone in elderly patients with untreated AML who are ineligible for intensive chemotherapy. ![]()
Adding cefepime to vancomycin improved MRSA bacteremia outcomes
NEW ORLEANS – Compared with vancomycin monotherapy, vancomycin combined with cefepime improved some outcomes for patients with methicillin-resistant Staphylococcus aureus (MRSA) bloodstream infections, a retrospective study of 109 patients revealed.
A lower likelihood of microbiological failure and fewer bloodstream infections persisting 7 days or more were the notable differences between treatment groups.
All patients had at least 72 hours of vancomycin therapy to treat MRSA bacteremia confirmed by blood culture. During 2008-2015, 38 adults received vancomycin monotherapy and 71 received vancomycin plus 24 hours or more of cefepime.
Compared with monotherapy, the combination treatment was associated with a nonsignificant reduction in the primary composite treatment failure outcome of 30-day all-cause mortality, in bacteremia duration of 7 days or more, and in 60-day bloodstream-infection recurrence: 55% for monotherapy versus 42% for combination therapy (P = .195). The difference was primarily associated with decreased duration of sepsis and fewer MRSA bloodstream infections persisting 7 days or more in the combination cohort.
Rates of bacteremia duration of 7 days or more were 42% in monotherapy patients and 20% in combination patients (P = .013). Differences in 60-day bloodstream-infection recurrence were nonsignificant, 8% versus 4%, respectively (P = .42).
Thirty-day mortality, however, was lower among monotherapy patients than combination patients – 13% vs. 25% – although the difference was nonsignificant (P = .21).
“From what I see here … it seems like they will have a lower duration of bacteremia, which is always great,” Ms. Atwan said. “You want to decrease length of stay in the hospital,” which will cut down on costs and on patients’ risks of getting more infections.
Although the primary outcome was a composite endpoint, “when we looked at them separately, we found the patients in the combination group had more mortality,” Ms. Atwan said at the annual meeting of the American Society for Microbiology. “That surprised me initially. But those patients are sicker and more likely to get dual coverage.”
The investigators confirmed the association between the severity of MRSA bacteremia and combination therapy by looking at Acute Physiology and Chronic Health Evaluation (APACHE II) scores. The median APACHE score was 23 in the combination group, compared with 13.5 in the monotherapy group (P = 0003). Higher APACHE scores were associated with greater odds of meeting the composite failure endpoint (adjusted odds ratio, 1.08) and of developing endocarditis (aOR, 3.6) in multivariate analyses.
More patients in the combination group had pneumonia as the primary source of infection than did patients in the monotherapy group: 54% vs. 29% (P = .016). Further, more of them had skin or soft tissue infections as the primary infection source: 29% vs. 13% (P = .036).
Although the exact mechanism remains unknown, synergy between the two agents could be caused by an increase in penicillin-binding proteins, Ms. Atwan said.
The study is still ongoing; Ms. Atwan hopes additional patients and data will lead to statistically significant differences between the outcomes of combination therapy and vancomycin monotherapy.
“I want to say that combination therapy is something you will always want to go to when you have a sicker patient, but I can’t really tell you that combination therapy is going to cause better outcomes for your patient,” she cautioned. “Hopefully, I can by the end of the study.”
In the meantime, “it looks like vancomycin and beta-lactams could be beneficial for MRSA bacteremia,” she added.
The researchers noted that although vancomycin monotherapy is a mainstay of treatment for MRSA bloodstream infections, emergence of reduced susceptibility and treatment failures warrants other therapeutic strategies.
Ms. Atwan had no relevant financial disclosures.
NEW ORLEANS – Compared with vancomycin monotherapy, vancomycin combined with cefepime improved some outcomes for patients with methicillin-resistant Staphylococcus aureus (MRSA) bloodstream infections, a retrospective study of 109 patients revealed.
A lower likelihood of microbiological failure and fewer bloodstream infections persisting 7 days or more were the notable differences between treatment groups.
All patients had at least 72 hours of vancomycin therapy to treat MRSA bacteremia confirmed by blood culture. During 2008-2015, 38 adults received vancomycin monotherapy and 71 received vancomycin plus 24 hours or more of cefepime.
Compared with monotherapy, the combination treatment was associated with a nonsignificant reduction in the primary composite treatment failure outcome of 30-day all-cause mortality, in bacteremia duration of 7 days or more, and in 60-day bloodstream-infection recurrence: 55% for monotherapy versus 42% for combination therapy (P = .195). The difference was primarily associated with decreased duration of sepsis and fewer MRSA bloodstream infections persisting 7 days or more in the combination cohort.
Rates of bacteremia duration of 7 days or more were 42% in monotherapy patients and 20% in combination patients (P = .013). Differences in 60-day bloodstream-infection recurrence were nonsignificant, 8% versus 4%, respectively (P = .42).
Thirty-day mortality, however, was lower among monotherapy patients than combination patients – 13% vs. 25% – although the difference was nonsignificant (P = .21).
“From what I see here … it seems like they will have a lower duration of bacteremia, which is always great,” Ms. Atwan said. “You want to decrease length of stay in the hospital,” which will cut down on costs and on patients’ risks of getting more infections.
Although the primary outcome was a composite endpoint, “when we looked at them separately, we found the patients in the combination group had more mortality,” Ms. Atwan said at the annual meeting of the American Society for Microbiology. “That surprised me initially. But those patients are sicker and more likely to get dual coverage.”
The investigators confirmed the association between the severity of MRSA bacteremia and combination therapy by looking at Acute Physiology and Chronic Health Evaluation (APACHE II) scores. The median APACHE score was 23 in the combination group, compared with 13.5 in the monotherapy group (P = 0003). Higher APACHE scores were associated with greater odds of meeting the composite failure endpoint (adjusted odds ratio, 1.08) and of developing endocarditis (aOR, 3.6) in multivariate analyses.
More patients in the combination group had pneumonia as the primary source of infection than did patients in the monotherapy group: 54% vs. 29% (P = .016). Further, more of them had skin or soft tissue infections as the primary infection source: 29% vs. 13% (P = .036).
Although the exact mechanism remains unknown, synergy between the two agents could be caused by an increase in penicillin-binding proteins, Ms. Atwan said.
The study is still ongoing; Ms. Atwan hopes additional patients and data will lead to statistically significant differences between the outcomes of combination therapy and vancomycin monotherapy.
“I want to say that combination therapy is something you will always want to go to when you have a sicker patient, but I can’t really tell you that combination therapy is going to cause better outcomes for your patient,” she cautioned. “Hopefully, I can by the end of the study.”
In the meantime, “it looks like vancomycin and beta-lactams could be beneficial for MRSA bacteremia,” she added.
The researchers noted that although vancomycin monotherapy is a mainstay of treatment for MRSA bloodstream infections, emergence of reduced susceptibility and treatment failures warrants other therapeutic strategies.
Ms. Atwan had no relevant financial disclosures.
NEW ORLEANS – Compared with vancomycin monotherapy, vancomycin combined with cefepime improved some outcomes for patients with methicillin-resistant Staphylococcus aureus (MRSA) bloodstream infections, a retrospective study of 109 patients revealed.
A lower likelihood of microbiological failure and fewer bloodstream infections persisting 7 days or more were the notable differences between treatment groups.
All patients had at least 72 hours of vancomycin therapy to treat MRSA bacteremia confirmed by blood culture. During 2008-2015, 38 adults received vancomycin monotherapy and 71 received vancomycin plus 24 hours or more of cefepime.
Compared with monotherapy, the combination treatment was associated with a nonsignificant reduction in the primary composite treatment failure outcome of 30-day all-cause mortality, in bacteremia duration of 7 days or more, and in 60-day bloodstream-infection recurrence: 55% for monotherapy versus 42% for combination therapy (P = .195). The difference was primarily associated with decreased duration of sepsis and fewer MRSA bloodstream infections persisting 7 days or more in the combination cohort.
Rates of bacteremia duration of 7 days or more were 42% in monotherapy patients and 20% in combination patients (P = .013). Differences in 60-day bloodstream-infection recurrence were nonsignificant, 8% versus 4%, respectively (P = .42).
Thirty-day mortality, however, was lower among monotherapy patients than combination patients – 13% vs. 25% – although the difference was nonsignificant (P = .21).
“From what I see here … it seems like they will have a lower duration of bacteremia, which is always great,” Ms. Atwan said. “You want to decrease length of stay in the hospital,” which will cut down on costs and on patients’ risks of getting more infections.
Although the primary outcome was a composite endpoint, “when we looked at them separately, we found the patients in the combination group had more mortality,” Ms. Atwan said at the annual meeting of the American Society for Microbiology. “That surprised me initially. But those patients are sicker and more likely to get dual coverage.”
The investigators confirmed the association between the severity of MRSA bacteremia and combination therapy by looking at Acute Physiology and Chronic Health Evaluation (APACHE II) scores. The median APACHE score was 23 in the combination group, compared with 13.5 in the monotherapy group (P = 0003). Higher APACHE scores were associated with greater odds of meeting the composite failure endpoint (adjusted odds ratio, 1.08) and of developing endocarditis (aOR, 3.6) in multivariate analyses.
More patients in the combination group had pneumonia as the primary source of infection than did patients in the monotherapy group: 54% vs. 29% (P = .016). Further, more of them had skin or soft tissue infections as the primary infection source: 29% vs. 13% (P = .036).
Although the exact mechanism remains unknown, synergy between the two agents could be caused by an increase in penicillin-binding proteins, Ms. Atwan said.
The study is still ongoing; Ms. Atwan hopes additional patients and data will lead to statistically significant differences between the outcomes of combination therapy and vancomycin monotherapy.
“I want to say that combination therapy is something you will always want to go to when you have a sicker patient, but I can’t really tell you that combination therapy is going to cause better outcomes for your patient,” she cautioned. “Hopefully, I can by the end of the study.”
In the meantime, “it looks like vancomycin and beta-lactams could be beneficial for MRSA bacteremia,” she added.
The researchers noted that although vancomycin monotherapy is a mainstay of treatment for MRSA bloodstream infections, emergence of reduced susceptibility and treatment failures warrants other therapeutic strategies.
Ms. Atwan had no relevant financial disclosures.
AT ASM MICROBE 2017
Key clinical point:
Major finding: Median duration of MRSA bacteremia was 4 days with combination therapy, versus 6 days with vancomycin alone.
Data source: A retrospective, single-center comparison of 109 patients treated with either vancomycin plus cefepime or vancomycin alone.
Disclosures: Safana M. Atwan had no relevant financial disclosures.
GOP health reform dead for now
Senate Republicans are scrambling to come up with another plan now that at least four member of their caucus have said that they would vote against moving forward with debate on the Better Care Reconciliation Act.
Support for the bill, which included dramatic Medicaid cuts and stripped many coverage provisions of the Affordable Care Act, was lacking after revisions were announced on July 13. At that time, conservative Sen. Ran Paul (R-Ky.) and moderate Susan Collins (R-Maine) voiced their opposition for different ideological reasons. They were joined by Sen. Mike Lee (R-Utah) and Sen. Jerry Moran (R-Kan.), who also declined to support the bill. Senate GOP leadership, with a slim 52-48 majority, could only afford to lose two votes (Vice President Mike Pence would have been the tie-breaking vote).
No new timeline has been revealed for the next steps.
“The health reform debate is by no means over,” David Barbe, MD, president of the American Medical Association, said in a statement. “Congress must begin a collaborative process that produces a bipartisan solution. ... Near-term action is needed to stabilize the individual/nongroup health insurance marketplace. In the long term, stakeholders and policymakers need to address the unsustainable trends in health care costs while achieving meaningful, affordable coverage for all Americans.”
Senate Republicans are scrambling to come up with another plan now that at least four member of their caucus have said that they would vote against moving forward with debate on the Better Care Reconciliation Act.
Support for the bill, which included dramatic Medicaid cuts and stripped many coverage provisions of the Affordable Care Act, was lacking after revisions were announced on July 13. At that time, conservative Sen. Ran Paul (R-Ky.) and moderate Susan Collins (R-Maine) voiced their opposition for different ideological reasons. They were joined by Sen. Mike Lee (R-Utah) and Sen. Jerry Moran (R-Kan.), who also declined to support the bill. Senate GOP leadership, with a slim 52-48 majority, could only afford to lose two votes (Vice President Mike Pence would have been the tie-breaking vote).
No new timeline has been revealed for the next steps.
“The health reform debate is by no means over,” David Barbe, MD, president of the American Medical Association, said in a statement. “Congress must begin a collaborative process that produces a bipartisan solution. ... Near-term action is needed to stabilize the individual/nongroup health insurance marketplace. In the long term, stakeholders and policymakers need to address the unsustainable trends in health care costs while achieving meaningful, affordable coverage for all Americans.”
Senate Republicans are scrambling to come up with another plan now that at least four member of their caucus have said that they would vote against moving forward with debate on the Better Care Reconciliation Act.
Support for the bill, which included dramatic Medicaid cuts and stripped many coverage provisions of the Affordable Care Act, was lacking after revisions were announced on July 13. At that time, conservative Sen. Ran Paul (R-Ky.) and moderate Susan Collins (R-Maine) voiced their opposition for different ideological reasons. They were joined by Sen. Mike Lee (R-Utah) and Sen. Jerry Moran (R-Kan.), who also declined to support the bill. Senate GOP leadership, with a slim 52-48 majority, could only afford to lose two votes (Vice President Mike Pence would have been the tie-breaking vote).
No new timeline has been revealed for the next steps.
“The health reform debate is by no means over,” David Barbe, MD, president of the American Medical Association, said in a statement. “Congress must begin a collaborative process that produces a bipartisan solution. ... Near-term action is needed to stabilize the individual/nongroup health insurance marketplace. In the long term, stakeholders and policymakers need to address the unsustainable trends in health care costs while achieving meaningful, affordable coverage for all Americans.”