User login
Algorithm-based trunk support system shows potential for recovery of walking ability
A multidirectional gravity-assist device that delivers precise trunk support to stroke and spinal cord injury (SCI) patients via an artificial intelligence algorithm has demonstrated significantly improved locomotor performance beyond treadmill-based systems in a new study.
The harness device used in the study adjusts patients’ balance while they stand still or walk by employing a unique, adaptive multidirectional gravity-assist (MGA) algorithm tailored to the specific needs of the patient, according to Jean-Baptiste Mignardot, PhD, of the Center for Neuroprosthetics and Brain Mind Institute, Swiss Federal Institute of Technology, Lausanne, Switzerland, and fellow investigators.
“The MGA establishes a safe and natural rehabilitation environment wherein individuals with neurological deficits can perform basic and skilled locomotor activities that would not be possible without robotic assistance,” according to the investigators. “The immediate and short-term ameliorations of gait performance during locomotion with MGA illustrate the potential of this environment to augment motor recovery.”
Current gait rehabilitation methods in stroke or SCI patients most commonly involves counterweight mechanisms or force-controlled equipment that apply upward support while walking on a treadmill. However Dr. Mignardot and his colleagues believe these methods are flawed.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
“Treadmill-restricted environments markedly differ from the rich repertoire of natural locomotor activities underlying daily living,” the investigators wrote. “Vertically restricted trunk support creates undesired forces that impede gait execution.”
To counteract these negative effects, the MGA adjusts both upward and forward forces on the patient’s body, re-creating a more naturally occurring gait posture, which investigators have likened to an inverted pendulum with a natural forward tilt. In order to create the algorithm, investigators ran through a series of procedures, starting with calibrations based on the gait of healthy subjects and adjusting for necessary upward and forward assistance for stroke and SCI patients.
The artificial neural network within the algorithm analyzes patients’ support needs, a job that therapists currently have to do based on visual observations. This opens a window to faster and more accurate estimations, according to the investigators (Sci Transl Med. 2017 Jul 19. doi: 10.1126/scitranslmed.aah3621).
Investigators tested the algorithm on 15 SCI patients and 12 stroke patients. The stroke patients had an average age of 51 years, with length of time after stroke varying from 8 to 235 months. The SCI patients had an average age of 47 years, with a length of time since injury ranging from 12 to 264 months. Most patients in both groups were male.
When tested, the algorithm showed varying success depending on the severity of the injury, according to the researchers.
“For example, the MGA enabled subjects who could not stand independently to walk overground with or without assistive device.” Subjects who were able to move around only with crutches or a walker progressed without the use of assistive devices and exhibited improved spatiotemporal gait features, according to Dr. Mignardot and fellow investigators. “Individuals with stroke exhibited similar or even superior amelioration of locomotor performance and showed that individuals who could only walk with crutches exhibited improved intralimb coordination.”
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
After initial efficacy tests, the researchers tested the MGA’s effectiveness in five SCI patients immediately after 1 hour of training with the device and found that their gait speed increased during the training. However, the improvements were not evident in a similar test 1 week later. Similar tests using treadmill-restricted step training without the MGA device did not show any improvement during either week of testing.
Although the study’s small sample size limited the conclusions that could be reached, the investigators were encouraged by the overall effects of the algorithm. They noted that further tests are required to test the potential sensitivity and accuracy of the software.
The study was supported by the European Commission’s Seventh Framework Programme, various foundations, and the Swiss National Science Foundation.
Investigators reported holding patents on the step-by-step procedure and use of the MGA algorithm in this study.
[email protected]
On Twitter @eaztweets
A multidirectional gravity-assist device that delivers precise trunk support to stroke and spinal cord injury (SCI) patients via an artificial intelligence algorithm has demonstrated significantly improved locomotor performance beyond treadmill-based systems in a new study.
The harness device used in the study adjusts patients’ balance while they stand still or walk by employing a unique, adaptive multidirectional gravity-assist (MGA) algorithm tailored to the specific needs of the patient, according to Jean-Baptiste Mignardot, PhD, of the Center for Neuroprosthetics and Brain Mind Institute, Swiss Federal Institute of Technology, Lausanne, Switzerland, and fellow investigators.
“The MGA establishes a safe and natural rehabilitation environment wherein individuals with neurological deficits can perform basic and skilled locomotor activities that would not be possible without robotic assistance,” according to the investigators. “The immediate and short-term ameliorations of gait performance during locomotion with MGA illustrate the potential of this environment to augment motor recovery.”
Current gait rehabilitation methods in stroke or SCI patients most commonly involves counterweight mechanisms or force-controlled equipment that apply upward support while walking on a treadmill. However Dr. Mignardot and his colleagues believe these methods are flawed.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
“Treadmill-restricted environments markedly differ from the rich repertoire of natural locomotor activities underlying daily living,” the investigators wrote. “Vertically restricted trunk support creates undesired forces that impede gait execution.”
To counteract these negative effects, the MGA adjusts both upward and forward forces on the patient’s body, re-creating a more naturally occurring gait posture, which investigators have likened to an inverted pendulum with a natural forward tilt. In order to create the algorithm, investigators ran through a series of procedures, starting with calibrations based on the gait of healthy subjects and adjusting for necessary upward and forward assistance for stroke and SCI patients.
The artificial neural network within the algorithm analyzes patients’ support needs, a job that therapists currently have to do based on visual observations. This opens a window to faster and more accurate estimations, according to the investigators (Sci Transl Med. 2017 Jul 19. doi: 10.1126/scitranslmed.aah3621).
Investigators tested the algorithm on 15 SCI patients and 12 stroke patients. The stroke patients had an average age of 51 years, with length of time after stroke varying from 8 to 235 months. The SCI patients had an average age of 47 years, with a length of time since injury ranging from 12 to 264 months. Most patients in both groups were male.
When tested, the algorithm showed varying success depending on the severity of the injury, according to the researchers.
“For example, the MGA enabled subjects who could not stand independently to walk overground with or without assistive device.” Subjects who were able to move around only with crutches or a walker progressed without the use of assistive devices and exhibited improved spatiotemporal gait features, according to Dr. Mignardot and fellow investigators. “Individuals with stroke exhibited similar or even superior amelioration of locomotor performance and showed that individuals who could only walk with crutches exhibited improved intralimb coordination.”
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
After initial efficacy tests, the researchers tested the MGA’s effectiveness in five SCI patients immediately after 1 hour of training with the device and found that their gait speed increased during the training. However, the improvements were not evident in a similar test 1 week later. Similar tests using treadmill-restricted step training without the MGA device did not show any improvement during either week of testing.
Although the study’s small sample size limited the conclusions that could be reached, the investigators were encouraged by the overall effects of the algorithm. They noted that further tests are required to test the potential sensitivity and accuracy of the software.
The study was supported by the European Commission’s Seventh Framework Programme, various foundations, and the Swiss National Science Foundation.
Investigators reported holding patents on the step-by-step procedure and use of the MGA algorithm in this study.
[email protected]
On Twitter @eaztweets
A multidirectional gravity-assist device that delivers precise trunk support to stroke and spinal cord injury (SCI) patients via an artificial intelligence algorithm has demonstrated significantly improved locomotor performance beyond treadmill-based systems in a new study.
The harness device used in the study adjusts patients’ balance while they stand still or walk by employing a unique, adaptive multidirectional gravity-assist (MGA) algorithm tailored to the specific needs of the patient, according to Jean-Baptiste Mignardot, PhD, of the Center for Neuroprosthetics and Brain Mind Institute, Swiss Federal Institute of Technology, Lausanne, Switzerland, and fellow investigators.
“The MGA establishes a safe and natural rehabilitation environment wherein individuals with neurological deficits can perform basic and skilled locomotor activities that would not be possible without robotic assistance,” according to the investigators. “The immediate and short-term ameliorations of gait performance during locomotion with MGA illustrate the potential of this environment to augment motor recovery.”
Current gait rehabilitation methods in stroke or SCI patients most commonly involves counterweight mechanisms or force-controlled equipment that apply upward support while walking on a treadmill. However Dr. Mignardot and his colleagues believe these methods are flawed.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
“Treadmill-restricted environments markedly differ from the rich repertoire of natural locomotor activities underlying daily living,” the investigators wrote. “Vertically restricted trunk support creates undesired forces that impede gait execution.”
To counteract these negative effects, the MGA adjusts both upward and forward forces on the patient’s body, re-creating a more naturally occurring gait posture, which investigators have likened to an inverted pendulum with a natural forward tilt. In order to create the algorithm, investigators ran through a series of procedures, starting with calibrations based on the gait of healthy subjects and adjusting for necessary upward and forward assistance for stroke and SCI patients.
The artificial neural network within the algorithm analyzes patients’ support needs, a job that therapists currently have to do based on visual observations. This opens a window to faster and more accurate estimations, according to the investigators (Sci Transl Med. 2017 Jul 19. doi: 10.1126/scitranslmed.aah3621).
Investigators tested the algorithm on 15 SCI patients and 12 stroke patients. The stroke patients had an average age of 51 years, with length of time after stroke varying from 8 to 235 months. The SCI patients had an average age of 47 years, with a length of time since injury ranging from 12 to 264 months. Most patients in both groups were male.
When tested, the algorithm showed varying success depending on the severity of the injury, according to the researchers.
“For example, the MGA enabled subjects who could not stand independently to walk overground with or without assistive device.” Subjects who were able to move around only with crutches or a walker progressed without the use of assistive devices and exhibited improved spatiotemporal gait features, according to Dr. Mignardot and fellow investigators. “Individuals with stroke exhibited similar or even superior amelioration of locomotor performance and showed that individuals who could only walk with crutches exhibited improved intralimb coordination.”
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
After initial efficacy tests, the researchers tested the MGA’s effectiveness in five SCI patients immediately after 1 hour of training with the device and found that their gait speed increased during the training. However, the improvements were not evident in a similar test 1 week later. Similar tests using treadmill-restricted step training without the MGA device did not show any improvement during either week of testing.
Although the study’s small sample size limited the conclusions that could be reached, the investigators were encouraged by the overall effects of the algorithm. They noted that further tests are required to test the potential sensitivity and accuracy of the software.
The study was supported by the European Commission’s Seventh Framework Programme, various foundations, and the Swiss National Science Foundation.
Investigators reported holding patents on the step-by-step procedure and use of the MGA algorithm in this study.
[email protected]
On Twitter @eaztweets
FROM SCIENCE TRANSLATIONAL MEDICINE
Ibrutinib and bleeding complications in Mohs surgery
Clinically significant bleeding events occurred in two elderly men who were taking ibrutinib and underwent Mohs micrographic surgery for squamous cell carcinomas, Cindy E. Parra and her colleagues reported in JAMA Dermatology.
On day 3 after his Mohs procedure, one 73-year-old man taking ibrutinib for Waldenstrom macroglobulinemia developed extensive bilateral periorbital ecchymosis that extended down to his upper chest. The other patient, an 88-year-old man taking ibrutinib for chronic lymphocytic leukemia, developed ecchymosis down to the chin. The first patient discontinued ibrutinib 3 days before his surgery; the second patient was taking ibrutinib at the time of his surgery.
“The increased incidence of nonmelanoma skin cancer and poorer outcomes in patients with non-Hodgkin lymphoma and CLL is well recognized, as is the importance of aggressive dermatologic management,” the researchers wrote (JAMA Dermatol. 2017 Jul 12. doi: 10.1001/jamadermatol.2017.1877). “It may be prudent to withhold ibrutinib treatment prior to dermatologic surgery to avoid potential bleeding complications.”
The findings argue for close collaboration between the dermatologic surgeon and the patient’s hematologist when scheduling extended-duration dermatologic procedures in patients taking ibrutinib.
Find the full summary here.
Clinically significant bleeding events occurred in two elderly men who were taking ibrutinib and underwent Mohs micrographic surgery for squamous cell carcinomas, Cindy E. Parra and her colleagues reported in JAMA Dermatology.
On day 3 after his Mohs procedure, one 73-year-old man taking ibrutinib for Waldenstrom macroglobulinemia developed extensive bilateral periorbital ecchymosis that extended down to his upper chest. The other patient, an 88-year-old man taking ibrutinib for chronic lymphocytic leukemia, developed ecchymosis down to the chin. The first patient discontinued ibrutinib 3 days before his surgery; the second patient was taking ibrutinib at the time of his surgery.
“The increased incidence of nonmelanoma skin cancer and poorer outcomes in patients with non-Hodgkin lymphoma and CLL is well recognized, as is the importance of aggressive dermatologic management,” the researchers wrote (JAMA Dermatol. 2017 Jul 12. doi: 10.1001/jamadermatol.2017.1877). “It may be prudent to withhold ibrutinib treatment prior to dermatologic surgery to avoid potential bleeding complications.”
The findings argue for close collaboration between the dermatologic surgeon and the patient’s hematologist when scheduling extended-duration dermatologic procedures in patients taking ibrutinib.
Find the full summary here.
Clinically significant bleeding events occurred in two elderly men who were taking ibrutinib and underwent Mohs micrographic surgery for squamous cell carcinomas, Cindy E. Parra and her colleagues reported in JAMA Dermatology.
On day 3 after his Mohs procedure, one 73-year-old man taking ibrutinib for Waldenstrom macroglobulinemia developed extensive bilateral periorbital ecchymosis that extended down to his upper chest. The other patient, an 88-year-old man taking ibrutinib for chronic lymphocytic leukemia, developed ecchymosis down to the chin. The first patient discontinued ibrutinib 3 days before his surgery; the second patient was taking ibrutinib at the time of his surgery.
“The increased incidence of nonmelanoma skin cancer and poorer outcomes in patients with non-Hodgkin lymphoma and CLL is well recognized, as is the importance of aggressive dermatologic management,” the researchers wrote (JAMA Dermatol. 2017 Jul 12. doi: 10.1001/jamadermatol.2017.1877). “It may be prudent to withhold ibrutinib treatment prior to dermatologic surgery to avoid potential bleeding complications.”
The findings argue for close collaboration between the dermatologic surgeon and the patient’s hematologist when scheduling extended-duration dermatologic procedures in patients taking ibrutinib.
Find the full summary here.
FROM JAMA DERMATOLOGY
Getting a Better Picture of Skin Cancer
A handheld detector that offers noninvasive real-time imaging can help dermatologic surgeons get a better idea of skin cancer dimensions before committing to surgery, according to researchers from National Skin Centre and Singapore Bioimaging Consortium, both in Singapore, and Technical University of Munich and iThera Medical GmbH, both in Germany.
Current imaging technologies can lead to excessive or incomplete removal of the cancer, the researchers say. The multispectral optoacoustic tomography (MSOT) allows the user to differentiate tissue chromophores (the chromophore is the part of the molecule responsible for its color) and exogenous contrast agents based on their spectral signatures.
The researchers performed MSOT imaging with 2- and 3-dimensional handheld scanners on 21 patients with nonmelanoma skin cancers. All the skin lesions had recognizable images on MSOT with both detectors, visualizing the shape and thickness of the lesions. The 2D and 3D detectors also offered images with well-resolved tissue chromophores. But the volumetric probe gave more accurate tumor dimensions compared with those from histology analysis.
Aggressive types of skin cancers can involve deeper structures, such as predominant deep blood vessels, the researchers note—another reason the MSOT detector could be useful. In one case, the depth of the basal cell carcinoma, which included its underlying vasculature, reached beyond 3 mm, which might have gone undetected by other imaging modalities, they say.
The fact that the device is also noninvasive and offers real-time imaging, the researchers suggest, makes volumetric MSOT “an ideal modality for longitudinal monitoring of skin diseases and treatment responses.”
Source:
Attia ABE, Chuah SY, Razansky D, et al. Photoacoustics. 2017;7:20-26.
doi: 10.1016/j.pacs.2017.05.003.
A handheld detector that offers noninvasive real-time imaging can help dermatologic surgeons get a better idea of skin cancer dimensions before committing to surgery, according to researchers from National Skin Centre and Singapore Bioimaging Consortium, both in Singapore, and Technical University of Munich and iThera Medical GmbH, both in Germany.
Current imaging technologies can lead to excessive or incomplete removal of the cancer, the researchers say. The multispectral optoacoustic tomography (MSOT) allows the user to differentiate tissue chromophores (the chromophore is the part of the molecule responsible for its color) and exogenous contrast agents based on their spectral signatures.
The researchers performed MSOT imaging with 2- and 3-dimensional handheld scanners on 21 patients with nonmelanoma skin cancers. All the skin lesions had recognizable images on MSOT with both detectors, visualizing the shape and thickness of the lesions. The 2D and 3D detectors also offered images with well-resolved tissue chromophores. But the volumetric probe gave more accurate tumor dimensions compared with those from histology analysis.
Aggressive types of skin cancers can involve deeper structures, such as predominant deep blood vessels, the researchers note—another reason the MSOT detector could be useful. In one case, the depth of the basal cell carcinoma, which included its underlying vasculature, reached beyond 3 mm, which might have gone undetected by other imaging modalities, they say.
The fact that the device is also noninvasive and offers real-time imaging, the researchers suggest, makes volumetric MSOT “an ideal modality for longitudinal monitoring of skin diseases and treatment responses.”
Source:
Attia ABE, Chuah SY, Razansky D, et al. Photoacoustics. 2017;7:20-26.
doi: 10.1016/j.pacs.2017.05.003.
A handheld detector that offers noninvasive real-time imaging can help dermatologic surgeons get a better idea of skin cancer dimensions before committing to surgery, according to researchers from National Skin Centre and Singapore Bioimaging Consortium, both in Singapore, and Technical University of Munich and iThera Medical GmbH, both in Germany.
Current imaging technologies can lead to excessive or incomplete removal of the cancer, the researchers say. The multispectral optoacoustic tomography (MSOT) allows the user to differentiate tissue chromophores (the chromophore is the part of the molecule responsible for its color) and exogenous contrast agents based on their spectral signatures.
The researchers performed MSOT imaging with 2- and 3-dimensional handheld scanners on 21 patients with nonmelanoma skin cancers. All the skin lesions had recognizable images on MSOT with both detectors, visualizing the shape and thickness of the lesions. The 2D and 3D detectors also offered images with well-resolved tissue chromophores. But the volumetric probe gave more accurate tumor dimensions compared with those from histology analysis.
Aggressive types of skin cancers can involve deeper structures, such as predominant deep blood vessels, the researchers note—another reason the MSOT detector could be useful. In one case, the depth of the basal cell carcinoma, which included its underlying vasculature, reached beyond 3 mm, which might have gone undetected by other imaging modalities, they say.
The fact that the device is also noninvasive and offers real-time imaging, the researchers suggest, makes volumetric MSOT “an ideal modality for longitudinal monitoring of skin diseases and treatment responses.”
Source:
Attia ABE, Chuah SY, Razansky D, et al. Photoacoustics. 2017;7:20-26.
doi: 10.1016/j.pacs.2017.05.003.
FDA Approves First New ALS Drug in Years
A new treatment for amyotrophic lateral sclerosis (ALS), Radicava (edaravone), is on the way for U.S. patients.
“After learning about the use of edaravone to treat ALS in Japan, we rapidly engaged with the drug developer about filing a marketing application in the United States,” said Eric Bastings, MD, deputy director of the Division of Neurology Products in the FDA’s Center for Drug Evaluation and Research. “This is the first new treatment approved by the FDA for ALS in many years, and we are pleased that people with ALS will now have an additional option.”
In a 6-month trial, 137 patients were randomly assigned to edaravone or placebo. At week 24, those receiving edaravone had declined less on a clinical assessment of daily functioning.
Edaravon is given intravenously daily for 14 days to start, followed by 14 days drug free. Subsequent treatment cycles consist of dosing on 10 of 14 days, followed by 14 days drug free.
The most common adverse reactions are bruising and gait disturbance. Edaravon also contains sodium bisulfite, which can cause serious allergic reactions in patients with sulfite sensitivity.
A new treatment for amyotrophic lateral sclerosis (ALS), Radicava (edaravone), is on the way for U.S. patients.
“After learning about the use of edaravone to treat ALS in Japan, we rapidly engaged with the drug developer about filing a marketing application in the United States,” said Eric Bastings, MD, deputy director of the Division of Neurology Products in the FDA’s Center for Drug Evaluation and Research. “This is the first new treatment approved by the FDA for ALS in many years, and we are pleased that people with ALS will now have an additional option.”
In a 6-month trial, 137 patients were randomly assigned to edaravone or placebo. At week 24, those receiving edaravone had declined less on a clinical assessment of daily functioning.
Edaravon is given intravenously daily for 14 days to start, followed by 14 days drug free. Subsequent treatment cycles consist of dosing on 10 of 14 days, followed by 14 days drug free.
The most common adverse reactions are bruising and gait disturbance. Edaravon also contains sodium bisulfite, which can cause serious allergic reactions in patients with sulfite sensitivity.
A new treatment for amyotrophic lateral sclerosis (ALS), Radicava (edaravone), is on the way for U.S. patients.
“After learning about the use of edaravone to treat ALS in Japan, we rapidly engaged with the drug developer about filing a marketing application in the United States,” said Eric Bastings, MD, deputy director of the Division of Neurology Products in the FDA’s Center for Drug Evaluation and Research. “This is the first new treatment approved by the FDA for ALS in many years, and we are pleased that people with ALS will now have an additional option.”
In a 6-month trial, 137 patients were randomly assigned to edaravone or placebo. At week 24, those receiving edaravone had declined less on a clinical assessment of daily functioning.
Edaravon is given intravenously daily for 14 days to start, followed by 14 days drug free. Subsequent treatment cycles consist of dosing on 10 of 14 days, followed by 14 days drug free.
The most common adverse reactions are bruising and gait disturbance. Edaravon also contains sodium bisulfite, which can cause serious allergic reactions in patients with sulfite sensitivity.
Predicting response to azacitidine in MDS
Research published in Cell Reports helps explain why some patients with myelodysplastic syndrome (MDS) do not respond to treatment with azacitidine.
The study showed that patients who were resistant to the drug had relatively quiescent hematopoietic progenitor cells (HPCs).
A smaller proportion of their HPCs were undergoing active cell-cycle progression when compared to the HPCs of patients who responded to azacitidine.
This discovery could provide the first method to identify non-responders to azacitidine early, according to study author Ashwin Unnikrishnan, PhD, of the University of New South Wales in Sydney, Australia.
“These are early days, but this could avoid what has really been a ‘wait and see’ approach with patients that sometimes results in them receiving futile treatment for 6 months,” Dr Unnikrishnan said.
“By that stage, the patient’s disease has progressed, and there’s no alternative for them.”
Dr Unnikrishnan and his colleagues also found the HPC quiescence in non-responders was mediated by integrin α5 (ITGA5) signaling, and the cells’ hematopoietic potential improved when the team combined azacitidine treatment with an ITGA5 inhibitor.
This suggests a potential avenue for future combination therapies that would improve azacitidine responsiveness.
Lastly, the researchers made discoveries that could explain why some patients who initially respond to azacitidine eventually relapse.
“All of the pernicious mutations that we associate with MDS never disappear in these patients, even after years of treatment,” Dr Unnikrishnan said. “From a clinical perspective, blood cell production is restored in patients, but they are a ticking time bomb, waiting to relapse.”
“[Azacitidine] is not a cure, and we are starting to understand why it does what it does. We need to find better treatments than azacitidine if we want a more durable therapy for MDS, and that’s the basis for our future work.” ![]()
Research published in Cell Reports helps explain why some patients with myelodysplastic syndrome (MDS) do not respond to treatment with azacitidine.
The study showed that patients who were resistant to the drug had relatively quiescent hematopoietic progenitor cells (HPCs).
A smaller proportion of their HPCs were undergoing active cell-cycle progression when compared to the HPCs of patients who responded to azacitidine.
This discovery could provide the first method to identify non-responders to azacitidine early, according to study author Ashwin Unnikrishnan, PhD, of the University of New South Wales in Sydney, Australia.
“These are early days, but this could avoid what has really been a ‘wait and see’ approach with patients that sometimes results in them receiving futile treatment for 6 months,” Dr Unnikrishnan said.
“By that stage, the patient’s disease has progressed, and there’s no alternative for them.”
Dr Unnikrishnan and his colleagues also found the HPC quiescence in non-responders was mediated by integrin α5 (ITGA5) signaling, and the cells’ hematopoietic potential improved when the team combined azacitidine treatment with an ITGA5 inhibitor.
This suggests a potential avenue for future combination therapies that would improve azacitidine responsiveness.
Lastly, the researchers made discoveries that could explain why some patients who initially respond to azacitidine eventually relapse.
“All of the pernicious mutations that we associate with MDS never disappear in these patients, even after years of treatment,” Dr Unnikrishnan said. “From a clinical perspective, blood cell production is restored in patients, but they are a ticking time bomb, waiting to relapse.”
“[Azacitidine] is not a cure, and we are starting to understand why it does what it does. We need to find better treatments than azacitidine if we want a more durable therapy for MDS, and that’s the basis for our future work.” ![]()
Research published in Cell Reports helps explain why some patients with myelodysplastic syndrome (MDS) do not respond to treatment with azacitidine.
The study showed that patients who were resistant to the drug had relatively quiescent hematopoietic progenitor cells (HPCs).
A smaller proportion of their HPCs were undergoing active cell-cycle progression when compared to the HPCs of patients who responded to azacitidine.
This discovery could provide the first method to identify non-responders to azacitidine early, according to study author Ashwin Unnikrishnan, PhD, of the University of New South Wales in Sydney, Australia.
“These are early days, but this could avoid what has really been a ‘wait and see’ approach with patients that sometimes results in them receiving futile treatment for 6 months,” Dr Unnikrishnan said.
“By that stage, the patient’s disease has progressed, and there’s no alternative for them.”
Dr Unnikrishnan and his colleagues also found the HPC quiescence in non-responders was mediated by integrin α5 (ITGA5) signaling, and the cells’ hematopoietic potential improved when the team combined azacitidine treatment with an ITGA5 inhibitor.
This suggests a potential avenue for future combination therapies that would improve azacitidine responsiveness.
Lastly, the researchers made discoveries that could explain why some patients who initially respond to azacitidine eventually relapse.
“All of the pernicious mutations that we associate with MDS never disappear in these patients, even after years of treatment,” Dr Unnikrishnan said. “From a clinical perspective, blood cell production is restored in patients, but they are a ticking time bomb, waiting to relapse.”
“[Azacitidine] is not a cure, and we are starting to understand why it does what it does. We need to find better treatments than azacitidine if we want a more durable therapy for MDS, and that’s the basis for our future work.” ![]()
Product can reduce, prevent bleeding in kids with FXD
BERLIN—Results of a phase 3 study suggest prophylaxis with plasma-derived factor X (pdFX, Coagadex®) prevents or reduces bleeding episodes in children with moderate to severe hereditary factor X deficiency (FXD).
pdFX was well tolerated in this study as well, with no treatment-related adverse events (AEs) reported.
“For the first time, we have data providing physicians with important evidence of the efficacy and safety of Coagadex as a prophylactic regimen for the reduction and prevention of bleeding episodes, as well as potential guidance on dosing for children with hereditary factor X deficiency,” said Michael Gattens, MBChB, a consultant pediatric hematologist at Cambridge University Hospitals in the UK and an investigator involved in this trial.
Data from the trial, known as TEN02, were presented in a poster (PB 818) at the International Society on Thrombosis and Haemostasis (ISTH) 2017 Congress. The trial was sponsored by Bio Products Laboratory Limited.
TEN02 was a prospective study conducted in children younger than 12 years with moderate or severe congenital FXD (basal plasma factor X activity <5 IU/dL at diagnosis) and either a history of severe bleeding or an F10 gene mutation causing a documented severe bleeding type.
The study enrolled 9 patients. Eight had severe FXD (FX:C <1 IU/dL), and 1 had moderate FXD (FX:C 1–5 IU/dL).
The patients’ mean age was 7.3 (range, 2.6 years to 11.9 years). Four patients were in the 0-5 age group, and 5 were in the 6-11 age group. Fifty-six percent of patients were female, 78% were Asian, and 22% were white.
The patients had a total of 21 prior bleeds, including overt (n=10), covert (n=9), and menorrhagic (n=2). The past bleeds were spontaneous (n=16) or caused by injury (n=2), menorrhagia (n=2), or surgery (n=2).
Treatment
Patients received 50 IU/kg of pdFX at visit 1 (baseline), followed 72 hours later by a second dose (visit 2). At the investigator’s discretion, visit 2 may have been conducted 48 hours after the first dose for subjects ages 0 to 5.
Following visit 2, patients used pdFX at 40-50 IU/kg as routine prophylaxis every 72 ± 2 hours or every 48 ± 2 hours for those age 0 to 5, at the investigator’s discretion. Dose and frequency were adjusted over the initial 6 weeks to maintain FX:C levels ≥5 IU/dL (with peak levels ≤120 IU/dL).
Treatment was continued for at least 26 weeks, with a minimum of 50 treatment days.
Following visit 2, patients returned to the study site for 3 additional visits (after 9-28, 29-42, and 50 treatment days/26 weeks; visits 3-5), during which time blood samples were collected to assess FX:C trough levels and vital signs and AEs were checked.
A final bolus dose of 50 IU/kg was administered during visit 5 to assess post-dose incremental recovery.
A dose of 25 IU/kg was recommended to treat minor bleeds, and 50 IU/kg was recommended for major bleeds. Treatment was to be repeated as often as necessary based on FX:C recovery levels and clinical need.
Nine patients completed 11 treatment cycles, including 2 patients who had 50 exposure days but less than 26 study weeks. These patients were re-screened and completed a second, per-protocol treatment cycle.
Results
A total of 537 prophylactic infusions were administered, with a mean dose of 38.6 IU/kg per patient.
Investigators rated the prophylactic efficacy of pdFX as “excellent.”
There were 10 bleeds in 3 patients. Four bleeds in 2 patients (both in the 6-11 age group) required treatment with a single infusion of pdFX (mean dose, 31.7±10.1 IU/kg).
The overall mean 30-minute incremental recovery was 1.66 IU/dL per IU/kg for patients’ baseline visit, 1.82 IU/dL per IU/kg for the end-of-study visit, and 1.74 IU/dL per IU/kg for both visits combined.
Incremental recovery was significantly lower in the 0-5 age group than the 6-11 age group:
- 1.45 vs 1.83 at baseline (P=0.027)
- 1.62 vs 1.99 at end of study (P=0.025)
- 1.53 vs 1.91 combined (P=0.001).
All patients had FX:C trough levels greater than 5% after visit 4 (days 29-42).
There were 28 AEs reported in 8 patients, but none of these events were considered related to treatment.
One patient did have 2 serious treatment-emergent AEs—lower respiratory tract infection and influenza.
There were no other serious AEs, no evidence of factor X inhibitor development, and no deaths. ![]()
BERLIN—Results of a phase 3 study suggest prophylaxis with plasma-derived factor X (pdFX, Coagadex®) prevents or reduces bleeding episodes in children with moderate to severe hereditary factor X deficiency (FXD).
pdFX was well tolerated in this study as well, with no treatment-related adverse events (AEs) reported.
“For the first time, we have data providing physicians with important evidence of the efficacy and safety of Coagadex as a prophylactic regimen for the reduction and prevention of bleeding episodes, as well as potential guidance on dosing for children with hereditary factor X deficiency,” said Michael Gattens, MBChB, a consultant pediatric hematologist at Cambridge University Hospitals in the UK and an investigator involved in this trial.
Data from the trial, known as TEN02, were presented in a poster (PB 818) at the International Society on Thrombosis and Haemostasis (ISTH) 2017 Congress. The trial was sponsored by Bio Products Laboratory Limited.
TEN02 was a prospective study conducted in children younger than 12 years with moderate or severe congenital FXD (basal plasma factor X activity <5 IU/dL at diagnosis) and either a history of severe bleeding or an F10 gene mutation causing a documented severe bleeding type.
The study enrolled 9 patients. Eight had severe FXD (FX:C <1 IU/dL), and 1 had moderate FXD (FX:C 1–5 IU/dL).
The patients’ mean age was 7.3 (range, 2.6 years to 11.9 years). Four patients were in the 0-5 age group, and 5 were in the 6-11 age group. Fifty-six percent of patients were female, 78% were Asian, and 22% were white.
The patients had a total of 21 prior bleeds, including overt (n=10), covert (n=9), and menorrhagic (n=2). The past bleeds were spontaneous (n=16) or caused by injury (n=2), menorrhagia (n=2), or surgery (n=2).
Treatment
Patients received 50 IU/kg of pdFX at visit 1 (baseline), followed 72 hours later by a second dose (visit 2). At the investigator’s discretion, visit 2 may have been conducted 48 hours after the first dose for subjects ages 0 to 5.
Following visit 2, patients used pdFX at 40-50 IU/kg as routine prophylaxis every 72 ± 2 hours or every 48 ± 2 hours for those age 0 to 5, at the investigator’s discretion. Dose and frequency were adjusted over the initial 6 weeks to maintain FX:C levels ≥5 IU/dL (with peak levels ≤120 IU/dL).
Treatment was continued for at least 26 weeks, with a minimum of 50 treatment days.
Following visit 2, patients returned to the study site for 3 additional visits (after 9-28, 29-42, and 50 treatment days/26 weeks; visits 3-5), during which time blood samples were collected to assess FX:C trough levels and vital signs and AEs were checked.
A final bolus dose of 50 IU/kg was administered during visit 5 to assess post-dose incremental recovery.
A dose of 25 IU/kg was recommended to treat minor bleeds, and 50 IU/kg was recommended for major bleeds. Treatment was to be repeated as often as necessary based on FX:C recovery levels and clinical need.
Nine patients completed 11 treatment cycles, including 2 patients who had 50 exposure days but less than 26 study weeks. These patients were re-screened and completed a second, per-protocol treatment cycle.
Results
A total of 537 prophylactic infusions were administered, with a mean dose of 38.6 IU/kg per patient.
Investigators rated the prophylactic efficacy of pdFX as “excellent.”
There were 10 bleeds in 3 patients. Four bleeds in 2 patients (both in the 6-11 age group) required treatment with a single infusion of pdFX (mean dose, 31.7±10.1 IU/kg).
The overall mean 30-minute incremental recovery was 1.66 IU/dL per IU/kg for patients’ baseline visit, 1.82 IU/dL per IU/kg for the end-of-study visit, and 1.74 IU/dL per IU/kg for both visits combined.
Incremental recovery was significantly lower in the 0-5 age group than the 6-11 age group:
- 1.45 vs 1.83 at baseline (P=0.027)
- 1.62 vs 1.99 at end of study (P=0.025)
- 1.53 vs 1.91 combined (P=0.001).
All patients had FX:C trough levels greater than 5% after visit 4 (days 29-42).
There were 28 AEs reported in 8 patients, but none of these events were considered related to treatment.
One patient did have 2 serious treatment-emergent AEs—lower respiratory tract infection and influenza.
There were no other serious AEs, no evidence of factor X inhibitor development, and no deaths. ![]()
BERLIN—Results of a phase 3 study suggest prophylaxis with plasma-derived factor X (pdFX, Coagadex®) prevents or reduces bleeding episodes in children with moderate to severe hereditary factor X deficiency (FXD).
pdFX was well tolerated in this study as well, with no treatment-related adverse events (AEs) reported.
“For the first time, we have data providing physicians with important evidence of the efficacy and safety of Coagadex as a prophylactic regimen for the reduction and prevention of bleeding episodes, as well as potential guidance on dosing for children with hereditary factor X deficiency,” said Michael Gattens, MBChB, a consultant pediatric hematologist at Cambridge University Hospitals in the UK and an investigator involved in this trial.
Data from the trial, known as TEN02, were presented in a poster (PB 818) at the International Society on Thrombosis and Haemostasis (ISTH) 2017 Congress. The trial was sponsored by Bio Products Laboratory Limited.
TEN02 was a prospective study conducted in children younger than 12 years with moderate or severe congenital FXD (basal plasma factor X activity <5 IU/dL at diagnosis) and either a history of severe bleeding or an F10 gene mutation causing a documented severe bleeding type.
The study enrolled 9 patients. Eight had severe FXD (FX:C <1 IU/dL), and 1 had moderate FXD (FX:C 1–5 IU/dL).
The patients’ mean age was 7.3 (range, 2.6 years to 11.9 years). Four patients were in the 0-5 age group, and 5 were in the 6-11 age group. Fifty-six percent of patients were female, 78% were Asian, and 22% were white.
The patients had a total of 21 prior bleeds, including overt (n=10), covert (n=9), and menorrhagic (n=2). The past bleeds were spontaneous (n=16) or caused by injury (n=2), menorrhagia (n=2), or surgery (n=2).
Treatment
Patients received 50 IU/kg of pdFX at visit 1 (baseline), followed 72 hours later by a second dose (visit 2). At the investigator’s discretion, visit 2 may have been conducted 48 hours after the first dose for subjects ages 0 to 5.
Following visit 2, patients used pdFX at 40-50 IU/kg as routine prophylaxis every 72 ± 2 hours or every 48 ± 2 hours for those age 0 to 5, at the investigator’s discretion. Dose and frequency were adjusted over the initial 6 weeks to maintain FX:C levels ≥5 IU/dL (with peak levels ≤120 IU/dL).
Treatment was continued for at least 26 weeks, with a minimum of 50 treatment days.
Following visit 2, patients returned to the study site for 3 additional visits (after 9-28, 29-42, and 50 treatment days/26 weeks; visits 3-5), during which time blood samples were collected to assess FX:C trough levels and vital signs and AEs were checked.
A final bolus dose of 50 IU/kg was administered during visit 5 to assess post-dose incremental recovery.
A dose of 25 IU/kg was recommended to treat minor bleeds, and 50 IU/kg was recommended for major bleeds. Treatment was to be repeated as often as necessary based on FX:C recovery levels and clinical need.
Nine patients completed 11 treatment cycles, including 2 patients who had 50 exposure days but less than 26 study weeks. These patients were re-screened and completed a second, per-protocol treatment cycle.
Results
A total of 537 prophylactic infusions were administered, with a mean dose of 38.6 IU/kg per patient.
Investigators rated the prophylactic efficacy of pdFX as “excellent.”
There were 10 bleeds in 3 patients. Four bleeds in 2 patients (both in the 6-11 age group) required treatment with a single infusion of pdFX (mean dose, 31.7±10.1 IU/kg).
The overall mean 30-minute incremental recovery was 1.66 IU/dL per IU/kg for patients’ baseline visit, 1.82 IU/dL per IU/kg for the end-of-study visit, and 1.74 IU/dL per IU/kg for both visits combined.
Incremental recovery was significantly lower in the 0-5 age group than the 6-11 age group:
- 1.45 vs 1.83 at baseline (P=0.027)
- 1.62 vs 1.99 at end of study (P=0.025)
- 1.53 vs 1.91 combined (P=0.001).
All patients had FX:C trough levels greater than 5% after visit 4 (days 29-42).
There were 28 AEs reported in 8 patients, but none of these events were considered related to treatment.
One patient did have 2 serious treatment-emergent AEs—lower respiratory tract infection and influenza.
There were no other serious AEs, no evidence of factor X inhibitor development, and no deaths. ![]()
Long-term maintenance deemed feasible in PV
MADRID—Long-term maintenance with ropeginterferon alfa-2b is feasible, effective, and well-tolerated in patients with polycythemia vera (PV), according to researchers.
In the ongoing phase 1/2 PEGINVERA study, patients have received ropeginterferon alfa-2b for a median of 4 years.
After the first 2 years, patients switched from bi-weekly dosing to receiving ropeginterferon alfa-2b once every 4 weeks.
None of the patients discontinued treatment after the switch, and many were able to maintain their best response.
Most adverse events (AEs) were mild, although there were several severe treatment-related AEs.
These results were presented in a poster (abstract P707) at the 22nd Congress of the European Hematology Association (EHA). The research was funded by AOP Orphan Pharmaceuticals AG.
The trial enrolled 51 patients, but the researchers reported results in the 29 patients who had completed 2 years of treatment and switched from bi-weekly dosing to receiving treatment once every 4 weeks.
All 29 patients remained on the 4-week schedule with a median observation period of roughly 2 years. The median monthly dose was 308 μg before the switch and 165 μg after.
At study entry, the patients’ median age was 58 (range, 40-80), and 76% were male. Their median spleen length was 12.8 cm (range, 8.0-22.0), and 34% of patients had prior treatment with hydroxyurea.
Patients’ median hematocrit was 45.40% (range, 36.9-53.8), their median platelet count was 431 G/L (range, 225-1016), their median leukocyte count was 11.1 G/L (range, 4.7-30.9), and their median JAKV617F allelic burden was 78% (range, 2-91.5).
Results
More than 80% of patients achieved a hematologic response, with more than 50% achieving a complete hematologic response. The same percentage of patients maintained their best hematologic response before and 6 months after switching to the 4-week schedule—51.7%.
More than 80% of patients achieved a molecular response, with nearly 20% achieving a complete molecular response. The percentage of patients maintaining their best molecular response was 62.1% before switching to the 4-week schedule and 58.6% 6 months after the switch.
The researchers said changes in hematocrit, platelet count, leukocyte count, and spleen size after the switch were “minimal and without clinical relevance.”
The median hematocrit changed from 42.3% to 42.6%, the median platelet count changed from 201.0 x 109/L to 211.9 x 109/L, the median leukocyte count changed from 5.0 x 109/L to 5.6 x 109/L, and the median spleen size changed from 12.8 cm to 12.4 cm.
The need for phlebotomy did not change, with 24.1% of patients requiring phlebotomy both before and 6 months after the switch.
The researchers also noted that ropeginterferon alfa-2b decreased mutant JAK2 allele burden in all of the patients over time, with the strongest effect observed in the second year of treatment.
After 2 years, most patients had a burden below 10%, and this was not affected by the change in dosing.
There were no cases of progression to myelofibrosis or leukemic transformation.
Seventy-one percent of AEs were mild, and 40.4% were considered likely related to ropeginterferon alfa-2b. The most frequent treatment-related AEs were arthralgia (29.4%) and fatigue (21.6%).
There were 34 severe AEs, 11 of which were related to ropeginterferon alfa-2b. ![]()
MADRID—Long-term maintenance with ropeginterferon alfa-2b is feasible, effective, and well-tolerated in patients with polycythemia vera (PV), according to researchers.
In the ongoing phase 1/2 PEGINVERA study, patients have received ropeginterferon alfa-2b for a median of 4 years.
After the first 2 years, patients switched from bi-weekly dosing to receiving ropeginterferon alfa-2b once every 4 weeks.
None of the patients discontinued treatment after the switch, and many were able to maintain their best response.
Most adverse events (AEs) were mild, although there were several severe treatment-related AEs.
These results were presented in a poster (abstract P707) at the 22nd Congress of the European Hematology Association (EHA). The research was funded by AOP Orphan Pharmaceuticals AG.
The trial enrolled 51 patients, but the researchers reported results in the 29 patients who had completed 2 years of treatment and switched from bi-weekly dosing to receiving treatment once every 4 weeks.
All 29 patients remained on the 4-week schedule with a median observation period of roughly 2 years. The median monthly dose was 308 μg before the switch and 165 μg after.
At study entry, the patients’ median age was 58 (range, 40-80), and 76% were male. Their median spleen length was 12.8 cm (range, 8.0-22.0), and 34% of patients had prior treatment with hydroxyurea.
Patients’ median hematocrit was 45.40% (range, 36.9-53.8), their median platelet count was 431 G/L (range, 225-1016), their median leukocyte count was 11.1 G/L (range, 4.7-30.9), and their median JAKV617F allelic burden was 78% (range, 2-91.5).
Results
More than 80% of patients achieved a hematologic response, with more than 50% achieving a complete hematologic response. The same percentage of patients maintained their best hematologic response before and 6 months after switching to the 4-week schedule—51.7%.
More than 80% of patients achieved a molecular response, with nearly 20% achieving a complete molecular response. The percentage of patients maintaining their best molecular response was 62.1% before switching to the 4-week schedule and 58.6% 6 months after the switch.
The researchers said changes in hematocrit, platelet count, leukocyte count, and spleen size after the switch were “minimal and without clinical relevance.”
The median hematocrit changed from 42.3% to 42.6%, the median platelet count changed from 201.0 x 109/L to 211.9 x 109/L, the median leukocyte count changed from 5.0 x 109/L to 5.6 x 109/L, and the median spleen size changed from 12.8 cm to 12.4 cm.
The need for phlebotomy did not change, with 24.1% of patients requiring phlebotomy both before and 6 months after the switch.
The researchers also noted that ropeginterferon alfa-2b decreased mutant JAK2 allele burden in all of the patients over time, with the strongest effect observed in the second year of treatment.
After 2 years, most patients had a burden below 10%, and this was not affected by the change in dosing.
There were no cases of progression to myelofibrosis or leukemic transformation.
Seventy-one percent of AEs were mild, and 40.4% were considered likely related to ropeginterferon alfa-2b. The most frequent treatment-related AEs were arthralgia (29.4%) and fatigue (21.6%).
There were 34 severe AEs, 11 of which were related to ropeginterferon alfa-2b. ![]()
MADRID—Long-term maintenance with ropeginterferon alfa-2b is feasible, effective, and well-tolerated in patients with polycythemia vera (PV), according to researchers.
In the ongoing phase 1/2 PEGINVERA study, patients have received ropeginterferon alfa-2b for a median of 4 years.
After the first 2 years, patients switched from bi-weekly dosing to receiving ropeginterferon alfa-2b once every 4 weeks.
None of the patients discontinued treatment after the switch, and many were able to maintain their best response.
Most adverse events (AEs) were mild, although there were several severe treatment-related AEs.
These results were presented in a poster (abstract P707) at the 22nd Congress of the European Hematology Association (EHA). The research was funded by AOP Orphan Pharmaceuticals AG.
The trial enrolled 51 patients, but the researchers reported results in the 29 patients who had completed 2 years of treatment and switched from bi-weekly dosing to receiving treatment once every 4 weeks.
All 29 patients remained on the 4-week schedule with a median observation period of roughly 2 years. The median monthly dose was 308 μg before the switch and 165 μg after.
At study entry, the patients’ median age was 58 (range, 40-80), and 76% were male. Their median spleen length was 12.8 cm (range, 8.0-22.0), and 34% of patients had prior treatment with hydroxyurea.
Patients’ median hematocrit was 45.40% (range, 36.9-53.8), their median platelet count was 431 G/L (range, 225-1016), their median leukocyte count was 11.1 G/L (range, 4.7-30.9), and their median JAKV617F allelic burden was 78% (range, 2-91.5).
Results
More than 80% of patients achieved a hematologic response, with more than 50% achieving a complete hematologic response. The same percentage of patients maintained their best hematologic response before and 6 months after switching to the 4-week schedule—51.7%.
More than 80% of patients achieved a molecular response, with nearly 20% achieving a complete molecular response. The percentage of patients maintaining their best molecular response was 62.1% before switching to the 4-week schedule and 58.6% 6 months after the switch.
The researchers said changes in hematocrit, platelet count, leukocyte count, and spleen size after the switch were “minimal and without clinical relevance.”
The median hematocrit changed from 42.3% to 42.6%, the median platelet count changed from 201.0 x 109/L to 211.9 x 109/L, the median leukocyte count changed from 5.0 x 109/L to 5.6 x 109/L, and the median spleen size changed from 12.8 cm to 12.4 cm.
The need for phlebotomy did not change, with 24.1% of patients requiring phlebotomy both before and 6 months after the switch.
The researchers also noted that ropeginterferon alfa-2b decreased mutant JAK2 allele burden in all of the patients over time, with the strongest effect observed in the second year of treatment.
After 2 years, most patients had a burden below 10%, and this was not affected by the change in dosing.
There were no cases of progression to myelofibrosis or leukemic transformation.
Seventy-one percent of AEs were mild, and 40.4% were considered likely related to ropeginterferon alfa-2b. The most frequent treatment-related AEs were arthralgia (29.4%) and fatigue (21.6%).
There were 34 severe AEs, 11 of which were related to ropeginterferon alfa-2b. ![]()
Itchy rash during pregnancy
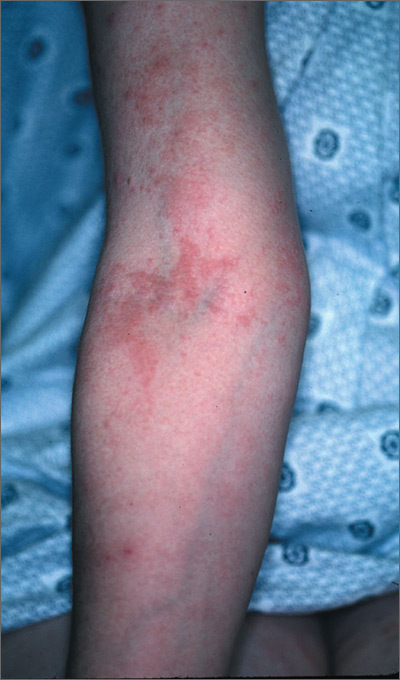
The FP diagnosed pruritic urticarial papules and plaques of pregnancy. PUPPP is usually diagnosed by its characteristic findings in the history and physical examination.
PUPPP typically presents as erythematous papules and plaques within striae (with periumbilical sparing) late in the third trimester. Extreme pruritus is a hallmark of PUPPP and is present in all patients. Abdominal striae are the most common initial symptoms. The lesions usually spread to the extremities and coalesce to form urticarial plaques.
General measures such as cool baths, frequent application of emollients, wet soaks, or cool packs applied to the skin provide some symptomatic relief. First-line pharmacologic therapy consists of topical steroids and oral antihistamines to alleviate symptoms.
In this case, the FP prescribed a mid-potency topical corticosteroid, 0.1% triamcinolone cream twice daily (pregnancy class B). The patient was given the choice of steroid vehicle and chose the cream over the ointment. Both vehicles work to treat PUPPP, but the one that will work best is the one the patient will actually use.
The FP also recommended over-the-counter diphenhydramine 25 mg for additional symptomatic relief of pruritus (pregnancy class B). At her following prenatal visit, the patient’s symptoms were about 80% better and she was tolerating the pruritus. She was relieved to learn that this condition would resolve after pregnancy.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux EJ. Pruritic urticarial papules and plaques of pregnancy. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013: 467-470.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com

The FP diagnosed pruritic urticarial papules and plaques of pregnancy. PUPPP is usually diagnosed by its characteristic findings in the history and physical examination.
PUPPP typically presents as erythematous papules and plaques within striae (with periumbilical sparing) late in the third trimester. Extreme pruritus is a hallmark of PUPPP and is present in all patients. Abdominal striae are the most common initial symptoms. The lesions usually spread to the extremities and coalesce to form urticarial plaques.
General measures such as cool baths, frequent application of emollients, wet soaks, or cool packs applied to the skin provide some symptomatic relief. First-line pharmacologic therapy consists of topical steroids and oral antihistamines to alleviate symptoms.
In this case, the FP prescribed a mid-potency topical corticosteroid, 0.1% triamcinolone cream twice daily (pregnancy class B). The patient was given the choice of steroid vehicle and chose the cream over the ointment. Both vehicles work to treat PUPPP, but the one that will work best is the one the patient will actually use.
The FP also recommended over-the-counter diphenhydramine 25 mg for additional symptomatic relief of pruritus (pregnancy class B). At her following prenatal visit, the patient’s symptoms were about 80% better and she was tolerating the pruritus. She was relieved to learn that this condition would resolve after pregnancy.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux EJ. Pruritic urticarial papules and plaques of pregnancy. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013: 467-470.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com

The FP diagnosed pruritic urticarial papules and plaques of pregnancy. PUPPP is usually diagnosed by its characteristic findings in the history and physical examination.
PUPPP typically presents as erythematous papules and plaques within striae (with periumbilical sparing) late in the third trimester. Extreme pruritus is a hallmark of PUPPP and is present in all patients. Abdominal striae are the most common initial symptoms. The lesions usually spread to the extremities and coalesce to form urticarial plaques.
General measures such as cool baths, frequent application of emollients, wet soaks, or cool packs applied to the skin provide some symptomatic relief. First-line pharmacologic therapy consists of topical steroids and oral antihistamines to alleviate symptoms.
In this case, the FP prescribed a mid-potency topical corticosteroid, 0.1% triamcinolone cream twice daily (pregnancy class B). The patient was given the choice of steroid vehicle and chose the cream over the ointment. Both vehicles work to treat PUPPP, but the one that will work best is the one the patient will actually use.
The FP also recommended over-the-counter diphenhydramine 25 mg for additional symptomatic relief of pruritus (pregnancy class B). At her following prenatal visit, the patient’s symptoms were about 80% better and she was tolerating the pruritus. She was relieved to learn that this condition would resolve after pregnancy.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux EJ. Pruritic urticarial papules and plaques of pregnancy. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013: 467-470.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
Factory contamination seen as likely source of postop endocarditis outbreak
Since 2013, over 100 cases of Mycobacterium chimaera prosthetic valve endocarditis and disseminated disease were detected in Europe and the United States, and these were presumptively linked to contaminated heater-cooler units (HCUs) used during cardiac surgery. A molecular epidemiological analysis of microbial isolate genomes detected a “remarkable clonality of isolates” in almost all of the assessed patients with M. chimaera disease, which “strongly points to a common source of infection,” as reported online in The Lancet Infectious Diseases.
The analysis comprised 250 whole-genome sequencing datasets: 24 isolates from 21 cardiac surgery–related patients in Switzerland, Germany, the Netherlands, and the United Kingdom; 36 from 35 unrelated patients; 126 from LivaNova HCUs in use (85 water cultures, 41 air cultures); 13 from LivaNova HCUs returned to the production site in Germany for disinfection; 4 from the LivaNova production site (3 from newly produced HCUs, 1 from a water source); 2 from Maquet extracorporeal membrane oxygenation (ECMO) devices in use; 14 from Maquet HCUs in use; 15 from new Maquet HCUs sampled at the production site; and 7 from hospital water supplies in Switzerland, Germany, and the Netherlands, plus one M. chimaera DSM 44623–type strain, and eight M. intracellulare strains (from four unrelated patients from Germany and four published genomes).
Isolates were analyzed by next-generation whole-genome sequencing and compared with published M. chimaera genomes, according to Jakko van Ingen, PhD, Radboud University Medical Center, Nijmegen, the Netherlands, and his colleagues. Phylogenetic analysis of these 250 isolates revealed two major M. chimaera groups. They found that all cardiac surgery–related patient isolates could be classified into group 1. They then did a subgroup analysis.
“Three distinct strains of M. chimaera appear to have contaminated the water systems of LivaNova HCUs at the production site, belonging to subgroups 1.1, 1.8, and 2.1,” the authors stated. However, most M. chimaera isolates from air samples taken near operating LivaNova HCUs and those of 23 of the 24 related patients belonged to subgroup 1.1.
“This finding further supports the presumed airborne transmission pathway leading to endocarditis, aortic graft infection, disseminated disease, and surgical site infections in the affected patients,” according to the authors (doi: 10.1016/S1473-3099[17]30324-9).
The results suggest “the possibility that the vast majority of cases of cardiothoracic surgery–related severe M. chimaera infections diagnosed in Switzerland, Germany, the Netherlands, the United Kingdom, the United States, and Australia resulted from a single common source of infection: LivaNova HCUs that were most likely contaminated during production in Germany,” the researchers concluded.
The study was partly funded by the EU Horizon 2020 program, its FP7 program, the German Center for Infection Research (DZIF), the Swiss National Science Foundation, the Swiss Federal Office of Public Health, and National Institute of Health Research Oxford Health Protection Research Units on Healthcare Associated Infection and Antimicrobial Resistance. The authors reported having no relevant conflicts.
Since 2013, over 100 cases of Mycobacterium chimaera prosthetic valve endocarditis and disseminated disease were detected in Europe and the United States, and these were presumptively linked to contaminated heater-cooler units (HCUs) used during cardiac surgery. A molecular epidemiological analysis of microbial isolate genomes detected a “remarkable clonality of isolates” in almost all of the assessed patients with M. chimaera disease, which “strongly points to a common source of infection,” as reported online in The Lancet Infectious Diseases.
The analysis comprised 250 whole-genome sequencing datasets: 24 isolates from 21 cardiac surgery–related patients in Switzerland, Germany, the Netherlands, and the United Kingdom; 36 from 35 unrelated patients; 126 from LivaNova HCUs in use (85 water cultures, 41 air cultures); 13 from LivaNova HCUs returned to the production site in Germany for disinfection; 4 from the LivaNova production site (3 from newly produced HCUs, 1 from a water source); 2 from Maquet extracorporeal membrane oxygenation (ECMO) devices in use; 14 from Maquet HCUs in use; 15 from new Maquet HCUs sampled at the production site; and 7 from hospital water supplies in Switzerland, Germany, and the Netherlands, plus one M. chimaera DSM 44623–type strain, and eight M. intracellulare strains (from four unrelated patients from Germany and four published genomes).
Isolates were analyzed by next-generation whole-genome sequencing and compared with published M. chimaera genomes, according to Jakko van Ingen, PhD, Radboud University Medical Center, Nijmegen, the Netherlands, and his colleagues. Phylogenetic analysis of these 250 isolates revealed two major M. chimaera groups. They found that all cardiac surgery–related patient isolates could be classified into group 1. They then did a subgroup analysis.
“Three distinct strains of M. chimaera appear to have contaminated the water systems of LivaNova HCUs at the production site, belonging to subgroups 1.1, 1.8, and 2.1,” the authors stated. However, most M. chimaera isolates from air samples taken near operating LivaNova HCUs and those of 23 of the 24 related patients belonged to subgroup 1.1.
“This finding further supports the presumed airborne transmission pathway leading to endocarditis, aortic graft infection, disseminated disease, and surgical site infections in the affected patients,” according to the authors (doi: 10.1016/S1473-3099[17]30324-9).
The results suggest “the possibility that the vast majority of cases of cardiothoracic surgery–related severe M. chimaera infections diagnosed in Switzerland, Germany, the Netherlands, the United Kingdom, the United States, and Australia resulted from a single common source of infection: LivaNova HCUs that were most likely contaminated during production in Germany,” the researchers concluded.
The study was partly funded by the EU Horizon 2020 program, its FP7 program, the German Center for Infection Research (DZIF), the Swiss National Science Foundation, the Swiss Federal Office of Public Health, and National Institute of Health Research Oxford Health Protection Research Units on Healthcare Associated Infection and Antimicrobial Resistance. The authors reported having no relevant conflicts.
Since 2013, over 100 cases of Mycobacterium chimaera prosthetic valve endocarditis and disseminated disease were detected in Europe and the United States, and these were presumptively linked to contaminated heater-cooler units (HCUs) used during cardiac surgery. A molecular epidemiological analysis of microbial isolate genomes detected a “remarkable clonality of isolates” in almost all of the assessed patients with M. chimaera disease, which “strongly points to a common source of infection,” as reported online in The Lancet Infectious Diseases.
The analysis comprised 250 whole-genome sequencing datasets: 24 isolates from 21 cardiac surgery–related patients in Switzerland, Germany, the Netherlands, and the United Kingdom; 36 from 35 unrelated patients; 126 from LivaNova HCUs in use (85 water cultures, 41 air cultures); 13 from LivaNova HCUs returned to the production site in Germany for disinfection; 4 from the LivaNova production site (3 from newly produced HCUs, 1 from a water source); 2 from Maquet extracorporeal membrane oxygenation (ECMO) devices in use; 14 from Maquet HCUs in use; 15 from new Maquet HCUs sampled at the production site; and 7 from hospital water supplies in Switzerland, Germany, and the Netherlands, plus one M. chimaera DSM 44623–type strain, and eight M. intracellulare strains (from four unrelated patients from Germany and four published genomes).
Isolates were analyzed by next-generation whole-genome sequencing and compared with published M. chimaera genomes, according to Jakko van Ingen, PhD, Radboud University Medical Center, Nijmegen, the Netherlands, and his colleagues. Phylogenetic analysis of these 250 isolates revealed two major M. chimaera groups. They found that all cardiac surgery–related patient isolates could be classified into group 1. They then did a subgroup analysis.
“Three distinct strains of M. chimaera appear to have contaminated the water systems of LivaNova HCUs at the production site, belonging to subgroups 1.1, 1.8, and 2.1,” the authors stated. However, most M. chimaera isolates from air samples taken near operating LivaNova HCUs and those of 23 of the 24 related patients belonged to subgroup 1.1.
“This finding further supports the presumed airborne transmission pathway leading to endocarditis, aortic graft infection, disseminated disease, and surgical site infections in the affected patients,” according to the authors (doi: 10.1016/S1473-3099[17]30324-9).
The results suggest “the possibility that the vast majority of cases of cardiothoracic surgery–related severe M. chimaera infections diagnosed in Switzerland, Germany, the Netherlands, the United Kingdom, the United States, and Australia resulted from a single common source of infection: LivaNova HCUs that were most likely contaminated during production in Germany,” the researchers concluded.
The study was partly funded by the EU Horizon 2020 program, its FP7 program, the German Center for Infection Research (DZIF), the Swiss National Science Foundation, the Swiss Federal Office of Public Health, and National Institute of Health Research Oxford Health Protection Research Units on Healthcare Associated Infection and Antimicrobial Resistance. The authors reported having no relevant conflicts.
FROM THE LANCET INFECTIOUS DISEASES
Key clinical point:
Major finding: Cardiac surgery–related patient isolates were all classified into the same group, in which all, except one, formed a distinct subgroup of Mycobacterium chimaera, which also comprised most isolates from LivaNova HCUs, and one from the equipment production site.
Data source: Phylogenetic analysis based on whole-genome sequencing of 250 M. chimaera isolates obtained from cardiac surgery patients, hospitals, and other sources.
Disclosures: Partly funded by the EU Horizon 2020 program and several German, Swiss, and U.K. infectious disease–related NGOs. The authors reported having no disclosures.
Depression in adolescence
As many as 20% of children and adolescents experience a psychiatric disorder, with 50% of all lifetime psychiatric illnesses occurring by the age of 14 years. ADHD and depression are among the most common. The National Institutes of Health estimate that, in 2015, 3 million 12- to 17-year-old American children experienced a major depressive episode. Any illness that affects over 10% of adolescents will present regularly in the primary care provider’s office. It is important to know whom to screen and how to start treatment when your patient appears to be suffering from this serious but treatable condition.
While there are many screening instruments, it is important to be ready to ask patients diagnostic questions when your clinical suspicion of depression is high. In addition to asking about mood, sleep, appetite, energy, and the other DSM5 criteria of a major depressive episode, it is important to remember that teens with depression might present with irritability as much as sadness. While they lose interest in school, sports, or hobbies, they still may be distracted or cheered up by friends. And
Explain to your patient (and their parents) that depression is very treatable, but most effective treatments take time. Psychotherapy usually works over several months, and even effective medications can take 6 weeks or more. But, without treatment, their symptoms may persist for over a year and can disrupt their healthy development.
This is also a good time to ask your patient about suicidal thoughts. Have they been imagining how their death would affect others? Wishing they could just sleep? Do they have a plan? Do they have access to a means of killing themselves? Do they feel attached or connected to family, friends, religion, or a goal? Explain to your patient that these thoughts are common symptoms of depression, and work with their parents to ensure that they are connected and safe when starting treatment.
Psychotherapy is considered the first line treatment for mild to moderate episodes of depression and should be used alongside medications in severe episodes. While structured therapies such as cognitive behavioral therapy or interpersonal therapy have a strong evidence base to support their use, the best predictor of an effective therapy appears to be a strong alliance between therapist and patient. So, help your patient to find a therapist, and explain the importance of finding someone with whom they feel comfortable. Suggest to your patients that they have three visits with a new therapist to see if it feels like a “good match,” before considering trying another.
Finally, antidepressant medications are first-line treatment for more severe episodes of depression and episodes in which significant suicidal ideation or functional impairment are present. If the symptoms are more severe, or if therapy alone has not been effective after 4-6 weeks, you might consider starting antidepressant treatment. Psychiatrists usually start with an selective serotonin reuptake inhibitor, typically of a medium half-life, at a low dose to minimize the chances of side effects. While real efficacy takes up to 6 weeks, there should be some improvement in energy within the first 2 weeks on an effective medication. If there is no change, the dose can be raised gradually as tolerated. It is important to tell patients and their families about common side effects (mild GI upset) and the more rare but dangerous ones (such as hypomania or an increase in the frequency or intensity of suicidal thoughts).
Even when you do not refer your patient to someone else for treatment of depression, it is important that you not be alone in their management. Work closely with their therapist or consider having a psychiatric social worker join your team to offer therapy in close connection with your management. You might also periodically consult with a child psychiatrist to address treatment and medication questions and identify needed resources. Staying in touch with parents or connected adults at school (with the appropriate permission) can be very useful with those patients you are more concerned about. The educated and attuned primary care provider can provide thoughtful first-line treatment of depression in young people and can be an important part of managing this public health challenge. It is always rewarding to help an adolescent overcome depression.
Dr. Swick is an attending psychiatrist in the division of child psychiatry at Massachusetts General Hospital, Boston, and director of the Parenting at a Challenging Time (PACT) Program at the Vernon Cancer Center at Newton Wellesley Hospital, also in Boston. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at [email protected].
As many as 20% of children and adolescents experience a psychiatric disorder, with 50% of all lifetime psychiatric illnesses occurring by the age of 14 years. ADHD and depression are among the most common. The National Institutes of Health estimate that, in 2015, 3 million 12- to 17-year-old American children experienced a major depressive episode. Any illness that affects over 10% of adolescents will present regularly in the primary care provider’s office. It is important to know whom to screen and how to start treatment when your patient appears to be suffering from this serious but treatable condition.
While there are many screening instruments, it is important to be ready to ask patients diagnostic questions when your clinical suspicion of depression is high. In addition to asking about mood, sleep, appetite, energy, and the other DSM5 criteria of a major depressive episode, it is important to remember that teens with depression might present with irritability as much as sadness. While they lose interest in school, sports, or hobbies, they still may be distracted or cheered up by friends. And
Explain to your patient (and their parents) that depression is very treatable, but most effective treatments take time. Psychotherapy usually works over several months, and even effective medications can take 6 weeks or more. But, without treatment, their symptoms may persist for over a year and can disrupt their healthy development.
This is also a good time to ask your patient about suicidal thoughts. Have they been imagining how their death would affect others? Wishing they could just sleep? Do they have a plan? Do they have access to a means of killing themselves? Do they feel attached or connected to family, friends, religion, or a goal? Explain to your patient that these thoughts are common symptoms of depression, and work with their parents to ensure that they are connected and safe when starting treatment.
Psychotherapy is considered the first line treatment for mild to moderate episodes of depression and should be used alongside medications in severe episodes. While structured therapies such as cognitive behavioral therapy or interpersonal therapy have a strong evidence base to support their use, the best predictor of an effective therapy appears to be a strong alliance between therapist and patient. So, help your patient to find a therapist, and explain the importance of finding someone with whom they feel comfortable. Suggest to your patients that they have three visits with a new therapist to see if it feels like a “good match,” before considering trying another.
Finally, antidepressant medications are first-line treatment for more severe episodes of depression and episodes in which significant suicidal ideation or functional impairment are present. If the symptoms are more severe, or if therapy alone has not been effective after 4-6 weeks, you might consider starting antidepressant treatment. Psychiatrists usually start with an selective serotonin reuptake inhibitor, typically of a medium half-life, at a low dose to minimize the chances of side effects. While real efficacy takes up to 6 weeks, there should be some improvement in energy within the first 2 weeks on an effective medication. If there is no change, the dose can be raised gradually as tolerated. It is important to tell patients and their families about common side effects (mild GI upset) and the more rare but dangerous ones (such as hypomania or an increase in the frequency or intensity of suicidal thoughts).
Even when you do not refer your patient to someone else for treatment of depression, it is important that you not be alone in their management. Work closely with their therapist or consider having a psychiatric social worker join your team to offer therapy in close connection with your management. You might also periodically consult with a child psychiatrist to address treatment and medication questions and identify needed resources. Staying in touch with parents or connected adults at school (with the appropriate permission) can be very useful with those patients you are more concerned about. The educated and attuned primary care provider can provide thoughtful first-line treatment of depression in young people and can be an important part of managing this public health challenge. It is always rewarding to help an adolescent overcome depression.
Dr. Swick is an attending psychiatrist in the division of child psychiatry at Massachusetts General Hospital, Boston, and director of the Parenting at a Challenging Time (PACT) Program at the Vernon Cancer Center at Newton Wellesley Hospital, also in Boston. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at [email protected].
As many as 20% of children and adolescents experience a psychiatric disorder, with 50% of all lifetime psychiatric illnesses occurring by the age of 14 years. ADHD and depression are among the most common. The National Institutes of Health estimate that, in 2015, 3 million 12- to 17-year-old American children experienced a major depressive episode. Any illness that affects over 10% of adolescents will present regularly in the primary care provider’s office. It is important to know whom to screen and how to start treatment when your patient appears to be suffering from this serious but treatable condition.
While there are many screening instruments, it is important to be ready to ask patients diagnostic questions when your clinical suspicion of depression is high. In addition to asking about mood, sleep, appetite, energy, and the other DSM5 criteria of a major depressive episode, it is important to remember that teens with depression might present with irritability as much as sadness. While they lose interest in school, sports, or hobbies, they still may be distracted or cheered up by friends. And
Explain to your patient (and their parents) that depression is very treatable, but most effective treatments take time. Psychotherapy usually works over several months, and even effective medications can take 6 weeks or more. But, without treatment, their symptoms may persist for over a year and can disrupt their healthy development.
This is also a good time to ask your patient about suicidal thoughts. Have they been imagining how their death would affect others? Wishing they could just sleep? Do they have a plan? Do they have access to a means of killing themselves? Do they feel attached or connected to family, friends, religion, or a goal? Explain to your patient that these thoughts are common symptoms of depression, and work with their parents to ensure that they are connected and safe when starting treatment.
Psychotherapy is considered the first line treatment for mild to moderate episodes of depression and should be used alongside medications in severe episodes. While structured therapies such as cognitive behavioral therapy or interpersonal therapy have a strong evidence base to support their use, the best predictor of an effective therapy appears to be a strong alliance between therapist and patient. So, help your patient to find a therapist, and explain the importance of finding someone with whom they feel comfortable. Suggest to your patients that they have three visits with a new therapist to see if it feels like a “good match,” before considering trying another.
Finally, antidepressant medications are first-line treatment for more severe episodes of depression and episodes in which significant suicidal ideation or functional impairment are present. If the symptoms are more severe, or if therapy alone has not been effective after 4-6 weeks, you might consider starting antidepressant treatment. Psychiatrists usually start with an selective serotonin reuptake inhibitor, typically of a medium half-life, at a low dose to minimize the chances of side effects. While real efficacy takes up to 6 weeks, there should be some improvement in energy within the first 2 weeks on an effective medication. If there is no change, the dose can be raised gradually as tolerated. It is important to tell patients and their families about common side effects (mild GI upset) and the more rare but dangerous ones (such as hypomania or an increase in the frequency or intensity of suicidal thoughts).
Even when you do not refer your patient to someone else for treatment of depression, it is important that you not be alone in their management. Work closely with their therapist or consider having a psychiatric social worker join your team to offer therapy in close connection with your management. You might also periodically consult with a child psychiatrist to address treatment and medication questions and identify needed resources. Staying in touch with parents or connected adults at school (with the appropriate permission) can be very useful with those patients you are more concerned about. The educated and attuned primary care provider can provide thoughtful first-line treatment of depression in young people and can be an important part of managing this public health challenge. It is always rewarding to help an adolescent overcome depression.
Dr. Swick is an attending psychiatrist in the division of child psychiatry at Massachusetts General Hospital, Boston, and director of the Parenting at a Challenging Time (PACT) Program at the Vernon Cancer Center at Newton Wellesley Hospital, also in Boston. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at [email protected].











