User login
Physician burnout common, not readily recognized
CHICAGO – Physician burnout is common, and occurs across specialties including gastroenterology, according to a discussion held at the annual Digestive Disease Week.
A number of studies and surveys have reported on physician burnout, including a large 2015 report from the Mayo Clinic, which found that 54% of the physicians surveyed had at least one symptom of burnout (Mayo Clin Proc. 2015 Dec;90[12]:1600-13).
Still, physicians often fail to recognize burnout symptoms in themselves. At the meeting, Laurie A. Keefer Levine, PhD, a GI health psychologist and the director of psychobehavioral research at the Icahn School of Medicine at Mount Sinai, New York, recounted the story of a medical student who jumped from her apartment building and killed herself.
One of the main questions that came out of this discussion was why burnout isn’t more readily recognized. “It had to do with our strength as medical professionals,” she explained. “One common strength is that the pressure is self generated, and we put a lot of pressure on ourselves to excel.”
Health care providers are passionate about their work and it is difficult to give up opportunities that are important to them. “We can delay gratification a really long time until research results come out, or wait a long time for that promotion,” Dr. Keefer said, “But burnout is a very slow and insidious process. A lot of the time we don’t recognize it, and we think ‘just as long as we publish that paper,’ everything will get better.”
Also as time goes on, and physicians become more secure in their work and take on more responsibility, and that becomes another avenue for burnout. But importantly, she emphasized, burnout can be confused with stress, and many people mistake encroaching burnout for stress.
There are pronounced differences between stress and burnout even though they can be co-occurring. The difference, Dr. Keefer explained, is that stress is a problem of too much – work, pressure, and so on, and it is an “overreaction” of the nervous system in that “we’ve got to get it done.”
There is damage associated with chronic stress, but burnout is very different. Instead, burnout is a problem of “not enough.”
“We do not have enough to mount necessary responses to deal with the stressors that we have,” she said. “We are disengaged, our emotions are blunted. We feel helpless or hopeless and lose our motivation. And don’t care about the things we were once passionate about. We don’t have it in us any longer to contribute.”
Physicians use any number of coping strategies, rather than recognizing the problem. The unhealthiest coping strategy is venting. “We all do it, and it feels great, and it is meant to make us feel better,” said Dr. Keefer. “But if continues to happen over and over again, I would encourage you to think it through – that you are engaging in a coping strategy and may be missing burnout.”
It is imperative that medical providers recognize burnout early on, and not wait until it is too late, when there may be major consequences, she said.
Arthur DeCross, MD, professor of medicine at the University of Rochester (N.Y.), discussed some of the subgroups of gastroenterologists who may be at the highest risk of burnout.
Gender plays a strong role, and female gastroenterologists were more likely to identify themselves as being burned out, compared to their male peers. “They may be at risk in the lower domain for a sense of personal accomplishment,” said Dr. DeCross.
“There are respect issues that may come into play, as the literature shows,” he said. “For example, women are more likely to be addressed by their first name by patients and their peers. Also, even at meetings such as this one, how many times is a female presenter simply introduced by her first name?”
There are implicit respect issues here, said Dr. DeCross. “How many times do we hear something like, ‘and now the lovely Millie will present her findings on …?’ ”
He noted that he didn’t think that this lack of respect is intentional, but that it is happening. In addition, there is an issue of wages, and reported data show that women gastroenterologists earn 15% less than their male peers, he noted.
Women are more likely to have competing elements of family and career that put them on the slower track to promotion, he added.
The duration of one’s career also figured into the equation. Burnout was more noticeable early in the career process, suggesting that physicians with young families may be facing more conflicts and stress, and this is an issue that needs to be further explored, he noted.
“Early in the career, there is also the stress of proving oneself,” said Dr. DeCross.
Another contributor to burnout is when physicians spend an increasing amount of time on weekends and holidays doing work-related activities, along with an increase in internal regulatory burdens in the workplace.
Digestive Disease Week® is jointly sponsored by the American Association for the Study of Liver Diseases (AASLD), the American Gastroenterological Association (AGA) Institute, the American Society for Gastrointestinal Endoscopy (ASGE), and the Society for Surgery of the Alimentary Tract (SSAT).
CHICAGO – Physician burnout is common, and occurs across specialties including gastroenterology, according to a discussion held at the annual Digestive Disease Week.
A number of studies and surveys have reported on physician burnout, including a large 2015 report from the Mayo Clinic, which found that 54% of the physicians surveyed had at least one symptom of burnout (Mayo Clin Proc. 2015 Dec;90[12]:1600-13).
Still, physicians often fail to recognize burnout symptoms in themselves. At the meeting, Laurie A. Keefer Levine, PhD, a GI health psychologist and the director of psychobehavioral research at the Icahn School of Medicine at Mount Sinai, New York, recounted the story of a medical student who jumped from her apartment building and killed herself.
One of the main questions that came out of this discussion was why burnout isn’t more readily recognized. “It had to do with our strength as medical professionals,” she explained. “One common strength is that the pressure is self generated, and we put a lot of pressure on ourselves to excel.”
Health care providers are passionate about their work and it is difficult to give up opportunities that are important to them. “We can delay gratification a really long time until research results come out, or wait a long time for that promotion,” Dr. Keefer said, “But burnout is a very slow and insidious process. A lot of the time we don’t recognize it, and we think ‘just as long as we publish that paper,’ everything will get better.”
Also as time goes on, and physicians become more secure in their work and take on more responsibility, and that becomes another avenue for burnout. But importantly, she emphasized, burnout can be confused with stress, and many people mistake encroaching burnout for stress.
There are pronounced differences between stress and burnout even though they can be co-occurring. The difference, Dr. Keefer explained, is that stress is a problem of too much – work, pressure, and so on, and it is an “overreaction” of the nervous system in that “we’ve got to get it done.”
There is damage associated with chronic stress, but burnout is very different. Instead, burnout is a problem of “not enough.”
“We do not have enough to mount necessary responses to deal with the stressors that we have,” she said. “We are disengaged, our emotions are blunted. We feel helpless or hopeless and lose our motivation. And don’t care about the things we were once passionate about. We don’t have it in us any longer to contribute.”
Physicians use any number of coping strategies, rather than recognizing the problem. The unhealthiest coping strategy is venting. “We all do it, and it feels great, and it is meant to make us feel better,” said Dr. Keefer. “But if continues to happen over and over again, I would encourage you to think it through – that you are engaging in a coping strategy and may be missing burnout.”
It is imperative that medical providers recognize burnout early on, and not wait until it is too late, when there may be major consequences, she said.
Arthur DeCross, MD, professor of medicine at the University of Rochester (N.Y.), discussed some of the subgroups of gastroenterologists who may be at the highest risk of burnout.
Gender plays a strong role, and female gastroenterologists were more likely to identify themselves as being burned out, compared to their male peers. “They may be at risk in the lower domain for a sense of personal accomplishment,” said Dr. DeCross.
“There are respect issues that may come into play, as the literature shows,” he said. “For example, women are more likely to be addressed by their first name by patients and their peers. Also, even at meetings such as this one, how many times is a female presenter simply introduced by her first name?”
There are implicit respect issues here, said Dr. DeCross. “How many times do we hear something like, ‘and now the lovely Millie will present her findings on …?’ ”
He noted that he didn’t think that this lack of respect is intentional, but that it is happening. In addition, there is an issue of wages, and reported data show that women gastroenterologists earn 15% less than their male peers, he noted.
Women are more likely to have competing elements of family and career that put them on the slower track to promotion, he added.
The duration of one’s career also figured into the equation. Burnout was more noticeable early in the career process, suggesting that physicians with young families may be facing more conflicts and stress, and this is an issue that needs to be further explored, he noted.
“Early in the career, there is also the stress of proving oneself,” said Dr. DeCross.
Another contributor to burnout is when physicians spend an increasing amount of time on weekends and holidays doing work-related activities, along with an increase in internal regulatory burdens in the workplace.
Digestive Disease Week® is jointly sponsored by the American Association for the Study of Liver Diseases (AASLD), the American Gastroenterological Association (AGA) Institute, the American Society for Gastrointestinal Endoscopy (ASGE), and the Society for Surgery of the Alimentary Tract (SSAT).
CHICAGO – Physician burnout is common, and occurs across specialties including gastroenterology, according to a discussion held at the annual Digestive Disease Week.
A number of studies and surveys have reported on physician burnout, including a large 2015 report from the Mayo Clinic, which found that 54% of the physicians surveyed had at least one symptom of burnout (Mayo Clin Proc. 2015 Dec;90[12]:1600-13).
Still, physicians often fail to recognize burnout symptoms in themselves. At the meeting, Laurie A. Keefer Levine, PhD, a GI health psychologist and the director of psychobehavioral research at the Icahn School of Medicine at Mount Sinai, New York, recounted the story of a medical student who jumped from her apartment building and killed herself.
One of the main questions that came out of this discussion was why burnout isn’t more readily recognized. “It had to do with our strength as medical professionals,” she explained. “One common strength is that the pressure is self generated, and we put a lot of pressure on ourselves to excel.”
Health care providers are passionate about their work and it is difficult to give up opportunities that are important to them. “We can delay gratification a really long time until research results come out, or wait a long time for that promotion,” Dr. Keefer said, “But burnout is a very slow and insidious process. A lot of the time we don’t recognize it, and we think ‘just as long as we publish that paper,’ everything will get better.”
Also as time goes on, and physicians become more secure in their work and take on more responsibility, and that becomes another avenue for burnout. But importantly, she emphasized, burnout can be confused with stress, and many people mistake encroaching burnout for stress.
There are pronounced differences between stress and burnout even though they can be co-occurring. The difference, Dr. Keefer explained, is that stress is a problem of too much – work, pressure, and so on, and it is an “overreaction” of the nervous system in that “we’ve got to get it done.”
There is damage associated with chronic stress, but burnout is very different. Instead, burnout is a problem of “not enough.”
“We do not have enough to mount necessary responses to deal with the stressors that we have,” she said. “We are disengaged, our emotions are blunted. We feel helpless or hopeless and lose our motivation. And don’t care about the things we were once passionate about. We don’t have it in us any longer to contribute.”
Physicians use any number of coping strategies, rather than recognizing the problem. The unhealthiest coping strategy is venting. “We all do it, and it feels great, and it is meant to make us feel better,” said Dr. Keefer. “But if continues to happen over and over again, I would encourage you to think it through – that you are engaging in a coping strategy and may be missing burnout.”
It is imperative that medical providers recognize burnout early on, and not wait until it is too late, when there may be major consequences, she said.
Arthur DeCross, MD, professor of medicine at the University of Rochester (N.Y.), discussed some of the subgroups of gastroenterologists who may be at the highest risk of burnout.
Gender plays a strong role, and female gastroenterologists were more likely to identify themselves as being burned out, compared to their male peers. “They may be at risk in the lower domain for a sense of personal accomplishment,” said Dr. DeCross.
“There are respect issues that may come into play, as the literature shows,” he said. “For example, women are more likely to be addressed by their first name by patients and their peers. Also, even at meetings such as this one, how many times is a female presenter simply introduced by her first name?”
There are implicit respect issues here, said Dr. DeCross. “How many times do we hear something like, ‘and now the lovely Millie will present her findings on …?’ ”
He noted that he didn’t think that this lack of respect is intentional, but that it is happening. In addition, there is an issue of wages, and reported data show that women gastroenterologists earn 15% less than their male peers, he noted.
Women are more likely to have competing elements of family and career that put them on the slower track to promotion, he added.
The duration of one’s career also figured into the equation. Burnout was more noticeable early in the career process, suggesting that physicians with young families may be facing more conflicts and stress, and this is an issue that needs to be further explored, he noted.
“Early in the career, there is also the stress of proving oneself,” said Dr. DeCross.
Another contributor to burnout is when physicians spend an increasing amount of time on weekends and holidays doing work-related activities, along with an increase in internal regulatory burdens in the workplace.
Digestive Disease Week® is jointly sponsored by the American Association for the Study of Liver Diseases (AASLD), the American Gastroenterological Association (AGA) Institute, the American Society for Gastrointestinal Endoscopy (ASGE), and the Society for Surgery of the Alimentary Tract (SSAT).
AT DDW
Physician burnout common, not readily recognized by sufferers
CHICAGO – Physician burnout is common, and occurs across specialties including gastroenterology, according to a discussion held at the annual Digestive Disease Week®.
A number of studies and surveys have reported on physician burnout, including a large 2015 report from the Mayo Clinic, which found that 54% of the physicians surveyed had at least one symptom of burnout (Mayo Clin Proc. 2015 Dec;90[12]:1600-13).
“While she is not the first medical student to kill herself, this was an opportunity for the medical students to sit down and talk with the faculty,” said Dr. Keefer Levine, noting that specifically it focused on how this young woman’s distress had been missed, what was going on with students, and what is missing in medical education.
One of the main questions that came out of this discussion was why burnout isn’t more readily recognized. “It had to do with our strength as medical professionals,” she explained. “One common strength is that the pressure is self-generated, and we put a lot of pressure on ourselves to excel.”
Health care providers are passionate about their work, and it is difficult to give up opportunities that are important to them. “We can delay gratification a really long time until research results come out, or wait a long time for that promotion,” Dr. Keefer Levine said, “But burnout is a very slow and insidious process. A lot of the time we don’t recognize it, and we think ‘just as long as we publish that paper,’ everything will get better.”
Also as time goes on, and physicians become more secure in their work and take on more responsibility, that becomes another avenue for burnout. But importantly, she emphasized, burnout can be confused with stress, and many people mistake encroaching burnout for stress.
There are pronounced differences between stress and burnout even though they can be co-occurring. The difference, Dr. Keefer Levine explained, is that stress is a problem of too much – work, pressure, and so on, and it is an “overreaction” of the nervous system in that “we’ve got to get it done.”
There is damage associated with chronic stress, but burnout is very different. Instead, burnout is a problem of “not enough.”
“We do not have enough to mount necessary responses to deal with the stressors that we have,” she said. “We are disengaged, our emotions are blunted. We feel helpless or hopeless and lose our motivation. And don’t care about the things we were once passionate about. We don’t have it in us any longer to contribute.”
Physicians use any number of coping strategies, rather than recognizing the problem. The unhealthiest coping strategy is venting. “We all do it, and it feels great, and it is meant to make us feel better,” said Dr. Keefer Levine. “But if continues to happen over and over again, I would encourage you to think it through – that you are engaging in a coping strategy and may be missing burnout.”
It is imperative that medical providers recognize burnout early on, and not wait until it is too late, when there may be major consequences, she said.
Arthur DeCross, MD, AGAF, professor of medicine at the University of Rochester (N.Y.), discussed some of the subgroups of gastroenterologists who may be at the highest risk of burnout.
Gender plays a strong role, and female gastroenterologists were more likely to identify themselves as being burned out, compared to their male peers. “They may be at risk in the lower domain for a sense of personal accomplishment,” said Dr. DeCross.
“There are respect issues that may come into play, as the literature shows,” he said. “For example, women are more likely to be addressed by their first name by patients and their peers. Also, even at meetings such as this one, how many times is a female presenter simply introduced by her first name?”
There are implicit respect issues here, said Dr. DeCross. “How many times do we hear something like, ‘and now the lovely Millie will present her findings on ...?’ ”
He noted that he didn’t think that this lack of respect is intentional, but that it is happening. In addition, there is an issue of wages, and reported data show that women gastroenterologists earn 15% less than their male peers, he noted.
Women are more likely to have competing elements of family and career that put them on the slower track to promotion, he added.
The duration of one’s career also figured into the equation. Burnout was more noticeable early in the career process, suggesting that physicians with young families may be facing more conflicts and stress, and this is an issue that needs to be further explored, he noted.
“Early in the career, there is also the stress of proving oneself,” said Dr. DeCross.
Another contributor to burnout is when physicians spend an increasing amount of time on weekends and holidays doing work-related activities, along with an increase in internal regulatory burdens in the workplace.
Dr. DeCross sat down with DDW TV to talk about the results of the survey, which you can watch at http://www.gastro.org/news_items/physician-burnout-amongst-gastroenterologists. Join your colleagues to discuss this important topic in the AGA Community at http://ow.Ly/aYyh30diuq3.
Digestive Disease Week® is jointly sponsored by the American Association for the Study of Liver Diseases (AASLD), the American Gastroenterological Association (AGA) Institute, the American Society for Gastrointestinal Endoscopy (ASGE), and the Society for Surgery of the Alimentary Tract (SSAT).
CHICAGO – Physician burnout is common, and occurs across specialties including gastroenterology, according to a discussion held at the annual Digestive Disease Week®.
A number of studies and surveys have reported on physician burnout, including a large 2015 report from the Mayo Clinic, which found that 54% of the physicians surveyed had at least one symptom of burnout (Mayo Clin Proc. 2015 Dec;90[12]:1600-13).
“While she is not the first medical student to kill herself, this was an opportunity for the medical students to sit down and talk with the faculty,” said Dr. Keefer Levine, noting that specifically it focused on how this young woman’s distress had been missed, what was going on with students, and what is missing in medical education.
One of the main questions that came out of this discussion was why burnout isn’t more readily recognized. “It had to do with our strength as medical professionals,” she explained. “One common strength is that the pressure is self-generated, and we put a lot of pressure on ourselves to excel.”
Health care providers are passionate about their work, and it is difficult to give up opportunities that are important to them. “We can delay gratification a really long time until research results come out, or wait a long time for that promotion,” Dr. Keefer Levine said, “But burnout is a very slow and insidious process. A lot of the time we don’t recognize it, and we think ‘just as long as we publish that paper,’ everything will get better.”
Also as time goes on, and physicians become more secure in their work and take on more responsibility, that becomes another avenue for burnout. But importantly, she emphasized, burnout can be confused with stress, and many people mistake encroaching burnout for stress.
There are pronounced differences between stress and burnout even though they can be co-occurring. The difference, Dr. Keefer Levine explained, is that stress is a problem of too much – work, pressure, and so on, and it is an “overreaction” of the nervous system in that “we’ve got to get it done.”
There is damage associated with chronic stress, but burnout is very different. Instead, burnout is a problem of “not enough.”
“We do not have enough to mount necessary responses to deal with the stressors that we have,” she said. “We are disengaged, our emotions are blunted. We feel helpless or hopeless and lose our motivation. And don’t care about the things we were once passionate about. We don’t have it in us any longer to contribute.”
Physicians use any number of coping strategies, rather than recognizing the problem. The unhealthiest coping strategy is venting. “We all do it, and it feels great, and it is meant to make us feel better,” said Dr. Keefer Levine. “But if continues to happen over and over again, I would encourage you to think it through – that you are engaging in a coping strategy and may be missing burnout.”
It is imperative that medical providers recognize burnout early on, and not wait until it is too late, when there may be major consequences, she said.
Arthur DeCross, MD, AGAF, professor of medicine at the University of Rochester (N.Y.), discussed some of the subgroups of gastroenterologists who may be at the highest risk of burnout.
Gender plays a strong role, and female gastroenterologists were more likely to identify themselves as being burned out, compared to their male peers. “They may be at risk in the lower domain for a sense of personal accomplishment,” said Dr. DeCross.
“There are respect issues that may come into play, as the literature shows,” he said. “For example, women are more likely to be addressed by their first name by patients and their peers. Also, even at meetings such as this one, how many times is a female presenter simply introduced by her first name?”
There are implicit respect issues here, said Dr. DeCross. “How many times do we hear something like, ‘and now the lovely Millie will present her findings on ...?’ ”
He noted that he didn’t think that this lack of respect is intentional, but that it is happening. In addition, there is an issue of wages, and reported data show that women gastroenterologists earn 15% less than their male peers, he noted.
Women are more likely to have competing elements of family and career that put them on the slower track to promotion, he added.
The duration of one’s career also figured into the equation. Burnout was more noticeable early in the career process, suggesting that physicians with young families may be facing more conflicts and stress, and this is an issue that needs to be further explored, he noted.
“Early in the career, there is also the stress of proving oneself,” said Dr. DeCross.
Another contributor to burnout is when physicians spend an increasing amount of time on weekends and holidays doing work-related activities, along with an increase in internal regulatory burdens in the workplace.
Dr. DeCross sat down with DDW TV to talk about the results of the survey, which you can watch at http://www.gastro.org/news_items/physician-burnout-amongst-gastroenterologists. Join your colleagues to discuss this important topic in the AGA Community at http://ow.Ly/aYyh30diuq3.
Digestive Disease Week® is jointly sponsored by the American Association for the Study of Liver Diseases (AASLD), the American Gastroenterological Association (AGA) Institute, the American Society for Gastrointestinal Endoscopy (ASGE), and the Society for Surgery of the Alimentary Tract (SSAT).
CHICAGO – Physician burnout is common, and occurs across specialties including gastroenterology, according to a discussion held at the annual Digestive Disease Week®.
A number of studies and surveys have reported on physician burnout, including a large 2015 report from the Mayo Clinic, which found that 54% of the physicians surveyed had at least one symptom of burnout (Mayo Clin Proc. 2015 Dec;90[12]:1600-13).
“While she is not the first medical student to kill herself, this was an opportunity for the medical students to sit down and talk with the faculty,” said Dr. Keefer Levine, noting that specifically it focused on how this young woman’s distress had been missed, what was going on with students, and what is missing in medical education.
One of the main questions that came out of this discussion was why burnout isn’t more readily recognized. “It had to do with our strength as medical professionals,” she explained. “One common strength is that the pressure is self-generated, and we put a lot of pressure on ourselves to excel.”
Health care providers are passionate about their work, and it is difficult to give up opportunities that are important to them. “We can delay gratification a really long time until research results come out, or wait a long time for that promotion,” Dr. Keefer Levine said, “But burnout is a very slow and insidious process. A lot of the time we don’t recognize it, and we think ‘just as long as we publish that paper,’ everything will get better.”
Also as time goes on, and physicians become more secure in their work and take on more responsibility, that becomes another avenue for burnout. But importantly, she emphasized, burnout can be confused with stress, and many people mistake encroaching burnout for stress.
There are pronounced differences between stress and burnout even though they can be co-occurring. The difference, Dr. Keefer Levine explained, is that stress is a problem of too much – work, pressure, and so on, and it is an “overreaction” of the nervous system in that “we’ve got to get it done.”
There is damage associated with chronic stress, but burnout is very different. Instead, burnout is a problem of “not enough.”
“We do not have enough to mount necessary responses to deal with the stressors that we have,” she said. “We are disengaged, our emotions are blunted. We feel helpless or hopeless and lose our motivation. And don’t care about the things we were once passionate about. We don’t have it in us any longer to contribute.”
Physicians use any number of coping strategies, rather than recognizing the problem. The unhealthiest coping strategy is venting. “We all do it, and it feels great, and it is meant to make us feel better,” said Dr. Keefer Levine. “But if continues to happen over and over again, I would encourage you to think it through – that you are engaging in a coping strategy and may be missing burnout.”
It is imperative that medical providers recognize burnout early on, and not wait until it is too late, when there may be major consequences, she said.
Arthur DeCross, MD, AGAF, professor of medicine at the University of Rochester (N.Y.), discussed some of the subgroups of gastroenterologists who may be at the highest risk of burnout.
Gender plays a strong role, and female gastroenterologists were more likely to identify themselves as being burned out, compared to their male peers. “They may be at risk in the lower domain for a sense of personal accomplishment,” said Dr. DeCross.
“There are respect issues that may come into play, as the literature shows,” he said. “For example, women are more likely to be addressed by their first name by patients and their peers. Also, even at meetings such as this one, how many times is a female presenter simply introduced by her first name?”
There are implicit respect issues here, said Dr. DeCross. “How many times do we hear something like, ‘and now the lovely Millie will present her findings on ...?’ ”
He noted that he didn’t think that this lack of respect is intentional, but that it is happening. In addition, there is an issue of wages, and reported data show that women gastroenterologists earn 15% less than their male peers, he noted.
Women are more likely to have competing elements of family and career that put them on the slower track to promotion, he added.
The duration of one’s career also figured into the equation. Burnout was more noticeable early in the career process, suggesting that physicians with young families may be facing more conflicts and stress, and this is an issue that needs to be further explored, he noted.
“Early in the career, there is also the stress of proving oneself,” said Dr. DeCross.
Another contributor to burnout is when physicians spend an increasing amount of time on weekends and holidays doing work-related activities, along with an increase in internal regulatory burdens in the workplace.
Dr. DeCross sat down with DDW TV to talk about the results of the survey, which you can watch at http://www.gastro.org/news_items/physician-burnout-amongst-gastroenterologists. Join your colleagues to discuss this important topic in the AGA Community at http://ow.Ly/aYyh30diuq3.
Digestive Disease Week® is jointly sponsored by the American Association for the Study of Liver Diseases (AASLD), the American Gastroenterological Association (AGA) Institute, the American Society for Gastrointestinal Endoscopy (ASGE), and the Society for Surgery of the Alimentary Tract (SSAT).
AT DDW
Fujifilm issues recall to update ED-530XT duodenoscopes
Fujifilm has issued an Urgent Medical Device Correction and Removal notification for all ED-530XT duodenoscopes, according to a Safety Alert from the Food and Drug Administration.
The recall, initiated voluntarily by Fujifilm, includes replacement of the ED-530XT forceps elevator mechanism including the O-ring seal, replacement of the distal end cap, and new operation manuals. The FDA authorized the changes on July 21, 2017.
“Reprocessing is a detailed, multistep process to clean and disinfect or sterilize reusable devices. The FDA has been working with duodenoscope manufacturers as they modify and validate their reprocessing instructions to further enhance the safety margin of their devices and show with a high degree of assurance that their reprocessing instructions, when followed correctly, effectively clean and disinfect the duodenoscopes,” the FDA said in the press release.
Find the full Safety Alert on the FDA website.
Fujifilm has issued an Urgent Medical Device Correction and Removal notification for all ED-530XT duodenoscopes, according to a Safety Alert from the Food and Drug Administration.
The recall, initiated voluntarily by Fujifilm, includes replacement of the ED-530XT forceps elevator mechanism including the O-ring seal, replacement of the distal end cap, and new operation manuals. The FDA authorized the changes on July 21, 2017.
“Reprocessing is a detailed, multistep process to clean and disinfect or sterilize reusable devices. The FDA has been working with duodenoscope manufacturers as they modify and validate their reprocessing instructions to further enhance the safety margin of their devices and show with a high degree of assurance that their reprocessing instructions, when followed correctly, effectively clean and disinfect the duodenoscopes,” the FDA said in the press release.
Find the full Safety Alert on the FDA website.
Fujifilm has issued an Urgent Medical Device Correction and Removal notification for all ED-530XT duodenoscopes, according to a Safety Alert from the Food and Drug Administration.
The recall, initiated voluntarily by Fujifilm, includes replacement of the ED-530XT forceps elevator mechanism including the O-ring seal, replacement of the distal end cap, and new operation manuals. The FDA authorized the changes on July 21, 2017.
“Reprocessing is a detailed, multistep process to clean and disinfect or sterilize reusable devices. The FDA has been working with duodenoscope manufacturers as they modify and validate their reprocessing instructions to further enhance the safety margin of their devices and show with a high degree of assurance that their reprocessing instructions, when followed correctly, effectively clean and disinfect the duodenoscopes,” the FDA said in the press release.
Find the full Safety Alert on the FDA website.
Increased risk of death seen in PPI users
Proton pump inhibitors (PPIs) are associated with a significantly higher risk of death than are H2-receptor antagonists, according to a 5-year longitudinal cohort study.
The study, published online in BMJ Open, found that increased risk of death was evident even in people without gastrointestinal conditions, and it increased with longer duration of use.
Yan Xie, MPH, of the VA Saint Louis Health Care System and coauthors, wrote that PPIs are linked to a range of serious adverse outcomes – such as acute interstitial nephritis, chronic kidney disease, incident dementia, and Clostridium difficile infection – each of which is associated with higher risk of mortality.

Researchers saw a 25% higher risk of death in the 275,977 participants treated with PPIs, compared with that in those who were treated with H2-receptor antagonists (95% confidence interval, 1.23-1.28), after adjusting for factors such as estimated glomerular filtration rate, age, hospitalizations, and a range of comorbidities, including gastrointestinal disorders.When PPI use was compared with no PPI use, there was a 15% increase in the risk of death (95% CI, 1.14-1.15). When compared with no known exposure to any acid suppression therapy, the increased risk of death was 23% (95% CI, 1.22-1.24).
In an attempt to look at the risk of death in a lower-risk cohort, the researchers analyzed a subgroup of participants who did not have the conditions for which PPIs are normally prescribed, such as gastroesophageal reflux disease, upper gastrointestinal tract bleeding, ulcer disease, Helicobacter pylori infection, and Barrett’s esophagus.
However, even in this lower-risk cohort, the study still showed a 24% increase in the risk of death with PPIs, compared with that in H2-receptor antagonists (95% CI, 1.21-1.27); a 19% increase with PPIs, compared with no PPIs; and a 22% increase with PPIs, compared with no acid suppression.
Duration of exposure to PPIs was also associated with increasing risk of death. Participants who had taken PPIs for fewer than 90 days in total had only a 5% increase in risk, while those taking them for 361-720 days had a 51% increased risk of death.
“Although our results should not deter prescription and use of PPIs where medically indicated, they may be used to encourage and promote pharmacovigilance and emphasize the need to exercise judicious use of PPIs and limit use and duration of therapy to instances where there is a clear medical indication and where benefit outweighs potential risk,” the authors wrote.“Standardized guidelines for initiating PPI prescription may lead to reduced overuse [and] regular review of prescription and over-the-counter medications, and deprescription, where a medical indication for PPI treatment ceases to exist, may be a meritorious approach.”
Examining possible physiologic mechanisms to explain the increased risk of death, the authors noted that animal studies suggested PPIs may limit the liver’s capacity to regenerate.
PPIs are also associated with increased activity of the heme oxygenase-1 enzyme in gastric and endothelial cells and impairment of lysosomal acidification and proteostasis and may alter gene expression in the cellular retinol metabolism pathway and the complement and coagulation cascades pathway.
However, the clinical mediator of the heightened risk of death was likely one of the adverse events linked to PPI use, they said.
The authors declared no relevant financial conflicts of interest.
Review AGA’s “Guide to Conversations About the Latest PPI Research Results” for tips on talking with your patients about this research study.
Proton pump inhibitors (PPIs) are associated with a significantly higher risk of death than are H2-receptor antagonists, according to a 5-year longitudinal cohort study.
The study, published online in BMJ Open, found that increased risk of death was evident even in people without gastrointestinal conditions, and it increased with longer duration of use.
Yan Xie, MPH, of the VA Saint Louis Health Care System and coauthors, wrote that PPIs are linked to a range of serious adverse outcomes – such as acute interstitial nephritis, chronic kidney disease, incident dementia, and Clostridium difficile infection – each of which is associated with higher risk of mortality.

Researchers saw a 25% higher risk of death in the 275,977 participants treated with PPIs, compared with that in those who were treated with H2-receptor antagonists (95% confidence interval, 1.23-1.28), after adjusting for factors such as estimated glomerular filtration rate, age, hospitalizations, and a range of comorbidities, including gastrointestinal disorders.When PPI use was compared with no PPI use, there was a 15% increase in the risk of death (95% CI, 1.14-1.15). When compared with no known exposure to any acid suppression therapy, the increased risk of death was 23% (95% CI, 1.22-1.24).
In an attempt to look at the risk of death in a lower-risk cohort, the researchers analyzed a subgroup of participants who did not have the conditions for which PPIs are normally prescribed, such as gastroesophageal reflux disease, upper gastrointestinal tract bleeding, ulcer disease, Helicobacter pylori infection, and Barrett’s esophagus.
However, even in this lower-risk cohort, the study still showed a 24% increase in the risk of death with PPIs, compared with that in H2-receptor antagonists (95% CI, 1.21-1.27); a 19% increase with PPIs, compared with no PPIs; and a 22% increase with PPIs, compared with no acid suppression.
Duration of exposure to PPIs was also associated with increasing risk of death. Participants who had taken PPIs for fewer than 90 days in total had only a 5% increase in risk, while those taking them for 361-720 days had a 51% increased risk of death.
“Although our results should not deter prescription and use of PPIs where medically indicated, they may be used to encourage and promote pharmacovigilance and emphasize the need to exercise judicious use of PPIs and limit use and duration of therapy to instances where there is a clear medical indication and where benefit outweighs potential risk,” the authors wrote.“Standardized guidelines for initiating PPI prescription may lead to reduced overuse [and] regular review of prescription and over-the-counter medications, and deprescription, where a medical indication for PPI treatment ceases to exist, may be a meritorious approach.”
Examining possible physiologic mechanisms to explain the increased risk of death, the authors noted that animal studies suggested PPIs may limit the liver’s capacity to regenerate.
PPIs are also associated with increased activity of the heme oxygenase-1 enzyme in gastric and endothelial cells and impairment of lysosomal acidification and proteostasis and may alter gene expression in the cellular retinol metabolism pathway and the complement and coagulation cascades pathway.
However, the clinical mediator of the heightened risk of death was likely one of the adverse events linked to PPI use, they said.
The authors declared no relevant financial conflicts of interest.
Review AGA’s “Guide to Conversations About the Latest PPI Research Results” for tips on talking with your patients about this research study.
Proton pump inhibitors (PPIs) are associated with a significantly higher risk of death than are H2-receptor antagonists, according to a 5-year longitudinal cohort study.
The study, published online in BMJ Open, found that increased risk of death was evident even in people without gastrointestinal conditions, and it increased with longer duration of use.
Yan Xie, MPH, of the VA Saint Louis Health Care System and coauthors, wrote that PPIs are linked to a range of serious adverse outcomes – such as acute interstitial nephritis, chronic kidney disease, incident dementia, and Clostridium difficile infection – each of which is associated with higher risk of mortality.

Researchers saw a 25% higher risk of death in the 275,977 participants treated with PPIs, compared with that in those who were treated with H2-receptor antagonists (95% confidence interval, 1.23-1.28), after adjusting for factors such as estimated glomerular filtration rate, age, hospitalizations, and a range of comorbidities, including gastrointestinal disorders.When PPI use was compared with no PPI use, there was a 15% increase in the risk of death (95% CI, 1.14-1.15). When compared with no known exposure to any acid suppression therapy, the increased risk of death was 23% (95% CI, 1.22-1.24).
In an attempt to look at the risk of death in a lower-risk cohort, the researchers analyzed a subgroup of participants who did not have the conditions for which PPIs are normally prescribed, such as gastroesophageal reflux disease, upper gastrointestinal tract bleeding, ulcer disease, Helicobacter pylori infection, and Barrett’s esophagus.
However, even in this lower-risk cohort, the study still showed a 24% increase in the risk of death with PPIs, compared with that in H2-receptor antagonists (95% CI, 1.21-1.27); a 19% increase with PPIs, compared with no PPIs; and a 22% increase with PPIs, compared with no acid suppression.
Duration of exposure to PPIs was also associated with increasing risk of death. Participants who had taken PPIs for fewer than 90 days in total had only a 5% increase in risk, while those taking them for 361-720 days had a 51% increased risk of death.
“Although our results should not deter prescription and use of PPIs where medically indicated, they may be used to encourage and promote pharmacovigilance and emphasize the need to exercise judicious use of PPIs and limit use and duration of therapy to instances where there is a clear medical indication and where benefit outweighs potential risk,” the authors wrote.“Standardized guidelines for initiating PPI prescription may lead to reduced overuse [and] regular review of prescription and over-the-counter medications, and deprescription, where a medical indication for PPI treatment ceases to exist, may be a meritorious approach.”
Examining possible physiologic mechanisms to explain the increased risk of death, the authors noted that animal studies suggested PPIs may limit the liver’s capacity to regenerate.
PPIs are also associated with increased activity of the heme oxygenase-1 enzyme in gastric and endothelial cells and impairment of lysosomal acidification and proteostasis and may alter gene expression in the cellular retinol metabolism pathway and the complement and coagulation cascades pathway.
However, the clinical mediator of the heightened risk of death was likely one of the adverse events linked to PPI use, they said.
The authors declared no relevant financial conflicts of interest.
Review AGA’s “Guide to Conversations About the Latest PPI Research Results” for tips on talking with your patients about this research study.
EUS beats MRCP on sensitivity for bile duct stones
Endoscopic ultrasound (EUS) beat magnetic resonance cholangiopancreatography (MRCP) on diagnostic accuracy for choledocholithiasis, but both provided similar specificity, according to data from a meta-analysis of head-to-head comparisons. The findings were published in Gastrointestinal Endoscopy.
EUS and MRCP can provide accurate diagnosis of choledocholithiasis with lower adverse event rates than the clinical standards of endoscopic retrograde cholangiopancreatography and intraoperative cholangiography, but a meta-analysis of the diagnostic test accuracy (DTA) of the two methods was lacking, wrote Mohammad Yaghoobi, MD, of McMaster University, Hamilton, Ont., and his colleagues (Gastrointest Endosc. 2017. doi: 10.1016/j.gie.2017.06.009).
“The methodology of a meta-analysis of DTA is different from a conventional meta-analysis in several aspects, including statistical analysis and quality assessment of the included trials,” the researchers wrote.
They reviewed data from studies conducted between 1980 and January 2017 and identified five studies of head-to-head comparisons of EUS and MRCP. Studies involving children, and those with insufficient data, no reference standards, and a gap of more than 48 hours between two index tests were excluded.
Overall, the diagnostic odds ratio was significantly higher for EUS than MRCP (P = .008). This difference was mainly a factor of the significantly higher sensitivity of EUS vs. MRCP (P = .006). Specificity was not significantly different between the two tests.
The results were limited by several factors, including inadequate data for a subgroup analysis based on stone size and the relatively small number of studies included in the analysis, but the strict inclusion criteria add to the strength of the findings, the researchers noted.
“EUS should be incorporated in the diagnostic algorithm in patients suspected for choledocholithiasis, whenever appropriate, given its reasonable safety profile,” the researchers said. “This might specially apply to the patients who need an esophagogastroduodenoscopy for investigating other alternative causes of abdominal pain,” they added. The researchers had no financial conflicts to disclose.
Endoscopic ultrasound (EUS) beat magnetic resonance cholangiopancreatography (MRCP) on diagnostic accuracy for choledocholithiasis, but both provided similar specificity, according to data from a meta-analysis of head-to-head comparisons. The findings were published in Gastrointestinal Endoscopy.
EUS and MRCP can provide accurate diagnosis of choledocholithiasis with lower adverse event rates than the clinical standards of endoscopic retrograde cholangiopancreatography and intraoperative cholangiography, but a meta-analysis of the diagnostic test accuracy (DTA) of the two methods was lacking, wrote Mohammad Yaghoobi, MD, of McMaster University, Hamilton, Ont., and his colleagues (Gastrointest Endosc. 2017. doi: 10.1016/j.gie.2017.06.009).
“The methodology of a meta-analysis of DTA is different from a conventional meta-analysis in several aspects, including statistical analysis and quality assessment of the included trials,” the researchers wrote.
They reviewed data from studies conducted between 1980 and January 2017 and identified five studies of head-to-head comparisons of EUS and MRCP. Studies involving children, and those with insufficient data, no reference standards, and a gap of more than 48 hours between two index tests were excluded.
Overall, the diagnostic odds ratio was significantly higher for EUS than MRCP (P = .008). This difference was mainly a factor of the significantly higher sensitivity of EUS vs. MRCP (P = .006). Specificity was not significantly different between the two tests.
The results were limited by several factors, including inadequate data for a subgroup analysis based on stone size and the relatively small number of studies included in the analysis, but the strict inclusion criteria add to the strength of the findings, the researchers noted.
“EUS should be incorporated in the diagnostic algorithm in patients suspected for choledocholithiasis, whenever appropriate, given its reasonable safety profile,” the researchers said. “This might specially apply to the patients who need an esophagogastroduodenoscopy for investigating other alternative causes of abdominal pain,” they added. The researchers had no financial conflicts to disclose.
Endoscopic ultrasound (EUS) beat magnetic resonance cholangiopancreatography (MRCP) on diagnostic accuracy for choledocholithiasis, but both provided similar specificity, according to data from a meta-analysis of head-to-head comparisons. The findings were published in Gastrointestinal Endoscopy.
EUS and MRCP can provide accurate diagnosis of choledocholithiasis with lower adverse event rates than the clinical standards of endoscopic retrograde cholangiopancreatography and intraoperative cholangiography, but a meta-analysis of the diagnostic test accuracy (DTA) of the two methods was lacking, wrote Mohammad Yaghoobi, MD, of McMaster University, Hamilton, Ont., and his colleagues (Gastrointest Endosc. 2017. doi: 10.1016/j.gie.2017.06.009).
“The methodology of a meta-analysis of DTA is different from a conventional meta-analysis in several aspects, including statistical analysis and quality assessment of the included trials,” the researchers wrote.
They reviewed data from studies conducted between 1980 and January 2017 and identified five studies of head-to-head comparisons of EUS and MRCP. Studies involving children, and those with insufficient data, no reference standards, and a gap of more than 48 hours between two index tests were excluded.
Overall, the diagnostic odds ratio was significantly higher for EUS than MRCP (P = .008). This difference was mainly a factor of the significantly higher sensitivity of EUS vs. MRCP (P = .006). Specificity was not significantly different between the two tests.
The results were limited by several factors, including inadequate data for a subgroup analysis based on stone size and the relatively small number of studies included in the analysis, but the strict inclusion criteria add to the strength of the findings, the researchers noted.
“EUS should be incorporated in the diagnostic algorithm in patients suspected for choledocholithiasis, whenever appropriate, given its reasonable safety profile,” the researchers said. “This might specially apply to the patients who need an esophagogastroduodenoscopy for investigating other alternative causes of abdominal pain,” they added. The researchers had no financial conflicts to disclose.
FROM GASTROINTESTINAL ENDOSCOPY
Key clinical point: EUS offers greater sensitivity and similar specificity compared with MRCP in detecting choledocholithiasis.
Major finding: The diagnostic odds ratio was significantly higher for EUS than MRCP (P = .008).
Data source: A meta-analysis of diagnostic test accuracy including five head-to-head studies.
Disclosures: The researchers had no financial conflicts to disclose.
Study finds family history, chocolate intake increases acne risk
Having two parents with a history of acne was associated with an eightfold higher risk of acne during adolescence and young adulthood, in a European study that surveyed people aged 15-24 years in seven European countries.
Researchers conducted an online population-based survey of 10,521 individuals aged 15-24 years in Belgium, Czech and Slovak republics, France, Italy, Poland, and Spain, with questions about the presence or absence of acne, sociodemographic characteristics, and lifestyle factors (such as diet, tobacco, cannabis or alcohol use, and family history). The results were published online on July 14.
The overall prevalence of self-reported acne was 57.8%, with the highest prevalence – 65.8% – found in the 15- to 17-years age group, and the lowest being 52.6% in the 21- to 24-years age group. Individuals who reported having one parent with a history of acne had a threefold greater incidence of acne compared to those without a history of paternal or maternal acne (P less than .0001 for both). Individuals whose parents both had acne had a nearly eightfold higher risk, which the authors noted was consistent with other studies showing a strong hereditary component of acne (J Eur Acad Dermatol Venereol. 2017 Jul 14. doi: 10.1111/jdv.14475).
“Previous studies have demonstrated an association between high glycemic index foods and acne, although in our study, only chocolate, and not pasta or sweets, was independently associated in multivariate analysis,” wrote Pierre Wolkenstein, MD, of the department of dermatology, Hôpital Henri Mondor, Créteil, France, and his coauthors.
“The relationship between smoking and acne is not clear. Some observational studies have found that smoking increases the prevalence of acne, others have found a negative association, and some have found no relationship,” they added.
The study also showed significant variation in the incidence of acne across different countries. Using Spain, which had a median prevalence of acne, as a reference point, the researchers found that respondents in the Czech and Slovak republics had a 96% higher incidence of acne, while those in Poland had a 55% lower incidence.
The authors cautioned that their results were based on self-report, rather than a physician diagnosis, but they noted that since acne is so common, false positive or false negative reports were unlikely. “An association between self-reported acne and chocolate consumption, and an apparent inverse relationship with smoking, need to be confirmed by additional studies,” they noted.
The survey was funded and supported by Pierre Fabre Dermatologie. Five authors declared fees as members of the European Severe Acne Board, supported by Pierre Fabre Dermatologie, and one author is an employee of the company.
Having two parents with a history of acne was associated with an eightfold higher risk of acne during adolescence and young adulthood, in a European study that surveyed people aged 15-24 years in seven European countries.
Researchers conducted an online population-based survey of 10,521 individuals aged 15-24 years in Belgium, Czech and Slovak republics, France, Italy, Poland, and Spain, with questions about the presence or absence of acne, sociodemographic characteristics, and lifestyle factors (such as diet, tobacco, cannabis or alcohol use, and family history). The results were published online on July 14.
The overall prevalence of self-reported acne was 57.8%, with the highest prevalence – 65.8% – found in the 15- to 17-years age group, and the lowest being 52.6% in the 21- to 24-years age group. Individuals who reported having one parent with a history of acne had a threefold greater incidence of acne compared to those without a history of paternal or maternal acne (P less than .0001 for both). Individuals whose parents both had acne had a nearly eightfold higher risk, which the authors noted was consistent with other studies showing a strong hereditary component of acne (J Eur Acad Dermatol Venereol. 2017 Jul 14. doi: 10.1111/jdv.14475).
“Previous studies have demonstrated an association between high glycemic index foods and acne, although in our study, only chocolate, and not pasta or sweets, was independently associated in multivariate analysis,” wrote Pierre Wolkenstein, MD, of the department of dermatology, Hôpital Henri Mondor, Créteil, France, and his coauthors.
“The relationship between smoking and acne is not clear. Some observational studies have found that smoking increases the prevalence of acne, others have found a negative association, and some have found no relationship,” they added.
The study also showed significant variation in the incidence of acne across different countries. Using Spain, which had a median prevalence of acne, as a reference point, the researchers found that respondents in the Czech and Slovak republics had a 96% higher incidence of acne, while those in Poland had a 55% lower incidence.
The authors cautioned that their results were based on self-report, rather than a physician diagnosis, but they noted that since acne is so common, false positive or false negative reports were unlikely. “An association between self-reported acne and chocolate consumption, and an apparent inverse relationship with smoking, need to be confirmed by additional studies,” they noted.
The survey was funded and supported by Pierre Fabre Dermatologie. Five authors declared fees as members of the European Severe Acne Board, supported by Pierre Fabre Dermatologie, and one author is an employee of the company.
Having two parents with a history of acne was associated with an eightfold higher risk of acne during adolescence and young adulthood, in a European study that surveyed people aged 15-24 years in seven European countries.
Researchers conducted an online population-based survey of 10,521 individuals aged 15-24 years in Belgium, Czech and Slovak republics, France, Italy, Poland, and Spain, with questions about the presence or absence of acne, sociodemographic characteristics, and lifestyle factors (such as diet, tobacco, cannabis or alcohol use, and family history). The results were published online on July 14.
The overall prevalence of self-reported acne was 57.8%, with the highest prevalence – 65.8% – found in the 15- to 17-years age group, and the lowest being 52.6% in the 21- to 24-years age group. Individuals who reported having one parent with a history of acne had a threefold greater incidence of acne compared to those without a history of paternal or maternal acne (P less than .0001 for both). Individuals whose parents both had acne had a nearly eightfold higher risk, which the authors noted was consistent with other studies showing a strong hereditary component of acne (J Eur Acad Dermatol Venereol. 2017 Jul 14. doi: 10.1111/jdv.14475).
“Previous studies have demonstrated an association between high glycemic index foods and acne, although in our study, only chocolate, and not pasta or sweets, was independently associated in multivariate analysis,” wrote Pierre Wolkenstein, MD, of the department of dermatology, Hôpital Henri Mondor, Créteil, France, and his coauthors.
“The relationship between smoking and acne is not clear. Some observational studies have found that smoking increases the prevalence of acne, others have found a negative association, and some have found no relationship,” they added.
The study also showed significant variation in the incidence of acne across different countries. Using Spain, which had a median prevalence of acne, as a reference point, the researchers found that respondents in the Czech and Slovak republics had a 96% higher incidence of acne, while those in Poland had a 55% lower incidence.
The authors cautioned that their results were based on self-report, rather than a physician diagnosis, but they noted that since acne is so common, false positive or false negative reports were unlikely. “An association between self-reported acne and chocolate consumption, and an apparent inverse relationship with smoking, need to be confirmed by additional studies,” they noted.
The survey was funded and supported by Pierre Fabre Dermatologie. Five authors declared fees as members of the European Severe Acne Board, supported by Pierre Fabre Dermatologie, and one author is an employee of the company.
FROM THE JOURNAL OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Key clinical point: A parental history of acne, higher levels of personal chocolate consumption, and younger age were significantly associated with an increased risk of acne.
Major finding: Adolescents and young adults with two parents with a history of acne have a nearly eightfold higher risk of the condition.
Data source: A population-based survey of 10,521 individuals aged 15-24 years in seven European countries.
Disclosures: Five authors declared fees as members of the European Severe Acne Board – supported by Pierre Fabre Dermatologie, which funded the survey – and one author is an employee of the company.
Get involved with AGA
We’re soliciting members to serve in AGA leadership positions – as committee and center members, as well as on the AGA Institute Governing Board. This is an opportunity to network with other physicians and scientists, pursue a special interest, and make an impact in an area that is important to you.
Join a committee or center
AGA and AGA Institute are seeking members to serve on several committees and centers that recommend and oversee new and existing policies and programs. Terms will start in June 2018; nominations must be received by Nov. 1, 2017. Members can either nominate themselves or other members. For more information on the positions available, take a look at the AGA Committee page, http://www.gastro.org/about/people/committees.
Serve in a leadership position
The AGA Nominating Committee is in the midst of identifying candidates to join the governing board – in the offices of vice president, clinical research councillor, and practice councillor – as well as 10 nominees for the 2018-2019 AGA Nominating Committee. To learn more, or to nominate yourself or a colleague, email [email protected]. Nominations are due by Oct. 1, 2017; earlier submissions are encouraged.
We’re soliciting members to serve in AGA leadership positions – as committee and center members, as well as on the AGA Institute Governing Board. This is an opportunity to network with other physicians and scientists, pursue a special interest, and make an impact in an area that is important to you.
Join a committee or center
AGA and AGA Institute are seeking members to serve on several committees and centers that recommend and oversee new and existing policies and programs. Terms will start in June 2018; nominations must be received by Nov. 1, 2017. Members can either nominate themselves or other members. For more information on the positions available, take a look at the AGA Committee page, http://www.gastro.org/about/people/committees.
Serve in a leadership position
The AGA Nominating Committee is in the midst of identifying candidates to join the governing board – in the offices of vice president, clinical research councillor, and practice councillor – as well as 10 nominees for the 2018-2019 AGA Nominating Committee. To learn more, or to nominate yourself or a colleague, email [email protected]. Nominations are due by Oct. 1, 2017; earlier submissions are encouraged.
We’re soliciting members to serve in AGA leadership positions – as committee and center members, as well as on the AGA Institute Governing Board. This is an opportunity to network with other physicians and scientists, pursue a special interest, and make an impact in an area that is important to you.
Join a committee or center
AGA and AGA Institute are seeking members to serve on several committees and centers that recommend and oversee new and existing policies and programs. Terms will start in June 2018; nominations must be received by Nov. 1, 2017. Members can either nominate themselves or other members. For more information on the positions available, take a look at the AGA Committee page, http://www.gastro.org/about/people/committees.
Serve in a leadership position
The AGA Nominating Committee is in the midst of identifying candidates to join the governing board – in the offices of vice president, clinical research councillor, and practice councillor – as well as 10 nominees for the 2018-2019 AGA Nominating Committee. To learn more, or to nominate yourself or a colleague, email [email protected]. Nominations are due by Oct. 1, 2017; earlier submissions are encouraged.
Mark your calendar: 2018 AGA grants cycle announced
The AGA Research Foundation is excited to announce the start of its 2018 Research Grants cycle. This year the foundation will be awarding over $2 million in funding to support researchers within gastroenterology and hepatology. Now is your chance to view upcoming opportunities and plan your applications. Learn more below about the first application due in August, and visit the AGA Research Funding website (www.gastro.org/research-funding) for the full list. Contact [email protected] with any questions.
Applications due: Aug. 4, 2017
AGA-R. Robert & Sally Funderburg Research Award in Gastric Cancer
This award provides $50,000 per year for 2 years to an established investigator working on novel approaches in gastric cancer research. For the past 25 years, this $100,000 award has enhanced the fundamental understanding of gastric cancer pathobiology toward ultimately developing a cure for the disease.
The AGA Research Foundation is excited to announce the start of its 2018 Research Grants cycle. This year the foundation will be awarding over $2 million in funding to support researchers within gastroenterology and hepatology. Now is your chance to view upcoming opportunities and plan your applications. Learn more below about the first application due in August, and visit the AGA Research Funding website (www.gastro.org/research-funding) for the full list. Contact [email protected] with any questions.
Applications due: Aug. 4, 2017
AGA-R. Robert & Sally Funderburg Research Award in Gastric Cancer
This award provides $50,000 per year for 2 years to an established investigator working on novel approaches in gastric cancer research. For the past 25 years, this $100,000 award has enhanced the fundamental understanding of gastric cancer pathobiology toward ultimately developing a cure for the disease.
The AGA Research Foundation is excited to announce the start of its 2018 Research Grants cycle. This year the foundation will be awarding over $2 million in funding to support researchers within gastroenterology and hepatology. Now is your chance to view upcoming opportunities and plan your applications. Learn more below about the first application due in August, and visit the AGA Research Funding website (www.gastro.org/research-funding) for the full list. Contact [email protected] with any questions.
Applications due: Aug. 4, 2017
AGA-R. Robert & Sally Funderburg Research Award in Gastric Cancer
This award provides $50,000 per year for 2 years to an established investigator working on novel approaches in gastric cancer research. For the past 25 years, this $100,000 award has enhanced the fundamental understanding of gastric cancer pathobiology toward ultimately developing a cure for the disease.
Physician compensation growing but at a slightly slower pace
Physicians working in large multispecialty groups saw their compensation increase in 2016, albeit at a slower pace than in 2015, according to survey results reported by AMGA.
The 2017 Medical Group Compensation and Productivity Survey shows that the overall weighted average increase in physician compensation for the calendar year 2016 was 2.9%, slightly lower than the 3.1% increase seen in 2015. Doctors in more than three-quarters (77%) of specialties saw increases in 2016.
Opthalmologic surgery saw the largest compensation increase at 7.7%, followed by cardiothoracic surgery (7.0%), hematology and medical oncology (6.7%), allergy/immunology (5.9%) and pulmonary disease (5.6%). Emergency medicine saw a decrease in compensation of 2.0% in 2016 after experiencing a 9.6% increase in 2015.
Value-based payment is beginning to factor into the growth in payment by specialty. Overall, about 8% of compensation is being linked to value-based pay, and that number is expected to rise, with some medical practice groups linking 15% or more of compensation to value-based metrics.
“In almost all of the groups that I have worked with in the last few years on [compensation] design, that has been one of the drivers of decision to do the compensation redesign is to allocate to the value-based metrics,” Wayne Hartley, vice president of AMGA Consulting, said in an interview. 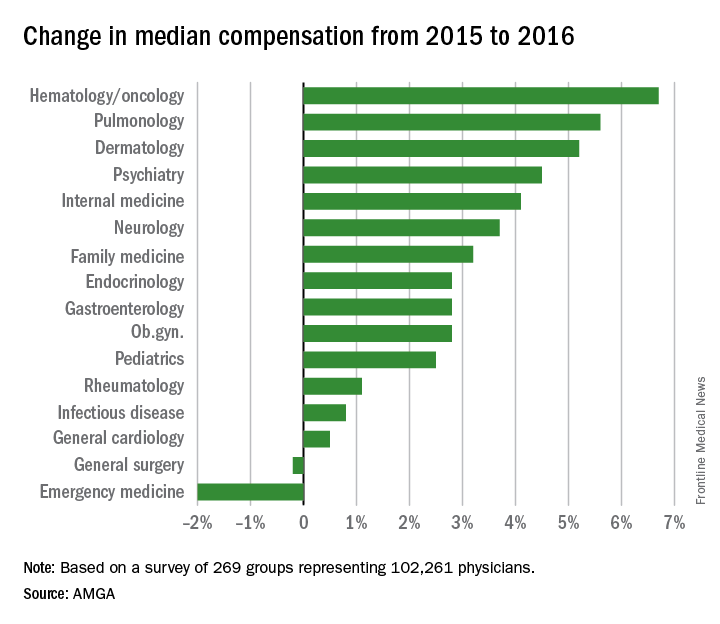
AMGA said that the data covers responses from 269 medical groups covering more than 102,000 providers and is representative of large multispecialty groups and integrated health systems that average 380 providers per group.
Physicians working in large multispecialty groups saw their compensation increase in 2016, albeit at a slower pace than in 2015, according to survey results reported by AMGA.
The 2017 Medical Group Compensation and Productivity Survey shows that the overall weighted average increase in physician compensation for the calendar year 2016 was 2.9%, slightly lower than the 3.1% increase seen in 2015. Doctors in more than three-quarters (77%) of specialties saw increases in 2016.
Opthalmologic surgery saw the largest compensation increase at 7.7%, followed by cardiothoracic surgery (7.0%), hematology and medical oncology (6.7%), allergy/immunology (5.9%) and pulmonary disease (5.6%). Emergency medicine saw a decrease in compensation of 2.0% in 2016 after experiencing a 9.6% increase in 2015.
Value-based payment is beginning to factor into the growth in payment by specialty. Overall, about 8% of compensation is being linked to value-based pay, and that number is expected to rise, with some medical practice groups linking 15% or more of compensation to value-based metrics.
“In almost all of the groups that I have worked with in the last few years on [compensation] design, that has been one of the drivers of decision to do the compensation redesign is to allocate to the value-based metrics,” Wayne Hartley, vice president of AMGA Consulting, said in an interview. 
AMGA said that the data covers responses from 269 medical groups covering more than 102,000 providers and is representative of large multispecialty groups and integrated health systems that average 380 providers per group.
Physicians working in large multispecialty groups saw their compensation increase in 2016, albeit at a slower pace than in 2015, according to survey results reported by AMGA.
The 2017 Medical Group Compensation and Productivity Survey shows that the overall weighted average increase in physician compensation for the calendar year 2016 was 2.9%, slightly lower than the 3.1% increase seen in 2015. Doctors in more than three-quarters (77%) of specialties saw increases in 2016.
Opthalmologic surgery saw the largest compensation increase at 7.7%, followed by cardiothoracic surgery (7.0%), hematology and medical oncology (6.7%), allergy/immunology (5.9%) and pulmonary disease (5.6%). Emergency medicine saw a decrease in compensation of 2.0% in 2016 after experiencing a 9.6% increase in 2015.
Value-based payment is beginning to factor into the growth in payment by specialty. Overall, about 8% of compensation is being linked to value-based pay, and that number is expected to rise, with some medical practice groups linking 15% or more of compensation to value-based metrics.
“In almost all of the groups that I have worked with in the last few years on [compensation] design, that has been one of the drivers of decision to do the compensation redesign is to allocate to the value-based metrics,” Wayne Hartley, vice president of AMGA Consulting, said in an interview. 
AMGA said that the data covers responses from 269 medical groups covering more than 102,000 providers and is representative of large multispecialty groups and integrated health systems that average 380 providers per group.
HHS eliminates funding for teen pregnancy prevention programs
The Trump administration’s sudden funding cut to the Teen Pregnancy Prevention Program will not only halt research and programming efforts at more than 80 institutions across the country, but also will likely unravel recent progress made in reducing teen pregnancies, physicians and program advocates say.
In early July, officials at the U.S. Department of Health & Human Services notified program grantees that the administration would be eliminating funding for the Teen Pregnancy Prevention Program (TPP Program) and that 5-year grants awarded under the Obama administration would be ending in June 2018, 2 years earlier than planned.
The TPP Program is a national, evidence-based initiative established in 2010 to fund medically accurate and age-appropriate programs that work to prevent teen pregnancy in the United States.
“Those programs serve many youth across the country, so of course the individuals in the programs are going to be impacted,” Dr. Oelschlager said in an interview. “It’s demoralizing for the educators, [and] the researchers, who are looking at innovative methods, to have their funding cut in the middle of evaluating whether their programs are effective.”
An HHS spokeswoman confirmed that on July 1, the agency informed TPP Program grantees that funding for the program would be eliminated as detailed in President Trump’s fiscal 2018 budget proposal. The HHS awarded 81 continuations for TPP Program Tier 1 and Tier 2 grant awards for a total of $89 million through June 30, 2018, according to the spokeswoman. The HHS informed the grantees of their June 30, 2018, end date “to give them an opportunity to adjust their programs and plan for an orderly closeout,” she said.
The University of Southern California, Los Angeles, is one of many institutions impacted by the funding cut. The primary objectives of the Keeping it Real Together project are to implement evidence-based sexual health education programs for youth in middle and alternative high schools and provide an education program for parents of middle school–aged youth, said Luanne Rohrbach, PhD, associate professor of clinical preventive medicine at the university’s Institute for Health Promotion & Disease Prevention and principal investigator for the program.
Dr. Rohrbach said she anticipated pushback from the new administration as far as receiving the full 5-year funding, but the sudden program elimination was unexpected.
In March, when President Trump announced his budget priorities for fiscal 2016 and 2017, it was clear that the TPP Program was on the chopping block, Dr. Rohrbach said. However, when Congress passed a continuing resolution for the remainder of fiscal 2016 and 2017, the program remained intact. In his budget recommendations for fiscal 2017 and 2018, the President proposed that the TPP Program be eliminated.
“Despite this recommendation, it was our understanding that the program would be debated in Congress as they developed their recommendations for FY 2017-18 funding,” she said. “That is, we expected there would at least be discussion about it in Congressional budget committees. We did not expect that the program would be eliminated by the Office of the Secretary of HHS.”
Haywood L. Brown, MD, president of the American Congress of Obstetricians and Gynecologists (ACOG) called the administration’s decision “highly unusual” and said it is a step backward for ensuring healthy moms and healthy babies.
“This program and others provide vital research and programming that successfully brought our nation to an all-time low rate of teen pregnancies – progress we cannot afford to jeopardize,” Dr. Brown said in a statement.
Federal data show that the teen pregnancy rate has steadily declined over the last decade. In 2015, the birth rate for girls and young women aged 15-19 years fell by nearly 8% from 2014, according to data from the National Center for Health Statistics. Since 1991, the rate has fallen by 64%. The rate for the younger half of the age group, girls aged 15-17 years, was down 9% from 2014.
Researchers attribute the decline to a combination of economic, cultural, and social factors, as well as a lower prevalence of sexual activity among youth, the use of more effective contraception, and the provision of more information about pregnancy prevention, Dr. Rohrbach said.
“The [TPP Program] has been focused on implementation of evidence-based, comprehensive sexual health education,” she said. “Elimination of these efforts endangers the progress that has been made in reducing teen pregnancies.”
Dr. Oelschlager noted that between 2008 and 2015, the teen birth rate in King County, Washington, declined by 55%. She attributed the reduction to multiple drivers, including a program called Flash, that incorporates a sexual health education curriculum in Seattle-area public schools. Some TPP programs in Northwest Seattle are using the FLASH curriculum, she said.
“This is a very comprehensive program, which includes discussion about reproductive health, sexual violence, prevention of teen pregnancy, sexually transmitted infections, [and] harm reduction,” she said. “That’s been a very effective method of educating our teens about these issues.”
[email protected]
On Twitter @legal_med
The Trump administration’s sudden funding cut to the Teen Pregnancy Prevention Program will not only halt research and programming efforts at more than 80 institutions across the country, but also will likely unravel recent progress made in reducing teen pregnancies, physicians and program advocates say.
In early July, officials at the U.S. Department of Health & Human Services notified program grantees that the administration would be eliminating funding for the Teen Pregnancy Prevention Program (TPP Program) and that 5-year grants awarded under the Obama administration would be ending in June 2018, 2 years earlier than planned.
The TPP Program is a national, evidence-based initiative established in 2010 to fund medically accurate and age-appropriate programs that work to prevent teen pregnancy in the United States.
“Those programs serve many youth across the country, so of course the individuals in the programs are going to be impacted,” Dr. Oelschlager said in an interview. “It’s demoralizing for the educators, [and] the researchers, who are looking at innovative methods, to have their funding cut in the middle of evaluating whether their programs are effective.”
An HHS spokeswoman confirmed that on July 1, the agency informed TPP Program grantees that funding for the program would be eliminated as detailed in President Trump’s fiscal 2018 budget proposal. The HHS awarded 81 continuations for TPP Program Tier 1 and Tier 2 grant awards for a total of $89 million through June 30, 2018, according to the spokeswoman. The HHS informed the grantees of their June 30, 2018, end date “to give them an opportunity to adjust their programs and plan for an orderly closeout,” she said.
The University of Southern California, Los Angeles, is one of many institutions impacted by the funding cut. The primary objectives of the Keeping it Real Together project are to implement evidence-based sexual health education programs for youth in middle and alternative high schools and provide an education program for parents of middle school–aged youth, said Luanne Rohrbach, PhD, associate professor of clinical preventive medicine at the university’s Institute for Health Promotion & Disease Prevention and principal investigator for the program.
Dr. Rohrbach said she anticipated pushback from the new administration as far as receiving the full 5-year funding, but the sudden program elimination was unexpected.
In March, when President Trump announced his budget priorities for fiscal 2016 and 2017, it was clear that the TPP Program was on the chopping block, Dr. Rohrbach said. However, when Congress passed a continuing resolution for the remainder of fiscal 2016 and 2017, the program remained intact. In his budget recommendations for fiscal 2017 and 2018, the President proposed that the TPP Program be eliminated.
“Despite this recommendation, it was our understanding that the program would be debated in Congress as they developed their recommendations for FY 2017-18 funding,” she said. “That is, we expected there would at least be discussion about it in Congressional budget committees. We did not expect that the program would be eliminated by the Office of the Secretary of HHS.”
Haywood L. Brown, MD, president of the American Congress of Obstetricians and Gynecologists (ACOG) called the administration’s decision “highly unusual” and said it is a step backward for ensuring healthy moms and healthy babies.
“This program and others provide vital research and programming that successfully brought our nation to an all-time low rate of teen pregnancies – progress we cannot afford to jeopardize,” Dr. Brown said in a statement.
Federal data show that the teen pregnancy rate has steadily declined over the last decade. In 2015, the birth rate for girls and young women aged 15-19 years fell by nearly 8% from 2014, according to data from the National Center for Health Statistics. Since 1991, the rate has fallen by 64%. The rate for the younger half of the age group, girls aged 15-17 years, was down 9% from 2014.
Researchers attribute the decline to a combination of economic, cultural, and social factors, as well as a lower prevalence of sexual activity among youth, the use of more effective contraception, and the provision of more information about pregnancy prevention, Dr. Rohrbach said.
“The [TPP Program] has been focused on implementation of evidence-based, comprehensive sexual health education,” she said. “Elimination of these efforts endangers the progress that has been made in reducing teen pregnancies.”
Dr. Oelschlager noted that between 2008 and 2015, the teen birth rate in King County, Washington, declined by 55%. She attributed the reduction to multiple drivers, including a program called Flash, that incorporates a sexual health education curriculum in Seattle-area public schools. Some TPP programs in Northwest Seattle are using the FLASH curriculum, she said.
“This is a very comprehensive program, which includes discussion about reproductive health, sexual violence, prevention of teen pregnancy, sexually transmitted infections, [and] harm reduction,” she said. “That’s been a very effective method of educating our teens about these issues.”
[email protected]
On Twitter @legal_med
The Trump administration’s sudden funding cut to the Teen Pregnancy Prevention Program will not only halt research and programming efforts at more than 80 institutions across the country, but also will likely unravel recent progress made in reducing teen pregnancies, physicians and program advocates say.
In early July, officials at the U.S. Department of Health & Human Services notified program grantees that the administration would be eliminating funding for the Teen Pregnancy Prevention Program (TPP Program) and that 5-year grants awarded under the Obama administration would be ending in June 2018, 2 years earlier than planned.
The TPP Program is a national, evidence-based initiative established in 2010 to fund medically accurate and age-appropriate programs that work to prevent teen pregnancy in the United States.
“Those programs serve many youth across the country, so of course the individuals in the programs are going to be impacted,” Dr. Oelschlager said in an interview. “It’s demoralizing for the educators, [and] the researchers, who are looking at innovative methods, to have their funding cut in the middle of evaluating whether their programs are effective.”
An HHS spokeswoman confirmed that on July 1, the agency informed TPP Program grantees that funding for the program would be eliminated as detailed in President Trump’s fiscal 2018 budget proposal. The HHS awarded 81 continuations for TPP Program Tier 1 and Tier 2 grant awards for a total of $89 million through June 30, 2018, according to the spokeswoman. The HHS informed the grantees of their June 30, 2018, end date “to give them an opportunity to adjust their programs and plan for an orderly closeout,” she said.
The University of Southern California, Los Angeles, is one of many institutions impacted by the funding cut. The primary objectives of the Keeping it Real Together project are to implement evidence-based sexual health education programs for youth in middle and alternative high schools and provide an education program for parents of middle school–aged youth, said Luanne Rohrbach, PhD, associate professor of clinical preventive medicine at the university’s Institute for Health Promotion & Disease Prevention and principal investigator for the program.
Dr. Rohrbach said she anticipated pushback from the new administration as far as receiving the full 5-year funding, but the sudden program elimination was unexpected.
In March, when President Trump announced his budget priorities for fiscal 2016 and 2017, it was clear that the TPP Program was on the chopping block, Dr. Rohrbach said. However, when Congress passed a continuing resolution for the remainder of fiscal 2016 and 2017, the program remained intact. In his budget recommendations for fiscal 2017 and 2018, the President proposed that the TPP Program be eliminated.
“Despite this recommendation, it was our understanding that the program would be debated in Congress as they developed their recommendations for FY 2017-18 funding,” she said. “That is, we expected there would at least be discussion about it in Congressional budget committees. We did not expect that the program would be eliminated by the Office of the Secretary of HHS.”
Haywood L. Brown, MD, president of the American Congress of Obstetricians and Gynecologists (ACOG) called the administration’s decision “highly unusual” and said it is a step backward for ensuring healthy moms and healthy babies.
“This program and others provide vital research and programming that successfully brought our nation to an all-time low rate of teen pregnancies – progress we cannot afford to jeopardize,” Dr. Brown said in a statement.
Federal data show that the teen pregnancy rate has steadily declined over the last decade. In 2015, the birth rate for girls and young women aged 15-19 years fell by nearly 8% from 2014, according to data from the National Center for Health Statistics. Since 1991, the rate has fallen by 64%. The rate for the younger half of the age group, girls aged 15-17 years, was down 9% from 2014.
Researchers attribute the decline to a combination of economic, cultural, and social factors, as well as a lower prevalence of sexual activity among youth, the use of more effective contraception, and the provision of more information about pregnancy prevention, Dr. Rohrbach said.
“The [TPP Program] has been focused on implementation of evidence-based, comprehensive sexual health education,” she said. “Elimination of these efforts endangers the progress that has been made in reducing teen pregnancies.”
Dr. Oelschlager noted that between 2008 and 2015, the teen birth rate in King County, Washington, declined by 55%. She attributed the reduction to multiple drivers, including a program called Flash, that incorporates a sexual health education curriculum in Seattle-area public schools. Some TPP programs in Northwest Seattle are using the FLASH curriculum, she said.
“This is a very comprehensive program, which includes discussion about reproductive health, sexual violence, prevention of teen pregnancy, sexually transmitted infections, [and] harm reduction,” she said. “That’s been a very effective method of educating our teens about these issues.”
[email protected]
On Twitter @legal_med











