User login
Hepatitis B elimination: Is it possible?
Despite the availability of safe and effective vaccines for more than three decades, the 2017 World Health Organization (WHO) Global Hepatitis Report estimated that worldwide more than 250 million persons are chronically infected with hepatitis B virus (www.who.int/hepatitis/publications/global-hepatitis-report2017/). In the United States, as many as 2.2 million persons may be chronically infected but only one-third are aware of their infection. In 2015, the WHO declared that hepatitis B and C should be eliminated as public health problems by the year 2030. In March 2017, the National Academies of Science, Engineering and Medicine (NASEM) set targets for HBV elimination in the United States by 2030 as follows: 50% reduction in deaths, 45% reduction in cirrhosis, and 33% reduction in hepatocellular carcinoma (HCC) compared to 2015. For these targets, 90% of persons chronically infected need to be diagnosed, 90% of those diagnosed linked to care, and treatment initiated in 80% of those with treatment indications. In addition, new infections among children should be eliminated through complete prevention of mother-to-child transmission.
For persons who are chronically infected, antiviral therapy can suppress HBV replication, reduce hepatic inflammation, reverse hepatic fibrosis, and prevent progression to cirrhosis, hepatic decompensation, and HCC. However, currently approved treatments are associated with low rates of hepatitis B surface antigen (HBsAg) clearance and decreased but continued risk of HCC. New treatments aimed at cure are desired but complete cure of HBV may not be feasible as HBV persists in the liver even in patients with serologic recovery after transient acute HBV infection.
Functional cure aimed at restoring chronic hepatitis B patients to a state akin to those with spontaneous HBsAg clearance might be a more realistic goal. With improved understanding of the biology of HBV, including recent identification of its entry receptor, better in vitro and animal models, and revival of interest in hepatitis B research, it is conceivable that combinations of antiviral targeting different steps in HBV life cyle and immunomodulatory therapies aimed to boost T-cell response to HBV and/or remove inhibitory signals can result in functional cure (HBsAg clearance) in a high percentage of patients after a finite course of treatment (Hepatology 2017; in press).
The HBV elimination goals set by WHO and NASEM are lofty, but as both organizations stated, these goals are feasible if all stakeholders make elimination of HBV a priority and allocate resources to make it happen.
Dr. Lok is the Alice Lohrman Andrews Research Professor in Hepatology in the department of internal medicine, University of Michigan Health System in Ann Arbor. Her comments were made during the AGA Institute Presidential Plenary at the Annual Digestive Disease Week.
Despite the availability of safe and effective vaccines for more than three decades, the 2017 World Health Organization (WHO) Global Hepatitis Report estimated that worldwide more than 250 million persons are chronically infected with hepatitis B virus (www.who.int/hepatitis/publications/global-hepatitis-report2017/). In the United States, as many as 2.2 million persons may be chronically infected but only one-third are aware of their infection. In 2015, the WHO declared that hepatitis B and C should be eliminated as public health problems by the year 2030. In March 2017, the National Academies of Science, Engineering and Medicine (NASEM) set targets for HBV elimination in the United States by 2030 as follows: 50% reduction in deaths, 45% reduction in cirrhosis, and 33% reduction in hepatocellular carcinoma (HCC) compared to 2015. For these targets, 90% of persons chronically infected need to be diagnosed, 90% of those diagnosed linked to care, and treatment initiated in 80% of those with treatment indications. In addition, new infections among children should be eliminated through complete prevention of mother-to-child transmission.
For persons who are chronically infected, antiviral therapy can suppress HBV replication, reduce hepatic inflammation, reverse hepatic fibrosis, and prevent progression to cirrhosis, hepatic decompensation, and HCC. However, currently approved treatments are associated with low rates of hepatitis B surface antigen (HBsAg) clearance and decreased but continued risk of HCC. New treatments aimed at cure are desired but complete cure of HBV may not be feasible as HBV persists in the liver even in patients with serologic recovery after transient acute HBV infection.
Functional cure aimed at restoring chronic hepatitis B patients to a state akin to those with spontaneous HBsAg clearance might be a more realistic goal. With improved understanding of the biology of HBV, including recent identification of its entry receptor, better in vitro and animal models, and revival of interest in hepatitis B research, it is conceivable that combinations of antiviral targeting different steps in HBV life cyle and immunomodulatory therapies aimed to boost T-cell response to HBV and/or remove inhibitory signals can result in functional cure (HBsAg clearance) in a high percentage of patients after a finite course of treatment (Hepatology 2017; in press).
The HBV elimination goals set by WHO and NASEM are lofty, but as both organizations stated, these goals are feasible if all stakeholders make elimination of HBV a priority and allocate resources to make it happen.
Dr. Lok is the Alice Lohrman Andrews Research Professor in Hepatology in the department of internal medicine, University of Michigan Health System in Ann Arbor. Her comments were made during the AGA Institute Presidential Plenary at the Annual Digestive Disease Week.
Despite the availability of safe and effective vaccines for more than three decades, the 2017 World Health Organization (WHO) Global Hepatitis Report estimated that worldwide more than 250 million persons are chronically infected with hepatitis B virus (www.who.int/hepatitis/publications/global-hepatitis-report2017/). In the United States, as many as 2.2 million persons may be chronically infected but only one-third are aware of their infection. In 2015, the WHO declared that hepatitis B and C should be eliminated as public health problems by the year 2030. In March 2017, the National Academies of Science, Engineering and Medicine (NASEM) set targets for HBV elimination in the United States by 2030 as follows: 50% reduction in deaths, 45% reduction in cirrhosis, and 33% reduction in hepatocellular carcinoma (HCC) compared to 2015. For these targets, 90% of persons chronically infected need to be diagnosed, 90% of those diagnosed linked to care, and treatment initiated in 80% of those with treatment indications. In addition, new infections among children should be eliminated through complete prevention of mother-to-child transmission.
For persons who are chronically infected, antiviral therapy can suppress HBV replication, reduce hepatic inflammation, reverse hepatic fibrosis, and prevent progression to cirrhosis, hepatic decompensation, and HCC. However, currently approved treatments are associated with low rates of hepatitis B surface antigen (HBsAg) clearance and decreased but continued risk of HCC. New treatments aimed at cure are desired but complete cure of HBV may not be feasible as HBV persists in the liver even in patients with serologic recovery after transient acute HBV infection.
Functional cure aimed at restoring chronic hepatitis B patients to a state akin to those with spontaneous HBsAg clearance might be a more realistic goal. With improved understanding of the biology of HBV, including recent identification of its entry receptor, better in vitro and animal models, and revival of interest in hepatitis B research, it is conceivable that combinations of antiviral targeting different steps in HBV life cyle and immunomodulatory therapies aimed to boost T-cell response to HBV and/or remove inhibitory signals can result in functional cure (HBsAg clearance) in a high percentage of patients after a finite course of treatment (Hepatology 2017; in press).
The HBV elimination goals set by WHO and NASEM are lofty, but as both organizations stated, these goals are feasible if all stakeholders make elimination of HBV a priority and allocate resources to make it happen.
Dr. Lok is the Alice Lohrman Andrews Research Professor in Hepatology in the department of internal medicine, University of Michigan Health System in Ann Arbor. Her comments were made during the AGA Institute Presidential Plenary at the Annual Digestive Disease Week.
Lupus classification criteria effort is going in the wrong direction
Editor’s note: This commentary relates to the story, “New classification system for systemic lupus erythematosus moves forward.”
While work to develop a new set of lupus classification criteria more suitable for clinical research is important, the Lupus Foundation of America believes the current work is going in the wrong direction.
Increasingly, key opinion leaders understand lupus to be a spectrum of disease, and there is ample justification of this from scientific evidence (Nat Rev Rheumatol. 2015;11[7]:385-6) The criteria being worked on by ACR and EULAR draws upon an archaic concept with the musty name of “systemic lupus erythematosus,” which relies on a 19th century approach to categorizing disease by counting the signs and symptoms instead of by modern concepts of pathophysiology and prognostic severity. This imposes a homogeneity on the population that simply does not exist. Attempts to re-order obsolescent arrays of organs and autoantibodies to classify lupus will be futile, especially if the aim is to improve the rationale for clinical trial recruitment. Recent clinical trials and modern immunologic methods have already demonstrated, beyond a doubt, that subsets of patients, definable by gene expression patterns or state-of-the-art pharmacodynamic responses, do or do not respond to individual targeted treatments. We now know that patients who require different treatments may well share many of their symptoms in many of the same organs, and this fact defies the outmoded, abacus-based approach to disease classification.
We are concerned that redefining SLE by weighting all disease in one organ as more or less impactful than all disease in another organ flies in the face of current scientific knowledge. If this is the direction in which the effort is going, there is the potential for a negative impact on drug development, clinical care, and access to treatment. The term itself, “SLE,” interferes with selecting appropriate lupus patients for participation in trials. Many people have moderate and even severe lupus syndromes who do not meet enough criteria to be labeled “SLE” (for example, immune thrombocytopenia, hemolytic anemia, discoid lupus, or subacute cutaneous lupus, which can cause severe rashes covering wide areas of the body). In this iteration, assigning less weight to cutaneous lupus as currently proposed is not just problematic; it will set the field back.
By not viewing cutaneous lupus as part of the lupus spectrum, we develop a false sense that this subset of people with lupus will not progress to “SLE,” yet many of them do. Even those who do not later develop features in other organs besides the skin may have more severe disease than other patients who do. Minimizing the “score” for cutaneous lupus will lead to individuals who carry a significant burden of disease being barred from trials, and from access to the treatments they need, once approved.
Conversely, people who do meet criteria for “SLE” under any algorithm, past or present, may have a range of severity, from very severe to very mild. The very mild patients (who may have, in their lifetime, met the multiorgan criteria) are entering trials in large numbers and contributing to the high placebo responses which have stopped many promising investigational treatments from further development. Additionally, the common misuse of the current classification criteria as diagnostic criteria has become an unacceptable norm in the lupus community. This leads us to believe that new criteria will continue to be misused, further disenfranchising a huge segment of the population who have lupus from access to state-of-the-art research and care.
Advancing the development of new classification criteria deserves a wider discussion among the field’s stakeholders, particularly those with expertise in clinical trial outcomes and the clinical care of the full spectrum of lupus patients.
Sandra C. Raymond is CEO and President, Leslie M. Hanrahan is VP of Education and Research, and Joan Merrill, MD, is the Chief Adviser for Clinical Development at the Lupus Foundation of America. Dr. Merrill is also the Oklahoma Medical Research Foundation Professor of Medicine at the University of Oklahoma, Oklahoma City.
Editor’s note: This commentary relates to the story, “New classification system for systemic lupus erythematosus moves forward.”
While work to develop a new set of lupus classification criteria more suitable for clinical research is important, the Lupus Foundation of America believes the current work is going in the wrong direction.
Increasingly, key opinion leaders understand lupus to be a spectrum of disease, and there is ample justification of this from scientific evidence (Nat Rev Rheumatol. 2015;11[7]:385-6) The criteria being worked on by ACR and EULAR draws upon an archaic concept with the musty name of “systemic lupus erythematosus,” which relies on a 19th century approach to categorizing disease by counting the signs and symptoms instead of by modern concepts of pathophysiology and prognostic severity. This imposes a homogeneity on the population that simply does not exist. Attempts to re-order obsolescent arrays of organs and autoantibodies to classify lupus will be futile, especially if the aim is to improve the rationale for clinical trial recruitment. Recent clinical trials and modern immunologic methods have already demonstrated, beyond a doubt, that subsets of patients, definable by gene expression patterns or state-of-the-art pharmacodynamic responses, do or do not respond to individual targeted treatments. We now know that patients who require different treatments may well share many of their symptoms in many of the same organs, and this fact defies the outmoded, abacus-based approach to disease classification.
We are concerned that redefining SLE by weighting all disease in one organ as more or less impactful than all disease in another organ flies in the face of current scientific knowledge. If this is the direction in which the effort is going, there is the potential for a negative impact on drug development, clinical care, and access to treatment. The term itself, “SLE,” interferes with selecting appropriate lupus patients for participation in trials. Many people have moderate and even severe lupus syndromes who do not meet enough criteria to be labeled “SLE” (for example, immune thrombocytopenia, hemolytic anemia, discoid lupus, or subacute cutaneous lupus, which can cause severe rashes covering wide areas of the body). In this iteration, assigning less weight to cutaneous lupus as currently proposed is not just problematic; it will set the field back.
By not viewing cutaneous lupus as part of the lupus spectrum, we develop a false sense that this subset of people with lupus will not progress to “SLE,” yet many of them do. Even those who do not later develop features in other organs besides the skin may have more severe disease than other patients who do. Minimizing the “score” for cutaneous lupus will lead to individuals who carry a significant burden of disease being barred from trials, and from access to the treatments they need, once approved.
Conversely, people who do meet criteria for “SLE” under any algorithm, past or present, may have a range of severity, from very severe to very mild. The very mild patients (who may have, in their lifetime, met the multiorgan criteria) are entering trials in large numbers and contributing to the high placebo responses which have stopped many promising investigational treatments from further development. Additionally, the common misuse of the current classification criteria as diagnostic criteria has become an unacceptable norm in the lupus community. This leads us to believe that new criteria will continue to be misused, further disenfranchising a huge segment of the population who have lupus from access to state-of-the-art research and care.
Advancing the development of new classification criteria deserves a wider discussion among the field’s stakeholders, particularly those with expertise in clinical trial outcomes and the clinical care of the full spectrum of lupus patients.
Sandra C. Raymond is CEO and President, Leslie M. Hanrahan is VP of Education and Research, and Joan Merrill, MD, is the Chief Adviser for Clinical Development at the Lupus Foundation of America. Dr. Merrill is also the Oklahoma Medical Research Foundation Professor of Medicine at the University of Oklahoma, Oklahoma City.
Editor’s note: This commentary relates to the story, “New classification system for systemic lupus erythematosus moves forward.”
While work to develop a new set of lupus classification criteria more suitable for clinical research is important, the Lupus Foundation of America believes the current work is going in the wrong direction.
Increasingly, key opinion leaders understand lupus to be a spectrum of disease, and there is ample justification of this from scientific evidence (Nat Rev Rheumatol. 2015;11[7]:385-6) The criteria being worked on by ACR and EULAR draws upon an archaic concept with the musty name of “systemic lupus erythematosus,” which relies on a 19th century approach to categorizing disease by counting the signs and symptoms instead of by modern concepts of pathophysiology and prognostic severity. This imposes a homogeneity on the population that simply does not exist. Attempts to re-order obsolescent arrays of organs and autoantibodies to classify lupus will be futile, especially if the aim is to improve the rationale for clinical trial recruitment. Recent clinical trials and modern immunologic methods have already demonstrated, beyond a doubt, that subsets of patients, definable by gene expression patterns or state-of-the-art pharmacodynamic responses, do or do not respond to individual targeted treatments. We now know that patients who require different treatments may well share many of their symptoms in many of the same organs, and this fact defies the outmoded, abacus-based approach to disease classification.
We are concerned that redefining SLE by weighting all disease in one organ as more or less impactful than all disease in another organ flies in the face of current scientific knowledge. If this is the direction in which the effort is going, there is the potential for a negative impact on drug development, clinical care, and access to treatment. The term itself, “SLE,” interferes with selecting appropriate lupus patients for participation in trials. Many people have moderate and even severe lupus syndromes who do not meet enough criteria to be labeled “SLE” (for example, immune thrombocytopenia, hemolytic anemia, discoid lupus, or subacute cutaneous lupus, which can cause severe rashes covering wide areas of the body). In this iteration, assigning less weight to cutaneous lupus as currently proposed is not just problematic; it will set the field back.
By not viewing cutaneous lupus as part of the lupus spectrum, we develop a false sense that this subset of people with lupus will not progress to “SLE,” yet many of them do. Even those who do not later develop features in other organs besides the skin may have more severe disease than other patients who do. Minimizing the “score” for cutaneous lupus will lead to individuals who carry a significant burden of disease being barred from trials, and from access to the treatments they need, once approved.
Conversely, people who do meet criteria for “SLE” under any algorithm, past or present, may have a range of severity, from very severe to very mild. The very mild patients (who may have, in their lifetime, met the multiorgan criteria) are entering trials in large numbers and contributing to the high placebo responses which have stopped many promising investigational treatments from further development. Additionally, the common misuse of the current classification criteria as diagnostic criteria has become an unacceptable norm in the lupus community. This leads us to believe that new criteria will continue to be misused, further disenfranchising a huge segment of the population who have lupus from access to state-of-the-art research and care.
Advancing the development of new classification criteria deserves a wider discussion among the field’s stakeholders, particularly those with expertise in clinical trial outcomes and the clinical care of the full spectrum of lupus patients.
Sandra C. Raymond is CEO and President, Leslie M. Hanrahan is VP of Education and Research, and Joan Merrill, MD, is the Chief Adviser for Clinical Development at the Lupus Foundation of America. Dr. Merrill is also the Oklahoma Medical Research Foundation Professor of Medicine at the University of Oklahoma, Oklahoma City.
Multidisciplinary bundle drives drop in colorectal SSIs
NEW YORK – Facing an “unacceptably high” rate of surgical site infections associated with colorectal surgery at their community hospital, surgeons searched for solutions. They created a perioperative bundle of interventions that ultimately dropped their infection rates enough to achieve the highest ranking in the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP).
“The Centers for Disease Control and Prevention recommends we use a robust surveillance program to monitor surgical site infection data. The system gives us feedback, and that will [help us] reduce surgical site infection (SSI) risk,” said Christopher Wolff, MD, a PGY4 resident at the Cleveland Clinic Akron General Hospital. “NSQIP and the National Healthcare Safety Network from the CDC are two programs that do just that.”
The effort paid off, with the number of SSIs going from 16 cases in 2013 to 10 cases in 2014 and then 5 cases in 2015. Since the bundle was implemented in the last quarter of 2014, “we’ve seen a consistent downtrend since that point in our total infections, and we kept that in the background of a consistent number of cases.
“We have good outcomes by incidence, but that is not the whole story,” Dr. Wolff said. “With respect to colorectal infections, we are now performing in the ‘exemplary’ category, compared with our peers” according to the ACS NSQIP data. In addition, “we are performing at or below the SIR [standardized infection ratio] or expected number consistently since the implementation.”
The bundle addresses actions in five domains: preoperative, anesthesia, operating room, post–anesthesia care unit, and postoperative floor interventions. Preoperative elements include patient education, use of chlorhexidine wipes before surgery, and antibiotics noted on the chart, for example. Additional features include prewarming preoperatively and maintaining normothermia, requiring all surgeons scrub traditionally instead of “foaming,” use of wound protectors in the OR, and close monitoring of blood glucose in diabetics postoperatively. “There also is education of floor nurses on how to take care of these patients specifically,” Dr. Wolff noted.
To identify these areas for improvement, Dr. Wolff and his colleagues initially reviewed the literature to find individual and bundle elements demonstrated to improve outcomes. Then, a surgeon group “think tank” discussed the possibilities. However, reaching agreement was not easy, Dr. Wolff said. “They had a hard time agreeing on best practices, even within our own specialty. We did finally come to a consensus.
“We took those bundled protocols through to other areas and said ‘here are the things we want you to work on, things we want you to improve.’ That did not necessarily go over so well,” Dr. Wolff said. Because of resistance from their colleagues, they changed strategies. “We brought other people to the table and changed our work groups from being surgeons only to [being] a multidisciplinary team.”
The process took months and months of deliberation. It’s important to have a champion behind the project, said Dr. Wolff. “I have to thank my chairman, Mark C. Horattas, MD, FACS, who had the vision to see this through.
“We implemented tried-and-true measures to reduce surgical site infections. We did so in a team manner and had multidisciplinary buy-in, and that created a culture change in our program over time,” Dr. Wolff said.
This study also shows, Dr. Wolff added, that “a successful multidisciplinary quality improvement program can be implemented in a community hospital setting.”
Going forward, continuous monitoring will identify any areas that need improvement over time. The preoperative bundle also will be integrated into an Enhanced Recovery After Surgery protocol.
The Akron Hospital is now ranked by ACS NSQIP in the top 10% of hospitals for their colorectal SSI rate. “It’s nice to meet someone in the first decile,” session moderator Timothy D. Jackson, MD, FACS, of the University of Toronto said after Dr. Wolff’s presentation. “I’ve never done that before, and I took notes for what to do at my hospital.”
Dr. Wolff had no relevant financial disclosures.
NEW YORK – Facing an “unacceptably high” rate of surgical site infections associated with colorectal surgery at their community hospital, surgeons searched for solutions. They created a perioperative bundle of interventions that ultimately dropped their infection rates enough to achieve the highest ranking in the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP).
“The Centers for Disease Control and Prevention recommends we use a robust surveillance program to monitor surgical site infection data. The system gives us feedback, and that will [help us] reduce surgical site infection (SSI) risk,” said Christopher Wolff, MD, a PGY4 resident at the Cleveland Clinic Akron General Hospital. “NSQIP and the National Healthcare Safety Network from the CDC are two programs that do just that.”
The effort paid off, with the number of SSIs going from 16 cases in 2013 to 10 cases in 2014 and then 5 cases in 2015. Since the bundle was implemented in the last quarter of 2014, “we’ve seen a consistent downtrend since that point in our total infections, and we kept that in the background of a consistent number of cases.
“We have good outcomes by incidence, but that is not the whole story,” Dr. Wolff said. “With respect to colorectal infections, we are now performing in the ‘exemplary’ category, compared with our peers” according to the ACS NSQIP data. In addition, “we are performing at or below the SIR [standardized infection ratio] or expected number consistently since the implementation.”
The bundle addresses actions in five domains: preoperative, anesthesia, operating room, post–anesthesia care unit, and postoperative floor interventions. Preoperative elements include patient education, use of chlorhexidine wipes before surgery, and antibiotics noted on the chart, for example. Additional features include prewarming preoperatively and maintaining normothermia, requiring all surgeons scrub traditionally instead of “foaming,” use of wound protectors in the OR, and close monitoring of blood glucose in diabetics postoperatively. “There also is education of floor nurses on how to take care of these patients specifically,” Dr. Wolff noted.
To identify these areas for improvement, Dr. Wolff and his colleagues initially reviewed the literature to find individual and bundle elements demonstrated to improve outcomes. Then, a surgeon group “think tank” discussed the possibilities. However, reaching agreement was not easy, Dr. Wolff said. “They had a hard time agreeing on best practices, even within our own specialty. We did finally come to a consensus.
“We took those bundled protocols through to other areas and said ‘here are the things we want you to work on, things we want you to improve.’ That did not necessarily go over so well,” Dr. Wolff said. Because of resistance from their colleagues, they changed strategies. “We brought other people to the table and changed our work groups from being surgeons only to [being] a multidisciplinary team.”
The process took months and months of deliberation. It’s important to have a champion behind the project, said Dr. Wolff. “I have to thank my chairman, Mark C. Horattas, MD, FACS, who had the vision to see this through.
“We implemented tried-and-true measures to reduce surgical site infections. We did so in a team manner and had multidisciplinary buy-in, and that created a culture change in our program over time,” Dr. Wolff said.
This study also shows, Dr. Wolff added, that “a successful multidisciplinary quality improvement program can be implemented in a community hospital setting.”
Going forward, continuous monitoring will identify any areas that need improvement over time. The preoperative bundle also will be integrated into an Enhanced Recovery After Surgery protocol.
The Akron Hospital is now ranked by ACS NSQIP in the top 10% of hospitals for their colorectal SSI rate. “It’s nice to meet someone in the first decile,” session moderator Timothy D. Jackson, MD, FACS, of the University of Toronto said after Dr. Wolff’s presentation. “I’ve never done that before, and I took notes for what to do at my hospital.”
Dr. Wolff had no relevant financial disclosures.
NEW YORK – Facing an “unacceptably high” rate of surgical site infections associated with colorectal surgery at their community hospital, surgeons searched for solutions. They created a perioperative bundle of interventions that ultimately dropped their infection rates enough to achieve the highest ranking in the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP).
“The Centers for Disease Control and Prevention recommends we use a robust surveillance program to monitor surgical site infection data. The system gives us feedback, and that will [help us] reduce surgical site infection (SSI) risk,” said Christopher Wolff, MD, a PGY4 resident at the Cleveland Clinic Akron General Hospital. “NSQIP and the National Healthcare Safety Network from the CDC are two programs that do just that.”
The effort paid off, with the number of SSIs going from 16 cases in 2013 to 10 cases in 2014 and then 5 cases in 2015. Since the bundle was implemented in the last quarter of 2014, “we’ve seen a consistent downtrend since that point in our total infections, and we kept that in the background of a consistent number of cases.
“We have good outcomes by incidence, but that is not the whole story,” Dr. Wolff said. “With respect to colorectal infections, we are now performing in the ‘exemplary’ category, compared with our peers” according to the ACS NSQIP data. In addition, “we are performing at or below the SIR [standardized infection ratio] or expected number consistently since the implementation.”
The bundle addresses actions in five domains: preoperative, anesthesia, operating room, post–anesthesia care unit, and postoperative floor interventions. Preoperative elements include patient education, use of chlorhexidine wipes before surgery, and antibiotics noted on the chart, for example. Additional features include prewarming preoperatively and maintaining normothermia, requiring all surgeons scrub traditionally instead of “foaming,” use of wound protectors in the OR, and close monitoring of blood glucose in diabetics postoperatively. “There also is education of floor nurses on how to take care of these patients specifically,” Dr. Wolff noted.
To identify these areas for improvement, Dr. Wolff and his colleagues initially reviewed the literature to find individual and bundle elements demonstrated to improve outcomes. Then, a surgeon group “think tank” discussed the possibilities. However, reaching agreement was not easy, Dr. Wolff said. “They had a hard time agreeing on best practices, even within our own specialty. We did finally come to a consensus.
“We took those bundled protocols through to other areas and said ‘here are the things we want you to work on, things we want you to improve.’ That did not necessarily go over so well,” Dr. Wolff said. Because of resistance from their colleagues, they changed strategies. “We brought other people to the table and changed our work groups from being surgeons only to [being] a multidisciplinary team.”
The process took months and months of deliberation. It’s important to have a champion behind the project, said Dr. Wolff. “I have to thank my chairman, Mark C. Horattas, MD, FACS, who had the vision to see this through.
“We implemented tried-and-true measures to reduce surgical site infections. We did so in a team manner and had multidisciplinary buy-in, and that created a culture change in our program over time,” Dr. Wolff said.
This study also shows, Dr. Wolff added, that “a successful multidisciplinary quality improvement program can be implemented in a community hospital setting.”
Going forward, continuous monitoring will identify any areas that need improvement over time. The preoperative bundle also will be integrated into an Enhanced Recovery After Surgery protocol.
The Akron Hospital is now ranked by ACS NSQIP in the top 10% of hospitals for their colorectal SSI rate. “It’s nice to meet someone in the first decile,” session moderator Timothy D. Jackson, MD, FACS, of the University of Toronto said after Dr. Wolff’s presentation. “I’ve never done that before, and I took notes for what to do at my hospital.”
Dr. Wolff had no relevant financial disclosures.
AT THE ACS QUALITY AND SAFETY CONFERENCE
Key clinical point: A multidisciplinary team initiative successfully reduced surgical site infections after colorectal surgery in a community hospital.
Major finding: The number of annual SSIs dropped from 16 in the calendar year before the intervention to 5 afterward.
Data source: Comparison of SSI rates before and after a bundled intervention in late 2014.
Disclosures: Dr. Wolff had no relevant financial disclosures.
Lupus classification criteria need input from dermatologists
Editor’s note: This commentary relates to the story, “ New classification system for systemic lupus erythematosus moves forward .”
The ACR/EULAR committee that is developing new classification criteria for systemic lupus erythematosus (SLE) has done the field a service by releasing its draft version in a presentation at the recent EULAR meeting. Releasing the draft version facilitates comments before the new classification criteria become finalized.
Many in the derm-rheum field, ourselves included, classify patients with skin-predominant lupus as lupus, but the new draft classification would place a significant percentage of these patients outside of lupus.
The presentation by Dr. Johnson at EULAR stated, “... a patient can’t be classified on skin findings alone. There is concern that skin findings by themselves may not be lupus, but something else, and some people even consider that cutaneous and systemic lupus are two different things.”
Abundant data indicate instead that lupus is a spectrum that includes skin-predominant lupus. For example, the histology is identical between discoid lupus erythematosus whether or not there is SLE. Moreover, we and others have published significant rates of progression of cutaneous lupus erythematosus (CLE) to SLE. By not viewing CLE in the lupus spectrum, we have a false sense that the patients won’t progress to SLE, yet many of them do.
Importantly, patients respond similarly to therapies when they have either CLE or SLE, so removing this subset of lupus hurts their inclusion in trials and access to new treatments.
When criteria are devised by one group without input from experts who see a specific subset of the disease, that is also a problem. We went down that path in dermatomyositis and missed a lot of patients with the disease when criteria were devised that said the patient had to have muscle involvement. Those criteria have now finally been revised as the ACR/EULAR myositis criteria.
We and others from the derm-rheum community would be happy to speak with the ACR/EULAR committee about these concerns.
Victoria P. Werth, MD, is professor of medicine and dermatology at the University of Pennsylvania, Philadelphia. Joseph F. Merola, MD, is codirector of the Center for Skin and Related Musculoskeletal Diseases at Brigham and Women’s Hospital, Boston. Andrew G. Franks, MD, is a clinical professor in the departments of medicine and dermatology at New York University. Benjamin F. Chong, MD, is an assistant professor of dermatology at the University of Texas, Dallas.
Editor’s note: This commentary relates to the story, “ New classification system for systemic lupus erythematosus moves forward .”
The ACR/EULAR committee that is developing new classification criteria for systemic lupus erythematosus (SLE) has done the field a service by releasing its draft version in a presentation at the recent EULAR meeting. Releasing the draft version facilitates comments before the new classification criteria become finalized.
Many in the derm-rheum field, ourselves included, classify patients with skin-predominant lupus as lupus, but the new draft classification would place a significant percentage of these patients outside of lupus.
The presentation by Dr. Johnson at EULAR stated, “... a patient can’t be classified on skin findings alone. There is concern that skin findings by themselves may not be lupus, but something else, and some people even consider that cutaneous and systemic lupus are two different things.”
Abundant data indicate instead that lupus is a spectrum that includes skin-predominant lupus. For example, the histology is identical between discoid lupus erythematosus whether or not there is SLE. Moreover, we and others have published significant rates of progression of cutaneous lupus erythematosus (CLE) to SLE. By not viewing CLE in the lupus spectrum, we have a false sense that the patients won’t progress to SLE, yet many of them do.
Importantly, patients respond similarly to therapies when they have either CLE or SLE, so removing this subset of lupus hurts their inclusion in trials and access to new treatments.
When criteria are devised by one group without input from experts who see a specific subset of the disease, that is also a problem. We went down that path in dermatomyositis and missed a lot of patients with the disease when criteria were devised that said the patient had to have muscle involvement. Those criteria have now finally been revised as the ACR/EULAR myositis criteria.
We and others from the derm-rheum community would be happy to speak with the ACR/EULAR committee about these concerns.
Victoria P. Werth, MD, is professor of medicine and dermatology at the University of Pennsylvania, Philadelphia. Joseph F. Merola, MD, is codirector of the Center for Skin and Related Musculoskeletal Diseases at Brigham and Women’s Hospital, Boston. Andrew G. Franks, MD, is a clinical professor in the departments of medicine and dermatology at New York University. Benjamin F. Chong, MD, is an assistant professor of dermatology at the University of Texas, Dallas.
Editor’s note: This commentary relates to the story, “ New classification system for systemic lupus erythematosus moves forward .”
The ACR/EULAR committee that is developing new classification criteria for systemic lupus erythematosus (SLE) has done the field a service by releasing its draft version in a presentation at the recent EULAR meeting. Releasing the draft version facilitates comments before the new classification criteria become finalized.
Many in the derm-rheum field, ourselves included, classify patients with skin-predominant lupus as lupus, but the new draft classification would place a significant percentage of these patients outside of lupus.
The presentation by Dr. Johnson at EULAR stated, “... a patient can’t be classified on skin findings alone. There is concern that skin findings by themselves may not be lupus, but something else, and some people even consider that cutaneous and systemic lupus are two different things.”
Abundant data indicate instead that lupus is a spectrum that includes skin-predominant lupus. For example, the histology is identical between discoid lupus erythematosus whether or not there is SLE. Moreover, we and others have published significant rates of progression of cutaneous lupus erythematosus (CLE) to SLE. By not viewing CLE in the lupus spectrum, we have a false sense that the patients won’t progress to SLE, yet many of them do.
Importantly, patients respond similarly to therapies when they have either CLE or SLE, so removing this subset of lupus hurts their inclusion in trials and access to new treatments.
When criteria are devised by one group without input from experts who see a specific subset of the disease, that is also a problem. We went down that path in dermatomyositis and missed a lot of patients with the disease when criteria were devised that said the patient had to have muscle involvement. Those criteria have now finally been revised as the ACR/EULAR myositis criteria.
We and others from the derm-rheum community would be happy to speak with the ACR/EULAR committee about these concerns.
Victoria P. Werth, MD, is professor of medicine and dermatology at the University of Pennsylvania, Philadelphia. Joseph F. Merola, MD, is codirector of the Center for Skin and Related Musculoskeletal Diseases at Brigham and Women’s Hospital, Boston. Andrew G. Franks, MD, is a clinical professor in the departments of medicine and dermatology at New York University. Benjamin F. Chong, MD, is an assistant professor of dermatology at the University of Texas, Dallas.
Clues to drug adulteration may lie skin deep
CHICAGO – Sometimes, the skin can provide the first clues that a patient has been exposed to a drug product that has been adulterated or an over-the-counter product illegally sold in this country that contains a prescription medication, according to pediatric dermatologist Scott Norton, MD.
Speaking at the World Congress of Pediatric Dermatology, he reviewed some of the reactions associated with exposure to counterfeit drugs, contraband drugs, as well as products, misrepresented as drugs that do not include any active pharmaceutical ingredients. The worldwide market for these products is a “hugely profitable industry,” and the scope of the problem should not be underestimated, said Dr. Norton, chief of dermatology at Children’s National Health System, Washington.
It’s particularly important to have a high index of suspicion for such products given an increasingly mobile worldwide population. Today, patients and their family members who travel out of the country – and even local shopkeepers – may bring in these sorts of products from outside the United States, many of which would require a prescription in the United States.
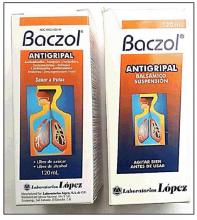
In the United States, there have been several reports of a mysterious fixed drug eruption in patients reported to have taken Baczol, a cold and flu remedy available over the counter in El Salvador for upper respiratory infections. Two of the ingredients listed on the Baczol label are sulfamethoxazole and trimethoprim, two prescription antibiotics. After determining that two Salvadoran American children with a suspected fixed drug eruption had taken a Baczol product, Dr. Norton, with the aid of medical students, was able to find Baczol containing trimethoprim-sulfamethoxazole for sale over the counter in more than one-third of the shops visited in the greater Washington area (MMWR Morb Mortal Wkly Rep. 2013 Nov 22;62[46]:914-6). Eventually, the Food and Drug Administration issued a consumer alert regarding certain Baczol products containing these ingredients, but Dr. Norton said he is still concerned about the possibility for more grave hypersensitivity reactions to these sulfa antibiotics in the Salvadoran product.
Sometimes, said Dr. Norton, the problem lies in the lack of an expected ingredient. He and his team at Children’s National Health System helped solve a medical mystery involving a skin ailment in very premature infants with cholestasis. An interdisciplinary team was convened after the neonatal intensive care unit at the hospital saw its third infant with severe blistering and erosions in an acral, perianal, and perioral pattern that did not respond to empiric treatment for herpes simplex virus and staphylococcal infection – a pattern reminiscent of zinc deficiency dermatitis. Dietitians reported that there was a nationwide shortage of sterile injectable zinc, so total parenteral nutrition was being formulated without zinc. All three of the premature infants were receiving total parenteral nutrition and were so premature that they had insufficient zinc stores. The problem was identified and corrected (MMWR 2014 Jan. 17;63[02];35-7).
A more pervasive issue, which has global significance, pertains to counterfeit vaccines prepared with absolutely no vaccine components, often made in China or Nigeria with high-quality and sophisticated packaging, said Dr. Norton.
Keeping a lid on counterfeit drugs is challenging since there are so many potential entry points into the supply chain, Dr. Norton pointed out. Weak points include mislabeled raw ingredients, packaging, storage, transportation, repackaging, and distribution. The proliferation of online pharmacies also makes regulation more difficult.
There is some international cooperation to detect and combat drug counterfeiting and adulteration: For example, Interpol, the International Coalition of Medicines Regulatory Authorities, the Pharmaceutical Security Institute, and even the United Nations are developing cooperative strategies to combat the problem.
In the meantime, he emphasized that physicians must maintain a high index of suspicion and keep in mind that the first signs of adulterated drugs or prescription drugs available OTC may appear on the skin.
Dr. Norton reported no conflicts of interest.
[email protected]
On Twitter @karioakes
CHICAGO – Sometimes, the skin can provide the first clues that a patient has been exposed to a drug product that has been adulterated or an over-the-counter product illegally sold in this country that contains a prescription medication, according to pediatric dermatologist Scott Norton, MD.
Speaking at the World Congress of Pediatric Dermatology, he reviewed some of the reactions associated with exposure to counterfeit drugs, contraband drugs, as well as products, misrepresented as drugs that do not include any active pharmaceutical ingredients. The worldwide market for these products is a “hugely profitable industry,” and the scope of the problem should not be underestimated, said Dr. Norton, chief of dermatology at Children’s National Health System, Washington.
It’s particularly important to have a high index of suspicion for such products given an increasingly mobile worldwide population. Today, patients and their family members who travel out of the country – and even local shopkeepers – may bring in these sorts of products from outside the United States, many of which would require a prescription in the United States.
In the United States, there have been several reports of a mysterious fixed drug eruption in patients reported to have taken Baczol, a cold and flu remedy available over the counter in El Salvador for upper respiratory infections. Two of the ingredients listed on the Baczol label are sulfamethoxazole and trimethoprim, two prescription antibiotics. After determining that two Salvadoran American children with a suspected fixed drug eruption had taken a Baczol product, Dr. Norton, with the aid of medical students, was able to find Baczol containing trimethoprim-sulfamethoxazole for sale over the counter in more than one-third of the shops visited in the greater Washington area (MMWR Morb Mortal Wkly Rep. 2013 Nov 22;62[46]:914-6). Eventually, the Food and Drug Administration issued a consumer alert regarding certain Baczol products containing these ingredients, but Dr. Norton said he is still concerned about the possibility for more grave hypersensitivity reactions to these sulfa antibiotics in the Salvadoran product.
Sometimes, said Dr. Norton, the problem lies in the lack of an expected ingredient. He and his team at Children’s National Health System helped solve a medical mystery involving a skin ailment in very premature infants with cholestasis. An interdisciplinary team was convened after the neonatal intensive care unit at the hospital saw its third infant with severe blistering and erosions in an acral, perianal, and perioral pattern that did not respond to empiric treatment for herpes simplex virus and staphylococcal infection – a pattern reminiscent of zinc deficiency dermatitis. Dietitians reported that there was a nationwide shortage of sterile injectable zinc, so total parenteral nutrition was being formulated without zinc. All three of the premature infants were receiving total parenteral nutrition and were so premature that they had insufficient zinc stores. The problem was identified and corrected (MMWR 2014 Jan. 17;63[02];35-7).
A more pervasive issue, which has global significance, pertains to counterfeit vaccines prepared with absolutely no vaccine components, often made in China or Nigeria with high-quality and sophisticated packaging, said Dr. Norton.
Keeping a lid on counterfeit drugs is challenging since there are so many potential entry points into the supply chain, Dr. Norton pointed out. Weak points include mislabeled raw ingredients, packaging, storage, transportation, repackaging, and distribution. The proliferation of online pharmacies also makes regulation more difficult.
There is some international cooperation to detect and combat drug counterfeiting and adulteration: For example, Interpol, the International Coalition of Medicines Regulatory Authorities, the Pharmaceutical Security Institute, and even the United Nations are developing cooperative strategies to combat the problem.
In the meantime, he emphasized that physicians must maintain a high index of suspicion and keep in mind that the first signs of adulterated drugs or prescription drugs available OTC may appear on the skin.
Dr. Norton reported no conflicts of interest.
[email protected]
On Twitter @karioakes
CHICAGO – Sometimes, the skin can provide the first clues that a patient has been exposed to a drug product that has been adulterated or an over-the-counter product illegally sold in this country that contains a prescription medication, according to pediatric dermatologist Scott Norton, MD.
Speaking at the World Congress of Pediatric Dermatology, he reviewed some of the reactions associated with exposure to counterfeit drugs, contraband drugs, as well as products, misrepresented as drugs that do not include any active pharmaceutical ingredients. The worldwide market for these products is a “hugely profitable industry,” and the scope of the problem should not be underestimated, said Dr. Norton, chief of dermatology at Children’s National Health System, Washington.
It’s particularly important to have a high index of suspicion for such products given an increasingly mobile worldwide population. Today, patients and their family members who travel out of the country – and even local shopkeepers – may bring in these sorts of products from outside the United States, many of which would require a prescription in the United States.
In the United States, there have been several reports of a mysterious fixed drug eruption in patients reported to have taken Baczol, a cold and flu remedy available over the counter in El Salvador for upper respiratory infections. Two of the ingredients listed on the Baczol label are sulfamethoxazole and trimethoprim, two prescription antibiotics. After determining that two Salvadoran American children with a suspected fixed drug eruption had taken a Baczol product, Dr. Norton, with the aid of medical students, was able to find Baczol containing trimethoprim-sulfamethoxazole for sale over the counter in more than one-third of the shops visited in the greater Washington area (MMWR Morb Mortal Wkly Rep. 2013 Nov 22;62[46]:914-6). Eventually, the Food and Drug Administration issued a consumer alert regarding certain Baczol products containing these ingredients, but Dr. Norton said he is still concerned about the possibility for more grave hypersensitivity reactions to these sulfa antibiotics in the Salvadoran product.
Sometimes, said Dr. Norton, the problem lies in the lack of an expected ingredient. He and his team at Children’s National Health System helped solve a medical mystery involving a skin ailment in very premature infants with cholestasis. An interdisciplinary team was convened after the neonatal intensive care unit at the hospital saw its third infant with severe blistering and erosions in an acral, perianal, and perioral pattern that did not respond to empiric treatment for herpes simplex virus and staphylococcal infection – a pattern reminiscent of zinc deficiency dermatitis. Dietitians reported that there was a nationwide shortage of sterile injectable zinc, so total parenteral nutrition was being formulated without zinc. All three of the premature infants were receiving total parenteral nutrition and were so premature that they had insufficient zinc stores. The problem was identified and corrected (MMWR 2014 Jan. 17;63[02];35-7).
A more pervasive issue, which has global significance, pertains to counterfeit vaccines prepared with absolutely no vaccine components, often made in China or Nigeria with high-quality and sophisticated packaging, said Dr. Norton.
Keeping a lid on counterfeit drugs is challenging since there are so many potential entry points into the supply chain, Dr. Norton pointed out. Weak points include mislabeled raw ingredients, packaging, storage, transportation, repackaging, and distribution. The proliferation of online pharmacies also makes regulation more difficult.
There is some international cooperation to detect and combat drug counterfeiting and adulteration: For example, Interpol, the International Coalition of Medicines Regulatory Authorities, the Pharmaceutical Security Institute, and even the United Nations are developing cooperative strategies to combat the problem.
In the meantime, he emphasized that physicians must maintain a high index of suspicion and keep in mind that the first signs of adulterated drugs or prescription drugs available OTC may appear on the skin.
Dr. Norton reported no conflicts of interest.
[email protected]
On Twitter @karioakes
EXPERT ANALYSIS FROM WCPD 2017
Small community hospitals need antibiotic stewardship programs
Antibiotic use and misuse is driving drug resistance. Each year in the United States, at least 2 million people become infected with bacteria that are resistant to antibiotics, and at least 23,000 people die each year as a result of these infections, according to the Centers for Disease Control and Prevention.
Over 70% of U.S. hospitals are small community hospitals with 200 beds or fewer; however, our understanding of antibiotic use in these facilities is extremely limited. Most of the existing data on antibiotic use rates come from larger academic medical centers. Describing antibiotic usage patterns in small facilities is a high priority, given they constitute the majority of acute care hospitals and national antibiotic stewardship is forthcoming.
Intermountain has a long history of antibiotic use measurements and digital data support. All facilities use an electronic medical record system that transmits data to a centralized enterprise data warehouse. Since 2011, antibiotic use reports have been collected from the data system and submitted to the CDC’s National Healthcare and Safety Network Antimicrobial Use (NHSN AU) module.
Using data from the NHSN AU module from January 2011 through December 2013, we calculated monthly and 3-year antibiotic use rates for each facility, care unit type, and antibiotic category. Data included in the NHSN AU modules include:
• Patient care location.
• Facility-wide antibiotic use.
• Use of individual antibiotics.
• Classes of antibiotics.
• Days of therapy.
• Patient-day data.
Antibiotic agents were categorized into five groups based on antibiotic spectrum and ability to treat multidrug-resistant organisms (MDROs). Category one antibiotics are narrower-spectrum agents, and category five antibiotics are the broadest-spectrum antibiotics or associated with treating MDROs. Categories four and five were classified as broad-spectrum antibiotics. Hospital care units were categorized as intensive care, medical/surgical, pediatric, or miscellaneous.
Antibiotic use rates, expressed as days of therapy per 1,000 patient-days (DOT/1000PD), were calculated for each small community hospital and compared with rates in large community hospitals. Negative-binomial regression was used to relate antibiotic use.
The key findings of the study include:
• Total antibiotic use rates varied widely across the 15 small community hospitals and were similar to rates in four large community hospitals.
• The proportion of patient-days spent in the respective care unit types varied substantially within small community hospitals and had a large impact on facility-level antibiotic use rates.
• Broad-spectrum antibiotics accounted for 26% of use in small community hospitals, similar to the proportion in large community hospitals.
• Significant predictors of antibiotic use include case mix index, proportion of patient-days in specific care unit types, and season.
• Small community hospitals need to become a focus of antibiotic stewardship efforts.
All hospitals in 2017 are required to have an antibiotic stewardship program in place according to Joint Commission guidelines. Small community hospitals in the United States face significant challenges meeting the national antibiotic stewardship requirements. These challenges include: limited access to infectious diseases physician and/or pharmacist leadership, limited information technology support, and lack of antibiotic guidance.
In order to holistically address the growing problem of antibiotic-resistant bacteria, the infectious disease community must respond to antibiotic use in ALL hospitals, not just the large academic medical facilities. Small hospitals are least likely to have stewardship programs even though antibiotic usage patterns are similar to larger facilities. We need to bring stewardship support to ALL hospitals, but the challenges come in knowing how to do that.
To address the challenges, researchers at Intermountain Healthcare are currently conducting a study to identify recommendations that will help build antibiotic stewardship programs for these facilities.
Eddie Stenehjem, MD, is an infectious disease physician and researcher at Intermountain Medical Center, Salt Lake City, the flagship facility for the Intermountain Healthcare system.
Antibiotic use and misuse is driving drug resistance. Each year in the United States, at least 2 million people become infected with bacteria that are resistant to antibiotics, and at least 23,000 people die each year as a result of these infections, according to the Centers for Disease Control and Prevention.
Over 70% of U.S. hospitals are small community hospitals with 200 beds or fewer; however, our understanding of antibiotic use in these facilities is extremely limited. Most of the existing data on antibiotic use rates come from larger academic medical centers. Describing antibiotic usage patterns in small facilities is a high priority, given they constitute the majority of acute care hospitals and national antibiotic stewardship is forthcoming.
Intermountain has a long history of antibiotic use measurements and digital data support. All facilities use an electronic medical record system that transmits data to a centralized enterprise data warehouse. Since 2011, antibiotic use reports have been collected from the data system and submitted to the CDC’s National Healthcare and Safety Network Antimicrobial Use (NHSN AU) module.
Using data from the NHSN AU module from January 2011 through December 2013, we calculated monthly and 3-year antibiotic use rates for each facility, care unit type, and antibiotic category. Data included in the NHSN AU modules include:
• Patient care location.
• Facility-wide antibiotic use.
• Use of individual antibiotics.
• Classes of antibiotics.
• Days of therapy.
• Patient-day data.
Antibiotic agents were categorized into five groups based on antibiotic spectrum and ability to treat multidrug-resistant organisms (MDROs). Category one antibiotics are narrower-spectrum agents, and category five antibiotics are the broadest-spectrum antibiotics or associated with treating MDROs. Categories four and five were classified as broad-spectrum antibiotics. Hospital care units were categorized as intensive care, medical/surgical, pediatric, or miscellaneous.
Antibiotic use rates, expressed as days of therapy per 1,000 patient-days (DOT/1000PD), were calculated for each small community hospital and compared with rates in large community hospitals. Negative-binomial regression was used to relate antibiotic use.
The key findings of the study include:
• Total antibiotic use rates varied widely across the 15 small community hospitals and were similar to rates in four large community hospitals.
• The proportion of patient-days spent in the respective care unit types varied substantially within small community hospitals and had a large impact on facility-level antibiotic use rates.
• Broad-spectrum antibiotics accounted for 26% of use in small community hospitals, similar to the proportion in large community hospitals.
• Significant predictors of antibiotic use include case mix index, proportion of patient-days in specific care unit types, and season.
• Small community hospitals need to become a focus of antibiotic stewardship efforts.
All hospitals in 2017 are required to have an antibiotic stewardship program in place according to Joint Commission guidelines. Small community hospitals in the United States face significant challenges meeting the national antibiotic stewardship requirements. These challenges include: limited access to infectious diseases physician and/or pharmacist leadership, limited information technology support, and lack of antibiotic guidance.
In order to holistically address the growing problem of antibiotic-resistant bacteria, the infectious disease community must respond to antibiotic use in ALL hospitals, not just the large academic medical facilities. Small hospitals are least likely to have stewardship programs even though antibiotic usage patterns are similar to larger facilities. We need to bring stewardship support to ALL hospitals, but the challenges come in knowing how to do that.
To address the challenges, researchers at Intermountain Healthcare are currently conducting a study to identify recommendations that will help build antibiotic stewardship programs for these facilities.
Eddie Stenehjem, MD, is an infectious disease physician and researcher at Intermountain Medical Center, Salt Lake City, the flagship facility for the Intermountain Healthcare system.
Antibiotic use and misuse is driving drug resistance. Each year in the United States, at least 2 million people become infected with bacteria that are resistant to antibiotics, and at least 23,000 people die each year as a result of these infections, according to the Centers for Disease Control and Prevention.
Over 70% of U.S. hospitals are small community hospitals with 200 beds or fewer; however, our understanding of antibiotic use in these facilities is extremely limited. Most of the existing data on antibiotic use rates come from larger academic medical centers. Describing antibiotic usage patterns in small facilities is a high priority, given they constitute the majority of acute care hospitals and national antibiotic stewardship is forthcoming.
Intermountain has a long history of antibiotic use measurements and digital data support. All facilities use an electronic medical record system that transmits data to a centralized enterprise data warehouse. Since 2011, antibiotic use reports have been collected from the data system and submitted to the CDC’s National Healthcare and Safety Network Antimicrobial Use (NHSN AU) module.
Using data from the NHSN AU module from January 2011 through December 2013, we calculated monthly and 3-year antibiotic use rates for each facility, care unit type, and antibiotic category. Data included in the NHSN AU modules include:
• Patient care location.
• Facility-wide antibiotic use.
• Use of individual antibiotics.
• Classes of antibiotics.
• Days of therapy.
• Patient-day data.
Antibiotic agents were categorized into five groups based on antibiotic spectrum and ability to treat multidrug-resistant organisms (MDROs). Category one antibiotics are narrower-spectrum agents, and category five antibiotics are the broadest-spectrum antibiotics or associated with treating MDROs. Categories four and five were classified as broad-spectrum antibiotics. Hospital care units were categorized as intensive care, medical/surgical, pediatric, or miscellaneous.
Antibiotic use rates, expressed as days of therapy per 1,000 patient-days (DOT/1000PD), were calculated for each small community hospital and compared with rates in large community hospitals. Negative-binomial regression was used to relate antibiotic use.
The key findings of the study include:
• Total antibiotic use rates varied widely across the 15 small community hospitals and were similar to rates in four large community hospitals.
• The proportion of patient-days spent in the respective care unit types varied substantially within small community hospitals and had a large impact on facility-level antibiotic use rates.
• Broad-spectrum antibiotics accounted for 26% of use in small community hospitals, similar to the proportion in large community hospitals.
• Significant predictors of antibiotic use include case mix index, proportion of patient-days in specific care unit types, and season.
• Small community hospitals need to become a focus of antibiotic stewardship efforts.
All hospitals in 2017 are required to have an antibiotic stewardship program in place according to Joint Commission guidelines. Small community hospitals in the United States face significant challenges meeting the national antibiotic stewardship requirements. These challenges include: limited access to infectious diseases physician and/or pharmacist leadership, limited information technology support, and lack of antibiotic guidance.
In order to holistically address the growing problem of antibiotic-resistant bacteria, the infectious disease community must respond to antibiotic use in ALL hospitals, not just the large academic medical facilities. Small hospitals are least likely to have stewardship programs even though antibiotic usage patterns are similar to larger facilities. We need to bring stewardship support to ALL hospitals, but the challenges come in knowing how to do that.
To address the challenges, researchers at Intermountain Healthcare are currently conducting a study to identify recommendations that will help build antibiotic stewardship programs for these facilities.
Eddie Stenehjem, MD, is an infectious disease physician and researcher at Intermountain Medical Center, Salt Lake City, the flagship facility for the Intermountain Healthcare system.
Attitudes and beliefs affecting methotrexate adherence identified
MADRID – Negative beliefs and uncertainty regarding treatment with methotrexate, as well as dislike for the drug, contribute the most to rheumatoid arthritis patients’ nonadherence to the therapy, with one study finding that about one-third were nonadherent at the time they were eligible to start biologic therapy.
The French cross-sectional survey of 244 patients who were not responding to methotrexate found that 34% actually had poor adherence, including 54% who skipped doses and 38% who temporarily stopped treatment without their doctors’ recommendation. In comparison, patients who were deemed adherent had a lower rate of skipping doses (15%) or temporarily stopped treatment without their doctors’ recommendation (4%), both of which were statistically significant differences, Catherine Beauvais, MD, reported at the European Congress of Rheumatology. Nonadherence was defined as taking less than 80% of doses, according to the CQR19 (Compliance Questionnaire for Rheumatology).
“We have identified profiles of adherence,” Dr. Beauvais of Saint-Antoine Hospital in Paris commented in an interview.
“Among nonadherent patients, there are two profiles,” she added. “We have patients who are not responding to methotrexate but they also have negative beliefs, low levels of support, and they have professional impairment. [Then,] there are patients who do not like their treatment [although it is being well tolerated].”
The other profiles identified were of patients with good adherence to methotrexate with a higher or lower impact on patient outcomes.
In a poster presentation, Dr. Beauvais and her coauthors suggested that the “detection of patients’ profiles may allow targeted strategies to improve or maintain adherence.”
The FORGET survey was conducted over a 3-month period starting in July 2016. A total of 78 rheumatologists recruited patients who were inadequately responding to methotrexate and, thus, eligible to start biologic treatment for rheumatoid arthritis. Both the rheumatologists and the patients completed questionnaires, with 200 questionnaires being completed by patients and their rheumatologist.
As might be suspected for an RA population, 72% of respondents were women, with a mean age of 54 years. Over half (58%) had at least one comorbidity, and the mean disease activity score in 28 joints at the time of the survey was 4.07.
Significant factors for nonadherence were feeling constrained about taking treatment, cited by 29% of respondents; feeling “less good” with a change in dosage (31%); and feeling that treatment was “doing me more harm than good” (19% of respondents).
Surprisingly, most rheumatologists seemed to be unaware of their patients’ lack of adherence to their medication, despite saying that they asked about adherence in more than 80% of their patients.
Rheumatologists proposed the addition of a biologic to methotrexate more often if patients were nonadherent than if patients showed good compliance (91% vs. 68%; P less than .01).
Effect of patient attitudes on compliance
A team of U.K. researchers evaluated how attitudes toward treatment with methotrexate affected patients’ compliance in a separate poster presentation at the meeting.
RAMS is a 1-year observational study of patients with RA who are starting treatment with methotrexate. Patients recruited into the study completed weekly diaries, noting whether they took methotrexate (adherence) and, if not, their reasons for not doing so. Patients were deemed nonadherent if they did not take methotrexate correctly for 90% of the time over a period of 6 months.
Lower adherence was significantly associated with negative or uncertain views about treatment, with an odds ratio of 2.7. Conversely, being positive or certain about treatment lowered patients’ odds of being nonadherent (OR, 0.32).
“People who are uncertain about how to attribute illness events are less likely to adhere within the first 6 months of starting methotrexate therapy,” Ms. Hope and her coauthors observed.
“Encouraging patients to actively monitor their progress with therapy and providing them with support to understand likely effects of methotrexate may help optimize disease-modifying antirheumatic drug use,” they concluded.
Ms. Hope and Dr. Beauvais had no conflicts of interest to disclose. The FORGET survey was funded by Chugai Pharma France.
MADRID – Negative beliefs and uncertainty regarding treatment with methotrexate, as well as dislike for the drug, contribute the most to rheumatoid arthritis patients’ nonadherence to the therapy, with one study finding that about one-third were nonadherent at the time they were eligible to start biologic therapy.
The French cross-sectional survey of 244 patients who were not responding to methotrexate found that 34% actually had poor adherence, including 54% who skipped doses and 38% who temporarily stopped treatment without their doctors’ recommendation. In comparison, patients who were deemed adherent had a lower rate of skipping doses (15%) or temporarily stopped treatment without their doctors’ recommendation (4%), both of which were statistically significant differences, Catherine Beauvais, MD, reported at the European Congress of Rheumatology. Nonadherence was defined as taking less than 80% of doses, according to the CQR19 (Compliance Questionnaire for Rheumatology).
“We have identified profiles of adherence,” Dr. Beauvais of Saint-Antoine Hospital in Paris commented in an interview.
“Among nonadherent patients, there are two profiles,” she added. “We have patients who are not responding to methotrexate but they also have negative beliefs, low levels of support, and they have professional impairment. [Then,] there are patients who do not like their treatment [although it is being well tolerated].”
The other profiles identified were of patients with good adherence to methotrexate with a higher or lower impact on patient outcomes.
In a poster presentation, Dr. Beauvais and her coauthors suggested that the “detection of patients’ profiles may allow targeted strategies to improve or maintain adherence.”
The FORGET survey was conducted over a 3-month period starting in July 2016. A total of 78 rheumatologists recruited patients who were inadequately responding to methotrexate and, thus, eligible to start biologic treatment for rheumatoid arthritis. Both the rheumatologists and the patients completed questionnaires, with 200 questionnaires being completed by patients and their rheumatologist.
As might be suspected for an RA population, 72% of respondents were women, with a mean age of 54 years. Over half (58%) had at least one comorbidity, and the mean disease activity score in 28 joints at the time of the survey was 4.07.
Significant factors for nonadherence were feeling constrained about taking treatment, cited by 29% of respondents; feeling “less good” with a change in dosage (31%); and feeling that treatment was “doing me more harm than good” (19% of respondents).
Surprisingly, most rheumatologists seemed to be unaware of their patients’ lack of adherence to their medication, despite saying that they asked about adherence in more than 80% of their patients.
Rheumatologists proposed the addition of a biologic to methotrexate more often if patients were nonadherent than if patients showed good compliance (91% vs. 68%; P less than .01).
Effect of patient attitudes on compliance
A team of U.K. researchers evaluated how attitudes toward treatment with methotrexate affected patients’ compliance in a separate poster presentation at the meeting.
RAMS is a 1-year observational study of patients with RA who are starting treatment with methotrexate. Patients recruited into the study completed weekly diaries, noting whether they took methotrexate (adherence) and, if not, their reasons for not doing so. Patients were deemed nonadherent if they did not take methotrexate correctly for 90% of the time over a period of 6 months.
Lower adherence was significantly associated with negative or uncertain views about treatment, with an odds ratio of 2.7. Conversely, being positive or certain about treatment lowered patients’ odds of being nonadherent (OR, 0.32).
“People who are uncertain about how to attribute illness events are less likely to adhere within the first 6 months of starting methotrexate therapy,” Ms. Hope and her coauthors observed.
“Encouraging patients to actively monitor their progress with therapy and providing them with support to understand likely effects of methotrexate may help optimize disease-modifying antirheumatic drug use,” they concluded.
Ms. Hope and Dr. Beauvais had no conflicts of interest to disclose. The FORGET survey was funded by Chugai Pharma France.
MADRID – Negative beliefs and uncertainty regarding treatment with methotrexate, as well as dislike for the drug, contribute the most to rheumatoid arthritis patients’ nonadherence to the therapy, with one study finding that about one-third were nonadherent at the time they were eligible to start biologic therapy.
The French cross-sectional survey of 244 patients who were not responding to methotrexate found that 34% actually had poor adherence, including 54% who skipped doses and 38% who temporarily stopped treatment without their doctors’ recommendation. In comparison, patients who were deemed adherent had a lower rate of skipping doses (15%) or temporarily stopped treatment without their doctors’ recommendation (4%), both of which were statistically significant differences, Catherine Beauvais, MD, reported at the European Congress of Rheumatology. Nonadherence was defined as taking less than 80% of doses, according to the CQR19 (Compliance Questionnaire for Rheumatology).
“We have identified profiles of adherence,” Dr. Beauvais of Saint-Antoine Hospital in Paris commented in an interview.
“Among nonadherent patients, there are two profiles,” she added. “We have patients who are not responding to methotrexate but they also have negative beliefs, low levels of support, and they have professional impairment. [Then,] there are patients who do not like their treatment [although it is being well tolerated].”
The other profiles identified were of patients with good adherence to methotrexate with a higher or lower impact on patient outcomes.
In a poster presentation, Dr. Beauvais and her coauthors suggested that the “detection of patients’ profiles may allow targeted strategies to improve or maintain adherence.”
The FORGET survey was conducted over a 3-month period starting in July 2016. A total of 78 rheumatologists recruited patients who were inadequately responding to methotrexate and, thus, eligible to start biologic treatment for rheumatoid arthritis. Both the rheumatologists and the patients completed questionnaires, with 200 questionnaires being completed by patients and their rheumatologist.
As might be suspected for an RA population, 72% of respondents were women, with a mean age of 54 years. Over half (58%) had at least one comorbidity, and the mean disease activity score in 28 joints at the time of the survey was 4.07.
Significant factors for nonadherence were feeling constrained about taking treatment, cited by 29% of respondents; feeling “less good” with a change in dosage (31%); and feeling that treatment was “doing me more harm than good” (19% of respondents).
Surprisingly, most rheumatologists seemed to be unaware of their patients’ lack of adherence to their medication, despite saying that they asked about adherence in more than 80% of their patients.
Rheumatologists proposed the addition of a biologic to methotrexate more often if patients were nonadherent than if patients showed good compliance (91% vs. 68%; P less than .01).
Effect of patient attitudes on compliance
A team of U.K. researchers evaluated how attitudes toward treatment with methotrexate affected patients’ compliance in a separate poster presentation at the meeting.
RAMS is a 1-year observational study of patients with RA who are starting treatment with methotrexate. Patients recruited into the study completed weekly diaries, noting whether they took methotrexate (adherence) and, if not, their reasons for not doing so. Patients were deemed nonadherent if they did not take methotrexate correctly for 90% of the time over a period of 6 months.
Lower adherence was significantly associated with negative or uncertain views about treatment, with an odds ratio of 2.7. Conversely, being positive or certain about treatment lowered patients’ odds of being nonadherent (OR, 0.32).
“People who are uncertain about how to attribute illness events are less likely to adhere within the first 6 months of starting methotrexate therapy,” Ms. Hope and her coauthors observed.
“Encouraging patients to actively monitor their progress with therapy and providing them with support to understand likely effects of methotrexate may help optimize disease-modifying antirheumatic drug use,” they concluded.
Ms. Hope and Dr. Beauvais had no conflicts of interest to disclose. The FORGET survey was funded by Chugai Pharma France.
AT THE EULAR 2017 CONGRESS
Key clinical point:
Major finding: One-third (34%) of RA patients were nonadherent to methotrexate at the initiation of biologic therapy; having negative and uncertain beliefs about the effects of treatment increased the odds of nonadherence.
Data source: The cross-sectional FORGET survey of 78 rheumatologists and 269 patients with rheumatoid arthritis conducted in France and the Rheumatoid Arthritis Medications Study (RAMS) of 200 adherent and nonadherent patients.
Disclosures: The authors had no conflicts of interest to disclose. The FORGET survey was funded by Chugai Pharma France.
Acute liver failure in the ED
Acute liver failure (ALF), is a life-threatening deterioration of liver function in people without preexisting cirrhosis. It can be caused by acetaminophen toxicity, pregnancy, ischemia, hepatitis A infection, and Wilson disease, among other things.
In emergency medicine, ALF can pose serious dilemmas. While transplantation has drastically improved survival rates in recent decades, it is not always required, and no firm criteria for transplantation exist.
But delays in the decision to go ahead with a liver transplant can lead to death.
A new literature review aims to distill the decision-making process for emergency medicine practitioners. Knowing which candidates will benefit and when to perform transplantation “is crucial in improving the likelihood of survival,” its authors say, because of the many factors involved.
In a paper published online in May in The American Journal of Emergency Medicine (2017 May. doi. 10.1016/j.ajem.2017.05.028), Hamid Shokoohi, MD, and his colleagues at George Washington University Medical Center in Washington say that establishing the cause of acute liver failure is essential to making treatment decisions, as some causes are associated with poorer prognosis without transplantation.
“We wanted to improve awareness among emergency medicine physicians, who are the first in the chain of command for transferring patients to a transplant site,” said Ali Pourmand, MD, of George Washington University, Washington, and the corresponding author of the study. “The high risk of early death among these cases makes it necessary for emergency physicians to consider coexisting etiology, be aware of indications and criteria available to determine the need for emergent transplantation, and be able to expedite patient transfer to a transplant center, when indicated.”
As patients presenting with ALF are likely too impaired be able to provide a history, and physical exam findings may be nonspecific, laboratory findings are key in establishing both severity and likely cause. ALF patients in general will have a prolonged prothrombin time, markedly elevated aminotransferase levels, elevated bilirubin, and low platelet count.
Patients with ALF caused by acetaminophen toxicity (the most common cause of ALF in the United States) are likely to present with very high aminotransferase levels, low bilirubin, and high international normalized ratio (INR). Those with viral causes of ALF, meanwhile, tend to have aminotransferase levels of 1,000-2,000 IU/L, and alanine transaminase higher than aspartate transaminase.
Prognosis without transplantation is considerably poorer in patients with severe ALF caused by Wilson disease, Budd-Chiari syndrome, or idiosyncratic drug reactions, compared with those who experience viral hepatitis or acetaminophen toxicity.
Dr. Shokoohi and his colleagues noted that two validated scoring systems can be used to assess prognosis for severe ALF. The King’s College Criteria can be used to establish prognosis for ALF caused by acetaminophen, and ALF from other causes, while the MELD score, recommended by the American Association for the Study of Liver Diseases, incorporates bilirubin, INR, sodium, and creatinine levels to predict prognosis. Both of these scoring systems can be used to inform decisions about transplantation.
Finally, the authors advised that patients with alcoholic liver disease be considered under the same criteria for transplantation as those with other causes of ALF. “Recent research has shown that only a minority of patients ... will have poor follow-up and noncompliance to therapy and/or will revert to heavy alcohol use or abuse after transplant,” they wrote in their analysis. The researchers disclosed no outside funding of conflicts of interest related to their article.
Acute liver failure (ALF), is a life-threatening deterioration of liver function in people without preexisting cirrhosis. It can be caused by acetaminophen toxicity, pregnancy, ischemia, hepatitis A infection, and Wilson disease, among other things.
In emergency medicine, ALF can pose serious dilemmas. While transplantation has drastically improved survival rates in recent decades, it is not always required, and no firm criteria for transplantation exist.
But delays in the decision to go ahead with a liver transplant can lead to death.
A new literature review aims to distill the decision-making process for emergency medicine practitioners. Knowing which candidates will benefit and when to perform transplantation “is crucial in improving the likelihood of survival,” its authors say, because of the many factors involved.
In a paper published online in May in The American Journal of Emergency Medicine (2017 May. doi. 10.1016/j.ajem.2017.05.028), Hamid Shokoohi, MD, and his colleagues at George Washington University Medical Center in Washington say that establishing the cause of acute liver failure is essential to making treatment decisions, as some causes are associated with poorer prognosis without transplantation.
“We wanted to improve awareness among emergency medicine physicians, who are the first in the chain of command for transferring patients to a transplant site,” said Ali Pourmand, MD, of George Washington University, Washington, and the corresponding author of the study. “The high risk of early death among these cases makes it necessary for emergency physicians to consider coexisting etiology, be aware of indications and criteria available to determine the need for emergent transplantation, and be able to expedite patient transfer to a transplant center, when indicated.”
As patients presenting with ALF are likely too impaired be able to provide a history, and physical exam findings may be nonspecific, laboratory findings are key in establishing both severity and likely cause. ALF patients in general will have a prolonged prothrombin time, markedly elevated aminotransferase levels, elevated bilirubin, and low platelet count.
Patients with ALF caused by acetaminophen toxicity (the most common cause of ALF in the United States) are likely to present with very high aminotransferase levels, low bilirubin, and high international normalized ratio (INR). Those with viral causes of ALF, meanwhile, tend to have aminotransferase levels of 1,000-2,000 IU/L, and alanine transaminase higher than aspartate transaminase.
Prognosis without transplantation is considerably poorer in patients with severe ALF caused by Wilson disease, Budd-Chiari syndrome, or idiosyncratic drug reactions, compared with those who experience viral hepatitis or acetaminophen toxicity.
Dr. Shokoohi and his colleagues noted that two validated scoring systems can be used to assess prognosis for severe ALF. The King’s College Criteria can be used to establish prognosis for ALF caused by acetaminophen, and ALF from other causes, while the MELD score, recommended by the American Association for the Study of Liver Diseases, incorporates bilirubin, INR, sodium, and creatinine levels to predict prognosis. Both of these scoring systems can be used to inform decisions about transplantation.
Finally, the authors advised that patients with alcoholic liver disease be considered under the same criteria for transplantation as those with other causes of ALF. “Recent research has shown that only a minority of patients ... will have poor follow-up and noncompliance to therapy and/or will revert to heavy alcohol use or abuse after transplant,” they wrote in their analysis. The researchers disclosed no outside funding of conflicts of interest related to their article.
Acute liver failure (ALF), is a life-threatening deterioration of liver function in people without preexisting cirrhosis. It can be caused by acetaminophen toxicity, pregnancy, ischemia, hepatitis A infection, and Wilson disease, among other things.
In emergency medicine, ALF can pose serious dilemmas. While transplantation has drastically improved survival rates in recent decades, it is not always required, and no firm criteria for transplantation exist.
But delays in the decision to go ahead with a liver transplant can lead to death.
A new literature review aims to distill the decision-making process for emergency medicine practitioners. Knowing which candidates will benefit and when to perform transplantation “is crucial in improving the likelihood of survival,” its authors say, because of the many factors involved.
In a paper published online in May in The American Journal of Emergency Medicine (2017 May. doi. 10.1016/j.ajem.2017.05.028), Hamid Shokoohi, MD, and his colleagues at George Washington University Medical Center in Washington say that establishing the cause of acute liver failure is essential to making treatment decisions, as some causes are associated with poorer prognosis without transplantation.
“We wanted to improve awareness among emergency medicine physicians, who are the first in the chain of command for transferring patients to a transplant site,” said Ali Pourmand, MD, of George Washington University, Washington, and the corresponding author of the study. “The high risk of early death among these cases makes it necessary for emergency physicians to consider coexisting etiology, be aware of indications and criteria available to determine the need for emergent transplantation, and be able to expedite patient transfer to a transplant center, when indicated.”
As patients presenting with ALF are likely too impaired be able to provide a history, and physical exam findings may be nonspecific, laboratory findings are key in establishing both severity and likely cause. ALF patients in general will have a prolonged prothrombin time, markedly elevated aminotransferase levels, elevated bilirubin, and low platelet count.
Patients with ALF caused by acetaminophen toxicity (the most common cause of ALF in the United States) are likely to present with very high aminotransferase levels, low bilirubin, and high international normalized ratio (INR). Those with viral causes of ALF, meanwhile, tend to have aminotransferase levels of 1,000-2,000 IU/L, and alanine transaminase higher than aspartate transaminase.
Prognosis without transplantation is considerably poorer in patients with severe ALF caused by Wilson disease, Budd-Chiari syndrome, or idiosyncratic drug reactions, compared with those who experience viral hepatitis or acetaminophen toxicity.
Dr. Shokoohi and his colleagues noted that two validated scoring systems can be used to assess prognosis for severe ALF. The King’s College Criteria can be used to establish prognosis for ALF caused by acetaminophen, and ALF from other causes, while the MELD score, recommended by the American Association for the Study of Liver Diseases, incorporates bilirubin, INR, sodium, and creatinine levels to predict prognosis. Both of these scoring systems can be used to inform decisions about transplantation.
Finally, the authors advised that patients with alcoholic liver disease be considered under the same criteria for transplantation as those with other causes of ALF. “Recent research has shown that only a minority of patients ... will have poor follow-up and noncompliance to therapy and/or will revert to heavy alcohol use or abuse after transplant,” they wrote in their analysis. The researchers disclosed no outside funding of conflicts of interest related to their article.
FROM THE AMERICAN JOURNAL OF EMERGENCY MEDICINE
Vaginal salpingo-oophorectomy: Tips and tricks

Visit the Society of Gynecologic Surgeons online: sgsonline.org
Additional videos from SGS are available here, including these recent offerings:

Visit the Society of Gynecologic Surgeons online: sgsonline.org
Additional videos from SGS are available here, including these recent offerings:

Visit the Society of Gynecologic Surgeons online: sgsonline.org
Additional videos from SGS are available here, including these recent offerings:
This video is brought to you by
Four drugs better than three for myeloma induction
MADRID – A four-drug induction regimen induced quicker and deeper remissions than sequential triplet regimens in patients with newly diagnosed multiple myeloma.
In addition, fast, deep remissions may lead to improved progression-free survival (PFS) following autologous stem cell transplantation (ASCT), said investigators from a U.K. Medical Research Council study.
In the phase 3 randomized, parallel group Myeloma XI study, very good partial responses (VGPR) or better were seen following induction in 79.5% of patients assigned to the quadruplet (KCRD) of carfilzomib (Kyprolis), cyclophosphamide, lenalidomide (Revlimid), and dexamethasone, compared with 60.8% for those assigned to cyclophosphamide, lenalidomide, and dexamethasone (CRD) and 52.8% for those assigned to cyclophosphamide, thalidomide, and dexamethasone (CTD), said Charlotte Pawlyn, MD, PhD, at the annual congress of the European Hematology Association.
“In our study, we see a very much deeper response after initial induction with the quadruplet regimen, compared with triplet regimens,” Dr. Pawlyn of the Institute of Cancer Research, London, said in an interview.
Medical Research Council investigators showed in the Myeloma IX study that among patients who had a less than VGPR to an immunomodulator-based triplet regimen such as CRD, a triplet regimen including the proteasome inhibitor bortezomib (Velcade) could improve both pre- and post-transplant response rates, and that the improved responses translated into improved PFS.
For the Myeloma XI study, the investigators employed the same response-adapted approach to compare outcomes following induction with the proteasome inhibitor–containing KCRD regimen and the lenalidomide- or thalidomide-based regimens.
They chose carfilzomib as the proteasome-inhibitor backbone of the quadruplet because of its selective, irreversible target binding, lower incidence of peripheral neuropathy (compared with bortezomib), and efficacy in both the frontline and relapsed/refractory setting, she said.
Asked why the comparator regimens did not contain a proteasome inhibitor, Dr. Pawlyn said that while the current standard for induction therapy in the United Kingdom is bortezomib, thalidomide, and dexamethasone, CTD was the standard of care at the time of study planning and initial enrollment.
The trial was open to all patients in the United Kingdom of all ages with newly diagnosed symptomatic multiple myeloma, with pathways for both transplant-eligible and transplant-ineligible patients. The only main exclusion criteria were for patients with dialysis-dependent renal failure and for those who had a prior or concurrent malignancy.
A total of 1,021 patients were assigned to each of the CTD and CRD cohorts, and 526 patients were assigned to the KCRD cohort. The cohorts were well balanced by sex, age, World Health Organization performance score, and other parameters.
Patients randomized to either CTD or CRD were assessed for response after a minimum of four induction cycles, with treatment continued until best response. Those with a VGPR or better went on to ASCT, while those with a partial response were randomized to either a second induction with cyclophosphamide, bortezomib, and dexamethasone (CVD) or no CVD, and then proceeded to transplant. Patients with stable disease or disease progression in either of these arms went on to CVD prior to ASCT.
In the KCRD arm, all patients went from induction to transplant. Following ASCT, patients were randomized to either observation or lenalidomide maintenance.
A higher proportion of patients assigned to KCRD completed the minimum of four induction cycles, and few patients in any trial arm had to stop induction therapy because of adverse events. Dose modifications were required in 63.9% of patients on KCRD, 56.3% of patients on CRD, and 82.2% of patients on CTD.
There was no significant cardiac signal seen in the study, and no difference in the incidence of venous thromboembolic events among the treatment arms.
As noted before, rates of VGPR or better after initial induction were highest in the KCRD arm, at 79.5%, compared with 60% for CRD, and 52.8% for CTD.
“The KCRD quadruplet achieved the highest speed and depth of response,” Dr. Pawlyn said.
The pattern of responses was similar across all cytogenetic risk groups, she added.
A higher proportion of patients treated with KCRD went on to ASCT, and the pattern of deeper responses among patients who underwent induction with KCRD persisted, with 92.1% of patients having a post-transplant VGPR or better, compared with 81.8% for CRD and 77.0% for CTD (statistical significance not shown).
Again, the pattern of responses post-transplant was similar across cytogenetic risk groups.
The investigators anticipate receiving PFS results from the Myeloma XI study in the third or fourth quarter of 2017.
The study was sponsored by the University of Leeds (England), with support from the U.K. National Cancer Research Institute, Cancer Research UK, and Myeloma UK, and collaboration with Celgene, Merck Sharp & Dohme, and Amgen. Dr. Pawlyn disclosed travel support from Celgene and Janssen, and honoraria from Celgene and Takeda.
MADRID – A four-drug induction regimen induced quicker and deeper remissions than sequential triplet regimens in patients with newly diagnosed multiple myeloma.
In addition, fast, deep remissions may lead to improved progression-free survival (PFS) following autologous stem cell transplantation (ASCT), said investigators from a U.K. Medical Research Council study.
In the phase 3 randomized, parallel group Myeloma XI study, very good partial responses (VGPR) or better were seen following induction in 79.5% of patients assigned to the quadruplet (KCRD) of carfilzomib (Kyprolis), cyclophosphamide, lenalidomide (Revlimid), and dexamethasone, compared with 60.8% for those assigned to cyclophosphamide, lenalidomide, and dexamethasone (CRD) and 52.8% for those assigned to cyclophosphamide, thalidomide, and dexamethasone (CTD), said Charlotte Pawlyn, MD, PhD, at the annual congress of the European Hematology Association.
“In our study, we see a very much deeper response after initial induction with the quadruplet regimen, compared with triplet regimens,” Dr. Pawlyn of the Institute of Cancer Research, London, said in an interview.
Medical Research Council investigators showed in the Myeloma IX study that among patients who had a less than VGPR to an immunomodulator-based triplet regimen such as CRD, a triplet regimen including the proteasome inhibitor bortezomib (Velcade) could improve both pre- and post-transplant response rates, and that the improved responses translated into improved PFS.
For the Myeloma XI study, the investigators employed the same response-adapted approach to compare outcomes following induction with the proteasome inhibitor–containing KCRD regimen and the lenalidomide- or thalidomide-based regimens.
They chose carfilzomib as the proteasome-inhibitor backbone of the quadruplet because of its selective, irreversible target binding, lower incidence of peripheral neuropathy (compared with bortezomib), and efficacy in both the frontline and relapsed/refractory setting, she said.
Asked why the comparator regimens did not contain a proteasome inhibitor, Dr. Pawlyn said that while the current standard for induction therapy in the United Kingdom is bortezomib, thalidomide, and dexamethasone, CTD was the standard of care at the time of study planning and initial enrollment.
The trial was open to all patients in the United Kingdom of all ages with newly diagnosed symptomatic multiple myeloma, with pathways for both transplant-eligible and transplant-ineligible patients. The only main exclusion criteria were for patients with dialysis-dependent renal failure and for those who had a prior or concurrent malignancy.
A total of 1,021 patients were assigned to each of the CTD and CRD cohorts, and 526 patients were assigned to the KCRD cohort. The cohorts were well balanced by sex, age, World Health Organization performance score, and other parameters.
Patients randomized to either CTD or CRD were assessed for response after a minimum of four induction cycles, with treatment continued until best response. Those with a VGPR or better went on to ASCT, while those with a partial response were randomized to either a second induction with cyclophosphamide, bortezomib, and dexamethasone (CVD) or no CVD, and then proceeded to transplant. Patients with stable disease or disease progression in either of these arms went on to CVD prior to ASCT.
In the KCRD arm, all patients went from induction to transplant. Following ASCT, patients were randomized to either observation or lenalidomide maintenance.
A higher proportion of patients assigned to KCRD completed the minimum of four induction cycles, and few patients in any trial arm had to stop induction therapy because of adverse events. Dose modifications were required in 63.9% of patients on KCRD, 56.3% of patients on CRD, and 82.2% of patients on CTD.
There was no significant cardiac signal seen in the study, and no difference in the incidence of venous thromboembolic events among the treatment arms.
As noted before, rates of VGPR or better after initial induction were highest in the KCRD arm, at 79.5%, compared with 60% for CRD, and 52.8% for CTD.
“The KCRD quadruplet achieved the highest speed and depth of response,” Dr. Pawlyn said.
The pattern of responses was similar across all cytogenetic risk groups, she added.
A higher proportion of patients treated with KCRD went on to ASCT, and the pattern of deeper responses among patients who underwent induction with KCRD persisted, with 92.1% of patients having a post-transplant VGPR or better, compared with 81.8% for CRD and 77.0% for CTD (statistical significance not shown).
Again, the pattern of responses post-transplant was similar across cytogenetic risk groups.
The investigators anticipate receiving PFS results from the Myeloma XI study in the third or fourth quarter of 2017.
The study was sponsored by the University of Leeds (England), with support from the U.K. National Cancer Research Institute, Cancer Research UK, and Myeloma UK, and collaboration with Celgene, Merck Sharp & Dohme, and Amgen. Dr. Pawlyn disclosed travel support from Celgene and Janssen, and honoraria from Celgene and Takeda.
MADRID – A four-drug induction regimen induced quicker and deeper remissions than sequential triplet regimens in patients with newly diagnosed multiple myeloma.
In addition, fast, deep remissions may lead to improved progression-free survival (PFS) following autologous stem cell transplantation (ASCT), said investigators from a U.K. Medical Research Council study.
In the phase 3 randomized, parallel group Myeloma XI study, very good partial responses (VGPR) or better were seen following induction in 79.5% of patients assigned to the quadruplet (KCRD) of carfilzomib (Kyprolis), cyclophosphamide, lenalidomide (Revlimid), and dexamethasone, compared with 60.8% for those assigned to cyclophosphamide, lenalidomide, and dexamethasone (CRD) and 52.8% for those assigned to cyclophosphamide, thalidomide, and dexamethasone (CTD), said Charlotte Pawlyn, MD, PhD, at the annual congress of the European Hematology Association.
“In our study, we see a very much deeper response after initial induction with the quadruplet regimen, compared with triplet regimens,” Dr. Pawlyn of the Institute of Cancer Research, London, said in an interview.
Medical Research Council investigators showed in the Myeloma IX study that among patients who had a less than VGPR to an immunomodulator-based triplet regimen such as CRD, a triplet regimen including the proteasome inhibitor bortezomib (Velcade) could improve both pre- and post-transplant response rates, and that the improved responses translated into improved PFS.
For the Myeloma XI study, the investigators employed the same response-adapted approach to compare outcomes following induction with the proteasome inhibitor–containing KCRD regimen and the lenalidomide- or thalidomide-based regimens.
They chose carfilzomib as the proteasome-inhibitor backbone of the quadruplet because of its selective, irreversible target binding, lower incidence of peripheral neuropathy (compared with bortezomib), and efficacy in both the frontline and relapsed/refractory setting, she said.
Asked why the comparator regimens did not contain a proteasome inhibitor, Dr. Pawlyn said that while the current standard for induction therapy in the United Kingdom is bortezomib, thalidomide, and dexamethasone, CTD was the standard of care at the time of study planning and initial enrollment.
The trial was open to all patients in the United Kingdom of all ages with newly diagnosed symptomatic multiple myeloma, with pathways for both transplant-eligible and transplant-ineligible patients. The only main exclusion criteria were for patients with dialysis-dependent renal failure and for those who had a prior or concurrent malignancy.
A total of 1,021 patients were assigned to each of the CTD and CRD cohorts, and 526 patients were assigned to the KCRD cohort. The cohorts were well balanced by sex, age, World Health Organization performance score, and other parameters.
Patients randomized to either CTD or CRD were assessed for response after a minimum of four induction cycles, with treatment continued until best response. Those with a VGPR or better went on to ASCT, while those with a partial response were randomized to either a second induction with cyclophosphamide, bortezomib, and dexamethasone (CVD) or no CVD, and then proceeded to transplant. Patients with stable disease or disease progression in either of these arms went on to CVD prior to ASCT.
In the KCRD arm, all patients went from induction to transplant. Following ASCT, patients were randomized to either observation or lenalidomide maintenance.
A higher proportion of patients assigned to KCRD completed the minimum of four induction cycles, and few patients in any trial arm had to stop induction therapy because of adverse events. Dose modifications were required in 63.9% of patients on KCRD, 56.3% of patients on CRD, and 82.2% of patients on CTD.
There was no significant cardiac signal seen in the study, and no difference in the incidence of venous thromboembolic events among the treatment arms.
As noted before, rates of VGPR or better after initial induction were highest in the KCRD arm, at 79.5%, compared with 60% for CRD, and 52.8% for CTD.
“The KCRD quadruplet achieved the highest speed and depth of response,” Dr. Pawlyn said.
The pattern of responses was similar across all cytogenetic risk groups, she added.
A higher proportion of patients treated with KCRD went on to ASCT, and the pattern of deeper responses among patients who underwent induction with KCRD persisted, with 92.1% of patients having a post-transplant VGPR or better, compared with 81.8% for CRD and 77.0% for CTD (statistical significance not shown).
Again, the pattern of responses post-transplant was similar across cytogenetic risk groups.
The investigators anticipate receiving PFS results from the Myeloma XI study in the third or fourth quarter of 2017.
The study was sponsored by the University of Leeds (England), with support from the U.K. National Cancer Research Institute, Cancer Research UK, and Myeloma UK, and collaboration with Celgene, Merck Sharp & Dohme, and Amgen. Dr. Pawlyn disclosed travel support from Celgene and Janssen, and honoraria from Celgene and Takeda.
AT THE EHA CONGRESS
Key clinical point:
Major finding: An induction quadruplet containing carfilzomib induced a higher rate of very good partial responses or better vs. regimens without a proteasome inhibitor.
Data source: A randomized, open-label, parallel group study of 2,568 patients with newly diagnosed multiple myeloma.
Disclosures: The study was sponsored by the University of Leeds (England), with support from the U.K. National Cancer Research Institute, Cancer Research UK, and Myeloma UK, and collaboration with Celgene, Merck Sharp & Dohme, and Amgen. Dr. Pawlyn disclosed travel support from Celgene and Janssen, and honoraria from Celgene and Takeda.