User login
When should you consider combining 2 long-acting injectable antipsychotics?
Ms. S, age 39, with a 15-year history of schizophrenia and severe paranoid delusions, is admitted after physically assaulting a staff member at a group home. She is receiving paliperidone palmitate, 234 mg every 4 weeks. This has reduced the severity of her symptoms, but she continues to have persistent delusions that affect her ability to accept redirection from staff. Ms. S frequently accuses staff and peers of sexual assault, says that she is pregnant, and does not adhere to treatment recommendations for laboratory monitoring because the “staff uses her blood for experiments.”
Ms. S frequently requires administration of oral and IM haloperidol, as needed, when she becomes aggressive with the staff. She has poor insight into her mental illness and does not believe that she needs medication. Ms. S has a long history of stopping her oral antipsychotic after a few days, reporting that it is “harming her baby.” Monotherapy has been tried with various long-acting injectable antipsychotics (LAIAs), but she still exhibits persistent delusions. The treatment team decides to add a second LAIA, haloperidol decanoate, 200 mg every 4 weeks, to her regimen.
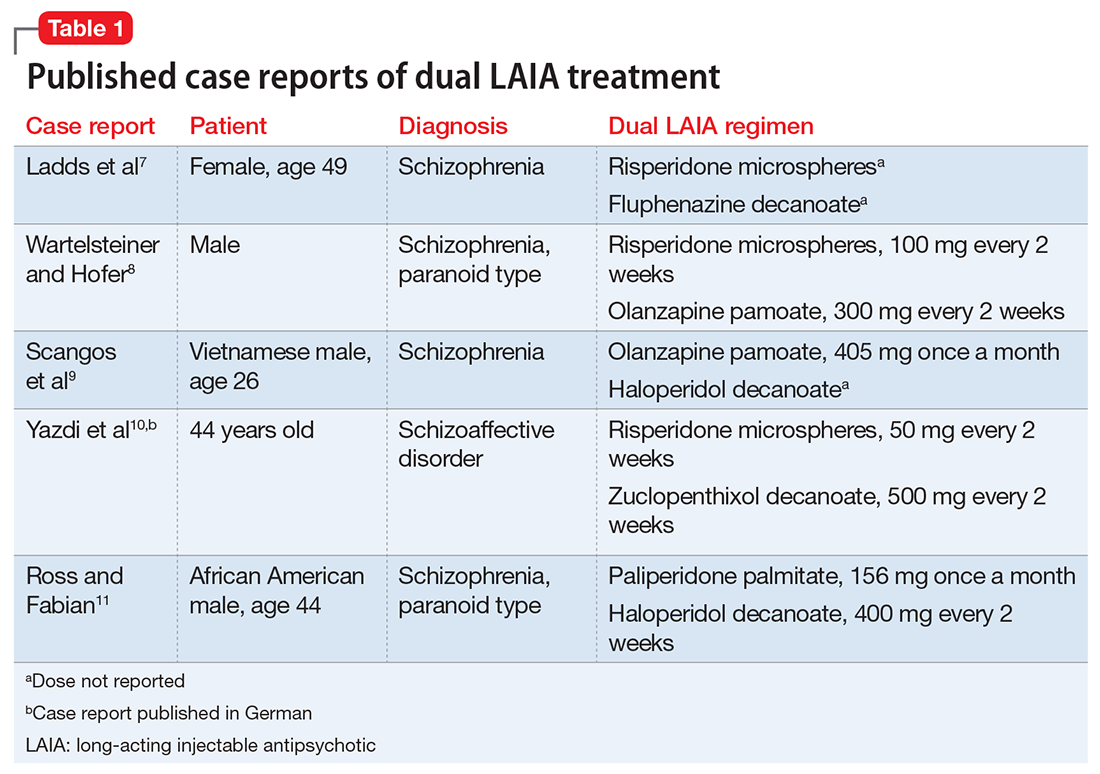
Ladds et al.7 A 49-year-old woman with schizophrenia who was hospitalized for aggressive and bizarre behavior and had been institutionalized for 20 years stopped taking her medication regimen.7 She started taking 8-hour showers with bleach, talking incoherently, and believing that someone was poisoning her. She had poor response to oral risperidone monotherapy; however, 2 months after adding oral fluphenazine and benztropine to her regimen, her symptoms substantially improved (doses not reported). Because she had impaired insight into the need for daily medication, she was started on depot fluphenazine decanoate and risperidone microspheres (doses not reported) before discharge. No substantial adverse effects were noted with this regimen.
Wartelsteiner and Hofer.8 A man who had been diagnosed with paranoid schizophrenia at age 20 presented with thought blocking, incoherence, persecutory delusions, and uncontrolled self-damaging behavior.8 He had been admitted 27 times over 7 years; during this time he received many antipsychotic monotherapies and combination regimens. A total of 8 oral antipsychotics (including clozapine) and 5 LAIAs had been administered during these trials. He significantly improved with the combination of olanzapine and risperidone. Both medications were switched to LAIA formulations to address medication nonadherence. His symptoms remained stable with risperidone microspheres, 100 mg, and olanzapine pamoate, 300 mg, each administered every 2 weeks. He did not experience any adverse effects with this combination therapy.
Scangos et al.9 A 26-year-old Vietnamese man with schizophrenia and an extensive history of unprovoked, psychotically driven assaults was given multiple antipsychotics (including clozapine) during hospitalizations, and his medication regimen consistently included 2 antipsychotics. After contracting viral gastroenteritis, he refused oral medications and required short-acting IM administration of both haloperidol, 5 mg, twice a day, and olanzapine, 10 mg, twice a day. Because of concerns about continuing this regimen, he was switched to haloperidol decanoate (dose not reported) and olanzapine pamoate, 405 mg, administered once per month. The injections were scheduled to alternate so that the patient would receive 1 injection every 2 weeks. The patient’s assaultive behavior was significantly reduced, and no adverse effects were reported.
Ross and Fabian.11 An African American man, age 44, was receiving haloperidol decanoate, 400 mg every 2 weeks, and oral haloperidol, 20 mg/d.11 Because of residual symptoms, a history of nonadherence, and concerns about increasing the haloperidol decanoate dose or frequency, oral haloperidol was discontinued and paliperidone palmitate, 156 mg every 4 weeks, was started. The patient was able to transition into a step-down unit, and no adverse effects were reported.
What to consider before initiating dual LAIA treatment
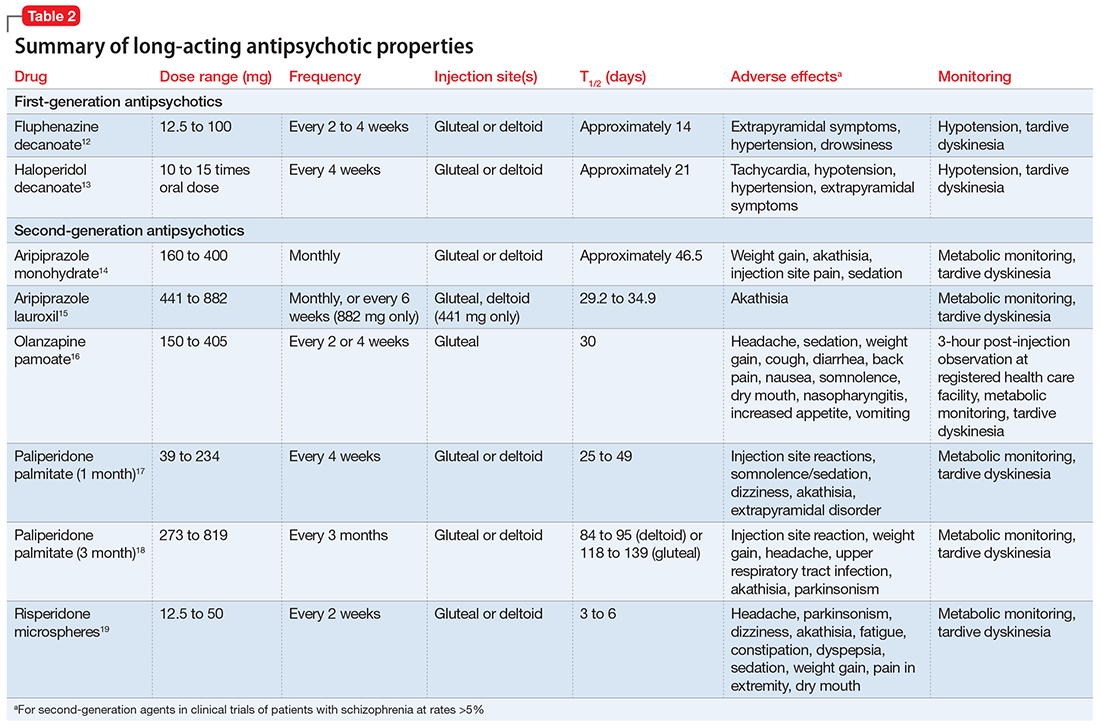
Evaluate the frequency of administration, flexibility of dosing, administration site, adverse effects, and monitoring requirements of each LAIA (Table 212-19) to ensure the patient’s optimal tolerability of the regimen. Previous tolerability of each medication must be confirmed by evaluating the patient’s medication history or oral or IM administration of each agent prior to initiating the LAIA.
When choosing 2 agents that are each administered once every 4 weeks, consider administering the medications together every 4 weeks or alternating administration so that the patient receives an injection every 2 weeks. Receiving an injection once every 2 weeks might be beneficial for patients who need close follow-up or are more sensitive to injection site reactions, whereas a regimen of once every 4 weeks might be beneficial for patients who are more resistant to receiving the injections, so there is potentially less time spent agitated or anxious leading up to the date of the injection.
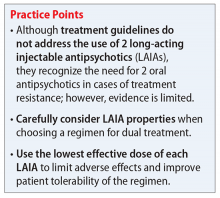
Use the lowest effective dose of each LAIA to limit adverse effects and improve tolerability of the regimen. Monitor patients closely for adverse reactions and discontinue the regimen as soon as possible if a severe adverse reaction occurs.
Cost may influence the decision to use 2 LAIAs. The majority of LAIAs in the United States are available only as branded formulations. Insurance companies may require prior authorization for the use of 2 LAIAs.
Although there are no treatment guidelines for combining 2 LAIAs, this practice has been used. A few case reports have described successful use of dual LAIA treatment, but one should consider the risk of the publication’s bias. Overall, the decision to use 2 LAIAs is difficult because there is lack of a large evidence base supporting the practice or direction from treatment guidelines. Because of this, dual LAIA treatment should not be used for most patients. In cases of treatment-resistant schizophrenia where clozapine is not an option and adherence is a concern, it is reasonable to consider this strategy on a case-by-case basis.
1. Kane J, Honigfeld G, Singer J, et al. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45(9):789-796.
2. Lehman A, Lieberman JA, Dixon LB, et al; American Psychiatric Association; Steering Committee on Practice Guidelines. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(suppl 2):1-56.
3. Hasan A, Falkai P, Wobrock T, et al; the WFSBP Task Force on Treatment Guidelines for Schizophrenia. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia, part 1: update 2012 on the acute treatment of schizophrenia and management of treatment resistance. World J Biol Psychiatry. 2012;13(5):318-78.
4. Barnes TR; Schizophrenia Consensus Group of British Association for Psychopharmacology. Evidence-based guidelines for the pharmacological treatment of schizophrenia: recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2011;25(5):567-620. 5. Hasan A, Falkai P, Wobrock T, et al; WFSBP Task Force on Treatment Guidelines for Schizophrenia. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia, part 2: update 2012 on the long-term treatment of schizophrenia and management of antipsychotic-induced side effects. World J Biol Psychiatry. 2013;14(1):2-44.
6. Kreyenbuhl J, Buchanan RW, Dickerson FB, et al; Schizophrenia Patient Outcomes Research Team (PORT). The Schizophrenia Patient Outcomes Research Team (PORT): updated treatment recommendations 2009. Schizophr Bull. 2010;36(1):94-103.
7. Ladds B, Cosme R, Rivera F. Concurrent use of two depot antipsychotic medications in schizophrenia. The Internet Journal of Psychiatry. 2009;1(1):1-3.
8. Wartelsteiner F, Hofer A. Treating schizophrenia with 2 long-acting injectable antipsychotic drugs: a case report. J Clin Psychopharmacol. 2015;35(4):474-475.
9. Scangos KW, Caton M, Newman WJ. Multiple long-acting injectable antipsychotics for treatment-resistant schizophrenia: case report. J Clin Psychopharmacol. 2016;36(3):283-285.
10. Yazdi K, Rosenleitner J, Pischinger B. Combination of two depot antipsychotic drugs [in German]. Nervenarzt. 2014;85(7):870-871.
11. Ross C, Fabian T. High dose haloperidol decanoate augmentation with paliperidone palmitate. Presented at: College of Psychiatric and Neurologic Pharmacists 16th Annual Meeting; April 21-24, 2013; Colorado Springs, CO.
12. Fluphenazine decanoate [package insert]. Schaumburg, IL: APP Pharmaceuticals, LLC; 2010.
13. Haloperidol decanoate [package insert]. Rockford, IL: Mylan; 2014.
14. Abilify Maintena [package insert]. Rockville, MD: Otsuka America Pharmaceutical, Inc.; 2016.
15. Aristada [package insert]. Waltham, MA: Alkermes; 2016.
16. Zyprexa Relprevv [package insert]. Indianapolis, IN: Lilly USA, LLC; 2016.
17. Invega Sustenna [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2009.
18. Invega Trinza [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2015.
19. Risperdal Consta [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2007.
Ms. S, age 39, with a 15-year history of schizophrenia and severe paranoid delusions, is admitted after physically assaulting a staff member at a group home. She is receiving paliperidone palmitate, 234 mg every 4 weeks. This has reduced the severity of her symptoms, but she continues to have persistent delusions that affect her ability to accept redirection from staff. Ms. S frequently accuses staff and peers of sexual assault, says that she is pregnant, and does not adhere to treatment recommendations for laboratory monitoring because the “staff uses her blood for experiments.”
Ms. S frequently requires administration of oral and IM haloperidol, as needed, when she becomes aggressive with the staff. She has poor insight into her mental illness and does not believe that she needs medication. Ms. S has a long history of stopping her oral antipsychotic after a few days, reporting that it is “harming her baby.” Monotherapy has been tried with various long-acting injectable antipsychotics (LAIAs), but she still exhibits persistent delusions. The treatment team decides to add a second LAIA, haloperidol decanoate, 200 mg every 4 weeks, to her regimen.

Ladds et al.7 A 49-year-old woman with schizophrenia who was hospitalized for aggressive and bizarre behavior and had been institutionalized for 20 years stopped taking her medication regimen.7 She started taking 8-hour showers with bleach, talking incoherently, and believing that someone was poisoning her. She had poor response to oral risperidone monotherapy; however, 2 months after adding oral fluphenazine and benztropine to her regimen, her symptoms substantially improved (doses not reported). Because she had impaired insight into the need for daily medication, she was started on depot fluphenazine decanoate and risperidone microspheres (doses not reported) before discharge. No substantial adverse effects were noted with this regimen.
Wartelsteiner and Hofer.8 A man who had been diagnosed with paranoid schizophrenia at age 20 presented with thought blocking, incoherence, persecutory delusions, and uncontrolled self-damaging behavior.8 He had been admitted 27 times over 7 years; during this time he received many antipsychotic monotherapies and combination regimens. A total of 8 oral antipsychotics (including clozapine) and 5 LAIAs had been administered during these trials. He significantly improved with the combination of olanzapine and risperidone. Both medications were switched to LAIA formulations to address medication nonadherence. His symptoms remained stable with risperidone microspheres, 100 mg, and olanzapine pamoate, 300 mg, each administered every 2 weeks. He did not experience any adverse effects with this combination therapy.
Scangos et al.9 A 26-year-old Vietnamese man with schizophrenia and an extensive history of unprovoked, psychotically driven assaults was given multiple antipsychotics (including clozapine) during hospitalizations, and his medication regimen consistently included 2 antipsychotics. After contracting viral gastroenteritis, he refused oral medications and required short-acting IM administration of both haloperidol, 5 mg, twice a day, and olanzapine, 10 mg, twice a day. Because of concerns about continuing this regimen, he was switched to haloperidol decanoate (dose not reported) and olanzapine pamoate, 405 mg, administered once per month. The injections were scheduled to alternate so that the patient would receive 1 injection every 2 weeks. The patient’s assaultive behavior was significantly reduced, and no adverse effects were reported.
Ross and Fabian.11 An African American man, age 44, was receiving haloperidol decanoate, 400 mg every 2 weeks, and oral haloperidol, 20 mg/d.11 Because of residual symptoms, a history of nonadherence, and concerns about increasing the haloperidol decanoate dose or frequency, oral haloperidol was discontinued and paliperidone palmitate, 156 mg every 4 weeks, was started. The patient was able to transition into a step-down unit, and no adverse effects were reported.
What to consider before initiating dual LAIA treatment
Evaluate the frequency of administration, flexibility of dosing, administration site, adverse effects, and monitoring requirements of each LAIA (Table 212-19) to ensure the patient’s optimal tolerability of the regimen. Previous tolerability of each medication must be confirmed by evaluating the patient’s medication history or oral or IM administration of each agent prior to initiating the LAIA.

When choosing 2 agents that are each administered once every 4 weeks, consider administering the medications together every 4 weeks or alternating administration so that the patient receives an injection every 2 weeks. Receiving an injection once every 2 weeks might be beneficial for patients who need close follow-up or are more sensitive to injection site reactions, whereas a regimen of once every 4 weeks might be beneficial for patients who are more resistant to receiving the injections, so there is potentially less time spent agitated or anxious leading up to the date of the injection.
Use the lowest effective dose of each LAIA to limit adverse effects and improve tolerability of the regimen. Monitor patients closely for adverse reactions and discontinue the regimen as soon as possible if a severe adverse reaction occurs.
Cost may influence the decision to use 2 LAIAs. The majority of LAIAs in the United States are available only as branded formulations. Insurance companies may require prior authorization for the use of 2 LAIAs.
Although there are no treatment guidelines for combining 2 LAIAs, this practice has been used. A few case reports have described successful use of dual LAIA treatment, but one should consider the risk of the publication’s bias. Overall, the decision to use 2 LAIAs is difficult because there is lack of a large evidence base supporting the practice or direction from treatment guidelines. Because of this, dual LAIA treatment should not be used for most patients. In cases of treatment-resistant schizophrenia where clozapine is not an option and adherence is a concern, it is reasonable to consider this strategy on a case-by-case basis.
Ms. S, age 39, with a 15-year history of schizophrenia and severe paranoid delusions, is admitted after physically assaulting a staff member at a group home. She is receiving paliperidone palmitate, 234 mg every 4 weeks. This has reduced the severity of her symptoms, but she continues to have persistent delusions that affect her ability to accept redirection from staff. Ms. S frequently accuses staff and peers of sexual assault, says that she is pregnant, and does not adhere to treatment recommendations for laboratory monitoring because the “staff uses her blood for experiments.”
Ms. S frequently requires administration of oral and IM haloperidol, as needed, when she becomes aggressive with the staff. She has poor insight into her mental illness and does not believe that she needs medication. Ms. S has a long history of stopping her oral antipsychotic after a few days, reporting that it is “harming her baby.” Monotherapy has been tried with various long-acting injectable antipsychotics (LAIAs), but she still exhibits persistent delusions. The treatment team decides to add a second LAIA, haloperidol decanoate, 200 mg every 4 weeks, to her regimen.

Ladds et al.7 A 49-year-old woman with schizophrenia who was hospitalized for aggressive and bizarre behavior and had been institutionalized for 20 years stopped taking her medication regimen.7 She started taking 8-hour showers with bleach, talking incoherently, and believing that someone was poisoning her. She had poor response to oral risperidone monotherapy; however, 2 months after adding oral fluphenazine and benztropine to her regimen, her symptoms substantially improved (doses not reported). Because she had impaired insight into the need for daily medication, she was started on depot fluphenazine decanoate and risperidone microspheres (doses not reported) before discharge. No substantial adverse effects were noted with this regimen.
Wartelsteiner and Hofer.8 A man who had been diagnosed with paranoid schizophrenia at age 20 presented with thought blocking, incoherence, persecutory delusions, and uncontrolled self-damaging behavior.8 He had been admitted 27 times over 7 years; during this time he received many antipsychotic monotherapies and combination regimens. A total of 8 oral antipsychotics (including clozapine) and 5 LAIAs had been administered during these trials. He significantly improved with the combination of olanzapine and risperidone. Both medications were switched to LAIA formulations to address medication nonadherence. His symptoms remained stable with risperidone microspheres, 100 mg, and olanzapine pamoate, 300 mg, each administered every 2 weeks. He did not experience any adverse effects with this combination therapy.
Scangos et al.9 A 26-year-old Vietnamese man with schizophrenia and an extensive history of unprovoked, psychotically driven assaults was given multiple antipsychotics (including clozapine) during hospitalizations, and his medication regimen consistently included 2 antipsychotics. After contracting viral gastroenteritis, he refused oral medications and required short-acting IM administration of both haloperidol, 5 mg, twice a day, and olanzapine, 10 mg, twice a day. Because of concerns about continuing this regimen, he was switched to haloperidol decanoate (dose not reported) and olanzapine pamoate, 405 mg, administered once per month. The injections were scheduled to alternate so that the patient would receive 1 injection every 2 weeks. The patient’s assaultive behavior was significantly reduced, and no adverse effects were reported.
Ross and Fabian.11 An African American man, age 44, was receiving haloperidol decanoate, 400 mg every 2 weeks, and oral haloperidol, 20 mg/d.11 Because of residual symptoms, a history of nonadherence, and concerns about increasing the haloperidol decanoate dose or frequency, oral haloperidol was discontinued and paliperidone palmitate, 156 mg every 4 weeks, was started. The patient was able to transition into a step-down unit, and no adverse effects were reported.
What to consider before initiating dual LAIA treatment
Evaluate the frequency of administration, flexibility of dosing, administration site, adverse effects, and monitoring requirements of each LAIA (Table 212-19) to ensure the patient’s optimal tolerability of the regimen. Previous tolerability of each medication must be confirmed by evaluating the patient’s medication history or oral or IM administration of each agent prior to initiating the LAIA.

When choosing 2 agents that are each administered once every 4 weeks, consider administering the medications together every 4 weeks or alternating administration so that the patient receives an injection every 2 weeks. Receiving an injection once every 2 weeks might be beneficial for patients who need close follow-up or are more sensitive to injection site reactions, whereas a regimen of once every 4 weeks might be beneficial for patients who are more resistant to receiving the injections, so there is potentially less time spent agitated or anxious leading up to the date of the injection.
Use the lowest effective dose of each LAIA to limit adverse effects and improve tolerability of the regimen. Monitor patients closely for adverse reactions and discontinue the regimen as soon as possible if a severe adverse reaction occurs.
Cost may influence the decision to use 2 LAIAs. The majority of LAIAs in the United States are available only as branded formulations. Insurance companies may require prior authorization for the use of 2 LAIAs.
Although there are no treatment guidelines for combining 2 LAIAs, this practice has been used. A few case reports have described successful use of dual LAIA treatment, but one should consider the risk of the publication’s bias. Overall, the decision to use 2 LAIAs is difficult because there is lack of a large evidence base supporting the practice or direction from treatment guidelines. Because of this, dual LAIA treatment should not be used for most patients. In cases of treatment-resistant schizophrenia where clozapine is not an option and adherence is a concern, it is reasonable to consider this strategy on a case-by-case basis.
1. Kane J, Honigfeld G, Singer J, et al. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45(9):789-796.
2. Lehman A, Lieberman JA, Dixon LB, et al; American Psychiatric Association; Steering Committee on Practice Guidelines. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(suppl 2):1-56.
3. Hasan A, Falkai P, Wobrock T, et al; the WFSBP Task Force on Treatment Guidelines for Schizophrenia. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia, part 1: update 2012 on the acute treatment of schizophrenia and management of treatment resistance. World J Biol Psychiatry. 2012;13(5):318-78.
4. Barnes TR; Schizophrenia Consensus Group of British Association for Psychopharmacology. Evidence-based guidelines for the pharmacological treatment of schizophrenia: recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2011;25(5):567-620. 5. Hasan A, Falkai P, Wobrock T, et al; WFSBP Task Force on Treatment Guidelines for Schizophrenia. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia, part 2: update 2012 on the long-term treatment of schizophrenia and management of antipsychotic-induced side effects. World J Biol Psychiatry. 2013;14(1):2-44.
6. Kreyenbuhl J, Buchanan RW, Dickerson FB, et al; Schizophrenia Patient Outcomes Research Team (PORT). The Schizophrenia Patient Outcomes Research Team (PORT): updated treatment recommendations 2009. Schizophr Bull. 2010;36(1):94-103.
7. Ladds B, Cosme R, Rivera F. Concurrent use of two depot antipsychotic medications in schizophrenia. The Internet Journal of Psychiatry. 2009;1(1):1-3.
8. Wartelsteiner F, Hofer A. Treating schizophrenia with 2 long-acting injectable antipsychotic drugs: a case report. J Clin Psychopharmacol. 2015;35(4):474-475.
9. Scangos KW, Caton M, Newman WJ. Multiple long-acting injectable antipsychotics for treatment-resistant schizophrenia: case report. J Clin Psychopharmacol. 2016;36(3):283-285.
10. Yazdi K, Rosenleitner J, Pischinger B. Combination of two depot antipsychotic drugs [in German]. Nervenarzt. 2014;85(7):870-871.
11. Ross C, Fabian T. High dose haloperidol decanoate augmentation with paliperidone palmitate. Presented at: College of Psychiatric and Neurologic Pharmacists 16th Annual Meeting; April 21-24, 2013; Colorado Springs, CO.
12. Fluphenazine decanoate [package insert]. Schaumburg, IL: APP Pharmaceuticals, LLC; 2010.
13. Haloperidol decanoate [package insert]. Rockford, IL: Mylan; 2014.
14. Abilify Maintena [package insert]. Rockville, MD: Otsuka America Pharmaceutical, Inc.; 2016.
15. Aristada [package insert]. Waltham, MA: Alkermes; 2016.
16. Zyprexa Relprevv [package insert]. Indianapolis, IN: Lilly USA, LLC; 2016.
17. Invega Sustenna [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2009.
18. Invega Trinza [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2015.
19. Risperdal Consta [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2007.
1. Kane J, Honigfeld G, Singer J, et al. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45(9):789-796.
2. Lehman A, Lieberman JA, Dixon LB, et al; American Psychiatric Association; Steering Committee on Practice Guidelines. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(suppl 2):1-56.
3. Hasan A, Falkai P, Wobrock T, et al; the WFSBP Task Force on Treatment Guidelines for Schizophrenia. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia, part 1: update 2012 on the acute treatment of schizophrenia and management of treatment resistance. World J Biol Psychiatry. 2012;13(5):318-78.
4. Barnes TR; Schizophrenia Consensus Group of British Association for Psychopharmacology. Evidence-based guidelines for the pharmacological treatment of schizophrenia: recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2011;25(5):567-620. 5. Hasan A, Falkai P, Wobrock T, et al; WFSBP Task Force on Treatment Guidelines for Schizophrenia. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia, part 2: update 2012 on the long-term treatment of schizophrenia and management of antipsychotic-induced side effects. World J Biol Psychiatry. 2013;14(1):2-44.
6. Kreyenbuhl J, Buchanan RW, Dickerson FB, et al; Schizophrenia Patient Outcomes Research Team (PORT). The Schizophrenia Patient Outcomes Research Team (PORT): updated treatment recommendations 2009. Schizophr Bull. 2010;36(1):94-103.
7. Ladds B, Cosme R, Rivera F. Concurrent use of two depot antipsychotic medications in schizophrenia. The Internet Journal of Psychiatry. 2009;1(1):1-3.
8. Wartelsteiner F, Hofer A. Treating schizophrenia with 2 long-acting injectable antipsychotic drugs: a case report. J Clin Psychopharmacol. 2015;35(4):474-475.
9. Scangos KW, Caton M, Newman WJ. Multiple long-acting injectable antipsychotics for treatment-resistant schizophrenia: case report. J Clin Psychopharmacol. 2016;36(3):283-285.
10. Yazdi K, Rosenleitner J, Pischinger B. Combination of two depot antipsychotic drugs [in German]. Nervenarzt. 2014;85(7):870-871.
11. Ross C, Fabian T. High dose haloperidol decanoate augmentation with paliperidone palmitate. Presented at: College of Psychiatric and Neurologic Pharmacists 16th Annual Meeting; April 21-24, 2013; Colorado Springs, CO.
12. Fluphenazine decanoate [package insert]. Schaumburg, IL: APP Pharmaceuticals, LLC; 2010.
13. Haloperidol decanoate [package insert]. Rockford, IL: Mylan; 2014.
14. Abilify Maintena [package insert]. Rockville, MD: Otsuka America Pharmaceutical, Inc.; 2016.
15. Aristada [package insert]. Waltham, MA: Alkermes; 2016.
16. Zyprexa Relprevv [package insert]. Indianapolis, IN: Lilly USA, LLC; 2016.
17. Invega Sustenna [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2009.
18. Invega Trinza [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2015.
19. Risperdal Consta [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2007.
The girl who couldn’t stop stealing
CASE A lifelong habit
Ms. B, age 14, has diagnoses of attention-deficit/hyperactive disorder (ADHD) and oppositional defiant disorder, and is taking extended-release (ER) methylphenidate, 36 mg/d. Her mother brings her to the hospital with concerns that Ms. B has been stealing small objects, such as money, toys, and pencils from home, school, and her peers, even though she does not need them and her family can afford to buy them for her. Ms. B’s mother routinely searches her daughter when she leaves the house and when she returns and frequently finds things in Ms. B’s possession that do not belong to her.
The mother reports that Ms. B’s stealing has been a lifelong habit that worsened after Ms. B’s father died in a car accident last year.
Ms. B does not volunteer any information about her stealing. She is admitted to a partial hospitalization program for further evaluation and treatment.
[polldaddy:9837962]
EVALUATION Continued stealing
A week later, Ms. B remains reluctant to talk about her stealing habit. However, once a therapeutic alliance is established, she reveals that she experiences increased anxiety before stealing and feels pleasure during the theft. Her methylphenidate ER dosage is increased to 54 mg/d in an attempt to address poor impulse control and subsequent stealing behavior. Her ADHD symptoms are controlled, and she does not exhibit poor impulse control in any situation other than stealing.
However, Ms. B continues to have poor insight and impaired judgment about her behavior. During treatment, Ms. B steals markers from the psychiatrist’s office, which later are found in her bag. When the staff convinces Ms. B to return the markers to the psychiatrist, she denies knowing how they got there. Behavioral interventions, including covert sensitization, systemic desensitization, positive reinforcement, body and bag search, and reminders, occur consistently as part of treatment, but have little effect on her symptoms.
The author’s observations
Risk-taking and novelty-seeking behaviors are common in adolescent patients. Impulsivity, instant reward-seeking behavior, and poor judgment can lead to stealing in this population, but this behavior is not necessarily indicative of kleptomania.
Kleptomania is the recurrent failure to resist impulses to steal objects.2 It differs from other forms of stealing in that the objects stolen by a patient with kleptomania are not needed for personal use or for their monetary value. Kleptomania usually begins in early adolescence, is found in about 0.5% of the general population, and is more common among females.3

There are 2 important theories to explain kleptomania:
- The psychoanalytical theory explains kleptomania as an immature defense against unconscious impulses, conflicts, and desires of destruction. By stealing, the individual protects the self from narcissistic injury and disintegration. The frantic search for objects helps to divert self-destructive aggressiveness and allows for the preservation of the self.4
- The biological model indicates that individuals with kleptomania have a significant deficit of white matter in inferior frontal regions and poor integrity of the tracts connecting the limbic system to the thalamus and to the prefrontal cortex.5 Reward system circuitry (ventral tegmental area–nucleus accumbens–orbital frontal cortex) is likely to be involved in impulse control disorders including kleptomania.6
Comorbidity. Kleptomania often is comorbid with substance use disorder (SUD), obsessive-compulsive disorder (OCD), and compulsive shopping, as well as depression, anxiety disorders, bulimia nervosa, and impulse control and conduct disorders.3,6
Kleptomania shares many characteristics with SUD, including continued engagement in a behavior despite negative consequences and the temporary reduction in urges after the behavior’s completion, followed by a return of the urge to steal. There also is a bidirectional relationship between OCD and kleptomania. Individuals with both disorders frequently engage in excessive and unnecessary rituals even when it is ego-dystonic. First-degree relatives of kleptomania patients have high rates of SUD and OCD.3
Serotonin, dopamine, and opioid pathways play a role in both kleptomania and other behavioral addictions.6 Clinicians should be cautious in treating comorbid disorders with stimulants. These agents may help patients with high impulsivity, but lead to disinhibition and worsen impulse control in patients with low impulsivity.7
TREATMENT Naltrexone
The psychiatrist discusses pharmacologic options to treat kleptomania with Ms. B and her mother. After considering the risks, benefits, adverse effects, and alternative treatments (including the option of no pharmacologic treatment), the mother consents and Ms. B assents to treatment with naltrexone, 25 mg/d. Before starting this medication, both the mother and Ms. B receive detailed psychoeducation describing naltrexone’s interactions with opioids. They are told that if Ms. B has a traumatic injury, they should inform the treatment team that she is taking naltrexone, which can acutely precipitate opiate withdrawal.
Before initiating pharmacotherapy, a comprehensive metabolic profile is obtained, and all values are within the normal range. After 1 week, naltrexone is increased to 50 mg/d. The medication is well tolerated, without any adverse effects.
[polldaddy:9837976]
The author’s observations
Behavioral interventions, such as covert sensitization and systemic desensitization, often are used to treat kleptomania.8 There are no FDA-approved medications for this condition. Opioid antagonists have been considered for the treatment of kleptomania.7
Mu-opioid receptors exist in highest concentrations in presynaptic neurons in the periaqueductal gray region and spinal cord and have high affinity for enkephalins and beta-endorphins. They also are involved in the reward and pleasure pathway. This neurocircuit is implicated in behavioral addiction.9
Naltrexone is an antagonist at μ-opioid receptors. It blocks the binding of endogenous and exogenous opioids at the receptors, particularly at the ventral tegmental area. By blocking the μ-receptor, naltrexone inhibits the processing of the reward and pleasure pathway involved in kleptomania. Naltrexone binds to these receptors, preventing the euphoric effects of behavioral addictions.10 This medication works best in conjunction with behavioral interventions.8
Naltrexone is a Schedule II drug. Use of naltrexone to treat kleptomania or other impulse control disorders is an off-label use of the medication. Naltrexone should not be prescribed to patients who are receiving opiates because it can cause acute opiate withdrawal.
Liver function tests should be monitored in all patients taking naltrexone. If liver function levels begin to rise, naltrexone should be discontinued. Naltrexone should be used with caution in patients with preexisting liver disease.11
OUTCOME Marked improvement
Ms. B’s K-SAS scores are evaluated 2 weeks after starting naltrexone. The results show a marked reduction in the urge to steal and in stealing behavior, and Ms. B’s mother reports no incidents of stealing in the previous week.
Ms. B is maintained on naltrexone, 50 mg/d, for 2 months. On repeated K-SAS scores, her mother rates Ms. B’s symptoms “very much improved” with “occasional” stealing. Ms. B is discharged from the intensive outpatient program.
1. Christianini AR, Conti MA, Hearst N, et al. Treating kleptomania: cross-cultural adaptation of the Kleptomania Symptom Assessment Scale and assessment of an outpatient program. Compr Psychiatry. 2015;56:289-294.
2. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
3. Talih FR. Kleptomania and potential exacerbating factors: a review and case report. Innov Clin Neurosci. 2011;8(10):35-39.
4. Cierpka M. Psychodynamics of neurotically-induced kleptomania [in German]. Psychiatr Prax. 1986;13(3):94-103.
5. Grant JE, Correia S, Brennan-Krohn T. White matter integrity in kleptomania: a pilot study. Psychiatry Res. 2006;147(2-3):233-237.
6. Grant JE, Odlaug BL, Kim SW. Kleptomania: clinical characteristics and relationship to substance use disorders. Am J Drug Alcohol Abuse. 2010;36(5):291-295.
7. Zack M, Poulos CX. Effects of the atypical stimulant modafinil on a brief gambling episode in pathological gamblers with high vs. low impulsivity. J Psychopharmacol. 2009;23(6):660-671.
8. Grant JE. Understanding and treating kleptomania: new models and new treatments. Isr J Psychiatry Relat Sci. 2006;43(2):81-87.
9. Potenza MN. Should addictive disorders include non-substance-related conditions? Addiction. 2006;101(suppl 1):142-151.
10. Grant JE, Kim SW. An open-label study of naltrexone in the treatment of kleptomania. J Clin Psychiatry. 2002;63(4):349-356.
11. Pfohl DN, Allen JI, Atkinson RL, et al. Naltrexone hydrochloride (Trexan): a review of serum transaminase elevations at high dosage. NIDA Res Monogr. 1986;67:66-72.
CASE A lifelong habit
Ms. B, age 14, has diagnoses of attention-deficit/hyperactive disorder (ADHD) and oppositional defiant disorder, and is taking extended-release (ER) methylphenidate, 36 mg/d. Her mother brings her to the hospital with concerns that Ms. B has been stealing small objects, such as money, toys, and pencils from home, school, and her peers, even though she does not need them and her family can afford to buy them for her. Ms. B’s mother routinely searches her daughter when she leaves the house and when she returns and frequently finds things in Ms. B’s possession that do not belong to her.
The mother reports that Ms. B’s stealing has been a lifelong habit that worsened after Ms. B’s father died in a car accident last year.
Ms. B does not volunteer any information about her stealing. She is admitted to a partial hospitalization program for further evaluation and treatment.
[polldaddy:9837962]
EVALUATION Continued stealing
A week later, Ms. B remains reluctant to talk about her stealing habit. However, once a therapeutic alliance is established, she reveals that she experiences increased anxiety before stealing and feels pleasure during the theft. Her methylphenidate ER dosage is increased to 54 mg/d in an attempt to address poor impulse control and subsequent stealing behavior. Her ADHD symptoms are controlled, and she does not exhibit poor impulse control in any situation other than stealing.
However, Ms. B continues to have poor insight and impaired judgment about her behavior. During treatment, Ms. B steals markers from the psychiatrist’s office, which later are found in her bag. When the staff convinces Ms. B to return the markers to the psychiatrist, she denies knowing how they got there. Behavioral interventions, including covert sensitization, systemic desensitization, positive reinforcement, body and bag search, and reminders, occur consistently as part of treatment, but have little effect on her symptoms.
The author’s observations
Risk-taking and novelty-seeking behaviors are common in adolescent patients. Impulsivity, instant reward-seeking behavior, and poor judgment can lead to stealing in this population, but this behavior is not necessarily indicative of kleptomania.
Kleptomania is the recurrent failure to resist impulses to steal objects.2 It differs from other forms of stealing in that the objects stolen by a patient with kleptomania are not needed for personal use or for their monetary value. Kleptomania usually begins in early adolescence, is found in about 0.5% of the general population, and is more common among females.3

There are 2 important theories to explain kleptomania:
- The psychoanalytical theory explains kleptomania as an immature defense against unconscious impulses, conflicts, and desires of destruction. By stealing, the individual protects the self from narcissistic injury and disintegration. The frantic search for objects helps to divert self-destructive aggressiveness and allows for the preservation of the self.4
- The biological model indicates that individuals with kleptomania have a significant deficit of white matter in inferior frontal regions and poor integrity of the tracts connecting the limbic system to the thalamus and to the prefrontal cortex.5 Reward system circuitry (ventral tegmental area–nucleus accumbens–orbital frontal cortex) is likely to be involved in impulse control disorders including kleptomania.6
Comorbidity. Kleptomania often is comorbid with substance use disorder (SUD), obsessive-compulsive disorder (OCD), and compulsive shopping, as well as depression, anxiety disorders, bulimia nervosa, and impulse control and conduct disorders.3,6
Kleptomania shares many characteristics with SUD, including continued engagement in a behavior despite negative consequences and the temporary reduction in urges after the behavior’s completion, followed by a return of the urge to steal. There also is a bidirectional relationship between OCD and kleptomania. Individuals with both disorders frequently engage in excessive and unnecessary rituals even when it is ego-dystonic. First-degree relatives of kleptomania patients have high rates of SUD and OCD.3
Serotonin, dopamine, and opioid pathways play a role in both kleptomania and other behavioral addictions.6 Clinicians should be cautious in treating comorbid disorders with stimulants. These agents may help patients with high impulsivity, but lead to disinhibition and worsen impulse control in patients with low impulsivity.7
TREATMENT Naltrexone
The psychiatrist discusses pharmacologic options to treat kleptomania with Ms. B and her mother. After considering the risks, benefits, adverse effects, and alternative treatments (including the option of no pharmacologic treatment), the mother consents and Ms. B assents to treatment with naltrexone, 25 mg/d. Before starting this medication, both the mother and Ms. B receive detailed psychoeducation describing naltrexone’s interactions with opioids. They are told that if Ms. B has a traumatic injury, they should inform the treatment team that she is taking naltrexone, which can acutely precipitate opiate withdrawal.
Before initiating pharmacotherapy, a comprehensive metabolic profile is obtained, and all values are within the normal range. After 1 week, naltrexone is increased to 50 mg/d. The medication is well tolerated, without any adverse effects.
[polldaddy:9837976]
The author’s observations
Behavioral interventions, such as covert sensitization and systemic desensitization, often are used to treat kleptomania.8 There are no FDA-approved medications for this condition. Opioid antagonists have been considered for the treatment of kleptomania.7
Mu-opioid receptors exist in highest concentrations in presynaptic neurons in the periaqueductal gray region and spinal cord and have high affinity for enkephalins and beta-endorphins. They also are involved in the reward and pleasure pathway. This neurocircuit is implicated in behavioral addiction.9
Naltrexone is an antagonist at μ-opioid receptors. It blocks the binding of endogenous and exogenous opioids at the receptors, particularly at the ventral tegmental area. By blocking the μ-receptor, naltrexone inhibits the processing of the reward and pleasure pathway involved in kleptomania. Naltrexone binds to these receptors, preventing the euphoric effects of behavioral addictions.10 This medication works best in conjunction with behavioral interventions.8
Naltrexone is a Schedule II drug. Use of naltrexone to treat kleptomania or other impulse control disorders is an off-label use of the medication. Naltrexone should not be prescribed to patients who are receiving opiates because it can cause acute opiate withdrawal.
Liver function tests should be monitored in all patients taking naltrexone. If liver function levels begin to rise, naltrexone should be discontinued. Naltrexone should be used with caution in patients with preexisting liver disease.11
OUTCOME Marked improvement
Ms. B’s K-SAS scores are evaluated 2 weeks after starting naltrexone. The results show a marked reduction in the urge to steal and in stealing behavior, and Ms. B’s mother reports no incidents of stealing in the previous week.
Ms. B is maintained on naltrexone, 50 mg/d, for 2 months. On repeated K-SAS scores, her mother rates Ms. B’s symptoms “very much improved” with “occasional” stealing. Ms. B is discharged from the intensive outpatient program.
CASE A lifelong habit
Ms. B, age 14, has diagnoses of attention-deficit/hyperactive disorder (ADHD) and oppositional defiant disorder, and is taking extended-release (ER) methylphenidate, 36 mg/d. Her mother brings her to the hospital with concerns that Ms. B has been stealing small objects, such as money, toys, and pencils from home, school, and her peers, even though she does not need them and her family can afford to buy them for her. Ms. B’s mother routinely searches her daughter when she leaves the house and when she returns and frequently finds things in Ms. B’s possession that do not belong to her.
The mother reports that Ms. B’s stealing has been a lifelong habit that worsened after Ms. B’s father died in a car accident last year.
Ms. B does not volunteer any information about her stealing. She is admitted to a partial hospitalization program for further evaluation and treatment.
[polldaddy:9837962]
EVALUATION Continued stealing
A week later, Ms. B remains reluctant to talk about her stealing habit. However, once a therapeutic alliance is established, she reveals that she experiences increased anxiety before stealing and feels pleasure during the theft. Her methylphenidate ER dosage is increased to 54 mg/d in an attempt to address poor impulse control and subsequent stealing behavior. Her ADHD symptoms are controlled, and she does not exhibit poor impulse control in any situation other than stealing.
However, Ms. B continues to have poor insight and impaired judgment about her behavior. During treatment, Ms. B steals markers from the psychiatrist’s office, which later are found in her bag. When the staff convinces Ms. B to return the markers to the psychiatrist, she denies knowing how they got there. Behavioral interventions, including covert sensitization, systemic desensitization, positive reinforcement, body and bag search, and reminders, occur consistently as part of treatment, but have little effect on her symptoms.
The author’s observations
Risk-taking and novelty-seeking behaviors are common in adolescent patients. Impulsivity, instant reward-seeking behavior, and poor judgment can lead to stealing in this population, but this behavior is not necessarily indicative of kleptomania.
Kleptomania is the recurrent failure to resist impulses to steal objects.2 It differs from other forms of stealing in that the objects stolen by a patient with kleptomania are not needed for personal use or for their monetary value. Kleptomania usually begins in early adolescence, is found in about 0.5% of the general population, and is more common among females.3

There are 2 important theories to explain kleptomania:
- The psychoanalytical theory explains kleptomania as an immature defense against unconscious impulses, conflicts, and desires of destruction. By stealing, the individual protects the self from narcissistic injury and disintegration. The frantic search for objects helps to divert self-destructive aggressiveness and allows for the preservation of the self.4
- The biological model indicates that individuals with kleptomania have a significant deficit of white matter in inferior frontal regions and poor integrity of the tracts connecting the limbic system to the thalamus and to the prefrontal cortex.5 Reward system circuitry (ventral tegmental area–nucleus accumbens–orbital frontal cortex) is likely to be involved in impulse control disorders including kleptomania.6
Comorbidity. Kleptomania often is comorbid with substance use disorder (SUD), obsessive-compulsive disorder (OCD), and compulsive shopping, as well as depression, anxiety disorders, bulimia nervosa, and impulse control and conduct disorders.3,6
Kleptomania shares many characteristics with SUD, including continued engagement in a behavior despite negative consequences and the temporary reduction in urges after the behavior’s completion, followed by a return of the urge to steal. There also is a bidirectional relationship between OCD and kleptomania. Individuals with both disorders frequently engage in excessive and unnecessary rituals even when it is ego-dystonic. First-degree relatives of kleptomania patients have high rates of SUD and OCD.3
Serotonin, dopamine, and opioid pathways play a role in both kleptomania and other behavioral addictions.6 Clinicians should be cautious in treating comorbid disorders with stimulants. These agents may help patients with high impulsivity, but lead to disinhibition and worsen impulse control in patients with low impulsivity.7
TREATMENT Naltrexone
The psychiatrist discusses pharmacologic options to treat kleptomania with Ms. B and her mother. After considering the risks, benefits, adverse effects, and alternative treatments (including the option of no pharmacologic treatment), the mother consents and Ms. B assents to treatment with naltrexone, 25 mg/d. Before starting this medication, both the mother and Ms. B receive detailed psychoeducation describing naltrexone’s interactions with opioids. They are told that if Ms. B has a traumatic injury, they should inform the treatment team that she is taking naltrexone, which can acutely precipitate opiate withdrawal.
Before initiating pharmacotherapy, a comprehensive metabolic profile is obtained, and all values are within the normal range. After 1 week, naltrexone is increased to 50 mg/d. The medication is well tolerated, without any adverse effects.
[polldaddy:9837976]
The author’s observations
Behavioral interventions, such as covert sensitization and systemic desensitization, often are used to treat kleptomania.8 There are no FDA-approved medications for this condition. Opioid antagonists have been considered for the treatment of kleptomania.7
Mu-opioid receptors exist in highest concentrations in presynaptic neurons in the periaqueductal gray region and spinal cord and have high affinity for enkephalins and beta-endorphins. They also are involved in the reward and pleasure pathway. This neurocircuit is implicated in behavioral addiction.9
Naltrexone is an antagonist at μ-opioid receptors. It blocks the binding of endogenous and exogenous opioids at the receptors, particularly at the ventral tegmental area. By blocking the μ-receptor, naltrexone inhibits the processing of the reward and pleasure pathway involved in kleptomania. Naltrexone binds to these receptors, preventing the euphoric effects of behavioral addictions.10 This medication works best in conjunction with behavioral interventions.8
Naltrexone is a Schedule II drug. Use of naltrexone to treat kleptomania or other impulse control disorders is an off-label use of the medication. Naltrexone should not be prescribed to patients who are receiving opiates because it can cause acute opiate withdrawal.
Liver function tests should be monitored in all patients taking naltrexone. If liver function levels begin to rise, naltrexone should be discontinued. Naltrexone should be used with caution in patients with preexisting liver disease.11
OUTCOME Marked improvement
Ms. B’s K-SAS scores are evaluated 2 weeks after starting naltrexone. The results show a marked reduction in the urge to steal and in stealing behavior, and Ms. B’s mother reports no incidents of stealing in the previous week.
Ms. B is maintained on naltrexone, 50 mg/d, for 2 months. On repeated K-SAS scores, her mother rates Ms. B’s symptoms “very much improved” with “occasional” stealing. Ms. B is discharged from the intensive outpatient program.
1. Christianini AR, Conti MA, Hearst N, et al. Treating kleptomania: cross-cultural adaptation of the Kleptomania Symptom Assessment Scale and assessment of an outpatient program. Compr Psychiatry. 2015;56:289-294.
2. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
3. Talih FR. Kleptomania and potential exacerbating factors: a review and case report. Innov Clin Neurosci. 2011;8(10):35-39.
4. Cierpka M. Psychodynamics of neurotically-induced kleptomania [in German]. Psychiatr Prax. 1986;13(3):94-103.
5. Grant JE, Correia S, Brennan-Krohn T. White matter integrity in kleptomania: a pilot study. Psychiatry Res. 2006;147(2-3):233-237.
6. Grant JE, Odlaug BL, Kim SW. Kleptomania: clinical characteristics and relationship to substance use disorders. Am J Drug Alcohol Abuse. 2010;36(5):291-295.
7. Zack M, Poulos CX. Effects of the atypical stimulant modafinil on a brief gambling episode in pathological gamblers with high vs. low impulsivity. J Psychopharmacol. 2009;23(6):660-671.
8. Grant JE. Understanding and treating kleptomania: new models and new treatments. Isr J Psychiatry Relat Sci. 2006;43(2):81-87.
9. Potenza MN. Should addictive disorders include non-substance-related conditions? Addiction. 2006;101(suppl 1):142-151.
10. Grant JE, Kim SW. An open-label study of naltrexone in the treatment of kleptomania. J Clin Psychiatry. 2002;63(4):349-356.
11. Pfohl DN, Allen JI, Atkinson RL, et al. Naltrexone hydrochloride (Trexan): a review of serum transaminase elevations at high dosage. NIDA Res Monogr. 1986;67:66-72.
1. Christianini AR, Conti MA, Hearst N, et al. Treating kleptomania: cross-cultural adaptation of the Kleptomania Symptom Assessment Scale and assessment of an outpatient program. Compr Psychiatry. 2015;56:289-294.
2. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
3. Talih FR. Kleptomania and potential exacerbating factors: a review and case report. Innov Clin Neurosci. 2011;8(10):35-39.
4. Cierpka M. Psychodynamics of neurotically-induced kleptomania [in German]. Psychiatr Prax. 1986;13(3):94-103.
5. Grant JE, Correia S, Brennan-Krohn T. White matter integrity in kleptomania: a pilot study. Psychiatry Res. 2006;147(2-3):233-237.
6. Grant JE, Odlaug BL, Kim SW. Kleptomania: clinical characteristics and relationship to substance use disorders. Am J Drug Alcohol Abuse. 2010;36(5):291-295.
7. Zack M, Poulos CX. Effects of the atypical stimulant modafinil on a brief gambling episode in pathological gamblers with high vs. low impulsivity. J Psychopharmacol. 2009;23(6):660-671.
8. Grant JE. Understanding and treating kleptomania: new models and new treatments. Isr J Psychiatry Relat Sci. 2006;43(2):81-87.
9. Potenza MN. Should addictive disorders include non-substance-related conditions? Addiction. 2006;101(suppl 1):142-151.
10. Grant JE, Kim SW. An open-label study of naltrexone in the treatment of kleptomania. J Clin Psychiatry. 2002;63(4):349-356.
11. Pfohl DN, Allen JI, Atkinson RL, et al. Naltrexone hydrochloride (Trexan): a review of serum transaminase elevations at high dosage. NIDA Res Monogr. 1986;67:66-72.
Physician impairment
Most physicians are likely familiar with guidelines relating to physician impairment, but they may not be aware that these guidelines typically conflict with the Americans with Disabilities Act (ADA), which protects all employees against unwarranted requests for mental health information or evaluations.
Under the ADA, employers cannot request mental health information from their employees or refer them for mental health evaluations without objective evidence showing that either the employee:
- is unable to perform essential job functions because of a mental health condition
- poses a high risk of substantial, imminent harm to himself (herself) or others in the workplace because of a mental health condition.1
Employers cannot rely on speculative evidence or generalizations about these conditions when making these determinations,1 and common mental disorders (eg, depressive disorders, anxiety disorders, attention-deficit/hyperactivity disorder, specific learning disorders, etc.) should almost never form the basis of such requests.2
In contrast, the American Medical Association (AMA) does not distinguish between the presence of a mental health condition and physician impairment,3,4 which may result in unwarranted requests and referrals for mental health evaluations. Some state laws on impairment, which all derive from AMA policies,5 even state outright that, “‘Impaired’ or ‘impairment’ means the presence of the diseases of alcoholism, drug abuse, or mental illness”6 and directly discriminate against physicians with these conditions.
State physician health programs (PHPs) also may describe impairment in problematic ways (eg, “Involvement in litigation against hospital”).7 Their descriptions also are overly inclusive in that they could be used to describe most physicians (N.D.L., J.W.B., unpublished data, 2017), and they rarely represent sufficient legal indications for a mental health evaluation under the ADA (N.D.L., J.W.B., unpublished data, 2017). Even the APA’s Clinical Guide to Psychiatric Ethics describes physician impairment as synonymous with mental illness.8
Requests for mental health information or evaluations not only can include referrals to state PHPs but also “suggestions” to see a psychologist, professional job coach, or any provider who may ask for mental health information. Under the ADA's guidelines, obtaining “voluntary” consent from an employee who could be fired for not cooperating does not change the involuntary nature of these requests.2,9
Employers who hire psychiatrists, physicians, and medical residents should comply with the ADA and disregard the AMA’s policies, state laws, PHPs, other institutional guidelines,10 and guidance from some articles published in
1. U.S. Equal Employment Opportunity Commission. EEOC enforcement guidance on the Americans with Disabilities Act and psychiatric disabilities. No. 915.002. http://www.eeoc.gov/policy/docs/psych.html. Updated March 9, 2009. Accessed July 20, 2017.
2. Lawson ND, Kalet AL. The administrative psychiatric evaluation. J Grad Med Educ. 2016;8(1):14-17.
3. American Medical Association. Physician impairment H-95.955: Drug Abuse. https://policy search.ama-assn.org/policyfinder/detail/physician%20impairment?uri=%2FAMADoc%2FHOD.xml-0-5334.xml. Updated 2009. Accessed April 20, 2017.
4. Myers MF, Gabbard GO. The physician as patient: a clinical handbook for mental health professionals. Arlington, VA: American Psychiatric Publishing, Inc.; 2008.
5. Sargent DA. The impaired physician movement: an interim report. Hosp Community Psychiatry. 1985;36(3):294-297.
6. Arkansas State Medical Board. Arkansas medical practices act and regulations. http://www.armedicalboard.org/professionals/pdf/mpa.pdf. Revised March 2017. Accessed July 11, 2017.
7. Oklahoma Health Professionals Program. Chemical dependency. https://www.okhpp.org/chemical-dependency. Accessed September 15, 2017.
8. Trockel M, Miller MN, Roberts LW. Clinician well-being and impairment. In: Roberts LW, ed. A clinical guide to psychiatric ethics. Arlington, VA: American Psychiatric Publishing, Inc.; 2016:223-236.
9. U.S. Equal Employment Opportunity Commission. Regulations under the Americans with Disabilities Act. Federal Register. https://www.gpo.gov/fdsys/pkg/FR-2016-05-17/pdf/2016-11558.pdf. Published May 17, 2016. Accessed August 2
10. Lawson ND. Comply with federal laws before checking institutional guidelines on resident referrals for psychiatric evaluations. J Grad Med Educ. In press.
11. Bright RP, Krahn L. Impaired physicians: how to recognize, when to report, and where to refer. Current Psychiatry. 2010;9(6):11-20.
12. Mossman D, Farrell HM. Physician impairment: when should you report? Current Psychiatry. 2011;10(9):67-71.
Most physicians are likely familiar with guidelines relating to physician impairment, but they may not be aware that these guidelines typically conflict with the Americans with Disabilities Act (ADA), which protects all employees against unwarranted requests for mental health information or evaluations.
Under the ADA, employers cannot request mental health information from their employees or refer them for mental health evaluations without objective evidence showing that either the employee:
- is unable to perform essential job functions because of a mental health condition
- poses a high risk of substantial, imminent harm to himself (herself) or others in the workplace because of a mental health condition.1
Employers cannot rely on speculative evidence or generalizations about these conditions when making these determinations,1 and common mental disorders (eg, depressive disorders, anxiety disorders, attention-deficit/hyperactivity disorder, specific learning disorders, etc.) should almost never form the basis of such requests.2
In contrast, the American Medical Association (AMA) does not distinguish between the presence of a mental health condition and physician impairment,3,4 which may result in unwarranted requests and referrals for mental health evaluations. Some state laws on impairment, which all derive from AMA policies,5 even state outright that, “‘Impaired’ or ‘impairment’ means the presence of the diseases of alcoholism, drug abuse, or mental illness”6 and directly discriminate against physicians with these conditions.
State physician health programs (PHPs) also may describe impairment in problematic ways (eg, “Involvement in litigation against hospital”).7 Their descriptions also are overly inclusive in that they could be used to describe most physicians (N.D.L., J.W.B., unpublished data, 2017), and they rarely represent sufficient legal indications for a mental health evaluation under the ADA (N.D.L., J.W.B., unpublished data, 2017). Even the APA’s Clinical Guide to Psychiatric Ethics describes physician impairment as synonymous with mental illness.8
Requests for mental health information or evaluations not only can include referrals to state PHPs but also “suggestions” to see a psychologist, professional job coach, or any provider who may ask for mental health information. Under the ADA's guidelines, obtaining “voluntary” consent from an employee who could be fired for not cooperating does not change the involuntary nature of these requests.2,9
Employers who hire psychiatrists, physicians, and medical residents should comply with the ADA and disregard the AMA’s policies, state laws, PHPs, other institutional guidelines,10 and guidance from some articles published in
Most physicians are likely familiar with guidelines relating to physician impairment, but they may not be aware that these guidelines typically conflict with the Americans with Disabilities Act (ADA), which protects all employees against unwarranted requests for mental health information or evaluations.
Under the ADA, employers cannot request mental health information from their employees or refer them for mental health evaluations without objective evidence showing that either the employee:
- is unable to perform essential job functions because of a mental health condition
- poses a high risk of substantial, imminent harm to himself (herself) or others in the workplace because of a mental health condition.1
Employers cannot rely on speculative evidence or generalizations about these conditions when making these determinations,1 and common mental disorders (eg, depressive disorders, anxiety disorders, attention-deficit/hyperactivity disorder, specific learning disorders, etc.) should almost never form the basis of such requests.2
In contrast, the American Medical Association (AMA) does not distinguish between the presence of a mental health condition and physician impairment,3,4 which may result in unwarranted requests and referrals for mental health evaluations. Some state laws on impairment, which all derive from AMA policies,5 even state outright that, “‘Impaired’ or ‘impairment’ means the presence of the diseases of alcoholism, drug abuse, or mental illness”6 and directly discriminate against physicians with these conditions.
State physician health programs (PHPs) also may describe impairment in problematic ways (eg, “Involvement in litigation against hospital”).7 Their descriptions also are overly inclusive in that they could be used to describe most physicians (N.D.L., J.W.B., unpublished data, 2017), and they rarely represent sufficient legal indications for a mental health evaluation under the ADA (N.D.L., J.W.B., unpublished data, 2017). Even the APA’s Clinical Guide to Psychiatric Ethics describes physician impairment as synonymous with mental illness.8
Requests for mental health information or evaluations not only can include referrals to state PHPs but also “suggestions” to see a psychologist, professional job coach, or any provider who may ask for mental health information. Under the ADA's guidelines, obtaining “voluntary” consent from an employee who could be fired for not cooperating does not change the involuntary nature of these requests.2,9
Employers who hire psychiatrists, physicians, and medical residents should comply with the ADA and disregard the AMA’s policies, state laws, PHPs, other institutional guidelines,10 and guidance from some articles published in
1. U.S. Equal Employment Opportunity Commission. EEOC enforcement guidance on the Americans with Disabilities Act and psychiatric disabilities. No. 915.002. http://www.eeoc.gov/policy/docs/psych.html. Updated March 9, 2009. Accessed July 20, 2017.
2. Lawson ND, Kalet AL. The administrative psychiatric evaluation. J Grad Med Educ. 2016;8(1):14-17.
3. American Medical Association. Physician impairment H-95.955: Drug Abuse. https://policy search.ama-assn.org/policyfinder/detail/physician%20impairment?uri=%2FAMADoc%2FHOD.xml-0-5334.xml. Updated 2009. Accessed April 20, 2017.
4. Myers MF, Gabbard GO. The physician as patient: a clinical handbook for mental health professionals. Arlington, VA: American Psychiatric Publishing, Inc.; 2008.
5. Sargent DA. The impaired physician movement: an interim report. Hosp Community Psychiatry. 1985;36(3):294-297.
6. Arkansas State Medical Board. Arkansas medical practices act and regulations. http://www.armedicalboard.org/professionals/pdf/mpa.pdf. Revised March 2017. Accessed July 11, 2017.
7. Oklahoma Health Professionals Program. Chemical dependency. https://www.okhpp.org/chemical-dependency. Accessed September 15, 2017.
8. Trockel M, Miller MN, Roberts LW. Clinician well-being and impairment. In: Roberts LW, ed. A clinical guide to psychiatric ethics. Arlington, VA: American Psychiatric Publishing, Inc.; 2016:223-236.
9. U.S. Equal Employment Opportunity Commission. Regulations under the Americans with Disabilities Act. Federal Register. https://www.gpo.gov/fdsys/pkg/FR-2016-05-17/pdf/2016-11558.pdf. Published May 17, 2016. Accessed August 2
10. Lawson ND. Comply with federal laws before checking institutional guidelines on resident referrals for psychiatric evaluations. J Grad Med Educ. In press.
11. Bright RP, Krahn L. Impaired physicians: how to recognize, when to report, and where to refer. Current Psychiatry. 2010;9(6):11-20.
12. Mossman D, Farrell HM. Physician impairment: when should you report? Current Psychiatry. 2011;10(9):67-71.
1. U.S. Equal Employment Opportunity Commission. EEOC enforcement guidance on the Americans with Disabilities Act and psychiatric disabilities. No. 915.002. http://www.eeoc.gov/policy/docs/psych.html. Updated March 9, 2009. Accessed July 20, 2017.
2. Lawson ND, Kalet AL. The administrative psychiatric evaluation. J Grad Med Educ. 2016;8(1):14-17.
3. American Medical Association. Physician impairment H-95.955: Drug Abuse. https://policy search.ama-assn.org/policyfinder/detail/physician%20impairment?uri=%2FAMADoc%2FHOD.xml-0-5334.xml. Updated 2009. Accessed April 20, 2017.
4. Myers MF, Gabbard GO. The physician as patient: a clinical handbook for mental health professionals. Arlington, VA: American Psychiatric Publishing, Inc.; 2008.
5. Sargent DA. The impaired physician movement: an interim report. Hosp Community Psychiatry. 1985;36(3):294-297.
6. Arkansas State Medical Board. Arkansas medical practices act and regulations. http://www.armedicalboard.org/professionals/pdf/mpa.pdf. Revised March 2017. Accessed July 11, 2017.
7. Oklahoma Health Professionals Program. Chemical dependency. https://www.okhpp.org/chemical-dependency. Accessed September 15, 2017.
8. Trockel M, Miller MN, Roberts LW. Clinician well-being and impairment. In: Roberts LW, ed. A clinical guide to psychiatric ethics. Arlington, VA: American Psychiatric Publishing, Inc.; 2016:223-236.
9. U.S. Equal Employment Opportunity Commission. Regulations under the Americans with Disabilities Act. Federal Register. https://www.gpo.gov/fdsys/pkg/FR-2016-05-17/pdf/2016-11558.pdf. Published May 17, 2016. Accessed August 2
10. Lawson ND. Comply with federal laws before checking institutional guidelines on resident referrals for psychiatric evaluations. J Grad Med Educ. In press.
11. Bright RP, Krahn L. Impaired physicians: how to recognize, when to report, and where to refer. Current Psychiatry. 2010;9(6):11-20.
12. Mossman D, Farrell HM. Physician impairment: when should you report? Current Psychiatry. 2011;10(9):67-71.
Self-disclosure as therapy: The benefits of expressive writing
As psychiatrists, we often provide our patients with a prescription in the hope that the medication will alleviate their symptoms. Perhaps we engage our patients in psychotherapy, encouraging them to reflect on their thoughts, behaviors, and emotions to alter their cognitions. We may remark that our goal is for the patient to “become their own therapist.” What if we encouraged our patients to express themselves in a less structured manner and become their own therapists through writing?
Benefits of expressive writing
Writing about an experienced traumatic event—specifically, to express emotions related to the event—has been associated with improved health outcomes.1,2 Many of these improvements are related to somatic health and basic function, including decreased use of health services, improved immune functioning, and a boost in grades or occupational performance.1 Patients who participate in expressive writing also have demonstrated improvements in distress, negative affect, depression, and posttraumatic stress disorder (PTSD) symptoms.1,2 Although improvement in PTSD symptoms with expressive writing has varied across studies, it appears that patients with PTSD who score high in trait negative emotion may receive the most benefit from the practice.3
Why does it work?
There are several theories regarding why expressive writing is an effective therapy. Originally, it was believed that the active inhibition of not talking about traumatic events was a form of physiological work and a long-term, low-lying stressor, and that writing about such events could reduce this stress. However, newer studies offer various explanations for its efficacy, including:
- repeat exposure to stressful or traumatic memories and consequent self-distancing
- creation of a narrative around the stressful event
- labeling of emotions
- self-affirmation and meaning-making related to the negative event.4
Rx writing
Encouraging your patients to use expressive writing is simple. You might ask a patient struggling with distress and negative affect following a traumatic experience to write about his (her) thoughts and feelings regarding the incident. For example:
Spend about 15 minutes writing your deepest thoughts and feelings about going through this traumatic experience. Discuss the ways it affected different areas of your life, including relationships with family and friends, school or work, or self-confidence and self-esteem. Don’t worry about spelling, grammar, or sentence structure.
Assure patients that you do not need to review their writing, but would like to hear about their experience writing. Many studies on expressive writing instructed participants to write for 3 to 5 consecutive days, 15 to 30 minutes each day.1,2 Patients may disclose a dramatic spectrum and intensity of experience and often are willing to do so.
Expressive writing is a simple, low-risk exercise that benefits many people. Perhaps by prescribing a course of writing, you will find your patients can benefit as well.
1. Baikie KA, Geerligs L, Wilhelm K. Expressive writing and positive writing for participants with mood disorders: an online randomized controlled trial. J Affect Disord. 2012;136(3):310-319.
2. Krpan KM, Kross E, Berman MG, et al. An everyday activity as a treatment for depression: the benefits of expressive writing for people diagnosed with major depressive disorder. J Affect Disord. 2013;150(3):1148-1151.
3. Hoyt T, Yeater EA. The effects of negative emotion and expressive writing on posttraumatic stress symptoms. J Soc Clin Psychol. 2011;30:549-569.
4. Niles AN, Byrne Haltom KE, Lieberman MD, et al. Writing content predicts benefit from written expressive disclosure: evidence for repeated exposure and self-affirmation. Cogn Emot. 2016;30(2):258-274.
As psychiatrists, we often provide our patients with a prescription in the hope that the medication will alleviate their symptoms. Perhaps we engage our patients in psychotherapy, encouraging them to reflect on their thoughts, behaviors, and emotions to alter their cognitions. We may remark that our goal is for the patient to “become their own therapist.” What if we encouraged our patients to express themselves in a less structured manner and become their own therapists through writing?
Benefits of expressive writing
Writing about an experienced traumatic event—specifically, to express emotions related to the event—has been associated with improved health outcomes.1,2 Many of these improvements are related to somatic health and basic function, including decreased use of health services, improved immune functioning, and a boost in grades or occupational performance.1 Patients who participate in expressive writing also have demonstrated improvements in distress, negative affect, depression, and posttraumatic stress disorder (PTSD) symptoms.1,2 Although improvement in PTSD symptoms with expressive writing has varied across studies, it appears that patients with PTSD who score high in trait negative emotion may receive the most benefit from the practice.3
Why does it work?
There are several theories regarding why expressive writing is an effective therapy. Originally, it was believed that the active inhibition of not talking about traumatic events was a form of physiological work and a long-term, low-lying stressor, and that writing about such events could reduce this stress. However, newer studies offer various explanations for its efficacy, including:
- repeat exposure to stressful or traumatic memories and consequent self-distancing
- creation of a narrative around the stressful event
- labeling of emotions
- self-affirmation and meaning-making related to the negative event.4
Rx writing
Encouraging your patients to use expressive writing is simple. You might ask a patient struggling with distress and negative affect following a traumatic experience to write about his (her) thoughts and feelings regarding the incident. For example:
Spend about 15 minutes writing your deepest thoughts and feelings about going through this traumatic experience. Discuss the ways it affected different areas of your life, including relationships with family and friends, school or work, or self-confidence and self-esteem. Don’t worry about spelling, grammar, or sentence structure.
Assure patients that you do not need to review their writing, but would like to hear about their experience writing. Many studies on expressive writing instructed participants to write for 3 to 5 consecutive days, 15 to 30 minutes each day.1,2 Patients may disclose a dramatic spectrum and intensity of experience and often are willing to do so.
Expressive writing is a simple, low-risk exercise that benefits many people. Perhaps by prescribing a course of writing, you will find your patients can benefit as well.
As psychiatrists, we often provide our patients with a prescription in the hope that the medication will alleviate their symptoms. Perhaps we engage our patients in psychotherapy, encouraging them to reflect on their thoughts, behaviors, and emotions to alter their cognitions. We may remark that our goal is for the patient to “become their own therapist.” What if we encouraged our patients to express themselves in a less structured manner and become their own therapists through writing?
Benefits of expressive writing
Writing about an experienced traumatic event—specifically, to express emotions related to the event—has been associated with improved health outcomes.1,2 Many of these improvements are related to somatic health and basic function, including decreased use of health services, improved immune functioning, and a boost in grades or occupational performance.1 Patients who participate in expressive writing also have demonstrated improvements in distress, negative affect, depression, and posttraumatic stress disorder (PTSD) symptoms.1,2 Although improvement in PTSD symptoms with expressive writing has varied across studies, it appears that patients with PTSD who score high in trait negative emotion may receive the most benefit from the practice.3
Why does it work?
There are several theories regarding why expressive writing is an effective therapy. Originally, it was believed that the active inhibition of not talking about traumatic events was a form of physiological work and a long-term, low-lying stressor, and that writing about such events could reduce this stress. However, newer studies offer various explanations for its efficacy, including:
- repeat exposure to stressful or traumatic memories and consequent self-distancing
- creation of a narrative around the stressful event
- labeling of emotions
- self-affirmation and meaning-making related to the negative event.4
Rx writing
Encouraging your patients to use expressive writing is simple. You might ask a patient struggling with distress and negative affect following a traumatic experience to write about his (her) thoughts and feelings regarding the incident. For example:
Spend about 15 minutes writing your deepest thoughts and feelings about going through this traumatic experience. Discuss the ways it affected different areas of your life, including relationships with family and friends, school or work, or self-confidence and self-esteem. Don’t worry about spelling, grammar, or sentence structure.
Assure patients that you do not need to review their writing, but would like to hear about their experience writing. Many studies on expressive writing instructed participants to write for 3 to 5 consecutive days, 15 to 30 minutes each day.1,2 Patients may disclose a dramatic spectrum and intensity of experience and often are willing to do so.
Expressive writing is a simple, low-risk exercise that benefits many people. Perhaps by prescribing a course of writing, you will find your patients can benefit as well.
1. Baikie KA, Geerligs L, Wilhelm K. Expressive writing and positive writing for participants with mood disorders: an online randomized controlled trial. J Affect Disord. 2012;136(3):310-319.
2. Krpan KM, Kross E, Berman MG, et al. An everyday activity as a treatment for depression: the benefits of expressive writing for people diagnosed with major depressive disorder. J Affect Disord. 2013;150(3):1148-1151.
3. Hoyt T, Yeater EA. The effects of negative emotion and expressive writing on posttraumatic stress symptoms. J Soc Clin Psychol. 2011;30:549-569.
4. Niles AN, Byrne Haltom KE, Lieberman MD, et al. Writing content predicts benefit from written expressive disclosure: evidence for repeated exposure and self-affirmation. Cogn Emot. 2016;30(2):258-274.
1. Baikie KA, Geerligs L, Wilhelm K. Expressive writing and positive writing for participants with mood disorders: an online randomized controlled trial. J Affect Disord. 2012;136(3):310-319.
2. Krpan KM, Kross E, Berman MG, et al. An everyday activity as a treatment for depression: the benefits of expressive writing for people diagnosed with major depressive disorder. J Affect Disord. 2013;150(3):1148-1151.
3. Hoyt T, Yeater EA. The effects of negative emotion and expressive writing on posttraumatic stress symptoms. J Soc Clin Psychol. 2011;30:549-569.
4. Niles AN, Byrne Haltom KE, Lieberman MD, et al. Writing content predicts benefit from written expressive disclosure: evidence for repeated exposure and self-affirmation. Cogn Emot. 2016;30(2):258-274.
Managing requests for gluten-, lactose-, and animal-free medications
Patients may ask their psychiatrist to prescribe gluten-, lactose-, or animal-free medications because of concerns about allergies, disease states, religious beliefs, or dietary preferences. Determining the source of non-active medication ingredients can be challenging and time-consuming, because ingredients vary across dosages and formulations of the same medication. We review how to address requests for gluten-, lactose-, and animal-free medications.
Gluten-free
Although the risk of a medication containing gluten is low,1 patients with Celiac disease must avoid gluten to prevent disease exacerbation. Therefore, physicians should thoroughly evaluate medication ingredients to prevent inadvertent gluten consumption.
Medication excipients that may contain gluten include:
- starch
- pregelatinized starch
- sodium starch glycolate.1
These starches can come from various sources, including corn, wheat, potato, and tapioca. Wheat-derived starch contains gluten and should be avoided by patients with Celiac disease. Advise patients to avoid any starch if its source cannot be determined.
Some sources may list sugar alcohols, such as mannitol and xylitol, as gluten–containing excipients because they may be extracted from starch sources, such as wheat; however, all gluten is removed during refinement and these products are safe.2
Lactose-free
How to respond to a patient’s request for lactose-free medication depends on whether the patient is lactose intolerant or has a milk allergy. The amount of lactose that patients with lactase deficiency can tolerate varies.3 Most medications are thought to contain minimal amounts of lactose. Case reports have described patients experiencing lactose intolerance symptoms after taking 1 or 2 medications, but this is rare.3 Therefore, it is reasonable to use lactose–containing products in patients with lactose intolerance. If such a patient develops symptoms after taking a medication that contains lactose, suggest that he (she):
- take the medication with food, if appropriate, to slow absorption and reduce symptoms
- take it with a lactase enzyme product
- substitute it with a medication that does not contain lactose (switch to a different product or formulation, as appropriate).
Compared with patients who are lactose intolerant, those with a milk allergy experience an immunoglobulin E–mediated reaction when they consume milk protein. Milk proteins typically are filtered out during manufacturing, but a small amount can remain. Although it has not been determined if oral medications containing milk protein can cause an allergic reaction, some researchers have hypothesized that these medications may be tolerated because acid and digestive enzymes break down the milk protein. However, because the respiratory tract lacks this protection, inhaled products that contain lactose may be more likely to cause an allergic reaction and should be avoided if possible. Because oral medications do not usually contain milk proteins, it may be reasonable to prescribe lactose–containing oral products to a patient with a milk allergy. If the patient experiences a reaction or wishes to avoid lactose, an alternative non-lactose–containing product or formulation may be prescribed.
Animal-free
Individuals who are members of certain religions, including Judaism, Islam, Orthodox Christianity, and the Seventh Day Adventist Church, typically avoid pork, and those who are Hindu or Buddhist may avoid beef products.4 Gelatin and stearic acid, which can be found in the gelatin shell of capsules and within extended-release (ER) tablets, frequently are derived from porcine or bovine sources. The source of gelatin and stearic acid may change from lot to lot, and manufacturers should be contacted to assist with identifying the source for a specific medication. Consider these options to reduce a patient’s exposure to animal-containing products:
- change from an ER to an immediate-release (IR) product (confirm that IR is gelatin- and stearic acid–free)
- use a non-capsule formulation
- remove the content of a capsule before ingestion, if appropriate
- try an alternative route of administration, such as transdermal.
How to best help patients
Before taking steps to accommodate a request for a gluten-, lactose-, or animal-free medication, which can be time-consuming, verify the reason for your patient’s request. It may be sufficient to explain to your patient that typically exposure to excipients within oral medications is small and does not cause problems for a patient with lactose intolerance or a milk allergy. The resources listed in the Table can help provide further education on these concerns; however, due to potential delays in updating a Web site, it may be necessary to contact the medication manufacturer directly to verify ingredients.
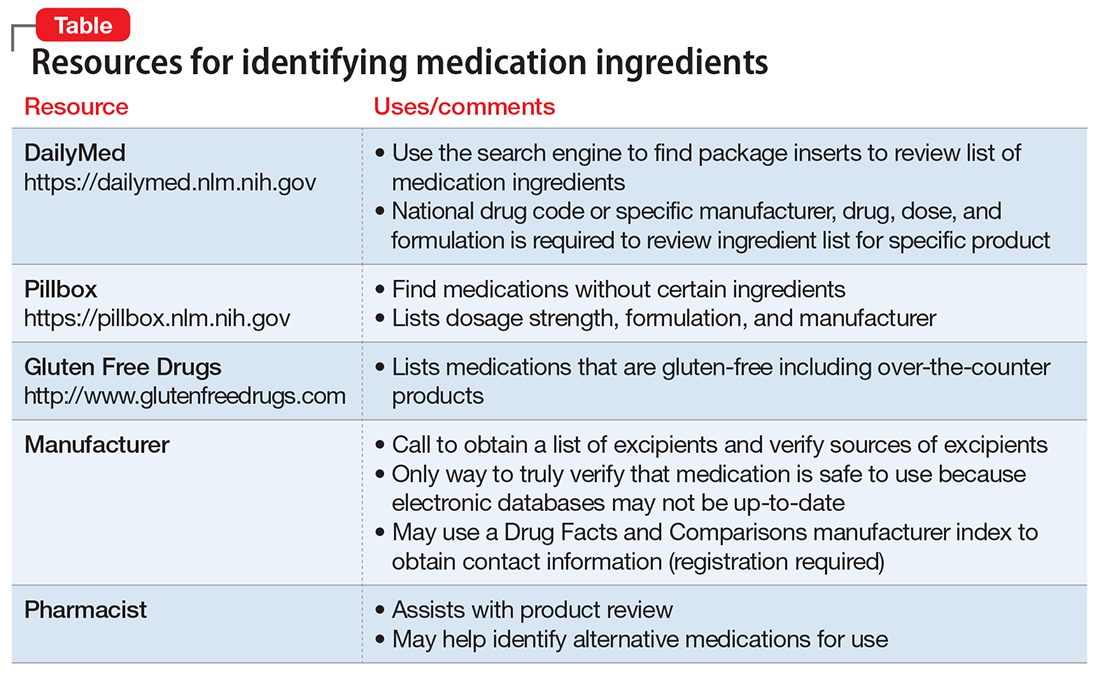
If your patient still has concerns about ingredients, consider the following steps:
- Use the National Library of Medicine’s Pillbox Web site (https://pillbox.nlm.nih.gov) to search for a medication, dose, or formulation that does not contain the concerning ingredients
- If the concerning ingredient is not listed, either prescribe the medication or contact the manufacturer for further information, depending on the patient’s reason for the request
- If the concerning ingredient is listed, work with the pharmacist to contact the manufacturer.
There are 2 additional points to consider regarding medication excipients. Be aware that generic medications are produced by multiple manufacturers, and each may use different excipients. Also, a manufacturer may not guarantee that a medication is gluten-free because of the potential for cross-contamination during manufacturing, although the risk is extremely low.
1. Plogsted S. Gluten in medication. Celiac Disease Foundation. https://celiac.org/live-gluten-free/glutenfreediet/gluten-medication. Accessed January 13, 2017.
2. Plogsted S. Gluten free drugs. http://www.glutenfreedrugs.com. Updated April 28, 2017. Accessed September 2, 2017.
3. Lactose in medications. Pharmacist’s Letter/Prescriber’s Letter. 2007;230779.
4. Sattar SP, Shakeel Ahmed M, Majeed F, et al. Inert medication ingredients causing nonadherence due to religious beliefs. Ann Pharmacother. 2004;38(4):621-624.
Patients may ask their psychiatrist to prescribe gluten-, lactose-, or animal-free medications because of concerns about allergies, disease states, religious beliefs, or dietary preferences. Determining the source of non-active medication ingredients can be challenging and time-consuming, because ingredients vary across dosages and formulations of the same medication. We review how to address requests for gluten-, lactose-, and animal-free medications.
Gluten-free
Although the risk of a medication containing gluten is low,1 patients with Celiac disease must avoid gluten to prevent disease exacerbation. Therefore, physicians should thoroughly evaluate medication ingredients to prevent inadvertent gluten consumption.
Medication excipients that may contain gluten include:
- starch
- pregelatinized starch
- sodium starch glycolate.1
These starches can come from various sources, including corn, wheat, potato, and tapioca. Wheat-derived starch contains gluten and should be avoided by patients with Celiac disease. Advise patients to avoid any starch if its source cannot be determined.
Some sources may list sugar alcohols, such as mannitol and xylitol, as gluten–containing excipients because they may be extracted from starch sources, such as wheat; however, all gluten is removed during refinement and these products are safe.2
Lactose-free
How to respond to a patient’s request for lactose-free medication depends on whether the patient is lactose intolerant or has a milk allergy. The amount of lactose that patients with lactase deficiency can tolerate varies.3 Most medications are thought to contain minimal amounts of lactose. Case reports have described patients experiencing lactose intolerance symptoms after taking 1 or 2 medications, but this is rare.3 Therefore, it is reasonable to use lactose–containing products in patients with lactose intolerance. If such a patient develops symptoms after taking a medication that contains lactose, suggest that he (she):
- take the medication with food, if appropriate, to slow absorption and reduce symptoms
- take it with a lactase enzyme product
- substitute it with a medication that does not contain lactose (switch to a different product or formulation, as appropriate).
Compared with patients who are lactose intolerant, those with a milk allergy experience an immunoglobulin E–mediated reaction when they consume milk protein. Milk proteins typically are filtered out during manufacturing, but a small amount can remain. Although it has not been determined if oral medications containing milk protein can cause an allergic reaction, some researchers have hypothesized that these medications may be tolerated because acid and digestive enzymes break down the milk protein. However, because the respiratory tract lacks this protection, inhaled products that contain lactose may be more likely to cause an allergic reaction and should be avoided if possible. Because oral medications do not usually contain milk proteins, it may be reasonable to prescribe lactose–containing oral products to a patient with a milk allergy. If the patient experiences a reaction or wishes to avoid lactose, an alternative non-lactose–containing product or formulation may be prescribed.
Animal-free
Individuals who are members of certain religions, including Judaism, Islam, Orthodox Christianity, and the Seventh Day Adventist Church, typically avoid pork, and those who are Hindu or Buddhist may avoid beef products.4 Gelatin and stearic acid, which can be found in the gelatin shell of capsules and within extended-release (ER) tablets, frequently are derived from porcine or bovine sources. The source of gelatin and stearic acid may change from lot to lot, and manufacturers should be contacted to assist with identifying the source for a specific medication. Consider these options to reduce a patient’s exposure to animal-containing products:
- change from an ER to an immediate-release (IR) product (confirm that IR is gelatin- and stearic acid–free)
- use a non-capsule formulation
- remove the content of a capsule before ingestion, if appropriate
- try an alternative route of administration, such as transdermal.
How to best help patients
Before taking steps to accommodate a request for a gluten-, lactose-, or animal-free medication, which can be time-consuming, verify the reason for your patient’s request. It may be sufficient to explain to your patient that typically exposure to excipients within oral medications is small and does not cause problems for a patient with lactose intolerance or a milk allergy. The resources listed in the Table can help provide further education on these concerns; however, due to potential delays in updating a Web site, it may be necessary to contact the medication manufacturer directly to verify ingredients.

If your patient still has concerns about ingredients, consider the following steps:
- Use the National Library of Medicine’s Pillbox Web site (https://pillbox.nlm.nih.gov) to search for a medication, dose, or formulation that does not contain the concerning ingredients
- If the concerning ingredient is not listed, either prescribe the medication or contact the manufacturer for further information, depending on the patient’s reason for the request
- If the concerning ingredient is listed, work with the pharmacist to contact the manufacturer.
There are 2 additional points to consider regarding medication excipients. Be aware that generic medications are produced by multiple manufacturers, and each may use different excipients. Also, a manufacturer may not guarantee that a medication is gluten-free because of the potential for cross-contamination during manufacturing, although the risk is extremely low.
Patients may ask their psychiatrist to prescribe gluten-, lactose-, or animal-free medications because of concerns about allergies, disease states, religious beliefs, or dietary preferences. Determining the source of non-active medication ingredients can be challenging and time-consuming, because ingredients vary across dosages and formulations of the same medication. We review how to address requests for gluten-, lactose-, and animal-free medications.
Gluten-free
Although the risk of a medication containing gluten is low,1 patients with Celiac disease must avoid gluten to prevent disease exacerbation. Therefore, physicians should thoroughly evaluate medication ingredients to prevent inadvertent gluten consumption.
Medication excipients that may contain gluten include:
- starch
- pregelatinized starch
- sodium starch glycolate.1
These starches can come from various sources, including corn, wheat, potato, and tapioca. Wheat-derived starch contains gluten and should be avoided by patients with Celiac disease. Advise patients to avoid any starch if its source cannot be determined.
Some sources may list sugar alcohols, such as mannitol and xylitol, as gluten–containing excipients because they may be extracted from starch sources, such as wheat; however, all gluten is removed during refinement and these products are safe.2
Lactose-free
How to respond to a patient’s request for lactose-free medication depends on whether the patient is lactose intolerant or has a milk allergy. The amount of lactose that patients with lactase deficiency can tolerate varies.3 Most medications are thought to contain minimal amounts of lactose. Case reports have described patients experiencing lactose intolerance symptoms after taking 1 or 2 medications, but this is rare.3 Therefore, it is reasonable to use lactose–containing products in patients with lactose intolerance. If such a patient develops symptoms after taking a medication that contains lactose, suggest that he (she):
- take the medication with food, if appropriate, to slow absorption and reduce symptoms
- take it with a lactase enzyme product
- substitute it with a medication that does not contain lactose (switch to a different product or formulation, as appropriate).
Compared with patients who are lactose intolerant, those with a milk allergy experience an immunoglobulin E–mediated reaction when they consume milk protein. Milk proteins typically are filtered out during manufacturing, but a small amount can remain. Although it has not been determined if oral medications containing milk protein can cause an allergic reaction, some researchers have hypothesized that these medications may be tolerated because acid and digestive enzymes break down the milk protein. However, because the respiratory tract lacks this protection, inhaled products that contain lactose may be more likely to cause an allergic reaction and should be avoided if possible. Because oral medications do not usually contain milk proteins, it may be reasonable to prescribe lactose–containing oral products to a patient with a milk allergy. If the patient experiences a reaction or wishes to avoid lactose, an alternative non-lactose–containing product or formulation may be prescribed.
Animal-free
Individuals who are members of certain religions, including Judaism, Islam, Orthodox Christianity, and the Seventh Day Adventist Church, typically avoid pork, and those who are Hindu or Buddhist may avoid beef products.4 Gelatin and stearic acid, which can be found in the gelatin shell of capsules and within extended-release (ER) tablets, frequently are derived from porcine or bovine sources. The source of gelatin and stearic acid may change from lot to lot, and manufacturers should be contacted to assist with identifying the source for a specific medication. Consider these options to reduce a patient’s exposure to animal-containing products:
- change from an ER to an immediate-release (IR) product (confirm that IR is gelatin- and stearic acid–free)
- use a non-capsule formulation
- remove the content of a capsule before ingestion, if appropriate
- try an alternative route of administration, such as transdermal.
How to best help patients
Before taking steps to accommodate a request for a gluten-, lactose-, or animal-free medication, which can be time-consuming, verify the reason for your patient’s request. It may be sufficient to explain to your patient that typically exposure to excipients within oral medications is small and does not cause problems for a patient with lactose intolerance or a milk allergy. The resources listed in the Table can help provide further education on these concerns; however, due to potential delays in updating a Web site, it may be necessary to contact the medication manufacturer directly to verify ingredients.

If your patient still has concerns about ingredients, consider the following steps:
- Use the National Library of Medicine’s Pillbox Web site (https://pillbox.nlm.nih.gov) to search for a medication, dose, or formulation that does not contain the concerning ingredients
- If the concerning ingredient is not listed, either prescribe the medication or contact the manufacturer for further information, depending on the patient’s reason for the request
- If the concerning ingredient is listed, work with the pharmacist to contact the manufacturer.
There are 2 additional points to consider regarding medication excipients. Be aware that generic medications are produced by multiple manufacturers, and each may use different excipients. Also, a manufacturer may not guarantee that a medication is gluten-free because of the potential for cross-contamination during manufacturing, although the risk is extremely low.
1. Plogsted S. Gluten in medication. Celiac Disease Foundation. https://celiac.org/live-gluten-free/glutenfreediet/gluten-medication. Accessed January 13, 2017.
2. Plogsted S. Gluten free drugs. http://www.glutenfreedrugs.com. Updated April 28, 2017. Accessed September 2, 2017.
3. Lactose in medications. Pharmacist’s Letter/Prescriber’s Letter. 2007;230779.
4. Sattar SP, Shakeel Ahmed M, Majeed F, et al. Inert medication ingredients causing nonadherence due to religious beliefs. Ann Pharmacother. 2004;38(4):621-624.
1. Plogsted S. Gluten in medication. Celiac Disease Foundation. https://celiac.org/live-gluten-free/glutenfreediet/gluten-medication. Accessed January 13, 2017.
2. Plogsted S. Gluten free drugs. http://www.glutenfreedrugs.com. Updated April 28, 2017. Accessed September 2, 2017.
3. Lactose in medications. Pharmacist’s Letter/Prescriber’s Letter. 2007;230779.
4. Sattar SP, Shakeel Ahmed M, Majeed F, et al. Inert medication ingredients causing nonadherence due to religious beliefs. Ann Pharmacother. 2004;38(4):621-624.
Improving our approach to discharge planning
Editor’s note: The Society of Hospital Medicine’s (SHM’s) Physician in Training Committee launched a scholarship program in 2015 for medical students to help transform health care and revolutionize patient care. The program has been expanded for the 2017-2018 year, offering two options for students to receive funding and engage in scholarly work during their first, second, and third years of medical school. As a part of the longitudinal (18-month) program, recipients are required to write about their experience on a monthly basis.
Since finishing up the initial planning phase of our project, my mentors and I have continued with even more planning as we head into the fall. Coming up with a good plan is the first step in making sure everything goes smoothly later on in a project. The same goes for coming up with a well-thought-out discharge plan when sending a patient to the next level of care.
Getting a patient out of the hospital and into their next destination – whether it’s a long-term acute care facility, skilled nursing facility, inpatient rehabilitation, home, or elsewhere – can approach the same level of complexity as the medical care received in the hospital. Getting a patient to any post-acute care facility can be time-consuming because it involves the coordination of two health care entities and their employees.
Discharge planning for post-acute care placement can take many forms and involve many resources. Some studies have shown that certain discharge planning interventions can reduce costs and 30-day readmissions. Many physicians think that discharge planning would help improve outcomes in most groups, but so far the aggregate data do not show that discharge planning account for much improvement in any of these outcomes. Targeting certain groups of hospitalized patients, however, could improve the effect that discharge planning has on these outcomes because more of these scarce resources might be devoted to the right patients earlier in their hospital stays.
A post-acute care placement prediction tool would help hospitalists determine how to allocate their discharge planning resources, including social work, case management, pharmacies, physical therapy, and occupational therapy. While we are working towards integrating this kind of tool in our own institution’s practice, we are also hopeful that we can create a generalizable tool that assists in helping care teams decide how to link patients to the right resources elsewhere.
Monisha Bhatia, a native of Nashville, Tenn., is a fourth-year medical student at Vanderbilt University in Nashville. She is hoping to pursue either a residency in internal medicine or a combined internal medicine/emergency medicine program. Prior to medical school, she completed a JD/MPH program at Boston University, and she hopes to use her legal training in working with regulatory authorities to improve access to health care for all Americans.
Editor’s note: The Society of Hospital Medicine’s (SHM’s) Physician in Training Committee launched a scholarship program in 2015 for medical students to help transform health care and revolutionize patient care. The program has been expanded for the 2017-2018 year, offering two options for students to receive funding and engage in scholarly work during their first, second, and third years of medical school. As a part of the longitudinal (18-month) program, recipients are required to write about their experience on a monthly basis.
Since finishing up the initial planning phase of our project, my mentors and I have continued with even more planning as we head into the fall. Coming up with a good plan is the first step in making sure everything goes smoothly later on in a project. The same goes for coming up with a well-thought-out discharge plan when sending a patient to the next level of care.
Getting a patient out of the hospital and into their next destination – whether it’s a long-term acute care facility, skilled nursing facility, inpatient rehabilitation, home, or elsewhere – can approach the same level of complexity as the medical care received in the hospital. Getting a patient to any post-acute care facility can be time-consuming because it involves the coordination of two health care entities and their employees.
Discharge planning for post-acute care placement can take many forms and involve many resources. Some studies have shown that certain discharge planning interventions can reduce costs and 30-day readmissions. Many physicians think that discharge planning would help improve outcomes in most groups, but so far the aggregate data do not show that discharge planning account for much improvement in any of these outcomes. Targeting certain groups of hospitalized patients, however, could improve the effect that discharge planning has on these outcomes because more of these scarce resources might be devoted to the right patients earlier in their hospital stays.
A post-acute care placement prediction tool would help hospitalists determine how to allocate their discharge planning resources, including social work, case management, pharmacies, physical therapy, and occupational therapy. While we are working towards integrating this kind of tool in our own institution’s practice, we are also hopeful that we can create a generalizable tool that assists in helping care teams decide how to link patients to the right resources elsewhere.
Monisha Bhatia, a native of Nashville, Tenn., is a fourth-year medical student at Vanderbilt University in Nashville. She is hoping to pursue either a residency in internal medicine or a combined internal medicine/emergency medicine program. Prior to medical school, she completed a JD/MPH program at Boston University, and she hopes to use her legal training in working with regulatory authorities to improve access to health care for all Americans.
Editor’s note: The Society of Hospital Medicine’s (SHM’s) Physician in Training Committee launched a scholarship program in 2015 for medical students to help transform health care and revolutionize patient care. The program has been expanded for the 2017-2018 year, offering two options for students to receive funding and engage in scholarly work during their first, second, and third years of medical school. As a part of the longitudinal (18-month) program, recipients are required to write about their experience on a monthly basis.
Since finishing up the initial planning phase of our project, my mentors and I have continued with even more planning as we head into the fall. Coming up with a good plan is the first step in making sure everything goes smoothly later on in a project. The same goes for coming up with a well-thought-out discharge plan when sending a patient to the next level of care.
Getting a patient out of the hospital and into their next destination – whether it’s a long-term acute care facility, skilled nursing facility, inpatient rehabilitation, home, or elsewhere – can approach the same level of complexity as the medical care received in the hospital. Getting a patient to any post-acute care facility can be time-consuming because it involves the coordination of two health care entities and their employees.
Discharge planning for post-acute care placement can take many forms and involve many resources. Some studies have shown that certain discharge planning interventions can reduce costs and 30-day readmissions. Many physicians think that discharge planning would help improve outcomes in most groups, but so far the aggregate data do not show that discharge planning account for much improvement in any of these outcomes. Targeting certain groups of hospitalized patients, however, could improve the effect that discharge planning has on these outcomes because more of these scarce resources might be devoted to the right patients earlier in their hospital stays.
A post-acute care placement prediction tool would help hospitalists determine how to allocate their discharge planning resources, including social work, case management, pharmacies, physical therapy, and occupational therapy. While we are working towards integrating this kind of tool in our own institution’s practice, we are also hopeful that we can create a generalizable tool that assists in helping care teams decide how to link patients to the right resources elsewhere.
Monisha Bhatia, a native of Nashville, Tenn., is a fourth-year medical student at Vanderbilt University in Nashville. She is hoping to pursue either a residency in internal medicine or a combined internal medicine/emergency medicine program. Prior to medical school, she completed a JD/MPH program at Boston University, and she hopes to use her legal training in working with regulatory authorities to improve access to health care for all Americans.
What to do after a patient assaults you
Physical assaults by patients are an occupational hazard of practicing medicine. Assaults can happen in any clinical setting, occur unexpectedly, and have a lasting impact on all involved. In an anonymous survey of 11,000 hospital workers, 18.8% reported being physically assaulted.1 Psychiatric clinicia
Remain calm. Although it may be difficult to do so immediately after being assaulted, remaining calm is essential. You may experience a myriad of emotions, such as anger, fear, vulnerability, shock, or guilt. Although these responses are normal, they can hinder your ability to accomplish subsequent tasks.
Recall the assault. Despite the unpleasantness of replaying the incident, recall as many details as you can and immediately write them down. Because of the copious amount of paperwork you may be required to file (eg, incident reports, employee health forms) and statements that you will likely repeat, having an accurate version of what happened is paramount to determining a course of action. You also may be required to give a statement to law enforcement officials.
Report the assault to your supervisor(s). Informing supervisors and colleagues of what happened could begin the implementation of corrective measures to decrease the risk of future assaults.
Talk about the incident with coworkers, supervisors, and friends to help process what happened, normalize what you are experiencing, and allow others to learn from you. Being assaulted can be traumatic and can result in experiencing post-assault symptoms, such as disruptions in sleep patterns, changes in appetite, and nightmares of the incident. These can be normal reactions to what is an abnormal situation. If necessary, seek medical assistance.
Evaluate the circumstances. Although you may not be at fault, consider if there may have been contributing factors:
- Were there signs of escalating aggressiveness in the patient’s behavior that you may have missed?
- Would the presence of a chaperone during interactions with the patient have reduced the risk of an assault?
- Did you maintain a safe distance from the patient?
- Were existing safety policies followed?
Examine your surroundings. Could the surroundings where the assault occurred have hindered your ability to escape? If so, can they be altered to increase your chance of escaping? Are there items that could be used as potential weapons and should be removed?Expect changes to processes and procedures as part of the reverberations after an assault. Your firsthand account of the assault can limit staff overreactions by analyzing whether existing policies were appropriately implemented, before deeming them ineffective and enacting new policies.
1. Pompeii LA, Schoenfisch AL, Lipscomb HJ, et al. Physical assault, physical threat, and verbal abuse perpetrated against hospital workers by patients or visitors in six U.S. hospitals. Am J Ind Med. 2015;58(11):1194-1204.
2. Phillips JP. Workplace violence against health care workers in the United States. N Engl J Med. 2016;374(17):1661-1669.
3. Privitera M, Weisman R, Cerulli C, et al. Violence toward mental health staff and safety in the work environment. Occup Med (Lond). 2005;55(6):480-486.
Physical assaults by patients are an occupational hazard of practicing medicine. Assaults can happen in any clinical setting, occur unexpectedly, and have a lasting impact on all involved. In an anonymous survey of 11,000 hospital workers, 18.8% reported being physically assaulted.1 Psychiatric clinicia
Remain calm. Although it may be difficult to do so immediately after being assaulted, remaining calm is essential. You may experience a myriad of emotions, such as anger, fear, vulnerability, shock, or guilt. Although these responses are normal, they can hinder your ability to accomplish subsequent tasks.
Recall the assault. Despite the unpleasantness of replaying the incident, recall as many details as you can and immediately write them down. Because of the copious amount of paperwork you may be required to file (eg, incident reports, employee health forms) and statements that you will likely repeat, having an accurate version of what happened is paramount to determining a course of action. You also may be required to give a statement to law enforcement officials.
Report the assault to your supervisor(s). Informing supervisors and colleagues of what happened could begin the implementation of corrective measures to decrease the risk of future assaults.
Talk about the incident with coworkers, supervisors, and friends to help process what happened, normalize what you are experiencing, and allow others to learn from you. Being assaulted can be traumatic and can result in experiencing post-assault symptoms, such as disruptions in sleep patterns, changes in appetite, and nightmares of the incident. These can be normal reactions to what is an abnormal situation. If necessary, seek medical assistance.
Evaluate the circumstances. Although you may not be at fault, consider if there may have been contributing factors:
- Were there signs of escalating aggressiveness in the patient’s behavior that you may have missed?
- Would the presence of a chaperone during interactions with the patient have reduced the risk of an assault?
- Did you maintain a safe distance from the patient?
- Were existing safety policies followed?
Examine your surroundings. Could the surroundings where the assault occurred have hindered your ability to escape? If so, can they be altered to increase your chance of escaping? Are there items that could be used as potential weapons and should be removed?Expect changes to processes and procedures as part of the reverberations after an assault. Your firsthand account of the assault can limit staff overreactions by analyzing whether existing policies were appropriately implemented, before deeming them ineffective and enacting new policies.
Physical assaults by patients are an occupational hazard of practicing medicine. Assaults can happen in any clinical setting, occur unexpectedly, and have a lasting impact on all involved. In an anonymous survey of 11,000 hospital workers, 18.8% reported being physically assaulted.1 Psychiatric clinicia
Remain calm. Although it may be difficult to do so immediately after being assaulted, remaining calm is essential. You may experience a myriad of emotions, such as anger, fear, vulnerability, shock, or guilt. Although these responses are normal, they can hinder your ability to accomplish subsequent tasks.
Recall the assault. Despite the unpleasantness of replaying the incident, recall as many details as you can and immediately write them down. Because of the copious amount of paperwork you may be required to file (eg, incident reports, employee health forms) and statements that you will likely repeat, having an accurate version of what happened is paramount to determining a course of action. You also may be required to give a statement to law enforcement officials.
Report the assault to your supervisor(s). Informing supervisors and colleagues of what happened could begin the implementation of corrective measures to decrease the risk of future assaults.
Talk about the incident with coworkers, supervisors, and friends to help process what happened, normalize what you are experiencing, and allow others to learn from you. Being assaulted can be traumatic and can result in experiencing post-assault symptoms, such as disruptions in sleep patterns, changes in appetite, and nightmares of the incident. These can be normal reactions to what is an abnormal situation. If necessary, seek medical assistance.
Evaluate the circumstances. Although you may not be at fault, consider if there may have been contributing factors:
- Were there signs of escalating aggressiveness in the patient’s behavior that you may have missed?
- Would the presence of a chaperone during interactions with the patient have reduced the risk of an assault?
- Did you maintain a safe distance from the patient?
- Were existing safety policies followed?
Examine your surroundings. Could the surroundings where the assault occurred have hindered your ability to escape? If so, can they be altered to increase your chance of escaping? Are there items that could be used as potential weapons and should be removed?Expect changes to processes and procedures as part of the reverberations after an assault. Your firsthand account of the assault can limit staff overreactions by analyzing whether existing policies were appropriately implemented, before deeming them ineffective and enacting new policies.
1. Pompeii LA, Schoenfisch AL, Lipscomb HJ, et al. Physical assault, physical threat, and verbal abuse perpetrated against hospital workers by patients or visitors in six U.S. hospitals. Am J Ind Med. 2015;58(11):1194-1204.
2. Phillips JP. Workplace violence against health care workers in the United States. N Engl J Med. 2016;374(17):1661-1669.
3. Privitera M, Weisman R, Cerulli C, et al. Violence toward mental health staff and safety in the work environment. Occup Med (Lond). 2005;55(6):480-486.
1. Pompeii LA, Schoenfisch AL, Lipscomb HJ, et al. Physical assault, physical threat, and verbal abuse perpetrated against hospital workers by patients or visitors in six U.S. hospitals. Am J Ind Med. 2015;58(11):1194-1204.
2. Phillips JP. Workplace violence against health care workers in the United States. N Engl J Med. 2016;374(17):1661-1669.
3. Privitera M, Weisman R, Cerulli C, et al. Violence toward mental health staff and safety in the work environment. Occup Med (Lond). 2005;55(6):480-486.
Doc advocates depression screening for cancer patients
SAN DIEGO—New research suggests a need for mental health screening among cancer patients.
The study revealed a 40% rate of depression among patients treated at an urban cancer center over a 3-year period.
Three-quarters of the depressed patients were previously undiagnosed.
Female patients and those who were unable to work due to disability were more likely to be depressed.
Jason Domogauer, PhD, of Rutgers New Jersey Medical School in Newark, New Jersey, presented these findings at ASTRO’s 59th Annual Meeting.
“Depression is widely recognized as an underdiagnosed disorder, particularly among older adults and cancer patients,” Dr Domogauer said. “Our findings point to a clear need for action, including depression screening during initial and continuing patient visits, initiation of mental health treatments for identified patients, and increased collaboration with mental health providers in cancer treatment centers. These efforts are particularly important for patients in urban centers, those who are female, and those who are unable to work because of their disease.”
Dr Domogauer and his colleagues studied 400 cancer patients who received treatment at Rutgers New Jersey Medical School/University Hospital Cancer Center between 2013 and 2016.
The average patient age was 55 (range, 20-86), and 53% of patients were female. Forty-eight percent of patients were African-American, 29% were non-Hispanic white, and 16% were Hispanic.
Nearly equal numbers of patients reported being able to work (49%) or unable to work due to disability (51%). Most patients (85%) received radiation as part of their cancer treatment.
The researchers assessed depression in the patients using a minimum score of 16 on the Center for Epidemiologic Studies Depression Scale.
In this way, 40% of the patients were diagnosed with depression. In 75% of these patients, depression was previously undiagnosed. This means roughly 30% of the overall patient population suffered from undiagnosed and untreated depression.
Depression was more common among females than males—47% and 32%, respectively (odds ratio [OR]=1.9, P=0.007).
Depression was also more likely among patients who were unable to work due to disability—48%, compared to 33% of those able to work (OR=1.9, P=0.005).
Depression prevalence did not differ significantly among racial/ethnic groups.
When the researchers looked specifically at patients who were previously not diagnosed with depression, the effects of being female or unable to work persisted.
In this subgroup, depression was more common among women than men—43% and 29%, respectively (OR=1.9, P=0.02)—and patients with disability compared to able patients—43% and 31%, respectively (OR=1.7, P=0.03). ![]()
SAN DIEGO—New research suggests a need for mental health screening among cancer patients.
The study revealed a 40% rate of depression among patients treated at an urban cancer center over a 3-year period.
Three-quarters of the depressed patients were previously undiagnosed.
Female patients and those who were unable to work due to disability were more likely to be depressed.
Jason Domogauer, PhD, of Rutgers New Jersey Medical School in Newark, New Jersey, presented these findings at ASTRO’s 59th Annual Meeting.
“Depression is widely recognized as an underdiagnosed disorder, particularly among older adults and cancer patients,” Dr Domogauer said. “Our findings point to a clear need for action, including depression screening during initial and continuing patient visits, initiation of mental health treatments for identified patients, and increased collaboration with mental health providers in cancer treatment centers. These efforts are particularly important for patients in urban centers, those who are female, and those who are unable to work because of their disease.”
Dr Domogauer and his colleagues studied 400 cancer patients who received treatment at Rutgers New Jersey Medical School/University Hospital Cancer Center between 2013 and 2016.
The average patient age was 55 (range, 20-86), and 53% of patients were female. Forty-eight percent of patients were African-American, 29% were non-Hispanic white, and 16% were Hispanic.
Nearly equal numbers of patients reported being able to work (49%) or unable to work due to disability (51%). Most patients (85%) received radiation as part of their cancer treatment.
The researchers assessed depression in the patients using a minimum score of 16 on the Center for Epidemiologic Studies Depression Scale.
In this way, 40% of the patients were diagnosed with depression. In 75% of these patients, depression was previously undiagnosed. This means roughly 30% of the overall patient population suffered from undiagnosed and untreated depression.
Depression was more common among females than males—47% and 32%, respectively (odds ratio [OR]=1.9, P=0.007).
Depression was also more likely among patients who were unable to work due to disability—48%, compared to 33% of those able to work (OR=1.9, P=0.005).
Depression prevalence did not differ significantly among racial/ethnic groups.
When the researchers looked specifically at patients who were previously not diagnosed with depression, the effects of being female or unable to work persisted.
In this subgroup, depression was more common among women than men—43% and 29%, respectively (OR=1.9, P=0.02)—and patients with disability compared to able patients—43% and 31%, respectively (OR=1.7, P=0.03). ![]()
SAN DIEGO—New research suggests a need for mental health screening among cancer patients.
The study revealed a 40% rate of depression among patients treated at an urban cancer center over a 3-year period.
Three-quarters of the depressed patients were previously undiagnosed.
Female patients and those who were unable to work due to disability were more likely to be depressed.
Jason Domogauer, PhD, of Rutgers New Jersey Medical School in Newark, New Jersey, presented these findings at ASTRO’s 59th Annual Meeting.
“Depression is widely recognized as an underdiagnosed disorder, particularly among older adults and cancer patients,” Dr Domogauer said. “Our findings point to a clear need for action, including depression screening during initial and continuing patient visits, initiation of mental health treatments for identified patients, and increased collaboration with mental health providers in cancer treatment centers. These efforts are particularly important for patients in urban centers, those who are female, and those who are unable to work because of their disease.”
Dr Domogauer and his colleagues studied 400 cancer patients who received treatment at Rutgers New Jersey Medical School/University Hospital Cancer Center between 2013 and 2016.
The average patient age was 55 (range, 20-86), and 53% of patients were female. Forty-eight percent of patients were African-American, 29% were non-Hispanic white, and 16% were Hispanic.
Nearly equal numbers of patients reported being able to work (49%) or unable to work due to disability (51%). Most patients (85%) received radiation as part of their cancer treatment.
The researchers assessed depression in the patients using a minimum score of 16 on the Center for Epidemiologic Studies Depression Scale.
In this way, 40% of the patients were diagnosed with depression. In 75% of these patients, depression was previously undiagnosed. This means roughly 30% of the overall patient population suffered from undiagnosed and untreated depression.
Depression was more common among females than males—47% and 32%, respectively (odds ratio [OR]=1.9, P=0.007).
Depression was also more likely among patients who were unable to work due to disability—48%, compared to 33% of those able to work (OR=1.9, P=0.005).
Depression prevalence did not differ significantly among racial/ethnic groups.
When the researchers looked specifically at patients who were previously not diagnosed with depression, the effects of being female or unable to work persisted.
In this subgroup, depression was more common among women than men—43% and 29%, respectively (OR=1.9, P=0.02)—and patients with disability compared to able patients—43% and 31%, respectively (OR=1.7, P=0.03). ![]()
Letter from an associate editor: Hurricane Harvey’s wrath
It seemed appropriate this month for me to step aside for the Editor’s commentary and provide a forum for one of our associate editors to talk about his experience during Hurricane Harvey.
John I. Allen, MD, MBA, AGAF
Editor in Chief
We knew that a powerful storm was coming, but very few anticipated the widespread destruction Hurricane Harvey would bring. Houston is no stranger to floods, but the amount of water that Harvey unleashed was record-breaking. Areas that had never flooded were underwater, evacuations were commonplace; the devastation was heart-breaking. In the midst of significant personal tragedy, Houston came together. Neighbors took in flooded colleagues, personal boats were used for rescues, and many braved impassable roads to donate clothes, food, labor and medical aid. Shelters across the city were assisted by volunteers; community groups collected and coordinated distribution of supplies. Medical teams were mobilized to treat chronically ill patients who evacuated without their medications or those injured while escaping the floods.
At one of the largest medical centers in the world, floodgates constructed after Tropical Storm Allison kept the waters at bay. And physicians, nurses, janitors, and other employees slept in hospitals for days to provide care to our patients during the worst of the floods. Those who relieved them worked long hours to see the many patients rescheduled in the aftermath of the storm. After-work crews of neighbors continue to go from house to house removing flooded floor boards and ripping out drywall. Houston came together.
Dr. Ketwaroo is an assistant professor in the division of gastroenterology and hepatology at Baylor College of Medicine, Houston, and an advanced endoscopist at the Michael E. Debakey VA Medical Center in Houston. He is an associate editor for GI & Hepatology News.
It seemed appropriate this month for me to step aside for the Editor’s commentary and provide a forum for one of our associate editors to talk about his experience during Hurricane Harvey.
John I. Allen, MD, MBA, AGAF
Editor in Chief
We knew that a powerful storm was coming, but very few anticipated the widespread destruction Hurricane Harvey would bring. Houston is no stranger to floods, but the amount of water that Harvey unleashed was record-breaking. Areas that had never flooded were underwater, evacuations were commonplace; the devastation was heart-breaking. In the midst of significant personal tragedy, Houston came together. Neighbors took in flooded colleagues, personal boats were used for rescues, and many braved impassable roads to donate clothes, food, labor and medical aid. Shelters across the city were assisted by volunteers; community groups collected and coordinated distribution of supplies. Medical teams were mobilized to treat chronically ill patients who evacuated without their medications or those injured while escaping the floods.
At one of the largest medical centers in the world, floodgates constructed after Tropical Storm Allison kept the waters at bay. And physicians, nurses, janitors, and other employees slept in hospitals for days to provide care to our patients during the worst of the floods. Those who relieved them worked long hours to see the many patients rescheduled in the aftermath of the storm. After-work crews of neighbors continue to go from house to house removing flooded floor boards and ripping out drywall. Houston came together.
Dr. Ketwaroo is an assistant professor in the division of gastroenterology and hepatology at Baylor College of Medicine, Houston, and an advanced endoscopist at the Michael E. Debakey VA Medical Center in Houston. He is an associate editor for GI & Hepatology News.
It seemed appropriate this month for me to step aside for the Editor’s commentary and provide a forum for one of our associate editors to talk about his experience during Hurricane Harvey.
John I. Allen, MD, MBA, AGAF
Editor in Chief
We knew that a powerful storm was coming, but very few anticipated the widespread destruction Hurricane Harvey would bring. Houston is no stranger to floods, but the amount of water that Harvey unleashed was record-breaking. Areas that had never flooded were underwater, evacuations were commonplace; the devastation was heart-breaking. In the midst of significant personal tragedy, Houston came together. Neighbors took in flooded colleagues, personal boats were used for rescues, and many braved impassable roads to donate clothes, food, labor and medical aid. Shelters across the city were assisted by volunteers; community groups collected and coordinated distribution of supplies. Medical teams were mobilized to treat chronically ill patients who evacuated without their medications or those injured while escaping the floods.
At one of the largest medical centers in the world, floodgates constructed after Tropical Storm Allison kept the waters at bay. And physicians, nurses, janitors, and other employees slept in hospitals for days to provide care to our patients during the worst of the floods. Those who relieved them worked long hours to see the many patients rescheduled in the aftermath of the storm. After-work crews of neighbors continue to go from house to house removing flooded floor boards and ripping out drywall. Houston came together.
Dr. Ketwaroo is an assistant professor in the division of gastroenterology and hepatology at Baylor College of Medicine, Houston, and an advanced endoscopist at the Michael E. Debakey VA Medical Center in Houston. He is an associate editor for GI & Hepatology News.
Pharmacologic Therapy for Acne: A Primer for Primary Care
CE/CME No: CR-1710
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Describe the main factors involved in the pathogenesis of acne.
• Assess acne severity and classify acne as mild, moderate, or severe.
• Describe available acne therapies, including their mechanisms of action, indications, and potential adverse effects.
• Identify strategies patients can employ to mitigate the adverse effects of acne treatments.
FACULTY
Janet Purath is an Associate Professor at Washington State University in Spokane, Washington. Theresa Coyner practices at Randall Dermatology, West Lafayette, Indiana.
The authors have no financial relationships to disclose.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid through September 30, 2018.
Article begins on next page >>
Many of the 50 million persons affected by acne in the United States present to primary care. Acne severity guides treatment choices, which include topical antibiotics and retinoids, hormonal agents, and systemic antibiotics and retinoids. Formulating a treatment plan requires a thorough understanding of the dosing, mechanism of action, and potential adverse effects of available medications.
Acne vulgaris (acne) is a common skin condition that is frequently encountered in primary care. Acne affects up to 50 million people in the United States, and about 85% of teenagers experience it at some point.1 Costs for treatment exceed $3 billion per year.2 Although commonly considered a condition of adolescence and young adults (85% prevalence), acne may persist in both men and women well into their 30s and 40s (43% prevalence). In fact, 5% of women ages 40 and older may experience acne.3
Acne is associated with considerable, long-lasting psychological sequelae, even in those with mild conditions, as many affected patients experience self-esteem issues and may avoid social interactions.4 Recognition of patients’ concerns about acne will help to promote a trusting patient-provider relationship. This article describes the pathophysiology and classifications of acne and reviews therapeutic options, enabling the practitioner to initiate treatment.
PRESENTATION AND ASSESSMENT
Acne lesions may occur on the face, neck, trunk, and extremities. The severity of acne is assessed based on lesion type, number, and size, and this grading is used to inform decisions about treatment options. Mild acne is characterized by plugging of the sebaceous gland (comedones), with small numbers of inflammatory papules and pustules. Moderate acne involves a larger number of inflammatory papules/pustules as well as the presence of small cystic nodules. Severe acne is marked by the presence of large numbers of noninflammatory and inflammatory lesions and cystic nodules or widespread involvement of these lesions.5 Examples of mild, moderate, and severe acne are shown in Figure 1. Assessment should include questions about the patient’s experiences with prior therapies.
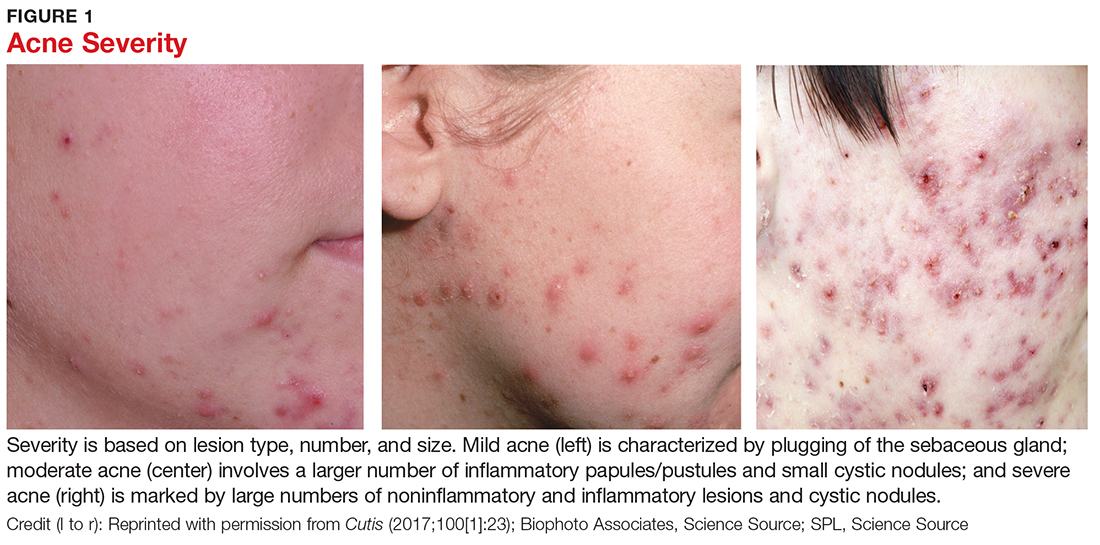
PATHOGENESIS
The pathogenesis of acne is a complex process involving multiple factors (see Figure 2). Knowledge about acne pathogenesis continues to evolve, but the current view is that a combination of simultaneous noninflammatory and inflammatory events involving pilosebaceous units (which consist of sebaceous glands and hair follicles) contribute to its development.6 Activation of the sebaceous glands is influenced by androgens, which increase sebum production and shedding of the keratinocytes lining the gland. Plugging of the pilosebaceous canal ensues, leading to the development of a microcomedone. Increased proliferation of Propionibacterium acnes occurs within the obstructed gland. The inflammatory response to this process includes a cascade of numerous cytokines, most notably toll-like receptor 2 (TLR-2).7 The plug at the opening of the sebaceous gland creates either an open comedone (blackhead) or a closed comedone (whitehead). Eventually, the follicular wall ruptures, leading to the formation of erythematous papules and pustules on the skin surface or deep-seated cystic structures under the skin surface. Current pharmacologic agents target one or more of these identified factors underlying acne pathogenesis.
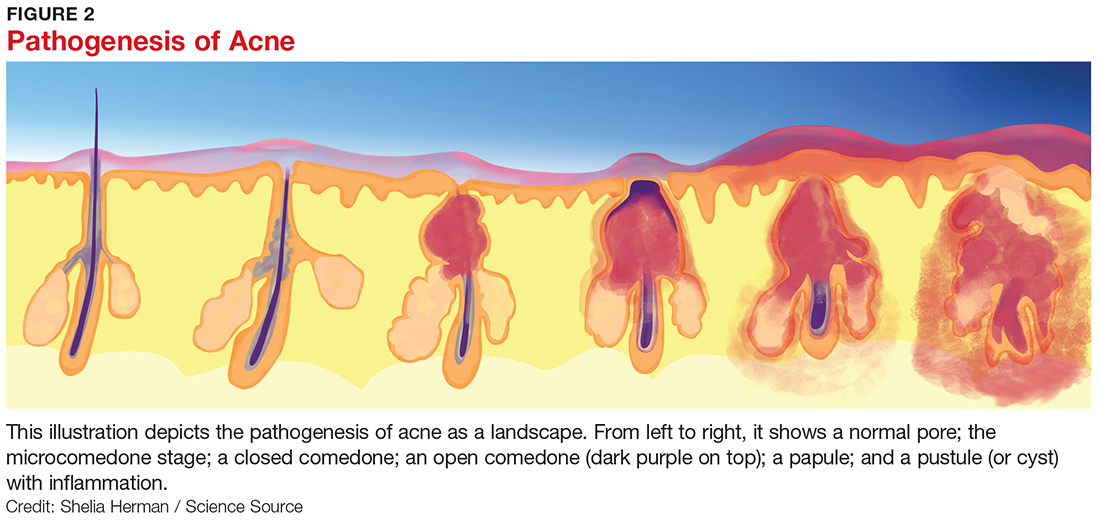
THERAPEUTIC OPTIONS
Pharmacologic treatment options for acne include topical, systemic, and hormonal agents. Topical and systemic therapies reduce inflammation and follicular plugging. Topical treatments include antibiotics, anti-inflammatories, and retinoids. Oral treatments include antibiotics, hormones, and retinoids. The clinician must have a thorough understanding of the actions, potential adverse reactions, and drug interactions of each proposed therapy prior to formulating a treatment plan.
Topical retinoids
Topical retinoids are the most effective comedolytic agents available.1 Since comedones are thought to be the precursor of all other acne lesions, retinoids are appropriate for cases in which comedones are seen.1 Retinoids belong to a class of compounds structurally related to vitamin A. Topical retinoids act by promoting normal follicular keratinocyte desquamation, which prevents obstruction of the pilosebaceous canal and thereby inhibits the formation of microcomedones.8
They also exhibit anti-inflammatory action via inhibition of TLR-2.9 The comedolytic and anti-inflammatory actions of topical retinoids make them a mainstay of acne treatment, although some patients are unable to tolerate their adverse effects, which include erythema and dryness related to increases in transepidermal water loss. Application of noncomedogenic emollients can improve these common effects.10 The newer micronized and time-release retinoid formulations may have less potential for irritation.8 Vehicle formulation and concentration also play a role in skin irritation, with gels and liquids and formulations with higher concentrations of retinoids generally causing more drying than creams and lower potency formulations.8 Table 1 summarizes the mechanisms of action, available formulations, and potential adverse effects of the topical retinoids and other topical agents.1,6,9-16
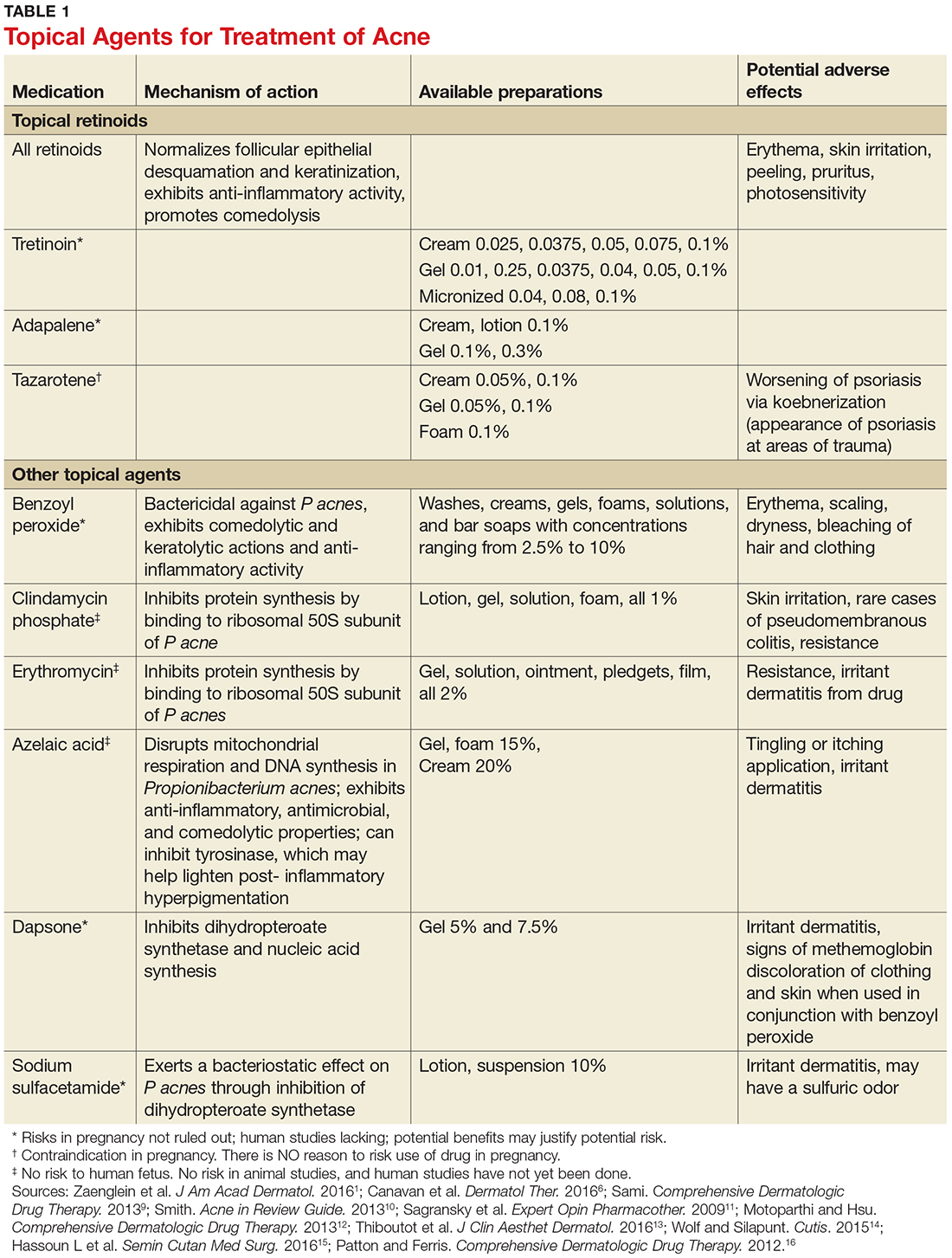
It is important to note that retinoids can adversely affect the developing fetus when absorbed in large quantities. Notably, tazarotene is assigned to pregnancy category X because when it is used to treat psoriasis, one of its approved indications, large surface areas may be treated, increasing absorption. Absorption amounts are extremely low when tazarotene is used to treat acne. Nevertheless, verification of a negative pregnancy test is recommended prior to initiating tazarotene therapy. Effective birth control measures should be utilized throughout therapy. Even though other commonly used retinoids (tretinoin and adapalene) are assigned to pregnancy category C, all topical retinoids should be avoided during pregnancy.9
As noted, patient education is key for increasing patient adherence to therapy. Patients should be instructed to use a small (pea-sized) amount of medication for the entire face. Providers should also inform patients that transient erythema and dryness can be expected, and that application of a noncomedolytic moisturizer may reduce irritation. Tretinoin is best used at night,1 and it is useful to advise that erythema and irritation associated with retinoid use can be reduced by initially using the medication every other night to every third night, gradually building up to nightly use.1
Topical antibiotic and anti-inflammatory agents
Topical agents used to treat inflammatory lesions include benzoyl peroxide, erythromycin, clindamycin, dapsone, azelaic acid, and sulfacetamide (Table 1).1,6,9-16 These topical agents are generally well tolerated, with most adverse reactions limited to facial irritation and erythema. They come in an array of vehicle formulations, including washes, creams, gels, solutions, foams, and lotions. Vehicle selection should be based upon patient preference and skin type. Gels and solutions have a drying effect, making them more appropriate for individuals with oily skin, whereas creams are moisturizing and appropriate for individuals with dry skin. Lotions are appropriate for all skin types.11
Benzoyl peroxide (BPO) has both keratolytic and comedolytic activity and is available in concentrations ranging from 2.5% to 10%. It is available OTC, as well as by prescription, and is thus readily accessed by the patient. Because BPO is bactericidal for P acnes, resistance to BPO among P acnes has not occurred.1 All concentrations are equally effective, but the higher concentrations are more likely to cause skin dryness and other adverse effects.12 Combination therapy with topical antibiotics, tretinoin, and BPO is more clinically effective than monotherapy.17 Combination products reduce the complexity of acne treatment and likely increase therapy adherence.11 Currently available combination products in various percentages are erythromycin with BPO, clindamycin with BPO, adapalene with BPO, and clindamycin with tretinoin.1
Oral antibiotics
Oral antibiotics should be reserved for use in situations where topical therapy is ineffective. All antibiotics are effective in treating acne due to their antimicrobial activity against P acnes.1 These agents play a key role in managing moderate to severe acne that is likely to scar, as well as in cases of widespread acne involving the face, arms, and trunk. Note that the use of oral antibiotics in acne treatment is controversial, as chronic use contributes to rising rates of bacterial resistance.18 For this reason, antibiotic therapy for acne should be limited to a duration of three months or less, and these agents should not be used as monotherapy.6 In particular, recent recommendations restrict the use of erythromycin for acne treatment due to an increase of P acnes resistance.1 Cephalosporins, macrolides, and penicillin class antibiotics are not routinely recommended due to lack of data regarding their clinical effectiveness in treating acne.1
Tetracycline class antibiotics are the most commonly used oral antibiotics for acne therapy, particularly doxycycline and minocycline.5 Common adverse effects include gastrointestinal upset, photosensitivity, and some pigmentation issues.19 Trimethoprim-sulfamethoxazole (TMP-SMX) is a folate synthesis inhibitor class antibiotic also used to treat acne. Its use should be reserved for individuals who are allergic to tetracyclines or in cases of acne resistant to other antimicrobials.1 Potential adverse reactions include photosensitivity and severe hypersensitivity conditions ranging from a mild rash to toxic epidermal necrolysis.19 Table 2 summarizes the dosage ranges, pregnancy category risk, and potential adverse effects of oral antibiotics used to treat acne.1,19,20
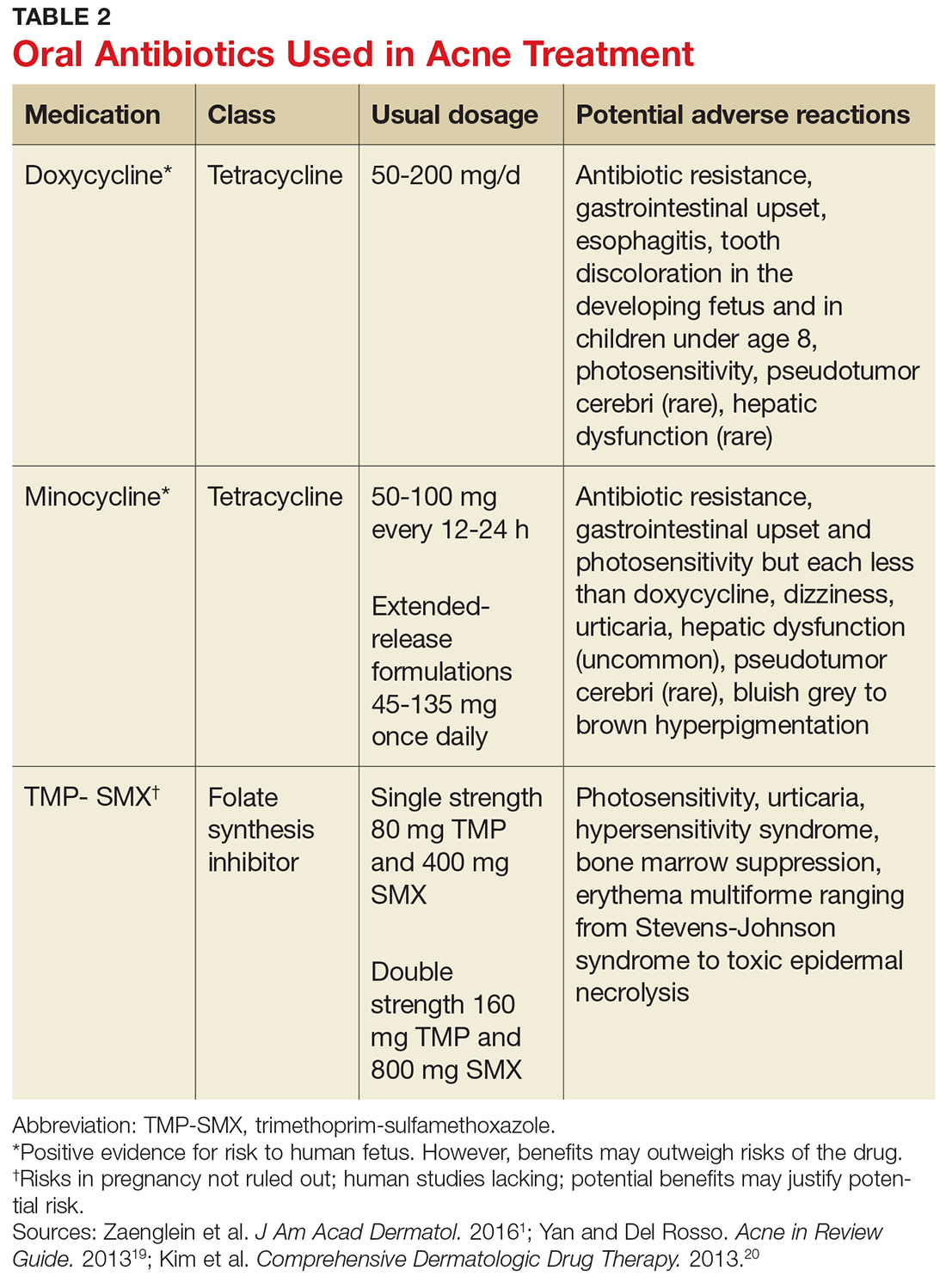
The firstline choice for treating moderate acne with papules and pustules is oral antibiotics with topical retinoids and BPO.5 Patients should be educated about potential adverse effects of these agents, including the development of antibiotic resistance.
Hormonal agents
Hormonal therapies should be reserved for females with acne lesions influenced by fluctuations in hormone levels.21 Pubertal changes initiate the production of adrenal dehydroepiandrosterone, which leads to increased testosterone production. Testosterone is converted to dihydrotestosterone (DHT), which binds to androgen receptors in the sebaceous glands, stimulating the glands and potentially increasing production of sebum. Hormonal agents act by reducing androgen activity in the sebaceous gland. Combined oral hormones, those containing both estrogen and progesterone, reduce the amount of free testosterone and ovarian androgens by suppressing ovulation.1 Hormonal therapy can be quite effective for females of childbearing age. Females who report acne flares with their menstrual cycles may be good candidates for hormonal therapy.1
The estrogen agent most frequently used in oral contraceptives is ethinyl estradiol. Numerous progesterone agents can also be used, but those with low
Spironolactone, a potassium-sparing diuretic, may also be appropriate for treating acne in women due to its antiandrogenic properties. The drug binds androgen receptors in the skin, which then blocks testosterone and DHT. Spironolactone can be an effective firstline agent in treating hormonal-pattern acne, which presents as inflammatory lesions located on the lower face and neck. In particular, it can be an appropriate choice for women with adult-onset acne.15 Spironolactone is not approved by the FDA for acne treatment, but it has been used successfully for many years.5 Spironolactone was found in rodent studies to cause feminization of the male rat fetus, so patients taking this drug should use reliable birth control methods. It can be used concomitantly with oral contraceptives.5 Common side effects include breast tenderness, diuretic effects, headaches, and menstrual irregularities. Although the risk for hypokalemia is low in healthy young women, it may be prudent to periodically assess potassium, sodium, and renal function in patients.1 Spironolactone should be avoided in patients with renal disease and those on other diuretics.15
Isotretinoin
Isotretinoin is an oral systemic retinoid that modulates nuclear receptors and regulates gene transcription in the epidermis.16 Isotretinoin’s mechanisms of action target the main pathogenic factors underlying acne, including reduction of follicular hyperkeratosis, comedogenesis, sebum production, and inflammation and suppression of P acnes.22 These combined actions make isotretinoin a highly effective treatment option for acne.
The drug is approved by the FDA for treatment of nodular acne refractory to traditional acne therapies.23 Isotretinoin is available in 10, 20, 25, 30, and 40 mg capsules, and the recommended dosing is 0.5 to 2.0 mg/kg/d. The usual course of therapy is 15 to 20 weeks or until an accumulative dosage of 120 to 150 mg/kg is attained.23 Patients should be instructed to take isotretinoin with meals, as oral availability is increased with high-fat foods.23
Isotretinoin has major adverse effects. It is a teratogenic medication that can cause congenital anomalies in exposed fetuses, including craniofacial, cardiac, and neurologic issues.16 Due to the seriousness of the congenital anomalies, all prescribers must be registered in the iPledge program, a computer-based risk management program instituted in 2006 by the FDA and the companies that manufacture isotretinoin to eliminate congenital risks associated with isotretinoin. All patients, both male and female, must sign an informed consent form when they register in the program.
Although the iPledge program does not mandate consistent condom use for male patients, they should be informed that minute amounts of isotretinoin can be found in semen. The risk for fathering a fetus with congenital anomalies when taking isotretinoin appears to be extremely low.16 Women of childbearing potential must commit to the use of two highly reliable forms of birth control when taking the medication, including one month before starting therapy and one month after completing therapy.16 Monthly pregnancy testing is mandatory throughout the course of treatment.24 Further information regarding the risk management program can be found at iPledgeprogram.com.
Isotretinoin is metabolized by the liver and may cause lipid abnormalities and hepatic enzyme elevations. Baseline and monthly laboratory monitoring of liver enzymes and cholesterol and triglyceride levels are recommended.24 The process of initiating and monitoring isotretinoin therapy is quite complex, and unless the practitioner plans to routinely prescribe this medication, patients needing isotretinoin therapy should be referred to a dermatology practice.
PATIENT EDUCATION
Patients are more likely to adhere to treatment when simplified regimens are used and when they have realistic expectations for therapy outcomes. Providers need to educate patients that all treatments may require at least two to three months of use before visible results occur. Initial and subsequent visits should include discussions about clear expectations and strategies to reduce potential adverse effects.
PUTTING IT ALL TOGETHER
Acne therapy starts with the use of a topical retinoid in mild acne cases, unless the patient is unable to tolerate the associated skin irritability. Addition of a topical antibiotic or anti-inflammatory agent, preferably BPO, either alone or with a combination product, is also recommended for mild to moderate acne. Patients with moderate to severe acne may benefit from a short course (three months or less) of antibiotics.
Oral hormones may be an excellent therapy choice when acne treatment is needed for women of childbearing age. Isotretinoin is indicated in select cases of severe acne resistant to other treatments.
1. Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74(5):945-973.
2. Bhate K, Williams HC. Epidemiology of acne vulgaris. Br J Dermatol. 2013;168(3):474-485.
3. Collier CN, Harper JC, Cafardi JA, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dertmatol. 2008;58(1):56-59.
4. Dunn LK, O’Neill JL, Feldman SR. Acne in adolescents: quality of life, self-esteem, mood, and psychological disorders. Dermatol Online J. 2011;17(1):1.
5. Baldwin HE, Zanglein AL, Leyden JJ, Webster GF. Pharmacologic treatment options in mild, moderate, and severe acne vulgaris. Semin Cutan Med Surg. 2015;34(supp5):S82-S85.
6. Canavan TN, Chen E, Elewski BE. Optimizing non-antibiotic treatments for patients with acne: a review. Dermatol Ther. 2016;6(4):555-578.
7. Bellew S, Thiboutot D, Del Rosso JQ. Pathogenesis of acne vulgaris: what’s new, what’s interesting and what may be clinically relevant. J Drugs Dermatol. 2011;10(6):582-585.
8. Russell JJ. Topical therapy for acne. Am Fam Phys. 2000; 61(2):357-365.
9. Sami N. Topical retinoids. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. St. Louis: Elsevier; 2013:505-517.
10. Smith RI. Treatments: Retinoids. In: Mancini AJ, Eichenfield LF, Del Rosso JQ, eds. Acne in Review Guide. New York: Educational Testing and Assessment Systems/SanovaWorks; 2013:59-63.
11. Sagransky M, Yentzer BA, Feldman SR. Benzoyl peroxide: a review of its current use in the treatment of acne vulgaris. Expert Opin Pharmacother. 2009;10(15):2555-2562.
12. Motoparthi K, Hsu S. Topical antibacterial agents. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. St Louis: Elsevier; 2013:452-459.
13. Thiboutot DM, Kircik L, McMichale A, et al. Efficacy, safety, and dermal tolerability of dapsone gel, 7.5% in patients with moderate acne vulgaris: a pooled analysis of two phase 3 trials. J Clin Aesthet Dermatol. 2016;9(10):18-27.
14. Wolf K, Silapunt S. The use of sodium sulfacetamide in dermatology. Cutis. 2015;96(2):128-130.
15. Hassoun LA, Chahal DS, Sivamani RK, Larsen LN. The use of hormonal agents in the treatment of acne. Semin Cutan Med Surg. 2016;35(2):68-73.
16. Patton TJ, Ferris LK. Systemic retinoids. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. St Louis: Elsevier; 2012:252-268.
17. Thiboutot D, Gollnick H, Bettoli V, et al. New insights into the management of acne: an update from the Global Alliance to Improve Outcomes in Acne group. J Am Acad Dermatol. 2009;60(5 Suppl):S1-S50.
18. Walsh TR, Efthimious J, Dreno B. Systematic review of antibiotic resistance in acne: an increasing topical and oral threat. Lancet Infect Dis. 2016;16(3):e22-32.
19. Yan AC, Del Rosso JQ. Prescription oral treatments: Antibiotics. In: Mancini AJ, Eichenfield LF, Del Rosso JQ, eds. Acne in Review Guide. New York: Educational Testing and Assessment Systems/SanovaWorks; 2013:77-87.
20. Kim S, Michaels BD, Kim GK, Del Rosso JQ. Systemic antibacterial agents. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. St Louis: Elsevier; 2013:62-67, 70-74, 77-85.
21. Hecht CT, Sidbury R, Del Rosso JQ. Prescription oral treatments: Hormonal therapies. In: Mancini AJ, Eichenfield LF, Del Rosso JQ, eds. Acne in Review Guide. New York: Educational Testing and Assessment Systems/SanovaWorks; 2013:89-95.
22. Webster GF, Leyden JJ, Baldwin HE, Zaenglein AL. Isotretinoin: mechanism of action and patient selection. Semin Cutan Med Surg. 2015;34(supp 5):S86-S88.
23. Leyden JJ, Del Rosso JQ, Baum EW. The use of isotretinoin in the treatment of acne vulgaris. J Clin and Aesthet Dermatol. 2014;7(2 Suppl):S3-S21.
24. Zane LT, Leyden WA, Marqueling AL, Manos MM. A population-based analysis of laboratory abnormalities during isotretinoin therapy for acne vulgaris. Arch Dermatol. 2006;142(8):1016-1022.
CE/CME No: CR-1710
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Describe the main factors involved in the pathogenesis of acne.
• Assess acne severity and classify acne as mild, moderate, or severe.
• Describe available acne therapies, including their mechanisms of action, indications, and potential adverse effects.
• Identify strategies patients can employ to mitigate the adverse effects of acne treatments.
FACULTY
Janet Purath is an Associate Professor at Washington State University in Spokane, Washington. Theresa Coyner practices at Randall Dermatology, West Lafayette, Indiana.
The authors have no financial relationships to disclose.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid through September 30, 2018.
Article begins on next page >>
Many of the 50 million persons affected by acne in the United States present to primary care. Acne severity guides treatment choices, which include topical antibiotics and retinoids, hormonal agents, and systemic antibiotics and retinoids. Formulating a treatment plan requires a thorough understanding of the dosing, mechanism of action, and potential adverse effects of available medications.
Acne vulgaris (acne) is a common skin condition that is frequently encountered in primary care. Acne affects up to 50 million people in the United States, and about 85% of teenagers experience it at some point.1 Costs for treatment exceed $3 billion per year.2 Although commonly considered a condition of adolescence and young adults (85% prevalence), acne may persist in both men and women well into their 30s and 40s (43% prevalence). In fact, 5% of women ages 40 and older may experience acne.3
Acne is associated with considerable, long-lasting psychological sequelae, even in those with mild conditions, as many affected patients experience self-esteem issues and may avoid social interactions.4 Recognition of patients’ concerns about acne will help to promote a trusting patient-provider relationship. This article describes the pathophysiology and classifications of acne and reviews therapeutic options, enabling the practitioner to initiate treatment.
PRESENTATION AND ASSESSMENT
Acne lesions may occur on the face, neck, trunk, and extremities. The severity of acne is assessed based on lesion type, number, and size, and this grading is used to inform decisions about treatment options. Mild acne is characterized by plugging of the sebaceous gland (comedones), with small numbers of inflammatory papules and pustules. Moderate acne involves a larger number of inflammatory papules/pustules as well as the presence of small cystic nodules. Severe acne is marked by the presence of large numbers of noninflammatory and inflammatory lesions and cystic nodules or widespread involvement of these lesions.5 Examples of mild, moderate, and severe acne are shown in Figure 1. Assessment should include questions about the patient’s experiences with prior therapies.

PATHOGENESIS
The pathogenesis of acne is a complex process involving multiple factors (see Figure 2). Knowledge about acne pathogenesis continues to evolve, but the current view is that a combination of simultaneous noninflammatory and inflammatory events involving pilosebaceous units (which consist of sebaceous glands and hair follicles) contribute to its development.6 Activation of the sebaceous glands is influenced by androgens, which increase sebum production and shedding of the keratinocytes lining the gland. Plugging of the pilosebaceous canal ensues, leading to the development of a microcomedone. Increased proliferation of Propionibacterium acnes occurs within the obstructed gland. The inflammatory response to this process includes a cascade of numerous cytokines, most notably toll-like receptor 2 (TLR-2).7 The plug at the opening of the sebaceous gland creates either an open comedone (blackhead) or a closed comedone (whitehead). Eventually, the follicular wall ruptures, leading to the formation of erythematous papules and pustules on the skin surface or deep-seated cystic structures under the skin surface. Current pharmacologic agents target one or more of these identified factors underlying acne pathogenesis.

THERAPEUTIC OPTIONS
Pharmacologic treatment options for acne include topical, systemic, and hormonal agents. Topical and systemic therapies reduce inflammation and follicular plugging. Topical treatments include antibiotics, anti-inflammatories, and retinoids. Oral treatments include antibiotics, hormones, and retinoids. The clinician must have a thorough understanding of the actions, potential adverse reactions, and drug interactions of each proposed therapy prior to formulating a treatment plan.
Topical retinoids
Topical retinoids are the most effective comedolytic agents available.1 Since comedones are thought to be the precursor of all other acne lesions, retinoids are appropriate for cases in which comedones are seen.1 Retinoids belong to a class of compounds structurally related to vitamin A. Topical retinoids act by promoting normal follicular keratinocyte desquamation, which prevents obstruction of the pilosebaceous canal and thereby inhibits the formation of microcomedones.8
They also exhibit anti-inflammatory action via inhibition of TLR-2.9 The comedolytic and anti-inflammatory actions of topical retinoids make them a mainstay of acne treatment, although some patients are unable to tolerate their adverse effects, which include erythema and dryness related to increases in transepidermal water loss. Application of noncomedogenic emollients can improve these common effects.10 The newer micronized and time-release retinoid formulations may have less potential for irritation.8 Vehicle formulation and concentration also play a role in skin irritation, with gels and liquids and formulations with higher concentrations of retinoids generally causing more drying than creams and lower potency formulations.8 Table 1 summarizes the mechanisms of action, available formulations, and potential adverse effects of the topical retinoids and other topical agents.1,6,9-16

It is important to note that retinoids can adversely affect the developing fetus when absorbed in large quantities. Notably, tazarotene is assigned to pregnancy category X because when it is used to treat psoriasis, one of its approved indications, large surface areas may be treated, increasing absorption. Absorption amounts are extremely low when tazarotene is used to treat acne. Nevertheless, verification of a negative pregnancy test is recommended prior to initiating tazarotene therapy. Effective birth control measures should be utilized throughout therapy. Even though other commonly used retinoids (tretinoin and adapalene) are assigned to pregnancy category C, all topical retinoids should be avoided during pregnancy.9
As noted, patient education is key for increasing patient adherence to therapy. Patients should be instructed to use a small (pea-sized) amount of medication for the entire face. Providers should also inform patients that transient erythema and dryness can be expected, and that application of a noncomedolytic moisturizer may reduce irritation. Tretinoin is best used at night,1 and it is useful to advise that erythema and irritation associated with retinoid use can be reduced by initially using the medication every other night to every third night, gradually building up to nightly use.1
Topical antibiotic and anti-inflammatory agents
Topical agents used to treat inflammatory lesions include benzoyl peroxide, erythromycin, clindamycin, dapsone, azelaic acid, and sulfacetamide (Table 1).1,6,9-16 These topical agents are generally well tolerated, with most adverse reactions limited to facial irritation and erythema. They come in an array of vehicle formulations, including washes, creams, gels, solutions, foams, and lotions. Vehicle selection should be based upon patient preference and skin type. Gels and solutions have a drying effect, making them more appropriate for individuals with oily skin, whereas creams are moisturizing and appropriate for individuals with dry skin. Lotions are appropriate for all skin types.11
Benzoyl peroxide (BPO) has both keratolytic and comedolytic activity and is available in concentrations ranging from 2.5% to 10%. It is available OTC, as well as by prescription, and is thus readily accessed by the patient. Because BPO is bactericidal for P acnes, resistance to BPO among P acnes has not occurred.1 All concentrations are equally effective, but the higher concentrations are more likely to cause skin dryness and other adverse effects.12 Combination therapy with topical antibiotics, tretinoin, and BPO is more clinically effective than monotherapy.17 Combination products reduce the complexity of acne treatment and likely increase therapy adherence.11 Currently available combination products in various percentages are erythromycin with BPO, clindamycin with BPO, adapalene with BPO, and clindamycin with tretinoin.1
Oral antibiotics
Oral antibiotics should be reserved for use in situations where topical therapy is ineffective. All antibiotics are effective in treating acne due to their antimicrobial activity against P acnes.1 These agents play a key role in managing moderate to severe acne that is likely to scar, as well as in cases of widespread acne involving the face, arms, and trunk. Note that the use of oral antibiotics in acne treatment is controversial, as chronic use contributes to rising rates of bacterial resistance.18 For this reason, antibiotic therapy for acne should be limited to a duration of three months or less, and these agents should not be used as monotherapy.6 In particular, recent recommendations restrict the use of erythromycin for acne treatment due to an increase of P acnes resistance.1 Cephalosporins, macrolides, and penicillin class antibiotics are not routinely recommended due to lack of data regarding their clinical effectiveness in treating acne.1
Tetracycline class antibiotics are the most commonly used oral antibiotics for acne therapy, particularly doxycycline and minocycline.5 Common adverse effects include gastrointestinal upset, photosensitivity, and some pigmentation issues.19 Trimethoprim-sulfamethoxazole (TMP-SMX) is a folate synthesis inhibitor class antibiotic also used to treat acne. Its use should be reserved for individuals who are allergic to tetracyclines or in cases of acne resistant to other antimicrobials.1 Potential adverse reactions include photosensitivity and severe hypersensitivity conditions ranging from a mild rash to toxic epidermal necrolysis.19 Table 2 summarizes the dosage ranges, pregnancy category risk, and potential adverse effects of oral antibiotics used to treat acne.1,19,20

The firstline choice for treating moderate acne with papules and pustules is oral antibiotics with topical retinoids and BPO.5 Patients should be educated about potential adverse effects of these agents, including the development of antibiotic resistance.
Hormonal agents
Hormonal therapies should be reserved for females with acne lesions influenced by fluctuations in hormone levels.21 Pubertal changes initiate the production of adrenal dehydroepiandrosterone, which leads to increased testosterone production. Testosterone is converted to dihydrotestosterone (DHT), which binds to androgen receptors in the sebaceous glands, stimulating the glands and potentially increasing production of sebum. Hormonal agents act by reducing androgen activity in the sebaceous gland. Combined oral hormones, those containing both estrogen and progesterone, reduce the amount of free testosterone and ovarian androgens by suppressing ovulation.1 Hormonal therapy can be quite effective for females of childbearing age. Females who report acne flares with their menstrual cycles may be good candidates for hormonal therapy.1
The estrogen agent most frequently used in oral contraceptives is ethinyl estradiol. Numerous progesterone agents can also be used, but those with low
Spironolactone, a potassium-sparing diuretic, may also be appropriate for treating acne in women due to its antiandrogenic properties. The drug binds androgen receptors in the skin, which then blocks testosterone and DHT. Spironolactone can be an effective firstline agent in treating hormonal-pattern acne, which presents as inflammatory lesions located on the lower face and neck. In particular, it can be an appropriate choice for women with adult-onset acne.15 Spironolactone is not approved by the FDA for acne treatment, but it has been used successfully for many years.5 Spironolactone was found in rodent studies to cause feminization of the male rat fetus, so patients taking this drug should use reliable birth control methods. It can be used concomitantly with oral contraceptives.5 Common side effects include breast tenderness, diuretic effects, headaches, and menstrual irregularities. Although the risk for hypokalemia is low in healthy young women, it may be prudent to periodically assess potassium, sodium, and renal function in patients.1 Spironolactone should be avoided in patients with renal disease and those on other diuretics.15
Isotretinoin
Isotretinoin is an oral systemic retinoid that modulates nuclear receptors and regulates gene transcription in the epidermis.16 Isotretinoin’s mechanisms of action target the main pathogenic factors underlying acne, including reduction of follicular hyperkeratosis, comedogenesis, sebum production, and inflammation and suppression of P acnes.22 These combined actions make isotretinoin a highly effective treatment option for acne.
The drug is approved by the FDA for treatment of nodular acne refractory to traditional acne therapies.23 Isotretinoin is available in 10, 20, 25, 30, and 40 mg capsules, and the recommended dosing is 0.5 to 2.0 mg/kg/d. The usual course of therapy is 15 to 20 weeks or until an accumulative dosage of 120 to 150 mg/kg is attained.23 Patients should be instructed to take isotretinoin with meals, as oral availability is increased with high-fat foods.23
Isotretinoin has major adverse effects. It is a teratogenic medication that can cause congenital anomalies in exposed fetuses, including craniofacial, cardiac, and neurologic issues.16 Due to the seriousness of the congenital anomalies, all prescribers must be registered in the iPledge program, a computer-based risk management program instituted in 2006 by the FDA and the companies that manufacture isotretinoin to eliminate congenital risks associated with isotretinoin. All patients, both male and female, must sign an informed consent form when they register in the program.
Although the iPledge program does not mandate consistent condom use for male patients, they should be informed that minute amounts of isotretinoin can be found in semen. The risk for fathering a fetus with congenital anomalies when taking isotretinoin appears to be extremely low.16 Women of childbearing potential must commit to the use of two highly reliable forms of birth control when taking the medication, including one month before starting therapy and one month after completing therapy.16 Monthly pregnancy testing is mandatory throughout the course of treatment.24 Further information regarding the risk management program can be found at iPledgeprogram.com.
Isotretinoin is metabolized by the liver and may cause lipid abnormalities and hepatic enzyme elevations. Baseline and monthly laboratory monitoring of liver enzymes and cholesterol and triglyceride levels are recommended.24 The process of initiating and monitoring isotretinoin therapy is quite complex, and unless the practitioner plans to routinely prescribe this medication, patients needing isotretinoin therapy should be referred to a dermatology practice.
PATIENT EDUCATION
Patients are more likely to adhere to treatment when simplified regimens are used and when they have realistic expectations for therapy outcomes. Providers need to educate patients that all treatments may require at least two to three months of use before visible results occur. Initial and subsequent visits should include discussions about clear expectations and strategies to reduce potential adverse effects.
PUTTING IT ALL TOGETHER
Acne therapy starts with the use of a topical retinoid in mild acne cases, unless the patient is unable to tolerate the associated skin irritability. Addition of a topical antibiotic or anti-inflammatory agent, preferably BPO, either alone or with a combination product, is also recommended for mild to moderate acne. Patients with moderate to severe acne may benefit from a short course (three months or less) of antibiotics.
Oral hormones may be an excellent therapy choice when acne treatment is needed for women of childbearing age. Isotretinoin is indicated in select cases of severe acne resistant to other treatments.
CE/CME No: CR-1710
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Describe the main factors involved in the pathogenesis of acne.
• Assess acne severity and classify acne as mild, moderate, or severe.
• Describe available acne therapies, including their mechanisms of action, indications, and potential adverse effects.
• Identify strategies patients can employ to mitigate the adverse effects of acne treatments.
FACULTY
Janet Purath is an Associate Professor at Washington State University in Spokane, Washington. Theresa Coyner practices at Randall Dermatology, West Lafayette, Indiana.
The authors have no financial relationships to disclose.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid through September 30, 2018.
Article begins on next page >>
Many of the 50 million persons affected by acne in the United States present to primary care. Acne severity guides treatment choices, which include topical antibiotics and retinoids, hormonal agents, and systemic antibiotics and retinoids. Formulating a treatment plan requires a thorough understanding of the dosing, mechanism of action, and potential adverse effects of available medications.
Acne vulgaris (acne) is a common skin condition that is frequently encountered in primary care. Acne affects up to 50 million people in the United States, and about 85% of teenagers experience it at some point.1 Costs for treatment exceed $3 billion per year.2 Although commonly considered a condition of adolescence and young adults (85% prevalence), acne may persist in both men and women well into their 30s and 40s (43% prevalence). In fact, 5% of women ages 40 and older may experience acne.3
Acne is associated with considerable, long-lasting psychological sequelae, even in those with mild conditions, as many affected patients experience self-esteem issues and may avoid social interactions.4 Recognition of patients’ concerns about acne will help to promote a trusting patient-provider relationship. This article describes the pathophysiology and classifications of acne and reviews therapeutic options, enabling the practitioner to initiate treatment.
PRESENTATION AND ASSESSMENT
Acne lesions may occur on the face, neck, trunk, and extremities. The severity of acne is assessed based on lesion type, number, and size, and this grading is used to inform decisions about treatment options. Mild acne is characterized by plugging of the sebaceous gland (comedones), with small numbers of inflammatory papules and pustules. Moderate acne involves a larger number of inflammatory papules/pustules as well as the presence of small cystic nodules. Severe acne is marked by the presence of large numbers of noninflammatory and inflammatory lesions and cystic nodules or widespread involvement of these lesions.5 Examples of mild, moderate, and severe acne are shown in Figure 1. Assessment should include questions about the patient’s experiences with prior therapies.

PATHOGENESIS
The pathogenesis of acne is a complex process involving multiple factors (see Figure 2). Knowledge about acne pathogenesis continues to evolve, but the current view is that a combination of simultaneous noninflammatory and inflammatory events involving pilosebaceous units (which consist of sebaceous glands and hair follicles) contribute to its development.6 Activation of the sebaceous glands is influenced by androgens, which increase sebum production and shedding of the keratinocytes lining the gland. Plugging of the pilosebaceous canal ensues, leading to the development of a microcomedone. Increased proliferation of Propionibacterium acnes occurs within the obstructed gland. The inflammatory response to this process includes a cascade of numerous cytokines, most notably toll-like receptor 2 (TLR-2).7 The plug at the opening of the sebaceous gland creates either an open comedone (blackhead) or a closed comedone (whitehead). Eventually, the follicular wall ruptures, leading to the formation of erythematous papules and pustules on the skin surface or deep-seated cystic structures under the skin surface. Current pharmacologic agents target one or more of these identified factors underlying acne pathogenesis.

THERAPEUTIC OPTIONS
Pharmacologic treatment options for acne include topical, systemic, and hormonal agents. Topical and systemic therapies reduce inflammation and follicular plugging. Topical treatments include antibiotics, anti-inflammatories, and retinoids. Oral treatments include antibiotics, hormones, and retinoids. The clinician must have a thorough understanding of the actions, potential adverse reactions, and drug interactions of each proposed therapy prior to formulating a treatment plan.
Topical retinoids
Topical retinoids are the most effective comedolytic agents available.1 Since comedones are thought to be the precursor of all other acne lesions, retinoids are appropriate for cases in which comedones are seen.1 Retinoids belong to a class of compounds structurally related to vitamin A. Topical retinoids act by promoting normal follicular keratinocyte desquamation, which prevents obstruction of the pilosebaceous canal and thereby inhibits the formation of microcomedones.8
They also exhibit anti-inflammatory action via inhibition of TLR-2.9 The comedolytic and anti-inflammatory actions of topical retinoids make them a mainstay of acne treatment, although some patients are unable to tolerate their adverse effects, which include erythema and dryness related to increases in transepidermal water loss. Application of noncomedogenic emollients can improve these common effects.10 The newer micronized and time-release retinoid formulations may have less potential for irritation.8 Vehicle formulation and concentration also play a role in skin irritation, with gels and liquids and formulations with higher concentrations of retinoids generally causing more drying than creams and lower potency formulations.8 Table 1 summarizes the mechanisms of action, available formulations, and potential adverse effects of the topical retinoids and other topical agents.1,6,9-16

It is important to note that retinoids can adversely affect the developing fetus when absorbed in large quantities. Notably, tazarotene is assigned to pregnancy category X because when it is used to treat psoriasis, one of its approved indications, large surface areas may be treated, increasing absorption. Absorption amounts are extremely low when tazarotene is used to treat acne. Nevertheless, verification of a negative pregnancy test is recommended prior to initiating tazarotene therapy. Effective birth control measures should be utilized throughout therapy. Even though other commonly used retinoids (tretinoin and adapalene) are assigned to pregnancy category C, all topical retinoids should be avoided during pregnancy.9
As noted, patient education is key for increasing patient adherence to therapy. Patients should be instructed to use a small (pea-sized) amount of medication for the entire face. Providers should also inform patients that transient erythema and dryness can be expected, and that application of a noncomedolytic moisturizer may reduce irritation. Tretinoin is best used at night,1 and it is useful to advise that erythema and irritation associated with retinoid use can be reduced by initially using the medication every other night to every third night, gradually building up to nightly use.1
Topical antibiotic and anti-inflammatory agents
Topical agents used to treat inflammatory lesions include benzoyl peroxide, erythromycin, clindamycin, dapsone, azelaic acid, and sulfacetamide (Table 1).1,6,9-16 These topical agents are generally well tolerated, with most adverse reactions limited to facial irritation and erythema. They come in an array of vehicle formulations, including washes, creams, gels, solutions, foams, and lotions. Vehicle selection should be based upon patient preference and skin type. Gels and solutions have a drying effect, making them more appropriate for individuals with oily skin, whereas creams are moisturizing and appropriate for individuals with dry skin. Lotions are appropriate for all skin types.11
Benzoyl peroxide (BPO) has both keratolytic and comedolytic activity and is available in concentrations ranging from 2.5% to 10%. It is available OTC, as well as by prescription, and is thus readily accessed by the patient. Because BPO is bactericidal for P acnes, resistance to BPO among P acnes has not occurred.1 All concentrations are equally effective, but the higher concentrations are more likely to cause skin dryness and other adverse effects.12 Combination therapy with topical antibiotics, tretinoin, and BPO is more clinically effective than monotherapy.17 Combination products reduce the complexity of acne treatment and likely increase therapy adherence.11 Currently available combination products in various percentages are erythromycin with BPO, clindamycin with BPO, adapalene with BPO, and clindamycin with tretinoin.1
Oral antibiotics
Oral antibiotics should be reserved for use in situations where topical therapy is ineffective. All antibiotics are effective in treating acne due to their antimicrobial activity against P acnes.1 These agents play a key role in managing moderate to severe acne that is likely to scar, as well as in cases of widespread acne involving the face, arms, and trunk. Note that the use of oral antibiotics in acne treatment is controversial, as chronic use contributes to rising rates of bacterial resistance.18 For this reason, antibiotic therapy for acne should be limited to a duration of three months or less, and these agents should not be used as monotherapy.6 In particular, recent recommendations restrict the use of erythromycin for acne treatment due to an increase of P acnes resistance.1 Cephalosporins, macrolides, and penicillin class antibiotics are not routinely recommended due to lack of data regarding their clinical effectiveness in treating acne.1
Tetracycline class antibiotics are the most commonly used oral antibiotics for acne therapy, particularly doxycycline and minocycline.5 Common adverse effects include gastrointestinal upset, photosensitivity, and some pigmentation issues.19 Trimethoprim-sulfamethoxazole (TMP-SMX) is a folate synthesis inhibitor class antibiotic also used to treat acne. Its use should be reserved for individuals who are allergic to tetracyclines or in cases of acne resistant to other antimicrobials.1 Potential adverse reactions include photosensitivity and severe hypersensitivity conditions ranging from a mild rash to toxic epidermal necrolysis.19 Table 2 summarizes the dosage ranges, pregnancy category risk, and potential adverse effects of oral antibiotics used to treat acne.1,19,20

The firstline choice for treating moderate acne with papules and pustules is oral antibiotics with topical retinoids and BPO.5 Patients should be educated about potential adverse effects of these agents, including the development of antibiotic resistance.
Hormonal agents
Hormonal therapies should be reserved for females with acne lesions influenced by fluctuations in hormone levels.21 Pubertal changes initiate the production of adrenal dehydroepiandrosterone, which leads to increased testosterone production. Testosterone is converted to dihydrotestosterone (DHT), which binds to androgen receptors in the sebaceous glands, stimulating the glands and potentially increasing production of sebum. Hormonal agents act by reducing androgen activity in the sebaceous gland. Combined oral hormones, those containing both estrogen and progesterone, reduce the amount of free testosterone and ovarian androgens by suppressing ovulation.1 Hormonal therapy can be quite effective for females of childbearing age. Females who report acne flares with their menstrual cycles may be good candidates for hormonal therapy.1
The estrogen agent most frequently used in oral contraceptives is ethinyl estradiol. Numerous progesterone agents can also be used, but those with low
Spironolactone, a potassium-sparing diuretic, may also be appropriate for treating acne in women due to its antiandrogenic properties. The drug binds androgen receptors in the skin, which then blocks testosterone and DHT. Spironolactone can be an effective firstline agent in treating hormonal-pattern acne, which presents as inflammatory lesions located on the lower face and neck. In particular, it can be an appropriate choice for women with adult-onset acne.15 Spironolactone is not approved by the FDA for acne treatment, but it has been used successfully for many years.5 Spironolactone was found in rodent studies to cause feminization of the male rat fetus, so patients taking this drug should use reliable birth control methods. It can be used concomitantly with oral contraceptives.5 Common side effects include breast tenderness, diuretic effects, headaches, and menstrual irregularities. Although the risk for hypokalemia is low in healthy young women, it may be prudent to periodically assess potassium, sodium, and renal function in patients.1 Spironolactone should be avoided in patients with renal disease and those on other diuretics.15
Isotretinoin
Isotretinoin is an oral systemic retinoid that modulates nuclear receptors and regulates gene transcription in the epidermis.16 Isotretinoin’s mechanisms of action target the main pathogenic factors underlying acne, including reduction of follicular hyperkeratosis, comedogenesis, sebum production, and inflammation and suppression of P acnes.22 These combined actions make isotretinoin a highly effective treatment option for acne.
The drug is approved by the FDA for treatment of nodular acne refractory to traditional acne therapies.23 Isotretinoin is available in 10, 20, 25, 30, and 40 mg capsules, and the recommended dosing is 0.5 to 2.0 mg/kg/d. The usual course of therapy is 15 to 20 weeks or until an accumulative dosage of 120 to 150 mg/kg is attained.23 Patients should be instructed to take isotretinoin with meals, as oral availability is increased with high-fat foods.23
Isotretinoin has major adverse effects. It is a teratogenic medication that can cause congenital anomalies in exposed fetuses, including craniofacial, cardiac, and neurologic issues.16 Due to the seriousness of the congenital anomalies, all prescribers must be registered in the iPledge program, a computer-based risk management program instituted in 2006 by the FDA and the companies that manufacture isotretinoin to eliminate congenital risks associated with isotretinoin. All patients, both male and female, must sign an informed consent form when they register in the program.
Although the iPledge program does not mandate consistent condom use for male patients, they should be informed that minute amounts of isotretinoin can be found in semen. The risk for fathering a fetus with congenital anomalies when taking isotretinoin appears to be extremely low.16 Women of childbearing potential must commit to the use of two highly reliable forms of birth control when taking the medication, including one month before starting therapy and one month after completing therapy.16 Monthly pregnancy testing is mandatory throughout the course of treatment.24 Further information regarding the risk management program can be found at iPledgeprogram.com.
Isotretinoin is metabolized by the liver and may cause lipid abnormalities and hepatic enzyme elevations. Baseline and monthly laboratory monitoring of liver enzymes and cholesterol and triglyceride levels are recommended.24 The process of initiating and monitoring isotretinoin therapy is quite complex, and unless the practitioner plans to routinely prescribe this medication, patients needing isotretinoin therapy should be referred to a dermatology practice.
PATIENT EDUCATION
Patients are more likely to adhere to treatment when simplified regimens are used and when they have realistic expectations for therapy outcomes. Providers need to educate patients that all treatments may require at least two to three months of use before visible results occur. Initial and subsequent visits should include discussions about clear expectations and strategies to reduce potential adverse effects.
PUTTING IT ALL TOGETHER
Acne therapy starts with the use of a topical retinoid in mild acne cases, unless the patient is unable to tolerate the associated skin irritability. Addition of a topical antibiotic or anti-inflammatory agent, preferably BPO, either alone or with a combination product, is also recommended for mild to moderate acne. Patients with moderate to severe acne may benefit from a short course (three months or less) of antibiotics.
Oral hormones may be an excellent therapy choice when acne treatment is needed for women of childbearing age. Isotretinoin is indicated in select cases of severe acne resistant to other treatments.
1. Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74(5):945-973.
2. Bhate K, Williams HC. Epidemiology of acne vulgaris. Br J Dermatol. 2013;168(3):474-485.
3. Collier CN, Harper JC, Cafardi JA, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dertmatol. 2008;58(1):56-59.
4. Dunn LK, O’Neill JL, Feldman SR. Acne in adolescents: quality of life, self-esteem, mood, and psychological disorders. Dermatol Online J. 2011;17(1):1.
5. Baldwin HE, Zanglein AL, Leyden JJ, Webster GF. Pharmacologic treatment options in mild, moderate, and severe acne vulgaris. Semin Cutan Med Surg. 2015;34(supp5):S82-S85.
6. Canavan TN, Chen E, Elewski BE. Optimizing non-antibiotic treatments for patients with acne: a review. Dermatol Ther. 2016;6(4):555-578.
7. Bellew S, Thiboutot D, Del Rosso JQ. Pathogenesis of acne vulgaris: what’s new, what’s interesting and what may be clinically relevant. J Drugs Dermatol. 2011;10(6):582-585.
8. Russell JJ. Topical therapy for acne. Am Fam Phys. 2000; 61(2):357-365.
9. Sami N. Topical retinoids. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. St. Louis: Elsevier; 2013:505-517.
10. Smith RI. Treatments: Retinoids. In: Mancini AJ, Eichenfield LF, Del Rosso JQ, eds. Acne in Review Guide. New York: Educational Testing and Assessment Systems/SanovaWorks; 2013:59-63.
11. Sagransky M, Yentzer BA, Feldman SR. Benzoyl peroxide: a review of its current use in the treatment of acne vulgaris. Expert Opin Pharmacother. 2009;10(15):2555-2562.
12. Motoparthi K, Hsu S. Topical antibacterial agents. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. St Louis: Elsevier; 2013:452-459.
13. Thiboutot DM, Kircik L, McMichale A, et al. Efficacy, safety, and dermal tolerability of dapsone gel, 7.5% in patients with moderate acne vulgaris: a pooled analysis of two phase 3 trials. J Clin Aesthet Dermatol. 2016;9(10):18-27.
14. Wolf K, Silapunt S. The use of sodium sulfacetamide in dermatology. Cutis. 2015;96(2):128-130.
15. Hassoun LA, Chahal DS, Sivamani RK, Larsen LN. The use of hormonal agents in the treatment of acne. Semin Cutan Med Surg. 2016;35(2):68-73.
16. Patton TJ, Ferris LK. Systemic retinoids. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. St Louis: Elsevier; 2012:252-268.
17. Thiboutot D, Gollnick H, Bettoli V, et al. New insights into the management of acne: an update from the Global Alliance to Improve Outcomes in Acne group. J Am Acad Dermatol. 2009;60(5 Suppl):S1-S50.
18. Walsh TR, Efthimious J, Dreno B. Systematic review of antibiotic resistance in acne: an increasing topical and oral threat. Lancet Infect Dis. 2016;16(3):e22-32.
19. Yan AC, Del Rosso JQ. Prescription oral treatments: Antibiotics. In: Mancini AJ, Eichenfield LF, Del Rosso JQ, eds. Acne in Review Guide. New York: Educational Testing and Assessment Systems/SanovaWorks; 2013:77-87.
20. Kim S, Michaels BD, Kim GK, Del Rosso JQ. Systemic antibacterial agents. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. St Louis: Elsevier; 2013:62-67, 70-74, 77-85.
21. Hecht CT, Sidbury R, Del Rosso JQ. Prescription oral treatments: Hormonal therapies. In: Mancini AJ, Eichenfield LF, Del Rosso JQ, eds. Acne in Review Guide. New York: Educational Testing and Assessment Systems/SanovaWorks; 2013:89-95.
22. Webster GF, Leyden JJ, Baldwin HE, Zaenglein AL. Isotretinoin: mechanism of action and patient selection. Semin Cutan Med Surg. 2015;34(supp 5):S86-S88.
23. Leyden JJ, Del Rosso JQ, Baum EW. The use of isotretinoin in the treatment of acne vulgaris. J Clin and Aesthet Dermatol. 2014;7(2 Suppl):S3-S21.
24. Zane LT, Leyden WA, Marqueling AL, Manos MM. A population-based analysis of laboratory abnormalities during isotretinoin therapy for acne vulgaris. Arch Dermatol. 2006;142(8):1016-1022.
1. Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74(5):945-973.
2. Bhate K, Williams HC. Epidemiology of acne vulgaris. Br J Dermatol. 2013;168(3):474-485.
3. Collier CN, Harper JC, Cafardi JA, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dertmatol. 2008;58(1):56-59.
4. Dunn LK, O’Neill JL, Feldman SR. Acne in adolescents: quality of life, self-esteem, mood, and psychological disorders. Dermatol Online J. 2011;17(1):1.
5. Baldwin HE, Zanglein AL, Leyden JJ, Webster GF. Pharmacologic treatment options in mild, moderate, and severe acne vulgaris. Semin Cutan Med Surg. 2015;34(supp5):S82-S85.
6. Canavan TN, Chen E, Elewski BE. Optimizing non-antibiotic treatments for patients with acne: a review. Dermatol Ther. 2016;6(4):555-578.
7. Bellew S, Thiboutot D, Del Rosso JQ. Pathogenesis of acne vulgaris: what’s new, what’s interesting and what may be clinically relevant. J Drugs Dermatol. 2011;10(6):582-585.
8. Russell JJ. Topical therapy for acne. Am Fam Phys. 2000; 61(2):357-365.
9. Sami N. Topical retinoids. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. St. Louis: Elsevier; 2013:505-517.
10. Smith RI. Treatments: Retinoids. In: Mancini AJ, Eichenfield LF, Del Rosso JQ, eds. Acne in Review Guide. New York: Educational Testing and Assessment Systems/SanovaWorks; 2013:59-63.
11. Sagransky M, Yentzer BA, Feldman SR. Benzoyl peroxide: a review of its current use in the treatment of acne vulgaris. Expert Opin Pharmacother. 2009;10(15):2555-2562.
12. Motoparthi K, Hsu S. Topical antibacterial agents. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. St Louis: Elsevier; 2013:452-459.
13. Thiboutot DM, Kircik L, McMichale A, et al. Efficacy, safety, and dermal tolerability of dapsone gel, 7.5% in patients with moderate acne vulgaris: a pooled analysis of two phase 3 trials. J Clin Aesthet Dermatol. 2016;9(10):18-27.
14. Wolf K, Silapunt S. The use of sodium sulfacetamide in dermatology. Cutis. 2015;96(2):128-130.
15. Hassoun LA, Chahal DS, Sivamani RK, Larsen LN. The use of hormonal agents in the treatment of acne. Semin Cutan Med Surg. 2016;35(2):68-73.
16. Patton TJ, Ferris LK. Systemic retinoids. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. St Louis: Elsevier; 2012:252-268.
17. Thiboutot D, Gollnick H, Bettoli V, et al. New insights into the management of acne: an update from the Global Alliance to Improve Outcomes in Acne group. J Am Acad Dermatol. 2009;60(5 Suppl):S1-S50.
18. Walsh TR, Efthimious J, Dreno B. Systematic review of antibiotic resistance in acne: an increasing topical and oral threat. Lancet Infect Dis. 2016;16(3):e22-32.
19. Yan AC, Del Rosso JQ. Prescription oral treatments: Antibiotics. In: Mancini AJ, Eichenfield LF, Del Rosso JQ, eds. Acne in Review Guide. New York: Educational Testing and Assessment Systems/SanovaWorks; 2013:77-87.
20. Kim S, Michaels BD, Kim GK, Del Rosso JQ. Systemic antibacterial agents. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. St Louis: Elsevier; 2013:62-67, 70-74, 77-85.
21. Hecht CT, Sidbury R, Del Rosso JQ. Prescription oral treatments: Hormonal therapies. In: Mancini AJ, Eichenfield LF, Del Rosso JQ, eds. Acne in Review Guide. New York: Educational Testing and Assessment Systems/SanovaWorks; 2013:89-95.
22. Webster GF, Leyden JJ, Baldwin HE, Zaenglein AL. Isotretinoin: mechanism of action and patient selection. Semin Cutan Med Surg. 2015;34(supp 5):S86-S88.
23. Leyden JJ, Del Rosso JQ, Baum EW. The use of isotretinoin in the treatment of acne vulgaris. J Clin and Aesthet Dermatol. 2014;7(2 Suppl):S3-S21.
24. Zane LT, Leyden WA, Marqueling AL, Manos MM. A population-based analysis of laboratory abnormalities during isotretinoin therapy for acne vulgaris. Arch Dermatol. 2006;142(8):1016-1022.