User login
Ideals of Facial Beauty
Several concepts of ideal aesthetic measurements can be traced back to ancient Greek and European Renaissance art. In examining canons of beauty, these classical ideals often are compared to modern-day standards, allowing clinicians to delineate the parameters of an attractive facial appearance and facilitate the planning of cosmetic procedures.
Given the growing number of available cosmetic interventions, dermatologists have a powerful ability to modify facial proportions; however, changes to individual structures should be made with a mindful approach to improving overall facial harmony. This article reviews the established parameters of facial beauty to assist the clinician in enhancing cosmetic outcomes.
Canons of Facial Aesthetics
Horizontal Thirds
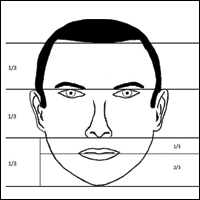
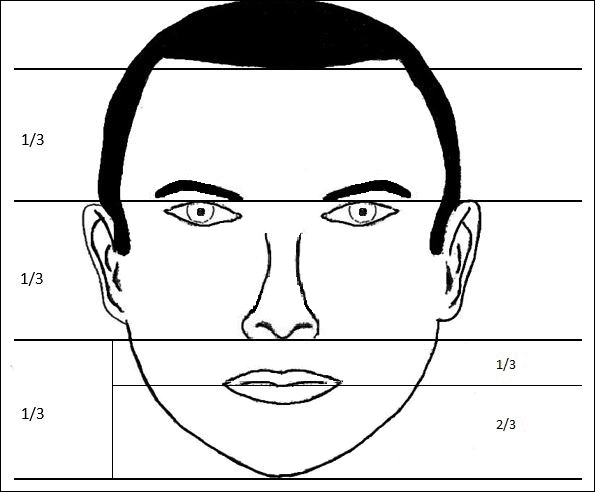
In his writings on human anatomy, Leonardo da Vinci described dividing the face into equal thirds (Figure 1). The upper third measures from the trichion (the midline point of the normal hairline) to the glabella (the smooth prominence between the eyebrows). The middle third measures from the glabella to the subnasale (the midline point where the nasal septum meets the upper lip). The lower third measures from the subnasale to the menton (the most inferior point of the chin).1
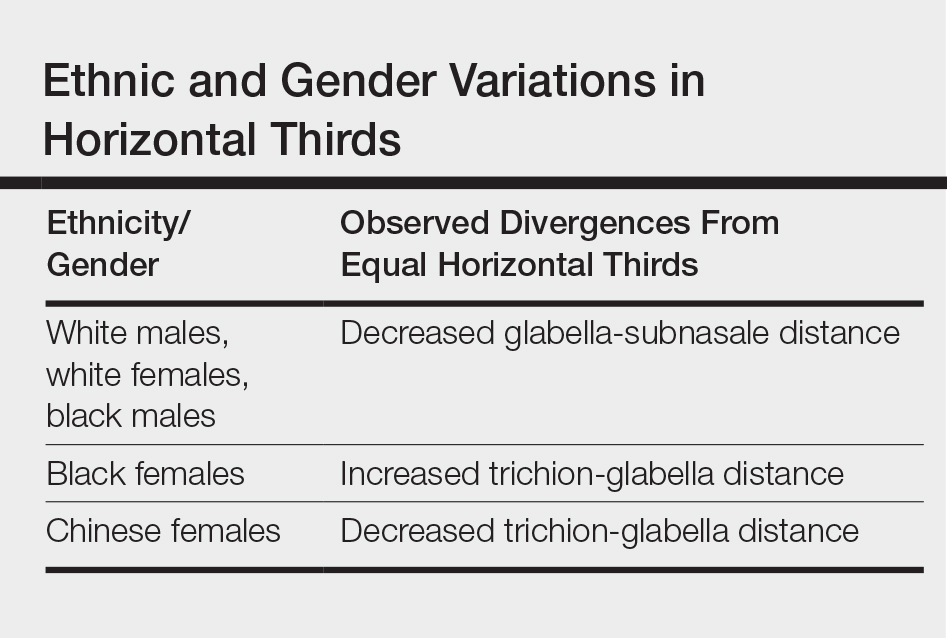
Although the validity of the canon is intended to apply across race and gender, these proportions may vary by ethnicity (Table). In white individuals, the middle third of the face tends to be shorter than the upper and lower thirds.2 This same relationship has been observed in black males.3 In Chinese females, the upper third commonly is shorter than the middle and lower thirds, correlating with a less prominent forehead. In contrast, black females tend to have a relatively longer upper third.4
The relationship between modern perceptions of attractiveness and the neoclassical norm of equal thirds remains a topic of interest. Milutinovic et al1 examined facial thirds in white female celebrities from beauty and fashion magazines and compared them to a group of anonymous white females from the general population. The group of anonymous females showed statistically significant (P<.05) differences between the sizes of the 3 facial segments, whereas the group of celebrity faces demonstrated uniformity between the facial thirds.1
The lower face can itself be divided into thirds, with the upper third measured from the subnasale to the stomion (the midline point of the oral fissure when the lips are closed), and the lower two-thirds measured from the stomion to the menton (Figure 1). Mommaerts and Moerenhout5 examined photographs of 105 attractive celebrity faces and compared their proportions to those of classical sculptures of gods and goddesses (antique faces). The authors identified an upper one-third to lower two-thirds ratio of 69.8% in celebrity females and 69.1% in celebrity males; these ratios were not significantly different from the 72.4% seen in antique females and 73.1% in antique males. The authors concluded that a 30% upper lip to 70% lower lip-chin proportion may be the most appropriate to describe contemporary standards.5
Vertical Fifths
In the vertical dimension, the neoclassical canon of facial proportions divides the face into equal fifths (Figure 2).6 The 2 most lateral fifths are measured from the lateral helix of each ear to the exocanthus of each eye. The eye fissure lengths (measured between the endocanthion and exocanthion of each eye) represent one-fifth. The middle fifth is measured between the medial canthi of both eyes (endocanthion to endocanthion). This distance is equal to the width of the nose, as measured between both alae. Finally, the width of the mouth represents 1.5-times the width of the nose. These ratios of the vertical fifths apply to both males and females.6
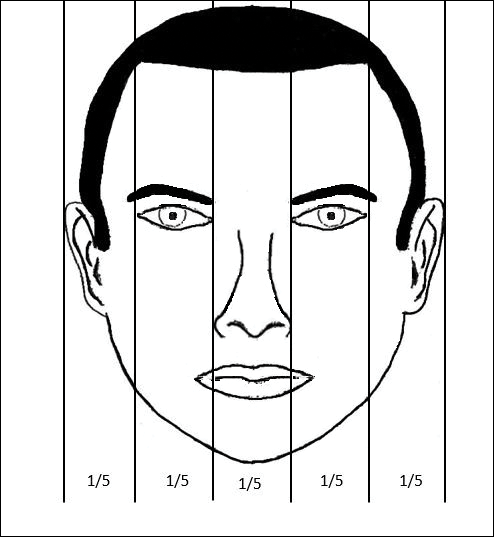
Anthropometric studies have examined deviations from the neoclassical canon according to ethnicity. Wang et al7 compared the measurements of North American white and Han Chinese patients to these standards. White patients demonstrated a greater ratio of mouth width to nose width relative to the canon. In contrast, Han Chinese patients demonstrated a relatively wider nose and narrower mouth.7
In black individuals, it has been observed that the dimensions of most facial segments correspond to the neoclassical standards; however, nose width is relatively wider in black individuals relative to the canon as well as relative to white individuals.8
Milutinovic et al1 also compared vertical fifths between white celebrities and anonymous females. In the anonymous female group, statistically significant (P<.05) variations were found between the sizes of the different facial components. In contrast, the celebrity female group showed balance between the widths of vertical fifths.1
Lips
In the lower facial third, the lips represent a key element of attractiveness. Recently, lip augmentation, aimed at creating fuller and plumper lips, has dominated the popular culture and social media landscape.9 Although the aesthetic ideal of lips continues to evolve over time, recent studies have aimed at quantifying modern notions of attractive lip appearance.
Popenko et al10 examined lip measurements using computer-generated images of white women with different variations of lip sizes and lower face proportions. Computer-generated faces were graded on attractiveness by more than 400 individuals from focus groups. An upper lip to lower lip ratio of 1:2 was judged to be the most attractive, while a ratio of 2:1 was judged to be the least attractive. Results also showed that the surface area of the most attractive lips comprised roughly 10% of the lower third of the face.10
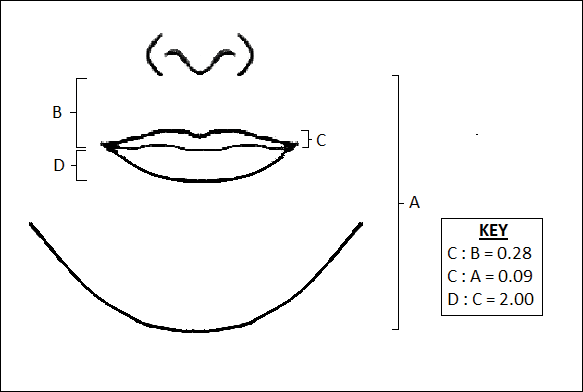
Penna et al11 analyzed various parameters of the lips and lower facial third using photographs of 176 white males and females that were judged on attractiveness by 250 volunteer evaluators. Faces were graded on a scale from 1 (absolutely attractive) to 7 (absolutely unattractive). Attractive males and females (grades 1 and 2) both demonstrated an average ratio of upper vermilion height to nose-mouth distance (measured from the subnasalae to the lower edge of the upper vermilion border) of 0.28, which was significantly greater than the average ratio observed in less attractive individuals (grades 6 or 7)(P<.05). In addition, attractive males and females demonstrated a ratio of upper vermilion height to nose-chin distance (measured from the subnasalae to the menton) of 0.09, which again was larger than the average ratio seen in less attractive individuals. Figure 3 demonstrates an aesthetic ideal of the lips derived from these 2 studies, though consideration should be given to the fact that these studies were based in white populations.
Golden Ratio
The golden ratio, also known as Phi, can be observed in nature, art, and architecture. Approximately equal to 1.618, the golden ratio also has been identified as a possible marker of beauty in the human face and has garnered attention in the lay press. The ratio has been applied to several proportions and structures in the face, such as the ratio of mouth width to nose width or the ratio of tooth height to tooth width, with investigation providing varying levels of validation about whether these ratios truly correlate with perceptions of beauty.12 Swift and Remington13 advocated for application of the golden ratio toward a comprehensive set of facial proportions. Marquardt14 used the golden ratio to create a 3-dimensional representation of an idealized face, known as the golden decagon mask. Although the golden ratio and the golden decagon mask have been proposed as analytic tools, their utility in clinical practice may be limited. Firstly, due to its popularity in the lay press, the golden ratio has been inconsistently applied to a wide range of facial ratios, which may undermine confidence in its representation as truth rather than coincidence. Secondly, although some authors have found validity of the golden decagon mask in representing unified ratios of attractiveness, others have asserted that it characterizes a masculinized white female and fails to account for ethnic differences.15-19
Age-Related Changes
In addition to the facial proportions guided by genetics, several changes occur with increased age. Over the course of a lifetime, predictable patterns emerge in the dimensions of the skin, soft tissue, and bone. These alterations in structural proportions may ultimately lead to an unevenness in facial aesthetics.
In skeletal structure, gradual bone resorption and expansion causes a reduction in facial height as well as an increase in facial width and depth.20 Fat atrophy and hypertrophy affect soft tissue proportions, visualized as hollowing at the temples, cheeks, and around the eyes, along with fullness in the submental region and jowls.21 Finally, decreases in skin elasticity and collagen exacerbate the appearance of rhytides and sagging. In older patients who desire a more youthful appearance, various applications of dermal fillers, fat grafting, liposuction, and skin tightening techniques can help to mitigate these changes.
Conclusion
Improving facial aesthetics relies on an understanding of the norms of facial proportions. Although cosmetic interventions commonly are advertised or described based on a single anatomical unit, it is important to appreciate the relationships between facial structures. Most notably, clinicians should be mindful of facial ratios when considering the introduction of filler materials or implants. Augmentation procedures at the temples, zygomatic arch, jaw, chin, and lips all have the possibility to alter facial ratios. Changes should therefore be considered in the context of improving overall facial harmony, with the clinician remaining cognizant of the ideal vertical and horizontal divisions of the face. Understanding such concepts and communicating them to patients can help in appropriately addressing all target areas, thereby leading to greater patient satisfaction.
- Milutinovic J, Zelic K, Nedeljkovic N. Evaluation of facial beauty using anthropometric proportions. ScientificWorldJournal. 2014;2014:428250. doi:10.1155/2014/428250.
- Farkas LG, Hreczko TA, Kolar JC, et al. Vertical and horizontal proportions of the face in young-adult North-American Caucasians: revision of neoclassical canons. Plast Reconstr Surg. 1985;75:328-338.
- Porter JP. The average African American male face: an anthropometric analysis. Arch Facial Plast Surg. 2004;6:78-81.
- Porter JP, Olson KL. Anthropometric facial analysis of the African American woman. Arch Facial Plast Surg. 2001;3:191-197.
- Mommaerts MY, Moerenhout BA. Ideal proportions in full face front view, contemporary versus antique. J Craniomaxillofac Surg. 2011;39:107-110.
- Vegter F, Hage JJ. Clinical anthropometry and canons of the face in historical perspective. Plast Reconstr Surg. 2000;106:1090-1096.
- Wang D, Qian G, Zhang M, et al. Differences in horizontal, neoclassical facial canons in Chinese (Han) and North American Caucasian populations. Aesthetic Plast Surg. 1997;21:265-269.
- Farkas LG, Forrest CR, Litsas L. Revision of neoclassical facial canons in young adult Afro-Americans. Aesthetic Plast Surg. 2000;24:179-184.
- Coleman GG, Lindauer SJ, Tüfekçi E, et al. Influence of chin prominence on esthetic lip profile preferences. Am J Orthod Dentofacial Orthop. 2007;132:36-42.
- Popenko NA, Tripathi PB, Devcic Z, et al. A quantitative approach to determining the ideal female lip aesthetic and its effect on facial attractiveness. JAMA Facial Plast Surg. 2017;19:261-267.
- Penna V, Fricke A, Iblher N, et al. The attractive lip: a photomorphometric analysis. J Plast Reconstr Aesthet Surg. 2015;68:920-929.
- Prokopakis EP, Vlastos IM, Picavet VA, et al. The golden ratio in facial symmetry. Rhinology. 2013;51:18-21.
- Swift A, Remington K. BeautiPHIcationTM: a global approach to facial beauty. Clin Plast Surg. 2011;38:247-277.
- Marquardt SR. Dr. Stephen R. Marquardt on the Golden Decagon and human facial beauty. interview by Dr. Gottlieb. J Clin Orthod. 2002;36:339-347.
- Veerala G, Gandikota CS, Yadagiri PK, et al. Marquardt’s facial Golden Decagon mask and its fitness with South Indian facial traits. J Clin Diagn Res. 2016;10:ZC49-ZC52.
- Holland E. Marquardt’s Phi mask: pitfalls of relying on fashion models and the golden ratio to describe a beautiful face. Aesthetic Plast Surg. 2008;32:200-208.
- Alam MK, Mohd Noor NF, Basri R, et al. Multiracial facial golden ratio and evaluation of facial appearance. PLoS One. 2015;10:e0142914.
- Kim YH. Easy facial analysis using the facial golden mask. J Craniofac Surg. 2007;18:643-649.
- Bashour M. An objective system for measuring facial attractiveness. Plast Reconstr Surg. 2006;118:757-774; discussion 775-776.
- Bartlett SP, Grossman R, Whitaker LA. Age-related changes of the craniofacial skeleton: an anthropometric and histologic analysis. Plast Reconstr Surg. 1992;90:592-600.
- Donofrio LM. Fat distribution: a morphologic study of the aging face. Dermatol Surg. 2000;26:1107-1112.
Several concepts of ideal aesthetic measurements can be traced back to ancient Greek and European Renaissance art. In examining canons of beauty, these classical ideals often are compared to modern-day standards, allowing clinicians to delineate the parameters of an attractive facial appearance and facilitate the planning of cosmetic procedures.
Given the growing number of available cosmetic interventions, dermatologists have a powerful ability to modify facial proportions; however, changes to individual structures should be made with a mindful approach to improving overall facial harmony. This article reviews the established parameters of facial beauty to assist the clinician in enhancing cosmetic outcomes.
Canons of Facial Aesthetics
Horizontal Thirds
In his writings on human anatomy, Leonardo da Vinci described dividing the face into equal thirds (Figure 1). The upper third measures from the trichion (the midline point of the normal hairline) to the glabella (the smooth prominence between the eyebrows). The middle third measures from the glabella to the subnasale (the midline point where the nasal septum meets the upper lip). The lower third measures from the subnasale to the menton (the most inferior point of the chin).1
Although the validity of the canon is intended to apply across race and gender, these proportions may vary by ethnicity (Table). In white individuals, the middle third of the face tends to be shorter than the upper and lower thirds.2 This same relationship has been observed in black males.3 In Chinese females, the upper third commonly is shorter than the middle and lower thirds, correlating with a less prominent forehead. In contrast, black females tend to have a relatively longer upper third.4

The relationship between modern perceptions of attractiveness and the neoclassical norm of equal thirds remains a topic of interest. Milutinovic et al1 examined facial thirds in white female celebrities from beauty and fashion magazines and compared them to a group of anonymous white females from the general population. The group of anonymous females showed statistically significant (P<.05) differences between the sizes of the 3 facial segments, whereas the group of celebrity faces demonstrated uniformity between the facial thirds.1
The lower face can itself be divided into thirds, with the upper third measured from the subnasale to the stomion (the midline point of the oral fissure when the lips are closed), and the lower two-thirds measured from the stomion to the menton (Figure 1). Mommaerts and Moerenhout5 examined photographs of 105 attractive celebrity faces and compared their proportions to those of classical sculptures of gods and goddesses (antique faces). The authors identified an upper one-third to lower two-thirds ratio of 69.8% in celebrity females and 69.1% in celebrity males; these ratios were not significantly different from the 72.4% seen in antique females and 73.1% in antique males. The authors concluded that a 30% upper lip to 70% lower lip-chin proportion may be the most appropriate to describe contemporary standards.5

Vertical Fifths
In the vertical dimension, the neoclassical canon of facial proportions divides the face into equal fifths (Figure 2).6 The 2 most lateral fifths are measured from the lateral helix of each ear to the exocanthus of each eye. The eye fissure lengths (measured between the endocanthion and exocanthion of each eye) represent one-fifth. The middle fifth is measured between the medial canthi of both eyes (endocanthion to endocanthion). This distance is equal to the width of the nose, as measured between both alae. Finally, the width of the mouth represents 1.5-times the width of the nose. These ratios of the vertical fifths apply to both males and females.6

Anthropometric studies have examined deviations from the neoclassical canon according to ethnicity. Wang et al7 compared the measurements of North American white and Han Chinese patients to these standards. White patients demonstrated a greater ratio of mouth width to nose width relative to the canon. In contrast, Han Chinese patients demonstrated a relatively wider nose and narrower mouth.7
In black individuals, it has been observed that the dimensions of most facial segments correspond to the neoclassical standards; however, nose width is relatively wider in black individuals relative to the canon as well as relative to white individuals.8
Milutinovic et al1 also compared vertical fifths between white celebrities and anonymous females. In the anonymous female group, statistically significant (P<.05) variations were found between the sizes of the different facial components. In contrast, the celebrity female group showed balance between the widths of vertical fifths.1
Lips
In the lower facial third, the lips represent a key element of attractiveness. Recently, lip augmentation, aimed at creating fuller and plumper lips, has dominated the popular culture and social media landscape.9 Although the aesthetic ideal of lips continues to evolve over time, recent studies have aimed at quantifying modern notions of attractive lip appearance.
Popenko et al10 examined lip measurements using computer-generated images of white women with different variations of lip sizes and lower face proportions. Computer-generated faces were graded on attractiveness by more than 400 individuals from focus groups. An upper lip to lower lip ratio of 1:2 was judged to be the most attractive, while a ratio of 2:1 was judged to be the least attractive. Results also showed that the surface area of the most attractive lips comprised roughly 10% of the lower third of the face.10
Penna et al11 analyzed various parameters of the lips and lower facial third using photographs of 176 white males and females that were judged on attractiveness by 250 volunteer evaluators. Faces were graded on a scale from 1 (absolutely attractive) to 7 (absolutely unattractive). Attractive males and females (grades 1 and 2) both demonstrated an average ratio of upper vermilion height to nose-mouth distance (measured from the subnasalae to the lower edge of the upper vermilion border) of 0.28, which was significantly greater than the average ratio observed in less attractive individuals (grades 6 or 7)(P<.05). In addition, attractive males and females demonstrated a ratio of upper vermilion height to nose-chin distance (measured from the subnasalae to the menton) of 0.09, which again was larger than the average ratio seen in less attractive individuals. Figure 3 demonstrates an aesthetic ideal of the lips derived from these 2 studies, though consideration should be given to the fact that these studies were based in white populations.

Golden Ratio
The golden ratio, also known as Phi, can be observed in nature, art, and architecture. Approximately equal to 1.618, the golden ratio also has been identified as a possible marker of beauty in the human face and has garnered attention in the lay press. The ratio has been applied to several proportions and structures in the face, such as the ratio of mouth width to nose width or the ratio of tooth height to tooth width, with investigation providing varying levels of validation about whether these ratios truly correlate with perceptions of beauty.12 Swift and Remington13 advocated for application of the golden ratio toward a comprehensive set of facial proportions. Marquardt14 used the golden ratio to create a 3-dimensional representation of an idealized face, known as the golden decagon mask. Although the golden ratio and the golden decagon mask have been proposed as analytic tools, their utility in clinical practice may be limited. Firstly, due to its popularity in the lay press, the golden ratio has been inconsistently applied to a wide range of facial ratios, which may undermine confidence in its representation as truth rather than coincidence. Secondly, although some authors have found validity of the golden decagon mask in representing unified ratios of attractiveness, others have asserted that it characterizes a masculinized white female and fails to account for ethnic differences.15-19
Age-Related Changes
In addition to the facial proportions guided by genetics, several changes occur with increased age. Over the course of a lifetime, predictable patterns emerge in the dimensions of the skin, soft tissue, and bone. These alterations in structural proportions may ultimately lead to an unevenness in facial aesthetics.
In skeletal structure, gradual bone resorption and expansion causes a reduction in facial height as well as an increase in facial width and depth.20 Fat atrophy and hypertrophy affect soft tissue proportions, visualized as hollowing at the temples, cheeks, and around the eyes, along with fullness in the submental region and jowls.21 Finally, decreases in skin elasticity and collagen exacerbate the appearance of rhytides and sagging. In older patients who desire a more youthful appearance, various applications of dermal fillers, fat grafting, liposuction, and skin tightening techniques can help to mitigate these changes.
Conclusion
Improving facial aesthetics relies on an understanding of the norms of facial proportions. Although cosmetic interventions commonly are advertised or described based on a single anatomical unit, it is important to appreciate the relationships between facial structures. Most notably, clinicians should be mindful of facial ratios when considering the introduction of filler materials or implants. Augmentation procedures at the temples, zygomatic arch, jaw, chin, and lips all have the possibility to alter facial ratios. Changes should therefore be considered in the context of improving overall facial harmony, with the clinician remaining cognizant of the ideal vertical and horizontal divisions of the face. Understanding such concepts and communicating them to patients can help in appropriately addressing all target areas, thereby leading to greater patient satisfaction.
Several concepts of ideal aesthetic measurements can be traced back to ancient Greek and European Renaissance art. In examining canons of beauty, these classical ideals often are compared to modern-day standards, allowing clinicians to delineate the parameters of an attractive facial appearance and facilitate the planning of cosmetic procedures.
Given the growing number of available cosmetic interventions, dermatologists have a powerful ability to modify facial proportions; however, changes to individual structures should be made with a mindful approach to improving overall facial harmony. This article reviews the established parameters of facial beauty to assist the clinician in enhancing cosmetic outcomes.
Canons of Facial Aesthetics
Horizontal Thirds
In his writings on human anatomy, Leonardo da Vinci described dividing the face into equal thirds (Figure 1). The upper third measures from the trichion (the midline point of the normal hairline) to the glabella (the smooth prominence between the eyebrows). The middle third measures from the glabella to the subnasale (the midline point where the nasal septum meets the upper lip). The lower third measures from the subnasale to the menton (the most inferior point of the chin).1
Although the validity of the canon is intended to apply across race and gender, these proportions may vary by ethnicity (Table). In white individuals, the middle third of the face tends to be shorter than the upper and lower thirds.2 This same relationship has been observed in black males.3 In Chinese females, the upper third commonly is shorter than the middle and lower thirds, correlating with a less prominent forehead. In contrast, black females tend to have a relatively longer upper third.4

The relationship between modern perceptions of attractiveness and the neoclassical norm of equal thirds remains a topic of interest. Milutinovic et al1 examined facial thirds in white female celebrities from beauty and fashion magazines and compared them to a group of anonymous white females from the general population. The group of anonymous females showed statistically significant (P<.05) differences between the sizes of the 3 facial segments, whereas the group of celebrity faces demonstrated uniformity between the facial thirds.1
The lower face can itself be divided into thirds, with the upper third measured from the subnasale to the stomion (the midline point of the oral fissure when the lips are closed), and the lower two-thirds measured from the stomion to the menton (Figure 1). Mommaerts and Moerenhout5 examined photographs of 105 attractive celebrity faces and compared their proportions to those of classical sculptures of gods and goddesses (antique faces). The authors identified an upper one-third to lower two-thirds ratio of 69.8% in celebrity females and 69.1% in celebrity males; these ratios were not significantly different from the 72.4% seen in antique females and 73.1% in antique males. The authors concluded that a 30% upper lip to 70% lower lip-chin proportion may be the most appropriate to describe contemporary standards.5

Vertical Fifths
In the vertical dimension, the neoclassical canon of facial proportions divides the face into equal fifths (Figure 2).6 The 2 most lateral fifths are measured from the lateral helix of each ear to the exocanthus of each eye. The eye fissure lengths (measured between the endocanthion and exocanthion of each eye) represent one-fifth. The middle fifth is measured between the medial canthi of both eyes (endocanthion to endocanthion). This distance is equal to the width of the nose, as measured between both alae. Finally, the width of the mouth represents 1.5-times the width of the nose. These ratios of the vertical fifths apply to both males and females.6

Anthropometric studies have examined deviations from the neoclassical canon according to ethnicity. Wang et al7 compared the measurements of North American white and Han Chinese patients to these standards. White patients demonstrated a greater ratio of mouth width to nose width relative to the canon. In contrast, Han Chinese patients demonstrated a relatively wider nose and narrower mouth.7
In black individuals, it has been observed that the dimensions of most facial segments correspond to the neoclassical standards; however, nose width is relatively wider in black individuals relative to the canon as well as relative to white individuals.8
Milutinovic et al1 also compared vertical fifths between white celebrities and anonymous females. In the anonymous female group, statistically significant (P<.05) variations were found between the sizes of the different facial components. In contrast, the celebrity female group showed balance between the widths of vertical fifths.1
Lips
In the lower facial third, the lips represent a key element of attractiveness. Recently, lip augmentation, aimed at creating fuller and plumper lips, has dominated the popular culture and social media landscape.9 Although the aesthetic ideal of lips continues to evolve over time, recent studies have aimed at quantifying modern notions of attractive lip appearance.
Popenko et al10 examined lip measurements using computer-generated images of white women with different variations of lip sizes and lower face proportions. Computer-generated faces were graded on attractiveness by more than 400 individuals from focus groups. An upper lip to lower lip ratio of 1:2 was judged to be the most attractive, while a ratio of 2:1 was judged to be the least attractive. Results also showed that the surface area of the most attractive lips comprised roughly 10% of the lower third of the face.10
Penna et al11 analyzed various parameters of the lips and lower facial third using photographs of 176 white males and females that were judged on attractiveness by 250 volunteer evaluators. Faces were graded on a scale from 1 (absolutely attractive) to 7 (absolutely unattractive). Attractive males and females (grades 1 and 2) both demonstrated an average ratio of upper vermilion height to nose-mouth distance (measured from the subnasalae to the lower edge of the upper vermilion border) of 0.28, which was significantly greater than the average ratio observed in less attractive individuals (grades 6 or 7)(P<.05). In addition, attractive males and females demonstrated a ratio of upper vermilion height to nose-chin distance (measured from the subnasalae to the menton) of 0.09, which again was larger than the average ratio seen in less attractive individuals. Figure 3 demonstrates an aesthetic ideal of the lips derived from these 2 studies, though consideration should be given to the fact that these studies were based in white populations.

Golden Ratio
The golden ratio, also known as Phi, can be observed in nature, art, and architecture. Approximately equal to 1.618, the golden ratio also has been identified as a possible marker of beauty in the human face and has garnered attention in the lay press. The ratio has been applied to several proportions and structures in the face, such as the ratio of mouth width to nose width or the ratio of tooth height to tooth width, with investigation providing varying levels of validation about whether these ratios truly correlate with perceptions of beauty.12 Swift and Remington13 advocated for application of the golden ratio toward a comprehensive set of facial proportions. Marquardt14 used the golden ratio to create a 3-dimensional representation of an idealized face, known as the golden decagon mask. Although the golden ratio and the golden decagon mask have been proposed as analytic tools, their utility in clinical practice may be limited. Firstly, due to its popularity in the lay press, the golden ratio has been inconsistently applied to a wide range of facial ratios, which may undermine confidence in its representation as truth rather than coincidence. Secondly, although some authors have found validity of the golden decagon mask in representing unified ratios of attractiveness, others have asserted that it characterizes a masculinized white female and fails to account for ethnic differences.15-19
Age-Related Changes
In addition to the facial proportions guided by genetics, several changes occur with increased age. Over the course of a lifetime, predictable patterns emerge in the dimensions of the skin, soft tissue, and bone. These alterations in structural proportions may ultimately lead to an unevenness in facial aesthetics.
In skeletal structure, gradual bone resorption and expansion causes a reduction in facial height as well as an increase in facial width and depth.20 Fat atrophy and hypertrophy affect soft tissue proportions, visualized as hollowing at the temples, cheeks, and around the eyes, along with fullness in the submental region and jowls.21 Finally, decreases in skin elasticity and collagen exacerbate the appearance of rhytides and sagging. In older patients who desire a more youthful appearance, various applications of dermal fillers, fat grafting, liposuction, and skin tightening techniques can help to mitigate these changes.
Conclusion
Improving facial aesthetics relies on an understanding of the norms of facial proportions. Although cosmetic interventions commonly are advertised or described based on a single anatomical unit, it is important to appreciate the relationships between facial structures. Most notably, clinicians should be mindful of facial ratios when considering the introduction of filler materials or implants. Augmentation procedures at the temples, zygomatic arch, jaw, chin, and lips all have the possibility to alter facial ratios. Changes should therefore be considered in the context of improving overall facial harmony, with the clinician remaining cognizant of the ideal vertical and horizontal divisions of the face. Understanding such concepts and communicating them to patients can help in appropriately addressing all target areas, thereby leading to greater patient satisfaction.
- Milutinovic J, Zelic K, Nedeljkovic N. Evaluation of facial beauty using anthropometric proportions. ScientificWorldJournal. 2014;2014:428250. doi:10.1155/2014/428250.
- Farkas LG, Hreczko TA, Kolar JC, et al. Vertical and horizontal proportions of the face in young-adult North-American Caucasians: revision of neoclassical canons. Plast Reconstr Surg. 1985;75:328-338.
- Porter JP. The average African American male face: an anthropometric analysis. Arch Facial Plast Surg. 2004;6:78-81.
- Porter JP, Olson KL. Anthropometric facial analysis of the African American woman. Arch Facial Plast Surg. 2001;3:191-197.
- Mommaerts MY, Moerenhout BA. Ideal proportions in full face front view, contemporary versus antique. J Craniomaxillofac Surg. 2011;39:107-110.
- Vegter F, Hage JJ. Clinical anthropometry and canons of the face in historical perspective. Plast Reconstr Surg. 2000;106:1090-1096.
- Wang D, Qian G, Zhang M, et al. Differences in horizontal, neoclassical facial canons in Chinese (Han) and North American Caucasian populations. Aesthetic Plast Surg. 1997;21:265-269.
- Farkas LG, Forrest CR, Litsas L. Revision of neoclassical facial canons in young adult Afro-Americans. Aesthetic Plast Surg. 2000;24:179-184.
- Coleman GG, Lindauer SJ, Tüfekçi E, et al. Influence of chin prominence on esthetic lip profile preferences. Am J Orthod Dentofacial Orthop. 2007;132:36-42.
- Popenko NA, Tripathi PB, Devcic Z, et al. A quantitative approach to determining the ideal female lip aesthetic and its effect on facial attractiveness. JAMA Facial Plast Surg. 2017;19:261-267.
- Penna V, Fricke A, Iblher N, et al. The attractive lip: a photomorphometric analysis. J Plast Reconstr Aesthet Surg. 2015;68:920-929.
- Prokopakis EP, Vlastos IM, Picavet VA, et al. The golden ratio in facial symmetry. Rhinology. 2013;51:18-21.
- Swift A, Remington K. BeautiPHIcationTM: a global approach to facial beauty. Clin Plast Surg. 2011;38:247-277.
- Marquardt SR. Dr. Stephen R. Marquardt on the Golden Decagon and human facial beauty. interview by Dr. Gottlieb. J Clin Orthod. 2002;36:339-347.
- Veerala G, Gandikota CS, Yadagiri PK, et al. Marquardt’s facial Golden Decagon mask and its fitness with South Indian facial traits. J Clin Diagn Res. 2016;10:ZC49-ZC52.
- Holland E. Marquardt’s Phi mask: pitfalls of relying on fashion models and the golden ratio to describe a beautiful face. Aesthetic Plast Surg. 2008;32:200-208.
- Alam MK, Mohd Noor NF, Basri R, et al. Multiracial facial golden ratio and evaluation of facial appearance. PLoS One. 2015;10:e0142914.
- Kim YH. Easy facial analysis using the facial golden mask. J Craniofac Surg. 2007;18:643-649.
- Bashour M. An objective system for measuring facial attractiveness. Plast Reconstr Surg. 2006;118:757-774; discussion 775-776.
- Bartlett SP, Grossman R, Whitaker LA. Age-related changes of the craniofacial skeleton: an anthropometric and histologic analysis. Plast Reconstr Surg. 1992;90:592-600.
- Donofrio LM. Fat distribution: a morphologic study of the aging face. Dermatol Surg. 2000;26:1107-1112.
- Milutinovic J, Zelic K, Nedeljkovic N. Evaluation of facial beauty using anthropometric proportions. ScientificWorldJournal. 2014;2014:428250. doi:10.1155/2014/428250.
- Farkas LG, Hreczko TA, Kolar JC, et al. Vertical and horizontal proportions of the face in young-adult North-American Caucasians: revision of neoclassical canons. Plast Reconstr Surg. 1985;75:328-338.
- Porter JP. The average African American male face: an anthropometric analysis. Arch Facial Plast Surg. 2004;6:78-81.
- Porter JP, Olson KL. Anthropometric facial analysis of the African American woman. Arch Facial Plast Surg. 2001;3:191-197.
- Mommaerts MY, Moerenhout BA. Ideal proportions in full face front view, contemporary versus antique. J Craniomaxillofac Surg. 2011;39:107-110.
- Vegter F, Hage JJ. Clinical anthropometry and canons of the face in historical perspective. Plast Reconstr Surg. 2000;106:1090-1096.
- Wang D, Qian G, Zhang M, et al. Differences in horizontal, neoclassical facial canons in Chinese (Han) and North American Caucasian populations. Aesthetic Plast Surg. 1997;21:265-269.
- Farkas LG, Forrest CR, Litsas L. Revision of neoclassical facial canons in young adult Afro-Americans. Aesthetic Plast Surg. 2000;24:179-184.
- Coleman GG, Lindauer SJ, Tüfekçi E, et al. Influence of chin prominence on esthetic lip profile preferences. Am J Orthod Dentofacial Orthop. 2007;132:36-42.
- Popenko NA, Tripathi PB, Devcic Z, et al. A quantitative approach to determining the ideal female lip aesthetic and its effect on facial attractiveness. JAMA Facial Plast Surg. 2017;19:261-267.
- Penna V, Fricke A, Iblher N, et al. The attractive lip: a photomorphometric analysis. J Plast Reconstr Aesthet Surg. 2015;68:920-929.
- Prokopakis EP, Vlastos IM, Picavet VA, et al. The golden ratio in facial symmetry. Rhinology. 2013;51:18-21.
- Swift A, Remington K. BeautiPHIcationTM: a global approach to facial beauty. Clin Plast Surg. 2011;38:247-277.
- Marquardt SR. Dr. Stephen R. Marquardt on the Golden Decagon and human facial beauty. interview by Dr. Gottlieb. J Clin Orthod. 2002;36:339-347.
- Veerala G, Gandikota CS, Yadagiri PK, et al. Marquardt’s facial Golden Decagon mask and its fitness with South Indian facial traits. J Clin Diagn Res. 2016;10:ZC49-ZC52.
- Holland E. Marquardt’s Phi mask: pitfalls of relying on fashion models and the golden ratio to describe a beautiful face. Aesthetic Plast Surg. 2008;32:200-208.
- Alam MK, Mohd Noor NF, Basri R, et al. Multiracial facial golden ratio and evaluation of facial appearance. PLoS One. 2015;10:e0142914.
- Kim YH. Easy facial analysis using the facial golden mask. J Craniofac Surg. 2007;18:643-649.
- Bashour M. An objective system for measuring facial attractiveness. Plast Reconstr Surg. 2006;118:757-774; discussion 775-776.
- Bartlett SP, Grossman R, Whitaker LA. Age-related changes of the craniofacial skeleton: an anthropometric and histologic analysis. Plast Reconstr Surg. 1992;90:592-600.
- Donofrio LM. Fat distribution: a morphologic study of the aging face. Dermatol Surg. 2000;26:1107-1112.
Practice Points
- Canons of ideal facial dimensions have existed since antiquity and remain relevant in modern times.
- Horizontal and vertical anatomical ratios can provide a useful framework for cosmetic interventions.
- To maximize aesthetic results, alterations to individual cosmetic units should be made with thoughtful consideration of overall facial harmony.
Do Infants Fed Rice and Rice Products Have an Increased Risk for Skin Cancer?
To the Editor:
Rice and rice products, such as rice cereal and rice snacks, contain inorganic arsenic. Exposure to arsenicin utero and during early life may be associated with adverse fetal growth, adverse infant and child immune response, and adverse neurodevelopmental outcomes. Therefore, the World Health Organization, the Food and Agriculture Organization of the United Nations, the European Union, and the US Food and Drug Administration have suggested maximum arsenic ingestion recommendations for infants: 100 ng/g for inorganic arsenic in products geared toward infants. However, infants consuming only a few servings of rice products may exceed the weekly tolerable intake of arsenic.
Karagas et al1 obtained dietary data on 759 infants who were enrolled in the New Hampshire Birth Cohort Study from 2011 to 2014. They noted that 80% of the infants had been introduced to rice cereal during the first year. Additional data on diet and total urinary arsenic at 12 months was available for 129 infants: 32.6% of these infants were fed rice snacks. In addition, the total urinary arsenic concentration was higher among infants who ate rice cereal or rice snacks as compared to infants who did not eat rice or rice products.
Chronic arsenic exposure can result in patchy dark brown hyperpigmentation with scattered pale spots referred to as “raindrops on a dusty road.” The axilla, eyelids, groin, neck, nipples, and temples often are affected. However, the hyperpigmentation can extend across the chest, abdomen, and back in severe cases.
Horizontal white lines across the nails (Mees lines) may develop. Keratoses, often on the palms (arsenic keratoses), may appear; they persist and may progress to skin cancers. In addition, patients with arsenic exposure are more susceptible to developing nonmelanoma skin cancers.2
It is unknown if exposure to inorganic arsenic in infancy predisposes these individuals to skin cancer when they become adults. Long-term longitudinal follow-up of the participants in this study may provide additional insight. Perhaps infants should not receive rice cereals and rice snacks or their parents should more carefully monitor the amount of rice and rice products that they ingest.
- Karagas MR, Punshon T, Sayarath V, et al. Association of rice and rice-product consumption with arsenic exposure early in life. JAMA Pediatr. 2016;170:609-616.
- Mayer JE, Goldman RH. Arsenic and skin cancer in the USA: the current evidence regarding arsenic-contaminated drinking water. Int J Dermatol. 2016;55;e585-e591.
To the Editor:
Rice and rice products, such as rice cereal and rice snacks, contain inorganic arsenic. Exposure to arsenicin utero and during early life may be associated with adverse fetal growth, adverse infant and child immune response, and adverse neurodevelopmental outcomes. Therefore, the World Health Organization, the Food and Agriculture Organization of the United Nations, the European Union, and the US Food and Drug Administration have suggested maximum arsenic ingestion recommendations for infants: 100 ng/g for inorganic arsenic in products geared toward infants. However, infants consuming only a few servings of rice products may exceed the weekly tolerable intake of arsenic.
Karagas et al1 obtained dietary data on 759 infants who were enrolled in the New Hampshire Birth Cohort Study from 2011 to 2014. They noted that 80% of the infants had been introduced to rice cereal during the first year. Additional data on diet and total urinary arsenic at 12 months was available for 129 infants: 32.6% of these infants were fed rice snacks. In addition, the total urinary arsenic concentration was higher among infants who ate rice cereal or rice snacks as compared to infants who did not eat rice or rice products.
Chronic arsenic exposure can result in patchy dark brown hyperpigmentation with scattered pale spots referred to as “raindrops on a dusty road.” The axilla, eyelids, groin, neck, nipples, and temples often are affected. However, the hyperpigmentation can extend across the chest, abdomen, and back in severe cases.
Horizontal white lines across the nails (Mees lines) may develop. Keratoses, often on the palms (arsenic keratoses), may appear; they persist and may progress to skin cancers. In addition, patients with arsenic exposure are more susceptible to developing nonmelanoma skin cancers.2
It is unknown if exposure to inorganic arsenic in infancy predisposes these individuals to skin cancer when they become adults. Long-term longitudinal follow-up of the participants in this study may provide additional insight. Perhaps infants should not receive rice cereals and rice snacks or their parents should more carefully monitor the amount of rice and rice products that they ingest.
To the Editor:
Rice and rice products, such as rice cereal and rice snacks, contain inorganic arsenic. Exposure to arsenicin utero and during early life may be associated with adverse fetal growth, adverse infant and child immune response, and adverse neurodevelopmental outcomes. Therefore, the World Health Organization, the Food and Agriculture Organization of the United Nations, the European Union, and the US Food and Drug Administration have suggested maximum arsenic ingestion recommendations for infants: 100 ng/g for inorganic arsenic in products geared toward infants. However, infants consuming only a few servings of rice products may exceed the weekly tolerable intake of arsenic.
Karagas et al1 obtained dietary data on 759 infants who were enrolled in the New Hampshire Birth Cohort Study from 2011 to 2014. They noted that 80% of the infants had been introduced to rice cereal during the first year. Additional data on diet and total urinary arsenic at 12 months was available for 129 infants: 32.6% of these infants were fed rice snacks. In addition, the total urinary arsenic concentration was higher among infants who ate rice cereal or rice snacks as compared to infants who did not eat rice or rice products.
Chronic arsenic exposure can result in patchy dark brown hyperpigmentation with scattered pale spots referred to as “raindrops on a dusty road.” The axilla, eyelids, groin, neck, nipples, and temples often are affected. However, the hyperpigmentation can extend across the chest, abdomen, and back in severe cases.
Horizontal white lines across the nails (Mees lines) may develop. Keratoses, often on the palms (arsenic keratoses), may appear; they persist and may progress to skin cancers. In addition, patients with arsenic exposure are more susceptible to developing nonmelanoma skin cancers.2
It is unknown if exposure to inorganic arsenic in infancy predisposes these individuals to skin cancer when they become adults. Long-term longitudinal follow-up of the participants in this study may provide additional insight. Perhaps infants should not receive rice cereals and rice snacks or their parents should more carefully monitor the amount of rice and rice products that they ingest.
- Karagas MR, Punshon T, Sayarath V, et al. Association of rice and rice-product consumption with arsenic exposure early in life. JAMA Pediatr. 2016;170:609-616.
- Mayer JE, Goldman RH. Arsenic and skin cancer in the USA: the current evidence regarding arsenic-contaminated drinking water. Int J Dermatol. 2016;55;e585-e591.
- Karagas MR, Punshon T, Sayarath V, et al. Association of rice and rice-product consumption with arsenic exposure early in life. JAMA Pediatr. 2016;170:609-616.
- Mayer JE, Goldman RH. Arsenic and skin cancer in the USA: the current evidence regarding arsenic-contaminated drinking water. Int J Dermatol. 2016;55;e585-e591.
Biologic may bring relief for children and adults with XLH syndrome
DENVER – Two studies provide hope for a new treatment of X-linked hypophosphatemia (XLH), a genetic disorder that leads to low phosphorus levels, which can cause rickets in children and a host of bone and other problems in adulthood.
The studies evaluated the use of burosumab, a monoclonal antibody that targets fibroblast growth factor 23 (FGF23). FGF23 is a hormone that reduces serum levels of phosphorus and vitamin D through its effects on the kidney.
“For the first time, this establishes the efficacy of any treatment in adults with XLH,” said Karl L. Insogna, MD, professor of medicine (endocrinology), at Yale University, New Haven, Conn., who presented the phase III study results during a poster session at the annual meeting of the American Society for Bone and Mineral Research. “Even in adults who have a lot of underlying disease burden, which is not likely to be completely reversed by this drug, you can address the underlying pathophysiology of the disease and show not only symptomatic improvement but also healing of fractures and pseudofractures,” he added.

XLH patients may be treated with calcitriol and phosphate, but this requires dosing 3-5 times a day, with side effects that can be onerous. “If you were dealing with a shot, you’d have 100% compliance and (fewer) side effects. It’s going to be a whole lot better,” he noted.
In the phase 3 adult trial, 134 patients were randomized to subcutaneous burosumab (at a dose of 1 mg/kg) or placebo every 4 weeks for 24 weeks. Among those treated with burosumab, 94.1% achieved serum phosphatase levels in the normal range, compared with 7.6% of those on placebo. Among patients taking burosumab, 36.9% of fractures and pseudofractures present at baseline had healed by the end of the study, compared with 9.9% of the fractures and pseudofractures in the placebo group (odds ratio, 7.76; P =.0001).
The two groups had similar safety profiles, with no differences in serum or urine calcium, serum intact parathyroid hormone, or nephrocalcinosis severity score.
In the phase 2 pediatric trial, 52 patients aged 5-12 years received subcutaneous burosumab every other week or once a month for 64 weeks. Although the patients had received vitamin D/phosphate therapy for an average of 7 years before enrollment, rickets was present at baseline (mean Thatcher Rickets Severity Score, 1.8). The dose of burosumab was titrated (maximum dose 2 mg/kg) to achieve age-appropriate fasting serum phosphate. All of the subjects achieved normal fasting serum phosphatase levels, but the values were more stable in the dose treated every other week.
The Thatcher RSS improved overall (–0.92; P less than .0001) in the group dosed every other week (–1.00; P less than .0001) and the group dosed monthly (–0.84; P less than .0001). These changes were more notable in patients with more severe rickets at baseline (RSS, 1.5 or higher), which had a change of –1.44 (P less than .0001).
Similar improvements were seen with the Radiographic Global Impression of Change (RGI-C). Among children with an RSS value of 1.5 or higher, substantial healing (an increase in RGI-C equal to or greater that 2) occurred in the group dosed every other week (82.4%) and the group dosed monthly (70.6%).
There was no evidence of hyperphosphatemia or hypercalcemia, and there were no clinically meaningful changes in urine calcium or serum intact parathyroid hormone levels.
The studies were funded by Ultragenyx and Kyowa Kirin International. Dr. Insogna reported having no financial disclosures.
DENVER – Two studies provide hope for a new treatment of X-linked hypophosphatemia (XLH), a genetic disorder that leads to low phosphorus levels, which can cause rickets in children and a host of bone and other problems in adulthood.
The studies evaluated the use of burosumab, a monoclonal antibody that targets fibroblast growth factor 23 (FGF23). FGF23 is a hormone that reduces serum levels of phosphorus and vitamin D through its effects on the kidney.
“For the first time, this establishes the efficacy of any treatment in adults with XLH,” said Karl L. Insogna, MD, professor of medicine (endocrinology), at Yale University, New Haven, Conn., who presented the phase III study results during a poster session at the annual meeting of the American Society for Bone and Mineral Research. “Even in adults who have a lot of underlying disease burden, which is not likely to be completely reversed by this drug, you can address the underlying pathophysiology of the disease and show not only symptomatic improvement but also healing of fractures and pseudofractures,” he added.

XLH patients may be treated with calcitriol and phosphate, but this requires dosing 3-5 times a day, with side effects that can be onerous. “If you were dealing with a shot, you’d have 100% compliance and (fewer) side effects. It’s going to be a whole lot better,” he noted.
In the phase 3 adult trial, 134 patients were randomized to subcutaneous burosumab (at a dose of 1 mg/kg) or placebo every 4 weeks for 24 weeks. Among those treated with burosumab, 94.1% achieved serum phosphatase levels in the normal range, compared with 7.6% of those on placebo. Among patients taking burosumab, 36.9% of fractures and pseudofractures present at baseline had healed by the end of the study, compared with 9.9% of the fractures and pseudofractures in the placebo group (odds ratio, 7.76; P =.0001).
The two groups had similar safety profiles, with no differences in serum or urine calcium, serum intact parathyroid hormone, or nephrocalcinosis severity score.
In the phase 2 pediatric trial, 52 patients aged 5-12 years received subcutaneous burosumab every other week or once a month for 64 weeks. Although the patients had received vitamin D/phosphate therapy for an average of 7 years before enrollment, rickets was present at baseline (mean Thatcher Rickets Severity Score, 1.8). The dose of burosumab was titrated (maximum dose 2 mg/kg) to achieve age-appropriate fasting serum phosphate. All of the subjects achieved normal fasting serum phosphatase levels, but the values were more stable in the dose treated every other week.
The Thatcher RSS improved overall (–0.92; P less than .0001) in the group dosed every other week (–1.00; P less than .0001) and the group dosed monthly (–0.84; P less than .0001). These changes were more notable in patients with more severe rickets at baseline (RSS, 1.5 or higher), which had a change of –1.44 (P less than .0001).
Similar improvements were seen with the Radiographic Global Impression of Change (RGI-C). Among children with an RSS value of 1.5 or higher, substantial healing (an increase in RGI-C equal to or greater that 2) occurred in the group dosed every other week (82.4%) and the group dosed monthly (70.6%).
There was no evidence of hyperphosphatemia or hypercalcemia, and there were no clinically meaningful changes in urine calcium or serum intact parathyroid hormone levels.
The studies were funded by Ultragenyx and Kyowa Kirin International. Dr. Insogna reported having no financial disclosures.
DENVER – Two studies provide hope for a new treatment of X-linked hypophosphatemia (XLH), a genetic disorder that leads to low phosphorus levels, which can cause rickets in children and a host of bone and other problems in adulthood.
The studies evaluated the use of burosumab, a monoclonal antibody that targets fibroblast growth factor 23 (FGF23). FGF23 is a hormone that reduces serum levels of phosphorus and vitamin D through its effects on the kidney.
“For the first time, this establishes the efficacy of any treatment in adults with XLH,” said Karl L. Insogna, MD, professor of medicine (endocrinology), at Yale University, New Haven, Conn., who presented the phase III study results during a poster session at the annual meeting of the American Society for Bone and Mineral Research. “Even in adults who have a lot of underlying disease burden, which is not likely to be completely reversed by this drug, you can address the underlying pathophysiology of the disease and show not only symptomatic improvement but also healing of fractures and pseudofractures,” he added.

XLH patients may be treated with calcitriol and phosphate, but this requires dosing 3-5 times a day, with side effects that can be onerous. “If you were dealing with a shot, you’d have 100% compliance and (fewer) side effects. It’s going to be a whole lot better,” he noted.
In the phase 3 adult trial, 134 patients were randomized to subcutaneous burosumab (at a dose of 1 mg/kg) or placebo every 4 weeks for 24 weeks. Among those treated with burosumab, 94.1% achieved serum phosphatase levels in the normal range, compared with 7.6% of those on placebo. Among patients taking burosumab, 36.9% of fractures and pseudofractures present at baseline had healed by the end of the study, compared with 9.9% of the fractures and pseudofractures in the placebo group (odds ratio, 7.76; P =.0001).
The two groups had similar safety profiles, with no differences in serum or urine calcium, serum intact parathyroid hormone, or nephrocalcinosis severity score.
In the phase 2 pediatric trial, 52 patients aged 5-12 years received subcutaneous burosumab every other week or once a month for 64 weeks. Although the patients had received vitamin D/phosphate therapy for an average of 7 years before enrollment, rickets was present at baseline (mean Thatcher Rickets Severity Score, 1.8). The dose of burosumab was titrated (maximum dose 2 mg/kg) to achieve age-appropriate fasting serum phosphate. All of the subjects achieved normal fasting serum phosphatase levels, but the values were more stable in the dose treated every other week.
The Thatcher RSS improved overall (–0.92; P less than .0001) in the group dosed every other week (–1.00; P less than .0001) and the group dosed monthly (–0.84; P less than .0001). These changes were more notable in patients with more severe rickets at baseline (RSS, 1.5 or higher), which had a change of –1.44 (P less than .0001).
Similar improvements were seen with the Radiographic Global Impression of Change (RGI-C). Among children with an RSS value of 1.5 or higher, substantial healing (an increase in RGI-C equal to or greater that 2) occurred in the group dosed every other week (82.4%) and the group dosed monthly (70.6%).
There was no evidence of hyperphosphatemia or hypercalcemia, and there were no clinically meaningful changes in urine calcium or serum intact parathyroid hormone levels.
The studies were funded by Ultragenyx and Kyowa Kirin International. Dr. Insogna reported having no financial disclosures.
AT ASBMR
Key clinical point: An investigational biologic targeting fibroblast growth factor 23 improved symptoms in both adults and children.
Major finding: Normal serum phosphatase levels were achieved in 94.1% of adults and in all children treated with burosumab
Data source: A prospective phase 2 trial in 52 children with XLH and a randomized, controlled phase 3 trial in 134 adults with XLH.
Disclosures: The studies were funded by Ultragenyx and Kyowa Kirin International. Dr. Insogna reported having no financial disclosures.
Clinical Trial Designs for Topical Antifungal Treatments of Onychomycosis and Implications on Clinical Practice
Onychomycosis is a fungal nail infection primarily caused by dermatophytes.1 If left untreated, the infection can cause nail destruction and deformities,1 resulting in pain and discomfort,2 impaired foot mobility,3 and an overall reduced quality of life.1 Onychomycosis is a chronic condition that requires long treatment periods due to the slow growth rates of toenails.1 To successfully cure the condition, fungal eradication must be achieved.
Prior to the US Food and Drug Administration (FDA) approval of tavaborole and efinaconazole, ciclopirox was the only approved topical treatment for onychomycosis.4 The recent approval of tavaborole and efinaconazole has increased treatment options available to patients and has started to pave the way for future topical treatments. This article discusses the 3 approved topical treatments for onychomycosis and focuses on the design of the phase 3 clinical trials that led to their approval.
Topical Agents Used to Treat Onychomycosis
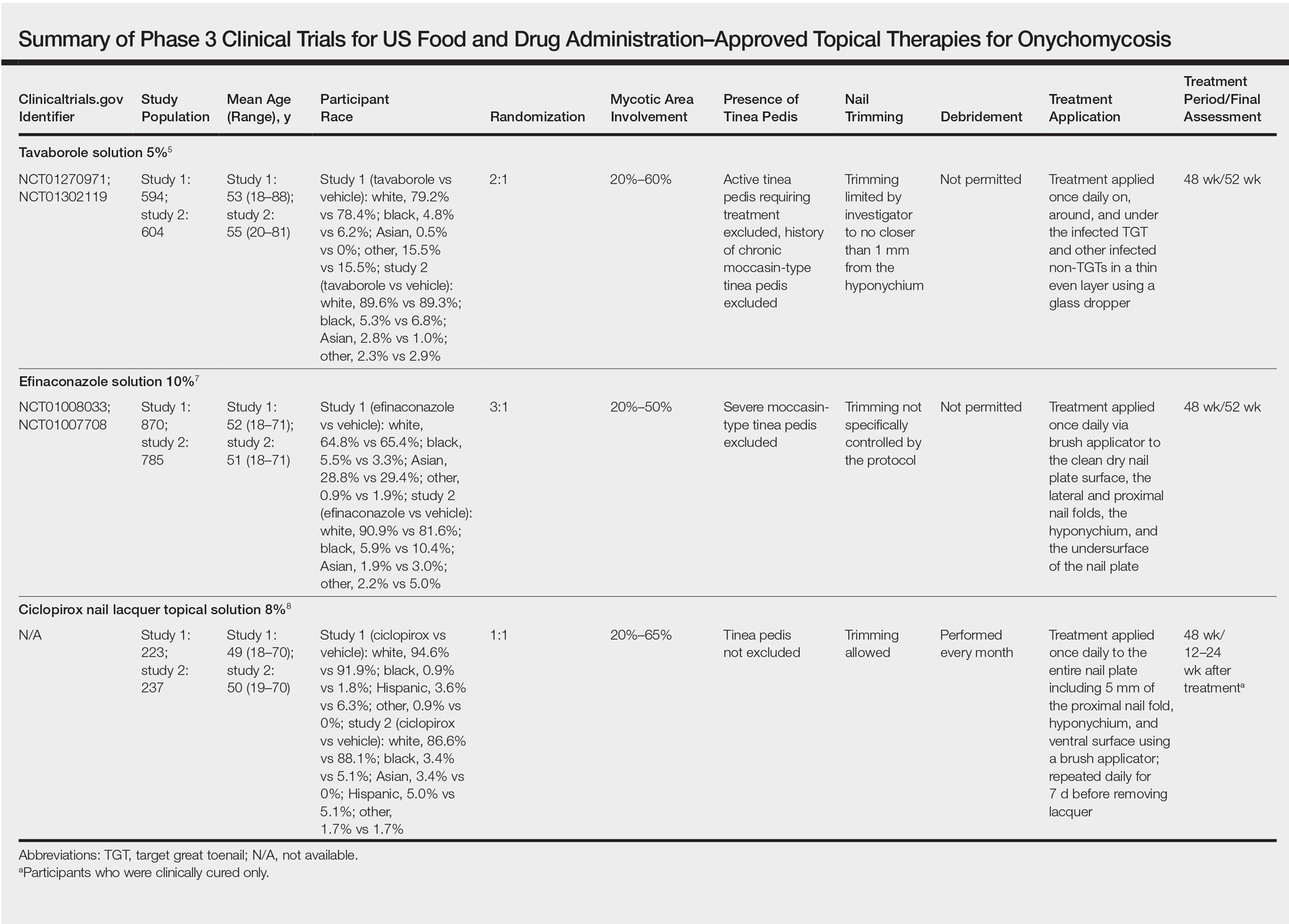
Tavaborole, efinaconazole, and ciclopirox have undergone extensive clinical investigation to receive FDA approval. Results from pivotal phase 3 studies establishing the efficacy and safety of each agent formed the basis for regulatory submission. Although it may seem intuitive to compare the relative performance of these agents based on their respective phase 3 clinical trial data, there are important differences in study methodology, conduct, and populations that prevent direct comparisons. The FDA provides limited guidance to the pharmaceutical industry on how to conduct clinical trials for potential onychomycosis treatments. Comparative efficacy and safety claims are limited based on cross-study comparisons. The details of the phase 3 trial designs are summarized in the Table.
Tavaborole
Tavaborole is a boron-based treatment with a novel mechanism of action.5 Tavaborole binds to the editing domain of leucyl–transfer ribonucleic acid synthetase via an integrated boron atom and inhibits fungal protein synthesis.6 Two identical randomized, double-blind, vehicle-controlled, parallel-group, phase 3 clinical trials evaluating tavaborole were performed.5 The first study (registered at www.clinicaltrials.gov with the identifier NCT01270971) included 594 participants from27 sites in the United States and Mexico and was conducted between December 2010 and November 2012. The second study (NCT01302119) included 604 participants from 32 sites in the United States and Canada and was conducted between February 2011 and January 2013.
Eligible participants 18 years and older had distal subungual onychomycosis (DSO) of the toenails affecting 20% to 60% of 1 or more target great toenails (TGTs), tested positive for fungus using potassium hydroxide (KOH) wet mounts and positive for Trichophyton rubrum and Trichophyton mentagrophytes on fungal culture diagnostic tests, had distal TGT thickness of 3 mm or less, and had 3 mm or more of clear nail between the proximal nail fold and the most proximal visible mycotic border.5 Those with active tinea pedis requiring treatment or with a history of chronic moccasin-type tinea pedis were excluded. Participants were randomized to receive either tavaborole or vehicle (2:1). Treatments were applied once daily to all infected toenails for a total of 48 weeks, and nail debridement (defined as partial or complete removal of the toenail) was not permitted. Notably, controlled trimming of the nail was allowed to 1 mm of the leading nail edge. Regular assessments of each toenail for disease involvement, onycholysis, and subungual hyperkeratosis were made at screening, baseline, week 2, week 6, and every 6 weeks thereafter until week 52. Subungual TGT samples were taken at screening and every 12 weeks during the study for examination at a mycology laboratory, which performed KOH and fungal culture tests. A follow-up assessment was made at week 52.5
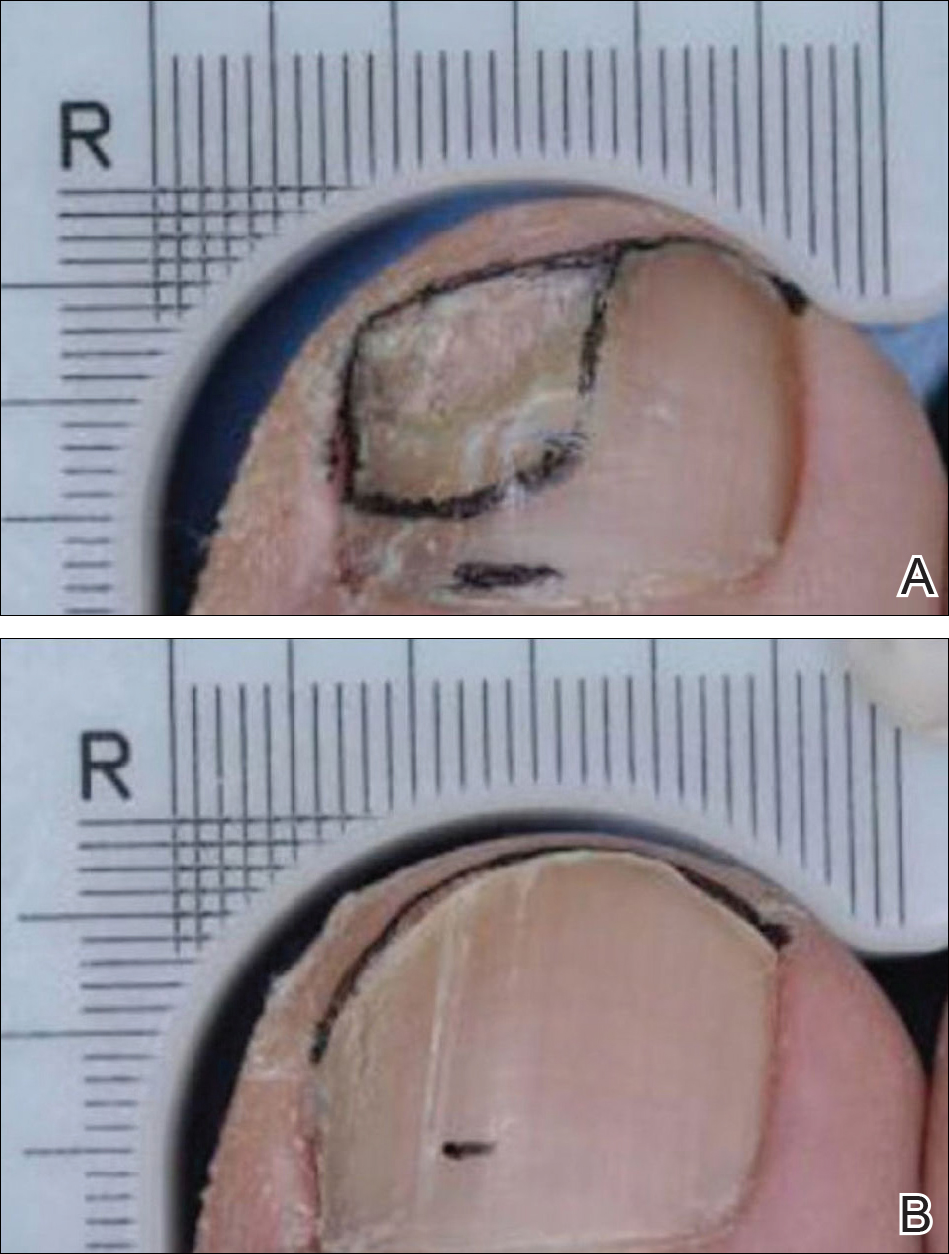
The primary end point was complete cure of the TGT at week 52, with secondary end points of completely or almost clear TGT nail (≤10% dystrophic nail), completely or almost clear TGT nail (≤10% dystrophic nail) plus negative mycology, and negative mycology of TGT.5 Examples of TGTs in participants who achieved complete cure and almost clear nails with negative mycology before and after treatment with tavaborole are shown in Figure 1. An example of a patient considered to have treatment failure is shown in Figure 2. This patient showed marked improvement in nail appearance and had a negative culture result but had a positive KOH test, which demonstrates the stringency in which topical agents are judged in onychomycosis trials.5
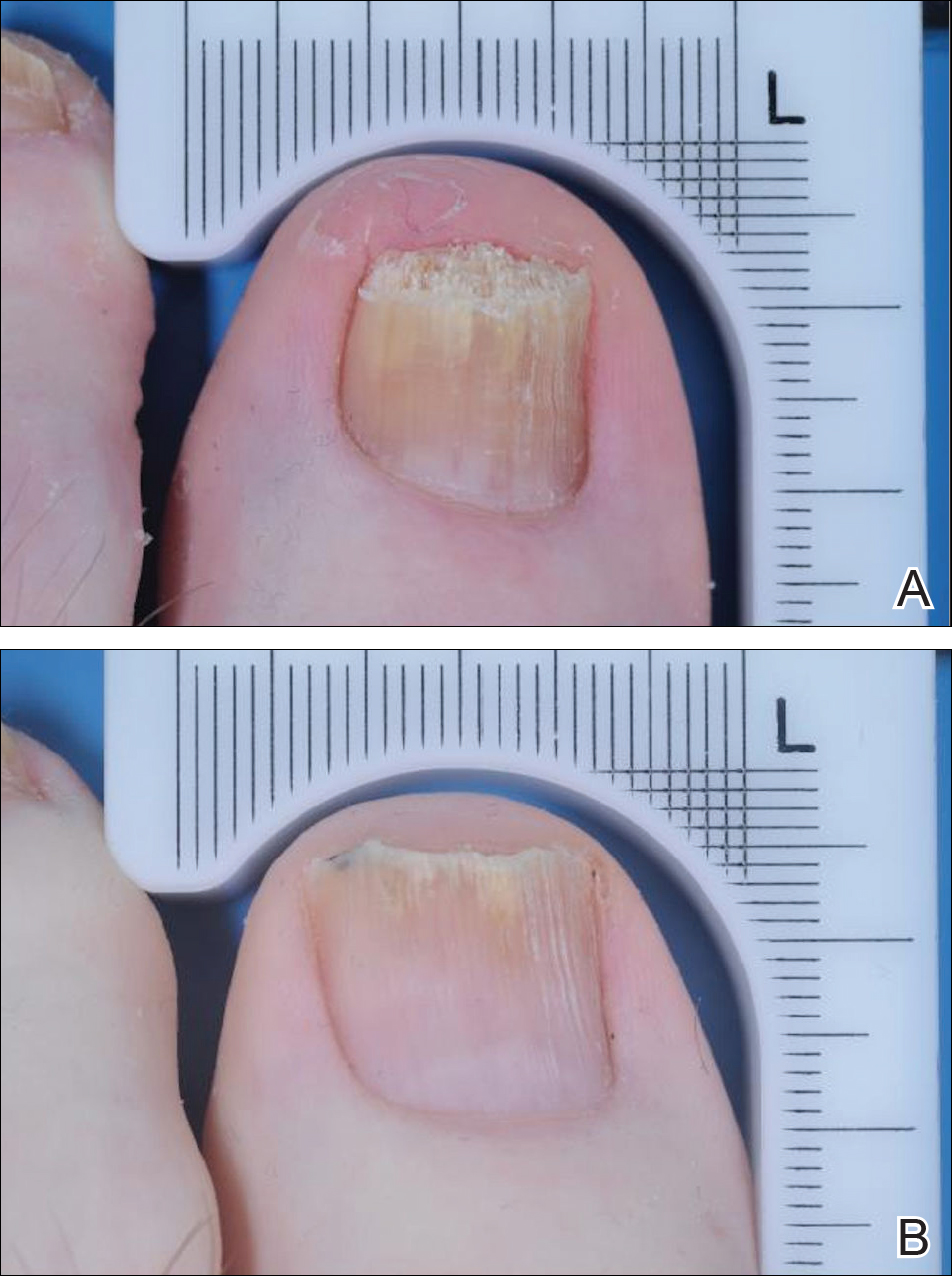
Efinaconazole
Efinaconazole is a topical triazole antifungal specifically indicated to treat onychomycosis. Two identical randomized, vehicle-controlled, double-blind, multicenter trials were performed to assess the safety and efficacy of efinaconazole solution 10%.7 The first study (NCT01008033) involved 870 participants and was conducted at a total of 74 sites in Japan (33 sites), Canada (7 sites), and the United States (34 sites) between December 2009 and September 2011. The second study (NCT01007708) had 785 participants and was conducted at 44 sites in Canada (8 sites) and the United States (36 sites) between December 2009 and October 2011.
Participants aged 18 to 70 years with a clinical diagnosis of DSO affecting 1 or more TGT were eligible to participate.7 Other eligibility criteria included an uninfected toenail length 3 mm or more from the proximal nail fold, a maximum toenail thickness of 3 mm, positive KOH wet mounts, and positive dermatophyte or mixed dermatophyte/candida cultures. Dermatophytes included T rubrum and T mentagrophytes. Those with severe moccasin-type tinea pedis were excluded. Participants were randomized to receive efinaconazole or vehicle (3:1). Once-daily treatments were self-applied to nails for 48 weeks. Clinical assessments were made at baseline and every 12 weeks until week 48, with a follow-up assessment at week 52. No nail trimming protocol was provided.7
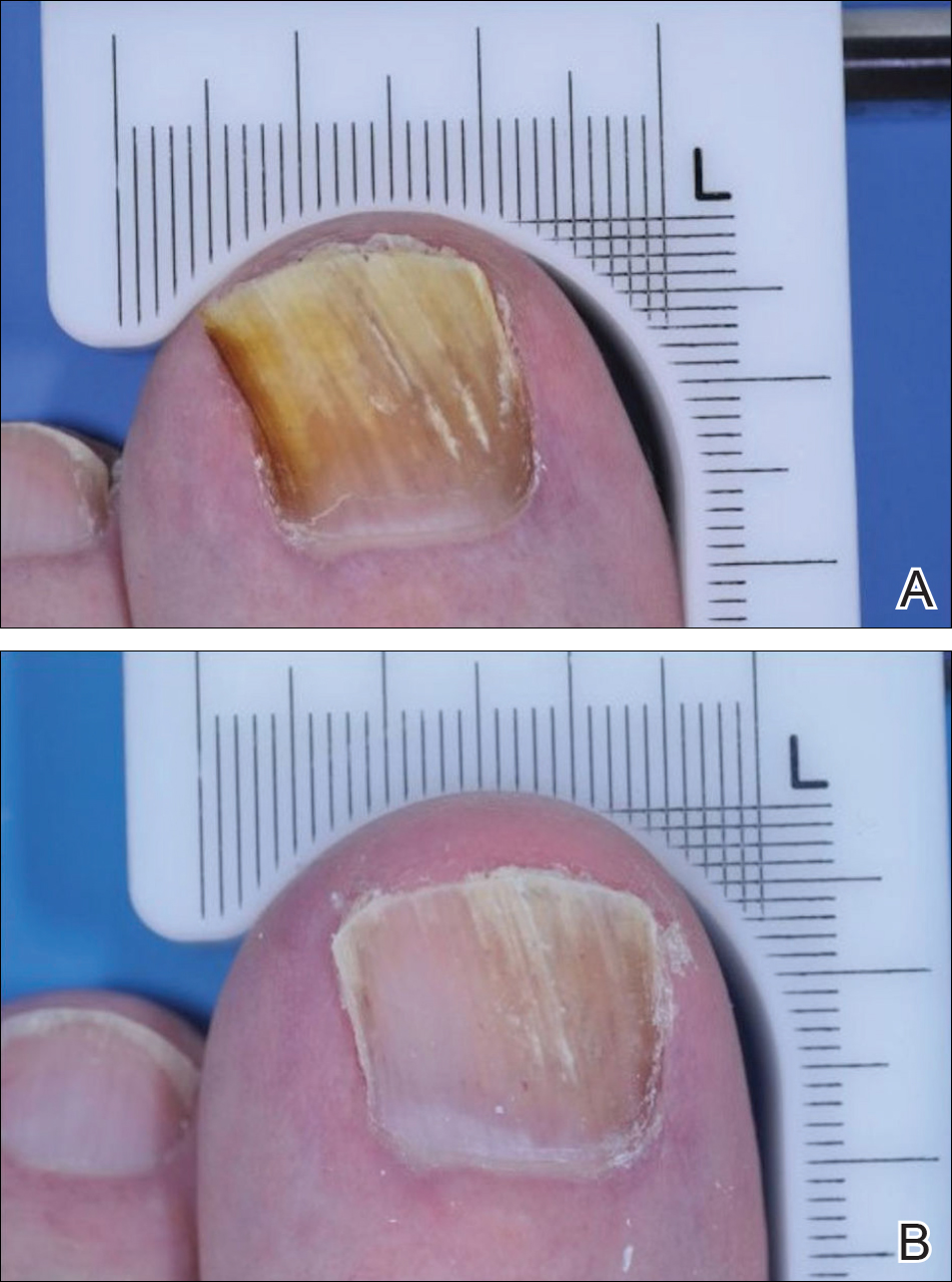
The primary end point of the efinaconazole phase 3 trials was complete cure at week 52, with secondary end points including mycologic cure, treatment success (≤5% mycotic nail), and complete or almost complete cure (negative culture and KOH, ≤5% mycotic nail). An example of a complete cure from baseline to week 52 is shown in Figure 3.7
Ciclopirox
Ciclopirox was the first topical therapy to be approved for the treatment of onychomycosis. Ciclopirox is a broad-spectrum antifungal agent that inhibits metal-dependent enzymes, which are responsible for the degradation of toxic peroxides in fungal cells. The safety and efficacy of ciclopirox nail lacquer topical solution 8% also was investigated in 2 identical phase 3 clinical trials.8 The first study was conducted at 9 sites in the United States between June 1994 and June 1996 and included 223 participants. The second study was conducted at 9 sites in the United States between July 1994 and April 1996 and included 237 participants.
Eligible participants were required to have DSO in at least one TGT, positive KOH wet mount with positive dermatophyte culture, and 20% to 65% nail involvement.8 Those with tinea pedis were not excluded. Participants were randomized to receive once-daily treatment with ciclopirox or vehicle (1:1)(applied to all toenails and affected fingernails) for 48 weeks. The product was to be removed by the patient with alcohol on a weekly basis. Trimming was allowed as necessary, and mechanical debridement by the physician could be performed monthly. Assessments were made every 4 weeks, and mycologic examinations were performed every 12 weeks. Participants who were clinically cured were assessed further in a 12- to 24-week posttreatment follow-up period.8
The primary end point of complete cure and secondary end points of treatment success (negative culture and KOH, ≤10% mycotic nail), mycologic cure, and negative mycologic culture were assessed at week 48.8
Phase 3 Clinical Trial Similarities and Differences
The phase 3 clinical trials used to investigate the safety and efficacy of tavaborole,5 efinaconazole,7 and ciclopirox8 were similar in their overall design. All trials were randomized, double-blind, vehicle-controlled studies in patients with DSO. Each agent was assessed using a once-daily application for a treatment period of 48 weeks.
Primary differences among study designs included the age range of participants, the range of mycotic nail involvement, the presence/absence of tinea pedis, and the nail trimming/debridement protocols used. Differences were observed in the patient eligibility criteria of these trials. Both mycotic area and participant age range were inconsistent for each agent (eTable). Participants with larger mycotic areas usually have a poorer prognosis, as they tend to have a greater fungal load.9 A baseline mycotic area of 20% to 60%,5 20% to 50%,7 and 20% to 65%8 at baseline was required for the tavaborole, efinaconazole, and ciclopirox trials, respectively. Variations in mycotic area between trials can affect treatment efficacy, as clinical cures can be reached quicker by patients with smaller areas of infection. Of note, the average mycotic area of involvement was not reported in the tavaborole studies but was 36% and 40% for the efinaconazole and ciclopirox studies, respectively.5,8 It also is more difficult to achieve complete cure in older patients, as they have poor circulation and reduced nail growth rates.1,10 The participant age range was 18 to 88 years in the tavaborole trials, with 8% of the participants older than 70 years,5 compared to 18 to 71 years in both the efinaconazole and ciclopirox trials.7,8 The average age of participants in each study was approximately 54, 51, and 50 years for tavaborole, efinaconazole, and ciclopirox, respectively. Because factors impacting treatment failure can increase with age, efficacy results can be confounded by differing age distributions across different studies.
Another important feature that differed between the clinical trials was the approach to nail trimming—defined as shortening of the free edge of the nail distal to the hyponychium—which varies from debridement in that the nail plate is removed or reduced in thickness proximal to the hyponychium. In the tavaborole trials, trimming was controlled to within 1 mm of the free edge of the nail,5 whereas the protocol used for the ciclopirox trials allowed nail trimming as necessary as well as moderate debridement before treatment application and on a monthly basis.8 Debridement is an important component in all ciclopirox trials, as it is used to reduce fungal load.11 No trimming control was provided during the efinaconazole trials; however, debridement was prohibited.7 These differences can dramatically affect the study results, as residual fungal elements and portions of infected nails are removed during the trimming process in an uncontrolled manner, which can affect mycologic testing results as well as the clinical efficacy results determined through investigator evaluation. Discrepancies regarding nail trimming approach inevitably makes the trial results difficult to compare, as mycologic cure is not translatable between studies.
Furthermore, somewhat unusually, complete cure rate variations were observed between different study centers in the efinaconazole trials. Japanese centers in the first efinaconazole study (NCT01008033) had higher complete cure rates in both the efinaconazole and vehicle treatment arms, which is notable because approximately 29% of participants in this study were Asian, mostly hailing from 33 Japanese centers. The reason for these confounding results is unknown and requires further analysis.
Lastly, the presence or absence of tinea pedis can affect the response to onychomycosis treatment. In the tavaborole trials, patients with active interdigital tinea pedis or exclusively plantar tinea pedis or chronic moccasin-type tinea pedis requiring treatment were excluded from the studies.5 In contrast, only patients with severe moccasin-type tinea pedis were excluded in efinaconazole trials.7 The ciclopirox studies had no exclusions based on presence of tinea pedis.8 These differences are noteworthy, as tinea pedis can serve as a reservoir for fungal infection if not treated and can lead to recurrence of onychomycosis.12
Conclusion
In recent years, disappointing efficacy has resulted in the failure of several topical agents for onychomycosis during their development; however, there are several aspects to consider when examining efficacy data in onychomycosis studies. Obtaining a complete cure in onychomycosis is difficult. Because patients applying treatments at home are unlikely to undergo mycologic testing to confirm complete cure, visual inspections are helpful to determine treatment efficacy.
Despite similar overall designs, notable differences in the study designs of the phase 3 clinical trials investigating tavaborole, efinaconazole, and ciclopirox are likely to have had an effect on the reported results, making the efficacy of the agents difficult to compare. It is particularly tempting to compare the primary end point results of each trial, especially considering tavaborole and efinaconazole had primary end points with the same parameters; however, there are several other factors (eg, age range of study population, extent of infection, nail trimming, patient demographics) that may have affected the outcomes of the studies and precluded a direct comparison of any end points. Without head-to-head investigations, there is room for prescribing clinicians to interpret results differently.
Acknowledgment
Writing and editorial assistance was provided by ApotheCom Associates, LLC, Yardley, Pennsylvania, and was supported by Sandoz, a Novartis division.
- Elewski BE. Onychomycosis: pathogenesis, diagnosis, and management. Clin Microbiol Rev. 1998;11:415-429.
- Thomas J, Jacobson GA, Narkowicz CK, et al. Toenail onychomycosis: an important global disease burden. J Clin Pharm Ther. 2010;35:497-519.
- Scher RK. Onychomycosis: a significant medical disorder. J Am Acad Dermatol. 1996;35(3, pt 2):S2-S5.
- Del Rosso JQ. The role of topical antifungal therapy for onychomycosis and the emergence of newer agents. J Clin Aesthet Dermatol. 2014;7:10-18.
- Elewski BE, Aly R, Baldwin SL, et al. Efficacy and safety of tavaborole topical solution, 5%, a novel boron-based antifungal agent, for the treatment of toenail onychomycosis: results from 2 randomized phase-III studies. J Am Acad Dermatol. 2015;73:62-69.
- Rock FL, Mao W, Yaremchuk A, et al. An antifungal agent inhibits an aminoacyl-tRNA synthetase by trapping tRNA in the editing site. Science. 2007;316:1759-1761.
- Elewski BE, Rich P, Pollak R, et al. Efinaconazole 10% solution in the treatment of toenail onychomycosis: two phase III multicenter, randomized, double-blind studies. J Am Acad Dermatol. 2013;68:600-608.
- Gupta AK, Joseph WS. Ciclopirox 8% nail lacquer in the treatment of onychomycosis of the toenails in the United States. J Am Pod Med Assoc. 2000;90:495-501.
- Carney C, Tosti A, Daniel R, et al. A new classification system for grading the severity of onychomycosis: Onychomycosis Severity Index. Arch Dermatol. 2011;147:1277-1282.
- Gupta AK. Onychomycosis in the elderly. Drugs Aging. 2000;16:397-407.
- Gupta AK, Malkin KF. Ciclopirox nail lacquer and podiatric practice. J Am Podiatr Med Assoc. 2000;90:502-507.
- Scher RK, Baran R. Onychomycosis in clinical practice: factors contributing to recurrence. Br J Dermatol. 2003;149(suppl 65):5-9.
Onychomycosis is a fungal nail infection primarily caused by dermatophytes.1 If left untreated, the infection can cause nail destruction and deformities,1 resulting in pain and discomfort,2 impaired foot mobility,3 and an overall reduced quality of life.1 Onychomycosis is a chronic condition that requires long treatment periods due to the slow growth rates of toenails.1 To successfully cure the condition, fungal eradication must be achieved.
Prior to the US Food and Drug Administration (FDA) approval of tavaborole and efinaconazole, ciclopirox was the only approved topical treatment for onychomycosis.4 The recent approval of tavaborole and efinaconazole has increased treatment options available to patients and has started to pave the way for future topical treatments. This article discusses the 3 approved topical treatments for onychomycosis and focuses on the design of the phase 3 clinical trials that led to their approval.
Topical Agents Used to Treat Onychomycosis
Tavaborole, efinaconazole, and ciclopirox have undergone extensive clinical investigation to receive FDA approval. Results from pivotal phase 3 studies establishing the efficacy and safety of each agent formed the basis for regulatory submission. Although it may seem intuitive to compare the relative performance of these agents based on their respective phase 3 clinical trial data, there are important differences in study methodology, conduct, and populations that prevent direct comparisons. The FDA provides limited guidance to the pharmaceutical industry on how to conduct clinical trials for potential onychomycosis treatments. Comparative efficacy and safety claims are limited based on cross-study comparisons. The details of the phase 3 trial designs are summarized in the Table.

Tavaborole
Tavaborole is a boron-based treatment with a novel mechanism of action.5 Tavaborole binds to the editing domain of leucyl–transfer ribonucleic acid synthetase via an integrated boron atom and inhibits fungal protein synthesis.6 Two identical randomized, double-blind, vehicle-controlled, parallel-group, phase 3 clinical trials evaluating tavaborole were performed.5 The first study (registered at www.clinicaltrials.gov with the identifier NCT01270971) included 594 participants from27 sites in the United States and Mexico and was conducted between December 2010 and November 2012. The second study (NCT01302119) included 604 participants from 32 sites in the United States and Canada and was conducted between February 2011 and January 2013.
Eligible participants 18 years and older had distal subungual onychomycosis (DSO) of the toenails affecting 20% to 60% of 1 or more target great toenails (TGTs), tested positive for fungus using potassium hydroxide (KOH) wet mounts and positive for Trichophyton rubrum and Trichophyton mentagrophytes on fungal culture diagnostic tests, had distal TGT thickness of 3 mm or less, and had 3 mm or more of clear nail between the proximal nail fold and the most proximal visible mycotic border.5 Those with active tinea pedis requiring treatment or with a history of chronic moccasin-type tinea pedis were excluded. Participants were randomized to receive either tavaborole or vehicle (2:1). Treatments were applied once daily to all infected toenails for a total of 48 weeks, and nail debridement (defined as partial or complete removal of the toenail) was not permitted. Notably, controlled trimming of the nail was allowed to 1 mm of the leading nail edge. Regular assessments of each toenail for disease involvement, onycholysis, and subungual hyperkeratosis were made at screening, baseline, week 2, week 6, and every 6 weeks thereafter until week 52. Subungual TGT samples were taken at screening and every 12 weeks during the study for examination at a mycology laboratory, which performed KOH and fungal culture tests. A follow-up assessment was made at week 52.5
The primary end point was complete cure of the TGT at week 52, with secondary end points of completely or almost clear TGT nail (≤10% dystrophic nail), completely or almost clear TGT nail (≤10% dystrophic nail) plus negative mycology, and negative mycology of TGT.5 Examples of TGTs in participants who achieved complete cure and almost clear nails with negative mycology before and after treatment with tavaborole are shown in Figure 1. An example of a patient considered to have treatment failure is shown in Figure 2. This patient showed marked improvement in nail appearance and had a negative culture result but had a positive KOH test, which demonstrates the stringency in which topical agents are judged in onychomycosis trials.5


Efinaconazole
Efinaconazole is a topical triazole antifungal specifically indicated to treat onychomycosis. Two identical randomized, vehicle-controlled, double-blind, multicenter trials were performed to assess the safety and efficacy of efinaconazole solution 10%.7 The first study (NCT01008033) involved 870 participants and was conducted at a total of 74 sites in Japan (33 sites), Canada (7 sites), and the United States (34 sites) between December 2009 and September 2011. The second study (NCT01007708) had 785 participants and was conducted at 44 sites in Canada (8 sites) and the United States (36 sites) between December 2009 and October 2011.
Participants aged 18 to 70 years with a clinical diagnosis of DSO affecting 1 or more TGT were eligible to participate.7 Other eligibility criteria included an uninfected toenail length 3 mm or more from the proximal nail fold, a maximum toenail thickness of 3 mm, positive KOH wet mounts, and positive dermatophyte or mixed dermatophyte/candida cultures. Dermatophytes included T rubrum and T mentagrophytes. Those with severe moccasin-type tinea pedis were excluded. Participants were randomized to receive efinaconazole or vehicle (3:1). Once-daily treatments were self-applied to nails for 48 weeks. Clinical assessments were made at baseline and every 12 weeks until week 48, with a follow-up assessment at week 52. No nail trimming protocol was provided.7
The primary end point of the efinaconazole phase 3 trials was complete cure at week 52, with secondary end points including mycologic cure, treatment success (≤5% mycotic nail), and complete or almost complete cure (negative culture and KOH, ≤5% mycotic nail). An example of a complete cure from baseline to week 52 is shown in Figure 3.7

Ciclopirox
Ciclopirox was the first topical therapy to be approved for the treatment of onychomycosis. Ciclopirox is a broad-spectrum antifungal agent that inhibits metal-dependent enzymes, which are responsible for the degradation of toxic peroxides in fungal cells. The safety and efficacy of ciclopirox nail lacquer topical solution 8% also was investigated in 2 identical phase 3 clinical trials.8 The first study was conducted at 9 sites in the United States between June 1994 and June 1996 and included 223 participants. The second study was conducted at 9 sites in the United States between July 1994 and April 1996 and included 237 participants.
Eligible participants were required to have DSO in at least one TGT, positive KOH wet mount with positive dermatophyte culture, and 20% to 65% nail involvement.8 Those with tinea pedis were not excluded. Participants were randomized to receive once-daily treatment with ciclopirox or vehicle (1:1)(applied to all toenails and affected fingernails) for 48 weeks. The product was to be removed by the patient with alcohol on a weekly basis. Trimming was allowed as necessary, and mechanical debridement by the physician could be performed monthly. Assessments were made every 4 weeks, and mycologic examinations were performed every 12 weeks. Participants who were clinically cured were assessed further in a 12- to 24-week posttreatment follow-up period.8
The primary end point of complete cure and secondary end points of treatment success (negative culture and KOH, ≤10% mycotic nail), mycologic cure, and negative mycologic culture were assessed at week 48.8
Phase 3 Clinical Trial Similarities and Differences
The phase 3 clinical trials used to investigate the safety and efficacy of tavaborole,5 efinaconazole,7 and ciclopirox8 were similar in their overall design. All trials were randomized, double-blind, vehicle-controlled studies in patients with DSO. Each agent was assessed using a once-daily application for a treatment period of 48 weeks.
Primary differences among study designs included the age range of participants, the range of mycotic nail involvement, the presence/absence of tinea pedis, and the nail trimming/debridement protocols used. Differences were observed in the patient eligibility criteria of these trials. Both mycotic area and participant age range were inconsistent for each agent (eTable). Participants with larger mycotic areas usually have a poorer prognosis, as they tend to have a greater fungal load.9 A baseline mycotic area of 20% to 60%,5 20% to 50%,7 and 20% to 65%8 at baseline was required for the tavaborole, efinaconazole, and ciclopirox trials, respectively. Variations in mycotic area between trials can affect treatment efficacy, as clinical cures can be reached quicker by patients with smaller areas of infection. Of note, the average mycotic area of involvement was not reported in the tavaborole studies but was 36% and 40% for the efinaconazole and ciclopirox studies, respectively.5,8 It also is more difficult to achieve complete cure in older patients, as they have poor circulation and reduced nail growth rates.1,10 The participant age range was 18 to 88 years in the tavaborole trials, with 8% of the participants older than 70 years,5 compared to 18 to 71 years in both the efinaconazole and ciclopirox trials.7,8 The average age of participants in each study was approximately 54, 51, and 50 years for tavaborole, efinaconazole, and ciclopirox, respectively. Because factors impacting treatment failure can increase with age, efficacy results can be confounded by differing age distributions across different studies.
Another important feature that differed between the clinical trials was the approach to nail trimming—defined as shortening of the free edge of the nail distal to the hyponychium—which varies from debridement in that the nail plate is removed or reduced in thickness proximal to the hyponychium. In the tavaborole trials, trimming was controlled to within 1 mm of the free edge of the nail,5 whereas the protocol used for the ciclopirox trials allowed nail trimming as necessary as well as moderate debridement before treatment application and on a monthly basis.8 Debridement is an important component in all ciclopirox trials, as it is used to reduce fungal load.11 No trimming control was provided during the efinaconazole trials; however, debridement was prohibited.7 These differences can dramatically affect the study results, as residual fungal elements and portions of infected nails are removed during the trimming process in an uncontrolled manner, which can affect mycologic testing results as well as the clinical efficacy results determined through investigator evaluation. Discrepancies regarding nail trimming approach inevitably makes the trial results difficult to compare, as mycologic cure is not translatable between studies.
Furthermore, somewhat unusually, complete cure rate variations were observed between different study centers in the efinaconazole trials. Japanese centers in the first efinaconazole study (NCT01008033) had higher complete cure rates in both the efinaconazole and vehicle treatment arms, which is notable because approximately 29% of participants in this study were Asian, mostly hailing from 33 Japanese centers. The reason for these confounding results is unknown and requires further analysis.
Lastly, the presence or absence of tinea pedis can affect the response to onychomycosis treatment. In the tavaborole trials, patients with active interdigital tinea pedis or exclusively plantar tinea pedis or chronic moccasin-type tinea pedis requiring treatment were excluded from the studies.5 In contrast, only patients with severe moccasin-type tinea pedis were excluded in efinaconazole trials.7 The ciclopirox studies had no exclusions based on presence of tinea pedis.8 These differences are noteworthy, as tinea pedis can serve as a reservoir for fungal infection if not treated and can lead to recurrence of onychomycosis.12
Conclusion
In recent years, disappointing efficacy has resulted in the failure of several topical agents for onychomycosis during their development; however, there are several aspects to consider when examining efficacy data in onychomycosis studies. Obtaining a complete cure in onychomycosis is difficult. Because patients applying treatments at home are unlikely to undergo mycologic testing to confirm complete cure, visual inspections are helpful to determine treatment efficacy.
Despite similar overall designs, notable differences in the study designs of the phase 3 clinical trials investigating tavaborole, efinaconazole, and ciclopirox are likely to have had an effect on the reported results, making the efficacy of the agents difficult to compare. It is particularly tempting to compare the primary end point results of each trial, especially considering tavaborole and efinaconazole had primary end points with the same parameters; however, there are several other factors (eg, age range of study population, extent of infection, nail trimming, patient demographics) that may have affected the outcomes of the studies and precluded a direct comparison of any end points. Without head-to-head investigations, there is room for prescribing clinicians to interpret results differently.
Acknowledgment
Writing and editorial assistance was provided by ApotheCom Associates, LLC, Yardley, Pennsylvania, and was supported by Sandoz, a Novartis division.
Onychomycosis is a fungal nail infection primarily caused by dermatophytes.1 If left untreated, the infection can cause nail destruction and deformities,1 resulting in pain and discomfort,2 impaired foot mobility,3 and an overall reduced quality of life.1 Onychomycosis is a chronic condition that requires long treatment periods due to the slow growth rates of toenails.1 To successfully cure the condition, fungal eradication must be achieved.
Prior to the US Food and Drug Administration (FDA) approval of tavaborole and efinaconazole, ciclopirox was the only approved topical treatment for onychomycosis.4 The recent approval of tavaborole and efinaconazole has increased treatment options available to patients and has started to pave the way for future topical treatments. This article discusses the 3 approved topical treatments for onychomycosis and focuses on the design of the phase 3 clinical trials that led to their approval.
Topical Agents Used to Treat Onychomycosis
Tavaborole, efinaconazole, and ciclopirox have undergone extensive clinical investigation to receive FDA approval. Results from pivotal phase 3 studies establishing the efficacy and safety of each agent formed the basis for regulatory submission. Although it may seem intuitive to compare the relative performance of these agents based on their respective phase 3 clinical trial data, there are important differences in study methodology, conduct, and populations that prevent direct comparisons. The FDA provides limited guidance to the pharmaceutical industry on how to conduct clinical trials for potential onychomycosis treatments. Comparative efficacy and safety claims are limited based on cross-study comparisons. The details of the phase 3 trial designs are summarized in the Table.

Tavaborole
Tavaborole is a boron-based treatment with a novel mechanism of action.5 Tavaborole binds to the editing domain of leucyl–transfer ribonucleic acid synthetase via an integrated boron atom and inhibits fungal protein synthesis.6 Two identical randomized, double-blind, vehicle-controlled, parallel-group, phase 3 clinical trials evaluating tavaborole were performed.5 The first study (registered at www.clinicaltrials.gov with the identifier NCT01270971) included 594 participants from27 sites in the United States and Mexico and was conducted between December 2010 and November 2012. The second study (NCT01302119) included 604 participants from 32 sites in the United States and Canada and was conducted between February 2011 and January 2013.
Eligible participants 18 years and older had distal subungual onychomycosis (DSO) of the toenails affecting 20% to 60% of 1 or more target great toenails (TGTs), tested positive for fungus using potassium hydroxide (KOH) wet mounts and positive for Trichophyton rubrum and Trichophyton mentagrophytes on fungal culture diagnostic tests, had distal TGT thickness of 3 mm or less, and had 3 mm or more of clear nail between the proximal nail fold and the most proximal visible mycotic border.5 Those with active tinea pedis requiring treatment or with a history of chronic moccasin-type tinea pedis were excluded. Participants were randomized to receive either tavaborole or vehicle (2:1). Treatments were applied once daily to all infected toenails for a total of 48 weeks, and nail debridement (defined as partial or complete removal of the toenail) was not permitted. Notably, controlled trimming of the nail was allowed to 1 mm of the leading nail edge. Regular assessments of each toenail for disease involvement, onycholysis, and subungual hyperkeratosis were made at screening, baseline, week 2, week 6, and every 6 weeks thereafter until week 52. Subungual TGT samples were taken at screening and every 12 weeks during the study for examination at a mycology laboratory, which performed KOH and fungal culture tests. A follow-up assessment was made at week 52.5
The primary end point was complete cure of the TGT at week 52, with secondary end points of completely or almost clear TGT nail (≤10% dystrophic nail), completely or almost clear TGT nail (≤10% dystrophic nail) plus negative mycology, and negative mycology of TGT.5 Examples of TGTs in participants who achieved complete cure and almost clear nails with negative mycology before and after treatment with tavaborole are shown in Figure 1. An example of a patient considered to have treatment failure is shown in Figure 2. This patient showed marked improvement in nail appearance and had a negative culture result but had a positive KOH test, which demonstrates the stringency in which topical agents are judged in onychomycosis trials.5


Efinaconazole
Efinaconazole is a topical triazole antifungal specifically indicated to treat onychomycosis. Two identical randomized, vehicle-controlled, double-blind, multicenter trials were performed to assess the safety and efficacy of efinaconazole solution 10%.7 The first study (NCT01008033) involved 870 participants and was conducted at a total of 74 sites in Japan (33 sites), Canada (7 sites), and the United States (34 sites) between December 2009 and September 2011. The second study (NCT01007708) had 785 participants and was conducted at 44 sites in Canada (8 sites) and the United States (36 sites) between December 2009 and October 2011.
Participants aged 18 to 70 years with a clinical diagnosis of DSO affecting 1 or more TGT were eligible to participate.7 Other eligibility criteria included an uninfected toenail length 3 mm or more from the proximal nail fold, a maximum toenail thickness of 3 mm, positive KOH wet mounts, and positive dermatophyte or mixed dermatophyte/candida cultures. Dermatophytes included T rubrum and T mentagrophytes. Those with severe moccasin-type tinea pedis were excluded. Participants were randomized to receive efinaconazole or vehicle (3:1). Once-daily treatments were self-applied to nails for 48 weeks. Clinical assessments were made at baseline and every 12 weeks until week 48, with a follow-up assessment at week 52. No nail trimming protocol was provided.7
The primary end point of the efinaconazole phase 3 trials was complete cure at week 52, with secondary end points including mycologic cure, treatment success (≤5% mycotic nail), and complete or almost complete cure (negative culture and KOH, ≤5% mycotic nail). An example of a complete cure from baseline to week 52 is shown in Figure 3.7

Ciclopirox
Ciclopirox was the first topical therapy to be approved for the treatment of onychomycosis. Ciclopirox is a broad-spectrum antifungal agent that inhibits metal-dependent enzymes, which are responsible for the degradation of toxic peroxides in fungal cells. The safety and efficacy of ciclopirox nail lacquer topical solution 8% also was investigated in 2 identical phase 3 clinical trials.8 The first study was conducted at 9 sites in the United States between June 1994 and June 1996 and included 223 participants. The second study was conducted at 9 sites in the United States between July 1994 and April 1996 and included 237 participants.
Eligible participants were required to have DSO in at least one TGT, positive KOH wet mount with positive dermatophyte culture, and 20% to 65% nail involvement.8 Those with tinea pedis were not excluded. Participants were randomized to receive once-daily treatment with ciclopirox or vehicle (1:1)(applied to all toenails and affected fingernails) for 48 weeks. The product was to be removed by the patient with alcohol on a weekly basis. Trimming was allowed as necessary, and mechanical debridement by the physician could be performed monthly. Assessments were made every 4 weeks, and mycologic examinations were performed every 12 weeks. Participants who were clinically cured were assessed further in a 12- to 24-week posttreatment follow-up period.8
The primary end point of complete cure and secondary end points of treatment success (negative culture and KOH, ≤10% mycotic nail), mycologic cure, and negative mycologic culture were assessed at week 48.8
Phase 3 Clinical Trial Similarities and Differences
The phase 3 clinical trials used to investigate the safety and efficacy of tavaborole,5 efinaconazole,7 and ciclopirox8 were similar in their overall design. All trials were randomized, double-blind, vehicle-controlled studies in patients with DSO. Each agent was assessed using a once-daily application for a treatment period of 48 weeks.
Primary differences among study designs included the age range of participants, the range of mycotic nail involvement, the presence/absence of tinea pedis, and the nail trimming/debridement protocols used. Differences were observed in the patient eligibility criteria of these trials. Both mycotic area and participant age range were inconsistent for each agent (eTable). Participants with larger mycotic areas usually have a poorer prognosis, as they tend to have a greater fungal load.9 A baseline mycotic area of 20% to 60%,5 20% to 50%,7 and 20% to 65%8 at baseline was required for the tavaborole, efinaconazole, and ciclopirox trials, respectively. Variations in mycotic area between trials can affect treatment efficacy, as clinical cures can be reached quicker by patients with smaller areas of infection. Of note, the average mycotic area of involvement was not reported in the tavaborole studies but was 36% and 40% for the efinaconazole and ciclopirox studies, respectively.5,8 It also is more difficult to achieve complete cure in older patients, as they have poor circulation and reduced nail growth rates.1,10 The participant age range was 18 to 88 years in the tavaborole trials, with 8% of the participants older than 70 years,5 compared to 18 to 71 years in both the efinaconazole and ciclopirox trials.7,8 The average age of participants in each study was approximately 54, 51, and 50 years for tavaborole, efinaconazole, and ciclopirox, respectively. Because factors impacting treatment failure can increase with age, efficacy results can be confounded by differing age distributions across different studies.
Another important feature that differed between the clinical trials was the approach to nail trimming—defined as shortening of the free edge of the nail distal to the hyponychium—which varies from debridement in that the nail plate is removed or reduced in thickness proximal to the hyponychium. In the tavaborole trials, trimming was controlled to within 1 mm of the free edge of the nail,5 whereas the protocol used for the ciclopirox trials allowed nail trimming as necessary as well as moderate debridement before treatment application and on a monthly basis.8 Debridement is an important component in all ciclopirox trials, as it is used to reduce fungal load.11 No trimming control was provided during the efinaconazole trials; however, debridement was prohibited.7 These differences can dramatically affect the study results, as residual fungal elements and portions of infected nails are removed during the trimming process in an uncontrolled manner, which can affect mycologic testing results as well as the clinical efficacy results determined through investigator evaluation. Discrepancies regarding nail trimming approach inevitably makes the trial results difficult to compare, as mycologic cure is not translatable between studies.
Furthermore, somewhat unusually, complete cure rate variations were observed between different study centers in the efinaconazole trials. Japanese centers in the first efinaconazole study (NCT01008033) had higher complete cure rates in both the efinaconazole and vehicle treatment arms, which is notable because approximately 29% of participants in this study were Asian, mostly hailing from 33 Japanese centers. The reason for these confounding results is unknown and requires further analysis.
Lastly, the presence or absence of tinea pedis can affect the response to onychomycosis treatment. In the tavaborole trials, patients with active interdigital tinea pedis or exclusively plantar tinea pedis or chronic moccasin-type tinea pedis requiring treatment were excluded from the studies.5 In contrast, only patients with severe moccasin-type tinea pedis were excluded in efinaconazole trials.7 The ciclopirox studies had no exclusions based on presence of tinea pedis.8 These differences are noteworthy, as tinea pedis can serve as a reservoir for fungal infection if not treated and can lead to recurrence of onychomycosis.12
Conclusion
In recent years, disappointing efficacy has resulted in the failure of several topical agents for onychomycosis during their development; however, there are several aspects to consider when examining efficacy data in onychomycosis studies. Obtaining a complete cure in onychomycosis is difficult. Because patients applying treatments at home are unlikely to undergo mycologic testing to confirm complete cure, visual inspections are helpful to determine treatment efficacy.
Despite similar overall designs, notable differences in the study designs of the phase 3 clinical trials investigating tavaborole, efinaconazole, and ciclopirox are likely to have had an effect on the reported results, making the efficacy of the agents difficult to compare. It is particularly tempting to compare the primary end point results of each trial, especially considering tavaborole and efinaconazole had primary end points with the same parameters; however, there are several other factors (eg, age range of study population, extent of infection, nail trimming, patient demographics) that may have affected the outcomes of the studies and precluded a direct comparison of any end points. Without head-to-head investigations, there is room for prescribing clinicians to interpret results differently.
Acknowledgment
Writing and editorial assistance was provided by ApotheCom Associates, LLC, Yardley, Pennsylvania, and was supported by Sandoz, a Novartis division.
- Elewski BE. Onychomycosis: pathogenesis, diagnosis, and management. Clin Microbiol Rev. 1998;11:415-429.
- Thomas J, Jacobson GA, Narkowicz CK, et al. Toenail onychomycosis: an important global disease burden. J Clin Pharm Ther. 2010;35:497-519.
- Scher RK. Onychomycosis: a significant medical disorder. J Am Acad Dermatol. 1996;35(3, pt 2):S2-S5.
- Del Rosso JQ. The role of topical antifungal therapy for onychomycosis and the emergence of newer agents. J Clin Aesthet Dermatol. 2014;7:10-18.
- Elewski BE, Aly R, Baldwin SL, et al. Efficacy and safety of tavaborole topical solution, 5%, a novel boron-based antifungal agent, for the treatment of toenail onychomycosis: results from 2 randomized phase-III studies. J Am Acad Dermatol. 2015;73:62-69.
- Rock FL, Mao W, Yaremchuk A, et al. An antifungal agent inhibits an aminoacyl-tRNA synthetase by trapping tRNA in the editing site. Science. 2007;316:1759-1761.
- Elewski BE, Rich P, Pollak R, et al. Efinaconazole 10% solution in the treatment of toenail onychomycosis: two phase III multicenter, randomized, double-blind studies. J Am Acad Dermatol. 2013;68:600-608.
- Gupta AK, Joseph WS. Ciclopirox 8% nail lacquer in the treatment of onychomycosis of the toenails in the United States. J Am Pod Med Assoc. 2000;90:495-501.
- Carney C, Tosti A, Daniel R, et al. A new classification system for grading the severity of onychomycosis: Onychomycosis Severity Index. Arch Dermatol. 2011;147:1277-1282.
- Gupta AK. Onychomycosis in the elderly. Drugs Aging. 2000;16:397-407.
- Gupta AK, Malkin KF. Ciclopirox nail lacquer and podiatric practice. J Am Podiatr Med Assoc. 2000;90:502-507.
- Scher RK, Baran R. Onychomycosis in clinical practice: factors contributing to recurrence. Br J Dermatol. 2003;149(suppl 65):5-9.
- Elewski BE. Onychomycosis: pathogenesis, diagnosis, and management. Clin Microbiol Rev. 1998;11:415-429.
- Thomas J, Jacobson GA, Narkowicz CK, et al. Toenail onychomycosis: an important global disease burden. J Clin Pharm Ther. 2010;35:497-519.
- Scher RK. Onychomycosis: a significant medical disorder. J Am Acad Dermatol. 1996;35(3, pt 2):S2-S5.
- Del Rosso JQ. The role of topical antifungal therapy for onychomycosis and the emergence of newer agents. J Clin Aesthet Dermatol. 2014;7:10-18.
- Elewski BE, Aly R, Baldwin SL, et al. Efficacy and safety of tavaborole topical solution, 5%, a novel boron-based antifungal agent, for the treatment of toenail onychomycosis: results from 2 randomized phase-III studies. J Am Acad Dermatol. 2015;73:62-69.
- Rock FL, Mao W, Yaremchuk A, et al. An antifungal agent inhibits an aminoacyl-tRNA synthetase by trapping tRNA in the editing site. Science. 2007;316:1759-1761.
- Elewski BE, Rich P, Pollak R, et al. Efinaconazole 10% solution in the treatment of toenail onychomycosis: two phase III multicenter, randomized, double-blind studies. J Am Acad Dermatol. 2013;68:600-608.
- Gupta AK, Joseph WS. Ciclopirox 8% nail lacquer in the treatment of onychomycosis of the toenails in the United States. J Am Pod Med Assoc. 2000;90:495-501.
- Carney C, Tosti A, Daniel R, et al. A new classification system for grading the severity of onychomycosis: Onychomycosis Severity Index. Arch Dermatol. 2011;147:1277-1282.
- Gupta AK. Onychomycosis in the elderly. Drugs Aging. 2000;16:397-407.
- Gupta AK, Malkin KF. Ciclopirox nail lacquer and podiatric practice. J Am Podiatr Med Assoc. 2000;90:502-507.
- Scher RK, Baran R. Onychomycosis in clinical practice: factors contributing to recurrence. Br J Dermatol. 2003;149(suppl 65):5-9.
Practice Points
- Despite similar overall designs, notable differences in the study designs of phase 3 clinical trials investigating tavaborole, efinaconazole, and ciclopirox for the treatment of onychomycosis are likely to have had an effect on the reported results, making the efficacy of these agents difficult to compare.
- The primary difference between studies for tavaborole, efinaconazole, and ciclopirox include the age range of participants, the range of mycotic nail involvement, the presence/absence of tinea pedis, and the nail trimming/debridement protocols used.
- Without head-to-head investigations, there is room for prescribing clinicians to interpret study results for these agents differently.
Must-Have Dermatology App for Skin Cancer Detection: Report From the Mount Sinai Fall Symposium
Imaging Overview: Report From the Mount Sinai Fall Symposium
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Update on Lasers and Radiofrequency: Report From the Mount Sinai Fall Symposium
Skin Cancer in Military Pilots: A Special Population With Special Risk Factors
Military dermatologists are charged with caring for a diverse population of active-duty members, civilian dependents, and military retirees. Although certain risk factors for cutaneous malignancies are common in all of these groups, the active-duty population experiences unique exposures to be considered when determining their risk for skin cancer. One subset that may be at a higher risk is military pilots who fly at high altitudes on irregular schedules in austere environments. Through the unparalleled comradeship inherent in many military units, pilots “hear” from their fellow pilots that they are at increased risk for skin cancer. Do their occupational exposures translate into increased risk for cutaneous malignancy? This article will survey the literature pertaining to pilots and skin cancer so that all dermatologists may better care for this unique population.
Epidemiology
Anecdotally, we have observed basal cell carcinoma in pilots in their 20s and early 30s, earlier than would be expected in an otherwise healthy prescreened military population.1 Woolley and Hughes2 published a case report of skin cancer in a young military aviator. The patient was a 32-year-old male helicopter pilot with Fitzpatrick skin type II and no personal or family history of skin cancer who was diagnosed with a periocular nodular basal cell carcinoma. He deployed to locations with high UV radiation (UVR) indices, and his vacation time also was spent in such areas.2 UV radiation exposure and Fitzpatrick skin type are known risk factors across occupations, but are there special exposures that come with military aviation service?
To better understand the risk for malignancy in this special population, the US Air Force examined the rates of all cancer types among a cohort of flying versus nonflying officers.3 Aviation personnel showed increased incidence of testicular, bladder, and all-site cancers combined. Noticeably absent was a statistically significant increased risk for malignant melanoma (MM) and nonmelanoma skin cancer (NMSC). Other epidemiological studies examined the incidence rates of MM in the US Armed Forces compared with age- and race-matched civilian populations and showed mixed results: 2 studies showed increased risk,4,5 while a third showed decreased risk.6 Despite finding opposite results of MM rates in military members versus the civilian population, 2 of these studies showed US Air Force members to have higher rates of MM than those in the US Army or Navy.4,6 Interestingly, the air force has the highest number of pilots among all the services, with 4000 more pilots than the army and navy.7 Further studies are needed to determine if the higher air force MM rates occur in pilots.
Although there are mixed and limited data pertaining to military flight crews, there is more robust literature concerning civilian flight personnel. One meta-analysis pooled studies related to cancer risk in cabin crews and civil and military pilots.8 In military pilots, they found a standardized incidence ratio (SIR) of 1.43 (95% confidence interval [CI], 1.09-1.87) for MM and 1.80 (95% CI, 1.25-2.80) for NMSC. The SIRs were higher for male cabin attendants (3.42 and 7.46, respectively) and civil pilots (2.18 and 1.88, respectively). They also found the most common cause of mortality in civilian cabin crews was AIDS, possibly explaining the higher SIRs for all types of malignancy in that population.8 In the United States, many civilian pilots previously were military pilots9 who likely served in the military for at least 10 years.10 A 2015 meta-analysis of 19 studies of more than 266,000 civil pilots and aircrew members found an SIR for MM of 2.22 (95% CI, 1.67-2.93) for civil pilots and 2.09 (95% CI, 1.67-2.62) for aircrews, stating the risk for MM is at least twice that of the general population.11
Risk Factors
UV Radiation
These studies suggest flight duties increase the risk for cutaneous malignancy. UV radiation is a known risk factor for skin cancer.12 The main body of the aircraft may protect the cabin’s crew and passengers from UVR, but pilots are exposed to more UVR, especially in aircraft with larger windshields. A government study in 2007 examined the transmittance of UVR through windscreens of 8 aircraft: 3 commercial jets, 2 commercial propeller planes, 1 private jet, and 2 small propeller planes.13 UVB was attenuated by all the windscreens (<1% transmittance), but 43% to 54% of UVA was transmitted, with plastic windshields attenuating more than glass. Sanlorenzo et al14 measured UVA irradiance at the pilot’s seat of a turboprop aircraft at 30,000-ft altitude. They compared this exposure to a UVA tanning bed and estimated that 57 minutes of flight at 30,000-ft altitude was equivalent to 20 minutes inside a UVA tanning booth, a startling finding.14
Cosmic Radiation
Cosmic radiation consists of neutrons and gamma rays that originate outside Earth’s atmosphere. Pilots are exposed to higher doses of cosmic radiation than nonpilots, but the health effects are difficult to study. Boice et al15 described how factors such as altitude, latitude, and flight time determine pilots’ cumulative exposure. With longer flight times at higher altitudes, a pilot’s exposure to cosmic radiation is increasing over the years.15 A 2012 review found that aircrews have low-level cosmic radiation exposure. Despite increases in MM and NMSC in pilots and increased rates of breast cancer in female aircrew, overall cancer-related mortality was lower in flying versus nonflying controls.16 Thus, cosmic radiation may not be as onerous of an occupational hazard for pilots as has been postulated.
Altered Circadian Rhythms
Aviation duties, especially in the military, require irregular work schedules that repeatedly interfere with normal sleep-wake cycles, disrupt circadian rhythms, and lead to reduced melatonin levels.8 Evidence suggests that low levels of melatonin could increase the risk for breast and prostate cancer—both cancers that occur more frequently in female aircrew and male pilots, respectively—by reducing melatonin’s natural protective role in such malignancies.17,18 A World Health Organization working group categorized shift work as “probably carcinogenic” and cited alterations of melatonin levels, changes in other circadian rhythm–related gene pathways, and relative immunosuppression as likely causative factors.19 In a 2011 study, exposing mice to UVR during times when nucleotide excision repair mechanisms were at their lowest activity caused an increased rate of skin cancers.20 A 2014 review discussed how epidemiological studies of shift workers such as nurses, firefighters, pilots, and flight crews found contradictory data, but molecular studies show that circadian rhythm–linked repair and tumorigenesis mechanisms are altered by aberrations in the normal sleep-wake cycle.21
Cockpit Instrumentation
Electromagnetic energy from the flight instruments in the cockpit also could influence malignancy risk. Nicholas et al22 found magnetic field measurements within the cockpit to be 2 to 10 times that experienced within the home or office. However, no studies examining the health effects of cockpit flight instruments and magnetic fields were found.
Final Thoughts
It is important to counsel pilots on the generally recognized, nonaviation-specific risk factors of family history, skin type, and UVR exposure in the development of skin cancer. Additionally, it is important to explain the possible role of exposure to UVR at higher altitudes, cosmic radiation, and electromagnetic energy from cockpit instruments, as well as altered sleep-wake cycles. A pilot’s risk for MM may be twice that of matched controls, and the risk for NMSC could be higher.8,11 Although the literature lacks specific recommendations for pilots, it is reasonable to screen pilots once per year to better assess their individual risk and encourage diligent use of sunscreen and sun-protective measures when flying. It also may be important to advocate for the development of engineering controls that decrease UVR transmittance through windscreens, particularly for aircraft flying at higher altitudes for longer flights. More research is needed to determine if changes in circadian rhythm and decreases in melatonin increase skin cancer risk, which could impact how pilots’ schedules are managed. Together, we can ensure adequate surveillance, diagnosis, and treatment in this at-risk population.
- Roewert‐Huber J, Lange-Asschenfeldt B, Stockfleth E, et al. Epidemiology and aetiology of basal cell carcinoma. Br J Dermatol. 2007;157(suppl 2):47-51.
- Woolley SD, Hughes C. A young military pilot presents with a periocular basal cell carcinoma: a case report. Travel Med Infect Dis. 2013;11:435-437.
- Grayson JK, Lyons TJ. Cancer incidence in United States Air Force aircrew, 1975-89. Aviat Space Environ Med. 1996;67:101-104.
- Lea CS, Efird JT, Toland AE, et al. Melanoma incidence rates in active duty military personnel compared with a population-based registry in the United States, 2000-2007. Mil Med. 2014;179:247-253.
- Garland FC, White MR, Garland CF, et al. Occupational sunlight exposure and melanoma in the US Navy. Arc Environ Health. 1990;45:261-267.
- Zhou J, Enewold L, Zahm SH, et al. Melanoma incidence rates among whites in the US military. Cancer Epidemiol Biomarkers Prev. 2011;20:318-323.
- Active Duty Master Personnel File: Active Duty Tactical Operations Officers. Seaside, CA: Defense Manpower Data Center; August 31, 2017. Accessed September 22, 2017.
- Buja A, Lange JH, Perissinotto E, et al. Cancer incidence among male military and civil pilots and flight attendants: an analysis on published data. Toxicol Ind Health. 2005;21:273-282.
- Jansen HS, Oster CV, eds. Taking Flight: Education and Training for Aviation Careers. Washington, DC: National Academy Press; 1997.
- About AFROTC Service Commitment. US Air Force ROTC website. https://www.afrotc.com/about/service. Accessed September 20, 2017.
- Sanlorenzo M, Wehner MR, Linos E, et al. The risk of melanoma in airline pilots and cabin crew: a meta-analysis. JAMA Dermatol. 2015;151:51-58.
- Ananthaswamy HN, Pierceall WE. Molecular mechanisms of ultraviolet radiation carcinogenesis. Photochem Photobiol. 1990;52:1119-1136.
- Nakagawara VB, Montgomery RW, Marshall WJ. Optical Radiation Transmittance of Aircraft Windscreens and Pilot Vision. Oklahoma City, OK: Federal Aviation Administration; 2007.
- Sanlorenzo M, Vujic I, Posch C, et al. The risk of melanoma in pilots and cabin crew: UV measurements in flying airplanes. JAMA Dermatol. 2015;151:450-452.
- Boice JD, Blettner M, Auvinen A. Epidemiologic studies of pilots and aircrew. Health Phys. 2000;79:576-584.
- Zeeb H, Hammer GP, Blettner M. Epidemiological investigations of aircrew: an occupational group with low-level cosmic radiation exposure [published online March 6, 2012]. J Radiol Prot. 2012;32:N15-N19.
- Stevens RG. Circadian disruption and breast cancer: from melatonin to clock genes. Epidemiology. 2005;16:254-258.
- Siu SW, Lau KW, Tam PC, et al. Melatonin and prostate cancer cell proliferation: interplay with castration, epidermal growth factor, and androgen sensitivity. Prostate. 2002;52:106-122.
- IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Painting, Firefighting, and Shiftwork. Lyon, France: World Health Organization International Agency for Research on Cancer; 2010.
- Gaddameedhi S, Selby CP, Kaufmann WK, et al. Control of skin cancer by the circadian rhythm. Proc Natl Acad Sci. 2011;108:18790-18795.
- Markova-Car EP, Jurišic´ D, Ilic´ N, et al. Running for time: circadian rhythms and melanoma. Tumour Biol. 2014;35:8359-8368.
- Nicholas JS, Lackland DT, Butler GC, et al. Cosmic radiation and magnetic field exposure to airline flight crews. Am J Ind Med. 1998;34:574-580.
Military dermatologists are charged with caring for a diverse population of active-duty members, civilian dependents, and military retirees. Although certain risk factors for cutaneous malignancies are common in all of these groups, the active-duty population experiences unique exposures to be considered when determining their risk for skin cancer. One subset that may be at a higher risk is military pilots who fly at high altitudes on irregular schedules in austere environments. Through the unparalleled comradeship inherent in many military units, pilots “hear” from their fellow pilots that they are at increased risk for skin cancer. Do their occupational exposures translate into increased risk for cutaneous malignancy? This article will survey the literature pertaining to pilots and skin cancer so that all dermatologists may better care for this unique population.
Epidemiology
Anecdotally, we have observed basal cell carcinoma in pilots in their 20s and early 30s, earlier than would be expected in an otherwise healthy prescreened military population.1 Woolley and Hughes2 published a case report of skin cancer in a young military aviator. The patient was a 32-year-old male helicopter pilot with Fitzpatrick skin type II and no personal or family history of skin cancer who was diagnosed with a periocular nodular basal cell carcinoma. He deployed to locations with high UV radiation (UVR) indices, and his vacation time also was spent in such areas.2 UV radiation exposure and Fitzpatrick skin type are known risk factors across occupations, but are there special exposures that come with military aviation service?
To better understand the risk for malignancy in this special population, the US Air Force examined the rates of all cancer types among a cohort of flying versus nonflying officers.3 Aviation personnel showed increased incidence of testicular, bladder, and all-site cancers combined. Noticeably absent was a statistically significant increased risk for malignant melanoma (MM) and nonmelanoma skin cancer (NMSC). Other epidemiological studies examined the incidence rates of MM in the US Armed Forces compared with age- and race-matched civilian populations and showed mixed results: 2 studies showed increased risk,4,5 while a third showed decreased risk.6 Despite finding opposite results of MM rates in military members versus the civilian population, 2 of these studies showed US Air Force members to have higher rates of MM than those in the US Army or Navy.4,6 Interestingly, the air force has the highest number of pilots among all the services, with 4000 more pilots than the army and navy.7 Further studies are needed to determine if the higher air force MM rates occur in pilots.
Although there are mixed and limited data pertaining to military flight crews, there is more robust literature concerning civilian flight personnel. One meta-analysis pooled studies related to cancer risk in cabin crews and civil and military pilots.8 In military pilots, they found a standardized incidence ratio (SIR) of 1.43 (95% confidence interval [CI], 1.09-1.87) for MM and 1.80 (95% CI, 1.25-2.80) for NMSC. The SIRs were higher for male cabin attendants (3.42 and 7.46, respectively) and civil pilots (2.18 and 1.88, respectively). They also found the most common cause of mortality in civilian cabin crews was AIDS, possibly explaining the higher SIRs for all types of malignancy in that population.8 In the United States, many civilian pilots previously were military pilots9 who likely served in the military for at least 10 years.10 A 2015 meta-analysis of 19 studies of more than 266,000 civil pilots and aircrew members found an SIR for MM of 2.22 (95% CI, 1.67-2.93) for civil pilots and 2.09 (95% CI, 1.67-2.62) for aircrews, stating the risk for MM is at least twice that of the general population.11
Risk Factors
UV Radiation
These studies suggest flight duties increase the risk for cutaneous malignancy. UV radiation is a known risk factor for skin cancer.12 The main body of the aircraft may protect the cabin’s crew and passengers from UVR, but pilots are exposed to more UVR, especially in aircraft with larger windshields. A government study in 2007 examined the transmittance of UVR through windscreens of 8 aircraft: 3 commercial jets, 2 commercial propeller planes, 1 private jet, and 2 small propeller planes.13 UVB was attenuated by all the windscreens (<1% transmittance), but 43% to 54% of UVA was transmitted, with plastic windshields attenuating more than glass. Sanlorenzo et al14 measured UVA irradiance at the pilot’s seat of a turboprop aircraft at 30,000-ft altitude. They compared this exposure to a UVA tanning bed and estimated that 57 minutes of flight at 30,000-ft altitude was equivalent to 20 minutes inside a UVA tanning booth, a startling finding.14
Cosmic Radiation
Cosmic radiation consists of neutrons and gamma rays that originate outside Earth’s atmosphere. Pilots are exposed to higher doses of cosmic radiation than nonpilots, but the health effects are difficult to study. Boice et al15 described how factors such as altitude, latitude, and flight time determine pilots’ cumulative exposure. With longer flight times at higher altitudes, a pilot’s exposure to cosmic radiation is increasing over the years.15 A 2012 review found that aircrews have low-level cosmic radiation exposure. Despite increases in MM and NMSC in pilots and increased rates of breast cancer in female aircrew, overall cancer-related mortality was lower in flying versus nonflying controls.16 Thus, cosmic radiation may not be as onerous of an occupational hazard for pilots as has been postulated.
Altered Circadian Rhythms
Aviation duties, especially in the military, require irregular work schedules that repeatedly interfere with normal sleep-wake cycles, disrupt circadian rhythms, and lead to reduced melatonin levels.8 Evidence suggests that low levels of melatonin could increase the risk for breast and prostate cancer—both cancers that occur more frequently in female aircrew and male pilots, respectively—by reducing melatonin’s natural protective role in such malignancies.17,18 A World Health Organization working group categorized shift work as “probably carcinogenic” and cited alterations of melatonin levels, changes in other circadian rhythm–related gene pathways, and relative immunosuppression as likely causative factors.19 In a 2011 study, exposing mice to UVR during times when nucleotide excision repair mechanisms were at their lowest activity caused an increased rate of skin cancers.20 A 2014 review discussed how epidemiological studies of shift workers such as nurses, firefighters, pilots, and flight crews found contradictory data, but molecular studies show that circadian rhythm–linked repair and tumorigenesis mechanisms are altered by aberrations in the normal sleep-wake cycle.21
Cockpit Instrumentation
Electromagnetic energy from the flight instruments in the cockpit also could influence malignancy risk. Nicholas et al22 found magnetic field measurements within the cockpit to be 2 to 10 times that experienced within the home or office. However, no studies examining the health effects of cockpit flight instruments and magnetic fields were found.
Final Thoughts
It is important to counsel pilots on the generally recognized, nonaviation-specific risk factors of family history, skin type, and UVR exposure in the development of skin cancer. Additionally, it is important to explain the possible role of exposure to UVR at higher altitudes, cosmic radiation, and electromagnetic energy from cockpit instruments, as well as altered sleep-wake cycles. A pilot’s risk for MM may be twice that of matched controls, and the risk for NMSC could be higher.8,11 Although the literature lacks specific recommendations for pilots, it is reasonable to screen pilots once per year to better assess their individual risk and encourage diligent use of sunscreen and sun-protective measures when flying. It also may be important to advocate for the development of engineering controls that decrease UVR transmittance through windscreens, particularly for aircraft flying at higher altitudes for longer flights. More research is needed to determine if changes in circadian rhythm and decreases in melatonin increase skin cancer risk, which could impact how pilots’ schedules are managed. Together, we can ensure adequate surveillance, diagnosis, and treatment in this at-risk population.
Military dermatologists are charged with caring for a diverse population of active-duty members, civilian dependents, and military retirees. Although certain risk factors for cutaneous malignancies are common in all of these groups, the active-duty population experiences unique exposures to be considered when determining their risk for skin cancer. One subset that may be at a higher risk is military pilots who fly at high altitudes on irregular schedules in austere environments. Through the unparalleled comradeship inherent in many military units, pilots “hear” from their fellow pilots that they are at increased risk for skin cancer. Do their occupational exposures translate into increased risk for cutaneous malignancy? This article will survey the literature pertaining to pilots and skin cancer so that all dermatologists may better care for this unique population.
Epidemiology
Anecdotally, we have observed basal cell carcinoma in pilots in their 20s and early 30s, earlier than would be expected in an otherwise healthy prescreened military population.1 Woolley and Hughes2 published a case report of skin cancer in a young military aviator. The patient was a 32-year-old male helicopter pilot with Fitzpatrick skin type II and no personal or family history of skin cancer who was diagnosed with a periocular nodular basal cell carcinoma. He deployed to locations with high UV radiation (UVR) indices, and his vacation time also was spent in such areas.2 UV radiation exposure and Fitzpatrick skin type are known risk factors across occupations, but are there special exposures that come with military aviation service?
To better understand the risk for malignancy in this special population, the US Air Force examined the rates of all cancer types among a cohort of flying versus nonflying officers.3 Aviation personnel showed increased incidence of testicular, bladder, and all-site cancers combined. Noticeably absent was a statistically significant increased risk for malignant melanoma (MM) and nonmelanoma skin cancer (NMSC). Other epidemiological studies examined the incidence rates of MM in the US Armed Forces compared with age- and race-matched civilian populations and showed mixed results: 2 studies showed increased risk,4,5 while a third showed decreased risk.6 Despite finding opposite results of MM rates in military members versus the civilian population, 2 of these studies showed US Air Force members to have higher rates of MM than those in the US Army or Navy.4,6 Interestingly, the air force has the highest number of pilots among all the services, with 4000 more pilots than the army and navy.7 Further studies are needed to determine if the higher air force MM rates occur in pilots.
Although there are mixed and limited data pertaining to military flight crews, there is more robust literature concerning civilian flight personnel. One meta-analysis pooled studies related to cancer risk in cabin crews and civil and military pilots.8 In military pilots, they found a standardized incidence ratio (SIR) of 1.43 (95% confidence interval [CI], 1.09-1.87) for MM and 1.80 (95% CI, 1.25-2.80) for NMSC. The SIRs were higher for male cabin attendants (3.42 and 7.46, respectively) and civil pilots (2.18 and 1.88, respectively). They also found the most common cause of mortality in civilian cabin crews was AIDS, possibly explaining the higher SIRs for all types of malignancy in that population.8 In the United States, many civilian pilots previously were military pilots9 who likely served in the military for at least 10 years.10 A 2015 meta-analysis of 19 studies of more than 266,000 civil pilots and aircrew members found an SIR for MM of 2.22 (95% CI, 1.67-2.93) for civil pilots and 2.09 (95% CI, 1.67-2.62) for aircrews, stating the risk for MM is at least twice that of the general population.11
Risk Factors
UV Radiation
These studies suggest flight duties increase the risk for cutaneous malignancy. UV radiation is a known risk factor for skin cancer.12 The main body of the aircraft may protect the cabin’s crew and passengers from UVR, but pilots are exposed to more UVR, especially in aircraft with larger windshields. A government study in 2007 examined the transmittance of UVR through windscreens of 8 aircraft: 3 commercial jets, 2 commercial propeller planes, 1 private jet, and 2 small propeller planes.13 UVB was attenuated by all the windscreens (<1% transmittance), but 43% to 54% of UVA was transmitted, with plastic windshields attenuating more than glass. Sanlorenzo et al14 measured UVA irradiance at the pilot’s seat of a turboprop aircraft at 30,000-ft altitude. They compared this exposure to a UVA tanning bed and estimated that 57 minutes of flight at 30,000-ft altitude was equivalent to 20 minutes inside a UVA tanning booth, a startling finding.14
Cosmic Radiation
Cosmic radiation consists of neutrons and gamma rays that originate outside Earth’s atmosphere. Pilots are exposed to higher doses of cosmic radiation than nonpilots, but the health effects are difficult to study. Boice et al15 described how factors such as altitude, latitude, and flight time determine pilots’ cumulative exposure. With longer flight times at higher altitudes, a pilot’s exposure to cosmic radiation is increasing over the years.15 A 2012 review found that aircrews have low-level cosmic radiation exposure. Despite increases in MM and NMSC in pilots and increased rates of breast cancer in female aircrew, overall cancer-related mortality was lower in flying versus nonflying controls.16 Thus, cosmic radiation may not be as onerous of an occupational hazard for pilots as has been postulated.
Altered Circadian Rhythms
Aviation duties, especially in the military, require irregular work schedules that repeatedly interfere with normal sleep-wake cycles, disrupt circadian rhythms, and lead to reduced melatonin levels.8 Evidence suggests that low levels of melatonin could increase the risk for breast and prostate cancer—both cancers that occur more frequently in female aircrew and male pilots, respectively—by reducing melatonin’s natural protective role in such malignancies.17,18 A World Health Organization working group categorized shift work as “probably carcinogenic” and cited alterations of melatonin levels, changes in other circadian rhythm–related gene pathways, and relative immunosuppression as likely causative factors.19 In a 2011 study, exposing mice to UVR during times when nucleotide excision repair mechanisms were at their lowest activity caused an increased rate of skin cancers.20 A 2014 review discussed how epidemiological studies of shift workers such as nurses, firefighters, pilots, and flight crews found contradictory data, but molecular studies show that circadian rhythm–linked repair and tumorigenesis mechanisms are altered by aberrations in the normal sleep-wake cycle.21
Cockpit Instrumentation
Electromagnetic energy from the flight instruments in the cockpit also could influence malignancy risk. Nicholas et al22 found magnetic field measurements within the cockpit to be 2 to 10 times that experienced within the home or office. However, no studies examining the health effects of cockpit flight instruments and magnetic fields were found.
Final Thoughts
It is important to counsel pilots on the generally recognized, nonaviation-specific risk factors of family history, skin type, and UVR exposure in the development of skin cancer. Additionally, it is important to explain the possible role of exposure to UVR at higher altitudes, cosmic radiation, and electromagnetic energy from cockpit instruments, as well as altered sleep-wake cycles. A pilot’s risk for MM may be twice that of matched controls, and the risk for NMSC could be higher.8,11 Although the literature lacks specific recommendations for pilots, it is reasonable to screen pilots once per year to better assess their individual risk and encourage diligent use of sunscreen and sun-protective measures when flying. It also may be important to advocate for the development of engineering controls that decrease UVR transmittance through windscreens, particularly for aircraft flying at higher altitudes for longer flights. More research is needed to determine if changes in circadian rhythm and decreases in melatonin increase skin cancer risk, which could impact how pilots’ schedules are managed. Together, we can ensure adequate surveillance, diagnosis, and treatment in this at-risk population.
- Roewert‐Huber J, Lange-Asschenfeldt B, Stockfleth E, et al. Epidemiology and aetiology of basal cell carcinoma. Br J Dermatol. 2007;157(suppl 2):47-51.
- Woolley SD, Hughes C. A young military pilot presents with a periocular basal cell carcinoma: a case report. Travel Med Infect Dis. 2013;11:435-437.
- Grayson JK, Lyons TJ. Cancer incidence in United States Air Force aircrew, 1975-89. Aviat Space Environ Med. 1996;67:101-104.
- Lea CS, Efird JT, Toland AE, et al. Melanoma incidence rates in active duty military personnel compared with a population-based registry in the United States, 2000-2007. Mil Med. 2014;179:247-253.
- Garland FC, White MR, Garland CF, et al. Occupational sunlight exposure and melanoma in the US Navy. Arc Environ Health. 1990;45:261-267.
- Zhou J, Enewold L, Zahm SH, et al. Melanoma incidence rates among whites in the US military. Cancer Epidemiol Biomarkers Prev. 2011;20:318-323.
- Active Duty Master Personnel File: Active Duty Tactical Operations Officers. Seaside, CA: Defense Manpower Data Center; August 31, 2017. Accessed September 22, 2017.
- Buja A, Lange JH, Perissinotto E, et al. Cancer incidence among male military and civil pilots and flight attendants: an analysis on published data. Toxicol Ind Health. 2005;21:273-282.
- Jansen HS, Oster CV, eds. Taking Flight: Education and Training for Aviation Careers. Washington, DC: National Academy Press; 1997.
- About AFROTC Service Commitment. US Air Force ROTC website. https://www.afrotc.com/about/service. Accessed September 20, 2017.
- Sanlorenzo M, Wehner MR, Linos E, et al. The risk of melanoma in airline pilots and cabin crew: a meta-analysis. JAMA Dermatol. 2015;151:51-58.
- Ananthaswamy HN, Pierceall WE. Molecular mechanisms of ultraviolet radiation carcinogenesis. Photochem Photobiol. 1990;52:1119-1136.
- Nakagawara VB, Montgomery RW, Marshall WJ. Optical Radiation Transmittance of Aircraft Windscreens and Pilot Vision. Oklahoma City, OK: Federal Aviation Administration; 2007.
- Sanlorenzo M, Vujic I, Posch C, et al. The risk of melanoma in pilots and cabin crew: UV measurements in flying airplanes. JAMA Dermatol. 2015;151:450-452.
- Boice JD, Blettner M, Auvinen A. Epidemiologic studies of pilots and aircrew. Health Phys. 2000;79:576-584.
- Zeeb H, Hammer GP, Blettner M. Epidemiological investigations of aircrew: an occupational group with low-level cosmic radiation exposure [published online March 6, 2012]. J Radiol Prot. 2012;32:N15-N19.
- Stevens RG. Circadian disruption and breast cancer: from melatonin to clock genes. Epidemiology. 2005;16:254-258.
- Siu SW, Lau KW, Tam PC, et al. Melatonin and prostate cancer cell proliferation: interplay with castration, epidermal growth factor, and androgen sensitivity. Prostate. 2002;52:106-122.
- IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Painting, Firefighting, and Shiftwork. Lyon, France: World Health Organization International Agency for Research on Cancer; 2010.
- Gaddameedhi S, Selby CP, Kaufmann WK, et al. Control of skin cancer by the circadian rhythm. Proc Natl Acad Sci. 2011;108:18790-18795.
- Markova-Car EP, Jurišic´ D, Ilic´ N, et al. Running for time: circadian rhythms and melanoma. Tumour Biol. 2014;35:8359-8368.
- Nicholas JS, Lackland DT, Butler GC, et al. Cosmic radiation and magnetic field exposure to airline flight crews. Am J Ind Med. 1998;34:574-580.
- Roewert‐Huber J, Lange-Asschenfeldt B, Stockfleth E, et al. Epidemiology and aetiology of basal cell carcinoma. Br J Dermatol. 2007;157(suppl 2):47-51.
- Woolley SD, Hughes C. A young military pilot presents with a periocular basal cell carcinoma: a case report. Travel Med Infect Dis. 2013;11:435-437.
- Grayson JK, Lyons TJ. Cancer incidence in United States Air Force aircrew, 1975-89. Aviat Space Environ Med. 1996;67:101-104.
- Lea CS, Efird JT, Toland AE, et al. Melanoma incidence rates in active duty military personnel compared with a population-based registry in the United States, 2000-2007. Mil Med. 2014;179:247-253.
- Garland FC, White MR, Garland CF, et al. Occupational sunlight exposure and melanoma in the US Navy. Arc Environ Health. 1990;45:261-267.
- Zhou J, Enewold L, Zahm SH, et al. Melanoma incidence rates among whites in the US military. Cancer Epidemiol Biomarkers Prev. 2011;20:318-323.
- Active Duty Master Personnel File: Active Duty Tactical Operations Officers. Seaside, CA: Defense Manpower Data Center; August 31, 2017. Accessed September 22, 2017.
- Buja A, Lange JH, Perissinotto E, et al. Cancer incidence among male military and civil pilots and flight attendants: an analysis on published data. Toxicol Ind Health. 2005;21:273-282.
- Jansen HS, Oster CV, eds. Taking Flight: Education and Training for Aviation Careers. Washington, DC: National Academy Press; 1997.
- About AFROTC Service Commitment. US Air Force ROTC website. https://www.afrotc.com/about/service. Accessed September 20, 2017.
- Sanlorenzo M, Wehner MR, Linos E, et al. The risk of melanoma in airline pilots and cabin crew: a meta-analysis. JAMA Dermatol. 2015;151:51-58.
- Ananthaswamy HN, Pierceall WE. Molecular mechanisms of ultraviolet radiation carcinogenesis. Photochem Photobiol. 1990;52:1119-1136.
- Nakagawara VB, Montgomery RW, Marshall WJ. Optical Radiation Transmittance of Aircraft Windscreens and Pilot Vision. Oklahoma City, OK: Federal Aviation Administration; 2007.
- Sanlorenzo M, Vujic I, Posch C, et al. The risk of melanoma in pilots and cabin crew: UV measurements in flying airplanes. JAMA Dermatol. 2015;151:450-452.
- Boice JD, Blettner M, Auvinen A. Epidemiologic studies of pilots and aircrew. Health Phys. 2000;79:576-584.
- Zeeb H, Hammer GP, Blettner M. Epidemiological investigations of aircrew: an occupational group with low-level cosmic radiation exposure [published online March 6, 2012]. J Radiol Prot. 2012;32:N15-N19.
- Stevens RG. Circadian disruption and breast cancer: from melatonin to clock genes. Epidemiology. 2005;16:254-258.
- Siu SW, Lau KW, Tam PC, et al. Melatonin and prostate cancer cell proliferation: interplay with castration, epidermal growth factor, and androgen sensitivity. Prostate. 2002;52:106-122.
- IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Painting, Firefighting, and Shiftwork. Lyon, France: World Health Organization International Agency for Research on Cancer; 2010.
- Gaddameedhi S, Selby CP, Kaufmann WK, et al. Control of skin cancer by the circadian rhythm. Proc Natl Acad Sci. 2011;108:18790-18795.
- Markova-Car EP, Jurišic´ D, Ilic´ N, et al. Running for time: circadian rhythms and melanoma. Tumour Biol. 2014;35:8359-8368.
- Nicholas JS, Lackland DT, Butler GC, et al. Cosmic radiation and magnetic field exposure to airline flight crews. Am J Ind Med. 1998;34:574-580.
Practice Points
- Military and civilian pilots have an increased risk for melanoma and nonmelanoma skin cancer, likely due to unique occupational exposures.
- We recommend annual skin cancer screening for all pilots to help assess their individual risk.
- Pilots should be educated on their increased risk for skin cancer and encouraged to use sun-protective measures during their flying duties and leisure activities.
Major Changes in the American Board of Dermatology’s Certification Examination
Older dermatologists may recall (or may have expunged from memory) taking the American Board of Dermatology (ABD) certification examination at the Holiday Inn in Rosemont, Illinois. I remember schlepping a borrowed microscope from Denver, Colorado; penciling in answers to questions about slides projected on a screen; and having a proctor escort me to the bathroom. On the flight home, the pilot kept my microscope in the cockpit for safekeeping.
Much has changed since then. Today’s examination takes 1 day instead of 2, is in July instead of October, and airline security would never allow me to stow a microscope in the pilot’s cabin. The content of the examination also has evolved. No longer does one have to identify yeasts and fungi in culture—a subject I spotted the ABD and hoped for the best—and surgery is a much more prominent part of the examination.
Nevertheless, over the years the examination continued to emphasize book knowledge and visual pattern recognition. Although they are essential components of being an effective dermatologist, there are other important factors. Many of these can be classified under the term clinical judgment, the ability to make good decisions that take into account the individual patient and situation.
In 2013, the ABD Board of Directors began the process of making fundamental changes in the certification examination with the goal of making it a better test of clinical competence. The process has included matters such as finding the correct technical consultant for examination development and psychometrics, writing and vetting new types of questions, gathering input from program directors, and building the electronic infrastructure to support these changes.
The structure of the new examination is based on a natural progression of learning, from mastering the basics, to acquiring more advanced knowledge, to applying that knowledge in clinical situations. It consists of the following:
- BASIC Exam, a test of fundamentals obtained during the first year of dermatology residency
- CORE Exam, a modular examination emphasizing the more comprehensive knowledge base obtained during the second and third years of residency
- APPLIED Exam, a case-based examination testing ability to apply knowledge appropriately in clinical situations
These new examinations will replace the In-Training Exam and the current certification examination, beginning with the cohort of residents entering dermatology training in July 2017.
The BASIC Exam is designed to test fundamentals such as visual recognition of common diseases, management of uncomplicated conditions, and familiarity with standard procedures. The purposes of the examination are to measure progress, to identify residents who are having difficulty, and to ensure that residents actually master the basics that we sometimes take for granted that they know. It is not a pass/fail examination and thus technically is not part of certification. A detailed content outline for the BASIC Exam can be found on the ABD website.1 Because it is a new examination, it is anticipated that the content will be modified as we gain experience with it and obtain feedback from program directors as to how its usefulness may be improved.
The CORE Exam is designed to test a more advanced, clinically relevant knowledge base. It is part of the certification
The APPLIED Exam is the centerpiece of the new examinations and tests the ability to apply knowledge appropriately in clinical situations. It is case based and ranges from straightforward (most likely diagnosis based on examination) to complex (how to manage pemphigus not responding to the initial treatment in a patient with multiple comorbidities). It is designed to test skills such as knowing when additional information is needed and when it is not, recognizing when referral is indicated, modifying management depending on response to therapy, and recognizing and managing complications. The unique characteristics of an individual patient including patient preferences, ability to comprehend and communicate, comorbidities, financial considerations, and other concerns, will need to be taken into account. The APPLIED Exam will be given in July following completion of residency.
Writing knowledge-based questions with straightforward answers in a psychometrically valid format is actually rather challenging, as first-time question writers discover. Writing items (questions) that test clinical judgment is considerably more difficult. One of the challenges is ensuring that there truly is agreement about the answers. To ensure that there is consensus, we have initiated a new process in item vetting. Rather than sit around a table and come to consensus, a process that could be dominated by experts in a particular area or those with the strongest opinions, committees first vet new questions through a blinded review. Each committee member takes the “test” from home without knowledge of what is supposed to be the correct answer. The responses are anonymous, so members feel free to respond candidly. Then, at the in-person meeting, the anonymous blinded review responses are evaluated and the items are discussed. We have found the blinded review to be invaluable, not just for items testing judgment but for all items.
An enormous amount of work has been put into preparing for the new examinations. Item-writing committees have been working enthusiastically to develop questions. There also is a great deal of work that goes on beyond the ABD. The ABD must contract with vendors for the electronic item bank, editing, psychometrics quality control and scoring, electronic publishing of the examination, virtual dermatopathology, website software for examination registration and reporting, and proctoring. Although developing new examinations is a costly enterprise, the ABD is committed not to increase the financial burden for residents and can use reserve funds to defray new examination development expenses. To keep expenses low during training, we will not charge residents an examination fee for the CORE modules, though they will pay a modest proctoring fee to the proctoring vendor. Also, instead of traveling to Tampa, Florida, in July, candidates will take the APPLIED Exam at a nearby Pearson VUE test center.
It will be the end of an era. Perhaps some of us will feel a little nostalgia for the Rosemont Holiday Inn and the fungal cultures, but I doubt it. Sample items for the 3 examinations, content overviews, frequently asked questions, and more information about the Exam of the Future can be found on the ABD website.2
- Exam of the Future: content outline and blueprint for BASIC exam. American Board of Dermatology website. https://dlpgnf31z4a6s.cloudfront.net/media/151102/basic-exam-content-outline-08132017.pdf. Updated August 13, 2017. Accessed September 12, 2017.
- Exam of the Future information center. American Board of Dermatology website. https://www.abderm.org/residents-and-fellows/exam-of-the-future-information-center.aspx. Accessed September 12, 2017.
Older dermatologists may recall (or may have expunged from memory) taking the American Board of Dermatology (ABD) certification examination at the Holiday Inn in Rosemont, Illinois. I remember schlepping a borrowed microscope from Denver, Colorado; penciling in answers to questions about slides projected on a screen; and having a proctor escort me to the bathroom. On the flight home, the pilot kept my microscope in the cockpit for safekeeping.
Much has changed since then. Today’s examination takes 1 day instead of 2, is in July instead of October, and airline security would never allow me to stow a microscope in the pilot’s cabin. The content of the examination also has evolved. No longer does one have to identify yeasts and fungi in culture—a subject I spotted the ABD and hoped for the best—and surgery is a much more prominent part of the examination.
Nevertheless, over the years the examination continued to emphasize book knowledge and visual pattern recognition. Although they are essential components of being an effective dermatologist, there are other important factors. Many of these can be classified under the term clinical judgment, the ability to make good decisions that take into account the individual patient and situation.
In 2013, the ABD Board of Directors began the process of making fundamental changes in the certification examination with the goal of making it a better test of clinical competence. The process has included matters such as finding the correct technical consultant for examination development and psychometrics, writing and vetting new types of questions, gathering input from program directors, and building the electronic infrastructure to support these changes.
The structure of the new examination is based on a natural progression of learning, from mastering the basics, to acquiring more advanced knowledge, to applying that knowledge in clinical situations. It consists of the following:
- BASIC Exam, a test of fundamentals obtained during the first year of dermatology residency
- CORE Exam, a modular examination emphasizing the more comprehensive knowledge base obtained during the second and third years of residency
- APPLIED Exam, a case-based examination testing ability to apply knowledge appropriately in clinical situations
These new examinations will replace the In-Training Exam and the current certification examination, beginning with the cohort of residents entering dermatology training in July 2017.
The BASIC Exam is designed to test fundamentals such as visual recognition of common diseases, management of uncomplicated conditions, and familiarity with standard procedures. The purposes of the examination are to measure progress, to identify residents who are having difficulty, and to ensure that residents actually master the basics that we sometimes take for granted that they know. It is not a pass/fail examination and thus technically is not part of certification. A detailed content outline for the BASIC Exam can be found on the ABD website.1 Because it is a new examination, it is anticipated that the content will be modified as we gain experience with it and obtain feedback from program directors as to how its usefulness may be improved.
The CORE Exam is designed to test a more advanced, clinically relevant knowledge base. It is part of the certification
The APPLIED Exam is the centerpiece of the new examinations and tests the ability to apply knowledge appropriately in clinical situations. It is case based and ranges from straightforward (most likely diagnosis based on examination) to complex (how to manage pemphigus not responding to the initial treatment in a patient with multiple comorbidities). It is designed to test skills such as knowing when additional information is needed and when it is not, recognizing when referral is indicated, modifying management depending on response to therapy, and recognizing and managing complications. The unique characteristics of an individual patient including patient preferences, ability to comprehend and communicate, comorbidities, financial considerations, and other concerns, will need to be taken into account. The APPLIED Exam will be given in July following completion of residency.
Writing knowledge-based questions with straightforward answers in a psychometrically valid format is actually rather challenging, as first-time question writers discover. Writing items (questions) that test clinical judgment is considerably more difficult. One of the challenges is ensuring that there truly is agreement about the answers. To ensure that there is consensus, we have initiated a new process in item vetting. Rather than sit around a table and come to consensus, a process that could be dominated by experts in a particular area or those with the strongest opinions, committees first vet new questions through a blinded review. Each committee member takes the “test” from home without knowledge of what is supposed to be the correct answer. The responses are anonymous, so members feel free to respond candidly. Then, at the in-person meeting, the anonymous blinded review responses are evaluated and the items are discussed. We have found the blinded review to be invaluable, not just for items testing judgment but for all items.
An enormous amount of work has been put into preparing for the new examinations. Item-writing committees have been working enthusiastically to develop questions. There also is a great deal of work that goes on beyond the ABD. The ABD must contract with vendors for the electronic item bank, editing, psychometrics quality control and scoring, electronic publishing of the examination, virtual dermatopathology, website software for examination registration and reporting, and proctoring. Although developing new examinations is a costly enterprise, the ABD is committed not to increase the financial burden for residents and can use reserve funds to defray new examination development expenses. To keep expenses low during training, we will not charge residents an examination fee for the CORE modules, though they will pay a modest proctoring fee to the proctoring vendor. Also, instead of traveling to Tampa, Florida, in July, candidates will take the APPLIED Exam at a nearby Pearson VUE test center.
It will be the end of an era. Perhaps some of us will feel a little nostalgia for the Rosemont Holiday Inn and the fungal cultures, but I doubt it. Sample items for the 3 examinations, content overviews, frequently asked questions, and more information about the Exam of the Future can be found on the ABD website.2
Older dermatologists may recall (or may have expunged from memory) taking the American Board of Dermatology (ABD) certification examination at the Holiday Inn in Rosemont, Illinois. I remember schlepping a borrowed microscope from Denver, Colorado; penciling in answers to questions about slides projected on a screen; and having a proctor escort me to the bathroom. On the flight home, the pilot kept my microscope in the cockpit for safekeeping.
Much has changed since then. Today’s examination takes 1 day instead of 2, is in July instead of October, and airline security would never allow me to stow a microscope in the pilot’s cabin. The content of the examination also has evolved. No longer does one have to identify yeasts and fungi in culture—a subject I spotted the ABD and hoped for the best—and surgery is a much more prominent part of the examination.
Nevertheless, over the years the examination continued to emphasize book knowledge and visual pattern recognition. Although they are essential components of being an effective dermatologist, there are other important factors. Many of these can be classified under the term clinical judgment, the ability to make good decisions that take into account the individual patient and situation.
In 2013, the ABD Board of Directors began the process of making fundamental changes in the certification examination with the goal of making it a better test of clinical competence. The process has included matters such as finding the correct technical consultant for examination development and psychometrics, writing and vetting new types of questions, gathering input from program directors, and building the electronic infrastructure to support these changes.
The structure of the new examination is based on a natural progression of learning, from mastering the basics, to acquiring more advanced knowledge, to applying that knowledge in clinical situations. It consists of the following:
- BASIC Exam, a test of fundamentals obtained during the first year of dermatology residency
- CORE Exam, a modular examination emphasizing the more comprehensive knowledge base obtained during the second and third years of residency
- APPLIED Exam, a case-based examination testing ability to apply knowledge appropriately in clinical situations
These new examinations will replace the In-Training Exam and the current certification examination, beginning with the cohort of residents entering dermatology training in July 2017.
The BASIC Exam is designed to test fundamentals such as visual recognition of common diseases, management of uncomplicated conditions, and familiarity with standard procedures. The purposes of the examination are to measure progress, to identify residents who are having difficulty, and to ensure that residents actually master the basics that we sometimes take for granted that they know. It is not a pass/fail examination and thus technically is not part of certification. A detailed content outline for the BASIC Exam can be found on the ABD website.1 Because it is a new examination, it is anticipated that the content will be modified as we gain experience with it and obtain feedback from program directors as to how its usefulness may be improved.
The CORE Exam is designed to test a more advanced, clinically relevant knowledge base. It is part of the certification
The APPLIED Exam is the centerpiece of the new examinations and tests the ability to apply knowledge appropriately in clinical situations. It is case based and ranges from straightforward (most likely diagnosis based on examination) to complex (how to manage pemphigus not responding to the initial treatment in a patient with multiple comorbidities). It is designed to test skills such as knowing when additional information is needed and when it is not, recognizing when referral is indicated, modifying management depending on response to therapy, and recognizing and managing complications. The unique characteristics of an individual patient including patient preferences, ability to comprehend and communicate, comorbidities, financial considerations, and other concerns, will need to be taken into account. The APPLIED Exam will be given in July following completion of residency.
Writing knowledge-based questions with straightforward answers in a psychometrically valid format is actually rather challenging, as first-time question writers discover. Writing items (questions) that test clinical judgment is considerably more difficult. One of the challenges is ensuring that there truly is agreement about the answers. To ensure that there is consensus, we have initiated a new process in item vetting. Rather than sit around a table and come to consensus, a process that could be dominated by experts in a particular area or those with the strongest opinions, committees first vet new questions through a blinded review. Each committee member takes the “test” from home without knowledge of what is supposed to be the correct answer. The responses are anonymous, so members feel free to respond candidly. Then, at the in-person meeting, the anonymous blinded review responses are evaluated and the items are discussed. We have found the blinded review to be invaluable, not just for items testing judgment but for all items.
An enormous amount of work has been put into preparing for the new examinations. Item-writing committees have been working enthusiastically to develop questions. There also is a great deal of work that goes on beyond the ABD. The ABD must contract with vendors for the electronic item bank, editing, psychometrics quality control and scoring, electronic publishing of the examination, virtual dermatopathology, website software for examination registration and reporting, and proctoring. Although developing new examinations is a costly enterprise, the ABD is committed not to increase the financial burden for residents and can use reserve funds to defray new examination development expenses. To keep expenses low during training, we will not charge residents an examination fee for the CORE modules, though they will pay a modest proctoring fee to the proctoring vendor. Also, instead of traveling to Tampa, Florida, in July, candidates will take the APPLIED Exam at a nearby Pearson VUE test center.
It will be the end of an era. Perhaps some of us will feel a little nostalgia for the Rosemont Holiday Inn and the fungal cultures, but I doubt it. Sample items for the 3 examinations, content overviews, frequently asked questions, and more information about the Exam of the Future can be found on the ABD website.2
- Exam of the Future: content outline and blueprint for BASIC exam. American Board of Dermatology website. https://dlpgnf31z4a6s.cloudfront.net/media/151102/basic-exam-content-outline-08132017.pdf. Updated August 13, 2017. Accessed September 12, 2017.
- Exam of the Future information center. American Board of Dermatology website. https://www.abderm.org/residents-and-fellows/exam-of-the-future-information-center.aspx. Accessed September 12, 2017.
- Exam of the Future: content outline and blueprint for BASIC exam. American Board of Dermatology website. https://dlpgnf31z4a6s.cloudfront.net/media/151102/basic-exam-content-outline-08132017.pdf. Updated August 13, 2017. Accessed September 12, 2017.
- Exam of the Future information center. American Board of Dermatology website. https://www.abderm.org/residents-and-fellows/exam-of-the-future-information-center.aspx. Accessed September 12, 2017.
Melanotrichoblastoma: A Rare Pigmented Variant of Trichoblastoma
Trichoblastomas are rare cutaneous tumors that recapitulate the germinative hair bulb and the surrounding mesenchyme. Although benign, they can present diagnostic difficulties for both the clinician and pathologist because of their rarity and overlap both clinically and microscopically with other follicular neoplasms as well as basal cell carcinoma (BCC). Several classification schemes for hair follicle neoplasms have been established based on the relative proportions of epithelial and mesenchymal components as well as stromal inductive change, but nomenclature continues to be problematic, as individual neoplasms show varying degrees of differentiation that do not always uniformly fit within these categories.1,2 One of these established categories is a pigmented trichoblastoma.3 An exceedingly rare variant of a pigmented trichoblastoma referred to as melanotrichoblastoma was first described in 20024 and has only been documented in 3 cases, according to a PubMed search of articles indexed for MEDLINE using the term melanotrichoblastoma.4-6 We report another case of this rare tumor and review the literature on this unique group of tumors.
Case Report
A 25-year-old white woman with a medical history of chronic migraines, myofascial syndrome, and Arnold-Chiari malformation type I presented to dermatology with a 1.5-cm, pedunculated, well-circumscribed tumor on the left side of the scalp (Figure 1). The tumor was grossly flesh colored with heterogeneous areas of dark pigmentation. Microscopic examination demonstrated that within the superficial and deep dermis were variable-sized nests of basaloid cells. Some of the nests had large central cystic spaces with brown pigment within some of these spaces and focal pigmentation of the basaloid cells (Figure 2A). Focal areas of keratinization were present. Mitotic figures were easily identified; however, no atypical mitotic figures were present. Areas of peripheral palisading were present but there was no retraction artifact. Connection to the overlying epidermis was not identified. Surrounding the basaloid nodules was a mildly cellular proliferation of cytologically bland spindle cells. Occasional pigment-laden macrophages were present in the dermis. Focal areas suggestive of papillary mesenchymal body formation were present (Figure 2B). Immunohistochemical staining for Melan-A was performed and demonstrated the presence of a prominent number of melanocytes in some of the nests (Figure 3) and minimal to no melanocytes in other nests. There was no evidence of a melanocytic lesion involving the overlying epidermis. Features of nevus sebaceus were not present. Immunohistochemical staining for cytokeratin (CK) 20 was performed and demonstrated no notable number of Merkel cells within the lesion.
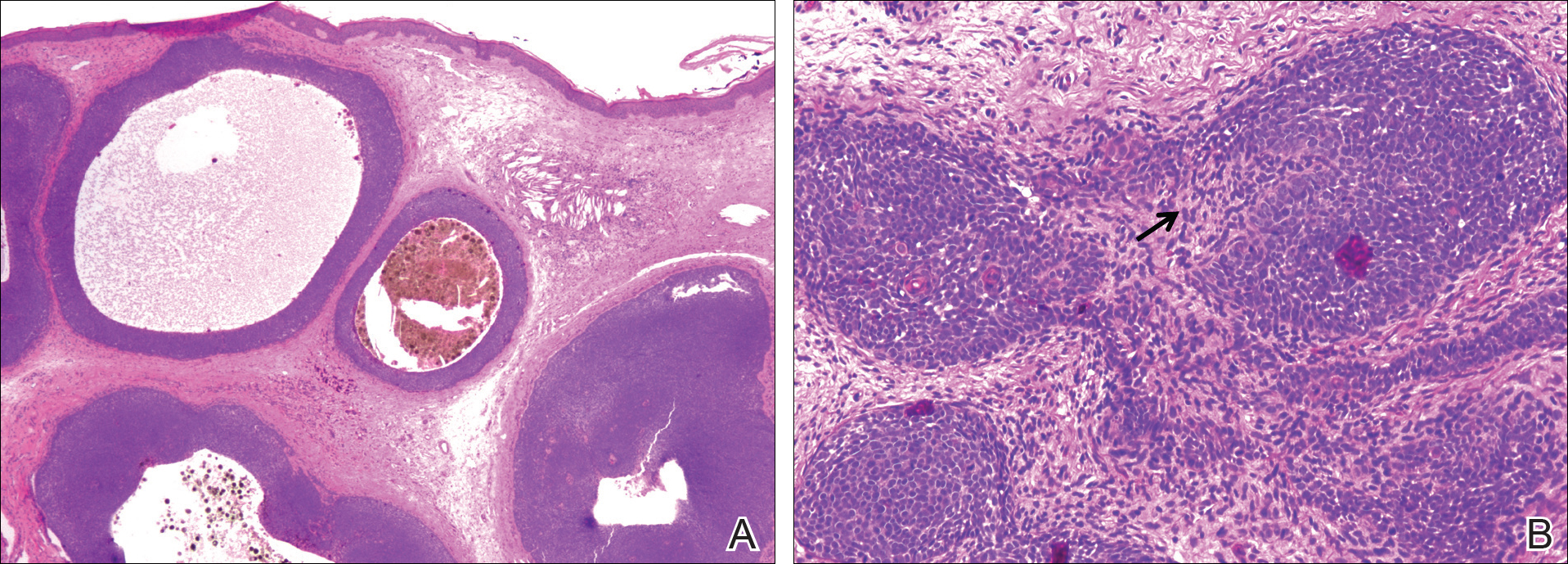
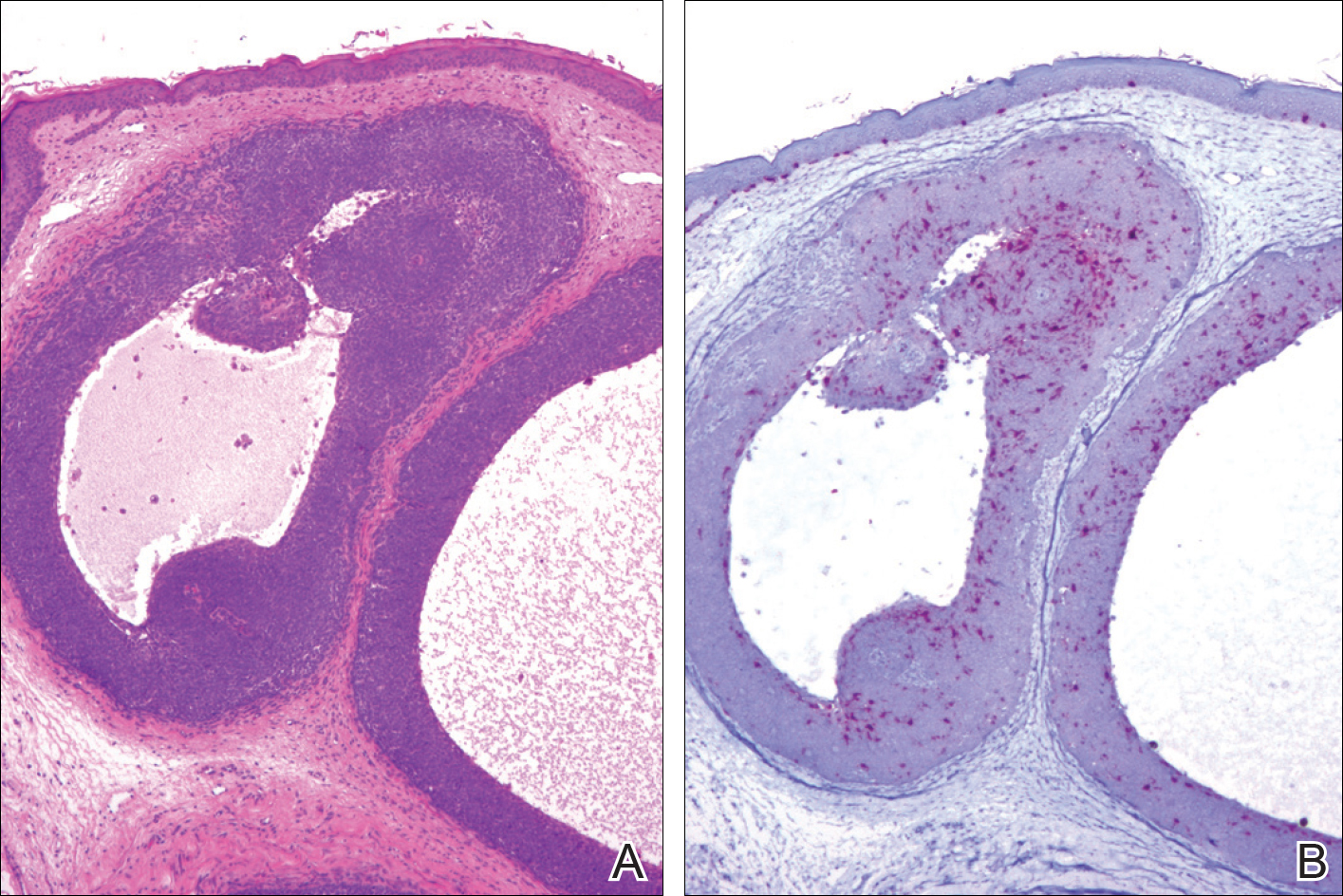
Comment
Overview of Trichoblastomas
Trichoblastomas most often present as solitary, flesh-colored, well-circumscribed, slow-growing tumors that usually progress in size over months to years. Although they may be present at any age, they most commonly occur in adults in the fifth to seventh decades of life and are equally distributed between males and females.7,8 They most often occur on the head and neck with a predilection for the scalp. Although they behave in a benign fashion, cases of malignant trichoblastomas have been reported.9
Histopathology
Histologically, these tumors are well circumscribed but unencapsulated and usually located within the deep dermis, often with extension into the subcutaneous tissue. An epidermal connection is not identified. The tumor typically is composed of variable-sized nests of basaloid cells surrounded by a variable cellular stromal component. Although peripheral palisading is present in the basaloid component, retraction artifact is not present. Several histologic variants of trichoblastomas have been reported including cribriform, racemiform, retiform, pigmented, giant, subcutaneous, rippled pattern, and clear cell.5 Pigmented trichoblastomas are histologically similar to typical trichoblastomas, except for the presence of large amounts of melanin deposited within and around the tumor nests.6 A melanotrichoblastoma is a rare variant of a pigmented trichoblastoma; pigment is present in the lesion and melanocytes are identified within the basaloid nests.
The stromal component of trichoblastomas may show areas of condensation associated with some of the basaloid cells, resembling an attempt at hair bulb formation. Staining for CD10 will be positive in these areas of papillary mesenchymal bodies.10
In an immunohistochemical study of 13 cases of trichoblastomas, there was diffuse positive staining for CK14 and CK17 in all cases (similar to BCC) and positive staining for CK19 in 70% (9/13) of cases compared to 21% (4/19) of BCC cases. Staining for CK8 and CK20 demonstrated the presence of numerous Merkel cells in all trichoblastomas but in none of the 19 cases of BCC tested.11 However, other studies have reported the presence of Merkel cells in only 42% to 70% of trichoblastomas.12,13 Despite the lack of Merkel cells in our case, the lesion was interpreted as a melanotrichoblastoma based on the histologic features in conjunction with the presence of the melanocytes.
Differential Diagnosis
The clinical and histologic differential diagnosis of trichoblastomas includes both trichoepithelioma and BCC. Clinically, all 3 lesions often are slow growing, dome shaped, and small in size (several millimeters), and are observed in the same anatomic distribution of the head and neck region. Furthermore, they often affect middle-aged to older individuals and those of Caucasian descent, though other ethnicities can be affected. Histologic evaluation often is necessary to differentiate between these 3 entities.
Histologically, trichoepitheliomas are composed of nodules of basaloid cells encircled by stromal spindle cells. Although there can be histologic overlap between trichoepitheliomas and trichoblastoma, trichoepitheliomas typically will display obvious features of hair follicle differentiation with the presence of small keratinous cysts and hair bulb structures, while trichoblastomas tend to display minimal changes suggestive of its hair follicle origin. Similar to trichoblastomas, BCC is composed of nests of basaloid cells; however, BCCs often demonstrate retraction artifact and connection to the overlying epidermis. In addition, BCCs typically demonstrate a fibromucinous stromal component that is distinct from the cellular stroma of trichoblastic tumors. Immunoperoxidase staining for androgen receptors has been reported to be positive in 78% (25/32) of BCCs and negative in trichoblastic tumors.14
Melanotrichoblastoma Differentiating Characteristics
An exceedingly rare variant of pigmented trichoblastoma is the melanotrichoblastoma. There are clinical and histologic similarities and differences between the reported cases. The first case, described by Kanitakis et al,4 reported a 32-year-old black woman with a 2-cm scalp mass that slowly enlarged over the course of 2 years. The second case, presented by Kim et al,5 described a 51-year-old Korean man with a subcutaneous 6-cm mass on the back that had been present and slowly enlarging over the course of 5 years. The third case, reported by Hung et al,6 described a 34-year-old Taiwanese man with a 1-cm, left-sided, temporal scalp mass present for 3 years, arising from a nevus sebaceous. Comparing these clinical findings with our case of a 25-year-old white woman with a 1.5-cm mass on the left side of the scalp, melanotrichoblastomas demonstrate a relatively similar age of onset in the early to middle-aged adult years. All 4 tumors were slow growing. Additionally, 3 of 4 cases demonstrated a predilection for the head, particularly the scalp, and grossly showed well-circumscribed lesions with notable pigmentation. Although age, size, location, and gross appearance were similar, a comparable ethnic and gender demographic was not identified.
Microscopic similarities between the 4 cases were present. Each case was characterized by a large, well-circumscribed, unencapsulated, basaloid tumor present in the lower dermis, with only 1 case having tumor cells occasionally reaching the undersurface of the epidermis. The tumor cells were monomorphic round-ovoid in appearance with scant cytoplasm. There was melanin pigment in the basaloid nests. The basaloid nests were surrounded by a proliferation of stromal cells. The mitotic rate was sparse in 2 cases, brisk in 1 case, and not discussed in 1 case. Melanocytes were identified in the basaloid nests in all 4 cases; however, in the current case, the melanocytes were seen in only some of the nests. None of the cases exhibited an overlying junctional melanocytic lesion, which would argue against a possible collision tumor or colonization of an epithelial lesion by a melanocytic lesion.
Although the histologic features of our cases are consistent with prior reports of melanotrichoblastoma, there is some question as to whether it represents a true variant of a pigmented trichoblastoma. There are relatively few articles in the literature that describe pigmented trichoblastomas, and of those, immunohistochemistry staining for melanocytes is uncommon. In one of the earliest descriptions of a pigmented trichoblastoma, dendritic melanocytes were present within the tumor lobules; however, the lesion was reported as a pigmented trichoblastoma and not a melanotrichoblastoma.3 It is possible that all pigmented trichoblastomas may contain some number of dendritic melanocytes, thus negating the existence of a melanotrichoblastoma as a true subtype of pigmented trichoblastomas. Additional study looking at multiple examples of pigmented trichoblastomas would be required to more definitively classify melanotrichoblastomas. It is important to appreciate that at least some cases of pigmented trichoblastomas may contain melanocytes and not to confuse the lesion as representing an example of colonization or collision tumor. A rare case of melanoma possibly arising from these dendritic melanocytes has been reported.15
Conclusion
Trichoblastomas are uncommon tumors of germinative hair bulb origin that can have several histologic variants. A well-documented subtype of trichoblastoma characterized by melanin deposits within and around tumor nests has been identified and classified as a pigmented trichoblastoma. Four cases of melanotrichoblastoma have been reported and represent a variant of a pigmented trichoblastoma characterized by the presence of melanocytes within the lesion. Whether they represent a true variant is of some debate and additional study is required. Although these tumors are exceedingly rare, it is important for the clinician and pathologist to be aware of this entity to prevent confusion with other similarly appearing follicular lesions, most notably BCCs, because of the difference in treatment and follow-up.
- Headington JT. Tumors of the hair follicle: a review. Am J Pathol. 1976; 85 : 479- 514 .
- Wong TY, Reed JA, Suster S, et al. Benign trichogenic tumors: a report of two cases supporting a simplified nomenclature. Histopathology. 1993;22:575-580.
- Aloi F, Tomasini C, Pippione M. Pigmented trichoblastoma. Am J Dermatopathol. 1992;14:345-349.
- Kanitakis J, Brutzkus A, Butnaru AC, et al. Melanotrichoblastoma: immunohistochemical study of a variant of pigmented trichoblastoma. Am J Dermatopathol. 2002;24:498-501.
- Kim DW, Lee JH, Kim I. Giant melanotrichoblastoma. Am J Dermatopathol. 2011;33:E37-E40.
- Hung CT, Chiang CP, Gao HW, et al. Ripple-pattern melanotrichoblastoma arising within nevus sebaceous. Indian J Dermatol Venereol Leprol. 2012;78:665.
- Sau P, Lupton GP, Graham JH. Trichogerminoma: report of 14 cases. J Cutan Pathol. 1992;19:357-365.
- Johnson TV, Wojno TH, Grossniklaus HE. Trichoblastoma of the eyelid. Ophthal Plast Reconstr Surg. 2011;27:E148-E149.
- Schulz T, Proske S, Hartschuh W, et al. High-grade trichoblasticcarcinoma arising in trichoblastoma: a rare adnexal neoplasm often showing metastatic spread. Am J Dermatopathol. 2005;27:9-16.
- Aslani FS, Akbarzadeh-Jahromi M, Jowkar F. Value of CD10 expression in differentiating cutaneous basal from squamous cell carcinomas and basal cell carcinoma from trichoepithelioma. Iran J Med Sci. 2013;38:100-106.
- Kurzen H, Esposito L, Langbein L, et al. Cytokeratins as markers of follicular differentiation: an immunohistochemical study of trichoblastoma and basal cell carcinoma. Am J Dermatopathol. 2001;23:501-509.
- Schulz T, Hartschuh W. Merkel cells are absent in basal cell carcinoma but frequently found in trichoblastomas. an immunohistochemical study. J Cutan Pathol. 1997;24:14-24.
- McNiff JM, Eisen RN, Glusac EJ. Immunohistochemical comparison of cutaneous lymphadenoma, trichoblastoma, and basal cell carcinoma: support for classification of lymphadenoma as a variant of trichoblastoma. J Cutan Pathol. 1999;26:119-124.
- Izikson L, Bhan A, Zembowicz A. Androgen receptor expression helps to differentiate basal cell carcinoma from benign trichoblastic tumors. Am J Dermatopathol. 2005;27:91-95.
- Benaim G, Castillo C, Houang M, et al. Melanoma arising from a long standing pigmented trichoblastoma: clinicopathologic study with complementary aCGH/mutation analysis. Am J Dermatopathol. 2014;36:E146-E151.
Trichoblastomas are rare cutaneous tumors that recapitulate the germinative hair bulb and the surrounding mesenchyme. Although benign, they can present diagnostic difficulties for both the clinician and pathologist because of their rarity and overlap both clinically and microscopically with other follicular neoplasms as well as basal cell carcinoma (BCC). Several classification schemes for hair follicle neoplasms have been established based on the relative proportions of epithelial and mesenchymal components as well as stromal inductive change, but nomenclature continues to be problematic, as individual neoplasms show varying degrees of differentiation that do not always uniformly fit within these categories.1,2 One of these established categories is a pigmented trichoblastoma.3 An exceedingly rare variant of a pigmented trichoblastoma referred to as melanotrichoblastoma was first described in 20024 and has only been documented in 3 cases, according to a PubMed search of articles indexed for MEDLINE using the term melanotrichoblastoma.4-6 We report another case of this rare tumor and review the literature on this unique group of tumors.
Case Report
A 25-year-old white woman with a medical history of chronic migraines, myofascial syndrome, and Arnold-Chiari malformation type I presented to dermatology with a 1.5-cm, pedunculated, well-circumscribed tumor on the left side of the scalp (Figure 1). The tumor was grossly flesh colored with heterogeneous areas of dark pigmentation. Microscopic examination demonstrated that within the superficial and deep dermis were variable-sized nests of basaloid cells. Some of the nests had large central cystic spaces with brown pigment within some of these spaces and focal pigmentation of the basaloid cells (Figure 2A). Focal areas of keratinization were present. Mitotic figures were easily identified; however, no atypical mitotic figures were present. Areas of peripheral palisading were present but there was no retraction artifact. Connection to the overlying epidermis was not identified. Surrounding the basaloid nodules was a mildly cellular proliferation of cytologically bland spindle cells. Occasional pigment-laden macrophages were present in the dermis. Focal areas suggestive of papillary mesenchymal body formation were present (Figure 2B). Immunohistochemical staining for Melan-A was performed and demonstrated the presence of a prominent number of melanocytes in some of the nests (Figure 3) and minimal to no melanocytes in other nests. There was no evidence of a melanocytic lesion involving the overlying epidermis. Features of nevus sebaceus were not present. Immunohistochemical staining for cytokeratin (CK) 20 was performed and demonstrated no notable number of Merkel cells within the lesion.



Comment
Overview of Trichoblastomas
Trichoblastomas most often present as solitary, flesh-colored, well-circumscribed, slow-growing tumors that usually progress in size over months to years. Although they may be present at any age, they most commonly occur in adults in the fifth to seventh decades of life and are equally distributed between males and females.7,8 They most often occur on the head and neck with a predilection for the scalp. Although they behave in a benign fashion, cases of malignant trichoblastomas have been reported.9
Histopathology
Histologically, these tumors are well circumscribed but unencapsulated and usually located within the deep dermis, often with extension into the subcutaneous tissue. An epidermal connection is not identified. The tumor typically is composed of variable-sized nests of basaloid cells surrounded by a variable cellular stromal component. Although peripheral palisading is present in the basaloid component, retraction artifact is not present. Several histologic variants of trichoblastomas have been reported including cribriform, racemiform, retiform, pigmented, giant, subcutaneous, rippled pattern, and clear cell.5 Pigmented trichoblastomas are histologically similar to typical trichoblastomas, except for the presence of large amounts of melanin deposited within and around the tumor nests.6 A melanotrichoblastoma is a rare variant of a pigmented trichoblastoma; pigment is present in the lesion and melanocytes are identified within the basaloid nests.
The stromal component of trichoblastomas may show areas of condensation associated with some of the basaloid cells, resembling an attempt at hair bulb formation. Staining for CD10 will be positive in these areas of papillary mesenchymal bodies.10
In an immunohistochemical study of 13 cases of trichoblastomas, there was diffuse positive staining for CK14 and CK17 in all cases (similar to BCC) and positive staining for CK19 in 70% (9/13) of cases compared to 21% (4/19) of BCC cases. Staining for CK8 and CK20 demonstrated the presence of numerous Merkel cells in all trichoblastomas but in none of the 19 cases of BCC tested.11 However, other studies have reported the presence of Merkel cells in only 42% to 70% of trichoblastomas.12,13 Despite the lack of Merkel cells in our case, the lesion was interpreted as a melanotrichoblastoma based on the histologic features in conjunction with the presence of the melanocytes.
Differential Diagnosis
The clinical and histologic differential diagnosis of trichoblastomas includes both trichoepithelioma and BCC. Clinically, all 3 lesions often are slow growing, dome shaped, and small in size (several millimeters), and are observed in the same anatomic distribution of the head and neck region. Furthermore, they often affect middle-aged to older individuals and those of Caucasian descent, though other ethnicities can be affected. Histologic evaluation often is necessary to differentiate between these 3 entities.
Histologically, trichoepitheliomas are composed of nodules of basaloid cells encircled by stromal spindle cells. Although there can be histologic overlap between trichoepitheliomas and trichoblastoma, trichoepitheliomas typically will display obvious features of hair follicle differentiation with the presence of small keratinous cysts and hair bulb structures, while trichoblastomas tend to display minimal changes suggestive of its hair follicle origin. Similar to trichoblastomas, BCC is composed of nests of basaloid cells; however, BCCs often demonstrate retraction artifact and connection to the overlying epidermis. In addition, BCCs typically demonstrate a fibromucinous stromal component that is distinct from the cellular stroma of trichoblastic tumors. Immunoperoxidase staining for androgen receptors has been reported to be positive in 78% (25/32) of BCCs and negative in trichoblastic tumors.14
Melanotrichoblastoma Differentiating Characteristics
An exceedingly rare variant of pigmented trichoblastoma is the melanotrichoblastoma. There are clinical and histologic similarities and differences between the reported cases. The first case, described by Kanitakis et al,4 reported a 32-year-old black woman with a 2-cm scalp mass that slowly enlarged over the course of 2 years. The second case, presented by Kim et al,5 described a 51-year-old Korean man with a subcutaneous 6-cm mass on the back that had been present and slowly enlarging over the course of 5 years. The third case, reported by Hung et al,6 described a 34-year-old Taiwanese man with a 1-cm, left-sided, temporal scalp mass present for 3 years, arising from a nevus sebaceous. Comparing these clinical findings with our case of a 25-year-old white woman with a 1.5-cm mass on the left side of the scalp, melanotrichoblastomas demonstrate a relatively similar age of onset in the early to middle-aged adult years. All 4 tumors were slow growing. Additionally, 3 of 4 cases demonstrated a predilection for the head, particularly the scalp, and grossly showed well-circumscribed lesions with notable pigmentation. Although age, size, location, and gross appearance were similar, a comparable ethnic and gender demographic was not identified.
Microscopic similarities between the 4 cases were present. Each case was characterized by a large, well-circumscribed, unencapsulated, basaloid tumor present in the lower dermis, with only 1 case having tumor cells occasionally reaching the undersurface of the epidermis. The tumor cells were monomorphic round-ovoid in appearance with scant cytoplasm. There was melanin pigment in the basaloid nests. The basaloid nests were surrounded by a proliferation of stromal cells. The mitotic rate was sparse in 2 cases, brisk in 1 case, and not discussed in 1 case. Melanocytes were identified in the basaloid nests in all 4 cases; however, in the current case, the melanocytes were seen in only some of the nests. None of the cases exhibited an overlying junctional melanocytic lesion, which would argue against a possible collision tumor or colonization of an epithelial lesion by a melanocytic lesion.
Although the histologic features of our cases are consistent with prior reports of melanotrichoblastoma, there is some question as to whether it represents a true variant of a pigmented trichoblastoma. There are relatively few articles in the literature that describe pigmented trichoblastomas, and of those, immunohistochemistry staining for melanocytes is uncommon. In one of the earliest descriptions of a pigmented trichoblastoma, dendritic melanocytes were present within the tumor lobules; however, the lesion was reported as a pigmented trichoblastoma and not a melanotrichoblastoma.3 It is possible that all pigmented trichoblastomas may contain some number of dendritic melanocytes, thus negating the existence of a melanotrichoblastoma as a true subtype of pigmented trichoblastomas. Additional study looking at multiple examples of pigmented trichoblastomas would be required to more definitively classify melanotrichoblastomas. It is important to appreciate that at least some cases of pigmented trichoblastomas may contain melanocytes and not to confuse the lesion as representing an example of colonization or collision tumor. A rare case of melanoma possibly arising from these dendritic melanocytes has been reported.15
Conclusion
Trichoblastomas are uncommon tumors of germinative hair bulb origin that can have several histologic variants. A well-documented subtype of trichoblastoma characterized by melanin deposits within and around tumor nests has been identified and classified as a pigmented trichoblastoma. Four cases of melanotrichoblastoma have been reported and represent a variant of a pigmented trichoblastoma characterized by the presence of melanocytes within the lesion. Whether they represent a true variant is of some debate and additional study is required. Although these tumors are exceedingly rare, it is important for the clinician and pathologist to be aware of this entity to prevent confusion with other similarly appearing follicular lesions, most notably BCCs, because of the difference in treatment and follow-up.
Trichoblastomas are rare cutaneous tumors that recapitulate the germinative hair bulb and the surrounding mesenchyme. Although benign, they can present diagnostic difficulties for both the clinician and pathologist because of their rarity and overlap both clinically and microscopically with other follicular neoplasms as well as basal cell carcinoma (BCC). Several classification schemes for hair follicle neoplasms have been established based on the relative proportions of epithelial and mesenchymal components as well as stromal inductive change, but nomenclature continues to be problematic, as individual neoplasms show varying degrees of differentiation that do not always uniformly fit within these categories.1,2 One of these established categories is a pigmented trichoblastoma.3 An exceedingly rare variant of a pigmented trichoblastoma referred to as melanotrichoblastoma was first described in 20024 and has only been documented in 3 cases, according to a PubMed search of articles indexed for MEDLINE using the term melanotrichoblastoma.4-6 We report another case of this rare tumor and review the literature on this unique group of tumors.
Case Report
A 25-year-old white woman with a medical history of chronic migraines, myofascial syndrome, and Arnold-Chiari malformation type I presented to dermatology with a 1.5-cm, pedunculated, well-circumscribed tumor on the left side of the scalp (Figure 1). The tumor was grossly flesh colored with heterogeneous areas of dark pigmentation. Microscopic examination demonstrated that within the superficial and deep dermis were variable-sized nests of basaloid cells. Some of the nests had large central cystic spaces with brown pigment within some of these spaces and focal pigmentation of the basaloid cells (Figure 2A). Focal areas of keratinization were present. Mitotic figures were easily identified; however, no atypical mitotic figures were present. Areas of peripheral palisading were present but there was no retraction artifact. Connection to the overlying epidermis was not identified. Surrounding the basaloid nodules was a mildly cellular proliferation of cytologically bland spindle cells. Occasional pigment-laden macrophages were present in the dermis. Focal areas suggestive of papillary mesenchymal body formation were present (Figure 2B). Immunohistochemical staining for Melan-A was performed and demonstrated the presence of a prominent number of melanocytes in some of the nests (Figure 3) and minimal to no melanocytes in other nests. There was no evidence of a melanocytic lesion involving the overlying epidermis. Features of nevus sebaceus were not present. Immunohistochemical staining for cytokeratin (CK) 20 was performed and demonstrated no notable number of Merkel cells within the lesion.



Comment
Overview of Trichoblastomas
Trichoblastomas most often present as solitary, flesh-colored, well-circumscribed, slow-growing tumors that usually progress in size over months to years. Although they may be present at any age, they most commonly occur in adults in the fifth to seventh decades of life and are equally distributed between males and females.7,8 They most often occur on the head and neck with a predilection for the scalp. Although they behave in a benign fashion, cases of malignant trichoblastomas have been reported.9
Histopathology
Histologically, these tumors are well circumscribed but unencapsulated and usually located within the deep dermis, often with extension into the subcutaneous tissue. An epidermal connection is not identified. The tumor typically is composed of variable-sized nests of basaloid cells surrounded by a variable cellular stromal component. Although peripheral palisading is present in the basaloid component, retraction artifact is not present. Several histologic variants of trichoblastomas have been reported including cribriform, racemiform, retiform, pigmented, giant, subcutaneous, rippled pattern, and clear cell.5 Pigmented trichoblastomas are histologically similar to typical trichoblastomas, except for the presence of large amounts of melanin deposited within and around the tumor nests.6 A melanotrichoblastoma is a rare variant of a pigmented trichoblastoma; pigment is present in the lesion and melanocytes are identified within the basaloid nests.
The stromal component of trichoblastomas may show areas of condensation associated with some of the basaloid cells, resembling an attempt at hair bulb formation. Staining for CD10 will be positive in these areas of papillary mesenchymal bodies.10
In an immunohistochemical study of 13 cases of trichoblastomas, there was diffuse positive staining for CK14 and CK17 in all cases (similar to BCC) and positive staining for CK19 in 70% (9/13) of cases compared to 21% (4/19) of BCC cases. Staining for CK8 and CK20 demonstrated the presence of numerous Merkel cells in all trichoblastomas but in none of the 19 cases of BCC tested.11 However, other studies have reported the presence of Merkel cells in only 42% to 70% of trichoblastomas.12,13 Despite the lack of Merkel cells in our case, the lesion was interpreted as a melanotrichoblastoma based on the histologic features in conjunction with the presence of the melanocytes.
Differential Diagnosis
The clinical and histologic differential diagnosis of trichoblastomas includes both trichoepithelioma and BCC. Clinically, all 3 lesions often are slow growing, dome shaped, and small in size (several millimeters), and are observed in the same anatomic distribution of the head and neck region. Furthermore, they often affect middle-aged to older individuals and those of Caucasian descent, though other ethnicities can be affected. Histologic evaluation often is necessary to differentiate between these 3 entities.
Histologically, trichoepitheliomas are composed of nodules of basaloid cells encircled by stromal spindle cells. Although there can be histologic overlap between trichoepitheliomas and trichoblastoma, trichoepitheliomas typically will display obvious features of hair follicle differentiation with the presence of small keratinous cysts and hair bulb structures, while trichoblastomas tend to display minimal changes suggestive of its hair follicle origin. Similar to trichoblastomas, BCC is composed of nests of basaloid cells; however, BCCs often demonstrate retraction artifact and connection to the overlying epidermis. In addition, BCCs typically demonstrate a fibromucinous stromal component that is distinct from the cellular stroma of trichoblastic tumors. Immunoperoxidase staining for androgen receptors has been reported to be positive in 78% (25/32) of BCCs and negative in trichoblastic tumors.14
Melanotrichoblastoma Differentiating Characteristics
An exceedingly rare variant of pigmented trichoblastoma is the melanotrichoblastoma. There are clinical and histologic similarities and differences between the reported cases. The first case, described by Kanitakis et al,4 reported a 32-year-old black woman with a 2-cm scalp mass that slowly enlarged over the course of 2 years. The second case, presented by Kim et al,5 described a 51-year-old Korean man with a subcutaneous 6-cm mass on the back that had been present and slowly enlarging over the course of 5 years. The third case, reported by Hung et al,6 described a 34-year-old Taiwanese man with a 1-cm, left-sided, temporal scalp mass present for 3 years, arising from a nevus sebaceous. Comparing these clinical findings with our case of a 25-year-old white woman with a 1.5-cm mass on the left side of the scalp, melanotrichoblastomas demonstrate a relatively similar age of onset in the early to middle-aged adult years. All 4 tumors were slow growing. Additionally, 3 of 4 cases demonstrated a predilection for the head, particularly the scalp, and grossly showed well-circumscribed lesions with notable pigmentation. Although age, size, location, and gross appearance were similar, a comparable ethnic and gender demographic was not identified.
Microscopic similarities between the 4 cases were present. Each case was characterized by a large, well-circumscribed, unencapsulated, basaloid tumor present in the lower dermis, with only 1 case having tumor cells occasionally reaching the undersurface of the epidermis. The tumor cells were monomorphic round-ovoid in appearance with scant cytoplasm. There was melanin pigment in the basaloid nests. The basaloid nests were surrounded by a proliferation of stromal cells. The mitotic rate was sparse in 2 cases, brisk in 1 case, and not discussed in 1 case. Melanocytes were identified in the basaloid nests in all 4 cases; however, in the current case, the melanocytes were seen in only some of the nests. None of the cases exhibited an overlying junctional melanocytic lesion, which would argue against a possible collision tumor or colonization of an epithelial lesion by a melanocytic lesion.
Although the histologic features of our cases are consistent with prior reports of melanotrichoblastoma, there is some question as to whether it represents a true variant of a pigmented trichoblastoma. There are relatively few articles in the literature that describe pigmented trichoblastomas, and of those, immunohistochemistry staining for melanocytes is uncommon. In one of the earliest descriptions of a pigmented trichoblastoma, dendritic melanocytes were present within the tumor lobules; however, the lesion was reported as a pigmented trichoblastoma and not a melanotrichoblastoma.3 It is possible that all pigmented trichoblastomas may contain some number of dendritic melanocytes, thus negating the existence of a melanotrichoblastoma as a true subtype of pigmented trichoblastomas. Additional study looking at multiple examples of pigmented trichoblastomas would be required to more definitively classify melanotrichoblastomas. It is important to appreciate that at least some cases of pigmented trichoblastomas may contain melanocytes and not to confuse the lesion as representing an example of colonization or collision tumor. A rare case of melanoma possibly arising from these dendritic melanocytes has been reported.15
Conclusion
Trichoblastomas are uncommon tumors of germinative hair bulb origin that can have several histologic variants. A well-documented subtype of trichoblastoma characterized by melanin deposits within and around tumor nests has been identified and classified as a pigmented trichoblastoma. Four cases of melanotrichoblastoma have been reported and represent a variant of a pigmented trichoblastoma characterized by the presence of melanocytes within the lesion. Whether they represent a true variant is of some debate and additional study is required. Although these tumors are exceedingly rare, it is important for the clinician and pathologist to be aware of this entity to prevent confusion with other similarly appearing follicular lesions, most notably BCCs, because of the difference in treatment and follow-up.
- Headington JT. Tumors of the hair follicle: a review. Am J Pathol. 1976; 85 : 479- 514 .
- Wong TY, Reed JA, Suster S, et al. Benign trichogenic tumors: a report of two cases supporting a simplified nomenclature. Histopathology. 1993;22:575-580.
- Aloi F, Tomasini C, Pippione M. Pigmented trichoblastoma. Am J Dermatopathol. 1992;14:345-349.
- Kanitakis J, Brutzkus A, Butnaru AC, et al. Melanotrichoblastoma: immunohistochemical study of a variant of pigmented trichoblastoma. Am J Dermatopathol. 2002;24:498-501.
- Kim DW, Lee JH, Kim I. Giant melanotrichoblastoma. Am J Dermatopathol. 2011;33:E37-E40.
- Hung CT, Chiang CP, Gao HW, et al. Ripple-pattern melanotrichoblastoma arising within nevus sebaceous. Indian J Dermatol Venereol Leprol. 2012;78:665.
- Sau P, Lupton GP, Graham JH. Trichogerminoma: report of 14 cases. J Cutan Pathol. 1992;19:357-365.
- Johnson TV, Wojno TH, Grossniklaus HE. Trichoblastoma of the eyelid. Ophthal Plast Reconstr Surg. 2011;27:E148-E149.
- Schulz T, Proske S, Hartschuh W, et al. High-grade trichoblasticcarcinoma arising in trichoblastoma: a rare adnexal neoplasm often showing metastatic spread. Am J Dermatopathol. 2005;27:9-16.
- Aslani FS, Akbarzadeh-Jahromi M, Jowkar F. Value of CD10 expression in differentiating cutaneous basal from squamous cell carcinomas and basal cell carcinoma from trichoepithelioma. Iran J Med Sci. 2013;38:100-106.
- Kurzen H, Esposito L, Langbein L, et al. Cytokeratins as markers of follicular differentiation: an immunohistochemical study of trichoblastoma and basal cell carcinoma. Am J Dermatopathol. 2001;23:501-509.
- Schulz T, Hartschuh W. Merkel cells are absent in basal cell carcinoma but frequently found in trichoblastomas. an immunohistochemical study. J Cutan Pathol. 1997;24:14-24.
- McNiff JM, Eisen RN, Glusac EJ. Immunohistochemical comparison of cutaneous lymphadenoma, trichoblastoma, and basal cell carcinoma: support for classification of lymphadenoma as a variant of trichoblastoma. J Cutan Pathol. 1999;26:119-124.
- Izikson L, Bhan A, Zembowicz A. Androgen receptor expression helps to differentiate basal cell carcinoma from benign trichoblastic tumors. Am J Dermatopathol. 2005;27:91-95.
- Benaim G, Castillo C, Houang M, et al. Melanoma arising from a long standing pigmented trichoblastoma: clinicopathologic study with complementary aCGH/mutation analysis. Am J Dermatopathol. 2014;36:E146-E151.
- Headington JT. Tumors of the hair follicle: a review. Am J Pathol. 1976; 85 : 479- 514 .
- Wong TY, Reed JA, Suster S, et al. Benign trichogenic tumors: a report of two cases supporting a simplified nomenclature. Histopathology. 1993;22:575-580.
- Aloi F, Tomasini C, Pippione M. Pigmented trichoblastoma. Am J Dermatopathol. 1992;14:345-349.
- Kanitakis J, Brutzkus A, Butnaru AC, et al. Melanotrichoblastoma: immunohistochemical study of a variant of pigmented trichoblastoma. Am J Dermatopathol. 2002;24:498-501.
- Kim DW, Lee JH, Kim I. Giant melanotrichoblastoma. Am J Dermatopathol. 2011;33:E37-E40.
- Hung CT, Chiang CP, Gao HW, et al. Ripple-pattern melanotrichoblastoma arising within nevus sebaceous. Indian J Dermatol Venereol Leprol. 2012;78:665.
- Sau P, Lupton GP, Graham JH. Trichogerminoma: report of 14 cases. J Cutan Pathol. 1992;19:357-365.
- Johnson TV, Wojno TH, Grossniklaus HE. Trichoblastoma of the eyelid. Ophthal Plast Reconstr Surg. 2011;27:E148-E149.
- Schulz T, Proske S, Hartschuh W, et al. High-grade trichoblasticcarcinoma arising in trichoblastoma: a rare adnexal neoplasm often showing metastatic spread. Am J Dermatopathol. 2005;27:9-16.
- Aslani FS, Akbarzadeh-Jahromi M, Jowkar F. Value of CD10 expression in differentiating cutaneous basal from squamous cell carcinomas and basal cell carcinoma from trichoepithelioma. Iran J Med Sci. 2013;38:100-106.
- Kurzen H, Esposito L, Langbein L, et al. Cytokeratins as markers of follicular differentiation: an immunohistochemical study of trichoblastoma and basal cell carcinoma. Am J Dermatopathol. 2001;23:501-509.
- Schulz T, Hartschuh W. Merkel cells are absent in basal cell carcinoma but frequently found in trichoblastomas. an immunohistochemical study. J Cutan Pathol. 1997;24:14-24.
- McNiff JM, Eisen RN, Glusac EJ. Immunohistochemical comparison of cutaneous lymphadenoma, trichoblastoma, and basal cell carcinoma: support for classification of lymphadenoma as a variant of trichoblastoma. J Cutan Pathol. 1999;26:119-124.
- Izikson L, Bhan A, Zembowicz A. Androgen receptor expression helps to differentiate basal cell carcinoma from benign trichoblastic tumors. Am J Dermatopathol. 2005;27:91-95.
- Benaim G, Castillo C, Houang M, et al. Melanoma arising from a long standing pigmented trichoblastoma: clinicopathologic study with complementary aCGH/mutation analysis. Am J Dermatopathol. 2014;36:E146-E151.
Practice Points
- Pigmented trichoblastoma is a histologic variant of trichoblastoma characterized by the presence of melanin pigment.
- At least some pigmented trichoblastomas contain melanocytes and have been referred to as melanotrichoblastomas.
- The presence of melanocytes within pigmented trichoblastomas should not be confused as representing an example of colonization or a collision tumor.