User login
SVS Coding Workshop is Oct. 13-14
Don't let the federal government keep money -- in the form of Medicare reimbursements -- to which you are entitled!
Learn all about coding and reimbursement, from the essentials to modifiers to future initiatives, at the SVS Coding and Reimbursement Workshop, Oct. 13-14, in Chicago. Instructors are Teri Romano, RN, MBA, CPC, CMDP; Sean P. Roddy, MD; Robert M. Zwolak, MD, PhD; and Sunita D. Srivastava, MD.
Friday topics are: coding and reimbursement essentials, global surgical packages, getting paid the first time when applying surgical modifiers and the Medicare rule on non-physician practitioner billing.
Saturday topics include an overview of Current Procedural Terminology, coding for a number of procedures and information on MACRA, MIPS and APMs. Future SVS CPT coding initiatives also will be discussed.
An optional half-day workshop, from 9 a.m. to noon Friday, Oct. 13, will focus on codes for evaluation and management (E&M), which physicians continue to misunderstand and misuse.
Cost is $880 for an SVS member or staff, $955 for a non-member and $250 for residents and trainees. Cost for the optional session is $100 for an SVS member or staff, $215 for a non-member and $50 for residents and trainees.
Learn more, register and access the full agenda here.
Don't let the federal government keep money -- in the form of Medicare reimbursements -- to which you are entitled!
Learn all about coding and reimbursement, from the essentials to modifiers to future initiatives, at the SVS Coding and Reimbursement Workshop, Oct. 13-14, in Chicago. Instructors are Teri Romano, RN, MBA, CPC, CMDP; Sean P. Roddy, MD; Robert M. Zwolak, MD, PhD; and Sunita D. Srivastava, MD.
Friday topics are: coding and reimbursement essentials, global surgical packages, getting paid the first time when applying surgical modifiers and the Medicare rule on non-physician practitioner billing.
Saturday topics include an overview of Current Procedural Terminology, coding for a number of procedures and information on MACRA, MIPS and APMs. Future SVS CPT coding initiatives also will be discussed.
An optional half-day workshop, from 9 a.m. to noon Friday, Oct. 13, will focus on codes for evaluation and management (E&M), which physicians continue to misunderstand and misuse.
Cost is $880 for an SVS member or staff, $955 for a non-member and $250 for residents and trainees. Cost for the optional session is $100 for an SVS member or staff, $215 for a non-member and $50 for residents and trainees.
Learn more, register and access the full agenda here.
Don't let the federal government keep money -- in the form of Medicare reimbursements -- to which you are entitled!
Learn all about coding and reimbursement, from the essentials to modifiers to future initiatives, at the SVS Coding and Reimbursement Workshop, Oct. 13-14, in Chicago. Instructors are Teri Romano, RN, MBA, CPC, CMDP; Sean P. Roddy, MD; Robert M. Zwolak, MD, PhD; and Sunita D. Srivastava, MD.
Friday topics are: coding and reimbursement essentials, global surgical packages, getting paid the first time when applying surgical modifiers and the Medicare rule on non-physician practitioner billing.
Saturday topics include an overview of Current Procedural Terminology, coding for a number of procedures and information on MACRA, MIPS and APMs. Future SVS CPT coding initiatives also will be discussed.
An optional half-day workshop, from 9 a.m. to noon Friday, Oct. 13, will focus on codes for evaluation and management (E&M), which physicians continue to misunderstand and misuse.
Cost is $880 for an SVS member or staff, $955 for a non-member and $250 for residents and trainees. Cost for the optional session is $100 for an SVS member or staff, $215 for a non-member and $50 for residents and trainees.
Learn more, register and access the full agenda here.
Granulomatous Pigmented Purpuric Dermatosis
Pigmented purpuric dermatoses (PPDs) are a spectrum of chronic disorders that present as speckled brown to purpuric lesions and orange-brown discoloration of the skin.1 Eruptions generally occur in middle-aged to elderly patients and commonly follow a chronic waxing and waning course.2 Lesions usually are found in a localized distribution on the legs. Histologically, PPD presents with perivascular infiltrates of lymphocytes and macrophages centered around the superficial small blood vessels with narrowing of the lumina. Extravasation of red blood cells and hemosiderin deposition are commonly seen in the absence of vasculitis.
The etiology of PPD is unknown; however, important cofactors include venous hypertension, exercise and gravitational dependency, capillary fragility, focal infections, and chemical ingestions.1 Drugs are the most important provoking factors, including acetaminophen, aspirin, adalin, carbromal, chlordiazepoxide, glipizide, glybuzole, hydralazine, meprobamate, dipyridamole, reserpine, thiamine, and interferon-alfa, as well as medroxyprogesterone acetate injection. Other phenomena include contact allergy and alcohol ingestion.1
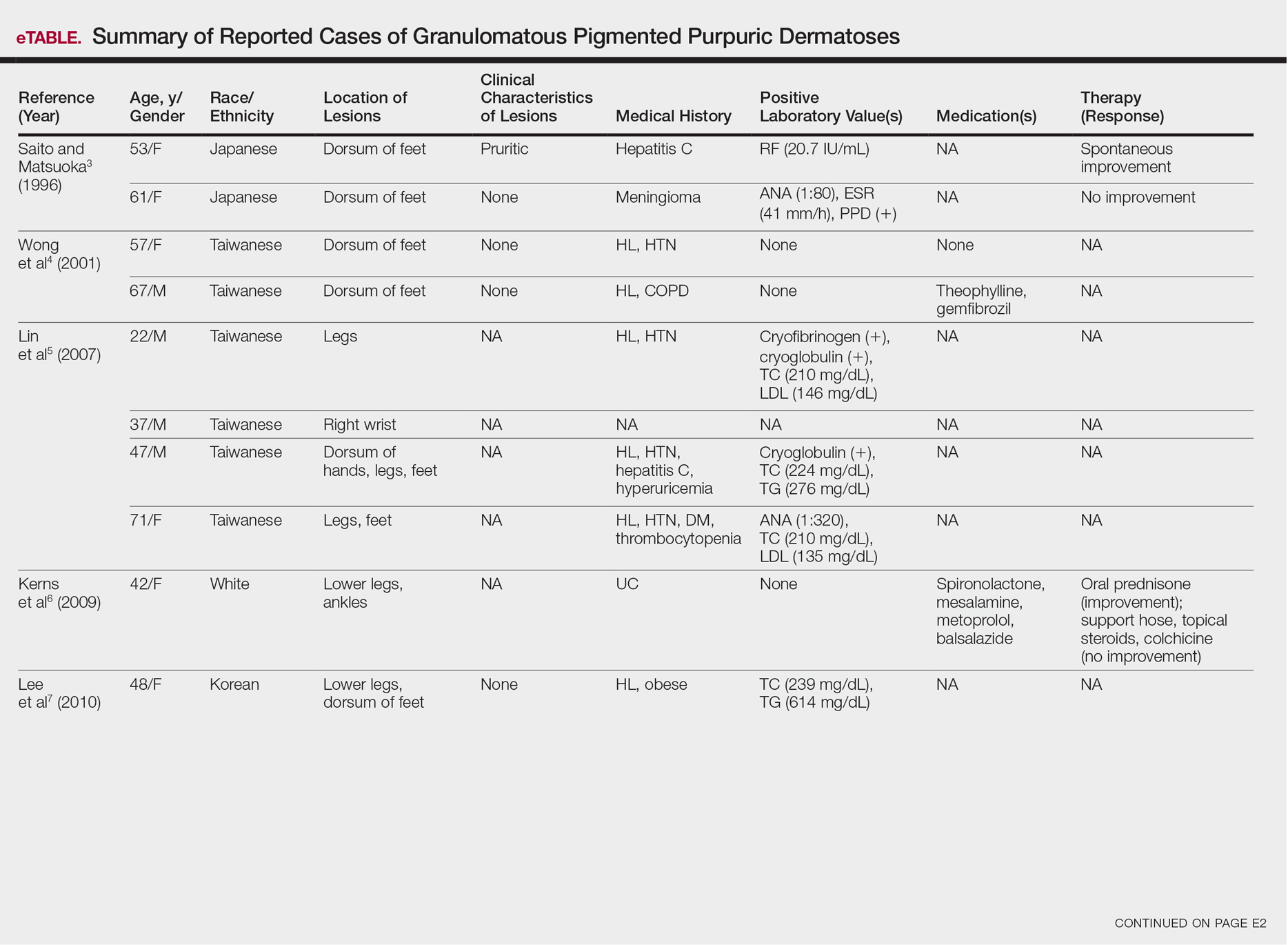
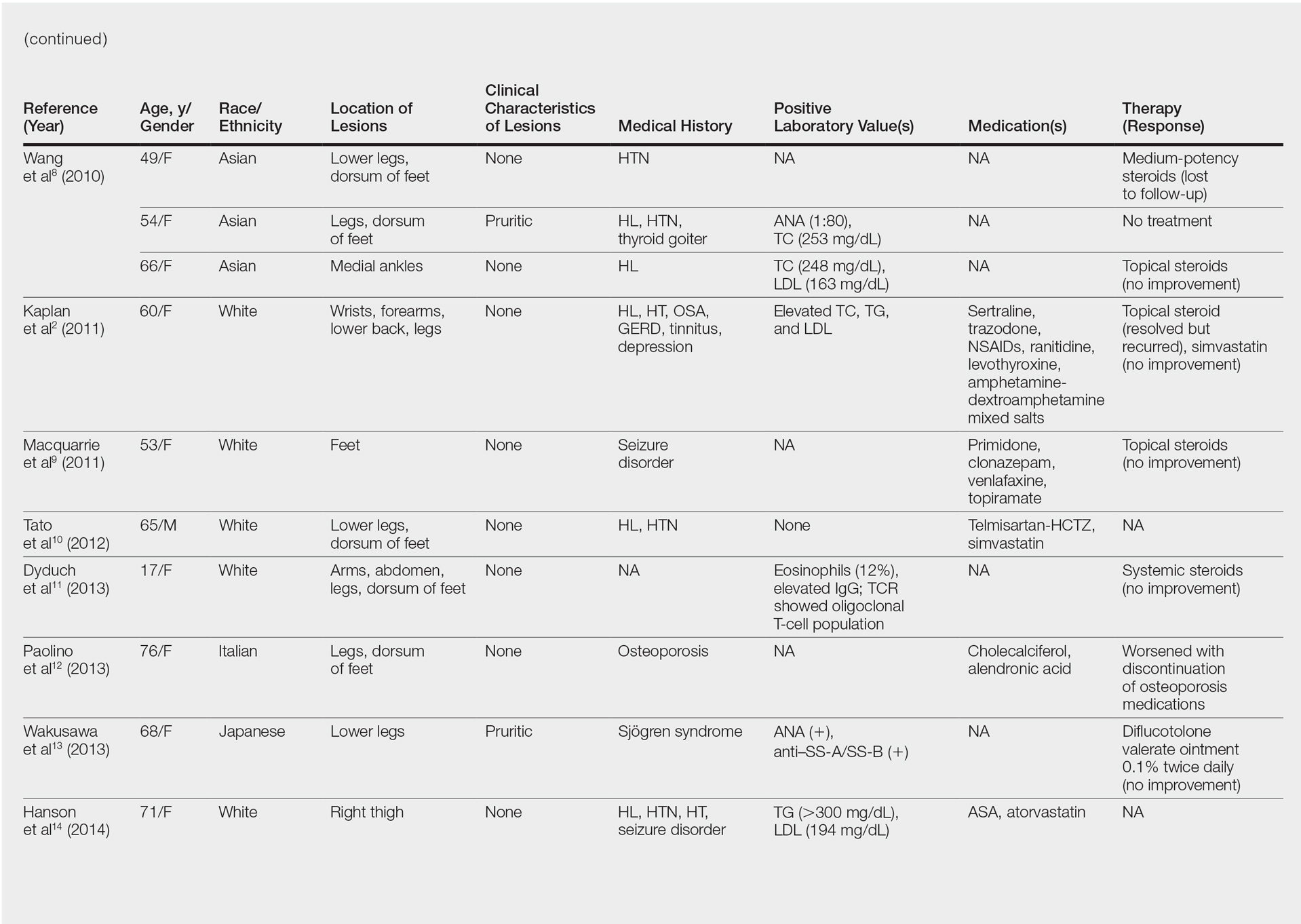
Although the diagnosis often is made clinically, many forms of PPD exist. The 4 main forms include Schaumberg disease, purpura annularis telangiectaticum of Majocchi, pigmented purpuric lichenoid dermatitis of Gougerot and Blum, and eczematoidlike purpura of Doucas and Kapetanakis. Less common variants include itching purpura of Lowenthal, lichen purpuricus, lichen aureus, granulomatous pigmented purpura, transitory pigmented purpuric dermatosis, and linear pigmented purpura.1Granulomatous PPD (GPPD) is a rare histologic variant of PPD. Clinically, it is indistinguishable from other forms of PPD but reveals itself histologically with granulomatous infiltrates superimposed on classic PPD. We report a case of GPPD and provide a thorough literature review focusing on epidemiology, clinical symptoms, and treatment.2-17 The eTable summarizes all reported cases of GPPD.
Case Report
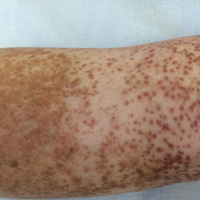
An 86-year-old white man with no remarkable medical history presented with an asymptomatic eruption over the bilateral shins extending up both thighs of 6 years’ duration (Figure 1). It began as a 15-cm patch on the right medial thigh that rapidly spread over 1 year to involve the majority of the legs. Physical examination revealed scattered 1- to 2-mm brown macules coalescing into patches on both legs. The patches increased in density distally and extended from the bilateral thighs to the ankles. Edema of the legs was absent, and lesions were nonblanchable and without scale or induration. The differential diagnoses included stasis dermatitis, vasculitis, and PPD. All laboratory values were within reference range, including complete blood cell count, comprehensive metabolic panel, urine analysis, and lipid profile.
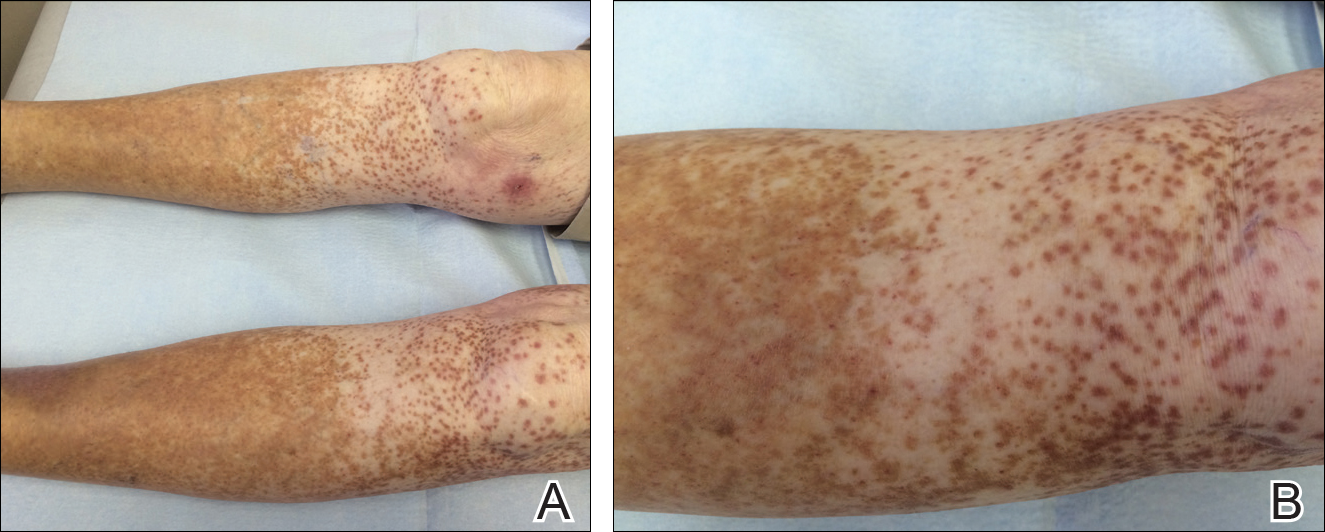
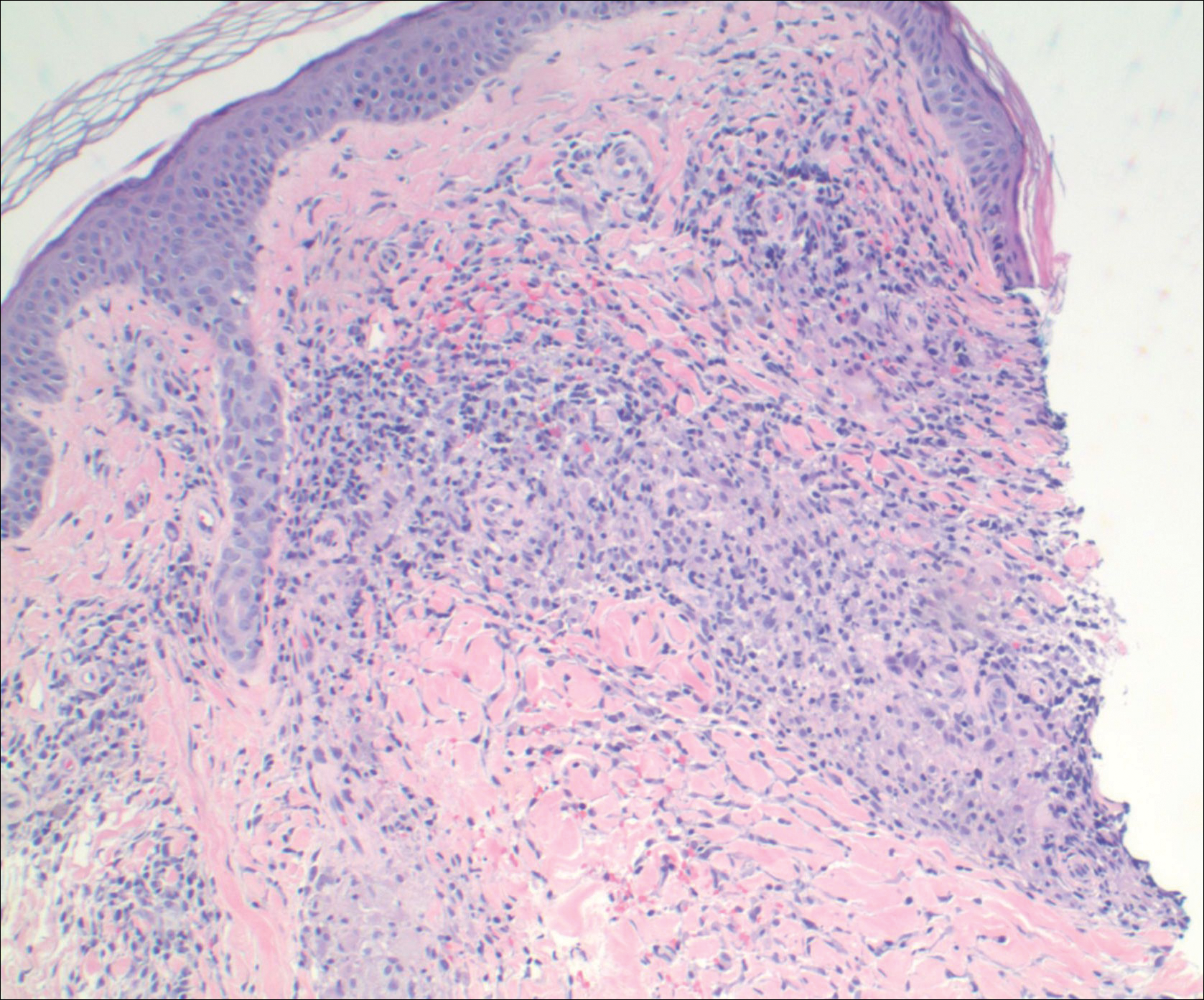
A punch biopsy from the distal right thigh revealed a superficial to mid dermal perivascular lymphocyte-predominant infiltrate with associated siderophages and a focal granulomatous infiltrate comprised of histiocytes (Figure 2). Periodic acid–Schiff, acid-fast bacilli, and Fite stains were negative for microorganisms. No eosinophils or leukocytoclasia were seen. The patient applied betamethasone dipropionate cream 0.05% twice daily for several weeks without improvement. Because the lesions were asymptomatic, he discontinued the topical medication.
Comment
Pathogenesis/Etiology of GPPD
Granulomatous PPD is a rare histological variant of PPD, which was first reported in 1996 by Saito and Matsuoka.3 Originally, GPPD was mainly thought to affect individuals in the Far East and be associated with the hepatitis C virus, antinuclear antibodies, or rheumatoid factor.3 Since its initial description, GPPD continues to predominantly be seen on the distal legs. According to a PubMed search of articles indexed for MEDLINE and the Michigan State University library database using the terms granulomatous pigmented purpuric dermatosis and pigmented purpuric dermatosis, 26 known cases including the current case (Asian, n=13; white, n=13) have been reported. The mean age of onset was 54.5 years and the female to male ratio was 2.5 to 1.
Currently, the etiology of GPPD is unknown; however, 13 reported cases have been associated with hyperlipidemia,2,4,5,7,8,10,14-16 which has led to the speculation that they may be related. Previous investigators have postulated that the granulomatous infiltrate is a response to lipid deposition in the endothelial cells or that the elevated lipid levels launch an incompetent helper T cell (TH1) response, leading to granuloma formation.5,7,8 Currently, hyperlipidemia is present in 50% of patients and appears to be trending downward as more cases present in the literature.
Medications have been implicated in the pathogenesis of PPD and may have a possible role in the development of the granulomatous variant.9 One case report noted preceding medication changes, alluding to the possibility of aminosalicylates being the culprit.6
Another case described GPPD appearing after an upper respiratory tract infection.11 Comorbidities are not uncommon in patients presenting with GPPD. Although the majority of cases are single reports, they include systemic derangements such as hepatitis C,3,5 Sjögren syndrome,13 hypertension,2,4,5,8,10,14,15 seizure disorder,9,14 ulcerative colitis,6 diabetes mellitus,5,15 meningioma,3 renal calculi,15 thrombocytopenia,5 chronic obstructive pulmonary disease,4 thyroid goiter,8 obstructive sleep apnea,2,15 osteoporosis,12 asthma,15 gastroesophageal reflux disease/Barrett esophagus,2,15 hypothyroidism,2,14,15 and hyperuricemia.5
Clinical Presentation
Clinically, GPPD commonly presents as asymptomatic petechiae and bronze discoloration of the lower legs. The clinical presentation can vary from a solitary lesion to a localized eruption typically on the lower legs or rarely a widespread eruption. A review of the literature revealed 5 cases presenting on the upper arms2,5,11,16 and 4 on the trunk.2,11,16,17 Four patients presented with pruritus3,8,13,16 and 1 described pain and photosensitive lesions.15 No other clinical signs of hyperlipidemia were described (eg, xanthomas). The duration of the disease has a wide spectrum, ranging from 3 weeks to 20 years.4,16
Histopathology
With the increasing trend toward dermatoscopic evaluation, 2 reviews evaluated dermatoscopic features of GPPD. These reports described scattered, round to oval, red dots, globules, and patches with a diffuse red-brown or coppery background of pigmentation.14,17
The granulomatous variant of PPD is characterized histopathologically by ill-defined, nonnecrotizing granulomas admixed with a lymphocytic infiltrate. Commonly, erythrocyte extravasation and hemosiderin are seen with granulomas superimposed on classic changes of PPD.15 Vasculitis features including endothelial swelling, fibrinoid necrosis, and leukocytoclasia are absent. Rarely, eosinophils are seen.6 Mild epidermal spongiosis and exocytosis of lymphocytes may be seen in all variants of PPD, except lichen aureus.1 This exocytosis was observed focally in one case of GPPD.4 Although loosely formed granulomas in the papillary dermis are characteristic, 7 cases have had a concomitant lichenoid infiltrate.2,9-11,15,16
Kaplan et al2 reported granulomatous and nongranulomatous PPD occurring together in different areas of the body. A new granulomatous variant was proposed in a 2015 report that revealed 2 patients with granulomatous infiltrates in the mid to deep dermis rather than the classic superficial dermis.15 One case of GPPD was suspicious for progression into mycosis fungoides (MF) and described a lichenoid infiltrate with mild atypical and small lymphocytes migrating into the epidermis.11 Follow-up biopsy lacked epidermotropism and quantitative representation of T-cell subsets. The diagnosis of early-phase MF was based on the progressive clinical course rather than immunohistologic and molecular findings.11 One other case exhibited minimal epidermotropism.15
Management of GPPD should require a lipid profile with other tests to assess cardiovascular risk.10 A thorough medication review and a punch rather than a shave biopsy should be performed, especially because granulomatous infiltrates have been found in the mid to deep dermis.15 With the lack of rebiopsies documented, follow-up and rebiopsy has been suggested if there is suspicion of MF; however, we favor rebiopsy at a later time to help reveal the course of this disease and rule out progression into MF.
Therapy
Thus far, therapy has mostly been with oral and topical steroids. Five case reports noted improvement,2,3,6,15,16 2 with oral and 3 with topical steroids. However, therapy has been discouraging, with clinical improvement being transient in most treatment-responsive patients. One case spontaneously resolved.3 Ten cases did not document therapy or follow-up.4,5,7,10,14,17 Only 1 case reported follow-up after treatment with simvastatin; unfortunately, the patient had no improvement.2 Our case revealed no improvement with topical steroids.
Conclusion
The exact pathogenesis of GPPD is unknown. The initial impression that GPPD was a disease in Far East Asians and patients with hyperlipidemia is becoming less clear. Based on the current literature including the addition of our case, the prevalence appears to be equal among white individuals and Asians, possibly due to increased awareness of this condition and documentation in the literature. Correlation with systemic disorders such as hyperlipidemia and hypertensive medications needs further review. Eight cases reported a medical history of hypertension.4,5,8,10,14 With antihypertensive medications being a potential culprit of PPD, this etiology should not be overlooked. A punch biopsy should be performed, especially because granulomatous infiltrates may be lurking in the mid to deep dermis.15 Granulomatous PPD has a chronic course with a disappointing response to therapy but appears to be benign in nature.12 A rebiopsy is recommended if MF is suspected. Evaluation of GPPD following therapy for hyperlipidemia is not well documented and should be pursued. Clinicians and pathologists should be aware of the suspected associations and consider this variant when dermal granulomatous infiltrates are present with a background of PPD.
- Sardana K, Sarkar R, Sehgal VN. Pigmented purpuric dermatoses: an overview. Int J Dermatol. 2004;43:482-488.
- Kaplan J, Burgin S, Sepehr A. Granulomatous pigmented purpura: report of a case and review of the literature. J Cutan Pathol. 2011;38:984-989.
- Saito R, Matsuoka Y. Granulomatous pigmented purpuric dermatosis. J Dermatol. 1996;23:551-555.
- Wong WR, Kuo TT, Chen MJ, et al. Granulomatous variant of chronic pigmented purpuric dermatosis: report of two cases. Br J Dermatol. 2001;145:162-164.
- Lin WL, Kuo TT, Shih PY, et al. Granulomatous variant of chronic pigmented purpuric dermatoses: report of four new cases and an association with hyperlipidaemia. Clin Exp Dermatol. 2007;32:513-515.
- Kerns MJ, Mallatt BD, Shamma HN. Granulomatous pigmented purpura: an unusual histological variant. Am J Dermatopathol. 2009;31:77-80.
- Lee SH, Kwon JE, Lee KG, et al. Granulomatous variant of chronic pigmented purpuric dermatosis associated with hyperlipidaemia. J Eur Acad Dermatol Venereol. 2010;24:1243-1245.
- Wang J, Wu Y, Hsiao P, et al. Granulomatous pigmented purpuric dermatoses: report of three cases and review of the literature. Dermatologica Sinica. 2010;28:77-81.
- Macquarrie EK, Pasternak S, Torok M, et al. Persistent pigmented purpuric dermatitis: granulomatous variant. J Cutan Pathol. 2011;38:979-983.
- Tato BP, Marinero Escobedo S, Pérez González YC, et al. Granulomatous variant of pigmented purpuric dermatosis. Am J Dermatopathol. 2012;34:746-748.
- Dyduch G, Zuber Z, Turowska-Heydel D, et al. Granulomatous pigmented purpura in an adolescent girl: a precursor of mycosis fungoides? Pol J Pathol. 2013;64:157-159; answer 160.
- Paolino S, Cinotti E, Merlo V, et al. Progressive petechial and pigmented macules and papules on the lower extremities. Am J Dermatopathol. 2013;35:370, 388.
- Wakusawa C, Fujimura T, Haga T, et al. Granulomatous pigmented purpuric dermatitis associated with primary Sjögren’s syndrome. Acta Derm Venereol. 2013;93:95-96.
- Hanson C, Fischer R, Fraga G, et al. Granulomatous pigmented purpuric dermatosis: an unusual variant associated with hyperlipidemia. Dermatol Online J. 2014;21. pii:13030/qt0tp272d1.
- Morrissey K, Rosenbach M, DeHoratius D, et al. Granulomatous changes associated with pigmented purpuric dermatosis. Cutis. 2014;94:197-202.
- Battle LR, Shalin SC, Gao L. Granulomatous pigmented purpuric dermatosis [published online December 18, 2014]. Clin Exp Dermatol. 2015;40:387-390.
- Mackenzie AI, Biswas A. Granulomatous pigmented purpuric dermatosis: report of a case with atypical clinical presentation including dermoscopic findings. Am J Dermatopathol. 2015;37:311-314.
Pigmented purpuric dermatoses (PPDs) are a spectrum of chronic disorders that present as speckled brown to purpuric lesions and orange-brown discoloration of the skin.1 Eruptions generally occur in middle-aged to elderly patients and commonly follow a chronic waxing and waning course.2 Lesions usually are found in a localized distribution on the legs. Histologically, PPD presents with perivascular infiltrates of lymphocytes and macrophages centered around the superficial small blood vessels with narrowing of the lumina. Extravasation of red blood cells and hemosiderin deposition are commonly seen in the absence of vasculitis.
The etiology of PPD is unknown; however, important cofactors include venous hypertension, exercise and gravitational dependency, capillary fragility, focal infections, and chemical ingestions.1 Drugs are the most important provoking factors, including acetaminophen, aspirin, adalin, carbromal, chlordiazepoxide, glipizide, glybuzole, hydralazine, meprobamate, dipyridamole, reserpine, thiamine, and interferon-alfa, as well as medroxyprogesterone acetate injection. Other phenomena include contact allergy and alcohol ingestion.1
Although the diagnosis often is made clinically, many forms of PPD exist. The 4 main forms include Schaumberg disease, purpura annularis telangiectaticum of Majocchi, pigmented purpuric lichenoid dermatitis of Gougerot and Blum, and eczematoidlike purpura of Doucas and Kapetanakis. Less common variants include itching purpura of Lowenthal, lichen purpuricus, lichen aureus, granulomatous pigmented purpura, transitory pigmented purpuric dermatosis, and linear pigmented purpura.1Granulomatous PPD (GPPD) is a rare histologic variant of PPD. Clinically, it is indistinguishable from other forms of PPD but reveals itself histologically with granulomatous infiltrates superimposed on classic PPD. We report a case of GPPD and provide a thorough literature review focusing on epidemiology, clinical symptoms, and treatment.2-17 The eTable summarizes all reported cases of GPPD.



Case Report
An 86-year-old white man with no remarkable medical history presented with an asymptomatic eruption over the bilateral shins extending up both thighs of 6 years’ duration (Figure 1). It began as a 15-cm patch on the right medial thigh that rapidly spread over 1 year to involve the majority of the legs. Physical examination revealed scattered 1- to 2-mm brown macules coalescing into patches on both legs. The patches increased in density distally and extended from the bilateral thighs to the ankles. Edema of the legs was absent, and lesions were nonblanchable and without scale or induration. The differential diagnoses included stasis dermatitis, vasculitis, and PPD. All laboratory values were within reference range, including complete blood cell count, comprehensive metabolic panel, urine analysis, and lipid profile.

A punch biopsy from the distal right thigh revealed a superficial to mid dermal perivascular lymphocyte-predominant infiltrate with associated siderophages and a focal granulomatous infiltrate comprised of histiocytes (Figure 2). Periodic acid–Schiff, acid-fast bacilli, and Fite stains were negative for microorganisms. No eosinophils or leukocytoclasia were seen. The patient applied betamethasone dipropionate cream 0.05% twice daily for several weeks without improvement. Because the lesions were asymptomatic, he discontinued the topical medication.

Comment
Pathogenesis/Etiology of GPPD
Granulomatous PPD is a rare histological variant of PPD, which was first reported in 1996 by Saito and Matsuoka.3 Originally, GPPD was mainly thought to affect individuals in the Far East and be associated with the hepatitis C virus, antinuclear antibodies, or rheumatoid factor.3 Since its initial description, GPPD continues to predominantly be seen on the distal legs. According to a PubMed search of articles indexed for MEDLINE and the Michigan State University library database using the terms granulomatous pigmented purpuric dermatosis and pigmented purpuric dermatosis, 26 known cases including the current case (Asian, n=13; white, n=13) have been reported. The mean age of onset was 54.5 years and the female to male ratio was 2.5 to 1.
Currently, the etiology of GPPD is unknown; however, 13 reported cases have been associated with hyperlipidemia,2,4,5,7,8,10,14-16 which has led to the speculation that they may be related. Previous investigators have postulated that the granulomatous infiltrate is a response to lipid deposition in the endothelial cells or that the elevated lipid levels launch an incompetent helper T cell (TH1) response, leading to granuloma formation.5,7,8 Currently, hyperlipidemia is present in 50% of patients and appears to be trending downward as more cases present in the literature.
Medications have been implicated in the pathogenesis of PPD and may have a possible role in the development of the granulomatous variant.9 One case report noted preceding medication changes, alluding to the possibility of aminosalicylates being the culprit.6
Another case described GPPD appearing after an upper respiratory tract infection.11 Comorbidities are not uncommon in patients presenting with GPPD. Although the majority of cases are single reports, they include systemic derangements such as hepatitis C,3,5 Sjögren syndrome,13 hypertension,2,4,5,8,10,14,15 seizure disorder,9,14 ulcerative colitis,6 diabetes mellitus,5,15 meningioma,3 renal calculi,15 thrombocytopenia,5 chronic obstructive pulmonary disease,4 thyroid goiter,8 obstructive sleep apnea,2,15 osteoporosis,12 asthma,15 gastroesophageal reflux disease/Barrett esophagus,2,15 hypothyroidism,2,14,15 and hyperuricemia.5
Clinical Presentation
Clinically, GPPD commonly presents as asymptomatic petechiae and bronze discoloration of the lower legs. The clinical presentation can vary from a solitary lesion to a localized eruption typically on the lower legs or rarely a widespread eruption. A review of the literature revealed 5 cases presenting on the upper arms2,5,11,16 and 4 on the trunk.2,11,16,17 Four patients presented with pruritus3,8,13,16 and 1 described pain and photosensitive lesions.15 No other clinical signs of hyperlipidemia were described (eg, xanthomas). The duration of the disease has a wide spectrum, ranging from 3 weeks to 20 years.4,16
Histopathology
With the increasing trend toward dermatoscopic evaluation, 2 reviews evaluated dermatoscopic features of GPPD. These reports described scattered, round to oval, red dots, globules, and patches with a diffuse red-brown or coppery background of pigmentation.14,17
The granulomatous variant of PPD is characterized histopathologically by ill-defined, nonnecrotizing granulomas admixed with a lymphocytic infiltrate. Commonly, erythrocyte extravasation and hemosiderin are seen with granulomas superimposed on classic changes of PPD.15 Vasculitis features including endothelial swelling, fibrinoid necrosis, and leukocytoclasia are absent. Rarely, eosinophils are seen.6 Mild epidermal spongiosis and exocytosis of lymphocytes may be seen in all variants of PPD, except lichen aureus.1 This exocytosis was observed focally in one case of GPPD.4 Although loosely formed granulomas in the papillary dermis are characteristic, 7 cases have had a concomitant lichenoid infiltrate.2,9-11,15,16
Kaplan et al2 reported granulomatous and nongranulomatous PPD occurring together in different areas of the body. A new granulomatous variant was proposed in a 2015 report that revealed 2 patients with granulomatous infiltrates in the mid to deep dermis rather than the classic superficial dermis.15 One case of GPPD was suspicious for progression into mycosis fungoides (MF) and described a lichenoid infiltrate with mild atypical and small lymphocytes migrating into the epidermis.11 Follow-up biopsy lacked epidermotropism and quantitative representation of T-cell subsets. The diagnosis of early-phase MF was based on the progressive clinical course rather than immunohistologic and molecular findings.11 One other case exhibited minimal epidermotropism.15
Management of GPPD should require a lipid profile with other tests to assess cardiovascular risk.10 A thorough medication review and a punch rather than a shave biopsy should be performed, especially because granulomatous infiltrates have been found in the mid to deep dermis.15 With the lack of rebiopsies documented, follow-up and rebiopsy has been suggested if there is suspicion of MF; however, we favor rebiopsy at a later time to help reveal the course of this disease and rule out progression into MF.
Therapy
Thus far, therapy has mostly been with oral and topical steroids. Five case reports noted improvement,2,3,6,15,16 2 with oral and 3 with topical steroids. However, therapy has been discouraging, with clinical improvement being transient in most treatment-responsive patients. One case spontaneously resolved.3 Ten cases did not document therapy or follow-up.4,5,7,10,14,17 Only 1 case reported follow-up after treatment with simvastatin; unfortunately, the patient had no improvement.2 Our case revealed no improvement with topical steroids.
Conclusion
The exact pathogenesis of GPPD is unknown. The initial impression that GPPD was a disease in Far East Asians and patients with hyperlipidemia is becoming less clear. Based on the current literature including the addition of our case, the prevalence appears to be equal among white individuals and Asians, possibly due to increased awareness of this condition and documentation in the literature. Correlation with systemic disorders such as hyperlipidemia and hypertensive medications needs further review. Eight cases reported a medical history of hypertension.4,5,8,10,14 With antihypertensive medications being a potential culprit of PPD, this etiology should not be overlooked. A punch biopsy should be performed, especially because granulomatous infiltrates may be lurking in the mid to deep dermis.15 Granulomatous PPD has a chronic course with a disappointing response to therapy but appears to be benign in nature.12 A rebiopsy is recommended if MF is suspected. Evaluation of GPPD following therapy for hyperlipidemia is not well documented and should be pursued. Clinicians and pathologists should be aware of the suspected associations and consider this variant when dermal granulomatous infiltrates are present with a background of PPD.
Pigmented purpuric dermatoses (PPDs) are a spectrum of chronic disorders that present as speckled brown to purpuric lesions and orange-brown discoloration of the skin.1 Eruptions generally occur in middle-aged to elderly patients and commonly follow a chronic waxing and waning course.2 Lesions usually are found in a localized distribution on the legs. Histologically, PPD presents with perivascular infiltrates of lymphocytes and macrophages centered around the superficial small blood vessels with narrowing of the lumina. Extravasation of red blood cells and hemosiderin deposition are commonly seen in the absence of vasculitis.
The etiology of PPD is unknown; however, important cofactors include venous hypertension, exercise and gravitational dependency, capillary fragility, focal infections, and chemical ingestions.1 Drugs are the most important provoking factors, including acetaminophen, aspirin, adalin, carbromal, chlordiazepoxide, glipizide, glybuzole, hydralazine, meprobamate, dipyridamole, reserpine, thiamine, and interferon-alfa, as well as medroxyprogesterone acetate injection. Other phenomena include contact allergy and alcohol ingestion.1
Although the diagnosis often is made clinically, many forms of PPD exist. The 4 main forms include Schaumberg disease, purpura annularis telangiectaticum of Majocchi, pigmented purpuric lichenoid dermatitis of Gougerot and Blum, and eczematoidlike purpura of Doucas and Kapetanakis. Less common variants include itching purpura of Lowenthal, lichen purpuricus, lichen aureus, granulomatous pigmented purpura, transitory pigmented purpuric dermatosis, and linear pigmented purpura.1Granulomatous PPD (GPPD) is a rare histologic variant of PPD. Clinically, it is indistinguishable from other forms of PPD but reveals itself histologically with granulomatous infiltrates superimposed on classic PPD. We report a case of GPPD and provide a thorough literature review focusing on epidemiology, clinical symptoms, and treatment.2-17 The eTable summarizes all reported cases of GPPD.



Case Report
An 86-year-old white man with no remarkable medical history presented with an asymptomatic eruption over the bilateral shins extending up both thighs of 6 years’ duration (Figure 1). It began as a 15-cm patch on the right medial thigh that rapidly spread over 1 year to involve the majority of the legs. Physical examination revealed scattered 1- to 2-mm brown macules coalescing into patches on both legs. The patches increased in density distally and extended from the bilateral thighs to the ankles. Edema of the legs was absent, and lesions were nonblanchable and without scale or induration. The differential diagnoses included stasis dermatitis, vasculitis, and PPD. All laboratory values were within reference range, including complete blood cell count, comprehensive metabolic panel, urine analysis, and lipid profile.

A punch biopsy from the distal right thigh revealed a superficial to mid dermal perivascular lymphocyte-predominant infiltrate with associated siderophages and a focal granulomatous infiltrate comprised of histiocytes (Figure 2). Periodic acid–Schiff, acid-fast bacilli, and Fite stains were negative for microorganisms. No eosinophils or leukocytoclasia were seen. The patient applied betamethasone dipropionate cream 0.05% twice daily for several weeks without improvement. Because the lesions were asymptomatic, he discontinued the topical medication.

Comment
Pathogenesis/Etiology of GPPD
Granulomatous PPD is a rare histological variant of PPD, which was first reported in 1996 by Saito and Matsuoka.3 Originally, GPPD was mainly thought to affect individuals in the Far East and be associated with the hepatitis C virus, antinuclear antibodies, or rheumatoid factor.3 Since its initial description, GPPD continues to predominantly be seen on the distal legs. According to a PubMed search of articles indexed for MEDLINE and the Michigan State University library database using the terms granulomatous pigmented purpuric dermatosis and pigmented purpuric dermatosis, 26 known cases including the current case (Asian, n=13; white, n=13) have been reported. The mean age of onset was 54.5 years and the female to male ratio was 2.5 to 1.
Currently, the etiology of GPPD is unknown; however, 13 reported cases have been associated with hyperlipidemia,2,4,5,7,8,10,14-16 which has led to the speculation that they may be related. Previous investigators have postulated that the granulomatous infiltrate is a response to lipid deposition in the endothelial cells or that the elevated lipid levels launch an incompetent helper T cell (TH1) response, leading to granuloma formation.5,7,8 Currently, hyperlipidemia is present in 50% of patients and appears to be trending downward as more cases present in the literature.
Medications have been implicated in the pathogenesis of PPD and may have a possible role in the development of the granulomatous variant.9 One case report noted preceding medication changes, alluding to the possibility of aminosalicylates being the culprit.6
Another case described GPPD appearing after an upper respiratory tract infection.11 Comorbidities are not uncommon in patients presenting with GPPD. Although the majority of cases are single reports, they include systemic derangements such as hepatitis C,3,5 Sjögren syndrome,13 hypertension,2,4,5,8,10,14,15 seizure disorder,9,14 ulcerative colitis,6 diabetes mellitus,5,15 meningioma,3 renal calculi,15 thrombocytopenia,5 chronic obstructive pulmonary disease,4 thyroid goiter,8 obstructive sleep apnea,2,15 osteoporosis,12 asthma,15 gastroesophageal reflux disease/Barrett esophagus,2,15 hypothyroidism,2,14,15 and hyperuricemia.5
Clinical Presentation
Clinically, GPPD commonly presents as asymptomatic petechiae and bronze discoloration of the lower legs. The clinical presentation can vary from a solitary lesion to a localized eruption typically on the lower legs or rarely a widespread eruption. A review of the literature revealed 5 cases presenting on the upper arms2,5,11,16 and 4 on the trunk.2,11,16,17 Four patients presented with pruritus3,8,13,16 and 1 described pain and photosensitive lesions.15 No other clinical signs of hyperlipidemia were described (eg, xanthomas). The duration of the disease has a wide spectrum, ranging from 3 weeks to 20 years.4,16
Histopathology
With the increasing trend toward dermatoscopic evaluation, 2 reviews evaluated dermatoscopic features of GPPD. These reports described scattered, round to oval, red dots, globules, and patches with a diffuse red-brown or coppery background of pigmentation.14,17
The granulomatous variant of PPD is characterized histopathologically by ill-defined, nonnecrotizing granulomas admixed with a lymphocytic infiltrate. Commonly, erythrocyte extravasation and hemosiderin are seen with granulomas superimposed on classic changes of PPD.15 Vasculitis features including endothelial swelling, fibrinoid necrosis, and leukocytoclasia are absent. Rarely, eosinophils are seen.6 Mild epidermal spongiosis and exocytosis of lymphocytes may be seen in all variants of PPD, except lichen aureus.1 This exocytosis was observed focally in one case of GPPD.4 Although loosely formed granulomas in the papillary dermis are characteristic, 7 cases have had a concomitant lichenoid infiltrate.2,9-11,15,16
Kaplan et al2 reported granulomatous and nongranulomatous PPD occurring together in different areas of the body. A new granulomatous variant was proposed in a 2015 report that revealed 2 patients with granulomatous infiltrates in the mid to deep dermis rather than the classic superficial dermis.15 One case of GPPD was suspicious for progression into mycosis fungoides (MF) and described a lichenoid infiltrate with mild atypical and small lymphocytes migrating into the epidermis.11 Follow-up biopsy lacked epidermotropism and quantitative representation of T-cell subsets. The diagnosis of early-phase MF was based on the progressive clinical course rather than immunohistologic and molecular findings.11 One other case exhibited minimal epidermotropism.15
Management of GPPD should require a lipid profile with other tests to assess cardiovascular risk.10 A thorough medication review and a punch rather than a shave biopsy should be performed, especially because granulomatous infiltrates have been found in the mid to deep dermis.15 With the lack of rebiopsies documented, follow-up and rebiopsy has been suggested if there is suspicion of MF; however, we favor rebiopsy at a later time to help reveal the course of this disease and rule out progression into MF.
Therapy
Thus far, therapy has mostly been with oral and topical steroids. Five case reports noted improvement,2,3,6,15,16 2 with oral and 3 with topical steroids. However, therapy has been discouraging, with clinical improvement being transient in most treatment-responsive patients. One case spontaneously resolved.3 Ten cases did not document therapy or follow-up.4,5,7,10,14,17 Only 1 case reported follow-up after treatment with simvastatin; unfortunately, the patient had no improvement.2 Our case revealed no improvement with topical steroids.
Conclusion
The exact pathogenesis of GPPD is unknown. The initial impression that GPPD was a disease in Far East Asians and patients with hyperlipidemia is becoming less clear. Based on the current literature including the addition of our case, the prevalence appears to be equal among white individuals and Asians, possibly due to increased awareness of this condition and documentation in the literature. Correlation with systemic disorders such as hyperlipidemia and hypertensive medications needs further review. Eight cases reported a medical history of hypertension.4,5,8,10,14 With antihypertensive medications being a potential culprit of PPD, this etiology should not be overlooked. A punch biopsy should be performed, especially because granulomatous infiltrates may be lurking in the mid to deep dermis.15 Granulomatous PPD has a chronic course with a disappointing response to therapy but appears to be benign in nature.12 A rebiopsy is recommended if MF is suspected. Evaluation of GPPD following therapy for hyperlipidemia is not well documented and should be pursued. Clinicians and pathologists should be aware of the suspected associations and consider this variant when dermal granulomatous infiltrates are present with a background of PPD.
- Sardana K, Sarkar R, Sehgal VN. Pigmented purpuric dermatoses: an overview. Int J Dermatol. 2004;43:482-488.
- Kaplan J, Burgin S, Sepehr A. Granulomatous pigmented purpura: report of a case and review of the literature. J Cutan Pathol. 2011;38:984-989.
- Saito R, Matsuoka Y. Granulomatous pigmented purpuric dermatosis. J Dermatol. 1996;23:551-555.
- Wong WR, Kuo TT, Chen MJ, et al. Granulomatous variant of chronic pigmented purpuric dermatosis: report of two cases. Br J Dermatol. 2001;145:162-164.
- Lin WL, Kuo TT, Shih PY, et al. Granulomatous variant of chronic pigmented purpuric dermatoses: report of four new cases and an association with hyperlipidaemia. Clin Exp Dermatol. 2007;32:513-515.
- Kerns MJ, Mallatt BD, Shamma HN. Granulomatous pigmented purpura: an unusual histological variant. Am J Dermatopathol. 2009;31:77-80.
- Lee SH, Kwon JE, Lee KG, et al. Granulomatous variant of chronic pigmented purpuric dermatosis associated with hyperlipidaemia. J Eur Acad Dermatol Venereol. 2010;24:1243-1245.
- Wang J, Wu Y, Hsiao P, et al. Granulomatous pigmented purpuric dermatoses: report of three cases and review of the literature. Dermatologica Sinica. 2010;28:77-81.
- Macquarrie EK, Pasternak S, Torok M, et al. Persistent pigmented purpuric dermatitis: granulomatous variant. J Cutan Pathol. 2011;38:979-983.
- Tato BP, Marinero Escobedo S, Pérez González YC, et al. Granulomatous variant of pigmented purpuric dermatosis. Am J Dermatopathol. 2012;34:746-748.
- Dyduch G, Zuber Z, Turowska-Heydel D, et al. Granulomatous pigmented purpura in an adolescent girl: a precursor of mycosis fungoides? Pol J Pathol. 2013;64:157-159; answer 160.
- Paolino S, Cinotti E, Merlo V, et al. Progressive petechial and pigmented macules and papules on the lower extremities. Am J Dermatopathol. 2013;35:370, 388.
- Wakusawa C, Fujimura T, Haga T, et al. Granulomatous pigmented purpuric dermatitis associated with primary Sjögren’s syndrome. Acta Derm Venereol. 2013;93:95-96.
- Hanson C, Fischer R, Fraga G, et al. Granulomatous pigmented purpuric dermatosis: an unusual variant associated with hyperlipidemia. Dermatol Online J. 2014;21. pii:13030/qt0tp272d1.
- Morrissey K, Rosenbach M, DeHoratius D, et al. Granulomatous changes associated with pigmented purpuric dermatosis. Cutis. 2014;94:197-202.
- Battle LR, Shalin SC, Gao L. Granulomatous pigmented purpuric dermatosis [published online December 18, 2014]. Clin Exp Dermatol. 2015;40:387-390.
- Mackenzie AI, Biswas A. Granulomatous pigmented purpuric dermatosis: report of a case with atypical clinical presentation including dermoscopic findings. Am J Dermatopathol. 2015;37:311-314.
- Sardana K, Sarkar R, Sehgal VN. Pigmented purpuric dermatoses: an overview. Int J Dermatol. 2004;43:482-488.
- Kaplan J, Burgin S, Sepehr A. Granulomatous pigmented purpura: report of a case and review of the literature. J Cutan Pathol. 2011;38:984-989.
- Saito R, Matsuoka Y. Granulomatous pigmented purpuric dermatosis. J Dermatol. 1996;23:551-555.
- Wong WR, Kuo TT, Chen MJ, et al. Granulomatous variant of chronic pigmented purpuric dermatosis: report of two cases. Br J Dermatol. 2001;145:162-164.
- Lin WL, Kuo TT, Shih PY, et al. Granulomatous variant of chronic pigmented purpuric dermatoses: report of four new cases and an association with hyperlipidaemia. Clin Exp Dermatol. 2007;32:513-515.
- Kerns MJ, Mallatt BD, Shamma HN. Granulomatous pigmented purpura: an unusual histological variant. Am J Dermatopathol. 2009;31:77-80.
- Lee SH, Kwon JE, Lee KG, et al. Granulomatous variant of chronic pigmented purpuric dermatosis associated with hyperlipidaemia. J Eur Acad Dermatol Venereol. 2010;24:1243-1245.
- Wang J, Wu Y, Hsiao P, et al. Granulomatous pigmented purpuric dermatoses: report of three cases and review of the literature. Dermatologica Sinica. 2010;28:77-81.
- Macquarrie EK, Pasternak S, Torok M, et al. Persistent pigmented purpuric dermatitis: granulomatous variant. J Cutan Pathol. 2011;38:979-983.
- Tato BP, Marinero Escobedo S, Pérez González YC, et al. Granulomatous variant of pigmented purpuric dermatosis. Am J Dermatopathol. 2012;34:746-748.
- Dyduch G, Zuber Z, Turowska-Heydel D, et al. Granulomatous pigmented purpura in an adolescent girl: a precursor of mycosis fungoides? Pol J Pathol. 2013;64:157-159; answer 160.
- Paolino S, Cinotti E, Merlo V, et al. Progressive petechial and pigmented macules and papules on the lower extremities. Am J Dermatopathol. 2013;35:370, 388.
- Wakusawa C, Fujimura T, Haga T, et al. Granulomatous pigmented purpuric dermatitis associated with primary Sjögren’s syndrome. Acta Derm Venereol. 2013;93:95-96.
- Hanson C, Fischer R, Fraga G, et al. Granulomatous pigmented purpuric dermatosis: an unusual variant associated with hyperlipidemia. Dermatol Online J. 2014;21. pii:13030/qt0tp272d1.
- Morrissey K, Rosenbach M, DeHoratius D, et al. Granulomatous changes associated with pigmented purpuric dermatosis. Cutis. 2014;94:197-202.
- Battle LR, Shalin SC, Gao L. Granulomatous pigmented purpuric dermatosis [published online December 18, 2014]. Clin Exp Dermatol. 2015;40:387-390.
- Mackenzie AI, Biswas A. Granulomatous pigmented purpuric dermatosis: report of a case with atypical clinical presentation including dermoscopic findings. Am J Dermatopathol. 2015;37:311-314.
Practice Points
- Granulomatous pigmented purpuric dermatosis is not only seen in Far East Asians and patients with hyperlipidemia.
- Suspected pigmented purpuric dermatoses should be managed with a punch biopsy to exclude the granulomatous variant.
Update on Coding Changes: Report From the Mount Sinai Fall Symposium
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Noninvasive Imaging: Report From the Mount Sinai Fall Symposium
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Color Wheel Approach to Diagnosing Skin Cancer: Report From the Mount Sinai Fall Symposium
Burnout for Dermatologists: Report From the Mount Sinai Fall Symposium
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
New Uses for Botulinum Toxins: Report From the Mount Sinai Fall Symposium
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Branch duct intraductal papillar mucinous neoplasms confer increased malignancy risk
Patients with branch duct intraductal papillary mucinous neoplasms were about 19 times more likely to develop malignancies over 5 years compared with the general population, although they lacked worrisome features of malignancy at baseline.
The overall risk of malignancy in this cohort approached 8% and persisted for 10 years or more, said Ilaria Pergolini, MD, of Massachusetts General Hospital, Boston, and her associates. The findings support surveillance of this population past 5 years, although cysts that remain 1.5 cm or smaller for more than 5 years might be regarded as unlikely to become malignant, they wrote. The report appears in the November issue of Gastroenterology (doi: 10.1053/j.gastro.2017.07.019).
Few studies have explored branch duct intraductal papillary mucinous neoplasms (BD-IPMNs), which the researchers defined as unilocular or multilocular pancreatic cysts with a nondilated main pancreatic duct (smaller than 5 mm). To begin filling this gap, they retrospectively studied 577 patients with suspected or presumed BD-IPMNs followed at Massachusetts General Hospital. Patients underwent cross-sectional imaging 3 months or more after initial diagnosis at least once thereafter. Standardized incidence ratios were calculated based on population-level data for the United States from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program.
Patients tended to be in their mid-60s at diagnosis (range, 21-90 years) and 59% were female, said the researchers. Median follow-up time was 82 months and ranged between 6 and 329 months, but 63% of patients were followed for at least 5 years (median, 107 months), and about one in five were followed for more than a decade. Fully 83% of patients were asymptomatic at initial diagnosis, of which 10% subsequently became symptomatic. Most patients underwent diagnostic CT, but nearly half underwent MRI/MRCP and about a third underwent endoscopic ultrasound. At diagnosis, median cyst size was 14 mm (range, 2-54 mm) and 9% of patients had cysts measuring at least 3 cm. By the end of follow-up, 55% had larger cysts than at baseline, and cysts grew by a median of 0.9 mm per year.
At diagnosis, only 1% of patients had high-risk stigmata while 12% had worrisome features such as acute pancreatitis, cysts measuring at least 3 cm, thickened or enhancing cyst walls, nonenhancing mural nodules, main pancreatic duct size of 5-9 mm, an abrupt change in caliber of the main pancreatic duct, and lymphadenopathy. During follow-up, another 13% of patients developed new worrisome features and 9% developed high-risk stigmata, while 2% experienced regression of a nodule. In all, 36% of patients had cysts with either worrisome features, high-risk stigmata, or both at some point during the study.
Among 363 patients followed for at least 5 years, 20 (5.5%) were diagnosed with high-risk dysplasia or invasive neoplasms and 4.4% developed invasive cancer, for a standardized incidence ratio of 18.8 (95% confidence interval, 9.7-32.8; P less than .001). Among 108 patients who had cysts measuring 1.5 cm or less, only one individual developed a distinct ductal adenocarcinoma during 5 or more years of follow-up. But of 255 patients with larger cysts, the 5-year rate of malignancy was 7.5% (P = .01).
“The absence of worrisome features or high-risk stigmata at a 5-year time point does not exclude the development of pancreatic malignancy, and the risk in these patients is 18.8 times higher than that of the general population,” the researchers concluded. “Because of this, we strongly support continued surveillance after 5 years from the initial diagnosis.” Cysts that remain 1.5 cm or smaller for at least 5 years are probably low risk, they said. “This is an important issue for further investigation, since it may help reduce costs related to surveillance and improve patients’ quality of life.”
The investigators did not disclose external funding sources. They reported having no conflicts of interest.
The appropriate surveillance strategy for branch duct IPMNs is a point of debate, and numerous guidelines have offered recommendations for managing these potentially malignant neoplasms. Among the contested topics is the appropriateness of ceasing imaging surveillance of lesions that are stable over years. In 2015, an American Gastroenterological Association guideline made a conditional recommendation for cessation of imaging surveillance of pancreatic cysts that have remained stable after 5 years, noting that only very low-quality evidence was available. Given the paucity of data on this topic, this recommendation has been debated.
Dr. Pergolini and colleagues shed new light on this question with this retrospective review. Their study demonstrates that a dramatically increased risk of developing pancreatic malignancy persists even when a branch duct IPMN demonstrates no worrisome features or growth after 5 years of imaging surveillance. In fact, in their cohort, the risk of malignancy not only persisted among patients with branch duct IPMNs compared to population-based controls, but in fact, the risk was even greater after 5 years of follow-up. The risk persisted even after 10 years of follow-up. This study lends credibility to the opinion that branch duct type IPMNs should undergo ongoing surveillance even after 5 years of stability on imaging. Furthermore, it invites further study on smaller (less than 1.5 cm) branch duct IPMNs that remain stable over 5 years, as they appear to be very low risk and may represent a category of IPMNs that do not require indefinite surveillance.
Anthony Gamboa, MD, is assistant professor of medicine, program director of advanced endoscopy fellowship, division of gastroenterology, hepatology and nutrition, Vanderbilt University, Nashville, Tenn. He has no conflicts of interest.
The appropriate surveillance strategy for branch duct IPMNs is a point of debate, and numerous guidelines have offered recommendations for managing these potentially malignant neoplasms. Among the contested topics is the appropriateness of ceasing imaging surveillance of lesions that are stable over years. In 2015, an American Gastroenterological Association guideline made a conditional recommendation for cessation of imaging surveillance of pancreatic cysts that have remained stable after 5 years, noting that only very low-quality evidence was available. Given the paucity of data on this topic, this recommendation has been debated.
Dr. Pergolini and colleagues shed new light on this question with this retrospective review. Their study demonstrates that a dramatically increased risk of developing pancreatic malignancy persists even when a branch duct IPMN demonstrates no worrisome features or growth after 5 years of imaging surveillance. In fact, in their cohort, the risk of malignancy not only persisted among patients with branch duct IPMNs compared to population-based controls, but in fact, the risk was even greater after 5 years of follow-up. The risk persisted even after 10 years of follow-up. This study lends credibility to the opinion that branch duct type IPMNs should undergo ongoing surveillance even after 5 years of stability on imaging. Furthermore, it invites further study on smaller (less than 1.5 cm) branch duct IPMNs that remain stable over 5 years, as they appear to be very low risk and may represent a category of IPMNs that do not require indefinite surveillance.
Anthony Gamboa, MD, is assistant professor of medicine, program director of advanced endoscopy fellowship, division of gastroenterology, hepatology and nutrition, Vanderbilt University, Nashville, Tenn. He has no conflicts of interest.
The appropriate surveillance strategy for branch duct IPMNs is a point of debate, and numerous guidelines have offered recommendations for managing these potentially malignant neoplasms. Among the contested topics is the appropriateness of ceasing imaging surveillance of lesions that are stable over years. In 2015, an American Gastroenterological Association guideline made a conditional recommendation for cessation of imaging surveillance of pancreatic cysts that have remained stable after 5 years, noting that only very low-quality evidence was available. Given the paucity of data on this topic, this recommendation has been debated.
Dr. Pergolini and colleagues shed new light on this question with this retrospective review. Their study demonstrates that a dramatically increased risk of developing pancreatic malignancy persists even when a branch duct IPMN demonstrates no worrisome features or growth after 5 years of imaging surveillance. In fact, in their cohort, the risk of malignancy not only persisted among patients with branch duct IPMNs compared to population-based controls, but in fact, the risk was even greater after 5 years of follow-up. The risk persisted even after 10 years of follow-up. This study lends credibility to the opinion that branch duct type IPMNs should undergo ongoing surveillance even after 5 years of stability on imaging. Furthermore, it invites further study on smaller (less than 1.5 cm) branch duct IPMNs that remain stable over 5 years, as they appear to be very low risk and may represent a category of IPMNs that do not require indefinite surveillance.
Anthony Gamboa, MD, is assistant professor of medicine, program director of advanced endoscopy fellowship, division of gastroenterology, hepatology and nutrition, Vanderbilt University, Nashville, Tenn. He has no conflicts of interest.
Patients with branch duct intraductal papillary mucinous neoplasms were about 19 times more likely to develop malignancies over 5 years compared with the general population, although they lacked worrisome features of malignancy at baseline.
The overall risk of malignancy in this cohort approached 8% and persisted for 10 years or more, said Ilaria Pergolini, MD, of Massachusetts General Hospital, Boston, and her associates. The findings support surveillance of this population past 5 years, although cysts that remain 1.5 cm or smaller for more than 5 years might be regarded as unlikely to become malignant, they wrote. The report appears in the November issue of Gastroenterology (doi: 10.1053/j.gastro.2017.07.019).
Few studies have explored branch duct intraductal papillary mucinous neoplasms (BD-IPMNs), which the researchers defined as unilocular or multilocular pancreatic cysts with a nondilated main pancreatic duct (smaller than 5 mm). To begin filling this gap, they retrospectively studied 577 patients with suspected or presumed BD-IPMNs followed at Massachusetts General Hospital. Patients underwent cross-sectional imaging 3 months or more after initial diagnosis at least once thereafter. Standardized incidence ratios were calculated based on population-level data for the United States from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program.
Patients tended to be in their mid-60s at diagnosis (range, 21-90 years) and 59% were female, said the researchers. Median follow-up time was 82 months and ranged between 6 and 329 months, but 63% of patients were followed for at least 5 years (median, 107 months), and about one in five were followed for more than a decade. Fully 83% of patients were asymptomatic at initial diagnosis, of which 10% subsequently became symptomatic. Most patients underwent diagnostic CT, but nearly half underwent MRI/MRCP and about a third underwent endoscopic ultrasound. At diagnosis, median cyst size was 14 mm (range, 2-54 mm) and 9% of patients had cysts measuring at least 3 cm. By the end of follow-up, 55% had larger cysts than at baseline, and cysts grew by a median of 0.9 mm per year.
At diagnosis, only 1% of patients had high-risk stigmata while 12% had worrisome features such as acute pancreatitis, cysts measuring at least 3 cm, thickened or enhancing cyst walls, nonenhancing mural nodules, main pancreatic duct size of 5-9 mm, an abrupt change in caliber of the main pancreatic duct, and lymphadenopathy. During follow-up, another 13% of patients developed new worrisome features and 9% developed high-risk stigmata, while 2% experienced regression of a nodule. In all, 36% of patients had cysts with either worrisome features, high-risk stigmata, or both at some point during the study.
Among 363 patients followed for at least 5 years, 20 (5.5%) were diagnosed with high-risk dysplasia or invasive neoplasms and 4.4% developed invasive cancer, for a standardized incidence ratio of 18.8 (95% confidence interval, 9.7-32.8; P less than .001). Among 108 patients who had cysts measuring 1.5 cm or less, only one individual developed a distinct ductal adenocarcinoma during 5 or more years of follow-up. But of 255 patients with larger cysts, the 5-year rate of malignancy was 7.5% (P = .01).
“The absence of worrisome features or high-risk stigmata at a 5-year time point does not exclude the development of pancreatic malignancy, and the risk in these patients is 18.8 times higher than that of the general population,” the researchers concluded. “Because of this, we strongly support continued surveillance after 5 years from the initial diagnosis.” Cysts that remain 1.5 cm or smaller for at least 5 years are probably low risk, they said. “This is an important issue for further investigation, since it may help reduce costs related to surveillance and improve patients’ quality of life.”
The investigators did not disclose external funding sources. They reported having no conflicts of interest.
Patients with branch duct intraductal papillary mucinous neoplasms were about 19 times more likely to develop malignancies over 5 years compared with the general population, although they lacked worrisome features of malignancy at baseline.
The overall risk of malignancy in this cohort approached 8% and persisted for 10 years or more, said Ilaria Pergolini, MD, of Massachusetts General Hospital, Boston, and her associates. The findings support surveillance of this population past 5 years, although cysts that remain 1.5 cm or smaller for more than 5 years might be regarded as unlikely to become malignant, they wrote. The report appears in the November issue of Gastroenterology (doi: 10.1053/j.gastro.2017.07.019).
Few studies have explored branch duct intraductal papillary mucinous neoplasms (BD-IPMNs), which the researchers defined as unilocular or multilocular pancreatic cysts with a nondilated main pancreatic duct (smaller than 5 mm). To begin filling this gap, they retrospectively studied 577 patients with suspected or presumed BD-IPMNs followed at Massachusetts General Hospital. Patients underwent cross-sectional imaging 3 months or more after initial diagnosis at least once thereafter. Standardized incidence ratios were calculated based on population-level data for the United States from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program.
Patients tended to be in their mid-60s at diagnosis (range, 21-90 years) and 59% were female, said the researchers. Median follow-up time was 82 months and ranged between 6 and 329 months, but 63% of patients were followed for at least 5 years (median, 107 months), and about one in five were followed for more than a decade. Fully 83% of patients were asymptomatic at initial diagnosis, of which 10% subsequently became symptomatic. Most patients underwent diagnostic CT, but nearly half underwent MRI/MRCP and about a third underwent endoscopic ultrasound. At diagnosis, median cyst size was 14 mm (range, 2-54 mm) and 9% of patients had cysts measuring at least 3 cm. By the end of follow-up, 55% had larger cysts than at baseline, and cysts grew by a median of 0.9 mm per year.
At diagnosis, only 1% of patients had high-risk stigmata while 12% had worrisome features such as acute pancreatitis, cysts measuring at least 3 cm, thickened or enhancing cyst walls, nonenhancing mural nodules, main pancreatic duct size of 5-9 mm, an abrupt change in caliber of the main pancreatic duct, and lymphadenopathy. During follow-up, another 13% of patients developed new worrisome features and 9% developed high-risk stigmata, while 2% experienced regression of a nodule. In all, 36% of patients had cysts with either worrisome features, high-risk stigmata, or both at some point during the study.
Among 363 patients followed for at least 5 years, 20 (5.5%) were diagnosed with high-risk dysplasia or invasive neoplasms and 4.4% developed invasive cancer, for a standardized incidence ratio of 18.8 (95% confidence interval, 9.7-32.8; P less than .001). Among 108 patients who had cysts measuring 1.5 cm or less, only one individual developed a distinct ductal adenocarcinoma during 5 or more years of follow-up. But of 255 patients with larger cysts, the 5-year rate of malignancy was 7.5% (P = .01).
“The absence of worrisome features or high-risk stigmata at a 5-year time point does not exclude the development of pancreatic malignancy, and the risk in these patients is 18.8 times higher than that of the general population,” the researchers concluded. “Because of this, we strongly support continued surveillance after 5 years from the initial diagnosis.” Cysts that remain 1.5 cm or smaller for at least 5 years are probably low risk, they said. “This is an important issue for further investigation, since it may help reduce costs related to surveillance and improve patients’ quality of life.”
The investigators did not disclose external funding sources. They reported having no conflicts of interest.
FROM GASTROENTEROLOGY
Key clinical point: Branch duct intraductal papillary mucinous neoplasms conferred a markedly increased risk of malignancy even when they lacked worrisome features at baseline.
Major finding: At 5 years, the standardized incidence ratio for malignancy was 18.8 compared with the general population.
Data source: A retrospective study of 577 patients with suspected branch duct intraductal papillary mucinous neoplasms.
Disclosures: The investigators did not disclose external funding sources. They reported having no relevant conflicts of interest.
Innovations: Quality, patient safety, and technology initiatives
Measuring hospital-acquired infection in a new way
Every day, hospitalists struggle with health care–associated infections, which 1 in 25 patients experiences, according to the Centers for Disease Control and Prevention.
These infections are often discussed in terms of the standardized infection ratio (SIR), but that measure may not assess overall performance, according to a study suggesting a new measure that could help large hospital systems better evaluate their infection outcomes by comparing them with those of their peers.
This gives hospitals a more current picture of how they’re doing, compared with the SIR, said Mohamad G. Fakih, MD, MPH, of Ascension Health, Grosse Pointe Woods, Mich., lead author of the study. “The SIR is a ratio based on a baseline that’s usually a few years prior; it’s not the year directly before. So, when we published this paper, some of the infections had a baseline of 2006 through 2008 for the central line infections.”
Another difference is that the ICS gives the six infections the same weight, rather than combining them. “So, if you add them up together and then you divide by six, you get a score that tells you how you’re doing for infection, compared [with] the whole system. If they have a problem that’s related to many infections, then you know the culture of infection prevention in that hospital is much worse. It’s not just one product. There’s something much more worrisome for that hospital.”
This simple score can be adjusted according to a particular hospital’s needs. “Let’s say you want to focus on additional infections that are publicly reported. You can add them to that score,” Dr. Fakih says. “And you can change the weight in a way depending on what you want to focus on, or, if you want to focus on something more than others, you can increase the weight.”
References
1. Centers for Disease Control and Prevention. Healthcare-associated infections. https://www.cdc.gov/hai/surveillance/. Accessed April 10, 2017.
2. Fakih MG, Skierczynski B, Bufalino A, et al. Taking advantage of public reporting: An infection composite score to assist evaluating hospital performance for infection prevention efforts. American Journal of Infection Control. (2016);44(12):1578-81.
Hospitalists lead in palliative care
According to a recent report, hospitalists made nearly half (48%) of all palliative care referrals in hospitals in 2015. The report comes from the Center to Advance Palliative Care and the National Palliative Care Research Center.
“The most important finding from this analysis is the near doubling of the number of people receiving palliative care services in U.S. hospital palliative care programs, from an average of 2.7% in 2009 to an average of 4.8% in 2015,” said Diane Meier, MD, director of the Center to Advance Palliative Care. “This suggests increasing recognition of the benefits of palliative care by health professionals and greater likelihood that those living with serious illness will receive state-of-the-art care.”
The report shows that hospitalists are the No. 1 source of referral to palliative care teams. “They see up close the suffering of their patients and families, their need for comprehensive whole-person care, and the beneficial impact of the added layer of support that palliative care provides,” she said.
“Hospitalists should work alongside their palliative care colleagues to develop standardized screening tools so that all patients and families who could benefit have access to the best quality of care during serious and complex illness,” Dr. Meier said. Hospitalists can also gain skills in communicating about prognosis and conducting family meetings, as well as safe and effective symptom management, through the online clinical training curriculum available at capc.org.
Reference
1. National Palliative Care Registry. How We Work: Trends and Insights in Hospital Palliative Care. https://registry.capc.org/wp-content/uploads/2017/02/How-We-Work-Trends-and-Insights-in-Hospital-Palliative-Care-2009-2015.pdf. Accessed April 7, 2017.
Improving outcomes for children with chronic conditions
Cincinnati Children’s Hospital Medical Center improved outcomes for 50% of pediatric patients by redesigning the way it cares for children with active chronic conditions, according to a new study.
The hospital implemented a Condition Outcomes Improvement Initiative, in which specialized clinical teams applied quality improvement principles to improve outcomes for pediatric patients with chronic illnesses.
Each improvement team focused on a specific chronic condition, such as juvenile arthritis, asthma, chronic kidney disease, or sickle cell disease. The improvement processes implemented included reviewing evidence to choose which outcomes to measure, developing condition-specific patient registries and data collection tools, classifying patients into defined risk groups, planning care before and after visits, and providing self-management and caregiver/parent support for patients and their families.
Study lead author Jennifer Lail, MD, FAAP, analyzed data from more than 27,000 pediatric patients from 18 improvement teams. Following implementation of the changes, half of patients had an improved outcome, and 11 of the 18 chronic condition teams achieved the goal of 20% improvement in their chosen clinical outcome, suggesting that clinical teams implementing quality improvement methods with multidisciplinary support can improve outcomes for populations with chronic conditions.
Reference
1. Lail J, et al. Applying the Chronic Care Model to Improve Care and Outcomes at a Pediatric Medical Center. Joint Commission Journal on Quality and Patient Safety. 2017;43(3):101-112.
FDA approves two new antibiotic tests
Hospitalists have two new FDA-approved tools available to help them make antibiotic treatment decisions.
The first is the expanded use of the Vidas Brahms PCT Assay, intended to be used in the hospital or emergency room. The test uses – for the first time – procalcitonin (PCT), a protein associated with the body’s response to a bacterial infection, as a biomarker that can help hospitalists make antibiotic management decisions in patients with those conditions. The results can help them determine if antibiotic treatment should be started or stopped in patients with lower respiratory tract infections (such as community-acquired pneumonia) and stopped in patients with sepsis.
The FDA has also allowed marketing of the PhenoTest BC Kit. This one is another first, the first test to identify organisms causing bloodstream infections and provide information about the antibiotics to which the organism is likely to respond.
The test can identify bacteria or yeast from a positive blood culture in approximately 1.5 hours (compared with traditional identification and antibiotic susceptibility tests, which can take one to two days). The test can identify 14 different species of bacteria and two species of yeast that cause bloodstream infections. It also provides antibiotic sensitivity information on 18 antibiotics. In addition, the test will identify the presence of two indicators of antibiotic resistance.
Quick byte
About a third of adverse events during hospitalizations involve a drug-related harm, resulting in longer hospital stays and increased costs, according to the New York Times. “The Institute of Medicine estimated that there are 400,000 preventable adverse drug events in hospitals each year, costing $3.5 billion. One-fifth of patients discharged from the hospital have a drug-related complication after returning home, many of which are preventable.”
Reference
1 Frakt A. How Many Pills Are Too Many? The New York Times. 2017 Apr 10. https://www.nytimes.com/2017/04/10/upshot/how-many-pills-are-too-many.html?rref=collection%2Fsectioncollection%2Fhealth&action=click&contentCollection=health®ion=stream&module=stream_unit&version=latest&contentPlacement=6&pgtype=sectionfront&_r=0. Accessed April 9, 2017.
Measuring hospital-acquired infection in a new way
Every day, hospitalists struggle with health care–associated infections, which 1 in 25 patients experiences, according to the Centers for Disease Control and Prevention.
These infections are often discussed in terms of the standardized infection ratio (SIR), but that measure may not assess overall performance, according to a study suggesting a new measure that could help large hospital systems better evaluate their infection outcomes by comparing them with those of their peers.
This gives hospitals a more current picture of how they’re doing, compared with the SIR, said Mohamad G. Fakih, MD, MPH, of Ascension Health, Grosse Pointe Woods, Mich., lead author of the study. “The SIR is a ratio based on a baseline that’s usually a few years prior; it’s not the year directly before. So, when we published this paper, some of the infections had a baseline of 2006 through 2008 for the central line infections.”
Another difference is that the ICS gives the six infections the same weight, rather than combining them. “So, if you add them up together and then you divide by six, you get a score that tells you how you’re doing for infection, compared [with] the whole system. If they have a problem that’s related to many infections, then you know the culture of infection prevention in that hospital is much worse. It’s not just one product. There’s something much more worrisome for that hospital.”
This simple score can be adjusted according to a particular hospital’s needs. “Let’s say you want to focus on additional infections that are publicly reported. You can add them to that score,” Dr. Fakih says. “And you can change the weight in a way depending on what you want to focus on, or, if you want to focus on something more than others, you can increase the weight.”
References
1. Centers for Disease Control and Prevention. Healthcare-associated infections. https://www.cdc.gov/hai/surveillance/. Accessed April 10, 2017.
2. Fakih MG, Skierczynski B, Bufalino A, et al. Taking advantage of public reporting: An infection composite score to assist evaluating hospital performance for infection prevention efforts. American Journal of Infection Control. (2016);44(12):1578-81.
Hospitalists lead in palliative care
According to a recent report, hospitalists made nearly half (48%) of all palliative care referrals in hospitals in 2015. The report comes from the Center to Advance Palliative Care and the National Palliative Care Research Center.
“The most important finding from this analysis is the near doubling of the number of people receiving palliative care services in U.S. hospital palliative care programs, from an average of 2.7% in 2009 to an average of 4.8% in 2015,” said Diane Meier, MD, director of the Center to Advance Palliative Care. “This suggests increasing recognition of the benefits of palliative care by health professionals and greater likelihood that those living with serious illness will receive state-of-the-art care.”
The report shows that hospitalists are the No. 1 source of referral to palliative care teams. “They see up close the suffering of their patients and families, their need for comprehensive whole-person care, and the beneficial impact of the added layer of support that palliative care provides,” she said.
“Hospitalists should work alongside their palliative care colleagues to develop standardized screening tools so that all patients and families who could benefit have access to the best quality of care during serious and complex illness,” Dr. Meier said. Hospitalists can also gain skills in communicating about prognosis and conducting family meetings, as well as safe and effective symptom management, through the online clinical training curriculum available at capc.org.
Reference
1. National Palliative Care Registry. How We Work: Trends and Insights in Hospital Palliative Care. https://registry.capc.org/wp-content/uploads/2017/02/How-We-Work-Trends-and-Insights-in-Hospital-Palliative-Care-2009-2015.pdf. Accessed April 7, 2017.
Improving outcomes for children with chronic conditions
Cincinnati Children’s Hospital Medical Center improved outcomes for 50% of pediatric patients by redesigning the way it cares for children with active chronic conditions, according to a new study.
The hospital implemented a Condition Outcomes Improvement Initiative, in which specialized clinical teams applied quality improvement principles to improve outcomes for pediatric patients with chronic illnesses.
Each improvement team focused on a specific chronic condition, such as juvenile arthritis, asthma, chronic kidney disease, or sickle cell disease. The improvement processes implemented included reviewing evidence to choose which outcomes to measure, developing condition-specific patient registries and data collection tools, classifying patients into defined risk groups, planning care before and after visits, and providing self-management and caregiver/parent support for patients and their families.
Study lead author Jennifer Lail, MD, FAAP, analyzed data from more than 27,000 pediatric patients from 18 improvement teams. Following implementation of the changes, half of patients had an improved outcome, and 11 of the 18 chronic condition teams achieved the goal of 20% improvement in their chosen clinical outcome, suggesting that clinical teams implementing quality improvement methods with multidisciplinary support can improve outcomes for populations with chronic conditions.
Reference
1. Lail J, et al. Applying the Chronic Care Model to Improve Care and Outcomes at a Pediatric Medical Center. Joint Commission Journal on Quality and Patient Safety. 2017;43(3):101-112.
FDA approves two new antibiotic tests
Hospitalists have two new FDA-approved tools available to help them make antibiotic treatment decisions.
The first is the expanded use of the Vidas Brahms PCT Assay, intended to be used in the hospital or emergency room. The test uses – for the first time – procalcitonin (PCT), a protein associated with the body’s response to a bacterial infection, as a biomarker that can help hospitalists make antibiotic management decisions in patients with those conditions. The results can help them determine if antibiotic treatment should be started or stopped in patients with lower respiratory tract infections (such as community-acquired pneumonia) and stopped in patients with sepsis.
The FDA has also allowed marketing of the PhenoTest BC Kit. This one is another first, the first test to identify organisms causing bloodstream infections and provide information about the antibiotics to which the organism is likely to respond.
The test can identify bacteria or yeast from a positive blood culture in approximately 1.5 hours (compared with traditional identification and antibiotic susceptibility tests, which can take one to two days). The test can identify 14 different species of bacteria and two species of yeast that cause bloodstream infections. It also provides antibiotic sensitivity information on 18 antibiotics. In addition, the test will identify the presence of two indicators of antibiotic resistance.
Quick byte
About a third of adverse events during hospitalizations involve a drug-related harm, resulting in longer hospital stays and increased costs, according to the New York Times. “The Institute of Medicine estimated that there are 400,000 preventable adverse drug events in hospitals each year, costing $3.5 billion. One-fifth of patients discharged from the hospital have a drug-related complication after returning home, many of which are preventable.”
Reference
1 Frakt A. How Many Pills Are Too Many? The New York Times. 2017 Apr 10. https://www.nytimes.com/2017/04/10/upshot/how-many-pills-are-too-many.html?rref=collection%2Fsectioncollection%2Fhealth&action=click&contentCollection=health®ion=stream&module=stream_unit&version=latest&contentPlacement=6&pgtype=sectionfront&_r=0. Accessed April 9, 2017.
Measuring hospital-acquired infection in a new way
Every day, hospitalists struggle with health care–associated infections, which 1 in 25 patients experiences, according to the Centers for Disease Control and Prevention.
These infections are often discussed in terms of the standardized infection ratio (SIR), but that measure may not assess overall performance, according to a study suggesting a new measure that could help large hospital systems better evaluate their infection outcomes by comparing them with those of their peers.
This gives hospitals a more current picture of how they’re doing, compared with the SIR, said Mohamad G. Fakih, MD, MPH, of Ascension Health, Grosse Pointe Woods, Mich., lead author of the study. “The SIR is a ratio based on a baseline that’s usually a few years prior; it’s not the year directly before. So, when we published this paper, some of the infections had a baseline of 2006 through 2008 for the central line infections.”
Another difference is that the ICS gives the six infections the same weight, rather than combining them. “So, if you add them up together and then you divide by six, you get a score that tells you how you’re doing for infection, compared [with] the whole system. If they have a problem that’s related to many infections, then you know the culture of infection prevention in that hospital is much worse. It’s not just one product. There’s something much more worrisome for that hospital.”
This simple score can be adjusted according to a particular hospital’s needs. “Let’s say you want to focus on additional infections that are publicly reported. You can add them to that score,” Dr. Fakih says. “And you can change the weight in a way depending on what you want to focus on, or, if you want to focus on something more than others, you can increase the weight.”
References
1. Centers for Disease Control and Prevention. Healthcare-associated infections. https://www.cdc.gov/hai/surveillance/. Accessed April 10, 2017.
2. Fakih MG, Skierczynski B, Bufalino A, et al. Taking advantage of public reporting: An infection composite score to assist evaluating hospital performance for infection prevention efforts. American Journal of Infection Control. (2016);44(12):1578-81.
Hospitalists lead in palliative care
According to a recent report, hospitalists made nearly half (48%) of all palliative care referrals in hospitals in 2015. The report comes from the Center to Advance Palliative Care and the National Palliative Care Research Center.
“The most important finding from this analysis is the near doubling of the number of people receiving palliative care services in U.S. hospital palliative care programs, from an average of 2.7% in 2009 to an average of 4.8% in 2015,” said Diane Meier, MD, director of the Center to Advance Palliative Care. “This suggests increasing recognition of the benefits of palliative care by health professionals and greater likelihood that those living with serious illness will receive state-of-the-art care.”
The report shows that hospitalists are the No. 1 source of referral to palliative care teams. “They see up close the suffering of their patients and families, their need for comprehensive whole-person care, and the beneficial impact of the added layer of support that palliative care provides,” she said.
“Hospitalists should work alongside their palliative care colleagues to develop standardized screening tools so that all patients and families who could benefit have access to the best quality of care during serious and complex illness,” Dr. Meier said. Hospitalists can also gain skills in communicating about prognosis and conducting family meetings, as well as safe and effective symptom management, through the online clinical training curriculum available at capc.org.
Reference
1. National Palliative Care Registry. How We Work: Trends and Insights in Hospital Palliative Care. https://registry.capc.org/wp-content/uploads/2017/02/How-We-Work-Trends-and-Insights-in-Hospital-Palliative-Care-2009-2015.pdf. Accessed April 7, 2017.
Improving outcomes for children with chronic conditions
Cincinnati Children’s Hospital Medical Center improved outcomes for 50% of pediatric patients by redesigning the way it cares for children with active chronic conditions, according to a new study.
The hospital implemented a Condition Outcomes Improvement Initiative, in which specialized clinical teams applied quality improvement principles to improve outcomes for pediatric patients with chronic illnesses.
Each improvement team focused on a specific chronic condition, such as juvenile arthritis, asthma, chronic kidney disease, or sickle cell disease. The improvement processes implemented included reviewing evidence to choose which outcomes to measure, developing condition-specific patient registries and data collection tools, classifying patients into defined risk groups, planning care before and after visits, and providing self-management and caregiver/parent support for patients and their families.
Study lead author Jennifer Lail, MD, FAAP, analyzed data from more than 27,000 pediatric patients from 18 improvement teams. Following implementation of the changes, half of patients had an improved outcome, and 11 of the 18 chronic condition teams achieved the goal of 20% improvement in their chosen clinical outcome, suggesting that clinical teams implementing quality improvement methods with multidisciplinary support can improve outcomes for populations with chronic conditions.
Reference
1. Lail J, et al. Applying the Chronic Care Model to Improve Care and Outcomes at a Pediatric Medical Center. Joint Commission Journal on Quality and Patient Safety. 2017;43(3):101-112.
FDA approves two new antibiotic tests
Hospitalists have two new FDA-approved tools available to help them make antibiotic treatment decisions.
The first is the expanded use of the Vidas Brahms PCT Assay, intended to be used in the hospital or emergency room. The test uses – for the first time – procalcitonin (PCT), a protein associated with the body’s response to a bacterial infection, as a biomarker that can help hospitalists make antibiotic management decisions in patients with those conditions. The results can help them determine if antibiotic treatment should be started or stopped in patients with lower respiratory tract infections (such as community-acquired pneumonia) and stopped in patients with sepsis.
The FDA has also allowed marketing of the PhenoTest BC Kit. This one is another first, the first test to identify organisms causing bloodstream infections and provide information about the antibiotics to which the organism is likely to respond.
The test can identify bacteria or yeast from a positive blood culture in approximately 1.5 hours (compared with traditional identification and antibiotic susceptibility tests, which can take one to two days). The test can identify 14 different species of bacteria and two species of yeast that cause bloodstream infections. It also provides antibiotic sensitivity information on 18 antibiotics. In addition, the test will identify the presence of two indicators of antibiotic resistance.
Quick byte
About a third of adverse events during hospitalizations involve a drug-related harm, resulting in longer hospital stays and increased costs, according to the New York Times. “The Institute of Medicine estimated that there are 400,000 preventable adverse drug events in hospitals each year, costing $3.5 billion. One-fifth of patients discharged from the hospital have a drug-related complication after returning home, many of which are preventable.”
Reference
1 Frakt A. How Many Pills Are Too Many? The New York Times. 2017 Apr 10. https://www.nytimes.com/2017/04/10/upshot/how-many-pills-are-too-many.html?rref=collection%2Fsectioncollection%2Fhealth&action=click&contentCollection=health®ion=stream&module=stream_unit&version=latest&contentPlacement=6&pgtype=sectionfront&_r=0. Accessed April 9, 2017.
Many years on metformin linked to anemia risk
LISBON – People with type 2 diabetes who take metformin for many years are more likely to develop anemia than are those who do not, according to the results of a large analysis of data from an observational, population-based study with 20 years of follow-up.
“Metformin treatment was associated with a 6% higher risk of anemia for every cumulative year of metformin exposure,” Louise Donnelly, PhD, and her associates reported in a poster presentation at the annual meeting of the European Association for the Study of Diabetes.
In an interview, Dr. Donnelly, a postdoctoral research assistant at the University of Dundee (Scotland), explained why they looked at the use of metformin and anemia risk in people with type 2 diabetes.
“The Diabetes Prevention Program (DPP) study showed that long-term metformin use in individuals with impaired glucose tolerance was associated with an increased risk of anemia, and this was independent of vitamin B12 status,” she said (J Clin Endocrinol Metab. 2016;101:1754-61). “Anemia is a common finding in people with type 2 diabetes, but the impact of long-term metformin use on anemia hasn’t been studied.”
Dr. Donnelly and her associates obtained detailed information on metformin prescribing and hematology measures from electronic patient medical records from the Genetics of Diabetes Audit and Research in Tayside and Scotland (GoDARTS) cohort, based in Scotland. This database contains information on individuals with type 2 diabetes and matching controls and is available to researchers worldwide.
For the analysis, the team looked for people diagnosed from 1996 onward who had a baseline hemoglobin measurement. Of 6,440 individuals with type 2 diabetes in the GoDARTS cohort, just over half had a hemoglobin measurement.
“We used a definition of ‘moderate’ anemia and we excluded patients with mild anemia or worse at diabetes diagnosis,” Dr. Donnelly observed. Anemia was considered to be a hemoglobin level of less than 12 g/dL in women and less than 13 g/dL in men. In all, 280 individuals with anemia were excluded from further analysis as the aim was to follow people until they developed anemia, died, left the area, or until the end of the follow-up period, which was set at September 30, 2015. A discrete-time failure analysis was used to model the effect of cumulative metformin exposure on anemia risk.
After a median follow-up of 8 years and a median number of 11 hemoglobin measurements per patient, 2,487 study subjects (71%) had some exposure to metformin and 1,458 of the whole sample (41.8%) had become anemic. Of those who developed anemia, 745 (51%) were current metformin users, 194 (13%) were former users, and 519 (36%) had never taken metformin.
“Cumulative metformin use was independently associated with an increased risk of anemia,” Dr. Donnelly noted (odds ratio [OR], 1.06; 95% confidence interval [CI], 1.02-1.09; P = .0006). This association was not seen when they examined the data based on sulfonylurea use (OR 1.0; 95% CI 0.97-1.04, P = .8), she added.
“Anemia risk was higher with age at diagnosis, duration of diabetes, lower hemoglobin at baseline, and lower eGFR [estimated glomerular filtration rate],” she observed. ORs for first anemia event were 1.03 (95% CI, 1.02-1.04) for every year of increasing age, 1.05 (95% CI, 1.03-1.08) for every additional year since diabetes diagnosis, 0.70 (95% CI, 0.66-0.74) per 1 g/dL of hemoglobin at diagnosis, and eGFR 0.98 (95% CI, 0.98-1.01) per additional 1 mL/min per 1.732 (P less than .0001 for all).
Why cumulative metformin use is associated with an increased of anemia is unclear, however, and Dr. Donnelly noted that this needs further investigation. “We do have data from two other clinical trials now, showing similar results, and maybe through those data we might be able to untangle it.”
The team does not think the anemia is related to B12 deficiency, however, as people who developed anemia while taking metformin were more likely to develop microcytic (12% vs. 7.3%) than macrocytic (7.6% vs. 12.3%), anemia, compared with people with anemia who were not exposed to metformin (P less than .0001).
“In terms of mechanism, we can only conjecture,” Ewan Pearson, MB, senior author of the study and professor of medicine at the University of Dundee, said during a discussion at the poster presentation. “It is important to stress that metformin is a great drug and we shouldn’t stop it because of a potentially increased risk of anemia.”
The Medical Research Council supported the work. Dr. Donnelly reported having no financial disclosures.
LISBON – People with type 2 diabetes who take metformin for many years are more likely to develop anemia than are those who do not, according to the results of a large analysis of data from an observational, population-based study with 20 years of follow-up.
“Metformin treatment was associated with a 6% higher risk of anemia for every cumulative year of metformin exposure,” Louise Donnelly, PhD, and her associates reported in a poster presentation at the annual meeting of the European Association for the Study of Diabetes.
In an interview, Dr. Donnelly, a postdoctoral research assistant at the University of Dundee (Scotland), explained why they looked at the use of metformin and anemia risk in people with type 2 diabetes.
“The Diabetes Prevention Program (DPP) study showed that long-term metformin use in individuals with impaired glucose tolerance was associated with an increased risk of anemia, and this was independent of vitamin B12 status,” she said (J Clin Endocrinol Metab. 2016;101:1754-61). “Anemia is a common finding in people with type 2 diabetes, but the impact of long-term metformin use on anemia hasn’t been studied.”
Dr. Donnelly and her associates obtained detailed information on metformin prescribing and hematology measures from electronic patient medical records from the Genetics of Diabetes Audit and Research in Tayside and Scotland (GoDARTS) cohort, based in Scotland. This database contains information on individuals with type 2 diabetes and matching controls and is available to researchers worldwide.
For the analysis, the team looked for people diagnosed from 1996 onward who had a baseline hemoglobin measurement. Of 6,440 individuals with type 2 diabetes in the GoDARTS cohort, just over half had a hemoglobin measurement.
“We used a definition of ‘moderate’ anemia and we excluded patients with mild anemia or worse at diabetes diagnosis,” Dr. Donnelly observed. Anemia was considered to be a hemoglobin level of less than 12 g/dL in women and less than 13 g/dL in men. In all, 280 individuals with anemia were excluded from further analysis as the aim was to follow people until they developed anemia, died, left the area, or until the end of the follow-up period, which was set at September 30, 2015. A discrete-time failure analysis was used to model the effect of cumulative metformin exposure on anemia risk.
After a median follow-up of 8 years and a median number of 11 hemoglobin measurements per patient, 2,487 study subjects (71%) had some exposure to metformin and 1,458 of the whole sample (41.8%) had become anemic. Of those who developed anemia, 745 (51%) were current metformin users, 194 (13%) were former users, and 519 (36%) had never taken metformin.
“Cumulative metformin use was independently associated with an increased risk of anemia,” Dr. Donnelly noted (odds ratio [OR], 1.06; 95% confidence interval [CI], 1.02-1.09; P = .0006). This association was not seen when they examined the data based on sulfonylurea use (OR 1.0; 95% CI 0.97-1.04, P = .8), she added.
“Anemia risk was higher with age at diagnosis, duration of diabetes, lower hemoglobin at baseline, and lower eGFR [estimated glomerular filtration rate],” she observed. ORs for first anemia event were 1.03 (95% CI, 1.02-1.04) for every year of increasing age, 1.05 (95% CI, 1.03-1.08) for every additional year since diabetes diagnosis, 0.70 (95% CI, 0.66-0.74) per 1 g/dL of hemoglobin at diagnosis, and eGFR 0.98 (95% CI, 0.98-1.01) per additional 1 mL/min per 1.732 (P less than .0001 for all).
Why cumulative metformin use is associated with an increased of anemia is unclear, however, and Dr. Donnelly noted that this needs further investigation. “We do have data from two other clinical trials now, showing similar results, and maybe through those data we might be able to untangle it.”
The team does not think the anemia is related to B12 deficiency, however, as people who developed anemia while taking metformin were more likely to develop microcytic (12% vs. 7.3%) than macrocytic (7.6% vs. 12.3%), anemia, compared with people with anemia who were not exposed to metformin (P less than .0001).
“In terms of mechanism, we can only conjecture,” Ewan Pearson, MB, senior author of the study and professor of medicine at the University of Dundee, said during a discussion at the poster presentation. “It is important to stress that metformin is a great drug and we shouldn’t stop it because of a potentially increased risk of anemia.”
The Medical Research Council supported the work. Dr. Donnelly reported having no financial disclosures.
LISBON – People with type 2 diabetes who take metformin for many years are more likely to develop anemia than are those who do not, according to the results of a large analysis of data from an observational, population-based study with 20 years of follow-up.
“Metformin treatment was associated with a 6% higher risk of anemia for every cumulative year of metformin exposure,” Louise Donnelly, PhD, and her associates reported in a poster presentation at the annual meeting of the European Association for the Study of Diabetes.
In an interview, Dr. Donnelly, a postdoctoral research assistant at the University of Dundee (Scotland), explained why they looked at the use of metformin and anemia risk in people with type 2 diabetes.
“The Diabetes Prevention Program (DPP) study showed that long-term metformin use in individuals with impaired glucose tolerance was associated with an increased risk of anemia, and this was independent of vitamin B12 status,” she said (J Clin Endocrinol Metab. 2016;101:1754-61). “Anemia is a common finding in people with type 2 diabetes, but the impact of long-term metformin use on anemia hasn’t been studied.”
Dr. Donnelly and her associates obtained detailed information on metformin prescribing and hematology measures from electronic patient medical records from the Genetics of Diabetes Audit and Research in Tayside and Scotland (GoDARTS) cohort, based in Scotland. This database contains information on individuals with type 2 diabetes and matching controls and is available to researchers worldwide.
For the analysis, the team looked for people diagnosed from 1996 onward who had a baseline hemoglobin measurement. Of 6,440 individuals with type 2 diabetes in the GoDARTS cohort, just over half had a hemoglobin measurement.
“We used a definition of ‘moderate’ anemia and we excluded patients with mild anemia or worse at diabetes diagnosis,” Dr. Donnelly observed. Anemia was considered to be a hemoglobin level of less than 12 g/dL in women and less than 13 g/dL in men. In all, 280 individuals with anemia were excluded from further analysis as the aim was to follow people until they developed anemia, died, left the area, or until the end of the follow-up period, which was set at September 30, 2015. A discrete-time failure analysis was used to model the effect of cumulative metformin exposure on anemia risk.
After a median follow-up of 8 years and a median number of 11 hemoglobin measurements per patient, 2,487 study subjects (71%) had some exposure to metformin and 1,458 of the whole sample (41.8%) had become anemic. Of those who developed anemia, 745 (51%) were current metformin users, 194 (13%) were former users, and 519 (36%) had never taken metformin.
“Cumulative metformin use was independently associated with an increased risk of anemia,” Dr. Donnelly noted (odds ratio [OR], 1.06; 95% confidence interval [CI], 1.02-1.09; P = .0006). This association was not seen when they examined the data based on sulfonylurea use (OR 1.0; 95% CI 0.97-1.04, P = .8), she added.
“Anemia risk was higher with age at diagnosis, duration of diabetes, lower hemoglobin at baseline, and lower eGFR [estimated glomerular filtration rate],” she observed. ORs for first anemia event were 1.03 (95% CI, 1.02-1.04) for every year of increasing age, 1.05 (95% CI, 1.03-1.08) for every additional year since diabetes diagnosis, 0.70 (95% CI, 0.66-0.74) per 1 g/dL of hemoglobin at diagnosis, and eGFR 0.98 (95% CI, 0.98-1.01) per additional 1 mL/min per 1.732 (P less than .0001 for all).
Why cumulative metformin use is associated with an increased of anemia is unclear, however, and Dr. Donnelly noted that this needs further investigation. “We do have data from two other clinical trials now, showing similar results, and maybe through those data we might be able to untangle it.”
The team does not think the anemia is related to B12 deficiency, however, as people who developed anemia while taking metformin were more likely to develop microcytic (12% vs. 7.3%) than macrocytic (7.6% vs. 12.3%), anemia, compared with people with anemia who were not exposed to metformin (P less than .0001).
“In terms of mechanism, we can only conjecture,” Ewan Pearson, MB, senior author of the study and professor of medicine at the University of Dundee, said during a discussion at the poster presentation. “It is important to stress that metformin is a great drug and we shouldn’t stop it because of a potentially increased risk of anemia.”
The Medical Research Council supported the work. Dr. Donnelly reported having no financial disclosures.
AT EASD 2017
Key clinical point: Cumulative metformin use was associated with an increased risk for anemia in patients with type 2 diabetes.
Major finding: For every additional year of metformin use, there was a 6% increase in the risk for anemia.
Data source: Data analysis of 3,435 individuals with type 2 diabetes who participated in a large observational, population-based study with almost 20 years of follow-up.
Disclosures: The Medical Research Council supported the work. Dr. Donnelly reported having no financial disclosures.