User login
Now missing in EHR charts: a good impression
I’ve previously written about “the monster note,” a creation of autofilled blanks with vital signs and test results that tells you very little about what’s really going on.
Recently, while on call, I discovered a new problem: the lack of a decent impression.
I was covering for another doctor, and a cardiologist rounding at a rehab center called to see if he could anticoagulate a recently discharged patient. It was certainly a reasonable question.
Unfortunately, not much was there. Most notes were the usual mishmash of test results, vital signs, and medication lists, with very little about the patient. So I scrolled down to the impressions to find out what the plan was.
Sadly, that area (which to me is the most critical part of a note) was also devoid of anything useful. Hoping for something like “embolic stroke, hoping to anticoagulate in future,” I instead found things like “To SNF or rehab soon” or “case discussed with family” as the entire impression and plan. That tells me nothing. The only note I found that had some sort of assessment and plan was the initial consult, which was done before any test results were in.
This seems to be the current state of things. Notes that actually give you some idea of the thinking and plan have become an endangered species. This helps no one, as most of us rely on other doctors’ notes to coordinate and plan care. While some of this is done through talking or texts, those things aren’t in the chart. So even though the doctors involved may have a good idea of what they’re doing (and I certainly hope they do), an outsider doesn’t.
In my opinion, that does nothing to improve patient care. I suppose it works if the same doctors are involved each day, but that’s not how American hospital medicine is any more. Hospitalists rotate in and out every few days and (as in my case) others cover call on nights, weekends, and holidays.
It’s also a gateway to legal challenges. A malpractice lawyer once told me that notes should be written so that if you have to read it 5 years later, you can get a pretty good idea of what your thinking was. If the details of the plan were carried in your head, or were in conversations with other doctors, those things aren’t going to help you. The written record is everything. If the issues these notes pose to patient care don’t worry you, maybe that thought should.
Back to my patient: It took me about 15-20 minutes of skimming through the note to find the answer I needed. And it wasn’t in any of the doctors’ notes at all.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I’ve previously written about “the monster note,” a creation of autofilled blanks with vital signs and test results that tells you very little about what’s really going on.
Recently, while on call, I discovered a new problem: the lack of a decent impression.
I was covering for another doctor, and a cardiologist rounding at a rehab center called to see if he could anticoagulate a recently discharged patient. It was certainly a reasonable question.
Unfortunately, not much was there. Most notes were the usual mishmash of test results, vital signs, and medication lists, with very little about the patient. So I scrolled down to the impressions to find out what the plan was.
Sadly, that area (which to me is the most critical part of a note) was also devoid of anything useful. Hoping for something like “embolic stroke, hoping to anticoagulate in future,” I instead found things like “To SNF or rehab soon” or “case discussed with family” as the entire impression and plan. That tells me nothing. The only note I found that had some sort of assessment and plan was the initial consult, which was done before any test results were in.
This seems to be the current state of things. Notes that actually give you some idea of the thinking and plan have become an endangered species. This helps no one, as most of us rely on other doctors’ notes to coordinate and plan care. While some of this is done through talking or texts, those things aren’t in the chart. So even though the doctors involved may have a good idea of what they’re doing (and I certainly hope they do), an outsider doesn’t.
In my opinion, that does nothing to improve patient care. I suppose it works if the same doctors are involved each day, but that’s not how American hospital medicine is any more. Hospitalists rotate in and out every few days and (as in my case) others cover call on nights, weekends, and holidays.
It’s also a gateway to legal challenges. A malpractice lawyer once told me that notes should be written so that if you have to read it 5 years later, you can get a pretty good idea of what your thinking was. If the details of the plan were carried in your head, or were in conversations with other doctors, those things aren’t going to help you. The written record is everything. If the issues these notes pose to patient care don’t worry you, maybe that thought should.
Back to my patient: It took me about 15-20 minutes of skimming through the note to find the answer I needed. And it wasn’t in any of the doctors’ notes at all.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I’ve previously written about “the monster note,” a creation of autofilled blanks with vital signs and test results that tells you very little about what’s really going on.
Recently, while on call, I discovered a new problem: the lack of a decent impression.
I was covering for another doctor, and a cardiologist rounding at a rehab center called to see if he could anticoagulate a recently discharged patient. It was certainly a reasonable question.
Unfortunately, not much was there. Most notes were the usual mishmash of test results, vital signs, and medication lists, with very little about the patient. So I scrolled down to the impressions to find out what the plan was.
Sadly, that area (which to me is the most critical part of a note) was also devoid of anything useful. Hoping for something like “embolic stroke, hoping to anticoagulate in future,” I instead found things like “To SNF or rehab soon” or “case discussed with family” as the entire impression and plan. That tells me nothing. The only note I found that had some sort of assessment and plan was the initial consult, which was done before any test results were in.
This seems to be the current state of things. Notes that actually give you some idea of the thinking and plan have become an endangered species. This helps no one, as most of us rely on other doctors’ notes to coordinate and plan care. While some of this is done through talking or texts, those things aren’t in the chart. So even though the doctors involved may have a good idea of what they’re doing (and I certainly hope they do), an outsider doesn’t.
In my opinion, that does nothing to improve patient care. I suppose it works if the same doctors are involved each day, but that’s not how American hospital medicine is any more. Hospitalists rotate in and out every few days and (as in my case) others cover call on nights, weekends, and holidays.
It’s also a gateway to legal challenges. A malpractice lawyer once told me that notes should be written so that if you have to read it 5 years later, you can get a pretty good idea of what your thinking was. If the details of the plan were carried in your head, or were in conversations with other doctors, those things aren’t going to help you. The written record is everything. If the issues these notes pose to patient care don’t worry you, maybe that thought should.
Back to my patient: It took me about 15-20 minutes of skimming through the note to find the answer I needed. And it wasn’t in any of the doctors’ notes at all.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Complications of Cosmetic Eye Whitening
First introduced in 2008 as a surgical treatment of chronic conjunctival injection, cosmetic eye whitening became popularized in South Kore
The procedure involves performing a localized conjunctivectomy with or without removal of the Tenon capsule.4 Brimonidine tartrate is given for vascular constriction. When conjunctivectomy is performed in the right eye, the medial conjunctiva is incised from the 2-o’clock to 5-o’clock positions and the lateral conjunctiva is incised from the 10-o’clock to 7-o’clock positions. After the conjunctiva and Tenon capsule are excised, hemostasis is achieved with electrocauterization. Postoperative management may consist of topical mitomycin C (MMC) 0.02% 4 times daily for 2 to 5 days along with topical steroids. The addition of bevacizumab 1.25 mg/mL also has been described.5
In this report, we provide a comprehensive review of the complications of cosmetic eye whitening based on a review of the literature. Clinicians in both aesthetic practice and ophthalmology should be aware of the potential complications to accurately educate their patients about the possible risks and benefits of this procedure.
Methods
A review of PubMed articles indexed for MEDLINE (January 2009 to July 2017) using the search terms cosmetic eye whitening, cosmetic wide conjunctivectomy, I-Brite, and chronic hyperemic conjuctiva was conducted to evaluate the number of reports of complications from cosmetic eye whitening. A total of 10 articles were included in the study based on a review of abstracts. Non–English-language abstracts were not reviewed.
Results
Based on a review of 10 articles commenting on the complications of cosmetic eye whitening, a total of 2400 patients had undergone a cosmetic conjunctivectomy with various postoperative complications and recurrences (Table).4-13 The most commonly recurring complications based on the reported frequencies in the articles included chronic conjunctival epithelial defects, scleral thinning, calcific plaques, dry eye syndrome, diplopia (sometimes requiring strabismus surgery), and elevated intraocular pressure.
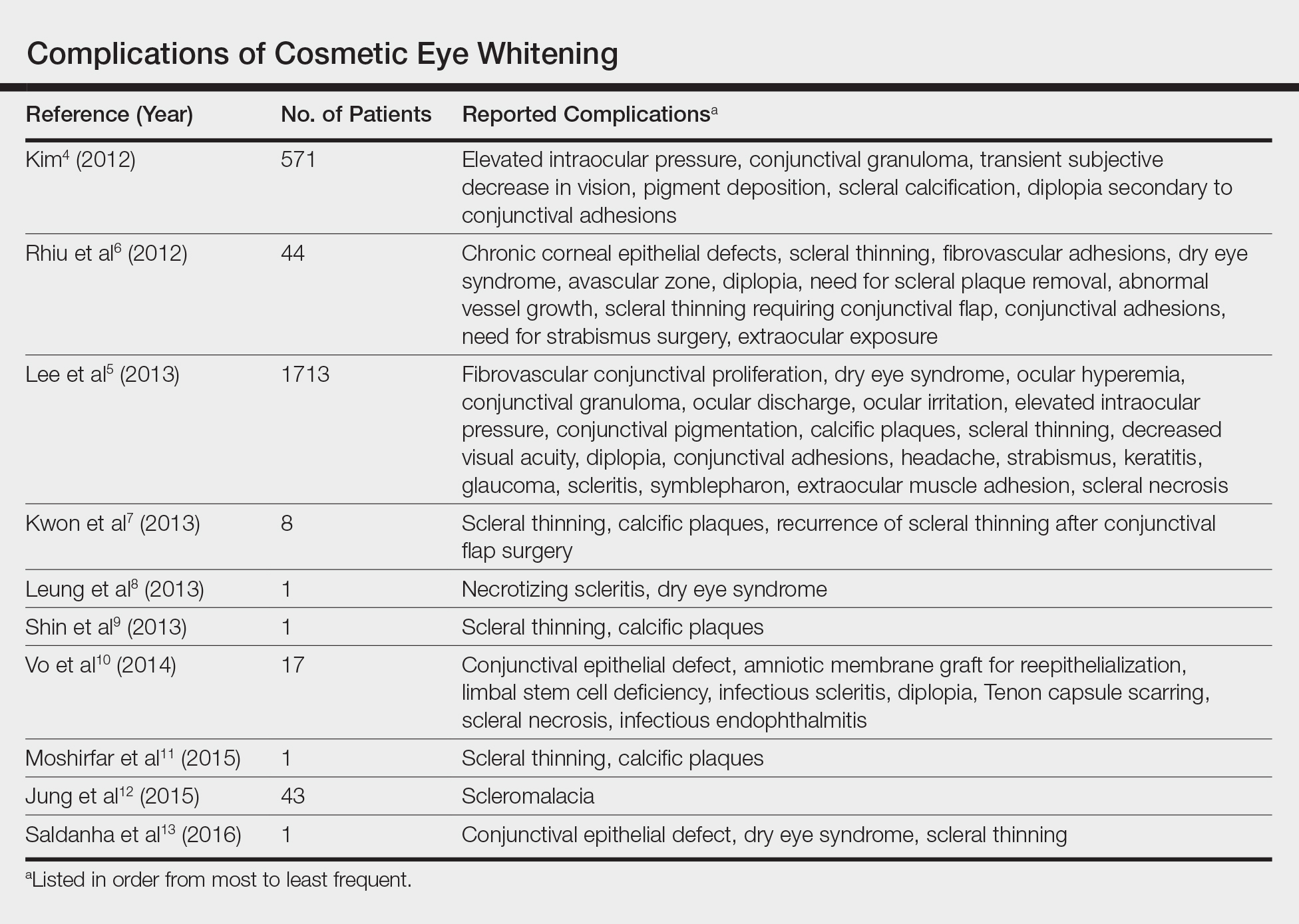
Kim4 was the first to report this surgical technique for irreversible hyperemic conjunctiva (N=1815). The reported success rate in South Korea was overwhelmingly high at 94.6%. In a mean (SD) follow-up time of 12.9 (7.8) months (range, 2–27 months), less than 20% of patients required surgical revision. During this time, the most common postoperative complications included elevation in intraocular pressure (17.2%), conjunctival granuloma (8.4%), transient vision decrease (7.5%), pigment deposition (5.3%), scleral calcifications (3.9%), and diplopia secondary to conjunctival adhesions (1.6%). No permanent defects were reported, and complications improved with surgical and medical management.4
Contrary to the findings of Kim,4 a large number of complications were seen; thus, on March 4, 2011, the Korean Ministry of Health & Welfare issued a declaration to discontinue the procedure under Article 49 of the Medical Service Act. Medical records from the single clinic in Korea from November 2007 to May 2010 were reviewed.5 One of the largest reviews of cosmetic eye whitening complications reviewed 1713 patients who underwent conjunctivectomy plus topical MMC with or without bevacizumab injection. Pterygium and chronic conjunctival hyperemia were the most common diagnoses that prompted patients to undergo treatment. Over an average follow-up period of 10.9 months, the overall complication rate was 82.9%, with severe complications being fibrovascular conjunctival proliferation (43.8%), recurrent hyperemic conjunctiva (28.1%), intraocular pressure (13.1%), scleral thinning with calcified plaques (6.2%), scleral thinning (4.4%), and diplopia (3.6%). A total of 56.9% of patients reported being satisfied with the cosmetic outcome of the surgery.5
In some of the smaller case series and case reports we reviewed, more vision-threatening complications have been described. Infectious endophthalmitis, infectious scleritis, and necrotizing scleritis have all been reported as complications of cosmetic eye whitening.8,10
Comment
The pathophysiology of the complications of cosmetic eye whitening stem from the disruption of the normal conjunctiva, destruction of the vascularization to the sclera, and loss of limbal stem cells. Mitomycin C is a topical antimetabolite antibiotic agent that inhibits DNA synthesis. This relatively safe and inexpensive product has decreased the recurrence rate in pterygium surgery as early as 1963.14,15 Complications of MMC in pterygium surgery include infectious scleritis, necrotizing scleritis, calcium formation, and even scleromalacia, occurring at incidence rates as low as 1.4%.16 These risks are balanced against the medical necessity of using MMC. Given the elective nature of cosmetic eye whitening, these complications in a cosmetic setting may not be justified.
The debate of the use of this procedure continues to occur in ophthalmologic societies. Both the Korean Ministry of Health & Welfare and the American Society of Cataract Refractive Surgery do not condone the use of regional conjunctivectomy for cosmetic eye whitening.5,17 Evidence shows that complications from cosmetic conjunctivectomy can be devastating and unnecessary given its elective nature. Although some complications (eg, dry eye syndrome, pain, discomfort) may be considered mild, the number of potentially serious complications brings the usefulness of the procedure into question.
This review is a launchpad to inform the medical community of the potential downside to conjunctivectomy for cosmetic eye whitening with the hope that it can initiate meaningful risk-benefit discussions between providers and physicians.
- Kim BH. Cosmetic eye whitening. Poster presented at: American Society of Cataract and Refractive Surgery; April 4-9, 2008; Chicago, IL.
- Kim BH. Cosmetic eye whitening by regional conjunctivectomy. Poster presented at: European Society of Cataract & Refractive Surgeons; September 13-17, 2008; Berlin, Germany.
- Raiskup F, Solomon A, Landau D, et al. Mitomycin C for pterygium: long term evaluation. Br J Ophthalmol. 2004;88:1425-1428.
- Kim BH. Regional conjunctivectomy with postoperative mitomycin C to treat chronic hyperemic conjunctiva. Cornea. 2012;31:236-244.
- Lee S, Go J, Rhiu S, et al. Cosmetic regional conjunctivectomy with postoperative mitomycin C application with or without bevacizumab injection [published online April 6, 2013]. Am J Ophthalmol. 2013;156:616-622.
- Rhiu S, Shim J, Kim EK, et al. Complications of cosmetic wide conjunctivectomy combined with postsurgical mitomycin C application. Cornea. 2012;31:245-252.
- Kwon HJ, Nam SM, Lee SY, et al. Conjunctival flap surgery for calcified scleromalacia after cosmetic conjunctivectomy. Cornea. 2013;32:821-825.
- Leung TG, Dunn JP, Akpek EK, et al. Necrotizing scleritis as a complication of cosmetic eye whitening procedure. J Ophthalmic Inflamm Infect. 2013;3:39.
- Shin HY, Kim MS, Chung SK. The development of scleromalacia after regional conjunctivectomy with the postoperative application of mitomycin C as an adjuvant therapy. Korean J Ophthalmol. 2013;27:208-210.
- Vo RC, Stafeeva K, Aldave AJ, et al. Complications related to a cosmetic eye-whitening procedure. Am J Ophthalmol. 2014;158:967-973.
- Moshirfar M, McCaughey MV, Fenzl CR, et al. Delayed manifestation of bilateral scleral thinning after I-BRITE® procedure and review of literature for cosmetic eye-whitening procedures. Clin Ophthalmol. 2015;9:445-451.
- Jung JW, Kwon KY, Choi DL, et al. Long-term clinical outcomes of conjunctival flap surgery for calcified scleromalacia after periocular surgery. Cornea. 2015;34:308-312.
- Saldanha MJ, Yang PT, Chan CC. Scleral thinning after I-BRITE procedure treated with amniotic membrane graft. Can J Ophthalmol. 2016;51:e115-e116.
- Seiler T, Schnelle B, Wollensak J. Pterygium excision using 193-nm excimer laser smoothing and topical mitomycin C. Ger J Ophthalmol. 1992;1:429-431.
- Singh G, Wilson MR, Foster CS. Long-term follow-up study of mitomycin eye drops as adjunctive treatment of pterygia and its comparison with conjunctival autograft transplantation. Cornea. 1990;9:331-334.
- Lam DS, Wong AK, Fan DS, et al. Intraoperative mitomycin C to prevent recurrence of pterygium after excision: a 30-month follow-up study. Ophthalmology. 1998;105:901-904; discussion 904-905.
- ASCRS Cornea Clinical Committee. Clinical alert: eye-whitening procedure: regional conjunctivectomy with mitomycin-C application [press release]. Fairfax, VA: American Society of Cataract and Refractive Surgery. http://www.ascrs.org/node/1352. Accessed January 22, 2015.
First introduced in 2008 as a surgical treatment of chronic conjunctival injection, cosmetic eye whitening became popularized in South Kore
The procedure involves performing a localized conjunctivectomy with or without removal of the Tenon capsule.4 Brimonidine tartrate is given for vascular constriction. When conjunctivectomy is performed in the right eye, the medial conjunctiva is incised from the 2-o’clock to 5-o’clock positions and the lateral conjunctiva is incised from the 10-o’clock to 7-o’clock positions. After the conjunctiva and Tenon capsule are excised, hemostasis is achieved with electrocauterization. Postoperative management may consist of topical mitomycin C (MMC) 0.02% 4 times daily for 2 to 5 days along with topical steroids. The addition of bevacizumab 1.25 mg/mL also has been described.5
In this report, we provide a comprehensive review of the complications of cosmetic eye whitening based on a review of the literature. Clinicians in both aesthetic practice and ophthalmology should be aware of the potential complications to accurately educate their patients about the possible risks and benefits of this procedure.
Methods
A review of PubMed articles indexed for MEDLINE (January 2009 to July 2017) using the search terms cosmetic eye whitening, cosmetic wide conjunctivectomy, I-Brite, and chronic hyperemic conjuctiva was conducted to evaluate the number of reports of complications from cosmetic eye whitening. A total of 10 articles were included in the study based on a review of abstracts. Non–English-language abstracts were not reviewed.
Results
Based on a review of 10 articles commenting on the complications of cosmetic eye whitening, a total of 2400 patients had undergone a cosmetic conjunctivectomy with various postoperative complications and recurrences (Table).4-13 The most commonly recurring complications based on the reported frequencies in the articles included chronic conjunctival epithelial defects, scleral thinning, calcific plaques, dry eye syndrome, diplopia (sometimes requiring strabismus surgery), and elevated intraocular pressure.

Kim4 was the first to report this surgical technique for irreversible hyperemic conjunctiva (N=1815). The reported success rate in South Korea was overwhelmingly high at 94.6%. In a mean (SD) follow-up time of 12.9 (7.8) months (range, 2–27 months), less than 20% of patients required surgical revision. During this time, the most common postoperative complications included elevation in intraocular pressure (17.2%), conjunctival granuloma (8.4%), transient vision decrease (7.5%), pigment deposition (5.3%), scleral calcifications (3.9%), and diplopia secondary to conjunctival adhesions (1.6%). No permanent defects were reported, and complications improved with surgical and medical management.4
Contrary to the findings of Kim,4 a large number of complications were seen; thus, on March 4, 2011, the Korean Ministry of Health & Welfare issued a declaration to discontinue the procedure under Article 49 of the Medical Service Act. Medical records from the single clinic in Korea from November 2007 to May 2010 were reviewed.5 One of the largest reviews of cosmetic eye whitening complications reviewed 1713 patients who underwent conjunctivectomy plus topical MMC with or without bevacizumab injection. Pterygium and chronic conjunctival hyperemia were the most common diagnoses that prompted patients to undergo treatment. Over an average follow-up period of 10.9 months, the overall complication rate was 82.9%, with severe complications being fibrovascular conjunctival proliferation (43.8%), recurrent hyperemic conjunctiva (28.1%), intraocular pressure (13.1%), scleral thinning with calcified plaques (6.2%), scleral thinning (4.4%), and diplopia (3.6%). A total of 56.9% of patients reported being satisfied with the cosmetic outcome of the surgery.5
In some of the smaller case series and case reports we reviewed, more vision-threatening complications have been described. Infectious endophthalmitis, infectious scleritis, and necrotizing scleritis have all been reported as complications of cosmetic eye whitening.8,10
Comment
The pathophysiology of the complications of cosmetic eye whitening stem from the disruption of the normal conjunctiva, destruction of the vascularization to the sclera, and loss of limbal stem cells. Mitomycin C is a topical antimetabolite antibiotic agent that inhibits DNA synthesis. This relatively safe and inexpensive product has decreased the recurrence rate in pterygium surgery as early as 1963.14,15 Complications of MMC in pterygium surgery include infectious scleritis, necrotizing scleritis, calcium formation, and even scleromalacia, occurring at incidence rates as low as 1.4%.16 These risks are balanced against the medical necessity of using MMC. Given the elective nature of cosmetic eye whitening, these complications in a cosmetic setting may not be justified.
The debate of the use of this procedure continues to occur in ophthalmologic societies. Both the Korean Ministry of Health & Welfare and the American Society of Cataract Refractive Surgery do not condone the use of regional conjunctivectomy for cosmetic eye whitening.5,17 Evidence shows that complications from cosmetic conjunctivectomy can be devastating and unnecessary given its elective nature. Although some complications (eg, dry eye syndrome, pain, discomfort) may be considered mild, the number of potentially serious complications brings the usefulness of the procedure into question.
This review is a launchpad to inform the medical community of the potential downside to conjunctivectomy for cosmetic eye whitening with the hope that it can initiate meaningful risk-benefit discussions between providers and physicians.
First introduced in 2008 as a surgical treatment of chronic conjunctival injection, cosmetic eye whitening became popularized in South Kore
The procedure involves performing a localized conjunctivectomy with or without removal of the Tenon capsule.4 Brimonidine tartrate is given for vascular constriction. When conjunctivectomy is performed in the right eye, the medial conjunctiva is incised from the 2-o’clock to 5-o’clock positions and the lateral conjunctiva is incised from the 10-o’clock to 7-o’clock positions. After the conjunctiva and Tenon capsule are excised, hemostasis is achieved with electrocauterization. Postoperative management may consist of topical mitomycin C (MMC) 0.02% 4 times daily for 2 to 5 days along with topical steroids. The addition of bevacizumab 1.25 mg/mL also has been described.5
In this report, we provide a comprehensive review of the complications of cosmetic eye whitening based on a review of the literature. Clinicians in both aesthetic practice and ophthalmology should be aware of the potential complications to accurately educate their patients about the possible risks and benefits of this procedure.
Methods
A review of PubMed articles indexed for MEDLINE (January 2009 to July 2017) using the search terms cosmetic eye whitening, cosmetic wide conjunctivectomy, I-Brite, and chronic hyperemic conjuctiva was conducted to evaluate the number of reports of complications from cosmetic eye whitening. A total of 10 articles were included in the study based on a review of abstracts. Non–English-language abstracts were not reviewed.
Results
Based on a review of 10 articles commenting on the complications of cosmetic eye whitening, a total of 2400 patients had undergone a cosmetic conjunctivectomy with various postoperative complications and recurrences (Table).4-13 The most commonly recurring complications based on the reported frequencies in the articles included chronic conjunctival epithelial defects, scleral thinning, calcific plaques, dry eye syndrome, diplopia (sometimes requiring strabismus surgery), and elevated intraocular pressure.

Kim4 was the first to report this surgical technique for irreversible hyperemic conjunctiva (N=1815). The reported success rate in South Korea was overwhelmingly high at 94.6%. In a mean (SD) follow-up time of 12.9 (7.8) months (range, 2–27 months), less than 20% of patients required surgical revision. During this time, the most common postoperative complications included elevation in intraocular pressure (17.2%), conjunctival granuloma (8.4%), transient vision decrease (7.5%), pigment deposition (5.3%), scleral calcifications (3.9%), and diplopia secondary to conjunctival adhesions (1.6%). No permanent defects were reported, and complications improved with surgical and medical management.4
Contrary to the findings of Kim,4 a large number of complications were seen; thus, on March 4, 2011, the Korean Ministry of Health & Welfare issued a declaration to discontinue the procedure under Article 49 of the Medical Service Act. Medical records from the single clinic in Korea from November 2007 to May 2010 were reviewed.5 One of the largest reviews of cosmetic eye whitening complications reviewed 1713 patients who underwent conjunctivectomy plus topical MMC with or without bevacizumab injection. Pterygium and chronic conjunctival hyperemia were the most common diagnoses that prompted patients to undergo treatment. Over an average follow-up period of 10.9 months, the overall complication rate was 82.9%, with severe complications being fibrovascular conjunctival proliferation (43.8%), recurrent hyperemic conjunctiva (28.1%), intraocular pressure (13.1%), scleral thinning with calcified plaques (6.2%), scleral thinning (4.4%), and diplopia (3.6%). A total of 56.9% of patients reported being satisfied with the cosmetic outcome of the surgery.5
In some of the smaller case series and case reports we reviewed, more vision-threatening complications have been described. Infectious endophthalmitis, infectious scleritis, and necrotizing scleritis have all been reported as complications of cosmetic eye whitening.8,10
Comment
The pathophysiology of the complications of cosmetic eye whitening stem from the disruption of the normal conjunctiva, destruction of the vascularization to the sclera, and loss of limbal stem cells. Mitomycin C is a topical antimetabolite antibiotic agent that inhibits DNA synthesis. This relatively safe and inexpensive product has decreased the recurrence rate in pterygium surgery as early as 1963.14,15 Complications of MMC in pterygium surgery include infectious scleritis, necrotizing scleritis, calcium formation, and even scleromalacia, occurring at incidence rates as low as 1.4%.16 These risks are balanced against the medical necessity of using MMC. Given the elective nature of cosmetic eye whitening, these complications in a cosmetic setting may not be justified.
The debate of the use of this procedure continues to occur in ophthalmologic societies. Both the Korean Ministry of Health & Welfare and the American Society of Cataract Refractive Surgery do not condone the use of regional conjunctivectomy for cosmetic eye whitening.5,17 Evidence shows that complications from cosmetic conjunctivectomy can be devastating and unnecessary given its elective nature. Although some complications (eg, dry eye syndrome, pain, discomfort) may be considered mild, the number of potentially serious complications brings the usefulness of the procedure into question.
This review is a launchpad to inform the medical community of the potential downside to conjunctivectomy for cosmetic eye whitening with the hope that it can initiate meaningful risk-benefit discussions between providers and physicians.
- Kim BH. Cosmetic eye whitening. Poster presented at: American Society of Cataract and Refractive Surgery; April 4-9, 2008; Chicago, IL.
- Kim BH. Cosmetic eye whitening by regional conjunctivectomy. Poster presented at: European Society of Cataract & Refractive Surgeons; September 13-17, 2008; Berlin, Germany.
- Raiskup F, Solomon A, Landau D, et al. Mitomycin C for pterygium: long term evaluation. Br J Ophthalmol. 2004;88:1425-1428.
- Kim BH. Regional conjunctivectomy with postoperative mitomycin C to treat chronic hyperemic conjunctiva. Cornea. 2012;31:236-244.
- Lee S, Go J, Rhiu S, et al. Cosmetic regional conjunctivectomy with postoperative mitomycin C application with or without bevacizumab injection [published online April 6, 2013]. Am J Ophthalmol. 2013;156:616-622.
- Rhiu S, Shim J, Kim EK, et al. Complications of cosmetic wide conjunctivectomy combined with postsurgical mitomycin C application. Cornea. 2012;31:245-252.
- Kwon HJ, Nam SM, Lee SY, et al. Conjunctival flap surgery for calcified scleromalacia after cosmetic conjunctivectomy. Cornea. 2013;32:821-825.
- Leung TG, Dunn JP, Akpek EK, et al. Necrotizing scleritis as a complication of cosmetic eye whitening procedure. J Ophthalmic Inflamm Infect. 2013;3:39.
- Shin HY, Kim MS, Chung SK. The development of scleromalacia after regional conjunctivectomy with the postoperative application of mitomycin C as an adjuvant therapy. Korean J Ophthalmol. 2013;27:208-210.
- Vo RC, Stafeeva K, Aldave AJ, et al. Complications related to a cosmetic eye-whitening procedure. Am J Ophthalmol. 2014;158:967-973.
- Moshirfar M, McCaughey MV, Fenzl CR, et al. Delayed manifestation of bilateral scleral thinning after I-BRITE® procedure and review of literature for cosmetic eye-whitening procedures. Clin Ophthalmol. 2015;9:445-451.
- Jung JW, Kwon KY, Choi DL, et al. Long-term clinical outcomes of conjunctival flap surgery for calcified scleromalacia after periocular surgery. Cornea. 2015;34:308-312.
- Saldanha MJ, Yang PT, Chan CC. Scleral thinning after I-BRITE procedure treated with amniotic membrane graft. Can J Ophthalmol. 2016;51:e115-e116.
- Seiler T, Schnelle B, Wollensak J. Pterygium excision using 193-nm excimer laser smoothing and topical mitomycin C. Ger J Ophthalmol. 1992;1:429-431.
- Singh G, Wilson MR, Foster CS. Long-term follow-up study of mitomycin eye drops as adjunctive treatment of pterygia and its comparison with conjunctival autograft transplantation. Cornea. 1990;9:331-334.
- Lam DS, Wong AK, Fan DS, et al. Intraoperative mitomycin C to prevent recurrence of pterygium after excision: a 30-month follow-up study. Ophthalmology. 1998;105:901-904; discussion 904-905.
- ASCRS Cornea Clinical Committee. Clinical alert: eye-whitening procedure: regional conjunctivectomy with mitomycin-C application [press release]. Fairfax, VA: American Society of Cataract and Refractive Surgery. http://www.ascrs.org/node/1352. Accessed January 22, 2015.
- Kim BH. Cosmetic eye whitening. Poster presented at: American Society of Cataract and Refractive Surgery; April 4-9, 2008; Chicago, IL.
- Kim BH. Cosmetic eye whitening by regional conjunctivectomy. Poster presented at: European Society of Cataract & Refractive Surgeons; September 13-17, 2008; Berlin, Germany.
- Raiskup F, Solomon A, Landau D, et al. Mitomycin C for pterygium: long term evaluation. Br J Ophthalmol. 2004;88:1425-1428.
- Kim BH. Regional conjunctivectomy with postoperative mitomycin C to treat chronic hyperemic conjunctiva. Cornea. 2012;31:236-244.
- Lee S, Go J, Rhiu S, et al. Cosmetic regional conjunctivectomy with postoperative mitomycin C application with or without bevacizumab injection [published online April 6, 2013]. Am J Ophthalmol. 2013;156:616-622.
- Rhiu S, Shim J, Kim EK, et al. Complications of cosmetic wide conjunctivectomy combined with postsurgical mitomycin C application. Cornea. 2012;31:245-252.
- Kwon HJ, Nam SM, Lee SY, et al. Conjunctival flap surgery for calcified scleromalacia after cosmetic conjunctivectomy. Cornea. 2013;32:821-825.
- Leung TG, Dunn JP, Akpek EK, et al. Necrotizing scleritis as a complication of cosmetic eye whitening procedure. J Ophthalmic Inflamm Infect. 2013;3:39.
- Shin HY, Kim MS, Chung SK. The development of scleromalacia after regional conjunctivectomy with the postoperative application of mitomycin C as an adjuvant therapy. Korean J Ophthalmol. 2013;27:208-210.
- Vo RC, Stafeeva K, Aldave AJ, et al. Complications related to a cosmetic eye-whitening procedure. Am J Ophthalmol. 2014;158:967-973.
- Moshirfar M, McCaughey MV, Fenzl CR, et al. Delayed manifestation of bilateral scleral thinning after I-BRITE® procedure and review of literature for cosmetic eye-whitening procedures. Clin Ophthalmol. 2015;9:445-451.
- Jung JW, Kwon KY, Choi DL, et al. Long-term clinical outcomes of conjunctival flap surgery for calcified scleromalacia after periocular surgery. Cornea. 2015;34:308-312.
- Saldanha MJ, Yang PT, Chan CC. Scleral thinning after I-BRITE procedure treated with amniotic membrane graft. Can J Ophthalmol. 2016;51:e115-e116.
- Seiler T, Schnelle B, Wollensak J. Pterygium excision using 193-nm excimer laser smoothing and topical mitomycin C. Ger J Ophthalmol. 1992;1:429-431.
- Singh G, Wilson MR, Foster CS. Long-term follow-up study of mitomycin eye drops as adjunctive treatment of pterygia and its comparison with conjunctival autograft transplantation. Cornea. 1990;9:331-334.
- Lam DS, Wong AK, Fan DS, et al. Intraoperative mitomycin C to prevent recurrence of pterygium after excision: a 30-month follow-up study. Ophthalmology. 1998;105:901-904; discussion 904-905.
- ASCRS Cornea Clinical Committee. Clinical alert: eye-whitening procedure: regional conjunctivectomy with mitomycin-C application [press release]. Fairfax, VA: American Society of Cataract and Refractive Surgery. http://www.ascrs.org/node/1352. Accessed January 22, 2015.
Resident Pearl
- Cosmetic eye whitening has severe and vision-threatening complications that should be aware to all cosmetic surgeons.
APOE affects tau pathology independent of amyloid-beta
Apolipoprotein E protein isoforms, particularly ApoE4, appear to accelerate brain-wide tau propagation that eventually leads to neuronal injury and death in a manner independent from amyloid-beta, according to findings from transgenic mouse model studies.
“We found ApoE itself, especially ApoE4, was essential to neuronal death,” wrote first author Yang Shi of Washington University, St. Louis, and her colleagues, led by David M. Holtzman, MD, in new research published in Nature. “With pathological tau accumulation, the presence of ApoE, especially ApoE4, may make neurons more susceptible to degeneration, whereas the absence of ApoE may protect neurons from death.”
The new research also illustrates a differential effect between the three APOE alleles. In the team’s in vivo study, tau-expressing mice with the APOE4 allele were most affected, and those with the E3 and E2 versions progressively less so. Mice that didn’t express the human gene at all showed no change in tau and no immune reaction (Nature. 2017;549:523-7).
This new picture of tauopathy – a common feature in Alzheimer’s, frontotemporal dementia, corticobasal degeneration, Pick disease, and progressive supranuclear palsy – suggests an expanded role for ApoE, which until now has been associated mostly with increased amyloid deposition in the Alzheimer’s disease (AD) brain.
“I think this paper is potentially very important, identifying what appear to be strong connections between tau and ApoE that we had no idea about before,” Dr. Wolfe said in an email. “While independent confirmation is needed, this new work is coming from a strong research team that has made other seminal discoveries in the field. Uncovering the molecular basis for ApoE’s effect on tau pathology and glial cell activation may suggest new targets for drug discovery for AD.”
Evidence of ApoE4’s greater impact
To examine ApoE’s effect on tau, the research team bred new lines of genetically modified mice, all of which expressed human tau. Some also expressed human ApoE4, E3, or E2 in place of mouse ApoE. A comparator mouse expressed tau, but not ApoE.
By the time the mice were 9 months old, the tau-E4 strain showed significantly more brain atrophy than did the tau-E3 and tau-E2 strains. The mice who expressed tau but were free of ApoE showed no brain changes.
A closer look showed that atrophy occurred primarily in areas associated with the cognitive changes seen in dementia: the hippocampus, piriform/entorhinal cortex, and amygdala. The ventricles were also enlarged.
“These results revealed an important role of ApoE in regulating tau-mediated neurodegeneration, with ApoE4 causing more severe damage and the absence of ApoE being protective,” the investigators wrote.
The E4/tau tango started early, too, the team noted. At 3 months old, tau-E4 mice had no obvious brain atrophy, but already had significantly more soluble tau than did any of the other strains. By 9 months, when tauopathy was obvious, the tau-E4 mice still had more of the protein, which had shifted from a soluble to an insoluble and hyperphosphorylated state. The tau-E4 mice didn’t appear to be making more tau than the others, though; rather, they were less able to clear it through the neurons’ clearing and recycling system of autophagy.
Drilling down further into the neurons’ pathophysiology, the team found that tau first appeared in the axons of dentate gyrus granule cells in the hippocampus, and then, at an early age, moved into the cell body. Again, there were APOE allele–specific patterns to tau propagation. The team saw four major tau staining patterns, which correlated with the level of brain atrophy. Types 1 and 2, associated with least atrophy, occurred most often in the tau-only, ApoE-negative mice; type 4, associated with the greatest atrophy, occurred most often in the tau-E4 mice.
“The featured distribution of these ... patterns, which either represent different tau structures or progressively more advanced pathological tau stages, indicate ApoE affects either tau conformation or tau pathology progression,” the investigators wrote.
Greatest neurodegeneration seen with ApoE4
Tau-mediated neurodegeneration initiated levels of inflammatory response that also depended on the type of ApoE isoform. Exposure to a culture of damaged neurons and mixed glial cells caused microglia to release a flood of inflammatory cytokines that called in a host of astrocytes to kill damaged tau-E4 neurons en masse, but attacked the tau-E3, tau-E2, and tau-only strains much less. This finding indicates that “ApoE itself was directly involved in inducing neurotoxicity in tau-expressing susceptible neurons,” the team wrote.
Finally, they investigated this model of neurodegeneration in postmortem brain samples of patients with corticobasal degeneration, Pick disease, and progressive supranuclear palsy – the three most common sporadic primary tauopathies. Patients with the E4 allele showed more severe neurodegeneration and a greater interaction of tau pathology and neurodegeneration. Amyloid deposition was associated with less severe neurodegeneration.
Taken together, the findings strongly suggest that the high-risk APOE4 allele is the linchpin that links neuroinflammation to neuronal death in the setting of tau pathology, the investigators concluded.
“The presence of degenerating neurons appeared to further induce neuroinflammation, which was augmented by ApoE4 owing to its inherently higher innate immune reactivity. While activated microglia may be protective to some extent in the setting of amyloid-beta pathology, by targeting plaques and reducing dystrophic neurites, they could be deleterious in tauopathy by directly targeting injured neurons and by activating toxic astrocytes. Enhanced neuroinflammation associated with ApoE4 may further exacerbate neurodegeneration.”
The study was funded by grants awarded to multiple investigators by the National Institutes of Health, the JPB Foundation, Cure Alzheimer’s Fund, AstraZeneca, the Consortium for Frontotemporal Dementia Research, the Tau Consortium, the National Multiple Sclerosis Society, the Nancy Davis Foundation, and the Amyotrophic Lateral Sclerosis Association. Dr. Holtzman cofounded and is on the scientific advisory board of C2N Diagnostics. He consults for Genentech, AbbVie, Eli Lilly, Proclara, GlaxoSmithKline, and Denali. Dr. Holtzman’s lab is funded by institutional research grants from C2N Diagnostics, Eli Lilly, AbbVie, and Denali.
[email protected]
On Twitter @Alz_Gal
The study by Shi et al. represents an important step forward in our understanding of Alzheimer’s disease (AD) pathogenesis and raises another challenge to the amyloid hypothesis: ApoE4 enhances tau pathology independent of amyloid in vivo, in vitro and – possibly – even in humans.
The discovery that the plaques seen in AD brains were composed of amyloid-beta (Abeta) peptide, and the discovery soon thereafter that mutations of genes involved in Abeta production caused dominantly inherited AD, made clear that there was something very important about amyloid and its relationship to AD.
ApoE4 is the next most potent genetic variant to predispose to AD, and much more prevalent than the dominant mutations. When first reported in the early 1990s, the gene was something of a mystery because it did not have an apparent link to amyloid. However, research suggests that ApoE4-mediated effects may be via either amyloid-dependent or amyloid-independent mechanisms. The latter have been particularly championed by investigators at the Gladstone Institute of Neurological Disease who are working to develop a structural modifier of the ApoE4 isoform to prevent the direct toxic effects of the intraneuronal carboxyl fragment that is generated during the production of Abeta. To date, no human trials have been conducted.
Turning to the study by Shi and colleagues, in addition to their convincing mouse and in vitro data, they present data from a collection of human tauopathy brains. These show that ApoE4 was associated with greater tau pathology in patients who died with corticobasal degeneration, Pick disease, and progressive supranuclear palsy. Incidental amyloid deposition in some members of this cohort was not associated with greater tauopathy burden, in contrast to what one might have predicted if Abeta was triggering tau hyperphosphorylation. For clinicians, the human observations are particularly intriguing and deserve replication and further study.
The study of Shi et al. does not invalidate the possibility that amyloid plays a key role in AD pathogenesis. Indeed, that relationship seems firm, since the dominantly inherited AD mutations all affect Abeta production or aggregation. However, it does raise questions as to whether the dominantly inherited cases are representative of the “sporadic” and ApoE4-associated cases. It also raises questions as to the exact role amyloid plays in AD pathogenesis. Abeta toxicity has been amply demonstrated, but that does not necessarily mean that Abeta toxicity is the cause of AD or the driving force behind disease progression.
Clearly, more work is needed to clarify what role amyloid is playing in AD pathogenesis beyond existing toxicity models. Hopefully, the study by Shi et al. will stimulate new models and therapeutic ideas.
Richard J. Caselli, MD, is professor of neurology at the Mayo Clinic in Scottsdale, Ariz. He also is associate director and clinical core director of the Arizona Alzheimer’s Disease Center.
The study by Shi et al. represents an important step forward in our understanding of Alzheimer’s disease (AD) pathogenesis and raises another challenge to the amyloid hypothesis: ApoE4 enhances tau pathology independent of amyloid in vivo, in vitro and – possibly – even in humans.
The discovery that the plaques seen in AD brains were composed of amyloid-beta (Abeta) peptide, and the discovery soon thereafter that mutations of genes involved in Abeta production caused dominantly inherited AD, made clear that there was something very important about amyloid and its relationship to AD.
ApoE4 is the next most potent genetic variant to predispose to AD, and much more prevalent than the dominant mutations. When first reported in the early 1990s, the gene was something of a mystery because it did not have an apparent link to amyloid. However, research suggests that ApoE4-mediated effects may be via either amyloid-dependent or amyloid-independent mechanisms. The latter have been particularly championed by investigators at the Gladstone Institute of Neurological Disease who are working to develop a structural modifier of the ApoE4 isoform to prevent the direct toxic effects of the intraneuronal carboxyl fragment that is generated during the production of Abeta. To date, no human trials have been conducted.
Turning to the study by Shi and colleagues, in addition to their convincing mouse and in vitro data, they present data from a collection of human tauopathy brains. These show that ApoE4 was associated with greater tau pathology in patients who died with corticobasal degeneration, Pick disease, and progressive supranuclear palsy. Incidental amyloid deposition in some members of this cohort was not associated with greater tauopathy burden, in contrast to what one might have predicted if Abeta was triggering tau hyperphosphorylation. For clinicians, the human observations are particularly intriguing and deserve replication and further study.
The study of Shi et al. does not invalidate the possibility that amyloid plays a key role in AD pathogenesis. Indeed, that relationship seems firm, since the dominantly inherited AD mutations all affect Abeta production or aggregation. However, it does raise questions as to whether the dominantly inherited cases are representative of the “sporadic” and ApoE4-associated cases. It also raises questions as to the exact role amyloid plays in AD pathogenesis. Abeta toxicity has been amply demonstrated, but that does not necessarily mean that Abeta toxicity is the cause of AD or the driving force behind disease progression.
Clearly, more work is needed to clarify what role amyloid is playing in AD pathogenesis beyond existing toxicity models. Hopefully, the study by Shi et al. will stimulate new models and therapeutic ideas.
Richard J. Caselli, MD, is professor of neurology at the Mayo Clinic in Scottsdale, Ariz. He also is associate director and clinical core director of the Arizona Alzheimer’s Disease Center.
The study by Shi et al. represents an important step forward in our understanding of Alzheimer’s disease (AD) pathogenesis and raises another challenge to the amyloid hypothesis: ApoE4 enhances tau pathology independent of amyloid in vivo, in vitro and – possibly – even in humans.
The discovery that the plaques seen in AD brains were composed of amyloid-beta (Abeta) peptide, and the discovery soon thereafter that mutations of genes involved in Abeta production caused dominantly inherited AD, made clear that there was something very important about amyloid and its relationship to AD.
ApoE4 is the next most potent genetic variant to predispose to AD, and much more prevalent than the dominant mutations. When first reported in the early 1990s, the gene was something of a mystery because it did not have an apparent link to amyloid. However, research suggests that ApoE4-mediated effects may be via either amyloid-dependent or amyloid-independent mechanisms. The latter have been particularly championed by investigators at the Gladstone Institute of Neurological Disease who are working to develop a structural modifier of the ApoE4 isoform to prevent the direct toxic effects of the intraneuronal carboxyl fragment that is generated during the production of Abeta. To date, no human trials have been conducted.
Turning to the study by Shi and colleagues, in addition to their convincing mouse and in vitro data, they present data from a collection of human tauopathy brains. These show that ApoE4 was associated with greater tau pathology in patients who died with corticobasal degeneration, Pick disease, and progressive supranuclear palsy. Incidental amyloid deposition in some members of this cohort was not associated with greater tauopathy burden, in contrast to what one might have predicted if Abeta was triggering tau hyperphosphorylation. For clinicians, the human observations are particularly intriguing and deserve replication and further study.
The study of Shi et al. does not invalidate the possibility that amyloid plays a key role in AD pathogenesis. Indeed, that relationship seems firm, since the dominantly inherited AD mutations all affect Abeta production or aggregation. However, it does raise questions as to whether the dominantly inherited cases are representative of the “sporadic” and ApoE4-associated cases. It also raises questions as to the exact role amyloid plays in AD pathogenesis. Abeta toxicity has been amply demonstrated, but that does not necessarily mean that Abeta toxicity is the cause of AD or the driving force behind disease progression.
Clearly, more work is needed to clarify what role amyloid is playing in AD pathogenesis beyond existing toxicity models. Hopefully, the study by Shi et al. will stimulate new models and therapeutic ideas.
Richard J. Caselli, MD, is professor of neurology at the Mayo Clinic in Scottsdale, Ariz. He also is associate director and clinical core director of the Arizona Alzheimer’s Disease Center.
Apolipoprotein E protein isoforms, particularly ApoE4, appear to accelerate brain-wide tau propagation that eventually leads to neuronal injury and death in a manner independent from amyloid-beta, according to findings from transgenic mouse model studies.
“We found ApoE itself, especially ApoE4, was essential to neuronal death,” wrote first author Yang Shi of Washington University, St. Louis, and her colleagues, led by David M. Holtzman, MD, in new research published in Nature. “With pathological tau accumulation, the presence of ApoE, especially ApoE4, may make neurons more susceptible to degeneration, whereas the absence of ApoE may protect neurons from death.”
The new research also illustrates a differential effect between the three APOE alleles. In the team’s in vivo study, tau-expressing mice with the APOE4 allele were most affected, and those with the E3 and E2 versions progressively less so. Mice that didn’t express the human gene at all showed no change in tau and no immune reaction (Nature. 2017;549:523-7).
This new picture of tauopathy – a common feature in Alzheimer’s, frontotemporal dementia, corticobasal degeneration, Pick disease, and progressive supranuclear palsy – suggests an expanded role for ApoE, which until now has been associated mostly with increased amyloid deposition in the Alzheimer’s disease (AD) brain.
“I think this paper is potentially very important, identifying what appear to be strong connections between tau and ApoE that we had no idea about before,” Dr. Wolfe said in an email. “While independent confirmation is needed, this new work is coming from a strong research team that has made other seminal discoveries in the field. Uncovering the molecular basis for ApoE’s effect on tau pathology and glial cell activation may suggest new targets for drug discovery for AD.”
Evidence of ApoE4’s greater impact
To examine ApoE’s effect on tau, the research team bred new lines of genetically modified mice, all of which expressed human tau. Some also expressed human ApoE4, E3, or E2 in place of mouse ApoE. A comparator mouse expressed tau, but not ApoE.
By the time the mice were 9 months old, the tau-E4 strain showed significantly more brain atrophy than did the tau-E3 and tau-E2 strains. The mice who expressed tau but were free of ApoE showed no brain changes.
A closer look showed that atrophy occurred primarily in areas associated with the cognitive changes seen in dementia: the hippocampus, piriform/entorhinal cortex, and amygdala. The ventricles were also enlarged.
“These results revealed an important role of ApoE in regulating tau-mediated neurodegeneration, with ApoE4 causing more severe damage and the absence of ApoE being protective,” the investigators wrote.
The E4/tau tango started early, too, the team noted. At 3 months old, tau-E4 mice had no obvious brain atrophy, but already had significantly more soluble tau than did any of the other strains. By 9 months, when tauopathy was obvious, the tau-E4 mice still had more of the protein, which had shifted from a soluble to an insoluble and hyperphosphorylated state. The tau-E4 mice didn’t appear to be making more tau than the others, though; rather, they were less able to clear it through the neurons’ clearing and recycling system of autophagy.
Drilling down further into the neurons’ pathophysiology, the team found that tau first appeared in the axons of dentate gyrus granule cells in the hippocampus, and then, at an early age, moved into the cell body. Again, there were APOE allele–specific patterns to tau propagation. The team saw four major tau staining patterns, which correlated with the level of brain atrophy. Types 1 and 2, associated with least atrophy, occurred most often in the tau-only, ApoE-negative mice; type 4, associated with the greatest atrophy, occurred most often in the tau-E4 mice.
“The featured distribution of these ... patterns, which either represent different tau structures or progressively more advanced pathological tau stages, indicate ApoE affects either tau conformation or tau pathology progression,” the investigators wrote.
Greatest neurodegeneration seen with ApoE4
Tau-mediated neurodegeneration initiated levels of inflammatory response that also depended on the type of ApoE isoform. Exposure to a culture of damaged neurons and mixed glial cells caused microglia to release a flood of inflammatory cytokines that called in a host of astrocytes to kill damaged tau-E4 neurons en masse, but attacked the tau-E3, tau-E2, and tau-only strains much less. This finding indicates that “ApoE itself was directly involved in inducing neurotoxicity in tau-expressing susceptible neurons,” the team wrote.
Finally, they investigated this model of neurodegeneration in postmortem brain samples of patients with corticobasal degeneration, Pick disease, and progressive supranuclear palsy – the three most common sporadic primary tauopathies. Patients with the E4 allele showed more severe neurodegeneration and a greater interaction of tau pathology and neurodegeneration. Amyloid deposition was associated with less severe neurodegeneration.
Taken together, the findings strongly suggest that the high-risk APOE4 allele is the linchpin that links neuroinflammation to neuronal death in the setting of tau pathology, the investigators concluded.
“The presence of degenerating neurons appeared to further induce neuroinflammation, which was augmented by ApoE4 owing to its inherently higher innate immune reactivity. While activated microglia may be protective to some extent in the setting of amyloid-beta pathology, by targeting plaques and reducing dystrophic neurites, they could be deleterious in tauopathy by directly targeting injured neurons and by activating toxic astrocytes. Enhanced neuroinflammation associated with ApoE4 may further exacerbate neurodegeneration.”
The study was funded by grants awarded to multiple investigators by the National Institutes of Health, the JPB Foundation, Cure Alzheimer’s Fund, AstraZeneca, the Consortium for Frontotemporal Dementia Research, the Tau Consortium, the National Multiple Sclerosis Society, the Nancy Davis Foundation, and the Amyotrophic Lateral Sclerosis Association. Dr. Holtzman cofounded and is on the scientific advisory board of C2N Diagnostics. He consults for Genentech, AbbVie, Eli Lilly, Proclara, GlaxoSmithKline, and Denali. Dr. Holtzman’s lab is funded by institutional research grants from C2N Diagnostics, Eli Lilly, AbbVie, and Denali.
[email protected]
On Twitter @Alz_Gal
Apolipoprotein E protein isoforms, particularly ApoE4, appear to accelerate brain-wide tau propagation that eventually leads to neuronal injury and death in a manner independent from amyloid-beta, according to findings from transgenic mouse model studies.
“We found ApoE itself, especially ApoE4, was essential to neuronal death,” wrote first author Yang Shi of Washington University, St. Louis, and her colleagues, led by David M. Holtzman, MD, in new research published in Nature. “With pathological tau accumulation, the presence of ApoE, especially ApoE4, may make neurons more susceptible to degeneration, whereas the absence of ApoE may protect neurons from death.”
The new research also illustrates a differential effect between the three APOE alleles. In the team’s in vivo study, tau-expressing mice with the APOE4 allele were most affected, and those with the E3 and E2 versions progressively less so. Mice that didn’t express the human gene at all showed no change in tau and no immune reaction (Nature. 2017;549:523-7).
This new picture of tauopathy – a common feature in Alzheimer’s, frontotemporal dementia, corticobasal degeneration, Pick disease, and progressive supranuclear palsy – suggests an expanded role for ApoE, which until now has been associated mostly with increased amyloid deposition in the Alzheimer’s disease (AD) brain.
“I think this paper is potentially very important, identifying what appear to be strong connections between tau and ApoE that we had no idea about before,” Dr. Wolfe said in an email. “While independent confirmation is needed, this new work is coming from a strong research team that has made other seminal discoveries in the field. Uncovering the molecular basis for ApoE’s effect on tau pathology and glial cell activation may suggest new targets for drug discovery for AD.”
Evidence of ApoE4’s greater impact
To examine ApoE’s effect on tau, the research team bred new lines of genetically modified mice, all of which expressed human tau. Some also expressed human ApoE4, E3, or E2 in place of mouse ApoE. A comparator mouse expressed tau, but not ApoE.
By the time the mice were 9 months old, the tau-E4 strain showed significantly more brain atrophy than did the tau-E3 and tau-E2 strains. The mice who expressed tau but were free of ApoE showed no brain changes.
A closer look showed that atrophy occurred primarily in areas associated with the cognitive changes seen in dementia: the hippocampus, piriform/entorhinal cortex, and amygdala. The ventricles were also enlarged.
“These results revealed an important role of ApoE in regulating tau-mediated neurodegeneration, with ApoE4 causing more severe damage and the absence of ApoE being protective,” the investigators wrote.
The E4/tau tango started early, too, the team noted. At 3 months old, tau-E4 mice had no obvious brain atrophy, but already had significantly more soluble tau than did any of the other strains. By 9 months, when tauopathy was obvious, the tau-E4 mice still had more of the protein, which had shifted from a soluble to an insoluble and hyperphosphorylated state. The tau-E4 mice didn’t appear to be making more tau than the others, though; rather, they were less able to clear it through the neurons’ clearing and recycling system of autophagy.
Drilling down further into the neurons’ pathophysiology, the team found that tau first appeared in the axons of dentate gyrus granule cells in the hippocampus, and then, at an early age, moved into the cell body. Again, there were APOE allele–specific patterns to tau propagation. The team saw four major tau staining patterns, which correlated with the level of brain atrophy. Types 1 and 2, associated with least atrophy, occurred most often in the tau-only, ApoE-negative mice; type 4, associated with the greatest atrophy, occurred most often in the tau-E4 mice.
“The featured distribution of these ... patterns, which either represent different tau structures or progressively more advanced pathological tau stages, indicate ApoE affects either tau conformation or tau pathology progression,” the investigators wrote.
Greatest neurodegeneration seen with ApoE4
Tau-mediated neurodegeneration initiated levels of inflammatory response that also depended on the type of ApoE isoform. Exposure to a culture of damaged neurons and mixed glial cells caused microglia to release a flood of inflammatory cytokines that called in a host of astrocytes to kill damaged tau-E4 neurons en masse, but attacked the tau-E3, tau-E2, and tau-only strains much less. This finding indicates that “ApoE itself was directly involved in inducing neurotoxicity in tau-expressing susceptible neurons,” the team wrote.
Finally, they investigated this model of neurodegeneration in postmortem brain samples of patients with corticobasal degeneration, Pick disease, and progressive supranuclear palsy – the three most common sporadic primary tauopathies. Patients with the E4 allele showed more severe neurodegeneration and a greater interaction of tau pathology and neurodegeneration. Amyloid deposition was associated with less severe neurodegeneration.
Taken together, the findings strongly suggest that the high-risk APOE4 allele is the linchpin that links neuroinflammation to neuronal death in the setting of tau pathology, the investigators concluded.
“The presence of degenerating neurons appeared to further induce neuroinflammation, which was augmented by ApoE4 owing to its inherently higher innate immune reactivity. While activated microglia may be protective to some extent in the setting of amyloid-beta pathology, by targeting plaques and reducing dystrophic neurites, they could be deleterious in tauopathy by directly targeting injured neurons and by activating toxic astrocytes. Enhanced neuroinflammation associated with ApoE4 may further exacerbate neurodegeneration.”
The study was funded by grants awarded to multiple investigators by the National Institutes of Health, the JPB Foundation, Cure Alzheimer’s Fund, AstraZeneca, the Consortium for Frontotemporal Dementia Research, the Tau Consortium, the National Multiple Sclerosis Society, the Nancy Davis Foundation, and the Amyotrophic Lateral Sclerosis Association. Dr. Holtzman cofounded and is on the scientific advisory board of C2N Diagnostics. He consults for Genentech, AbbVie, Eli Lilly, Proclara, GlaxoSmithKline, and Denali. Dr. Holtzman’s lab is funded by institutional research grants from C2N Diagnostics, Eli Lilly, AbbVie, and Denali.
[email protected]
On Twitter @Alz_Gal
FROM NATURE
Key clinical point:
Major finding: The presence of the e4 allele was associated with brain atrophy and neurodegeneration in a transgenic mouse model, while the absence of any APOE allele was neuroprotective.
Data source: The in vivo study employed transgenic mice that expressed the human tau protein along with human ApoE variants.
Disclosures: The study was funded by grants awarded to multiple investigators by the National Institutes of Health, the JPB Foundation, Cure Alzheimer’s Fund, AstraZeneca, the Consortium for Frontotemporal Dementia Research, the Tau Consortium, the National Multiple Sclerosis Society, the Nancy Davis Foundation, and the Amyotrophic Lateral Sclerosis Association. Dr. Holtzman cofounded and is on the scientific advisory board of C2N Diagnostics. He consults for Genentech, AbbVie, Eli Lilly, Proclara, GlaxoSmithKline, and Denali. Dr. Holtzman’s lab is funded by institutional research grants from C2N Diagnostics, Eli Lilly, AbbVie, and Denali.
U.S. House passes 20-week abortion ban
The U.S. House of Representatives has passed legislation that would ban abortions starting at 20 weeks.
This isn’t the first time in recent years that the Republican-controlled House has passed a 20-week ban. What’s different this time is that it has the support of the White House. Despite the Trump administration’s support for the bill, it’s unlikely to garner the support necessary to come up for a vote in the U.S. Senate.
The American Congress of Obstetricians and Gynecologists (ACOG), which opposes the bill, called it a “cruel ban” that would leave many women without treatment options.
“Many women seek abortion later in pregnancy because restrictive state laws or the lack of abortion providers made it impossible for them to access abortion earlier in their pregnancies,” ACOG said in a statement. “Additionally, many women are delayed in their ability to access abortion care because they need time to raise or save enough money to pay for it.”
Currently, 17 states have enacted their own 20-week abortion bans, according to ACOG.
[email protected]
On Twitter @maryellenny
The U.S. House of Representatives has passed legislation that would ban abortions starting at 20 weeks.
This isn’t the first time in recent years that the Republican-controlled House has passed a 20-week ban. What’s different this time is that it has the support of the White House. Despite the Trump administration’s support for the bill, it’s unlikely to garner the support necessary to come up for a vote in the U.S. Senate.
The American Congress of Obstetricians and Gynecologists (ACOG), which opposes the bill, called it a “cruel ban” that would leave many women without treatment options.
“Many women seek abortion later in pregnancy because restrictive state laws or the lack of abortion providers made it impossible for them to access abortion earlier in their pregnancies,” ACOG said in a statement. “Additionally, many women are delayed in their ability to access abortion care because they need time to raise or save enough money to pay for it.”
Currently, 17 states have enacted their own 20-week abortion bans, according to ACOG.
[email protected]
On Twitter @maryellenny
The U.S. House of Representatives has passed legislation that would ban abortions starting at 20 weeks.
This isn’t the first time in recent years that the Republican-controlled House has passed a 20-week ban. What’s different this time is that it has the support of the White House. Despite the Trump administration’s support for the bill, it’s unlikely to garner the support necessary to come up for a vote in the U.S. Senate.
The American Congress of Obstetricians and Gynecologists (ACOG), which opposes the bill, called it a “cruel ban” that would leave many women without treatment options.
“Many women seek abortion later in pregnancy because restrictive state laws or the lack of abortion providers made it impossible for them to access abortion earlier in their pregnancies,” ACOG said in a statement. “Additionally, many women are delayed in their ability to access abortion care because they need time to raise or save enough money to pay for it.”
Currently, 17 states have enacted their own 20-week abortion bans, according to ACOG.
[email protected]
On Twitter @maryellenny
What can happen if you fail to check the PDMP?
Nearly all states now have a prescription drug monitoring program (PDMP) that requires physicians to report patient data and track patient histories before prescribing controlled substances, but who is watching to ensure such reporting occurs, and what can happen if physicians fail to check the database?
The answer depends on the state.
The agencies that monitor state PDMPs vary from medical boards or health departments to pharmacy boards and law enforcement agencies. How and when the data can be accessed and by whom depends on the jurisdiction.
“States vary on who can access the information,” said Natalia Mazina, a San Francisco–based attorney who specializes in health and pharmacy law. In some cases, only licensing boards can access the data. In other states, only mental health professionals, the department of corrections, or insurers can access the data, she said.
Complaints that lead to investigations are a top reason that licensing or regulatory boards may review PDMP patient data (or lack thereof) from a specific physician. In many states, law enforcement agencies also can review PDMP data if there is an active investigation involving a health provider or patient.
In at least 10 states, law enforcement officers must have probable cause, a search warrant, or a subpoena to search the database, according to a state summary by the National Alliance for Model State Drug Laws. In Oklahoma and Virginia, judicial officials can review PDMP data only with approval from a grand jury.
Some states, such as Arizona, New Mexico, North Dakota, and Wyoming, allow unsolicited PDMP reports to prescribers, dispensers, licensing boards, and law enforcement agencies, while other states allow unsolicited reports only to one entity or a smaller combination. Other groups that may be able to access PDMP information per state law include:
• State Medicaid programs for Medicaid member or provider reviews.
• State medical examiners or coroners for cause-of-death investigations.
• Research organizations that may be provided de-identified data for analysis and research.
• Patients who are the subject of data and parents of minors prescribed controlled substances.
Case law shapes PDMP access
Recent court rulings also are shaping when PDMP data can be accessed and how physicians can come under the radar of state and federal authorities.
For example, the California Supreme Court ruled in June that a medical board was justified in obtaining a physician’s prescription records through the state’s PDMP without a warrant or subpoena. The case started when a patient made a complaint against Burbank, Calif.–based internist Alwin Carl Lewis, MD, regarding medical advice he offered her about losing weight. The complaint was not related to the prescribing of controlled substances. During the course of the investigation, a Medical Board of California investigator obtained a prescriber report on Dr. Lewis from the state’s CURES database, which contained prescription information for hundreds of his patients.
As a result of the investigation, the medical board charged Dr. Lewis with several violations related to the original patient who complained, as well as five additional patients who were prescribed controlled substances. Charges included unprofessional conduct, prescribing dangerous drugs without an appropriate examination, excessive prescribing, and failure to maintain adequate and accurate medical records, according to court documents.
Dr. Lewis sued the state, arguing that the medical board violated his patients’ privacy rights by obtaining the CURES reports without a warrant, subpoena, or good cause. A lower court found in favor of the board and the state supreme court agreed, ruling the government’s need to protect public safety outweighed any intrusion into privacy.
A similar decision was recently issued in Oregon when the 9th U.S. Circuit Court of Appeals ruled that the federal Drug Enforcement Administration (DEA) does not need a warrant to subpoena prescription drug information from Oregon’s PDMP.
In that case, the DEA sought patient-specific information from Oregon’s PDMP through the use of a federal administrative subpoena, which does not involve judicial review or a showing of probable cause. The Oregon PDMP refused to comply on the grounds that doing so violates Oregon state law, which requires a court order based on probable cause before patient data in the PDMP can be disclosed. Oregon internist James Roe, MD, and several patients sued the DEA, arguing that the request violated patient privacy rights, but the appeals court in June ruled that federal law that grants subpoena power to the agency trumps Oregon law, which requires a court order.
In a court brief, the American Medical Association and eight other medical associations expressed concern that allowing the DEA access to PDMP records without a court order violates patient privacy and jeopardizes the integrity of the patient-physician relationship.
“To the extent that the Drug Enforcement Administration asserts an unfettered right to access data from the PDMP without probable cause or judicial oversight and approval, that not only takes improper advantage of the health care data system – which by its terms in Oregon prohibits such access – but undermines the health care purposes that the state PDMPs were set up to serve,” the associations stated in a court brief.
PDMP violations – what happens next?
Failing to register, report to, or check your state’s PDMP can result in a license suspension, disciplinary action by a state agency, or potential criminal charges if the violation is serious enough.
“Their license is at stake, [and] their business is at stake,” Ms. Mazina said. “Overprescribing [and] overdispensing is the number one reason for administrative actions.”
Physicians also may face civil fines for failing to register with their state PDMP or for violating program regulations. In Kentucky, for example, doctors can be fined $250 for each prescription written while the doctor is not properly registered. In Maine, prescribers and dispensers face a civil fine of up to $5,000 for violating PDMP rules. Violations that lead to criminal investigations also may result in arrest or prosecution by federal authorities.
Education is key to avoiding PDMP penalties and ensuring compliance with state rules, Ms. Mazina stressed. Learn your state’s PDMP regulations, ensure staff members are trained on the proper protocols, and keep up to date on changes.
[email protected] On Twitter @legal_med
Nearly all states now have a prescription drug monitoring program (PDMP) that requires physicians to report patient data and track patient histories before prescribing controlled substances, but who is watching to ensure such reporting occurs, and what can happen if physicians fail to check the database?
The answer depends on the state.
The agencies that monitor state PDMPs vary from medical boards or health departments to pharmacy boards and law enforcement agencies. How and when the data can be accessed and by whom depends on the jurisdiction.
“States vary on who can access the information,” said Natalia Mazina, a San Francisco–based attorney who specializes in health and pharmacy law. In some cases, only licensing boards can access the data. In other states, only mental health professionals, the department of corrections, or insurers can access the data, she said.
Complaints that lead to investigations are a top reason that licensing or regulatory boards may review PDMP patient data (or lack thereof) from a specific physician. In many states, law enforcement agencies also can review PDMP data if there is an active investigation involving a health provider or patient.
In at least 10 states, law enforcement officers must have probable cause, a search warrant, or a subpoena to search the database, according to a state summary by the National Alliance for Model State Drug Laws. In Oklahoma and Virginia, judicial officials can review PDMP data only with approval from a grand jury.
Some states, such as Arizona, New Mexico, North Dakota, and Wyoming, allow unsolicited PDMP reports to prescribers, dispensers, licensing boards, and law enforcement agencies, while other states allow unsolicited reports only to one entity or a smaller combination. Other groups that may be able to access PDMP information per state law include:
• State Medicaid programs for Medicaid member or provider reviews.
• State medical examiners or coroners for cause-of-death investigations.
• Research organizations that may be provided de-identified data for analysis and research.
• Patients who are the subject of data and parents of minors prescribed controlled substances.
Case law shapes PDMP access
Recent court rulings also are shaping when PDMP data can be accessed and how physicians can come under the radar of state and federal authorities.
For example, the California Supreme Court ruled in June that a medical board was justified in obtaining a physician’s prescription records through the state’s PDMP without a warrant or subpoena. The case started when a patient made a complaint against Burbank, Calif.–based internist Alwin Carl Lewis, MD, regarding medical advice he offered her about losing weight. The complaint was not related to the prescribing of controlled substances. During the course of the investigation, a Medical Board of California investigator obtained a prescriber report on Dr. Lewis from the state’s CURES database, which contained prescription information for hundreds of his patients.
As a result of the investigation, the medical board charged Dr. Lewis with several violations related to the original patient who complained, as well as five additional patients who were prescribed controlled substances. Charges included unprofessional conduct, prescribing dangerous drugs without an appropriate examination, excessive prescribing, and failure to maintain adequate and accurate medical records, according to court documents.
Dr. Lewis sued the state, arguing that the medical board violated his patients’ privacy rights by obtaining the CURES reports without a warrant, subpoena, or good cause. A lower court found in favor of the board and the state supreme court agreed, ruling the government’s need to protect public safety outweighed any intrusion into privacy.
A similar decision was recently issued in Oregon when the 9th U.S. Circuit Court of Appeals ruled that the federal Drug Enforcement Administration (DEA) does not need a warrant to subpoena prescription drug information from Oregon’s PDMP.
In that case, the DEA sought patient-specific information from Oregon’s PDMP through the use of a federal administrative subpoena, which does not involve judicial review or a showing of probable cause. The Oregon PDMP refused to comply on the grounds that doing so violates Oregon state law, which requires a court order based on probable cause before patient data in the PDMP can be disclosed. Oregon internist James Roe, MD, and several patients sued the DEA, arguing that the request violated patient privacy rights, but the appeals court in June ruled that federal law that grants subpoena power to the agency trumps Oregon law, which requires a court order.
In a court brief, the American Medical Association and eight other medical associations expressed concern that allowing the DEA access to PDMP records without a court order violates patient privacy and jeopardizes the integrity of the patient-physician relationship.
“To the extent that the Drug Enforcement Administration asserts an unfettered right to access data from the PDMP without probable cause or judicial oversight and approval, that not only takes improper advantage of the health care data system – which by its terms in Oregon prohibits such access – but undermines the health care purposes that the state PDMPs were set up to serve,” the associations stated in a court brief.
PDMP violations – what happens next?
Failing to register, report to, or check your state’s PDMP can result in a license suspension, disciplinary action by a state agency, or potential criminal charges if the violation is serious enough.
“Their license is at stake, [and] their business is at stake,” Ms. Mazina said. “Overprescribing [and] overdispensing is the number one reason for administrative actions.”
Physicians also may face civil fines for failing to register with their state PDMP or for violating program regulations. In Kentucky, for example, doctors can be fined $250 for each prescription written while the doctor is not properly registered. In Maine, prescribers and dispensers face a civil fine of up to $5,000 for violating PDMP rules. Violations that lead to criminal investigations also may result in arrest or prosecution by federal authorities.
Education is key to avoiding PDMP penalties and ensuring compliance with state rules, Ms. Mazina stressed. Learn your state’s PDMP regulations, ensure staff members are trained on the proper protocols, and keep up to date on changes.
[email protected] On Twitter @legal_med
Nearly all states now have a prescription drug monitoring program (PDMP) that requires physicians to report patient data and track patient histories before prescribing controlled substances, but who is watching to ensure such reporting occurs, and what can happen if physicians fail to check the database?
The answer depends on the state.
The agencies that monitor state PDMPs vary from medical boards or health departments to pharmacy boards and law enforcement agencies. How and when the data can be accessed and by whom depends on the jurisdiction.
“States vary on who can access the information,” said Natalia Mazina, a San Francisco–based attorney who specializes in health and pharmacy law. In some cases, only licensing boards can access the data. In other states, only mental health professionals, the department of corrections, or insurers can access the data, she said.
Complaints that lead to investigations are a top reason that licensing or regulatory boards may review PDMP patient data (or lack thereof) from a specific physician. In many states, law enforcement agencies also can review PDMP data if there is an active investigation involving a health provider or patient.
In at least 10 states, law enforcement officers must have probable cause, a search warrant, or a subpoena to search the database, according to a state summary by the National Alliance for Model State Drug Laws. In Oklahoma and Virginia, judicial officials can review PDMP data only with approval from a grand jury.
Some states, such as Arizona, New Mexico, North Dakota, and Wyoming, allow unsolicited PDMP reports to prescribers, dispensers, licensing boards, and law enforcement agencies, while other states allow unsolicited reports only to one entity or a smaller combination. Other groups that may be able to access PDMP information per state law include:
• State Medicaid programs for Medicaid member or provider reviews.
• State medical examiners or coroners for cause-of-death investigations.
• Research organizations that may be provided de-identified data for analysis and research.
• Patients who are the subject of data and parents of minors prescribed controlled substances.
Case law shapes PDMP access
Recent court rulings also are shaping when PDMP data can be accessed and how physicians can come under the radar of state and federal authorities.
For example, the California Supreme Court ruled in June that a medical board was justified in obtaining a physician’s prescription records through the state’s PDMP without a warrant or subpoena. The case started when a patient made a complaint against Burbank, Calif.–based internist Alwin Carl Lewis, MD, regarding medical advice he offered her about losing weight. The complaint was not related to the prescribing of controlled substances. During the course of the investigation, a Medical Board of California investigator obtained a prescriber report on Dr. Lewis from the state’s CURES database, which contained prescription information for hundreds of his patients.
As a result of the investigation, the medical board charged Dr. Lewis with several violations related to the original patient who complained, as well as five additional patients who were prescribed controlled substances. Charges included unprofessional conduct, prescribing dangerous drugs without an appropriate examination, excessive prescribing, and failure to maintain adequate and accurate medical records, according to court documents.
Dr. Lewis sued the state, arguing that the medical board violated his patients’ privacy rights by obtaining the CURES reports without a warrant, subpoena, or good cause. A lower court found in favor of the board and the state supreme court agreed, ruling the government’s need to protect public safety outweighed any intrusion into privacy.
A similar decision was recently issued in Oregon when the 9th U.S. Circuit Court of Appeals ruled that the federal Drug Enforcement Administration (DEA) does not need a warrant to subpoena prescription drug information from Oregon’s PDMP.
In that case, the DEA sought patient-specific information from Oregon’s PDMP through the use of a federal administrative subpoena, which does not involve judicial review or a showing of probable cause. The Oregon PDMP refused to comply on the grounds that doing so violates Oregon state law, which requires a court order based on probable cause before patient data in the PDMP can be disclosed. Oregon internist James Roe, MD, and several patients sued the DEA, arguing that the request violated patient privacy rights, but the appeals court in June ruled that federal law that grants subpoena power to the agency trumps Oregon law, which requires a court order.
In a court brief, the American Medical Association and eight other medical associations expressed concern that allowing the DEA access to PDMP records without a court order violates patient privacy and jeopardizes the integrity of the patient-physician relationship.
“To the extent that the Drug Enforcement Administration asserts an unfettered right to access data from the PDMP without probable cause or judicial oversight and approval, that not only takes improper advantage of the health care data system – which by its terms in Oregon prohibits such access – but undermines the health care purposes that the state PDMPs were set up to serve,” the associations stated in a court brief.
PDMP violations – what happens next?
Failing to register, report to, or check your state’s PDMP can result in a license suspension, disciplinary action by a state agency, or potential criminal charges if the violation is serious enough.
“Their license is at stake, [and] their business is at stake,” Ms. Mazina said. “Overprescribing [and] overdispensing is the number one reason for administrative actions.”
Physicians also may face civil fines for failing to register with their state PDMP or for violating program regulations. In Kentucky, for example, doctors can be fined $250 for each prescription written while the doctor is not properly registered. In Maine, prescribers and dispensers face a civil fine of up to $5,000 for violating PDMP rules. Violations that lead to criminal investigations also may result in arrest or prosecution by federal authorities.
Education is key to avoiding PDMP penalties and ensuring compliance with state rules, Ms. Mazina stressed. Learn your state’s PDMP regulations, ensure staff members are trained on the proper protocols, and keep up to date on changes.
[email protected] On Twitter @legal_med
Prescription drug monitoring programs improve, but challenges remain
When Wisconsin introduced its first prescription drug monitoring program (PDMP) system in 2013, doctors found the system clunky and cumbersome to navigate, recalled Noel Deep, MD, an Antigo, Wis.–based internist and president of the Wisconsin Medical Society.
Physicians had to click through several screens and were then directed to log into another website to enter patient information and scan records.
The state’s PDMP is much improved today, Dr. Deep said. The Wisconsin Department of Safety and Professional Services launched an enhanced version of the PDMP in January, giving doctors time to learn the new system before its use became mandatory for prescribers in April. The system takes fewer clicks and communicates with practices’ electronic medical record.
“It is very easy, compared with what it was before,” Dr. Deep said in an interview. “I was one of those people who were skeptical, but I’ve been happy with the PDMP. It’s extremely quick. I know it’s a few more clicks, but it has also shown that, in Wisconsin, this has significantly decreased the use of prescription [opioid] medications.”
Across the country, physicians are experiencing similar ups and downs with state PDMPs as they work to manage the systems, juggle patient caseloads, and make the best prescribing decisions. Currently, 49 states, the District of Columbia, and Guam have operational PDMPs. Most databases generally require that Schedule II, III, and IV prescriptions be reported, explained Natalia Mazina, a San Francisco–based attorney who specializes in health and pharmacy law.
“Some states also require Schedule V, and some states even require certain noncontrolled substances to be reported,” she said in an interview. “That’s the biggest difference. There are also different agencies responsible for enforcement.”
States differ in the time allowed for prescribers and dispensers to report data, Ms. Mazina added. States such as New York and Utah, for instance, require real-time reporting, while Alabama and Louisiana require daily PDMP reporting. South Dakota requires reporting within 7 days of a prescription, while Alaska allows for monthly reporting. Training hours required for PDMP participation also vary by state.
More states are moving toward mandatory rather than voluntary PDMP systems. At least 34 states now specify circumstances in which prescribers, dispensers, or both must access a patient’s PDMP prescription history, according to a summary by the National Alliance for Model State Drug Laws. For doctors, the push toward mandated PDMPs has generated mixed feelings and led to legislative battles in some states.
“From a safety standpoint, [PDMPs are] a good thing,” Dr. Deep said. “From a physician standpoint, [some] people feel this is restricting the physician-patient relationship and dictating how much we can prescribe, when we can prescribe, and what we can do. That’s one of the downsides.”
Beneficial or burdensome?
When Georgia legislators proposed a law earlier this year that would tighten reporting requirements for their state’s PDMP, physician leaders fought back against what they viewed as excessive regulations.
An initial bill included reporting requirements not only for standard controlled substances but for stimulants such as Adderall, Vyvanse, Focalin, and Ritalin, and all other nonopioid controlled drugs. The early version of the bill also recommended civil and criminal penalties for physicians who violated the regulations.
The American College of Physicians Georgia Chapter and the Medical Association of Georgia successfully advocated for the two provisions to be removed from the bill’s final version, said W. Cody McClatchey, MD, chair of the ACP Georgia Chapter’s health and public policy committee.
“We are in the midst of an opioid epidemic,” Dr. McClatchey said in an interview. “It would have been unreasonable and costly for state government to mandate that prescribers check PDMP for controlled drugs not related to the opioid epidemic. [In addition], I felt strongly that prescribers should not be subject to criminal penalties for not checking the PDMP. We are already subject to civil and criminal penalties for intentionally or knowingly overprescribing controlled drugs. That is adequate protection for patients.”
Georgia’s law, signed in May, requires physicians and up to two certified staff to seek and review information from the PDMP every 90 days for any prescription outlined in the law that exceeds 3 days/26 pills for medical care, or 10 days/40 pills for surgical care. In addition, physicians must make a notation in the patient’s medical record stating the date and time upon which such inquiry was made, among other requirements.
The new requirements mean it will take longer to manage patients with chronic pain, anxiety, and depression who may need opioids and benzodiazepines because of the time necessary to access the PDMP, document that it was reviewed, and properly counsel the patient, Dr. McClatchey said. However, he noted that the additional time may allow physicians to charge a higher level of evaluation and management services. Doctors can minimize the impact of the rules by delegating many of the tasks to certified medical assistants and using “smartphrases” to document completion, he said.
“In my opinion, the final version of HB 249 strikes a fair balance between the needs of patients and the administrative burden on physicians,” he said. “Most physicians do not prescribe opioids and benzodiazepines on a continuing basis to many patients. Physicians who prescribe chronic opioids or benzodiazepines now have the ability to more accurately know when patients may be abusing opioids and benzodiazepines, which can be a matter of life or death.”
‘Well-designed regs keep patients safe’
In California, prescribers are encouraged but not yet required to check the Controlled Substance Utilization Review and Evaluation System (CURES) database before prescribing controlled substances. Under state law, checking the database will become mandatory 6 months after the California Department of Justice certifies that the CURES system is ready for statewide use and the department has adequate staff to handle the technical and administrative workload. When that will happen remains unclear.
It’s too early to know how well CURES will work once fully implemented, said Patricia Salber, MD, a Larkspur, Calif.–based internist and founder of the blog TheDoctorWeighsIn.com, but access to statewide data about patients’ medical and drug history is a positive for doctors.
“As a former emergency physician who has taken care of many drug-seeking patients, having access to statewide data about an individual’s drug use will be a valuable tool to help stem the tide of scheduled prescription drug abuse,” she said. “Given our mobile society, I would also like to eventually see a nationwide system.”
As new systems roll out, it’s important for physicians to give the databases a chance and advocate fixing the bugs, rather than condemning them because of logistics or initial glitches, Dr. Salber added.

“I think sometimes people blame rules and regulations for making their lives difficult, when in fact it is clunky design and implementation of the regulations that cause the problem,” she said. “Well-designed regulations can keep our patients safe, for example, by requiring adequate testing of the safety and efficacy of therapeutics. If regulations are found to be effective but burdensome, I believe the first response should not be to overturn the regulation, but rather to improve the way the regulation is carried out.”
For example, she noted that the California Medical Association successfully fought for state law language requiring the CURES process to be certified ready before requiring physicians to use the system.
Are PDMPs working?
Although PDMPs may be causing headaches for some, data show that they are having effective results against opioid abuse and overprescribing.
In Florida, opioid prescriptions decreased in 80% of counties from 2010 to 2015 after the state established a PDMP in combination with tighter regulation of pain clinics. In the first month after implementation of Florida’s PDMP, oxycodone deaths dropped by 25%, according to a 2015 study published in Drug and Alcohol Dependence (2015 May 1. doi: 10.1016/j.drugalcdep.2015.02.010).
Opioid prescriptions in Kentucky, New York, and Tennessee dropped after mandates that prescribers check their state PDMPs, according to a summary by the PDMP Center of Excellence at Brandeis University. In Kentucky, doses dispensed declined for hydrocodone (–10.3%), oxycodone (–11.6%), and oxymorphone (–35%), while in Tennessee the number of opioid prescriptions fell by 7%. In New York, total opioid prescriptions have dropped by more than 9% since the state’s PDMP went into effect.
In Wisconsin, an analysis after enactment of the state’s PDMP found a nearly 12% reduction in opioid prescriptions and a 13% reduction in opioid doses dispensed between the fourth quarter of 2015 and the fourth quarter of 2016, according to a report by the Wisconsin Department of Safety and Professional Services.
PDMPs also have altered physicians’ prescribing behaviors and changed patient care decisions, studies show. A review of medical providers in Ohio emergency departments found that 41% of those given PDMP data altered their prescribing for patients receiving multiple simultaneous narcotics prescriptions, according to the Brandeis University summary. Of those Ohio providers, 61% prescribed no narcotics or fewer narcotics than originally planned.
A survey of prescribers in Rhode Island and Connecticut found that those who used PDMP data were more likely than nonusers to take clinically appropriate action in response to suspected cases of prescription drug abuse or diversion by patients, such as conducting drug screens or referring them to substance abuse treatment.
Despite the positive impacts, however, challenges for PDMPs remain.
Dr. Deep noted that physicians in solo and small practices may have a harder time than employed physicians when it comes to checking databases, recording data, and delegating duties. In addition, differing PDMP regulations may not catch prescription drug abusers who go across state lines.
Most states with PDMPs share their PDMP data with other state PDMPs or share data with authorized users in other states. Florida can receive PDMP data from other jurisdictions and provide that data to authorized users in Florida, but it does not share its data with other states. Oregon allows only prescribers in California, Idaho, Nevada, and Washington state to access its database information.
PDMPs also are limited in what they tell physicians about patients, said Gregory A. Hood, MD, an internist in Lexington, Ky., and former governor of the American College of Physicians, Kentucky Chapter.
“PDMP is only helpful to a point,” he said in an interview. “Any PDMP has the inherent limitation that it only reports what is reported to it. This doesn’t tell us about whether the patient actually takes the medicine, gives or sells it away, or whether they use it appropriately or not. Patients can overuse for 3 of 4 weeks, buy a week on the street, or from someone they know, and we’re none the wiser, absent an informant.”
Kentucky’s database, called the Kentucky All Schedule Prescription Electronic Reporting System (KASPER), requires that prescribers and dispensers of controlled substances query the state’s electronic monitoring system before issuing new prescriptions or refills. To track illicit use of opiates, Kentucky also recently made gabapentin a Schedule V controlled substance, Dr. Hood said.
Whether PDMPs have a positive effect depends on what doctors do with the information they learn from the database, Dr. Hood said.
“Generally, PDMPs can help identify at least some who are seeking adverse gain,” he said. “Properly identifying someone with a medical issue and arranging proper care is a positive. Rote dropping of someone with a ‘dirty’ PDMP – as has been known to happen in some primary care and specialty offices – is difficult to view as a positive, particularly given shortages in both primary care and in pain management.”
[email protected] On Twitter @legal_med
When Wisconsin introduced its first prescription drug monitoring program (PDMP) system in 2013, doctors found the system clunky and cumbersome to navigate, recalled Noel Deep, MD, an Antigo, Wis.–based internist and president of the Wisconsin Medical Society.
Physicians had to click through several screens and were then directed to log into another website to enter patient information and scan records.
The state’s PDMP is much improved today, Dr. Deep said. The Wisconsin Department of Safety and Professional Services launched an enhanced version of the PDMP in January, giving doctors time to learn the new system before its use became mandatory for prescribers in April. The system takes fewer clicks and communicates with practices’ electronic medical record.
“It is very easy, compared with what it was before,” Dr. Deep said in an interview. “I was one of those people who were skeptical, but I’ve been happy with the PDMP. It’s extremely quick. I know it’s a few more clicks, but it has also shown that, in Wisconsin, this has significantly decreased the use of prescription [opioid] medications.”
Across the country, physicians are experiencing similar ups and downs with state PDMPs as they work to manage the systems, juggle patient caseloads, and make the best prescribing decisions. Currently, 49 states, the District of Columbia, and Guam have operational PDMPs. Most databases generally require that Schedule II, III, and IV prescriptions be reported, explained Natalia Mazina, a San Francisco–based attorney who specializes in health and pharmacy law.
“Some states also require Schedule V, and some states even require certain noncontrolled substances to be reported,” she said in an interview. “That’s the biggest difference. There are also different agencies responsible for enforcement.”
States differ in the time allowed for prescribers and dispensers to report data, Ms. Mazina added. States such as New York and Utah, for instance, require real-time reporting, while Alabama and Louisiana require daily PDMP reporting. South Dakota requires reporting within 7 days of a prescription, while Alaska allows for monthly reporting. Training hours required for PDMP participation also vary by state.
More states are moving toward mandatory rather than voluntary PDMP systems. At least 34 states now specify circumstances in which prescribers, dispensers, or both must access a patient’s PDMP prescription history, according to a summary by the National Alliance for Model State Drug Laws. For doctors, the push toward mandated PDMPs has generated mixed feelings and led to legislative battles in some states.
“From a safety standpoint, [PDMPs are] a good thing,” Dr. Deep said. “From a physician standpoint, [some] people feel this is restricting the physician-patient relationship and dictating how much we can prescribe, when we can prescribe, and what we can do. That’s one of the downsides.”
Beneficial or burdensome?
When Georgia legislators proposed a law earlier this year that would tighten reporting requirements for their state’s PDMP, physician leaders fought back against what they viewed as excessive regulations.
An initial bill included reporting requirements not only for standard controlled substances but for stimulants such as Adderall, Vyvanse, Focalin, and Ritalin, and all other nonopioid controlled drugs. The early version of the bill also recommended civil and criminal penalties for physicians who violated the regulations.
The American College of Physicians Georgia Chapter and the Medical Association of Georgia successfully advocated for the two provisions to be removed from the bill’s final version, said W. Cody McClatchey, MD, chair of the ACP Georgia Chapter’s health and public policy committee.
“We are in the midst of an opioid epidemic,” Dr. McClatchey said in an interview. “It would have been unreasonable and costly for state government to mandate that prescribers check PDMP for controlled drugs not related to the opioid epidemic. [In addition], I felt strongly that prescribers should not be subject to criminal penalties for not checking the PDMP. We are already subject to civil and criminal penalties for intentionally or knowingly overprescribing controlled drugs. That is adequate protection for patients.”
Georgia’s law, signed in May, requires physicians and up to two certified staff to seek and review information from the PDMP every 90 days for any prescription outlined in the law that exceeds 3 days/26 pills for medical care, or 10 days/40 pills for surgical care. In addition, physicians must make a notation in the patient’s medical record stating the date and time upon which such inquiry was made, among other requirements.
The new requirements mean it will take longer to manage patients with chronic pain, anxiety, and depression who may need opioids and benzodiazepines because of the time necessary to access the PDMP, document that it was reviewed, and properly counsel the patient, Dr. McClatchey said. However, he noted that the additional time may allow physicians to charge a higher level of evaluation and management services. Doctors can minimize the impact of the rules by delegating many of the tasks to certified medical assistants and using “smartphrases” to document completion, he said.
“In my opinion, the final version of HB 249 strikes a fair balance between the needs of patients and the administrative burden on physicians,” he said. “Most physicians do not prescribe opioids and benzodiazepines on a continuing basis to many patients. Physicians who prescribe chronic opioids or benzodiazepines now have the ability to more accurately know when patients may be abusing opioids and benzodiazepines, which can be a matter of life or death.”
‘Well-designed regs keep patients safe’
In California, prescribers are encouraged but not yet required to check the Controlled Substance Utilization Review and Evaluation System (CURES) database before prescribing controlled substances. Under state law, checking the database will become mandatory 6 months after the California Department of Justice certifies that the CURES system is ready for statewide use and the department has adequate staff to handle the technical and administrative workload. When that will happen remains unclear.
It’s too early to know how well CURES will work once fully implemented, said Patricia Salber, MD, a Larkspur, Calif.–based internist and founder of the blog TheDoctorWeighsIn.com, but access to statewide data about patients’ medical and drug history is a positive for doctors.
“As a former emergency physician who has taken care of many drug-seeking patients, having access to statewide data about an individual’s drug use will be a valuable tool to help stem the tide of scheduled prescription drug abuse,” she said. “Given our mobile society, I would also like to eventually see a nationwide system.”
As new systems roll out, it’s important for physicians to give the databases a chance and advocate fixing the bugs, rather than condemning them because of logistics or initial glitches, Dr. Salber added.

“I think sometimes people blame rules and regulations for making their lives difficult, when in fact it is clunky design and implementation of the regulations that cause the problem,” she said. “Well-designed regulations can keep our patients safe, for example, by requiring adequate testing of the safety and efficacy of therapeutics. If regulations are found to be effective but burdensome, I believe the first response should not be to overturn the regulation, but rather to improve the way the regulation is carried out.”
For example, she noted that the California Medical Association successfully fought for state law language requiring the CURES process to be certified ready before requiring physicians to use the system.
Are PDMPs working?
Although PDMPs may be causing headaches for some, data show that they are having effective results against opioid abuse and overprescribing.
In Florida, opioid prescriptions decreased in 80% of counties from 2010 to 2015 after the state established a PDMP in combination with tighter regulation of pain clinics. In the first month after implementation of Florida’s PDMP, oxycodone deaths dropped by 25%, according to a 2015 study published in Drug and Alcohol Dependence (2015 May 1. doi: 10.1016/j.drugalcdep.2015.02.010).
Opioid prescriptions in Kentucky, New York, and Tennessee dropped after mandates that prescribers check their state PDMPs, according to a summary by the PDMP Center of Excellence at Brandeis University. In Kentucky, doses dispensed declined for hydrocodone (–10.3%), oxycodone (–11.6%), and oxymorphone (–35%), while in Tennessee the number of opioid prescriptions fell by 7%. In New York, total opioid prescriptions have dropped by more than 9% since the state’s PDMP went into effect.
In Wisconsin, an analysis after enactment of the state’s PDMP found a nearly 12% reduction in opioid prescriptions and a 13% reduction in opioid doses dispensed between the fourth quarter of 2015 and the fourth quarter of 2016, according to a report by the Wisconsin Department of Safety and Professional Services.
PDMPs also have altered physicians’ prescribing behaviors and changed patient care decisions, studies show. A review of medical providers in Ohio emergency departments found that 41% of those given PDMP data altered their prescribing for patients receiving multiple simultaneous narcotics prescriptions, according to the Brandeis University summary. Of those Ohio providers, 61% prescribed no narcotics or fewer narcotics than originally planned.
A survey of prescribers in Rhode Island and Connecticut found that those who used PDMP data were more likely than nonusers to take clinically appropriate action in response to suspected cases of prescription drug abuse or diversion by patients, such as conducting drug screens or referring them to substance abuse treatment.
Despite the positive impacts, however, challenges for PDMPs remain.
Dr. Deep noted that physicians in solo and small practices may have a harder time than employed physicians when it comes to checking databases, recording data, and delegating duties. In addition, differing PDMP regulations may not catch prescription drug abusers who go across state lines.
Most states with PDMPs share their PDMP data with other state PDMPs or share data with authorized users in other states. Florida can receive PDMP data from other jurisdictions and provide that data to authorized users in Florida, but it does not share its data with other states. Oregon allows only prescribers in California, Idaho, Nevada, and Washington state to access its database information.
PDMPs also are limited in what they tell physicians about patients, said Gregory A. Hood, MD, an internist in Lexington, Ky., and former governor of the American College of Physicians, Kentucky Chapter.
“PDMP is only helpful to a point,” he said in an interview. “Any PDMP has the inherent limitation that it only reports what is reported to it. This doesn’t tell us about whether the patient actually takes the medicine, gives or sells it away, or whether they use it appropriately or not. Patients can overuse for 3 of 4 weeks, buy a week on the street, or from someone they know, and we’re none the wiser, absent an informant.”
Kentucky’s database, called the Kentucky All Schedule Prescription Electronic Reporting System (KASPER), requires that prescribers and dispensers of controlled substances query the state’s electronic monitoring system before issuing new prescriptions or refills. To track illicit use of opiates, Kentucky also recently made gabapentin a Schedule V controlled substance, Dr. Hood said.
Whether PDMPs have a positive effect depends on what doctors do with the information they learn from the database, Dr. Hood said.
“Generally, PDMPs can help identify at least some who are seeking adverse gain,” he said. “Properly identifying someone with a medical issue and arranging proper care is a positive. Rote dropping of someone with a ‘dirty’ PDMP – as has been known to happen in some primary care and specialty offices – is difficult to view as a positive, particularly given shortages in both primary care and in pain management.”
[email protected] On Twitter @legal_med
When Wisconsin introduced its first prescription drug monitoring program (PDMP) system in 2013, doctors found the system clunky and cumbersome to navigate, recalled Noel Deep, MD, an Antigo, Wis.–based internist and president of the Wisconsin Medical Society.
Physicians had to click through several screens and were then directed to log into another website to enter patient information and scan records.
The state’s PDMP is much improved today, Dr. Deep said. The Wisconsin Department of Safety and Professional Services launched an enhanced version of the PDMP in January, giving doctors time to learn the new system before its use became mandatory for prescribers in April. The system takes fewer clicks and communicates with practices’ electronic medical record.
“It is very easy, compared with what it was before,” Dr. Deep said in an interview. “I was one of those people who were skeptical, but I’ve been happy with the PDMP. It’s extremely quick. I know it’s a few more clicks, but it has also shown that, in Wisconsin, this has significantly decreased the use of prescription [opioid] medications.”
Across the country, physicians are experiencing similar ups and downs with state PDMPs as they work to manage the systems, juggle patient caseloads, and make the best prescribing decisions. Currently, 49 states, the District of Columbia, and Guam have operational PDMPs. Most databases generally require that Schedule II, III, and IV prescriptions be reported, explained Natalia Mazina, a San Francisco–based attorney who specializes in health and pharmacy law.
“Some states also require Schedule V, and some states even require certain noncontrolled substances to be reported,” she said in an interview. “That’s the biggest difference. There are also different agencies responsible for enforcement.”
States differ in the time allowed for prescribers and dispensers to report data, Ms. Mazina added. States such as New York and Utah, for instance, require real-time reporting, while Alabama and Louisiana require daily PDMP reporting. South Dakota requires reporting within 7 days of a prescription, while Alaska allows for monthly reporting. Training hours required for PDMP participation also vary by state.
More states are moving toward mandatory rather than voluntary PDMP systems. At least 34 states now specify circumstances in which prescribers, dispensers, or both must access a patient’s PDMP prescription history, according to a summary by the National Alliance for Model State Drug Laws. For doctors, the push toward mandated PDMPs has generated mixed feelings and led to legislative battles in some states.
“From a safety standpoint, [PDMPs are] a good thing,” Dr. Deep said. “From a physician standpoint, [some] people feel this is restricting the physician-patient relationship and dictating how much we can prescribe, when we can prescribe, and what we can do. That’s one of the downsides.”
Beneficial or burdensome?
When Georgia legislators proposed a law earlier this year that would tighten reporting requirements for their state’s PDMP, physician leaders fought back against what they viewed as excessive regulations.
An initial bill included reporting requirements not only for standard controlled substances but for stimulants such as Adderall, Vyvanse, Focalin, and Ritalin, and all other nonopioid controlled drugs. The early version of the bill also recommended civil and criminal penalties for physicians who violated the regulations.
The American College of Physicians Georgia Chapter and the Medical Association of Georgia successfully advocated for the two provisions to be removed from the bill’s final version, said W. Cody McClatchey, MD, chair of the ACP Georgia Chapter’s health and public policy committee.
“We are in the midst of an opioid epidemic,” Dr. McClatchey said in an interview. “It would have been unreasonable and costly for state government to mandate that prescribers check PDMP for controlled drugs not related to the opioid epidemic. [In addition], I felt strongly that prescribers should not be subject to criminal penalties for not checking the PDMP. We are already subject to civil and criminal penalties for intentionally or knowingly overprescribing controlled drugs. That is adequate protection for patients.”
Georgia’s law, signed in May, requires physicians and up to two certified staff to seek and review information from the PDMP every 90 days for any prescription outlined in the law that exceeds 3 days/26 pills for medical care, or 10 days/40 pills for surgical care. In addition, physicians must make a notation in the patient’s medical record stating the date and time upon which such inquiry was made, among other requirements.
The new requirements mean it will take longer to manage patients with chronic pain, anxiety, and depression who may need opioids and benzodiazepines because of the time necessary to access the PDMP, document that it was reviewed, and properly counsel the patient, Dr. McClatchey said. However, he noted that the additional time may allow physicians to charge a higher level of evaluation and management services. Doctors can minimize the impact of the rules by delegating many of the tasks to certified medical assistants and using “smartphrases” to document completion, he said.
“In my opinion, the final version of HB 249 strikes a fair balance between the needs of patients and the administrative burden on physicians,” he said. “Most physicians do not prescribe opioids and benzodiazepines on a continuing basis to many patients. Physicians who prescribe chronic opioids or benzodiazepines now have the ability to more accurately know when patients may be abusing opioids and benzodiazepines, which can be a matter of life or death.”
‘Well-designed regs keep patients safe’
In California, prescribers are encouraged but not yet required to check the Controlled Substance Utilization Review and Evaluation System (CURES) database before prescribing controlled substances. Under state law, checking the database will become mandatory 6 months after the California Department of Justice certifies that the CURES system is ready for statewide use and the department has adequate staff to handle the technical and administrative workload. When that will happen remains unclear.
It’s too early to know how well CURES will work once fully implemented, said Patricia Salber, MD, a Larkspur, Calif.–based internist and founder of the blog TheDoctorWeighsIn.com, but access to statewide data about patients’ medical and drug history is a positive for doctors.
“As a former emergency physician who has taken care of many drug-seeking patients, having access to statewide data about an individual’s drug use will be a valuable tool to help stem the tide of scheduled prescription drug abuse,” she said. “Given our mobile society, I would also like to eventually see a nationwide system.”
As new systems roll out, it’s important for physicians to give the databases a chance and advocate fixing the bugs, rather than condemning them because of logistics or initial glitches, Dr. Salber added.

“I think sometimes people blame rules and regulations for making their lives difficult, when in fact it is clunky design and implementation of the regulations that cause the problem,” she said. “Well-designed regulations can keep our patients safe, for example, by requiring adequate testing of the safety and efficacy of therapeutics. If regulations are found to be effective but burdensome, I believe the first response should not be to overturn the regulation, but rather to improve the way the regulation is carried out.”
For example, she noted that the California Medical Association successfully fought for state law language requiring the CURES process to be certified ready before requiring physicians to use the system.
Are PDMPs working?
Although PDMPs may be causing headaches for some, data show that they are having effective results against opioid abuse and overprescribing.
In Florida, opioid prescriptions decreased in 80% of counties from 2010 to 2015 after the state established a PDMP in combination with tighter regulation of pain clinics. In the first month after implementation of Florida’s PDMP, oxycodone deaths dropped by 25%, according to a 2015 study published in Drug and Alcohol Dependence (2015 May 1. doi: 10.1016/j.drugalcdep.2015.02.010).
Opioid prescriptions in Kentucky, New York, and Tennessee dropped after mandates that prescribers check their state PDMPs, according to a summary by the PDMP Center of Excellence at Brandeis University. In Kentucky, doses dispensed declined for hydrocodone (–10.3%), oxycodone (–11.6%), and oxymorphone (–35%), while in Tennessee the number of opioid prescriptions fell by 7%. In New York, total opioid prescriptions have dropped by more than 9% since the state’s PDMP went into effect.
In Wisconsin, an analysis after enactment of the state’s PDMP found a nearly 12% reduction in opioid prescriptions and a 13% reduction in opioid doses dispensed between the fourth quarter of 2015 and the fourth quarter of 2016, according to a report by the Wisconsin Department of Safety and Professional Services.
PDMPs also have altered physicians’ prescribing behaviors and changed patient care decisions, studies show. A review of medical providers in Ohio emergency departments found that 41% of those given PDMP data altered their prescribing for patients receiving multiple simultaneous narcotics prescriptions, according to the Brandeis University summary. Of those Ohio providers, 61% prescribed no narcotics or fewer narcotics than originally planned.
A survey of prescribers in Rhode Island and Connecticut found that those who used PDMP data were more likely than nonusers to take clinically appropriate action in response to suspected cases of prescription drug abuse or diversion by patients, such as conducting drug screens or referring them to substance abuse treatment.
Despite the positive impacts, however, challenges for PDMPs remain.
Dr. Deep noted that physicians in solo and small practices may have a harder time than employed physicians when it comes to checking databases, recording data, and delegating duties. In addition, differing PDMP regulations may not catch prescription drug abusers who go across state lines.
Most states with PDMPs share their PDMP data with other state PDMPs or share data with authorized users in other states. Florida can receive PDMP data from other jurisdictions and provide that data to authorized users in Florida, but it does not share its data with other states. Oregon allows only prescribers in California, Idaho, Nevada, and Washington state to access its database information.
PDMPs also are limited in what they tell physicians about patients, said Gregory A. Hood, MD, an internist in Lexington, Ky., and former governor of the American College of Physicians, Kentucky Chapter.
“PDMP is only helpful to a point,” he said in an interview. “Any PDMP has the inherent limitation that it only reports what is reported to it. This doesn’t tell us about whether the patient actually takes the medicine, gives or sells it away, or whether they use it appropriately or not. Patients can overuse for 3 of 4 weeks, buy a week on the street, or from someone they know, and we’re none the wiser, absent an informant.”
Kentucky’s database, called the Kentucky All Schedule Prescription Electronic Reporting System (KASPER), requires that prescribers and dispensers of controlled substances query the state’s electronic monitoring system before issuing new prescriptions or refills. To track illicit use of opiates, Kentucky also recently made gabapentin a Schedule V controlled substance, Dr. Hood said.
Whether PDMPs have a positive effect depends on what doctors do with the information they learn from the database, Dr. Hood said.
“Generally, PDMPs can help identify at least some who are seeking adverse gain,” he said. “Properly identifying someone with a medical issue and arranging proper care is a positive. Rote dropping of someone with a ‘dirty’ PDMP – as has been known to happen in some primary care and specialty offices – is difficult to view as a positive, particularly given shortages in both primary care and in pain management.”
[email protected] On Twitter @legal_med
Smoking and Food Insecurity: How to Solve a Dual Challenge?
Smoking and poor nutrition—2 of the leading preventable causes of death—are reciprocally linked in many ways, multiplying the public health challenges. For instance, smokers are less likely to eat healthful foods and food insecurity is independently associated with smoking. Researchers from the University at Albany, State University of New York, who conducted both a health interview survey and a food environment assessment with 1,917 adults, found that each indicator of food distress was significantly associated with current smoking. Respondents who consumed ≤ 1 serving of fruits and vegetables per day had significantly higher odds of current smoking, compared with those who consumed ≥ 5 servings. Similarly, the odds of current smoking were significantly higher among respondents who were food insecure, used a food pantry, and received Supplemental Nutrition Assistance Program benefits. Living in a neighborhood with low access to healthful food doubled the prevalence of smoking.
Respondents shopped for food often at a corner store (convenience store), dollar store, or drug store. That highlights one of the challenges: All the convenience stores, drug stores, and about 63% of the dollar stores also were tobacco retailers, and nearly all of those had tobacco advertising.
The researchers note that research on the link between smoking and food distress is “limited.” A common explanation for it, they say, is the “opportunity cost” argument. Smokers spend up to 24% of their income on cigarettes—leaving less money for food. Other research also has found that smokers tend to have less appetite than do nonsmokers (smoking may alter hunger-satiety sensation). On the other hand, chronic hunger, imbalanced diet and not having enough money to buy adequate food naturally may cause stress and anxiety and can increase dependence on nicotine. Moreover, food-insecure people may smoke to suppress hunger.
The researchers suggest ways to help solve the problem. One would be to disseminate smoking-related educational materials in food pantries and other community nutrition assistance resources. Another would be to prioritize smoking-cessation interventions for stores in “food deserts.” Only a few policy-based interventions exist, the researchers say. They point to a California city that enacted a citywide “healthy corner store” policy that rewards local small business for offering healthful foods and imposes tobacco-control measures, such as eliminating visible tobacco displays at checkout counters.
Smoking and poor nutrition—2 of the leading preventable causes of death—are reciprocally linked in many ways, multiplying the public health challenges. For instance, smokers are less likely to eat healthful foods and food insecurity is independently associated with smoking. Researchers from the University at Albany, State University of New York, who conducted both a health interview survey and a food environment assessment with 1,917 adults, found that each indicator of food distress was significantly associated with current smoking. Respondents who consumed ≤ 1 serving of fruits and vegetables per day had significantly higher odds of current smoking, compared with those who consumed ≥ 5 servings. Similarly, the odds of current smoking were significantly higher among respondents who were food insecure, used a food pantry, and received Supplemental Nutrition Assistance Program benefits. Living in a neighborhood with low access to healthful food doubled the prevalence of smoking.
Respondents shopped for food often at a corner store (convenience store), dollar store, or drug store. That highlights one of the challenges: All the convenience stores, drug stores, and about 63% of the dollar stores also were tobacco retailers, and nearly all of those had tobacco advertising.
The researchers note that research on the link between smoking and food distress is “limited.” A common explanation for it, they say, is the “opportunity cost” argument. Smokers spend up to 24% of their income on cigarettes—leaving less money for food. Other research also has found that smokers tend to have less appetite than do nonsmokers (smoking may alter hunger-satiety sensation). On the other hand, chronic hunger, imbalanced diet and not having enough money to buy adequate food naturally may cause stress and anxiety and can increase dependence on nicotine. Moreover, food-insecure people may smoke to suppress hunger.
The researchers suggest ways to help solve the problem. One would be to disseminate smoking-related educational materials in food pantries and other community nutrition assistance resources. Another would be to prioritize smoking-cessation interventions for stores in “food deserts.” Only a few policy-based interventions exist, the researchers say. They point to a California city that enacted a citywide “healthy corner store” policy that rewards local small business for offering healthful foods and imposes tobacco-control measures, such as eliminating visible tobacco displays at checkout counters.
Smoking and poor nutrition—2 of the leading preventable causes of death—are reciprocally linked in many ways, multiplying the public health challenges. For instance, smokers are less likely to eat healthful foods and food insecurity is independently associated with smoking. Researchers from the University at Albany, State University of New York, who conducted both a health interview survey and a food environment assessment with 1,917 adults, found that each indicator of food distress was significantly associated with current smoking. Respondents who consumed ≤ 1 serving of fruits and vegetables per day had significantly higher odds of current smoking, compared with those who consumed ≥ 5 servings. Similarly, the odds of current smoking were significantly higher among respondents who were food insecure, used a food pantry, and received Supplemental Nutrition Assistance Program benefits. Living in a neighborhood with low access to healthful food doubled the prevalence of smoking.
Respondents shopped for food often at a corner store (convenience store), dollar store, or drug store. That highlights one of the challenges: All the convenience stores, drug stores, and about 63% of the dollar stores also were tobacco retailers, and nearly all of those had tobacco advertising.
The researchers note that research on the link between smoking and food distress is “limited.” A common explanation for it, they say, is the “opportunity cost” argument. Smokers spend up to 24% of their income on cigarettes—leaving less money for food. Other research also has found that smokers tend to have less appetite than do nonsmokers (smoking may alter hunger-satiety sensation). On the other hand, chronic hunger, imbalanced diet and not having enough money to buy adequate food naturally may cause stress and anxiety and can increase dependence on nicotine. Moreover, food-insecure people may smoke to suppress hunger.
The researchers suggest ways to help solve the problem. One would be to disseminate smoking-related educational materials in food pantries and other community nutrition assistance resources. Another would be to prioritize smoking-cessation interventions for stores in “food deserts.” Only a few policy-based interventions exist, the researchers say. They point to a California city that enacted a citywide “healthy corner store” policy that rewards local small business for offering healthful foods and imposes tobacco-control measures, such as eliminating visible tobacco displays at checkout counters.
Drugs may increase risk of bleeding with NOACs
Concurrent use of non-vitamin K oral anticoagulants (NOACs) and drugs that share metabolic pathways with NOACs may affect the risk of major bleeding in patients with nonvalvular atrial fibrillation (NVAF), according to a study published in JAMA.
Researchers studied more than 91,000 Taiwanese patients with NVAF who were taking dabigatran, rivaroxaban, or apixaban.
Concurrent use of these NOACs with amiodarone, fluconazole, rifampin, or phenytoin was associated with a significant increase in major bleeding, when compared to use of NOACs alone.
On the other hand, major bleeding was significantly decreased for patients concurrently receiving NOACs and atorvastatin, digoxin, or erythromycin/clarithromycin.
Chang-Fu Kuo, MD, PhD, of Chang Gung Memorial Hospital in Taoyuan, Taiwan, and colleagues conducted this study.
The team used data from the Taiwan National Health Insurance database to study 91,330 patients with NVAF who received at least 1 NOAC prescription of dabigatran (n=45,347), rivaroxaban (n=54,006), or apixaban (n=12,886). The patients’ mean age was 74.7, and 55.8% were male.
The researchers estimated the bleeding risk associated with or without the concurrent use of commonly prescribed medications that share metabolic pathways with NOACs—atorvastatin, digoxin, verapamil, diltiazem, amiodarone, cyclosporine, rifampin, phenytoin, dronedarone, erythromycin/clarithromycin, fluconazole, and other azoles (ketoconazole, itraconazole, voriconazole, or posaconazole).
There were 4770 major bleeding events in the patient population.
The data showed that concurrent use of amiodarone, fluconazole, rifampin, and phenytoin with NOACs significantly increased the incidence rates of major bleeding. The adjusted incidence rates per 1000 person-years were:
- 38.09 for NOAC use alone vs 52.04 for amiodarone (difference=13.94, adjusted rate ratio [aRR]=1.37, P<0.01)
- 102.77 for NOAC alone vs 241.92 for fluconazole (difference=138.46, aRR=2.35, P<0.01)
- 65.66 for NOAC alone vs 103.14 for rifampin (difference=36.90, aRR=1.57, P<0.01)
- 56.07 for NOAC alone vs 108.52 for phenytoin (difference=52.31, aRR=1.94, P<0.01).
The incidence rate for major bleeding was significantly lower with concurrent use of atorvastatin, digoxin, and erythromycin or clarithromycin. The adjusted incidence rates per 1000 person-years were:
- 48.96 for NOAC alone vs 34.57 for atorvastatin (difference=-14.38, aRR=0.71, P<0.01)
- 50.14 for NOAC alone vs 45.69 for digoxin (difference=-4.46, aRR=0.91, P<0.01)
- 99.28 for NOAC alone vs 59.38 for erythromycin/clarithromycin (difference=-39.78, aRR=0.60, P<0.01).
The incidence rates of major bleeding were not significantly different for NOAC use alone and concurrent use of NOACs with verapamil, diltiazem, cyclosporine, dronedarone, and ketoconazole, itraconazole, voriconazole, or posaconazole.
The researchers noted that this study has some limitations, including that bleeding risk and anticoagulant treatment are different in Asian and Western populations. Therefore, the external generalizability of these results, particularly to a Western population, may be limited.
Still, the team said physicians prescribing NOACs should consider the potential risks associated with concomitant use of other drugs. ![]()
Concurrent use of non-vitamin K oral anticoagulants (NOACs) and drugs that share metabolic pathways with NOACs may affect the risk of major bleeding in patients with nonvalvular atrial fibrillation (NVAF), according to a study published in JAMA.
Researchers studied more than 91,000 Taiwanese patients with NVAF who were taking dabigatran, rivaroxaban, or apixaban.
Concurrent use of these NOACs with amiodarone, fluconazole, rifampin, or phenytoin was associated with a significant increase in major bleeding, when compared to use of NOACs alone.
On the other hand, major bleeding was significantly decreased for patients concurrently receiving NOACs and atorvastatin, digoxin, or erythromycin/clarithromycin.
Chang-Fu Kuo, MD, PhD, of Chang Gung Memorial Hospital in Taoyuan, Taiwan, and colleagues conducted this study.
The team used data from the Taiwan National Health Insurance database to study 91,330 patients with NVAF who received at least 1 NOAC prescription of dabigatran (n=45,347), rivaroxaban (n=54,006), or apixaban (n=12,886). The patients’ mean age was 74.7, and 55.8% were male.
The researchers estimated the bleeding risk associated with or without the concurrent use of commonly prescribed medications that share metabolic pathways with NOACs—atorvastatin, digoxin, verapamil, diltiazem, amiodarone, cyclosporine, rifampin, phenytoin, dronedarone, erythromycin/clarithromycin, fluconazole, and other azoles (ketoconazole, itraconazole, voriconazole, or posaconazole).
There were 4770 major bleeding events in the patient population.
The data showed that concurrent use of amiodarone, fluconazole, rifampin, and phenytoin with NOACs significantly increased the incidence rates of major bleeding. The adjusted incidence rates per 1000 person-years were:
- 38.09 for NOAC use alone vs 52.04 for amiodarone (difference=13.94, adjusted rate ratio [aRR]=1.37, P<0.01)
- 102.77 for NOAC alone vs 241.92 for fluconazole (difference=138.46, aRR=2.35, P<0.01)
- 65.66 for NOAC alone vs 103.14 for rifampin (difference=36.90, aRR=1.57, P<0.01)
- 56.07 for NOAC alone vs 108.52 for phenytoin (difference=52.31, aRR=1.94, P<0.01).
The incidence rate for major bleeding was significantly lower with concurrent use of atorvastatin, digoxin, and erythromycin or clarithromycin. The adjusted incidence rates per 1000 person-years were:
- 48.96 for NOAC alone vs 34.57 for atorvastatin (difference=-14.38, aRR=0.71, P<0.01)
- 50.14 for NOAC alone vs 45.69 for digoxin (difference=-4.46, aRR=0.91, P<0.01)
- 99.28 for NOAC alone vs 59.38 for erythromycin/clarithromycin (difference=-39.78, aRR=0.60, P<0.01).
The incidence rates of major bleeding were not significantly different for NOAC use alone and concurrent use of NOACs with verapamil, diltiazem, cyclosporine, dronedarone, and ketoconazole, itraconazole, voriconazole, or posaconazole.
The researchers noted that this study has some limitations, including that bleeding risk and anticoagulant treatment are different in Asian and Western populations. Therefore, the external generalizability of these results, particularly to a Western population, may be limited.
Still, the team said physicians prescribing NOACs should consider the potential risks associated with concomitant use of other drugs. ![]()
Concurrent use of non-vitamin K oral anticoagulants (NOACs) and drugs that share metabolic pathways with NOACs may affect the risk of major bleeding in patients with nonvalvular atrial fibrillation (NVAF), according to a study published in JAMA.
Researchers studied more than 91,000 Taiwanese patients with NVAF who were taking dabigatran, rivaroxaban, or apixaban.
Concurrent use of these NOACs with amiodarone, fluconazole, rifampin, or phenytoin was associated with a significant increase in major bleeding, when compared to use of NOACs alone.
On the other hand, major bleeding was significantly decreased for patients concurrently receiving NOACs and atorvastatin, digoxin, or erythromycin/clarithromycin.
Chang-Fu Kuo, MD, PhD, of Chang Gung Memorial Hospital in Taoyuan, Taiwan, and colleagues conducted this study.
The team used data from the Taiwan National Health Insurance database to study 91,330 patients with NVAF who received at least 1 NOAC prescription of dabigatran (n=45,347), rivaroxaban (n=54,006), or apixaban (n=12,886). The patients’ mean age was 74.7, and 55.8% were male.
The researchers estimated the bleeding risk associated with or without the concurrent use of commonly prescribed medications that share metabolic pathways with NOACs—atorvastatin, digoxin, verapamil, diltiazem, amiodarone, cyclosporine, rifampin, phenytoin, dronedarone, erythromycin/clarithromycin, fluconazole, and other azoles (ketoconazole, itraconazole, voriconazole, or posaconazole).
There were 4770 major bleeding events in the patient population.
The data showed that concurrent use of amiodarone, fluconazole, rifampin, and phenytoin with NOACs significantly increased the incidence rates of major bleeding. The adjusted incidence rates per 1000 person-years were:
- 38.09 for NOAC use alone vs 52.04 for amiodarone (difference=13.94, adjusted rate ratio [aRR]=1.37, P<0.01)
- 102.77 for NOAC alone vs 241.92 for fluconazole (difference=138.46, aRR=2.35, P<0.01)
- 65.66 for NOAC alone vs 103.14 for rifampin (difference=36.90, aRR=1.57, P<0.01)
- 56.07 for NOAC alone vs 108.52 for phenytoin (difference=52.31, aRR=1.94, P<0.01).
The incidence rate for major bleeding was significantly lower with concurrent use of atorvastatin, digoxin, and erythromycin or clarithromycin. The adjusted incidence rates per 1000 person-years were:
- 48.96 for NOAC alone vs 34.57 for atorvastatin (difference=-14.38, aRR=0.71, P<0.01)
- 50.14 for NOAC alone vs 45.69 for digoxin (difference=-4.46, aRR=0.91, P<0.01)
- 99.28 for NOAC alone vs 59.38 for erythromycin/clarithromycin (difference=-39.78, aRR=0.60, P<0.01).
The incidence rates of major bleeding were not significantly different for NOAC use alone and concurrent use of NOACs with verapamil, diltiazem, cyclosporine, dronedarone, and ketoconazole, itraconazole, voriconazole, or posaconazole.
The researchers noted that this study has some limitations, including that bleeding risk and anticoagulant treatment are different in Asian and Western populations. Therefore, the external generalizability of these results, particularly to a Western population, may be limited.
Still, the team said physicians prescribing NOACs should consider the potential risks associated with concomitant use of other drugs. ![]()
How Abl ‘shape-shifts’ in drug-resistant CML
Researchers say they have determined how the structure of Abl kinase regulates its activity, enabling the enzyme to switch itself on and off.
The team believes these findings will pave the way to new treatment strategies that can overcome drug resistance in chronic myeloid leukemia (CML) and other malignancies.
Charalampos Kalodimos, PhD, of St Jude Children’s Research Hospital in Memphis, Tennessee, and his colleagues described this research in Nature Structural & Molecular Biology.
The researchers sought to understand how Abl manages to switch itself on and off by altering its shape. Abl controls this switching through allosteric regulation, in which a part of the molecule distant from its kinase domain somehow inhibits or activates Abl.
“We knew we had these 2 functional states, but we had no idea about the conditions under which Abl switched from one to another,” Dr Kalodimos said.
“We also didn’t understand how external molecules that regulate Abl acted on these 2 states. Nor did we understand how mutations that confer drug resistance affected the states.”
To investigate, the researchers used NMR spectroscopy to view Abl’s structure and watch the kinase change. The team explored how the region of Abl called the allosteric regulatory module interacted with the kinase domain to control it.
The research revealed that, in its shape-shifting, Abl was precisely balanced between its inhibition and activation states.
“We saw this very fast ‘breathing’ motion of several thousand times a second, in which the molecule goes on and off, on and off,” Dr Kalodimos said. “This motion is important because it allows other molecules that regulate Abl to adjust its activity one way or the other in a graded manner—like turning a rheostat up or down.”
Such regulation would involve pushing the Abl molecule toward either the inhibited or activated state, Dr Kalodimos said.
Newfound activator region
The researchers also discovered new details about how Abl’s structure affects its activation state. For example, the team’s experiments revealed a previously unknown activator region within Abl.
The researchers noted that the Abl regulatory module consists of 5 regions:
- An unstructured N-terminal region called the cap (residues 1–80)
- The SH3 domain (residues 85–138)
- A short linker called the connectorSH3/2 (residues 139–152), which links the SH3 and SH2 domains
- The SH2 domain (residues 153–237)
- A linker (linkerSH2–KD; residues 238–250) that connects SH2 to the kinase domain (residues 255–534).
The previously unknown activator region the researchers identified is part of the cap region comprising residues 14 to 20 (capPxxP), which carries a PxxP sequence motif, a preferred binding site of the SH3 domain.
The team found that capPxxP is an SH3-binding site that can compete with and displace the linkerSH2–KD from the SH3 domain, thereby destabilizing the inhibiting state.
The researchers said they believe the recently reported A19V drug-resistance mutation exerts its function by promoting the activated state of Abl by means of capPxxP.
Implications for treatment
The researchers also analyzed mutations in Bcr-Abl that allow it to become resistant to imatinib. The drug has proven effective in treating CML by plugging into the kinase domain of the over-activated Abl enzyme and shutting it down. However, in many patients, a mutation in the gene that produces Abl renders it drug-resistant.
While many of the mutations block imatinib from plugging into the kinase domain, others appear to interfere with the allosteric regulation. In effect, they may “warp” the enzyme to keep it activated.
In analyzing the structure of these allosteric mutants, Dr Kalodimos and his colleagues discovered the mutants altered Abl’s shape to activate it and did not interfere with how imatinib plugs into the kinase domain.
This finding points the way to new treatments to overcome such resistance, according to Dr Kalodimos.
“There is now a new generation of drugs that bind to the allosteric pocket to inhibit its activity,” he said. “These could be combined with [imatinib] to overcome allosteric mutations to shift Abl into an inhibited state.”
Dr Kalodimos said that treatment strategy could also be applied to other forms of leukemia that have uncontrolled Bcr-Abl activity. And this new basic understanding of Abl regulation will yield insight into similar enzymes in which allosteric regulation controls a kinase domain. ![]()
Researchers say they have determined how the structure of Abl kinase regulates its activity, enabling the enzyme to switch itself on and off.
The team believes these findings will pave the way to new treatment strategies that can overcome drug resistance in chronic myeloid leukemia (CML) and other malignancies.
Charalampos Kalodimos, PhD, of St Jude Children’s Research Hospital in Memphis, Tennessee, and his colleagues described this research in Nature Structural & Molecular Biology.
The researchers sought to understand how Abl manages to switch itself on and off by altering its shape. Abl controls this switching through allosteric regulation, in which a part of the molecule distant from its kinase domain somehow inhibits or activates Abl.
“We knew we had these 2 functional states, but we had no idea about the conditions under which Abl switched from one to another,” Dr Kalodimos said.
“We also didn’t understand how external molecules that regulate Abl acted on these 2 states. Nor did we understand how mutations that confer drug resistance affected the states.”
To investigate, the researchers used NMR spectroscopy to view Abl’s structure and watch the kinase change. The team explored how the region of Abl called the allosteric regulatory module interacted with the kinase domain to control it.
The research revealed that, in its shape-shifting, Abl was precisely balanced between its inhibition and activation states.
“We saw this very fast ‘breathing’ motion of several thousand times a second, in which the molecule goes on and off, on and off,” Dr Kalodimos said. “This motion is important because it allows other molecules that regulate Abl to adjust its activity one way or the other in a graded manner—like turning a rheostat up or down.”
Such regulation would involve pushing the Abl molecule toward either the inhibited or activated state, Dr Kalodimos said.
Newfound activator region
The researchers also discovered new details about how Abl’s structure affects its activation state. For example, the team’s experiments revealed a previously unknown activator region within Abl.
The researchers noted that the Abl regulatory module consists of 5 regions:
- An unstructured N-terminal region called the cap (residues 1–80)
- The SH3 domain (residues 85–138)
- A short linker called the connectorSH3/2 (residues 139–152), which links the SH3 and SH2 domains
- The SH2 domain (residues 153–237)
- A linker (linkerSH2–KD; residues 238–250) that connects SH2 to the kinase domain (residues 255–534).
The previously unknown activator region the researchers identified is part of the cap region comprising residues 14 to 20 (capPxxP), which carries a PxxP sequence motif, a preferred binding site of the SH3 domain.
The team found that capPxxP is an SH3-binding site that can compete with and displace the linkerSH2–KD from the SH3 domain, thereby destabilizing the inhibiting state.
The researchers said they believe the recently reported A19V drug-resistance mutation exerts its function by promoting the activated state of Abl by means of capPxxP.
Implications for treatment
The researchers also analyzed mutations in Bcr-Abl that allow it to become resistant to imatinib. The drug has proven effective in treating CML by plugging into the kinase domain of the over-activated Abl enzyme and shutting it down. However, in many patients, a mutation in the gene that produces Abl renders it drug-resistant.
While many of the mutations block imatinib from plugging into the kinase domain, others appear to interfere with the allosteric regulation. In effect, they may “warp” the enzyme to keep it activated.
In analyzing the structure of these allosteric mutants, Dr Kalodimos and his colleagues discovered the mutants altered Abl’s shape to activate it and did not interfere with how imatinib plugs into the kinase domain.
This finding points the way to new treatments to overcome such resistance, according to Dr Kalodimos.
“There is now a new generation of drugs that bind to the allosteric pocket to inhibit its activity,” he said. “These could be combined with [imatinib] to overcome allosteric mutations to shift Abl into an inhibited state.”
Dr Kalodimos said that treatment strategy could also be applied to other forms of leukemia that have uncontrolled Bcr-Abl activity. And this new basic understanding of Abl regulation will yield insight into similar enzymes in which allosteric regulation controls a kinase domain. ![]()
Researchers say they have determined how the structure of Abl kinase regulates its activity, enabling the enzyme to switch itself on and off.
The team believes these findings will pave the way to new treatment strategies that can overcome drug resistance in chronic myeloid leukemia (CML) and other malignancies.
Charalampos Kalodimos, PhD, of St Jude Children’s Research Hospital in Memphis, Tennessee, and his colleagues described this research in Nature Structural & Molecular Biology.
The researchers sought to understand how Abl manages to switch itself on and off by altering its shape. Abl controls this switching through allosteric regulation, in which a part of the molecule distant from its kinase domain somehow inhibits or activates Abl.
“We knew we had these 2 functional states, but we had no idea about the conditions under which Abl switched from one to another,” Dr Kalodimos said.
“We also didn’t understand how external molecules that regulate Abl acted on these 2 states. Nor did we understand how mutations that confer drug resistance affected the states.”
To investigate, the researchers used NMR spectroscopy to view Abl’s structure and watch the kinase change. The team explored how the region of Abl called the allosteric regulatory module interacted with the kinase domain to control it.
The research revealed that, in its shape-shifting, Abl was precisely balanced between its inhibition and activation states.
“We saw this very fast ‘breathing’ motion of several thousand times a second, in which the molecule goes on and off, on and off,” Dr Kalodimos said. “This motion is important because it allows other molecules that regulate Abl to adjust its activity one way or the other in a graded manner—like turning a rheostat up or down.”
Such regulation would involve pushing the Abl molecule toward either the inhibited or activated state, Dr Kalodimos said.
Newfound activator region
The researchers also discovered new details about how Abl’s structure affects its activation state. For example, the team’s experiments revealed a previously unknown activator region within Abl.
The researchers noted that the Abl regulatory module consists of 5 regions:
- An unstructured N-terminal region called the cap (residues 1–80)
- The SH3 domain (residues 85–138)
- A short linker called the connectorSH3/2 (residues 139–152), which links the SH3 and SH2 domains
- The SH2 domain (residues 153–237)
- A linker (linkerSH2–KD; residues 238–250) that connects SH2 to the kinase domain (residues 255–534).
The previously unknown activator region the researchers identified is part of the cap region comprising residues 14 to 20 (capPxxP), which carries a PxxP sequence motif, a preferred binding site of the SH3 domain.
The team found that capPxxP is an SH3-binding site that can compete with and displace the linkerSH2–KD from the SH3 domain, thereby destabilizing the inhibiting state.
The researchers said they believe the recently reported A19V drug-resistance mutation exerts its function by promoting the activated state of Abl by means of capPxxP.
Implications for treatment
The researchers also analyzed mutations in Bcr-Abl that allow it to become resistant to imatinib. The drug has proven effective in treating CML by plugging into the kinase domain of the over-activated Abl enzyme and shutting it down. However, in many patients, a mutation in the gene that produces Abl renders it drug-resistant.
While many of the mutations block imatinib from plugging into the kinase domain, others appear to interfere with the allosteric regulation. In effect, they may “warp” the enzyme to keep it activated.
In analyzing the structure of these allosteric mutants, Dr Kalodimos and his colleagues discovered the mutants altered Abl’s shape to activate it and did not interfere with how imatinib plugs into the kinase domain.
This finding points the way to new treatments to overcome such resistance, according to Dr Kalodimos.
“There is now a new generation of drugs that bind to the allosteric pocket to inhibit its activity,” he said. “These could be combined with [imatinib] to overcome allosteric mutations to shift Abl into an inhibited state.”
Dr Kalodimos said that treatment strategy could also be applied to other forms of leukemia that have uncontrolled Bcr-Abl activity. And this new basic understanding of Abl regulation will yield insight into similar enzymes in which allosteric regulation controls a kinase domain. ![]()
Fostamatinib elicits responses in AIHA
The oral SYK inhibitor fostamatinib can produce responses in patients with warm antibody autoimmune hemolytic anemia (AIHA), according to Rigel Pharmaceuticals, Inc.
The company reported topline results from the first stage of the phase 2 SOAR study, which showed that fostamatinib produced a 35% response rate.
A response was defined as achieving a hemoglobin level of greater than 10 g/dL and at least a 2 g/dL increase from baseline.
“Many patients with AIHA suffer from severe, debilitating disease that negatively affects their quality of life,” said David J. Kuter, MD, the director for the Center of Hematology at Massachusetts General Hospital in Boston and the lead investigator of the SOAR study.
“There are no FDA-approved medications for the treatment of AIHA, which means that those living with the condition are in need of new and effective therapeutic options.”
In the SOAR study, Dr Kuter and his colleagues are evaluating fostamatinib in patients with warm antibody AIHA who previously received at least 1 treatment but did not have a meaningful benefit and are still anemic.
The study utilizes an open-label, Simon 2-stage design to evaluate fostamatinib given at 150 mg twice daily.
Stage 1 has enrolled 17 patients who have had at least 1 post-baseline hemoglobin measure.
Four of these patients responded to fostamatinib during the 12-week evaluation period, and an additional 2 patients met response criteria in the extension study after 12 weeks of dosing.
So the overall response rate was 35% (6/17), although Rigel Pharmaceuticals said these data are preliminary and require further verification.
Two patients withdrew from the study early due to non-safety-related reasons and will be replaced per the study protocol.
According to Rigel Pharmaceuticals, the treatment-emergent adverse events (AEs) in this trial were consistent with the prior clinical experience with fostamatinib.
Treatment-emergent AEs—which included diarrhea, elevated liver function tests, and hypertension—were manageable and mostly mild or moderate in nature.
There were 2 deaths reported during the trial. Both were due to non-treatment-related serious AEs, according to investigators. One patient had skin necrosis and infection. The other was an elderly patient who had pneumonia and was immunosuppressed due to prior chronic lymphocytic leukemia and steroid use.
A third patient experienced a non-treatment-related serious AE but recovered and continued on treatment.
Rigel Pharmaceuticals said a comprehensive analysis of the data will continue and will be presented at a future scientific conference.
The company also intends to begin enrollment for stage 2 of this study, in which 20 patients will be enrolled under the same protocol. ![]()
The oral SYK inhibitor fostamatinib can produce responses in patients with warm antibody autoimmune hemolytic anemia (AIHA), according to Rigel Pharmaceuticals, Inc.
The company reported topline results from the first stage of the phase 2 SOAR study, which showed that fostamatinib produced a 35% response rate.
A response was defined as achieving a hemoglobin level of greater than 10 g/dL and at least a 2 g/dL increase from baseline.
“Many patients with AIHA suffer from severe, debilitating disease that negatively affects their quality of life,” said David J. Kuter, MD, the director for the Center of Hematology at Massachusetts General Hospital in Boston and the lead investigator of the SOAR study.
“There are no FDA-approved medications for the treatment of AIHA, which means that those living with the condition are in need of new and effective therapeutic options.”
In the SOAR study, Dr Kuter and his colleagues are evaluating fostamatinib in patients with warm antibody AIHA who previously received at least 1 treatment but did not have a meaningful benefit and are still anemic.
The study utilizes an open-label, Simon 2-stage design to evaluate fostamatinib given at 150 mg twice daily.
Stage 1 has enrolled 17 patients who have had at least 1 post-baseline hemoglobin measure.
Four of these patients responded to fostamatinib during the 12-week evaluation period, and an additional 2 patients met response criteria in the extension study after 12 weeks of dosing.
So the overall response rate was 35% (6/17), although Rigel Pharmaceuticals said these data are preliminary and require further verification.
Two patients withdrew from the study early due to non-safety-related reasons and will be replaced per the study protocol.
According to Rigel Pharmaceuticals, the treatment-emergent adverse events (AEs) in this trial were consistent with the prior clinical experience with fostamatinib.
Treatment-emergent AEs—which included diarrhea, elevated liver function tests, and hypertension—were manageable and mostly mild or moderate in nature.
There were 2 deaths reported during the trial. Both were due to non-treatment-related serious AEs, according to investigators. One patient had skin necrosis and infection. The other was an elderly patient who had pneumonia and was immunosuppressed due to prior chronic lymphocytic leukemia and steroid use.
A third patient experienced a non-treatment-related serious AE but recovered and continued on treatment.
Rigel Pharmaceuticals said a comprehensive analysis of the data will continue and will be presented at a future scientific conference.
The company also intends to begin enrollment for stage 2 of this study, in which 20 patients will be enrolled under the same protocol. ![]()
The oral SYK inhibitor fostamatinib can produce responses in patients with warm antibody autoimmune hemolytic anemia (AIHA), according to Rigel Pharmaceuticals, Inc.
The company reported topline results from the first stage of the phase 2 SOAR study, which showed that fostamatinib produced a 35% response rate.
A response was defined as achieving a hemoglobin level of greater than 10 g/dL and at least a 2 g/dL increase from baseline.
“Many patients with AIHA suffer from severe, debilitating disease that negatively affects their quality of life,” said David J. Kuter, MD, the director for the Center of Hematology at Massachusetts General Hospital in Boston and the lead investigator of the SOAR study.
“There are no FDA-approved medications for the treatment of AIHA, which means that those living with the condition are in need of new and effective therapeutic options.”
In the SOAR study, Dr Kuter and his colleagues are evaluating fostamatinib in patients with warm antibody AIHA who previously received at least 1 treatment but did not have a meaningful benefit and are still anemic.
The study utilizes an open-label, Simon 2-stage design to evaluate fostamatinib given at 150 mg twice daily.
Stage 1 has enrolled 17 patients who have had at least 1 post-baseline hemoglobin measure.
Four of these patients responded to fostamatinib during the 12-week evaluation period, and an additional 2 patients met response criteria in the extension study after 12 weeks of dosing.
So the overall response rate was 35% (6/17), although Rigel Pharmaceuticals said these data are preliminary and require further verification.
Two patients withdrew from the study early due to non-safety-related reasons and will be replaced per the study protocol.
According to Rigel Pharmaceuticals, the treatment-emergent adverse events (AEs) in this trial were consistent with the prior clinical experience with fostamatinib.
Treatment-emergent AEs—which included diarrhea, elevated liver function tests, and hypertension—were manageable and mostly mild or moderate in nature.
There were 2 deaths reported during the trial. Both were due to non-treatment-related serious AEs, according to investigators. One patient had skin necrosis and infection. The other was an elderly patient who had pneumonia and was immunosuppressed due to prior chronic lymphocytic leukemia and steroid use.
A third patient experienced a non-treatment-related serious AE but recovered and continued on treatment.
Rigel Pharmaceuticals said a comprehensive analysis of the data will continue and will be presented at a future scientific conference.
The company also intends to begin enrollment for stage 2 of this study, in which 20 patients will be enrolled under the same protocol. ![]()



















