User login
ANA 2017 offers career development sessions at all levels
Professional developments courses that will take place each morning during the annual meeting of the American Neurological Association in San Diego offer special opportunities to learn about the rewards and challenges of different career options for neurologists and researchers in academic neurology from those who have gone down a variety of careers paths themselves. Three courses each morning are geared specifically for students, residents, post-docs, and fellows, as well as for professionals at early-, mid-, and university-chair career levels.
For students, residents, post-docs, and fellows
On Oct. 15, young neurology scholars will begin the course with a discussion on the transition from neurology resident to fellow, and then career pathways as a basic scientist, a clinical scientist/researcher, and clinician-administrator. Last year’s recipients of the Derek Denny-Brown Young Neurological Scholar Award also will provide insights on successful early careers in academic neurology.
Global Health has many opportunities for individuals who are looking at potential career paths in neurology to conduct research, clinical care, or educate others in low- to middle-income countries. Speakers on Oct. 16 will share their experiences and discuss emerging trends in global health in neurology and ways in which individuals might pursue careers or opportunities that complement pursuits in academic neurology.
A workshop on Oct. 17 will focus on the essential skills needed for a successful job-seeking experience in academic neurology. There will be opportunities to practice interviewing and negotiating skills, as well as to learn how to market your research and abilities when the opportunity strikes and time is limited.
For early- to mid-career levels
Finding a niche and a successful career trajectory takes a good deal of planning and taking opportunities when the time is right. On Oct. 15, several mid-career level academic neurologists will share their career trajectories and alternative methods they have used for obtaining support and funding for their clinical research, educational enterprises, and curricular development.
The Oct. 16 career development course will outline methods for setting milestones for career development and finding the resources and mentoring individuals you will need for career advancement.
To learn skills and discover tools that will help to write successful grant proposals, particularly for the National Institutes of Health, come to the session on Oct. 17. You can learn how to develop your grant application, respond to critiques of your application, and hear about a variety of sources of funding.
For university chairs of neurology
At a time in which funding for salaries is hampered by many factors, salary disparities are changing across subspecialties as well as in different faculty positions, with some disparities widening and others shrinking. Come to the career development course on Oct. 15 to learn about novel revenue sources and ways in which you can best subsidize the salaries of faculties in your department as well as offer nonmonetary compensation.
Given the current uncertainties of the fates of Affordable Care Act, Medicare, and other major systems supporting health care, it is important to know the best approach to take in advocating for academic neurology and your department’s needs. At the course on Oct. 16, come learn about current health policy issues and the political climate and determine how and when to be politically active.
To understand the impact that the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA) requirements will have on academic neurology, come to the session on Oct. 17. You’ll learn how population health measures and value-based care can be brought into an academic neurology practice, how to report on quality measures in the Merit-based Incentive Payment System, and how to participate in Advanced Alternative Payment Models.
Professional developments courses that will take place each morning during the annual meeting of the American Neurological Association in San Diego offer special opportunities to learn about the rewards and challenges of different career options for neurologists and researchers in academic neurology from those who have gone down a variety of careers paths themselves. Three courses each morning are geared specifically for students, residents, post-docs, and fellows, as well as for professionals at early-, mid-, and university-chair career levels.
For students, residents, post-docs, and fellows
On Oct. 15, young neurology scholars will begin the course with a discussion on the transition from neurology resident to fellow, and then career pathways as a basic scientist, a clinical scientist/researcher, and clinician-administrator. Last year’s recipients of the Derek Denny-Brown Young Neurological Scholar Award also will provide insights on successful early careers in academic neurology.
Global Health has many opportunities for individuals who are looking at potential career paths in neurology to conduct research, clinical care, or educate others in low- to middle-income countries. Speakers on Oct. 16 will share their experiences and discuss emerging trends in global health in neurology and ways in which individuals might pursue careers or opportunities that complement pursuits in academic neurology.
A workshop on Oct. 17 will focus on the essential skills needed for a successful job-seeking experience in academic neurology. There will be opportunities to practice interviewing and negotiating skills, as well as to learn how to market your research and abilities when the opportunity strikes and time is limited.
For early- to mid-career levels
Finding a niche and a successful career trajectory takes a good deal of planning and taking opportunities when the time is right. On Oct. 15, several mid-career level academic neurologists will share their career trajectories and alternative methods they have used for obtaining support and funding for their clinical research, educational enterprises, and curricular development.
The Oct. 16 career development course will outline methods for setting milestones for career development and finding the resources and mentoring individuals you will need for career advancement.
To learn skills and discover tools that will help to write successful grant proposals, particularly for the National Institutes of Health, come to the session on Oct. 17. You can learn how to develop your grant application, respond to critiques of your application, and hear about a variety of sources of funding.
For university chairs of neurology
At a time in which funding for salaries is hampered by many factors, salary disparities are changing across subspecialties as well as in different faculty positions, with some disparities widening and others shrinking. Come to the career development course on Oct. 15 to learn about novel revenue sources and ways in which you can best subsidize the salaries of faculties in your department as well as offer nonmonetary compensation.
Given the current uncertainties of the fates of Affordable Care Act, Medicare, and other major systems supporting health care, it is important to know the best approach to take in advocating for academic neurology and your department’s needs. At the course on Oct. 16, come learn about current health policy issues and the political climate and determine how and when to be politically active.
To understand the impact that the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA) requirements will have on academic neurology, come to the session on Oct. 17. You’ll learn how population health measures and value-based care can be brought into an academic neurology practice, how to report on quality measures in the Merit-based Incentive Payment System, and how to participate in Advanced Alternative Payment Models.
Professional developments courses that will take place each morning during the annual meeting of the American Neurological Association in San Diego offer special opportunities to learn about the rewards and challenges of different career options for neurologists and researchers in academic neurology from those who have gone down a variety of careers paths themselves. Three courses each morning are geared specifically for students, residents, post-docs, and fellows, as well as for professionals at early-, mid-, and university-chair career levels.
For students, residents, post-docs, and fellows
On Oct. 15, young neurology scholars will begin the course with a discussion on the transition from neurology resident to fellow, and then career pathways as a basic scientist, a clinical scientist/researcher, and clinician-administrator. Last year’s recipients of the Derek Denny-Brown Young Neurological Scholar Award also will provide insights on successful early careers in academic neurology.
Global Health has many opportunities for individuals who are looking at potential career paths in neurology to conduct research, clinical care, or educate others in low- to middle-income countries. Speakers on Oct. 16 will share their experiences and discuss emerging trends in global health in neurology and ways in which individuals might pursue careers or opportunities that complement pursuits in academic neurology.
A workshop on Oct. 17 will focus on the essential skills needed for a successful job-seeking experience in academic neurology. There will be opportunities to practice interviewing and negotiating skills, as well as to learn how to market your research and abilities when the opportunity strikes and time is limited.
For early- to mid-career levels
Finding a niche and a successful career trajectory takes a good deal of planning and taking opportunities when the time is right. On Oct. 15, several mid-career level academic neurologists will share their career trajectories and alternative methods they have used for obtaining support and funding for their clinical research, educational enterprises, and curricular development.
The Oct. 16 career development course will outline methods for setting milestones for career development and finding the resources and mentoring individuals you will need for career advancement.
To learn skills and discover tools that will help to write successful grant proposals, particularly for the National Institutes of Health, come to the session on Oct. 17. You can learn how to develop your grant application, respond to critiques of your application, and hear about a variety of sources of funding.
For university chairs of neurology
At a time in which funding for salaries is hampered by many factors, salary disparities are changing across subspecialties as well as in different faculty positions, with some disparities widening and others shrinking. Come to the career development course on Oct. 15 to learn about novel revenue sources and ways in which you can best subsidize the salaries of faculties in your department as well as offer nonmonetary compensation.
Given the current uncertainties of the fates of Affordable Care Act, Medicare, and other major systems supporting health care, it is important to know the best approach to take in advocating for academic neurology and your department’s needs. At the course on Oct. 16, come learn about current health policy issues and the political climate and determine how and when to be politically active.
To understand the impact that the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA) requirements will have on academic neurology, come to the session on Oct. 17. You’ll learn how population health measures and value-based care can be brought into an academic neurology practice, how to report on quality measures in the Merit-based Incentive Payment System, and how to participate in Advanced Alternative Payment Models.
Here’s what’s trending at SHM – Oct. 2017
Don’t miss pre-courses at HM18
Enrich your educational experience and earn additional CME credit and MOC points with pre-courses at Hospital Medicine 2018 (HM18), to be held from April 8-11, 2018, at the Orlando (Fla.) World Center Marriott.
Broaden your skills, fine-tune your practice, and immerse yourself in a day of learning by enrolling in one of the following:
• Bedside procedures for the hospitalist
• Essentials of perioperative medicine and comanagement for the hospitalist
• Hospitalist practice management: How to thrive in a time of intense change
• Sepsis: New insights into detection and management
• Keep your finger on the pulse – cardiology update for the hospitalist
• Maintenance of certification and board prep
• Point-of-care ultrasound for the hospitalist
Pre-course day is Sunday, April 8, 2018. Learn more and register at shmannualconference.org/precourse.
Improve quality at your institution with SHM
The National Association for Healthcare Quality’s (NAHQ) Healthcare Quality Week is Oct. 15-21, 2017, a week dedicated to celebrating the contributions professionals have made in the field and bringing awareness to the profession of health care quality. SHM’s Center for Quality Improvement provides a variety of resources, tools, and programs to address quality and patient safety issues at your institution. Learn more at hospitalmedicine.org/QI.
Distinguish yourself as a Class of 2018 Fellow in Hospital Medicine
SHM’s Fellows designation is a prestigious way to differentiate yourself in the rapidly growing profession of hospital medicine. There are currently over 2,000 hospitalists who have earned the Fellow in Hospital Medicine (FHM) or Senior Fellow in Hospital Medicine (SFHM) designation by demonstrating the core values of leadership, teamwork, and quality improvement.
Apply now and learn how you can join this prestigious group of hospitalists at hospitalmedicine.org/fellows. Applications officially close on Nov. 30, 2017.
Critical care for the hospitalist: Now on the SHM Learning Portal
Many hospitalists provide critical care services without adequate support or training, putting patients at risk and exposing hospitalists to medical liability. Don’t miss the newest SHM Learning Portal series, Critical Care for the Hospitalist. The four courses in this educational series cover common or high-risk clinical scenarios that hospitalists encounter in and out of the intensive care unit, including:
1. Airway management for the hospitalist
2. Noninvasive positive pressure ventilation for the hospitalist
3. Arrhythmias
4. High-risk pulmonary embolism
This series is free for SHM members and $45 per module for nonmembers. Earn 0.75 AMA PRA Category 1 Credit™ and ABIM MOC points per each module. Visit shmlearningportal.org to get started today.
Connect with SHM locally at a chapter meeting near you
Attend a chapter meeting to experience SHM at the local level. Chapter meetings provide focused educational topics through keynote speakers, presentations, and opportunities to network with other hospitalists in your area. Find a chapter meeting close to you at hospitalmedicine.org/chapters.
Stay on top of trending topics in practice management
SHM recently released white papers on trending topics in practice management: Hospitalist Perspectives on EMRs, Telemedicine in Hospital Medicine, and the Evolution of Co-Management in Hospital Medicine. These resources are free to download to members and can be found at hospitalmedicine.org under the Practice Management tab.
Enhance your coding skills and earn CME
SHM’s Clinical Documentation & Coding for Hospitalists (formerly CODE-H) recently launched an updated program with all-new content that offers hospitalists the latest information on best practices in coding, documentation, and compliance from national experts. It provides eight recorded webinar sessions presented by expert faculty, downloadable resources, and an interactive discussion forum on SHM’s online community.
CME credits are offered through an evaluation following the webinars. Each participant is eligible for CME credits for completion of the series.
To learn more, visit hospitalmedicine.org/CODEH. If you have questions on the new program, please contact [email protected].
Mr. Radler is marketing communications manager at the Society of Hospital Medicine.
Don’t miss pre-courses at HM18
Enrich your educational experience and earn additional CME credit and MOC points with pre-courses at Hospital Medicine 2018 (HM18), to be held from April 8-11, 2018, at the Orlando (Fla.) World Center Marriott.
Broaden your skills, fine-tune your practice, and immerse yourself in a day of learning by enrolling in one of the following:
• Bedside procedures for the hospitalist
• Essentials of perioperative medicine and comanagement for the hospitalist
• Hospitalist practice management: How to thrive in a time of intense change
• Sepsis: New insights into detection and management
• Keep your finger on the pulse – cardiology update for the hospitalist
• Maintenance of certification and board prep
• Point-of-care ultrasound for the hospitalist
Pre-course day is Sunday, April 8, 2018. Learn more and register at shmannualconference.org/precourse.
Improve quality at your institution with SHM
The National Association for Healthcare Quality’s (NAHQ) Healthcare Quality Week is Oct. 15-21, 2017, a week dedicated to celebrating the contributions professionals have made in the field and bringing awareness to the profession of health care quality. SHM’s Center for Quality Improvement provides a variety of resources, tools, and programs to address quality and patient safety issues at your institution. Learn more at hospitalmedicine.org/QI.
Distinguish yourself as a Class of 2018 Fellow in Hospital Medicine
SHM’s Fellows designation is a prestigious way to differentiate yourself in the rapidly growing profession of hospital medicine. There are currently over 2,000 hospitalists who have earned the Fellow in Hospital Medicine (FHM) or Senior Fellow in Hospital Medicine (SFHM) designation by demonstrating the core values of leadership, teamwork, and quality improvement.
Apply now and learn how you can join this prestigious group of hospitalists at hospitalmedicine.org/fellows. Applications officially close on Nov. 30, 2017.
Critical care for the hospitalist: Now on the SHM Learning Portal
Many hospitalists provide critical care services without adequate support or training, putting patients at risk and exposing hospitalists to medical liability. Don’t miss the newest SHM Learning Portal series, Critical Care for the Hospitalist. The four courses in this educational series cover common or high-risk clinical scenarios that hospitalists encounter in and out of the intensive care unit, including:
1. Airway management for the hospitalist
2. Noninvasive positive pressure ventilation for the hospitalist
3. Arrhythmias
4. High-risk pulmonary embolism
This series is free for SHM members and $45 per module for nonmembers. Earn 0.75 AMA PRA Category 1 Credit™ and ABIM MOC points per each module. Visit shmlearningportal.org to get started today.
Connect with SHM locally at a chapter meeting near you
Attend a chapter meeting to experience SHM at the local level. Chapter meetings provide focused educational topics through keynote speakers, presentations, and opportunities to network with other hospitalists in your area. Find a chapter meeting close to you at hospitalmedicine.org/chapters.
Stay on top of trending topics in practice management
SHM recently released white papers on trending topics in practice management: Hospitalist Perspectives on EMRs, Telemedicine in Hospital Medicine, and the Evolution of Co-Management in Hospital Medicine. These resources are free to download to members and can be found at hospitalmedicine.org under the Practice Management tab.
Enhance your coding skills and earn CME
SHM’s Clinical Documentation & Coding for Hospitalists (formerly CODE-H) recently launched an updated program with all-new content that offers hospitalists the latest information on best practices in coding, documentation, and compliance from national experts. It provides eight recorded webinar sessions presented by expert faculty, downloadable resources, and an interactive discussion forum on SHM’s online community.
CME credits are offered through an evaluation following the webinars. Each participant is eligible for CME credits for completion of the series.
To learn more, visit hospitalmedicine.org/CODEH. If you have questions on the new program, please contact [email protected].
Mr. Radler is marketing communications manager at the Society of Hospital Medicine.
Don’t miss pre-courses at HM18
Enrich your educational experience and earn additional CME credit and MOC points with pre-courses at Hospital Medicine 2018 (HM18), to be held from April 8-11, 2018, at the Orlando (Fla.) World Center Marriott.
Broaden your skills, fine-tune your practice, and immerse yourself in a day of learning by enrolling in one of the following:
• Bedside procedures for the hospitalist
• Essentials of perioperative medicine and comanagement for the hospitalist
• Hospitalist practice management: How to thrive in a time of intense change
• Sepsis: New insights into detection and management
• Keep your finger on the pulse – cardiology update for the hospitalist
• Maintenance of certification and board prep
• Point-of-care ultrasound for the hospitalist
Pre-course day is Sunday, April 8, 2018. Learn more and register at shmannualconference.org/precourse.
Improve quality at your institution with SHM
The National Association for Healthcare Quality’s (NAHQ) Healthcare Quality Week is Oct. 15-21, 2017, a week dedicated to celebrating the contributions professionals have made in the field and bringing awareness to the profession of health care quality. SHM’s Center for Quality Improvement provides a variety of resources, tools, and programs to address quality and patient safety issues at your institution. Learn more at hospitalmedicine.org/QI.
Distinguish yourself as a Class of 2018 Fellow in Hospital Medicine
SHM’s Fellows designation is a prestigious way to differentiate yourself in the rapidly growing profession of hospital medicine. There are currently over 2,000 hospitalists who have earned the Fellow in Hospital Medicine (FHM) or Senior Fellow in Hospital Medicine (SFHM) designation by demonstrating the core values of leadership, teamwork, and quality improvement.
Apply now and learn how you can join this prestigious group of hospitalists at hospitalmedicine.org/fellows. Applications officially close on Nov. 30, 2017.
Critical care for the hospitalist: Now on the SHM Learning Portal
Many hospitalists provide critical care services without adequate support or training, putting patients at risk and exposing hospitalists to medical liability. Don’t miss the newest SHM Learning Portal series, Critical Care for the Hospitalist. The four courses in this educational series cover common or high-risk clinical scenarios that hospitalists encounter in and out of the intensive care unit, including:
1. Airway management for the hospitalist
2. Noninvasive positive pressure ventilation for the hospitalist
3. Arrhythmias
4. High-risk pulmonary embolism
This series is free for SHM members and $45 per module for nonmembers. Earn 0.75 AMA PRA Category 1 Credit™ and ABIM MOC points per each module. Visit shmlearningportal.org to get started today.
Connect with SHM locally at a chapter meeting near you
Attend a chapter meeting to experience SHM at the local level. Chapter meetings provide focused educational topics through keynote speakers, presentations, and opportunities to network with other hospitalists in your area. Find a chapter meeting close to you at hospitalmedicine.org/chapters.
Stay on top of trending topics in practice management
SHM recently released white papers on trending topics in practice management: Hospitalist Perspectives on EMRs, Telemedicine in Hospital Medicine, and the Evolution of Co-Management in Hospital Medicine. These resources are free to download to members and can be found at hospitalmedicine.org under the Practice Management tab.
Enhance your coding skills and earn CME
SHM’s Clinical Documentation & Coding for Hospitalists (formerly CODE-H) recently launched an updated program with all-new content that offers hospitalists the latest information on best practices in coding, documentation, and compliance from national experts. It provides eight recorded webinar sessions presented by expert faculty, downloadable resources, and an interactive discussion forum on SHM’s online community.
CME credits are offered through an evaluation following the webinars. Each participant is eligible for CME credits for completion of the series.
To learn more, visit hospitalmedicine.org/CODEH. If you have questions on the new program, please contact [email protected].
Mr. Radler is marketing communications manager at the Society of Hospital Medicine.
Blood donation intervals of 8 weeks safe for most donors
Reducing the interval between blood donations from every 12 weeks to every 8 weeks increases blood units collected with little ill effects on donor quality of life other than symptoms related to iron deficiency, based on results reported by the INTERVAL Trial Group.
In the United States, men and women can donate blood every 8 weeks; the Food and Drug Administration and the AABB (formerly the American Association of Blood Banks) have considered lengthening the 8-week minimum inter-donation interval to reduce the risk of iron deficiency. The current practice in the United Kingdom is to allow men to donate every 12 weeks and women every 16 weeks, the authors noted.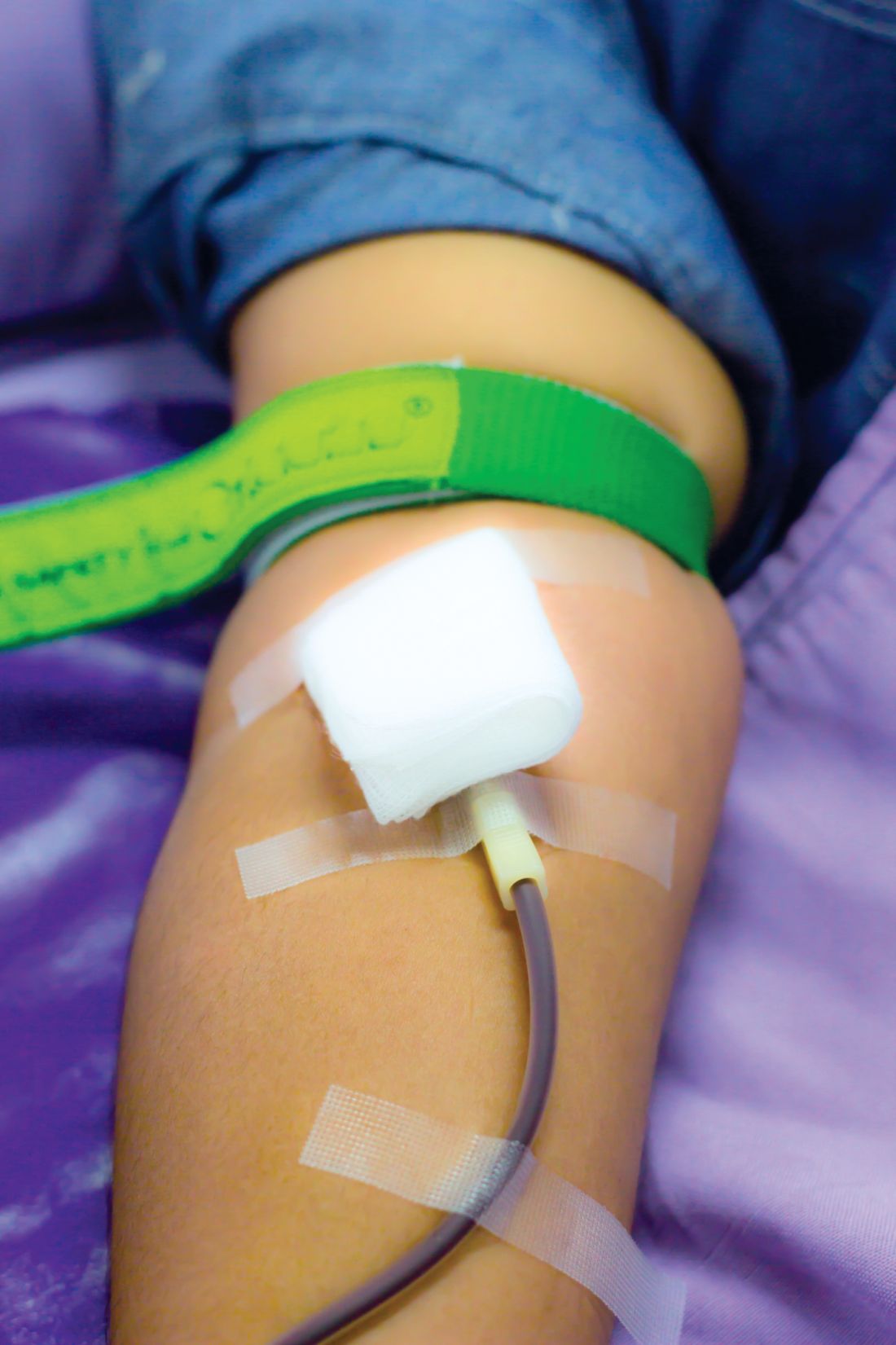
The INTERVAL study examined hemoglobin levels, ferritin levels, and self-reported symptoms as well as the number of blood donations over a 2-year period among 22,466 men randomly assigned to either 8-, 10-, or 12-week blood donation intervals and 22,797 women randomly assigned to donation intervals of 12, 14, and 16 weeks.
Men in the 8-week interval group donated significantly more blood on a per-donor basis, contributing 1.69 and 7.9 units more than the men in the 10- and 12-week interval groups, respectively. Similarly, women in the 12-week interval group contributed approximately 0.84 and 0.46 units more than did women in the 14- and 16-week interval groups.
Hemoglobin and ferritin concentrations stayed relatively stable throughout the study in men and women in all donation interval groups. The baseline hemoglobin and ferritin averages were 149.7 g/L and 44.9 mcg/L in men, and 133.9 g/L and 24.6 mcg/L in women, respectively. Ultimately, mean hemoglobin level decreased by 1%-2%. Ferritin level decreased by 15%-30%, indicating that it is a more accurate marker of iron depletion, the study authors wrote. The report was published online in The Lancet.
The researchers also analyzed self-reported symptoms associated with blood donations derived from donor follow-up surveys sent at 6-, 12-, and 18-month intervals. At the end of the 2-year study, donors underwent a series of cognitive function tests and answered a physical activity questionnaire. Increased donation rates correlated with increased symptoms of iron deficiency such as tiredness, restless legs, and dizziness. This effect was reported more strongly in men.
About 10% of participants were allowed to donate despite having baseline hemoglobin concentrations below the minimum regulatory threshold, said the authors, who said that the screening method used in the United Kingdom to test eligibility to donate needs to be reviewed.
“In response to these findings, we have started the COMPARE study (ISRCTN90871183) to provide a systematic, within-person comparison of the relative merits of different haemoglobin screening methods,” they wrote
The study was funded by National Health Service Blood and Transplant and the National Institute for Health Research Blood and Transplant Research Unit in Donor Health and Genomics (NIHR BTRU-2014-10024).
Donating blood serves an important function in medicine, but the practices associated with blood donations do not consider the effect it has on the donors. The amount of blood that most donors give is around 500 mL, or about 10% of total blood volume. The ratio of iron to blood is 1:1, meaning that for each milligram of blood drawn, one milligram of iron is lost. Considering that women only have 250 mg of iron reserves and men have 1,000 mg of reserves, many frequent donors are at risk of developing iron deficiency.
This study found that the total units of blood collected increased with shorter inter-donation intervals, while secondary safety outcomes of hemoglobin and ferritin levels remained similar between short and long inter-donation interval groups. While overall health did not decline, short inter-donation donors reported symptoms consistent with iron deficiency, including dizziness, tiredness, and restless legs. Researchers found that “about 25% of men and women at the most frequent inter-donation interval had iron deficiency and a third had at least one deferral for low hemoglobin.”
Iron deficiency can be mitigated by iron supplements or by lengthening the time between donations, but this does not fully correct the issue. Some blood centers have already introduced ferritin screening and lengthened the inter-donation interval for donors found to have low ferritin concentrations. Given the advances in automated laboratory testing, information technology, and the high compliance of blood donors, individualized approaches for prevention of iron deficiency could be feasible.
Alan E. Mast, MD , is the medical director for the Blood Center of Wisconsin, and an associate professor of pathology and cell biology at the Medical College of Wisconsin, Milwaukee. Edward L. Murphy, MD , is resident professor of laboratory medicine at University of California, San Francisco, and an affiliate of the Blood Systems Research Institute. They made their comments in an editorial that accompanied the study.
Donating blood serves an important function in medicine, but the practices associated with blood donations do not consider the effect it has on the donors. The amount of blood that most donors give is around 500 mL, or about 10% of total blood volume. The ratio of iron to blood is 1:1, meaning that for each milligram of blood drawn, one milligram of iron is lost. Considering that women only have 250 mg of iron reserves and men have 1,000 mg of reserves, many frequent donors are at risk of developing iron deficiency.
This study found that the total units of blood collected increased with shorter inter-donation intervals, while secondary safety outcomes of hemoglobin and ferritin levels remained similar between short and long inter-donation interval groups. While overall health did not decline, short inter-donation donors reported symptoms consistent with iron deficiency, including dizziness, tiredness, and restless legs. Researchers found that “about 25% of men and women at the most frequent inter-donation interval had iron deficiency and a third had at least one deferral for low hemoglobin.”
Iron deficiency can be mitigated by iron supplements or by lengthening the time between donations, but this does not fully correct the issue. Some blood centers have already introduced ferritin screening and lengthened the inter-donation interval for donors found to have low ferritin concentrations. Given the advances in automated laboratory testing, information technology, and the high compliance of blood donors, individualized approaches for prevention of iron deficiency could be feasible.
Alan E. Mast, MD , is the medical director for the Blood Center of Wisconsin, and an associate professor of pathology and cell biology at the Medical College of Wisconsin, Milwaukee. Edward L. Murphy, MD , is resident professor of laboratory medicine at University of California, San Francisco, and an affiliate of the Blood Systems Research Institute. They made their comments in an editorial that accompanied the study.
Donating blood serves an important function in medicine, but the practices associated with blood donations do not consider the effect it has on the donors. The amount of blood that most donors give is around 500 mL, or about 10% of total blood volume. The ratio of iron to blood is 1:1, meaning that for each milligram of blood drawn, one milligram of iron is lost. Considering that women only have 250 mg of iron reserves and men have 1,000 mg of reserves, many frequent donors are at risk of developing iron deficiency.
This study found that the total units of blood collected increased with shorter inter-donation intervals, while secondary safety outcomes of hemoglobin and ferritin levels remained similar between short and long inter-donation interval groups. While overall health did not decline, short inter-donation donors reported symptoms consistent with iron deficiency, including dizziness, tiredness, and restless legs. Researchers found that “about 25% of men and women at the most frequent inter-donation interval had iron deficiency and a third had at least one deferral for low hemoglobin.”
Iron deficiency can be mitigated by iron supplements or by lengthening the time between donations, but this does not fully correct the issue. Some blood centers have already introduced ferritin screening and lengthened the inter-donation interval for donors found to have low ferritin concentrations. Given the advances in automated laboratory testing, information technology, and the high compliance of blood donors, individualized approaches for prevention of iron deficiency could be feasible.
Alan E. Mast, MD , is the medical director for the Blood Center of Wisconsin, and an associate professor of pathology and cell biology at the Medical College of Wisconsin, Milwaukee. Edward L. Murphy, MD , is resident professor of laboratory medicine at University of California, San Francisco, and an affiliate of the Blood Systems Research Institute. They made their comments in an editorial that accompanied the study.
Reducing the interval between blood donations from every 12 weeks to every 8 weeks increases blood units collected with little ill effects on donor quality of life other than symptoms related to iron deficiency, based on results reported by the INTERVAL Trial Group.
In the United States, men and women can donate blood every 8 weeks; the Food and Drug Administration and the AABB (formerly the American Association of Blood Banks) have considered lengthening the 8-week minimum inter-donation interval to reduce the risk of iron deficiency. The current practice in the United Kingdom is to allow men to donate every 12 weeks and women every 16 weeks, the authors noted.
The INTERVAL study examined hemoglobin levels, ferritin levels, and self-reported symptoms as well as the number of blood donations over a 2-year period among 22,466 men randomly assigned to either 8-, 10-, or 12-week blood donation intervals and 22,797 women randomly assigned to donation intervals of 12, 14, and 16 weeks.
Men in the 8-week interval group donated significantly more blood on a per-donor basis, contributing 1.69 and 7.9 units more than the men in the 10- and 12-week interval groups, respectively. Similarly, women in the 12-week interval group contributed approximately 0.84 and 0.46 units more than did women in the 14- and 16-week interval groups.
Hemoglobin and ferritin concentrations stayed relatively stable throughout the study in men and women in all donation interval groups. The baseline hemoglobin and ferritin averages were 149.7 g/L and 44.9 mcg/L in men, and 133.9 g/L and 24.6 mcg/L in women, respectively. Ultimately, mean hemoglobin level decreased by 1%-2%. Ferritin level decreased by 15%-30%, indicating that it is a more accurate marker of iron depletion, the study authors wrote. The report was published online in The Lancet.
The researchers also analyzed self-reported symptoms associated with blood donations derived from donor follow-up surveys sent at 6-, 12-, and 18-month intervals. At the end of the 2-year study, donors underwent a series of cognitive function tests and answered a physical activity questionnaire. Increased donation rates correlated with increased symptoms of iron deficiency such as tiredness, restless legs, and dizziness. This effect was reported more strongly in men.
About 10% of participants were allowed to donate despite having baseline hemoglobin concentrations below the minimum regulatory threshold, said the authors, who said that the screening method used in the United Kingdom to test eligibility to donate needs to be reviewed.
“In response to these findings, we have started the COMPARE study (ISRCTN90871183) to provide a systematic, within-person comparison of the relative merits of different haemoglobin screening methods,” they wrote
The study was funded by National Health Service Blood and Transplant and the National Institute for Health Research Blood and Transplant Research Unit in Donor Health and Genomics (NIHR BTRU-2014-10024).
Reducing the interval between blood donations from every 12 weeks to every 8 weeks increases blood units collected with little ill effects on donor quality of life other than symptoms related to iron deficiency, based on results reported by the INTERVAL Trial Group.
In the United States, men and women can donate blood every 8 weeks; the Food and Drug Administration and the AABB (formerly the American Association of Blood Banks) have considered lengthening the 8-week minimum inter-donation interval to reduce the risk of iron deficiency. The current practice in the United Kingdom is to allow men to donate every 12 weeks and women every 16 weeks, the authors noted.
The INTERVAL study examined hemoglobin levels, ferritin levels, and self-reported symptoms as well as the number of blood donations over a 2-year period among 22,466 men randomly assigned to either 8-, 10-, or 12-week blood donation intervals and 22,797 women randomly assigned to donation intervals of 12, 14, and 16 weeks.
Men in the 8-week interval group donated significantly more blood on a per-donor basis, contributing 1.69 and 7.9 units more than the men in the 10- and 12-week interval groups, respectively. Similarly, women in the 12-week interval group contributed approximately 0.84 and 0.46 units more than did women in the 14- and 16-week interval groups.
Hemoglobin and ferritin concentrations stayed relatively stable throughout the study in men and women in all donation interval groups. The baseline hemoglobin and ferritin averages were 149.7 g/L and 44.9 mcg/L in men, and 133.9 g/L and 24.6 mcg/L in women, respectively. Ultimately, mean hemoglobin level decreased by 1%-2%. Ferritin level decreased by 15%-30%, indicating that it is a more accurate marker of iron depletion, the study authors wrote. The report was published online in The Lancet.
The researchers also analyzed self-reported symptoms associated with blood donations derived from donor follow-up surveys sent at 6-, 12-, and 18-month intervals. At the end of the 2-year study, donors underwent a series of cognitive function tests and answered a physical activity questionnaire. Increased donation rates correlated with increased symptoms of iron deficiency such as tiredness, restless legs, and dizziness. This effect was reported more strongly in men.
About 10% of participants were allowed to donate despite having baseline hemoglobin concentrations below the minimum regulatory threshold, said the authors, who said that the screening method used in the United Kingdom to test eligibility to donate needs to be reviewed.
“In response to these findings, we have started the COMPARE study (ISRCTN90871183) to provide a systematic, within-person comparison of the relative merits of different haemoglobin screening methods,” they wrote
The study was funded by National Health Service Blood and Transplant and the National Institute for Health Research Blood and Transplant Research Unit in Donor Health and Genomics (NIHR BTRU-2014-10024).
FROM THE LANCET
Key clinical point:
Major finding: Reducing inter-donation intervals increases blood collections by 33% in men and 24% in women.
Data source: Parallel group, pragmatic, randomized trial of 22,466 men and 22,797 women from the 25 donor centers of National Health Service Blood and Transplant.
Disclosures: The study was funded by National Health Service Blood and Transplant and the National Institute for Health Research Blood and Transplant Research Unit in Donor Health and Genomics.
Oral anticoagulation ‘reasonable’ in advanced kidney disease with A-fib
BARCELONA – Oral anticoagulation had a net overall benefit for patients with atrial fibrillation and advanced chronic kidney disease, based on results of a large observational study reported at the annual congress of the European Society of Cardiology.
The novel direct-acting oral anticoagulants (NOACs) and warfarin were all similarly effective in this study of 39,241 patients who had stage 4 or 5 chronic kidney disease (CKD), atrial fibrillation, and were not on dialysis. Compared with no oral anticoagulation, the drugs cut in half the risk of stroke or systemic embolism, with no increased risk of major bleeding.
“In patients with advanced CKD, it appears that OACs [oral anticoagulants] are reasonable,” concluded Peter A. Noseworthy, MD, of the Mayo Clinic in Rochester, Minn.
This is a potentially practice-changing finding given the “striking underutilization” of OACs in advanced CKD, he noted. Indeed, only one-third of the patients in this study were prescribed an OAC and picked up their prescriptions. And while the study has the limitations inherent to an observational study reliant upon data from a large U.S. administrative database – chiefly, the potential for residual confounding because of factors that couldn’t be adjusted for statistically – these real-world data may be as good as it gets, since patients with advanced CKD were excluded from the pivotal trials of the NOACs.
Apixaban (Eliquis) was the winner in this study: It separated itself from the pack by reducing the major bleeding risk by 57%, compared with warfarin, although it wasn’t significantly more effective than the other drugs in terms of stroke prevention. In contrast, the major bleeding rates for dabigatran (Pradaxa) and rivaroxaban (Xarelto) weren’t significantly different from warfarin in this challenging patient population.
In a related analysis of 10,712 patients with atrial fibrillation and advanced CKD who were on dialysis, use of an OAC was once again a winning strategy: It resulted not only in an impressive 58% reduction in the risk of stroke or systemic embolism, but also a 26% reduction in the risk of major bleeding, compared with no OAC.
Here again, apixaban was arguably the drug of choice. None of the 125 dialysis patients on apixaban experienced a stroke or systemic embolism. In contrast, dabigatran and rivaroxaban were associated with greater than threefold higher stroke rates than in patients on warfarin, although these differences didn’t achieve statistical significance because of small numbers, just 36 patients on dabigatran and 56 on rivaroxaban, the cardiologist continued.
For these analyses of the relationship between OAC exposure and stroke and bleeding outcomes, Dr. Noseworthy and his coinvestigators used propensity scores based upon 59 clinical and sociodemographic characteristics.
Asked why rates of utilization of OACs are so low in patients with advanced CKD, Dr. Noseworthy replied that he didn’t find that particularly surprising.
“Even if you look only at patients without renal dysfunction, there is incredible undertreatment of atrial fibrillation with OACs. And adherence is very poor,” he observed.
Moreover, in talking with nephrologists, he finds many of them have legitimate reservations about prescribing OACs for patients with end-stage renal disease on hemodialysis.
“They’re undergoing a lot of procedures. They’re having a ton of lines placed; they’re having fistulas revised; and they have very high rates of GI bleeding. In some studies the annual risk of bleeding is 20%-40% in this population. And they’re a frail population with frequent falls,” Dr. Noseworthy said.
He reported having no financial conflicts of interest regarding his study, which was conducted free of commercial support.
BARCELONA – Oral anticoagulation had a net overall benefit for patients with atrial fibrillation and advanced chronic kidney disease, based on results of a large observational study reported at the annual congress of the European Society of Cardiology.
The novel direct-acting oral anticoagulants (NOACs) and warfarin were all similarly effective in this study of 39,241 patients who had stage 4 or 5 chronic kidney disease (CKD), atrial fibrillation, and were not on dialysis. Compared with no oral anticoagulation, the drugs cut in half the risk of stroke or systemic embolism, with no increased risk of major bleeding.
“In patients with advanced CKD, it appears that OACs [oral anticoagulants] are reasonable,” concluded Peter A. Noseworthy, MD, of the Mayo Clinic in Rochester, Minn.
This is a potentially practice-changing finding given the “striking underutilization” of OACs in advanced CKD, he noted. Indeed, only one-third of the patients in this study were prescribed an OAC and picked up their prescriptions. And while the study has the limitations inherent to an observational study reliant upon data from a large U.S. administrative database – chiefly, the potential for residual confounding because of factors that couldn’t be adjusted for statistically – these real-world data may be as good as it gets, since patients with advanced CKD were excluded from the pivotal trials of the NOACs.
Apixaban (Eliquis) was the winner in this study: It separated itself from the pack by reducing the major bleeding risk by 57%, compared with warfarin, although it wasn’t significantly more effective than the other drugs in terms of stroke prevention. In contrast, the major bleeding rates for dabigatran (Pradaxa) and rivaroxaban (Xarelto) weren’t significantly different from warfarin in this challenging patient population.
In a related analysis of 10,712 patients with atrial fibrillation and advanced CKD who were on dialysis, use of an OAC was once again a winning strategy: It resulted not only in an impressive 58% reduction in the risk of stroke or systemic embolism, but also a 26% reduction in the risk of major bleeding, compared with no OAC.
Here again, apixaban was arguably the drug of choice. None of the 125 dialysis patients on apixaban experienced a stroke or systemic embolism. In contrast, dabigatran and rivaroxaban were associated with greater than threefold higher stroke rates than in patients on warfarin, although these differences didn’t achieve statistical significance because of small numbers, just 36 patients on dabigatran and 56 on rivaroxaban, the cardiologist continued.
For these analyses of the relationship between OAC exposure and stroke and bleeding outcomes, Dr. Noseworthy and his coinvestigators used propensity scores based upon 59 clinical and sociodemographic characteristics.
Asked why rates of utilization of OACs are so low in patients with advanced CKD, Dr. Noseworthy replied that he didn’t find that particularly surprising.
“Even if you look only at patients without renal dysfunction, there is incredible undertreatment of atrial fibrillation with OACs. And adherence is very poor,” he observed.
Moreover, in talking with nephrologists, he finds many of them have legitimate reservations about prescribing OACs for patients with end-stage renal disease on hemodialysis.
“They’re undergoing a lot of procedures. They’re having a ton of lines placed; they’re having fistulas revised; and they have very high rates of GI bleeding. In some studies the annual risk of bleeding is 20%-40% in this population. And they’re a frail population with frequent falls,” Dr. Noseworthy said.
He reported having no financial conflicts of interest regarding his study, which was conducted free of commercial support.
BARCELONA – Oral anticoagulation had a net overall benefit for patients with atrial fibrillation and advanced chronic kidney disease, based on results of a large observational study reported at the annual congress of the European Society of Cardiology.
The novel direct-acting oral anticoagulants (NOACs) and warfarin were all similarly effective in this study of 39,241 patients who had stage 4 or 5 chronic kidney disease (CKD), atrial fibrillation, and were not on dialysis. Compared with no oral anticoagulation, the drugs cut in half the risk of stroke or systemic embolism, with no increased risk of major bleeding.
“In patients with advanced CKD, it appears that OACs [oral anticoagulants] are reasonable,” concluded Peter A. Noseworthy, MD, of the Mayo Clinic in Rochester, Minn.
This is a potentially practice-changing finding given the “striking underutilization” of OACs in advanced CKD, he noted. Indeed, only one-third of the patients in this study were prescribed an OAC and picked up their prescriptions. And while the study has the limitations inherent to an observational study reliant upon data from a large U.S. administrative database – chiefly, the potential for residual confounding because of factors that couldn’t be adjusted for statistically – these real-world data may be as good as it gets, since patients with advanced CKD were excluded from the pivotal trials of the NOACs.
Apixaban (Eliquis) was the winner in this study: It separated itself from the pack by reducing the major bleeding risk by 57%, compared with warfarin, although it wasn’t significantly more effective than the other drugs in terms of stroke prevention. In contrast, the major bleeding rates for dabigatran (Pradaxa) and rivaroxaban (Xarelto) weren’t significantly different from warfarin in this challenging patient population.
In a related analysis of 10,712 patients with atrial fibrillation and advanced CKD who were on dialysis, use of an OAC was once again a winning strategy: It resulted not only in an impressive 58% reduction in the risk of stroke or systemic embolism, but also a 26% reduction in the risk of major bleeding, compared with no OAC.
Here again, apixaban was arguably the drug of choice. None of the 125 dialysis patients on apixaban experienced a stroke or systemic embolism. In contrast, dabigatran and rivaroxaban were associated with greater than threefold higher stroke rates than in patients on warfarin, although these differences didn’t achieve statistical significance because of small numbers, just 36 patients on dabigatran and 56 on rivaroxaban, the cardiologist continued.
For these analyses of the relationship between OAC exposure and stroke and bleeding outcomes, Dr. Noseworthy and his coinvestigators used propensity scores based upon 59 clinical and sociodemographic characteristics.
Asked why rates of utilization of OACs are so low in patients with advanced CKD, Dr. Noseworthy replied that he didn’t find that particularly surprising.
“Even if you look only at patients without renal dysfunction, there is incredible undertreatment of atrial fibrillation with OACs. And adherence is very poor,” he observed.
Moreover, in talking with nephrologists, he finds many of them have legitimate reservations about prescribing OACs for patients with end-stage renal disease on hemodialysis.
“They’re undergoing a lot of procedures. They’re having a ton of lines placed; they’re having fistulas revised; and they have very high rates of GI bleeding. In some studies the annual risk of bleeding is 20%-40% in this population. And they’re a frail population with frequent falls,” Dr. Noseworthy said.
He reported having no financial conflicts of interest regarding his study, which was conducted free of commercial support.
AT THE ESC CONGRESS 2017
Key clinical point:
Major finding: The risk of stroke/systemic embolism in patients with advanced chronic kidney disease who were on oral anticoagulation was reduced by 49% among those not on hemodialysis and by 58% in those who were, compared with similar patients not on oral anticoagulation.
Data source: This was an observational study of nearly 50,000 patients with atrial fibrillation and stage 4 or 5 chronic kidney disease in a large U.S. administrative database.
Disclosures: The presenter reported having no financial conflicts of interest regarding his study, which was conducted free of commercial support.
Hospital mortality for emergency bowel resection linked to failure to rescue
BALTIMORE – The variation among hospitals in mortality for emergent bowel resection may be explained in part by failure-to-rescue (FTR) rates, according to a study presented at the American Association for Surgery of Trauma annual meeting.
Ambar Mehta, a medical student at Johns Hopkins University School of Medicine in Baltimore, and a team of colleagues reviewed case data on 105,925 bowel resections that occurred between 2010 and 2013 using the Agency for Healthcare Research and Quality Nationwide Inpatient Sample.
Hospitals included were ranked by mortality for emergency bowel resection with the top quintile representing lowest mortality (1.5%) and bottom quintile representing the highest (14.9%).
Failure to rescue was defined as death after a major postoperative complication, according to Mr. Mehta. Overall failure-to-rescue rates were 9.8-fold higher in the bottom quintile hospitals, compared with those in the top quintile (33.4% vs. 3.4%). Patients studied were majority white (78.9%), female (53.9%), and younger than 65 years (52%).
Although FTR rates were significantly higher in the bottom quintile hospitals, complication rates were comparable (41.6% for bottom vs. 33.3% for top), suggesting that it was not complications per se that drove mortality but other postop factors.
Complications measured were acute renal failure, pulmonary failure, pneumonia, hemorrhage, gastrointestinal bleeding, pulmonary embolism, surgical site infections, and myocardial infarction.
Risk ratios for failure to rescue showed a similar increasing trend in correlation with mortality, with the hospitals in the lowest quintile showing a risk of 13 (P less than .01), compared with a risk of 3.2 (P less than .01) among hospitals in the highest quintile.
Correlation between failure to rescue and mortality was still evident when investigators adjusted analysis to compare hospitals that conducted more than 10 resections annually, causing investigators to suggest a need for changes in resection procedures.
“Our data suggest rates of failure to rescue correlate to rates of hospital mortality and we believe that system-level initiatives such as focusing on teamwork and team culture can reduce nationwide variations in mortality,” said Mr. Mehta.
Discussant Andrew Peitzman, MD, FACS, vice president for Trauma and Surgical Services at the University of Pittsburgh, acknowledged the link between mortality and failure to rescue among bowel resection patients and brought up the question of how to avoid such issues in the first place.
“This study validates the principle that a patient will generally tolerate an operation but not the first complication,” said Dr. Peitzman. “How do we avoid the first complication [and] what do you recommend in our acute care surgery practices and hospital structures to rescue our patients?”
Understanding why the complications happen at all is the first step to preventing them, Mr. Mehta said. Emergency general surgery–specific programs or mentorship programs may be a good start to cutting down on the inherent risk increase of emergent procedures.
Having greater than 20 beds designated to the intensive care unit and having a greater ratio of nursing staff to patients may be other viable solutions, according to a study Mr. Mehta cited; however, he asserted, focusing on team protocols seems to be the most successful course.
When asked by audience members about the idea of regionalizing care, Dr. Mehta said more data would be needed. “Regionalization has definitely shown benefits in a trauma setting,” said Mr. Mehta. “Copying a model of that idea for nontrauma [emergency general surgery] procedures may work, but it would require studies in a multicenter program.”
The study was limited by the use of administrative claims data, including being unable to determine if deaths were caused by a failure to rescue or whether families determined to end care after an initial complication. Investigators were also unable to identify which surgical diagnoses led to the procedure, nor could they adjust for varying hospital resources.
Investigators reported no relevant financial disclosures.
[email protected]
On Twitter @eaztweets
BALTIMORE – The variation among hospitals in mortality for emergent bowel resection may be explained in part by failure-to-rescue (FTR) rates, according to a study presented at the American Association for Surgery of Trauma annual meeting.
Ambar Mehta, a medical student at Johns Hopkins University School of Medicine in Baltimore, and a team of colleagues reviewed case data on 105,925 bowel resections that occurred between 2010 and 2013 using the Agency for Healthcare Research and Quality Nationwide Inpatient Sample.
Hospitals included were ranked by mortality for emergency bowel resection with the top quintile representing lowest mortality (1.5%) and bottom quintile representing the highest (14.9%).
Failure to rescue was defined as death after a major postoperative complication, according to Mr. Mehta. Overall failure-to-rescue rates were 9.8-fold higher in the bottom quintile hospitals, compared with those in the top quintile (33.4% vs. 3.4%). Patients studied were majority white (78.9%), female (53.9%), and younger than 65 years (52%).
Although FTR rates were significantly higher in the bottom quintile hospitals, complication rates were comparable (41.6% for bottom vs. 33.3% for top), suggesting that it was not complications per se that drove mortality but other postop factors.
Complications measured were acute renal failure, pulmonary failure, pneumonia, hemorrhage, gastrointestinal bleeding, pulmonary embolism, surgical site infections, and myocardial infarction.
Risk ratios for failure to rescue showed a similar increasing trend in correlation with mortality, with the hospitals in the lowest quintile showing a risk of 13 (P less than .01), compared with a risk of 3.2 (P less than .01) among hospitals in the highest quintile.
Correlation between failure to rescue and mortality was still evident when investigators adjusted analysis to compare hospitals that conducted more than 10 resections annually, causing investigators to suggest a need for changes in resection procedures.
“Our data suggest rates of failure to rescue correlate to rates of hospital mortality and we believe that system-level initiatives such as focusing on teamwork and team culture can reduce nationwide variations in mortality,” said Mr. Mehta.
Discussant Andrew Peitzman, MD, FACS, vice president for Trauma and Surgical Services at the University of Pittsburgh, acknowledged the link between mortality and failure to rescue among bowel resection patients and brought up the question of how to avoid such issues in the first place.
“This study validates the principle that a patient will generally tolerate an operation but not the first complication,” said Dr. Peitzman. “How do we avoid the first complication [and] what do you recommend in our acute care surgery practices and hospital structures to rescue our patients?”
Understanding why the complications happen at all is the first step to preventing them, Mr. Mehta said. Emergency general surgery–specific programs or mentorship programs may be a good start to cutting down on the inherent risk increase of emergent procedures.
Having greater than 20 beds designated to the intensive care unit and having a greater ratio of nursing staff to patients may be other viable solutions, according to a study Mr. Mehta cited; however, he asserted, focusing on team protocols seems to be the most successful course.
When asked by audience members about the idea of regionalizing care, Dr. Mehta said more data would be needed. “Regionalization has definitely shown benefits in a trauma setting,” said Mr. Mehta. “Copying a model of that idea for nontrauma [emergency general surgery] procedures may work, but it would require studies in a multicenter program.”
The study was limited by the use of administrative claims data, including being unable to determine if deaths were caused by a failure to rescue or whether families determined to end care after an initial complication. Investigators were also unable to identify which surgical diagnoses led to the procedure, nor could they adjust for varying hospital resources.
Investigators reported no relevant financial disclosures.
[email protected]
On Twitter @eaztweets
BALTIMORE – The variation among hospitals in mortality for emergent bowel resection may be explained in part by failure-to-rescue (FTR) rates, according to a study presented at the American Association for Surgery of Trauma annual meeting.
Ambar Mehta, a medical student at Johns Hopkins University School of Medicine in Baltimore, and a team of colleagues reviewed case data on 105,925 bowel resections that occurred between 2010 and 2013 using the Agency for Healthcare Research and Quality Nationwide Inpatient Sample.
Hospitals included were ranked by mortality for emergency bowel resection with the top quintile representing lowest mortality (1.5%) and bottom quintile representing the highest (14.9%).
Failure to rescue was defined as death after a major postoperative complication, according to Mr. Mehta. Overall failure-to-rescue rates were 9.8-fold higher in the bottom quintile hospitals, compared with those in the top quintile (33.4% vs. 3.4%). Patients studied were majority white (78.9%), female (53.9%), and younger than 65 years (52%).
Although FTR rates were significantly higher in the bottom quintile hospitals, complication rates were comparable (41.6% for bottom vs. 33.3% for top), suggesting that it was not complications per se that drove mortality but other postop factors.
Complications measured were acute renal failure, pulmonary failure, pneumonia, hemorrhage, gastrointestinal bleeding, pulmonary embolism, surgical site infections, and myocardial infarction.
Risk ratios for failure to rescue showed a similar increasing trend in correlation with mortality, with the hospitals in the lowest quintile showing a risk of 13 (P less than .01), compared with a risk of 3.2 (P less than .01) among hospitals in the highest quintile.
Correlation between failure to rescue and mortality was still evident when investigators adjusted analysis to compare hospitals that conducted more than 10 resections annually, causing investigators to suggest a need for changes in resection procedures.
“Our data suggest rates of failure to rescue correlate to rates of hospital mortality and we believe that system-level initiatives such as focusing on teamwork and team culture can reduce nationwide variations in mortality,” said Mr. Mehta.
Discussant Andrew Peitzman, MD, FACS, vice president for Trauma and Surgical Services at the University of Pittsburgh, acknowledged the link between mortality and failure to rescue among bowel resection patients and brought up the question of how to avoid such issues in the first place.
“This study validates the principle that a patient will generally tolerate an operation but not the first complication,” said Dr. Peitzman. “How do we avoid the first complication [and] what do you recommend in our acute care surgery practices and hospital structures to rescue our patients?”
Understanding why the complications happen at all is the first step to preventing them, Mr. Mehta said. Emergency general surgery–specific programs or mentorship programs may be a good start to cutting down on the inherent risk increase of emergent procedures.
Having greater than 20 beds designated to the intensive care unit and having a greater ratio of nursing staff to patients may be other viable solutions, according to a study Mr. Mehta cited; however, he asserted, focusing on team protocols seems to be the most successful course.
When asked by audience members about the idea of regionalizing care, Dr. Mehta said more data would be needed. “Regionalization has definitely shown benefits in a trauma setting,” said Mr. Mehta. “Copying a model of that idea for nontrauma [emergency general surgery] procedures may work, but it would require studies in a multicenter program.”
The study was limited by the use of administrative claims data, including being unable to determine if deaths were caused by a failure to rescue or whether families determined to end care after an initial complication. Investigators were also unable to identify which surgical diagnoses led to the procedure, nor could they adjust for varying hospital resources.
Investigators reported no relevant financial disclosures.
[email protected]
On Twitter @eaztweets
FROM AAST
Key clinical point:
Major finding: Risk-adjusted failure-to-rescue rates were 9.8 times higher in hospitals with the highest mortality than in those with the lowest (33.4% vs. 3.1%).
Data source: Study of 105,925 bowel resections that occurred between 2010 and 2013 collected from the AHRQ Nationwide Inpatient Sample.
Disclosures: Investigators reported no relevant financial disclosures.
Increase in Kids Who Are Getting the HPV Vaccine
The HPV vaccine has led to “dramatic declines” in HPV infections, according to the CDC. Since the first HPV vaccine was introduced 10 years ago, the percentage of infections that cause cancers and genital warts has dropped by 71% among teenage girls and 61% among young women. According to the annual National Immunization Survey-Teen report, 60% of teens aged 13 to 17 years received ≥ 1 doses of HPV vaccine in 2016, up 4 percentage points from 2015.
More boys are getting the vaccine, too. About 56% of boys received their first dose (although that is still less than the 65% seen in girls)—representing a 6% increase from 2015; rates for girls remained stable.
As encouraging as those numbers are, there is more work to do, the CDC says. Although most adolescents have received the first dose, only 43% are up-to-date on all the recommended doses. The CDC recommends that 11- to 12-year-olds get 2 doses of HPV vaccine at least 6 months apart. The CDC updated its HPV vaccine recommendations in 2016 when new evidence showed that 2 doses of the vaccine provided levels of protection similar to those seen for 3 doses in older adolescents and young adults.
Parents can get the vaccine for their child during any doctor’s visit, but the CDC recommends that adolescents get the HPV vaccine during the same visit that they get whooping cough and meningitis vaccine.
The HPV vaccine has led to “dramatic declines” in HPV infections, according to the CDC. Since the first HPV vaccine was introduced 10 years ago, the percentage of infections that cause cancers and genital warts has dropped by 71% among teenage girls and 61% among young women. According to the annual National Immunization Survey-Teen report, 60% of teens aged 13 to 17 years received ≥ 1 doses of HPV vaccine in 2016, up 4 percentage points from 2015.
More boys are getting the vaccine, too. About 56% of boys received their first dose (although that is still less than the 65% seen in girls)—representing a 6% increase from 2015; rates for girls remained stable.
As encouraging as those numbers are, there is more work to do, the CDC says. Although most adolescents have received the first dose, only 43% are up-to-date on all the recommended doses. The CDC recommends that 11- to 12-year-olds get 2 doses of HPV vaccine at least 6 months apart. The CDC updated its HPV vaccine recommendations in 2016 when new evidence showed that 2 doses of the vaccine provided levels of protection similar to those seen for 3 doses in older adolescents and young adults.
Parents can get the vaccine for their child during any doctor’s visit, but the CDC recommends that adolescents get the HPV vaccine during the same visit that they get whooping cough and meningitis vaccine.
The HPV vaccine has led to “dramatic declines” in HPV infections, according to the CDC. Since the first HPV vaccine was introduced 10 years ago, the percentage of infections that cause cancers and genital warts has dropped by 71% among teenage girls and 61% among young women. According to the annual National Immunization Survey-Teen report, 60% of teens aged 13 to 17 years received ≥ 1 doses of HPV vaccine in 2016, up 4 percentage points from 2015.
More boys are getting the vaccine, too. About 56% of boys received their first dose (although that is still less than the 65% seen in girls)—representing a 6% increase from 2015; rates for girls remained stable.
As encouraging as those numbers are, there is more work to do, the CDC says. Although most adolescents have received the first dose, only 43% are up-to-date on all the recommended doses. The CDC recommends that 11- to 12-year-olds get 2 doses of HPV vaccine at least 6 months apart. The CDC updated its HPV vaccine recommendations in 2016 when new evidence showed that 2 doses of the vaccine provided levels of protection similar to those seen for 3 doses in older adolescents and young adults.
Parents can get the vaccine for their child during any doctor’s visit, but the CDC recommends that adolescents get the HPV vaccine during the same visit that they get whooping cough and meningitis vaccine.
Onodera’s Prognostic Nutritional Index in soft tissue sarcoma patients as a predictor of wound complications
Wound complications after pre- or post-operative radiation for soft tissue sarcomas are well established.1 The ability to predict who will have a wound complication remains difficult. Some studies have looked at risk factors such as smoking, and the preoperative nutritional status of patients has been identified as a risk factor for wound complication in patients with elective orthopedic surgical procedures.2 One validated method of measuring preoperative nutritional status in patients with gastrointestinal malignant tumors has been with Onodera’s Prognostic Nutritional Index (OPNI). It uses the patient’s preoperative albumin (g/dL) and absolute lymphocyte values (per mm3). The prognostic value of the OPNI has been demonstrated in patients with colorectal, esophageal, and gastric cancers, and has been shown to be prognostic for postoperative wound healing and overall prognosis.3-5 In this study, we investigate the significance of preoperative nutritional status, measured by OPNI, as a predictor of wound complications in patients treated with pre- or postoperative radiation for soft tissue sarcoma.
Methods
After receiving Institutional Review Board approval for the study, we conducted a retrospective review of consecutive patients treated during July 2012-April 2016 for a soft tissue sarcoma by the orthopedic oncology division at Cooper University Hospital in Camden, New Jersey. Inclusion criteria were patients with biopsy-proven soft tissue sarcoma, who were older than 18 years, had received pre- or postoperative radiation, and who had a recorded preoperative albumin and total lymphocyte count. A minimum follow-up of 3 months was required to assess for postoperative wound complications. Exclusion criteria included patients who had a bone sarcoma, had not received radiation therapy, or had a missing preoperative albumin or total lymphocyte count.
All of the surgeries were performed by 2 fellowshiptrained orthopedic oncologists. Patients received either pre- or postoperative radiation therapy by multiple radiation oncologists.
The OPNI was calculated based on the published formula OPNI = (10*albumin level [g/dL]) + (0.005*total lymphocyte count [per mm3]). The albumin level and total lymphocyte counts closest to the index operation were chosen.
Demographic information including gender, age at diagnosis, height, and weight were recorded. Data related to the patients’ pathologic diagnosis, stage at presentation, radiation therapy, and surgical resection were collected. A minor wound complication was defined as a wound problem that did not require operative intervention. Major wound complication was defined as a complication requiring operative intervention with or without flap reconstruction. Wound complications occurring within the 3-month postoperative period were considered.
Univariate and multiple variable analysis was performed. A P value <.05 was considered significant. A receiver operating curve as well as recursive partitioning was performed for OPNI and age to determine the best cut-off point to use in the analysis. The Sobel test was used to evaluate mediation. All statistical analysis was performed using SAS v9.4 and JMP10. (SAS Institute, Cary, NC).
Results
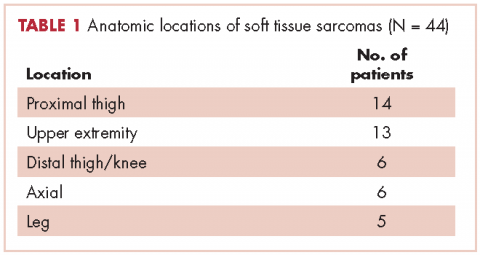
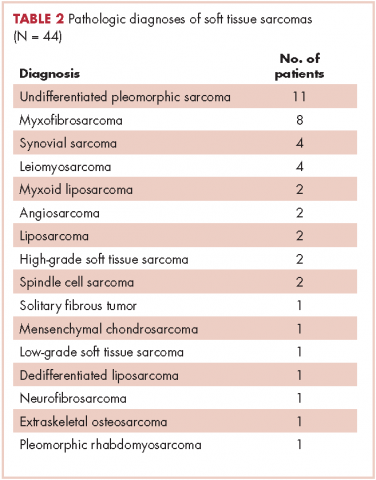
In all, 44 patients (28 men, 16 women) were included in the study. Their mean age was 61.2 years (range, 19-94). The average size of the tumors was 8.5 cm in greatest dimension (range, 1.2-27.4 cm), and all of the patients had nonmetastatic disease at the time of surgical resection; 37 patients had R0 resections, and 7 patients had a positive margin from an outside hospital, but obtained R0 resections on a subsequent resection (Table 1 and Table 2).
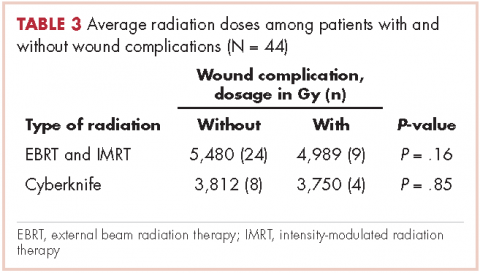
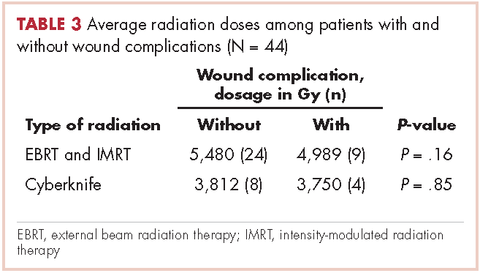
In all, 30 patients received preoperative radiation, 14 patients received postoperative radiation, 32 patients received external beam radiation, 8 received Cyberknife treatment, and information for 4 patients was not unavailable. Mean preoperative external beam radiation and Cyberknife dose was 4,931 Gy and 3,750 Gy, respectively. Mean postoperative external beam and Cyberknife radiation dose was 6,077 Gy and 4,000 Gy, respectively. When evaluating radiation dose delivered between those who had wound complications and those who did not, there was no significant difference (Table 3).
Of the total, 13 patients had a wound complication (30%). Ten patients had preoperative radiation, and 3 had postoperative radiation. Ten patients had major wound complications requiring a combined 27 surgeries. Three patients had minor wound complications, which resolved with conservative management. One patient had a major wound complication in the group that had an initial R1 resection.
The OPNI was calculated based on the aforementioned formula. When the univariate analysis was performed, only age and OPNI were statistically significant. Patients older than 72.6 years had a 6.8 times higher risk of a wound complication (P = .01; 95% confidence interval [CI], 1.6-28.7). When the OPNI value of 45.4 was used as the threshold, a patient with a preoperative OPNI value of <45.4 had a 7.5 times increased risk of developing a wound complication (P = .005; 95% CI, 1.8-31.0).
When the receiver operating curve and recursive partitioning was performed, an OPNI value of 45.4 showed a sensitivity of 62% and specificity of 82% in predicting wound complications (Figure 1).
When a multiple variable analysis was performed, OPNI and age were not statistically significant (P = .06 and P = .11, respectively). A test for mediation was performed, and the OPNI seemed to mediate the effect age has on wound complications, accounting for 36% of the total effect (Sobel test statistic, 1.79; P = .07).
Discussion
Wound complications after pre- and postoperative radiation for soft tissue sarcomas are well known. The best study to date to demonstrate that relationship was a randomized controlled trial performed in Canada, which showed that preoperative radiation resulted in 37% wound complications, compared with 17% for postoperative radiation.6 In that study, of the wound complications in both radiation types, more than 50%-60% required a secondary surgical procedure, designating it as a major wound complication. Other variables that have been shown to contribute to wound complications include being older than 40 years and/or having large tumors, diabetes, peripheral vascular disease, and begin a smoker.7-10
In our study, we applied OPNI to orthopedic oncology and showed that the patient’s age and preoperative nutritional status were significant predictors of developing a wound complication. An OPNI of <45.4 increased the chance of a wound complication by 7.5 times. Being older than 73 years increased the risk of a wound complication by 6.8 times. Most of these wound complications were major and required surgical intervention.
In general surgical oncology, the evaluation of nutritional status has had a significant impact on the care of patients, especially for those patients undergoing gastrointestinal surgery. The OPNI was initially designed to assess the nutritional and immunological statuses of patients undergoing gastrointestinal surgery.11 Preoperative OPNI has been shown to be a good predictor of postoperative complications and survival in patients with colorectal cancer, malignant mesothelioma, hepatocellular carcinoma and in patients who undergo total gastrectomy.12-15 Chen and colleagues evaluated the significance of OPNI in patients with colorectal cancer. They found an optimal cut-off value of 45. An OPNI value <45 has a sensitivity and specificity of 85% and 69%, respectively, in predicting 5-year overall survival.16 Hong and colleagues noted that an OPNI cut-off value of 52.6 as a predictor of overall survival.17
Poor preoperative nutritional status has been shown to have a negative impact on wound healing. In patients who underwent emergency laparotomy, a low OPNI had significantly higher rates of wound dehiscence and infection.18 This happens because protein deficiency leads to decreased wound tensile strength, decreased T-cell function, decreased phagocytic activity, which ultimately diminish the patient’s ability to heal and defend against wound infections.19-21
In soft tissue sarcoma patients, poor preoperative nutritional status is further compromised by radiation therapy to the wound. Gu and colleagues showed that radiation to wounds in mice showed early inhibition of the inflammatory phase, injury and inhibition of fibroblasts, and collagen formation, and then prolonged re-epithelialization.22 This “double hit” with radiation onto host tissue that is already nutritionally compromised could be an important cause of why wound complications occur at such high rates in our soft tissue sarcoma patients.
There are several limitations to this study. First, the study has a small sample size, which was a direct result of the number of patients who were excluded because an OPNI value could not be calculated for them. Second, we could not determine if the OPNI was more valuable in patients who underwent pre- or postoperative radiation. This study did not look at other nutritional indices such as prealbumin and vitamin levels. Third, the radiation was provided by different providers, so technique was variable, but the patients received nearly equivalent doses and variability in technique is likely limited. Fourth, we were not able to meaningfully analyze the role of chemotherapy in this patient population because there was a significant heterogeneity of patients receiving pre- and postoperative chemotherapy.
Our findings strongly suggest that a preoperative OPNI of <45.4 and being older than 73 years are strong predictors of patients who will experience a wound complication after radiation therapy for soft tissue sarcomas. This study has led us to start measuring preoperative albumin levels and assess complete metabolic panels. Our goal is to identify patients who are at high risk of wound complication and perform interventions to improve nutrition, then to study whether the interventions help lower the rates of wound complications.
1. Ormsby MV, Hilaris BS, Nori D, Brennan MF. Wound complications of adjuvant radiation therapy in patients with soft-tissue sarcomas. Ann Surg. 1989;210(1):93-99.
2. Greene KA, Wilde AH, Stulberg BN. Preoperative nutritional status of total joint patients: relationship to postoperative wound complications. J Arthroplasty. 1991;6(4):321-325.
3. Nozoe T, Kimura Y, Ishida M, Saeki H, Korenaga D, Sugimachi K. Correlation of pre-operative nutritional condition with post-operative complications in surgical treatment for oesophageal carcinoma. Eur J Surg Oncol. 2002;28(4):396-400.
4. Nozoe T, Kohno M, Iguchi T, et al. The prognostic nutritional index can be a prognostic indicator in colorectal carcinoma. Surg Today. 2012;42(6):532-535.
5. Nozoe T, Ninomiya M, Maeda T, Matsukuma A, Nakashima H, Ezaki T. Prognostic nutritional index: a tool to predict the biological aggressiveness of gastric carcinoma. Surg Today. 2010;40(5):440-443.
6. O’Sullivan B, Davis AM, Turcotte R, Bell R, Catton C, Chabot P, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet. 2002;359(9325):2235-2241.
7. Peat BG, Bell RS, Davis A, et al. Wound-healing complications after soft-tissue sarcoma surgery. Plast Reconstr Surg. 1994;93(5):980-987.
8. Kunisada T, Ngan SY, Powell G, Choong PF. Wound complications following pre-operative radiotherapy for soft tissue sarcoma. Eur J Surg Oncol. 2002;28(1):75-79.
9. Saddegh MK, Bauer HC. Wound complication in surgery of soft tissue sarcoma: analysis of 103 consecutive patients managed without adjuvant therapy. Clin Orthop Relat Res. 1993;289:247-253.
10. Tseng JF, Ballo MT, Langstein HN, et al. The effect of preoperative radiotherapy and reconstructive surgery on wound complications after resection of extremity soft-tissue sarcomas. Ann Surg Oncol. 2006;13(9):1209-1215.
11. Smale BF, Mullen JL, Buzby GP, Rosato EF. The efficacy of nutritional assessment and support in cancer surgery. Cancer. 1981;47(10):2375-2381.
12. Mohri Y, Inoue Y, Tanaka K, Hiro J, Uchida K, Kusunoki M. Prognostic nutritional index predicts postoperative outcome in colorectal cancer. World J Surg. 2013;37(11):2688-2692.
13. Jiang N, Deng JY, Ding XW, et al. Prognostic nutritional index predicts postoperative complications and long-term outcomes of gastric cancer. World J Gastroenterol. 2014;20(30):10537-10544.
14. Pinato DJ, North BV, Sharma R. A novel, externally validated inflammation-based prognostic algorithm in hepatocellular carcinoma: the prognostic nutritional index (PNI). Brit J Cancer. 2012;106(8):1439-1445.
15. Yao ZH, Tian GY, Wan YY, et al. Prognostic nutritional index predicts outcomes of malignant pleural mesothelioma. J Cancer Res Clin Oncol. 2013;139(12):2117-2123.
16. Jian-Hui C, Iskandar EA, Cai Sh I, et al. Significance of Onodera’s prognostic nutritional index in patients with colorectal cancer: a large cohort study in a single Chinese institution. Tumour Biol. 2016;37(3):3277-3283.
17. Hong S, Zhou T, Fang W, et al. The prognostic nutritional index (PNI) predicts overall survival of small-cell lung cancer patients. Tumour Biol. 2015;36(5):3389-9337.
18. Mohil RS, Agarwal A, Singh N, Arora J, Bhatnagar D. Does nutritional status play a role in patients undergoing emergency laparotomy? E Spen Eur E J Clin Nutr Metab. 2008;3(5):e226-e231.
19. Kay SP, Moreland JR, Schmitter E. Nutritional status and wound healing in lower extremity amputations. Clin Orthop Relat Res. 1987;(217):253-256.
20. Dickhaut SC, DeLee JC, Page CP. Nutritional status: importance in predicting wound-healing after amputation. J Bone Joint Surg Am. 1984;66(1):71-75.
21. Casey J, Flinn WR, Yao JS, Fahey V, Pawlowski J, Bergan JJ. Correlation of immune and nutritional status with wound complications in patients undergoing vascular operations. Surgery. 1983;93(6):822-827.
22. Gu Q, Wang D, Cui C, Gao Y, Xia G, Cui X. Effects of radiation on wound healing. J Environ Pathol Toxicol Oncol. 1998;17(2):117-123.
Wound complications after pre- or post-operative radiation for soft tissue sarcomas are well established.1 The ability to predict who will have a wound complication remains difficult. Some studies have looked at risk factors such as smoking, and the preoperative nutritional status of patients has been identified as a risk factor for wound complication in patients with elective orthopedic surgical procedures.2 One validated method of measuring preoperative nutritional status in patients with gastrointestinal malignant tumors has been with Onodera’s Prognostic Nutritional Index (OPNI). It uses the patient’s preoperative albumin (g/dL) and absolute lymphocyte values (per mm3). The prognostic value of the OPNI has been demonstrated in patients with colorectal, esophageal, and gastric cancers, and has been shown to be prognostic for postoperative wound healing and overall prognosis.3-5 In this study, we investigate the significance of preoperative nutritional status, measured by OPNI, as a predictor of wound complications in patients treated with pre- or postoperative radiation for soft tissue sarcoma.
Methods
After receiving Institutional Review Board approval for the study, we conducted a retrospective review of consecutive patients treated during July 2012-April 2016 for a soft tissue sarcoma by the orthopedic oncology division at Cooper University Hospital in Camden, New Jersey. Inclusion criteria were patients with biopsy-proven soft tissue sarcoma, who were older than 18 years, had received pre- or postoperative radiation, and who had a recorded preoperative albumin and total lymphocyte count. A minimum follow-up of 3 months was required to assess for postoperative wound complications. Exclusion criteria included patients who had a bone sarcoma, had not received radiation therapy, or had a missing preoperative albumin or total lymphocyte count.
All of the surgeries were performed by 2 fellowshiptrained orthopedic oncologists. Patients received either pre- or postoperative radiation therapy by multiple radiation oncologists.
The OPNI was calculated based on the published formula OPNI = (10*albumin level [g/dL]) + (0.005*total lymphocyte count [per mm3]). The albumin level and total lymphocyte counts closest to the index operation were chosen.
Demographic information including gender, age at diagnosis, height, and weight were recorded. Data related to the patients’ pathologic diagnosis, stage at presentation, radiation therapy, and surgical resection were collected. A minor wound complication was defined as a wound problem that did not require operative intervention. Major wound complication was defined as a complication requiring operative intervention with or without flap reconstruction. Wound complications occurring within the 3-month postoperative period were considered.
Univariate and multiple variable analysis was performed. A P value <.05 was considered significant. A receiver operating curve as well as recursive partitioning was performed for OPNI and age to determine the best cut-off point to use in the analysis. The Sobel test was used to evaluate mediation. All statistical analysis was performed using SAS v9.4 and JMP10. (SAS Institute, Cary, NC).
Results
In all, 44 patients (28 men, 16 women) were included in the study. Their mean age was 61.2 years (range, 19-94). The average size of the tumors was 8.5 cm in greatest dimension (range, 1.2-27.4 cm), and all of the patients had nonmetastatic disease at the time of surgical resection; 37 patients had R0 resections, and 7 patients had a positive margin from an outside hospital, but obtained R0 resections on a subsequent resection (Table 1 and Table 2).
In all, 30 patients received preoperative radiation, 14 patients received postoperative radiation, 32 patients received external beam radiation, 8 received Cyberknife treatment, and information for 4 patients was not unavailable. Mean preoperative external beam radiation and Cyberknife dose was 4,931 Gy and 3,750 Gy, respectively. Mean postoperative external beam and Cyberknife radiation dose was 6,077 Gy and 4,000 Gy, respectively. When evaluating radiation dose delivered between those who had wound complications and those who did not, there was no significant difference (Table 3).
Of the total, 13 patients had a wound complication (30%). Ten patients had preoperative radiation, and 3 had postoperative radiation. Ten patients had major wound complications requiring a combined 27 surgeries. Three patients had minor wound complications, which resolved with conservative management. One patient had a major wound complication in the group that had an initial R1 resection.
The OPNI was calculated based on the aforementioned formula. When the univariate analysis was performed, only age and OPNI were statistically significant. Patients older than 72.6 years had a 6.8 times higher risk of a wound complication (P = .01; 95% confidence interval [CI], 1.6-28.7). When the OPNI value of 45.4 was used as the threshold, a patient with a preoperative OPNI value of <45.4 had a 7.5 times increased risk of developing a wound complication (P = .005; 95% CI, 1.8-31.0).
When the receiver operating curve and recursive partitioning was performed, an OPNI value of 45.4 showed a sensitivity of 62% and specificity of 82% in predicting wound complications (Figure 1).
When a multiple variable analysis was performed, OPNI and age were not statistically significant (P = .06 and P = .11, respectively). A test for mediation was performed, and the OPNI seemed to mediate the effect age has on wound complications, accounting for 36% of the total effect (Sobel test statistic, 1.79; P = .07).
Discussion
Wound complications after pre- and postoperative radiation for soft tissue sarcomas are well known. The best study to date to demonstrate that relationship was a randomized controlled trial performed in Canada, which showed that preoperative radiation resulted in 37% wound complications, compared with 17% for postoperative radiation.6 In that study, of the wound complications in both radiation types, more than 50%-60% required a secondary surgical procedure, designating it as a major wound complication. Other variables that have been shown to contribute to wound complications include being older than 40 years and/or having large tumors, diabetes, peripheral vascular disease, and begin a smoker.7-10
In our study, we applied OPNI to orthopedic oncology and showed that the patient’s age and preoperative nutritional status were significant predictors of developing a wound complication. An OPNI of <45.4 increased the chance of a wound complication by 7.5 times. Being older than 73 years increased the risk of a wound complication by 6.8 times. Most of these wound complications were major and required surgical intervention.
In general surgical oncology, the evaluation of nutritional status has had a significant impact on the care of patients, especially for those patients undergoing gastrointestinal surgery. The OPNI was initially designed to assess the nutritional and immunological statuses of patients undergoing gastrointestinal surgery.11 Preoperative OPNI has been shown to be a good predictor of postoperative complications and survival in patients with colorectal cancer, malignant mesothelioma, hepatocellular carcinoma and in patients who undergo total gastrectomy.12-15 Chen and colleagues evaluated the significance of OPNI in patients with colorectal cancer. They found an optimal cut-off value of 45. An OPNI value <45 has a sensitivity and specificity of 85% and 69%, respectively, in predicting 5-year overall survival.16 Hong and colleagues noted that an OPNI cut-off value of 52.6 as a predictor of overall survival.17
Poor preoperative nutritional status has been shown to have a negative impact on wound healing. In patients who underwent emergency laparotomy, a low OPNI had significantly higher rates of wound dehiscence and infection.18 This happens because protein deficiency leads to decreased wound tensile strength, decreased T-cell function, decreased phagocytic activity, which ultimately diminish the patient’s ability to heal and defend against wound infections.19-21
In soft tissue sarcoma patients, poor preoperative nutritional status is further compromised by radiation therapy to the wound. Gu and colleagues showed that radiation to wounds in mice showed early inhibition of the inflammatory phase, injury and inhibition of fibroblasts, and collagen formation, and then prolonged re-epithelialization.22 This “double hit” with radiation onto host tissue that is already nutritionally compromised could be an important cause of why wound complications occur at such high rates in our soft tissue sarcoma patients.
There are several limitations to this study. First, the study has a small sample size, which was a direct result of the number of patients who were excluded because an OPNI value could not be calculated for them. Second, we could not determine if the OPNI was more valuable in patients who underwent pre- or postoperative radiation. This study did not look at other nutritional indices such as prealbumin and vitamin levels. Third, the radiation was provided by different providers, so technique was variable, but the patients received nearly equivalent doses and variability in technique is likely limited. Fourth, we were not able to meaningfully analyze the role of chemotherapy in this patient population because there was a significant heterogeneity of patients receiving pre- and postoperative chemotherapy.
Our findings strongly suggest that a preoperative OPNI of <45.4 and being older than 73 years are strong predictors of patients who will experience a wound complication after radiation therapy for soft tissue sarcomas. This study has led us to start measuring preoperative albumin levels and assess complete metabolic panels. Our goal is to identify patients who are at high risk of wound complication and perform interventions to improve nutrition, then to study whether the interventions help lower the rates of wound complications.
Wound complications after pre- or post-operative radiation for soft tissue sarcomas are well established.1 The ability to predict who will have a wound complication remains difficult. Some studies have looked at risk factors such as smoking, and the preoperative nutritional status of patients has been identified as a risk factor for wound complication in patients with elective orthopedic surgical procedures.2 One validated method of measuring preoperative nutritional status in patients with gastrointestinal malignant tumors has been with Onodera’s Prognostic Nutritional Index (OPNI). It uses the patient’s preoperative albumin (g/dL) and absolute lymphocyte values (per mm3). The prognostic value of the OPNI has been demonstrated in patients with colorectal, esophageal, and gastric cancers, and has been shown to be prognostic for postoperative wound healing and overall prognosis.3-5 In this study, we investigate the significance of preoperative nutritional status, measured by OPNI, as a predictor of wound complications in patients treated with pre- or postoperative radiation for soft tissue sarcoma.
Methods
After receiving Institutional Review Board approval for the study, we conducted a retrospective review of consecutive patients treated during July 2012-April 2016 for a soft tissue sarcoma by the orthopedic oncology division at Cooper University Hospital in Camden, New Jersey. Inclusion criteria were patients with biopsy-proven soft tissue sarcoma, who were older than 18 years, had received pre- or postoperative radiation, and who had a recorded preoperative albumin and total lymphocyte count. A minimum follow-up of 3 months was required to assess for postoperative wound complications. Exclusion criteria included patients who had a bone sarcoma, had not received radiation therapy, or had a missing preoperative albumin or total lymphocyte count.
All of the surgeries were performed by 2 fellowshiptrained orthopedic oncologists. Patients received either pre- or postoperative radiation therapy by multiple radiation oncologists.
The OPNI was calculated based on the published formula OPNI = (10*albumin level [g/dL]) + (0.005*total lymphocyte count [per mm3]). The albumin level and total lymphocyte counts closest to the index operation were chosen.
Demographic information including gender, age at diagnosis, height, and weight were recorded. Data related to the patients’ pathologic diagnosis, stage at presentation, radiation therapy, and surgical resection were collected. A minor wound complication was defined as a wound problem that did not require operative intervention. Major wound complication was defined as a complication requiring operative intervention with or without flap reconstruction. Wound complications occurring within the 3-month postoperative period were considered.
Univariate and multiple variable analysis was performed. A P value <.05 was considered significant. A receiver operating curve as well as recursive partitioning was performed for OPNI and age to determine the best cut-off point to use in the analysis. The Sobel test was used to evaluate mediation. All statistical analysis was performed using SAS v9.4 and JMP10. (SAS Institute, Cary, NC).
Results
In all, 44 patients (28 men, 16 women) were included in the study. Their mean age was 61.2 years (range, 19-94). The average size of the tumors was 8.5 cm in greatest dimension (range, 1.2-27.4 cm), and all of the patients had nonmetastatic disease at the time of surgical resection; 37 patients had R0 resections, and 7 patients had a positive margin from an outside hospital, but obtained R0 resections on a subsequent resection (Table 1 and Table 2).
In all, 30 patients received preoperative radiation, 14 patients received postoperative radiation, 32 patients received external beam radiation, 8 received Cyberknife treatment, and information for 4 patients was not unavailable. Mean preoperative external beam radiation and Cyberknife dose was 4,931 Gy and 3,750 Gy, respectively. Mean postoperative external beam and Cyberknife radiation dose was 6,077 Gy and 4,000 Gy, respectively. When evaluating radiation dose delivered between those who had wound complications and those who did not, there was no significant difference (Table 3).
Of the total, 13 patients had a wound complication (30%). Ten patients had preoperative radiation, and 3 had postoperative radiation. Ten patients had major wound complications requiring a combined 27 surgeries. Three patients had minor wound complications, which resolved with conservative management. One patient had a major wound complication in the group that had an initial R1 resection.
The OPNI was calculated based on the aforementioned formula. When the univariate analysis was performed, only age and OPNI were statistically significant. Patients older than 72.6 years had a 6.8 times higher risk of a wound complication (P = .01; 95% confidence interval [CI], 1.6-28.7). When the OPNI value of 45.4 was used as the threshold, a patient with a preoperative OPNI value of <45.4 had a 7.5 times increased risk of developing a wound complication (P = .005; 95% CI, 1.8-31.0).
When the receiver operating curve and recursive partitioning was performed, an OPNI value of 45.4 showed a sensitivity of 62% and specificity of 82% in predicting wound complications (Figure 1).
When a multiple variable analysis was performed, OPNI and age were not statistically significant (P = .06 and P = .11, respectively). A test for mediation was performed, and the OPNI seemed to mediate the effect age has on wound complications, accounting for 36% of the total effect (Sobel test statistic, 1.79; P = .07).
Discussion
Wound complications after pre- and postoperative radiation for soft tissue sarcomas are well known. The best study to date to demonstrate that relationship was a randomized controlled trial performed in Canada, which showed that preoperative radiation resulted in 37% wound complications, compared with 17% for postoperative radiation.6 In that study, of the wound complications in both radiation types, more than 50%-60% required a secondary surgical procedure, designating it as a major wound complication. Other variables that have been shown to contribute to wound complications include being older than 40 years and/or having large tumors, diabetes, peripheral vascular disease, and begin a smoker.7-10
In our study, we applied OPNI to orthopedic oncology and showed that the patient’s age and preoperative nutritional status were significant predictors of developing a wound complication. An OPNI of <45.4 increased the chance of a wound complication by 7.5 times. Being older than 73 years increased the risk of a wound complication by 6.8 times. Most of these wound complications were major and required surgical intervention.
In general surgical oncology, the evaluation of nutritional status has had a significant impact on the care of patients, especially for those patients undergoing gastrointestinal surgery. The OPNI was initially designed to assess the nutritional and immunological statuses of patients undergoing gastrointestinal surgery.11 Preoperative OPNI has been shown to be a good predictor of postoperative complications and survival in patients with colorectal cancer, malignant mesothelioma, hepatocellular carcinoma and in patients who undergo total gastrectomy.12-15 Chen and colleagues evaluated the significance of OPNI in patients with colorectal cancer. They found an optimal cut-off value of 45. An OPNI value <45 has a sensitivity and specificity of 85% and 69%, respectively, in predicting 5-year overall survival.16 Hong and colleagues noted that an OPNI cut-off value of 52.6 as a predictor of overall survival.17
Poor preoperative nutritional status has been shown to have a negative impact on wound healing. In patients who underwent emergency laparotomy, a low OPNI had significantly higher rates of wound dehiscence and infection.18 This happens because protein deficiency leads to decreased wound tensile strength, decreased T-cell function, decreased phagocytic activity, which ultimately diminish the patient’s ability to heal and defend against wound infections.19-21
In soft tissue sarcoma patients, poor preoperative nutritional status is further compromised by radiation therapy to the wound. Gu and colleagues showed that radiation to wounds in mice showed early inhibition of the inflammatory phase, injury and inhibition of fibroblasts, and collagen formation, and then prolonged re-epithelialization.22 This “double hit” with radiation onto host tissue that is already nutritionally compromised could be an important cause of why wound complications occur at such high rates in our soft tissue sarcoma patients.
There are several limitations to this study. First, the study has a small sample size, which was a direct result of the number of patients who were excluded because an OPNI value could not be calculated for them. Second, we could not determine if the OPNI was more valuable in patients who underwent pre- or postoperative radiation. This study did not look at other nutritional indices such as prealbumin and vitamin levels. Third, the radiation was provided by different providers, so technique was variable, but the patients received nearly equivalent doses and variability in technique is likely limited. Fourth, we were not able to meaningfully analyze the role of chemotherapy in this patient population because there was a significant heterogeneity of patients receiving pre- and postoperative chemotherapy.
Our findings strongly suggest that a preoperative OPNI of <45.4 and being older than 73 years are strong predictors of patients who will experience a wound complication after radiation therapy for soft tissue sarcomas. This study has led us to start measuring preoperative albumin levels and assess complete metabolic panels. Our goal is to identify patients who are at high risk of wound complication and perform interventions to improve nutrition, then to study whether the interventions help lower the rates of wound complications.
1. Ormsby MV, Hilaris BS, Nori D, Brennan MF. Wound complications of adjuvant radiation therapy in patients with soft-tissue sarcomas. Ann Surg. 1989;210(1):93-99.
2. Greene KA, Wilde AH, Stulberg BN. Preoperative nutritional status of total joint patients: relationship to postoperative wound complications. J Arthroplasty. 1991;6(4):321-325.
3. Nozoe T, Kimura Y, Ishida M, Saeki H, Korenaga D, Sugimachi K. Correlation of pre-operative nutritional condition with post-operative complications in surgical treatment for oesophageal carcinoma. Eur J Surg Oncol. 2002;28(4):396-400.
4. Nozoe T, Kohno M, Iguchi T, et al. The prognostic nutritional index can be a prognostic indicator in colorectal carcinoma. Surg Today. 2012;42(6):532-535.
5. Nozoe T, Ninomiya M, Maeda T, Matsukuma A, Nakashima H, Ezaki T. Prognostic nutritional index: a tool to predict the biological aggressiveness of gastric carcinoma. Surg Today. 2010;40(5):440-443.
6. O’Sullivan B, Davis AM, Turcotte R, Bell R, Catton C, Chabot P, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet. 2002;359(9325):2235-2241.
7. Peat BG, Bell RS, Davis A, et al. Wound-healing complications after soft-tissue sarcoma surgery. Plast Reconstr Surg. 1994;93(5):980-987.
8. Kunisada T, Ngan SY, Powell G, Choong PF. Wound complications following pre-operative radiotherapy for soft tissue sarcoma. Eur J Surg Oncol. 2002;28(1):75-79.
9. Saddegh MK, Bauer HC. Wound complication in surgery of soft tissue sarcoma: analysis of 103 consecutive patients managed without adjuvant therapy. Clin Orthop Relat Res. 1993;289:247-253.
10. Tseng JF, Ballo MT, Langstein HN, et al. The effect of preoperative radiotherapy and reconstructive surgery on wound complications after resection of extremity soft-tissue sarcomas. Ann Surg Oncol. 2006;13(9):1209-1215.
11. Smale BF, Mullen JL, Buzby GP, Rosato EF. The efficacy of nutritional assessment and support in cancer surgery. Cancer. 1981;47(10):2375-2381.
12. Mohri Y, Inoue Y, Tanaka K, Hiro J, Uchida K, Kusunoki M. Prognostic nutritional index predicts postoperative outcome in colorectal cancer. World J Surg. 2013;37(11):2688-2692.
13. Jiang N, Deng JY, Ding XW, et al. Prognostic nutritional index predicts postoperative complications and long-term outcomes of gastric cancer. World J Gastroenterol. 2014;20(30):10537-10544.
14. Pinato DJ, North BV, Sharma R. A novel, externally validated inflammation-based prognostic algorithm in hepatocellular carcinoma: the prognostic nutritional index (PNI). Brit J Cancer. 2012;106(8):1439-1445.
15. Yao ZH, Tian GY, Wan YY, et al. Prognostic nutritional index predicts outcomes of malignant pleural mesothelioma. J Cancer Res Clin Oncol. 2013;139(12):2117-2123.
16. Jian-Hui C, Iskandar EA, Cai Sh I, et al. Significance of Onodera’s prognostic nutritional index in patients with colorectal cancer: a large cohort study in a single Chinese institution. Tumour Biol. 2016;37(3):3277-3283.
17. Hong S, Zhou T, Fang W, et al. The prognostic nutritional index (PNI) predicts overall survival of small-cell lung cancer patients. Tumour Biol. 2015;36(5):3389-9337.
18. Mohil RS, Agarwal A, Singh N, Arora J, Bhatnagar D. Does nutritional status play a role in patients undergoing emergency laparotomy? E Spen Eur E J Clin Nutr Metab. 2008;3(5):e226-e231.
19. Kay SP, Moreland JR, Schmitter E. Nutritional status and wound healing in lower extremity amputations. Clin Orthop Relat Res. 1987;(217):253-256.
20. Dickhaut SC, DeLee JC, Page CP. Nutritional status: importance in predicting wound-healing after amputation. J Bone Joint Surg Am. 1984;66(1):71-75.
21. Casey J, Flinn WR, Yao JS, Fahey V, Pawlowski J, Bergan JJ. Correlation of immune and nutritional status with wound complications in patients undergoing vascular operations. Surgery. 1983;93(6):822-827.
22. Gu Q, Wang D, Cui C, Gao Y, Xia G, Cui X. Effects of radiation on wound healing. J Environ Pathol Toxicol Oncol. 1998;17(2):117-123.
1. Ormsby MV, Hilaris BS, Nori D, Brennan MF. Wound complications of adjuvant radiation therapy in patients with soft-tissue sarcomas. Ann Surg. 1989;210(1):93-99.
2. Greene KA, Wilde AH, Stulberg BN. Preoperative nutritional status of total joint patients: relationship to postoperative wound complications. J Arthroplasty. 1991;6(4):321-325.
3. Nozoe T, Kimura Y, Ishida M, Saeki H, Korenaga D, Sugimachi K. Correlation of pre-operative nutritional condition with post-operative complications in surgical treatment for oesophageal carcinoma. Eur J Surg Oncol. 2002;28(4):396-400.
4. Nozoe T, Kohno M, Iguchi T, et al. The prognostic nutritional index can be a prognostic indicator in colorectal carcinoma. Surg Today. 2012;42(6):532-535.
5. Nozoe T, Ninomiya M, Maeda T, Matsukuma A, Nakashima H, Ezaki T. Prognostic nutritional index: a tool to predict the biological aggressiveness of gastric carcinoma. Surg Today. 2010;40(5):440-443.
6. O’Sullivan B, Davis AM, Turcotte R, Bell R, Catton C, Chabot P, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet. 2002;359(9325):2235-2241.
7. Peat BG, Bell RS, Davis A, et al. Wound-healing complications after soft-tissue sarcoma surgery. Plast Reconstr Surg. 1994;93(5):980-987.
8. Kunisada T, Ngan SY, Powell G, Choong PF. Wound complications following pre-operative radiotherapy for soft tissue sarcoma. Eur J Surg Oncol. 2002;28(1):75-79.
9. Saddegh MK, Bauer HC. Wound complication in surgery of soft tissue sarcoma: analysis of 103 consecutive patients managed without adjuvant therapy. Clin Orthop Relat Res. 1993;289:247-253.
10. Tseng JF, Ballo MT, Langstein HN, et al. The effect of preoperative radiotherapy and reconstructive surgery on wound complications after resection of extremity soft-tissue sarcomas. Ann Surg Oncol. 2006;13(9):1209-1215.
11. Smale BF, Mullen JL, Buzby GP, Rosato EF. The efficacy of nutritional assessment and support in cancer surgery. Cancer. 1981;47(10):2375-2381.
12. Mohri Y, Inoue Y, Tanaka K, Hiro J, Uchida K, Kusunoki M. Prognostic nutritional index predicts postoperative outcome in colorectal cancer. World J Surg. 2013;37(11):2688-2692.
13. Jiang N, Deng JY, Ding XW, et al. Prognostic nutritional index predicts postoperative complications and long-term outcomes of gastric cancer. World J Gastroenterol. 2014;20(30):10537-10544.
14. Pinato DJ, North BV, Sharma R. A novel, externally validated inflammation-based prognostic algorithm in hepatocellular carcinoma: the prognostic nutritional index (PNI). Brit J Cancer. 2012;106(8):1439-1445.
15. Yao ZH, Tian GY, Wan YY, et al. Prognostic nutritional index predicts outcomes of malignant pleural mesothelioma. J Cancer Res Clin Oncol. 2013;139(12):2117-2123.
16. Jian-Hui C, Iskandar EA, Cai Sh I, et al. Significance of Onodera’s prognostic nutritional index in patients with colorectal cancer: a large cohort study in a single Chinese institution. Tumour Biol. 2016;37(3):3277-3283.
17. Hong S, Zhou T, Fang W, et al. The prognostic nutritional index (PNI) predicts overall survival of small-cell lung cancer patients. Tumour Biol. 2015;36(5):3389-9337.
18. Mohil RS, Agarwal A, Singh N, Arora J, Bhatnagar D. Does nutritional status play a role in patients undergoing emergency laparotomy? E Spen Eur E J Clin Nutr Metab. 2008;3(5):e226-e231.
19. Kay SP, Moreland JR, Schmitter E. Nutritional status and wound healing in lower extremity amputations. Clin Orthop Relat Res. 1987;(217):253-256.
20. Dickhaut SC, DeLee JC, Page CP. Nutritional status: importance in predicting wound-healing after amputation. J Bone Joint Surg Am. 1984;66(1):71-75.
21. Casey J, Flinn WR, Yao JS, Fahey V, Pawlowski J, Bergan JJ. Correlation of immune and nutritional status with wound complications in patients undergoing vascular operations. Surgery. 1983;93(6):822-827.
22. Gu Q, Wang D, Cui C, Gao Y, Xia G, Cui X. Effects of radiation on wound healing. J Environ Pathol Toxicol Oncol. 1998;17(2):117-123.
Team discovers oncogenic driver of T-ALL
Preclinical research suggests the TOX protein is an oncogenic driver of T-cell acute lymphoblastic leukemia (T-ALL).
Results indicate that TOX may be expressed in as many as 95% of human T-ALL cases, and the protein is required for the cancer’s growth and persistence.
“A major role for TOX in T-ALL is to elicit defects in DNA repair, leading to genetic changes that drive normal cells into cancer,” said study author David Langenau, PhD, of Massachusetts General Hospital in Boston.
“TOX then continues to be expressed within leukemic cells and is required for continued tumor growth. That means that, if we can successfully target TOX with small molecules in the future, the 95% of T-ALL patients whose tumors express TOX would have new treatment options for this aggressive leukemia.”
Dr Langenau and his colleagues described this new role for TOX in Cancer Discovery.
The team noted that T-ALL has several molecular subtypes, many of which are driven by common oncogenes such as MYC and NOTCH. However, evidence has suggested the cancer’s initiation is likely driven by aberrations in DNA repair.
To identify genes that might help drive T-ALL, the researchers performed a transgenic screen in zebrafish.
The team found that TOX collaborates with known oncogene pathways to transform T-cell precursors into leukemia cells by altering DNA repair and then expanding the population of transformed cells.
In human T-ALL cells, TOX was shown to suppress non-homologous end joining (NHEJ) repair, a pathway required for repairing double-strand DNA breaks that, when disrupted, is known to cause errant DNA repair and genomic instability.
Nearly all of the human T-ALL samples the researchers tested were found to express TOX. And TOX proved essential for the proliferation and survival of T-ALL.
Dr Langenau explained that TOX is known to have important roles in the development and maturation of several types of immune cells, yet its roles in leukemia initiation and genomic instability were not described until this work.
TOX belongs to a group of proteins known to regulate the configuration or expression of genes by binding to DNA molecules, yet its mechanism in T-ALL—blocking NHEJ repair by binding to DNA repair proteins rather than directly to DNA—was totally unexpected.
The researchers believe that, in addition to better understanding how TOX regulates the continued growth of T-ALL, it will be important to determine whether related proteins have similar molecular functions in other cancers. ![]()
Preclinical research suggests the TOX protein is an oncogenic driver of T-cell acute lymphoblastic leukemia (T-ALL).
Results indicate that TOX may be expressed in as many as 95% of human T-ALL cases, and the protein is required for the cancer’s growth and persistence.
“A major role for TOX in T-ALL is to elicit defects in DNA repair, leading to genetic changes that drive normal cells into cancer,” said study author David Langenau, PhD, of Massachusetts General Hospital in Boston.
“TOX then continues to be expressed within leukemic cells and is required for continued tumor growth. That means that, if we can successfully target TOX with small molecules in the future, the 95% of T-ALL patients whose tumors express TOX would have new treatment options for this aggressive leukemia.”
Dr Langenau and his colleagues described this new role for TOX in Cancer Discovery.
The team noted that T-ALL has several molecular subtypes, many of which are driven by common oncogenes such as MYC and NOTCH. However, evidence has suggested the cancer’s initiation is likely driven by aberrations in DNA repair.
To identify genes that might help drive T-ALL, the researchers performed a transgenic screen in zebrafish.
The team found that TOX collaborates with known oncogene pathways to transform T-cell precursors into leukemia cells by altering DNA repair and then expanding the population of transformed cells.
In human T-ALL cells, TOX was shown to suppress non-homologous end joining (NHEJ) repair, a pathway required for repairing double-strand DNA breaks that, when disrupted, is known to cause errant DNA repair and genomic instability.
Nearly all of the human T-ALL samples the researchers tested were found to express TOX. And TOX proved essential for the proliferation and survival of T-ALL.
Dr Langenau explained that TOX is known to have important roles in the development and maturation of several types of immune cells, yet its roles in leukemia initiation and genomic instability were not described until this work.
TOX belongs to a group of proteins known to regulate the configuration or expression of genes by binding to DNA molecules, yet its mechanism in T-ALL—blocking NHEJ repair by binding to DNA repair proteins rather than directly to DNA—was totally unexpected.
The researchers believe that, in addition to better understanding how TOX regulates the continued growth of T-ALL, it will be important to determine whether related proteins have similar molecular functions in other cancers. ![]()
Preclinical research suggests the TOX protein is an oncogenic driver of T-cell acute lymphoblastic leukemia (T-ALL).
Results indicate that TOX may be expressed in as many as 95% of human T-ALL cases, and the protein is required for the cancer’s growth and persistence.
“A major role for TOX in T-ALL is to elicit defects in DNA repair, leading to genetic changes that drive normal cells into cancer,” said study author David Langenau, PhD, of Massachusetts General Hospital in Boston.
“TOX then continues to be expressed within leukemic cells and is required for continued tumor growth. That means that, if we can successfully target TOX with small molecules in the future, the 95% of T-ALL patients whose tumors express TOX would have new treatment options for this aggressive leukemia.”
Dr Langenau and his colleagues described this new role for TOX in Cancer Discovery.
The team noted that T-ALL has several molecular subtypes, many of which are driven by common oncogenes such as MYC and NOTCH. However, evidence has suggested the cancer’s initiation is likely driven by aberrations in DNA repair.
To identify genes that might help drive T-ALL, the researchers performed a transgenic screen in zebrafish.
The team found that TOX collaborates with known oncogene pathways to transform T-cell precursors into leukemia cells by altering DNA repair and then expanding the population of transformed cells.
In human T-ALL cells, TOX was shown to suppress non-homologous end joining (NHEJ) repair, a pathway required for repairing double-strand DNA breaks that, when disrupted, is known to cause errant DNA repair and genomic instability.
Nearly all of the human T-ALL samples the researchers tested were found to express TOX. And TOX proved essential for the proliferation and survival of T-ALL.
Dr Langenau explained that TOX is known to have important roles in the development and maturation of several types of immune cells, yet its roles in leukemia initiation and genomic instability were not described until this work.
TOX belongs to a group of proteins known to regulate the configuration or expression of genes by binding to DNA molecules, yet its mechanism in T-ALL—blocking NHEJ repair by binding to DNA repair proteins rather than directly to DNA—was totally unexpected.
The researchers believe that, in addition to better understanding how TOX regulates the continued growth of T-ALL, it will be important to determine whether related proteins have similar molecular functions in other cancers. ![]()
Some cancers linked to weight are on the rise in the US
Cancers associated with being overweight or obese accounted for about 40% of all cancers diagnosed in the US in 2014, according to the latest Vital Signs report by the US Centers for Disease Control and Prevention.
The International Agency for Research on Cancer has identified 13 cancers associated with overweight and obesity—multiple myeloma (MM), meningioma, adenocarcinoma of the esophagus, and thyroid, postmenopausal breast, gallbladder, stomach, liver, pancreatic, kidney, ovarian, uterine, and colorectal cancers.
For the Vital Signs report, researchers reviewed US data from 2005 to 2014 to determine trends for cancers associated with being overweight (having a body mass index [BMI] of 25 to 29.9 kg/m2) or obese (having a BMI of 30 kg/m2 and higher).
In 2014, roughly 631,000 people were diagnosed with cancers associated with overweight and obesity, which represents 40% of all cancers diagnosed.
Fifty-five percent of all cancers diagnosed in women and 24% of those diagnosed in men were associated with overweight and obesity.
Incidence rates of the 13 cancers combined were highest in non-Hispanic blacks, followed by non-Hispanic whites, American Indians/Alaska Natives, Hispanics, and Asians/Pacific Islanders.
Incidence over time
Overall, the incidence of cancers associated with overweight and obesity decreased 2% from 2005 to 2014.
However, when colorectal cancer was excluded, the incidence of the other 12 cancers combined increased 7% from 2005 to 2014. The incidence of colorectal cancer decreased 23% during that time. Researchers said this was due, in large part, to screening.
The incidence of cancers not associated with overweight and obesity decreased 13% from 2005 to 2014.
The incidence of MM increased over the period studied, but it was not a significant increase. The incidence rate of MM was 5.6 per 100,000 persons (age-adjusted to the 2000 US standard population) in 2005 and 6.0 per 100,000 persons in 2014.
So overall, there was an 8% increase in MM incidence rate from 2005 to 2014, or a 1.1% average annual increase. There was a 2% increase in the risk of MM per 1 kg/m2 increase in BMI.
Like MM, 3 other cancers had fairly stable incidence rates over the study period—adenocarcinoma of the esophagus, gallbladder cancer, and postmenopausal breast cancer.
However, incidence rates increased significantly each year for thyroid cancer (4.0% per year), liver cancer (2.9%), gastric cardia cancer (1.2%), endometrial cancer (1.1%), pancreatic cancer (0.8%), and kidney cancer (0.7%).
And incidence rates decreased significantly each year for meningioma (-3.8%), colorectal cancer (-2.9%), and ovarian cancer (-2.0%). ![]()
Cancers associated with being overweight or obese accounted for about 40% of all cancers diagnosed in the US in 2014, according to the latest Vital Signs report by the US Centers for Disease Control and Prevention.
The International Agency for Research on Cancer has identified 13 cancers associated with overweight and obesity—multiple myeloma (MM), meningioma, adenocarcinoma of the esophagus, and thyroid, postmenopausal breast, gallbladder, stomach, liver, pancreatic, kidney, ovarian, uterine, and colorectal cancers.
For the Vital Signs report, researchers reviewed US data from 2005 to 2014 to determine trends for cancers associated with being overweight (having a body mass index [BMI] of 25 to 29.9 kg/m2) or obese (having a BMI of 30 kg/m2 and higher).
In 2014, roughly 631,000 people were diagnosed with cancers associated with overweight and obesity, which represents 40% of all cancers diagnosed.
Fifty-five percent of all cancers diagnosed in women and 24% of those diagnosed in men were associated with overweight and obesity.
Incidence rates of the 13 cancers combined were highest in non-Hispanic blacks, followed by non-Hispanic whites, American Indians/Alaska Natives, Hispanics, and Asians/Pacific Islanders.
Incidence over time
Overall, the incidence of cancers associated with overweight and obesity decreased 2% from 2005 to 2014.
However, when colorectal cancer was excluded, the incidence of the other 12 cancers combined increased 7% from 2005 to 2014. The incidence of colorectal cancer decreased 23% during that time. Researchers said this was due, in large part, to screening.
The incidence of cancers not associated with overweight and obesity decreased 13% from 2005 to 2014.
The incidence of MM increased over the period studied, but it was not a significant increase. The incidence rate of MM was 5.6 per 100,000 persons (age-adjusted to the 2000 US standard population) in 2005 and 6.0 per 100,000 persons in 2014.
So overall, there was an 8% increase in MM incidence rate from 2005 to 2014, or a 1.1% average annual increase. There was a 2% increase in the risk of MM per 1 kg/m2 increase in BMI.
Like MM, 3 other cancers had fairly stable incidence rates over the study period—adenocarcinoma of the esophagus, gallbladder cancer, and postmenopausal breast cancer.
However, incidence rates increased significantly each year for thyroid cancer (4.0% per year), liver cancer (2.9%), gastric cardia cancer (1.2%), endometrial cancer (1.1%), pancreatic cancer (0.8%), and kidney cancer (0.7%).
And incidence rates decreased significantly each year for meningioma (-3.8%), colorectal cancer (-2.9%), and ovarian cancer (-2.0%). ![]()
Cancers associated with being overweight or obese accounted for about 40% of all cancers diagnosed in the US in 2014, according to the latest Vital Signs report by the US Centers for Disease Control and Prevention.
The International Agency for Research on Cancer has identified 13 cancers associated with overweight and obesity—multiple myeloma (MM), meningioma, adenocarcinoma of the esophagus, and thyroid, postmenopausal breast, gallbladder, stomach, liver, pancreatic, kidney, ovarian, uterine, and colorectal cancers.
For the Vital Signs report, researchers reviewed US data from 2005 to 2014 to determine trends for cancers associated with being overweight (having a body mass index [BMI] of 25 to 29.9 kg/m2) or obese (having a BMI of 30 kg/m2 and higher).
In 2014, roughly 631,000 people were diagnosed with cancers associated with overweight and obesity, which represents 40% of all cancers diagnosed.
Fifty-five percent of all cancers diagnosed in women and 24% of those diagnosed in men were associated with overweight and obesity.
Incidence rates of the 13 cancers combined were highest in non-Hispanic blacks, followed by non-Hispanic whites, American Indians/Alaska Natives, Hispanics, and Asians/Pacific Islanders.
Incidence over time
Overall, the incidence of cancers associated with overweight and obesity decreased 2% from 2005 to 2014.
However, when colorectal cancer was excluded, the incidence of the other 12 cancers combined increased 7% from 2005 to 2014. The incidence of colorectal cancer decreased 23% during that time. Researchers said this was due, in large part, to screening.
The incidence of cancers not associated with overweight and obesity decreased 13% from 2005 to 2014.
The incidence of MM increased over the period studied, but it was not a significant increase. The incidence rate of MM was 5.6 per 100,000 persons (age-adjusted to the 2000 US standard population) in 2005 and 6.0 per 100,000 persons in 2014.
So overall, there was an 8% increase in MM incidence rate from 2005 to 2014, or a 1.1% average annual increase. There was a 2% increase in the risk of MM per 1 kg/m2 increase in BMI.
Like MM, 3 other cancers had fairly stable incidence rates over the study period—adenocarcinoma of the esophagus, gallbladder cancer, and postmenopausal breast cancer.
However, incidence rates increased significantly each year for thyroid cancer (4.0% per year), liver cancer (2.9%), gastric cardia cancer (1.2%), endometrial cancer (1.1%), pancreatic cancer (0.8%), and kidney cancer (0.7%).
And incidence rates decreased significantly each year for meningioma (-3.8%), colorectal cancer (-2.9%), and ovarian cancer (-2.0%). ![]()
Device could improve anemia detection
A new microfluidic device could improve testing for anemia, according to researchers.
The team said the device can detect the level of hemoglobin in a whole blood sample using optical absorbance.
The device is portable, requires only a few drops of blood for analysis, and eliminates the need for chemical preparations.
In addition, researchers believe the device could be integrated with other microfluidic approaches to blood analysis.
The researchers described their device in AIP Advances.
The team noted that blood analyzers currently on the market measure hemoglobin via hemolysis. This requires hands-on expertise to prepare and run a sample, limiting the ability to monitor anemia in many parts of the world.
“The most exciting aspect to this analyzer is that it uses whole blood and does not require the additional steps and reagents to prepare a sample,” said study author Nathan Sniadecki, PhD, of the University of Washington in Seattle.
“You just run blood into the channel, and that’s it,” added Nikita Taparia, a doctoral candidate in Sniadecki’s lab. “It can be used anywhere.”
The analyzer takes advantage of the optical properties of blood, such as absorption and scattering, to measure hemoglobin concentration. Anemic blood transmits more light than normal blood, so the severity of anemia can be measured as a ratio of transmitted to original light intensity.
To simulate anemia, the researchers diluted blood samples with a buffer solution. The blood analyzer was effective at predicting cases of moderate to severe anemia, defined as less than 10 g/dL of hemoglobin in a sample. The analyzer did not produce any false-negative results.
The optical density of samples did not increase linearly, so a higher concentration of hemoglobin defines the upper limit of detection for the device.
The researchers said the device could be integrated with other microfluidic devices to analyze whole blood samples in parallel to diagnose anemia and other underlying factors that could contribute to the condition.
The team said such an integrated diagnostic tool would “aid the global health community in their continued surveillance of anemia and its etiology in high-risk subpopulations.” ![]()
A new microfluidic device could improve testing for anemia, according to researchers.
The team said the device can detect the level of hemoglobin in a whole blood sample using optical absorbance.
The device is portable, requires only a few drops of blood for analysis, and eliminates the need for chemical preparations.
In addition, researchers believe the device could be integrated with other microfluidic approaches to blood analysis.
The researchers described their device in AIP Advances.
The team noted that blood analyzers currently on the market measure hemoglobin via hemolysis. This requires hands-on expertise to prepare and run a sample, limiting the ability to monitor anemia in many parts of the world.
“The most exciting aspect to this analyzer is that it uses whole blood and does not require the additional steps and reagents to prepare a sample,” said study author Nathan Sniadecki, PhD, of the University of Washington in Seattle.
“You just run blood into the channel, and that’s it,” added Nikita Taparia, a doctoral candidate in Sniadecki’s lab. “It can be used anywhere.”
The analyzer takes advantage of the optical properties of blood, such as absorption and scattering, to measure hemoglobin concentration. Anemic blood transmits more light than normal blood, so the severity of anemia can be measured as a ratio of transmitted to original light intensity.
To simulate anemia, the researchers diluted blood samples with a buffer solution. The blood analyzer was effective at predicting cases of moderate to severe anemia, defined as less than 10 g/dL of hemoglobin in a sample. The analyzer did not produce any false-negative results.
The optical density of samples did not increase linearly, so a higher concentration of hemoglobin defines the upper limit of detection for the device.
The researchers said the device could be integrated with other microfluidic devices to analyze whole blood samples in parallel to diagnose anemia and other underlying factors that could contribute to the condition.
The team said such an integrated diagnostic tool would “aid the global health community in their continued surveillance of anemia and its etiology in high-risk subpopulations.” ![]()
A new microfluidic device could improve testing for anemia, according to researchers.
The team said the device can detect the level of hemoglobin in a whole blood sample using optical absorbance.
The device is portable, requires only a few drops of blood for analysis, and eliminates the need for chemical preparations.
In addition, researchers believe the device could be integrated with other microfluidic approaches to blood analysis.
The researchers described their device in AIP Advances.
The team noted that blood analyzers currently on the market measure hemoglobin via hemolysis. This requires hands-on expertise to prepare and run a sample, limiting the ability to monitor anemia in many parts of the world.
“The most exciting aspect to this analyzer is that it uses whole blood and does not require the additional steps and reagents to prepare a sample,” said study author Nathan Sniadecki, PhD, of the University of Washington in Seattle.
“You just run blood into the channel, and that’s it,” added Nikita Taparia, a doctoral candidate in Sniadecki’s lab. “It can be used anywhere.”
The analyzer takes advantage of the optical properties of blood, such as absorption and scattering, to measure hemoglobin concentration. Anemic blood transmits more light than normal blood, so the severity of anemia can be measured as a ratio of transmitted to original light intensity.
To simulate anemia, the researchers diluted blood samples with a buffer solution. The blood analyzer was effective at predicting cases of moderate to severe anemia, defined as less than 10 g/dL of hemoglobin in a sample. The analyzer did not produce any false-negative results.
The optical density of samples did not increase linearly, so a higher concentration of hemoglobin defines the upper limit of detection for the device.
The researchers said the device could be integrated with other microfluidic devices to analyze whole blood samples in parallel to diagnose anemia and other underlying factors that could contribute to the condition.
The team said such an integrated diagnostic tool would “aid the global health community in their continued surveillance of anemia and its etiology in high-risk subpopulations.” ![]()