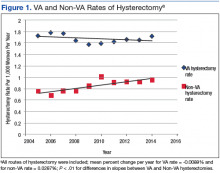
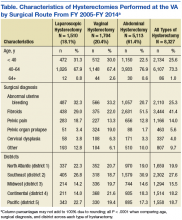
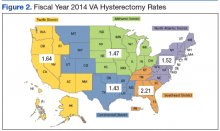
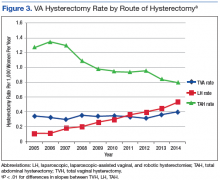
User login
Malpractice Counsel: A Pain in the…Scrotum
Case
A 52-year-old man presented to the ED for evaluation of right scrotal pain and swelling. The patient stated that the pain started several hours prior to presentation and had gradually worsened. He denied any trauma or inciting event to the affected area; he further denied abdominal pain, nausea, vomiting, dysuria, polyuria, or fever. The patient’s remote medical history was significant for type 2 diabetes mellitus (DM), which he managed through dietary modification-only as he had refused pharmacological therapy. The patient admitted to smoking one half-pack of cigarettes per week, but denied alcohol or illicit drug use.
At presentation, the patient’s vital signs were all within normal range. The physical examination was remarkable only for right testicular tenderness and mild scrotal swelling, and there were no hernias or lymphadenopathy present.
The emergency physician (EP) ordered a urinalysis and color-flow Doppler ultrasound study of both testes, which the radiologist interpreted as an enlarged right epididymis with hyperemia; the left testicle was normal. The urinalysis was normal.
The patient was diagnosed with epididymitis and discharged home with a prescription for oral levofloxacin 500 mg daily for 10 days. He also was instructed to take ibuprofen for pain, apply ice to the affected area, keep the scrotal area elevated, and follow-up with a urologist in 1 week.
Approximately 8 hours after discharge, the patient returned to the same ED with complaints of increasing right testicular pain and swelling. The history and physical examination at this visit were essentially unchanged from his initial presentation. No laboratory evaluation, imaging studies, or other tests were ordered at the second visit.
The patient was discharged home with a prescription for a narcotic analgesic, which he was instructed to take in addition to the ibuprofen; he was also instructed to follow-up with a urologist within the next 2 to 3 days, instead of in 1 week.
The patient returned the following morning to the same ED with complaints of increased swelling and pain of the right testicle. In addition to the worsening testicular pain and swelling, he also had right inguinal pain, nausea, vomiting, and fever. Vital signs at this third presentation were: blood pressure (BP), 124/64 mm Hg; heart rate (HR), 110 beats/min; respiratory rate, 20 breaths/min; and temperature, 99.8o F. Oxygen saturation was 98% on room air.
The patient was tachycardic on heart examination, but with regular rhythm and no murmurs, rubs, or gallops. The lung and abdominal examinations were normal. The genital examination revealed marked right scrotal swelling and tenderness, as well as tender right inguinal lymphadenopathy.
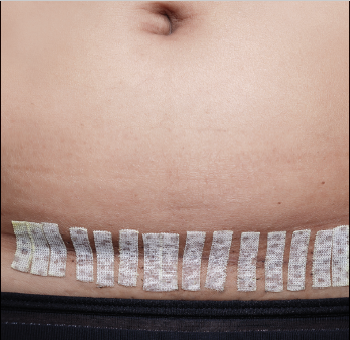
The EP ordered an intravenous (IV) bolus of 1 L normal saline and laboratory studies, which included lactic acid, blood cultures, urinalysis, and urine culture and sensitivity. The EP was concerned for a scrotal abscess and ordered a testicular Doppler color-flow ultrasound study. The laboratory studies revealed an elevated white blood count of 16.5 K/uL, elevated blood glucose of 364 mg/dL, and elevated lactate of 2.8 mg/dL. As demonstrated on the ultrasound study performed at the patient’s first presentation, the ultrasound again showed an enlarged right epididymis, but without orchitis or abscess. The scrotal wall had significant thickening, consistent with cellulitis. The EP ordered broad spectrum IV antibiotics and admitted the patient to the hospitalist with a consult request for urology services.
The patient continued to receive IV fluids and antibiotics throughout the evening. In the morning, he was seen by the same hospitalist/admitting physician from the previous evening. Upon physical examination, the hospitalist noted tenderness, swelling, and erythema in the patient’s perineal area. The patient’s BP had dropped to 100/60 mm Hg, and his HR had increased to 115 beats/min despite receiving nearly 2 L of normal saline IV throughout the previous evening and night.
The urologist examined the patient soon after the consult request and diagnosed him with Fournier’s gangrene. He started the patient on aggressive IV fluid resuscitation, after which the patient was immediately taken to the operating room for extensive surgical debridement and scrotectomy. The patient’s postoperative course was complicated by acute kidney injury, respiratory failure requiring ventilator support, and sepsis. After a lengthy hospital stay, the patient was discharged home, but required a scrotal skin graft, and experienced erectile dysfunction and depression.
The patient sued all of the EPs involved in his care, the hospital, the hospitalist/admitting physician, and the urologist for negligence. The plaintiff’s attorney argued that since the patient progressively deteriorated over the 24 to 36 hours during his three presentations to the ED, urology services should have been consulted earlier, and that the urologist should have seen the patient immediately at the time of hospital admission.
The attorneys for the defendants claimed the patient denied dysuria, penile lesions, or urethral discharge and that the history, physical examination, and testicular ultrasound were all consistent with the diagnosis of epididymitis. For this reason, they argued, there was no indication for an emergent consultation with urology services. The jury returned a defense verdict.
Discussion
It is easy for a busy EP to have a differential diagnosis of only two disorders when evaluating a patient for unilateral testicular pain and swelling—in this case, testicular torsion and epididymitis. While these are the most common causes of testicular pain and swelling, this case emphasizes the need to also consider Fournier’s gangrene in the differential. A thorough history and physical examination, coupled with appropriate testing, will usually identify the correct diagnosis. While the differential diagnosis is broader than just these three disease processes (see the Box), we will review the evaluation and management of the three most serious: epididymitis, testicular torsion, and Fournier’s gangrene.
Noninfectious and Bacterial Epididymitis
Epididymitis is the most common cause of acute scrotal pain among US adults, accounting for approximately 600,000 cases each year.1 Infectious epididymitis is typically classified as acute (symptom duration of <6 weeks) or chronic (symptom duration of ≥6 weeks).2
Cases of noninfectious epididymitis are typically due to a chronic condition, such as autoimmune disease, cancer, or vasculitis. Although not as common, noninfectious epididymitis can also occur due to testicular trauma or amiodarone therapy.3,4
Patients with acute bacterial epididymitis typically present with scrotal pain and swelling ranging from mild to marked. These patients may also exhibit fever and chills, along with dysuria, frequency, and urgency, if associated with a urinary tract infection.2 The chronic presentation is more common though, and usually not associated with voiding issues.
Chronic epididymis is frequently seen in postpubertal boys and men following sexual activity, heavy physical exertion, and bicycle/motorcycle riding.2 On physical examination, palpation reveals induration and swelling of the involved epididymis with exquisite tenderness.2 Testicular swelling and pain, along with scrotal wall erythema, may be present in more advanced cases.2 The cremasteric reflex should be intact (ie, scratching the medial proximal thigh will cause ipsilateral testicle retraction). Similarly, the lie of both testicles while the patient is standing should be equal and symmetrical—ie, both testicles descended equally. However, in the presence of moderate-to-severe scrotal swelling, both of these physical findings may be impossible to confirm.
A urinalysis and urine culture should be ordered if there is any suspicion of epididymitis; pyuria will be present in approximately 50% of cases. However, since pyuria is neither sensitive nor specific for epididymitis, in most cases, a testicular ultrasound with Doppler flow is required to exclude testicular torsion. In cases of epididymitis, ultrasound usually demonstrates increased flow on the affected side, whereas in testicular torsion, there is decreased or absent blood flow.
The treatment for epididymitis involves antibiotics and symptomatic care. If epididymitis from chlamydia and/or gonorrhea is the suspected cause, or if the patient is younger than age 35 years, he should be given ceftriaxone 250 mg intramuscularly plus oral doxycycline 100 mg twice a day for 10 days. Patients who practice insertive anal sex should be treated with ceftriaxone, plus either oral ofloxacin 300 mg twice a day or oral levofloxacin 500 mg daily for 10 days.
In cases in which enteric organisms are suspected, the patient is older than age 35 years, or if patient status is posturinary tract instrumentation or vasectomy, he should be treated with either oral ofloxacin 300 mg twice a day or oral levofloxacin 500 mg daily for 10 days.2
For symptomatic relief, scrotal elevation, ice application, and nonsteroidal anti-inflammatory drugs are recommended.
Patients with epididymitis, regardless of etiology, should be instructed to follow-up with a urologist within 1 week. If the patient appears ill, septic, or in significant pain, admission to the hospital with IV antibiotics, IV fluids, and an urgent consult with urology services is required.
Testicular Torsion
Testicular torsion is a time-sensitive issue, requiring early diagnosis and rapid treatment to preserve the patient’s fertility. Most clinicians recommend detorsion within 6 hours of torsion onset because salvage rates are excellent when performed within this timeframe; after 12 hours, the testis will likely suffer irreversible damage due to ischemia.5,6
Testicular torsion can occur at any age, but is most commonly seen in a bimodal distribution—ie, neonates and postpubertal boys. The prevalence of testicular torsion in adult patients hospitalized with acute scrotal pain is approximately 25% to 50%.2
Patients with testicular torsion usually describe a sudden onset of severe, acute pain. The pain frequently occurs a few hours after vigorous physical activity or minor testicular trauma.2 Occasionally, the patient may complain of lower quadrant abdominal pain rather than testicular or scrotal pain. Nausea with vomiting can also be present.
On physical examination, significant testicular swelling is usually present. Examining the patient in the standing position will often reveal an asymmetrical, high-riding testis with a transverse lie on the affected side. The cremasteric reflex is usually absent in patients with testicular torsion.
Because of the significant overlap in history and physical examination findings for epididymitis and testicular torsion, a testicular ultrasound with color Doppler should be ordered. Multiple studies have confirmed the high sensitivity and specificity of ultrasound in the diagnosis of testicular torsion.
The treatment for suspected or confirmed testicular torsion is immediate surgical exploration with intraoperative detorsion and fixation of the testes. The EP can attempt manual detorsion (ie, performed in a medial to lateral motion, similar to opening a book). However, this should not delay the EP from consulting with urology services.
Pediatric patients with testicular torsion usually have a more favorable outcome than do adults. In one retrospective study, patients younger than age 21 years had a 70% testicular salvage rate compared to only 41% of patients aged 21 years and older.7 Regardless of age, better outcomes are associated with shorter periods of torsion.
Fournier’s Gangrene
Fournier’s gangrene is a polymicrobial necrotizing fasciitis of the perineum and scrotum that typically develops initially as a benign infection or abscess but quickly spreads. Risk factors for Fournier’s gangrene include DM, alcohol abuse, and any immunocompromised state (eg, HIV, cancer).
If the patient presents early in onset, there may be only mild tenderness, erythema, or swelling of the affected area; however, this infection progresses rapidly. Later findings include marked tenderness, swelling, crepitus, blisters, and ecchymoses. Patients with Fournier’s gangrene also develop systemic signs of infection, including fever, tachycardia, tachypnea, and hypotension. The key to diagnosis is careful examination of the perineal and scrotal area in any patient presenting with acute scrotal pain.
In the majority of cases, the diagnosis of Fournier’s gangrene is made clinically. Once the diagnosis is made, patients require immediate and aggressive IV fluid resuscitation, broad-spectrum IV antibiotics (typically vancomycin and piperacillin/tazobactam), and emergent evaluation by a urologist. It is essential that these patients undergo early and aggressive surgical exploration and debridement of necrotic tissue.2 Antibiotic therapy alone is associated with a 100% mortality rate, emphasizing the need for urgent surgery.2 Even with optimal medical and surgical management, the mortality rate remains significant.
Summary
This case emphasizes several important teaching points. The EP should be mindful of the patient who keeps returning to the ED with the same complaint—despite “appropriate” treatment—as the initial diagnosis may not be the correct one. Such returning patients require greater, not less, scrutiny. As with any patient, the EP should always take a complete history and perform a thorough physical examination at each presentation—as one would with a de novo patient. Finally, the EP should consider Fournier’s gangrene in addition to testicular torsion and epididymitis in the differential diagnosis for acute scrotal pain.
1. Trojian TH, Lishnak TS, Heiman D. Epididymitis and orchitis: an overview. Am Fam Physician. 2009;79(7):583-587.
2. Eyre RC. Evaluation of acute scrotal pain in adults. UpToDate Web site. https://www.uptodate.com/contents/evaluation-of-acute-scrotal-pain-in-adults. Updated July 31, 2017. Accessed September 7, 2017.
3. Shen Y, Liu H, Cheng J, Bu P. Amiodarone-induced epididymitis: a pathologically confirmed case report and review of the literature. Cardiology. 2014;128(4):349-351. doi:10.1159/000361038.
4. Tracy CR, Steers WD, Costabile R. Diagnosis and management of epididymitis. Urol Clin North Am. 2008;35(1):101-108. doi:10.1016/j.ucl.2007.09.013.
5. Wampler SM, Llanes M. Common scrotal and testicular problems. Prim Care. 2010;37(3):613-626. doi:10.1016/j.pop.2010.04.009.
6. Dunne PJ, O’Loughlin BS. Testicular torsion: time is the enemy. Aust NZ J Surg. 2000;70(6):441-442.
7. Cummings JM, Boullier JA, Sekhon D, Bose K. Adult testicular torsion. J Urol. 2002;167(5):2109-2110.
Case
A 52-year-old man presented to the ED for evaluation of right scrotal pain and swelling. The patient stated that the pain started several hours prior to presentation and had gradually worsened. He denied any trauma or inciting event to the affected area; he further denied abdominal pain, nausea, vomiting, dysuria, polyuria, or fever. The patient’s remote medical history was significant for type 2 diabetes mellitus (DM), which he managed through dietary modification-only as he had refused pharmacological therapy. The patient admitted to smoking one half-pack of cigarettes per week, but denied alcohol or illicit drug use.
At presentation, the patient’s vital signs were all within normal range. The physical examination was remarkable only for right testicular tenderness and mild scrotal swelling, and there were no hernias or lymphadenopathy present.
The emergency physician (EP) ordered a urinalysis and color-flow Doppler ultrasound study of both testes, which the radiologist interpreted as an enlarged right epididymis with hyperemia; the left testicle was normal. The urinalysis was normal.
The patient was diagnosed with epididymitis and discharged home with a prescription for oral levofloxacin 500 mg daily for 10 days. He also was instructed to take ibuprofen for pain, apply ice to the affected area, keep the scrotal area elevated, and follow-up with a urologist in 1 week.
Approximately 8 hours after discharge, the patient returned to the same ED with complaints of increasing right testicular pain and swelling. The history and physical examination at this visit were essentially unchanged from his initial presentation. No laboratory evaluation, imaging studies, or other tests were ordered at the second visit.
The patient was discharged home with a prescription for a narcotic analgesic, which he was instructed to take in addition to the ibuprofen; he was also instructed to follow-up with a urologist within the next 2 to 3 days, instead of in 1 week.
The patient returned the following morning to the same ED with complaints of increased swelling and pain of the right testicle. In addition to the worsening testicular pain and swelling, he also had right inguinal pain, nausea, vomiting, and fever. Vital signs at this third presentation were: blood pressure (BP), 124/64 mm Hg; heart rate (HR), 110 beats/min; respiratory rate, 20 breaths/min; and temperature, 99.8o F. Oxygen saturation was 98% on room air.
The patient was tachycardic on heart examination, but with regular rhythm and no murmurs, rubs, or gallops. The lung and abdominal examinations were normal. The genital examination revealed marked right scrotal swelling and tenderness, as well as tender right inguinal lymphadenopathy.
The EP ordered an intravenous (IV) bolus of 1 L normal saline and laboratory studies, which included lactic acid, blood cultures, urinalysis, and urine culture and sensitivity. The EP was concerned for a scrotal abscess and ordered a testicular Doppler color-flow ultrasound study. The laboratory studies revealed an elevated white blood count of 16.5 K/uL, elevated blood glucose of 364 mg/dL, and elevated lactate of 2.8 mg/dL. As demonstrated on the ultrasound study performed at the patient’s first presentation, the ultrasound again showed an enlarged right epididymis, but without orchitis or abscess. The scrotal wall had significant thickening, consistent with cellulitis. The EP ordered broad spectrum IV antibiotics and admitted the patient to the hospitalist with a consult request for urology services.
The patient continued to receive IV fluids and antibiotics throughout the evening. In the morning, he was seen by the same hospitalist/admitting physician from the previous evening. Upon physical examination, the hospitalist noted tenderness, swelling, and erythema in the patient’s perineal area. The patient’s BP had dropped to 100/60 mm Hg, and his HR had increased to 115 beats/min despite receiving nearly 2 L of normal saline IV throughout the previous evening and night.
The urologist examined the patient soon after the consult request and diagnosed him with Fournier’s gangrene. He started the patient on aggressive IV fluid resuscitation, after which the patient was immediately taken to the operating room for extensive surgical debridement and scrotectomy. The patient’s postoperative course was complicated by acute kidney injury, respiratory failure requiring ventilator support, and sepsis. After a lengthy hospital stay, the patient was discharged home, but required a scrotal skin graft, and experienced erectile dysfunction and depression.
The patient sued all of the EPs involved in his care, the hospital, the hospitalist/admitting physician, and the urologist for negligence. The plaintiff’s attorney argued that since the patient progressively deteriorated over the 24 to 36 hours during his three presentations to the ED, urology services should have been consulted earlier, and that the urologist should have seen the patient immediately at the time of hospital admission.
The attorneys for the defendants claimed the patient denied dysuria, penile lesions, or urethral discharge and that the history, physical examination, and testicular ultrasound were all consistent with the diagnosis of epididymitis. For this reason, they argued, there was no indication for an emergent consultation with urology services. The jury returned a defense verdict.
Discussion
It is easy for a busy EP to have a differential diagnosis of only two disorders when evaluating a patient for unilateral testicular pain and swelling—in this case, testicular torsion and epididymitis. While these are the most common causes of testicular pain and swelling, this case emphasizes the need to also consider Fournier’s gangrene in the differential. A thorough history and physical examination, coupled with appropriate testing, will usually identify the correct diagnosis. While the differential diagnosis is broader than just these three disease processes (see the Box), we will review the evaluation and management of the three most serious: epididymitis, testicular torsion, and Fournier’s gangrene.
Noninfectious and Bacterial Epididymitis
Epididymitis is the most common cause of acute scrotal pain among US adults, accounting for approximately 600,000 cases each year.1 Infectious epididymitis is typically classified as acute (symptom duration of <6 weeks) or chronic (symptom duration of ≥6 weeks).2
Cases of noninfectious epididymitis are typically due to a chronic condition, such as autoimmune disease, cancer, or vasculitis. Although not as common, noninfectious epididymitis can also occur due to testicular trauma or amiodarone therapy.3,4
Patients with acute bacterial epididymitis typically present with scrotal pain and swelling ranging from mild to marked. These patients may also exhibit fever and chills, along with dysuria, frequency, and urgency, if associated with a urinary tract infection.2 The chronic presentation is more common though, and usually not associated with voiding issues.
Chronic epididymis is frequently seen in postpubertal boys and men following sexual activity, heavy physical exertion, and bicycle/motorcycle riding.2 On physical examination, palpation reveals induration and swelling of the involved epididymis with exquisite tenderness.2 Testicular swelling and pain, along with scrotal wall erythema, may be present in more advanced cases.2 The cremasteric reflex should be intact (ie, scratching the medial proximal thigh will cause ipsilateral testicle retraction). Similarly, the lie of both testicles while the patient is standing should be equal and symmetrical—ie, both testicles descended equally. However, in the presence of moderate-to-severe scrotal swelling, both of these physical findings may be impossible to confirm.
A urinalysis and urine culture should be ordered if there is any suspicion of epididymitis; pyuria will be present in approximately 50% of cases. However, since pyuria is neither sensitive nor specific for epididymitis, in most cases, a testicular ultrasound with Doppler flow is required to exclude testicular torsion. In cases of epididymitis, ultrasound usually demonstrates increased flow on the affected side, whereas in testicular torsion, there is decreased or absent blood flow.
The treatment for epididymitis involves antibiotics and symptomatic care. If epididymitis from chlamydia and/or gonorrhea is the suspected cause, or if the patient is younger than age 35 years, he should be given ceftriaxone 250 mg intramuscularly plus oral doxycycline 100 mg twice a day for 10 days. Patients who practice insertive anal sex should be treated with ceftriaxone, plus either oral ofloxacin 300 mg twice a day or oral levofloxacin 500 mg daily for 10 days.
In cases in which enteric organisms are suspected, the patient is older than age 35 years, or if patient status is posturinary tract instrumentation or vasectomy, he should be treated with either oral ofloxacin 300 mg twice a day or oral levofloxacin 500 mg daily for 10 days.2
For symptomatic relief, scrotal elevation, ice application, and nonsteroidal anti-inflammatory drugs are recommended.
Patients with epididymitis, regardless of etiology, should be instructed to follow-up with a urologist within 1 week. If the patient appears ill, septic, or in significant pain, admission to the hospital with IV antibiotics, IV fluids, and an urgent consult with urology services is required.
Testicular Torsion
Testicular torsion is a time-sensitive issue, requiring early diagnosis and rapid treatment to preserve the patient’s fertility. Most clinicians recommend detorsion within 6 hours of torsion onset because salvage rates are excellent when performed within this timeframe; after 12 hours, the testis will likely suffer irreversible damage due to ischemia.5,6
Testicular torsion can occur at any age, but is most commonly seen in a bimodal distribution—ie, neonates and postpubertal boys. The prevalence of testicular torsion in adult patients hospitalized with acute scrotal pain is approximately 25% to 50%.2
Patients with testicular torsion usually describe a sudden onset of severe, acute pain. The pain frequently occurs a few hours after vigorous physical activity or minor testicular trauma.2 Occasionally, the patient may complain of lower quadrant abdominal pain rather than testicular or scrotal pain. Nausea with vomiting can also be present.
On physical examination, significant testicular swelling is usually present. Examining the patient in the standing position will often reveal an asymmetrical, high-riding testis with a transverse lie on the affected side. The cremasteric reflex is usually absent in patients with testicular torsion.
Because of the significant overlap in history and physical examination findings for epididymitis and testicular torsion, a testicular ultrasound with color Doppler should be ordered. Multiple studies have confirmed the high sensitivity and specificity of ultrasound in the diagnosis of testicular torsion.
The treatment for suspected or confirmed testicular torsion is immediate surgical exploration with intraoperative detorsion and fixation of the testes. The EP can attempt manual detorsion (ie, performed in a medial to lateral motion, similar to opening a book). However, this should not delay the EP from consulting with urology services.
Pediatric patients with testicular torsion usually have a more favorable outcome than do adults. In one retrospective study, patients younger than age 21 years had a 70% testicular salvage rate compared to only 41% of patients aged 21 years and older.7 Regardless of age, better outcomes are associated with shorter periods of torsion.
Fournier’s Gangrene
Fournier’s gangrene is a polymicrobial necrotizing fasciitis of the perineum and scrotum that typically develops initially as a benign infection or abscess but quickly spreads. Risk factors for Fournier’s gangrene include DM, alcohol abuse, and any immunocompromised state (eg, HIV, cancer).
If the patient presents early in onset, there may be only mild tenderness, erythema, or swelling of the affected area; however, this infection progresses rapidly. Later findings include marked tenderness, swelling, crepitus, blisters, and ecchymoses. Patients with Fournier’s gangrene also develop systemic signs of infection, including fever, tachycardia, tachypnea, and hypotension. The key to diagnosis is careful examination of the perineal and scrotal area in any patient presenting with acute scrotal pain.
In the majority of cases, the diagnosis of Fournier’s gangrene is made clinically. Once the diagnosis is made, patients require immediate and aggressive IV fluid resuscitation, broad-spectrum IV antibiotics (typically vancomycin and piperacillin/tazobactam), and emergent evaluation by a urologist. It is essential that these patients undergo early and aggressive surgical exploration and debridement of necrotic tissue.2 Antibiotic therapy alone is associated with a 100% mortality rate, emphasizing the need for urgent surgery.2 Even with optimal medical and surgical management, the mortality rate remains significant.
Summary
This case emphasizes several important teaching points. The EP should be mindful of the patient who keeps returning to the ED with the same complaint—despite “appropriate” treatment—as the initial diagnosis may not be the correct one. Such returning patients require greater, not less, scrutiny. As with any patient, the EP should always take a complete history and perform a thorough physical examination at each presentation—as one would with a de novo patient. Finally, the EP should consider Fournier’s gangrene in addition to testicular torsion and epididymitis in the differential diagnosis for acute scrotal pain.
Case
A 52-year-old man presented to the ED for evaluation of right scrotal pain and swelling. The patient stated that the pain started several hours prior to presentation and had gradually worsened. He denied any trauma or inciting event to the affected area; he further denied abdominal pain, nausea, vomiting, dysuria, polyuria, or fever. The patient’s remote medical history was significant for type 2 diabetes mellitus (DM), which he managed through dietary modification-only as he had refused pharmacological therapy. The patient admitted to smoking one half-pack of cigarettes per week, but denied alcohol or illicit drug use.
At presentation, the patient’s vital signs were all within normal range. The physical examination was remarkable only for right testicular tenderness and mild scrotal swelling, and there were no hernias or lymphadenopathy present.
The emergency physician (EP) ordered a urinalysis and color-flow Doppler ultrasound study of both testes, which the radiologist interpreted as an enlarged right epididymis with hyperemia; the left testicle was normal. The urinalysis was normal.
The patient was diagnosed with epididymitis and discharged home with a prescription for oral levofloxacin 500 mg daily for 10 days. He also was instructed to take ibuprofen for pain, apply ice to the affected area, keep the scrotal area elevated, and follow-up with a urologist in 1 week.
Approximately 8 hours after discharge, the patient returned to the same ED with complaints of increasing right testicular pain and swelling. The history and physical examination at this visit were essentially unchanged from his initial presentation. No laboratory evaluation, imaging studies, or other tests were ordered at the second visit.
The patient was discharged home with a prescription for a narcotic analgesic, which he was instructed to take in addition to the ibuprofen; he was also instructed to follow-up with a urologist within the next 2 to 3 days, instead of in 1 week.
The patient returned the following morning to the same ED with complaints of increased swelling and pain of the right testicle. In addition to the worsening testicular pain and swelling, he also had right inguinal pain, nausea, vomiting, and fever. Vital signs at this third presentation were: blood pressure (BP), 124/64 mm Hg; heart rate (HR), 110 beats/min; respiratory rate, 20 breaths/min; and temperature, 99.8o F. Oxygen saturation was 98% on room air.
The patient was tachycardic on heart examination, but with regular rhythm and no murmurs, rubs, or gallops. The lung and abdominal examinations were normal. The genital examination revealed marked right scrotal swelling and tenderness, as well as tender right inguinal lymphadenopathy.
The EP ordered an intravenous (IV) bolus of 1 L normal saline and laboratory studies, which included lactic acid, blood cultures, urinalysis, and urine culture and sensitivity. The EP was concerned for a scrotal abscess and ordered a testicular Doppler color-flow ultrasound study. The laboratory studies revealed an elevated white blood count of 16.5 K/uL, elevated blood glucose of 364 mg/dL, and elevated lactate of 2.8 mg/dL. As demonstrated on the ultrasound study performed at the patient’s first presentation, the ultrasound again showed an enlarged right epididymis, but without orchitis or abscess. The scrotal wall had significant thickening, consistent with cellulitis. The EP ordered broad spectrum IV antibiotics and admitted the patient to the hospitalist with a consult request for urology services.
The patient continued to receive IV fluids and antibiotics throughout the evening. In the morning, he was seen by the same hospitalist/admitting physician from the previous evening. Upon physical examination, the hospitalist noted tenderness, swelling, and erythema in the patient’s perineal area. The patient’s BP had dropped to 100/60 mm Hg, and his HR had increased to 115 beats/min despite receiving nearly 2 L of normal saline IV throughout the previous evening and night.
The urologist examined the patient soon after the consult request and diagnosed him with Fournier’s gangrene. He started the patient on aggressive IV fluid resuscitation, after which the patient was immediately taken to the operating room for extensive surgical debridement and scrotectomy. The patient’s postoperative course was complicated by acute kidney injury, respiratory failure requiring ventilator support, and sepsis. After a lengthy hospital stay, the patient was discharged home, but required a scrotal skin graft, and experienced erectile dysfunction and depression.
The patient sued all of the EPs involved in his care, the hospital, the hospitalist/admitting physician, and the urologist for negligence. The plaintiff’s attorney argued that since the patient progressively deteriorated over the 24 to 36 hours during his three presentations to the ED, urology services should have been consulted earlier, and that the urologist should have seen the patient immediately at the time of hospital admission.
The attorneys for the defendants claimed the patient denied dysuria, penile lesions, or urethral discharge and that the history, physical examination, and testicular ultrasound were all consistent with the diagnosis of epididymitis. For this reason, they argued, there was no indication for an emergent consultation with urology services. The jury returned a defense verdict.
Discussion
It is easy for a busy EP to have a differential diagnosis of only two disorders when evaluating a patient for unilateral testicular pain and swelling—in this case, testicular torsion and epididymitis. While these are the most common causes of testicular pain and swelling, this case emphasizes the need to also consider Fournier’s gangrene in the differential. A thorough history and physical examination, coupled with appropriate testing, will usually identify the correct diagnosis. While the differential diagnosis is broader than just these three disease processes (see the Box), we will review the evaluation and management of the three most serious: epididymitis, testicular torsion, and Fournier’s gangrene.
Noninfectious and Bacterial Epididymitis
Epididymitis is the most common cause of acute scrotal pain among US adults, accounting for approximately 600,000 cases each year.1 Infectious epididymitis is typically classified as acute (symptom duration of <6 weeks) or chronic (symptom duration of ≥6 weeks).2
Cases of noninfectious epididymitis are typically due to a chronic condition, such as autoimmune disease, cancer, or vasculitis. Although not as common, noninfectious epididymitis can also occur due to testicular trauma or amiodarone therapy.3,4
Patients with acute bacterial epididymitis typically present with scrotal pain and swelling ranging from mild to marked. These patients may also exhibit fever and chills, along with dysuria, frequency, and urgency, if associated with a urinary tract infection.2 The chronic presentation is more common though, and usually not associated with voiding issues.
Chronic epididymis is frequently seen in postpubertal boys and men following sexual activity, heavy physical exertion, and bicycle/motorcycle riding.2 On physical examination, palpation reveals induration and swelling of the involved epididymis with exquisite tenderness.2 Testicular swelling and pain, along with scrotal wall erythema, may be present in more advanced cases.2 The cremasteric reflex should be intact (ie, scratching the medial proximal thigh will cause ipsilateral testicle retraction). Similarly, the lie of both testicles while the patient is standing should be equal and symmetrical—ie, both testicles descended equally. However, in the presence of moderate-to-severe scrotal swelling, both of these physical findings may be impossible to confirm.
A urinalysis and urine culture should be ordered if there is any suspicion of epididymitis; pyuria will be present in approximately 50% of cases. However, since pyuria is neither sensitive nor specific for epididymitis, in most cases, a testicular ultrasound with Doppler flow is required to exclude testicular torsion. In cases of epididymitis, ultrasound usually demonstrates increased flow on the affected side, whereas in testicular torsion, there is decreased or absent blood flow.
The treatment for epididymitis involves antibiotics and symptomatic care. If epididymitis from chlamydia and/or gonorrhea is the suspected cause, or if the patient is younger than age 35 years, he should be given ceftriaxone 250 mg intramuscularly plus oral doxycycline 100 mg twice a day for 10 days. Patients who practice insertive anal sex should be treated with ceftriaxone, plus either oral ofloxacin 300 mg twice a day or oral levofloxacin 500 mg daily for 10 days.
In cases in which enteric organisms are suspected, the patient is older than age 35 years, or if patient status is posturinary tract instrumentation or vasectomy, he should be treated with either oral ofloxacin 300 mg twice a day or oral levofloxacin 500 mg daily for 10 days.2
For symptomatic relief, scrotal elevation, ice application, and nonsteroidal anti-inflammatory drugs are recommended.
Patients with epididymitis, regardless of etiology, should be instructed to follow-up with a urologist within 1 week. If the patient appears ill, septic, or in significant pain, admission to the hospital with IV antibiotics, IV fluids, and an urgent consult with urology services is required.
Testicular Torsion
Testicular torsion is a time-sensitive issue, requiring early diagnosis and rapid treatment to preserve the patient’s fertility. Most clinicians recommend detorsion within 6 hours of torsion onset because salvage rates are excellent when performed within this timeframe; after 12 hours, the testis will likely suffer irreversible damage due to ischemia.5,6
Testicular torsion can occur at any age, but is most commonly seen in a bimodal distribution—ie, neonates and postpubertal boys. The prevalence of testicular torsion in adult patients hospitalized with acute scrotal pain is approximately 25% to 50%.2
Patients with testicular torsion usually describe a sudden onset of severe, acute pain. The pain frequently occurs a few hours after vigorous physical activity or minor testicular trauma.2 Occasionally, the patient may complain of lower quadrant abdominal pain rather than testicular or scrotal pain. Nausea with vomiting can also be present.
On physical examination, significant testicular swelling is usually present. Examining the patient in the standing position will often reveal an asymmetrical, high-riding testis with a transverse lie on the affected side. The cremasteric reflex is usually absent in patients with testicular torsion.
Because of the significant overlap in history and physical examination findings for epididymitis and testicular torsion, a testicular ultrasound with color Doppler should be ordered. Multiple studies have confirmed the high sensitivity and specificity of ultrasound in the diagnosis of testicular torsion.
The treatment for suspected or confirmed testicular torsion is immediate surgical exploration with intraoperative detorsion and fixation of the testes. The EP can attempt manual detorsion (ie, performed in a medial to lateral motion, similar to opening a book). However, this should not delay the EP from consulting with urology services.
Pediatric patients with testicular torsion usually have a more favorable outcome than do adults. In one retrospective study, patients younger than age 21 years had a 70% testicular salvage rate compared to only 41% of patients aged 21 years and older.7 Regardless of age, better outcomes are associated with shorter periods of torsion.
Fournier’s Gangrene
Fournier’s gangrene is a polymicrobial necrotizing fasciitis of the perineum and scrotum that typically develops initially as a benign infection or abscess but quickly spreads. Risk factors for Fournier’s gangrene include DM, alcohol abuse, and any immunocompromised state (eg, HIV, cancer).
If the patient presents early in onset, there may be only mild tenderness, erythema, or swelling of the affected area; however, this infection progresses rapidly. Later findings include marked tenderness, swelling, crepitus, blisters, and ecchymoses. Patients with Fournier’s gangrene also develop systemic signs of infection, including fever, tachycardia, tachypnea, and hypotension. The key to diagnosis is careful examination of the perineal and scrotal area in any patient presenting with acute scrotal pain.
In the majority of cases, the diagnosis of Fournier’s gangrene is made clinically. Once the diagnosis is made, patients require immediate and aggressive IV fluid resuscitation, broad-spectrum IV antibiotics (typically vancomycin and piperacillin/tazobactam), and emergent evaluation by a urologist. It is essential that these patients undergo early and aggressive surgical exploration and debridement of necrotic tissue.2 Antibiotic therapy alone is associated with a 100% mortality rate, emphasizing the need for urgent surgery.2 Even with optimal medical and surgical management, the mortality rate remains significant.
Summary
This case emphasizes several important teaching points. The EP should be mindful of the patient who keeps returning to the ED with the same complaint—despite “appropriate” treatment—as the initial diagnosis may not be the correct one. Such returning patients require greater, not less, scrutiny. As with any patient, the EP should always take a complete history and perform a thorough physical examination at each presentation—as one would with a de novo patient. Finally, the EP should consider Fournier’s gangrene in addition to testicular torsion and epididymitis in the differential diagnosis for acute scrotal pain.
1. Trojian TH, Lishnak TS, Heiman D. Epididymitis and orchitis: an overview. Am Fam Physician. 2009;79(7):583-587.
2. Eyre RC. Evaluation of acute scrotal pain in adults. UpToDate Web site. https://www.uptodate.com/contents/evaluation-of-acute-scrotal-pain-in-adults. Updated July 31, 2017. Accessed September 7, 2017.
3. Shen Y, Liu H, Cheng J, Bu P. Amiodarone-induced epididymitis: a pathologically confirmed case report and review of the literature. Cardiology. 2014;128(4):349-351. doi:10.1159/000361038.
4. Tracy CR, Steers WD, Costabile R. Diagnosis and management of epididymitis. Urol Clin North Am. 2008;35(1):101-108. doi:10.1016/j.ucl.2007.09.013.
5. Wampler SM, Llanes M. Common scrotal and testicular problems. Prim Care. 2010;37(3):613-626. doi:10.1016/j.pop.2010.04.009.
6. Dunne PJ, O’Loughlin BS. Testicular torsion: time is the enemy. Aust NZ J Surg. 2000;70(6):441-442.
7. Cummings JM, Boullier JA, Sekhon D, Bose K. Adult testicular torsion. J Urol. 2002;167(5):2109-2110.
1. Trojian TH, Lishnak TS, Heiman D. Epididymitis and orchitis: an overview. Am Fam Physician. 2009;79(7):583-587.
2. Eyre RC. Evaluation of acute scrotal pain in adults. UpToDate Web site. https://www.uptodate.com/contents/evaluation-of-acute-scrotal-pain-in-adults. Updated July 31, 2017. Accessed September 7, 2017.
3. Shen Y, Liu H, Cheng J, Bu P. Amiodarone-induced epididymitis: a pathologically confirmed case report and review of the literature. Cardiology. 2014;128(4):349-351. doi:10.1159/000361038.
4. Tracy CR, Steers WD, Costabile R. Diagnosis and management of epididymitis. Urol Clin North Am. 2008;35(1):101-108. doi:10.1016/j.ucl.2007.09.013.
5. Wampler SM, Llanes M. Common scrotal and testicular problems. Prim Care. 2010;37(3):613-626. doi:10.1016/j.pop.2010.04.009.
6. Dunne PJ, O’Loughlin BS. Testicular torsion: time is the enemy. Aust NZ J Surg. 2000;70(6):441-442.
7. Cummings JM, Boullier JA, Sekhon D, Bose K. Adult testicular torsion. J Urol. 2002;167(5):2109-2110.
Case Studies in Toxicology: Always Cook Your Boba
Case
A 45-year-old Chinese man with no known medical history presented to the ED with right-sided facial spasm and cheek swelling, which began immediately after he bit into a piece of taro root, approximately 2 hours prior to presentation. The patient stated that the root was an ingredient in a soup that a relative had made. According to the patient, after biting into the root, he immediately experienced a burning pain on the right side of his mouth. He further noted that he swallowed less than two bites of the root and stopped eating because the act of chewing was too painful.
Initial vital signs at presentation were: blood pressure, 140/100 mm Hg; heart rate, 84 beats/min; respiratory rate, 14 beats/min; and temperature, 97.6°F. Oxygen saturation was 98% on room air. The patient’s physical examination was remarkable for pain upon opening the mouth, as well as right-sided cheek and lip swelling and tenderness. The tongue and oropharynx were not erythematous or swollen. The patient was only able to speak in short sentences, secondary to oropharyngeal pain, but he was in no respiratory distress. No urticaria, pruritus, wheezing, or stridor was present.
During the patient’s workup, his 40-year-old wife also presented to the same ED for evaluation of burning pain and spasm on the left side of her mouth, which she stated also developed immediately after she bit into a piece of taro root contained in the same soup as that ingested by the patient.
The wife’s vital signs were unremarkable, and she was in no respiratory distress. Her physical examination was remarkable only for left-sided cheek and lip swelling and tenderness, associated with an erythematous oropharynx and pain with speaking.
What is taro? What are the manifestations of taro toxicity?
Taro commonly refers to plants from the Araceae family, usually Colocasia esculenta.1 Taro is ubiquitous in Southern Asia and Southeast India. It is a widely naturalized and perennial tropical plant primarily grown as a root vegetable, and is a common flavor in boba (bubble) tea. All members of Araceae contain calcium oxalate crystals in the form of raphides, sharp needle-shaped crystals packaged in idioblasts and contained within the waxy leaf.2 Pressure on the idioblasts, such as from mastication, triggers the release of the raphides. The needles pierce the surface of any tissue with which they come into contact, creating a gateway for proteolytic enzymes to enter the consumer.3 The leaves and root of Araceae must be cooked before eating to inactivate the raphides.
Oral exposure to uncooked taro leaves or taro root can result in mouth irritation and swelling that can progress to angioedema and airway obstruction. Although the traditional method of removing taro raphides is to soak the root in cold water overnight,4,5 this does not fully remove all of the raphides. Instead, taro root should be thoroughly cooked in boiling water to draw-out oxalates from the root into the cooking water, which must then be discarded. Consuming taro with warm milk also reduces the effect of the oxalates by about 80%.6
Many other plants of the Araceae family, such as Dieffenbachia (dumbcane), share similar toxicity and are commonly kept in the home and office.
Patients with oral exposure to taro may experience a delayed (also termed biphasic) anaphylactic reaction, ie, the development of anaphylactic symptoms more than 4 hours after the inciting event. Delayed anaphylaxis is distinct from delayed hypersensitivity, though both may be immunoglobulin E-mediated. Delayed hypersensitivity presents later (2-14 days) and with less immediately life-threatening effects, most commonly dermatitis (eg, poison ivy dermatitis).
While both of the patients in this case presented with mild symptoms, life-threatening angioedema of the oropharynx, anaphylaxis, and hypocalcemia have been reported7,8 and should be considered in any symptomatic patient with exposure to taro.
What is the differential diagnosis of plant-related mouth pain?
The oral mucosa is composed of superficial layers of mucin and epithelial cells that lie over the dermis and connective tissue. Local immune cells, including mast cells and Langerhans cells, reside in the deeper layers. The differential diagnosis of plant-based mouth pain can be divided into mechanical, chemical, and thermal causes.
Mechanical Causes. Causes of mechanical plant-based oral pain include structural damage when foreign matter, such as barbs, sharp leaves, or hard seeds, pierce the layers of the oral mucosa.
Chemical Causes. Chemical-related causes of oral pain include caustic ingestion, for example from detergents or cleaning agents that contaminate the broth. Araceae, such as taro or arum, have sharp calcium oxalate crystals tipped with phospholipases and proteases that cause mechanical pain on piercing mucous membranes, and chemical pain by enzymatically degrading epithelium and mucosa. Both chemical and mechanical irritation can lead to an inflammatory response. Raw taro can cause irritant contact stomatitis as the raphides pierce the oral mucosa. It can also cause allergic stomatitis if antigens related to the phospholipases or proteases are presented to Langerhans cells.9
Thermal Causes. The hot temperature of the ingested broth could cause thermal injury, but the injury is likely to be more diffuse.
How common is taro exposure, and how is it treated?
From 1995 to 1999, 15 cases of taro poisoning were reported to the Drug and Toxicology Information service in Zimbabwe.10 From 2005 to 2009, 21 out of 31 cases reported to the Hong Kong Poison Control Center involving gastrointestinal irritation involved the consumption of Colocasia fallax, a form of taro more common in Tibet, the Himalayas, and northern Indochina.7 Of the 31 cases, six patients were treated with diphenhydramine, epinephrine, and dexamethasone for angioedema.
From 2011 to 2013, two cases of mouth irritation and swelling after eating raw taro leaves were reported to the British Columbia Poison Control Center.11 Those two patients were observed for 6 hours without specific treatment and discharged.
Case Conclusion
Due to concerns of the potential for anaphylaxis, both patients were treated intravenously with 50 mg diphenhydramine and 10 mg dexamethasone. The husband was also given 650 mg acetaminophen orally for pain relief; his wife declined pain medication. Laboratory evaluation, including a complete blood count, basic metabolic panel, liver function panel, and urinalysis were ordered for both patients; all results were within normal limits for both patients.
After an uneventful 6-hour observation period, both patients were discharged home with instructions to return to the ED if they develop any signs of allergic reaction and to call emergency medical services for any sign of anaphylaxis.
1. Rao RV, Matthews PJ, Eyzaguirre PB, Hunter D, eds. 2010. The Global Diversity of Taro: Ethnobotany and Conservation. Rome, Italy; Biouniversity International; 2010. http://www.bioversityinternational.org/fileadmin/user_upload/online_library/publications/pdfs/1402.pdf#page=11. Accessed September 15, 2017.
2. Franceschi VR, Nakata PA. Calcium oxalate in plants: formation and function. Annu Rev Plant Biol. 2005;56:41-71. doi:10.1146/annurev.arplant.56.032604.144106.
3. Herbert DA. Stinging crystals in plants. Science. 1924;60(1548):204-205. doi:10.1126/science.60.1548.204-a.
4. Njintang YN, Mbofung CMF. Effect of precooking time and drying temperature on the physico-chemical characteristics and in-vitro carbohydrate digestibility of taro flour. LWT – Food Sci and Tech. 2006;39(6):684-691. doi.org/10.1016/j.lwt.2005.03.022.
5. Savage GP, Dubois M. The effect of soaking and cooking on the oxalate content of taro leaves. Int J Food Sci Nutr. 2006;57(5-6):376-381. doi:10.1080/09637480600855239.
6. Oscarsson, KV. Savage GP. Composition and availability of soluble and insoluble oxalates in raw and cooked taro (Colocasia esculenta var. Schott) leaves. Food Chem 101. 2007;101(2):559-562. doi:10.1016/j.foodchem.2006.02.014.
7. Pang CT, Ng HW, Lau FL. Oral mucosal irritating plant ingestion in Hong Kong, epidemiology and its clinical presentation. Hong Kong J Emerg Med. 2010;17(5):477-481.
8. Yuen E. Upper airway obstruction as a presentation of Taro poisoning. Hong Kong J Emerg Med. 2001;8(3):163-165.
9. Davis CC, Squier CA, Lilly GE. Irritant contact stomatitis: a review of the condition. J Periodontol. 1998;69(6):620-631. doi:10.1902/jop.1998.69.6.620.
10 Tagwireyi D, Ball DE. The management of Elephant’s Ear poisoning. Hum Exp Toxicol. 2001;20(4):189-192. doi:10.1191/096032701678766822.
11. Omura JD, Blake C, McIntyre L, Li D, Kosatsky T. Two cases of poisoning by raw taro leaf and how a poison control centre, food safety inspectors, and a specialty supermarket chain found a solution.” Environ Health Rev. 2014;57(3):59-64. doi.org/10.5864/d2014-027.
Case
A 45-year-old Chinese man with no known medical history presented to the ED with right-sided facial spasm and cheek swelling, which began immediately after he bit into a piece of taro root, approximately 2 hours prior to presentation. The patient stated that the root was an ingredient in a soup that a relative had made. According to the patient, after biting into the root, he immediately experienced a burning pain on the right side of his mouth. He further noted that he swallowed less than two bites of the root and stopped eating because the act of chewing was too painful.
Initial vital signs at presentation were: blood pressure, 140/100 mm Hg; heart rate, 84 beats/min; respiratory rate, 14 beats/min; and temperature, 97.6°F. Oxygen saturation was 98% on room air. The patient’s physical examination was remarkable for pain upon opening the mouth, as well as right-sided cheek and lip swelling and tenderness. The tongue and oropharynx were not erythematous or swollen. The patient was only able to speak in short sentences, secondary to oropharyngeal pain, but he was in no respiratory distress. No urticaria, pruritus, wheezing, or stridor was present.
During the patient’s workup, his 40-year-old wife also presented to the same ED for evaluation of burning pain and spasm on the left side of her mouth, which she stated also developed immediately after she bit into a piece of taro root contained in the same soup as that ingested by the patient.
The wife’s vital signs were unremarkable, and she was in no respiratory distress. Her physical examination was remarkable only for left-sided cheek and lip swelling and tenderness, associated with an erythematous oropharynx and pain with speaking.
What is taro? What are the manifestations of taro toxicity?
Taro commonly refers to plants from the Araceae family, usually Colocasia esculenta.1 Taro is ubiquitous in Southern Asia and Southeast India. It is a widely naturalized and perennial tropical plant primarily grown as a root vegetable, and is a common flavor in boba (bubble) tea. All members of Araceae contain calcium oxalate crystals in the form of raphides, sharp needle-shaped crystals packaged in idioblasts and contained within the waxy leaf.2 Pressure on the idioblasts, such as from mastication, triggers the release of the raphides. The needles pierce the surface of any tissue with which they come into contact, creating a gateway for proteolytic enzymes to enter the consumer.3 The leaves and root of Araceae must be cooked before eating to inactivate the raphides.
Oral exposure to uncooked taro leaves or taro root can result in mouth irritation and swelling that can progress to angioedema and airway obstruction. Although the traditional method of removing taro raphides is to soak the root in cold water overnight,4,5 this does not fully remove all of the raphides. Instead, taro root should be thoroughly cooked in boiling water to draw-out oxalates from the root into the cooking water, which must then be discarded. Consuming taro with warm milk also reduces the effect of the oxalates by about 80%.6
Many other plants of the Araceae family, such as Dieffenbachia (dumbcane), share similar toxicity and are commonly kept in the home and office.
Patients with oral exposure to taro may experience a delayed (also termed biphasic) anaphylactic reaction, ie, the development of anaphylactic symptoms more than 4 hours after the inciting event. Delayed anaphylaxis is distinct from delayed hypersensitivity, though both may be immunoglobulin E-mediated. Delayed hypersensitivity presents later (2-14 days) and with less immediately life-threatening effects, most commonly dermatitis (eg, poison ivy dermatitis).
While both of the patients in this case presented with mild symptoms, life-threatening angioedema of the oropharynx, anaphylaxis, and hypocalcemia have been reported7,8 and should be considered in any symptomatic patient with exposure to taro.
What is the differential diagnosis of plant-related mouth pain?
The oral mucosa is composed of superficial layers of mucin and epithelial cells that lie over the dermis and connective tissue. Local immune cells, including mast cells and Langerhans cells, reside in the deeper layers. The differential diagnosis of plant-based mouth pain can be divided into mechanical, chemical, and thermal causes.
Mechanical Causes. Causes of mechanical plant-based oral pain include structural damage when foreign matter, such as barbs, sharp leaves, or hard seeds, pierce the layers of the oral mucosa.
Chemical Causes. Chemical-related causes of oral pain include caustic ingestion, for example from detergents or cleaning agents that contaminate the broth. Araceae, such as taro or arum, have sharp calcium oxalate crystals tipped with phospholipases and proteases that cause mechanical pain on piercing mucous membranes, and chemical pain by enzymatically degrading epithelium and mucosa. Both chemical and mechanical irritation can lead to an inflammatory response. Raw taro can cause irritant contact stomatitis as the raphides pierce the oral mucosa. It can also cause allergic stomatitis if antigens related to the phospholipases or proteases are presented to Langerhans cells.9
Thermal Causes. The hot temperature of the ingested broth could cause thermal injury, but the injury is likely to be more diffuse.
How common is taro exposure, and how is it treated?
From 1995 to 1999, 15 cases of taro poisoning were reported to the Drug and Toxicology Information service in Zimbabwe.10 From 2005 to 2009, 21 out of 31 cases reported to the Hong Kong Poison Control Center involving gastrointestinal irritation involved the consumption of Colocasia fallax, a form of taro more common in Tibet, the Himalayas, and northern Indochina.7 Of the 31 cases, six patients were treated with diphenhydramine, epinephrine, and dexamethasone for angioedema.
From 2011 to 2013, two cases of mouth irritation and swelling after eating raw taro leaves were reported to the British Columbia Poison Control Center.11 Those two patients were observed for 6 hours without specific treatment and discharged.
Case Conclusion
Due to concerns of the potential for anaphylaxis, both patients were treated intravenously with 50 mg diphenhydramine and 10 mg dexamethasone. The husband was also given 650 mg acetaminophen orally for pain relief; his wife declined pain medication. Laboratory evaluation, including a complete blood count, basic metabolic panel, liver function panel, and urinalysis were ordered for both patients; all results were within normal limits for both patients.
After an uneventful 6-hour observation period, both patients were discharged home with instructions to return to the ED if they develop any signs of allergic reaction and to call emergency medical services for any sign of anaphylaxis.
Case
A 45-year-old Chinese man with no known medical history presented to the ED with right-sided facial spasm and cheek swelling, which began immediately after he bit into a piece of taro root, approximately 2 hours prior to presentation. The patient stated that the root was an ingredient in a soup that a relative had made. According to the patient, after biting into the root, he immediately experienced a burning pain on the right side of his mouth. He further noted that he swallowed less than two bites of the root and stopped eating because the act of chewing was too painful.
Initial vital signs at presentation were: blood pressure, 140/100 mm Hg; heart rate, 84 beats/min; respiratory rate, 14 beats/min; and temperature, 97.6°F. Oxygen saturation was 98% on room air. The patient’s physical examination was remarkable for pain upon opening the mouth, as well as right-sided cheek and lip swelling and tenderness. The tongue and oropharynx were not erythematous or swollen. The patient was only able to speak in short sentences, secondary to oropharyngeal pain, but he was in no respiratory distress. No urticaria, pruritus, wheezing, or stridor was present.
During the patient’s workup, his 40-year-old wife also presented to the same ED for evaluation of burning pain and spasm on the left side of her mouth, which she stated also developed immediately after she bit into a piece of taro root contained in the same soup as that ingested by the patient.
The wife’s vital signs were unremarkable, and she was in no respiratory distress. Her physical examination was remarkable only for left-sided cheek and lip swelling and tenderness, associated with an erythematous oropharynx and pain with speaking.
What is taro? What are the manifestations of taro toxicity?
Taro commonly refers to plants from the Araceae family, usually Colocasia esculenta.1 Taro is ubiquitous in Southern Asia and Southeast India. It is a widely naturalized and perennial tropical plant primarily grown as a root vegetable, and is a common flavor in boba (bubble) tea. All members of Araceae contain calcium oxalate crystals in the form of raphides, sharp needle-shaped crystals packaged in idioblasts and contained within the waxy leaf.2 Pressure on the idioblasts, such as from mastication, triggers the release of the raphides. The needles pierce the surface of any tissue with which they come into contact, creating a gateway for proteolytic enzymes to enter the consumer.3 The leaves and root of Araceae must be cooked before eating to inactivate the raphides.
Oral exposure to uncooked taro leaves or taro root can result in mouth irritation and swelling that can progress to angioedema and airway obstruction. Although the traditional method of removing taro raphides is to soak the root in cold water overnight,4,5 this does not fully remove all of the raphides. Instead, taro root should be thoroughly cooked in boiling water to draw-out oxalates from the root into the cooking water, which must then be discarded. Consuming taro with warm milk also reduces the effect of the oxalates by about 80%.6
Many other plants of the Araceae family, such as Dieffenbachia (dumbcane), share similar toxicity and are commonly kept in the home and office.
Patients with oral exposure to taro may experience a delayed (also termed biphasic) anaphylactic reaction, ie, the development of anaphylactic symptoms more than 4 hours after the inciting event. Delayed anaphylaxis is distinct from delayed hypersensitivity, though both may be immunoglobulin E-mediated. Delayed hypersensitivity presents later (2-14 days) and with less immediately life-threatening effects, most commonly dermatitis (eg, poison ivy dermatitis).
While both of the patients in this case presented with mild symptoms, life-threatening angioedema of the oropharynx, anaphylaxis, and hypocalcemia have been reported7,8 and should be considered in any symptomatic patient with exposure to taro.
What is the differential diagnosis of plant-related mouth pain?
The oral mucosa is composed of superficial layers of mucin and epithelial cells that lie over the dermis and connective tissue. Local immune cells, including mast cells and Langerhans cells, reside in the deeper layers. The differential diagnosis of plant-based mouth pain can be divided into mechanical, chemical, and thermal causes.
Mechanical Causes. Causes of mechanical plant-based oral pain include structural damage when foreign matter, such as barbs, sharp leaves, or hard seeds, pierce the layers of the oral mucosa.
Chemical Causes. Chemical-related causes of oral pain include caustic ingestion, for example from detergents or cleaning agents that contaminate the broth. Araceae, such as taro or arum, have sharp calcium oxalate crystals tipped with phospholipases and proteases that cause mechanical pain on piercing mucous membranes, and chemical pain by enzymatically degrading epithelium and mucosa. Both chemical and mechanical irritation can lead to an inflammatory response. Raw taro can cause irritant contact stomatitis as the raphides pierce the oral mucosa. It can also cause allergic stomatitis if antigens related to the phospholipases or proteases are presented to Langerhans cells.9
Thermal Causes. The hot temperature of the ingested broth could cause thermal injury, but the injury is likely to be more diffuse.
How common is taro exposure, and how is it treated?
From 1995 to 1999, 15 cases of taro poisoning were reported to the Drug and Toxicology Information service in Zimbabwe.10 From 2005 to 2009, 21 out of 31 cases reported to the Hong Kong Poison Control Center involving gastrointestinal irritation involved the consumption of Colocasia fallax, a form of taro more common in Tibet, the Himalayas, and northern Indochina.7 Of the 31 cases, six patients were treated with diphenhydramine, epinephrine, and dexamethasone for angioedema.
From 2011 to 2013, two cases of mouth irritation and swelling after eating raw taro leaves were reported to the British Columbia Poison Control Center.11 Those two patients were observed for 6 hours without specific treatment and discharged.
Case Conclusion
Due to concerns of the potential for anaphylaxis, both patients were treated intravenously with 50 mg diphenhydramine and 10 mg dexamethasone. The husband was also given 650 mg acetaminophen orally for pain relief; his wife declined pain medication. Laboratory evaluation, including a complete blood count, basic metabolic panel, liver function panel, and urinalysis were ordered for both patients; all results were within normal limits for both patients.
After an uneventful 6-hour observation period, both patients were discharged home with instructions to return to the ED if they develop any signs of allergic reaction and to call emergency medical services for any sign of anaphylaxis.
1. Rao RV, Matthews PJ, Eyzaguirre PB, Hunter D, eds. 2010. The Global Diversity of Taro: Ethnobotany and Conservation. Rome, Italy; Biouniversity International; 2010. http://www.bioversityinternational.org/fileadmin/user_upload/online_library/publications/pdfs/1402.pdf#page=11. Accessed September 15, 2017.
2. Franceschi VR, Nakata PA. Calcium oxalate in plants: formation and function. Annu Rev Plant Biol. 2005;56:41-71. doi:10.1146/annurev.arplant.56.032604.144106.
3. Herbert DA. Stinging crystals in plants. Science. 1924;60(1548):204-205. doi:10.1126/science.60.1548.204-a.
4. Njintang YN, Mbofung CMF. Effect of precooking time and drying temperature on the physico-chemical characteristics and in-vitro carbohydrate digestibility of taro flour. LWT – Food Sci and Tech. 2006;39(6):684-691. doi.org/10.1016/j.lwt.2005.03.022.
5. Savage GP, Dubois M. The effect of soaking and cooking on the oxalate content of taro leaves. Int J Food Sci Nutr. 2006;57(5-6):376-381. doi:10.1080/09637480600855239.
6. Oscarsson, KV. Savage GP. Composition and availability of soluble and insoluble oxalates in raw and cooked taro (Colocasia esculenta var. Schott) leaves. Food Chem 101. 2007;101(2):559-562. doi:10.1016/j.foodchem.2006.02.014.
7. Pang CT, Ng HW, Lau FL. Oral mucosal irritating plant ingestion in Hong Kong, epidemiology and its clinical presentation. Hong Kong J Emerg Med. 2010;17(5):477-481.
8. Yuen E. Upper airway obstruction as a presentation of Taro poisoning. Hong Kong J Emerg Med. 2001;8(3):163-165.
9. Davis CC, Squier CA, Lilly GE. Irritant contact stomatitis: a review of the condition. J Periodontol. 1998;69(6):620-631. doi:10.1902/jop.1998.69.6.620.
10 Tagwireyi D, Ball DE. The management of Elephant’s Ear poisoning. Hum Exp Toxicol. 2001;20(4):189-192. doi:10.1191/096032701678766822.
11. Omura JD, Blake C, McIntyre L, Li D, Kosatsky T. Two cases of poisoning by raw taro leaf and how a poison control centre, food safety inspectors, and a specialty supermarket chain found a solution.” Environ Health Rev. 2014;57(3):59-64. doi.org/10.5864/d2014-027.
1. Rao RV, Matthews PJ, Eyzaguirre PB, Hunter D, eds. 2010. The Global Diversity of Taro: Ethnobotany and Conservation. Rome, Italy; Biouniversity International; 2010. http://www.bioversityinternational.org/fileadmin/user_upload/online_library/publications/pdfs/1402.pdf#page=11. Accessed September 15, 2017.
2. Franceschi VR, Nakata PA. Calcium oxalate in plants: formation and function. Annu Rev Plant Biol. 2005;56:41-71. doi:10.1146/annurev.arplant.56.032604.144106.
3. Herbert DA. Stinging crystals in plants. Science. 1924;60(1548):204-205. doi:10.1126/science.60.1548.204-a.
4. Njintang YN, Mbofung CMF. Effect of precooking time and drying temperature on the physico-chemical characteristics and in-vitro carbohydrate digestibility of taro flour. LWT – Food Sci and Tech. 2006;39(6):684-691. doi.org/10.1016/j.lwt.2005.03.022.
5. Savage GP, Dubois M. The effect of soaking and cooking on the oxalate content of taro leaves. Int J Food Sci Nutr. 2006;57(5-6):376-381. doi:10.1080/09637480600855239.
6. Oscarsson, KV. Savage GP. Composition and availability of soluble and insoluble oxalates in raw and cooked taro (Colocasia esculenta var. Schott) leaves. Food Chem 101. 2007;101(2):559-562. doi:10.1016/j.foodchem.2006.02.014.
7. Pang CT, Ng HW, Lau FL. Oral mucosal irritating plant ingestion in Hong Kong, epidemiology and its clinical presentation. Hong Kong J Emerg Med. 2010;17(5):477-481.
8. Yuen E. Upper airway obstruction as a presentation of Taro poisoning. Hong Kong J Emerg Med. 2001;8(3):163-165.
9. Davis CC, Squier CA, Lilly GE. Irritant contact stomatitis: a review of the condition. J Periodontol. 1998;69(6):620-631. doi:10.1902/jop.1998.69.6.620.
10 Tagwireyi D, Ball DE. The management of Elephant’s Ear poisoning. Hum Exp Toxicol. 2001;20(4):189-192. doi:10.1191/096032701678766822.
11. Omura JD, Blake C, McIntyre L, Li D, Kosatsky T. Two cases of poisoning by raw taro leaf and how a poison control centre, food safety inspectors, and a specialty supermarket chain found a solution.” Environ Health Rev. 2014;57(3):59-64. doi.org/10.5864/d2014-027.
Heart Failure in the Emergency Department
Patients with heart failure (HF) present daily to busy EDs. An estimated 6.5 million Americans are living with this diagnosis, and the number is predicted to grow to 8 million by 2023.1 Most HF patients (82.1%) who present to EDs are hospitalized, while a selected minority are either managed in the ED and discharged (11.6%) or managed in observation units (OU) (6.3%).2 The prognosis after HF is initially diagnosed is poor, with a 5-year mortality of 50%,3 and after a single HF hospitalization, 29% will die within 1 year.4
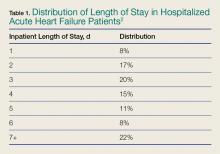
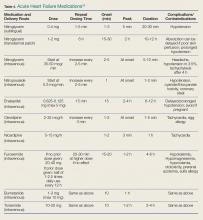
One-third of the total Medicare budget is spent on HF, despite the fact that HF represents only 10.5% of the Medicare population.2 Up to 80% of HF costs are for hospitalizations, which cost an average of $11,840 per inpatient admission.5,6 The high costs are due to an average length of stay (LOS) of 5.2 days7 (Table 1).
Adding to hospital costs is the degree of “reactivism,” with approximately 20% of patients discharged from the ED returning within 2 weeks, of whom nearly 50% will be hospitalized.11 Following HF hospitalization and discharge, the 30-day readmission rate is 26.2%,2 increasing to 36% by 90 days.12 The Centers for Medicare & Medicaid Services (CMS) has incentivized hospitals and providers to reduce admissions, but penalize hospitals that do not. Overall, CMS will reduce payments by up to 3% to hospitals with excess readmissions for select conditions, including HF.13
Causes of Heart Failure
Heart failure represents a final common pathway, which in the United States is most often due to coronary artery disease (CAD). Many types of pathology ultimately result in left ventricular (LV) dysfunction, and much of its rising prevalence is a result of the success we now have in managing historically fatal cardiovascular (CV) conditions. These include hypertension, diabetes mellitus (DM), CAD, and valvular and other CV structural conditions.
Heart failure is caused by either a dilated ventricle with a reduced ejection fraction (HFrEF) and inability to eject volume, or a stiffened ventricle with a preserved EF (HFpEF) that is unable to receive increased venous return. Both conditions acutely decompensate pulmonary congestion. A preserved EF is defined as an EF at or greater than 50%, whereas a reduced EF is at or less than 40%, with the 41% to 49% range considered as borderline preserved EF.3
While there are important differences in the treatment of chronic and subacute HF, driven by the EF, the effect of EF on early decision-making and treatment in the ED is negligible: Although the probability of HFpEF increases with increasing initial ED systolic blood pressure (SBP), clinical presentation and treatment in the ED are initially identical—regardless of the EF.
Noninvasive continuous transcutaneous hemodynamic monitoring is available for ED use, and may provide further insight into the underlying pathophysiology. A study of 127 acute heart failure (AHF) ED patients identified three hemodynamic AHF phenotypes. These include normal cardiac index (CI) and systemic vascular resistance index (SVRI), low CI and SVRI, and low CI and elevated SVRI.14 While it is attractive to suggest therapeutic interventions based on these measurements, outcome data are lacking.
Presentation
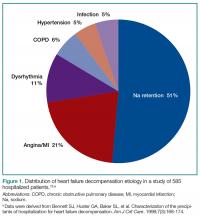
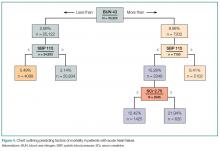
The most common ED presentation of patients suffering from AHF is dyspnea secondary to volume overload, or as the result of acute hypertension with relatively less volume overload. However, regardless of the cause of dyspnea, it is not only the most common resulting complaint, but one that requires immediate treatment. Ultimately, 59% of all HF admissions are attributed to volume overload and dyspnea (Figure 1).15
Heart failure can also present in a more protean manner, with cough, fatigue, and edema, as well as more subtle symptoms predominating and resulting in a complicated differential diagnosis (Table 2).16
Because HF is a disease that most significantly affects older patients who frequently have concomitant morbidities (eg, myocardial ischemia, chronic obstructive pulmonary disease [COPD] exacerbation, uncontrolled DM), other less clinically obvious disease presentations may actually be the cause of the AHF exacerbation.
Diagnosis
A focused history and physical examination that is part of all ED evaluations should be expedited whenever there is evidence of hemodynamic instability or respiratory compromise. An early working diagnosis is essential to avoid a delay in appropriate treatment, which is associated with increased mortality.
When HF is likely, the potential etiology and precipitants for decompensation must be considered. This list is long, but medication noncompliance and dietary indiscretion are the most common causes.
Symptoms and Prior History of HF
The classic symptoms for AHF include dyspnea at rest or exertion, and orthopnea, both of which unfortunately have poor sensitivity and specificity for AHF. As an isolated symptom, dyspnea is of marginal diagnostic utility (sensitivity and specificity for an HF diagnosis is 56% and 53%, respectively), and orthopnea is only slightly better (sensitivity and specificity 77% and 50%, respectively). A prior HF diagnosis makes repeat presentations much more likely (sensitivity and specificity 60% and 90%, respectively).17
Physical Examination
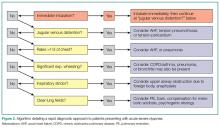
Simple observation and a directed examination can rapidly point to the diagnosis (Figure 2).
Electrocardiography
Because CAD is one of the most common underlying AHF etiologies, an electrocardiogram (ECG) should always be obtained early for a patient presenting with potential AHF. Although the ECG does not usually contribute to ED management, the identification of new ST-segment changes or a malignant arrhythmia will guide critical management decisions.
Imaging Studies
Chest X-ray Imaging. A chest X-ray (CXR) study must be considered early when a patient presents with signs and symptoms suggestive of AHF. Although the classic findings of HF (eg, Kerley B lines [short horizontal lines perpendicular to the pleural surface],18 interstitial congestion, pulmonary effusion) can lag behind the clinical presentation, and also be nondiagnostic in the setting of mild HF, the CXR is an effective aid in identifying other causes of dyspnea such as pneumonia. Ultimately, the utility of the CXR for diagnosis is similar to that of the history and physical examination in that it will be diagnostic when positive but cannot exclude AHF if normal.
Ultrasound. Because it is fast, inexpensive, noninvasive, and readily available in the ED, ultrasound is frequently used to evaluate potential HF patients. Several studies have demonstrated that the presence of B lines in two or more regions is specific for AHF (specificity 75%-100%), although the sensitivity may be limited (40%-91%).19-21 The presence of inferior vena cava (IVC) dilation is also associated with adverse outcomes.22 In 80 patients hospitalized with acute decompensated HF (ADHF), a dilated IVC (≥1.9 cm) at admission was associated with higher 90-day mortality (25.4% vs 3.4%, P = 0.009).23 These findings may be considered in groups: In an evaluation of the combination of LV EF, IVC collapsibility, and B lines for an HF diagnosis, the combination of all three had a poor sensitivity (36%) but an excellent specificity (100%), and any two of the three had a specificity of at least 93%.24
Laboratory Evaluation
Myocardial Strain: BNP/NTproBNP. Natriuretic peptides (NPs) are not AHF-specific, but rather they are synthesized and released by the myocardium in the setting of myocardial pressure or volume stress. They are manufactured as preproBNP, then enzymatically cleaved into the active BNP and the inactive fragment N-terminal proBNP (NTproBNP). The predominant hormonal effects of BNP are vasodilation and natriuresis, as well as antagonism of the hormones associated with sodium retention (aldosterone) and vasoconstriction (endothelin, norepinephrine).
As AHF results in myocardial stress, NP elevation provides diagnostic and prognostic information. Clinical judgment supported by a BNP greater than 100 pg/mL is a better predictor of AHF than clinical judgment alone (accuracy 81% vs 74%, respectively).25 While low levels (BNP <100 pg/mL or NTproBNP <300 pg/mL) reliably exclude the diagnosis of HF (sensitivities >95%), higher levels (BNP >500 pg/mL, NTproBNP >900 pg/mL) are useful as “rule-in” markers, with specificity greater than 95%. The NTproBNP also requires adjustment for patients older than age 75 years, with a higher level (>1,800 pg/mL) to rule-in HF. The NP grey zone (BNP 100-500 pg/mL, NTproBNP 300-900 pg/mL)requires additional testing for accurate diagnoses (Figure 3).25-29
There are several confounders to the interpretation of NP results: NPs are negatively confounded by the presence of obesity, resulting in a lowering of the value as compared to the clinical presentation.Thus, the measured BNP level should be doubled if the patient’s body mass index exceeds 35 kg/m2.30 Secondly, because NP metabolism is partially renal dependent, elevated levels may not reflect AHF in the presence of renal failure. If the estimated glomerular filtration rate is less than 60 mL/min, measured BNP levels should be halved.31
AHF vs Myocardial Ischemia: Troponin Levels. Large registry data using contemporary troponin assays clearly identify the association between elevated troponin levels (>99th percentile in a healthy population) and increased short-term risk. With the US Food and Drug Association (FDA) approval of a high-sensitivity troponin (hs-cTnT) assay, a greater frequency of elevated cardiac troponin T (cTnT) and cardiac troponin I (cTnl) will be identified in AHF patients in the ED.
In one retrospective study of 4,705 AHF patients in the ED, hs-cTnT were elevated in 48.4% of cases (25.3% in cTnI, 37.9% in cTnT, and 82.2% in hs-cTnT). Although 1-year mortality was higher in those with elevated troponin (adjusted heart rate [HR] 1.61; CI 95% 1.38-1.88), elevated troponin was not associated with 30-day revisits to the ED (1.01; 0.87-1.19) and high sensitive elevations less than double the reference value had no impact on outcomes.32 Thus, in terms of management of AHF in the ED, slightly elevated stable serial troponins are more consistent with underlying HF, and should be managed as such. This is not true of rising/falling troponin levels, which should still engender concern for underlying myocardial ischemia and a different management pathway.
Renal function. Comprised renal function is an important predictor of AHF outcome. Large registry data from hospitalized HF patients demonstrate that a presenting blood urea nitrogen level greater than 43 mg/dL is one of the most important predictors of increased acute mortality,33 and levels below 30 mg/dL identify a cohort likely to be successfully managed in an observation environment.34 Creatinine is a helpful lagging indicator of mortality, with higher levels (>2.75 mg/dL) associated with increased short-term adverse outcomes and decreased therapeutic responsiveness (Figure 4).
For patients presenting with ADHF, a newer test recently approved by the FDA uses the product of the urine markers tissue inhibitor of metalloproteinase-2 and insulin-like growth factor-binding protein 7, to generate a score predictive of acute kidney injury.35 While promising, no studies of ED outcomes are currently available.
Volume Assessment
Objective volume assessment is useful for diagnosis and prognosis in AHF. Bioimpedance vector analysis (BIVA) is a rapid, inexpensive, noninvasive technique that measures total body water by placing a pair of electrodes on the wrist and ipsilateral ankle. The BIVA measurements have strong correlations with the gold standard volume-assessment technique of deuterium dilution (r > 0.99).36 In HF, BIVA can assess volume depletion37 and overload,38 and identifies differences in hydration status between 90-day survivors and non-survivors (P < 0.01).39
Used in combination with BNP, one prospective study of 292 dyspneic patients found that, while BIVA was a strong predictor of AHF (c-statistic 0.93, P = 0.016), the most accurate volume status determination was the combination of both (c-statistic, 0.99; P = 0.005), for which the combined accuracy exceeded either alone.40 Finally, in 166 hospitalized HF patients discharged by BNP and BIVA parameters, vs 149 discharged based on clinical impressions, those assessed with BNP and BIVA had lower 6-month readmissions (23% vs 35%, P = 0.02) and overall cost of care.41
Combination Technologies
Obviously, EPs may consider multiple technologies to arrive at an accurate diagnosis. One prospective evaluation enrolled 236 patients to determine the diagnostic accuracy for AHF in the ED and reported lung ultrasound, CXR, and NTproBNP had a sensitivity of 57.7% and 88.0%, 74.5% and 86.3%, and a specificity of 97.6% and 28.0%. The best overall combination was the CXR with lung ultrasound (sensitivity 84.7%, specificity 77.7%).42
Another prospective study evaluated IVC diameter, bioelectrical impedance analysis (BIA), and NTproBNP in 96 elderly patients. ADHF patients had higher IVC diameters and lower collapsibility index, lower resistance and reactance, and higher NTproBNP levels. While all had high and statistically similar C-statistics (range 0.8 to 0.9) for an ADHF diagnosis, they concluded that IVC ultrasonography and BIA were as useful as NT-proBNP for diagnosing ADHF. 24
Diagnostic Scoring Systems
A scoring system has been proposed to improve diagnosis in the ED. Unfortunately, the value over clinical impression has not been clearly proven, though one randomized, controlled trial did not show statistically significant improvement in diagnostic accuracy when compared to standard care (77% vs 74%, P = 0.77).43
Differential Diagnosis
The differential diagnosis for acute dyspnea is long and potentially arcane. Efforts should focus on excluding non-HF causes of dyspnea, while considering the high risk of alternative etiologies for signs and symptoms. These include asthma, COPD, pneumonia, and pulmonary embolism, which may represent the primary pathologies in a patient with a history of HF, or be the cause of a HF exacerbation. Additional causes of noncardiogenic pulmonary edema should also be considered (eg, acute respiratory distress syndrome, toxins, etc). Acute coronary syndrome and dyspnea may be angina equivalents—one important consideration.
Treatment and Management
Airway Management
Treatment of CH in the ED must always start with an immediate airway evaluation, with the possible need for endotracheal intubation preceding all diagnostic or other management considerations. Intubation is a decision most successfully based on physician clinical assessment, including oxygen (O2) selection rather than waiting for the results of objective measures such as arterial blood gas analysis.
Oxygen
Supplemental O2 should be administered to maintain an O2 saturation above 95%, but obviously is unnecessary in the absence of hypoxia.
Noninvasive Ventilation
Two kinds of noninvasive ventilation (NIV) are available, continuous positive airway pressure and bilevel positive airway pressure ventilation. The physiological differences between these types of NIV have little bearing on ED treatment.
Noninvasive ventilation has not been clearly shown to provide long-term mortality benefit. Large registry data44 report that outcomes are no worse than the alternative of endotracheal intubation, while multiple systematic reviews,45,46meta-analysis,47and Cochrane reviews48,49have established NIV as an acute pulmonary edema intervention that provides reductions in hospital mortality (numbers needed to treat [NNT] 13) and intubation (NNT 8), the prospective randomized C3PO (Congenital Cardiac Catheterization Project on Outcomes) trial50 failed to demonstrate any mortality reduction.
In patients with severe respiratory distress, NIV is a reasonable strategy during the aggressive administration of medical therapy in an attempt to avoid endotracheal intubation. However, NIV is not a stand-alone therapy and though its use may obviate the need for immediate intubation, its implementation should not be considered definitive management.
Correction of Abnormal Vital Signs: Abnormal SBP
Vital signs are an important determinant of therapy, driving treatment strategies. Interventions for HF are based on the patient’s SBP, in particular correction of symptomatic hypotension and hypertensive HF (Table 3).51
Symptomatic Hypotension. The presence of symptomatic hypotension is an extremely poor prognostic finding in AHF. Inotrope therapy may be considered, but it does not reduce mortality except as a bridge to mechanical interventions (LV assist device or transplant).52-54 Temporary inotropic support is recommended for cardiogenic shock to maintain systemic perfusion and prevent end organ damage.3 The inotropic support includes administration of dopamine, dobutamine, or milrinone, though none have been proven to be superior over the other. The lowest possible dose of the selected inotrope should be used to limit arrhythmogenic effects. Inotropic agents should not be used in the absence of severe systolic dysfunction, or low BP, or impaired perfusion, or evidence of significantly decreased cardiac output.
Hypertensive Heart Failure. Defined as the rapid onset of pulmonary congestion with an SBP greater than 140 mm Hg, and commonly greater than 160 mm Hg, these patients may have profound dyspnea, requiring endotracheal intubation. However, in this situation, aggressive vasodilation is typically rapidly effective. Overall, patients presenting with an elevated SBP have lower rates of in-hospital mortality, 30-day myocardial infarction (MI), death, or rehospitalization, and a greater likelihood of discharge within 24 hours—as long as the elevated SBP is aggressively and rapidly treated.
Pharmacological Therapy
Pharmacological management is the mainstay for treating HF. No other acute therapy (eg, NIV) has demonstrated a morality benefit (See Table 4 for specific dose and administration strategies).55 The time to initiate pharmacological therapy and whether an aggressive approach is indicated must be based on the severity of the clinical symptoms and objective risk stratification measures (eg, NP, troponin levels).
Furosemide. Except for hypertensive HF—in which case BP lowering is the most important goal—diuretics are a mainstay of AHF treatment, and consensus guidelines provide a class I recommendation for their use.3 The DOSE (Diuretic Strategies in Patients with ADHF) trial56 prospectively evaluated diuretics in 308 hospitalized AHF patients and found no outcome differences in administration route (bolus or continuous infusion) or dose (high vs low dose). This study reported trends toward greater improvement with higher furosemide dosing, as well as greater diuresis, but at a cost of transient worsening of renal function.
In general, diuretics should be administered in an intravenous (IV) dose equal to 1 to 2.5 times the patient’s usual daily oral dose. For patients who are diuretic-naïve, a dose of 40 mg IV furosemide or 1 mg IV bumetanide, with subsequent dosing titrated to urine output, is recommended.
Vasodilators. In patients with both AHF and even mildly elevated BP, vasodilators can be extremely effective in achieving symptom improvement. The choice of vasodilator, and how aggressive to increase dosing, depends upon symptom severity. The purpose of vasodilators is to lower BP and therefore, should not be used in the setting of hypotension or signs of hypoperfusion. Flow-limiting, preload-dependent CV states (eg, right ventricular infarction) increase the risk of hypotension, and are relative contraindications to the use of vasodilators. For patients who are severely dyspneic and with critical presentations, the emergency physician (EP) should preclude a detailed history and examination to initiate immediate therapy with short-acting agents that can be terminated rapidly in the case of an adverse event (eg, unexpected hypotension) are preferred.
Nitroglycerin. Nitroglycerin is the vasodilation agent of choice for hypertensive AHF. It is a short-acting, rapid-onset, venous and arterial dilator that decreases BP by preload reduction, and by afterload reduction in higher doses. Nitroglycerin has coronary vasodilatory effects associated with decreased ischemia, but should be avoided in patients taking phosphodiesterase inhibitors.55 Its most common side effect is headache, and hypotension occurs in about 3.5% of patients.57
Commonly given as a continuous infusion at IV doses up to 400 mcg/min, nitroglycerin may be associated with higher costs and longer LOS.58 Some authors suggest that bolus nitroglycerin therapy may be superior: In a retrospective study of 395 patients, an IV bolus of nitroglycerin 0.5 mg was superior to both an infusion, or a combination of bolus and infusion, as demonstrated by lower rates of ICU admission (48% vs 67% and 79%, respectively, P = 0.006) and shorter hospital stays (4.4 vs 6.3 and 7.3 days, respectively, P = 0.01). In all cohorts, adverse event rates were similar for hypotension, troponin elevation, and creatinine increase over 48 hours.59 Nitroprusside. Nitroprusside is a potent arterial and venous dilator that causes rapid decrease in BP and LV-filling pressures. It is usually considered more effective than nitroglycerin, despite a small study showing similar hemodynamic responses.60
Initial dosing of nitroprusside starts at 0.3 µg/kg/min IV, and is increased every 5 minutes to a maximum of 10 mcg/kg/min, based on BP and clinical response. The most common acute complication of nitroprusside infusions is hypotension. Cyanide toxicity may occur with prolonged use, high doses, or in patients with renal failure.55
Nesiritide. Exogenously administered, the B-type NP nesiritide is effective in lowering BP and improving dyspnea in AHF,55 although large prospective studies showed it had little long-term advantage over standard care.61 In a small, randomized, controlled trial, nesiritide reduced 30-day revisit LOS when given in an OU.62 The 22-minute half-life of nesiritide is longer than that of the nitrates, and its side effect is predominately hypotension, which occurs at rates similar to those of other vasodilators.55
Angiotensin Converting Enzyme Inhibitors. Because angiotensin converting enzyme inhibitors (ACEIs) have chronic mortality reduction benefits, their use in the acute setting is theoretically attractive, however, this has been poorly proven in AHF ED patients. In a retrospective review of 103 patients with elevated NTproBNP levels receiving bolus IV enalaprilat within 3 hours of presentation, the mean SBP decreased by 30 mm Hg, with only 2% of patients developing hypotension.63 However, with the longer half-life of ACEIs, if hypotension occurs, the potential for a prolonged BP-lowering effect exists.
Calcium Channel Blockers. Clevidipine and nicardipine are rapidly acting IV calcium channel blockers that lower BP by selective arteriolar vasodilation and increased cardiac output as vascular resistance declines.55 Because these agents have no negative inotropic or chronotropic effects, they may be beneficial in hypertensive AHF. In an open-label trial of 104 hypertensive AHF patients, clevidipine was more effective than standard care for the rapid control of BP and relief of dyspnea.64
Morphine. Large registry analyses have demonstrated potential harm with the routine use of morphine,65 as do recent propensity score matched analyses.66 Until there are studies demonstrating benefit, the use of morphine at present should be reserved for palliative care.
Time to Treatment
Although a randomized controlled trial on the importance of time to treatment of AHF is unlikely to ever be completed, data suggest that, as in the case of MI, delayed AHF therapy is associated with adverse outcomes. In a study of 499 suspected AHF patients transferred by ambulance, patients randomized to immediate therapy vs those whose therapy was not initiated until hospital arrival (mean delay of 36 minutes), had a 251% increase in survival (P < 0.01).67
Furthermore, the delayed administration of vasoactive agents, defined as medication administered to alter hemodynamics (eg, dobutamine, dopamine, nitroglycerin, nesiritide) is also associated with harm,68 and registry studies demonstrate increased death rates (n = 35,700).69 Finally, another registry (n = 14,900) study demonstrated early IV furosemide is associated with decreased mortality.70 This latter finding was also validated in a prospective observational cohort study (mortality 2.3 vs 6.0 in early vs delayed therapy groups, respectively).71
Patient Disposition
One of the unique features of emergency medicine is the need to determine, with very limited information and time, a patient’s very short-term clinical trajectory. Few physicians are required to have greater accuracy with less information or time than do EPs. Several studies report objective data points and risk scores to assist in this task, but none has been universally adopted, reflecting the challenge of applying population data to individuals.
Short-term Prognosis
In 1,638 patients evaluated for 14-day outcomes, an HR lower than 50% maximal HR (MHR), and an SBP greater than 140 mm Hg were associated with the lowest rate of serious adverse events (SAEs) (6%) and hospitalization (38%).72 An MHR over 75% was associated with the highest SAE rate, although SAEs decreased as SBP increased (30%, 24%, and 21% with SBPs < 120 mm Hg, 120-140 mm Hg, and > 140 mm Hg, respectively).72
Risk Scores
In a prospective, observational cohort study of 1,100 ED patients, the Ottawa Heart Failure Risk Scale, combined with NTproBNP values, had a sensitivity of 95.8%—at the cost of increasing the admission rate (from 60.8% to 88%)—for serious adverse events (defined as death within 30 days), admission to a monitored unit, intubation, NIV, MI, or relapse resulting in hospital admission within 14 days.73
Observation Unit
Overall, 44% of in-patient HF admissions are for less than 3 days (Table 1),2 supporting the practice of managing selected patients in shorter clinical-care environments than in inpatient units. Further, ED patients presenting with moderate dyspnea require both a diagnosis and an evaluation of their therapeutic response to determine the need for hospitalization. However, evaluating therapeutic response requires more time than is available in the typical ED. Thus, an ED OU offers the following:
(1) The OU provides the EP with a longer evaluation time, and therefore a more accurate disposition may be effected;
(2) Costs are significantly lower in patients managed in an ED OU; and
(3) Patient satisfaction may be improved, as most patients prefer home management over hospitalization.
All three of these opportunities are supported by a number of studies,74-78 with validated entry and exclusion criteria, treatment algorithms and discharge metrics. Most recently, in a registry of hospitals in Spain registry, patients presenting to hospitals that had OUs had a 2.2-day shorter LOS, lower 30-day ED revisit rate, and similar mortality rates compared to those in institutions without OUs—although these beneficial effects occurred at the cost of an 8.9% higher admission rate.79
Patient Education
Intuitively, it would be expected that patient education would reduce return visits, 30-day hospitalizations, and AHF-related mortality. Unfortunately, it has not been demonstrated that patient education results in a consistent benefit at hospital discharge, or in the outpatient environment.80-85
Although AHF education in the ED has been poorly studied, areas that have shown promise are education occurring before ED management (ie, in the ED waiting area) in underinsured patients,86 and during ED care for patients with poor health care literacy.87 As educational interventions are both inexpensive and unlikely to result in harm, their implementation should be considered.
Conclusion
The spectrum of HF is a common presentation in the ED. Because HF generally appears as dyspnea, in a cohort with multiple comorbidities, the diagnosis can be challenging. This is complicated by the fact that patients with severe presentations may require life-saving interventions long before a clinical evaluation is completed (or even initiated). The skill of the EP, and his or her ability to improve the clinical condition before intubation is required, will determine the patient’s trajectory. Conversely, as a chronic condition, HF may present with moderate symptoms for which a short diuretic “tune-up” in an observation environment may be appropriate.
How these decisions are made will depend upon the local environment, the availability of outpatient resources, and individual patient choices. There are few chronic diseases that are more complex, are seen more often in the ED, or that require more skill and finesse in management.
1. Benjamin EJ, Blaha MJ, Chiuve SE, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation. 2017;135(10):e146-e603.
2. Fitch KV, Engel T, Lauet J. The cost burden of worsening heart failure in the Medicare fee for service population: an actuarial analysis. Milliman Web site. 2017. http://www.milliman.com/insight/2017/The-cost-burden-of-worsening-heart-failure-in-the-Medicare-fee-for-service-population-An-actuarial-analysis/ Published April 3, 2017. Accessed June 1, 2017.
3. Yancy CW, Jessup M, Bozkurt B, et al; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013. J Am Coll Cardiol. 2013;62(16):e147-e239. doi:10.1016/j.jacc.2013.05.019.
4. Chen J, Normand SL, Wang Y, Krumholz HM. National and regional trends in heart failure hospitalization and mortality rates for Medicare beneficiaries, 1998-2008. JAMA. 2011;306(15):1669-1678. doi:10.1001/jama.2011.1474.
5. Heidenreich PA, Albert NM, Allen LA, et al; American Heart Association Advocacy Coordinating Committee; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Stroke Council. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6(3):606-619. doi:10.1161/HHF.0b013e318291329a.
6. Dunlay SM, Shah ND, Shi Q, et al. Lifetime costs of medical care after heart failure diagnosis. Circ Cardiovasc Qual Outcomes. 2011;4(1):68-75. doi:10.1161/CIRCOUTCOMES.110.957225.
7. HCUP National Inpatient Sample (NIS); 2014 Agency for Healthcare Research and Quality (AHRQ). HCUPnet Healthcare Cost and Utilization Project. http://bit.ly/2u2ymFA. Published 2014. Accessed July 27, 2017.
8. FY 2017 Final Rule and Correction Notice Tables. CMS 2014 data based on DRGs, Table 5:List of MS-DRGs, Relative Weighting Factors and Geometric and Arithmetic Mean Length of Stay. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/FY2017-IPPS-Final-Rule-Home-Page-Items/FY2017-IPPS-Final-Rule-Tables.html. Published 2017. Accessed August 28, 2017.
9. Hospital Adjusted Expenses per Inpatient Day by Ownership. Kaiser Family Foundation. http://www.kff.org/health-costs/state-indicator/expenses-per-inpatient-day-by-ownership/?currentTimeframe=0&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22asc%22%7D. Accessed August 2, 2017
10. Ellison, A. Average cost per inpatient day across 50 states. Becker’s Hospital CFO Report. http://www.beckershospitalreview.com/finance/average-cost-per-inpatient-day-across-50-states-2016.html. Published January 13, 2016. Accessed August 2, 2017
11. Claret PG, Calder LA, Stiell IG, et al. Rates and predictive factors of return to the emergency department following an initial release by the emergency department for acute heart failure. [published online ahead of print April 3, 2017]. CJEM. 1-8. doi:10.1017/cem.2017.14.
12. Yam FK, Lew T, Eraly SA, Lin HW, Hirsch JD, Devor M. Changes in medication regimen complexity and the risk for 90-day hospital readmission and/or emergency department visits in U.S. Veterans with heart failure. Res Social Adm Pharm. 2016;12(5):713-721. doi:10.1016/j.sapharm.2015.10.004.
13. Readmissions Reduction Program. CMS. https://www.cms.gov/medicare/medicare-fee-for-service-payment/acuteinpatientpps/readmissions-reduction-program.html Updated April 18, 2016. Accessed July 13, 2017.
14. Nowak RM, Reed BP, DiSomma S, et al. Presenting phenotypes of acute heart failure patients in the ED: Identification and implications. Am J Emerg Med. 2017;35(4):536-542. doi:10.1016/j.ajem.2016.12.003.
15. Bennett SJ, Huster GA, Baker SL, et al. Characterization of the precipitants of hospitalization for heart failure decompensation. Am J Crit Care. 1998;7(3):168-174.
16. Kuo DC, Peacock WF. Diagnosing and managing acute heart failure in the emergency department. Clin Exp Emerg Med. 2015;2(3):141-149. eCollection 2015 Sep. doi:10.15441/ceem.15.007.
17. Wang CS, FitzGerald JM, Schulzer M, Mak E, Ayas NT. Does this dyspneic patient in the emergency department have congestive heart failure? JAMA. 2005;294:1944-1956.
18. Koga T, Fujimoto K. Images in clinical medicine. Kerley’s A, B, and C line. N Engl J Med. 2009;360(15):1539. doi:10.1056/NEJMicm0708489.
19. Glöckner E, Christ M, Geier F, et al. Accuracy of point-of-care B-Line lung ultrasound in comparison to NT-ProBNP for screening acute heart failure. Ultrasound Int Open. 2016;2(3):e90-e92. doi:10.1055/s-0042-108343.
20. Bitar Z, Maadarani O, Almerri K. Sonographic chest B-lines anticipate elevated B-type natriuretic peptide level, irrespective of ejection fraction. Ann Intensive Care. 2015;5(1): 56. doi:10.1186/s13613-015-0100-x.
21. Miglioranza MH, Gargani L, Sant’Anna RT, et al. Lung ultrasound for the evaluation of pulmonary congestion in outpatients: a comparison with clinical assessment, natriuretic peptides, and echocardiography. JACC Cardiovasc Imaging. 2013;6(11):1141-1151. doi:10.1016/j.jcmg.2013.08.004.
22. Anderson KL, Jenq KY, Fields JM, Panebianco NL, Dean AJ. Diagnosing heart failure among acutely dyspneic patients with cardiac, inferior vena cava, and lung ultrasonography. Am J Emerg Med. 2013;31(8):1208-1214. doi:10.1016/j.ajem.2013.05.007.
23. Cubo-Romano P, Torres-Macho J, Soni NJ, et al. Admission inferior vena cava measurements are associated with mortality after hospitalization for acute decompensated heart failure. J Hosp Med. 2016;11(11):778-784. doi:10.1002/jhm.2620.
24. Martínez PG, Martínez DM, García JC, Loidi JC. Amino-terminal pro–B-type natriuretic peptide, inferior vena cava ultrasound, and bioelectrical impedance analysis for the diagnosis of acute decompensated CHF. Am J Emerg Med. 2016;34(9): 1817–1822. doi:10.1016/j.ajem.2016.06.043.

25. Maisel AS, Krishnaswamy P, Nowak RM, et al; Breathing Not Properly Multinational Study Investigators. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002;347(3):161-167.
26. Van Kimmenade RR, Pinto YM, Bayes-Genis A, Lainchbury JG, Richards AM, Januzzi JL Jr. Usefulness of intermediate amino-terminal pro-brain natriuretic peptide concentrations for diagnosis and prognosis of acute heart failure. Am J Cardiol. 2006;98(3):386-390.
27. Moe GW, Howlett J, Januzzi JL, Zowall H; Canadian Multicenter Improved Management of Patients With Congestive Heart Failure (IMPROVE-CHF) Study Investigators. N-terminal pro-B-type natriuretic peptide testing improves the management of patients with suspected acute heart failure: primary results of the Canadian prospective randomized multicenter IMPROVE-CHF study. Circulation. 2007;115(24):3103-3110.
28. Mayo DD, Colletti JE, Kuo DC. Brain natriuretic peptide (BNP) testing in the emergency department. J Emerg Med. 2006;31(2):201-210.
29. Mueller C, Scholer A, Laule-Kilian K, et al. Use of B-type natriuretic peptide in the evaluation and management of acute dyspnea. N Engl J Med. 2004;350(7):647-654.
30. Krauser DG, Lloyd-Jones DM, Chae CU, et al. Effect of body mass index on natriuretic peptide levels in patients with acute congestive heart failure: a ProBNP Investigation of Dyspnea in the Emergency Department (PRIDE) substudy. Am Heart J. 2005;149(4):744-750.
31. McCullough PA, Duc P, Omland T, et al. B-type natriuretic peptide and renal function in the diagnosis of heart failure: an analysis from the Breathing Not Properly Multinational Study. Am J Kidney Dis. 2003;41(3):571-579.
32. Jacob J, Roset A, Miró Ò, et al; ICA-SEMES Research Group. EAHFE - TROPICA2 study. Prognostic value of troponin in patients with acute heart failure treated in Spanish hospital emergency departments. Biomarkers. 2017;22(3-4):337-344. doi:10.1080/1354750X.2016.1265006.
33. Fonarow GC, Adams KF Jr, Abraham WT, et al; ADHERE Scientific Advisory Committee, Study Group, and Investigators. Risk stratification for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. JAMA. 2005;293(5):572-580.
34. Burkhardt J, Peacock WF, Emerman CL. Predictors of emergency department observation unit outcomes. Acad Emerg Med. 2005;12(9):869-874.
35. Schanz M, Shi J, Wasser C, Alscher MD, Kimmel M. Urinary [TIMP-2] × [IGFBP7] for risk prediction of acute kidney injury in decompensated heart failure. Clin Cardiol. 2017;40(7):485-491. doi:10.1002/clc.22683.
36. Kushner RF, Schoeller DA, Fjeld CR, Danford L: Is the impedance index (ht2/R) significant in predicting total body water? Am J Clin Nutr. 1992;56(5): 835-839.
37. Ackland GL, Singh-Ranger D, Fox S, et al. Assessment of preoperative fluid depletion using bioimpedance analysis. Br J Anaesth. 2004;92(1): 134-136.
38. Uszko-Lencer NH, Bothmer F, van Pol PE, Schols AM. Measuring body composition in chronic heart failure: a comparison of methods. Eur J Heart Fail. 2006;8(2): 208-214.
39. Santarelli S, Russo V, Lalle I, et al; GREAT network. Usefulness of combining admission brain natriuretic peptide (BNP) plus hospital discharge bioelectrical impedance vector analysis (BIVA) in predicting 90 days cardiovascular mortality in patients with acute heart failure. Intern Emerg Med. 2017;12(4):445-451. doi:10.1007/s11739-016-1581-9.
40. Parrinello G, Paterna S, Di Pasquale P, et al. The usefulness of bioelectrical impedance analysis in differentiating dyspnea due to decompensated heart failure. J Card Fail. 2008;14(8): 676-686. doi:10.1016/j.cardfail.2008.04.005.
41. Valle R, Aspromonte N, Carbonieri E, et al. Fall in readmission rate for heart failure after implementation of B-type natriuretic peptide testing for discharge decision: a retrospective study. Int J Cardiol. 2008;126(3): 400-406.
42. Sartini S, Frizzi J, Borselli M, et al. Which method is best for an early accurate diagnosis of acute heart failure? Comparison between lung ultrasound, chest X-ray and NT pro-BNP performance: a prospective study. [published online ahead of print July 11, 2016]. Intern Emerg Med. doi:10.1007/s11739-016-1498-3.
43. Steinhart BD, Levy P, Vandenberghe H, et al. A randomized control trial using a validated prediction model for diagnosing acute heart failure in undifferentiated dyspneic emergency department patients-results of the GASP4Ar study. J Card Fail. 2017;23(2):145-152. doi:10.1016/j.cardfail.2016.08.007.
44. Tallman TA, Peacock WF, Emerman CL, et al; ADHERE Registry. Noninvasive ventilation outcomes in 2,430 acute decompensated heart failure patients: an ADHERE registry analysis. Acad EM. 2008;15(4):355–362. doi:10.1111/j.1553-2712.2008.00059.x.
45. Pang D, Keenan SP, Cook DJ, Sibbald WJ. The effect of positive pressure airway support on mortality and the need for intubation in cardiogenic pulmonary edema: a systematic review. Chest. 1998;114(4):1185-1192.
46. Peter JV, Moran JL, Phillips-Hughes J, Graham P, Bersten AD. Effect of non-invasive positive pressure ventilation (NIPPV) on mortality in patients with acute cardiogenic pulmonary oedema: a meta-analysis. Lancet. 2006;367(9517):1155-1163.
47. Weng CL, Zhao YT, Liu QH, et al. Meta-analysis: Noninvasive ventilation in acute cardiogenic pulmonary edema. Ann Intern Med. 2010;152(9):590-600
48. Vital FM, Saconato H, Ladeira MT, et al. Noninvasive positive pressure ventilation (CPAP or bilevel NPPV) for cardiogenic pulmonary edema. Cochrane Database Syst Rev. 2008;(3):CD005351. doi:10.1002/14651858.CD005351.pub2.
49. Vital FM, Ladeira MT, Atallah AN. Non-invasive positive pressure ventilation (CPAP or bilevel NPPV) for cardiogenic pulmonary oedema. Cochrane Database Syst Rev. 2013;(5):CD005351. doi:10.1002/14651858.CD005351.pub3.
50. Gray A, Goodacre S, Seah M, Tilley S. Diuretic, opiate and nitrate use in severe acidotic acute cardiogenic pulmonary oedema: analysis from the 3CPO trial. QJM. 2010;103(8):573-581. doi:10.1093/qjmed/hcq077.
51. Collins SP, Storrow AB, Levy PD, et al. Early management of patients with acute heart failure: state of the art and future directions—a consensus document from the SAEM/HFSA acute heart failure working group. Acad Emerg Med. 2015;22(1):94-112. doi:10.1111/acem.12538.]
52. O’Connor CM, Gattis WA, Uretsky BF, et al. Continuous intravenous dobutamine is associated with an increased risk of death in patients with advanced heart failure: insights from the Flolan International Randomized Survival Trial (FIRST). Am Heart J. 1999;138(1 Pt 1):78-86.
53. Hershberger RE, Nauman D, Walker TL, Dutton D, Burgess D. Care processes and clinical outcomes of continuous outpatient support with inotropes (COSI) in patients with refractory endstage heart failure. J Card Fail. 2003;(9):180-187.
54. Gorodeski EZ, Chu EC, Reese JR, Shishehbor MH, Hsich E, Starling RC. Prognosis on chronic dobutamine or milrinone infusions for stage D heart failure. Circ Heart Fail. 2009;2(4):320-324. doi:10.1161/CIRCHEARTFAILURE.108.839076.
55. Collins SP, Levy PD, Martindale JL, et al. Clinical and research considerations for patients with hypertensive acute heart failure: A consensus statement from the Society for Academic Emergency Medicine and the Heart Failure Society of America Acute Heart Failure Working Group. Acad Emerg Med. 2016;23(8):922-931. doi:10.1111/acem.13025.
56. Felker GM, Lee KL, Bull DA, et al. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med. 2011; 364(9):797-805. doi:10.1056/NEJMoa1005419.

57. Publication Committee for the VMAC Investigators (Vasodilatation in the Management of Acute CHF). Intravenous nesiritide vs nitroglycerin for treatment of decompensated congestive heart failure: a randomized controlled trial. JAMA. 2002;287(12):1531-1540.
58. Gradman AH, Vekeman F, Eldar-Lissai A, Trahey A, Ong SH, Duh MS. Is addition of vasodilators to loop diuretics of value in the care of hospitalized acute heart failure patients? Real-world evidence from a retrospective analysis of a large United States hospital database. J Card Fail. 2014;20(11):853-863. doi:10.1016/j.cardfail.2014.08.006.
59. Wilson SS, Kwiatkowski GM, Millis SR, Purakal JD, Mahajan AP, Levy PD. Use of nitroglycerin by bolus prevents intensive care unit admission in patients with acute hypertensive heart failure. Am J Emerg Med. 2017;35(1):126-131. doi:10.1016/j.ajem.2016.10.038.
60. Eryonucu B, Guler N, Guntekin U, Tuncer M. Comparison of the effects of nitroglycerin and nitroprusside on transmitral Doppler flow parameters in patients with hypertensive urgency. Ann Pharmacother. 2005;39(6):997–1001.
61. O’Connor CM, Starling RC, Hernandez AF, et al. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med. 2011;365(1):32-43. doi:10.1056/NEJMoa1100171.
62. Peacock WF 4th, Holland R, Gyarmathy R, et al. Observation unit treatment of heart failure with nesiritide: results from the proaction trial. J Emerg Med. 2005;29(3):243-252.
63. Ayaz SI, Sharkey CM, Kwiatkowski GM, et al. Intravenous enalaprilat for treatment of acute hypertensive heart failure in the emergency department. Int J Emerg Med. 2016;9(1):28. doi:10.1186/s12245-016-0125-4.
64. Peacock WF 4th, Chandra A, Char D, et al. Clevidipine in acute heart failure: results of the A study of BP control in acute heart failure-a pilot study (PRONTO). Am Heart J. 2014;167(4):529-536. doi:10.1016/j.ahj.2013.12.023.
65. Peacock WF 4th, Hollander JE, Diercks DB, et al. Morphine and outcomes in acute decompensated heart failure: an ADHERE analysis. Emerg Med J. 2008;25(4):205-209. doi:10.1136/emj.2007.050419.
66. Miró Ò, Gil V, Martín-Sánchez FJ, Herrero-Puente P, Jet al; ICA-SEMES Research Group. Morphine use in the ED and outcomes of patients with acute heart failure: a propensity score-matching analysis based on the EAHFE registry. [published ahead of print April 12, 2017] Chest. pii:S0012-3692(17)30707-9. doi:10.1016/j.chest.2017.03.037.
67. Wuerz RC, Meador SA. Effects of prehospital medications on mortality and length of stay in congestive heart failure. Ann Emerg Med. 1992;21(6):669-674.
68. Peacock WF 4th, Fonarow GC, Emerman CL, Mills RM, Wynne J; ADHERE Scientific Advisory Committee and Investigators; Adhere Study Group. Impact of early initiation of intravenous therapy for acute decompensated heart failure on outcomes in ADHERE. Cardiology. 2007; 107(1):44-51. doi:10.1159/000093612.
69. Peacock WF, Emerman C, Costanzo MR, Diercks DB, Lopatin M, Fonarow GC. Early vasoactive drugs improve heart failure outcomes. Congest Heart Fail. 2009;15(6): 256-264. doi:10.1111/j.1751-7133.2009.00112.x.
70. Maisel AS, Peacock WF, McMullin N, et al. Timing of immunoreactive B-type natriuretic peptide levels and treatment delay in acute decompensated heart failure: an ADHERE (Acute Decompensated Heart Failure National Registry) analysis. J Am Coll Cardiol. 2008;52(7):534-540. doi:10.1016/j.jacc.2008.05.010.
71. Matsue Y, Damman K, Voors AA, et al. Time-to-Furosemide Treatment and Mortality in Patients Hospitalized With Acute Heart Failure. J Am Coll Cardiol. 2017 Jun 27;69(25):3042-3051. doi:10.1016/j.jacc.2017.04.042.
72. Claret PG, Stiell IG, Yan JW, et al. Hemodynamic, management, and outcomes of patients admitted to emergency department with heart failure. Scand J Trauma Resusc Emerg Med. 2016;24(1):132.
73. Stiell IG, Perry JJ, Clement CM, et al. Prospective and explicit clinical validation of the Ottawa Heart Failure Risk Scale, with and without use of quantitative NT-proBNP. Acad Emerg Med. 2017;24(3):316-327. doi:10.1111/acem.13141.
74. Pang PS, Jesse R, Collins SP, Maisel A. Patients with acute heart failure in the emergency department: do they all need to be admitted? J Card Fail. 2012;18:900-903. doi:10.1016/j.cardfail.2012.10.014.
75. Peacock WF 4th, Young J, Collins S, Emerman C, Diercks D. Heart failure observation units: optimizing care. Ann Emerg Med. 2006;47(1):22-33.
76. Storrow AB, Collins SP, Lyons MS, Wagoner LE, Gibler WB, Lindsell CJ. Emergency department observation of heart failure: preliminary analysis of safety and cost. Congest Heart Fail. 2005;11(2):68-72.
77. Peacock WF 4th, Remer EE, Aponte J, Moffa DA, Emerman CE, Albert NM. Effective observation unit treatment of decompensated heart failure. Congest Heart Fail. 2002;8(2):68 -73.
78. Peacock WF 4th, Albert NM. Observation unit management of heart failure. Emerg Med Clin North Am. 2001;19(1):209-232.
79. Miró O, Carbajosa V, Peacock WF 4th, et al; ICA-SEMES group. The effect of a short-stay unit on hospital admission and length of stay in acute heart failure: REDUCE-AHF study. Eur J Intern Med. 2017;40:30-36. doi:10.1016/j.ejim.2017.01.015.
80. Ekman I, Andersson B, Ehnfors M, Matejka G, Persson B, Fagerberg B. Feasibility of a nurse-monitored, outpatient-care programme for elderly patients with moderate-to-severe, chronic heart failure. Eur Heart J. 1998;19(8):1254-1260.
81. Riegel B, Carlson B, Kopp Z, LePetri B, Glaser D, Unger A. Effect of a standardized nurse case-management telephone intervention on resource use in patients with chronic heart failure. Arch Intern Med. 2002;162(6):705-712.
82. Laramee AS, Levinsky SK, Sargent J, Ross R, Callas P. Case management in a heterogeneous congestive heart failure population: a randomized controlled trial. Arch Intern Med. 2003;163(7):809-817.
83. Stewart S, Pearson S, Horowitz JD. Effects of a home-based intervention among patients with congestive heart failure discharged from acute hospital care. Arch Intern Med. 1998;158(10):1067-1072.
84. Stewart S, Marley JE, Horowitz JD. Effects of a multidisciplinary, home-based intervention on unplanned readmissions and survival among patients with chronic congestive heart failure: a randomised controlled study. Lancet. 1999;354(9184):1077-1083.
85. Weinberger M, Oddone EZ, Henderson WG; Veterans Affairs Cooperative Study Group. Does increased access to primary care reduce hospital readmissions? Veterans Affairs Cooperative Study Group on primary care and hospital readmission. N Engl J Med. 1996;334(22):1441-1447.
86. Asthana V, Sundararajan M, Karun V, et al. Educational strategy for management of heart failure markedly reduces 90-day emergency department and hospital readmissions in un- and underinsured patients. J Am Coll Cardiol. 2017;69(11Suppl): 780. doi:10.1016/S0735-1097(17)34169-4.
87. Bell SP, Schnipper JL, Goggins K, et al; Pharmacist Intervention for Low Literacy in Cardiovascular Disease (PILL-CVD) Study Group. Effect of pharmacist counseling intervention on health care utilization following hospital discharge: a randomized control trial. J Gen Intern Med. 2016;31(5):470-477. doi:10.1007/s11606-016-3596-3.
Patients with heart failure (HF) present daily to busy EDs. An estimated 6.5 million Americans are living with this diagnosis, and the number is predicted to grow to 8 million by 2023.1 Most HF patients (82.1%) who present to EDs are hospitalized, while a selected minority are either managed in the ED and discharged (11.6%) or managed in observation units (OU) (6.3%).2 The prognosis after HF is initially diagnosed is poor, with a 5-year mortality of 50%,3 and after a single HF hospitalization, 29% will die within 1 year.4
One-third of the total Medicare budget is spent on HF, despite the fact that HF represents only 10.5% of the Medicare population.2 Up to 80% of HF costs are for hospitalizations, which cost an average of $11,840 per inpatient admission.5,6 The high costs are due to an average length of stay (LOS) of 5.2 days7 (Table 1).
Adding to hospital costs is the degree of “reactivism,” with approximately 20% of patients discharged from the ED returning within 2 weeks, of whom nearly 50% will be hospitalized.11 Following HF hospitalization and discharge, the 30-day readmission rate is 26.2%,2 increasing to 36% by 90 days.12 The Centers for Medicare & Medicaid Services (CMS) has incentivized hospitals and providers to reduce admissions, but penalize hospitals that do not. Overall, CMS will reduce payments by up to 3% to hospitals with excess readmissions for select conditions, including HF.13
Causes of Heart Failure
Heart failure represents a final common pathway, which in the United States is most often due to coronary artery disease (CAD). Many types of pathology ultimately result in left ventricular (LV) dysfunction, and much of its rising prevalence is a result of the success we now have in managing historically fatal cardiovascular (CV) conditions. These include hypertension, diabetes mellitus (DM), CAD, and valvular and other CV structural conditions.
Heart failure is caused by either a dilated ventricle with a reduced ejection fraction (HFrEF) and inability to eject volume, or a stiffened ventricle with a preserved EF (HFpEF) that is unable to receive increased venous return. Both conditions acutely decompensate pulmonary congestion. A preserved EF is defined as an EF at or greater than 50%, whereas a reduced EF is at or less than 40%, with the 41% to 49% range considered as borderline preserved EF.3
While there are important differences in the treatment of chronic and subacute HF, driven by the EF, the effect of EF on early decision-making and treatment in the ED is negligible: Although the probability of HFpEF increases with increasing initial ED systolic blood pressure (SBP), clinical presentation and treatment in the ED are initially identical—regardless of the EF.
Noninvasive continuous transcutaneous hemodynamic monitoring is available for ED use, and may provide further insight into the underlying pathophysiology. A study of 127 acute heart failure (AHF) ED patients identified three hemodynamic AHF phenotypes. These include normal cardiac index (CI) and systemic vascular resistance index (SVRI), low CI and SVRI, and low CI and elevated SVRI.14 While it is attractive to suggest therapeutic interventions based on these measurements, outcome data are lacking.
Presentation
The most common ED presentation of patients suffering from AHF is dyspnea secondary to volume overload, or as the result of acute hypertension with relatively less volume overload. However, regardless of the cause of dyspnea, it is not only the most common resulting complaint, but one that requires immediate treatment. Ultimately, 59% of all HF admissions are attributed to volume overload and dyspnea (Figure 1).15
Heart failure can also present in a more protean manner, with cough, fatigue, and edema, as well as more subtle symptoms predominating and resulting in a complicated differential diagnosis (Table 2).16
Because HF is a disease that most significantly affects older patients who frequently have concomitant morbidities (eg, myocardial ischemia, chronic obstructive pulmonary disease [COPD] exacerbation, uncontrolled DM), other less clinically obvious disease presentations may actually be the cause of the AHF exacerbation.
Diagnosis
A focused history and physical examination that is part of all ED evaluations should be expedited whenever there is evidence of hemodynamic instability or respiratory compromise. An early working diagnosis is essential to avoid a delay in appropriate treatment, which is associated with increased mortality.
When HF is likely, the potential etiology and precipitants for decompensation must be considered. This list is long, but medication noncompliance and dietary indiscretion are the most common causes.
Symptoms and Prior History of HF
The classic symptoms for AHF include dyspnea at rest or exertion, and orthopnea, both of which unfortunately have poor sensitivity and specificity for AHF. As an isolated symptom, dyspnea is of marginal diagnostic utility (sensitivity and specificity for an HF diagnosis is 56% and 53%, respectively), and orthopnea is only slightly better (sensitivity and specificity 77% and 50%, respectively). A prior HF diagnosis makes repeat presentations much more likely (sensitivity and specificity 60% and 90%, respectively).17
Physical Examination
Simple observation and a directed examination can rapidly point to the diagnosis (Figure 2).
Electrocardiography
Because CAD is one of the most common underlying AHF etiologies, an electrocardiogram (ECG) should always be obtained early for a patient presenting with potential AHF. Although the ECG does not usually contribute to ED management, the identification of new ST-segment changes or a malignant arrhythmia will guide critical management decisions.
Imaging Studies
Chest X-ray Imaging. A chest X-ray (CXR) study must be considered early when a patient presents with signs and symptoms suggestive of AHF. Although the classic findings of HF (eg, Kerley B lines [short horizontal lines perpendicular to the pleural surface],18 interstitial congestion, pulmonary effusion) can lag behind the clinical presentation, and also be nondiagnostic in the setting of mild HF, the CXR is an effective aid in identifying other causes of dyspnea such as pneumonia. Ultimately, the utility of the CXR for diagnosis is similar to that of the history and physical examination in that it will be diagnostic when positive but cannot exclude AHF if normal.
Ultrasound. Because it is fast, inexpensive, noninvasive, and readily available in the ED, ultrasound is frequently used to evaluate potential HF patients. Several studies have demonstrated that the presence of B lines in two or more regions is specific for AHF (specificity 75%-100%), although the sensitivity may be limited (40%-91%).19-21 The presence of inferior vena cava (IVC) dilation is also associated with adverse outcomes.22 In 80 patients hospitalized with acute decompensated HF (ADHF), a dilated IVC (≥1.9 cm) at admission was associated with higher 90-day mortality (25.4% vs 3.4%, P = 0.009).23 These findings may be considered in groups: In an evaluation of the combination of LV EF, IVC collapsibility, and B lines for an HF diagnosis, the combination of all three had a poor sensitivity (36%) but an excellent specificity (100%), and any two of the three had a specificity of at least 93%.24
Laboratory Evaluation
Myocardial Strain: BNP/NTproBNP. Natriuretic peptides (NPs) are not AHF-specific, but rather they are synthesized and released by the myocardium in the setting of myocardial pressure or volume stress. They are manufactured as preproBNP, then enzymatically cleaved into the active BNP and the inactive fragment N-terminal proBNP (NTproBNP). The predominant hormonal effects of BNP are vasodilation and natriuresis, as well as antagonism of the hormones associated with sodium retention (aldosterone) and vasoconstriction (endothelin, norepinephrine).
As AHF results in myocardial stress, NP elevation provides diagnostic and prognostic information. Clinical judgment supported by a BNP greater than 100 pg/mL is a better predictor of AHF than clinical judgment alone (accuracy 81% vs 74%, respectively).25 While low levels (BNP <100 pg/mL or NTproBNP <300 pg/mL) reliably exclude the diagnosis of HF (sensitivities >95%), higher levels (BNP >500 pg/mL, NTproBNP >900 pg/mL) are useful as “rule-in” markers, with specificity greater than 95%. The NTproBNP also requires adjustment for patients older than age 75 years, with a higher level (>1,800 pg/mL) to rule-in HF. The NP grey zone (BNP 100-500 pg/mL, NTproBNP 300-900 pg/mL)requires additional testing for accurate diagnoses (Figure 3).25-29
There are several confounders to the interpretation of NP results: NPs are negatively confounded by the presence of obesity, resulting in a lowering of the value as compared to the clinical presentation.Thus, the measured BNP level should be doubled if the patient’s body mass index exceeds 35 kg/m2.30 Secondly, because NP metabolism is partially renal dependent, elevated levels may not reflect AHF in the presence of renal failure. If the estimated glomerular filtration rate is less than 60 mL/min, measured BNP levels should be halved.31
AHF vs Myocardial Ischemia: Troponin Levels. Large registry data using contemporary troponin assays clearly identify the association between elevated troponin levels (>99th percentile in a healthy population) and increased short-term risk. With the US Food and Drug Association (FDA) approval of a high-sensitivity troponin (hs-cTnT) assay, a greater frequency of elevated cardiac troponin T (cTnT) and cardiac troponin I (cTnl) will be identified in AHF patients in the ED.
In one retrospective study of 4,705 AHF patients in the ED, hs-cTnT were elevated in 48.4% of cases (25.3% in cTnI, 37.9% in cTnT, and 82.2% in hs-cTnT). Although 1-year mortality was higher in those with elevated troponin (adjusted heart rate [HR] 1.61; CI 95% 1.38-1.88), elevated troponin was not associated with 30-day revisits to the ED (1.01; 0.87-1.19) and high sensitive elevations less than double the reference value had no impact on outcomes.32 Thus, in terms of management of AHF in the ED, slightly elevated stable serial troponins are more consistent with underlying HF, and should be managed as such. This is not true of rising/falling troponin levels, which should still engender concern for underlying myocardial ischemia and a different management pathway.
Renal function. Comprised renal function is an important predictor of AHF outcome. Large registry data from hospitalized HF patients demonstrate that a presenting blood urea nitrogen level greater than 43 mg/dL is one of the most important predictors of increased acute mortality,33 and levels below 30 mg/dL identify a cohort likely to be successfully managed in an observation environment.34 Creatinine is a helpful lagging indicator of mortality, with higher levels (>2.75 mg/dL) associated with increased short-term adverse outcomes and decreased therapeutic responsiveness (Figure 4).
For patients presenting with ADHF, a newer test recently approved by the FDA uses the product of the urine markers tissue inhibitor of metalloproteinase-2 and insulin-like growth factor-binding protein 7, to generate a score predictive of acute kidney injury.35 While promising, no studies of ED outcomes are currently available.
Volume Assessment
Objective volume assessment is useful for diagnosis and prognosis in AHF. Bioimpedance vector analysis (BIVA) is a rapid, inexpensive, noninvasive technique that measures total body water by placing a pair of electrodes on the wrist and ipsilateral ankle. The BIVA measurements have strong correlations with the gold standard volume-assessment technique of deuterium dilution (r > 0.99).36 In HF, BIVA can assess volume depletion37 and overload,38 and identifies differences in hydration status between 90-day survivors and non-survivors (P < 0.01).39
Used in combination with BNP, one prospective study of 292 dyspneic patients found that, while BIVA was a strong predictor of AHF (c-statistic 0.93, P = 0.016), the most accurate volume status determination was the combination of both (c-statistic, 0.99; P = 0.005), for which the combined accuracy exceeded either alone.40 Finally, in 166 hospitalized HF patients discharged by BNP and BIVA parameters, vs 149 discharged based on clinical impressions, those assessed with BNP and BIVA had lower 6-month readmissions (23% vs 35%, P = 0.02) and overall cost of care.41
Combination Technologies
Obviously, EPs may consider multiple technologies to arrive at an accurate diagnosis. One prospective evaluation enrolled 236 patients to determine the diagnostic accuracy for AHF in the ED and reported lung ultrasound, CXR, and NTproBNP had a sensitivity of 57.7% and 88.0%, 74.5% and 86.3%, and a specificity of 97.6% and 28.0%. The best overall combination was the CXR with lung ultrasound (sensitivity 84.7%, specificity 77.7%).42
Another prospective study evaluated IVC diameter, bioelectrical impedance analysis (BIA), and NTproBNP in 96 elderly patients. ADHF patients had higher IVC diameters and lower collapsibility index, lower resistance and reactance, and higher NTproBNP levels. While all had high and statistically similar C-statistics (range 0.8 to 0.9) for an ADHF diagnosis, they concluded that IVC ultrasonography and BIA were as useful as NT-proBNP for diagnosing ADHF. 24
Diagnostic Scoring Systems
A scoring system has been proposed to improve diagnosis in the ED. Unfortunately, the value over clinical impression has not been clearly proven, though one randomized, controlled trial did not show statistically significant improvement in diagnostic accuracy when compared to standard care (77% vs 74%, P = 0.77).43
Differential Diagnosis
The differential diagnosis for acute dyspnea is long and potentially arcane. Efforts should focus on excluding non-HF causes of dyspnea, while considering the high risk of alternative etiologies for signs and symptoms. These include asthma, COPD, pneumonia, and pulmonary embolism, which may represent the primary pathologies in a patient with a history of HF, or be the cause of a HF exacerbation. Additional causes of noncardiogenic pulmonary edema should also be considered (eg, acute respiratory distress syndrome, toxins, etc). Acute coronary syndrome and dyspnea may be angina equivalents—one important consideration.
Treatment and Management
Airway Management
Treatment of CH in the ED must always start with an immediate airway evaluation, with the possible need for endotracheal intubation preceding all diagnostic or other management considerations. Intubation is a decision most successfully based on physician clinical assessment, including oxygen (O2) selection rather than waiting for the results of objective measures such as arterial blood gas analysis.
Oxygen
Supplemental O2 should be administered to maintain an O2 saturation above 95%, but obviously is unnecessary in the absence of hypoxia.
Noninvasive Ventilation
Two kinds of noninvasive ventilation (NIV) are available, continuous positive airway pressure and bilevel positive airway pressure ventilation. The physiological differences between these types of NIV have little bearing on ED treatment.
Noninvasive ventilation has not been clearly shown to provide long-term mortality benefit. Large registry data44 report that outcomes are no worse than the alternative of endotracheal intubation, while multiple systematic reviews,45,46meta-analysis,47and Cochrane reviews48,49have established NIV as an acute pulmonary edema intervention that provides reductions in hospital mortality (numbers needed to treat [NNT] 13) and intubation (NNT 8), the prospective randomized C3PO (Congenital Cardiac Catheterization Project on Outcomes) trial50 failed to demonstrate any mortality reduction.
In patients with severe respiratory distress, NIV is a reasonable strategy during the aggressive administration of medical therapy in an attempt to avoid endotracheal intubation. However, NIV is not a stand-alone therapy and though its use may obviate the need for immediate intubation, its implementation should not be considered definitive management.
Correction of Abnormal Vital Signs: Abnormal SBP
Vital signs are an important determinant of therapy, driving treatment strategies. Interventions for HF are based on the patient’s SBP, in particular correction of symptomatic hypotension and hypertensive HF (Table 3).51
Symptomatic Hypotension. The presence of symptomatic hypotension is an extremely poor prognostic finding in AHF. Inotrope therapy may be considered, but it does not reduce mortality except as a bridge to mechanical interventions (LV assist device or transplant).52-54 Temporary inotropic support is recommended for cardiogenic shock to maintain systemic perfusion and prevent end organ damage.3 The inotropic support includes administration of dopamine, dobutamine, or milrinone, though none have been proven to be superior over the other. The lowest possible dose of the selected inotrope should be used to limit arrhythmogenic effects. Inotropic agents should not be used in the absence of severe systolic dysfunction, or low BP, or impaired perfusion, or evidence of significantly decreased cardiac output.
Hypertensive Heart Failure. Defined as the rapid onset of pulmonary congestion with an SBP greater than 140 mm Hg, and commonly greater than 160 mm Hg, these patients may have profound dyspnea, requiring endotracheal intubation. However, in this situation, aggressive vasodilation is typically rapidly effective. Overall, patients presenting with an elevated SBP have lower rates of in-hospital mortality, 30-day myocardial infarction (MI), death, or rehospitalization, and a greater likelihood of discharge within 24 hours—as long as the elevated SBP is aggressively and rapidly treated.
Pharmacological Therapy
Pharmacological management is the mainstay for treating HF. No other acute therapy (eg, NIV) has demonstrated a morality benefit (See Table 4 for specific dose and administration strategies).55 The time to initiate pharmacological therapy and whether an aggressive approach is indicated must be based on the severity of the clinical symptoms and objective risk stratification measures (eg, NP, troponin levels).
Furosemide. Except for hypertensive HF—in which case BP lowering is the most important goal—diuretics are a mainstay of AHF treatment, and consensus guidelines provide a class I recommendation for their use.3 The DOSE (Diuretic Strategies in Patients with ADHF) trial56 prospectively evaluated diuretics in 308 hospitalized AHF patients and found no outcome differences in administration route (bolus or continuous infusion) or dose (high vs low dose). This study reported trends toward greater improvement with higher furosemide dosing, as well as greater diuresis, but at a cost of transient worsening of renal function.
In general, diuretics should be administered in an intravenous (IV) dose equal to 1 to 2.5 times the patient’s usual daily oral dose. For patients who are diuretic-naïve, a dose of 40 mg IV furosemide or 1 mg IV bumetanide, with subsequent dosing titrated to urine output, is recommended.
Vasodilators. In patients with both AHF and even mildly elevated BP, vasodilators can be extremely effective in achieving symptom improvement. The choice of vasodilator, and how aggressive to increase dosing, depends upon symptom severity. The purpose of vasodilators is to lower BP and therefore, should not be used in the setting of hypotension or signs of hypoperfusion. Flow-limiting, preload-dependent CV states (eg, right ventricular infarction) increase the risk of hypotension, and are relative contraindications to the use of vasodilators. For patients who are severely dyspneic and with critical presentations, the emergency physician (EP) should preclude a detailed history and examination to initiate immediate therapy with short-acting agents that can be terminated rapidly in the case of an adverse event (eg, unexpected hypotension) are preferred.
Nitroglycerin. Nitroglycerin is the vasodilation agent of choice for hypertensive AHF. It is a short-acting, rapid-onset, venous and arterial dilator that decreases BP by preload reduction, and by afterload reduction in higher doses. Nitroglycerin has coronary vasodilatory effects associated with decreased ischemia, but should be avoided in patients taking phosphodiesterase inhibitors.55 Its most common side effect is headache, and hypotension occurs in about 3.5% of patients.57
Commonly given as a continuous infusion at IV doses up to 400 mcg/min, nitroglycerin may be associated with higher costs and longer LOS.58 Some authors suggest that bolus nitroglycerin therapy may be superior: In a retrospective study of 395 patients, an IV bolus of nitroglycerin 0.5 mg was superior to both an infusion, or a combination of bolus and infusion, as demonstrated by lower rates of ICU admission (48% vs 67% and 79%, respectively, P = 0.006) and shorter hospital stays (4.4 vs 6.3 and 7.3 days, respectively, P = 0.01). In all cohorts, adverse event rates were similar for hypotension, troponin elevation, and creatinine increase over 48 hours.59 Nitroprusside. Nitroprusside is a potent arterial and venous dilator that causes rapid decrease in BP and LV-filling pressures. It is usually considered more effective than nitroglycerin, despite a small study showing similar hemodynamic responses.60
Initial dosing of nitroprusside starts at 0.3 µg/kg/min IV, and is increased every 5 minutes to a maximum of 10 mcg/kg/min, based on BP and clinical response. The most common acute complication of nitroprusside infusions is hypotension. Cyanide toxicity may occur with prolonged use, high doses, or in patients with renal failure.55
Nesiritide. Exogenously administered, the B-type NP nesiritide is effective in lowering BP and improving dyspnea in AHF,55 although large prospective studies showed it had little long-term advantage over standard care.61 In a small, randomized, controlled trial, nesiritide reduced 30-day revisit LOS when given in an OU.62 The 22-minute half-life of nesiritide is longer than that of the nitrates, and its side effect is predominately hypotension, which occurs at rates similar to those of other vasodilators.55
Angiotensin Converting Enzyme Inhibitors. Because angiotensin converting enzyme inhibitors (ACEIs) have chronic mortality reduction benefits, their use in the acute setting is theoretically attractive, however, this has been poorly proven in AHF ED patients. In a retrospective review of 103 patients with elevated NTproBNP levels receiving bolus IV enalaprilat within 3 hours of presentation, the mean SBP decreased by 30 mm Hg, with only 2% of patients developing hypotension.63 However, with the longer half-life of ACEIs, if hypotension occurs, the potential for a prolonged BP-lowering effect exists.
Calcium Channel Blockers. Clevidipine and nicardipine are rapidly acting IV calcium channel blockers that lower BP by selective arteriolar vasodilation and increased cardiac output as vascular resistance declines.55 Because these agents have no negative inotropic or chronotropic effects, they may be beneficial in hypertensive AHF. In an open-label trial of 104 hypertensive AHF patients, clevidipine was more effective than standard care for the rapid control of BP and relief of dyspnea.64
Morphine. Large registry analyses have demonstrated potential harm with the routine use of morphine,65 as do recent propensity score matched analyses.66 Until there are studies demonstrating benefit, the use of morphine at present should be reserved for palliative care.
Time to Treatment
Although a randomized controlled trial on the importance of time to treatment of AHF is unlikely to ever be completed, data suggest that, as in the case of MI, delayed AHF therapy is associated with adverse outcomes. In a study of 499 suspected AHF patients transferred by ambulance, patients randomized to immediate therapy vs those whose therapy was not initiated until hospital arrival (mean delay of 36 minutes), had a 251% increase in survival (P < 0.01).67
Furthermore, the delayed administration of vasoactive agents, defined as medication administered to alter hemodynamics (eg, dobutamine, dopamine, nitroglycerin, nesiritide) is also associated with harm,68 and registry studies demonstrate increased death rates (n = 35,700).69 Finally, another registry (n = 14,900) study demonstrated early IV furosemide is associated with decreased mortality.70 This latter finding was also validated in a prospective observational cohort study (mortality 2.3 vs 6.0 in early vs delayed therapy groups, respectively).71
Patient Disposition
One of the unique features of emergency medicine is the need to determine, with very limited information and time, a patient’s very short-term clinical trajectory. Few physicians are required to have greater accuracy with less information or time than do EPs. Several studies report objective data points and risk scores to assist in this task, but none has been universally adopted, reflecting the challenge of applying population data to individuals.
Short-term Prognosis
In 1,638 patients evaluated for 14-day outcomes, an HR lower than 50% maximal HR (MHR), and an SBP greater than 140 mm Hg were associated with the lowest rate of serious adverse events (SAEs) (6%) and hospitalization (38%).72 An MHR over 75% was associated with the highest SAE rate, although SAEs decreased as SBP increased (30%, 24%, and 21% with SBPs < 120 mm Hg, 120-140 mm Hg, and > 140 mm Hg, respectively).72
Risk Scores
In a prospective, observational cohort study of 1,100 ED patients, the Ottawa Heart Failure Risk Scale, combined with NTproBNP values, had a sensitivity of 95.8%—at the cost of increasing the admission rate (from 60.8% to 88%)—for serious adverse events (defined as death within 30 days), admission to a monitored unit, intubation, NIV, MI, or relapse resulting in hospital admission within 14 days.73
Observation Unit
Overall, 44% of in-patient HF admissions are for less than 3 days (Table 1),2 supporting the practice of managing selected patients in shorter clinical-care environments than in inpatient units. Further, ED patients presenting with moderate dyspnea require both a diagnosis and an evaluation of their therapeutic response to determine the need for hospitalization. However, evaluating therapeutic response requires more time than is available in the typical ED. Thus, an ED OU offers the following:
(1) The OU provides the EP with a longer evaluation time, and therefore a more accurate disposition may be effected;
(2) Costs are significantly lower in patients managed in an ED OU; and
(3) Patient satisfaction may be improved, as most patients prefer home management over hospitalization.
All three of these opportunities are supported by a number of studies,74-78 with validated entry and exclusion criteria, treatment algorithms and discharge metrics. Most recently, in a registry of hospitals in Spain registry, patients presenting to hospitals that had OUs had a 2.2-day shorter LOS, lower 30-day ED revisit rate, and similar mortality rates compared to those in institutions without OUs—although these beneficial effects occurred at the cost of an 8.9% higher admission rate.79
Patient Education
Intuitively, it would be expected that patient education would reduce return visits, 30-day hospitalizations, and AHF-related mortality. Unfortunately, it has not been demonstrated that patient education results in a consistent benefit at hospital discharge, or in the outpatient environment.80-85
Although AHF education in the ED has been poorly studied, areas that have shown promise are education occurring before ED management (ie, in the ED waiting area) in underinsured patients,86 and during ED care for patients with poor health care literacy.87 As educational interventions are both inexpensive and unlikely to result in harm, their implementation should be considered.
Conclusion
The spectrum of HF is a common presentation in the ED. Because HF generally appears as dyspnea, in a cohort with multiple comorbidities, the diagnosis can be challenging. This is complicated by the fact that patients with severe presentations may require life-saving interventions long before a clinical evaluation is completed (or even initiated). The skill of the EP, and his or her ability to improve the clinical condition before intubation is required, will determine the patient’s trajectory. Conversely, as a chronic condition, HF may present with moderate symptoms for which a short diuretic “tune-up” in an observation environment may be appropriate.
How these decisions are made will depend upon the local environment, the availability of outpatient resources, and individual patient choices. There are few chronic diseases that are more complex, are seen more often in the ED, or that require more skill and finesse in management.
Patients with heart failure (HF) present daily to busy EDs. An estimated 6.5 million Americans are living with this diagnosis, and the number is predicted to grow to 8 million by 2023.1 Most HF patients (82.1%) who present to EDs are hospitalized, while a selected minority are either managed in the ED and discharged (11.6%) or managed in observation units (OU) (6.3%).2 The prognosis after HF is initially diagnosed is poor, with a 5-year mortality of 50%,3 and after a single HF hospitalization, 29% will die within 1 year.4
One-third of the total Medicare budget is spent on HF, despite the fact that HF represents only 10.5% of the Medicare population.2 Up to 80% of HF costs are for hospitalizations, which cost an average of $11,840 per inpatient admission.5,6 The high costs are due to an average length of stay (LOS) of 5.2 days7 (Table 1).
Adding to hospital costs is the degree of “reactivism,” with approximately 20% of patients discharged from the ED returning within 2 weeks, of whom nearly 50% will be hospitalized.11 Following HF hospitalization and discharge, the 30-day readmission rate is 26.2%,2 increasing to 36% by 90 days.12 The Centers for Medicare & Medicaid Services (CMS) has incentivized hospitals and providers to reduce admissions, but penalize hospitals that do not. Overall, CMS will reduce payments by up to 3% to hospitals with excess readmissions for select conditions, including HF.13
Causes of Heart Failure
Heart failure represents a final common pathway, which in the United States is most often due to coronary artery disease (CAD). Many types of pathology ultimately result in left ventricular (LV) dysfunction, and much of its rising prevalence is a result of the success we now have in managing historically fatal cardiovascular (CV) conditions. These include hypertension, diabetes mellitus (DM), CAD, and valvular and other CV structural conditions.
Heart failure is caused by either a dilated ventricle with a reduced ejection fraction (HFrEF) and inability to eject volume, or a stiffened ventricle with a preserved EF (HFpEF) that is unable to receive increased venous return. Both conditions acutely decompensate pulmonary congestion. A preserved EF is defined as an EF at or greater than 50%, whereas a reduced EF is at or less than 40%, with the 41% to 49% range considered as borderline preserved EF.3
While there are important differences in the treatment of chronic and subacute HF, driven by the EF, the effect of EF on early decision-making and treatment in the ED is negligible: Although the probability of HFpEF increases with increasing initial ED systolic blood pressure (SBP), clinical presentation and treatment in the ED are initially identical—regardless of the EF.
Noninvasive continuous transcutaneous hemodynamic monitoring is available for ED use, and may provide further insight into the underlying pathophysiology. A study of 127 acute heart failure (AHF) ED patients identified three hemodynamic AHF phenotypes. These include normal cardiac index (CI) and systemic vascular resistance index (SVRI), low CI and SVRI, and low CI and elevated SVRI.14 While it is attractive to suggest therapeutic interventions based on these measurements, outcome data are lacking.
Presentation
The most common ED presentation of patients suffering from AHF is dyspnea secondary to volume overload, or as the result of acute hypertension with relatively less volume overload. However, regardless of the cause of dyspnea, it is not only the most common resulting complaint, but one that requires immediate treatment. Ultimately, 59% of all HF admissions are attributed to volume overload and dyspnea (Figure 1).15
Heart failure can also present in a more protean manner, with cough, fatigue, and edema, as well as more subtle symptoms predominating and resulting in a complicated differential diagnosis (Table 2).16
Because HF is a disease that most significantly affects older patients who frequently have concomitant morbidities (eg, myocardial ischemia, chronic obstructive pulmonary disease [COPD] exacerbation, uncontrolled DM), other less clinically obvious disease presentations may actually be the cause of the AHF exacerbation.
Diagnosis
A focused history and physical examination that is part of all ED evaluations should be expedited whenever there is evidence of hemodynamic instability or respiratory compromise. An early working diagnosis is essential to avoid a delay in appropriate treatment, which is associated with increased mortality.
When HF is likely, the potential etiology and precipitants for decompensation must be considered. This list is long, but medication noncompliance and dietary indiscretion are the most common causes.
Symptoms and Prior History of HF
The classic symptoms for AHF include dyspnea at rest or exertion, and orthopnea, both of which unfortunately have poor sensitivity and specificity for AHF. As an isolated symptom, dyspnea is of marginal diagnostic utility (sensitivity and specificity for an HF diagnosis is 56% and 53%, respectively), and orthopnea is only slightly better (sensitivity and specificity 77% and 50%, respectively). A prior HF diagnosis makes repeat presentations much more likely (sensitivity and specificity 60% and 90%, respectively).17
Physical Examination
Simple observation and a directed examination can rapidly point to the diagnosis (Figure 2).
Electrocardiography
Because CAD is one of the most common underlying AHF etiologies, an electrocardiogram (ECG) should always be obtained early for a patient presenting with potential AHF. Although the ECG does not usually contribute to ED management, the identification of new ST-segment changes or a malignant arrhythmia will guide critical management decisions.
Imaging Studies
Chest X-ray Imaging. A chest X-ray (CXR) study must be considered early when a patient presents with signs and symptoms suggestive of AHF. Although the classic findings of HF (eg, Kerley B lines [short horizontal lines perpendicular to the pleural surface],18 interstitial congestion, pulmonary effusion) can lag behind the clinical presentation, and also be nondiagnostic in the setting of mild HF, the CXR is an effective aid in identifying other causes of dyspnea such as pneumonia. Ultimately, the utility of the CXR for diagnosis is similar to that of the history and physical examination in that it will be diagnostic when positive but cannot exclude AHF if normal.
Ultrasound. Because it is fast, inexpensive, noninvasive, and readily available in the ED, ultrasound is frequently used to evaluate potential HF patients. Several studies have demonstrated that the presence of B lines in two or more regions is specific for AHF (specificity 75%-100%), although the sensitivity may be limited (40%-91%).19-21 The presence of inferior vena cava (IVC) dilation is also associated with adverse outcomes.22 In 80 patients hospitalized with acute decompensated HF (ADHF), a dilated IVC (≥1.9 cm) at admission was associated with higher 90-day mortality (25.4% vs 3.4%, P = 0.009).23 These findings may be considered in groups: In an evaluation of the combination of LV EF, IVC collapsibility, and B lines for an HF diagnosis, the combination of all three had a poor sensitivity (36%) but an excellent specificity (100%), and any two of the three had a specificity of at least 93%.24
Laboratory Evaluation
Myocardial Strain: BNP/NTproBNP. Natriuretic peptides (NPs) are not AHF-specific, but rather they are synthesized and released by the myocardium in the setting of myocardial pressure or volume stress. They are manufactured as preproBNP, then enzymatically cleaved into the active BNP and the inactive fragment N-terminal proBNP (NTproBNP). The predominant hormonal effects of BNP are vasodilation and natriuresis, as well as antagonism of the hormones associated with sodium retention (aldosterone) and vasoconstriction (endothelin, norepinephrine).
As AHF results in myocardial stress, NP elevation provides diagnostic and prognostic information. Clinical judgment supported by a BNP greater than 100 pg/mL is a better predictor of AHF than clinical judgment alone (accuracy 81% vs 74%, respectively).25 While low levels (BNP <100 pg/mL or NTproBNP <300 pg/mL) reliably exclude the diagnosis of HF (sensitivities >95%), higher levels (BNP >500 pg/mL, NTproBNP >900 pg/mL) are useful as “rule-in” markers, with specificity greater than 95%. The NTproBNP also requires adjustment for patients older than age 75 years, with a higher level (>1,800 pg/mL) to rule-in HF. The NP grey zone (BNP 100-500 pg/mL, NTproBNP 300-900 pg/mL)requires additional testing for accurate diagnoses (Figure 3).25-29
There are several confounders to the interpretation of NP results: NPs are negatively confounded by the presence of obesity, resulting in a lowering of the value as compared to the clinical presentation.Thus, the measured BNP level should be doubled if the patient’s body mass index exceeds 35 kg/m2.30 Secondly, because NP metabolism is partially renal dependent, elevated levels may not reflect AHF in the presence of renal failure. If the estimated glomerular filtration rate is less than 60 mL/min, measured BNP levels should be halved.31
AHF vs Myocardial Ischemia: Troponin Levels. Large registry data using contemporary troponin assays clearly identify the association between elevated troponin levels (>99th percentile in a healthy population) and increased short-term risk. With the US Food and Drug Association (FDA) approval of a high-sensitivity troponin (hs-cTnT) assay, a greater frequency of elevated cardiac troponin T (cTnT) and cardiac troponin I (cTnl) will be identified in AHF patients in the ED.
In one retrospective study of 4,705 AHF patients in the ED, hs-cTnT were elevated in 48.4% of cases (25.3% in cTnI, 37.9% in cTnT, and 82.2% in hs-cTnT). Although 1-year mortality was higher in those with elevated troponin (adjusted heart rate [HR] 1.61; CI 95% 1.38-1.88), elevated troponin was not associated with 30-day revisits to the ED (1.01; 0.87-1.19) and high sensitive elevations less than double the reference value had no impact on outcomes.32 Thus, in terms of management of AHF in the ED, slightly elevated stable serial troponins are more consistent with underlying HF, and should be managed as such. This is not true of rising/falling troponin levels, which should still engender concern for underlying myocardial ischemia and a different management pathway.
Renal function. Comprised renal function is an important predictor of AHF outcome. Large registry data from hospitalized HF patients demonstrate that a presenting blood urea nitrogen level greater than 43 mg/dL is one of the most important predictors of increased acute mortality,33 and levels below 30 mg/dL identify a cohort likely to be successfully managed in an observation environment.34 Creatinine is a helpful lagging indicator of mortality, with higher levels (>2.75 mg/dL) associated with increased short-term adverse outcomes and decreased therapeutic responsiveness (Figure 4).
For patients presenting with ADHF, a newer test recently approved by the FDA uses the product of the urine markers tissue inhibitor of metalloproteinase-2 and insulin-like growth factor-binding protein 7, to generate a score predictive of acute kidney injury.35 While promising, no studies of ED outcomes are currently available.
Volume Assessment
Objective volume assessment is useful for diagnosis and prognosis in AHF. Bioimpedance vector analysis (BIVA) is a rapid, inexpensive, noninvasive technique that measures total body water by placing a pair of electrodes on the wrist and ipsilateral ankle. The BIVA measurements have strong correlations with the gold standard volume-assessment technique of deuterium dilution (r > 0.99).36 In HF, BIVA can assess volume depletion37 and overload,38 and identifies differences in hydration status between 90-day survivors and non-survivors (P < 0.01).39
Used in combination with BNP, one prospective study of 292 dyspneic patients found that, while BIVA was a strong predictor of AHF (c-statistic 0.93, P = 0.016), the most accurate volume status determination was the combination of both (c-statistic, 0.99; P = 0.005), for which the combined accuracy exceeded either alone.40 Finally, in 166 hospitalized HF patients discharged by BNP and BIVA parameters, vs 149 discharged based on clinical impressions, those assessed with BNP and BIVA had lower 6-month readmissions (23% vs 35%, P = 0.02) and overall cost of care.41
Combination Technologies
Obviously, EPs may consider multiple technologies to arrive at an accurate diagnosis. One prospective evaluation enrolled 236 patients to determine the diagnostic accuracy for AHF in the ED and reported lung ultrasound, CXR, and NTproBNP had a sensitivity of 57.7% and 88.0%, 74.5% and 86.3%, and a specificity of 97.6% and 28.0%. The best overall combination was the CXR with lung ultrasound (sensitivity 84.7%, specificity 77.7%).42
Another prospective study evaluated IVC diameter, bioelectrical impedance analysis (BIA), and NTproBNP in 96 elderly patients. ADHF patients had higher IVC diameters and lower collapsibility index, lower resistance and reactance, and higher NTproBNP levels. While all had high and statistically similar C-statistics (range 0.8 to 0.9) for an ADHF diagnosis, they concluded that IVC ultrasonography and BIA were as useful as NT-proBNP for diagnosing ADHF. 24
Diagnostic Scoring Systems
A scoring system has been proposed to improve diagnosis in the ED. Unfortunately, the value over clinical impression has not been clearly proven, though one randomized, controlled trial did not show statistically significant improvement in diagnostic accuracy when compared to standard care (77% vs 74%, P = 0.77).43
Differential Diagnosis
The differential diagnosis for acute dyspnea is long and potentially arcane. Efforts should focus on excluding non-HF causes of dyspnea, while considering the high risk of alternative etiologies for signs and symptoms. These include asthma, COPD, pneumonia, and pulmonary embolism, which may represent the primary pathologies in a patient with a history of HF, or be the cause of a HF exacerbation. Additional causes of noncardiogenic pulmonary edema should also be considered (eg, acute respiratory distress syndrome, toxins, etc). Acute coronary syndrome and dyspnea may be angina equivalents—one important consideration.
Treatment and Management
Airway Management
Treatment of CH in the ED must always start with an immediate airway evaluation, with the possible need for endotracheal intubation preceding all diagnostic or other management considerations. Intubation is a decision most successfully based on physician clinical assessment, including oxygen (O2) selection rather than waiting for the results of objective measures such as arterial blood gas analysis.
Oxygen
Supplemental O2 should be administered to maintain an O2 saturation above 95%, but obviously is unnecessary in the absence of hypoxia.
Noninvasive Ventilation
Two kinds of noninvasive ventilation (NIV) are available, continuous positive airway pressure and bilevel positive airway pressure ventilation. The physiological differences between these types of NIV have little bearing on ED treatment.
Noninvasive ventilation has not been clearly shown to provide long-term mortality benefit. Large registry data44 report that outcomes are no worse than the alternative of endotracheal intubation, while multiple systematic reviews,45,46meta-analysis,47and Cochrane reviews48,49have established NIV as an acute pulmonary edema intervention that provides reductions in hospital mortality (numbers needed to treat [NNT] 13) and intubation (NNT 8), the prospective randomized C3PO (Congenital Cardiac Catheterization Project on Outcomes) trial50 failed to demonstrate any mortality reduction.
In patients with severe respiratory distress, NIV is a reasonable strategy during the aggressive administration of medical therapy in an attempt to avoid endotracheal intubation. However, NIV is not a stand-alone therapy and though its use may obviate the need for immediate intubation, its implementation should not be considered definitive management.
Correction of Abnormal Vital Signs: Abnormal SBP
Vital signs are an important determinant of therapy, driving treatment strategies. Interventions for HF are based on the patient’s SBP, in particular correction of symptomatic hypotension and hypertensive HF (Table 3).51
Symptomatic Hypotension. The presence of symptomatic hypotension is an extremely poor prognostic finding in AHF. Inotrope therapy may be considered, but it does not reduce mortality except as a bridge to mechanical interventions (LV assist device or transplant).52-54 Temporary inotropic support is recommended for cardiogenic shock to maintain systemic perfusion and prevent end organ damage.3 The inotropic support includes administration of dopamine, dobutamine, or milrinone, though none have been proven to be superior over the other. The lowest possible dose of the selected inotrope should be used to limit arrhythmogenic effects. Inotropic agents should not be used in the absence of severe systolic dysfunction, or low BP, or impaired perfusion, or evidence of significantly decreased cardiac output.
Hypertensive Heart Failure. Defined as the rapid onset of pulmonary congestion with an SBP greater than 140 mm Hg, and commonly greater than 160 mm Hg, these patients may have profound dyspnea, requiring endotracheal intubation. However, in this situation, aggressive vasodilation is typically rapidly effective. Overall, patients presenting with an elevated SBP have lower rates of in-hospital mortality, 30-day myocardial infarction (MI), death, or rehospitalization, and a greater likelihood of discharge within 24 hours—as long as the elevated SBP is aggressively and rapidly treated.
Pharmacological Therapy
Pharmacological management is the mainstay for treating HF. No other acute therapy (eg, NIV) has demonstrated a morality benefit (See Table 4 for specific dose and administration strategies).55 The time to initiate pharmacological therapy and whether an aggressive approach is indicated must be based on the severity of the clinical symptoms and objective risk stratification measures (eg, NP, troponin levels).
Furosemide. Except for hypertensive HF—in which case BP lowering is the most important goal—diuretics are a mainstay of AHF treatment, and consensus guidelines provide a class I recommendation for their use.3 The DOSE (Diuretic Strategies in Patients with ADHF) trial56 prospectively evaluated diuretics in 308 hospitalized AHF patients and found no outcome differences in administration route (bolus or continuous infusion) or dose (high vs low dose). This study reported trends toward greater improvement with higher furosemide dosing, as well as greater diuresis, but at a cost of transient worsening of renal function.
In general, diuretics should be administered in an intravenous (IV) dose equal to 1 to 2.5 times the patient’s usual daily oral dose. For patients who are diuretic-naïve, a dose of 40 mg IV furosemide or 1 mg IV bumetanide, with subsequent dosing titrated to urine output, is recommended.
Vasodilators. In patients with both AHF and even mildly elevated BP, vasodilators can be extremely effective in achieving symptom improvement. The choice of vasodilator, and how aggressive to increase dosing, depends upon symptom severity. The purpose of vasodilators is to lower BP and therefore, should not be used in the setting of hypotension or signs of hypoperfusion. Flow-limiting, preload-dependent CV states (eg, right ventricular infarction) increase the risk of hypotension, and are relative contraindications to the use of vasodilators. For patients who are severely dyspneic and with critical presentations, the emergency physician (EP) should preclude a detailed history and examination to initiate immediate therapy with short-acting agents that can be terminated rapidly in the case of an adverse event (eg, unexpected hypotension) are preferred.
Nitroglycerin. Nitroglycerin is the vasodilation agent of choice for hypertensive AHF. It is a short-acting, rapid-onset, venous and arterial dilator that decreases BP by preload reduction, and by afterload reduction in higher doses. Nitroglycerin has coronary vasodilatory effects associated with decreased ischemia, but should be avoided in patients taking phosphodiesterase inhibitors.55 Its most common side effect is headache, and hypotension occurs in about 3.5% of patients.57
Commonly given as a continuous infusion at IV doses up to 400 mcg/min, nitroglycerin may be associated with higher costs and longer LOS.58 Some authors suggest that bolus nitroglycerin therapy may be superior: In a retrospective study of 395 patients, an IV bolus of nitroglycerin 0.5 mg was superior to both an infusion, or a combination of bolus and infusion, as demonstrated by lower rates of ICU admission (48% vs 67% and 79%, respectively, P = 0.006) and shorter hospital stays (4.4 vs 6.3 and 7.3 days, respectively, P = 0.01). In all cohorts, adverse event rates were similar for hypotension, troponin elevation, and creatinine increase over 48 hours.59 Nitroprusside. Nitroprusside is a potent arterial and venous dilator that causes rapid decrease in BP and LV-filling pressures. It is usually considered more effective than nitroglycerin, despite a small study showing similar hemodynamic responses.60
Initial dosing of nitroprusside starts at 0.3 µg/kg/min IV, and is increased every 5 minutes to a maximum of 10 mcg/kg/min, based on BP and clinical response. The most common acute complication of nitroprusside infusions is hypotension. Cyanide toxicity may occur with prolonged use, high doses, or in patients with renal failure.55
Nesiritide. Exogenously administered, the B-type NP nesiritide is effective in lowering BP and improving dyspnea in AHF,55 although large prospective studies showed it had little long-term advantage over standard care.61 In a small, randomized, controlled trial, nesiritide reduced 30-day revisit LOS when given in an OU.62 The 22-minute half-life of nesiritide is longer than that of the nitrates, and its side effect is predominately hypotension, which occurs at rates similar to those of other vasodilators.55
Angiotensin Converting Enzyme Inhibitors. Because angiotensin converting enzyme inhibitors (ACEIs) have chronic mortality reduction benefits, their use in the acute setting is theoretically attractive, however, this has been poorly proven in AHF ED patients. In a retrospective review of 103 patients with elevated NTproBNP levels receiving bolus IV enalaprilat within 3 hours of presentation, the mean SBP decreased by 30 mm Hg, with only 2% of patients developing hypotension.63 However, with the longer half-life of ACEIs, if hypotension occurs, the potential for a prolonged BP-lowering effect exists.
Calcium Channel Blockers. Clevidipine and nicardipine are rapidly acting IV calcium channel blockers that lower BP by selective arteriolar vasodilation and increased cardiac output as vascular resistance declines.55 Because these agents have no negative inotropic or chronotropic effects, they may be beneficial in hypertensive AHF. In an open-label trial of 104 hypertensive AHF patients, clevidipine was more effective than standard care for the rapid control of BP and relief of dyspnea.64
Morphine. Large registry analyses have demonstrated potential harm with the routine use of morphine,65 as do recent propensity score matched analyses.66 Until there are studies demonstrating benefit, the use of morphine at present should be reserved for palliative care.
Time to Treatment
Although a randomized controlled trial on the importance of time to treatment of AHF is unlikely to ever be completed, data suggest that, as in the case of MI, delayed AHF therapy is associated with adverse outcomes. In a study of 499 suspected AHF patients transferred by ambulance, patients randomized to immediate therapy vs those whose therapy was not initiated until hospital arrival (mean delay of 36 minutes), had a 251% increase in survival (P < 0.01).67
Furthermore, the delayed administration of vasoactive agents, defined as medication administered to alter hemodynamics (eg, dobutamine, dopamine, nitroglycerin, nesiritide) is also associated with harm,68 and registry studies demonstrate increased death rates (n = 35,700).69 Finally, another registry (n = 14,900) study demonstrated early IV furosemide is associated with decreased mortality.70 This latter finding was also validated in a prospective observational cohort study (mortality 2.3 vs 6.0 in early vs delayed therapy groups, respectively).71
Patient Disposition
One of the unique features of emergency medicine is the need to determine, with very limited information and time, a patient’s very short-term clinical trajectory. Few physicians are required to have greater accuracy with less information or time than do EPs. Several studies report objective data points and risk scores to assist in this task, but none has been universally adopted, reflecting the challenge of applying population data to individuals.
Short-term Prognosis
In 1,638 patients evaluated for 14-day outcomes, an HR lower than 50% maximal HR (MHR), and an SBP greater than 140 mm Hg were associated with the lowest rate of serious adverse events (SAEs) (6%) and hospitalization (38%).72 An MHR over 75% was associated with the highest SAE rate, although SAEs decreased as SBP increased (30%, 24%, and 21% with SBPs < 120 mm Hg, 120-140 mm Hg, and > 140 mm Hg, respectively).72
Risk Scores
In a prospective, observational cohort study of 1,100 ED patients, the Ottawa Heart Failure Risk Scale, combined with NTproBNP values, had a sensitivity of 95.8%—at the cost of increasing the admission rate (from 60.8% to 88%)—for serious adverse events (defined as death within 30 days), admission to a monitored unit, intubation, NIV, MI, or relapse resulting in hospital admission within 14 days.73
Observation Unit
Overall, 44% of in-patient HF admissions are for less than 3 days (Table 1),2 supporting the practice of managing selected patients in shorter clinical-care environments than in inpatient units. Further, ED patients presenting with moderate dyspnea require both a diagnosis and an evaluation of their therapeutic response to determine the need for hospitalization. However, evaluating therapeutic response requires more time than is available in the typical ED. Thus, an ED OU offers the following:
(1) The OU provides the EP with a longer evaluation time, and therefore a more accurate disposition may be effected;
(2) Costs are significantly lower in patients managed in an ED OU; and
(3) Patient satisfaction may be improved, as most patients prefer home management over hospitalization.
All three of these opportunities are supported by a number of studies,74-78 with validated entry and exclusion criteria, treatment algorithms and discharge metrics. Most recently, in a registry of hospitals in Spain registry, patients presenting to hospitals that had OUs had a 2.2-day shorter LOS, lower 30-day ED revisit rate, and similar mortality rates compared to those in institutions without OUs—although these beneficial effects occurred at the cost of an 8.9% higher admission rate.79
Patient Education
Intuitively, it would be expected that patient education would reduce return visits, 30-day hospitalizations, and AHF-related mortality. Unfortunately, it has not been demonstrated that patient education results in a consistent benefit at hospital discharge, or in the outpatient environment.80-85
Although AHF education in the ED has been poorly studied, areas that have shown promise are education occurring before ED management (ie, in the ED waiting area) in underinsured patients,86 and during ED care for patients with poor health care literacy.87 As educational interventions are both inexpensive and unlikely to result in harm, their implementation should be considered.
Conclusion
The spectrum of HF is a common presentation in the ED. Because HF generally appears as dyspnea, in a cohort with multiple comorbidities, the diagnosis can be challenging. This is complicated by the fact that patients with severe presentations may require life-saving interventions long before a clinical evaluation is completed (or even initiated). The skill of the EP, and his or her ability to improve the clinical condition before intubation is required, will determine the patient’s trajectory. Conversely, as a chronic condition, HF may present with moderate symptoms for which a short diuretic “tune-up” in an observation environment may be appropriate.
How these decisions are made will depend upon the local environment, the availability of outpatient resources, and individual patient choices. There are few chronic diseases that are more complex, are seen more often in the ED, or that require more skill and finesse in management.
1. Benjamin EJ, Blaha MJ, Chiuve SE, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation. 2017;135(10):e146-e603.
2. Fitch KV, Engel T, Lauet J. The cost burden of worsening heart failure in the Medicare fee for service population: an actuarial analysis. Milliman Web site. 2017. http://www.milliman.com/insight/2017/The-cost-burden-of-worsening-heart-failure-in-the-Medicare-fee-for-service-population-An-actuarial-analysis/ Published April 3, 2017. Accessed June 1, 2017.
3. Yancy CW, Jessup M, Bozkurt B, et al; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013. J Am Coll Cardiol. 2013;62(16):e147-e239. doi:10.1016/j.jacc.2013.05.019.
4. Chen J, Normand SL, Wang Y, Krumholz HM. National and regional trends in heart failure hospitalization and mortality rates for Medicare beneficiaries, 1998-2008. JAMA. 2011;306(15):1669-1678. doi:10.1001/jama.2011.1474.
5. Heidenreich PA, Albert NM, Allen LA, et al; American Heart Association Advocacy Coordinating Committee; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Stroke Council. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6(3):606-619. doi:10.1161/HHF.0b013e318291329a.
6. Dunlay SM, Shah ND, Shi Q, et al. Lifetime costs of medical care after heart failure diagnosis. Circ Cardiovasc Qual Outcomes. 2011;4(1):68-75. doi:10.1161/CIRCOUTCOMES.110.957225.
7. HCUP National Inpatient Sample (NIS); 2014 Agency for Healthcare Research and Quality (AHRQ). HCUPnet Healthcare Cost and Utilization Project. http://bit.ly/2u2ymFA. Published 2014. Accessed July 27, 2017.
8. FY 2017 Final Rule and Correction Notice Tables. CMS 2014 data based on DRGs, Table 5:List of MS-DRGs, Relative Weighting Factors and Geometric and Arithmetic Mean Length of Stay. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/FY2017-IPPS-Final-Rule-Home-Page-Items/FY2017-IPPS-Final-Rule-Tables.html. Published 2017. Accessed August 28, 2017.
9. Hospital Adjusted Expenses per Inpatient Day by Ownership. Kaiser Family Foundation. http://www.kff.org/health-costs/state-indicator/expenses-per-inpatient-day-by-ownership/?currentTimeframe=0&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22asc%22%7D. Accessed August 2, 2017
10. Ellison, A. Average cost per inpatient day across 50 states. Becker’s Hospital CFO Report. http://www.beckershospitalreview.com/finance/average-cost-per-inpatient-day-across-50-states-2016.html. Published January 13, 2016. Accessed August 2, 2017
11. Claret PG, Calder LA, Stiell IG, et al. Rates and predictive factors of return to the emergency department following an initial release by the emergency department for acute heart failure. [published online ahead of print April 3, 2017]. CJEM. 1-8. doi:10.1017/cem.2017.14.
12. Yam FK, Lew T, Eraly SA, Lin HW, Hirsch JD, Devor M. Changes in medication regimen complexity and the risk for 90-day hospital readmission and/or emergency department visits in U.S. Veterans with heart failure. Res Social Adm Pharm. 2016;12(5):713-721. doi:10.1016/j.sapharm.2015.10.004.
13. Readmissions Reduction Program. CMS. https://www.cms.gov/medicare/medicare-fee-for-service-payment/acuteinpatientpps/readmissions-reduction-program.html Updated April 18, 2016. Accessed July 13, 2017.
14. Nowak RM, Reed BP, DiSomma S, et al. Presenting phenotypes of acute heart failure patients in the ED: Identification and implications. Am J Emerg Med. 2017;35(4):536-542. doi:10.1016/j.ajem.2016.12.003.
15. Bennett SJ, Huster GA, Baker SL, et al. Characterization of the precipitants of hospitalization for heart failure decompensation. Am J Crit Care. 1998;7(3):168-174.
16. Kuo DC, Peacock WF. Diagnosing and managing acute heart failure in the emergency department. Clin Exp Emerg Med. 2015;2(3):141-149. eCollection 2015 Sep. doi:10.15441/ceem.15.007.
17. Wang CS, FitzGerald JM, Schulzer M, Mak E, Ayas NT. Does this dyspneic patient in the emergency department have congestive heart failure? JAMA. 2005;294:1944-1956.
18. Koga T, Fujimoto K. Images in clinical medicine. Kerley’s A, B, and C line. N Engl J Med. 2009;360(15):1539. doi:10.1056/NEJMicm0708489.
19. Glöckner E, Christ M, Geier F, et al. Accuracy of point-of-care B-Line lung ultrasound in comparison to NT-ProBNP for screening acute heart failure. Ultrasound Int Open. 2016;2(3):e90-e92. doi:10.1055/s-0042-108343.
20. Bitar Z, Maadarani O, Almerri K. Sonographic chest B-lines anticipate elevated B-type natriuretic peptide level, irrespective of ejection fraction. Ann Intensive Care. 2015;5(1): 56. doi:10.1186/s13613-015-0100-x.
21. Miglioranza MH, Gargani L, Sant’Anna RT, et al. Lung ultrasound for the evaluation of pulmonary congestion in outpatients: a comparison with clinical assessment, natriuretic peptides, and echocardiography. JACC Cardiovasc Imaging. 2013;6(11):1141-1151. doi:10.1016/j.jcmg.2013.08.004.
22. Anderson KL, Jenq KY, Fields JM, Panebianco NL, Dean AJ. Diagnosing heart failure among acutely dyspneic patients with cardiac, inferior vena cava, and lung ultrasonography. Am J Emerg Med. 2013;31(8):1208-1214. doi:10.1016/j.ajem.2013.05.007.
23. Cubo-Romano P, Torres-Macho J, Soni NJ, et al. Admission inferior vena cava measurements are associated with mortality after hospitalization for acute decompensated heart failure. J Hosp Med. 2016;11(11):778-784. doi:10.1002/jhm.2620.
24. Martínez PG, Martínez DM, García JC, Loidi JC. Amino-terminal pro–B-type natriuretic peptide, inferior vena cava ultrasound, and bioelectrical impedance analysis for the diagnosis of acute decompensated CHF. Am J Emerg Med. 2016;34(9): 1817–1822. doi:10.1016/j.ajem.2016.06.043.

25. Maisel AS, Krishnaswamy P, Nowak RM, et al; Breathing Not Properly Multinational Study Investigators. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002;347(3):161-167.
26. Van Kimmenade RR, Pinto YM, Bayes-Genis A, Lainchbury JG, Richards AM, Januzzi JL Jr. Usefulness of intermediate amino-terminal pro-brain natriuretic peptide concentrations for diagnosis and prognosis of acute heart failure. Am J Cardiol. 2006;98(3):386-390.
27. Moe GW, Howlett J, Januzzi JL, Zowall H; Canadian Multicenter Improved Management of Patients With Congestive Heart Failure (IMPROVE-CHF) Study Investigators. N-terminal pro-B-type natriuretic peptide testing improves the management of patients with suspected acute heart failure: primary results of the Canadian prospective randomized multicenter IMPROVE-CHF study. Circulation. 2007;115(24):3103-3110.
28. Mayo DD, Colletti JE, Kuo DC. Brain natriuretic peptide (BNP) testing in the emergency department. J Emerg Med. 2006;31(2):201-210.
29. Mueller C, Scholer A, Laule-Kilian K, et al. Use of B-type natriuretic peptide in the evaluation and management of acute dyspnea. N Engl J Med. 2004;350(7):647-654.
30. Krauser DG, Lloyd-Jones DM, Chae CU, et al. Effect of body mass index on natriuretic peptide levels in patients with acute congestive heart failure: a ProBNP Investigation of Dyspnea in the Emergency Department (PRIDE) substudy. Am Heart J. 2005;149(4):744-750.
31. McCullough PA, Duc P, Omland T, et al. B-type natriuretic peptide and renal function in the diagnosis of heart failure: an analysis from the Breathing Not Properly Multinational Study. Am J Kidney Dis. 2003;41(3):571-579.
32. Jacob J, Roset A, Miró Ò, et al; ICA-SEMES Research Group. EAHFE - TROPICA2 study. Prognostic value of troponin in patients with acute heart failure treated in Spanish hospital emergency departments. Biomarkers. 2017;22(3-4):337-344. doi:10.1080/1354750X.2016.1265006.
33. Fonarow GC, Adams KF Jr, Abraham WT, et al; ADHERE Scientific Advisory Committee, Study Group, and Investigators. Risk stratification for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. JAMA. 2005;293(5):572-580.
34. Burkhardt J, Peacock WF, Emerman CL. Predictors of emergency department observation unit outcomes. Acad Emerg Med. 2005;12(9):869-874.
35. Schanz M, Shi J, Wasser C, Alscher MD, Kimmel M. Urinary [TIMP-2] × [IGFBP7] for risk prediction of acute kidney injury in decompensated heart failure. Clin Cardiol. 2017;40(7):485-491. doi:10.1002/clc.22683.
36. Kushner RF, Schoeller DA, Fjeld CR, Danford L: Is the impedance index (ht2/R) significant in predicting total body water? Am J Clin Nutr. 1992;56(5): 835-839.
37. Ackland GL, Singh-Ranger D, Fox S, et al. Assessment of preoperative fluid depletion using bioimpedance analysis. Br J Anaesth. 2004;92(1): 134-136.
38. Uszko-Lencer NH, Bothmer F, van Pol PE, Schols AM. Measuring body composition in chronic heart failure: a comparison of methods. Eur J Heart Fail. 2006;8(2): 208-214.
39. Santarelli S, Russo V, Lalle I, et al; GREAT network. Usefulness of combining admission brain natriuretic peptide (BNP) plus hospital discharge bioelectrical impedance vector analysis (BIVA) in predicting 90 days cardiovascular mortality in patients with acute heart failure. Intern Emerg Med. 2017;12(4):445-451. doi:10.1007/s11739-016-1581-9.
40. Parrinello G, Paterna S, Di Pasquale P, et al. The usefulness of bioelectrical impedance analysis in differentiating dyspnea due to decompensated heart failure. J Card Fail. 2008;14(8): 676-686. doi:10.1016/j.cardfail.2008.04.005.
41. Valle R, Aspromonte N, Carbonieri E, et al. Fall in readmission rate for heart failure after implementation of B-type natriuretic peptide testing for discharge decision: a retrospective study. Int J Cardiol. 2008;126(3): 400-406.
42. Sartini S, Frizzi J, Borselli M, et al. Which method is best for an early accurate diagnosis of acute heart failure? Comparison between lung ultrasound, chest X-ray and NT pro-BNP performance: a prospective study. [published online ahead of print July 11, 2016]. Intern Emerg Med. doi:10.1007/s11739-016-1498-3.
43. Steinhart BD, Levy P, Vandenberghe H, et al. A randomized control trial using a validated prediction model for diagnosing acute heart failure in undifferentiated dyspneic emergency department patients-results of the GASP4Ar study. J Card Fail. 2017;23(2):145-152. doi:10.1016/j.cardfail.2016.08.007.
44. Tallman TA, Peacock WF, Emerman CL, et al; ADHERE Registry. Noninvasive ventilation outcomes in 2,430 acute decompensated heart failure patients: an ADHERE registry analysis. Acad EM. 2008;15(4):355–362. doi:10.1111/j.1553-2712.2008.00059.x.
45. Pang D, Keenan SP, Cook DJ, Sibbald WJ. The effect of positive pressure airway support on mortality and the need for intubation in cardiogenic pulmonary edema: a systematic review. Chest. 1998;114(4):1185-1192.
46. Peter JV, Moran JL, Phillips-Hughes J, Graham P, Bersten AD. Effect of non-invasive positive pressure ventilation (NIPPV) on mortality in patients with acute cardiogenic pulmonary oedema: a meta-analysis. Lancet. 2006;367(9517):1155-1163.
47. Weng CL, Zhao YT, Liu QH, et al. Meta-analysis: Noninvasive ventilation in acute cardiogenic pulmonary edema. Ann Intern Med. 2010;152(9):590-600
48. Vital FM, Saconato H, Ladeira MT, et al. Noninvasive positive pressure ventilation (CPAP or bilevel NPPV) for cardiogenic pulmonary edema. Cochrane Database Syst Rev. 2008;(3):CD005351. doi:10.1002/14651858.CD005351.pub2.
49. Vital FM, Ladeira MT, Atallah AN. Non-invasive positive pressure ventilation (CPAP or bilevel NPPV) for cardiogenic pulmonary oedema. Cochrane Database Syst Rev. 2013;(5):CD005351. doi:10.1002/14651858.CD005351.pub3.
50. Gray A, Goodacre S, Seah M, Tilley S. Diuretic, opiate and nitrate use in severe acidotic acute cardiogenic pulmonary oedema: analysis from the 3CPO trial. QJM. 2010;103(8):573-581. doi:10.1093/qjmed/hcq077.
51. Collins SP, Storrow AB, Levy PD, et al. Early management of patients with acute heart failure: state of the art and future directions—a consensus document from the SAEM/HFSA acute heart failure working group. Acad Emerg Med. 2015;22(1):94-112. doi:10.1111/acem.12538.]
52. O’Connor CM, Gattis WA, Uretsky BF, et al. Continuous intravenous dobutamine is associated with an increased risk of death in patients with advanced heart failure: insights from the Flolan International Randomized Survival Trial (FIRST). Am Heart J. 1999;138(1 Pt 1):78-86.
53. Hershberger RE, Nauman D, Walker TL, Dutton D, Burgess D. Care processes and clinical outcomes of continuous outpatient support with inotropes (COSI) in patients with refractory endstage heart failure. J Card Fail. 2003;(9):180-187.
54. Gorodeski EZ, Chu EC, Reese JR, Shishehbor MH, Hsich E, Starling RC. Prognosis on chronic dobutamine or milrinone infusions for stage D heart failure. Circ Heart Fail. 2009;2(4):320-324. doi:10.1161/CIRCHEARTFAILURE.108.839076.
55. Collins SP, Levy PD, Martindale JL, et al. Clinical and research considerations for patients with hypertensive acute heart failure: A consensus statement from the Society for Academic Emergency Medicine and the Heart Failure Society of America Acute Heart Failure Working Group. Acad Emerg Med. 2016;23(8):922-931. doi:10.1111/acem.13025.
56. Felker GM, Lee KL, Bull DA, et al. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med. 2011; 364(9):797-805. doi:10.1056/NEJMoa1005419.

57. Publication Committee for the VMAC Investigators (Vasodilatation in the Management of Acute CHF). Intravenous nesiritide vs nitroglycerin for treatment of decompensated congestive heart failure: a randomized controlled trial. JAMA. 2002;287(12):1531-1540.
58. Gradman AH, Vekeman F, Eldar-Lissai A, Trahey A, Ong SH, Duh MS. Is addition of vasodilators to loop diuretics of value in the care of hospitalized acute heart failure patients? Real-world evidence from a retrospective analysis of a large United States hospital database. J Card Fail. 2014;20(11):853-863. doi:10.1016/j.cardfail.2014.08.006.
59. Wilson SS, Kwiatkowski GM, Millis SR, Purakal JD, Mahajan AP, Levy PD. Use of nitroglycerin by bolus prevents intensive care unit admission in patients with acute hypertensive heart failure. Am J Emerg Med. 2017;35(1):126-131. doi:10.1016/j.ajem.2016.10.038.
60. Eryonucu B, Guler N, Guntekin U, Tuncer M. Comparison of the effects of nitroglycerin and nitroprusside on transmitral Doppler flow parameters in patients with hypertensive urgency. Ann Pharmacother. 2005;39(6):997–1001.
61. O’Connor CM, Starling RC, Hernandez AF, et al. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med. 2011;365(1):32-43. doi:10.1056/NEJMoa1100171.
62. Peacock WF 4th, Holland R, Gyarmathy R, et al. Observation unit treatment of heart failure with nesiritide: results from the proaction trial. J Emerg Med. 2005;29(3):243-252.
63. Ayaz SI, Sharkey CM, Kwiatkowski GM, et al. Intravenous enalaprilat for treatment of acute hypertensive heart failure in the emergency department. Int J Emerg Med. 2016;9(1):28. doi:10.1186/s12245-016-0125-4.
64. Peacock WF 4th, Chandra A, Char D, et al. Clevidipine in acute heart failure: results of the A study of BP control in acute heart failure-a pilot study (PRONTO). Am Heart J. 2014;167(4):529-536. doi:10.1016/j.ahj.2013.12.023.
65. Peacock WF 4th, Hollander JE, Diercks DB, et al. Morphine and outcomes in acute decompensated heart failure: an ADHERE analysis. Emerg Med J. 2008;25(4):205-209. doi:10.1136/emj.2007.050419.
66. Miró Ò, Gil V, Martín-Sánchez FJ, Herrero-Puente P, Jet al; ICA-SEMES Research Group. Morphine use in the ED and outcomes of patients with acute heart failure: a propensity score-matching analysis based on the EAHFE registry. [published ahead of print April 12, 2017] Chest. pii:S0012-3692(17)30707-9. doi:10.1016/j.chest.2017.03.037.
67. Wuerz RC, Meador SA. Effects of prehospital medications on mortality and length of stay in congestive heart failure. Ann Emerg Med. 1992;21(6):669-674.
68. Peacock WF 4th, Fonarow GC, Emerman CL, Mills RM, Wynne J; ADHERE Scientific Advisory Committee and Investigators; Adhere Study Group. Impact of early initiation of intravenous therapy for acute decompensated heart failure on outcomes in ADHERE. Cardiology. 2007; 107(1):44-51. doi:10.1159/000093612.
69. Peacock WF, Emerman C, Costanzo MR, Diercks DB, Lopatin M, Fonarow GC. Early vasoactive drugs improve heart failure outcomes. Congest Heart Fail. 2009;15(6): 256-264. doi:10.1111/j.1751-7133.2009.00112.x.
70. Maisel AS, Peacock WF, McMullin N, et al. Timing of immunoreactive B-type natriuretic peptide levels and treatment delay in acute decompensated heart failure: an ADHERE (Acute Decompensated Heart Failure National Registry) analysis. J Am Coll Cardiol. 2008;52(7):534-540. doi:10.1016/j.jacc.2008.05.010.
71. Matsue Y, Damman K, Voors AA, et al. Time-to-Furosemide Treatment and Mortality in Patients Hospitalized With Acute Heart Failure. J Am Coll Cardiol. 2017 Jun 27;69(25):3042-3051. doi:10.1016/j.jacc.2017.04.042.
72. Claret PG, Stiell IG, Yan JW, et al. Hemodynamic, management, and outcomes of patients admitted to emergency department with heart failure. Scand J Trauma Resusc Emerg Med. 2016;24(1):132.
73. Stiell IG, Perry JJ, Clement CM, et al. Prospective and explicit clinical validation of the Ottawa Heart Failure Risk Scale, with and without use of quantitative NT-proBNP. Acad Emerg Med. 2017;24(3):316-327. doi:10.1111/acem.13141.
74. Pang PS, Jesse R, Collins SP, Maisel A. Patients with acute heart failure in the emergency department: do they all need to be admitted? J Card Fail. 2012;18:900-903. doi:10.1016/j.cardfail.2012.10.014.
75. Peacock WF 4th, Young J, Collins S, Emerman C, Diercks D. Heart failure observation units: optimizing care. Ann Emerg Med. 2006;47(1):22-33.
76. Storrow AB, Collins SP, Lyons MS, Wagoner LE, Gibler WB, Lindsell CJ. Emergency department observation of heart failure: preliminary analysis of safety and cost. Congest Heart Fail. 2005;11(2):68-72.
77. Peacock WF 4th, Remer EE, Aponte J, Moffa DA, Emerman CE, Albert NM. Effective observation unit treatment of decompensated heart failure. Congest Heart Fail. 2002;8(2):68 -73.
78. Peacock WF 4th, Albert NM. Observation unit management of heart failure. Emerg Med Clin North Am. 2001;19(1):209-232.
79. Miró O, Carbajosa V, Peacock WF 4th, et al; ICA-SEMES group. The effect of a short-stay unit on hospital admission and length of stay in acute heart failure: REDUCE-AHF study. Eur J Intern Med. 2017;40:30-36. doi:10.1016/j.ejim.2017.01.015.
80. Ekman I, Andersson B, Ehnfors M, Matejka G, Persson B, Fagerberg B. Feasibility of a nurse-monitored, outpatient-care programme for elderly patients with moderate-to-severe, chronic heart failure. Eur Heart J. 1998;19(8):1254-1260.
81. Riegel B, Carlson B, Kopp Z, LePetri B, Glaser D, Unger A. Effect of a standardized nurse case-management telephone intervention on resource use in patients with chronic heart failure. Arch Intern Med. 2002;162(6):705-712.
82. Laramee AS, Levinsky SK, Sargent J, Ross R, Callas P. Case management in a heterogeneous congestive heart failure population: a randomized controlled trial. Arch Intern Med. 2003;163(7):809-817.
83. Stewart S, Pearson S, Horowitz JD. Effects of a home-based intervention among patients with congestive heart failure discharged from acute hospital care. Arch Intern Med. 1998;158(10):1067-1072.
84. Stewart S, Marley JE, Horowitz JD. Effects of a multidisciplinary, home-based intervention on unplanned readmissions and survival among patients with chronic congestive heart failure: a randomised controlled study. Lancet. 1999;354(9184):1077-1083.
85. Weinberger M, Oddone EZ, Henderson WG; Veterans Affairs Cooperative Study Group. Does increased access to primary care reduce hospital readmissions? Veterans Affairs Cooperative Study Group on primary care and hospital readmission. N Engl J Med. 1996;334(22):1441-1447.
86. Asthana V, Sundararajan M, Karun V, et al. Educational strategy for management of heart failure markedly reduces 90-day emergency department and hospital readmissions in un- and underinsured patients. J Am Coll Cardiol. 2017;69(11Suppl): 780. doi:10.1016/S0735-1097(17)34169-4.
87. Bell SP, Schnipper JL, Goggins K, et al; Pharmacist Intervention for Low Literacy in Cardiovascular Disease (PILL-CVD) Study Group. Effect of pharmacist counseling intervention on health care utilization following hospital discharge: a randomized control trial. J Gen Intern Med. 2016;31(5):470-477. doi:10.1007/s11606-016-3596-3.
1. Benjamin EJ, Blaha MJ, Chiuve SE, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation. 2017;135(10):e146-e603.
2. Fitch KV, Engel T, Lauet J. The cost burden of worsening heart failure in the Medicare fee for service population: an actuarial analysis. Milliman Web site. 2017. http://www.milliman.com/insight/2017/The-cost-burden-of-worsening-heart-failure-in-the-Medicare-fee-for-service-population-An-actuarial-analysis/ Published April 3, 2017. Accessed June 1, 2017.
3. Yancy CW, Jessup M, Bozkurt B, et al; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013. J Am Coll Cardiol. 2013;62(16):e147-e239. doi:10.1016/j.jacc.2013.05.019.
4. Chen J, Normand SL, Wang Y, Krumholz HM. National and regional trends in heart failure hospitalization and mortality rates for Medicare beneficiaries, 1998-2008. JAMA. 2011;306(15):1669-1678. doi:10.1001/jama.2011.1474.
5. Heidenreich PA, Albert NM, Allen LA, et al; American Heart Association Advocacy Coordinating Committee; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Stroke Council. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6(3):606-619. doi:10.1161/HHF.0b013e318291329a.
6. Dunlay SM, Shah ND, Shi Q, et al. Lifetime costs of medical care after heart failure diagnosis. Circ Cardiovasc Qual Outcomes. 2011;4(1):68-75. doi:10.1161/CIRCOUTCOMES.110.957225.
7. HCUP National Inpatient Sample (NIS); 2014 Agency for Healthcare Research and Quality (AHRQ). HCUPnet Healthcare Cost and Utilization Project. http://bit.ly/2u2ymFA. Published 2014. Accessed July 27, 2017.
8. FY 2017 Final Rule and Correction Notice Tables. CMS 2014 data based on DRGs, Table 5:List of MS-DRGs, Relative Weighting Factors and Geometric and Arithmetic Mean Length of Stay. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/FY2017-IPPS-Final-Rule-Home-Page-Items/FY2017-IPPS-Final-Rule-Tables.html. Published 2017. Accessed August 28, 2017.
9. Hospital Adjusted Expenses per Inpatient Day by Ownership. Kaiser Family Foundation. http://www.kff.org/health-costs/state-indicator/expenses-per-inpatient-day-by-ownership/?currentTimeframe=0&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22asc%22%7D. Accessed August 2, 2017
10. Ellison, A. Average cost per inpatient day across 50 states. Becker’s Hospital CFO Report. http://www.beckershospitalreview.com/finance/average-cost-per-inpatient-day-across-50-states-2016.html. Published January 13, 2016. Accessed August 2, 2017
11. Claret PG, Calder LA, Stiell IG, et al. Rates and predictive factors of return to the emergency department following an initial release by the emergency department for acute heart failure. [published online ahead of print April 3, 2017]. CJEM. 1-8. doi:10.1017/cem.2017.14.
12. Yam FK, Lew T, Eraly SA, Lin HW, Hirsch JD, Devor M. Changes in medication regimen complexity and the risk for 90-day hospital readmission and/or emergency department visits in U.S. Veterans with heart failure. Res Social Adm Pharm. 2016;12(5):713-721. doi:10.1016/j.sapharm.2015.10.004.
13. Readmissions Reduction Program. CMS. https://www.cms.gov/medicare/medicare-fee-for-service-payment/acuteinpatientpps/readmissions-reduction-program.html Updated April 18, 2016. Accessed July 13, 2017.
14. Nowak RM, Reed BP, DiSomma S, et al. Presenting phenotypes of acute heart failure patients in the ED: Identification and implications. Am J Emerg Med. 2017;35(4):536-542. doi:10.1016/j.ajem.2016.12.003.
15. Bennett SJ, Huster GA, Baker SL, et al. Characterization of the precipitants of hospitalization for heart failure decompensation. Am J Crit Care. 1998;7(3):168-174.
16. Kuo DC, Peacock WF. Diagnosing and managing acute heart failure in the emergency department. Clin Exp Emerg Med. 2015;2(3):141-149. eCollection 2015 Sep. doi:10.15441/ceem.15.007.
17. Wang CS, FitzGerald JM, Schulzer M, Mak E, Ayas NT. Does this dyspneic patient in the emergency department have congestive heart failure? JAMA. 2005;294:1944-1956.
18. Koga T, Fujimoto K. Images in clinical medicine. Kerley’s A, B, and C line. N Engl J Med. 2009;360(15):1539. doi:10.1056/NEJMicm0708489.
19. Glöckner E, Christ M, Geier F, et al. Accuracy of point-of-care B-Line lung ultrasound in comparison to NT-ProBNP for screening acute heart failure. Ultrasound Int Open. 2016;2(3):e90-e92. doi:10.1055/s-0042-108343.
20. Bitar Z, Maadarani O, Almerri K. Sonographic chest B-lines anticipate elevated B-type natriuretic peptide level, irrespective of ejection fraction. Ann Intensive Care. 2015;5(1): 56. doi:10.1186/s13613-015-0100-x.
21. Miglioranza MH, Gargani L, Sant’Anna RT, et al. Lung ultrasound for the evaluation of pulmonary congestion in outpatients: a comparison with clinical assessment, natriuretic peptides, and echocardiography. JACC Cardiovasc Imaging. 2013;6(11):1141-1151. doi:10.1016/j.jcmg.2013.08.004.
22. Anderson KL, Jenq KY, Fields JM, Panebianco NL, Dean AJ. Diagnosing heart failure among acutely dyspneic patients with cardiac, inferior vena cava, and lung ultrasonography. Am J Emerg Med. 2013;31(8):1208-1214. doi:10.1016/j.ajem.2013.05.007.
23. Cubo-Romano P, Torres-Macho J, Soni NJ, et al. Admission inferior vena cava measurements are associated with mortality after hospitalization for acute decompensated heart failure. J Hosp Med. 2016;11(11):778-784. doi:10.1002/jhm.2620.
24. Martínez PG, Martínez DM, García JC, Loidi JC. Amino-terminal pro–B-type natriuretic peptide, inferior vena cava ultrasound, and bioelectrical impedance analysis for the diagnosis of acute decompensated CHF. Am J Emerg Med. 2016;34(9): 1817–1822. doi:10.1016/j.ajem.2016.06.043.

25. Maisel AS, Krishnaswamy P, Nowak RM, et al; Breathing Not Properly Multinational Study Investigators. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002;347(3):161-167.
26. Van Kimmenade RR, Pinto YM, Bayes-Genis A, Lainchbury JG, Richards AM, Januzzi JL Jr. Usefulness of intermediate amino-terminal pro-brain natriuretic peptide concentrations for diagnosis and prognosis of acute heart failure. Am J Cardiol. 2006;98(3):386-390.
27. Moe GW, Howlett J, Januzzi JL, Zowall H; Canadian Multicenter Improved Management of Patients With Congestive Heart Failure (IMPROVE-CHF) Study Investigators. N-terminal pro-B-type natriuretic peptide testing improves the management of patients with suspected acute heart failure: primary results of the Canadian prospective randomized multicenter IMPROVE-CHF study. Circulation. 2007;115(24):3103-3110.
28. Mayo DD, Colletti JE, Kuo DC. Brain natriuretic peptide (BNP) testing in the emergency department. J Emerg Med. 2006;31(2):201-210.
29. Mueller C, Scholer A, Laule-Kilian K, et al. Use of B-type natriuretic peptide in the evaluation and management of acute dyspnea. N Engl J Med. 2004;350(7):647-654.
30. Krauser DG, Lloyd-Jones DM, Chae CU, et al. Effect of body mass index on natriuretic peptide levels in patients with acute congestive heart failure: a ProBNP Investigation of Dyspnea in the Emergency Department (PRIDE) substudy. Am Heart J. 2005;149(4):744-750.
31. McCullough PA, Duc P, Omland T, et al. B-type natriuretic peptide and renal function in the diagnosis of heart failure: an analysis from the Breathing Not Properly Multinational Study. Am J Kidney Dis. 2003;41(3):571-579.
32. Jacob J, Roset A, Miró Ò, et al; ICA-SEMES Research Group. EAHFE - TROPICA2 study. Prognostic value of troponin in patients with acute heart failure treated in Spanish hospital emergency departments. Biomarkers. 2017;22(3-4):337-344. doi:10.1080/1354750X.2016.1265006.
33. Fonarow GC, Adams KF Jr, Abraham WT, et al; ADHERE Scientific Advisory Committee, Study Group, and Investigators. Risk stratification for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. JAMA. 2005;293(5):572-580.
34. Burkhardt J, Peacock WF, Emerman CL. Predictors of emergency department observation unit outcomes. Acad Emerg Med. 2005;12(9):869-874.
35. Schanz M, Shi J, Wasser C, Alscher MD, Kimmel M. Urinary [TIMP-2] × [IGFBP7] for risk prediction of acute kidney injury in decompensated heart failure. Clin Cardiol. 2017;40(7):485-491. doi:10.1002/clc.22683.
36. Kushner RF, Schoeller DA, Fjeld CR, Danford L: Is the impedance index (ht2/R) significant in predicting total body water? Am J Clin Nutr. 1992;56(5): 835-839.
37. Ackland GL, Singh-Ranger D, Fox S, et al. Assessment of preoperative fluid depletion using bioimpedance analysis. Br J Anaesth. 2004;92(1): 134-136.
38. Uszko-Lencer NH, Bothmer F, van Pol PE, Schols AM. Measuring body composition in chronic heart failure: a comparison of methods. Eur J Heart Fail. 2006;8(2): 208-214.
39. Santarelli S, Russo V, Lalle I, et al; GREAT network. Usefulness of combining admission brain natriuretic peptide (BNP) plus hospital discharge bioelectrical impedance vector analysis (BIVA) in predicting 90 days cardiovascular mortality in patients with acute heart failure. Intern Emerg Med. 2017;12(4):445-451. doi:10.1007/s11739-016-1581-9.
40. Parrinello G, Paterna S, Di Pasquale P, et al. The usefulness of bioelectrical impedance analysis in differentiating dyspnea due to decompensated heart failure. J Card Fail. 2008;14(8): 676-686. doi:10.1016/j.cardfail.2008.04.005.
41. Valle R, Aspromonte N, Carbonieri E, et al. Fall in readmission rate for heart failure after implementation of B-type natriuretic peptide testing for discharge decision: a retrospective study. Int J Cardiol. 2008;126(3): 400-406.
42. Sartini S, Frizzi J, Borselli M, et al. Which method is best for an early accurate diagnosis of acute heart failure? Comparison between lung ultrasound, chest X-ray and NT pro-BNP performance: a prospective study. [published online ahead of print July 11, 2016]. Intern Emerg Med. doi:10.1007/s11739-016-1498-3.
43. Steinhart BD, Levy P, Vandenberghe H, et al. A randomized control trial using a validated prediction model for diagnosing acute heart failure in undifferentiated dyspneic emergency department patients-results of the GASP4Ar study. J Card Fail. 2017;23(2):145-152. doi:10.1016/j.cardfail.2016.08.007.
44. Tallman TA, Peacock WF, Emerman CL, et al; ADHERE Registry. Noninvasive ventilation outcomes in 2,430 acute decompensated heart failure patients: an ADHERE registry analysis. Acad EM. 2008;15(4):355–362. doi:10.1111/j.1553-2712.2008.00059.x.
45. Pang D, Keenan SP, Cook DJ, Sibbald WJ. The effect of positive pressure airway support on mortality and the need for intubation in cardiogenic pulmonary edema: a systematic review. Chest. 1998;114(4):1185-1192.
46. Peter JV, Moran JL, Phillips-Hughes J, Graham P, Bersten AD. Effect of non-invasive positive pressure ventilation (NIPPV) on mortality in patients with acute cardiogenic pulmonary oedema: a meta-analysis. Lancet. 2006;367(9517):1155-1163.
47. Weng CL, Zhao YT, Liu QH, et al. Meta-analysis: Noninvasive ventilation in acute cardiogenic pulmonary edema. Ann Intern Med. 2010;152(9):590-600
48. Vital FM, Saconato H, Ladeira MT, et al. Noninvasive positive pressure ventilation (CPAP or bilevel NPPV) for cardiogenic pulmonary edema. Cochrane Database Syst Rev. 2008;(3):CD005351. doi:10.1002/14651858.CD005351.pub2.
49. Vital FM, Ladeira MT, Atallah AN. Non-invasive positive pressure ventilation (CPAP or bilevel NPPV) for cardiogenic pulmonary oedema. Cochrane Database Syst Rev. 2013;(5):CD005351. doi:10.1002/14651858.CD005351.pub3.
50. Gray A, Goodacre S, Seah M, Tilley S. Diuretic, opiate and nitrate use in severe acidotic acute cardiogenic pulmonary oedema: analysis from the 3CPO trial. QJM. 2010;103(8):573-581. doi:10.1093/qjmed/hcq077.
51. Collins SP, Storrow AB, Levy PD, et al. Early management of patients with acute heart failure: state of the art and future directions—a consensus document from the SAEM/HFSA acute heart failure working group. Acad Emerg Med. 2015;22(1):94-112. doi:10.1111/acem.12538.]
52. O’Connor CM, Gattis WA, Uretsky BF, et al. Continuous intravenous dobutamine is associated with an increased risk of death in patients with advanced heart failure: insights from the Flolan International Randomized Survival Trial (FIRST). Am Heart J. 1999;138(1 Pt 1):78-86.
53. Hershberger RE, Nauman D, Walker TL, Dutton D, Burgess D. Care processes and clinical outcomes of continuous outpatient support with inotropes (COSI) in patients with refractory endstage heart failure. J Card Fail. 2003;(9):180-187.
54. Gorodeski EZ, Chu EC, Reese JR, Shishehbor MH, Hsich E, Starling RC. Prognosis on chronic dobutamine or milrinone infusions for stage D heart failure. Circ Heart Fail. 2009;2(4):320-324. doi:10.1161/CIRCHEARTFAILURE.108.839076.
55. Collins SP, Levy PD, Martindale JL, et al. Clinical and research considerations for patients with hypertensive acute heart failure: A consensus statement from the Society for Academic Emergency Medicine and the Heart Failure Society of America Acute Heart Failure Working Group. Acad Emerg Med. 2016;23(8):922-931. doi:10.1111/acem.13025.
56. Felker GM, Lee KL, Bull DA, et al. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med. 2011; 364(9):797-805. doi:10.1056/NEJMoa1005419.

57. Publication Committee for the VMAC Investigators (Vasodilatation in the Management of Acute CHF). Intravenous nesiritide vs nitroglycerin for treatment of decompensated congestive heart failure: a randomized controlled trial. JAMA. 2002;287(12):1531-1540.
58. Gradman AH, Vekeman F, Eldar-Lissai A, Trahey A, Ong SH, Duh MS. Is addition of vasodilators to loop diuretics of value in the care of hospitalized acute heart failure patients? Real-world evidence from a retrospective analysis of a large United States hospital database. J Card Fail. 2014;20(11):853-863. doi:10.1016/j.cardfail.2014.08.006.
59. Wilson SS, Kwiatkowski GM, Millis SR, Purakal JD, Mahajan AP, Levy PD. Use of nitroglycerin by bolus prevents intensive care unit admission in patients with acute hypertensive heart failure. Am J Emerg Med. 2017;35(1):126-131. doi:10.1016/j.ajem.2016.10.038.
60. Eryonucu B, Guler N, Guntekin U, Tuncer M. Comparison of the effects of nitroglycerin and nitroprusside on transmitral Doppler flow parameters in patients with hypertensive urgency. Ann Pharmacother. 2005;39(6):997–1001.
61. O’Connor CM, Starling RC, Hernandez AF, et al. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med. 2011;365(1):32-43. doi:10.1056/NEJMoa1100171.
62. Peacock WF 4th, Holland R, Gyarmathy R, et al. Observation unit treatment of heart failure with nesiritide: results from the proaction trial. J Emerg Med. 2005;29(3):243-252.
63. Ayaz SI, Sharkey CM, Kwiatkowski GM, et al. Intravenous enalaprilat for treatment of acute hypertensive heart failure in the emergency department. Int J Emerg Med. 2016;9(1):28. doi:10.1186/s12245-016-0125-4.
64. Peacock WF 4th, Chandra A, Char D, et al. Clevidipine in acute heart failure: results of the A study of BP control in acute heart failure-a pilot study (PRONTO). Am Heart J. 2014;167(4):529-536. doi:10.1016/j.ahj.2013.12.023.
65. Peacock WF 4th, Hollander JE, Diercks DB, et al. Morphine and outcomes in acute decompensated heart failure: an ADHERE analysis. Emerg Med J. 2008;25(4):205-209. doi:10.1136/emj.2007.050419.
66. Miró Ò, Gil V, Martín-Sánchez FJ, Herrero-Puente P, Jet al; ICA-SEMES Research Group. Morphine use in the ED and outcomes of patients with acute heart failure: a propensity score-matching analysis based on the EAHFE registry. [published ahead of print April 12, 2017] Chest. pii:S0012-3692(17)30707-9. doi:10.1016/j.chest.2017.03.037.
67. Wuerz RC, Meador SA. Effects of prehospital medications on mortality and length of stay in congestive heart failure. Ann Emerg Med. 1992;21(6):669-674.
68. Peacock WF 4th, Fonarow GC, Emerman CL, Mills RM, Wynne J; ADHERE Scientific Advisory Committee and Investigators; Adhere Study Group. Impact of early initiation of intravenous therapy for acute decompensated heart failure on outcomes in ADHERE. Cardiology. 2007; 107(1):44-51. doi:10.1159/000093612.
69. Peacock WF, Emerman C, Costanzo MR, Diercks DB, Lopatin M, Fonarow GC. Early vasoactive drugs improve heart failure outcomes. Congest Heart Fail. 2009;15(6): 256-264. doi:10.1111/j.1751-7133.2009.00112.x.
70. Maisel AS, Peacock WF, McMullin N, et al. Timing of immunoreactive B-type natriuretic peptide levels and treatment delay in acute decompensated heart failure: an ADHERE (Acute Decompensated Heart Failure National Registry) analysis. J Am Coll Cardiol. 2008;52(7):534-540. doi:10.1016/j.jacc.2008.05.010.
71. Matsue Y, Damman K, Voors AA, et al. Time-to-Furosemide Treatment and Mortality in Patients Hospitalized With Acute Heart Failure. J Am Coll Cardiol. 2017 Jun 27;69(25):3042-3051. doi:10.1016/j.jacc.2017.04.042.
72. Claret PG, Stiell IG, Yan JW, et al. Hemodynamic, management, and outcomes of patients admitted to emergency department with heart failure. Scand J Trauma Resusc Emerg Med. 2016;24(1):132.
73. Stiell IG, Perry JJ, Clement CM, et al. Prospective and explicit clinical validation of the Ottawa Heart Failure Risk Scale, with and without use of quantitative NT-proBNP. Acad Emerg Med. 2017;24(3):316-327. doi:10.1111/acem.13141.
74. Pang PS, Jesse R, Collins SP, Maisel A. Patients with acute heart failure in the emergency department: do they all need to be admitted? J Card Fail. 2012;18:900-903. doi:10.1016/j.cardfail.2012.10.014.
75. Peacock WF 4th, Young J, Collins S, Emerman C, Diercks D. Heart failure observation units: optimizing care. Ann Emerg Med. 2006;47(1):22-33.
76. Storrow AB, Collins SP, Lyons MS, Wagoner LE, Gibler WB, Lindsell CJ. Emergency department observation of heart failure: preliminary analysis of safety and cost. Congest Heart Fail. 2005;11(2):68-72.
77. Peacock WF 4th, Remer EE, Aponte J, Moffa DA, Emerman CE, Albert NM. Effective observation unit treatment of decompensated heart failure. Congest Heart Fail. 2002;8(2):68 -73.
78. Peacock WF 4th, Albert NM. Observation unit management of heart failure. Emerg Med Clin North Am. 2001;19(1):209-232.
79. Miró O, Carbajosa V, Peacock WF 4th, et al; ICA-SEMES group. The effect of a short-stay unit on hospital admission and length of stay in acute heart failure: REDUCE-AHF study. Eur J Intern Med. 2017;40:30-36. doi:10.1016/j.ejim.2017.01.015.
80. Ekman I, Andersson B, Ehnfors M, Matejka G, Persson B, Fagerberg B. Feasibility of a nurse-monitored, outpatient-care programme for elderly patients with moderate-to-severe, chronic heart failure. Eur Heart J. 1998;19(8):1254-1260.
81. Riegel B, Carlson B, Kopp Z, LePetri B, Glaser D, Unger A. Effect of a standardized nurse case-management telephone intervention on resource use in patients with chronic heart failure. Arch Intern Med. 2002;162(6):705-712.
82. Laramee AS, Levinsky SK, Sargent J, Ross R, Callas P. Case management in a heterogeneous congestive heart failure population: a randomized controlled trial. Arch Intern Med. 2003;163(7):809-817.
83. Stewart S, Pearson S, Horowitz JD. Effects of a home-based intervention among patients with congestive heart failure discharged from acute hospital care. Arch Intern Med. 1998;158(10):1067-1072.
84. Stewart S, Marley JE, Horowitz JD. Effects of a multidisciplinary, home-based intervention on unplanned readmissions and survival among patients with chronic congestive heart failure: a randomised controlled study. Lancet. 1999;354(9184):1077-1083.
85. Weinberger M, Oddone EZ, Henderson WG; Veterans Affairs Cooperative Study Group. Does increased access to primary care reduce hospital readmissions? Veterans Affairs Cooperative Study Group on primary care and hospital readmission. N Engl J Med. 1996;334(22):1441-1447.
86. Asthana V, Sundararajan M, Karun V, et al. Educational strategy for management of heart failure markedly reduces 90-day emergency department and hospital readmissions in un- and underinsured patients. J Am Coll Cardiol. 2017;69(11Suppl): 780. doi:10.1016/S0735-1097(17)34169-4.
87. Bell SP, Schnipper JL, Goggins K, et al; Pharmacist Intervention for Low Literacy in Cardiovascular Disease (PILL-CVD) Study Group. Effect of pharmacist counseling intervention on health care utilization following hospital discharge: a randomized control trial. J Gen Intern Med. 2016;31(5):470-477. doi:10.1007/s11606-016-3596-3.
First EDition: New Assay Helps Distinguish Viral and Bacterial Infections in Children, more
New Assay Helps Distinguish Viral and Bacterial Infections in Children
BY IAN LACY
An assay testing the presence of three blood-borne host-proteins shows promise in accurately identifying viral and bacterial infections in febrile children, a validation study found.
The three proteins that the ImmunoXpert assay uses to differentiate between viral and bacterial infections are: viral-induced tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), interferon gamma-induced protein-10 (IP-10), and bacterial-induced C-reactive protein (CRP). While TRAIL and IP-10 are novel identifiers, CRP has been used in traditional bacterial detecting assays, Isaac Srugo, MD, and colleagues reported.
The investigators identified 597 potential stored patient serum samples from patients admitted to multiple pediatric EDs and wards in Israel and Switzerland, and ultimately, 361 samples were selected for assay testing.1
Of the 361 patients whose samples were selected for testing, the assay identified 209 patients (58%) with a viral infection, 99 patients (27%) with a bacterial infection, and the remaining 53 patients (15%) with an equivocal outcome, according to Dr Srugo of the Ruth and Bruce Rappaport Faculty of Medicine, Technion-Israel Institute of Technology, Haifa, Israel, and his colleagues. The 307 patients with a bacterial or viral diagnosis had sensitivity of 93.8% (95% confidence interval [CI], 87.8%-99.8%) and specificity of 89.8% (CI, 85.6%-94.0%). There were four false-negative and 21 false-positive findings.
The study found that TRAIL and IP-10 were present in higher levels in children with viral infections compared to children with bacterial infections. The opposite was true of CRP results, with significantly lower levels of CRP in children with viral infections compared to those in children with bacterial infections.
“Notably, among the indeterminate diagnosis patients without a reference standard, the assay gave a bacterial or viral outcome for 69% of the cases (the rest were equivocal), with half of these yielding a score associated with a particularly high degree of assay diagnostic confidence,” investigators said. “This finding suggests that the assay may be applicable to ‘harder-to-diagnose’ cases in real-life clinical settings.”
Also, the assay “exhibits consistent performance across a wide range of ages [3 months to 18 years], time from symptom onset, and clinical syndromes,” Dr Srugo and his associates said.
1. Srugo I, Klein A, Stein M, et al. Validation of a novel assay to distinguish bacterial and viral infections. Pediatrics. 2017 Sep 13. doi:10.1542/peds.2016-3453 [Epub ahead of print]
Emergency Medicine Associate Editor-in-Chief, Francis L. Counselman, MD, to Receive ACEP Award for Outstanding Contribution in Education
Francis L. Counselman, MD, Associate Editor-in-Chief of Emergency Medicine will receive the 2017 American College of Emergency Physicians (ACEP) Award for Outstanding Contribution in Education, at the ACEP Scientific Assembly this month. This award is “presented to an ACEP member who has made a significant contribution to the educational aspects of the specialty,” and is the highest award ACEP gives annually regarding education and teaching.
Dr. Counselman is the founding Chairman of the Department of Emergency Medicine at Eastern Virginia Medical School (EVMS) in Norfolk, Virginia. Founded in 1992, he continues to serve as Chairman. He served as Program Director of the Emergency Medicine residency program at EVMS from 1990 to 2010. In 1999, he was made the EVMS Distinguished Professor of Emergency Medicine. He is the recipient of numerous teaching awards, including the Accreditation Council for Graduate Medical Education (ACGME) Parker Palmer “Courage to Teach” Award; the Emergency Medicine Residents’ Association (EMRA) Residency Director of the Year Award; and the EVMS Award for Outstanding Faculty Achievement. He has served as President of the Association of Academic Chairs of Emergency Medicine (AACEM) and the American Board of Emergency Medicine (ABEM). He is also a frequently invited speaker at state and national meetings. Dr Counselman has served on the Editorial Board of Emergency Medicine since 1999; and has been the Associate Editor-in-Chief since 2006.
Soccer-Playing Girls Five Times More Likely to Return to Same-Day Play After Concussion
BY KARI OAKES
Soccer-playing girls were five times more likely to return to play on the day of a concussion as were their male peers, according to a study presented at the annual meeting of the American Academy of Pediatrics.
Records from 87 soccer players aged 7 to 18 years (median age, 14 years) were examined in a retrospective review of patients seen over a 2-year period by a single physician at a pediatric sports medicine center. Of these, two thirds (n = 58) were girls.
Thirty of the 58 girls (51.7%) reported that they returned to play on the day of their concussion, compared to five of 29 boys (17.2%; P = .002). This difference in reporting yielded an odds ratio of 5.14 (95% CI, 1.72-15.3) for girls returning to same-day play, compared with boys who sustained a concussion.
The soccer players included children participating in recreational, club, and school-sponsored soccer, said senior author Shane M. Miller, MD, in an interview. All patients were assessed according to a standardized concussion protocol that involved a neurological examination and validated concussion evaluation tests, including the Immediate Post-Concussion Assessment and Cognitive Test and the Sports Concussion Assessment Tool.
As soccer has grown in popularity as a youth sport, so has the number of reported concussions. “The incidence of reported concussions has increased 1,600% from 1990 to 2014,” wrote Dr Miller and his coauthors in the abstract accompanying the presentation. Dr Miller said that girls are 1.5 times more likely than boys to sustain a concussion while playing soccer.
While seeing the patients who were the subject of the study, Dr Miller realized that most of the soccer players had not come out of play for evaluation after the head impact. Rather, they had continued to play, only later reporting concussion symptoms to coaches, trainers, or parents.
“The athletes may have chosen not to say anything because they didn’t want to come out of the game,” said Dr Miller, a sports medicine physician at Texas Scottish Rite Hospital for Children, Dallas.
“I was surprised by the significant degree of difference” between male and female soccer players, said Dr Miller. The study was not designed to get at the reason for the discrepancy, so Dr Miller could not say with certainty whether awareness of concussion symptoms is significantly lower for female athletes, or whether the athletic culture more strongly encourages minimization of symptoms for girls than boys. In any case, he said, there is room for education of players, coaches, and families to raise awareness of the importance of recognizing and reporting concussion, and then removing the affected athlete from play,
Dr Miller said that future research directions include collaboration with other facilities to conduct prospective research using a concussion registry. This will allow more robust statistical analysis and help ascertain the degree of regional variation in pediatric sports concussion management.
“Current education efforts may not be enough to help athletes, parents, and coaches identify concussion symptoms, know the guidelines for immediate removal from play, and understand the risks of returning to play after an injury. More research is needed on how to better spread this message intended to protect the health of young athletes….” Aaron Zynda, the study’s first author and clinical research coordinator at Texas Scottish Rite, said in a press release accompanying the abstract. “Concussion recognition and identification is a team effort,” he said.
Opioid Management Protocol Reduced Trauma Patient Pain Medication Use
BY ELI ZIMMERMAN
A pain management protocol implemented in a trauma service reduced opioid intake in trauma patients while improving patient satisfaction, according to a retrospective study.
The opioid epidemic continues to grow every day, partly as a result of irresponsible overprescribing of opioid medication, according to Jessica Gross, MB BAO BCh, a trauma surgeon from Wake Forest Baptist Health, North Carolina, at the American Association for the Surgery of Trauma annual meeting. Dr Gross and her colleagues developed a pain management protocol (PMP) to provide adequate pain control while using fewer opioids in the postdischarge setting. They tested their PMP through a retrospective chart review of 498 patients admitted to the trauma service between January 2015 and December 2016, half of whom were admitted before the PMP was initiated and half of whom were admitted afterward.
The PMP involved a stepped approach to treating pain, with acetaminophen or ibuprofen as needed for mild pain, one 5-mg tablet of oxycodone/acetaminophen every 6 hours for moderate pain, two tablets for severe pain, and 50 to 100 mg of tramadol every 6 hours for breakthrough pain.
Counseling services for patients who were found to be in danger of substance abuse were provided in the hospital, and at discharge, patients received a weaning plan for their medication, according to Dr Gross.
If the short-acting medications were found to be inadequate to control pain, patients were given slow-release pain medication as needed.
The average total medication, which included medication given at discharge and for refills, prescribed after PMP initiation was 1,242 morphine milligram equivalents (MME), compared with 2,421 MME prior to implementation of the protocol (P < .0001).
After the protocol was implemented, Dr Gross and her colleagues found the number of patients for whom a refill was prescribed dropped from 39.7% to 28.1%, and the size of those refills dropped from 1,032 MME to 213 MME on average.
“By having a comprehensive pain management protocol, we can reduce the amount of pain medications we prescribe for outpatient use, after discharge from the trauma service,” said Dr Gross, “…not only by decreasing the number of refills we were providing, but also the amount of pain medications that was prescribed within these refills.”
A Press Ganey survey analysis of patients during the month before and the month after the PMP implementation, found a significant increase in overall patient satisfaction and satisfaction with pain management, according to Dr Gross.
Certain limitations include not being able to confirm whether patients received prescription medication elsewhere, nor any concrete data on patient satisfaction after discharge other than an inference based on fewer refills and lower refill MME.
New Assay Helps Distinguish Viral and Bacterial Infections in Children
BY IAN LACY
An assay testing the presence of three blood-borne host-proteins shows promise in accurately identifying viral and bacterial infections in febrile children, a validation study found.
The three proteins that the ImmunoXpert assay uses to differentiate between viral and bacterial infections are: viral-induced tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), interferon gamma-induced protein-10 (IP-10), and bacterial-induced C-reactive protein (CRP). While TRAIL and IP-10 are novel identifiers, CRP has been used in traditional bacterial detecting assays, Isaac Srugo, MD, and colleagues reported.
The investigators identified 597 potential stored patient serum samples from patients admitted to multiple pediatric EDs and wards in Israel and Switzerland, and ultimately, 361 samples were selected for assay testing.1
Of the 361 patients whose samples were selected for testing, the assay identified 209 patients (58%) with a viral infection, 99 patients (27%) with a bacterial infection, and the remaining 53 patients (15%) with an equivocal outcome, according to Dr Srugo of the Ruth and Bruce Rappaport Faculty of Medicine, Technion-Israel Institute of Technology, Haifa, Israel, and his colleagues. The 307 patients with a bacterial or viral diagnosis had sensitivity of 93.8% (95% confidence interval [CI], 87.8%-99.8%) and specificity of 89.8% (CI, 85.6%-94.0%). There were four false-negative and 21 false-positive findings.
The study found that TRAIL and IP-10 were present in higher levels in children with viral infections compared to children with bacterial infections. The opposite was true of CRP results, with significantly lower levels of CRP in children with viral infections compared to those in children with bacterial infections.
“Notably, among the indeterminate diagnosis patients without a reference standard, the assay gave a bacterial or viral outcome for 69% of the cases (the rest were equivocal), with half of these yielding a score associated with a particularly high degree of assay diagnostic confidence,” investigators said. “This finding suggests that the assay may be applicable to ‘harder-to-diagnose’ cases in real-life clinical settings.”
Also, the assay “exhibits consistent performance across a wide range of ages [3 months to 18 years], time from symptom onset, and clinical syndromes,” Dr Srugo and his associates said.
1. Srugo I, Klein A, Stein M, et al. Validation of a novel assay to distinguish bacterial and viral infections. Pediatrics. 2017 Sep 13. doi:10.1542/peds.2016-3453 [Epub ahead of print]
Emergency Medicine Associate Editor-in-Chief, Francis L. Counselman, MD, to Receive ACEP Award for Outstanding Contribution in Education
Francis L. Counselman, MD, Associate Editor-in-Chief of Emergency Medicine will receive the 2017 American College of Emergency Physicians (ACEP) Award for Outstanding Contribution in Education, at the ACEP Scientific Assembly this month. This award is “presented to an ACEP member who has made a significant contribution to the educational aspects of the specialty,” and is the highest award ACEP gives annually regarding education and teaching.
Dr. Counselman is the founding Chairman of the Department of Emergency Medicine at Eastern Virginia Medical School (EVMS) in Norfolk, Virginia. Founded in 1992, he continues to serve as Chairman. He served as Program Director of the Emergency Medicine residency program at EVMS from 1990 to 2010. In 1999, he was made the EVMS Distinguished Professor of Emergency Medicine. He is the recipient of numerous teaching awards, including the Accreditation Council for Graduate Medical Education (ACGME) Parker Palmer “Courage to Teach” Award; the Emergency Medicine Residents’ Association (EMRA) Residency Director of the Year Award; and the EVMS Award for Outstanding Faculty Achievement. He has served as President of the Association of Academic Chairs of Emergency Medicine (AACEM) and the American Board of Emergency Medicine (ABEM). He is also a frequently invited speaker at state and national meetings. Dr Counselman has served on the Editorial Board of Emergency Medicine since 1999; and has been the Associate Editor-in-Chief since 2006.
Soccer-Playing Girls Five Times More Likely to Return to Same-Day Play After Concussion
BY KARI OAKES
Soccer-playing girls were five times more likely to return to play on the day of a concussion as were their male peers, according to a study presented at the annual meeting of the American Academy of Pediatrics.
Records from 87 soccer players aged 7 to 18 years (median age, 14 years) were examined in a retrospective review of patients seen over a 2-year period by a single physician at a pediatric sports medicine center. Of these, two thirds (n = 58) were girls.
Thirty of the 58 girls (51.7%) reported that they returned to play on the day of their concussion, compared to five of 29 boys (17.2%; P = .002). This difference in reporting yielded an odds ratio of 5.14 (95% CI, 1.72-15.3) for girls returning to same-day play, compared with boys who sustained a concussion.
The soccer players included children participating in recreational, club, and school-sponsored soccer, said senior author Shane M. Miller, MD, in an interview. All patients were assessed according to a standardized concussion protocol that involved a neurological examination and validated concussion evaluation tests, including the Immediate Post-Concussion Assessment and Cognitive Test and the Sports Concussion Assessment Tool.
As soccer has grown in popularity as a youth sport, so has the number of reported concussions. “The incidence of reported concussions has increased 1,600% from 1990 to 2014,” wrote Dr Miller and his coauthors in the abstract accompanying the presentation. Dr Miller said that girls are 1.5 times more likely than boys to sustain a concussion while playing soccer.
While seeing the patients who were the subject of the study, Dr Miller realized that most of the soccer players had not come out of play for evaluation after the head impact. Rather, they had continued to play, only later reporting concussion symptoms to coaches, trainers, or parents.
“The athletes may have chosen not to say anything because they didn’t want to come out of the game,” said Dr Miller, a sports medicine physician at Texas Scottish Rite Hospital for Children, Dallas.
“I was surprised by the significant degree of difference” between male and female soccer players, said Dr Miller. The study was not designed to get at the reason for the discrepancy, so Dr Miller could not say with certainty whether awareness of concussion symptoms is significantly lower for female athletes, or whether the athletic culture more strongly encourages minimization of symptoms for girls than boys. In any case, he said, there is room for education of players, coaches, and families to raise awareness of the importance of recognizing and reporting concussion, and then removing the affected athlete from play,
Dr Miller said that future research directions include collaboration with other facilities to conduct prospective research using a concussion registry. This will allow more robust statistical analysis and help ascertain the degree of regional variation in pediatric sports concussion management.
“Current education efforts may not be enough to help athletes, parents, and coaches identify concussion symptoms, know the guidelines for immediate removal from play, and understand the risks of returning to play after an injury. More research is needed on how to better spread this message intended to protect the health of young athletes….” Aaron Zynda, the study’s first author and clinical research coordinator at Texas Scottish Rite, said in a press release accompanying the abstract. “Concussion recognition and identification is a team effort,” he said.
Opioid Management Protocol Reduced Trauma Patient Pain Medication Use
BY ELI ZIMMERMAN
A pain management protocol implemented in a trauma service reduced opioid intake in trauma patients while improving patient satisfaction, according to a retrospective study.
The opioid epidemic continues to grow every day, partly as a result of irresponsible overprescribing of opioid medication, according to Jessica Gross, MB BAO BCh, a trauma surgeon from Wake Forest Baptist Health, North Carolina, at the American Association for the Surgery of Trauma annual meeting. Dr Gross and her colleagues developed a pain management protocol (PMP) to provide adequate pain control while using fewer opioids in the postdischarge setting. They tested their PMP through a retrospective chart review of 498 patients admitted to the trauma service between January 2015 and December 2016, half of whom were admitted before the PMP was initiated and half of whom were admitted afterward.
The PMP involved a stepped approach to treating pain, with acetaminophen or ibuprofen as needed for mild pain, one 5-mg tablet of oxycodone/acetaminophen every 6 hours for moderate pain, two tablets for severe pain, and 50 to 100 mg of tramadol every 6 hours for breakthrough pain.
Counseling services for patients who were found to be in danger of substance abuse were provided in the hospital, and at discharge, patients received a weaning plan for their medication, according to Dr Gross.
If the short-acting medications were found to be inadequate to control pain, patients were given slow-release pain medication as needed.
The average total medication, which included medication given at discharge and for refills, prescribed after PMP initiation was 1,242 morphine milligram equivalents (MME), compared with 2,421 MME prior to implementation of the protocol (P < .0001).
After the protocol was implemented, Dr Gross and her colleagues found the number of patients for whom a refill was prescribed dropped from 39.7% to 28.1%, and the size of those refills dropped from 1,032 MME to 213 MME on average.
“By having a comprehensive pain management protocol, we can reduce the amount of pain medications we prescribe for outpatient use, after discharge from the trauma service,” said Dr Gross, “…not only by decreasing the number of refills we were providing, but also the amount of pain medications that was prescribed within these refills.”
A Press Ganey survey analysis of patients during the month before and the month after the PMP implementation, found a significant increase in overall patient satisfaction and satisfaction with pain management, according to Dr Gross.
Certain limitations include not being able to confirm whether patients received prescription medication elsewhere, nor any concrete data on patient satisfaction after discharge other than an inference based on fewer refills and lower refill MME.
New Assay Helps Distinguish Viral and Bacterial Infections in Children
BY IAN LACY
An assay testing the presence of three blood-borne host-proteins shows promise in accurately identifying viral and bacterial infections in febrile children, a validation study found.
The three proteins that the ImmunoXpert assay uses to differentiate between viral and bacterial infections are: viral-induced tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), interferon gamma-induced protein-10 (IP-10), and bacterial-induced C-reactive protein (CRP). While TRAIL and IP-10 are novel identifiers, CRP has been used in traditional bacterial detecting assays, Isaac Srugo, MD, and colleagues reported.
The investigators identified 597 potential stored patient serum samples from patients admitted to multiple pediatric EDs and wards in Israel and Switzerland, and ultimately, 361 samples were selected for assay testing.1
Of the 361 patients whose samples were selected for testing, the assay identified 209 patients (58%) with a viral infection, 99 patients (27%) with a bacterial infection, and the remaining 53 patients (15%) with an equivocal outcome, according to Dr Srugo of the Ruth and Bruce Rappaport Faculty of Medicine, Technion-Israel Institute of Technology, Haifa, Israel, and his colleagues. The 307 patients with a bacterial or viral diagnosis had sensitivity of 93.8% (95% confidence interval [CI], 87.8%-99.8%) and specificity of 89.8% (CI, 85.6%-94.0%). There were four false-negative and 21 false-positive findings.
The study found that TRAIL and IP-10 were present in higher levels in children with viral infections compared to children with bacterial infections. The opposite was true of CRP results, with significantly lower levels of CRP in children with viral infections compared to those in children with bacterial infections.
“Notably, among the indeterminate diagnosis patients without a reference standard, the assay gave a bacterial or viral outcome for 69% of the cases (the rest were equivocal), with half of these yielding a score associated with a particularly high degree of assay diagnostic confidence,” investigators said. “This finding suggests that the assay may be applicable to ‘harder-to-diagnose’ cases in real-life clinical settings.”
Also, the assay “exhibits consistent performance across a wide range of ages [3 months to 18 years], time from symptom onset, and clinical syndromes,” Dr Srugo and his associates said.
1. Srugo I, Klein A, Stein M, et al. Validation of a novel assay to distinguish bacterial and viral infections. Pediatrics. 2017 Sep 13. doi:10.1542/peds.2016-3453 [Epub ahead of print]
Emergency Medicine Associate Editor-in-Chief, Francis L. Counselman, MD, to Receive ACEP Award for Outstanding Contribution in Education
Francis L. Counselman, MD, Associate Editor-in-Chief of Emergency Medicine will receive the 2017 American College of Emergency Physicians (ACEP) Award for Outstanding Contribution in Education, at the ACEP Scientific Assembly this month. This award is “presented to an ACEP member who has made a significant contribution to the educational aspects of the specialty,” and is the highest award ACEP gives annually regarding education and teaching.
Dr. Counselman is the founding Chairman of the Department of Emergency Medicine at Eastern Virginia Medical School (EVMS) in Norfolk, Virginia. Founded in 1992, he continues to serve as Chairman. He served as Program Director of the Emergency Medicine residency program at EVMS from 1990 to 2010. In 1999, he was made the EVMS Distinguished Professor of Emergency Medicine. He is the recipient of numerous teaching awards, including the Accreditation Council for Graduate Medical Education (ACGME) Parker Palmer “Courage to Teach” Award; the Emergency Medicine Residents’ Association (EMRA) Residency Director of the Year Award; and the EVMS Award for Outstanding Faculty Achievement. He has served as President of the Association of Academic Chairs of Emergency Medicine (AACEM) and the American Board of Emergency Medicine (ABEM). He is also a frequently invited speaker at state and national meetings. Dr Counselman has served on the Editorial Board of Emergency Medicine since 1999; and has been the Associate Editor-in-Chief since 2006.
Soccer-Playing Girls Five Times More Likely to Return to Same-Day Play After Concussion
BY KARI OAKES
Soccer-playing girls were five times more likely to return to play on the day of a concussion as were their male peers, according to a study presented at the annual meeting of the American Academy of Pediatrics.
Records from 87 soccer players aged 7 to 18 years (median age, 14 years) were examined in a retrospective review of patients seen over a 2-year period by a single physician at a pediatric sports medicine center. Of these, two thirds (n = 58) were girls.
Thirty of the 58 girls (51.7%) reported that they returned to play on the day of their concussion, compared to five of 29 boys (17.2%; P = .002). This difference in reporting yielded an odds ratio of 5.14 (95% CI, 1.72-15.3) for girls returning to same-day play, compared with boys who sustained a concussion.
The soccer players included children participating in recreational, club, and school-sponsored soccer, said senior author Shane M. Miller, MD, in an interview. All patients were assessed according to a standardized concussion protocol that involved a neurological examination and validated concussion evaluation tests, including the Immediate Post-Concussion Assessment and Cognitive Test and the Sports Concussion Assessment Tool.
As soccer has grown in popularity as a youth sport, so has the number of reported concussions. “The incidence of reported concussions has increased 1,600% from 1990 to 2014,” wrote Dr Miller and his coauthors in the abstract accompanying the presentation. Dr Miller said that girls are 1.5 times more likely than boys to sustain a concussion while playing soccer.
While seeing the patients who were the subject of the study, Dr Miller realized that most of the soccer players had not come out of play for evaluation after the head impact. Rather, they had continued to play, only later reporting concussion symptoms to coaches, trainers, or parents.
“The athletes may have chosen not to say anything because they didn’t want to come out of the game,” said Dr Miller, a sports medicine physician at Texas Scottish Rite Hospital for Children, Dallas.
“I was surprised by the significant degree of difference” between male and female soccer players, said Dr Miller. The study was not designed to get at the reason for the discrepancy, so Dr Miller could not say with certainty whether awareness of concussion symptoms is significantly lower for female athletes, or whether the athletic culture more strongly encourages minimization of symptoms for girls than boys. In any case, he said, there is room for education of players, coaches, and families to raise awareness of the importance of recognizing and reporting concussion, and then removing the affected athlete from play,
Dr Miller said that future research directions include collaboration with other facilities to conduct prospective research using a concussion registry. This will allow more robust statistical analysis and help ascertain the degree of regional variation in pediatric sports concussion management.
“Current education efforts may not be enough to help athletes, parents, and coaches identify concussion symptoms, know the guidelines for immediate removal from play, and understand the risks of returning to play after an injury. More research is needed on how to better spread this message intended to protect the health of young athletes….” Aaron Zynda, the study’s first author and clinical research coordinator at Texas Scottish Rite, said in a press release accompanying the abstract. “Concussion recognition and identification is a team effort,” he said.
Opioid Management Protocol Reduced Trauma Patient Pain Medication Use
BY ELI ZIMMERMAN
A pain management protocol implemented in a trauma service reduced opioid intake in trauma patients while improving patient satisfaction, according to a retrospective study.
The opioid epidemic continues to grow every day, partly as a result of irresponsible overprescribing of opioid medication, according to Jessica Gross, MB BAO BCh, a trauma surgeon from Wake Forest Baptist Health, North Carolina, at the American Association for the Surgery of Trauma annual meeting. Dr Gross and her colleagues developed a pain management protocol (PMP) to provide adequate pain control while using fewer opioids in the postdischarge setting. They tested their PMP through a retrospective chart review of 498 patients admitted to the trauma service between January 2015 and December 2016, half of whom were admitted before the PMP was initiated and half of whom were admitted afterward.
The PMP involved a stepped approach to treating pain, with acetaminophen or ibuprofen as needed for mild pain, one 5-mg tablet of oxycodone/acetaminophen every 6 hours for moderate pain, two tablets for severe pain, and 50 to 100 mg of tramadol every 6 hours for breakthrough pain.
Counseling services for patients who were found to be in danger of substance abuse were provided in the hospital, and at discharge, patients received a weaning plan for their medication, according to Dr Gross.
If the short-acting medications were found to be inadequate to control pain, patients were given slow-release pain medication as needed.
The average total medication, which included medication given at discharge and for refills, prescribed after PMP initiation was 1,242 morphine milligram equivalents (MME), compared with 2,421 MME prior to implementation of the protocol (P < .0001).
After the protocol was implemented, Dr Gross and her colleagues found the number of patients for whom a refill was prescribed dropped from 39.7% to 28.1%, and the size of those refills dropped from 1,032 MME to 213 MME on average.
“By having a comprehensive pain management protocol, we can reduce the amount of pain medications we prescribe for outpatient use, after discharge from the trauma service,” said Dr Gross, “…not only by decreasing the number of refills we were providing, but also the amount of pain medications that was prescribed within these refills.”
A Press Ganey survey analysis of patients during the month before and the month after the PMP implementation, found a significant increase in overall patient satisfaction and satisfaction with pain management, according to Dr Gross.
Certain limitations include not being able to confirm whether patients received prescription medication elsewhere, nor any concrete data on patient satisfaction after discharge other than an inference based on fewer refills and lower refill MME.
Oh No, Not Again!
Between August 30 and September 16, 2017, Hurricane Irma wreaked havoc in the Caribbean and throughout Florida. In the days after the Rehabilitation Center at Hollywood Hills, Florida, lost its transformer and air conditioning due to the storm, 12 residents ranging in age from 57 to 99 years died* with body temperatures as high as 109.9°F, even though the hospital across the street continued to have full power. The nursing home staff tried in vain several times to get Florida Power and Light to restore full power. They also called a “personal” cell phone number provided by the governor for storm victims in need of help, but their voicemail messages went unanswered. Apparently, no one called 911 or tried to have the patients moved across the street before they were in extremis or began to die.
Large numbers of casualties are not an inevitable sequela of natural disasters. In August 1973 (the second month of my internship), a late summer heat wave in New York City sent 12 patients with heat stroke and heat exhaustion from nearby non-air-conditioned nursing homes to the Albert Einstein/Jacobi Hospital emergency department in just a few hours. After being packed in ice until their temperatures dropped, all but one of the patients survived, while the 12th patient died of her underlying illnesses (see “Sheldon Jacobson, MD 1938-2009,” July 2009 EM).
On August 14, 2003, a hot (92.5°F), humid day in NYC, a power outage affecting the entire northeast and northwest United States trapped many New Yorkers in elevators, subways, and train cars. Residents were also trapped in high-rise apartments with only limited battery power for respirators and other essential electrical equipment. Within a few hours, first responders had reportedly evacuated everyone from stalled elevators in about 800 buildings, and over 600 subway and commuter train cars. Others were safely evacuated from their high-rise residences and taken to EDs powered by emergency generators. Upon arrival, their life support equipment and devices were plugged into electrical outlets, while they were examined, given medications as necessary, and later returned to their homes when power was restored.
First responders and emergency physicians have become quite adept at managing heat stroke and heat-related conditions, but only in patients who are still alive. In the aftermath of Hurricane Katrina, which devastated New Orleans on August 29, 2005, 215 bodies were found in New Orleans hospitals and nursing homes—including those from 40 post-storm deaths in one hospital alone (See Sheri Fink. Five Days at Memorial: Life and Death in a Storm-Ravaged Hospital. New York, NY: Crown Publishers; 2013). The tragic events following Katrina should have been a wake-up call for all health facilities and regulators in the US to anticipate and adequately prepare for loss of power, water, and severe heat conditions. Instead, a 2006 Florida bill that would have required adequate generators in all nursing homes was defeated, reportedly due to industry lobbying efforts.
The number of casualties and deaths due to natural disasters in this country may be fewer than those caused by such man-made incidents as the June 12, 2016 Pulse Nightclub shooting in Orlando, FL (see “The Orlando Nightclub Shooting: Firsthand Accounts and Lessons Learned,” August 2016 EM) and now the mass shooting in Las Vegas, NV on October 1, 2017, as this issue of Emergency Medicine was going to press. But natural disasters such as hurricanes, tornadoes, earthquakes, etc, are far more predictable and will reoccur with a 100% certainty in areas prone to or previously affected by such events. In these incidents, loss of life is preventable.
To quote the famous aphorism of George Santayana, “Those who cannot remember the past are condemned to repeat it.” More inexcusably, it often seems that those who can remember the past are also condemned to repeat it.
*As of October 11, 2017, this number rose to 14 deaths.
Between August 30 and September 16, 2017, Hurricane Irma wreaked havoc in the Caribbean and throughout Florida. In the days after the Rehabilitation Center at Hollywood Hills, Florida, lost its transformer and air conditioning due to the storm, 12 residents ranging in age from 57 to 99 years died* with body temperatures as high as 109.9°F, even though the hospital across the street continued to have full power. The nursing home staff tried in vain several times to get Florida Power and Light to restore full power. They also called a “personal” cell phone number provided by the governor for storm victims in need of help, but their voicemail messages went unanswered. Apparently, no one called 911 or tried to have the patients moved across the street before they were in extremis or began to die.
Large numbers of casualties are not an inevitable sequela of natural disasters. In August 1973 (the second month of my internship), a late summer heat wave in New York City sent 12 patients with heat stroke and heat exhaustion from nearby non-air-conditioned nursing homes to the Albert Einstein/Jacobi Hospital emergency department in just a few hours. After being packed in ice until their temperatures dropped, all but one of the patients survived, while the 12th patient died of her underlying illnesses (see “Sheldon Jacobson, MD 1938-2009,” July 2009 EM).
On August 14, 2003, a hot (92.5°F), humid day in NYC, a power outage affecting the entire northeast and northwest United States trapped many New Yorkers in elevators, subways, and train cars. Residents were also trapped in high-rise apartments with only limited battery power for respirators and other essential electrical equipment. Within a few hours, first responders had reportedly evacuated everyone from stalled elevators in about 800 buildings, and over 600 subway and commuter train cars. Others were safely evacuated from their high-rise residences and taken to EDs powered by emergency generators. Upon arrival, their life support equipment and devices were plugged into electrical outlets, while they were examined, given medications as necessary, and later returned to their homes when power was restored.
First responders and emergency physicians have become quite adept at managing heat stroke and heat-related conditions, but only in patients who are still alive. In the aftermath of Hurricane Katrina, which devastated New Orleans on August 29, 2005, 215 bodies were found in New Orleans hospitals and nursing homes—including those from 40 post-storm deaths in one hospital alone (See Sheri Fink. Five Days at Memorial: Life and Death in a Storm-Ravaged Hospital. New York, NY: Crown Publishers; 2013). The tragic events following Katrina should have been a wake-up call for all health facilities and regulators in the US to anticipate and adequately prepare for loss of power, water, and severe heat conditions. Instead, a 2006 Florida bill that would have required adequate generators in all nursing homes was defeated, reportedly due to industry lobbying efforts.
The number of casualties and deaths due to natural disasters in this country may be fewer than those caused by such man-made incidents as the June 12, 2016 Pulse Nightclub shooting in Orlando, FL (see “The Orlando Nightclub Shooting: Firsthand Accounts and Lessons Learned,” August 2016 EM) and now the mass shooting in Las Vegas, NV on October 1, 2017, as this issue of Emergency Medicine was going to press. But natural disasters such as hurricanes, tornadoes, earthquakes, etc, are far more predictable and will reoccur with a 100% certainty in areas prone to or previously affected by such events. In these incidents, loss of life is preventable.
To quote the famous aphorism of George Santayana, “Those who cannot remember the past are condemned to repeat it.” More inexcusably, it often seems that those who can remember the past are also condemned to repeat it.
*As of October 11, 2017, this number rose to 14 deaths.
Between August 30 and September 16, 2017, Hurricane Irma wreaked havoc in the Caribbean and throughout Florida. In the days after the Rehabilitation Center at Hollywood Hills, Florida, lost its transformer and air conditioning due to the storm, 12 residents ranging in age from 57 to 99 years died* with body temperatures as high as 109.9°F, even though the hospital across the street continued to have full power. The nursing home staff tried in vain several times to get Florida Power and Light to restore full power. They also called a “personal” cell phone number provided by the governor for storm victims in need of help, but their voicemail messages went unanswered. Apparently, no one called 911 or tried to have the patients moved across the street before they were in extremis or began to die.
Large numbers of casualties are not an inevitable sequela of natural disasters. In August 1973 (the second month of my internship), a late summer heat wave in New York City sent 12 patients with heat stroke and heat exhaustion from nearby non-air-conditioned nursing homes to the Albert Einstein/Jacobi Hospital emergency department in just a few hours. After being packed in ice until their temperatures dropped, all but one of the patients survived, while the 12th patient died of her underlying illnesses (see “Sheldon Jacobson, MD 1938-2009,” July 2009 EM).
On August 14, 2003, a hot (92.5°F), humid day in NYC, a power outage affecting the entire northeast and northwest United States trapped many New Yorkers in elevators, subways, and train cars. Residents were also trapped in high-rise apartments with only limited battery power for respirators and other essential electrical equipment. Within a few hours, first responders had reportedly evacuated everyone from stalled elevators in about 800 buildings, and over 600 subway and commuter train cars. Others were safely evacuated from their high-rise residences and taken to EDs powered by emergency generators. Upon arrival, their life support equipment and devices were plugged into electrical outlets, while they were examined, given medications as necessary, and later returned to their homes when power was restored.
First responders and emergency physicians have become quite adept at managing heat stroke and heat-related conditions, but only in patients who are still alive. In the aftermath of Hurricane Katrina, which devastated New Orleans on August 29, 2005, 215 bodies were found in New Orleans hospitals and nursing homes—including those from 40 post-storm deaths in one hospital alone (See Sheri Fink. Five Days at Memorial: Life and Death in a Storm-Ravaged Hospital. New York, NY: Crown Publishers; 2013). The tragic events following Katrina should have been a wake-up call for all health facilities and regulators in the US to anticipate and adequately prepare for loss of power, water, and severe heat conditions. Instead, a 2006 Florida bill that would have required adequate generators in all nursing homes was defeated, reportedly due to industry lobbying efforts.
The number of casualties and deaths due to natural disasters in this country may be fewer than those caused by such man-made incidents as the June 12, 2016 Pulse Nightclub shooting in Orlando, FL (see “The Orlando Nightclub Shooting: Firsthand Accounts and Lessons Learned,” August 2016 EM) and now the mass shooting in Las Vegas, NV on October 1, 2017, as this issue of Emergency Medicine was going to press. But natural disasters such as hurricanes, tornadoes, earthquakes, etc, are far more predictable and will reoccur with a 100% certainty in areas prone to or previously affected by such events. In these incidents, loss of life is preventable.
To quote the famous aphorism of George Santayana, “Those who cannot remember the past are condemned to repeat it.” More inexcusably, it often seems that those who can remember the past are also condemned to repeat it.
*As of October 11, 2017, this number rose to 14 deaths.
Optimal empiric treatment for uncomplicated cellulitis
Clinical question: Is empiric MRSA coverage for nonpurulent cellulitis necessary?
Background: Most nonpurulent skin and soft tissue infections are caused by beta-hemolytic streptococci and methicillin-susceptible Staphylococcus aureus. However, there is a growing incidence of community-acquired methicillin-resistant S. aureus infections. The authors of this study attempted to answer whether adding empiric methicillin-resistant S. aureus coverage reduces the risk of treatment failure.
Setting: Five emergency departments in the United States.
Synopsis: The authors of this study randomized 500 patients with cellulitis without purulent drainage or evidence of abscess as confirmed by sonography to receive a 7-day course of either cephalexin with placebo or cephalexin plus trimethoprimsulfamethoxazole. When analyzing those patients who took most of the prescribed pills (greater than 75% of doses) according to treatment protocol, there was no significant difference in clinical cure rate between the two arms of the study, reaffirming current guidelines that advocate against empiric methicillin-resistant S. aureus coverage for uncomplicated cellulitis.
When the authors analyzed the result of their data with the assumption that patients who were lost to follow-up had treatment failure, there was a trend favoring the addition of trimethoprim-sulfamethoxazole with cephalexin over monotherapy with cephalexin (P = .07). Although the authors concluded that this finding may warrant further investigation, this was essentially a negative study.
Bottom line: Empirically adding community-acquired methicillin-resistant S. aureus coverage with trimethoprim-sulfamethoxazole to uncomplicated cellulitis did not statistically improve a clinical cure, compared with empiric treatment with monotherapy with cephalexin.
Citation: Moran GJ, Krishnadasan A, Mower WR, et al. Effect of cephalexin plus trimethoprim-sulfamethoxazole vs. cephalexin alone on clinical cure of uncomplicated cellulitis. JAMA. 2017;317(20):2088-96.
Dr. Ramee is a hospitalist at Ochsner Health System, New Orleans.
Clinical question: Is empiric MRSA coverage for nonpurulent cellulitis necessary?
Background: Most nonpurulent skin and soft tissue infections are caused by beta-hemolytic streptococci and methicillin-susceptible Staphylococcus aureus. However, there is a growing incidence of community-acquired methicillin-resistant S. aureus infections. The authors of this study attempted to answer whether adding empiric methicillin-resistant S. aureus coverage reduces the risk of treatment failure.
Setting: Five emergency departments in the United States.
Synopsis: The authors of this study randomized 500 patients with cellulitis without purulent drainage or evidence of abscess as confirmed by sonography to receive a 7-day course of either cephalexin with placebo or cephalexin plus trimethoprimsulfamethoxazole. When analyzing those patients who took most of the prescribed pills (greater than 75% of doses) according to treatment protocol, there was no significant difference in clinical cure rate between the two arms of the study, reaffirming current guidelines that advocate against empiric methicillin-resistant S. aureus coverage for uncomplicated cellulitis.
When the authors analyzed the result of their data with the assumption that patients who were lost to follow-up had treatment failure, there was a trend favoring the addition of trimethoprim-sulfamethoxazole with cephalexin over monotherapy with cephalexin (P = .07). Although the authors concluded that this finding may warrant further investigation, this was essentially a negative study.
Bottom line: Empirically adding community-acquired methicillin-resistant S. aureus coverage with trimethoprim-sulfamethoxazole to uncomplicated cellulitis did not statistically improve a clinical cure, compared with empiric treatment with monotherapy with cephalexin.
Citation: Moran GJ, Krishnadasan A, Mower WR, et al. Effect of cephalexin plus trimethoprim-sulfamethoxazole vs. cephalexin alone on clinical cure of uncomplicated cellulitis. JAMA. 2017;317(20):2088-96.
Dr. Ramee is a hospitalist at Ochsner Health System, New Orleans.
Clinical question: Is empiric MRSA coverage for nonpurulent cellulitis necessary?
Background: Most nonpurulent skin and soft tissue infections are caused by beta-hemolytic streptococci and methicillin-susceptible Staphylococcus aureus. However, there is a growing incidence of community-acquired methicillin-resistant S. aureus infections. The authors of this study attempted to answer whether adding empiric methicillin-resistant S. aureus coverage reduces the risk of treatment failure.
Setting: Five emergency departments in the United States.
Synopsis: The authors of this study randomized 500 patients with cellulitis without purulent drainage or evidence of abscess as confirmed by sonography to receive a 7-day course of either cephalexin with placebo or cephalexin plus trimethoprimsulfamethoxazole. When analyzing those patients who took most of the prescribed pills (greater than 75% of doses) according to treatment protocol, there was no significant difference in clinical cure rate between the two arms of the study, reaffirming current guidelines that advocate against empiric methicillin-resistant S. aureus coverage for uncomplicated cellulitis.
When the authors analyzed the result of their data with the assumption that patients who were lost to follow-up had treatment failure, there was a trend favoring the addition of trimethoprim-sulfamethoxazole with cephalexin over monotherapy with cephalexin (P = .07). Although the authors concluded that this finding may warrant further investigation, this was essentially a negative study.
Bottom line: Empirically adding community-acquired methicillin-resistant S. aureus coverage with trimethoprim-sulfamethoxazole to uncomplicated cellulitis did not statistically improve a clinical cure, compared with empiric treatment with monotherapy with cephalexin.
Citation: Moran GJ, Krishnadasan A, Mower WR, et al. Effect of cephalexin plus trimethoprim-sulfamethoxazole vs. cephalexin alone on clinical cure of uncomplicated cellulitis. JAMA. 2017;317(20):2088-96.
Dr. Ramee is a hospitalist at Ochsner Health System, New Orleans.
Trends in Hysterectomy Rates and Approaches in the VA
The VA operates the largest integrated health care system in the country, consisting of 144 hospitals and 1,221 outpatient clinics. This system provides medical care for about 22 million veterans. In 2015, women accounted for nearly 10% of the veteran population and are expected to increase to about 16% by 2040.1 With an expected population increase of 18,000 per year over the next 10 years, women are the fastest growing group of veterans.
The VA acknowledges that women are an integral part of the veteran community and that a paradigm shift must occur to meet their unique health needs. Although clinical services specific to women veterans’ health needs have been introduced within the VA, gynecologic surgical services must be addressed in order to improve access and provide comprehensive women’s health care within the VA system.
About 600,000 hysterectomies are performed annually in the U.S., making this procedure one of the most commonly performed in women.2 Over the past 30 years, technologic advances have allowed surgeons to perform more hysterectomies via minimally invasive methods. Both the American Congress of Obstetricians and Gynecologists and American Association of Gynecologic Laparoscopists have published consensus statements that minimally invasive hysterectomy should be the standard of care.3,4 Studies in non-VA facilities have shown that practice patterns in the route of hysterectomy have evolved with the advancement of surgical equipment and techniques.
It is uncertain, however, whether these changes in practice patterns exist in the VA, because there are limited published data. Given the frequency of hysterectomies in the U.S., the rate and route of this procedure are easily identifiable measures that can be evaluated and utilized as a comparison model for health care received within the VA vs the civilian sector.
The aim of this study was to assess the changes in rate and surgical approach to benign hysterectomy for women veterans at VAMCs and referrals to non-VA facilities over a 10-year period. The authors’ hypothesis was that a minimally invasive approach would be more common in recent years. This study also compares published national data to evaluate whether the VA is offering comparable surgical services to the civilian sector.
Methods
The institutional review boards of Indiana University and the Richard L. Roudebush VAMC in Indianapolis, Indiana, approved this retrospective cross-sectional study. The VHA Support Service Center (VSSC) authorized access to VA database information.
All women veterans who underwent hysterectomy for benign indications from fiscal years (FY) 2005 to 2014 were included. In order to identify this group, the authors queried the VA Corporate Data Warehouse (CDW) and the Non-VA Care Cube for all hysterectomy current procedural terminology (CPT) codes typically performed for benign indications, including 58150, 58152, 58180, 58260, 58262, 58263, 58267, 58270, 58290, 58291, 58292, 58293, 58294, 58541, 58542, 58543, 58544, 58550, 58552, 58553, 58554, 58570, 58571, 58572, and 58573. For each patient identified, the following variables were collected: date of the procedure, facility location, primary CPT code, primary ICD-9 code, and patient age. Patients whose primary ICD-9 code was for a malignancy of gynecologic origin were excluded from the study.
The CDW is a national database collected by the VA Office of Information and Technology to provide clinical data for VA analytical purposes. The Non-VA Care Cube identifies services purchased for veterans with non-VA care dollars and, therefore, captures women veterans who were referred outside the VA for a hysterectomy. Additional data collected include age, gender, hospital complexity, place of care, payment location, primary CPT, primary ICD-9, and several other parameters. The annual number of women veterans accessing VA health care was extracted from the VSSC Unique Patients Cube.
Laparoscopic hysterectomy was defined as total laparoscopic hysterectomy, laparoscopic-assisted vaginal hysterectomy, laparoscopic-supracervical hysterectomy, and robotic-assisted laparoscopic hysterectomy. Minimally invasive hysterectomy was defined as all laparoscopic and vaginal hysterectomies.
Frequency distributions between categoric variables were compared using chi-squared tests. The population-adjusted hysterectomy rates were estimated by dividing the total number of hysterectomies by the number of women veterans accessing VA medical care. Hysterectomy rates are reported as rate per 1,000 women per year. A time trend analysis was performed with linear regression to evaluate the slopes of trends for each route of hysterectomy, using Microsoft Excel 2010 (Redmond, WA). The authors analyzed the relationship between route of hysterectomy and fiscal year, using a multivariable logistic regression that was adjusted for age, district, and surgical diagnosis. The adjusted relative risk (RR) for each type of hysterectomy was reported with 95% confidence intervals (CI). All statistical analyses were performed using SPSS 12.0 (Chicago, IL) with P < .05 defined as being statistically significant.To ensure the accuracy of the CDW data, the documented CPT and ICD-9 codes were compared between the CDW and the VA electronic medical records (EMR) for 400 charts selected at random. This cohort represents about 5% of the total charts and was felt to be an adequate measure of the entire sample since the CPT and ICD-9 codes were verified and matched 100% of the time. Demographic and descriptive data regarding body mass index, level of education, race, smoking status, medical comorbidities, and surgical history were excluded from the study because it was either not available or not consistently reported within the CDW.
Results
A retrospective query of the CDW identified 8,327 hysterectomies performed at the VA for benign indications from fiscal year (FY) 2005 to FY 2014. The total number of annual hysterectomies at the VA increased 30.7% from 710 in FY 2004 to 1,025 in FY 2014. The annual number of women veterans who accessed VA health care increased 30.8% from 412,271 to 596,011 during the same time frame. Thus, the population adjusted hysterectomy rate remained stable at 1.72 (Figure 1).
The authors also analyzed the VA data by district and decided to highlight the most recent data trends, as this is most applicable to how the VA currently operates. During FY 2014, the VA hysterectomy rates were as follows: district 1 (North Atlantic) 1.52; district 2 (Southeast) 2.21; district 3 (Midwest) 1.47; district 4 (Continental) 1.43; and district 5 (Pacific) 1.64 (Figure 2)
During the study period, calculated hysterectomy rates based on route at the VA showed that the laparoscopic hysterectomy rate increased from 0.11 to 0.53, the vaginal hysterectomy rate remained relatively stable at 0.34 to 0.37, and the abdominal hysterectomy rate declined from 1.28 to 0.8 (Figure 3).
Discussion
Although the total hysterectomy rate within the VA remained stable during the study period, the minimally invasive hysterectomy rate increased significantly. In FY 2014, the majority of hysterectomies at the VA were performed via a minimally invasive approach. Minimally invasive hysterectomy has many recognized advantages over abdominal hysterectomy as it offers a significant reduction in postoperative pain, narcotic use, length of stay, intraoperative blood loss, fever, deep venous thrombosis, and a faster recovery with return to baseline functioning thus improving overall quality of life.5-7 Previous literature of VA hysterectomy data from 1991 to 1997 reported an abdominal hysterectomy percentage of 74%, vaginal hysterectomy percentage of 22%, and laparoscopic assisted vaginal hysterectomy percentage of 4%.8
Additionally, previous literature of the civilian sector reported a national laparoscopic hysterectomy percentage of 32.4% in 2012, which is comparable to the laparoscopic hysterectomy percentage found in this study.9,10 These data highlight the growth of laparoscopic hysterectomy at the VA, which is comparable to that of the civilian sector.The Nationwide Inpatient Sample reported an abdominal hysterectomy percentage of 66.1% in 2003 and 52.8% in 2012.9,10 The authors observed a similar decline in the abdominal hysterectomy rate at the VA over the period studied. Although many factors may have contributed to this decline, the growth of laparoscopic hysterectomy was a possible contributing factor since the vaginal hysterectomy rate remained stable over the study period. Future studies are needed to evaluate surgical complications and readmission rates in order to more accurately assess the quality of gynecologic surgical care provided by the VA compared with the civilian sector.
Strengths and Limitations
This study has several important strengths. First, the large sample size from VA nationwide databases included information from all VAMC performing hysterectomies. Second, this study included 10 years of data, with the latest data from 2014, allowing for depiction of both long-term and recent trends.
Potential issues with large databases such as the CDW and the Non-VA Care Cube included inaccurate coding of procedures and diagnoses as well as missing data. This possible limitation was addressed by randomly selecting 400 patients in the database to verify the database information against the patient’s EMR, which matched 100% of the time. In addition, the authors calculated the hysterectomy rates using a denominator based on all women veterans accessing VA health care, which included women who had previously had a hysterectomy. Therefore, the true hysterectomy rate may have been underestimated.
Conclusion
The VA operates the largest health care system in the U.S. with more than 500,000 women veterans currently utilizing VA health care.11 The VA provides services to women veterans living in urban, suburban, and rural areas. The breadth of geographic locations, the declining number of VA facilities offering gynecologic surgical services, and the growing population of female veterans present unique challenges to providing accessible and comparable health care to these female patients.
VA district 4 (Continental) has the lowest population density as well as the lowest VA hysterectomy rate in FY 2014, which may be attributable to the aforementioned challenges. As a result of these challenges, an increasing number of gynecologic surgical referrals to non-VA facilities was observed during the study period. The VA has made considerable progress in supporting and promoting health care for women by strategically enhancing services and access for women veterans. Although the number of hysterectomies has increased across VA facilities offering gynecologic surgical services, about 1 in 3 women veterans are referred to non-VA facilities for their gynecologic surgical needs. The VA has a challenging opportunity to expand gynecologic surgical services and improve access for the growing population of women veterans. To accommodate this growth, the VA may consider strategically increasing the number of facilities providing gynecologic surgical services or expanding established gynecologic surgical departments.
1. U.S. Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. Unique veteran users profile FY 2015. https://www.va.gov/vetdata/docs/SpecialReports/Profile_of_Unique_Veteran_Users_2015.pdf. Published December 2016. Accessed August 24, 2017.
2. Centers for Disease Control and Prevention. Hysterectomy surveillance - United States, 1994-1999. Malaria surveillance - United States, 2000. MMWR Morb Mortal Wkly Rep; 2002;55(SS-5):1-28. https://stacks.cdc.gov/view/cdc/13513/Share. Published July 12, 2002. Accessed August 24, 2017.
3. AAGL Advancing Minimally Invasive Gynecology Worldwide. AAGL position statement: route of hysterectomy to treat benign uterine disease. J Minim Invasive Gynecol. 2011;18(1):1-3.
4. [No authors listed]. ACOG Committee Opinion No. 444: choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2009;114(5):1156-1158.
5. Garry R, Fountain J, Mason S, et al. The eVALuate study: two parallel randomised trials, one comparing laparoscopic with abdominal hysterectomy, the other comparing laparoscopic with vaginal hysterectomy. BMJ. 2004;328:129.
6. Marana R, Busacca M, Zupi E, Garcea N, Paparella P, Catalano GF. Laparoscopically assisted vaginal hysterectomy versus total abdominal hysterectomy: a prospective, randomized, multicenter study. Am J Obstet Gynecol. 1999;180(2, pt 1):270-275.
7. Aarts JW, Nieboer TE, Johnson N, et al. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst Rev. 2015(8):CD003677.
8. Weaver F, Hynes D, Goldberg JM, Khuri S, Daley J, Henderson W. Hysterectomy in Veterans Affairs medical centers. Obstet Gynecol. 2001;97(6):88-94.
9. Desai VB, Xu X. An update on inpatient hysterectomy routes in the United States. Am J Obstet Gynecol. 2015;213(5):742-743.
10. Wu JM, Wechter ME, Geller EJ, Nguyen TV, Visco AG. Hysterectomy rates in the United States, 2003. Obstet Gynecol. 2007;110(5):1091-1095.
11. U.S. Department of Veterans Affairs. Study of barriers for women veterans to VA health care. Final report 2015. http://www.womenshealth.va.gov/WOMENSHEALTH/docs/Womens%20Health%20Services_Barriers%20to%20Care%20Final%20Report_April2015.pdf. Published April 2015. Accessed August 24, 2017
The VA operates the largest integrated health care system in the country, consisting of 144 hospitals and 1,221 outpatient clinics. This system provides medical care for about 22 million veterans. In 2015, women accounted for nearly 10% of the veteran population and are expected to increase to about 16% by 2040.1 With an expected population increase of 18,000 per year over the next 10 years, women are the fastest growing group of veterans.
The VA acknowledges that women are an integral part of the veteran community and that a paradigm shift must occur to meet their unique health needs. Although clinical services specific to women veterans’ health needs have been introduced within the VA, gynecologic surgical services must be addressed in order to improve access and provide comprehensive women’s health care within the VA system.
About 600,000 hysterectomies are performed annually in the U.S., making this procedure one of the most commonly performed in women.2 Over the past 30 years, technologic advances have allowed surgeons to perform more hysterectomies via minimally invasive methods. Both the American Congress of Obstetricians and Gynecologists and American Association of Gynecologic Laparoscopists have published consensus statements that minimally invasive hysterectomy should be the standard of care.3,4 Studies in non-VA facilities have shown that practice patterns in the route of hysterectomy have evolved with the advancement of surgical equipment and techniques.
It is uncertain, however, whether these changes in practice patterns exist in the VA, because there are limited published data. Given the frequency of hysterectomies in the U.S., the rate and route of this procedure are easily identifiable measures that can be evaluated and utilized as a comparison model for health care received within the VA vs the civilian sector.
The aim of this study was to assess the changes in rate and surgical approach to benign hysterectomy for women veterans at VAMCs and referrals to non-VA facilities over a 10-year period. The authors’ hypothesis was that a minimally invasive approach would be more common in recent years. This study also compares published national data to evaluate whether the VA is offering comparable surgical services to the civilian sector.
Methods
The institutional review boards of Indiana University and the Richard L. Roudebush VAMC in Indianapolis, Indiana, approved this retrospective cross-sectional study. The VHA Support Service Center (VSSC) authorized access to VA database information.
All women veterans who underwent hysterectomy for benign indications from fiscal years (FY) 2005 to 2014 were included. In order to identify this group, the authors queried the VA Corporate Data Warehouse (CDW) and the Non-VA Care Cube for all hysterectomy current procedural terminology (CPT) codes typically performed for benign indications, including 58150, 58152, 58180, 58260, 58262, 58263, 58267, 58270, 58290, 58291, 58292, 58293, 58294, 58541, 58542, 58543, 58544, 58550, 58552, 58553, 58554, 58570, 58571, 58572, and 58573. For each patient identified, the following variables were collected: date of the procedure, facility location, primary CPT code, primary ICD-9 code, and patient age. Patients whose primary ICD-9 code was for a malignancy of gynecologic origin were excluded from the study.
The CDW is a national database collected by the VA Office of Information and Technology to provide clinical data for VA analytical purposes. The Non-VA Care Cube identifies services purchased for veterans with non-VA care dollars and, therefore, captures women veterans who were referred outside the VA for a hysterectomy. Additional data collected include age, gender, hospital complexity, place of care, payment location, primary CPT, primary ICD-9, and several other parameters. The annual number of women veterans accessing VA health care was extracted from the VSSC Unique Patients Cube.
Laparoscopic hysterectomy was defined as total laparoscopic hysterectomy, laparoscopic-assisted vaginal hysterectomy, laparoscopic-supracervical hysterectomy, and robotic-assisted laparoscopic hysterectomy. Minimally invasive hysterectomy was defined as all laparoscopic and vaginal hysterectomies.
Frequency distributions between categoric variables were compared using chi-squared tests. The population-adjusted hysterectomy rates were estimated by dividing the total number of hysterectomies by the number of women veterans accessing VA medical care. Hysterectomy rates are reported as rate per 1,000 women per year. A time trend analysis was performed with linear regression to evaluate the slopes of trends for each route of hysterectomy, using Microsoft Excel 2010 (Redmond, WA). The authors analyzed the relationship between route of hysterectomy and fiscal year, using a multivariable logistic regression that was adjusted for age, district, and surgical diagnosis. The adjusted relative risk (RR) for each type of hysterectomy was reported with 95% confidence intervals (CI). All statistical analyses were performed using SPSS 12.0 (Chicago, IL) with P < .05 defined as being statistically significant.To ensure the accuracy of the CDW data, the documented CPT and ICD-9 codes were compared between the CDW and the VA electronic medical records (EMR) for 400 charts selected at random. This cohort represents about 5% of the total charts and was felt to be an adequate measure of the entire sample since the CPT and ICD-9 codes were verified and matched 100% of the time. Demographic and descriptive data regarding body mass index, level of education, race, smoking status, medical comorbidities, and surgical history were excluded from the study because it was either not available or not consistently reported within the CDW.
Results
A retrospective query of the CDW identified 8,327 hysterectomies performed at the VA for benign indications from fiscal year (FY) 2005 to FY 2014. The total number of annual hysterectomies at the VA increased 30.7% from 710 in FY 2004 to 1,025 in FY 2014. The annual number of women veterans who accessed VA health care increased 30.8% from 412,271 to 596,011 during the same time frame. Thus, the population adjusted hysterectomy rate remained stable at 1.72 (Figure 1).
The authors also analyzed the VA data by district and decided to highlight the most recent data trends, as this is most applicable to how the VA currently operates. During FY 2014, the VA hysterectomy rates were as follows: district 1 (North Atlantic) 1.52; district 2 (Southeast) 2.21; district 3 (Midwest) 1.47; district 4 (Continental) 1.43; and district 5 (Pacific) 1.64 (Figure 2)
During the study period, calculated hysterectomy rates based on route at the VA showed that the laparoscopic hysterectomy rate increased from 0.11 to 0.53, the vaginal hysterectomy rate remained relatively stable at 0.34 to 0.37, and the abdominal hysterectomy rate declined from 1.28 to 0.8 (Figure 3).
Discussion
Although the total hysterectomy rate within the VA remained stable during the study period, the minimally invasive hysterectomy rate increased significantly. In FY 2014, the majority of hysterectomies at the VA were performed via a minimally invasive approach. Minimally invasive hysterectomy has many recognized advantages over abdominal hysterectomy as it offers a significant reduction in postoperative pain, narcotic use, length of stay, intraoperative blood loss, fever, deep venous thrombosis, and a faster recovery with return to baseline functioning thus improving overall quality of life.5-7 Previous literature of VA hysterectomy data from 1991 to 1997 reported an abdominal hysterectomy percentage of 74%, vaginal hysterectomy percentage of 22%, and laparoscopic assisted vaginal hysterectomy percentage of 4%.8
Additionally, previous literature of the civilian sector reported a national laparoscopic hysterectomy percentage of 32.4% in 2012, which is comparable to the laparoscopic hysterectomy percentage found in this study.9,10 These data highlight the growth of laparoscopic hysterectomy at the VA, which is comparable to that of the civilian sector.The Nationwide Inpatient Sample reported an abdominal hysterectomy percentage of 66.1% in 2003 and 52.8% in 2012.9,10 The authors observed a similar decline in the abdominal hysterectomy rate at the VA over the period studied. Although many factors may have contributed to this decline, the growth of laparoscopic hysterectomy was a possible contributing factor since the vaginal hysterectomy rate remained stable over the study period. Future studies are needed to evaluate surgical complications and readmission rates in order to more accurately assess the quality of gynecologic surgical care provided by the VA compared with the civilian sector.
Strengths and Limitations
This study has several important strengths. First, the large sample size from VA nationwide databases included information from all VAMC performing hysterectomies. Second, this study included 10 years of data, with the latest data from 2014, allowing for depiction of both long-term and recent trends.
Potential issues with large databases such as the CDW and the Non-VA Care Cube included inaccurate coding of procedures and diagnoses as well as missing data. This possible limitation was addressed by randomly selecting 400 patients in the database to verify the database information against the patient’s EMR, which matched 100% of the time. In addition, the authors calculated the hysterectomy rates using a denominator based on all women veterans accessing VA health care, which included women who had previously had a hysterectomy. Therefore, the true hysterectomy rate may have been underestimated.
Conclusion
The VA operates the largest health care system in the U.S. with more than 500,000 women veterans currently utilizing VA health care.11 The VA provides services to women veterans living in urban, suburban, and rural areas. The breadth of geographic locations, the declining number of VA facilities offering gynecologic surgical services, and the growing population of female veterans present unique challenges to providing accessible and comparable health care to these female patients.
VA district 4 (Continental) has the lowest population density as well as the lowest VA hysterectomy rate in FY 2014, which may be attributable to the aforementioned challenges. As a result of these challenges, an increasing number of gynecologic surgical referrals to non-VA facilities was observed during the study period. The VA has made considerable progress in supporting and promoting health care for women by strategically enhancing services and access for women veterans. Although the number of hysterectomies has increased across VA facilities offering gynecologic surgical services, about 1 in 3 women veterans are referred to non-VA facilities for their gynecologic surgical needs. The VA has a challenging opportunity to expand gynecologic surgical services and improve access for the growing population of women veterans. To accommodate this growth, the VA may consider strategically increasing the number of facilities providing gynecologic surgical services or expanding established gynecologic surgical departments.
The VA operates the largest integrated health care system in the country, consisting of 144 hospitals and 1,221 outpatient clinics. This system provides medical care for about 22 million veterans. In 2015, women accounted for nearly 10% of the veteran population and are expected to increase to about 16% by 2040.1 With an expected population increase of 18,000 per year over the next 10 years, women are the fastest growing group of veterans.
The VA acknowledges that women are an integral part of the veteran community and that a paradigm shift must occur to meet their unique health needs. Although clinical services specific to women veterans’ health needs have been introduced within the VA, gynecologic surgical services must be addressed in order to improve access and provide comprehensive women’s health care within the VA system.
About 600,000 hysterectomies are performed annually in the U.S., making this procedure one of the most commonly performed in women.2 Over the past 30 years, technologic advances have allowed surgeons to perform more hysterectomies via minimally invasive methods. Both the American Congress of Obstetricians and Gynecologists and American Association of Gynecologic Laparoscopists have published consensus statements that minimally invasive hysterectomy should be the standard of care.3,4 Studies in non-VA facilities have shown that practice patterns in the route of hysterectomy have evolved with the advancement of surgical equipment and techniques.
It is uncertain, however, whether these changes in practice patterns exist in the VA, because there are limited published data. Given the frequency of hysterectomies in the U.S., the rate and route of this procedure are easily identifiable measures that can be evaluated and utilized as a comparison model for health care received within the VA vs the civilian sector.
The aim of this study was to assess the changes in rate and surgical approach to benign hysterectomy for women veterans at VAMCs and referrals to non-VA facilities over a 10-year period. The authors’ hypothesis was that a minimally invasive approach would be more common in recent years. This study also compares published national data to evaluate whether the VA is offering comparable surgical services to the civilian sector.
Methods
The institutional review boards of Indiana University and the Richard L. Roudebush VAMC in Indianapolis, Indiana, approved this retrospective cross-sectional study. The VHA Support Service Center (VSSC) authorized access to VA database information.
All women veterans who underwent hysterectomy for benign indications from fiscal years (FY) 2005 to 2014 were included. In order to identify this group, the authors queried the VA Corporate Data Warehouse (CDW) and the Non-VA Care Cube for all hysterectomy current procedural terminology (CPT) codes typically performed for benign indications, including 58150, 58152, 58180, 58260, 58262, 58263, 58267, 58270, 58290, 58291, 58292, 58293, 58294, 58541, 58542, 58543, 58544, 58550, 58552, 58553, 58554, 58570, 58571, 58572, and 58573. For each patient identified, the following variables were collected: date of the procedure, facility location, primary CPT code, primary ICD-9 code, and patient age. Patients whose primary ICD-9 code was for a malignancy of gynecologic origin were excluded from the study.
The CDW is a national database collected by the VA Office of Information and Technology to provide clinical data for VA analytical purposes. The Non-VA Care Cube identifies services purchased for veterans with non-VA care dollars and, therefore, captures women veterans who were referred outside the VA for a hysterectomy. Additional data collected include age, gender, hospital complexity, place of care, payment location, primary CPT, primary ICD-9, and several other parameters. The annual number of women veterans accessing VA health care was extracted from the VSSC Unique Patients Cube.
Laparoscopic hysterectomy was defined as total laparoscopic hysterectomy, laparoscopic-assisted vaginal hysterectomy, laparoscopic-supracervical hysterectomy, and robotic-assisted laparoscopic hysterectomy. Minimally invasive hysterectomy was defined as all laparoscopic and vaginal hysterectomies.
Frequency distributions between categoric variables were compared using chi-squared tests. The population-adjusted hysterectomy rates were estimated by dividing the total number of hysterectomies by the number of women veterans accessing VA medical care. Hysterectomy rates are reported as rate per 1,000 women per year. A time trend analysis was performed with linear regression to evaluate the slopes of trends for each route of hysterectomy, using Microsoft Excel 2010 (Redmond, WA). The authors analyzed the relationship between route of hysterectomy and fiscal year, using a multivariable logistic regression that was adjusted for age, district, and surgical diagnosis. The adjusted relative risk (RR) for each type of hysterectomy was reported with 95% confidence intervals (CI). All statistical analyses were performed using SPSS 12.0 (Chicago, IL) with P < .05 defined as being statistically significant.To ensure the accuracy of the CDW data, the documented CPT and ICD-9 codes were compared between the CDW and the VA electronic medical records (EMR) for 400 charts selected at random. This cohort represents about 5% of the total charts and was felt to be an adequate measure of the entire sample since the CPT and ICD-9 codes were verified and matched 100% of the time. Demographic and descriptive data regarding body mass index, level of education, race, smoking status, medical comorbidities, and surgical history were excluded from the study because it was either not available or not consistently reported within the CDW.
Results
A retrospective query of the CDW identified 8,327 hysterectomies performed at the VA for benign indications from fiscal year (FY) 2005 to FY 2014. The total number of annual hysterectomies at the VA increased 30.7% from 710 in FY 2004 to 1,025 in FY 2014. The annual number of women veterans who accessed VA health care increased 30.8% from 412,271 to 596,011 during the same time frame. Thus, the population adjusted hysterectomy rate remained stable at 1.72 (Figure 1).
The authors also analyzed the VA data by district and decided to highlight the most recent data trends, as this is most applicable to how the VA currently operates. During FY 2014, the VA hysterectomy rates were as follows: district 1 (North Atlantic) 1.52; district 2 (Southeast) 2.21; district 3 (Midwest) 1.47; district 4 (Continental) 1.43; and district 5 (Pacific) 1.64 (Figure 2)
During the study period, calculated hysterectomy rates based on route at the VA showed that the laparoscopic hysterectomy rate increased from 0.11 to 0.53, the vaginal hysterectomy rate remained relatively stable at 0.34 to 0.37, and the abdominal hysterectomy rate declined from 1.28 to 0.8 (Figure 3).
Discussion
Although the total hysterectomy rate within the VA remained stable during the study period, the minimally invasive hysterectomy rate increased significantly. In FY 2014, the majority of hysterectomies at the VA were performed via a minimally invasive approach. Minimally invasive hysterectomy has many recognized advantages over abdominal hysterectomy as it offers a significant reduction in postoperative pain, narcotic use, length of stay, intraoperative blood loss, fever, deep venous thrombosis, and a faster recovery with return to baseline functioning thus improving overall quality of life.5-7 Previous literature of VA hysterectomy data from 1991 to 1997 reported an abdominal hysterectomy percentage of 74%, vaginal hysterectomy percentage of 22%, and laparoscopic assisted vaginal hysterectomy percentage of 4%.8
Additionally, previous literature of the civilian sector reported a national laparoscopic hysterectomy percentage of 32.4% in 2012, which is comparable to the laparoscopic hysterectomy percentage found in this study.9,10 These data highlight the growth of laparoscopic hysterectomy at the VA, which is comparable to that of the civilian sector.The Nationwide Inpatient Sample reported an abdominal hysterectomy percentage of 66.1% in 2003 and 52.8% in 2012.9,10 The authors observed a similar decline in the abdominal hysterectomy rate at the VA over the period studied. Although many factors may have contributed to this decline, the growth of laparoscopic hysterectomy was a possible contributing factor since the vaginal hysterectomy rate remained stable over the study period. Future studies are needed to evaluate surgical complications and readmission rates in order to more accurately assess the quality of gynecologic surgical care provided by the VA compared with the civilian sector.
Strengths and Limitations
This study has several important strengths. First, the large sample size from VA nationwide databases included information from all VAMC performing hysterectomies. Second, this study included 10 years of data, with the latest data from 2014, allowing for depiction of both long-term and recent trends.
Potential issues with large databases such as the CDW and the Non-VA Care Cube included inaccurate coding of procedures and diagnoses as well as missing data. This possible limitation was addressed by randomly selecting 400 patients in the database to verify the database information against the patient’s EMR, which matched 100% of the time. In addition, the authors calculated the hysterectomy rates using a denominator based on all women veterans accessing VA health care, which included women who had previously had a hysterectomy. Therefore, the true hysterectomy rate may have been underestimated.
Conclusion
The VA operates the largest health care system in the U.S. with more than 500,000 women veterans currently utilizing VA health care.11 The VA provides services to women veterans living in urban, suburban, and rural areas. The breadth of geographic locations, the declining number of VA facilities offering gynecologic surgical services, and the growing population of female veterans present unique challenges to providing accessible and comparable health care to these female patients.
VA district 4 (Continental) has the lowest population density as well as the lowest VA hysterectomy rate in FY 2014, which may be attributable to the aforementioned challenges. As a result of these challenges, an increasing number of gynecologic surgical referrals to non-VA facilities was observed during the study period. The VA has made considerable progress in supporting and promoting health care for women by strategically enhancing services and access for women veterans. Although the number of hysterectomies has increased across VA facilities offering gynecologic surgical services, about 1 in 3 women veterans are referred to non-VA facilities for their gynecologic surgical needs. The VA has a challenging opportunity to expand gynecologic surgical services and improve access for the growing population of women veterans. To accommodate this growth, the VA may consider strategically increasing the number of facilities providing gynecologic surgical services or expanding established gynecologic surgical departments.
1. U.S. Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. Unique veteran users profile FY 2015. https://www.va.gov/vetdata/docs/SpecialReports/Profile_of_Unique_Veteran_Users_2015.pdf. Published December 2016. Accessed August 24, 2017.
2. Centers for Disease Control and Prevention. Hysterectomy surveillance - United States, 1994-1999. Malaria surveillance - United States, 2000. MMWR Morb Mortal Wkly Rep; 2002;55(SS-5):1-28. https://stacks.cdc.gov/view/cdc/13513/Share. Published July 12, 2002. Accessed August 24, 2017.
3. AAGL Advancing Minimally Invasive Gynecology Worldwide. AAGL position statement: route of hysterectomy to treat benign uterine disease. J Minim Invasive Gynecol. 2011;18(1):1-3.
4. [No authors listed]. ACOG Committee Opinion No. 444: choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2009;114(5):1156-1158.
5. Garry R, Fountain J, Mason S, et al. The eVALuate study: two parallel randomised trials, one comparing laparoscopic with abdominal hysterectomy, the other comparing laparoscopic with vaginal hysterectomy. BMJ. 2004;328:129.
6. Marana R, Busacca M, Zupi E, Garcea N, Paparella P, Catalano GF. Laparoscopically assisted vaginal hysterectomy versus total abdominal hysterectomy: a prospective, randomized, multicenter study. Am J Obstet Gynecol. 1999;180(2, pt 1):270-275.
7. Aarts JW, Nieboer TE, Johnson N, et al. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst Rev. 2015(8):CD003677.
8. Weaver F, Hynes D, Goldberg JM, Khuri S, Daley J, Henderson W. Hysterectomy in Veterans Affairs medical centers. Obstet Gynecol. 2001;97(6):88-94.
9. Desai VB, Xu X. An update on inpatient hysterectomy routes in the United States. Am J Obstet Gynecol. 2015;213(5):742-743.
10. Wu JM, Wechter ME, Geller EJ, Nguyen TV, Visco AG. Hysterectomy rates in the United States, 2003. Obstet Gynecol. 2007;110(5):1091-1095.
11. U.S. Department of Veterans Affairs. Study of barriers for women veterans to VA health care. Final report 2015. http://www.womenshealth.va.gov/WOMENSHEALTH/docs/Womens%20Health%20Services_Barriers%20to%20Care%20Final%20Report_April2015.pdf. Published April 2015. Accessed August 24, 2017
1. U.S. Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. Unique veteran users profile FY 2015. https://www.va.gov/vetdata/docs/SpecialReports/Profile_of_Unique_Veteran_Users_2015.pdf. Published December 2016. Accessed August 24, 2017.
2. Centers for Disease Control and Prevention. Hysterectomy surveillance - United States, 1994-1999. Malaria surveillance - United States, 2000. MMWR Morb Mortal Wkly Rep; 2002;55(SS-5):1-28. https://stacks.cdc.gov/view/cdc/13513/Share. Published July 12, 2002. Accessed August 24, 2017.
3. AAGL Advancing Minimally Invasive Gynecology Worldwide. AAGL position statement: route of hysterectomy to treat benign uterine disease. J Minim Invasive Gynecol. 2011;18(1):1-3.
4. [No authors listed]. ACOG Committee Opinion No. 444: choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2009;114(5):1156-1158.
5. Garry R, Fountain J, Mason S, et al. The eVALuate study: two parallel randomised trials, one comparing laparoscopic with abdominal hysterectomy, the other comparing laparoscopic with vaginal hysterectomy. BMJ. 2004;328:129.
6. Marana R, Busacca M, Zupi E, Garcea N, Paparella P, Catalano GF. Laparoscopically assisted vaginal hysterectomy versus total abdominal hysterectomy: a prospective, randomized, multicenter study. Am J Obstet Gynecol. 1999;180(2, pt 1):270-275.
7. Aarts JW, Nieboer TE, Johnson N, et al. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst Rev. 2015(8):CD003677.
8. Weaver F, Hynes D, Goldberg JM, Khuri S, Daley J, Henderson W. Hysterectomy in Veterans Affairs medical centers. Obstet Gynecol. 2001;97(6):88-94.
9. Desai VB, Xu X. An update on inpatient hysterectomy routes in the United States. Am J Obstet Gynecol. 2015;213(5):742-743.
10. Wu JM, Wechter ME, Geller EJ, Nguyen TV, Visco AG. Hysterectomy rates in the United States, 2003. Obstet Gynecol. 2007;110(5):1091-1095.
11. U.S. Department of Veterans Affairs. Study of barriers for women veterans to VA health care. Final report 2015. http://www.womenshealth.va.gov/WOMENSHEALTH/docs/Womens%20Health%20Services_Barriers%20to%20Care%20Final%20Report_April2015.pdf. Published April 2015. Accessed August 24, 2017
Newer blood cancer drugs may not improve OS, QOL
A study of cancer drugs approved by the European Commission from 2009 to 2013 showed that few hematology drugs were known to provide a benefit in overall survival (OS) or quality of life (QOL) over existing treatments.
Of 12 drugs approved for 17 hematology indications, 3 drugs had been shown to provide a benefit in OS (for 3 indications) at the time of approval.
None of the other hematology drugs were known to provide an OS benefit even after a median follow-up of 5.4 years.
Two hematology drugs were shown to provide a benefit in QOL (for 2 indications) after approval, but none of the drugs were known to provide a QOL benefit at the time of approval.
These findings were published in The BMJ alongside a related editorial, feature article, and patient commentary.
All cancer drugs
Researchers analyzed reports on all cancer drug approvals by the European Commission from 2009 to 2013.
There were 48 drugs approved for 68 cancer indications during this period. Fifty-one of the indications were for solid tumor malignancies, and 17 were for hematologic malignancies.
For 24 indications (35%), research had demonstrated a significant improvement in OS at the time of the drugs’ approval. For 3 indications, an improvement in OS was demonstrated after approval.
There was a known improvement in QOL for 7 of the indications (10%) at the time of approval and for 5 indications after approval.
The median follow-up was 5.4 years (range, 3.3 years to 8.1 years).
Overall, there was a significant improvement in OS or QOL during the study period for 51% of the indications (35/68). For the other half (49%, n=33), it wasn’t clear if the drugs provide any benefits in OS or QOL.
All cancer trials
The 68 approvals of cancer drugs were supported by 72 clinical trials.
Sixty approvals (88%) were supported by at least 1 randomized, controlled trial. Eight approvals (12%) were based on a single-arm study. This included 6 of 10 conditional marketing authorizations and 2 of 58 regular marketing authorizations.
Eighteen of the approvals (26%) were supported by a pivotal study powered to evaluate OS as the primary endpoint. And 37 of the approvals (54%) had a supporting pivotal trial evaluating QOL, but results were not reported for 2 of these trials.
Hematology trials and drugs
Of the 12 drugs approved for 17 hematology indications, 4 were regular approvals, 5 were conditional approvals, and 8 had orphan drug designation.
The approvals were supported by data from 18 trials—13 randomized and 5 single-arm trials.
The study drug was compared to an active comparator in 9 of the trials. The drug was evaluated as an add-on treatment in 4 trials. And the drug was not compared to anything in 5 trials (the single-arm trials).
OS was the primary endpoint in 1 of the trials, and 17 trials had OS or QOL as a secondary endpoint.
There were 3 drugs that had demonstrated an OS benefit at the time of approval but no QOL benefit at any time:
- Decitabine used for first-line treatment of acute myeloid leukemia in adults 65 and older who are ineligible for chemotherapy
- Pomalidomide in combination with dexamethasone as third-line therapy for relapsed/refractory multiple myeloma (MM)
- Rituximab plus chemotherapy for first-line treatment of chronic lymphocytic leukemia (CLL).
There were 2 drugs that had demonstrated a QOL benefit, only after approval, but they were not known to provide an OS benefit at any time:
- Nilotinib as a treatment for adults with newly diagnosed, chronic phase, Ph+ chronic myeloid leukemia (CML)
- Ofatumumab for CLL that is refractory to fludarabine and alemtuzumab
For the remaining drugs, there was no evidence of an OS or QOL benefit at any time during the period studied. The drugs included:
- Bortezomib given alone or in combination with doxorubicin or dexamethasone as second-line therapy for MM patients ineligible for hematopoietic stem cell transplant (HSCT)
- Bortezomib plus dexamethasone with or without thalidomide as first-line therapy in MM patients eligible for HSCT
- Bosutinib as second- or third-line treatment of Ph+ CML (any phase)
- Brentuximab vedotin for relapsed or refractory systemic anaplastic large-cell lymphoma
- Brentuximab vedotin for relapsed or refractory, CD30+ Hodgkin lymphoma after autologous HSCT or as third-line treatment for patients ineligible for autologous HSCT
- Dasatinib for first-line treatment of chronic phase, Ph+ CML
- Pixantrone for multiply relapsed or refractory B-cell non-Hodgkin lymphoma
- Ponatinib for patients with Ph+ acute lymphoblastic leukemia who are ineligible for imatinib or have disease that is resistant or intolerant to dasatinib or characterized by T315I mutation
- Ponatinib for patients with any phase of CML who are ineligible for imatinib or have disease that is resistant or intolerant to dasatinib/nilotinib or characterized by T315I mutation
- Rituximab as maintenance after induction for patients with follicular lymphoma
- Rituximab plus chemotherapy for relapsed or refractory CLL
- Temsirolimus for relapsed or refractory mantle cell lymphoma.

A study of cancer drugs approved by the European Commission from 2009 to 2013 showed that few hematology drugs were known to provide a benefit in overall survival (OS) or quality of life (QOL) over existing treatments.
Of 12 drugs approved for 17 hematology indications, 3 drugs had been shown to provide a benefit in OS (for 3 indications) at the time of approval.
None of the other hematology drugs were known to provide an OS benefit even after a median follow-up of 5.4 years.
Two hematology drugs were shown to provide a benefit in QOL (for 2 indications) after approval, but none of the drugs were known to provide a QOL benefit at the time of approval.
These findings were published in The BMJ alongside a related editorial, feature article, and patient commentary.
All cancer drugs
Researchers analyzed reports on all cancer drug approvals by the European Commission from 2009 to 2013.
There were 48 drugs approved for 68 cancer indications during this period. Fifty-one of the indications were for solid tumor malignancies, and 17 were for hematologic malignancies.
For 24 indications (35%), research had demonstrated a significant improvement in OS at the time of the drugs’ approval. For 3 indications, an improvement in OS was demonstrated after approval.
There was a known improvement in QOL for 7 of the indications (10%) at the time of approval and for 5 indications after approval.
The median follow-up was 5.4 years (range, 3.3 years to 8.1 years).
Overall, there was a significant improvement in OS or QOL during the study period for 51% of the indications (35/68). For the other half (49%, n=33), it wasn’t clear if the drugs provide any benefits in OS or QOL.
All cancer trials
The 68 approvals of cancer drugs were supported by 72 clinical trials.
Sixty approvals (88%) were supported by at least 1 randomized, controlled trial. Eight approvals (12%) were based on a single-arm study. This included 6 of 10 conditional marketing authorizations and 2 of 58 regular marketing authorizations.
Eighteen of the approvals (26%) were supported by a pivotal study powered to evaluate OS as the primary endpoint. And 37 of the approvals (54%) had a supporting pivotal trial evaluating QOL, but results were not reported for 2 of these trials.
Hematology trials and drugs
Of the 12 drugs approved for 17 hematology indications, 4 were regular approvals, 5 were conditional approvals, and 8 had orphan drug designation.
The approvals were supported by data from 18 trials—13 randomized and 5 single-arm trials.
The study drug was compared to an active comparator in 9 of the trials. The drug was evaluated as an add-on treatment in 4 trials. And the drug was not compared to anything in 5 trials (the single-arm trials).
OS was the primary endpoint in 1 of the trials, and 17 trials had OS or QOL as a secondary endpoint.
There were 3 drugs that had demonstrated an OS benefit at the time of approval but no QOL benefit at any time:
- Decitabine used for first-line treatment of acute myeloid leukemia in adults 65 and older who are ineligible for chemotherapy
- Pomalidomide in combination with dexamethasone as third-line therapy for relapsed/refractory multiple myeloma (MM)
- Rituximab plus chemotherapy for first-line treatment of chronic lymphocytic leukemia (CLL).
There were 2 drugs that had demonstrated a QOL benefit, only after approval, but they were not known to provide an OS benefit at any time:
- Nilotinib as a treatment for adults with newly diagnosed, chronic phase, Ph+ chronic myeloid leukemia (CML)
- Ofatumumab for CLL that is refractory to fludarabine and alemtuzumab
For the remaining drugs, there was no evidence of an OS or QOL benefit at any time during the period studied. The drugs included:
- Bortezomib given alone or in combination with doxorubicin or dexamethasone as second-line therapy for MM patients ineligible for hematopoietic stem cell transplant (HSCT)
- Bortezomib plus dexamethasone with or without thalidomide as first-line therapy in MM patients eligible for HSCT
- Bosutinib as second- or third-line treatment of Ph+ CML (any phase)
- Brentuximab vedotin for relapsed or refractory systemic anaplastic large-cell lymphoma
- Brentuximab vedotin for relapsed or refractory, CD30+ Hodgkin lymphoma after autologous HSCT or as third-line treatment for patients ineligible for autologous HSCT
- Dasatinib for first-line treatment of chronic phase, Ph+ CML
- Pixantrone for multiply relapsed or refractory B-cell non-Hodgkin lymphoma
- Ponatinib for patients with Ph+ acute lymphoblastic leukemia who are ineligible for imatinib or have disease that is resistant or intolerant to dasatinib or characterized by T315I mutation
- Ponatinib for patients with any phase of CML who are ineligible for imatinib or have disease that is resistant or intolerant to dasatinib/nilotinib or characterized by T315I mutation
- Rituximab as maintenance after induction for patients with follicular lymphoma
- Rituximab plus chemotherapy for relapsed or refractory CLL
- Temsirolimus for relapsed or refractory mantle cell lymphoma.

A study of cancer drugs approved by the European Commission from 2009 to 2013 showed that few hematology drugs were known to provide a benefit in overall survival (OS) or quality of life (QOL) over existing treatments.
Of 12 drugs approved for 17 hematology indications, 3 drugs had been shown to provide a benefit in OS (for 3 indications) at the time of approval.
None of the other hematology drugs were known to provide an OS benefit even after a median follow-up of 5.4 years.
Two hematology drugs were shown to provide a benefit in QOL (for 2 indications) after approval, but none of the drugs were known to provide a QOL benefit at the time of approval.
These findings were published in The BMJ alongside a related editorial, feature article, and patient commentary.
All cancer drugs
Researchers analyzed reports on all cancer drug approvals by the European Commission from 2009 to 2013.
There were 48 drugs approved for 68 cancer indications during this period. Fifty-one of the indications were for solid tumor malignancies, and 17 were for hematologic malignancies.
For 24 indications (35%), research had demonstrated a significant improvement in OS at the time of the drugs’ approval. For 3 indications, an improvement in OS was demonstrated after approval.
There was a known improvement in QOL for 7 of the indications (10%) at the time of approval and for 5 indications after approval.
The median follow-up was 5.4 years (range, 3.3 years to 8.1 years).
Overall, there was a significant improvement in OS or QOL during the study period for 51% of the indications (35/68). For the other half (49%, n=33), it wasn’t clear if the drugs provide any benefits in OS or QOL.
All cancer trials
The 68 approvals of cancer drugs were supported by 72 clinical trials.
Sixty approvals (88%) were supported by at least 1 randomized, controlled trial. Eight approvals (12%) were based on a single-arm study. This included 6 of 10 conditional marketing authorizations and 2 of 58 regular marketing authorizations.
Eighteen of the approvals (26%) were supported by a pivotal study powered to evaluate OS as the primary endpoint. And 37 of the approvals (54%) had a supporting pivotal trial evaluating QOL, but results were not reported for 2 of these trials.
Hematology trials and drugs
Of the 12 drugs approved for 17 hematology indications, 4 were regular approvals, 5 were conditional approvals, and 8 had orphan drug designation.
The approvals were supported by data from 18 trials—13 randomized and 5 single-arm trials.
The study drug was compared to an active comparator in 9 of the trials. The drug was evaluated as an add-on treatment in 4 trials. And the drug was not compared to anything in 5 trials (the single-arm trials).
OS was the primary endpoint in 1 of the trials, and 17 trials had OS or QOL as a secondary endpoint.
There were 3 drugs that had demonstrated an OS benefit at the time of approval but no QOL benefit at any time:
- Decitabine used for first-line treatment of acute myeloid leukemia in adults 65 and older who are ineligible for chemotherapy
- Pomalidomide in combination with dexamethasone as third-line therapy for relapsed/refractory multiple myeloma (MM)
- Rituximab plus chemotherapy for first-line treatment of chronic lymphocytic leukemia (CLL).
There were 2 drugs that had demonstrated a QOL benefit, only after approval, but they were not known to provide an OS benefit at any time:
- Nilotinib as a treatment for adults with newly diagnosed, chronic phase, Ph+ chronic myeloid leukemia (CML)
- Ofatumumab for CLL that is refractory to fludarabine and alemtuzumab
For the remaining drugs, there was no evidence of an OS or QOL benefit at any time during the period studied. The drugs included:
- Bortezomib given alone or in combination with doxorubicin or dexamethasone as second-line therapy for MM patients ineligible for hematopoietic stem cell transplant (HSCT)
- Bortezomib plus dexamethasone with or without thalidomide as first-line therapy in MM patients eligible for HSCT
- Bosutinib as second- or third-line treatment of Ph+ CML (any phase)
- Brentuximab vedotin for relapsed or refractory systemic anaplastic large-cell lymphoma
- Brentuximab vedotin for relapsed or refractory, CD30+ Hodgkin lymphoma after autologous HSCT or as third-line treatment for patients ineligible for autologous HSCT
- Dasatinib for first-line treatment of chronic phase, Ph+ CML
- Pixantrone for multiply relapsed or refractory B-cell non-Hodgkin lymphoma
- Ponatinib for patients with Ph+ acute lymphoblastic leukemia who are ineligible for imatinib or have disease that is resistant or intolerant to dasatinib or characterized by T315I mutation
- Ponatinib for patients with any phase of CML who are ineligible for imatinib or have disease that is resistant or intolerant to dasatinib/nilotinib or characterized by T315I mutation
- Rituximab as maintenance after induction for patients with follicular lymphoma
- Rituximab plus chemotherapy for relapsed or refractory CLL
- Temsirolimus for relapsed or refractory mantle cell lymphoma.

FDA approves first test for detecting Zika in blood donations
The US Food and Drug Administration (FDA) has approved the first test designed to screen blood donations for Zika virus.
The cobas Zika test is a qualitative nucleic acid test that can detect Zika virus RNA in individual plasma specimens obtained from donors of whole blood and blood components and from living organ donors.
The test is not intended for the individual diagnosis of Zika virus infection.
The cobas Zika test is intended for use on the fully automated cobas 6800 and cobas 8800 systems. These systems and the test are manufactured by Roche Molecular Systems, Inc.
“Today’s action represents the first approval of a Zika virus detection test for use with screening the nation’s blood supply,” said Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research.
“Screening blood donations for the Zika virus is critical to preventing infected donations from entering the US blood supply. Today’s approval is the result of a commitment by the manufacturer to work rapidly and collaboratively with the FDA and the blood collection industry to respond to a public health crisis and ensure the safety of blood in the US and its territories.”
In August 2016, the FDA issued a guidance document recommending that all states and territories screen individual units of whole blood and blood components with an investigational blood screening test available under an investigational new drug (IND) application, or use an approved test when available.
Several blood collection establishments have used the cobas Zika test under an IND. The data collected from this testing and additional studies performed by the manufacturer demonstrated that the test is effective in screening blood donors for Zika virus infection, according to the FDA.
The test’s clinical specificity was evaluated by testing individual samples from blood donations at 5 external laboratory sites, and the specificity exceeded 99%. ![]()
The US Food and Drug Administration (FDA) has approved the first test designed to screen blood donations for Zika virus.
The cobas Zika test is a qualitative nucleic acid test that can detect Zika virus RNA in individual plasma specimens obtained from donors of whole blood and blood components and from living organ donors.
The test is not intended for the individual diagnosis of Zika virus infection.
The cobas Zika test is intended for use on the fully automated cobas 6800 and cobas 8800 systems. These systems and the test are manufactured by Roche Molecular Systems, Inc.
“Today’s action represents the first approval of a Zika virus detection test for use with screening the nation’s blood supply,” said Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research.
“Screening blood donations for the Zika virus is critical to preventing infected donations from entering the US blood supply. Today’s approval is the result of a commitment by the manufacturer to work rapidly and collaboratively with the FDA and the blood collection industry to respond to a public health crisis and ensure the safety of blood in the US and its territories.”
In August 2016, the FDA issued a guidance document recommending that all states and territories screen individual units of whole blood and blood components with an investigational blood screening test available under an investigational new drug (IND) application, or use an approved test when available.
Several blood collection establishments have used the cobas Zika test under an IND. The data collected from this testing and additional studies performed by the manufacturer demonstrated that the test is effective in screening blood donors for Zika virus infection, according to the FDA.
The test’s clinical specificity was evaluated by testing individual samples from blood donations at 5 external laboratory sites, and the specificity exceeded 99%. ![]()
The US Food and Drug Administration (FDA) has approved the first test designed to screen blood donations for Zika virus.
The cobas Zika test is a qualitative nucleic acid test that can detect Zika virus RNA in individual plasma specimens obtained from donors of whole blood and blood components and from living organ donors.
The test is not intended for the individual diagnosis of Zika virus infection.
The cobas Zika test is intended for use on the fully automated cobas 6800 and cobas 8800 systems. These systems and the test are manufactured by Roche Molecular Systems, Inc.
“Today’s action represents the first approval of a Zika virus detection test for use with screening the nation’s blood supply,” said Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research.
“Screening blood donations for the Zika virus is critical to preventing infected donations from entering the US blood supply. Today’s approval is the result of a commitment by the manufacturer to work rapidly and collaboratively with the FDA and the blood collection industry to respond to a public health crisis and ensure the safety of blood in the US and its territories.”
In August 2016, the FDA issued a guidance document recommending that all states and territories screen individual units of whole blood and blood components with an investigational blood screening test available under an investigational new drug (IND) application, or use an approved test when available.
Several blood collection establishments have used the cobas Zika test under an IND. The data collected from this testing and additional studies performed by the manufacturer demonstrated that the test is effective in screening blood donors for Zika virus infection, according to the FDA.
The test’s clinical specificity was evaluated by testing individual samples from blood donations at 5 external laboratory sites, and the specificity exceeded 99%. ![]()
Sperm banking may be underused by young cancer patients
New research suggests sperm banking may be underutilized by adolescent and young adult males with cancer who are at risk of infertility.
However, the study also showed that patients were more likely to attempt sperm banking if they were physically mature, met with fertility specialists, or their parents recommended sperm banking.
These findings were published in the Journal of Clinical Oncology.
“Research has found that the majority of males who survive childhood cancer desire biological children,” said study author James Klosky, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
“Fertility preservation is also associated with a variety of benefits for survivors, including increased optimism about the future. While sperm banking is not for everyone, it is an effective method for preserving male fertility. Yet this study shows that sperm banking remains underutilized by at-risk patients with cancer.”
Dr Klosky and his colleagues surveyed 146 young males with cancer who were at risk of infertility. The researchers also surveyed 144 parents or guardians and 52 oncologists and other healthcare providers.
The patients’ mean age was 16.49 (range, 13.0-21.99). Diagnoses included leukemia and lymphoma (56.2%), solid tumor malignancies (37.7%), and brain tumors (6.2%).
Slightly more than half of the patients (53.4%, n=78) attempted sperm banking prior to starting treatment. Sixty-two, or 82.1%, of those who attempted sperm banking were successful.
In all, 43.8% of the patients successfully banked sperm.
Of the 68 patients who did not attempt sperm banking, 29 reported discussing the option with their families but deciding against it. Twenty-six patients indicated they did not believe sperm banking was necessary, and 9 patients were unsure what it was.
There were several factors that influenced the likelihood of patients making sperm collection attempts as well as successfully banking sperm.
In a multivariable analysis, the following factors were associated with an increased likelihood of attempting to bank sperm:
- Meeting with a fertility specialist (odds ratio[OR]=29.96; 95% CI, 2.48 to 361.41; P=0.007)
- Parent recommending banking (OR=12.30; 95% CI, 2.01 to 75.94; P=0.007)
- Higher Tanner stage (OR=5.42; 95% CI, 1.75 to 16.78; P=0.003).
In another multivariable analysis, successful sperm banking was associated with:
- Patient history of masturbation (OR=5.99; 95% CI, 1.25 to 28.50; P=0.025)
- Higher self-efficacy for banking coordination (OR=1.23; 95% CI, 1.05 to 1.45; P=0.012)
- Medical team member recommending banking (OR=4.26; 95% CI, 1.45 to 12.43; P=0.008)
- Parent recommending banking (OR=4.62; 95% CI, 1.46 to 14.73; P=0.010).
“These results highlight factors that providers can target to empower adolescents to actively participate in their own healthcare,” Dr Klosky said. “These decisions, which are typically made at the time of diagnosis, have high potential to affect their lives as survivors.” ![]()
New research suggests sperm banking may be underutilized by adolescent and young adult males with cancer who are at risk of infertility.
However, the study also showed that patients were more likely to attempt sperm banking if they were physically mature, met with fertility specialists, or their parents recommended sperm banking.
These findings were published in the Journal of Clinical Oncology.
“Research has found that the majority of males who survive childhood cancer desire biological children,” said study author James Klosky, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
“Fertility preservation is also associated with a variety of benefits for survivors, including increased optimism about the future. While sperm banking is not for everyone, it is an effective method for preserving male fertility. Yet this study shows that sperm banking remains underutilized by at-risk patients with cancer.”
Dr Klosky and his colleagues surveyed 146 young males with cancer who were at risk of infertility. The researchers also surveyed 144 parents or guardians and 52 oncologists and other healthcare providers.
The patients’ mean age was 16.49 (range, 13.0-21.99). Diagnoses included leukemia and lymphoma (56.2%), solid tumor malignancies (37.7%), and brain tumors (6.2%).
Slightly more than half of the patients (53.4%, n=78) attempted sperm banking prior to starting treatment. Sixty-two, or 82.1%, of those who attempted sperm banking were successful.
In all, 43.8% of the patients successfully banked sperm.
Of the 68 patients who did not attempt sperm banking, 29 reported discussing the option with their families but deciding against it. Twenty-six patients indicated they did not believe sperm banking was necessary, and 9 patients were unsure what it was.
There were several factors that influenced the likelihood of patients making sperm collection attempts as well as successfully banking sperm.
In a multivariable analysis, the following factors were associated with an increased likelihood of attempting to bank sperm:
- Meeting with a fertility specialist (odds ratio[OR]=29.96; 95% CI, 2.48 to 361.41; P=0.007)
- Parent recommending banking (OR=12.30; 95% CI, 2.01 to 75.94; P=0.007)
- Higher Tanner stage (OR=5.42; 95% CI, 1.75 to 16.78; P=0.003).
In another multivariable analysis, successful sperm banking was associated with:
- Patient history of masturbation (OR=5.99; 95% CI, 1.25 to 28.50; P=0.025)
- Higher self-efficacy for banking coordination (OR=1.23; 95% CI, 1.05 to 1.45; P=0.012)
- Medical team member recommending banking (OR=4.26; 95% CI, 1.45 to 12.43; P=0.008)
- Parent recommending banking (OR=4.62; 95% CI, 1.46 to 14.73; P=0.010).
“These results highlight factors that providers can target to empower adolescents to actively participate in their own healthcare,” Dr Klosky said. “These decisions, which are typically made at the time of diagnosis, have high potential to affect their lives as survivors.” ![]()
New research suggests sperm banking may be underutilized by adolescent and young adult males with cancer who are at risk of infertility.
However, the study also showed that patients were more likely to attempt sperm banking if they were physically mature, met with fertility specialists, or their parents recommended sperm banking.
These findings were published in the Journal of Clinical Oncology.
“Research has found that the majority of males who survive childhood cancer desire biological children,” said study author James Klosky, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
“Fertility preservation is also associated with a variety of benefits for survivors, including increased optimism about the future. While sperm banking is not for everyone, it is an effective method for preserving male fertility. Yet this study shows that sperm banking remains underutilized by at-risk patients with cancer.”
Dr Klosky and his colleagues surveyed 146 young males with cancer who were at risk of infertility. The researchers also surveyed 144 parents or guardians and 52 oncologists and other healthcare providers.
The patients’ mean age was 16.49 (range, 13.0-21.99). Diagnoses included leukemia and lymphoma (56.2%), solid tumor malignancies (37.7%), and brain tumors (6.2%).
Slightly more than half of the patients (53.4%, n=78) attempted sperm banking prior to starting treatment. Sixty-two, or 82.1%, of those who attempted sperm banking were successful.
In all, 43.8% of the patients successfully banked sperm.
Of the 68 patients who did not attempt sperm banking, 29 reported discussing the option with their families but deciding against it. Twenty-six patients indicated they did not believe sperm banking was necessary, and 9 patients were unsure what it was.
There were several factors that influenced the likelihood of patients making sperm collection attempts as well as successfully banking sperm.
In a multivariable analysis, the following factors were associated with an increased likelihood of attempting to bank sperm:
- Meeting with a fertility specialist (odds ratio[OR]=29.96; 95% CI, 2.48 to 361.41; P=0.007)
- Parent recommending banking (OR=12.30; 95% CI, 2.01 to 75.94; P=0.007)
- Higher Tanner stage (OR=5.42; 95% CI, 1.75 to 16.78; P=0.003).
In another multivariable analysis, successful sperm banking was associated with:
- Patient history of masturbation (OR=5.99; 95% CI, 1.25 to 28.50; P=0.025)
- Higher self-efficacy for banking coordination (OR=1.23; 95% CI, 1.05 to 1.45; P=0.012)
- Medical team member recommending banking (OR=4.26; 95% CI, 1.45 to 12.43; P=0.008)
- Parent recommending banking (OR=4.62; 95% CI, 1.46 to 14.73; P=0.010).
“These results highlight factors that providers can target to empower adolescents to actively participate in their own healthcare,” Dr Klosky said. “These decisions, which are typically made at the time of diagnosis, have high potential to affect their lives as survivors.” ![]()