User login
Universal paternal Rh screening is cost effective in IVF
SAN ANTONIO – Implementing universal paternal Rh screening would be a cost-effective safety strategy among patients receiving in vitro fertilization, according to a model that used up-to-date data and accounted for ethnic variations in the prevalence of the Rh (D) antibody.
Using a universal Rh screening strategy for semen donors to Rh (D) negative women undergoing in vitro fertilization would result in a cost savings of $11.01 per patient, or $1,120,000 per 100,000 Rh negative IVF pregnancies, according to Pietro Bortoletto, MD, and his coauthors. Their findings were presented during a poster session at the annual meeting of the American Society for Reproductive Medicine.
If paternal Rh factor status is unknown, vaginal bleeding during pregnancy prompts maternal administration of anti-D immune globulin if the mother is Rh (D) negative to prevent hemolytic disease of the fetus and newborn. However, wrote Dr. Bortoletto and his colleagues, “in the IVF population, where paternity status is presumed to be certain, the Rh (D) status of the male partner can be used to triage Rh (D) negative women to more appropriate administration of anti-D globulin.”
To see whether a universal paternal Rh screening strategy would be cost effective, Dr. Bortoletto, a resident physician in obstetrics and gynecology at Harvard Medical School and Brigham and Women’s Hospital, both in Boston, and his collaborators constructed a decision tree to estimate cost savings. The model compared a universal paternal-screening strategy for Rh (D) negative women undergoing IVF with the current standard of practice, which does not involve routine Rh (D) screening for the sperm donor.
In constructing the model, the investigators drew on published data showing that first trimester bleeding is more common in women who undergo IVF: It occurs in one-third of these women, compared with about 20% of the general pregnant population.
They also established probability estimates of pregnancy loss before 20 weeks, with and without first trimester bleeding (0.34 and 0.18, respectively); third trimester bleeding, with and without first trimester bleeding (0.05 and 0.02); and trauma in pregnancy (0.08). They estimated the overall probability of the pregnancy producing an Rh positive neonate at 0.62.
An additional factor that Dr. Bortoletto and his collaborators took into account was the variable prevalence of Rh factor by ethnicity; in the United States, it’s most common in white men and least common in Asian men, with intermediate prevalence in African American and Hispanic men.
When paternal ethnicity was included in the analysis, savings were greatest with white sperm donors, at $1,889,000 per 100,000 Rh negative IVF pregnancies. The lower prevalence of Rh factor in Asian men meant that the strategy was not cost effective in this population since it would cost a net $2,323,000 per 100,000 Rh negative IVF pregnancies.
“A targeted screening approach, by paternal ethnicity, may be a targeted strategy for cost reduction,” wrote Dr. Bortoletto and his colleagues.
Figures for cost estimates were drawn from data from the Centers for Medicare & Medicaid (CMS) using 2017 dollars. The cost for tests to determine blood type and Rh status ranged from $6 to $11, so the investigators set the cost estimate of $8.20. The cost for an antibody screen was estimated at $5.25 (range, $4-$7).
The cost for a 300 mcg dose of anti-D immune globulin was estimated at $93.93 (range, $79-$109), and administration costs were $27.04 (range, $25-$28). Kleihauer-Betke testing to determine the amount of fetal blood in maternal circulation was pinned at $10.61 (range, $8-$14).
Even when the lowest end of the cost range of anti-D immune globulin was used, a universal screening model would still realize a cost savings of $820,000 per 100,000 Rh negative IVF pregnancies. When Rh screening cost was set at $11 – at the high end of the range – “the strategy still remained favorable at $981,000,” wrote Dr. Bortoletto and his colleagues.
“Universal paternal Rh screening provides a cost saving intervention by preventing nonindicated and costly administration of anti-D immune globulin in the IVF population presenting with bleeding or trauma in pregnancy,” they wrote.
Dr. Bortoletto reported no outside sources of funding and no conflicts of interest.
[email protected]
On Twitter @karioakes
SAN ANTONIO – Implementing universal paternal Rh screening would be a cost-effective safety strategy among patients receiving in vitro fertilization, according to a model that used up-to-date data and accounted for ethnic variations in the prevalence of the Rh (D) antibody.
Using a universal Rh screening strategy for semen donors to Rh (D) negative women undergoing in vitro fertilization would result in a cost savings of $11.01 per patient, or $1,120,000 per 100,000 Rh negative IVF pregnancies, according to Pietro Bortoletto, MD, and his coauthors. Their findings were presented during a poster session at the annual meeting of the American Society for Reproductive Medicine.
If paternal Rh factor status is unknown, vaginal bleeding during pregnancy prompts maternal administration of anti-D immune globulin if the mother is Rh (D) negative to prevent hemolytic disease of the fetus and newborn. However, wrote Dr. Bortoletto and his colleagues, “in the IVF population, where paternity status is presumed to be certain, the Rh (D) status of the male partner can be used to triage Rh (D) negative women to more appropriate administration of anti-D globulin.”
To see whether a universal paternal Rh screening strategy would be cost effective, Dr. Bortoletto, a resident physician in obstetrics and gynecology at Harvard Medical School and Brigham and Women’s Hospital, both in Boston, and his collaborators constructed a decision tree to estimate cost savings. The model compared a universal paternal-screening strategy for Rh (D) negative women undergoing IVF with the current standard of practice, which does not involve routine Rh (D) screening for the sperm donor.
In constructing the model, the investigators drew on published data showing that first trimester bleeding is more common in women who undergo IVF: It occurs in one-third of these women, compared with about 20% of the general pregnant population.
They also established probability estimates of pregnancy loss before 20 weeks, with and without first trimester bleeding (0.34 and 0.18, respectively); third trimester bleeding, with and without first trimester bleeding (0.05 and 0.02); and trauma in pregnancy (0.08). They estimated the overall probability of the pregnancy producing an Rh positive neonate at 0.62.
An additional factor that Dr. Bortoletto and his collaborators took into account was the variable prevalence of Rh factor by ethnicity; in the United States, it’s most common in white men and least common in Asian men, with intermediate prevalence in African American and Hispanic men.
When paternal ethnicity was included in the analysis, savings were greatest with white sperm donors, at $1,889,000 per 100,000 Rh negative IVF pregnancies. The lower prevalence of Rh factor in Asian men meant that the strategy was not cost effective in this population since it would cost a net $2,323,000 per 100,000 Rh negative IVF pregnancies.
“A targeted screening approach, by paternal ethnicity, may be a targeted strategy for cost reduction,” wrote Dr. Bortoletto and his colleagues.
Figures for cost estimates were drawn from data from the Centers for Medicare & Medicaid (CMS) using 2017 dollars. The cost for tests to determine blood type and Rh status ranged from $6 to $11, so the investigators set the cost estimate of $8.20. The cost for an antibody screen was estimated at $5.25 (range, $4-$7).
The cost for a 300 mcg dose of anti-D immune globulin was estimated at $93.93 (range, $79-$109), and administration costs were $27.04 (range, $25-$28). Kleihauer-Betke testing to determine the amount of fetal blood in maternal circulation was pinned at $10.61 (range, $8-$14).
Even when the lowest end of the cost range of anti-D immune globulin was used, a universal screening model would still realize a cost savings of $820,000 per 100,000 Rh negative IVF pregnancies. When Rh screening cost was set at $11 – at the high end of the range – “the strategy still remained favorable at $981,000,” wrote Dr. Bortoletto and his colleagues.
“Universal paternal Rh screening provides a cost saving intervention by preventing nonindicated and costly administration of anti-D immune globulin in the IVF population presenting with bleeding or trauma in pregnancy,” they wrote.
Dr. Bortoletto reported no outside sources of funding and no conflicts of interest.
[email protected]
On Twitter @karioakes
SAN ANTONIO – Implementing universal paternal Rh screening would be a cost-effective safety strategy among patients receiving in vitro fertilization, according to a model that used up-to-date data and accounted for ethnic variations in the prevalence of the Rh (D) antibody.
Using a universal Rh screening strategy for semen donors to Rh (D) negative women undergoing in vitro fertilization would result in a cost savings of $11.01 per patient, or $1,120,000 per 100,000 Rh negative IVF pregnancies, according to Pietro Bortoletto, MD, and his coauthors. Their findings were presented during a poster session at the annual meeting of the American Society for Reproductive Medicine.
If paternal Rh factor status is unknown, vaginal bleeding during pregnancy prompts maternal administration of anti-D immune globulin if the mother is Rh (D) negative to prevent hemolytic disease of the fetus and newborn. However, wrote Dr. Bortoletto and his colleagues, “in the IVF population, where paternity status is presumed to be certain, the Rh (D) status of the male partner can be used to triage Rh (D) negative women to more appropriate administration of anti-D globulin.”
To see whether a universal paternal Rh screening strategy would be cost effective, Dr. Bortoletto, a resident physician in obstetrics and gynecology at Harvard Medical School and Brigham and Women’s Hospital, both in Boston, and his collaborators constructed a decision tree to estimate cost savings. The model compared a universal paternal-screening strategy for Rh (D) negative women undergoing IVF with the current standard of practice, which does not involve routine Rh (D) screening for the sperm donor.
In constructing the model, the investigators drew on published data showing that first trimester bleeding is more common in women who undergo IVF: It occurs in one-third of these women, compared with about 20% of the general pregnant population.
They also established probability estimates of pregnancy loss before 20 weeks, with and without first trimester bleeding (0.34 and 0.18, respectively); third trimester bleeding, with and without first trimester bleeding (0.05 and 0.02); and trauma in pregnancy (0.08). They estimated the overall probability of the pregnancy producing an Rh positive neonate at 0.62.
An additional factor that Dr. Bortoletto and his collaborators took into account was the variable prevalence of Rh factor by ethnicity; in the United States, it’s most common in white men and least common in Asian men, with intermediate prevalence in African American and Hispanic men.
When paternal ethnicity was included in the analysis, savings were greatest with white sperm donors, at $1,889,000 per 100,000 Rh negative IVF pregnancies. The lower prevalence of Rh factor in Asian men meant that the strategy was not cost effective in this population since it would cost a net $2,323,000 per 100,000 Rh negative IVF pregnancies.
“A targeted screening approach, by paternal ethnicity, may be a targeted strategy for cost reduction,” wrote Dr. Bortoletto and his colleagues.
Figures for cost estimates were drawn from data from the Centers for Medicare & Medicaid (CMS) using 2017 dollars. The cost for tests to determine blood type and Rh status ranged from $6 to $11, so the investigators set the cost estimate of $8.20. The cost for an antibody screen was estimated at $5.25 (range, $4-$7).
The cost for a 300 mcg dose of anti-D immune globulin was estimated at $93.93 (range, $79-$109), and administration costs were $27.04 (range, $25-$28). Kleihauer-Betke testing to determine the amount of fetal blood in maternal circulation was pinned at $10.61 (range, $8-$14).
Even when the lowest end of the cost range of anti-D immune globulin was used, a universal screening model would still realize a cost savings of $820,000 per 100,000 Rh negative IVF pregnancies. When Rh screening cost was set at $11 – at the high end of the range – “the strategy still remained favorable at $981,000,” wrote Dr. Bortoletto and his colleagues.
“Universal paternal Rh screening provides a cost saving intervention by preventing nonindicated and costly administration of anti-D immune globulin in the IVF population presenting with bleeding or trauma in pregnancy,” they wrote.
Dr. Bortoletto reported no outside sources of funding and no conflicts of interest.
[email protected]
On Twitter @karioakes
At ASRM 2017
Key clinical point:
Major finding: Universal screening would save $1,120,000 per 100,000 Rh negative IVF pregnancies.
Data source: Decision tree incorporating Rh (D) prevalence, bleeding risk, and cost data.
Disclosures: Dr. Bortoletto reported having no relevant disclosures and no outside funding.
Online Conference Library Available
For 44 years, the latest pharmacologic, radiologic, surgical, and endovascular techniques and technologies have been presented at the VEITHsymposium, along with discussions of when these treatments are indicated. Updates on clinical trials and opportunities for dialogue with experts in the field provide insight along with the latest results of the various treatment modalities.
This information is packed into a single meeting with as many short presentations as possible - some of them concurrent. Access has been facilitated by providing electronically archived material, including talks, slides, and panels after the meeting.
To avoid the conundrum of having to choose between concurrent sessions, meeting attendees can access them through this year’s online library, which can be found at www.veithondemand.com. For individuals unable to attend the meeting, the online library is CME accredited so that they may receive credit for their educational viewing experience.
VEITHsymposium has partnered with Edge Creek Media, Inc. to digitally capture the presentations from this year’s event and make them available online for cross-platform and mobile device viewing. Each webcast presentation will be produced with synchronous presenter audio, and slide content to reproduce the presentations.
The online library will contain over 1,200 webcast presentations and will include presentations from approximately 600 expert speakers. This library will recreate the remarkable educational experience of attending in person. It will also provide an invaluable resource of ongoing vascular information.
Edge Creek Media is a specialized multimedia production company that offers custom live event recording and webcast streaming solutions. Other core services include mobile app development, live event production, audio/video post production, and multimedia web development.
For more information, visit www.edgecreekmedia.com or contact the company at [email protected]. ■
For 44 years, the latest pharmacologic, radiologic, surgical, and endovascular techniques and technologies have been presented at the VEITHsymposium, along with discussions of when these treatments are indicated. Updates on clinical trials and opportunities for dialogue with experts in the field provide insight along with the latest results of the various treatment modalities.
This information is packed into a single meeting with as many short presentations as possible - some of them concurrent. Access has been facilitated by providing electronically archived material, including talks, slides, and panels after the meeting.
To avoid the conundrum of having to choose between concurrent sessions, meeting attendees can access them through this year’s online library, which can be found at www.veithondemand.com. For individuals unable to attend the meeting, the online library is CME accredited so that they may receive credit for their educational viewing experience.
VEITHsymposium has partnered with Edge Creek Media, Inc. to digitally capture the presentations from this year’s event and make them available online for cross-platform and mobile device viewing. Each webcast presentation will be produced with synchronous presenter audio, and slide content to reproduce the presentations.
The online library will contain over 1,200 webcast presentations and will include presentations from approximately 600 expert speakers. This library will recreate the remarkable educational experience of attending in person. It will also provide an invaluable resource of ongoing vascular information.
Edge Creek Media is a specialized multimedia production company that offers custom live event recording and webcast streaming solutions. Other core services include mobile app development, live event production, audio/video post production, and multimedia web development.
For more information, visit www.edgecreekmedia.com or contact the company at [email protected]. ■
For 44 years, the latest pharmacologic, radiologic, surgical, and endovascular techniques and technologies have been presented at the VEITHsymposium, along with discussions of when these treatments are indicated. Updates on clinical trials and opportunities for dialogue with experts in the field provide insight along with the latest results of the various treatment modalities.
This information is packed into a single meeting with as many short presentations as possible - some of them concurrent. Access has been facilitated by providing electronically archived material, including talks, slides, and panels after the meeting.
To avoid the conundrum of having to choose between concurrent sessions, meeting attendees can access them through this year’s online library, which can be found at www.veithondemand.com. For individuals unable to attend the meeting, the online library is CME accredited so that they may receive credit for their educational viewing experience.
VEITHsymposium has partnered with Edge Creek Media, Inc. to digitally capture the presentations from this year’s event and make them available online for cross-platform and mobile device viewing. Each webcast presentation will be produced with synchronous presenter audio, and slide content to reproduce the presentations.
The online library will contain over 1,200 webcast presentations and will include presentations from approximately 600 expert speakers. This library will recreate the remarkable educational experience of attending in person. It will also provide an invaluable resource of ongoing vascular information.
Edge Creek Media is a specialized multimedia production company that offers custom live event recording and webcast streaming solutions. Other core services include mobile app development, live event production, audio/video post production, and multimedia web development.
For more information, visit www.edgecreekmedia.com or contact the company at [email protected]. ■
Stenting improved outcomes for treating chronic IVC obstruction
Stenting improved outcomes in patients with chronic obstruction of the inferior vena cava (IVC), according to a retrospective analysis of medical records from March 2010 to September 2015.
In the study, 19 of 20 patients with chronic IVC obstruction had successful stent placement, with most resulting in improved and sustained clinical outcomes. The study, conducted by Ole Jørgen Grøtta, MD, and colleagues, was reported in the European Journal of Vascular and Endovascular Surgery (2017;54:620-8).
Dr. Grøtta and colleagues investigated 11 men and 9 women patients, with a median age 43years, and with venography-verified chronic IVC obstruction. Most patients also were screened and found to be positive for thrombophilia. Median follow-up was 25 months (range, 3-70 months).
The researchers reported on 13 patients with IVC occlusion and 7 with stenosis. Patient clinical status and symptom severity was assessed according to standardized CEAP (clinical, etiology, anatomy and pathophysiology) classification and the venous clinical severity score (VCSS), respectively. Sixteen patients presented chronic venous disease (CVD) symptoms in their lower limbs; of these, nine exhibited symptoms of an acute thrombosis and, based on their symptoms, seven were assigned to a specific CEAP category, ranging from C3-C6. The additional four patients included in the study had been referred to receive endovascular treatment intervention based on symptoms consistent with physical activity–related reduced cardiac preload.
A total of 13 of 19 (68%) patients showed sustained and significant clinical improvement, seen as a shift from the baseline VCSS score of 8.5 down to 7.0 (P = .007) at final follow-up. This included all four of the patients identified as having reduced cardiac preload problems. The authors also reported that here were no periprocedural or long-term complications.
The need for an alternative treatment for patients with chronic obstruction of the IVC is exemplified by typically poor sustained clinical improvement outcomes and/or gradual clinical deterioration when using the traditional conservative approach of anticoagulants combined with compression stockings, according to the authors.
“The endovascular approach with stent placement for chronic IVC obstructions is a safe treatment alternative that should be offered to patients who otherwise have little opportunity for clinical improvement,” they concluded.
The authors reported that they had no conflicts of interest.
Stenting improved outcomes in patients with chronic obstruction of the inferior vena cava (IVC), according to a retrospective analysis of medical records from March 2010 to September 2015.
In the study, 19 of 20 patients with chronic IVC obstruction had successful stent placement, with most resulting in improved and sustained clinical outcomes. The study, conducted by Ole Jørgen Grøtta, MD, and colleagues, was reported in the European Journal of Vascular and Endovascular Surgery (2017;54:620-8).
Dr. Grøtta and colleagues investigated 11 men and 9 women patients, with a median age 43years, and with venography-verified chronic IVC obstruction. Most patients also were screened and found to be positive for thrombophilia. Median follow-up was 25 months (range, 3-70 months).
The researchers reported on 13 patients with IVC occlusion and 7 with stenosis. Patient clinical status and symptom severity was assessed according to standardized CEAP (clinical, etiology, anatomy and pathophysiology) classification and the venous clinical severity score (VCSS), respectively. Sixteen patients presented chronic venous disease (CVD) symptoms in their lower limbs; of these, nine exhibited symptoms of an acute thrombosis and, based on their symptoms, seven were assigned to a specific CEAP category, ranging from C3-C6. The additional four patients included in the study had been referred to receive endovascular treatment intervention based on symptoms consistent with physical activity–related reduced cardiac preload.
A total of 13 of 19 (68%) patients showed sustained and significant clinical improvement, seen as a shift from the baseline VCSS score of 8.5 down to 7.0 (P = .007) at final follow-up. This included all four of the patients identified as having reduced cardiac preload problems. The authors also reported that here were no periprocedural or long-term complications.
The need for an alternative treatment for patients with chronic obstruction of the IVC is exemplified by typically poor sustained clinical improvement outcomes and/or gradual clinical deterioration when using the traditional conservative approach of anticoagulants combined with compression stockings, according to the authors.
“The endovascular approach with stent placement for chronic IVC obstructions is a safe treatment alternative that should be offered to patients who otherwise have little opportunity for clinical improvement,” they concluded.
The authors reported that they had no conflicts of interest.
Stenting improved outcomes in patients with chronic obstruction of the inferior vena cava (IVC), according to a retrospective analysis of medical records from March 2010 to September 2015.
In the study, 19 of 20 patients with chronic IVC obstruction had successful stent placement, with most resulting in improved and sustained clinical outcomes. The study, conducted by Ole Jørgen Grøtta, MD, and colleagues, was reported in the European Journal of Vascular and Endovascular Surgery (2017;54:620-8).
Dr. Grøtta and colleagues investigated 11 men and 9 women patients, with a median age 43years, and with venography-verified chronic IVC obstruction. Most patients also were screened and found to be positive for thrombophilia. Median follow-up was 25 months (range, 3-70 months).
The researchers reported on 13 patients with IVC occlusion and 7 with stenosis. Patient clinical status and symptom severity was assessed according to standardized CEAP (clinical, etiology, anatomy and pathophysiology) classification and the venous clinical severity score (VCSS), respectively. Sixteen patients presented chronic venous disease (CVD) symptoms in their lower limbs; of these, nine exhibited symptoms of an acute thrombosis and, based on their symptoms, seven were assigned to a specific CEAP category, ranging from C3-C6. The additional four patients included in the study had been referred to receive endovascular treatment intervention based on symptoms consistent with physical activity–related reduced cardiac preload.
A total of 13 of 19 (68%) patients showed sustained and significant clinical improvement, seen as a shift from the baseline VCSS score of 8.5 down to 7.0 (P = .007) at final follow-up. This included all four of the patients identified as having reduced cardiac preload problems. The authors also reported that here were no periprocedural or long-term complications.
The need for an alternative treatment for patients with chronic obstruction of the IVC is exemplified by typically poor sustained clinical improvement outcomes and/or gradual clinical deterioration when using the traditional conservative approach of anticoagulants combined with compression stockings, according to the authors.
“The endovascular approach with stent placement for chronic IVC obstructions is a safe treatment alternative that should be offered to patients who otherwise have little opportunity for clinical improvement,” they concluded.
The authors reported that they had no conflicts of interest.
FROM THE EUROPEAN JOURNAL OF VASCULAR AND ENDOVASCULAR SURGERY
Key clinical point:
Major finding: Most patients showed a significant improvement from the baseline score of 8.5 score down to 7.0 (P = .007).
Data source: A retrospective analysis of 20 patients referred to the Norwegian National Unit for Reconstructive Deep Venous Surgery for stenting.
Disclosures: The authors reported they had no conflict of interest.
Day-Long Program to Provide Comprehensive Overview of Hemodialysis Issues
Optimizing care of dialysis patients will be the focus of a comprehensive program with five sessions, “New Developments in Vascular Access for Hemodialysis,” taking place all day Saturday.
“Chronic kidney disease (CKD) has become an epidemic in the United States. Medicare spending for patients with CKD ages 65 and older exceeded $50 billion in 2013 and represented 20% of all Medicare spending for this age group. This epidemic has been driven by the rise in diabetes, hypertension and obesity and has resulted in a staggering increase in the number of patients requiring hemodialysis,” stated program organizer Dr. Larry A. Scher, professor of clinical surgery at Albert Einstein College of Medicine and attending surgeon at Montefiore Medical Center.
“Providing functioning vascular access for these patients has become a significant challenge for vascular surgeons, transplant surgeons, interventional nephrologists, and interventional radiologists along with nephrologists, nurses, dialysis technicians, and others interested in optimizing the care of dialysis patients,” he continued. “These practitioners are the target audience for this program, which will address many important topics in hemodialysis access.”
There will be five sessions covering issues in the field, optimization of outcomes, political, economic and legal topics, new technologies and concepts, and updates on clinical challenges.
The first sessions will cover important issues in the field and outcome optimization. Experts will address topics such as fistula maturation, use of ultrasound for access planning and cannulation, importance of dialysis maturation, significance of dialysis blood flow, and the use of stent grafts and drug-eluting balloons. Other talks will address cognitive function in patients with chronic kidney disease, measuring cardiac output in the dialysis improve patient safety, review of significant contributions to the literature, and an update on the mission of Kidney Health International.
“We are honored to have Harald C. Ott, MD, principal investigator at the Ott Laboratory for Organ Engineering and Regeneration at Massachusetts General Hospital as our guest speaker,” said Dr. Scher. “There is a critical shortage of kidneys available for transplantation, and Dr. Ott has performed important research on reengineered organs.” His presentation will be on the revolution in renal replacement therapy, specifically the current status of the bio-artificial kidney.
“The talk should be of great interest to medical professionals interested in improving care for our patients with end-stage renal disease,” said Dr. Scher. Other session talks will discuss Medicare costs for patients on hemodialysis, changes in reimbursement for outpatient procedures, and training of vascular access surgeons.
“The segment on new technologies and concepts will present updated results of several important clinical trials, including efforts aimed at improving fistula maturation with elastase, sirolimus, and the VasQ device,” explained Dr. Scher. Results will be presented of trials of minimally invasive technologies for creating hemodialysis access. Also covered will be a unique sensor capable of providing remote monitoring of AV fistulas and grafts, as well as the RADAR technique, which emphasizes the importance of hemodynamics in arteriovenous fistula maturation.
“The final session will delve into clinical issues in hemodialysis access,” said Dr. Scher. “There will be several talks about achieving successful access in challenging patient populations including obese, elderly and hypercoagulable patients, as well as patients with implantable cardiac devices.” Subject areas will include the role of biologic grafts in hemodialysis access and management of dialysis access complications, including steal syndrome, high flow fistula, central venous stenosis, aneurysms, and infection.
“We have assembled an expert faculty that will offer a comprehensive overview of a wide-range of topics of interest to physicians and allied professionals who care for patients with end-stage renal disease,” said Dr. Scher. “Panel discussions will further enhance the program, allowing attendees to not only interact with faculty, but also discuss topics of interest and concern to their clinical practices.” ■
Optimizing care of dialysis patients will be the focus of a comprehensive program with five sessions, “New Developments in Vascular Access for Hemodialysis,” taking place all day Saturday.
“Chronic kidney disease (CKD) has become an epidemic in the United States. Medicare spending for patients with CKD ages 65 and older exceeded $50 billion in 2013 and represented 20% of all Medicare spending for this age group. This epidemic has been driven by the rise in diabetes, hypertension and obesity and has resulted in a staggering increase in the number of patients requiring hemodialysis,” stated program organizer Dr. Larry A. Scher, professor of clinical surgery at Albert Einstein College of Medicine and attending surgeon at Montefiore Medical Center.
“Providing functioning vascular access for these patients has become a significant challenge for vascular surgeons, transplant surgeons, interventional nephrologists, and interventional radiologists along with nephrologists, nurses, dialysis technicians, and others interested in optimizing the care of dialysis patients,” he continued. “These practitioners are the target audience for this program, which will address many important topics in hemodialysis access.”
There will be five sessions covering issues in the field, optimization of outcomes, political, economic and legal topics, new technologies and concepts, and updates on clinical challenges.
The first sessions will cover important issues in the field and outcome optimization. Experts will address topics such as fistula maturation, use of ultrasound for access planning and cannulation, importance of dialysis maturation, significance of dialysis blood flow, and the use of stent grafts and drug-eluting balloons. Other talks will address cognitive function in patients with chronic kidney disease, measuring cardiac output in the dialysis improve patient safety, review of significant contributions to the literature, and an update on the mission of Kidney Health International.
“We are honored to have Harald C. Ott, MD, principal investigator at the Ott Laboratory for Organ Engineering and Regeneration at Massachusetts General Hospital as our guest speaker,” said Dr. Scher. “There is a critical shortage of kidneys available for transplantation, and Dr. Ott has performed important research on reengineered organs.” His presentation will be on the revolution in renal replacement therapy, specifically the current status of the bio-artificial kidney.
“The talk should be of great interest to medical professionals interested in improving care for our patients with end-stage renal disease,” said Dr. Scher. Other session talks will discuss Medicare costs for patients on hemodialysis, changes in reimbursement for outpatient procedures, and training of vascular access surgeons.
“The segment on new technologies and concepts will present updated results of several important clinical trials, including efforts aimed at improving fistula maturation with elastase, sirolimus, and the VasQ device,” explained Dr. Scher. Results will be presented of trials of minimally invasive technologies for creating hemodialysis access. Also covered will be a unique sensor capable of providing remote monitoring of AV fistulas and grafts, as well as the RADAR technique, which emphasizes the importance of hemodynamics in arteriovenous fistula maturation.
“The final session will delve into clinical issues in hemodialysis access,” said Dr. Scher. “There will be several talks about achieving successful access in challenging patient populations including obese, elderly and hypercoagulable patients, as well as patients with implantable cardiac devices.” Subject areas will include the role of biologic grafts in hemodialysis access and management of dialysis access complications, including steal syndrome, high flow fistula, central venous stenosis, aneurysms, and infection.
“We have assembled an expert faculty that will offer a comprehensive overview of a wide-range of topics of interest to physicians and allied professionals who care for patients with end-stage renal disease,” said Dr. Scher. “Panel discussions will further enhance the program, allowing attendees to not only interact with faculty, but also discuss topics of interest and concern to their clinical practices.” ■
Optimizing care of dialysis patients will be the focus of a comprehensive program with five sessions, “New Developments in Vascular Access for Hemodialysis,” taking place all day Saturday.
“Chronic kidney disease (CKD) has become an epidemic in the United States. Medicare spending for patients with CKD ages 65 and older exceeded $50 billion in 2013 and represented 20% of all Medicare spending for this age group. This epidemic has been driven by the rise in diabetes, hypertension and obesity and has resulted in a staggering increase in the number of patients requiring hemodialysis,” stated program organizer Dr. Larry A. Scher, professor of clinical surgery at Albert Einstein College of Medicine and attending surgeon at Montefiore Medical Center.
“Providing functioning vascular access for these patients has become a significant challenge for vascular surgeons, transplant surgeons, interventional nephrologists, and interventional radiologists along with nephrologists, nurses, dialysis technicians, and others interested in optimizing the care of dialysis patients,” he continued. “These practitioners are the target audience for this program, which will address many important topics in hemodialysis access.”
There will be five sessions covering issues in the field, optimization of outcomes, political, economic and legal topics, new technologies and concepts, and updates on clinical challenges.
The first sessions will cover important issues in the field and outcome optimization. Experts will address topics such as fistula maturation, use of ultrasound for access planning and cannulation, importance of dialysis maturation, significance of dialysis blood flow, and the use of stent grafts and drug-eluting balloons. Other talks will address cognitive function in patients with chronic kidney disease, measuring cardiac output in the dialysis improve patient safety, review of significant contributions to the literature, and an update on the mission of Kidney Health International.
“We are honored to have Harald C. Ott, MD, principal investigator at the Ott Laboratory for Organ Engineering and Regeneration at Massachusetts General Hospital as our guest speaker,” said Dr. Scher. “There is a critical shortage of kidneys available for transplantation, and Dr. Ott has performed important research on reengineered organs.” His presentation will be on the revolution in renal replacement therapy, specifically the current status of the bio-artificial kidney.
“The talk should be of great interest to medical professionals interested in improving care for our patients with end-stage renal disease,” said Dr. Scher. Other session talks will discuss Medicare costs for patients on hemodialysis, changes in reimbursement for outpatient procedures, and training of vascular access surgeons.
“The segment on new technologies and concepts will present updated results of several important clinical trials, including efforts aimed at improving fistula maturation with elastase, sirolimus, and the VasQ device,” explained Dr. Scher. Results will be presented of trials of minimally invasive technologies for creating hemodialysis access. Also covered will be a unique sensor capable of providing remote monitoring of AV fistulas and grafts, as well as the RADAR technique, which emphasizes the importance of hemodynamics in arteriovenous fistula maturation.
“The final session will delve into clinical issues in hemodialysis access,” said Dr. Scher. “There will be several talks about achieving successful access in challenging patient populations including obese, elderly and hypercoagulable patients, as well as patients with implantable cardiac devices.” Subject areas will include the role of biologic grafts in hemodialysis access and management of dialysis access complications, including steal syndrome, high flow fistula, central venous stenosis, aneurysms, and infection.
“We have assembled an expert faculty that will offer a comprehensive overview of a wide-range of topics of interest to physicians and allied professionals who care for patients with end-stage renal disease,” said Dr. Scher. “Panel discussions will further enhance the program, allowing attendees to not only interact with faculty, but also discuss topics of interest and concern to their clinical practices.” ■
FDA approves obinutuzumab for follicular lymphoma
The Food and Drug Administration has approved obinutuzumab in combination with chemotherapy, followed by obinutuzumab alone in those who responded, for people with previously untreated advanced follicular lymphoma (stage II bulky, III or IV).
The most common adverse events associated with obinutuzumab were infusion reactions, low white blood cell count, upper respiratory tract infection, cough, constipation, and diarrhea. The most common significant adverse events are low white blood cell count, low white blood cell count with fever, and low platelet count.
Obinutuzumab is marketed as Gazyva by Genentech.
“Today’s Gazyva approval is an important advance for the thousands of people diagnosed each year with follicular lymphoma who hope to delay disease progression for as long as possible,” said Sarah Horning, MD, chief medical officer and head of global product development at Genentech, in the company press release.
The Food and Drug Administration has approved obinutuzumab in combination with chemotherapy, followed by obinutuzumab alone in those who responded, for people with previously untreated advanced follicular lymphoma (stage II bulky, III or IV).
The most common adverse events associated with obinutuzumab were infusion reactions, low white blood cell count, upper respiratory tract infection, cough, constipation, and diarrhea. The most common significant adverse events are low white blood cell count, low white blood cell count with fever, and low platelet count.
Obinutuzumab is marketed as Gazyva by Genentech.
“Today’s Gazyva approval is an important advance for the thousands of people diagnosed each year with follicular lymphoma who hope to delay disease progression for as long as possible,” said Sarah Horning, MD, chief medical officer and head of global product development at Genentech, in the company press release.
The Food and Drug Administration has approved obinutuzumab in combination with chemotherapy, followed by obinutuzumab alone in those who responded, for people with previously untreated advanced follicular lymphoma (stage II bulky, III or IV).
The most common adverse events associated with obinutuzumab were infusion reactions, low white blood cell count, upper respiratory tract infection, cough, constipation, and diarrhea. The most common significant adverse events are low white blood cell count, low white blood cell count with fever, and low platelet count.
Obinutuzumab is marketed as Gazyva by Genentech.
“Today’s Gazyva approval is an important advance for the thousands of people diagnosed each year with follicular lymphoma who hope to delay disease progression for as long as possible,” said Sarah Horning, MD, chief medical officer and head of global product development at Genentech, in the company press release.
Special Focus on Management of Superficial Vein Thrombosis
The options for treatment and management of superficial vein thrombosis will be the focus of “Venous Imaging, Thrombophilia” on Saturday morning.
“This session will include talks on management of superficial vein thrombosis using direct oral anticoagulants, balancing anticoagulation with bleeding risk after surgery, and predicting patients at risk of post-thrombotic syndrome,” said Dr. Ian J. Franklin of the Imperial College and London Vascular Clinic. Dr. Franklin is co-moderator of the second half of the morning session. “There is much variation in practice in these areas, which will be addressed during the presentations,” he said.
“We have a fairly decent grasp regarding the optimal management of some aspects of venous disease,” added co-moderator Dr. Timothy K. Liem, professor of surgery at Oregon Health & Science University and codirector for quality at the Knight Cardiovascular Institute. “For example, in patients with proximal deep vein thrombosis or pulmonary embolism, the vast majority of clinicians would administer therapeutic anticoagulation for at least 3 months. However, when it comes to other very common venous problems and scenarios, such as superficial vein thrombosis (with or without the presence of venous reflux), there are still significant knowledge gaps with regard to optimal care. The same goes for perioperative management of anticoagulation and prevention of post-thrombotic syndrome,” he continued. “This has led to significant variability in the ways patients are treated. Attendees will learn more about these issues and ways to better manage their patients.”
Dr. Franklin and Dr. Liem will each be making several presentations.
“Trial evidence is consistent in showing that risk of venous thromboembolism (VTE) in patients with superficial vein thrombosis is reduced significantly by prolonged treatment with anticoagulants, but the number needed to prevent one VTE episode is more than 80,” explained Dr. Franklin. “This presents problems relating to cost and clinical effectiveness, which will be discussed in the session.” In one talk, Dr. Franklin will be discussing the grading of severity of venous thrombophlebitis and variation of treatment between primary and secondary care. He will also be covering treatment options: anticoagulation, compression, and follow-up.
Dr. Liem will be highlighting anticoagulation issues, looking at the use of direct oral anticoagulants in one talk and management of anticoagulation to avoid postoperative hemorrhage in another. “The presentations will allow attendees who specialize in venous disease to understand when to anticoagulate and when to administer compression for patients with superficial vein thrombosis,” he stated. “It will also allow these physicians to better identify patients who are at increased risk of developing post-thrombotic syndrome.” In addition, he noted, “we hope to provide a better understanding regarding optimal strategies for managing coagulation that minimize the risk of postoperative hemorrhage while reducing the risk for recurrent thromboembolism during surgery or other invasive procedures.”
Dr. Tomasz Urbanek of the Medical University of Silesia, Katowice, Poland, will present the final talk on the predictive factors of post-thrombotic syndrome. When asked how the session might influence the practices of those in attendance, Dr. Franklin replied, “Hopefully, it will result in more rational use of anticoagulation treatment for patients with superficial vein thrombosis, better use of direct oral anticoagulants as a treatment option, and safer surgery on anticoagulated patients.”
Dr. Liem concluded, “These sessions will have the goal of helping clinicians standardize as much of our care as possible.” Dr. Franklin added, “The take-home message is better risk stratification may help rationalize treatment.” ■
The options for treatment and management of superficial vein thrombosis will be the focus of “Venous Imaging, Thrombophilia” on Saturday morning.
“This session will include talks on management of superficial vein thrombosis using direct oral anticoagulants, balancing anticoagulation with bleeding risk after surgery, and predicting patients at risk of post-thrombotic syndrome,” said Dr. Ian J. Franklin of the Imperial College and London Vascular Clinic. Dr. Franklin is co-moderator of the second half of the morning session. “There is much variation in practice in these areas, which will be addressed during the presentations,” he said.
“We have a fairly decent grasp regarding the optimal management of some aspects of venous disease,” added co-moderator Dr. Timothy K. Liem, professor of surgery at Oregon Health & Science University and codirector for quality at the Knight Cardiovascular Institute. “For example, in patients with proximal deep vein thrombosis or pulmonary embolism, the vast majority of clinicians would administer therapeutic anticoagulation for at least 3 months. However, when it comes to other very common venous problems and scenarios, such as superficial vein thrombosis (with or without the presence of venous reflux), there are still significant knowledge gaps with regard to optimal care. The same goes for perioperative management of anticoagulation and prevention of post-thrombotic syndrome,” he continued. “This has led to significant variability in the ways patients are treated. Attendees will learn more about these issues and ways to better manage their patients.”
Dr. Franklin and Dr. Liem will each be making several presentations.
“Trial evidence is consistent in showing that risk of venous thromboembolism (VTE) in patients with superficial vein thrombosis is reduced significantly by prolonged treatment with anticoagulants, but the number needed to prevent one VTE episode is more than 80,” explained Dr. Franklin. “This presents problems relating to cost and clinical effectiveness, which will be discussed in the session.” In one talk, Dr. Franklin will be discussing the grading of severity of venous thrombophlebitis and variation of treatment between primary and secondary care. He will also be covering treatment options: anticoagulation, compression, and follow-up.
Dr. Liem will be highlighting anticoagulation issues, looking at the use of direct oral anticoagulants in one talk and management of anticoagulation to avoid postoperative hemorrhage in another. “The presentations will allow attendees who specialize in venous disease to understand when to anticoagulate and when to administer compression for patients with superficial vein thrombosis,” he stated. “It will also allow these physicians to better identify patients who are at increased risk of developing post-thrombotic syndrome.” In addition, he noted, “we hope to provide a better understanding regarding optimal strategies for managing coagulation that minimize the risk of postoperative hemorrhage while reducing the risk for recurrent thromboembolism during surgery or other invasive procedures.”
Dr. Tomasz Urbanek of the Medical University of Silesia, Katowice, Poland, will present the final talk on the predictive factors of post-thrombotic syndrome. When asked how the session might influence the practices of those in attendance, Dr. Franklin replied, “Hopefully, it will result in more rational use of anticoagulation treatment for patients with superficial vein thrombosis, better use of direct oral anticoagulants as a treatment option, and safer surgery on anticoagulated patients.”
Dr. Liem concluded, “These sessions will have the goal of helping clinicians standardize as much of our care as possible.” Dr. Franklin added, “The take-home message is better risk stratification may help rationalize treatment.” ■
The options for treatment and management of superficial vein thrombosis will be the focus of “Venous Imaging, Thrombophilia” on Saturday morning.
“This session will include talks on management of superficial vein thrombosis using direct oral anticoagulants, balancing anticoagulation with bleeding risk after surgery, and predicting patients at risk of post-thrombotic syndrome,” said Dr. Ian J. Franklin of the Imperial College and London Vascular Clinic. Dr. Franklin is co-moderator of the second half of the morning session. “There is much variation in practice in these areas, which will be addressed during the presentations,” he said.
“We have a fairly decent grasp regarding the optimal management of some aspects of venous disease,” added co-moderator Dr. Timothy K. Liem, professor of surgery at Oregon Health & Science University and codirector for quality at the Knight Cardiovascular Institute. “For example, in patients with proximal deep vein thrombosis or pulmonary embolism, the vast majority of clinicians would administer therapeutic anticoagulation for at least 3 months. However, when it comes to other very common venous problems and scenarios, such as superficial vein thrombosis (with or without the presence of venous reflux), there are still significant knowledge gaps with regard to optimal care. The same goes for perioperative management of anticoagulation and prevention of post-thrombotic syndrome,” he continued. “This has led to significant variability in the ways patients are treated. Attendees will learn more about these issues and ways to better manage their patients.”
Dr. Franklin and Dr. Liem will each be making several presentations.
“Trial evidence is consistent in showing that risk of venous thromboembolism (VTE) in patients with superficial vein thrombosis is reduced significantly by prolonged treatment with anticoagulants, but the number needed to prevent one VTE episode is more than 80,” explained Dr. Franklin. “This presents problems relating to cost and clinical effectiveness, which will be discussed in the session.” In one talk, Dr. Franklin will be discussing the grading of severity of venous thrombophlebitis and variation of treatment between primary and secondary care. He will also be covering treatment options: anticoagulation, compression, and follow-up.
Dr. Liem will be highlighting anticoagulation issues, looking at the use of direct oral anticoagulants in one talk and management of anticoagulation to avoid postoperative hemorrhage in another. “The presentations will allow attendees who specialize in venous disease to understand when to anticoagulate and when to administer compression for patients with superficial vein thrombosis,” he stated. “It will also allow these physicians to better identify patients who are at increased risk of developing post-thrombotic syndrome.” In addition, he noted, “we hope to provide a better understanding regarding optimal strategies for managing coagulation that minimize the risk of postoperative hemorrhage while reducing the risk for recurrent thromboembolism during surgery or other invasive procedures.”
Dr. Tomasz Urbanek of the Medical University of Silesia, Katowice, Poland, will present the final talk on the predictive factors of post-thrombotic syndrome. When asked how the session might influence the practices of those in attendance, Dr. Franklin replied, “Hopefully, it will result in more rational use of anticoagulation treatment for patients with superficial vein thrombosis, better use of direct oral anticoagulants as a treatment option, and safer surgery on anticoagulated patients.”
Dr. Liem concluded, “These sessions will have the goal of helping clinicians standardize as much of our care as possible.” Dr. Franklin added, “The take-home message is better risk stratification may help rationalize treatment.” ■
Restrictive transfusion strategy safe in cardiac surgery
ANAHEIM, CALIF. – Waiting to transfuse heart surgery patients until their hemoglobin drops below 7.5 g/dL is just as safe as transfusing them when their hemoglobin drops below 9.5 g/dL, and it saves a lot of blood, according to the TRICS III randomized, noninferiority trial of nearly 5,000 patients undergoing cardiac surgery with cardiopulmonary bypass.
Cardiac surgeons have been moving to more restrictive transfusion policies following reports of worse postoperative survival when patients are transfused. However, there are concerns about safety and uncertainty over whether it’s the transfusions themselves that are problematic or whether transfused patients do worse because they are sicker to begin with. The Transfusion Requirements in Cardiac Surgery (TRICS) III trial removes some of the doubt: “A restrictive transfusion strategy is as effective and safe as a liberal strategy in patients undergoing cardiac surgery,” said lead investigator C. David Mazer, MD, a professor in the department of anesthesia at the University of Toronto.
Overall, 11.4% in the restrictive-threshold group and 12.5% in the liberal-threshold group met the study’s composite primary outcome of death from any cause, MI, stroke, and new-onset renal failure with dialysis (P less than .001 for noninferiority). There were no statistically significant between-group differences in the individual components of the composite outcome. Mortality was 3% in the restrictive group and 3.6% in the liberal group, a 15% reduction for the restrictive group.
About 52% of the patients in the restrictive arm, compared with 72.6% in the liberal arm, were transfused. When transfused, patients in the restrictive arm received a median of 2 units of red cells; liberal-arm patients received a median of 3 units. The overall cost difference was roughly $3 million, Dr. Mazer said at the American Heart Association scientific sessions.
There were no statistically significant differences in secondary outcomes. Restrictive patients were on mechanical ventilation for a median of 0.38 days and in the ICU for a median of 2.1 days; patients in the liberal arm were ventilated for a median of 0.36 days and in the ICU for a median of 1.9 days. The median hospital stay was 8 days in both groups.
Unexpectedly, patients 75 years and older did better with the restrictive transfusion strategy, with a 30% lower risk of the composite outcome. “Many people think the older you are, the higher your hemoglobin should be, and the more liberal you should be with transfusions. We didn’t find that. [It] challenges current beliefs and may be considered to be hypothesis generating; at a minimum, it highlights that a restrictive transfusion strategy appears to be safe in elderly patients,” Dr. Mazer said.
The participants were a mean of 72 years old, and 35% were women. The majority in both arms underwent coronary artery bypass surgery, valve surgery, or both. Heart transplants were excluded from the study. The trial was conducted in 19 countries, including China and India, but “the results were remarkably consistent independent of where the sites were,” he said.
Results of the TRICS III trial were published simultaneously with Dr. Mazer’s presentation (N Engl J Med. 2017 Nov 12. doi: 10.1056/NEJMoa1711818).
The trial was funded by the Canadian Institutes of Health Research, among others. Dr. Mazer reported personal fees from Amgen, Boehringer Ingelheim, Octapharma, and Pharmascience, as well as grants and personal fees from Fresenius Kabi.
This is an extremely important study. There have been multiple other trials, and, unfortunately, results have been quite equivocal. It’s incumbent upon us to figure out the best transfusion strategy, especially in cardiac surgery, since it is associated with a large amount of blood utilization. Also, there’ve been projections for a significant lack of blood supply in the future.
While the overall results showed no significant difference in outcomes between the groups, there was a numerical benefit evident in the restrictive group for the composite outcome, as well as all components of the main primary outcome except MI. This is not entirely unexpected, but we are really looking at the short-term effects here. I’m hoping that the longer-term outcomes will be evaluated, because they are extremely important.
Frank Sellke, MD , is chief of cardiothoracic surgery at Brown University in Providence, R.I. He made his comments after the study was presented at the American Heart Association scientific sessions. He was not involved with the work.
This is an extremely important study. There have been multiple other trials, and, unfortunately, results have been quite equivocal. It’s incumbent upon us to figure out the best transfusion strategy, especially in cardiac surgery, since it is associated with a large amount of blood utilization. Also, there’ve been projections for a significant lack of blood supply in the future.
While the overall results showed no significant difference in outcomes between the groups, there was a numerical benefit evident in the restrictive group for the composite outcome, as well as all components of the main primary outcome except MI. This is not entirely unexpected, but we are really looking at the short-term effects here. I’m hoping that the longer-term outcomes will be evaluated, because they are extremely important.
Frank Sellke, MD , is chief of cardiothoracic surgery at Brown University in Providence, R.I. He made his comments after the study was presented at the American Heart Association scientific sessions. He was not involved with the work.
This is an extremely important study. There have been multiple other trials, and, unfortunately, results have been quite equivocal. It’s incumbent upon us to figure out the best transfusion strategy, especially in cardiac surgery, since it is associated with a large amount of blood utilization. Also, there’ve been projections for a significant lack of blood supply in the future.
While the overall results showed no significant difference in outcomes between the groups, there was a numerical benefit evident in the restrictive group for the composite outcome, as well as all components of the main primary outcome except MI. This is not entirely unexpected, but we are really looking at the short-term effects here. I’m hoping that the longer-term outcomes will be evaluated, because they are extremely important.
Frank Sellke, MD , is chief of cardiothoracic surgery at Brown University in Providence, R.I. He made his comments after the study was presented at the American Heart Association scientific sessions. He was not involved with the work.
ANAHEIM, CALIF. – Waiting to transfuse heart surgery patients until their hemoglobin drops below 7.5 g/dL is just as safe as transfusing them when their hemoglobin drops below 9.5 g/dL, and it saves a lot of blood, according to the TRICS III randomized, noninferiority trial of nearly 5,000 patients undergoing cardiac surgery with cardiopulmonary bypass.
Cardiac surgeons have been moving to more restrictive transfusion policies following reports of worse postoperative survival when patients are transfused. However, there are concerns about safety and uncertainty over whether it’s the transfusions themselves that are problematic or whether transfused patients do worse because they are sicker to begin with. The Transfusion Requirements in Cardiac Surgery (TRICS) III trial removes some of the doubt: “A restrictive transfusion strategy is as effective and safe as a liberal strategy in patients undergoing cardiac surgery,” said lead investigator C. David Mazer, MD, a professor in the department of anesthesia at the University of Toronto.
Overall, 11.4% in the restrictive-threshold group and 12.5% in the liberal-threshold group met the study’s composite primary outcome of death from any cause, MI, stroke, and new-onset renal failure with dialysis (P less than .001 for noninferiority). There were no statistically significant between-group differences in the individual components of the composite outcome. Mortality was 3% in the restrictive group and 3.6% in the liberal group, a 15% reduction for the restrictive group.
About 52% of the patients in the restrictive arm, compared with 72.6% in the liberal arm, were transfused. When transfused, patients in the restrictive arm received a median of 2 units of red cells; liberal-arm patients received a median of 3 units. The overall cost difference was roughly $3 million, Dr. Mazer said at the American Heart Association scientific sessions.
There were no statistically significant differences in secondary outcomes. Restrictive patients were on mechanical ventilation for a median of 0.38 days and in the ICU for a median of 2.1 days; patients in the liberal arm were ventilated for a median of 0.36 days and in the ICU for a median of 1.9 days. The median hospital stay was 8 days in both groups.
Unexpectedly, patients 75 years and older did better with the restrictive transfusion strategy, with a 30% lower risk of the composite outcome. “Many people think the older you are, the higher your hemoglobin should be, and the more liberal you should be with transfusions. We didn’t find that. [It] challenges current beliefs and may be considered to be hypothesis generating; at a minimum, it highlights that a restrictive transfusion strategy appears to be safe in elderly patients,” Dr. Mazer said.
The participants were a mean of 72 years old, and 35% were women. The majority in both arms underwent coronary artery bypass surgery, valve surgery, or both. Heart transplants were excluded from the study. The trial was conducted in 19 countries, including China and India, but “the results were remarkably consistent independent of where the sites were,” he said.
Results of the TRICS III trial were published simultaneously with Dr. Mazer’s presentation (N Engl J Med. 2017 Nov 12. doi: 10.1056/NEJMoa1711818).
The trial was funded by the Canadian Institutes of Health Research, among others. Dr. Mazer reported personal fees from Amgen, Boehringer Ingelheim, Octapharma, and Pharmascience, as well as grants and personal fees from Fresenius Kabi.
ANAHEIM, CALIF. – Waiting to transfuse heart surgery patients until their hemoglobin drops below 7.5 g/dL is just as safe as transfusing them when their hemoglobin drops below 9.5 g/dL, and it saves a lot of blood, according to the TRICS III randomized, noninferiority trial of nearly 5,000 patients undergoing cardiac surgery with cardiopulmonary bypass.
Cardiac surgeons have been moving to more restrictive transfusion policies following reports of worse postoperative survival when patients are transfused. However, there are concerns about safety and uncertainty over whether it’s the transfusions themselves that are problematic or whether transfused patients do worse because they are sicker to begin with. The Transfusion Requirements in Cardiac Surgery (TRICS) III trial removes some of the doubt: “A restrictive transfusion strategy is as effective and safe as a liberal strategy in patients undergoing cardiac surgery,” said lead investigator C. David Mazer, MD, a professor in the department of anesthesia at the University of Toronto.
Overall, 11.4% in the restrictive-threshold group and 12.5% in the liberal-threshold group met the study’s composite primary outcome of death from any cause, MI, stroke, and new-onset renal failure with dialysis (P less than .001 for noninferiority). There were no statistically significant between-group differences in the individual components of the composite outcome. Mortality was 3% in the restrictive group and 3.6% in the liberal group, a 15% reduction for the restrictive group.
About 52% of the patients in the restrictive arm, compared with 72.6% in the liberal arm, were transfused. When transfused, patients in the restrictive arm received a median of 2 units of red cells; liberal-arm patients received a median of 3 units. The overall cost difference was roughly $3 million, Dr. Mazer said at the American Heart Association scientific sessions.
There were no statistically significant differences in secondary outcomes. Restrictive patients were on mechanical ventilation for a median of 0.38 days and in the ICU for a median of 2.1 days; patients in the liberal arm were ventilated for a median of 0.36 days and in the ICU for a median of 1.9 days. The median hospital stay was 8 days in both groups.
Unexpectedly, patients 75 years and older did better with the restrictive transfusion strategy, with a 30% lower risk of the composite outcome. “Many people think the older you are, the higher your hemoglobin should be, and the more liberal you should be with transfusions. We didn’t find that. [It] challenges current beliefs and may be considered to be hypothesis generating; at a minimum, it highlights that a restrictive transfusion strategy appears to be safe in elderly patients,” Dr. Mazer said.
The participants were a mean of 72 years old, and 35% were women. The majority in both arms underwent coronary artery bypass surgery, valve surgery, or both. Heart transplants were excluded from the study. The trial was conducted in 19 countries, including China and India, but “the results were remarkably consistent independent of where the sites were,” he said.
Results of the TRICS III trial were published simultaneously with Dr. Mazer’s presentation (N Engl J Med. 2017 Nov 12. doi: 10.1056/NEJMoa1711818).
The trial was funded by the Canadian Institutes of Health Research, among others. Dr. Mazer reported personal fees from Amgen, Boehringer Ingelheim, Octapharma, and Pharmascience, as well as grants and personal fees from Fresenius Kabi.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point:
Major finding: Overall, 11.4% in the restrictive-threshold group, versus 12.5% in the liberal-threshold group, met the study’s composite primary outcome of death from any cause, myocardial infarction, stroke, or new-onset renal failure with dialysis.
Data source: TRICS III, a randomized, noninferiority trial with almost 5,000 participants
Disclosures: TRICS III was funded by the Canadian Institutes of Health Research, among others. The lead investigator reported personal fees from Amgen, Boehringer Ingelheim, Octapharma, and Pharmascience, as well as grants and personal fees from Fresenius Kabi.
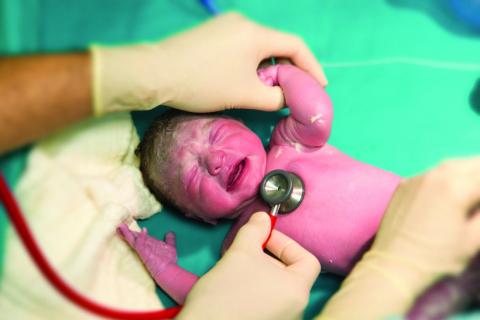
Two changes are made to resuscitation practice in delivery room
CHICAGO – , according to Gary M. Weiner, MD, of the department of pediatrics and neonatal-perinatal medicine at the University of Michigan and C.S. Mott Children’s Hospital in Ann Arbor.
One is recommending an electronic cardiac (EC) monitor to assess heart rate during resuscitation instead of relying on pulse oximetry, and the other is no longer recommending routine tracheal suction in nonvigorous babies with meconium-stained fluid, he told attendees at the American Academy of Pediatrics annual meeting.
About two-thirds of all births have a risk factor for needing resuscitation, and about 10%-20% of babies with a risk factor will need positive pressure ventilation (PPV). But risk factors do not identify all newborns who will need it. The risk is greatest for newborns less than 36 weeks’ or greater than 40 weeks’ gestational age, but 7% of term newborns will need PPV despite having no risk factors.
Situations in which there is the highest risk for advanced resuscitation include the following:
- Fetal bradycardia: 24-fold greater odds.
- Intrauterine growth restriction (IUGR): 20-fold greater odds.
- Clinical chorioamnionitis: 17-fold greater odds.
- Forceps or vacuum: 17-fold greater odds.
- Meconium-stained amniotic fluid (MSAF): 17-fold greater odds.
- Gestational diabetes: 16-fold greater odds.
- Abruption: 12-fold greater odds.
- General anesthesia: 11-fold greater odds.
These risks were determined in a prospective multicenter, case-control study of 61,593 births (Arch Dis Child Fetal Neonatal Ed. 2017 Jan;102[1]:F44-F50).
Assembling a team and using checklists
Teamwork and communication are key in delivery room emergencies, and teams should debrief afterward, ideally having videotaped the resuscitation, if possible, Dr. Weiner said.
He discussed preparation for a very-low-birth-weight birth, a “routine emergency” requiring many tasks in a short period of time: 130 tasks in the first hour and 40 in the first 3 minutes.
“Decisions made during the first hour have long-term implications, so you need multiple caregivers and a high-performance team,” Dr. Weiner said. In addition to a thorough understanding of the clinical situation, a high-performance team should have both effective leadership, and clearly defined roles and responsibilities for each member. Clinicians on the team need highly developed technical skills that they reliably and consistently execute with precision. “Practice, refine, practice, refine,” he emphasized.
It’s also important to make use of preset protocols, scripts, and checklists, Dr. Weiner said. These tools assure consistency, facilitate communication among team members, and improve outcomes. Research has shown that use of protocols, scripts, and checklists leads to improved stroke and trauma care, decreased complications during intubation, fewer central-line complications, and decreased perioperative mortality and complications.
He also recommended implementing a standardized equipment check and team briefing “time-out,” similar to a surgical time-out. This time-out gives teams an opportunity to identify a team leader, define member roles and responsibilities, check all equipment and supplies, discuss risk factors and possible scenarios, talk with the obstetrician and, if possible, introduce the leader or another team member to the parents.
In a study from University of California, San Diego, Medical Center, using checklists as part of resuscitation of potentially high-risk infants reduced the occurrence of communication problems from 24% to 4% of resuscitations (P less than 0.001) over a 3-year period (Resuscitation. 2013 Nov;84[11]:1552-7).
Delayed cord clamping
Dr. Weiner also discussed the benefits of placental transfusion. The fetal-placental unit includes approximately 110 mL/kg of blood, and about one-third of its volume remains in the placenta immediately after birth. Immediate cord clamping means a loss of 10-20 mL/kg of “potential” newborn blood volume, and could contribute to unstable pulmonary blood flow or a carotid artery pressure spike (Matern Health Neonatol Perinatol. 2016. doi: 10.1186/s40748-016-0032-y).
“Umbilical blood flow is complex,” he said. Blood flows toward the baby via the umbilical vein during inhalation, but stops or reverses during crying. The umbilical artery primarily carries blood to the placenta, and flow stops after about 4 minutes in more than half of infants. Gravity’s role in blood flow is controversial (Lancet. 2014 Jul 19;384[9939]:235-40).
The two options for placental transfusion are delayed cord clamping and milking the umbilical cord (also called “stripping”). In vaginal births, delayed clamping allows 20 mL/kg blood to transfer to the baby by 3 minutes after birth, with 90% of that reaching the baby in the first minute (Lancet. 1969 Oct 25;294[7626]:871-3).
Blood transfer is less efficient in cesarean births, so milking may be more efficient than simply delaying clamping, according to a small randomized controlled trial of preterm infants around 28 weeks’ gestational age. No difference between the methods was seen in vaginal births. To milk the cord, pinch it near the placenta and squeeze it toward the newborn for 2 seconds; then release, refill and repeat.
The biggest benefits in delayed cord clamping or milking occur among preterm infants: decreased mortality, higher mean arterial pressure on day 1, and a lower risk of blood transfusion, necrotizing enterocolitis, and a Bayley Motor score below 85 at 18-22 months. Term babies also get benefits, though: increased hemoglobin at birth (approximately 2 g/dL), a 0.5- to 5-point average increase in boys’ Ages & Stages fine motor and social domain scores at age 4 years, and among high-risk infants, a lower risk of iron deficiency anemia at age 1 year (JAMA Pediatr. 2017;171[3]:264-70).
According to current guidelines from the American Academy of Pediatrics, “delayed cord clamping longer than 30 seconds is reasonable for both term and preterm infants who do not require resuscitation at birth,” but “there is insufficient evidence to recommend an approach to cord clamping for infants who require resuscitation.” They also recommend against routine milking for newborns less than 29 weeks’ gestation (Pediatrics. 2015 Nov;136 Suppl 2:S196-218).
Meconium-related complications
Meconium-stained amniotic fluid (MSAF) is common, occurring in about 8% of deliveries and increasing with gestational age, but meconium aspiration syndrome (MAS) is less common, occurring in about 2% of all MSAF cases (Int J Pediatr. 2012. doi: 10.1155/2012/321545).
Risk factors for severe MAS include thick meconium and an abnormal fetal heart rate. But about two-thirds of MAS cases are mild, not requiring ventilation or continuous positive airway pressure (CPAP), Dr. Weiner said. Practice should be driven by evidence from randomized controlled trials (RCTs).
“Nonrandomized observational studies can be misleading, and rational conjecture has led to many mistakes in medicine,” he said. “Be willing to challenge conventional wisdom.”
For example, the standard of care in the 1970s, based on two nonrandomized retrospective reviews of 175 babies, included orapharyngeal and nasopharyngeal suction by the obstetrician and endotracheal tube (ETT) suction by the pediatrician. In the 2000s, however, an RCT of 2,500 infants found no benefit from orapharyngeal and nasopharyngeal suction, even with thick MSAF, (Lancet. 2004 Aug 14-20;364[9434]:597-602) and another RCT with 2,100 infants found no benefit from ETT suction (Pediatrics. 2000 Jan;105[1 Pt 1]:1-7).
More recent, smaller studies have confirmed those conclusions and found similar lack of benefit from ETT in non-vigorous infants, contributing to the new recommendation (Resuscitation. 2016 Aug;105:79-84; Indian J Pediatr. 2016 Oct;83[10]:1125-30).
“Routine tracheal suction is no longer recommended for nonvigorous babies with meconium stained fluid,” Dr. Weiner said. Since MSAF is risk factor for resuscitation, though, at least two clinicians with Neonatal Resuscitation Program (NRP) training should be present, as well as a full team if resuscitation is expected.
Heart rate assessment and tracking
“The baby’s heart rate needs to be monitored during PPV [positive pressure ventilation] because a prompt increase in the baby’s heart rate is the most important indicator of effective PPV,” Dr. Weiner said in an interview. “Half of errors made during NRP [Neonatal Resuscitation Program] simulations are the result of incorrect heart rate assessment.”
Recent evidence comparing pulse oximetry to an EC monitor favored the latter for tracking heart rate, leading to the other new recommendation.
“The baby’s heart rate can be monitored using the pulse oximeter,” Dr. Weiner said. “However, health providers should consider using an electronic cardiac monitor in addition to pulse oximetry because studies show that it achieves a reliable signal faster.” He cited a study of 20 newborns that showed an EC monitor determined the heart rate in a median 34 seconds, compared with 122 seconds with the pulse oximeter (Pediatr Int. 2012 Apr;54[2]:205-7).
Pulse oximetry takes 90-120 seconds to attain a reliable signal and may not work if there’s poor perfusion, but an EC monitor provides continuous heart rate monitoring even with poor perfusion. So an initial heart rate assessment by auscultation is fine, but if PPV begins, EC monitoring may be better and is the preferred method with anticipated resuscitation or chest compressions.
However, pulse oximetry is still recommended “whenever positive pressure ventilation is started or oxygen is administered in order to guide the appropriate amount of oxygen supplementation,” Dr. Weiner noted.
He added that “preliminary studies suggest that handheld Doppler fetal heart monitors correlate well with ECG, provide a rapid audible heart rate and may be a promising alternative in the future” (Pediatr Int. 2017 Oct;59[10]:1069-73).
Correct ventilation techniques
“Ventilation of the lungs is the single most important and most effective step in cardiopulmonary resuscitation of the compromised newborn,” Dr. Weiner said. “If the heart rate is not rapidly increasing, ask if the chest is moving.”
He emphasized that no compressions should occur until after at least 30 seconds of PPV that moves the chest. He provided a “MR. SOPA” acronym: Mask adjustment, Reposition airway, Suction, Open mouth, Pressure increase, Alternative airway.
You also should be aware of possible leaking or obstruction around the mask, which is common, he said, so monitor pressure instead of volume.
“We are not good at identifying leak, obstruction, or adequate tidal volume,” Dr. Weiner said. “A colorimetric CO2 detector attached to the mask is a simple indicator of gas exchange” (Resuscitation. 2014 Nov;85[11]:1568-72).
He also strongly recommended inserting an alternative airway before starting chest compressions with either intubation or a laryngeal mask.
Dr. Weiner concluded with the following list of clinical practice changes you may consider:
- Use a standardized equipment checklist.
- Develop and practice standardized scripts.
- Debrief after all resuscitations; use videotape if you can.
- Delay cord clamping for most term and preterm babies.
- Do not routinely intubate/suction nonvigorous newborns with MSAF. Initiate resuscitation.
- Use an electronic cardiac monitor if resuscitation is required.
- Use a colorimetric CO2 detector with PPV.
- Intubate or place a laryngeal mask before starting compressions.
Dr. Weiner reported having no disclosures, and no external funding was used for the presentation.
CHICAGO – , according to Gary M. Weiner, MD, of the department of pediatrics and neonatal-perinatal medicine at the University of Michigan and C.S. Mott Children’s Hospital in Ann Arbor.
One is recommending an electronic cardiac (EC) monitor to assess heart rate during resuscitation instead of relying on pulse oximetry, and the other is no longer recommending routine tracheal suction in nonvigorous babies with meconium-stained fluid, he told attendees at the American Academy of Pediatrics annual meeting.
About two-thirds of all births have a risk factor for needing resuscitation, and about 10%-20% of babies with a risk factor will need positive pressure ventilation (PPV). But risk factors do not identify all newborns who will need it. The risk is greatest for newborns less than 36 weeks’ or greater than 40 weeks’ gestational age, but 7% of term newborns will need PPV despite having no risk factors.
Situations in which there is the highest risk for advanced resuscitation include the following:
- Fetal bradycardia: 24-fold greater odds.
- Intrauterine growth restriction (IUGR): 20-fold greater odds.
- Clinical chorioamnionitis: 17-fold greater odds.
- Forceps or vacuum: 17-fold greater odds.
- Meconium-stained amniotic fluid (MSAF): 17-fold greater odds.
- Gestational diabetes: 16-fold greater odds.
- Abruption: 12-fold greater odds.
- General anesthesia: 11-fold greater odds.
These risks were determined in a prospective multicenter, case-control study of 61,593 births (Arch Dis Child Fetal Neonatal Ed. 2017 Jan;102[1]:F44-F50).
Assembling a team and using checklists
Teamwork and communication are key in delivery room emergencies, and teams should debrief afterward, ideally having videotaped the resuscitation, if possible, Dr. Weiner said.
He discussed preparation for a very-low-birth-weight birth, a “routine emergency” requiring many tasks in a short period of time: 130 tasks in the first hour and 40 in the first 3 minutes.
“Decisions made during the first hour have long-term implications, so you need multiple caregivers and a high-performance team,” Dr. Weiner said. In addition to a thorough understanding of the clinical situation, a high-performance team should have both effective leadership, and clearly defined roles and responsibilities for each member. Clinicians on the team need highly developed technical skills that they reliably and consistently execute with precision. “Practice, refine, practice, refine,” he emphasized.
It’s also important to make use of preset protocols, scripts, and checklists, Dr. Weiner said. These tools assure consistency, facilitate communication among team members, and improve outcomes. Research has shown that use of protocols, scripts, and checklists leads to improved stroke and trauma care, decreased complications during intubation, fewer central-line complications, and decreased perioperative mortality and complications.
He also recommended implementing a standardized equipment check and team briefing “time-out,” similar to a surgical time-out. This time-out gives teams an opportunity to identify a team leader, define member roles and responsibilities, check all equipment and supplies, discuss risk factors and possible scenarios, talk with the obstetrician and, if possible, introduce the leader or another team member to the parents.
In a study from University of California, San Diego, Medical Center, using checklists as part of resuscitation of potentially high-risk infants reduced the occurrence of communication problems from 24% to 4% of resuscitations (P less than 0.001) over a 3-year period (Resuscitation. 2013 Nov;84[11]:1552-7).
Delayed cord clamping
Dr. Weiner also discussed the benefits of placental transfusion. The fetal-placental unit includes approximately 110 mL/kg of blood, and about one-third of its volume remains in the placenta immediately after birth. Immediate cord clamping means a loss of 10-20 mL/kg of “potential” newborn blood volume, and could contribute to unstable pulmonary blood flow or a carotid artery pressure spike (Matern Health Neonatol Perinatol. 2016. doi: 10.1186/s40748-016-0032-y).
“Umbilical blood flow is complex,” he said. Blood flows toward the baby via the umbilical vein during inhalation, but stops or reverses during crying. The umbilical artery primarily carries blood to the placenta, and flow stops after about 4 minutes in more than half of infants. Gravity’s role in blood flow is controversial (Lancet. 2014 Jul 19;384[9939]:235-40).
The two options for placental transfusion are delayed cord clamping and milking the umbilical cord (also called “stripping”). In vaginal births, delayed clamping allows 20 mL/kg blood to transfer to the baby by 3 minutes after birth, with 90% of that reaching the baby in the first minute (Lancet. 1969 Oct 25;294[7626]:871-3).
Blood transfer is less efficient in cesarean births, so milking may be more efficient than simply delaying clamping, according to a small randomized controlled trial of preterm infants around 28 weeks’ gestational age. No difference between the methods was seen in vaginal births. To milk the cord, pinch it near the placenta and squeeze it toward the newborn for 2 seconds; then release, refill and repeat.
The biggest benefits in delayed cord clamping or milking occur among preterm infants: decreased mortality, higher mean arterial pressure on day 1, and a lower risk of blood transfusion, necrotizing enterocolitis, and a Bayley Motor score below 85 at 18-22 months. Term babies also get benefits, though: increased hemoglobin at birth (approximately 2 g/dL), a 0.5- to 5-point average increase in boys’ Ages & Stages fine motor and social domain scores at age 4 years, and among high-risk infants, a lower risk of iron deficiency anemia at age 1 year (JAMA Pediatr. 2017;171[3]:264-70).
According to current guidelines from the American Academy of Pediatrics, “delayed cord clamping longer than 30 seconds is reasonable for both term and preterm infants who do not require resuscitation at birth,” but “there is insufficient evidence to recommend an approach to cord clamping for infants who require resuscitation.” They also recommend against routine milking for newborns less than 29 weeks’ gestation (Pediatrics. 2015 Nov;136 Suppl 2:S196-218).
Meconium-related complications
Meconium-stained amniotic fluid (MSAF) is common, occurring in about 8% of deliveries and increasing with gestational age, but meconium aspiration syndrome (MAS) is less common, occurring in about 2% of all MSAF cases (Int J Pediatr. 2012. doi: 10.1155/2012/321545).
Risk factors for severe MAS include thick meconium and an abnormal fetal heart rate. But about two-thirds of MAS cases are mild, not requiring ventilation or continuous positive airway pressure (CPAP), Dr. Weiner said. Practice should be driven by evidence from randomized controlled trials (RCTs).
“Nonrandomized observational studies can be misleading, and rational conjecture has led to many mistakes in medicine,” he said. “Be willing to challenge conventional wisdom.”
For example, the standard of care in the 1970s, based on two nonrandomized retrospective reviews of 175 babies, included orapharyngeal and nasopharyngeal suction by the obstetrician and endotracheal tube (ETT) suction by the pediatrician. In the 2000s, however, an RCT of 2,500 infants found no benefit from orapharyngeal and nasopharyngeal suction, even with thick MSAF, (Lancet. 2004 Aug 14-20;364[9434]:597-602) and another RCT with 2,100 infants found no benefit from ETT suction (Pediatrics. 2000 Jan;105[1 Pt 1]:1-7).
More recent, smaller studies have confirmed those conclusions and found similar lack of benefit from ETT in non-vigorous infants, contributing to the new recommendation (Resuscitation. 2016 Aug;105:79-84; Indian J Pediatr. 2016 Oct;83[10]:1125-30).
“Routine tracheal suction is no longer recommended for nonvigorous babies with meconium stained fluid,” Dr. Weiner said. Since MSAF is risk factor for resuscitation, though, at least two clinicians with Neonatal Resuscitation Program (NRP) training should be present, as well as a full team if resuscitation is expected.
Heart rate assessment and tracking
“The baby’s heart rate needs to be monitored during PPV [positive pressure ventilation] because a prompt increase in the baby’s heart rate is the most important indicator of effective PPV,” Dr. Weiner said in an interview. “Half of errors made during NRP [Neonatal Resuscitation Program] simulations are the result of incorrect heart rate assessment.”
Recent evidence comparing pulse oximetry to an EC monitor favored the latter for tracking heart rate, leading to the other new recommendation.
“The baby’s heart rate can be monitored using the pulse oximeter,” Dr. Weiner said. “However, health providers should consider using an electronic cardiac monitor in addition to pulse oximetry because studies show that it achieves a reliable signal faster.” He cited a study of 20 newborns that showed an EC monitor determined the heart rate in a median 34 seconds, compared with 122 seconds with the pulse oximeter (Pediatr Int. 2012 Apr;54[2]:205-7).
Pulse oximetry takes 90-120 seconds to attain a reliable signal and may not work if there’s poor perfusion, but an EC monitor provides continuous heart rate monitoring even with poor perfusion. So an initial heart rate assessment by auscultation is fine, but if PPV begins, EC monitoring may be better and is the preferred method with anticipated resuscitation or chest compressions.
However, pulse oximetry is still recommended “whenever positive pressure ventilation is started or oxygen is administered in order to guide the appropriate amount of oxygen supplementation,” Dr. Weiner noted.
He added that “preliminary studies suggest that handheld Doppler fetal heart monitors correlate well with ECG, provide a rapid audible heart rate and may be a promising alternative in the future” (Pediatr Int. 2017 Oct;59[10]:1069-73).
Correct ventilation techniques
“Ventilation of the lungs is the single most important and most effective step in cardiopulmonary resuscitation of the compromised newborn,” Dr. Weiner said. “If the heart rate is not rapidly increasing, ask if the chest is moving.”
He emphasized that no compressions should occur until after at least 30 seconds of PPV that moves the chest. He provided a “MR. SOPA” acronym: Mask adjustment, Reposition airway, Suction, Open mouth, Pressure increase, Alternative airway.
You also should be aware of possible leaking or obstruction around the mask, which is common, he said, so monitor pressure instead of volume.
“We are not good at identifying leak, obstruction, or adequate tidal volume,” Dr. Weiner said. “A colorimetric CO2 detector attached to the mask is a simple indicator of gas exchange” (Resuscitation. 2014 Nov;85[11]:1568-72).
He also strongly recommended inserting an alternative airway before starting chest compressions with either intubation or a laryngeal mask.
Dr. Weiner concluded with the following list of clinical practice changes you may consider:
- Use a standardized equipment checklist.
- Develop and practice standardized scripts.
- Debrief after all resuscitations; use videotape if you can.
- Delay cord clamping for most term and preterm babies.
- Do not routinely intubate/suction nonvigorous newborns with MSAF. Initiate resuscitation.
- Use an electronic cardiac monitor if resuscitation is required.
- Use a colorimetric CO2 detector with PPV.
- Intubate or place a laryngeal mask before starting compressions.
Dr. Weiner reported having no disclosures, and no external funding was used for the presentation.
CHICAGO – , according to Gary M. Weiner, MD, of the department of pediatrics and neonatal-perinatal medicine at the University of Michigan and C.S. Mott Children’s Hospital in Ann Arbor.
One is recommending an electronic cardiac (EC) monitor to assess heart rate during resuscitation instead of relying on pulse oximetry, and the other is no longer recommending routine tracheal suction in nonvigorous babies with meconium-stained fluid, he told attendees at the American Academy of Pediatrics annual meeting.
About two-thirds of all births have a risk factor for needing resuscitation, and about 10%-20% of babies with a risk factor will need positive pressure ventilation (PPV). But risk factors do not identify all newborns who will need it. The risk is greatest for newborns less than 36 weeks’ or greater than 40 weeks’ gestational age, but 7% of term newborns will need PPV despite having no risk factors.
Situations in which there is the highest risk for advanced resuscitation include the following:
- Fetal bradycardia: 24-fold greater odds.
- Intrauterine growth restriction (IUGR): 20-fold greater odds.
- Clinical chorioamnionitis: 17-fold greater odds.
- Forceps or vacuum: 17-fold greater odds.
- Meconium-stained amniotic fluid (MSAF): 17-fold greater odds.
- Gestational diabetes: 16-fold greater odds.
- Abruption: 12-fold greater odds.
- General anesthesia: 11-fold greater odds.
These risks were determined in a prospective multicenter, case-control study of 61,593 births (Arch Dis Child Fetal Neonatal Ed. 2017 Jan;102[1]:F44-F50).
Assembling a team and using checklists
Teamwork and communication are key in delivery room emergencies, and teams should debrief afterward, ideally having videotaped the resuscitation, if possible, Dr. Weiner said.
He discussed preparation for a very-low-birth-weight birth, a “routine emergency” requiring many tasks in a short period of time: 130 tasks in the first hour and 40 in the first 3 minutes.
“Decisions made during the first hour have long-term implications, so you need multiple caregivers and a high-performance team,” Dr. Weiner said. In addition to a thorough understanding of the clinical situation, a high-performance team should have both effective leadership, and clearly defined roles and responsibilities for each member. Clinicians on the team need highly developed technical skills that they reliably and consistently execute with precision. “Practice, refine, practice, refine,” he emphasized.
It’s also important to make use of preset protocols, scripts, and checklists, Dr. Weiner said. These tools assure consistency, facilitate communication among team members, and improve outcomes. Research has shown that use of protocols, scripts, and checklists leads to improved stroke and trauma care, decreased complications during intubation, fewer central-line complications, and decreased perioperative mortality and complications.
He also recommended implementing a standardized equipment check and team briefing “time-out,” similar to a surgical time-out. This time-out gives teams an opportunity to identify a team leader, define member roles and responsibilities, check all equipment and supplies, discuss risk factors and possible scenarios, talk with the obstetrician and, if possible, introduce the leader or another team member to the parents.
In a study from University of California, San Diego, Medical Center, using checklists as part of resuscitation of potentially high-risk infants reduced the occurrence of communication problems from 24% to 4% of resuscitations (P less than 0.001) over a 3-year period (Resuscitation. 2013 Nov;84[11]:1552-7).
Delayed cord clamping
Dr. Weiner also discussed the benefits of placental transfusion. The fetal-placental unit includes approximately 110 mL/kg of blood, and about one-third of its volume remains in the placenta immediately after birth. Immediate cord clamping means a loss of 10-20 mL/kg of “potential” newborn blood volume, and could contribute to unstable pulmonary blood flow or a carotid artery pressure spike (Matern Health Neonatol Perinatol. 2016. doi: 10.1186/s40748-016-0032-y).
“Umbilical blood flow is complex,” he said. Blood flows toward the baby via the umbilical vein during inhalation, but stops or reverses during crying. The umbilical artery primarily carries blood to the placenta, and flow stops after about 4 minutes in more than half of infants. Gravity’s role in blood flow is controversial (Lancet. 2014 Jul 19;384[9939]:235-40).
The two options for placental transfusion are delayed cord clamping and milking the umbilical cord (also called “stripping”). In vaginal births, delayed clamping allows 20 mL/kg blood to transfer to the baby by 3 minutes after birth, with 90% of that reaching the baby in the first minute (Lancet. 1969 Oct 25;294[7626]:871-3).
Blood transfer is less efficient in cesarean births, so milking may be more efficient than simply delaying clamping, according to a small randomized controlled trial of preterm infants around 28 weeks’ gestational age. No difference between the methods was seen in vaginal births. To milk the cord, pinch it near the placenta and squeeze it toward the newborn for 2 seconds; then release, refill and repeat.
The biggest benefits in delayed cord clamping or milking occur among preterm infants: decreased mortality, higher mean arterial pressure on day 1, and a lower risk of blood transfusion, necrotizing enterocolitis, and a Bayley Motor score below 85 at 18-22 months. Term babies also get benefits, though: increased hemoglobin at birth (approximately 2 g/dL), a 0.5- to 5-point average increase in boys’ Ages & Stages fine motor and social domain scores at age 4 years, and among high-risk infants, a lower risk of iron deficiency anemia at age 1 year (JAMA Pediatr. 2017;171[3]:264-70).
According to current guidelines from the American Academy of Pediatrics, “delayed cord clamping longer than 30 seconds is reasonable for both term and preterm infants who do not require resuscitation at birth,” but “there is insufficient evidence to recommend an approach to cord clamping for infants who require resuscitation.” They also recommend against routine milking for newborns less than 29 weeks’ gestation (Pediatrics. 2015 Nov;136 Suppl 2:S196-218).
Meconium-related complications
Meconium-stained amniotic fluid (MSAF) is common, occurring in about 8% of deliveries and increasing with gestational age, but meconium aspiration syndrome (MAS) is less common, occurring in about 2% of all MSAF cases (Int J Pediatr. 2012. doi: 10.1155/2012/321545).
Risk factors for severe MAS include thick meconium and an abnormal fetal heart rate. But about two-thirds of MAS cases are mild, not requiring ventilation or continuous positive airway pressure (CPAP), Dr. Weiner said. Practice should be driven by evidence from randomized controlled trials (RCTs).
“Nonrandomized observational studies can be misleading, and rational conjecture has led to many mistakes in medicine,” he said. “Be willing to challenge conventional wisdom.”
For example, the standard of care in the 1970s, based on two nonrandomized retrospective reviews of 175 babies, included orapharyngeal and nasopharyngeal suction by the obstetrician and endotracheal tube (ETT) suction by the pediatrician. In the 2000s, however, an RCT of 2,500 infants found no benefit from orapharyngeal and nasopharyngeal suction, even with thick MSAF, (Lancet. 2004 Aug 14-20;364[9434]:597-602) and another RCT with 2,100 infants found no benefit from ETT suction (Pediatrics. 2000 Jan;105[1 Pt 1]:1-7).
More recent, smaller studies have confirmed those conclusions and found similar lack of benefit from ETT in non-vigorous infants, contributing to the new recommendation (Resuscitation. 2016 Aug;105:79-84; Indian J Pediatr. 2016 Oct;83[10]:1125-30).
“Routine tracheal suction is no longer recommended for nonvigorous babies with meconium stained fluid,” Dr. Weiner said. Since MSAF is risk factor for resuscitation, though, at least two clinicians with Neonatal Resuscitation Program (NRP) training should be present, as well as a full team if resuscitation is expected.
Heart rate assessment and tracking
“The baby’s heart rate needs to be monitored during PPV [positive pressure ventilation] because a prompt increase in the baby’s heart rate is the most important indicator of effective PPV,” Dr. Weiner said in an interview. “Half of errors made during NRP [Neonatal Resuscitation Program] simulations are the result of incorrect heart rate assessment.”
Recent evidence comparing pulse oximetry to an EC monitor favored the latter for tracking heart rate, leading to the other new recommendation.
“The baby’s heart rate can be monitored using the pulse oximeter,” Dr. Weiner said. “However, health providers should consider using an electronic cardiac monitor in addition to pulse oximetry because studies show that it achieves a reliable signal faster.” He cited a study of 20 newborns that showed an EC monitor determined the heart rate in a median 34 seconds, compared with 122 seconds with the pulse oximeter (Pediatr Int. 2012 Apr;54[2]:205-7).
Pulse oximetry takes 90-120 seconds to attain a reliable signal and may not work if there’s poor perfusion, but an EC monitor provides continuous heart rate monitoring even with poor perfusion. So an initial heart rate assessment by auscultation is fine, but if PPV begins, EC monitoring may be better and is the preferred method with anticipated resuscitation or chest compressions.
However, pulse oximetry is still recommended “whenever positive pressure ventilation is started or oxygen is administered in order to guide the appropriate amount of oxygen supplementation,” Dr. Weiner noted.
He added that “preliminary studies suggest that handheld Doppler fetal heart monitors correlate well with ECG, provide a rapid audible heart rate and may be a promising alternative in the future” (Pediatr Int. 2017 Oct;59[10]:1069-73).
Correct ventilation techniques
“Ventilation of the lungs is the single most important and most effective step in cardiopulmonary resuscitation of the compromised newborn,” Dr. Weiner said. “If the heart rate is not rapidly increasing, ask if the chest is moving.”
He emphasized that no compressions should occur until after at least 30 seconds of PPV that moves the chest. He provided a “MR. SOPA” acronym: Mask adjustment, Reposition airway, Suction, Open mouth, Pressure increase, Alternative airway.
You also should be aware of possible leaking or obstruction around the mask, which is common, he said, so monitor pressure instead of volume.
“We are not good at identifying leak, obstruction, or adequate tidal volume,” Dr. Weiner said. “A colorimetric CO2 detector attached to the mask is a simple indicator of gas exchange” (Resuscitation. 2014 Nov;85[11]:1568-72).
He also strongly recommended inserting an alternative airway before starting chest compressions with either intubation or a laryngeal mask.
Dr. Weiner concluded with the following list of clinical practice changes you may consider:
- Use a standardized equipment checklist.
- Develop and practice standardized scripts.
- Debrief after all resuscitations; use videotape if you can.
- Delay cord clamping for most term and preterm babies.
- Do not routinely intubate/suction nonvigorous newborns with MSAF. Initiate resuscitation.
- Use an electronic cardiac monitor if resuscitation is required.
- Use a colorimetric CO2 detector with PPV.
- Intubate or place a laryngeal mask before starting compressions.
Dr. Weiner reported having no disclosures, and no external funding was used for the presentation.
EXPERT ANALYSIS FROM AAP 2017
Chinese school-based flu vaccination program reduced outbreaks
, said Yang Pan, PhD, of the Institute for Infectious Disease and Endemic Disease Control, Beijing Center for Disease Prevention and Control, and associates.
School-based trivalent inactivated influenza vaccination programs generally occurred Oct. 15-Nov. 30 each year since 2007, with greater than 50% vaccination coverage. In an 11-year retrospective study of school outbreaks of influenza in elementary, middle, and high schools in the Beijing area during Sept. 1, 2006-March 31, 2017, there were 286 febrile outbreaks in schools, involving 6,863 children.
During the 11 years, a mismatch between circulating strains and vaccine strains was identified in two influenza seasons, such as “the A(H3N2) 3C.1 (vaccine strain)-A(H3N2) 3C.3a (circulating strains) mismatch in 2014-2015, the B(Yamagata) Clade 2 (vaccine strain)-B(Yamagata) Clade 3 (circulating strain) mismatch in the 2014-2015 influenza season, and B(Yamagata) (vaccine strain)-B(Victoria) (circulating strains) mismatch in 2015-2016,” they reported.
A combination of high flu vaccine coverage because of school-based vaccinations and a good vaccine match reduced influenza outbreaks in schools by 89% (odds ratio, 0.111), Dr. Pan and associates concluded.
“The school-based influenza vaccination program has been in operation for nearly 10 years in the Beijing area, is unique in China, and is one of the few school-based influenza programs in the world,” the researchers explained. “These data can inform and improve vaccination policy locally and nationally.”
Read more in Vaccine (2017 Nov 8. doi: 10.1016/j.vaccine.2017.10.096).
, said Yang Pan, PhD, of the Institute for Infectious Disease and Endemic Disease Control, Beijing Center for Disease Prevention and Control, and associates.
School-based trivalent inactivated influenza vaccination programs generally occurred Oct. 15-Nov. 30 each year since 2007, with greater than 50% vaccination coverage. In an 11-year retrospective study of school outbreaks of influenza in elementary, middle, and high schools in the Beijing area during Sept. 1, 2006-March 31, 2017, there were 286 febrile outbreaks in schools, involving 6,863 children.
During the 11 years, a mismatch between circulating strains and vaccine strains was identified in two influenza seasons, such as “the A(H3N2) 3C.1 (vaccine strain)-A(H3N2) 3C.3a (circulating strains) mismatch in 2014-2015, the B(Yamagata) Clade 2 (vaccine strain)-B(Yamagata) Clade 3 (circulating strain) mismatch in the 2014-2015 influenza season, and B(Yamagata) (vaccine strain)-B(Victoria) (circulating strains) mismatch in 2015-2016,” they reported.
A combination of high flu vaccine coverage because of school-based vaccinations and a good vaccine match reduced influenza outbreaks in schools by 89% (odds ratio, 0.111), Dr. Pan and associates concluded.
“The school-based influenza vaccination program has been in operation for nearly 10 years in the Beijing area, is unique in China, and is one of the few school-based influenza programs in the world,” the researchers explained. “These data can inform and improve vaccination policy locally and nationally.”
Read more in Vaccine (2017 Nov 8. doi: 10.1016/j.vaccine.2017.10.096).
, said Yang Pan, PhD, of the Institute for Infectious Disease and Endemic Disease Control, Beijing Center for Disease Prevention and Control, and associates.
School-based trivalent inactivated influenza vaccination programs generally occurred Oct. 15-Nov. 30 each year since 2007, with greater than 50% vaccination coverage. In an 11-year retrospective study of school outbreaks of influenza in elementary, middle, and high schools in the Beijing area during Sept. 1, 2006-March 31, 2017, there were 286 febrile outbreaks in schools, involving 6,863 children.
During the 11 years, a mismatch between circulating strains and vaccine strains was identified in two influenza seasons, such as “the A(H3N2) 3C.1 (vaccine strain)-A(H3N2) 3C.3a (circulating strains) mismatch in 2014-2015, the B(Yamagata) Clade 2 (vaccine strain)-B(Yamagata) Clade 3 (circulating strain) mismatch in the 2014-2015 influenza season, and B(Yamagata) (vaccine strain)-B(Victoria) (circulating strains) mismatch in 2015-2016,” they reported.
A combination of high flu vaccine coverage because of school-based vaccinations and a good vaccine match reduced influenza outbreaks in schools by 89% (odds ratio, 0.111), Dr. Pan and associates concluded.
“The school-based influenza vaccination program has been in operation for nearly 10 years in the Beijing area, is unique in China, and is one of the few school-based influenza programs in the world,” the researchers explained. “These data can inform and improve vaccination policy locally and nationally.”
Read more in Vaccine (2017 Nov 8. doi: 10.1016/j.vaccine.2017.10.096).
FROM VACCINE
New opportunities for gastroenterology leadership in the evolving payment reform landscape
This year’s Congressional debate over repealing or reforming key provisions of the Affordable Care Act was contentious in large part because of the high and rising costs of health care. Though a new health care reform bill is now unlikely, it remains critical to continue the discussion on how to deliver and pay for care in a way that addresses these high costs and makes coverage more affordable through more efficient and high-quality approaches.1
Illustrating the bipartisan nature of payment reforms, the Medicare Access and CHIP Reauthorization Act (MACRA) passed with more than 90% support in both the House and Senate in 2015. MACRA provides a 5% bonus payment for physicians who receive a significant part of their Medicare payments in an advanced APM, which involves some downside financial risk. In addition, any physician who participates significantly in a broader range of Medicare APMs, including many without downside risk, receives an exception from the reporting requirements for the new Merit-Based Incentive Payment System (MIPS) and would report on APM performance measures instead.
However, the details of payment reform are challenging and will benefit from engagement and leadership by physicians – including in gastroenterology. A new survey shows that the Department of Health and Human Services has achieved its goal of having 30% of health care payments tied to APMs by the end of 2016.3 It hopes to have 50% by the end of 2018.
Physician-Focused Payment Model Technical Advisory Committee’s role in recommending new payment models
The paucity of APMs was one reason the MACRA law established the Physician-Focused Payment Model Technical Advisory Committee (PTAC). Organizations can submit proposals for new Medicare payment models to PTAC, which then are reviewed according to 10 established criteria. The criteria place particular emphasis on the scope of the APM, the APM’s ability to increase quality while maintaining or decreasing costs, and whether the payment methodology improves on current policy. PTAC then makes recommendations to CMS for full implementation of a proposal, limited testing (a pilot program), or no implementation.
The fate of the two GI APMs offers broad insight on the path forward for new specialized-care models. Although PTAC focuses on physician payment, its criteria and critiques emphasize that the primary focus of any APM should be on the full spectrum of patient care. Project Sonar likely received a positive recommendation because it focused on shifting payment to improving chronic care and avoiding complications. Although the colonoscopy proposal was withdrawn, we can gain a sense of PTAC’s concerns through the preliminary review.5 The review argues the proposal did not sufficiently address how it would lead to a more efficient, better integrated, and higher quality screening that improves patient health. More specifically, the review criticized the proposal for focusing primarily on a site-of-service shift and offering fewer details on how the APM would reduce overutilization.
Overall, PTAC’s deliberations at both its April and September meetings suggest that it will deeply scrutinize models focusing only on a single procedure or specialty, or ones that it believes do not sufficiently coordinate with primary care or other specialties, because it does not believe that such models have a sufficiently comprehensive patient focus. These PTAC reviews also suggest that the Committee will recommend programs with ideas they find viable, even if committee members have expressed concerns about certain aspects. Indeed, despite preliminary recommendations against 6 initial proposals, the full Committee has approved 3 of them for limited testing. The Committee was receptive to the argument that without testing APMs in the real world, even if those programs have limitations, the field cannot move forward.
Implications for gastrointestinal practice and planning
Despite the many challenges in payment model development, the broader march toward APMs will continue, driven by increasing pressures to provide access to quality care while controlling costs. Further developments in several areas bear watching because they could accelerate opportunities for gastroenterologists.
Most notable is the considerable payment model innovation underway in private health insurance plans and state Medicaid plans, models that could develop into PTAC submissions. Project Sonar was first implemented in collaboration with a private payer in Illinois. Similarly, the inflammatory bowel disease specialty medical home was developed at the University of Pittsburgh. Both successfully have achieved the Triple Aim, improving patient experience and population health while decreasing medical costs.6 The private sector can serve as a testing ground for new APMs and the new administration’s desire to support innovative private sector models of care reform makes CMS likely to take further steps to support these approaches.
Second, working with both private and public payers, gastroenterologists could expand the concept of a specialty medical home or a primary-specialty coordinated medical home by incorporating more aspects of GI care. Chronic liver disease, chronic pancreatitis, and irritable bowel syndrome all could benefit from these approaches.7 Medical home models generally include a shift from fee-for-service payments by providing per-patient payments (potentially risk-adjusted) to the coordinating physician for a period of time. That per-member per-month payment may enable additional patient-centric services such as extending access to care, regular patient outreach to monitor changes in health status, and partnering with primary care and other providers to help patients access treatment for comorbid conditions.
Third, as evidenced by the PTAC critique on the Comprehensive Colonoscopy APM, a revised approach is needed for bundled episode payment reforms to better support endoscopists focused on performing high-quality procedures. Given their procedural focus, these physicians will need to show the value of endoscopic services in well-coordinated patient care. Site-of-service shifts are helpful where appropriate, but bundle proposals also must consider coordination with primary care providers on appropriate referrals, encouragement of non-endoscopic approaches, preparation technique to minimize the number of procedures that have to be repeated, and reducing anesthesia care for low-risk patients. These considerations generally suggest a broader episode payment model related to the goals of the procedure, rather than endoscopy-based bundles alone.
For example, a bundled payment for colorectal cancer screening, covering a full episode of treatment beyond a single colonoscopy, would make it easier for gastroenterologists to work more effectively with primary care providers to reduce gaps in colorectal cancer screening rates at the lowest possible overall cost. This bundle could be implemented by a specialized GI practice in conjunction with a primary care medical home or an ACO. If such a broad bundle is too much of a practice shift, an endoscopy-based episode payment could include performance measures and limited additional payments related to these same patient-focused objectives.
The kinds of reforms described earlier could work well with both primary care–focused and ACO models. However, there are technical challenges in dealing with overlapping payment reforms, and gastroenterologists should look for further guidance from CMS on how bundled episode payments and other specialized-care payment reforms will interact with APMs for primary care, such as ACOs and the Project Sonar model recommended by PTAC.8
Despite the broader shift toward APMs, it remains likely that many gastroenterologists will participate in the fee-for-service–based MIPS program in the near term. These physicians still will face fundamental pressures to deliver better value. Here, there may be opportunities to improve coordination in the MIPS program through additional care coordination payments for chronic disease, complementing the chronic care management payments that primary care physicians receive. Such payments would encourage further development and testing of more meaningful and outcome-oriented performance measures related to GI care.
Finally, GI care would benefit from better evidence for all GI-related payment reforms. Many of these reforms will be implemented outside of Medicare, but do not have results reported in a manner that make it easy to assess their impact and potential for broader implementation. Building an evidence base is feasible without imposing large costs or additional burdens on practices, especially when evaluations are implemented along with payment reforms, and offers the best way for organizations to learn and improve based on what works and what does not.9
Conclusions
Though the health care debate has ended in Congress for now, the march toward payment reform will continue. To accelerate progress, continued leadership from gastroenterologists is needed, especially in finding solutions that move beyond traditional GI practice. Collaborative incremental models that advance population health and are feasible to implement will provide the best opportunity for practice reform. Effective partnerships with primary care are particularly important to help avoid traditional gatekeeper approaches, and move toward a patient-centric model of shared accountability in which specialists function as a key partner in a medical neighborhood.10 Gastroenterologists can shape these steps, not only through PTAC and Medicare APMs, but through the other steps described earlier, and have a unique role in developing new models that leverage their specialty expertise. However, these models cannot be developed in isolation, and increased collaboration with primary care and other medical and nonmedical specialists will be critical. Physicians should start identifying opportunities to improve their practices and build these relationships now. These investments will allow them to thrive as new payment models come online.
References
1. Dzau V.J., McClellan M.B., McGinnis J.M. Vital directions for health and health care: priorities from a National Academy of Medicine initiative. JAMA 2017;317:1461-70.
2. Alternative Payment Model Framework Progress Tracking Work Group. Alternative payment model (APM) framework. Health
Care Payment Learning and Action Network. Available from: https://hcp-lan.org/workproducts/apm-whitepaper.pdf. Accessed: January 12, 2016.
3. Health Care Payment Learning and Action Network. APM Measurement: Progress of Alternative Payment Models. Available from: http://hcp-lan.org/workproducts/measurement_discussion%20article_2017.pdf Accessed November 2, 2017.
4. McClellan M., McStay F., Saunders R. The roadmap to physician payment reform: what it will take for all clinicians to succeed
under MACRA. Health Affairs Blog. Available from: http://healthaffairs.org/blog/2016/08/30/the-roadmap-to-physicianpayment-reform-what-it-will-take-for-all-clinicians-to-succeedunder-macra/. Accessed: August 30, 2016.
5. Medows R., Casale P., Berenson R. Preliminary review team report to the physician-focused Payment Model Technical Advisory Committee (PTAC). Physician-Focused Payment Model Technical Advisory Committee. Available from: https://aspe.hhs.gov/system/files/pdf/255906/DHNPRTReport.pdf. Accessed: March 22, 2017.
6. Regueiro M., Click B., Holder D., et al. Constructing an inflammatory bowel disease patient–centered medical home. Clin Gastroenterol Hepatol. 2017;15:1148-53.
7. Meier S.K., Shah N.D., Talwalkar J.A., et al. Adapting the patient-centered specialty practice model for populations with cirrhosis. Clin Gastroenterol Hepatol. 2016;14:492-6.
8. Pham H., Chernew M., Shrank W., et al. Market momentum, spillover effects, and evidence-based decision making on payment reform. Health Affairs Blog. Available from: http:// healthaffairs.org/blog/2017/05/24/market-momentum-spillovereffects-and-evidence-based-decision-making-on-paymentreform/. Accessed: May 24, 2017.
9. McClellan M., Richards R., Japinga M. Evidence on payment reform: where are the gaps? Health Affairs Blog. Available from: http://healthaffairs.org/blog/2017/04/25/evidence-onpayment-reform-where-are-the-gaps/. Accessed: April 25, 2017.
10. Huang X., Rosenthal M.B. Transforming specialty practice – the patient-centered medical neighborhood. N Engl J Med 2014;370:1376-9.
Mr. Japinga, Dr. Saunders, and Dr. McClellan are at the Duke-Margolis Center for Health Policy, Washington; Dr. Gellad is in the division of gastroenterology at the Duke University School of Medicine and at the Durham VA Medical Center, Durham, N.C. Dr. Gellad was supported by a Career Development Award from Veterans Affairs Health Services Research (CDA 14-158). The authors had no conflicts of interest.
This year’s Congressional debate over repealing or reforming key provisions of the Affordable Care Act was contentious in large part because of the high and rising costs of health care. Though a new health care reform bill is now unlikely, it remains critical to continue the discussion on how to deliver and pay for care in a way that addresses these high costs and makes coverage more affordable through more efficient and high-quality approaches.1
Illustrating the bipartisan nature of payment reforms, the Medicare Access and CHIP Reauthorization Act (MACRA) passed with more than 90% support in both the House and Senate in 2015. MACRA provides a 5% bonus payment for physicians who receive a significant part of their Medicare payments in an advanced APM, which involves some downside financial risk. In addition, any physician who participates significantly in a broader range of Medicare APMs, including many without downside risk, receives an exception from the reporting requirements for the new Merit-Based Incentive Payment System (MIPS) and would report on APM performance measures instead.
However, the details of payment reform are challenging and will benefit from engagement and leadership by physicians – including in gastroenterology. A new survey shows that the Department of Health and Human Services has achieved its goal of having 30% of health care payments tied to APMs by the end of 2016.3 It hopes to have 50% by the end of 2018.
Physician-Focused Payment Model Technical Advisory Committee’s role in recommending new payment models
The paucity of APMs was one reason the MACRA law established the Physician-Focused Payment Model Technical Advisory Committee (PTAC). Organizations can submit proposals for new Medicare payment models to PTAC, which then are reviewed according to 10 established criteria. The criteria place particular emphasis on the scope of the APM, the APM’s ability to increase quality while maintaining or decreasing costs, and whether the payment methodology improves on current policy. PTAC then makes recommendations to CMS for full implementation of a proposal, limited testing (a pilot program), or no implementation.
The fate of the two GI APMs offers broad insight on the path forward for new specialized-care models. Although PTAC focuses on physician payment, its criteria and critiques emphasize that the primary focus of any APM should be on the full spectrum of patient care. Project Sonar likely received a positive recommendation because it focused on shifting payment to improving chronic care and avoiding complications. Although the colonoscopy proposal was withdrawn, we can gain a sense of PTAC’s concerns through the preliminary review.5 The review argues the proposal did not sufficiently address how it would lead to a more efficient, better integrated, and higher quality screening that improves patient health. More specifically, the review criticized the proposal for focusing primarily on a site-of-service shift and offering fewer details on how the APM would reduce overutilization.
Overall, PTAC’s deliberations at both its April and September meetings suggest that it will deeply scrutinize models focusing only on a single procedure or specialty, or ones that it believes do not sufficiently coordinate with primary care or other specialties, because it does not believe that such models have a sufficiently comprehensive patient focus. These PTAC reviews also suggest that the Committee will recommend programs with ideas they find viable, even if committee members have expressed concerns about certain aspects. Indeed, despite preliminary recommendations against 6 initial proposals, the full Committee has approved 3 of them for limited testing. The Committee was receptive to the argument that without testing APMs in the real world, even if those programs have limitations, the field cannot move forward.
Implications for gastrointestinal practice and planning
Despite the many challenges in payment model development, the broader march toward APMs will continue, driven by increasing pressures to provide access to quality care while controlling costs. Further developments in several areas bear watching because they could accelerate opportunities for gastroenterologists.
Most notable is the considerable payment model innovation underway in private health insurance plans and state Medicaid plans, models that could develop into PTAC submissions. Project Sonar was first implemented in collaboration with a private payer in Illinois. Similarly, the inflammatory bowel disease specialty medical home was developed at the University of Pittsburgh. Both successfully have achieved the Triple Aim, improving patient experience and population health while decreasing medical costs.6 The private sector can serve as a testing ground for new APMs and the new administration’s desire to support innovative private sector models of care reform makes CMS likely to take further steps to support these approaches.
Second, working with both private and public payers, gastroenterologists could expand the concept of a specialty medical home or a primary-specialty coordinated medical home by incorporating more aspects of GI care. Chronic liver disease, chronic pancreatitis, and irritable bowel syndrome all could benefit from these approaches.7 Medical home models generally include a shift from fee-for-service payments by providing per-patient payments (potentially risk-adjusted) to the coordinating physician for a period of time. That per-member per-month payment may enable additional patient-centric services such as extending access to care, regular patient outreach to monitor changes in health status, and partnering with primary care and other providers to help patients access treatment for comorbid conditions.
Third, as evidenced by the PTAC critique on the Comprehensive Colonoscopy APM, a revised approach is needed for bundled episode payment reforms to better support endoscopists focused on performing high-quality procedures. Given their procedural focus, these physicians will need to show the value of endoscopic services in well-coordinated patient care. Site-of-service shifts are helpful where appropriate, but bundle proposals also must consider coordination with primary care providers on appropriate referrals, encouragement of non-endoscopic approaches, preparation technique to minimize the number of procedures that have to be repeated, and reducing anesthesia care for low-risk patients. These considerations generally suggest a broader episode payment model related to the goals of the procedure, rather than endoscopy-based bundles alone.
For example, a bundled payment for colorectal cancer screening, covering a full episode of treatment beyond a single colonoscopy, would make it easier for gastroenterologists to work more effectively with primary care providers to reduce gaps in colorectal cancer screening rates at the lowest possible overall cost. This bundle could be implemented by a specialized GI practice in conjunction with a primary care medical home or an ACO. If such a broad bundle is too much of a practice shift, an endoscopy-based episode payment could include performance measures and limited additional payments related to these same patient-focused objectives.
The kinds of reforms described earlier could work well with both primary care–focused and ACO models. However, there are technical challenges in dealing with overlapping payment reforms, and gastroenterologists should look for further guidance from CMS on how bundled episode payments and other specialized-care payment reforms will interact with APMs for primary care, such as ACOs and the Project Sonar model recommended by PTAC.8
Despite the broader shift toward APMs, it remains likely that many gastroenterologists will participate in the fee-for-service–based MIPS program in the near term. These physicians still will face fundamental pressures to deliver better value. Here, there may be opportunities to improve coordination in the MIPS program through additional care coordination payments for chronic disease, complementing the chronic care management payments that primary care physicians receive. Such payments would encourage further development and testing of more meaningful and outcome-oriented performance measures related to GI care.
Finally, GI care would benefit from better evidence for all GI-related payment reforms. Many of these reforms will be implemented outside of Medicare, but do not have results reported in a manner that make it easy to assess their impact and potential for broader implementation. Building an evidence base is feasible without imposing large costs or additional burdens on practices, especially when evaluations are implemented along with payment reforms, and offers the best way for organizations to learn and improve based on what works and what does not.9
Conclusions
Though the health care debate has ended in Congress for now, the march toward payment reform will continue. To accelerate progress, continued leadership from gastroenterologists is needed, especially in finding solutions that move beyond traditional GI practice. Collaborative incremental models that advance population health and are feasible to implement will provide the best opportunity for practice reform. Effective partnerships with primary care are particularly important to help avoid traditional gatekeeper approaches, and move toward a patient-centric model of shared accountability in which specialists function as a key partner in a medical neighborhood.10 Gastroenterologists can shape these steps, not only through PTAC and Medicare APMs, but through the other steps described earlier, and have a unique role in developing new models that leverage their specialty expertise. However, these models cannot be developed in isolation, and increased collaboration with primary care and other medical and nonmedical specialists will be critical. Physicians should start identifying opportunities to improve their practices and build these relationships now. These investments will allow them to thrive as new payment models come online.
References
1. Dzau V.J., McClellan M.B., McGinnis J.M. Vital directions for health and health care: priorities from a National Academy of Medicine initiative. JAMA 2017;317:1461-70.
2. Alternative Payment Model Framework Progress Tracking Work Group. Alternative payment model (APM) framework. Health
Care Payment Learning and Action Network. Available from: https://hcp-lan.org/workproducts/apm-whitepaper.pdf. Accessed: January 12, 2016.
3. Health Care Payment Learning and Action Network. APM Measurement: Progress of Alternative Payment Models. Available from: http://hcp-lan.org/workproducts/measurement_discussion%20article_2017.pdf Accessed November 2, 2017.
4. McClellan M., McStay F., Saunders R. The roadmap to physician payment reform: what it will take for all clinicians to succeed
under MACRA. Health Affairs Blog. Available from: http://healthaffairs.org/blog/2016/08/30/the-roadmap-to-physicianpayment-reform-what-it-will-take-for-all-clinicians-to-succeedunder-macra/. Accessed: August 30, 2016.
5. Medows R., Casale P., Berenson R. Preliminary review team report to the physician-focused Payment Model Technical Advisory Committee (PTAC). Physician-Focused Payment Model Technical Advisory Committee. Available from: https://aspe.hhs.gov/system/files/pdf/255906/DHNPRTReport.pdf. Accessed: March 22, 2017.
6. Regueiro M., Click B., Holder D., et al. Constructing an inflammatory bowel disease patient–centered medical home. Clin Gastroenterol Hepatol. 2017;15:1148-53.
7. Meier S.K., Shah N.D., Talwalkar J.A., et al. Adapting the patient-centered specialty practice model for populations with cirrhosis. Clin Gastroenterol Hepatol. 2016;14:492-6.
8. Pham H., Chernew M., Shrank W., et al. Market momentum, spillover effects, and evidence-based decision making on payment reform. Health Affairs Blog. Available from: http:// healthaffairs.org/blog/2017/05/24/market-momentum-spillovereffects-and-evidence-based-decision-making-on-paymentreform/. Accessed: May 24, 2017.
9. McClellan M., Richards R., Japinga M. Evidence on payment reform: where are the gaps? Health Affairs Blog. Available from: http://healthaffairs.org/blog/2017/04/25/evidence-onpayment-reform-where-are-the-gaps/. Accessed: April 25, 2017.
10. Huang X., Rosenthal M.B. Transforming specialty practice – the patient-centered medical neighborhood. N Engl J Med 2014;370:1376-9.
Mr. Japinga, Dr. Saunders, and Dr. McClellan are at the Duke-Margolis Center for Health Policy, Washington; Dr. Gellad is in the division of gastroenterology at the Duke University School of Medicine and at the Durham VA Medical Center, Durham, N.C. Dr. Gellad was supported by a Career Development Award from Veterans Affairs Health Services Research (CDA 14-158). The authors had no conflicts of interest.
This year’s Congressional debate over repealing or reforming key provisions of the Affordable Care Act was contentious in large part because of the high and rising costs of health care. Though a new health care reform bill is now unlikely, it remains critical to continue the discussion on how to deliver and pay for care in a way that addresses these high costs and makes coverage more affordable through more efficient and high-quality approaches.1
Illustrating the bipartisan nature of payment reforms, the Medicare Access and CHIP Reauthorization Act (MACRA) passed with more than 90% support in both the House and Senate in 2015. MACRA provides a 5% bonus payment for physicians who receive a significant part of their Medicare payments in an advanced APM, which involves some downside financial risk. In addition, any physician who participates significantly in a broader range of Medicare APMs, including many without downside risk, receives an exception from the reporting requirements for the new Merit-Based Incentive Payment System (MIPS) and would report on APM performance measures instead.
However, the details of payment reform are challenging and will benefit from engagement and leadership by physicians – including in gastroenterology. A new survey shows that the Department of Health and Human Services has achieved its goal of having 30% of health care payments tied to APMs by the end of 2016.3 It hopes to have 50% by the end of 2018.
Physician-Focused Payment Model Technical Advisory Committee’s role in recommending new payment models
The paucity of APMs was one reason the MACRA law established the Physician-Focused Payment Model Technical Advisory Committee (PTAC). Organizations can submit proposals for new Medicare payment models to PTAC, which then are reviewed according to 10 established criteria. The criteria place particular emphasis on the scope of the APM, the APM’s ability to increase quality while maintaining or decreasing costs, and whether the payment methodology improves on current policy. PTAC then makes recommendations to CMS for full implementation of a proposal, limited testing (a pilot program), or no implementation.
The fate of the two GI APMs offers broad insight on the path forward for new specialized-care models. Although PTAC focuses on physician payment, its criteria and critiques emphasize that the primary focus of any APM should be on the full spectrum of patient care. Project Sonar likely received a positive recommendation because it focused on shifting payment to improving chronic care and avoiding complications. Although the colonoscopy proposal was withdrawn, we can gain a sense of PTAC’s concerns through the preliminary review.5 The review argues the proposal did not sufficiently address how it would lead to a more efficient, better integrated, and higher quality screening that improves patient health. More specifically, the review criticized the proposal for focusing primarily on a site-of-service shift and offering fewer details on how the APM would reduce overutilization.
Overall, PTAC’s deliberations at both its April and September meetings suggest that it will deeply scrutinize models focusing only on a single procedure or specialty, or ones that it believes do not sufficiently coordinate with primary care or other specialties, because it does not believe that such models have a sufficiently comprehensive patient focus. These PTAC reviews also suggest that the Committee will recommend programs with ideas they find viable, even if committee members have expressed concerns about certain aspects. Indeed, despite preliminary recommendations against 6 initial proposals, the full Committee has approved 3 of them for limited testing. The Committee was receptive to the argument that without testing APMs in the real world, even if those programs have limitations, the field cannot move forward.
Implications for gastrointestinal practice and planning
Despite the many challenges in payment model development, the broader march toward APMs will continue, driven by increasing pressures to provide access to quality care while controlling costs. Further developments in several areas bear watching because they could accelerate opportunities for gastroenterologists.
Most notable is the considerable payment model innovation underway in private health insurance plans and state Medicaid plans, models that could develop into PTAC submissions. Project Sonar was first implemented in collaboration with a private payer in Illinois. Similarly, the inflammatory bowel disease specialty medical home was developed at the University of Pittsburgh. Both successfully have achieved the Triple Aim, improving patient experience and population health while decreasing medical costs.6 The private sector can serve as a testing ground for new APMs and the new administration’s desire to support innovative private sector models of care reform makes CMS likely to take further steps to support these approaches.
Second, working with both private and public payers, gastroenterologists could expand the concept of a specialty medical home or a primary-specialty coordinated medical home by incorporating more aspects of GI care. Chronic liver disease, chronic pancreatitis, and irritable bowel syndrome all could benefit from these approaches.7 Medical home models generally include a shift from fee-for-service payments by providing per-patient payments (potentially risk-adjusted) to the coordinating physician for a period of time. That per-member per-month payment may enable additional patient-centric services such as extending access to care, regular patient outreach to monitor changes in health status, and partnering with primary care and other providers to help patients access treatment for comorbid conditions.
Third, as evidenced by the PTAC critique on the Comprehensive Colonoscopy APM, a revised approach is needed for bundled episode payment reforms to better support endoscopists focused on performing high-quality procedures. Given their procedural focus, these physicians will need to show the value of endoscopic services in well-coordinated patient care. Site-of-service shifts are helpful where appropriate, but bundle proposals also must consider coordination with primary care providers on appropriate referrals, encouragement of non-endoscopic approaches, preparation technique to minimize the number of procedures that have to be repeated, and reducing anesthesia care for low-risk patients. These considerations generally suggest a broader episode payment model related to the goals of the procedure, rather than endoscopy-based bundles alone.
For example, a bundled payment for colorectal cancer screening, covering a full episode of treatment beyond a single colonoscopy, would make it easier for gastroenterologists to work more effectively with primary care providers to reduce gaps in colorectal cancer screening rates at the lowest possible overall cost. This bundle could be implemented by a specialized GI practice in conjunction with a primary care medical home or an ACO. If such a broad bundle is too much of a practice shift, an endoscopy-based episode payment could include performance measures and limited additional payments related to these same patient-focused objectives.
The kinds of reforms described earlier could work well with both primary care–focused and ACO models. However, there are technical challenges in dealing with overlapping payment reforms, and gastroenterologists should look for further guidance from CMS on how bundled episode payments and other specialized-care payment reforms will interact with APMs for primary care, such as ACOs and the Project Sonar model recommended by PTAC.8
Despite the broader shift toward APMs, it remains likely that many gastroenterologists will participate in the fee-for-service–based MIPS program in the near term. These physicians still will face fundamental pressures to deliver better value. Here, there may be opportunities to improve coordination in the MIPS program through additional care coordination payments for chronic disease, complementing the chronic care management payments that primary care physicians receive. Such payments would encourage further development and testing of more meaningful and outcome-oriented performance measures related to GI care.
Finally, GI care would benefit from better evidence for all GI-related payment reforms. Many of these reforms will be implemented outside of Medicare, but do not have results reported in a manner that make it easy to assess their impact and potential for broader implementation. Building an evidence base is feasible without imposing large costs or additional burdens on practices, especially when evaluations are implemented along with payment reforms, and offers the best way for organizations to learn and improve based on what works and what does not.9
Conclusions
Though the health care debate has ended in Congress for now, the march toward payment reform will continue. To accelerate progress, continued leadership from gastroenterologists is needed, especially in finding solutions that move beyond traditional GI practice. Collaborative incremental models that advance population health and are feasible to implement will provide the best opportunity for practice reform. Effective partnerships with primary care are particularly important to help avoid traditional gatekeeper approaches, and move toward a patient-centric model of shared accountability in which specialists function as a key partner in a medical neighborhood.10 Gastroenterologists can shape these steps, not only through PTAC and Medicare APMs, but through the other steps described earlier, and have a unique role in developing new models that leverage their specialty expertise. However, these models cannot be developed in isolation, and increased collaboration with primary care and other medical and nonmedical specialists will be critical. Physicians should start identifying opportunities to improve their practices and build these relationships now. These investments will allow them to thrive as new payment models come online.
References
1. Dzau V.J., McClellan M.B., McGinnis J.M. Vital directions for health and health care: priorities from a National Academy of Medicine initiative. JAMA 2017;317:1461-70.
2. Alternative Payment Model Framework Progress Tracking Work Group. Alternative payment model (APM) framework. Health
Care Payment Learning and Action Network. Available from: https://hcp-lan.org/workproducts/apm-whitepaper.pdf. Accessed: January 12, 2016.
3. Health Care Payment Learning and Action Network. APM Measurement: Progress of Alternative Payment Models. Available from: http://hcp-lan.org/workproducts/measurement_discussion%20article_2017.pdf Accessed November 2, 2017.
4. McClellan M., McStay F., Saunders R. The roadmap to physician payment reform: what it will take for all clinicians to succeed
under MACRA. Health Affairs Blog. Available from: http://healthaffairs.org/blog/2016/08/30/the-roadmap-to-physicianpayment-reform-what-it-will-take-for-all-clinicians-to-succeedunder-macra/. Accessed: August 30, 2016.
5. Medows R., Casale P., Berenson R. Preliminary review team report to the physician-focused Payment Model Technical Advisory Committee (PTAC). Physician-Focused Payment Model Technical Advisory Committee. Available from: https://aspe.hhs.gov/system/files/pdf/255906/DHNPRTReport.pdf. Accessed: March 22, 2017.
6. Regueiro M., Click B., Holder D., et al. Constructing an inflammatory bowel disease patient–centered medical home. Clin Gastroenterol Hepatol. 2017;15:1148-53.
7. Meier S.K., Shah N.D., Talwalkar J.A., et al. Adapting the patient-centered specialty practice model for populations with cirrhosis. Clin Gastroenterol Hepatol. 2016;14:492-6.
8. Pham H., Chernew M., Shrank W., et al. Market momentum, spillover effects, and evidence-based decision making on payment reform. Health Affairs Blog. Available from: http:// healthaffairs.org/blog/2017/05/24/market-momentum-spillovereffects-and-evidence-based-decision-making-on-paymentreform/. Accessed: May 24, 2017.
9. McClellan M., Richards R., Japinga M. Evidence on payment reform: where are the gaps? Health Affairs Blog. Available from: http://healthaffairs.org/blog/2017/04/25/evidence-onpayment-reform-where-are-the-gaps/. Accessed: April 25, 2017.
10. Huang X., Rosenthal M.B. Transforming specialty practice – the patient-centered medical neighborhood. N Engl J Med 2014;370:1376-9.
Mr. Japinga, Dr. Saunders, and Dr. McClellan are at the Duke-Margolis Center for Health Policy, Washington; Dr. Gellad is in the division of gastroenterology at the Duke University School of Medicine and at the Durham VA Medical Center, Durham, N.C. Dr. Gellad was supported by a Career Development Award from Veterans Affairs Health Services Research (CDA 14-158). The authors had no conflicts of interest.