User login
Tau imaging predicts looming cognitive decline in cognitively normal elderly
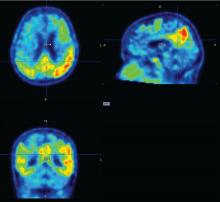
BOSTON – Progressive tau accumulation in the temporal lobe of cognitively normal older adults was associated with cognitive decline over time in a prospective, longitudinal study presented at the Clinical Trials on Alzheimer’s Disease conference.
This track of cognitive impairment following tau pathology in a preclinical Alzheimer’s disease (AD) population suggests two roles for serial positron emission tomography (PET) scans with a tau binding agent, Bernard Hanseeuw, MD, PhD, said at the meeting. In the near future, they could be used to track therapeutic response in clinical trials. Farther out, if future validation studies confirm these preliminary results, they might be a useful clinical tool for predicting how fast an individual Alzheimer’s patient will progress, he said in an interview.
Serial tau scans, however, would, he said.
“Every patient with Alzheimer’s disease is different, with a different disease course. Amyloid scans can tell us if someone is on the wrong path, but tau scans could tell us how fast they are going. If you have Alzheimer’s, it’s important to know if you may not be able to live in your own home in a year. With tau PET, we could track the disease and predict how fast it might evolve. That is very clinically relevant,” said Dr. Hanseeuw.
Tau imaging remains investigational only. Several tau imaging agents are being developed, but none has yet been approved in the United States or in Europe.
To investigate the correlation of tau and cognitive decline in preclinical Alzheimer’s, Dr. Hanseeuw examined serial tau and amyloid PET scans conducted on 60 clinically normal older adults with a mean age of 75 years. About one-third of the cohort was positive for the APOE4 allele. All of them had a baseline Clinical Dementia Rating (CDR) score of 0 and a mean Mini-Mental State Exam score of at least 27. They also scored in the normal range on the Preclinical Alzheimer’s Cognitive Composite (PACC) test. This relatively new cognitive scale is an increasingly popular item in clinical trials. The PACC is a composite of the WAIS-R Digit Symbol Substitution Test, Mini-Mental State Exam, Free and Cued Selective Reminding Test, and Logical Memory IIA Delayed Recall, and correlates well with amyloid accumulation in the brain.
The study included up to 4 years of data on cognition and amyloid PET imaging, and up to 3 years of tau PET imaging data. The investigators assessed amyloid as a whole-brain aggregate and tau in the bilateral inferior temporal neocortex. “This is where the change is most happening in patients, and it’s a place where relatively few normal elderly would have tau,” Dr. Hanseeuw said. All of the analyses controlled for age, sex, and years of education.
Baseline amyloid levels were low in 36 participants and high in 24. At least some tau was present in all of the subjects. This is not an unexpected finding, since tau accumulates with age, Dr. Hanseeuw said. Over the study period, six subjects progressed to a CDR of 0.5 – a rating consistent with mild cognitive impairment. At baseline, high tau and high amyloid levels were both associated with a progressive decline in PACC scores in the following years. However, the rate of change in tau predicted change in cognition better than did the baseline measurements. In contrast, the rate of change in amyloid was not associated with cognitive decline.
“What is interesting here is that tau changed four times faster than amyloid,” Dr. Hanseeuw said. “The average subject needed 5 years to change 1 standard deviation in tau, but would have needed 20 years to change 1 standard deviation in amyloid.”
Fast-changing outcomes are important to accelerate drug assessment in clinical trials. Currently, it takes 3-5 years to conduct most anti-AD trials, he added.
Dr. Hanseeuw had no relevant financial disclosures.
BOSTON – Progressive tau accumulation in the temporal lobe of cognitively normal older adults was associated with cognitive decline over time in a prospective, longitudinal study presented at the Clinical Trials on Alzheimer’s Disease conference.
This track of cognitive impairment following tau pathology in a preclinical Alzheimer’s disease (AD) population suggests two roles for serial positron emission tomography (PET) scans with a tau binding agent, Bernard Hanseeuw, MD, PhD, said at the meeting. In the near future, they could be used to track therapeutic response in clinical trials. Farther out, if future validation studies confirm these preliminary results, they might be a useful clinical tool for predicting how fast an individual Alzheimer’s patient will progress, he said in an interview.
Serial tau scans, however, would, he said.
“Every patient with Alzheimer’s disease is different, with a different disease course. Amyloid scans can tell us if someone is on the wrong path, but tau scans could tell us how fast they are going. If you have Alzheimer’s, it’s important to know if you may not be able to live in your own home in a year. With tau PET, we could track the disease and predict how fast it might evolve. That is very clinically relevant,” said Dr. Hanseeuw.
Tau imaging remains investigational only. Several tau imaging agents are being developed, but none has yet been approved in the United States or in Europe.
To investigate the correlation of tau and cognitive decline in preclinical Alzheimer’s, Dr. Hanseeuw examined serial tau and amyloid PET scans conducted on 60 clinically normal older adults with a mean age of 75 years. About one-third of the cohort was positive for the APOE4 allele. All of them had a baseline Clinical Dementia Rating (CDR) score of 0 and a mean Mini-Mental State Exam score of at least 27. They also scored in the normal range on the Preclinical Alzheimer’s Cognitive Composite (PACC) test. This relatively new cognitive scale is an increasingly popular item in clinical trials. The PACC is a composite of the WAIS-R Digit Symbol Substitution Test, Mini-Mental State Exam, Free and Cued Selective Reminding Test, and Logical Memory IIA Delayed Recall, and correlates well with amyloid accumulation in the brain.
The study included up to 4 years of data on cognition and amyloid PET imaging, and up to 3 years of tau PET imaging data. The investigators assessed amyloid as a whole-brain aggregate and tau in the bilateral inferior temporal neocortex. “This is where the change is most happening in patients, and it’s a place where relatively few normal elderly would have tau,” Dr. Hanseeuw said. All of the analyses controlled for age, sex, and years of education.
Baseline amyloid levels were low in 36 participants and high in 24. At least some tau was present in all of the subjects. This is not an unexpected finding, since tau accumulates with age, Dr. Hanseeuw said. Over the study period, six subjects progressed to a CDR of 0.5 – a rating consistent with mild cognitive impairment. At baseline, high tau and high amyloid levels were both associated with a progressive decline in PACC scores in the following years. However, the rate of change in tau predicted change in cognition better than did the baseline measurements. In contrast, the rate of change in amyloid was not associated with cognitive decline.
“What is interesting here is that tau changed four times faster than amyloid,” Dr. Hanseeuw said. “The average subject needed 5 years to change 1 standard deviation in tau, but would have needed 20 years to change 1 standard deviation in amyloid.”
Fast-changing outcomes are important to accelerate drug assessment in clinical trials. Currently, it takes 3-5 years to conduct most anti-AD trials, he added.
Dr. Hanseeuw had no relevant financial disclosures.
BOSTON – Progressive tau accumulation in the temporal lobe of cognitively normal older adults was associated with cognitive decline over time in a prospective, longitudinal study presented at the Clinical Trials on Alzheimer’s Disease conference.
This track of cognitive impairment following tau pathology in a preclinical Alzheimer’s disease (AD) population suggests two roles for serial positron emission tomography (PET) scans with a tau binding agent, Bernard Hanseeuw, MD, PhD, said at the meeting. In the near future, they could be used to track therapeutic response in clinical trials. Farther out, if future validation studies confirm these preliminary results, they might be a useful clinical tool for predicting how fast an individual Alzheimer’s patient will progress, he said in an interview.
Serial tau scans, however, would, he said.
“Every patient with Alzheimer’s disease is different, with a different disease course. Amyloid scans can tell us if someone is on the wrong path, but tau scans could tell us how fast they are going. If you have Alzheimer’s, it’s important to know if you may not be able to live in your own home in a year. With tau PET, we could track the disease and predict how fast it might evolve. That is very clinically relevant,” said Dr. Hanseeuw.
Tau imaging remains investigational only. Several tau imaging agents are being developed, but none has yet been approved in the United States or in Europe.
To investigate the correlation of tau and cognitive decline in preclinical Alzheimer’s, Dr. Hanseeuw examined serial tau and amyloid PET scans conducted on 60 clinically normal older adults with a mean age of 75 years. About one-third of the cohort was positive for the APOE4 allele. All of them had a baseline Clinical Dementia Rating (CDR) score of 0 and a mean Mini-Mental State Exam score of at least 27. They also scored in the normal range on the Preclinical Alzheimer’s Cognitive Composite (PACC) test. This relatively new cognitive scale is an increasingly popular item in clinical trials. The PACC is a composite of the WAIS-R Digit Symbol Substitution Test, Mini-Mental State Exam, Free and Cued Selective Reminding Test, and Logical Memory IIA Delayed Recall, and correlates well with amyloid accumulation in the brain.
The study included up to 4 years of data on cognition and amyloid PET imaging, and up to 3 years of tau PET imaging data. The investigators assessed amyloid as a whole-brain aggregate and tau in the bilateral inferior temporal neocortex. “This is where the change is most happening in patients, and it’s a place where relatively few normal elderly would have tau,” Dr. Hanseeuw said. All of the analyses controlled for age, sex, and years of education.
Baseline amyloid levels were low in 36 participants and high in 24. At least some tau was present in all of the subjects. This is not an unexpected finding, since tau accumulates with age, Dr. Hanseeuw said. Over the study period, six subjects progressed to a CDR of 0.5 – a rating consistent with mild cognitive impairment. At baseline, high tau and high amyloid levels were both associated with a progressive decline in PACC scores in the following years. However, the rate of change in tau predicted change in cognition better than did the baseline measurements. In contrast, the rate of change in amyloid was not associated with cognitive decline.
“What is interesting here is that tau changed four times faster than amyloid,” Dr. Hanseeuw said. “The average subject needed 5 years to change 1 standard deviation in tau, but would have needed 20 years to change 1 standard deviation in amyloid.”
Fast-changing outcomes are important to accelerate drug assessment in clinical trials. Currently, it takes 3-5 years to conduct most anti-AD trials, he added.
Dr. Hanseeuw had no relevant financial disclosures.
REPORTING FROM CTAD
Key clinical point:
Major finding: Tau levels changed twice as fast as cognition, suggesting that the protein is a significant marker of future cognitive change.
Data source: A prospective, longitudinal study of 60 cognitively normal subjects.
Disclosures: Dr. Hanseeuw had no relevant financial disclosures.
Source: Hanseeuw B et al. CTAD 2017 Abstract OC2.
Retinal changes may reflect brain changes in preclinical Alzheimer’s
BOSTON – Changes in the retina seem to mirror changes that begin to reshape the brain in preclinical Alzheimer’s disease.
Manifested as a reduction in volume in the retinal nerve fiber layer, these changes appear to track the aggregation of beta amyloid brain plaques well before cognitive problems arise – and can be easily measured with a piece of equipment already in many optometry offices, Peter J. Snyder, PhD, said at the Clinical Trials in Alzheimer’s Disease conference.
“If we are lucky enough to live past age 45, then it’s a given that we’re all going to develop some presbyopia. So we all have to go to the optometrist sometime, and that may become a point of entry for broad screening and to track changes over time, to keep an eye on at-risk patients, and to refer those with retinal changes that fit the preclinical AD profile to specialty care for more comprehensive diagnostic evaluations.”
The retina begins to form in the third week of embryologic life, arising from the neural tube cells that also form the brain and spinal cord. It makes sense then that very early neuronal changes in Alzheimer’s disease could be occurring in the retina as well, said Dr. Snyder, professor of neurology and surgery (ophthalmology) at Rhode Island Hospital and Brown University, Providence.
“The retina is really a protrusion of the brain, and it is part and parcel of the central nervous system. In terms of the neuronal structure, the retina develops in layers with very specific cell types that are neurochemically and physiologically the same as the nervous tissue in the brain. That’s why it is, potentially, literally a window that could let us see what’s happening in the brain in early Alzheimer’s disease.”
Other researchers have explored amyloid in the lens and retina as a possible early Alzheimer’s identification tool. But Dr. Snyder’s study is the first to demonstrate a longitudinal association between neuronal changes in the eye and amyloid burden in the brain among clinically normal subjects.
For 27 months, he followed 56 people who had normal cognition but were beginning to experience subjective memory complaints. All subjects had at least one parent with Alzheimer’s disease. Everyone underwent an amyloid PET scan at baseline. Of the cohort, 15 had PET imaging evidence of abnormal beta-amyloid protein aggregation in the neocortex. This group was deemed to have preclinical Alzheimer’s disease, while the remainder served as a control group.
Dr. Snyder imaged each subject’s retinas twice – once at baseline and once at 27 months, when everyone underwent a second amyloid PET scan as well. He examined the retina with spectral domain optical coherence tomography, a relatively new method of imaging the retina.
These scanners are becoming increasingly more common in optometry practices, Dr. Snyder said. “Graduate optometrists tell me they would not want to be in a practice without one.” The scanners are typically used to detect retinal and ocular changes associated with diabetes, macular degeneration, glaucoma and multiple sclerosis.
Dr. Snyder used the scanner to examine the optic nerve head and macula at both baseline and 27 months in his cohort. He was looking for volumetric changes in several of the retinal layers: the peripapillary retinal nerve fiber layer (pRNFL), macular RNFL (mRNFL), ganglion cell layer (GCL), inner plexiform layer (IPL), outer nuclear layer (ONL), outer plexiform layer (OPL), and inner nuclear layer (INL). He also computed changes in total retinal volume.
Even at baseline, he found a significant difference between the groups. Among the amyloid-positive subjects, the inner plexiform layer was slightly larger in volume. “This seems a bit counterintuitive, but I think it suggests that there may be some inflammatory processes going on in this early stage and that we are catching that inflammation.”
Dr. Snyder noted that this finding has recently been replicated by an independent research group in Perth, Australia – with a much larger sample of participants – and will be reported at international conferences this coming year.
At 27 months, both the total retinal volume and the macular retinal nerve fiber layer volume were significantly lower in the preclinical AD group than in the control group. There was also a volume reduction in the peripapillary retinal nerve fiber layer, although the between-group difference was not statistically significant.
In a multivariate linear regression model that controlled for age and total amyloid burden, the mean volume change in the macular retinal nerve fiber layer accounted for about 10% of the variation in PET binding to brain amyloid by 27 months. Volume reductions in all the other layers appeared to be associated only with age, representing normal age-related changes in the eye.
Dr. Snyder said this volume loss in the retinal nerve fiber layer probably represents early demyelination and/or degeneration of the axons coursing from the cell bodies in the ganglion cell layer, which project to the optic nerve head.
“This finding in the retina appears analogous, and possibly directly related to, a similar loss of white matter that is readily observable in the early stages of Alzheimer’s disease. At the same time, patients are beginning to experience both cholinergic changes in the basal forebrain and the abnormal aggregation of fibrillar beta-amyloid plaques. I don’t know to what extent these changes are mechanistically dependent on each other, but they appear to also be happening, in the earliest stages of the disease course, in the retina.”
There is a lot of work left to be done before retinal scanning could be employed as a risk-assessment tool, however. With every new biomarker – and especially with imaging – the ability to measure change occurs far in advance of an understanding of what those changes mean, and how to judge them accurately.
“Every time we have a major advance in imaging, the technical engineering breakthroughs precede our detailed understanding of what we’re looking at and what to measure. This is where we are right now with retinal imaging. Biologically, it makes sense to be looking at this as a marker of risk in those who are clinically healthy, and maybe later as a marker of disease progression. But there is a lot of work to be done here yet.”
Dr. Snyder’s project was funded in part by a research award from Pfizer, with PET imaging supported in part by a grant from Avid Radiopharmaceuticals. He has no financial ties to the company, or other financial interest related to the study.
[email protected]
On Twitter @Alz_Gal
BOSTON – Changes in the retina seem to mirror changes that begin to reshape the brain in preclinical Alzheimer’s disease.
Manifested as a reduction in volume in the retinal nerve fiber layer, these changes appear to track the aggregation of beta amyloid brain plaques well before cognitive problems arise – and can be easily measured with a piece of equipment already in many optometry offices, Peter J. Snyder, PhD, said at the Clinical Trials in Alzheimer’s Disease conference.
“If we are lucky enough to live past age 45, then it’s a given that we’re all going to develop some presbyopia. So we all have to go to the optometrist sometime, and that may become a point of entry for broad screening and to track changes over time, to keep an eye on at-risk patients, and to refer those with retinal changes that fit the preclinical AD profile to specialty care for more comprehensive diagnostic evaluations.”
The retina begins to form in the third week of embryologic life, arising from the neural tube cells that also form the brain and spinal cord. It makes sense then that very early neuronal changes in Alzheimer’s disease could be occurring in the retina as well, said Dr. Snyder, professor of neurology and surgery (ophthalmology) at Rhode Island Hospital and Brown University, Providence.
“The retina is really a protrusion of the brain, and it is part and parcel of the central nervous system. In terms of the neuronal structure, the retina develops in layers with very specific cell types that are neurochemically and physiologically the same as the nervous tissue in the brain. That’s why it is, potentially, literally a window that could let us see what’s happening in the brain in early Alzheimer’s disease.”
Other researchers have explored amyloid in the lens and retina as a possible early Alzheimer’s identification tool. But Dr. Snyder’s study is the first to demonstrate a longitudinal association between neuronal changes in the eye and amyloid burden in the brain among clinically normal subjects.
For 27 months, he followed 56 people who had normal cognition but were beginning to experience subjective memory complaints. All subjects had at least one parent with Alzheimer’s disease. Everyone underwent an amyloid PET scan at baseline. Of the cohort, 15 had PET imaging evidence of abnormal beta-amyloid protein aggregation in the neocortex. This group was deemed to have preclinical Alzheimer’s disease, while the remainder served as a control group.
Dr. Snyder imaged each subject’s retinas twice – once at baseline and once at 27 months, when everyone underwent a second amyloid PET scan as well. He examined the retina with spectral domain optical coherence tomography, a relatively new method of imaging the retina.
These scanners are becoming increasingly more common in optometry practices, Dr. Snyder said. “Graduate optometrists tell me they would not want to be in a practice without one.” The scanners are typically used to detect retinal and ocular changes associated with diabetes, macular degeneration, glaucoma and multiple sclerosis.
Dr. Snyder used the scanner to examine the optic nerve head and macula at both baseline and 27 months in his cohort. He was looking for volumetric changes in several of the retinal layers: the peripapillary retinal nerve fiber layer (pRNFL), macular RNFL (mRNFL), ganglion cell layer (GCL), inner plexiform layer (IPL), outer nuclear layer (ONL), outer plexiform layer (OPL), and inner nuclear layer (INL). He also computed changes in total retinal volume.
Even at baseline, he found a significant difference between the groups. Among the amyloid-positive subjects, the inner plexiform layer was slightly larger in volume. “This seems a bit counterintuitive, but I think it suggests that there may be some inflammatory processes going on in this early stage and that we are catching that inflammation.”
Dr. Snyder noted that this finding has recently been replicated by an independent research group in Perth, Australia – with a much larger sample of participants – and will be reported at international conferences this coming year.
At 27 months, both the total retinal volume and the macular retinal nerve fiber layer volume were significantly lower in the preclinical AD group than in the control group. There was also a volume reduction in the peripapillary retinal nerve fiber layer, although the between-group difference was not statistically significant.
In a multivariate linear regression model that controlled for age and total amyloid burden, the mean volume change in the macular retinal nerve fiber layer accounted for about 10% of the variation in PET binding to brain amyloid by 27 months. Volume reductions in all the other layers appeared to be associated only with age, representing normal age-related changes in the eye.
Dr. Snyder said this volume loss in the retinal nerve fiber layer probably represents early demyelination and/or degeneration of the axons coursing from the cell bodies in the ganglion cell layer, which project to the optic nerve head.
“This finding in the retina appears analogous, and possibly directly related to, a similar loss of white matter that is readily observable in the early stages of Alzheimer’s disease. At the same time, patients are beginning to experience both cholinergic changes in the basal forebrain and the abnormal aggregation of fibrillar beta-amyloid plaques. I don’t know to what extent these changes are mechanistically dependent on each other, but they appear to also be happening, in the earliest stages of the disease course, in the retina.”
There is a lot of work left to be done before retinal scanning could be employed as a risk-assessment tool, however. With every new biomarker – and especially with imaging – the ability to measure change occurs far in advance of an understanding of what those changes mean, and how to judge them accurately.
“Every time we have a major advance in imaging, the technical engineering breakthroughs precede our detailed understanding of what we’re looking at and what to measure. This is where we are right now with retinal imaging. Biologically, it makes sense to be looking at this as a marker of risk in those who are clinically healthy, and maybe later as a marker of disease progression. But there is a lot of work to be done here yet.”
Dr. Snyder’s project was funded in part by a research award from Pfizer, with PET imaging supported in part by a grant from Avid Radiopharmaceuticals. He has no financial ties to the company, or other financial interest related to the study.
[email protected]
On Twitter @Alz_Gal
BOSTON – Changes in the retina seem to mirror changes that begin to reshape the brain in preclinical Alzheimer’s disease.
Manifested as a reduction in volume in the retinal nerve fiber layer, these changes appear to track the aggregation of beta amyloid brain plaques well before cognitive problems arise – and can be easily measured with a piece of equipment already in many optometry offices, Peter J. Snyder, PhD, said at the Clinical Trials in Alzheimer’s Disease conference.
“If we are lucky enough to live past age 45, then it’s a given that we’re all going to develop some presbyopia. So we all have to go to the optometrist sometime, and that may become a point of entry for broad screening and to track changes over time, to keep an eye on at-risk patients, and to refer those with retinal changes that fit the preclinical AD profile to specialty care for more comprehensive diagnostic evaluations.”
The retina begins to form in the third week of embryologic life, arising from the neural tube cells that also form the brain and spinal cord. It makes sense then that very early neuronal changes in Alzheimer’s disease could be occurring in the retina as well, said Dr. Snyder, professor of neurology and surgery (ophthalmology) at Rhode Island Hospital and Brown University, Providence.
“The retina is really a protrusion of the brain, and it is part and parcel of the central nervous system. In terms of the neuronal structure, the retina develops in layers with very specific cell types that are neurochemically and physiologically the same as the nervous tissue in the brain. That’s why it is, potentially, literally a window that could let us see what’s happening in the brain in early Alzheimer’s disease.”
Other researchers have explored amyloid in the lens and retina as a possible early Alzheimer’s identification tool. But Dr. Snyder’s study is the first to demonstrate a longitudinal association between neuronal changes in the eye and amyloid burden in the brain among clinically normal subjects.
For 27 months, he followed 56 people who had normal cognition but were beginning to experience subjective memory complaints. All subjects had at least one parent with Alzheimer’s disease. Everyone underwent an amyloid PET scan at baseline. Of the cohort, 15 had PET imaging evidence of abnormal beta-amyloid protein aggregation in the neocortex. This group was deemed to have preclinical Alzheimer’s disease, while the remainder served as a control group.
Dr. Snyder imaged each subject’s retinas twice – once at baseline and once at 27 months, when everyone underwent a second amyloid PET scan as well. He examined the retina with spectral domain optical coherence tomography, a relatively new method of imaging the retina.
These scanners are becoming increasingly more common in optometry practices, Dr. Snyder said. “Graduate optometrists tell me they would not want to be in a practice without one.” The scanners are typically used to detect retinal and ocular changes associated with diabetes, macular degeneration, glaucoma and multiple sclerosis.
Dr. Snyder used the scanner to examine the optic nerve head and macula at both baseline and 27 months in his cohort. He was looking for volumetric changes in several of the retinal layers: the peripapillary retinal nerve fiber layer (pRNFL), macular RNFL (mRNFL), ganglion cell layer (GCL), inner plexiform layer (IPL), outer nuclear layer (ONL), outer plexiform layer (OPL), and inner nuclear layer (INL). He also computed changes in total retinal volume.
Even at baseline, he found a significant difference between the groups. Among the amyloid-positive subjects, the inner plexiform layer was slightly larger in volume. “This seems a bit counterintuitive, but I think it suggests that there may be some inflammatory processes going on in this early stage and that we are catching that inflammation.”
Dr. Snyder noted that this finding has recently been replicated by an independent research group in Perth, Australia – with a much larger sample of participants – and will be reported at international conferences this coming year.
At 27 months, both the total retinal volume and the macular retinal nerve fiber layer volume were significantly lower in the preclinical AD group than in the control group. There was also a volume reduction in the peripapillary retinal nerve fiber layer, although the between-group difference was not statistically significant.
In a multivariate linear regression model that controlled for age and total amyloid burden, the mean volume change in the macular retinal nerve fiber layer accounted for about 10% of the variation in PET binding to brain amyloid by 27 months. Volume reductions in all the other layers appeared to be associated only with age, representing normal age-related changes in the eye.
Dr. Snyder said this volume loss in the retinal nerve fiber layer probably represents early demyelination and/or degeneration of the axons coursing from the cell bodies in the ganglion cell layer, which project to the optic nerve head.
“This finding in the retina appears analogous, and possibly directly related to, a similar loss of white matter that is readily observable in the early stages of Alzheimer’s disease. At the same time, patients are beginning to experience both cholinergic changes in the basal forebrain and the abnormal aggregation of fibrillar beta-amyloid plaques. I don’t know to what extent these changes are mechanistically dependent on each other, but they appear to also be happening, in the earliest stages of the disease course, in the retina.”
There is a lot of work left to be done before retinal scanning could be employed as a risk-assessment tool, however. With every new biomarker – and especially with imaging – the ability to measure change occurs far in advance of an understanding of what those changes mean, and how to judge them accurately.
“Every time we have a major advance in imaging, the technical engineering breakthroughs precede our detailed understanding of what we’re looking at and what to measure. This is where we are right now with retinal imaging. Biologically, it makes sense to be looking at this as a marker of risk in those who are clinically healthy, and maybe later as a marker of disease progression. But there is a lot of work to be done here yet.”
Dr. Snyder’s project was funded in part by a research award from Pfizer, with PET imaging supported in part by a grant from Avid Radiopharmaceuticals. He has no financial ties to the company, or other financial interest related to the study.
[email protected]
On Twitter @Alz_Gal
AT CTAD
Key clinical point: Retinal scans might eventually be an easy, noninvasive, and inexpensive way to tag people who may be at elevated risk for Alzheimer’s disease.
Major finding: At 27 months, both the total retinal volume and the macular retinal nerve fiber layer volume were significantly lower in 15 patients in the preclinical AD group than in the 41 patients in the control group.
Data source: A follow-up study of 56 people who had at least one parent with Alzheimer’s disease and were beginning to experience subjective memory complaints.
Disclosures: Dr. Snyder’s project was funded in part by a research award from Pfizer, with PET imaging supported in part by a grant from Avid Radiopharmaceuticals. He has no financial ties to the company, or other financial interest related to the study.
Walking has beneficial cognitive effects in amyloid-positive older adults
BOSTON – Walking appears to moderate cognitive decline in people with elevated brain amyloid, a 4-year observational study has determined.
Among a group of cognitively normal older adults with beta-amyloid brain plaques, those who walked the most experienced significantly less decline in memory and thinking than those who didn’t walk much, Dylan Kirn reported at the Clinical Trials on Alzheimer’s Disease conference. Walking didn’t affect any of the hallmark biomarkers of Alzheimer’s disease, such as brain glucose utilization, amyloid accumulation, or hippocampal volume, but it was associated with significantly better cognitive scores on a composite measure of memory over time.
“We should be careful in interpreting these data, because this is an observational cohort and we can’t make claims regarding causality or the mechanism by which physical activity may be influencing cognitive decline,” said Mr. Kirn of the Athinoula A. Martinos Center for Biomedical Imaging at Massachusetts General Hospital, Boston. “But I find these results interesting and novel, and I think they support further investigation.”
The walking study comprised 255 subjects with a mean age of 73 years. They were highly educated, with a mean of 16 years’ schooling. About 24% were amyloid-positive by PET imaging. All were cognitively normal, with a Clinical Dementia Rating scale score of 0. Activity was established at baseline with a pedometer, which was worn for 7 consecutive days; only those who walked at least 100 steps per day were included in the analysis.
In addition to amyloid PET imaging, subjects also underwent a 18F-fluorodeoxyglucose (FDG) PET scan to assess brain glucose utilization, and MRI to measure hippocampal volume changes and assess white matter hyperintensities (WMHs). Changes in all of these biomarkers can herald the onset of Alzheimer’s.
The primary outcome was the relationship between physical activity as measured by number of walking steps per day and changes on the Preclinical Alzheimer’s Cognitive Composite (PACC) test. This relatively new cognitive scale is an increasingly popular item in clinical trials. The PACC is a composite of the Digit Symbol Substitution Test score from the Wechsler Adult Intelligence Scale–Revised, the Mini Mental State Exam, the Total Recall score from the Free and Cued Selective Reminding Test, and the Delayed Recall score on the Logical Memory IIa sub-test from the Wechsler Memory Scale. It correlates well with amyloid accumulation in the brain, Mr. Kirn said.
The cohort was followed for up to 6 years (median of 4), and PACC scores were calculated annually. The investigators looked at the relationship between walking at baseline and PACC decline over the study period in two multivariate models: One controlling for age, sex, and years of education, and the second for those variables plus the biomarkers of cortical WMHs, bilateral hippocampal volume (HV), and FDG-PET in brain regions typically affected by Alzheimer’s.
Physical activity was divided into tertiles by the average number of steps per day over the 7-day measuring period: Mean (5,616 steps), one standard deviation above mean (high; 8,482 steps), and one standard deviation below mean (low, 2,751 steps). Amyloid-positive patients were further divided into those with high brain amyloid load and those with low amyloid brain load.
There were no significant relationships between any of the biomarkers and any level of physical activity in either of the analyses, Mr. Kirn said. However, when looking at the time-linked changes in the PACC, significant differences did emerge. Subjects who walked at least the mean number of steps per day were much more likely to maintain a stable cognitive score, while those who walked the fewest steps declined about a quarter of a point on the PACC. The difference in decline between the high activity and low activity subjects was statistically significant, even when the investigators controlled for amyloid burden and the other hallmark Alzheimer’s biomarkers.
The level of physical activity at baseline was a particularly strong predictor of cognitive health among amyloid-positive subjects. Those in the high-activity group maintained a steady score on the PACC. Those in the mean activity group declined slightly, and those in the low activity group showed a sharp decline, losing almost a full point on the PACC by the end of follow-up.
In the amyloid-negative group, there was no association between cognition and activity. All the groups improved their PACC scores over the study period, probably reflecting a practice effect, Mr. Kirn said.
Finally, he split the amyloid-positive group into subjects with low and high brain amyloid levels. “We observed that physical activity was significantly predictive of cognitive decline in high-amyloid participants, but not in low-amyloid participants,” he said. “Individuals with high amyloid and low physical activity at baseline had the steepest decline in cognition over time. But in those with high amyloid and high physical activity at baseline, we didn’t see a tremendous amount of decline.”
The study suggests that pedometers may have a place in stratifying patients for clinical trials, or assessing cognitive risk in elderly subjects. “Most studies that have looked at physical activity and dementia use a self-reported activity level, so the results have been varied,” Mr. Kirn said. “These findings support consideration of objectively measured physical activity in clinical research, and perhaps in stratification for risk of cognitive decline.”
He had no financial disclosures.
[email protected]
On Twitter @Alz_Gal
BOSTON – Walking appears to moderate cognitive decline in people with elevated brain amyloid, a 4-year observational study has determined.
Among a group of cognitively normal older adults with beta-amyloid brain plaques, those who walked the most experienced significantly less decline in memory and thinking than those who didn’t walk much, Dylan Kirn reported at the Clinical Trials on Alzheimer’s Disease conference. Walking didn’t affect any of the hallmark biomarkers of Alzheimer’s disease, such as brain glucose utilization, amyloid accumulation, or hippocampal volume, but it was associated with significantly better cognitive scores on a composite measure of memory over time.
“We should be careful in interpreting these data, because this is an observational cohort and we can’t make claims regarding causality or the mechanism by which physical activity may be influencing cognitive decline,” said Mr. Kirn of the Athinoula A. Martinos Center for Biomedical Imaging at Massachusetts General Hospital, Boston. “But I find these results interesting and novel, and I think they support further investigation.”
The walking study comprised 255 subjects with a mean age of 73 years. They were highly educated, with a mean of 16 years’ schooling. About 24% were amyloid-positive by PET imaging. All were cognitively normal, with a Clinical Dementia Rating scale score of 0. Activity was established at baseline with a pedometer, which was worn for 7 consecutive days; only those who walked at least 100 steps per day were included in the analysis.
In addition to amyloid PET imaging, subjects also underwent a 18F-fluorodeoxyglucose (FDG) PET scan to assess brain glucose utilization, and MRI to measure hippocampal volume changes and assess white matter hyperintensities (WMHs). Changes in all of these biomarkers can herald the onset of Alzheimer’s.
The primary outcome was the relationship between physical activity as measured by number of walking steps per day and changes on the Preclinical Alzheimer’s Cognitive Composite (PACC) test. This relatively new cognitive scale is an increasingly popular item in clinical trials. The PACC is a composite of the Digit Symbol Substitution Test score from the Wechsler Adult Intelligence Scale–Revised, the Mini Mental State Exam, the Total Recall score from the Free and Cued Selective Reminding Test, and the Delayed Recall score on the Logical Memory IIa sub-test from the Wechsler Memory Scale. It correlates well with amyloid accumulation in the brain, Mr. Kirn said.
The cohort was followed for up to 6 years (median of 4), and PACC scores were calculated annually. The investigators looked at the relationship between walking at baseline and PACC decline over the study period in two multivariate models: One controlling for age, sex, and years of education, and the second for those variables plus the biomarkers of cortical WMHs, bilateral hippocampal volume (HV), and FDG-PET in brain regions typically affected by Alzheimer’s.
Physical activity was divided into tertiles by the average number of steps per day over the 7-day measuring period: Mean (5,616 steps), one standard deviation above mean (high; 8,482 steps), and one standard deviation below mean (low, 2,751 steps). Amyloid-positive patients were further divided into those with high brain amyloid load and those with low amyloid brain load.
There were no significant relationships between any of the biomarkers and any level of physical activity in either of the analyses, Mr. Kirn said. However, when looking at the time-linked changes in the PACC, significant differences did emerge. Subjects who walked at least the mean number of steps per day were much more likely to maintain a stable cognitive score, while those who walked the fewest steps declined about a quarter of a point on the PACC. The difference in decline between the high activity and low activity subjects was statistically significant, even when the investigators controlled for amyloid burden and the other hallmark Alzheimer’s biomarkers.
The level of physical activity at baseline was a particularly strong predictor of cognitive health among amyloid-positive subjects. Those in the high-activity group maintained a steady score on the PACC. Those in the mean activity group declined slightly, and those in the low activity group showed a sharp decline, losing almost a full point on the PACC by the end of follow-up.
In the amyloid-negative group, there was no association between cognition and activity. All the groups improved their PACC scores over the study period, probably reflecting a practice effect, Mr. Kirn said.
Finally, he split the amyloid-positive group into subjects with low and high brain amyloid levels. “We observed that physical activity was significantly predictive of cognitive decline in high-amyloid participants, but not in low-amyloid participants,” he said. “Individuals with high amyloid and low physical activity at baseline had the steepest decline in cognition over time. But in those with high amyloid and high physical activity at baseline, we didn’t see a tremendous amount of decline.”
The study suggests that pedometers may have a place in stratifying patients for clinical trials, or assessing cognitive risk in elderly subjects. “Most studies that have looked at physical activity and dementia use a self-reported activity level, so the results have been varied,” Mr. Kirn said. “These findings support consideration of objectively measured physical activity in clinical research, and perhaps in stratification for risk of cognitive decline.”
He had no financial disclosures.
[email protected]
On Twitter @Alz_Gal
BOSTON – Walking appears to moderate cognitive decline in people with elevated brain amyloid, a 4-year observational study has determined.
Among a group of cognitively normal older adults with beta-amyloid brain plaques, those who walked the most experienced significantly less decline in memory and thinking than those who didn’t walk much, Dylan Kirn reported at the Clinical Trials on Alzheimer’s Disease conference. Walking didn’t affect any of the hallmark biomarkers of Alzheimer’s disease, such as brain glucose utilization, amyloid accumulation, or hippocampal volume, but it was associated with significantly better cognitive scores on a composite measure of memory over time.
“We should be careful in interpreting these data, because this is an observational cohort and we can’t make claims regarding causality or the mechanism by which physical activity may be influencing cognitive decline,” said Mr. Kirn of the Athinoula A. Martinos Center for Biomedical Imaging at Massachusetts General Hospital, Boston. “But I find these results interesting and novel, and I think they support further investigation.”
The walking study comprised 255 subjects with a mean age of 73 years. They were highly educated, with a mean of 16 years’ schooling. About 24% were amyloid-positive by PET imaging. All were cognitively normal, with a Clinical Dementia Rating scale score of 0. Activity was established at baseline with a pedometer, which was worn for 7 consecutive days; only those who walked at least 100 steps per day were included in the analysis.
In addition to amyloid PET imaging, subjects also underwent a 18F-fluorodeoxyglucose (FDG) PET scan to assess brain glucose utilization, and MRI to measure hippocampal volume changes and assess white matter hyperintensities (WMHs). Changes in all of these biomarkers can herald the onset of Alzheimer’s.
The primary outcome was the relationship between physical activity as measured by number of walking steps per day and changes on the Preclinical Alzheimer’s Cognitive Composite (PACC) test. This relatively new cognitive scale is an increasingly popular item in clinical trials. The PACC is a composite of the Digit Symbol Substitution Test score from the Wechsler Adult Intelligence Scale–Revised, the Mini Mental State Exam, the Total Recall score from the Free and Cued Selective Reminding Test, and the Delayed Recall score on the Logical Memory IIa sub-test from the Wechsler Memory Scale. It correlates well with amyloid accumulation in the brain, Mr. Kirn said.
The cohort was followed for up to 6 years (median of 4), and PACC scores were calculated annually. The investigators looked at the relationship between walking at baseline and PACC decline over the study period in two multivariate models: One controlling for age, sex, and years of education, and the second for those variables plus the biomarkers of cortical WMHs, bilateral hippocampal volume (HV), and FDG-PET in brain regions typically affected by Alzheimer’s.
Physical activity was divided into tertiles by the average number of steps per day over the 7-day measuring period: Mean (5,616 steps), one standard deviation above mean (high; 8,482 steps), and one standard deviation below mean (low, 2,751 steps). Amyloid-positive patients were further divided into those with high brain amyloid load and those with low amyloid brain load.
There were no significant relationships between any of the biomarkers and any level of physical activity in either of the analyses, Mr. Kirn said. However, when looking at the time-linked changes in the PACC, significant differences did emerge. Subjects who walked at least the mean number of steps per day were much more likely to maintain a stable cognitive score, while those who walked the fewest steps declined about a quarter of a point on the PACC. The difference in decline between the high activity and low activity subjects was statistically significant, even when the investigators controlled for amyloid burden and the other hallmark Alzheimer’s biomarkers.
The level of physical activity at baseline was a particularly strong predictor of cognitive health among amyloid-positive subjects. Those in the high-activity group maintained a steady score on the PACC. Those in the mean activity group declined slightly, and those in the low activity group showed a sharp decline, losing almost a full point on the PACC by the end of follow-up.
In the amyloid-negative group, there was no association between cognition and activity. All the groups improved their PACC scores over the study period, probably reflecting a practice effect, Mr. Kirn said.
Finally, he split the amyloid-positive group into subjects with low and high brain amyloid levels. “We observed that physical activity was significantly predictive of cognitive decline in high-amyloid participants, but not in low-amyloid participants,” he said. “Individuals with high amyloid and low physical activity at baseline had the steepest decline in cognition over time. But in those with high amyloid and high physical activity at baseline, we didn’t see a tremendous amount of decline.”
The study suggests that pedometers may have a place in stratifying patients for clinical trials, or assessing cognitive risk in elderly subjects. “Most studies that have looked at physical activity and dementia use a self-reported activity level, so the results have been varied,” Mr. Kirn said. “These findings support consideration of objectively measured physical activity in clinical research, and perhaps in stratification for risk of cognitive decline.”
He had no financial disclosures.
[email protected]
On Twitter @Alz_Gal
AT CTAD
Key clinical point:
Major finding: Subjects with high amyloid burden who walked the least declined by almost 1 point on the PACC score; high-amyloid subjects who walked the most stayed at their baseline score.
Data source: A prospective, observational study comprising 255 elderly subjects with normal cognition.
Disclosures: The presenter had no financial disclosures.
Intepirdine flops in phase 3 study of mild to moderate Alzheimer’s patients
BOSTON – An investigational Alzheimer’s drug intended to potentiate acetylcholine release didn’t pass muster in a global phase 3 study, despite a successful phase 2 trial.
Intepirdine on a background of stable-dose donepezil failed to confer any cognitive or functional benefit in patients with mild to moderate Alzheimer’s disease, Ilise Lombardo, PhD, said at the Clinical Trials on Alzheimer’s Disease conference.
Intepirdine blocks the 5-hydroxytryptamine receptor 6 and increases the release of acetylcholine, according to Dr. Lombardo. By giving it on a stable background of an acetylcholinesterase inhibitor, researchers hoped to improve cognition by increasing acetylcholine signaling between neurons. In a modestly successful phase 2b study reported out last summer, intepirdine did confer some cognitive and functional benefits.
The study was scheduled to be presented at the Alzheimer’s Association International Conference, but was pulled at the last minute when Axovant announced its initial public offering of stock shortly before the July meeting. However, Dr. Lombardo did review study 866 at CTAD. It randomized 269 patients with mild to moderate AD to placebo or intepirdine at 15 mg or 35 mg/day for 24 weeks. By 12 weeks, patients taking the 35-mg dose had declined 1.6 points less than those on placebo on the Alzheimer’s Disease Assessment Scale–cognitive subscale (ADAS-cog) and 1.94 points on the Alzheimer’s Disease Cooperative Study–Activities of Daily Living (ADCS-ADL). Both differences were statistically significant. The drug moved into a phase 3 study, dubbed MINDSET, at 35 mg.
MINDSET enrolled 1, 315 patients with mild to moderate Alzheimer’s disease and randomized them to placebo or 35 mg intepirdine for 24 weeks. All patients were on stable background donepezil. Again, the coprimary endpoints were the ADAS-cog and the ADCS-ADL at the end of the study. There were three secondary endpoints: the Clinician Interview-Based Impression of Change plus caregiver interview (CIBIC+), the Dependence Scale, and the Neuropsychiatric Inventory.
Patients were a mean of 70 years old with a mean Mini-Mental State Exam (MMSE) score of 18.5. The mean ADAS-cog score was 24 and the mean ADCS-ADL score, 58.
On the ADAS-cog, both groups exhibited a positive placebo response in the first 6 weeks, followed by a mean decline of 0.36 points. There was no statistically significant between-group difference. The story was much the same on the ADCS-ADL measure. Both groups had a brief placebo response, followed by a mean decline of 1.06 points. Again, the intepirdine and placebo groups declined similarly. There were no significant differences on either measure when the groups were broken down into patients with mild and moderate disease.
The CIBIC+ score was the only positive response among the secondary endpoints. Compared with those taking placebo, those taking intepirdine were more likely to be rated as minimally improved or unchanged.
The drug was safe however, with virtually no between-group difference in the occurrence of or the type of serious adverse events (about 6% in each group). Five patents died during the study; none of the deaths were related to the study drug.
Although Axovant no longer lists intepirdine as a potential Alzheimer’s treatment, investigation continues in a phase 3 placebo-controlled study in patients with Lewy body dementia. HEADWAY is testing a 70-mg dose, Dr. Lombardo said.
“Enrollment is finished and we are looking forward to results,” in early 2018. Answering a question about why the company went with 35 mg instead of 70 mg in MINDSET, she said that Axovant wanted to recreate the success of study 866.
“Since we had statistical significance even at 12 weeks with 35 mg, that’s what we went with,” she said. However, reviewing the adverse events told investigators that “we were not even near the maximum tolerated dose” at 35 mg. “Certainly we are now faced with the question of whether there will be a better response with 70 mg, and we are looking forward to answering that question with HEADWAY.”
Dr. Lombardo is senior vice president for clinical research at Axovant Sciences.
[email protected]
On Twitter @alz_gal
BOSTON – An investigational Alzheimer’s drug intended to potentiate acetylcholine release didn’t pass muster in a global phase 3 study, despite a successful phase 2 trial.
Intepirdine on a background of stable-dose donepezil failed to confer any cognitive or functional benefit in patients with mild to moderate Alzheimer’s disease, Ilise Lombardo, PhD, said at the Clinical Trials on Alzheimer’s Disease conference.
Intepirdine blocks the 5-hydroxytryptamine receptor 6 and increases the release of acetylcholine, according to Dr. Lombardo. By giving it on a stable background of an acetylcholinesterase inhibitor, researchers hoped to improve cognition by increasing acetylcholine signaling between neurons. In a modestly successful phase 2b study reported out last summer, intepirdine did confer some cognitive and functional benefits.
The study was scheduled to be presented at the Alzheimer’s Association International Conference, but was pulled at the last minute when Axovant announced its initial public offering of stock shortly before the July meeting. However, Dr. Lombardo did review study 866 at CTAD. It randomized 269 patients with mild to moderate AD to placebo or intepirdine at 15 mg or 35 mg/day for 24 weeks. By 12 weeks, patients taking the 35-mg dose had declined 1.6 points less than those on placebo on the Alzheimer’s Disease Assessment Scale–cognitive subscale (ADAS-cog) and 1.94 points on the Alzheimer’s Disease Cooperative Study–Activities of Daily Living (ADCS-ADL). Both differences were statistically significant. The drug moved into a phase 3 study, dubbed MINDSET, at 35 mg.
MINDSET enrolled 1, 315 patients with mild to moderate Alzheimer’s disease and randomized them to placebo or 35 mg intepirdine for 24 weeks. All patients were on stable background donepezil. Again, the coprimary endpoints were the ADAS-cog and the ADCS-ADL at the end of the study. There were three secondary endpoints: the Clinician Interview-Based Impression of Change plus caregiver interview (CIBIC+), the Dependence Scale, and the Neuropsychiatric Inventory.
Patients were a mean of 70 years old with a mean Mini-Mental State Exam (MMSE) score of 18.5. The mean ADAS-cog score was 24 and the mean ADCS-ADL score, 58.
On the ADAS-cog, both groups exhibited a positive placebo response in the first 6 weeks, followed by a mean decline of 0.36 points. There was no statistically significant between-group difference. The story was much the same on the ADCS-ADL measure. Both groups had a brief placebo response, followed by a mean decline of 1.06 points. Again, the intepirdine and placebo groups declined similarly. There were no significant differences on either measure when the groups were broken down into patients with mild and moderate disease.
The CIBIC+ score was the only positive response among the secondary endpoints. Compared with those taking placebo, those taking intepirdine were more likely to be rated as minimally improved or unchanged.
The drug was safe however, with virtually no between-group difference in the occurrence of or the type of serious adverse events (about 6% in each group). Five patents died during the study; none of the deaths were related to the study drug.
Although Axovant no longer lists intepirdine as a potential Alzheimer’s treatment, investigation continues in a phase 3 placebo-controlled study in patients with Lewy body dementia. HEADWAY is testing a 70-mg dose, Dr. Lombardo said.
“Enrollment is finished and we are looking forward to results,” in early 2018. Answering a question about why the company went with 35 mg instead of 70 mg in MINDSET, she said that Axovant wanted to recreate the success of study 866.
“Since we had statistical significance even at 12 weeks with 35 mg, that’s what we went with,” she said. However, reviewing the adverse events told investigators that “we were not even near the maximum tolerated dose” at 35 mg. “Certainly we are now faced with the question of whether there will be a better response with 70 mg, and we are looking forward to answering that question with HEADWAY.”
Dr. Lombardo is senior vice president for clinical research at Axovant Sciences.
[email protected]
On Twitter @alz_gal
BOSTON – An investigational Alzheimer’s drug intended to potentiate acetylcholine release didn’t pass muster in a global phase 3 study, despite a successful phase 2 trial.
Intepirdine on a background of stable-dose donepezil failed to confer any cognitive or functional benefit in patients with mild to moderate Alzheimer’s disease, Ilise Lombardo, PhD, said at the Clinical Trials on Alzheimer’s Disease conference.
Intepirdine blocks the 5-hydroxytryptamine receptor 6 and increases the release of acetylcholine, according to Dr. Lombardo. By giving it on a stable background of an acetylcholinesterase inhibitor, researchers hoped to improve cognition by increasing acetylcholine signaling between neurons. In a modestly successful phase 2b study reported out last summer, intepirdine did confer some cognitive and functional benefits.
The study was scheduled to be presented at the Alzheimer’s Association International Conference, but was pulled at the last minute when Axovant announced its initial public offering of stock shortly before the July meeting. However, Dr. Lombardo did review study 866 at CTAD. It randomized 269 patients with mild to moderate AD to placebo or intepirdine at 15 mg or 35 mg/day for 24 weeks. By 12 weeks, patients taking the 35-mg dose had declined 1.6 points less than those on placebo on the Alzheimer’s Disease Assessment Scale–cognitive subscale (ADAS-cog) and 1.94 points on the Alzheimer’s Disease Cooperative Study–Activities of Daily Living (ADCS-ADL). Both differences were statistically significant. The drug moved into a phase 3 study, dubbed MINDSET, at 35 mg.
MINDSET enrolled 1, 315 patients with mild to moderate Alzheimer’s disease and randomized them to placebo or 35 mg intepirdine for 24 weeks. All patients were on stable background donepezil. Again, the coprimary endpoints were the ADAS-cog and the ADCS-ADL at the end of the study. There were three secondary endpoints: the Clinician Interview-Based Impression of Change plus caregiver interview (CIBIC+), the Dependence Scale, and the Neuropsychiatric Inventory.
Patients were a mean of 70 years old with a mean Mini-Mental State Exam (MMSE) score of 18.5. The mean ADAS-cog score was 24 and the mean ADCS-ADL score, 58.
On the ADAS-cog, both groups exhibited a positive placebo response in the first 6 weeks, followed by a mean decline of 0.36 points. There was no statistically significant between-group difference. The story was much the same on the ADCS-ADL measure. Both groups had a brief placebo response, followed by a mean decline of 1.06 points. Again, the intepirdine and placebo groups declined similarly. There were no significant differences on either measure when the groups were broken down into patients with mild and moderate disease.
The CIBIC+ score was the only positive response among the secondary endpoints. Compared with those taking placebo, those taking intepirdine were more likely to be rated as minimally improved or unchanged.
The drug was safe however, with virtually no between-group difference in the occurrence of or the type of serious adverse events (about 6% in each group). Five patents died during the study; none of the deaths were related to the study drug.
Although Axovant no longer lists intepirdine as a potential Alzheimer’s treatment, investigation continues in a phase 3 placebo-controlled study in patients with Lewy body dementia. HEADWAY is testing a 70-mg dose, Dr. Lombardo said.
“Enrollment is finished and we are looking forward to results,” in early 2018. Answering a question about why the company went with 35 mg instead of 70 mg in MINDSET, she said that Axovant wanted to recreate the success of study 866.
“Since we had statistical significance even at 12 weeks with 35 mg, that’s what we went with,” she said. However, reviewing the adverse events told investigators that “we were not even near the maximum tolerated dose” at 35 mg. “Certainly we are now faced with the question of whether there will be a better response with 70 mg, and we are looking forward to answering that question with HEADWAY.”
Dr. Lombardo is senior vice president for clinical research at Axovant Sciences.
[email protected]
On Twitter @alz_gal
AT CTAD
Key clinical point:
Major finding: On the ADAS-cog at 24 weeks, there was a mean decline of 0.36 points; on the ADCS-ADL, the mean decline was 1.06 points. There were no between-group differences, either overall or in the mild to moderate groups separately.
Data source: The placebo-controlled study randomized 1,315 patients to placebo or 35 mg daily intepirdine.
Disclosures: Dr. Lombardo is senior vice president for clinical research at Axovant Sciences, which is developing the drug.
Development of a sigma 1 receptor agonist for Alzheimer’s proceeds based on 2-year phase 2 data
BOSTON – A reputedly neuroprotective compound will advance along its developmental pathway after developers said it exerted a blood level–dependent relationship with both cognitive and functional measures in patients with mild to moderate Alzheimer’s.
Most of the 26 patients left in the ongoing phase 2a extension study of ANAVEX 2-73 declined cognitively and functionally by varying degrees over 1 year. But a few, most of whom had high drug plasma levels, did experience cognitive and functional changes for the better. Some maintained stability, and some improved on both measures over 109 weeks, according to Christopher U. Missling, PhD, president and chief executive officer of Anavex.
“Improvement of cognition or function was a rare occurrence, but our analysis showed that patients who had a plasma concentration of 4-12 ng/mL had the best chance of experiencing this,” Dr. Missling said in an interview.
Mohammad Afshar, MD, PhD, presented these data at the Clinical Trials in Alzheimer’s Disease conference. He is the head of Ariana Pharmaceuticals, a firm hired to perform an independent analysis of the Anavex data. Concentrating on the pharmacodynamics data, he said ANAVEX 2-73 exhibits a clear drug concentration–clinical response relationship, which supports taking the drug into a phase 2/3 study.
“When focusing on the best responders at week 57, they continued to perform well. Patients with a score of greater than 20 on the Mini-Mental State Exam at baseline tended to respond better, as well as those with the highest plasma concentration,” he said.
The concentration-response picture is not completely clear, however. While five patients with high levels did improve on the MMSE at 57 weeks, one patient with a low plasma level also improved, and four patients with high levels declined. Functional results appeared more clearly related to drug levels: All of the high-level patients except one improved, as did about half of those with moderate plasma levels. Everyone with a low level declined.
During a later interview, Dr. Missling reviewed the data with an eye toward understatement. The study’s primary endpoints are safety and tolerability, as well as dosing and pharmacokinetics, he noted – cognitive and functional endpoints are exploratory measures. The study never had a control arm, other than several historical cohorts that served as reference points for decline in typically managed Alzheimer’s patients. And of course, he said, the numbers are very, very small.
And yet, the results are a source of “cautious optimism,” Dr. Missling said.
“While we think this is remarkable – because no drug has yet shown this long a response in non-decline among Alzheimer’s patients – we want to be very cautious. We are only looking at six patients here. We can’t overinterpret this.”
Dr. Missling and his colleagues are trying to find commonalities in these patients’ characteristics and clinical responses, hoping to enroll a phase 2/3 cohort of similar profile – whatever that may be. “We’re looking at the patients who improved to try and identify characteristics and be sure to enroll people who match them, to try and maximize our chance of success in phase 2/3,” which he said should be announced by the end of this year.
Just as important, he said, is to scrutinize the outliers – patients whose high and low plasma levels didn’t line up with the group’s overall response curve. “We’re looking at them in depth,” Dr. Missling said, adding that every patient in the study will undergo a full genomic profile, along with both an RNA and gut microbiota profile.
ANAVEX 2-73 is a sigma 1 receptor agonist. A chaperone protein, sigma 1 is activated in response to acute and chronic cellular stressors, several of which are important in neurodegeneration. The sigma 1 receptor is found on neurons and glia in many areas of the central nervous system. It modulates numerous processes implicated in neurodegenerative diseases, including glutamate and calcium activity, reaction to oxidative stress, and mitochondrial function. There is some evidence that sigma 1 receptor activation can induce neuronal regrowth and functional recovery after stroke. Sigma 1 also appears to play a role in helping cells clear misfolded proteins – a pathway that makes it an attractive drug target in Alzheimer’s disease, as well as other neurodegenerative diseases with aberrant proteins, such as Parkinson’s and Huntington’s diseases.
“Sigma 1 is never used except in times of trouble,” Dr. Missling said. “It’s only needed if we have a serious dysfunction in cells. By giving an agonist, we are increasing the expression of this protein, so it’s a bit like immune stimulation in oncology. We’re using the body’s own mechanism,” to fight neurodegeneration.
ANAVEX 2-73’s phase 2 development started with a 5-week crossover trial of 32 patients; they were a mean of 71 years old, with a mean MMSE of 21. The initial phase was followed by a 52-week, open-label trial of 10, 20, 30, and 50 mg/day orally, titrating each patient to the maximum tolerated dose.
Dr. Afshar presented 57-week data for the 26 patients who were still in the trial at that point and 109-week data for the six patients who had the best response at 57 weeks. Patients in both analyses were grouped by plasma level, not by their dosage level, although Dr. Missling said higher dosage generally correlated with higher plasma levels.
At 57 weeks, six patients had improved on the MMSE: four with high plasma levels and two with low plasma levels – patients identified as “outliers.” One patient with a high level remained stable. The rest of the cohort declined: seven with low levels, eight with moderate levels, and four with high levels, who were also identified as outliers. Also at 57 weeks, 24 patients had full data on the functional measure, the Alzheimer’s Disease Cooperative Study-Activities of Daily Living (ADCS-ADL). Nine patients had improved: five with high plasma levels, three with moderate levels, and one with a low level, identified as an outlier. One patient, with a moderate level, remained stable. The remaining 14 patients declined: nine with moderate levels, four with low levels, and one with a high level, dubbed an outlier.
Dr. Afshar reported more detailed data on the six best-performing patients. These included the two patients with low plasma levels who were characterized in the 57-week MMSE data as outliers. Their mean baseline MMSE went from 23.2 to 25.7 at 57 weeks; their mean functional score on the ADCS-ADL scale rose from 72 to 73.7.
He then showed each of these patients’ 109-week changes. Each was identified with a unique number in both the 57- and 109-week datasets. Numbers here are estimates drawn from the company’s data slides, which are publicly available on the Anavex website.
The ADCS-ADL is a caregiver-rated questionnaire of 23 items, with possible scores over a range of 0-78, where 78 implies full functioning with no impairment. On the 30-point MMSE scale, a score greater of 24 or higher indicates a normal cognition.
• 101009 (low plasma level)
MMSE: 20-29 ADCS-ADL: 73-71
• 101011 (low plasma level)
MMSE: 20-21 ADCS-ADL: 72-70
•101013 (high plasma level)
MMSE: 22-18 ADCS-ADL: 73-57
• 101014 (high plasma level)
MMSE: 20-25 ADCS-ADL: 75-74
• 102006 (high plasma level)
MMSE: 25-28 ADCS-ADL: 74-78
• 102010 (high plasma level)
MMSE: 25-28 ADCS-ADL: 64-68
Dr. Missling said ANAVEX 2-73 still appears safe and well tolerated. In the initial study phase, 98% of the subjects did have an adverse event; only one was considered serious. This was a case of delirium that developed in a patient who had several risk factors for the disorder, including a prior episode, and was not considered related to the study drug. Three patients withdrew because of an adverse event in the first 57 weeks; no one has withdrawn because of a side effect since then.
Dizziness was the most common adverse event (20 incidents in 15 patients), followed by headache (16 in 10 patients). Most (94%) occurred in the first week of treatment. All were mild or moderate. Headache and dizziness are considered signs that patients are approaching their maximum tolerated dose, Dr. Missling said.
The company’s task now is to find a standard minimum dose that is strong enough to get patients to at least 4 ng/mL plasma level, without inducing these side effects. The extension study will close out in November 2018. Anavex hopes to begin the drug’s next phase of development before then, with a phase 2/3 study of about 200 patients.
“We’re trying to gather as much data as possible so we can do this properly,” Dr. Missling said. “You can make the case that some developers have rushed into these studies after interpreting early results the wrong way. They said the glass was half-full when it was really half-empty. For us, it’s very important not to do that. We won’t go ahead with this until we are completely comfortable with what we see.”
[email protected]
On Twitter @alz_gal
BOSTON – A reputedly neuroprotective compound will advance along its developmental pathway after developers said it exerted a blood level–dependent relationship with both cognitive and functional measures in patients with mild to moderate Alzheimer’s.
Most of the 26 patients left in the ongoing phase 2a extension study of ANAVEX 2-73 declined cognitively and functionally by varying degrees over 1 year. But a few, most of whom had high drug plasma levels, did experience cognitive and functional changes for the better. Some maintained stability, and some improved on both measures over 109 weeks, according to Christopher U. Missling, PhD, president and chief executive officer of Anavex.
“Improvement of cognition or function was a rare occurrence, but our analysis showed that patients who had a plasma concentration of 4-12 ng/mL had the best chance of experiencing this,” Dr. Missling said in an interview.
Mohammad Afshar, MD, PhD, presented these data at the Clinical Trials in Alzheimer’s Disease conference. He is the head of Ariana Pharmaceuticals, a firm hired to perform an independent analysis of the Anavex data. Concentrating on the pharmacodynamics data, he said ANAVEX 2-73 exhibits a clear drug concentration–clinical response relationship, which supports taking the drug into a phase 2/3 study.
“When focusing on the best responders at week 57, they continued to perform well. Patients with a score of greater than 20 on the Mini-Mental State Exam at baseline tended to respond better, as well as those with the highest plasma concentration,” he said.
The concentration-response picture is not completely clear, however. While five patients with high levels did improve on the MMSE at 57 weeks, one patient with a low plasma level also improved, and four patients with high levels declined. Functional results appeared more clearly related to drug levels: All of the high-level patients except one improved, as did about half of those with moderate plasma levels. Everyone with a low level declined.
During a later interview, Dr. Missling reviewed the data with an eye toward understatement. The study’s primary endpoints are safety and tolerability, as well as dosing and pharmacokinetics, he noted – cognitive and functional endpoints are exploratory measures. The study never had a control arm, other than several historical cohorts that served as reference points for decline in typically managed Alzheimer’s patients. And of course, he said, the numbers are very, very small.
And yet, the results are a source of “cautious optimism,” Dr. Missling said.
“While we think this is remarkable – because no drug has yet shown this long a response in non-decline among Alzheimer’s patients – we want to be very cautious. We are only looking at six patients here. We can’t overinterpret this.”
Dr. Missling and his colleagues are trying to find commonalities in these patients’ characteristics and clinical responses, hoping to enroll a phase 2/3 cohort of similar profile – whatever that may be. “We’re looking at the patients who improved to try and identify characteristics and be sure to enroll people who match them, to try and maximize our chance of success in phase 2/3,” which he said should be announced by the end of this year.
Just as important, he said, is to scrutinize the outliers – patients whose high and low plasma levels didn’t line up with the group’s overall response curve. “We’re looking at them in depth,” Dr. Missling said, adding that every patient in the study will undergo a full genomic profile, along with both an RNA and gut microbiota profile.
ANAVEX 2-73 is a sigma 1 receptor agonist. A chaperone protein, sigma 1 is activated in response to acute and chronic cellular stressors, several of which are important in neurodegeneration. The sigma 1 receptor is found on neurons and glia in many areas of the central nervous system. It modulates numerous processes implicated in neurodegenerative diseases, including glutamate and calcium activity, reaction to oxidative stress, and mitochondrial function. There is some evidence that sigma 1 receptor activation can induce neuronal regrowth and functional recovery after stroke. Sigma 1 also appears to play a role in helping cells clear misfolded proteins – a pathway that makes it an attractive drug target in Alzheimer’s disease, as well as other neurodegenerative diseases with aberrant proteins, such as Parkinson’s and Huntington’s diseases.
“Sigma 1 is never used except in times of trouble,” Dr. Missling said. “It’s only needed if we have a serious dysfunction in cells. By giving an agonist, we are increasing the expression of this protein, so it’s a bit like immune stimulation in oncology. We’re using the body’s own mechanism,” to fight neurodegeneration.
ANAVEX 2-73’s phase 2 development started with a 5-week crossover trial of 32 patients; they were a mean of 71 years old, with a mean MMSE of 21. The initial phase was followed by a 52-week, open-label trial of 10, 20, 30, and 50 mg/day orally, titrating each patient to the maximum tolerated dose.
Dr. Afshar presented 57-week data for the 26 patients who were still in the trial at that point and 109-week data for the six patients who had the best response at 57 weeks. Patients in both analyses were grouped by plasma level, not by their dosage level, although Dr. Missling said higher dosage generally correlated with higher plasma levels.
At 57 weeks, six patients had improved on the MMSE: four with high plasma levels and two with low plasma levels – patients identified as “outliers.” One patient with a high level remained stable. The rest of the cohort declined: seven with low levels, eight with moderate levels, and four with high levels, who were also identified as outliers. Also at 57 weeks, 24 patients had full data on the functional measure, the Alzheimer’s Disease Cooperative Study-Activities of Daily Living (ADCS-ADL). Nine patients had improved: five with high plasma levels, three with moderate levels, and one with a low level, identified as an outlier. One patient, with a moderate level, remained stable. The remaining 14 patients declined: nine with moderate levels, four with low levels, and one with a high level, dubbed an outlier.
Dr. Afshar reported more detailed data on the six best-performing patients. These included the two patients with low plasma levels who were characterized in the 57-week MMSE data as outliers. Their mean baseline MMSE went from 23.2 to 25.7 at 57 weeks; their mean functional score on the ADCS-ADL scale rose from 72 to 73.7.
He then showed each of these patients’ 109-week changes. Each was identified with a unique number in both the 57- and 109-week datasets. Numbers here are estimates drawn from the company’s data slides, which are publicly available on the Anavex website.
The ADCS-ADL is a caregiver-rated questionnaire of 23 items, with possible scores over a range of 0-78, where 78 implies full functioning with no impairment. On the 30-point MMSE scale, a score greater of 24 or higher indicates a normal cognition.
• 101009 (low plasma level)
MMSE: 20-29 ADCS-ADL: 73-71
• 101011 (low plasma level)
MMSE: 20-21 ADCS-ADL: 72-70
•101013 (high plasma level)
MMSE: 22-18 ADCS-ADL: 73-57
• 101014 (high plasma level)
MMSE: 20-25 ADCS-ADL: 75-74
• 102006 (high plasma level)
MMSE: 25-28 ADCS-ADL: 74-78
• 102010 (high plasma level)
MMSE: 25-28 ADCS-ADL: 64-68
Dr. Missling said ANAVEX 2-73 still appears safe and well tolerated. In the initial study phase, 98% of the subjects did have an adverse event; only one was considered serious. This was a case of delirium that developed in a patient who had several risk factors for the disorder, including a prior episode, and was not considered related to the study drug. Three patients withdrew because of an adverse event in the first 57 weeks; no one has withdrawn because of a side effect since then.
Dizziness was the most common adverse event (20 incidents in 15 patients), followed by headache (16 in 10 patients). Most (94%) occurred in the first week of treatment. All were mild or moderate. Headache and dizziness are considered signs that patients are approaching their maximum tolerated dose, Dr. Missling said.
The company’s task now is to find a standard minimum dose that is strong enough to get patients to at least 4 ng/mL plasma level, without inducing these side effects. The extension study will close out in November 2018. Anavex hopes to begin the drug’s next phase of development before then, with a phase 2/3 study of about 200 patients.
“We’re trying to gather as much data as possible so we can do this properly,” Dr. Missling said. “You can make the case that some developers have rushed into these studies after interpreting early results the wrong way. They said the glass was half-full when it was really half-empty. For us, it’s very important not to do that. We won’t go ahead with this until we are completely comfortable with what we see.”
[email protected]
On Twitter @alz_gal
BOSTON – A reputedly neuroprotective compound will advance along its developmental pathway after developers said it exerted a blood level–dependent relationship with both cognitive and functional measures in patients with mild to moderate Alzheimer’s.
Most of the 26 patients left in the ongoing phase 2a extension study of ANAVEX 2-73 declined cognitively and functionally by varying degrees over 1 year. But a few, most of whom had high drug plasma levels, did experience cognitive and functional changes for the better. Some maintained stability, and some improved on both measures over 109 weeks, according to Christopher U. Missling, PhD, president and chief executive officer of Anavex.
“Improvement of cognition or function was a rare occurrence, but our analysis showed that patients who had a plasma concentration of 4-12 ng/mL had the best chance of experiencing this,” Dr. Missling said in an interview.
Mohammad Afshar, MD, PhD, presented these data at the Clinical Trials in Alzheimer’s Disease conference. He is the head of Ariana Pharmaceuticals, a firm hired to perform an independent analysis of the Anavex data. Concentrating on the pharmacodynamics data, he said ANAVEX 2-73 exhibits a clear drug concentration–clinical response relationship, which supports taking the drug into a phase 2/3 study.
“When focusing on the best responders at week 57, they continued to perform well. Patients with a score of greater than 20 on the Mini-Mental State Exam at baseline tended to respond better, as well as those with the highest plasma concentration,” he said.
The concentration-response picture is not completely clear, however. While five patients with high levels did improve on the MMSE at 57 weeks, one patient with a low plasma level also improved, and four patients with high levels declined. Functional results appeared more clearly related to drug levels: All of the high-level patients except one improved, as did about half of those with moderate plasma levels. Everyone with a low level declined.
During a later interview, Dr. Missling reviewed the data with an eye toward understatement. The study’s primary endpoints are safety and tolerability, as well as dosing and pharmacokinetics, he noted – cognitive and functional endpoints are exploratory measures. The study never had a control arm, other than several historical cohorts that served as reference points for decline in typically managed Alzheimer’s patients. And of course, he said, the numbers are very, very small.
And yet, the results are a source of “cautious optimism,” Dr. Missling said.
“While we think this is remarkable – because no drug has yet shown this long a response in non-decline among Alzheimer’s patients – we want to be very cautious. We are only looking at six patients here. We can’t overinterpret this.”
Dr. Missling and his colleagues are trying to find commonalities in these patients’ characteristics and clinical responses, hoping to enroll a phase 2/3 cohort of similar profile – whatever that may be. “We’re looking at the patients who improved to try and identify characteristics and be sure to enroll people who match them, to try and maximize our chance of success in phase 2/3,” which he said should be announced by the end of this year.
Just as important, he said, is to scrutinize the outliers – patients whose high and low plasma levels didn’t line up with the group’s overall response curve. “We’re looking at them in depth,” Dr. Missling said, adding that every patient in the study will undergo a full genomic profile, along with both an RNA and gut microbiota profile.
ANAVEX 2-73 is a sigma 1 receptor agonist. A chaperone protein, sigma 1 is activated in response to acute and chronic cellular stressors, several of which are important in neurodegeneration. The sigma 1 receptor is found on neurons and glia in many areas of the central nervous system. It modulates numerous processes implicated in neurodegenerative diseases, including glutamate and calcium activity, reaction to oxidative stress, and mitochondrial function. There is some evidence that sigma 1 receptor activation can induce neuronal regrowth and functional recovery after stroke. Sigma 1 also appears to play a role in helping cells clear misfolded proteins – a pathway that makes it an attractive drug target in Alzheimer’s disease, as well as other neurodegenerative diseases with aberrant proteins, such as Parkinson’s and Huntington’s diseases.
“Sigma 1 is never used except in times of trouble,” Dr. Missling said. “It’s only needed if we have a serious dysfunction in cells. By giving an agonist, we are increasing the expression of this protein, so it’s a bit like immune stimulation in oncology. We’re using the body’s own mechanism,” to fight neurodegeneration.
ANAVEX 2-73’s phase 2 development started with a 5-week crossover trial of 32 patients; they were a mean of 71 years old, with a mean MMSE of 21. The initial phase was followed by a 52-week, open-label trial of 10, 20, 30, and 50 mg/day orally, titrating each patient to the maximum tolerated dose.
Dr. Afshar presented 57-week data for the 26 patients who were still in the trial at that point and 109-week data for the six patients who had the best response at 57 weeks. Patients in both analyses were grouped by plasma level, not by their dosage level, although Dr. Missling said higher dosage generally correlated with higher plasma levels.
At 57 weeks, six patients had improved on the MMSE: four with high plasma levels and two with low plasma levels – patients identified as “outliers.” One patient with a high level remained stable. The rest of the cohort declined: seven with low levels, eight with moderate levels, and four with high levels, who were also identified as outliers. Also at 57 weeks, 24 patients had full data on the functional measure, the Alzheimer’s Disease Cooperative Study-Activities of Daily Living (ADCS-ADL). Nine patients had improved: five with high plasma levels, three with moderate levels, and one with a low level, identified as an outlier. One patient, with a moderate level, remained stable. The remaining 14 patients declined: nine with moderate levels, four with low levels, and one with a high level, dubbed an outlier.
Dr. Afshar reported more detailed data on the six best-performing patients. These included the two patients with low plasma levels who were characterized in the 57-week MMSE data as outliers. Their mean baseline MMSE went from 23.2 to 25.7 at 57 weeks; their mean functional score on the ADCS-ADL scale rose from 72 to 73.7.
He then showed each of these patients’ 109-week changes. Each was identified with a unique number in both the 57- and 109-week datasets. Numbers here are estimates drawn from the company’s data slides, which are publicly available on the Anavex website.
The ADCS-ADL is a caregiver-rated questionnaire of 23 items, with possible scores over a range of 0-78, where 78 implies full functioning with no impairment. On the 30-point MMSE scale, a score greater of 24 or higher indicates a normal cognition.
• 101009 (low plasma level)
MMSE: 20-29 ADCS-ADL: 73-71
• 101011 (low plasma level)
MMSE: 20-21 ADCS-ADL: 72-70
•101013 (high plasma level)
MMSE: 22-18 ADCS-ADL: 73-57
• 101014 (high plasma level)
MMSE: 20-25 ADCS-ADL: 75-74
• 102006 (high plasma level)
MMSE: 25-28 ADCS-ADL: 74-78
• 102010 (high plasma level)
MMSE: 25-28 ADCS-ADL: 64-68
Dr. Missling said ANAVEX 2-73 still appears safe and well tolerated. In the initial study phase, 98% of the subjects did have an adverse event; only one was considered serious. This was a case of delirium that developed in a patient who had several risk factors for the disorder, including a prior episode, and was not considered related to the study drug. Three patients withdrew because of an adverse event in the first 57 weeks; no one has withdrawn because of a side effect since then.
Dizziness was the most common adverse event (20 incidents in 15 patients), followed by headache (16 in 10 patients). Most (94%) occurred in the first week of treatment. All were mild or moderate. Headache and dizziness are considered signs that patients are approaching their maximum tolerated dose, Dr. Missling said.
The company’s task now is to find a standard minimum dose that is strong enough to get patients to at least 4 ng/mL plasma level, without inducing these side effects. The extension study will close out in November 2018. Anavex hopes to begin the drug’s next phase of development before then, with a phase 2/3 study of about 200 patients.
“We’re trying to gather as much data as possible so we can do this properly,” Dr. Missling said. “You can make the case that some developers have rushed into these studies after interpreting early results the wrong way. They said the glass was half-full when it was really half-empty. For us, it’s very important not to do that. We won’t go ahead with this until we are completely comfortable with what we see.”
[email protected]
On Twitter @alz_gal
AT CTAD
Key clinical point:
Major finding: Among the six best responders, the mean baseline MMSE went from 23.2 to 25.7; the mean functional score on the Alzheimer’s Disease Cooperative Study-activities of daily living scale rose from 72 to 73.7.
Data source: The phase 2a study is following 26 patients.
Disclosures: Dr. Christopher Missling is the chief executive officer of Anavex, which is developing 2-73. Dr. Mohammed Afshar is the CEO of Ariana Pharmaceuticals, which was hired to perform an independent data analysis of the study.
Long-term cholinesterase inhibition may slow cognitive decline – and more
BOSTON – Long-term use of cholinesterase inhibitors appears to confer a number of benefits, including protection from heart attack, stroke, diabetes-related mortality, and – according to a large new observational study – an annual 30% slowing of the cognitive decline associated with Alzheimer’s disease.
Long-term follow-up of thousands of patients in the Swedish Dementia Registry (SveDem) finds consistent, dose-dependent benefits associated with the drugs, Maria Eriksdotter, MD, said at the Clinical Trials on Alzheimer’s Disease conference. Most recently, her 3-year analysis of 29,000 patients showed that each year, those taking the drugs lost almost a point less on the Mini Mental State Exam (MMSE) than did nontreated patients.
“These drugs do reduce mortality and cardiovascular events, and improve cognitive decline,” she said in an interview. “It can be a difficult discussion to have with families, because yes, the patient is still declining, although more slowly. But combined with these other benefits that we are showing, I would say there is no reason not to get patients on a cholinesterase inhibitor as soon as possible. You want to get those benefits online as early as possible.”
The findings also strongly argue for cholinesterase inhibition to stay part of the standard treatment picture, even after disease-modifying medications do come on board.
“One could say you gain 30% of the decline back, and that 30% is something we could start from when we eventually begin talking about these therapies,” Dr. Eriksdotter said.
SveDem, launched in 2007, is a national project to improve the quality of diagnostics, treatment, and care of Swedish patients with dementia disorders. Patients newly diagnosed with a dementia disorder are registered and followed up yearly. Currently, it contains information on close to 70,000 patients.
The cognition study comprised almost 29,000 of those (CTAD abstract OC15). Dr. Eriksdotter and her colleagues categorized them as using or not using cholinesterase inhibitors (ChEI), and then examined the 3-year curves of cognitive decline, adjusting for age, sex, and a propensity score of whether or not they got a ChEI at baseline. The primary endpoint was MMSE decline by 3 years
At baseline, patients were a median of 80 years old. Alzheimer’s dementia was the most common diagnosis (63%), and 63% were taking a ChEI. Prescribing increased during each year of follow-up; by year 3, 91% were taking a ChEI. The median baseline MMSE was 22; this declined over time, to a median of 18. Patients taking the drugs were significantly younger than those not taking them (79 vs. 83 years), and less cognitively impaired (MMSE of 22 vs. 20). Most patients taking them had a diagnosis of Alzheimer’s dementia (70%), while others had a mix of vascular, frontotemporal, and Parkinson’s disease dementia.
Patients in both groups declined cognitively over the 3-year period, but the curves of decline were significantly different. The median MMSE decline regardless of treatment was 2.89 points. But in the fully adjusted model, those taking the drugs declined by about 0.85-point less each year than did those not taking them. This translated into an annual 30% reduction in cognitive decline, compared with untreated patients, Dr. Eriksdotter said.
She briefly discussed additional SveDem data supporting the drugs’ benefits in cardiovascular disorders and diabetes.
In 2013, she and her colleagues examined the link between ChEIs and heart attack in more than 7,000 SveDem registrants over a period of up to 5 years (Eur Heart J. 2013 Sep;34[33]:2585-91). After adjustment for confounding factors, the team found that ChEIs conferred a 34% risk reduction for a composite endpoint of myocardial infarction or death, compared with nonusers. The differences in the individual endpoints were also significant: a 36% lower risk of heart attack and 38% lower risk of death. She also found a dose-dependent response, with patients taking the highest recommended doses having the lowest risk of heart attack (hazard ratio, 0.35) and death (HR, 0.54).
Data from a similar study on stroke risk have been submitted for publication, Dr. Eriksdotter noted. This observational study comprised 22,300 patients followed for up to 5 years. Those who had ever taken a ChEI had a 33% decreased risk of ischemic stroke and 38% decreased risk of all-cause mortality. While the benefit was seen with the use of donepezil, rivastigmine, and galantamine, it was most pronounced with galantamine (30% decreased stroke risk, 32% decreased mortality risk). The study further determined that only patients taking high-dose ChEIs experienced the significant stroke benefit (HR, 0.59). The decreased risk of death was significant for all doses, but showed a dose-dependent benefit (low: HR, 0.85; medium: HR, 0.73; high: HR, 0.57).
Finally, Dr. Eriksdotter discussed a study in preparation comprising about 7,000 Alzheimer’s disease patients who also had diabetes. Of these, about 1,600 were taking a ChEI. In a fully adjusted model, the drugs were associated with a 16% decreased risk of death – exactly the same mortality benefit conferred by metformin. As an interesting side note, insulin use in this population was associated with a significant 15% increase in the risk of death, after full adjustment for confounding factors. None of the other antidiabetes medications (thiazolidinediones, dipeptidyl peptidase-4 inhibitors, or any newer drugs) showed any significant mortality benefit.
The protective mechanisms at work in these studies aren’t fully elucidated, Dr. Eriksdotter said. “But we do know that cholinesterase inhibitors exert an anti-inflammatory effect, and inflammation is definitely part of the cardiovascular disease and diabetes pictures. They also tend to slow heart rate, which has been found to have survival benefit in animal models.”
She had no financial disclosures.
[email protected]
On Twitter @Alz_Gal
The cholinesterase inhibitors are widely used for patients with Alzheimer’s disease, and to some extent other forms of cognitive impairment (rightly or wrongly). Their observed symptomatic benefits are modest and families frequently question whether the drugs are having any beneficial effect. So the idea that they may have long-term benefits is an extremely encouraging thought.
We do not have all the information that may be available at this time, and all or many of these issues undoubtedly occurred to the investigators who may have controlled for them, or been able to at least partially control for them, so that for now, I want to believe and am hoping the investigators are able to justify that belief with further data.
Richard J. Caselli, MD, is professor of neurology and medical director for service at the Mayo Clinic, Scottsdale, Ariz., and is associate director and clinical core director of the Arizona Alzheimer’s Disease Center. He has no relevant disclosures.
The cholinesterase inhibitors are widely used for patients with Alzheimer’s disease, and to some extent other forms of cognitive impairment (rightly or wrongly). Their observed symptomatic benefits are modest and families frequently question whether the drugs are having any beneficial effect. So the idea that they may have long-term benefits is an extremely encouraging thought.
We do not have all the information that may be available at this time, and all or many of these issues undoubtedly occurred to the investigators who may have controlled for them, or been able to at least partially control for them, so that for now, I want to believe and am hoping the investigators are able to justify that belief with further data.
Richard J. Caselli, MD, is professor of neurology and medical director for service at the Mayo Clinic, Scottsdale, Ariz., and is associate director and clinical core director of the Arizona Alzheimer’s Disease Center. He has no relevant disclosures.
The cholinesterase inhibitors are widely used for patients with Alzheimer’s disease, and to some extent other forms of cognitive impairment (rightly or wrongly). Their observed symptomatic benefits are modest and families frequently question whether the drugs are having any beneficial effect. So the idea that they may have long-term benefits is an extremely encouraging thought.
We do not have all the information that may be available at this time, and all or many of these issues undoubtedly occurred to the investigators who may have controlled for them, or been able to at least partially control for them, so that for now, I want to believe and am hoping the investigators are able to justify that belief with further data.
Richard J. Caselli, MD, is professor of neurology and medical director for service at the Mayo Clinic, Scottsdale, Ariz., and is associate director and clinical core director of the Arizona Alzheimer’s Disease Center. He has no relevant disclosures.
BOSTON – Long-term use of cholinesterase inhibitors appears to confer a number of benefits, including protection from heart attack, stroke, diabetes-related mortality, and – according to a large new observational study – an annual 30% slowing of the cognitive decline associated with Alzheimer’s disease.
Long-term follow-up of thousands of patients in the Swedish Dementia Registry (SveDem) finds consistent, dose-dependent benefits associated with the drugs, Maria Eriksdotter, MD, said at the Clinical Trials on Alzheimer’s Disease conference. Most recently, her 3-year analysis of 29,000 patients showed that each year, those taking the drugs lost almost a point less on the Mini Mental State Exam (MMSE) than did nontreated patients.
“These drugs do reduce mortality and cardiovascular events, and improve cognitive decline,” she said in an interview. “It can be a difficult discussion to have with families, because yes, the patient is still declining, although more slowly. But combined with these other benefits that we are showing, I would say there is no reason not to get patients on a cholinesterase inhibitor as soon as possible. You want to get those benefits online as early as possible.”
The findings also strongly argue for cholinesterase inhibition to stay part of the standard treatment picture, even after disease-modifying medications do come on board.
“One could say you gain 30% of the decline back, and that 30% is something we could start from when we eventually begin talking about these therapies,” Dr. Eriksdotter said.
SveDem, launched in 2007, is a national project to improve the quality of diagnostics, treatment, and care of Swedish patients with dementia disorders. Patients newly diagnosed with a dementia disorder are registered and followed up yearly. Currently, it contains information on close to 70,000 patients.
The cognition study comprised almost 29,000 of those (CTAD abstract OC15). Dr. Eriksdotter and her colleagues categorized them as using or not using cholinesterase inhibitors (ChEI), and then examined the 3-year curves of cognitive decline, adjusting for age, sex, and a propensity score of whether or not they got a ChEI at baseline. The primary endpoint was MMSE decline by 3 years
At baseline, patients were a median of 80 years old. Alzheimer’s dementia was the most common diagnosis (63%), and 63% were taking a ChEI. Prescribing increased during each year of follow-up; by year 3, 91% were taking a ChEI. The median baseline MMSE was 22; this declined over time, to a median of 18. Patients taking the drugs were significantly younger than those not taking them (79 vs. 83 years), and less cognitively impaired (MMSE of 22 vs. 20). Most patients taking them had a diagnosis of Alzheimer’s dementia (70%), while others had a mix of vascular, frontotemporal, and Parkinson’s disease dementia.
Patients in both groups declined cognitively over the 3-year period, but the curves of decline were significantly different. The median MMSE decline regardless of treatment was 2.89 points. But in the fully adjusted model, those taking the drugs declined by about 0.85-point less each year than did those not taking them. This translated into an annual 30% reduction in cognitive decline, compared with untreated patients, Dr. Eriksdotter said.
She briefly discussed additional SveDem data supporting the drugs’ benefits in cardiovascular disorders and diabetes.
In 2013, she and her colleagues examined the link between ChEIs and heart attack in more than 7,000 SveDem registrants over a period of up to 5 years (Eur Heart J. 2013 Sep;34[33]:2585-91). After adjustment for confounding factors, the team found that ChEIs conferred a 34% risk reduction for a composite endpoint of myocardial infarction or death, compared with nonusers. The differences in the individual endpoints were also significant: a 36% lower risk of heart attack and 38% lower risk of death. She also found a dose-dependent response, with patients taking the highest recommended doses having the lowest risk of heart attack (hazard ratio, 0.35) and death (HR, 0.54).
Data from a similar study on stroke risk have been submitted for publication, Dr. Eriksdotter noted. This observational study comprised 22,300 patients followed for up to 5 years. Those who had ever taken a ChEI had a 33% decreased risk of ischemic stroke and 38% decreased risk of all-cause mortality. While the benefit was seen with the use of donepezil, rivastigmine, and galantamine, it was most pronounced with galantamine (30% decreased stroke risk, 32% decreased mortality risk). The study further determined that only patients taking high-dose ChEIs experienced the significant stroke benefit (HR, 0.59). The decreased risk of death was significant for all doses, but showed a dose-dependent benefit (low: HR, 0.85; medium: HR, 0.73; high: HR, 0.57).
Finally, Dr. Eriksdotter discussed a study in preparation comprising about 7,000 Alzheimer’s disease patients who also had diabetes. Of these, about 1,600 were taking a ChEI. In a fully adjusted model, the drugs were associated with a 16% decreased risk of death – exactly the same mortality benefit conferred by metformin. As an interesting side note, insulin use in this population was associated with a significant 15% increase in the risk of death, after full adjustment for confounding factors. None of the other antidiabetes medications (thiazolidinediones, dipeptidyl peptidase-4 inhibitors, or any newer drugs) showed any significant mortality benefit.
The protective mechanisms at work in these studies aren’t fully elucidated, Dr. Eriksdotter said. “But we do know that cholinesterase inhibitors exert an anti-inflammatory effect, and inflammation is definitely part of the cardiovascular disease and diabetes pictures. They also tend to slow heart rate, which has been found to have survival benefit in animal models.”
She had no financial disclosures.
[email protected]
On Twitter @Alz_Gal
BOSTON – Long-term use of cholinesterase inhibitors appears to confer a number of benefits, including protection from heart attack, stroke, diabetes-related mortality, and – according to a large new observational study – an annual 30% slowing of the cognitive decline associated with Alzheimer’s disease.
Long-term follow-up of thousands of patients in the Swedish Dementia Registry (SveDem) finds consistent, dose-dependent benefits associated with the drugs, Maria Eriksdotter, MD, said at the Clinical Trials on Alzheimer’s Disease conference. Most recently, her 3-year analysis of 29,000 patients showed that each year, those taking the drugs lost almost a point less on the Mini Mental State Exam (MMSE) than did nontreated patients.
“These drugs do reduce mortality and cardiovascular events, and improve cognitive decline,” she said in an interview. “It can be a difficult discussion to have with families, because yes, the patient is still declining, although more slowly. But combined with these other benefits that we are showing, I would say there is no reason not to get patients on a cholinesterase inhibitor as soon as possible. You want to get those benefits online as early as possible.”
The findings also strongly argue for cholinesterase inhibition to stay part of the standard treatment picture, even after disease-modifying medications do come on board.
“One could say you gain 30% of the decline back, and that 30% is something we could start from when we eventually begin talking about these therapies,” Dr. Eriksdotter said.
SveDem, launched in 2007, is a national project to improve the quality of diagnostics, treatment, and care of Swedish patients with dementia disorders. Patients newly diagnosed with a dementia disorder are registered and followed up yearly. Currently, it contains information on close to 70,000 patients.
The cognition study comprised almost 29,000 of those (CTAD abstract OC15). Dr. Eriksdotter and her colleagues categorized them as using or not using cholinesterase inhibitors (ChEI), and then examined the 3-year curves of cognitive decline, adjusting for age, sex, and a propensity score of whether or not they got a ChEI at baseline. The primary endpoint was MMSE decline by 3 years
At baseline, patients were a median of 80 years old. Alzheimer’s dementia was the most common diagnosis (63%), and 63% were taking a ChEI. Prescribing increased during each year of follow-up; by year 3, 91% were taking a ChEI. The median baseline MMSE was 22; this declined over time, to a median of 18. Patients taking the drugs were significantly younger than those not taking them (79 vs. 83 years), and less cognitively impaired (MMSE of 22 vs. 20). Most patients taking them had a diagnosis of Alzheimer’s dementia (70%), while others had a mix of vascular, frontotemporal, and Parkinson’s disease dementia.
Patients in both groups declined cognitively over the 3-year period, but the curves of decline were significantly different. The median MMSE decline regardless of treatment was 2.89 points. But in the fully adjusted model, those taking the drugs declined by about 0.85-point less each year than did those not taking them. This translated into an annual 30% reduction in cognitive decline, compared with untreated patients, Dr. Eriksdotter said.
She briefly discussed additional SveDem data supporting the drugs’ benefits in cardiovascular disorders and diabetes.
In 2013, she and her colleagues examined the link between ChEIs and heart attack in more than 7,000 SveDem registrants over a period of up to 5 years (Eur Heart J. 2013 Sep;34[33]:2585-91). After adjustment for confounding factors, the team found that ChEIs conferred a 34% risk reduction for a composite endpoint of myocardial infarction or death, compared with nonusers. The differences in the individual endpoints were also significant: a 36% lower risk of heart attack and 38% lower risk of death. She also found a dose-dependent response, with patients taking the highest recommended doses having the lowest risk of heart attack (hazard ratio, 0.35) and death (HR, 0.54).
Data from a similar study on stroke risk have been submitted for publication, Dr. Eriksdotter noted. This observational study comprised 22,300 patients followed for up to 5 years. Those who had ever taken a ChEI had a 33% decreased risk of ischemic stroke and 38% decreased risk of all-cause mortality. While the benefit was seen with the use of donepezil, rivastigmine, and galantamine, it was most pronounced with galantamine (30% decreased stroke risk, 32% decreased mortality risk). The study further determined that only patients taking high-dose ChEIs experienced the significant stroke benefit (HR, 0.59). The decreased risk of death was significant for all doses, but showed a dose-dependent benefit (low: HR, 0.85; medium: HR, 0.73; high: HR, 0.57).
Finally, Dr. Eriksdotter discussed a study in preparation comprising about 7,000 Alzheimer’s disease patients who also had diabetes. Of these, about 1,600 were taking a ChEI. In a fully adjusted model, the drugs were associated with a 16% decreased risk of death – exactly the same mortality benefit conferred by metformin. As an interesting side note, insulin use in this population was associated with a significant 15% increase in the risk of death, after full adjustment for confounding factors. None of the other antidiabetes medications (thiazolidinediones, dipeptidyl peptidase-4 inhibitors, or any newer drugs) showed any significant mortality benefit.
The protective mechanisms at work in these studies aren’t fully elucidated, Dr. Eriksdotter said. “But we do know that cholinesterase inhibitors exert an anti-inflammatory effect, and inflammation is definitely part of the cardiovascular disease and diabetes pictures. They also tend to slow heart rate, which has been found to have survival benefit in animal models.”
She had no financial disclosures.
[email protected]
On Twitter @Alz_Gal
AT CTAD
Key clinical point:
Major finding: Patients taking the drugs lost almost 1 point less on the Mini Mental State Exam each year than did those who didn’t take them.
Data source: The 3-year observational study comprised almost 29,000 patients in the Swedish Dementia Registry.
Disclosures: Dr. Eriksdotter had no financial disclosures.
Pimavanserin found modestly effective in phase 2 Alzheimer’s psychosis study
BOSTON – Pimavanserin, an atypical antipsychotic approved for use in psychosis associated with Parkinson’s disease, was modestly effective in treating psychosis associated with Alzheimer’s dementia in a phase 2 study.
The study of 181 patients showed that pimavanserin (Nuplazid) was associated with a statistically significant 3.76-point improvement on the Neuropsychiatric Inventory–Nursing Home Version (NPI-NH) psychosis score, Clive Ballard, MD, reported at the Clinical Trials on Alzheimer’s Disease conference. But although pimavanserin was significantly more effective than placebo at 6 weeks, it lost its statistical edge by the trial’s end at 12 weeks, largely because the placebo group improved over the study period.
A key finding was that pimavanserin was more effective in a subset of patients with severe symptoms, reducing those by more than 4 points on the NPI scale, said Dr. Ballard, codirector of the Biomedical Research Unit for Dementia in the Institute for Psychiatry at King’s College London. “A 4-point change is the difference from having moderate symptoms daily to having them weekly. I think this is the most clinically relevant finding.”
The drug seemed to largely spare cognition, which is another notch in its clinical belt, said Richard J. Caselli, MD, professor of neurology at the Mayo Clinic, Scottsdale, Ariz.
“The relative preservation of cognition as seen in an absence of adverse cognitive effects is encouraging, and something pimavanserin may have over its antipsychotic rivals,” Dr. Caselli said in an interview. “The improved scores on the NPI seem modest as does the relative percentage of responders, defined as at least 30% improved NPI-NH score. But at least it is a positive result. One concern is that the company advises it may take 4-6 weeks to see an improvement, which is not the kind of timeline one has with acutely and severely agitated patients. So I suspect antipsychotic drugs, which work more quickly, are likely not going away.”
The 12-week, phase 2 study randomized 181 patients with advanced Alzheimer’s dementia to placebo or 40 mg pimavanserin. They were a mean of 86 years old. The mean baseline NPI-NH psychosis score was 9.8 and the mean Mini–Mental State Exam score was 10.
By 6 weeks, the psychosis score had improved significantly more in the pimavanserin group than in the placebo group (–3.76 vs. –1.93 points; P = .0451). The drug was more effective among patients with severe psychosis at baseline, defined as an NPI-NH psychosis score of at least 12. Among this group, the score improved by 4.43 points. The results were slightly, but not significantly, better in patients who had responded to prior antipsychotic medications and among those who were also taking a selective serotonin reuptake inhibitor. A responder analysis also favored treatment, with 90% of those taking pimavanserin experiencing at least a 30% improvement on the NPI-NH psychosis score, compared with 43% of those taking placebo.
At 12 weeks, however, the overall between-group difference was no longer statistically significant, because the placebo group continued to improve over the treatment period.
Safety and tolerability were important considerations in such an elderly and cognitively compromised group, Dr. Ballard noted. In this respect, pimavanserin performed relatively well. There were more serious adverse events in the treated group (16.7% vs. 11%). These included respiratory infections (5 vs. 2) and urinary tract infection (2 vs. 0). Falls and fractures were similar in both groups. There was one fall in the active group, with one laceration, one hip fracture, and one femoral neck fracture. In the placebo group, there was one fall, one upper limb fracture, one wrist fracture, and one vertebral fracture. Among treated patients, there was also one heart attack and one case of renal failure. Four patients in each group died during the study.
Psychiatric events were more common in the pimavanserin group, most notably agitation (21% vs. 14%). Other psychiatric adverse events included aggression (10% vs. 4%), anxiety (5.6% vs. 2.2%), and dementia-related behavioral symptoms (5.6% vs. 2%). The drug had no effect on Mini–Mental State Exam score.
Pimavanserin was associated with a mean change of 9.4 ms in the heart rate-corrected QT interval, and was more likely to induce a weight loss of 7% or more.
Dr. Ballard had no financial disclosures with regard to pimavanserin or Acadia Pharmaceuticals, which sponsored the trial.
[email protected]
On Twitter @Alz_Gal
BOSTON – Pimavanserin, an atypical antipsychotic approved for use in psychosis associated with Parkinson’s disease, was modestly effective in treating psychosis associated with Alzheimer’s dementia in a phase 2 study.
The study of 181 patients showed that pimavanserin (Nuplazid) was associated with a statistically significant 3.76-point improvement on the Neuropsychiatric Inventory–Nursing Home Version (NPI-NH) psychosis score, Clive Ballard, MD, reported at the Clinical Trials on Alzheimer’s Disease conference. But although pimavanserin was significantly more effective than placebo at 6 weeks, it lost its statistical edge by the trial’s end at 12 weeks, largely because the placebo group improved over the study period.
A key finding was that pimavanserin was more effective in a subset of patients with severe symptoms, reducing those by more than 4 points on the NPI scale, said Dr. Ballard, codirector of the Biomedical Research Unit for Dementia in the Institute for Psychiatry at King’s College London. “A 4-point change is the difference from having moderate symptoms daily to having them weekly. I think this is the most clinically relevant finding.”
The drug seemed to largely spare cognition, which is another notch in its clinical belt, said Richard J. Caselli, MD, professor of neurology at the Mayo Clinic, Scottsdale, Ariz.
“The relative preservation of cognition as seen in an absence of adverse cognitive effects is encouraging, and something pimavanserin may have over its antipsychotic rivals,” Dr. Caselli said in an interview. “The improved scores on the NPI seem modest as does the relative percentage of responders, defined as at least 30% improved NPI-NH score. But at least it is a positive result. One concern is that the company advises it may take 4-6 weeks to see an improvement, which is not the kind of timeline one has with acutely and severely agitated patients. So I suspect antipsychotic drugs, which work more quickly, are likely not going away.”
The 12-week, phase 2 study randomized 181 patients with advanced Alzheimer’s dementia to placebo or 40 mg pimavanserin. They were a mean of 86 years old. The mean baseline NPI-NH psychosis score was 9.8 and the mean Mini–Mental State Exam score was 10.
By 6 weeks, the psychosis score had improved significantly more in the pimavanserin group than in the placebo group (–3.76 vs. –1.93 points; P = .0451). The drug was more effective among patients with severe psychosis at baseline, defined as an NPI-NH psychosis score of at least 12. Among this group, the score improved by 4.43 points. The results were slightly, but not significantly, better in patients who had responded to prior antipsychotic medications and among those who were also taking a selective serotonin reuptake inhibitor. A responder analysis also favored treatment, with 90% of those taking pimavanserin experiencing at least a 30% improvement on the NPI-NH psychosis score, compared with 43% of those taking placebo.
At 12 weeks, however, the overall between-group difference was no longer statistically significant, because the placebo group continued to improve over the treatment period.
Safety and tolerability were important considerations in such an elderly and cognitively compromised group, Dr. Ballard noted. In this respect, pimavanserin performed relatively well. There were more serious adverse events in the treated group (16.7% vs. 11%). These included respiratory infections (5 vs. 2) and urinary tract infection (2 vs. 0). Falls and fractures were similar in both groups. There was one fall in the active group, with one laceration, one hip fracture, and one femoral neck fracture. In the placebo group, there was one fall, one upper limb fracture, one wrist fracture, and one vertebral fracture. Among treated patients, there was also one heart attack and one case of renal failure. Four patients in each group died during the study.
Psychiatric events were more common in the pimavanserin group, most notably agitation (21% vs. 14%). Other psychiatric adverse events included aggression (10% vs. 4%), anxiety (5.6% vs. 2.2%), and dementia-related behavioral symptoms (5.6% vs. 2%). The drug had no effect on Mini–Mental State Exam score.
Pimavanserin was associated with a mean change of 9.4 ms in the heart rate-corrected QT interval, and was more likely to induce a weight loss of 7% or more.
Dr. Ballard had no financial disclosures with regard to pimavanserin or Acadia Pharmaceuticals, which sponsored the trial.
[email protected]
On Twitter @Alz_Gal
BOSTON – Pimavanserin, an atypical antipsychotic approved for use in psychosis associated with Parkinson’s disease, was modestly effective in treating psychosis associated with Alzheimer’s dementia in a phase 2 study.
The study of 181 patients showed that pimavanserin (Nuplazid) was associated with a statistically significant 3.76-point improvement on the Neuropsychiatric Inventory–Nursing Home Version (NPI-NH) psychosis score, Clive Ballard, MD, reported at the Clinical Trials on Alzheimer’s Disease conference. But although pimavanserin was significantly more effective than placebo at 6 weeks, it lost its statistical edge by the trial’s end at 12 weeks, largely because the placebo group improved over the study period.
A key finding was that pimavanserin was more effective in a subset of patients with severe symptoms, reducing those by more than 4 points on the NPI scale, said Dr. Ballard, codirector of the Biomedical Research Unit for Dementia in the Institute for Psychiatry at King’s College London. “A 4-point change is the difference from having moderate symptoms daily to having them weekly. I think this is the most clinically relevant finding.”
The drug seemed to largely spare cognition, which is another notch in its clinical belt, said Richard J. Caselli, MD, professor of neurology at the Mayo Clinic, Scottsdale, Ariz.
“The relative preservation of cognition as seen in an absence of adverse cognitive effects is encouraging, and something pimavanserin may have over its antipsychotic rivals,” Dr. Caselli said in an interview. “The improved scores on the NPI seem modest as does the relative percentage of responders, defined as at least 30% improved NPI-NH score. But at least it is a positive result. One concern is that the company advises it may take 4-6 weeks to see an improvement, which is not the kind of timeline one has with acutely and severely agitated patients. So I suspect antipsychotic drugs, which work more quickly, are likely not going away.”
The 12-week, phase 2 study randomized 181 patients with advanced Alzheimer’s dementia to placebo or 40 mg pimavanserin. They were a mean of 86 years old. The mean baseline NPI-NH psychosis score was 9.8 and the mean Mini–Mental State Exam score was 10.
By 6 weeks, the psychosis score had improved significantly more in the pimavanserin group than in the placebo group (–3.76 vs. –1.93 points; P = .0451). The drug was more effective among patients with severe psychosis at baseline, defined as an NPI-NH psychosis score of at least 12. Among this group, the score improved by 4.43 points. The results were slightly, but not significantly, better in patients who had responded to prior antipsychotic medications and among those who were also taking a selective serotonin reuptake inhibitor. A responder analysis also favored treatment, with 90% of those taking pimavanserin experiencing at least a 30% improvement on the NPI-NH psychosis score, compared with 43% of those taking placebo.
At 12 weeks, however, the overall between-group difference was no longer statistically significant, because the placebo group continued to improve over the treatment period.
Safety and tolerability were important considerations in such an elderly and cognitively compromised group, Dr. Ballard noted. In this respect, pimavanserin performed relatively well. There were more serious adverse events in the treated group (16.7% vs. 11%). These included respiratory infections (5 vs. 2) and urinary tract infection (2 vs. 0). Falls and fractures were similar in both groups. There was one fall in the active group, with one laceration, one hip fracture, and one femoral neck fracture. In the placebo group, there was one fall, one upper limb fracture, one wrist fracture, and one vertebral fracture. Among treated patients, there was also one heart attack and one case of renal failure. Four patients in each group died during the study.
Psychiatric events were more common in the pimavanserin group, most notably agitation (21% vs. 14%). Other psychiatric adverse events included aggression (10% vs. 4%), anxiety (5.6% vs. 2.2%), and dementia-related behavioral symptoms (5.6% vs. 2%). The drug had no effect on Mini–Mental State Exam score.
Pimavanserin was associated with a mean change of 9.4 ms in the heart rate-corrected QT interval, and was more likely to induce a weight loss of 7% or more.
Dr. Ballard had no financial disclosures with regard to pimavanserin or Acadia Pharmaceuticals, which sponsored the trial.
[email protected]
On Twitter @Alz_Gal
AT CTAD
Key clinical point:
Major finding: The psychosis score at 6 weeks improved significantly more in patients taking pimavanserin than in those taking placebo (–3.76 vs. –1.93 points; P = .0451).
Data source: The randomized, placebo-controlled study enrolled 181 patients.
Disclosures: Acadia Pharmaceuticals makes pimavanserin and sponsored the trial. Dr. Ballard has no financial relationship with the company.
Unblinded data show extent of verubecestat’s failure in mild-moderate Alzheimer’s
BOSTON – The Alzheimer’s research community absorbed yet another downer recently, when Merck scientists revealed the unblinded efficacy and safety data of EPOCH, the company’s failed verubecestat trial: The BACE inhibitor didn’t score in any endpoint, no matter how the data were sliced and diced.
Merck halted the study last February, when an interim analysis determined there was no chance of a positive outcome. No safety data played into the decision, officials said. At the time of discontinuation, Merck had not yet examined the unblinded data, which were released to a packed audience at the Clinical Trials on Alzheimer’s Disease conference in Boston.
Compared with placebo, the nonselective beta-secretase (BACE) inhibitor conferred no cognitive or functional benefit upon patients with mild-moderate Alzheimer’s disease, either in the overall analysis or in any age, disease stage, or genetic subgroup, Michael Egan, MD, said during a panel discussion. And although there was plenty of biomarker evidence that the drug did block beta amyloid production, there was also a plethora of concerning adverse events.
However, the failure of yet another antiamyloid drug doesn’t mean that researchers should abandon amyloid as a therapeutic target, said Dr. Egan, Merck’s associate vice president of clinical neuroscience. Verubecestat is still being investigated in the APECS study of patients with mild cognitive impairment, and a number of antiamyloid antibodies are still going forward in patients whose disease stages run from preclinical to moderate.
“It’s still possible that we may see a clinical benefit in some of these studies, so we have to keep an open mind,” Dr. Egan said.
EPOCH, a pivotal phase 2/3 trial, randomized about 1,200 patients with mild-moderate Alzheimer’s to either placebo or verubecestat 12 mg or 40 mg daily for 18 months. None of the patients had amyloid PET imaging, but in subsets of patients who had the imaging or lumbar puncture for Abeta levels, 90% were amyloid-positive. The primary efficacy outcomes were the change from baseline in the Alzheimer’s Disease Assessment Scale Cognitive Subscale (ADAS-Cog) score and the change from baseline in the Alzheimer’s Disease Cooperative Study–Activities of Daily Living (ADCS-ADL) score.
There were a number of secondary endpoints, including the Clinical Dementia Rating–Sum of Boxes (CDR-sb); total hippocampal volume; cerebrospinal fluid total and phosphorylated tau; changes on the Neuropsychiatric Index and Mini Mental State Exam; and brain amyloid burden.
In a nutshell, Dr. Egan said, there was virtually no efficacy signal on any of the primary or secondary endpoints. On the ADAS-Cog, all patients, regardless of treatment group, lost 7-8 points over the trial. The same was true for the ADCS-ADL, with everyone declining about 8 points over time. On the CDR-sb, all groups declined about 2 points.
“We also looked at a number of subgroups: younger subjects, those with earlier disease, and those who we knew were amyloid-positive, including all of our ApoE4 carriers. We found no evidence of efficacy in any subgroup.”
It is “worth noting,” Dr. Egan said, that in the amyloid PET substudy, there were treatment-related reductions in plaque burden. While those taking placebo showed no changes in brain amyloid, the 12-mg group experienced a mean 2% reduction in amyloid, and the 40-mg group, a 4% reduction. “While this is modest, it does show target engagement,” Dr. Egan said – an important and positive finding in light of the ongoing APECS study.
The study of cerebrospinal fluid showed no effect on either tau protein, but marked, dose-related reductions in Abeta1-40 and soluble amyloid precursor protein – both products of BACE cleavage of the amyloid precursor protein. “We saw a 70% reduction in the 12-mg group and more than an 80% reduction in the 40-mg group, but no change in the placebo group. This is an important finding, demonstrating that the drug got into the brain and turned off production of Abeta. EPOCH is the first phase 3 study of an antiamyloid agent where target engagement of this magnitude has been demonstrated.”
Verubecestat also had its share of adverse events, Dr. Egan said. The most common was rash, which developed in about 10%; 20% of those who developed rash discontinued treatment for that reason. More concerning were falls and injuries; diarrhea and weight loss; and a variety of neuropsychiatric events, including insomnia and sleep disorders, anxiety, depression, and suicidal ideation.*
The drug was also associated with more loss of hippocampal volume, compared with placebo (5.7% vs. 5%), Dr. Egan said in an interview. The etiology isn’t clear; he suggested that it could be related to amyloid plaque removal, resolution of neuroinflammation, or an actual worsening of neurodegeneration. “That is a concerning possibility, although if that were the case we would expect to see worsening cognition, which we did not.”
Falls and injuries occurred in 15% of the placebo group and 20%-23% of the active groups. A detailed analysis didn’t turn up any specific risk factors, though. The episodes of suicidal ideation were passive and more common in the first 6 months of treatment and among patients who had a history of depression or prior suicidal ideation. Four patients discontinued due to that side effect.
Verubecestat is a nonselective inhibitor of both BACE1 and BACE2, and it’s not clear if that wide-ranging inhibition increased the likelihood of adverse events over what might be seen with a more selective compound. “It’s difficult to attribute them to BACE2 over BACE1,” Dr. Egan said. “Any BACE inhibitor could potentially have similar side effects.”
Only time will provide those answers; BACE inhibition is an area of active investigation among several large companies. The newly announced Generation studies will test a selective BACE1 inhibitor called CNP520.
Eli Lilly is recruiting for a phase 2 study of its BACE1 inhibitor, dubbed LY3202626. AstraZeneca is also looking at BACE1 inhibition with its candidate, lanabecestat.
Dr. Egan remains hopeful, though, and said that Merck retains its commitment to bringing an effective Alzheimer’s treatment to market.
“It’s natural to get discouraged with negative trials, and there certainly have been a lot of them. But I think we have to continue to work very hard to try and find something to help patients, and we have more and more knowledge every year about how to do that. I believe BACE inhibition continues to offer the possibility that if we treat earlier that there could be benefit, but for those with dementia, BACE inhibition is just too late.”
Dr. Egan is employed by Merck Sharp & Dohme, which sponsored EPOCH.
This article was updated 11/16/17.
Correction, 11/20/17: An earlier version of this article misstated the percentage of patients who experienced rash.
[email protected]
On Twitter @Alz_Gal
BOSTON – The Alzheimer’s research community absorbed yet another downer recently, when Merck scientists revealed the unblinded efficacy and safety data of EPOCH, the company’s failed verubecestat trial: The BACE inhibitor didn’t score in any endpoint, no matter how the data were sliced and diced.
Merck halted the study last February, when an interim analysis determined there was no chance of a positive outcome. No safety data played into the decision, officials said. At the time of discontinuation, Merck had not yet examined the unblinded data, which were released to a packed audience at the Clinical Trials on Alzheimer’s Disease conference in Boston.
Compared with placebo, the nonselective beta-secretase (BACE) inhibitor conferred no cognitive or functional benefit upon patients with mild-moderate Alzheimer’s disease, either in the overall analysis or in any age, disease stage, or genetic subgroup, Michael Egan, MD, said during a panel discussion. And although there was plenty of biomarker evidence that the drug did block beta amyloid production, there was also a plethora of concerning adverse events.
However, the failure of yet another antiamyloid drug doesn’t mean that researchers should abandon amyloid as a therapeutic target, said Dr. Egan, Merck’s associate vice president of clinical neuroscience. Verubecestat is still being investigated in the APECS study of patients with mild cognitive impairment, and a number of antiamyloid antibodies are still going forward in patients whose disease stages run from preclinical to moderate.
“It’s still possible that we may see a clinical benefit in some of these studies, so we have to keep an open mind,” Dr. Egan said.
EPOCH, a pivotal phase 2/3 trial, randomized about 1,200 patients with mild-moderate Alzheimer’s to either placebo or verubecestat 12 mg or 40 mg daily for 18 months. None of the patients had amyloid PET imaging, but in subsets of patients who had the imaging or lumbar puncture for Abeta levels, 90% were amyloid-positive. The primary efficacy outcomes were the change from baseline in the Alzheimer’s Disease Assessment Scale Cognitive Subscale (ADAS-Cog) score and the change from baseline in the Alzheimer’s Disease Cooperative Study–Activities of Daily Living (ADCS-ADL) score.
There were a number of secondary endpoints, including the Clinical Dementia Rating–Sum of Boxes (CDR-sb); total hippocampal volume; cerebrospinal fluid total and phosphorylated tau; changes on the Neuropsychiatric Index and Mini Mental State Exam; and brain amyloid burden.
In a nutshell, Dr. Egan said, there was virtually no efficacy signal on any of the primary or secondary endpoints. On the ADAS-Cog, all patients, regardless of treatment group, lost 7-8 points over the trial. The same was true for the ADCS-ADL, with everyone declining about 8 points over time. On the CDR-sb, all groups declined about 2 points.
“We also looked at a number of subgroups: younger subjects, those with earlier disease, and those who we knew were amyloid-positive, including all of our ApoE4 carriers. We found no evidence of efficacy in any subgroup.”
It is “worth noting,” Dr. Egan said, that in the amyloid PET substudy, there were treatment-related reductions in plaque burden. While those taking placebo showed no changes in brain amyloid, the 12-mg group experienced a mean 2% reduction in amyloid, and the 40-mg group, a 4% reduction. “While this is modest, it does show target engagement,” Dr. Egan said – an important and positive finding in light of the ongoing APECS study.
The study of cerebrospinal fluid showed no effect on either tau protein, but marked, dose-related reductions in Abeta1-40 and soluble amyloid precursor protein – both products of BACE cleavage of the amyloid precursor protein. “We saw a 70% reduction in the 12-mg group and more than an 80% reduction in the 40-mg group, but no change in the placebo group. This is an important finding, demonstrating that the drug got into the brain and turned off production of Abeta. EPOCH is the first phase 3 study of an antiamyloid agent where target engagement of this magnitude has been demonstrated.”
Verubecestat also had its share of adverse events, Dr. Egan said. The most common was rash, which developed in about 10%; 20% of those who developed rash discontinued treatment for that reason. More concerning were falls and injuries; diarrhea and weight loss; and a variety of neuropsychiatric events, including insomnia and sleep disorders, anxiety, depression, and suicidal ideation.*
The drug was also associated with more loss of hippocampal volume, compared with placebo (5.7% vs. 5%), Dr. Egan said in an interview. The etiology isn’t clear; he suggested that it could be related to amyloid plaque removal, resolution of neuroinflammation, or an actual worsening of neurodegeneration. “That is a concerning possibility, although if that were the case we would expect to see worsening cognition, which we did not.”
Falls and injuries occurred in 15% of the placebo group and 20%-23% of the active groups. A detailed analysis didn’t turn up any specific risk factors, though. The episodes of suicidal ideation were passive and more common in the first 6 months of treatment and among patients who had a history of depression or prior suicidal ideation. Four patients discontinued due to that side effect.
Verubecestat is a nonselective inhibitor of both BACE1 and BACE2, and it’s not clear if that wide-ranging inhibition increased the likelihood of adverse events over what might be seen with a more selective compound. “It’s difficult to attribute them to BACE2 over BACE1,” Dr. Egan said. “Any BACE inhibitor could potentially have similar side effects.”
Only time will provide those answers; BACE inhibition is an area of active investigation among several large companies. The newly announced Generation studies will test a selective BACE1 inhibitor called CNP520.
Eli Lilly is recruiting for a phase 2 study of its BACE1 inhibitor, dubbed LY3202626. AstraZeneca is also looking at BACE1 inhibition with its candidate, lanabecestat.
Dr. Egan remains hopeful, though, and said that Merck retains its commitment to bringing an effective Alzheimer’s treatment to market.
“It’s natural to get discouraged with negative trials, and there certainly have been a lot of them. But I think we have to continue to work very hard to try and find something to help patients, and we have more and more knowledge every year about how to do that. I believe BACE inhibition continues to offer the possibility that if we treat earlier that there could be benefit, but for those with dementia, BACE inhibition is just too late.”
Dr. Egan is employed by Merck Sharp & Dohme, which sponsored EPOCH.
This article was updated 11/16/17.
Correction, 11/20/17: An earlier version of this article misstated the percentage of patients who experienced rash.
[email protected]
On Twitter @Alz_Gal
BOSTON – The Alzheimer’s research community absorbed yet another downer recently, when Merck scientists revealed the unblinded efficacy and safety data of EPOCH, the company’s failed verubecestat trial: The BACE inhibitor didn’t score in any endpoint, no matter how the data were sliced and diced.
Merck halted the study last February, when an interim analysis determined there was no chance of a positive outcome. No safety data played into the decision, officials said. At the time of discontinuation, Merck had not yet examined the unblinded data, which were released to a packed audience at the Clinical Trials on Alzheimer’s Disease conference in Boston.
Compared with placebo, the nonselective beta-secretase (BACE) inhibitor conferred no cognitive or functional benefit upon patients with mild-moderate Alzheimer’s disease, either in the overall analysis or in any age, disease stage, or genetic subgroup, Michael Egan, MD, said during a panel discussion. And although there was plenty of biomarker evidence that the drug did block beta amyloid production, there was also a plethora of concerning adverse events.
However, the failure of yet another antiamyloid drug doesn’t mean that researchers should abandon amyloid as a therapeutic target, said Dr. Egan, Merck’s associate vice president of clinical neuroscience. Verubecestat is still being investigated in the APECS study of patients with mild cognitive impairment, and a number of antiamyloid antibodies are still going forward in patients whose disease stages run from preclinical to moderate.
“It’s still possible that we may see a clinical benefit in some of these studies, so we have to keep an open mind,” Dr. Egan said.
EPOCH, a pivotal phase 2/3 trial, randomized about 1,200 patients with mild-moderate Alzheimer’s to either placebo or verubecestat 12 mg or 40 mg daily for 18 months. None of the patients had amyloid PET imaging, but in subsets of patients who had the imaging or lumbar puncture for Abeta levels, 90% were amyloid-positive. The primary efficacy outcomes were the change from baseline in the Alzheimer’s Disease Assessment Scale Cognitive Subscale (ADAS-Cog) score and the change from baseline in the Alzheimer’s Disease Cooperative Study–Activities of Daily Living (ADCS-ADL) score.
There were a number of secondary endpoints, including the Clinical Dementia Rating–Sum of Boxes (CDR-sb); total hippocampal volume; cerebrospinal fluid total and phosphorylated tau; changes on the Neuropsychiatric Index and Mini Mental State Exam; and brain amyloid burden.
In a nutshell, Dr. Egan said, there was virtually no efficacy signal on any of the primary or secondary endpoints. On the ADAS-Cog, all patients, regardless of treatment group, lost 7-8 points over the trial. The same was true for the ADCS-ADL, with everyone declining about 8 points over time. On the CDR-sb, all groups declined about 2 points.
“We also looked at a number of subgroups: younger subjects, those with earlier disease, and those who we knew were amyloid-positive, including all of our ApoE4 carriers. We found no evidence of efficacy in any subgroup.”
It is “worth noting,” Dr. Egan said, that in the amyloid PET substudy, there were treatment-related reductions in plaque burden. While those taking placebo showed no changes in brain amyloid, the 12-mg group experienced a mean 2% reduction in amyloid, and the 40-mg group, a 4% reduction. “While this is modest, it does show target engagement,” Dr. Egan said – an important and positive finding in light of the ongoing APECS study.
The study of cerebrospinal fluid showed no effect on either tau protein, but marked, dose-related reductions in Abeta1-40 and soluble amyloid precursor protein – both products of BACE cleavage of the amyloid precursor protein. “We saw a 70% reduction in the 12-mg group and more than an 80% reduction in the 40-mg group, but no change in the placebo group. This is an important finding, demonstrating that the drug got into the brain and turned off production of Abeta. EPOCH is the first phase 3 study of an antiamyloid agent where target engagement of this magnitude has been demonstrated.”
Verubecestat also had its share of adverse events, Dr. Egan said. The most common was rash, which developed in about 10%; 20% of those who developed rash discontinued treatment for that reason. More concerning were falls and injuries; diarrhea and weight loss; and a variety of neuropsychiatric events, including insomnia and sleep disorders, anxiety, depression, and suicidal ideation.*
The drug was also associated with more loss of hippocampal volume, compared with placebo (5.7% vs. 5%), Dr. Egan said in an interview. The etiology isn’t clear; he suggested that it could be related to amyloid plaque removal, resolution of neuroinflammation, or an actual worsening of neurodegeneration. “That is a concerning possibility, although if that were the case we would expect to see worsening cognition, which we did not.”
Falls and injuries occurred in 15% of the placebo group and 20%-23% of the active groups. A detailed analysis didn’t turn up any specific risk factors, though. The episodes of suicidal ideation were passive and more common in the first 6 months of treatment and among patients who had a history of depression or prior suicidal ideation. Four patients discontinued due to that side effect.
Verubecestat is a nonselective inhibitor of both BACE1 and BACE2, and it’s not clear if that wide-ranging inhibition increased the likelihood of adverse events over what might be seen with a more selective compound. “It’s difficult to attribute them to BACE2 over BACE1,” Dr. Egan said. “Any BACE inhibitor could potentially have similar side effects.”
Only time will provide those answers; BACE inhibition is an area of active investigation among several large companies. The newly announced Generation studies will test a selective BACE1 inhibitor called CNP520.
Eli Lilly is recruiting for a phase 2 study of its BACE1 inhibitor, dubbed LY3202626. AstraZeneca is also looking at BACE1 inhibition with its candidate, lanabecestat.
Dr. Egan remains hopeful, though, and said that Merck retains its commitment to bringing an effective Alzheimer’s treatment to market.
“It’s natural to get discouraged with negative trials, and there certainly have been a lot of them. But I think we have to continue to work very hard to try and find something to help patients, and we have more and more knowledge every year about how to do that. I believe BACE inhibition continues to offer the possibility that if we treat earlier that there could be benefit, but for those with dementia, BACE inhibition is just too late.”
Dr. Egan is employed by Merck Sharp & Dohme, which sponsored EPOCH.
This article was updated 11/16/17.
Correction, 11/20/17: An earlier version of this article misstated the percentage of patients who experienced rash.
[email protected]
On Twitter @Alz_Gal
AT CTAD
Key clinical point:
Major finding: On the ADAS-Cog score, all patients, regardless of treatment group, lost 7-8 points over the trial. The same was true for the ADCS-ADL score, with everyone declining about 8 points over time. On the CDR-sb, all groups declined about 2 points.
Data source: EPOCH, a pivotal phase 2/3 trial, randomized about 1,200 patients with mild-moderate Alzheimer’s to either placebo or verubecestat 12 mg or 40 mg daily for 18 months.
Disclosures: Dr. Egan is employed by Merck Sharp & Dohme, which sponsored EPOCH.
Amyloid imaging changed management for 80% of patients with uncertain dementia diagnosis
BOSTON – Amyloid imaging with the PET agent florbetaben changed the clinical management of 80% of dementia patients with a complicated or uncertain presentation, Mathieu Ceccaldi, MD, reported at the Clinical Trials on Alzheimer’s Disease conference.
The French real-world study also found that 51% of the patients had a medication change after their amyloid imaging study, said Dr. Ceccaldi, a neurologist at the Timone Hospital in Marseille, France.
“In daily practice, we are sometimes faced with complex presentations and diagnostic uncertainty in patients who have early onset dementia, atypical nonamnestic dementia, unusual behavioral symptoms, or an unexpected rate of progression,” he said. “Amyloid imaging can help resolve those issues.”
The results echo – and even exceed – those returned in an interim analysis of the large U.S.-based Imaging Dementia–Evidence for Amyloid Scanning study. The IDEAS study is examining how amyloid imaging with the PET agent florbetapir may change clinical management of dementia patients. According to data presented at last summer’s Alzheimer’s Association International Conference, knowledge of patients’ brain amyloid status changed clinical management in 68% of cases.
In France, as in the United States, amyloid imaging is not routinely available outside of clinical research programs. French patients normally undergo a lumbar puncture (LP) to obtain amyloid biomarkers. However, if an LP isn’t feasible because of the patient’s clinical condition, it’s attempted and fails, the patient refuses, or the results are unclear, then imaging may be employed.
Dr. Ceccaldi’s study comprised 205 such patients seen in any of 18 memory clinics for dementia of uncertain etiology. The study evaluated how often amyloid PET imaging with florbetaben changed the patient’s diagnosis or management and how often it contributed to improving diagnostic uncertainty.
The patients were a mean of 71 years old, with a mean Mini-Mental State Exam score of 22. An LP had been performed in 42%, but those results were either ambiguous or inconsistent with the patient’s clinical presentation. Other patients (37%) refused the procedure, and the rest had a medical contraindication to LP or had a failed procedure.
The most common diagnosis at baseline was Alzheimer’s dementia (72%), which included typical and atypical sporadic AD, young-onset AD, and rapidly progressing AD. In 16% of the cohort, the diagnosis was non-Alzheimer’s dementia, including frontotemporal dementia, primary progressive aphasia, Lewy body dementia, corticobasal degeneration, semantic dementia, and Parkinson’s disease dementia. The diagnosis was mixed dementia in 8% and nonneurodegenerative dementia in the remainder – a catchall that included vascular dementia, psychiatric disorders, and other forms of dementia.
Imaging determined that 73 patients were amyloid negative and that 132 patients were amyloid positive. These results changed diagnosis in 67% of cases overall, in 58% of amyloid-positive patients, and in 84% of amyloid-negative patients.
In the absence of cerebrospinal fluid data, florbetaben imaging significantly improved diagnostic confidence in 81% of the entire cohort (167 patients). Before imaging, clinicians rated fewer than 5% of their diagnoses as very highly confident; this rose to nearly 100% after imaging. Similarly, before imaging, about 40% of clinicians said they had weak confidence in their initial diagnoses; this dropped to fewer than 5% after imaging.
Of the 67% with a changed diagnosis (137 patients), 76 were amyloid positive, and 61 were amyloid negative. Positive scans reclassified 18 patients as having AD; negative scans removed an AD diagnosis for 38 patients.
AD was the most commonly altered diagnosis, dropping from 72% to 62% of the entire cohort. The proportion of those with non-AD dementia increased from 16% before imaging to 18% after. Mixed dementias decreased from 8% to 5%, and the number diagnosed with nonneurodegenerative dementias increased from 5% to 17%.
In particular, Dr. Ceccaldi noted, the number of patients with potentially treatable nonneurodegenerative dementia rose from 10 to 35. These included revised diagnoses for those with psychiatric disorders (from 3 patients before imaging to 11 patients after), vascular dementias (from 2 to 8 patients), and other treatable causes of dementia (from 5 to 16 patients).
All of these altered diagnoses changed management in 80% of both positive and negative patients. Among these changes were the addition of a new medication (50% of amyloid-positive patients, 18% of amyloid-negative patients), the withdrawal of a medication (15% of amyloid-negative cases), additional testing (5% of amyloid-positive cases, 20% of amyloid-negative cases), and referral to another specialist (5% of amyloid-positive patients, 20% of amyloid-negative patients).
“Our results highlight the significant utility of amyloid PET for patients with complex dementia presentations in the context of the existing work-up,” Dr. Ceccaldi said.
He reported financial relationships with a number of pharmaceutical companies, including Piramal, which developed and manufactures florbetaben.
[email protected]
On Twitter @alz_gal
BOSTON – Amyloid imaging with the PET agent florbetaben changed the clinical management of 80% of dementia patients with a complicated or uncertain presentation, Mathieu Ceccaldi, MD, reported at the Clinical Trials on Alzheimer’s Disease conference.
The French real-world study also found that 51% of the patients had a medication change after their amyloid imaging study, said Dr. Ceccaldi, a neurologist at the Timone Hospital in Marseille, France.
“In daily practice, we are sometimes faced with complex presentations and diagnostic uncertainty in patients who have early onset dementia, atypical nonamnestic dementia, unusual behavioral symptoms, or an unexpected rate of progression,” he said. “Amyloid imaging can help resolve those issues.”
The results echo – and even exceed – those returned in an interim analysis of the large U.S.-based Imaging Dementia–Evidence for Amyloid Scanning study. The IDEAS study is examining how amyloid imaging with the PET agent florbetapir may change clinical management of dementia patients. According to data presented at last summer’s Alzheimer’s Association International Conference, knowledge of patients’ brain amyloid status changed clinical management in 68% of cases.
In France, as in the United States, amyloid imaging is not routinely available outside of clinical research programs. French patients normally undergo a lumbar puncture (LP) to obtain amyloid biomarkers. However, if an LP isn’t feasible because of the patient’s clinical condition, it’s attempted and fails, the patient refuses, or the results are unclear, then imaging may be employed.
Dr. Ceccaldi’s study comprised 205 such patients seen in any of 18 memory clinics for dementia of uncertain etiology. The study evaluated how often amyloid PET imaging with florbetaben changed the patient’s diagnosis or management and how often it contributed to improving diagnostic uncertainty.
The patients were a mean of 71 years old, with a mean Mini-Mental State Exam score of 22. An LP had been performed in 42%, but those results were either ambiguous or inconsistent with the patient’s clinical presentation. Other patients (37%) refused the procedure, and the rest had a medical contraindication to LP or had a failed procedure.
The most common diagnosis at baseline was Alzheimer’s dementia (72%), which included typical and atypical sporadic AD, young-onset AD, and rapidly progressing AD. In 16% of the cohort, the diagnosis was non-Alzheimer’s dementia, including frontotemporal dementia, primary progressive aphasia, Lewy body dementia, corticobasal degeneration, semantic dementia, and Parkinson’s disease dementia. The diagnosis was mixed dementia in 8% and nonneurodegenerative dementia in the remainder – a catchall that included vascular dementia, psychiatric disorders, and other forms of dementia.
Imaging determined that 73 patients were amyloid negative and that 132 patients were amyloid positive. These results changed diagnosis in 67% of cases overall, in 58% of amyloid-positive patients, and in 84% of amyloid-negative patients.
In the absence of cerebrospinal fluid data, florbetaben imaging significantly improved diagnostic confidence in 81% of the entire cohort (167 patients). Before imaging, clinicians rated fewer than 5% of their diagnoses as very highly confident; this rose to nearly 100% after imaging. Similarly, before imaging, about 40% of clinicians said they had weak confidence in their initial diagnoses; this dropped to fewer than 5% after imaging.
Of the 67% with a changed diagnosis (137 patients), 76 were amyloid positive, and 61 were amyloid negative. Positive scans reclassified 18 patients as having AD; negative scans removed an AD diagnosis for 38 patients.
AD was the most commonly altered diagnosis, dropping from 72% to 62% of the entire cohort. The proportion of those with non-AD dementia increased from 16% before imaging to 18% after. Mixed dementias decreased from 8% to 5%, and the number diagnosed with nonneurodegenerative dementias increased from 5% to 17%.
In particular, Dr. Ceccaldi noted, the number of patients with potentially treatable nonneurodegenerative dementia rose from 10 to 35. These included revised diagnoses for those with psychiatric disorders (from 3 patients before imaging to 11 patients after), vascular dementias (from 2 to 8 patients), and other treatable causes of dementia (from 5 to 16 patients).
All of these altered diagnoses changed management in 80% of both positive and negative patients. Among these changes were the addition of a new medication (50% of amyloid-positive patients, 18% of amyloid-negative patients), the withdrawal of a medication (15% of amyloid-negative cases), additional testing (5% of amyloid-positive cases, 20% of amyloid-negative cases), and referral to another specialist (5% of amyloid-positive patients, 20% of amyloid-negative patients).
“Our results highlight the significant utility of amyloid PET for patients with complex dementia presentations in the context of the existing work-up,” Dr. Ceccaldi said.
He reported financial relationships with a number of pharmaceutical companies, including Piramal, which developed and manufactures florbetaben.
[email protected]
On Twitter @alz_gal
BOSTON – Amyloid imaging with the PET agent florbetaben changed the clinical management of 80% of dementia patients with a complicated or uncertain presentation, Mathieu Ceccaldi, MD, reported at the Clinical Trials on Alzheimer’s Disease conference.
The French real-world study also found that 51% of the patients had a medication change after their amyloid imaging study, said Dr. Ceccaldi, a neurologist at the Timone Hospital in Marseille, France.
“In daily practice, we are sometimes faced with complex presentations and diagnostic uncertainty in patients who have early onset dementia, atypical nonamnestic dementia, unusual behavioral symptoms, or an unexpected rate of progression,” he said. “Amyloid imaging can help resolve those issues.”
The results echo – and even exceed – those returned in an interim analysis of the large U.S.-based Imaging Dementia–Evidence for Amyloid Scanning study. The IDEAS study is examining how amyloid imaging with the PET agent florbetapir may change clinical management of dementia patients. According to data presented at last summer’s Alzheimer’s Association International Conference, knowledge of patients’ brain amyloid status changed clinical management in 68% of cases.
In France, as in the United States, amyloid imaging is not routinely available outside of clinical research programs. French patients normally undergo a lumbar puncture (LP) to obtain amyloid biomarkers. However, if an LP isn’t feasible because of the patient’s clinical condition, it’s attempted and fails, the patient refuses, or the results are unclear, then imaging may be employed.
Dr. Ceccaldi’s study comprised 205 such patients seen in any of 18 memory clinics for dementia of uncertain etiology. The study evaluated how often amyloid PET imaging with florbetaben changed the patient’s diagnosis or management and how often it contributed to improving diagnostic uncertainty.
The patients were a mean of 71 years old, with a mean Mini-Mental State Exam score of 22. An LP had been performed in 42%, but those results were either ambiguous or inconsistent with the patient’s clinical presentation. Other patients (37%) refused the procedure, and the rest had a medical contraindication to LP or had a failed procedure.
The most common diagnosis at baseline was Alzheimer’s dementia (72%), which included typical and atypical sporadic AD, young-onset AD, and rapidly progressing AD. In 16% of the cohort, the diagnosis was non-Alzheimer’s dementia, including frontotemporal dementia, primary progressive aphasia, Lewy body dementia, corticobasal degeneration, semantic dementia, and Parkinson’s disease dementia. The diagnosis was mixed dementia in 8% and nonneurodegenerative dementia in the remainder – a catchall that included vascular dementia, psychiatric disorders, and other forms of dementia.
Imaging determined that 73 patients were amyloid negative and that 132 patients were amyloid positive. These results changed diagnosis in 67% of cases overall, in 58% of amyloid-positive patients, and in 84% of amyloid-negative patients.
In the absence of cerebrospinal fluid data, florbetaben imaging significantly improved diagnostic confidence in 81% of the entire cohort (167 patients). Before imaging, clinicians rated fewer than 5% of their diagnoses as very highly confident; this rose to nearly 100% after imaging. Similarly, before imaging, about 40% of clinicians said they had weak confidence in their initial diagnoses; this dropped to fewer than 5% after imaging.
Of the 67% with a changed diagnosis (137 patients), 76 were amyloid positive, and 61 were amyloid negative. Positive scans reclassified 18 patients as having AD; negative scans removed an AD diagnosis for 38 patients.
AD was the most commonly altered diagnosis, dropping from 72% to 62% of the entire cohort. The proportion of those with non-AD dementia increased from 16% before imaging to 18% after. Mixed dementias decreased from 8% to 5%, and the number diagnosed with nonneurodegenerative dementias increased from 5% to 17%.
In particular, Dr. Ceccaldi noted, the number of patients with potentially treatable nonneurodegenerative dementia rose from 10 to 35. These included revised diagnoses for those with psychiatric disorders (from 3 patients before imaging to 11 patients after), vascular dementias (from 2 to 8 patients), and other treatable causes of dementia (from 5 to 16 patients).
All of these altered diagnoses changed management in 80% of both positive and negative patients. Among these changes were the addition of a new medication (50% of amyloid-positive patients, 18% of amyloid-negative patients), the withdrawal of a medication (15% of amyloid-negative cases), additional testing (5% of amyloid-positive cases, 20% of amyloid-negative cases), and referral to another specialist (5% of amyloid-positive patients, 20% of amyloid-negative patients).
“Our results highlight the significant utility of amyloid PET for patients with complex dementia presentations in the context of the existing work-up,” Dr. Ceccaldi said.
He reported financial relationships with a number of pharmaceutical companies, including Piramal, which developed and manufactures florbetaben.
[email protected]
On Twitter @alz_gal
AT CTAD
Key clinical point:
Major finding: A majority of patients (80%) experienced a change in management, including drugs added or withdrawn, or referral to another specialist.
Data source: A naturalistic, clinic-based study comprising 205 patients.
Disclosures: Dr. Ceccaldi reported financial relationships with several pharmaceutical companies, including Piramal, which developed and manufactures florbetaben.
New BACE1 study launches in the shadow of verubecestat’s demise
BOSTON – Undeterred by a failed BACE inhibitor study with worrisome adverse events, an ambitious new clinical trial will investigate a different BACE-inhibiting molecule as an Alzheimer’s preventive in people at high genetic risk of Alzheimer’s disease.
The global Generation 2 trial intends to recruit about 3,300 cognitively normal subjects aged 60-75 years, who have either one or two copies of the apolipoprotein e4 (ApoE4) allele, said Pierre Tariot, MD, who announced the new study during the Clinical Trials on Alzheimer’s Disease 2017 meeting.
The Alzheimer Preventive Initiative and industry partners Novartis and Amgen are sponsoring the trial, which is a sister study to Generation 1. Generation 1, now ongoing, targets cognitively normal ApoE4 subjects only. It is a four-armed trial, comparing placebo to both CNP520 and an active immunotherapy called CAD106. Together, the studies comprise the Generation Program.
Both trials are event-driven, and will run 5-8 years. Generation 2 has a dual primary endpoint – a successful trial will find either a delay in progression to mild cognitive impairment or dementia relative to placebo, or significant differences from baseline in cognitive change as measured by the Alzheimer’s Preclinical Composite Cognitive test, or both.
The study partners are enthusiastic about CNP520, which performed well in its safety and tolerability studies. In an interview, Dr. Tariot called the CNP520 “likely a best-in-class molecule.”
“The only adverse events we saw in the entire safety program were a few cases of itchy skin,” said Dr. Tariot, director of the Banner Alzheimer Institute in Phoenix. “One of the reasons we chose Novartis as a study partner is that this drug of theirs looked remarkably clean – so clean that when we were deciding what doses to look at, the decision related only to what degree of BACE inhibition we wanted, not safety.”
The drug seemed very well-tolerated at every dose tested (1 mg, 10 mg, 25 mg, and 75 mg). Generation 1 employs a 50-mg oral dose per day. Generation 2 will investigate both 50 mg and 15 mg.
Site investigators are solidly behind the choice, said Anton Porsteinsson, MD, a Generation investigator at the University of Rochester (N.Y.) Medical Center, despite the unveiling at CTAD of worrisome adverse events seen in Merck’s failed EPOCH trial.
“I am even more convinced now that BACE inhibitors are drugs that need to be used early and that are probably highly indicated for this population,” he said in an interview. Dr. Porsteinsson wasn’t overly disturbed by data that Merck released during the meeting on its BACE inhibitor, verubecestat. In February, the company pulled the plug on its EPOCH trial investigating verubecestat in patients with mild-moderate AD. An interim analysis determined that there was no chance of success with the molecule.
Despite the general disappointment of yet another rainy-day parade in late-stage AD drug trials, researchers who heard the EPOCH post-mortem during CTAD expressed considerable concern over the unexpected, wide-ranging, and serious adverse events the trial accumulated.
Although there were no cases of Amyloid-Related Imaging Abnormalities (ARIA), a number of adverse events occurred significantly more often in the active groups than the placebo group. These included rash, falls and injuries, insomnia, headache, anxiety, suicidal ideation, diarrhea, dizziness, and weight loss.
At least some of these were hinted at in the bench science that brought verubecestat to late-stage clinical development. BACE is important for proper muscle function, and some BACE-knockout mice displayed a decrease in muscle spindles, receptors that sense changes in the length of muscle fibers. Other peculiarities in the mice have included axon targeting errors, reduced myelination, memory impairment, neurochemical abnormalities, alterations in neurogenesis and astrogenesis, increased age-related neurodegeneration, reduced spine density, retinal pathology, endophenotypes of schizophrenia, and seizures.
But the verubecestat findings aren’t a show-stopper for the more target-specific CNP520, Dr. Porsteinsson said. Verubecestat inhibited both BACE1 and BACE2; CNP520, only BACE1. And Dr. Porsteinsson, like most researchers, believes that preventing the accumulation of neurotoxic AB species will probably be much more effective clinically than trying to dissolve large stubborn brain plaques, which have already wreaked cognitive havoc.
In EPOCH, “even in people with advanced disease and a high load of insoluble plaques; they saw an 80% reduction in the production of AB and a 4% decrease in plaque burden. To me that signals this is not the class to use in moderate patients, or even mild, but in these very early stages where you don’t have full saturation, it might just be perfect,” he said.
“Do the side effects give me pause? Obviously and maybe mostly because we don’t know exactly what the driver was. But what we do is keep a close eye on everyone, maybe do extra safety monitoring, Most importantly, we picked a drug that shows less issues with any of these problems.”
Cognitively normal ApoE4 carriers – especially homozygotes – are an extremely important population to study, both in terms of clinical and scientific need. The gene is the single largest genetic risk factor for Alzheimer’s; those who carry two copies are 60% more likely than the general population to develop Alzheimer’s.
“These people truly need an effective intervention,” said Dr. Porsteinsson. “At the preclinical stage, they don’t truly have a disease yet; they are living their lives unimpaired. But something is percolating, and the results won’t be good. Even early on, there are fairly significant changes going on in the brain: a buildup of amyloid and tau, excess oxidative damage, inflammation. And if you don’t deal with it early on, it’s like trying to cure cancer once it’s metastasized.”
In the larger scientific picture, success in a primary prevention trial would finally put to rest questions about the amyloid cascade hypothesis and explain the long string of anti-amyloid drug trial failures, all of which were targeted at people with more advanced disease.
To enroll the entire cohort, Generation 2 will need to screen about 30,000 people. This sounds like a daunting task, but a new digital platform makes it eminently do-able, Dr. Tariot said. Both Generation studies are enrolling online. The consumer-friendly website offers detailed information about Alzheimer’s disease in general, ApoE4 risk, the studies’ structure, and the investigational medications. Most importantly, Dr. Tariot said, the website has a direct link to GeneMatch. Hosted by Banner Alzheimer Institute, GeneMatch prescreens potential trial participants (U.S. residents aged 55-75 years) by taking baseline demographic information, and mailing out free, simple-to-use cheek swab kits for genetic analysis. The program then stores and sorts the information, matching volunteers with appropriate trials according to location, age, interest, and genetic status. People don’t necessarily learn their ApoE4 status, unless they are recruited into a genetics-driven trial.
Launched 2 years ago, GeneMatch has already accrued more than 280,000 volunteers – a pretty remarkable achievement in itself, Dr. Tariot said. “People are very motivated to help find a cure for Alzheimer’s. Many of them have a family member who has the disease. Others are concerned about themselves, and many people just want to be part of something important.”
Dr. Porsteinsson agreed, relaying a startling interaction he had with a patient, who found out he was not qualified for a Generation study, meaning, of course, that he was ApoE4 negative.
“He told me he was actually kind of disappointed, because he believes so much in this study, and wanted to be part of doing something important for humanity,” Dr. Porsteinsson said. “Of course, I didn’t let him off the hook. I directed him to a bunch of other studies he was qualified for. We need everyone’s help to solve this.”
Both Dr. Tariot and Dr. Porsteinsson have reported financial relationships with numerous pharmaceutical companies.
* This story was updated 11/7/17.
[email protected]
On Twitter @Alz_Gal
BOSTON – Undeterred by a failed BACE inhibitor study with worrisome adverse events, an ambitious new clinical trial will investigate a different BACE-inhibiting molecule as an Alzheimer’s preventive in people at high genetic risk of Alzheimer’s disease.
The global Generation 2 trial intends to recruit about 3,300 cognitively normal subjects aged 60-75 years, who have either one or two copies of the apolipoprotein e4 (ApoE4) allele, said Pierre Tariot, MD, who announced the new study during the Clinical Trials on Alzheimer’s Disease 2017 meeting.
The Alzheimer Preventive Initiative and industry partners Novartis and Amgen are sponsoring the trial, which is a sister study to Generation 1. Generation 1, now ongoing, targets cognitively normal ApoE4 subjects only. It is a four-armed trial, comparing placebo to both CNP520 and an active immunotherapy called CAD106. Together, the studies comprise the Generation Program.
Both trials are event-driven, and will run 5-8 years. Generation 2 has a dual primary endpoint – a successful trial will find either a delay in progression to mild cognitive impairment or dementia relative to placebo, or significant differences from baseline in cognitive change as measured by the Alzheimer’s Preclinical Composite Cognitive test, or both.
The study partners are enthusiastic about CNP520, which performed well in its safety and tolerability studies. In an interview, Dr. Tariot called the CNP520 “likely a best-in-class molecule.”
“The only adverse events we saw in the entire safety program were a few cases of itchy skin,” said Dr. Tariot, director of the Banner Alzheimer Institute in Phoenix. “One of the reasons we chose Novartis as a study partner is that this drug of theirs looked remarkably clean – so clean that when we were deciding what doses to look at, the decision related only to what degree of BACE inhibition we wanted, not safety.”
The drug seemed very well-tolerated at every dose tested (1 mg, 10 mg, 25 mg, and 75 mg). Generation 1 employs a 50-mg oral dose per day. Generation 2 will investigate both 50 mg and 15 mg.
Site investigators are solidly behind the choice, said Anton Porsteinsson, MD, a Generation investigator at the University of Rochester (N.Y.) Medical Center, despite the unveiling at CTAD of worrisome adverse events seen in Merck’s failed EPOCH trial.
“I am even more convinced now that BACE inhibitors are drugs that need to be used early and that are probably highly indicated for this population,” he said in an interview. Dr. Porsteinsson wasn’t overly disturbed by data that Merck released during the meeting on its BACE inhibitor, verubecestat. In February, the company pulled the plug on its EPOCH trial investigating verubecestat in patients with mild-moderate AD. An interim analysis determined that there was no chance of success with the molecule.
Despite the general disappointment of yet another rainy-day parade in late-stage AD drug trials, researchers who heard the EPOCH post-mortem during CTAD expressed considerable concern over the unexpected, wide-ranging, and serious adverse events the trial accumulated.
Although there were no cases of Amyloid-Related Imaging Abnormalities (ARIA), a number of adverse events occurred significantly more often in the active groups than the placebo group. These included rash, falls and injuries, insomnia, headache, anxiety, suicidal ideation, diarrhea, dizziness, and weight loss.
At least some of these were hinted at in the bench science that brought verubecestat to late-stage clinical development. BACE is important for proper muscle function, and some BACE-knockout mice displayed a decrease in muscle spindles, receptors that sense changes in the length of muscle fibers. Other peculiarities in the mice have included axon targeting errors, reduced myelination, memory impairment, neurochemical abnormalities, alterations in neurogenesis and astrogenesis, increased age-related neurodegeneration, reduced spine density, retinal pathology, endophenotypes of schizophrenia, and seizures.
But the verubecestat findings aren’t a show-stopper for the more target-specific CNP520, Dr. Porsteinsson said. Verubecestat inhibited both BACE1 and BACE2; CNP520, only BACE1. And Dr. Porsteinsson, like most researchers, believes that preventing the accumulation of neurotoxic AB species will probably be much more effective clinically than trying to dissolve large stubborn brain plaques, which have already wreaked cognitive havoc.
In EPOCH, “even in people with advanced disease and a high load of insoluble plaques; they saw an 80% reduction in the production of AB and a 4% decrease in plaque burden. To me that signals this is not the class to use in moderate patients, or even mild, but in these very early stages where you don’t have full saturation, it might just be perfect,” he said.
“Do the side effects give me pause? Obviously and maybe mostly because we don’t know exactly what the driver was. But what we do is keep a close eye on everyone, maybe do extra safety monitoring, Most importantly, we picked a drug that shows less issues with any of these problems.”
Cognitively normal ApoE4 carriers – especially homozygotes – are an extremely important population to study, both in terms of clinical and scientific need. The gene is the single largest genetic risk factor for Alzheimer’s; those who carry two copies are 60% more likely than the general population to develop Alzheimer’s.
“These people truly need an effective intervention,” said Dr. Porsteinsson. “At the preclinical stage, they don’t truly have a disease yet; they are living their lives unimpaired. But something is percolating, and the results won’t be good. Even early on, there are fairly significant changes going on in the brain: a buildup of amyloid and tau, excess oxidative damage, inflammation. And if you don’t deal with it early on, it’s like trying to cure cancer once it’s metastasized.”
In the larger scientific picture, success in a primary prevention trial would finally put to rest questions about the amyloid cascade hypothesis and explain the long string of anti-amyloid drug trial failures, all of which were targeted at people with more advanced disease.
To enroll the entire cohort, Generation 2 will need to screen about 30,000 people. This sounds like a daunting task, but a new digital platform makes it eminently do-able, Dr. Tariot said. Both Generation studies are enrolling online. The consumer-friendly website offers detailed information about Alzheimer’s disease in general, ApoE4 risk, the studies’ structure, and the investigational medications. Most importantly, Dr. Tariot said, the website has a direct link to GeneMatch. Hosted by Banner Alzheimer Institute, GeneMatch prescreens potential trial participants (U.S. residents aged 55-75 years) by taking baseline demographic information, and mailing out free, simple-to-use cheek swab kits for genetic analysis. The program then stores and sorts the information, matching volunteers with appropriate trials according to location, age, interest, and genetic status. People don’t necessarily learn their ApoE4 status, unless they are recruited into a genetics-driven trial.
Launched 2 years ago, GeneMatch has already accrued more than 280,000 volunteers – a pretty remarkable achievement in itself, Dr. Tariot said. “People are very motivated to help find a cure for Alzheimer’s. Many of them have a family member who has the disease. Others are concerned about themselves, and many people just want to be part of something important.”
Dr. Porsteinsson agreed, relaying a startling interaction he had with a patient, who found out he was not qualified for a Generation study, meaning, of course, that he was ApoE4 negative.
“He told me he was actually kind of disappointed, because he believes so much in this study, and wanted to be part of doing something important for humanity,” Dr. Porsteinsson said. “Of course, I didn’t let him off the hook. I directed him to a bunch of other studies he was qualified for. We need everyone’s help to solve this.”
Both Dr. Tariot and Dr. Porsteinsson have reported financial relationships with numerous pharmaceutical companies.
* This story was updated 11/7/17.
[email protected]
On Twitter @Alz_Gal
BOSTON – Undeterred by a failed BACE inhibitor study with worrisome adverse events, an ambitious new clinical trial will investigate a different BACE-inhibiting molecule as an Alzheimer’s preventive in people at high genetic risk of Alzheimer’s disease.
The global Generation 2 trial intends to recruit about 3,300 cognitively normal subjects aged 60-75 years, who have either one or two copies of the apolipoprotein e4 (ApoE4) allele, said Pierre Tariot, MD, who announced the new study during the Clinical Trials on Alzheimer’s Disease 2017 meeting.
The Alzheimer Preventive Initiative and industry partners Novartis and Amgen are sponsoring the trial, which is a sister study to Generation 1. Generation 1, now ongoing, targets cognitively normal ApoE4 subjects only. It is a four-armed trial, comparing placebo to both CNP520 and an active immunotherapy called CAD106. Together, the studies comprise the Generation Program.
Both trials are event-driven, and will run 5-8 years. Generation 2 has a dual primary endpoint – a successful trial will find either a delay in progression to mild cognitive impairment or dementia relative to placebo, or significant differences from baseline in cognitive change as measured by the Alzheimer’s Preclinical Composite Cognitive test, or both.
The study partners are enthusiastic about CNP520, which performed well in its safety and tolerability studies. In an interview, Dr. Tariot called the CNP520 “likely a best-in-class molecule.”
“The only adverse events we saw in the entire safety program were a few cases of itchy skin,” said Dr. Tariot, director of the Banner Alzheimer Institute in Phoenix. “One of the reasons we chose Novartis as a study partner is that this drug of theirs looked remarkably clean – so clean that when we were deciding what doses to look at, the decision related only to what degree of BACE inhibition we wanted, not safety.”
The drug seemed very well-tolerated at every dose tested (1 mg, 10 mg, 25 mg, and 75 mg). Generation 1 employs a 50-mg oral dose per day. Generation 2 will investigate both 50 mg and 15 mg.
Site investigators are solidly behind the choice, said Anton Porsteinsson, MD, a Generation investigator at the University of Rochester (N.Y.) Medical Center, despite the unveiling at CTAD of worrisome adverse events seen in Merck’s failed EPOCH trial.
“I am even more convinced now that BACE inhibitors are drugs that need to be used early and that are probably highly indicated for this population,” he said in an interview. Dr. Porsteinsson wasn’t overly disturbed by data that Merck released during the meeting on its BACE inhibitor, verubecestat. In February, the company pulled the plug on its EPOCH trial investigating verubecestat in patients with mild-moderate AD. An interim analysis determined that there was no chance of success with the molecule.
Despite the general disappointment of yet another rainy-day parade in late-stage AD drug trials, researchers who heard the EPOCH post-mortem during CTAD expressed considerable concern over the unexpected, wide-ranging, and serious adverse events the trial accumulated.
Although there were no cases of Amyloid-Related Imaging Abnormalities (ARIA), a number of adverse events occurred significantly more often in the active groups than the placebo group. These included rash, falls and injuries, insomnia, headache, anxiety, suicidal ideation, diarrhea, dizziness, and weight loss.
At least some of these were hinted at in the bench science that brought verubecestat to late-stage clinical development. BACE is important for proper muscle function, and some BACE-knockout mice displayed a decrease in muscle spindles, receptors that sense changes in the length of muscle fibers. Other peculiarities in the mice have included axon targeting errors, reduced myelination, memory impairment, neurochemical abnormalities, alterations in neurogenesis and astrogenesis, increased age-related neurodegeneration, reduced spine density, retinal pathology, endophenotypes of schizophrenia, and seizures.
But the verubecestat findings aren’t a show-stopper for the more target-specific CNP520, Dr. Porsteinsson said. Verubecestat inhibited both BACE1 and BACE2; CNP520, only BACE1. And Dr. Porsteinsson, like most researchers, believes that preventing the accumulation of neurotoxic AB species will probably be much more effective clinically than trying to dissolve large stubborn brain plaques, which have already wreaked cognitive havoc.
In EPOCH, “even in people with advanced disease and a high load of insoluble plaques; they saw an 80% reduction in the production of AB and a 4% decrease in plaque burden. To me that signals this is not the class to use in moderate patients, or even mild, but in these very early stages where you don’t have full saturation, it might just be perfect,” he said.
“Do the side effects give me pause? Obviously and maybe mostly because we don’t know exactly what the driver was. But what we do is keep a close eye on everyone, maybe do extra safety monitoring, Most importantly, we picked a drug that shows less issues with any of these problems.”
Cognitively normal ApoE4 carriers – especially homozygotes – are an extremely important population to study, both in terms of clinical and scientific need. The gene is the single largest genetic risk factor for Alzheimer’s; those who carry two copies are 60% more likely than the general population to develop Alzheimer’s.
“These people truly need an effective intervention,” said Dr. Porsteinsson. “At the preclinical stage, they don’t truly have a disease yet; they are living their lives unimpaired. But something is percolating, and the results won’t be good. Even early on, there are fairly significant changes going on in the brain: a buildup of amyloid and tau, excess oxidative damage, inflammation. And if you don’t deal with it early on, it’s like trying to cure cancer once it’s metastasized.”
In the larger scientific picture, success in a primary prevention trial would finally put to rest questions about the amyloid cascade hypothesis and explain the long string of anti-amyloid drug trial failures, all of which were targeted at people with more advanced disease.
To enroll the entire cohort, Generation 2 will need to screen about 30,000 people. This sounds like a daunting task, but a new digital platform makes it eminently do-able, Dr. Tariot said. Both Generation studies are enrolling online. The consumer-friendly website offers detailed information about Alzheimer’s disease in general, ApoE4 risk, the studies’ structure, and the investigational medications. Most importantly, Dr. Tariot said, the website has a direct link to GeneMatch. Hosted by Banner Alzheimer Institute, GeneMatch prescreens potential trial participants (U.S. residents aged 55-75 years) by taking baseline demographic information, and mailing out free, simple-to-use cheek swab kits for genetic analysis. The program then stores and sorts the information, matching volunteers with appropriate trials according to location, age, interest, and genetic status. People don’t necessarily learn their ApoE4 status, unless they are recruited into a genetics-driven trial.
Launched 2 years ago, GeneMatch has already accrued more than 280,000 volunteers – a pretty remarkable achievement in itself, Dr. Tariot said. “People are very motivated to help find a cure for Alzheimer’s. Many of them have a family member who has the disease. Others are concerned about themselves, and many people just want to be part of something important.”
Dr. Porsteinsson agreed, relaying a startling interaction he had with a patient, who found out he was not qualified for a Generation study, meaning, of course, that he was ApoE4 negative.
“He told me he was actually kind of disappointed, because he believes so much in this study, and wanted to be part of doing something important for humanity,” Dr. Porsteinsson said. “Of course, I didn’t let him off the hook. I directed him to a bunch of other studies he was qualified for. We need everyone’s help to solve this.”
Both Dr. Tariot and Dr. Porsteinsson have reported financial relationships with numerous pharmaceutical companies.
* This story was updated 11/7/17.
[email protected]
On Twitter @Alz_Gal
AT CTAD