User login
BOSTON – Progressive tau accumulation in the temporal lobe of cognitively normal older adults was associated with cognitive decline over time in a prospective, longitudinal study presented at the Clinical Trials on Alzheimer’s Disease conference.
This track of cognitive impairment following tau pathology in a preclinical Alzheimer’s disease (AD) population suggests two roles for serial positron emission tomography (PET) scans with a tau binding agent, Bernard Hanseeuw, MD, PhD, said at the meeting. In the near future, they could be used to track therapeutic response in clinical trials. Farther out, if future validation studies confirm these preliminary results, they might be a useful clinical tool for predicting how fast an individual Alzheimer’s patient will progress, he said in an interview.
Serial tau scans, however, would, he said.
“Every patient with Alzheimer’s disease is different, with a different disease course. Amyloid scans can tell us if someone is on the wrong path, but tau scans could tell us how fast they are going. If you have Alzheimer’s, it’s important to know if you may not be able to live in your own home in a year. With tau PET, we could track the disease and predict how fast it might evolve. That is very clinically relevant,” said Dr. Hanseeuw.
Tau imaging remains investigational only. Several tau imaging agents are being developed, but none has yet been approved in the United States or in Europe.
To investigate the correlation of tau and cognitive decline in preclinical Alzheimer’s, Dr. Hanseeuw examined serial tau and amyloid PET scans conducted on 60 clinically normal older adults with a mean age of 75 years. About one-third of the cohort was positive for the APOE4 allele. All of them had a baseline Clinical Dementia Rating (CDR) score of 0 and a mean Mini-Mental State Exam score of at least 27. They also scored in the normal range on the Preclinical Alzheimer’s Cognitive Composite (PACC) test. This relatively new cognitive scale is an increasingly popular item in clinical trials. The PACC is a composite of the WAIS-R Digit Symbol Substitution Test, Mini-Mental State Exam, Free and Cued Selective Reminding Test, and Logical Memory IIA Delayed Recall, and correlates well with amyloid accumulation in the brain.
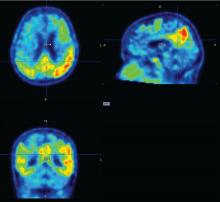
The study included up to 4 years of data on cognition and amyloid PET imaging, and up to 3 years of tau PET imaging data. The investigators assessed amyloid as a whole-brain aggregate and tau in the bilateral inferior temporal neocortex. “This is where the change is most happening in patients, and it’s a place where relatively few normal elderly would have tau,” Dr. Hanseeuw said. All of the analyses controlled for age, sex, and years of education.
Baseline amyloid levels were low in 36 participants and high in 24. At least some tau was present in all of the subjects. This is not an unexpected finding, since tau accumulates with age, Dr. Hanseeuw said. Over the study period, six subjects progressed to a CDR of 0.5 – a rating consistent with mild cognitive impairment. At baseline, high tau and high amyloid levels were both associated with a progressive decline in PACC scores in the following years. However, the rate of change in tau predicted change in cognition better than did the baseline measurements. In contrast, the rate of change in amyloid was not associated with cognitive decline.
“What is interesting here is that tau changed four times faster than amyloid,” Dr. Hanseeuw said. “The average subject needed 5 years to change 1 standard deviation in tau, but would have needed 20 years to change 1 standard deviation in amyloid.”
Fast-changing outcomes are important to accelerate drug assessment in clinical trials. Currently, it takes 3-5 years to conduct most anti-AD trials, he added.
Dr. Hanseeuw had no relevant financial disclosures.
BOSTON – Progressive tau accumulation in the temporal lobe of cognitively normal older adults was associated with cognitive decline over time in a prospective, longitudinal study presented at the Clinical Trials on Alzheimer’s Disease conference.
This track of cognitive impairment following tau pathology in a preclinical Alzheimer’s disease (AD) population suggests two roles for serial positron emission tomography (PET) scans with a tau binding agent, Bernard Hanseeuw, MD, PhD, said at the meeting. In the near future, they could be used to track therapeutic response in clinical trials. Farther out, if future validation studies confirm these preliminary results, they might be a useful clinical tool for predicting how fast an individual Alzheimer’s patient will progress, he said in an interview.
Serial tau scans, however, would, he said.
“Every patient with Alzheimer’s disease is different, with a different disease course. Amyloid scans can tell us if someone is on the wrong path, but tau scans could tell us how fast they are going. If you have Alzheimer’s, it’s important to know if you may not be able to live in your own home in a year. With tau PET, we could track the disease and predict how fast it might evolve. That is very clinically relevant,” said Dr. Hanseeuw.
Tau imaging remains investigational only. Several tau imaging agents are being developed, but none has yet been approved in the United States or in Europe.
To investigate the correlation of tau and cognitive decline in preclinical Alzheimer’s, Dr. Hanseeuw examined serial tau and amyloid PET scans conducted on 60 clinically normal older adults with a mean age of 75 years. About one-third of the cohort was positive for the APOE4 allele. All of them had a baseline Clinical Dementia Rating (CDR) score of 0 and a mean Mini-Mental State Exam score of at least 27. They also scored in the normal range on the Preclinical Alzheimer’s Cognitive Composite (PACC) test. This relatively new cognitive scale is an increasingly popular item in clinical trials. The PACC is a composite of the WAIS-R Digit Symbol Substitution Test, Mini-Mental State Exam, Free and Cued Selective Reminding Test, and Logical Memory IIA Delayed Recall, and correlates well with amyloid accumulation in the brain.
The study included up to 4 years of data on cognition and amyloid PET imaging, and up to 3 years of tau PET imaging data. The investigators assessed amyloid as a whole-brain aggregate and tau in the bilateral inferior temporal neocortex. “This is where the change is most happening in patients, and it’s a place where relatively few normal elderly would have tau,” Dr. Hanseeuw said. All of the analyses controlled for age, sex, and years of education.
Baseline amyloid levels were low in 36 participants and high in 24. At least some tau was present in all of the subjects. This is not an unexpected finding, since tau accumulates with age, Dr. Hanseeuw said. Over the study period, six subjects progressed to a CDR of 0.5 – a rating consistent with mild cognitive impairment. At baseline, high tau and high amyloid levels were both associated with a progressive decline in PACC scores in the following years. However, the rate of change in tau predicted change in cognition better than did the baseline measurements. In contrast, the rate of change in amyloid was not associated with cognitive decline.
“What is interesting here is that tau changed four times faster than amyloid,” Dr. Hanseeuw said. “The average subject needed 5 years to change 1 standard deviation in tau, but would have needed 20 years to change 1 standard deviation in amyloid.”
Fast-changing outcomes are important to accelerate drug assessment in clinical trials. Currently, it takes 3-5 years to conduct most anti-AD trials, he added.
Dr. Hanseeuw had no relevant financial disclosures.
BOSTON – Progressive tau accumulation in the temporal lobe of cognitively normal older adults was associated with cognitive decline over time in a prospective, longitudinal study presented at the Clinical Trials on Alzheimer’s Disease conference.
This track of cognitive impairment following tau pathology in a preclinical Alzheimer’s disease (AD) population suggests two roles for serial positron emission tomography (PET) scans with a tau binding agent, Bernard Hanseeuw, MD, PhD, said at the meeting. In the near future, they could be used to track therapeutic response in clinical trials. Farther out, if future validation studies confirm these preliminary results, they might be a useful clinical tool for predicting how fast an individual Alzheimer’s patient will progress, he said in an interview.
Serial tau scans, however, would, he said.
“Every patient with Alzheimer’s disease is different, with a different disease course. Amyloid scans can tell us if someone is on the wrong path, but tau scans could tell us how fast they are going. If you have Alzheimer’s, it’s important to know if you may not be able to live in your own home in a year. With tau PET, we could track the disease and predict how fast it might evolve. That is very clinically relevant,” said Dr. Hanseeuw.
Tau imaging remains investigational only. Several tau imaging agents are being developed, but none has yet been approved in the United States or in Europe.
To investigate the correlation of tau and cognitive decline in preclinical Alzheimer’s, Dr. Hanseeuw examined serial tau and amyloid PET scans conducted on 60 clinically normal older adults with a mean age of 75 years. About one-third of the cohort was positive for the APOE4 allele. All of them had a baseline Clinical Dementia Rating (CDR) score of 0 and a mean Mini-Mental State Exam score of at least 27. They also scored in the normal range on the Preclinical Alzheimer’s Cognitive Composite (PACC) test. This relatively new cognitive scale is an increasingly popular item in clinical trials. The PACC is a composite of the WAIS-R Digit Symbol Substitution Test, Mini-Mental State Exam, Free and Cued Selective Reminding Test, and Logical Memory IIA Delayed Recall, and correlates well with amyloid accumulation in the brain.
The study included up to 4 years of data on cognition and amyloid PET imaging, and up to 3 years of tau PET imaging data. The investigators assessed amyloid as a whole-brain aggregate and tau in the bilateral inferior temporal neocortex. “This is where the change is most happening in patients, and it’s a place where relatively few normal elderly would have tau,” Dr. Hanseeuw said. All of the analyses controlled for age, sex, and years of education.
Baseline amyloid levels were low in 36 participants and high in 24. At least some tau was present in all of the subjects. This is not an unexpected finding, since tau accumulates with age, Dr. Hanseeuw said. Over the study period, six subjects progressed to a CDR of 0.5 – a rating consistent with mild cognitive impairment. At baseline, high tau and high amyloid levels were both associated with a progressive decline in PACC scores in the following years. However, the rate of change in tau predicted change in cognition better than did the baseline measurements. In contrast, the rate of change in amyloid was not associated with cognitive decline.
“What is interesting here is that tau changed four times faster than amyloid,” Dr. Hanseeuw said. “The average subject needed 5 years to change 1 standard deviation in tau, but would have needed 20 years to change 1 standard deviation in amyloid.”
Fast-changing outcomes are important to accelerate drug assessment in clinical trials. Currently, it takes 3-5 years to conduct most anti-AD trials, he added.
Dr. Hanseeuw had no relevant financial disclosures.
REPORTING FROM CTAD
Key clinical point:
Major finding: Tau levels changed twice as fast as cognition, suggesting that the protein is a significant marker of future cognitive change.
Data source: A prospective, longitudinal study of 60 cognitively normal subjects.
Disclosures: Dr. Hanseeuw had no relevant financial disclosures.
Source: Hanseeuw B et al. CTAD 2017 Abstract OC2.