User login
Smartphone app detects voice quality changes indicating worsening heart failure
Worsening heart failure is accompanied by a build-up of fluid in the lungs. An AI smartphone app that picks up changes in a heart failure patient’s voice quality caused by this fluid accumulation and then alerts the physician about them – nearly 3 weeks before that ongoing decompensation would necessitate hospitalization and/or lead the physician to urgently introduce intravenous diuretics – is getting experts to sit up and take notice.
“In this incredibly prevalent waxing and waning condition, finding ways to identify worsening heart failure to prevent hospitalization and progressive disease is incredibly important,” observed American Heart Association (AHA)-appointed discussant David Ouyang, MD, assistant professor, Smidt Heart Institute, Division of Artificial Intelligence in Medicine, Cedars Sinai, Los Angeles. “Heart failure remains among the most common causes of hospitalization for older adults in the United States.
“The other standout feature is that we all use our cell phones on a daily basis,” Dr. Ouyang said at a late-breaking trial press briefing at the AHA 2023 annual meeting where results of the HearO Community Study were presented. “The ability to capture data from routine speech (patients speak five sentences into their phones every morning) is remarkable ... The HearO® technology was able to detect a substantial proportion of worsening heart failure events, with an average per individual of only three false positives over the course of a year. And, adherence to the study protocol was 81%. That’s higher than in many other kinds of routine patient monitoring studies,” he added.
Accumulating fluid changes speech
(e.g., pharynx, velum, tongue, and vocal folds). In the Israeli study, investigators enrolled 416 adults (75% were male, average age was 68 years) whose New York Heart Association (NYHA) 2-3 heart failure with either reduced or preserved ejection fraction was stable but placed them at-risk for heart failure events. The study goal was to analyze their speech data using the HearO® system to refine and test its ability to detect impending heart failure deterioration. Patients recorded five sentences in their native language (Hebrew, Russian, Arabic, or English) into the smartphone app daily. In a training phase of the study, distinct speech measures from 263 participants were used to develop the AI algorithm. Then, the algorithm was used in the remaining 153 participants to validate the tool’s effectiveness. In its ultimate form, once a deviation from the patient’s predefined baseline is detected, the app will generate a notice and send it to the health care practitioners.
Lead study author William T. Abraham, MD, FAHA, professor of medicine, physiology, and cell biology; and a College of Medicine Distinguished Professor in the division of cardiovascular medicine at The Ohio State University in Columbus, reported that between Mar. 27, 2018, and Nov. 30, 2021, subjects in the training phase made recordings on 83% of days. They were followed for up to 44 months. The test group made recordings on 81% of days between Feb. 1, 2020, and Apr. 30, 2023, and were followed for up to 31 months. Heart failure events were defined as hospitalization or outpatient intravenous diuretic treatment for worsening heart failure.
In the training phase, the app accurately predicted 44 of 58 heart failure events (76%) and 81% of first events (n = 35) on average 24 days before hospitalization or need for intravenous fluids. In the validation phase, the app was 71% accurate in detecting 10 of 14 heart failure events and 77% of first events (n = 10) on average 26 days in advance of events. In both periods, the app generated about 3 unnecessary alerts per patient year.
Dr. Abraham concluded, “This technology has the potential to improve patient outcomes, keeping patients well and out of the hospital, through the implementation of proactive, outpatient care in response to voice changes.”
The HearO® technology is being evaluated in an ongoing pivotal trial in the United State4s, Dr. Abraham said. The study is limited, he added, by the small number of patients and heart failure events, particularly in the test group.
“We continue to struggle with the burden of heart failure morbidity,” observed AHA press briefing moderator (and past AHA president) Clyde Yancy, MD, Magerstadt Professor at Northwestern University, Chicago. “So any tool that we can utilize and further refine that helps us address the need for hospitalization becomes very important. The idea that speech evaluation might give us sufficient early warning to forestall any admissions – and consider the cost savings attributable to that – is a very credible goal that we should continue to follow.” He pointed out that the technology enables assessments in the home environment for older patients who are less mobile.
In response to a press briefing question about the potential for physicians to be trained to hear early subtle voice changes on their own, Dr. Abraham stated, “I guess that is unknown, but the important difference is the system’s ability to take data in every day from patients and then process it automatically with AI.”
Joining in, Dr. Yancy said, “You know, this is interesting because even if you saw a patient once a month, which is an incredible frequency for any practice, there’s still 353 days that you haven’t seen the patient.” He noted that the AHA had just announced a multi-million dollar program to more deeply understand telemanagement. “So I think this is here to stay,” Dr. Yancy said.
Dr. Ouyang posed a further question. “Like with most AI recognition tools, we can now identify individuals at risk. How do we get from that step of identifying those at risk to improving their outcomes? This has been a critical question about heart failure, remote management, and remote monitoring, and I think it is a critical question for many of our AI tools.”
Dr. Abraham disclosed that he has received personal fees from Cordio Medical. Dr. Ouyang said that he had no disclosures relevant to this presentation.
Worsening heart failure is accompanied by a build-up of fluid in the lungs. An AI smartphone app that picks up changes in a heart failure patient’s voice quality caused by this fluid accumulation and then alerts the physician about them – nearly 3 weeks before that ongoing decompensation would necessitate hospitalization and/or lead the physician to urgently introduce intravenous diuretics – is getting experts to sit up and take notice.
“In this incredibly prevalent waxing and waning condition, finding ways to identify worsening heart failure to prevent hospitalization and progressive disease is incredibly important,” observed American Heart Association (AHA)-appointed discussant David Ouyang, MD, assistant professor, Smidt Heart Institute, Division of Artificial Intelligence in Medicine, Cedars Sinai, Los Angeles. “Heart failure remains among the most common causes of hospitalization for older adults in the United States.
“The other standout feature is that we all use our cell phones on a daily basis,” Dr. Ouyang said at a late-breaking trial press briefing at the AHA 2023 annual meeting where results of the HearO Community Study were presented. “The ability to capture data from routine speech (patients speak five sentences into their phones every morning) is remarkable ... The HearO® technology was able to detect a substantial proportion of worsening heart failure events, with an average per individual of only three false positives over the course of a year. And, adherence to the study protocol was 81%. That’s higher than in many other kinds of routine patient monitoring studies,” he added.
Accumulating fluid changes speech
(e.g., pharynx, velum, tongue, and vocal folds). In the Israeli study, investigators enrolled 416 adults (75% were male, average age was 68 years) whose New York Heart Association (NYHA) 2-3 heart failure with either reduced or preserved ejection fraction was stable but placed them at-risk for heart failure events. The study goal was to analyze their speech data using the HearO® system to refine and test its ability to detect impending heart failure deterioration. Patients recorded five sentences in their native language (Hebrew, Russian, Arabic, or English) into the smartphone app daily. In a training phase of the study, distinct speech measures from 263 participants were used to develop the AI algorithm. Then, the algorithm was used in the remaining 153 participants to validate the tool’s effectiveness. In its ultimate form, once a deviation from the patient’s predefined baseline is detected, the app will generate a notice and send it to the health care practitioners.
Lead study author William T. Abraham, MD, FAHA, professor of medicine, physiology, and cell biology; and a College of Medicine Distinguished Professor in the division of cardiovascular medicine at The Ohio State University in Columbus, reported that between Mar. 27, 2018, and Nov. 30, 2021, subjects in the training phase made recordings on 83% of days. They were followed for up to 44 months. The test group made recordings on 81% of days between Feb. 1, 2020, and Apr. 30, 2023, and were followed for up to 31 months. Heart failure events were defined as hospitalization or outpatient intravenous diuretic treatment for worsening heart failure.
In the training phase, the app accurately predicted 44 of 58 heart failure events (76%) and 81% of first events (n = 35) on average 24 days before hospitalization or need for intravenous fluids. In the validation phase, the app was 71% accurate in detecting 10 of 14 heart failure events and 77% of first events (n = 10) on average 26 days in advance of events. In both periods, the app generated about 3 unnecessary alerts per patient year.
Dr. Abraham concluded, “This technology has the potential to improve patient outcomes, keeping patients well and out of the hospital, through the implementation of proactive, outpatient care in response to voice changes.”
The HearO® technology is being evaluated in an ongoing pivotal trial in the United State4s, Dr. Abraham said. The study is limited, he added, by the small number of patients and heart failure events, particularly in the test group.
“We continue to struggle with the burden of heart failure morbidity,” observed AHA press briefing moderator (and past AHA president) Clyde Yancy, MD, Magerstadt Professor at Northwestern University, Chicago. “So any tool that we can utilize and further refine that helps us address the need for hospitalization becomes very important. The idea that speech evaluation might give us sufficient early warning to forestall any admissions – and consider the cost savings attributable to that – is a very credible goal that we should continue to follow.” He pointed out that the technology enables assessments in the home environment for older patients who are less mobile.
In response to a press briefing question about the potential for physicians to be trained to hear early subtle voice changes on their own, Dr. Abraham stated, “I guess that is unknown, but the important difference is the system’s ability to take data in every day from patients and then process it automatically with AI.”
Joining in, Dr. Yancy said, “You know, this is interesting because even if you saw a patient once a month, which is an incredible frequency for any practice, there’s still 353 days that you haven’t seen the patient.” He noted that the AHA had just announced a multi-million dollar program to more deeply understand telemanagement. “So I think this is here to stay,” Dr. Yancy said.
Dr. Ouyang posed a further question. “Like with most AI recognition tools, we can now identify individuals at risk. How do we get from that step of identifying those at risk to improving their outcomes? This has been a critical question about heart failure, remote management, and remote monitoring, and I think it is a critical question for many of our AI tools.”
Dr. Abraham disclosed that he has received personal fees from Cordio Medical. Dr. Ouyang said that he had no disclosures relevant to this presentation.
Worsening heart failure is accompanied by a build-up of fluid in the lungs. An AI smartphone app that picks up changes in a heart failure patient’s voice quality caused by this fluid accumulation and then alerts the physician about them – nearly 3 weeks before that ongoing decompensation would necessitate hospitalization and/or lead the physician to urgently introduce intravenous diuretics – is getting experts to sit up and take notice.
“In this incredibly prevalent waxing and waning condition, finding ways to identify worsening heart failure to prevent hospitalization and progressive disease is incredibly important,” observed American Heart Association (AHA)-appointed discussant David Ouyang, MD, assistant professor, Smidt Heart Institute, Division of Artificial Intelligence in Medicine, Cedars Sinai, Los Angeles. “Heart failure remains among the most common causes of hospitalization for older adults in the United States.
“The other standout feature is that we all use our cell phones on a daily basis,” Dr. Ouyang said at a late-breaking trial press briefing at the AHA 2023 annual meeting where results of the HearO Community Study were presented. “The ability to capture data from routine speech (patients speak five sentences into their phones every morning) is remarkable ... The HearO® technology was able to detect a substantial proportion of worsening heart failure events, with an average per individual of only three false positives over the course of a year. And, adherence to the study protocol was 81%. That’s higher than in many other kinds of routine patient monitoring studies,” he added.
Accumulating fluid changes speech
(e.g., pharynx, velum, tongue, and vocal folds). In the Israeli study, investigators enrolled 416 adults (75% were male, average age was 68 years) whose New York Heart Association (NYHA) 2-3 heart failure with either reduced or preserved ejection fraction was stable but placed them at-risk for heart failure events. The study goal was to analyze their speech data using the HearO® system to refine and test its ability to detect impending heart failure deterioration. Patients recorded five sentences in their native language (Hebrew, Russian, Arabic, or English) into the smartphone app daily. In a training phase of the study, distinct speech measures from 263 participants were used to develop the AI algorithm. Then, the algorithm was used in the remaining 153 participants to validate the tool’s effectiveness. In its ultimate form, once a deviation from the patient’s predefined baseline is detected, the app will generate a notice and send it to the health care practitioners.
Lead study author William T. Abraham, MD, FAHA, professor of medicine, physiology, and cell biology; and a College of Medicine Distinguished Professor in the division of cardiovascular medicine at The Ohio State University in Columbus, reported that between Mar. 27, 2018, and Nov. 30, 2021, subjects in the training phase made recordings on 83% of days. They were followed for up to 44 months. The test group made recordings on 81% of days between Feb. 1, 2020, and Apr. 30, 2023, and were followed for up to 31 months. Heart failure events were defined as hospitalization or outpatient intravenous diuretic treatment for worsening heart failure.
In the training phase, the app accurately predicted 44 of 58 heart failure events (76%) and 81% of first events (n = 35) on average 24 days before hospitalization or need for intravenous fluids. In the validation phase, the app was 71% accurate in detecting 10 of 14 heart failure events and 77% of first events (n = 10) on average 26 days in advance of events. In both periods, the app generated about 3 unnecessary alerts per patient year.
Dr. Abraham concluded, “This technology has the potential to improve patient outcomes, keeping patients well and out of the hospital, through the implementation of proactive, outpatient care in response to voice changes.”
The HearO® technology is being evaluated in an ongoing pivotal trial in the United State4s, Dr. Abraham said. The study is limited, he added, by the small number of patients and heart failure events, particularly in the test group.
“We continue to struggle with the burden of heart failure morbidity,” observed AHA press briefing moderator (and past AHA president) Clyde Yancy, MD, Magerstadt Professor at Northwestern University, Chicago. “So any tool that we can utilize and further refine that helps us address the need for hospitalization becomes very important. The idea that speech evaluation might give us sufficient early warning to forestall any admissions – and consider the cost savings attributable to that – is a very credible goal that we should continue to follow.” He pointed out that the technology enables assessments in the home environment for older patients who are less mobile.
In response to a press briefing question about the potential for physicians to be trained to hear early subtle voice changes on their own, Dr. Abraham stated, “I guess that is unknown, but the important difference is the system’s ability to take data in every day from patients and then process it automatically with AI.”
Joining in, Dr. Yancy said, “You know, this is interesting because even if you saw a patient once a month, which is an incredible frequency for any practice, there’s still 353 days that you haven’t seen the patient.” He noted that the AHA had just announced a multi-million dollar program to more deeply understand telemanagement. “So I think this is here to stay,” Dr. Yancy said.
Dr. Ouyang posed a further question. “Like with most AI recognition tools, we can now identify individuals at risk. How do we get from that step of identifying those at risk to improving their outcomes? This has been a critical question about heart failure, remote management, and remote monitoring, and I think it is a critical question for many of our AI tools.”
Dr. Abraham disclosed that he has received personal fees from Cordio Medical. Dr. Ouyang said that he had no disclosures relevant to this presentation.
FROM AHA 2023
Promising first results with DNA editing to lower LDL
PHILADELPHIA –
While one of four patients in the highest-dose groups had a myocardial infarction the day after getting the treatment, investigators have enough confidence to go forward with the next phase of study.
“The HEART-1trial demonstrated the first human proof of concept for in vivo DNA-based editing,” said Andrew Bellinger, MD, PhD, chief scientific officer of Verve Therapeutics, the company developing the treatment. “We saw dose-dependent–based reductions in LDL and the PCSK9 protein.”
The HEART-1 study was a phase 1b trial of VERVE-101, a CRISPR-based gene editing mechanism designed to inactivate the liver gene PCSK9, which contributes to raising cholesterol. “Human genetics suggest that turning off the cholesterol-raising gene PCSK9 in the liver will durably reduce LDL cholesterol,” Dr. Bellinger said in presenting the results at the annual scientific sessions of the American Heart Association.
Lipid nanoparticle
VERVE-101 is designed to be a single-course treatment to specifically treat HeFH, Dr. Bellinger said. He explained how the therapy, given by intravenous infusion, differs from adeno-associated virus vectors that have dominated gene therapy platforms.
“It’s a lipid nanoparticle encapsulating two RNA nanoparticles that are taken up by hepatocytes in the liver from the blood by the LDL receptor,” he explained. “Then the A-to-G–based editor protein and the guide mRNA protein together find the PCSK9 gene in the liver.” That single DNA-base change in one position of the PCSK9 gene is able to turn off PCSK9 production in those liver cells.
Dr. Bellinger presented interim results of the first 10 patients treated in the open-label, single ascending dose study. The patients were male and female, ages 18-75, with HeFH, established atherosclerotic cardiovascular disease and uncontrolled hypercholesterolemia despite being on maximally tolerated lipid-lowering therapy.
They received four different doses: Three patients each received 0.1, 0.3, and 0.45 mg/kg; and one patient received 0.6 mg/kg.
Reductions in blood PCSK9 levels were measured across all dosing groups at 4 weeks, but they were most pronounced in the two highest groups, Dr. Bellinger said. Two patients in the 0.45-mg/kg group had reductions of 59% and 84%. The sole patient in the 0.6-mg/kg arm had a reduction of 47%.
Regarding the 84% reduction in one individual, Dr. Bellinger said, “Roughly 85% of PCSK9 comes from the liver. These data suggest that we have successfully made a single base pair change in both copies of the PCSK9 gene in nearly every hepatocyte in the liver of this individual.”
Those benefits carried over to LDL cholesterol measures, with the highest-dose patients registering 39%, 48% and 55% reductions.
Safety outcomes
Two patients had serious cardiovascular (CV) events. One in the 0.3-mg/kg arm died from cardiac arrest 5 weeks after receiving the infusion. A patient in the 0.45-mg/kg arm had a myocardial infarction a day after getting the infusion and then nonsustained ventricular tachycardia (NSVT) 4 weeks later. Dr. Bellinger said an independent review panel determined that the CV events were in line with outcomes for high-risk patients and weren’t directly related to treatment.
He added, “Increased liver transaminases were seen in patients treated in the higher-dose cohorts. It’s transient, asymptomatic, and it resolved quickly.”
The next step involves pursuing only the 0.45- and 0.6-mg/kg doses in the next dose-escalation phase and enrolling an expansion cohort in 2024, Dr. Bellinger said, with a plan to initiate a randomized, placebo-controlled phase 2 trial in 2025.
First, do no harm
Karol Watson, MD, PhD, a women’s cardiovascular disease specialist at UCLA, said the promise of gene therapy was “revolutionary,” but that proving safety was critical going forward.
“You’re changing the genome forever,” she said. “Safety is going to be of the utmost importance especially because there are currently safe and efficacious strategies available for lipid lowering. This is a strategy that could be revolutionary, but we have to make sure that it’s safe.”
She pointed to a multinational study from earlier this year that warned about pathogenic consequences from CRISPR-based gene editing. “There are concerns about gene editing,” Dr. Watson said. “This was a whole-genome analysis showing atypical nonhomologous on-target effects of genome editing. Of course this is a very different strategy from what we heard today, but, again, we have to know that this is safe.”
Despite the small sample size from the two highest-dose groups in the study, Dr. Watson said the investigators have reason for going forward. “I think the preclinical data supports moving forward, but the next studies will have to be scrutinized carefully,” she said. “This is a preventive therapy; the first tenet is to do no harm.”
Dr. Bellinger is an employee of Verve Therapeutics, which sponsored the trial. Dr. Watson disclosed relationships with Boehringer-Ingelheim, Amgen, Lilly and Novartis.
PHILADELPHIA –
While one of four patients in the highest-dose groups had a myocardial infarction the day after getting the treatment, investigators have enough confidence to go forward with the next phase of study.
“The HEART-1trial demonstrated the first human proof of concept for in vivo DNA-based editing,” said Andrew Bellinger, MD, PhD, chief scientific officer of Verve Therapeutics, the company developing the treatment. “We saw dose-dependent–based reductions in LDL and the PCSK9 protein.”
The HEART-1 study was a phase 1b trial of VERVE-101, a CRISPR-based gene editing mechanism designed to inactivate the liver gene PCSK9, which contributes to raising cholesterol. “Human genetics suggest that turning off the cholesterol-raising gene PCSK9 in the liver will durably reduce LDL cholesterol,” Dr. Bellinger said in presenting the results at the annual scientific sessions of the American Heart Association.
Lipid nanoparticle
VERVE-101 is designed to be a single-course treatment to specifically treat HeFH, Dr. Bellinger said. He explained how the therapy, given by intravenous infusion, differs from adeno-associated virus vectors that have dominated gene therapy platforms.
“It’s a lipid nanoparticle encapsulating two RNA nanoparticles that are taken up by hepatocytes in the liver from the blood by the LDL receptor,” he explained. “Then the A-to-G–based editor protein and the guide mRNA protein together find the PCSK9 gene in the liver.” That single DNA-base change in one position of the PCSK9 gene is able to turn off PCSK9 production in those liver cells.
Dr. Bellinger presented interim results of the first 10 patients treated in the open-label, single ascending dose study. The patients were male and female, ages 18-75, with HeFH, established atherosclerotic cardiovascular disease and uncontrolled hypercholesterolemia despite being on maximally tolerated lipid-lowering therapy.
They received four different doses: Three patients each received 0.1, 0.3, and 0.45 mg/kg; and one patient received 0.6 mg/kg.
Reductions in blood PCSK9 levels were measured across all dosing groups at 4 weeks, but they were most pronounced in the two highest groups, Dr. Bellinger said. Two patients in the 0.45-mg/kg group had reductions of 59% and 84%. The sole patient in the 0.6-mg/kg arm had a reduction of 47%.
Regarding the 84% reduction in one individual, Dr. Bellinger said, “Roughly 85% of PCSK9 comes from the liver. These data suggest that we have successfully made a single base pair change in both copies of the PCSK9 gene in nearly every hepatocyte in the liver of this individual.”
Those benefits carried over to LDL cholesterol measures, with the highest-dose patients registering 39%, 48% and 55% reductions.
Safety outcomes
Two patients had serious cardiovascular (CV) events. One in the 0.3-mg/kg arm died from cardiac arrest 5 weeks after receiving the infusion. A patient in the 0.45-mg/kg arm had a myocardial infarction a day after getting the infusion and then nonsustained ventricular tachycardia (NSVT) 4 weeks later. Dr. Bellinger said an independent review panel determined that the CV events were in line with outcomes for high-risk patients and weren’t directly related to treatment.
He added, “Increased liver transaminases were seen in patients treated in the higher-dose cohorts. It’s transient, asymptomatic, and it resolved quickly.”
The next step involves pursuing only the 0.45- and 0.6-mg/kg doses in the next dose-escalation phase and enrolling an expansion cohort in 2024, Dr. Bellinger said, with a plan to initiate a randomized, placebo-controlled phase 2 trial in 2025.
First, do no harm
Karol Watson, MD, PhD, a women’s cardiovascular disease specialist at UCLA, said the promise of gene therapy was “revolutionary,” but that proving safety was critical going forward.
“You’re changing the genome forever,” she said. “Safety is going to be of the utmost importance especially because there are currently safe and efficacious strategies available for lipid lowering. This is a strategy that could be revolutionary, but we have to make sure that it’s safe.”
She pointed to a multinational study from earlier this year that warned about pathogenic consequences from CRISPR-based gene editing. “There are concerns about gene editing,” Dr. Watson said. “This was a whole-genome analysis showing atypical nonhomologous on-target effects of genome editing. Of course this is a very different strategy from what we heard today, but, again, we have to know that this is safe.”
Despite the small sample size from the two highest-dose groups in the study, Dr. Watson said the investigators have reason for going forward. “I think the preclinical data supports moving forward, but the next studies will have to be scrutinized carefully,” she said. “This is a preventive therapy; the first tenet is to do no harm.”
Dr. Bellinger is an employee of Verve Therapeutics, which sponsored the trial. Dr. Watson disclosed relationships with Boehringer-Ingelheim, Amgen, Lilly and Novartis.
PHILADELPHIA –
While one of four patients in the highest-dose groups had a myocardial infarction the day after getting the treatment, investigators have enough confidence to go forward with the next phase of study.
“The HEART-1trial demonstrated the first human proof of concept for in vivo DNA-based editing,” said Andrew Bellinger, MD, PhD, chief scientific officer of Verve Therapeutics, the company developing the treatment. “We saw dose-dependent–based reductions in LDL and the PCSK9 protein.”
The HEART-1 study was a phase 1b trial of VERVE-101, a CRISPR-based gene editing mechanism designed to inactivate the liver gene PCSK9, which contributes to raising cholesterol. “Human genetics suggest that turning off the cholesterol-raising gene PCSK9 in the liver will durably reduce LDL cholesterol,” Dr. Bellinger said in presenting the results at the annual scientific sessions of the American Heart Association.
Lipid nanoparticle
VERVE-101 is designed to be a single-course treatment to specifically treat HeFH, Dr. Bellinger said. He explained how the therapy, given by intravenous infusion, differs from adeno-associated virus vectors that have dominated gene therapy platforms.
“It’s a lipid nanoparticle encapsulating two RNA nanoparticles that are taken up by hepatocytes in the liver from the blood by the LDL receptor,” he explained. “Then the A-to-G–based editor protein and the guide mRNA protein together find the PCSK9 gene in the liver.” That single DNA-base change in one position of the PCSK9 gene is able to turn off PCSK9 production in those liver cells.
Dr. Bellinger presented interim results of the first 10 patients treated in the open-label, single ascending dose study. The patients were male and female, ages 18-75, with HeFH, established atherosclerotic cardiovascular disease and uncontrolled hypercholesterolemia despite being on maximally tolerated lipid-lowering therapy.
They received four different doses: Three patients each received 0.1, 0.3, and 0.45 mg/kg; and one patient received 0.6 mg/kg.
Reductions in blood PCSK9 levels were measured across all dosing groups at 4 weeks, but they were most pronounced in the two highest groups, Dr. Bellinger said. Two patients in the 0.45-mg/kg group had reductions of 59% and 84%. The sole patient in the 0.6-mg/kg arm had a reduction of 47%.
Regarding the 84% reduction in one individual, Dr. Bellinger said, “Roughly 85% of PCSK9 comes from the liver. These data suggest that we have successfully made a single base pair change in both copies of the PCSK9 gene in nearly every hepatocyte in the liver of this individual.”
Those benefits carried over to LDL cholesterol measures, with the highest-dose patients registering 39%, 48% and 55% reductions.
Safety outcomes
Two patients had serious cardiovascular (CV) events. One in the 0.3-mg/kg arm died from cardiac arrest 5 weeks after receiving the infusion. A patient in the 0.45-mg/kg arm had a myocardial infarction a day after getting the infusion and then nonsustained ventricular tachycardia (NSVT) 4 weeks later. Dr. Bellinger said an independent review panel determined that the CV events were in line with outcomes for high-risk patients and weren’t directly related to treatment.
He added, “Increased liver transaminases were seen in patients treated in the higher-dose cohorts. It’s transient, asymptomatic, and it resolved quickly.”
The next step involves pursuing only the 0.45- and 0.6-mg/kg doses in the next dose-escalation phase and enrolling an expansion cohort in 2024, Dr. Bellinger said, with a plan to initiate a randomized, placebo-controlled phase 2 trial in 2025.
First, do no harm
Karol Watson, MD, PhD, a women’s cardiovascular disease specialist at UCLA, said the promise of gene therapy was “revolutionary,” but that proving safety was critical going forward.
“You’re changing the genome forever,” she said. “Safety is going to be of the utmost importance especially because there are currently safe and efficacious strategies available for lipid lowering. This is a strategy that could be revolutionary, but we have to make sure that it’s safe.”
She pointed to a multinational study from earlier this year that warned about pathogenic consequences from CRISPR-based gene editing. “There are concerns about gene editing,” Dr. Watson said. “This was a whole-genome analysis showing atypical nonhomologous on-target effects of genome editing. Of course this is a very different strategy from what we heard today, but, again, we have to know that this is safe.”
Despite the small sample size from the two highest-dose groups in the study, Dr. Watson said the investigators have reason for going forward. “I think the preclinical data supports moving forward, but the next studies will have to be scrutinized carefully,” she said. “This is a preventive therapy; the first tenet is to do no harm.”
Dr. Bellinger is an employee of Verve Therapeutics, which sponsored the trial. Dr. Watson disclosed relationships with Boehringer-Ingelheim, Amgen, Lilly and Novartis.
AT AHA 2023
Novel blood test can detect RA
SAN DIEGO – Researchers say they’ve developed a novel blood-based assay that can differentiate patients with seropositive or seronegative rheumatoid arthritis from healthy people and those with other inflammatory diseases or osteoarthritis.
While cautioning that the results need to be confirmed, University of Oxford (England) rheumatologist Peter Taylor, PhD, MA, told an audience at the annual meeting of the American College of Rheumatology that the test has an overall mean sensitivity of 90.8% (standard deviation, 0.94%; 95% confidence interval, 83.2%-95.4%) and mean specificity of 96.1% (SD, 0.64%; 95% CI, 92.7%-97.9%). The mean area under the curve (AUC) is 0.991 (SD, 0.001; 95% CI, 97.2%-99.6%).
“That is to say that it can correctly identify 96% of people without rheumatoid arthritis, and it can correctly identify over 90% of those who do have rheumatoid arthritis,” Dr. Taylor said. In the big picture, he said, “we’ve developed a blood-based assay that detects both organ-specific and systemic biological processes in patients with rheumatoid arthritis.”
Rheumatologist Kevin W. Byram, MD, of Vanderbilt University, Nashville, Tenn., who did not take part in the study but is familiar with its findings, noted in an interview that “there is a constant search for biomarkers to help aide in more accurate and faster diagnosis of all rheumatic conditions, including RA.”
He added that “a common clinical scenario for the rheumatologist is a patient presenting with painful and/or swollen joints and other features that might suggest a few different diagnoses. A diagnostic assay like this might help distinguish patients with RA from other inflammatory conditions or non-inflammatory conditions that might mimic RA.”
In his presentation, Dr. Taylor noted that “we haven’t yet integrated precision medicine into routine clinical practice in rheumatology.” While blood-based assays are prevalent in other types of clinical diagnostics, rheumatology relies on synovial biopsies that are “rarely used in routine clinical practice,” he said.
The new test is a “non-invasive DNA capture assay that can identify specific gene expression from synovium-specific signatures in blood plasma of patients with rheumatoid arthritis,” Dr. Taylor said. Specifically, it focuses on the “unique patterns and sizes of cell-free DNA,” he said. “Analysis of [long] fragments has the potential to give us a great deal of information about disease progression, potentially about customizing treatments and even evaluating the effectiveness of therapies.”
For the new study, researchers examined 229 samples from 191 patients, of whom 63.3% were White and 67.9% were female, with a median age of 56. A total of 89 patients with RA provided 89 samples and 102 without RA provided 140 samples, including 29 healthy controls (66 samples) and others with conditions such as psoriatic arthritis, ulcerative colitis, and osteoarthritis.
The machine learning model “identified 3,425 epigenetic features with statistically significant discrimination between the patients with and without rheumatoid arthritis,” Dr. Taylor said. These features were mapped to 929 genes which had some overlap with known blood pathway genes.
“Over and above that, there’s a whole set of these epigenetic features which represent novel pathways and potentially rich hunting ground for therapeutic targets and other translational investigation,” he said.
For seronegative cases, mean AUC was 0.971 (SD, 0.001; 95% CI, 93.8%-99.2%), sensitivity was 83.7% (SD, 2.03; 95% CI, 63.3%-91.8%) and specificity was 95.4% (SD, 0.69; 95% CI, 90.8%-97.5%).
Specificity for RA versus healthy controls was 100 (SD, 0; 95% CI, 94.4-100.0).
Dr. Byram described the study as small but intriguing. He cautioned that “there is always some likelihood that the actual components of the test are just recognizing some combination of things we are already testing in the clinic,” he said. Details about the patients in the study can offer insight into “whether the assay is actually just recognizing something about patients with RA that is truly different, or rather is it recognizing how a common factor among patients with RA is transcribed by the cell.”
Moving forward, “it is important to get a grasp of how these biomarkers might perform in various settings,” he said.
Dr. Taylor did not discuss the potential cost of the assay in his presentation. “Tests like these have to strike a real balance in being useful and cost-effective and, since they are still made by commercial companies with commercial interests, also make a margin for their owner,” Dr. Byram said. “Turnaround time is also an important factor to think about.”
Aqtual funded the study. Dr. Taylor reports consulting for AbbVie, Aqtual, Biogen, Fresenius Kabi, Galapagos, Gilead, GlaxoSmithKline, Janssen, Lilly, Nordic Pharma, Pfizer, Sanofi, and UCB and receiving grant support from Galapagos. The other study authors all have relationships with Aqtual, and some report various other disclosures. Dr. Byram has no disclosures.
SAN DIEGO – Researchers say they’ve developed a novel blood-based assay that can differentiate patients with seropositive or seronegative rheumatoid arthritis from healthy people and those with other inflammatory diseases or osteoarthritis.
While cautioning that the results need to be confirmed, University of Oxford (England) rheumatologist Peter Taylor, PhD, MA, told an audience at the annual meeting of the American College of Rheumatology that the test has an overall mean sensitivity of 90.8% (standard deviation, 0.94%; 95% confidence interval, 83.2%-95.4%) and mean specificity of 96.1% (SD, 0.64%; 95% CI, 92.7%-97.9%). The mean area under the curve (AUC) is 0.991 (SD, 0.001; 95% CI, 97.2%-99.6%).
“That is to say that it can correctly identify 96% of people without rheumatoid arthritis, and it can correctly identify over 90% of those who do have rheumatoid arthritis,” Dr. Taylor said. In the big picture, he said, “we’ve developed a blood-based assay that detects both organ-specific and systemic biological processes in patients with rheumatoid arthritis.”
Rheumatologist Kevin W. Byram, MD, of Vanderbilt University, Nashville, Tenn., who did not take part in the study but is familiar with its findings, noted in an interview that “there is a constant search for biomarkers to help aide in more accurate and faster diagnosis of all rheumatic conditions, including RA.”
He added that “a common clinical scenario for the rheumatologist is a patient presenting with painful and/or swollen joints and other features that might suggest a few different diagnoses. A diagnostic assay like this might help distinguish patients with RA from other inflammatory conditions or non-inflammatory conditions that might mimic RA.”
In his presentation, Dr. Taylor noted that “we haven’t yet integrated precision medicine into routine clinical practice in rheumatology.” While blood-based assays are prevalent in other types of clinical diagnostics, rheumatology relies on synovial biopsies that are “rarely used in routine clinical practice,” he said.
The new test is a “non-invasive DNA capture assay that can identify specific gene expression from synovium-specific signatures in blood plasma of patients with rheumatoid arthritis,” Dr. Taylor said. Specifically, it focuses on the “unique patterns and sizes of cell-free DNA,” he said. “Analysis of [long] fragments has the potential to give us a great deal of information about disease progression, potentially about customizing treatments and even evaluating the effectiveness of therapies.”
For the new study, researchers examined 229 samples from 191 patients, of whom 63.3% were White and 67.9% were female, with a median age of 56. A total of 89 patients with RA provided 89 samples and 102 without RA provided 140 samples, including 29 healthy controls (66 samples) and others with conditions such as psoriatic arthritis, ulcerative colitis, and osteoarthritis.
The machine learning model “identified 3,425 epigenetic features with statistically significant discrimination between the patients with and without rheumatoid arthritis,” Dr. Taylor said. These features were mapped to 929 genes which had some overlap with known blood pathway genes.
“Over and above that, there’s a whole set of these epigenetic features which represent novel pathways and potentially rich hunting ground for therapeutic targets and other translational investigation,” he said.
For seronegative cases, mean AUC was 0.971 (SD, 0.001; 95% CI, 93.8%-99.2%), sensitivity was 83.7% (SD, 2.03; 95% CI, 63.3%-91.8%) and specificity was 95.4% (SD, 0.69; 95% CI, 90.8%-97.5%).
Specificity for RA versus healthy controls was 100 (SD, 0; 95% CI, 94.4-100.0).
Dr. Byram described the study as small but intriguing. He cautioned that “there is always some likelihood that the actual components of the test are just recognizing some combination of things we are already testing in the clinic,” he said. Details about the patients in the study can offer insight into “whether the assay is actually just recognizing something about patients with RA that is truly different, or rather is it recognizing how a common factor among patients with RA is transcribed by the cell.”
Moving forward, “it is important to get a grasp of how these biomarkers might perform in various settings,” he said.
Dr. Taylor did not discuss the potential cost of the assay in his presentation. “Tests like these have to strike a real balance in being useful and cost-effective and, since they are still made by commercial companies with commercial interests, also make a margin for their owner,” Dr. Byram said. “Turnaround time is also an important factor to think about.”
Aqtual funded the study. Dr. Taylor reports consulting for AbbVie, Aqtual, Biogen, Fresenius Kabi, Galapagos, Gilead, GlaxoSmithKline, Janssen, Lilly, Nordic Pharma, Pfizer, Sanofi, and UCB and receiving grant support from Galapagos. The other study authors all have relationships with Aqtual, and some report various other disclosures. Dr. Byram has no disclosures.
SAN DIEGO – Researchers say they’ve developed a novel blood-based assay that can differentiate patients with seropositive or seronegative rheumatoid arthritis from healthy people and those with other inflammatory diseases or osteoarthritis.
While cautioning that the results need to be confirmed, University of Oxford (England) rheumatologist Peter Taylor, PhD, MA, told an audience at the annual meeting of the American College of Rheumatology that the test has an overall mean sensitivity of 90.8% (standard deviation, 0.94%; 95% confidence interval, 83.2%-95.4%) and mean specificity of 96.1% (SD, 0.64%; 95% CI, 92.7%-97.9%). The mean area under the curve (AUC) is 0.991 (SD, 0.001; 95% CI, 97.2%-99.6%).
“That is to say that it can correctly identify 96% of people without rheumatoid arthritis, and it can correctly identify over 90% of those who do have rheumatoid arthritis,” Dr. Taylor said. In the big picture, he said, “we’ve developed a blood-based assay that detects both organ-specific and systemic biological processes in patients with rheumatoid arthritis.”
Rheumatologist Kevin W. Byram, MD, of Vanderbilt University, Nashville, Tenn., who did not take part in the study but is familiar with its findings, noted in an interview that “there is a constant search for biomarkers to help aide in more accurate and faster diagnosis of all rheumatic conditions, including RA.”
He added that “a common clinical scenario for the rheumatologist is a patient presenting with painful and/or swollen joints and other features that might suggest a few different diagnoses. A diagnostic assay like this might help distinguish patients with RA from other inflammatory conditions or non-inflammatory conditions that might mimic RA.”
In his presentation, Dr. Taylor noted that “we haven’t yet integrated precision medicine into routine clinical practice in rheumatology.” While blood-based assays are prevalent in other types of clinical diagnostics, rheumatology relies on synovial biopsies that are “rarely used in routine clinical practice,” he said.
The new test is a “non-invasive DNA capture assay that can identify specific gene expression from synovium-specific signatures in blood plasma of patients with rheumatoid arthritis,” Dr. Taylor said. Specifically, it focuses on the “unique patterns and sizes of cell-free DNA,” he said. “Analysis of [long] fragments has the potential to give us a great deal of information about disease progression, potentially about customizing treatments and even evaluating the effectiveness of therapies.”
For the new study, researchers examined 229 samples from 191 patients, of whom 63.3% were White and 67.9% were female, with a median age of 56. A total of 89 patients with RA provided 89 samples and 102 without RA provided 140 samples, including 29 healthy controls (66 samples) and others with conditions such as psoriatic arthritis, ulcerative colitis, and osteoarthritis.
The machine learning model “identified 3,425 epigenetic features with statistically significant discrimination between the patients with and without rheumatoid arthritis,” Dr. Taylor said. These features were mapped to 929 genes which had some overlap with known blood pathway genes.
“Over and above that, there’s a whole set of these epigenetic features which represent novel pathways and potentially rich hunting ground for therapeutic targets and other translational investigation,” he said.
For seronegative cases, mean AUC was 0.971 (SD, 0.001; 95% CI, 93.8%-99.2%), sensitivity was 83.7% (SD, 2.03; 95% CI, 63.3%-91.8%) and specificity was 95.4% (SD, 0.69; 95% CI, 90.8%-97.5%).
Specificity for RA versus healthy controls was 100 (SD, 0; 95% CI, 94.4-100.0).
Dr. Byram described the study as small but intriguing. He cautioned that “there is always some likelihood that the actual components of the test are just recognizing some combination of things we are already testing in the clinic,” he said. Details about the patients in the study can offer insight into “whether the assay is actually just recognizing something about patients with RA that is truly different, or rather is it recognizing how a common factor among patients with RA is transcribed by the cell.”
Moving forward, “it is important to get a grasp of how these biomarkers might perform in various settings,” he said.
Dr. Taylor did not discuss the potential cost of the assay in his presentation. “Tests like these have to strike a real balance in being useful and cost-effective and, since they are still made by commercial companies with commercial interests, also make a margin for their owner,” Dr. Byram said. “Turnaround time is also an important factor to think about.”
Aqtual funded the study. Dr. Taylor reports consulting for AbbVie, Aqtual, Biogen, Fresenius Kabi, Galapagos, Gilead, GlaxoSmithKline, Janssen, Lilly, Nordic Pharma, Pfizer, Sanofi, and UCB and receiving grant support from Galapagos. The other study authors all have relationships with Aqtual, and some report various other disclosures. Dr. Byram has no disclosures.
AT ACR 2023
Bilateral facial swelling
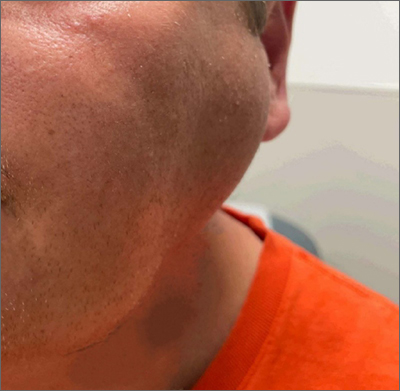
The patient was given a diagnosis of sialadenosis (also known as sialosis), a noninflammatory, non-neoplastic enlargement of the parotid glands. It can often manifest as fatty degeneration of the parotid glands, which may be associated with underlying conditions such as hypertriglyceridemia, diabetes, and metabolic syndrome.1-3
Ultrasonography and a subsequent computed tomography with contrast demonstrated fatty hypertrophy of the parotid glands without any concerning parotid mass or enlarged cervical lymph nodes. No abnormalities of the ductal system (eg, stricture or obstruction with stone) were noted, so sialography and sialendoscopy were not indicated.
Evaluation for inflammatory, autoimmune, and granulomatous diseases was negative, including negative anti-Ro/SSA and anti-La/SSB antibodies and negative HIV screen. However, our patient had an elevated serum triglyceride level of 589 mg/dL (reference range, < 150 mg/dL), while serum total cholesterol was within the reference range (< 200 mg/dL). (Interestingly, his triglycerides were normal a year earlier.) The patient’s A1c level was normal.
The differential diagnosis for this patient included Sjögren syndrome, abscess, viral infection (eg, mumps, HIV sialopathy), Kimura disease, sarcoidosis, masseter hypertrophy, and tumors of the parotid gland (eg, Warthin tumor and pleomorphic adenoma). Drug-induced sialadenitis was another possibility, as several drugs may be associated with salivary gland enlargement.4 However, no association was found for our patient.
Primary management is focused on treating the underlying disorder. The application of heat, massage, and sialagogues (eg, pilocarpine 5 mg orally tid) can be used to stimulate salivation, which may help reduce the swelling. Bilateral parotid gland swelling in patients with increased triglyceride levels often resolves after treatment of hypertriglyceridemia.3,5 Less common modalities include botulinum neurotoxin injection, tympanic neurectomy, and parotidectomy.6
The treatment plan for this patient included aggressive dietary modification and increasing his current dosage of atorvastatin from 20 mg to 80 mg at bedtime. Increasing the dosage of statin was preferred over adding another agent (such as fibrates) to decrease the risk of myopathy. Fine-needle aspiration biopsy may be considered if the swelling does not resolve after correction of lipid abnormalities, which can take between 6 months and 3 years.3
Photo courtesy of Faryal Tahir, MD. Text courtesy of Faryal Tahir, MD, Assistant Professor, and Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University, Homer Stryker, MD School of Medicine, Kalamazoo.
1. Garcia DS, Bussoloti Filho I. Fat deposition of parotid glands. Braz J Otorhinolaryngol. 2013;79:173-176
2. Hida A, Akahoshi M, Takagi Y, et al. Lipid infiltration in the parotid glands: a clinical manifestation of metabolic syndrome. Exp Clin Endocrinol Diabetes. 2012;120:110-115. doi: 10.1055/s-0031-1291315
3. Sheikh JS, Sharma M, Kunath A, et al. Reversible parotid enlargement and pseudo-Sjögren's syndrome secondary to hypertriglyceridemia. J Rheumatol. 1996;23:1288-1291
4. Vinayak V, Annigeri RG, Patel HA, et al. Adverse effects of drugs on saliva and salivary glands. J Orofac Sci. 2013;5:15-20. doi: 10.4103/0975-8844.113684
5. Kaltreider HB, Talal N. Bilateral parotid gland enlargement and hyperlipoproteinemia. JAMA. 1969;210:2067-2070. doi:10.1001/jama.1969.03160370051010
6. Davis AB, Hoffman HT. Management options for sialadenosis. Otolaryngol Clin North Am. 2021;54:605-611. doi: 10.1016/j.otc.2021.02.005

The patient was given a diagnosis of sialadenosis (also known as sialosis), a noninflammatory, non-neoplastic enlargement of the parotid glands. It can often manifest as fatty degeneration of the parotid glands, which may be associated with underlying conditions such as hypertriglyceridemia, diabetes, and metabolic syndrome.1-3
Ultrasonography and a subsequent computed tomography with contrast demonstrated fatty hypertrophy of the parotid glands without any concerning parotid mass or enlarged cervical lymph nodes. No abnormalities of the ductal system (eg, stricture or obstruction with stone) were noted, so sialography and sialendoscopy were not indicated.
Evaluation for inflammatory, autoimmune, and granulomatous diseases was negative, including negative anti-Ro/SSA and anti-La/SSB antibodies and negative HIV screen. However, our patient had an elevated serum triglyceride level of 589 mg/dL (reference range, < 150 mg/dL), while serum total cholesterol was within the reference range (< 200 mg/dL). (Interestingly, his triglycerides were normal a year earlier.) The patient’s A1c level was normal.
The differential diagnosis for this patient included Sjögren syndrome, abscess, viral infection (eg, mumps, HIV sialopathy), Kimura disease, sarcoidosis, masseter hypertrophy, and tumors of the parotid gland (eg, Warthin tumor and pleomorphic adenoma). Drug-induced sialadenitis was another possibility, as several drugs may be associated with salivary gland enlargement.4 However, no association was found for our patient.
Primary management is focused on treating the underlying disorder. The application of heat, massage, and sialagogues (eg, pilocarpine 5 mg orally tid) can be used to stimulate salivation, which may help reduce the swelling. Bilateral parotid gland swelling in patients with increased triglyceride levels often resolves after treatment of hypertriglyceridemia.3,5 Less common modalities include botulinum neurotoxin injection, tympanic neurectomy, and parotidectomy.6
The treatment plan for this patient included aggressive dietary modification and increasing his current dosage of atorvastatin from 20 mg to 80 mg at bedtime. Increasing the dosage of statin was preferred over adding another agent (such as fibrates) to decrease the risk of myopathy. Fine-needle aspiration biopsy may be considered if the swelling does not resolve after correction of lipid abnormalities, which can take between 6 months and 3 years.3
Photo courtesy of Faryal Tahir, MD. Text courtesy of Faryal Tahir, MD, Assistant Professor, and Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University, Homer Stryker, MD School of Medicine, Kalamazoo.

The patient was given a diagnosis of sialadenosis (also known as sialosis), a noninflammatory, non-neoplastic enlargement of the parotid glands. It can often manifest as fatty degeneration of the parotid glands, which may be associated with underlying conditions such as hypertriglyceridemia, diabetes, and metabolic syndrome.1-3
Ultrasonography and a subsequent computed tomography with contrast demonstrated fatty hypertrophy of the parotid glands without any concerning parotid mass or enlarged cervical lymph nodes. No abnormalities of the ductal system (eg, stricture or obstruction with stone) were noted, so sialography and sialendoscopy were not indicated.
Evaluation for inflammatory, autoimmune, and granulomatous diseases was negative, including negative anti-Ro/SSA and anti-La/SSB antibodies and negative HIV screen. However, our patient had an elevated serum triglyceride level of 589 mg/dL (reference range, < 150 mg/dL), while serum total cholesterol was within the reference range (< 200 mg/dL). (Interestingly, his triglycerides were normal a year earlier.) The patient’s A1c level was normal.
The differential diagnosis for this patient included Sjögren syndrome, abscess, viral infection (eg, mumps, HIV sialopathy), Kimura disease, sarcoidosis, masseter hypertrophy, and tumors of the parotid gland (eg, Warthin tumor and pleomorphic adenoma). Drug-induced sialadenitis was another possibility, as several drugs may be associated with salivary gland enlargement.4 However, no association was found for our patient.
Primary management is focused on treating the underlying disorder. The application of heat, massage, and sialagogues (eg, pilocarpine 5 mg orally tid) can be used to stimulate salivation, which may help reduce the swelling. Bilateral parotid gland swelling in patients with increased triglyceride levels often resolves after treatment of hypertriglyceridemia.3,5 Less common modalities include botulinum neurotoxin injection, tympanic neurectomy, and parotidectomy.6
The treatment plan for this patient included aggressive dietary modification and increasing his current dosage of atorvastatin from 20 mg to 80 mg at bedtime. Increasing the dosage of statin was preferred over adding another agent (such as fibrates) to decrease the risk of myopathy. Fine-needle aspiration biopsy may be considered if the swelling does not resolve after correction of lipid abnormalities, which can take between 6 months and 3 years.3
Photo courtesy of Faryal Tahir, MD. Text courtesy of Faryal Tahir, MD, Assistant Professor, and Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University, Homer Stryker, MD School of Medicine, Kalamazoo.
1. Garcia DS, Bussoloti Filho I. Fat deposition of parotid glands. Braz J Otorhinolaryngol. 2013;79:173-176
2. Hida A, Akahoshi M, Takagi Y, et al. Lipid infiltration in the parotid glands: a clinical manifestation of metabolic syndrome. Exp Clin Endocrinol Diabetes. 2012;120:110-115. doi: 10.1055/s-0031-1291315
3. Sheikh JS, Sharma M, Kunath A, et al. Reversible parotid enlargement and pseudo-Sjögren's syndrome secondary to hypertriglyceridemia. J Rheumatol. 1996;23:1288-1291
4. Vinayak V, Annigeri RG, Patel HA, et al. Adverse effects of drugs on saliva and salivary glands. J Orofac Sci. 2013;5:15-20. doi: 10.4103/0975-8844.113684
5. Kaltreider HB, Talal N. Bilateral parotid gland enlargement and hyperlipoproteinemia. JAMA. 1969;210:2067-2070. doi:10.1001/jama.1969.03160370051010
6. Davis AB, Hoffman HT. Management options for sialadenosis. Otolaryngol Clin North Am. 2021;54:605-611. doi: 10.1016/j.otc.2021.02.005
1. Garcia DS, Bussoloti Filho I. Fat deposition of parotid glands. Braz J Otorhinolaryngol. 2013;79:173-176
2. Hida A, Akahoshi M, Takagi Y, et al. Lipid infiltration in the parotid glands: a clinical manifestation of metabolic syndrome. Exp Clin Endocrinol Diabetes. 2012;120:110-115. doi: 10.1055/s-0031-1291315
3. Sheikh JS, Sharma M, Kunath A, et al. Reversible parotid enlargement and pseudo-Sjögren's syndrome secondary to hypertriglyceridemia. J Rheumatol. 1996;23:1288-1291
4. Vinayak V, Annigeri RG, Patel HA, et al. Adverse effects of drugs on saliva and salivary glands. J Orofac Sci. 2013;5:15-20. doi: 10.4103/0975-8844.113684
5. Kaltreider HB, Talal N. Bilateral parotid gland enlargement and hyperlipoproteinemia. JAMA. 1969;210:2067-2070. doi:10.1001/jama.1969.03160370051010
6. Davis AB, Hoffman HT. Management options for sialadenosis. Otolaryngol Clin North Am. 2021;54:605-611. doi: 10.1016/j.otc.2021.02.005
Split-dose methotrexate speeds RA response over single dose
SAN DIEGO – A split dose of methotrexate (MTX) given orally once per week showed significantly higher efficacy in patients with rheumatoid arthritis at 16 weeks, compared with a single MTX dose weekly, according to new research. By 24 weeks, efficacy measures were similar for both groups.
However, fewer patients in the split-dose group needed additional disease-modifying antirheumatic drugs (DMARDs) to control disease activity.
MTX is a highly utilized, inexpensive drug for RA, but only about 30% of patients can achieve low disease activity or remission on MTX monotherapy, said Varun Dhir, MD, MBBS, of the Postgraduate Institute of Medical Education and Research, Chandigarh, India. He co-authored and presented the research at the annual meeting of the American College of Rheumatology.
Part of the problem is that “oral methotrexate absorption from the gut reduces as the doses go up,” Dr. Dhir noted, because the transport mechanism gets saturated. MTX delivered subcutaneously is one way to improve efficacy, but patients can be needle-averse, and in some countries, like India, pre-filled syringes are not available, he said.
There is pharmacokinetic data dating back 20 years that suggest split-dose MTX could be more efficacious. “However, there are no randomized controlled trials to date, and the guidelines therefore are silent on this approach,” Dr. Dhir said.
To address this question, Dr. Dhir and colleagues recruited patients with RA from six centers across India. Patients were aged 18-60 years, seropositive (rheumatoid factor or anti-citrullinated protein antibodies), and had a disease duration of 5 years or fewer. Patients had active disease, defined as at least four tender joints and at least two swollen joints, and were not taking any DMARDs except for hydroxychloroquine and/or low-dose prednisolone.
A total of 253 patients were randomly assigned to a single 25-mg dose or a split-dose of MTX once weekly (10 mg in the morning and 15 mg in the evening on the same day). The primary outcome was a European Alliance of Associations for Rheumatology (EULAR) good response at 24 weeks. At the 16-week mark, if patients had not achieved low disease activity based on a 28-joint Disease Activity Score (DAS28) greater than 3.2, a blinded assessor could add either leflunomide or sulfasalazine to the continued MTX therapy.
At baseline, there was no difference between the groups’ DAS28, but after 16 weeks, DAS28 was significantly lower in the split-dose group, compared with the single-dose group (4.4 vs. 5.1; P < .001), and a higher percentage of patients in the split-dose group had a EULAR good response.
About three-quarters (76.6%) of patients in the split-dose group experienced an improvement of at least 20% in ACR response criteria (ACR20), compared with 52% in the single-dose group. The split-dose group also had higher proportion of patients achieving ACR50 and ACR70.
About one-third of the split-dose group (35%) added an additional DMARD at 16 weeks, compared with 54.5% of the single-dose group (P = .005).
After 24 weeks, DAS28 scores remained lower in the split-dose group (4.1 vs. 4.5; P = .03), but there were no other differences in treatment responses. Health Assessment Questionnaire scores were the same between both groups at 16 and 24 weeks.
The primary outcome was not met, although Dr. Dhir noted a flaw in the study design that could have affected the results. By allowing patients to add additional DMARDs at 16 weeks, “there were two factors which were affecting the primary outcome” at 24 weeks, he told this news organization. “I feel there was a robust result at least at 16 weeks.”
While there were no major adverse events, the split-dose group had higher rates of transaminitis (elevated liver enzymes) during the study, and low white blood cell count was higher in the single-dose group at 24 weeks. There was no difference in MTX intolerance between the two groups.
“It looks like [the split-dose group] gets out of the block faster. It’s a faster effect,” although the other group did catch up, Janet Pope, MD, MPH, of Western University, London, Ont., said in an interview. She was not involved with the research. Two positive results were the earlier ACR responses in the split-dose group as well as fewer patients in that same group needing to add another DMARD to therapy.
“In my opinion, if it’s equal cost, why not try it and see?” she said.
In a separate presentation referring to the abstract, Joan Bathon, MD, director of rheumatology at Columbia University, New York City, noted that these results align with ACR 2021 recommendations. Dr. Bathon was not involved with this study but was on the writing committee establishing those 2021 guidelines.
“The recommendation – with low certainty of evidence – was that for patients who are intolerant to MTX, that split-dose of oral MTX is worth trying before you switch to a different DMARD,” she said. “I think these data support that concept.”
Dr. Dhir and Dr. Bathon had no relevant financial relationships. Dr. Pope disclosed financial relationships with AbbVie/Abbott, Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Fresenius Kabi, GlaxoSmithKline, Janssen, Mallinckrodt, Novartis, Organon, Pfizer, Sandoz, and Viatris.
A version of this article first appeared on Medscape.com.
SAN DIEGO – A split dose of methotrexate (MTX) given orally once per week showed significantly higher efficacy in patients with rheumatoid arthritis at 16 weeks, compared with a single MTX dose weekly, according to new research. By 24 weeks, efficacy measures were similar for both groups.
However, fewer patients in the split-dose group needed additional disease-modifying antirheumatic drugs (DMARDs) to control disease activity.
MTX is a highly utilized, inexpensive drug for RA, but only about 30% of patients can achieve low disease activity or remission on MTX monotherapy, said Varun Dhir, MD, MBBS, of the Postgraduate Institute of Medical Education and Research, Chandigarh, India. He co-authored and presented the research at the annual meeting of the American College of Rheumatology.
Part of the problem is that “oral methotrexate absorption from the gut reduces as the doses go up,” Dr. Dhir noted, because the transport mechanism gets saturated. MTX delivered subcutaneously is one way to improve efficacy, but patients can be needle-averse, and in some countries, like India, pre-filled syringes are not available, he said.
There is pharmacokinetic data dating back 20 years that suggest split-dose MTX could be more efficacious. “However, there are no randomized controlled trials to date, and the guidelines therefore are silent on this approach,” Dr. Dhir said.
To address this question, Dr. Dhir and colleagues recruited patients with RA from six centers across India. Patients were aged 18-60 years, seropositive (rheumatoid factor or anti-citrullinated protein antibodies), and had a disease duration of 5 years or fewer. Patients had active disease, defined as at least four tender joints and at least two swollen joints, and were not taking any DMARDs except for hydroxychloroquine and/or low-dose prednisolone.
A total of 253 patients were randomly assigned to a single 25-mg dose or a split-dose of MTX once weekly (10 mg in the morning and 15 mg in the evening on the same day). The primary outcome was a European Alliance of Associations for Rheumatology (EULAR) good response at 24 weeks. At the 16-week mark, if patients had not achieved low disease activity based on a 28-joint Disease Activity Score (DAS28) greater than 3.2, a blinded assessor could add either leflunomide or sulfasalazine to the continued MTX therapy.
At baseline, there was no difference between the groups’ DAS28, but after 16 weeks, DAS28 was significantly lower in the split-dose group, compared with the single-dose group (4.4 vs. 5.1; P < .001), and a higher percentage of patients in the split-dose group had a EULAR good response.
About three-quarters (76.6%) of patients in the split-dose group experienced an improvement of at least 20% in ACR response criteria (ACR20), compared with 52% in the single-dose group. The split-dose group also had higher proportion of patients achieving ACR50 and ACR70.
About one-third of the split-dose group (35%) added an additional DMARD at 16 weeks, compared with 54.5% of the single-dose group (P = .005).
After 24 weeks, DAS28 scores remained lower in the split-dose group (4.1 vs. 4.5; P = .03), but there were no other differences in treatment responses. Health Assessment Questionnaire scores were the same between both groups at 16 and 24 weeks.
The primary outcome was not met, although Dr. Dhir noted a flaw in the study design that could have affected the results. By allowing patients to add additional DMARDs at 16 weeks, “there were two factors which were affecting the primary outcome” at 24 weeks, he told this news organization. “I feel there was a robust result at least at 16 weeks.”
While there were no major adverse events, the split-dose group had higher rates of transaminitis (elevated liver enzymes) during the study, and low white blood cell count was higher in the single-dose group at 24 weeks. There was no difference in MTX intolerance between the two groups.
“It looks like [the split-dose group] gets out of the block faster. It’s a faster effect,” although the other group did catch up, Janet Pope, MD, MPH, of Western University, London, Ont., said in an interview. She was not involved with the research. Two positive results were the earlier ACR responses in the split-dose group as well as fewer patients in that same group needing to add another DMARD to therapy.
“In my opinion, if it’s equal cost, why not try it and see?” she said.
In a separate presentation referring to the abstract, Joan Bathon, MD, director of rheumatology at Columbia University, New York City, noted that these results align with ACR 2021 recommendations. Dr. Bathon was not involved with this study but was on the writing committee establishing those 2021 guidelines.
“The recommendation – with low certainty of evidence – was that for patients who are intolerant to MTX, that split-dose of oral MTX is worth trying before you switch to a different DMARD,” she said. “I think these data support that concept.”
Dr. Dhir and Dr. Bathon had no relevant financial relationships. Dr. Pope disclosed financial relationships with AbbVie/Abbott, Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Fresenius Kabi, GlaxoSmithKline, Janssen, Mallinckrodt, Novartis, Organon, Pfizer, Sandoz, and Viatris.
A version of this article first appeared on Medscape.com.
SAN DIEGO – A split dose of methotrexate (MTX) given orally once per week showed significantly higher efficacy in patients with rheumatoid arthritis at 16 weeks, compared with a single MTX dose weekly, according to new research. By 24 weeks, efficacy measures were similar for both groups.
However, fewer patients in the split-dose group needed additional disease-modifying antirheumatic drugs (DMARDs) to control disease activity.
MTX is a highly utilized, inexpensive drug for RA, but only about 30% of patients can achieve low disease activity or remission on MTX monotherapy, said Varun Dhir, MD, MBBS, of the Postgraduate Institute of Medical Education and Research, Chandigarh, India. He co-authored and presented the research at the annual meeting of the American College of Rheumatology.
Part of the problem is that “oral methotrexate absorption from the gut reduces as the doses go up,” Dr. Dhir noted, because the transport mechanism gets saturated. MTX delivered subcutaneously is one way to improve efficacy, but patients can be needle-averse, and in some countries, like India, pre-filled syringes are not available, he said.
There is pharmacokinetic data dating back 20 years that suggest split-dose MTX could be more efficacious. “However, there are no randomized controlled trials to date, and the guidelines therefore are silent on this approach,” Dr. Dhir said.
To address this question, Dr. Dhir and colleagues recruited patients with RA from six centers across India. Patients were aged 18-60 years, seropositive (rheumatoid factor or anti-citrullinated protein antibodies), and had a disease duration of 5 years or fewer. Patients had active disease, defined as at least four tender joints and at least two swollen joints, and were not taking any DMARDs except for hydroxychloroquine and/or low-dose prednisolone.
A total of 253 patients were randomly assigned to a single 25-mg dose or a split-dose of MTX once weekly (10 mg in the morning and 15 mg in the evening on the same day). The primary outcome was a European Alliance of Associations for Rheumatology (EULAR) good response at 24 weeks. At the 16-week mark, if patients had not achieved low disease activity based on a 28-joint Disease Activity Score (DAS28) greater than 3.2, a blinded assessor could add either leflunomide or sulfasalazine to the continued MTX therapy.
At baseline, there was no difference between the groups’ DAS28, but after 16 weeks, DAS28 was significantly lower in the split-dose group, compared with the single-dose group (4.4 vs. 5.1; P < .001), and a higher percentage of patients in the split-dose group had a EULAR good response.
About three-quarters (76.6%) of patients in the split-dose group experienced an improvement of at least 20% in ACR response criteria (ACR20), compared with 52% in the single-dose group. The split-dose group also had higher proportion of patients achieving ACR50 and ACR70.
About one-third of the split-dose group (35%) added an additional DMARD at 16 weeks, compared with 54.5% of the single-dose group (P = .005).
After 24 weeks, DAS28 scores remained lower in the split-dose group (4.1 vs. 4.5; P = .03), but there were no other differences in treatment responses. Health Assessment Questionnaire scores were the same between both groups at 16 and 24 weeks.
The primary outcome was not met, although Dr. Dhir noted a flaw in the study design that could have affected the results. By allowing patients to add additional DMARDs at 16 weeks, “there were two factors which were affecting the primary outcome” at 24 weeks, he told this news organization. “I feel there was a robust result at least at 16 weeks.”
While there were no major adverse events, the split-dose group had higher rates of transaminitis (elevated liver enzymes) during the study, and low white blood cell count was higher in the single-dose group at 24 weeks. There was no difference in MTX intolerance between the two groups.
“It looks like [the split-dose group] gets out of the block faster. It’s a faster effect,” although the other group did catch up, Janet Pope, MD, MPH, of Western University, London, Ont., said in an interview. She was not involved with the research. Two positive results were the earlier ACR responses in the split-dose group as well as fewer patients in that same group needing to add another DMARD to therapy.
“In my opinion, if it’s equal cost, why not try it and see?” she said.
In a separate presentation referring to the abstract, Joan Bathon, MD, director of rheumatology at Columbia University, New York City, noted that these results align with ACR 2021 recommendations. Dr. Bathon was not involved with this study but was on the writing committee establishing those 2021 guidelines.
“The recommendation – with low certainty of evidence – was that for patients who are intolerant to MTX, that split-dose of oral MTX is worth trying before you switch to a different DMARD,” she said. “I think these data support that concept.”
Dr. Dhir and Dr. Bathon had no relevant financial relationships. Dr. Pope disclosed financial relationships with AbbVie/Abbott, Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Fresenius Kabi, GlaxoSmithKline, Janssen, Mallinckrodt, Novartis, Organon, Pfizer, Sandoz, and Viatris.
A version of this article first appeared on Medscape.com.
AT ACR 2023
Lebrikizumab gets European nod for treating moderate-to-severe atopic dermatitis
The , according to a press release from the manufacturer.
Lebrikizumab, which selectively targets interleukin-13 and inhibits its signaling pathway, will first be available in Germany, with a rollout in other European countries expected through 2024, according to Almirall, the manufacturer.
The European approval of lebrikizumab (Ebglyss) was based on data from a trio of pivotal phase 3 studies including ADvocate1 and ADvocate2, which evaluated lebrikizumab as monotherapy, and ADhere, which evaluated lebrikizumab in combination with topical corticosteroids. All three trials included adult and adolescent patients aged 12 years and older with moderate-to-severe AD.
In the two ADvocate studies, published in the New England Journal of Medicine, participants were randomized to a 250-mg injection of lebrikizumab or placebo every 2 weeks. The primary outcome was a score of clear or almost clear skin based on the Investigator’s Global Assessment with at least a 2-point reduction from baseline to 16 weeks.
Compared with placebo, lebrikizumab showed significant clinical efficacy in both studies. In study 1, 43.1% of 283 patients treated with lebrikizumab versus 12.7% of 141 patients on placebo met the primary endpoint (P < .001), as did 33.2% of the 281 patients on lebrikizumab and 10.8% of 146 patients on placebo in study 2 (P < .001). In addition, 58.8% and 52.1% of patients on lebrikizumab in studies 1 and 2, respectively, met the secondary endpoint of a 75% reduction in the Eczema Area and Severity Index score (EASI-75), versus 16.2% and 18.1% of patients on placebo in study 1 and 2, respectively (P < .001 for both).
In the ADhere study, published in JAMA Dermatology, 41.2% of patients receiving a lebrikizumab/corticosteroid combination and 22.1% of those randomized to a placebo/corticosteroid combination met the primary endpoint of IGA scores of 0 or 1 at 16 weeks, and nearly 70% patients treated with a combination of lebrikizumab and topical corticosteroids achieved EASI-75, compared with 42% of those on the combination.
Nearly 80% of patients who responded at 16 weeks and continued treatment with lebrikizumab as monotherapy or combination therapy showed sustained results up to 52 weeks with maintenance monthly dosing, according to the Almirall press release.
Most adverse events across the studies were mild or moderate and were not associated with treatment discontinuation. The most common adverse reactions were conjunctivitis, injection site reactions, allergic conjunctivitis, and dry eye.
Further research has shown showed clinical efficacy and safety in patients who used lebrikizumab for up to 2 years, either as monotherapy or in combination with topical corticosteroids, according to the manufacturer.
Lebrikizumab remains under review in the United States after the Food and Drug Administration issued a complete response letter in October regarding findings made during an inspection of a third-party contract manufacturer that included the “monoclonal antibody drug substance” for lebrikizumab, although no concerns about clinical data or safety were raised, Eli Lilly announced in October. Eli Lilly has the rights to develop lebrikizumab in the United States and the rest of the world excluding Europe.
The , according to a press release from the manufacturer.
Lebrikizumab, which selectively targets interleukin-13 and inhibits its signaling pathway, will first be available in Germany, with a rollout in other European countries expected through 2024, according to Almirall, the manufacturer.
The European approval of lebrikizumab (Ebglyss) was based on data from a trio of pivotal phase 3 studies including ADvocate1 and ADvocate2, which evaluated lebrikizumab as monotherapy, and ADhere, which evaluated lebrikizumab in combination with topical corticosteroids. All three trials included adult and adolescent patients aged 12 years and older with moderate-to-severe AD.
In the two ADvocate studies, published in the New England Journal of Medicine, participants were randomized to a 250-mg injection of lebrikizumab or placebo every 2 weeks. The primary outcome was a score of clear or almost clear skin based on the Investigator’s Global Assessment with at least a 2-point reduction from baseline to 16 weeks.
Compared with placebo, lebrikizumab showed significant clinical efficacy in both studies. In study 1, 43.1% of 283 patients treated with lebrikizumab versus 12.7% of 141 patients on placebo met the primary endpoint (P < .001), as did 33.2% of the 281 patients on lebrikizumab and 10.8% of 146 patients on placebo in study 2 (P < .001). In addition, 58.8% and 52.1% of patients on lebrikizumab in studies 1 and 2, respectively, met the secondary endpoint of a 75% reduction in the Eczema Area and Severity Index score (EASI-75), versus 16.2% and 18.1% of patients on placebo in study 1 and 2, respectively (P < .001 for both).
In the ADhere study, published in JAMA Dermatology, 41.2% of patients receiving a lebrikizumab/corticosteroid combination and 22.1% of those randomized to a placebo/corticosteroid combination met the primary endpoint of IGA scores of 0 or 1 at 16 weeks, and nearly 70% patients treated with a combination of lebrikizumab and topical corticosteroids achieved EASI-75, compared with 42% of those on the combination.
Nearly 80% of patients who responded at 16 weeks and continued treatment with lebrikizumab as monotherapy or combination therapy showed sustained results up to 52 weeks with maintenance monthly dosing, according to the Almirall press release.
Most adverse events across the studies were mild or moderate and were not associated with treatment discontinuation. The most common adverse reactions were conjunctivitis, injection site reactions, allergic conjunctivitis, and dry eye.
Further research has shown showed clinical efficacy and safety in patients who used lebrikizumab for up to 2 years, either as monotherapy or in combination with topical corticosteroids, according to the manufacturer.
Lebrikizumab remains under review in the United States after the Food and Drug Administration issued a complete response letter in October regarding findings made during an inspection of a third-party contract manufacturer that included the “monoclonal antibody drug substance” for lebrikizumab, although no concerns about clinical data or safety were raised, Eli Lilly announced in October. Eli Lilly has the rights to develop lebrikizumab in the United States and the rest of the world excluding Europe.
The , according to a press release from the manufacturer.
Lebrikizumab, which selectively targets interleukin-13 and inhibits its signaling pathway, will first be available in Germany, with a rollout in other European countries expected through 2024, according to Almirall, the manufacturer.
The European approval of lebrikizumab (Ebglyss) was based on data from a trio of pivotal phase 3 studies including ADvocate1 and ADvocate2, which evaluated lebrikizumab as monotherapy, and ADhere, which evaluated lebrikizumab in combination with topical corticosteroids. All three trials included adult and adolescent patients aged 12 years and older with moderate-to-severe AD.
In the two ADvocate studies, published in the New England Journal of Medicine, participants were randomized to a 250-mg injection of lebrikizumab or placebo every 2 weeks. The primary outcome was a score of clear or almost clear skin based on the Investigator’s Global Assessment with at least a 2-point reduction from baseline to 16 weeks.
Compared with placebo, lebrikizumab showed significant clinical efficacy in both studies. In study 1, 43.1% of 283 patients treated with lebrikizumab versus 12.7% of 141 patients on placebo met the primary endpoint (P < .001), as did 33.2% of the 281 patients on lebrikizumab and 10.8% of 146 patients on placebo in study 2 (P < .001). In addition, 58.8% and 52.1% of patients on lebrikizumab in studies 1 and 2, respectively, met the secondary endpoint of a 75% reduction in the Eczema Area and Severity Index score (EASI-75), versus 16.2% and 18.1% of patients on placebo in study 1 and 2, respectively (P < .001 for both).
In the ADhere study, published in JAMA Dermatology, 41.2% of patients receiving a lebrikizumab/corticosteroid combination and 22.1% of those randomized to a placebo/corticosteroid combination met the primary endpoint of IGA scores of 0 or 1 at 16 weeks, and nearly 70% patients treated with a combination of lebrikizumab and topical corticosteroids achieved EASI-75, compared with 42% of those on the combination.
Nearly 80% of patients who responded at 16 weeks and continued treatment with lebrikizumab as monotherapy or combination therapy showed sustained results up to 52 weeks with maintenance monthly dosing, according to the Almirall press release.
Most adverse events across the studies were mild or moderate and were not associated with treatment discontinuation. The most common adverse reactions were conjunctivitis, injection site reactions, allergic conjunctivitis, and dry eye.
Further research has shown showed clinical efficacy and safety in patients who used lebrikizumab for up to 2 years, either as monotherapy or in combination with topical corticosteroids, according to the manufacturer.
Lebrikizumab remains under review in the United States after the Food and Drug Administration issued a complete response letter in October regarding findings made during an inspection of a third-party contract manufacturer that included the “monoclonal antibody drug substance” for lebrikizumab, although no concerns about clinical data or safety were raised, Eli Lilly announced in October. Eli Lilly has the rights to develop lebrikizumab in the United States and the rest of the world excluding Europe.
Can a Mediterranean diet reduce breast cancer recurrence?
TOPLINE:
However, women at high risk for recurrence who made the greatest improvements in their diet quality demonstrated a 41% lower risk for recurrence, compared with peers who made the fewest improvements.
METHODOLOGY:
- A growing body of evidence suggests that a better dietary quality may improve survival among patients with breast cancer, but whether diet impacts breast cancer–specific mortality remains controversial.
- To better understand the relationship between diet and breast cancer outcomes, investigators recruited 1,542 women with breast cancer who had undergone surgical resection in the past 5 years and were considered high risk for recurrence.
- All women received general recommendations for cancer prevention, while the intervention group received active support to adhere to a macro–Mediterranean-style diet, which encourages mainly consuming whole grains, legumes, and high-fiber vegetables and discourages eating foods high in saturated and trans fats, processed meats, and foods and beverages high in sugar.
- Diet was assessed at baseline, 1 year, and every few months in subsequent years via food frequency diaries. Compliance with dietary recommendations for the whole cohort was assessed using a dietary index developed for the trial.
- In addition to diet, women in the diet intervention group were encouraged to maintain moderate to intense physical activity – 30 minutes, on average, each day – and received pedometers to track steps, aiming for 10,000 per day.
TAKEAWAY:
- Over 5 years of follow-up, the rate of breast cancer recurrence did not differ between women in the diet intervention group and those in the control group. Overall, 95 of 769 women in the intervention group and 98 of 773 in the control group had a breast cancer recurrence (hazard ratio, 0.99).
- When evaluating outcomes in the entire cohort, looking at everyone’s level of compliance with dietary recommendations, women who adhered the most to the dietary guidelines had a 41% lower recurrence risk compared with women who adhered the least (HR, 0.59).
- The greatest protective effect among women who demonstrated high compliance occurred in those with ER-positive cancers (HR, 0.42) and those with ER-positive cancers who received tamoxifen (HR, 0.30).
IN PRACTICE:
This intervention trial “did not confirm the hypothesis that a comprehensive dietary modification reduces breast cancer recurrence and metastases,” but when looking at compliance to the Mediterranean diet overall, the analysis did find “a significantly better prognosis” for women with the best adherence.
SOURCE:
The study, with first author Franco Berrino, MD, PhD, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, was published online in Clinical Cancer Research.
LIMITATIONS:
The study relied on self-reported dietary data. No dietary instrument was used to estimate nutrient intake and the dietary index developed for the trial remains unvalidated.
DISCLOSURES:
The study was supported by the Italian Department of Health, the Associazione Italiana per la Ricerca sul Cancro, and the Vita e Salute Foundation. The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
However, women at high risk for recurrence who made the greatest improvements in their diet quality demonstrated a 41% lower risk for recurrence, compared with peers who made the fewest improvements.
METHODOLOGY:
- A growing body of evidence suggests that a better dietary quality may improve survival among patients with breast cancer, but whether diet impacts breast cancer–specific mortality remains controversial.
- To better understand the relationship between diet and breast cancer outcomes, investigators recruited 1,542 women with breast cancer who had undergone surgical resection in the past 5 years and were considered high risk for recurrence.
- All women received general recommendations for cancer prevention, while the intervention group received active support to adhere to a macro–Mediterranean-style diet, which encourages mainly consuming whole grains, legumes, and high-fiber vegetables and discourages eating foods high in saturated and trans fats, processed meats, and foods and beverages high in sugar.
- Diet was assessed at baseline, 1 year, and every few months in subsequent years via food frequency diaries. Compliance with dietary recommendations for the whole cohort was assessed using a dietary index developed for the trial.
- In addition to diet, women in the diet intervention group were encouraged to maintain moderate to intense physical activity – 30 minutes, on average, each day – and received pedometers to track steps, aiming for 10,000 per day.
TAKEAWAY:
- Over 5 years of follow-up, the rate of breast cancer recurrence did not differ between women in the diet intervention group and those in the control group. Overall, 95 of 769 women in the intervention group and 98 of 773 in the control group had a breast cancer recurrence (hazard ratio, 0.99).
- When evaluating outcomes in the entire cohort, looking at everyone’s level of compliance with dietary recommendations, women who adhered the most to the dietary guidelines had a 41% lower recurrence risk compared with women who adhered the least (HR, 0.59).
- The greatest protective effect among women who demonstrated high compliance occurred in those with ER-positive cancers (HR, 0.42) and those with ER-positive cancers who received tamoxifen (HR, 0.30).
IN PRACTICE:
This intervention trial “did not confirm the hypothesis that a comprehensive dietary modification reduces breast cancer recurrence and metastases,” but when looking at compliance to the Mediterranean diet overall, the analysis did find “a significantly better prognosis” for women with the best adherence.
SOURCE:
The study, with first author Franco Berrino, MD, PhD, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, was published online in Clinical Cancer Research.
LIMITATIONS:
The study relied on self-reported dietary data. No dietary instrument was used to estimate nutrient intake and the dietary index developed for the trial remains unvalidated.
DISCLOSURES:
The study was supported by the Italian Department of Health, the Associazione Italiana per la Ricerca sul Cancro, and the Vita e Salute Foundation. The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
However, women at high risk for recurrence who made the greatest improvements in their diet quality demonstrated a 41% lower risk for recurrence, compared with peers who made the fewest improvements.
METHODOLOGY:
- A growing body of evidence suggests that a better dietary quality may improve survival among patients with breast cancer, but whether diet impacts breast cancer–specific mortality remains controversial.
- To better understand the relationship between diet and breast cancer outcomes, investigators recruited 1,542 women with breast cancer who had undergone surgical resection in the past 5 years and were considered high risk for recurrence.
- All women received general recommendations for cancer prevention, while the intervention group received active support to adhere to a macro–Mediterranean-style diet, which encourages mainly consuming whole grains, legumes, and high-fiber vegetables and discourages eating foods high in saturated and trans fats, processed meats, and foods and beverages high in sugar.
- Diet was assessed at baseline, 1 year, and every few months in subsequent years via food frequency diaries. Compliance with dietary recommendations for the whole cohort was assessed using a dietary index developed for the trial.
- In addition to diet, women in the diet intervention group were encouraged to maintain moderate to intense physical activity – 30 minutes, on average, each day – and received pedometers to track steps, aiming for 10,000 per day.
TAKEAWAY:
- Over 5 years of follow-up, the rate of breast cancer recurrence did not differ between women in the diet intervention group and those in the control group. Overall, 95 of 769 women in the intervention group and 98 of 773 in the control group had a breast cancer recurrence (hazard ratio, 0.99).
- When evaluating outcomes in the entire cohort, looking at everyone’s level of compliance with dietary recommendations, women who adhered the most to the dietary guidelines had a 41% lower recurrence risk compared with women who adhered the least (HR, 0.59).
- The greatest protective effect among women who demonstrated high compliance occurred in those with ER-positive cancers (HR, 0.42) and those with ER-positive cancers who received tamoxifen (HR, 0.30).
IN PRACTICE:
This intervention trial “did not confirm the hypothesis that a comprehensive dietary modification reduces breast cancer recurrence and metastases,” but when looking at compliance to the Mediterranean diet overall, the analysis did find “a significantly better prognosis” for women with the best adherence.
SOURCE:
The study, with first author Franco Berrino, MD, PhD, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, was published online in Clinical Cancer Research.
LIMITATIONS:
The study relied on self-reported dietary data. No dietary instrument was used to estimate nutrient intake and the dietary index developed for the trial remains unvalidated.
DISCLOSURES:
The study was supported by the Italian Department of Health, the Associazione Italiana per la Ricerca sul Cancro, and the Vita e Salute Foundation. The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FDA OKs capivasertib for certain advanced breast cancers
Specifically, the first-in-class AKT kinase inhibitor approval is for patients with one or more PIK3CA/AKT1/PTEN alterations, as detected by an FDA-approved test, whose metastatic disease progressed on at least one endocrine-based regimen or who experienced recurrence on or within 12 months of completing adjuvant therapy, according to the FDA approval announcement.
The FDA also approved a companion diagnostic device, the FoundationOne CDx assay, to identify patients who are eligible for treatment with capivasertib.
Approval of capivasertib was based on findings from the randomized, placebo-controlled, phase 3 CAPItello-291 trial, which involved 708 patients with locally advanced or metastatic HR-positive, HER2-negative breast cancer, including 289 whose tumors had PIK3CA/AKT1/PTEN alterations. All had progressed on aromatase inhibitor–based treatment and may have received up to two prior lines of endocrine therapy and up to one line of chemotherapy.
Patients were randomized to either 400 mg of oral capivasertib or placebo twice daily for 4 days, followed by 3 days off treatment each week over a 28-day treatment cycle. Patients in both arms received 500 mg intramuscular fulvestrant on cycle 1 days 1 and 15, and then every 28 days thereafter. Treatment continued until disease progression or unacceptable toxicity.
In the 289 patients with PIK3CA/AKT1/PTEN–altered tumors, median progression-free survival (PFS) in the capivasertib arm was 7.3 months versus 3.1 months in the placebo group (hazard ratio, 0.50).
An exploratory analysis of PFS in the 313 (44%) patients whose tumors did not have a PIK3CA/AKT1/PTEN alteration demonstrated a less notable benefit to the combination (HR, 0.79; 95% confidence interval, 0.61-1.02), indicating that “the difference in the overall population was primarily attributed to the results seen in the population of patients whose tumors have PIK3CA/AKT1/PTEN alteration,” the FDA explained.
Adverse reactions occurring in at least 20% of patients included decreased lymphocytes, leukocytes, hemoglobin, and neutrophils; increased fasting glucose, creatinine, and triglycerides; and diarrhea, nausea, fatigue, vomiting, and stomatitis.
The recommended capivasertib dose is 400 mg orally twice daily, given about 12 hours apart with or without food, for 4 days followed by 3 off days until disease progression or unacceptable toxicity, according to the prescribing information.
A version of this article first appeared on Medscape.com.
Specifically, the first-in-class AKT kinase inhibitor approval is for patients with one or more PIK3CA/AKT1/PTEN alterations, as detected by an FDA-approved test, whose metastatic disease progressed on at least one endocrine-based regimen or who experienced recurrence on or within 12 months of completing adjuvant therapy, according to the FDA approval announcement.
The FDA also approved a companion diagnostic device, the FoundationOne CDx assay, to identify patients who are eligible for treatment with capivasertib.
Approval of capivasertib was based on findings from the randomized, placebo-controlled, phase 3 CAPItello-291 trial, which involved 708 patients with locally advanced or metastatic HR-positive, HER2-negative breast cancer, including 289 whose tumors had PIK3CA/AKT1/PTEN alterations. All had progressed on aromatase inhibitor–based treatment and may have received up to two prior lines of endocrine therapy and up to one line of chemotherapy.
Patients were randomized to either 400 mg of oral capivasertib or placebo twice daily for 4 days, followed by 3 days off treatment each week over a 28-day treatment cycle. Patients in both arms received 500 mg intramuscular fulvestrant on cycle 1 days 1 and 15, and then every 28 days thereafter. Treatment continued until disease progression or unacceptable toxicity.
In the 289 patients with PIK3CA/AKT1/PTEN–altered tumors, median progression-free survival (PFS) in the capivasertib arm was 7.3 months versus 3.1 months in the placebo group (hazard ratio, 0.50).
An exploratory analysis of PFS in the 313 (44%) patients whose tumors did not have a PIK3CA/AKT1/PTEN alteration demonstrated a less notable benefit to the combination (HR, 0.79; 95% confidence interval, 0.61-1.02), indicating that “the difference in the overall population was primarily attributed to the results seen in the population of patients whose tumors have PIK3CA/AKT1/PTEN alteration,” the FDA explained.
Adverse reactions occurring in at least 20% of patients included decreased lymphocytes, leukocytes, hemoglobin, and neutrophils; increased fasting glucose, creatinine, and triglycerides; and diarrhea, nausea, fatigue, vomiting, and stomatitis.
The recommended capivasertib dose is 400 mg orally twice daily, given about 12 hours apart with or without food, for 4 days followed by 3 off days until disease progression or unacceptable toxicity, according to the prescribing information.
A version of this article first appeared on Medscape.com.
Specifically, the first-in-class AKT kinase inhibitor approval is for patients with one or more PIK3CA/AKT1/PTEN alterations, as detected by an FDA-approved test, whose metastatic disease progressed on at least one endocrine-based regimen or who experienced recurrence on or within 12 months of completing adjuvant therapy, according to the FDA approval announcement.
The FDA also approved a companion diagnostic device, the FoundationOne CDx assay, to identify patients who are eligible for treatment with capivasertib.
Approval of capivasertib was based on findings from the randomized, placebo-controlled, phase 3 CAPItello-291 trial, which involved 708 patients with locally advanced or metastatic HR-positive, HER2-negative breast cancer, including 289 whose tumors had PIK3CA/AKT1/PTEN alterations. All had progressed on aromatase inhibitor–based treatment and may have received up to two prior lines of endocrine therapy and up to one line of chemotherapy.
Patients were randomized to either 400 mg of oral capivasertib or placebo twice daily for 4 days, followed by 3 days off treatment each week over a 28-day treatment cycle. Patients in both arms received 500 mg intramuscular fulvestrant on cycle 1 days 1 and 15, and then every 28 days thereafter. Treatment continued until disease progression or unacceptable toxicity.
In the 289 patients with PIK3CA/AKT1/PTEN–altered tumors, median progression-free survival (PFS) in the capivasertib arm was 7.3 months versus 3.1 months in the placebo group (hazard ratio, 0.50).
An exploratory analysis of PFS in the 313 (44%) patients whose tumors did not have a PIK3CA/AKT1/PTEN alteration demonstrated a less notable benefit to the combination (HR, 0.79; 95% confidence interval, 0.61-1.02), indicating that “the difference in the overall population was primarily attributed to the results seen in the population of patients whose tumors have PIK3CA/AKT1/PTEN alteration,” the FDA explained.
Adverse reactions occurring in at least 20% of patients included decreased lymphocytes, leukocytes, hemoglobin, and neutrophils; increased fasting glucose, creatinine, and triglycerides; and diarrhea, nausea, fatigue, vomiting, and stomatitis.
The recommended capivasertib dose is 400 mg orally twice daily, given about 12 hours apart with or without food, for 4 days followed by 3 off days until disease progression or unacceptable toxicity, according to the prescribing information.
A version of this article first appeared on Medscape.com.
Compensation is key to fixing primary care shortage
Money talks.
The United States faces a serious shortage of primary care physicians for many reasons, but one, in particular, is inescapable: compensation.
Substantial disparities between what primary care physicians earn relative to specialists like orthopedists and cardiologists can weigh into medical students’ decisions about which field to choose. Plus, the system that Medicare and other health plans use to pay doctors generally places more value on doing procedures like replacing a knee or inserting a stent than on delivering the whole-person, long-term health care management that primary care physicians provide.
As a result of those pay disparities, and the punishing workload typically faced by primary care physicians, more new doctors are becoming specialists, often leaving patients with fewer choices for primary care.
“There is a public out there that is dissatisfied with the lack of access to a routine source of care,” said Christopher Koller, president of the Milbank Memorial Fund, a foundation that focuses on improving population health and health equity. “That’s not going to be addressed until we pay for it.”
Primary care is the foundation of our health care system, the only area in which providing more services – such as childhood vaccines and regular blood pressure screenings – is linked to better population health and more equitable outcomes, according to the National Academies of Sciences, Engineering, and Medicine, in a recently published report on how to rebuild primary care. Without it, the national academies wrote, “minor health problems can spiral into chronic disease,” with poor disease management, ED overuse, and unsustainable costs. Yet for decades, the United States has underinvested in primary care. It accounted for less than 5% of health care spending in 2020 – significantly less than the average spending by countries that are members of the Organization for Economic Cooperation and Development, according to the report.
A $26 billion piece of bipartisan legislation proposed last month by Sen. Bernie Sanders (I-Vt.), chair of the Senate Health, Education, Labor, and Pensions Committee, and Sen. Roger Marshall (R-Kan.) would bolster primary care by increasing training opportunities for doctors and nurses and expanding access to community health centers. Policy experts say the bill would provide important support, but it’s not enough. It doesn’t touch compensation.
“We need primary care to be paid differently and to be paid more, and that starts with Medicare,” Mr. Koller said.
How Medicare drives payment
Medicare, which covers 65 million people who are 65 and older or who have certain long-term disabilities, finances more than a fifth of all health care spending — giving it significant muscle in the health care market. Private health plans typically base their payment amounts on the Medicare system, so what Medicare pays is crucial.
Under the Medicare payment system, the amount the program pays for a medical service is determined by three geographically weighted components: a physician’s work, including time and intensity; the practice’s expense, such as overhead and equipment; and professional insurance. It tends to reward specialties that emphasize procedures, such as repairing a hernia or removing a tumor, more than primary care, where the focus is on talking with patients, answering questions, and educating them about managing their chronic conditions.
Medical students may not be familiar with the particulars of how the payment system works, but their clinical training exposes them to a punishing workload and burnout that is contributing to the shortage of primary care physicians, projected to reach up to 48,000 by 2034, according to estimates from the Association of American Medical Colleges.
The earnings differential between primary care and other specialists is also not lost on them. Average annual compensation for doctors who focus on primary care – family medicine, internists, and pediatricians – ranges from an average of about $250,000 to $275,000, according to Medscape’s annual physician compensation report. Many specialists make more than twice as much: Plastic surgeons top the compensation list at $619,000 annually, followed by orthopedists ($573,000) and cardiologists ($507,000).
“I think the major issues in terms of the primary care physician pipeline are the compensation and the work of primary care,” said Russ Phillips, MD, an internist and the director of the Harvard Medical School Center for Primary Care, Boston. “You have to really want to be a primary care physician when that student will make one-third of what students going into dermatology will make.”
According to statistics from the National Resident Matching Program, which tracks the number of residency slots available for graduating medical students and the number of slots filled, 89% of 5,088 family medicine residency slots were filled in 2023, compared with a 93% residency fill rate overall. Internists had a higher fill rate, 96%, but a significant proportion of internal medicine residents eventually practice in a specialty area rather than in primary care.
No one would claim that doctors are poorly paid, but with the average medical student graduating with just over $200,000 in medical school debt, making a good salary matters.
Not in it for the money
Still, it’s a misperception that student debt always drives the decision whether to go into primary care, said Len Marquez, senior director of government relations and legislative advocacy at the Association of American Medical Colleges.
For Anitza Quintero, 24, a second-year medical student at the Geisinger Commonwealth School of Medicine in rural Pennsylvania, primary care is a logical extension of her interest in helping children and immigrants. Ms. Quintero’s family came to the United States on a raft from Cuba before she was born. She plans to focus on internal medicine and pediatrics.
“I want to keep going to help my family and other families,” Ms. Quintero said. “There’s obviously something attractive about having a specialty and a high pay grade,” Still, she wants to work “where the whole body is involved,” she said, adding that long-term doctor-patient relationships are “also attractive.”
Ms. Quintero is part of the Abigail Geisinger Scholars Program, which aims to recruit primary care physicians and psychiatrists to the rural health system in part with a promise of medical school loan forgiveness. Health care shortages tend to be more acute in rural areas.
These students’ education costs are covered, and they receive a $2,000 monthly stipend. They can do their residency elsewhere, but upon completing it they return to Geisinger for a primary care job with the health care system. Every year of work there erases one year of the debt covered by their award. If they don’t take a job with the health care system, they must repay the amount they received.
Payment imbalances a source of tension
In recent years, the Centers for Medicare & Medicaid Services, which administers the Medicare program, has made changes to address some of the payment imbalances between primary care and specialist services. The agency has expanded the office visit services for which providers can bill to manage their patients, including adding nonprocedural billing codes for providing transitional care, chronic care management, and advance care planning.
In 2024’s final physician fee schedule, the agency plans to allow another new code to take effect, G2211. It would let physicians bill for complex patient evaluation and management services. Any physician could use the code, but it is expected that primary care physicians would use it more frequently than specialists. Congress has delayed implementation of the code since 2021.
The new code is a tiny piece of overall payment reform, “but it is critically important, and it is our top priority on the Hill right now,” said Shari Erickson, chief advocacy officer for the American College of Physicians.
It also triggered a tussle that highlights ongoing tension in Medicare physician payment rules.
The American College of Surgeons and 18 other specialty groups published a statement describing the new code as “unnecessary.” They oppose its implementation because it would primarily benefit primary care providers who, they say, already have the flexibility to bill more for more complex visits.
But the real issue is that, under federal law, changes to Medicare physician payments must preserve budget neutrality, a zero-sum arrangement in which payment increases for primary care providers mean payment decreases elsewhere.
“If they want to keep it, they need to pay for it,” said Christian Shalgian, director of the division of advocacy and health policy for the American College of Surgeons, noting that his organization will continue to oppose implementation otherwise.
Still, there’s general agreement that strengthening the primary care system through payment reform won’t be accomplished by tinkering with billing codes.
The current fee-for-service system doesn’t fully accommodate the time and effort primary care physicians put into “small-ticket” activities like emails and phone calls, reviews of lab results, and consultation reports. A better arrangement, they say, would be to pay primary care physicians a set monthly amount per patient to provide all their care, a system called capitation.
“We’re much better off paying on a per capita basis, get that monthly payment paid in advance plus some extra amount for other things,” said Paul Ginsburg, PhD, a senior fellow at the University of Southern California Schaeffer Center for Health Policy and Economics and former commissioner of the Medicare Payment Advisory Commission.
But if adding a single five-character code to Medicare’s payment rules has proved challenging, imagine the heavy lift involved in overhauling the program’s entire physician payment system. MedPAC and the national academies, both of which provide advice to Congress, have weighed in on the broad outlines of what such a transformation might look like. And there are targeted efforts in Congress: For instance, a bill that would add an annual inflation update to Medicare physician payments and a proposal to address budget neutrality. But it’s unclear whether lawmakers have strong interest in taking action.
“The fact that Medicare has been squeezing physician payment rates for 2 decades is making reforming their structure more difficult,” said Dr. Ginsburg. “The losers are more sensitive to reductions in the rates for the procedures they do.”
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF—an independent source of health policy research, polling, and journalism. Learn more about KFF.
Money talks.
The United States faces a serious shortage of primary care physicians for many reasons, but one, in particular, is inescapable: compensation.
Substantial disparities between what primary care physicians earn relative to specialists like orthopedists and cardiologists can weigh into medical students’ decisions about which field to choose. Plus, the system that Medicare and other health plans use to pay doctors generally places more value on doing procedures like replacing a knee or inserting a stent than on delivering the whole-person, long-term health care management that primary care physicians provide.
As a result of those pay disparities, and the punishing workload typically faced by primary care physicians, more new doctors are becoming specialists, often leaving patients with fewer choices for primary care.
“There is a public out there that is dissatisfied with the lack of access to a routine source of care,” said Christopher Koller, president of the Milbank Memorial Fund, a foundation that focuses on improving population health and health equity. “That’s not going to be addressed until we pay for it.”
Primary care is the foundation of our health care system, the only area in which providing more services – such as childhood vaccines and regular blood pressure screenings – is linked to better population health and more equitable outcomes, according to the National Academies of Sciences, Engineering, and Medicine, in a recently published report on how to rebuild primary care. Without it, the national academies wrote, “minor health problems can spiral into chronic disease,” with poor disease management, ED overuse, and unsustainable costs. Yet for decades, the United States has underinvested in primary care. It accounted for less than 5% of health care spending in 2020 – significantly less than the average spending by countries that are members of the Organization for Economic Cooperation and Development, according to the report.
A $26 billion piece of bipartisan legislation proposed last month by Sen. Bernie Sanders (I-Vt.), chair of the Senate Health, Education, Labor, and Pensions Committee, and Sen. Roger Marshall (R-Kan.) would bolster primary care by increasing training opportunities for doctors and nurses and expanding access to community health centers. Policy experts say the bill would provide important support, but it’s not enough. It doesn’t touch compensation.
“We need primary care to be paid differently and to be paid more, and that starts with Medicare,” Mr. Koller said.
How Medicare drives payment
Medicare, which covers 65 million people who are 65 and older or who have certain long-term disabilities, finances more than a fifth of all health care spending — giving it significant muscle in the health care market. Private health plans typically base their payment amounts on the Medicare system, so what Medicare pays is crucial.
Under the Medicare payment system, the amount the program pays for a medical service is determined by three geographically weighted components: a physician’s work, including time and intensity; the practice’s expense, such as overhead and equipment; and professional insurance. It tends to reward specialties that emphasize procedures, such as repairing a hernia or removing a tumor, more than primary care, where the focus is on talking with patients, answering questions, and educating them about managing their chronic conditions.
Medical students may not be familiar with the particulars of how the payment system works, but their clinical training exposes them to a punishing workload and burnout that is contributing to the shortage of primary care physicians, projected to reach up to 48,000 by 2034, according to estimates from the Association of American Medical Colleges.
The earnings differential between primary care and other specialists is also not lost on them. Average annual compensation for doctors who focus on primary care – family medicine, internists, and pediatricians – ranges from an average of about $250,000 to $275,000, according to Medscape’s annual physician compensation report. Many specialists make more than twice as much: Plastic surgeons top the compensation list at $619,000 annually, followed by orthopedists ($573,000) and cardiologists ($507,000).
“I think the major issues in terms of the primary care physician pipeline are the compensation and the work of primary care,” said Russ Phillips, MD, an internist and the director of the Harvard Medical School Center for Primary Care, Boston. “You have to really want to be a primary care physician when that student will make one-third of what students going into dermatology will make.”
According to statistics from the National Resident Matching Program, which tracks the number of residency slots available for graduating medical students and the number of slots filled, 89% of 5,088 family medicine residency slots were filled in 2023, compared with a 93% residency fill rate overall. Internists had a higher fill rate, 96%, but a significant proportion of internal medicine residents eventually practice in a specialty area rather than in primary care.
No one would claim that doctors are poorly paid, but with the average medical student graduating with just over $200,000 in medical school debt, making a good salary matters.
Not in it for the money
Still, it’s a misperception that student debt always drives the decision whether to go into primary care, said Len Marquez, senior director of government relations and legislative advocacy at the Association of American Medical Colleges.
For Anitza Quintero, 24, a second-year medical student at the Geisinger Commonwealth School of Medicine in rural Pennsylvania, primary care is a logical extension of her interest in helping children and immigrants. Ms. Quintero’s family came to the United States on a raft from Cuba before she was born. She plans to focus on internal medicine and pediatrics.
“I want to keep going to help my family and other families,” Ms. Quintero said. “There’s obviously something attractive about having a specialty and a high pay grade,” Still, she wants to work “where the whole body is involved,” she said, adding that long-term doctor-patient relationships are “also attractive.”
Ms. Quintero is part of the Abigail Geisinger Scholars Program, which aims to recruit primary care physicians and psychiatrists to the rural health system in part with a promise of medical school loan forgiveness. Health care shortages tend to be more acute in rural areas.
These students’ education costs are covered, and they receive a $2,000 monthly stipend. They can do their residency elsewhere, but upon completing it they return to Geisinger for a primary care job with the health care system. Every year of work there erases one year of the debt covered by their award. If they don’t take a job with the health care system, they must repay the amount they received.
Payment imbalances a source of tension
In recent years, the Centers for Medicare & Medicaid Services, which administers the Medicare program, has made changes to address some of the payment imbalances between primary care and specialist services. The agency has expanded the office visit services for which providers can bill to manage their patients, including adding nonprocedural billing codes for providing transitional care, chronic care management, and advance care planning.
In 2024’s final physician fee schedule, the agency plans to allow another new code to take effect, G2211. It would let physicians bill for complex patient evaluation and management services. Any physician could use the code, but it is expected that primary care physicians would use it more frequently than specialists. Congress has delayed implementation of the code since 2021.
The new code is a tiny piece of overall payment reform, “but it is critically important, and it is our top priority on the Hill right now,” said Shari Erickson, chief advocacy officer for the American College of Physicians.
It also triggered a tussle that highlights ongoing tension in Medicare physician payment rules.
The American College of Surgeons and 18 other specialty groups published a statement describing the new code as “unnecessary.” They oppose its implementation because it would primarily benefit primary care providers who, they say, already have the flexibility to bill more for more complex visits.
But the real issue is that, under federal law, changes to Medicare physician payments must preserve budget neutrality, a zero-sum arrangement in which payment increases for primary care providers mean payment decreases elsewhere.
“If they want to keep it, they need to pay for it,” said Christian Shalgian, director of the division of advocacy and health policy for the American College of Surgeons, noting that his organization will continue to oppose implementation otherwise.
Still, there’s general agreement that strengthening the primary care system through payment reform won’t be accomplished by tinkering with billing codes.
The current fee-for-service system doesn’t fully accommodate the time and effort primary care physicians put into “small-ticket” activities like emails and phone calls, reviews of lab results, and consultation reports. A better arrangement, they say, would be to pay primary care physicians a set monthly amount per patient to provide all their care, a system called capitation.
“We’re much better off paying on a per capita basis, get that monthly payment paid in advance plus some extra amount for other things,” said Paul Ginsburg, PhD, a senior fellow at the University of Southern California Schaeffer Center for Health Policy and Economics and former commissioner of the Medicare Payment Advisory Commission.
But if adding a single five-character code to Medicare’s payment rules has proved challenging, imagine the heavy lift involved in overhauling the program’s entire physician payment system. MedPAC and the national academies, both of which provide advice to Congress, have weighed in on the broad outlines of what such a transformation might look like. And there are targeted efforts in Congress: For instance, a bill that would add an annual inflation update to Medicare physician payments and a proposal to address budget neutrality. But it’s unclear whether lawmakers have strong interest in taking action.
“The fact that Medicare has been squeezing physician payment rates for 2 decades is making reforming their structure more difficult,” said Dr. Ginsburg. “The losers are more sensitive to reductions in the rates for the procedures they do.”
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF—an independent source of health policy research, polling, and journalism. Learn more about KFF.
Money talks.
The United States faces a serious shortage of primary care physicians for many reasons, but one, in particular, is inescapable: compensation.
Substantial disparities between what primary care physicians earn relative to specialists like orthopedists and cardiologists can weigh into medical students’ decisions about which field to choose. Plus, the system that Medicare and other health plans use to pay doctors generally places more value on doing procedures like replacing a knee or inserting a stent than on delivering the whole-person, long-term health care management that primary care physicians provide.
As a result of those pay disparities, and the punishing workload typically faced by primary care physicians, more new doctors are becoming specialists, often leaving patients with fewer choices for primary care.
“There is a public out there that is dissatisfied with the lack of access to a routine source of care,” said Christopher Koller, president of the Milbank Memorial Fund, a foundation that focuses on improving population health and health equity. “That’s not going to be addressed until we pay for it.”
Primary care is the foundation of our health care system, the only area in which providing more services – such as childhood vaccines and regular blood pressure screenings – is linked to better population health and more equitable outcomes, according to the National Academies of Sciences, Engineering, and Medicine, in a recently published report on how to rebuild primary care. Without it, the national academies wrote, “minor health problems can spiral into chronic disease,” with poor disease management, ED overuse, and unsustainable costs. Yet for decades, the United States has underinvested in primary care. It accounted for less than 5% of health care spending in 2020 – significantly less than the average spending by countries that are members of the Organization for Economic Cooperation and Development, according to the report.
A $26 billion piece of bipartisan legislation proposed last month by Sen. Bernie Sanders (I-Vt.), chair of the Senate Health, Education, Labor, and Pensions Committee, and Sen. Roger Marshall (R-Kan.) would bolster primary care by increasing training opportunities for doctors and nurses and expanding access to community health centers. Policy experts say the bill would provide important support, but it’s not enough. It doesn’t touch compensation.
“We need primary care to be paid differently and to be paid more, and that starts with Medicare,” Mr. Koller said.
How Medicare drives payment
Medicare, which covers 65 million people who are 65 and older or who have certain long-term disabilities, finances more than a fifth of all health care spending — giving it significant muscle in the health care market. Private health plans typically base their payment amounts on the Medicare system, so what Medicare pays is crucial.
Under the Medicare payment system, the amount the program pays for a medical service is determined by three geographically weighted components: a physician’s work, including time and intensity; the practice’s expense, such as overhead and equipment; and professional insurance. It tends to reward specialties that emphasize procedures, such as repairing a hernia or removing a tumor, more than primary care, where the focus is on talking with patients, answering questions, and educating them about managing their chronic conditions.
Medical students may not be familiar with the particulars of how the payment system works, but their clinical training exposes them to a punishing workload and burnout that is contributing to the shortage of primary care physicians, projected to reach up to 48,000 by 2034, according to estimates from the Association of American Medical Colleges.
The earnings differential between primary care and other specialists is also not lost on them. Average annual compensation for doctors who focus on primary care – family medicine, internists, and pediatricians – ranges from an average of about $250,000 to $275,000, according to Medscape’s annual physician compensation report. Many specialists make more than twice as much: Plastic surgeons top the compensation list at $619,000 annually, followed by orthopedists ($573,000) and cardiologists ($507,000).
“I think the major issues in terms of the primary care physician pipeline are the compensation and the work of primary care,” said Russ Phillips, MD, an internist and the director of the Harvard Medical School Center for Primary Care, Boston. “You have to really want to be a primary care physician when that student will make one-third of what students going into dermatology will make.”
According to statistics from the National Resident Matching Program, which tracks the number of residency slots available for graduating medical students and the number of slots filled, 89% of 5,088 family medicine residency slots were filled in 2023, compared with a 93% residency fill rate overall. Internists had a higher fill rate, 96%, but a significant proportion of internal medicine residents eventually practice in a specialty area rather than in primary care.
No one would claim that doctors are poorly paid, but with the average medical student graduating with just over $200,000 in medical school debt, making a good salary matters.
Not in it for the money
Still, it’s a misperception that student debt always drives the decision whether to go into primary care, said Len Marquez, senior director of government relations and legislative advocacy at the Association of American Medical Colleges.
For Anitza Quintero, 24, a second-year medical student at the Geisinger Commonwealth School of Medicine in rural Pennsylvania, primary care is a logical extension of her interest in helping children and immigrants. Ms. Quintero’s family came to the United States on a raft from Cuba before she was born. She plans to focus on internal medicine and pediatrics.
“I want to keep going to help my family and other families,” Ms. Quintero said. “There’s obviously something attractive about having a specialty and a high pay grade,” Still, she wants to work “where the whole body is involved,” she said, adding that long-term doctor-patient relationships are “also attractive.”
Ms. Quintero is part of the Abigail Geisinger Scholars Program, which aims to recruit primary care physicians and psychiatrists to the rural health system in part with a promise of medical school loan forgiveness. Health care shortages tend to be more acute in rural areas.
These students’ education costs are covered, and they receive a $2,000 monthly stipend. They can do their residency elsewhere, but upon completing it they return to Geisinger for a primary care job with the health care system. Every year of work there erases one year of the debt covered by their award. If they don’t take a job with the health care system, they must repay the amount they received.
Payment imbalances a source of tension
In recent years, the Centers for Medicare & Medicaid Services, which administers the Medicare program, has made changes to address some of the payment imbalances between primary care and specialist services. The agency has expanded the office visit services for which providers can bill to manage their patients, including adding nonprocedural billing codes for providing transitional care, chronic care management, and advance care planning.
In 2024’s final physician fee schedule, the agency plans to allow another new code to take effect, G2211. It would let physicians bill for complex patient evaluation and management services. Any physician could use the code, but it is expected that primary care physicians would use it more frequently than specialists. Congress has delayed implementation of the code since 2021.
The new code is a tiny piece of overall payment reform, “but it is critically important, and it is our top priority on the Hill right now,” said Shari Erickson, chief advocacy officer for the American College of Physicians.
It also triggered a tussle that highlights ongoing tension in Medicare physician payment rules.
The American College of Surgeons and 18 other specialty groups published a statement describing the new code as “unnecessary.” They oppose its implementation because it would primarily benefit primary care providers who, they say, already have the flexibility to bill more for more complex visits.
But the real issue is that, under federal law, changes to Medicare physician payments must preserve budget neutrality, a zero-sum arrangement in which payment increases for primary care providers mean payment decreases elsewhere.
“If they want to keep it, they need to pay for it,” said Christian Shalgian, director of the division of advocacy and health policy for the American College of Surgeons, noting that his organization will continue to oppose implementation otherwise.
Still, there’s general agreement that strengthening the primary care system through payment reform won’t be accomplished by tinkering with billing codes.
The current fee-for-service system doesn’t fully accommodate the time and effort primary care physicians put into “small-ticket” activities like emails and phone calls, reviews of lab results, and consultation reports. A better arrangement, they say, would be to pay primary care physicians a set monthly amount per patient to provide all their care, a system called capitation.
“We’re much better off paying on a per capita basis, get that monthly payment paid in advance plus some extra amount for other things,” said Paul Ginsburg, PhD, a senior fellow at the University of Southern California Schaeffer Center for Health Policy and Economics and former commissioner of the Medicare Payment Advisory Commission.
But if adding a single five-character code to Medicare’s payment rules has proved challenging, imagine the heavy lift involved in overhauling the program’s entire physician payment system. MedPAC and the national academies, both of which provide advice to Congress, have weighed in on the broad outlines of what such a transformation might look like. And there are targeted efforts in Congress: For instance, a bill that would add an annual inflation update to Medicare physician payments and a proposal to address budget neutrality. But it’s unclear whether lawmakers have strong interest in taking action.
“The fact that Medicare has been squeezing physician payment rates for 2 decades is making reforming their structure more difficult,” said Dr. Ginsburg. “The losers are more sensitive to reductions in the rates for the procedures they do.”
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF—an independent source of health policy research, polling, and journalism. Learn more about KFF.
Diagnosing patients with sarcoidosis
A 40-year-old women is evaluated for liver abnormalities. She had elevated transaminases and alkaline phosphatase. A liver ultrasound showed multiple lesions. She underwent liver biopsy, which showed granulomas. What test results, if abnormal, would be most suggestive of sarcoidosis?
A. Erythrocyte sedimentation rate
B. C-reactive protein
C. Lymphocyte count
D. Antinuclear antibodies
The correct answer here is lymphocyte count. Sarcoidosis is in just about every differential diagnosis, as it can involve every organ system. I will share with you a few pearls I have learned over 30 years of taking care of patients with sarcoidosis. Lymphocyte counts drop with active sarcoidosis. Sarcoidosis should always be part of the differential when you see lymphopenia. El Jammal et al. studied 90 patients referred for possible granulomatous hepatitis.1 Seventy-three patients had a final diagnosis of granulomatous hepatitis, and 38 of those patients had sarcoidosis. Lymphopenia had a high specificity (85.7%) for the diagnosis of sarcoidosis, with a specificity of 100% in the patients under 50 years old.
Morell and colleagues looked at whether low lymphocyte counts and low lymphocyte percentage were markers of active sarcoidosis.2 Forty patients with biopsy-proven sarcoidosis were prospectively evaluated every 6 months. A low lymphocyte count and a low lymphocyte percentage (< 20%) were detected more frequently in patients with active sarcoidosis than in the patients with asymptomatic sarcoidosis (P < .02 and P < .0001).
Jones et al. looked at lymphopenia as a marker of sarcoidosis in patients presenting with uveitis.3 The study was a retrospective case-control study (112 patients with sarcoidosis-associated uveitis and 398 controls with other forms of uveitis). The mean lymphocyte count for patients with sarcoidosis was 1.43 vs. 2.04 for other causes of uveitis (P ≤ .0001).
Patients with sarcoidosis are at risk of hypercalciuria, hypercalcemia, and kidney stones. These are common in patients with sarcoidosis, with up to 50% of such patients having hypercalciuria. This is because in sarcoidosis patients 25(OH) vitamin D is converted in granulomas by activated macrophages to 1,25(OH)2 vitamin D, which is the active form of vitamin D.
Several studies have looked at the diagnostic utility of 1,25(OH)2 vitamin D levels in patients with suspected sarcoidosis. Rohmer and colleagues looked at whether 1,25(OH)2 vitamin D levels could help with the diagnosis of sarcoidosis as the cause of uveitis.4 They found that the level of 25(OH) vitamin D in sarcoidosis patients with uveitis was lower than in patients with uveitis without sarcoidosis, 34 vs. 43 nmol/mL (P < .02), whereas the 1,25(OH)2 vitamin D level was higher in patients with sarcoidosis than in those with uveitis without sarcoidosis, 132 vs. 108 pmol/L (P = .02). They looked at the 1,25(OH)2D/25(OH)D ratio; a ratio > 3.5 was strongly associated with an abnormal chest CT-scan (OR = 5.7, P = .003) and granulomas on bronchial biopsy (OR = 14.7, P = .007).
Kavathia et al. looked at whether elevated 1,25(OH)2 vitamin D levels predicted chronicity of sarcoidosis.5 A total of 59 sarcoidosis patients were recruited for the study. Higher serum 1,25(OH)2 vitamin D levels were associated with patients requiring repeated systemic immunosuppressive therapy or > 1 year of therapy. Increasing quartiles of serum 1,25(OH)2 vitamin D level were associated with increased odds of patients having chronic sarcoidosis (OR = 1.82; 95% CI, 1.11-2.99, P = .019).
Because of the higher activated vitamin D levels in sarcoidosis patients, they are at risk for problems with vitamin D supplementation. I have seen two patients develop large numbers of kidney stones after receiving high-dose vitamin D. Sodhi and Aldrich reported on a cohort of 196 sarcoidosis patients who had received vitamin D and compared them with 196 control patients with sarcoidosis who were not receiving vitamin D.6 Hypercalcemia was more frequent in the group that received vitamin D (42.3%) than in the group that did not (18.3%, P < .0001). In this study, only a minority (23%) of patients receiving vitamin D had their 1,25(OH)2 vitamin D level checked.
Pearl: Lymphocyte count and 1,25(OH)2 vitamin D levels can be helpful tests in assessing sarcoidosis activity. Patients with sarcoidosis who receive vitamin D should have their 1.25(OH)2 vitamin D levels monitored.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. El Jammal et al. Sarcoidosis Vasc Diffuse Lung Dis. 2023 Sep 13;40(3):e2023031.
2. Morell F et al. Chest. 2002 Apr;121(4):1239-44.
3. Jones NP et al. Br J Ophthalmol. 2016 Oct;100(10):1393-6.
4. Rohmer J et al. Ocul Immunol Inflamm. 2020 Apr 2;28(3):341-7.
5. Kavathia D et al. Respir Med. 2010 Apr;104(4):564–70.
6. Sodhi A and Aldrich T. Am J Med Sci. 2016 Sep;352(3):252-7.
A 40-year-old women is evaluated for liver abnormalities. She had elevated transaminases and alkaline phosphatase. A liver ultrasound showed multiple lesions. She underwent liver biopsy, which showed granulomas. What test results, if abnormal, would be most suggestive of sarcoidosis?
A. Erythrocyte sedimentation rate
B. C-reactive protein
C. Lymphocyte count
D. Antinuclear antibodies
The correct answer here is lymphocyte count. Sarcoidosis is in just about every differential diagnosis, as it can involve every organ system. I will share with you a few pearls I have learned over 30 years of taking care of patients with sarcoidosis. Lymphocyte counts drop with active sarcoidosis. Sarcoidosis should always be part of the differential when you see lymphopenia. El Jammal et al. studied 90 patients referred for possible granulomatous hepatitis.1 Seventy-three patients had a final diagnosis of granulomatous hepatitis, and 38 of those patients had sarcoidosis. Lymphopenia had a high specificity (85.7%) for the diagnosis of sarcoidosis, with a specificity of 100% in the patients under 50 years old.
Morell and colleagues looked at whether low lymphocyte counts and low lymphocyte percentage were markers of active sarcoidosis.2 Forty patients with biopsy-proven sarcoidosis were prospectively evaluated every 6 months. A low lymphocyte count and a low lymphocyte percentage (< 20%) were detected more frequently in patients with active sarcoidosis than in the patients with asymptomatic sarcoidosis (P < .02 and P < .0001).
Jones et al. looked at lymphopenia as a marker of sarcoidosis in patients presenting with uveitis.3 The study was a retrospective case-control study (112 patients with sarcoidosis-associated uveitis and 398 controls with other forms of uveitis). The mean lymphocyte count for patients with sarcoidosis was 1.43 vs. 2.04 for other causes of uveitis (P ≤ .0001).
Patients with sarcoidosis are at risk of hypercalciuria, hypercalcemia, and kidney stones. These are common in patients with sarcoidosis, with up to 50% of such patients having hypercalciuria. This is because in sarcoidosis patients 25(OH) vitamin D is converted in granulomas by activated macrophages to 1,25(OH)2 vitamin D, which is the active form of vitamin D.
Several studies have looked at the diagnostic utility of 1,25(OH)2 vitamin D levels in patients with suspected sarcoidosis. Rohmer and colleagues looked at whether 1,25(OH)2 vitamin D levels could help with the diagnosis of sarcoidosis as the cause of uveitis.4 They found that the level of 25(OH) vitamin D in sarcoidosis patients with uveitis was lower than in patients with uveitis without sarcoidosis, 34 vs. 43 nmol/mL (P < .02), whereas the 1,25(OH)2 vitamin D level was higher in patients with sarcoidosis than in those with uveitis without sarcoidosis, 132 vs. 108 pmol/L (P = .02). They looked at the 1,25(OH)2D/25(OH)D ratio; a ratio > 3.5 was strongly associated with an abnormal chest CT-scan (OR = 5.7, P = .003) and granulomas on bronchial biopsy (OR = 14.7, P = .007).
Kavathia et al. looked at whether elevated 1,25(OH)2 vitamin D levels predicted chronicity of sarcoidosis.5 A total of 59 sarcoidosis patients were recruited for the study. Higher serum 1,25(OH)2 vitamin D levels were associated with patients requiring repeated systemic immunosuppressive therapy or > 1 year of therapy. Increasing quartiles of serum 1,25(OH)2 vitamin D level were associated with increased odds of patients having chronic sarcoidosis (OR = 1.82; 95% CI, 1.11-2.99, P = .019).
Because of the higher activated vitamin D levels in sarcoidosis patients, they are at risk for problems with vitamin D supplementation. I have seen two patients develop large numbers of kidney stones after receiving high-dose vitamin D. Sodhi and Aldrich reported on a cohort of 196 sarcoidosis patients who had received vitamin D and compared them with 196 control patients with sarcoidosis who were not receiving vitamin D.6 Hypercalcemia was more frequent in the group that received vitamin D (42.3%) than in the group that did not (18.3%, P < .0001). In this study, only a minority (23%) of patients receiving vitamin D had their 1,25(OH)2 vitamin D level checked.
Pearl: Lymphocyte count and 1,25(OH)2 vitamin D levels can be helpful tests in assessing sarcoidosis activity. Patients with sarcoidosis who receive vitamin D should have their 1.25(OH)2 vitamin D levels monitored.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. El Jammal et al. Sarcoidosis Vasc Diffuse Lung Dis. 2023 Sep 13;40(3):e2023031.
2. Morell F et al. Chest. 2002 Apr;121(4):1239-44.
3. Jones NP et al. Br J Ophthalmol. 2016 Oct;100(10):1393-6.
4. Rohmer J et al. Ocul Immunol Inflamm. 2020 Apr 2;28(3):341-7.
5. Kavathia D et al. Respir Med. 2010 Apr;104(4):564–70.
6. Sodhi A and Aldrich T. Am J Med Sci. 2016 Sep;352(3):252-7.
A 40-year-old women is evaluated for liver abnormalities. She had elevated transaminases and alkaline phosphatase. A liver ultrasound showed multiple lesions. She underwent liver biopsy, which showed granulomas. What test results, if abnormal, would be most suggestive of sarcoidosis?
A. Erythrocyte sedimentation rate
B. C-reactive protein
C. Lymphocyte count
D. Antinuclear antibodies
The correct answer here is lymphocyte count. Sarcoidosis is in just about every differential diagnosis, as it can involve every organ system. I will share with you a few pearls I have learned over 30 years of taking care of patients with sarcoidosis. Lymphocyte counts drop with active sarcoidosis. Sarcoidosis should always be part of the differential when you see lymphopenia. El Jammal et al. studied 90 patients referred for possible granulomatous hepatitis.1 Seventy-three patients had a final diagnosis of granulomatous hepatitis, and 38 of those patients had sarcoidosis. Lymphopenia had a high specificity (85.7%) for the diagnosis of sarcoidosis, with a specificity of 100% in the patients under 50 years old.
Morell and colleagues looked at whether low lymphocyte counts and low lymphocyte percentage were markers of active sarcoidosis.2 Forty patients with biopsy-proven sarcoidosis were prospectively evaluated every 6 months. A low lymphocyte count and a low lymphocyte percentage (< 20%) were detected more frequently in patients with active sarcoidosis than in the patients with asymptomatic sarcoidosis (P < .02 and P < .0001).
Jones et al. looked at lymphopenia as a marker of sarcoidosis in patients presenting with uveitis.3 The study was a retrospective case-control study (112 patients with sarcoidosis-associated uveitis and 398 controls with other forms of uveitis). The mean lymphocyte count for patients with sarcoidosis was 1.43 vs. 2.04 for other causes of uveitis (P ≤ .0001).
Patients with sarcoidosis are at risk of hypercalciuria, hypercalcemia, and kidney stones. These are common in patients with sarcoidosis, with up to 50% of such patients having hypercalciuria. This is because in sarcoidosis patients 25(OH) vitamin D is converted in granulomas by activated macrophages to 1,25(OH)2 vitamin D, which is the active form of vitamin D.
Several studies have looked at the diagnostic utility of 1,25(OH)2 vitamin D levels in patients with suspected sarcoidosis. Rohmer and colleagues looked at whether 1,25(OH)2 vitamin D levels could help with the diagnosis of sarcoidosis as the cause of uveitis.4 They found that the level of 25(OH) vitamin D in sarcoidosis patients with uveitis was lower than in patients with uveitis without sarcoidosis, 34 vs. 43 nmol/mL (P < .02), whereas the 1,25(OH)2 vitamin D level was higher in patients with sarcoidosis than in those with uveitis without sarcoidosis, 132 vs. 108 pmol/L (P = .02). They looked at the 1,25(OH)2D/25(OH)D ratio; a ratio > 3.5 was strongly associated with an abnormal chest CT-scan (OR = 5.7, P = .003) and granulomas on bronchial biopsy (OR = 14.7, P = .007).
Kavathia et al. looked at whether elevated 1,25(OH)2 vitamin D levels predicted chronicity of sarcoidosis.5 A total of 59 sarcoidosis patients were recruited for the study. Higher serum 1,25(OH)2 vitamin D levels were associated with patients requiring repeated systemic immunosuppressive therapy or > 1 year of therapy. Increasing quartiles of serum 1,25(OH)2 vitamin D level were associated with increased odds of patients having chronic sarcoidosis (OR = 1.82; 95% CI, 1.11-2.99, P = .019).
Because of the higher activated vitamin D levels in sarcoidosis patients, they are at risk for problems with vitamin D supplementation. I have seen two patients develop large numbers of kidney stones after receiving high-dose vitamin D. Sodhi and Aldrich reported on a cohort of 196 sarcoidosis patients who had received vitamin D and compared them with 196 control patients with sarcoidosis who were not receiving vitamin D.6 Hypercalcemia was more frequent in the group that received vitamin D (42.3%) than in the group that did not (18.3%, P < .0001). In this study, only a minority (23%) of patients receiving vitamin D had their 1,25(OH)2 vitamin D level checked.
Pearl: Lymphocyte count and 1,25(OH)2 vitamin D levels can be helpful tests in assessing sarcoidosis activity. Patients with sarcoidosis who receive vitamin D should have their 1.25(OH)2 vitamin D levels monitored.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. El Jammal et al. Sarcoidosis Vasc Diffuse Lung Dis. 2023 Sep 13;40(3):e2023031.
2. Morell F et al. Chest. 2002 Apr;121(4):1239-44.
3. Jones NP et al. Br J Ophthalmol. 2016 Oct;100(10):1393-6.
4. Rohmer J et al. Ocul Immunol Inflamm. 2020 Apr 2;28(3):341-7.
5. Kavathia D et al. Respir Med. 2010 Apr;104(4):564–70.
6. Sodhi A and Aldrich T. Am J Med Sci. 2016 Sep;352(3):252-7.