User login
Paradoxical Reaction to TNF-α Inhibitor Therapy in a Patient With Hidradenitis Suppurativa
To the Editor:
Hidradenitis suppurativa (HS) is a chronic inflammatory condition of the pilosebaceous unit that occurs in concert with elevations of various cytokines, including tumor necrosis factor α (TNF-α), IL-1β, IL-10, and IL-17.1,2 Adalimumab is a TNF-α inhibitor approved by the US Food and Drug Administration for the treatment of HS. Although TNF-α inhibitors are effective for many immune-mediated inflammatory disorders, paradoxical drug reactions have been reported following treatment with these agents.3-6 True paradoxical drug reactions likely are immune mediated and directly lead to new onset of a pathologic condition that would otherwise respond to that drug. For example, there are reports of rheumatoid arthritis patients who were treated with a TNF-α inhibitor and developed psoriatic skin lesions.3,6 Paradoxical drug reactions also have been reported with acute-onset inflammatory bowel disease and HS or less commonly pyoderma gangrenosum (PG), uveitis, granulomatous reactions, and vasculitis.4,5 We present the case of a patient with HS who was treated with a TNF-α inhibitor and developed 2 distinct paradoxical drug reactions. We also provide an overview of paradoxical drug reactions associated with TNF-α inhibitors.
A 38-year-old woman developed a painful “boil” on the right leg that was previously treated in the emergency department with incision and drainage as well as oral clindamycin for 7 days, but the lesion spread and continued to worsen. She had a history of HS in the axillae and groin region that had been present since 12 years of age. The condition was poorly controlled despite multiple courses of oral antibiotics and surgical resections. An oral contraceptive also was attempted, but the patient discontinued treatment when liver enzyme levels became elevated. The patient had no other notable medical history, including skin disease. There was a family history of HS in her father and a sibling. Seeking more effective treatment, the patient was offered adalimumab approximately 4 months prior to clinical presentation and agreed to start a course of the drug. She received a loading dose of 160 mg on day 1 and 80 mg on day 15 followed by a maintenance dosage of 40 mg weekly. She experienced improvement in HS symptoms after 3 months on adalimumab; however, she developed scaly pruritic patches on the scalp, arms, and legs that were consistent with psoriasis. Because of the absence of a personal or family history of psoriasis, the patient was informed of the probability of paradoxical psoriasis resulting from adalimumab. She elected to continue adalimumab because of the improvement in HS symptoms, and the psoriatic lesions were mild and adequately controlled with a topical steroid.
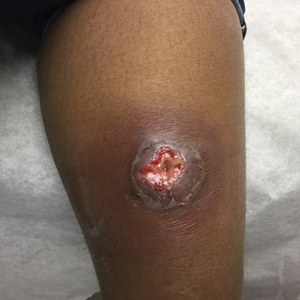
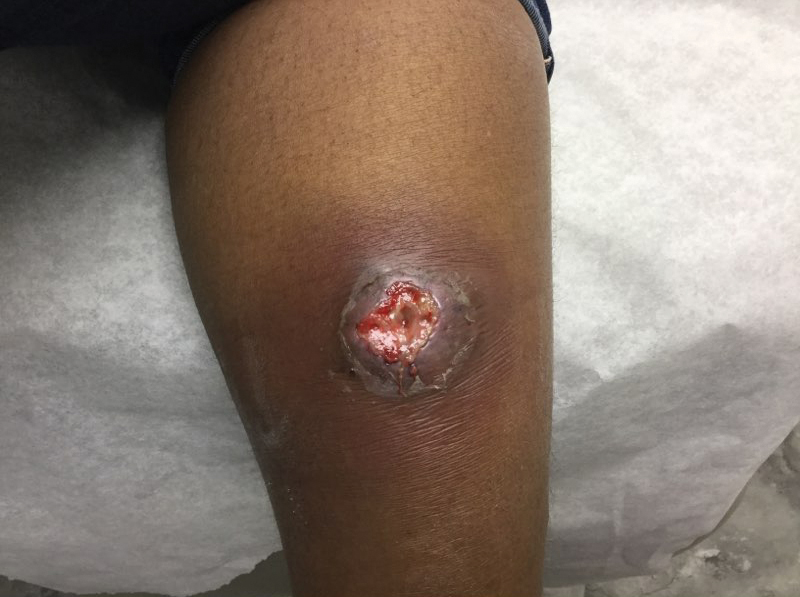
At the current presentation 1 month later, physical examination revealed a large indurated and ulcerated area with jagged edges at the incision and drainage site (Figure 1). Pyoderma gangrenosum was clinically suspected; a biopsy was performed, and the patient was started on oral prednisone. At 2-week follow-up, the ulcer was found to be rapidly resolving with prednisone and healing with cribriform scarring (Figure 2). Histopathology revealed an undermining neutrophilic inflammatory process that was consistent with PG. A diagnosis of PG was made based on previously published criteria7 and the following major/minor criteria in the patient: pathology; absence of infection on histologic analysis; history of pathergy related to worsening ulceration at the site of incision and drainage of the initial boil; clinical findings of an ulcer with peripheral violaceous erythema; undermined borders and tenderness at the site; and rapid resolution of the ulcer with prednisone.
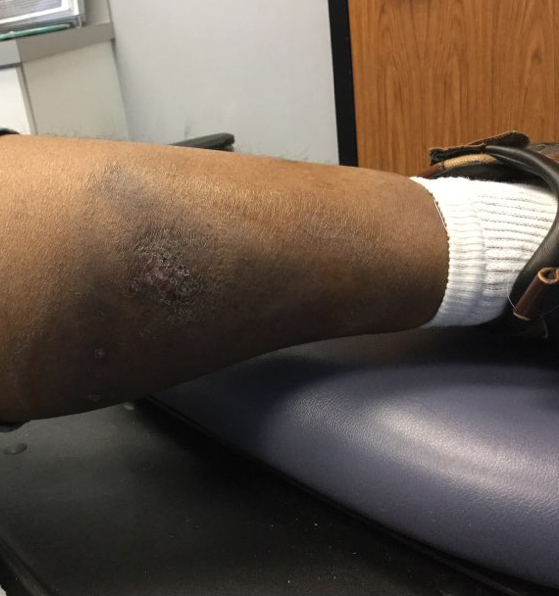
Cessation of adalimumab gradually led to clearance of both psoriasiform lesions and PG; however, HS lesions persisted.
Although the precise pathogenesis of HS is unclear, both genetic abnormalities of the pilosebaceous unit and a dysregulated immune reaction appear to lead to the clinical characteristics of chronic inflammation and scarring seen in HS. A key effector appears to be helper T-cell (TH17) lymphocyte activation, with increased secretion of TNF-α, IL-1β, and IL-17.1,2 In turn, IL-17 induces higher expression of TNF-α, leading to a persistent cycle of inflammation. Peripheral recruitment of IL-17–producing neutrophils also may contribute to chronic inflammation.8
Adalimumab is the only US Food and Drug Administration–approved biologic indicated for the treatment of HS. Our patient initially responded to adalimumab with improvement of HS; however, treatment had to be discontinued because of the unusual occurrence of 2 distinct paradoxical reactions in a short span of time. Psoriasis and PG are both considered true paradoxical reactions because primary occurrences of both diseases usually are responsive to treatment with adalimumab.
Tumor necrosis factor α inhibitor–induced psoriasis arises de novo and is estimated to occur in approximately 5% of patients with rheumatoid arthritis.3,6 Palmoplantar pustular psoriasiform reactions are the most common form of paradoxical psoriasis. Topical medications can be used to treat skin lesions, but systemic treatment is required in many cases. Switching to an alternate class of a biologic, such as an IL-17, IL-12/23, or IL-23 inhibitor, can improve the skin reaction; however, such treatment is inconsistently successful, and paradoxical drug reactions also have been seen with these other classes of biologics.4,9
Recent studies support distinct immune causes for classical and paradoxical psoriasis. In classical psoriasis, plasmacytoid dendritic cells (pDCs) produce IFN-α, which stimulates conventional dendritic cells to produce TNF-α. However, TNF-α matures both pDCs and conventional dendritic cells; upon maturation, both types of dendritic cells lose the ability to produce IFN-α, thus allowing TNF-α to become dominant.10 The blockade of TNF-α prevents pDC maturation, leading to uninhibited IFN-α, which appears to drive inflammation in paradoxical psoriasis. In classical psoriasis, oligoclonal dermal CD4+ T cells and epidermal CD8+ T cells remain, even in resolved skin lesions, and can cause disease recurrence through reactivation of skin-resident memory T cells.11 No relapse of paradoxical psoriasis occurs with discontinuation of anti-TNF-α therapy, which supports the notion of an absence of memory T cells.
The incidence of paradoxical psoriasis in patients receiving a TNF-α inhibitor for HS is unclear.12 There are case series in which patients who had concurrent psoriasis and HS were successfully treated with a TNF-α inhibitor.13 A recently recognized condition—PASH syndrome—encompasses the clinical triad of PG, acne, and HS.10
Our patient had no history of acne or PG, only a long-standing history of HS. New-onset PG occurred only after a TNF-α inhibitor was initiated. Notably, PASH syndrome has been successfully treated with TNF-α inhibitors, highlighting the shared inflammatory etiology of HS and PG.14 In patients with concurrent PG and HS, TNF-α inhibitors were more effective for treating PG than for HS.
Pyoderma gangrenosum is an inflammatory disorder that often occurs concomitantly with other conditions, such as inflammatory bowel disease. The exact underlying cause of PG is unclear, but there appears to be both neutrophil and T-cell dysfunction in PG, with excess inflammatory cytokine production (eg, IL-1β, TNF-α, IL-17).15
The mainstay of treatment of PG is systemic corticosteroids and immunosuppressives, such as cyclosporine. Tumor necrosis factor α inhibitors as well as other interleukin inhibitors are increasingly utilized as potential therapeutic alternatives for PG.16,17
Unlike paradoxical psoriasis, the underlying cause of paradoxical PG is unclear.18,19 A similar mechanism may be postulated whereby inhibition of TNF-α leads to excessive activation of alternative inflammatory pathways that result in paradoxical PG. In one study, the prevalence of PG among 68,232 patients with HS was 0.18% compared with 0.01% among those without HS; therefore, patients with HS appear to be more predisposed to PG.20
This case illustrates the complex, often conflicting effects of cytokine inhibition in the paradoxical elicitation of alternative inflammatory disorders as an unintended consequence of the initial cytokine blockade. It is likely that genetic predisposition allows for paradoxical reactions in some patients when there is predominant inhibition of one cytokine in the inflammatory pathway. In rare cases, multiple paradoxical reactions are possible.
1. Vossen ARJV, van der Zee HH, Prens EP. Hidradenitis suppurativa: a systematic review integrating inflammatory pathways into a cohesive pathogenic model. Front Immunol. 2018;9:2965. doi:10.3389/fimmu.2018.02965
2. Goldburg SR, Strober BE, Payette MJ. Hidradenitis suppurativa: epidemiology, clinical presentation and pathogenesis. J Am Acad Dermatol. 2020; 82:1045-1058. doi:10.1016/j.jaad.2019.08.090
3. Brown G, Wang E, Leon A, et al. Tumor necrosis factor-α inhibitor-induced psoriasis: systematic review of clinical features, histopathological findings, and management experience. J Am Acad Dermatol. 2017;76:334-341. doi:10.1016/j.jaad.2016.08.012
4. Puig L. Paradoxical reactions: anti-tumor necrosis factor alpha agents, ustekinumab, secukinumab, ixekizumab and others. Curr Prob Dermatol. 2018;53:49-63. doi:10.1159/000479475
5. Faivre C, Villani AP, Aubin F, et al; . Hidradenitis suppurativa (HS): an unrecognized paradoxical effect of biologic agents (BA) used in chronic inflammatory diseases. J Am Acad Dermatol. 2016;74:1153-1159. doi:10.1016/j.jaad.2016.01.018
6. Ko JM, Gottlieb AB, Kerbleski JF. Induction and exacerbation of psoriasis with TNF-blockade therapy: a review and analysis of 127 cases. J Dermatolog Treat. 2009;20:100-108. doi:10.1080/09546630802441234
7. Maverakis E, Ma C, Shinkai K, et al. Diagnostic criteria of ulcerative pyoderma gangrenosum: a delphi consensus of international experts. JAMA Dermatol. 2018;154:461-466. doi:10.1001/jamadermatol.2017.5980
8. Lima AL, Karl I, Giner T, et al. Keratinocytes and neutrophils are important sources of proinflammatory molecules in hidradenitis suppurativa. Br J Dermatol. 2016;174:514-521. doi:10.1111/bjd.14214
9. Li SJ, Perez-Chada LM, Merola JF. TNF inhibitor-induced psoriasis: proposed algorithm for treatment and management. J Psoriasis Psoriatic Arthritis. 2019;4:70-80. doi:10.1177/2475530318810851
10. Conrad C, Di Domizio J, Mylonas A, et al. TNF blockade induces a dysregulated type I interferon response without autoimmunity in paradoxical psoriasis. Nat Commun. 2018;9:25. doi:10.1038/s41467-017-02466-4
11. Matos TR, O’Malley JT, Lowry EL, et al. Clinically resolved psoriatic lesions contain psoriasis-specific IL-17-producing αβ T cell clones. J Clin Invest. 2017;127:4031-4041. doi:10.1172/JCI93396
12. Faivre C, Villani AP, Aubin F, et al. Hidradenitis suppurativa (HS): an unrecognized paradoxical effect of biologic agents (BA) used in chronic inflammatory diseases. J Am Acad Dermatol. 2016;74:1153-1159. doi:10.1016/j.jaad.2016.01.018
13. Marzano AV, Damiani G, Ceccherini I, et al. Autoinflammation in pyoderma gangrenosum and its syndromic form (pyoderma gangrenosum, acne and suppurative hidradenitis). Br J Dermatol. 2017;176:1588-1598. doi:10.1111/bjd.15226
14. Cugno M, Borghi A, Marzano AV. PAPA, PASH, PAPASH syndromes: pathophysiology, presentation and treatment. Am J Clin Dermatol. 2017;18:555-562. doi:10.1007/s40257-017-0265-1
15. Wang EA, Steel A, Luxardi G, et al. Classic ulcerative pyoderma gangrenosum is a T cell-mediated disease targeting follicular adnexal structures: a hypothesis based on molecular and clinicopathologic studies. Front Immunol. 2018;8:1980. doi:10.3389/fimmu.2017.01980
16. Patel F, Fitzmaurice S, Duong C, et al. Effective strategies for the management of pyoderma gangrenosum: a comprehensive review. Acta Derm Venereol. 2015;95:525-531. doi:10.2340/00015555-2008
17. Partridge ACR, Bai JW, Rosen CF, et al. Effectiveness of systemic treatments for pyoderma gangrenosum: a systematic review of observational studies and clinical trials. Br J Dermatol. 2018;179:290-295. doi:10.1111/bjd.16485
18. Benzaquen M, Monnier J, Beaussault Y, et al. Pyoderma gangrenosum arising during treatment of psoriasis with adalimumab: effectiveness of ustekinumab. Australas J Dermatol. 2017;58:e270-e271. doi:10.1111/ajd.12545
19. Fujimoto N, Yamasaki Y, Watanabe RJ. Paradoxical uveitis and pyoderma gangrenosum in a patient with psoriatic arthritis under infliximab treatment. J Dtsch Dermatol Ges. 2018;16:1139-1140. doi:10.1111/ddg.13632
20. Tannenbaum R, Strunk A, Garg A. Overall and subgroup prevalence of pyoderma gangrenosum among patients with hidradenitis suppurativa: a population-based analysis in the United States. J Am Acad Dermatol. 2019;80:1533-1537. doi:10.1016/j.jaad.2019.02.004
To the Editor:
Hidradenitis suppurativa (HS) is a chronic inflammatory condition of the pilosebaceous unit that occurs in concert with elevations of various cytokines, including tumor necrosis factor α (TNF-α), IL-1β, IL-10, and IL-17.1,2 Adalimumab is a TNF-α inhibitor approved by the US Food and Drug Administration for the treatment of HS. Although TNF-α inhibitors are effective for many immune-mediated inflammatory disorders, paradoxical drug reactions have been reported following treatment with these agents.3-6 True paradoxical drug reactions likely are immune mediated and directly lead to new onset of a pathologic condition that would otherwise respond to that drug. For example, there are reports of rheumatoid arthritis patients who were treated with a TNF-α inhibitor and developed psoriatic skin lesions.3,6 Paradoxical drug reactions also have been reported with acute-onset inflammatory bowel disease and HS or less commonly pyoderma gangrenosum (PG), uveitis, granulomatous reactions, and vasculitis.4,5 We present the case of a patient with HS who was treated with a TNF-α inhibitor and developed 2 distinct paradoxical drug reactions. We also provide an overview of paradoxical drug reactions associated with TNF-α inhibitors.
A 38-year-old woman developed a painful “boil” on the right leg that was previously treated in the emergency department with incision and drainage as well as oral clindamycin for 7 days, but the lesion spread and continued to worsen. She had a history of HS in the axillae and groin region that had been present since 12 years of age. The condition was poorly controlled despite multiple courses of oral antibiotics and surgical resections. An oral contraceptive also was attempted, but the patient discontinued treatment when liver enzyme levels became elevated. The patient had no other notable medical history, including skin disease. There was a family history of HS in her father and a sibling. Seeking more effective treatment, the patient was offered adalimumab approximately 4 months prior to clinical presentation and agreed to start a course of the drug. She received a loading dose of 160 mg on day 1 and 80 mg on day 15 followed by a maintenance dosage of 40 mg weekly. She experienced improvement in HS symptoms after 3 months on adalimumab; however, she developed scaly pruritic patches on the scalp, arms, and legs that were consistent with psoriasis. Because of the absence of a personal or family history of psoriasis, the patient was informed of the probability of paradoxical psoriasis resulting from adalimumab. She elected to continue adalimumab because of the improvement in HS symptoms, and the psoriatic lesions were mild and adequately controlled with a topical steroid.
At the current presentation 1 month later, physical examination revealed a large indurated and ulcerated area with jagged edges at the incision and drainage site (Figure 1). Pyoderma gangrenosum was clinically suspected; a biopsy was performed, and the patient was started on oral prednisone. At 2-week follow-up, the ulcer was found to be rapidly resolving with prednisone and healing with cribriform scarring (Figure 2). Histopathology revealed an undermining neutrophilic inflammatory process that was consistent with PG. A diagnosis of PG was made based on previously published criteria7 and the following major/minor criteria in the patient: pathology; absence of infection on histologic analysis; history of pathergy related to worsening ulceration at the site of incision and drainage of the initial boil; clinical findings of an ulcer with peripheral violaceous erythema; undermined borders and tenderness at the site; and rapid resolution of the ulcer with prednisone.

Cessation of adalimumab gradually led to clearance of both psoriasiform lesions and PG; however, HS lesions persisted.

Although the precise pathogenesis of HS is unclear, both genetic abnormalities of the pilosebaceous unit and a dysregulated immune reaction appear to lead to the clinical characteristics of chronic inflammation and scarring seen in HS. A key effector appears to be helper T-cell (TH17) lymphocyte activation, with increased secretion of TNF-α, IL-1β, and IL-17.1,2 In turn, IL-17 induces higher expression of TNF-α, leading to a persistent cycle of inflammation. Peripheral recruitment of IL-17–producing neutrophils also may contribute to chronic inflammation.8
Adalimumab is the only US Food and Drug Administration–approved biologic indicated for the treatment of HS. Our patient initially responded to adalimumab with improvement of HS; however, treatment had to be discontinued because of the unusual occurrence of 2 distinct paradoxical reactions in a short span of time. Psoriasis and PG are both considered true paradoxical reactions because primary occurrences of both diseases usually are responsive to treatment with adalimumab.
Tumor necrosis factor α inhibitor–induced psoriasis arises de novo and is estimated to occur in approximately 5% of patients with rheumatoid arthritis.3,6 Palmoplantar pustular psoriasiform reactions are the most common form of paradoxical psoriasis. Topical medications can be used to treat skin lesions, but systemic treatment is required in many cases. Switching to an alternate class of a biologic, such as an IL-17, IL-12/23, or IL-23 inhibitor, can improve the skin reaction; however, such treatment is inconsistently successful, and paradoxical drug reactions also have been seen with these other classes of biologics.4,9
Recent studies support distinct immune causes for classical and paradoxical psoriasis. In classical psoriasis, plasmacytoid dendritic cells (pDCs) produce IFN-α, which stimulates conventional dendritic cells to produce TNF-α. However, TNF-α matures both pDCs and conventional dendritic cells; upon maturation, both types of dendritic cells lose the ability to produce IFN-α, thus allowing TNF-α to become dominant.10 The blockade of TNF-α prevents pDC maturation, leading to uninhibited IFN-α, which appears to drive inflammation in paradoxical psoriasis. In classical psoriasis, oligoclonal dermal CD4+ T cells and epidermal CD8+ T cells remain, even in resolved skin lesions, and can cause disease recurrence through reactivation of skin-resident memory T cells.11 No relapse of paradoxical psoriasis occurs with discontinuation of anti-TNF-α therapy, which supports the notion of an absence of memory T cells.
The incidence of paradoxical psoriasis in patients receiving a TNF-α inhibitor for HS is unclear.12 There are case series in which patients who had concurrent psoriasis and HS were successfully treated with a TNF-α inhibitor.13 A recently recognized condition—PASH syndrome—encompasses the clinical triad of PG, acne, and HS.10
Our patient had no history of acne or PG, only a long-standing history of HS. New-onset PG occurred only after a TNF-α inhibitor was initiated. Notably, PASH syndrome has been successfully treated with TNF-α inhibitors, highlighting the shared inflammatory etiology of HS and PG.14 In patients with concurrent PG and HS, TNF-α inhibitors were more effective for treating PG than for HS.
Pyoderma gangrenosum is an inflammatory disorder that often occurs concomitantly with other conditions, such as inflammatory bowel disease. The exact underlying cause of PG is unclear, but there appears to be both neutrophil and T-cell dysfunction in PG, with excess inflammatory cytokine production (eg, IL-1β, TNF-α, IL-17).15
The mainstay of treatment of PG is systemic corticosteroids and immunosuppressives, such as cyclosporine. Tumor necrosis factor α inhibitors as well as other interleukin inhibitors are increasingly utilized as potential therapeutic alternatives for PG.16,17
Unlike paradoxical psoriasis, the underlying cause of paradoxical PG is unclear.18,19 A similar mechanism may be postulated whereby inhibition of TNF-α leads to excessive activation of alternative inflammatory pathways that result in paradoxical PG. In one study, the prevalence of PG among 68,232 patients with HS was 0.18% compared with 0.01% among those without HS; therefore, patients with HS appear to be more predisposed to PG.20
This case illustrates the complex, often conflicting effects of cytokine inhibition in the paradoxical elicitation of alternative inflammatory disorders as an unintended consequence of the initial cytokine blockade. It is likely that genetic predisposition allows for paradoxical reactions in some patients when there is predominant inhibition of one cytokine in the inflammatory pathway. In rare cases, multiple paradoxical reactions are possible.
To the Editor:
Hidradenitis suppurativa (HS) is a chronic inflammatory condition of the pilosebaceous unit that occurs in concert with elevations of various cytokines, including tumor necrosis factor α (TNF-α), IL-1β, IL-10, and IL-17.1,2 Adalimumab is a TNF-α inhibitor approved by the US Food and Drug Administration for the treatment of HS. Although TNF-α inhibitors are effective for many immune-mediated inflammatory disorders, paradoxical drug reactions have been reported following treatment with these agents.3-6 True paradoxical drug reactions likely are immune mediated and directly lead to new onset of a pathologic condition that would otherwise respond to that drug. For example, there are reports of rheumatoid arthritis patients who were treated with a TNF-α inhibitor and developed psoriatic skin lesions.3,6 Paradoxical drug reactions also have been reported with acute-onset inflammatory bowel disease and HS or less commonly pyoderma gangrenosum (PG), uveitis, granulomatous reactions, and vasculitis.4,5 We present the case of a patient with HS who was treated with a TNF-α inhibitor and developed 2 distinct paradoxical drug reactions. We also provide an overview of paradoxical drug reactions associated with TNF-α inhibitors.
A 38-year-old woman developed a painful “boil” on the right leg that was previously treated in the emergency department with incision and drainage as well as oral clindamycin for 7 days, but the lesion spread and continued to worsen. She had a history of HS in the axillae and groin region that had been present since 12 years of age. The condition was poorly controlled despite multiple courses of oral antibiotics and surgical resections. An oral contraceptive also was attempted, but the patient discontinued treatment when liver enzyme levels became elevated. The patient had no other notable medical history, including skin disease. There was a family history of HS in her father and a sibling. Seeking more effective treatment, the patient was offered adalimumab approximately 4 months prior to clinical presentation and agreed to start a course of the drug. She received a loading dose of 160 mg on day 1 and 80 mg on day 15 followed by a maintenance dosage of 40 mg weekly. She experienced improvement in HS symptoms after 3 months on adalimumab; however, she developed scaly pruritic patches on the scalp, arms, and legs that were consistent with psoriasis. Because of the absence of a personal or family history of psoriasis, the patient was informed of the probability of paradoxical psoriasis resulting from adalimumab. She elected to continue adalimumab because of the improvement in HS symptoms, and the psoriatic lesions were mild and adequately controlled with a topical steroid.
At the current presentation 1 month later, physical examination revealed a large indurated and ulcerated area with jagged edges at the incision and drainage site (Figure 1). Pyoderma gangrenosum was clinically suspected; a biopsy was performed, and the patient was started on oral prednisone. At 2-week follow-up, the ulcer was found to be rapidly resolving with prednisone and healing with cribriform scarring (Figure 2). Histopathology revealed an undermining neutrophilic inflammatory process that was consistent with PG. A diagnosis of PG was made based on previously published criteria7 and the following major/minor criteria in the patient: pathology; absence of infection on histologic analysis; history of pathergy related to worsening ulceration at the site of incision and drainage of the initial boil; clinical findings of an ulcer with peripheral violaceous erythema; undermined borders and tenderness at the site; and rapid resolution of the ulcer with prednisone.

Cessation of adalimumab gradually led to clearance of both psoriasiform lesions and PG; however, HS lesions persisted.

Although the precise pathogenesis of HS is unclear, both genetic abnormalities of the pilosebaceous unit and a dysregulated immune reaction appear to lead to the clinical characteristics of chronic inflammation and scarring seen in HS. A key effector appears to be helper T-cell (TH17) lymphocyte activation, with increased secretion of TNF-α, IL-1β, and IL-17.1,2 In turn, IL-17 induces higher expression of TNF-α, leading to a persistent cycle of inflammation. Peripheral recruitment of IL-17–producing neutrophils also may contribute to chronic inflammation.8
Adalimumab is the only US Food and Drug Administration–approved biologic indicated for the treatment of HS. Our patient initially responded to adalimumab with improvement of HS; however, treatment had to be discontinued because of the unusual occurrence of 2 distinct paradoxical reactions in a short span of time. Psoriasis and PG are both considered true paradoxical reactions because primary occurrences of both diseases usually are responsive to treatment with adalimumab.
Tumor necrosis factor α inhibitor–induced psoriasis arises de novo and is estimated to occur in approximately 5% of patients with rheumatoid arthritis.3,6 Palmoplantar pustular psoriasiform reactions are the most common form of paradoxical psoriasis. Topical medications can be used to treat skin lesions, but systemic treatment is required in many cases. Switching to an alternate class of a biologic, such as an IL-17, IL-12/23, or IL-23 inhibitor, can improve the skin reaction; however, such treatment is inconsistently successful, and paradoxical drug reactions also have been seen with these other classes of biologics.4,9
Recent studies support distinct immune causes for classical and paradoxical psoriasis. In classical psoriasis, plasmacytoid dendritic cells (pDCs) produce IFN-α, which stimulates conventional dendritic cells to produce TNF-α. However, TNF-α matures both pDCs and conventional dendritic cells; upon maturation, both types of dendritic cells lose the ability to produce IFN-α, thus allowing TNF-α to become dominant.10 The blockade of TNF-α prevents pDC maturation, leading to uninhibited IFN-α, which appears to drive inflammation in paradoxical psoriasis. In classical psoriasis, oligoclonal dermal CD4+ T cells and epidermal CD8+ T cells remain, even in resolved skin lesions, and can cause disease recurrence through reactivation of skin-resident memory T cells.11 No relapse of paradoxical psoriasis occurs with discontinuation of anti-TNF-α therapy, which supports the notion of an absence of memory T cells.
The incidence of paradoxical psoriasis in patients receiving a TNF-α inhibitor for HS is unclear.12 There are case series in which patients who had concurrent psoriasis and HS were successfully treated with a TNF-α inhibitor.13 A recently recognized condition—PASH syndrome—encompasses the clinical triad of PG, acne, and HS.10
Our patient had no history of acne or PG, only a long-standing history of HS. New-onset PG occurred only after a TNF-α inhibitor was initiated. Notably, PASH syndrome has been successfully treated with TNF-α inhibitors, highlighting the shared inflammatory etiology of HS and PG.14 In patients with concurrent PG and HS, TNF-α inhibitors were more effective for treating PG than for HS.
Pyoderma gangrenosum is an inflammatory disorder that often occurs concomitantly with other conditions, such as inflammatory bowel disease. The exact underlying cause of PG is unclear, but there appears to be both neutrophil and T-cell dysfunction in PG, with excess inflammatory cytokine production (eg, IL-1β, TNF-α, IL-17).15
The mainstay of treatment of PG is systemic corticosteroids and immunosuppressives, such as cyclosporine. Tumor necrosis factor α inhibitors as well as other interleukin inhibitors are increasingly utilized as potential therapeutic alternatives for PG.16,17
Unlike paradoxical psoriasis, the underlying cause of paradoxical PG is unclear.18,19 A similar mechanism may be postulated whereby inhibition of TNF-α leads to excessive activation of alternative inflammatory pathways that result in paradoxical PG. In one study, the prevalence of PG among 68,232 patients with HS was 0.18% compared with 0.01% among those without HS; therefore, patients with HS appear to be more predisposed to PG.20
This case illustrates the complex, often conflicting effects of cytokine inhibition in the paradoxical elicitation of alternative inflammatory disorders as an unintended consequence of the initial cytokine blockade. It is likely that genetic predisposition allows for paradoxical reactions in some patients when there is predominant inhibition of one cytokine in the inflammatory pathway. In rare cases, multiple paradoxical reactions are possible.
1. Vossen ARJV, van der Zee HH, Prens EP. Hidradenitis suppurativa: a systematic review integrating inflammatory pathways into a cohesive pathogenic model. Front Immunol. 2018;9:2965. doi:10.3389/fimmu.2018.02965
2. Goldburg SR, Strober BE, Payette MJ. Hidradenitis suppurativa: epidemiology, clinical presentation and pathogenesis. J Am Acad Dermatol. 2020; 82:1045-1058. doi:10.1016/j.jaad.2019.08.090
3. Brown G, Wang E, Leon A, et al. Tumor necrosis factor-α inhibitor-induced psoriasis: systematic review of clinical features, histopathological findings, and management experience. J Am Acad Dermatol. 2017;76:334-341. doi:10.1016/j.jaad.2016.08.012
4. Puig L. Paradoxical reactions: anti-tumor necrosis factor alpha agents, ustekinumab, secukinumab, ixekizumab and others. Curr Prob Dermatol. 2018;53:49-63. doi:10.1159/000479475
5. Faivre C, Villani AP, Aubin F, et al; . Hidradenitis suppurativa (HS): an unrecognized paradoxical effect of biologic agents (BA) used in chronic inflammatory diseases. J Am Acad Dermatol. 2016;74:1153-1159. doi:10.1016/j.jaad.2016.01.018
6. Ko JM, Gottlieb AB, Kerbleski JF. Induction and exacerbation of psoriasis with TNF-blockade therapy: a review and analysis of 127 cases. J Dermatolog Treat. 2009;20:100-108. doi:10.1080/09546630802441234
7. Maverakis E, Ma C, Shinkai K, et al. Diagnostic criteria of ulcerative pyoderma gangrenosum: a delphi consensus of international experts. JAMA Dermatol. 2018;154:461-466. doi:10.1001/jamadermatol.2017.5980
8. Lima AL, Karl I, Giner T, et al. Keratinocytes and neutrophils are important sources of proinflammatory molecules in hidradenitis suppurativa. Br J Dermatol. 2016;174:514-521. doi:10.1111/bjd.14214
9. Li SJ, Perez-Chada LM, Merola JF. TNF inhibitor-induced psoriasis: proposed algorithm for treatment and management. J Psoriasis Psoriatic Arthritis. 2019;4:70-80. doi:10.1177/2475530318810851
10. Conrad C, Di Domizio J, Mylonas A, et al. TNF blockade induces a dysregulated type I interferon response without autoimmunity in paradoxical psoriasis. Nat Commun. 2018;9:25. doi:10.1038/s41467-017-02466-4
11. Matos TR, O’Malley JT, Lowry EL, et al. Clinically resolved psoriatic lesions contain psoriasis-specific IL-17-producing αβ T cell clones. J Clin Invest. 2017;127:4031-4041. doi:10.1172/JCI93396
12. Faivre C, Villani AP, Aubin F, et al. Hidradenitis suppurativa (HS): an unrecognized paradoxical effect of biologic agents (BA) used in chronic inflammatory diseases. J Am Acad Dermatol. 2016;74:1153-1159. doi:10.1016/j.jaad.2016.01.018
13. Marzano AV, Damiani G, Ceccherini I, et al. Autoinflammation in pyoderma gangrenosum and its syndromic form (pyoderma gangrenosum, acne and suppurative hidradenitis). Br J Dermatol. 2017;176:1588-1598. doi:10.1111/bjd.15226
14. Cugno M, Borghi A, Marzano AV. PAPA, PASH, PAPASH syndromes: pathophysiology, presentation and treatment. Am J Clin Dermatol. 2017;18:555-562. doi:10.1007/s40257-017-0265-1
15. Wang EA, Steel A, Luxardi G, et al. Classic ulcerative pyoderma gangrenosum is a T cell-mediated disease targeting follicular adnexal structures: a hypothesis based on molecular and clinicopathologic studies. Front Immunol. 2018;8:1980. doi:10.3389/fimmu.2017.01980
16. Patel F, Fitzmaurice S, Duong C, et al. Effective strategies for the management of pyoderma gangrenosum: a comprehensive review. Acta Derm Venereol. 2015;95:525-531. doi:10.2340/00015555-2008
17. Partridge ACR, Bai JW, Rosen CF, et al. Effectiveness of systemic treatments for pyoderma gangrenosum: a systematic review of observational studies and clinical trials. Br J Dermatol. 2018;179:290-295. doi:10.1111/bjd.16485
18. Benzaquen M, Monnier J, Beaussault Y, et al. Pyoderma gangrenosum arising during treatment of psoriasis with adalimumab: effectiveness of ustekinumab. Australas J Dermatol. 2017;58:e270-e271. doi:10.1111/ajd.12545
19. Fujimoto N, Yamasaki Y, Watanabe RJ. Paradoxical uveitis and pyoderma gangrenosum in a patient with psoriatic arthritis under infliximab treatment. J Dtsch Dermatol Ges. 2018;16:1139-1140. doi:10.1111/ddg.13632
20. Tannenbaum R, Strunk A, Garg A. Overall and subgroup prevalence of pyoderma gangrenosum among patients with hidradenitis suppurativa: a population-based analysis in the United States. J Am Acad Dermatol. 2019;80:1533-1537. doi:10.1016/j.jaad.2019.02.004
1. Vossen ARJV, van der Zee HH, Prens EP. Hidradenitis suppurativa: a systematic review integrating inflammatory pathways into a cohesive pathogenic model. Front Immunol. 2018;9:2965. doi:10.3389/fimmu.2018.02965
2. Goldburg SR, Strober BE, Payette MJ. Hidradenitis suppurativa: epidemiology, clinical presentation and pathogenesis. J Am Acad Dermatol. 2020; 82:1045-1058. doi:10.1016/j.jaad.2019.08.090
3. Brown G, Wang E, Leon A, et al. Tumor necrosis factor-α inhibitor-induced psoriasis: systematic review of clinical features, histopathological findings, and management experience. J Am Acad Dermatol. 2017;76:334-341. doi:10.1016/j.jaad.2016.08.012
4. Puig L. Paradoxical reactions: anti-tumor necrosis factor alpha agents, ustekinumab, secukinumab, ixekizumab and others. Curr Prob Dermatol. 2018;53:49-63. doi:10.1159/000479475
5. Faivre C, Villani AP, Aubin F, et al; . Hidradenitis suppurativa (HS): an unrecognized paradoxical effect of biologic agents (BA) used in chronic inflammatory diseases. J Am Acad Dermatol. 2016;74:1153-1159. doi:10.1016/j.jaad.2016.01.018
6. Ko JM, Gottlieb AB, Kerbleski JF. Induction and exacerbation of psoriasis with TNF-blockade therapy: a review and analysis of 127 cases. J Dermatolog Treat. 2009;20:100-108. doi:10.1080/09546630802441234
7. Maverakis E, Ma C, Shinkai K, et al. Diagnostic criteria of ulcerative pyoderma gangrenosum: a delphi consensus of international experts. JAMA Dermatol. 2018;154:461-466. doi:10.1001/jamadermatol.2017.5980
8. Lima AL, Karl I, Giner T, et al. Keratinocytes and neutrophils are important sources of proinflammatory molecules in hidradenitis suppurativa. Br J Dermatol. 2016;174:514-521. doi:10.1111/bjd.14214
9. Li SJ, Perez-Chada LM, Merola JF. TNF inhibitor-induced psoriasis: proposed algorithm for treatment and management. J Psoriasis Psoriatic Arthritis. 2019;4:70-80. doi:10.1177/2475530318810851
10. Conrad C, Di Domizio J, Mylonas A, et al. TNF blockade induces a dysregulated type I interferon response without autoimmunity in paradoxical psoriasis. Nat Commun. 2018;9:25. doi:10.1038/s41467-017-02466-4
11. Matos TR, O’Malley JT, Lowry EL, et al. Clinically resolved psoriatic lesions contain psoriasis-specific IL-17-producing αβ T cell clones. J Clin Invest. 2017;127:4031-4041. doi:10.1172/JCI93396
12. Faivre C, Villani AP, Aubin F, et al. Hidradenitis suppurativa (HS): an unrecognized paradoxical effect of biologic agents (BA) used in chronic inflammatory diseases. J Am Acad Dermatol. 2016;74:1153-1159. doi:10.1016/j.jaad.2016.01.018
13. Marzano AV, Damiani G, Ceccherini I, et al. Autoinflammation in pyoderma gangrenosum and its syndromic form (pyoderma gangrenosum, acne and suppurative hidradenitis). Br J Dermatol. 2017;176:1588-1598. doi:10.1111/bjd.15226
14. Cugno M, Borghi A, Marzano AV. PAPA, PASH, PAPASH syndromes: pathophysiology, presentation and treatment. Am J Clin Dermatol. 2017;18:555-562. doi:10.1007/s40257-017-0265-1
15. Wang EA, Steel A, Luxardi G, et al. Classic ulcerative pyoderma gangrenosum is a T cell-mediated disease targeting follicular adnexal structures: a hypothesis based on molecular and clinicopathologic studies. Front Immunol. 2018;8:1980. doi:10.3389/fimmu.2017.01980
16. Patel F, Fitzmaurice S, Duong C, et al. Effective strategies for the management of pyoderma gangrenosum: a comprehensive review. Acta Derm Venereol. 2015;95:525-531. doi:10.2340/00015555-2008
17. Partridge ACR, Bai JW, Rosen CF, et al. Effectiveness of systemic treatments for pyoderma gangrenosum: a systematic review of observational studies and clinical trials. Br J Dermatol. 2018;179:290-295. doi:10.1111/bjd.16485
18. Benzaquen M, Monnier J, Beaussault Y, et al. Pyoderma gangrenosum arising during treatment of psoriasis with adalimumab: effectiveness of ustekinumab. Australas J Dermatol. 2017;58:e270-e271. doi:10.1111/ajd.12545
19. Fujimoto N, Yamasaki Y, Watanabe RJ. Paradoxical uveitis and pyoderma gangrenosum in a patient with psoriatic arthritis under infliximab treatment. J Dtsch Dermatol Ges. 2018;16:1139-1140. doi:10.1111/ddg.13632
20. Tannenbaum R, Strunk A, Garg A. Overall and subgroup prevalence of pyoderma gangrenosum among patients with hidradenitis suppurativa: a population-based analysis in the United States. J Am Acad Dermatol. 2019;80:1533-1537. doi:10.1016/j.jaad.2019.02.004
Practice Points
- Clinicians need to be aware of the potential risk for a paradoxical reaction in patients receiving a tumor necrosis factor α (TNF-α) inhibitor for hidradenitis suppurativa.
- Although uncommon, developing more than 1 type of paradoxical skin reaction is possible with a TNF-α inhibitor.
- Early recognition and appropriate management of these paradoxical reactions are critical.
First referral guide issued for axial spondyloarthritis
SAN DIEGO – The Spondyloarthritis Research and Treatment Network (SPARTAN) has created the first referral recommendations for axial spondyloarthritis (axSpA).
The draft recommendations use a points scoring system, with the goal that at least one in three patients referred would be diagnosed with axSpA, an inflammatory arthritis that affects the central skeleton and shares a genetic overlap with skin psoriasis, inflammatory bowel disease, and inflammatory eye disease.
Patients with axSpA can wait 10 years after symptom onset to be diagnosed with the condition. There are currently no guidelines to advise clinicians on when to refer to a rheumatologist, and with the rheumatology workforce shortage, “it is impossible for rheumatologists to evaluate the 20% of adults in the U.S. who have chronic back pain,” said Maureen Dubreuil, MD, a rheumatologist at Boston University. She presented the work at the annual meeting of the American College of Rheumatology.
To address this issue, Dr. Dubreuil and colleagues conducted a literature review to determine how predictive different spondyloarthritis features were of eventual axSpA diagnosis. The interdisciplinary team identified 38 studies published before March 2022, and uncovered 28 individual potential features associated with axSpA, including pain sites, family history of axSpA and related conditions, blood markers of inflammation, genetic testing, and imaging findings.
Inflammatory back pain elements had the lower predictive values, with positive likelihood ratios (LR+) ranging from 1.15 to 2.32, while imaging findings were the most predictive (LR+s from 6.40 to 10.02).
Using a Delphi exercise and discrete choice experiments, members narrowed the checklist down to 10 features. These 10 features were assigned points, with a score of 3 points qualifying for a referral of adults 45 years or younger with chronic pain (3 or more months) in the back, hip, or buttock.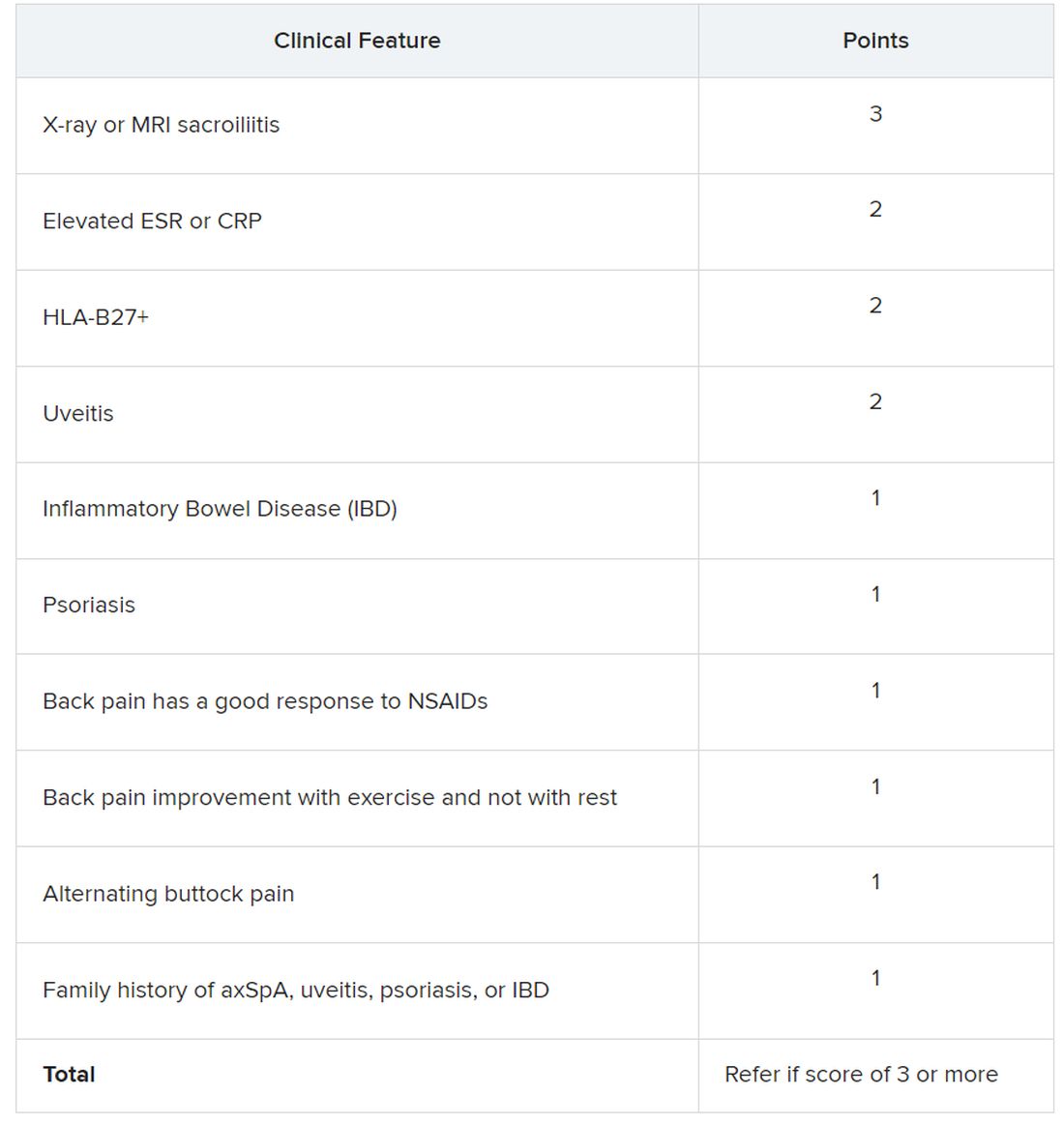
Sacroiliitis seen on imaging, either by x-ray or MRI, received the highest score of 3 points. Dr. Dubreuil emphasized that imaging was not required for a referral, but if a patient has received imaging “that shows sacroiliitis, that is sufficient for referral to a rheumatologist,” she said in her presentation.
Elevated erythrocyte sedimentation rate or C-reactive protein, HLA-B27 positivity, and uveitis score 2 points. Inflammatory bowel disease; psoriasis; back pain with good response to NSAIDs; back pain improvement with exercise and not with rest; alternating buttock pain; and family history of axial spondyloarthritis, uveitis, psoriasis, or IBD score 1 point.
Dr. Dubreuil and colleagues expect that these criteria for referral will result in about one in three referred adults aged 45 years or younger with chronic back pain being diagnosed with axSpA. They also say additional research is necessary to understand if these recommendations increase probability of axSpA diagnosis and reduce diagnostic delays.
“We’re now getting to the stage where we are creating this screening tool, but [testing the] performance of the screening tool is going to be the major next step,” said Mark Hwang, MD, of UTHealth Houston in an interview with this news organization. He is a member of SPARTAN but was not involved with authoring the recommendations. “Will the screening tool enhance the ability on the back end to identify axSpA? We don’t know yet.”
Jon Chan, MD, a rheumatologist at the University of British Columbia, Vancouver, agreed that these recommendations “are a good first step,” but that more awareness about axSpA from nonrheumatologists would also be helpful in identifying new axSpA patients. He is also a member of SPARTAN and comoderated with Dr. Hwang the session where the new recommendations were presented. “I think other diseases like rheumatoid arthritis or lupus have a lot more recognition in the nonrheumatology community,” he told this news organization.
Connecting with other health professionals who see a lot of patients with back pain – physiotherapists, chiropractors, and chronic pain physicians – could also be helpful, he added. “A lot of times, patients go straight to a physio and circumvent the doctor,” he said.
Dr. Chan reports success in educating other departments. “I put up a poster in the emergency department saying, ‘If you’re young with back pain and uveitis, you need to be seen by rheumatology,’ and we’ve identified a ton of axSpA patients that way,” he said. “Maybe their uveitis was very mild, but their back pain was quite severe, and no one really clued in.”
Dr. Dubreuil disclosed financial relationships with Amgen, Pfizer, and UCB Pharma. Her abstract coauthors disclosed financial relationships with multiple pharmaceutical companies. Dr. Hwang consults for UCB and has received research support from Janssen. Dr. Chan has relationships with AbbVie/Abbott, Eli Lilly, Janssen, Novartis, and UCB.
SAN DIEGO – The Spondyloarthritis Research and Treatment Network (SPARTAN) has created the first referral recommendations for axial spondyloarthritis (axSpA).
The draft recommendations use a points scoring system, with the goal that at least one in three patients referred would be diagnosed with axSpA, an inflammatory arthritis that affects the central skeleton and shares a genetic overlap with skin psoriasis, inflammatory bowel disease, and inflammatory eye disease.
Patients with axSpA can wait 10 years after symptom onset to be diagnosed with the condition. There are currently no guidelines to advise clinicians on when to refer to a rheumatologist, and with the rheumatology workforce shortage, “it is impossible for rheumatologists to evaluate the 20% of adults in the U.S. who have chronic back pain,” said Maureen Dubreuil, MD, a rheumatologist at Boston University. She presented the work at the annual meeting of the American College of Rheumatology.
To address this issue, Dr. Dubreuil and colleagues conducted a literature review to determine how predictive different spondyloarthritis features were of eventual axSpA diagnosis. The interdisciplinary team identified 38 studies published before March 2022, and uncovered 28 individual potential features associated with axSpA, including pain sites, family history of axSpA and related conditions, blood markers of inflammation, genetic testing, and imaging findings.
Inflammatory back pain elements had the lower predictive values, with positive likelihood ratios (LR+) ranging from 1.15 to 2.32, while imaging findings were the most predictive (LR+s from 6.40 to 10.02).
Using a Delphi exercise and discrete choice experiments, members narrowed the checklist down to 10 features. These 10 features were assigned points, with a score of 3 points qualifying for a referral of adults 45 years or younger with chronic pain (3 or more months) in the back, hip, or buttock.
Sacroiliitis seen on imaging, either by x-ray or MRI, received the highest score of 3 points. Dr. Dubreuil emphasized that imaging was not required for a referral, but if a patient has received imaging “that shows sacroiliitis, that is sufficient for referral to a rheumatologist,” she said in her presentation.
Elevated erythrocyte sedimentation rate or C-reactive protein, HLA-B27 positivity, and uveitis score 2 points. Inflammatory bowel disease; psoriasis; back pain with good response to NSAIDs; back pain improvement with exercise and not with rest; alternating buttock pain; and family history of axial spondyloarthritis, uveitis, psoriasis, or IBD score 1 point.
Dr. Dubreuil and colleagues expect that these criteria for referral will result in about one in three referred adults aged 45 years or younger with chronic back pain being diagnosed with axSpA. They also say additional research is necessary to understand if these recommendations increase probability of axSpA diagnosis and reduce diagnostic delays.
“We’re now getting to the stage where we are creating this screening tool, but [testing the] performance of the screening tool is going to be the major next step,” said Mark Hwang, MD, of UTHealth Houston in an interview with this news organization. He is a member of SPARTAN but was not involved with authoring the recommendations. “Will the screening tool enhance the ability on the back end to identify axSpA? We don’t know yet.”
Jon Chan, MD, a rheumatologist at the University of British Columbia, Vancouver, agreed that these recommendations “are a good first step,” but that more awareness about axSpA from nonrheumatologists would also be helpful in identifying new axSpA patients. He is also a member of SPARTAN and comoderated with Dr. Hwang the session where the new recommendations were presented. “I think other diseases like rheumatoid arthritis or lupus have a lot more recognition in the nonrheumatology community,” he told this news organization.
Connecting with other health professionals who see a lot of patients with back pain – physiotherapists, chiropractors, and chronic pain physicians – could also be helpful, he added. “A lot of times, patients go straight to a physio and circumvent the doctor,” he said.
Dr. Chan reports success in educating other departments. “I put up a poster in the emergency department saying, ‘If you’re young with back pain and uveitis, you need to be seen by rheumatology,’ and we’ve identified a ton of axSpA patients that way,” he said. “Maybe their uveitis was very mild, but their back pain was quite severe, and no one really clued in.”
Dr. Dubreuil disclosed financial relationships with Amgen, Pfizer, and UCB Pharma. Her abstract coauthors disclosed financial relationships with multiple pharmaceutical companies. Dr. Hwang consults for UCB and has received research support from Janssen. Dr. Chan has relationships with AbbVie/Abbott, Eli Lilly, Janssen, Novartis, and UCB.
SAN DIEGO – The Spondyloarthritis Research and Treatment Network (SPARTAN) has created the first referral recommendations for axial spondyloarthritis (axSpA).
The draft recommendations use a points scoring system, with the goal that at least one in three patients referred would be diagnosed with axSpA, an inflammatory arthritis that affects the central skeleton and shares a genetic overlap with skin psoriasis, inflammatory bowel disease, and inflammatory eye disease.
Patients with axSpA can wait 10 years after symptom onset to be diagnosed with the condition. There are currently no guidelines to advise clinicians on when to refer to a rheumatologist, and with the rheumatology workforce shortage, “it is impossible for rheumatologists to evaluate the 20% of adults in the U.S. who have chronic back pain,” said Maureen Dubreuil, MD, a rheumatologist at Boston University. She presented the work at the annual meeting of the American College of Rheumatology.
To address this issue, Dr. Dubreuil and colleagues conducted a literature review to determine how predictive different spondyloarthritis features were of eventual axSpA diagnosis. The interdisciplinary team identified 38 studies published before March 2022, and uncovered 28 individual potential features associated with axSpA, including pain sites, family history of axSpA and related conditions, blood markers of inflammation, genetic testing, and imaging findings.
Inflammatory back pain elements had the lower predictive values, with positive likelihood ratios (LR+) ranging from 1.15 to 2.32, while imaging findings were the most predictive (LR+s from 6.40 to 10.02).
Using a Delphi exercise and discrete choice experiments, members narrowed the checklist down to 10 features. These 10 features were assigned points, with a score of 3 points qualifying for a referral of adults 45 years or younger with chronic pain (3 or more months) in the back, hip, or buttock.
Sacroiliitis seen on imaging, either by x-ray or MRI, received the highest score of 3 points. Dr. Dubreuil emphasized that imaging was not required for a referral, but if a patient has received imaging “that shows sacroiliitis, that is sufficient for referral to a rheumatologist,” she said in her presentation.
Elevated erythrocyte sedimentation rate or C-reactive protein, HLA-B27 positivity, and uveitis score 2 points. Inflammatory bowel disease; psoriasis; back pain with good response to NSAIDs; back pain improvement with exercise and not with rest; alternating buttock pain; and family history of axial spondyloarthritis, uveitis, psoriasis, or IBD score 1 point.
Dr. Dubreuil and colleagues expect that these criteria for referral will result in about one in three referred adults aged 45 years or younger with chronic back pain being diagnosed with axSpA. They also say additional research is necessary to understand if these recommendations increase probability of axSpA diagnosis and reduce diagnostic delays.
“We’re now getting to the stage where we are creating this screening tool, but [testing the] performance of the screening tool is going to be the major next step,” said Mark Hwang, MD, of UTHealth Houston in an interview with this news organization. He is a member of SPARTAN but was not involved with authoring the recommendations. “Will the screening tool enhance the ability on the back end to identify axSpA? We don’t know yet.”
Jon Chan, MD, a rheumatologist at the University of British Columbia, Vancouver, agreed that these recommendations “are a good first step,” but that more awareness about axSpA from nonrheumatologists would also be helpful in identifying new axSpA patients. He is also a member of SPARTAN and comoderated with Dr. Hwang the session where the new recommendations were presented. “I think other diseases like rheumatoid arthritis or lupus have a lot more recognition in the nonrheumatology community,” he told this news organization.
Connecting with other health professionals who see a lot of patients with back pain – physiotherapists, chiropractors, and chronic pain physicians – could also be helpful, he added. “A lot of times, patients go straight to a physio and circumvent the doctor,” he said.
Dr. Chan reports success in educating other departments. “I put up a poster in the emergency department saying, ‘If you’re young with back pain and uveitis, you need to be seen by rheumatology,’ and we’ve identified a ton of axSpA patients that way,” he said. “Maybe their uveitis was very mild, but their back pain was quite severe, and no one really clued in.”
Dr. Dubreuil disclosed financial relationships with Amgen, Pfizer, and UCB Pharma. Her abstract coauthors disclosed financial relationships with multiple pharmaceutical companies. Dr. Hwang consults for UCB and has received research support from Janssen. Dr. Chan has relationships with AbbVie/Abbott, Eli Lilly, Janssen, Novartis, and UCB.
AT ACR 2023
Abstracts from the Neurology Exchange 2023, a virtual event held September 19-21, 2023
T2D: Real benefits of new oral antidiabetic drugs
Cardiovascular disease is the most common cause of death in people living with type 2 diabetes (T2D). It is true that patient prognoses have improved with the use of metformin and by addressing cardiovascular risk factors. But the new oral antidiabetic drugs, SGLT2 (sodium glucose cotransporter-2) inhibitors (SGLT2i), and glucagon-like peptide-1 receptor agonists (GLP-1Ra) offer fresh therapeutic approaches.
A cohort of more than 2 million patients with T2D
What about in the real world, far away from the ideal conditions of randomized trials? Could combining SGLT2 inhibitors with GLP-1R agonists be even more effective?
These are the questions answered by a large retrospective cohort study in which 2.2 million patients with T2D receiving insulin were initially enrolled and monitored at 85 specialist centers spread throughout three countries (Denmark, the United Kingdom, and the United States).
Three groups were formed from this cohort according to whether they received monotherapy or combination treatments: SGLT2i (n = 143,600), GLP-1Ra (n = 186,841), and SGLT2i + GLP-1Ra (n = 108,5040). A control group received none of these treatments.
Propensity score matching took into account the following relevant variables: age, sex, ischemic heart disease, hypertension, chronic kidney disease, heart failure, and glycated hemoglobin. The data was analyzed using the Cox’s proportional hazards model, with follow-up at 5 years.
Real-world benefits – Even better when combined
The inter-group comparison suggests that oral antidiabetic agents are effective when taking into account three major events:
All-cause mortality: SGLT2i (hazard ratio, 0.49; confidence interval 95% 0.48-0.50); GLP-1Ra (HR, 0.47; CI 95% 0.46-0.48); SGLT2i + GLP-1Ra (HR, 0.25; CI 95% 0.24-0.26).
Admissions rate: respectively HR: 0.73 (0.72-0.74); 0.69 (0.68-0.69); 0.60 (0.59-0.61).
Myocardial infarction rate: respectively HR: 0.75 (0.72-0.78); 0.70 (0.68-0.73); 0.63 (0.60-0.66).
A complementary sub-analysis also revealed a more significant reduction in all-cause mortality in the event of exposure to the combination of two antidiabetic drugs versus SGLT2i alone (HR, 0.53 [0.50-0.55]) and GLP-1Ra as monotherapy (HR, 0.56 [0.54-0.59]).
This real-world retrospective cohort study involves a large sample size: more than 400,000 patients with T2D treated with new oral antidiabetic drugs and as many control patients. It suggests that SGLT2 inhibitors and GLP-1R agonists have a significant effect on overall mortality, as well as on the risk of myocardial infarction and the admissions rate. Yes, it is retrospective, but its findings are in line with those from the most recent and conclusive randomized trials that suggest a cardio- and nephroprotective effect, at least with regard to SGLT2 inhibitors.
This article was translated from JIM and a version appeared on Medscape.com.
Cardiovascular disease is the most common cause of death in people living with type 2 diabetes (T2D). It is true that patient prognoses have improved with the use of metformin and by addressing cardiovascular risk factors. But the new oral antidiabetic drugs, SGLT2 (sodium glucose cotransporter-2) inhibitors (SGLT2i), and glucagon-like peptide-1 receptor agonists (GLP-1Ra) offer fresh therapeutic approaches.
A cohort of more than 2 million patients with T2D
What about in the real world, far away from the ideal conditions of randomized trials? Could combining SGLT2 inhibitors with GLP-1R agonists be even more effective?
These are the questions answered by a large retrospective cohort study in which 2.2 million patients with T2D receiving insulin were initially enrolled and monitored at 85 specialist centers spread throughout three countries (Denmark, the United Kingdom, and the United States).
Three groups were formed from this cohort according to whether they received monotherapy or combination treatments: SGLT2i (n = 143,600), GLP-1Ra (n = 186,841), and SGLT2i + GLP-1Ra (n = 108,5040). A control group received none of these treatments.
Propensity score matching took into account the following relevant variables: age, sex, ischemic heart disease, hypertension, chronic kidney disease, heart failure, and glycated hemoglobin. The data was analyzed using the Cox’s proportional hazards model, with follow-up at 5 years.
Real-world benefits – Even better when combined
The inter-group comparison suggests that oral antidiabetic agents are effective when taking into account three major events:
All-cause mortality: SGLT2i (hazard ratio, 0.49; confidence interval 95% 0.48-0.50); GLP-1Ra (HR, 0.47; CI 95% 0.46-0.48); SGLT2i + GLP-1Ra (HR, 0.25; CI 95% 0.24-0.26).
Admissions rate: respectively HR: 0.73 (0.72-0.74); 0.69 (0.68-0.69); 0.60 (0.59-0.61).
Myocardial infarction rate: respectively HR: 0.75 (0.72-0.78); 0.70 (0.68-0.73); 0.63 (0.60-0.66).
A complementary sub-analysis also revealed a more significant reduction in all-cause mortality in the event of exposure to the combination of two antidiabetic drugs versus SGLT2i alone (HR, 0.53 [0.50-0.55]) and GLP-1Ra as monotherapy (HR, 0.56 [0.54-0.59]).
This real-world retrospective cohort study involves a large sample size: more than 400,000 patients with T2D treated with new oral antidiabetic drugs and as many control patients. It suggests that SGLT2 inhibitors and GLP-1R agonists have a significant effect on overall mortality, as well as on the risk of myocardial infarction and the admissions rate. Yes, it is retrospective, but its findings are in line with those from the most recent and conclusive randomized trials that suggest a cardio- and nephroprotective effect, at least with regard to SGLT2 inhibitors.
This article was translated from JIM and a version appeared on Medscape.com.
Cardiovascular disease is the most common cause of death in people living with type 2 diabetes (T2D). It is true that patient prognoses have improved with the use of metformin and by addressing cardiovascular risk factors. But the new oral antidiabetic drugs, SGLT2 (sodium glucose cotransporter-2) inhibitors (SGLT2i), and glucagon-like peptide-1 receptor agonists (GLP-1Ra) offer fresh therapeutic approaches.
A cohort of more than 2 million patients with T2D
What about in the real world, far away from the ideal conditions of randomized trials? Could combining SGLT2 inhibitors with GLP-1R agonists be even more effective?
These are the questions answered by a large retrospective cohort study in which 2.2 million patients with T2D receiving insulin were initially enrolled and monitored at 85 specialist centers spread throughout three countries (Denmark, the United Kingdom, and the United States).
Three groups were formed from this cohort according to whether they received monotherapy or combination treatments: SGLT2i (n = 143,600), GLP-1Ra (n = 186,841), and SGLT2i + GLP-1Ra (n = 108,5040). A control group received none of these treatments.
Propensity score matching took into account the following relevant variables: age, sex, ischemic heart disease, hypertension, chronic kidney disease, heart failure, and glycated hemoglobin. The data was analyzed using the Cox’s proportional hazards model, with follow-up at 5 years.
Real-world benefits – Even better when combined
The inter-group comparison suggests that oral antidiabetic agents are effective when taking into account three major events:
All-cause mortality: SGLT2i (hazard ratio, 0.49; confidence interval 95% 0.48-0.50); GLP-1Ra (HR, 0.47; CI 95% 0.46-0.48); SGLT2i + GLP-1Ra (HR, 0.25; CI 95% 0.24-0.26).
Admissions rate: respectively HR: 0.73 (0.72-0.74); 0.69 (0.68-0.69); 0.60 (0.59-0.61).
Myocardial infarction rate: respectively HR: 0.75 (0.72-0.78); 0.70 (0.68-0.73); 0.63 (0.60-0.66).
A complementary sub-analysis also revealed a more significant reduction in all-cause mortality in the event of exposure to the combination of two antidiabetic drugs versus SGLT2i alone (HR, 0.53 [0.50-0.55]) and GLP-1Ra as monotherapy (HR, 0.56 [0.54-0.59]).
This real-world retrospective cohort study involves a large sample size: more than 400,000 patients with T2D treated with new oral antidiabetic drugs and as many control patients. It suggests that SGLT2 inhibitors and GLP-1R agonists have a significant effect on overall mortality, as well as on the risk of myocardial infarction and the admissions rate. Yes, it is retrospective, but its findings are in line with those from the most recent and conclusive randomized trials that suggest a cardio- and nephroprotective effect, at least with regard to SGLT2 inhibitors.
This article was translated from JIM and a version appeared on Medscape.com.
Pharmacist-based strategy places more patients on statins
Visit-based strategy has more modest effect
PHILADELPHIA – In two studies run in parallel fashion to test different strategies, one that employed automatic referral to a pharmacist appeared to be superior to one using alerts from the electronic health record (EHR) in increasing the number of at-risk patients receiving a prescription for statins.
, reported Alexander C. Faranoff, MD, assistant professor of cardiovascular medicine at Penn Medicine, Philadelphia.
The parallel studies were part of the SUPER LIPID program, created to generate evidence-based strategies for increasing the proportion of at-risk patients on statins. Dr. Faranoff said current data show that at least 50% of patients indicated for high-intensity statins in the United States are not taking them.
The two studies were presented together in a late breaking presentation at the American Heart Association scientific sessions.
EHR algorithm identifies statin candidates
The candidates for statin therapy were identified through an EHR algorithm for both studies. Both compared the impact of the intervention against a baseline period of usual care, although the study of EHR alerts also randomized physicians to provide usual care for 3 months or 6 months prior to intervention.
Dr. Faranoff described these interventions as non–visit related and visit related.
In the study of the non–visit-related strategy, referrals were generated by EHR and sent directly to the pharmacist. Upon receipt, the pharmacist verified the order was appropriate and called the patient directly to discuss starting therapy. Patients agreeing to start a statin were provided with a prescription and followed by the pharmacist.
In the study of the patient-visit approach, physicians seeing EHR-identified candidates received interruptive pop-up alerts during patient encounters. The physicians were randomized to provide usual care for 3 or 6 months before they began receiving alerts. The alerts recommended referral to a pharmacist.
During usual care in the non–visit-related study, only 15.2% of the 975 candidates for statins received a prescription. During the intervention period, the rate climbed to 31.6%. Statistically, the intervention more than doubled the odds ratio (OR) of receiving a statin prescription relative to usual care (OR 2.22; 95% confidence interval [CI] 1.47-3.37).
In addition, the proportion of patients receiving an appropriate dose of statins climbed from 7.7% in the period of usual care to 24.8% in the intervention period (OR 6.79; 95% CI 4.00-11.53).
Visit-based study also randomized
In the study evaluating a visit-based intervention, 16 physicians were randomized to deliver usual care for 3 or 6 months. Of physicians randomized to 3 months, 970 candidates for statins were treated during the 6-month intervention period. The physicians randomized to usual care for 6 months treated 672 candidates for statins during a 3-month intervention period,
More than 3,000 alerts were sent to both groups of physicians over the intervention period. Only 165 (4.6%) were associated with a prescription.
For the group randomized to 3 months of usual care, the proportion of candidates for statins who received a prescription rose from 14.9% during the period of usual care to 17.6% in the first 3 months of intervention and then fell slightly to 15.5% in the second 3 months.
For the group randomized to usual care for 6 months, the proportion of candidates for statins who received a prescription rose from about 11% during the period of usual care to 14.6%. Combining data from both arms, the small gain in prescriptions was significant but modest (OR 1.43; 95% CI 1.02-2.00).
In addition, the visit-based EHR notifications failed to yield a significant gain in the proportion of patients on an appropriate statin dose. During the intervention period, this proportion was only about 9% of patients treated by either of the two groups of randomized physicians,
The SUPER LIPID program involved 11 internal medicine and family medicine clinics in rural Pennsylvania. In the visit-based intervention, 16 primary care physicians (PCPs) were randomized. In the asynchronous intervention, 10 primary care practices participated. The EHR identified a total of 1,950 candidates for a statin.
Although the gain in statin prescriptions was disappointing for the visit-based intervention, the strategy of using the EHR to refer statin-eligible patients to pharmacists “could be an effective adjunct to visit-based clinical interactions in increasing statin prescribing for high-risk patients,” Dr. Faranoff maintained.
Overcoming clinical inertia a challenge
The greater efficacy of a pharmacist-based approach did not surprise the AHA-invited discussant, Benjamin M. Scirica, MD, associate professor of medicine at Harvard Medical School, Boston.
Pointing out that the pharmacist-based strategy of increasing statin prescriptions is more complicated and more costly, he said, “You get what you pay for.” In his opinion, simple solutions are unlikely ever to be effective due to the complex reasons for clinical inertia. Overall, he thinks a multifaceted approach to placing more patients who need statins on therapy is essential.
“Implementation science is hard,” Dr. Scirica said. Even though the referral-to-a-pharmacist approach ended up putting more patients on statins and putting them on an appropriate dose, he said even this more effective strategy “is still not getting to the majority of patients.”
This does not mean that this approach is without merit or should not be one of many strategies employed, but Dr. Scirica said “there is so much more to be done,” and that it should be employed along with other initiatives.
Faranoff reports no potential conflicts of interest. Dr. Scirica reports financial relationships with AbbVie, Aktiia, AstraZeneca, Better Therapeutics, Boehringer-Ingelheim, Eisai, GlaxoSmithKline, Hanmi, Lexicon, Merck, Novartis, Novo Nordisk, Pfizer, and Sanofi.
Visit-based strategy has more modest effect
Visit-based strategy has more modest effect
PHILADELPHIA – In two studies run in parallel fashion to test different strategies, one that employed automatic referral to a pharmacist appeared to be superior to one using alerts from the electronic health record (EHR) in increasing the number of at-risk patients receiving a prescription for statins.
, reported Alexander C. Faranoff, MD, assistant professor of cardiovascular medicine at Penn Medicine, Philadelphia.
The parallel studies were part of the SUPER LIPID program, created to generate evidence-based strategies for increasing the proportion of at-risk patients on statins. Dr. Faranoff said current data show that at least 50% of patients indicated for high-intensity statins in the United States are not taking them.
The two studies were presented together in a late breaking presentation at the American Heart Association scientific sessions.
EHR algorithm identifies statin candidates
The candidates for statin therapy were identified through an EHR algorithm for both studies. Both compared the impact of the intervention against a baseline period of usual care, although the study of EHR alerts also randomized physicians to provide usual care for 3 months or 6 months prior to intervention.
Dr. Faranoff described these interventions as non–visit related and visit related.
In the study of the non–visit-related strategy, referrals were generated by EHR and sent directly to the pharmacist. Upon receipt, the pharmacist verified the order was appropriate and called the patient directly to discuss starting therapy. Patients agreeing to start a statin were provided with a prescription and followed by the pharmacist.
In the study of the patient-visit approach, physicians seeing EHR-identified candidates received interruptive pop-up alerts during patient encounters. The physicians were randomized to provide usual care for 3 or 6 months before they began receiving alerts. The alerts recommended referral to a pharmacist.
During usual care in the non–visit-related study, only 15.2% of the 975 candidates for statins received a prescription. During the intervention period, the rate climbed to 31.6%. Statistically, the intervention more than doubled the odds ratio (OR) of receiving a statin prescription relative to usual care (OR 2.22; 95% confidence interval [CI] 1.47-3.37).
In addition, the proportion of patients receiving an appropriate dose of statins climbed from 7.7% in the period of usual care to 24.8% in the intervention period (OR 6.79; 95% CI 4.00-11.53).
Visit-based study also randomized
In the study evaluating a visit-based intervention, 16 physicians were randomized to deliver usual care for 3 or 6 months. Of physicians randomized to 3 months, 970 candidates for statins were treated during the 6-month intervention period. The physicians randomized to usual care for 6 months treated 672 candidates for statins during a 3-month intervention period,
More than 3,000 alerts were sent to both groups of physicians over the intervention period. Only 165 (4.6%) were associated with a prescription.
For the group randomized to 3 months of usual care, the proportion of candidates for statins who received a prescription rose from 14.9% during the period of usual care to 17.6% in the first 3 months of intervention and then fell slightly to 15.5% in the second 3 months.
For the group randomized to usual care for 6 months, the proportion of candidates for statins who received a prescription rose from about 11% during the period of usual care to 14.6%. Combining data from both arms, the small gain in prescriptions was significant but modest (OR 1.43; 95% CI 1.02-2.00).
In addition, the visit-based EHR notifications failed to yield a significant gain in the proportion of patients on an appropriate statin dose. During the intervention period, this proportion was only about 9% of patients treated by either of the two groups of randomized physicians,
The SUPER LIPID program involved 11 internal medicine and family medicine clinics in rural Pennsylvania. In the visit-based intervention, 16 primary care physicians (PCPs) were randomized. In the asynchronous intervention, 10 primary care practices participated. The EHR identified a total of 1,950 candidates for a statin.
Although the gain in statin prescriptions was disappointing for the visit-based intervention, the strategy of using the EHR to refer statin-eligible patients to pharmacists “could be an effective adjunct to visit-based clinical interactions in increasing statin prescribing for high-risk patients,” Dr. Faranoff maintained.
Overcoming clinical inertia a challenge
The greater efficacy of a pharmacist-based approach did not surprise the AHA-invited discussant, Benjamin M. Scirica, MD, associate professor of medicine at Harvard Medical School, Boston.
Pointing out that the pharmacist-based strategy of increasing statin prescriptions is more complicated and more costly, he said, “You get what you pay for.” In his opinion, simple solutions are unlikely ever to be effective due to the complex reasons for clinical inertia. Overall, he thinks a multifaceted approach to placing more patients who need statins on therapy is essential.
“Implementation science is hard,” Dr. Scirica said. Even though the referral-to-a-pharmacist approach ended up putting more patients on statins and putting them on an appropriate dose, he said even this more effective strategy “is still not getting to the majority of patients.”
This does not mean that this approach is without merit or should not be one of many strategies employed, but Dr. Scirica said “there is so much more to be done,” and that it should be employed along with other initiatives.
Faranoff reports no potential conflicts of interest. Dr. Scirica reports financial relationships with AbbVie, Aktiia, AstraZeneca, Better Therapeutics, Boehringer-Ingelheim, Eisai, GlaxoSmithKline, Hanmi, Lexicon, Merck, Novartis, Novo Nordisk, Pfizer, and Sanofi.
PHILADELPHIA – In two studies run in parallel fashion to test different strategies, one that employed automatic referral to a pharmacist appeared to be superior to one using alerts from the electronic health record (EHR) in increasing the number of at-risk patients receiving a prescription for statins.
, reported Alexander C. Faranoff, MD, assistant professor of cardiovascular medicine at Penn Medicine, Philadelphia.
The parallel studies were part of the SUPER LIPID program, created to generate evidence-based strategies for increasing the proportion of at-risk patients on statins. Dr. Faranoff said current data show that at least 50% of patients indicated for high-intensity statins in the United States are not taking them.
The two studies were presented together in a late breaking presentation at the American Heart Association scientific sessions.
EHR algorithm identifies statin candidates
The candidates for statin therapy were identified through an EHR algorithm for both studies. Both compared the impact of the intervention against a baseline period of usual care, although the study of EHR alerts also randomized physicians to provide usual care for 3 months or 6 months prior to intervention.
Dr. Faranoff described these interventions as non–visit related and visit related.
In the study of the non–visit-related strategy, referrals were generated by EHR and sent directly to the pharmacist. Upon receipt, the pharmacist verified the order was appropriate and called the patient directly to discuss starting therapy. Patients agreeing to start a statin were provided with a prescription and followed by the pharmacist.
In the study of the patient-visit approach, physicians seeing EHR-identified candidates received interruptive pop-up alerts during patient encounters. The physicians were randomized to provide usual care for 3 or 6 months before they began receiving alerts. The alerts recommended referral to a pharmacist.
During usual care in the non–visit-related study, only 15.2% of the 975 candidates for statins received a prescription. During the intervention period, the rate climbed to 31.6%. Statistically, the intervention more than doubled the odds ratio (OR) of receiving a statin prescription relative to usual care (OR 2.22; 95% confidence interval [CI] 1.47-3.37).
In addition, the proportion of patients receiving an appropriate dose of statins climbed from 7.7% in the period of usual care to 24.8% in the intervention period (OR 6.79; 95% CI 4.00-11.53).
Visit-based study also randomized
In the study evaluating a visit-based intervention, 16 physicians were randomized to deliver usual care for 3 or 6 months. Of physicians randomized to 3 months, 970 candidates for statins were treated during the 6-month intervention period. The physicians randomized to usual care for 6 months treated 672 candidates for statins during a 3-month intervention period,
More than 3,000 alerts were sent to both groups of physicians over the intervention period. Only 165 (4.6%) were associated with a prescription.
For the group randomized to 3 months of usual care, the proportion of candidates for statins who received a prescription rose from 14.9% during the period of usual care to 17.6% in the first 3 months of intervention and then fell slightly to 15.5% in the second 3 months.
For the group randomized to usual care for 6 months, the proportion of candidates for statins who received a prescription rose from about 11% during the period of usual care to 14.6%. Combining data from both arms, the small gain in prescriptions was significant but modest (OR 1.43; 95% CI 1.02-2.00).
In addition, the visit-based EHR notifications failed to yield a significant gain in the proportion of patients on an appropriate statin dose. During the intervention period, this proportion was only about 9% of patients treated by either of the two groups of randomized physicians,
The SUPER LIPID program involved 11 internal medicine and family medicine clinics in rural Pennsylvania. In the visit-based intervention, 16 primary care physicians (PCPs) were randomized. In the asynchronous intervention, 10 primary care practices participated. The EHR identified a total of 1,950 candidates for a statin.
Although the gain in statin prescriptions was disappointing for the visit-based intervention, the strategy of using the EHR to refer statin-eligible patients to pharmacists “could be an effective adjunct to visit-based clinical interactions in increasing statin prescribing for high-risk patients,” Dr. Faranoff maintained.
Overcoming clinical inertia a challenge
The greater efficacy of a pharmacist-based approach did not surprise the AHA-invited discussant, Benjamin M. Scirica, MD, associate professor of medicine at Harvard Medical School, Boston.
Pointing out that the pharmacist-based strategy of increasing statin prescriptions is more complicated and more costly, he said, “You get what you pay for.” In his opinion, simple solutions are unlikely ever to be effective due to the complex reasons for clinical inertia. Overall, he thinks a multifaceted approach to placing more patients who need statins on therapy is essential.
“Implementation science is hard,” Dr. Scirica said. Even though the referral-to-a-pharmacist approach ended up putting more patients on statins and putting them on an appropriate dose, he said even this more effective strategy “is still not getting to the majority of patients.”
This does not mean that this approach is without merit or should not be one of many strategies employed, but Dr. Scirica said “there is so much more to be done,” and that it should be employed along with other initiatives.
Faranoff reports no potential conflicts of interest. Dr. Scirica reports financial relationships with AbbVie, Aktiia, AstraZeneca, Better Therapeutics, Boehringer-Ingelheim, Eisai, GlaxoSmithKline, Hanmi, Lexicon, Merck, Novartis, Novo Nordisk, Pfizer, and Sanofi.
AT AHA 2023
Spinal cord stimulator restores Parkinson patient’s gait
The neuroprosthesis involves targeted epidural electrical stimulation of areas of the lumbosacral spinal cord that produce walking.
This new therapeutic tool offers hope to patients with PD and, combined with existing approaches, may alleviate a motor sign in PD for which there is currently “no real solution,” study investigator Eduardo Martin Moraud, PhD, who leads PD research at the Defitech Center for Interventional Neurotherapies (NeuroRestore), Lausanne, Switzerland, said in an interview.
“This is exciting for the many patients that develop gait deficits and experience frequent falls, who can only rely on physical therapy to try and minimize the consequences,” he added.
The findings were published online in Nature Medicine.
Personalized stimulation
About 90% of people with advanced PD experience gait and balance problems or freezing-of-gait episodes. These locomotor deficits typically don’t respond well to dopamine replacement therapy or deep brain stimulation (DBS) of the subthalamic nucleus, possibly because the neural origins of these motor problems involve brain circuits not related to dopamine, said Dr. Moraud.
Continuous electrical stimulation over the cervical or thoracic segments of the spinal cord reduces locomotor deficits in some people with PD, but the broader application of this strategy has led to variable and unsatisfying outcomes.
The new approach focuses on correcting abnormal activation of circuits in the lumbar spinal cord, a region that hosts all the neurons that control activation of the leg muscles used for walking.
The stimulating device is placed on the lumbar region of the spinal cord, which sends messages to leg muscles. It is wired to a small impulse generator implanted under the skin of the abdomen. Sensors placed in shoes align the stimulation to the patient’s movement.
The system can detect the beginning of a movement, immediately activate the appropriate electrode, and so facilitate the necessary movement, be that leg flexion, extension, or propulsion, said Dr. Moraud. “This allows for increased walking symmetry, reinforced balance, and increased length of steps.”
The concept of this neuroprosthesis is similar to that used to allow patients with a spinal cord injury (SCI) to walk. But unlike patients with SCI, those with PD can move their legs, indicating that there is a descending command from the brain that needs to interact with the stimulation of the spinal cord, and patients with PD can feel the stimulation.
“Both these elements imply that amplitudes of stimulation need to be much lower in PD than SCI, and that stimulation needs to be fully personalized in PD to synergistically interact with the descending commands from the brain.”
After fine-tuning this new neuroprosthesis in animal models, researchers implanted the device in a 62-year-old man with a 30-year history of PD who presented with severe gait impairments, including marked gait asymmetry, reduced stride length, and balance problems.
Gait restored to near normal
The patient had frequent freezing-of-gait episodes when turning and passing through narrow paths, which led to multiple falls a day. This was despite being treated with DBS and dopaminergic replacement therapies.
But after getting used to the neuroprosthesis, the patient now walks with a gait akin to that of people without PD.
“Our experience in the preclinical animal models and this first patient is that gait can be restored to an almost healthy level, but this, of course, may vary across patients, depending on the severity of their disease progression, and their other motor deficits,” said Dr. Moraud.
When the neuroprosthesis is turned on, freezing of gait nearly vanishes, both with and without DBS.
In addition, the neuroprosthesis augmented the impact of the patient’s rehabilitation program, which involved a variety of regular exercises, including walking on basic and complex terrains, navigating outdoors in community settings, balance training, and basic physical therapy.
Frequent use of the neuroprosthesis during gait rehabilitation also translated into “highly improved” quality of life as reported by the patient (and his wife), said Dr. Moraud.
The patient has now been using the neuroprosthesis about 8 hours a day for nearly 2 years, only switching it off when sitting for long periods of time or while sleeping.
“He regained the capacity to walk in complex or crowded environments such as shops, airports, or his own home, without falling,” said Dr. Moraud. “He went from falling five to six times per day to one or two [falls] every couple of weeks. He’s also much more confident. He can walk for many miles, run, and go on holidays, without the constant fear of falling and having related injuries.”
Dr. Moraud stressed that the device does not replace DBS, which is a “key therapy” that addresses other deficits in PD, such as rigidity or slowness of movement. “What we propose here is a fully complementary approach for the gait problems that are not well addressed by DBS.”
One of the next steps will be to evaluate the efficacy of this approach across a wider spectrum of patient profiles to fully define the best responders, said Dr. Moraud.
A ‘tour de force’
In a comment, Michael S. Okun, MD, director of the Norman Fixel Institute for Neurological Diseases, University of Florida, Gainesville, and medical director of the Parkinson’s Foundation, noted that the researchers used “a smarter device” than past approaches that failed to adequately address progressive walking challenges of patients with PD.
Although it’s “tempting to get excited” about the findings, it’s important to consider that the study included only one human subject and did not target circuits for both walking and balance, said Dr. Okun. “It’s possible that even if future studies revealed a benefit for walking, the device may or may not address falling.”
In an accompanying editorial, Aviv Mizrahi-Kliger, MD, PhD, department of neurology, University of California, San Francisco, and Karunesh Ganguly, MD, PhD, Neurology and Rehabilitation Service, San Francisco Veterans Affairs Health Care System, called the study an “impressive tour de force,” with data from the nonhuman primate model and the individual with PD “jointly” indicating that epidural electrical stimulation (EES) “is a very promising treatment for several aspects of gait, posture and balance impairments in PD.”
But although the effect in the single patient “is quite impressive,” the “next crucial step” is to test this approach in a larger cohort of patients, they said.
They noted the nonhuman model does not exhibit freezing of gait, “which precluded the ability to corroborate or further study the role of EES in alleviating this symptom of PD in an animal model.”
In addition, stimulation parameters in the patient with PD “had to rely on estimated normal activity patterns, owing to the inability to measure pre-disease patterns at the individual level,” they wrote.
The study received funding from the Defitech Foundation, ONWARD Medical, CAMS Innovation Fund for Medical Sciences, National Natural Science Foundation of China, Parkinson Schweiz Foundation, European Community’s Seventh Framework Program (NeuWalk), European Research Council, Wyss Center for Bio and Neuroengineering, Bertarelli Foundation, and Swiss National Science Foundation. Dr. Moraud and other study authors hold various patents or applications in relation to the present work. Dr. Mizrahi-Kliger has no relevant conflicts of interest; Dr. Ganguly has a patent for modulation of sensory inputs to improve motor recovery from stroke and has been a consultant to Cala Health.
A version of this article first appeared on Medscape.com.
The neuroprosthesis involves targeted epidural electrical stimulation of areas of the lumbosacral spinal cord that produce walking.
This new therapeutic tool offers hope to patients with PD and, combined with existing approaches, may alleviate a motor sign in PD for which there is currently “no real solution,” study investigator Eduardo Martin Moraud, PhD, who leads PD research at the Defitech Center for Interventional Neurotherapies (NeuroRestore), Lausanne, Switzerland, said in an interview.
“This is exciting for the many patients that develop gait deficits and experience frequent falls, who can only rely on physical therapy to try and minimize the consequences,” he added.
The findings were published online in Nature Medicine.
Personalized stimulation
About 90% of people with advanced PD experience gait and balance problems or freezing-of-gait episodes. These locomotor deficits typically don’t respond well to dopamine replacement therapy or deep brain stimulation (DBS) of the subthalamic nucleus, possibly because the neural origins of these motor problems involve brain circuits not related to dopamine, said Dr. Moraud.
Continuous electrical stimulation over the cervical or thoracic segments of the spinal cord reduces locomotor deficits in some people with PD, but the broader application of this strategy has led to variable and unsatisfying outcomes.
The new approach focuses on correcting abnormal activation of circuits in the lumbar spinal cord, a region that hosts all the neurons that control activation of the leg muscles used for walking.
The stimulating device is placed on the lumbar region of the spinal cord, which sends messages to leg muscles. It is wired to a small impulse generator implanted under the skin of the abdomen. Sensors placed in shoes align the stimulation to the patient’s movement.
The system can detect the beginning of a movement, immediately activate the appropriate electrode, and so facilitate the necessary movement, be that leg flexion, extension, or propulsion, said Dr. Moraud. “This allows for increased walking symmetry, reinforced balance, and increased length of steps.”
The concept of this neuroprosthesis is similar to that used to allow patients with a spinal cord injury (SCI) to walk. But unlike patients with SCI, those with PD can move their legs, indicating that there is a descending command from the brain that needs to interact with the stimulation of the spinal cord, and patients with PD can feel the stimulation.
“Both these elements imply that amplitudes of stimulation need to be much lower in PD than SCI, and that stimulation needs to be fully personalized in PD to synergistically interact with the descending commands from the brain.”
After fine-tuning this new neuroprosthesis in animal models, researchers implanted the device in a 62-year-old man with a 30-year history of PD who presented with severe gait impairments, including marked gait asymmetry, reduced stride length, and balance problems.
Gait restored to near normal
The patient had frequent freezing-of-gait episodes when turning and passing through narrow paths, which led to multiple falls a day. This was despite being treated with DBS and dopaminergic replacement therapies.
But after getting used to the neuroprosthesis, the patient now walks with a gait akin to that of people without PD.
“Our experience in the preclinical animal models and this first patient is that gait can be restored to an almost healthy level, but this, of course, may vary across patients, depending on the severity of their disease progression, and their other motor deficits,” said Dr. Moraud.
When the neuroprosthesis is turned on, freezing of gait nearly vanishes, both with and without DBS.
In addition, the neuroprosthesis augmented the impact of the patient’s rehabilitation program, which involved a variety of regular exercises, including walking on basic and complex terrains, navigating outdoors in community settings, balance training, and basic physical therapy.
Frequent use of the neuroprosthesis during gait rehabilitation also translated into “highly improved” quality of life as reported by the patient (and his wife), said Dr. Moraud.
The patient has now been using the neuroprosthesis about 8 hours a day for nearly 2 years, only switching it off when sitting for long periods of time or while sleeping.
“He regained the capacity to walk in complex or crowded environments such as shops, airports, or his own home, without falling,” said Dr. Moraud. “He went from falling five to six times per day to one or two [falls] every couple of weeks. He’s also much more confident. He can walk for many miles, run, and go on holidays, without the constant fear of falling and having related injuries.”
Dr. Moraud stressed that the device does not replace DBS, which is a “key therapy” that addresses other deficits in PD, such as rigidity or slowness of movement. “What we propose here is a fully complementary approach for the gait problems that are not well addressed by DBS.”
One of the next steps will be to evaluate the efficacy of this approach across a wider spectrum of patient profiles to fully define the best responders, said Dr. Moraud.
A ‘tour de force’
In a comment, Michael S. Okun, MD, director of the Norman Fixel Institute for Neurological Diseases, University of Florida, Gainesville, and medical director of the Parkinson’s Foundation, noted that the researchers used “a smarter device” than past approaches that failed to adequately address progressive walking challenges of patients with PD.
Although it’s “tempting to get excited” about the findings, it’s important to consider that the study included only one human subject and did not target circuits for both walking and balance, said Dr. Okun. “It’s possible that even if future studies revealed a benefit for walking, the device may or may not address falling.”
In an accompanying editorial, Aviv Mizrahi-Kliger, MD, PhD, department of neurology, University of California, San Francisco, and Karunesh Ganguly, MD, PhD, Neurology and Rehabilitation Service, San Francisco Veterans Affairs Health Care System, called the study an “impressive tour de force,” with data from the nonhuman primate model and the individual with PD “jointly” indicating that epidural electrical stimulation (EES) “is a very promising treatment for several aspects of gait, posture and balance impairments in PD.”
But although the effect in the single patient “is quite impressive,” the “next crucial step” is to test this approach in a larger cohort of patients, they said.
They noted the nonhuman model does not exhibit freezing of gait, “which precluded the ability to corroborate or further study the role of EES in alleviating this symptom of PD in an animal model.”
In addition, stimulation parameters in the patient with PD “had to rely on estimated normal activity patterns, owing to the inability to measure pre-disease patterns at the individual level,” they wrote.
The study received funding from the Defitech Foundation, ONWARD Medical, CAMS Innovation Fund for Medical Sciences, National Natural Science Foundation of China, Parkinson Schweiz Foundation, European Community’s Seventh Framework Program (NeuWalk), European Research Council, Wyss Center for Bio and Neuroengineering, Bertarelli Foundation, and Swiss National Science Foundation. Dr. Moraud and other study authors hold various patents or applications in relation to the present work. Dr. Mizrahi-Kliger has no relevant conflicts of interest; Dr. Ganguly has a patent for modulation of sensory inputs to improve motor recovery from stroke and has been a consultant to Cala Health.
A version of this article first appeared on Medscape.com.
The neuroprosthesis involves targeted epidural electrical stimulation of areas of the lumbosacral spinal cord that produce walking.
This new therapeutic tool offers hope to patients with PD and, combined with existing approaches, may alleviate a motor sign in PD for which there is currently “no real solution,” study investigator Eduardo Martin Moraud, PhD, who leads PD research at the Defitech Center for Interventional Neurotherapies (NeuroRestore), Lausanne, Switzerland, said in an interview.
“This is exciting for the many patients that develop gait deficits and experience frequent falls, who can only rely on physical therapy to try and minimize the consequences,” he added.
The findings were published online in Nature Medicine.
Personalized stimulation
About 90% of people with advanced PD experience gait and balance problems or freezing-of-gait episodes. These locomotor deficits typically don’t respond well to dopamine replacement therapy or deep brain stimulation (DBS) of the subthalamic nucleus, possibly because the neural origins of these motor problems involve brain circuits not related to dopamine, said Dr. Moraud.
Continuous electrical stimulation over the cervical or thoracic segments of the spinal cord reduces locomotor deficits in some people with PD, but the broader application of this strategy has led to variable and unsatisfying outcomes.
The new approach focuses on correcting abnormal activation of circuits in the lumbar spinal cord, a region that hosts all the neurons that control activation of the leg muscles used for walking.
The stimulating device is placed on the lumbar region of the spinal cord, which sends messages to leg muscles. It is wired to a small impulse generator implanted under the skin of the abdomen. Sensors placed in shoes align the stimulation to the patient’s movement.
The system can detect the beginning of a movement, immediately activate the appropriate electrode, and so facilitate the necessary movement, be that leg flexion, extension, or propulsion, said Dr. Moraud. “This allows for increased walking symmetry, reinforced balance, and increased length of steps.”
The concept of this neuroprosthesis is similar to that used to allow patients with a spinal cord injury (SCI) to walk. But unlike patients with SCI, those with PD can move their legs, indicating that there is a descending command from the brain that needs to interact with the stimulation of the spinal cord, and patients with PD can feel the stimulation.
“Both these elements imply that amplitudes of stimulation need to be much lower in PD than SCI, and that stimulation needs to be fully personalized in PD to synergistically interact with the descending commands from the brain.”
After fine-tuning this new neuroprosthesis in animal models, researchers implanted the device in a 62-year-old man with a 30-year history of PD who presented with severe gait impairments, including marked gait asymmetry, reduced stride length, and balance problems.
Gait restored to near normal
The patient had frequent freezing-of-gait episodes when turning and passing through narrow paths, which led to multiple falls a day. This was despite being treated with DBS and dopaminergic replacement therapies.
But after getting used to the neuroprosthesis, the patient now walks with a gait akin to that of people without PD.
“Our experience in the preclinical animal models and this first patient is that gait can be restored to an almost healthy level, but this, of course, may vary across patients, depending on the severity of their disease progression, and their other motor deficits,” said Dr. Moraud.
When the neuroprosthesis is turned on, freezing of gait nearly vanishes, both with and without DBS.
In addition, the neuroprosthesis augmented the impact of the patient’s rehabilitation program, which involved a variety of regular exercises, including walking on basic and complex terrains, navigating outdoors in community settings, balance training, and basic physical therapy.
Frequent use of the neuroprosthesis during gait rehabilitation also translated into “highly improved” quality of life as reported by the patient (and his wife), said Dr. Moraud.
The patient has now been using the neuroprosthesis about 8 hours a day for nearly 2 years, only switching it off when sitting for long periods of time or while sleeping.
“He regained the capacity to walk in complex or crowded environments such as shops, airports, or his own home, without falling,” said Dr. Moraud. “He went from falling five to six times per day to one or two [falls] every couple of weeks. He’s also much more confident. He can walk for many miles, run, and go on holidays, without the constant fear of falling and having related injuries.”
Dr. Moraud stressed that the device does not replace DBS, which is a “key therapy” that addresses other deficits in PD, such as rigidity or slowness of movement. “What we propose here is a fully complementary approach for the gait problems that are not well addressed by DBS.”
One of the next steps will be to evaluate the efficacy of this approach across a wider spectrum of patient profiles to fully define the best responders, said Dr. Moraud.
A ‘tour de force’
In a comment, Michael S. Okun, MD, director of the Norman Fixel Institute for Neurological Diseases, University of Florida, Gainesville, and medical director of the Parkinson’s Foundation, noted that the researchers used “a smarter device” than past approaches that failed to adequately address progressive walking challenges of patients with PD.
Although it’s “tempting to get excited” about the findings, it’s important to consider that the study included only one human subject and did not target circuits for both walking and balance, said Dr. Okun. “It’s possible that even if future studies revealed a benefit for walking, the device may or may not address falling.”
In an accompanying editorial, Aviv Mizrahi-Kliger, MD, PhD, department of neurology, University of California, San Francisco, and Karunesh Ganguly, MD, PhD, Neurology and Rehabilitation Service, San Francisco Veterans Affairs Health Care System, called the study an “impressive tour de force,” with data from the nonhuman primate model and the individual with PD “jointly” indicating that epidural electrical stimulation (EES) “is a very promising treatment for several aspects of gait, posture and balance impairments in PD.”
But although the effect in the single patient “is quite impressive,” the “next crucial step” is to test this approach in a larger cohort of patients, they said.
They noted the nonhuman model does not exhibit freezing of gait, “which precluded the ability to corroborate or further study the role of EES in alleviating this symptom of PD in an animal model.”
In addition, stimulation parameters in the patient with PD “had to rely on estimated normal activity patterns, owing to the inability to measure pre-disease patterns at the individual level,” they wrote.
The study received funding from the Defitech Foundation, ONWARD Medical, CAMS Innovation Fund for Medical Sciences, National Natural Science Foundation of China, Parkinson Schweiz Foundation, European Community’s Seventh Framework Program (NeuWalk), European Research Council, Wyss Center for Bio and Neuroengineering, Bertarelli Foundation, and Swiss National Science Foundation. Dr. Moraud and other study authors hold various patents or applications in relation to the present work. Dr. Mizrahi-Kliger has no relevant conflicts of interest; Dr. Ganguly has a patent for modulation of sensory inputs to improve motor recovery from stroke and has been a consultant to Cala Health.
A version of this article first appeared on Medscape.com.
FROM NATURE MEDICINE
Does laughter offer better blood glucose control?
David S. Greene, PhD, associate professor in the department of rehabilitation and human services at the University of Northern Colorado, Greeley, has pioneered research into the field, one previously overlooked when it comes to diabetes management.
This news organization caught up with Dr. Greene to ask about his research along with the implications for diabetes care and for patient well-being.
Question: What prompted you to research the link between humor and diabetes control?
Answer: I was diagnosed with type 1 diabetes in 1966 and consequently have lived with, and studied, various aspects of diabetes for the past 57 years.
For a time, I maintained a small private practice counseling people with diabetes. There I noticed, anecdotally, that my clients’ ability to laugh at, and see the humor in, their diabetes correlated with their emotional adjustment to living with their condition.
While I could find research confirming the physical and psychological benefits of humor in general, I was unable to find any research specifically relating to humor and diabetes.
My new research agenda was born.
Q: What did your research reveal?
A: My first study, published in 2020, found that people living with diabetes displayed the same level of both types of positive, or affiliative and self-enhancing, humor as the norm group used in developing the Humor Styles Questionnaire.
This was a surprising finding given that individuals with diabetes are dealing with a life-altering, chronic illness, with higher rates of depression and anxiety. Moreover, positive humor scores are known to be negatively correlated with depression and anxiety.
While levels of aggressive humor were not altered in my study, people with diabetes did have higher levels of self-defeating humor in my study, which is correlated with psychiatric and somatic concerns, and symptomatology, and so is to be expected.
My second study, published in 2021, examined whether there was a difference between people with diabetes who had an hemoglobin A1c level of 6.99% or less versus those with levels of at least 7% on four disparate types of humor.
The A1c of 6.99% or less group scored significantly higher for both affiliative humor and self-enhancing humor. This implies that better diabetes control is associated with positive humor. This was expected, as was the finding that negative humor was not associated with better control.
On the other hand, there was no significant difference between participants on either type of negative humor, whether aggressive or self-defeating.
Finally, my third study, published in 2023 [with coauthor Nancy D. King, PhD], found that offering humor training to people with type 1 diabetes can strengthen both their affiliative and self-enhancing sense of humor, while leaving their aggressive or self-defeating humor unaffected.
Q: What is ‘positive humor,’ and why do you think it is associated with diabetes control?
A: Both affiliative and self-enhancing humor enhance a person’s sense of self and their relationships with others.
Affiliative humor has been shown to be positively correlated with self-esteem, psychological well-being, social intimacy, and emotional stability. It is also negatively correlated with depression and anxiety.
Self-enhancing humor involves the ability to maintain a humorous outlook on life and to use humor to regulate emotions and as a coping strategy. Like affiliative humor, it is correlated with cheerfulness, self-esteem, optimism, psychological well-being, and life satisfaction. It is negatively related to depression and anxiety.
The preponderance of literature suggests positive humor specifically is associated with buffering many of the risk factors and complications associated with diabetes.
Q: What could underlie the associations between humor and diabetes control?
A: Unfortunately, none of my studies have been able to determine cause and effect, although the most recent one came the closest.
While not reaching statistical significance, the mean reduction in A1c levels from 7.12% at baseline to 6.75% at the post–humor training assessment may very well imply a practical and psychological significance to patients.
I believe, with a larger sample size, significance will be achieved, and that the relationship between positive humor and diabetes control will be shown to be bidirectional, with positive humor improving diabetes control, and improved control improving positive humor.
I hypothesize this will also bring psychological and physiological benefits. After all, humor has already been associated with reduced blood glucose levels, reduced microvascular complications, improved heart health, reduced blood pressure, decreased levels of depression and anxiety.
Humor also helps us deal with stress and trauma, so a cause-and-effect relationship makes sense.
Q: Can a positive sense of humor be taught?
A: Absolutely. There is evidence that humor can be developed and strengthened. Paul McGhee, PhD, developed a seven-step humor training program that has been effective in elevating measures of humor across a number of studies.
Others have successfully developed their own protocols, and of course my third study demonstrated a significant increase in both affiliative and self-enhancing humor with training.
Q: Do you think humor training could be incorporated into diabetes care?
A: Humor training programs are easily accessible, incur low to no cost, and are easy to implement. Furthermore, once a person is trained, access to ongoing humor is free, readily available, and fun.
Several diabetes educators have also reported that humor can promote connections, encourage and support diabetes management, galvanize effectiveness, and increase an audience’s attention during education programs.
A version of this article appeared on Medscape.com.
David S. Greene, PhD, associate professor in the department of rehabilitation and human services at the University of Northern Colorado, Greeley, has pioneered research into the field, one previously overlooked when it comes to diabetes management.
This news organization caught up with Dr. Greene to ask about his research along with the implications for diabetes care and for patient well-being.
Question: What prompted you to research the link between humor and diabetes control?
Answer: I was diagnosed with type 1 diabetes in 1966 and consequently have lived with, and studied, various aspects of diabetes for the past 57 years.
For a time, I maintained a small private practice counseling people with diabetes. There I noticed, anecdotally, that my clients’ ability to laugh at, and see the humor in, their diabetes correlated with their emotional adjustment to living with their condition.
While I could find research confirming the physical and psychological benefits of humor in general, I was unable to find any research specifically relating to humor and diabetes.
My new research agenda was born.
Q: What did your research reveal?
A: My first study, published in 2020, found that people living with diabetes displayed the same level of both types of positive, or affiliative and self-enhancing, humor as the norm group used in developing the Humor Styles Questionnaire.
This was a surprising finding given that individuals with diabetes are dealing with a life-altering, chronic illness, with higher rates of depression and anxiety. Moreover, positive humor scores are known to be negatively correlated with depression and anxiety.
While levels of aggressive humor were not altered in my study, people with diabetes did have higher levels of self-defeating humor in my study, which is correlated with psychiatric and somatic concerns, and symptomatology, and so is to be expected.
My second study, published in 2021, examined whether there was a difference between people with diabetes who had an hemoglobin A1c level of 6.99% or less versus those with levels of at least 7% on four disparate types of humor.
The A1c of 6.99% or less group scored significantly higher for both affiliative humor and self-enhancing humor. This implies that better diabetes control is associated with positive humor. This was expected, as was the finding that negative humor was not associated with better control.
On the other hand, there was no significant difference between participants on either type of negative humor, whether aggressive or self-defeating.
Finally, my third study, published in 2023 [with coauthor Nancy D. King, PhD], found that offering humor training to people with type 1 diabetes can strengthen both their affiliative and self-enhancing sense of humor, while leaving their aggressive or self-defeating humor unaffected.
Q: What is ‘positive humor,’ and why do you think it is associated with diabetes control?
A: Both affiliative and self-enhancing humor enhance a person’s sense of self and their relationships with others.
Affiliative humor has been shown to be positively correlated with self-esteem, psychological well-being, social intimacy, and emotional stability. It is also negatively correlated with depression and anxiety.
Self-enhancing humor involves the ability to maintain a humorous outlook on life and to use humor to regulate emotions and as a coping strategy. Like affiliative humor, it is correlated with cheerfulness, self-esteem, optimism, psychological well-being, and life satisfaction. It is negatively related to depression and anxiety.
The preponderance of literature suggests positive humor specifically is associated with buffering many of the risk factors and complications associated with diabetes.
Q: What could underlie the associations between humor and diabetes control?
A: Unfortunately, none of my studies have been able to determine cause and effect, although the most recent one came the closest.
While not reaching statistical significance, the mean reduction in A1c levels from 7.12% at baseline to 6.75% at the post–humor training assessment may very well imply a practical and psychological significance to patients.
I believe, with a larger sample size, significance will be achieved, and that the relationship between positive humor and diabetes control will be shown to be bidirectional, with positive humor improving diabetes control, and improved control improving positive humor.
I hypothesize this will also bring psychological and physiological benefits. After all, humor has already been associated with reduced blood glucose levels, reduced microvascular complications, improved heart health, reduced blood pressure, decreased levels of depression and anxiety.
Humor also helps us deal with stress and trauma, so a cause-and-effect relationship makes sense.
Q: Can a positive sense of humor be taught?
A: Absolutely. There is evidence that humor can be developed and strengthened. Paul McGhee, PhD, developed a seven-step humor training program that has been effective in elevating measures of humor across a number of studies.
Others have successfully developed their own protocols, and of course my third study demonstrated a significant increase in both affiliative and self-enhancing humor with training.
Q: Do you think humor training could be incorporated into diabetes care?
A: Humor training programs are easily accessible, incur low to no cost, and are easy to implement. Furthermore, once a person is trained, access to ongoing humor is free, readily available, and fun.
Several diabetes educators have also reported that humor can promote connections, encourage and support diabetes management, galvanize effectiveness, and increase an audience’s attention during education programs.
A version of this article appeared on Medscape.com.
David S. Greene, PhD, associate professor in the department of rehabilitation and human services at the University of Northern Colorado, Greeley, has pioneered research into the field, one previously overlooked when it comes to diabetes management.
This news organization caught up with Dr. Greene to ask about his research along with the implications for diabetes care and for patient well-being.
Question: What prompted you to research the link between humor and diabetes control?
Answer: I was diagnosed with type 1 diabetes in 1966 and consequently have lived with, and studied, various aspects of diabetes for the past 57 years.
For a time, I maintained a small private practice counseling people with diabetes. There I noticed, anecdotally, that my clients’ ability to laugh at, and see the humor in, their diabetes correlated with their emotional adjustment to living with their condition.
While I could find research confirming the physical and psychological benefits of humor in general, I was unable to find any research specifically relating to humor and diabetes.
My new research agenda was born.
Q: What did your research reveal?
A: My first study, published in 2020, found that people living with diabetes displayed the same level of both types of positive, or affiliative and self-enhancing, humor as the norm group used in developing the Humor Styles Questionnaire.
This was a surprising finding given that individuals with diabetes are dealing with a life-altering, chronic illness, with higher rates of depression and anxiety. Moreover, positive humor scores are known to be negatively correlated with depression and anxiety.
While levels of aggressive humor were not altered in my study, people with diabetes did have higher levels of self-defeating humor in my study, which is correlated with psychiatric and somatic concerns, and symptomatology, and so is to be expected.
My second study, published in 2021, examined whether there was a difference between people with diabetes who had an hemoglobin A1c level of 6.99% or less versus those with levels of at least 7% on four disparate types of humor.
The A1c of 6.99% or less group scored significantly higher for both affiliative humor and self-enhancing humor. This implies that better diabetes control is associated with positive humor. This was expected, as was the finding that negative humor was not associated with better control.
On the other hand, there was no significant difference between participants on either type of negative humor, whether aggressive or self-defeating.
Finally, my third study, published in 2023 [with coauthor Nancy D. King, PhD], found that offering humor training to people with type 1 diabetes can strengthen both their affiliative and self-enhancing sense of humor, while leaving their aggressive or self-defeating humor unaffected.
Q: What is ‘positive humor,’ and why do you think it is associated with diabetes control?
A: Both affiliative and self-enhancing humor enhance a person’s sense of self and their relationships with others.
Affiliative humor has been shown to be positively correlated with self-esteem, psychological well-being, social intimacy, and emotional stability. It is also negatively correlated with depression and anxiety.
Self-enhancing humor involves the ability to maintain a humorous outlook on life and to use humor to regulate emotions and as a coping strategy. Like affiliative humor, it is correlated with cheerfulness, self-esteem, optimism, psychological well-being, and life satisfaction. It is negatively related to depression and anxiety.
The preponderance of literature suggests positive humor specifically is associated with buffering many of the risk factors and complications associated with diabetes.
Q: What could underlie the associations between humor and diabetes control?
A: Unfortunately, none of my studies have been able to determine cause and effect, although the most recent one came the closest.
While not reaching statistical significance, the mean reduction in A1c levels from 7.12% at baseline to 6.75% at the post–humor training assessment may very well imply a practical and psychological significance to patients.
I believe, with a larger sample size, significance will be achieved, and that the relationship between positive humor and diabetes control will be shown to be bidirectional, with positive humor improving diabetes control, and improved control improving positive humor.
I hypothesize this will also bring psychological and physiological benefits. After all, humor has already been associated with reduced blood glucose levels, reduced microvascular complications, improved heart health, reduced blood pressure, decreased levels of depression and anxiety.
Humor also helps us deal with stress and trauma, so a cause-and-effect relationship makes sense.
Q: Can a positive sense of humor be taught?
A: Absolutely. There is evidence that humor can be developed and strengthened. Paul McGhee, PhD, developed a seven-step humor training program that has been effective in elevating measures of humor across a number of studies.
Others have successfully developed their own protocols, and of course my third study demonstrated a significant increase in both affiliative and self-enhancing humor with training.
Q: Do you think humor training could be incorporated into diabetes care?
A: Humor training programs are easily accessible, incur low to no cost, and are easy to implement. Furthermore, once a person is trained, access to ongoing humor is free, readily available, and fun.
Several diabetes educators have also reported that humor can promote connections, encourage and support diabetes management, galvanize effectiveness, and increase an audience’s attention during education programs.
A version of this article appeared on Medscape.com.
Low-salt diet cut BP by 6 mm Hg in 1 week: CARDIA-SSBP
The CARDIA-SSBP trial involved 213 individuals aged 50-75 years, including those with and those without hypertension, and showed that the decline in blood pressure brought about by a low-salt diet was independent of hypertension status and antihypertensive medication use. It was also generally consistent across subgroups and did not result in excess adverse events.
“The blood pressure reduction we see here is meaningful, and comparable to that produced by one antihypertensive medication,” lead investigator Deepak Gupta, MD, Vanderbilt University Medical Center, Nashville, Tenn., said in an interview.
Dr. Gupta presented the CARDIA-SSBP study on Nov. 11 at the American Heart Association scientific sessions, held in Philadelphia. The study was published online in JAMA. The exact menus used in the study are available in a supplement to the JAMA paper.
“In order to live a healthy lifestyle, understanding what we eat has important health effects. Raised blood pressure contributes to one out of every eight deaths worldwide,” Dr. Gupta noted. “If people want to lower their blood pressure, attention to dietary sodium is one part of that. If individuals can stick with a low sodium diet, they may be able to stop taking one of their antihypertensive medications, and those who are normotensive will be less likely to develop hypertension.”
Commentators said the study had significant implications for public health, but they pointed out that maintaining a low-sodium diet over the long term is challenging, given the high salt content of generally available foods.
Dr. Gupta noted that the study did use commercially available products in the low-sodium diets and the menus are available for people to follow, making it more accessible than some diets used in previous studies.
“What may also be attractive to people is that you don’t have to wait for months to see an effect. If you start to consume a low-sodium diet, you can see results on blood pressure rapidly, within a week,” he said.
The diet in this study brought about a large reduction in dietary sodium, but Dr. Gupta says any reduction in dietary sodium is likely to be beneficial.
“If you go to the level that we got to, you could expect to see a reduction of around 6 mm Hg. But it’s like walking – you don’t necessarily need to get to 10,000 steps every day. Any amount of walking or physical activity is of benefit. The same is probably true for salt: Any reduction that you can make is probably of benefit.”
For the study, participants had their blood pressure measured by 24-hour ambulatory monitoring while on their usual diets. They were then randomly assigned to either a high-sodium diet or a low-sodium diet for 1 week. Participants then crossed over to the opposite diet for 1 week, with blood pressure measured over a 24-hour period on the last day of each diet.
As assessed by 24-hour urine excretion, the usual diet of participants was found to already be high in sodium (median, 4.45 g/d). This increased to a median of 5.00 g/d when on the high-sodium diet in the study and decreased to 1.27 g/d while on the low-sodium diet.
Results found participants had a median systolic blood pressure of 125 mm Hg on their usual diets. This was raised to 126 mm Hg on the high-sodium diet and lowered to 119 mm Hg on the low-sodium diet.
The researchers also reported that 75% of individuals showed a blood pressure reduction on the low-sodium diet and are thus defined as “salt-sensitive.” This is a higher percentage than found in previous studies.
“Of those that didn’t show a blood pressure reduction with a low-sodium diet in this study, it appears that they may not have been so adherent to the diet as those who did show a blood pressure reduction,” Dr. Gupta said.
He noted that hypertension is the most common chronic disease condition worldwide, with about 1.3 billion people affected, and although it has been known for some time that dietary sodium affects blood pressure, there have been some gaps in previous studies.
For example, many studies have excluded individuals who were already taking antihypertensive medications and people with diabetes, and they have generally not included many older individuals. The current study found that all of these groups showed significant blood pressure reductions by reducing dietary sodium.
Large effect in people with diabetes
Subgroup analysis largely showed consistent results across the population, regardless of age, sex, race, and body mass index and whether participants were taking antihypertensive medication or not, but there were a couple of exceptions. Individuals with higher blood pressure at baseline seemed to have a greater effect of lowering dietary sodium, although those who were normotensive at baseline still showed significant blood pressure reduction, Dr. Gupta reported.
The researchers found a particularly large reduction in blood pressure from lowering sodium intake in people with diabetes, who made up about 21% of the overall cohort. Their average reduction in systolic blood pressure between the high and low sodium diet was close to 17 mm Hg rather than the 7-8 mm Hg in the whole cohort.
Dr. Gupta said that the results are applicable to most of the population.
“The people who will be most motivated to follow a low-sodium diet are those with hypertension. But even in normotensive individuals, there is likely to be benefit.”
To help people follow a low-sodium diet, Dr. Gupta says education campaigns are needed “to show people that they can do it and make it work.” But there are bigger structural issues that need to be addressed at policy and governmental levels.
“Most of our food available in grocery stores and restaurants is high in salt. We now have a preponderance of evidence showing us that we need to change what’s available in the food supply,” he said. “There is a push going on for this now, and the U.S. has introduced some guidelines for the food industry on sodium content of foods. These are voluntary at this point, but it’s a start.”
Difficult to maintain long term
Commenting on the study, Paul Whelton, MD, chair in global public health at Tulane University, New Orleans, noted that sodium reduction is known to reduce blood pressure, with greater sodium reductions giving greater blood pressure decreases, and that some people are more sensitive to the effects of sodium than others.
He described CARDIA-SSBP as a “well-done study.”
“They managed to get a very low sodium intake and a large difference between the two groups, which translated into a big reduction in systolic blood pressure,” Dr. Whelton said. “However, the problem with these sorts of trials where the diets are provided to the participants is that although they show proof of concept, it is difficult to generalize because we can’t normally provide patients with their meals. In this type of ‘feeding’ study, we find it difficult to maintain people on their behavioral intervention over the long term.”
Dr. Whelton said that he was more excited about this trial knowing that the food given was commercially available. “That makes it more practical, but you still have to be quite motivated to follow a diet like this. Buying low-sodium products in the supermarket does require quite a lot of work to read the labels, and sometimes the low-sodium foods are specialty products and are more expensive.”
He pointed out that older people in higher socioeconomic classes are more likely to attempt this and do better from behavioral interventions in general. “Unfortunately, people who don’t do well from behavioral interventions like this are those from lower socioeconomic groups, who are ones at most at risk for cardiovascular disease.”
Dr. Whelton noted that the food industry has been reluctant to lower sodium content because high-salt foods sell better. “Unfortunately, foods high in saturated fat and salt taste good to most people. We are generally attuned to a high salt intake. But when people have been following a low-salt diet for a while, they generally don’t like high-salt foods anymore. They become attuned to lower-sodium diet,” he added.
New U.S. sodium reduction guidelines
Discussant of the CARDIA-SSBP study at the AHA meeting, Cheryl Anderson, MD, University of California, San Diego, said that the findings were important and consistent with prior studies.
“These studies have global implications because salt is ubiquitous in the food supply in much of the world,” she noted, adding that, “Americans consume almost 50% more sodium than recommended, and there has been a persistent lack of adherence to healthy diet recommendations for reductions in salt, sugar, and fats.”
Dr. Anderson pointed out that in 2021, the Food and Drug Administration issued guidance for voluntary sodium reduction, which uses a gradual approach, with targets to reach a population goal of 3,000 mg/d of sodium by 2023 and 2,300 mg/d by 2031.
“These targets apply to 150 categories of food that are sales-weighted to focus on dominant sellers in each category. They apply to food manufacturers, restaurants and food service operations,” she concluded. “These targets serve as a basis for continued dialogue. The research community eagerly awaits the review of population-based data to help refine this approach and goals.”
This study was supported by grants from the National Heart, Lung, and Blood Institute, the National Institutes of Health, the American Heart Association, and the National Center for Advancing Translational Sciences. The authors report no disclosures.
A version of this article appeared on Medscape.com.
The CARDIA-SSBP trial involved 213 individuals aged 50-75 years, including those with and those without hypertension, and showed that the decline in blood pressure brought about by a low-salt diet was independent of hypertension status and antihypertensive medication use. It was also generally consistent across subgroups and did not result in excess adverse events.
“The blood pressure reduction we see here is meaningful, and comparable to that produced by one antihypertensive medication,” lead investigator Deepak Gupta, MD, Vanderbilt University Medical Center, Nashville, Tenn., said in an interview.
Dr. Gupta presented the CARDIA-SSBP study on Nov. 11 at the American Heart Association scientific sessions, held in Philadelphia. The study was published online in JAMA. The exact menus used in the study are available in a supplement to the JAMA paper.
“In order to live a healthy lifestyle, understanding what we eat has important health effects. Raised blood pressure contributes to one out of every eight deaths worldwide,” Dr. Gupta noted. “If people want to lower their blood pressure, attention to dietary sodium is one part of that. If individuals can stick with a low sodium diet, they may be able to stop taking one of their antihypertensive medications, and those who are normotensive will be less likely to develop hypertension.”
Commentators said the study had significant implications for public health, but they pointed out that maintaining a low-sodium diet over the long term is challenging, given the high salt content of generally available foods.
Dr. Gupta noted that the study did use commercially available products in the low-sodium diets and the menus are available for people to follow, making it more accessible than some diets used in previous studies.
“What may also be attractive to people is that you don’t have to wait for months to see an effect. If you start to consume a low-sodium diet, you can see results on blood pressure rapidly, within a week,” he said.
The diet in this study brought about a large reduction in dietary sodium, but Dr. Gupta says any reduction in dietary sodium is likely to be beneficial.
“If you go to the level that we got to, you could expect to see a reduction of around 6 mm Hg. But it’s like walking – you don’t necessarily need to get to 10,000 steps every day. Any amount of walking or physical activity is of benefit. The same is probably true for salt: Any reduction that you can make is probably of benefit.”
For the study, participants had their blood pressure measured by 24-hour ambulatory monitoring while on their usual diets. They were then randomly assigned to either a high-sodium diet or a low-sodium diet for 1 week. Participants then crossed over to the opposite diet for 1 week, with blood pressure measured over a 24-hour period on the last day of each diet.
As assessed by 24-hour urine excretion, the usual diet of participants was found to already be high in sodium (median, 4.45 g/d). This increased to a median of 5.00 g/d when on the high-sodium diet in the study and decreased to 1.27 g/d while on the low-sodium diet.
Results found participants had a median systolic blood pressure of 125 mm Hg on their usual diets. This was raised to 126 mm Hg on the high-sodium diet and lowered to 119 mm Hg on the low-sodium diet.
The researchers also reported that 75% of individuals showed a blood pressure reduction on the low-sodium diet and are thus defined as “salt-sensitive.” This is a higher percentage than found in previous studies.
“Of those that didn’t show a blood pressure reduction with a low-sodium diet in this study, it appears that they may not have been so adherent to the diet as those who did show a blood pressure reduction,” Dr. Gupta said.
He noted that hypertension is the most common chronic disease condition worldwide, with about 1.3 billion people affected, and although it has been known for some time that dietary sodium affects blood pressure, there have been some gaps in previous studies.
For example, many studies have excluded individuals who were already taking antihypertensive medications and people with diabetes, and they have generally not included many older individuals. The current study found that all of these groups showed significant blood pressure reductions by reducing dietary sodium.
Large effect in people with diabetes
Subgroup analysis largely showed consistent results across the population, regardless of age, sex, race, and body mass index and whether participants were taking antihypertensive medication or not, but there were a couple of exceptions. Individuals with higher blood pressure at baseline seemed to have a greater effect of lowering dietary sodium, although those who were normotensive at baseline still showed significant blood pressure reduction, Dr. Gupta reported.
The researchers found a particularly large reduction in blood pressure from lowering sodium intake in people with diabetes, who made up about 21% of the overall cohort. Their average reduction in systolic blood pressure between the high and low sodium diet was close to 17 mm Hg rather than the 7-8 mm Hg in the whole cohort.
Dr. Gupta said that the results are applicable to most of the population.
“The people who will be most motivated to follow a low-sodium diet are those with hypertension. But even in normotensive individuals, there is likely to be benefit.”
To help people follow a low-sodium diet, Dr. Gupta says education campaigns are needed “to show people that they can do it and make it work.” But there are bigger structural issues that need to be addressed at policy and governmental levels.
“Most of our food available in grocery stores and restaurants is high in salt. We now have a preponderance of evidence showing us that we need to change what’s available in the food supply,” he said. “There is a push going on for this now, and the U.S. has introduced some guidelines for the food industry on sodium content of foods. These are voluntary at this point, but it’s a start.”
Difficult to maintain long term
Commenting on the study, Paul Whelton, MD, chair in global public health at Tulane University, New Orleans, noted that sodium reduction is known to reduce blood pressure, with greater sodium reductions giving greater blood pressure decreases, and that some people are more sensitive to the effects of sodium than others.
He described CARDIA-SSBP as a “well-done study.”
“They managed to get a very low sodium intake and a large difference between the two groups, which translated into a big reduction in systolic blood pressure,” Dr. Whelton said. “However, the problem with these sorts of trials where the diets are provided to the participants is that although they show proof of concept, it is difficult to generalize because we can’t normally provide patients with their meals. In this type of ‘feeding’ study, we find it difficult to maintain people on their behavioral intervention over the long term.”
Dr. Whelton said that he was more excited about this trial knowing that the food given was commercially available. “That makes it more practical, but you still have to be quite motivated to follow a diet like this. Buying low-sodium products in the supermarket does require quite a lot of work to read the labels, and sometimes the low-sodium foods are specialty products and are more expensive.”
He pointed out that older people in higher socioeconomic classes are more likely to attempt this and do better from behavioral interventions in general. “Unfortunately, people who don’t do well from behavioral interventions like this are those from lower socioeconomic groups, who are ones at most at risk for cardiovascular disease.”
Dr. Whelton noted that the food industry has been reluctant to lower sodium content because high-salt foods sell better. “Unfortunately, foods high in saturated fat and salt taste good to most people. We are generally attuned to a high salt intake. But when people have been following a low-salt diet for a while, they generally don’t like high-salt foods anymore. They become attuned to lower-sodium diet,” he added.
New U.S. sodium reduction guidelines
Discussant of the CARDIA-SSBP study at the AHA meeting, Cheryl Anderson, MD, University of California, San Diego, said that the findings were important and consistent with prior studies.
“These studies have global implications because salt is ubiquitous in the food supply in much of the world,” she noted, adding that, “Americans consume almost 50% more sodium than recommended, and there has been a persistent lack of adherence to healthy diet recommendations for reductions in salt, sugar, and fats.”
Dr. Anderson pointed out that in 2021, the Food and Drug Administration issued guidance for voluntary sodium reduction, which uses a gradual approach, with targets to reach a population goal of 3,000 mg/d of sodium by 2023 and 2,300 mg/d by 2031.
“These targets apply to 150 categories of food that are sales-weighted to focus on dominant sellers in each category. They apply to food manufacturers, restaurants and food service operations,” she concluded. “These targets serve as a basis for continued dialogue. The research community eagerly awaits the review of population-based data to help refine this approach and goals.”
This study was supported by grants from the National Heart, Lung, and Blood Institute, the National Institutes of Health, the American Heart Association, and the National Center for Advancing Translational Sciences. The authors report no disclosures.
A version of this article appeared on Medscape.com.
The CARDIA-SSBP trial involved 213 individuals aged 50-75 years, including those with and those without hypertension, and showed that the decline in blood pressure brought about by a low-salt diet was independent of hypertension status and antihypertensive medication use. It was also generally consistent across subgroups and did not result in excess adverse events.
“The blood pressure reduction we see here is meaningful, and comparable to that produced by one antihypertensive medication,” lead investigator Deepak Gupta, MD, Vanderbilt University Medical Center, Nashville, Tenn., said in an interview.
Dr. Gupta presented the CARDIA-SSBP study on Nov. 11 at the American Heart Association scientific sessions, held in Philadelphia. The study was published online in JAMA. The exact menus used in the study are available in a supplement to the JAMA paper.
“In order to live a healthy lifestyle, understanding what we eat has important health effects. Raised blood pressure contributes to one out of every eight deaths worldwide,” Dr. Gupta noted. “If people want to lower their blood pressure, attention to dietary sodium is one part of that. If individuals can stick with a low sodium diet, they may be able to stop taking one of their antihypertensive medications, and those who are normotensive will be less likely to develop hypertension.”
Commentators said the study had significant implications for public health, but they pointed out that maintaining a low-sodium diet over the long term is challenging, given the high salt content of generally available foods.
Dr. Gupta noted that the study did use commercially available products in the low-sodium diets and the menus are available for people to follow, making it more accessible than some diets used in previous studies.
“What may also be attractive to people is that you don’t have to wait for months to see an effect. If you start to consume a low-sodium diet, you can see results on blood pressure rapidly, within a week,” he said.
The diet in this study brought about a large reduction in dietary sodium, but Dr. Gupta says any reduction in dietary sodium is likely to be beneficial.
“If you go to the level that we got to, you could expect to see a reduction of around 6 mm Hg. But it’s like walking – you don’t necessarily need to get to 10,000 steps every day. Any amount of walking or physical activity is of benefit. The same is probably true for salt: Any reduction that you can make is probably of benefit.”
For the study, participants had their blood pressure measured by 24-hour ambulatory monitoring while on their usual diets. They were then randomly assigned to either a high-sodium diet or a low-sodium diet for 1 week. Participants then crossed over to the opposite diet for 1 week, with blood pressure measured over a 24-hour period on the last day of each diet.
As assessed by 24-hour urine excretion, the usual diet of participants was found to already be high in sodium (median, 4.45 g/d). This increased to a median of 5.00 g/d when on the high-sodium diet in the study and decreased to 1.27 g/d while on the low-sodium diet.
Results found participants had a median systolic blood pressure of 125 mm Hg on their usual diets. This was raised to 126 mm Hg on the high-sodium diet and lowered to 119 mm Hg on the low-sodium diet.
The researchers also reported that 75% of individuals showed a blood pressure reduction on the low-sodium diet and are thus defined as “salt-sensitive.” This is a higher percentage than found in previous studies.
“Of those that didn’t show a blood pressure reduction with a low-sodium diet in this study, it appears that they may not have been so adherent to the diet as those who did show a blood pressure reduction,” Dr. Gupta said.
He noted that hypertension is the most common chronic disease condition worldwide, with about 1.3 billion people affected, and although it has been known for some time that dietary sodium affects blood pressure, there have been some gaps in previous studies.
For example, many studies have excluded individuals who were already taking antihypertensive medications and people with diabetes, and they have generally not included many older individuals. The current study found that all of these groups showed significant blood pressure reductions by reducing dietary sodium.
Large effect in people with diabetes
Subgroup analysis largely showed consistent results across the population, regardless of age, sex, race, and body mass index and whether participants were taking antihypertensive medication or not, but there were a couple of exceptions. Individuals with higher blood pressure at baseline seemed to have a greater effect of lowering dietary sodium, although those who were normotensive at baseline still showed significant blood pressure reduction, Dr. Gupta reported.
The researchers found a particularly large reduction in blood pressure from lowering sodium intake in people with diabetes, who made up about 21% of the overall cohort. Their average reduction in systolic blood pressure between the high and low sodium diet was close to 17 mm Hg rather than the 7-8 mm Hg in the whole cohort.
Dr. Gupta said that the results are applicable to most of the population.
“The people who will be most motivated to follow a low-sodium diet are those with hypertension. But even in normotensive individuals, there is likely to be benefit.”
To help people follow a low-sodium diet, Dr. Gupta says education campaigns are needed “to show people that they can do it and make it work.” But there are bigger structural issues that need to be addressed at policy and governmental levels.
“Most of our food available in grocery stores and restaurants is high in salt. We now have a preponderance of evidence showing us that we need to change what’s available in the food supply,” he said. “There is a push going on for this now, and the U.S. has introduced some guidelines for the food industry on sodium content of foods. These are voluntary at this point, but it’s a start.”
Difficult to maintain long term
Commenting on the study, Paul Whelton, MD, chair in global public health at Tulane University, New Orleans, noted that sodium reduction is known to reduce blood pressure, with greater sodium reductions giving greater blood pressure decreases, and that some people are more sensitive to the effects of sodium than others.
He described CARDIA-SSBP as a “well-done study.”
“They managed to get a very low sodium intake and a large difference between the two groups, which translated into a big reduction in systolic blood pressure,” Dr. Whelton said. “However, the problem with these sorts of trials where the diets are provided to the participants is that although they show proof of concept, it is difficult to generalize because we can’t normally provide patients with their meals. In this type of ‘feeding’ study, we find it difficult to maintain people on their behavioral intervention over the long term.”
Dr. Whelton said that he was more excited about this trial knowing that the food given was commercially available. “That makes it more practical, but you still have to be quite motivated to follow a diet like this. Buying low-sodium products in the supermarket does require quite a lot of work to read the labels, and sometimes the low-sodium foods are specialty products and are more expensive.”
He pointed out that older people in higher socioeconomic classes are more likely to attempt this and do better from behavioral interventions in general. “Unfortunately, people who don’t do well from behavioral interventions like this are those from lower socioeconomic groups, who are ones at most at risk for cardiovascular disease.”
Dr. Whelton noted that the food industry has been reluctant to lower sodium content because high-salt foods sell better. “Unfortunately, foods high in saturated fat and salt taste good to most people. We are generally attuned to a high salt intake. But when people have been following a low-salt diet for a while, they generally don’t like high-salt foods anymore. They become attuned to lower-sodium diet,” he added.
New U.S. sodium reduction guidelines
Discussant of the CARDIA-SSBP study at the AHA meeting, Cheryl Anderson, MD, University of California, San Diego, said that the findings were important and consistent with prior studies.
“These studies have global implications because salt is ubiquitous in the food supply in much of the world,” she noted, adding that, “Americans consume almost 50% more sodium than recommended, and there has been a persistent lack of adherence to healthy diet recommendations for reductions in salt, sugar, and fats.”
Dr. Anderson pointed out that in 2021, the Food and Drug Administration issued guidance for voluntary sodium reduction, which uses a gradual approach, with targets to reach a population goal of 3,000 mg/d of sodium by 2023 and 2,300 mg/d by 2031.
“These targets apply to 150 categories of food that are sales-weighted to focus on dominant sellers in each category. They apply to food manufacturers, restaurants and food service operations,” she concluded. “These targets serve as a basis for continued dialogue. The research community eagerly awaits the review of population-based data to help refine this approach and goals.”
This study was supported by grants from the National Heart, Lung, and Blood Institute, the National Institutes of Health, the American Heart Association, and the National Center for Advancing Translational Sciences. The authors report no disclosures.
A version of this article appeared on Medscape.com.
FROM AHA 2023
Life in the woods
“I went to the woods because I wished to live deliberately, to front only the essential facts of life, and see if I could not learn what it had to teach.” – Henry David Thoreau
I have many patients like Maxine. Tall, with a shock of white hair. Old, but still in charge. When you try to make eye contact, she looks right through you. First with her left eye. Then her right. Her face is inscrutable. What’s she thinking? Unlike many of my patients, however, this Maxine was a llama. Every morning my daughter and I tried to coax her into moving as we leaned on the cold steel gate that kept her in her pasture. We were visiting family in October and chose to stay on a working New England farm. The kids will love the animals, we thought, and we’ll appreciate the extra bedrooms.
Airbnb helped us find this charming fiber-farm in Rhode Island where they raise Leicester Longwool sheep, a historic breed that once roamed George Washington’s pastures, along with a few goats, ducks, chickens, and Maxine. It’s situated deep in the woods, which were yellow, orange, and red that week. As it happens, we were just a short drive due south of Walden Pond where Henry David Thoreau spent 2 years, 2 months and 2 days escaping “overcivilization” nearly 175 years ago. Hoisting our overweight bags over the uneven granite stone steps when we arrived, I realized this was going to be more like the Thoreau experiment than I intended. The farmhouse dated to the 1790s. There were wide, creaky floorboards, low ceilings, one staircase to the bedrooms (which could have aptly been called a ladder) and loads of book-laden shelves. Instructions posted in the kitchen warned that the heat is tricky to regulate – a redundant admonition as we watched our 3-year-old putting on her socks and shoes as she got into bed.
Now, if you’ve ever been on vacation with little kids, you know that it’s basically just childcare in a novel location. After barricading the staircase with luggage and unplugging lamps from their dicey outlets we set out to feed the chickens and try to pet a sheep. Walking the perimeter of the farm we saw stone walls that needed mending and stumbled across two ancient cemeteries, one had been for family, the other for slaves. I wondered how many farmers and weavers and menders had walked this trail with their kids over the generations.
The next morning, we learned that roosters do not in fact crow at dawn, they crow before dawn (which could also aptly be called nighttime). There were no commutes or late patients here. But there was work to be done. Chickens don’t care that it’s Sunday. It downpoured. Watching the sheep from the kitchen as I sipped my coffee, they didn’t seem to mind. Nor did our farmer hosts who trudged past them in tall boots, just as they had every other day of their farmer lives.
By the fifth day, we had fallen into the rhythms of the homestead. We cracked the blue, green, and brown eggs that our hosts placed outside our door in the early hours and made omelets that were as orange as the foliage. We finally learned to adjust the heat so we neither got chilblains nor had to open the windows and strip naked to cool down. The sky was a brilliant blue that last morning and Sloan ran around trying to catch leaves as they blew off the trees. She had no objective. No counting. No contest. Just chasing leaves as they fell. It was the ultimate atelic activity, done just for doing it. I joined her and found I was no better at this than a 3-year-old.
We might all benefit from a little time in the woods.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
“I went to the woods because I wished to live deliberately, to front only the essential facts of life, and see if I could not learn what it had to teach.” – Henry David Thoreau
I have many patients like Maxine. Tall, with a shock of white hair. Old, but still in charge. When you try to make eye contact, she looks right through you. First with her left eye. Then her right. Her face is inscrutable. What’s she thinking? Unlike many of my patients, however, this Maxine was a llama. Every morning my daughter and I tried to coax her into moving as we leaned on the cold steel gate that kept her in her pasture. We were visiting family in October and chose to stay on a working New England farm. The kids will love the animals, we thought, and we’ll appreciate the extra bedrooms.
Airbnb helped us find this charming fiber-farm in Rhode Island where they raise Leicester Longwool sheep, a historic breed that once roamed George Washington’s pastures, along with a few goats, ducks, chickens, and Maxine. It’s situated deep in the woods, which were yellow, orange, and red that week. As it happens, we were just a short drive due south of Walden Pond where Henry David Thoreau spent 2 years, 2 months and 2 days escaping “overcivilization” nearly 175 years ago. Hoisting our overweight bags over the uneven granite stone steps when we arrived, I realized this was going to be more like the Thoreau experiment than I intended. The farmhouse dated to the 1790s. There were wide, creaky floorboards, low ceilings, one staircase to the bedrooms (which could have aptly been called a ladder) and loads of book-laden shelves. Instructions posted in the kitchen warned that the heat is tricky to regulate – a redundant admonition as we watched our 3-year-old putting on her socks and shoes as she got into bed.
Now, if you’ve ever been on vacation with little kids, you know that it’s basically just childcare in a novel location. After barricading the staircase with luggage and unplugging lamps from their dicey outlets we set out to feed the chickens and try to pet a sheep. Walking the perimeter of the farm we saw stone walls that needed mending and stumbled across two ancient cemeteries, one had been for family, the other for slaves. I wondered how many farmers and weavers and menders had walked this trail with their kids over the generations.
The next morning, we learned that roosters do not in fact crow at dawn, they crow before dawn (which could also aptly be called nighttime). There were no commutes or late patients here. But there was work to be done. Chickens don’t care that it’s Sunday. It downpoured. Watching the sheep from the kitchen as I sipped my coffee, they didn’t seem to mind. Nor did our farmer hosts who trudged past them in tall boots, just as they had every other day of their farmer lives.
By the fifth day, we had fallen into the rhythms of the homestead. We cracked the blue, green, and brown eggs that our hosts placed outside our door in the early hours and made omelets that were as orange as the foliage. We finally learned to adjust the heat so we neither got chilblains nor had to open the windows and strip naked to cool down. The sky was a brilliant blue that last morning and Sloan ran around trying to catch leaves as they blew off the trees. She had no objective. No counting. No contest. Just chasing leaves as they fell. It was the ultimate atelic activity, done just for doing it. I joined her and found I was no better at this than a 3-year-old.
We might all benefit from a little time in the woods.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
“I went to the woods because I wished to live deliberately, to front only the essential facts of life, and see if I could not learn what it had to teach.” – Henry David Thoreau
I have many patients like Maxine. Tall, with a shock of white hair. Old, but still in charge. When you try to make eye contact, she looks right through you. First with her left eye. Then her right. Her face is inscrutable. What’s she thinking? Unlike many of my patients, however, this Maxine was a llama. Every morning my daughter and I tried to coax her into moving as we leaned on the cold steel gate that kept her in her pasture. We were visiting family in October and chose to stay on a working New England farm. The kids will love the animals, we thought, and we’ll appreciate the extra bedrooms.
Airbnb helped us find this charming fiber-farm in Rhode Island where they raise Leicester Longwool sheep, a historic breed that once roamed George Washington’s pastures, along with a few goats, ducks, chickens, and Maxine. It’s situated deep in the woods, which were yellow, orange, and red that week. As it happens, we were just a short drive due south of Walden Pond where Henry David Thoreau spent 2 years, 2 months and 2 days escaping “overcivilization” nearly 175 years ago. Hoisting our overweight bags over the uneven granite stone steps when we arrived, I realized this was going to be more like the Thoreau experiment than I intended. The farmhouse dated to the 1790s. There were wide, creaky floorboards, low ceilings, one staircase to the bedrooms (which could have aptly been called a ladder) and loads of book-laden shelves. Instructions posted in the kitchen warned that the heat is tricky to regulate – a redundant admonition as we watched our 3-year-old putting on her socks and shoes as she got into bed.
Now, if you’ve ever been on vacation with little kids, you know that it’s basically just childcare in a novel location. After barricading the staircase with luggage and unplugging lamps from their dicey outlets we set out to feed the chickens and try to pet a sheep. Walking the perimeter of the farm we saw stone walls that needed mending and stumbled across two ancient cemeteries, one had been for family, the other for slaves. I wondered how many farmers and weavers and menders had walked this trail with their kids over the generations.
The next morning, we learned that roosters do not in fact crow at dawn, they crow before dawn (which could also aptly be called nighttime). There were no commutes or late patients here. But there was work to be done. Chickens don’t care that it’s Sunday. It downpoured. Watching the sheep from the kitchen as I sipped my coffee, they didn’t seem to mind. Nor did our farmer hosts who trudged past them in tall boots, just as they had every other day of their farmer lives.
By the fifth day, we had fallen into the rhythms of the homestead. We cracked the blue, green, and brown eggs that our hosts placed outside our door in the early hours and made omelets that were as orange as the foliage. We finally learned to adjust the heat so we neither got chilblains nor had to open the windows and strip naked to cool down. The sky was a brilliant blue that last morning and Sloan ran around trying to catch leaves as they blew off the trees. She had no objective. No counting. No contest. Just chasing leaves as they fell. It was the ultimate atelic activity, done just for doing it. I joined her and found I was no better at this than a 3-year-old.
We might all benefit from a little time in the woods.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
False-positive mammography results and risk for cancer death
False-positive mammography results are common, but
Women with a false-positive mammography result had 61% greater risk of developing breast cancer and an 84% greater risk of dying of breast cancer, compared with those who did not have a false-positive result.
However, the investigators also found that the risk for breast cancer varied by individual characteristics such as age and breast density.
The analysis provides clues about which patients with false-positive mammography results will go on to develop breast cancer and “can be used to develop individualized risk-based breast cancer screening,” said the investigators, led by Xinhe Mao, MSc, of Karolinska Institute, Stockholm.
The findings were published online in JAMA Oncology.
About 11% of women in the United States and 2.5% in Europe will receive a false-positive result after a single mammography screening, and previous research shows that these women have a higher risk of developing breast cancer, compared with women without false-positive results. Still, whether this risk for breast cancer varies by individual characteristics and whether an association between a false-positive mammography result and mortality exists remain unclear.
To assess long-term outcomes after a false-positive result, the study investigators compared 45,213 women who had a false-positive mammography result between 1991 and 2017 with 452,130 controls matched for age, calendar year of mammography, and screening history. These data came from the Stockholm Mammography Screening program and Swedish nationwide registers. The analysis also included 1,113 women with a false-positive result and 11,130 matched controls with information on mammographic breast density from the Karolinska Mammography Project for Risk Prediction of Breast Cancer study.
Among women with a false-positive result, the 20-year cumulative breast cancer incidence was 11.3% compared with 7.3% among those without a false-positive (adjusted hazard ratio, 1.61).
Breast cancer risk was higher in older women – those aged 60-75 years (HR, 2.02) – vs younger women aged 40-49 years (HR, 1.38). Breast cancer risk was also higher among women with less dense breasts (HR, 4.65) vs more dense breasts (HR, 1.60) and those who underwent a biopsy during recall (HR, 1.77) vs those who did not (HR, 1.51).
After a false-positive result, cancers were more likely to occur on the ipsilateral side to the false-positive result (HR, 1.92) versus the contralateral (HR, 1.28) and were more common during the first 4 years of follow-up (HR, 2.57 in the first 2 years and 1.93 between 2 and 4 years). No statistical differences were observed based on tumor characteristics, aside from tumor size (HR, 1.78 for tumors ≥ 20 mm vs. 1.47 for smaller tumors).
The prognosis of patients with breast cancer did not differ on the basis of whether they had false-positive results before diagnosis (HR, 1.05 for a false-positive result versus no false-positive result; 95% CI, 0.89-1.25).
This study is the first to show that “women with a false-positive result are at increased risk of death from breast cancer,” Ms. Mao and colleagues concluded. This finding is “most probably associated with the increased breast cancer incidence,” given that the prognosis of patients with breast cancer was similar among those who had a false-positive result versus those who did not.
The authors noted that the increased risk for breast cancer after a false-positive result could suggest that false positives indicate the presence of small tumors that were missed or generally indicate a higher risk for breast cancer. Other factors, such as hormones or genetics, may be at play as well, but would need to be investigated in further studies, Ms. Mao and colleagues noted.
When individualizing surveillance after a false-positive result, age and breast density should be considered, the authors explained. Clinicians may also want to provide more intensive surveillance in the years after a false-positive result as well as education to patients about the risks associated with a false-positive result.
Overall, the findings indicate that clinicians “ should stress the importance of continued screening in women with false-positive results, given their higher risk of cancer, especially within the first 5 or so years after a false-positive result,” Diana L. Miglioretti, PhD, professor and division chief of biostatistics at the University of California, Davis, said in an interview.
Dr. Miglioretti, who has led research on false-positive mammography results and approaches to reduce false positives, noted that “this is a very important study confirming prior work by the Breast Cancer Surveillance Consortium showing individuals with false-positive screening mammography results are at increased risk of developing breast cancer in the future.”
The new evidence demonstrated an increased risk for death from breast cancer in patients who have a false-positive result is particularly worrisome because some studies suggest that women with false-positive results are less likely to return for screening, perhaps because of their negative experience, Dr. Miglioretti said.
However, her own research has shown that providing immediate screening mammography interpretation and same-day diagnostic workup to individuals who have not had a mammogram in the past 5 years and to younger women could prevent 40% of people from needing to return for diagnostic workup later and potentially reduce time to diagnosis for those with cancer.
It is “important that radiology facilities find ways to reduce false-positive results and the anxiety associated with these results,” Dr. Miglioretti said.
This study was supported by grants from the Swedish Research Council, the Swedish Cancer Society, the Stockholm County Council, and FORTE. Ms. Mao is supported by a grant from the China Scholarship Council. Dr. Miglioretti received funding from PCORI and NCI and royalties from Elsevier.
A version of this article first appeared on Medscape.com.
False-positive mammography results are common, but
Women with a false-positive mammography result had 61% greater risk of developing breast cancer and an 84% greater risk of dying of breast cancer, compared with those who did not have a false-positive result.
However, the investigators also found that the risk for breast cancer varied by individual characteristics such as age and breast density.
The analysis provides clues about which patients with false-positive mammography results will go on to develop breast cancer and “can be used to develop individualized risk-based breast cancer screening,” said the investigators, led by Xinhe Mao, MSc, of Karolinska Institute, Stockholm.
The findings were published online in JAMA Oncology.
About 11% of women in the United States and 2.5% in Europe will receive a false-positive result after a single mammography screening, and previous research shows that these women have a higher risk of developing breast cancer, compared with women without false-positive results. Still, whether this risk for breast cancer varies by individual characteristics and whether an association between a false-positive mammography result and mortality exists remain unclear.
To assess long-term outcomes after a false-positive result, the study investigators compared 45,213 women who had a false-positive mammography result between 1991 and 2017 with 452,130 controls matched for age, calendar year of mammography, and screening history. These data came from the Stockholm Mammography Screening program and Swedish nationwide registers. The analysis also included 1,113 women with a false-positive result and 11,130 matched controls with information on mammographic breast density from the Karolinska Mammography Project for Risk Prediction of Breast Cancer study.
Among women with a false-positive result, the 20-year cumulative breast cancer incidence was 11.3% compared with 7.3% among those without a false-positive (adjusted hazard ratio, 1.61).
Breast cancer risk was higher in older women – those aged 60-75 years (HR, 2.02) – vs younger women aged 40-49 years (HR, 1.38). Breast cancer risk was also higher among women with less dense breasts (HR, 4.65) vs more dense breasts (HR, 1.60) and those who underwent a biopsy during recall (HR, 1.77) vs those who did not (HR, 1.51).
After a false-positive result, cancers were more likely to occur on the ipsilateral side to the false-positive result (HR, 1.92) versus the contralateral (HR, 1.28) and were more common during the first 4 years of follow-up (HR, 2.57 in the first 2 years and 1.93 between 2 and 4 years). No statistical differences were observed based on tumor characteristics, aside from tumor size (HR, 1.78 for tumors ≥ 20 mm vs. 1.47 for smaller tumors).
The prognosis of patients with breast cancer did not differ on the basis of whether they had false-positive results before diagnosis (HR, 1.05 for a false-positive result versus no false-positive result; 95% CI, 0.89-1.25).
This study is the first to show that “women with a false-positive result are at increased risk of death from breast cancer,” Ms. Mao and colleagues concluded. This finding is “most probably associated with the increased breast cancer incidence,” given that the prognosis of patients with breast cancer was similar among those who had a false-positive result versus those who did not.
The authors noted that the increased risk for breast cancer after a false-positive result could suggest that false positives indicate the presence of small tumors that were missed or generally indicate a higher risk for breast cancer. Other factors, such as hormones or genetics, may be at play as well, but would need to be investigated in further studies, Ms. Mao and colleagues noted.
When individualizing surveillance after a false-positive result, age and breast density should be considered, the authors explained. Clinicians may also want to provide more intensive surveillance in the years after a false-positive result as well as education to patients about the risks associated with a false-positive result.
Overall, the findings indicate that clinicians “ should stress the importance of continued screening in women with false-positive results, given their higher risk of cancer, especially within the first 5 or so years after a false-positive result,” Diana L. Miglioretti, PhD, professor and division chief of biostatistics at the University of California, Davis, said in an interview.
Dr. Miglioretti, who has led research on false-positive mammography results and approaches to reduce false positives, noted that “this is a very important study confirming prior work by the Breast Cancer Surveillance Consortium showing individuals with false-positive screening mammography results are at increased risk of developing breast cancer in the future.”
The new evidence demonstrated an increased risk for death from breast cancer in patients who have a false-positive result is particularly worrisome because some studies suggest that women with false-positive results are less likely to return for screening, perhaps because of their negative experience, Dr. Miglioretti said.
However, her own research has shown that providing immediate screening mammography interpretation and same-day diagnostic workup to individuals who have not had a mammogram in the past 5 years and to younger women could prevent 40% of people from needing to return for diagnostic workup later and potentially reduce time to diagnosis for those with cancer.
It is “important that radiology facilities find ways to reduce false-positive results and the anxiety associated with these results,” Dr. Miglioretti said.
This study was supported by grants from the Swedish Research Council, the Swedish Cancer Society, the Stockholm County Council, and FORTE. Ms. Mao is supported by a grant from the China Scholarship Council. Dr. Miglioretti received funding from PCORI and NCI and royalties from Elsevier.
A version of this article first appeared on Medscape.com.
False-positive mammography results are common, but
Women with a false-positive mammography result had 61% greater risk of developing breast cancer and an 84% greater risk of dying of breast cancer, compared with those who did not have a false-positive result.
However, the investigators also found that the risk for breast cancer varied by individual characteristics such as age and breast density.
The analysis provides clues about which patients with false-positive mammography results will go on to develop breast cancer and “can be used to develop individualized risk-based breast cancer screening,” said the investigators, led by Xinhe Mao, MSc, of Karolinska Institute, Stockholm.
The findings were published online in JAMA Oncology.
About 11% of women in the United States and 2.5% in Europe will receive a false-positive result after a single mammography screening, and previous research shows that these women have a higher risk of developing breast cancer, compared with women without false-positive results. Still, whether this risk for breast cancer varies by individual characteristics and whether an association between a false-positive mammography result and mortality exists remain unclear.
To assess long-term outcomes after a false-positive result, the study investigators compared 45,213 women who had a false-positive mammography result between 1991 and 2017 with 452,130 controls matched for age, calendar year of mammography, and screening history. These data came from the Stockholm Mammography Screening program and Swedish nationwide registers. The analysis also included 1,113 women with a false-positive result and 11,130 matched controls with information on mammographic breast density from the Karolinska Mammography Project for Risk Prediction of Breast Cancer study.
Among women with a false-positive result, the 20-year cumulative breast cancer incidence was 11.3% compared with 7.3% among those without a false-positive (adjusted hazard ratio, 1.61).
Breast cancer risk was higher in older women – those aged 60-75 years (HR, 2.02) – vs younger women aged 40-49 years (HR, 1.38). Breast cancer risk was also higher among women with less dense breasts (HR, 4.65) vs more dense breasts (HR, 1.60) and those who underwent a biopsy during recall (HR, 1.77) vs those who did not (HR, 1.51).
After a false-positive result, cancers were more likely to occur on the ipsilateral side to the false-positive result (HR, 1.92) versus the contralateral (HR, 1.28) and were more common during the first 4 years of follow-up (HR, 2.57 in the first 2 years and 1.93 between 2 and 4 years). No statistical differences were observed based on tumor characteristics, aside from tumor size (HR, 1.78 for tumors ≥ 20 mm vs. 1.47 for smaller tumors).
The prognosis of patients with breast cancer did not differ on the basis of whether they had false-positive results before diagnosis (HR, 1.05 for a false-positive result versus no false-positive result; 95% CI, 0.89-1.25).
This study is the first to show that “women with a false-positive result are at increased risk of death from breast cancer,” Ms. Mao and colleagues concluded. This finding is “most probably associated with the increased breast cancer incidence,” given that the prognosis of patients with breast cancer was similar among those who had a false-positive result versus those who did not.
The authors noted that the increased risk for breast cancer after a false-positive result could suggest that false positives indicate the presence of small tumors that were missed or generally indicate a higher risk for breast cancer. Other factors, such as hormones or genetics, may be at play as well, but would need to be investigated in further studies, Ms. Mao and colleagues noted.
When individualizing surveillance after a false-positive result, age and breast density should be considered, the authors explained. Clinicians may also want to provide more intensive surveillance in the years after a false-positive result as well as education to patients about the risks associated with a false-positive result.
Overall, the findings indicate that clinicians “ should stress the importance of continued screening in women with false-positive results, given their higher risk of cancer, especially within the first 5 or so years after a false-positive result,” Diana L. Miglioretti, PhD, professor and division chief of biostatistics at the University of California, Davis, said in an interview.
Dr. Miglioretti, who has led research on false-positive mammography results and approaches to reduce false positives, noted that “this is a very important study confirming prior work by the Breast Cancer Surveillance Consortium showing individuals with false-positive screening mammography results are at increased risk of developing breast cancer in the future.”
The new evidence demonstrated an increased risk for death from breast cancer in patients who have a false-positive result is particularly worrisome because some studies suggest that women with false-positive results are less likely to return for screening, perhaps because of their negative experience, Dr. Miglioretti said.
However, her own research has shown that providing immediate screening mammography interpretation and same-day diagnostic workup to individuals who have not had a mammogram in the past 5 years and to younger women could prevent 40% of people from needing to return for diagnostic workup later and potentially reduce time to diagnosis for those with cancer.
It is “important that radiology facilities find ways to reduce false-positive results and the anxiety associated with these results,” Dr. Miglioretti said.
This study was supported by grants from the Swedish Research Council, the Swedish Cancer Society, the Stockholm County Council, and FORTE. Ms. Mao is supported by a grant from the China Scholarship Council. Dr. Miglioretti received funding from PCORI and NCI and royalties from Elsevier.
A version of this article first appeared on Medscape.com.
FROM JAMA ONCOLOGY