User login
Study takes fine-grained look at MACE risk with glucocorticoids in RA
SAN DIEGO – Even when taken at low doses and over short periods, glucocorticoids (GCs) were linked to a higher risk of major adverse cardiovascular events (MACE) over the long term in a Veterans Affairs population of older, mostly male patients with rheumatoid arthritis, a new retrospective cohort study has found.
The analysis of nearly 19,000 patients, presented by rheumatologist Beth Wallace, MD, MSc, at the annual meeting of the American College of Rheumatology, showed that the level of risk for MACE rose with the dose, duration, and recency of GC use, in which risk increased significantly at prednisone-equivalent doses as low as 5 mg/day, durations as short as 30 days, and with last use as long as 1 year before MACE.
“Up to half of RA patients in the United States use long-term glucocorticoids despite previous work suggesting they increase MACE in a dose-dependent way,” said Dr. Wallace, assistant professor of medicine at the University of Michigan, Ann Arbor, and a rheumatologist at the VA Ann Arbor Healthcare Center. “Our group previously presented work suggesting that less than 14 days of glucocorticoid use in a 6-month period is associated with a two-thirds increase in odds of MACE over the following 6 months, with 90 days of use associated with more than twofold increase.”
In recent years, researchers such as Dr. Wallace have focused attention on the risks of GCs in RA. The American College of Rheumatology and the European Alliance of Associations for Rheumatology emphasize avoiding long-term use of GCs in RA and keeping doses as small and over the shortest amount of time as possible.
When Dr. Wallace and colleagues looked at the clinical pattern of GC use for patients with RA during the past 2 years, those who took 5 mg, 7.5 mg, and 10 mg daily doses for 30 days and had stopped at least a year before had risk for MACE that rose significantly by 3%, 5%, and 7%, respectively, compared with those who didn’t take GCs in the past 2 years.
While those increases were small, risk for MACE rose even more for those who took the same daily doses for 90 days, increasing 10%, 15%, and 21%, respectively. Researchers linked current ongoing use of GCs for the past 90 days to a 13%, 19%, and 27% higher risk for MACE at those respective doses.
The findings “add to the literature suggesting that there is some risk even with low-dose steroids,” said Michael George, MD, assistant professor of rheumatology and epidemiology at the University of Pennsylvania, Philadelphia, who did not take part in the research but is familiar with the findings.
“We can see that even glucocorticoids taken several years ago may affect cardiovascular risk but that recent use has a bigger effect on risk,” Dr. George said in an interview. “This study also suggests that very low-dose use affects risk.”
For the new study, Dr. Wallace and colleagues examined a Veterans Affairs database and identified 18,882 patients with RA (mean age, 62.5 years; 84% male; 66% GC users) who met the criteria of being > 40 and < 90 years old. The subjects had an initial VA rheumatology visit during 2010-2018 and were excluded if they had a non-RA rheumatologic disorder, prior MACE, or heart failure. MACE was defined as MI, stroke/TIA, cardiac arrest, coronary revascularization, or death from CV cause.
A total of 16% of the cohort had the largest exposure to GCs, defined as use for 90 days or more; 23% had exposure of 14-89 days, and 14% had exposure of 1-13 days.
The median 5-year MACE risk at baseline was 5.3%, and 3,754 patients (19.9%) had high baseline MACE risk. Incident MACE occurred in 4.1% of patients, and the median time to MACE was 2.67 years (interquartile ratio, 1.26-4.45 years).
Covariates included factors such as age, race, sex, body mass index, smoking status, adjusted Elixhauser index, VA risk score for cardiovascular disease, cancer, hospitalization for infection, number of rheumatology clinic visits, and use of lipid-lowering drugs, opioids, methotrexate, biologics, and hydroxychloroquine.
Dr. Wallace noted limitations including the possibility of residual confounding and the influence of background cardiovascular risk. The study didn’t examine the clinical value of taking GCs or compare that to the potential risk. Nor did it examine cost or the risks and benefits of alternative therapeutic options.
A study released earlier this year suggested that patients taking daily prednisolone doses under 5 mg do not have a higher risk of MACE. Previous studies had reached conflicting results.
“Glucocorticoids can provide major benefits to patients, but these benefits must be balanced with the potential risks,” Dr. George said. At low doses, these risks may be small, but they are present. In many cases, escalating DMARD [disease-modifying antirheumatic drug] therapy may be safer than continuing glucocorticoids.”
He added that the risks of GCs may be especially high in older patients and in those who have cardiovascular risk factors: “Often biologics are avoided in these higher-risk patients. But in fact, in many cases biologics may be the safer choice.”
No study funding was reported. Dr. Wallace reported no relevant financial relationships, and some of the other authors reported various ties with industry. Dr. George reported research funding from GlaxoSmithKline and Janssen and consulting fees from AbbVie.
SAN DIEGO – Even when taken at low doses and over short periods, glucocorticoids (GCs) were linked to a higher risk of major adverse cardiovascular events (MACE) over the long term in a Veterans Affairs population of older, mostly male patients with rheumatoid arthritis, a new retrospective cohort study has found.
The analysis of nearly 19,000 patients, presented by rheumatologist Beth Wallace, MD, MSc, at the annual meeting of the American College of Rheumatology, showed that the level of risk for MACE rose with the dose, duration, and recency of GC use, in which risk increased significantly at prednisone-equivalent doses as low as 5 mg/day, durations as short as 30 days, and with last use as long as 1 year before MACE.
“Up to half of RA patients in the United States use long-term glucocorticoids despite previous work suggesting they increase MACE in a dose-dependent way,” said Dr. Wallace, assistant professor of medicine at the University of Michigan, Ann Arbor, and a rheumatologist at the VA Ann Arbor Healthcare Center. “Our group previously presented work suggesting that less than 14 days of glucocorticoid use in a 6-month period is associated with a two-thirds increase in odds of MACE over the following 6 months, with 90 days of use associated with more than twofold increase.”
In recent years, researchers such as Dr. Wallace have focused attention on the risks of GCs in RA. The American College of Rheumatology and the European Alliance of Associations for Rheumatology emphasize avoiding long-term use of GCs in RA and keeping doses as small and over the shortest amount of time as possible.
When Dr. Wallace and colleagues looked at the clinical pattern of GC use for patients with RA during the past 2 years, those who took 5 mg, 7.5 mg, and 10 mg daily doses for 30 days and had stopped at least a year before had risk for MACE that rose significantly by 3%, 5%, and 7%, respectively, compared with those who didn’t take GCs in the past 2 years.
While those increases were small, risk for MACE rose even more for those who took the same daily doses for 90 days, increasing 10%, 15%, and 21%, respectively. Researchers linked current ongoing use of GCs for the past 90 days to a 13%, 19%, and 27% higher risk for MACE at those respective doses.
The findings “add to the literature suggesting that there is some risk even with low-dose steroids,” said Michael George, MD, assistant professor of rheumatology and epidemiology at the University of Pennsylvania, Philadelphia, who did not take part in the research but is familiar with the findings.
“We can see that even glucocorticoids taken several years ago may affect cardiovascular risk but that recent use has a bigger effect on risk,” Dr. George said in an interview. “This study also suggests that very low-dose use affects risk.”
For the new study, Dr. Wallace and colleagues examined a Veterans Affairs database and identified 18,882 patients with RA (mean age, 62.5 years; 84% male; 66% GC users) who met the criteria of being > 40 and < 90 years old. The subjects had an initial VA rheumatology visit during 2010-2018 and were excluded if they had a non-RA rheumatologic disorder, prior MACE, or heart failure. MACE was defined as MI, stroke/TIA, cardiac arrest, coronary revascularization, or death from CV cause.
A total of 16% of the cohort had the largest exposure to GCs, defined as use for 90 days or more; 23% had exposure of 14-89 days, and 14% had exposure of 1-13 days.
The median 5-year MACE risk at baseline was 5.3%, and 3,754 patients (19.9%) had high baseline MACE risk. Incident MACE occurred in 4.1% of patients, and the median time to MACE was 2.67 years (interquartile ratio, 1.26-4.45 years).
Covariates included factors such as age, race, sex, body mass index, smoking status, adjusted Elixhauser index, VA risk score for cardiovascular disease, cancer, hospitalization for infection, number of rheumatology clinic visits, and use of lipid-lowering drugs, opioids, methotrexate, biologics, and hydroxychloroquine.
Dr. Wallace noted limitations including the possibility of residual confounding and the influence of background cardiovascular risk. The study didn’t examine the clinical value of taking GCs or compare that to the potential risk. Nor did it examine cost or the risks and benefits of alternative therapeutic options.
A study released earlier this year suggested that patients taking daily prednisolone doses under 5 mg do not have a higher risk of MACE. Previous studies had reached conflicting results.
“Glucocorticoids can provide major benefits to patients, but these benefits must be balanced with the potential risks,” Dr. George said. At low doses, these risks may be small, but they are present. In many cases, escalating DMARD [disease-modifying antirheumatic drug] therapy may be safer than continuing glucocorticoids.”
He added that the risks of GCs may be especially high in older patients and in those who have cardiovascular risk factors: “Often biologics are avoided in these higher-risk patients. But in fact, in many cases biologics may be the safer choice.”
No study funding was reported. Dr. Wallace reported no relevant financial relationships, and some of the other authors reported various ties with industry. Dr. George reported research funding from GlaxoSmithKline and Janssen and consulting fees from AbbVie.
SAN DIEGO – Even when taken at low doses and over short periods, glucocorticoids (GCs) were linked to a higher risk of major adverse cardiovascular events (MACE) over the long term in a Veterans Affairs population of older, mostly male patients with rheumatoid arthritis, a new retrospective cohort study has found.
The analysis of nearly 19,000 patients, presented by rheumatologist Beth Wallace, MD, MSc, at the annual meeting of the American College of Rheumatology, showed that the level of risk for MACE rose with the dose, duration, and recency of GC use, in which risk increased significantly at prednisone-equivalent doses as low as 5 mg/day, durations as short as 30 days, and with last use as long as 1 year before MACE.
“Up to half of RA patients in the United States use long-term glucocorticoids despite previous work suggesting they increase MACE in a dose-dependent way,” said Dr. Wallace, assistant professor of medicine at the University of Michigan, Ann Arbor, and a rheumatologist at the VA Ann Arbor Healthcare Center. “Our group previously presented work suggesting that less than 14 days of glucocorticoid use in a 6-month period is associated with a two-thirds increase in odds of MACE over the following 6 months, with 90 days of use associated with more than twofold increase.”
In recent years, researchers such as Dr. Wallace have focused attention on the risks of GCs in RA. The American College of Rheumatology and the European Alliance of Associations for Rheumatology emphasize avoiding long-term use of GCs in RA and keeping doses as small and over the shortest amount of time as possible.
When Dr. Wallace and colleagues looked at the clinical pattern of GC use for patients with RA during the past 2 years, those who took 5 mg, 7.5 mg, and 10 mg daily doses for 30 days and had stopped at least a year before had risk for MACE that rose significantly by 3%, 5%, and 7%, respectively, compared with those who didn’t take GCs in the past 2 years.
While those increases were small, risk for MACE rose even more for those who took the same daily doses for 90 days, increasing 10%, 15%, and 21%, respectively. Researchers linked current ongoing use of GCs for the past 90 days to a 13%, 19%, and 27% higher risk for MACE at those respective doses.
The findings “add to the literature suggesting that there is some risk even with low-dose steroids,” said Michael George, MD, assistant professor of rheumatology and epidemiology at the University of Pennsylvania, Philadelphia, who did not take part in the research but is familiar with the findings.
“We can see that even glucocorticoids taken several years ago may affect cardiovascular risk but that recent use has a bigger effect on risk,” Dr. George said in an interview. “This study also suggests that very low-dose use affects risk.”
For the new study, Dr. Wallace and colleagues examined a Veterans Affairs database and identified 18,882 patients with RA (mean age, 62.5 years; 84% male; 66% GC users) who met the criteria of being > 40 and < 90 years old. The subjects had an initial VA rheumatology visit during 2010-2018 and were excluded if they had a non-RA rheumatologic disorder, prior MACE, or heart failure. MACE was defined as MI, stroke/TIA, cardiac arrest, coronary revascularization, or death from CV cause.
A total of 16% of the cohort had the largest exposure to GCs, defined as use for 90 days or more; 23% had exposure of 14-89 days, and 14% had exposure of 1-13 days.
The median 5-year MACE risk at baseline was 5.3%, and 3,754 patients (19.9%) had high baseline MACE risk. Incident MACE occurred in 4.1% of patients, and the median time to MACE was 2.67 years (interquartile ratio, 1.26-4.45 years).
Covariates included factors such as age, race, sex, body mass index, smoking status, adjusted Elixhauser index, VA risk score for cardiovascular disease, cancer, hospitalization for infection, number of rheumatology clinic visits, and use of lipid-lowering drugs, opioids, methotrexate, biologics, and hydroxychloroquine.
Dr. Wallace noted limitations including the possibility of residual confounding and the influence of background cardiovascular risk. The study didn’t examine the clinical value of taking GCs or compare that to the potential risk. Nor did it examine cost or the risks and benefits of alternative therapeutic options.
A study released earlier this year suggested that patients taking daily prednisolone doses under 5 mg do not have a higher risk of MACE. Previous studies had reached conflicting results.
“Glucocorticoids can provide major benefits to patients, but these benefits must be balanced with the potential risks,” Dr. George said. At low doses, these risks may be small, but they are present. In many cases, escalating DMARD [disease-modifying antirheumatic drug] therapy may be safer than continuing glucocorticoids.”
He added that the risks of GCs may be especially high in older patients and in those who have cardiovascular risk factors: “Often biologics are avoided in these higher-risk patients. But in fact, in many cases biologics may be the safer choice.”
No study funding was reported. Dr. Wallace reported no relevant financial relationships, and some of the other authors reported various ties with industry. Dr. George reported research funding from GlaxoSmithKline and Janssen and consulting fees from AbbVie.
AT ACR 2023
Impact of the COVID-19 Pandemic on Care for Patients With Atopic Dermatitis
To the Editor:
Atopic dermatitis (AD) is a widely prevalent dermatologic condition that can severely impact a patient’s quality of life.1 Individuals with AD have been substantially affected during the COVID-19 pandemic due to the increased use of irritants, decreased access to care, and rise in psychological stress.1,2 These factors have resulted in lower quality of life and worsening dermatologic symptoms for many AD patients over the last few years.1 One major potential contributory component of these findings is decreased accessibility to in-office care during the pandemic, with a shift to telemedicine instead. Accessibility to care during the COVID-19 pandemic for AD patients compared to those without AD remains unknown. Therefore, we explored the impact of the COVID-19 pandemic on care for patients with AD in a large US population.
Using anonymous survey data from the 2021 National Health Interview Survey,3 we conducted a population-based, cross-sectional study to evaluate access to care during the COVID-19 pandemic for patients with AD compared to those without AD. We assigned the following 3 survey questions as outcome variables to assess access to care: delayed medical care due to COVID-19 pandemic (yes/no), did not get care due to COVID-19 pandemic (yes/no), and virtual medical appointment in the last 12 months (yes/no). In Table 1, numerous categorical survey variables, including sex, health insurance status, race/ethnicity, education, US citizenship, birth in the United States, public assistance/welfare, and region, were analyzed using χ2 testing to evaluate for differences among individuals with and without AD. Multivariable logistic regression models evaluating the relationship between AD and access to care were constructed using Stata/MP 17 (StataCorp LLC). In our analysis we controlled for age, sex, health insurance status, race/ethnicity, education, US citizenship, birth in the United States, public assistance/welfare, and region.
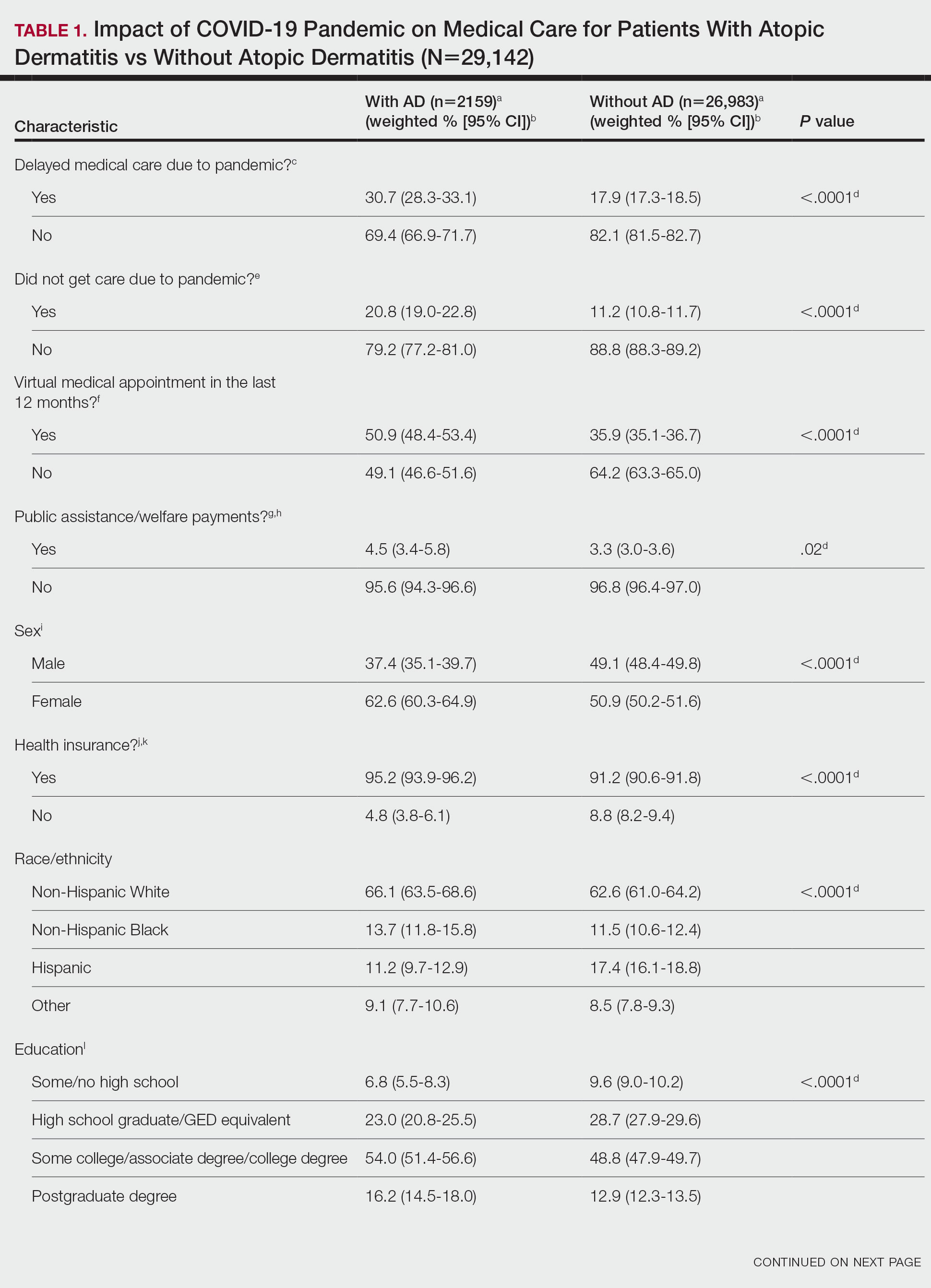
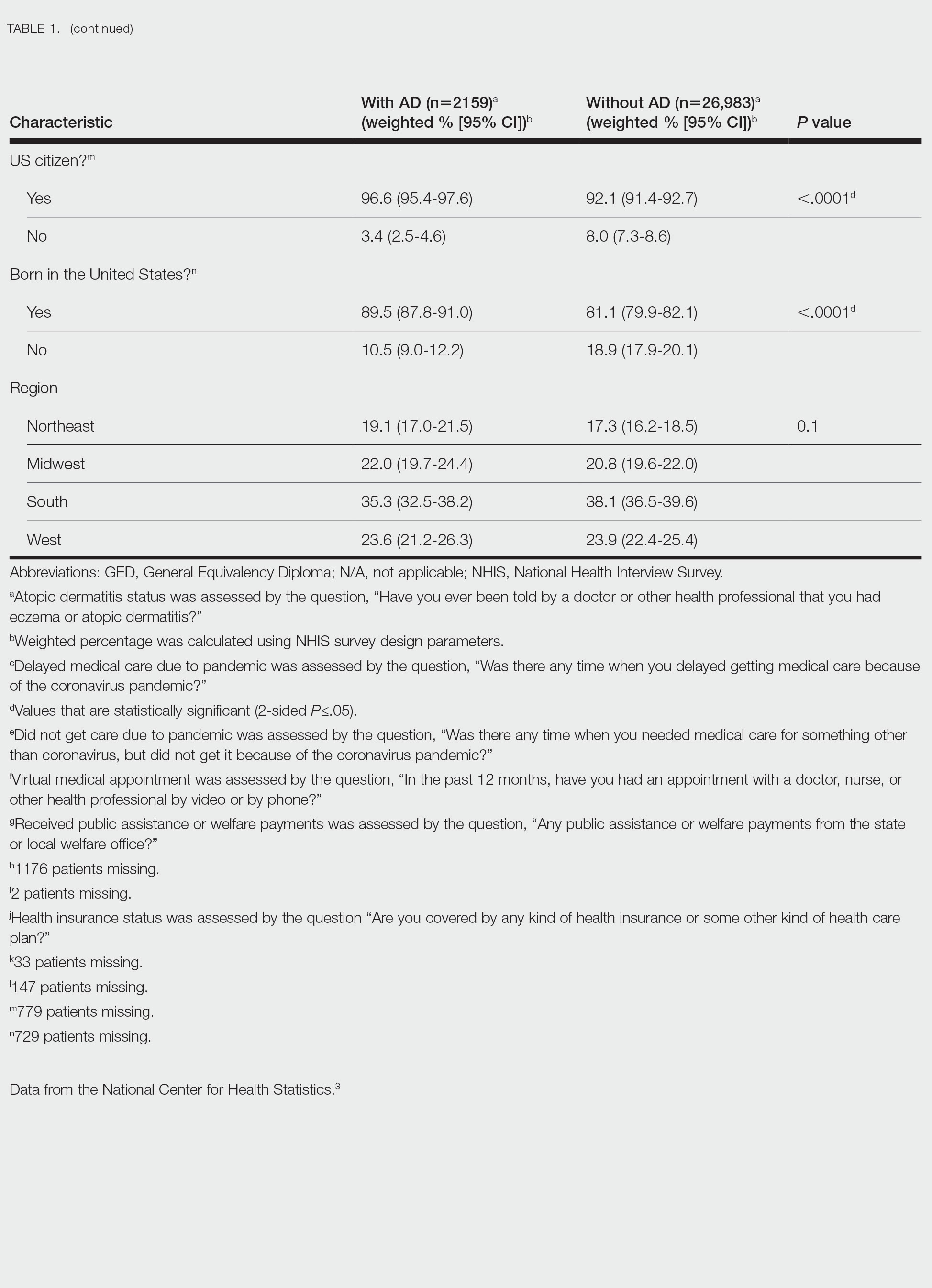
There were 29,142 adult patients (aged ≥18 years) included in our analysis. Approximately 7.4% (weighted) of individuals had AD (Table 1). After adjusting for confounding variables, patients with AD had a higher odds of delaying medical care due to the COVID-19 pandemic (adjusted odds ratio [AOR], 1.91; 95% CI, 1.69-2.16; P<.001), not receiving care due to the COVID-19 pandemic (AOR, 1.94; 95% CI, 1.71-2.22; P<.001), and having a virtual medical visit in the last 12 months (AOR, 1.72; 95% CI, 1.54-1.93; P<.001)(Table 2) compared with patients without AD.
Our findings support the association between AD and decreased access to in-person care due to the COVID-19 pandemic. Moreover, telemedicine was utilized more among individuals with AD, possibly due to the accessibility of diagnostic tools for dermatologic diagnoses, such as high-quality photographs.4 According to Trinidad et al,4 telemedicine became an invaluable tool for dermatology hospitalists during the COVID-19 pandemic, as many physicians were able to comfortably diagnose patients with cutaneous diseases without an in-person visit. Utilizing telemedicine for patient care can help reduce the risk for COVID-19 transmission while also providing quality care for individuals living in rural areas.5 Chiricozzi et al6 discussed the importance of telemedicine in Italy during the pandemic, as many AD patients were able to maintain control of their disease while on systemic treatments.
Limitations of this study include self-reported measures; inability to compare patients with AD to individuals with other cutaneous diseases; and additional potential confounders, such as chronic comorbidities. Future studies should evaluate the use of telemedicine and access to care among individuals with other common skin diseases and help determine why such discrepancies exist. Understanding the difficulties in access to care and the viable alternatives in place may increase awareness and assist clinicians with adequate management of patients with AD.
1. Sieniawska J, Lesiak A, Cia˛z˙yn´ski K, et al. Impact of the COVID-19 pandemic on atopic dermatitis patients. Int J Environ Res Public Health. 2022;19:1734. doi:10.3390/ijerph19031734
2. Pourani MR, Ganji R, Dashti T, et al. Impact of COVID-19 pandemic on patients with atopic dermatitis [in Spanish]. Actas Dermosifiliogr. 2022;113:T286-T293. doi:10.1016/j.ad.2021.08.004
3. National Center for Health Statistics. NHIS Data, Questionnaires and Related Documentation. Centers for Disease Control and Prevention website. Accessed February 1, 2023. https://www.cdc.gov/nchs/nhis/data-questionnaires-documentation.htm
4. Trinidad J, Gabel CK, Han JJ, et al. Telemedicine and dermatology hospital consultations during the COVID-19 pandemic: a multi-centre observational study on resource utilization and conversion to in-person consultations during the COVID-19 pandemic. J Eur Acad Dermatol Venereol. 2022;36:E323-E325. doi:10.1111/jdv.17898
5. Marasca C, Annunziata MC, Camela E, et al. Teledermatology and inflammatory skin conditions during COVID-19 era: new perspectives and applications. J Clin Med. 2022;11:1511. doi:10.3390/jcm11061511
6. Chiricozzi A, Talamonti M, De Simone C, et al. Management of patients with atopic dermatitis undergoing systemic therapy during COVID-19 pandemic in Italy: data from the DA-COVID-19 registry. Allergy. 2021;76:1813-1824. doi:10.1111/all.14767
To the Editor:
Atopic dermatitis (AD) is a widely prevalent dermatologic condition that can severely impact a patient’s quality of life.1 Individuals with AD have been substantially affected during the COVID-19 pandemic due to the increased use of irritants, decreased access to care, and rise in psychological stress.1,2 These factors have resulted in lower quality of life and worsening dermatologic symptoms for many AD patients over the last few years.1 One major potential contributory component of these findings is decreased accessibility to in-office care during the pandemic, with a shift to telemedicine instead. Accessibility to care during the COVID-19 pandemic for AD patients compared to those without AD remains unknown. Therefore, we explored the impact of the COVID-19 pandemic on care for patients with AD in a large US population.
Using anonymous survey data from the 2021 National Health Interview Survey,3 we conducted a population-based, cross-sectional study to evaluate access to care during the COVID-19 pandemic for patients with AD compared to those without AD. We assigned the following 3 survey questions as outcome variables to assess access to care: delayed medical care due to COVID-19 pandemic (yes/no), did not get care due to COVID-19 pandemic (yes/no), and virtual medical appointment in the last 12 months (yes/no). In Table 1, numerous categorical survey variables, including sex, health insurance status, race/ethnicity, education, US citizenship, birth in the United States, public assistance/welfare, and region, were analyzed using χ2 testing to evaluate for differences among individuals with and without AD. Multivariable logistic regression models evaluating the relationship between AD and access to care were constructed using Stata/MP 17 (StataCorp LLC). In our analysis we controlled for age, sex, health insurance status, race/ethnicity, education, US citizenship, birth in the United States, public assistance/welfare, and region.


There were 29,142 adult patients (aged ≥18 years) included in our analysis. Approximately 7.4% (weighted) of individuals had AD (Table 1). After adjusting for confounding variables, patients with AD had a higher odds of delaying medical care due to the COVID-19 pandemic (adjusted odds ratio [AOR], 1.91; 95% CI, 1.69-2.16; P<.001), not receiving care due to the COVID-19 pandemic (AOR, 1.94; 95% CI, 1.71-2.22; P<.001), and having a virtual medical visit in the last 12 months (AOR, 1.72; 95% CI, 1.54-1.93; P<.001)(Table 2) compared with patients without AD.

Our findings support the association between AD and decreased access to in-person care due to the COVID-19 pandemic. Moreover, telemedicine was utilized more among individuals with AD, possibly due to the accessibility of diagnostic tools for dermatologic diagnoses, such as high-quality photographs.4 According to Trinidad et al,4 telemedicine became an invaluable tool for dermatology hospitalists during the COVID-19 pandemic, as many physicians were able to comfortably diagnose patients with cutaneous diseases without an in-person visit. Utilizing telemedicine for patient care can help reduce the risk for COVID-19 transmission while also providing quality care for individuals living in rural areas.5 Chiricozzi et al6 discussed the importance of telemedicine in Italy during the pandemic, as many AD patients were able to maintain control of their disease while on systemic treatments.
Limitations of this study include self-reported measures; inability to compare patients with AD to individuals with other cutaneous diseases; and additional potential confounders, such as chronic comorbidities. Future studies should evaluate the use of telemedicine and access to care among individuals with other common skin diseases and help determine why such discrepancies exist. Understanding the difficulties in access to care and the viable alternatives in place may increase awareness and assist clinicians with adequate management of patients with AD.
To the Editor:
Atopic dermatitis (AD) is a widely prevalent dermatologic condition that can severely impact a patient’s quality of life.1 Individuals with AD have been substantially affected during the COVID-19 pandemic due to the increased use of irritants, decreased access to care, and rise in psychological stress.1,2 These factors have resulted in lower quality of life and worsening dermatologic symptoms for many AD patients over the last few years.1 One major potential contributory component of these findings is decreased accessibility to in-office care during the pandemic, with a shift to telemedicine instead. Accessibility to care during the COVID-19 pandemic for AD patients compared to those without AD remains unknown. Therefore, we explored the impact of the COVID-19 pandemic on care for patients with AD in a large US population.
Using anonymous survey data from the 2021 National Health Interview Survey,3 we conducted a population-based, cross-sectional study to evaluate access to care during the COVID-19 pandemic for patients with AD compared to those without AD. We assigned the following 3 survey questions as outcome variables to assess access to care: delayed medical care due to COVID-19 pandemic (yes/no), did not get care due to COVID-19 pandemic (yes/no), and virtual medical appointment in the last 12 months (yes/no). In Table 1, numerous categorical survey variables, including sex, health insurance status, race/ethnicity, education, US citizenship, birth in the United States, public assistance/welfare, and region, were analyzed using χ2 testing to evaluate for differences among individuals with and without AD. Multivariable logistic regression models evaluating the relationship between AD and access to care were constructed using Stata/MP 17 (StataCorp LLC). In our analysis we controlled for age, sex, health insurance status, race/ethnicity, education, US citizenship, birth in the United States, public assistance/welfare, and region.


There were 29,142 adult patients (aged ≥18 years) included in our analysis. Approximately 7.4% (weighted) of individuals had AD (Table 1). After adjusting for confounding variables, patients with AD had a higher odds of delaying medical care due to the COVID-19 pandemic (adjusted odds ratio [AOR], 1.91; 95% CI, 1.69-2.16; P<.001), not receiving care due to the COVID-19 pandemic (AOR, 1.94; 95% CI, 1.71-2.22; P<.001), and having a virtual medical visit in the last 12 months (AOR, 1.72; 95% CI, 1.54-1.93; P<.001)(Table 2) compared with patients without AD.

Our findings support the association between AD and decreased access to in-person care due to the COVID-19 pandemic. Moreover, telemedicine was utilized more among individuals with AD, possibly due to the accessibility of diagnostic tools for dermatologic diagnoses, such as high-quality photographs.4 According to Trinidad et al,4 telemedicine became an invaluable tool for dermatology hospitalists during the COVID-19 pandemic, as many physicians were able to comfortably diagnose patients with cutaneous diseases without an in-person visit. Utilizing telemedicine for patient care can help reduce the risk for COVID-19 transmission while also providing quality care for individuals living in rural areas.5 Chiricozzi et al6 discussed the importance of telemedicine in Italy during the pandemic, as many AD patients were able to maintain control of their disease while on systemic treatments.
Limitations of this study include self-reported measures; inability to compare patients with AD to individuals with other cutaneous diseases; and additional potential confounders, such as chronic comorbidities. Future studies should evaluate the use of telemedicine and access to care among individuals with other common skin diseases and help determine why such discrepancies exist. Understanding the difficulties in access to care and the viable alternatives in place may increase awareness and assist clinicians with adequate management of patients with AD.
1. Sieniawska J, Lesiak A, Cia˛z˙yn´ski K, et al. Impact of the COVID-19 pandemic on atopic dermatitis patients. Int J Environ Res Public Health. 2022;19:1734. doi:10.3390/ijerph19031734
2. Pourani MR, Ganji R, Dashti T, et al. Impact of COVID-19 pandemic on patients with atopic dermatitis [in Spanish]. Actas Dermosifiliogr. 2022;113:T286-T293. doi:10.1016/j.ad.2021.08.004
3. National Center for Health Statistics. NHIS Data, Questionnaires and Related Documentation. Centers for Disease Control and Prevention website. Accessed February 1, 2023. https://www.cdc.gov/nchs/nhis/data-questionnaires-documentation.htm
4. Trinidad J, Gabel CK, Han JJ, et al. Telemedicine and dermatology hospital consultations during the COVID-19 pandemic: a multi-centre observational study on resource utilization and conversion to in-person consultations during the COVID-19 pandemic. J Eur Acad Dermatol Venereol. 2022;36:E323-E325. doi:10.1111/jdv.17898
5. Marasca C, Annunziata MC, Camela E, et al. Teledermatology and inflammatory skin conditions during COVID-19 era: new perspectives and applications. J Clin Med. 2022;11:1511. doi:10.3390/jcm11061511
6. Chiricozzi A, Talamonti M, De Simone C, et al. Management of patients with atopic dermatitis undergoing systemic therapy during COVID-19 pandemic in Italy: data from the DA-COVID-19 registry. Allergy. 2021;76:1813-1824. doi:10.1111/all.14767
1. Sieniawska J, Lesiak A, Cia˛z˙yn´ski K, et al. Impact of the COVID-19 pandemic on atopic dermatitis patients. Int J Environ Res Public Health. 2022;19:1734. doi:10.3390/ijerph19031734
2. Pourani MR, Ganji R, Dashti T, et al. Impact of COVID-19 pandemic on patients with atopic dermatitis [in Spanish]. Actas Dermosifiliogr. 2022;113:T286-T293. doi:10.1016/j.ad.2021.08.004
3. National Center for Health Statistics. NHIS Data, Questionnaires and Related Documentation. Centers for Disease Control and Prevention website. Accessed February 1, 2023. https://www.cdc.gov/nchs/nhis/data-questionnaires-documentation.htm
4. Trinidad J, Gabel CK, Han JJ, et al. Telemedicine and dermatology hospital consultations during the COVID-19 pandemic: a multi-centre observational study on resource utilization and conversion to in-person consultations during the COVID-19 pandemic. J Eur Acad Dermatol Venereol. 2022;36:E323-E325. doi:10.1111/jdv.17898
5. Marasca C, Annunziata MC, Camela E, et al. Teledermatology and inflammatory skin conditions during COVID-19 era: new perspectives and applications. J Clin Med. 2022;11:1511. doi:10.3390/jcm11061511
6. Chiricozzi A, Talamonti M, De Simone C, et al. Management of patients with atopic dermatitis undergoing systemic therapy during COVID-19 pandemic in Italy: data from the DA-COVID-19 registry. Allergy. 2021;76:1813-1824. doi:10.1111/all.14767
Practice Points
- The landscape of dermatology has seen major changes due to the COVID-19 pandemic, as many patients now utilize telemedicine to receive care.
- Understanding accessibility to in-person care for patients with atopic dermatitis during the COVID-19 pandemic can assist with the development of methods to enhance management.
Aberrant Expression of CD56 in Metastatic Malignant Melanoma
To the Editor:
Many types of neoplasms can show aberrant immunoreactivity or unexpected expression of markers.1 Malignant melanoma is a tumor that can show not only aberrant immunohistochemical staining patterns but also notable histologic diversity,1,2 which often makes the diagnosis of melanoma challenging and ultimately can lead to diagnostic uncertainty.2
The incidence of malignant melanoma continues to grow.3 Maintaining a high degree of suspicion for this disease, recognizing its heterogeneity and divergent differentiation, and knowing potential aberrant immunohistochemical staining patterns are imperative for accurate diagnosis.
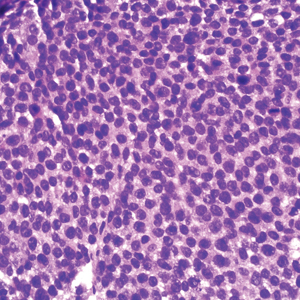
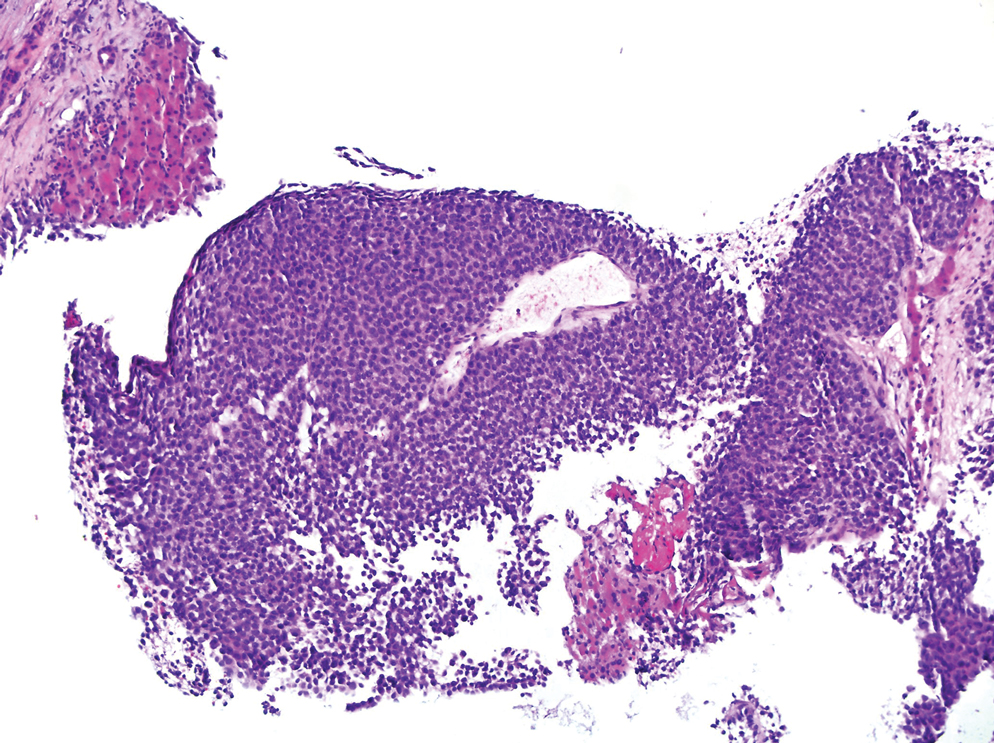
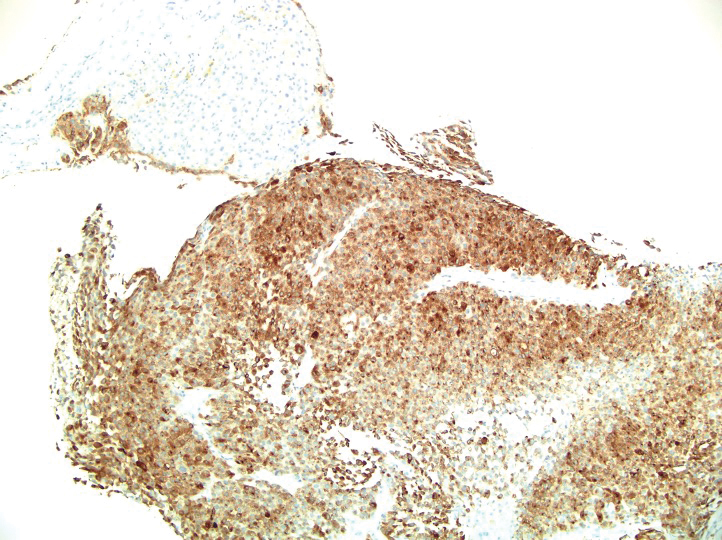
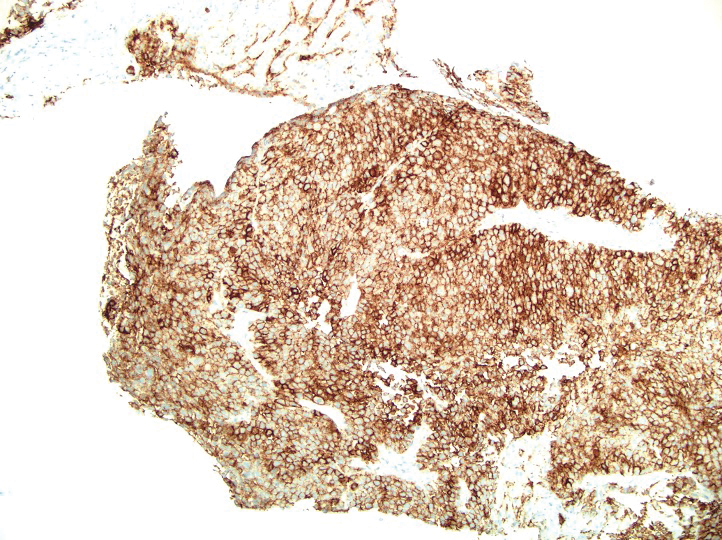
A 36-year-old man presented to a primary care physician with right-sided chest pain, upper and lower back aches, bilateral hip pain, neck pain, headache, night sweats, chills, and nausea. After infectious causes were ruled out, he was placed on a steroid taper without improvement. He presented to the emergency department a few days later with muscle spasms and was found to also have diffuse abdominal tenderness and guarding. The patient’s medical history was noncontributory; he was a lifelong nonsmoker. Laboratory studies revealed elevated levels of alanine aminotransferase and C-reactive protein. Computed tomography of the chest and abdomen revealed innumerable liver and lung lesions that were suspicious for metastatic malignancy. A liver biopsy revealed nests and sheets of metastatic tumor with pleomorphic nuclei, inconspicuous nucleoli, and areas of intranuclear clearing (Figures 1 and 2). Immunohistochemical staining was performed to further characterize the tumor. Neoplastic cells were positive for MART-1 (also known as Melan-A and melanoma-associated antigen recognized by T cells)(Figure 3), SOX10, S-100, HMB-45, and vimentin. Nonspecific staining with CD56 (Figure 4), a neuroendocrine marker, also was noted; however, the neoplasm was negative for synaptophysin, another neuroendocrine marker. Other markers for which staining was negative included pan-keratin, CD138 (syndecan-1), desmin, placental alkaline phosphatase (PLAP), inhibin, OCT-4, cytokeratin 7, and cytokeratin 20. This staining pattern was compatible with metastatic melanoma with aberrant CD56 expression.
BRAF V600E immunohistochemical staining also was performed and showed strong and diffuse positivity within neoplastic cells. A subsequent positron emission tomography scan revealed widespread metastatic disease involving the lungs, liver, spleen, and bones. The patient did not have a history of an excised skin lesion; no primary cutaneous or mucosal lesions were identified.

The patient was started on targeted therapy with trametinib, a mitogen-activated extracellular signal-related kinase kinase (MEK) inhibitor, and dabrafenib, a BRAF inhibitor. The disease continued to progress; he developed extensive leptomeningeal metastatic disease for which palliative radiation therapy was administered. The patient died 4 months after the initial diagnosis.
More than 90% of melanoma cases are of cutaneous origin; however, 4% to 8% of cases present as a metastatic lesion in the absence of an identified primary lesion,4 similar to our patient. The diagnosis of melanoma often is challenging; the tumor can show notable histologic diversity and has the potential to express aberrant immunophenotypes.1,2 The histologic diversity of melanoma includes a variety of architectural patterns (eg, nests, trabeculae, fascicular, pseudoglandular, pseudopapillary, or pseudorosette patterns), cytomorphologic features, and stromal changes. Cytomorphologic features of melanoma can be large pleomorphic cells; small cells; spindle cells; clear cells; signet-ring cells; and rhabdoid, plasmacytoid, and balloon cells.5
Melanoma can mimic carcinoma, sarcoma, lymphoma, benign stromal tumors, plasmacytoma, and germ-cell tumors.5 Nuclei can binucleated, multinucleated, or lobated and may contain inclusions or grooves. Stroma may become myxoid or desmoplastic in appearance or rarely show granulomatous inflammation or osteoclastic giant cells.5 These variations render the diagnosis of melanoma challenging and ultimately can lead to diagnostic uncertainty.
Melanomas typically express MART-1, HMB-45, S-100, tyrosinase, NK1C3, vimentin, and neuron-specific enolase. However, melanoma is among the many neoplasms that sometimes exhibit aberrant immunoreactivity and differentiation toward nonmelanocytic elements.6 The most commonly expressed immunophenotypic aberration is cytokeratin, especially the low-molecular-weight keratin marker CAM5.2.5 CAM5.2 positivity also is seen more often in metastatic melanoma. Melanomas rarely express other intermediate filaments, including desmin, neurofilament protein, and glial fibrillary acidic protein; expression of smooth-muscle actin is rare.5
Only a few cases of melanoma showing expression of neuroendocrine markers have been reported. However, one study reported synaptophysin positivity in 29% (10/34) of cases of primary and metastatic melanoma, making the stain a relatively common finding.1
In contrast, expression of CD56 (also known as neural-cell adhesion molecule 1) in melanoma has been reported only rarely. CD56 is a nonspecific neuroendocrine marker that normally is expressed on neurons, glial tissue, skeletal muscle, and natural killer cells. Riddle and Bui7 reported a case of metastatic malignant melanoma with focal CD56 positivity and no expression of other neuroendocrine markers, similar to our patient. Suzuki and colleagues4 also reported a case of melanoma metastatic to bone marrow that showed CD56 expression in true nonhematologic tumor cells and negative immunoreactivity with synaptophysin and chromogranin A.
It is important to document cases of melanoma that express neuroendocrine markers to prevent an incorrect diagnosis of a neuroendocrine tumor.1 In some cases, distinguishing amelanotic melanoma from poorly differentiated squamous cell carcinoma, neuroendocrine tumor, and lymphoma can be difficult.5
The term neuroendocrine differentiation is reserved for cases of melanoma that show areas of ultrastructural change consistent with a neuroendocrine tumor.2 Neuroendocrine differentiation in melanoma is not common; its prognostic significance is unknown.8 We do not consider our case to be true neuroendocrine differentiation, as the tumor lacked the morphologic changes of a neuroendocrine tumor. Furthermore, CD56 is a nonspecific neuroendocrine marker, and the tumor was negative for synaptophysin.
Melanoma has the potential to show notable histologic diversity as well as aberrant immunohistochemical staining patterns.1,2 Our patient had metastatic melanoma with aberrant neuroendocrine expression of CD56, which could have been a potential diagnostic pitfall. Because expression of CD56 in melanoma is rare, it is imperative to recognize this potential aberrant staining pattern to ensure the accurate diagnosis of melanoma and appropriate provision of care.
1. Romano RC, Carter JM, Folpe AL. Aberrant intermediate filament and synaptophysin expression is a frequent event in malignant melanoma: an immunohistochemical study of 73 cases. Mod Pathol. 2015;28:1033-1042. doi:10.1038/modpathol.2015.62
2. Eyden B, Pandit D, Banerjee SS. Malignant melanoma with neuroendocrine differentiation: clinical, histological, immunohistochemical and ultrastructural features of three cases. Histopathology. 2005;47:402-409. doi:10.1111/j.1365-2559.2005.02240.x
3. Katerji H, Childs JM, Bratton LE, et al. Primary esophageal melanoma with aberrant CD56 expression: a potential diagnostic pitfall. Case Rep Pathol. 2017;2017:9052637. doi:10.1155/2017/9052637
4. Suzuki T, Kusumoto S, Iida S, et al. Amelanotic malignant melanoma of unknown primary origin metastasizing to the bone marrow: a case report and review of the literature. Intern Med. 2014;53:325-328. doi:10.2169/internalmedicine.53.1412
5. Banerjee SS, Harris M. Morphological and immunophenotypic variations in malignant melanoma. Histopathology. 2000;36:387-402. doi:10.1046/j.1365-2559.2000.00894.x
6. Banerjee SS, Eyden B. Divergent differentiation in malignant melanomas: a review. Histopathology. 2008;52:119-129. doi:10.1111/j.1365-2559.2007.02823.x
7. Riddle ND, Bui MM. When melanoma is negative for S100: diagnostic pitfalls. Arch Pathol Lab Med. 2012;136:237-239. doi:10.5858/arpa.2011-0405-LE
8. Ilardi G, Caroppo D, Varricchio S, et al. Anal melanoma with neuroendocrine differentiation: report of a case. Int J Surg Pathol. 2015;23:329-332. doi:10.1177/1066896915573568
To the Editor:
Many types of neoplasms can show aberrant immunoreactivity or unexpected expression of markers.1 Malignant melanoma is a tumor that can show not only aberrant immunohistochemical staining patterns but also notable histologic diversity,1,2 which often makes the diagnosis of melanoma challenging and ultimately can lead to diagnostic uncertainty.2
The incidence of malignant melanoma continues to grow.3 Maintaining a high degree of suspicion for this disease, recognizing its heterogeneity and divergent differentiation, and knowing potential aberrant immunohistochemical staining patterns are imperative for accurate diagnosis.
A 36-year-old man presented to a primary care physician with right-sided chest pain, upper and lower back aches, bilateral hip pain, neck pain, headache, night sweats, chills, and nausea. After infectious causes were ruled out, he was placed on a steroid taper without improvement. He presented to the emergency department a few days later with muscle spasms and was found to also have diffuse abdominal tenderness and guarding. The patient’s medical history was noncontributory; he was a lifelong nonsmoker. Laboratory studies revealed elevated levels of alanine aminotransferase and C-reactive protein. Computed tomography of the chest and abdomen revealed innumerable liver and lung lesions that were suspicious for metastatic malignancy. A liver biopsy revealed nests and sheets of metastatic tumor with pleomorphic nuclei, inconspicuous nucleoli, and areas of intranuclear clearing (Figures 1 and 2). Immunohistochemical staining was performed to further characterize the tumor. Neoplastic cells were positive for MART-1 (also known as Melan-A and melanoma-associated antigen recognized by T cells)(Figure 3), SOX10, S-100, HMB-45, and vimentin. Nonspecific staining with CD56 (Figure 4), a neuroendocrine marker, also was noted; however, the neoplasm was negative for synaptophysin, another neuroendocrine marker. Other markers for which staining was negative included pan-keratin, CD138 (syndecan-1), desmin, placental alkaline phosphatase (PLAP), inhibin, OCT-4, cytokeratin 7, and cytokeratin 20. This staining pattern was compatible with metastatic melanoma with aberrant CD56 expression.

BRAF V600E immunohistochemical staining also was performed and showed strong and diffuse positivity within neoplastic cells. A subsequent positron emission tomography scan revealed widespread metastatic disease involving the lungs, liver, spleen, and bones. The patient did not have a history of an excised skin lesion; no primary cutaneous or mucosal lesions were identified.

The patient was started on targeted therapy with trametinib, a mitogen-activated extracellular signal-related kinase kinase (MEK) inhibitor, and dabrafenib, a BRAF inhibitor. The disease continued to progress; he developed extensive leptomeningeal metastatic disease for which palliative radiation therapy was administered. The patient died 4 months after the initial diagnosis.

More than 90% of melanoma cases are of cutaneous origin; however, 4% to 8% of cases present as a metastatic lesion in the absence of an identified primary lesion,4 similar to our patient. The diagnosis of melanoma often is challenging; the tumor can show notable histologic diversity and has the potential to express aberrant immunophenotypes.1,2 The histologic diversity of melanoma includes a variety of architectural patterns (eg, nests, trabeculae, fascicular, pseudoglandular, pseudopapillary, or pseudorosette patterns), cytomorphologic features, and stromal changes. Cytomorphologic features of melanoma can be large pleomorphic cells; small cells; spindle cells; clear cells; signet-ring cells; and rhabdoid, plasmacytoid, and balloon cells.5

Melanoma can mimic carcinoma, sarcoma, lymphoma, benign stromal tumors, plasmacytoma, and germ-cell tumors.5 Nuclei can binucleated, multinucleated, or lobated and may contain inclusions or grooves. Stroma may become myxoid or desmoplastic in appearance or rarely show granulomatous inflammation or osteoclastic giant cells.5 These variations render the diagnosis of melanoma challenging and ultimately can lead to diagnostic uncertainty.
Melanomas typically express MART-1, HMB-45, S-100, tyrosinase, NK1C3, vimentin, and neuron-specific enolase. However, melanoma is among the many neoplasms that sometimes exhibit aberrant immunoreactivity and differentiation toward nonmelanocytic elements.6 The most commonly expressed immunophenotypic aberration is cytokeratin, especially the low-molecular-weight keratin marker CAM5.2.5 CAM5.2 positivity also is seen more often in metastatic melanoma. Melanomas rarely express other intermediate filaments, including desmin, neurofilament protein, and glial fibrillary acidic protein; expression of smooth-muscle actin is rare.5
Only a few cases of melanoma showing expression of neuroendocrine markers have been reported. However, one study reported synaptophysin positivity in 29% (10/34) of cases of primary and metastatic melanoma, making the stain a relatively common finding.1
In contrast, expression of CD56 (also known as neural-cell adhesion molecule 1) in melanoma has been reported only rarely. CD56 is a nonspecific neuroendocrine marker that normally is expressed on neurons, glial tissue, skeletal muscle, and natural killer cells. Riddle and Bui7 reported a case of metastatic malignant melanoma with focal CD56 positivity and no expression of other neuroendocrine markers, similar to our patient. Suzuki and colleagues4 also reported a case of melanoma metastatic to bone marrow that showed CD56 expression in true nonhematologic tumor cells and negative immunoreactivity with synaptophysin and chromogranin A.
It is important to document cases of melanoma that express neuroendocrine markers to prevent an incorrect diagnosis of a neuroendocrine tumor.1 In some cases, distinguishing amelanotic melanoma from poorly differentiated squamous cell carcinoma, neuroendocrine tumor, and lymphoma can be difficult.5
The term neuroendocrine differentiation is reserved for cases of melanoma that show areas of ultrastructural change consistent with a neuroendocrine tumor.2 Neuroendocrine differentiation in melanoma is not common; its prognostic significance is unknown.8 We do not consider our case to be true neuroendocrine differentiation, as the tumor lacked the morphologic changes of a neuroendocrine tumor. Furthermore, CD56 is a nonspecific neuroendocrine marker, and the tumor was negative for synaptophysin.
Melanoma has the potential to show notable histologic diversity as well as aberrant immunohistochemical staining patterns.1,2 Our patient had metastatic melanoma with aberrant neuroendocrine expression of CD56, which could have been a potential diagnostic pitfall. Because expression of CD56 in melanoma is rare, it is imperative to recognize this potential aberrant staining pattern to ensure the accurate diagnosis of melanoma and appropriate provision of care.
To the Editor:
Many types of neoplasms can show aberrant immunoreactivity or unexpected expression of markers.1 Malignant melanoma is a tumor that can show not only aberrant immunohistochemical staining patterns but also notable histologic diversity,1,2 which often makes the diagnosis of melanoma challenging and ultimately can lead to diagnostic uncertainty.2
The incidence of malignant melanoma continues to grow.3 Maintaining a high degree of suspicion for this disease, recognizing its heterogeneity and divergent differentiation, and knowing potential aberrant immunohistochemical staining patterns are imperative for accurate diagnosis.
A 36-year-old man presented to a primary care physician with right-sided chest pain, upper and lower back aches, bilateral hip pain, neck pain, headache, night sweats, chills, and nausea. After infectious causes were ruled out, he was placed on a steroid taper without improvement. He presented to the emergency department a few days later with muscle spasms and was found to also have diffuse abdominal tenderness and guarding. The patient’s medical history was noncontributory; he was a lifelong nonsmoker. Laboratory studies revealed elevated levels of alanine aminotransferase and C-reactive protein. Computed tomography of the chest and abdomen revealed innumerable liver and lung lesions that were suspicious for metastatic malignancy. A liver biopsy revealed nests and sheets of metastatic tumor with pleomorphic nuclei, inconspicuous nucleoli, and areas of intranuclear clearing (Figures 1 and 2). Immunohistochemical staining was performed to further characterize the tumor. Neoplastic cells were positive for MART-1 (also known as Melan-A and melanoma-associated antigen recognized by T cells)(Figure 3), SOX10, S-100, HMB-45, and vimentin. Nonspecific staining with CD56 (Figure 4), a neuroendocrine marker, also was noted; however, the neoplasm was negative for synaptophysin, another neuroendocrine marker. Other markers for which staining was negative included pan-keratin, CD138 (syndecan-1), desmin, placental alkaline phosphatase (PLAP), inhibin, OCT-4, cytokeratin 7, and cytokeratin 20. This staining pattern was compatible with metastatic melanoma with aberrant CD56 expression.

BRAF V600E immunohistochemical staining also was performed and showed strong and diffuse positivity within neoplastic cells. A subsequent positron emission tomography scan revealed widespread metastatic disease involving the lungs, liver, spleen, and bones. The patient did not have a history of an excised skin lesion; no primary cutaneous or mucosal lesions were identified.

The patient was started on targeted therapy with trametinib, a mitogen-activated extracellular signal-related kinase kinase (MEK) inhibitor, and dabrafenib, a BRAF inhibitor. The disease continued to progress; he developed extensive leptomeningeal metastatic disease for which palliative radiation therapy was administered. The patient died 4 months after the initial diagnosis.

More than 90% of melanoma cases are of cutaneous origin; however, 4% to 8% of cases present as a metastatic lesion in the absence of an identified primary lesion,4 similar to our patient. The diagnosis of melanoma often is challenging; the tumor can show notable histologic diversity and has the potential to express aberrant immunophenotypes.1,2 The histologic diversity of melanoma includes a variety of architectural patterns (eg, nests, trabeculae, fascicular, pseudoglandular, pseudopapillary, or pseudorosette patterns), cytomorphologic features, and stromal changes. Cytomorphologic features of melanoma can be large pleomorphic cells; small cells; spindle cells; clear cells; signet-ring cells; and rhabdoid, plasmacytoid, and balloon cells.5

Melanoma can mimic carcinoma, sarcoma, lymphoma, benign stromal tumors, plasmacytoma, and germ-cell tumors.5 Nuclei can binucleated, multinucleated, or lobated and may contain inclusions or grooves. Stroma may become myxoid or desmoplastic in appearance or rarely show granulomatous inflammation or osteoclastic giant cells.5 These variations render the diagnosis of melanoma challenging and ultimately can lead to diagnostic uncertainty.
Melanomas typically express MART-1, HMB-45, S-100, tyrosinase, NK1C3, vimentin, and neuron-specific enolase. However, melanoma is among the many neoplasms that sometimes exhibit aberrant immunoreactivity and differentiation toward nonmelanocytic elements.6 The most commonly expressed immunophenotypic aberration is cytokeratin, especially the low-molecular-weight keratin marker CAM5.2.5 CAM5.2 positivity also is seen more often in metastatic melanoma. Melanomas rarely express other intermediate filaments, including desmin, neurofilament protein, and glial fibrillary acidic protein; expression of smooth-muscle actin is rare.5
Only a few cases of melanoma showing expression of neuroendocrine markers have been reported. However, one study reported synaptophysin positivity in 29% (10/34) of cases of primary and metastatic melanoma, making the stain a relatively common finding.1
In contrast, expression of CD56 (also known as neural-cell adhesion molecule 1) in melanoma has been reported only rarely. CD56 is a nonspecific neuroendocrine marker that normally is expressed on neurons, glial tissue, skeletal muscle, and natural killer cells. Riddle and Bui7 reported a case of metastatic malignant melanoma with focal CD56 positivity and no expression of other neuroendocrine markers, similar to our patient. Suzuki and colleagues4 also reported a case of melanoma metastatic to bone marrow that showed CD56 expression in true nonhematologic tumor cells and negative immunoreactivity with synaptophysin and chromogranin A.
It is important to document cases of melanoma that express neuroendocrine markers to prevent an incorrect diagnosis of a neuroendocrine tumor.1 In some cases, distinguishing amelanotic melanoma from poorly differentiated squamous cell carcinoma, neuroendocrine tumor, and lymphoma can be difficult.5
The term neuroendocrine differentiation is reserved for cases of melanoma that show areas of ultrastructural change consistent with a neuroendocrine tumor.2 Neuroendocrine differentiation in melanoma is not common; its prognostic significance is unknown.8 We do not consider our case to be true neuroendocrine differentiation, as the tumor lacked the morphologic changes of a neuroendocrine tumor. Furthermore, CD56 is a nonspecific neuroendocrine marker, and the tumor was negative for synaptophysin.
Melanoma has the potential to show notable histologic diversity as well as aberrant immunohistochemical staining patterns.1,2 Our patient had metastatic melanoma with aberrant neuroendocrine expression of CD56, which could have been a potential diagnostic pitfall. Because expression of CD56 in melanoma is rare, it is imperative to recognize this potential aberrant staining pattern to ensure the accurate diagnosis of melanoma and appropriate provision of care.
1. Romano RC, Carter JM, Folpe AL. Aberrant intermediate filament and synaptophysin expression is a frequent event in malignant melanoma: an immunohistochemical study of 73 cases. Mod Pathol. 2015;28:1033-1042. doi:10.1038/modpathol.2015.62
2. Eyden B, Pandit D, Banerjee SS. Malignant melanoma with neuroendocrine differentiation: clinical, histological, immunohistochemical and ultrastructural features of three cases. Histopathology. 2005;47:402-409. doi:10.1111/j.1365-2559.2005.02240.x
3. Katerji H, Childs JM, Bratton LE, et al. Primary esophageal melanoma with aberrant CD56 expression: a potential diagnostic pitfall. Case Rep Pathol. 2017;2017:9052637. doi:10.1155/2017/9052637
4. Suzuki T, Kusumoto S, Iida S, et al. Amelanotic malignant melanoma of unknown primary origin metastasizing to the bone marrow: a case report and review of the literature. Intern Med. 2014;53:325-328. doi:10.2169/internalmedicine.53.1412
5. Banerjee SS, Harris M. Morphological and immunophenotypic variations in malignant melanoma. Histopathology. 2000;36:387-402. doi:10.1046/j.1365-2559.2000.00894.x
6. Banerjee SS, Eyden B. Divergent differentiation in malignant melanomas: a review. Histopathology. 2008;52:119-129. doi:10.1111/j.1365-2559.2007.02823.x
7. Riddle ND, Bui MM. When melanoma is negative for S100: diagnostic pitfalls. Arch Pathol Lab Med. 2012;136:237-239. doi:10.5858/arpa.2011-0405-LE
8. Ilardi G, Caroppo D, Varricchio S, et al. Anal melanoma with neuroendocrine differentiation: report of a case. Int J Surg Pathol. 2015;23:329-332. doi:10.1177/1066896915573568
1. Romano RC, Carter JM, Folpe AL. Aberrant intermediate filament and synaptophysin expression is a frequent event in malignant melanoma: an immunohistochemical study of 73 cases. Mod Pathol. 2015;28:1033-1042. doi:10.1038/modpathol.2015.62
2. Eyden B, Pandit D, Banerjee SS. Malignant melanoma with neuroendocrine differentiation: clinical, histological, immunohistochemical and ultrastructural features of three cases. Histopathology. 2005;47:402-409. doi:10.1111/j.1365-2559.2005.02240.x
3. Katerji H, Childs JM, Bratton LE, et al. Primary esophageal melanoma with aberrant CD56 expression: a potential diagnostic pitfall. Case Rep Pathol. 2017;2017:9052637. doi:10.1155/2017/9052637
4. Suzuki T, Kusumoto S, Iida S, et al. Amelanotic malignant melanoma of unknown primary origin metastasizing to the bone marrow: a case report and review of the literature. Intern Med. 2014;53:325-328. doi:10.2169/internalmedicine.53.1412
5. Banerjee SS, Harris M. Morphological and immunophenotypic variations in malignant melanoma. Histopathology. 2000;36:387-402. doi:10.1046/j.1365-2559.2000.00894.x
6. Banerjee SS, Eyden B. Divergent differentiation in malignant melanomas: a review. Histopathology. 2008;52:119-129. doi:10.1111/j.1365-2559.2007.02823.x
7. Riddle ND, Bui MM. When melanoma is negative for S100: diagnostic pitfalls. Arch Pathol Lab Med. 2012;136:237-239. doi:10.5858/arpa.2011-0405-LE
8. Ilardi G, Caroppo D, Varricchio S, et al. Anal melanoma with neuroendocrine differentiation: report of a case. Int J Surg Pathol. 2015;23:329-332. doi:10.1177/1066896915573568
Practice Points
- The diagnosis of melanoma often is challenging as tumors can show notable histologic diversity and have the potential to express aberrant immunophenotypes including CD56 expression.
- Because expression of CD56 in melanoma is rare, it is important to be aware of this potential aberrant staining pattern.
- Recognizing this heterogeneity and divergent differentiation as well as knowing potential aberrant immunohistochemical staining patterns are imperative for accurate and timely diagnosis.
People with diabetes have a higher risk of colon cancer: Study
Getting a colonoscopy dramatically reduced the risk, the results showed.
The findings, published in JAMA Network Open, suggest that colonoscopies are particularly important for people with diabetes. People diagnosed with diabetes within the past 5 years have the greatest colorectal cancer risk, the study found, suggesting screening should be part of a person’s health care after they’re diagnosed with diabetes.
Researchers analyzed data for 54,597 people who contributed at least 2 years of health data as part of a study that recruited people from 12 Southeastern states between 2002 and 2009. The people self-reported their diabetes status, and although researchers tried to only include people with type 2 diabetes, it’s possible that some people in the study had type 1 diabetes. The average age of those in the study was 51 years old; 64% were women; more than half of them had an income of less than $15,000 per year; and 66% of them were African American.
Among the people in the study who had diabetes, the risk of having colorectal cancer was not strongly impacted by their race or ethnicity, gender, weight, or income level, the study showed.
While race didn’t predict whether people with diabetes would get colorectal cancer, the findings are particularly important because most of the people in the study were African American. Diabetes and colorectal cancer disproportionately affect African American people, the authors noted. Medical research studies often struggle to recruit people of color, resulting in a lack of data to help guide health care priorities and decision-making.
The study also provided important guidance for people newly diagnosed with diabetes. People who were diagnosed with diabetes within the past 5 years were at a particularly increased risk of getting colorectal cancer, compared to people who had been diagnosed for 5-10 years.
The authors concluded that increased referrals for colonoscopies among people with diabetes, particularly among those newly diagnosed, could greatly reduce the impact of colorectal cancer. Current guidelines suggest most people should begin colorectal cancer screenings at age 45, according to the Centers for Disease Control and Prevention.
The study was supported by the National Cancer Institute and the University of Wisconsin, Madison. The study authors reported no relevant conflicts of interest.
A version of this article first appeared on WebMD.com.
Getting a colonoscopy dramatically reduced the risk, the results showed.
The findings, published in JAMA Network Open, suggest that colonoscopies are particularly important for people with diabetes. People diagnosed with diabetes within the past 5 years have the greatest colorectal cancer risk, the study found, suggesting screening should be part of a person’s health care after they’re diagnosed with diabetes.
Researchers analyzed data for 54,597 people who contributed at least 2 years of health data as part of a study that recruited people from 12 Southeastern states between 2002 and 2009. The people self-reported their diabetes status, and although researchers tried to only include people with type 2 diabetes, it’s possible that some people in the study had type 1 diabetes. The average age of those in the study was 51 years old; 64% were women; more than half of them had an income of less than $15,000 per year; and 66% of them were African American.
Among the people in the study who had diabetes, the risk of having colorectal cancer was not strongly impacted by their race or ethnicity, gender, weight, or income level, the study showed.
While race didn’t predict whether people with diabetes would get colorectal cancer, the findings are particularly important because most of the people in the study were African American. Diabetes and colorectal cancer disproportionately affect African American people, the authors noted. Medical research studies often struggle to recruit people of color, resulting in a lack of data to help guide health care priorities and decision-making.
The study also provided important guidance for people newly diagnosed with diabetes. People who were diagnosed with diabetes within the past 5 years were at a particularly increased risk of getting colorectal cancer, compared to people who had been diagnosed for 5-10 years.
The authors concluded that increased referrals for colonoscopies among people with diabetes, particularly among those newly diagnosed, could greatly reduce the impact of colorectal cancer. Current guidelines suggest most people should begin colorectal cancer screenings at age 45, according to the Centers for Disease Control and Prevention.
The study was supported by the National Cancer Institute and the University of Wisconsin, Madison. The study authors reported no relevant conflicts of interest.
A version of this article first appeared on WebMD.com.
Getting a colonoscopy dramatically reduced the risk, the results showed.
The findings, published in JAMA Network Open, suggest that colonoscopies are particularly important for people with diabetes. People diagnosed with diabetes within the past 5 years have the greatest colorectal cancer risk, the study found, suggesting screening should be part of a person’s health care after they’re diagnosed with diabetes.
Researchers analyzed data for 54,597 people who contributed at least 2 years of health data as part of a study that recruited people from 12 Southeastern states between 2002 and 2009. The people self-reported their diabetes status, and although researchers tried to only include people with type 2 diabetes, it’s possible that some people in the study had type 1 diabetes. The average age of those in the study was 51 years old; 64% were women; more than half of them had an income of less than $15,000 per year; and 66% of them were African American.
Among the people in the study who had diabetes, the risk of having colorectal cancer was not strongly impacted by their race or ethnicity, gender, weight, or income level, the study showed.
While race didn’t predict whether people with diabetes would get colorectal cancer, the findings are particularly important because most of the people in the study were African American. Diabetes and colorectal cancer disproportionately affect African American people, the authors noted. Medical research studies often struggle to recruit people of color, resulting in a lack of data to help guide health care priorities and decision-making.
The study also provided important guidance for people newly diagnosed with diabetes. People who were diagnosed with diabetes within the past 5 years were at a particularly increased risk of getting colorectal cancer, compared to people who had been diagnosed for 5-10 years.
The authors concluded that increased referrals for colonoscopies among people with diabetes, particularly among those newly diagnosed, could greatly reduce the impact of colorectal cancer. Current guidelines suggest most people should begin colorectal cancer screenings at age 45, according to the Centers for Disease Control and Prevention.
The study was supported by the National Cancer Institute and the University of Wisconsin, Madison. The study authors reported no relevant conflicts of interest.
A version of this article first appeared on WebMD.com.
FROM JAMA NETWORK OPEN
FTC considers proposals on mergers and noncompete clauses
Changes may be in store for how physicians do business based on pending proposals from the Federal Trade Commission to ban noncompete clauses and monitor potential merger monopolies.
In January 2023, the FTC announced a rule that would ban noncompete clauses, stating that such clauses reduce workers’ wages and stifle new businesses. Simply put, the rule would ban employers from entering into noncompete clauses with workers, including independent contractors.
Aspects of the rule include whether it should pertain to franchisees, whether senior executives should be exempted, and whether low-wage and high-wage workers should be treated differently.
According to the FTC, banning noncompete clauses would increase workers’ earnings by approximately $300 billion per year, save consumers as much as $148 billion in health care costs, and double the number of companies founded by former workers in the same field.
In June 2023, the FTC and the Department of Justice proposed changes to rules governing mergers, including changes to prenotification forms that would promote more efficient screening of potential mergers. According to a press release from the FTC, the proposed changes include provision of details about investments or corporate relationships, product and services, projected revenue streams, and previous acquisitions.
The proposal also includes a waiting period during which agencies would assess the risk that a merger would lessen competition or tend to create a monopoly.
What the FTC proposals mean for physicians
FTC Chair Lina M. Khan addressed attendees at the American College of Physicians at their annual meeting in October.
In March 2023, ACEP wrote to Ms. Khan in support of the banning of noncompete clauses. The ACEP also stated that the FTC should monitor the effect of a ban on the ability to recruit and maintain a stable physician workforce in rural and underserved areas “and should examine the potential impacts should nonprofit health systems be exempt from a ban.”
However, the American Medical Group Association, a nonprofit trade organization that supports multispecialty medical groups, opposes the ban. In a press release issued in March 2023, AMGA noted that, “As employers, AMGA members rely in part on noncompete agreements to build strong, sustainable care teams that work together to coordinate care for their patients. These care teams emphasize the importance of the doctor-patient relationship, which reasonable noncompete agreements help support.”
The American Medical Association supports the ban on noncompete clauses, detailed in an official AMA policy statement as, “support[ing] policies, regulations, and legislation that prohibits covenants not-to-compete for all physicians in clinical practice who hold employment contracts with for-profit or nonprofit hospital, hospital system, or staffing company employers.”
In regard to the merger guidelines, ACEP wrote a separate letter to Ms. Khan identifying some of the unique aspects of emergency medicine practice. The ACEP stressed the need for caution as the consolidation of medical practices continues, many under the umbrella of private equity investment companies.
“Unchecked mergers that substantially lessen competition in the labor market for emergency physicians, in which the employer is the buyer and the physician is the seller, can impact physicians directly by lowering wages or slowing wage growth, worsening benefits or working conditions, or contributing to other degradations in workplace quality,” according to ACEP.
The AMA also supports the FTC’s draft merger guidelines as protective of physicians and their working environments.
In September 2023, the AMA sent a letter to the FTC commending the agency on the proposed guidelines: “It is our strong contention that the agencies must have merger guidelines that protect physicians against health insurer mergers that may substantially lessen competition for the purchase of physician services and that degrade physician working conditions,” according to the AMA letter.
According the FTC, the proposed changes represent an expansion and reorganization of information along with the addition of new document requirements and represents the first comprehensive review of the Hart-Scott-Rodino Antitrust Improvements Act since 1978.
After soliciting public comments, the FTC is reviewing the proposals, and no specific date for a final vote has been announced.
More specifics on the potential changes to premerger notification, reporting, and waiting period requirements are available on the FTC website.
A version of this article appeared on Medscape.com.
Changes may be in store for how physicians do business based on pending proposals from the Federal Trade Commission to ban noncompete clauses and monitor potential merger monopolies.
In January 2023, the FTC announced a rule that would ban noncompete clauses, stating that such clauses reduce workers’ wages and stifle new businesses. Simply put, the rule would ban employers from entering into noncompete clauses with workers, including independent contractors.
Aspects of the rule include whether it should pertain to franchisees, whether senior executives should be exempted, and whether low-wage and high-wage workers should be treated differently.
According to the FTC, banning noncompete clauses would increase workers’ earnings by approximately $300 billion per year, save consumers as much as $148 billion in health care costs, and double the number of companies founded by former workers in the same field.
In June 2023, the FTC and the Department of Justice proposed changes to rules governing mergers, including changes to prenotification forms that would promote more efficient screening of potential mergers. According to a press release from the FTC, the proposed changes include provision of details about investments or corporate relationships, product and services, projected revenue streams, and previous acquisitions.
The proposal also includes a waiting period during which agencies would assess the risk that a merger would lessen competition or tend to create a monopoly.
What the FTC proposals mean for physicians
FTC Chair Lina M. Khan addressed attendees at the American College of Physicians at their annual meeting in October.
In March 2023, ACEP wrote to Ms. Khan in support of the banning of noncompete clauses. The ACEP also stated that the FTC should monitor the effect of a ban on the ability to recruit and maintain a stable physician workforce in rural and underserved areas “and should examine the potential impacts should nonprofit health systems be exempt from a ban.”
However, the American Medical Group Association, a nonprofit trade organization that supports multispecialty medical groups, opposes the ban. In a press release issued in March 2023, AMGA noted that, “As employers, AMGA members rely in part on noncompete agreements to build strong, sustainable care teams that work together to coordinate care for their patients. These care teams emphasize the importance of the doctor-patient relationship, which reasonable noncompete agreements help support.”
The American Medical Association supports the ban on noncompete clauses, detailed in an official AMA policy statement as, “support[ing] policies, regulations, and legislation that prohibits covenants not-to-compete for all physicians in clinical practice who hold employment contracts with for-profit or nonprofit hospital, hospital system, or staffing company employers.”
In regard to the merger guidelines, ACEP wrote a separate letter to Ms. Khan identifying some of the unique aspects of emergency medicine practice. The ACEP stressed the need for caution as the consolidation of medical practices continues, many under the umbrella of private equity investment companies.
“Unchecked mergers that substantially lessen competition in the labor market for emergency physicians, in which the employer is the buyer and the physician is the seller, can impact physicians directly by lowering wages or slowing wage growth, worsening benefits or working conditions, or contributing to other degradations in workplace quality,” according to ACEP.
The AMA also supports the FTC’s draft merger guidelines as protective of physicians and their working environments.
In September 2023, the AMA sent a letter to the FTC commending the agency on the proposed guidelines: “It is our strong contention that the agencies must have merger guidelines that protect physicians against health insurer mergers that may substantially lessen competition for the purchase of physician services and that degrade physician working conditions,” according to the AMA letter.
According the FTC, the proposed changes represent an expansion and reorganization of information along with the addition of new document requirements and represents the first comprehensive review of the Hart-Scott-Rodino Antitrust Improvements Act since 1978.
After soliciting public comments, the FTC is reviewing the proposals, and no specific date for a final vote has been announced.
More specifics on the potential changes to premerger notification, reporting, and waiting period requirements are available on the FTC website.
A version of this article appeared on Medscape.com.
Changes may be in store for how physicians do business based on pending proposals from the Federal Trade Commission to ban noncompete clauses and monitor potential merger monopolies.
In January 2023, the FTC announced a rule that would ban noncompete clauses, stating that such clauses reduce workers’ wages and stifle new businesses. Simply put, the rule would ban employers from entering into noncompete clauses with workers, including independent contractors.
Aspects of the rule include whether it should pertain to franchisees, whether senior executives should be exempted, and whether low-wage and high-wage workers should be treated differently.
According to the FTC, banning noncompete clauses would increase workers’ earnings by approximately $300 billion per year, save consumers as much as $148 billion in health care costs, and double the number of companies founded by former workers in the same field.
In June 2023, the FTC and the Department of Justice proposed changes to rules governing mergers, including changes to prenotification forms that would promote more efficient screening of potential mergers. According to a press release from the FTC, the proposed changes include provision of details about investments or corporate relationships, product and services, projected revenue streams, and previous acquisitions.
The proposal also includes a waiting period during which agencies would assess the risk that a merger would lessen competition or tend to create a monopoly.
What the FTC proposals mean for physicians
FTC Chair Lina M. Khan addressed attendees at the American College of Physicians at their annual meeting in October.
In March 2023, ACEP wrote to Ms. Khan in support of the banning of noncompete clauses. The ACEP also stated that the FTC should monitor the effect of a ban on the ability to recruit and maintain a stable physician workforce in rural and underserved areas “and should examine the potential impacts should nonprofit health systems be exempt from a ban.”
However, the American Medical Group Association, a nonprofit trade organization that supports multispecialty medical groups, opposes the ban. In a press release issued in March 2023, AMGA noted that, “As employers, AMGA members rely in part on noncompete agreements to build strong, sustainable care teams that work together to coordinate care for their patients. These care teams emphasize the importance of the doctor-patient relationship, which reasonable noncompete agreements help support.”
The American Medical Association supports the ban on noncompete clauses, detailed in an official AMA policy statement as, “support[ing] policies, regulations, and legislation that prohibits covenants not-to-compete for all physicians in clinical practice who hold employment contracts with for-profit or nonprofit hospital, hospital system, or staffing company employers.”
In regard to the merger guidelines, ACEP wrote a separate letter to Ms. Khan identifying some of the unique aspects of emergency medicine practice. The ACEP stressed the need for caution as the consolidation of medical practices continues, many under the umbrella of private equity investment companies.
“Unchecked mergers that substantially lessen competition in the labor market for emergency physicians, in which the employer is the buyer and the physician is the seller, can impact physicians directly by lowering wages or slowing wage growth, worsening benefits or working conditions, or contributing to other degradations in workplace quality,” according to ACEP.
The AMA also supports the FTC’s draft merger guidelines as protective of physicians and their working environments.
In September 2023, the AMA sent a letter to the FTC commending the agency on the proposed guidelines: “It is our strong contention that the agencies must have merger guidelines that protect physicians against health insurer mergers that may substantially lessen competition for the purchase of physician services and that degrade physician working conditions,” according to the AMA letter.
According the FTC, the proposed changes represent an expansion and reorganization of information along with the addition of new document requirements and represents the first comprehensive review of the Hart-Scott-Rodino Antitrust Improvements Act since 1978.
After soliciting public comments, the FTC is reviewing the proposals, and no specific date for a final vote has been announced.
More specifics on the potential changes to premerger notification, reporting, and waiting period requirements are available on the FTC website.
A version of this article appeared on Medscape.com.
Asymptomatic Hair Loss in a Patient With Systemic Lupus Erythematosus
The Diagnosis: Tinea Capitis
Dermoscopy revealed many black spot signs with broken, corkscrew, and comma hairs, as well as increased single hair follicles and focal polymorphic vascular distribution in the scalp (Figure 1). Fungal microscopy showed large round spores within the hair. A fungal culture demonstrated Trichophyton tonsurans growth in the broken hair. Based on the clinical presentation and laboratory findings, a diagnosis of tinea capitis was rendered. Oral terbinafine 250 mg/d was prescribed. At 4-week follow-up, the patient did not report worsening or new symptoms, and there was visible evidence of hair regrowth (Figure 2). There has been no sign of recurrence.

According to the most recent set of classification criteria published by the Systemic Lupus Erythematosus (SLE) International Collaborating Clinics, nonscarring alopecia is now a diagnostic criterion for SLE that has a specificity of 95.7%.1 Although discoid lupus erythematosus presents with diffuse scarring alopecia, SLE manifests as nonscarring alopecia in 1 of 3 patterns: diffuse, patchy, or “lupus hair.”2 It is commonly believed that lupus-related alopecia is a nonspecific symptom of SLE exacerbation and signals that the disease is active.3 Our patient had a history of SLE with no pruritus or pain accompanying the hair loss; however, we considered hair loss due to SLE disease activity, and dermoscopic examination was performed to further rule out the likelihood of SLE alopecia. The dermoscopic characteristics of lupus-related alopecia and tinea capitis vary. For lupusrelated alopecia, alterations to the hair shaft are visible with dermoscopy, including a reduced number or smaller diameter of hairs, hypopigmentation, the black dot sign, brown scattered pigmentation, blue-gray pigmentation, and thick dendritic capillaries.2 Tinea capitis typically displays characteristic dermoscopic manifestations, such as comma, corkscrew, Morse code–like, or jagged hair; black spots; and broken hair.4
Included in the differential diagnosis, androgenetic alopecia dermoscopic findings include hair diameter diversity, perifollicular pigmentation/peripilar sign, and yellow dots.5 The most common vascular patterns present in seborrheic dermatitis are arborizing red lines, twisted red loops, atypical vessels, and glomerular vessels. Perifollicular scaling may be white or yellow and oily.6 There are no specific dermoscopic findings for telogen effluvium; however, the presence of hair regrowth and the predominance of follicular openings with a single sprouting hair shaft may suggest this condition.7 Therefore, dermoscopy can assist clinicians in correctly diagnosing a patient’s condition and determining the its etiology, allowing for early and effective treatment.
Tinea capitis is a typical superficial dermatophyte infection that commonly occurs in prepubescent children and is uncommon in adults because the pH level of the scalp shifts during puberty and the amount of sebum that contains saturated fatty acids increases.8 The risk for developing tinea capitis is higher in certain individuals with comorbid systemic immune diseases, such as SLE and diabetes mellitus, among others, as well as in immunocompromised individuals, such as those with AIDS, organ transplant recipients, or patients receiving high doses of steroids or immunosuppressive drugs.9 The type of dermatophyte entering the hair, the level of host resistance, and the intensity of the inflammatory reaction all affect the clinical picture of tinea capitis in adults, which is pleomorphic and atypical.10 Although tinea capitis is not highly prevalent in adults, the fact that our patient had SLE and had been on immunosuppressive therapy to keep the condition stable increased the chance of contracting tinea capitis, underscoring the need for clinicians to be alert for fungal infections in this patient population.
Trichophyton tonsurans is the most prevalent form of microorganism that causes tinea capitis in the United States, the United Kingdom, and France. However, T tonsurans causing tinea capitis is uncommon in China, with one study reporting only 6 cases from 2000 to 2019.11 Tinea capitis caused by T tonsurans typically presents as black spot alopecia with inflammatory erythema and scaling of the scalp.12 Because most T tonsurans infections have few clinical symptoms, it is challenging to make a clinical diagnosis.13 Although not performed in our patient, a potassium hydroxide preparation and direct microscopic inspection of the afflicted hair and scales can help in quickly identifying and treating these infections. Additional fungal cultures can precisely identify the strain and trace its epidemiology, which is clinically significant not only to identify the potential infection source but also to direct the selection of an organized treatment plan.
- Petri M, Orbai AM, Alarcón GS, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64:2677-2686. doi:10.1002/art.34473
- Desai K, Miteva M. Recent insight on the management of lupus erythematosus alopecia. Clin Cosmet Investig Dermatol. 2021;14:333-347. doi:10.2147/CCID.S269288
- Wysenbeek AJ, Leibovici L, Amit M, et al. Alopecia in systemic lupus erythematosus. relation to disease manifestations. J Rheumatol. 1991;18:1185-1186.
- Lekkas D, Ioannides D, Lazaridou E, et al. Dermatoscopy in tinea capitis: can it provide clues for the responsible fungi? J Eur Acad Dermatol Venereol. 2021;35:E85-E87. doi:10.1111/jdv.16825
- Inui S. Trichoscopy for common hair loss diseases: algorithmic method for diagnosis. J Dermatol. 2011;38:71-75. doi:10.1111/j .1346-8138.2010.01119.x
- Golin´ska J, Sar-Pomian M, Rudnicka L. Diagnostic accuracy of trichoscopy in inflammatory scalp diseases: a systematic review. Dermatology. 2022;238:412-421. doi:10.1159/000517516
- Fernández-Domper L, Ballesteros-Redondo M, Vañó-Galván S. Trichoscopy: an update. Actas Dermosifiliogr. 2023;114:327-333. doi:10.1016/j.ad.2022.12.003
- He M, Zeng J, Mao Y, et al. Aetiological changes of tinea capitis in the Hubei area in 60 years: focus on adult tinea capitis. Mycoses. 2021;64:1527-1534. doi:10.1111/myc.13305
- Khosravi AR, Shokri H, Vahedi G. Factors in etiology and predisposition of adult tinea capitis and review of published literature. Mycopathologia. 2016;181:371-378. doi:10.1007/s11046 -016-0004-9
- Gianni C, Betti R, Perotta E, et al. Tinea capitis in adults. Mycoses. 1995;38:329-331. doi:10.1111/j.1439-0507.1995.tb00417.x
- Liang G, Zheng X, Song G, et al. Adult tinea capitis in China: a retrospective analysis from 2000 to 2019. Mycoses. 2020;63:876-888. doi:10.1111/myc.13102
- Zalewski A, Goldust M, Szepietowski JC. Tinea gladiatorum: epidemiology, clinical aspects, and management. J Clin Med. 2022;11:4066. doi:10.3390/jcm11144066
- Hiruma J, Ogawa Y, Hiruma M. Trichophyton tonsurans infection in Japan: epidemiology, clinical features, diagnosis and infection control. J Dermatol. 2015;42:245-249. doi:10.1111 /1346-8138.12678
The Diagnosis: Tinea Capitis
Dermoscopy revealed many black spot signs with broken, corkscrew, and comma hairs, as well as increased single hair follicles and focal polymorphic vascular distribution in the scalp (Figure 1). Fungal microscopy showed large round spores within the hair. A fungal culture demonstrated Trichophyton tonsurans growth in the broken hair. Based on the clinical presentation and laboratory findings, a diagnosis of tinea capitis was rendered. Oral terbinafine 250 mg/d was prescribed. At 4-week follow-up, the patient did not report worsening or new symptoms, and there was visible evidence of hair regrowth (Figure 2). There has been no sign of recurrence.

According to the most recent set of classification criteria published by the Systemic Lupus Erythematosus (SLE) International Collaborating Clinics, nonscarring alopecia is now a diagnostic criterion for SLE that has a specificity of 95.7%.1 Although discoid lupus erythematosus presents with diffuse scarring alopecia, SLE manifests as nonscarring alopecia in 1 of 3 patterns: diffuse, patchy, or “lupus hair.”2 It is commonly believed that lupus-related alopecia is a nonspecific symptom of SLE exacerbation and signals that the disease is active.3 Our patient had a history of SLE with no pruritus or pain accompanying the hair loss; however, we considered hair loss due to SLE disease activity, and dermoscopic examination was performed to further rule out the likelihood of SLE alopecia. The dermoscopic characteristics of lupus-related alopecia and tinea capitis vary. For lupusrelated alopecia, alterations to the hair shaft are visible with dermoscopy, including a reduced number or smaller diameter of hairs, hypopigmentation, the black dot sign, brown scattered pigmentation, blue-gray pigmentation, and thick dendritic capillaries.2 Tinea capitis typically displays characteristic dermoscopic manifestations, such as comma, corkscrew, Morse code–like, or jagged hair; black spots; and broken hair.4

Included in the differential diagnosis, androgenetic alopecia dermoscopic findings include hair diameter diversity, perifollicular pigmentation/peripilar sign, and yellow dots.5 The most common vascular patterns present in seborrheic dermatitis are arborizing red lines, twisted red loops, atypical vessels, and glomerular vessels. Perifollicular scaling may be white or yellow and oily.6 There are no specific dermoscopic findings for telogen effluvium; however, the presence of hair regrowth and the predominance of follicular openings with a single sprouting hair shaft may suggest this condition.7 Therefore, dermoscopy can assist clinicians in correctly diagnosing a patient’s condition and determining the its etiology, allowing for early and effective treatment.
Tinea capitis is a typical superficial dermatophyte infection that commonly occurs in prepubescent children and is uncommon in adults because the pH level of the scalp shifts during puberty and the amount of sebum that contains saturated fatty acids increases.8 The risk for developing tinea capitis is higher in certain individuals with comorbid systemic immune diseases, such as SLE and diabetes mellitus, among others, as well as in immunocompromised individuals, such as those with AIDS, organ transplant recipients, or patients receiving high doses of steroids or immunosuppressive drugs.9 The type of dermatophyte entering the hair, the level of host resistance, and the intensity of the inflammatory reaction all affect the clinical picture of tinea capitis in adults, which is pleomorphic and atypical.10 Although tinea capitis is not highly prevalent in adults, the fact that our patient had SLE and had been on immunosuppressive therapy to keep the condition stable increased the chance of contracting tinea capitis, underscoring the need for clinicians to be alert for fungal infections in this patient population.
Trichophyton tonsurans is the most prevalent form of microorganism that causes tinea capitis in the United States, the United Kingdom, and France. However, T tonsurans causing tinea capitis is uncommon in China, with one study reporting only 6 cases from 2000 to 2019.11 Tinea capitis caused by T tonsurans typically presents as black spot alopecia with inflammatory erythema and scaling of the scalp.12 Because most T tonsurans infections have few clinical symptoms, it is challenging to make a clinical diagnosis.13 Although not performed in our patient, a potassium hydroxide preparation and direct microscopic inspection of the afflicted hair and scales can help in quickly identifying and treating these infections. Additional fungal cultures can precisely identify the strain and trace its epidemiology, which is clinically significant not only to identify the potential infection source but also to direct the selection of an organized treatment plan.
The Diagnosis: Tinea Capitis
Dermoscopy revealed many black spot signs with broken, corkscrew, and comma hairs, as well as increased single hair follicles and focal polymorphic vascular distribution in the scalp (Figure 1). Fungal microscopy showed large round spores within the hair. A fungal culture demonstrated Trichophyton tonsurans growth in the broken hair. Based on the clinical presentation and laboratory findings, a diagnosis of tinea capitis was rendered. Oral terbinafine 250 mg/d was prescribed. At 4-week follow-up, the patient did not report worsening or new symptoms, and there was visible evidence of hair regrowth (Figure 2). There has been no sign of recurrence.

According to the most recent set of classification criteria published by the Systemic Lupus Erythematosus (SLE) International Collaborating Clinics, nonscarring alopecia is now a diagnostic criterion for SLE that has a specificity of 95.7%.1 Although discoid lupus erythematosus presents with diffuse scarring alopecia, SLE manifests as nonscarring alopecia in 1 of 3 patterns: diffuse, patchy, or “lupus hair.”2 It is commonly believed that lupus-related alopecia is a nonspecific symptom of SLE exacerbation and signals that the disease is active.3 Our patient had a history of SLE with no pruritus or pain accompanying the hair loss; however, we considered hair loss due to SLE disease activity, and dermoscopic examination was performed to further rule out the likelihood of SLE alopecia. The dermoscopic characteristics of lupus-related alopecia and tinea capitis vary. For lupusrelated alopecia, alterations to the hair shaft are visible with dermoscopy, including a reduced number or smaller diameter of hairs, hypopigmentation, the black dot sign, brown scattered pigmentation, blue-gray pigmentation, and thick dendritic capillaries.2 Tinea capitis typically displays characteristic dermoscopic manifestations, such as comma, corkscrew, Morse code–like, or jagged hair; black spots; and broken hair.4

Included in the differential diagnosis, androgenetic alopecia dermoscopic findings include hair diameter diversity, perifollicular pigmentation/peripilar sign, and yellow dots.5 The most common vascular patterns present in seborrheic dermatitis are arborizing red lines, twisted red loops, atypical vessels, and glomerular vessels. Perifollicular scaling may be white or yellow and oily.6 There are no specific dermoscopic findings for telogen effluvium; however, the presence of hair regrowth and the predominance of follicular openings with a single sprouting hair shaft may suggest this condition.7 Therefore, dermoscopy can assist clinicians in correctly diagnosing a patient’s condition and determining the its etiology, allowing for early and effective treatment.
Tinea capitis is a typical superficial dermatophyte infection that commonly occurs in prepubescent children and is uncommon in adults because the pH level of the scalp shifts during puberty and the amount of sebum that contains saturated fatty acids increases.8 The risk for developing tinea capitis is higher in certain individuals with comorbid systemic immune diseases, such as SLE and diabetes mellitus, among others, as well as in immunocompromised individuals, such as those with AIDS, organ transplant recipients, or patients receiving high doses of steroids or immunosuppressive drugs.9 The type of dermatophyte entering the hair, the level of host resistance, and the intensity of the inflammatory reaction all affect the clinical picture of tinea capitis in adults, which is pleomorphic and atypical.10 Although tinea capitis is not highly prevalent in adults, the fact that our patient had SLE and had been on immunosuppressive therapy to keep the condition stable increased the chance of contracting tinea capitis, underscoring the need for clinicians to be alert for fungal infections in this patient population.
Trichophyton tonsurans is the most prevalent form of microorganism that causes tinea capitis in the United States, the United Kingdom, and France. However, T tonsurans causing tinea capitis is uncommon in China, with one study reporting only 6 cases from 2000 to 2019.11 Tinea capitis caused by T tonsurans typically presents as black spot alopecia with inflammatory erythema and scaling of the scalp.12 Because most T tonsurans infections have few clinical symptoms, it is challenging to make a clinical diagnosis.13 Although not performed in our patient, a potassium hydroxide preparation and direct microscopic inspection of the afflicted hair and scales can help in quickly identifying and treating these infections. Additional fungal cultures can precisely identify the strain and trace its epidemiology, which is clinically significant not only to identify the potential infection source but also to direct the selection of an organized treatment plan.
- Petri M, Orbai AM, Alarcón GS, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64:2677-2686. doi:10.1002/art.34473
- Desai K, Miteva M. Recent insight on the management of lupus erythematosus alopecia. Clin Cosmet Investig Dermatol. 2021;14:333-347. doi:10.2147/CCID.S269288
- Wysenbeek AJ, Leibovici L, Amit M, et al. Alopecia in systemic lupus erythematosus. relation to disease manifestations. J Rheumatol. 1991;18:1185-1186.
- Lekkas D, Ioannides D, Lazaridou E, et al. Dermatoscopy in tinea capitis: can it provide clues for the responsible fungi? J Eur Acad Dermatol Venereol. 2021;35:E85-E87. doi:10.1111/jdv.16825
- Inui S. Trichoscopy for common hair loss diseases: algorithmic method for diagnosis. J Dermatol. 2011;38:71-75. doi:10.1111/j .1346-8138.2010.01119.x
- Golin´ska J, Sar-Pomian M, Rudnicka L. Diagnostic accuracy of trichoscopy in inflammatory scalp diseases: a systematic review. Dermatology. 2022;238:412-421. doi:10.1159/000517516
- Fernández-Domper L, Ballesteros-Redondo M, Vañó-Galván S. Trichoscopy: an update. Actas Dermosifiliogr. 2023;114:327-333. doi:10.1016/j.ad.2022.12.003
- He M, Zeng J, Mao Y, et al. Aetiological changes of tinea capitis in the Hubei area in 60 years: focus on adult tinea capitis. Mycoses. 2021;64:1527-1534. doi:10.1111/myc.13305
- Khosravi AR, Shokri H, Vahedi G. Factors in etiology and predisposition of adult tinea capitis and review of published literature. Mycopathologia. 2016;181:371-378. doi:10.1007/s11046 -016-0004-9
- Gianni C, Betti R, Perotta E, et al. Tinea capitis in adults. Mycoses. 1995;38:329-331. doi:10.1111/j.1439-0507.1995.tb00417.x
- Liang G, Zheng X, Song G, et al. Adult tinea capitis in China: a retrospective analysis from 2000 to 2019. Mycoses. 2020;63:876-888. doi:10.1111/myc.13102
- Zalewski A, Goldust M, Szepietowski JC. Tinea gladiatorum: epidemiology, clinical aspects, and management. J Clin Med. 2022;11:4066. doi:10.3390/jcm11144066
- Hiruma J, Ogawa Y, Hiruma M. Trichophyton tonsurans infection in Japan: epidemiology, clinical features, diagnosis and infection control. J Dermatol. 2015;42:245-249. doi:10.1111 /1346-8138.12678
- Petri M, Orbai AM, Alarcón GS, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64:2677-2686. doi:10.1002/art.34473
- Desai K, Miteva M. Recent insight on the management of lupus erythematosus alopecia. Clin Cosmet Investig Dermatol. 2021;14:333-347. doi:10.2147/CCID.S269288
- Wysenbeek AJ, Leibovici L, Amit M, et al. Alopecia in systemic lupus erythematosus. relation to disease manifestations. J Rheumatol. 1991;18:1185-1186.
- Lekkas D, Ioannides D, Lazaridou E, et al. Dermatoscopy in tinea capitis: can it provide clues for the responsible fungi? J Eur Acad Dermatol Venereol. 2021;35:E85-E87. doi:10.1111/jdv.16825
- Inui S. Trichoscopy for common hair loss diseases: algorithmic method for diagnosis. J Dermatol. 2011;38:71-75. doi:10.1111/j .1346-8138.2010.01119.x
- Golin´ska J, Sar-Pomian M, Rudnicka L. Diagnostic accuracy of trichoscopy in inflammatory scalp diseases: a systematic review. Dermatology. 2022;238:412-421. doi:10.1159/000517516
- Fernández-Domper L, Ballesteros-Redondo M, Vañó-Galván S. Trichoscopy: an update. Actas Dermosifiliogr. 2023;114:327-333. doi:10.1016/j.ad.2022.12.003
- He M, Zeng J, Mao Y, et al. Aetiological changes of tinea capitis in the Hubei area in 60 years: focus on adult tinea capitis. Mycoses. 2021;64:1527-1534. doi:10.1111/myc.13305
- Khosravi AR, Shokri H, Vahedi G. Factors in etiology and predisposition of adult tinea capitis and review of published literature. Mycopathologia. 2016;181:371-378. doi:10.1007/s11046 -016-0004-9
- Gianni C, Betti R, Perotta E, et al. Tinea capitis in adults. Mycoses. 1995;38:329-331. doi:10.1111/j.1439-0507.1995.tb00417.x
- Liang G, Zheng X, Song G, et al. Adult tinea capitis in China: a retrospective analysis from 2000 to 2019. Mycoses. 2020;63:876-888. doi:10.1111/myc.13102
- Zalewski A, Goldust M, Szepietowski JC. Tinea gladiatorum: epidemiology, clinical aspects, and management. J Clin Med. 2022;11:4066. doi:10.3390/jcm11144066
- Hiruma J, Ogawa Y, Hiruma M. Trichophyton tonsurans infection in Japan: epidemiology, clinical features, diagnosis and infection control. J Dermatol. 2015;42:245-249. doi:10.1111 /1346-8138.12678
A 51-year-old woman residing in the Hainan Province, China, was referred to our hospital for treatment of recurrent joint pain that could not be controlled at the local hospital. She had a history of systemic lupus erythematosus with a Systemic Lupus Erythematosus Disease Activity Index score of 8 (mild activity). Physical examination revealed irregular patches of hair loss on the head. There also were remnants of hair in some areas with black dots at the follicular opening and perifollicular keratotic papules interspersed as well as a few pale erythematous spots and white adherent scales.
Patients with MCL more prone to develop secondary malignancies
Key clinical point: Survivors of mantle cell lymphoma (MCL), particularly those treated with rituximab plus bendamustine (R-bendamustine), have an increased risk for secondary malignancies (SM).
Major finding: Patients with MCL vs lymphoma-free comparators had significantly higher rates of SM (adjusted hazard ratio [aHR] 1.6; 95% CI 1.4-1.8), with higher rates being observed across all primary treatment groups, ie, the Nordic-MCL2 protocol; rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone (R-CHOP); R-bendamustine; ibrutinib; lenalidomide; and R-CHOP/cytarabine groups. Treatment with R-bendamustine vs Nordic-MCL2 was independently associated with an increased risk for SM (aHR 2.0; 95% CI 1.3-3.2).
Study details: This population-based retrospective study included adult patients with MCL (n = 1452), each of whom was matched with ≤10 lymphoma-free comparators from the general population (n = 13,992).
Disclosures: This study was funded by the Swedish Cancer Society. I Glimelius and S Eloranta declared receiving research grants, contracts, or support for attending meetings from various sources, including the Swedish Cancer Society. The other authors declared no conflicts of interest.
Source: Abalo KD et al. Secondary malignancies among mantle cell lymphoma patients. Eur J Cancer. 2023;195:113403 (Oct 28). doi: 10.1016/j.ejca.2023.113403
Key clinical point: Survivors of mantle cell lymphoma (MCL), particularly those treated with rituximab plus bendamustine (R-bendamustine), have an increased risk for secondary malignancies (SM).
Major finding: Patients with MCL vs lymphoma-free comparators had significantly higher rates of SM (adjusted hazard ratio [aHR] 1.6; 95% CI 1.4-1.8), with higher rates being observed across all primary treatment groups, ie, the Nordic-MCL2 protocol; rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone (R-CHOP); R-bendamustine; ibrutinib; lenalidomide; and R-CHOP/cytarabine groups. Treatment with R-bendamustine vs Nordic-MCL2 was independently associated with an increased risk for SM (aHR 2.0; 95% CI 1.3-3.2).
Study details: This population-based retrospective study included adult patients with MCL (n = 1452), each of whom was matched with ≤10 lymphoma-free comparators from the general population (n = 13,992).
Disclosures: This study was funded by the Swedish Cancer Society. I Glimelius and S Eloranta declared receiving research grants, contracts, or support for attending meetings from various sources, including the Swedish Cancer Society. The other authors declared no conflicts of interest.
Source: Abalo KD et al. Secondary malignancies among mantle cell lymphoma patients. Eur J Cancer. 2023;195:113403 (Oct 28). doi: 10.1016/j.ejca.2023.113403
Key clinical point: Survivors of mantle cell lymphoma (MCL), particularly those treated with rituximab plus bendamustine (R-bendamustine), have an increased risk for secondary malignancies (SM).
Major finding: Patients with MCL vs lymphoma-free comparators had significantly higher rates of SM (adjusted hazard ratio [aHR] 1.6; 95% CI 1.4-1.8), with higher rates being observed across all primary treatment groups, ie, the Nordic-MCL2 protocol; rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone (R-CHOP); R-bendamustine; ibrutinib; lenalidomide; and R-CHOP/cytarabine groups. Treatment with R-bendamustine vs Nordic-MCL2 was independently associated with an increased risk for SM (aHR 2.0; 95% CI 1.3-3.2).
Study details: This population-based retrospective study included adult patients with MCL (n = 1452), each of whom was matched with ≤10 lymphoma-free comparators from the general population (n = 13,992).
Disclosures: This study was funded by the Swedish Cancer Society. I Glimelius and S Eloranta declared receiving research grants, contracts, or support for attending meetings from various sources, including the Swedish Cancer Society. The other authors declared no conflicts of interest.
Source: Abalo KD et al. Secondary malignancies among mantle cell lymphoma patients. Eur J Cancer. 2023;195:113403 (Oct 28). doi: 10.1016/j.ejca.2023.113403
Combination of time-limited ibrutinib and chimeric antigen receptor T-cells shows promise in r/r MCL
Key clinical point: The combination of tisagenlecleucel and time-limited ibrutinib improved outcomes and could be safely administered to patients with relapsed or refractory mantle cell lymphoma (r/r MCL), irrespective of prior covalent Bruton tyrosine kinase inhibitor (BTKi) exposure.
Major finding: At 4 months post infusion, the overall and complete response rates were 80% each. Patients with and without prior BTKi exposure had complete response rates of 90% and 70%, respectively. Grades 1-2 and grade 3 cytokine release syndrome rates were 55% and 20%, respectively.
Study details: This phase 2 study, TARMAC, included 20 patients having r/r MCL after ≥1 prior lines of therapy with (n = 10) or without (n = 10) a BTKi who were infused with tisagenlecleucel and commenced ibrutinib before leukapheresis and continued it for ≥6 months post infusion.
Disclosures: The study was sponsored by Peter MacCallum Cancer Centre, Australia. Several authors declared being members of the advisory committee, board of directors, or speakers’ bureau of or receiving honoraria or research funding from various sources.
Source: Minson AG et al. CAR T-cells and time-limited ibrutinib as treatment for relapsed/refractory mantle cell lymphoma: Phase II TARMAC study. Blood. 2023 (Oct 26). doi: 10.1182/blood.2023021306
Key clinical point: The combination of tisagenlecleucel and time-limited ibrutinib improved outcomes and could be safely administered to patients with relapsed or refractory mantle cell lymphoma (r/r MCL), irrespective of prior covalent Bruton tyrosine kinase inhibitor (BTKi) exposure.
Major finding: At 4 months post infusion, the overall and complete response rates were 80% each. Patients with and without prior BTKi exposure had complete response rates of 90% and 70%, respectively. Grades 1-2 and grade 3 cytokine release syndrome rates were 55% and 20%, respectively.
Study details: This phase 2 study, TARMAC, included 20 patients having r/r MCL after ≥1 prior lines of therapy with (n = 10) or without (n = 10) a BTKi who were infused with tisagenlecleucel and commenced ibrutinib before leukapheresis and continued it for ≥6 months post infusion.
Disclosures: The study was sponsored by Peter MacCallum Cancer Centre, Australia. Several authors declared being members of the advisory committee, board of directors, or speakers’ bureau of or receiving honoraria or research funding from various sources.
Source: Minson AG et al. CAR T-cells and time-limited ibrutinib as treatment for relapsed/refractory mantle cell lymphoma: Phase II TARMAC study. Blood. 2023 (Oct 26). doi: 10.1182/blood.2023021306
Key clinical point: The combination of tisagenlecleucel and time-limited ibrutinib improved outcomes and could be safely administered to patients with relapsed or refractory mantle cell lymphoma (r/r MCL), irrespective of prior covalent Bruton tyrosine kinase inhibitor (BTKi) exposure.
Major finding: At 4 months post infusion, the overall and complete response rates were 80% each. Patients with and without prior BTKi exposure had complete response rates of 90% and 70%, respectively. Grades 1-2 and grade 3 cytokine release syndrome rates were 55% and 20%, respectively.
Study details: This phase 2 study, TARMAC, included 20 patients having r/r MCL after ≥1 prior lines of therapy with (n = 10) or without (n = 10) a BTKi who were infused with tisagenlecleucel and commenced ibrutinib before leukapheresis and continued it for ≥6 months post infusion.
Disclosures: The study was sponsored by Peter MacCallum Cancer Centre, Australia. Several authors declared being members of the advisory committee, board of directors, or speakers’ bureau of or receiving honoraria or research funding from various sources.
Source: Minson AG et al. CAR T-cells and time-limited ibrutinib as treatment for relapsed/refractory mantle cell lymphoma: Phase II TARMAC study. Blood. 2023 (Oct 26). doi: 10.1182/blood.2023021306
Axi-cel vs tisagenlecleucel improves efficacy but may cause higher neurologic toxicity in LBCL
Key clinical point: Compared with tisagenlecleucel, axicabtagene ciloleucel (axi-cel) was associated with improved treatment outcomes but increased the risk for grade ≥ 3 neurologic events in patients with relapsed or refractory large B-cell lymphoma (LBCL) in real-world settings.
Major finding: Axi-cel vs tisagenlecleucel improved the overall survival (adjusted hazard ratio [aHR] 0.60; 95% CI 0.47-0.77), progression-free survival (aHR 0.67; 95% CI 0.57-0.78), and overall response rate (odds ratio 2.05; 95% CI 1.76-2.40). However, it was associated with a higher incidence of grade ≥ 3 immune effector cell-associated neurotoxicity syndrome (odds ratio 3.95; 95% CI 3.05-5.11).
Study details: This comparative meta-analysis of 14 real-world cohorts included patients with relapsed or refractory LBCL who received axi-cel (n = 2432) or tisagenlecleucel (n = 1514) chimeric antigen receptor T-cell therapy.
Disclosures: This study was funded by Kite, a Gilead Company. Six authors declared being employees of or holding leadership positions and stocks in Kite or Gilead. Several authors reported receiving honoraria, travel fees, research funding, etc., from various sources, including Kite.
Source: Jacobson CA et al. Real-world outcomes with CAR T-cell therapies in large B-cell lymphoma: A systematic review and meta-analysis. Transplant Cell Ther. 2023 (Oct 25). doi: 10.1016/j.jtct.2023.10.017
Key clinical point: Compared with tisagenlecleucel, axicabtagene ciloleucel (axi-cel) was associated with improved treatment outcomes but increased the risk for grade ≥ 3 neurologic events in patients with relapsed or refractory large B-cell lymphoma (LBCL) in real-world settings.
Major finding: Axi-cel vs tisagenlecleucel improved the overall survival (adjusted hazard ratio [aHR] 0.60; 95% CI 0.47-0.77), progression-free survival (aHR 0.67; 95% CI 0.57-0.78), and overall response rate (odds ratio 2.05; 95% CI 1.76-2.40). However, it was associated with a higher incidence of grade ≥ 3 immune effector cell-associated neurotoxicity syndrome (odds ratio 3.95; 95% CI 3.05-5.11).
Study details: This comparative meta-analysis of 14 real-world cohorts included patients with relapsed or refractory LBCL who received axi-cel (n = 2432) or tisagenlecleucel (n = 1514) chimeric antigen receptor T-cell therapy.
Disclosures: This study was funded by Kite, a Gilead Company. Six authors declared being employees of or holding leadership positions and stocks in Kite or Gilead. Several authors reported receiving honoraria, travel fees, research funding, etc., from various sources, including Kite.
Source: Jacobson CA et al. Real-world outcomes with CAR T-cell therapies in large B-cell lymphoma: A systematic review and meta-analysis. Transplant Cell Ther. 2023 (Oct 25). doi: 10.1016/j.jtct.2023.10.017
Key clinical point: Compared with tisagenlecleucel, axicabtagene ciloleucel (axi-cel) was associated with improved treatment outcomes but increased the risk for grade ≥ 3 neurologic events in patients with relapsed or refractory large B-cell lymphoma (LBCL) in real-world settings.
Major finding: Axi-cel vs tisagenlecleucel improved the overall survival (adjusted hazard ratio [aHR] 0.60; 95% CI 0.47-0.77), progression-free survival (aHR 0.67; 95% CI 0.57-0.78), and overall response rate (odds ratio 2.05; 95% CI 1.76-2.40). However, it was associated with a higher incidence of grade ≥ 3 immune effector cell-associated neurotoxicity syndrome (odds ratio 3.95; 95% CI 3.05-5.11).
Study details: This comparative meta-analysis of 14 real-world cohorts included patients with relapsed or refractory LBCL who received axi-cel (n = 2432) or tisagenlecleucel (n = 1514) chimeric antigen receptor T-cell therapy.
Disclosures: This study was funded by Kite, a Gilead Company. Six authors declared being employees of or holding leadership positions and stocks in Kite or Gilead. Several authors reported receiving honoraria, travel fees, research funding, etc., from various sources, including Kite.
Source: Jacobson CA et al. Real-world outcomes with CAR T-cell therapies in large B-cell lymphoma: A systematic review and meta-analysis. Transplant Cell Ther. 2023 (Oct 25). doi: 10.1016/j.jtct.2023.10.017
PET/CT-biomarkers hold prognostic value in DLBCL
Key clinical point: Total metabolic tumor volume (MTV) is an independent prognostic factor for treatment response and survival in patients receiving loncastuximab tesirine for relapsed or refractory diffuse large B-cell lymphoma (DLBCL) treated with ≥2 prior systemic therapy lines.
Major finding: An MTV ≥ 96 mL was significantly associated with failure to achieve a complete metabolic response (adjusted odds ratio 5.42; P = .002). Patients with an MTV ≥ 96 mL vs < 96 mL had shorter progression-free survival (adjusted hazard ratio [aHR] 2.68; P = .002) and overall survival (aHR 3.09; P < .0001).L
Study details: This post hoc analysis reviewed the screening PET/CT scans of 138 patients with relapsed or refractory DLBCL treated with ≥2 prior systemic therapy lines who received loncastuximab tesirine in LOTIS-2.
Disclosures: This study was supported by ADC Therapeutics, SA, and the Sylvester Comprehensive Cancer Center, Miami. Some authors declared serving as consultants, advisors, etc., for or receiving research funding or honoraria from ADC Therapeutics and others. J Radford declared owing stocks in ADC Therapeutics.
Source: Alderuccio JP et al. PET/CT-biomarkers enable risk stratification of patients with relapsed/refractory diffuse large B-cell lymphoma enrolled in the LOTIS-2 clinical trial. Clin Cancer Res. 2023 (Oct 19). doi: 10.1158/1078-0432.CCR-23-1561
Key clinical point: Total metabolic tumor volume (MTV) is an independent prognostic factor for treatment response and survival in patients receiving loncastuximab tesirine for relapsed or refractory diffuse large B-cell lymphoma (DLBCL) treated with ≥2 prior systemic therapy lines.
Major finding: An MTV ≥ 96 mL was significantly associated with failure to achieve a complete metabolic response (adjusted odds ratio 5.42; P = .002). Patients with an MTV ≥ 96 mL vs < 96 mL had shorter progression-free survival (adjusted hazard ratio [aHR] 2.68; P = .002) and overall survival (aHR 3.09; P < .0001).L
Study details: This post hoc analysis reviewed the screening PET/CT scans of 138 patients with relapsed or refractory DLBCL treated with ≥2 prior systemic therapy lines who received loncastuximab tesirine in LOTIS-2.
Disclosures: This study was supported by ADC Therapeutics, SA, and the Sylvester Comprehensive Cancer Center, Miami. Some authors declared serving as consultants, advisors, etc., for or receiving research funding or honoraria from ADC Therapeutics and others. J Radford declared owing stocks in ADC Therapeutics.
Source: Alderuccio JP et al. PET/CT-biomarkers enable risk stratification of patients with relapsed/refractory diffuse large B-cell lymphoma enrolled in the LOTIS-2 clinical trial. Clin Cancer Res. 2023 (Oct 19). doi: 10.1158/1078-0432.CCR-23-1561
Key clinical point: Total metabolic tumor volume (MTV) is an independent prognostic factor for treatment response and survival in patients receiving loncastuximab tesirine for relapsed or refractory diffuse large B-cell lymphoma (DLBCL) treated with ≥2 prior systemic therapy lines.
Major finding: An MTV ≥ 96 mL was significantly associated with failure to achieve a complete metabolic response (adjusted odds ratio 5.42; P = .002). Patients with an MTV ≥ 96 mL vs < 96 mL had shorter progression-free survival (adjusted hazard ratio [aHR] 2.68; P = .002) and overall survival (aHR 3.09; P < .0001).L
Study details: This post hoc analysis reviewed the screening PET/CT scans of 138 patients with relapsed or refractory DLBCL treated with ≥2 prior systemic therapy lines who received loncastuximab tesirine in LOTIS-2.
Disclosures: This study was supported by ADC Therapeutics, SA, and the Sylvester Comprehensive Cancer Center, Miami. Some authors declared serving as consultants, advisors, etc., for or receiving research funding or honoraria from ADC Therapeutics and others. J Radford declared owing stocks in ADC Therapeutics.
Source: Alderuccio JP et al. PET/CT-biomarkers enable risk stratification of patients with relapsed/refractory diffuse large B-cell lymphoma enrolled in the LOTIS-2 clinical trial. Clin Cancer Res. 2023 (Oct 19). doi: 10.1158/1078-0432.CCR-23-1561