User login
ASCO expands recommendations on bone-modifying agents in myeloma
Bisphosphonates should be prescribed for any patient receiving treatment for active multiple myeloma, regardless of whether or not there is evidence of lytic bone destruction or spinal compression fracture, according to updated guidelines from the American Society of Clinical Oncology.
Previous guidelines from the society, last updated in 2007, recommended the use of intravenous bisphosphonates for patients with myeloma with evidence of bone disease, according to the expert panel that drafted the update.
“Fewer adverse events related to renal toxicity have been noted with denosumab, compared with zoledronic acid,” and “this may be preferred in patients with compromised renal function,” wrote the expert panel, led by cochairs Kenneth C. Anderson, MD, of Dana-Farber Cancer Institute, Boston, and Robert A. Kyle, MD, of Mayo Clinic, Rochester, Minn.
ASCO guidelines on bisphosphonates in myeloma were first drafted in 2002 and then updated in 2007. The new recommendations on bone-modifying therapy in myeloma are based on review of an additional 35 publications. The new guidelines are “consistent with the previous recommendations” while updating indications for therapy and information on denosumab, according to the expert panel.
Evidence that myeloma patients without lytic bone disease will benefit from intravenous bisphosphonates comes from the randomized MRC IX trial, in which patients who received zoledronic acid had reduced skeletal-related events at relapse and improved progression-free survival.
Denosumab, a receptor activator of nuclear factor kappa-B ligand (RANKL) inhibitor, was noninferior to zoledronic acid for prevention of skeletal-related events in a randomized phase 3 clinical trial; however, it is “more expensive than zoledronic acid or pamidronate and must be considered in treatment decisions,” the guidelines authors wrote.
The total price in the United States for a 1-year treatment cycle of denosumab is just under $26,000, according to data included in the ASCO guideline. By comparison, the 1-year treatment cycle price for the bisphosphonates ranges from $214 to $697, depending on the regimen.
When intravenous bisphosphonate therapy is warranted, the guideline-recommended schedule is infusion of zoledronic acid 4 mg over at least 15 minutes, or pamidronate 90 mg over 2 hours, every 3-4 weeks.
The guidelines also address osteonecrosis of the jaw (ONJ), a major complication observed not only with the potent bisphosphonates pamidronate and zoledronic acid, but also with denosumab.
The panel said they were in agreement with revised labels from the Food and Drug Administration for zoledronic acid and pamidronate, among other papers or statements addressing ONJ and noted that patients need a comprehensive dental exam and preventive dentistry as appropriate before starting bone-modifying therapy.
“The risk of ONJ has prompted the use of less-frequent dosing of zoledronic acid, which may be an option for patients,” they said in their report.
Guideline authors reported ties to Amgen, Celgene, Millennium Pharmaceuticals, Gilead Sciences, Bristol-Myers Squibb, Novartis, Pfizer, and others.
SOURCE: Anderson K et al. J Clin Oncol. 2018 Jan 17:JCO2017766402. doi: 10.1200/JCO.2017.76.6402.
Bisphosphonates should be prescribed for any patient receiving treatment for active multiple myeloma, regardless of whether or not there is evidence of lytic bone destruction or spinal compression fracture, according to updated guidelines from the American Society of Clinical Oncology.
Previous guidelines from the society, last updated in 2007, recommended the use of intravenous bisphosphonates for patients with myeloma with evidence of bone disease, according to the expert panel that drafted the update.
“Fewer adverse events related to renal toxicity have been noted with denosumab, compared with zoledronic acid,” and “this may be preferred in patients with compromised renal function,” wrote the expert panel, led by cochairs Kenneth C. Anderson, MD, of Dana-Farber Cancer Institute, Boston, and Robert A. Kyle, MD, of Mayo Clinic, Rochester, Minn.
ASCO guidelines on bisphosphonates in myeloma were first drafted in 2002 and then updated in 2007. The new recommendations on bone-modifying therapy in myeloma are based on review of an additional 35 publications. The new guidelines are “consistent with the previous recommendations” while updating indications for therapy and information on denosumab, according to the expert panel.
Evidence that myeloma patients without lytic bone disease will benefit from intravenous bisphosphonates comes from the randomized MRC IX trial, in which patients who received zoledronic acid had reduced skeletal-related events at relapse and improved progression-free survival.
Denosumab, a receptor activator of nuclear factor kappa-B ligand (RANKL) inhibitor, was noninferior to zoledronic acid for prevention of skeletal-related events in a randomized phase 3 clinical trial; however, it is “more expensive than zoledronic acid or pamidronate and must be considered in treatment decisions,” the guidelines authors wrote.
The total price in the United States for a 1-year treatment cycle of denosumab is just under $26,000, according to data included in the ASCO guideline. By comparison, the 1-year treatment cycle price for the bisphosphonates ranges from $214 to $697, depending on the regimen.
When intravenous bisphosphonate therapy is warranted, the guideline-recommended schedule is infusion of zoledronic acid 4 mg over at least 15 minutes, or pamidronate 90 mg over 2 hours, every 3-4 weeks.
The guidelines also address osteonecrosis of the jaw (ONJ), a major complication observed not only with the potent bisphosphonates pamidronate and zoledronic acid, but also with denosumab.
The panel said they were in agreement with revised labels from the Food and Drug Administration for zoledronic acid and pamidronate, among other papers or statements addressing ONJ and noted that patients need a comprehensive dental exam and preventive dentistry as appropriate before starting bone-modifying therapy.
“The risk of ONJ has prompted the use of less-frequent dosing of zoledronic acid, which may be an option for patients,” they said in their report.
Guideline authors reported ties to Amgen, Celgene, Millennium Pharmaceuticals, Gilead Sciences, Bristol-Myers Squibb, Novartis, Pfizer, and others.
SOURCE: Anderson K et al. J Clin Oncol. 2018 Jan 17:JCO2017766402. doi: 10.1200/JCO.2017.76.6402.
Bisphosphonates should be prescribed for any patient receiving treatment for active multiple myeloma, regardless of whether or not there is evidence of lytic bone destruction or spinal compression fracture, according to updated guidelines from the American Society of Clinical Oncology.
Previous guidelines from the society, last updated in 2007, recommended the use of intravenous bisphosphonates for patients with myeloma with evidence of bone disease, according to the expert panel that drafted the update.
“Fewer adverse events related to renal toxicity have been noted with denosumab, compared with zoledronic acid,” and “this may be preferred in patients with compromised renal function,” wrote the expert panel, led by cochairs Kenneth C. Anderson, MD, of Dana-Farber Cancer Institute, Boston, and Robert A. Kyle, MD, of Mayo Clinic, Rochester, Minn.
ASCO guidelines on bisphosphonates in myeloma were first drafted in 2002 and then updated in 2007. The new recommendations on bone-modifying therapy in myeloma are based on review of an additional 35 publications. The new guidelines are “consistent with the previous recommendations” while updating indications for therapy and information on denosumab, according to the expert panel.
Evidence that myeloma patients without lytic bone disease will benefit from intravenous bisphosphonates comes from the randomized MRC IX trial, in which patients who received zoledronic acid had reduced skeletal-related events at relapse and improved progression-free survival.
Denosumab, a receptor activator of nuclear factor kappa-B ligand (RANKL) inhibitor, was noninferior to zoledronic acid for prevention of skeletal-related events in a randomized phase 3 clinical trial; however, it is “more expensive than zoledronic acid or pamidronate and must be considered in treatment decisions,” the guidelines authors wrote.
The total price in the United States for a 1-year treatment cycle of denosumab is just under $26,000, according to data included in the ASCO guideline. By comparison, the 1-year treatment cycle price for the bisphosphonates ranges from $214 to $697, depending on the regimen.
When intravenous bisphosphonate therapy is warranted, the guideline-recommended schedule is infusion of zoledronic acid 4 mg over at least 15 minutes, or pamidronate 90 mg over 2 hours, every 3-4 weeks.
The guidelines also address osteonecrosis of the jaw (ONJ), a major complication observed not only with the potent bisphosphonates pamidronate and zoledronic acid, but also with denosumab.
The panel said they were in agreement with revised labels from the Food and Drug Administration for zoledronic acid and pamidronate, among other papers or statements addressing ONJ and noted that patients need a comprehensive dental exam and preventive dentistry as appropriate before starting bone-modifying therapy.
“The risk of ONJ has prompted the use of less-frequent dosing of zoledronic acid, which may be an option for patients,” they said in their report.
Guideline authors reported ties to Amgen, Celgene, Millennium Pharmaceuticals, Gilead Sciences, Bristol-Myers Squibb, Novartis, Pfizer, and others.
SOURCE: Anderson K et al. J Clin Oncol. 2018 Jan 17:JCO2017766402. doi: 10.1200/JCO.2017.76.6402.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
nPEP for HIV: Updated CDC guidelines available for primary care physicians
In 2016, the Centers for Disease Control and Prevention provided health care providers with updated recommendations for nonoccupational postexposure prophylaxis (nPEP) with antiretroviral drugs to prevent transmission of HIV following sexual interaction, injection-drug use, or other nonoccupational exposures.1 The new recommendations include the use of more effective and more tolerable drug regimens that employ antiretroviral medications that were approved since the previous guidelines came out in 2005; they also provide updated guidance on exposure assessment, baseline and follow-up HIV testing, and longer-term prevention measures, such as pre-exposure prophylaxis (PrEP).
Screening for HIV infection has been expanding broadly in all health care settings over the past decade, so primary care physicians play an increasingly vital role in preventing HIV infection. Today, primary care physicians are also often the most likely “go-to” health care provider when patients think they may have been exposed to HIV. Clinically, this is an emergency situation, so time is of the essence: Treatment with three powerful antiretrovirals must be initiated within a few hours of – but no later than 72 hours after – an isolated exposure to blood, genital secretions, or other potentially infectious body fluids that may contain HIV.
The key issue for primary care physicians, especially those who have never prescribed PEP before, is advance planning. What you do up front, in terms of organizing materials and training staff, is worth the effort because there is so much at stake – for your patients and for society. The good news is that once you have an established nPEP protocol in place, it stays in place. When a patient asks for help, the protocol kicks in automatically.
Getting ready for nPEP
Prepare your staff:
- Educate your whole staff about the urgency of seeing potential nPEP patients immediately.
- Choose the staff person in your office who will submit requests for PEP medications to the pharmacy and/or pharmaceutical companies; your financial reimbursement staff person is likely a good candidate for this job.
- Learn about patient assistance programs (for uninsured or underinsured patients) and crime victims compensation programs (reimbursement or emergency awards for victims of violent crimes, including rape, for various out-of-pocket expenses including medical expenses).
Keep paperwork and materials on hand:
- Have information and forms for patient assistance programs for pharmaceutical companies supplying the drugs. Pharmaceutical companies are aware of the urgency for nPEP medications and are ready to respond immediately. They may mail the medication so it arrives the next day or, more likely, fax a voucher or other information for the patient to present to a local pharmacist who will fill the prescription.
- Have information on your state’s crime victims compensation program available.
- Consider keeping nPEP Starter Packs (with an initial 3-7 days’ worth of medication) readily available in your office.
Rapid evaluation of patients seeking care after potential exposure to HIV
Effective delivery of nPEP requires prompt initial evaluation of patients and assessment of HIV transmission risk. Take a methodical, step-by-step history of the exposure to address the following basic questions:
- Date and time of exposure? nPEP should be initiated as soon as possible after HIV exposure; it is unlikely to be effective if not initiated within 72 hours or less.
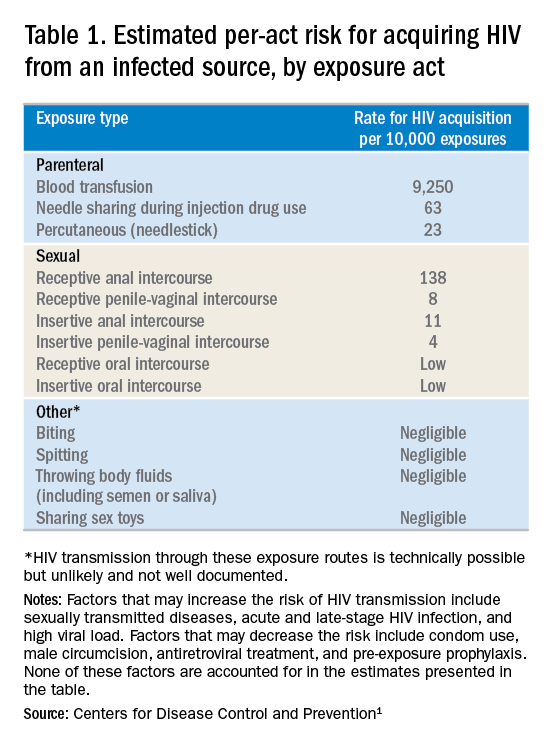
- Frequency of exposure? Type/route of exposure? nPEP is generally reserved for isolated or infrequent exposures that present a substantial risk for HIV acquisition (see Table 1 on HIV acquisition risk below).
- HIV status of exposure source? If the source is positive, is the source person on HIV treatment with antiretroviral therapy? If unknown, is the source person an injecting drug user or a man who has sex with men (MSM)?
Based on the initial evaluation, is nPEP recommended?
Answers to the questions asked during the initial evaluation of the patient will determine whether nPEP is indicated. Along with its updated recommendations, the CDC provided an algorithm to help guide evaluation and treatment.
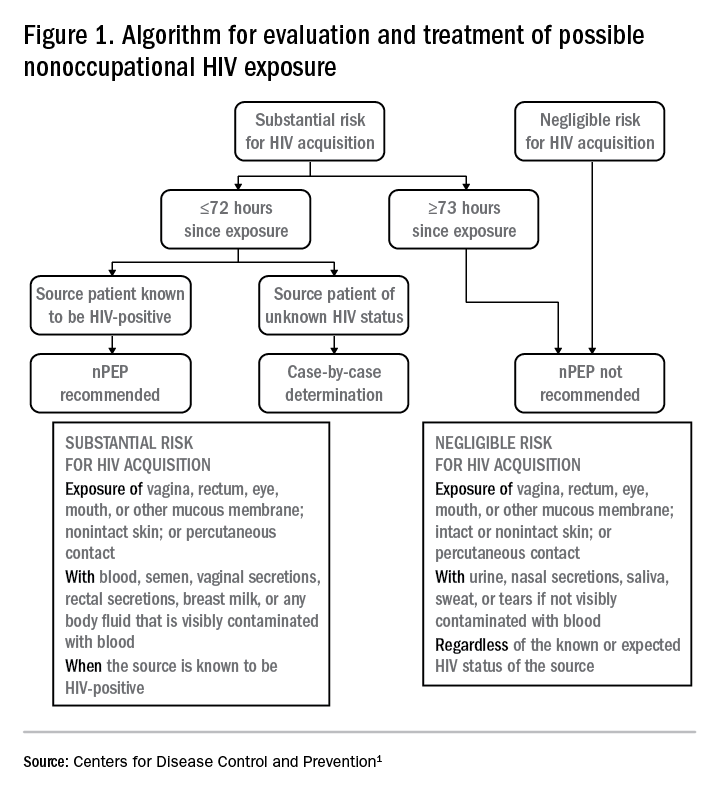
Preferred HIV test
Administer an HIV test to all patients considered for nPEP, preferably the rapid combined antigen and antibody test (Ag/Ab), or just the antibody test if the Ag/Ab test is not available. nPEP is indicated only for persons without HIV infections. However, if results are not available during the initial evaluation, assume the patient is not infected. If indicated and started, nPEP can be discontinued if tests later shown the patient already has an HIV infection.
Laboratory testing
If nPEP is indicated, conduct laboratory testing. Lab testing is required to document the patient’s HIV status (and that of the source person, when available), identify and manage other conditions potentially resulting from exposure, identify conditions that may affect the nPEP medication regimen, and monitor safety or toxicities to the prescribed regimen.
nPEP treatment regimen for otherwise healthy adults and adolescents
In the absence of randomized clinical trials, data from a case/control study demonstrating an 81% reduction of HIV transmission after use of occupational PEP among hospital workers remains the strongest evidence for the benefit of nPEP.1,2 For patients offered nPEP, recommended treatment includes prescribing either of the following regimens for 28 days:
- Preferred regimen: tenofovir disoproxil fumarate (TDF) (300 mg) with emtricitabine (FTC) (200 mg) once daily plus either raltegravir (RAL) 400 mg twice daily or dolutegravir (DTG) 50 mg daily.
- Alternative regimen: TDF (300 mg) with FTC (200 mg) once daily plus darunavir (DRV) (800 mg) and ritonavir (RTV) (100 mg) once daily.
Additional considerations and nPEP treatment regimens for children, patients with decreased renal function, and pregnant women are included in the CDC guidelines.
Crucial Information for Patients on nPEP
Emphasize the importance of proper dosing and adherence.
Review the patient information for each drug in the regimen, specifically the black boxes, warnings, and side effects, and counsel your patients accordingly.
Transitioning from nPEP to PrEP or from PrEP to nPEP
If you have a patient who engages in behavior that places them at risk for frequent, recurrent exposures to HIV, consider transitioning them to PrEP (pre-exposure prophylaxis) following their 28-day course of nPEP.3 PrEP is a two-drug regimen taken daily on an ongoing basis.
Additionally, for patients who are already on PrEP but who have not taken their medications within a week before the possible exposure, consider initiating nPEP for 28 days and then reintroducing PrEP if their HIV status is negative and the problems with adherence can be addressed moving forward.
Raising Awareness About nPEP
Many people never expect to be exposed to HIV and may not know about the availability of PEP in an emergency situation. You can help raise awareness by making educational materials available in your waiting rooms and exam rooms. Brochures and other HIV/AIDS educational materials for patients are available from the CDC Act Against AIDS campaign.
Summary
Dr. Dominguez is a Captain, U.S. Public Health Service, epidemiology branch, division of HIV/AIDS prevention, CDC.
Additional resources
- The CDC recommends that everyone between the ages of 13 and 64 get tested for HIV at least once as part of routine health care. As part of its Act Against AIDS initiative, the CDC developed the HIV Screening Standard Care program, which provides free tools and resources to help clinicians and nurses incorporate routine HIV screening into primary care settings.
- HIV guidelines and recommendations .
- Postexposure prophylaxis (PEP)
- Pre-Exposure prophylaxis (PrEP)
References
1. Centers for Disease Control and Prevention. Updated guidelines for antiretroviral postexposure prophylaxis after sexual, injection drug use, or other nonoccupational exposure to HIV. United States, 2016. Accessed March 6, 2017.
2. Cardo DM et al. New Engl J Med. 1997;337(21):1485-90.
3. Centers for Disease Control and Prevention. Preexposure prophylaxis for the prevention of HIV infection in the United States–2014: a clinical practice guideline. Accessed March 6, 2017.
In 2016, the Centers for Disease Control and Prevention provided health care providers with updated recommendations for nonoccupational postexposure prophylaxis (nPEP) with antiretroviral drugs to prevent transmission of HIV following sexual interaction, injection-drug use, or other nonoccupational exposures.1 The new recommendations include the use of more effective and more tolerable drug regimens that employ antiretroviral medications that were approved since the previous guidelines came out in 2005; they also provide updated guidance on exposure assessment, baseline and follow-up HIV testing, and longer-term prevention measures, such as pre-exposure prophylaxis (PrEP).
Screening for HIV infection has been expanding broadly in all health care settings over the past decade, so primary care physicians play an increasingly vital role in preventing HIV infection. Today, primary care physicians are also often the most likely “go-to” health care provider when patients think they may have been exposed to HIV. Clinically, this is an emergency situation, so time is of the essence: Treatment with three powerful antiretrovirals must be initiated within a few hours of – but no later than 72 hours after – an isolated exposure to blood, genital secretions, or other potentially infectious body fluids that may contain HIV.
The key issue for primary care physicians, especially those who have never prescribed PEP before, is advance planning. What you do up front, in terms of organizing materials and training staff, is worth the effort because there is so much at stake – for your patients and for society. The good news is that once you have an established nPEP protocol in place, it stays in place. When a patient asks for help, the protocol kicks in automatically.
Getting ready for nPEP
Prepare your staff:
- Educate your whole staff about the urgency of seeing potential nPEP patients immediately.
- Choose the staff person in your office who will submit requests for PEP medications to the pharmacy and/or pharmaceutical companies; your financial reimbursement staff person is likely a good candidate for this job.
- Learn about patient assistance programs (for uninsured or underinsured patients) and crime victims compensation programs (reimbursement or emergency awards for victims of violent crimes, including rape, for various out-of-pocket expenses including medical expenses).
Keep paperwork and materials on hand:
- Have information and forms for patient assistance programs for pharmaceutical companies supplying the drugs. Pharmaceutical companies are aware of the urgency for nPEP medications and are ready to respond immediately. They may mail the medication so it arrives the next day or, more likely, fax a voucher or other information for the patient to present to a local pharmacist who will fill the prescription.
- Have information on your state’s crime victims compensation program available.
- Consider keeping nPEP Starter Packs (with an initial 3-7 days’ worth of medication) readily available in your office.
Rapid evaluation of patients seeking care after potential exposure to HIV
Effective delivery of nPEP requires prompt initial evaluation of patients and assessment of HIV transmission risk. Take a methodical, step-by-step history of the exposure to address the following basic questions:
- Date and time of exposure? nPEP should be initiated as soon as possible after HIV exposure; it is unlikely to be effective if not initiated within 72 hours or less.
- Frequency of exposure? Type/route of exposure? nPEP is generally reserved for isolated or infrequent exposures that present a substantial risk for HIV acquisition (see Table 1 on HIV acquisition risk below).
- HIV status of exposure source? If the source is positive, is the source person on HIV treatment with antiretroviral therapy? If unknown, is the source person an injecting drug user or a man who has sex with men (MSM)?

Based on the initial evaluation, is nPEP recommended?
Answers to the questions asked during the initial evaluation of the patient will determine whether nPEP is indicated. Along with its updated recommendations, the CDC provided an algorithm to help guide evaluation and treatment.

Preferred HIV test
Administer an HIV test to all patients considered for nPEP, preferably the rapid combined antigen and antibody test (Ag/Ab), or just the antibody test if the Ag/Ab test is not available. nPEP is indicated only for persons without HIV infections. However, if results are not available during the initial evaluation, assume the patient is not infected. If indicated and started, nPEP can be discontinued if tests later shown the patient already has an HIV infection.
Laboratory testing
If nPEP is indicated, conduct laboratory testing. Lab testing is required to document the patient’s HIV status (and that of the source person, when available), identify and manage other conditions potentially resulting from exposure, identify conditions that may affect the nPEP medication regimen, and monitor safety or toxicities to the prescribed regimen.

nPEP treatment regimen for otherwise healthy adults and adolescents
In the absence of randomized clinical trials, data from a case/control study demonstrating an 81% reduction of HIV transmission after use of occupational PEP among hospital workers remains the strongest evidence for the benefit of nPEP.1,2 For patients offered nPEP, recommended treatment includes prescribing either of the following regimens for 28 days:
- Preferred regimen: tenofovir disoproxil fumarate (TDF) (300 mg) with emtricitabine (FTC) (200 mg) once daily plus either raltegravir (RAL) 400 mg twice daily or dolutegravir (DTG) 50 mg daily.
- Alternative regimen: TDF (300 mg) with FTC (200 mg) once daily plus darunavir (DRV) (800 mg) and ritonavir (RTV) (100 mg) once daily.
Additional considerations and nPEP treatment regimens for children, patients with decreased renal function, and pregnant women are included in the CDC guidelines.
Crucial Information for Patients on nPEP
Emphasize the importance of proper dosing and adherence.
Review the patient information for each drug in the regimen, specifically the black boxes, warnings, and side effects, and counsel your patients accordingly.
Transitioning from nPEP to PrEP or from PrEP to nPEP
If you have a patient who engages in behavior that places them at risk for frequent, recurrent exposures to HIV, consider transitioning them to PrEP (pre-exposure prophylaxis) following their 28-day course of nPEP.3 PrEP is a two-drug regimen taken daily on an ongoing basis.
Additionally, for patients who are already on PrEP but who have not taken their medications within a week before the possible exposure, consider initiating nPEP for 28 days and then reintroducing PrEP if their HIV status is negative and the problems with adherence can be addressed moving forward.
Raising Awareness About nPEP
Many people never expect to be exposed to HIV and may not know about the availability of PEP in an emergency situation. You can help raise awareness by making educational materials available in your waiting rooms and exam rooms. Brochures and other HIV/AIDS educational materials for patients are available from the CDC Act Against AIDS campaign.
Summary
Dr. Dominguez is a Captain, U.S. Public Health Service, epidemiology branch, division of HIV/AIDS prevention, CDC.
Additional resources
- The CDC recommends that everyone between the ages of 13 and 64 get tested for HIV at least once as part of routine health care. As part of its Act Against AIDS initiative, the CDC developed the HIV Screening Standard Care program, which provides free tools and resources to help clinicians and nurses incorporate routine HIV screening into primary care settings.
- HIV guidelines and recommendations .
- Postexposure prophylaxis (PEP)
- Pre-Exposure prophylaxis (PrEP)
References
1. Centers for Disease Control and Prevention. Updated guidelines for antiretroviral postexposure prophylaxis after sexual, injection drug use, or other nonoccupational exposure to HIV. United States, 2016. Accessed March 6, 2017.
2. Cardo DM et al. New Engl J Med. 1997;337(21):1485-90.
3. Centers for Disease Control and Prevention. Preexposure prophylaxis for the prevention of HIV infection in the United States–2014: a clinical practice guideline. Accessed March 6, 2017.
In 2016, the Centers for Disease Control and Prevention provided health care providers with updated recommendations for nonoccupational postexposure prophylaxis (nPEP) with antiretroviral drugs to prevent transmission of HIV following sexual interaction, injection-drug use, or other nonoccupational exposures.1 The new recommendations include the use of more effective and more tolerable drug regimens that employ antiretroviral medications that were approved since the previous guidelines came out in 2005; they also provide updated guidance on exposure assessment, baseline and follow-up HIV testing, and longer-term prevention measures, such as pre-exposure prophylaxis (PrEP).
Screening for HIV infection has been expanding broadly in all health care settings over the past decade, so primary care physicians play an increasingly vital role in preventing HIV infection. Today, primary care physicians are also often the most likely “go-to” health care provider when patients think they may have been exposed to HIV. Clinically, this is an emergency situation, so time is of the essence: Treatment with three powerful antiretrovirals must be initiated within a few hours of – but no later than 72 hours after – an isolated exposure to blood, genital secretions, or other potentially infectious body fluids that may contain HIV.
The key issue for primary care physicians, especially those who have never prescribed PEP before, is advance planning. What you do up front, in terms of organizing materials and training staff, is worth the effort because there is so much at stake – for your patients and for society. The good news is that once you have an established nPEP protocol in place, it stays in place. When a patient asks for help, the protocol kicks in automatically.
Getting ready for nPEP
Prepare your staff:
- Educate your whole staff about the urgency of seeing potential nPEP patients immediately.
- Choose the staff person in your office who will submit requests for PEP medications to the pharmacy and/or pharmaceutical companies; your financial reimbursement staff person is likely a good candidate for this job.
- Learn about patient assistance programs (for uninsured or underinsured patients) and crime victims compensation programs (reimbursement or emergency awards for victims of violent crimes, including rape, for various out-of-pocket expenses including medical expenses).
Keep paperwork and materials on hand:
- Have information and forms for patient assistance programs for pharmaceutical companies supplying the drugs. Pharmaceutical companies are aware of the urgency for nPEP medications and are ready to respond immediately. They may mail the medication so it arrives the next day or, more likely, fax a voucher or other information for the patient to present to a local pharmacist who will fill the prescription.
- Have information on your state’s crime victims compensation program available.
- Consider keeping nPEP Starter Packs (with an initial 3-7 days’ worth of medication) readily available in your office.
Rapid evaluation of patients seeking care after potential exposure to HIV
Effective delivery of nPEP requires prompt initial evaluation of patients and assessment of HIV transmission risk. Take a methodical, step-by-step history of the exposure to address the following basic questions:
- Date and time of exposure? nPEP should be initiated as soon as possible after HIV exposure; it is unlikely to be effective if not initiated within 72 hours or less.
- Frequency of exposure? Type/route of exposure? nPEP is generally reserved for isolated or infrequent exposures that present a substantial risk for HIV acquisition (see Table 1 on HIV acquisition risk below).
- HIV status of exposure source? If the source is positive, is the source person on HIV treatment with antiretroviral therapy? If unknown, is the source person an injecting drug user or a man who has sex with men (MSM)?

Based on the initial evaluation, is nPEP recommended?
Answers to the questions asked during the initial evaluation of the patient will determine whether nPEP is indicated. Along with its updated recommendations, the CDC provided an algorithm to help guide evaluation and treatment.

Preferred HIV test
Administer an HIV test to all patients considered for nPEP, preferably the rapid combined antigen and antibody test (Ag/Ab), or just the antibody test if the Ag/Ab test is not available. nPEP is indicated only for persons without HIV infections. However, if results are not available during the initial evaluation, assume the patient is not infected. If indicated and started, nPEP can be discontinued if tests later shown the patient already has an HIV infection.
Laboratory testing
If nPEP is indicated, conduct laboratory testing. Lab testing is required to document the patient’s HIV status (and that of the source person, when available), identify and manage other conditions potentially resulting from exposure, identify conditions that may affect the nPEP medication regimen, and monitor safety or toxicities to the prescribed regimen.

nPEP treatment regimen for otherwise healthy adults and adolescents
In the absence of randomized clinical trials, data from a case/control study demonstrating an 81% reduction of HIV transmission after use of occupational PEP among hospital workers remains the strongest evidence for the benefit of nPEP.1,2 For patients offered nPEP, recommended treatment includes prescribing either of the following regimens for 28 days:
- Preferred regimen: tenofovir disoproxil fumarate (TDF) (300 mg) with emtricitabine (FTC) (200 mg) once daily plus either raltegravir (RAL) 400 mg twice daily or dolutegravir (DTG) 50 mg daily.
- Alternative regimen: TDF (300 mg) with FTC (200 mg) once daily plus darunavir (DRV) (800 mg) and ritonavir (RTV) (100 mg) once daily.
Additional considerations and nPEP treatment regimens for children, patients with decreased renal function, and pregnant women are included in the CDC guidelines.
Crucial Information for Patients on nPEP
Emphasize the importance of proper dosing and adherence.
Review the patient information for each drug in the regimen, specifically the black boxes, warnings, and side effects, and counsel your patients accordingly.
Transitioning from nPEP to PrEP or from PrEP to nPEP
If you have a patient who engages in behavior that places them at risk for frequent, recurrent exposures to HIV, consider transitioning them to PrEP (pre-exposure prophylaxis) following their 28-day course of nPEP.3 PrEP is a two-drug regimen taken daily on an ongoing basis.
Additionally, for patients who are already on PrEP but who have not taken their medications within a week before the possible exposure, consider initiating nPEP for 28 days and then reintroducing PrEP if their HIV status is negative and the problems with adherence can be addressed moving forward.
Raising Awareness About nPEP
Many people never expect to be exposed to HIV and may not know about the availability of PEP in an emergency situation. You can help raise awareness by making educational materials available in your waiting rooms and exam rooms. Brochures and other HIV/AIDS educational materials for patients are available from the CDC Act Against AIDS campaign.
Summary
Dr. Dominguez is a Captain, U.S. Public Health Service, epidemiology branch, division of HIV/AIDS prevention, CDC.
Additional resources
- The CDC recommends that everyone between the ages of 13 and 64 get tested for HIV at least once as part of routine health care. As part of its Act Against AIDS initiative, the CDC developed the HIV Screening Standard Care program, which provides free tools and resources to help clinicians and nurses incorporate routine HIV screening into primary care settings.
- HIV guidelines and recommendations .
- Postexposure prophylaxis (PEP)
- Pre-Exposure prophylaxis (PrEP)
References
1. Centers for Disease Control and Prevention. Updated guidelines for antiretroviral postexposure prophylaxis after sexual, injection drug use, or other nonoccupational exposure to HIV. United States, 2016. Accessed March 6, 2017.
2. Cardo DM et al. New Engl J Med. 1997;337(21):1485-90.
3. Centers for Disease Control and Prevention. Preexposure prophylaxis for the prevention of HIV infection in the United States–2014: a clinical practice guideline. Accessed March 6, 2017.
VIDEO: Retinal infarctions get missed as stroke harbingers
LOS ANGELES – Retinal infarctions are often going missed as important red flags for future ischemic strokes.
Among U.S. Medicare beneficiaries older than 65 years who had a retinal infarction (RI), “only one-third underwent adequate stroke risk factor evaluation,” Alexander E. Merkler, MD, reported in a poster presented at the International Stroke Conference sponsored by the American Heart Association. And fewer than 10% underwent assessment by a neurologist, based on a review of 5,688 of these older Medicare beneficiaries who had a RI sometime during 2009-2015.
The high-risk profile of these patients was affirmed by a 1% ischemic stroke incidence during the 90 days following their RI diagnosis, a rate roughly fourfold higher than in similar patients without a recent RI.
“A lot of people don’t recognize that a retinal infarction is a type of stroke,” Dr. Merkler said in a video interview. To test this hypothesis, Dr. Merkler and his associates examined the follow-up run on elderly Medicare beneficiaries following a RI diagnosis.”The guidelines recommend evaluating why these patients had a stroke [a retinal infarction] and treating risk factors to reduce the risk of a future stroke,” said Dr. Merkler, a neurologist at Weill Cornell Medicine in New York.
The review showed that 34% of the RI patients underwent cervical carotid imaging, 29% had heart rhythm monitoring, 23% underwent echocardiography, and 8% had assessment by a neurologist.
Dr. Merkler had no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SOURCE: Merkler A et al. ISC 2018 Abstract TMP76 (Stroke. 2018 Jan;49[Suppl 1]:ATMP76).
LOS ANGELES – Retinal infarctions are often going missed as important red flags for future ischemic strokes.
Among U.S. Medicare beneficiaries older than 65 years who had a retinal infarction (RI), “only one-third underwent adequate stroke risk factor evaluation,” Alexander E. Merkler, MD, reported in a poster presented at the International Stroke Conference sponsored by the American Heart Association. And fewer than 10% underwent assessment by a neurologist, based on a review of 5,688 of these older Medicare beneficiaries who had a RI sometime during 2009-2015.
The high-risk profile of these patients was affirmed by a 1% ischemic stroke incidence during the 90 days following their RI diagnosis, a rate roughly fourfold higher than in similar patients without a recent RI.
“A lot of people don’t recognize that a retinal infarction is a type of stroke,” Dr. Merkler said in a video interview. To test this hypothesis, Dr. Merkler and his associates examined the follow-up run on elderly Medicare beneficiaries following a RI diagnosis.”The guidelines recommend evaluating why these patients had a stroke [a retinal infarction] and treating risk factors to reduce the risk of a future stroke,” said Dr. Merkler, a neurologist at Weill Cornell Medicine in New York.
The review showed that 34% of the RI patients underwent cervical carotid imaging, 29% had heart rhythm monitoring, 23% underwent echocardiography, and 8% had assessment by a neurologist.
Dr. Merkler had no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SOURCE: Merkler A et al. ISC 2018 Abstract TMP76 (Stroke. 2018 Jan;49[Suppl 1]:ATMP76).
LOS ANGELES – Retinal infarctions are often going missed as important red flags for future ischemic strokes.
Among U.S. Medicare beneficiaries older than 65 years who had a retinal infarction (RI), “only one-third underwent adequate stroke risk factor evaluation,” Alexander E. Merkler, MD, reported in a poster presented at the International Stroke Conference sponsored by the American Heart Association. And fewer than 10% underwent assessment by a neurologist, based on a review of 5,688 of these older Medicare beneficiaries who had a RI sometime during 2009-2015.
The high-risk profile of these patients was affirmed by a 1% ischemic stroke incidence during the 90 days following their RI diagnosis, a rate roughly fourfold higher than in similar patients without a recent RI.
“A lot of people don’t recognize that a retinal infarction is a type of stroke,” Dr. Merkler said in a video interview. To test this hypothesis, Dr. Merkler and his associates examined the follow-up run on elderly Medicare beneficiaries following a RI diagnosis.”The guidelines recommend evaluating why these patients had a stroke [a retinal infarction] and treating risk factors to reduce the risk of a future stroke,” said Dr. Merkler, a neurologist at Weill Cornell Medicine in New York.
The review showed that 34% of the RI patients underwent cervical carotid imaging, 29% had heart rhythm monitoring, 23% underwent echocardiography, and 8% had assessment by a neurologist.
Dr. Merkler had no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SOURCE: Merkler A et al. ISC 2018 Abstract TMP76 (Stroke. 2018 Jan;49[Suppl 1]:ATMP76).
REPORTING FROM ISC 2018
Key clinical point: Retinal infarction patients often fail to undergo stroke assessment.
Major finding: One-third of Medicare beneficiaries with retinal infarction received adequate evaluation for stroke risk factors.
Study details: Review of 5,688 Medicare patients with a retinal infarction during 2009-2015.
Disclosures: Dr. Merkler had no disclosures.
Source: Merkler A et al. ISC 2018 Abstract TMP76 (Stroke. 2018 Jan;49[Suppl 1]:ATMP76).
Smallpox Vaccine Complications: The Dermatologist’s Role in Diagnosis and Management
The practice of variolation, or inoculation of the smallpox virus from a pustule into a healthy person, was described as early as 1500
Immunization
Vaccinia is an orthopoxvirus, distinct from the smallpox virus variola, with cross-protective immunity after infection. The smallpox vaccine that is available today is a second-generation vaccinia virus derived from plaque purification cloning from the first-generation version originally licensed in 1932, which was central to eradication.5 Today’s vaccine is administered using a bifurcated needle to puncture the epidermis 15 times. Ideally, a papule forms at the inoculation site 3 to 5 days later, progresses to a vesicle and then a pustule, and finally crusts and reaches maximum size by day 10. The crust separates from the skin at 14 to 21 days, at which time the virus can no longer be isolated from the wound. United States Department of Defense surveillance of the first 450,000 vaccinated personnel noted 1% of recipients developed cutaneous eruptions beyond the vaccination site, 5% developed a localized rash, and 1% experienced a generalized eruption.2 Adverse reactions included generalized vaccinia, erythema multiforme (EM), autoinoculation (including ocular vaccinia), and contact vaccinia. There were no cases of eczema vaccinatum (EV) or progressive vaccinia (PV) reported, and no deaths were attributed to these initial vaccines.2
Immunologic Response
Vaccinia replicates in keratinocytes, spreading from cell to cell, resulting in necrosis and vesicle formation. Components of both cellular and humoral immune responses are in place by 10 days after immunization. Deficiencies in these responses result in vaccine complications secondary to vaccine escape and replication beyond the inoculation site.6 A helper T cell TH2-predominant cytokine response in atopic individuals is the likely pathogenesis required for the rapid viral spread for EV.7 Similarly, patients with cell-mediated immunity deficiencies cannot sufficiently produce enough cytotoxic T cells to eliminate an established infection, which can result in PV. Despite the effectiveness of intravenous vaccinia immunoglobulins (VIGIVs) when administered to patients with certain vaccine complications, observations that children with severe X-linked agammaglobulinemia (Bruton disease) have normal responses to vaccination suggest that antibody production is least important in viral control.8 Simian models also suggest that B-cell depletion has no impact on lesion dissemination, as lesion size is inversely correlated with T-cell count.9
Eczema Vaccinatum
A national survey estimated the prevalence of eczema in the United States at 31.6 million individuals,10 with 2- to 3-fold increases in incidence since the 1970s.11 Due to the risk for developing EV, the Advisory Committee on Immunization Practices considers personal history of eczema or contact with a family member who has eczema (either currently or in the past) contraindications to nonemergency administration of the vaccine.12,13 However, atopic conditions in general are underrecognized, with only approximately one-third of patients carrying an official diagnosis from a physician.10 Despite a large atopic and vaccinated population, EV remains relatively uncommon at 10 to 39 cases per million vaccines.6
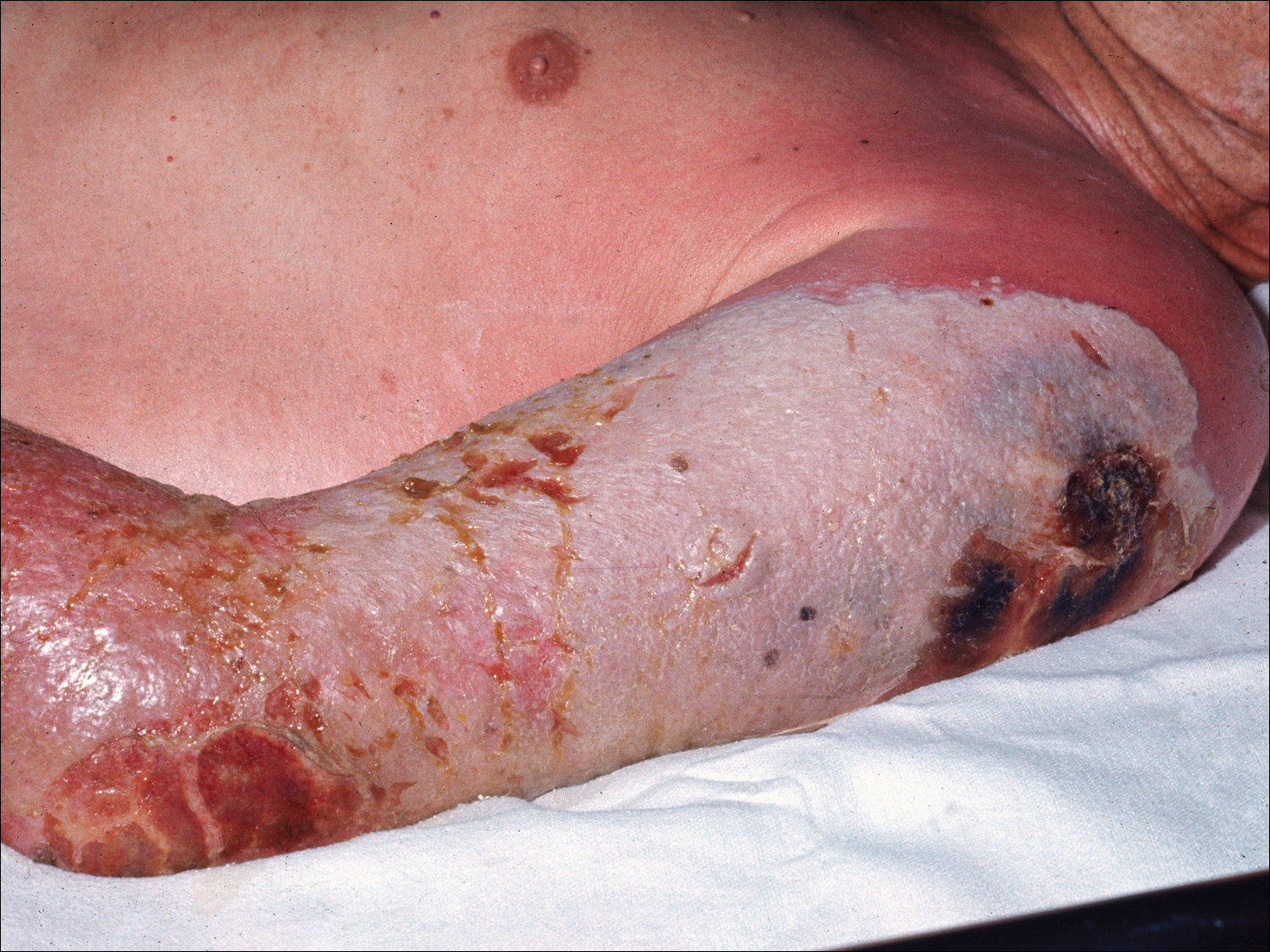
The EV rash classically involves the midface, neck, and antecubital and popliteal fossae but can present in any location. The lesions start as papules that quickly progress to vesicles and pustules with crusting on an erythematous base. Given the extent of denudation of the epidermis, impetiginization can occur. Death rates as high as 30% have been reported14 but have only occurred in instances of secondary contact transmission with no deaths occurring in the primary vaccinees.15 In a case published in 2008, a 2-year-old boy developed the first documented EV case under the new program after exposure to his father’s predeployment vaccine.16 A similar rash is shown in Figure 1 with notable vesicles and pustules. The child required burn patient–type management, VIGIV, and treatment with cidofovir and an investigational antiorthopox agent. He was discharged from the hospital after 48 days without sequelae or considerable scarring.16 If a family member has a contraindication barring secondary contact with the vaccine, the US Department of Defense’s policy defers vaccination in active-duty members until they reach their deployment destination, at which point the inoculation is administered.
Progressive Vaccinia
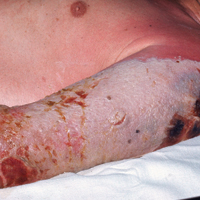
Progressive vaccinia is also known as vaccinia necrosum or vaccinia gangrenosum. It is a dreaded but uncommon complication, occurring once in every 1 million vaccinations. It carries an overall case fatality rate of 15%,17 but it nearly always is fatal in patients with severe T-cell defects.18 Progressive vaccinia occurs exclusively in patients with cell-mediated immunodeficiency, with the severity of the acute illness correlating with the severity of immunodeficiency. In patients with cell-mediated immunodeficiency but intact humoral immunity, progression can be limited to expansion of the lesion, as it is thought that antibody production restricts viremia.18 Progressive vaccinia should be suspected in a patient if the vaccine site shows no signs of improvement by 14 days.19 The PV lesions do not heal and may progress or recur in patients with signs of prior healing. The leading edge has confluent vesicles, and the center of the lesion develops necrosis with thick black eschar formation. Most specifically, there is no surrounding inflammation; however, inflammation can develop later as a response to treatment or secondary infection. Figure 2 shows a PV lesion with black eschar and a transition to intact dermis without inflammation.
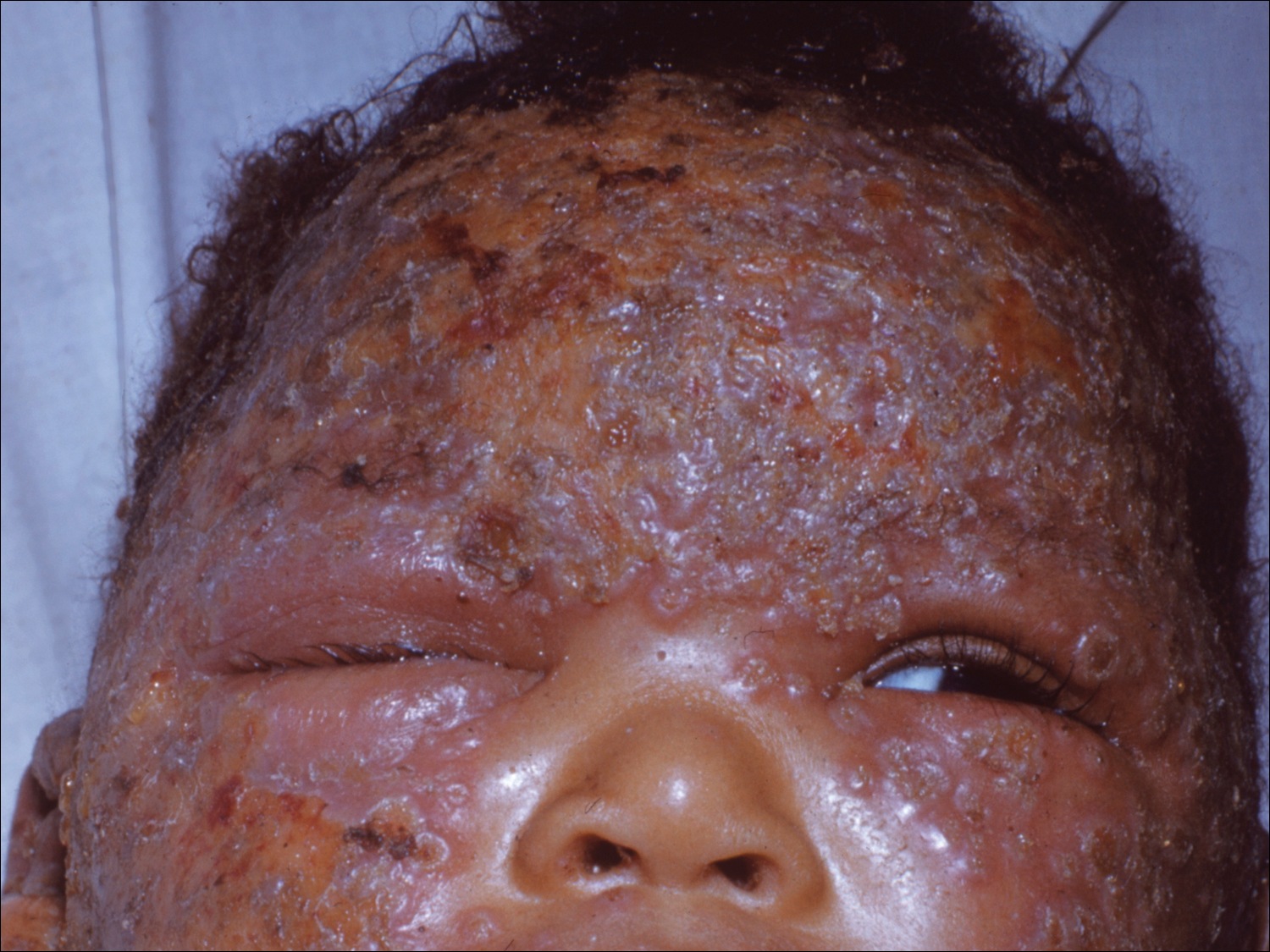
The first known case of PV since the 1960s vaccination campaign occurred in an active-duty Marine vaccinated with vaccinia before a diagnosis of acute myelogenous leukemia was recognized 2 weeks later.19 The vaccine site was stable in size and crusted when he received neutropenia-inducing chemotherapy 6.5 weeks after vaccination. The site then progressed in a manner typical for PV with central necrosis and a lack of inflammation at the expanding painless wound edge.19 This classic appearance with progression of satellite lesions prompted the treatment team to obtain wound and serum samples, which yielded the orthopox virus from polymerase chain reaction and viral culture. He required 2 months of care in an intensive care unit and received treatment with topical imiquimod, VIGIV, a topical and intravenous antiorthopox agent, and a second investigational antiorthopox agent; the patient ultimately survived.17,20
Generalized Vaccinia
Generalized vaccinia (GV) typically is a benign vaccine complication resulting from viremic spread from the initial inoculation site and is most commonly seen in healthy patients. Generalized vaccinia is only life threatening in immunocompromised patients. The incidence of GV is 23.4 to 241.5 patients per million vaccines.6 The majority of GV cases occur 5 to 12 days after vaccination when small distant pustules or vesicles appear on any part of the body, including the palms and soles. The lesions usually are smaller than the primary vaccination site and resolve more quickly. Generalized vaccinia can have a few to several hundred pocks, though the rash is rarely as diffuse as EV presentations.3 Given that EV can present diffusely on skin unaffected by atopic dermatitis, GV can be difficult to distinguish from EV. Features more common to EV include more systemically ill patients, increased numbers of lesions, and lesions that become confluent in an atopic distribution. It has been suggested that GV can be differentiated from vesicular or vesiculopapular EM because GV does not develop flaccid bullae and EM typically has targetoid lesions.18 Mild GV disease requires no treatment, but VIGIV can be used in more extensive cases.
Localized Reactions Due to Viral Replication
Accidental autoinoculation can occur when patients touch the vaccination site and then themselves, transferring virus particles to areas of compromised skin integrity, most commonly on the face, eyes, hands, genitalia, anus, or any other broken skin. Autoinoculation happens with some frequency and is of limited clinical concern unless there is ocular involvement. Keratitis develops in 6% of ocular vaccinia cases, and VIGIV is contraindicated, as rabbit models suggest that antigen-antibody precipitates in the cornea can cause scarring.21 Instead, trifluorothymidine is an effective topical treatment available for ocular vaccinia.
A robust response or “take” is defined as a reaction having redness, swelling, and warmth more than 3 inches in diameter at the inoculation site, peaking 6 to 12 days after inoculation with spontaneous regression occurring 1 to 3 days after.22,23 A robust take frequently is of concern to the clinician, as it can be difficult to discern from secondary infection. Secondary infections are uncommon, and a robust take is secondary to viral, not bacterial, cellulitis. Unfortunately, there are no diagnostics that have utility in distinguishing between the two, and the decision to administer empiric antibiotics might be unavoidable in light of the consequences of an untreated, rapidly progressive bacterial cellulitis. Milder cases in the setting of no constitutional symptoms could be safely monitored if close follow-up is assured.
Generalized Skin Reactions Without Viral Replication
Development of erythematous, pruritic, urticarial, and diffuse targetlike lesions of EM is common in first-time vaccinees. Often misdiagnosed as GV, EM is an immunologically mediated, not virally mediated, process. The most common infectious cause prompting EM is herpes simplex virus type 1. In the setting of a live-virus vaccine, it is difficult to determine if the vaccine prompted herpes simplex virus type 1 viral shedding and associated EM or if the vaccinia vaccine is more directly the cause of EM.24 Symptoms typically are mild, but more severe reactions may require treatment with corticosteroids. Stevens-Johnson syndrome with a severe bullous eruption has been linked to vaccinia24 but fortunately is rare. Morbilliform eruptions, urticaria, and angioedema also can occur.
Final Thoughts
Given current world events and ongoing bioterrorism threats, the smallpox vaccine program continues indefinitely. With a brisk military deployment tempo, a larger population of new vaccinees naturally will yield more cutaneous reactions. Military members, civilian health care workers, and members of the National Guard and National Reserves will develop complications and present to dermatologists for care. The historical pool of providers accustomed to seeing these complications from the 1960s eradication campaign is scant. Military and civilian dermatologists alike are uniquely poised to be the experts on protean manifestations of vaccinia reactions.
- Voigt EA, Kennedy RB, Poland GA. Defending against smallpox: a focus on vaccines. Expert Rev Vaccines. 2016;15:1197-1211.
- Grabenstein J, Wikenwerder W Jr. US military smallpox vaccination program experience. JAMA. 2003;289:3278-3282.
- Kelly CD, Egan C, Davis SW, et al. Laboratory confirmation of generalized vaccinia following smallpox vaccination. J Clin Microbiol. 2004;42:1373-1375.
- Slike BM, Creegan M, Marovich M, et al. Humoral immunity to primary smallpox vaccination: impact of childhood versus adult immunization on vaccinia vector vaccine development in military populations. PLoS One. 2017;12:E0169247.
- Notice to readers: newly licensed vaccine to replace old smallpox vaccine. MMWR. 2008;57:207-208.
- Bray M. Pathogenesis and potential antiviral therapy of complications of smallpox vaccination. Antiviral Res. 2003;58:101-114.
- Engler R, Kenner J, Leung D. Smallpox vaccination: risk considerations for patients with atopic dermatitis. J Allergy Clin Immunol. 2002;110:357-365.
- Bray M, Wright ME. Progressive vaccinia. Clin Infect Dis. 2003;36:766-774.
- Gordon S, Cecchinato V, Andresen V, et al. Smallpox vaccine safety is dependent on T cells and not B cells. J Infect Dis. 2011;203:1043-1053.
- Hanifin J, Reed M. A population-based survey of eczema prevalence in the United States. Dermatitis. 2007;82:82-91.
- Avena-Woods C. Overview of atopic dermatitis. Am J Manag Care. 2017;23(8 suppl):S115-S123.
- Wharton M, Strikas RA, Harpaz R, et al; Advisory Committee on Immunization Practices; Healthcare Infection Control Practices Advisory Committee. Recommendations for using smallpox vaccine in a pre-event vaccination program. Supplemental recommendations of the Advisory Committee on Immunization Practices (ACIP) and the Healthcare Infection Control Practices Advisory Committee (HICPAC). MMWR Recomm Rep. 2003;52:1-16.
- Petersen BW, Harms TJ, Reynolds MG, et al. Use of vaccinia virus smallpox vaccine in laboratory and health care personnel at risk for occupation exposure to orthopoxviruses—recommendations of the Advisory Committee on Immunizations Practices (ACIP), 2015. MMWR Morb Mortal Wkly Rep. 2016;65:257-262.
- Nell P, Kohl KS, Graham PL, et al; Brighton Collaboration Vaccinia Virus Vaccine Adverse Event Working Group for Eczema Vaccinatum. Eczema vaccinatum as an adverse event following exposure to vaccinia virus: case definition and guidelines of data collection analysis, and presentation of immunization safety data. Vaccine. 2007:25;5725-5734.
- Aragón TJ, Ulrich S, Fernyak S, et al. Risks of serious complications and death from smallpox vaccination: a systematic review of the United States experience, 1963-1968. BMC Public Health. 2003;3:26.
- Vora S, Damon I, Fulginiti V, et al. Severe eczema vaccinatum in a household contact of a smallpox vaccinee. Clin Infect Dis. 2008;46:1555-1561.
- Centers for Disease Control and Prevention (CDC). Progressive vaccinia in a military smallpox vaccinee—United States 2009. MMWR Morb Mortal Wkly Rep. 2009;58:532-536.
- Fulginiti VA, Papier A, Lane M, et al. Smallpox vaccination: a review, part II. adverse events. Clin Infect Dis. 2003;37:251-271.
- Nell P, Kohl KS, Graham PL, et al; Brighton Collaboration Vaccinia Virus Vaccine Adverse Event Working Group for Progressive Vaccinia. Progressive vaccinia as an adverse event following exposure to vaccinia virus: case definition and guidelines of data collection, analysis, and presentation of immunization safety data. Vaccine. 2007;25:5735-5744.
- Lederman ER, Davidson W, Groff HL, et al. Progressive vaccinia: case description and laboratory-guided therapy with vaccinia immune globulin, ST-246, and CMX001. J Infect Dis. 2012;206:E1372-E1385.
- Lane ML, Goldstein J. Adverse events occurring after smallpox vaccination. Semin Ped Infect Dis. 2003;14:189-195.
- Vaccine adverse events. CDC website. http://www.cdc.gov/smallpox/clinicians/vaccine-adverse-events5.html. Accessed January 3, 2018.
- Cono J, Casey CG, Bell DM. Smallpox vaccination and adversereactions, guidance for clinicians. CDC website. http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5204a1.htm. Accessed January 3, 2018.
- Rosenblatt AE, Stein SL. Cutaneous reactions to vaccinations. Clin Dermatol. 2015;33:327-332.
The practice of variolation, or inoculation of the smallpox virus from a pustule into a healthy person, was described as early as 1500
Immunization
Vaccinia is an orthopoxvirus, distinct from the smallpox virus variola, with cross-protective immunity after infection. The smallpox vaccine that is available today is a second-generation vaccinia virus derived from plaque purification cloning from the first-generation version originally licensed in 1932, which was central to eradication.5 Today’s vaccine is administered using a bifurcated needle to puncture the epidermis 15 times. Ideally, a papule forms at the inoculation site 3 to 5 days later, progresses to a vesicle and then a pustule, and finally crusts and reaches maximum size by day 10. The crust separates from the skin at 14 to 21 days, at which time the virus can no longer be isolated from the wound. United States Department of Defense surveillance of the first 450,000 vaccinated personnel noted 1% of recipients developed cutaneous eruptions beyond the vaccination site, 5% developed a localized rash, and 1% experienced a generalized eruption.2 Adverse reactions included generalized vaccinia, erythema multiforme (EM), autoinoculation (including ocular vaccinia), and contact vaccinia. There were no cases of eczema vaccinatum (EV) or progressive vaccinia (PV) reported, and no deaths were attributed to these initial vaccines.2
Immunologic Response
Vaccinia replicates in keratinocytes, spreading from cell to cell, resulting in necrosis and vesicle formation. Components of both cellular and humoral immune responses are in place by 10 days after immunization. Deficiencies in these responses result in vaccine complications secondary to vaccine escape and replication beyond the inoculation site.6 A helper T cell TH2-predominant cytokine response in atopic individuals is the likely pathogenesis required for the rapid viral spread for EV.7 Similarly, patients with cell-mediated immunity deficiencies cannot sufficiently produce enough cytotoxic T cells to eliminate an established infection, which can result in PV. Despite the effectiveness of intravenous vaccinia immunoglobulins (VIGIVs) when administered to patients with certain vaccine complications, observations that children with severe X-linked agammaglobulinemia (Bruton disease) have normal responses to vaccination suggest that antibody production is least important in viral control.8 Simian models also suggest that B-cell depletion has no impact on lesion dissemination, as lesion size is inversely correlated with T-cell count.9
Eczema Vaccinatum
A national survey estimated the prevalence of eczema in the United States at 31.6 million individuals,10 with 2- to 3-fold increases in incidence since the 1970s.11 Due to the risk for developing EV, the Advisory Committee on Immunization Practices considers personal history of eczema or contact with a family member who has eczema (either currently or in the past) contraindications to nonemergency administration of the vaccine.12,13 However, atopic conditions in general are underrecognized, with only approximately one-third of patients carrying an official diagnosis from a physician.10 Despite a large atopic and vaccinated population, EV remains relatively uncommon at 10 to 39 cases per million vaccines.6
The EV rash classically involves the midface, neck, and antecubital and popliteal fossae but can present in any location. The lesions start as papules that quickly progress to vesicles and pustules with crusting on an erythematous base. Given the extent of denudation of the epidermis, impetiginization can occur. Death rates as high as 30% have been reported14 but have only occurred in instances of secondary contact transmission with no deaths occurring in the primary vaccinees.15 In a case published in 2008, a 2-year-old boy developed the first documented EV case under the new program after exposure to his father’s predeployment vaccine.16 A similar rash is shown in Figure 1 with notable vesicles and pustules. The child required burn patient–type management, VIGIV, and treatment with cidofovir and an investigational antiorthopox agent. He was discharged from the hospital after 48 days without sequelae or considerable scarring.16 If a family member has a contraindication barring secondary contact with the vaccine, the US Department of Defense’s policy defers vaccination in active-duty members until they reach their deployment destination, at which point the inoculation is administered.

Progressive Vaccinia
Progressive vaccinia is also known as vaccinia necrosum or vaccinia gangrenosum. It is a dreaded but uncommon complication, occurring once in every 1 million vaccinations. It carries an overall case fatality rate of 15%,17 but it nearly always is fatal in patients with severe T-cell defects.18 Progressive vaccinia occurs exclusively in patients with cell-mediated immunodeficiency, with the severity of the acute illness correlating with the severity of immunodeficiency. In patients with cell-mediated immunodeficiency but intact humoral immunity, progression can be limited to expansion of the lesion, as it is thought that antibody production restricts viremia.18 Progressive vaccinia should be suspected in a patient if the vaccine site shows no signs of improvement by 14 days.19 The PV lesions do not heal and may progress or recur in patients with signs of prior healing. The leading edge has confluent vesicles, and the center of the lesion develops necrosis with thick black eschar formation. Most specifically, there is no surrounding inflammation; however, inflammation can develop later as a response to treatment or secondary infection. Figure 2 shows a PV lesion with black eschar and a transition to intact dermis without inflammation.

The first known case of PV since the 1960s vaccination campaign occurred in an active-duty Marine vaccinated with vaccinia before a diagnosis of acute myelogenous leukemia was recognized 2 weeks later.19 The vaccine site was stable in size and crusted when he received neutropenia-inducing chemotherapy 6.5 weeks after vaccination. The site then progressed in a manner typical for PV with central necrosis and a lack of inflammation at the expanding painless wound edge.19 This classic appearance with progression of satellite lesions prompted the treatment team to obtain wound and serum samples, which yielded the orthopox virus from polymerase chain reaction and viral culture. He required 2 months of care in an intensive care unit and received treatment with topical imiquimod, VIGIV, a topical and intravenous antiorthopox agent, and a second investigational antiorthopox agent; the patient ultimately survived.17,20
Generalized Vaccinia
Generalized vaccinia (GV) typically is a benign vaccine complication resulting from viremic spread from the initial inoculation site and is most commonly seen in healthy patients. Generalized vaccinia is only life threatening in immunocompromised patients. The incidence of GV is 23.4 to 241.5 patients per million vaccines.6 The majority of GV cases occur 5 to 12 days after vaccination when small distant pustules or vesicles appear on any part of the body, including the palms and soles. The lesions usually are smaller than the primary vaccination site and resolve more quickly. Generalized vaccinia can have a few to several hundred pocks, though the rash is rarely as diffuse as EV presentations.3 Given that EV can present diffusely on skin unaffected by atopic dermatitis, GV can be difficult to distinguish from EV. Features more common to EV include more systemically ill patients, increased numbers of lesions, and lesions that become confluent in an atopic distribution. It has been suggested that GV can be differentiated from vesicular or vesiculopapular EM because GV does not develop flaccid bullae and EM typically has targetoid lesions.18 Mild GV disease requires no treatment, but VIGIV can be used in more extensive cases.
Localized Reactions Due to Viral Replication
Accidental autoinoculation can occur when patients touch the vaccination site and then themselves, transferring virus particles to areas of compromised skin integrity, most commonly on the face, eyes, hands, genitalia, anus, or any other broken skin. Autoinoculation happens with some frequency and is of limited clinical concern unless there is ocular involvement. Keratitis develops in 6% of ocular vaccinia cases, and VIGIV is contraindicated, as rabbit models suggest that antigen-antibody precipitates in the cornea can cause scarring.21 Instead, trifluorothymidine is an effective topical treatment available for ocular vaccinia.
A robust response or “take” is defined as a reaction having redness, swelling, and warmth more than 3 inches in diameter at the inoculation site, peaking 6 to 12 days after inoculation with spontaneous regression occurring 1 to 3 days after.22,23 A robust take frequently is of concern to the clinician, as it can be difficult to discern from secondary infection. Secondary infections are uncommon, and a robust take is secondary to viral, not bacterial, cellulitis. Unfortunately, there are no diagnostics that have utility in distinguishing between the two, and the decision to administer empiric antibiotics might be unavoidable in light of the consequences of an untreated, rapidly progressive bacterial cellulitis. Milder cases in the setting of no constitutional symptoms could be safely monitored if close follow-up is assured.
Generalized Skin Reactions Without Viral Replication
Development of erythematous, pruritic, urticarial, and diffuse targetlike lesions of EM is common in first-time vaccinees. Often misdiagnosed as GV, EM is an immunologically mediated, not virally mediated, process. The most common infectious cause prompting EM is herpes simplex virus type 1. In the setting of a live-virus vaccine, it is difficult to determine if the vaccine prompted herpes simplex virus type 1 viral shedding and associated EM or if the vaccinia vaccine is more directly the cause of EM.24 Symptoms typically are mild, but more severe reactions may require treatment with corticosteroids. Stevens-Johnson syndrome with a severe bullous eruption has been linked to vaccinia24 but fortunately is rare. Morbilliform eruptions, urticaria, and angioedema also can occur.
Final Thoughts
Given current world events and ongoing bioterrorism threats, the smallpox vaccine program continues indefinitely. With a brisk military deployment tempo, a larger population of new vaccinees naturally will yield more cutaneous reactions. Military members, civilian health care workers, and members of the National Guard and National Reserves will develop complications and present to dermatologists for care. The historical pool of providers accustomed to seeing these complications from the 1960s eradication campaign is scant. Military and civilian dermatologists alike are uniquely poised to be the experts on protean manifestations of vaccinia reactions.
The practice of variolation, or inoculation of the smallpox virus from a pustule into a healthy person, was described as early as 1500
Immunization
Vaccinia is an orthopoxvirus, distinct from the smallpox virus variola, with cross-protective immunity after infection. The smallpox vaccine that is available today is a second-generation vaccinia virus derived from plaque purification cloning from the first-generation version originally licensed in 1932, which was central to eradication.5 Today’s vaccine is administered using a bifurcated needle to puncture the epidermis 15 times. Ideally, a papule forms at the inoculation site 3 to 5 days later, progresses to a vesicle and then a pustule, and finally crusts and reaches maximum size by day 10. The crust separates from the skin at 14 to 21 days, at which time the virus can no longer be isolated from the wound. United States Department of Defense surveillance of the first 450,000 vaccinated personnel noted 1% of recipients developed cutaneous eruptions beyond the vaccination site, 5% developed a localized rash, and 1% experienced a generalized eruption.2 Adverse reactions included generalized vaccinia, erythema multiforme (EM), autoinoculation (including ocular vaccinia), and contact vaccinia. There were no cases of eczema vaccinatum (EV) or progressive vaccinia (PV) reported, and no deaths were attributed to these initial vaccines.2
Immunologic Response
Vaccinia replicates in keratinocytes, spreading from cell to cell, resulting in necrosis and vesicle formation. Components of both cellular and humoral immune responses are in place by 10 days after immunization. Deficiencies in these responses result in vaccine complications secondary to vaccine escape and replication beyond the inoculation site.6 A helper T cell TH2-predominant cytokine response in atopic individuals is the likely pathogenesis required for the rapid viral spread for EV.7 Similarly, patients with cell-mediated immunity deficiencies cannot sufficiently produce enough cytotoxic T cells to eliminate an established infection, which can result in PV. Despite the effectiveness of intravenous vaccinia immunoglobulins (VIGIVs) when administered to patients with certain vaccine complications, observations that children with severe X-linked agammaglobulinemia (Bruton disease) have normal responses to vaccination suggest that antibody production is least important in viral control.8 Simian models also suggest that B-cell depletion has no impact on lesion dissemination, as lesion size is inversely correlated with T-cell count.9
Eczema Vaccinatum
A national survey estimated the prevalence of eczema in the United States at 31.6 million individuals,10 with 2- to 3-fold increases in incidence since the 1970s.11 Due to the risk for developing EV, the Advisory Committee on Immunization Practices considers personal history of eczema or contact with a family member who has eczema (either currently or in the past) contraindications to nonemergency administration of the vaccine.12,13 However, atopic conditions in general are underrecognized, with only approximately one-third of patients carrying an official diagnosis from a physician.10 Despite a large atopic and vaccinated population, EV remains relatively uncommon at 10 to 39 cases per million vaccines.6
The EV rash classically involves the midface, neck, and antecubital and popliteal fossae but can present in any location. The lesions start as papules that quickly progress to vesicles and pustules with crusting on an erythematous base. Given the extent of denudation of the epidermis, impetiginization can occur. Death rates as high as 30% have been reported14 but have only occurred in instances of secondary contact transmission with no deaths occurring in the primary vaccinees.15 In a case published in 2008, a 2-year-old boy developed the first documented EV case under the new program after exposure to his father’s predeployment vaccine.16 A similar rash is shown in Figure 1 with notable vesicles and pustules. The child required burn patient–type management, VIGIV, and treatment with cidofovir and an investigational antiorthopox agent. He was discharged from the hospital after 48 days without sequelae or considerable scarring.16 If a family member has a contraindication barring secondary contact with the vaccine, the US Department of Defense’s policy defers vaccination in active-duty members until they reach their deployment destination, at which point the inoculation is administered.

Progressive Vaccinia
Progressive vaccinia is also known as vaccinia necrosum or vaccinia gangrenosum. It is a dreaded but uncommon complication, occurring once in every 1 million vaccinations. It carries an overall case fatality rate of 15%,17 but it nearly always is fatal in patients with severe T-cell defects.18 Progressive vaccinia occurs exclusively in patients with cell-mediated immunodeficiency, with the severity of the acute illness correlating with the severity of immunodeficiency. In patients with cell-mediated immunodeficiency but intact humoral immunity, progression can be limited to expansion of the lesion, as it is thought that antibody production restricts viremia.18 Progressive vaccinia should be suspected in a patient if the vaccine site shows no signs of improvement by 14 days.19 The PV lesions do not heal and may progress or recur in patients with signs of prior healing. The leading edge has confluent vesicles, and the center of the lesion develops necrosis with thick black eschar formation. Most specifically, there is no surrounding inflammation; however, inflammation can develop later as a response to treatment or secondary infection. Figure 2 shows a PV lesion with black eschar and a transition to intact dermis without inflammation.

The first known case of PV since the 1960s vaccination campaign occurred in an active-duty Marine vaccinated with vaccinia before a diagnosis of acute myelogenous leukemia was recognized 2 weeks later.19 The vaccine site was stable in size and crusted when he received neutropenia-inducing chemotherapy 6.5 weeks after vaccination. The site then progressed in a manner typical for PV with central necrosis and a lack of inflammation at the expanding painless wound edge.19 This classic appearance with progression of satellite lesions prompted the treatment team to obtain wound and serum samples, which yielded the orthopox virus from polymerase chain reaction and viral culture. He required 2 months of care in an intensive care unit and received treatment with topical imiquimod, VIGIV, a topical and intravenous antiorthopox agent, and a second investigational antiorthopox agent; the patient ultimately survived.17,20
Generalized Vaccinia
Generalized vaccinia (GV) typically is a benign vaccine complication resulting from viremic spread from the initial inoculation site and is most commonly seen in healthy patients. Generalized vaccinia is only life threatening in immunocompromised patients. The incidence of GV is 23.4 to 241.5 patients per million vaccines.6 The majority of GV cases occur 5 to 12 days after vaccination when small distant pustules or vesicles appear on any part of the body, including the palms and soles. The lesions usually are smaller than the primary vaccination site and resolve more quickly. Generalized vaccinia can have a few to several hundred pocks, though the rash is rarely as diffuse as EV presentations.3 Given that EV can present diffusely on skin unaffected by atopic dermatitis, GV can be difficult to distinguish from EV. Features more common to EV include more systemically ill patients, increased numbers of lesions, and lesions that become confluent in an atopic distribution. It has been suggested that GV can be differentiated from vesicular or vesiculopapular EM because GV does not develop flaccid bullae and EM typically has targetoid lesions.18 Mild GV disease requires no treatment, but VIGIV can be used in more extensive cases.
Localized Reactions Due to Viral Replication
Accidental autoinoculation can occur when patients touch the vaccination site and then themselves, transferring virus particles to areas of compromised skin integrity, most commonly on the face, eyes, hands, genitalia, anus, or any other broken skin. Autoinoculation happens with some frequency and is of limited clinical concern unless there is ocular involvement. Keratitis develops in 6% of ocular vaccinia cases, and VIGIV is contraindicated, as rabbit models suggest that antigen-antibody precipitates in the cornea can cause scarring.21 Instead, trifluorothymidine is an effective topical treatment available for ocular vaccinia.
A robust response or “take” is defined as a reaction having redness, swelling, and warmth more than 3 inches in diameter at the inoculation site, peaking 6 to 12 days after inoculation with spontaneous regression occurring 1 to 3 days after.22,23 A robust take frequently is of concern to the clinician, as it can be difficult to discern from secondary infection. Secondary infections are uncommon, and a robust take is secondary to viral, not bacterial, cellulitis. Unfortunately, there are no diagnostics that have utility in distinguishing between the two, and the decision to administer empiric antibiotics might be unavoidable in light of the consequences of an untreated, rapidly progressive bacterial cellulitis. Milder cases in the setting of no constitutional symptoms could be safely monitored if close follow-up is assured.
Generalized Skin Reactions Without Viral Replication
Development of erythematous, pruritic, urticarial, and diffuse targetlike lesions of EM is common in first-time vaccinees. Often misdiagnosed as GV, EM is an immunologically mediated, not virally mediated, process. The most common infectious cause prompting EM is herpes simplex virus type 1. In the setting of a live-virus vaccine, it is difficult to determine if the vaccine prompted herpes simplex virus type 1 viral shedding and associated EM or if the vaccinia vaccine is more directly the cause of EM.24 Symptoms typically are mild, but more severe reactions may require treatment with corticosteroids. Stevens-Johnson syndrome with a severe bullous eruption has been linked to vaccinia24 but fortunately is rare. Morbilliform eruptions, urticaria, and angioedema also can occur.
Final Thoughts
Given current world events and ongoing bioterrorism threats, the smallpox vaccine program continues indefinitely. With a brisk military deployment tempo, a larger population of new vaccinees naturally will yield more cutaneous reactions. Military members, civilian health care workers, and members of the National Guard and National Reserves will develop complications and present to dermatologists for care. The historical pool of providers accustomed to seeing these complications from the 1960s eradication campaign is scant. Military and civilian dermatologists alike are uniquely poised to be the experts on protean manifestations of vaccinia reactions.
- Voigt EA, Kennedy RB, Poland GA. Defending against smallpox: a focus on vaccines. Expert Rev Vaccines. 2016;15:1197-1211.
- Grabenstein J, Wikenwerder W Jr. US military smallpox vaccination program experience. JAMA. 2003;289:3278-3282.
- Kelly CD, Egan C, Davis SW, et al. Laboratory confirmation of generalized vaccinia following smallpox vaccination. J Clin Microbiol. 2004;42:1373-1375.
- Slike BM, Creegan M, Marovich M, et al. Humoral immunity to primary smallpox vaccination: impact of childhood versus adult immunization on vaccinia vector vaccine development in military populations. PLoS One. 2017;12:E0169247.
- Notice to readers: newly licensed vaccine to replace old smallpox vaccine. MMWR. 2008;57:207-208.
- Bray M. Pathogenesis and potential antiviral therapy of complications of smallpox vaccination. Antiviral Res. 2003;58:101-114.
- Engler R, Kenner J, Leung D. Smallpox vaccination: risk considerations for patients with atopic dermatitis. J Allergy Clin Immunol. 2002;110:357-365.
- Bray M, Wright ME. Progressive vaccinia. Clin Infect Dis. 2003;36:766-774.
- Gordon S, Cecchinato V, Andresen V, et al. Smallpox vaccine safety is dependent on T cells and not B cells. J Infect Dis. 2011;203:1043-1053.
- Hanifin J, Reed M. A population-based survey of eczema prevalence in the United States. Dermatitis. 2007;82:82-91.
- Avena-Woods C. Overview of atopic dermatitis. Am J Manag Care. 2017;23(8 suppl):S115-S123.
- Wharton M, Strikas RA, Harpaz R, et al; Advisory Committee on Immunization Practices; Healthcare Infection Control Practices Advisory Committee. Recommendations for using smallpox vaccine in a pre-event vaccination program. Supplemental recommendations of the Advisory Committee on Immunization Practices (ACIP) and the Healthcare Infection Control Practices Advisory Committee (HICPAC). MMWR Recomm Rep. 2003;52:1-16.
- Petersen BW, Harms TJ, Reynolds MG, et al. Use of vaccinia virus smallpox vaccine in laboratory and health care personnel at risk for occupation exposure to orthopoxviruses—recommendations of the Advisory Committee on Immunizations Practices (ACIP), 2015. MMWR Morb Mortal Wkly Rep. 2016;65:257-262.
- Nell P, Kohl KS, Graham PL, et al; Brighton Collaboration Vaccinia Virus Vaccine Adverse Event Working Group for Eczema Vaccinatum. Eczema vaccinatum as an adverse event following exposure to vaccinia virus: case definition and guidelines of data collection analysis, and presentation of immunization safety data. Vaccine. 2007:25;5725-5734.
- Aragón TJ, Ulrich S, Fernyak S, et al. Risks of serious complications and death from smallpox vaccination: a systematic review of the United States experience, 1963-1968. BMC Public Health. 2003;3:26.
- Vora S, Damon I, Fulginiti V, et al. Severe eczema vaccinatum in a household contact of a smallpox vaccinee. Clin Infect Dis. 2008;46:1555-1561.
- Centers for Disease Control and Prevention (CDC). Progressive vaccinia in a military smallpox vaccinee—United States 2009. MMWR Morb Mortal Wkly Rep. 2009;58:532-536.
- Fulginiti VA, Papier A, Lane M, et al. Smallpox vaccination: a review, part II. adverse events. Clin Infect Dis. 2003;37:251-271.
- Nell P, Kohl KS, Graham PL, et al; Brighton Collaboration Vaccinia Virus Vaccine Adverse Event Working Group for Progressive Vaccinia. Progressive vaccinia as an adverse event following exposure to vaccinia virus: case definition and guidelines of data collection, analysis, and presentation of immunization safety data. Vaccine. 2007;25:5735-5744.
- Lederman ER, Davidson W, Groff HL, et al. Progressive vaccinia: case description and laboratory-guided therapy with vaccinia immune globulin, ST-246, and CMX001. J Infect Dis. 2012;206:E1372-E1385.
- Lane ML, Goldstein J. Adverse events occurring after smallpox vaccination. Semin Ped Infect Dis. 2003;14:189-195.
- Vaccine adverse events. CDC website. http://www.cdc.gov/smallpox/clinicians/vaccine-adverse-events5.html. Accessed January 3, 2018.
- Cono J, Casey CG, Bell DM. Smallpox vaccination and adversereactions, guidance for clinicians. CDC website. http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5204a1.htm. Accessed January 3, 2018.
- Rosenblatt AE, Stein SL. Cutaneous reactions to vaccinations. Clin Dermatol. 2015;33:327-332.
- Voigt EA, Kennedy RB, Poland GA. Defending against smallpox: a focus on vaccines. Expert Rev Vaccines. 2016;15:1197-1211.
- Grabenstein J, Wikenwerder W Jr. US military smallpox vaccination program experience. JAMA. 2003;289:3278-3282.
- Kelly CD, Egan C, Davis SW, et al. Laboratory confirmation of generalized vaccinia following smallpox vaccination. J Clin Microbiol. 2004;42:1373-1375.
- Slike BM, Creegan M, Marovich M, et al. Humoral immunity to primary smallpox vaccination: impact of childhood versus adult immunization on vaccinia vector vaccine development in military populations. PLoS One. 2017;12:E0169247.
- Notice to readers: newly licensed vaccine to replace old smallpox vaccine. MMWR. 2008;57:207-208.
- Bray M. Pathogenesis and potential antiviral therapy of complications of smallpox vaccination. Antiviral Res. 2003;58:101-114.
- Engler R, Kenner J, Leung D. Smallpox vaccination: risk considerations for patients with atopic dermatitis. J Allergy Clin Immunol. 2002;110:357-365.
- Bray M, Wright ME. Progressive vaccinia. Clin Infect Dis. 2003;36:766-774.
- Gordon S, Cecchinato V, Andresen V, et al. Smallpox vaccine safety is dependent on T cells and not B cells. J Infect Dis. 2011;203:1043-1053.
- Hanifin J, Reed M. A population-based survey of eczema prevalence in the United States. Dermatitis. 2007;82:82-91.
- Avena-Woods C. Overview of atopic dermatitis. Am J Manag Care. 2017;23(8 suppl):S115-S123.
- Wharton M, Strikas RA, Harpaz R, et al; Advisory Committee on Immunization Practices; Healthcare Infection Control Practices Advisory Committee. Recommendations for using smallpox vaccine in a pre-event vaccination program. Supplemental recommendations of the Advisory Committee on Immunization Practices (ACIP) and the Healthcare Infection Control Practices Advisory Committee (HICPAC). MMWR Recomm Rep. 2003;52:1-16.
- Petersen BW, Harms TJ, Reynolds MG, et al. Use of vaccinia virus smallpox vaccine in laboratory and health care personnel at risk for occupation exposure to orthopoxviruses—recommendations of the Advisory Committee on Immunizations Practices (ACIP), 2015. MMWR Morb Mortal Wkly Rep. 2016;65:257-262.
- Nell P, Kohl KS, Graham PL, et al; Brighton Collaboration Vaccinia Virus Vaccine Adverse Event Working Group for Eczema Vaccinatum. Eczema vaccinatum as an adverse event following exposure to vaccinia virus: case definition and guidelines of data collection analysis, and presentation of immunization safety data. Vaccine. 2007:25;5725-5734.
- Aragón TJ, Ulrich S, Fernyak S, et al. Risks of serious complications and death from smallpox vaccination: a systematic review of the United States experience, 1963-1968. BMC Public Health. 2003;3:26.
- Vora S, Damon I, Fulginiti V, et al. Severe eczema vaccinatum in a household contact of a smallpox vaccinee. Clin Infect Dis. 2008;46:1555-1561.
- Centers for Disease Control and Prevention (CDC). Progressive vaccinia in a military smallpox vaccinee—United States 2009. MMWR Morb Mortal Wkly Rep. 2009;58:532-536.
- Fulginiti VA, Papier A, Lane M, et al. Smallpox vaccination: a review, part II. adverse events. Clin Infect Dis. 2003;37:251-271.
- Nell P, Kohl KS, Graham PL, et al; Brighton Collaboration Vaccinia Virus Vaccine Adverse Event Working Group for Progressive Vaccinia. Progressive vaccinia as an adverse event following exposure to vaccinia virus: case definition and guidelines of data collection, analysis, and presentation of immunization safety data. Vaccine. 2007;25:5735-5744.
- Lederman ER, Davidson W, Groff HL, et al. Progressive vaccinia: case description and laboratory-guided therapy with vaccinia immune globulin, ST-246, and CMX001. J Infect Dis. 2012;206:E1372-E1385.
- Lane ML, Goldstein J. Adverse events occurring after smallpox vaccination. Semin Ped Infect Dis. 2003;14:189-195.
- Vaccine adverse events. CDC website. http://www.cdc.gov/smallpox/clinicians/vaccine-adverse-events5.html. Accessed January 3, 2018.
- Cono J, Casey CG, Bell DM. Smallpox vaccination and adversereactions, guidance for clinicians. CDC website. http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5204a1.htm. Accessed January 3, 2018.
- Rosenblatt AE, Stein SL. Cutaneous reactions to vaccinations. Clin Dermatol. 2015;33:327-332.
Practice Points
- Dermatologists should be aware that smallpox vaccinations are being administered to patients and may present with a myriad of cutaneous complications.
- Progressive vaccinia should be suspected if a smallpox inoculation has not healed after 14 days and, most specifically, if there is no inflammation surrounding the site.
- Generalized vaccinia generally is a benign condition seen in otherwise healthy patients and usually requires no treatment.
Atopic patients should be educated to avoid receiving routine smallpox vaccinations if they would be considered at risk for requiring the inoculation.
What’s Eating You? Sand Flies
Identification
Phlebotomine sand flies are the only member of the Psychodidae family that are capable of taking blood.1 The mouthparts of the sand fly are toothed distally, and the maxilla and mandible are utilized in a sawtooth fashion to take a bloodmeal.2 The flies are very small (ie, only 1.5–3.5 mm in length), which makes their identification difficult.1 Sand flies can be distinguished by the appearance of their wings, which often are covered in hair and extend across the back in a V shape.3 The adult sand fly is hairy with a 6- to 8-segmented abdomen, and the color can range from gray to yellow to brown.2 Phlebotomine sand flies can be further identified by their long antennae, dark eyes, and small heads (Figure).2

As is the case with all Diptera, the sand fly goes through 4 complete life stages from egg to larva to pupa to adult.3 Female sand flies will lay their eggs following a blood meal and have been found to take multiple blood meals in a single cycle.2 On average, the eggs will hatch in 6 to 17 days but are temperature dependent.3 The subsequent larvae and pupa stages last 20 to 30 days and 6 to 13 days, respectively.1 The larvae are white in color with short antennae and dark heads.4 Sand flies prefer to lay their eggs in areas where adequate resting places are available and where their larvae will thrive.4,5 The larvae require warm moist environments to succeed and thus are commonly found in animal burrows.3 Once fully developed, the adult sand fly can live up to 6 weeks.2
Sand Fly Vector
Although it is more common in rural forested areas, the sand fly also can be found in urban areas, including heavily populated cities in Brazil.6 Sand flies are most active during hot humid seasons but depending on the local climate may remain active year-round.1,7 For example, in tropical regions of Asia, the number of sand flies increases substantially during the monsoon season compared to the dry season.2 Phlebotomine sand flies are most active at dusk and during the night5 but may become agitated during the daytime if their environment is disturbed.1
Host selection usually is broad and includes a wide variety of vertebrates.2 In the United States, host species are thought to include small rodents, foxes, armadillos, and opossums.8 One study found that visceral leishmaniasis in foxhounds is able to develop fully in sand flies, thus posing an emerging risk to the American population.9
Distribution
The Phlebotominae family contains approximately 700 different species of sand flies but only 21 are known vectors of disease.10 The great majority belong to 1 of 3 genuses: Phlebotomus, Sergentomyia, and Lutzomyia.11 The vectors are commonly divided into Old World species, dominated by the Phlebotomus genus, and New World species, which exclusively refers to the Lutzomyia genus.3 The Old World and New World distinction helps to classify the various vectors and subsequently the diseases they transmit. Old World refers to those vectors found in Southwest and Central Asia, the Indian subcontinent, the Middle East, and East Africa, as well as Southern Europe.6 New World refers to vectors found predominantly in Brazil and other parts of Latin America but also Mexico and the United States.6 Sand flies are found to be endemic in 90 countries and on each continent, except Australia.5 Although the vector can be found in a variety of environments, sand flies prefer moist environments that typify tropical and subtropical climates, thus it is not surprising that the highest diversity of Phlebotominae in the world can be found in the Amazon basin.12
Disease Transmission
Leishmania refers to a genus of intracellular protozoa found in both the Old World and the New World that causes a variety of clinical syndromes.5 Approximately 20 Leishmania species are known to cause human disease that includes localized cutaneous, diffuse cutaneous, mucosal cutaneous, and visceral infections.13 Cases of all forms of leishmaniasis worldwide have increased rapidly over the last few decades from multiple factors including war in endemic regions, increased numbers of immunodeficient individuals, and increased travel to endemic areas.14 In the United States, leishmaniasis is caused by both imported and autochthonous forms of transmission and often mirrors recent travel and immigration patterns.14,15
Sand flies also serve as vectors for sandfly fever, also known as Pappataci fever. Although sandfly fever commonly causes a mild febrile illness, it has been shown to be a considerable cause of aseptic meningitis.16 A number of novel Phleboviruses have been isolated as causes of sandfly fever, including Massilia virus, Granada virus, and Punique virus.16-18 A form of sandfly fever caused by the Toscana virus has a predilection for the nervous system and can cause encephalitis.19 Sandfly fever can be found in both the Old World and New World and thus poses a global risk.2 Additionally, Phlebotominae also have been found to transmit the Changuinola virus, a type of bunyavirus that is known to cause febrile illness in Panama.20 Vesicular stomatitis, also carried by sand flies, is a known cause of febrile disease in North and South America, including the United States.2 In 2013, the Niakha virus, a novel type of Rhabdoviridae, was isolated from Phlebotominae in Senegal.21 The sand fly is noted to transmit another type of Rhabdoviridae in India and Africa, known as the Chandipura virus.22 Although originally thought to cause mild febrile disease, it was the primary cause of multiple outbreaks of fatal encephalitis in India in 200323,24 and again in 2012.22
Sand flies also are known to serve as vectors for the bacterium Bartonella bacilliformis, which is responsible for bartonellosis.25 The disease is divided into 2 forms, which can occur separately or in succession, and is endemic to the Andes region of Peru, Ecuador, and Colombia. The first form is Oroya fever, an acute febrile hemolytic anemia that is fatal in 40% to 88% of cases without intervention.25 This bacterium also causes verruga peruana, an endemic form of bacillary angiomatosis that can persist for years.2 Two reports suggested that bartonellosis also can be caused by Bartonella rochalimae and Candidatus Bartonella ancashi.26,27
Vector Control
Prevention is key to reducing the risk of the various diseases caused by the Phlebotominae vector. Vector control often falls into a few categories, including residual sprays, barriers, and topical repellants.3 It appears that residual sprays applied to houses and animal shelters are the most utilized and effective form of control, with the pyrethroid insecticides having the highest sand fly–specific toxicity.3,28 Insecticides also have been applied to animal burrows where sand flies are known to reproduce; one study in Kenya showed a 90% reduction in the sand fly population following treatment of termite and animal burrows with a pyrethroid spray.29 Studies by Perich et al30,31 in 1995 and 2003 showed that using barrier sprays can be an effective protective measure. The investigators applied a 100-m barrier using a pyrethroid spray on vegetation and reported a notable decrease in sand flies for over an 80-day period.30,31
For personal protection, barrier methods are important adjunct methods of preventing individual exposures. Due to the small size of sand flies, ordinary bed nets are not effective and those treated with insecticides should be used,15 which may ultimately prove to be the most sustainable way to prevent sand fly–borne disease.32 Protective attire also should be worn, as sand flies are not able to penetrate clothing.2 N,N-diethyl-meta-toluamide (DEET)–based repellants should be applied to exposed skin.15 Finally, it is important to avoid exposure from dusk to dawn when sand flies are most active.15
Rise in Autochthonous Cutaneous Leishmaniasis in the United States
With the increased amount of worldwide tourism, especially to endemic areas, providers will continue to see rising numbers of leishmaniasis in the United States. It is difficult to determine the incidence of the disease in the United States, but one study has shown that leishmaniasis accounts for 143 of every 1000 dermatologic diseases acquired by South American tourists.33,34 In addition, the number of autochthonous cases reported in the United States continues to grow. Although only 29 cases were reported between 1903 and 1996, 13 cases were reported between 2000 and 2008.35 Another report in 2013 described an additional 3 cases in the states of Texas and Oklahoma.35 The cases have continued to move in a northeasterly pattern, suggesting a possible shift in the location of sand fly populations. Each of these cases in which a specific species of Leishmania was identified showed transmission of Leishmania mexicana.35 Most cases of cutaneous disease have occurred in Texas and Oklahoma. The first known case outside of this region was reported in 2014 in North Dakota.8 Leishmania donovani, brought into the United States with European foxhounds, also is spreading.8 One species of sand fly, Leishmania shannoni, has now been discovered in 16 states,36-42 where it serves as a potential vector for L mexicana.43,44
- European Centre for Disease Prevention and Control. Phlebotomine sand flies—factsheet for experts. https://ecdc.europa.eu/en/disease-vectors/facts/phlebotomine-sand-flies. Accessed January 24, 2018.
- Durden L, Mullen G. Moth flies and sand flies (Psychodidae). Medical And Veterinary Entomology. San Diego, CA: Academic Press; 2002.
- Claborn DM. The biology and control of leishmaniasis vectors. J Glob Infect Dis. 2010;2:127-134.
- Young DG, Duncan MA. Guide to the identification and geographic distribution of Lutzomyia sand flies in Mexico, the West Indies, Central and South America (Diptera: Psychodidae). Mem Am Entomol Inst. 1994;54:1-881.
- Wolff K, Johnson R, Saavedra AP. Systemic parasitic infections. In: Wolff K, Johnson R, Saavedra AP, eds. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 7th ed. New York, NY: McGraw-Hill; 2013.
- Herwaldt BL, Magill AJ. Leishmaniasis, visceral. In: Centers for Disease Control and Prevention. CDC Yellow Book. https://wwwnc.cdc.gov/travel/yellowbook/2018/infectious-diseases-related-to-travel/leishmaniasis-visceral. Updated May 31, 2017. Accessed January 24, 2018.
- Lawyer PG, Perkins PV. Leishmaniasis and trypanosomiasis. In: Eldridge BF, Edman JD, eds. Medical Entomology. Dordrecht, Netherlands: Kluwer Academic; 2000.
- Douvoyiannis M, Khromachou T, Byers N, et al. Cutaneous leishmaniasis in North Dakota. Clin Infect Dis. 2014;59:73-75.
- Schaut RG, Robles-Murguia M, Juelsgaard R, et al. Vectorborne transmission of Leishmania infantum from hounds, United States. Emerg Infect Dis. 2015;21:2209-2212 .
- Hennings C, Bloch K, Miller J, et al. What is your diagnosis? New World cutaneous leishmaniasis. Cutis. 2015;95:208, 229-230.
- Lewis DJ. Phlebotomid sandflies. Bull World Health Organ. 1971;44:535-551.
- Alves VR, Freitas RA, Santos FL, et al. Sand flies (Diptera, Psychodidae, Phlebotominae) from Central Amazonia and four new records for the Amazonas state, Brazil. Rev Bras Entomol. 2012;56:220-227.
- Hashiguchi Y, Gomez EL, Kato H, et al. Diffuse and disseminated cutaneous leishmaniasis: clinical cases experienced in Ecuador and a brief review. Trop Med Health. 2016;44:2.
- Shaw J. The leishmaniases—survival and expansion in a changing world. a mini-review. Mem Inst Oswaldo Cruz. 2007;102:541-547.
- Centers for Disease Control and Prevention. CDC Health Information for International Travel 2016. New York, NY: Oxford University Press; 2016.
- Zhioua E, Moureau G, Chelbi I, et al. Punique virus, a novel phlebovirus, related to sandfly fever Naples virus, isolated from sandflies collected in Tunisia. J Gen Virol. 2010;91:1275-1283.
- Charrel RN, Moureau G, Temmam S, et al. Massilia virus, a novel phlebovirus (Bunyaviridae) isolated from sandflies in the Mediterranean. Vector Borne Zoonotic Dis. 2009;9:519-530.
- Collao X, Palacios G, de Ory F, et al. SecoGranada virus: a natural phlebovirus reassortant of the sandfly fever Naples serocomplex with low seroprevalence in humans. Am J Trop Med Hyg. 2010;83:760-765.
- Alkan C, Bichaud L, de Lamballerie X, et al. Sandfly-borne phleboviruses of Eurasia and Africa: epidemiology, genetic diversity, geographic range, control measures. Antiviral Res. 2013;100:54-74.
- Travassos da Rosa AP, Tesh RB, Pinheiro FP, et al. Characterization of the Changuinola serogroup viruses (Reoviridae: Orbivirus). Intervirology. 1984;21:38-49.
- Vasilakis N, Widen S, Mayer SV, et al. Niakha virus: a novel member of the family Rhabdoviridae isolated from phlebotomine sandflies in Senegal. Virology. 2013;444:80-89.
- Sudeep AB, Bondre VP, Gurav YK, et al. Isolation of Chandipura virus (Vesiculovirus: Rhabdoviridae) from Sergentomyia species of sandflies from Nagpur, Maharashtra, India. Indian J Med Res. 2014;139:769-772.
- Rao BL, Basu A, Wairagkar NS, et al. A large outbreak of acute encephalitis with high fatality rate in children in Andhra Pradesh, India, in 2003, associated with Chandipura virus. Lancet. 2004;364:869-874.
- Chadha MS, Arankalle VA, Jadi RS, et al. An outbreak of Chandipura virus encephalitis in the eastern districts of Gujarat state, India. Am J Trop Med Hyg. 2005;73:566-570.
- Minnick MF, Anderson BE, Lima A, et al. Oroya fever and verruga peruana: bartonelloses unique to South America. PLoS Negl Trop Dis. 2014;8:E2919.
- Eremeeva ME, Gerns HL, Lydy SL, et al. Bacteremia, fever, and splenomegaly caused by a newly recognized bartonella species. N Engl J Med. 2007;356:2381-2387.
- Blazes DL, Mullins K, Smoak BL, et al. Novel bartonella agent as cause of verruga peruana. Emerg Infect Dis. 2013;19:1111-1114.
- Tetreault GE, Zayed AB, Hanafi HA, et al. Suseptibility of sand flies to selected insecticides in North Africa and the Middle East. J Am Mosq Control Assoc. 2001;17:23-27.
- Robert LL, Perich MJ. Phlebotomine sand fly (Diptera:Psychodidae) control using a residual pyrethroid insecticide. J Am Mosq Control Assoc. 1995;11:195-199.
- Perich MJ, Hoch AL, Rizzo N, et al. Insecticide barrier spraying for the control of sandfly vectors of cutaneous leishmaniasis in rural Guatemala. Am J Trop Med Hyg. 1995;52:485-488.
- Perich MJ, Kardec A, Braga IA, et al. Field evaluation of a lethal ovitrap against dengue vectors in Brazil. Med Vet Entomol. 2003;17:205-210.
- Alexander B, Maroli M. Control of phlebotomine sandflies. Medical and Veterinary Entomology. 2003;17:1-18.
- Freedman DO, Weld LH, Kozarsky PE, et al. Spectrum of disease and relation to place of exposure among ill returned travelers. New Engl J Med. 2006;354:119-130.
- Ergen EN, King AH, Tull M. Cutaneous leishmaniasis: an emerging infectious disease in travelers. Cutis. 2015;96:E22-E26.
- Clarke CF, Bradley KK, Wright JH, et al. Emergence of autochthonous cutaneous leishmaniasis in northeastern Texas and southeastern Oklahoma. Am J Trop Med Hyg. 2013;88:157-161.
- Young DG, Perkins PV. Phlebotomine sand flies of North America (Diptera:Psychodidae). Mosq News. 1984;44:263-304.
- Comer JA, Tesh RB, Modi GB, et al. Vesicular stomatitis virus, New Jersey serotype: replication in and transmission by Lutzomyia shannoni (Diptera: Psychodidae). Am J Trop Med Hyg. 1990;42:483-490.
- Haddow A, Curler G, Moulton J. New records of Lutzomyia shannoni and Lutzomyia vexator (Diptera: Psychodidae) in eastern Tennessee. J Vector Ecol. 2008;33:393-396.
- Claborn DM, Rowton ED, Lawyer PG, et al. Species diversity and relative abundance of phlebotomine sand flies (Diptera: Psychodidae) on three Army installations in the southern United States and susceptibility of a domestic sand fly to infection with Old World Leishmania major. Mil Med. 2009;174:1203-1208.
- Minter L, Kovacic B, Claborn DM, et al. New state records for Lutzomyia shannoni (Dyar) and Lutzomyia vexator (Coquillett). J Med Entomol. 2009;46:965-968.
- Price DC, Gunther DE, Gaugler R. First collection records of phlebotomine sand flies (Diptera: Psychodidae) from New Jersey. J Med Entomol. 2011;48:476-478.
- Weng J, Young SL, Gordon DM, et al. First report of phlebotomine sand flies (Diptera: Psychodidae) in Kansas and Missouri, and a PCR method to distinguish Lutzomyia shannoni from Lutzomyia vexator. J Med Entomol. 2012;49:1460-1465.
- Pech-May A, Escobedo-Ortegón FJ, Berzunza-Cruz M, et al. Incrimination of four sandfly species previously unrecognized as vectors of leishmania parasites in Mexico. Med Vet Entomol. 2010;24:150-161.
- González C, Rebollar-Téllez EA, Ibáñez-Bernal S, et al. Current knowledge of leishmania vectors in Mexico: how geographic distributions of species relate to transmission areas. Am J Trop Med Hyg. 2011;85:839-846.
Identification
Phlebotomine sand flies are the only member of the Psychodidae family that are capable of taking blood.1 The mouthparts of the sand fly are toothed distally, and the maxilla and mandible are utilized in a sawtooth fashion to take a bloodmeal.2 The flies are very small (ie, only 1.5–3.5 mm in length), which makes their identification difficult.1 Sand flies can be distinguished by the appearance of their wings, which often are covered in hair and extend across the back in a V shape.3 The adult sand fly is hairy with a 6- to 8-segmented abdomen, and the color can range from gray to yellow to brown.2 Phlebotomine sand flies can be further identified by their long antennae, dark eyes, and small heads (Figure).2

As is the case with all Diptera, the sand fly goes through 4 complete life stages from egg to larva to pupa to adult.3 Female sand flies will lay their eggs following a blood meal and have been found to take multiple blood meals in a single cycle.2 On average, the eggs will hatch in 6 to 17 days but are temperature dependent.3 The subsequent larvae and pupa stages last 20 to 30 days and 6 to 13 days, respectively.1 The larvae are white in color with short antennae and dark heads.4 Sand flies prefer to lay their eggs in areas where adequate resting places are available and where their larvae will thrive.4,5 The larvae require warm moist environments to succeed and thus are commonly found in animal burrows.3 Once fully developed, the adult sand fly can live up to 6 weeks.2
Sand Fly Vector
Although it is more common in rural forested areas, the sand fly also can be found in urban areas, including heavily populated cities in Brazil.6 Sand flies are most active during hot humid seasons but depending on the local climate may remain active year-round.1,7 For example, in tropical regions of Asia, the number of sand flies increases substantially during the monsoon season compared to the dry season.2 Phlebotomine sand flies are most active at dusk and during the night5 but may become agitated during the daytime if their environment is disturbed.1
Host selection usually is broad and includes a wide variety of vertebrates.2 In the United States, host species are thought to include small rodents, foxes, armadillos, and opossums.8 One study found that visceral leishmaniasis in foxhounds is able to develop fully in sand flies, thus posing an emerging risk to the American population.9
Distribution
The Phlebotominae family contains approximately 700 different species of sand flies but only 21 are known vectors of disease.10 The great majority belong to 1 of 3 genuses: Phlebotomus, Sergentomyia, and Lutzomyia.11 The vectors are commonly divided into Old World species, dominated by the Phlebotomus genus, and New World species, which exclusively refers to the Lutzomyia genus.3 The Old World and New World distinction helps to classify the various vectors and subsequently the diseases they transmit. Old World refers to those vectors found in Southwest and Central Asia, the Indian subcontinent, the Middle East, and East Africa, as well as Southern Europe.6 New World refers to vectors found predominantly in Brazil and other parts of Latin America but also Mexico and the United States.6 Sand flies are found to be endemic in 90 countries and on each continent, except Australia.5 Although the vector can be found in a variety of environments, sand flies prefer moist environments that typify tropical and subtropical climates, thus it is not surprising that the highest diversity of Phlebotominae in the world can be found in the Amazon basin.12
Disease Transmission
Leishmania refers to a genus of intracellular protozoa found in both the Old World and the New World that causes a variety of clinical syndromes.5 Approximately 20 Leishmania species are known to cause human disease that includes localized cutaneous, diffuse cutaneous, mucosal cutaneous, and visceral infections.13 Cases of all forms of leishmaniasis worldwide have increased rapidly over the last few decades from multiple factors including war in endemic regions, increased numbers of immunodeficient individuals, and increased travel to endemic areas.14 In the United States, leishmaniasis is caused by both imported and autochthonous forms of transmission and often mirrors recent travel and immigration patterns.14,15
Sand flies also serve as vectors for sandfly fever, also known as Pappataci fever. Although sandfly fever commonly causes a mild febrile illness, it has been shown to be a considerable cause of aseptic meningitis.16 A number of novel Phleboviruses have been isolated as causes of sandfly fever, including Massilia virus, Granada virus, and Punique virus.16-18 A form of sandfly fever caused by the Toscana virus has a predilection for the nervous system and can cause encephalitis.19 Sandfly fever can be found in both the Old World and New World and thus poses a global risk.2 Additionally, Phlebotominae also have been found to transmit the Changuinola virus, a type of bunyavirus that is known to cause febrile illness in Panama.20 Vesicular stomatitis, also carried by sand flies, is a known cause of febrile disease in North and South America, including the United States.2 In 2013, the Niakha virus, a novel type of Rhabdoviridae, was isolated from Phlebotominae in Senegal.21 The sand fly is noted to transmit another type of Rhabdoviridae in India and Africa, known as the Chandipura virus.22 Although originally thought to cause mild febrile disease, it was the primary cause of multiple outbreaks of fatal encephalitis in India in 200323,24 and again in 2012.22
Sand flies also are known to serve as vectors for the bacterium Bartonella bacilliformis, which is responsible for bartonellosis.25 The disease is divided into 2 forms, which can occur separately or in succession, and is endemic to the Andes region of Peru, Ecuador, and Colombia. The first form is Oroya fever, an acute febrile hemolytic anemia that is fatal in 40% to 88% of cases without intervention.25 This bacterium also causes verruga peruana, an endemic form of bacillary angiomatosis that can persist for years.2 Two reports suggested that bartonellosis also can be caused by Bartonella rochalimae and Candidatus Bartonella ancashi.26,27
Vector Control
Prevention is key to reducing the risk of the various diseases caused by the Phlebotominae vector. Vector control often falls into a few categories, including residual sprays, barriers, and topical repellants.3 It appears that residual sprays applied to houses and animal shelters are the most utilized and effective form of control, with the pyrethroid insecticides having the highest sand fly–specific toxicity.3,28 Insecticides also have been applied to animal burrows where sand flies are known to reproduce; one study in Kenya showed a 90% reduction in the sand fly population following treatment of termite and animal burrows with a pyrethroid spray.29 Studies by Perich et al30,31 in 1995 and 2003 showed that using barrier sprays can be an effective protective measure. The investigators applied a 100-m barrier using a pyrethroid spray on vegetation and reported a notable decrease in sand flies for over an 80-day period.30,31
For personal protection, barrier methods are important adjunct methods of preventing individual exposures. Due to the small size of sand flies, ordinary bed nets are not effective and those treated with insecticides should be used,15 which may ultimately prove to be the most sustainable way to prevent sand fly–borne disease.32 Protective attire also should be worn, as sand flies are not able to penetrate clothing.2 N,N-diethyl-meta-toluamide (DEET)–based repellants should be applied to exposed skin.15 Finally, it is important to avoid exposure from dusk to dawn when sand flies are most active.15
Rise in Autochthonous Cutaneous Leishmaniasis in the United States
With the increased amount of worldwide tourism, especially to endemic areas, providers will continue to see rising numbers of leishmaniasis in the United States. It is difficult to determine the incidence of the disease in the United States, but one study has shown that leishmaniasis accounts for 143 of every 1000 dermatologic diseases acquired by South American tourists.33,34 In addition, the number of autochthonous cases reported in the United States continues to grow. Although only 29 cases were reported between 1903 and 1996, 13 cases were reported between 2000 and 2008.35 Another report in 2013 described an additional 3 cases in the states of Texas and Oklahoma.35 The cases have continued to move in a northeasterly pattern, suggesting a possible shift in the location of sand fly populations. Each of these cases in which a specific species of Leishmania was identified showed transmission of Leishmania mexicana.35 Most cases of cutaneous disease have occurred in Texas and Oklahoma. The first known case outside of this region was reported in 2014 in North Dakota.8 Leishmania donovani, brought into the United States with European foxhounds, also is spreading.8 One species of sand fly, Leishmania shannoni, has now been discovered in 16 states,36-42 where it serves as a potential vector for L mexicana.43,44
Identification
Phlebotomine sand flies are the only member of the Psychodidae family that are capable of taking blood.1 The mouthparts of the sand fly are toothed distally, and the maxilla and mandible are utilized in a sawtooth fashion to take a bloodmeal.2 The flies are very small (ie, only 1.5–3.5 mm in length), which makes their identification difficult.1 Sand flies can be distinguished by the appearance of their wings, which often are covered in hair and extend across the back in a V shape.3 The adult sand fly is hairy with a 6- to 8-segmented abdomen, and the color can range from gray to yellow to brown.2 Phlebotomine sand flies can be further identified by their long antennae, dark eyes, and small heads (Figure).2

As is the case with all Diptera, the sand fly goes through 4 complete life stages from egg to larva to pupa to adult.3 Female sand flies will lay their eggs following a blood meal and have been found to take multiple blood meals in a single cycle.2 On average, the eggs will hatch in 6 to 17 days but are temperature dependent.3 The subsequent larvae and pupa stages last 20 to 30 days and 6 to 13 days, respectively.1 The larvae are white in color with short antennae and dark heads.4 Sand flies prefer to lay their eggs in areas where adequate resting places are available and where their larvae will thrive.4,5 The larvae require warm moist environments to succeed and thus are commonly found in animal burrows.3 Once fully developed, the adult sand fly can live up to 6 weeks.2
Sand Fly Vector
Although it is more common in rural forested areas, the sand fly also can be found in urban areas, including heavily populated cities in Brazil.6 Sand flies are most active during hot humid seasons but depending on the local climate may remain active year-round.1,7 For example, in tropical regions of Asia, the number of sand flies increases substantially during the monsoon season compared to the dry season.2 Phlebotomine sand flies are most active at dusk and during the night5 but may become agitated during the daytime if their environment is disturbed.1
Host selection usually is broad and includes a wide variety of vertebrates.2 In the United States, host species are thought to include small rodents, foxes, armadillos, and opossums.8 One study found that visceral leishmaniasis in foxhounds is able to develop fully in sand flies, thus posing an emerging risk to the American population.9
Distribution
The Phlebotominae family contains approximately 700 different species of sand flies but only 21 are known vectors of disease.10 The great majority belong to 1 of 3 genuses: Phlebotomus, Sergentomyia, and Lutzomyia.11 The vectors are commonly divided into Old World species, dominated by the Phlebotomus genus, and New World species, which exclusively refers to the Lutzomyia genus.3 The Old World and New World distinction helps to classify the various vectors and subsequently the diseases they transmit. Old World refers to those vectors found in Southwest and Central Asia, the Indian subcontinent, the Middle East, and East Africa, as well as Southern Europe.6 New World refers to vectors found predominantly in Brazil and other parts of Latin America but also Mexico and the United States.6 Sand flies are found to be endemic in 90 countries and on each continent, except Australia.5 Although the vector can be found in a variety of environments, sand flies prefer moist environments that typify tropical and subtropical climates, thus it is not surprising that the highest diversity of Phlebotominae in the world can be found in the Amazon basin.12
Disease Transmission
Leishmania refers to a genus of intracellular protozoa found in both the Old World and the New World that causes a variety of clinical syndromes.5 Approximately 20 Leishmania species are known to cause human disease that includes localized cutaneous, diffuse cutaneous, mucosal cutaneous, and visceral infections.13 Cases of all forms of leishmaniasis worldwide have increased rapidly over the last few decades from multiple factors including war in endemic regions, increased numbers of immunodeficient individuals, and increased travel to endemic areas.14 In the United States, leishmaniasis is caused by both imported and autochthonous forms of transmission and often mirrors recent travel and immigration patterns.14,15
Sand flies also serve as vectors for sandfly fever, also known as Pappataci fever. Although sandfly fever commonly causes a mild febrile illness, it has been shown to be a considerable cause of aseptic meningitis.16 A number of novel Phleboviruses have been isolated as causes of sandfly fever, including Massilia virus, Granada virus, and Punique virus.16-18 A form of sandfly fever caused by the Toscana virus has a predilection for the nervous system and can cause encephalitis.19 Sandfly fever can be found in both the Old World and New World and thus poses a global risk.2 Additionally, Phlebotominae also have been found to transmit the Changuinola virus, a type of bunyavirus that is known to cause febrile illness in Panama.20 Vesicular stomatitis, also carried by sand flies, is a known cause of febrile disease in North and South America, including the United States.2 In 2013, the Niakha virus, a novel type of Rhabdoviridae, was isolated from Phlebotominae in Senegal.21 The sand fly is noted to transmit another type of Rhabdoviridae in India and Africa, known as the Chandipura virus.22 Although originally thought to cause mild febrile disease, it was the primary cause of multiple outbreaks of fatal encephalitis in India in 200323,24 and again in 2012.22
Sand flies also are known to serve as vectors for the bacterium Bartonella bacilliformis, which is responsible for bartonellosis.25 The disease is divided into 2 forms, which can occur separately or in succession, and is endemic to the Andes region of Peru, Ecuador, and Colombia. The first form is Oroya fever, an acute febrile hemolytic anemia that is fatal in 40% to 88% of cases without intervention.25 This bacterium also causes verruga peruana, an endemic form of bacillary angiomatosis that can persist for years.2 Two reports suggested that bartonellosis also can be caused by Bartonella rochalimae and Candidatus Bartonella ancashi.26,27
Vector Control
Prevention is key to reducing the risk of the various diseases caused by the Phlebotominae vector. Vector control often falls into a few categories, including residual sprays, barriers, and topical repellants.3 It appears that residual sprays applied to houses and animal shelters are the most utilized and effective form of control, with the pyrethroid insecticides having the highest sand fly–specific toxicity.3,28 Insecticides also have been applied to animal burrows where sand flies are known to reproduce; one study in Kenya showed a 90% reduction in the sand fly population following treatment of termite and animal burrows with a pyrethroid spray.29 Studies by Perich et al30,31 in 1995 and 2003 showed that using barrier sprays can be an effective protective measure. The investigators applied a 100-m barrier using a pyrethroid spray on vegetation and reported a notable decrease in sand flies for over an 80-day period.30,31
For personal protection, barrier methods are important adjunct methods of preventing individual exposures. Due to the small size of sand flies, ordinary bed nets are not effective and those treated with insecticides should be used,15 which may ultimately prove to be the most sustainable way to prevent sand fly–borne disease.32 Protective attire also should be worn, as sand flies are not able to penetrate clothing.2 N,N-diethyl-meta-toluamide (DEET)–based repellants should be applied to exposed skin.15 Finally, it is important to avoid exposure from dusk to dawn when sand flies are most active.15
Rise in Autochthonous Cutaneous Leishmaniasis in the United States
With the increased amount of worldwide tourism, especially to endemic areas, providers will continue to see rising numbers of leishmaniasis in the United States. It is difficult to determine the incidence of the disease in the United States, but one study has shown that leishmaniasis accounts for 143 of every 1000 dermatologic diseases acquired by South American tourists.33,34 In addition, the number of autochthonous cases reported in the United States continues to grow. Although only 29 cases were reported between 1903 and 1996, 13 cases were reported between 2000 and 2008.35 Another report in 2013 described an additional 3 cases in the states of Texas and Oklahoma.35 The cases have continued to move in a northeasterly pattern, suggesting a possible shift in the location of sand fly populations. Each of these cases in which a specific species of Leishmania was identified showed transmission of Leishmania mexicana.35 Most cases of cutaneous disease have occurred in Texas and Oklahoma. The first known case outside of this region was reported in 2014 in North Dakota.8 Leishmania donovani, brought into the United States with European foxhounds, also is spreading.8 One species of sand fly, Leishmania shannoni, has now been discovered in 16 states,36-42 where it serves as a potential vector for L mexicana.43,44
- European Centre for Disease Prevention and Control. Phlebotomine sand flies—factsheet for experts. https://ecdc.europa.eu/en/disease-vectors/facts/phlebotomine-sand-flies. Accessed January 24, 2018.
- Durden L, Mullen G. Moth flies and sand flies (Psychodidae). Medical And Veterinary Entomology. San Diego, CA: Academic Press; 2002.
- Claborn DM. The biology and control of leishmaniasis vectors. J Glob Infect Dis. 2010;2:127-134.
- Young DG, Duncan MA. Guide to the identification and geographic distribution of Lutzomyia sand flies in Mexico, the West Indies, Central and South America (Diptera: Psychodidae). Mem Am Entomol Inst. 1994;54:1-881.
- Wolff K, Johnson R, Saavedra AP. Systemic parasitic infections. In: Wolff K, Johnson R, Saavedra AP, eds. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 7th ed. New York, NY: McGraw-Hill; 2013.
- Herwaldt BL, Magill AJ. Leishmaniasis, visceral. In: Centers for Disease Control and Prevention. CDC Yellow Book. https://wwwnc.cdc.gov/travel/yellowbook/2018/infectious-diseases-related-to-travel/leishmaniasis-visceral. Updated May 31, 2017. Accessed January 24, 2018.
- Lawyer PG, Perkins PV. Leishmaniasis and trypanosomiasis. In: Eldridge BF, Edman JD, eds. Medical Entomology. Dordrecht, Netherlands: Kluwer Academic; 2000.
- Douvoyiannis M, Khromachou T, Byers N, et al. Cutaneous leishmaniasis in North Dakota. Clin Infect Dis. 2014;59:73-75.
- Schaut RG, Robles-Murguia M, Juelsgaard R, et al. Vectorborne transmission of Leishmania infantum from hounds, United States. Emerg Infect Dis. 2015;21:2209-2212 .
- Hennings C, Bloch K, Miller J, et al. What is your diagnosis? New World cutaneous leishmaniasis. Cutis. 2015;95:208, 229-230.
- Lewis DJ. Phlebotomid sandflies. Bull World Health Organ. 1971;44:535-551.
- Alves VR, Freitas RA, Santos FL, et al. Sand flies (Diptera, Psychodidae, Phlebotominae) from Central Amazonia and four new records for the Amazonas state, Brazil. Rev Bras Entomol. 2012;56:220-227.
- Hashiguchi Y, Gomez EL, Kato H, et al. Diffuse and disseminated cutaneous leishmaniasis: clinical cases experienced in Ecuador and a brief review. Trop Med Health. 2016;44:2.
- Shaw J. The leishmaniases—survival and expansion in a changing world. a mini-review. Mem Inst Oswaldo Cruz. 2007;102:541-547.
- Centers for Disease Control and Prevention. CDC Health Information for International Travel 2016. New York, NY: Oxford University Press; 2016.
- Zhioua E, Moureau G, Chelbi I, et al. Punique virus, a novel phlebovirus, related to sandfly fever Naples virus, isolated from sandflies collected in Tunisia. J Gen Virol. 2010;91:1275-1283.
- Charrel RN, Moureau G, Temmam S, et al. Massilia virus, a novel phlebovirus (Bunyaviridae) isolated from sandflies in the Mediterranean. Vector Borne Zoonotic Dis. 2009;9:519-530.
- Collao X, Palacios G, de Ory F, et al. SecoGranada virus: a natural phlebovirus reassortant of the sandfly fever Naples serocomplex with low seroprevalence in humans. Am J Trop Med Hyg. 2010;83:760-765.
- Alkan C, Bichaud L, de Lamballerie X, et al. Sandfly-borne phleboviruses of Eurasia and Africa: epidemiology, genetic diversity, geographic range, control measures. Antiviral Res. 2013;100:54-74.
- Travassos da Rosa AP, Tesh RB, Pinheiro FP, et al. Characterization of the Changuinola serogroup viruses (Reoviridae: Orbivirus). Intervirology. 1984;21:38-49.
- Vasilakis N, Widen S, Mayer SV, et al. Niakha virus: a novel member of the family Rhabdoviridae isolated from phlebotomine sandflies in Senegal. Virology. 2013;444:80-89.
- Sudeep AB, Bondre VP, Gurav YK, et al. Isolation of Chandipura virus (Vesiculovirus: Rhabdoviridae) from Sergentomyia species of sandflies from Nagpur, Maharashtra, India. Indian J Med Res. 2014;139:769-772.
- Rao BL, Basu A, Wairagkar NS, et al. A large outbreak of acute encephalitis with high fatality rate in children in Andhra Pradesh, India, in 2003, associated with Chandipura virus. Lancet. 2004;364:869-874.
- Chadha MS, Arankalle VA, Jadi RS, et al. An outbreak of Chandipura virus encephalitis in the eastern districts of Gujarat state, India. Am J Trop Med Hyg. 2005;73:566-570.
- Minnick MF, Anderson BE, Lima A, et al. Oroya fever and verruga peruana: bartonelloses unique to South America. PLoS Negl Trop Dis. 2014;8:E2919.
- Eremeeva ME, Gerns HL, Lydy SL, et al. Bacteremia, fever, and splenomegaly caused by a newly recognized bartonella species. N Engl J Med. 2007;356:2381-2387.
- Blazes DL, Mullins K, Smoak BL, et al. Novel bartonella agent as cause of verruga peruana. Emerg Infect Dis. 2013;19:1111-1114.
- Tetreault GE, Zayed AB, Hanafi HA, et al. Suseptibility of sand flies to selected insecticides in North Africa and the Middle East. J Am Mosq Control Assoc. 2001;17:23-27.
- Robert LL, Perich MJ. Phlebotomine sand fly (Diptera:Psychodidae) control using a residual pyrethroid insecticide. J Am Mosq Control Assoc. 1995;11:195-199.
- Perich MJ, Hoch AL, Rizzo N, et al. Insecticide barrier spraying for the control of sandfly vectors of cutaneous leishmaniasis in rural Guatemala. Am J Trop Med Hyg. 1995;52:485-488.
- Perich MJ, Kardec A, Braga IA, et al. Field evaluation of a lethal ovitrap against dengue vectors in Brazil. Med Vet Entomol. 2003;17:205-210.
- Alexander B, Maroli M. Control of phlebotomine sandflies. Medical and Veterinary Entomology. 2003;17:1-18.
- Freedman DO, Weld LH, Kozarsky PE, et al. Spectrum of disease and relation to place of exposure among ill returned travelers. New Engl J Med. 2006;354:119-130.
- Ergen EN, King AH, Tull M. Cutaneous leishmaniasis: an emerging infectious disease in travelers. Cutis. 2015;96:E22-E26.
- Clarke CF, Bradley KK, Wright JH, et al. Emergence of autochthonous cutaneous leishmaniasis in northeastern Texas and southeastern Oklahoma. Am J Trop Med Hyg. 2013;88:157-161.
- Young DG, Perkins PV. Phlebotomine sand flies of North America (Diptera:Psychodidae). Mosq News. 1984;44:263-304.
- Comer JA, Tesh RB, Modi GB, et al. Vesicular stomatitis virus, New Jersey serotype: replication in and transmission by Lutzomyia shannoni (Diptera: Psychodidae). Am J Trop Med Hyg. 1990;42:483-490.
- Haddow A, Curler G, Moulton J. New records of Lutzomyia shannoni and Lutzomyia vexator (Diptera: Psychodidae) in eastern Tennessee. J Vector Ecol. 2008;33:393-396.
- Claborn DM, Rowton ED, Lawyer PG, et al. Species diversity and relative abundance of phlebotomine sand flies (Diptera: Psychodidae) on three Army installations in the southern United States and susceptibility of a domestic sand fly to infection with Old World Leishmania major. Mil Med. 2009;174:1203-1208.
- Minter L, Kovacic B, Claborn DM, et al. New state records for Lutzomyia shannoni (Dyar) and Lutzomyia vexator (Coquillett). J Med Entomol. 2009;46:965-968.
- Price DC, Gunther DE, Gaugler R. First collection records of phlebotomine sand flies (Diptera: Psychodidae) from New Jersey. J Med Entomol. 2011;48:476-478.
- Weng J, Young SL, Gordon DM, et al. First report of phlebotomine sand flies (Diptera: Psychodidae) in Kansas and Missouri, and a PCR method to distinguish Lutzomyia shannoni from Lutzomyia vexator. J Med Entomol. 2012;49:1460-1465.
- Pech-May A, Escobedo-Ortegón FJ, Berzunza-Cruz M, et al. Incrimination of four sandfly species previously unrecognized as vectors of leishmania parasites in Mexico. Med Vet Entomol. 2010;24:150-161.
- González C, Rebollar-Téllez EA, Ibáñez-Bernal S, et al. Current knowledge of leishmania vectors in Mexico: how geographic distributions of species relate to transmission areas. Am J Trop Med Hyg. 2011;85:839-846.
- European Centre for Disease Prevention and Control. Phlebotomine sand flies—factsheet for experts. https://ecdc.europa.eu/en/disease-vectors/facts/phlebotomine-sand-flies. Accessed January 24, 2018.
- Durden L, Mullen G. Moth flies and sand flies (Psychodidae). Medical And Veterinary Entomology. San Diego, CA: Academic Press; 2002.
- Claborn DM. The biology and control of leishmaniasis vectors. J Glob Infect Dis. 2010;2:127-134.
- Young DG, Duncan MA. Guide to the identification and geographic distribution of Lutzomyia sand flies in Mexico, the West Indies, Central and South America (Diptera: Psychodidae). Mem Am Entomol Inst. 1994;54:1-881.
- Wolff K, Johnson R, Saavedra AP. Systemic parasitic infections. In: Wolff K, Johnson R, Saavedra AP, eds. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 7th ed. New York, NY: McGraw-Hill; 2013.
- Herwaldt BL, Magill AJ. Leishmaniasis, visceral. In: Centers for Disease Control and Prevention. CDC Yellow Book. https://wwwnc.cdc.gov/travel/yellowbook/2018/infectious-diseases-related-to-travel/leishmaniasis-visceral. Updated May 31, 2017. Accessed January 24, 2018.
- Lawyer PG, Perkins PV. Leishmaniasis and trypanosomiasis. In: Eldridge BF, Edman JD, eds. Medical Entomology. Dordrecht, Netherlands: Kluwer Academic; 2000.
- Douvoyiannis M, Khromachou T, Byers N, et al. Cutaneous leishmaniasis in North Dakota. Clin Infect Dis. 2014;59:73-75.
- Schaut RG, Robles-Murguia M, Juelsgaard R, et al. Vectorborne transmission of Leishmania infantum from hounds, United States. Emerg Infect Dis. 2015;21:2209-2212 .
- Hennings C, Bloch K, Miller J, et al. What is your diagnosis? New World cutaneous leishmaniasis. Cutis. 2015;95:208, 229-230.
- Lewis DJ. Phlebotomid sandflies. Bull World Health Organ. 1971;44:535-551.
- Alves VR, Freitas RA, Santos FL, et al. Sand flies (Diptera, Psychodidae, Phlebotominae) from Central Amazonia and four new records for the Amazonas state, Brazil. Rev Bras Entomol. 2012;56:220-227.
- Hashiguchi Y, Gomez EL, Kato H, et al. Diffuse and disseminated cutaneous leishmaniasis: clinical cases experienced in Ecuador and a brief review. Trop Med Health. 2016;44:2.
- Shaw J. The leishmaniases—survival and expansion in a changing world. a mini-review. Mem Inst Oswaldo Cruz. 2007;102:541-547.
- Centers for Disease Control and Prevention. CDC Health Information for International Travel 2016. New York, NY: Oxford University Press; 2016.
- Zhioua E, Moureau G, Chelbi I, et al. Punique virus, a novel phlebovirus, related to sandfly fever Naples virus, isolated from sandflies collected in Tunisia. J Gen Virol. 2010;91:1275-1283.
- Charrel RN, Moureau G, Temmam S, et al. Massilia virus, a novel phlebovirus (Bunyaviridae) isolated from sandflies in the Mediterranean. Vector Borne Zoonotic Dis. 2009;9:519-530.
- Collao X, Palacios G, de Ory F, et al. SecoGranada virus: a natural phlebovirus reassortant of the sandfly fever Naples serocomplex with low seroprevalence in humans. Am J Trop Med Hyg. 2010;83:760-765.
- Alkan C, Bichaud L, de Lamballerie X, et al. Sandfly-borne phleboviruses of Eurasia and Africa: epidemiology, genetic diversity, geographic range, control measures. Antiviral Res. 2013;100:54-74.
- Travassos da Rosa AP, Tesh RB, Pinheiro FP, et al. Characterization of the Changuinola serogroup viruses (Reoviridae: Orbivirus). Intervirology. 1984;21:38-49.
- Vasilakis N, Widen S, Mayer SV, et al. Niakha virus: a novel member of the family Rhabdoviridae isolated from phlebotomine sandflies in Senegal. Virology. 2013;444:80-89.
- Sudeep AB, Bondre VP, Gurav YK, et al. Isolation of Chandipura virus (Vesiculovirus: Rhabdoviridae) from Sergentomyia species of sandflies from Nagpur, Maharashtra, India. Indian J Med Res. 2014;139:769-772.
- Rao BL, Basu A, Wairagkar NS, et al. A large outbreak of acute encephalitis with high fatality rate in children in Andhra Pradesh, India, in 2003, associated with Chandipura virus. Lancet. 2004;364:869-874.
- Chadha MS, Arankalle VA, Jadi RS, et al. An outbreak of Chandipura virus encephalitis in the eastern districts of Gujarat state, India. Am J Trop Med Hyg. 2005;73:566-570.
- Minnick MF, Anderson BE, Lima A, et al. Oroya fever and verruga peruana: bartonelloses unique to South America. PLoS Negl Trop Dis. 2014;8:E2919.
- Eremeeva ME, Gerns HL, Lydy SL, et al. Bacteremia, fever, and splenomegaly caused by a newly recognized bartonella species. N Engl J Med. 2007;356:2381-2387.
- Blazes DL, Mullins K, Smoak BL, et al. Novel bartonella agent as cause of verruga peruana. Emerg Infect Dis. 2013;19:1111-1114.
- Tetreault GE, Zayed AB, Hanafi HA, et al. Suseptibility of sand flies to selected insecticides in North Africa and the Middle East. J Am Mosq Control Assoc. 2001;17:23-27.
- Robert LL, Perich MJ. Phlebotomine sand fly (Diptera:Psychodidae) control using a residual pyrethroid insecticide. J Am Mosq Control Assoc. 1995;11:195-199.
- Perich MJ, Hoch AL, Rizzo N, et al. Insecticide barrier spraying for the control of sandfly vectors of cutaneous leishmaniasis in rural Guatemala. Am J Trop Med Hyg. 1995;52:485-488.
- Perich MJ, Kardec A, Braga IA, et al. Field evaluation of a lethal ovitrap against dengue vectors in Brazil. Med Vet Entomol. 2003;17:205-210.
- Alexander B, Maroli M. Control of phlebotomine sandflies. Medical and Veterinary Entomology. 2003;17:1-18.
- Freedman DO, Weld LH, Kozarsky PE, et al. Spectrum of disease and relation to place of exposure among ill returned travelers. New Engl J Med. 2006;354:119-130.
- Ergen EN, King AH, Tull M. Cutaneous leishmaniasis: an emerging infectious disease in travelers. Cutis. 2015;96:E22-E26.
- Clarke CF, Bradley KK, Wright JH, et al. Emergence of autochthonous cutaneous leishmaniasis in northeastern Texas and southeastern Oklahoma. Am J Trop Med Hyg. 2013;88:157-161.
- Young DG, Perkins PV. Phlebotomine sand flies of North America (Diptera:Psychodidae). Mosq News. 1984;44:263-304.
- Comer JA, Tesh RB, Modi GB, et al. Vesicular stomatitis virus, New Jersey serotype: replication in and transmission by Lutzomyia shannoni (Diptera: Psychodidae). Am J Trop Med Hyg. 1990;42:483-490.
- Haddow A, Curler G, Moulton J. New records of Lutzomyia shannoni and Lutzomyia vexator (Diptera: Psychodidae) in eastern Tennessee. J Vector Ecol. 2008;33:393-396.
- Claborn DM, Rowton ED, Lawyer PG, et al. Species diversity and relative abundance of phlebotomine sand flies (Diptera: Psychodidae) on three Army installations in the southern United States and susceptibility of a domestic sand fly to infection with Old World Leishmania major. Mil Med. 2009;174:1203-1208.
- Minter L, Kovacic B, Claborn DM, et al. New state records for Lutzomyia shannoni (Dyar) and Lutzomyia vexator (Coquillett). J Med Entomol. 2009;46:965-968.
- Price DC, Gunther DE, Gaugler R. First collection records of phlebotomine sand flies (Diptera: Psychodidae) from New Jersey. J Med Entomol. 2011;48:476-478.
- Weng J, Young SL, Gordon DM, et al. First report of phlebotomine sand flies (Diptera: Psychodidae) in Kansas and Missouri, and a PCR method to distinguish Lutzomyia shannoni from Lutzomyia vexator. J Med Entomol. 2012;49:1460-1465.
- Pech-May A, Escobedo-Ortegón FJ, Berzunza-Cruz M, et al. Incrimination of four sandfly species previously unrecognized as vectors of leishmania parasites in Mexico. Med Vet Entomol. 2010;24:150-161.
- González C, Rebollar-Téllez EA, Ibáñez-Bernal S, et al. Current knowledge of leishmania vectors in Mexico: how geographic distributions of species relate to transmission areas. Am J Trop Med Hyg. 2011;85:839-846.
Practice Points
- Sand flies cause a wide array of cutaneous and systemic diseases worldwide.
- Identification and treatment of leishmaniasis and other diseases transmitted by sand flies requires a high degree of clinical suspicion.
- With the increase in global travel and the rise of autochthonous disease in the United States, American physicians must increase their awareness of diseases for which sand flies serve as vectors.
Can Corynebacterium in the Gut Trigger Parkinson’s Disease?
SAN DIEGO—The presence of Corynebacterium in the gut microbiome of people with two G alleles at the rs356219 single nucleotide polymorphism locus of the alpha-synuclein gene was associated with 100% probability of having Parkinson’s disease in a study conducted by the NeuroGenetics Research Consortium.
If the finding is replicated, it may mean that Corynebacterium triggers Parkinson’s disease in people with the GG genotype. The GG signature at rs356219 is the strongest genetic risk factor for Parkinson’s disease identified to date, but it is not necessarily strong enough to cause the disease on its own. “It definitely needs a trigger,” and there is a good chance that Corynebacterium is it, said senior investigator Haydeh Payami, PhD, Professor of Neurology and Genomics at the University of Alabama, Birmingham.
Genotypes and Triggers
The finding, which was presented at the 142nd Annual Meeting of the American Neurological Association, may begin to clarify the link between the dozens of genetic risk factors for Parkinson’s disease and environmental triggers that lead to the disease. Different bacteria may be associated with different genetic risk factors. Eventually, the researchers aim to map out which genetic susceptibilities are associated with which elements of the microbiome and which genotypes are associated with other environmental factors, such as pesticides, Dr. Payami, leader of the multicenter neurogenetics research collaboration, said.
Her team genotyped SNCA rs356219 from blood samples in 197 middle-aged patients with Parkinson’s disease and 115 age-matched controls. They also extracted DNA from stool samples to see what bacteria were in their guts and then looked for interactions between rs356219 genotype, gut microbiome, and Parkinson’s disease risk.
The medical literature has been full of hints that Parkinson’s disease might be set off by something going wrong in the gastrointestinal (GI) tract. Colonic inflammation, alpha-synuclein pathology in the gut, and dysbiosis of the gut microbiome in Parkinson’s disease are among the many clues. The goal of the work was to find the link between Parkinson’s disease and its GI aberrations.
Ninety genera were identified in the stool samples, but “no matter how you looked at the data, whichever method you used, one [genus] kept coming up” for interaction with the rs356219 genotype, “and that was Corynebacterium,” Dr. Payami said.
Heightened Risk
As in past studies, the rs356219 AA genotype did not increase the odds of Parkinson’s disease, and there was no difference in microbiome abundance between patients with Parkinson’s disease and controls. The GA genotype increased the odds slightly without Corynebacterium, but it increased the odds more than fivefold when Corynebacterium was in the gut (odds ratio, 5.9). If people had GG plus Corynebacterium, however, developing Parkinson’s disease was a certainty.
Corynebacterium was more abundant in GA subjects with Parkinson’s disease than in GA subjects without Parkinson’s disease, but it was by far the most abundant in GG subjects, and every person who had the GG genotype and gut Corynebacterium also had Parkinson’s disease.
Corynebacteria are gram-positive, aerobic bacilli commonly found on the skin. Some members of the genus are opportunistic pathogens. It is not clear how they get incorporated into the gut microbiome, or if they can be wiped out selectively in the gut with antibiotics or probiotics.
Perhaps Corynebacterium in the GI tract induces expression of alpha-synuclein protein, a major component of Parkinson’s disease Lewy bodies that is known to travel from the gut to the brain. Maybe the amount expressed depends on how many Gs people have in rs356219. Perhaps “if you have two Gs, you get so much alpha-synuclein that there is no turning back, and it is enough to cause Parkinson’s disease,” Dr. Payami said.
The study was led by Zachary Wallen, a PhD candidate in Dr. Payami’s lab. The study was supported by the NIH.
—M. Alexander Otto
SAN DIEGO—The presence of Corynebacterium in the gut microbiome of people with two G alleles at the rs356219 single nucleotide polymorphism locus of the alpha-synuclein gene was associated with 100% probability of having Parkinson’s disease in a study conducted by the NeuroGenetics Research Consortium.
If the finding is replicated, it may mean that Corynebacterium triggers Parkinson’s disease in people with the GG genotype. The GG signature at rs356219 is the strongest genetic risk factor for Parkinson’s disease identified to date, but it is not necessarily strong enough to cause the disease on its own. “It definitely needs a trigger,” and there is a good chance that Corynebacterium is it, said senior investigator Haydeh Payami, PhD, Professor of Neurology and Genomics at the University of Alabama, Birmingham.
Genotypes and Triggers
The finding, which was presented at the 142nd Annual Meeting of the American Neurological Association, may begin to clarify the link between the dozens of genetic risk factors for Parkinson’s disease and environmental triggers that lead to the disease. Different bacteria may be associated with different genetic risk factors. Eventually, the researchers aim to map out which genetic susceptibilities are associated with which elements of the microbiome and which genotypes are associated with other environmental factors, such as pesticides, Dr. Payami, leader of the multicenter neurogenetics research collaboration, said.
Her team genotyped SNCA rs356219 from blood samples in 197 middle-aged patients with Parkinson’s disease and 115 age-matched controls. They also extracted DNA from stool samples to see what bacteria were in their guts and then looked for interactions between rs356219 genotype, gut microbiome, and Parkinson’s disease risk.
The medical literature has been full of hints that Parkinson’s disease might be set off by something going wrong in the gastrointestinal (GI) tract. Colonic inflammation, alpha-synuclein pathology in the gut, and dysbiosis of the gut microbiome in Parkinson’s disease are among the many clues. The goal of the work was to find the link between Parkinson’s disease and its GI aberrations.
Ninety genera were identified in the stool samples, but “no matter how you looked at the data, whichever method you used, one [genus] kept coming up” for interaction with the rs356219 genotype, “and that was Corynebacterium,” Dr. Payami said.
Heightened Risk
As in past studies, the rs356219 AA genotype did not increase the odds of Parkinson’s disease, and there was no difference in microbiome abundance between patients with Parkinson’s disease and controls. The GA genotype increased the odds slightly without Corynebacterium, but it increased the odds more than fivefold when Corynebacterium was in the gut (odds ratio, 5.9). If people had GG plus Corynebacterium, however, developing Parkinson’s disease was a certainty.
Corynebacterium was more abundant in GA subjects with Parkinson’s disease than in GA subjects without Parkinson’s disease, but it was by far the most abundant in GG subjects, and every person who had the GG genotype and gut Corynebacterium also had Parkinson’s disease.
Corynebacteria are gram-positive, aerobic bacilli commonly found on the skin. Some members of the genus are opportunistic pathogens. It is not clear how they get incorporated into the gut microbiome, or if they can be wiped out selectively in the gut with antibiotics or probiotics.
Perhaps Corynebacterium in the GI tract induces expression of alpha-synuclein protein, a major component of Parkinson’s disease Lewy bodies that is known to travel from the gut to the brain. Maybe the amount expressed depends on how many Gs people have in rs356219. Perhaps “if you have two Gs, you get so much alpha-synuclein that there is no turning back, and it is enough to cause Parkinson’s disease,” Dr. Payami said.
The study was led by Zachary Wallen, a PhD candidate in Dr. Payami’s lab. The study was supported by the NIH.
—M. Alexander Otto
SAN DIEGO—The presence of Corynebacterium in the gut microbiome of people with two G alleles at the rs356219 single nucleotide polymorphism locus of the alpha-synuclein gene was associated with 100% probability of having Parkinson’s disease in a study conducted by the NeuroGenetics Research Consortium.
If the finding is replicated, it may mean that Corynebacterium triggers Parkinson’s disease in people with the GG genotype. The GG signature at rs356219 is the strongest genetic risk factor for Parkinson’s disease identified to date, but it is not necessarily strong enough to cause the disease on its own. “It definitely needs a trigger,” and there is a good chance that Corynebacterium is it, said senior investigator Haydeh Payami, PhD, Professor of Neurology and Genomics at the University of Alabama, Birmingham.
Genotypes and Triggers
The finding, which was presented at the 142nd Annual Meeting of the American Neurological Association, may begin to clarify the link between the dozens of genetic risk factors for Parkinson’s disease and environmental triggers that lead to the disease. Different bacteria may be associated with different genetic risk factors. Eventually, the researchers aim to map out which genetic susceptibilities are associated with which elements of the microbiome and which genotypes are associated with other environmental factors, such as pesticides, Dr. Payami, leader of the multicenter neurogenetics research collaboration, said.
Her team genotyped SNCA rs356219 from blood samples in 197 middle-aged patients with Parkinson’s disease and 115 age-matched controls. They also extracted DNA from stool samples to see what bacteria were in their guts and then looked for interactions between rs356219 genotype, gut microbiome, and Parkinson’s disease risk.
The medical literature has been full of hints that Parkinson’s disease might be set off by something going wrong in the gastrointestinal (GI) tract. Colonic inflammation, alpha-synuclein pathology in the gut, and dysbiosis of the gut microbiome in Parkinson’s disease are among the many clues. The goal of the work was to find the link between Parkinson’s disease and its GI aberrations.
Ninety genera were identified in the stool samples, but “no matter how you looked at the data, whichever method you used, one [genus] kept coming up” for interaction with the rs356219 genotype, “and that was Corynebacterium,” Dr. Payami said.
Heightened Risk
As in past studies, the rs356219 AA genotype did not increase the odds of Parkinson’s disease, and there was no difference in microbiome abundance between patients with Parkinson’s disease and controls. The GA genotype increased the odds slightly without Corynebacterium, but it increased the odds more than fivefold when Corynebacterium was in the gut (odds ratio, 5.9). If people had GG plus Corynebacterium, however, developing Parkinson’s disease was a certainty.
Corynebacterium was more abundant in GA subjects with Parkinson’s disease than in GA subjects without Parkinson’s disease, but it was by far the most abundant in GG subjects, and every person who had the GG genotype and gut Corynebacterium also had Parkinson’s disease.
Corynebacteria are gram-positive, aerobic bacilli commonly found on the skin. Some members of the genus are opportunistic pathogens. It is not clear how they get incorporated into the gut microbiome, or if they can be wiped out selectively in the gut with antibiotics or probiotics.
Perhaps Corynebacterium in the GI tract induces expression of alpha-synuclein protein, a major component of Parkinson’s disease Lewy bodies that is known to travel from the gut to the brain. Maybe the amount expressed depends on how many Gs people have in rs356219. Perhaps “if you have two Gs, you get so much alpha-synuclein that there is no turning back, and it is enough to cause Parkinson’s disease,” Dr. Payami said.
The study was led by Zachary Wallen, a PhD candidate in Dr. Payami’s lab. The study was supported by the NIH.
—M. Alexander Otto
Hypertension Guideline Lowers Threshold to 130/80 mm Hg
ANAHEIM, CA—Thirty million Americans became hypertensive overnight with the introduction of a new high blood pressure guideline from the American College of Cardiology (ACC) and American Heart Association (AHA).
The guideline changes the definition of adult hypertension from the long-standing threshold of 140/90 mm Hg to 130/80 mm Hg. As a result, adult prevalence of hypertension in the United States increased from roughly 32% to 46%, bringing the national hypertensive population to 103 million. The guideline was presented at the AHA’s 2017 Scientific Sessions and published in the Journal of the American College of Cardiology and Hypertension.
In addition to those with hypertension, another 12% of American adults have what the new guideline calls elevated blood pressure—a systolic pressure of 120–129 mm Hg with a diastolic pressure of less than 80 mm Hg. This group warrants lifestyle interventions to arrest progression, according to the guideline. In selected subgroups, the prevalence of hypertension is even greater. Among African American men and women, for example, approximately 55% have hypertension under the new guideline. And among men and women ages 65 and older, more than three-quarters now have hypertension.
Goal Is to Transform Care
Beyond the guideline’s epidemiologic implications, it includes 106 recommendations for preventing, detecting, evaluating, and managing adult hypertension. The guideline addresses every aspect of blood pressure in American medical practice, from how it is measured to how medical systems can try to ensure that every person with a blood pressure outside the redefined limits gets a comprehensive package of interventions.
The guideline includes a risk-based approach to making treatment decisions, a reduced treatment target of less than 130/80 mm Hg, and strategies to improve treatment efficacy, said Paul K. Whelton, MD, chair of the guidelines task force and Professor of Global Health at Tulane University in New Orleans.
Some of the recommendations represent “seismic changes,” said Lawrence J. Appel, MD, Professor of Epidemiology at Johns Hopkins University in Baltimore, who was not involved in writing the guideline. In particular, the new classification of stage 1 hypertension, the emphasis on using out-of-office blood pressure measurement to confirm a diagnosis, and having the same blood pressure goal of less than 130/80 mm Hg for all patients with hypertension, regardless of age, as long as they remain ambulatory and community dwelling, are major changes, he said.
One Goal for All Adults
“The systolic blood pressure goal for older people has gone from 140 mm Hg to 150 mm Hg and now to 130 mm Hg” within a few years, commented Dr. Appel. In fact, the guideline simplifies the treatment goal to less than 130/80 mm Hg for all adults, including patients with diabetes, those with chronic kidney disease, and the elderly.
“It will be clearer and easier now that everyone should be less than 130/80 mm Hg. You will not need to remember a second target,” said Sandra J. Taler, MD, a nephrologist and Professor of Medicine at the Mayo Clinic in Rochester, Minnesota, and a member of the guidelines task force. “Some people may be upset that we changed the rules on them. They had normal blood pressure yesterday, and today it is high. But it is a good awakening, especially for using lifestyle interventions.”
Preferred Intervention: Lifestyle, Not Drugs
The guideline cites lifestyle optimization as the cornerstone of intervention for everyone and as the only endorsed intervention for patients with hypertens
The guideline may encourage “a recommitment to lifestyle changes” for preventing and managing hypertension, said the task force’s vice chair, Robert M. Carey, MD, Professor of Medicine at the University of Virginia in Charlottesville.
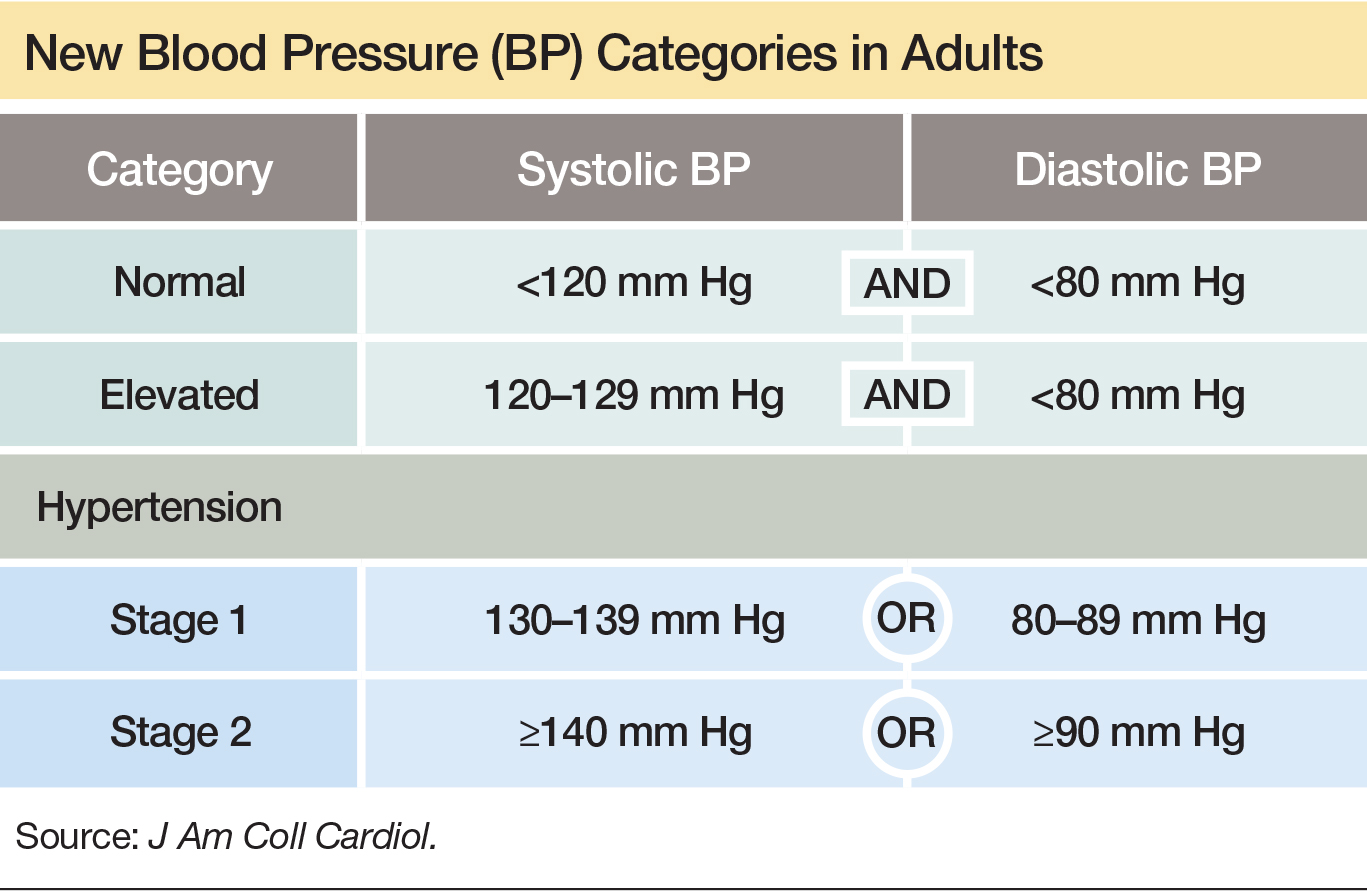
Team-Based Care Is Essential
The guideline emphasizes a team-based management approach that includes nurses, nurse practitioners, pharmacists, dietitians, and other clinicians, allowing for more frequent and focused care. Dr. Whelton and others cited the VA Health System and Kaiser-Permanente as operating team-based and system-driven blood pressure management programs that have resulted in control rates for more than 90% of patients with hypertension. The team-based approach is a key component of Target:BP, a program founded by the AHA and American Medical Association to promote implementation of the new guideline, Dr. Carey said. Another systems recommendation in the guideline is that every patient with hypertension should have a “clear, detailed, and current evidence-based plan of care.”
“Using nurse practitioners, physician assistants, and pharmacists has been shown to improve blood pressure levels,” and health systems that take this approach have had “great success,” commented Donald M. Lloyd-Jones, MD, Professor and Chairman of Preventive Medicine at Northwestern University in Chicago, who was not part of the guidelines task force. Financial penalties and incentives from payers exist to push for higher levels of blood pressure control, and the alignment of financial and health incentives should result in big changes, Dr. Lloyd-Jones predicted.
—Mitchel L. Zoler
Suggested Reading
Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection,evaluation, and management of high bood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2017 Nov 7 [Epub ahead of print].
ANAHEIM, CA—Thirty million Americans became hypertensive overnight with the introduction of a new high blood pressure guideline from the American College of Cardiology (ACC) and American Heart Association (AHA).
The guideline changes the definition of adult hypertension from the long-standing threshold of 140/90 mm Hg to 130/80 mm Hg. As a result, adult prevalence of hypertension in the United States increased from roughly 32% to 46%, bringing the national hypertensive population to 103 million. The guideline was presented at the AHA’s 2017 Scientific Sessions and published in the Journal of the American College of Cardiology and Hypertension.
In addition to those with hypertension, another 12% of American adults have what the new guideline calls elevated blood pressure—a systolic pressure of 120–129 mm Hg with a diastolic pressure of less than 80 mm Hg. This group warrants lifestyle interventions to arrest progression, according to the guideline. In selected subgroups, the prevalence of hypertension is even greater. Among African American men and women, for example, approximately 55% have hypertension under the new guideline. And among men and women ages 65 and older, more than three-quarters now have hypertension.
Goal Is to Transform Care
Beyond the guideline’s epidemiologic implications, it includes 106 recommendations for preventing, detecting, evaluating, and managing adult hypertension. The guideline addresses every aspect of blood pressure in American medical practice, from how it is measured to how medical systems can try to ensure that every person with a blood pressure outside the redefined limits gets a comprehensive package of interventions.
The guideline includes a risk-based approach to making treatment decisions, a reduced treatment target of less than 130/80 mm Hg, and strategies to improve treatment efficacy, said Paul K. Whelton, MD, chair of the guidelines task force and Professor of Global Health at Tulane University in New Orleans.
Some of the recommendations represent “seismic changes,” said Lawrence J. Appel, MD, Professor of Epidemiology at Johns Hopkins University in Baltimore, who was not involved in writing the guideline. In particular, the new classification of stage 1 hypertension, the emphasis on using out-of-office blood pressure measurement to confirm a diagnosis, and having the same blood pressure goal of less than 130/80 mm Hg for all patients with hypertension, regardless of age, as long as they remain ambulatory and community dwelling, are major changes, he said.
One Goal for All Adults
“The systolic blood pressure goal for older people has gone from 140 mm Hg to 150 mm Hg and now to 130 mm Hg” within a few years, commented Dr. Appel. In fact, the guideline simplifies the treatment goal to less than 130/80 mm Hg for all adults, including patients with diabetes, those with chronic kidney disease, and the elderly.
“It will be clearer and easier now that everyone should be less than 130/80 mm Hg. You will not need to remember a second target,” said Sandra J. Taler, MD, a nephrologist and Professor of Medicine at the Mayo Clinic in Rochester, Minnesota, and a member of the guidelines task force. “Some people may be upset that we changed the rules on them. They had normal blood pressure yesterday, and today it is high. But it is a good awakening, especially for using lifestyle interventions.”
Preferred Intervention: Lifestyle, Not Drugs
The guideline cites lifestyle optimization as the cornerstone of intervention for everyone and as the only endorsed intervention for patients with hypertens
The guideline may encourage “a recommitment to lifestyle changes” for preventing and managing hypertension, said the task force’s vice chair, Robert M. Carey, MD, Professor of Medicine at the University of Virginia in Charlottesville.

Team-Based Care Is Essential
The guideline emphasizes a team-based management approach that includes nurses, nurse practitioners, pharmacists, dietitians, and other clinicians, allowing for more frequent and focused care. Dr. Whelton and others cited the VA Health System and Kaiser-Permanente as operating team-based and system-driven blood pressure management programs that have resulted in control rates for more than 90% of patients with hypertension. The team-based approach is a key component of Target:BP, a program founded by the AHA and American Medical Association to promote implementation of the new guideline, Dr. Carey said. Another systems recommendation in the guideline is that every patient with hypertension should have a “clear, detailed, and current evidence-based plan of care.”
“Using nurse practitioners, physician assistants, and pharmacists has been shown to improve blood pressure levels,” and health systems that take this approach have had “great success,” commented Donald M. Lloyd-Jones, MD, Professor and Chairman of Preventive Medicine at Northwestern University in Chicago, who was not part of the guidelines task force. Financial penalties and incentives from payers exist to push for higher levels of blood pressure control, and the alignment of financial and health incentives should result in big changes, Dr. Lloyd-Jones predicted.
—Mitchel L. Zoler
Suggested Reading
Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection,evaluation, and management of high bood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2017 Nov 7 [Epub ahead of print].
ANAHEIM, CA—Thirty million Americans became hypertensive overnight with the introduction of a new high blood pressure guideline from the American College of Cardiology (ACC) and American Heart Association (AHA).
The guideline changes the definition of adult hypertension from the long-standing threshold of 140/90 mm Hg to 130/80 mm Hg. As a result, adult prevalence of hypertension in the United States increased from roughly 32% to 46%, bringing the national hypertensive population to 103 million. The guideline was presented at the AHA’s 2017 Scientific Sessions and published in the Journal of the American College of Cardiology and Hypertension.
In addition to those with hypertension, another 12% of American adults have what the new guideline calls elevated blood pressure—a systolic pressure of 120–129 mm Hg with a diastolic pressure of less than 80 mm Hg. This group warrants lifestyle interventions to arrest progression, according to the guideline. In selected subgroups, the prevalence of hypertension is even greater. Among African American men and women, for example, approximately 55% have hypertension under the new guideline. And among men and women ages 65 and older, more than three-quarters now have hypertension.
Goal Is to Transform Care
Beyond the guideline’s epidemiologic implications, it includes 106 recommendations for preventing, detecting, evaluating, and managing adult hypertension. The guideline addresses every aspect of blood pressure in American medical practice, from how it is measured to how medical systems can try to ensure that every person with a blood pressure outside the redefined limits gets a comprehensive package of interventions.
The guideline includes a risk-based approach to making treatment decisions, a reduced treatment target of less than 130/80 mm Hg, and strategies to improve treatment efficacy, said Paul K. Whelton, MD, chair of the guidelines task force and Professor of Global Health at Tulane University in New Orleans.
Some of the recommendations represent “seismic changes,” said Lawrence J. Appel, MD, Professor of Epidemiology at Johns Hopkins University in Baltimore, who was not involved in writing the guideline. In particular, the new classification of stage 1 hypertension, the emphasis on using out-of-office blood pressure measurement to confirm a diagnosis, and having the same blood pressure goal of less than 130/80 mm Hg for all patients with hypertension, regardless of age, as long as they remain ambulatory and community dwelling, are major changes, he said.
One Goal for All Adults
“The systolic blood pressure goal for older people has gone from 140 mm Hg to 150 mm Hg and now to 130 mm Hg” within a few years, commented Dr. Appel. In fact, the guideline simplifies the treatment goal to less than 130/80 mm Hg for all adults, including patients with diabetes, those with chronic kidney disease, and the elderly.
“It will be clearer and easier now that everyone should be less than 130/80 mm Hg. You will not need to remember a second target,” said Sandra J. Taler, MD, a nephrologist and Professor of Medicine at the Mayo Clinic in Rochester, Minnesota, and a member of the guidelines task force. “Some people may be upset that we changed the rules on them. They had normal blood pressure yesterday, and today it is high. But it is a good awakening, especially for using lifestyle interventions.”
Preferred Intervention: Lifestyle, Not Drugs
The guideline cites lifestyle optimization as the cornerstone of intervention for everyone and as the only endorsed intervention for patients with hypertens
The guideline may encourage “a recommitment to lifestyle changes” for preventing and managing hypertension, said the task force’s vice chair, Robert M. Carey, MD, Professor of Medicine at the University of Virginia in Charlottesville.

Team-Based Care Is Essential
The guideline emphasizes a team-based management approach that includes nurses, nurse practitioners, pharmacists, dietitians, and other clinicians, allowing for more frequent and focused care. Dr. Whelton and others cited the VA Health System and Kaiser-Permanente as operating team-based and system-driven blood pressure management programs that have resulted in control rates for more than 90% of patients with hypertension. The team-based approach is a key component of Target:BP, a program founded by the AHA and American Medical Association to promote implementation of the new guideline, Dr. Carey said. Another systems recommendation in the guideline is that every patient with hypertension should have a “clear, detailed, and current evidence-based plan of care.”
“Using nurse practitioners, physician assistants, and pharmacists has been shown to improve blood pressure levels,” and health systems that take this approach have had “great success,” commented Donald M. Lloyd-Jones, MD, Professor and Chairman of Preventive Medicine at Northwestern University in Chicago, who was not part of the guidelines task force. Financial penalties and incentives from payers exist to push for higher levels of blood pressure control, and the alignment of financial and health incentives should result in big changes, Dr. Lloyd-Jones predicted.
—Mitchel L. Zoler
Suggested Reading
Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection,evaluation, and management of high bood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2017 Nov 7 [Epub ahead of print].
Mass Psychogenic Illness: Risk Factors and Treatment
KANSAS CITY, MO—Mass psychogenic illness is a condition where signs and symptoms spread rapidly between members of a cohesive group. The illness may entail loss or alteration of function, and patients unconsciously manifest physical symptoms. “Our brains are wired to be empathetic and to pick up on the symptoms of others,” said Jonathan W. Mink, MD, PhD, at the 46th Annual Meeting of the Child Neurology Society. “In mass psychogenic illness, those symptoms persist, and there is that contagion from individual to individual.”
A recent occurrence of mass psychogenic illness in Le Roy, New York, suggests that news media attention and patients’ use of social media may play a role in the spread and perpetuation of symptoms. Few descriptions of mass psychogenic illness have been published in pediatric neurology journals, but prior cases may provide useful information about the condition, its risk factors, and treatment, Dr. Mink said.
“It has been argued whether this is a subcategory of conversion disorder. There has been some discussion of whether some of this is … factitious or malingering. I would argue that it does not really matter,” said Dr. Mink, Professor of Pediatric Neurology at the University of Rochester in New York. “What matters is that we understand that this is an entity. Exactly why [it occurs] may not be relevant to treatment.”
Teens With Tic-Like Movements
Between August 2011 and January 2012, 19 teenage students at Le Roy Junior–Senior High School developed a sudden onset of tic-like movements. Two had a prior diagnosis of a tic disorder: one had Tourette syndrome, and one had chronic motor tic disorder. Eighteen of the 19 were girls. Six of the 19 had additional symptoms (eg, syncope and paroxysmal nonepileptic attacks), and 10 had clearly identified significant life stressors. All of the patients had otherwise normal neurologic exams. The movements “were not tics,” Dr. Mink said. “They had no premonitory urge. They were not suppressible. They were not stereotyped. But they were often referred to as tic-like.”
The cases drew national and local media attention. News reports portrayed the cases as mysterious and suggested that the symptoms could have autoimmune or environmental causes. Furthermore, social media may have contributed to the spread of symptoms. “It has often been said that mass psychogenic illness, or what was formerly called mass hysteria, was conveyed by line-of-sight transmission. It was seeing the symptoms of other people,” Dr. Mink said. “Many of these girls had posted videos or detailed descriptions of their symptoms.”
Most of the patients were seen at the Dent Neurologic Institute in Buffalo. To help confirm the diagnosis, a majority of the patients also were seen at Dr. Mink’s center in Rochester.
Insights From Prior Research
In 2004, Roach and Langley described an occurrence of mass psychogenic illness among a cohort of 10 teenage girls at a school in rural North Carolina. The patients developed paroxysmal episodes that resembled epilepsy or syncope. The episodes were relatively infrequent and typically occurred between classes. Four patients underwent video-EEG monitoring, which showed that the seizures were not epileptic. Symptoms mostly resolved after a two-week holiday break from school. “More than half of them had been treated with one or more antiepileptic medications,” Dr. Mink said.
A study of environmental chemical incidents in the United Kingdom found that a substantial minority of cases could involve mass psychogenic illness. Page et al examined incidents over a 15-month period between 2007 and 2008. Of 965 total incidents, 747 were eligible for inclusion in the study, and 280 were selected randomly for detailed evaluation.
The British researchers’ criteria for diagnosing mass psychogenic illness included the presence of somatic symptoms, a preexisting social connection between two or more of the affected people, the spread of symptoms from person to person, and the attribution of symptoms by affected individuals or by their parents or caregivers to a threatening external agent of a physical or spiritual nature. Finally, the symptoms and signs were not compatible with environmental exposures that reasonably could have been expected to be present at the time.
Nineteen of the 280 incidents were classified as probable or highly probable mass psychogenic illness (six highly probable and 13 probable), which represented 7% of the incidents and 16% of incidents in which people reported symptoms that were attributed to the chemical incident. Factors that were more common among cases of mass psychogenic illness included the presence of a nonsmoke odor and occurrence in a school or health care facility.
Experimental Induction of Mass Psychogenic Illness
Broderick et al in 2011 described a randomized controlled experimental induction of mass psychogenic illness. Their study included 39 healthy adults with a mean age of 42. A little more than half were women, half were college graduates, and almost 80% were Caucasian.
A control group sat in a room and engaged in quiet activity, while two psychogenic illness induction groups received a pill. One of the induction groups was shown a video (ie, the pill-plus-media group).
Researchers told participants in the pill groups that the study was designed to further evaluate the side effects of a new carrier compound for an antiviral medication. The participants were told that the compound contained only cellulose and did not produce serious side effects.
In the pill groups, confederates (one man and one woman) feigned illness (eg, nausea, dizziness, and headache) about 20 minutes after participants took the pills. Nurses attended to the confederates and to any participant who simulated or experienced symptoms, by taking their blood pressure and pulse, providing cool cloths for their foreheads, supplying bowls for potential vomiting, and sometimes putting participants with symptoms on gurneys outside the room within view of the other participants. An hour after taking the pills, the pill-plus-media group watched a public television documentary about the 1918 flu pandemic.
All three groups were assessed at baseline, at one hour, and at two hours. Participants rated their current symptoms by questionnaire, and nurses measured participants’ heart rate and blood pressure. Researchers debriefed all participants after the third assessment, and participants completed a psychosocial risk factor battery within a week of the initial experiment.
The primary outcome was symptom score. Participants in all three groups had some symptoms at baseline (eg, elevated heart rate and slight discomfort). At one hour, the control group had reduced symptoms, whereas the pill groups had increased symptom scores. At two hours, the control group still had few symptoms, but the psychogenic illness induction groups’ symptoms increased further. Symptoms did not differ between the pill-only and pill-plus-media groups, however.
An analysis of the psychosocial risk factor questionnaires found that participants’ total number of traumatic life events was positively associated with increased symptoms, but this relationship was not particularly strong. “It seems that the most important thing is … being exposed to people who are feigning symptoms or displaying symptoms in the setting of being told that there is a potential agent that might cause those symptoms,” Dr. Mink said.
Outcomes in Le Roy
Amid media coverage of the cases in Le Roy, Dr. Mink declined daily invitations to appear on television. “The last time I talked to the producer, I said, ‘You know, you would do these girls a big favor if you would just leave them alone,’” he said. “While the media attention persisted for another week, the improvement of their symptoms did coincide with reduced media attention, so one can speculate that that might have played a role.”
The patients in Le Roy received varying therapies, including cognitive behavioral therapy and supportive psychotherapy. They all received education about functional neurologic disorders. Some received pharmacothe
At last follow-up about two years ago, five of the 19 no longer had symptoms. Six of the 19 had experienced a greater than 85% improvement. A couple of patients continued to have symptoms, including the patients with a prior diagnosis of a chronic tic disorder. Two patients who had not improved sued the school district and alleged environmental toxins as the cause of their symptoms. The other patients were lost to follow up.
Treatment Principles
When treating patients with mass psychogenic illness, “First of all, you have to be an ally of your patient and not challenge the veracity,” Dr. Mink said. “For some of these girls, I think that there was some factitious component,” but this factor was not especially relevant to treatment recommendations.
“For mass psychogenic illness, reducing attention from social media groups and perhaps finding a way to disrupt the social cohesiveness of that group, at least until the symptoms can improve,” may be beneficial, he said. “Regular follow-up is helpful for all conversion disorders…. You do not want the patient to have to get worse to be able to see their doctor.”
Once neurologists have confirmed the diagnosis of mass psychogenic illness, they should “reinforce the certainty of the diagnosis” to occupational therapists, physical therapists, psychotherapists, and others on the health care team who treat these patients, Dr. Mink said. In addition,“It is important to prepare the therapist, particularly in an unusual situation like this where there has been a lot of media attention.”
—Jake Remaly
Suggested Reading
Bartholomew RE, Wessely S, Rubin GJ. Mass psychogenic illness and the social network: is it changing the pattern of outbreaks? J R Soc Med. 2012;105(12):509-512.
Broderick JE, Kaplan-Liss E, Bass E. Experimental induction of psychogenic illness in the context of a medical event and media exposure. Am J Disaster Med. 2011;6(3):163-172.
Page LA, Keshishian C, Leonardi G, et al. Frequency and predictors of mass psychogenic illness. Epidemiology. 2010;21(5):744-747.
Roach ES, Langley RL. Episodic neurological dysfunction due to mass hysteria. Arch Neurol. 2004;61(8):1269-1272.
KANSAS CITY, MO—Mass psychogenic illness is a condition where signs and symptoms spread rapidly between members of a cohesive group. The illness may entail loss or alteration of function, and patients unconsciously manifest physical symptoms. “Our brains are wired to be empathetic and to pick up on the symptoms of others,” said Jonathan W. Mink, MD, PhD, at the 46th Annual Meeting of the Child Neurology Society. “In mass psychogenic illness, those symptoms persist, and there is that contagion from individual to individual.”
A recent occurrence of mass psychogenic illness in Le Roy, New York, suggests that news media attention and patients’ use of social media may play a role in the spread and perpetuation of symptoms. Few descriptions of mass psychogenic illness have been published in pediatric neurology journals, but prior cases may provide useful information about the condition, its risk factors, and treatment, Dr. Mink said.
“It has been argued whether this is a subcategory of conversion disorder. There has been some discussion of whether some of this is … factitious or malingering. I would argue that it does not really matter,” said Dr. Mink, Professor of Pediatric Neurology at the University of Rochester in New York. “What matters is that we understand that this is an entity. Exactly why [it occurs] may not be relevant to treatment.”
Teens With Tic-Like Movements
Between August 2011 and January 2012, 19 teenage students at Le Roy Junior–Senior High School developed a sudden onset of tic-like movements. Two had a prior diagnosis of a tic disorder: one had Tourette syndrome, and one had chronic motor tic disorder. Eighteen of the 19 were girls. Six of the 19 had additional symptoms (eg, syncope and paroxysmal nonepileptic attacks), and 10 had clearly identified significant life stressors. All of the patients had otherwise normal neurologic exams. The movements “were not tics,” Dr. Mink said. “They had no premonitory urge. They were not suppressible. They were not stereotyped. But they were often referred to as tic-like.”
The cases drew national and local media attention. News reports portrayed the cases as mysterious and suggested that the symptoms could have autoimmune or environmental causes. Furthermore, social media may have contributed to the spread of symptoms. “It has often been said that mass psychogenic illness, or what was formerly called mass hysteria, was conveyed by line-of-sight transmission. It was seeing the symptoms of other people,” Dr. Mink said. “Many of these girls had posted videos or detailed descriptions of their symptoms.”
Most of the patients were seen at the Dent Neurologic Institute in Buffalo. To help confirm the diagnosis, a majority of the patients also were seen at Dr. Mink’s center in Rochester.
Insights From Prior Research
In 2004, Roach and Langley described an occurrence of mass psychogenic illness among a cohort of 10 teenage girls at a school in rural North Carolina. The patients developed paroxysmal episodes that resembled epilepsy or syncope. The episodes were relatively infrequent and typically occurred between classes. Four patients underwent video-EEG monitoring, which showed that the seizures were not epileptic. Symptoms mostly resolved after a two-week holiday break from school. “More than half of them had been treated with one or more antiepileptic medications,” Dr. Mink said.
A study of environmental chemical incidents in the United Kingdom found that a substantial minority of cases could involve mass psychogenic illness. Page et al examined incidents over a 15-month period between 2007 and 2008. Of 965 total incidents, 747 were eligible for inclusion in the study, and 280 were selected randomly for detailed evaluation.
The British researchers’ criteria for diagnosing mass psychogenic illness included the presence of somatic symptoms, a preexisting social connection between two or more of the affected people, the spread of symptoms from person to person, and the attribution of symptoms by affected individuals or by their parents or caregivers to a threatening external agent of a physical or spiritual nature. Finally, the symptoms and signs were not compatible with environmental exposures that reasonably could have been expected to be present at the time.
Nineteen of the 280 incidents were classified as probable or highly probable mass psychogenic illness (six highly probable and 13 probable), which represented 7% of the incidents and 16% of incidents in which people reported symptoms that were attributed to the chemical incident. Factors that were more common among cases of mass psychogenic illness included the presence of a nonsmoke odor and occurrence in a school or health care facility.
Experimental Induction of Mass Psychogenic Illness
Broderick et al in 2011 described a randomized controlled experimental induction of mass psychogenic illness. Their study included 39 healthy adults with a mean age of 42. A little more than half were women, half were college graduates, and almost 80% were Caucasian.
A control group sat in a room and engaged in quiet activity, while two psychogenic illness induction groups received a pill. One of the induction groups was shown a video (ie, the pill-plus-media group).
Researchers told participants in the pill groups that the study was designed to further evaluate the side effects of a new carrier compound for an antiviral medication. The participants were told that the compound contained only cellulose and did not produce serious side effects.
In the pill groups, confederates (one man and one woman) feigned illness (eg, nausea, dizziness, and headache) about 20 minutes after participants took the pills. Nurses attended to the confederates and to any participant who simulated or experienced symptoms, by taking their blood pressure and pulse, providing cool cloths for their foreheads, supplying bowls for potential vomiting, and sometimes putting participants with symptoms on gurneys outside the room within view of the other participants. An hour after taking the pills, the pill-plus-media group watched a public television documentary about the 1918 flu pandemic.
All three groups were assessed at baseline, at one hour, and at two hours. Participants rated their current symptoms by questionnaire, and nurses measured participants’ heart rate and blood pressure. Researchers debriefed all participants after the third assessment, and participants completed a psychosocial risk factor battery within a week of the initial experiment.
The primary outcome was symptom score. Participants in all three groups had some symptoms at baseline (eg, elevated heart rate and slight discomfort). At one hour, the control group had reduced symptoms, whereas the pill groups had increased symptom scores. At two hours, the control group still had few symptoms, but the psychogenic illness induction groups’ symptoms increased further. Symptoms did not differ between the pill-only and pill-plus-media groups, however.
An analysis of the psychosocial risk factor questionnaires found that participants’ total number of traumatic life events was positively associated with increased symptoms, but this relationship was not particularly strong. “It seems that the most important thing is … being exposed to people who are feigning symptoms or displaying symptoms in the setting of being told that there is a potential agent that might cause those symptoms,” Dr. Mink said.
Outcomes in Le Roy
Amid media coverage of the cases in Le Roy, Dr. Mink declined daily invitations to appear on television. “The last time I talked to the producer, I said, ‘You know, you would do these girls a big favor if you would just leave them alone,’” he said. “While the media attention persisted for another week, the improvement of their symptoms did coincide with reduced media attention, so one can speculate that that might have played a role.”
The patients in Le Roy received varying therapies, including cognitive behavioral therapy and supportive psychotherapy. They all received education about functional neurologic disorders. Some received pharmacothe
At last follow-up about two years ago, five of the 19 no longer had symptoms. Six of the 19 had experienced a greater than 85% improvement. A couple of patients continued to have symptoms, including the patients with a prior diagnosis of a chronic tic disorder. Two patients who had not improved sued the school district and alleged environmental toxins as the cause of their symptoms. The other patients were lost to follow up.
Treatment Principles
When treating patients with mass psychogenic illness, “First of all, you have to be an ally of your patient and not challenge the veracity,” Dr. Mink said. “For some of these girls, I think that there was some factitious component,” but this factor was not especially relevant to treatment recommendations.
“For mass psychogenic illness, reducing attention from social media groups and perhaps finding a way to disrupt the social cohesiveness of that group, at least until the symptoms can improve,” may be beneficial, he said. “Regular follow-up is helpful for all conversion disorders…. You do not want the patient to have to get worse to be able to see their doctor.”
Once neurologists have confirmed the diagnosis of mass psychogenic illness, they should “reinforce the certainty of the diagnosis” to occupational therapists, physical therapists, psychotherapists, and others on the health care team who treat these patients, Dr. Mink said. In addition,“It is important to prepare the therapist, particularly in an unusual situation like this where there has been a lot of media attention.”
—Jake Remaly
Suggested Reading
Bartholomew RE, Wessely S, Rubin GJ. Mass psychogenic illness and the social network: is it changing the pattern of outbreaks? J R Soc Med. 2012;105(12):509-512.
Broderick JE, Kaplan-Liss E, Bass E. Experimental induction of psychogenic illness in the context of a medical event and media exposure. Am J Disaster Med. 2011;6(3):163-172.
Page LA, Keshishian C, Leonardi G, et al. Frequency and predictors of mass psychogenic illness. Epidemiology. 2010;21(5):744-747.
Roach ES, Langley RL. Episodic neurological dysfunction due to mass hysteria. Arch Neurol. 2004;61(8):1269-1272.
KANSAS CITY, MO—Mass psychogenic illness is a condition where signs and symptoms spread rapidly between members of a cohesive group. The illness may entail loss or alteration of function, and patients unconsciously manifest physical symptoms. “Our brains are wired to be empathetic and to pick up on the symptoms of others,” said Jonathan W. Mink, MD, PhD, at the 46th Annual Meeting of the Child Neurology Society. “In mass psychogenic illness, those symptoms persist, and there is that contagion from individual to individual.”
A recent occurrence of mass psychogenic illness in Le Roy, New York, suggests that news media attention and patients’ use of social media may play a role in the spread and perpetuation of symptoms. Few descriptions of mass psychogenic illness have been published in pediatric neurology journals, but prior cases may provide useful information about the condition, its risk factors, and treatment, Dr. Mink said.
“It has been argued whether this is a subcategory of conversion disorder. There has been some discussion of whether some of this is … factitious or malingering. I would argue that it does not really matter,” said Dr. Mink, Professor of Pediatric Neurology at the University of Rochester in New York. “What matters is that we understand that this is an entity. Exactly why [it occurs] may not be relevant to treatment.”
Teens With Tic-Like Movements
Between August 2011 and January 2012, 19 teenage students at Le Roy Junior–Senior High School developed a sudden onset of tic-like movements. Two had a prior diagnosis of a tic disorder: one had Tourette syndrome, and one had chronic motor tic disorder. Eighteen of the 19 were girls. Six of the 19 had additional symptoms (eg, syncope and paroxysmal nonepileptic attacks), and 10 had clearly identified significant life stressors. All of the patients had otherwise normal neurologic exams. The movements “were not tics,” Dr. Mink said. “They had no premonitory urge. They were not suppressible. They were not stereotyped. But they were often referred to as tic-like.”
The cases drew national and local media attention. News reports portrayed the cases as mysterious and suggested that the symptoms could have autoimmune or environmental causes. Furthermore, social media may have contributed to the spread of symptoms. “It has often been said that mass psychogenic illness, or what was formerly called mass hysteria, was conveyed by line-of-sight transmission. It was seeing the symptoms of other people,” Dr. Mink said. “Many of these girls had posted videos or detailed descriptions of their symptoms.”
Most of the patients were seen at the Dent Neurologic Institute in Buffalo. To help confirm the diagnosis, a majority of the patients also were seen at Dr. Mink’s center in Rochester.
Insights From Prior Research
In 2004, Roach and Langley described an occurrence of mass psychogenic illness among a cohort of 10 teenage girls at a school in rural North Carolina. The patients developed paroxysmal episodes that resembled epilepsy or syncope. The episodes were relatively infrequent and typically occurred between classes. Four patients underwent video-EEG monitoring, which showed that the seizures were not epileptic. Symptoms mostly resolved after a two-week holiday break from school. “More than half of them had been treated with one or more antiepileptic medications,” Dr. Mink said.
A study of environmental chemical incidents in the United Kingdom found that a substantial minority of cases could involve mass psychogenic illness. Page et al examined incidents over a 15-month period between 2007 and 2008. Of 965 total incidents, 747 were eligible for inclusion in the study, and 280 were selected randomly for detailed evaluation.
The British researchers’ criteria for diagnosing mass psychogenic illness included the presence of somatic symptoms, a preexisting social connection between two or more of the affected people, the spread of symptoms from person to person, and the attribution of symptoms by affected individuals or by their parents or caregivers to a threatening external agent of a physical or spiritual nature. Finally, the symptoms and signs were not compatible with environmental exposures that reasonably could have been expected to be present at the time.
Nineteen of the 280 incidents were classified as probable or highly probable mass psychogenic illness (six highly probable and 13 probable), which represented 7% of the incidents and 16% of incidents in which people reported symptoms that were attributed to the chemical incident. Factors that were more common among cases of mass psychogenic illness included the presence of a nonsmoke odor and occurrence in a school or health care facility.
Experimental Induction of Mass Psychogenic Illness
Broderick et al in 2011 described a randomized controlled experimental induction of mass psychogenic illness. Their study included 39 healthy adults with a mean age of 42. A little more than half were women, half were college graduates, and almost 80% were Caucasian.
A control group sat in a room and engaged in quiet activity, while two psychogenic illness induction groups received a pill. One of the induction groups was shown a video (ie, the pill-plus-media group).
Researchers told participants in the pill groups that the study was designed to further evaluate the side effects of a new carrier compound for an antiviral medication. The participants were told that the compound contained only cellulose and did not produce serious side effects.
In the pill groups, confederates (one man and one woman) feigned illness (eg, nausea, dizziness, and headache) about 20 minutes after participants took the pills. Nurses attended to the confederates and to any participant who simulated or experienced symptoms, by taking their blood pressure and pulse, providing cool cloths for their foreheads, supplying bowls for potential vomiting, and sometimes putting participants with symptoms on gurneys outside the room within view of the other participants. An hour after taking the pills, the pill-plus-media group watched a public television documentary about the 1918 flu pandemic.
All three groups were assessed at baseline, at one hour, and at two hours. Participants rated their current symptoms by questionnaire, and nurses measured participants’ heart rate and blood pressure. Researchers debriefed all participants after the third assessment, and participants completed a psychosocial risk factor battery within a week of the initial experiment.
The primary outcome was symptom score. Participants in all three groups had some symptoms at baseline (eg, elevated heart rate and slight discomfort). At one hour, the control group had reduced symptoms, whereas the pill groups had increased symptom scores. At two hours, the control group still had few symptoms, but the psychogenic illness induction groups’ symptoms increased further. Symptoms did not differ between the pill-only and pill-plus-media groups, however.
An analysis of the psychosocial risk factor questionnaires found that participants’ total number of traumatic life events was positively associated with increased symptoms, but this relationship was not particularly strong. “It seems that the most important thing is … being exposed to people who are feigning symptoms or displaying symptoms in the setting of being told that there is a potential agent that might cause those symptoms,” Dr. Mink said.
Outcomes in Le Roy
Amid media coverage of the cases in Le Roy, Dr. Mink declined daily invitations to appear on television. “The last time I talked to the producer, I said, ‘You know, you would do these girls a big favor if you would just leave them alone,’” he said. “While the media attention persisted for another week, the improvement of their symptoms did coincide with reduced media attention, so one can speculate that that might have played a role.”
The patients in Le Roy received varying therapies, including cognitive behavioral therapy and supportive psychotherapy. They all received education about functional neurologic disorders. Some received pharmacothe
At last follow-up about two years ago, five of the 19 no longer had symptoms. Six of the 19 had experienced a greater than 85% improvement. A couple of patients continued to have symptoms, including the patients with a prior diagnosis of a chronic tic disorder. Two patients who had not improved sued the school district and alleged environmental toxins as the cause of their symptoms. The other patients were lost to follow up.
Treatment Principles
When treating patients with mass psychogenic illness, “First of all, you have to be an ally of your patient and not challenge the veracity,” Dr. Mink said. “For some of these girls, I think that there was some factitious component,” but this factor was not especially relevant to treatment recommendations.
“For mass psychogenic illness, reducing attention from social media groups and perhaps finding a way to disrupt the social cohesiveness of that group, at least until the symptoms can improve,” may be beneficial, he said. “Regular follow-up is helpful for all conversion disorders…. You do not want the patient to have to get worse to be able to see their doctor.”
Once neurologists have confirmed the diagnosis of mass psychogenic illness, they should “reinforce the certainty of the diagnosis” to occupational therapists, physical therapists, psychotherapists, and others on the health care team who treat these patients, Dr. Mink said. In addition,“It is important to prepare the therapist, particularly in an unusual situation like this where there has been a lot of media attention.”
—Jake Remaly
Suggested Reading
Bartholomew RE, Wessely S, Rubin GJ. Mass psychogenic illness and the social network: is it changing the pattern of outbreaks? J R Soc Med. 2012;105(12):509-512.
Broderick JE, Kaplan-Liss E, Bass E. Experimental induction of psychogenic illness in the context of a medical event and media exposure. Am J Disaster Med. 2011;6(3):163-172.
Page LA, Keshishian C, Leonardi G, et al. Frequency and predictors of mass psychogenic illness. Epidemiology. 2010;21(5):744-747.
Roach ES, Langley RL. Episodic neurological dysfunction due to mass hysteria. Arch Neurol. 2004;61(8):1269-1272.
MS: Past, Present, and Future
Stuart D. Cook, MD, and Abdul Rahman Alchaki
Dr. Cook is the Ruth Dunietz Kushner and Michael Jay Serwitz Professor of Neurology/Neurosciences at Rutgers, the State University of New Jersey, Newark. Dr. Alchaki is a resident in the Deptartment of Neurology/Neurosciences at Rutgers, the State University of New Jersey, Newark.
Disclosure: Stuart Cook has received honoraria for lectures from Bayer HealthCare and Merck Serono. He has served as a consultant for Merck Serono, Bayer HealthCare, Teva, Novartis, Sanofi-Aventis, Biogen Idec, and Actinobac Biomed. He has served on steering committees for the BEYOND and CLARITY Studies and as a member of Advisory Boards for Merck Serono, Bayer HealthCare, Teva, Biogen Idec, Sanofi Aventis, and Actinobac Biomed.
The Initial Years (1838 to 1930s)
The earliest recognition of MS clinical features and pathology was attributed to Jean-Martin Charcot, Robert Carswell, and Jean Cruveilhier in Europe from 1838 to 1868. Beyond those early descriptions, relatively few MS breakthroughs occurred until the 1930s, when Thomas Rivers discovered experimental autoimmune encephalomyelitis (EAE), a demyelinating disease, in animals. His insightful concepts were widely cited and ultimately contributed to undestanding of the immune mechanisms of MS and acute disseminated encephalomyelitis (ADEM).
Advances in Diagnosis (1965 to 1992)
In 1965, Schumacher et al provided the essential clinical criteria for MS diagnosis. Poser et al refined these criteria in 1983. In 2001, McDonald et al added neuroimaging, CSF analysis, and evoked potentials to further complement MS clinical diagnosis. For the first time, the disease could generally be recognized.
Early Treatments
Various treatments for MS were tried over the years, without great success. However, in 1953, a small descriptive trial by Miller and Gibbons reported clinical benefits in patients using intramuscular (IM) adrenocorticotropic hormone (ACTH) for MS and disseminated encephalomyelitis. This was followed in 1970 by a Cooperative Study of IM ACTH versus placebo by Rose et al, which resulted in ACTH, and subsequently oral corticosteroids, being widely used to treat MS, particularly for acute exacerbations of the disease. However, robust evidence of long-term steroids remain limited, even to the present.
High-Dose Steroids
By 1980, the initial descriptive treatment of high-dose intravenous (IV) steroids for demyelinating diseases, including MS and transverse myelitis, by Dowling et al resulted in rapid clinical improvement in some patients. This result was ultimately confirmed by others. High-dose IV steroids became the gold standard for acute attacks, particularly those aggressive in nature. In the mid 1980s, work by Troiano et al, as well as others, showed that the rapid use of high-dose IV as well as oral steroids showed similar effects, with reduction or elimination of CT contrast-enhancing lesions within as few as eight hours, while lower doses or alternative-day treatments were less effective. In addition, descriptive studies of immune modulatory and immunosuppressive drugs, as well as small randomized studies, were published. These agents did not receive FDA approval.
The Golden Age of Therapy (1993 to 2018)
A remarkable era in MS prognosis and treatment began with immunomodulation injections of Betaseron (INFβ-1b), Avonex (INFβ-1a), and Copaxone (glatiramer acetate). This can be attributed, at least in part, to advances in molecular biology, genetics, and neuroimaging, and support by corporate, private, and public funding. Since the initial FDA approval of INFβ-1b, 15 MS therapies have become clinically available, including eight injectables, three orals, and four infusion treatments (see Table 1). In addition, two other drugs have been FDA approved for uses other than MS: rituximab (approved for lymphoma) and cladribine (for hairy cell leukemia), with the latter now approved by the European Medicines Agency for MS. Table 1 depicts characteristics of these therapies approved by US or European agencies (or for other disorders increasingly used off label for MS) in an attempt to compare annual relapse rates (ARR) and decreases in the percent of gadolinium-enhancing MS lesions versus placebo. This information was chosen because ARR has been uniformly selected and defined for such trials, while percent decrease of gadolinium-enhancing lesions on MRI has been the most sensitive barometer available for assessing acute clinical activity. As a result, risk-benefit considerations have been critical in evaluating these drug treatments, with efficacy improving greatly over time, whereas risks have been more variable.
Disease Categories
In 1996, Lublin and Reingold provided a new classification, not specifically for the diagnosis of MS, but rather for the clinical course of the disease. Initially, there were four categories—relapsing-remitting MS, secondary progressive MS, primary progressive MS, and progressive-relapsing MS—that were universally identified. These were thought to be relatively distinct clinical categories, but over time it became clear that the classification did not fully distinguish MS disease activity within these categories. For that reason, it was subsequently recommended, by Lincoln et al in 2009 and Cook et al in 2012, to include MRI, a vastly more sensitive modality, as well as clinical data in assessing disease activity.
On another note, MS and neuromyelitis optica (NMO), although having similar features, were clearly identified as different diseases by Lennon et al in 2004. Differences in pathology, clinical characteristics, immunology, and therapy separate the two disorders.
MRI in MS
Work by Young et al in 1981 established the central role of MRI brain imaging in MS diagnosis and therapeutic considerations. Since then it has become ubiquitous.
An example of a sensitive and highly productive MRI protocol is the BECOME study of MS and clinically isolated syndrome by Cadavid et al from 2009 to 2017. In this study, IFNβ-1b was compared with glatiramer acetate treatment. Cadavid et al used a 3T scanner with triple-dose gadolinium, performed monthly for as long as 24 consecutive months. This unique study brought about a virtual gold mine of valuable research and clinical information. This included proof that gadolinium-enhancing lesions persisted for six months or more, evidence of a 30:1 ratio of new MRI brain lesions to clinical activity, and documentation that 96% of T2 lesions and black holes derive from prior gadolinium-enhancing lesions. It was further noted that 80% to 90% of acute black holes disappeared with treatment and 75% to 80% of patients taking IFNβ-1b or glatiramer acetate had new MRI lesions despite continuing treatment. Perhaps most interestingly, monthly MRIs could predict relapse and disability in a relatively small number of patients, depending upon the frequency and activity of MRI lesions. In 2017, Brown et al documented that magnetization transfer ratio recovery in MS brain lesions occurred more significantly with glatiramer acetate than with IFNβ-1b, whereas more chronic black hole lesions were found with glatiramer acetate. Also in 2017, Maranzano et al found evidence of acute inflammatory leukocortical lesions, which were not as well recognized previously.
In summary, it has become increasingly clear that MRI is the most sen
The Future of MS
While it is not yet a curable disease, there is growing evidence that MS prognosis has improved and will continue to improve. This is based on incremental decreases in acute MS exacerbations, progressive disability, and MRI lesion activity, as well as a combination of the three—no evidence of disease activity (NEDA).
Not only are drug therapies becoming more effective, but patients and physicians now have many more treatment options to carefully consider with regard to efficacy, side effect profiles, treatment frequency, route of administration, cost, and quality of life. Newer drugs with different mechanisms of action such as cladribine, now approved in Europe, fulfill most of these beneficial criteria (see Giovannoni et al, 2010). More promising MS treatments, including long-acting induction therapies, are still being evaluated. As with other complex diseases, multiple therapies are likely to be used as well.
In summary, compared with the time before 1993, MS will be much less likely to be a progressive disease, and quality of life will be much improved. In my opinion, patients will be less fearful about their prognosis than ever before, and with appropriate evaluations and treatments, we may realize that disabling MS will be far less common.
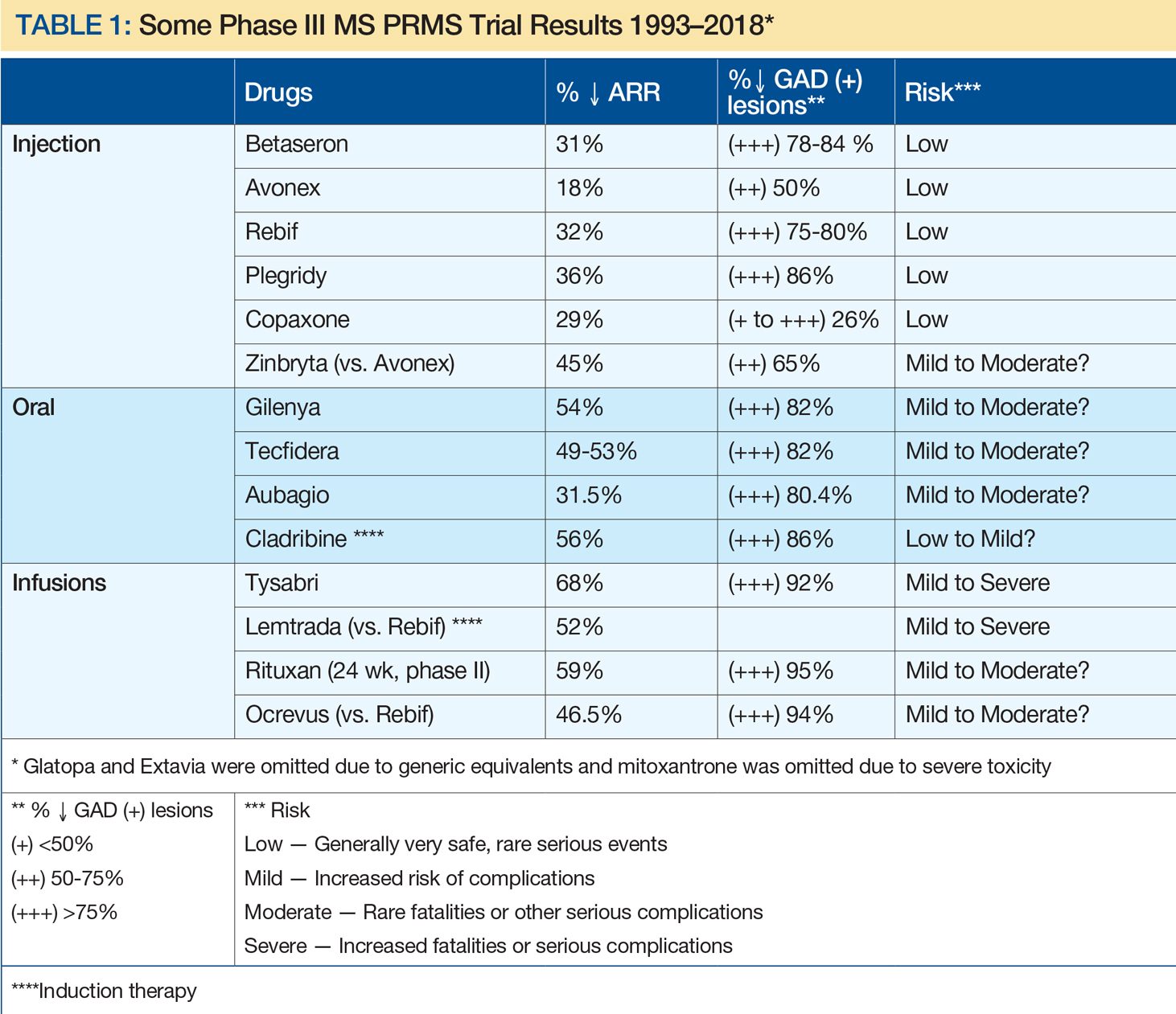
Suggested Reading
Brown JW, Pardini M, Brownlee WJ, et al. An abnormal periventricular magnetization transfer ratio gradient occurs early in multiple sclerosis. Brain. 2017;140(2):387-398.
Cadavid D, Wolansky LJ, Skurnick J, et al. Efficacy of treatment of MS with IFNbeta-1b or glatiramer acetate by monthly brain MRI in the BECOME study. Neurology. 2009;72(23):1976-1983.
Cook SD, Dhib-Jalbut S, Dowling P, et al. Use of magnetic resonance imaging as well as clinical disease activity in the clinical classification of multiple sclerosis and assessment of its course: a report from an international CMSC consensus conference, March 5-7, 2010. Int J MS Care. 2012;14(3):105-114.
Dowling PC, Bosch VV, Cook SD. Possible beneficial effect of high-dose intravenous steroid therapy in acute demyelinating disease and transverse myelitis. Neurology. 1980;30(7 Pt 2):33-36.
Giovannoni G, Comi G, Cook S, et al. A placebo-controlled trial of oral cladribine for relapsing multiple sclerosis. N Engl J Med. 2010;362(5):416-426.
Lennon VA, Wingerchuk DM, Kryzer TJ, et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet. 2004;364(9451):2106-2112.
Lincoln JA, Cadavid D, Pollard J, et al. We should use magnetic resonance imaging to classify and monitor the course of multiple sclerosis. Arch Neurol. 2009;66(3):412-414.
Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology. 1996;46(4):907-911.
Maranzano J, Rudko DA, Nakamura K, et al. MRI evidence of acute inflammation in leukocortical lesions of patients with early multiple sclerosis. Neurology. 2017;89(7):714-721.
McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50(1):121-127.
Miller HG, Gibbons JL. Acute disseminated encephalomyelitis and acute disseminated sclerosis; results of treatment with A.C.T.H. Br Med J. 1953;2(4850):1345-1348.
Poser CM, Paty DW, Scheinberg L, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983;13(3):227-231.
Rose AS, Kuzma JW, Kurtzke JF, et al. Cooperative study in the evaluation of therapy in multiple sclerosis. ACTH vs. placebo--final report. Neurology. 1970;20(5):1-59.
Troiano R, Hafstein M, Ruderman M, et al. Effect of high-dose intravenous steroid administration on contrast-enhancing computed tomographic scan lesions in multiple sclerosis. Ann Neurol. 1984;15(3):257-263.
Troiano RA, Hafstein MP, Zito G, et al. The effect of oral corticosteroid dosage on CT enhancing multiple sclerosis plaques. J Neurol Sci. 1985;70(1):67-72.
Young IR, Hall AS, Pallis A, et al. Nuclear magnetic resonance imaging of the brain in multiple sclerosis. Lancet. 1981;2(8255):1063-1066.
Stuart D. Cook, MD, and Abdul Rahman Alchaki
Dr. Cook is the Ruth Dunietz Kushner and Michael Jay Serwitz Professor of Neurology/Neurosciences at Rutgers, the State University of New Jersey, Newark. Dr. Alchaki is a resident in the Deptartment of Neurology/Neurosciences at Rutgers, the State University of New Jersey, Newark.
Disclosure: Stuart Cook has received honoraria for lectures from Bayer HealthCare and Merck Serono. He has served as a consultant for Merck Serono, Bayer HealthCare, Teva, Novartis, Sanofi-Aventis, Biogen Idec, and Actinobac Biomed. He has served on steering committees for the BEYOND and CLARITY Studies and as a member of Advisory Boards for Merck Serono, Bayer HealthCare, Teva, Biogen Idec, Sanofi Aventis, and Actinobac Biomed.
The Initial Years (1838 to 1930s)
The earliest recognition of MS clinical features and pathology was attributed to Jean-Martin Charcot, Robert Carswell, and Jean Cruveilhier in Europe from 1838 to 1868. Beyond those early descriptions, relatively few MS breakthroughs occurred until the 1930s, when Thomas Rivers discovered experimental autoimmune encephalomyelitis (EAE), a demyelinating disease, in animals. His insightful concepts were widely cited and ultimately contributed to undestanding of the immune mechanisms of MS and acute disseminated encephalomyelitis (ADEM).
Advances in Diagnosis (1965 to 1992)
In 1965, Schumacher et al provided the essential clinical criteria for MS diagnosis. Poser et al refined these criteria in 1983. In 2001, McDonald et al added neuroimaging, CSF analysis, and evoked potentials to further complement MS clinical diagnosis. For the first time, the disease could generally be recognized.
Early Treatments
Various treatments for MS were tried over the years, without great success. However, in 1953, a small descriptive trial by Miller and Gibbons reported clinical benefits in patients using intramuscular (IM) adrenocorticotropic hormone (ACTH) for MS and disseminated encephalomyelitis. This was followed in 1970 by a Cooperative Study of IM ACTH versus placebo by Rose et al, which resulted in ACTH, and subsequently oral corticosteroids, being widely used to treat MS, particularly for acute exacerbations of the disease. However, robust evidence of long-term steroids remain limited, even to the present.
High-Dose Steroids
By 1980, the initial descriptive treatment of high-dose intravenous (IV) steroids for demyelinating diseases, including MS and transverse myelitis, by Dowling et al resulted in rapid clinical improvement in some patients. This result was ultimately confirmed by others. High-dose IV steroids became the gold standard for acute attacks, particularly those aggressive in nature. In the mid 1980s, work by Troiano et al, as well as others, showed that the rapid use of high-dose IV as well as oral steroids showed similar effects, with reduction or elimination of CT contrast-enhancing lesions within as few as eight hours, while lower doses or alternative-day treatments were less effective. In addition, descriptive studies of immune modulatory and immunosuppressive drugs, as well as small randomized studies, were published. These agents did not receive FDA approval.
The Golden Age of Therapy (1993 to 2018)
A remarkable era in MS prognosis and treatment began with immunomodulation injections of Betaseron (INFβ-1b), Avonex (INFβ-1a), and Copaxone (glatiramer acetate). This can be attributed, at least in part, to advances in molecular biology, genetics, and neuroimaging, and support by corporate, private, and public funding. Since the initial FDA approval of INFβ-1b, 15 MS therapies have become clinically available, including eight injectables, three orals, and four infusion treatments (see Table 1). In addition, two other drugs have been FDA approved for uses other than MS: rituximab (approved for lymphoma) and cladribine (for hairy cell leukemia), with the latter now approved by the European Medicines Agency for MS. Table 1 depicts characteristics of these therapies approved by US or European agencies (or for other disorders increasingly used off label for MS) in an attempt to compare annual relapse rates (ARR) and decreases in the percent of gadolinium-enhancing MS lesions versus placebo. This information was chosen because ARR has been uniformly selected and defined for such trials, while percent decrease of gadolinium-enhancing lesions on MRI has been the most sensitive barometer available for assessing acute clinical activity. As a result, risk-benefit considerations have been critical in evaluating these drug treatments, with efficacy improving greatly over time, whereas risks have been more variable.
Disease Categories
In 1996, Lublin and Reingold provided a new classification, not specifically for the diagnosis of MS, but rather for the clinical course of the disease. Initially, there were four categories—relapsing-remitting MS, secondary progressive MS, primary progressive MS, and progressive-relapsing MS—that were universally identified. These were thought to be relatively distinct clinical categories, but over time it became clear that the classification did not fully distinguish MS disease activity within these categories. For that reason, it was subsequently recommended, by Lincoln et al in 2009 and Cook et al in 2012, to include MRI, a vastly more sensitive modality, as well as clinical data in assessing disease activity.
On another note, MS and neuromyelitis optica (NMO), although having similar features, were clearly identified as different diseases by Lennon et al in 2004. Differences in pathology, clinical characteristics, immunology, and therapy separate the two disorders.
MRI in MS
Work by Young et al in 1981 established the central role of MRI brain imaging in MS diagnosis and therapeutic considerations. Since then it has become ubiquitous.
An example of a sensitive and highly productive MRI protocol is the BECOME study of MS and clinically isolated syndrome by Cadavid et al from 2009 to 2017. In this study, IFNβ-1b was compared with glatiramer acetate treatment. Cadavid et al used a 3T scanner with triple-dose gadolinium, performed monthly for as long as 24 consecutive months. This unique study brought about a virtual gold mine of valuable research and clinical information. This included proof that gadolinium-enhancing lesions persisted for six months or more, evidence of a 30:1 ratio of new MRI brain lesions to clinical activity, and documentation that 96% of T2 lesions and black holes derive from prior gadolinium-enhancing lesions. It was further noted that 80% to 90% of acute black holes disappeared with treatment and 75% to 80% of patients taking IFNβ-1b or glatiramer acetate had new MRI lesions despite continuing treatment. Perhaps most interestingly, monthly MRIs could predict relapse and disability in a relatively small number of patients, depending upon the frequency and activity of MRI lesions. In 2017, Brown et al documented that magnetization transfer ratio recovery in MS brain lesions occurred more significantly with glatiramer acetate than with IFNβ-1b, whereas more chronic black hole lesions were found with glatiramer acetate. Also in 2017, Maranzano et al found evidence of acute inflammatory leukocortical lesions, which were not as well recognized previously.
In summary, it has become increasingly clear that MRI is the most sen
The Future of MS
While it is not yet a curable disease, there is growing evidence that MS prognosis has improved and will continue to improve. This is based on incremental decreases in acute MS exacerbations, progressive disability, and MRI lesion activity, as well as a combination of the three—no evidence of disease activity (NEDA).
Not only are drug therapies becoming more effective, but patients and physicians now have many more treatment options to carefully consider with regard to efficacy, side effect profiles, treatment frequency, route of administration, cost, and quality of life. Newer drugs with different mechanisms of action such as cladribine, now approved in Europe, fulfill most of these beneficial criteria (see Giovannoni et al, 2010). More promising MS treatments, including long-acting induction therapies, are still being evaluated. As with other complex diseases, multiple therapies are likely to be used as well.
In summary, compared with the time before 1993, MS will be much less likely to be a progressive disease, and quality of life will be much improved. In my opinion, patients will be less fearful about their prognosis than ever before, and with appropriate evaluations and treatments, we may realize that disabling MS will be far less common.

Suggested Reading
Brown JW, Pardini M, Brownlee WJ, et al. An abnormal periventricular magnetization transfer ratio gradient occurs early in multiple sclerosis. Brain. 2017;140(2):387-398.
Cadavid D, Wolansky LJ, Skurnick J, et al. Efficacy of treatment of MS with IFNbeta-1b or glatiramer acetate by monthly brain MRI in the BECOME study. Neurology. 2009;72(23):1976-1983.
Cook SD, Dhib-Jalbut S, Dowling P, et al. Use of magnetic resonance imaging as well as clinical disease activity in the clinical classification of multiple sclerosis and assessment of its course: a report from an international CMSC consensus conference, March 5-7, 2010. Int J MS Care. 2012;14(3):105-114.
Dowling PC, Bosch VV, Cook SD. Possible beneficial effect of high-dose intravenous steroid therapy in acute demyelinating disease and transverse myelitis. Neurology. 1980;30(7 Pt 2):33-36.
Giovannoni G, Comi G, Cook S, et al. A placebo-controlled trial of oral cladribine for relapsing multiple sclerosis. N Engl J Med. 2010;362(5):416-426.
Lennon VA, Wingerchuk DM, Kryzer TJ, et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet. 2004;364(9451):2106-2112.
Lincoln JA, Cadavid D, Pollard J, et al. We should use magnetic resonance imaging to classify and monitor the course of multiple sclerosis. Arch Neurol. 2009;66(3):412-414.
Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology. 1996;46(4):907-911.
Maranzano J, Rudko DA, Nakamura K, et al. MRI evidence of acute inflammation in leukocortical lesions of patients with early multiple sclerosis. Neurology. 2017;89(7):714-721.
McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50(1):121-127.
Miller HG, Gibbons JL. Acute disseminated encephalomyelitis and acute disseminated sclerosis; results of treatment with A.C.T.H. Br Med J. 1953;2(4850):1345-1348.
Poser CM, Paty DW, Scheinberg L, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983;13(3):227-231.
Rose AS, Kuzma JW, Kurtzke JF, et al. Cooperative study in the evaluation of therapy in multiple sclerosis. ACTH vs. placebo--final report. Neurology. 1970;20(5):1-59.
Troiano R, Hafstein M, Ruderman M, et al. Effect of high-dose intravenous steroid administration on contrast-enhancing computed tomographic scan lesions in multiple sclerosis. Ann Neurol. 1984;15(3):257-263.
Troiano RA, Hafstein MP, Zito G, et al. The effect of oral corticosteroid dosage on CT enhancing multiple sclerosis plaques. J Neurol Sci. 1985;70(1):67-72.
Young IR, Hall AS, Pallis A, et al. Nuclear magnetic resonance imaging of the brain in multiple sclerosis. Lancet. 1981;2(8255):1063-1066.
Stuart D. Cook, MD, and Abdul Rahman Alchaki
Dr. Cook is the Ruth Dunietz Kushner and Michael Jay Serwitz Professor of Neurology/Neurosciences at Rutgers, the State University of New Jersey, Newark. Dr. Alchaki is a resident in the Deptartment of Neurology/Neurosciences at Rutgers, the State University of New Jersey, Newark.
Disclosure: Stuart Cook has received honoraria for lectures from Bayer HealthCare and Merck Serono. He has served as a consultant for Merck Serono, Bayer HealthCare, Teva, Novartis, Sanofi-Aventis, Biogen Idec, and Actinobac Biomed. He has served on steering committees for the BEYOND and CLARITY Studies and as a member of Advisory Boards for Merck Serono, Bayer HealthCare, Teva, Biogen Idec, Sanofi Aventis, and Actinobac Biomed.
The Initial Years (1838 to 1930s)
The earliest recognition of MS clinical features and pathology was attributed to Jean-Martin Charcot, Robert Carswell, and Jean Cruveilhier in Europe from 1838 to 1868. Beyond those early descriptions, relatively few MS breakthroughs occurred until the 1930s, when Thomas Rivers discovered experimental autoimmune encephalomyelitis (EAE), a demyelinating disease, in animals. His insightful concepts were widely cited and ultimately contributed to undestanding of the immune mechanisms of MS and acute disseminated encephalomyelitis (ADEM).
Advances in Diagnosis (1965 to 1992)
In 1965, Schumacher et al provided the essential clinical criteria for MS diagnosis. Poser et al refined these criteria in 1983. In 2001, McDonald et al added neuroimaging, CSF analysis, and evoked potentials to further complement MS clinical diagnosis. For the first time, the disease could generally be recognized.
Early Treatments
Various treatments for MS were tried over the years, without great success. However, in 1953, a small descriptive trial by Miller and Gibbons reported clinical benefits in patients using intramuscular (IM) adrenocorticotropic hormone (ACTH) for MS and disseminated encephalomyelitis. This was followed in 1970 by a Cooperative Study of IM ACTH versus placebo by Rose et al, which resulted in ACTH, and subsequently oral corticosteroids, being widely used to treat MS, particularly for acute exacerbations of the disease. However, robust evidence of long-term steroids remain limited, even to the present.
High-Dose Steroids
By 1980, the initial descriptive treatment of high-dose intravenous (IV) steroids for demyelinating diseases, including MS and transverse myelitis, by Dowling et al resulted in rapid clinical improvement in some patients. This result was ultimately confirmed by others. High-dose IV steroids became the gold standard for acute attacks, particularly those aggressive in nature. In the mid 1980s, work by Troiano et al, as well as others, showed that the rapid use of high-dose IV as well as oral steroids showed similar effects, with reduction or elimination of CT contrast-enhancing lesions within as few as eight hours, while lower doses or alternative-day treatments were less effective. In addition, descriptive studies of immune modulatory and immunosuppressive drugs, as well as small randomized studies, were published. These agents did not receive FDA approval.
The Golden Age of Therapy (1993 to 2018)
A remarkable era in MS prognosis and treatment began with immunomodulation injections of Betaseron (INFβ-1b), Avonex (INFβ-1a), and Copaxone (glatiramer acetate). This can be attributed, at least in part, to advances in molecular biology, genetics, and neuroimaging, and support by corporate, private, and public funding. Since the initial FDA approval of INFβ-1b, 15 MS therapies have become clinically available, including eight injectables, three orals, and four infusion treatments (see Table 1). In addition, two other drugs have been FDA approved for uses other than MS: rituximab (approved for lymphoma) and cladribine (for hairy cell leukemia), with the latter now approved by the European Medicines Agency for MS. Table 1 depicts characteristics of these therapies approved by US or European agencies (or for other disorders increasingly used off label for MS) in an attempt to compare annual relapse rates (ARR) and decreases in the percent of gadolinium-enhancing MS lesions versus placebo. This information was chosen because ARR has been uniformly selected and defined for such trials, while percent decrease of gadolinium-enhancing lesions on MRI has been the most sensitive barometer available for assessing acute clinical activity. As a result, risk-benefit considerations have been critical in evaluating these drug treatments, with efficacy improving greatly over time, whereas risks have been more variable.
Disease Categories
In 1996, Lublin and Reingold provided a new classification, not specifically for the diagnosis of MS, but rather for the clinical course of the disease. Initially, there were four categories—relapsing-remitting MS, secondary progressive MS, primary progressive MS, and progressive-relapsing MS—that were universally identified. These were thought to be relatively distinct clinical categories, but over time it became clear that the classification did not fully distinguish MS disease activity within these categories. For that reason, it was subsequently recommended, by Lincoln et al in 2009 and Cook et al in 2012, to include MRI, a vastly more sensitive modality, as well as clinical data in assessing disease activity.
On another note, MS and neuromyelitis optica (NMO), although having similar features, were clearly identified as different diseases by Lennon et al in 2004. Differences in pathology, clinical characteristics, immunology, and therapy separate the two disorders.
MRI in MS
Work by Young et al in 1981 established the central role of MRI brain imaging in MS diagnosis and therapeutic considerations. Since then it has become ubiquitous.
An example of a sensitive and highly productive MRI protocol is the BECOME study of MS and clinically isolated syndrome by Cadavid et al from 2009 to 2017. In this study, IFNβ-1b was compared with glatiramer acetate treatment. Cadavid et al used a 3T scanner with triple-dose gadolinium, performed monthly for as long as 24 consecutive months. This unique study brought about a virtual gold mine of valuable research and clinical information. This included proof that gadolinium-enhancing lesions persisted for six months or more, evidence of a 30:1 ratio of new MRI brain lesions to clinical activity, and documentation that 96% of T2 lesions and black holes derive from prior gadolinium-enhancing lesions. It was further noted that 80% to 90% of acute black holes disappeared with treatment and 75% to 80% of patients taking IFNβ-1b or glatiramer acetate had new MRI lesions despite continuing treatment. Perhaps most interestingly, monthly MRIs could predict relapse and disability in a relatively small number of patients, depending upon the frequency and activity of MRI lesions. In 2017, Brown et al documented that magnetization transfer ratio recovery in MS brain lesions occurred more significantly with glatiramer acetate than with IFNβ-1b, whereas more chronic black hole lesions were found with glatiramer acetate. Also in 2017, Maranzano et al found evidence of acute inflammatory leukocortical lesions, which were not as well recognized previously.
In summary, it has become increasingly clear that MRI is the most sen
The Future of MS
While it is not yet a curable disease, there is growing evidence that MS prognosis has improved and will continue to improve. This is based on incremental decreases in acute MS exacerbations, progressive disability, and MRI lesion activity, as well as a combination of the three—no evidence of disease activity (NEDA).
Not only are drug therapies becoming more effective, but patients and physicians now have many more treatment options to carefully consider with regard to efficacy, side effect profiles, treatment frequency, route of administration, cost, and quality of life. Newer drugs with different mechanisms of action such as cladribine, now approved in Europe, fulfill most of these beneficial criteria (see Giovannoni et al, 2010). More promising MS treatments, including long-acting induction therapies, are still being evaluated. As with other complex diseases, multiple therapies are likely to be used as well.
In summary, compared with the time before 1993, MS will be much less likely to be a progressive disease, and quality of life will be much improved. In my opinion, patients will be less fearful about their prognosis than ever before, and with appropriate evaluations and treatments, we may realize that disabling MS will be far less common.

Suggested Reading
Brown JW, Pardini M, Brownlee WJ, et al. An abnormal periventricular magnetization transfer ratio gradient occurs early in multiple sclerosis. Brain. 2017;140(2):387-398.
Cadavid D, Wolansky LJ, Skurnick J, et al. Efficacy of treatment of MS with IFNbeta-1b or glatiramer acetate by monthly brain MRI in the BECOME study. Neurology. 2009;72(23):1976-1983.
Cook SD, Dhib-Jalbut S, Dowling P, et al. Use of magnetic resonance imaging as well as clinical disease activity in the clinical classification of multiple sclerosis and assessment of its course: a report from an international CMSC consensus conference, March 5-7, 2010. Int J MS Care. 2012;14(3):105-114.
Dowling PC, Bosch VV, Cook SD. Possible beneficial effect of high-dose intravenous steroid therapy in acute demyelinating disease and transverse myelitis. Neurology. 1980;30(7 Pt 2):33-36.
Giovannoni G, Comi G, Cook S, et al. A placebo-controlled trial of oral cladribine for relapsing multiple sclerosis. N Engl J Med. 2010;362(5):416-426.
Lennon VA, Wingerchuk DM, Kryzer TJ, et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet. 2004;364(9451):2106-2112.
Lincoln JA, Cadavid D, Pollard J, et al. We should use magnetic resonance imaging to classify and monitor the course of multiple sclerosis. Arch Neurol. 2009;66(3):412-414.
Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology. 1996;46(4):907-911.
Maranzano J, Rudko DA, Nakamura K, et al. MRI evidence of acute inflammation in leukocortical lesions of patients with early multiple sclerosis. Neurology. 2017;89(7):714-721.
McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50(1):121-127.
Miller HG, Gibbons JL. Acute disseminated encephalomyelitis and acute disseminated sclerosis; results of treatment with A.C.T.H. Br Med J. 1953;2(4850):1345-1348.
Poser CM, Paty DW, Scheinberg L, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983;13(3):227-231.
Rose AS, Kuzma JW, Kurtzke JF, et al. Cooperative study in the evaluation of therapy in multiple sclerosis. ACTH vs. placebo--final report. Neurology. 1970;20(5):1-59.
Troiano R, Hafstein M, Ruderman M, et al. Effect of high-dose intravenous steroid administration on contrast-enhancing computed tomographic scan lesions in multiple sclerosis. Ann Neurol. 1984;15(3):257-263.
Troiano RA, Hafstein MP, Zito G, et al. The effect of oral corticosteroid dosage on CT enhancing multiple sclerosis plaques. J Neurol Sci. 1985;70(1):67-72.
Young IR, Hall AS, Pallis A, et al. Nuclear magnetic resonance imaging of the brain in multiple sclerosis. Lancet. 1981;2(8255):1063-1066.
What Is the Impact of Binge Drinking in Patients With Epilepsy?
WASHINGTON, DC—Alcohol is a major seizure precipitant in the context of hazardous drinking and withdrawal, according to a study presented at the 71st Annual Meeting of the American Epilepsy Society. Occasional social drinking, however, is an uncommon cause of seizure breakthrough in predominantly focal epilepsy, said the researchers.
The seizure-inducing effect of alcohol withdrawal in chronic alcohol abuse is apparent, but the effect of binge drinking and modest social drinking among patients with epilepsy is less clear. Christian Samsonsen, MD, a neurologist at St. Olav’s University Hospital in Trondheim, Norway, and colleagues conducted a prospective, observational cross-over study to examine the relationship between alcohol and seizure disorders in acutely hospitalized patients. They also examined the clinical characteristics of patients with alcohol-related seizures and their drinking patterns.
Evaluating Drinking Patterns
The study included 134 consecutive patients with seizures. Ninety-two patients had epilepsy, and 42 patients had isolated seizures not diagnosed as epilepsy. At hospital admission, researchers conducted a semistructured interview and applied the Alcohol Use Disorders Identification Test (AUDIT).
Investigators defined withdrawal seizure as having an AUDIT score of 8 or greater and alcohol intake within the last two days. They defined binge drinking as drinking more than four units in one session for females and more than five units in one session for males. They defined social drinking as having an AUDIT score of less than 8 and not drinking more than 12 units in one day.
The researchers recorded daily alcohol consumption during the five days prior to the seizure, as well as sleep time during the prior three days. Researchers then performed a follow-up telephone interview on a seizure-free day at least four weeks later.
Seizures Were More Common on Sunday
In all, 28% of patients had an AUDIT score of 8 or greater (ie, hazardous drinking), including 22% of patients with epilepsy and 43% of patients with isolated seizures. Alcohol consumption and nonfocal seizures were increased in isolated seizures, suggesting withdrawal.
One in five patients with epilepsy had been binge drinking, and 59 (64%) patients with epilepsy had been socially drinking. Among the patients who had been socially drinking, alcohol intake was not different prior to seizure, compared with follow-up, downgrading the role of modest social drinking as a seizure precipitant, said the researchers. Among the 19 patients with idiopathic generalized epilepsy, however, “even social drinking two days prior to seizure was associated with seizures,” the researchers said. Patients with epilepsy were more likely to have had their seizures on a Sunday (21%) or Monday (23%)than on other days. Patients with single seizures were more likely to have had their seizures on a Monday (29%) than on other days. Seizures associated with binge drinking were more common on Sunday.
Overall, binge drinking was associated with loss of seizure control in people with epilepsy; however, “alcohol alone should not always be blamed,” said the researchers. “In people with epilepsy, alcohol intake is often combined with other seizure precipitants,” such as sleep loss, they concluded.
—Erica Tricarico
Suggested Reading
Samsonsen C, Sand T, Brathen G, et al. The impact of sleep loss on the facilitation of seizures: A prospective case-crossover study. Epilepsy Res. 2016;127:260-266.
WASHINGTON, DC—Alcohol is a major seizure precipitant in the context of hazardous drinking and withdrawal, according to a study presented at the 71st Annual Meeting of the American Epilepsy Society. Occasional social drinking, however, is an uncommon cause of seizure breakthrough in predominantly focal epilepsy, said the researchers.
The seizure-inducing effect of alcohol withdrawal in chronic alcohol abuse is apparent, but the effect of binge drinking and modest social drinking among patients with epilepsy is less clear. Christian Samsonsen, MD, a neurologist at St. Olav’s University Hospital in Trondheim, Norway, and colleagues conducted a prospective, observational cross-over study to examine the relationship between alcohol and seizure disorders in acutely hospitalized patients. They also examined the clinical characteristics of patients with alcohol-related seizures and their drinking patterns.
Evaluating Drinking Patterns
The study included 134 consecutive patients with seizures. Ninety-two patients had epilepsy, and 42 patients had isolated seizures not diagnosed as epilepsy. At hospital admission, researchers conducted a semistructured interview and applied the Alcohol Use Disorders Identification Test (AUDIT).
Investigators defined withdrawal seizure as having an AUDIT score of 8 or greater and alcohol intake within the last two days. They defined binge drinking as drinking more than four units in one session for females and more than five units in one session for males. They defined social drinking as having an AUDIT score of less than 8 and not drinking more than 12 units in one day.
The researchers recorded daily alcohol consumption during the five days prior to the seizure, as well as sleep time during the prior three days. Researchers then performed a follow-up telephone interview on a seizure-free day at least four weeks later.
Seizures Were More Common on Sunday
In all, 28% of patients had an AUDIT score of 8 or greater (ie, hazardous drinking), including 22% of patients with epilepsy and 43% of patients with isolated seizures. Alcohol consumption and nonfocal seizures were increased in isolated seizures, suggesting withdrawal.
One in five patients with epilepsy had been binge drinking, and 59 (64%) patients with epilepsy had been socially drinking. Among the patients who had been socially drinking, alcohol intake was not different prior to seizure, compared with follow-up, downgrading the role of modest social drinking as a seizure precipitant, said the researchers. Among the 19 patients with idiopathic generalized epilepsy, however, “even social drinking two days prior to seizure was associated with seizures,” the researchers said. Patients with epilepsy were more likely to have had their seizures on a Sunday (21%) or Monday (23%)than on other days. Patients with single seizures were more likely to have had their seizures on a Monday (29%) than on other days. Seizures associated with binge drinking were more common on Sunday.
Overall, binge drinking was associated with loss of seizure control in people with epilepsy; however, “alcohol alone should not always be blamed,” said the researchers. “In people with epilepsy, alcohol intake is often combined with other seizure precipitants,” such as sleep loss, they concluded.
—Erica Tricarico
Suggested Reading
Samsonsen C, Sand T, Brathen G, et al. The impact of sleep loss on the facilitation of seizures: A prospective case-crossover study. Epilepsy Res. 2016;127:260-266.
WASHINGTON, DC—Alcohol is a major seizure precipitant in the context of hazardous drinking and withdrawal, according to a study presented at the 71st Annual Meeting of the American Epilepsy Society. Occasional social drinking, however, is an uncommon cause of seizure breakthrough in predominantly focal epilepsy, said the researchers.
The seizure-inducing effect of alcohol withdrawal in chronic alcohol abuse is apparent, but the effect of binge drinking and modest social drinking among patients with epilepsy is less clear. Christian Samsonsen, MD, a neurologist at St. Olav’s University Hospital in Trondheim, Norway, and colleagues conducted a prospective, observational cross-over study to examine the relationship between alcohol and seizure disorders in acutely hospitalized patients. They also examined the clinical characteristics of patients with alcohol-related seizures and their drinking patterns.
Evaluating Drinking Patterns
The study included 134 consecutive patients with seizures. Ninety-two patients had epilepsy, and 42 patients had isolated seizures not diagnosed as epilepsy. At hospital admission, researchers conducted a semistructured interview and applied the Alcohol Use Disorders Identification Test (AUDIT).
Investigators defined withdrawal seizure as having an AUDIT score of 8 or greater and alcohol intake within the last two days. They defined binge drinking as drinking more than four units in one session for females and more than five units in one session for males. They defined social drinking as having an AUDIT score of less than 8 and not drinking more than 12 units in one day.
The researchers recorded daily alcohol consumption during the five days prior to the seizure, as well as sleep time during the prior three days. Researchers then performed a follow-up telephone interview on a seizure-free day at least four weeks later.
Seizures Were More Common on Sunday
In all, 28% of patients had an AUDIT score of 8 or greater (ie, hazardous drinking), including 22% of patients with epilepsy and 43% of patients with isolated seizures. Alcohol consumption and nonfocal seizures were increased in isolated seizures, suggesting withdrawal.
One in five patients with epilepsy had been binge drinking, and 59 (64%) patients with epilepsy had been socially drinking. Among the patients who had been socially drinking, alcohol intake was not different prior to seizure, compared with follow-up, downgrading the role of modest social drinking as a seizure precipitant, said the researchers. Among the 19 patients with idiopathic generalized epilepsy, however, “even social drinking two days prior to seizure was associated with seizures,” the researchers said. Patients with epilepsy were more likely to have had their seizures on a Sunday (21%) or Monday (23%)than on other days. Patients with single seizures were more likely to have had their seizures on a Monday (29%) than on other days. Seizures associated with binge drinking were more common on Sunday.
Overall, binge drinking was associated with loss of seizure control in people with epilepsy; however, “alcohol alone should not always be blamed,” said the researchers. “In people with epilepsy, alcohol intake is often combined with other seizure precipitants,” such as sleep loss, they concluded.
—Erica Tricarico
Suggested Reading
Samsonsen C, Sand T, Brathen G, et al. The impact of sleep loss on the facilitation of seizures: A prospective case-crossover study. Epilepsy Res. 2016;127:260-266.