User login
Can a Model Predict Pediatric SUDEP Risk?
WASHINGTON, DC—In a pediatric epilepsy population, number of antiepileptic drugs (AEDs) used and prior epilepsy surgery may predict sudden unexpected death in epilepsy (SUDEP), according to a study presented at the 71st Annual Meeting of the American Epilepsy Society.
Kishore Vedala, a medical student at the Medical College of Georgia at Augusta University, and colleagues sought to develop a predictive model for at-risk pediatric patients by conducting a matched case–control study.
Using records from the Medical College of Georgia, the researchers identified 11 SUDEP cases that occurred between 2007 and 2017. They compared the cases with 53 controls matched for age, epilepsy duration, and gender. The researchers used a conditional logistic regression model to evaluate the following nine potential predictor variables: mental retardation, seizure frequency, seizure type, prior status epilepticus, number of AEDs, prior epilepsy surgery, vagus nerve stimulator therapy, seizure progression, and awake interictal heart rate variability. They identified the optimum predictor models using Akaike’s information criterion and evaluated model performance using receiver operating characteristic area under the curve (AUC).
Prior status epilepticus (ie, having had a seizure that lasted longer than five minutes), prior epilepsy surgery, and having received three or more AEDs at the same time were significant predictors of SUDEP (odds ratios, 7.83, 4.23, and 4.7, respectively), whereas the other variables were not. The best model used number of AEDs and prior epilepsy surgery (AUC, 0.855). The second-best model used number of AEDs and prior status epilepticus (AUC, 0.839). The third-best model used number of AEDs alone (AUC, 0.807).
The risk factors likely reflect epilepsy that is difficult to control.
—Jake Remaly
WASHINGTON, DC—In a pediatric epilepsy population, number of antiepileptic drugs (AEDs) used and prior epilepsy surgery may predict sudden unexpected death in epilepsy (SUDEP), according to a study presented at the 71st Annual Meeting of the American Epilepsy Society.
Kishore Vedala, a medical student at the Medical College of Georgia at Augusta University, and colleagues sought to develop a predictive model for at-risk pediatric patients by conducting a matched case–control study.
Using records from the Medical College of Georgia, the researchers identified 11 SUDEP cases that occurred between 2007 and 2017. They compared the cases with 53 controls matched for age, epilepsy duration, and gender. The researchers used a conditional logistic regression model to evaluate the following nine potential predictor variables: mental retardation, seizure frequency, seizure type, prior status epilepticus, number of AEDs, prior epilepsy surgery, vagus nerve stimulator therapy, seizure progression, and awake interictal heart rate variability. They identified the optimum predictor models using Akaike’s information criterion and evaluated model performance using receiver operating characteristic area under the curve (AUC).
Prior status epilepticus (ie, having had a seizure that lasted longer than five minutes), prior epilepsy surgery, and having received three or more AEDs at the same time were significant predictors of SUDEP (odds ratios, 7.83, 4.23, and 4.7, respectively), whereas the other variables were not. The best model used number of AEDs and prior epilepsy surgery (AUC, 0.855). The second-best model used number of AEDs and prior status epilepticus (AUC, 0.839). The third-best model used number of AEDs alone (AUC, 0.807).
The risk factors likely reflect epilepsy that is difficult to control.
—Jake Remaly
WASHINGTON, DC—In a pediatric epilepsy population, number of antiepileptic drugs (AEDs) used and prior epilepsy surgery may predict sudden unexpected death in epilepsy (SUDEP), according to a study presented at the 71st Annual Meeting of the American Epilepsy Society.
Kishore Vedala, a medical student at the Medical College of Georgia at Augusta University, and colleagues sought to develop a predictive model for at-risk pediatric patients by conducting a matched case–control study.
Using records from the Medical College of Georgia, the researchers identified 11 SUDEP cases that occurred between 2007 and 2017. They compared the cases with 53 controls matched for age, epilepsy duration, and gender. The researchers used a conditional logistic regression model to evaluate the following nine potential predictor variables: mental retardation, seizure frequency, seizure type, prior status epilepticus, number of AEDs, prior epilepsy surgery, vagus nerve stimulator therapy, seizure progression, and awake interictal heart rate variability. They identified the optimum predictor models using Akaike’s information criterion and evaluated model performance using receiver operating characteristic area under the curve (AUC).
Prior status epilepticus (ie, having had a seizure that lasted longer than five minutes), prior epilepsy surgery, and having received three or more AEDs at the same time were significant predictors of SUDEP (odds ratios, 7.83, 4.23, and 4.7, respectively), whereas the other variables were not. The best model used number of AEDs and prior epilepsy surgery (AUC, 0.855). The second-best model used number of AEDs and prior status epilepticus (AUC, 0.839). The third-best model used number of AEDs alone (AUC, 0.807).
The risk factors likely reflect epilepsy that is difficult to control.
—Jake Remaly
Rituximab May Provide Greater Benefits Than Other First-Line MS Therapies
Rituximab is associated with a lower drug discontinuation rate than all other commonly prescribed disease-modifying treatments (DMTs) used as initial therapy for relapsing-remitting multiple sclerosis (MS), according to a retrospective study of patient data from a Swedish MS registry.
In addition, relapse rates were lower with rituximab (Rituxan) than they were with injectable DMTs and dimethyl fumarate (Tecfidera), according to the study, which was published online ahead of print January 8 in JAMA Neurology.
The results suggest that rituximab “can be considered an option” for treatment-naive patients with relapsing-remitting MS, according to Mathias Granqvist, MD, a graduate student in the Department of Clinical Neuroscience at the Karolinska Institute in Stockholm, and his coauthors.
Anti-CD20 agents such as rituximab are “likely to become an additional treatment option” for relapsing-remitting MS, and off-label use of rituximab for this indication has “increased considerably” in Sweden in recent years, the investigators said. Relapsing-remitting MS is not an approved indication for rituximab in the United States, but in Sweden “there is no difference in reimbursement policy ... because all DMTs are covered by the national health insurance, including off-label medications.”
The emergence of new DMTs for relapsing-remitting MS has changed the treatment landscape recently, although in real-world practice, there is a lack of “detailed knowledge about how to tailor therapy,” the authors said. They noted that the majority of patients discontinue traditional first-line treatment with injectable DMTs (ie, interferon beta and glatiramer acetate) within two years, suggesting a need for better treatment options.
To evaluate the real-world effectiveness of rituximab in this setting, Dr. Granqvist and his colleagues selected patient registry data for two Swedish counties that included 494 participants who received a diagnosis of relapsing-remitting MS between January 1, 2012, and October 31, 2015.
The largest subset of patients (215) received injectable DMTs, while 120 received rituximab, 86 received dimethyl fumarate, 50 received natalizumab (Tysabri), 17 received fingolimod (Gilenya), and six received other treatments, according to the authors.
The proportion of patients who stayed on treatment was significantly higher for rituximab versus all other DMTs. Compared with rituximab, the hazard ratios for drug discontinuation, after adjusting for covariates and propensity score, were 11.4 for injectable DMTs, 15.1 for dimethyl fumarate, 5.9 for fingolimod, and 11.3 for natalizumab.
Rituximab-treated patients also had lower rates of clinical relapse, neuroradiologic disease activity, and adverse events, compared with patients who received injectable DMTs or dimethyl fumarate, according to the investigators.
In comparison with fingolimod and natalizumab, rituximab was associated with lower relapse rates and fewer gadolinium-enhancing lesions, but those differences did not reach statistical significance in all analyses.
The study was funded by the Swedish Medical Research Council, among other sources. Study authors reported conflicts of interest related to Biogen, Novartis, and Genzyme.
—Andrew D. Bowser
Suggested Reading
Granqvist M, Boremalm M, Poorghobad A, et al. Comparative effectiveness of rituximab and other initial treatment choices for multiple sclerosis. JAMA Neurol. 2018 Jan 8 [Epub ahead of print].
Rituximab is associated with a lower drug discontinuation rate than all other commonly prescribed disease-modifying treatments (DMTs) used as initial therapy for relapsing-remitting multiple sclerosis (MS), according to a retrospective study of patient data from a Swedish MS registry.
In addition, relapse rates were lower with rituximab (Rituxan) than they were with injectable DMTs and dimethyl fumarate (Tecfidera), according to the study, which was published online ahead of print January 8 in JAMA Neurology.
The results suggest that rituximab “can be considered an option” for treatment-naive patients with relapsing-remitting MS, according to Mathias Granqvist, MD, a graduate student in the Department of Clinical Neuroscience at the Karolinska Institute in Stockholm, and his coauthors.
Anti-CD20 agents such as rituximab are “likely to become an additional treatment option” for relapsing-remitting MS, and off-label use of rituximab for this indication has “increased considerably” in Sweden in recent years, the investigators said. Relapsing-remitting MS is not an approved indication for rituximab in the United States, but in Sweden “there is no difference in reimbursement policy ... because all DMTs are covered by the national health insurance, including off-label medications.”
The emergence of new DMTs for relapsing-remitting MS has changed the treatment landscape recently, although in real-world practice, there is a lack of “detailed knowledge about how to tailor therapy,” the authors said. They noted that the majority of patients discontinue traditional first-line treatment with injectable DMTs (ie, interferon beta and glatiramer acetate) within two years, suggesting a need for better treatment options.
To evaluate the real-world effectiveness of rituximab in this setting, Dr. Granqvist and his colleagues selected patient registry data for two Swedish counties that included 494 participants who received a diagnosis of relapsing-remitting MS between January 1, 2012, and October 31, 2015.
The largest subset of patients (215) received injectable DMTs, while 120 received rituximab, 86 received dimethyl fumarate, 50 received natalizumab (Tysabri), 17 received fingolimod (Gilenya), and six received other treatments, according to the authors.
The proportion of patients who stayed on treatment was significantly higher for rituximab versus all other DMTs. Compared with rituximab, the hazard ratios for drug discontinuation, after adjusting for covariates and propensity score, were 11.4 for injectable DMTs, 15.1 for dimethyl fumarate, 5.9 for fingolimod, and 11.3 for natalizumab.
Rituximab-treated patients also had lower rates of clinical relapse, neuroradiologic disease activity, and adverse events, compared with patients who received injectable DMTs or dimethyl fumarate, according to the investigators.
In comparison with fingolimod and natalizumab, rituximab was associated with lower relapse rates and fewer gadolinium-enhancing lesions, but those differences did not reach statistical significance in all analyses.
The study was funded by the Swedish Medical Research Council, among other sources. Study authors reported conflicts of interest related to Biogen, Novartis, and Genzyme.
—Andrew D. Bowser
Suggested Reading
Granqvist M, Boremalm M, Poorghobad A, et al. Comparative effectiveness of rituximab and other initial treatment choices for multiple sclerosis. JAMA Neurol. 2018 Jan 8 [Epub ahead of print].
Rituximab is associated with a lower drug discontinuation rate than all other commonly prescribed disease-modifying treatments (DMTs) used as initial therapy for relapsing-remitting multiple sclerosis (MS), according to a retrospective study of patient data from a Swedish MS registry.
In addition, relapse rates were lower with rituximab (Rituxan) than they were with injectable DMTs and dimethyl fumarate (Tecfidera), according to the study, which was published online ahead of print January 8 in JAMA Neurology.
The results suggest that rituximab “can be considered an option” for treatment-naive patients with relapsing-remitting MS, according to Mathias Granqvist, MD, a graduate student in the Department of Clinical Neuroscience at the Karolinska Institute in Stockholm, and his coauthors.
Anti-CD20 agents such as rituximab are “likely to become an additional treatment option” for relapsing-remitting MS, and off-label use of rituximab for this indication has “increased considerably” in Sweden in recent years, the investigators said. Relapsing-remitting MS is not an approved indication for rituximab in the United States, but in Sweden “there is no difference in reimbursement policy ... because all DMTs are covered by the national health insurance, including off-label medications.”
The emergence of new DMTs for relapsing-remitting MS has changed the treatment landscape recently, although in real-world practice, there is a lack of “detailed knowledge about how to tailor therapy,” the authors said. They noted that the majority of patients discontinue traditional first-line treatment with injectable DMTs (ie, interferon beta and glatiramer acetate) within two years, suggesting a need for better treatment options.
To evaluate the real-world effectiveness of rituximab in this setting, Dr. Granqvist and his colleagues selected patient registry data for two Swedish counties that included 494 participants who received a diagnosis of relapsing-remitting MS between January 1, 2012, and October 31, 2015.
The largest subset of patients (215) received injectable DMTs, while 120 received rituximab, 86 received dimethyl fumarate, 50 received natalizumab (Tysabri), 17 received fingolimod (Gilenya), and six received other treatments, according to the authors.
The proportion of patients who stayed on treatment was significantly higher for rituximab versus all other DMTs. Compared with rituximab, the hazard ratios for drug discontinuation, after adjusting for covariates and propensity score, were 11.4 for injectable DMTs, 15.1 for dimethyl fumarate, 5.9 for fingolimod, and 11.3 for natalizumab.
Rituximab-treated patients also had lower rates of clinical relapse, neuroradiologic disease activity, and adverse events, compared with patients who received injectable DMTs or dimethyl fumarate, according to the investigators.
In comparison with fingolimod and natalizumab, rituximab was associated with lower relapse rates and fewer gadolinium-enhancing lesions, but those differences did not reach statistical significance in all analyses.
The study was funded by the Swedish Medical Research Council, among other sources. Study authors reported conflicts of interest related to Biogen, Novartis, and Genzyme.
—Andrew D. Bowser
Suggested Reading
Granqvist M, Boremalm M, Poorghobad A, et al. Comparative effectiveness of rituximab and other initial treatment choices for multiple sclerosis. JAMA Neurol. 2018 Jan 8 [Epub ahead of print].
Growing Nodule on the Arm
The Diagnosis: Primary Cutaneous Anaplastic Large Cell Lymphoma
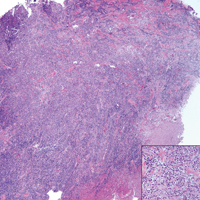
Primary cutaneous CD30+ lymphoproliferative disorders encompass lymphomatoid papulosis and primary cutaneous anaplastic large cell lymphoma (PCALCL) as well as borderline cases. Primary cutaneous anaplastic large cell lymphoma is a rare disease that is more common in white patients with slight male predominance and median age at diagnosis of 61 years.1 Prognosis is excellent, with a 90% survival rate at 10 years. Although lesions spontaneously regress in 6% to 22% of cases, complete resolution is rare.2 Clinically, the classic presentation is a solitary, rapidly growing, flesh-colored, erythematous nodule or plaque on the arms and legs or trunk, often with ulceration. Proper diagnosis requires clinical, histopathologic, and immunophenotypic correlation.
Histopathologic examination of PCALCL typically reveals large, atypical, Reed-Sternberg-like cells most commonly with anaplastic cytomorphology, but pleomorphic or immunoblastic morphology is not uncommon. Cells are in sheets or nodules, diffusely occupying the dermis and often the subcutaneous fat, with more than 75% of large cells expressing CD30.3 In addition to CD30 positivity, immunophenotype is classically CD4+, cutaneous lymphocyte-associated antigen positive, epithelial membrane antigen negative, and anaplastic lymphoma kinase negative; CD2, CD5, and CD3 expression is variable. Interestingly, in our case, there was a minor population of CD8+ cells. CD8 expression is seen in less than 5% of PCALCL cases; this phenotype is associated with an indolent disease with favorable prognosis.3 Of note, anaplastic lymphoma kinase positivity corresponding to a t(2;5) translocation is more suggestive of systemic anaplastic large cell lymphoma with secondary skin involvement and more commonly is seen in children. For reasons possibly related to mediators such as epidermal growth factor or transforming growth factor α from CD30+ cells, epidermal hyperplasia can be seen in PCALCL.4 The subsequent hyperkeratosis, crusting, and ulceration can be difficult to distinguish from lesions such as pyoderma gangrenosum, squamous cell carcinoma, arthropod bite, leukemia cutis, Merkel cell carcinoma (MCC), and metastatic breast cancer.
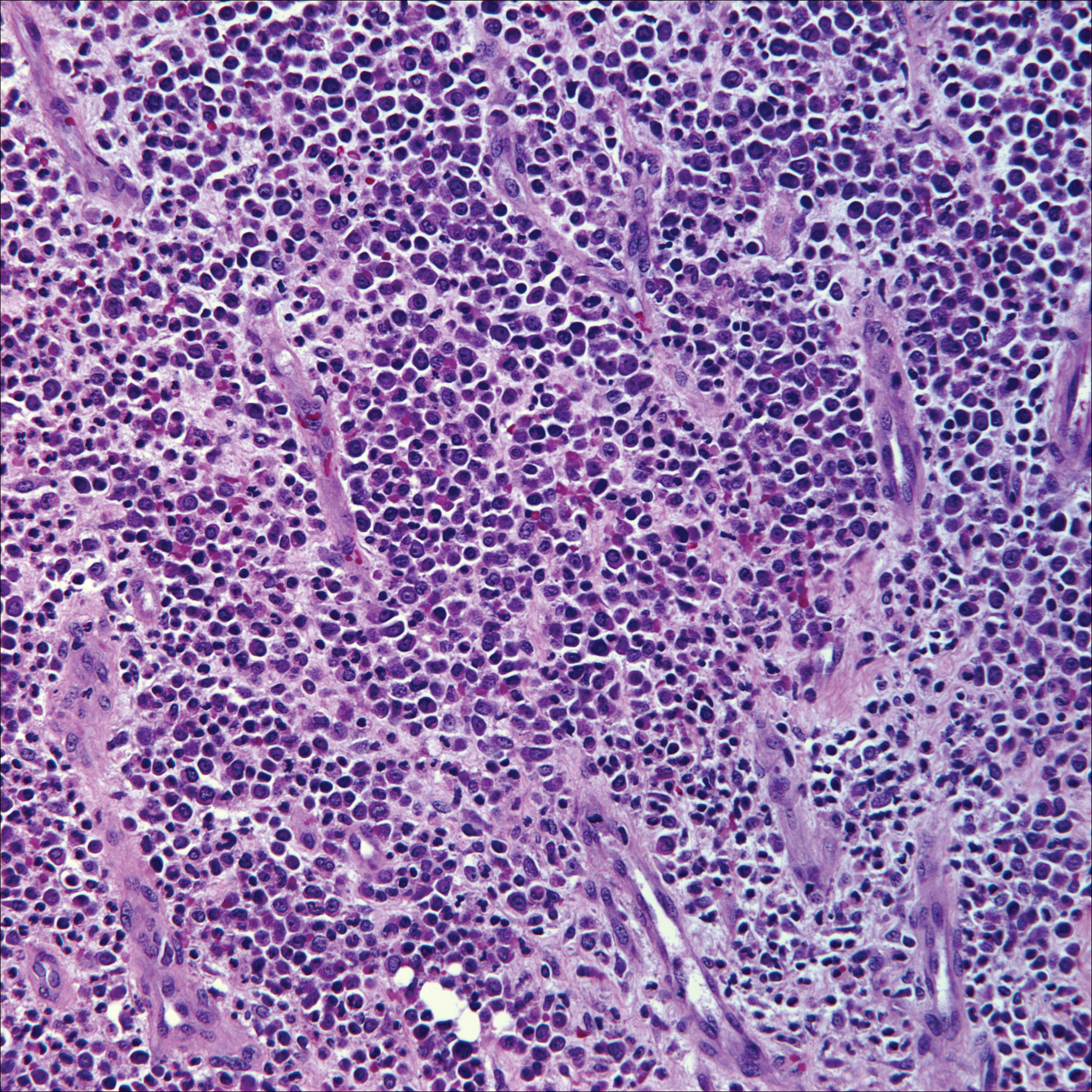
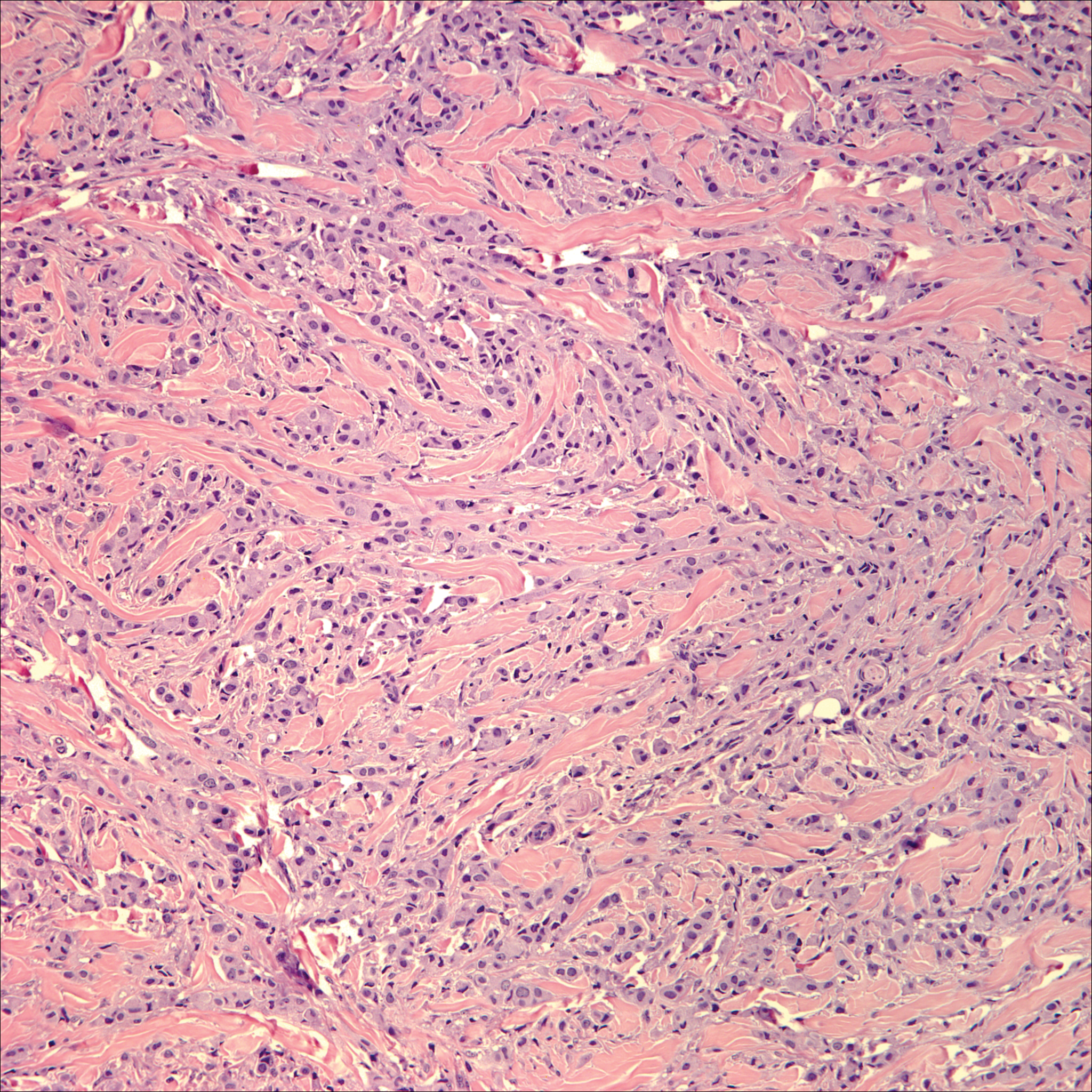
Skin involvement with leukemia is rare but most commonly is seen in acute myelogenous leukemia, specifically more mature forms such as acute myelomonocytic leukemia and acute monocytic leukemia. Approximately 10% to 20% of acute myelomonocytic leukemia cases have cutaneous involvement.5 Although there is a variety of potential skin lesions, the most common is a red-purple papule or nodule, sometimes with hemorrhage or ulceration, on the head, neck, and trunk. Leukemic infiltrates may arise from sites of prior trauma. Histopathology depends on the type of leukemia; however, general features include a normal epidermis without epidermotropism and perivascular, nodular, or diffuse infiltrate of neoplastic cells in the dermis, often with a Grenz zone (Figure 1). Compared to PCALCL, leukemia cutis shows sparing of the papillary dermis (Grenz zone), and the cells have more cytoplasm and show a different immunophenotype. The cells often are fragile and show crush artifact. Acute myelogenous leukemia often will show cytoplasmic granules; however, immature precursor cells may not have granules. The myeloid cells will stain with myeloperoxidase and chloroacetate. Positivity is seen for CD13, CD33, and CD68. Clinical correlation is important because other diseases with nodular or diffuse infiltrates of small cell infiltrates, such as extramedullary hematopoiesis and lymphoma, appear similar. Acute myelogenous leukemia is associated with neutrophilic dermatoses such as Sweet syndrome and pyoderma gangrenosum. Cutaneous eruption resolves with successful treatment of the leukemia.
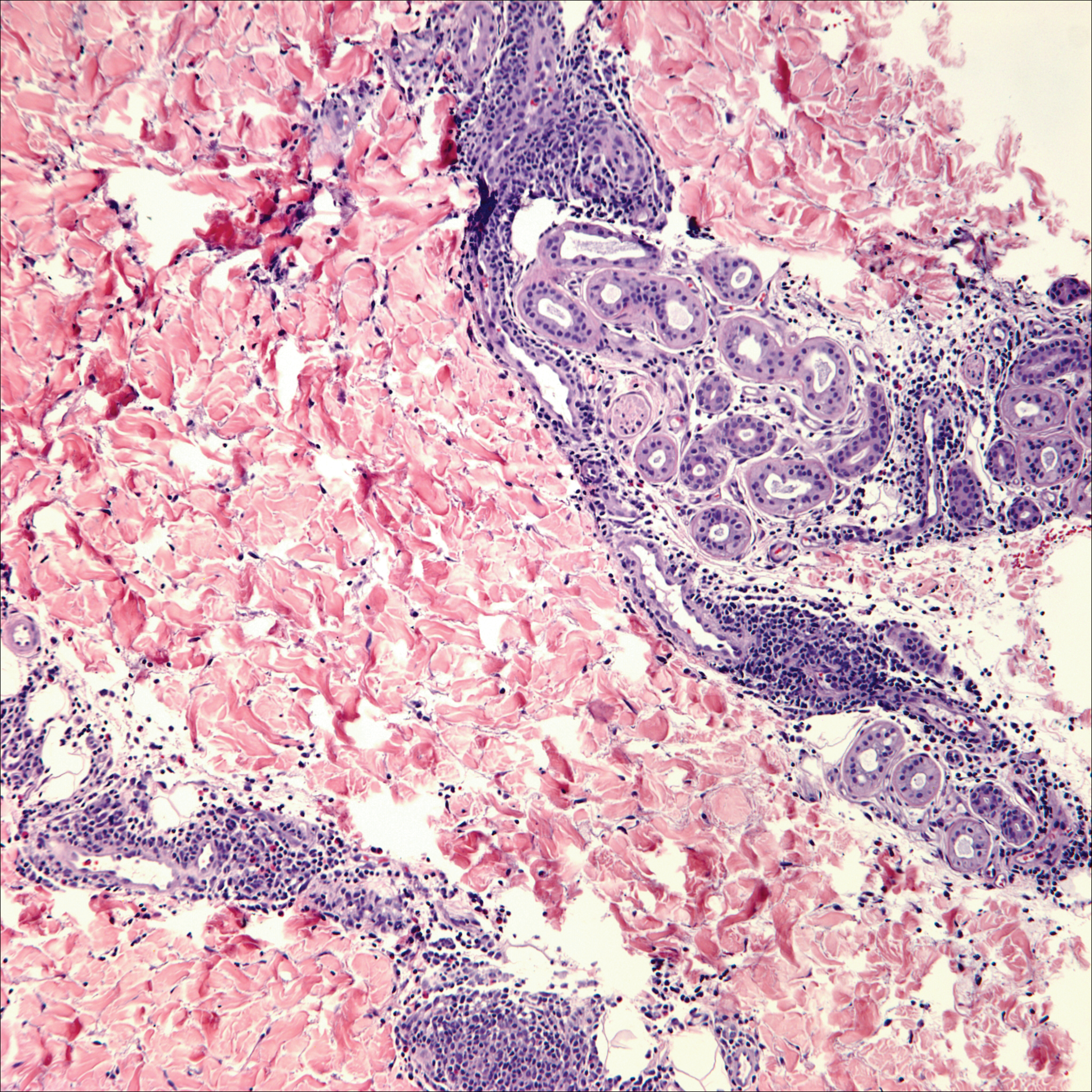
Breast cancer is the most common cancer to metastasize to the skin in women, accounting for 73% of cutaneous metastases, followed by melanoma, which is responsible for 11%.5 The classic presentation is an erythematous patch with spreading borders or a nodule on the trunk. Many cases of metastatic breast cancer with skin involvement may represent direct extension of the cancer into the skin. General histologic clues to cutaneous metastasis include well-circumscribed dermal or subcutaneous nodules of atypical cells with an increase in mitotic activity without connection to the epidermis. Tumor cells may show diffuse, nodular, or single file pattern and may exhibit areas of necrosis. Ductal carcinoma additionally may show ductal or glandular differentiation with surrounding desmoplasia (Figure 2). Immunohistochemistry typically is positive for cytokeratin (CK) 7, estrogen receptor/progesterone receptor, mammaglobin, and gross cystic disease fluid protein-15, and negative for CK20, CK5/6, and thyroid transcription factor-1.
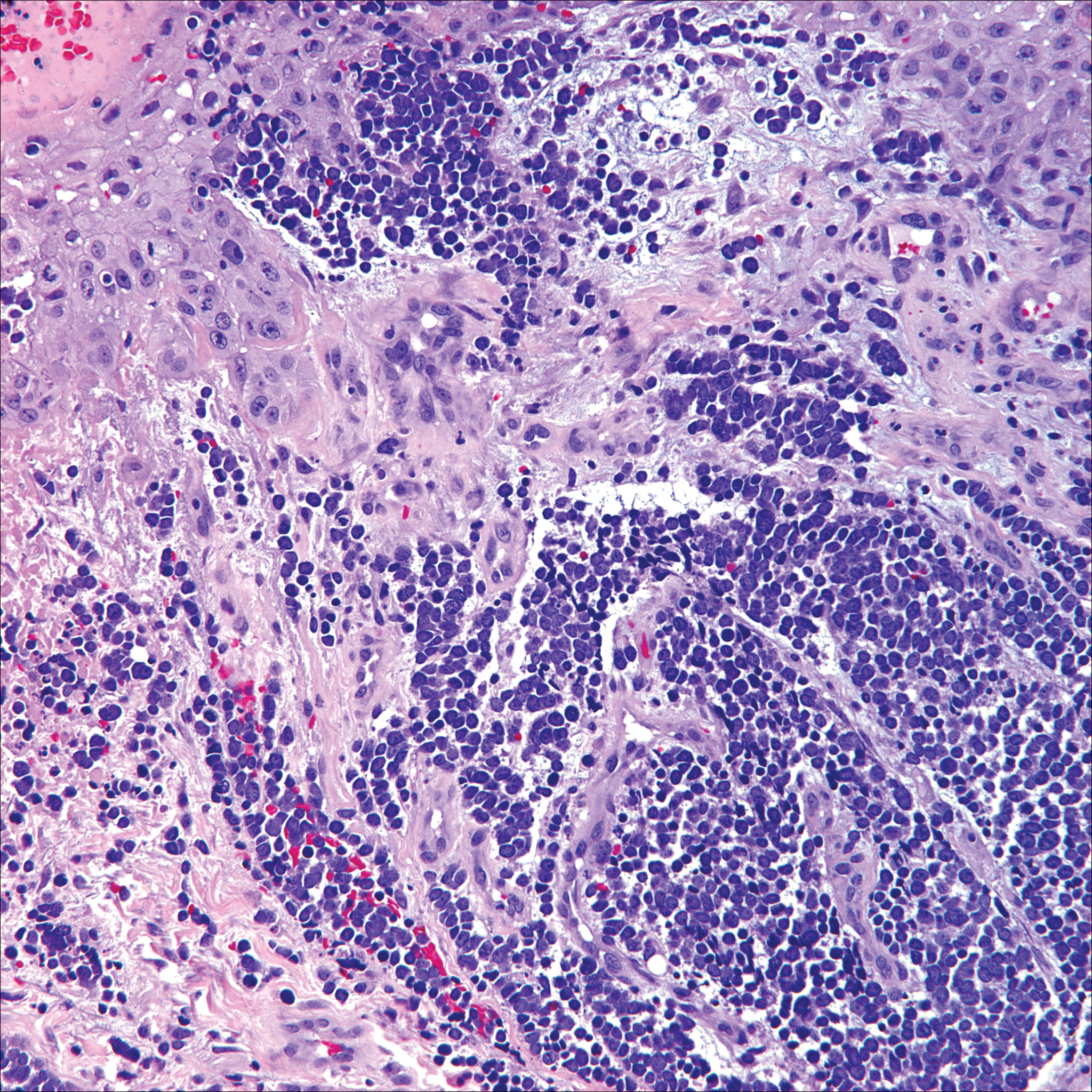
Papulovesicular and nodular lesions appearing as an arthropod bite have been noted in hematologic malignancies, underscoring the importance of histopathology and clinical correlation. Arthropod bites commonly present as red papules, nodules, vesicles, or pustules at the site of the bite. Pseudolymphomatous nodules occasionally develop. Excoriations and further progression to persistent prurigo also may occur. Histopathology shows variable epidermal features including spongiosis, acanthosis, parakeratosis, dermal edema, and superficial and deep perivascular neutrophils (Figure 3). Additionally, lymphocytes sometimes with CD30 positivity may be seen. The presence of eosinophils in interstitial areas, especially in the deep dermis, is a useful clue.
Lack of staining for epithelial and neuroendocrine markers differentiates PCALCL from MCC; specifically CK20, an epithelial marker positive in more than 90% of MCC cases, excludes lymphoma.6 Merkel cell carcinoma presents as a solitary, quickly growing, red and often ulcerated nodule or plaque on the head, neck, or legs of elderly patients. The lesions often are in areas of sun damage. Histopathology classically shows a diffuse dermal infiltrate of monotonous round blue cells with a scant cytoplasmic rim and multiple inconspicuous nucleoli in nests, rosettes, or strands in the dermis. There are frequent mitotic figures. The cells are uniform and 2 to 3 times larger than mature lymphocytes. Single-cell necrosis and crush artifact is common. Epidermotropism or coexisting Bowenoid change also may be observed (Figure 4). The term primary neuroendocrine carcinoma of the skin is preferred over Merkel cell carcinoma because the tumor cells share similar morphology to the specialized touch receptor of the basal layer (Merkel cell), but no direct histogenetic relationship has been established.7,8
Immunohistochemistry is key to diagnosis because MCC stains for both epithelial and neuroendocrine markers. Positivity is seen for neuron-specific enolase, epithelial membrane antigen, neurofilament, synaptophysin, and chromogranin. Because the histology of MCC may resemble small cell carcinoma of the lung, staining for low-molecular-weight keratin such as CK20 and CK7 help to distinguish MCC. Merkel cell carcinoma typically is CK20+ and CK7-, while small cell carcinoma of the lung is the opposite.9 The tumor grows aggressively and metastasis is common, thus surgery is the primary approach, but adjuvant chemotherapy and radiation often are given in addition.
- Yu J, Blitzblau R, Decker R, et al. Analysis of primary CD30+ cutaneous lymphoproliferative disease and survival from the Surveillance, Epidemiology, and End Results database. J Clin Oncol. 2008;26:1483-1488.
- Liu HL, Hoppe RT, Kohler S, et al. CD30+ cutaneous lymphoproliferative disorders: the Stanford experience in lymphomatoid papulosis and primary cutaneous anaplastic large cell lymphoma. J Am Acad Dermatol. 2003;49:1049-1058.
- Nasit JG, Patel SC. Primary cutaneous CD8(+) CD30(+) anaplastic large cell lymphoma: an unusual case with a high Ki-67 index--a short review. Indian J Dermatol. 2015;60:373-377.
- Park J, Lee J, Lim Y, et al. Synchronous occurrence of primary cutaneous anaplastic large cell lymphoma and squamous cell carcinoma. Ann Dermatol. 2016;28:491-494.
- Marks JG Jr, Miller JJ. Lookingbill and Marks' Principles of Dermatology. 5th ed. Philadelphia, PA: Elsevier Saunders; 2013.
- Kudchadkar R, Gonzalez R, Lewis K, et al. A case of Merkel cell carcinoma. Oncology. 2008;22:322-328.
- Ratner D, Nelson BR, Brown MD, et al. Merkel cell carcinoma. J Am Acad Dermatol. 1993;29:143-156.
- Zur Hausen A, Rennspiess D, Winnepenninckx V, et al. Early B-cell differentiation in Merkel cell carcinomas: clues to cellular ancestry [published online April 10, 2013]. Cancer Res. 2013;73:4982-4987.
- Sidiropoulos M, Hanna W, Raphael SJ, et al. Expression of TdT in Merkel cell carcinoma and small cell lung carcinoma. Am J Clin Pathol. 2011;135:831-838.
The Diagnosis: Primary Cutaneous Anaplastic Large Cell Lymphoma
Primary cutaneous CD30+ lymphoproliferative disorders encompass lymphomatoid papulosis and primary cutaneous anaplastic large cell lymphoma (PCALCL) as well as borderline cases. Primary cutaneous anaplastic large cell lymphoma is a rare disease that is more common in white patients with slight male predominance and median age at diagnosis of 61 years.1 Prognosis is excellent, with a 90% survival rate at 10 years. Although lesions spontaneously regress in 6% to 22% of cases, complete resolution is rare.2 Clinically, the classic presentation is a solitary, rapidly growing, flesh-colored, erythematous nodule or plaque on the arms and legs or trunk, often with ulceration. Proper diagnosis requires clinical, histopathologic, and immunophenotypic correlation.
Histopathologic examination of PCALCL typically reveals large, atypical, Reed-Sternberg-like cells most commonly with anaplastic cytomorphology, but pleomorphic or immunoblastic morphology is not uncommon. Cells are in sheets or nodules, diffusely occupying the dermis and often the subcutaneous fat, with more than 75% of large cells expressing CD30.3 In addition to CD30 positivity, immunophenotype is classically CD4+, cutaneous lymphocyte-associated antigen positive, epithelial membrane antigen negative, and anaplastic lymphoma kinase negative; CD2, CD5, and CD3 expression is variable. Interestingly, in our case, there was a minor population of CD8+ cells. CD8 expression is seen in less than 5% of PCALCL cases; this phenotype is associated with an indolent disease with favorable prognosis.3 Of note, anaplastic lymphoma kinase positivity corresponding to a t(2;5) translocation is more suggestive of systemic anaplastic large cell lymphoma with secondary skin involvement and more commonly is seen in children. For reasons possibly related to mediators such as epidermal growth factor or transforming growth factor α from CD30+ cells, epidermal hyperplasia can be seen in PCALCL.4 The subsequent hyperkeratosis, crusting, and ulceration can be difficult to distinguish from lesions such as pyoderma gangrenosum, squamous cell carcinoma, arthropod bite, leukemia cutis, Merkel cell carcinoma (MCC), and metastatic breast cancer.
Skin involvement with leukemia is rare but most commonly is seen in acute myelogenous leukemia, specifically more mature forms such as acute myelomonocytic leukemia and acute monocytic leukemia. Approximately 10% to 20% of acute myelomonocytic leukemia cases have cutaneous involvement.5 Although there is a variety of potential skin lesions, the most common is a red-purple papule or nodule, sometimes with hemorrhage or ulceration, on the head, neck, and trunk. Leukemic infiltrates may arise from sites of prior trauma. Histopathology depends on the type of leukemia; however, general features include a normal epidermis without epidermotropism and perivascular, nodular, or diffuse infiltrate of neoplastic cells in the dermis, often with a Grenz zone (Figure 1). Compared to PCALCL, leukemia cutis shows sparing of the papillary dermis (Grenz zone), and the cells have more cytoplasm and show a different immunophenotype. The cells often are fragile and show crush artifact. Acute myelogenous leukemia often will show cytoplasmic granules; however, immature precursor cells may not have granules. The myeloid cells will stain with myeloperoxidase and chloroacetate. Positivity is seen for CD13, CD33, and CD68. Clinical correlation is important because other diseases with nodular or diffuse infiltrates of small cell infiltrates, such as extramedullary hematopoiesis and lymphoma, appear similar. Acute myelogenous leukemia is associated with neutrophilic dermatoses such as Sweet syndrome and pyoderma gangrenosum. Cutaneous eruption resolves with successful treatment of the leukemia.

Breast cancer is the most common cancer to metastasize to the skin in women, accounting for 73% of cutaneous metastases, followed by melanoma, which is responsible for 11%.5 The classic presentation is an erythematous patch with spreading borders or a nodule on the trunk. Many cases of metastatic breast cancer with skin involvement may represent direct extension of the cancer into the skin. General histologic clues to cutaneous metastasis include well-circumscribed dermal or subcutaneous nodules of atypical cells with an increase in mitotic activity without connection to the epidermis. Tumor cells may show diffuse, nodular, or single file pattern and may exhibit areas of necrosis. Ductal carcinoma additionally may show ductal or glandular differentiation with surrounding desmoplasia (Figure 2). Immunohistochemistry typically is positive for cytokeratin (CK) 7, estrogen receptor/progesterone receptor, mammaglobin, and gross cystic disease fluid protein-15, and negative for CK20, CK5/6, and thyroid transcription factor-1.

Papulovesicular and nodular lesions appearing as an arthropod bite have been noted in hematologic malignancies, underscoring the importance of histopathology and clinical correlation. Arthropod bites commonly present as red papules, nodules, vesicles, or pustules at the site of the bite. Pseudolymphomatous nodules occasionally develop. Excoriations and further progression to persistent prurigo also may occur. Histopathology shows variable epidermal features including spongiosis, acanthosis, parakeratosis, dermal edema, and superficial and deep perivascular neutrophils (Figure 3). Additionally, lymphocytes sometimes with CD30 positivity may be seen. The presence of eosinophils in interstitial areas, especially in the deep dermis, is a useful clue.

Lack of staining for epithelial and neuroendocrine markers differentiates PCALCL from MCC; specifically CK20, an epithelial marker positive in more than 90% of MCC cases, excludes lymphoma.6 Merkel cell carcinoma presents as a solitary, quickly growing, red and often ulcerated nodule or plaque on the head, neck, or legs of elderly patients. The lesions often are in areas of sun damage. Histopathology classically shows a diffuse dermal infiltrate of monotonous round blue cells with a scant cytoplasmic rim and multiple inconspicuous nucleoli in nests, rosettes, or strands in the dermis. There are frequent mitotic figures. The cells are uniform and 2 to 3 times larger than mature lymphocytes. Single-cell necrosis and crush artifact is common. Epidermotropism or coexisting Bowenoid change also may be observed (Figure 4). The term primary neuroendocrine carcinoma of the skin is preferred over Merkel cell carcinoma because the tumor cells share similar morphology to the specialized touch receptor of the basal layer (Merkel cell), but no direct histogenetic relationship has been established.7,8

Immunohistochemistry is key to diagnosis because MCC stains for both epithelial and neuroendocrine markers. Positivity is seen for neuron-specific enolase, epithelial membrane antigen, neurofilament, synaptophysin, and chromogranin. Because the histology of MCC may resemble small cell carcinoma of the lung, staining for low-molecular-weight keratin such as CK20 and CK7 help to distinguish MCC. Merkel cell carcinoma typically is CK20+ and CK7-, while small cell carcinoma of the lung is the opposite.9 The tumor grows aggressively and metastasis is common, thus surgery is the primary approach, but adjuvant chemotherapy and radiation often are given in addition.
The Diagnosis: Primary Cutaneous Anaplastic Large Cell Lymphoma
Primary cutaneous CD30+ lymphoproliferative disorders encompass lymphomatoid papulosis and primary cutaneous anaplastic large cell lymphoma (PCALCL) as well as borderline cases. Primary cutaneous anaplastic large cell lymphoma is a rare disease that is more common in white patients with slight male predominance and median age at diagnosis of 61 years.1 Prognosis is excellent, with a 90% survival rate at 10 years. Although lesions spontaneously regress in 6% to 22% of cases, complete resolution is rare.2 Clinically, the classic presentation is a solitary, rapidly growing, flesh-colored, erythematous nodule or plaque on the arms and legs or trunk, often with ulceration. Proper diagnosis requires clinical, histopathologic, and immunophenotypic correlation.
Histopathologic examination of PCALCL typically reveals large, atypical, Reed-Sternberg-like cells most commonly with anaplastic cytomorphology, but pleomorphic or immunoblastic morphology is not uncommon. Cells are in sheets or nodules, diffusely occupying the dermis and often the subcutaneous fat, with more than 75% of large cells expressing CD30.3 In addition to CD30 positivity, immunophenotype is classically CD4+, cutaneous lymphocyte-associated antigen positive, epithelial membrane antigen negative, and anaplastic lymphoma kinase negative; CD2, CD5, and CD3 expression is variable. Interestingly, in our case, there was a minor population of CD8+ cells. CD8 expression is seen in less than 5% of PCALCL cases; this phenotype is associated with an indolent disease with favorable prognosis.3 Of note, anaplastic lymphoma kinase positivity corresponding to a t(2;5) translocation is more suggestive of systemic anaplastic large cell lymphoma with secondary skin involvement and more commonly is seen in children. For reasons possibly related to mediators such as epidermal growth factor or transforming growth factor α from CD30+ cells, epidermal hyperplasia can be seen in PCALCL.4 The subsequent hyperkeratosis, crusting, and ulceration can be difficult to distinguish from lesions such as pyoderma gangrenosum, squamous cell carcinoma, arthropod bite, leukemia cutis, Merkel cell carcinoma (MCC), and metastatic breast cancer.
Skin involvement with leukemia is rare but most commonly is seen in acute myelogenous leukemia, specifically more mature forms such as acute myelomonocytic leukemia and acute monocytic leukemia. Approximately 10% to 20% of acute myelomonocytic leukemia cases have cutaneous involvement.5 Although there is a variety of potential skin lesions, the most common is a red-purple papule or nodule, sometimes with hemorrhage or ulceration, on the head, neck, and trunk. Leukemic infiltrates may arise from sites of prior trauma. Histopathology depends on the type of leukemia; however, general features include a normal epidermis without epidermotropism and perivascular, nodular, or diffuse infiltrate of neoplastic cells in the dermis, often with a Grenz zone (Figure 1). Compared to PCALCL, leukemia cutis shows sparing of the papillary dermis (Grenz zone), and the cells have more cytoplasm and show a different immunophenotype. The cells often are fragile and show crush artifact. Acute myelogenous leukemia often will show cytoplasmic granules; however, immature precursor cells may not have granules. The myeloid cells will stain with myeloperoxidase and chloroacetate. Positivity is seen for CD13, CD33, and CD68. Clinical correlation is important because other diseases with nodular or diffuse infiltrates of small cell infiltrates, such as extramedullary hematopoiesis and lymphoma, appear similar. Acute myelogenous leukemia is associated with neutrophilic dermatoses such as Sweet syndrome and pyoderma gangrenosum. Cutaneous eruption resolves with successful treatment of the leukemia.

Breast cancer is the most common cancer to metastasize to the skin in women, accounting for 73% of cutaneous metastases, followed by melanoma, which is responsible for 11%.5 The classic presentation is an erythematous patch with spreading borders or a nodule on the trunk. Many cases of metastatic breast cancer with skin involvement may represent direct extension of the cancer into the skin. General histologic clues to cutaneous metastasis include well-circumscribed dermal or subcutaneous nodules of atypical cells with an increase in mitotic activity without connection to the epidermis. Tumor cells may show diffuse, nodular, or single file pattern and may exhibit areas of necrosis. Ductal carcinoma additionally may show ductal or glandular differentiation with surrounding desmoplasia (Figure 2). Immunohistochemistry typically is positive for cytokeratin (CK) 7, estrogen receptor/progesterone receptor, mammaglobin, and gross cystic disease fluid protein-15, and negative for CK20, CK5/6, and thyroid transcription factor-1.

Papulovesicular and nodular lesions appearing as an arthropod bite have been noted in hematologic malignancies, underscoring the importance of histopathology and clinical correlation. Arthropod bites commonly present as red papules, nodules, vesicles, or pustules at the site of the bite. Pseudolymphomatous nodules occasionally develop. Excoriations and further progression to persistent prurigo also may occur. Histopathology shows variable epidermal features including spongiosis, acanthosis, parakeratosis, dermal edema, and superficial and deep perivascular neutrophils (Figure 3). Additionally, lymphocytes sometimes with CD30 positivity may be seen. The presence of eosinophils in interstitial areas, especially in the deep dermis, is a useful clue.

Lack of staining for epithelial and neuroendocrine markers differentiates PCALCL from MCC; specifically CK20, an epithelial marker positive in more than 90% of MCC cases, excludes lymphoma.6 Merkel cell carcinoma presents as a solitary, quickly growing, red and often ulcerated nodule or plaque on the head, neck, or legs of elderly patients. The lesions often are in areas of sun damage. Histopathology classically shows a diffuse dermal infiltrate of monotonous round blue cells with a scant cytoplasmic rim and multiple inconspicuous nucleoli in nests, rosettes, or strands in the dermis. There are frequent mitotic figures. The cells are uniform and 2 to 3 times larger than mature lymphocytes. Single-cell necrosis and crush artifact is common. Epidermotropism or coexisting Bowenoid change also may be observed (Figure 4). The term primary neuroendocrine carcinoma of the skin is preferred over Merkel cell carcinoma because the tumor cells share similar morphology to the specialized touch receptor of the basal layer (Merkel cell), but no direct histogenetic relationship has been established.7,8

Immunohistochemistry is key to diagnosis because MCC stains for both epithelial and neuroendocrine markers. Positivity is seen for neuron-specific enolase, epithelial membrane antigen, neurofilament, synaptophysin, and chromogranin. Because the histology of MCC may resemble small cell carcinoma of the lung, staining for low-molecular-weight keratin such as CK20 and CK7 help to distinguish MCC. Merkel cell carcinoma typically is CK20+ and CK7-, while small cell carcinoma of the lung is the opposite.9 The tumor grows aggressively and metastasis is common, thus surgery is the primary approach, but adjuvant chemotherapy and radiation often are given in addition.
- Yu J, Blitzblau R, Decker R, et al. Analysis of primary CD30+ cutaneous lymphoproliferative disease and survival from the Surveillance, Epidemiology, and End Results database. J Clin Oncol. 2008;26:1483-1488.
- Liu HL, Hoppe RT, Kohler S, et al. CD30+ cutaneous lymphoproliferative disorders: the Stanford experience in lymphomatoid papulosis and primary cutaneous anaplastic large cell lymphoma. J Am Acad Dermatol. 2003;49:1049-1058.
- Nasit JG, Patel SC. Primary cutaneous CD8(+) CD30(+) anaplastic large cell lymphoma: an unusual case with a high Ki-67 index--a short review. Indian J Dermatol. 2015;60:373-377.
- Park J, Lee J, Lim Y, et al. Synchronous occurrence of primary cutaneous anaplastic large cell lymphoma and squamous cell carcinoma. Ann Dermatol. 2016;28:491-494.
- Marks JG Jr, Miller JJ. Lookingbill and Marks' Principles of Dermatology. 5th ed. Philadelphia, PA: Elsevier Saunders; 2013.
- Kudchadkar R, Gonzalez R, Lewis K, et al. A case of Merkel cell carcinoma. Oncology. 2008;22:322-328.
- Ratner D, Nelson BR, Brown MD, et al. Merkel cell carcinoma. J Am Acad Dermatol. 1993;29:143-156.
- Zur Hausen A, Rennspiess D, Winnepenninckx V, et al. Early B-cell differentiation in Merkel cell carcinomas: clues to cellular ancestry [published online April 10, 2013]. Cancer Res. 2013;73:4982-4987.
- Sidiropoulos M, Hanna W, Raphael SJ, et al. Expression of TdT in Merkel cell carcinoma and small cell lung carcinoma. Am J Clin Pathol. 2011;135:831-838.
- Yu J, Blitzblau R, Decker R, et al. Analysis of primary CD30+ cutaneous lymphoproliferative disease and survival from the Surveillance, Epidemiology, and End Results database. J Clin Oncol. 2008;26:1483-1488.
- Liu HL, Hoppe RT, Kohler S, et al. CD30+ cutaneous lymphoproliferative disorders: the Stanford experience in lymphomatoid papulosis and primary cutaneous anaplastic large cell lymphoma. J Am Acad Dermatol. 2003;49:1049-1058.
- Nasit JG, Patel SC. Primary cutaneous CD8(+) CD30(+) anaplastic large cell lymphoma: an unusual case with a high Ki-67 index--a short review. Indian J Dermatol. 2015;60:373-377.
- Park J, Lee J, Lim Y, et al. Synchronous occurrence of primary cutaneous anaplastic large cell lymphoma and squamous cell carcinoma. Ann Dermatol. 2016;28:491-494.
- Marks JG Jr, Miller JJ. Lookingbill and Marks' Principles of Dermatology. 5th ed. Philadelphia, PA: Elsevier Saunders; 2013.
- Kudchadkar R, Gonzalez R, Lewis K, et al. A case of Merkel cell carcinoma. Oncology. 2008;22:322-328.
- Ratner D, Nelson BR, Brown MD, et al. Merkel cell carcinoma. J Am Acad Dermatol. 1993;29:143-156.
- Zur Hausen A, Rennspiess D, Winnepenninckx V, et al. Early B-cell differentiation in Merkel cell carcinomas: clues to cellular ancestry [published online April 10, 2013]. Cancer Res. 2013;73:4982-4987.
- Sidiropoulos M, Hanna W, Raphael SJ, et al. Expression of TdT in Merkel cell carcinoma and small cell lung carcinoma. Am J Clin Pathol. 2011;135:831-838.
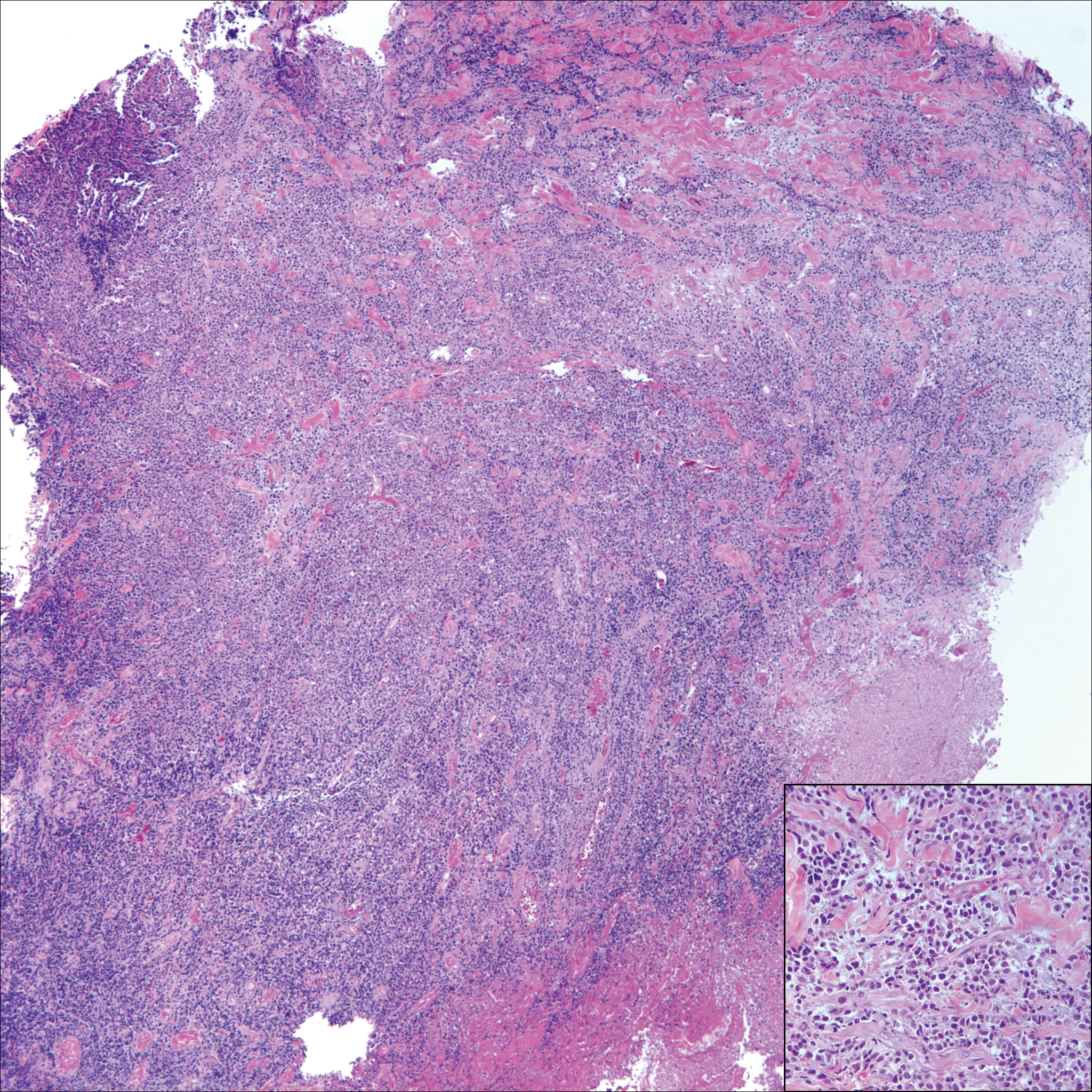
A 65-year-old white woman presented with an asymptomatic bump on the left upper arm of 4 months' duration that arose following a cat scratch. Physical examination was notable for a 35×30-mm, firm, ulcerated, exophytic nodule. Histologic examination demonstrated an ulcerated epidermis and a dense basophilic infiltrate occupying the entire dermis and extending to the subcutaneous tissue. Higher magnification (inset) demonstrated a pleomorphic population of medium- to large-sized discohesive round cells containing variable amounts of slightly eosinophilic cytoplasm, irregular nuclear contours, and prominent nucleoli. Scattered atypical mitotic figures were identified. CD30, CD4, leukocyte common antigen, and Ki-67 immunostains were strongly and diffusely positive. Notable negative stains included anaplastic lymphoma kinase, synaptophysin, epithelial membrane antigen, neuron-specific enolase, CD20, and S-100.
VIDEO: What to monitor during isotretinoin treatment
KAUAI, HAWAII – Hilary Baldwin, MD, medical director of the Acne Treatment and Research Center, Morristown, N.J., recently changed how she monitors patients on isotretinoin.
The latest research indicates that ongoing CBCs really aren’t necessary, and that GGT (gamma-glutamyl transferase), which is liver specific, is a far better option than ALT/AST to keep tabs on the liver. Creatine kinase can’t be ignored, either, especially in young, athletic patients, because of the risk of rhabdomyolysis.
In a video interview at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation, Dr. Baldwin explained the thinking behind her new approach, plus what else needs to be monitored and for how long – and the level of creatine kinase that should raise a red flag for clinicians.
Dr. Baldwin is a speaker, advisor, and/or investigator for a number of companies, including Allergan, Galderma, and La Roche Posay.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
KAUAI, HAWAII – Hilary Baldwin, MD, medical director of the Acne Treatment and Research Center, Morristown, N.J., recently changed how she monitors patients on isotretinoin.
The latest research indicates that ongoing CBCs really aren’t necessary, and that GGT (gamma-glutamyl transferase), which is liver specific, is a far better option than ALT/AST to keep tabs on the liver. Creatine kinase can’t be ignored, either, especially in young, athletic patients, because of the risk of rhabdomyolysis.
In a video interview at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation, Dr. Baldwin explained the thinking behind her new approach, plus what else needs to be monitored and for how long – and the level of creatine kinase that should raise a red flag for clinicians.
Dr. Baldwin is a speaker, advisor, and/or investigator for a number of companies, including Allergan, Galderma, and La Roche Posay.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
KAUAI, HAWAII – Hilary Baldwin, MD, medical director of the Acne Treatment and Research Center, Morristown, N.J., recently changed how she monitors patients on isotretinoin.
The latest research indicates that ongoing CBCs really aren’t necessary, and that GGT (gamma-glutamyl transferase), which is liver specific, is a far better option than ALT/AST to keep tabs on the liver. Creatine kinase can’t be ignored, either, especially in young, athletic patients, because of the risk of rhabdomyolysis.
In a video interview at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation, Dr. Baldwin explained the thinking behind her new approach, plus what else needs to be monitored and for how long – and the level of creatine kinase that should raise a red flag for clinicians.
Dr. Baldwin is a speaker, advisor, and/or investigator for a number of companies, including Allergan, Galderma, and La Roche Posay.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
REPORTING FROM SDEF HAWAII DERMATOLOGY SEMINAR
Mobile Medical Apps for Patient Education: A Graded Review of Available Dermatology Apps
According to industry estimates, roughly 64% of US adults were smartphone users in 2015.1 Smartphones enable users to utilize mobile applications (apps) that can perform a variety of functions in many categories, including business, music, photography, entertainment, education, social networking, travel, and lifestyle. The widespread adoption and use of mobile apps has implications for medical practice. Mobile apps have the capability to serve as information sources for patients, educational tools for students, and diagnostic aids for physicians.2 Consequently, a number of medical and health care–oriented apps have already been developed3 and are increasingly utilized by patients and providers.4
Given its visual nature, dermatology is particularly amenable to the integration of mobile medical apps. A study by Brewer et al5 identified more than 229 dermatology-related apps in categories ranging from general dermatology reference, self-surveillance and diagnosis, disease guides, educational aids, sunscreen and UV recommendations, and teledermatology. Patients served as the target audience and principal consumers of more than half of these dermatology apps.5
Mobile medical and health care apps demonstrate great potential for serving as valuable information sources for patients with dermatologic conditions; however, the content, functions, accuracy, and educational value of dermatology mobile apps are not well characterized, making it difficult for patients and health care providers to select and recommend appropriate apps.6 In this study, we created a rubric to objectively grade 44 publicly available mobile dermatology apps with the primary focus of patient education.
Methods
We conducted a search of dermatology-related educational mobile apps that were publicly available via the App Store (Apple Inc) from January 2016 to November 2016. (The pricing, availability, and other features of these apps may have changed since the study period.) The following search terms were used: dermatology, dermoscopy, melanoma, skin cancer, psoriasis, rosacea, acne, eczema, dermal fillers, and Mohs surgery. We excluded apps that were not in English; had a solely commercial focus; were mobile textbooks or scientific journals; were used to provide teledermatology services with no educational purpose; were solely focused on homeopathic, alternative, and/or complementary medicine; or were intended primarily as a reference for students or health care professionals. Our search yielded 44 apps with patient education as a primary objective. The apps were divided into 6 categories based on their focus: general dermatology, cosmetic dermatology, acne, eczema, psoriasis, and skin cancer.
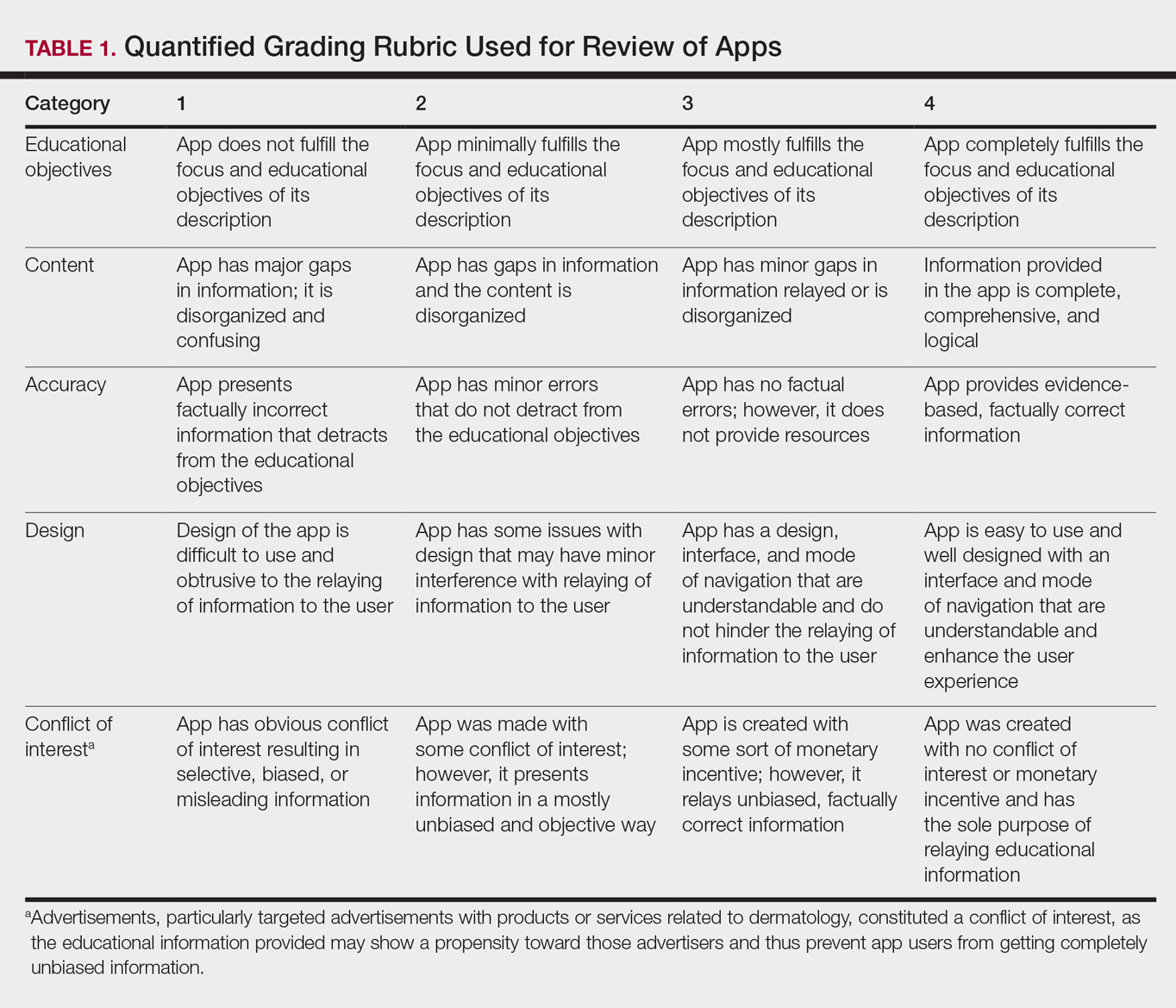
Each app was reviewed using a quantified grading rubric developed by the researchers. In a prior evaluation, Handel7 reviewed 35 health and wellness mobile apps utilizing the categories of ease of use, reliability, quality, scope of information, and aesthetics.4 These criteria were modified and adapted for the purposes of this study, and a 4-point scale was applied to each criterion. The final criteria were (1) educational objectives, (2) content, (3) accuracy, (4) design, and (5) conflict of interest. The quantified grading rubric is described in Table 1.
Results
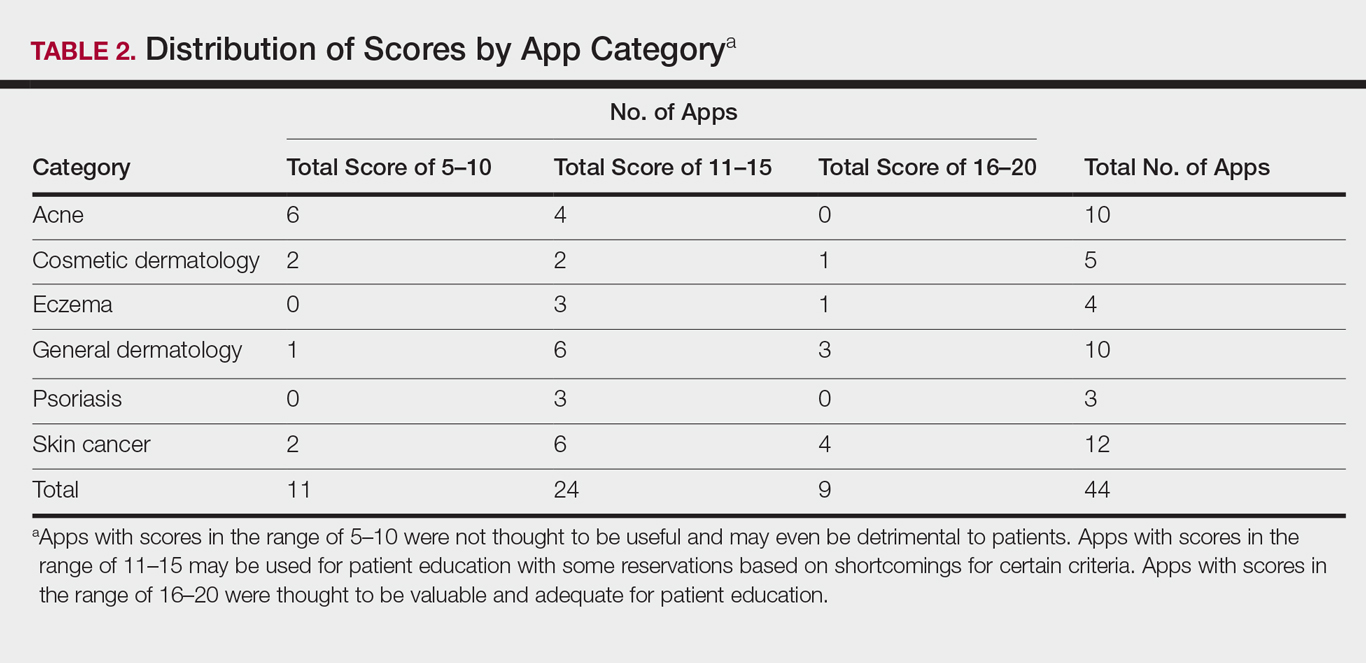
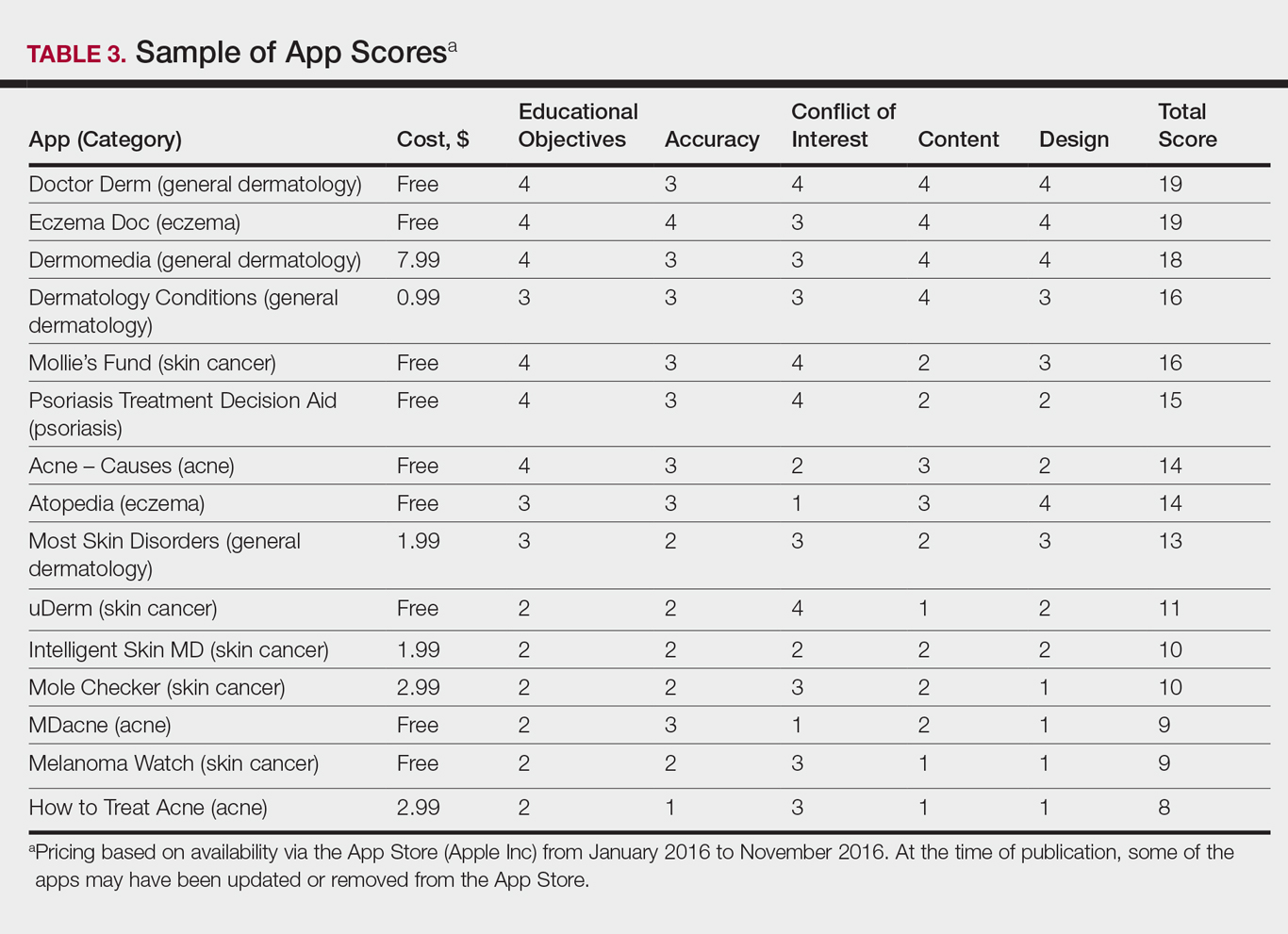
The possible range of scores based on the grading rubric was 5 to 20. The actual range of scores was 8 to 19 (Table 2). The 44 reviewed apps were categorized by topic as acne, cosmetic dermatology, eczema, general dermatology, psoriasis, or skin cancer. A sample of 15 apps selected to represent the distribution of scores and their grading on the rubric are presented in Table 3.
Comment
The number of dermatology-related apps available to mobile users continues to grow at an increasing rate.8 The apps vary in many aspects, including their purpose, scope, intended audience, and goals of the app publisher. In turn, more individuals are turning to mobile apps for medical information,4 especially in dermatology, thus it is necessary to create a systematic way to evaluate the quality and utility of each app to assist users in making informed decisions about which apps will best meet their needs in the midst of a wide array of choices.
For the purpose of this study, an objective rubric was created that can be used to evaluate the quality of medical apps for patient education in dermatology. An app’s adequacy and usefulness for patient education was thought to depend on 3 possible score ranges into which the app could fall based on the grading rubric. An app with a total score in the range of 5 to 10 was not thought to be useful and may even be detrimental to patients. An app with a total score in the range of 11 to 15 may be used for patient education with some reservations based on shortcomings for certain criteria. An app with a score in the range of 16 to 20 was thought to be valuable and adequate for patient education. For example, the How to Treat Acne app received a total score of 8 and therefore would not be recommended to patients based on the grading rubric used in this study. This particular app provided sparse and sometimes inaccurate information, had a confusing user interface, and contained many obstructive advertisements. In contrast, the Eczema Doc app received a total score of 19, which indicates a quality app deemed to be useful for patient information based on the established rubric. This app met all the objectives that it advertised, contained accurate information with verified citation of sources, and was very easy for users to navigate.
Of the 44 graded apps, only 9 (20.5%) received scores in the highest range of 16 to 20, which indicates a need for improvements in mobile dermatology apps intended for patient education. Adopting the grading rubric developed in this study as a standard in the creation of medical apps could have beneficial implications in disseminating accurate, safe, unbiased, and easy-to-understand information to patients.
- Smith A. U.S. smartphone use in 2015. Pew Research Center website. http://www.pewinternet.org/2015/04/01/us-smartphone-use-in-2015. Published April 1, 2015. Accessed August 29, 2017.
- Nilsen W, Kumar S, Shar A, et al. Advancing the science of mHealth. J Health Commun. 2012;17(suppl 1):5-10.
- West DM. How mobile devices are transforming healthcare issues in technology innovation. Issues Technol Innov. 2012;18:1-14.
- Boudreaux ED, Waring ME, Hayes RB, et al. Evaluating and selecting mobile health apps: strategies for healthcare providers and healthcare organizations. Transl Behav Med. 2014;4:363-371.
- Brewer AC, Endly DC, Henley J, et al. Mobile applications in dermatology. JAMA Dermatol. 2013;149:1300-1304.
- Cummings E, Borycki E, Roehrer E. Issues and considerations for healthcare consumers using mobile applications. Stud Health Technol Inform. 2013;183:227-231.
- Handel MJ. mHealth (mobile health)-using apps for health and wellness. Explore. 2011;7:256-261.
- Boulos MN, Brewer AC, Karimkhani C, et al. Mobile medical and health apps: state of the art, concerns, regulatory control and certification. Online J Public Health Inform. 2014;5:229.
According to industry estimates, roughly 64% of US adults were smartphone users in 2015.1 Smartphones enable users to utilize mobile applications (apps) that can perform a variety of functions in many categories, including business, music, photography, entertainment, education, social networking, travel, and lifestyle. The widespread adoption and use of mobile apps has implications for medical practice. Mobile apps have the capability to serve as information sources for patients, educational tools for students, and diagnostic aids for physicians.2 Consequently, a number of medical and health care–oriented apps have already been developed3 and are increasingly utilized by patients and providers.4
Given its visual nature, dermatology is particularly amenable to the integration of mobile medical apps. A study by Brewer et al5 identified more than 229 dermatology-related apps in categories ranging from general dermatology reference, self-surveillance and diagnosis, disease guides, educational aids, sunscreen and UV recommendations, and teledermatology. Patients served as the target audience and principal consumers of more than half of these dermatology apps.5
Mobile medical and health care apps demonstrate great potential for serving as valuable information sources for patients with dermatologic conditions; however, the content, functions, accuracy, and educational value of dermatology mobile apps are not well characterized, making it difficult for patients and health care providers to select and recommend appropriate apps.6 In this study, we created a rubric to objectively grade 44 publicly available mobile dermatology apps with the primary focus of patient education.
Methods
We conducted a search of dermatology-related educational mobile apps that were publicly available via the App Store (Apple Inc) from January 2016 to November 2016. (The pricing, availability, and other features of these apps may have changed since the study period.) The following search terms were used: dermatology, dermoscopy, melanoma, skin cancer, psoriasis, rosacea, acne, eczema, dermal fillers, and Mohs surgery. We excluded apps that were not in English; had a solely commercial focus; were mobile textbooks or scientific journals; were used to provide teledermatology services with no educational purpose; were solely focused on homeopathic, alternative, and/or complementary medicine; or were intended primarily as a reference for students or health care professionals. Our search yielded 44 apps with patient education as a primary objective. The apps were divided into 6 categories based on their focus: general dermatology, cosmetic dermatology, acne, eczema, psoriasis, and skin cancer.
Each app was reviewed using a quantified grading rubric developed by the researchers. In a prior evaluation, Handel7 reviewed 35 health and wellness mobile apps utilizing the categories of ease of use, reliability, quality, scope of information, and aesthetics.4 These criteria were modified and adapted for the purposes of this study, and a 4-point scale was applied to each criterion. The final criteria were (1) educational objectives, (2) content, (3) accuracy, (4) design, and (5) conflict of interest. The quantified grading rubric is described in Table 1.

Results
The possible range of scores based on the grading rubric was 5 to 20. The actual range of scores was 8 to 19 (Table 2). The 44 reviewed apps were categorized by topic as acne, cosmetic dermatology, eczema, general dermatology, psoriasis, or skin cancer. A sample of 15 apps selected to represent the distribution of scores and their grading on the rubric are presented in Table 3.


Comment
The number of dermatology-related apps available to mobile users continues to grow at an increasing rate.8 The apps vary in many aspects, including their purpose, scope, intended audience, and goals of the app publisher. In turn, more individuals are turning to mobile apps for medical information,4 especially in dermatology, thus it is necessary to create a systematic way to evaluate the quality and utility of each app to assist users in making informed decisions about which apps will best meet their needs in the midst of a wide array of choices.
For the purpose of this study, an objective rubric was created that can be used to evaluate the quality of medical apps for patient education in dermatology. An app’s adequacy and usefulness for patient education was thought to depend on 3 possible score ranges into which the app could fall based on the grading rubric. An app with a total score in the range of 5 to 10 was not thought to be useful and may even be detrimental to patients. An app with a total score in the range of 11 to 15 may be used for patient education with some reservations based on shortcomings for certain criteria. An app with a score in the range of 16 to 20 was thought to be valuable and adequate for patient education. For example, the How to Treat Acne app received a total score of 8 and therefore would not be recommended to patients based on the grading rubric used in this study. This particular app provided sparse and sometimes inaccurate information, had a confusing user interface, and contained many obstructive advertisements. In contrast, the Eczema Doc app received a total score of 19, which indicates a quality app deemed to be useful for patient information based on the established rubric. This app met all the objectives that it advertised, contained accurate information with verified citation of sources, and was very easy for users to navigate.
Of the 44 graded apps, only 9 (20.5%) received scores in the highest range of 16 to 20, which indicates a need for improvements in mobile dermatology apps intended for patient education. Adopting the grading rubric developed in this study as a standard in the creation of medical apps could have beneficial implications in disseminating accurate, safe, unbiased, and easy-to-understand information to patients.
According to industry estimates, roughly 64% of US adults were smartphone users in 2015.1 Smartphones enable users to utilize mobile applications (apps) that can perform a variety of functions in many categories, including business, music, photography, entertainment, education, social networking, travel, and lifestyle. The widespread adoption and use of mobile apps has implications for medical practice. Mobile apps have the capability to serve as information sources for patients, educational tools for students, and diagnostic aids for physicians.2 Consequently, a number of medical and health care–oriented apps have already been developed3 and are increasingly utilized by patients and providers.4
Given its visual nature, dermatology is particularly amenable to the integration of mobile medical apps. A study by Brewer et al5 identified more than 229 dermatology-related apps in categories ranging from general dermatology reference, self-surveillance and diagnosis, disease guides, educational aids, sunscreen and UV recommendations, and teledermatology. Patients served as the target audience and principal consumers of more than half of these dermatology apps.5
Mobile medical and health care apps demonstrate great potential for serving as valuable information sources for patients with dermatologic conditions; however, the content, functions, accuracy, and educational value of dermatology mobile apps are not well characterized, making it difficult for patients and health care providers to select and recommend appropriate apps.6 In this study, we created a rubric to objectively grade 44 publicly available mobile dermatology apps with the primary focus of patient education.
Methods
We conducted a search of dermatology-related educational mobile apps that were publicly available via the App Store (Apple Inc) from January 2016 to November 2016. (The pricing, availability, and other features of these apps may have changed since the study period.) The following search terms were used: dermatology, dermoscopy, melanoma, skin cancer, psoriasis, rosacea, acne, eczema, dermal fillers, and Mohs surgery. We excluded apps that were not in English; had a solely commercial focus; were mobile textbooks or scientific journals; were used to provide teledermatology services with no educational purpose; were solely focused on homeopathic, alternative, and/or complementary medicine; or were intended primarily as a reference for students or health care professionals. Our search yielded 44 apps with patient education as a primary objective. The apps were divided into 6 categories based on their focus: general dermatology, cosmetic dermatology, acne, eczema, psoriasis, and skin cancer.
Each app was reviewed using a quantified grading rubric developed by the researchers. In a prior evaluation, Handel7 reviewed 35 health and wellness mobile apps utilizing the categories of ease of use, reliability, quality, scope of information, and aesthetics.4 These criteria were modified and adapted for the purposes of this study, and a 4-point scale was applied to each criterion. The final criteria were (1) educational objectives, (2) content, (3) accuracy, (4) design, and (5) conflict of interest. The quantified grading rubric is described in Table 1.

Results
The possible range of scores based on the grading rubric was 5 to 20. The actual range of scores was 8 to 19 (Table 2). The 44 reviewed apps were categorized by topic as acne, cosmetic dermatology, eczema, general dermatology, psoriasis, or skin cancer. A sample of 15 apps selected to represent the distribution of scores and their grading on the rubric are presented in Table 3.


Comment
The number of dermatology-related apps available to mobile users continues to grow at an increasing rate.8 The apps vary in many aspects, including their purpose, scope, intended audience, and goals of the app publisher. In turn, more individuals are turning to mobile apps for medical information,4 especially in dermatology, thus it is necessary to create a systematic way to evaluate the quality and utility of each app to assist users in making informed decisions about which apps will best meet their needs in the midst of a wide array of choices.
For the purpose of this study, an objective rubric was created that can be used to evaluate the quality of medical apps for patient education in dermatology. An app’s adequacy and usefulness for patient education was thought to depend on 3 possible score ranges into which the app could fall based on the grading rubric. An app with a total score in the range of 5 to 10 was not thought to be useful and may even be detrimental to patients. An app with a total score in the range of 11 to 15 may be used for patient education with some reservations based on shortcomings for certain criteria. An app with a score in the range of 16 to 20 was thought to be valuable and adequate for patient education. For example, the How to Treat Acne app received a total score of 8 and therefore would not be recommended to patients based on the grading rubric used in this study. This particular app provided sparse and sometimes inaccurate information, had a confusing user interface, and contained many obstructive advertisements. In contrast, the Eczema Doc app received a total score of 19, which indicates a quality app deemed to be useful for patient information based on the established rubric. This app met all the objectives that it advertised, contained accurate information with verified citation of sources, and was very easy for users to navigate.
Of the 44 graded apps, only 9 (20.5%) received scores in the highest range of 16 to 20, which indicates a need for improvements in mobile dermatology apps intended for patient education. Adopting the grading rubric developed in this study as a standard in the creation of medical apps could have beneficial implications in disseminating accurate, safe, unbiased, and easy-to-understand information to patients.
- Smith A. U.S. smartphone use in 2015. Pew Research Center website. http://www.pewinternet.org/2015/04/01/us-smartphone-use-in-2015. Published April 1, 2015. Accessed August 29, 2017.
- Nilsen W, Kumar S, Shar A, et al. Advancing the science of mHealth. J Health Commun. 2012;17(suppl 1):5-10.
- West DM. How mobile devices are transforming healthcare issues in technology innovation. Issues Technol Innov. 2012;18:1-14.
- Boudreaux ED, Waring ME, Hayes RB, et al. Evaluating and selecting mobile health apps: strategies for healthcare providers and healthcare organizations. Transl Behav Med. 2014;4:363-371.
- Brewer AC, Endly DC, Henley J, et al. Mobile applications in dermatology. JAMA Dermatol. 2013;149:1300-1304.
- Cummings E, Borycki E, Roehrer E. Issues and considerations for healthcare consumers using mobile applications. Stud Health Technol Inform. 2013;183:227-231.
- Handel MJ. mHealth (mobile health)-using apps for health and wellness. Explore. 2011;7:256-261.
- Boulos MN, Brewer AC, Karimkhani C, et al. Mobile medical and health apps: state of the art, concerns, regulatory control and certification. Online J Public Health Inform. 2014;5:229.
- Smith A. U.S. smartphone use in 2015. Pew Research Center website. http://www.pewinternet.org/2015/04/01/us-smartphone-use-in-2015. Published April 1, 2015. Accessed August 29, 2017.
- Nilsen W, Kumar S, Shar A, et al. Advancing the science of mHealth. J Health Commun. 2012;17(suppl 1):5-10.
- West DM. How mobile devices are transforming healthcare issues in technology innovation. Issues Technol Innov. 2012;18:1-14.
- Boudreaux ED, Waring ME, Hayes RB, et al. Evaluating and selecting mobile health apps: strategies for healthcare providers and healthcare organizations. Transl Behav Med. 2014;4:363-371.
- Brewer AC, Endly DC, Henley J, et al. Mobile applications in dermatology. JAMA Dermatol. 2013;149:1300-1304.
- Cummings E, Borycki E, Roehrer E. Issues and considerations for healthcare consumers using mobile applications. Stud Health Technol Inform. 2013;183:227-231.
- Handel MJ. mHealth (mobile health)-using apps for health and wellness. Explore. 2011;7:256-261.
- Boulos MN, Brewer AC, Karimkhani C, et al. Mobile medical and health apps: state of the art, concerns, regulatory control and certification. Online J Public Health Inform. 2014;5:229.
Practice Points
- Mobile dermatology apps for educational purposes should be objectively reviewed before being used by patients.
- In our study, only 9 (20.5%) of the 44 dermatology apps evaluated were considered adequate for patient information based on our grading criteria.
VIDEO: Rivaroxaban plus aspirin halves ischemic strokes
LOS ANGELES – Combined treatment with a low dosage of the anticoagulant rivaroxaban plus aspirin cut the incidence of ischemic strokes nearly in half, compared with aspirin alone, in a multicenter, randomized trial of more than 27,000 patients with stable atherosclerotic vascular disease.
This dramatic reduction in ischemic strokes as well as in all-cause strokes by adding low-dose rivaroxaban(Xarelto) occurred without any significant increase in hemorrhagic strokes but with a small increase in total major bleeding events, such as gastrointestinal bleeds, Mike Sharma, MD, said at the International Stroke Conference, sponsored by the American Heart Association.
“There was a consistent effect across all strata of stroke risk. For patients who had a prior stroke, it’s pretty clear to use rivaroxaban plus aspirin because it had a big benefit” with no increase in intracranial hemorrhages, Dr. Sharma said in a video interview.
“We think these results will fundamentally change how we approach stroke prevention,” added Dr. Sharma, a stroke neurologist in the Population Health Research Institute of McMaster University in Hamilton, Ont.
The results he reported came from a secondary analysis of data collected in the COMPASS (Rivaroxaban for the Prevention of Major Cardiovascular Events in Coronary or Peripheral Artery Disease) trial, which enrolled 27,395 patients with stable coronary or peripheral artery disease at 602 centers in 33 countries.
The primary outcome of the trial, reported in 2017, was the combined rate of cardiovascular death, MI, or stroke during an average 23 months of follow-up, which occurred in 4.1% of patients treated with 2.5 mg rivaroxaban twice daily plus 100 mg aspirin once daily, 4.9% of patients who received 5.0 mg rivaroxaban twice daily, and 5.4% in patients who received 100 mg aspirin daily, a statistically significant 24% relative risk reduction in the combined treatment group, compared with aspirin only. The rivaroxaban only–treated patients did not significantly differ from the control patients who received only aspirin (N Engl J Med. 2017 Oct 5;377[14]:1319-30). The main results showed a 1.2% increase in the rate of major bleeds in patients treated with rivaroxaban plus aspirin, compared with aspirin only, but the rate of nonfatal symptomatic intracranial hemorrhages was identical in the two treatment groups.
The new results Dr. Sharma reported at the conference focused on various measures of stroke. The rate of all strokes was 42% lower among the patients treated with rivaroxaban plus aspirin, compared with the aspirin alone patients, and ischemic strokes were 49% lower with the dual therapy, compared with aspirin only. Both differences were statistically significant. In contrast, the rivaroxaban alone regimen did not significantly reduce all-cause strokes. It did significantly reduce ischemic strokes, compared with aspirin only, but it also significantly increased hemorrhagic strokes, compared with aspirin only, an adverse effect not caused by the combination of low-dose rivaroxaban plus aspirin.
Rivaroxaban plus aspirin surpassed aspirin alone for preventing both mild and severe strokes and for preventing strokes both in patients with a history of a prior stroke and in those who never had a prior stroke. The stroke reduction produced by rivaroxaban plus aspirin was greatest in the highest risk patients – those with a prior stroke. On the combined regimen, these patients had an average stroke incidence of 0.7% per year, compared with an annual 3.4% rate among the patients on aspirin only, a 2.7% absolute reduction by using rivaroxaban plus aspirin that translated into a number needed to treat of 37 patients with a history of stroke to prevent one new stroke per year.
The 2017 report of the main COMPASS results included a net clinical benefit analysis that factored together the primary endpoint events and major bleeding events. The net rate of all these events was 4.7% with rivaroxaban plus aspirin and 5.9% with aspirin only, a statistically significant 20% relative risk reduction for all adverse outcomes with dual therapy. This net clinical benefit suggests that adding rivaroxaban has a cost-effective benefit. Assessment of rivaroxaban’s cost benefit in COMPASS is in process, Dr. Sharma said.
Rivaroxaban received Food and Drug Administration marketing approval in 2011 for preventing deep vein thrombosis and preventing stroke in patients with atrial fibrillation at dosages higher than what was used in COMPASS. The approved rivaroxaban dosage for preventing deep vein thrombosis is 10 mg/day, and 20 mg/day for preventing stroke in atrial fibrillation patients. The 2.5-mg formulation of rivaroxaban that was given twice daily had the best safety and efficacy in COMPASS, but it is not available now on the U.S. market, although it is available in Europe. Johnson & Johnson, which markets rivaroxaban globally with Bayer, submitted an application to the FDA in December for marketing approval of the 2.5-mg formulation in twice-daily dosing for use as in the COMPASS trial.
COMPASS was sponsored by Bayer, the company that markets rivaroxaban in collaboration with Johnson & Johnson. Dr. Sharma has been a consultant or adviser to Bayer, Bristol-Myers Squibb, Boehringer Ingelheim, and Daiichi-Sankyo.
SOURCE: Sharma M et al. ISC 2018, Abstract LB7.
LOS ANGELES – Combined treatment with a low dosage of the anticoagulant rivaroxaban plus aspirin cut the incidence of ischemic strokes nearly in half, compared with aspirin alone, in a multicenter, randomized trial of more than 27,000 patients with stable atherosclerotic vascular disease.
This dramatic reduction in ischemic strokes as well as in all-cause strokes by adding low-dose rivaroxaban(Xarelto) occurred without any significant increase in hemorrhagic strokes but with a small increase in total major bleeding events, such as gastrointestinal bleeds, Mike Sharma, MD, said at the International Stroke Conference, sponsored by the American Heart Association.
“There was a consistent effect across all strata of stroke risk. For patients who had a prior stroke, it’s pretty clear to use rivaroxaban plus aspirin because it had a big benefit” with no increase in intracranial hemorrhages, Dr. Sharma said in a video interview.
“We think these results will fundamentally change how we approach stroke prevention,” added Dr. Sharma, a stroke neurologist in the Population Health Research Institute of McMaster University in Hamilton, Ont.
The results he reported came from a secondary analysis of data collected in the COMPASS (Rivaroxaban for the Prevention of Major Cardiovascular Events in Coronary or Peripheral Artery Disease) trial, which enrolled 27,395 patients with stable coronary or peripheral artery disease at 602 centers in 33 countries.
The primary outcome of the trial, reported in 2017, was the combined rate of cardiovascular death, MI, or stroke during an average 23 months of follow-up, which occurred in 4.1% of patients treated with 2.5 mg rivaroxaban twice daily plus 100 mg aspirin once daily, 4.9% of patients who received 5.0 mg rivaroxaban twice daily, and 5.4% in patients who received 100 mg aspirin daily, a statistically significant 24% relative risk reduction in the combined treatment group, compared with aspirin only. The rivaroxaban only–treated patients did not significantly differ from the control patients who received only aspirin (N Engl J Med. 2017 Oct 5;377[14]:1319-30). The main results showed a 1.2% increase in the rate of major bleeds in patients treated with rivaroxaban plus aspirin, compared with aspirin only, but the rate of nonfatal symptomatic intracranial hemorrhages was identical in the two treatment groups.
The new results Dr. Sharma reported at the conference focused on various measures of stroke. The rate of all strokes was 42% lower among the patients treated with rivaroxaban plus aspirin, compared with the aspirin alone patients, and ischemic strokes were 49% lower with the dual therapy, compared with aspirin only. Both differences were statistically significant. In contrast, the rivaroxaban alone regimen did not significantly reduce all-cause strokes. It did significantly reduce ischemic strokes, compared with aspirin only, but it also significantly increased hemorrhagic strokes, compared with aspirin only, an adverse effect not caused by the combination of low-dose rivaroxaban plus aspirin.
Rivaroxaban plus aspirin surpassed aspirin alone for preventing both mild and severe strokes and for preventing strokes both in patients with a history of a prior stroke and in those who never had a prior stroke. The stroke reduction produced by rivaroxaban plus aspirin was greatest in the highest risk patients – those with a prior stroke. On the combined regimen, these patients had an average stroke incidence of 0.7% per year, compared with an annual 3.4% rate among the patients on aspirin only, a 2.7% absolute reduction by using rivaroxaban plus aspirin that translated into a number needed to treat of 37 patients with a history of stroke to prevent one new stroke per year.
The 2017 report of the main COMPASS results included a net clinical benefit analysis that factored together the primary endpoint events and major bleeding events. The net rate of all these events was 4.7% with rivaroxaban plus aspirin and 5.9% with aspirin only, a statistically significant 20% relative risk reduction for all adverse outcomes with dual therapy. This net clinical benefit suggests that adding rivaroxaban has a cost-effective benefit. Assessment of rivaroxaban’s cost benefit in COMPASS is in process, Dr. Sharma said.
Rivaroxaban received Food and Drug Administration marketing approval in 2011 for preventing deep vein thrombosis and preventing stroke in patients with atrial fibrillation at dosages higher than what was used in COMPASS. The approved rivaroxaban dosage for preventing deep vein thrombosis is 10 mg/day, and 20 mg/day for preventing stroke in atrial fibrillation patients. The 2.5-mg formulation of rivaroxaban that was given twice daily had the best safety and efficacy in COMPASS, but it is not available now on the U.S. market, although it is available in Europe. Johnson & Johnson, which markets rivaroxaban globally with Bayer, submitted an application to the FDA in December for marketing approval of the 2.5-mg formulation in twice-daily dosing for use as in the COMPASS trial.
COMPASS was sponsored by Bayer, the company that markets rivaroxaban in collaboration with Johnson & Johnson. Dr. Sharma has been a consultant or adviser to Bayer, Bristol-Myers Squibb, Boehringer Ingelheim, and Daiichi-Sankyo.
SOURCE: Sharma M et al. ISC 2018, Abstract LB7.
LOS ANGELES – Combined treatment with a low dosage of the anticoagulant rivaroxaban plus aspirin cut the incidence of ischemic strokes nearly in half, compared with aspirin alone, in a multicenter, randomized trial of more than 27,000 patients with stable atherosclerotic vascular disease.
This dramatic reduction in ischemic strokes as well as in all-cause strokes by adding low-dose rivaroxaban(Xarelto) occurred without any significant increase in hemorrhagic strokes but with a small increase in total major bleeding events, such as gastrointestinal bleeds, Mike Sharma, MD, said at the International Stroke Conference, sponsored by the American Heart Association.
“There was a consistent effect across all strata of stroke risk. For patients who had a prior stroke, it’s pretty clear to use rivaroxaban plus aspirin because it had a big benefit” with no increase in intracranial hemorrhages, Dr. Sharma said in a video interview.
“We think these results will fundamentally change how we approach stroke prevention,” added Dr. Sharma, a stroke neurologist in the Population Health Research Institute of McMaster University in Hamilton, Ont.
The results he reported came from a secondary analysis of data collected in the COMPASS (Rivaroxaban for the Prevention of Major Cardiovascular Events in Coronary or Peripheral Artery Disease) trial, which enrolled 27,395 patients with stable coronary or peripheral artery disease at 602 centers in 33 countries.
The primary outcome of the trial, reported in 2017, was the combined rate of cardiovascular death, MI, or stroke during an average 23 months of follow-up, which occurred in 4.1% of patients treated with 2.5 mg rivaroxaban twice daily plus 100 mg aspirin once daily, 4.9% of patients who received 5.0 mg rivaroxaban twice daily, and 5.4% in patients who received 100 mg aspirin daily, a statistically significant 24% relative risk reduction in the combined treatment group, compared with aspirin only. The rivaroxaban only–treated patients did not significantly differ from the control patients who received only aspirin (N Engl J Med. 2017 Oct 5;377[14]:1319-30). The main results showed a 1.2% increase in the rate of major bleeds in patients treated with rivaroxaban plus aspirin, compared with aspirin only, but the rate of nonfatal symptomatic intracranial hemorrhages was identical in the two treatment groups.
The new results Dr. Sharma reported at the conference focused on various measures of stroke. The rate of all strokes was 42% lower among the patients treated with rivaroxaban plus aspirin, compared with the aspirin alone patients, and ischemic strokes were 49% lower with the dual therapy, compared with aspirin only. Both differences were statistically significant. In contrast, the rivaroxaban alone regimen did not significantly reduce all-cause strokes. It did significantly reduce ischemic strokes, compared with aspirin only, but it also significantly increased hemorrhagic strokes, compared with aspirin only, an adverse effect not caused by the combination of low-dose rivaroxaban plus aspirin.
Rivaroxaban plus aspirin surpassed aspirin alone for preventing both mild and severe strokes and for preventing strokes both in patients with a history of a prior stroke and in those who never had a prior stroke. The stroke reduction produced by rivaroxaban plus aspirin was greatest in the highest risk patients – those with a prior stroke. On the combined regimen, these patients had an average stroke incidence of 0.7% per year, compared with an annual 3.4% rate among the patients on aspirin only, a 2.7% absolute reduction by using rivaroxaban plus aspirin that translated into a number needed to treat of 37 patients with a history of stroke to prevent one new stroke per year.
The 2017 report of the main COMPASS results included a net clinical benefit analysis that factored together the primary endpoint events and major bleeding events. The net rate of all these events was 4.7% with rivaroxaban plus aspirin and 5.9% with aspirin only, a statistically significant 20% relative risk reduction for all adverse outcomes with dual therapy. This net clinical benefit suggests that adding rivaroxaban has a cost-effective benefit. Assessment of rivaroxaban’s cost benefit in COMPASS is in process, Dr. Sharma said.
Rivaroxaban received Food and Drug Administration marketing approval in 2011 for preventing deep vein thrombosis and preventing stroke in patients with atrial fibrillation at dosages higher than what was used in COMPASS. The approved rivaroxaban dosage for preventing deep vein thrombosis is 10 mg/day, and 20 mg/day for preventing stroke in atrial fibrillation patients. The 2.5-mg formulation of rivaroxaban that was given twice daily had the best safety and efficacy in COMPASS, but it is not available now on the U.S. market, although it is available in Europe. Johnson & Johnson, which markets rivaroxaban globally with Bayer, submitted an application to the FDA in December for marketing approval of the 2.5-mg formulation in twice-daily dosing for use as in the COMPASS trial.
COMPASS was sponsored by Bayer, the company that markets rivaroxaban in collaboration with Johnson & Johnson. Dr. Sharma has been a consultant or adviser to Bayer, Bristol-Myers Squibb, Boehringer Ingelheim, and Daiichi-Sankyo.
SOURCE: Sharma M et al. ISC 2018, Abstract LB7.
REPORTING FROM ISC 2018
Key clinical point: Rivaroxaban plus aspirin cuts strokes in patients with stable atherosclerotic vascular disease.
Major finding: Rivaroxaban plus aspirin cut the rate of ischemic strokes by 49%, compared with aspirin only.
Study details: Secondary analysis from the COMPASS trial, a multicenter, randomized trial with 27,395 patients.
Disclosures: COMPASS was sponsored by Bayer, the company that markets rivaroxaban in collaboration with Johnson & Johnson. Dr. Sharma has been a consultant or adviser to Bayer, Bristol-Myers Squibb, Boehringer Ingelheim, and Daiichi-Sankyo.
Source: Sharma M et al. ISC 2018, Abstract LB7.
US Dermatology Residency Program Rankings Based on Academic Achievement
Rankings of US residency programs based on academic achievement are a resource for fourth-year medical students applying for residency through the National Resident Matching Program. They also highlight the leading academic training programs in each medical specialty. Currently, the Doximity Residency Navigator (https://residency.doximity.com) provides rankings of US residency programs based on either subjective or objective criteria. The subjective rankings utilize current resident and recent alumni satisfaction surveys as well as nominations from board-certified Doximity members who were asked to nominate up to 5 residency programs in their specialty that offer the best clinical training. The objective rankings are based on measurement of research output, which is calculated from the collective h-index of publications authored by graduating alumni within the last 15 years as well as the amount of research funding awarded.1
Aquino et al2 provided a ranking of US dermatology residency programs using alternative objective data measures (as of December 31, 2008) from the Doximity algorithm, including National Institutes of Health (NIH) and Dermatology Foundation (DF) funding, number of publications by full-time faculty members, number of faculty lectures given at annual meetings of 5 societies, and number of full-time faculty members serving on the editorial boards of 6 dermatology journals. The current study is an update to those rankings utilizing data from 2014.
Methods
The following data for each dermatology residency program were obtained to formulate the rankings: number of full-time faculty members, amount of NIH funding received in 2014 (https://report.nih.gov/), number of publications by full-time faculty members in 2014 (http://www.ncbi.nlm.nih.gov/pubmed/), and the number of faculty lectures given at annual meetings of 5 societies in 2014 (American Academy of Dermatology, the Society for Investigative Dermatology, the American Society of Dermatopathology, the Society for Pediatric Dermatology, and the American Society for Dermatologic Surgery). This study was approved by the institutional review board at Kaiser Permanente Southern California.
The names of all US dermatology residency programs were obtained as of December 31, 2014, from FREIDA Online using the search term dermatology. An email was sent to a representative from each residency program (eg, residency program coordinator, program director, full-time faculty member) requesting confirmation of a list of full-time faculty members in the program, excluding part-time and volunteer faculty. If a response was not obtained or the representative declined to participate, a list was compiled using available information from that residency program’s website.
National Institutes of Health funding for 2014 was obtained for individual faculty members from the NIH Research Portfolio Online Reporting Tools expenditures and reports (https://projectreporter.nih.gov/reporter.cfm) by searching the first and last name of each full-time faculty member along with their affiliated institution. The search results were filtered to only include NIH funding for full-time faculty members listed as principal investigators rather than as coinvestigators. The fiscal year total cost by institute/center for each full-time faculty member’s projects was summated to obtain the total NIH funding for the program.
The total number of publications by full-time faculty members in 2014 was obtained utilizing a PubMed search of articles indexed for MEDLINE using each faculty member’s first and last name. The authors’ affiliations were verified for each publication, and the number of publications was summed for all full-time faculty members at each residency program. If multiple authors from the same program coauthored an article, it was only counted once toward the total number of faculty publications from that program.
Program brochures for the 2014 meetings of the 5 societies were reviewed to quantify the number of lectures given by full-time faculty members in each program.
Each residency program was assigned a score from 0 to 1.0 for each of the 4 factors of academic achievement analyzed. The program with the highest number of faculty publications was assigned a score of 1.0 and the program with the lowest number of publications was assigned a score of 0. The programs in between were subsequently assigned scores from 0 to 1.0 based on the number of publications as a percentage of the number of publications from the program with the most publications.
A weighted ranking scheme was used to rank residency programs based on the relative importance of each factor. There were 3 factors that were deemed to be the most reflective of academic achievement among dermatology residency programs: amount of NIH funding received in 2014, number of publications by full-time faculty members in 2014, and number of faculty lectures given at society meetings in 2014; thus, these factors were given a weight of 1.0. The remaining factor— total number of full-time faculty members—was given a weight of 0.5. Values were totaled and programs were ranked based on the sum of these values. All quantitative analyses were performed using an electronic spreadsheet program.
Results
The overall ranking of the top 20 US dermatology residency programs in 2014 is presented in Table 1. The top 5 programs based on each of the 3 factors most reflective of academic achievement used in the weighted ranking algorithm are presented in Tables 2 through 4.

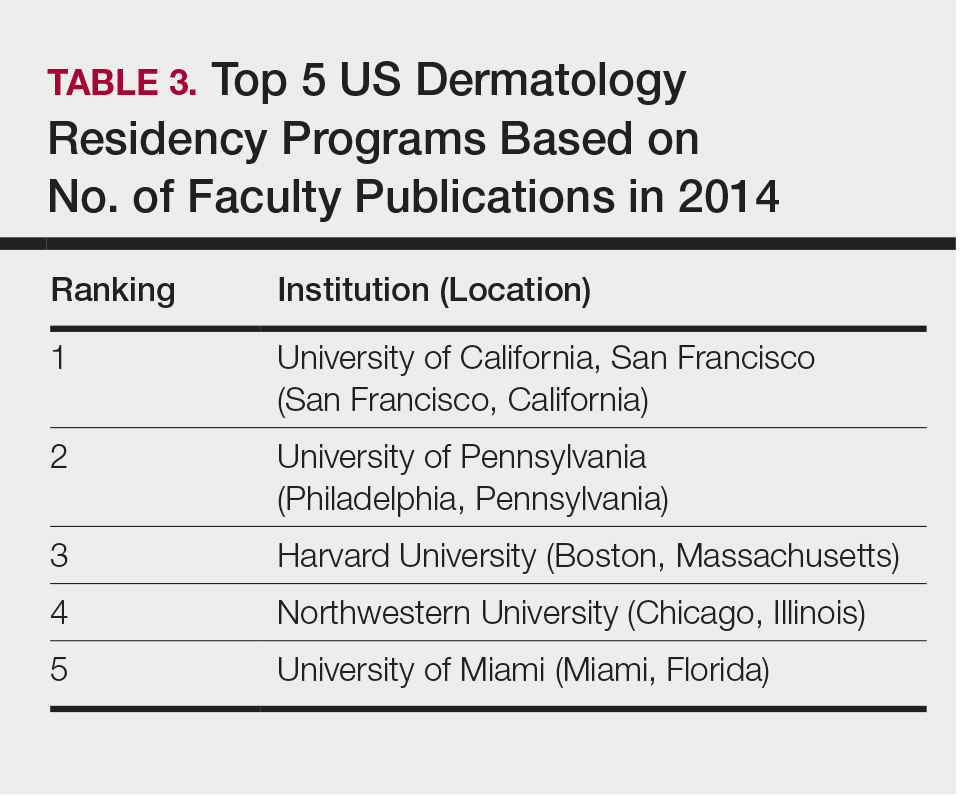
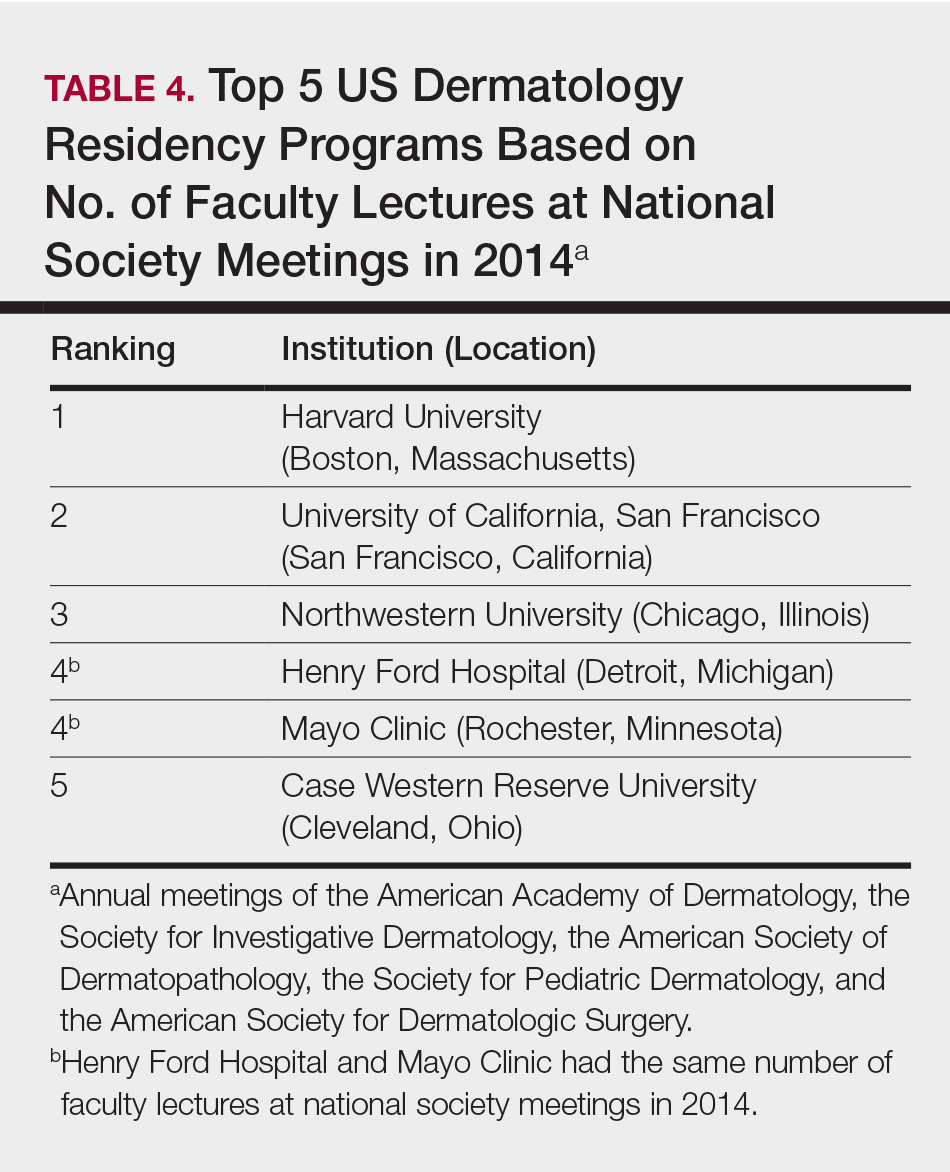
Comment
The ranking of US residency programs involves using data in an unbiased manner while also accounting for important subjective measures. In a 2015 survey of residency applicants (n=6285), the 5 most important factors for applicants in selecting a program were the program’s ability to prepare residents for future training or position, resident esprit de corps, faculty availability and involvement in teaching, depth and breadth of faculty, and variety of patients and clinical resources.3 However, these subjective measures are difficult to quantify in a standardized fashion. In its ranking of residency programs, the Doximity Residency Navigator utilizes surveys of current residents and recent alumni as well as nominations from board-certified Doximity members.1
One of the main issues in utilizing survey data to rank residency programs is the inherent bias that most residents and alumni possess toward their own program. Moreover, the question arises whether most residents, faculty members, or recent alumni of residency programs have sufficient knowledge of other programs to rank them in a well-informed manner.
Wu et al4 used data from 2004 to perform the first algorithmic ranking of US dermatology programs, which was based on publications in 2001 to 2004, the amount of NIH funding in 2004, DF grants in 2001 to 2004, faculty lectures delivered at national conferences in 2004, and number of full-time faculty members on the editorial boards of the top 3 US dermatology journals and the top 4 subspecialty journals. Aquino et al2 provided updated rankings that utilized a weighted algorithm to collect data from 2008 related to a number of factors, including annual amount of NIH and DF funding received, number of publications by full-time faculty members, number of faculty lectures given at 5 annual society meetings, and number of full-time faculty members who were on the editorial boards of 6 dermatology journals with the highest impact factors. The top 5 ranked programs based on the 2008 data were the University of California, San Francisco (San Francisco, California); Northwestern University (Chicago, Illinois); University of Pennsylvania (Philadelphia, Pennsylvania); Yale University (New Haven, Connecticut); and Stanford University (Stanford, California).2
The current ranking algorithm is more indicative of a residency program’s commitment to research and scholarship, with an assumption that successful clinical training is offered. Leading researchers in the field also are usually known to be clinical experts, but the current data does not take into account the frequency, quality, or methodology of teaching provided to residents. Perhaps the most objective measure reflecting the quality of resident education would be American Board of Dermatology examination scores, but these data are not publically available. Additional factors such as the percentage of residents who received fellowship positions; diversity of the patient population; and number and extent of surgical, cosmetic, or laser procedures performed also are not readily available. Doximity provides board pass rates for each residency program, but these data are self-reported and are not taken into account in their rankings.1
The current study aimed to utilize publicly available data to rank US dermatology residency programs based on objective measures of academic achievement. A recent study showed that 531 of 793 applicants (67%) to emergency medicine residency programs were aware of the Doximity residency rankings.One-quarter of these applicants made changes to their rank list based on this data, demonstrating that residency rankings may impact applicant decision-making.5 In the future, the most accurate and unbiased rankings may be performed if each residency program joins a cooperative effort to provide more objective data about the training they provide and utilizes a standardized survey system for current residents and recent graduates to evaluate important subjective measures.
Conclusion
Based on our weighted ranking algorithm, the top 5 dermatology residency programs in 2014 were Harvard University (Boston, Massachusetts); University of California, San Francisco (San Francisco, California); Stanford University (Stanford, California); University of Pennsylvania (Philadelphia, Pennsylvania); and Emory University (Atlanta, Georgia).
Acknowledgments
We thank all of the program coordinators, full-time faculty members, program directors, and chairs who provided responses to our inquiries for additional information about their residency programs.
- Residency navigator 2017-2018. Doximity website. https://residency.doximity.com. Accessed January 19, 2018.
- Aquino LL, Wen G, Wu JJ. US dermatology residency program rankings. Cutis. 2014;94:189-194.
- Phitayakorn R, Macklin EA, Goldsmith J, et al. Applicants’ self-reported priorities in selecting a residency program. J Grad Med Educ. 2015;7:21-26.
- Wu JJ, Ramirez CC, Alonso CA, et al. Ranking the dermatology programs based on measurements of academic achievement. Dermatol Online J. 2007;13:3.
- Peterson WJ, Hopson LR, Khandelwal S. Impact of Doximity residency rankings on emergency medicine applicant rank lists [published online May 5, 2016]. West J Emerg Med. 2016;17:350-354.
Rankings of US residency programs based on academic achievement are a resource for fourth-year medical students applying for residency through the National Resident Matching Program. They also highlight the leading academic training programs in each medical specialty. Currently, the Doximity Residency Navigator (https://residency.doximity.com) provides rankings of US residency programs based on either subjective or objective criteria. The subjective rankings utilize current resident and recent alumni satisfaction surveys as well as nominations from board-certified Doximity members who were asked to nominate up to 5 residency programs in their specialty that offer the best clinical training. The objective rankings are based on measurement of research output, which is calculated from the collective h-index of publications authored by graduating alumni within the last 15 years as well as the amount of research funding awarded.1
Aquino et al2 provided a ranking of US dermatology residency programs using alternative objective data measures (as of December 31, 2008) from the Doximity algorithm, including National Institutes of Health (NIH) and Dermatology Foundation (DF) funding, number of publications by full-time faculty members, number of faculty lectures given at annual meetings of 5 societies, and number of full-time faculty members serving on the editorial boards of 6 dermatology journals. The current study is an update to those rankings utilizing data from 2014.
Methods
The following data for each dermatology residency program were obtained to formulate the rankings: number of full-time faculty members, amount of NIH funding received in 2014 (https://report.nih.gov/), number of publications by full-time faculty members in 2014 (http://www.ncbi.nlm.nih.gov/pubmed/), and the number of faculty lectures given at annual meetings of 5 societies in 2014 (American Academy of Dermatology, the Society for Investigative Dermatology, the American Society of Dermatopathology, the Society for Pediatric Dermatology, and the American Society for Dermatologic Surgery). This study was approved by the institutional review board at Kaiser Permanente Southern California.
The names of all US dermatology residency programs were obtained as of December 31, 2014, from FREIDA Online using the search term dermatology. An email was sent to a representative from each residency program (eg, residency program coordinator, program director, full-time faculty member) requesting confirmation of a list of full-time faculty members in the program, excluding part-time and volunteer faculty. If a response was not obtained or the representative declined to participate, a list was compiled using available information from that residency program’s website.
National Institutes of Health funding for 2014 was obtained for individual faculty members from the NIH Research Portfolio Online Reporting Tools expenditures and reports (https://projectreporter.nih.gov/reporter.cfm) by searching the first and last name of each full-time faculty member along with their affiliated institution. The search results were filtered to only include NIH funding for full-time faculty members listed as principal investigators rather than as coinvestigators. The fiscal year total cost by institute/center for each full-time faculty member’s projects was summated to obtain the total NIH funding for the program.
The total number of publications by full-time faculty members in 2014 was obtained utilizing a PubMed search of articles indexed for MEDLINE using each faculty member’s first and last name. The authors’ affiliations were verified for each publication, and the number of publications was summed for all full-time faculty members at each residency program. If multiple authors from the same program coauthored an article, it was only counted once toward the total number of faculty publications from that program.
Program brochures for the 2014 meetings of the 5 societies were reviewed to quantify the number of lectures given by full-time faculty members in each program.
Each residency program was assigned a score from 0 to 1.0 for each of the 4 factors of academic achievement analyzed. The program with the highest number of faculty publications was assigned a score of 1.0 and the program with the lowest number of publications was assigned a score of 0. The programs in between were subsequently assigned scores from 0 to 1.0 based on the number of publications as a percentage of the number of publications from the program with the most publications.
A weighted ranking scheme was used to rank residency programs based on the relative importance of each factor. There were 3 factors that were deemed to be the most reflective of academic achievement among dermatology residency programs: amount of NIH funding received in 2014, number of publications by full-time faculty members in 2014, and number of faculty lectures given at society meetings in 2014; thus, these factors were given a weight of 1.0. The remaining factor— total number of full-time faculty members—was given a weight of 0.5. Values were totaled and programs were ranked based on the sum of these values. All quantitative analyses were performed using an electronic spreadsheet program.
Results
The overall ranking of the top 20 US dermatology residency programs in 2014 is presented in Table 1. The top 5 programs based on each of the 3 factors most reflective of academic achievement used in the weighted ranking algorithm are presented in Tables 2 through 4.




Comment
The ranking of US residency programs involves using data in an unbiased manner while also accounting for important subjective measures. In a 2015 survey of residency applicants (n=6285), the 5 most important factors for applicants in selecting a program were the program’s ability to prepare residents for future training or position, resident esprit de corps, faculty availability and involvement in teaching, depth and breadth of faculty, and variety of patients and clinical resources.3 However, these subjective measures are difficult to quantify in a standardized fashion. In its ranking of residency programs, the Doximity Residency Navigator utilizes surveys of current residents and recent alumni as well as nominations from board-certified Doximity members.1
One of the main issues in utilizing survey data to rank residency programs is the inherent bias that most residents and alumni possess toward their own program. Moreover, the question arises whether most residents, faculty members, or recent alumni of residency programs have sufficient knowledge of other programs to rank them in a well-informed manner.
Wu et al4 used data from 2004 to perform the first algorithmic ranking of US dermatology programs, which was based on publications in 2001 to 2004, the amount of NIH funding in 2004, DF grants in 2001 to 2004, faculty lectures delivered at national conferences in 2004, and number of full-time faculty members on the editorial boards of the top 3 US dermatology journals and the top 4 subspecialty journals. Aquino et al2 provided updated rankings that utilized a weighted algorithm to collect data from 2008 related to a number of factors, including annual amount of NIH and DF funding received, number of publications by full-time faculty members, number of faculty lectures given at 5 annual society meetings, and number of full-time faculty members who were on the editorial boards of 6 dermatology journals with the highest impact factors. The top 5 ranked programs based on the 2008 data were the University of California, San Francisco (San Francisco, California); Northwestern University (Chicago, Illinois); University of Pennsylvania (Philadelphia, Pennsylvania); Yale University (New Haven, Connecticut); and Stanford University (Stanford, California).2
The current ranking algorithm is more indicative of a residency program’s commitment to research and scholarship, with an assumption that successful clinical training is offered. Leading researchers in the field also are usually known to be clinical experts, but the current data does not take into account the frequency, quality, or methodology of teaching provided to residents. Perhaps the most objective measure reflecting the quality of resident education would be American Board of Dermatology examination scores, but these data are not publically available. Additional factors such as the percentage of residents who received fellowship positions; diversity of the patient population; and number and extent of surgical, cosmetic, or laser procedures performed also are not readily available. Doximity provides board pass rates for each residency program, but these data are self-reported and are not taken into account in their rankings.1
The current study aimed to utilize publicly available data to rank US dermatology residency programs based on objective measures of academic achievement. A recent study showed that 531 of 793 applicants (67%) to emergency medicine residency programs were aware of the Doximity residency rankings.One-quarter of these applicants made changes to their rank list based on this data, demonstrating that residency rankings may impact applicant decision-making.5 In the future, the most accurate and unbiased rankings may be performed if each residency program joins a cooperative effort to provide more objective data about the training they provide and utilizes a standardized survey system for current residents and recent graduates to evaluate important subjective measures.
Conclusion
Based on our weighted ranking algorithm, the top 5 dermatology residency programs in 2014 were Harvard University (Boston, Massachusetts); University of California, San Francisco (San Francisco, California); Stanford University (Stanford, California); University of Pennsylvania (Philadelphia, Pennsylvania); and Emory University (Atlanta, Georgia).
Acknowledgments
We thank all of the program coordinators, full-time faculty members, program directors, and chairs who provided responses to our inquiries for additional information about their residency programs.
Rankings of US residency programs based on academic achievement are a resource for fourth-year medical students applying for residency through the National Resident Matching Program. They also highlight the leading academic training programs in each medical specialty. Currently, the Doximity Residency Navigator (https://residency.doximity.com) provides rankings of US residency programs based on either subjective or objective criteria. The subjective rankings utilize current resident and recent alumni satisfaction surveys as well as nominations from board-certified Doximity members who were asked to nominate up to 5 residency programs in their specialty that offer the best clinical training. The objective rankings are based on measurement of research output, which is calculated from the collective h-index of publications authored by graduating alumni within the last 15 years as well as the amount of research funding awarded.1
Aquino et al2 provided a ranking of US dermatology residency programs using alternative objective data measures (as of December 31, 2008) from the Doximity algorithm, including National Institutes of Health (NIH) and Dermatology Foundation (DF) funding, number of publications by full-time faculty members, number of faculty lectures given at annual meetings of 5 societies, and number of full-time faculty members serving on the editorial boards of 6 dermatology journals. The current study is an update to those rankings utilizing data from 2014.
Methods
The following data for each dermatology residency program were obtained to formulate the rankings: number of full-time faculty members, amount of NIH funding received in 2014 (https://report.nih.gov/), number of publications by full-time faculty members in 2014 (http://www.ncbi.nlm.nih.gov/pubmed/), and the number of faculty lectures given at annual meetings of 5 societies in 2014 (American Academy of Dermatology, the Society for Investigative Dermatology, the American Society of Dermatopathology, the Society for Pediatric Dermatology, and the American Society for Dermatologic Surgery). This study was approved by the institutional review board at Kaiser Permanente Southern California.
The names of all US dermatology residency programs were obtained as of December 31, 2014, from FREIDA Online using the search term dermatology. An email was sent to a representative from each residency program (eg, residency program coordinator, program director, full-time faculty member) requesting confirmation of a list of full-time faculty members in the program, excluding part-time and volunteer faculty. If a response was not obtained or the representative declined to participate, a list was compiled using available information from that residency program’s website.
National Institutes of Health funding for 2014 was obtained for individual faculty members from the NIH Research Portfolio Online Reporting Tools expenditures and reports (https://projectreporter.nih.gov/reporter.cfm) by searching the first and last name of each full-time faculty member along with their affiliated institution. The search results were filtered to only include NIH funding for full-time faculty members listed as principal investigators rather than as coinvestigators. The fiscal year total cost by institute/center for each full-time faculty member’s projects was summated to obtain the total NIH funding for the program.
The total number of publications by full-time faculty members in 2014 was obtained utilizing a PubMed search of articles indexed for MEDLINE using each faculty member’s first and last name. The authors’ affiliations were verified for each publication, and the number of publications was summed for all full-time faculty members at each residency program. If multiple authors from the same program coauthored an article, it was only counted once toward the total number of faculty publications from that program.
Program brochures for the 2014 meetings of the 5 societies were reviewed to quantify the number of lectures given by full-time faculty members in each program.
Each residency program was assigned a score from 0 to 1.0 for each of the 4 factors of academic achievement analyzed. The program with the highest number of faculty publications was assigned a score of 1.0 and the program with the lowest number of publications was assigned a score of 0. The programs in between were subsequently assigned scores from 0 to 1.0 based on the number of publications as a percentage of the number of publications from the program with the most publications.
A weighted ranking scheme was used to rank residency programs based on the relative importance of each factor. There were 3 factors that were deemed to be the most reflective of academic achievement among dermatology residency programs: amount of NIH funding received in 2014, number of publications by full-time faculty members in 2014, and number of faculty lectures given at society meetings in 2014; thus, these factors were given a weight of 1.0. The remaining factor— total number of full-time faculty members—was given a weight of 0.5. Values were totaled and programs were ranked based on the sum of these values. All quantitative analyses were performed using an electronic spreadsheet program.
Results
The overall ranking of the top 20 US dermatology residency programs in 2014 is presented in Table 1. The top 5 programs based on each of the 3 factors most reflective of academic achievement used in the weighted ranking algorithm are presented in Tables 2 through 4.




Comment
The ranking of US residency programs involves using data in an unbiased manner while also accounting for important subjective measures. In a 2015 survey of residency applicants (n=6285), the 5 most important factors for applicants in selecting a program were the program’s ability to prepare residents for future training or position, resident esprit de corps, faculty availability and involvement in teaching, depth and breadth of faculty, and variety of patients and clinical resources.3 However, these subjective measures are difficult to quantify in a standardized fashion. In its ranking of residency programs, the Doximity Residency Navigator utilizes surveys of current residents and recent alumni as well as nominations from board-certified Doximity members.1
One of the main issues in utilizing survey data to rank residency programs is the inherent bias that most residents and alumni possess toward their own program. Moreover, the question arises whether most residents, faculty members, or recent alumni of residency programs have sufficient knowledge of other programs to rank them in a well-informed manner.
Wu et al4 used data from 2004 to perform the first algorithmic ranking of US dermatology programs, which was based on publications in 2001 to 2004, the amount of NIH funding in 2004, DF grants in 2001 to 2004, faculty lectures delivered at national conferences in 2004, and number of full-time faculty members on the editorial boards of the top 3 US dermatology journals and the top 4 subspecialty journals. Aquino et al2 provided updated rankings that utilized a weighted algorithm to collect data from 2008 related to a number of factors, including annual amount of NIH and DF funding received, number of publications by full-time faculty members, number of faculty lectures given at 5 annual society meetings, and number of full-time faculty members who were on the editorial boards of 6 dermatology journals with the highest impact factors. The top 5 ranked programs based on the 2008 data were the University of California, San Francisco (San Francisco, California); Northwestern University (Chicago, Illinois); University of Pennsylvania (Philadelphia, Pennsylvania); Yale University (New Haven, Connecticut); and Stanford University (Stanford, California).2
The current ranking algorithm is more indicative of a residency program’s commitment to research and scholarship, with an assumption that successful clinical training is offered. Leading researchers in the field also are usually known to be clinical experts, but the current data does not take into account the frequency, quality, or methodology of teaching provided to residents. Perhaps the most objective measure reflecting the quality of resident education would be American Board of Dermatology examination scores, but these data are not publically available. Additional factors such as the percentage of residents who received fellowship positions; diversity of the patient population; and number and extent of surgical, cosmetic, or laser procedures performed also are not readily available. Doximity provides board pass rates for each residency program, but these data are self-reported and are not taken into account in their rankings.1
The current study aimed to utilize publicly available data to rank US dermatology residency programs based on objective measures of academic achievement. A recent study showed that 531 of 793 applicants (67%) to emergency medicine residency programs were aware of the Doximity residency rankings.One-quarter of these applicants made changes to their rank list based on this data, demonstrating that residency rankings may impact applicant decision-making.5 In the future, the most accurate and unbiased rankings may be performed if each residency program joins a cooperative effort to provide more objective data about the training they provide and utilizes a standardized survey system for current residents and recent graduates to evaluate important subjective measures.
Conclusion
Based on our weighted ranking algorithm, the top 5 dermatology residency programs in 2014 were Harvard University (Boston, Massachusetts); University of California, San Francisco (San Francisco, California); Stanford University (Stanford, California); University of Pennsylvania (Philadelphia, Pennsylvania); and Emory University (Atlanta, Georgia).
Acknowledgments
We thank all of the program coordinators, full-time faculty members, program directors, and chairs who provided responses to our inquiries for additional information about their residency programs.
- Residency navigator 2017-2018. Doximity website. https://residency.doximity.com. Accessed January 19, 2018.
- Aquino LL, Wen G, Wu JJ. US dermatology residency program rankings. Cutis. 2014;94:189-194.
- Phitayakorn R, Macklin EA, Goldsmith J, et al. Applicants’ self-reported priorities in selecting a residency program. J Grad Med Educ. 2015;7:21-26.
- Wu JJ, Ramirez CC, Alonso CA, et al. Ranking the dermatology programs based on measurements of academic achievement. Dermatol Online J. 2007;13:3.
- Peterson WJ, Hopson LR, Khandelwal S. Impact of Doximity residency rankings on emergency medicine applicant rank lists [published online May 5, 2016]. West J Emerg Med. 2016;17:350-354.
- Residency navigator 2017-2018. Doximity website. https://residency.doximity.com. Accessed January 19, 2018.
- Aquino LL, Wen G, Wu JJ. US dermatology residency program rankings. Cutis. 2014;94:189-194.
- Phitayakorn R, Macklin EA, Goldsmith J, et al. Applicants’ self-reported priorities in selecting a residency program. J Grad Med Educ. 2015;7:21-26.
- Wu JJ, Ramirez CC, Alonso CA, et al. Ranking the dermatology programs based on measurements of academic achievement. Dermatol Online J. 2007;13:3.
- Peterson WJ, Hopson LR, Khandelwal S. Impact of Doximity residency rankings on emergency medicine applicant rank lists [published online May 5, 2016]. West J Emerg Med. 2016;17:350-354.
Practice Points
- Dermatology is not among the many hospital-based adult specialties that are routinely ranked annually by US News & World Report.
- In the current study, US dermatology residency programs were ranked based on various academic factors, including the number of full-time faculty members, amount of National Institutes of Health funding received in 2014, number of publications by full-time faculty members in 2014, and the number of faculty lectures given at annual meetings of 5 societies in 2014.
VIDEO: Dupilumab or cyclosporine for treating atopic dermatitis?
KAUAI, HAWAII – Sometimes, older is better, according to Eric Simpson, MD, professor of dermatology at Oregon Health & Science University, Portland.
Dr. Simpson was a key investigator in trials that were the basis of dupilumab’s approval in 2017 for adults with moderate to severe atopic dermatitis (AD), but there’s still a role for cyclosporine and other old standbys, he said in a video interview at the Hawaii Dermatology Seminar, provided by Global Academy for Medical Education/Skin Disease Education Foundation.
He said he’s asked all the time how to pick a systemic treatment for AD when topicals aren’t doing the trick. In the interview, he explained how dupilumab (Dupixent) fits into the picture, and how to select the right systemic therapy for the right patient. There are not a lot of data yet pointing to one option over the others for first-line treatment; a lot of it comes down to clinical smarts and patient preference.
Dr. Simpson is a consultant and/or investigator for a number of companies, including Eli Lilly, Pfizer, Novartis, and dupilumab manufacturer, Regeneron.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
KAUAI, HAWAII – Sometimes, older is better, according to Eric Simpson, MD, professor of dermatology at Oregon Health & Science University, Portland.
Dr. Simpson was a key investigator in trials that were the basis of dupilumab’s approval in 2017 for adults with moderate to severe atopic dermatitis (AD), but there’s still a role for cyclosporine and other old standbys, he said in a video interview at the Hawaii Dermatology Seminar, provided by Global Academy for Medical Education/Skin Disease Education Foundation.
He said he’s asked all the time how to pick a systemic treatment for AD when topicals aren’t doing the trick. In the interview, he explained how dupilumab (Dupixent) fits into the picture, and how to select the right systemic therapy for the right patient. There are not a lot of data yet pointing to one option over the others for first-line treatment; a lot of it comes down to clinical smarts and patient preference.
Dr. Simpson is a consultant and/or investigator for a number of companies, including Eli Lilly, Pfizer, Novartis, and dupilumab manufacturer, Regeneron.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
KAUAI, HAWAII – Sometimes, older is better, according to Eric Simpson, MD, professor of dermatology at Oregon Health & Science University, Portland.
Dr. Simpson was a key investigator in trials that were the basis of dupilumab’s approval in 2017 for adults with moderate to severe atopic dermatitis (AD), but there’s still a role for cyclosporine and other old standbys, he said in a video interview at the Hawaii Dermatology Seminar, provided by Global Academy for Medical Education/Skin Disease Education Foundation.
He said he’s asked all the time how to pick a systemic treatment for AD when topicals aren’t doing the trick. In the interview, he explained how dupilumab (Dupixent) fits into the picture, and how to select the right systemic therapy for the right patient. There are not a lot of data yet pointing to one option over the others for first-line treatment; a lot of it comes down to clinical smarts and patient preference.
Dr. Simpson is a consultant and/or investigator for a number of companies, including Eli Lilly, Pfizer, Novartis, and dupilumab manufacturer, Regeneron.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
REPORTING FROM SDEF HAWAII DERMATOLOGY SEMINAR
Pain-Minimizing Strategies for Nail Surgery
Nail surgery is an important part of dermatologic training and clinical practice, both for diagnosis and treatment of nail disorders as well as benign and malignant nail tumors. Patient comfort is essential prior to the procedure and while administering local anesthetics. Effective anesthesia facilitates nail unit biopsies, excisions, and other surgical nail procedures. Pain management immediately following the procedure and during the postoperative period are equally important.
Patients who undergo nail surgery may experience anxiety due to fear of a cancer diagnosis, pain during the surgery, or disfigurement from the procedure. This anxiety may lead to increased blood pressure, a decreased pain threshold, and mental and physical discomfort.1 A detailed explanation of the procedure itself as well as expectations following the surgery are helpful in diminishing these fears. Administration of a fast-acting benzodiazepine also may be helpful in these patients to decrease anxiety prior to the procedure.2
Attaining adequate anesthesia requires an understanding of digital anatomy, particularly innervation. Innervation of the digits is supplied by the volar and dorsal nerves, which divide into 3 branches at the distal interphalangeal joint, innervating the nail bed, the digital tip, and the pulp.3 Pacinian and Ruffini corpuscles and free-ended nociceptors activate nerve fibers that transmit pain impulses.4,5 Local anesthetics block pain transmission by impeding voltage-gated sodium channels located at free nerve endings. Pain from anesthesia may be due to both needle insertion and fluid infiltration.
Simple measures can maximize patient comfort during digital anesthesia. Both audiovisual distraction and interpersonal interaction can help to put the patient at ease.6,7 Application of topical anesthetic cream (1–2 hours prior to the procedure under occlusion),8 ice (at least 6 minutes),9 or an ethyl chloride spray can be applied to the nail folds prior to needle insertion to alleviate injection pain, but these methods do little for infiltration pain. Use of an ethyl chloride spray may be the preferred technique due to the rapidity of the analgesic effects (Figure).10 A vibrating massager also can be applied in close proximity to the site of needle insertion.11
Proper anesthetic preparation and technique also can minimize pain during injection. Because lidocaine 1% is acidic (pH, 6.09), buffering with sodium bicarbonate 8.4% can result in decreased injection pain and faster onset of action.6,12 Warming the anesthetic using a water bath, incubator, or autoclave can decrease pain without degradation of lidocaine or epinephrine.13 At a minimum, 30-gauge needles are preferred to minimize pain from needle insertion. Use of 33-gauge needles has shown benefit for injecting the face and scalp and may prove to be helpful injecting sensitive areas such as the digits.14 A slow injection technique is more comfortable for the patient, as rapid injection causes tissue distention.11
The ideal anesthetic for nail surgery would have a fast onset and a long duration of action, which would allow for shorter operation time as well as alleviation of pain postprocedure and some degree of vasoconstriction to help maintain a bloodless field. Lidocaine has the fastest time of onset (<1–3 minutes) but a short duration of action (30–120 minutes) and a vasodilatory effect. Bupivacaine takes 2 to 5 minutes to take effect and has a long duration of action (120–240 minutes) but a risk for cardiotoxicity. Ropivacaine is the preferred anesthetic by some nail surgeons because of its intermediate time of onset (1–15 minutes), long duration of action (120–360 minutes), and the benefit of some vasoconstriction.5,15 The addition of epinephrine has 2 main advantages: vasoconstriction and prolongation of anesthetic effects; the latter may help to alleviate postoperative pain. If there are no contraindications to its use (ie, severe hypertension, Raynaud phenomenon), it can be used safely in digital anesthesia without risk for ischemia or infarction.11
Digital anesthesia can be achieved by infiltration or using nerve blocks. One major difference between these 2 approaches is the time of onset of anesthesia, with the former being nearly instantaneous and the latter taking up to 15 minutes.16 There also usually is more prolonged pain at the site of needle insertion with nerve blocks compared to infiltration. The type of nail surgery being performed, the digit involved, and surgeon preference will determine the anesthetic method of choice.17
Pain management immediately following the procedure and for several days after is essential. Use of a longer-acting anesthetic, such as bupivacaine or ropivacaine, will provide anesthesia for several hours. A well-padded dressing serves to absorb blood and protect the nail and distal digit from trauma, as even minor trauma can exacerbate pain and bleeding. The patient should be instructed to apply ice to the surgical site and keep the ipsilateral extremity elevated for the next 2 days to reduce edema and pain.15 Written instructions are helpful, as anxiety during and after the procedure may limit the patient’s understanding and recollection of the verbal postoperative instructions. To maximize readability of the information, the National Institutes of Health and American Medical Association recommend that the instructions be written at a fourth- to sixth-grade reading level.18,19
A single dose of ibuprofen (400 mg) or acetaminophen (500 mg to 1 g) immediately before or after the procedure can reduce opioid use and postoperative pain.20 Gabapentin (300–1200 mg) given 1 to 2 hours before surgery may be considered in patients who are at high risk for postsurgical pain.21 Acetaminophen or nonsteroidal anti-inflammatory drugs (eg, ibuprofen [200–400 mg]) administered every 4 to 6 hours provides considerable pain reduction postprocedure. Nonsteroidal anti-inflammatory drugs may be superior to acetaminophen for pain control22 and carry a low risk for postoperative bleeding.23 Additionally, a combination of acetaminophen with a nonsteroidal anti-inflammatory drug for 3 doses may be more effective than either drug alone.24 Some patients may require an opioid combination, such as codeine plus acetaminophen, for a short time (up to 3 days) for pain relief following surgery. Excessive pain or pain lasting than more than 3 days is not normal or expected; in these cases, patients should return to the office to rule out ischemia or infection.
It is important to implement pain-minimizing strategies for nail surgeries. Because many of these approaches are derived from other surgical specialties, well-controlled clinical trials in patients undergoing nail surgery will be necessary to improve outcomes.
- Goktay F, Altan ZM, Talas A, et al. Anxiety among patients undergoing nail surgery and skin punch biopsy: effects of age, gender, educational status, and previous experience. J Cutan Med Surg. 2016;20:35-39.
- Ravitskiy L, Phillips PK, Roenigk RK, et al. The use of oral midazolam for perioperative anxiolysis of healthy patients undergoing Mohs surgery: conclusions from randomized controlled and prospective studies. J Am Acad Dermatol. 2011;64:310-322.
- Richert B. Anesthesia of the nail apparatus. In: Richert B, Di Chiacchio N, Haneke E, eds. Nail Surgery. New York, NY: Informa Healthcare; 2010:24-30.
- Egekvist H, Bjerring P, Arendt-Nielsen L. Pain and mechanical injury of human skin following needle insertions. Eur J Pain. 1999;3:41-49.
- Soriano TT, Beynet DP. Anesthesia and analgesia. In: Robinson J, Hanke CW, Siegel D, et al, eds. Surgery of the Skin. 2nd ed. New York, NY: Elsevier; 2010:43-63.
- Strazar AR, Leynes PG, Lalonde DH. Minimizing the pain of local anesthesia injection. Plast Reconstr Surg. 2013;132:675-684.
- Drahota A, Galloway E, Stores R, et al. Audiovisual distraction as an adjunct to pain and anxiety relief during minor surgery. Foot (Edinb). 2008;18:211-219.
- Browne J, Fung M, Donnelly M, et al. The use of EMLA reduces the pain associated with digital ring block for ingrowing toenail correction. Eur J Anaesthesiol. 2000;17:182-184.
- Hayward SC, Landorf KB, Redmond AC. Ice reduces needle-stick pain associated with a digital nerve block of the hallux. Foot. 2006;16:145-148.
- Kose O, Saylan S, Ediz N, et al. Effects of topical alkane vapocoolant spray on pain intensity prior to digital nerve block for ingrown nail surgery. Foot Ankle Spec. 2010;3:73-75.
- Jellinek NJ, Velez NF. Nail surgery: best way to obtain effective anesthesia. Dermatol Clin. 2015;33:265-271.
- Strazar R, Lalonde D. Minimizing injection pain in local anesthesia. CMAJ. 2012;184:2016.
- Hogan ME, vanderVaart S, Perampaladas K, et al. Systematic review and meta-analysis of the effect of warming local anesthetics on injection pain. Ann Emerg Med. 2011;58:86-98.e1.
- Zelickson BR, Goldberg LH, Rubenzik MK, et al. Finer needles reduce pain associated with injection of local anesthetic using a minimal insertion injection technique [published online October 6, 2017]. Dermatol Surg. doi:10.1097/DSS.0000000000001279.
- Haneke E. Nail surgery. Clin Dermatol. 2013;31:516-525.
- Vinycomb TI, Sahhar LJ. Comparison of local anesthetics for digital nerve blocks: a systematic review. J Hand Surg Am. 2014;39:744-51.e5.
- Jellinek NJ. Nail surgery: practical tips and treatment options. Dermatol Ther. 2007;20:68-74.
- How to write easy-to-read health materials. Medline Plus website. https://medlineplus.gov/etr.html. Updated June 28, 2017.
Accessed January 29, 2018. - Weis BD. Health Literacy: A Manual for Clinicians. Chicago, IL: American Medical Foundation, American Medical Association; 2003.
- Rosero EB, Joshi GP. Preemptive, preventive, multimodal analgesia: what do they really mean? Plast Reconstr Surg. 2014;134(4 suppl 2):85S-93S.
- Straube S, Derry S, Moore RA, et al. Single dose oral gabapentin for established acute postoperative pain in adults [published online May 12 2010]. Cochrane Database Syst Rev. doi:10.1002/14651858.CD008183.pub2.
- Bailey E, Worthington H, Coulthard P. Ibuprofen and/or paracetamol (acetaminophen) for pain relief after surgical removal of lower wisdom teeth, a Cochrane systematic review. Br Dent J. 2014;216:451-455.
- Glass JS, Hardy CL, Meeks NM, et al. Acute pain management in dermatology: risk assessment and treatment. J Am Acad Dermatol. 2015;73:543-560; quiz 561-562.
- Sniezek PJ, Brodland DG, Zitelli JA. A randomized controlled trial comparing acetaminophen, acetaminophen and ibuprofen, and acetaminophen and codeine for postoperative pain relief after Mohs surgery and cutaneous reconstruction. Dermatol Surg. 2011;37:1007-1013.
Nail surgery is an important part of dermatologic training and clinical practice, both for diagnosis and treatment of nail disorders as well as benign and malignant nail tumors. Patient comfort is essential prior to the procedure and while administering local anesthetics. Effective anesthesia facilitates nail unit biopsies, excisions, and other surgical nail procedures. Pain management immediately following the procedure and during the postoperative period are equally important.
Patients who undergo nail surgery may experience anxiety due to fear of a cancer diagnosis, pain during the surgery, or disfigurement from the procedure. This anxiety may lead to increased blood pressure, a decreased pain threshold, and mental and physical discomfort.1 A detailed explanation of the procedure itself as well as expectations following the surgery are helpful in diminishing these fears. Administration of a fast-acting benzodiazepine also may be helpful in these patients to decrease anxiety prior to the procedure.2
Attaining adequate anesthesia requires an understanding of digital anatomy, particularly innervation. Innervation of the digits is supplied by the volar and dorsal nerves, which divide into 3 branches at the distal interphalangeal joint, innervating the nail bed, the digital tip, and the pulp.3 Pacinian and Ruffini corpuscles and free-ended nociceptors activate nerve fibers that transmit pain impulses.4,5 Local anesthetics block pain transmission by impeding voltage-gated sodium channels located at free nerve endings. Pain from anesthesia may be due to both needle insertion and fluid infiltration.
Simple measures can maximize patient comfort during digital anesthesia. Both audiovisual distraction and interpersonal interaction can help to put the patient at ease.6,7 Application of topical anesthetic cream (1–2 hours prior to the procedure under occlusion),8 ice (at least 6 minutes),9 or an ethyl chloride spray can be applied to the nail folds prior to needle insertion to alleviate injection pain, but these methods do little for infiltration pain. Use of an ethyl chloride spray may be the preferred technique due to the rapidity of the analgesic effects (Figure).10 A vibrating massager also can be applied in close proximity to the site of needle insertion.11

Proper anesthetic preparation and technique also can minimize pain during injection. Because lidocaine 1% is acidic (pH, 6.09), buffering with sodium bicarbonate 8.4% can result in decreased injection pain and faster onset of action.6,12 Warming the anesthetic using a water bath, incubator, or autoclave can decrease pain without degradation of lidocaine or epinephrine.13 At a minimum, 30-gauge needles are preferred to minimize pain from needle insertion. Use of 33-gauge needles has shown benefit for injecting the face and scalp and may prove to be helpful injecting sensitive areas such as the digits.14 A slow injection technique is more comfortable for the patient, as rapid injection causes tissue distention.11
The ideal anesthetic for nail surgery would have a fast onset and a long duration of action, which would allow for shorter operation time as well as alleviation of pain postprocedure and some degree of vasoconstriction to help maintain a bloodless field. Lidocaine has the fastest time of onset (<1–3 minutes) but a short duration of action (30–120 minutes) and a vasodilatory effect. Bupivacaine takes 2 to 5 minutes to take effect and has a long duration of action (120–240 minutes) but a risk for cardiotoxicity. Ropivacaine is the preferred anesthetic by some nail surgeons because of its intermediate time of onset (1–15 minutes), long duration of action (120–360 minutes), and the benefit of some vasoconstriction.5,15 The addition of epinephrine has 2 main advantages: vasoconstriction and prolongation of anesthetic effects; the latter may help to alleviate postoperative pain. If there are no contraindications to its use (ie, severe hypertension, Raynaud phenomenon), it can be used safely in digital anesthesia without risk for ischemia or infarction.11
Digital anesthesia can be achieved by infiltration or using nerve blocks. One major difference between these 2 approaches is the time of onset of anesthesia, with the former being nearly instantaneous and the latter taking up to 15 minutes.16 There also usually is more prolonged pain at the site of needle insertion with nerve blocks compared to infiltration. The type of nail surgery being performed, the digit involved, and surgeon preference will determine the anesthetic method of choice.17
Pain management immediately following the procedure and for several days after is essential. Use of a longer-acting anesthetic, such as bupivacaine or ropivacaine, will provide anesthesia for several hours. A well-padded dressing serves to absorb blood and protect the nail and distal digit from trauma, as even minor trauma can exacerbate pain and bleeding. The patient should be instructed to apply ice to the surgical site and keep the ipsilateral extremity elevated for the next 2 days to reduce edema and pain.15 Written instructions are helpful, as anxiety during and after the procedure may limit the patient’s understanding and recollection of the verbal postoperative instructions. To maximize readability of the information, the National Institutes of Health and American Medical Association recommend that the instructions be written at a fourth- to sixth-grade reading level.18,19
A single dose of ibuprofen (400 mg) or acetaminophen (500 mg to 1 g) immediately before or after the procedure can reduce opioid use and postoperative pain.20 Gabapentin (300–1200 mg) given 1 to 2 hours before surgery may be considered in patients who are at high risk for postsurgical pain.21 Acetaminophen or nonsteroidal anti-inflammatory drugs (eg, ibuprofen [200–400 mg]) administered every 4 to 6 hours provides considerable pain reduction postprocedure. Nonsteroidal anti-inflammatory drugs may be superior to acetaminophen for pain control22 and carry a low risk for postoperative bleeding.23 Additionally, a combination of acetaminophen with a nonsteroidal anti-inflammatory drug for 3 doses may be more effective than either drug alone.24 Some patients may require an opioid combination, such as codeine plus acetaminophen, for a short time (up to 3 days) for pain relief following surgery. Excessive pain or pain lasting than more than 3 days is not normal or expected; in these cases, patients should return to the office to rule out ischemia or infection.
It is important to implement pain-minimizing strategies for nail surgeries. Because many of these approaches are derived from other surgical specialties, well-controlled clinical trials in patients undergoing nail surgery will be necessary to improve outcomes.
Nail surgery is an important part of dermatologic training and clinical practice, both for diagnosis and treatment of nail disorders as well as benign and malignant nail tumors. Patient comfort is essential prior to the procedure and while administering local anesthetics. Effective anesthesia facilitates nail unit biopsies, excisions, and other surgical nail procedures. Pain management immediately following the procedure and during the postoperative period are equally important.
Patients who undergo nail surgery may experience anxiety due to fear of a cancer diagnosis, pain during the surgery, or disfigurement from the procedure. This anxiety may lead to increased blood pressure, a decreased pain threshold, and mental and physical discomfort.1 A detailed explanation of the procedure itself as well as expectations following the surgery are helpful in diminishing these fears. Administration of a fast-acting benzodiazepine also may be helpful in these patients to decrease anxiety prior to the procedure.2
Attaining adequate anesthesia requires an understanding of digital anatomy, particularly innervation. Innervation of the digits is supplied by the volar and dorsal nerves, which divide into 3 branches at the distal interphalangeal joint, innervating the nail bed, the digital tip, and the pulp.3 Pacinian and Ruffini corpuscles and free-ended nociceptors activate nerve fibers that transmit pain impulses.4,5 Local anesthetics block pain transmission by impeding voltage-gated sodium channels located at free nerve endings. Pain from anesthesia may be due to both needle insertion and fluid infiltration.
Simple measures can maximize patient comfort during digital anesthesia. Both audiovisual distraction and interpersonal interaction can help to put the patient at ease.6,7 Application of topical anesthetic cream (1–2 hours prior to the procedure under occlusion),8 ice (at least 6 minutes),9 or an ethyl chloride spray can be applied to the nail folds prior to needle insertion to alleviate injection pain, but these methods do little for infiltration pain. Use of an ethyl chloride spray may be the preferred technique due to the rapidity of the analgesic effects (Figure).10 A vibrating massager also can be applied in close proximity to the site of needle insertion.11

Proper anesthetic preparation and technique also can minimize pain during injection. Because lidocaine 1% is acidic (pH, 6.09), buffering with sodium bicarbonate 8.4% can result in decreased injection pain and faster onset of action.6,12 Warming the anesthetic using a water bath, incubator, or autoclave can decrease pain without degradation of lidocaine or epinephrine.13 At a minimum, 30-gauge needles are preferred to minimize pain from needle insertion. Use of 33-gauge needles has shown benefit for injecting the face and scalp and may prove to be helpful injecting sensitive areas such as the digits.14 A slow injection technique is more comfortable for the patient, as rapid injection causes tissue distention.11
The ideal anesthetic for nail surgery would have a fast onset and a long duration of action, which would allow for shorter operation time as well as alleviation of pain postprocedure and some degree of vasoconstriction to help maintain a bloodless field. Lidocaine has the fastest time of onset (<1–3 minutes) but a short duration of action (30–120 minutes) and a vasodilatory effect. Bupivacaine takes 2 to 5 minutes to take effect and has a long duration of action (120–240 minutes) but a risk for cardiotoxicity. Ropivacaine is the preferred anesthetic by some nail surgeons because of its intermediate time of onset (1–15 minutes), long duration of action (120–360 minutes), and the benefit of some vasoconstriction.5,15 The addition of epinephrine has 2 main advantages: vasoconstriction and prolongation of anesthetic effects; the latter may help to alleviate postoperative pain. If there are no contraindications to its use (ie, severe hypertension, Raynaud phenomenon), it can be used safely in digital anesthesia without risk for ischemia or infarction.11
Digital anesthesia can be achieved by infiltration or using nerve blocks. One major difference between these 2 approaches is the time of onset of anesthesia, with the former being nearly instantaneous and the latter taking up to 15 minutes.16 There also usually is more prolonged pain at the site of needle insertion with nerve blocks compared to infiltration. The type of nail surgery being performed, the digit involved, and surgeon preference will determine the anesthetic method of choice.17
Pain management immediately following the procedure and for several days after is essential. Use of a longer-acting anesthetic, such as bupivacaine or ropivacaine, will provide anesthesia for several hours. A well-padded dressing serves to absorb blood and protect the nail and distal digit from trauma, as even minor trauma can exacerbate pain and bleeding. The patient should be instructed to apply ice to the surgical site and keep the ipsilateral extremity elevated for the next 2 days to reduce edema and pain.15 Written instructions are helpful, as anxiety during and after the procedure may limit the patient’s understanding and recollection of the verbal postoperative instructions. To maximize readability of the information, the National Institutes of Health and American Medical Association recommend that the instructions be written at a fourth- to sixth-grade reading level.18,19
A single dose of ibuprofen (400 mg) or acetaminophen (500 mg to 1 g) immediately before or after the procedure can reduce opioid use and postoperative pain.20 Gabapentin (300–1200 mg) given 1 to 2 hours before surgery may be considered in patients who are at high risk for postsurgical pain.21 Acetaminophen or nonsteroidal anti-inflammatory drugs (eg, ibuprofen [200–400 mg]) administered every 4 to 6 hours provides considerable pain reduction postprocedure. Nonsteroidal anti-inflammatory drugs may be superior to acetaminophen for pain control22 and carry a low risk for postoperative bleeding.23 Additionally, a combination of acetaminophen with a nonsteroidal anti-inflammatory drug for 3 doses may be more effective than either drug alone.24 Some patients may require an opioid combination, such as codeine plus acetaminophen, for a short time (up to 3 days) for pain relief following surgery. Excessive pain or pain lasting than more than 3 days is not normal or expected; in these cases, patients should return to the office to rule out ischemia or infection.
It is important to implement pain-minimizing strategies for nail surgeries. Because many of these approaches are derived from other surgical specialties, well-controlled clinical trials in patients undergoing nail surgery will be necessary to improve outcomes.
- Goktay F, Altan ZM, Talas A, et al. Anxiety among patients undergoing nail surgery and skin punch biopsy: effects of age, gender, educational status, and previous experience. J Cutan Med Surg. 2016;20:35-39.
- Ravitskiy L, Phillips PK, Roenigk RK, et al. The use of oral midazolam for perioperative anxiolysis of healthy patients undergoing Mohs surgery: conclusions from randomized controlled and prospective studies. J Am Acad Dermatol. 2011;64:310-322.
- Richert B. Anesthesia of the nail apparatus. In: Richert B, Di Chiacchio N, Haneke E, eds. Nail Surgery. New York, NY: Informa Healthcare; 2010:24-30.
- Egekvist H, Bjerring P, Arendt-Nielsen L. Pain and mechanical injury of human skin following needle insertions. Eur J Pain. 1999;3:41-49.
- Soriano TT, Beynet DP. Anesthesia and analgesia. In: Robinson J, Hanke CW, Siegel D, et al, eds. Surgery of the Skin. 2nd ed. New York, NY: Elsevier; 2010:43-63.
- Strazar AR, Leynes PG, Lalonde DH. Minimizing the pain of local anesthesia injection. Plast Reconstr Surg. 2013;132:675-684.
- Drahota A, Galloway E, Stores R, et al. Audiovisual distraction as an adjunct to pain and anxiety relief during minor surgery. Foot (Edinb). 2008;18:211-219.
- Browne J, Fung M, Donnelly M, et al. The use of EMLA reduces the pain associated with digital ring block for ingrowing toenail correction. Eur J Anaesthesiol. 2000;17:182-184.
- Hayward SC, Landorf KB, Redmond AC. Ice reduces needle-stick pain associated with a digital nerve block of the hallux. Foot. 2006;16:145-148.
- Kose O, Saylan S, Ediz N, et al. Effects of topical alkane vapocoolant spray on pain intensity prior to digital nerve block for ingrown nail surgery. Foot Ankle Spec. 2010;3:73-75.
- Jellinek NJ, Velez NF. Nail surgery: best way to obtain effective anesthesia. Dermatol Clin. 2015;33:265-271.
- Strazar R, Lalonde D. Minimizing injection pain in local anesthesia. CMAJ. 2012;184:2016.
- Hogan ME, vanderVaart S, Perampaladas K, et al. Systematic review and meta-analysis of the effect of warming local anesthetics on injection pain. Ann Emerg Med. 2011;58:86-98.e1.
- Zelickson BR, Goldberg LH, Rubenzik MK, et al. Finer needles reduce pain associated with injection of local anesthetic using a minimal insertion injection technique [published online October 6, 2017]. Dermatol Surg. doi:10.1097/DSS.0000000000001279.
- Haneke E. Nail surgery. Clin Dermatol. 2013;31:516-525.
- Vinycomb TI, Sahhar LJ. Comparison of local anesthetics for digital nerve blocks: a systematic review. J Hand Surg Am. 2014;39:744-51.e5.
- Jellinek NJ. Nail surgery: practical tips and treatment options. Dermatol Ther. 2007;20:68-74.
- How to write easy-to-read health materials. Medline Plus website. https://medlineplus.gov/etr.html. Updated June 28, 2017.
Accessed January 29, 2018. - Weis BD. Health Literacy: A Manual for Clinicians. Chicago, IL: American Medical Foundation, American Medical Association; 2003.
- Rosero EB, Joshi GP. Preemptive, preventive, multimodal analgesia: what do they really mean? Plast Reconstr Surg. 2014;134(4 suppl 2):85S-93S.
- Straube S, Derry S, Moore RA, et al. Single dose oral gabapentin for established acute postoperative pain in adults [published online May 12 2010]. Cochrane Database Syst Rev. doi:10.1002/14651858.CD008183.pub2.
- Bailey E, Worthington H, Coulthard P. Ibuprofen and/or paracetamol (acetaminophen) for pain relief after surgical removal of lower wisdom teeth, a Cochrane systematic review. Br Dent J. 2014;216:451-455.
- Glass JS, Hardy CL, Meeks NM, et al. Acute pain management in dermatology: risk assessment and treatment. J Am Acad Dermatol. 2015;73:543-560; quiz 561-562.
- Sniezek PJ, Brodland DG, Zitelli JA. A randomized controlled trial comparing acetaminophen, acetaminophen and ibuprofen, and acetaminophen and codeine for postoperative pain relief after Mohs surgery and cutaneous reconstruction. Dermatol Surg. 2011;37:1007-1013.
- Goktay F, Altan ZM, Talas A, et al. Anxiety among patients undergoing nail surgery and skin punch biopsy: effects of age, gender, educational status, and previous experience. J Cutan Med Surg. 2016;20:35-39.
- Ravitskiy L, Phillips PK, Roenigk RK, et al. The use of oral midazolam for perioperative anxiolysis of healthy patients undergoing Mohs surgery: conclusions from randomized controlled and prospective studies. J Am Acad Dermatol. 2011;64:310-322.
- Richert B. Anesthesia of the nail apparatus. In: Richert B, Di Chiacchio N, Haneke E, eds. Nail Surgery. New York, NY: Informa Healthcare; 2010:24-30.
- Egekvist H, Bjerring P, Arendt-Nielsen L. Pain and mechanical injury of human skin following needle insertions. Eur J Pain. 1999;3:41-49.
- Soriano TT, Beynet DP. Anesthesia and analgesia. In: Robinson J, Hanke CW, Siegel D, et al, eds. Surgery of the Skin. 2nd ed. New York, NY: Elsevier; 2010:43-63.
- Strazar AR, Leynes PG, Lalonde DH. Minimizing the pain of local anesthesia injection. Plast Reconstr Surg. 2013;132:675-684.
- Drahota A, Galloway E, Stores R, et al. Audiovisual distraction as an adjunct to pain and anxiety relief during minor surgery. Foot (Edinb). 2008;18:211-219.
- Browne J, Fung M, Donnelly M, et al. The use of EMLA reduces the pain associated with digital ring block for ingrowing toenail correction. Eur J Anaesthesiol. 2000;17:182-184.
- Hayward SC, Landorf KB, Redmond AC. Ice reduces needle-stick pain associated with a digital nerve block of the hallux. Foot. 2006;16:145-148.
- Kose O, Saylan S, Ediz N, et al. Effects of topical alkane vapocoolant spray on pain intensity prior to digital nerve block for ingrown nail surgery. Foot Ankle Spec. 2010;3:73-75.
- Jellinek NJ, Velez NF. Nail surgery: best way to obtain effective anesthesia. Dermatol Clin. 2015;33:265-271.
- Strazar R, Lalonde D. Minimizing injection pain in local anesthesia. CMAJ. 2012;184:2016.
- Hogan ME, vanderVaart S, Perampaladas K, et al. Systematic review and meta-analysis of the effect of warming local anesthetics on injection pain. Ann Emerg Med. 2011;58:86-98.e1.
- Zelickson BR, Goldberg LH, Rubenzik MK, et al. Finer needles reduce pain associated with injection of local anesthetic using a minimal insertion injection technique [published online October 6, 2017]. Dermatol Surg. doi:10.1097/DSS.0000000000001279.
- Haneke E. Nail surgery. Clin Dermatol. 2013;31:516-525.
- Vinycomb TI, Sahhar LJ. Comparison of local anesthetics for digital nerve blocks: a systematic review. J Hand Surg Am. 2014;39:744-51.e5.
- Jellinek NJ. Nail surgery: practical tips and treatment options. Dermatol Ther. 2007;20:68-74.
- How to write easy-to-read health materials. Medline Plus website. https://medlineplus.gov/etr.html. Updated June 28, 2017.
Accessed January 29, 2018. - Weis BD. Health Literacy: A Manual for Clinicians. Chicago, IL: American Medical Foundation, American Medical Association; 2003.
- Rosero EB, Joshi GP. Preemptive, preventive, multimodal analgesia: what do they really mean? Plast Reconstr Surg. 2014;134(4 suppl 2):85S-93S.
- Straube S, Derry S, Moore RA, et al. Single dose oral gabapentin for established acute postoperative pain in adults [published online May 12 2010]. Cochrane Database Syst Rev. doi:10.1002/14651858.CD008183.pub2.
- Bailey E, Worthington H, Coulthard P. Ibuprofen and/or paracetamol (acetaminophen) for pain relief after surgical removal of lower wisdom teeth, a Cochrane systematic review. Br Dent J. 2014;216:451-455.
- Glass JS, Hardy CL, Meeks NM, et al. Acute pain management in dermatology: risk assessment and treatment. J Am Acad Dermatol. 2015;73:543-560; quiz 561-562.
- Sniezek PJ, Brodland DG, Zitelli JA. A randomized controlled trial comparing acetaminophen, acetaminophen and ibuprofen, and acetaminophen and codeine for postoperative pain relief after Mohs surgery and cutaneous reconstruction. Dermatol Surg. 2011;37:1007-1013.
MDedge Daily News: Flu set to break hospital records
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Hospitals race toward a new flu record, the drug pipeline is filling up for inflammatory bowel disease, there’s bad news for cardiovascular prevention in type 2 diabetes, and research points to elective induction at 39 weeks.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Hospitals race toward a new flu record, the drug pipeline is filling up for inflammatory bowel disease, there’s bad news for cardiovascular prevention in type 2 diabetes, and research points to elective induction at 39 weeks.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Hospitals race toward a new flu record, the drug pipeline is filling up for inflammatory bowel disease, there’s bad news for cardiovascular prevention in type 2 diabetes, and research points to elective induction at 39 weeks.
Listen to the MDedge Daily News podcast for all the details on today’s top news.