User login
ACS Clinical Congress Report 2017
The American College of Surgeons (ACS) Clinical Congress is designed to offer a broad range of substantive opportunities for surgeons to interact with colleagues, participate in discussions, and learn about the latest innovations in our profession. The 2017 ACS Clinical Congress met all of these expectations.
The ACS Clinical Congress Report is a collection of news articles and videos from the meeting. This sampling of reportage by ACS Surgery News is meant to convey the essence of the meeting: new ideas, intense debate, and a profound commitment to professional development. We are especially proud to highlight the many substantive presentations by surgical trainees.
We hope this collection of articles will serve as a reminder of what the Clinical Congress is all about. The 2018 ACS Clinical Congress will be held in Boston, Oct. 21-25. Surgeons will be offered another great opportunity hear important presentations and updates, meet with colleagues, and witness firsthand the breaking news and unveiling of discoveries in our field. We hope you are inspired to attend.
Click on the PDF Download link above to read the report!
Karen E. Deveney, MD, FACS
Tyler G. Hughes, MD, FACS
Co-Editors, ACS Surgery News
Therese Borden
Managing Editor, ACS Surgery News
The American College of Surgeons (ACS) Clinical Congress is designed to offer a broad range of substantive opportunities for surgeons to interact with colleagues, participate in discussions, and learn about the latest innovations in our profession. The 2017 ACS Clinical Congress met all of these expectations.
The ACS Clinical Congress Report is a collection of news articles and videos from the meeting. This sampling of reportage by ACS Surgery News is meant to convey the essence of the meeting: new ideas, intense debate, and a profound commitment to professional development. We are especially proud to highlight the many substantive presentations by surgical trainees.
We hope this collection of articles will serve as a reminder of what the Clinical Congress is all about. The 2018 ACS Clinical Congress will be held in Boston, Oct. 21-25. Surgeons will be offered another great opportunity hear important presentations and updates, meet with colleagues, and witness firsthand the breaking news and unveiling of discoveries in our field. We hope you are inspired to attend.
Click on the PDF Download link above to read the report!
Karen E. Deveney, MD, FACS
Tyler G. Hughes, MD, FACS
Co-Editors, ACS Surgery News
Therese Borden
Managing Editor, ACS Surgery News
The American College of Surgeons (ACS) Clinical Congress is designed to offer a broad range of substantive opportunities for surgeons to interact with colleagues, participate in discussions, and learn about the latest innovations in our profession. The 2017 ACS Clinical Congress met all of these expectations.
The ACS Clinical Congress Report is a collection of news articles and videos from the meeting. This sampling of reportage by ACS Surgery News is meant to convey the essence of the meeting: new ideas, intense debate, and a profound commitment to professional development. We are especially proud to highlight the many substantive presentations by surgical trainees.
We hope this collection of articles will serve as a reminder of what the Clinical Congress is all about. The 2018 ACS Clinical Congress will be held in Boston, Oct. 21-25. Surgeons will be offered another great opportunity hear important presentations and updates, meet with colleagues, and witness firsthand the breaking news and unveiling of discoveries in our field. We hope you are inspired to attend.
Click on the PDF Download link above to read the report!
Karen E. Deveney, MD, FACS
Tyler G. Hughes, MD, FACS
Co-Editors, ACS Surgery News
Therese Borden
Managing Editor, ACS Surgery News
How Do Cognitively Normal Adults Understand Elevated Brain Amyloid Results?
Most cognitively normal adults with elevated brain amyloid in an Alzheimer’s disease prevention trial “understood that elevated amyloid conferred an increased but uncertain risk of developing Alzheimer’s disease,” according to research published in the January issue of JAMA Neurology. Some participants, however, “desired clarification of the term ‘elevated’ beyond its being a categorical result enabling trial entry eligibility.”
“Clinicians should be prepared to explain how and why a dimensional biomarker, in this case amyloid-β as measured using PET, is converted to a categorical state … and what the result means in terms of a person’s risk for developing Alzheimer disease dementia,” the researchers said.
Trials in Asymptomatic Populations
Advances in understanding the pathophysiology of Alzheimer’s disease have led to clinical trials in cognitively normal adults with evidence of Alzheimer’s disease biomarkers, but data are limited regarding how cognitively normal adults, comprehend biomarker results. A person’s knowledge of his or her amyloid status “may generate clinical and ethical problems, including the potential for misunderstanding, discrimination, stigma, depression, anxiety, and, in the most extreme cases, suicide in the face of a debilitating disease with no treatment,” the researchers said. “There is no consensus … about whether and how to return Alzheimer’s disease biomarker results to cognitively normal adults, given the prognostic uncertainty and absence of available treatments.”
To determine participants’ comprehension of an elevated amyloid PET biomarker result, Jessica Mozersky, PhD, of the Department of Medical Ethics and Health Policy at Perelman School of Medicine, University of Pennsylvania in Philadelphia, and colleagues conducted the Study of Knowledge and Reactions to Amyloid Testing (SOKRATES).
The researchers conducted two semistructured telephone interviews with 80 participants from nine study sites. Fifty of the participants had elevated amyloid PET scan results and 30 had not-elevated amyloid PET scan results. Researchers conducted the interviews about four to 12 weeks after result disclosure and one year later. The present study examined the first interviews of 50 participants with elevated amyloid results.
Of the 50 participants, 49 (98%) were white, 40 (80%) had a family history of Alzheimer’s disease, and 30 (60%) had a postgraduate educational level. Patients were between ages 65 and 85, and half were men. Data were collected between November 5, 2014, and December 10, 2015.
The A4 Study and SOKRATES
SOKRATES is a substudy of the Anti-Amyloid Treatment in Asymptomatic Alzheimer (A4) study, which is testing whether solanezumab, compared with placebo, affects the rate of cognitive decline in cognitively normal adults with elevated amyloid.
Clinicians informed all A4 study participants of their amyloid PET scan result through a disclosure process that was designed to maximize safety and effectiveness. The process included a depression and anxiety prescreen, an educational session, a teach-back exercise to check comprehension, an in-person disclosure by a trained clinician, and a telephone follow-up to assess mood. In addition, a study guide provided information about the amyloid PET scan, its purpose, possible results, and limitations. “The study guide describes ‘preclinical’ or ‘asymptomatic’ Alzheimer’s disease as a new concept in development and explains that elevated amyloid ‘does not necessarily mean you will develop Alzheimer’s disease-related memory loss’ but can be associated with an increased risk, and individual risk estimates are not possible. It further explains that ‘not elevated’ does not mean you will never develop Alzheimer’s disease or ‘elevated amyloid’ in the future,” the researchers said.
A4 trial sites provided participants with materials describing the SOKRATES substudy, and interested participants contacted the SOKRATES researchers at the University of Pennsylvania.
The researchers examined comprehension of an elevated amyloid PET scan result by analyzing participants’ responses to the following questions: “What was the result of your amyloid PET scan?” (followed by “Can you tell me in your own words what that means?” or “How would you explain it to a friend?”), “Was it the result you expected?” and “Did the result teach you anything or clarify anything for you?”
Understanding Risk
When asked, “What was your result,” 64% used the word “amyloid” or “amyloids,” and the remaining participants primarily referred to the presence of “plaques” or to the result being “positive.” Two answers suggested misunderstanding. Six participants used the word “elevated” to describe their amyloid result, while most participants used other words (eg, “increased,” “higher levels,” or “excessive”). Nineteen participants described the result in terms of qualifying for the A4 study (eg, “enough amyloid buildup to qualify for the study”).
More than half of the participants (54%) expected their elevated result due to a family history of Alzheimer’s disease or subjective memory problems. Fifteen participants (30%) were unsure what result they had expected or were prepared for either result. Eight participants (16%) expected not to have elevated amyloid because they had led a healthy lifestyle, had no family history of Alzheimer’s disease, and had no subjective memory concerns.
When asked about the meaning of the result, 94% responded with their understanding of the risk of developing Alzheimer’s disease conferred by elevated amyloid. Thirty-one participants (62%) interpreted the result as signaling an increased risk of developing Alzheimer’s disease, while 10 participants (20%) perceived the risk conferred by elevated amyloid to be equivocal. Six participants (12%) perceived elevated amyloid to mean that Alzheimer’s disease was imminent or that it was diagnostic of Alzheimer’s disease.
Twenty participants (40%) were dissatisfied “with the lack of specificity regarding the meaning of ‘elevated,’” the researchers said. Some wanted “a granular result describing the degree of amyloid elevation … and how close they were to the threshold for study entry,” and some participants expressed frustration at the lack of detail.
Implications
The results suggest that some people who are cognitively normal but have subjective memory concerns “will use an Alzheimer’s disease biomarker test to explain their memory concerns, potentially pathologizing normal and nondisease-related cognitive aging,” the researchers said. In addition, people without a family history of Alzheimer’s disease or subjective memory concerns “may be unprepared to receive their biomarker results.”
A desire for more specific and detailed information about the results may be especially relevant to cognitively normal adults. “Such specific information is likely less relevant for symptomatic individuals who receive a binary result that either confirms or rules out a diagnosis that explains their history of cognitive decline,” the researchers said.
Because most participants were highly educated and had a family history of Alzheimer’s disease, the applicability of the results to other populations is limited. In future studies, the researchers plan to assess how elevated versus not-elevated brain amyloid results influenced SOKRATES participants’ sense of self, social relationships, and behaviors.
Potential Interventions and Ethical Concerns
The long prodromal period of Alzheimer’s disease “represents our greatest hope for effective therapeutic intervention as well as a domain of serious ethical concern,” said Winston Chiong, MD, PhD, of the Memory and Aging Center, Weill Institute for Neurosciences, University of California, San Francisco, in an accompanying editorial. “Prevailing appropriate use criteria advise against clinical use of amyloid imaging in asymptomatic individuals, citing ‘a significant potential for patients and families to make inaccurate assumptions about risk and future outcomes on the basis of amyloid PET results.’” The A4 trial, however, “not only requires that amyloid imaging be performed but also effectively requires that the results of such imaging be disclosed to participants.”
Qualitative research may elicit unanticipated beliefs or concerns, and in SOKRATES, “a sizable proportion of participants … expressed dissatisfaction with the categorical characterization of results.”
“Overall, the findings of Mozersky and colleagues are broadly reassuring regarding research participants’ ability to understand the prognostic uncertainty of amyloid imaging. But as the authors note, caution is needed in generalizing their results,” Dr. Chiong said. “These participants represent a selected subpopulation of an already rarified group: prospective participants in the A4 study were provided with study materials for this substudy, and interested participants themselves contacted the study investigators. These participants were thus likely to be particularly supportive of the Alzheimer’s disease research enterprise, and given their family histories and high educational attainment
Suggested Reading
Chiong W. Challenges in communicating and understanding predictive biomarker imaging for Alzheimer disease. JAMA Neurol. 2018;75(1):18-19.
Mozersky J, Sankar P, Harkins K, et al. Comprehension of an elevated amyloid positron emission tomography biomarker result by cognitively normal older adults. JAMA Neurol. 2018;75(1):44-50.
Most cognitively normal adults with elevated brain amyloid in an Alzheimer’s disease prevention trial “understood that elevated amyloid conferred an increased but uncertain risk of developing Alzheimer’s disease,” according to research published in the January issue of JAMA Neurology. Some participants, however, “desired clarification of the term ‘elevated’ beyond its being a categorical result enabling trial entry eligibility.”
“Clinicians should be prepared to explain how and why a dimensional biomarker, in this case amyloid-β as measured using PET, is converted to a categorical state … and what the result means in terms of a person’s risk for developing Alzheimer disease dementia,” the researchers said.
Trials in Asymptomatic Populations
Advances in understanding the pathophysiology of Alzheimer’s disease have led to clinical trials in cognitively normal adults with evidence of Alzheimer’s disease biomarkers, but data are limited regarding how cognitively normal adults, comprehend biomarker results. A person’s knowledge of his or her amyloid status “may generate clinical and ethical problems, including the potential for misunderstanding, discrimination, stigma, depression, anxiety, and, in the most extreme cases, suicide in the face of a debilitating disease with no treatment,” the researchers said. “There is no consensus … about whether and how to return Alzheimer’s disease biomarker results to cognitively normal adults, given the prognostic uncertainty and absence of available treatments.”
To determine participants’ comprehension of an elevated amyloid PET biomarker result, Jessica Mozersky, PhD, of the Department of Medical Ethics and Health Policy at Perelman School of Medicine, University of Pennsylvania in Philadelphia, and colleagues conducted the Study of Knowledge and Reactions to Amyloid Testing (SOKRATES).
The researchers conducted two semistructured telephone interviews with 80 participants from nine study sites. Fifty of the participants had elevated amyloid PET scan results and 30 had not-elevated amyloid PET scan results. Researchers conducted the interviews about four to 12 weeks after result disclosure and one year later. The present study examined the first interviews of 50 participants with elevated amyloid results.
Of the 50 participants, 49 (98%) were white, 40 (80%) had a family history of Alzheimer’s disease, and 30 (60%) had a postgraduate educational level. Patients were between ages 65 and 85, and half were men. Data were collected between November 5, 2014, and December 10, 2015.
The A4 Study and SOKRATES
SOKRATES is a substudy of the Anti-Amyloid Treatment in Asymptomatic Alzheimer (A4) study, which is testing whether solanezumab, compared with placebo, affects the rate of cognitive decline in cognitively normal adults with elevated amyloid.
Clinicians informed all A4 study participants of their amyloid PET scan result through a disclosure process that was designed to maximize safety and effectiveness. The process included a depression and anxiety prescreen, an educational session, a teach-back exercise to check comprehension, an in-person disclosure by a trained clinician, and a telephone follow-up to assess mood. In addition, a study guide provided information about the amyloid PET scan, its purpose, possible results, and limitations. “The study guide describes ‘preclinical’ or ‘asymptomatic’ Alzheimer’s disease as a new concept in development and explains that elevated amyloid ‘does not necessarily mean you will develop Alzheimer’s disease-related memory loss’ but can be associated with an increased risk, and individual risk estimates are not possible. It further explains that ‘not elevated’ does not mean you will never develop Alzheimer’s disease or ‘elevated amyloid’ in the future,” the researchers said.
A4 trial sites provided participants with materials describing the SOKRATES substudy, and interested participants contacted the SOKRATES researchers at the University of Pennsylvania.
The researchers examined comprehension of an elevated amyloid PET scan result by analyzing participants’ responses to the following questions: “What was the result of your amyloid PET scan?” (followed by “Can you tell me in your own words what that means?” or “How would you explain it to a friend?”), “Was it the result you expected?” and “Did the result teach you anything or clarify anything for you?”
Understanding Risk
When asked, “What was your result,” 64% used the word “amyloid” or “amyloids,” and the remaining participants primarily referred to the presence of “plaques” or to the result being “positive.” Two answers suggested misunderstanding. Six participants used the word “elevated” to describe their amyloid result, while most participants used other words (eg, “increased,” “higher levels,” or “excessive”). Nineteen participants described the result in terms of qualifying for the A4 study (eg, “enough amyloid buildup to qualify for the study”).
More than half of the participants (54%) expected their elevated result due to a family history of Alzheimer’s disease or subjective memory problems. Fifteen participants (30%) were unsure what result they had expected or were prepared for either result. Eight participants (16%) expected not to have elevated amyloid because they had led a healthy lifestyle, had no family history of Alzheimer’s disease, and had no subjective memory concerns.
When asked about the meaning of the result, 94% responded with their understanding of the risk of developing Alzheimer’s disease conferred by elevated amyloid. Thirty-one participants (62%) interpreted the result as signaling an increased risk of developing Alzheimer’s disease, while 10 participants (20%) perceived the risk conferred by elevated amyloid to be equivocal. Six participants (12%) perceived elevated amyloid to mean that Alzheimer’s disease was imminent or that it was diagnostic of Alzheimer’s disease.
Twenty participants (40%) were dissatisfied “with the lack of specificity regarding the meaning of ‘elevated,’” the researchers said. Some wanted “a granular result describing the degree of amyloid elevation … and how close they were to the threshold for study entry,” and some participants expressed frustration at the lack of detail.
Implications
The results suggest that some people who are cognitively normal but have subjective memory concerns “will use an Alzheimer’s disease biomarker test to explain their memory concerns, potentially pathologizing normal and nondisease-related cognitive aging,” the researchers said. In addition, people without a family history of Alzheimer’s disease or subjective memory concerns “may be unprepared to receive their biomarker results.”
A desire for more specific and detailed information about the results may be especially relevant to cognitively normal adults. “Such specific information is likely less relevant for symptomatic individuals who receive a binary result that either confirms or rules out a diagnosis that explains their history of cognitive decline,” the researchers said.
Because most participants were highly educated and had a family history of Alzheimer’s disease, the applicability of the results to other populations is limited. In future studies, the researchers plan to assess how elevated versus not-elevated brain amyloid results influenced SOKRATES participants’ sense of self, social relationships, and behaviors.
Potential Interventions and Ethical Concerns
The long prodromal period of Alzheimer’s disease “represents our greatest hope for effective therapeutic intervention as well as a domain of serious ethical concern,” said Winston Chiong, MD, PhD, of the Memory and Aging Center, Weill Institute for Neurosciences, University of California, San Francisco, in an accompanying editorial. “Prevailing appropriate use criteria advise against clinical use of amyloid imaging in asymptomatic individuals, citing ‘a significant potential for patients and families to make inaccurate assumptions about risk and future outcomes on the basis of amyloid PET results.’” The A4 trial, however, “not only requires that amyloid imaging be performed but also effectively requires that the results of such imaging be disclosed to participants.”
Qualitative research may elicit unanticipated beliefs or concerns, and in SOKRATES, “a sizable proportion of participants … expressed dissatisfaction with the categorical characterization of results.”
“Overall, the findings of Mozersky and colleagues are broadly reassuring regarding research participants’ ability to understand the prognostic uncertainty of amyloid imaging. But as the authors note, caution is needed in generalizing their results,” Dr. Chiong said. “These participants represent a selected subpopulation of an already rarified group: prospective participants in the A4 study were provided with study materials for this substudy, and interested participants themselves contacted the study investigators. These participants were thus likely to be particularly supportive of the Alzheimer’s disease research enterprise, and given their family histories and high educational attainment
Suggested Reading
Chiong W. Challenges in communicating and understanding predictive biomarker imaging for Alzheimer disease. JAMA Neurol. 2018;75(1):18-19.
Mozersky J, Sankar P, Harkins K, et al. Comprehension of an elevated amyloid positron emission tomography biomarker result by cognitively normal older adults. JAMA Neurol. 2018;75(1):44-50.
Most cognitively normal adults with elevated brain amyloid in an Alzheimer’s disease prevention trial “understood that elevated amyloid conferred an increased but uncertain risk of developing Alzheimer’s disease,” according to research published in the January issue of JAMA Neurology. Some participants, however, “desired clarification of the term ‘elevated’ beyond its being a categorical result enabling trial entry eligibility.”
“Clinicians should be prepared to explain how and why a dimensional biomarker, in this case amyloid-β as measured using PET, is converted to a categorical state … and what the result means in terms of a person’s risk for developing Alzheimer disease dementia,” the researchers said.
Trials in Asymptomatic Populations
Advances in understanding the pathophysiology of Alzheimer’s disease have led to clinical trials in cognitively normal adults with evidence of Alzheimer’s disease biomarkers, but data are limited regarding how cognitively normal adults, comprehend biomarker results. A person’s knowledge of his or her amyloid status “may generate clinical and ethical problems, including the potential for misunderstanding, discrimination, stigma, depression, anxiety, and, in the most extreme cases, suicide in the face of a debilitating disease with no treatment,” the researchers said. “There is no consensus … about whether and how to return Alzheimer’s disease biomarker results to cognitively normal adults, given the prognostic uncertainty and absence of available treatments.”
To determine participants’ comprehension of an elevated amyloid PET biomarker result, Jessica Mozersky, PhD, of the Department of Medical Ethics and Health Policy at Perelman School of Medicine, University of Pennsylvania in Philadelphia, and colleagues conducted the Study of Knowledge and Reactions to Amyloid Testing (SOKRATES).
The researchers conducted two semistructured telephone interviews with 80 participants from nine study sites. Fifty of the participants had elevated amyloid PET scan results and 30 had not-elevated amyloid PET scan results. Researchers conducted the interviews about four to 12 weeks after result disclosure and one year later. The present study examined the first interviews of 50 participants with elevated amyloid results.
Of the 50 participants, 49 (98%) were white, 40 (80%) had a family history of Alzheimer’s disease, and 30 (60%) had a postgraduate educational level. Patients were between ages 65 and 85, and half were men. Data were collected between November 5, 2014, and December 10, 2015.
The A4 Study and SOKRATES
SOKRATES is a substudy of the Anti-Amyloid Treatment in Asymptomatic Alzheimer (A4) study, which is testing whether solanezumab, compared with placebo, affects the rate of cognitive decline in cognitively normal adults with elevated amyloid.
Clinicians informed all A4 study participants of their amyloid PET scan result through a disclosure process that was designed to maximize safety and effectiveness. The process included a depression and anxiety prescreen, an educational session, a teach-back exercise to check comprehension, an in-person disclosure by a trained clinician, and a telephone follow-up to assess mood. In addition, a study guide provided information about the amyloid PET scan, its purpose, possible results, and limitations. “The study guide describes ‘preclinical’ or ‘asymptomatic’ Alzheimer’s disease as a new concept in development and explains that elevated amyloid ‘does not necessarily mean you will develop Alzheimer’s disease-related memory loss’ but can be associated with an increased risk, and individual risk estimates are not possible. It further explains that ‘not elevated’ does not mean you will never develop Alzheimer’s disease or ‘elevated amyloid’ in the future,” the researchers said.
A4 trial sites provided participants with materials describing the SOKRATES substudy, and interested participants contacted the SOKRATES researchers at the University of Pennsylvania.
The researchers examined comprehension of an elevated amyloid PET scan result by analyzing participants’ responses to the following questions: “What was the result of your amyloid PET scan?” (followed by “Can you tell me in your own words what that means?” or “How would you explain it to a friend?”), “Was it the result you expected?” and “Did the result teach you anything or clarify anything for you?”
Understanding Risk
When asked, “What was your result,” 64% used the word “amyloid” or “amyloids,” and the remaining participants primarily referred to the presence of “plaques” or to the result being “positive.” Two answers suggested misunderstanding. Six participants used the word “elevated” to describe their amyloid result, while most participants used other words (eg, “increased,” “higher levels,” or “excessive”). Nineteen participants described the result in terms of qualifying for the A4 study (eg, “enough amyloid buildup to qualify for the study”).
More than half of the participants (54%) expected their elevated result due to a family history of Alzheimer’s disease or subjective memory problems. Fifteen participants (30%) were unsure what result they had expected or were prepared for either result. Eight participants (16%) expected not to have elevated amyloid because they had led a healthy lifestyle, had no family history of Alzheimer’s disease, and had no subjective memory concerns.
When asked about the meaning of the result, 94% responded with their understanding of the risk of developing Alzheimer’s disease conferred by elevated amyloid. Thirty-one participants (62%) interpreted the result as signaling an increased risk of developing Alzheimer’s disease, while 10 participants (20%) perceived the risk conferred by elevated amyloid to be equivocal. Six participants (12%) perceived elevated amyloid to mean that Alzheimer’s disease was imminent or that it was diagnostic of Alzheimer’s disease.
Twenty participants (40%) were dissatisfied “with the lack of specificity regarding the meaning of ‘elevated,’” the researchers said. Some wanted “a granular result describing the degree of amyloid elevation … and how close they were to the threshold for study entry,” and some participants expressed frustration at the lack of detail.
Implications
The results suggest that some people who are cognitively normal but have subjective memory concerns “will use an Alzheimer’s disease biomarker test to explain their memory concerns, potentially pathologizing normal and nondisease-related cognitive aging,” the researchers said. In addition, people without a family history of Alzheimer’s disease or subjective memory concerns “may be unprepared to receive their biomarker results.”
A desire for more specific and detailed information about the results may be especially relevant to cognitively normal adults. “Such specific information is likely less relevant for symptomatic individuals who receive a binary result that either confirms or rules out a diagnosis that explains their history of cognitive decline,” the researchers said.
Because most participants were highly educated and had a family history of Alzheimer’s disease, the applicability of the results to other populations is limited. In future studies, the researchers plan to assess how elevated versus not-elevated brain amyloid results influenced SOKRATES participants’ sense of self, social relationships, and behaviors.
Potential Interventions and Ethical Concerns
The long prodromal period of Alzheimer’s disease “represents our greatest hope for effective therapeutic intervention as well as a domain of serious ethical concern,” said Winston Chiong, MD, PhD, of the Memory and Aging Center, Weill Institute for Neurosciences, University of California, San Francisco, in an accompanying editorial. “Prevailing appropriate use criteria advise against clinical use of amyloid imaging in asymptomatic individuals, citing ‘a significant potential for patients and families to make inaccurate assumptions about risk and future outcomes on the basis of amyloid PET results.’” The A4 trial, however, “not only requires that amyloid imaging be performed but also effectively requires that the results of such imaging be disclosed to participants.”
Qualitative research may elicit unanticipated beliefs or concerns, and in SOKRATES, “a sizable proportion of participants … expressed dissatisfaction with the categorical characterization of results.”
“Overall, the findings of Mozersky and colleagues are broadly reassuring regarding research participants’ ability to understand the prognostic uncertainty of amyloid imaging. But as the authors note, caution is needed in generalizing their results,” Dr. Chiong said. “These participants represent a selected subpopulation of an already rarified group: prospective participants in the A4 study were provided with study materials for this substudy, and interested participants themselves contacted the study investigators. These participants were thus likely to be particularly supportive of the Alzheimer’s disease research enterprise, and given their family histories and high educational attainment
Suggested Reading
Chiong W. Challenges in communicating and understanding predictive biomarker imaging for Alzheimer disease. JAMA Neurol. 2018;75(1):18-19.
Mozersky J, Sankar P, Harkins K, et al. Comprehension of an elevated amyloid positron emission tomography biomarker result by cognitively normal older adults. JAMA Neurol. 2018;75(1):44-50.
Enlarging Red Papulonodule on the Chest
The Diagnosis: Metastatic Renal Cell Carcinoma
Histopathologic examination of the punch biopsy demonstrated epithelioid cells with abundant clear cytoplasm and numerous chicken wire-like vascular channels consistent with a diagnosis of cutaneous metastasis of renal cell carcinoma (RCC)(Figure). Collateral history revealed that 8 years prior, the patient had been diagnosed with clear cell RCC, stage III (T3aN0M0). At that time, he was treated with radical nephrectomy, which was considered curative. He remained disease free until several months prior to the development of the cutaneous lesion when he was found to have pulmonary and cerebral metastases with biopsies showing metastatic RCC. He was treated with lobectomy and Gamma Knife radiation for the lung and cerebral metastases, respectively. His oncologist planned to initiate therapy with the multikinase inhibitor sunitinib, which inhibits vascular endothelial growth factor (VEGF) signaling. Unfortunately, the patient died prior to treatment due to overwhelming tumor burden.
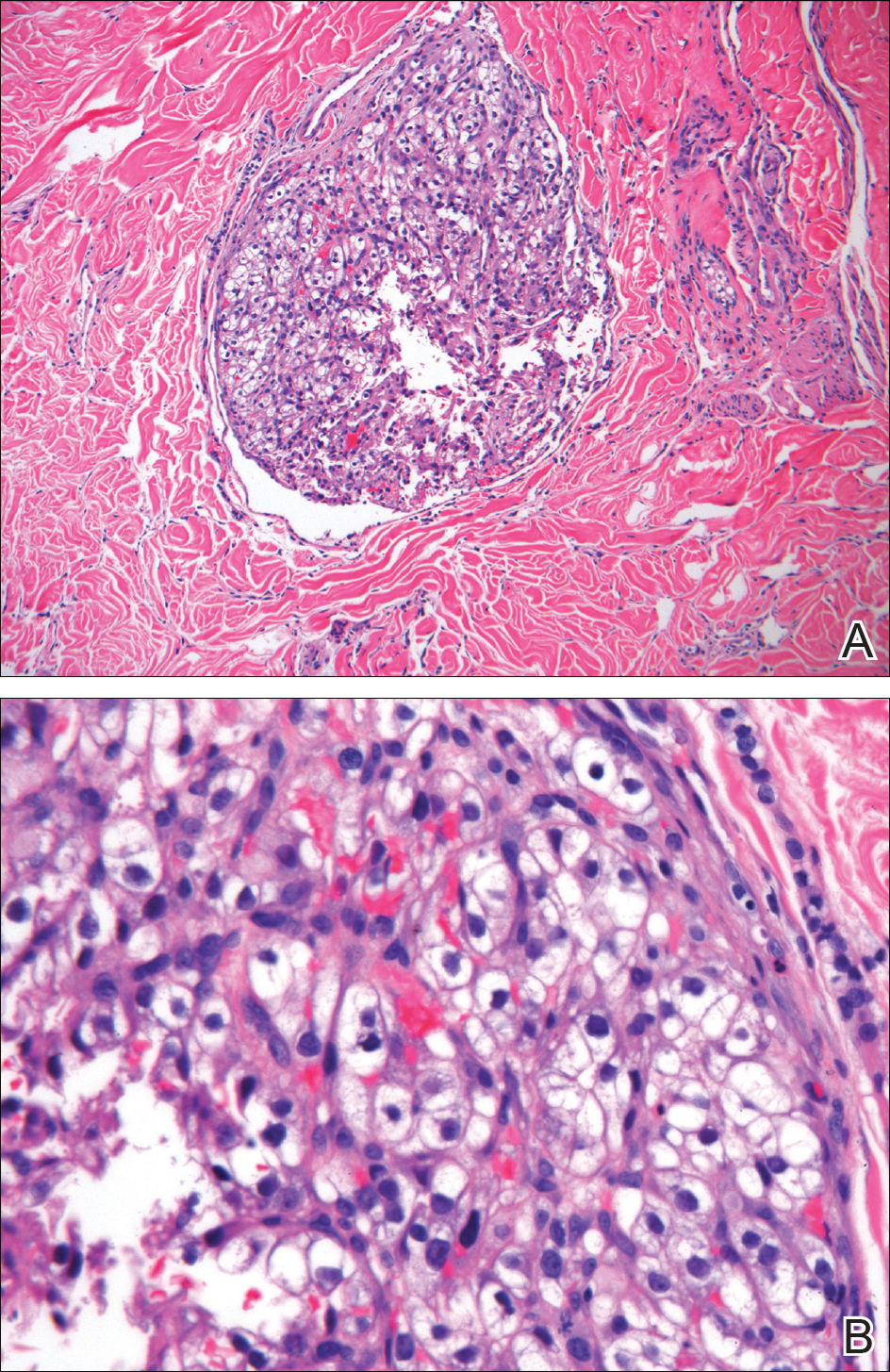
Clear cell RCC, the most common renal malignancy, presents with metastatic disease at the time of diagnosis in 21% of patients.1 An additional 20% of patients with localized disease develop metastases within several years of receiving a nephrectomy without adjuvant therapy, which is standard treatment for stage I to stage III disease.1,2 Metastatic RCC most frequently targets the lungs, bone, liver, and brain, though virtually any organ can be involved. Cutaneous involvement is estimated to occur in 3.3% of RCC cases,3 accounting for only 1.4% of cutaneous metastases overall.4 The risk for developing cutaneous metastases is greatest within 3 years following nephrectomy.3 However, our patient demonstrates that metastasis of RCC to skin can be long delayed (>5 years) despite an initial diagnosis of localized disease.
Cutaneous RCC classically presents as a painless firm papulonodule with a deep red or purple color due to its high vascularity.4 Several retrospective studies have identified the scalp as the most frequent site of cutaneous involvement, followed by the chest, abdomen, and nephrectomy scar.3,4 The differential diagnosis includes other vascular lesions such as pyogenic granuloma, hemangioma, angiosarcoma, bacillary angiomatosis, and Kaposi sarcoma. Diagnosis usually is easily confirmed histologically. Proliferative nests of epithelioid cells with clear cell morphology are surrounded by delicately branching vessels referred to as chicken wire-like vasculature. Immunohistochemical studies demonstrate positivity for pan-cytokeratin, vimentin, and CD-10, and negativity for p63 and cytokeratins 5 and 6, helping to confirm the diagnosis in more challenging cases, especially when there is no known history of primary RCC.5
If cutaneous metastasis of RCC is diagnosed, a chest and abdominal computed tomography scan as well as serum alkaline phosphatase test are warranted, as up to 90% of patients with RCC in the skin have additional lesions in at least 1 other site such as the lungs, bones, or liver.3 Management of metastatic RCC includes surgical excision if a single metastasis is found and either immunotherapy with high-dose IL-2 or an anti-programmed cell death inhibitor. Patients with progressive disease also may receive targeted anti-VEGF inhibitors (eg, axitinib, pazopanib, sunitinib), which have been shown to increase progression-free survival in metastatic RCC.6-8 Interestingly, some evidence suggests severely delayed recurrence of RCC (>5 years following nephrectomy) may predict better response to systemic therapy.9
This case of severely delayed metastasis of RCC 8 years after nephrectomy raises the question of whether routine surveillance for RCC recurrence should continue beyond 5 years. It also underscores the need for further studies to determine the utility of postsurgical adjuvant therapy for localized disease (stages I-III). A randomized clinical trial showed no significant difference in disease-free survival when the multikinase inhibitors sunitinib and sorafenib were used as adjuvant therapy.10 The randomized, placebo-controlled PROTECT trial showed no significant difference in disease-free survival between the VEGF inhibitor pazopanib and placebo when used as adjuvant therapy.11 However, trials are ongoing to investigate a potential survival advantage of adjuvant therapy with the VEGF receptor inhibitor axitinib and the mammalian target of rapamycin inhibitor everolimus.
- Dabestani S, Thorstenson A, Lindblad P, et al. Renal cell carcinoma recurrences and metastases in primary non-metastatic patients: a population-based study. World J Urol. 2016;34:1081-1086.
- Ljungberg B, Campbell SC, Choi HY, et al. The epidemiology of renal cell carcinoma. Eur Urol. 2011;60:615-621.
- Dorairajan LN, Hemal AK, Aron M, et al. Cutaneous metastases in renal cell carcinoma. Urol Int. 1999;63:164-167.
- Lookingbill DP, Spangler N, Helm KF. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29(2, pt 1):228-236.
- Sariya D, Ruth K, Adams-McDonnell R, et al. Clinicopathologic correlation of cutaneous metastases: experience from a cancer center. Arch Dermatol. 2007;143:613-620.
- Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28:1061-1068.
- Motzer RJ, Hutson TE, Tomczak P, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:3584-3590.
- Rini BI, Grunwald V, Fishman MN, et al. Axitinib for first-line metastatic renal cell carcinoma (mRCC): overall efficacy and pharmacokinetic (PK) analyses from a randomized phase II study. J Clin Oncol. 2012;30(suppl). doi:10.1200/jco.2012.30.15_suppl.4503.
- Ficarra V, Novara G. Characterizing late recurrence of renal cell carcinoma. Nat Rev Urol. 2013;10:687-689.
- Haas NB, Manola J, Uzzo RG, et al. Adjuvant sunitinib or sorafenib for high-risk, non-metastatic renal-cell carcinoma (ECOG-ACRIN E2805): a double-blind, placebo-controlled, randomised, phase 3 trial [published online March 9, 2016]. Lancet. 2016;387:2008-2016.
- Motzer RJ, Haas NB, Donskov F, et al; PROTECT investigators. Randomized phase III trial of adjuvant pazopanib versus placebo after nephrectomy in patients with localized or locally advanced renal cell carcinoma [published online September 13, 2017]. J Clin Oncol. 2017;35:3916-3923.
The Diagnosis: Metastatic Renal Cell Carcinoma
Histopathologic examination of the punch biopsy demonstrated epithelioid cells with abundant clear cytoplasm and numerous chicken wire-like vascular channels consistent with a diagnosis of cutaneous metastasis of renal cell carcinoma (RCC)(Figure). Collateral history revealed that 8 years prior, the patient had been diagnosed with clear cell RCC, stage III (T3aN0M0). At that time, he was treated with radical nephrectomy, which was considered curative. He remained disease free until several months prior to the development of the cutaneous lesion when he was found to have pulmonary and cerebral metastases with biopsies showing metastatic RCC. He was treated with lobectomy and Gamma Knife radiation for the lung and cerebral metastases, respectively. His oncologist planned to initiate therapy with the multikinase inhibitor sunitinib, which inhibits vascular endothelial growth factor (VEGF) signaling. Unfortunately, the patient died prior to treatment due to overwhelming tumor burden.

Clear cell RCC, the most common renal malignancy, presents with metastatic disease at the time of diagnosis in 21% of patients.1 An additional 20% of patients with localized disease develop metastases within several years of receiving a nephrectomy without adjuvant therapy, which is standard treatment for stage I to stage III disease.1,2 Metastatic RCC most frequently targets the lungs, bone, liver, and brain, though virtually any organ can be involved. Cutaneous involvement is estimated to occur in 3.3% of RCC cases,3 accounting for only 1.4% of cutaneous metastases overall.4 The risk for developing cutaneous metastases is greatest within 3 years following nephrectomy.3 However, our patient demonstrates that metastasis of RCC to skin can be long delayed (>5 years) despite an initial diagnosis of localized disease.
Cutaneous RCC classically presents as a painless firm papulonodule with a deep red or purple color due to its high vascularity.4 Several retrospective studies have identified the scalp as the most frequent site of cutaneous involvement, followed by the chest, abdomen, and nephrectomy scar.3,4 The differential diagnosis includes other vascular lesions such as pyogenic granuloma, hemangioma, angiosarcoma, bacillary angiomatosis, and Kaposi sarcoma. Diagnosis usually is easily confirmed histologically. Proliferative nests of epithelioid cells with clear cell morphology are surrounded by delicately branching vessels referred to as chicken wire-like vasculature. Immunohistochemical studies demonstrate positivity for pan-cytokeratin, vimentin, and CD-10, and negativity for p63 and cytokeratins 5 and 6, helping to confirm the diagnosis in more challenging cases, especially when there is no known history of primary RCC.5
If cutaneous metastasis of RCC is diagnosed, a chest and abdominal computed tomography scan as well as serum alkaline phosphatase test are warranted, as up to 90% of patients with RCC in the skin have additional lesions in at least 1 other site such as the lungs, bones, or liver.3 Management of metastatic RCC includes surgical excision if a single metastasis is found and either immunotherapy with high-dose IL-2 or an anti-programmed cell death inhibitor. Patients with progressive disease also may receive targeted anti-VEGF inhibitors (eg, axitinib, pazopanib, sunitinib), which have been shown to increase progression-free survival in metastatic RCC.6-8 Interestingly, some evidence suggests severely delayed recurrence of RCC (>5 years following nephrectomy) may predict better response to systemic therapy.9
This case of severely delayed metastasis of RCC 8 years after nephrectomy raises the question of whether routine surveillance for RCC recurrence should continue beyond 5 years. It also underscores the need for further studies to determine the utility of postsurgical adjuvant therapy for localized disease (stages I-III). A randomized clinical trial showed no significant difference in disease-free survival when the multikinase inhibitors sunitinib and sorafenib were used as adjuvant therapy.10 The randomized, placebo-controlled PROTECT trial showed no significant difference in disease-free survival between the VEGF inhibitor pazopanib and placebo when used as adjuvant therapy.11 However, trials are ongoing to investigate a potential survival advantage of adjuvant therapy with the VEGF receptor inhibitor axitinib and the mammalian target of rapamycin inhibitor everolimus.
The Diagnosis: Metastatic Renal Cell Carcinoma
Histopathologic examination of the punch biopsy demonstrated epithelioid cells with abundant clear cytoplasm and numerous chicken wire-like vascular channels consistent with a diagnosis of cutaneous metastasis of renal cell carcinoma (RCC)(Figure). Collateral history revealed that 8 years prior, the patient had been diagnosed with clear cell RCC, stage III (T3aN0M0). At that time, he was treated with radical nephrectomy, which was considered curative. He remained disease free until several months prior to the development of the cutaneous lesion when he was found to have pulmonary and cerebral metastases with biopsies showing metastatic RCC. He was treated with lobectomy and Gamma Knife radiation for the lung and cerebral metastases, respectively. His oncologist planned to initiate therapy with the multikinase inhibitor sunitinib, which inhibits vascular endothelial growth factor (VEGF) signaling. Unfortunately, the patient died prior to treatment due to overwhelming tumor burden.

Clear cell RCC, the most common renal malignancy, presents with metastatic disease at the time of diagnosis in 21% of patients.1 An additional 20% of patients with localized disease develop metastases within several years of receiving a nephrectomy without adjuvant therapy, which is standard treatment for stage I to stage III disease.1,2 Metastatic RCC most frequently targets the lungs, bone, liver, and brain, though virtually any organ can be involved. Cutaneous involvement is estimated to occur in 3.3% of RCC cases,3 accounting for only 1.4% of cutaneous metastases overall.4 The risk for developing cutaneous metastases is greatest within 3 years following nephrectomy.3 However, our patient demonstrates that metastasis of RCC to skin can be long delayed (>5 years) despite an initial diagnosis of localized disease.
Cutaneous RCC classically presents as a painless firm papulonodule with a deep red or purple color due to its high vascularity.4 Several retrospective studies have identified the scalp as the most frequent site of cutaneous involvement, followed by the chest, abdomen, and nephrectomy scar.3,4 The differential diagnosis includes other vascular lesions such as pyogenic granuloma, hemangioma, angiosarcoma, bacillary angiomatosis, and Kaposi sarcoma. Diagnosis usually is easily confirmed histologically. Proliferative nests of epithelioid cells with clear cell morphology are surrounded by delicately branching vessels referred to as chicken wire-like vasculature. Immunohistochemical studies demonstrate positivity for pan-cytokeratin, vimentin, and CD-10, and negativity for p63 and cytokeratins 5 and 6, helping to confirm the diagnosis in more challenging cases, especially when there is no known history of primary RCC.5
If cutaneous metastasis of RCC is diagnosed, a chest and abdominal computed tomography scan as well as serum alkaline phosphatase test are warranted, as up to 90% of patients with RCC in the skin have additional lesions in at least 1 other site such as the lungs, bones, or liver.3 Management of metastatic RCC includes surgical excision if a single metastasis is found and either immunotherapy with high-dose IL-2 or an anti-programmed cell death inhibitor. Patients with progressive disease also may receive targeted anti-VEGF inhibitors (eg, axitinib, pazopanib, sunitinib), which have been shown to increase progression-free survival in metastatic RCC.6-8 Interestingly, some evidence suggests severely delayed recurrence of RCC (>5 years following nephrectomy) may predict better response to systemic therapy.9
This case of severely delayed metastasis of RCC 8 years after nephrectomy raises the question of whether routine surveillance for RCC recurrence should continue beyond 5 years. It also underscores the need for further studies to determine the utility of postsurgical adjuvant therapy for localized disease (stages I-III). A randomized clinical trial showed no significant difference in disease-free survival when the multikinase inhibitors sunitinib and sorafenib were used as adjuvant therapy.10 The randomized, placebo-controlled PROTECT trial showed no significant difference in disease-free survival between the VEGF inhibitor pazopanib and placebo when used as adjuvant therapy.11 However, trials are ongoing to investigate a potential survival advantage of adjuvant therapy with the VEGF receptor inhibitor axitinib and the mammalian target of rapamycin inhibitor everolimus.
- Dabestani S, Thorstenson A, Lindblad P, et al. Renal cell carcinoma recurrences and metastases in primary non-metastatic patients: a population-based study. World J Urol. 2016;34:1081-1086.
- Ljungberg B, Campbell SC, Choi HY, et al. The epidemiology of renal cell carcinoma. Eur Urol. 2011;60:615-621.
- Dorairajan LN, Hemal AK, Aron M, et al. Cutaneous metastases in renal cell carcinoma. Urol Int. 1999;63:164-167.
- Lookingbill DP, Spangler N, Helm KF. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29(2, pt 1):228-236.
- Sariya D, Ruth K, Adams-McDonnell R, et al. Clinicopathologic correlation of cutaneous metastases: experience from a cancer center. Arch Dermatol. 2007;143:613-620.
- Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28:1061-1068.
- Motzer RJ, Hutson TE, Tomczak P, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:3584-3590.
- Rini BI, Grunwald V, Fishman MN, et al. Axitinib for first-line metastatic renal cell carcinoma (mRCC): overall efficacy and pharmacokinetic (PK) analyses from a randomized phase II study. J Clin Oncol. 2012;30(suppl). doi:10.1200/jco.2012.30.15_suppl.4503.
- Ficarra V, Novara G. Characterizing late recurrence of renal cell carcinoma. Nat Rev Urol. 2013;10:687-689.
- Haas NB, Manola J, Uzzo RG, et al. Adjuvant sunitinib or sorafenib for high-risk, non-metastatic renal-cell carcinoma (ECOG-ACRIN E2805): a double-blind, placebo-controlled, randomised, phase 3 trial [published online March 9, 2016]. Lancet. 2016;387:2008-2016.
- Motzer RJ, Haas NB, Donskov F, et al; PROTECT investigators. Randomized phase III trial of adjuvant pazopanib versus placebo after nephrectomy in patients with localized or locally advanced renal cell carcinoma [published online September 13, 2017]. J Clin Oncol. 2017;35:3916-3923.
- Dabestani S, Thorstenson A, Lindblad P, et al. Renal cell carcinoma recurrences and metastases in primary non-metastatic patients: a population-based study. World J Urol. 2016;34:1081-1086.
- Ljungberg B, Campbell SC, Choi HY, et al. The epidemiology of renal cell carcinoma. Eur Urol. 2011;60:615-621.
- Dorairajan LN, Hemal AK, Aron M, et al. Cutaneous metastases in renal cell carcinoma. Urol Int. 1999;63:164-167.
- Lookingbill DP, Spangler N, Helm KF. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29(2, pt 1):228-236.
- Sariya D, Ruth K, Adams-McDonnell R, et al. Clinicopathologic correlation of cutaneous metastases: experience from a cancer center. Arch Dermatol. 2007;143:613-620.
- Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28:1061-1068.
- Motzer RJ, Hutson TE, Tomczak P, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:3584-3590.
- Rini BI, Grunwald V, Fishman MN, et al. Axitinib for first-line metastatic renal cell carcinoma (mRCC): overall efficacy and pharmacokinetic (PK) analyses from a randomized phase II study. J Clin Oncol. 2012;30(suppl). doi:10.1200/jco.2012.30.15_suppl.4503.
- Ficarra V, Novara G. Characterizing late recurrence of renal cell carcinoma. Nat Rev Urol. 2013;10:687-689.
- Haas NB, Manola J, Uzzo RG, et al. Adjuvant sunitinib or sorafenib for high-risk, non-metastatic renal-cell carcinoma (ECOG-ACRIN E2805): a double-blind, placebo-controlled, randomised, phase 3 trial [published online March 9, 2016]. Lancet. 2016;387:2008-2016.
- Motzer RJ, Haas NB, Donskov F, et al; PROTECT investigators. Randomized phase III trial of adjuvant pazopanib versus placebo after nephrectomy in patients with localized or locally advanced renal cell carcinoma [published online September 13, 2017]. J Clin Oncol. 2017;35:3916-3923.
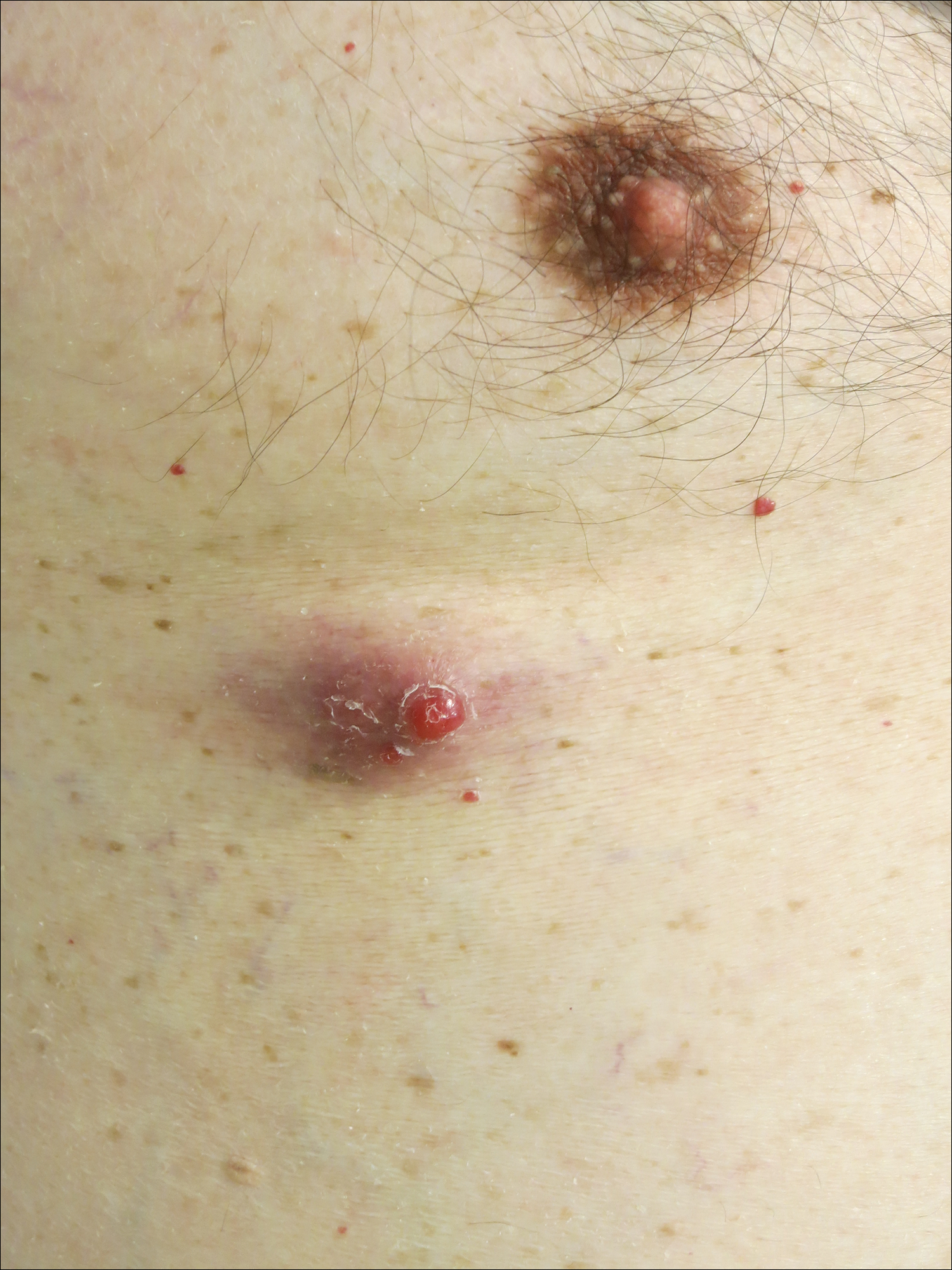
A man in his 60s presented with a subcutaneous nodule on the right side of the chest. Due to impaired mental status, he was unable to describe the precise age of the lesion, but his wife reported it had been present at least several weeks. She recently noted a new, bright red growth on top of the nodule. The lesion was asymptomatic but seemed to be growing in size. Physical examination revealed a 3-cm firm fixed nodule on the right side of the chest with an overlying, exophytic bright red papule. No similar lesions were found elsewhere on physical examination. A punch biopsy of the lesion was performed.
MDedge Daily News: More Medicare money for E&M visits
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The eyes may be windows into a coming stroke, E&M visits may soon mean more money, many doctors still reach for the wrong atopic dermatitis tool, and misfolded proteins may plant the seed for multiple sclerosis.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The eyes may be windows into a coming stroke, E&M visits may soon mean more money, many doctors still reach for the wrong atopic dermatitis tool, and misfolded proteins may plant the seed for multiple sclerosis.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The eyes may be windows into a coming stroke, E&M visits may soon mean more money, many doctors still reach for the wrong atopic dermatitis tool, and misfolded proteins may plant the seed for multiple sclerosis.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
A New Era for Physician-Patient Communication in Dermatology
The physician-patient relationship is an important component of patient care. In the last few years a new paradigm has emerged of instant communication. Because dermatologic diagnosis is visual, many patients feel that making a correct diagnosis is as easy as taking a quick look. The availability of smartphone photography and easy ways to get in touch with dermatologists have created a new reality in physician-patient communication, which sometimes may be abused. We conducted an email survey to assess the attitudes of Chilean dermatologists regarding new methods of communication with their patients.
A survey of 16 questions was distributed to all 343 members of the Chilean Society of Dermatology and Venerology from July 2016 to August 2016. A total of 147 (42.9%) dermatologists completed the survey. When asked if they use personal and direct communication with their patients outside of an office visit, 39% of respondents said always, 41% said sometimes, 17% said only in some circumstances, and 3% said never. Regarding the method of communication, 79% used personal email, 59% used mobile phones, 35% used corporate email, and 34% used text messages. Among respondents who gave their personal email address and phone number to patients, the primary reason stated was to be available for any kind of emergency (67%), for patient follow-up (57%), and for patients to feel close to their dermatologist (28%).
Sixty-nine percent of respondents said patients occasionally have requested to receive a diagnosis via a mobile messaging application, social networks, and email. Of them, 22% said they were very annoyed by these requests. When dermatologists were asked if these instant types of communication improved their relationship with patients, 30% said it does help and 36% said it does not; 30% said they do not know and 4% did not respond. If patients used personal methods of communication to contact their dermatologist that was considered outside of physician-patient boundaries, 63% of physician respondents said they kindly directed patients to formal ways of communication and 15% did not respond to such requests; 22% responded by informal methods of communication. Eighty-one percent of all respondents felt the limits of formal communication between physicians and patients have been surpassed.
To improve the quality of health care, many clinicians use modern methods of communication with their patients. Today, patients can turn to their physicians for medical advice by mobile phone or email. We attempted to characterize the attitudes of Chilean dermatologists regarding new ways of communicating with patients. Our results are similar to other studies. One analysis of primary care physicians in Geneva, Switzerland (N=372), showed that 72% gave their personal email address and 74% gave their mobile phone number to patients. The latter is higher than what was found in our study (59%), which may be explained by the fact that primary care physicians may need to maintain closer contact with their patients.1
In another study performed in primary care physicians in Israel, physicians preferred to provide their mobile phone number rather than their personal email address because they felt that email communication was more likely to lead miscommunication than a phone call.2 There are few reports on this subject in the international literature, and we believe cultural differences may be important when physicians confront these issues.
In general, patient satisfaction is high when patients can contact their physician by phone or email; however, new immediate forms of communication may lead to physician burnout, as patients expect immediate responses and solutions to their requests and healthy physician-patient boundaries may be surpassed. It is important to educate both patients and physicians on how these new tools may be properly used on both sides. New boundaries must be set.
- Dash J, Haller DM, Sommer J, et al. Use of email, cell phone and text message between patients and primary-care physicians: cross-sectional study in a French-speaking part of Switzerland. BMC Health Serv Res. 2016;16:549.
- Peleg R, Avdalimov A, Freud T. Providing cell phone numbers and email addresses to patients: the physician’s perspective. BMC Res Notes. 2011;4:76.
The physician-patient relationship is an important component of patient care. In the last few years a new paradigm has emerged of instant communication. Because dermatologic diagnosis is visual, many patients feel that making a correct diagnosis is as easy as taking a quick look. The availability of smartphone photography and easy ways to get in touch with dermatologists have created a new reality in physician-patient communication, which sometimes may be abused. We conducted an email survey to assess the attitudes of Chilean dermatologists regarding new methods of communication with their patients.
A survey of 16 questions was distributed to all 343 members of the Chilean Society of Dermatology and Venerology from July 2016 to August 2016. A total of 147 (42.9%) dermatologists completed the survey. When asked if they use personal and direct communication with their patients outside of an office visit, 39% of respondents said always, 41% said sometimes, 17% said only in some circumstances, and 3% said never. Regarding the method of communication, 79% used personal email, 59% used mobile phones, 35% used corporate email, and 34% used text messages. Among respondents who gave their personal email address and phone number to patients, the primary reason stated was to be available for any kind of emergency (67%), for patient follow-up (57%), and for patients to feel close to their dermatologist (28%).
Sixty-nine percent of respondents said patients occasionally have requested to receive a diagnosis via a mobile messaging application, social networks, and email. Of them, 22% said they were very annoyed by these requests. When dermatologists were asked if these instant types of communication improved their relationship with patients, 30% said it does help and 36% said it does not; 30% said they do not know and 4% did not respond. If patients used personal methods of communication to contact their dermatologist that was considered outside of physician-patient boundaries, 63% of physician respondents said they kindly directed patients to formal ways of communication and 15% did not respond to such requests; 22% responded by informal methods of communication. Eighty-one percent of all respondents felt the limits of formal communication between physicians and patients have been surpassed.
To improve the quality of health care, many clinicians use modern methods of communication with their patients. Today, patients can turn to their physicians for medical advice by mobile phone or email. We attempted to characterize the attitudes of Chilean dermatologists regarding new ways of communicating with patients. Our results are similar to other studies. One analysis of primary care physicians in Geneva, Switzerland (N=372), showed that 72% gave their personal email address and 74% gave their mobile phone number to patients. The latter is higher than what was found in our study (59%), which may be explained by the fact that primary care physicians may need to maintain closer contact with their patients.1
In another study performed in primary care physicians in Israel, physicians preferred to provide their mobile phone number rather than their personal email address because they felt that email communication was more likely to lead miscommunication than a phone call.2 There are few reports on this subject in the international literature, and we believe cultural differences may be important when physicians confront these issues.
In general, patient satisfaction is high when patients can contact their physician by phone or email; however, new immediate forms of communication may lead to physician burnout, as patients expect immediate responses and solutions to their requests and healthy physician-patient boundaries may be surpassed. It is important to educate both patients and physicians on how these new tools may be properly used on both sides. New boundaries must be set.
The physician-patient relationship is an important component of patient care. In the last few years a new paradigm has emerged of instant communication. Because dermatologic diagnosis is visual, many patients feel that making a correct diagnosis is as easy as taking a quick look. The availability of smartphone photography and easy ways to get in touch with dermatologists have created a new reality in physician-patient communication, which sometimes may be abused. We conducted an email survey to assess the attitudes of Chilean dermatologists regarding new methods of communication with their patients.
A survey of 16 questions was distributed to all 343 members of the Chilean Society of Dermatology and Venerology from July 2016 to August 2016. A total of 147 (42.9%) dermatologists completed the survey. When asked if they use personal and direct communication with their patients outside of an office visit, 39% of respondents said always, 41% said sometimes, 17% said only in some circumstances, and 3% said never. Regarding the method of communication, 79% used personal email, 59% used mobile phones, 35% used corporate email, and 34% used text messages. Among respondents who gave their personal email address and phone number to patients, the primary reason stated was to be available for any kind of emergency (67%), for patient follow-up (57%), and for patients to feel close to their dermatologist (28%).
Sixty-nine percent of respondents said patients occasionally have requested to receive a diagnosis via a mobile messaging application, social networks, and email. Of them, 22% said they were very annoyed by these requests. When dermatologists were asked if these instant types of communication improved their relationship with patients, 30% said it does help and 36% said it does not; 30% said they do not know and 4% did not respond. If patients used personal methods of communication to contact their dermatologist that was considered outside of physician-patient boundaries, 63% of physician respondents said they kindly directed patients to formal ways of communication and 15% did not respond to such requests; 22% responded by informal methods of communication. Eighty-one percent of all respondents felt the limits of formal communication between physicians and patients have been surpassed.
To improve the quality of health care, many clinicians use modern methods of communication with their patients. Today, patients can turn to their physicians for medical advice by mobile phone or email. We attempted to characterize the attitudes of Chilean dermatologists regarding new ways of communicating with patients. Our results are similar to other studies. One analysis of primary care physicians in Geneva, Switzerland (N=372), showed that 72% gave their personal email address and 74% gave their mobile phone number to patients. The latter is higher than what was found in our study (59%), which may be explained by the fact that primary care physicians may need to maintain closer contact with their patients.1
In another study performed in primary care physicians in Israel, physicians preferred to provide their mobile phone number rather than their personal email address because they felt that email communication was more likely to lead miscommunication than a phone call.2 There are few reports on this subject in the international literature, and we believe cultural differences may be important when physicians confront these issues.
In general, patient satisfaction is high when patients can contact their physician by phone or email; however, new immediate forms of communication may lead to physician burnout, as patients expect immediate responses and solutions to their requests and healthy physician-patient boundaries may be surpassed. It is important to educate both patients and physicians on how these new tools may be properly used on both sides. New boundaries must be set.
- Dash J, Haller DM, Sommer J, et al. Use of email, cell phone and text message between patients and primary-care physicians: cross-sectional study in a French-speaking part of Switzerland. BMC Health Serv Res. 2016;16:549.
- Peleg R, Avdalimov A, Freud T. Providing cell phone numbers and email addresses to patients: the physician’s perspective. BMC Res Notes. 2011;4:76.
- Dash J, Haller DM, Sommer J, et al. Use of email, cell phone and text message between patients and primary-care physicians: cross-sectional study in a French-speaking part of Switzerland. BMC Health Serv Res. 2016;16:549.
- Peleg R, Avdalimov A, Freud T. Providing cell phone numbers and email addresses to patients: the physician’s perspective. BMC Res Notes. 2011;4:76.
Polycythemia Vera and Essential Thrombocythemia: Current Management
Introduction
Polycythemia vera (PV) and essential thrombocythemia (ET), along with primary myelofibrosis (PMF), belong to the group of Philadelphia-negative myeloproliferative neoplasms (MPN). All these malignancies arise from the clonal proliferation of an aberrant hematopoietic stem cell, but are characterized by distinct clinical phenotypes.1,2 Although the clinical course of PV and ET is indolent, it can be complicated by thrombohemorrhagic episodes and/or evolution into myelofibrosis and/or acute myeloid leukemia (AML).3 Since vascular events are the most frequent life-threatening complications of PV and ET, therapeutic strategies are aimed at reducing this risk. Treatment may also help control other disease-associated symptoms.4 No therapy has been shown to prevent evolution of PV or ET into myelofibrosis or AML. The discovery of the Janus kinase 2 (JAK2)/V617F mutation in most patients with PV and over half of those with ET (and PMF)5,6 has opened new avenues of research and led to the development of targeted therapies, such as the JAK1/2 inhibitor ruxolitinib, for patients with MPN.7,8
Epidemiology
PV and ET are typically diagnosed in the fifth to seventh decade of life.9 Although these disorders are generally associated with a long clinical course, survival of patients with PV or ET may be shorter than that of the general population.10–13 Estimating the incidence and prevalence of MPN is a challenge because most patients remain asymptomatic for long periods of time and do not seek medical attention.13 The annual incidence rates of PV and ET are estimated at 0.01 to 2.61 and 0.21 to 2.53 per 100,000, respectively. PV occurs slightly more frequently in males, whereas ET has a predilection for females.14 Given the long course and low mortality associated with these disorders, the prevalence of PV and ET are significantly higher than the respective incidence: up to 47 and 57 per 100,000, respectively.15–17
Molecular Pathogenesis
In 2005 researchers discovered a gain-of-function mutation of the JAK2 gene in nearly all patients with PV and more than half of those with ET and PMF.5,6,18,19 JAK2 is a non-receptor tyrosine kinase that plays a central role in normal hematopoiesis. Substitution of a valine for a phenylalanine at codon 617 (ie, V617F) leads to its constitutive activation and signaling through the JAK-STAT pathway.5,6,18,19 More rarely (and exclusively in patients with PV), JAK2 mutations involve exon 12.20–22 The vast majority of JAK2-negative ET patients harbor mutations in either the myeloproliferative leukemia (MPL) gene, which encodes the thrombopoietin receptor,23–25 or the calreticulin (CALR) gene,26,27 which encodes for a chaperone protein that plays a role in cellular proliferation, differentiation, and apoptosis.28 Both the MPL and CALR mutations ultimately result in the constitutive activation of the JAK-STAT pathway. Thus, JAK2, MPL, and CALR alterations are collectively referred to as driver mutations. Moreover, because these mutations affect the same oncogenic pathway (ie, JAK-STAT), they are almost always mutually exclusive in a given patient. Patients with ET (or myelofibrosis) who are wild-type for JAK2, MPL, and CALR are referred to as having “triple-negative” disease. Many recurrent non-driver mutations are also found in patients with MPN that are not exclusive of each other (ie, patients may have many at the same time), and involve for example ten-eleven translocation-2 (TET2), additional sex combs like 1 (ASXL1), enhancer of zeste homolog 2 (EZH2), isocitrate dehydrogenase 1 and isocitrate dehydrogenase 2 (IDH1/2), and DNA methyltransferase 3A (DNMT3A) genes, among others.29 The biologic and prognostic significance of these non-driver alterations remain to be fully defined in ET and PV.
Diagnosis and Risk Assessment
Case Presentations
Patient A is a 68-year-old man with a history of gouty arthritis who presents with a 6-month history of recurrent headaches and itching that increases after a hot shower. Over the past 2 months, he has also noticed worsening fatigue and redness of his face. He is a nonsmoker. Physical exam reveals erythromelalgia (ie, erythema, edema, and warmth) of the upper and lower extremities, scattered scratch marks, and splenomegaly 4 cm below the costal margin. Complete blood count (CBC) shows a white blood cell (WBC) count of 8100/µL, hemoglobin 194 g/L, and platelets 582 × 103/µL. Serum erythropoietin level is decreased at 2 mU/mL. Peripheral blood testing reveals a JAK2V617F mutation.
Patient B is a 51-year-old woman with a history of severe depression treated with sertraline and hypertension controlled with lisinopril and amlodipine who presents to her primary care physician for her “50-year-old physical.” She denies symptoms and is a nonsmoker. Physical exam is unrevealing. CBC shows a WBC count of 7400/µL (normal differential), hemoglobin 135 g/L, and platelets 1282 × 103/µL. A bone marrow biopsy shows normal cellularity with clusters of large, hyperlobulated megakaryocytes. Reverse transcriptase-polymerase chain reaction fails to reveal a BCR-ABL fusion product. The patient is diagnosed with ET.
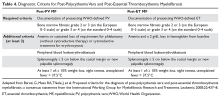
Diagnostic Criteria
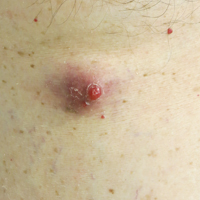
Diagnostic criteria for PV and ET according to the World Health Organization (WHO) classification30 are summarized in Table 1. Criteria for the diagnosis of prefibrotic myelofibrosis are included as well since this entity was formally recognized as separate from ET and part of the PMF spectrum in the 2016 WHO classification of myeloid tumors.30
Risk Stratification
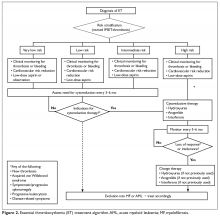
Thrombohemorrhagic events, evolution into myelofibrosis, and leukemic transformation are the most serious complications in the course of PV or ET. Only thrombohemorrhagic events are, at least partially, preventable. Arterial or venous thrombotic complications are observed at rates of 1.8 to 10.9 per 100 patient-years in PV (arterial thrombosis being more common than venous) and 0.74 to 7.7 per 100 patient-years in ET, depending on the risk group35 and the presence of other factors (see below).
Thrombosis Risk Stratification in PV
The risk stratification of patients with PV is based on 2 factors: age ≥ 60 years and prior history of thrombosis. If either is present the patient is assigned to the high-risk category, whereas if none is present the patient is considered at low risk.36 In addition, high hematocrit37 and high WBC,38 but not thrombocytosis, have been associated with the development of vascular complications. In one study, the risk of new arterial thrombosis was increased by the presence of leukoerythroblastosis, hypertension, and prior arterial thrombosis, while karyotypic abnormalities and prior venous thrombosis were predictors of new venous thrombosis.39 Another emerging risk factor for thrombosis in patients with PV is high JAK2 allele burden (ie, the normal-to-mutated gene product ratio), although the evidence supporting this conclusion is equivocal.40
Thrombosis Risk Stratification in ET
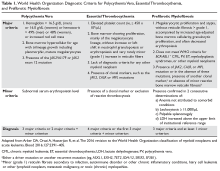
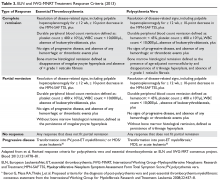
Traditionally, in ET patients, thrombotic risk was assessed using the same 2 factors (age ≥ 60 years and prior history of thrombosis), separating patients into low- and high-risk groups. However, the prognostication of ET patients has been refined recently with the identification of new relevant factors. In particular, the impact of JAK2 mutations on thrombotic risk has been thoroughly studied. Clinically, the presence of JAK2V617F is associated with older age, higher hemoglobin and hematocrit, lower platelet counts, more frequent need for cytoreductive treatment, and greater tendency to evolve into PV (a rare event).41,42 Many,41,43–46 but not all,47–51 studies suggested a correlation between JAK2 mutation and risk of both arterial and venous thrombosis. Although infrequent, a JAK2V617F homozygous state (ie, the mutation is present in both alleles) might confer an even higher thrombotic risk.52 Moreover, the impact of the JAK2 mutation on vascular events persists over time,53 particularly in patients with high or unstable mutation burden.54 Based on JAK2V617F’s influence on the thrombotic risk of ET patients, a new prognostic score was proposed, the International Prognostic Score for ET (IPSET)-thrombosis (Table 2). The revised version of this model is currently endorsed by the National Comprehensive Cancer Network and divides patients into 4 risk groups: high, intermediate, low, and very low. Treatment recommendations vary according to the risk group (as described below).55
Other thrombotic risk factors have been identified, but deemed not significant enough to be included in the model. Cardiovascular risk factors (hypercholesterolemia, hypertension, smoking, diabetes mellitus) can increase the risk of vascular events,56–59 as can splenomegaly60 and baseline or persistent leukocytosis.61–63 Thrombocytosis has been correlated with thrombotic risk in some studies,64–68 whereas others did not support this conclusion and/or suggested a lower rate of thrombosis and, in some cases, increased risk of bleeding in ET patients with platelet counts greater than 1000 × 103/µL (due to acquired von Willebrand syndrome).56,61,63,68,69
CALR mutations tend to occur in younger males with lower hemoglobin and WBC count, higher platelet count, and greater marrow megakaryocytic predominance as compared to JAK2 mutations.26,27,70–72 The associated incidence of thrombosis was less than 10% at 15 years in patients with CALR mutations, lower than the incidence reported for ET patients with JAK2V617F mutations.73 The presence of the mutation per se does not appear to affect the thrombotic risk.74–76 Information on the thrombotic risk associated with MPL mutations or a triple-negative state is scarce. In both instances, however, the risk appears to be lower than with the JAK2 mutation.73,77–79
Venous thromboembolism in patients with PV or ET may occur at unusual sites, such as the splanchnic or cerebral venous systems.80 Risk factors for unusual venous thromboembolism include younger age,81 female gender (especially with concomitant use of oral contraceptive pills),82 and splenomegaly/splenectomy.83JAK2 mutation has also been associated with thrombosis at unusual sites. However, the prevalence of MPN or JAK2V617F in patients presenting with splanchnic venous thromboembolism has varied.80 In addition, MPN may be occult (ie, no clinical or laboratory abnormalities) in around 15% of patients.84 Screening for JAK2V617F and underlying MPN is recommended in patients presenting with isolated unexplained splanchnic venous thromboembolism. Treatment entails long-term anticoagulation therapy. JAK2V617F screening in patients with nonsplanchnic venous thromboembolism is not recommended, as its prevalence in this group is low (< 3%).85,86
Treatment
Cases Continued
Patient A is diagnosed with PV based on the presence of 2 major criteria (elevated hemoglobin and presence of the JAK2V617F mutation) and 1 minor criterion (low erythropoietin level). Given his age, he belongs to the high-risk disease category. He is now seeking advice regarding the management of his newly diagnosed PV.
Patient B presents to the emergency department with right lower extremity swelling and is found to have deep femoral thrombosis extending to the iliac vein. Five days after being discharged from the emergency department, she presents for follow-up. She is taking warfarin compliantly and her INR is within therapeutic range. The patient now has high-risk ET and would like to know more about thrombosis in her condition and how to best manage her risk.
Risk-Adapted Therapy
Low-Risk PV
All patients with PV should receive counseling to mitigate cardiovascular risk factors, including smoking cessation, lifestyle modifications, and lipid-lowering therapy, as indicated. Furthermore, all PV patients should receive acetylsalicylic acid (ASA) to decrease their risk for thrombosis and control vasomotor symptoms.55,87 Aspirin 81 to 100 mg daily is the preferred regimen because it provides adequate antithrombotic effect without the associated bleeding risk of higher-dose aspirin.88 Low-risk PV patients should also receive periodic phlebotomies to reduce and maintain their hematocrit below 45%. This recommendation is based on the results of the Cytoreductive Therapy in Polycythemia Vera (CYTO PV) randomized controlled trial. In the CYTO PV study, patients receiving more intense therapy to maintain the hematocrit below 45% had a lower incidence of cardiovascular-related deaths or major thrombotic events than those with hematocrit goals of 45% to 50% (2.7% versus 9.8%).89 Cytoreduction is an option for low-risk patients who do not tolerate phlebotomy or require frequent phlebotomy, or who have disease-related bleeding, severe symptoms, symptomatic splenomegaly, or progressive leukocytosis.38
High-Risk PV
Patients older than 60 years and/or with a history of thrombosis should be considered for cytoreductive therapy in addition to the above measures. Front-line cytoreductive therapies include hydroxyurea or interferon (IFN)- alfa.87 Hydroxyurea is a potent ribonucleotide reductase inhibitor that interferes with DNA repair and is the treatment of choice for most high-risk patients with PV.90 In a small trial hydroxyurea reduced the risk of thrombosis compared with historical controls treated with phlebotomy alone.91 Hydroxyurea is generally well tolerated; common side effects include cytopenias, nail changes, and mucosal and/or skin ulcers. Although never formally proven to be leukemogenic, this agent should be used with caution in younger patients.87 Indeed, in the original study, the rates of transformation were 5.9% and 1.5% for patients receiving hydroxyurea and phlebotomy alone,92 respectively, although an independent role for hydroxyurea in leukemic transformation was not supported in the much larger European Collaboration on Low-dose Aspirin in Polycythemia Vera (ECLAP) study.93 About 70% of patients will have a sustained response to hydroxyurea,94 while the remaining patients become resistant to or intolerant of the drug. Resistant individuals have a higher risk of progression to acute leukemia and death.95
IFN alfa is a pleiotropic antitumor agent that has found application in many types of malignancies96 and is sometimes employed as treatment for patients with newly diagnosed high-risk PV. Early studies showed responses in up to 100% of cases,97,98 albeit at the expense of a high discontinuation rate due to adverse events, such as flu-like symptoms, fatigue, and neuropsychiatric manifestations.99 A newer formulation of the drug obtained by adding a polyethylene glycol (PEG) moiety to the native IFN alfa molecule (PEG-IFN alfa) was shown to have a longer half-life, greater stability, less immunogenicity, and, potentially, better tolerability.100 Pilot phase 2 trials of PEG-IFN alfa-2a demonstrated its remarkable activity, with symptomatic and hematologic responses seen in the majority of patients (which, in some cases, persisted beyond discontinuation), and reasonable tolerability, with long-term discontinuation rates of around 20% to 30%.101–103 In some patients JAK2V617F became undetectable over time.104 Results of 2 ongoing trials, MDP-RC111 (single-arm study, PEG-IFN alfa-2a in high-risk PV or ET [NCT01259817]) and MPD-RC112 (randomized controlled trial, PEG-IFN alfa-2a versus hydroxyurea in the same population [NCT01258856]), will shed light on the role of PEG-IFN alfa in the management of patients with high-risk PV or ET. In 2 phase 2 studies of PEG-IFN alfa-2b, complete responses were seen in 70% to 100% of patients and discontinuation occurred in around a third of cases.105,106 A new, longer-acting formulation of PEG-IFN alfa-2a (peg-proline INF alfa-2b, AOP2014) is also undergoing clinical development.107,108
The approach to treatment of PV based on thrombotic risk level is illustrated in Figure 1.
Very Low- and Low-Risk ET
Like patients with PV, individuals with ET should undergo rigorous cardiovascular risk management and generally receive ASA to decrease their thrombotic risk and improve symptom control. Antiplatelet therapy may not be warranted in patients with documented
Intermediate-Risk ET
This category includes patients older than 60 years but without thrombosis or JAK2 mutations. These individuals would have been considered high risk (and thus candidates for cytoreductive therapy) according to the traditional risk stratification. Guidelines currently recommend ASA as the sole therapy for these patients, while reserving cytoreduction for those who experience thrombosis (ie, become high-risk) or have uncontrolled vasomotor or general symptoms, symptomatic splenomegaly, symptomatic thrombocytosis, or progressive leukocytosis.
High-Risk ET
For patients with ET in need of cytoreductive therapy (ie, those with prior thrombosis or older than 60 years with a JAK2V617F mutation), first-line options include hydroxyurea, IFN, and anagrelide. Hydroxyurea remains the treatment of choice in the majority of patients.110 In a seminal study, 114 patients with ET were randomly assigned to either observation or hydroxyurea treatment with the goal of maintaining the platelet count below 600 × 103/µL. At a median follow-up of 27 months, patients in the hydroxyurea group had a lower thrombosis rate (3.6% versus 24%, P = 0.003) and longer thrombosis-free survival, regardless of the use of antiplatelet drugs.64
Anagrelide, a selective inhibitor of megakaryocytic differentiation and proliferation, was compared with hydroxyurea in patients with ET in 2 randomized trials. In the first (N = 809), the group receiving anagrelide had a higher risk of arterial thrombosis, major bleeding, and fibrotic evolution, but lower incidence of venous thrombosis. Hydroxyurea was better tolerated, mainly due to anagrelide-related cardiovascular adverse events.111 As a result of this study, hydroxyurea is often preferred to anagrelide as front-line therapy for patients with newly diagnosed high-risk ET. In the second, more recent study (N = 259), however, the 2 agents proved equivalent in terms of major or minor arterial or venous thrombosis, as well as discontinuation rate.112 The discrepancy between the 2 trials may be partly explained by the different ET diagnostic criteria used, with the latter only enrolling patients with WHO-defined true ET, while the former utilized Polycythemia Vera Study Group-ET diagnostic criteria that included patients with increases in other blood counts or varying degrees of marrow fibrosis.
Interferons were studied in ET in parallel with PV. PEG-IFN alfa-2a proved effective in patients with ET, with responses observed in 80% of patients.103 PEG-IFN alfa-2b produced similar results, with responses in 70% to 90% of patients in small studies and discontinuation observed in 20% to 38% of cases.105,106,113 Because the very long-term leukemogenic potential of hydroxyurea has remained somewhat uncertain, anagrelide or IFN might be preferable choices in younger patients.
The approach to treatment of ET based on thrombotic risk level is illustrated in Figure 2.
Assessing Response to Therapy
For both patients with PV and ET the endpoint of treatment set forth for clinical trials has been the achievement of a clinicohematologic response. However, studies have failed to show a correlation between response and reduction of the thrombohemorrhagic risk.114 Therefore, proposed clinical trial response criteria were revised to include absence of hemorrhagic or thrombotic events as part of the definition of response (Table 3).94
Cases Continued
Patient A was initially treated with phlebotomies and his blood counts were subsequently controlled with hydroxyurea, which he took uninterruptedly at an average dose of 2.5 g daily. He also took ASA daily throughout. Now, 18 months after the start of therapy, he presents with a complaint of fatigue for the past 3 months, which more recently has been associated with recurrent itching. A repeat CBC shows a WBC count of 17,200/µL, hemoglobin 181 g/L, and platelets 940 × 103/µL.
Patient B presents for scheduled follow-up. She has had no further thrombotic episodes. However, she spontaneously discontinued hydroxyurea 1 month ago because of worsening mouth ulcers that impaired her ability to eat even small meals. She seeks recommendations for further treatment options.
Approach to Patients Refractory to or Intolerant of First-Line Therapy
According to the European LeukemiaNet recommendations, an inadequate response to hydroxyurea in patients with PV (or myelofibrosis) is defined as a need for phlebotomy to maintain hematocrit below < 45%, platelet count > 400 × 103/µL, and a WBC count > 10,000/µL, or failure to reduce splenomegaly > 10 cm by > 50% at a dose of ≥ 2 g/day or maximum tolerated dose. Historically, treatment options for patients with PV or ET who failed first-line therapy (most commonly hydroxyurea) have included alkylating agents, such as busulfan, chlorambucil, or pipobroman, and phosphorus (P)-32. However, the use of these drugs is limited by the associated risk of leukemic transformation.93,115,116 The use of IFN (or anagrelide for ET) is often considered in patients previously treated with hydroxyurea, and vice versa.
Ruxolitinib is a JAK1 and JAK2 inhibitor currently approved for the treatment of PV patients refractory to or intolerant of hydroxyurea.7 Following promising results of a phase 2 trial,117 ruxolitinib 10 mg twice daily was compared with best available therapy in the pivotal RESPONSE trial (N = 222). Ruxolitinib proved superior in achieving hematocrit control, reduction of spleen volume, and improvement of symptoms. Grade 3-4 hematologic toxicity was infrequent and similar in the 2 arms.118 In addition, longer follow-up of that study suggested a lower rate of thrombotic events in patients receiving ruxolitinib (1.8 versus 8.2 per 100 patient-years).119 In a similarly designed randomized phase 3 study in PV patients without splenomegaly (RESPONSE-2), more patients in the ruxolitinib arm had hematocrit reduction without an increase in toxicity. Based on the results of the above studies, ruxolitinib can be considered a standard of care for second-line therapy in this post-hydroxyurea patient population.120
Ruxolitinib is also being tested in patients with high-risk ET who have become resistant to, or were intolerant of hydroxyurea, but currently has no approved indication in this setting.121,122 Common side effects of ruxolitinib include cytopenias (especially anemia), increased risk of infections, hyperlipidemia, and increased risk of non-melanoma skin cancer.
Novel Agents
Novel agents that have been studied in patients with PV and ET are histone deacetylase inhibitors, murine double minute 2 (MDM2, or HDM2 for their human counterpart) inhibitors (which restore the function of p53), Bcl-2 homology domain 3 mimetics such as navitoclax and venetoclax, and, for patients with ET, the telomerase inhibitor imetelstat.123
Disease Evolution
Cases Continued
Patient A’s PV has been well controlled with PEG-IFN alfa-2a 90 μg subcutaneously weekly. However, he now presents with a complaint of worsening fatigue and early satiety. On exam the patient appears ill and splenomegaly is appreciated 12 cm below the costal margin. CBC shows a WBC count of 2600/µL, hemoglobin 73 g/L, and platelets 122 × 103/µL. Peripheral blood smear reveals leukoerythroblastosis and dacrocytosis. CBC 6 months ago was normal. A bone marrow biopsy is consistent with myelofibrosis.
After discontinuing hydroxyurea, patient B’s ET has been well controlled with anagrelide. However, for the past 4 weeks she has complained of severe fatigue and easy bruising. Physical exam reveals a pale, ill-appearing woman with scattered bruises. CBC shows a WBC count of 14,600/µL with 44% myeloblasts, hemoglobin 73 g/L, and platelets 22 × 103/µL. CBC 6 months ago was normal. A bone marrow biopsy is consistent with leukemic transformation of ET.
Post-PV/Post-ET Myelofibrosis
Diagnostic criteria for post-PV and post-ET myelofibrosis are outlined in Table 4.
Leukemic Transformation
The presence of more than 20% blasts in peripheral blood or bone marrow in a patient with MPN defines leukemic transformation. This occurs in up to 5% to 10% of patients and may or may not be preceded by a myelofibrosis phase.126 In cases of extramedullary transformation, a lower percentage of blasts can be seen in the bone marrow compared to the peripheral blood. The pathogenesis of leukemic transformation has remained elusive, but it is believed to be associated with genetic instability, which facilitates the acquisition of additional mutations, including those of TET2, ASXL1, EZH2 and DNMT3, IDH1/2, and TP53.127
Clinical risk factors for leukemic transformation include advanced age, karyotypic abnormalities, prior therapy with alkylating agents or P-32, splenectomy, increased peripheral blood or bone marrow blasts, leukocytosis, anemia, thrombocytopenia, and cytogenetic abnormalities. Hydroxyurea, interferon, and ruxolitinib have not been shown to have leukemogenic potential thus far. Prognosis of leukemic transformation is uniformly poor and patient survival rarely exceeds 6 months.
There is no standard of care for leukemic transformation of MPN (MPN-LT). Treatment options range from low-intensity regimens to more aggressive AML-type induction chemotherapy. No strategy appears clearly superior to others.128 Hematopoietic stem cell transplantation is the only therapy that provides clinically meaningful benefit to patients,129 but it is applicable only to a minority of patients with chemosensitive disease and good performance status.130 Notable experimental approaches to MPN–LT include hypomethylating agents, such as decitabine131 or azacitidine,132 with or without ruxolitinib.133-135
Conclusion
PV and ET are rare, chronic myeloid disorders. Patients typically experience a long clinical course and enjoy near-normal quality of life if properly managed. The 2 most important life-limiting complications of PV and ET are thrombohemorrhagic events and myelofibrosis/AML transformation. Vascular events are at least in part preventable with counseling on risk factors, phlebotomy (for patients with PV), antiplatelet therapy, and cytoreduction with hydroxyurea, IFNs, or anagrelide (for patients with ET). In addition, ruxolitinib was recently approved for PV patients after hydroxyurea failure. PV/ET transformation in myelofibrosis or AML is part of the natural history of the disease and no therapy has been shown to prevent it. Treatment follows recommendations set forth for PMF and AML, but results are generally poorer and novel strategies are needed to improve patients’ outcomes.
1. Swerdlow SH, Campo E, Harris NL, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon: IARC; 2008.
2. Alvarez-Larran A, Pereira A, Arellano-Rodrigo E, et al. Cytoreduction plus low-dose aspirin versus cytoreduction alone as primary prophylaxis of thrombosis in patients with high-risk essential thrombocythaemia: an observational study. Br J Haematol 2013;161:865–71.
3. Tefferi A. Polycythemia vera and essential thrombocythemia: 2013 update on diagnosis, risk-stratification, and management. Am J Hematol 2013;88:507–16.
4. Michiels JJ, Berneman Z, Schroyens W, et al. Platelet-mediated erythromelalgic, cerebral, ocular and coronary microvascular ischemic and thrombotic manifestations in patients with essential thrombocythemia and polycythemia vera: a distinct aspirin-responsive and coumadin-resistant arterial thrombophilia. Platelets 2006;17:528–44.
5. James C, Ugo V, Couedic J-PL, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature 2005;434:1144–8.
6. Kralovics R, Passamonti F, Buser A, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders.N Engl J Med 2005;352:1779–90.
7. Verstovsek S, Mesa R, Gotlib J, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med 2012;366:799–807.
8. Harrison C, Kiladjian J, Al-Ali H, et al. JAK Inhibition with ruxolitinib versus best available therapy for myelofibrosis.N Engl J Med 2012;366:787–98.
9. Berglund S, Zettervall O. Incidence of polycythemia vera in a defined population. Eur J Haematol 1992;48:20–6.
10. Rozman C, Giralt M, Feliu E, et al. Life expectancy of patients with chronic nonleukemic myeloproliferative disorders. Cancer 1991;67:2658–63.
11. Passamonti F, Rumi E, Pungolino E, et al. Life expectancy and prognostic factors for survival in patients with polycythemia vera and essential thrombocythemia. Am J Med 2004;117:755–61.
12. Hultcrantz M, Kristinsson SY, Andersson TM, et al. Patterns of survival among patients with myeloproliferative neoplasms diagnosed in Sweden from 1973 to 2008: a population-based study. J Clin Oncol 2012;30:2995–3001.
13. Wolanskyj AP, Schwager SM, et al. Essential thrombocythemia beyond the first decade: life expectancy, long-term complication rates, and prognostic factors. Mayo Clin Proc 2006;81:159–66.
14. Srour SA, Devesa SS, Morton LM, et al. Incidence and patient survival of myeloproliferative neoplasms and myelodysplastic/myeloproliferative neoplasms in the United States, 2001-12. Br J Haematol 2016;174:382–96.
15. Titmarsh GJ, Duncombe AS, McMullin MF, et al. How common are myeloproliferative neoplasms? A systematic review and meta-analysis. Am J Hematol 2014;89:581–7.
16. Moulard O, Mehta J, Fryzek J, et al. Epidemiology of myelofibrosis, essential thrombocythemia, and polycythemia vera in the European Union. Eur J Haematol 2014;92:289–97.
17. Stein BL, Gotlib J, Arcasoy M, et al. Historical views, conventional approaches, and evolving management strategies for myeloproliferative neoplasms. J Natl Compr Canc Netw 2015;13:424–34.
18. Levine RL, Wadleigh M, Cools J, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell 2005;7:387–97.
19. Baxter EJ, Scott LM, Campbell PJ, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet 2005;365:1054–61.
20. Scott LM, Tong W, Levine RL, et al. JAK2 exon 12 mutations in polycythemia vera and idiopathic erythrocytosis. N Engl J Med 2007;356:459–68.
21. Pardanani A, Lasho TL, Finke C, et al. Prevalence and clinicopathologic correlates of JAK2 exon 12 mutations in JAK2V617F-negative polycythemia vera. Leukemia 2007;21:1960–3.
22. Passamonti F, Elena C, Schnittger S, et al. Molecular and clinical features of the myeloproliferative neoplasm associated with JAK2 exon 12 mutations. Blood 2011;117:2813–6.
23. Abe M, Suzuki K, Inagaki O, et al. A novel MPL point mutation resulting in thrombopoietin-independent activation. Leukemia 2002;16:1500–6.
24. Pikman Y, Lee BH, Mercher T, et al. MPLW515L is a novel somatic activating mutation in myelofibrosis with myeloid metaplasia. PLoS Med 2006;3:e270.
25. Pardanani AD, Levine RL, Lasho T, et al. MPL515 mutations in myeloproliferative and other myeloid disorders: a study of 1182 patients. Blood 2006;108:3472–6.
26. Nangalia J, Massie CE, Baxter EJ, et al. Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. N Engl J Med 2013;369:2391–405.
27. Klampfl T, Gisslinger H, Harutyunyan AS, et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N Engl J Med 2013;369:2379–90.
28. Wang WA, Groenendyk J, Michalak M. Calreticulin signaling in health and disease. Int J Biochem Cell Biol 2012;44:842–6.
29. Saeidi K. Myeloproliferative neoplasms: Current molecular biology and genetics. Crit Rev Oncol Hematol 2016;98:375–89.
30. Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016;127:2391–405.
31. Mesa RA, Niblack J, Wadleigh M, et al. The burden of fatigue and quality of life in myeloproliferative disorders (MPDs): an international Internet-based survey of 1179 MPD patients. Cancer 2007;109:68–76.
32. Marchioli R, Finazzi G, Landolfi R, et al. Vascular and neoplastic risk in a large cohort of patients with polycythemia vera. J Clin Oncol 2005;23:2224–32.
33. Scherber R, Dueck AC, Johansson P, et al. The Myeloproliferative Neoplasm Symptom Assessment Form (MPN-SAF): international prospective validation and reliability trial in 402 patients. Blood 2011;118:401–8.
34. Emanuel RM, Dueck AC, Geyer HL, et al. Myeloproliferative neoplasm (MPN) symptom assessment form total symptom score: prospective international assessment of an abbreviated symptom burden scoring system among patients with MPNs. J Clin Oncol 2012;30:4098–103.
35. Casini A, Fontana P, Lecompte TP. Thrombotic complications of myeloproliferative neoplasms: risk assessment and risk-guided management. J Thromb Haemost 2013;11:1215–27.
36. Barbui T, Finazzi G, Falanga A. Myeloproliferative neoplasms and thrombosis. Blood 2013;122:2176-–84.
37. Pearson TC, Wetherley-Mein G. Vascular occlusive episodes and venous haematocrit in primary myeloproliferative polychytemia. Lancet 1978;2:1219–22.
38. Landolfi R, Di Gennaro L, Barbui T, et al. Leukocytosis as a major thrombotic risk factor in patients with polycythemia vera. Blood 2007;109:2446–52.
39. Tefferi A, Rumi E, Finazzi G, et al. Survival and prognosis among 1545 patients with contemporary polycythemia vera: an international study. Leukemia 2013;27:1874–81.
40. Barbui T, Falanga A. Molecular biomarkers of thrombosis in myeloproliferative neoplasms. Thromb Res 2016;140 Suppl 1:S71–75.
41. Campbell PJ, Scott LM, Buck G, et al. Definition of subtypes of essential thrombocythaemia and relation to polycythaemia vera based on JAK2 V617F mutation status: a prospective study. Lancet 2005;366:1945–53.
42. Tefferi A, Guglielmelli P, Larson DR, et al. Long-term survival and blast transformation in molecularly annotated essential thrombocythemia, polycythemia vera, and myelofibrosis. Blood 2014;124:2507–13.
43. Qin Y, Wang X, Zhao C, et al. The impact of JAK2V617F mutation on different types of thrombosis risk in patients with essential thrombocythemia: a meta-analysis. Int J Hematol 2015;102:170–80.
44. Ziakas PD. Effect of JAK2 V617F on thrombotic risk in patients with essential thrombocythemia: measuring the uncertain. Haematologica 2008;93:1412–4.
45. Lussana F, Dentali F, Abbate R, et al. Screening for thrombophilia and antithrombotic prophylaxis in pregnancy: Guidelines of the Italian Society for Haemostasis and Thrombosis (SISET). Thromb Res 2009;124:e19–25.
46. Dahabreh IJ, Zoi K, Giannouli S, et al. Is JAK2 V617F mutation more than a diagnostic index? A meta-analysis of clinical outcomes in essential thrombocythemia. Leuk Res 2009;33:67–73.
47. Cho YU, Chi HS, Lee EH, et al. Comparison of clinicopathologic findings according to JAK2 V617F mutation in patients with essential thrombocythemia. Int J Hematol 2009;89:39–44.
48. Wolanskyj AP, Lasho TL, Schwager SM, et al. JAK2 mutation in essential thrombocythaemia: clinical associations and long-term prognostic relevance. Br J Haematol 2005;131:208–13.
49. Antonioli E, Guglielmelli P, Pancrazzi A, et al. Clinical implications of the JAK2 V617F mutation in essential thrombocythemia. Leukemia 2005;19:1847–9.
50. Palandri F, Catani L, Testoni N, et al. Long-term follow-up of 386 consecutive patients with essential thrombocythemia: safety of cytoreductive therapy. Am J Hematol 2009;84:215–20.
51. Chim CS, Sim JP, Chan CC, et al. Impact of JAK2V617F mutation on thrombosis and myeloid transformation in essential thrombocythemia: a multivariate analysis by Cox regression in 141 patients. Hematology 2010;15:187–92.
52. Vannucchi AM, Antonioli E, Guglielmelli P, et al. Clinical profile of homozygous JAK2 617V>F mutation in patients with polycythemia vera or essential thrombocythemia. Blood 2007;110:840–6.
53. Carobbio A, Finazzi G, Antonioli E, et al. JAK2V617F allele burden and thrombosis: a direct comparison in essential thrombocythemia and polycythemia vera. Exp Hematol 2009;37:1016–21.
54. Alvarez-Larran A, Bellosillo B, Pereira A, et al. JAK2V617F monitoring in polycythemia vera and essential thrombocythemia: clinical usefulness for predicting myelofibrotic transformation and thrombotic events. Am J Hematol 2014;89:517–23.
55. Barbui T, Vannucchi AM, Buxhofer-Ausch V, et al. Practice-relevant revision of IPSET-thrombosis based on 1019 patients with WHO-defined essential thrombocythemia. Blood Cancer J 2015;5:e369.
56. Carobbio A, Thiele J, Passamonti F, et al. Risk factors for arterial and venous thrombosis in WHO-defined essential thrombocythemia: an international study of 891 patients. Blood 2011;117:5857–9.
57. Alvarez-Larran A, Cervantes F, Bellosillo B, et al. Essential thrombocythemia in young individuals: frequency and risk factors for vascular events and evolution to myelofibrosis in 126 patients. Leukemia 2007;21:1218–23.
58. Jantunen R, Juvonen E, Ikkala E, et al. The predictive value of vascular risk factors and gender for the development of thrombotic complications in essential thrombocythemia. Ann Hematol 2001;80:74–8.
59. Besses C, Cervantes F, Pereira A, et al. Major vascular complications in essential thrombocythemia: a study of the predictive factors in a series of 148 patients. Leukemia 1999;13:150–4.
60. Haider M, Gangat N, Hanson C, Tefferi A. Splenomegaly and thrombosis risk in essential thrombocythemia: the mayo clinic experience. Am J Hematol 2016;91:E296–297.
61. Carobbio A, Finazzi G, Antonioli E, et al. Thrombocytosis and leukocytosis interaction in vascular complications of essential thrombocythemia. Blood 2008;112:3135–7.
62. Palandri F, Polverelli N, Catani L, et al. Impact of leukocytosis on thrombotic risk and survival in 532 patients with essential thrombocythemia: a retrospective study. Ann Hematol 2011;90:933–8.
63. Campbell PJ, MacLean C, Beer PA, et al. Correlation of blood counts with vascular complications in essential thrombocythemia: analysis of the prospective PT1 cohort. Blood 2012;120:1409–11.
64. Cortelazzo S, Finazzi G, Ruggeri M, et al. Hydroxyurea for patients with essential thrombocythemia and a high risk of thrombosis. N Engl J Med 1995;332:1132–6.
65. van Genderen PJ, Mulder PG, Waleboer M, et al. Prevention and treatment of thrombotic complications in essential thrombocythaemia: efficacy and safety of aspirin. Br J Haematol 1997;97:179–84.
66. Storen EC, Tefferi A. Long-term use of anagrelide in young patients with essential thrombocythemia. Blood 2001;97:863–6.
67. De Stefano V, Za T, Rossi E, et al. Recurrent thrombosis in patients with polycythemia vera and essential thrombocythemia: incidence, risk factors, and effect of treatments. Haematologica 2008;93:372–80.
68. Alvarez-Larran A, Cervantes F, Pereira A, et al. Observation versus antiplatelet therapy as primary prophylaxis for thrombosis in low-risk essential thrombocythemia. Blood 2010;116:1205–10.
69. Palandri F, Polverelli N, Catani L, et al. Bleeding in essential thrombocythaemia: a retrospective analysis on 565 patients. Br J Haematol 2012;156:281–4.
70. Rotunno G, Mannarelli C, Guglielmelli P, et al. Impact of calreticulin mutations on clinical and hematological phenotype and outcome in essential thrombocythemia. Blood 2014;123:1552–5.
71. Tefferi A, Wassie EA, Lasho TL, et al. Calreticulin mutations and long-term survival in essential thrombocythemia. Leukemia 2014;28:2300–3.
72. Rumi E, Pietra D, Ferretti V, et al. JAK2 or CALR mutation status defines subtypes of essential thrombocythemia with substantially different clinical course and outcomes. Blood 2014;123:1544–51.
73. Palandri F, Latagliata R, Polverelli N, et al. Mutations and long-term outcome of 217 young patients with essential thrombocythemia or early primary myelofibrosis. Leukemia 2015;29:1344–9.
74. Fu R, Xuan M, Zhou Y, et al. Analysis of calreticulin mutations in Chinese patients with essential thrombocythemia: clinical implications in diagnosis, prognosis and treatment. Leukemia 2014;28:1912–4.
75. Tefferi A, Wassie EA, Guglielmelli P, et al. Type 1 versus Type 2 calreticulin mutations in essential thrombocythemia: a collaborative study of 1027 patients. Am J Hematol 2014;89:E121–4.
76. Pietra D, Rumi E, Ferretti VV, et al. Differential clinical effects of different mutation subtypes in CALR-mutant myeloproliferative neoplasms. Leukemia 2016;30:431–8.
77. Rumi E, Pietra D, Guglielmelli P, et al. Acquired copy-neutral loss of heterozygosity of chromosome 1p as a molecular event associated with marrow fibrosis in MPL-mutated myeloproliferative neoplasms. Blood 2013;121:4388–95.
78. Beer PA, Campbell PJ, Scott LM, et al. MPL mutations in myeloproliferative disorders: analysis of the PT-1 cohort. Blood 2008;112:141–9.
79. Gangat N, Wassie EA, Lasho TL, et al. Mutations and thrombosis in essential thrombocythemia: prognostic interaction with age and thrombosis history. Eur J Haematol 2015;94:31–6.
80. Sekhar M, McVinnie K, Burroughs AK. Splanchnic vein thrombosis in myeloproliferative neoplasms. Br J Haematol 2013;162:730–47.
81. Stein BL, Saraf S, Sobol U, et al. Age-related differences in disease characteristics and clinical outcomes in polycythemia vera. Leuk Lymph 2013;54:1989–95.
82. Landolfi R, Di Gennaro L, Nicolazzi MA, et al. Polycythemia vera: gender-related phenotypic differences. Intern Emerg Med 2012;7:509–15.
83. Winslow ER, Brunt LM, Drebin JA, et al. Portal vein thrombosis after splenectomy. Am J Surg 2002;184:631–6.
84. Smalberg JH, Arends LR, Valla DC, et al. Myeloproliferative neoplasms in Budd-Chiari syndrome and portal vein thrombosis: a meta-analysis. Blood 2012;120:4921–8.
85. Dentali F, Squizzato A, Brivio L, et al. JAK2V617F mutation for the early diagnosis of Ph- myeloproliferative neoplasms in patients with venous thromboembolism: a meta-analysis. Blood 2009;113:5617–23.
86. Pardanani A, Lasho TL, Hussein K, et al. JAK2V617F mutation screening as part of the hypercoagulable work-up in the absence of splanchnic venous thrombosis or overt myeloproliferative neoplasm: assessment of value in a series of 664 consecutive patients. Mayo Clin Proc 2008;83:457–9.
87. Barbui T, Barosi G, Birgegard G, et al. Philadelphia-negative classical myeloproliferative neoplasms: critical concepts and management recommendations from European LeukemiaNet. J Clin Oncol 2011;29:761–70.
88. Landolfi R, Marchioli R, Kutti J, et al. Efficacy and safety of low-dose aspirin in polycythemia vera. N Engl J Med 2004;350:114–24.
89. Marchioli R, Finazzi G, Specchia G, et al. Cardiovascular events and intensity of treatment in polycythemia vera. N Engl J Med 2013;368:22–33.
90. Kiladjian JJ, Chevret S, Dosquet C, et al. Treatment of polycythemia vera with hydroxyurea and pipobroman: final results of a randomized trial initiated in 1980. J Clin Oncol 2011;29:3907–13.
91. Kaplan ME, Mack K, Goldberg JD, et al. Long-term management of polycythemia vera with hydroxyurea: a progress report. Semin Hematol 1986;23:167–71.
92. Fruchtman SM, Mack K, Kaplan ME, et al. From efficacy to safety: a Polycythemia Vera Study group report on hydroxyurea in patients with polycythemia vera. Semin Hematol 1997;34:17–23.
93. Finazzi G, Caruso V, Marchioli R, et al. Acute leukemia in polycythemia vera: an analysis of 1638 patients enrolled in a prospective observational study. Blood 2005;105:2664–70.
94. Barosi G, Mesa R, Finazzi G, et al. Revised response criteria for polycythemia vera and essential thrombocythemia: an ELN and IWG-MRT consensus project. Blood 2013;121:4778–81.
95. Alvarez-Larran A, Pereira A, Cervantes F, et al. Assessment and prognostic value of the European LeukemiaNet criteria for clinicohematologic response, resistance, and intolerance to hydroxyurea in polycythemia vera. Blood 2012;119:1363–9.
96. Stein BL, Tiu RV. Biological rationale and clinical use of interferon in the classical BCR-ABL-negative myeloproliferative neoplasms. J Interferon Cytokine Res 2013;33:145–53.
97. Ludwig H, Cortelezzi A, Van Camp BG, et al. Treatment with recombinant interferon-alpha-2C: multiple myeloma and thrombocythaemia in myeloproliferative diseases. Oncology 1985;42 Suppl 1:19–25.
98. Silver RT. Long-term effects of the treatment of polycythemia vera with recombinant interferon-alpha. Cancer 2006;107:451–8.
99. Kiladjian JJ, Mesa RA, Hoffman R. The renaissance of interferon therapy for the treatment of myeloid malignancies. Blood 2011;117:4706–15.
100. Veronese FM, Mero A. The impact of PEGylation on biological therapies. BioDrugs 2008;22:315–29.
101. Kiladjian JJ, Cassinat B, Chevret S, et al. Pegylated interferon-alfa-2a induces complete hematologic and molecular responses with low toxicity in polycythemia vera. Blood 2008;112:3065–72.
102. Turlure P, Cambier N, Roussel M, et al. Complete hematological, molecular and histological remissions without cytoreductive treatment lasting after pegylated-interferon {alpha}-2a (peg-IFN{alpha}-2a) therapy in polycythemia vera (PV): long term results of a phase 2 trial [abstract]. Blood 2011;118(21). Abstract 280.
103. Quintas-Cardama A, Kantarjian H, Manshouri T, et al. Pegylated interferon alfa-2a yields high rates of hematologic and molecular response in patients with advanced essential thrombocythemia and polycythemia vera. J Clin Oncol 2009;27:5418–24.
104. Quintas-Cardama A, Abdel-Wahab O, Manshouri T, et al. Molecular analysis of patients with polycythemia vera or essential thrombocythemia receiving pegylated interferon a-2a. Blood 2013;122:893–901.
105. Samuelsson J, Hasselbalch H, Bruserud O, et al. A phase II trial of pegylated interferon alpha-2b therapy for polycythemia vera and essential thrombocythemia: feasibility, clinical and biologic effects, and impact on quality of life. Cancer 2006;106:2397–405.
106. Jabbour E, Kantarjian H, Cortes J, et al. PEG-IFN-alpha-2b therapy in BCR-ABL-negative myeloproliferative disorders: final result of a phase 2 study. Cancer 2007;110:2012–18.
107. Them NC, Bagienski K, Berg T, et al. Molecular responses and chromosomal aberrations in patients with polycythemia vera treated with peg-proline-interferon alpha-2b. Am J Hematol 2015;90:288–94.
108. Gisslinger H, Klade C, Georgiev P, et al. Final results from PROUD-PV a randomized controlled phase 3 trial comparing ropeginterferon alfa-2b to hydroxyurea in polycythemia vera patients [abstract]. Blood 2016;128(suppl 22). Abstract 475.
109. van Genderen PJ, van Vliet HH, Prins FJ, et al. Excessive prolongation of the bleeding time by aspirin in essential thrombocythemia is related to a decrease of large von Willebrand factor multimers in plasma. Ann Hematol 1997;75:215–20.
110. Cortelazzo S, Finazzi G, Ruggeri M, et al. Hydroxyurea for patients with essential thrombocythemia and a high risk of thrombosis. N Engl J Med 1995;332:1132–7.
111. Harrison CN, Campbell PJ, Buck G, et al. Hydroxyurea compared with anagrelide in high-risk essential thrombocythemia. N Engl J Med 2005;353:33–45.
112. Gisslinger H, Gotic M, Holowiecki J, et al. Anagrelide compared with hydroxyurea in WHO-classified essential thrombocythemia: the ANAHYDRET Study, a randomized controlled trial. Blood 2013;121:1720–8.
113. Alvarado Y, Cortes J, Verstovsek S, et al. Pilot study of pegylated interferon-alpha 2b in patients with essential thrombocythemia. Cancer Chemother Pharmacol 2003;51:81–6.
114. Barosi G, Tefferi A, Barbui T, ad hoc committee ‘Definition of clinically relevant outcomes for contemporarily clinical trials in Ph-neg M. Do current response criteria in classical Ph-negative myeloproliferative neoplasms capture benefit for patients? Leukemia 2012;26:1148–9.
115. Bjorkholm M, Derolf AR, Hultcrantz M, et al. Treatment-related risk factors for transformation to acute myeloid leukemia and myelodysplastic syndromes in myeloproliferative neoplasms. J Clin Oncol 2011;29:2410–5.
116. Alvarez-Larran A, Martinez-Aviles L, Hernandez-Boluda JC, et al. Busulfan in patients with polycythemia vera or essential thrombocythemia refractory or intolerant to hydroxyurea. Ann Hematol 2014;93:2037–43.
117. Verstovsek S, Passamonti F, Rambaldi A, et al. A phase 2 study of ruxolitinib, an oral JAK1 and JAK2 Inhibitor, in patients with advanced polycythemia vera who are refractory or intolerant to hydroxyurea. Cancer 2014;120:513–20.
118. Vannucchi AM, Kiladjian JJ, Griesshammer M, et al. Ruxolitinib in polycythemia vera resistant to or intolerant of hydroxyurea. N Engl J Med 2015; 372:426–35.
119. Verstovsek S, Vannucchi AM, Griesshammer M, et al. Ruxolitinib versus best available therapy in patients with polycythemia vera: 80-week follow-up from the RESPONSE trial. Haematologica 2016;101:821–9.
120. Passamonti F, Griesshammer M, Palandri F, et al. Ruxolitinib for the treatment of inadequately controlled polycythaemia vera without splenomegaly (RESPONSE-2): a randomised, open-label, phase 3b study. Lancet Oncol 2017;18:88–99.
121. Verstovsek S, Passamonti F, Rambaldi A, et al. Long-term results from a phase II open-label study of ruxolitinib in patients with essential thrombocythemia refractory to or intolerant of hydroxyurea [abstract]. Blood 2014;124. Abstract 1847.
122. Harrison CN, Mead AJ, Panchal A, et al. Ruxolitinib versus best available therapy for ET intolerant or resistant to hydroxycarbamide in a randomized trial. Blood 2017 Aug 9. pii: blood-2017-05-785790 .
123. Bose P, Verstovsek S. Drug development pipeline for myeloproliferative neoplasms: potential future impact on guidelines and management. J Natl Compr Canc Netw 2016;14:1613–24.
124. Cerquozzi S, Teffieri A. Blast transformation and fibrotic progression in polycythemia vera and essential thrombocythemia: a literature review of incidence and risk factors. Blood Cancer J 2015;Nov 13;5:e366.
125. Passamonti F, Rumi E, Caramella M, et al. A dynamic prognostic model to predict survival in post-polycythemia vera myelofibrosis. Blood 2008;111:3383–7.
126. Mesa RA, Verstovsek S, Cervantes F, et al. Primary myelofibrosis (PMF), post polycythemia vera myelofibrosis (post-PV MF), post essential thrombocythemia myelofibrosis (post-ET MF), blast phase PMF (PMF-BP): Consensus on terminology by the international working group for myelofibrosis research and treatment (IWG-MRT). Leuk Res 2007;31:737–40.
127. Rampal R, Mascarenhas J. Pathogenesis and management of acute myeloid leukemia that has evolved from a myeloproliferative neoplasm. Curr Opin Hematol 2014;21:65–71.
128. Chihara D, Kantarjian HM, Newberry KJ, et al. Survival outcome of patients with acute myeloid leukemia transformed from myeloproliferative neoplasms [abstract]. Blood 2016;128. Abstract 1940.
129. Tam CS, Nussenzveig RM, Popat U, et al. The natural history and treatment outcome of blast phase BCR-ABL- myeloproliferative neoplasms. Blood 2008;112:1628–37.
130. Kundranda MN, Tibes R, Mesa RA. Transformation of a chronic myeloproliferative neoplasm to acute myelogenous leukemia: does anything work? Curr Hematol Malig Rep 2012;7:78–86.
131. Badar T, Kantarjian HM, Ravandi F, et al. Therapeutic benefit of decitabine, a hypomethylating agent, in patients with high-risk primary myelofibrosis and myeloproliferative neoplasm in accelerated or blastic/acute myeloid leukemia phase. Leuk Res 2015;39:950–6.
132. Thepot S, Itzykson R, Seegers V, et al. Treatment of progression of Philadelphia-negative myeloproliferative neoplasms to myelodysplastic syndrome or acute myeloid leukemia by azacitidine: a report on 54 cases on the behalf of the Groupe Francophone des Myelodysplasies (GFM). Blood 2010;116:3735–42.
133. Pemmaraju N, Kantarjian H, Kadia T, et al. A phase I/II study of the Janus kinase (JAK)1 and 2 inhibitor ruxolitinib in patients with relapsed or refractory acute myeloid leukemia. Clin Lymphoma Myeloma Leuk 2015;15:171–6.
134. Rampal RK, Mascarenhas JO, Kosiorek HE, et al. Safety and efficacy of combined ruxolitinib and decitabine in patients with blast-phase MPN and post-MPN AML: results of a phase I study (Myeloproliferative Disorders Research Consortium 109 trial) [abstract]. Blood 2016;128. Abstract 1124.
135. Bose P, Verstovsek S, Gasior Y, et al. Phase I/II study of ruxolitinib (RUX) with decitabine (DAC) in patients with post-myeloproliferative neoplasm acute myeloid leukemia (post-MPN AML): phase I results [abstract]. Blood 2016;128. Abstract 4262.
Introduction
Polycythemia vera (PV) and essential thrombocythemia (ET), along with primary myelofibrosis (PMF), belong to the group of Philadelphia-negative myeloproliferative neoplasms (MPN). All these malignancies arise from the clonal proliferation of an aberrant hematopoietic stem cell, but are characterized by distinct clinical phenotypes.1,2 Although the clinical course of PV and ET is indolent, it can be complicated by thrombohemorrhagic episodes and/or evolution into myelofibrosis and/or acute myeloid leukemia (AML).3 Since vascular events are the most frequent life-threatening complications of PV and ET, therapeutic strategies are aimed at reducing this risk. Treatment may also help control other disease-associated symptoms.4 No therapy has been shown to prevent evolution of PV or ET into myelofibrosis or AML. The discovery of the Janus kinase 2 (JAK2)/V617F mutation in most patients with PV and over half of those with ET (and PMF)5,6 has opened new avenues of research and led to the development of targeted therapies, such as the JAK1/2 inhibitor ruxolitinib, for patients with MPN.7,8
Epidemiology
PV and ET are typically diagnosed in the fifth to seventh decade of life.9 Although these disorders are generally associated with a long clinical course, survival of patients with PV or ET may be shorter than that of the general population.10–13 Estimating the incidence and prevalence of MPN is a challenge because most patients remain asymptomatic for long periods of time and do not seek medical attention.13 The annual incidence rates of PV and ET are estimated at 0.01 to 2.61 and 0.21 to 2.53 per 100,000, respectively. PV occurs slightly more frequently in males, whereas ET has a predilection for females.14 Given the long course and low mortality associated with these disorders, the prevalence of PV and ET are significantly higher than the respective incidence: up to 47 and 57 per 100,000, respectively.15–17
Molecular Pathogenesis
In 2005 researchers discovered a gain-of-function mutation of the JAK2 gene in nearly all patients with PV and more than half of those with ET and PMF.5,6,18,19 JAK2 is a non-receptor tyrosine kinase that plays a central role in normal hematopoiesis. Substitution of a valine for a phenylalanine at codon 617 (ie, V617F) leads to its constitutive activation and signaling through the JAK-STAT pathway.5,6,18,19 More rarely (and exclusively in patients with PV), JAK2 mutations involve exon 12.20–22 The vast majority of JAK2-negative ET patients harbor mutations in either the myeloproliferative leukemia (MPL) gene, which encodes the thrombopoietin receptor,23–25 or the calreticulin (CALR) gene,26,27 which encodes for a chaperone protein that plays a role in cellular proliferation, differentiation, and apoptosis.28 Both the MPL and CALR mutations ultimately result in the constitutive activation of the JAK-STAT pathway. Thus, JAK2, MPL, and CALR alterations are collectively referred to as driver mutations. Moreover, because these mutations affect the same oncogenic pathway (ie, JAK-STAT), they are almost always mutually exclusive in a given patient. Patients with ET (or myelofibrosis) who are wild-type for JAK2, MPL, and CALR are referred to as having “triple-negative” disease. Many recurrent non-driver mutations are also found in patients with MPN that are not exclusive of each other (ie, patients may have many at the same time), and involve for example ten-eleven translocation-2 (TET2), additional sex combs like 1 (ASXL1), enhancer of zeste homolog 2 (EZH2), isocitrate dehydrogenase 1 and isocitrate dehydrogenase 2 (IDH1/2), and DNA methyltransferase 3A (DNMT3A) genes, among others.29 The biologic and prognostic significance of these non-driver alterations remain to be fully defined in ET and PV.
Diagnosis and Risk Assessment
Case Presentations
Patient A is a 68-year-old man with a history of gouty arthritis who presents with a 6-month history of recurrent headaches and itching that increases after a hot shower. Over the past 2 months, he has also noticed worsening fatigue and redness of his face. He is a nonsmoker. Physical exam reveals erythromelalgia (ie, erythema, edema, and warmth) of the upper and lower extremities, scattered scratch marks, and splenomegaly 4 cm below the costal margin. Complete blood count (CBC) shows a white blood cell (WBC) count of 8100/µL, hemoglobin 194 g/L, and platelets 582 × 103/µL. Serum erythropoietin level is decreased at 2 mU/mL. Peripheral blood testing reveals a JAK2V617F mutation.
Patient B is a 51-year-old woman with a history of severe depression treated with sertraline and hypertension controlled with lisinopril and amlodipine who presents to her primary care physician for her “50-year-old physical.” She denies symptoms and is a nonsmoker. Physical exam is unrevealing. CBC shows a WBC count of 7400/µL (normal differential), hemoglobin 135 g/L, and platelets 1282 × 103/µL. A bone marrow biopsy shows normal cellularity with clusters of large, hyperlobulated megakaryocytes. Reverse transcriptase-polymerase chain reaction fails to reveal a BCR-ABL fusion product. The patient is diagnosed with ET.
Diagnostic Criteria
Diagnostic criteria for PV and ET according to the World Health Organization (WHO) classification30 are summarized in Table 1. Criteria for the diagnosis of prefibrotic myelofibrosis are included as well since this entity was formally recognized as separate from ET and part of the PMF spectrum in the 2016 WHO classification of myeloid tumors.30
Risk Stratification
Thrombohemorrhagic events, evolution into myelofibrosis, and leukemic transformation are the most serious complications in the course of PV or ET. Only thrombohemorrhagic events are, at least partially, preventable. Arterial or venous thrombotic complications are observed at rates of 1.8 to 10.9 per 100 patient-years in PV (arterial thrombosis being more common than venous) and 0.74 to 7.7 per 100 patient-years in ET, depending on the risk group35 and the presence of other factors (see below).
Thrombosis Risk Stratification in PV
The risk stratification of patients with PV is based on 2 factors: age ≥ 60 years and prior history of thrombosis. If either is present the patient is assigned to the high-risk category, whereas if none is present the patient is considered at low risk.36 In addition, high hematocrit37 and high WBC,38 but not thrombocytosis, have been associated with the development of vascular complications. In one study, the risk of new arterial thrombosis was increased by the presence of leukoerythroblastosis, hypertension, and prior arterial thrombosis, while karyotypic abnormalities and prior venous thrombosis were predictors of new venous thrombosis.39 Another emerging risk factor for thrombosis in patients with PV is high JAK2 allele burden (ie, the normal-to-mutated gene product ratio), although the evidence supporting this conclusion is equivocal.40
Thrombosis Risk Stratification in ET
Traditionally, in ET patients, thrombotic risk was assessed using the same 2 factors (age ≥ 60 years and prior history of thrombosis), separating patients into low- and high-risk groups. However, the prognostication of ET patients has been refined recently with the identification of new relevant factors. In particular, the impact of JAK2 mutations on thrombotic risk has been thoroughly studied. Clinically, the presence of JAK2V617F is associated with older age, higher hemoglobin and hematocrit, lower platelet counts, more frequent need for cytoreductive treatment, and greater tendency to evolve into PV (a rare event).41,42 Many,41,43–46 but not all,47–51 studies suggested a correlation between JAK2 mutation and risk of both arterial and venous thrombosis. Although infrequent, a JAK2V617F homozygous state (ie, the mutation is present in both alleles) might confer an even higher thrombotic risk.52 Moreover, the impact of the JAK2 mutation on vascular events persists over time,53 particularly in patients with high or unstable mutation burden.54 Based on JAK2V617F’s influence on the thrombotic risk of ET patients, a new prognostic score was proposed, the International Prognostic Score for ET (IPSET)-thrombosis (Table 2). The revised version of this model is currently endorsed by the National Comprehensive Cancer Network and divides patients into 4 risk groups: high, intermediate, low, and very low. Treatment recommendations vary according to the risk group (as described below).55
Other thrombotic risk factors have been identified, but deemed not significant enough to be included in the model. Cardiovascular risk factors (hypercholesterolemia, hypertension, smoking, diabetes mellitus) can increase the risk of vascular events,56–59 as can splenomegaly60 and baseline or persistent leukocytosis.61–63 Thrombocytosis has been correlated with thrombotic risk in some studies,64–68 whereas others did not support this conclusion and/or suggested a lower rate of thrombosis and, in some cases, increased risk of bleeding in ET patients with platelet counts greater than 1000 × 103/µL (due to acquired von Willebrand syndrome).56,61,63,68,69
CALR mutations tend to occur in younger males with lower hemoglobin and WBC count, higher platelet count, and greater marrow megakaryocytic predominance as compared to JAK2 mutations.26,27,70–72 The associated incidence of thrombosis was less than 10% at 15 years in patients with CALR mutations, lower than the incidence reported for ET patients with JAK2V617F mutations.73 The presence of the mutation per se does not appear to affect the thrombotic risk.74–76 Information on the thrombotic risk associated with MPL mutations or a triple-negative state is scarce. In both instances, however, the risk appears to be lower than with the JAK2 mutation.73,77–79
Venous thromboembolism in patients with PV or ET may occur at unusual sites, such as the splanchnic or cerebral venous systems.80 Risk factors for unusual venous thromboembolism include younger age,81 female gender (especially with concomitant use of oral contraceptive pills),82 and splenomegaly/splenectomy.83JAK2 mutation has also been associated with thrombosis at unusual sites. However, the prevalence of MPN or JAK2V617F in patients presenting with splanchnic venous thromboembolism has varied.80 In addition, MPN may be occult (ie, no clinical or laboratory abnormalities) in around 15% of patients.84 Screening for JAK2V617F and underlying MPN is recommended in patients presenting with isolated unexplained splanchnic venous thromboembolism. Treatment entails long-term anticoagulation therapy. JAK2V617F screening in patients with nonsplanchnic venous thromboembolism is not recommended, as its prevalence in this group is low (< 3%).85,86
Treatment
Cases Continued
Patient A is diagnosed with PV based on the presence of 2 major criteria (elevated hemoglobin and presence of the JAK2V617F mutation) and 1 minor criterion (low erythropoietin level). Given his age, he belongs to the high-risk disease category. He is now seeking advice regarding the management of his newly diagnosed PV.
Patient B presents to the emergency department with right lower extremity swelling and is found to have deep femoral thrombosis extending to the iliac vein. Five days after being discharged from the emergency department, she presents for follow-up. She is taking warfarin compliantly and her INR is within therapeutic range. The patient now has high-risk ET and would like to know more about thrombosis in her condition and how to best manage her risk.
Risk-Adapted Therapy
Low-Risk PV
All patients with PV should receive counseling to mitigate cardiovascular risk factors, including smoking cessation, lifestyle modifications, and lipid-lowering therapy, as indicated. Furthermore, all PV patients should receive acetylsalicylic acid (ASA) to decrease their risk for thrombosis and control vasomotor symptoms.55,87 Aspirin 81 to 100 mg daily is the preferred regimen because it provides adequate antithrombotic effect without the associated bleeding risk of higher-dose aspirin.88 Low-risk PV patients should also receive periodic phlebotomies to reduce and maintain their hematocrit below 45%. This recommendation is based on the results of the Cytoreductive Therapy in Polycythemia Vera (CYTO PV) randomized controlled trial. In the CYTO PV study, patients receiving more intense therapy to maintain the hematocrit below 45% had a lower incidence of cardiovascular-related deaths or major thrombotic events than those with hematocrit goals of 45% to 50% (2.7% versus 9.8%).89 Cytoreduction is an option for low-risk patients who do not tolerate phlebotomy or require frequent phlebotomy, or who have disease-related bleeding, severe symptoms, symptomatic splenomegaly, or progressive leukocytosis.38
High-Risk PV
Patients older than 60 years and/or with a history of thrombosis should be considered for cytoreductive therapy in addition to the above measures. Front-line cytoreductive therapies include hydroxyurea or interferon (IFN)- alfa.87 Hydroxyurea is a potent ribonucleotide reductase inhibitor that interferes with DNA repair and is the treatment of choice for most high-risk patients with PV.90 In a small trial hydroxyurea reduced the risk of thrombosis compared with historical controls treated with phlebotomy alone.91 Hydroxyurea is generally well tolerated; common side effects include cytopenias, nail changes, and mucosal and/or skin ulcers. Although never formally proven to be leukemogenic, this agent should be used with caution in younger patients.87 Indeed, in the original study, the rates of transformation were 5.9% and 1.5% for patients receiving hydroxyurea and phlebotomy alone,92 respectively, although an independent role for hydroxyurea in leukemic transformation was not supported in the much larger European Collaboration on Low-dose Aspirin in Polycythemia Vera (ECLAP) study.93 About 70% of patients will have a sustained response to hydroxyurea,94 while the remaining patients become resistant to or intolerant of the drug. Resistant individuals have a higher risk of progression to acute leukemia and death.95
IFN alfa is a pleiotropic antitumor agent that has found application in many types of malignancies96 and is sometimes employed as treatment for patients with newly diagnosed high-risk PV. Early studies showed responses in up to 100% of cases,97,98 albeit at the expense of a high discontinuation rate due to adverse events, such as flu-like symptoms, fatigue, and neuropsychiatric manifestations.99 A newer formulation of the drug obtained by adding a polyethylene glycol (PEG) moiety to the native IFN alfa molecule (PEG-IFN alfa) was shown to have a longer half-life, greater stability, less immunogenicity, and, potentially, better tolerability.100 Pilot phase 2 trials of PEG-IFN alfa-2a demonstrated its remarkable activity, with symptomatic and hematologic responses seen in the majority of patients (which, in some cases, persisted beyond discontinuation), and reasonable tolerability, with long-term discontinuation rates of around 20% to 30%.101–103 In some patients JAK2V617F became undetectable over time.104 Results of 2 ongoing trials, MDP-RC111 (single-arm study, PEG-IFN alfa-2a in high-risk PV or ET [NCT01259817]) and MPD-RC112 (randomized controlled trial, PEG-IFN alfa-2a versus hydroxyurea in the same population [NCT01258856]), will shed light on the role of PEG-IFN alfa in the management of patients with high-risk PV or ET. In 2 phase 2 studies of PEG-IFN alfa-2b, complete responses were seen in 70% to 100% of patients and discontinuation occurred in around a third of cases.105,106 A new, longer-acting formulation of PEG-IFN alfa-2a (peg-proline INF alfa-2b, AOP2014) is also undergoing clinical development.107,108
The approach to treatment of PV based on thrombotic risk level is illustrated in Figure 1.
Very Low- and Low-Risk ET
Like patients with PV, individuals with ET should undergo rigorous cardiovascular risk management and generally receive ASA to decrease their thrombotic risk and improve symptom control. Antiplatelet therapy may not be warranted in patients with documented
Intermediate-Risk ET
This category includes patients older than 60 years but without thrombosis or JAK2 mutations. These individuals would have been considered high risk (and thus candidates for cytoreductive therapy) according to the traditional risk stratification. Guidelines currently recommend ASA as the sole therapy for these patients, while reserving cytoreduction for those who experience thrombosis (ie, become high-risk) or have uncontrolled vasomotor or general symptoms, symptomatic splenomegaly, symptomatic thrombocytosis, or progressive leukocytosis.
High-Risk ET
For patients with ET in need of cytoreductive therapy (ie, those with prior thrombosis or older than 60 years with a JAK2V617F mutation), first-line options include hydroxyurea, IFN, and anagrelide. Hydroxyurea remains the treatment of choice in the majority of patients.110 In a seminal study, 114 patients with ET were randomly assigned to either observation or hydroxyurea treatment with the goal of maintaining the platelet count below 600 × 103/µL. At a median follow-up of 27 months, patients in the hydroxyurea group had a lower thrombosis rate (3.6% versus 24%, P = 0.003) and longer thrombosis-free survival, regardless of the use of antiplatelet drugs.64
Anagrelide, a selective inhibitor of megakaryocytic differentiation and proliferation, was compared with hydroxyurea in patients with ET in 2 randomized trials. In the first (N = 809), the group receiving anagrelide had a higher risk of arterial thrombosis, major bleeding, and fibrotic evolution, but lower incidence of venous thrombosis. Hydroxyurea was better tolerated, mainly due to anagrelide-related cardiovascular adverse events.111 As a result of this study, hydroxyurea is often preferred to anagrelide as front-line therapy for patients with newly diagnosed high-risk ET. In the second, more recent study (N = 259), however, the 2 agents proved equivalent in terms of major or minor arterial or venous thrombosis, as well as discontinuation rate.112 The discrepancy between the 2 trials may be partly explained by the different ET diagnostic criteria used, with the latter only enrolling patients with WHO-defined true ET, while the former utilized Polycythemia Vera Study Group-ET diagnostic criteria that included patients with increases in other blood counts or varying degrees of marrow fibrosis.
Interferons were studied in ET in parallel with PV. PEG-IFN alfa-2a proved effective in patients with ET, with responses observed in 80% of patients.103 PEG-IFN alfa-2b produced similar results, with responses in 70% to 90% of patients in small studies and discontinuation observed in 20% to 38% of cases.105,106,113 Because the very long-term leukemogenic potential of hydroxyurea has remained somewhat uncertain, anagrelide or IFN might be preferable choices in younger patients.
The approach to treatment of ET based on thrombotic risk level is illustrated in Figure 2.
Assessing Response to Therapy
For both patients with PV and ET the endpoint of treatment set forth for clinical trials has been the achievement of a clinicohematologic response. However, studies have failed to show a correlation between response and reduction of the thrombohemorrhagic risk.114 Therefore, proposed clinical trial response criteria were revised to include absence of hemorrhagic or thrombotic events as part of the definition of response (Table 3).94
Cases Continued
Patient A was initially treated with phlebotomies and his blood counts were subsequently controlled with hydroxyurea, which he took uninterruptedly at an average dose of 2.5 g daily. He also took ASA daily throughout. Now, 18 months after the start of therapy, he presents with a complaint of fatigue for the past 3 months, which more recently has been associated with recurrent itching. A repeat CBC shows a WBC count of 17,200/µL, hemoglobin 181 g/L, and platelets 940 × 103/µL.
Patient B presents for scheduled follow-up. She has had no further thrombotic episodes. However, she spontaneously discontinued hydroxyurea 1 month ago because of worsening mouth ulcers that impaired her ability to eat even small meals. She seeks recommendations for further treatment options.
Approach to Patients Refractory to or Intolerant of First-Line Therapy
According to the European LeukemiaNet recommendations, an inadequate response to hydroxyurea in patients with PV (or myelofibrosis) is defined as a need for phlebotomy to maintain hematocrit below < 45%, platelet count > 400 × 103/µL, and a WBC count > 10,000/µL, or failure to reduce splenomegaly > 10 cm by > 50% at a dose of ≥ 2 g/day or maximum tolerated dose. Historically, treatment options for patients with PV or ET who failed first-line therapy (most commonly hydroxyurea) have included alkylating agents, such as busulfan, chlorambucil, or pipobroman, and phosphorus (P)-32. However, the use of these drugs is limited by the associated risk of leukemic transformation.93,115,116 The use of IFN (or anagrelide for ET) is often considered in patients previously treated with hydroxyurea, and vice versa.
Ruxolitinib is a JAK1 and JAK2 inhibitor currently approved for the treatment of PV patients refractory to or intolerant of hydroxyurea.7 Following promising results of a phase 2 trial,117 ruxolitinib 10 mg twice daily was compared with best available therapy in the pivotal RESPONSE trial (N = 222). Ruxolitinib proved superior in achieving hematocrit control, reduction of spleen volume, and improvement of symptoms. Grade 3-4 hematologic toxicity was infrequent and similar in the 2 arms.118 In addition, longer follow-up of that study suggested a lower rate of thrombotic events in patients receiving ruxolitinib (1.8 versus 8.2 per 100 patient-years).119 In a similarly designed randomized phase 3 study in PV patients without splenomegaly (RESPONSE-2), more patients in the ruxolitinib arm had hematocrit reduction without an increase in toxicity. Based on the results of the above studies, ruxolitinib can be considered a standard of care for second-line therapy in this post-hydroxyurea patient population.120
Ruxolitinib is also being tested in patients with high-risk ET who have become resistant to, or were intolerant of hydroxyurea, but currently has no approved indication in this setting.121,122 Common side effects of ruxolitinib include cytopenias (especially anemia), increased risk of infections, hyperlipidemia, and increased risk of non-melanoma skin cancer.
Novel Agents
Novel agents that have been studied in patients with PV and ET are histone deacetylase inhibitors, murine double minute 2 (MDM2, or HDM2 for their human counterpart) inhibitors (which restore the function of p53), Bcl-2 homology domain 3 mimetics such as navitoclax and venetoclax, and, for patients with ET, the telomerase inhibitor imetelstat.123
Disease Evolution
Cases Continued
Patient A’s PV has been well controlled with PEG-IFN alfa-2a 90 μg subcutaneously weekly. However, he now presents with a complaint of worsening fatigue and early satiety. On exam the patient appears ill and splenomegaly is appreciated 12 cm below the costal margin. CBC shows a WBC count of 2600/µL, hemoglobin 73 g/L, and platelets 122 × 103/µL. Peripheral blood smear reveals leukoerythroblastosis and dacrocytosis. CBC 6 months ago was normal. A bone marrow biopsy is consistent with myelofibrosis.
After discontinuing hydroxyurea, patient B’s ET has been well controlled with anagrelide. However, for the past 4 weeks she has complained of severe fatigue and easy bruising. Physical exam reveals a pale, ill-appearing woman with scattered bruises. CBC shows a WBC count of 14,600/µL with 44% myeloblasts, hemoglobin 73 g/L, and platelets 22 × 103/µL. CBC 6 months ago was normal. A bone marrow biopsy is consistent with leukemic transformation of ET.
Post-PV/Post-ET Myelofibrosis
Diagnostic criteria for post-PV and post-ET myelofibrosis are outlined in Table 4.
Leukemic Transformation
The presence of more than 20% blasts in peripheral blood or bone marrow in a patient with MPN defines leukemic transformation. This occurs in up to 5% to 10% of patients and may or may not be preceded by a myelofibrosis phase.126 In cases of extramedullary transformation, a lower percentage of blasts can be seen in the bone marrow compared to the peripheral blood. The pathogenesis of leukemic transformation has remained elusive, but it is believed to be associated with genetic instability, which facilitates the acquisition of additional mutations, including those of TET2, ASXL1, EZH2 and DNMT3, IDH1/2, and TP53.127
Clinical risk factors for leukemic transformation include advanced age, karyotypic abnormalities, prior therapy with alkylating agents or P-32, splenectomy, increased peripheral blood or bone marrow blasts, leukocytosis, anemia, thrombocytopenia, and cytogenetic abnormalities. Hydroxyurea, interferon, and ruxolitinib have not been shown to have leukemogenic potential thus far. Prognosis of leukemic transformation is uniformly poor and patient survival rarely exceeds 6 months.
There is no standard of care for leukemic transformation of MPN (MPN-LT). Treatment options range from low-intensity regimens to more aggressive AML-type induction chemotherapy. No strategy appears clearly superior to others.128 Hematopoietic stem cell transplantation is the only therapy that provides clinically meaningful benefit to patients,129 but it is applicable only to a minority of patients with chemosensitive disease and good performance status.130 Notable experimental approaches to MPN–LT include hypomethylating agents, such as decitabine131 or azacitidine,132 with or without ruxolitinib.133-135
Conclusion
PV and ET are rare, chronic myeloid disorders. Patients typically experience a long clinical course and enjoy near-normal quality of life if properly managed. The 2 most important life-limiting complications of PV and ET are thrombohemorrhagic events and myelofibrosis/AML transformation. Vascular events are at least in part preventable with counseling on risk factors, phlebotomy (for patients with PV), antiplatelet therapy, and cytoreduction with hydroxyurea, IFNs, or anagrelide (for patients with ET). In addition, ruxolitinib was recently approved for PV patients after hydroxyurea failure. PV/ET transformation in myelofibrosis or AML is part of the natural history of the disease and no therapy has been shown to prevent it. Treatment follows recommendations set forth for PMF and AML, but results are generally poorer and novel strategies are needed to improve patients’ outcomes.
Introduction
Polycythemia vera (PV) and essential thrombocythemia (ET), along with primary myelofibrosis (PMF), belong to the group of Philadelphia-negative myeloproliferative neoplasms (MPN). All these malignancies arise from the clonal proliferation of an aberrant hematopoietic stem cell, but are characterized by distinct clinical phenotypes.1,2 Although the clinical course of PV and ET is indolent, it can be complicated by thrombohemorrhagic episodes and/or evolution into myelofibrosis and/or acute myeloid leukemia (AML).3 Since vascular events are the most frequent life-threatening complications of PV and ET, therapeutic strategies are aimed at reducing this risk. Treatment may also help control other disease-associated symptoms.4 No therapy has been shown to prevent evolution of PV or ET into myelofibrosis or AML. The discovery of the Janus kinase 2 (JAK2)/V617F mutation in most patients with PV and over half of those with ET (and PMF)5,6 has opened new avenues of research and led to the development of targeted therapies, such as the JAK1/2 inhibitor ruxolitinib, for patients with MPN.7,8
Epidemiology
PV and ET are typically diagnosed in the fifth to seventh decade of life.9 Although these disorders are generally associated with a long clinical course, survival of patients with PV or ET may be shorter than that of the general population.10–13 Estimating the incidence and prevalence of MPN is a challenge because most patients remain asymptomatic for long periods of time and do not seek medical attention.13 The annual incidence rates of PV and ET are estimated at 0.01 to 2.61 and 0.21 to 2.53 per 100,000, respectively. PV occurs slightly more frequently in males, whereas ET has a predilection for females.14 Given the long course and low mortality associated with these disorders, the prevalence of PV and ET are significantly higher than the respective incidence: up to 47 and 57 per 100,000, respectively.15–17
Molecular Pathogenesis
In 2005 researchers discovered a gain-of-function mutation of the JAK2 gene in nearly all patients with PV and more than half of those with ET and PMF.5,6,18,19 JAK2 is a non-receptor tyrosine kinase that plays a central role in normal hematopoiesis. Substitution of a valine for a phenylalanine at codon 617 (ie, V617F) leads to its constitutive activation and signaling through the JAK-STAT pathway.5,6,18,19 More rarely (and exclusively in patients with PV), JAK2 mutations involve exon 12.20–22 The vast majority of JAK2-negative ET patients harbor mutations in either the myeloproliferative leukemia (MPL) gene, which encodes the thrombopoietin receptor,23–25 or the calreticulin (CALR) gene,26,27 which encodes for a chaperone protein that plays a role in cellular proliferation, differentiation, and apoptosis.28 Both the MPL and CALR mutations ultimately result in the constitutive activation of the JAK-STAT pathway. Thus, JAK2, MPL, and CALR alterations are collectively referred to as driver mutations. Moreover, because these mutations affect the same oncogenic pathway (ie, JAK-STAT), they are almost always mutually exclusive in a given patient. Patients with ET (or myelofibrosis) who are wild-type for JAK2, MPL, and CALR are referred to as having “triple-negative” disease. Many recurrent non-driver mutations are also found in patients with MPN that are not exclusive of each other (ie, patients may have many at the same time), and involve for example ten-eleven translocation-2 (TET2), additional sex combs like 1 (ASXL1), enhancer of zeste homolog 2 (EZH2), isocitrate dehydrogenase 1 and isocitrate dehydrogenase 2 (IDH1/2), and DNA methyltransferase 3A (DNMT3A) genes, among others.29 The biologic and prognostic significance of these non-driver alterations remain to be fully defined in ET and PV.
Diagnosis and Risk Assessment
Case Presentations
Patient A is a 68-year-old man with a history of gouty arthritis who presents with a 6-month history of recurrent headaches and itching that increases after a hot shower. Over the past 2 months, he has also noticed worsening fatigue and redness of his face. He is a nonsmoker. Physical exam reveals erythromelalgia (ie, erythema, edema, and warmth) of the upper and lower extremities, scattered scratch marks, and splenomegaly 4 cm below the costal margin. Complete blood count (CBC) shows a white blood cell (WBC) count of 8100/µL, hemoglobin 194 g/L, and platelets 582 × 103/µL. Serum erythropoietin level is decreased at 2 mU/mL. Peripheral blood testing reveals a JAK2V617F mutation.
Patient B is a 51-year-old woman with a history of severe depression treated with sertraline and hypertension controlled with lisinopril and amlodipine who presents to her primary care physician for her “50-year-old physical.” She denies symptoms and is a nonsmoker. Physical exam is unrevealing. CBC shows a WBC count of 7400/µL (normal differential), hemoglobin 135 g/L, and platelets 1282 × 103/µL. A bone marrow biopsy shows normal cellularity with clusters of large, hyperlobulated megakaryocytes. Reverse transcriptase-polymerase chain reaction fails to reveal a BCR-ABL fusion product. The patient is diagnosed with ET.
Diagnostic Criteria
Diagnostic criteria for PV and ET according to the World Health Organization (WHO) classification30 are summarized in Table 1. Criteria for the diagnosis of prefibrotic myelofibrosis are included as well since this entity was formally recognized as separate from ET and part of the PMF spectrum in the 2016 WHO classification of myeloid tumors.30
Risk Stratification
Thrombohemorrhagic events, evolution into myelofibrosis, and leukemic transformation are the most serious complications in the course of PV or ET. Only thrombohemorrhagic events are, at least partially, preventable. Arterial or venous thrombotic complications are observed at rates of 1.8 to 10.9 per 100 patient-years in PV (arterial thrombosis being more common than venous) and 0.74 to 7.7 per 100 patient-years in ET, depending on the risk group35 and the presence of other factors (see below).
Thrombosis Risk Stratification in PV
The risk stratification of patients with PV is based on 2 factors: age ≥ 60 years and prior history of thrombosis. If either is present the patient is assigned to the high-risk category, whereas if none is present the patient is considered at low risk.36 In addition, high hematocrit37 and high WBC,38 but not thrombocytosis, have been associated with the development of vascular complications. In one study, the risk of new arterial thrombosis was increased by the presence of leukoerythroblastosis, hypertension, and prior arterial thrombosis, while karyotypic abnormalities and prior venous thrombosis were predictors of new venous thrombosis.39 Another emerging risk factor for thrombosis in patients with PV is high JAK2 allele burden (ie, the normal-to-mutated gene product ratio), although the evidence supporting this conclusion is equivocal.40
Thrombosis Risk Stratification in ET
Traditionally, in ET patients, thrombotic risk was assessed using the same 2 factors (age ≥ 60 years and prior history of thrombosis), separating patients into low- and high-risk groups. However, the prognostication of ET patients has been refined recently with the identification of new relevant factors. In particular, the impact of JAK2 mutations on thrombotic risk has been thoroughly studied. Clinically, the presence of JAK2V617F is associated with older age, higher hemoglobin and hematocrit, lower platelet counts, more frequent need for cytoreductive treatment, and greater tendency to evolve into PV (a rare event).41,42 Many,41,43–46 but not all,47–51 studies suggested a correlation between JAK2 mutation and risk of both arterial and venous thrombosis. Although infrequent, a JAK2V617F homozygous state (ie, the mutation is present in both alleles) might confer an even higher thrombotic risk.52 Moreover, the impact of the JAK2 mutation on vascular events persists over time,53 particularly in patients with high or unstable mutation burden.54 Based on JAK2V617F’s influence on the thrombotic risk of ET patients, a new prognostic score was proposed, the International Prognostic Score for ET (IPSET)-thrombosis (Table 2). The revised version of this model is currently endorsed by the National Comprehensive Cancer Network and divides patients into 4 risk groups: high, intermediate, low, and very low. Treatment recommendations vary according to the risk group (as described below).55
Other thrombotic risk factors have been identified, but deemed not significant enough to be included in the model. Cardiovascular risk factors (hypercholesterolemia, hypertension, smoking, diabetes mellitus) can increase the risk of vascular events,56–59 as can splenomegaly60 and baseline or persistent leukocytosis.61–63 Thrombocytosis has been correlated with thrombotic risk in some studies,64–68 whereas others did not support this conclusion and/or suggested a lower rate of thrombosis and, in some cases, increased risk of bleeding in ET patients with platelet counts greater than 1000 × 103/µL (due to acquired von Willebrand syndrome).56,61,63,68,69
CALR mutations tend to occur in younger males with lower hemoglobin and WBC count, higher platelet count, and greater marrow megakaryocytic predominance as compared to JAK2 mutations.26,27,70–72 The associated incidence of thrombosis was less than 10% at 15 years in patients with CALR mutations, lower than the incidence reported for ET patients with JAK2V617F mutations.73 The presence of the mutation per se does not appear to affect the thrombotic risk.74–76 Information on the thrombotic risk associated with MPL mutations or a triple-negative state is scarce. In both instances, however, the risk appears to be lower than with the JAK2 mutation.73,77–79
Venous thromboembolism in patients with PV or ET may occur at unusual sites, such as the splanchnic or cerebral venous systems.80 Risk factors for unusual venous thromboembolism include younger age,81 female gender (especially with concomitant use of oral contraceptive pills),82 and splenomegaly/splenectomy.83JAK2 mutation has also been associated with thrombosis at unusual sites. However, the prevalence of MPN or JAK2V617F in patients presenting with splanchnic venous thromboembolism has varied.80 In addition, MPN may be occult (ie, no clinical or laboratory abnormalities) in around 15% of patients.84 Screening for JAK2V617F and underlying MPN is recommended in patients presenting with isolated unexplained splanchnic venous thromboembolism. Treatment entails long-term anticoagulation therapy. JAK2V617F screening in patients with nonsplanchnic venous thromboembolism is not recommended, as its prevalence in this group is low (< 3%).85,86
Treatment
Cases Continued
Patient A is diagnosed with PV based on the presence of 2 major criteria (elevated hemoglobin and presence of the JAK2V617F mutation) and 1 minor criterion (low erythropoietin level). Given his age, he belongs to the high-risk disease category. He is now seeking advice regarding the management of his newly diagnosed PV.
Patient B presents to the emergency department with right lower extremity swelling and is found to have deep femoral thrombosis extending to the iliac vein. Five days after being discharged from the emergency department, she presents for follow-up. She is taking warfarin compliantly and her INR is within therapeutic range. The patient now has high-risk ET and would like to know more about thrombosis in her condition and how to best manage her risk.
Risk-Adapted Therapy
Low-Risk PV
All patients with PV should receive counseling to mitigate cardiovascular risk factors, including smoking cessation, lifestyle modifications, and lipid-lowering therapy, as indicated. Furthermore, all PV patients should receive acetylsalicylic acid (ASA) to decrease their risk for thrombosis and control vasomotor symptoms.55,87 Aspirin 81 to 100 mg daily is the preferred regimen because it provides adequate antithrombotic effect without the associated bleeding risk of higher-dose aspirin.88 Low-risk PV patients should also receive periodic phlebotomies to reduce and maintain their hematocrit below 45%. This recommendation is based on the results of the Cytoreductive Therapy in Polycythemia Vera (CYTO PV) randomized controlled trial. In the CYTO PV study, patients receiving more intense therapy to maintain the hematocrit below 45% had a lower incidence of cardiovascular-related deaths or major thrombotic events than those with hematocrit goals of 45% to 50% (2.7% versus 9.8%).89 Cytoreduction is an option for low-risk patients who do not tolerate phlebotomy or require frequent phlebotomy, or who have disease-related bleeding, severe symptoms, symptomatic splenomegaly, or progressive leukocytosis.38
High-Risk PV
Patients older than 60 years and/or with a history of thrombosis should be considered for cytoreductive therapy in addition to the above measures. Front-line cytoreductive therapies include hydroxyurea or interferon (IFN)- alfa.87 Hydroxyurea is a potent ribonucleotide reductase inhibitor that interferes with DNA repair and is the treatment of choice for most high-risk patients with PV.90 In a small trial hydroxyurea reduced the risk of thrombosis compared with historical controls treated with phlebotomy alone.91 Hydroxyurea is generally well tolerated; common side effects include cytopenias, nail changes, and mucosal and/or skin ulcers. Although never formally proven to be leukemogenic, this agent should be used with caution in younger patients.87 Indeed, in the original study, the rates of transformation were 5.9% and 1.5% for patients receiving hydroxyurea and phlebotomy alone,92 respectively, although an independent role for hydroxyurea in leukemic transformation was not supported in the much larger European Collaboration on Low-dose Aspirin in Polycythemia Vera (ECLAP) study.93 About 70% of patients will have a sustained response to hydroxyurea,94 while the remaining patients become resistant to or intolerant of the drug. Resistant individuals have a higher risk of progression to acute leukemia and death.95
IFN alfa is a pleiotropic antitumor agent that has found application in many types of malignancies96 and is sometimes employed as treatment for patients with newly diagnosed high-risk PV. Early studies showed responses in up to 100% of cases,97,98 albeit at the expense of a high discontinuation rate due to adverse events, such as flu-like symptoms, fatigue, and neuropsychiatric manifestations.99 A newer formulation of the drug obtained by adding a polyethylene glycol (PEG) moiety to the native IFN alfa molecule (PEG-IFN alfa) was shown to have a longer half-life, greater stability, less immunogenicity, and, potentially, better tolerability.100 Pilot phase 2 trials of PEG-IFN alfa-2a demonstrated its remarkable activity, with symptomatic and hematologic responses seen in the majority of patients (which, in some cases, persisted beyond discontinuation), and reasonable tolerability, with long-term discontinuation rates of around 20% to 30%.101–103 In some patients JAK2V617F became undetectable over time.104 Results of 2 ongoing trials, MDP-RC111 (single-arm study, PEG-IFN alfa-2a in high-risk PV or ET [NCT01259817]) and MPD-RC112 (randomized controlled trial, PEG-IFN alfa-2a versus hydroxyurea in the same population [NCT01258856]), will shed light on the role of PEG-IFN alfa in the management of patients with high-risk PV or ET. In 2 phase 2 studies of PEG-IFN alfa-2b, complete responses were seen in 70% to 100% of patients and discontinuation occurred in around a third of cases.105,106 A new, longer-acting formulation of PEG-IFN alfa-2a (peg-proline INF alfa-2b, AOP2014) is also undergoing clinical development.107,108
The approach to treatment of PV based on thrombotic risk level is illustrated in Figure 1.
Very Low- and Low-Risk ET
Like patients with PV, individuals with ET should undergo rigorous cardiovascular risk management and generally receive ASA to decrease their thrombotic risk and improve symptom control. Antiplatelet therapy may not be warranted in patients with documented
Intermediate-Risk ET
This category includes patients older than 60 years but without thrombosis or JAK2 mutations. These individuals would have been considered high risk (and thus candidates for cytoreductive therapy) according to the traditional risk stratification. Guidelines currently recommend ASA as the sole therapy for these patients, while reserving cytoreduction for those who experience thrombosis (ie, become high-risk) or have uncontrolled vasomotor or general symptoms, symptomatic splenomegaly, symptomatic thrombocytosis, or progressive leukocytosis.
High-Risk ET
For patients with ET in need of cytoreductive therapy (ie, those with prior thrombosis or older than 60 years with a JAK2V617F mutation), first-line options include hydroxyurea, IFN, and anagrelide. Hydroxyurea remains the treatment of choice in the majority of patients.110 In a seminal study, 114 patients with ET were randomly assigned to either observation or hydroxyurea treatment with the goal of maintaining the platelet count below 600 × 103/µL. At a median follow-up of 27 months, patients in the hydroxyurea group had a lower thrombosis rate (3.6% versus 24%, P = 0.003) and longer thrombosis-free survival, regardless of the use of antiplatelet drugs.64
Anagrelide, a selective inhibitor of megakaryocytic differentiation and proliferation, was compared with hydroxyurea in patients with ET in 2 randomized trials. In the first (N = 809), the group receiving anagrelide had a higher risk of arterial thrombosis, major bleeding, and fibrotic evolution, but lower incidence of venous thrombosis. Hydroxyurea was better tolerated, mainly due to anagrelide-related cardiovascular adverse events.111 As a result of this study, hydroxyurea is often preferred to anagrelide as front-line therapy for patients with newly diagnosed high-risk ET. In the second, more recent study (N = 259), however, the 2 agents proved equivalent in terms of major or minor arterial or venous thrombosis, as well as discontinuation rate.112 The discrepancy between the 2 trials may be partly explained by the different ET diagnostic criteria used, with the latter only enrolling patients with WHO-defined true ET, while the former utilized Polycythemia Vera Study Group-ET diagnostic criteria that included patients with increases in other blood counts or varying degrees of marrow fibrosis.
Interferons were studied in ET in parallel with PV. PEG-IFN alfa-2a proved effective in patients with ET, with responses observed in 80% of patients.103 PEG-IFN alfa-2b produced similar results, with responses in 70% to 90% of patients in small studies and discontinuation observed in 20% to 38% of cases.105,106,113 Because the very long-term leukemogenic potential of hydroxyurea has remained somewhat uncertain, anagrelide or IFN might be preferable choices in younger patients.
The approach to treatment of ET based on thrombotic risk level is illustrated in Figure 2.
Assessing Response to Therapy
For both patients with PV and ET the endpoint of treatment set forth for clinical trials has been the achievement of a clinicohematologic response. However, studies have failed to show a correlation between response and reduction of the thrombohemorrhagic risk.114 Therefore, proposed clinical trial response criteria were revised to include absence of hemorrhagic or thrombotic events as part of the definition of response (Table 3).94
Cases Continued
Patient A was initially treated with phlebotomies and his blood counts were subsequently controlled with hydroxyurea, which he took uninterruptedly at an average dose of 2.5 g daily. He also took ASA daily throughout. Now, 18 months after the start of therapy, he presents with a complaint of fatigue for the past 3 months, which more recently has been associated with recurrent itching. A repeat CBC shows a WBC count of 17,200/µL, hemoglobin 181 g/L, and platelets 940 × 103/µL.
Patient B presents for scheduled follow-up. She has had no further thrombotic episodes. However, she spontaneously discontinued hydroxyurea 1 month ago because of worsening mouth ulcers that impaired her ability to eat even small meals. She seeks recommendations for further treatment options.
Approach to Patients Refractory to or Intolerant of First-Line Therapy
According to the European LeukemiaNet recommendations, an inadequate response to hydroxyurea in patients with PV (or myelofibrosis) is defined as a need for phlebotomy to maintain hematocrit below < 45%, platelet count > 400 × 103/µL, and a WBC count > 10,000/µL, or failure to reduce splenomegaly > 10 cm by > 50% at a dose of ≥ 2 g/day or maximum tolerated dose. Historically, treatment options for patients with PV or ET who failed first-line therapy (most commonly hydroxyurea) have included alkylating agents, such as busulfan, chlorambucil, or pipobroman, and phosphorus (P)-32. However, the use of these drugs is limited by the associated risk of leukemic transformation.93,115,116 The use of IFN (or anagrelide for ET) is often considered in patients previously treated with hydroxyurea, and vice versa.
Ruxolitinib is a JAK1 and JAK2 inhibitor currently approved for the treatment of PV patients refractory to or intolerant of hydroxyurea.7 Following promising results of a phase 2 trial,117 ruxolitinib 10 mg twice daily was compared with best available therapy in the pivotal RESPONSE trial (N = 222). Ruxolitinib proved superior in achieving hematocrit control, reduction of spleen volume, and improvement of symptoms. Grade 3-4 hematologic toxicity was infrequent and similar in the 2 arms.118 In addition, longer follow-up of that study suggested a lower rate of thrombotic events in patients receiving ruxolitinib (1.8 versus 8.2 per 100 patient-years).119 In a similarly designed randomized phase 3 study in PV patients without splenomegaly (RESPONSE-2), more patients in the ruxolitinib arm had hematocrit reduction without an increase in toxicity. Based on the results of the above studies, ruxolitinib can be considered a standard of care for second-line therapy in this post-hydroxyurea patient population.120
Ruxolitinib is also being tested in patients with high-risk ET who have become resistant to, or were intolerant of hydroxyurea, but currently has no approved indication in this setting.121,122 Common side effects of ruxolitinib include cytopenias (especially anemia), increased risk of infections, hyperlipidemia, and increased risk of non-melanoma skin cancer.
Novel Agents
Novel agents that have been studied in patients with PV and ET are histone deacetylase inhibitors, murine double minute 2 (MDM2, or HDM2 for their human counterpart) inhibitors (which restore the function of p53), Bcl-2 homology domain 3 mimetics such as navitoclax and venetoclax, and, for patients with ET, the telomerase inhibitor imetelstat.123
Disease Evolution
Cases Continued
Patient A’s PV has been well controlled with PEG-IFN alfa-2a 90 μg subcutaneously weekly. However, he now presents with a complaint of worsening fatigue and early satiety. On exam the patient appears ill and splenomegaly is appreciated 12 cm below the costal margin. CBC shows a WBC count of 2600/µL, hemoglobin 73 g/L, and platelets 122 × 103/µL. Peripheral blood smear reveals leukoerythroblastosis and dacrocytosis. CBC 6 months ago was normal. A bone marrow biopsy is consistent with myelofibrosis.
After discontinuing hydroxyurea, patient B’s ET has been well controlled with anagrelide. However, for the past 4 weeks she has complained of severe fatigue and easy bruising. Physical exam reveals a pale, ill-appearing woman with scattered bruises. CBC shows a WBC count of 14,600/µL with 44% myeloblasts, hemoglobin 73 g/L, and platelets 22 × 103/µL. CBC 6 months ago was normal. A bone marrow biopsy is consistent with leukemic transformation of ET.
Post-PV/Post-ET Myelofibrosis
Diagnostic criteria for post-PV and post-ET myelofibrosis are outlined in Table 4.
Leukemic Transformation
The presence of more than 20% blasts in peripheral blood or bone marrow in a patient with MPN defines leukemic transformation. This occurs in up to 5% to 10% of patients and may or may not be preceded by a myelofibrosis phase.126 In cases of extramedullary transformation, a lower percentage of blasts can be seen in the bone marrow compared to the peripheral blood. The pathogenesis of leukemic transformation has remained elusive, but it is believed to be associated with genetic instability, which facilitates the acquisition of additional mutations, including those of TET2, ASXL1, EZH2 and DNMT3, IDH1/2, and TP53.127
Clinical risk factors for leukemic transformation include advanced age, karyotypic abnormalities, prior therapy with alkylating agents or P-32, splenectomy, increased peripheral blood or bone marrow blasts, leukocytosis, anemia, thrombocytopenia, and cytogenetic abnormalities. Hydroxyurea, interferon, and ruxolitinib have not been shown to have leukemogenic potential thus far. Prognosis of leukemic transformation is uniformly poor and patient survival rarely exceeds 6 months.
There is no standard of care for leukemic transformation of MPN (MPN-LT). Treatment options range from low-intensity regimens to more aggressive AML-type induction chemotherapy. No strategy appears clearly superior to others.128 Hematopoietic stem cell transplantation is the only therapy that provides clinically meaningful benefit to patients,129 but it is applicable only to a minority of patients with chemosensitive disease and good performance status.130 Notable experimental approaches to MPN–LT include hypomethylating agents, such as decitabine131 or azacitidine,132 with or without ruxolitinib.133-135
Conclusion
PV and ET are rare, chronic myeloid disorders. Patients typically experience a long clinical course and enjoy near-normal quality of life if properly managed. The 2 most important life-limiting complications of PV and ET are thrombohemorrhagic events and myelofibrosis/AML transformation. Vascular events are at least in part preventable with counseling on risk factors, phlebotomy (for patients with PV), antiplatelet therapy, and cytoreduction with hydroxyurea, IFNs, or anagrelide (for patients with ET). In addition, ruxolitinib was recently approved for PV patients after hydroxyurea failure. PV/ET transformation in myelofibrosis or AML is part of the natural history of the disease and no therapy has been shown to prevent it. Treatment follows recommendations set forth for PMF and AML, but results are generally poorer and novel strategies are needed to improve patients’ outcomes.
1. Swerdlow SH, Campo E, Harris NL, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon: IARC; 2008.
2. Alvarez-Larran A, Pereira A, Arellano-Rodrigo E, et al. Cytoreduction plus low-dose aspirin versus cytoreduction alone as primary prophylaxis of thrombosis in patients with high-risk essential thrombocythaemia: an observational study. Br J Haematol 2013;161:865–71.
3. Tefferi A. Polycythemia vera and essential thrombocythemia: 2013 update on diagnosis, risk-stratification, and management. Am J Hematol 2013;88:507–16.
4. Michiels JJ, Berneman Z, Schroyens W, et al. Platelet-mediated erythromelalgic, cerebral, ocular and coronary microvascular ischemic and thrombotic manifestations in patients with essential thrombocythemia and polycythemia vera: a distinct aspirin-responsive and coumadin-resistant arterial thrombophilia. Platelets 2006;17:528–44.
5. James C, Ugo V, Couedic J-PL, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature 2005;434:1144–8.
6. Kralovics R, Passamonti F, Buser A, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders.N Engl J Med 2005;352:1779–90.
7. Verstovsek S, Mesa R, Gotlib J, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med 2012;366:799–807.
8. Harrison C, Kiladjian J, Al-Ali H, et al. JAK Inhibition with ruxolitinib versus best available therapy for myelofibrosis.N Engl J Med 2012;366:787–98.
9. Berglund S, Zettervall O. Incidence of polycythemia vera in a defined population. Eur J Haematol 1992;48:20–6.
10. Rozman C, Giralt M, Feliu E, et al. Life expectancy of patients with chronic nonleukemic myeloproliferative disorders. Cancer 1991;67:2658–63.
11. Passamonti F, Rumi E, Pungolino E, et al. Life expectancy and prognostic factors for survival in patients with polycythemia vera and essential thrombocythemia. Am J Med 2004;117:755–61.
12. Hultcrantz M, Kristinsson SY, Andersson TM, et al. Patterns of survival among patients with myeloproliferative neoplasms diagnosed in Sweden from 1973 to 2008: a population-based study. J Clin Oncol 2012;30:2995–3001.
13. Wolanskyj AP, Schwager SM, et al. Essential thrombocythemia beyond the first decade: life expectancy, long-term complication rates, and prognostic factors. Mayo Clin Proc 2006;81:159–66.
14. Srour SA, Devesa SS, Morton LM, et al. Incidence and patient survival of myeloproliferative neoplasms and myelodysplastic/myeloproliferative neoplasms in the United States, 2001-12. Br J Haematol 2016;174:382–96.
15. Titmarsh GJ, Duncombe AS, McMullin MF, et al. How common are myeloproliferative neoplasms? A systematic review and meta-analysis. Am J Hematol 2014;89:581–7.
16. Moulard O, Mehta J, Fryzek J, et al. Epidemiology of myelofibrosis, essential thrombocythemia, and polycythemia vera in the European Union. Eur J Haematol 2014;92:289–97.
17. Stein BL, Gotlib J, Arcasoy M, et al. Historical views, conventional approaches, and evolving management strategies for myeloproliferative neoplasms. J Natl Compr Canc Netw 2015;13:424–34.
18. Levine RL, Wadleigh M, Cools J, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell 2005;7:387–97.
19. Baxter EJ, Scott LM, Campbell PJ, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet 2005;365:1054–61.
20. Scott LM, Tong W, Levine RL, et al. JAK2 exon 12 mutations in polycythemia vera and idiopathic erythrocytosis. N Engl J Med 2007;356:459–68.
21. Pardanani A, Lasho TL, Finke C, et al. Prevalence and clinicopathologic correlates of JAK2 exon 12 mutations in JAK2V617F-negative polycythemia vera. Leukemia 2007;21:1960–3.
22. Passamonti F, Elena C, Schnittger S, et al. Molecular and clinical features of the myeloproliferative neoplasm associated with JAK2 exon 12 mutations. Blood 2011;117:2813–6.
23. Abe M, Suzuki K, Inagaki O, et al. A novel MPL point mutation resulting in thrombopoietin-independent activation. Leukemia 2002;16:1500–6.
24. Pikman Y, Lee BH, Mercher T, et al. MPLW515L is a novel somatic activating mutation in myelofibrosis with myeloid metaplasia. PLoS Med 2006;3:e270.
25. Pardanani AD, Levine RL, Lasho T, et al. MPL515 mutations in myeloproliferative and other myeloid disorders: a study of 1182 patients. Blood 2006;108:3472–6.
26. Nangalia J, Massie CE, Baxter EJ, et al. Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. N Engl J Med 2013;369:2391–405.
27. Klampfl T, Gisslinger H, Harutyunyan AS, et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N Engl J Med 2013;369:2379–90.
28. Wang WA, Groenendyk J, Michalak M. Calreticulin signaling in health and disease. Int J Biochem Cell Biol 2012;44:842–6.
29. Saeidi K. Myeloproliferative neoplasms: Current molecular biology and genetics. Crit Rev Oncol Hematol 2016;98:375–89.
30. Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016;127:2391–405.
31. Mesa RA, Niblack J, Wadleigh M, et al. The burden of fatigue and quality of life in myeloproliferative disorders (MPDs): an international Internet-based survey of 1179 MPD patients. Cancer 2007;109:68–76.
32. Marchioli R, Finazzi G, Landolfi R, et al. Vascular and neoplastic risk in a large cohort of patients with polycythemia vera. J Clin Oncol 2005;23:2224–32.
33. Scherber R, Dueck AC, Johansson P, et al. The Myeloproliferative Neoplasm Symptom Assessment Form (MPN-SAF): international prospective validation and reliability trial in 402 patients. Blood 2011;118:401–8.
34. Emanuel RM, Dueck AC, Geyer HL, et al. Myeloproliferative neoplasm (MPN) symptom assessment form total symptom score: prospective international assessment of an abbreviated symptom burden scoring system among patients with MPNs. J Clin Oncol 2012;30:4098–103.
35. Casini A, Fontana P, Lecompte TP. Thrombotic complications of myeloproliferative neoplasms: risk assessment and risk-guided management. J Thromb Haemost 2013;11:1215–27.
36. Barbui T, Finazzi G, Falanga A. Myeloproliferative neoplasms and thrombosis. Blood 2013;122:2176-–84.
37. Pearson TC, Wetherley-Mein G. Vascular occlusive episodes and venous haematocrit in primary myeloproliferative polychytemia. Lancet 1978;2:1219–22.
38. Landolfi R, Di Gennaro L, Barbui T, et al. Leukocytosis as a major thrombotic risk factor in patients with polycythemia vera. Blood 2007;109:2446–52.
39. Tefferi A, Rumi E, Finazzi G, et al. Survival and prognosis among 1545 patients with contemporary polycythemia vera: an international study. Leukemia 2013;27:1874–81.
40. Barbui T, Falanga A. Molecular biomarkers of thrombosis in myeloproliferative neoplasms. Thromb Res 2016;140 Suppl 1:S71–75.
41. Campbell PJ, Scott LM, Buck G, et al. Definition of subtypes of essential thrombocythaemia and relation to polycythaemia vera based on JAK2 V617F mutation status: a prospective study. Lancet 2005;366:1945–53.
42. Tefferi A, Guglielmelli P, Larson DR, et al. Long-term survival and blast transformation in molecularly annotated essential thrombocythemia, polycythemia vera, and myelofibrosis. Blood 2014;124:2507–13.
43. Qin Y, Wang X, Zhao C, et al. The impact of JAK2V617F mutation on different types of thrombosis risk in patients with essential thrombocythemia: a meta-analysis. Int J Hematol 2015;102:170–80.
44. Ziakas PD. Effect of JAK2 V617F on thrombotic risk in patients with essential thrombocythemia: measuring the uncertain. Haematologica 2008;93:1412–4.
45. Lussana F, Dentali F, Abbate R, et al. Screening for thrombophilia and antithrombotic prophylaxis in pregnancy: Guidelines of the Italian Society for Haemostasis and Thrombosis (SISET). Thromb Res 2009;124:e19–25.
46. Dahabreh IJ, Zoi K, Giannouli S, et al. Is JAK2 V617F mutation more than a diagnostic index? A meta-analysis of clinical outcomes in essential thrombocythemia. Leuk Res 2009;33:67–73.
47. Cho YU, Chi HS, Lee EH, et al. Comparison of clinicopathologic findings according to JAK2 V617F mutation in patients with essential thrombocythemia. Int J Hematol 2009;89:39–44.
48. Wolanskyj AP, Lasho TL, Schwager SM, et al. JAK2 mutation in essential thrombocythaemia: clinical associations and long-term prognostic relevance. Br J Haematol 2005;131:208–13.
49. Antonioli E, Guglielmelli P, Pancrazzi A, et al. Clinical implications of the JAK2 V617F mutation in essential thrombocythemia. Leukemia 2005;19:1847–9.
50. Palandri F, Catani L, Testoni N, et al. Long-term follow-up of 386 consecutive patients with essential thrombocythemia: safety of cytoreductive therapy. Am J Hematol 2009;84:215–20.
51. Chim CS, Sim JP, Chan CC, et al. Impact of JAK2V617F mutation on thrombosis and myeloid transformation in essential thrombocythemia: a multivariate analysis by Cox regression in 141 patients. Hematology 2010;15:187–92.
52. Vannucchi AM, Antonioli E, Guglielmelli P, et al. Clinical profile of homozygous JAK2 617V>F mutation in patients with polycythemia vera or essential thrombocythemia. Blood 2007;110:840–6.
53. Carobbio A, Finazzi G, Antonioli E, et al. JAK2V617F allele burden and thrombosis: a direct comparison in essential thrombocythemia and polycythemia vera. Exp Hematol 2009;37:1016–21.
54. Alvarez-Larran A, Bellosillo B, Pereira A, et al. JAK2V617F monitoring in polycythemia vera and essential thrombocythemia: clinical usefulness for predicting myelofibrotic transformation and thrombotic events. Am J Hematol 2014;89:517–23.
55. Barbui T, Vannucchi AM, Buxhofer-Ausch V, et al. Practice-relevant revision of IPSET-thrombosis based on 1019 patients with WHO-defined essential thrombocythemia. Blood Cancer J 2015;5:e369.
56. Carobbio A, Thiele J, Passamonti F, et al. Risk factors for arterial and venous thrombosis in WHO-defined essential thrombocythemia: an international study of 891 patients. Blood 2011;117:5857–9.
57. Alvarez-Larran A, Cervantes F, Bellosillo B, et al. Essential thrombocythemia in young individuals: frequency and risk factors for vascular events and evolution to myelofibrosis in 126 patients. Leukemia 2007;21:1218–23.
58. Jantunen R, Juvonen E, Ikkala E, et al. The predictive value of vascular risk factors and gender for the development of thrombotic complications in essential thrombocythemia. Ann Hematol 2001;80:74–8.
59. Besses C, Cervantes F, Pereira A, et al. Major vascular complications in essential thrombocythemia: a study of the predictive factors in a series of 148 patients. Leukemia 1999;13:150–4.
60. Haider M, Gangat N, Hanson C, Tefferi A. Splenomegaly and thrombosis risk in essential thrombocythemia: the mayo clinic experience. Am J Hematol 2016;91:E296–297.
61. Carobbio A, Finazzi G, Antonioli E, et al. Thrombocytosis and leukocytosis interaction in vascular complications of essential thrombocythemia. Blood 2008;112:3135–7.
62. Palandri F, Polverelli N, Catani L, et al. Impact of leukocytosis on thrombotic risk and survival in 532 patients with essential thrombocythemia: a retrospective study. Ann Hematol 2011;90:933–8.
63. Campbell PJ, MacLean C, Beer PA, et al. Correlation of blood counts with vascular complications in essential thrombocythemia: analysis of the prospective PT1 cohort. Blood 2012;120:1409–11.
64. Cortelazzo S, Finazzi G, Ruggeri M, et al. Hydroxyurea for patients with essential thrombocythemia and a high risk of thrombosis. N Engl J Med 1995;332:1132–6.
65. van Genderen PJ, Mulder PG, Waleboer M, et al. Prevention and treatment of thrombotic complications in essential thrombocythaemia: efficacy and safety of aspirin. Br J Haematol 1997;97:179–84.
66. Storen EC, Tefferi A. Long-term use of anagrelide in young patients with essential thrombocythemia. Blood 2001;97:863–6.
67. De Stefano V, Za T, Rossi E, et al. Recurrent thrombosis in patients with polycythemia vera and essential thrombocythemia: incidence, risk factors, and effect of treatments. Haematologica 2008;93:372–80.
68. Alvarez-Larran A, Cervantes F, Pereira A, et al. Observation versus antiplatelet therapy as primary prophylaxis for thrombosis in low-risk essential thrombocythemia. Blood 2010;116:1205–10.
69. Palandri F, Polverelli N, Catani L, et al. Bleeding in essential thrombocythaemia: a retrospective analysis on 565 patients. Br J Haematol 2012;156:281–4.
70. Rotunno G, Mannarelli C, Guglielmelli P, et al. Impact of calreticulin mutations on clinical and hematological phenotype and outcome in essential thrombocythemia. Blood 2014;123:1552–5.
71. Tefferi A, Wassie EA, Lasho TL, et al. Calreticulin mutations and long-term survival in essential thrombocythemia. Leukemia 2014;28:2300–3.
72. Rumi E, Pietra D, Ferretti V, et al. JAK2 or CALR mutation status defines subtypes of essential thrombocythemia with substantially different clinical course and outcomes. Blood 2014;123:1544–51.
73. Palandri F, Latagliata R, Polverelli N, et al. Mutations and long-term outcome of 217 young patients with essential thrombocythemia or early primary myelofibrosis. Leukemia 2015;29:1344–9.
74. Fu R, Xuan M, Zhou Y, et al. Analysis of calreticulin mutations in Chinese patients with essential thrombocythemia: clinical implications in diagnosis, prognosis and treatment. Leukemia 2014;28:1912–4.
75. Tefferi A, Wassie EA, Guglielmelli P, et al. Type 1 versus Type 2 calreticulin mutations in essential thrombocythemia: a collaborative study of 1027 patients. Am J Hematol 2014;89:E121–4.
76. Pietra D, Rumi E, Ferretti VV, et al. Differential clinical effects of different mutation subtypes in CALR-mutant myeloproliferative neoplasms. Leukemia 2016;30:431–8.
77. Rumi E, Pietra D, Guglielmelli P, et al. Acquired copy-neutral loss of heterozygosity of chromosome 1p as a molecular event associated with marrow fibrosis in MPL-mutated myeloproliferative neoplasms. Blood 2013;121:4388–95.
78. Beer PA, Campbell PJ, Scott LM, et al. MPL mutations in myeloproliferative disorders: analysis of the PT-1 cohort. Blood 2008;112:141–9.
79. Gangat N, Wassie EA, Lasho TL, et al. Mutations and thrombosis in essential thrombocythemia: prognostic interaction with age and thrombosis history. Eur J Haematol 2015;94:31–6.
80. Sekhar M, McVinnie K, Burroughs AK. Splanchnic vein thrombosis in myeloproliferative neoplasms. Br J Haematol 2013;162:730–47.
81. Stein BL, Saraf S, Sobol U, et al. Age-related differences in disease characteristics and clinical outcomes in polycythemia vera. Leuk Lymph 2013;54:1989–95.
82. Landolfi R, Di Gennaro L, Nicolazzi MA, et al. Polycythemia vera: gender-related phenotypic differences. Intern Emerg Med 2012;7:509–15.
83. Winslow ER, Brunt LM, Drebin JA, et al. Portal vein thrombosis after splenectomy. Am J Surg 2002;184:631–6.
84. Smalberg JH, Arends LR, Valla DC, et al. Myeloproliferative neoplasms in Budd-Chiari syndrome and portal vein thrombosis: a meta-analysis. Blood 2012;120:4921–8.
85. Dentali F, Squizzato A, Brivio L, et al. JAK2V617F mutation for the early diagnosis of Ph- myeloproliferative neoplasms in patients with venous thromboembolism: a meta-analysis. Blood 2009;113:5617–23.
86. Pardanani A, Lasho TL, Hussein K, et al. JAK2V617F mutation screening as part of the hypercoagulable work-up in the absence of splanchnic venous thrombosis or overt myeloproliferative neoplasm: assessment of value in a series of 664 consecutive patients. Mayo Clin Proc 2008;83:457–9.
87. Barbui T, Barosi G, Birgegard G, et al. Philadelphia-negative classical myeloproliferative neoplasms: critical concepts and management recommendations from European LeukemiaNet. J Clin Oncol 2011;29:761–70.
88. Landolfi R, Marchioli R, Kutti J, et al. Efficacy and safety of low-dose aspirin in polycythemia vera. N Engl J Med 2004;350:114–24.
89. Marchioli R, Finazzi G, Specchia G, et al. Cardiovascular events and intensity of treatment in polycythemia vera. N Engl J Med 2013;368:22–33.
90. Kiladjian JJ, Chevret S, Dosquet C, et al. Treatment of polycythemia vera with hydroxyurea and pipobroman: final results of a randomized trial initiated in 1980. J Clin Oncol 2011;29:3907–13.
91. Kaplan ME, Mack K, Goldberg JD, et al. Long-term management of polycythemia vera with hydroxyurea: a progress report. Semin Hematol 1986;23:167–71.
92. Fruchtman SM, Mack K, Kaplan ME, et al. From efficacy to safety: a Polycythemia Vera Study group report on hydroxyurea in patients with polycythemia vera. Semin Hematol 1997;34:17–23.
93. Finazzi G, Caruso V, Marchioli R, et al. Acute leukemia in polycythemia vera: an analysis of 1638 patients enrolled in a prospective observational study. Blood 2005;105:2664–70.
94. Barosi G, Mesa R, Finazzi G, et al. Revised response criteria for polycythemia vera and essential thrombocythemia: an ELN and IWG-MRT consensus project. Blood 2013;121:4778–81.
95. Alvarez-Larran A, Pereira A, Cervantes F, et al. Assessment and prognostic value of the European LeukemiaNet criteria for clinicohematologic response, resistance, and intolerance to hydroxyurea in polycythemia vera. Blood 2012;119:1363–9.
96. Stein BL, Tiu RV. Biological rationale and clinical use of interferon in the classical BCR-ABL-negative myeloproliferative neoplasms. J Interferon Cytokine Res 2013;33:145–53.
97. Ludwig H, Cortelezzi A, Van Camp BG, et al. Treatment with recombinant interferon-alpha-2C: multiple myeloma and thrombocythaemia in myeloproliferative diseases. Oncology 1985;42 Suppl 1:19–25.
98. Silver RT. Long-term effects of the treatment of polycythemia vera with recombinant interferon-alpha. Cancer 2006;107:451–8.
99. Kiladjian JJ, Mesa RA, Hoffman R. The renaissance of interferon therapy for the treatment of myeloid malignancies. Blood 2011;117:4706–15.
100. Veronese FM, Mero A. The impact of PEGylation on biological therapies. BioDrugs 2008;22:315–29.
101. Kiladjian JJ, Cassinat B, Chevret S, et al. Pegylated interferon-alfa-2a induces complete hematologic and molecular responses with low toxicity in polycythemia vera. Blood 2008;112:3065–72.
102. Turlure P, Cambier N, Roussel M, et al. Complete hematological, molecular and histological remissions without cytoreductive treatment lasting after pegylated-interferon {alpha}-2a (peg-IFN{alpha}-2a) therapy in polycythemia vera (PV): long term results of a phase 2 trial [abstract]. Blood 2011;118(21). Abstract 280.
103. Quintas-Cardama A, Kantarjian H, Manshouri T, et al. Pegylated interferon alfa-2a yields high rates of hematologic and molecular response in patients with advanced essential thrombocythemia and polycythemia vera. J Clin Oncol 2009;27:5418–24.
104. Quintas-Cardama A, Abdel-Wahab O, Manshouri T, et al. Molecular analysis of patients with polycythemia vera or essential thrombocythemia receiving pegylated interferon a-2a. Blood 2013;122:893–901.
105. Samuelsson J, Hasselbalch H, Bruserud O, et al. A phase II trial of pegylated interferon alpha-2b therapy for polycythemia vera and essential thrombocythemia: feasibility, clinical and biologic effects, and impact on quality of life. Cancer 2006;106:2397–405.
106. Jabbour E, Kantarjian H, Cortes J, et al. PEG-IFN-alpha-2b therapy in BCR-ABL-negative myeloproliferative disorders: final result of a phase 2 study. Cancer 2007;110:2012–18.
107. Them NC, Bagienski K, Berg T, et al. Molecular responses and chromosomal aberrations in patients with polycythemia vera treated with peg-proline-interferon alpha-2b. Am J Hematol 2015;90:288–94.
108. Gisslinger H, Klade C, Georgiev P, et al. Final results from PROUD-PV a randomized controlled phase 3 trial comparing ropeginterferon alfa-2b to hydroxyurea in polycythemia vera patients [abstract]. Blood 2016;128(suppl 22). Abstract 475.
109. van Genderen PJ, van Vliet HH, Prins FJ, et al. Excessive prolongation of the bleeding time by aspirin in essential thrombocythemia is related to a decrease of large von Willebrand factor multimers in plasma. Ann Hematol 1997;75:215–20.
110. Cortelazzo S, Finazzi G, Ruggeri M, et al. Hydroxyurea for patients with essential thrombocythemia and a high risk of thrombosis. N Engl J Med 1995;332:1132–7.
111. Harrison CN, Campbell PJ, Buck G, et al. Hydroxyurea compared with anagrelide in high-risk essential thrombocythemia. N Engl J Med 2005;353:33–45.
112. Gisslinger H, Gotic M, Holowiecki J, et al. Anagrelide compared with hydroxyurea in WHO-classified essential thrombocythemia: the ANAHYDRET Study, a randomized controlled trial. Blood 2013;121:1720–8.
113. Alvarado Y, Cortes J, Verstovsek S, et al. Pilot study of pegylated interferon-alpha 2b in patients with essential thrombocythemia. Cancer Chemother Pharmacol 2003;51:81–6.
114. Barosi G, Tefferi A, Barbui T, ad hoc committee ‘Definition of clinically relevant outcomes for contemporarily clinical trials in Ph-neg M. Do current response criteria in classical Ph-negative myeloproliferative neoplasms capture benefit for patients? Leukemia 2012;26:1148–9.
115. Bjorkholm M, Derolf AR, Hultcrantz M, et al. Treatment-related risk factors for transformation to acute myeloid leukemia and myelodysplastic syndromes in myeloproliferative neoplasms. J Clin Oncol 2011;29:2410–5.
116. Alvarez-Larran A, Martinez-Aviles L, Hernandez-Boluda JC, et al. Busulfan in patients with polycythemia vera or essential thrombocythemia refractory or intolerant to hydroxyurea. Ann Hematol 2014;93:2037–43.
117. Verstovsek S, Passamonti F, Rambaldi A, et al. A phase 2 study of ruxolitinib, an oral JAK1 and JAK2 Inhibitor, in patients with advanced polycythemia vera who are refractory or intolerant to hydroxyurea. Cancer 2014;120:513–20.
118. Vannucchi AM, Kiladjian JJ, Griesshammer M, et al. Ruxolitinib in polycythemia vera resistant to or intolerant of hydroxyurea. N Engl J Med 2015; 372:426–35.
119. Verstovsek S, Vannucchi AM, Griesshammer M, et al. Ruxolitinib versus best available therapy in patients with polycythemia vera: 80-week follow-up from the RESPONSE trial. Haematologica 2016;101:821–9.
120. Passamonti F, Griesshammer M, Palandri F, et al. Ruxolitinib for the treatment of inadequately controlled polycythaemia vera without splenomegaly (RESPONSE-2): a randomised, open-label, phase 3b study. Lancet Oncol 2017;18:88–99.
121. Verstovsek S, Passamonti F, Rambaldi A, et al. Long-term results from a phase II open-label study of ruxolitinib in patients with essential thrombocythemia refractory to or intolerant of hydroxyurea [abstract]. Blood 2014;124. Abstract 1847.
122. Harrison CN, Mead AJ, Panchal A, et al. Ruxolitinib versus best available therapy for ET intolerant or resistant to hydroxycarbamide in a randomized trial. Blood 2017 Aug 9. pii: blood-2017-05-785790 .
123. Bose P, Verstovsek S. Drug development pipeline for myeloproliferative neoplasms: potential future impact on guidelines and management. J Natl Compr Canc Netw 2016;14:1613–24.
124. Cerquozzi S, Teffieri A. Blast transformation and fibrotic progression in polycythemia vera and essential thrombocythemia: a literature review of incidence and risk factors. Blood Cancer J 2015;Nov 13;5:e366.
125. Passamonti F, Rumi E, Caramella M, et al. A dynamic prognostic model to predict survival in post-polycythemia vera myelofibrosis. Blood 2008;111:3383–7.
126. Mesa RA, Verstovsek S, Cervantes F, et al. Primary myelofibrosis (PMF), post polycythemia vera myelofibrosis (post-PV MF), post essential thrombocythemia myelofibrosis (post-ET MF), blast phase PMF (PMF-BP): Consensus on terminology by the international working group for myelofibrosis research and treatment (IWG-MRT). Leuk Res 2007;31:737–40.
127. Rampal R, Mascarenhas J. Pathogenesis and management of acute myeloid leukemia that has evolved from a myeloproliferative neoplasm. Curr Opin Hematol 2014;21:65–71.
128. Chihara D, Kantarjian HM, Newberry KJ, et al. Survival outcome of patients with acute myeloid leukemia transformed from myeloproliferative neoplasms [abstract]. Blood 2016;128. Abstract 1940.
129. Tam CS, Nussenzveig RM, Popat U, et al. The natural history and treatment outcome of blast phase BCR-ABL- myeloproliferative neoplasms. Blood 2008;112:1628–37.
130. Kundranda MN, Tibes R, Mesa RA. Transformation of a chronic myeloproliferative neoplasm to acute myelogenous leukemia: does anything work? Curr Hematol Malig Rep 2012;7:78–86.
131. Badar T, Kantarjian HM, Ravandi F, et al. Therapeutic benefit of decitabine, a hypomethylating agent, in patients with high-risk primary myelofibrosis and myeloproliferative neoplasm in accelerated or blastic/acute myeloid leukemia phase. Leuk Res 2015;39:950–6.
132. Thepot S, Itzykson R, Seegers V, et al. Treatment of progression of Philadelphia-negative myeloproliferative neoplasms to myelodysplastic syndrome or acute myeloid leukemia by azacitidine: a report on 54 cases on the behalf of the Groupe Francophone des Myelodysplasies (GFM). Blood 2010;116:3735–42.
133. Pemmaraju N, Kantarjian H, Kadia T, et al. A phase I/II study of the Janus kinase (JAK)1 and 2 inhibitor ruxolitinib in patients with relapsed or refractory acute myeloid leukemia. Clin Lymphoma Myeloma Leuk 2015;15:171–6.
134. Rampal RK, Mascarenhas JO, Kosiorek HE, et al. Safety and efficacy of combined ruxolitinib and decitabine in patients with blast-phase MPN and post-MPN AML: results of a phase I study (Myeloproliferative Disorders Research Consortium 109 trial) [abstract]. Blood 2016;128. Abstract 1124.
135. Bose P, Verstovsek S, Gasior Y, et al. Phase I/II study of ruxolitinib (RUX) with decitabine (DAC) in patients with post-myeloproliferative neoplasm acute myeloid leukemia (post-MPN AML): phase I results [abstract]. Blood 2016;128. Abstract 4262.
1. Swerdlow SH, Campo E, Harris NL, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon: IARC; 2008.
2. Alvarez-Larran A, Pereira A, Arellano-Rodrigo E, et al. Cytoreduction plus low-dose aspirin versus cytoreduction alone as primary prophylaxis of thrombosis in patients with high-risk essential thrombocythaemia: an observational study. Br J Haematol 2013;161:865–71.
3. Tefferi A. Polycythemia vera and essential thrombocythemia: 2013 update on diagnosis, risk-stratification, and management. Am J Hematol 2013;88:507–16.
4. Michiels JJ, Berneman Z, Schroyens W, et al. Platelet-mediated erythromelalgic, cerebral, ocular and coronary microvascular ischemic and thrombotic manifestations in patients with essential thrombocythemia and polycythemia vera: a distinct aspirin-responsive and coumadin-resistant arterial thrombophilia. Platelets 2006;17:528–44.
5. James C, Ugo V, Couedic J-PL, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature 2005;434:1144–8.
6. Kralovics R, Passamonti F, Buser A, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders.N Engl J Med 2005;352:1779–90.
7. Verstovsek S, Mesa R, Gotlib J, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med 2012;366:799–807.
8. Harrison C, Kiladjian J, Al-Ali H, et al. JAK Inhibition with ruxolitinib versus best available therapy for myelofibrosis.N Engl J Med 2012;366:787–98.
9. Berglund S, Zettervall O. Incidence of polycythemia vera in a defined population. Eur J Haematol 1992;48:20–6.
10. Rozman C, Giralt M, Feliu E, et al. Life expectancy of patients with chronic nonleukemic myeloproliferative disorders. Cancer 1991;67:2658–63.
11. Passamonti F, Rumi E, Pungolino E, et al. Life expectancy and prognostic factors for survival in patients with polycythemia vera and essential thrombocythemia. Am J Med 2004;117:755–61.
12. Hultcrantz M, Kristinsson SY, Andersson TM, et al. Patterns of survival among patients with myeloproliferative neoplasms diagnosed in Sweden from 1973 to 2008: a population-based study. J Clin Oncol 2012;30:2995–3001.
13. Wolanskyj AP, Schwager SM, et al. Essential thrombocythemia beyond the first decade: life expectancy, long-term complication rates, and prognostic factors. Mayo Clin Proc 2006;81:159–66.
14. Srour SA, Devesa SS, Morton LM, et al. Incidence and patient survival of myeloproliferative neoplasms and myelodysplastic/myeloproliferative neoplasms in the United States, 2001-12. Br J Haematol 2016;174:382–96.
15. Titmarsh GJ, Duncombe AS, McMullin MF, et al. How common are myeloproliferative neoplasms? A systematic review and meta-analysis. Am J Hematol 2014;89:581–7.
16. Moulard O, Mehta J, Fryzek J, et al. Epidemiology of myelofibrosis, essential thrombocythemia, and polycythemia vera in the European Union. Eur J Haematol 2014;92:289–97.
17. Stein BL, Gotlib J, Arcasoy M, et al. Historical views, conventional approaches, and evolving management strategies for myeloproliferative neoplasms. J Natl Compr Canc Netw 2015;13:424–34.
18. Levine RL, Wadleigh M, Cools J, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell 2005;7:387–97.
19. Baxter EJ, Scott LM, Campbell PJ, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet 2005;365:1054–61.
20. Scott LM, Tong W, Levine RL, et al. JAK2 exon 12 mutations in polycythemia vera and idiopathic erythrocytosis. N Engl J Med 2007;356:459–68.
21. Pardanani A, Lasho TL, Finke C, et al. Prevalence and clinicopathologic correlates of JAK2 exon 12 mutations in JAK2V617F-negative polycythemia vera. Leukemia 2007;21:1960–3.
22. Passamonti F, Elena C, Schnittger S, et al. Molecular and clinical features of the myeloproliferative neoplasm associated with JAK2 exon 12 mutations. Blood 2011;117:2813–6.
23. Abe M, Suzuki K, Inagaki O, et al. A novel MPL point mutation resulting in thrombopoietin-independent activation. Leukemia 2002;16:1500–6.
24. Pikman Y, Lee BH, Mercher T, et al. MPLW515L is a novel somatic activating mutation in myelofibrosis with myeloid metaplasia. PLoS Med 2006;3:e270.
25. Pardanani AD, Levine RL, Lasho T, et al. MPL515 mutations in myeloproliferative and other myeloid disorders: a study of 1182 patients. Blood 2006;108:3472–6.
26. Nangalia J, Massie CE, Baxter EJ, et al. Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. N Engl J Med 2013;369:2391–405.
27. Klampfl T, Gisslinger H, Harutyunyan AS, et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N Engl J Med 2013;369:2379–90.
28. Wang WA, Groenendyk J, Michalak M. Calreticulin signaling in health and disease. Int J Biochem Cell Biol 2012;44:842–6.
29. Saeidi K. Myeloproliferative neoplasms: Current molecular biology and genetics. Crit Rev Oncol Hematol 2016;98:375–89.
30. Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016;127:2391–405.
31. Mesa RA, Niblack J, Wadleigh M, et al. The burden of fatigue and quality of life in myeloproliferative disorders (MPDs): an international Internet-based survey of 1179 MPD patients. Cancer 2007;109:68–76.
32. Marchioli R, Finazzi G, Landolfi R, et al. Vascular and neoplastic risk in a large cohort of patients with polycythemia vera. J Clin Oncol 2005;23:2224–32.
33. Scherber R, Dueck AC, Johansson P, et al. The Myeloproliferative Neoplasm Symptom Assessment Form (MPN-SAF): international prospective validation and reliability trial in 402 patients. Blood 2011;118:401–8.
34. Emanuel RM, Dueck AC, Geyer HL, et al. Myeloproliferative neoplasm (MPN) symptom assessment form total symptom score: prospective international assessment of an abbreviated symptom burden scoring system among patients with MPNs. J Clin Oncol 2012;30:4098–103.
35. Casini A, Fontana P, Lecompte TP. Thrombotic complications of myeloproliferative neoplasms: risk assessment and risk-guided management. J Thromb Haemost 2013;11:1215–27.
36. Barbui T, Finazzi G, Falanga A. Myeloproliferative neoplasms and thrombosis. Blood 2013;122:2176-–84.
37. Pearson TC, Wetherley-Mein G. Vascular occlusive episodes and venous haematocrit in primary myeloproliferative polychytemia. Lancet 1978;2:1219–22.
38. Landolfi R, Di Gennaro L, Barbui T, et al. Leukocytosis as a major thrombotic risk factor in patients with polycythemia vera. Blood 2007;109:2446–52.
39. Tefferi A, Rumi E, Finazzi G, et al. Survival and prognosis among 1545 patients with contemporary polycythemia vera: an international study. Leukemia 2013;27:1874–81.
40. Barbui T, Falanga A. Molecular biomarkers of thrombosis in myeloproliferative neoplasms. Thromb Res 2016;140 Suppl 1:S71–75.
41. Campbell PJ, Scott LM, Buck G, et al. Definition of subtypes of essential thrombocythaemia and relation to polycythaemia vera based on JAK2 V617F mutation status: a prospective study. Lancet 2005;366:1945–53.
42. Tefferi A, Guglielmelli P, Larson DR, et al. Long-term survival and blast transformation in molecularly annotated essential thrombocythemia, polycythemia vera, and myelofibrosis. Blood 2014;124:2507–13.
43. Qin Y, Wang X, Zhao C, et al. The impact of JAK2V617F mutation on different types of thrombosis risk in patients with essential thrombocythemia: a meta-analysis. Int J Hematol 2015;102:170–80.
44. Ziakas PD. Effect of JAK2 V617F on thrombotic risk in patients with essential thrombocythemia: measuring the uncertain. Haematologica 2008;93:1412–4.
45. Lussana F, Dentali F, Abbate R, et al. Screening for thrombophilia and antithrombotic prophylaxis in pregnancy: Guidelines of the Italian Society for Haemostasis and Thrombosis (SISET). Thromb Res 2009;124:e19–25.
46. Dahabreh IJ, Zoi K, Giannouli S, et al. Is JAK2 V617F mutation more than a diagnostic index? A meta-analysis of clinical outcomes in essential thrombocythemia. Leuk Res 2009;33:67–73.
47. Cho YU, Chi HS, Lee EH, et al. Comparison of clinicopathologic findings according to JAK2 V617F mutation in patients with essential thrombocythemia. Int J Hematol 2009;89:39–44.
48. Wolanskyj AP, Lasho TL, Schwager SM, et al. JAK2 mutation in essential thrombocythaemia: clinical associations and long-term prognostic relevance. Br J Haematol 2005;131:208–13.
49. Antonioli E, Guglielmelli P, Pancrazzi A, et al. Clinical implications of the JAK2 V617F mutation in essential thrombocythemia. Leukemia 2005;19:1847–9.
50. Palandri F, Catani L, Testoni N, et al. Long-term follow-up of 386 consecutive patients with essential thrombocythemia: safety of cytoreductive therapy. Am J Hematol 2009;84:215–20.
51. Chim CS, Sim JP, Chan CC, et al. Impact of JAK2V617F mutation on thrombosis and myeloid transformation in essential thrombocythemia: a multivariate analysis by Cox regression in 141 patients. Hematology 2010;15:187–92.
52. Vannucchi AM, Antonioli E, Guglielmelli P, et al. Clinical profile of homozygous JAK2 617V>F mutation in patients with polycythemia vera or essential thrombocythemia. Blood 2007;110:840–6.
53. Carobbio A, Finazzi G, Antonioli E, et al. JAK2V617F allele burden and thrombosis: a direct comparison in essential thrombocythemia and polycythemia vera. Exp Hematol 2009;37:1016–21.
54. Alvarez-Larran A, Bellosillo B, Pereira A, et al. JAK2V617F monitoring in polycythemia vera and essential thrombocythemia: clinical usefulness for predicting myelofibrotic transformation and thrombotic events. Am J Hematol 2014;89:517–23.
55. Barbui T, Vannucchi AM, Buxhofer-Ausch V, et al. Practice-relevant revision of IPSET-thrombosis based on 1019 patients with WHO-defined essential thrombocythemia. Blood Cancer J 2015;5:e369.
56. Carobbio A, Thiele J, Passamonti F, et al. Risk factors for arterial and venous thrombosis in WHO-defined essential thrombocythemia: an international study of 891 patients. Blood 2011;117:5857–9.
57. Alvarez-Larran A, Cervantes F, Bellosillo B, et al. Essential thrombocythemia in young individuals: frequency and risk factors for vascular events and evolution to myelofibrosis in 126 patients. Leukemia 2007;21:1218–23.
58. Jantunen R, Juvonen E, Ikkala E, et al. The predictive value of vascular risk factors and gender for the development of thrombotic complications in essential thrombocythemia. Ann Hematol 2001;80:74–8.
59. Besses C, Cervantes F, Pereira A, et al. Major vascular complications in essential thrombocythemia: a study of the predictive factors in a series of 148 patients. Leukemia 1999;13:150–4.
60. Haider M, Gangat N, Hanson C, Tefferi A. Splenomegaly and thrombosis risk in essential thrombocythemia: the mayo clinic experience. Am J Hematol 2016;91:E296–297.
61. Carobbio A, Finazzi G, Antonioli E, et al. Thrombocytosis and leukocytosis interaction in vascular complications of essential thrombocythemia. Blood 2008;112:3135–7.
62. Palandri F, Polverelli N, Catani L, et al. Impact of leukocytosis on thrombotic risk and survival in 532 patients with essential thrombocythemia: a retrospective study. Ann Hematol 2011;90:933–8.
63. Campbell PJ, MacLean C, Beer PA, et al. Correlation of blood counts with vascular complications in essential thrombocythemia: analysis of the prospective PT1 cohort. Blood 2012;120:1409–11.
64. Cortelazzo S, Finazzi G, Ruggeri M, et al. Hydroxyurea for patients with essential thrombocythemia and a high risk of thrombosis. N Engl J Med 1995;332:1132–6.
65. van Genderen PJ, Mulder PG, Waleboer M, et al. Prevention and treatment of thrombotic complications in essential thrombocythaemia: efficacy and safety of aspirin. Br J Haematol 1997;97:179–84.
66. Storen EC, Tefferi A. Long-term use of anagrelide in young patients with essential thrombocythemia. Blood 2001;97:863–6.
67. De Stefano V, Za T, Rossi E, et al. Recurrent thrombosis in patients with polycythemia vera and essential thrombocythemia: incidence, risk factors, and effect of treatments. Haematologica 2008;93:372–80.
68. Alvarez-Larran A, Cervantes F, Pereira A, et al. Observation versus antiplatelet therapy as primary prophylaxis for thrombosis in low-risk essential thrombocythemia. Blood 2010;116:1205–10.
69. Palandri F, Polverelli N, Catani L, et al. Bleeding in essential thrombocythaemia: a retrospective analysis on 565 patients. Br J Haematol 2012;156:281–4.
70. Rotunno G, Mannarelli C, Guglielmelli P, et al. Impact of calreticulin mutations on clinical and hematological phenotype and outcome in essential thrombocythemia. Blood 2014;123:1552–5.
71. Tefferi A, Wassie EA, Lasho TL, et al. Calreticulin mutations and long-term survival in essential thrombocythemia. Leukemia 2014;28:2300–3.
72. Rumi E, Pietra D, Ferretti V, et al. JAK2 or CALR mutation status defines subtypes of essential thrombocythemia with substantially different clinical course and outcomes. Blood 2014;123:1544–51.
73. Palandri F, Latagliata R, Polverelli N, et al. Mutations and long-term outcome of 217 young patients with essential thrombocythemia or early primary myelofibrosis. Leukemia 2015;29:1344–9.
74. Fu R, Xuan M, Zhou Y, et al. Analysis of calreticulin mutations in Chinese patients with essential thrombocythemia: clinical implications in diagnosis, prognosis and treatment. Leukemia 2014;28:1912–4.
75. Tefferi A, Wassie EA, Guglielmelli P, et al. Type 1 versus Type 2 calreticulin mutations in essential thrombocythemia: a collaborative study of 1027 patients. Am J Hematol 2014;89:E121–4.
76. Pietra D, Rumi E, Ferretti VV, et al. Differential clinical effects of different mutation subtypes in CALR-mutant myeloproliferative neoplasms. Leukemia 2016;30:431–8.
77. Rumi E, Pietra D, Guglielmelli P, et al. Acquired copy-neutral loss of heterozygosity of chromosome 1p as a molecular event associated with marrow fibrosis in MPL-mutated myeloproliferative neoplasms. Blood 2013;121:4388–95.
78. Beer PA, Campbell PJ, Scott LM, et al. MPL mutations in myeloproliferative disorders: analysis of the PT-1 cohort. Blood 2008;112:141–9.
79. Gangat N, Wassie EA, Lasho TL, et al. Mutations and thrombosis in essential thrombocythemia: prognostic interaction with age and thrombosis history. Eur J Haematol 2015;94:31–6.
80. Sekhar M, McVinnie K, Burroughs AK. Splanchnic vein thrombosis in myeloproliferative neoplasms. Br J Haematol 2013;162:730–47.
81. Stein BL, Saraf S, Sobol U, et al. Age-related differences in disease characteristics and clinical outcomes in polycythemia vera. Leuk Lymph 2013;54:1989–95.
82. Landolfi R, Di Gennaro L, Nicolazzi MA, et al. Polycythemia vera: gender-related phenotypic differences. Intern Emerg Med 2012;7:509–15.
83. Winslow ER, Brunt LM, Drebin JA, et al. Portal vein thrombosis after splenectomy. Am J Surg 2002;184:631–6.
84. Smalberg JH, Arends LR, Valla DC, et al. Myeloproliferative neoplasms in Budd-Chiari syndrome and portal vein thrombosis: a meta-analysis. Blood 2012;120:4921–8.
85. Dentali F, Squizzato A, Brivio L, et al. JAK2V617F mutation for the early diagnosis of Ph- myeloproliferative neoplasms in patients with venous thromboembolism: a meta-analysis. Blood 2009;113:5617–23.
86. Pardanani A, Lasho TL, Hussein K, et al. JAK2V617F mutation screening as part of the hypercoagulable work-up in the absence of splanchnic venous thrombosis or overt myeloproliferative neoplasm: assessment of value in a series of 664 consecutive patients. Mayo Clin Proc 2008;83:457–9.
87. Barbui T, Barosi G, Birgegard G, et al. Philadelphia-negative classical myeloproliferative neoplasms: critical concepts and management recommendations from European LeukemiaNet. J Clin Oncol 2011;29:761–70.
88. Landolfi R, Marchioli R, Kutti J, et al. Efficacy and safety of low-dose aspirin in polycythemia vera. N Engl J Med 2004;350:114–24.
89. Marchioli R, Finazzi G, Specchia G, et al. Cardiovascular events and intensity of treatment in polycythemia vera. N Engl J Med 2013;368:22–33.
90. Kiladjian JJ, Chevret S, Dosquet C, et al. Treatment of polycythemia vera with hydroxyurea and pipobroman: final results of a randomized trial initiated in 1980. J Clin Oncol 2011;29:3907–13.
91. Kaplan ME, Mack K, Goldberg JD, et al. Long-term management of polycythemia vera with hydroxyurea: a progress report. Semin Hematol 1986;23:167–71.
92. Fruchtman SM, Mack K, Kaplan ME, et al. From efficacy to safety: a Polycythemia Vera Study group report on hydroxyurea in patients with polycythemia vera. Semin Hematol 1997;34:17–23.
93. Finazzi G, Caruso V, Marchioli R, et al. Acute leukemia in polycythemia vera: an analysis of 1638 patients enrolled in a prospective observational study. Blood 2005;105:2664–70.
94. Barosi G, Mesa R, Finazzi G, et al. Revised response criteria for polycythemia vera and essential thrombocythemia: an ELN and IWG-MRT consensus project. Blood 2013;121:4778–81.
95. Alvarez-Larran A, Pereira A, Cervantes F, et al. Assessment and prognostic value of the European LeukemiaNet criteria for clinicohematologic response, resistance, and intolerance to hydroxyurea in polycythemia vera. Blood 2012;119:1363–9.
96. Stein BL, Tiu RV. Biological rationale and clinical use of interferon in the classical BCR-ABL-negative myeloproliferative neoplasms. J Interferon Cytokine Res 2013;33:145–53.
97. Ludwig H, Cortelezzi A, Van Camp BG, et al. Treatment with recombinant interferon-alpha-2C: multiple myeloma and thrombocythaemia in myeloproliferative diseases. Oncology 1985;42 Suppl 1:19–25.
98. Silver RT. Long-term effects of the treatment of polycythemia vera with recombinant interferon-alpha. Cancer 2006;107:451–8.
99. Kiladjian JJ, Mesa RA, Hoffman R. The renaissance of interferon therapy for the treatment of myeloid malignancies. Blood 2011;117:4706–15.
100. Veronese FM, Mero A. The impact of PEGylation on biological therapies. BioDrugs 2008;22:315–29.
101. Kiladjian JJ, Cassinat B, Chevret S, et al. Pegylated interferon-alfa-2a induces complete hematologic and molecular responses with low toxicity in polycythemia vera. Blood 2008;112:3065–72.
102. Turlure P, Cambier N, Roussel M, et al. Complete hematological, molecular and histological remissions without cytoreductive treatment lasting after pegylated-interferon {alpha}-2a (peg-IFN{alpha}-2a) therapy in polycythemia vera (PV): long term results of a phase 2 trial [abstract]. Blood 2011;118(21). Abstract 280.
103. Quintas-Cardama A, Kantarjian H, Manshouri T, et al. Pegylated interferon alfa-2a yields high rates of hematologic and molecular response in patients with advanced essential thrombocythemia and polycythemia vera. J Clin Oncol 2009;27:5418–24.
104. Quintas-Cardama A, Abdel-Wahab O, Manshouri T, et al. Molecular analysis of patients with polycythemia vera or essential thrombocythemia receiving pegylated interferon a-2a. Blood 2013;122:893–901.
105. Samuelsson J, Hasselbalch H, Bruserud O, et al. A phase II trial of pegylated interferon alpha-2b therapy for polycythemia vera and essential thrombocythemia: feasibility, clinical and biologic effects, and impact on quality of life. Cancer 2006;106:2397–405.
106. Jabbour E, Kantarjian H, Cortes J, et al. PEG-IFN-alpha-2b therapy in BCR-ABL-negative myeloproliferative disorders: final result of a phase 2 study. Cancer 2007;110:2012–18.
107. Them NC, Bagienski K, Berg T, et al. Molecular responses and chromosomal aberrations in patients with polycythemia vera treated with peg-proline-interferon alpha-2b. Am J Hematol 2015;90:288–94.
108. Gisslinger H, Klade C, Georgiev P, et al. Final results from PROUD-PV a randomized controlled phase 3 trial comparing ropeginterferon alfa-2b to hydroxyurea in polycythemia vera patients [abstract]. Blood 2016;128(suppl 22). Abstract 475.
109. van Genderen PJ, van Vliet HH, Prins FJ, et al. Excessive prolongation of the bleeding time by aspirin in essential thrombocythemia is related to a decrease of large von Willebrand factor multimers in plasma. Ann Hematol 1997;75:215–20.
110. Cortelazzo S, Finazzi G, Ruggeri M, et al. Hydroxyurea for patients with essential thrombocythemia and a high risk of thrombosis. N Engl J Med 1995;332:1132–7.
111. Harrison CN, Campbell PJ, Buck G, et al. Hydroxyurea compared with anagrelide in high-risk essential thrombocythemia. N Engl J Med 2005;353:33–45.
112. Gisslinger H, Gotic M, Holowiecki J, et al. Anagrelide compared with hydroxyurea in WHO-classified essential thrombocythemia: the ANAHYDRET Study, a randomized controlled trial. Blood 2013;121:1720–8.
113. Alvarado Y, Cortes J, Verstovsek S, et al. Pilot study of pegylated interferon-alpha 2b in patients with essential thrombocythemia. Cancer Chemother Pharmacol 2003;51:81–6.
114. Barosi G, Tefferi A, Barbui T, ad hoc committee ‘Definition of clinically relevant outcomes for contemporarily clinical trials in Ph-neg M. Do current response criteria in classical Ph-negative myeloproliferative neoplasms capture benefit for patients? Leukemia 2012;26:1148–9.
115. Bjorkholm M, Derolf AR, Hultcrantz M, et al. Treatment-related risk factors for transformation to acute myeloid leukemia and myelodysplastic syndromes in myeloproliferative neoplasms. J Clin Oncol 2011;29:2410–5.
116. Alvarez-Larran A, Martinez-Aviles L, Hernandez-Boluda JC, et al. Busulfan in patients with polycythemia vera or essential thrombocythemia refractory or intolerant to hydroxyurea. Ann Hematol 2014;93:2037–43.
117. Verstovsek S, Passamonti F, Rambaldi A, et al. A phase 2 study of ruxolitinib, an oral JAK1 and JAK2 Inhibitor, in patients with advanced polycythemia vera who are refractory or intolerant to hydroxyurea. Cancer 2014;120:513–20.
118. Vannucchi AM, Kiladjian JJ, Griesshammer M, et al. Ruxolitinib in polycythemia vera resistant to or intolerant of hydroxyurea. N Engl J Med 2015; 372:426–35.
119. Verstovsek S, Vannucchi AM, Griesshammer M, et al. Ruxolitinib versus best available therapy in patients with polycythemia vera: 80-week follow-up from the RESPONSE trial. Haematologica 2016;101:821–9.
120. Passamonti F, Griesshammer M, Palandri F, et al. Ruxolitinib for the treatment of inadequately controlled polycythaemia vera without splenomegaly (RESPONSE-2): a randomised, open-label, phase 3b study. Lancet Oncol 2017;18:88–99.
121. Verstovsek S, Passamonti F, Rambaldi A, et al. Long-term results from a phase II open-label study of ruxolitinib in patients with essential thrombocythemia refractory to or intolerant of hydroxyurea [abstract]. Blood 2014;124. Abstract 1847.
122. Harrison CN, Mead AJ, Panchal A, et al. Ruxolitinib versus best available therapy for ET intolerant or resistant to hydroxycarbamide in a randomized trial. Blood 2017 Aug 9. pii: blood-2017-05-785790 .
123. Bose P, Verstovsek S. Drug development pipeline for myeloproliferative neoplasms: potential future impact on guidelines and management. J Natl Compr Canc Netw 2016;14:1613–24.
124. Cerquozzi S, Teffieri A. Blast transformation and fibrotic progression in polycythemia vera and essential thrombocythemia: a literature review of incidence and risk factors. Blood Cancer J 2015;Nov 13;5:e366.
125. Passamonti F, Rumi E, Caramella M, et al. A dynamic prognostic model to predict survival in post-polycythemia vera myelofibrosis. Blood 2008;111:3383–7.
126. Mesa RA, Verstovsek S, Cervantes F, et al. Primary myelofibrosis (PMF), post polycythemia vera myelofibrosis (post-PV MF), post essential thrombocythemia myelofibrosis (post-ET MF), blast phase PMF (PMF-BP): Consensus on terminology by the international working group for myelofibrosis research and treatment (IWG-MRT). Leuk Res 2007;31:737–40.
127. Rampal R, Mascarenhas J. Pathogenesis and management of acute myeloid leukemia that has evolved from a myeloproliferative neoplasm. Curr Opin Hematol 2014;21:65–71.
128. Chihara D, Kantarjian HM, Newberry KJ, et al. Survival outcome of patients with acute myeloid leukemia transformed from myeloproliferative neoplasms [abstract]. Blood 2016;128. Abstract 1940.
129. Tam CS, Nussenzveig RM, Popat U, et al. The natural history and treatment outcome of blast phase BCR-ABL- myeloproliferative neoplasms. Blood 2008;112:1628–37.
130. Kundranda MN, Tibes R, Mesa RA. Transformation of a chronic myeloproliferative neoplasm to acute myelogenous leukemia: does anything work? Curr Hematol Malig Rep 2012;7:78–86.
131. Badar T, Kantarjian HM, Ravandi F, et al. Therapeutic benefit of decitabine, a hypomethylating agent, in patients with high-risk primary myelofibrosis and myeloproliferative neoplasm in accelerated or blastic/acute myeloid leukemia phase. Leuk Res 2015;39:950–6.
132. Thepot S, Itzykson R, Seegers V, et al. Treatment of progression of Philadelphia-negative myeloproliferative neoplasms to myelodysplastic syndrome or acute myeloid leukemia by azacitidine: a report on 54 cases on the behalf of the Groupe Francophone des Myelodysplasies (GFM). Blood 2010;116:3735–42.
133. Pemmaraju N, Kantarjian H, Kadia T, et al. A phase I/II study of the Janus kinase (JAK)1 and 2 inhibitor ruxolitinib in patients with relapsed or refractory acute myeloid leukemia. Clin Lymphoma Myeloma Leuk 2015;15:171–6.
134. Rampal RK, Mascarenhas JO, Kosiorek HE, et al. Safety and efficacy of combined ruxolitinib and decitabine in patients with blast-phase MPN and post-MPN AML: results of a phase I study (Myeloproliferative Disorders Research Consortium 109 trial) [abstract]. Blood 2016;128. Abstract 1124.
135. Bose P, Verstovsek S, Gasior Y, et al. Phase I/II study of ruxolitinib (RUX) with decitabine (DAC) in patients with post-myeloproliferative neoplasm acute myeloid leukemia (post-MPN AML): phase I results [abstract]. Blood 2016;128. Abstract 4262.
Omalizumab Helps Relieve Food Allergies
More than 80% of children who were given omalizumab with oral immunotherapy (OIT) for 36 weeks could safely consume portions of at least 2 foods they were causing an allergic reaction, according to findings from a phase 2 study funded by the National Institute of Allergy and Infectious Diseases. Omalizumab, an injectable antibody drug approved for moderate-to-severe allergic asthma, blocks the activity of IgE.
Researchers from Stanford University School of Medicine in California enrolled 48 children aged 4 years to 15 years with confirmed allergy to multiple foods, such as milk, egg, wheat, soy, sesame seeds, peanuts, and tree nuts. The children received omalizumab or placebo injections for the first 16 weeks. At week 8, all participants began eating small, gradually increasing amounts of an allergenic food. They continued OIT until week 36, when they underwent an oral food challenge.
Of the 36 children who received omalizumab, 30 were able to eat at least 2 grams of ≥ 2 allergenic foods, compared with that of only 4 of 12 children (33%) who received placebo. Children who received omalizumab also had fewer adverse events from OIT
More than 80% of children who were given omalizumab with oral immunotherapy (OIT) for 36 weeks could safely consume portions of at least 2 foods they were causing an allergic reaction, according to findings from a phase 2 study funded by the National Institute of Allergy and Infectious Diseases. Omalizumab, an injectable antibody drug approved for moderate-to-severe allergic asthma, blocks the activity of IgE.
Researchers from Stanford University School of Medicine in California enrolled 48 children aged 4 years to 15 years with confirmed allergy to multiple foods, such as milk, egg, wheat, soy, sesame seeds, peanuts, and tree nuts. The children received omalizumab or placebo injections for the first 16 weeks. At week 8, all participants began eating small, gradually increasing amounts of an allergenic food. They continued OIT until week 36, when they underwent an oral food challenge.
Of the 36 children who received omalizumab, 30 were able to eat at least 2 grams of ≥ 2 allergenic foods, compared with that of only 4 of 12 children (33%) who received placebo. Children who received omalizumab also had fewer adverse events from OIT
More than 80% of children who were given omalizumab with oral immunotherapy (OIT) for 36 weeks could safely consume portions of at least 2 foods they were causing an allergic reaction, according to findings from a phase 2 study funded by the National Institute of Allergy and Infectious Diseases. Omalizumab, an injectable antibody drug approved for moderate-to-severe allergic asthma, blocks the activity of IgE.
Researchers from Stanford University School of Medicine in California enrolled 48 children aged 4 years to 15 years with confirmed allergy to multiple foods, such as milk, egg, wheat, soy, sesame seeds, peanuts, and tree nuts. The children received omalizumab or placebo injections for the first 16 weeks. At week 8, all participants began eating small, gradually increasing amounts of an allergenic food. They continued OIT until week 36, when they underwent an oral food challenge.
Of the 36 children who received omalizumab, 30 were able to eat at least 2 grams of ≥ 2 allergenic foods, compared with that of only 4 of 12 children (33%) who received placebo. Children who received omalizumab also had fewer adverse events from OIT
Analysis reveals potential MM risk variants
Researchers have used high-risk pedigrees (HRPs) to identify gene variants that may cause multiple myeloma (MM).
The team’s analysis revealed shared genomic segments harboring genes with potential MM risk variants.
These single nucleotide variants (SNVs) are in USP45, a gene involved in DNA repair, and ARID1A, a gene in the SWI/SNF chromatin remodeling complex.
Nicola Camp, PhD, of the University of Utah School of Medicine in Salt Lake City, and her colleagues reported these findings in PLOS Genetics.
The researchers developed a new method to analyze HRPs (large, multi-generational families with more affected members than would be expected by chance) to identify shared regions of the genome that likely harbor MM risk variants.
The team applied the method using pedigrees from 11 Utah families at risk of MM as well as whole-exome sequencing of shared genomic segments in 1063 patients with MM or monoclonal gammopathy of undetermined significance (MGUS) and 964 control subjects.
The analysis revealed 2 regions that may contribute to MM. One was a 1.8 Mb segment at 6q16 with SNVs in USP45, and the other was a 1.2 Mb segment at 1p36.11 with SNVs in ARID1A.
One of the SNVs in USP45 was a stop gain—p.Gln691*—which was shared by 3 siblings, 1 with MM and 2 with MGUS. The other was a missense SNV—p.Gln621Glu—which was shared by 2 of 4 siblings in a family, 1 with MM and 1 with MGUS.
One of the missense SNVs in ARID1A—rs752026201, p. Ser90Gly—was shared by 3 MM cases. The other missense SNV—rs140664170, p.Met890Val—was shared by 2 cousins with MM.
The researchers believe these findings show that HRPs can be effective for identifying risk variants in complex diseases.
“We are very encouraged by the new method,” Dr Camp said. “It certainly plays to the strengths of the large Utah pedigrees, revitalizing the family design for complex diseases. As we did in this study, the focused regions can be further investigated in smaller families to find genes and specific mutations. The method can be used for any complex disease.”
“We are already pursuing large pedigrees in several other domains, including other cancers, psychiatric disorders, birth defects, and pre-term birth phenotypes, with several more genome-wide significant regions found. We’re excited about the potential.” ![]()
Researchers have used high-risk pedigrees (HRPs) to identify gene variants that may cause multiple myeloma (MM).
The team’s analysis revealed shared genomic segments harboring genes with potential MM risk variants.
These single nucleotide variants (SNVs) are in USP45, a gene involved in DNA repair, and ARID1A, a gene in the SWI/SNF chromatin remodeling complex.
Nicola Camp, PhD, of the University of Utah School of Medicine in Salt Lake City, and her colleagues reported these findings in PLOS Genetics.
The researchers developed a new method to analyze HRPs (large, multi-generational families with more affected members than would be expected by chance) to identify shared regions of the genome that likely harbor MM risk variants.
The team applied the method using pedigrees from 11 Utah families at risk of MM as well as whole-exome sequencing of shared genomic segments in 1063 patients with MM or monoclonal gammopathy of undetermined significance (MGUS) and 964 control subjects.
The analysis revealed 2 regions that may contribute to MM. One was a 1.8 Mb segment at 6q16 with SNVs in USP45, and the other was a 1.2 Mb segment at 1p36.11 with SNVs in ARID1A.
One of the SNVs in USP45 was a stop gain—p.Gln691*—which was shared by 3 siblings, 1 with MM and 2 with MGUS. The other was a missense SNV—p.Gln621Glu—which was shared by 2 of 4 siblings in a family, 1 with MM and 1 with MGUS.
One of the missense SNVs in ARID1A—rs752026201, p. Ser90Gly—was shared by 3 MM cases. The other missense SNV—rs140664170, p.Met890Val—was shared by 2 cousins with MM.
The researchers believe these findings show that HRPs can be effective for identifying risk variants in complex diseases.
“We are very encouraged by the new method,” Dr Camp said. “It certainly plays to the strengths of the large Utah pedigrees, revitalizing the family design for complex diseases. As we did in this study, the focused regions can be further investigated in smaller families to find genes and specific mutations. The method can be used for any complex disease.”
“We are already pursuing large pedigrees in several other domains, including other cancers, psychiatric disorders, birth defects, and pre-term birth phenotypes, with several more genome-wide significant regions found. We’re excited about the potential.” ![]()
Researchers have used high-risk pedigrees (HRPs) to identify gene variants that may cause multiple myeloma (MM).
The team’s analysis revealed shared genomic segments harboring genes with potential MM risk variants.
These single nucleotide variants (SNVs) are in USP45, a gene involved in DNA repair, and ARID1A, a gene in the SWI/SNF chromatin remodeling complex.
Nicola Camp, PhD, of the University of Utah School of Medicine in Salt Lake City, and her colleagues reported these findings in PLOS Genetics.
The researchers developed a new method to analyze HRPs (large, multi-generational families with more affected members than would be expected by chance) to identify shared regions of the genome that likely harbor MM risk variants.
The team applied the method using pedigrees from 11 Utah families at risk of MM as well as whole-exome sequencing of shared genomic segments in 1063 patients with MM or monoclonal gammopathy of undetermined significance (MGUS) and 964 control subjects.
The analysis revealed 2 regions that may contribute to MM. One was a 1.8 Mb segment at 6q16 with SNVs in USP45, and the other was a 1.2 Mb segment at 1p36.11 with SNVs in ARID1A.
One of the SNVs in USP45 was a stop gain—p.Gln691*—which was shared by 3 siblings, 1 with MM and 2 with MGUS. The other was a missense SNV—p.Gln621Glu—which was shared by 2 of 4 siblings in a family, 1 with MM and 1 with MGUS.
One of the missense SNVs in ARID1A—rs752026201, p. Ser90Gly—was shared by 3 MM cases. The other missense SNV—rs140664170, p.Met890Val—was shared by 2 cousins with MM.
The researchers believe these findings show that HRPs can be effective for identifying risk variants in complex diseases.
“We are very encouraged by the new method,” Dr Camp said. “It certainly plays to the strengths of the large Utah pedigrees, revitalizing the family design for complex diseases. As we did in this study, the focused regions can be further investigated in smaller families to find genes and specific mutations. The method can be used for any complex disease.”
“We are already pursuing large pedigrees in several other domains, including other cancers, psychiatric disorders, birth defects, and pre-term birth phenotypes, with several more genome-wide significant regions found. We’re excited about the potential.” ![]()
FDA investigating VTEs related to ECP
The US Food and Drug Administration (FDA) says it is evaluating reports of venous thromboembolism (VTE) in patients treated with the CELLEX Photopheresis System by Therakos, Inc.
This extracorporeal photopheresis (ECP) device system is FDA-approved for use in patients with cutaneous T-cell lymphoma (CTCL).
The system is used to perform ultraviolet-A irradiation of a patient’s own leukocyte-enriched blood that is then used as palliative treatment for skin manifestations of CTCL that are unresponsive to other forms of treatment.
The CELLEX Photopheresis System is also used to treat graft-vs-host disease (GVHD) that is resistant to standard immunosuppressive therapy and acute cardiac allograft rejection that is resistant to standard immunosuppressive therapy.
The CELLEX Photopheresis System uses methoxsalen as a photosensitizing agent and heparin as an anticoagulant.
Since 2012, the FDA has received 7 reports of pulmonary embolism (PE) and 2 reports of deep vein thrombosis (DVT) occurring during or soon after treatment with the CELLEX Photopheresis System.
Two of the patients who developed a PE died, although it’s not clear whether PE was the cause of death.
Four of the 7 PEs occurred in patients undergoing treatment for GVHD, including the 2 patients who died. Both DVTs occurred in patients undergoing treatment for GVHD as well.
The FDA is recommending that healthcare providers inform patients, clinical staff, and technicians that PE and DVT can occur during or after an ECP procedure.
The agency also recommends that healthcare providers consult device labeling regarding anticoagulation and use clinical judgment in adjusting a patient’s heparin dosage.
Finally, providers should report VTEs related to ECP procedures to the FDA’s MedWatch Safety Information and Adverse Event Reporting Program.
If possible, reports should include the following:
- The indication for ECP therapy
- Comorbidities that may predispose a patient to increased coagulation and history of DVT or PE
- The anticoagulation regimen used
- The number of ECP sessions the patient underwent prior to VTE onset, including the date of the first treatment session, frequency of treatment sessions, and timing of the final treatment
- Timing of the VTE in relation to the most recent treatment session
- Interventions required to manage the VTE.

The US Food and Drug Administration (FDA) says it is evaluating reports of venous thromboembolism (VTE) in patients treated with the CELLEX Photopheresis System by Therakos, Inc.
This extracorporeal photopheresis (ECP) device system is FDA-approved for use in patients with cutaneous T-cell lymphoma (CTCL).
The system is used to perform ultraviolet-A irradiation of a patient’s own leukocyte-enriched blood that is then used as palliative treatment for skin manifestations of CTCL that are unresponsive to other forms of treatment.
The CELLEX Photopheresis System is also used to treat graft-vs-host disease (GVHD) that is resistant to standard immunosuppressive therapy and acute cardiac allograft rejection that is resistant to standard immunosuppressive therapy.
The CELLEX Photopheresis System uses methoxsalen as a photosensitizing agent and heparin as an anticoagulant.
Since 2012, the FDA has received 7 reports of pulmonary embolism (PE) and 2 reports of deep vein thrombosis (DVT) occurring during or soon after treatment with the CELLEX Photopheresis System.
Two of the patients who developed a PE died, although it’s not clear whether PE was the cause of death.
Four of the 7 PEs occurred in patients undergoing treatment for GVHD, including the 2 patients who died. Both DVTs occurred in patients undergoing treatment for GVHD as well.
The FDA is recommending that healthcare providers inform patients, clinical staff, and technicians that PE and DVT can occur during or after an ECP procedure.
The agency also recommends that healthcare providers consult device labeling regarding anticoagulation and use clinical judgment in adjusting a patient’s heparin dosage.
Finally, providers should report VTEs related to ECP procedures to the FDA’s MedWatch Safety Information and Adverse Event Reporting Program.
If possible, reports should include the following:
- The indication for ECP therapy
- Comorbidities that may predispose a patient to increased coagulation and history of DVT or PE
- The anticoagulation regimen used
- The number of ECP sessions the patient underwent prior to VTE onset, including the date of the first treatment session, frequency of treatment sessions, and timing of the final treatment
- Timing of the VTE in relation to the most recent treatment session
- Interventions required to manage the VTE.

The US Food and Drug Administration (FDA) says it is evaluating reports of venous thromboembolism (VTE) in patients treated with the CELLEX Photopheresis System by Therakos, Inc.
This extracorporeal photopheresis (ECP) device system is FDA-approved for use in patients with cutaneous T-cell lymphoma (CTCL).
The system is used to perform ultraviolet-A irradiation of a patient’s own leukocyte-enriched blood that is then used as palliative treatment for skin manifestations of CTCL that are unresponsive to other forms of treatment.
The CELLEX Photopheresis System is also used to treat graft-vs-host disease (GVHD) that is resistant to standard immunosuppressive therapy and acute cardiac allograft rejection that is resistant to standard immunosuppressive therapy.
The CELLEX Photopheresis System uses methoxsalen as a photosensitizing agent and heparin as an anticoagulant.
Since 2012, the FDA has received 7 reports of pulmonary embolism (PE) and 2 reports of deep vein thrombosis (DVT) occurring during or soon after treatment with the CELLEX Photopheresis System.
Two of the patients who developed a PE died, although it’s not clear whether PE was the cause of death.
Four of the 7 PEs occurred in patients undergoing treatment for GVHD, including the 2 patients who died. Both DVTs occurred in patients undergoing treatment for GVHD as well.
The FDA is recommending that healthcare providers inform patients, clinical staff, and technicians that PE and DVT can occur during or after an ECP procedure.
The agency also recommends that healthcare providers consult device labeling regarding anticoagulation and use clinical judgment in adjusting a patient’s heparin dosage.
Finally, providers should report VTEs related to ECP procedures to the FDA’s MedWatch Safety Information and Adverse Event Reporting Program.
If possible, reports should include the following:
- The indication for ECP therapy
- Comorbidities that may predispose a patient to increased coagulation and history of DVT or PE
- The anticoagulation regimen used
- The number of ECP sessions the patient underwent prior to VTE onset, including the date of the first treatment session, frequency of treatment sessions, and timing of the final treatment
- Timing of the VTE in relation to the most recent treatment session
- Interventions required to manage the VTE.

The Gut Microbiome in Type 2 Diabetes
The surfaces of the human body exposed to the environment are colonized by microbes—the majority of which reside in the intestinal tract. Collectively, the microbial cells that live in and on us (bacteria, eukaryotes, viruses, fungi, and archaea) make up our microbiota, and their genetic material constitutes our microbiome. There are at least 100 times more genes in the human microbiome than in the human genome.1,2
With the help of recent technologic advances in genetic sequencing, we’re beginning to understand more about this vast biological habitat. We know that the microbiota plays a role in vitamin production, energy harvest and storage, and fermentation and absorption of undigested carbohydrates. It also has bidirectional influence on the central nervous system and neuropsychologic health and is involved in the maturation and development of the immune system.
A healthy biome is characterized by bacterial diversity and richness. Gut microbiota is mostly comprised of Firmicutes (64%), Bacteroidetes (23%), Proteobacteria (8%), and Actinobacteria (3%).2 The distribution of these bacteria is largely determined by diet; individuals who follow a diet high in animal fat have a Bacteroides-dominant pattern, whereas those who follow a carbohydrate-rich diet tend toward a Prevotella-dominant pattern.1-3
Lack of bacterial diversity and overgrowth of pathobacteria results in dysbiosis, an imbalance in the gut’s microbial composition. Alterations in the proportions of bacteria are thought to result in metabolic disease. As such, dysbiosis is correlated with obesity and diabetes, as well as other diseases (eg, inflammatory bowel disease, multiple sclerosis, Crohn disease, and rheumatoid arthritis).1-3 At this time, however, it is unclear whether these bacterial imbalances cause or result from disease.
ROLE IN TYPE 2 DIABETES
The microbiome of patients with type 2 diabetes (T2DM) is characterized by reduced levels of Firmicutes and Clostridia and an increased ratio of Bacteroidetes:Firmicutes (this ratio correlates with plasma glucose concentration).4,5 Interestingly, although T2DM and obesity are closely related, available data indicate that gut microbiome changes are not always identical between these two patient populations. In some studies, the microbiome of obese individuals involves a decreased Bacteroidetes:Firmicutes ratio, in contrast to the increase seen with T2DM—which raises the question of whether the same or different factors cause these two entities.1,5-7
Patients with T2DM also have decreased amounts of butyrate-producing bacteria in their microbiomes. Butyrate, acetate, and propionate are short-chain fatty acids (SCFAs) fermented in the large intestine by bacteria from dietary fiber. These SCFAs play an important role in energy metabolism and are critical for modulating immune responses and tumorigenesis in the gut. Butyrate, in particular, provides energy for colonic epithelial cells. By feeding colonic cells, butyrate helps to maintain intestinal integrity and prevent translocation—a process that moves gram-negative intestinal bacteria across the lumen of the gut, causing endotoxemia. Endotoxemia triggers a low-grade inflammatory response, and low-grade inflammation is thought to underlie T2DM.2,5,6
Therapeutic interventions—such as dietary modifications, prebiotics, probiotics, antibiotics, metformin, fecal transplantation, and bariatric surgery—can effectively alter the composition of gut bacteria. It has been proposed that these interventions could be harnessed to prevent and treat T2DM in the future.2 So, what might these interventions have (or not have) to offer?
ANTIBIOTICS
Antibiotics are useful for eradicating pathogenic bacteria, but they can also destroy beneficial intestinal commensals in the process. Therefore, concern about the widespread use of antibiotics in humans and livestock has increased. Subtherapeutic use of antibiotics, which has been common in farm animals throughout the past 50 years to increase growth and food production, has been shown to affect metabolic pathways—particularly with respect to SCFAs—in mouse studies.6
Recent data on humans have linked antibiotic treatment in early infancy to long-term effects on microbial diversity and childhood overweight. Similarly, long-term use of IV vancomycin in adults has been linked to an increased obesity risk. But it’s not just long-term exposure that poses a threat; even short courses of oral antibiotics can have profound and irreversible effects on intestinal microbial diversity and composition. For example, short-term use of oral vancomycin was found to impair peripheral insulin sensitivity in males with metabolic syndrome associated with altered gut microbiota, while amoxicillin did not.6
PREBIOTICS AND PROBIOTICS
Prebiotics are indigestible carbohydrates that improve host health by stimulating the growth and activity of colonic bacteria. Most prebiotics are oligosaccharides, which can travel through the upper GI system undigested. When they reach the colon, they are fermented to produce SCFAs that stimulate the growth of microbes that reside there. Prebiotics come from a wide variety of food sources, including asparagus, barley, garlic, onions, and wheat bran.2,3 Pickled and fermented foods (eg, kimchi, sauerkraut, yogurt, miso) are good sources of both prebiotics and probiotics.2
Bifidobacteria and lactobacilli are the most commonly used strains in foods and supplements containing probiotics. These live microorganisms bring about specific changes in the composition and activity of gut microbiota: they secrete antimicrobial substances, compete with pathogenic bacteria, strengthen the intestinal barrier, and modulate the immune system.2,3,6 Research on human and animal models suggests that administering probiotics may help manage diabetes.2
DIETARY MODULATION
Dietary changes have been shown to modify the bacterial metabolic activity of the human gut. In one study, obese adults with T2DM were placed on either a fat- or carbohydrate-restricted diet, and it was found that their levels of Bacteroidetes increased and Firmicutes decreased.7
In another study, patients with T2DM adhered to one of two calorie-controlled diets: a high-fiber macrobiotic diet or a Mediterranean-style (control) diet. The macrobiotic diet was high in complex carbohydrates, legumes, fermented products, sea salt, and green tea and was free of animal protein, fat, and added sugar. Both diets were effective at improving dysbiosis—ecosystem diversity increased, and health-promoting SCFA producers were replenished. However, the macrobiotic diet was more effective than the control diet at reducing fasting and postprandial glucose, A1C, serum cholesterol, insulin resistance, BMI, and waist and hip circumferences; and only the macrobiotic diet counteracted the inflammation-producing bacterial groups.8
METFORMIN
Metformin has therapeutic effects on microbial composition and SCFA synthesis. In a microbiome comparison study, patients with T2DM treated with metformin had more butyrate-producing bacteria than their untreated counterparts. The trend toward increased Lactobacillus seen in the context of T2DM was reduced or reversed by metformin treatment. Researchers were able to tell which patients were (and were not) treated based on their gut microbiome taxonomic signature.9
FECAL MICROBIOTA TRANSPLANT
Fecal microbiota transplant, also known as stool transplant or bacteriotherapy, is the process of transferring fecal bacteria from a healthy individual into a recipient. It is used in the treatment of recurrent Clostridium difficile colitis to replenish beneficial bacteria in the digestive tract following use of wide-spectrum antibiotics. In a double-blind randomized controlled trial, insulin-resistant men received either autologous (reinfusion of one’s collected feces) or allogenic (feces from a lean donor) infusions. Allogenic transplantation resulted in significantly increased intestinal microbial diversity and increased levels of butyrate-producing species, accompanied by significantly improved peripheral muscle sensitivity to insulin.1,6
BARIATRIC SURGERY
Bariatric surgery, specifically Roux-en-Y gastric bypass (RYGBP), is a powerful tool used to treat obesity. In six patients (five of whom had diabetes) treated with RYGBP,
CONCLUSION
We are just beginning to understand the microbiome and its relationship to health and disease. For patients with T2DM, a variety of interventions may be used to return the gut microbiota to health. Dietary interventions, prebiotics and probiotics, fecal microbial transplant, and bariatric surgery can influence gut microbial composition, with the goal of preventing and/or treating disease. In the future, gut microbial signatures may serve as early diagnostic markers.
1. Clemente JC, Ursell LK, Parfrey LW, Knight R. The impact of the gut microbiota on human health: an integrative view. Cell. 2012;148(6):1258-1270.
2. Barengolts E. Gut microbiota, prebiotics, probiotics and synbiotics in management of obesity and prediabetes: review of randomized controlled trials. Endod Prac. 2016;22(10):1224-1234.
3. Fujimura KE, Slusher NA, Cabana MD, Lynch SV. Role of the gut microbiota in defining human health. Expert Rev Anti Infect Ther. 2010;8(4):435-454.
4. Graessler J, Qin Y, Zhong H, et al. Metagenomic sequencing of the human gut microbiome before and after bariatric surgery in obese patients with type 2 diabetes: correlation with inflammatory and metabolic parameters. Pharmacogenomics J. 2013;13(6):514-522.
5. Larsen N, Vogensen F, van den Berg F, et al. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PloS One. 2010;5(2):e9085.
6. Hartsra AV, Bouter KEC, Backhed F, Nieuwdorp M. Insights into the role of the microbiome in obesity and type 2 diabetes. Diabetes Care. 2015;38(1):159-165.
7. Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022-1023.
8. Candela M, Biagi E, Soverini M, et al. Modulation of gut microbiota dysbioses in type 2 diabetic patients by macrobiotic Ma-Pi 2 diet. Br J Nutr. 2016;116(1):80-93.
9. Forslund K, Hildebrand F, Nielsen T, et al. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature. 2015;528(7581):262-266.
The surfaces of the human body exposed to the environment are colonized by microbes—the majority of which reside in the intestinal tract. Collectively, the microbial cells that live in and on us (bacteria, eukaryotes, viruses, fungi, and archaea) make up our microbiota, and their genetic material constitutes our microbiome. There are at least 100 times more genes in the human microbiome than in the human genome.1,2
With the help of recent technologic advances in genetic sequencing, we’re beginning to understand more about this vast biological habitat. We know that the microbiota plays a role in vitamin production, energy harvest and storage, and fermentation and absorption of undigested carbohydrates. It also has bidirectional influence on the central nervous system and neuropsychologic health and is involved in the maturation and development of the immune system.
A healthy biome is characterized by bacterial diversity and richness. Gut microbiota is mostly comprised of Firmicutes (64%), Bacteroidetes (23%), Proteobacteria (8%), and Actinobacteria (3%).2 The distribution of these bacteria is largely determined by diet; individuals who follow a diet high in animal fat have a Bacteroides-dominant pattern, whereas those who follow a carbohydrate-rich diet tend toward a Prevotella-dominant pattern.1-3
Lack of bacterial diversity and overgrowth of pathobacteria results in dysbiosis, an imbalance in the gut’s microbial composition. Alterations in the proportions of bacteria are thought to result in metabolic disease. As such, dysbiosis is correlated with obesity and diabetes, as well as other diseases (eg, inflammatory bowel disease, multiple sclerosis, Crohn disease, and rheumatoid arthritis).1-3 At this time, however, it is unclear whether these bacterial imbalances cause or result from disease.
ROLE IN TYPE 2 DIABETES
The microbiome of patients with type 2 diabetes (T2DM) is characterized by reduced levels of Firmicutes and Clostridia and an increased ratio of Bacteroidetes:Firmicutes (this ratio correlates with plasma glucose concentration).4,5 Interestingly, although T2DM and obesity are closely related, available data indicate that gut microbiome changes are not always identical between these two patient populations. In some studies, the microbiome of obese individuals involves a decreased Bacteroidetes:Firmicutes ratio, in contrast to the increase seen with T2DM—which raises the question of whether the same or different factors cause these two entities.1,5-7
Patients with T2DM also have decreased amounts of butyrate-producing bacteria in their microbiomes. Butyrate, acetate, and propionate are short-chain fatty acids (SCFAs) fermented in the large intestine by bacteria from dietary fiber. These SCFAs play an important role in energy metabolism and are critical for modulating immune responses and tumorigenesis in the gut. Butyrate, in particular, provides energy for colonic epithelial cells. By feeding colonic cells, butyrate helps to maintain intestinal integrity and prevent translocation—a process that moves gram-negative intestinal bacteria across the lumen of the gut, causing endotoxemia. Endotoxemia triggers a low-grade inflammatory response, and low-grade inflammation is thought to underlie T2DM.2,5,6
Therapeutic interventions—such as dietary modifications, prebiotics, probiotics, antibiotics, metformin, fecal transplantation, and bariatric surgery—can effectively alter the composition of gut bacteria. It has been proposed that these interventions could be harnessed to prevent and treat T2DM in the future.2 So, what might these interventions have (or not have) to offer?
ANTIBIOTICS
Antibiotics are useful for eradicating pathogenic bacteria, but they can also destroy beneficial intestinal commensals in the process. Therefore, concern about the widespread use of antibiotics in humans and livestock has increased. Subtherapeutic use of antibiotics, which has been common in farm animals throughout the past 50 years to increase growth and food production, has been shown to affect metabolic pathways—particularly with respect to SCFAs—in mouse studies.6
Recent data on humans have linked antibiotic treatment in early infancy to long-term effects on microbial diversity and childhood overweight. Similarly, long-term use of IV vancomycin in adults has been linked to an increased obesity risk. But it’s not just long-term exposure that poses a threat; even short courses of oral antibiotics can have profound and irreversible effects on intestinal microbial diversity and composition. For example, short-term use of oral vancomycin was found to impair peripheral insulin sensitivity in males with metabolic syndrome associated with altered gut microbiota, while amoxicillin did not.6
PREBIOTICS AND PROBIOTICS
Prebiotics are indigestible carbohydrates that improve host health by stimulating the growth and activity of colonic bacteria. Most prebiotics are oligosaccharides, which can travel through the upper GI system undigested. When they reach the colon, they are fermented to produce SCFAs that stimulate the growth of microbes that reside there. Prebiotics come from a wide variety of food sources, including asparagus, barley, garlic, onions, and wheat bran.2,3 Pickled and fermented foods (eg, kimchi, sauerkraut, yogurt, miso) are good sources of both prebiotics and probiotics.2
Bifidobacteria and lactobacilli are the most commonly used strains in foods and supplements containing probiotics. These live microorganisms bring about specific changes in the composition and activity of gut microbiota: they secrete antimicrobial substances, compete with pathogenic bacteria, strengthen the intestinal barrier, and modulate the immune system.2,3,6 Research on human and animal models suggests that administering probiotics may help manage diabetes.2
DIETARY MODULATION
Dietary changes have been shown to modify the bacterial metabolic activity of the human gut. In one study, obese adults with T2DM were placed on either a fat- or carbohydrate-restricted diet, and it was found that their levels of Bacteroidetes increased and Firmicutes decreased.7
In another study, patients with T2DM adhered to one of two calorie-controlled diets: a high-fiber macrobiotic diet or a Mediterranean-style (control) diet. The macrobiotic diet was high in complex carbohydrates, legumes, fermented products, sea salt, and green tea and was free of animal protein, fat, and added sugar. Both diets were effective at improving dysbiosis—ecosystem diversity increased, and health-promoting SCFA producers were replenished. However, the macrobiotic diet was more effective than the control diet at reducing fasting and postprandial glucose, A1C, serum cholesterol, insulin resistance, BMI, and waist and hip circumferences; and only the macrobiotic diet counteracted the inflammation-producing bacterial groups.8
METFORMIN
Metformin has therapeutic effects on microbial composition and SCFA synthesis. In a microbiome comparison study, patients with T2DM treated with metformin had more butyrate-producing bacteria than their untreated counterparts. The trend toward increased Lactobacillus seen in the context of T2DM was reduced or reversed by metformin treatment. Researchers were able to tell which patients were (and were not) treated based on their gut microbiome taxonomic signature.9
FECAL MICROBIOTA TRANSPLANT
Fecal microbiota transplant, also known as stool transplant or bacteriotherapy, is the process of transferring fecal bacteria from a healthy individual into a recipient. It is used in the treatment of recurrent Clostridium difficile colitis to replenish beneficial bacteria in the digestive tract following use of wide-spectrum antibiotics. In a double-blind randomized controlled trial, insulin-resistant men received either autologous (reinfusion of one’s collected feces) or allogenic (feces from a lean donor) infusions. Allogenic transplantation resulted in significantly increased intestinal microbial diversity and increased levels of butyrate-producing species, accompanied by significantly improved peripheral muscle sensitivity to insulin.1,6
BARIATRIC SURGERY
Bariatric surgery, specifically Roux-en-Y gastric bypass (RYGBP), is a powerful tool used to treat obesity. In six patients (five of whom had diabetes) treated with RYGBP,
CONCLUSION
We are just beginning to understand the microbiome and its relationship to health and disease. For patients with T2DM, a variety of interventions may be used to return the gut microbiota to health. Dietary interventions, prebiotics and probiotics, fecal microbial transplant, and bariatric surgery can influence gut microbial composition, with the goal of preventing and/or treating disease. In the future, gut microbial signatures may serve as early diagnostic markers.
The surfaces of the human body exposed to the environment are colonized by microbes—the majority of which reside in the intestinal tract. Collectively, the microbial cells that live in and on us (bacteria, eukaryotes, viruses, fungi, and archaea) make up our microbiota, and their genetic material constitutes our microbiome. There are at least 100 times more genes in the human microbiome than in the human genome.1,2
With the help of recent technologic advances in genetic sequencing, we’re beginning to understand more about this vast biological habitat. We know that the microbiota plays a role in vitamin production, energy harvest and storage, and fermentation and absorption of undigested carbohydrates. It also has bidirectional influence on the central nervous system and neuropsychologic health and is involved in the maturation and development of the immune system.
A healthy biome is characterized by bacterial diversity and richness. Gut microbiota is mostly comprised of Firmicutes (64%), Bacteroidetes (23%), Proteobacteria (8%), and Actinobacteria (3%).2 The distribution of these bacteria is largely determined by diet; individuals who follow a diet high in animal fat have a Bacteroides-dominant pattern, whereas those who follow a carbohydrate-rich diet tend toward a Prevotella-dominant pattern.1-3
Lack of bacterial diversity and overgrowth of pathobacteria results in dysbiosis, an imbalance in the gut’s microbial composition. Alterations in the proportions of bacteria are thought to result in metabolic disease. As such, dysbiosis is correlated with obesity and diabetes, as well as other diseases (eg, inflammatory bowel disease, multiple sclerosis, Crohn disease, and rheumatoid arthritis).1-3 At this time, however, it is unclear whether these bacterial imbalances cause or result from disease.
ROLE IN TYPE 2 DIABETES
The microbiome of patients with type 2 diabetes (T2DM) is characterized by reduced levels of Firmicutes and Clostridia and an increased ratio of Bacteroidetes:Firmicutes (this ratio correlates with plasma glucose concentration).4,5 Interestingly, although T2DM and obesity are closely related, available data indicate that gut microbiome changes are not always identical between these two patient populations. In some studies, the microbiome of obese individuals involves a decreased Bacteroidetes:Firmicutes ratio, in contrast to the increase seen with T2DM—which raises the question of whether the same or different factors cause these two entities.1,5-7
Patients with T2DM also have decreased amounts of butyrate-producing bacteria in their microbiomes. Butyrate, acetate, and propionate are short-chain fatty acids (SCFAs) fermented in the large intestine by bacteria from dietary fiber. These SCFAs play an important role in energy metabolism and are critical for modulating immune responses and tumorigenesis in the gut. Butyrate, in particular, provides energy for colonic epithelial cells. By feeding colonic cells, butyrate helps to maintain intestinal integrity and prevent translocation—a process that moves gram-negative intestinal bacteria across the lumen of the gut, causing endotoxemia. Endotoxemia triggers a low-grade inflammatory response, and low-grade inflammation is thought to underlie T2DM.2,5,6
Therapeutic interventions—such as dietary modifications, prebiotics, probiotics, antibiotics, metformin, fecal transplantation, and bariatric surgery—can effectively alter the composition of gut bacteria. It has been proposed that these interventions could be harnessed to prevent and treat T2DM in the future.2 So, what might these interventions have (or not have) to offer?
ANTIBIOTICS
Antibiotics are useful for eradicating pathogenic bacteria, but they can also destroy beneficial intestinal commensals in the process. Therefore, concern about the widespread use of antibiotics in humans and livestock has increased. Subtherapeutic use of antibiotics, which has been common in farm animals throughout the past 50 years to increase growth and food production, has been shown to affect metabolic pathways—particularly with respect to SCFAs—in mouse studies.6
Recent data on humans have linked antibiotic treatment in early infancy to long-term effects on microbial diversity and childhood overweight. Similarly, long-term use of IV vancomycin in adults has been linked to an increased obesity risk. But it’s not just long-term exposure that poses a threat; even short courses of oral antibiotics can have profound and irreversible effects on intestinal microbial diversity and composition. For example, short-term use of oral vancomycin was found to impair peripheral insulin sensitivity in males with metabolic syndrome associated with altered gut microbiota, while amoxicillin did not.6
PREBIOTICS AND PROBIOTICS
Prebiotics are indigestible carbohydrates that improve host health by stimulating the growth and activity of colonic bacteria. Most prebiotics are oligosaccharides, which can travel through the upper GI system undigested. When they reach the colon, they are fermented to produce SCFAs that stimulate the growth of microbes that reside there. Prebiotics come from a wide variety of food sources, including asparagus, barley, garlic, onions, and wheat bran.2,3 Pickled and fermented foods (eg, kimchi, sauerkraut, yogurt, miso) are good sources of both prebiotics and probiotics.2
Bifidobacteria and lactobacilli are the most commonly used strains in foods and supplements containing probiotics. These live microorganisms bring about specific changes in the composition and activity of gut microbiota: they secrete antimicrobial substances, compete with pathogenic bacteria, strengthen the intestinal barrier, and modulate the immune system.2,3,6 Research on human and animal models suggests that administering probiotics may help manage diabetes.2
DIETARY MODULATION
Dietary changes have been shown to modify the bacterial metabolic activity of the human gut. In one study, obese adults with T2DM were placed on either a fat- or carbohydrate-restricted diet, and it was found that their levels of Bacteroidetes increased and Firmicutes decreased.7
In another study, patients with T2DM adhered to one of two calorie-controlled diets: a high-fiber macrobiotic diet or a Mediterranean-style (control) diet. The macrobiotic diet was high in complex carbohydrates, legumes, fermented products, sea salt, and green tea and was free of animal protein, fat, and added sugar. Both diets were effective at improving dysbiosis—ecosystem diversity increased, and health-promoting SCFA producers were replenished. However, the macrobiotic diet was more effective than the control diet at reducing fasting and postprandial glucose, A1C, serum cholesterol, insulin resistance, BMI, and waist and hip circumferences; and only the macrobiotic diet counteracted the inflammation-producing bacterial groups.8
METFORMIN
Metformin has therapeutic effects on microbial composition and SCFA synthesis. In a microbiome comparison study, patients with T2DM treated with metformin had more butyrate-producing bacteria than their untreated counterparts. The trend toward increased Lactobacillus seen in the context of T2DM was reduced or reversed by metformin treatment. Researchers were able to tell which patients were (and were not) treated based on their gut microbiome taxonomic signature.9
FECAL MICROBIOTA TRANSPLANT
Fecal microbiota transplant, also known as stool transplant or bacteriotherapy, is the process of transferring fecal bacteria from a healthy individual into a recipient. It is used in the treatment of recurrent Clostridium difficile colitis to replenish beneficial bacteria in the digestive tract following use of wide-spectrum antibiotics. In a double-blind randomized controlled trial, insulin-resistant men received either autologous (reinfusion of one’s collected feces) or allogenic (feces from a lean donor) infusions. Allogenic transplantation resulted in significantly increased intestinal microbial diversity and increased levels of butyrate-producing species, accompanied by significantly improved peripheral muscle sensitivity to insulin.1,6
BARIATRIC SURGERY
Bariatric surgery, specifically Roux-en-Y gastric bypass (RYGBP), is a powerful tool used to treat obesity. In six patients (five of whom had diabetes) treated with RYGBP,
CONCLUSION
We are just beginning to understand the microbiome and its relationship to health and disease. For patients with T2DM, a variety of interventions may be used to return the gut microbiota to health. Dietary interventions, prebiotics and probiotics, fecal microbial transplant, and bariatric surgery can influence gut microbial composition, with the goal of preventing and/or treating disease. In the future, gut microbial signatures may serve as early diagnostic markers.
1. Clemente JC, Ursell LK, Parfrey LW, Knight R. The impact of the gut microbiota on human health: an integrative view. Cell. 2012;148(6):1258-1270.
2. Barengolts E. Gut microbiota, prebiotics, probiotics and synbiotics in management of obesity and prediabetes: review of randomized controlled trials. Endod Prac. 2016;22(10):1224-1234.
3. Fujimura KE, Slusher NA, Cabana MD, Lynch SV. Role of the gut microbiota in defining human health. Expert Rev Anti Infect Ther. 2010;8(4):435-454.
4. Graessler J, Qin Y, Zhong H, et al. Metagenomic sequencing of the human gut microbiome before and after bariatric surgery in obese patients with type 2 diabetes: correlation with inflammatory and metabolic parameters. Pharmacogenomics J. 2013;13(6):514-522.
5. Larsen N, Vogensen F, van den Berg F, et al. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PloS One. 2010;5(2):e9085.
6. Hartsra AV, Bouter KEC, Backhed F, Nieuwdorp M. Insights into the role of the microbiome in obesity and type 2 diabetes. Diabetes Care. 2015;38(1):159-165.
7. Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022-1023.
8. Candela M, Biagi E, Soverini M, et al. Modulation of gut microbiota dysbioses in type 2 diabetic patients by macrobiotic Ma-Pi 2 diet. Br J Nutr. 2016;116(1):80-93.
9. Forslund K, Hildebrand F, Nielsen T, et al. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature. 2015;528(7581):262-266.
1. Clemente JC, Ursell LK, Parfrey LW, Knight R. The impact of the gut microbiota on human health: an integrative view. Cell. 2012;148(6):1258-1270.
2. Barengolts E. Gut microbiota, prebiotics, probiotics and synbiotics in management of obesity and prediabetes: review of randomized controlled trials. Endod Prac. 2016;22(10):1224-1234.
3. Fujimura KE, Slusher NA, Cabana MD, Lynch SV. Role of the gut microbiota in defining human health. Expert Rev Anti Infect Ther. 2010;8(4):435-454.
4. Graessler J, Qin Y, Zhong H, et al. Metagenomic sequencing of the human gut microbiome before and after bariatric surgery in obese patients with type 2 diabetes: correlation with inflammatory and metabolic parameters. Pharmacogenomics J. 2013;13(6):514-522.
5. Larsen N, Vogensen F, van den Berg F, et al. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PloS One. 2010;5(2):e9085.
6. Hartsra AV, Bouter KEC, Backhed F, Nieuwdorp M. Insights into the role of the microbiome in obesity and type 2 diabetes. Diabetes Care. 2015;38(1):159-165.
7. Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022-1023.
8. Candela M, Biagi E, Soverini M, et al. Modulation of gut microbiota dysbioses in type 2 diabetic patients by macrobiotic Ma-Pi 2 diet. Br J Nutr. 2016;116(1):80-93.
9. Forslund K, Hildebrand F, Nielsen T, et al. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature. 2015;528(7581):262-266.