User login
‘Clean and sober’ ex-prisoners have better HIV suppression
BOSTON – Prisoners living with HIV who have drug- or alcohol-abuse disorders and are given extended-release naltrexone prior to release are significantly more likely to have improved viral suppression at 6 months, compared with fellow HIV+ prisoners who do not, investigators in two parallel clinical trials reported.
“A medication that can be used for reduction of alcohol and opiate use could also help stabilize patients coming out of prison and jail, and help maintain or achieve viral suppression,” said Sandra Springer, MD, from Yale University, New Haven, Conn.
“The United States incarcerates more individuals than any other country in the world, and our prevalence rates in prison of HIV are three times greater, and if we’re trying to achieve the 90-90-90 goals in 2 years, we have to pay special attention to this population that has significant substance-use disorders, in particular opiate- and alcohol-use disorders,” she said at a briefing following her presentation of the data in an oral abstract session.
Dr. Springer and her colleagues had shown in a previous study that, although 59% of HIV-positive prisoners treated with ART while incarcerated attained viral suppression, the percentage who retained suppression dropped to 18% just 3 months after they were released. The investigators also found that relapse to drug and alcohol use occurs quickly after release, and that relapses are associated with loss of viral suppression.
In the studies reported at CROI 2018, Dr. Springer and her colleagues evaluated the effects of treatment with XR-NTX on HIV viral suppression among HIV-positive prisoners and jail detainees with either opioid-use disorders (NEW HOPE study) or alcohol-use disorders (INSPIRE study) after they are released to the community.
Both studies were double-blind, placebo-controlled, randomized trials. Detainees were recruited, enrolled, and randomized while imprisoned to receive either placebo or XR-NTX in six monthly injections, with the first performed in prison, and the subsequent five injections performed in the community.
The participants were all HIV-seropositive prisoners aged 18 years or older returning to communities in Connecticut and western Massachusetts who met DSM-IV criteria for either alcohol- or opioid-use disorder.
The investigators found that among opioid users in NEW HOPE, viral suppression levels (fewer than 50 copies/mL) improved from 37.9% at baseline to 60.6% at 6 months among 66 individuals who received XR-NTX (P = .002). In contrast, viral suppression among 27 placebo users dropped from 55.6% at baseline to 40.7%, although this decline was not statistically significant.
In multivariate analysis controlling for treatment arm, cocaine-use disorder, homelessness, or number of injections received, the only significant predictor for viral suppression at 6 months was XR-NTX vs. placebo (odds ratio, 2.90; P = .043). There were no serious adverse events in this study.
Among those with alcohol-use disorders in the INSPIRE study, the changes in viral suppression were similar to those in the NEW HOPE study, improving from 31% at baseline to 56.7% at 6 months among 67 participants in the XR-NTX arm (P = .001), compared with a decline from 42% to 30.3% among 33 participants in the placebo arm, although again this difference was not significant.
In the INSPIRE study, significant predictors of viral suppression at 6 months included naltrexone XR (OR, 4.54; P = .009), three or more injections (OR, 6.34; P = .001), white vs. black or Hispanic (OR, 5.37; P = .040), and alcohol improvement score, a composite measure of drinking parameters (OR, 1.43; P =.033).
Dr. Springer said in the briefing that inclusion criteria in the studies were broad enough to allow overlap between alcohol-use and drug-use disorders,
She emphasized that persons living with HIV who have drug- or alcohol-use disorders and are being released from incarcerations should be strongly considered for antiopioid and/or antialcohol pharmacotherapy in addition to ART.
The study was supported by National Institutes of Health grants, and by Alkermes, which supplied XR-NTX and placebo to investigators. Dr. Springer disclosed research grants from the National Institute on Drug Abuse.
SOURCE: Springer S et al. CROI Abstract 96
BOSTON – Prisoners living with HIV who have drug- or alcohol-abuse disorders and are given extended-release naltrexone prior to release are significantly more likely to have improved viral suppression at 6 months, compared with fellow HIV+ prisoners who do not, investigators in two parallel clinical trials reported.
“A medication that can be used for reduction of alcohol and opiate use could also help stabilize patients coming out of prison and jail, and help maintain or achieve viral suppression,” said Sandra Springer, MD, from Yale University, New Haven, Conn.
“The United States incarcerates more individuals than any other country in the world, and our prevalence rates in prison of HIV are three times greater, and if we’re trying to achieve the 90-90-90 goals in 2 years, we have to pay special attention to this population that has significant substance-use disorders, in particular opiate- and alcohol-use disorders,” she said at a briefing following her presentation of the data in an oral abstract session.
Dr. Springer and her colleagues had shown in a previous study that, although 59% of HIV-positive prisoners treated with ART while incarcerated attained viral suppression, the percentage who retained suppression dropped to 18% just 3 months after they were released. The investigators also found that relapse to drug and alcohol use occurs quickly after release, and that relapses are associated with loss of viral suppression.
In the studies reported at CROI 2018, Dr. Springer and her colleagues evaluated the effects of treatment with XR-NTX on HIV viral suppression among HIV-positive prisoners and jail detainees with either opioid-use disorders (NEW HOPE study) or alcohol-use disorders (INSPIRE study) after they are released to the community.
Both studies were double-blind, placebo-controlled, randomized trials. Detainees were recruited, enrolled, and randomized while imprisoned to receive either placebo or XR-NTX in six monthly injections, with the first performed in prison, and the subsequent five injections performed in the community.
The participants were all HIV-seropositive prisoners aged 18 years or older returning to communities in Connecticut and western Massachusetts who met DSM-IV criteria for either alcohol- or opioid-use disorder.
The investigators found that among opioid users in NEW HOPE, viral suppression levels (fewer than 50 copies/mL) improved from 37.9% at baseline to 60.6% at 6 months among 66 individuals who received XR-NTX (P = .002). In contrast, viral suppression among 27 placebo users dropped from 55.6% at baseline to 40.7%, although this decline was not statistically significant.
In multivariate analysis controlling for treatment arm, cocaine-use disorder, homelessness, or number of injections received, the only significant predictor for viral suppression at 6 months was XR-NTX vs. placebo (odds ratio, 2.90; P = .043). There were no serious adverse events in this study.
Among those with alcohol-use disorders in the INSPIRE study, the changes in viral suppression were similar to those in the NEW HOPE study, improving from 31% at baseline to 56.7% at 6 months among 67 participants in the XR-NTX arm (P = .001), compared with a decline from 42% to 30.3% among 33 participants in the placebo arm, although again this difference was not significant.
In the INSPIRE study, significant predictors of viral suppression at 6 months included naltrexone XR (OR, 4.54; P = .009), three or more injections (OR, 6.34; P = .001), white vs. black or Hispanic (OR, 5.37; P = .040), and alcohol improvement score, a composite measure of drinking parameters (OR, 1.43; P =.033).
Dr. Springer said in the briefing that inclusion criteria in the studies were broad enough to allow overlap between alcohol-use and drug-use disorders,
She emphasized that persons living with HIV who have drug- or alcohol-use disorders and are being released from incarcerations should be strongly considered for antiopioid and/or antialcohol pharmacotherapy in addition to ART.
The study was supported by National Institutes of Health grants, and by Alkermes, which supplied XR-NTX and placebo to investigators. Dr. Springer disclosed research grants from the National Institute on Drug Abuse.
SOURCE: Springer S et al. CROI Abstract 96
BOSTON – Prisoners living with HIV who have drug- or alcohol-abuse disorders and are given extended-release naltrexone prior to release are significantly more likely to have improved viral suppression at 6 months, compared with fellow HIV+ prisoners who do not, investigators in two parallel clinical trials reported.
“A medication that can be used for reduction of alcohol and opiate use could also help stabilize patients coming out of prison and jail, and help maintain or achieve viral suppression,” said Sandra Springer, MD, from Yale University, New Haven, Conn.
“The United States incarcerates more individuals than any other country in the world, and our prevalence rates in prison of HIV are three times greater, and if we’re trying to achieve the 90-90-90 goals in 2 years, we have to pay special attention to this population that has significant substance-use disorders, in particular opiate- and alcohol-use disorders,” she said at a briefing following her presentation of the data in an oral abstract session.
Dr. Springer and her colleagues had shown in a previous study that, although 59% of HIV-positive prisoners treated with ART while incarcerated attained viral suppression, the percentage who retained suppression dropped to 18% just 3 months after they were released. The investigators also found that relapse to drug and alcohol use occurs quickly after release, and that relapses are associated with loss of viral suppression.
In the studies reported at CROI 2018, Dr. Springer and her colleagues evaluated the effects of treatment with XR-NTX on HIV viral suppression among HIV-positive prisoners and jail detainees with either opioid-use disorders (NEW HOPE study) or alcohol-use disorders (INSPIRE study) after they are released to the community.
Both studies were double-blind, placebo-controlled, randomized trials. Detainees were recruited, enrolled, and randomized while imprisoned to receive either placebo or XR-NTX in six monthly injections, with the first performed in prison, and the subsequent five injections performed in the community.
The participants were all HIV-seropositive prisoners aged 18 years or older returning to communities in Connecticut and western Massachusetts who met DSM-IV criteria for either alcohol- or opioid-use disorder.
The investigators found that among opioid users in NEW HOPE, viral suppression levels (fewer than 50 copies/mL) improved from 37.9% at baseline to 60.6% at 6 months among 66 individuals who received XR-NTX (P = .002). In contrast, viral suppression among 27 placebo users dropped from 55.6% at baseline to 40.7%, although this decline was not statistically significant.
In multivariate analysis controlling for treatment arm, cocaine-use disorder, homelessness, or number of injections received, the only significant predictor for viral suppression at 6 months was XR-NTX vs. placebo (odds ratio, 2.90; P = .043). There were no serious adverse events in this study.
Among those with alcohol-use disorders in the INSPIRE study, the changes in viral suppression were similar to those in the NEW HOPE study, improving from 31% at baseline to 56.7% at 6 months among 67 participants in the XR-NTX arm (P = .001), compared with a decline from 42% to 30.3% among 33 participants in the placebo arm, although again this difference was not significant.
In the INSPIRE study, significant predictors of viral suppression at 6 months included naltrexone XR (OR, 4.54; P = .009), three or more injections (OR, 6.34; P = .001), white vs. black or Hispanic (OR, 5.37; P = .040), and alcohol improvement score, a composite measure of drinking parameters (OR, 1.43; P =.033).
Dr. Springer said in the briefing that inclusion criteria in the studies were broad enough to allow overlap between alcohol-use and drug-use disorders,
She emphasized that persons living with HIV who have drug- or alcohol-use disorders and are being released from incarcerations should be strongly considered for antiopioid and/or antialcohol pharmacotherapy in addition to ART.
The study was supported by National Institutes of Health grants, and by Alkermes, which supplied XR-NTX and placebo to investigators. Dr. Springer disclosed research grants from the National Institute on Drug Abuse.
SOURCE: Springer S et al. CROI Abstract 96
REPORTING FROM CROI
Key clinical point: Among the HIV-positive prison population, drug and alcohol use are predictors for lower viral suppression rates.
Major finding: Viral suppression levels at 6 months were significantly higher among HIV+ drug or alcohol abusers treated with monthly naltrexone extended release.
Data source: Prospective, double-blind randomized trials in 93 HIV+ prisoners with opioid-use disorder (NEW HOPE) and 100 with alcohol-use disorder (INSPIRE).
Disclosures: The study was supported by National Institutes of Health grants, and by Alkermes, which supplied XR-NTX and placebo to investigators. Dr. Springer disclosed research grants from the National Institute on Drug Abuse.
Source: Springer S et al. CROI, Abstract 96.
MDedge Daily News: High deductibles harm breast cancer care
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
How high deductibles could harm breast cancer care, CMS pushes back on Arkansas’s proposed Medicaid rollback, opioid deaths spill into the nation’s emergency departments, and why opioids aren’t the better choice for chronic pain.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
How high deductibles could harm breast cancer care, CMS pushes back on Arkansas’s proposed Medicaid rollback, opioid deaths spill into the nation’s emergency departments, and why opioids aren’t the better choice for chronic pain.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
How high deductibles could harm breast cancer care, CMS pushes back on Arkansas’s proposed Medicaid rollback, opioid deaths spill into the nation’s emergency departments, and why opioids aren’t the better choice for chronic pain.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
Researchers question validity of NCCN guidelines
New research suggests guidelines from the National Comprehensive Cancer Network (NCCN) may sometimes be supported by low-quality evidence or no evidence at all.
Researchers compared NCCN recommendations for cancer drugs to US cancer drug approvals over a 5-year period.
Thirty-nine percent of NCCN’s treatment recommendations did not coincide with uses approved by the US Food and Drug Administration (FDA).
For most of these recommendations (84%), NCCN did not provide supporting data from randomized, phase 3 trials.
For 36% of the recommendations, NCCN gave no supporting evidence.
Vinay Prasad, MD, of Oregon Health & Science University in Portland, Oregon, and his colleagues reported these findings in The BMJ.
Dr Prasad and his colleagues compared FDA approvals of cancer drugs between 2011 and 2015 with NCCN recommendations as of March 25, 2016.
When NCCN made recommendations beyond FDA approvals, the researchers evaluated the evidence used to support those recommendations.
Forty-seven new cancer drugs were approved by the FDA for 69 indications between 2011 and 2015. NCCN recommended the 47 drugs for 113 indications, including the 69 FDA-approved indications.
So 39% (n=44) of NCCN’s recommendations were not approved by the FDA, and NCCN gave the following evidence to support these recommendations:
- No evidence—36% (n=16)
- Phase 2 trial without randomization—30% (n=13)
- Randomized, phase 3 trial—16% (n=7)
- Phase 2 trial with randomization—7% (n=3)
- Case report or series of less than 5 patients—5% (n=2)
- Book chapter or review article—2% (n=1)
- Phase 1 trial—2% (n=1)
- Ongoing trial—2% (n=1).
Dr Prasad and his colleagues did point out that not all FDA approvals are supported by randomized, phase 3 trials.
And when the team followed-up 21 months after their initial analysis, they found that 6 of the 44 (14%) additional recommendations by NCCN had received FDA approval.
The researchers also noted that they did not search for independent evidence to support NCCN recommendations beyond the references NCCN provided. So some of the recommendations may have had more or better supporting evidence than what was provided.
Still, the team said these results suggest NCCN “frequently” makes recommendations that go beyond FDA approvals and “often fails to cite evidence or relies on low levels of evidence.” Therefore, NCCN should cite all evidence used to formulate its recommendations.
NCCN argues that it does provide ample evidence to support the recommendations in its guidelines.
“The NCCN guidelines contain more than 24,500 references to inform users of the evidence used in making its decisions,” said Robert W. Carlson, MD, chief executive officer of NCCN.
“These data are supplemented by the analysis of the available evidence by expert clinician researchers and patient advocates who evaluate each recommendation and come to consensus. Each recommendation is labeled with a Category of Evidence, and the vast majority of those for systemic therapies are accompanied by Evidence Blocks, which outline, on 1-5 scales, the efficacy, safety, quality of the evidence, consistency of the evidence, and affordability of the treatment.”
New research suggests guidelines from the National Comprehensive Cancer Network (NCCN) may sometimes be supported by low-quality evidence or no evidence at all.
Researchers compared NCCN recommendations for cancer drugs to US cancer drug approvals over a 5-year period.
Thirty-nine percent of NCCN’s treatment recommendations did not coincide with uses approved by the US Food and Drug Administration (FDA).
For most of these recommendations (84%), NCCN did not provide supporting data from randomized, phase 3 trials.
For 36% of the recommendations, NCCN gave no supporting evidence.
Vinay Prasad, MD, of Oregon Health & Science University in Portland, Oregon, and his colleagues reported these findings in The BMJ.
Dr Prasad and his colleagues compared FDA approvals of cancer drugs between 2011 and 2015 with NCCN recommendations as of March 25, 2016.
When NCCN made recommendations beyond FDA approvals, the researchers evaluated the evidence used to support those recommendations.
Forty-seven new cancer drugs were approved by the FDA for 69 indications between 2011 and 2015. NCCN recommended the 47 drugs for 113 indications, including the 69 FDA-approved indications.
So 39% (n=44) of NCCN’s recommendations were not approved by the FDA, and NCCN gave the following evidence to support these recommendations:
- No evidence—36% (n=16)
- Phase 2 trial without randomization—30% (n=13)
- Randomized, phase 3 trial—16% (n=7)
- Phase 2 trial with randomization—7% (n=3)
- Case report or series of less than 5 patients—5% (n=2)
- Book chapter or review article—2% (n=1)
- Phase 1 trial—2% (n=1)
- Ongoing trial—2% (n=1).
Dr Prasad and his colleagues did point out that not all FDA approvals are supported by randomized, phase 3 trials.
And when the team followed-up 21 months after their initial analysis, they found that 6 of the 44 (14%) additional recommendations by NCCN had received FDA approval.
The researchers also noted that they did not search for independent evidence to support NCCN recommendations beyond the references NCCN provided. So some of the recommendations may have had more or better supporting evidence than what was provided.
Still, the team said these results suggest NCCN “frequently” makes recommendations that go beyond FDA approvals and “often fails to cite evidence or relies on low levels of evidence.” Therefore, NCCN should cite all evidence used to formulate its recommendations.
NCCN argues that it does provide ample evidence to support the recommendations in its guidelines.
“The NCCN guidelines contain more than 24,500 references to inform users of the evidence used in making its decisions,” said Robert W. Carlson, MD, chief executive officer of NCCN.
“These data are supplemented by the analysis of the available evidence by expert clinician researchers and patient advocates who evaluate each recommendation and come to consensus. Each recommendation is labeled with a Category of Evidence, and the vast majority of those for systemic therapies are accompanied by Evidence Blocks, which outline, on 1-5 scales, the efficacy, safety, quality of the evidence, consistency of the evidence, and affordability of the treatment.”
New research suggests guidelines from the National Comprehensive Cancer Network (NCCN) may sometimes be supported by low-quality evidence or no evidence at all.
Researchers compared NCCN recommendations for cancer drugs to US cancer drug approvals over a 5-year period.
Thirty-nine percent of NCCN’s treatment recommendations did not coincide with uses approved by the US Food and Drug Administration (FDA).
For most of these recommendations (84%), NCCN did not provide supporting data from randomized, phase 3 trials.
For 36% of the recommendations, NCCN gave no supporting evidence.
Vinay Prasad, MD, of Oregon Health & Science University in Portland, Oregon, and his colleagues reported these findings in The BMJ.
Dr Prasad and his colleagues compared FDA approvals of cancer drugs between 2011 and 2015 with NCCN recommendations as of March 25, 2016.
When NCCN made recommendations beyond FDA approvals, the researchers evaluated the evidence used to support those recommendations.
Forty-seven new cancer drugs were approved by the FDA for 69 indications between 2011 and 2015. NCCN recommended the 47 drugs for 113 indications, including the 69 FDA-approved indications.
So 39% (n=44) of NCCN’s recommendations were not approved by the FDA, and NCCN gave the following evidence to support these recommendations:
- No evidence—36% (n=16)
- Phase 2 trial without randomization—30% (n=13)
- Randomized, phase 3 trial—16% (n=7)
- Phase 2 trial with randomization—7% (n=3)
- Case report or series of less than 5 patients—5% (n=2)
- Book chapter or review article—2% (n=1)
- Phase 1 trial—2% (n=1)
- Ongoing trial—2% (n=1).
Dr Prasad and his colleagues did point out that not all FDA approvals are supported by randomized, phase 3 trials.
And when the team followed-up 21 months after their initial analysis, they found that 6 of the 44 (14%) additional recommendations by NCCN had received FDA approval.
The researchers also noted that they did not search for independent evidence to support NCCN recommendations beyond the references NCCN provided. So some of the recommendations may have had more or better supporting evidence than what was provided.
Still, the team said these results suggest NCCN “frequently” makes recommendations that go beyond FDA approvals and “often fails to cite evidence or relies on low levels of evidence.” Therefore, NCCN should cite all evidence used to formulate its recommendations.
NCCN argues that it does provide ample evidence to support the recommendations in its guidelines.
“The NCCN guidelines contain more than 24,500 references to inform users of the evidence used in making its decisions,” said Robert W. Carlson, MD, chief executive officer of NCCN.
“These data are supplemented by the analysis of the available evidence by expert clinician researchers and patient advocates who evaluate each recommendation and come to consensus. Each recommendation is labeled with a Category of Evidence, and the vast majority of those for systemic therapies are accompanied by Evidence Blocks, which outline, on 1-5 scales, the efficacy, safety, quality of the evidence, consistency of the evidence, and affordability of the treatment.”
FDA approves first donor screening tests for Babesia
The US Food and Drug Administration (FDA) has approved the first tests to screen blood donors for Babesia parasites.
The Imugen Babesia microti Arrayed Fluorescent Immunoassay (AFIA) is approved for the detection of antibodies to Babesia microti in plasma samples, and the Imugen Babesia microti Nucleic Acid Test (NAT) is approved for the detection of Babesia microti DNA in whole blood samples.
These tests are intended to be used on samples from volunteer donors of whole blood and blood components as well as living organ and tissue donors.
The tests are not intended for use in the diagnosis of babesiosis infections.
The approval of the Imugen Babesia microti AFIA and NAT tests was granted to Oxford Immunotec, Inc. Both assays are in-house tests that can only be performed at the Norwood, Massachusetts facility.
The applications for the tests were granted priority review. The FDA aims to take action on a priority review application within 6 months of receiving it, rather than the standard 10 months.
The FDA grants priority review to applications for products expected to significantly improve the safety or effectiveness of treating, diagnosing, or preventing a serious condition.
“While babesiosis is both preventable and treatable, until today, there was no way to screen for infections amongst blood donors,” said Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research.
“Today’s actions represent the first approvals of Babesia detection tests for use in screening donors of whole blood and blood components, and other living donors.”
About babesiosis
Babesiosis is caused by Babesia parasites that are transmitted by Ixodes scapularis ticks, also known as blacklegged or deer ticks. Babesia microti is the main species of parasite that causes infection in the US.
There are about 1000 to 2000 cases of babesiosis reported in the US each year, with the majority reported from states in the Northeast and upper Midwest.
Most people infected with Babesia microti do not have symptoms and are never diagnosed. Some people develop flu-like symptoms, such as fever, headache, and body aches.
For certain people, especially those with a weak immune system, babesiosis can be a severe, life-threatening disease. And although blood-borne transmission of babesiosis is thought to be uncommon, it is the most frequently reported transfusion-transmitted parasitic infection in the US.
At present, there is no FDA guidance for the testing of donor samples for Babesia. However, the FDA is planning to issue a draft guidance later this year that will include recommendations for reducing the risk of transfusion-transmitted babesiosis.
The US Food and Drug Administration (FDA) has approved the first tests to screen blood donors for Babesia parasites.
The Imugen Babesia microti Arrayed Fluorescent Immunoassay (AFIA) is approved for the detection of antibodies to Babesia microti in plasma samples, and the Imugen Babesia microti Nucleic Acid Test (NAT) is approved for the detection of Babesia microti DNA in whole blood samples.
These tests are intended to be used on samples from volunteer donors of whole blood and blood components as well as living organ and tissue donors.
The tests are not intended for use in the diagnosis of babesiosis infections.
The approval of the Imugen Babesia microti AFIA and NAT tests was granted to Oxford Immunotec, Inc. Both assays are in-house tests that can only be performed at the Norwood, Massachusetts facility.
The applications for the tests were granted priority review. The FDA aims to take action on a priority review application within 6 months of receiving it, rather than the standard 10 months.
The FDA grants priority review to applications for products expected to significantly improve the safety or effectiveness of treating, diagnosing, or preventing a serious condition.
“While babesiosis is both preventable and treatable, until today, there was no way to screen for infections amongst blood donors,” said Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research.
“Today’s actions represent the first approvals of Babesia detection tests for use in screening donors of whole blood and blood components, and other living donors.”
About babesiosis
Babesiosis is caused by Babesia parasites that are transmitted by Ixodes scapularis ticks, also known as blacklegged or deer ticks. Babesia microti is the main species of parasite that causes infection in the US.
There are about 1000 to 2000 cases of babesiosis reported in the US each year, with the majority reported from states in the Northeast and upper Midwest.
Most people infected with Babesia microti do not have symptoms and are never diagnosed. Some people develop flu-like symptoms, such as fever, headache, and body aches.
For certain people, especially those with a weak immune system, babesiosis can be a severe, life-threatening disease. And although blood-borne transmission of babesiosis is thought to be uncommon, it is the most frequently reported transfusion-transmitted parasitic infection in the US.
At present, there is no FDA guidance for the testing of donor samples for Babesia. However, the FDA is planning to issue a draft guidance later this year that will include recommendations for reducing the risk of transfusion-transmitted babesiosis.
The US Food and Drug Administration (FDA) has approved the first tests to screen blood donors for Babesia parasites.
The Imugen Babesia microti Arrayed Fluorescent Immunoassay (AFIA) is approved for the detection of antibodies to Babesia microti in plasma samples, and the Imugen Babesia microti Nucleic Acid Test (NAT) is approved for the detection of Babesia microti DNA in whole blood samples.
These tests are intended to be used on samples from volunteer donors of whole blood and blood components as well as living organ and tissue donors.
The tests are not intended for use in the diagnosis of babesiosis infections.
The approval of the Imugen Babesia microti AFIA and NAT tests was granted to Oxford Immunotec, Inc. Both assays are in-house tests that can only be performed at the Norwood, Massachusetts facility.
The applications for the tests were granted priority review. The FDA aims to take action on a priority review application within 6 months of receiving it, rather than the standard 10 months.
The FDA grants priority review to applications for products expected to significantly improve the safety or effectiveness of treating, diagnosing, or preventing a serious condition.
“While babesiosis is both preventable and treatable, until today, there was no way to screen for infections amongst blood donors,” said Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research.
“Today’s actions represent the first approvals of Babesia detection tests for use in screening donors of whole blood and blood components, and other living donors.”
About babesiosis
Babesiosis is caused by Babesia parasites that are transmitted by Ixodes scapularis ticks, also known as blacklegged or deer ticks. Babesia microti is the main species of parasite that causes infection in the US.
There are about 1000 to 2000 cases of babesiosis reported in the US each year, with the majority reported from states in the Northeast and upper Midwest.
Most people infected with Babesia microti do not have symptoms and are never diagnosed. Some people develop flu-like symptoms, such as fever, headache, and body aches.
For certain people, especially those with a weak immune system, babesiosis can be a severe, life-threatening disease. And although blood-borne transmission of babesiosis is thought to be uncommon, it is the most frequently reported transfusion-transmitted parasitic infection in the US.
At present, there is no FDA guidance for the testing of donor samples for Babesia. However, the FDA is planning to issue a draft guidance later this year that will include recommendations for reducing the risk of transfusion-transmitted babesiosis.
Drug nets orphan designation for beta-thalassemia
The US Food and Drug Administration (FDA) has granted orphan drug designation to PTG-300, a subcutaneous injectable hepcidin mimetic, for the treatment of beta-thalassemia.
Protagonist Therapeutics, the company developing PTG-300, recently completed a phase 1 trial of the drug.
In this study, healthy volunteers treated with PTG-300 achieved dose-related and sustained reductions in serum iron levels.
In addition, PTG-300 was considered well tolerated, producing no serious adverse events or dose-limiting toxicities.
Protagonist Therapeutics intends to initiate a global clinical trial of PTG-300 in patients with beta-thalassemia following upcoming meetings with the FDA and European Medicines Agency.
“Beta-thalassemia is a rare genetic blood disorder that is characterized by impaired red blood cell production that can result in life-threatening chronic anemia, usually requiring regular and life-long blood transfusions for survival,” said David Y. Liu, PhD, chief scientific officer and head of research and development at Protagonist Therapeutics.
“Over time, these transfusions can lead to excessive iron levels in the body, which can be toxic and consequently lead to end-stage damage to vital organs such as the liver and the heart. As a hepcidin mimetic, PTG-300 is designed to help reduce these excessive iron levels, and, thereby, it may lead to improvements in anemia and decreased need for blood transfusions and chelation therapy.”
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
The US Food and Drug Administration (FDA) has granted orphan drug designation to PTG-300, a subcutaneous injectable hepcidin mimetic, for the treatment of beta-thalassemia.
Protagonist Therapeutics, the company developing PTG-300, recently completed a phase 1 trial of the drug.
In this study, healthy volunteers treated with PTG-300 achieved dose-related and sustained reductions in serum iron levels.
In addition, PTG-300 was considered well tolerated, producing no serious adverse events or dose-limiting toxicities.
Protagonist Therapeutics intends to initiate a global clinical trial of PTG-300 in patients with beta-thalassemia following upcoming meetings with the FDA and European Medicines Agency.
“Beta-thalassemia is a rare genetic blood disorder that is characterized by impaired red blood cell production that can result in life-threatening chronic anemia, usually requiring regular and life-long blood transfusions for survival,” said David Y. Liu, PhD, chief scientific officer and head of research and development at Protagonist Therapeutics.
“Over time, these transfusions can lead to excessive iron levels in the body, which can be toxic and consequently lead to end-stage damage to vital organs such as the liver and the heart. As a hepcidin mimetic, PTG-300 is designed to help reduce these excessive iron levels, and, thereby, it may lead to improvements in anemia and decreased need for blood transfusions and chelation therapy.”
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
The US Food and Drug Administration (FDA) has granted orphan drug designation to PTG-300, a subcutaneous injectable hepcidin mimetic, for the treatment of beta-thalassemia.
Protagonist Therapeutics, the company developing PTG-300, recently completed a phase 1 trial of the drug.
In this study, healthy volunteers treated with PTG-300 achieved dose-related and sustained reductions in serum iron levels.
In addition, PTG-300 was considered well tolerated, producing no serious adverse events or dose-limiting toxicities.
Protagonist Therapeutics intends to initiate a global clinical trial of PTG-300 in patients with beta-thalassemia following upcoming meetings with the FDA and European Medicines Agency.
“Beta-thalassemia is a rare genetic blood disorder that is characterized by impaired red blood cell production that can result in life-threatening chronic anemia, usually requiring regular and life-long blood transfusions for survival,” said David Y. Liu, PhD, chief scientific officer and head of research and development at Protagonist Therapeutics.
“Over time, these transfusions can lead to excessive iron levels in the body, which can be toxic and consequently lead to end-stage damage to vital organs such as the liver and the heart. As a hepcidin mimetic, PTG-300 is designed to help reduce these excessive iron levels, and, thereby, it may lead to improvements in anemia and decreased need for blood transfusions and chelation therapy.”
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
FDA approves label update for nivolumab
The US Food and Drug Administration (FDA) has updated the label for nivolumab (Opdivo®) to include new dosing and administration information.
Nivolumab can now be given at 480 mg infused every 4 weeks for most approved indications, in addition to the previously approved dosing schedule of 240 mg every 2 weeks.
The FDA also approved a shorter 30-minute infusion across all approved indications of nivolumab.
The 480 mg dose option can be used for nearly all approved indications of nivolumab. The exceptions are patients with microsatellite instability-high or mismatch repair-deficient metastatic colorectal cancer.
Nivolumab is FDA-approved for the following indications:
- To treat adults with classical Hodgkin lymphoma that has relapsed or progressed after autologous hematopoietic stem cell transplant (HSCT) and brentuximab vedotin or after 3 or more lines of systemic therapy that includes autologous HSCT.
- As monotherapy for patients with BRAF V600 mutation-positive unresectable or metastatic melanoma as well as BRAF V600 wild-type unresectable or metastatic melanoma.
- In combination with ipilimumab for the treatment of patients with unresectable or metastatic melanoma.
- To treat patients with metastatic non-small cell lung cancer with progression on or after platinum-based chemotherapy. Patients with EGFR or ALK genomic tumor aberrations should have disease progression on FDA-approved therapy for these aberrations prior to receiving nivolumab.
- For patients with advanced renal cell carcinoma who have received prior anti-angiogenic therapy.
- To treat patients with recurrent or metastatic squamous cell carcinoma of the head and neck with disease progression on or after platinum-based therapy.
- For patients with locally advanced or metastatic urothelial carcinoma who have disease progression during or after platinum-containing chemotherapy or have disease progression within 12 months of neoadjuvant or adjuvant treatment with platinum-containing chemotherapy.
- To treat adult and pediatric (12 years and older) patients with microsatellite instability-high or mismatch repair-deficient metastatic colorectal cancer that has progressed following treatment with a fluoropyrimidine, oxaliplatin, and irinotecan.
- For patients with hepatocellular carcinoma who have been previously treated with sorafenib.
- For the adjuvant treatment of patients with melanoma with involvement of lymph nodes or metastatic disease who have undergone complete resection.
The US Food and Drug Administration (FDA) has updated the label for nivolumab (Opdivo®) to include new dosing and administration information.
Nivolumab can now be given at 480 mg infused every 4 weeks for most approved indications, in addition to the previously approved dosing schedule of 240 mg every 2 weeks.
The FDA also approved a shorter 30-minute infusion across all approved indications of nivolumab.
The 480 mg dose option can be used for nearly all approved indications of nivolumab. The exceptions are patients with microsatellite instability-high or mismatch repair-deficient metastatic colorectal cancer.
Nivolumab is FDA-approved for the following indications:
- To treat adults with classical Hodgkin lymphoma that has relapsed or progressed after autologous hematopoietic stem cell transplant (HSCT) and brentuximab vedotin or after 3 or more lines of systemic therapy that includes autologous HSCT.
- As monotherapy for patients with BRAF V600 mutation-positive unresectable or metastatic melanoma as well as BRAF V600 wild-type unresectable or metastatic melanoma.
- In combination with ipilimumab for the treatment of patients with unresectable or metastatic melanoma.
- To treat patients with metastatic non-small cell lung cancer with progression on or after platinum-based chemotherapy. Patients with EGFR or ALK genomic tumor aberrations should have disease progression on FDA-approved therapy for these aberrations prior to receiving nivolumab.
- For patients with advanced renal cell carcinoma who have received prior anti-angiogenic therapy.
- To treat patients with recurrent or metastatic squamous cell carcinoma of the head and neck with disease progression on or after platinum-based therapy.
- For patients with locally advanced or metastatic urothelial carcinoma who have disease progression during or after platinum-containing chemotherapy or have disease progression within 12 months of neoadjuvant or adjuvant treatment with platinum-containing chemotherapy.
- To treat adult and pediatric (12 years and older) patients with microsatellite instability-high or mismatch repair-deficient metastatic colorectal cancer that has progressed following treatment with a fluoropyrimidine, oxaliplatin, and irinotecan.
- For patients with hepatocellular carcinoma who have been previously treated with sorafenib.
- For the adjuvant treatment of patients with melanoma with involvement of lymph nodes or metastatic disease who have undergone complete resection.
The US Food and Drug Administration (FDA) has updated the label for nivolumab (Opdivo®) to include new dosing and administration information.
Nivolumab can now be given at 480 mg infused every 4 weeks for most approved indications, in addition to the previously approved dosing schedule of 240 mg every 2 weeks.
The FDA also approved a shorter 30-minute infusion across all approved indications of nivolumab.
The 480 mg dose option can be used for nearly all approved indications of nivolumab. The exceptions are patients with microsatellite instability-high or mismatch repair-deficient metastatic colorectal cancer.
Nivolumab is FDA-approved for the following indications:
- To treat adults with classical Hodgkin lymphoma that has relapsed or progressed after autologous hematopoietic stem cell transplant (HSCT) and brentuximab vedotin or after 3 or more lines of systemic therapy that includes autologous HSCT.
- As monotherapy for patients with BRAF V600 mutation-positive unresectable or metastatic melanoma as well as BRAF V600 wild-type unresectable or metastatic melanoma.
- In combination with ipilimumab for the treatment of patients with unresectable or metastatic melanoma.
- To treat patients with metastatic non-small cell lung cancer with progression on or after platinum-based chemotherapy. Patients with EGFR or ALK genomic tumor aberrations should have disease progression on FDA-approved therapy for these aberrations prior to receiving nivolumab.
- For patients with advanced renal cell carcinoma who have received prior anti-angiogenic therapy.
- To treat patients with recurrent or metastatic squamous cell carcinoma of the head and neck with disease progression on or after platinum-based therapy.
- For patients with locally advanced or metastatic urothelial carcinoma who have disease progression during or after platinum-containing chemotherapy or have disease progression within 12 months of neoadjuvant or adjuvant treatment with platinum-containing chemotherapy.
- To treat adult and pediatric (12 years and older) patients with microsatellite instability-high or mismatch repair-deficient metastatic colorectal cancer that has progressed following treatment with a fluoropyrimidine, oxaliplatin, and irinotecan.
- For patients with hepatocellular carcinoma who have been previously treated with sorafenib.
- For the adjuvant treatment of patients with melanoma with involvement of lymph nodes or metastatic disease who have undergone complete resection.
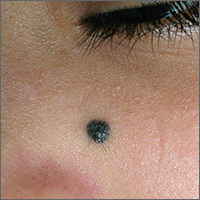
Dark mole on face
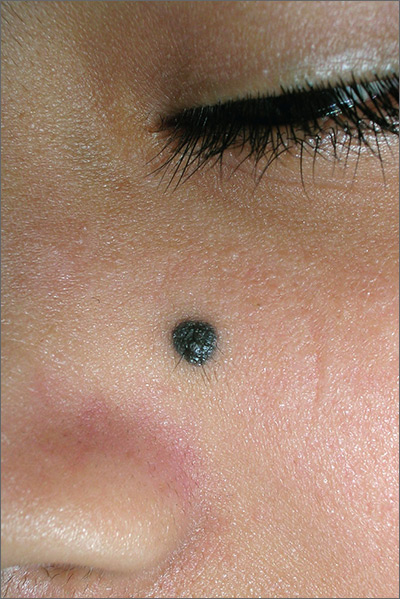
The physician suspected that this was a benign blue nevus because it had a regular border and was uniformly dark in color. He also recognized that melanoma is very rare at age 19. That said, it is hard to ignore a changing mole that is so black in color.
The patient wanted it removed, so a 5-mm punch biopsy was performed. (See the Watch and Learn video on punch biopsy.)
When the punch core was removed, the physician noted that the pigment was visible in the deep dermis (as expected with a blue nevus). A single suture was placed, and the patient was scheduled for follow-up in one week. The pathology report came back as a blue nevus, which is completely benign.
While many blue nevi actually appear blue because of the Tyndall effect causing the dark melanin in the deep dermis to create a blue coloration, some will appear black (as was seen in this case). On the follow-up visit, the suture was removed and the incision was healing well. The patient was reassured that this was a benign mole and was happy with the cosmetic result.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Smith M, Usatine R. Benign nevi. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:945-952.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.

The physician suspected that this was a benign blue nevus because it had a regular border and was uniformly dark in color. He also recognized that melanoma is very rare at age 19. That said, it is hard to ignore a changing mole that is so black in color.
The patient wanted it removed, so a 5-mm punch biopsy was performed. (See the Watch and Learn video on punch biopsy.)
When the punch core was removed, the physician noted that the pigment was visible in the deep dermis (as expected with a blue nevus). A single suture was placed, and the patient was scheduled for follow-up in one week. The pathology report came back as a blue nevus, which is completely benign.
While many blue nevi actually appear blue because of the Tyndall effect causing the dark melanin in the deep dermis to create a blue coloration, some will appear black (as was seen in this case). On the follow-up visit, the suture was removed and the incision was healing well. The patient was reassured that this was a benign mole and was happy with the cosmetic result.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Smith M, Usatine R. Benign nevi. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:945-952.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.

The physician suspected that this was a benign blue nevus because it had a regular border and was uniformly dark in color. He also recognized that melanoma is very rare at age 19. That said, it is hard to ignore a changing mole that is so black in color.
The patient wanted it removed, so a 5-mm punch biopsy was performed. (See the Watch and Learn video on punch biopsy.)
When the punch core was removed, the physician noted that the pigment was visible in the deep dermis (as expected with a blue nevus). A single suture was placed, and the patient was scheduled for follow-up in one week. The pathology report came back as a blue nevus, which is completely benign.
While many blue nevi actually appear blue because of the Tyndall effect causing the dark melanin in the deep dermis to create a blue coloration, some will appear black (as was seen in this case). On the follow-up visit, the suture was removed and the incision was healing well. The patient was reassured that this was a benign mole and was happy with the cosmetic result.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Smith M, Usatine R. Benign nevi. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:945-952.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.
Hypertension guidelines unlikely to hold sway with PCPs
A new and long-anticipated guideline for diagnosing and managing hypertension by the American College of Cardiology, the American Heart Association, and nine other collaborating societies was finally released last fall. What happens now?
Will the top-line, seismic change that the new guidelines called for – treating many patients with hypertension to a blood pressure below 130/80 mm Hg – become the standard of care for U.S. medicine? And what about the several other novel steps the guideline calls for including more careful and methodical measurement of blood pressure with greater emphasis on out-of-office blood pressure monitoring, increased reliance on lifestyle interventions, running a formal calculation of a patient’s cardiovascular disease risk to identify patients who warrant drug treatment, development and attention to a care plan for each hypertensive patient, and a team approach to management?
In this two-part feature, a potential revolution in care will be explored.
ACC/AHA guidelines: More than just numbers
The ACC and AHA guideline (J Am Coll Cardiol. 2017 Nov 13. doi: 10.1016/j.jacc.2017.11.006) is “comprehensive. It addresses many issues around hypertension, not just what is a good [blood pressure] number and a bad number. This is important because this type of document has not existed for quite some time,” said Donald E. Casey Jr., MD, a member of the guideline-writing panel, an internal medicine physician, and chief clinical affairs officer at Medecision in Wayne, Pa.
“This is the first time that a [blood pressure] guideline has explicitly led with a strategy of calculating a person’s risk using the ACC/AHA risk calculator. It’s not ‘what is your blood pressure?’ but ‘what is your risk?’ This is not a one-size-fits-all guideline.”
The biggest wild card when handicapping the odds that the new guideline will take hold is how U.S. primary care physicians, the clinicians who stand on the front line of U.S. medicine for diagnosing and treating hypertension, respond. Their reaction remained an open issue during the weeks following release of the new guideline, in large part because its headline feature, the treatment target of less than 130/80 mm Hg, is at odds with what a competing guideline released just 8 months earlier from the American College of Physicians (ACP) and the American Academy of Family Practice (AAFP) called for, namely, a hypertension treatment target of less than 150/90 mm Hg in patients aged 60 years or older who need drug intervention (Ann Int Med. 2017 March 21; 166[6]:430-7).
Primary care pushes back
Although a significant part of the ACC/AHA guideline has either received endorsement from or is likely acceptable to the primary care organizations, including recommendations for improved blood pressure measurement and the key role for healthy lifestyle interventions, these two sides seem irreconcilable on the core issue of what is the target blood pressure for many patients when they are on antihypertensive drugs.
That inference turned into a reality in mid-December when the AAFP released a scathing critique of the ACC/AHA guidelines, and reaffirmed its endorsement of the controversial blood pressure target set by the panel appointed to the Eighth Joint National Committee (JNC 8), which in 2014 recommended a treatment target when using antihypertensive medications of less than 150/90 mm Hg for patients aged 60 years and older (JAMA 2014 Feb 5;311[5]:507-20).
A few weeks later, in late January, the ACP issued its own rejection of the core blood pressure target of the ACC/AHA guideline (Ann Int Med. 2018 Jan 23. doi: 10.7326/M17-3293). “Are the harms, costs, and complexity of care associated with this new target justified by the presumed benefits of labeling nearly half the U.S. [adult] population as unwell and subjecting them to treatment? We think not,” declared the statement from the ACP’s Clinical Guidelines Committee.
Although this ACP statement praised the ACC/AHA guideline in its “emphasis on the importance of blood pressure measurement technique and lifestyle changes,” it added that the guideline also “falls short in weighing the potential benefits against potential harms, costs, and anticipated variation in individual patient preferences.”
The statement went on to cite three specific flaws in the notion of using antihypertensive drugs to treat patients to less than 130/80 mm Hg: 1. Clinical trial results have not shown consistent evidence for benefit from a systolic blood pressure target of less than 130 mm Hg in older adults with diabetes or kidney disease. 2. Benefits are often overestimated and harms often underestimated when trial findings are applied to broad primary care populations. 3. No evidence from randomized controlled trials support a diastolic blood pressure target of less than 80 mm Hg.
Dr. Cohen said she agreed with a general target blood pressure of less than 130 mm Hg, while qualifying it with some caveats, and added that she did not agree that most patients should be treated to less than 80 mm Hg diastolic. “I will be less inclined to push for aggressive management in patients who are poorly functional, or those with many drug side effects or issues with polypharmacy. And given the evidence from ACCORD (N Engl J Med. 2010 April 29;362[17]:1575-85), I do not plan to apply stricter blood pressure control in patients with diabetes, and also not in patients with nonproteinuric chronic kidney disease.”
Dr. Ioannidis said in his JAMA commentary that the panel that wrote the ACC/AHA guidelines had “no conflicts of interest.” In the interview, he took issue with the AAFP’s objection to intellectual conflicts among some panel members.
“The concern raised by the AAFP is legitimate. Most cardiovascular medicine guidelines until now have been problematic because of overt financial conflicts of interest. Here is a guideline with no obvious financial conflicts of interest.” The ACC/AHA guideline document shows that across the entire list of guideline panel members no one had any financial disclosures.
“Intellectual conflict exists in every person who knows something about a certain field,” continued Dr. Ioannidis. “The only way to get rid of intellectual conflicts is to recruit people who know nothing about the subject. That’s not advisable. The problem is not an intellectual conflict in any one person – everyone has an intellectual conflict. The problem is stacking the membership of a guideline panel in a way that all or almost all have expressed a strong preference for some policy or strategy so that the guideline is bound to stick with this approach. While several members of the ACC/AHA had an intellectual conflict, I doubt there is a case for saying the entire committee was stacked.”
Goals for older adults: Irreconcilable differences?
How was it that the ACC/AHA guideline and the ACP/AAFP guideline reached such disparate conclusions about the appropriate blood pressure treatment target for patients aged 60 years or older?
“A critical difference between the two guidelines stems from how potential [treatment] harms and patient values and preferences were considered. Our review and the ACP/AAFP guidelines extensively considered harms and treatment burden associated with lower blood pressure treatment targets. Lower targets did not increase the risk for fractures, falls, or cognitive declines, but they were associated with more symptomatic hypotension, syncope, and greater medication burden. The ACP/AAFP believed that there is likely to be significant variation in how individual patients might weigh the small potential benefit of aggressive blood pressure control against the potential harms and treatment burden. The ACC/AHA guidelines and the systematic review on which they were based did not include an assessment of harms,” Dr. Kansagara said in an interview. Dr. Casey maintained that the ACC/AHA literature review did consider potential harms from treatment.
“The ACC/AHA guidelines also considered results from observational studies. The ACP/AAFP did not. A number of studies show that progressively higher levels of blood pressure are associated with higher rates of cardiovascular disease events and mortality. But some observational studies showed that blood pressures in the low to low-normal range are associated with higher mortality. The problem with observational studies is that there are many reasons why a population with higher blood pressure may have worse outcomes. That does not mean that using medication to reduce blood pressure will improve outcomes.”
Other experts noted that while the ACP/AAFP data review and guideline took the SPRINT results into account, their review occurred too soon to also include two other important analyses that came down in favor of the less than 130/80 mm Hg target and were included in the ACC/AHA review: A meta-analysis of 42 trials with more than 144,000 patients that showed patients treated to a systolic blood pressure of 120-124 mm Hg had significantly fewer deaths and cardiovascular disease events compared with patients with higher achieved blood pressures (JAMA Cardiology, 2017 July;2[7]:775-81); and a second meta-analysis of 17 trials with more than 55,000 patients that showed a target systolic pressure of less than 130 mm Hg produced the best balance of efficacy and safety (Am J Med. 2017 June;130[6]:707-19).
The bottom line, said Dr. Kansagara, is that regardless of which guideline a physician follows, the publication of both last year will mean that “patients and their providers will likely have more conversations about blood pressure treatment. Both guidelines underscore the need to at least consider lower blood pressure targets in patients at high cardiovascular disease risk or in those who have had a cardiovascular disease event.” As a result of the two 2017 guidelines “I think PCPs will pay more attention to blood pressure as a modifiable risk factor.”
Dr. Casey, Dr. Whelton, Dr. Cohen, Dr. Kansagara, and Dr. Ioannidis had no disclosures.
This is part one of a two-part series. Part two will explore how the approach to diagnosis and management of hypertension spelled out in the ACC/AHA guidelines fits into the protocol-driven, data-monitored, team-delivered primary care model that has come to dominate U.S. primary care in the decade following passage of the Affordable Care Act.
A new and long-anticipated guideline for diagnosing and managing hypertension by the American College of Cardiology, the American Heart Association, and nine other collaborating societies was finally released last fall. What happens now?
Will the top-line, seismic change that the new guidelines called for – treating many patients with hypertension to a blood pressure below 130/80 mm Hg – become the standard of care for U.S. medicine? And what about the several other novel steps the guideline calls for including more careful and methodical measurement of blood pressure with greater emphasis on out-of-office blood pressure monitoring, increased reliance on lifestyle interventions, running a formal calculation of a patient’s cardiovascular disease risk to identify patients who warrant drug treatment, development and attention to a care plan for each hypertensive patient, and a team approach to management?
In this two-part feature, a potential revolution in care will be explored.
ACC/AHA guidelines: More than just numbers
The ACC and AHA guideline (J Am Coll Cardiol. 2017 Nov 13. doi: 10.1016/j.jacc.2017.11.006) is “comprehensive. It addresses many issues around hypertension, not just what is a good [blood pressure] number and a bad number. This is important because this type of document has not existed for quite some time,” said Donald E. Casey Jr., MD, a member of the guideline-writing panel, an internal medicine physician, and chief clinical affairs officer at Medecision in Wayne, Pa.
“This is the first time that a [blood pressure] guideline has explicitly led with a strategy of calculating a person’s risk using the ACC/AHA risk calculator. It’s not ‘what is your blood pressure?’ but ‘what is your risk?’ This is not a one-size-fits-all guideline.”
The biggest wild card when handicapping the odds that the new guideline will take hold is how U.S. primary care physicians, the clinicians who stand on the front line of U.S. medicine for diagnosing and treating hypertension, respond. Their reaction remained an open issue during the weeks following release of the new guideline, in large part because its headline feature, the treatment target of less than 130/80 mm Hg, is at odds with what a competing guideline released just 8 months earlier from the American College of Physicians (ACP) and the American Academy of Family Practice (AAFP) called for, namely, a hypertension treatment target of less than 150/90 mm Hg in patients aged 60 years or older who need drug intervention (Ann Int Med. 2017 March 21; 166[6]:430-7).
Primary care pushes back
Although a significant part of the ACC/AHA guideline has either received endorsement from or is likely acceptable to the primary care organizations, including recommendations for improved blood pressure measurement and the key role for healthy lifestyle interventions, these two sides seem irreconcilable on the core issue of what is the target blood pressure for many patients when they are on antihypertensive drugs.
That inference turned into a reality in mid-December when the AAFP released a scathing critique of the ACC/AHA guidelines, and reaffirmed its endorsement of the controversial blood pressure target set by the panel appointed to the Eighth Joint National Committee (JNC 8), which in 2014 recommended a treatment target when using antihypertensive medications of less than 150/90 mm Hg for patients aged 60 years and older (JAMA 2014 Feb 5;311[5]:507-20).
A few weeks later, in late January, the ACP issued its own rejection of the core blood pressure target of the ACC/AHA guideline (Ann Int Med. 2018 Jan 23. doi: 10.7326/M17-3293). “Are the harms, costs, and complexity of care associated with this new target justified by the presumed benefits of labeling nearly half the U.S. [adult] population as unwell and subjecting them to treatment? We think not,” declared the statement from the ACP’s Clinical Guidelines Committee.
Although this ACP statement praised the ACC/AHA guideline in its “emphasis on the importance of blood pressure measurement technique and lifestyle changes,” it added that the guideline also “falls short in weighing the potential benefits against potential harms, costs, and anticipated variation in individual patient preferences.”
The statement went on to cite three specific flaws in the notion of using antihypertensive drugs to treat patients to less than 130/80 mm Hg: 1. Clinical trial results have not shown consistent evidence for benefit from a systolic blood pressure target of less than 130 mm Hg in older adults with diabetes or kidney disease. 2. Benefits are often overestimated and harms often underestimated when trial findings are applied to broad primary care populations. 3. No evidence from randomized controlled trials support a diastolic blood pressure target of less than 80 mm Hg.
Dr. Cohen said she agreed with a general target blood pressure of less than 130 mm Hg, while qualifying it with some caveats, and added that she did not agree that most patients should be treated to less than 80 mm Hg diastolic. “I will be less inclined to push for aggressive management in patients who are poorly functional, or those with many drug side effects or issues with polypharmacy. And given the evidence from ACCORD (N Engl J Med. 2010 April 29;362[17]:1575-85), I do not plan to apply stricter blood pressure control in patients with diabetes, and also not in patients with nonproteinuric chronic kidney disease.”
Dr. Ioannidis said in his JAMA commentary that the panel that wrote the ACC/AHA guidelines had “no conflicts of interest.” In the interview, he took issue with the AAFP’s objection to intellectual conflicts among some panel members.
“The concern raised by the AAFP is legitimate. Most cardiovascular medicine guidelines until now have been problematic because of overt financial conflicts of interest. Here is a guideline with no obvious financial conflicts of interest.” The ACC/AHA guideline document shows that across the entire list of guideline panel members no one had any financial disclosures.
“Intellectual conflict exists in every person who knows something about a certain field,” continued Dr. Ioannidis. “The only way to get rid of intellectual conflicts is to recruit people who know nothing about the subject. That’s not advisable. The problem is not an intellectual conflict in any one person – everyone has an intellectual conflict. The problem is stacking the membership of a guideline panel in a way that all or almost all have expressed a strong preference for some policy or strategy so that the guideline is bound to stick with this approach. While several members of the ACC/AHA had an intellectual conflict, I doubt there is a case for saying the entire committee was stacked.”
Goals for older adults: Irreconcilable differences?
How was it that the ACC/AHA guideline and the ACP/AAFP guideline reached such disparate conclusions about the appropriate blood pressure treatment target for patients aged 60 years or older?
“A critical difference between the two guidelines stems from how potential [treatment] harms and patient values and preferences were considered. Our review and the ACP/AAFP guidelines extensively considered harms and treatment burden associated with lower blood pressure treatment targets. Lower targets did not increase the risk for fractures, falls, or cognitive declines, but they were associated with more symptomatic hypotension, syncope, and greater medication burden. The ACP/AAFP believed that there is likely to be significant variation in how individual patients might weigh the small potential benefit of aggressive blood pressure control against the potential harms and treatment burden. The ACC/AHA guidelines and the systematic review on which they were based did not include an assessment of harms,” Dr. Kansagara said in an interview. Dr. Casey maintained that the ACC/AHA literature review did consider potential harms from treatment.
“The ACC/AHA guidelines also considered results from observational studies. The ACP/AAFP did not. A number of studies show that progressively higher levels of blood pressure are associated with higher rates of cardiovascular disease events and mortality. But some observational studies showed that blood pressures in the low to low-normal range are associated with higher mortality. The problem with observational studies is that there are many reasons why a population with higher blood pressure may have worse outcomes. That does not mean that using medication to reduce blood pressure will improve outcomes.”
Other experts noted that while the ACP/AAFP data review and guideline took the SPRINT results into account, their review occurred too soon to also include two other important analyses that came down in favor of the less than 130/80 mm Hg target and were included in the ACC/AHA review: A meta-analysis of 42 trials with more than 144,000 patients that showed patients treated to a systolic blood pressure of 120-124 mm Hg had significantly fewer deaths and cardiovascular disease events compared with patients with higher achieved blood pressures (JAMA Cardiology, 2017 July;2[7]:775-81); and a second meta-analysis of 17 trials with more than 55,000 patients that showed a target systolic pressure of less than 130 mm Hg produced the best balance of efficacy and safety (Am J Med. 2017 June;130[6]:707-19).
The bottom line, said Dr. Kansagara, is that regardless of which guideline a physician follows, the publication of both last year will mean that “patients and their providers will likely have more conversations about blood pressure treatment. Both guidelines underscore the need to at least consider lower blood pressure targets in patients at high cardiovascular disease risk or in those who have had a cardiovascular disease event.” As a result of the two 2017 guidelines “I think PCPs will pay more attention to blood pressure as a modifiable risk factor.”
Dr. Casey, Dr. Whelton, Dr. Cohen, Dr. Kansagara, and Dr. Ioannidis had no disclosures.
This is part one of a two-part series. Part two will explore how the approach to diagnosis and management of hypertension spelled out in the ACC/AHA guidelines fits into the protocol-driven, data-monitored, team-delivered primary care model that has come to dominate U.S. primary care in the decade following passage of the Affordable Care Act.
A new and long-anticipated guideline for diagnosing and managing hypertension by the American College of Cardiology, the American Heart Association, and nine other collaborating societies was finally released last fall. What happens now?
Will the top-line, seismic change that the new guidelines called for – treating many patients with hypertension to a blood pressure below 130/80 mm Hg – become the standard of care for U.S. medicine? And what about the several other novel steps the guideline calls for including more careful and methodical measurement of blood pressure with greater emphasis on out-of-office blood pressure monitoring, increased reliance on lifestyle interventions, running a formal calculation of a patient’s cardiovascular disease risk to identify patients who warrant drug treatment, development and attention to a care plan for each hypertensive patient, and a team approach to management?
In this two-part feature, a potential revolution in care will be explored.
ACC/AHA guidelines: More than just numbers
The ACC and AHA guideline (J Am Coll Cardiol. 2017 Nov 13. doi: 10.1016/j.jacc.2017.11.006) is “comprehensive. It addresses many issues around hypertension, not just what is a good [blood pressure] number and a bad number. This is important because this type of document has not existed for quite some time,” said Donald E. Casey Jr., MD, a member of the guideline-writing panel, an internal medicine physician, and chief clinical affairs officer at Medecision in Wayne, Pa.
“This is the first time that a [blood pressure] guideline has explicitly led with a strategy of calculating a person’s risk using the ACC/AHA risk calculator. It’s not ‘what is your blood pressure?’ but ‘what is your risk?’ This is not a one-size-fits-all guideline.”
The biggest wild card when handicapping the odds that the new guideline will take hold is how U.S. primary care physicians, the clinicians who stand on the front line of U.S. medicine for diagnosing and treating hypertension, respond. Their reaction remained an open issue during the weeks following release of the new guideline, in large part because its headline feature, the treatment target of less than 130/80 mm Hg, is at odds with what a competing guideline released just 8 months earlier from the American College of Physicians (ACP) and the American Academy of Family Practice (AAFP) called for, namely, a hypertension treatment target of less than 150/90 mm Hg in patients aged 60 years or older who need drug intervention (Ann Int Med. 2017 March 21; 166[6]:430-7).
Primary care pushes back
Although a significant part of the ACC/AHA guideline has either received endorsement from or is likely acceptable to the primary care organizations, including recommendations for improved blood pressure measurement and the key role for healthy lifestyle interventions, these two sides seem irreconcilable on the core issue of what is the target blood pressure for many patients when they are on antihypertensive drugs.
That inference turned into a reality in mid-December when the AAFP released a scathing critique of the ACC/AHA guidelines, and reaffirmed its endorsement of the controversial blood pressure target set by the panel appointed to the Eighth Joint National Committee (JNC 8), which in 2014 recommended a treatment target when using antihypertensive medications of less than 150/90 mm Hg for patients aged 60 years and older (JAMA 2014 Feb 5;311[5]:507-20).
A few weeks later, in late January, the ACP issued its own rejection of the core blood pressure target of the ACC/AHA guideline (Ann Int Med. 2018 Jan 23. doi: 10.7326/M17-3293). “Are the harms, costs, and complexity of care associated with this new target justified by the presumed benefits of labeling nearly half the U.S. [adult] population as unwell and subjecting them to treatment? We think not,” declared the statement from the ACP’s Clinical Guidelines Committee.
Although this ACP statement praised the ACC/AHA guideline in its “emphasis on the importance of blood pressure measurement technique and lifestyle changes,” it added that the guideline also “falls short in weighing the potential benefits against potential harms, costs, and anticipated variation in individual patient preferences.”
The statement went on to cite three specific flaws in the notion of using antihypertensive drugs to treat patients to less than 130/80 mm Hg: 1. Clinical trial results have not shown consistent evidence for benefit from a systolic blood pressure target of less than 130 mm Hg in older adults with diabetes or kidney disease. 2. Benefits are often overestimated and harms often underestimated when trial findings are applied to broad primary care populations. 3. No evidence from randomized controlled trials support a diastolic blood pressure target of less than 80 mm Hg.
Dr. Cohen said she agreed with a general target blood pressure of less than 130 mm Hg, while qualifying it with some caveats, and added that she did not agree that most patients should be treated to less than 80 mm Hg diastolic. “I will be less inclined to push for aggressive management in patients who are poorly functional, or those with many drug side effects or issues with polypharmacy. And given the evidence from ACCORD (N Engl J Med. 2010 April 29;362[17]:1575-85), I do not plan to apply stricter blood pressure control in patients with diabetes, and also not in patients with nonproteinuric chronic kidney disease.”
Dr. Ioannidis said in his JAMA commentary that the panel that wrote the ACC/AHA guidelines had “no conflicts of interest.” In the interview, he took issue with the AAFP’s objection to intellectual conflicts among some panel members.
“The concern raised by the AAFP is legitimate. Most cardiovascular medicine guidelines until now have been problematic because of overt financial conflicts of interest. Here is a guideline with no obvious financial conflicts of interest.” The ACC/AHA guideline document shows that across the entire list of guideline panel members no one had any financial disclosures.
“Intellectual conflict exists in every person who knows something about a certain field,” continued Dr. Ioannidis. “The only way to get rid of intellectual conflicts is to recruit people who know nothing about the subject. That’s not advisable. The problem is not an intellectual conflict in any one person – everyone has an intellectual conflict. The problem is stacking the membership of a guideline panel in a way that all or almost all have expressed a strong preference for some policy or strategy so that the guideline is bound to stick with this approach. While several members of the ACC/AHA had an intellectual conflict, I doubt there is a case for saying the entire committee was stacked.”
Goals for older adults: Irreconcilable differences?
How was it that the ACC/AHA guideline and the ACP/AAFP guideline reached such disparate conclusions about the appropriate blood pressure treatment target for patients aged 60 years or older?
“A critical difference between the two guidelines stems from how potential [treatment] harms and patient values and preferences were considered. Our review and the ACP/AAFP guidelines extensively considered harms and treatment burden associated with lower blood pressure treatment targets. Lower targets did not increase the risk for fractures, falls, or cognitive declines, but they were associated with more symptomatic hypotension, syncope, and greater medication burden. The ACP/AAFP believed that there is likely to be significant variation in how individual patients might weigh the small potential benefit of aggressive blood pressure control against the potential harms and treatment burden. The ACC/AHA guidelines and the systematic review on which they were based did not include an assessment of harms,” Dr. Kansagara said in an interview. Dr. Casey maintained that the ACC/AHA literature review did consider potential harms from treatment.
“The ACC/AHA guidelines also considered results from observational studies. The ACP/AAFP did not. A number of studies show that progressively higher levels of blood pressure are associated with higher rates of cardiovascular disease events and mortality. But some observational studies showed that blood pressures in the low to low-normal range are associated with higher mortality. The problem with observational studies is that there are many reasons why a population with higher blood pressure may have worse outcomes. That does not mean that using medication to reduce blood pressure will improve outcomes.”
Other experts noted that while the ACP/AAFP data review and guideline took the SPRINT results into account, their review occurred too soon to also include two other important analyses that came down in favor of the less than 130/80 mm Hg target and were included in the ACC/AHA review: A meta-analysis of 42 trials with more than 144,000 patients that showed patients treated to a systolic blood pressure of 120-124 mm Hg had significantly fewer deaths and cardiovascular disease events compared with patients with higher achieved blood pressures (JAMA Cardiology, 2017 July;2[7]:775-81); and a second meta-analysis of 17 trials with more than 55,000 patients that showed a target systolic pressure of less than 130 mm Hg produced the best balance of efficacy and safety (Am J Med. 2017 June;130[6]:707-19).
The bottom line, said Dr. Kansagara, is that regardless of which guideline a physician follows, the publication of both last year will mean that “patients and their providers will likely have more conversations about blood pressure treatment. Both guidelines underscore the need to at least consider lower blood pressure targets in patients at high cardiovascular disease risk or in those who have had a cardiovascular disease event.” As a result of the two 2017 guidelines “I think PCPs will pay more attention to blood pressure as a modifiable risk factor.”
Dr. Casey, Dr. Whelton, Dr. Cohen, Dr. Kansagara, and Dr. Ioannidis had no disclosures.
This is part one of a two-part series. Part two will explore how the approach to diagnosis and management of hypertension spelled out in the ACC/AHA guidelines fits into the protocol-driven, data-monitored, team-delivered primary care model that has come to dominate U.S. primary care in the decade following passage of the Affordable Care Act.
FDA approves tests to screen for tickborne parasite in blood supply
The Food and Drug Administration has approved two new tests to screen whole blood and plasma supplies for the tickborne parasite Babesia microti.
The Imugen Babesia microti Arrayed Fluorescent Immunoassay (AFIA) detects antibodies to B. microti in human plasma samples and the Imugen Babesia microti Nucleic Acid Test (NAT) detects B. microti DNA in human whole blood samples. The tests can be used as donor screening tests on individual samples from donors of whole blood and blood components, as well as living organ and tissue donors, according to the FDA. The tests are not intended as a way to diagnose babesiosis infections.
The new screening tests are the first approvals for B. microti detection. The tests have been available under investigational use since 2012. The tests are manufactured by Oxford Immunotec and are currently only available as in-house tests at the company’s Norwood, Mass., facility.
There is no FDA guidance for testing blood and plasma samples for B. microti, but the agency is planning to issue draft guidance later in 2018.
The Food and Drug Administration has approved two new tests to screen whole blood and plasma supplies for the tickborne parasite Babesia microti.
The Imugen Babesia microti Arrayed Fluorescent Immunoassay (AFIA) detects antibodies to B. microti in human plasma samples and the Imugen Babesia microti Nucleic Acid Test (NAT) detects B. microti DNA in human whole blood samples. The tests can be used as donor screening tests on individual samples from donors of whole blood and blood components, as well as living organ and tissue donors, according to the FDA. The tests are not intended as a way to diagnose babesiosis infections.
The new screening tests are the first approvals for B. microti detection. The tests have been available under investigational use since 2012. The tests are manufactured by Oxford Immunotec and are currently only available as in-house tests at the company’s Norwood, Mass., facility.
There is no FDA guidance for testing blood and plasma samples for B. microti, but the agency is planning to issue draft guidance later in 2018.
The Food and Drug Administration has approved two new tests to screen whole blood and plasma supplies for the tickborne parasite Babesia microti.
The Imugen Babesia microti Arrayed Fluorescent Immunoassay (AFIA) detects antibodies to B. microti in human plasma samples and the Imugen Babesia microti Nucleic Acid Test (NAT) detects B. microti DNA in human whole blood samples. The tests can be used as donor screening tests on individual samples from donors of whole blood and blood components, as well as living organ and tissue donors, according to the FDA. The tests are not intended as a way to diagnose babesiosis infections.
The new screening tests are the first approvals for B. microti detection. The tests have been available under investigational use since 2012. The tests are manufactured by Oxford Immunotec and are currently only available as in-house tests at the company’s Norwood, Mass., facility.
There is no FDA guidance for testing blood and plasma samples for B. microti, but the agency is planning to issue draft guidance later in 2018.
Tenofovir didn’t prevent hepatitis B transmission to newborns
Prenatal tenofovir didn’t reduce the rate of hepatitis B among infants born to women infected with the virus.
Among 322 6-month-olds, the rate of HBV transmission was 0 in those whose mothers received the antiviral during pregnancy and 2% among those whose mothers received placebo – not a statistically significant difference, Gonzague Jourdain, MD, and his colleagues reported in the New England Journal of Medicine.
The study randomized 331 pregnant women with proven HBV infections to either tenofovir or placebo from 28 weeks’ gestation to 2 months post partum. All infants received HBV immune globulin at birth, and HBV vaccine at birth and at 1, 2, 4, and 6 months. The primary endpoint was confirmed HBV infection in the infant at 6 months.
Women were a mean of 28 weeks pregnant at baseline, with a mean viral load of 8.0 log10 IU/mL. Most women (about 90% of each group) had an HBV DNA of more than 200,000 IU/mL – a level associated with an increased risk of perinatal HBV infection despite vaccination.
There were 322 deliveries, resulting in 319 singletons, two pairs of twins, and one stillbirth. Postpartum infant treatment was quick, with a median of 1.3 hours from birth to administration of immune globulin and a median of 1.2 hours to administration of the first dose of the vaccine.
At 6 months, there were no HBV infections in the tenofovir-exposed group and 3 (2%) in the placebo group – a nonsignificant difference (P = .12).
Tenofovir was safe for both mother and fetus, with no significant adverse events in either group. The incidence of elevated maternal alanine aminotransferase level (more than 300 IU/L) was 6% in the tenofovir group and 3% in the placebo group, also a nonsignificant finding.
Dr. Jourdain and his colleagues noted that the 2% transmission rate in the placebo group is considerably lower than the 7% seen in similar studies and could be related to the rapid postpartum administration of HBV immune globulin and vaccine. If this is the case, prenatal antivirals could be more effective in countries where postpartum treatment is delayed or inconsistent.
“Maternal use of tenofovir may prevent transmissions that would occur when the birth dose is delayed, but its exact timing has not been reported consistently in previous perinatal studies,” the team said.
Another question is whether the stringent, 5-dose infant HBV vaccine series required in Thailand is simply more effective than schedules that have fewer doses or are combined with other vaccines and delivered later.
“It remains unclear whether the administration of more vaccine doses is more efficacious than the administration of the three vaccine doses that is recommended in the United States and by the World Health Organization.”
Dr. Jourdain had no financial disclosures relevant to the study, which was sponsored by the National Institute of Child Health and Human Development.
SOURCE: Jourdain G et al. N Engl J Med. 2018;378:911-23.
The trial by Jourdain et al. – although described by the authors as negative – “puts down an intriguing marker attesting to the possibility that rapidly phasing in the timely administration of a safe monovalent HBV vaccine within a few hours after birth could contribute to the interruption of mother-to-child transmission and avert preventable HBV infections in childhood,” Geoffrey Dusheiko, MD, wrote in an accompanying editorial (N Engl J Med. 2018;378:952-3).
The World Health Organization supports HBV vaccine schedules of three or four doses, which are usually given as part of a combination immunization protocol beginning at 6 weeks of age. “Currently, HBV vaccination is most frequently administered as a pentavalent or hexavalent vaccine as part of the Expanded Program on Immunization, typically in combination with vaccines against diphtheria, tetanus, pertussis, polio, and Haemophilus influenzae type B,” wrote Dr. Dusheiko. “Paradoxically, support for combination vaccines within an integrated EPI schedule has unwittingly but undesirably shifted thinking and policy away from HBV vaccination at birth. This gap in vaccine strategy is disadvantageous.”
While the study doesn’t support tenofovir for maternal prophylaxis, it does imply value for treating the infant with immune globulin and HBV vaccination soon after birth. Delivering this kind of care globally will be challenging, but it’s entirely feasible, Dr. Dusheiko said.
“It is necessary to analyze regional data to assess the requirements for implementing vaccination at birth, including ... the training of otherwise unskilled birth attendants to deliver monovalent HBV vaccine at the same time as the vaccines against polio and bacille Calmette–Guérin. Importantly, the use of monovalent HBV vaccine would also require governmental or nongovernmental support. HBV vaccination at birth, despite the challenges for poverty-affected countries to deliver vaccination in rural and isolated locales, is feasible.”
Dr. Dusheiko is a hepatologist at the University College London School of Medicine and King’s College Hospital, London.
The trial by Jourdain et al. – although described by the authors as negative – “puts down an intriguing marker attesting to the possibility that rapidly phasing in the timely administration of a safe monovalent HBV vaccine within a few hours after birth could contribute to the interruption of mother-to-child transmission and avert preventable HBV infections in childhood,” Geoffrey Dusheiko, MD, wrote in an accompanying editorial (N Engl J Med. 2018;378:952-3).
The World Health Organization supports HBV vaccine schedules of three or four doses, which are usually given as part of a combination immunization protocol beginning at 6 weeks of age. “Currently, HBV vaccination is most frequently administered as a pentavalent or hexavalent vaccine as part of the Expanded Program on Immunization, typically in combination with vaccines against diphtheria, tetanus, pertussis, polio, and Haemophilus influenzae type B,” wrote Dr. Dusheiko. “Paradoxically, support for combination vaccines within an integrated EPI schedule has unwittingly but undesirably shifted thinking and policy away from HBV vaccination at birth. This gap in vaccine strategy is disadvantageous.”
While the study doesn’t support tenofovir for maternal prophylaxis, it does imply value for treating the infant with immune globulin and HBV vaccination soon after birth. Delivering this kind of care globally will be challenging, but it’s entirely feasible, Dr. Dusheiko said.
“It is necessary to analyze regional data to assess the requirements for implementing vaccination at birth, including ... the training of otherwise unskilled birth attendants to deliver monovalent HBV vaccine at the same time as the vaccines against polio and bacille Calmette–Guérin. Importantly, the use of monovalent HBV vaccine would also require governmental or nongovernmental support. HBV vaccination at birth, despite the challenges for poverty-affected countries to deliver vaccination in rural and isolated locales, is feasible.”
Dr. Dusheiko is a hepatologist at the University College London School of Medicine and King’s College Hospital, London.
The trial by Jourdain et al. – although described by the authors as negative – “puts down an intriguing marker attesting to the possibility that rapidly phasing in the timely administration of a safe monovalent HBV vaccine within a few hours after birth could contribute to the interruption of mother-to-child transmission and avert preventable HBV infections in childhood,” Geoffrey Dusheiko, MD, wrote in an accompanying editorial (N Engl J Med. 2018;378:952-3).
The World Health Organization supports HBV vaccine schedules of three or four doses, which are usually given as part of a combination immunization protocol beginning at 6 weeks of age. “Currently, HBV vaccination is most frequently administered as a pentavalent or hexavalent vaccine as part of the Expanded Program on Immunization, typically in combination with vaccines against diphtheria, tetanus, pertussis, polio, and Haemophilus influenzae type B,” wrote Dr. Dusheiko. “Paradoxically, support for combination vaccines within an integrated EPI schedule has unwittingly but undesirably shifted thinking and policy away from HBV vaccination at birth. This gap in vaccine strategy is disadvantageous.”
While the study doesn’t support tenofovir for maternal prophylaxis, it does imply value for treating the infant with immune globulin and HBV vaccination soon after birth. Delivering this kind of care globally will be challenging, but it’s entirely feasible, Dr. Dusheiko said.
“It is necessary to analyze regional data to assess the requirements for implementing vaccination at birth, including ... the training of otherwise unskilled birth attendants to deliver monovalent HBV vaccine at the same time as the vaccines against polio and bacille Calmette–Guérin. Importantly, the use of monovalent HBV vaccine would also require governmental or nongovernmental support. HBV vaccination at birth, despite the challenges for poverty-affected countries to deliver vaccination in rural and isolated locales, is feasible.”
Dr. Dusheiko is a hepatologist at the University College London School of Medicine and King’s College Hospital, London.
Prenatal tenofovir didn’t reduce the rate of hepatitis B among infants born to women infected with the virus.
Among 322 6-month-olds, the rate of HBV transmission was 0 in those whose mothers received the antiviral during pregnancy and 2% among those whose mothers received placebo – not a statistically significant difference, Gonzague Jourdain, MD, and his colleagues reported in the New England Journal of Medicine.
The study randomized 331 pregnant women with proven HBV infections to either tenofovir or placebo from 28 weeks’ gestation to 2 months post partum. All infants received HBV immune globulin at birth, and HBV vaccine at birth and at 1, 2, 4, and 6 months. The primary endpoint was confirmed HBV infection in the infant at 6 months.
Women were a mean of 28 weeks pregnant at baseline, with a mean viral load of 8.0 log10 IU/mL. Most women (about 90% of each group) had an HBV DNA of more than 200,000 IU/mL – a level associated with an increased risk of perinatal HBV infection despite vaccination.
There were 322 deliveries, resulting in 319 singletons, two pairs of twins, and one stillbirth. Postpartum infant treatment was quick, with a median of 1.3 hours from birth to administration of immune globulin and a median of 1.2 hours to administration of the first dose of the vaccine.
At 6 months, there were no HBV infections in the tenofovir-exposed group and 3 (2%) in the placebo group – a nonsignificant difference (P = .12).
Tenofovir was safe for both mother and fetus, with no significant adverse events in either group. The incidence of elevated maternal alanine aminotransferase level (more than 300 IU/L) was 6% in the tenofovir group and 3% in the placebo group, also a nonsignificant finding.
Dr. Jourdain and his colleagues noted that the 2% transmission rate in the placebo group is considerably lower than the 7% seen in similar studies and could be related to the rapid postpartum administration of HBV immune globulin and vaccine. If this is the case, prenatal antivirals could be more effective in countries where postpartum treatment is delayed or inconsistent.
“Maternal use of tenofovir may prevent transmissions that would occur when the birth dose is delayed, but its exact timing has not been reported consistently in previous perinatal studies,” the team said.
Another question is whether the stringent, 5-dose infant HBV vaccine series required in Thailand is simply more effective than schedules that have fewer doses or are combined with other vaccines and delivered later.
“It remains unclear whether the administration of more vaccine doses is more efficacious than the administration of the three vaccine doses that is recommended in the United States and by the World Health Organization.”
Dr. Jourdain had no financial disclosures relevant to the study, which was sponsored by the National Institute of Child Health and Human Development.
SOURCE: Jourdain G et al. N Engl J Med. 2018;378:911-23.
Prenatal tenofovir didn’t reduce the rate of hepatitis B among infants born to women infected with the virus.
Among 322 6-month-olds, the rate of HBV transmission was 0 in those whose mothers received the antiviral during pregnancy and 2% among those whose mothers received placebo – not a statistically significant difference, Gonzague Jourdain, MD, and his colleagues reported in the New England Journal of Medicine.
The study randomized 331 pregnant women with proven HBV infections to either tenofovir or placebo from 28 weeks’ gestation to 2 months post partum. All infants received HBV immune globulin at birth, and HBV vaccine at birth and at 1, 2, 4, and 6 months. The primary endpoint was confirmed HBV infection in the infant at 6 months.
Women were a mean of 28 weeks pregnant at baseline, with a mean viral load of 8.0 log10 IU/mL. Most women (about 90% of each group) had an HBV DNA of more than 200,000 IU/mL – a level associated with an increased risk of perinatal HBV infection despite vaccination.
There were 322 deliveries, resulting in 319 singletons, two pairs of twins, and one stillbirth. Postpartum infant treatment was quick, with a median of 1.3 hours from birth to administration of immune globulin and a median of 1.2 hours to administration of the first dose of the vaccine.
At 6 months, there were no HBV infections in the tenofovir-exposed group and 3 (2%) in the placebo group – a nonsignificant difference (P = .12).
Tenofovir was safe for both mother and fetus, with no significant adverse events in either group. The incidence of elevated maternal alanine aminotransferase level (more than 300 IU/L) was 6% in the tenofovir group and 3% in the placebo group, also a nonsignificant finding.
Dr. Jourdain and his colleagues noted that the 2% transmission rate in the placebo group is considerably lower than the 7% seen in similar studies and could be related to the rapid postpartum administration of HBV immune globulin and vaccine. If this is the case, prenatal antivirals could be more effective in countries where postpartum treatment is delayed or inconsistent.
“Maternal use of tenofovir may prevent transmissions that would occur when the birth dose is delayed, but its exact timing has not been reported consistently in previous perinatal studies,” the team said.
Another question is whether the stringent, 5-dose infant HBV vaccine series required in Thailand is simply more effective than schedules that have fewer doses or are combined with other vaccines and delivered later.
“It remains unclear whether the administration of more vaccine doses is more efficacious than the administration of the three vaccine doses that is recommended in the United States and by the World Health Organization.”
Dr. Jourdain had no financial disclosures relevant to the study, which was sponsored by the National Institute of Child Health and Human Development.
SOURCE: Jourdain G et al. N Engl J Med. 2018;378:911-23.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Tenofovir was no different than placebo in preventing HBV transmission to newborns.
Major finding: The transmission rate was 0% in the tenofovir group and 2% in the placebo group (P = .12).
Study details: The study randomized 331 pregnant women to tenofovir or placebo from gestational week 28 to 2 months postpartum.
Disclosures: Dr. Jourdain had no financial disclosures; the National Institute of Child Health and Development sponsored the study.
Source: Jourdain G et al. N Engl J Med. 2018;378:911-23.