User login
Opioid prescriptions got shorter in 2017
Use of opioids was down among enrollees of all types of payers in 2017, while the use of drugs to treat opioid dependence went up for three of the four payer categories, according to pharmacy benefits manager Express Scripts.
The days’ worth of opioids dispensed per person per year was down 16.6% from 2016 to 2017 for enrollees with plans on the health exchanges received managed by Express Scripts. Medicaid patients received 16% fewer days’ worth, patients with commercial plans received 10.3% fewer days’ worth, and Medicare patients received 4.9% fewer days’ worth, Express Scripts said in its 2017 Drug Trend Report, which was based on data for 34.3 million members of pharmacy benefits plans the company administers.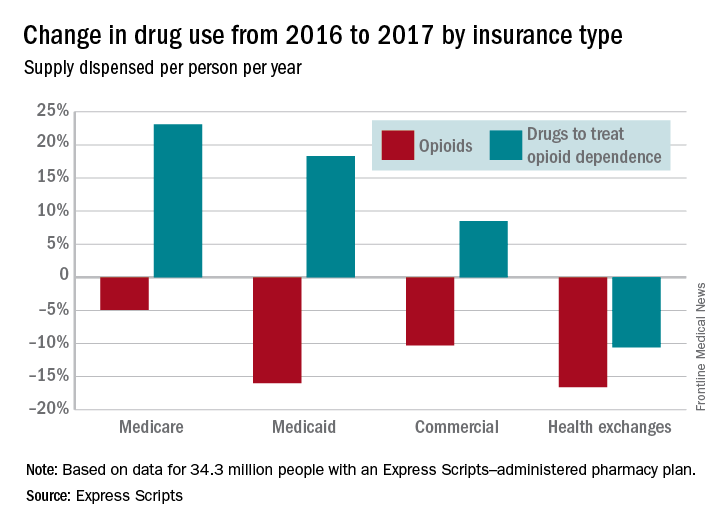
Plans that participated in Express Scripts’ Advanced Opioid Management solution, which was launched in September, experienced “a 60% reduction in the average days’ supply per initial fill, from 18.6 days to just 7.5 days,” according to the report.
Use of opioids was down among enrollees of all types of payers in 2017, while the use of drugs to treat opioid dependence went up for three of the four payer categories, according to pharmacy benefits manager Express Scripts.
The days’ worth of opioids dispensed per person per year was down 16.6% from 2016 to 2017 for enrollees with plans on the health exchanges received managed by Express Scripts. Medicaid patients received 16% fewer days’ worth, patients with commercial plans received 10.3% fewer days’ worth, and Medicare patients received 4.9% fewer days’ worth, Express Scripts said in its 2017 Drug Trend Report, which was based on data for 34.3 million members of pharmacy benefits plans the company administers.
Plans that participated in Express Scripts’ Advanced Opioid Management solution, which was launched in September, experienced “a 60% reduction in the average days’ supply per initial fill, from 18.6 days to just 7.5 days,” according to the report.
Use of opioids was down among enrollees of all types of payers in 2017, while the use of drugs to treat opioid dependence went up for three of the four payer categories, according to pharmacy benefits manager Express Scripts.
The days’ worth of opioids dispensed per person per year was down 16.6% from 2016 to 2017 for enrollees with plans on the health exchanges received managed by Express Scripts. Medicaid patients received 16% fewer days’ worth, patients with commercial plans received 10.3% fewer days’ worth, and Medicare patients received 4.9% fewer days’ worth, Express Scripts said in its 2017 Drug Trend Report, which was based on data for 34.3 million members of pharmacy benefits plans the company administers.
Plans that participated in Express Scripts’ Advanced Opioid Management solution, which was launched in September, experienced “a 60% reduction in the average days’ supply per initial fill, from 18.6 days to just 7.5 days,” according to the report.
Hemophilia A treatment gains approval in Europe
The European Commission has approved emicizumab for routine prophylaxis of bleeding episodes in adults and children with hemophilia A with factor VIII inhibitors.
The European Medicines Agency’s Committee for Medicinal Products for Human Use recommended granting marketing authorization to emicizumab (Hemlibra) in January 2018 and it was approved by the Food and Drug Administration in November 2017.
The approvals are based on findings from the HAVEN 1 trial (in 109 adults and adolescents) and the HAVEN 2 trial (in children younger than age 12 years), according to the drug’s maker, Roche. In HAVEN 1 (NCT02622321), emicizumab showed a significant reduction in treatment bleeds (87%), compared with no prophylaxis. And the drug reduced treated bleeds by 79%, compared with previous treatment with bypassing agent prophylaxis. An interim analysis of HAVEN 2 (NCT02795767) of 23 children, showed that 87% of children who received emicizumab prophylaxis had zero treated bleeds, according to Roche.
The European Commission has approved emicizumab for routine prophylaxis of bleeding episodes in adults and children with hemophilia A with factor VIII inhibitors.
The European Medicines Agency’s Committee for Medicinal Products for Human Use recommended granting marketing authorization to emicizumab (Hemlibra) in January 2018 and it was approved by the Food and Drug Administration in November 2017.
The approvals are based on findings from the HAVEN 1 trial (in 109 adults and adolescents) and the HAVEN 2 trial (in children younger than age 12 years), according to the drug’s maker, Roche. In HAVEN 1 (NCT02622321), emicizumab showed a significant reduction in treatment bleeds (87%), compared with no prophylaxis. And the drug reduced treated bleeds by 79%, compared with previous treatment with bypassing agent prophylaxis. An interim analysis of HAVEN 2 (NCT02795767) of 23 children, showed that 87% of children who received emicizumab prophylaxis had zero treated bleeds, according to Roche.
The European Commission has approved emicizumab for routine prophylaxis of bleeding episodes in adults and children with hemophilia A with factor VIII inhibitors.
The European Medicines Agency’s Committee for Medicinal Products for Human Use recommended granting marketing authorization to emicizumab (Hemlibra) in January 2018 and it was approved by the Food and Drug Administration in November 2017.
The approvals are based on findings from the HAVEN 1 trial (in 109 adults and adolescents) and the HAVEN 2 trial (in children younger than age 12 years), according to the drug’s maker, Roche. In HAVEN 1 (NCT02622321), emicizumab showed a significant reduction in treatment bleeds (87%), compared with no prophylaxis. And the drug reduced treated bleeds by 79%, compared with previous treatment with bypassing agent prophylaxis. An interim analysis of HAVEN 2 (NCT02795767) of 23 children, showed that 87% of children who received emicizumab prophylaxis had zero treated bleeds, according to Roche.
Finding a groove helps patients move
Listening to uptempo music significantly improved patients’ compared with patients who didn’t listen to music, according to data from a randomized trial of 127 patients.
Exercise stress tests are frequently recommended to evaluate patients for heart disease, but many patients don’t work hard enough to reach a useful level of exertion, Waseem Shami, MD, of Texas Tech University in El Paso, said in a web briefing in advance of the annual meeting of the American College of Cardiology.
The group of patients that listened to lively music averaged 55 seconds longer exercise time, compared with the no-music control group, Dr. Shami said. The average exercise time was 505.8 seconds in the music group and 455.2 in the control group (P = .045).
Dr. Shami and his colleagues randomized 67 adults scheduled for cardiac stress tests to listen to music during the test and 60 controls to undergo the test without music. The average age of the patients was 53 years, and 61% and 67% of those in the music and control groups, respectively, were women. Demographic characteristics and variables, including resting heart rate and blood pressure, were similar between the music and control groups.
In a clinical setting, the use of music may help reduce unnecessary stress testing, which is deemed unsuccessful if the patient doesn’t exercise hard enough to achieve a target heart rate. “Perhaps this motivational tool can help us make stress testing more valuable,” said Dr. Shami. The results were limited by the relatively small study population and the fact that the researchers, not the patients, chose the music, Dr. Shami said.
More research is needed in a larger trial, and “allowing patients to choose their own music might make an even bigger difference,” he noted. Also, patients’ discomfort with exercising in general and exercising in public may have impacted the results, he said.
Moderator Martha Gulati, MD, of the University of Arizona, Phoenix, noted that an area for future research might be to do a cardiac stress test without and without music on the same person, with the patient serving as his or her own control.
Dr. Shami had no relevant financial conflicts to disclose.
Listening to uptempo music significantly improved patients’ compared with patients who didn’t listen to music, according to data from a randomized trial of 127 patients.
Exercise stress tests are frequently recommended to evaluate patients for heart disease, but many patients don’t work hard enough to reach a useful level of exertion, Waseem Shami, MD, of Texas Tech University in El Paso, said in a web briefing in advance of the annual meeting of the American College of Cardiology.
The group of patients that listened to lively music averaged 55 seconds longer exercise time, compared with the no-music control group, Dr. Shami said. The average exercise time was 505.8 seconds in the music group and 455.2 in the control group (P = .045).
Dr. Shami and his colleagues randomized 67 adults scheduled for cardiac stress tests to listen to music during the test and 60 controls to undergo the test without music. The average age of the patients was 53 years, and 61% and 67% of those in the music and control groups, respectively, were women. Demographic characteristics and variables, including resting heart rate and blood pressure, were similar between the music and control groups.
In a clinical setting, the use of music may help reduce unnecessary stress testing, which is deemed unsuccessful if the patient doesn’t exercise hard enough to achieve a target heart rate. “Perhaps this motivational tool can help us make stress testing more valuable,” said Dr. Shami. The results were limited by the relatively small study population and the fact that the researchers, not the patients, chose the music, Dr. Shami said.
More research is needed in a larger trial, and “allowing patients to choose their own music might make an even bigger difference,” he noted. Also, patients’ discomfort with exercising in general and exercising in public may have impacted the results, he said.
Moderator Martha Gulati, MD, of the University of Arizona, Phoenix, noted that an area for future research might be to do a cardiac stress test without and without music on the same person, with the patient serving as his or her own control.
Dr. Shami had no relevant financial conflicts to disclose.
Listening to uptempo music significantly improved patients’ compared with patients who didn’t listen to music, according to data from a randomized trial of 127 patients.
Exercise stress tests are frequently recommended to evaluate patients for heart disease, but many patients don’t work hard enough to reach a useful level of exertion, Waseem Shami, MD, of Texas Tech University in El Paso, said in a web briefing in advance of the annual meeting of the American College of Cardiology.
The group of patients that listened to lively music averaged 55 seconds longer exercise time, compared with the no-music control group, Dr. Shami said. The average exercise time was 505.8 seconds in the music group and 455.2 in the control group (P = .045).
Dr. Shami and his colleagues randomized 67 adults scheduled for cardiac stress tests to listen to music during the test and 60 controls to undergo the test without music. The average age of the patients was 53 years, and 61% and 67% of those in the music and control groups, respectively, were women. Demographic characteristics and variables, including resting heart rate and blood pressure, were similar between the music and control groups.
In a clinical setting, the use of music may help reduce unnecessary stress testing, which is deemed unsuccessful if the patient doesn’t exercise hard enough to achieve a target heart rate. “Perhaps this motivational tool can help us make stress testing more valuable,” said Dr. Shami. The results were limited by the relatively small study population and the fact that the researchers, not the patients, chose the music, Dr. Shami said.
More research is needed in a larger trial, and “allowing patients to choose their own music might make an even bigger difference,” he noted. Also, patients’ discomfort with exercising in general and exercising in public may have impacted the results, he said.
Moderator Martha Gulati, MD, of the University of Arizona, Phoenix, noted that an area for future research might be to do a cardiac stress test without and without music on the same person, with the patient serving as his or her own control.
Dr. Shami had no relevant financial conflicts to disclose.
FROM ACC 18
ACC Day 3: Finishing strong in Late-Breaking Clinical Trial Sessions
On the third and final day of the annual meeting of the American College of Cardiology in Orlando, two very different studies were emphasized by the meeting’s vice chair, Andrew Kates, MD, in a media briefing: Blood Pressure Reductions in Black Barbershops and ANNEXA 4.
Blood Pressure Reductions in Black Barbershops
In the study, barbers in those 53 shops were trained to measure clients’ blood pressure and refer those with high readings to a community pharmacist for confirmation of uncontrolled high blood pressure and development of a hypertension management plan. The primary outcome is a 6-month blood pressure reading of less than 135/85 mm Hg.
“This stood out for highlighting because we’re now realizing the importance of barbershops in the community” in education and perhaps improving treatment. The data may reinforce the use of barbershops to improve hypertension,” said Dr. Kates, professor of medicine, Washington University, St. Louis.
ANNEXA 4
An interim analysis of ANNEXA 4 will shed light on the safety of andexanet alfa, a novel potential reversal agent for patients on direct oral anticoagulants who are experiencing an acute major bleed.
ANNEXA 4 (Prospective, Open-Label Study of Andexanet Alfa in Patients Receiving a Factor Xa Inhibitor Who Have Acute Major Bleeding) is an ongoing phase 3b/4 trial of the novel agent, a modified form of the human factor Xa molecule. Interim results showed that, at 12 hours after infusion, 37 of 47 patients in the efficacy analysis achieved excellent or good hemostasis.
The Food and Drug Administration is currently reviewing andexanet alfa, and a decision is expected this May.
Stuart J. Connolly, MD, of McMaster University, Hamilton, Ont., will present the report at the Fifth Late-Breaking Clinical Trial Session on Monday, 10:45 a.m.-11:45 a.m., in the Main Tent.
On the third and final day of the annual meeting of the American College of Cardiology in Orlando, two very different studies were emphasized by the meeting’s vice chair, Andrew Kates, MD, in a media briefing: Blood Pressure Reductions in Black Barbershops and ANNEXA 4.
Blood Pressure Reductions in Black Barbershops
In the study, barbers in those 53 shops were trained to measure clients’ blood pressure and refer those with high readings to a community pharmacist for confirmation of uncontrolled high blood pressure and development of a hypertension management plan. The primary outcome is a 6-month blood pressure reading of less than 135/85 mm Hg.
“This stood out for highlighting because we’re now realizing the importance of barbershops in the community” in education and perhaps improving treatment. The data may reinforce the use of barbershops to improve hypertension,” said Dr. Kates, professor of medicine, Washington University, St. Louis.
ANNEXA 4
An interim analysis of ANNEXA 4 will shed light on the safety of andexanet alfa, a novel potential reversal agent for patients on direct oral anticoagulants who are experiencing an acute major bleed.
ANNEXA 4 (Prospective, Open-Label Study of Andexanet Alfa in Patients Receiving a Factor Xa Inhibitor Who Have Acute Major Bleeding) is an ongoing phase 3b/4 trial of the novel agent, a modified form of the human factor Xa molecule. Interim results showed that, at 12 hours after infusion, 37 of 47 patients in the efficacy analysis achieved excellent or good hemostasis.
The Food and Drug Administration is currently reviewing andexanet alfa, and a decision is expected this May.
Stuart J. Connolly, MD, of McMaster University, Hamilton, Ont., will present the report at the Fifth Late-Breaking Clinical Trial Session on Monday, 10:45 a.m.-11:45 a.m., in the Main Tent.
On the third and final day of the annual meeting of the American College of Cardiology in Orlando, two very different studies were emphasized by the meeting’s vice chair, Andrew Kates, MD, in a media briefing: Blood Pressure Reductions in Black Barbershops and ANNEXA 4.
Blood Pressure Reductions in Black Barbershops
In the study, barbers in those 53 shops were trained to measure clients’ blood pressure and refer those with high readings to a community pharmacist for confirmation of uncontrolled high blood pressure and development of a hypertension management plan. The primary outcome is a 6-month blood pressure reading of less than 135/85 mm Hg.
“This stood out for highlighting because we’re now realizing the importance of barbershops in the community” in education and perhaps improving treatment. The data may reinforce the use of barbershops to improve hypertension,” said Dr. Kates, professor of medicine, Washington University, St. Louis.
ANNEXA 4
An interim analysis of ANNEXA 4 will shed light on the safety of andexanet alfa, a novel potential reversal agent for patients on direct oral anticoagulants who are experiencing an acute major bleed.
ANNEXA 4 (Prospective, Open-Label Study of Andexanet Alfa in Patients Receiving a Factor Xa Inhibitor Who Have Acute Major Bleeding) is an ongoing phase 3b/4 trial of the novel agent, a modified form of the human factor Xa molecule. Interim results showed that, at 12 hours after infusion, 37 of 47 patients in the efficacy analysis achieved excellent or good hemostasis.
The Food and Drug Administration is currently reviewing andexanet alfa, and a decision is expected this May.
Stuart J. Connolly, MD, of McMaster University, Hamilton, Ont., will present the report at the Fifth Late-Breaking Clinical Trial Session on Monday, 10:45 a.m.-11:45 a.m., in the Main Tent.
Heart attacks soar in young IBD patients
Inflammatory bowel disease significantly increases the risk of a heart attack in adults, but especially young adults aged 18-24 years, and in women compared with men across all age groups, according to data from about 200,000 IBD patients.
The odds ratio for heart attack in IBD patients vs. controls remained a significant 1.2 after adjustment for traditional cardiovascular risk factors, Muhammad S. Panhwar, MD, said in a media briefing in advance of the annual meeting of the American College of Cardiology.
“Chronic inflammation has been recognized as having an important role in the development of heart disease,” he noted.
Although other chronic inflammatory conditions are associated with increased heart attack risk, the link between heart attacks and IBD has not been well studied, despite its high prevalence in the United States (about 3 million adults, according to the Centers for Disease Control and Prevention), said Dr. Panhwar, an internal medicine resident at Case Western Reserve University in Cleveland. He and his colleagues reviewed a nationwide medical records database of 17.5 million adults aged 18-65 years for diagnoses of IBD between 2013 and 2017. Overall, 1.2% of the patients (211,870) had IBD, and most of the patients in the IBD group were younger, female, and white, Dr. Panhwar noted.
The relative risk of myocardial infarction was roughly twice as high in IBD patients as that of controls without IBD (5.9% vs. 3.5%), Dr. Panhwar said. That risk was highest in patients aged 20-25 years, with a relative risk of 20.5, occurring mostly in women, and decreased to 1.8 by age 60-64 (both P less than .001).
In addition, IBD patients tended to have a higher prevalence of common cardiovascular risk factors such as high blood pressure, obesity, and smoking.
The IBD patients’ higher prevalence of smoking – 21%, vs. 12% of the controls – is not a surprise, said Martha Gulati, MD, who moderated the briefing. Many people with IBD smoke, particularly those with Crohn’s disease, because it seems to reduce the number of flares, said Dr. Gulati, chief of cardiology at the University of Arizona, Phoenix.
The findings may be affected by the increased inflammation often observed in younger individuals with IBD and younger women with IBD, who may not present with traditional cardiovascular risk factors, the researchers noted.
“Clinicians who care for patients with traditional cardiovascular risk factors who also have IBD should recognize IBD as an independent risk factor as well, and treat appropriately,” Dr. Panhwar said.
Dr. Panhwar had no relevant financial conflicts to disclose.
SOURCE: Panhwar M. ACC 18.
Inflammatory bowel disease significantly increases the risk of a heart attack in adults, but especially young adults aged 18-24 years, and in women compared with men across all age groups, according to data from about 200,000 IBD patients.
The odds ratio for heart attack in IBD patients vs. controls remained a significant 1.2 after adjustment for traditional cardiovascular risk factors, Muhammad S. Panhwar, MD, said in a media briefing in advance of the annual meeting of the American College of Cardiology.
“Chronic inflammation has been recognized as having an important role in the development of heart disease,” he noted.
Although other chronic inflammatory conditions are associated with increased heart attack risk, the link between heart attacks and IBD has not been well studied, despite its high prevalence in the United States (about 3 million adults, according to the Centers for Disease Control and Prevention), said Dr. Panhwar, an internal medicine resident at Case Western Reserve University in Cleveland. He and his colleagues reviewed a nationwide medical records database of 17.5 million adults aged 18-65 years for diagnoses of IBD between 2013 and 2017. Overall, 1.2% of the patients (211,870) had IBD, and most of the patients in the IBD group were younger, female, and white, Dr. Panhwar noted.
The relative risk of myocardial infarction was roughly twice as high in IBD patients as that of controls without IBD (5.9% vs. 3.5%), Dr. Panhwar said. That risk was highest in patients aged 20-25 years, with a relative risk of 20.5, occurring mostly in women, and decreased to 1.8 by age 60-64 (both P less than .001).
In addition, IBD patients tended to have a higher prevalence of common cardiovascular risk factors such as high blood pressure, obesity, and smoking.
The IBD patients’ higher prevalence of smoking – 21%, vs. 12% of the controls – is not a surprise, said Martha Gulati, MD, who moderated the briefing. Many people with IBD smoke, particularly those with Crohn’s disease, because it seems to reduce the number of flares, said Dr. Gulati, chief of cardiology at the University of Arizona, Phoenix.
The findings may be affected by the increased inflammation often observed in younger individuals with IBD and younger women with IBD, who may not present with traditional cardiovascular risk factors, the researchers noted.
“Clinicians who care for patients with traditional cardiovascular risk factors who also have IBD should recognize IBD as an independent risk factor as well, and treat appropriately,” Dr. Panhwar said.
Dr. Panhwar had no relevant financial conflicts to disclose.
SOURCE: Panhwar M. ACC 18.
Inflammatory bowel disease significantly increases the risk of a heart attack in adults, but especially young adults aged 18-24 years, and in women compared with men across all age groups, according to data from about 200,000 IBD patients.
The odds ratio for heart attack in IBD patients vs. controls remained a significant 1.2 after adjustment for traditional cardiovascular risk factors, Muhammad S. Panhwar, MD, said in a media briefing in advance of the annual meeting of the American College of Cardiology.
“Chronic inflammation has been recognized as having an important role in the development of heart disease,” he noted.
Although other chronic inflammatory conditions are associated with increased heart attack risk, the link between heart attacks and IBD has not been well studied, despite its high prevalence in the United States (about 3 million adults, according to the Centers for Disease Control and Prevention), said Dr. Panhwar, an internal medicine resident at Case Western Reserve University in Cleveland. He and his colleagues reviewed a nationwide medical records database of 17.5 million adults aged 18-65 years for diagnoses of IBD between 2013 and 2017. Overall, 1.2% of the patients (211,870) had IBD, and most of the patients in the IBD group were younger, female, and white, Dr. Panhwar noted.
The relative risk of myocardial infarction was roughly twice as high in IBD patients as that of controls without IBD (5.9% vs. 3.5%), Dr. Panhwar said. That risk was highest in patients aged 20-25 years, with a relative risk of 20.5, occurring mostly in women, and decreased to 1.8 by age 60-64 (both P less than .001).
In addition, IBD patients tended to have a higher prevalence of common cardiovascular risk factors such as high blood pressure, obesity, and smoking.
The IBD patients’ higher prevalence of smoking – 21%, vs. 12% of the controls – is not a surprise, said Martha Gulati, MD, who moderated the briefing. Many people with IBD smoke, particularly those with Crohn’s disease, because it seems to reduce the number of flares, said Dr. Gulati, chief of cardiology at the University of Arizona, Phoenix.
The findings may be affected by the increased inflammation often observed in younger individuals with IBD and younger women with IBD, who may not present with traditional cardiovascular risk factors, the researchers noted.
“Clinicians who care for patients with traditional cardiovascular risk factors who also have IBD should recognize IBD as an independent risk factor as well, and treat appropriately,” Dr. Panhwar said.
Dr. Panhwar had no relevant financial conflicts to disclose.
SOURCE: Panhwar M. ACC 18.
FROM ACC 18
Key clinical point:
Major finding: The relative risk of MI was roughly twice as high in IBD patients compared with controls without IBD (5.9% vs. 3.5%).
Study details: Review of a nationwide medical records database of 17.5 million adults aged 18-65 years.
Disclosures: Dr. Panhwar had no relevant financial conflicts to disclose.
Source: Panhwar M. ACC 18.
VIDEO: Device-based therapy for onychomycosis
REPORTING FROM AAD 18
SAN DIEGO – which has been studied in two clinical trials and case series, Shari Lipner, MD, PhD, said in a video interview at the annual meeting of the American Academy of Dermatology, where she presented on this topic.
“Something that we’re looking at is plasma treatment of onychomycosis basically using ionized gas,” which has been shown to inhibit the growth of Trichophyton rubrum in vitro, added Dr. Lipner of the department of dermatology, Cornell University, New York.
In a pilot study of 19 patients with onychomycosis, she and her associates found that the clinical cure with nonthermal plasma was about 50% and the mycological cure rate was 15%, “and we’re now trying to improve efficacy using this device,” she said (Clin Exp Dermatol. 2017 Apr;42[3]:295-8). With a dielectric insulator, “nonthermal plasma is created by short pulses (about 10 ns) of strong (about 20 kV/mm peak) electric field that ionizes air molecules, creating ions and electrons, as well as ozone, hydroxyl radicals and nitric oxide,” according to the description in the study.
Other device-based therapies include iontophoresis, using electrical currents to increase drug delivery, and creating small punch biopsies or using a device to create “microholes” in the nails to increase delivery of topical medication across the nail, Dr. Lipner said.
Patients often ask about another device-based treatment, laser therapy, which she pointed out is not approved by the Food and Drug Administration for cure, but for a temporary increase in clear nail in patients with onychomycosis, “very different” than the criteria used for topical and systemic medications, making it difficult to compare efficacy data between lasers and medications, she noted.
Dr. Lipner reported receiving grants/research funding from MOE Medical Devices.
REPORTING FROM AAD 18
SAN DIEGO – which has been studied in two clinical trials and case series, Shari Lipner, MD, PhD, said in a video interview at the annual meeting of the American Academy of Dermatology, where she presented on this topic.
“Something that we’re looking at is plasma treatment of onychomycosis basically using ionized gas,” which has been shown to inhibit the growth of Trichophyton rubrum in vitro, added Dr. Lipner of the department of dermatology, Cornell University, New York.
In a pilot study of 19 patients with onychomycosis, she and her associates found that the clinical cure with nonthermal plasma was about 50% and the mycological cure rate was 15%, “and we’re now trying to improve efficacy using this device,” she said (Clin Exp Dermatol. 2017 Apr;42[3]:295-8). With a dielectric insulator, “nonthermal plasma is created by short pulses (about 10 ns) of strong (about 20 kV/mm peak) electric field that ionizes air molecules, creating ions and electrons, as well as ozone, hydroxyl radicals and nitric oxide,” according to the description in the study.
Other device-based therapies include iontophoresis, using electrical currents to increase drug delivery, and creating small punch biopsies or using a device to create “microholes” in the nails to increase delivery of topical medication across the nail, Dr. Lipner said.
Patients often ask about another device-based treatment, laser therapy, which she pointed out is not approved by the Food and Drug Administration for cure, but for a temporary increase in clear nail in patients with onychomycosis, “very different” than the criteria used for topical and systemic medications, making it difficult to compare efficacy data between lasers and medications, she noted.
Dr. Lipner reported receiving grants/research funding from MOE Medical Devices.
REPORTING FROM AAD 18
SAN DIEGO – which has been studied in two clinical trials and case series, Shari Lipner, MD, PhD, said in a video interview at the annual meeting of the American Academy of Dermatology, where she presented on this topic.
“Something that we’re looking at is plasma treatment of onychomycosis basically using ionized gas,” which has been shown to inhibit the growth of Trichophyton rubrum in vitro, added Dr. Lipner of the department of dermatology, Cornell University, New York.
In a pilot study of 19 patients with onychomycosis, she and her associates found that the clinical cure with nonthermal plasma was about 50% and the mycological cure rate was 15%, “and we’re now trying to improve efficacy using this device,” she said (Clin Exp Dermatol. 2017 Apr;42[3]:295-8). With a dielectric insulator, “nonthermal plasma is created by short pulses (about 10 ns) of strong (about 20 kV/mm peak) electric field that ionizes air molecules, creating ions and electrons, as well as ozone, hydroxyl radicals and nitric oxide,” according to the description in the study.
Other device-based therapies include iontophoresis, using electrical currents to increase drug delivery, and creating small punch biopsies or using a device to create “microholes” in the nails to increase delivery of topical medication across the nail, Dr. Lipner said.
Patients often ask about another device-based treatment, laser therapy, which she pointed out is not approved by the Food and Drug Administration for cure, but for a temporary increase in clear nail in patients with onychomycosis, “very different” than the criteria used for topical and systemic medications, making it difficult to compare efficacy data between lasers and medications, she noted.
Dr. Lipner reported receiving grants/research funding from MOE Medical Devices.
Strategies to reduce colorectal surgery complications
LAS VEGAS – Colorectal surgery is rife with potential complications, but there are steps that surgeons can take to improve outcomes, and factors to consider to reduce complications. These strategies and considerations were the focus of a talk by Matthew G. Mutch, MD, at the Annual Minimally Invasive Surgery Symposium by Global Academy for Medical Education.
Prehabilitation
The approach to improve outcomes can begin with prehabilitation – preparing the patient for the difficult process of surgery. “If somebody is going to fight a 15-round heavyweight bout, they train for 6 or 8 weeks before a fight. Why not bring that concept to surgery?” said Dr. Mutch, chief of colon and rectal surgery at the Washington University, St. Louis.
Prehabilitation can include lifestyle changes, such as quitting smoking, but can also incorporate aerobic and/or resistance exercise, dietary counseling and protein supplementation, anxiety reduction, and medical education to prepare the patient for the challenges ahead. “Preoperatively, we try to identify factors to see if we can make meaningful lifestyle changes, because that’s really the grassroots level where a lot of this [improvement in outcomes] is going to occur,” said Dr. Mutch.
Frailty
Frailty is a factor driving complications in colorectal surgery. A meta-analysis of 20 studies showed that frailty and prefrailty were associated with worse all-cause mortality during follow-up among older cancer patients. More striking, it showed that frail patients were nearly five times more likely to be intolerant of cancer treatment (odds ratio, 4.86) and more likely to experience postoperative complications (30-day hazard ratio, 3.19) (Ann Oncol. 2015;26[6]:1091-1101).
Hemoglobin A1c
Dr. Mutch went on to discuss hemoglobin A1c (HbA1c) levels as a risk factor in colorectal surgery. HbA1c levels higher than 6 are associated with worse outcomes, but tight postoperative control is associated with hypoglycemia. “What you want to do is set that patient up before surgery. HbA1c has a half-life of about a month, so if you start modifying their risk factors 4-6 weeks before you get them into surgery, by 1 month you can see a 50% reduction, and at 2 months a 75% reduction. If you do these things in a preoperative setting it makes a difference,” said Dr. Mutch.
Smoking cessation
Smoking cessation is another key strategy. Two weeks of cessation should lead to a decline in coughing, but a minimum of 4 weeks is needed to significantly reduce overall complications. Lifestyle changes need to be long term. “These are not measures that you’re going to do over a short period of time, and then when surgery is over throw it out the window,” said Dr. Mutch.
Anastomotic leak
Another factor is the detection of anastomotic leak, which can be challenging because its definitions vary significantly, and its causes can be multifactorial. Studies show that predictions of anastomotic leak are not especially successful, Dr. Mutch said, but routine leak testing improves outcomes. In a study of left-side anastomoses in Washington State, hospitals that performed leak tests had lower leak rates at least 90% of the time (OR, 0.23), and hospitals that later implemented leak tests experienced a significant reduction (Arch Surg. 2012:147[4]:345-51).
Venous thromboembolic events
Venous thromboembolic events (VTE), are the leading cause of operative mortality in colorectal surgery patients. This complication can be greatly reduced with prophylaxis, but requires screening for risk factors. Major surgery raises the risk of deep vein thrombosis in 20% of all hospitalized patients to 40%-80%, depending on the surgery type. “We have a lot of room to improve,” said Dr. Mutch.
Timing
One factor that may have an impact on complications appears to be timing of surgery, at least at Washington University, where Dr. Mutch practices. The institution found that patients who had surgery the same day they were admitted had a 2.5% VTE risk, compared with 11% in patients who had surgery 5 or more days after admission.
Postop ambulation
Postsurgical ambulation was another critical complication factor. Dr. Mutch cited a study showing that ambulation on the day after surgery was associated with a 1% VTE risk, compared to 6.9% in patients who waited until day 2.
Dr. Mutch had no disclosures. Global Academy for Medical Education and this news organization are owned by the same parent company.
LAS VEGAS – Colorectal surgery is rife with potential complications, but there are steps that surgeons can take to improve outcomes, and factors to consider to reduce complications. These strategies and considerations were the focus of a talk by Matthew G. Mutch, MD, at the Annual Minimally Invasive Surgery Symposium by Global Academy for Medical Education.
Prehabilitation
The approach to improve outcomes can begin with prehabilitation – preparing the patient for the difficult process of surgery. “If somebody is going to fight a 15-round heavyweight bout, they train for 6 or 8 weeks before a fight. Why not bring that concept to surgery?” said Dr. Mutch, chief of colon and rectal surgery at the Washington University, St. Louis.
Prehabilitation can include lifestyle changes, such as quitting smoking, but can also incorporate aerobic and/or resistance exercise, dietary counseling and protein supplementation, anxiety reduction, and medical education to prepare the patient for the challenges ahead. “Preoperatively, we try to identify factors to see if we can make meaningful lifestyle changes, because that’s really the grassroots level where a lot of this [improvement in outcomes] is going to occur,” said Dr. Mutch.
Frailty
Frailty is a factor driving complications in colorectal surgery. A meta-analysis of 20 studies showed that frailty and prefrailty were associated with worse all-cause mortality during follow-up among older cancer patients. More striking, it showed that frail patients were nearly five times more likely to be intolerant of cancer treatment (odds ratio, 4.86) and more likely to experience postoperative complications (30-day hazard ratio, 3.19) (Ann Oncol. 2015;26[6]:1091-1101).
Hemoglobin A1c
Dr. Mutch went on to discuss hemoglobin A1c (HbA1c) levels as a risk factor in colorectal surgery. HbA1c levels higher than 6 are associated with worse outcomes, but tight postoperative control is associated with hypoglycemia. “What you want to do is set that patient up before surgery. HbA1c has a half-life of about a month, so if you start modifying their risk factors 4-6 weeks before you get them into surgery, by 1 month you can see a 50% reduction, and at 2 months a 75% reduction. If you do these things in a preoperative setting it makes a difference,” said Dr. Mutch.
Smoking cessation
Smoking cessation is another key strategy. Two weeks of cessation should lead to a decline in coughing, but a minimum of 4 weeks is needed to significantly reduce overall complications. Lifestyle changes need to be long term. “These are not measures that you’re going to do over a short period of time, and then when surgery is over throw it out the window,” said Dr. Mutch.
Anastomotic leak
Another factor is the detection of anastomotic leak, which can be challenging because its definitions vary significantly, and its causes can be multifactorial. Studies show that predictions of anastomotic leak are not especially successful, Dr. Mutch said, but routine leak testing improves outcomes. In a study of left-side anastomoses in Washington State, hospitals that performed leak tests had lower leak rates at least 90% of the time (OR, 0.23), and hospitals that later implemented leak tests experienced a significant reduction (Arch Surg. 2012:147[4]:345-51).
Venous thromboembolic events
Venous thromboembolic events (VTE), are the leading cause of operative mortality in colorectal surgery patients. This complication can be greatly reduced with prophylaxis, but requires screening for risk factors. Major surgery raises the risk of deep vein thrombosis in 20% of all hospitalized patients to 40%-80%, depending on the surgery type. “We have a lot of room to improve,” said Dr. Mutch.
Timing
One factor that may have an impact on complications appears to be timing of surgery, at least at Washington University, where Dr. Mutch practices. The institution found that patients who had surgery the same day they were admitted had a 2.5% VTE risk, compared with 11% in patients who had surgery 5 or more days after admission.
Postop ambulation
Postsurgical ambulation was another critical complication factor. Dr. Mutch cited a study showing that ambulation on the day after surgery was associated with a 1% VTE risk, compared to 6.9% in patients who waited until day 2.
Dr. Mutch had no disclosures. Global Academy for Medical Education and this news organization are owned by the same parent company.
LAS VEGAS – Colorectal surgery is rife with potential complications, but there are steps that surgeons can take to improve outcomes, and factors to consider to reduce complications. These strategies and considerations were the focus of a talk by Matthew G. Mutch, MD, at the Annual Minimally Invasive Surgery Symposium by Global Academy for Medical Education.
Prehabilitation
The approach to improve outcomes can begin with prehabilitation – preparing the patient for the difficult process of surgery. “If somebody is going to fight a 15-round heavyweight bout, they train for 6 or 8 weeks before a fight. Why not bring that concept to surgery?” said Dr. Mutch, chief of colon and rectal surgery at the Washington University, St. Louis.
Prehabilitation can include lifestyle changes, such as quitting smoking, but can also incorporate aerobic and/or resistance exercise, dietary counseling and protein supplementation, anxiety reduction, and medical education to prepare the patient for the challenges ahead. “Preoperatively, we try to identify factors to see if we can make meaningful lifestyle changes, because that’s really the grassroots level where a lot of this [improvement in outcomes] is going to occur,” said Dr. Mutch.
Frailty
Frailty is a factor driving complications in colorectal surgery. A meta-analysis of 20 studies showed that frailty and prefrailty were associated with worse all-cause mortality during follow-up among older cancer patients. More striking, it showed that frail patients were nearly five times more likely to be intolerant of cancer treatment (odds ratio, 4.86) and more likely to experience postoperative complications (30-day hazard ratio, 3.19) (Ann Oncol. 2015;26[6]:1091-1101).
Hemoglobin A1c
Dr. Mutch went on to discuss hemoglobin A1c (HbA1c) levels as a risk factor in colorectal surgery. HbA1c levels higher than 6 are associated with worse outcomes, but tight postoperative control is associated with hypoglycemia. “What you want to do is set that patient up before surgery. HbA1c has a half-life of about a month, so if you start modifying their risk factors 4-6 weeks before you get them into surgery, by 1 month you can see a 50% reduction, and at 2 months a 75% reduction. If you do these things in a preoperative setting it makes a difference,” said Dr. Mutch.
Smoking cessation
Smoking cessation is another key strategy. Two weeks of cessation should lead to a decline in coughing, but a minimum of 4 weeks is needed to significantly reduce overall complications. Lifestyle changes need to be long term. “These are not measures that you’re going to do over a short period of time, and then when surgery is over throw it out the window,” said Dr. Mutch.
Anastomotic leak
Another factor is the detection of anastomotic leak, which can be challenging because its definitions vary significantly, and its causes can be multifactorial. Studies show that predictions of anastomotic leak are not especially successful, Dr. Mutch said, but routine leak testing improves outcomes. In a study of left-side anastomoses in Washington State, hospitals that performed leak tests had lower leak rates at least 90% of the time (OR, 0.23), and hospitals that later implemented leak tests experienced a significant reduction (Arch Surg. 2012:147[4]:345-51).
Venous thromboembolic events
Venous thromboembolic events (VTE), are the leading cause of operative mortality in colorectal surgery patients. This complication can be greatly reduced with prophylaxis, but requires screening for risk factors. Major surgery raises the risk of deep vein thrombosis in 20% of all hospitalized patients to 40%-80%, depending on the surgery type. “We have a lot of room to improve,” said Dr. Mutch.
Timing
One factor that may have an impact on complications appears to be timing of surgery, at least at Washington University, where Dr. Mutch practices. The institution found that patients who had surgery the same day they were admitted had a 2.5% VTE risk, compared with 11% in patients who had surgery 5 or more days after admission.
Postop ambulation
Postsurgical ambulation was another critical complication factor. Dr. Mutch cited a study showing that ambulation on the day after surgery was associated with a 1% VTE risk, compared to 6.9% in patients who waited until day 2.
Dr. Mutch had no disclosures. Global Academy for Medical Education and this news organization are owned by the same parent company.
REPORTING FROM MISS
Measuring high-value care practices
Because health care in the United States is extremely expensive, it’s driving an increased focus on high-value care (HVC), said Carolyn D. Sy, MD. And, she added, while hospitalists and other physicians are the ones responsible for translating HVC from formalized settings (lectures, modules, etc.) to the bedside, there are few instruments designed to measure the success of HVC practices.
So Dr. Sy, director of the University of Washington Medical Center Hospital Medicine Service in Seattle and her colleagues developed an HVC Rounding Tool, which allows users to empirically assess the discussion of HVC topics at the bedside. They divided 10 HVC topics into three domains (quality, cost, patient values) to create an observational tool and tested its validity.
“It addresses an important educational gap in translating HVC from theoretical knowledge to bedside practice,” she said.
The tool is designed to capture multidisciplinary participation: involvement from faculty, fellows or trainees, nurses, pharmacists, families, and other members of the health care team.
It has multidisciplinary benefits too. “The HVC Rounding Tool provides an opportunity for faculty development through peer observation and feedback on the integration and role modeling of HVC at the bedside,” Dr. Sy said. “It also is an instrument to help assess the educational efficacy of formal HVC curriculum and translation into bedside practice. Lastly, it is a tool that could be used to measure the relationship between HVC behaviors and actual patient outcomes, such as length of stay, readmissions, and cost of hospitalization – a feature with increasing importance given our move towards value-based health care.”
Reference
1. Sy CD et al. The development and validation of a high-value care rounding tool using the Delphi method. J Hosp Med. 2017;12(suppl 2). Accessed Oct 10, 2017.
Because health care in the United States is extremely expensive, it’s driving an increased focus on high-value care (HVC), said Carolyn D. Sy, MD. And, she added, while hospitalists and other physicians are the ones responsible for translating HVC from formalized settings (lectures, modules, etc.) to the bedside, there are few instruments designed to measure the success of HVC practices.
So Dr. Sy, director of the University of Washington Medical Center Hospital Medicine Service in Seattle and her colleagues developed an HVC Rounding Tool, which allows users to empirically assess the discussion of HVC topics at the bedside. They divided 10 HVC topics into three domains (quality, cost, patient values) to create an observational tool and tested its validity.
“It addresses an important educational gap in translating HVC from theoretical knowledge to bedside practice,” she said.
The tool is designed to capture multidisciplinary participation: involvement from faculty, fellows or trainees, nurses, pharmacists, families, and other members of the health care team.
It has multidisciplinary benefits too. “The HVC Rounding Tool provides an opportunity for faculty development through peer observation and feedback on the integration and role modeling of HVC at the bedside,” Dr. Sy said. “It also is an instrument to help assess the educational efficacy of formal HVC curriculum and translation into bedside practice. Lastly, it is a tool that could be used to measure the relationship between HVC behaviors and actual patient outcomes, such as length of stay, readmissions, and cost of hospitalization – a feature with increasing importance given our move towards value-based health care.”
Reference
1. Sy CD et al. The development and validation of a high-value care rounding tool using the Delphi method. J Hosp Med. 2017;12(suppl 2). Accessed Oct 10, 2017.
Because health care in the United States is extremely expensive, it’s driving an increased focus on high-value care (HVC), said Carolyn D. Sy, MD. And, she added, while hospitalists and other physicians are the ones responsible for translating HVC from formalized settings (lectures, modules, etc.) to the bedside, there are few instruments designed to measure the success of HVC practices.
So Dr. Sy, director of the University of Washington Medical Center Hospital Medicine Service in Seattle and her colleagues developed an HVC Rounding Tool, which allows users to empirically assess the discussion of HVC topics at the bedside. They divided 10 HVC topics into three domains (quality, cost, patient values) to create an observational tool and tested its validity.
“It addresses an important educational gap in translating HVC from theoretical knowledge to bedside practice,” she said.
The tool is designed to capture multidisciplinary participation: involvement from faculty, fellows or trainees, nurses, pharmacists, families, and other members of the health care team.
It has multidisciplinary benefits too. “The HVC Rounding Tool provides an opportunity for faculty development through peer observation and feedback on the integration and role modeling of HVC at the bedside,” Dr. Sy said. “It also is an instrument to help assess the educational efficacy of formal HVC curriculum and translation into bedside practice. Lastly, it is a tool that could be used to measure the relationship between HVC behaviors and actual patient outcomes, such as length of stay, readmissions, and cost of hospitalization – a feature with increasing importance given our move towards value-based health care.”
Reference
1. Sy CD et al. The development and validation of a high-value care rounding tool using the Delphi method. J Hosp Med. 2017;12(suppl 2). Accessed Oct 10, 2017.
Hidden costs
By the time you reach age 55, 18% of your pediatrician colleagues – or you yourself – will have been sued for malpractice. As many as 64% of your brother and sister obstetrician-gynecologists will have suffered the same fate. These numbers come from a pool of recent data released by the American Medical Association that suggest that nearly half of physicians in this country will be sued for malpractice by age 55 years.
I have read the AMA’s press release and the two studies several times and didn’t see a single reference to the emotional toll taken on the health care providers who have been sued. Of course, although it is easy to imagine that the human cost of a malpractice suit is probably high, it is not one of those figures that the number crunchers can find in their data sets and spreadsheets.
Although a case that goes to trial is unusual (7%), most cases like ours are won by defendants (87.5%). That number was of little or no reassurance to me and my codefendants. Every waking hour during the 7 years that it took our case to play out was darkened by the threat to my professional career. Every trip to the mailbox was an exercise in courage. Would there be another piece of voluminous communication to remind me of the terrible reality?
Thanks to my wife and a lawyer who was always concerned about how I was doing emotionally, my story had a happy ending. I survived with my solo practice and my marriage intact. But I know that others have not been as lucky. Chemical dependency, divorce, poor productivity, and early retirement are just part of the underreported collateral damage that can come in the wake of a malpractice case, even one that is resolved with a relatively small financial cost.
Beyond the emotional cost, there is the staggering expense of defensive medicine. It has been going on for so long that many physicians practicing today don’t realize they are practicing defensive medicine because that’s the way they have been taught by several generations of gun-shy mentors.
And, of course, the risk-averse mentality is one of the drivers of the tsunami of computerization that threatens to drown our health care delivery system. The fallacious legal argument that if it wasn’t documented, it didn’t happen, has resulted in a glut of computer-enhanced “documentation” that even the most casual observer realizes isn’t worth the electrons it takes to light up the pixels on a computer screen.
Even if we ignore their incalculable emotional costs, malpractice suits and the litigious climate in which we practice are eating away at our health care delivery system. I certainly don’t have the answers to rein in the costs. I only can recommend that you practice the best medicine you know how, and do it in a manner that demonstrates you care about the patient. And hope you have good lawyer who cares about you more than his fee.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
By the time you reach age 55, 18% of your pediatrician colleagues – or you yourself – will have been sued for malpractice. As many as 64% of your brother and sister obstetrician-gynecologists will have suffered the same fate. These numbers come from a pool of recent data released by the American Medical Association that suggest that nearly half of physicians in this country will be sued for malpractice by age 55 years.
I have read the AMA’s press release and the two studies several times and didn’t see a single reference to the emotional toll taken on the health care providers who have been sued. Of course, although it is easy to imagine that the human cost of a malpractice suit is probably high, it is not one of those figures that the number crunchers can find in their data sets and spreadsheets.
Although a case that goes to trial is unusual (7%), most cases like ours are won by defendants (87.5%). That number was of little or no reassurance to me and my codefendants. Every waking hour during the 7 years that it took our case to play out was darkened by the threat to my professional career. Every trip to the mailbox was an exercise in courage. Would there be another piece of voluminous communication to remind me of the terrible reality?
Thanks to my wife and a lawyer who was always concerned about how I was doing emotionally, my story had a happy ending. I survived with my solo practice and my marriage intact. But I know that others have not been as lucky. Chemical dependency, divorce, poor productivity, and early retirement are just part of the underreported collateral damage that can come in the wake of a malpractice case, even one that is resolved with a relatively small financial cost.
Beyond the emotional cost, there is the staggering expense of defensive medicine. It has been going on for so long that many physicians practicing today don’t realize they are practicing defensive medicine because that’s the way they have been taught by several generations of gun-shy mentors.
And, of course, the risk-averse mentality is one of the drivers of the tsunami of computerization that threatens to drown our health care delivery system. The fallacious legal argument that if it wasn’t documented, it didn’t happen, has resulted in a glut of computer-enhanced “documentation” that even the most casual observer realizes isn’t worth the electrons it takes to light up the pixels on a computer screen.
Even if we ignore their incalculable emotional costs, malpractice suits and the litigious climate in which we practice are eating away at our health care delivery system. I certainly don’t have the answers to rein in the costs. I only can recommend that you practice the best medicine you know how, and do it in a manner that demonstrates you care about the patient. And hope you have good lawyer who cares about you more than his fee.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
By the time you reach age 55, 18% of your pediatrician colleagues – or you yourself – will have been sued for malpractice. As many as 64% of your brother and sister obstetrician-gynecologists will have suffered the same fate. These numbers come from a pool of recent data released by the American Medical Association that suggest that nearly half of physicians in this country will be sued for malpractice by age 55 years.
I have read the AMA’s press release and the two studies several times and didn’t see a single reference to the emotional toll taken on the health care providers who have been sued. Of course, although it is easy to imagine that the human cost of a malpractice suit is probably high, it is not one of those figures that the number crunchers can find in their data sets and spreadsheets.
Although a case that goes to trial is unusual (7%), most cases like ours are won by defendants (87.5%). That number was of little or no reassurance to me and my codefendants. Every waking hour during the 7 years that it took our case to play out was darkened by the threat to my professional career. Every trip to the mailbox was an exercise in courage. Would there be another piece of voluminous communication to remind me of the terrible reality?
Thanks to my wife and a lawyer who was always concerned about how I was doing emotionally, my story had a happy ending. I survived with my solo practice and my marriage intact. But I know that others have not been as lucky. Chemical dependency, divorce, poor productivity, and early retirement are just part of the underreported collateral damage that can come in the wake of a malpractice case, even one that is resolved with a relatively small financial cost.
Beyond the emotional cost, there is the staggering expense of defensive medicine. It has been going on for so long that many physicians practicing today don’t realize they are practicing defensive medicine because that’s the way they have been taught by several generations of gun-shy mentors.
And, of course, the risk-averse mentality is one of the drivers of the tsunami of computerization that threatens to drown our health care delivery system. The fallacious legal argument that if it wasn’t documented, it didn’t happen, has resulted in a glut of computer-enhanced “documentation” that even the most casual observer realizes isn’t worth the electrons it takes to light up the pixels on a computer screen.
Even if we ignore their incalculable emotional costs, malpractice suits and the litigious climate in which we practice are eating away at our health care delivery system. I certainly don’t have the answers to rein in the costs. I only can recommend that you practice the best medicine you know how, and do it in a manner that demonstrates you care about the patient. And hope you have good lawyer who cares about you more than his fee.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
ACC Day 2: Late-Breaking Clinical Trial highlights
Of the late-breaking clinical trials to be presented on Sunday, March 11, at the annual meeting of the American College of Cardiology in Orlando, the meeting’s vice chair, Andrew Kates, MD, highlighted two in a press briefing: ARTEMIS and MOMENTUM 3.
ARTEMIS
The investigators of this unique trial sought to determine whether reducing patients’ cost burden for their medication would improve adherence and, in turn, improve patient outcomes.
Specifically, ARTEMIS (Affordability and Real-World Antiplatelet Treatment Effectiveness After Myocardial Infarction Study) evaluated whether eliminating copayments for patients after acute MI could optimize antiplatelet therapy selection and long-term adherence, as well as patient outcomes and overall cost of care following acute MI.
Dr. Kates, professor of medicine, Washington University, St. Louis, said ARTEMIS would go far in answering the question, “If costs are lowered, are guidelines followed?”
Tracy Wang, MD, of Duke University, Durham, N.C., will present the results on Sunday in a joint Late-Breaking Clinical Trial session with JAMA, being held at 8 a.m.-9 a.m. in the Main Tent (Hall C).
MOMENTUM 3
Last year, HeartMate 3, a fully magnetically levitated centrifugal-flow circulatory pump, showed better outcomes at 6 months than did HEARTMATE 2, an axial-flow pump, in the primary results of MOMENTUM 3 (Multicenter Study of MagLev Technology in Patients Undergoing Mechanical Circulatory Support Therapy With HeartMate 3).
Most impressive was the complete elimination of pump thrombosis in the patients with the smaller HeartMate 3 device. On Sunday, the 2-year results will reveal whether longer-term use of the novel technology will carry similar benefits, Dr. Kates said.
MOMENTUM 3 will be presented at the third Late-Breaking Clinical Trials session, at 10:45-11:45 a.m., in the Main Tent (Hall C) by Mandeep R. Mehra, MD, professor of medicine at Harvard Medical School and medical director of the Heart and Vascular Center of Brigham and Women’s Hospital, both in Boston.
Of the late-breaking clinical trials to be presented on Sunday, March 11, at the annual meeting of the American College of Cardiology in Orlando, the meeting’s vice chair, Andrew Kates, MD, highlighted two in a press briefing: ARTEMIS and MOMENTUM 3.
ARTEMIS
The investigators of this unique trial sought to determine whether reducing patients’ cost burden for their medication would improve adherence and, in turn, improve patient outcomes.
Specifically, ARTEMIS (Affordability and Real-World Antiplatelet Treatment Effectiveness After Myocardial Infarction Study) evaluated whether eliminating copayments for patients after acute MI could optimize antiplatelet therapy selection and long-term adherence, as well as patient outcomes and overall cost of care following acute MI.
Dr. Kates, professor of medicine, Washington University, St. Louis, said ARTEMIS would go far in answering the question, “If costs are lowered, are guidelines followed?”
Tracy Wang, MD, of Duke University, Durham, N.C., will present the results on Sunday in a joint Late-Breaking Clinical Trial session with JAMA, being held at 8 a.m.-9 a.m. in the Main Tent (Hall C).
MOMENTUM 3
Last year, HeartMate 3, a fully magnetically levitated centrifugal-flow circulatory pump, showed better outcomes at 6 months than did HEARTMATE 2, an axial-flow pump, in the primary results of MOMENTUM 3 (Multicenter Study of MagLev Technology in Patients Undergoing Mechanical Circulatory Support Therapy With HeartMate 3).
Most impressive was the complete elimination of pump thrombosis in the patients with the smaller HeartMate 3 device. On Sunday, the 2-year results will reveal whether longer-term use of the novel technology will carry similar benefits, Dr. Kates said.
MOMENTUM 3 will be presented at the third Late-Breaking Clinical Trials session, at 10:45-11:45 a.m., in the Main Tent (Hall C) by Mandeep R. Mehra, MD, professor of medicine at Harvard Medical School and medical director of the Heart and Vascular Center of Brigham and Women’s Hospital, both in Boston.
Of the late-breaking clinical trials to be presented on Sunday, March 11, at the annual meeting of the American College of Cardiology in Orlando, the meeting’s vice chair, Andrew Kates, MD, highlighted two in a press briefing: ARTEMIS and MOMENTUM 3.
ARTEMIS
The investigators of this unique trial sought to determine whether reducing patients’ cost burden for their medication would improve adherence and, in turn, improve patient outcomes.
Specifically, ARTEMIS (Affordability and Real-World Antiplatelet Treatment Effectiveness After Myocardial Infarction Study) evaluated whether eliminating copayments for patients after acute MI could optimize antiplatelet therapy selection and long-term adherence, as well as patient outcomes and overall cost of care following acute MI.
Dr. Kates, professor of medicine, Washington University, St. Louis, said ARTEMIS would go far in answering the question, “If costs are lowered, are guidelines followed?”
Tracy Wang, MD, of Duke University, Durham, N.C., will present the results on Sunday in a joint Late-Breaking Clinical Trial session with JAMA, being held at 8 a.m.-9 a.m. in the Main Tent (Hall C).
MOMENTUM 3
Last year, HeartMate 3, a fully magnetically levitated centrifugal-flow circulatory pump, showed better outcomes at 6 months than did HEARTMATE 2, an axial-flow pump, in the primary results of MOMENTUM 3 (Multicenter Study of MagLev Technology in Patients Undergoing Mechanical Circulatory Support Therapy With HeartMate 3).
Most impressive was the complete elimination of pump thrombosis in the patients with the smaller HeartMate 3 device. On Sunday, the 2-year results will reveal whether longer-term use of the novel technology will carry similar benefits, Dr. Kates said.
MOMENTUM 3 will be presented at the third Late-Breaking Clinical Trials session, at 10:45-11:45 a.m., in the Main Tent (Hall C) by Mandeep R. Mehra, MD, professor of medicine at Harvard Medical School and medical director of the Heart and Vascular Center of Brigham and Women’s Hospital, both in Boston.