User login
‘Aggressive’ new advance directive would let dementia patients refuse food
Treading into ethically and legally uncertain territory, .
The directive, finalized this month by the board for End of Life Choices New York, aims to provide patients a way to hasten death in late-stage dementia, if they choose.
Dementia is a terminal illness, but even in the seven U.S. jurisdictions that allow medical aid-in-dying, it’s not a condition covered by the laws. Increasingly, patients are seeking other options, said Timothy Quill, MD, a palliative care expert at the University of Rochester (N.Y.) and an advocate of the practice.
“Developing incapacitating dementia is certainly my and a lot of people’s worst nightmare,” he said. “This is an aggressive document. It’s a way of addressing a real problem, which is the prospect of advanced dementia.”
The document offers two options: one that requests “comfort feeding” – providing oral food and water if a patient appears to enjoy or allows it during the final stages of the disease – and one that would halt all assisted eating and drinking, even if a patient seems willing to accept it.
Supporters say it’s the strongest effort to date to allow people who want to avoid the ravages of advanced dementia to make their final wishes known – while they still have the ability to do so.
“They do not want their dying prolonged,” said Judith Schwarz, who drafted the document as clinical director for the advocacy group. “This is an informed and thoughtful choice that needs a great deal of reflection and discussion.”
But critics say it’s a disturbing effort to allow withdrawal of basic sustenance from the most vulnerable in society.
“I think oral feeding is basic care,” said Richard Doerflinger, an associate scholar with the Charlotte Lozier Institute, which opposes abortion and euthanasia. “It’s what they want here and now that matters. If they start taking food, you give them food.”
Advance directives are legally recognized documents that specify care if a person is incapacitated. They can confirm that a patient doesn’t want to be resuscitated or kept on life support, such as a ventilator or feeding tube, if they have a terminal condition from which they’re not likely to recover.
However, the documents typically say nothing about withdrawing hand-feeding of food or fluids.
The New York directive, in contrast, offers option A, which allows refusal of all oral assisted feeding. Option B permits comfort-focused feeding.
Both options would be invoked only when a patient is diagnosed with moderate or severe dementia, defined as stages 6 or 7 of a widely used test known as the Functional Assessment Staging Test (FAST). At those stages, patients would be unable to feed themselves or make health care decisions.
The new form goes further than a similar dementia directive introduced last year by another group that supports aid-in-dying, End of Life Washington. That document says that a person with dementia who accepts food or drink should receive oral nourishment until he or she is unwilling or unable to do so.
The New York document says, “My instructions are that I do NOT want to be fed by hand even if I appear to cooperate in being fed by opening my mouth.”
Whether the new directive will be honored in New York – or anywhere else – is unclear. Legal scholars and ethicists say directives withdrawing oral assisted feeding are prohibited in several states. Many care facilities are unlikely to cooperate, said Thaddeus Pope, director of the Health Law Institute at Hamline University, St. Paul, Minn., and an expert on end-of-life law. Doctors have a duty to honor patient wishes, but they can refuse if they have medical or moral qualms.
“Even solidly legal advance directives do not and cannot ENSURE that wishes are respected,” Pope said in an email. “They can only ‘help assure’ that.”
Directors at End of Life Choices New York consider the document “legally sturdy,” Schwarz said, adding, “Of course it’s going to end up in court.”
Whether assisted feeding can be withdrawn was at the center of recent high-profile cases in which patients with dementia were spoon-fed against their documented wishes because they continued to open their mouths. In a case in Canada, a court ruled that such feeding is basic care that can’t be withdrawn.
People who fill out the directives may be more likely to have them honored if they remain at home, Schwarz said. She stressed that patients should make their wishes known far in advance and choose health care agents who will be strong advocates. Legal experts say the documents should be updated regularly.
Doerflinger, however, said creating the directive and making it available misses a crucial point: People who don’t have dementia now can’t know how they’ll feel later, yet they’re deciding in advance to forgo nourishment.
“The question is: Do we, the able-bodied, have a right to discriminate against the disabled people we will later become?” Doerflinger said.
Already, though, Schwarz has heard from people determined to put the new directive in place.
Janet Dwyer, 59, of New York, said her family was horrified by her father’s lingering death after a heart attack 4 years ago and mindful of a family history of dementia. When Dwyer learned there was a directive to address terminal illness and dementia, she signed it. So did her husband, John Harney, also 59.
“Judith informed me of the Option A or Option B scenarios,” said Dwyer, who opted for A. “I said, ‘Well, that is just perfect.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of the Kaiser Family Foundation, which is not affiliated with Kaiser Permanente. KHN’s coverage of end-of-life and serious illness issues is supported in part by the Gordon and Betty Moore Foundation.
Treading into ethically and legally uncertain territory, .
The directive, finalized this month by the board for End of Life Choices New York, aims to provide patients a way to hasten death in late-stage dementia, if they choose.
Dementia is a terminal illness, but even in the seven U.S. jurisdictions that allow medical aid-in-dying, it’s not a condition covered by the laws. Increasingly, patients are seeking other options, said Timothy Quill, MD, a palliative care expert at the University of Rochester (N.Y.) and an advocate of the practice.
“Developing incapacitating dementia is certainly my and a lot of people’s worst nightmare,” he said. “This is an aggressive document. It’s a way of addressing a real problem, which is the prospect of advanced dementia.”
The document offers two options: one that requests “comfort feeding” – providing oral food and water if a patient appears to enjoy or allows it during the final stages of the disease – and one that would halt all assisted eating and drinking, even if a patient seems willing to accept it.
Supporters say it’s the strongest effort to date to allow people who want to avoid the ravages of advanced dementia to make their final wishes known – while they still have the ability to do so.
“They do not want their dying prolonged,” said Judith Schwarz, who drafted the document as clinical director for the advocacy group. “This is an informed and thoughtful choice that needs a great deal of reflection and discussion.”
But critics say it’s a disturbing effort to allow withdrawal of basic sustenance from the most vulnerable in society.
“I think oral feeding is basic care,” said Richard Doerflinger, an associate scholar with the Charlotte Lozier Institute, which opposes abortion and euthanasia. “It’s what they want here and now that matters. If they start taking food, you give them food.”
Advance directives are legally recognized documents that specify care if a person is incapacitated. They can confirm that a patient doesn’t want to be resuscitated or kept on life support, such as a ventilator or feeding tube, if they have a terminal condition from which they’re not likely to recover.
However, the documents typically say nothing about withdrawing hand-feeding of food or fluids.
The New York directive, in contrast, offers option A, which allows refusal of all oral assisted feeding. Option B permits comfort-focused feeding.
Both options would be invoked only when a patient is diagnosed with moderate or severe dementia, defined as stages 6 or 7 of a widely used test known as the Functional Assessment Staging Test (FAST). At those stages, patients would be unable to feed themselves or make health care decisions.
The new form goes further than a similar dementia directive introduced last year by another group that supports aid-in-dying, End of Life Washington. That document says that a person with dementia who accepts food or drink should receive oral nourishment until he or she is unwilling or unable to do so.
The New York document says, “My instructions are that I do NOT want to be fed by hand even if I appear to cooperate in being fed by opening my mouth.”
Whether the new directive will be honored in New York – or anywhere else – is unclear. Legal scholars and ethicists say directives withdrawing oral assisted feeding are prohibited in several states. Many care facilities are unlikely to cooperate, said Thaddeus Pope, director of the Health Law Institute at Hamline University, St. Paul, Minn., and an expert on end-of-life law. Doctors have a duty to honor patient wishes, but they can refuse if they have medical or moral qualms.
“Even solidly legal advance directives do not and cannot ENSURE that wishes are respected,” Pope said in an email. “They can only ‘help assure’ that.”
Directors at End of Life Choices New York consider the document “legally sturdy,” Schwarz said, adding, “Of course it’s going to end up in court.”
Whether assisted feeding can be withdrawn was at the center of recent high-profile cases in which patients with dementia were spoon-fed against their documented wishes because they continued to open their mouths. In a case in Canada, a court ruled that such feeding is basic care that can’t be withdrawn.
People who fill out the directives may be more likely to have them honored if they remain at home, Schwarz said. She stressed that patients should make their wishes known far in advance and choose health care agents who will be strong advocates. Legal experts say the documents should be updated regularly.
Doerflinger, however, said creating the directive and making it available misses a crucial point: People who don’t have dementia now can’t know how they’ll feel later, yet they’re deciding in advance to forgo nourishment.
“The question is: Do we, the able-bodied, have a right to discriminate against the disabled people we will later become?” Doerflinger said.
Already, though, Schwarz has heard from people determined to put the new directive in place.
Janet Dwyer, 59, of New York, said her family was horrified by her father’s lingering death after a heart attack 4 years ago and mindful of a family history of dementia. When Dwyer learned there was a directive to address terminal illness and dementia, she signed it. So did her husband, John Harney, also 59.
“Judith informed me of the Option A or Option B scenarios,” said Dwyer, who opted for A. “I said, ‘Well, that is just perfect.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of the Kaiser Family Foundation, which is not affiliated with Kaiser Permanente. KHN’s coverage of end-of-life and serious illness issues is supported in part by the Gordon and Betty Moore Foundation.
Treading into ethically and legally uncertain territory, .
The directive, finalized this month by the board for End of Life Choices New York, aims to provide patients a way to hasten death in late-stage dementia, if they choose.
Dementia is a terminal illness, but even in the seven U.S. jurisdictions that allow medical aid-in-dying, it’s not a condition covered by the laws. Increasingly, patients are seeking other options, said Timothy Quill, MD, a palliative care expert at the University of Rochester (N.Y.) and an advocate of the practice.
“Developing incapacitating dementia is certainly my and a lot of people’s worst nightmare,” he said. “This is an aggressive document. It’s a way of addressing a real problem, which is the prospect of advanced dementia.”
The document offers two options: one that requests “comfort feeding” – providing oral food and water if a patient appears to enjoy or allows it during the final stages of the disease – and one that would halt all assisted eating and drinking, even if a patient seems willing to accept it.
Supporters say it’s the strongest effort to date to allow people who want to avoid the ravages of advanced dementia to make their final wishes known – while they still have the ability to do so.
“They do not want their dying prolonged,” said Judith Schwarz, who drafted the document as clinical director for the advocacy group. “This is an informed and thoughtful choice that needs a great deal of reflection and discussion.”
But critics say it’s a disturbing effort to allow withdrawal of basic sustenance from the most vulnerable in society.
“I think oral feeding is basic care,” said Richard Doerflinger, an associate scholar with the Charlotte Lozier Institute, which opposes abortion and euthanasia. “It’s what they want here and now that matters. If they start taking food, you give them food.”
Advance directives are legally recognized documents that specify care if a person is incapacitated. They can confirm that a patient doesn’t want to be resuscitated or kept on life support, such as a ventilator or feeding tube, if they have a terminal condition from which they’re not likely to recover.
However, the documents typically say nothing about withdrawing hand-feeding of food or fluids.
The New York directive, in contrast, offers option A, which allows refusal of all oral assisted feeding. Option B permits comfort-focused feeding.
Both options would be invoked only when a patient is diagnosed with moderate or severe dementia, defined as stages 6 or 7 of a widely used test known as the Functional Assessment Staging Test (FAST). At those stages, patients would be unable to feed themselves or make health care decisions.
The new form goes further than a similar dementia directive introduced last year by another group that supports aid-in-dying, End of Life Washington. That document says that a person with dementia who accepts food or drink should receive oral nourishment until he or she is unwilling or unable to do so.
The New York document says, “My instructions are that I do NOT want to be fed by hand even if I appear to cooperate in being fed by opening my mouth.”
Whether the new directive will be honored in New York – or anywhere else – is unclear. Legal scholars and ethicists say directives withdrawing oral assisted feeding are prohibited in several states. Many care facilities are unlikely to cooperate, said Thaddeus Pope, director of the Health Law Institute at Hamline University, St. Paul, Minn., and an expert on end-of-life law. Doctors have a duty to honor patient wishes, but they can refuse if they have medical or moral qualms.
“Even solidly legal advance directives do not and cannot ENSURE that wishes are respected,” Pope said in an email. “They can only ‘help assure’ that.”
Directors at End of Life Choices New York consider the document “legally sturdy,” Schwarz said, adding, “Of course it’s going to end up in court.”
Whether assisted feeding can be withdrawn was at the center of recent high-profile cases in which patients with dementia were spoon-fed against their documented wishes because they continued to open their mouths. In a case in Canada, a court ruled that such feeding is basic care that can’t be withdrawn.
People who fill out the directives may be more likely to have them honored if they remain at home, Schwarz said. She stressed that patients should make their wishes known far in advance and choose health care agents who will be strong advocates. Legal experts say the documents should be updated regularly.
Doerflinger, however, said creating the directive and making it available misses a crucial point: People who don’t have dementia now can’t know how they’ll feel later, yet they’re deciding in advance to forgo nourishment.
“The question is: Do we, the able-bodied, have a right to discriminate against the disabled people we will later become?” Doerflinger said.
Already, though, Schwarz has heard from people determined to put the new directive in place.
Janet Dwyer, 59, of New York, said her family was horrified by her father’s lingering death after a heart attack 4 years ago and mindful of a family history of dementia. When Dwyer learned there was a directive to address terminal illness and dementia, she signed it. So did her husband, John Harney, also 59.
“Judith informed me of the Option A or Option B scenarios,” said Dwyer, who opted for A. “I said, ‘Well, that is just perfect.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of the Kaiser Family Foundation, which is not affiliated with Kaiser Permanente. KHN’s coverage of end-of-life and serious illness issues is supported in part by the Gordon and Betty Moore Foundation.
A public health approach to gun violence
In 2014, 33,594 people were killed by firearms in the United States. More than 21,000 of these deaths were suicides. The rest were primarily homicides and accidental shootings. Meanwhile, firearm deaths represented nearly 17% of injury deaths that year.1,2
In a 2015 Perspective published in the New England Journal of Medicine, author Chana Sacks, MD, pointed out that 20 children and adolescents are sent to the hospital daily for firearm injuries and 2,000 people each year suffer gunshot-related spinal cord injuries and “become lifelong patients.”3
At the same time, Federal Bureau of Investigation data show that the number of active shooter situations rose between 2000 and 2013, with an average of 6.4 incidents a year for the first 7 years of the study, conducted in 2013, and an average of 16.4 in the last 7 years of the study. More than 1,000 people were wounded or killed across 160 active shooter incidents, defined as an individual or individuals actively engaged in killing or trying to kill people in a populous area.4
“Gun violence is undeniably a public health issue,” said Dr. Sacks, a hospitalist at Massachusetts General Hospital and instructor at Harvard Medical School, both in Boston, and a vocal proponent of addressing firearms in the public health sphere. Her cousin’s 7-year-old son, Daniel Barden, was fatally shot at Sandy Hook Elementary School in Newtown, Conn., in December 2012.
Yet, the notion of firearm injuries and deaths as a public health issue is, in America, an issue of contention. How can hospitalists and other health care providers avoid wading into the political thicket while also looking out for their patients?
For one, it’s not the only controversial issue with which providers are confronted, Dr. Sacks and others say. From taking sexual histories, counseling patients about abortion and adoption, and discussing end-of-life issues, clinicians may routinely face uncomfortable interactions in the name of patient care.
“It’s not a question about their right to a weapon; it’s about how individuals can stay as safe as possible and keep their families as safe as possible,” said Dr. Sacks, who also wrote in a January 2017 opinion for the American Medical Association that: “Counseling about gun safety is not political – no more so than a physician counseling a patient about cutting down on sugary beverages is an act of declaring support for New York City’s attempted ban on large-sized sodas.”5
This idea is echoed by David Hemenway, PhD, director of the Harvard Injury Control Research Center, Boston. “You can talk about wearing your seat belt without advocating for mandatory seat belt laws,” he said.
Yet in a 2014 survey of internist members of the American College of Physicians, only 66% of respondents said they believed physicians have the right to counsel patients on gun violence prevention and 58% said they never ask patients about guns in their home. That same survey showed the public is also split: While two-thirds of respondents said it was at least sometimes appropriate for providers to ask about firearms during a visit, one-third believed it was never appropriate.6
In fact, Barbara Meyer, MD, MPH, a family physician in Seattle, said she once had a patient walk out of the office when he encountered a question about firearms on the intake forms for the health system at which she was employed at the time. Today, at NeighborCare Health, the presence of firearms in the home is a question in the well-child electronic health record.
The Harvard Injury Control Research Center runs a campaign called Means Matter, designed to address suicide by firearm, the most common method of suicide in America. The campaign – backed by decades of some of the best research available – reports that people die of suicide by gun more than all other methods combined, that suicide attempts using a firearm are almost always fatal, and that firearms used by youths who commit suicide almost always belong to a parent.
“Suicide is often an impulsive act,” said Dr. Sacks, which means preventing access to firearms for patients at risk can be a matter of life and death. “There is potential for intervention there … what can be more clearly medical than suicide prevention?”
For her, that means eliminating the partisan component and equipping providers with the best evidence-based research available and with best practices. Reliable studies show that having guns at home increases the danger to families, said Dr. Hemenway, and places with fewer guns and stronger gun laws are correlated with fewer gun fatalities.7,8
“In accordance with guidelines and the best evidence out there, we should be screening patients who might be at risk for gun violence,” he said. “In some cases, interventions can be as simple and straightforward as informing patients where to get gun locks and talking to them about how to store firearms safely.”
At Massachusetts General Hospital, Dr. Sacks helped found the Gun Violence Prevention Coalition, an interdisciplinary group of physicians, nurses, physical therapists, and others committed to raising awareness and preparing providers to address gun violence. She believes strongly that physicians can act locally to help address the issue.
In Seattle, Dr. Meyer has been involved with a local group called Washington Ceasefire, prompted both by her experience as a resident in Detroit – where she was routinely exposed to the traumas of gun violence – as well as a shooting that occurred outside her daughter’s high school in Seattle years ago. The group has recently begun advocating for smart guns, which are designed to be fired only by an authorized user.
Indeed, Dr. Hemenway said research by his group suggests 300,000-500,000 guns are stolen every year, though he points out that we know almost nothing about “who, what, when, why, and where.” That’s largely because of an effective ban on gun violence research, enacted by Congress in the 1990s.9
“It’s not like there’s no evidence, but compared to the size of the problem, you want good evidence,” Dr. Hemenway said. “America has lots of guns. How can we learn to live with them?”
Gun violence affects not just those shot and killed by firearms, but also those affected by the trauma it can leave in its wake. Dr. Sacks recounts a recent visit to Massachusetts General by survivors of the Pulse Nightclub shooting in Orlando, Fla., which took place on June 12, 2016.
“It was a moving, intense event where we all sat around and talked about this issue,” Dr. Sacks said. “The number of people dying is horrific enough, but it’s not just that. Here were a number of young people who survived and yet whose lives will never be the same. We are undercounting the number of people affected by gun violence.”
Studies also estimate the cost of medical care related to gun violence to be roughly $620 million per year, averaging between $9,000 and $18,000 per patient in 2014.10
Despite some arguments to the contrary, addressing gun violence as a public health issue is not a distraction from other important public health issues such as opioid abuse. “It is entirely a false choice that we must only take on one issue or another,” Dr. Sacks said.
Nor should efforts to address gun violence focus only on individuals, said Dr. Hemenway, who told the Harvard T.H. Chan School of Public Health in October 2017 that: “A lesson from public health is that it is usually more effective to change the environment than to try to change people. The U.S. should use the same harm reduction approach to gun violence that it uses to treat other public health threats, like automobile crashes or air pollution, employing a wide variety of methods to reduce the problem.”
The issue must be reframed, said Dr. Sacks. This remains one of her biggest goals. “If we can find a way to act and intervene and lower [the] number [of people affected by gun violence], what could be more fundamentally in line with what we try to do every day as physicians?” she asked. “How can we reduce morbidity and mortality? That’s an answerable question and we can make sure we have pathways and approaches we can put in place to understand this. This is a solvable problem.”
1. Centers for Disease Control and Prevention, National Center for Health Statistics. FastStats. Injuries. https://www.cdc.gov/nchs/fastats/injury.htm. Accessed Nov 20, 2017.
2. Centers for Disease Control and Prevention, National Center for Health Statistics. FastStats. Suicide. https://www.cdc.gov/nchs/fastats/suicide.htm. Accessed Nov 20, 2017.
3. Sacks CA. In memory of Daniel – Reviving research to prevent gun violence. N Engl J Med. 2015; 372:800-801. doi: 10.1056/NEJMp1415128.
4. U.S. Department of Justice, Federal Bureau of Investigation. A study of active shooter incidents in the United States between 2000 and 2013. Published Sept 16, 2013. Accessed Nov 20, 2017.
5. Sacks CA. The role of physicians in preventing firearm suicides. JAMA Int Med. doi: 10.001/jamainternmed.2016.6715. Published Nov 14, 2016. Accessed Nov 20, 2017.
6. Butkus R, Weissman A. Internists’ attitudes toward prevention of firearm injury. Ann Intern Med. 2014;160(12):821-827. doi: 10.7326/M13-1960.
7. Fleegler EW, Lee LK, Monuteaux MC, et al. Firearm Legislation and Firearm-Related Fatalities in the United States. JAMA Intern Med. 2013; 173(9):732-740. doi: 10.1001/jamaimternmed.2013.1286.
8. American Academy of Pediatricians. Addressing gun violence. The federal level. https://www.aap.org/en-us/advocacy-and-policy/aap-health-initiatives/Pages/Gun-Violence-Matrix--Intentional-(Federal).aspx. Accessed Nov 20, 2017.
9. Rubin R. Tale of 2 agencies: CDC avoids gun violence research bit NIH funds it. JAMA. 2016;315(16):1689-1692. doi:10.1001/jama.2016.1707.
10. Howell E and Gangopadhyaya A. State variation in the hospital costs of gun violence, 2010 and 2014. The Urban Institute, Health Policy Center.
In 2014, 33,594 people were killed by firearms in the United States. More than 21,000 of these deaths were suicides. The rest were primarily homicides and accidental shootings. Meanwhile, firearm deaths represented nearly 17% of injury deaths that year.1,2
In a 2015 Perspective published in the New England Journal of Medicine, author Chana Sacks, MD, pointed out that 20 children and adolescents are sent to the hospital daily for firearm injuries and 2,000 people each year suffer gunshot-related spinal cord injuries and “become lifelong patients.”3
At the same time, Federal Bureau of Investigation data show that the number of active shooter situations rose between 2000 and 2013, with an average of 6.4 incidents a year for the first 7 years of the study, conducted in 2013, and an average of 16.4 in the last 7 years of the study. More than 1,000 people were wounded or killed across 160 active shooter incidents, defined as an individual or individuals actively engaged in killing or trying to kill people in a populous area.4
“Gun violence is undeniably a public health issue,” said Dr. Sacks, a hospitalist at Massachusetts General Hospital and instructor at Harvard Medical School, both in Boston, and a vocal proponent of addressing firearms in the public health sphere. Her cousin’s 7-year-old son, Daniel Barden, was fatally shot at Sandy Hook Elementary School in Newtown, Conn., in December 2012.
Yet, the notion of firearm injuries and deaths as a public health issue is, in America, an issue of contention. How can hospitalists and other health care providers avoid wading into the political thicket while also looking out for their patients?
For one, it’s not the only controversial issue with which providers are confronted, Dr. Sacks and others say. From taking sexual histories, counseling patients about abortion and adoption, and discussing end-of-life issues, clinicians may routinely face uncomfortable interactions in the name of patient care.
“It’s not a question about their right to a weapon; it’s about how individuals can stay as safe as possible and keep their families as safe as possible,” said Dr. Sacks, who also wrote in a January 2017 opinion for the American Medical Association that: “Counseling about gun safety is not political – no more so than a physician counseling a patient about cutting down on sugary beverages is an act of declaring support for New York City’s attempted ban on large-sized sodas.”5
This idea is echoed by David Hemenway, PhD, director of the Harvard Injury Control Research Center, Boston. “You can talk about wearing your seat belt without advocating for mandatory seat belt laws,” he said.
Yet in a 2014 survey of internist members of the American College of Physicians, only 66% of respondents said they believed physicians have the right to counsel patients on gun violence prevention and 58% said they never ask patients about guns in their home. That same survey showed the public is also split: While two-thirds of respondents said it was at least sometimes appropriate for providers to ask about firearms during a visit, one-third believed it was never appropriate.6
In fact, Barbara Meyer, MD, MPH, a family physician in Seattle, said she once had a patient walk out of the office when he encountered a question about firearms on the intake forms for the health system at which she was employed at the time. Today, at NeighborCare Health, the presence of firearms in the home is a question in the well-child electronic health record.
The Harvard Injury Control Research Center runs a campaign called Means Matter, designed to address suicide by firearm, the most common method of suicide in America. The campaign – backed by decades of some of the best research available – reports that people die of suicide by gun more than all other methods combined, that suicide attempts using a firearm are almost always fatal, and that firearms used by youths who commit suicide almost always belong to a parent.
“Suicide is often an impulsive act,” said Dr. Sacks, which means preventing access to firearms for patients at risk can be a matter of life and death. “There is potential for intervention there … what can be more clearly medical than suicide prevention?”
For her, that means eliminating the partisan component and equipping providers with the best evidence-based research available and with best practices. Reliable studies show that having guns at home increases the danger to families, said Dr. Hemenway, and places with fewer guns and stronger gun laws are correlated with fewer gun fatalities.7,8
“In accordance with guidelines and the best evidence out there, we should be screening patients who might be at risk for gun violence,” he said. “In some cases, interventions can be as simple and straightforward as informing patients where to get gun locks and talking to them about how to store firearms safely.”
At Massachusetts General Hospital, Dr. Sacks helped found the Gun Violence Prevention Coalition, an interdisciplinary group of physicians, nurses, physical therapists, and others committed to raising awareness and preparing providers to address gun violence. She believes strongly that physicians can act locally to help address the issue.
In Seattle, Dr. Meyer has been involved with a local group called Washington Ceasefire, prompted both by her experience as a resident in Detroit – where she was routinely exposed to the traumas of gun violence – as well as a shooting that occurred outside her daughter’s high school in Seattle years ago. The group has recently begun advocating for smart guns, which are designed to be fired only by an authorized user.
Indeed, Dr. Hemenway said research by his group suggests 300,000-500,000 guns are stolen every year, though he points out that we know almost nothing about “who, what, when, why, and where.” That’s largely because of an effective ban on gun violence research, enacted by Congress in the 1990s.9
“It’s not like there’s no evidence, but compared to the size of the problem, you want good evidence,” Dr. Hemenway said. “America has lots of guns. How can we learn to live with them?”
Gun violence affects not just those shot and killed by firearms, but also those affected by the trauma it can leave in its wake. Dr. Sacks recounts a recent visit to Massachusetts General by survivors of the Pulse Nightclub shooting in Orlando, Fla., which took place on June 12, 2016.
“It was a moving, intense event where we all sat around and talked about this issue,” Dr. Sacks said. “The number of people dying is horrific enough, but it’s not just that. Here were a number of young people who survived and yet whose lives will never be the same. We are undercounting the number of people affected by gun violence.”
Studies also estimate the cost of medical care related to gun violence to be roughly $620 million per year, averaging between $9,000 and $18,000 per patient in 2014.10
Despite some arguments to the contrary, addressing gun violence as a public health issue is not a distraction from other important public health issues such as opioid abuse. “It is entirely a false choice that we must only take on one issue or another,” Dr. Sacks said.
Nor should efforts to address gun violence focus only on individuals, said Dr. Hemenway, who told the Harvard T.H. Chan School of Public Health in October 2017 that: “A lesson from public health is that it is usually more effective to change the environment than to try to change people. The U.S. should use the same harm reduction approach to gun violence that it uses to treat other public health threats, like automobile crashes or air pollution, employing a wide variety of methods to reduce the problem.”
The issue must be reframed, said Dr. Sacks. This remains one of her biggest goals. “If we can find a way to act and intervene and lower [the] number [of people affected by gun violence], what could be more fundamentally in line with what we try to do every day as physicians?” she asked. “How can we reduce morbidity and mortality? That’s an answerable question and we can make sure we have pathways and approaches we can put in place to understand this. This is a solvable problem.”
In 2014, 33,594 people were killed by firearms in the United States. More than 21,000 of these deaths were suicides. The rest were primarily homicides and accidental shootings. Meanwhile, firearm deaths represented nearly 17% of injury deaths that year.1,2
In a 2015 Perspective published in the New England Journal of Medicine, author Chana Sacks, MD, pointed out that 20 children and adolescents are sent to the hospital daily for firearm injuries and 2,000 people each year suffer gunshot-related spinal cord injuries and “become lifelong patients.”3
At the same time, Federal Bureau of Investigation data show that the number of active shooter situations rose between 2000 and 2013, with an average of 6.4 incidents a year for the first 7 years of the study, conducted in 2013, and an average of 16.4 in the last 7 years of the study. More than 1,000 people were wounded or killed across 160 active shooter incidents, defined as an individual or individuals actively engaged in killing or trying to kill people in a populous area.4
“Gun violence is undeniably a public health issue,” said Dr. Sacks, a hospitalist at Massachusetts General Hospital and instructor at Harvard Medical School, both in Boston, and a vocal proponent of addressing firearms in the public health sphere. Her cousin’s 7-year-old son, Daniel Barden, was fatally shot at Sandy Hook Elementary School in Newtown, Conn., in December 2012.
Yet, the notion of firearm injuries and deaths as a public health issue is, in America, an issue of contention. How can hospitalists and other health care providers avoid wading into the political thicket while also looking out for their patients?
For one, it’s not the only controversial issue with which providers are confronted, Dr. Sacks and others say. From taking sexual histories, counseling patients about abortion and adoption, and discussing end-of-life issues, clinicians may routinely face uncomfortable interactions in the name of patient care.
“It’s not a question about their right to a weapon; it’s about how individuals can stay as safe as possible and keep their families as safe as possible,” said Dr. Sacks, who also wrote in a January 2017 opinion for the American Medical Association that: “Counseling about gun safety is not political – no more so than a physician counseling a patient about cutting down on sugary beverages is an act of declaring support for New York City’s attempted ban on large-sized sodas.”5
This idea is echoed by David Hemenway, PhD, director of the Harvard Injury Control Research Center, Boston. “You can talk about wearing your seat belt without advocating for mandatory seat belt laws,” he said.
Yet in a 2014 survey of internist members of the American College of Physicians, only 66% of respondents said they believed physicians have the right to counsel patients on gun violence prevention and 58% said they never ask patients about guns in their home. That same survey showed the public is also split: While two-thirds of respondents said it was at least sometimes appropriate for providers to ask about firearms during a visit, one-third believed it was never appropriate.6
In fact, Barbara Meyer, MD, MPH, a family physician in Seattle, said she once had a patient walk out of the office when he encountered a question about firearms on the intake forms for the health system at which she was employed at the time. Today, at NeighborCare Health, the presence of firearms in the home is a question in the well-child electronic health record.
The Harvard Injury Control Research Center runs a campaign called Means Matter, designed to address suicide by firearm, the most common method of suicide in America. The campaign – backed by decades of some of the best research available – reports that people die of suicide by gun more than all other methods combined, that suicide attempts using a firearm are almost always fatal, and that firearms used by youths who commit suicide almost always belong to a parent.
“Suicide is often an impulsive act,” said Dr. Sacks, which means preventing access to firearms for patients at risk can be a matter of life and death. “There is potential for intervention there … what can be more clearly medical than suicide prevention?”
For her, that means eliminating the partisan component and equipping providers with the best evidence-based research available and with best practices. Reliable studies show that having guns at home increases the danger to families, said Dr. Hemenway, and places with fewer guns and stronger gun laws are correlated with fewer gun fatalities.7,8
“In accordance with guidelines and the best evidence out there, we should be screening patients who might be at risk for gun violence,” he said. “In some cases, interventions can be as simple and straightforward as informing patients where to get gun locks and talking to them about how to store firearms safely.”
At Massachusetts General Hospital, Dr. Sacks helped found the Gun Violence Prevention Coalition, an interdisciplinary group of physicians, nurses, physical therapists, and others committed to raising awareness and preparing providers to address gun violence. She believes strongly that physicians can act locally to help address the issue.
In Seattle, Dr. Meyer has been involved with a local group called Washington Ceasefire, prompted both by her experience as a resident in Detroit – where she was routinely exposed to the traumas of gun violence – as well as a shooting that occurred outside her daughter’s high school in Seattle years ago. The group has recently begun advocating for smart guns, which are designed to be fired only by an authorized user.
Indeed, Dr. Hemenway said research by his group suggests 300,000-500,000 guns are stolen every year, though he points out that we know almost nothing about “who, what, when, why, and where.” That’s largely because of an effective ban on gun violence research, enacted by Congress in the 1990s.9
“It’s not like there’s no evidence, but compared to the size of the problem, you want good evidence,” Dr. Hemenway said. “America has lots of guns. How can we learn to live with them?”
Gun violence affects not just those shot and killed by firearms, but also those affected by the trauma it can leave in its wake. Dr. Sacks recounts a recent visit to Massachusetts General by survivors of the Pulse Nightclub shooting in Orlando, Fla., which took place on June 12, 2016.
“It was a moving, intense event where we all sat around and talked about this issue,” Dr. Sacks said. “The number of people dying is horrific enough, but it’s not just that. Here were a number of young people who survived and yet whose lives will never be the same. We are undercounting the number of people affected by gun violence.”
Studies also estimate the cost of medical care related to gun violence to be roughly $620 million per year, averaging between $9,000 and $18,000 per patient in 2014.10
Despite some arguments to the contrary, addressing gun violence as a public health issue is not a distraction from other important public health issues such as opioid abuse. “It is entirely a false choice that we must only take on one issue or another,” Dr. Sacks said.
Nor should efforts to address gun violence focus only on individuals, said Dr. Hemenway, who told the Harvard T.H. Chan School of Public Health in October 2017 that: “A lesson from public health is that it is usually more effective to change the environment than to try to change people. The U.S. should use the same harm reduction approach to gun violence that it uses to treat other public health threats, like automobile crashes or air pollution, employing a wide variety of methods to reduce the problem.”
The issue must be reframed, said Dr. Sacks. This remains one of her biggest goals. “If we can find a way to act and intervene and lower [the] number [of people affected by gun violence], what could be more fundamentally in line with what we try to do every day as physicians?” she asked. “How can we reduce morbidity and mortality? That’s an answerable question and we can make sure we have pathways and approaches we can put in place to understand this. This is a solvable problem.”
1. Centers for Disease Control and Prevention, National Center for Health Statistics. FastStats. Injuries. https://www.cdc.gov/nchs/fastats/injury.htm. Accessed Nov 20, 2017.
2. Centers for Disease Control and Prevention, National Center for Health Statistics. FastStats. Suicide. https://www.cdc.gov/nchs/fastats/suicide.htm. Accessed Nov 20, 2017.
3. Sacks CA. In memory of Daniel – Reviving research to prevent gun violence. N Engl J Med. 2015; 372:800-801. doi: 10.1056/NEJMp1415128.
4. U.S. Department of Justice, Federal Bureau of Investigation. A study of active shooter incidents in the United States between 2000 and 2013. Published Sept 16, 2013. Accessed Nov 20, 2017.
5. Sacks CA. The role of physicians in preventing firearm suicides. JAMA Int Med. doi: 10.001/jamainternmed.2016.6715. Published Nov 14, 2016. Accessed Nov 20, 2017.
6. Butkus R, Weissman A. Internists’ attitudes toward prevention of firearm injury. Ann Intern Med. 2014;160(12):821-827. doi: 10.7326/M13-1960.
7. Fleegler EW, Lee LK, Monuteaux MC, et al. Firearm Legislation and Firearm-Related Fatalities in the United States. JAMA Intern Med. 2013; 173(9):732-740. doi: 10.1001/jamaimternmed.2013.1286.
8. American Academy of Pediatricians. Addressing gun violence. The federal level. https://www.aap.org/en-us/advocacy-and-policy/aap-health-initiatives/Pages/Gun-Violence-Matrix--Intentional-(Federal).aspx. Accessed Nov 20, 2017.
9. Rubin R. Tale of 2 agencies: CDC avoids gun violence research bit NIH funds it. JAMA. 2016;315(16):1689-1692. doi:10.1001/jama.2016.1707.
10. Howell E and Gangopadhyaya A. State variation in the hospital costs of gun violence, 2010 and 2014. The Urban Institute, Health Policy Center.
1. Centers for Disease Control and Prevention, National Center for Health Statistics. FastStats. Injuries. https://www.cdc.gov/nchs/fastats/injury.htm. Accessed Nov 20, 2017.
2. Centers for Disease Control and Prevention, National Center for Health Statistics. FastStats. Suicide. https://www.cdc.gov/nchs/fastats/suicide.htm. Accessed Nov 20, 2017.
3. Sacks CA. In memory of Daniel – Reviving research to prevent gun violence. N Engl J Med. 2015; 372:800-801. doi: 10.1056/NEJMp1415128.
4. U.S. Department of Justice, Federal Bureau of Investigation. A study of active shooter incidents in the United States between 2000 and 2013. Published Sept 16, 2013. Accessed Nov 20, 2017.
5. Sacks CA. The role of physicians in preventing firearm suicides. JAMA Int Med. doi: 10.001/jamainternmed.2016.6715. Published Nov 14, 2016. Accessed Nov 20, 2017.
6. Butkus R, Weissman A. Internists’ attitudes toward prevention of firearm injury. Ann Intern Med. 2014;160(12):821-827. doi: 10.7326/M13-1960.
7. Fleegler EW, Lee LK, Monuteaux MC, et al. Firearm Legislation and Firearm-Related Fatalities in the United States. JAMA Intern Med. 2013; 173(9):732-740. doi: 10.1001/jamaimternmed.2013.1286.
8. American Academy of Pediatricians. Addressing gun violence. The federal level. https://www.aap.org/en-us/advocacy-and-policy/aap-health-initiatives/Pages/Gun-Violence-Matrix--Intentional-(Federal).aspx. Accessed Nov 20, 2017.
9. Rubin R. Tale of 2 agencies: CDC avoids gun violence research bit NIH funds it. JAMA. 2016;315(16):1689-1692. doi:10.1001/jama.2016.1707.
10. Howell E and Gangopadhyaya A. State variation in the hospital costs of gun violence, 2010 and 2014. The Urban Institute, Health Policy Center.
Bedside Ultrasound for Pulsatile Hand Mass
Case
A 23-year-old man presented to an outside hospital’s ED for evaluation of a wound on his right hand, which he sustained after he accidentally stabbed himself with a steak knife. At presentation, the patient’s vital signs were: heart rate, 90 beats/min; respiratory rate, 16 breaths/min; blood pressure, 150/92 mm Hg; and temperature, 98.1°F. Oxygen saturation was 98% on room air. Examination revealed a laceration on the patient’s right hand measuring 2 cm in length. The emergency physician (EP) closed the wound using four nylon sutures and administered a Boostrix shot. The patient was discharged home with a prescription for cephalexin capsule 500 mg to be taken four times daily for 5 days. He was instructed to return in 10 days for suture removal, but failed to follow-up.
The patient presented to our ED two months after the initial injury for evaluation of a 1.5-cm round pulsatile mass on his right palm, at the base of the middle finger, from which exuded a small amount of sanguineous fluid. The patient complained of numbness and difficulty extending his right index and middle fingers.
Discussion
Palmar Pseudoaneurysms
A pseudoaneurysm, also referred to as a traumatic aneurysm, develops when a tear of the vessel wall and hemorrhage is contained by a thin-walled capsule, typically following traumatic perforation of the arterial wall. Unlike a true aneurysm, a pseudoaneurysm does not contain all three layers of intima, media, and adventitia. Thin walls lead to inevitable expansion over time; in some cases, a patient will present with a soft-tissue mass years after the initial injury. Compression of nearby structures can cause neuropathy, peripheral edema, venous thrombosis, arterial occlusion or emboli, and even bone erosion.1,2
Hand pseudoaneurysms are more likely to occur on the palmar surface, involving the superficial palmar arch,3 and are due to a penetrating injury or repetitive microtrauma. Hypothenar hammer syndrome occurs when repetitive microtrauma is applied to the ulnar artery as it passes under the hook of the hamate bone into the hand. This condition is also referred to as “hammer hand syndrome” because it frequently occurs in laborers such as mechanics, carpenters, and machinists as a result of repetitive palm trauma. Cases have also been reported in baseball players and cooks who also expose their hands to repetitive trauma.3 Likewise, elderly patients who use walking canes can also present with bilateral hammer hand syndrome,3 and patients who need crutches for a prolonged period of time may also develop axillary artery aneurysms.1,2
Although rare, there have also been cases of spontaneous hand pseudoaneurysms in patients on anticoagulation therapy;4,5 however, pseudoaneurysms are not an absolute contraindication to initiating or continuing use of anticoagulants.
Evaluation
Physical Examination. The patient’s mass in this case was clearly pulsatile on examination, but physical examination alone is not a reliable indicator of pseudoaneurysm, as patients may present only with soft-tissue swelling, pain, erythema, or neurological symptoms.3,6,7
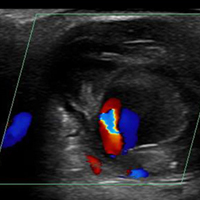
Ultrasound Imaging. In the emergency setting, POC ultrasound should be performed to evaluate any soft-tissue hand mass, especially in the context of trauma or any neurovascular findings, since palmar pseudoaneurysms can easily be confused with an abscess, foreign body, cyst, or even a tendon tear.6 Ultrasound studies using the linear vascular probe should always be done before any attempt to incise and drain the mass.
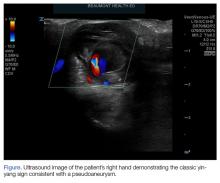
Three ultrasound characteristics of pseudoaneurysms include expansile pulsatility, turbulent flow with a classic yin-yang sign on Doppler, and a hematoma with variable echogenicity. Variable echogenicity may represent separate episodes of bleeding and rebleeding.8 A “to-and-fro” spectral waveform is pathognomonic for palmar pseudoaneurysms.8
Computed Tomography and Magnetic Resonance Angiography. Definitive imaging for operative management includes computed tomography or magnetic resonance angiography to assess for the exact location and presence of collateral circulation.
Treatment
Treatment of pseudoaneurysms includes conservative compression therapy, surgical excision, or anastomosis, and more recently, ultrasound-guided thrombin injection (UGTI).
Compression Therapy. Compression therapy is often used for femoral artery pseudoaneurysms that develop after iatrogenic injury. However, this technique is time consuming, is uncomfortable for patients, is not effective in treating large pseudoaneurysms, and is contraindicated in patients on anticoagulation therapy. Compression therapy also has a high-failure rate of resolving pseudoaneurysms. Traditionally, surgical excision or anastomosis has been the definitive treatment for palmar pseudoaneurysms.
Ultrasound-Guided Thrombin Injection. A more recent treatment option is UGTI, which is usually performed by an interventional radiologist. Although there is no consensus on exact dose of thrombin for this procedure, the literature describes UGTI to treat both the radial and ulnar arteries.9,10 One study of 83 pseudoaneurysms demonstrated a relationship between the size of the palmar pseudoaneurysm and the number of thrombin injections required to resolve it. Depending on the size of the palmar pseudoaneurysm, the effective thrombin doses ranged from 200 to 2,500 U. Regarding adverse effects and events from treatment, this study reported one case of transient distal ischemia.11
Intravascular balloon occlusion of the pseudoaneurysm neck has also been recommended for UGTI in the femoral artery if the neck is greater than 1 mm, but there is currently nothing in the literature describing its use in palmar pseudoaneurysms.12
Complications
There are more descriptions of palmar, radial, and ulnar pseudoaneurysms in critical care patients due to the frequent, but necessary, use of invasive lines. Emergency physicians frequently place radial or femoral arterial lines for hemodynamic monitoring in critically ill patients. However, the incidence of pseudoaneurysms and its sequelae from these lines are not usually observed in the ED setting.
Radial arterial lines may cause thrombosis in 19% to 57% of cases, and local infection in 1% to 18% of cases.10 In a study of 12,500 patients with radial artery catheters, the rate of radial pseudoaneurysm was only 0.05%.11 Although this is a small complication rate, pseudoaneurysms can lead to significant loss of function. To decrease the number of attempts and penetrating injuries to the arteries, ultrasound guidance for these procedures in the ED is strongly recommended. In addition to decreasing the risk of developing a pseudoaneurysm, ultrasound-guidance decreases the discomfort level of the patient and reduces the risk of bleeding, hematoma formation, and infection. Arterial line placement in the ED using ultrasound guidance decreases the risk of developing pseudoaneurysms and their sequelae, such as distal embolization.
Case Conclusion
The patient in this case underwent an arterial duplex study, which found a partially thrombosed right superficial palmar arch pseudoaneurysm measuring 1.91 cm x 2.08 cm, with an active flow area measuring 0.58 cm x 0.68 cm. The flow to the index finger medial artery and middle finger lateral artery was also diminished. The patient was discharged home with a bulky soft dressing and underwent excision and repair by hand surgery 3 days later. At the 1-month postoperative follow-up visit, the patient had full sensation but mildly decreased range of motion in his fingers.
Summary
Hand pseudoaneurysms are often associated with penetrating injuries—as demonstrated in our case—or repetitive microtrauma. Hand pseudoaneurysms can present with minimal findings such as isolated soft-tissue swelling, pain, or neuropathy. The EP should consider vascular pathology in the differential for patients who present with posttraumatic neuropathy. Regarding imaging studies, ultrasound is the best imaging modality to assess for pseudoaneurysms, and EPs should have a low threshold for its use at bedside—especially prior to attempting any invasive procedure. Patients with a confirmed pseudoaneurysm should be referred to a hand or vascular surgeon for surgical repair, or to an interventional radiologist for UGTI.
1. Newton EJ, Arora S. Peripheral vascular injury. In: Marx JA, Hockberger RS, Walls RM, et al, eds. Rosen’s Emergency Medicine Concepts and Clinical Practice. Vol 1. 8th ed. Philadelphia, PA: Elsevier Saunders; 2014:502.
2. Aufderheide TP. Peripheral arteriovascular disease. In: Marx JA, Hockberger RS, Walls RM, et al, eds. Rosen’s Emergency Medicine Concepts and Clinical Practice. Vol 1. 8th ed. 2014:1147-1149.
3. Anderson SE, De Monaco D, Buechler U, et al. Imaging features of pseudoaneurysms of the hand in children and adults. AJR Am J Roentgenol. 2003;180(3):659-664. doi:10.2214/ajr.180.3.1800659.
4. Shah S, Powell-Brett S, Garnham A. Pseudoaneurysm: an unusual cause of post-traumatic hand swelling. BMJ Case Rep. 2015;2015. pii: bcr2014208750. doi:10.1136/bcr-2014-208750.
5. Kitamura A, Mukohara N. Spontaneous pseudoaneurysm of the hand. Ann Vasc Surg. 2014;28(3):739.e1-e3. doi:10.1016/j.avsg.2013.04.033.
6. Huang SW, Wei TS, Liu SY, Wang WT. Spontaneous totally thrombosed pseudoaneurysm mimicking a tendon tear of the wrist. Orthopedics. 2010;33(10):776. doi:10.3928/01477447-20100826-23.
7. Belyayev L, Rich NM, McKay P, Nesti L, Wind G. Traumatic ulnar artery pseudoaneurysm following a grenade blast: report of a case. Mil Med. 2015;180(6):e725-e727. doi:10.7205/MILMED-D-14-00400.
8. Pero T, Herrick J. Pseudoaneurysm of the radial artery diagnosed by bedside ultrasound. West J Emerg Med. 2009;10(2):89-91.
9. Bosman A, Veger HTC, Doornink F, Hedeman Joosten PPA. A pseudoaneurysm of the deep palmar arch after penetrating trauma to the hand: successful exclusion by ultrasound guided percutaneous thrombin injection. EJVES Short Rep. 2016;31:9-11. doi:10.1016/j.ejvssr.2016.03.002.
10. Komorowska-Timek E, Teruya TH, Abou-Zamzam AM Jr, Papa D, Ballard JL. Treatment of radial and ulnar artery pseudoaneurysms using percutaneous thrombin injection. J Hand Surg. 2004;29A(5):936-942. doi:10.1016/j.jhsa.2004.05.009.
11. Falk PS, Scuderi PE, Sherertz RJ, Motsinger SM. Infected radial artery pseudoaneurysms occurring after percutaneous cannulation. Chest. 1992;101(2):490-495.
12. Kang SS, Labropoulos N, Mansour MA, et al. Expanded indications for ultrasound-guided thrombin injection of pseudoaneurysms. J Vasc Surg. 2000;31(2):289-298.
Case
A 23-year-old man presented to an outside hospital’s ED for evaluation of a wound on his right hand, which he sustained after he accidentally stabbed himself with a steak knife. At presentation, the patient’s vital signs were: heart rate, 90 beats/min; respiratory rate, 16 breaths/min; blood pressure, 150/92 mm Hg; and temperature, 98.1°F. Oxygen saturation was 98% on room air. Examination revealed a laceration on the patient’s right hand measuring 2 cm in length. The emergency physician (EP) closed the wound using four nylon sutures and administered a Boostrix shot. The patient was discharged home with a prescription for cephalexin capsule 500 mg to be taken four times daily for 5 days. He was instructed to return in 10 days for suture removal, but failed to follow-up.
The patient presented to our ED two months after the initial injury for evaluation of a 1.5-cm round pulsatile mass on his right palm, at the base of the middle finger, from which exuded a small amount of sanguineous fluid. The patient complained of numbness and difficulty extending his right index and middle fingers.
Discussion
Palmar Pseudoaneurysms
A pseudoaneurysm, also referred to as a traumatic aneurysm, develops when a tear of the vessel wall and hemorrhage is contained by a thin-walled capsule, typically following traumatic perforation of the arterial wall. Unlike a true aneurysm, a pseudoaneurysm does not contain all three layers of intima, media, and adventitia. Thin walls lead to inevitable expansion over time; in some cases, a patient will present with a soft-tissue mass years after the initial injury. Compression of nearby structures can cause neuropathy, peripheral edema, venous thrombosis, arterial occlusion or emboli, and even bone erosion.1,2
Hand pseudoaneurysms are more likely to occur on the palmar surface, involving the superficial palmar arch,3 and are due to a penetrating injury or repetitive microtrauma. Hypothenar hammer syndrome occurs when repetitive microtrauma is applied to the ulnar artery as it passes under the hook of the hamate bone into the hand. This condition is also referred to as “hammer hand syndrome” because it frequently occurs in laborers such as mechanics, carpenters, and machinists as a result of repetitive palm trauma. Cases have also been reported in baseball players and cooks who also expose their hands to repetitive trauma.3 Likewise, elderly patients who use walking canes can also present with bilateral hammer hand syndrome,3 and patients who need crutches for a prolonged period of time may also develop axillary artery aneurysms.1,2
Although rare, there have also been cases of spontaneous hand pseudoaneurysms in patients on anticoagulation therapy;4,5 however, pseudoaneurysms are not an absolute contraindication to initiating or continuing use of anticoagulants.
Evaluation
Physical Examination. The patient’s mass in this case was clearly pulsatile on examination, but physical examination alone is not a reliable indicator of pseudoaneurysm, as patients may present only with soft-tissue swelling, pain, erythema, or neurological symptoms.3,6,7
Ultrasound Imaging. In the emergency setting, POC ultrasound should be performed to evaluate any soft-tissue hand mass, especially in the context of trauma or any neurovascular findings, since palmar pseudoaneurysms can easily be confused with an abscess, foreign body, cyst, or even a tendon tear.6 Ultrasound studies using the linear vascular probe should always be done before any attempt to incise and drain the mass.
Three ultrasound characteristics of pseudoaneurysms include expansile pulsatility, turbulent flow with a classic yin-yang sign on Doppler, and a hematoma with variable echogenicity. Variable echogenicity may represent separate episodes of bleeding and rebleeding.8 A “to-and-fro” spectral waveform is pathognomonic for palmar pseudoaneurysms.8
Computed Tomography and Magnetic Resonance Angiography. Definitive imaging for operative management includes computed tomography or magnetic resonance angiography to assess for the exact location and presence of collateral circulation.
Treatment
Treatment of pseudoaneurysms includes conservative compression therapy, surgical excision, or anastomosis, and more recently, ultrasound-guided thrombin injection (UGTI).
Compression Therapy. Compression therapy is often used for femoral artery pseudoaneurysms that develop after iatrogenic injury. However, this technique is time consuming, is uncomfortable for patients, is not effective in treating large pseudoaneurysms, and is contraindicated in patients on anticoagulation therapy. Compression therapy also has a high-failure rate of resolving pseudoaneurysms. Traditionally, surgical excision or anastomosis has been the definitive treatment for palmar pseudoaneurysms.
Ultrasound-Guided Thrombin Injection. A more recent treatment option is UGTI, which is usually performed by an interventional radiologist. Although there is no consensus on exact dose of thrombin for this procedure, the literature describes UGTI to treat both the radial and ulnar arteries.9,10 One study of 83 pseudoaneurysms demonstrated a relationship between the size of the palmar pseudoaneurysm and the number of thrombin injections required to resolve it. Depending on the size of the palmar pseudoaneurysm, the effective thrombin doses ranged from 200 to 2,500 U. Regarding adverse effects and events from treatment, this study reported one case of transient distal ischemia.11
Intravascular balloon occlusion of the pseudoaneurysm neck has also been recommended for UGTI in the femoral artery if the neck is greater than 1 mm, but there is currently nothing in the literature describing its use in palmar pseudoaneurysms.12
Complications
There are more descriptions of palmar, radial, and ulnar pseudoaneurysms in critical care patients due to the frequent, but necessary, use of invasive lines. Emergency physicians frequently place radial or femoral arterial lines for hemodynamic monitoring in critically ill patients. However, the incidence of pseudoaneurysms and its sequelae from these lines are not usually observed in the ED setting.
Radial arterial lines may cause thrombosis in 19% to 57% of cases, and local infection in 1% to 18% of cases.10 In a study of 12,500 patients with radial artery catheters, the rate of radial pseudoaneurysm was only 0.05%.11 Although this is a small complication rate, pseudoaneurysms can lead to significant loss of function. To decrease the number of attempts and penetrating injuries to the arteries, ultrasound guidance for these procedures in the ED is strongly recommended. In addition to decreasing the risk of developing a pseudoaneurysm, ultrasound-guidance decreases the discomfort level of the patient and reduces the risk of bleeding, hematoma formation, and infection. Arterial line placement in the ED using ultrasound guidance decreases the risk of developing pseudoaneurysms and their sequelae, such as distal embolization.
Case Conclusion
The patient in this case underwent an arterial duplex study, which found a partially thrombosed right superficial palmar arch pseudoaneurysm measuring 1.91 cm x 2.08 cm, with an active flow area measuring 0.58 cm x 0.68 cm. The flow to the index finger medial artery and middle finger lateral artery was also diminished. The patient was discharged home with a bulky soft dressing and underwent excision and repair by hand surgery 3 days later. At the 1-month postoperative follow-up visit, the patient had full sensation but mildly decreased range of motion in his fingers.
Summary
Hand pseudoaneurysms are often associated with penetrating injuries—as demonstrated in our case—or repetitive microtrauma. Hand pseudoaneurysms can present with minimal findings such as isolated soft-tissue swelling, pain, or neuropathy. The EP should consider vascular pathology in the differential for patients who present with posttraumatic neuropathy. Regarding imaging studies, ultrasound is the best imaging modality to assess for pseudoaneurysms, and EPs should have a low threshold for its use at bedside—especially prior to attempting any invasive procedure. Patients with a confirmed pseudoaneurysm should be referred to a hand or vascular surgeon for surgical repair, or to an interventional radiologist for UGTI.
Case
A 23-year-old man presented to an outside hospital’s ED for evaluation of a wound on his right hand, which he sustained after he accidentally stabbed himself with a steak knife. At presentation, the patient’s vital signs were: heart rate, 90 beats/min; respiratory rate, 16 breaths/min; blood pressure, 150/92 mm Hg; and temperature, 98.1°F. Oxygen saturation was 98% on room air. Examination revealed a laceration on the patient’s right hand measuring 2 cm in length. The emergency physician (EP) closed the wound using four nylon sutures and administered a Boostrix shot. The patient was discharged home with a prescription for cephalexin capsule 500 mg to be taken four times daily for 5 days. He was instructed to return in 10 days for suture removal, but failed to follow-up.
The patient presented to our ED two months after the initial injury for evaluation of a 1.5-cm round pulsatile mass on his right palm, at the base of the middle finger, from which exuded a small amount of sanguineous fluid. The patient complained of numbness and difficulty extending his right index and middle fingers.
Discussion
Palmar Pseudoaneurysms
A pseudoaneurysm, also referred to as a traumatic aneurysm, develops when a tear of the vessel wall and hemorrhage is contained by a thin-walled capsule, typically following traumatic perforation of the arterial wall. Unlike a true aneurysm, a pseudoaneurysm does not contain all three layers of intima, media, and adventitia. Thin walls lead to inevitable expansion over time; in some cases, a patient will present with a soft-tissue mass years after the initial injury. Compression of nearby structures can cause neuropathy, peripheral edema, venous thrombosis, arterial occlusion or emboli, and even bone erosion.1,2
Hand pseudoaneurysms are more likely to occur on the palmar surface, involving the superficial palmar arch,3 and are due to a penetrating injury or repetitive microtrauma. Hypothenar hammer syndrome occurs when repetitive microtrauma is applied to the ulnar artery as it passes under the hook of the hamate bone into the hand. This condition is also referred to as “hammer hand syndrome” because it frequently occurs in laborers such as mechanics, carpenters, and machinists as a result of repetitive palm trauma. Cases have also been reported in baseball players and cooks who also expose their hands to repetitive trauma.3 Likewise, elderly patients who use walking canes can also present with bilateral hammer hand syndrome,3 and patients who need crutches for a prolonged period of time may also develop axillary artery aneurysms.1,2
Although rare, there have also been cases of spontaneous hand pseudoaneurysms in patients on anticoagulation therapy;4,5 however, pseudoaneurysms are not an absolute contraindication to initiating or continuing use of anticoagulants.
Evaluation
Physical Examination. The patient’s mass in this case was clearly pulsatile on examination, but physical examination alone is not a reliable indicator of pseudoaneurysm, as patients may present only with soft-tissue swelling, pain, erythema, or neurological symptoms.3,6,7
Ultrasound Imaging. In the emergency setting, POC ultrasound should be performed to evaluate any soft-tissue hand mass, especially in the context of trauma or any neurovascular findings, since palmar pseudoaneurysms can easily be confused with an abscess, foreign body, cyst, or even a tendon tear.6 Ultrasound studies using the linear vascular probe should always be done before any attempt to incise and drain the mass.
Three ultrasound characteristics of pseudoaneurysms include expansile pulsatility, turbulent flow with a classic yin-yang sign on Doppler, and a hematoma with variable echogenicity. Variable echogenicity may represent separate episodes of bleeding and rebleeding.8 A “to-and-fro” spectral waveform is pathognomonic for palmar pseudoaneurysms.8
Computed Tomography and Magnetic Resonance Angiography. Definitive imaging for operative management includes computed tomography or magnetic resonance angiography to assess for the exact location and presence of collateral circulation.
Treatment
Treatment of pseudoaneurysms includes conservative compression therapy, surgical excision, or anastomosis, and more recently, ultrasound-guided thrombin injection (UGTI).
Compression Therapy. Compression therapy is often used for femoral artery pseudoaneurysms that develop after iatrogenic injury. However, this technique is time consuming, is uncomfortable for patients, is not effective in treating large pseudoaneurysms, and is contraindicated in patients on anticoagulation therapy. Compression therapy also has a high-failure rate of resolving pseudoaneurysms. Traditionally, surgical excision or anastomosis has been the definitive treatment for palmar pseudoaneurysms.
Ultrasound-Guided Thrombin Injection. A more recent treatment option is UGTI, which is usually performed by an interventional radiologist. Although there is no consensus on exact dose of thrombin for this procedure, the literature describes UGTI to treat both the radial and ulnar arteries.9,10 One study of 83 pseudoaneurysms demonstrated a relationship between the size of the palmar pseudoaneurysm and the number of thrombin injections required to resolve it. Depending on the size of the palmar pseudoaneurysm, the effective thrombin doses ranged from 200 to 2,500 U. Regarding adverse effects and events from treatment, this study reported one case of transient distal ischemia.11
Intravascular balloon occlusion of the pseudoaneurysm neck has also been recommended for UGTI in the femoral artery if the neck is greater than 1 mm, but there is currently nothing in the literature describing its use in palmar pseudoaneurysms.12
Complications
There are more descriptions of palmar, radial, and ulnar pseudoaneurysms in critical care patients due to the frequent, but necessary, use of invasive lines. Emergency physicians frequently place radial or femoral arterial lines for hemodynamic monitoring in critically ill patients. However, the incidence of pseudoaneurysms and its sequelae from these lines are not usually observed in the ED setting.
Radial arterial lines may cause thrombosis in 19% to 57% of cases, and local infection in 1% to 18% of cases.10 In a study of 12,500 patients with radial artery catheters, the rate of radial pseudoaneurysm was only 0.05%.11 Although this is a small complication rate, pseudoaneurysms can lead to significant loss of function. To decrease the number of attempts and penetrating injuries to the arteries, ultrasound guidance for these procedures in the ED is strongly recommended. In addition to decreasing the risk of developing a pseudoaneurysm, ultrasound-guidance decreases the discomfort level of the patient and reduces the risk of bleeding, hematoma formation, and infection. Arterial line placement in the ED using ultrasound guidance decreases the risk of developing pseudoaneurysms and their sequelae, such as distal embolization.
Case Conclusion
The patient in this case underwent an arterial duplex study, which found a partially thrombosed right superficial palmar arch pseudoaneurysm measuring 1.91 cm x 2.08 cm, with an active flow area measuring 0.58 cm x 0.68 cm. The flow to the index finger medial artery and middle finger lateral artery was also diminished. The patient was discharged home with a bulky soft dressing and underwent excision and repair by hand surgery 3 days later. At the 1-month postoperative follow-up visit, the patient had full sensation but mildly decreased range of motion in his fingers.
Summary
Hand pseudoaneurysms are often associated with penetrating injuries—as demonstrated in our case—or repetitive microtrauma. Hand pseudoaneurysms can present with minimal findings such as isolated soft-tissue swelling, pain, or neuropathy. The EP should consider vascular pathology in the differential for patients who present with posttraumatic neuropathy. Regarding imaging studies, ultrasound is the best imaging modality to assess for pseudoaneurysms, and EPs should have a low threshold for its use at bedside—especially prior to attempting any invasive procedure. Patients with a confirmed pseudoaneurysm should be referred to a hand or vascular surgeon for surgical repair, or to an interventional radiologist for UGTI.
1. Newton EJ, Arora S. Peripheral vascular injury. In: Marx JA, Hockberger RS, Walls RM, et al, eds. Rosen’s Emergency Medicine Concepts and Clinical Practice. Vol 1. 8th ed. Philadelphia, PA: Elsevier Saunders; 2014:502.
2. Aufderheide TP. Peripheral arteriovascular disease. In: Marx JA, Hockberger RS, Walls RM, et al, eds. Rosen’s Emergency Medicine Concepts and Clinical Practice. Vol 1. 8th ed. 2014:1147-1149.
3. Anderson SE, De Monaco D, Buechler U, et al. Imaging features of pseudoaneurysms of the hand in children and adults. AJR Am J Roentgenol. 2003;180(3):659-664. doi:10.2214/ajr.180.3.1800659.
4. Shah S, Powell-Brett S, Garnham A. Pseudoaneurysm: an unusual cause of post-traumatic hand swelling. BMJ Case Rep. 2015;2015. pii: bcr2014208750. doi:10.1136/bcr-2014-208750.
5. Kitamura A, Mukohara N. Spontaneous pseudoaneurysm of the hand. Ann Vasc Surg. 2014;28(3):739.e1-e3. doi:10.1016/j.avsg.2013.04.033.
6. Huang SW, Wei TS, Liu SY, Wang WT. Spontaneous totally thrombosed pseudoaneurysm mimicking a tendon tear of the wrist. Orthopedics. 2010;33(10):776. doi:10.3928/01477447-20100826-23.
7. Belyayev L, Rich NM, McKay P, Nesti L, Wind G. Traumatic ulnar artery pseudoaneurysm following a grenade blast: report of a case. Mil Med. 2015;180(6):e725-e727. doi:10.7205/MILMED-D-14-00400.
8. Pero T, Herrick J. Pseudoaneurysm of the radial artery diagnosed by bedside ultrasound. West J Emerg Med. 2009;10(2):89-91.
9. Bosman A, Veger HTC, Doornink F, Hedeman Joosten PPA. A pseudoaneurysm of the deep palmar arch after penetrating trauma to the hand: successful exclusion by ultrasound guided percutaneous thrombin injection. EJVES Short Rep. 2016;31:9-11. doi:10.1016/j.ejvssr.2016.03.002.
10. Komorowska-Timek E, Teruya TH, Abou-Zamzam AM Jr, Papa D, Ballard JL. Treatment of radial and ulnar artery pseudoaneurysms using percutaneous thrombin injection. J Hand Surg. 2004;29A(5):936-942. doi:10.1016/j.jhsa.2004.05.009.
11. Falk PS, Scuderi PE, Sherertz RJ, Motsinger SM. Infected radial artery pseudoaneurysms occurring after percutaneous cannulation. Chest. 1992;101(2):490-495.
12. Kang SS, Labropoulos N, Mansour MA, et al. Expanded indications for ultrasound-guided thrombin injection of pseudoaneurysms. J Vasc Surg. 2000;31(2):289-298.
1. Newton EJ, Arora S. Peripheral vascular injury. In: Marx JA, Hockberger RS, Walls RM, et al, eds. Rosen’s Emergency Medicine Concepts and Clinical Practice. Vol 1. 8th ed. Philadelphia, PA: Elsevier Saunders; 2014:502.
2. Aufderheide TP. Peripheral arteriovascular disease. In: Marx JA, Hockberger RS, Walls RM, et al, eds. Rosen’s Emergency Medicine Concepts and Clinical Practice. Vol 1. 8th ed. 2014:1147-1149.
3. Anderson SE, De Monaco D, Buechler U, et al. Imaging features of pseudoaneurysms of the hand in children and adults. AJR Am J Roentgenol. 2003;180(3):659-664. doi:10.2214/ajr.180.3.1800659.
4. Shah S, Powell-Brett S, Garnham A. Pseudoaneurysm: an unusual cause of post-traumatic hand swelling. BMJ Case Rep. 2015;2015. pii: bcr2014208750. doi:10.1136/bcr-2014-208750.
5. Kitamura A, Mukohara N. Spontaneous pseudoaneurysm of the hand. Ann Vasc Surg. 2014;28(3):739.e1-e3. doi:10.1016/j.avsg.2013.04.033.
6. Huang SW, Wei TS, Liu SY, Wang WT. Spontaneous totally thrombosed pseudoaneurysm mimicking a tendon tear of the wrist. Orthopedics. 2010;33(10):776. doi:10.3928/01477447-20100826-23.
7. Belyayev L, Rich NM, McKay P, Nesti L, Wind G. Traumatic ulnar artery pseudoaneurysm following a grenade blast: report of a case. Mil Med. 2015;180(6):e725-e727. doi:10.7205/MILMED-D-14-00400.
8. Pero T, Herrick J. Pseudoaneurysm of the radial artery diagnosed by bedside ultrasound. West J Emerg Med. 2009;10(2):89-91.
9. Bosman A, Veger HTC, Doornink F, Hedeman Joosten PPA. A pseudoaneurysm of the deep palmar arch after penetrating trauma to the hand: successful exclusion by ultrasound guided percutaneous thrombin injection. EJVES Short Rep. 2016;31:9-11. doi:10.1016/j.ejvssr.2016.03.002.
10. Komorowska-Timek E, Teruya TH, Abou-Zamzam AM Jr, Papa D, Ballard JL. Treatment of radial and ulnar artery pseudoaneurysms using percutaneous thrombin injection. J Hand Surg. 2004;29A(5):936-942. doi:10.1016/j.jhsa.2004.05.009.
11. Falk PS, Scuderi PE, Sherertz RJ, Motsinger SM. Infected radial artery pseudoaneurysms occurring after percutaneous cannulation. Chest. 1992;101(2):490-495.
12. Kang SS, Labropoulos N, Mansour MA, et al. Expanded indications for ultrasound-guided thrombin injection of pseudoaneurysms. J Vasc Surg. 2000;31(2):289-298.
Balloon pulmonary angioplasty for CTEPH improves heart failure symptoms
WASHINGTON – Balloon pulmonary angioplasty provides meaningful improvements in functional capacity for patients with chronic thromboembolic pulmonary hypertension (CTEPH) who are not candidates for surgical pulmonary thromboendarterectomy, according to a single-center experience with 15 consecutive patients that was presented at CRT 2018 sponsored by the Cardiovascular Research Institute at Washington Hospital Center.
The treatment of choice for CTEPH is pulmonary thromboendarterectomy (PTE), but “a huge percentage of the population with CTEPH” is not eligible or does not undergo surgical treatment, which was the impetus to initiate this intervention, reported Riyaz Bashir, MD, director of vascular and endovascular medicine at Temple University Hospital and professor of medicine at Temple University, Philadelphia.
There have now been 15 CTEPH patients treated with BPA by Dr. Bashir and his team at Temple University. He reported 6-month outcome data on the first 13 patients, all of whom had a history of pulmonary embolism. Three of the patients had a prior PTE.
The primary outcome of interest in this series was functional improvement. Unlike PTE, immediate improvement in hemodynamics is not typically observed immediately after the procedure, but these measures do improve incrementally over time, Dr. Bashir reported. This is reflected in progressive improvements in the 6-minute walk test and in New York Heart Association (NYHA) functional class.
Of the 13 patients treated so far, 6 (46%) were in NYHA class IV and only 2 (15%) were in NYHA class II prior to BPA. Six months after BPA, the proportions had reversed. At that point, seven patients (54%) were in class II and two (15%) in class IV. The remaining patients at both time points were in NYHA class III. Similar improvements were seen in the 6-minute walk test, which typically tracks with NYHA class.
Describing the first case, performed about 2 years ago, Dr. Bashir explained that a tight stenosis in the right lower pulmonary artery of a 44-year-old woman was reached with a multipurpose guiding catheter through femoral access. A 5-mm balloon was used to dilate the stenosis and create a pulsatile flow.
“The goal is not to raise the mean arterial pressure above 35 mm Hg, because this has been associated with significant peripheral edema,” Dr. Bashir explained.
In this patient, progressive improvements in pulmonary pressure, cardiac index, and other hemodynamics were associated with progressive shrinking of the right ventricle over 6 months of follow-up. The walk test improved from 216 m prior to BPA to 421 m at her most recent evaluation.
The average age in the 13 patients treated so far was 55 years, and 75% are males. The mean left ventricular ejection fraction (LVEF) was 55%. According to Dr. Bashir, most patients required at least two treatment sessions and some have required up to four.
There have been two complications – one patient developed hemoptysis that required a brief intubation and the other involved perfusion edema – and no deaths in this series so far, he said.
The outcomes so far, which Dr. Bashir characterized as “an early experience,” provide evidence that BPA is “safe and feasible” for patients with CTEPH who are not surgical candidates. At Dr. Bashir’s institution, where PTE is commonly performed in patients with CTEPH (Dr. Bashir reported that 134 cases were performed over the period of time in which these 15 cases of BPA were performed), there is a plan to compare functional outcomes in CTEPH patients managed with these two different approaches.
Dr. Bashir reported no financial relationships relevant to this study.
SOURCE: Bashir R. CRT 2018
WASHINGTON – Balloon pulmonary angioplasty provides meaningful improvements in functional capacity for patients with chronic thromboembolic pulmonary hypertension (CTEPH) who are not candidates for surgical pulmonary thromboendarterectomy, according to a single-center experience with 15 consecutive patients that was presented at CRT 2018 sponsored by the Cardiovascular Research Institute at Washington Hospital Center.
The treatment of choice for CTEPH is pulmonary thromboendarterectomy (PTE), but “a huge percentage of the population with CTEPH” is not eligible or does not undergo surgical treatment, which was the impetus to initiate this intervention, reported Riyaz Bashir, MD, director of vascular and endovascular medicine at Temple University Hospital and professor of medicine at Temple University, Philadelphia.
There have now been 15 CTEPH patients treated with BPA by Dr. Bashir and his team at Temple University. He reported 6-month outcome data on the first 13 patients, all of whom had a history of pulmonary embolism. Three of the patients had a prior PTE.
The primary outcome of interest in this series was functional improvement. Unlike PTE, immediate improvement in hemodynamics is not typically observed immediately after the procedure, but these measures do improve incrementally over time, Dr. Bashir reported. This is reflected in progressive improvements in the 6-minute walk test and in New York Heart Association (NYHA) functional class.
Of the 13 patients treated so far, 6 (46%) were in NYHA class IV and only 2 (15%) were in NYHA class II prior to BPA. Six months after BPA, the proportions had reversed. At that point, seven patients (54%) were in class II and two (15%) in class IV. The remaining patients at both time points were in NYHA class III. Similar improvements were seen in the 6-minute walk test, which typically tracks with NYHA class.
Describing the first case, performed about 2 years ago, Dr. Bashir explained that a tight stenosis in the right lower pulmonary artery of a 44-year-old woman was reached with a multipurpose guiding catheter through femoral access. A 5-mm balloon was used to dilate the stenosis and create a pulsatile flow.
“The goal is not to raise the mean arterial pressure above 35 mm Hg, because this has been associated with significant peripheral edema,” Dr. Bashir explained.
In this patient, progressive improvements in pulmonary pressure, cardiac index, and other hemodynamics were associated with progressive shrinking of the right ventricle over 6 months of follow-up. The walk test improved from 216 m prior to BPA to 421 m at her most recent evaluation.
The average age in the 13 patients treated so far was 55 years, and 75% are males. The mean left ventricular ejection fraction (LVEF) was 55%. According to Dr. Bashir, most patients required at least two treatment sessions and some have required up to four.
There have been two complications – one patient developed hemoptysis that required a brief intubation and the other involved perfusion edema – and no deaths in this series so far, he said.
The outcomes so far, which Dr. Bashir characterized as “an early experience,” provide evidence that BPA is “safe and feasible” for patients with CTEPH who are not surgical candidates. At Dr. Bashir’s institution, where PTE is commonly performed in patients with CTEPH (Dr. Bashir reported that 134 cases were performed over the period of time in which these 15 cases of BPA were performed), there is a plan to compare functional outcomes in CTEPH patients managed with these two different approaches.
Dr. Bashir reported no financial relationships relevant to this study.
SOURCE: Bashir R. CRT 2018
WASHINGTON – Balloon pulmonary angioplasty provides meaningful improvements in functional capacity for patients with chronic thromboembolic pulmonary hypertension (CTEPH) who are not candidates for surgical pulmonary thromboendarterectomy, according to a single-center experience with 15 consecutive patients that was presented at CRT 2018 sponsored by the Cardiovascular Research Institute at Washington Hospital Center.
The treatment of choice for CTEPH is pulmonary thromboendarterectomy (PTE), but “a huge percentage of the population with CTEPH” is not eligible or does not undergo surgical treatment, which was the impetus to initiate this intervention, reported Riyaz Bashir, MD, director of vascular and endovascular medicine at Temple University Hospital and professor of medicine at Temple University, Philadelphia.
There have now been 15 CTEPH patients treated with BPA by Dr. Bashir and his team at Temple University. He reported 6-month outcome data on the first 13 patients, all of whom had a history of pulmonary embolism. Three of the patients had a prior PTE.
The primary outcome of interest in this series was functional improvement. Unlike PTE, immediate improvement in hemodynamics is not typically observed immediately after the procedure, but these measures do improve incrementally over time, Dr. Bashir reported. This is reflected in progressive improvements in the 6-minute walk test and in New York Heart Association (NYHA) functional class.
Of the 13 patients treated so far, 6 (46%) were in NYHA class IV and only 2 (15%) were in NYHA class II prior to BPA. Six months after BPA, the proportions had reversed. At that point, seven patients (54%) were in class II and two (15%) in class IV. The remaining patients at both time points were in NYHA class III. Similar improvements were seen in the 6-minute walk test, which typically tracks with NYHA class.
Describing the first case, performed about 2 years ago, Dr. Bashir explained that a tight stenosis in the right lower pulmonary artery of a 44-year-old woman was reached with a multipurpose guiding catheter through femoral access. A 5-mm balloon was used to dilate the stenosis and create a pulsatile flow.
“The goal is not to raise the mean arterial pressure above 35 mm Hg, because this has been associated with significant peripheral edema,” Dr. Bashir explained.
In this patient, progressive improvements in pulmonary pressure, cardiac index, and other hemodynamics were associated with progressive shrinking of the right ventricle over 6 months of follow-up. The walk test improved from 216 m prior to BPA to 421 m at her most recent evaluation.
The average age in the 13 patients treated so far was 55 years, and 75% are males. The mean left ventricular ejection fraction (LVEF) was 55%. According to Dr. Bashir, most patients required at least two treatment sessions and some have required up to four.
There have been two complications – one patient developed hemoptysis that required a brief intubation and the other involved perfusion edema – and no deaths in this series so far, he said.
The outcomes so far, which Dr. Bashir characterized as “an early experience,” provide evidence that BPA is “safe and feasible” for patients with CTEPH who are not surgical candidates. At Dr. Bashir’s institution, where PTE is commonly performed in patients with CTEPH (Dr. Bashir reported that 134 cases were performed over the period of time in which these 15 cases of BPA were performed), there is a plan to compare functional outcomes in CTEPH patients managed with these two different approaches.
Dr. Bashir reported no financial relationships relevant to this study.
SOURCE: Bashir R. CRT 2018
REPORTING FROM CRT 2018
Key clinical point: Balloon pulmonary angioplasty has clinical benefits in chronic thromboembolic pulmonary hypertension (CTEPH) patients not candidates for surgery.
Major finding: At baseline, only 30% of CTEPH patients were at or below NYHA class II, rising to 65% 6 months after balloon pulmonary angioplasty.
Data source: Single-center review of 15 patients.
Disclosures: Dr. Bashir reports no financial relationships relevant to this study.
Source: Bashir R. CRT 2018
Failure to find cancer earlier; patient dies: $4.69M verdict
Failure to find cancer earlier; patient dies: $4.69M verdict
On July 19, a 26-year-old woman presented to the emergency department (ED) with abnormal vaginal bleeding 3 months after giving birth. She was found to have endometrial thickening and an elevated ß human chorionic gonadotropin level. An ObGyn (Dr. A) assumed that the patient was having a miscarriage and sent her home.
On July 30, when the patient returned to the ED with continued bleeding, lesions on her cervix and urethra were discovered. A second ObGyn, Dr. B, addressed the bleeding, removed the lesion, and ordered testing. On August 17, the patient saw a third ObGyn (Dr. C), who did not conduct an examination.
Days later, the patient suffered a brain hemorrhage that was suspicious for hemorrhagic metastasis. After that, stage IV choriocarcinoma was identified. Although she underwent chemotherapy, the patient died 18 months later.
ESTATE'S CLAIM: All 3 ObGyns failed to take a proper history, conduct adequate examinations, and order appropriate testing. Even at stage IV, 75% of patients with choriocarcinoma survive past 5 years. The stroke rendered chemotherapy less effective and substantially contributed to the patient's death. Failure to diagnose the cancer before the stroke allowed the disease to progress beyond the point at which the patient's life could be saved.
DEFENDANTS' DEFENSE: The ObGyns and hospital claimed that appropriate care was provided and that they were not negligent in failing to consider the diagnosis of a very rare form of cancer.
VERDICT: A $4.69 million New Jersey verdict was returned, with all 3 physicians held partially liable.
Hot speculum burns patient: $547,090 award
A 54-year-old woman underwent a hysterectomy performed at a government-operated hospital. After she was anesthetized and unconscious, a second-year resident took a speculum that had been placed in the sterile field by a nurse, and inserted it in the patient's vagina.
When the patient awoke from surgery, she discovered significant burns to her vaginal area, perineum, anus, and buttocks.
PATIENT'S CLAIM: The speculum had just been removed from the autoclave and was very hot. The patient incurred substantial medical bills to treat her injuries and was unable to work for several months. She sued the hospital and resident, alleging error by the nurse in placing the hot speculum in the sterile field without cooling it or advising the resident that it was still hot. The resident was blamed for using the speculum without confirming that it was hot.
DEFENDANTS' DEFENSE: The resident claimed that she reasonably relied on the nurse to not place a hot instrument in the surgical field without first cooling it. The hospital, representing the nurse, denied fault, blaming the resident for not checking the speculum.
VERDICT: A $547,090 Louisiana verdict was awarded by a judge against the resident and the hospital, but it was halved by comparative fault to $273,545.
Surgeon's breast exam insufficient: $375,000 verdict
After a woman in her early 40s found a lump in her left breast, she underwent a radiographic study, which a radiologist interpreted as showing a 3-mm cyst. Without performing additional tests, a general surgeon immediately scheduled her for surgery.
On May 17, the radiologist performed an ultrasound-guided needle-localized biopsy and found a nodule. The patient was immediately sent to the operating room where the surgeon performed a segmental resection of the nodule.
On May 24, the patient presented to the surgeon's office for a postoperative visit. She told the nurse that the palpable mass was still there. The nurse examined the mass, told the patient that the incision was healing nicely, and suggested follow-up in a month.
Four months later, the patient sought a second opinion. On September 15, she underwent a diagnostic mammogram, ultrasound, and biopsy. The biopsy was positive for invasive ductal carcinoma. On September 30, magnetic resonance imaging and a second biopsy further confirmed the diagnosis. On November 2, she underwent a segmental mastectomy with sentinel lymph node biopsy. The pathology report noted a 3-cm invasive ductal carcinoma with necrosis. The patient underwent chemotherapy and radiation treatment.
PATIENT'S CLAIM: She sued the general surgeon, radiologist, and surgical center, alleging that her breast cancer went undiagnosed. Prior to trial, the radiologist and surgical center were dismissed from the case.
The surgeon failed to perform a thorough physical examination and nodal evaluation of the left breast and axilla. His substandard methods to diagnose and treat the patient's breast cancer delayed proper treatment and significantly altered the outcome.
PHYSICIAN'S CLAIM: The surgeon's treatment met the standard of care. The outcome and treatment were not significantly changed by the delay.
VERDICT: A $375,000 Pennsylvania verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Failure to find cancer earlier; patient dies: $4.69M verdict
On July 19, a 26-year-old woman presented to the emergency department (ED) with abnormal vaginal bleeding 3 months after giving birth. She was found to have endometrial thickening and an elevated ß human chorionic gonadotropin level. An ObGyn (Dr. A) assumed that the patient was having a miscarriage and sent her home.
On July 30, when the patient returned to the ED with continued bleeding, lesions on her cervix and urethra were discovered. A second ObGyn, Dr. B, addressed the bleeding, removed the lesion, and ordered testing. On August 17, the patient saw a third ObGyn (Dr. C), who did not conduct an examination.
Days later, the patient suffered a brain hemorrhage that was suspicious for hemorrhagic metastasis. After that, stage IV choriocarcinoma was identified. Although she underwent chemotherapy, the patient died 18 months later.
ESTATE'S CLAIM: All 3 ObGyns failed to take a proper history, conduct adequate examinations, and order appropriate testing. Even at stage IV, 75% of patients with choriocarcinoma survive past 5 years. The stroke rendered chemotherapy less effective and substantially contributed to the patient's death. Failure to diagnose the cancer before the stroke allowed the disease to progress beyond the point at which the patient's life could be saved.
DEFENDANTS' DEFENSE: The ObGyns and hospital claimed that appropriate care was provided and that they were not negligent in failing to consider the diagnosis of a very rare form of cancer.
VERDICT: A $4.69 million New Jersey verdict was returned, with all 3 physicians held partially liable.
Hot speculum burns patient: $547,090 award
A 54-year-old woman underwent a hysterectomy performed at a government-operated hospital. After she was anesthetized and unconscious, a second-year resident took a speculum that had been placed in the sterile field by a nurse, and inserted it in the patient's vagina.
When the patient awoke from surgery, she discovered significant burns to her vaginal area, perineum, anus, and buttocks.
PATIENT'S CLAIM: The speculum had just been removed from the autoclave and was very hot. The patient incurred substantial medical bills to treat her injuries and was unable to work for several months. She sued the hospital and resident, alleging error by the nurse in placing the hot speculum in the sterile field without cooling it or advising the resident that it was still hot. The resident was blamed for using the speculum without confirming that it was hot.
DEFENDANTS' DEFENSE: The resident claimed that she reasonably relied on the nurse to not place a hot instrument in the surgical field without first cooling it. The hospital, representing the nurse, denied fault, blaming the resident for not checking the speculum.
VERDICT: A $547,090 Louisiana verdict was awarded by a judge against the resident and the hospital, but it was halved by comparative fault to $273,545.
Surgeon's breast exam insufficient: $375,000 verdict
After a woman in her early 40s found a lump in her left breast, she underwent a radiographic study, which a radiologist interpreted as showing a 3-mm cyst. Without performing additional tests, a general surgeon immediately scheduled her for surgery.
On May 17, the radiologist performed an ultrasound-guided needle-localized biopsy and found a nodule. The patient was immediately sent to the operating room where the surgeon performed a segmental resection of the nodule.
On May 24, the patient presented to the surgeon's office for a postoperative visit. She told the nurse that the palpable mass was still there. The nurse examined the mass, told the patient that the incision was healing nicely, and suggested follow-up in a month.
Four months later, the patient sought a second opinion. On September 15, she underwent a diagnostic mammogram, ultrasound, and biopsy. The biopsy was positive for invasive ductal carcinoma. On September 30, magnetic resonance imaging and a second biopsy further confirmed the diagnosis. On November 2, she underwent a segmental mastectomy with sentinel lymph node biopsy. The pathology report noted a 3-cm invasive ductal carcinoma with necrosis. The patient underwent chemotherapy and radiation treatment.
PATIENT'S CLAIM: She sued the general surgeon, radiologist, and surgical center, alleging that her breast cancer went undiagnosed. Prior to trial, the radiologist and surgical center were dismissed from the case.
The surgeon failed to perform a thorough physical examination and nodal evaluation of the left breast and axilla. His substandard methods to diagnose and treat the patient's breast cancer delayed proper treatment and significantly altered the outcome.
PHYSICIAN'S CLAIM: The surgeon's treatment met the standard of care. The outcome and treatment were not significantly changed by the delay.
VERDICT: A $375,000 Pennsylvania verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Failure to find cancer earlier; patient dies: $4.69M verdict
On July 19, a 26-year-old woman presented to the emergency department (ED) with abnormal vaginal bleeding 3 months after giving birth. She was found to have endometrial thickening and an elevated ß human chorionic gonadotropin level. An ObGyn (Dr. A) assumed that the patient was having a miscarriage and sent her home.
On July 30, when the patient returned to the ED with continued bleeding, lesions on her cervix and urethra were discovered. A second ObGyn, Dr. B, addressed the bleeding, removed the lesion, and ordered testing. On August 17, the patient saw a third ObGyn (Dr. C), who did not conduct an examination.
Days later, the patient suffered a brain hemorrhage that was suspicious for hemorrhagic metastasis. After that, stage IV choriocarcinoma was identified. Although she underwent chemotherapy, the patient died 18 months later.
ESTATE'S CLAIM: All 3 ObGyns failed to take a proper history, conduct adequate examinations, and order appropriate testing. Even at stage IV, 75% of patients with choriocarcinoma survive past 5 years. The stroke rendered chemotherapy less effective and substantially contributed to the patient's death. Failure to diagnose the cancer before the stroke allowed the disease to progress beyond the point at which the patient's life could be saved.
DEFENDANTS' DEFENSE: The ObGyns and hospital claimed that appropriate care was provided and that they were not negligent in failing to consider the diagnosis of a very rare form of cancer.
VERDICT: A $4.69 million New Jersey verdict was returned, with all 3 physicians held partially liable.
Hot speculum burns patient: $547,090 award
A 54-year-old woman underwent a hysterectomy performed at a government-operated hospital. After she was anesthetized and unconscious, a second-year resident took a speculum that had been placed in the sterile field by a nurse, and inserted it in the patient's vagina.
When the patient awoke from surgery, she discovered significant burns to her vaginal area, perineum, anus, and buttocks.
PATIENT'S CLAIM: The speculum had just been removed from the autoclave and was very hot. The patient incurred substantial medical bills to treat her injuries and was unable to work for several months. She sued the hospital and resident, alleging error by the nurse in placing the hot speculum in the sterile field without cooling it or advising the resident that it was still hot. The resident was blamed for using the speculum without confirming that it was hot.
DEFENDANTS' DEFENSE: The resident claimed that she reasonably relied on the nurse to not place a hot instrument in the surgical field without first cooling it. The hospital, representing the nurse, denied fault, blaming the resident for not checking the speculum.
VERDICT: A $547,090 Louisiana verdict was awarded by a judge against the resident and the hospital, but it was halved by comparative fault to $273,545.
Surgeon's breast exam insufficient: $375,000 verdict
After a woman in her early 40s found a lump in her left breast, she underwent a radiographic study, which a radiologist interpreted as showing a 3-mm cyst. Without performing additional tests, a general surgeon immediately scheduled her for surgery.
On May 17, the radiologist performed an ultrasound-guided needle-localized biopsy and found a nodule. The patient was immediately sent to the operating room where the surgeon performed a segmental resection of the nodule.
On May 24, the patient presented to the surgeon's office for a postoperative visit. She told the nurse that the palpable mass was still there. The nurse examined the mass, told the patient that the incision was healing nicely, and suggested follow-up in a month.
Four months later, the patient sought a second opinion. On September 15, she underwent a diagnostic mammogram, ultrasound, and biopsy. The biopsy was positive for invasive ductal carcinoma. On September 30, magnetic resonance imaging and a second biopsy further confirmed the diagnosis. On November 2, she underwent a segmental mastectomy with sentinel lymph node biopsy. The pathology report noted a 3-cm invasive ductal carcinoma with necrosis. The patient underwent chemotherapy and radiation treatment.
PATIENT'S CLAIM: She sued the general surgeon, radiologist, and surgical center, alleging that her breast cancer went undiagnosed. Prior to trial, the radiologist and surgical center were dismissed from the case.
The surgeon failed to perform a thorough physical examination and nodal evaluation of the left breast and axilla. His substandard methods to diagnose and treat the patient's breast cancer delayed proper treatment and significantly altered the outcome.
PHYSICIAN'S CLAIM: The surgeon's treatment met the standard of care. The outcome and treatment were not significantly changed by the delay.
VERDICT: A $375,000 Pennsylvania verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Checkpoint inhibition less toxic than antiangiogenic therapy in NSCLC
ORLANDO – , a systematic review and meta-analysis suggests.
In 16,810 patients from 37 trials included in the analysis, first-line treatment with nivolumab or pembrolizumab, compared with first-line sorafenib plus platinum doublets, for example, was associated with less combined direct and indirect toxicity (odds ratios, 0.08 and 0.12, respectively), Chin-Chuan Hung, MD, and her colleagues reported in a poster at the annual conference of the National Comprehensive Cancer Network.
For subsequent therapy, nivolumab showed lower risk than most antiangiogenic therapies, particularly combination ramucirumab and docetaxel (OR, 0.06), said Dr. Hung of China Medical University Hospital in Taichung, Taiwan.
The findings are notable because tolerability is an essential selection criterion for patients with advanced stage disease, and while checkpoint inhibitors – including nivolumab, pembrolizumab, and atezolizumab – and antiangiogenic agents – including bevacizumab, ramucirumab, and nintedanib – have become the treatments of choice, direct comparisons with respect to tolerability are lacking, she noted.
The investigators performed a systematic review using Bayesian-model network meta-analysis of studies conducted through July 2017 comparing first-line and subsequent regimens containing chemotherapy, antiangiogenic therapy, and/or immune checkpoint inhibitors. Chemotherapy agents studied included cisplatin, carboplatin, oxaliplatin, gemcitabine, paclitaxel, docetaxel, and pemetrexed; antiangiogenic agents included bevacizumab, aflibercept, ramucirumab, nintedanib, axitinib, sorafenib, vandetanib, and sunitinib; and immune checkpoint inhibitors included ipilimumab, pembrolizumab, nivolumab, and atezolizumab.
Direct and indirect data for all grade 3-5 adverse events were combined using random-effects network meta-analysis.
“The results indicated that [checkpoint] inhibitors can be preferred choices for less toxicity to treat advanced stage NSCLC compared with antiangiogenic therapies in first-line and subsequent settings,” Dr. Hung and her associates concluded.
This study was supported by the China Medical University Beigang Hospital.
SOURCE: Hsu C et al. NCCN poster 13
ORLANDO – , a systematic review and meta-analysis suggests.
In 16,810 patients from 37 trials included in the analysis, first-line treatment with nivolumab or pembrolizumab, compared with first-line sorafenib plus platinum doublets, for example, was associated with less combined direct and indirect toxicity (odds ratios, 0.08 and 0.12, respectively), Chin-Chuan Hung, MD, and her colleagues reported in a poster at the annual conference of the National Comprehensive Cancer Network.
For subsequent therapy, nivolumab showed lower risk than most antiangiogenic therapies, particularly combination ramucirumab and docetaxel (OR, 0.06), said Dr. Hung of China Medical University Hospital in Taichung, Taiwan.
The findings are notable because tolerability is an essential selection criterion for patients with advanced stage disease, and while checkpoint inhibitors – including nivolumab, pembrolizumab, and atezolizumab – and antiangiogenic agents – including bevacizumab, ramucirumab, and nintedanib – have become the treatments of choice, direct comparisons with respect to tolerability are lacking, she noted.
The investigators performed a systematic review using Bayesian-model network meta-analysis of studies conducted through July 2017 comparing first-line and subsequent regimens containing chemotherapy, antiangiogenic therapy, and/or immune checkpoint inhibitors. Chemotherapy agents studied included cisplatin, carboplatin, oxaliplatin, gemcitabine, paclitaxel, docetaxel, and pemetrexed; antiangiogenic agents included bevacizumab, aflibercept, ramucirumab, nintedanib, axitinib, sorafenib, vandetanib, and sunitinib; and immune checkpoint inhibitors included ipilimumab, pembrolizumab, nivolumab, and atezolizumab.
Direct and indirect data for all grade 3-5 adverse events were combined using random-effects network meta-analysis.
“The results indicated that [checkpoint] inhibitors can be preferred choices for less toxicity to treat advanced stage NSCLC compared with antiangiogenic therapies in first-line and subsequent settings,” Dr. Hung and her associates concluded.
This study was supported by the China Medical University Beigang Hospital.
SOURCE: Hsu C et al. NCCN poster 13
ORLANDO – , a systematic review and meta-analysis suggests.
In 16,810 patients from 37 trials included in the analysis, first-line treatment with nivolumab or pembrolizumab, compared with first-line sorafenib plus platinum doublets, for example, was associated with less combined direct and indirect toxicity (odds ratios, 0.08 and 0.12, respectively), Chin-Chuan Hung, MD, and her colleagues reported in a poster at the annual conference of the National Comprehensive Cancer Network.
For subsequent therapy, nivolumab showed lower risk than most antiangiogenic therapies, particularly combination ramucirumab and docetaxel (OR, 0.06), said Dr. Hung of China Medical University Hospital in Taichung, Taiwan.
The findings are notable because tolerability is an essential selection criterion for patients with advanced stage disease, and while checkpoint inhibitors – including nivolumab, pembrolizumab, and atezolizumab – and antiangiogenic agents – including bevacizumab, ramucirumab, and nintedanib – have become the treatments of choice, direct comparisons with respect to tolerability are lacking, she noted.
The investigators performed a systematic review using Bayesian-model network meta-analysis of studies conducted through July 2017 comparing first-line and subsequent regimens containing chemotherapy, antiangiogenic therapy, and/or immune checkpoint inhibitors. Chemotherapy agents studied included cisplatin, carboplatin, oxaliplatin, gemcitabine, paclitaxel, docetaxel, and pemetrexed; antiangiogenic agents included bevacizumab, aflibercept, ramucirumab, nintedanib, axitinib, sorafenib, vandetanib, and sunitinib; and immune checkpoint inhibitors included ipilimumab, pembrolizumab, nivolumab, and atezolizumab.
Direct and indirect data for all grade 3-5 adverse events were combined using random-effects network meta-analysis.
“The results indicated that [checkpoint] inhibitors can be preferred choices for less toxicity to treat advanced stage NSCLC compared with antiangiogenic therapies in first-line and subsequent settings,” Dr. Hung and her associates concluded.
This study was supported by the China Medical University Beigang Hospital.
SOURCE: Hsu C et al. NCCN poster 13
REPORTING FROM THE NCCN ANNUAL CONFERENCE
Key clinical point: Checkpoint blockade appears less toxic than antiangiogenic therapies in advanced NSCLC
Major finding: Less toxicity was seen with first-line nivolumab or pembrolizumab vs. sorafenib + platinum doublets (odds ratios, 0.08 and 0.12, respectively).
Study details: A systematic review and meta-analysis of 37 trials involving 16,810 patients.
Disclosures: The study was supported by the China Medical University Beigang Hospital.
Source: Hsu C et al. NCCN poster 13.
Obesity in adults continues to rise
according to data from the National Health and Nutrition Examination Survey.
The age-standardized prevalence of obesity – defined as a body mass index of 30 or more – among adults aged 20 years and over increased from 33.7% for the 2-year period of 2007-2008 to 39.6% in 2015-2016, while the prevalence of severe obesity – defined as a body mass index of 40 kg/m2 or more – went from 5.7% to 7.7% over that same period, Craig M. Hales, MD, and his associates at the Centers for Disease Control and Prevention in Hyattsville, Md., and Atlanta said in a research letter published in JAMA.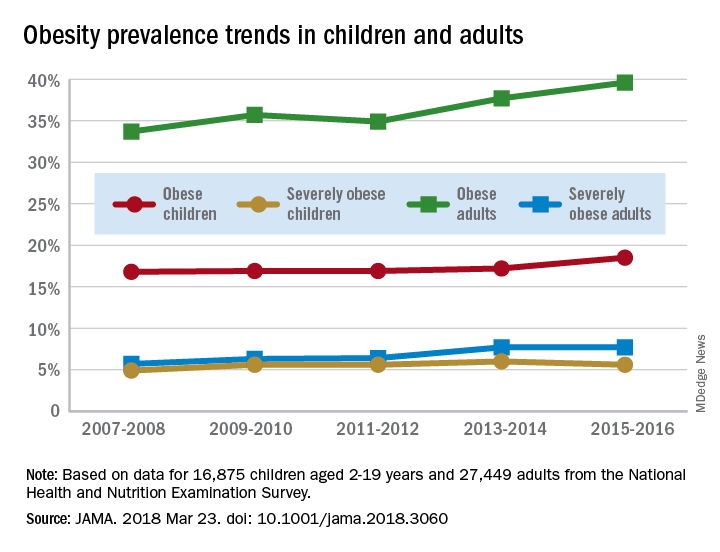
For the most recent reporting period, boys were more likely than girls to be obese (19.1% vs. 17.8%) and severely obese (6.3% vs. 4.9%), and both obesity and severe obesity were more common with increasing age. Obesity prevalence went from 13.9% in those aged 2-5 years to 20.6% in 12- to 19-year-olds, and severe obesity was 1.8% in the youngest group and 7.7% in the oldest, with the middle-age group (6-11 years) in the middle in both categories, they said
Among the adults, obesity was more common in women than men (41.1% vs. 37.9%) for 2015-2016, as was severe obesity (9.7% vs. 5.6%). Obesity and severe obesity were both highest in those aged 40-59 years, but obesity prevalence was lowest in the younger group (20-39 years) and severe obesity was least common in the older group (60 years and older), Dr. Hales and his associates said.
The analysis involved 16,875 children and 27,449 adults over the 10-year period. The investigators did not report any conflicts of interest.
AGA patient education materials can help your patients better understand how to manage and discuss obesity, including lifestyle, pharmacological and endoscopic treatment options. Learn more at www.gastro.org/patientInfo/topic/obesity.
SOURCE: Hales CM et al. JAMA 2018 Mar 23. doi: 10.1001/jama.2018.3060.
according to data from the National Health and Nutrition Examination Survey.
The age-standardized prevalence of obesity – defined as a body mass index of 30 or more – among adults aged 20 years and over increased from 33.7% for the 2-year period of 2007-2008 to 39.6% in 2015-2016, while the prevalence of severe obesity – defined as a body mass index of 40 kg/m2 or more – went from 5.7% to 7.7% over that same period, Craig M. Hales, MD, and his associates at the Centers for Disease Control and Prevention in Hyattsville, Md., and Atlanta said in a research letter published in JAMA.
For the most recent reporting period, boys were more likely than girls to be obese (19.1% vs. 17.8%) and severely obese (6.3% vs. 4.9%), and both obesity and severe obesity were more common with increasing age. Obesity prevalence went from 13.9% in those aged 2-5 years to 20.6% in 12- to 19-year-olds, and severe obesity was 1.8% in the youngest group and 7.7% in the oldest, with the middle-age group (6-11 years) in the middle in both categories, they said
Among the adults, obesity was more common in women than men (41.1% vs. 37.9%) for 2015-2016, as was severe obesity (9.7% vs. 5.6%). Obesity and severe obesity were both highest in those aged 40-59 years, but obesity prevalence was lowest in the younger group (20-39 years) and severe obesity was least common in the older group (60 years and older), Dr. Hales and his associates said.
The analysis involved 16,875 children and 27,449 adults over the 10-year period. The investigators did not report any conflicts of interest.
AGA patient education materials can help your patients better understand how to manage and discuss obesity, including lifestyle, pharmacological and endoscopic treatment options. Learn more at www.gastro.org/patientInfo/topic/obesity.
SOURCE: Hales CM et al. JAMA 2018 Mar 23. doi: 10.1001/jama.2018.3060.
according to data from the National Health and Nutrition Examination Survey.
The age-standardized prevalence of obesity – defined as a body mass index of 30 or more – among adults aged 20 years and over increased from 33.7% for the 2-year period of 2007-2008 to 39.6% in 2015-2016, while the prevalence of severe obesity – defined as a body mass index of 40 kg/m2 or more – went from 5.7% to 7.7% over that same period, Craig M. Hales, MD, and his associates at the Centers for Disease Control and Prevention in Hyattsville, Md., and Atlanta said in a research letter published in JAMA.
For the most recent reporting period, boys were more likely than girls to be obese (19.1% vs. 17.8%) and severely obese (6.3% vs. 4.9%), and both obesity and severe obesity were more common with increasing age. Obesity prevalence went from 13.9% in those aged 2-5 years to 20.6% in 12- to 19-year-olds, and severe obesity was 1.8% in the youngest group and 7.7% in the oldest, with the middle-age group (6-11 years) in the middle in both categories, they said
Among the adults, obesity was more common in women than men (41.1% vs. 37.9%) for 2015-2016, as was severe obesity (9.7% vs. 5.6%). Obesity and severe obesity were both highest in those aged 40-59 years, but obesity prevalence was lowest in the younger group (20-39 years) and severe obesity was least common in the older group (60 years and older), Dr. Hales and his associates said.
The analysis involved 16,875 children and 27,449 adults over the 10-year period. The investigators did not report any conflicts of interest.
AGA patient education materials can help your patients better understand how to manage and discuss obesity, including lifestyle, pharmacological and endoscopic treatment options. Learn more at www.gastro.org/patientInfo/topic/obesity.
SOURCE: Hales CM et al. JAMA 2018 Mar 23. doi: 10.1001/jama.2018.3060.
FROM JAMA
Greater Occipital Nerve Block Proves Effective
Greater occipital nerve (GON) block seems to be an effective option for acute management of migraine headache, with promising reductions in pain scores, a recent study found. This retrospective cohort study was undertaken between January 2009 and August 2014 and included patients who underwent at least 1 GON block and attended at least 1 follow-up appointment. Change in the 11-point numeric pain rating scale (NPRS) was used to assess the response to GON block. A total of 562 patients met inclusion criteria; 423 were women (75%); mean age was 58.6 ± 16.7 years. Response was defined as “minimal” (less than 30% NPRS point reduction), “moderate” (31% to 50% NPRS point reduction), or “significant” ( greater than 50% NPRS point reduction). Researchers found:
- Of total patients, 459 (82%) rated their response to GON block as moderate or significant.
- No statistically significant relationship existed between previous treatment regimens and response to GON block.
- GON block was equally effective across the different age and sex groups.
Greater occipital nerve block for acute treatment of migraine headache: A large retrospective cohort study. J Am Board Fam Med. 2018;31(2):211-218. doi:10.3122/jabfm.2018.02.170188.
Greater occipital nerve (GON) block seems to be an effective option for acute management of migraine headache, with promising reductions in pain scores, a recent study found. This retrospective cohort study was undertaken between January 2009 and August 2014 and included patients who underwent at least 1 GON block and attended at least 1 follow-up appointment. Change in the 11-point numeric pain rating scale (NPRS) was used to assess the response to GON block. A total of 562 patients met inclusion criteria; 423 were women (75%); mean age was 58.6 ± 16.7 years. Response was defined as “minimal” (less than 30% NPRS point reduction), “moderate” (31% to 50% NPRS point reduction), or “significant” ( greater than 50% NPRS point reduction). Researchers found:
- Of total patients, 459 (82%) rated their response to GON block as moderate or significant.
- No statistically significant relationship existed between previous treatment regimens and response to GON block.
- GON block was equally effective across the different age and sex groups.
Greater occipital nerve block for acute treatment of migraine headache: A large retrospective cohort study. J Am Board Fam Med. 2018;31(2):211-218. doi:10.3122/jabfm.2018.02.170188.
Greater occipital nerve (GON) block seems to be an effective option for acute management of migraine headache, with promising reductions in pain scores, a recent study found. This retrospective cohort study was undertaken between January 2009 and August 2014 and included patients who underwent at least 1 GON block and attended at least 1 follow-up appointment. Change in the 11-point numeric pain rating scale (NPRS) was used to assess the response to GON block. A total of 562 patients met inclusion criteria; 423 were women (75%); mean age was 58.6 ± 16.7 years. Response was defined as “minimal” (less than 30% NPRS point reduction), “moderate” (31% to 50% NPRS point reduction), or “significant” ( greater than 50% NPRS point reduction). Researchers found:
- Of total patients, 459 (82%) rated their response to GON block as moderate or significant.
- No statistically significant relationship existed between previous treatment regimens and response to GON block.
- GON block was equally effective across the different age and sex groups.
Greater occipital nerve block for acute treatment of migraine headache: A large retrospective cohort study. J Am Board Fam Med. 2018;31(2):211-218. doi:10.3122/jabfm.2018.02.170188.
Brain Diffusion Abnormalities in Children Examined
A recent study identifies early cerebral diffusion changes in children with tension-type and migraine-type headaches compared with controls. The hypothesized mechanisms of nociception in migraine-type and tension-type headaches may explain the findings as a precursor to structural changes seen in adult patients with chronic headache. Patients evaluated for tension-type or migraine-type headache without aura from May 2014 to July 2016 in a single center were retrospectively reviewed. Thirty-two patients with tension-type headache and 23 with migraine-type headache at an average of 4 months after diagnosis were enrolled. All patients underwent diffusion weighted imaging at 3T before the start of pharmacotherapy. Researchers found:
- There were no significant differences in regional brain volumes between the groups.
- Patients with tension-type and migraine-type headaches showed significantly increased apparent diffusion coefficient (ADC) in the hippocampus and brain stem compared with controls.
- Additionally, only patients with migraine-type headache showed significantly increased ADC in the thalamus and a trend toward increased ADC in the amygdala compared with controls.
Brain diffusion abnormalities in children with tension-type and migraine-type headaches. [Published online ahead of print March 15, 2018]. AJNR Am J Neuroradiol. doi:10.3174/ajnr.A5582.
A recent study identifies early cerebral diffusion changes in children with tension-type and migraine-type headaches compared with controls. The hypothesized mechanisms of nociception in migraine-type and tension-type headaches may explain the findings as a precursor to structural changes seen in adult patients with chronic headache. Patients evaluated for tension-type or migraine-type headache without aura from May 2014 to July 2016 in a single center were retrospectively reviewed. Thirty-two patients with tension-type headache and 23 with migraine-type headache at an average of 4 months after diagnosis were enrolled. All patients underwent diffusion weighted imaging at 3T before the start of pharmacotherapy. Researchers found:
- There were no significant differences in regional brain volumes between the groups.
- Patients with tension-type and migraine-type headaches showed significantly increased apparent diffusion coefficient (ADC) in the hippocampus and brain stem compared with controls.
- Additionally, only patients with migraine-type headache showed significantly increased ADC in the thalamus and a trend toward increased ADC in the amygdala compared with controls.
Brain diffusion abnormalities in children with tension-type and migraine-type headaches. [Published online ahead of print March 15, 2018]. AJNR Am J Neuroradiol. doi:10.3174/ajnr.A5582.
A recent study identifies early cerebral diffusion changes in children with tension-type and migraine-type headaches compared with controls. The hypothesized mechanisms of nociception in migraine-type and tension-type headaches may explain the findings as a precursor to structural changes seen in adult patients with chronic headache. Patients evaluated for tension-type or migraine-type headache without aura from May 2014 to July 2016 in a single center were retrospectively reviewed. Thirty-two patients with tension-type headache and 23 with migraine-type headache at an average of 4 months after diagnosis were enrolled. All patients underwent diffusion weighted imaging at 3T before the start of pharmacotherapy. Researchers found:
- There were no significant differences in regional brain volumes between the groups.
- Patients with tension-type and migraine-type headaches showed significantly increased apparent diffusion coefficient (ADC) in the hippocampus and brain stem compared with controls.
- Additionally, only patients with migraine-type headache showed significantly increased ADC in the thalamus and a trend toward increased ADC in the amygdala compared with controls.
Brain diffusion abnormalities in children with tension-type and migraine-type headaches. [Published online ahead of print March 15, 2018]. AJNR Am J Neuroradiol. doi:10.3174/ajnr.A5582.
Migraine Linked with Otolaryngologic Symptoms
Migraine disease has a higher prevalence in an otolaryngologic cohort than in the general population, according to a recent study, presenting with a high rate of sinonasal and otologic symptoms that may be due to or exacerbated by migraines. In a cross-sectional study utilizing the CHEER (Creating Healthcare Excellence through Education and Research) network, patients were recruited in 14 CHEER sites between June 2015 and March 2017. Those included were aged 18 years or older and had been seen for any concern that was not head and neck cancer. Patients with any history of brain abnormality or headaches that began within 2 weeks of a medical illness, trauma, or head injury were excluded. If they screened positive on the Migraine Assessment Tool (MAT+), the subjects also filled out questionnaires for sinonasal, otologic, and migraine-specific symptoms. Researchers found:
- Of 1458 patients screened, 235 (16.1%) screened positive for migraine (MAT+), which is higher than the general population (13%).
- The MAT+ group was significantly younger (47.2 vs 55.6 years of age) and predominantly women (80.0% vs 55.9%).
Patterns of migraine disease in otolaryngology: A CHEER network study. [Published online ahead of print March 20, 2018]. Otolaryngol Head Neck Surg. doi:10.1177/0194599818764387.
Migraine disease has a higher prevalence in an otolaryngologic cohort than in the general population, according to a recent study, presenting with a high rate of sinonasal and otologic symptoms that may be due to or exacerbated by migraines. In a cross-sectional study utilizing the CHEER (Creating Healthcare Excellence through Education and Research) network, patients were recruited in 14 CHEER sites between June 2015 and March 2017. Those included were aged 18 years or older and had been seen for any concern that was not head and neck cancer. Patients with any history of brain abnormality or headaches that began within 2 weeks of a medical illness, trauma, or head injury were excluded. If they screened positive on the Migraine Assessment Tool (MAT+), the subjects also filled out questionnaires for sinonasal, otologic, and migraine-specific symptoms. Researchers found:
- Of 1458 patients screened, 235 (16.1%) screened positive for migraine (MAT+), which is higher than the general population (13%).
- The MAT+ group was significantly younger (47.2 vs 55.6 years of age) and predominantly women (80.0% vs 55.9%).
Patterns of migraine disease in otolaryngology: A CHEER network study. [Published online ahead of print March 20, 2018]. Otolaryngol Head Neck Surg. doi:10.1177/0194599818764387.
Migraine disease has a higher prevalence in an otolaryngologic cohort than in the general population, according to a recent study, presenting with a high rate of sinonasal and otologic symptoms that may be due to or exacerbated by migraines. In a cross-sectional study utilizing the CHEER (Creating Healthcare Excellence through Education and Research) network, patients were recruited in 14 CHEER sites between June 2015 and March 2017. Those included were aged 18 years or older and had been seen for any concern that was not head and neck cancer. Patients with any history of brain abnormality or headaches that began within 2 weeks of a medical illness, trauma, or head injury were excluded. If they screened positive on the Migraine Assessment Tool (MAT+), the subjects also filled out questionnaires for sinonasal, otologic, and migraine-specific symptoms. Researchers found:
- Of 1458 patients screened, 235 (16.1%) screened positive for migraine (MAT+), which is higher than the general population (13%).
- The MAT+ group was significantly younger (47.2 vs 55.6 years of age) and predominantly women (80.0% vs 55.9%).
Patterns of migraine disease in otolaryngology: A CHEER network study. [Published online ahead of print March 20, 2018]. Otolaryngol Head Neck Surg. doi:10.1177/0194599818764387.