User login
GDM, subsequent diabetes predictive of later renal damage
Women with a history of gestational diabetes mellitus who later develop diabetes had an elevated urinary albumin-to-creatinine ratio an average of 13 years later, indicating renal damage, according to a new study.
Those with a history of gestational diabetes mellitus (GDM) – but no subsequent development of diabetes – did not show an increased urinary albumin-to-creatinine ratio (UACR) but did have a higher estimated glomerular filtration rate (eGFR), hinting at early stages of glomerular hyperfiltration and renal damage.
“Our findings suggest that in women with a history of GDM, deterioration of renal function may potentially precede the development of overt diabetes, although clinically relevant outcomes such as elevated UACR may manifest only after progression to diabetes,” wrote Shristi Rawal, PhD, a postdoctoral fellow at the National Institute of Child Health and Human Development and her associates.
“These findings suggest that women with GDM-complicated pregnancies may represent a high-risk group that could benefit from regular monitoring for early-stage renal damage, timely detection of which may help clinicians initiate treatment to prevent or delay further disease progression,” they wrote.
The investigators compared outcomes among 1,226 Danish women 9-16 years after their index pregnancy during 1996-2002; a predominantly white study population, which limited the study’s generalizability to other demographic groups, the authors acknowledged. A total of 607 women had had GDM during their first pregnancy, 183 of whom developed type 1 or 2 diabetes. Of the 619 women who did not have GDM, 9 developed diabetes.
Serum creatinine and urinary albumin and creatinine measurements were taken to determine eGFR and UACR. Women with a previous GDM diagnosis had higher eGFR and UACR than women without previous GDM. The higher eGFR remained significant after adjustments for age at first pregnancy, completion of high school, smoking during pregnancy, a family history of diabetes, prepregnancy hypertension, and prepregnancy body mass index (BMI). UACR differences were not significant after adjustment.
Women with GDM and subsequent diabetes had a significantly higher UACR than women without either and had more than twice the risk of an elevated UACR of at least 20 mg/g (adjusted relative risk, 2.3), even after confounder adjustment.
The association with increased UACR was significant with the combination of a GDM history and a subsequent diabetes diagnosis, but not individually. The increased eGFR, however, remained significant after adjustment even for women with only a history of GDM, regardless of whether they later developed diabetes or even prediabetes.
“The independent association of GDM with eGFR also remained significant when we excluded women with conditions that might influence renal function markers at follow-up, including type 1 diabetes, preeclampsia/eclampsia or any hypertension complication during the index pregnancy, regular use of cholesterol-lowering drugs, or recent use of ACE inhibitors, diuretics, or H2 blockers,” the authors reported.
“Furthermore, no effect modification was observed when we stratified the analyses by clinical and lifestyle characteristics at follow-up, including current BMI, smoking, antihypertension medication use, family history of diabetes, physical activity, and median time since index pregnancy. Associations in some strata became statistically insignificant due to reduced sample size [all P for interaction = .05],” they wrote.
The research was funded by the National Institute of Child Health and Human Development at the National Institutes of Health, the Innovation Fund Denmark, March of Dimes Birth Defects Foundation, Health Foundation, Heart Foundation and European Union.
Coauthor Allan Vaag, MD, PhD is a vice president at AstraZeneca. No other authors had disclosures.
SOURCE: Rawal S et al. Diabetes Care. 2018 May 4. doi: 10.2337/dc17-2629.
Women with a history of gestational diabetes mellitus who later develop diabetes had an elevated urinary albumin-to-creatinine ratio an average of 13 years later, indicating renal damage, according to a new study.
Those with a history of gestational diabetes mellitus (GDM) – but no subsequent development of diabetes – did not show an increased urinary albumin-to-creatinine ratio (UACR) but did have a higher estimated glomerular filtration rate (eGFR), hinting at early stages of glomerular hyperfiltration and renal damage.
“Our findings suggest that in women with a history of GDM, deterioration of renal function may potentially precede the development of overt diabetes, although clinically relevant outcomes such as elevated UACR may manifest only after progression to diabetes,” wrote Shristi Rawal, PhD, a postdoctoral fellow at the National Institute of Child Health and Human Development and her associates.
“These findings suggest that women with GDM-complicated pregnancies may represent a high-risk group that could benefit from regular monitoring for early-stage renal damage, timely detection of which may help clinicians initiate treatment to prevent or delay further disease progression,” they wrote.
The investigators compared outcomes among 1,226 Danish women 9-16 years after their index pregnancy during 1996-2002; a predominantly white study population, which limited the study’s generalizability to other demographic groups, the authors acknowledged. A total of 607 women had had GDM during their first pregnancy, 183 of whom developed type 1 or 2 diabetes. Of the 619 women who did not have GDM, 9 developed diabetes.
Serum creatinine and urinary albumin and creatinine measurements were taken to determine eGFR and UACR. Women with a previous GDM diagnosis had higher eGFR and UACR than women without previous GDM. The higher eGFR remained significant after adjustments for age at first pregnancy, completion of high school, smoking during pregnancy, a family history of diabetes, prepregnancy hypertension, and prepregnancy body mass index (BMI). UACR differences were not significant after adjustment.
Women with GDM and subsequent diabetes had a significantly higher UACR than women without either and had more than twice the risk of an elevated UACR of at least 20 mg/g (adjusted relative risk, 2.3), even after confounder adjustment.
The association with increased UACR was significant with the combination of a GDM history and a subsequent diabetes diagnosis, but not individually. The increased eGFR, however, remained significant after adjustment even for women with only a history of GDM, regardless of whether they later developed diabetes or even prediabetes.
“The independent association of GDM with eGFR also remained significant when we excluded women with conditions that might influence renal function markers at follow-up, including type 1 diabetes, preeclampsia/eclampsia or any hypertension complication during the index pregnancy, regular use of cholesterol-lowering drugs, or recent use of ACE inhibitors, diuretics, or H2 blockers,” the authors reported.
“Furthermore, no effect modification was observed when we stratified the analyses by clinical and lifestyle characteristics at follow-up, including current BMI, smoking, antihypertension medication use, family history of diabetes, physical activity, and median time since index pregnancy. Associations in some strata became statistically insignificant due to reduced sample size [all P for interaction = .05],” they wrote.
The research was funded by the National Institute of Child Health and Human Development at the National Institutes of Health, the Innovation Fund Denmark, March of Dimes Birth Defects Foundation, Health Foundation, Heart Foundation and European Union.
Coauthor Allan Vaag, MD, PhD is a vice president at AstraZeneca. No other authors had disclosures.
SOURCE: Rawal S et al. Diabetes Care. 2018 May 4. doi: 10.2337/dc17-2629.
Women with a history of gestational diabetes mellitus who later develop diabetes had an elevated urinary albumin-to-creatinine ratio an average of 13 years later, indicating renal damage, according to a new study.
Those with a history of gestational diabetes mellitus (GDM) – but no subsequent development of diabetes – did not show an increased urinary albumin-to-creatinine ratio (UACR) but did have a higher estimated glomerular filtration rate (eGFR), hinting at early stages of glomerular hyperfiltration and renal damage.
“Our findings suggest that in women with a history of GDM, deterioration of renal function may potentially precede the development of overt diabetes, although clinically relevant outcomes such as elevated UACR may manifest only after progression to diabetes,” wrote Shristi Rawal, PhD, a postdoctoral fellow at the National Institute of Child Health and Human Development and her associates.
“These findings suggest that women with GDM-complicated pregnancies may represent a high-risk group that could benefit from regular monitoring for early-stage renal damage, timely detection of which may help clinicians initiate treatment to prevent or delay further disease progression,” they wrote.
The investigators compared outcomes among 1,226 Danish women 9-16 years after their index pregnancy during 1996-2002; a predominantly white study population, which limited the study’s generalizability to other demographic groups, the authors acknowledged. A total of 607 women had had GDM during their first pregnancy, 183 of whom developed type 1 or 2 diabetes. Of the 619 women who did not have GDM, 9 developed diabetes.
Serum creatinine and urinary albumin and creatinine measurements were taken to determine eGFR and UACR. Women with a previous GDM diagnosis had higher eGFR and UACR than women without previous GDM. The higher eGFR remained significant after adjustments for age at first pregnancy, completion of high school, smoking during pregnancy, a family history of diabetes, prepregnancy hypertension, and prepregnancy body mass index (BMI). UACR differences were not significant after adjustment.
Women with GDM and subsequent diabetes had a significantly higher UACR than women without either and had more than twice the risk of an elevated UACR of at least 20 mg/g (adjusted relative risk, 2.3), even after confounder adjustment.
The association with increased UACR was significant with the combination of a GDM history and a subsequent diabetes diagnosis, but not individually. The increased eGFR, however, remained significant after adjustment even for women with only a history of GDM, regardless of whether they later developed diabetes or even prediabetes.
“The independent association of GDM with eGFR also remained significant when we excluded women with conditions that might influence renal function markers at follow-up, including type 1 diabetes, preeclampsia/eclampsia or any hypertension complication during the index pregnancy, regular use of cholesterol-lowering drugs, or recent use of ACE inhibitors, diuretics, or H2 blockers,” the authors reported.
“Furthermore, no effect modification was observed when we stratified the analyses by clinical and lifestyle characteristics at follow-up, including current BMI, smoking, antihypertension medication use, family history of diabetes, physical activity, and median time since index pregnancy. Associations in some strata became statistically insignificant due to reduced sample size [all P for interaction = .05],” they wrote.
The research was funded by the National Institute of Child Health and Human Development at the National Institutes of Health, the Innovation Fund Denmark, March of Dimes Birth Defects Foundation, Health Foundation, Heart Foundation and European Union.
Coauthor Allan Vaag, MD, PhD is a vice president at AstraZeneca. No other authors had disclosures.
SOURCE: Rawal S et al. Diabetes Care. 2018 May 4. doi: 10.2337/dc17-2629.
FROM DIABETES CARE
Key clinical point: Gestational diabetes mellitus may be a risk factor for future development of renal damage.
Major finding: Women with previous GDM and a subsequent diagnosis of diabetes were over twice as likely to show evidence of existing renal damage.
Data source: The findings are based on 9-16 years of prospective follow-up of 607 women with and 619 women without a history of GDM.
Disclosures: The research was funded by the National Institute of Child Health and Human Development at the National Institutes of Health, the Innovation Fund Denmark, March of Dimes Birth Defects Foundation, Health Foundation, Heart Foundation and European Union. Coauthor Allan Vaag is an employee of AstraZeneca. No other authors had disclosures.
Source: Rawal S et al. Diabetes Care. 2018 May 4. doi: 10.2337/dc17-2629.
Magnetic Resonance Imaging Evaluation of the Distal Biceps Tendon
ABSTRACT
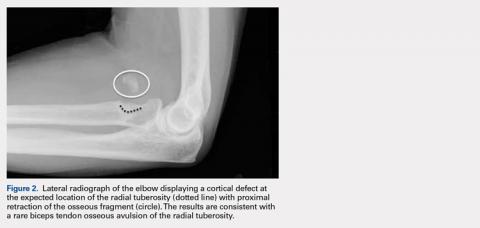
Injuries to the distal biceps occur at the tendinous insertion at the radial tuberosity. Distal biceps injuries range from tendinosis to partial tears to non-retracted and retracted complete tears. Acute and chronic complete tears result from a tendinous avulsion at the radial tuberosity. Acute tears result from a strong force exerted on an eccentric biceps contraction, leading to tendon injury.
Distal biceps tendon injuries are uncommon (1.2 per 100,000 patients in one study).1 An underlying degenerative component is involved in all distal biceps tendon tears and tendinosis.2 Partial tears can be caused by the same mechanism or by no particular inciting event.3 Magnetic resonance imaging (MRI) is the optimal imaging modality for distal tendon tears because of its excellent specificity and sensitivity in the detection of complete tears.4,5 Imaging also accurately diagnoses and characterizes partial tears and tendinosis.5 On MRI, fast spin-echo intermediate-weighted and T2-weighted or short tau inversion recovery (STIR) sequences are normally obtained to assess tendon integrity. Along with standard axial and sagittal views, the FABS (flexed elbow, abducted shoulder, supinated forearm) view is an important tool in the diagnosis of distal biceps tendon tears.6 The FABS view is obtained with the patient prone with the shoulder abducted 180° (above the head), with the elbow flexed to 90°, and the forearm supinated. This position allows a longitudinal view of along the entire length of the distal tendon.
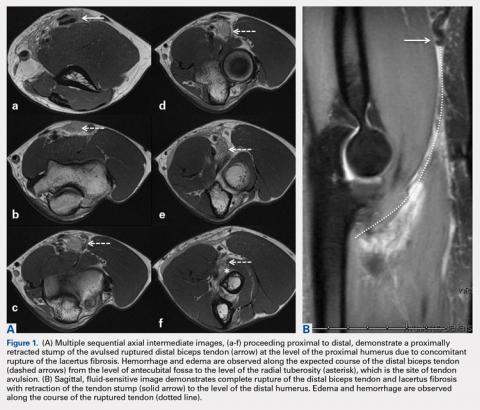
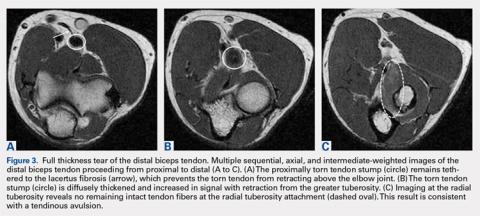
Complete distal biceps tears can usually be diagnosed by history and physical examinations. However, imaging can be helpful when intact brachialis function can compensate for a completely torn tendon. MRI is also useful in the setting of a complete tear to locate the torn tendon stump, and assess the degree of retraction for tendon retrieval7,8 and quality of the tendon stump for repair. For associated rupture of the lacertus, the degree of proximal tendon retraction can be significant (Figures 1A, 1B).
Continue to: Partial distal bicep tears...
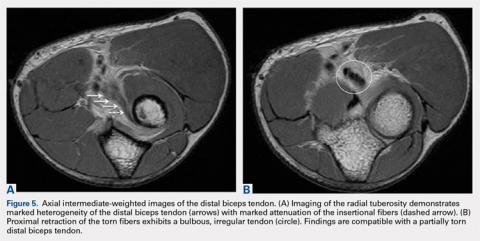
Partial distal bicep tears are characterized on MRI by focal or partial detachment of the tendon at the radial tuberosity with fluid filling the site of the tear. The degree of partial tearing can be assessed on MRI (Figures 5A, 5B).
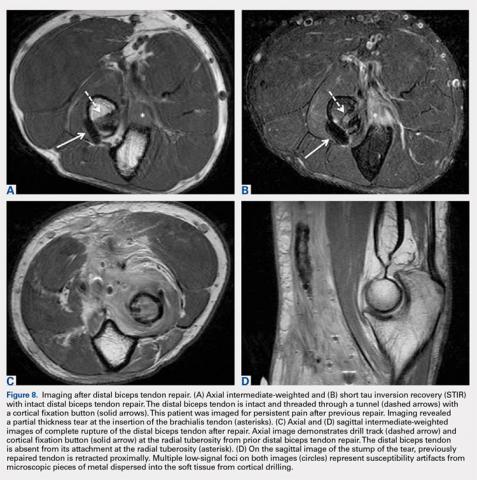
MRI is useful in assessing the distal biceps tendon in the postoperative setting to evaluate the integrity of a repaired tendon. Cortical fixation button technique for repair creates minimal susceptibility artifacts on MRI. Postoperative MRI typically demonstrates a transverse hole drilled through the proximal radius at the site of the tuberosity with a cortical fixation button flush against the posterior radial cortex (Figures 8A-8D).
1. Safran M, Graham S. Distal biceps tendon ruptures. Clin Orthop Relat Res. 2002;404:275-283.
2. Kannus P, Józsa L. Histopathological changes preceding spontaneous rupture of a tendon. A controlled study of 891 patients. J Bone Joint Surg Am. 1991;73(10):1507-1525. doi:10.2106/00004623-199173100-00009.
3. Frazier M, Boardman M, Westland M, Imbriglia J. Surgical treatment of partial distal biceps tendon ruptures. J Hand Surg Am. 2010;35(7):1111-1114. doi:10.1016/j.jhsa.2010.04.024.
4. Festa A, Mulieri P, Newman J, Spitz D, Leslie B. Effectiveness of magnetic resonance imaging in detecting partial and complete distal biceps tendon rupture. J Hand Surg Am. 2010;35(1):77-83. doi:10.1016/j.jhsa.2009.08.016.
5. O'Driscoll S, Goncalves L, Dietz P. The hook test for distal biceps tendon avulsion. Am J Sports Med. 2007;35(11):1865-1869. doi:10.1177/0363546507305016.
6. Giuffrè B, Moss M. Optimal positioning for MRI of the distal biceps brachii tendon: flexed abducted supinated view. Am J Roentgenol. 2004;182(4):944-946. doi:10.2214/ajr.182.4.1820944.
7. Falchook F, Zlatkin M, Erbacher G, Moulton J, Bisset G. Murphy B. Rupture of the distal biceps tendon: evaluation with MR imaging. Radiology. 1994;190(3):659-663. doi:10.1148/radiology.190.3.8115606.
8. Fitzgerald S, Curry D, Erickson S, Quinn S, Friedman H. Distal biceps tendon injury: MR imaging diagnosis. Radiology. 1994;191(1):203-206. doi:10.1148/radiology.191.1.8134571.
9. Lehuec J, Zipoli B, Liquois F, Moinard M, Chauveaux D, Le Rebeller A. Distal rupture of the biceps tendon MRI evaluation and surgical repair. J Shoulder Elbow Surg. 1996;5(2):S49.
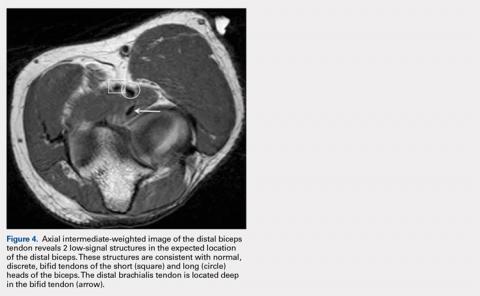
10. Dirim B, Brouha S, Pretterklieber M, et al. Terminal bifurcation of the biceps brachii muscle and tendon: anatomic considerations and clinical implications. Am J Roentgenol. 2008;191(6):W248-W255. doi:10.2214/AJR.08.1048.
11. Quach T, Jazayeri R, Sherman O, Rosen J. Distal biceps tendon injuries--current treatment options. Bull NYU Hosp Jt Dis. 2010;68(2):103-111.
ABSTRACT
Injuries to the distal biceps occur at the tendinous insertion at the radial tuberosity. Distal biceps injuries range from tendinosis to partial tears to non-retracted and retracted complete tears. Acute and chronic complete tears result from a tendinous avulsion at the radial tuberosity. Acute tears result from a strong force exerted on an eccentric biceps contraction, leading to tendon injury.
Distal biceps tendon injuries are uncommon (1.2 per 100,000 patients in one study).1 An underlying degenerative component is involved in all distal biceps tendon tears and tendinosis.2 Partial tears can be caused by the same mechanism or by no particular inciting event.3 Magnetic resonance imaging (MRI) is the optimal imaging modality for distal tendon tears because of its excellent specificity and sensitivity in the detection of complete tears.4,5 Imaging also accurately diagnoses and characterizes partial tears and tendinosis.5 On MRI, fast spin-echo intermediate-weighted and T2-weighted or short tau inversion recovery (STIR) sequences are normally obtained to assess tendon integrity. Along with standard axial and sagittal views, the FABS (flexed elbow, abducted shoulder, supinated forearm) view is an important tool in the diagnosis of distal biceps tendon tears.6 The FABS view is obtained with the patient prone with the shoulder abducted 180° (above the head), with the elbow flexed to 90°, and the forearm supinated. This position allows a longitudinal view of along the entire length of the distal tendon.
Complete distal biceps tears can usually be diagnosed by history and physical examinations. However, imaging can be helpful when intact brachialis function can compensate for a completely torn tendon. MRI is also useful in the setting of a complete tear to locate the torn tendon stump, and assess the degree of retraction for tendon retrieval7,8 and quality of the tendon stump for repair. For associated rupture of the lacertus, the degree of proximal tendon retraction can be significant (Figures 1A, 1B).
Continue to: Partial distal bicep tears...
Partial distal bicep tears are characterized on MRI by focal or partial detachment of the tendon at the radial tuberosity with fluid filling the site of the tear. The degree of partial tearing can be assessed on MRI (Figures 5A, 5B).
MRI is useful in assessing the distal biceps tendon in the postoperative setting to evaluate the integrity of a repaired tendon. Cortical fixation button technique for repair creates minimal susceptibility artifacts on MRI. Postoperative MRI typically demonstrates a transverse hole drilled through the proximal radius at the site of the tuberosity with a cortical fixation button flush against the posterior radial cortex (Figures 8A-8D).
ABSTRACT
Injuries to the distal biceps occur at the tendinous insertion at the radial tuberosity. Distal biceps injuries range from tendinosis to partial tears to non-retracted and retracted complete tears. Acute and chronic complete tears result from a tendinous avulsion at the radial tuberosity. Acute tears result from a strong force exerted on an eccentric biceps contraction, leading to tendon injury.
Distal biceps tendon injuries are uncommon (1.2 per 100,000 patients in one study).1 An underlying degenerative component is involved in all distal biceps tendon tears and tendinosis.2 Partial tears can be caused by the same mechanism or by no particular inciting event.3 Magnetic resonance imaging (MRI) is the optimal imaging modality for distal tendon tears because of its excellent specificity and sensitivity in the detection of complete tears.4,5 Imaging also accurately diagnoses and characterizes partial tears and tendinosis.5 On MRI, fast spin-echo intermediate-weighted and T2-weighted or short tau inversion recovery (STIR) sequences are normally obtained to assess tendon integrity. Along with standard axial and sagittal views, the FABS (flexed elbow, abducted shoulder, supinated forearm) view is an important tool in the diagnosis of distal biceps tendon tears.6 The FABS view is obtained with the patient prone with the shoulder abducted 180° (above the head), with the elbow flexed to 90°, and the forearm supinated. This position allows a longitudinal view of along the entire length of the distal tendon.
Complete distal biceps tears can usually be diagnosed by history and physical examinations. However, imaging can be helpful when intact brachialis function can compensate for a completely torn tendon. MRI is also useful in the setting of a complete tear to locate the torn tendon stump, and assess the degree of retraction for tendon retrieval7,8 and quality of the tendon stump for repair. For associated rupture of the lacertus, the degree of proximal tendon retraction can be significant (Figures 1A, 1B).
Continue to: Partial distal bicep tears...
Partial distal bicep tears are characterized on MRI by focal or partial detachment of the tendon at the radial tuberosity with fluid filling the site of the tear. The degree of partial tearing can be assessed on MRI (Figures 5A, 5B).
MRI is useful in assessing the distal biceps tendon in the postoperative setting to evaluate the integrity of a repaired tendon. Cortical fixation button technique for repair creates minimal susceptibility artifacts on MRI. Postoperative MRI typically demonstrates a transverse hole drilled through the proximal radius at the site of the tuberosity with a cortical fixation button flush against the posterior radial cortex (Figures 8A-8D).
1. Safran M, Graham S. Distal biceps tendon ruptures. Clin Orthop Relat Res. 2002;404:275-283.
2. Kannus P, Józsa L. Histopathological changes preceding spontaneous rupture of a tendon. A controlled study of 891 patients. J Bone Joint Surg Am. 1991;73(10):1507-1525. doi:10.2106/00004623-199173100-00009.
3. Frazier M, Boardman M, Westland M, Imbriglia J. Surgical treatment of partial distal biceps tendon ruptures. J Hand Surg Am. 2010;35(7):1111-1114. doi:10.1016/j.jhsa.2010.04.024.
4. Festa A, Mulieri P, Newman J, Spitz D, Leslie B. Effectiveness of magnetic resonance imaging in detecting partial and complete distal biceps tendon rupture. J Hand Surg Am. 2010;35(1):77-83. doi:10.1016/j.jhsa.2009.08.016.
5. O'Driscoll S, Goncalves L, Dietz P. The hook test for distal biceps tendon avulsion. Am J Sports Med. 2007;35(11):1865-1869. doi:10.1177/0363546507305016.
6. Giuffrè B, Moss M. Optimal positioning for MRI of the distal biceps brachii tendon: flexed abducted supinated view. Am J Roentgenol. 2004;182(4):944-946. doi:10.2214/ajr.182.4.1820944.
7. Falchook F, Zlatkin M, Erbacher G, Moulton J, Bisset G. Murphy B. Rupture of the distal biceps tendon: evaluation with MR imaging. Radiology. 1994;190(3):659-663. doi:10.1148/radiology.190.3.8115606.
8. Fitzgerald S, Curry D, Erickson S, Quinn S, Friedman H. Distal biceps tendon injury: MR imaging diagnosis. Radiology. 1994;191(1):203-206. doi:10.1148/radiology.191.1.8134571.
9. Lehuec J, Zipoli B, Liquois F, Moinard M, Chauveaux D, Le Rebeller A. Distal rupture of the biceps tendon MRI evaluation and surgical repair. J Shoulder Elbow Surg. 1996;5(2):S49.
10. Dirim B, Brouha S, Pretterklieber M, et al. Terminal bifurcation of the biceps brachii muscle and tendon: anatomic considerations and clinical implications. Am J Roentgenol. 2008;191(6):W248-W255. doi:10.2214/AJR.08.1048.
11. Quach T, Jazayeri R, Sherman O, Rosen J. Distal biceps tendon injuries--current treatment options. Bull NYU Hosp Jt Dis. 2010;68(2):103-111.
1. Safran M, Graham S. Distal biceps tendon ruptures. Clin Orthop Relat Res. 2002;404:275-283.
2. Kannus P, Józsa L. Histopathological changes preceding spontaneous rupture of a tendon. A controlled study of 891 patients. J Bone Joint Surg Am. 1991;73(10):1507-1525. doi:10.2106/00004623-199173100-00009.
3. Frazier M, Boardman M, Westland M, Imbriglia J. Surgical treatment of partial distal biceps tendon ruptures. J Hand Surg Am. 2010;35(7):1111-1114. doi:10.1016/j.jhsa.2010.04.024.
4. Festa A, Mulieri P, Newman J, Spitz D, Leslie B. Effectiveness of magnetic resonance imaging in detecting partial and complete distal biceps tendon rupture. J Hand Surg Am. 2010;35(1):77-83. doi:10.1016/j.jhsa.2009.08.016.
5. O'Driscoll S, Goncalves L, Dietz P. The hook test for distal biceps tendon avulsion. Am J Sports Med. 2007;35(11):1865-1869. doi:10.1177/0363546507305016.
6. Giuffrè B, Moss M. Optimal positioning for MRI of the distal biceps brachii tendon: flexed abducted supinated view. Am J Roentgenol. 2004;182(4):944-946. doi:10.2214/ajr.182.4.1820944.
7. Falchook F, Zlatkin M, Erbacher G, Moulton J, Bisset G. Murphy B. Rupture of the distal biceps tendon: evaluation with MR imaging. Radiology. 1994;190(3):659-663. doi:10.1148/radiology.190.3.8115606.
8. Fitzgerald S, Curry D, Erickson S, Quinn S, Friedman H. Distal biceps tendon injury: MR imaging diagnosis. Radiology. 1994;191(1):203-206. doi:10.1148/radiology.191.1.8134571.
9. Lehuec J, Zipoli B, Liquois F, Moinard M, Chauveaux D, Le Rebeller A. Distal rupture of the biceps tendon MRI evaluation and surgical repair. J Shoulder Elbow Surg. 1996;5(2):S49.
10. Dirim B, Brouha S, Pretterklieber M, et al. Terminal bifurcation of the biceps brachii muscle and tendon: anatomic considerations and clinical implications. Am J Roentgenol. 2008;191(6):W248-W255. doi:10.2214/AJR.08.1048.
11. Quach T, Jazayeri R, Sherman O, Rosen J. Distal biceps tendon injuries--current treatment options. Bull NYU Hosp Jt Dis. 2010;68(2):103-111.
TAKE-HOME POINTS
- There are a variety of injuries to the distal biceps tendon.
- Injuries vary from tendinosis to full thickness, retracted tears.
- The degree of retraction of full thickness tears depends on the integrity of the lacertus fibrosis.
- The FABS view allows for MRI of the entire length of the distal biceps tendon.
- MRI is the most useful imaging modality to determine the integrity of the postoperative biceps tendon.
FDA recalls HeartMate 3 LV assist device
The HeartMate 3 left ventricular assist device received a class 1 recall from the Food and Drug Administration on May 17, an action the agency publicly announced on May 22.
The FDA’s step formalized a “corrective action” that the HeartMate 3’s manufacturer, Abbott, first issued in early April and then announced in early May, which said the device used to treat patients with severe advanced heart failure was subject to “outflow graft twist occlusions” that trigger a persistent low-flow alarm and “can result in serious adverse events such as hemodynamic compromise, thrombus, and death,” according to the FDA’s May 17 statement.
The class 1 recall category the FDA applied means the agency rates the danger posed as a “situation where there is a reasonable chance that a product will cause serious health problems or death.” However, in its statements, the agency has not suggested removing a well-functioning device from patients, nor has the agency called for discontinuing new placements of the HeartMate 3. The FDA gave a full endorsement to the approach Abbott suggested in its April 5 “Dear Physician” letter and then followed with a second letter on May 21.
The first letter attributed development of these twists in the tube that directs blood out from the device to accumulated mechanical forces from heartbeats, respiration, and activity, and also provided some management guidance that Abbott then expanded in its second letter.
The FDA cited some of the key steps Abbott recommended clinicians take with patients who receive a HeartMate 3. This included regular surveillance with transthoracic echocardiography, although echo is not considered definitive for identifying a graft twist obstruction and hence other investigations may also be needed. If the low-flow alarm sounds, a CT scan is “urgently” needed – as long as it’s not contraindicated – to identify a possible outflow twist. Abbott noted that surgical intervention may be needed to correct a twist.
Researchers recently reported 2-year follow-up data from 257 patients in MOMENTUM 3, the randomized pivotal trial for the HeartMate 3 that compared this fully magnetically levitated centrifugal-flow pump to the prior-generation, axial-flow pump. The composite endpoint of freedom from death, stroke, or need for repeat surgery after 2 years was 80% in the HeartMate 3 recipients and 60% among patients in the control arm who received the older-model device (N Engl J Med. 2018 April 12; 378[15]:1386-95).
The HeartMate 3 left ventricular assist device received a class 1 recall from the Food and Drug Administration on May 17, an action the agency publicly announced on May 22.
The FDA’s step formalized a “corrective action” that the HeartMate 3’s manufacturer, Abbott, first issued in early April and then announced in early May, which said the device used to treat patients with severe advanced heart failure was subject to “outflow graft twist occlusions” that trigger a persistent low-flow alarm and “can result in serious adverse events such as hemodynamic compromise, thrombus, and death,” according to the FDA’s May 17 statement.
The class 1 recall category the FDA applied means the agency rates the danger posed as a “situation where there is a reasonable chance that a product will cause serious health problems or death.” However, in its statements, the agency has not suggested removing a well-functioning device from patients, nor has the agency called for discontinuing new placements of the HeartMate 3. The FDA gave a full endorsement to the approach Abbott suggested in its April 5 “Dear Physician” letter and then followed with a second letter on May 21.
The first letter attributed development of these twists in the tube that directs blood out from the device to accumulated mechanical forces from heartbeats, respiration, and activity, and also provided some management guidance that Abbott then expanded in its second letter.
The FDA cited some of the key steps Abbott recommended clinicians take with patients who receive a HeartMate 3. This included regular surveillance with transthoracic echocardiography, although echo is not considered definitive for identifying a graft twist obstruction and hence other investigations may also be needed. If the low-flow alarm sounds, a CT scan is “urgently” needed – as long as it’s not contraindicated – to identify a possible outflow twist. Abbott noted that surgical intervention may be needed to correct a twist.
Researchers recently reported 2-year follow-up data from 257 patients in MOMENTUM 3, the randomized pivotal trial for the HeartMate 3 that compared this fully magnetically levitated centrifugal-flow pump to the prior-generation, axial-flow pump. The composite endpoint of freedom from death, stroke, or need for repeat surgery after 2 years was 80% in the HeartMate 3 recipients and 60% among patients in the control arm who received the older-model device (N Engl J Med. 2018 April 12; 378[15]:1386-95).
The HeartMate 3 left ventricular assist device received a class 1 recall from the Food and Drug Administration on May 17, an action the agency publicly announced on May 22.
The FDA’s step formalized a “corrective action” that the HeartMate 3’s manufacturer, Abbott, first issued in early April and then announced in early May, which said the device used to treat patients with severe advanced heart failure was subject to “outflow graft twist occlusions” that trigger a persistent low-flow alarm and “can result in serious adverse events such as hemodynamic compromise, thrombus, and death,” according to the FDA’s May 17 statement.
The class 1 recall category the FDA applied means the agency rates the danger posed as a “situation where there is a reasonable chance that a product will cause serious health problems or death.” However, in its statements, the agency has not suggested removing a well-functioning device from patients, nor has the agency called for discontinuing new placements of the HeartMate 3. The FDA gave a full endorsement to the approach Abbott suggested in its April 5 “Dear Physician” letter and then followed with a second letter on May 21.
The first letter attributed development of these twists in the tube that directs blood out from the device to accumulated mechanical forces from heartbeats, respiration, and activity, and also provided some management guidance that Abbott then expanded in its second letter.
The FDA cited some of the key steps Abbott recommended clinicians take with patients who receive a HeartMate 3. This included regular surveillance with transthoracic echocardiography, although echo is not considered definitive for identifying a graft twist obstruction and hence other investigations may also be needed. If the low-flow alarm sounds, a CT scan is “urgently” needed – as long as it’s not contraindicated – to identify a possible outflow twist. Abbott noted that surgical intervention may be needed to correct a twist.
Researchers recently reported 2-year follow-up data from 257 patients in MOMENTUM 3, the randomized pivotal trial for the HeartMate 3 that compared this fully magnetically levitated centrifugal-flow pump to the prior-generation, axial-flow pump. The composite endpoint of freedom from death, stroke, or need for repeat surgery after 2 years was 80% in the HeartMate 3 recipients and 60% among patients in the control arm who received the older-model device (N Engl J Med. 2018 April 12; 378[15]:1386-95).
Drugmakers blamed for blocking generics have cost U.S. billions
Makers of brand-name drugs called out by the Trump administration for potentially stalling generic competition have hiked their prices by double-digit percentages since 2012 and cost Medicare and Medicaid nearly $12 billion in 2016, a Kaiser Health News analysis has found.
As part of President Donald Trump’s promise to curb high drug prices, the Food and Drug Administration posted a list of pharmaceutical companies that makers of generics allege refused to let them buy the drug samples needed to develop their products. For approval, the FDA requires so-called bioequivalence testing using samples to demonstrate that generics are the same as their branded counterparts.
The analysis shows that drug companies that may have engaged in what FDA Commissioner Scott Gottlieb, MD, called “shenanigans” to delay the entrance of cheaper competitors onto the market have indeed raised prices and cost taxpayers more money over time.
The FDA listed more than 50 drugs whose manufacturers have withheld or refused to sell samples and cited 164 inquiries for help obtaining them. Thirteen of these pleas from makers of generics pertained to Celgene’s blockbuster cancer drug Revlimid (lenalidomide), which accounted for 63% of Celgene’s revenue in the first quarter of 2018, according to a company press release.
The brand-name drug companies “wouldn’t put so much effort into fighting off competition if these weren’t [such] lucrative sources of revenue,” said Ameet Sarpatwari, JD, PhD, of Harvard Medical School in Boston. “In the case of a blockbuster drug, that can be hundreds of millions of dollars of revenue for the brand-name drugs and almost the same cost to the health care system.”
Indeed, a KHN analysis found that 47 of the drugs cost Medicare and Medicaid almost $12 billion in 2016. The spending totals don’t include rebates, which drugmakers return to the government after paying for the drugs upfront but are not public. The rebates ranged from 9.5% to 26.3% for Medicare Part D in 2014, the most recent year that data are available.
The remaining drugs do not appear in the Medicare and Medicaid data.
By delaying development of generics, drugmakers can maintain their monopolies and keep prices high. Most of the drugs cost Medicare Part D more in 2016 than they did in 2012, for an average spending increase of about 60% more per unit. This excludes drugs that don’t appear in the 2012 Medicare Part D data.
Revlimid cost Medicare Part D $2.7 billion in 2016, trailing only Harvoni (ledipasvir and sofosbuvir), which treats hepatitis C and is not on the FDA’s new list. The cost of Revlimid, which faces no competition from generics, has jumped 40% per unit in just 4 years, the Medicare data show, and cost $75,200/beneficiary in 2016.
Some drugs on the FDA’s list, including Celgene’s, are part of a safety program that can require restricted distribution of brand-name drugs that have serious risks or addictive qualities. Drugmakers with products in the safety program sometimes say they can’t provide samples unless the generics manufacturer jumps through a series of hoops “that generic companies find hard or impossible to comply with,” Dr. Gottlieb said in a statement.
The Department of Health & Human Services Office of Inspector General issued a report in 2013 that said the FDA couldn’t prove that the program actually improved safety, and Dr. Sarpatwari said there’s evidence drugmakers are abusing it to stave off competition from generics.
Dr. Gottlieb said the FDA will be notifying the Federal Trade Commission about pleas for help from would-be generics manufacturers about obtaining samples, and he encouraged the manufacturers to do the same if they suspect they’re being thwarted by anticompetitive practices.
Celgene spokesman Greg Geissman said the company has sold samples to generics manufacturers and will continue to do so. He stressed maintaining a balance of innovation, generic competition, and safety.
“Even a single dose of thalidomide, the active ingredient in Thalomid, can cause irreversible, debilitating birth defects if not properly handled and dispensed. Revlimid and Pomalyst (pomalidomide) are believed to have similar risks,” Mr. Geissman said.
The highest number of pleas for help related to Actelion Pharmaceuticals’ pulmonary hypertension drug Tracleer (bosentan). In 2016, that drug cost Medicare $90,700/patient and more than $304 million overall. Meanwhile, spending per unit jumped 52% from 2012 through 2016.
Actelion was acquired by Johnson & Johnson’s pharmaceutical arm, Janssen, in 2017.
Actelion spokeswoman Colleen Wilson said that the company “cooperate[s]” with makers of generic drugs and “has responded to all requests it has received directly from generic manufacturers seeking access to its medications for bioequivalence testing.”
PhRMA, the trade group for makers of brand-name pharmaceuticals, said the FDA’s list was somewhat unfair because it lacked context and responses from those it represents.
“While we must continue to foster a competitive marketplace, PhRMA is concerned that FDA’s release of the ‘inquiries’ it has received lacks proper context and conflates a number of divergent scenarios,” said PhRMA spokesman Andrew Powaleny.
Congress is considering the CREATES Act, which stands for “Creating and Restoring Equal Access to Equivalent Samples” and would foster competition in part by allowing generics manufacturers to sue brand-name drug manufacturers to compel them to provide samples.
The bill’s sponsor, Sen. Patrick Leahy (D-Vt.), said more transparency from the FDA is helpful, but more work from the agency is needed to end the anticompetitive tactics. “With billions of dollars at stake, a database alone will not stop this behavior,” Sen. Leahy said.
Cosponsor Sen. Chuck Grassley (R-Iowa), chairman of the Judiciary Committee, expressed similar sentiments, telling KHN: “The CREATES Act is necessary because it would serve as a strong deterrent to pharmaceutical companies that engage in anticompetitive practices to keep low-cost generic drugs off the market.”
The FDA hasn’t come out in support of CREATES. “They should know that this is going to require a legislative solution,” Dr. Sarpatwari said. “Why are they not stepping into this arena and saying that?”
KHN’s coverage of prescription drug development, costs and pricing is supported by the Laura and John Arnold Foundation. Kaiser Health News (hyperlink to khn.org) is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Makers of brand-name drugs called out by the Trump administration for potentially stalling generic competition have hiked their prices by double-digit percentages since 2012 and cost Medicare and Medicaid nearly $12 billion in 2016, a Kaiser Health News analysis has found.
As part of President Donald Trump’s promise to curb high drug prices, the Food and Drug Administration posted a list of pharmaceutical companies that makers of generics allege refused to let them buy the drug samples needed to develop their products. For approval, the FDA requires so-called bioequivalence testing using samples to demonstrate that generics are the same as their branded counterparts.
The analysis shows that drug companies that may have engaged in what FDA Commissioner Scott Gottlieb, MD, called “shenanigans” to delay the entrance of cheaper competitors onto the market have indeed raised prices and cost taxpayers more money over time.
The FDA listed more than 50 drugs whose manufacturers have withheld or refused to sell samples and cited 164 inquiries for help obtaining them. Thirteen of these pleas from makers of generics pertained to Celgene’s blockbuster cancer drug Revlimid (lenalidomide), which accounted for 63% of Celgene’s revenue in the first quarter of 2018, according to a company press release.
The brand-name drug companies “wouldn’t put so much effort into fighting off competition if these weren’t [such] lucrative sources of revenue,” said Ameet Sarpatwari, JD, PhD, of Harvard Medical School in Boston. “In the case of a blockbuster drug, that can be hundreds of millions of dollars of revenue for the brand-name drugs and almost the same cost to the health care system.”
Indeed, a KHN analysis found that 47 of the drugs cost Medicare and Medicaid almost $12 billion in 2016. The spending totals don’t include rebates, which drugmakers return to the government after paying for the drugs upfront but are not public. The rebates ranged from 9.5% to 26.3% for Medicare Part D in 2014, the most recent year that data are available.
The remaining drugs do not appear in the Medicare and Medicaid data.
By delaying development of generics, drugmakers can maintain their monopolies and keep prices high. Most of the drugs cost Medicare Part D more in 2016 than they did in 2012, for an average spending increase of about 60% more per unit. This excludes drugs that don’t appear in the 2012 Medicare Part D data.
Revlimid cost Medicare Part D $2.7 billion in 2016, trailing only Harvoni (ledipasvir and sofosbuvir), which treats hepatitis C and is not on the FDA’s new list. The cost of Revlimid, which faces no competition from generics, has jumped 40% per unit in just 4 years, the Medicare data show, and cost $75,200/beneficiary in 2016.
Some drugs on the FDA’s list, including Celgene’s, are part of a safety program that can require restricted distribution of brand-name drugs that have serious risks or addictive qualities. Drugmakers with products in the safety program sometimes say they can’t provide samples unless the generics manufacturer jumps through a series of hoops “that generic companies find hard or impossible to comply with,” Dr. Gottlieb said in a statement.
The Department of Health & Human Services Office of Inspector General issued a report in 2013 that said the FDA couldn’t prove that the program actually improved safety, and Dr. Sarpatwari said there’s evidence drugmakers are abusing it to stave off competition from generics.
Dr. Gottlieb said the FDA will be notifying the Federal Trade Commission about pleas for help from would-be generics manufacturers about obtaining samples, and he encouraged the manufacturers to do the same if they suspect they’re being thwarted by anticompetitive practices.
Celgene spokesman Greg Geissman said the company has sold samples to generics manufacturers and will continue to do so. He stressed maintaining a balance of innovation, generic competition, and safety.
“Even a single dose of thalidomide, the active ingredient in Thalomid, can cause irreversible, debilitating birth defects if not properly handled and dispensed. Revlimid and Pomalyst (pomalidomide) are believed to have similar risks,” Mr. Geissman said.
The highest number of pleas for help related to Actelion Pharmaceuticals’ pulmonary hypertension drug Tracleer (bosentan). In 2016, that drug cost Medicare $90,700/patient and more than $304 million overall. Meanwhile, spending per unit jumped 52% from 2012 through 2016.
Actelion was acquired by Johnson & Johnson’s pharmaceutical arm, Janssen, in 2017.
Actelion spokeswoman Colleen Wilson said that the company “cooperate[s]” with makers of generic drugs and “has responded to all requests it has received directly from generic manufacturers seeking access to its medications for bioequivalence testing.”
PhRMA, the trade group for makers of brand-name pharmaceuticals, said the FDA’s list was somewhat unfair because it lacked context and responses from those it represents.
“While we must continue to foster a competitive marketplace, PhRMA is concerned that FDA’s release of the ‘inquiries’ it has received lacks proper context and conflates a number of divergent scenarios,” said PhRMA spokesman Andrew Powaleny.
Congress is considering the CREATES Act, which stands for “Creating and Restoring Equal Access to Equivalent Samples” and would foster competition in part by allowing generics manufacturers to sue brand-name drug manufacturers to compel them to provide samples.
The bill’s sponsor, Sen. Patrick Leahy (D-Vt.), said more transparency from the FDA is helpful, but more work from the agency is needed to end the anticompetitive tactics. “With billions of dollars at stake, a database alone will not stop this behavior,” Sen. Leahy said.
Cosponsor Sen. Chuck Grassley (R-Iowa), chairman of the Judiciary Committee, expressed similar sentiments, telling KHN: “The CREATES Act is necessary because it would serve as a strong deterrent to pharmaceutical companies that engage in anticompetitive practices to keep low-cost generic drugs off the market.”
The FDA hasn’t come out in support of CREATES. “They should know that this is going to require a legislative solution,” Dr. Sarpatwari said. “Why are they not stepping into this arena and saying that?”
KHN’s coverage of prescription drug development, costs and pricing is supported by the Laura and John Arnold Foundation. Kaiser Health News (hyperlink to khn.org) is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Makers of brand-name drugs called out by the Trump administration for potentially stalling generic competition have hiked their prices by double-digit percentages since 2012 and cost Medicare and Medicaid nearly $12 billion in 2016, a Kaiser Health News analysis has found.
As part of President Donald Trump’s promise to curb high drug prices, the Food and Drug Administration posted a list of pharmaceutical companies that makers of generics allege refused to let them buy the drug samples needed to develop their products. For approval, the FDA requires so-called bioequivalence testing using samples to demonstrate that generics are the same as their branded counterparts.
The analysis shows that drug companies that may have engaged in what FDA Commissioner Scott Gottlieb, MD, called “shenanigans” to delay the entrance of cheaper competitors onto the market have indeed raised prices and cost taxpayers more money over time.
The FDA listed more than 50 drugs whose manufacturers have withheld or refused to sell samples and cited 164 inquiries for help obtaining them. Thirteen of these pleas from makers of generics pertained to Celgene’s blockbuster cancer drug Revlimid (lenalidomide), which accounted for 63% of Celgene’s revenue in the first quarter of 2018, according to a company press release.
The brand-name drug companies “wouldn’t put so much effort into fighting off competition if these weren’t [such] lucrative sources of revenue,” said Ameet Sarpatwari, JD, PhD, of Harvard Medical School in Boston. “In the case of a blockbuster drug, that can be hundreds of millions of dollars of revenue for the brand-name drugs and almost the same cost to the health care system.”
Indeed, a KHN analysis found that 47 of the drugs cost Medicare and Medicaid almost $12 billion in 2016. The spending totals don’t include rebates, which drugmakers return to the government after paying for the drugs upfront but are not public. The rebates ranged from 9.5% to 26.3% for Medicare Part D in 2014, the most recent year that data are available.
The remaining drugs do not appear in the Medicare and Medicaid data.
By delaying development of generics, drugmakers can maintain their monopolies and keep prices high. Most of the drugs cost Medicare Part D more in 2016 than they did in 2012, for an average spending increase of about 60% more per unit. This excludes drugs that don’t appear in the 2012 Medicare Part D data.
Revlimid cost Medicare Part D $2.7 billion in 2016, trailing only Harvoni (ledipasvir and sofosbuvir), which treats hepatitis C and is not on the FDA’s new list. The cost of Revlimid, which faces no competition from generics, has jumped 40% per unit in just 4 years, the Medicare data show, and cost $75,200/beneficiary in 2016.
Some drugs on the FDA’s list, including Celgene’s, are part of a safety program that can require restricted distribution of brand-name drugs that have serious risks or addictive qualities. Drugmakers with products in the safety program sometimes say they can’t provide samples unless the generics manufacturer jumps through a series of hoops “that generic companies find hard or impossible to comply with,” Dr. Gottlieb said in a statement.
The Department of Health & Human Services Office of Inspector General issued a report in 2013 that said the FDA couldn’t prove that the program actually improved safety, and Dr. Sarpatwari said there’s evidence drugmakers are abusing it to stave off competition from generics.
Dr. Gottlieb said the FDA will be notifying the Federal Trade Commission about pleas for help from would-be generics manufacturers about obtaining samples, and he encouraged the manufacturers to do the same if they suspect they’re being thwarted by anticompetitive practices.
Celgene spokesman Greg Geissman said the company has sold samples to generics manufacturers and will continue to do so. He stressed maintaining a balance of innovation, generic competition, and safety.
“Even a single dose of thalidomide, the active ingredient in Thalomid, can cause irreversible, debilitating birth defects if not properly handled and dispensed. Revlimid and Pomalyst (pomalidomide) are believed to have similar risks,” Mr. Geissman said.
The highest number of pleas for help related to Actelion Pharmaceuticals’ pulmonary hypertension drug Tracleer (bosentan). In 2016, that drug cost Medicare $90,700/patient and more than $304 million overall. Meanwhile, spending per unit jumped 52% from 2012 through 2016.
Actelion was acquired by Johnson & Johnson’s pharmaceutical arm, Janssen, in 2017.
Actelion spokeswoman Colleen Wilson said that the company “cooperate[s]” with makers of generic drugs and “has responded to all requests it has received directly from generic manufacturers seeking access to its medications for bioequivalence testing.”
PhRMA, the trade group for makers of brand-name pharmaceuticals, said the FDA’s list was somewhat unfair because it lacked context and responses from those it represents.
“While we must continue to foster a competitive marketplace, PhRMA is concerned that FDA’s release of the ‘inquiries’ it has received lacks proper context and conflates a number of divergent scenarios,” said PhRMA spokesman Andrew Powaleny.
Congress is considering the CREATES Act, which stands for “Creating and Restoring Equal Access to Equivalent Samples” and would foster competition in part by allowing generics manufacturers to sue brand-name drug manufacturers to compel them to provide samples.
The bill’s sponsor, Sen. Patrick Leahy (D-Vt.), said more transparency from the FDA is helpful, but more work from the agency is needed to end the anticompetitive tactics. “With billions of dollars at stake, a database alone will not stop this behavior,” Sen. Leahy said.
Cosponsor Sen. Chuck Grassley (R-Iowa), chairman of the Judiciary Committee, expressed similar sentiments, telling KHN: “The CREATES Act is necessary because it would serve as a strong deterrent to pharmaceutical companies that engage in anticompetitive practices to keep low-cost generic drugs off the market.”
The FDA hasn’t come out in support of CREATES. “They should know that this is going to require a legislative solution,” Dr. Sarpatwari said. “Why are they not stepping into this arena and saying that?”
KHN’s coverage of prescription drug development, costs and pricing is supported by the Laura and John Arnold Foundation. Kaiser Health News (hyperlink to khn.org) is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Bismuth subgallate cuts stool smell after duodenal switch
SEATTLE – Bismuth subgallate (Devrom) is a big help with an embarrassing and underappreciated problem after loop duodenal switch: smelly flatulence and stool.
Bismuth subgallate, an over the counter product that’s been on the market for decades, has been primarily studied to eliminate the odor of flatulence and bowel movements in ostomates, according to Walter Medlin, MD, a surgeon at the Bariatric Medicine Institute in Salt Lake City.
“A lot of patients have this complaint, but they tend not to talk to their physicians about it,” Dr. Medlin said at the World Congress of Endoscopic Surgery hosted by SAGES & CAGS.
Enter bismuth subgallate. Dr. Medlin and his team randomized 36 LDS patients to 200-mg capsules, two per meal, or to placebo for a week. Patients then underwent a one-week washout period, then crossed over to bismuth subgallate or placebo for another week. Patients and surgeons were blinded to treatment groups.
Subjects were at least 6 months out from LDS to give their gut a chance to adapt to the surgery. Patients with GI infections and those on confounding medications were among those excluded from the study. The mean age of the patients was 48 years, and there were slightly more women than men.
Subjects filled out the Gastrointestinal Quality of Life Index questionnaire at baseline and after both treatment periods. The index assesses digestive symptoms, physical status, emotional status, social performance, and treatment effects. Additional measures were added: Patients rated stool smell, flatulence smell, and concerns about each on a 4-point scale.
Twenty-nine patients completed the study; five were lost to follow-up, and two withdrew. With bismuth subgallate, scores improved by about 1.5 points on all four questions about stool and flatulence odor.
“Most of these patients had complaints of ‘all the time’ or ‘very frequent’ odor issues, and this really takes [those complaints] down to ‘occasional’ or ‘rare.’ It’s a pretty big change,” Dr. Medlin said.
Total Gastrointestinal Quality of Life Index scores improved as well, from a mean at baseline of 93 points up to 109 points out of a possible score of 160 points. Scores on the digestive portion improved from 49 to 60 points. Bismuth subgallate outperformed placebo significantly on both measures. There were trends toward improvement in other domains as well.
Stools darkened in one patient, and the tongue darkened in another; both are well-known side effects. There were no drug toxicities.
The study “is an important contribution. Duodenal switch is the most effective [bariatric] operation we do, but a lot of patients aren’t utilizing it because of this concern [about flatulence smell],” said the moderator of Dr. Medlin’s presentation, John Morton, MD, chief of bariatric and minimally invasive surgery at Stanford (Calif.) University.
Perhaps the biggest problem with bismuth subgallate is getting a hold of it, as Dr. Medlin and others noted. It’s not sold in stores but can be purchased online, including from its maker Parthenon at about $14 for a hundred capsules. The product is also available as a chewable.
The product probably helps by blocking bacterial breakdown of food residues in the colon, among other actions. “It really is an intestinal deodorant. I find patients are interested in having access to this tool” and might not need as much as in the trial, said Dr. Medlin, who stocks it in his office.
The study was funded by an unrestricted education grant from Parthenon. The investigators had no relevant disclosures.
SOURCE: Zaveri H et al. SAGES 2018, Abstract S028.
SEATTLE – Bismuth subgallate (Devrom) is a big help with an embarrassing and underappreciated problem after loop duodenal switch: smelly flatulence and stool.
Bismuth subgallate, an over the counter product that’s been on the market for decades, has been primarily studied to eliminate the odor of flatulence and bowel movements in ostomates, according to Walter Medlin, MD, a surgeon at the Bariatric Medicine Institute in Salt Lake City.
“A lot of patients have this complaint, but they tend not to talk to their physicians about it,” Dr. Medlin said at the World Congress of Endoscopic Surgery hosted by SAGES & CAGS.
Enter bismuth subgallate. Dr. Medlin and his team randomized 36 LDS patients to 200-mg capsules, two per meal, or to placebo for a week. Patients then underwent a one-week washout period, then crossed over to bismuth subgallate or placebo for another week. Patients and surgeons were blinded to treatment groups.
Subjects were at least 6 months out from LDS to give their gut a chance to adapt to the surgery. Patients with GI infections and those on confounding medications were among those excluded from the study. The mean age of the patients was 48 years, and there were slightly more women than men.
Subjects filled out the Gastrointestinal Quality of Life Index questionnaire at baseline and after both treatment periods. The index assesses digestive symptoms, physical status, emotional status, social performance, and treatment effects. Additional measures were added: Patients rated stool smell, flatulence smell, and concerns about each on a 4-point scale.
Twenty-nine patients completed the study; five were lost to follow-up, and two withdrew. With bismuth subgallate, scores improved by about 1.5 points on all four questions about stool and flatulence odor.
“Most of these patients had complaints of ‘all the time’ or ‘very frequent’ odor issues, and this really takes [those complaints] down to ‘occasional’ or ‘rare.’ It’s a pretty big change,” Dr. Medlin said.
Total Gastrointestinal Quality of Life Index scores improved as well, from a mean at baseline of 93 points up to 109 points out of a possible score of 160 points. Scores on the digestive portion improved from 49 to 60 points. Bismuth subgallate outperformed placebo significantly on both measures. There were trends toward improvement in other domains as well.
Stools darkened in one patient, and the tongue darkened in another; both are well-known side effects. There were no drug toxicities.
The study “is an important contribution. Duodenal switch is the most effective [bariatric] operation we do, but a lot of patients aren’t utilizing it because of this concern [about flatulence smell],” said the moderator of Dr. Medlin’s presentation, John Morton, MD, chief of bariatric and minimally invasive surgery at Stanford (Calif.) University.
Perhaps the biggest problem with bismuth subgallate is getting a hold of it, as Dr. Medlin and others noted. It’s not sold in stores but can be purchased online, including from its maker Parthenon at about $14 for a hundred capsules. The product is also available as a chewable.
The product probably helps by blocking bacterial breakdown of food residues in the colon, among other actions. “It really is an intestinal deodorant. I find patients are interested in having access to this tool” and might not need as much as in the trial, said Dr. Medlin, who stocks it in his office.
The study was funded by an unrestricted education grant from Parthenon. The investigators had no relevant disclosures.
SOURCE: Zaveri H et al. SAGES 2018, Abstract S028.
SEATTLE – Bismuth subgallate (Devrom) is a big help with an embarrassing and underappreciated problem after loop duodenal switch: smelly flatulence and stool.
Bismuth subgallate, an over the counter product that’s been on the market for decades, has been primarily studied to eliminate the odor of flatulence and bowel movements in ostomates, according to Walter Medlin, MD, a surgeon at the Bariatric Medicine Institute in Salt Lake City.
“A lot of patients have this complaint, but they tend not to talk to their physicians about it,” Dr. Medlin said at the World Congress of Endoscopic Surgery hosted by SAGES & CAGS.
Enter bismuth subgallate. Dr. Medlin and his team randomized 36 LDS patients to 200-mg capsules, two per meal, or to placebo for a week. Patients then underwent a one-week washout period, then crossed over to bismuth subgallate or placebo for another week. Patients and surgeons were blinded to treatment groups.
Subjects were at least 6 months out from LDS to give their gut a chance to adapt to the surgery. Patients with GI infections and those on confounding medications were among those excluded from the study. The mean age of the patients was 48 years, and there were slightly more women than men.
Subjects filled out the Gastrointestinal Quality of Life Index questionnaire at baseline and after both treatment periods. The index assesses digestive symptoms, physical status, emotional status, social performance, and treatment effects. Additional measures were added: Patients rated stool smell, flatulence smell, and concerns about each on a 4-point scale.
Twenty-nine patients completed the study; five were lost to follow-up, and two withdrew. With bismuth subgallate, scores improved by about 1.5 points on all four questions about stool and flatulence odor.
“Most of these patients had complaints of ‘all the time’ or ‘very frequent’ odor issues, and this really takes [those complaints] down to ‘occasional’ or ‘rare.’ It’s a pretty big change,” Dr. Medlin said.
Total Gastrointestinal Quality of Life Index scores improved as well, from a mean at baseline of 93 points up to 109 points out of a possible score of 160 points. Scores on the digestive portion improved from 49 to 60 points. Bismuth subgallate outperformed placebo significantly on both measures. There were trends toward improvement in other domains as well.
Stools darkened in one patient, and the tongue darkened in another; both are well-known side effects. There were no drug toxicities.
The study “is an important contribution. Duodenal switch is the most effective [bariatric] operation we do, but a lot of patients aren’t utilizing it because of this concern [about flatulence smell],” said the moderator of Dr. Medlin’s presentation, John Morton, MD, chief of bariatric and minimally invasive surgery at Stanford (Calif.) University.
Perhaps the biggest problem with bismuth subgallate is getting a hold of it, as Dr. Medlin and others noted. It’s not sold in stores but can be purchased online, including from its maker Parthenon at about $14 for a hundred capsules. The product is also available as a chewable.
The product probably helps by blocking bacterial breakdown of food residues in the colon, among other actions. “It really is an intestinal deodorant. I find patients are interested in having access to this tool” and might not need as much as in the trial, said Dr. Medlin, who stocks it in his office.
The study was funded by an unrestricted education grant from Parthenon. The investigators had no relevant disclosures.
SOURCE: Zaveri H et al. SAGES 2018, Abstract S028.
REPORTING FROM SAGES 2018
Key clinical point: Bismuth subgallate (Devrom) is a big help for an embarrassing and underappreciated problem after loop duodenal switch: stool odor.
Major finding: Patients reported about a 1.5-point improvement on 4-point scales rating stool and flatulence odor and their concerns about them.
Study details: Randomized, placebo-controlled trial with 36 patients
Disclosures: The investigators reported an unrestricted educational grant from Parthenon, the makers of the tested product.
Source: Zaveri H et al. SAGES 2018, Abstract S028.
Clinical Trial: Study looks at GI tract recovery after hernia repair
A randomized, multi-center, double-blinded trial is underway to study or placebo.
One group will be given a 12-mg dose of alvimopan 30-90 minutes before the scheduled start of surgery on Day 0, and twice daily beginning on postoperative Day 1 after nasogastric tube (NGT) removal until hospital discharge or for a maximum of 7 days (up to 15 doses). The other group will be given a similarly colored 12-mg placebo capsule 30-90 minutes on the same schedule.
Primary outcomes will include the length of time (hrs/days) for recovery of the GI tract, measured by time to first flatus and time to first bowel movement, both measured twice daily. In addition, toleration of diet and oral pain medication will be measured. Variables such as NGT insertion, diet restriction or reductions, episodes of emesis, bloating, and pain will be recorded twice daily.
Secondary outcomes include length of hospital stay, 30-day morbidity and hospital readmission. Postoperative pain scores will be obtained during the hospital stay, and 2 weeks and 30 days postoperatively and the Hernia-Related Quality-of-Life Survey (HerQLes) will also be administered postoperatively.
The study is sponsored by the Medical College of Wisconsin in collaboration with Merck Sharp and Dohme Corp.
Find more information on the “Gastrointestinal Tract Recovery in Patients Undergoing Open Ventral Hernia Repair (NCT02379858),” at www.clincaltrials.gov.
A randomized, multi-center, double-blinded trial is underway to study or placebo.
One group will be given a 12-mg dose of alvimopan 30-90 minutes before the scheduled start of surgery on Day 0, and twice daily beginning on postoperative Day 1 after nasogastric tube (NGT) removal until hospital discharge or for a maximum of 7 days (up to 15 doses). The other group will be given a similarly colored 12-mg placebo capsule 30-90 minutes on the same schedule.
Primary outcomes will include the length of time (hrs/days) for recovery of the GI tract, measured by time to first flatus and time to first bowel movement, both measured twice daily. In addition, toleration of diet and oral pain medication will be measured. Variables such as NGT insertion, diet restriction or reductions, episodes of emesis, bloating, and pain will be recorded twice daily.
Secondary outcomes include length of hospital stay, 30-day morbidity and hospital readmission. Postoperative pain scores will be obtained during the hospital stay, and 2 weeks and 30 days postoperatively and the Hernia-Related Quality-of-Life Survey (HerQLes) will also be administered postoperatively.
The study is sponsored by the Medical College of Wisconsin in collaboration with Merck Sharp and Dohme Corp.
Find more information on the “Gastrointestinal Tract Recovery in Patients Undergoing Open Ventral Hernia Repair (NCT02379858),” at www.clincaltrials.gov.
A randomized, multi-center, double-blinded trial is underway to study or placebo.
One group will be given a 12-mg dose of alvimopan 30-90 minutes before the scheduled start of surgery on Day 0, and twice daily beginning on postoperative Day 1 after nasogastric tube (NGT) removal until hospital discharge or for a maximum of 7 days (up to 15 doses). The other group will be given a similarly colored 12-mg placebo capsule 30-90 minutes on the same schedule.
Primary outcomes will include the length of time (hrs/days) for recovery of the GI tract, measured by time to first flatus and time to first bowel movement, both measured twice daily. In addition, toleration of diet and oral pain medication will be measured. Variables such as NGT insertion, diet restriction or reductions, episodes of emesis, bloating, and pain will be recorded twice daily.
Secondary outcomes include length of hospital stay, 30-day morbidity and hospital readmission. Postoperative pain scores will be obtained during the hospital stay, and 2 weeks and 30 days postoperatively and the Hernia-Related Quality-of-Life Survey (HerQLes) will also be administered postoperatively.
The study is sponsored by the Medical College of Wisconsin in collaboration with Merck Sharp and Dohme Corp.
Find more information on the “Gastrointestinal Tract Recovery in Patients Undergoing Open Ventral Hernia Repair (NCT02379858),” at www.clincaltrials.gov.
FROM CLINICALTRIALS.COM
Psoriatic arthritis patients’ cardiovascular risks aren’t spurring increased management
CAMBRIDGE, MASS. – Patients with psoriatic arthritis (PsA) receive risk factor management and follow-up for atherosclerotic cardiovascular disease (ASCVD) that is similar to the population at large, according to research presented in a poster session at the annual meeting of the Spondyloarthritis Research and Treatment Network (SPARTAN).
The researchers found that patients with PsA in their single-center study did not receive more intensive management and follow-up despite having a higher risk score for ASCVD. This may be caused in part by the fact that there are no specific guidelines for ASCVD management in patients with PsA, according to study authors Linh Truong, MD, and Nicole Ridolfi, DO, internal medicine residents at the University of California, Irvine.
Even though this study and others have shown that patients with psoriatic arthritis have a higher risk for ASCVD, “currently, patients with PsA are being managed exactly the same way as everyone else,” Dr. Truong said.
In addition to assessing ASCVD morbidity in patients with PsA, Dr. Truong and Dr. Ridolfi also wanted to investigate how management of the known ASCVD risk in these patients in a primary care settings compared with national guidelines. Their research came in two parts. In the first part, they calculated 10-year ASCVD risk using a risk score estimator from the American Heart Association/American College of Cardiology. They then calculated an odds ratio for relevant atherosclerotic cardiovascular disease events, such as MI, heart failure, and cerebrovascular accidents. In the second part, they evaluated how the risk factors for ASCVD in patients with PsA were managed in the primary care setting.
The researchers compared data from a single center for 103 patients – 61 with PsA and 42 matching controls. Patients in both groups had an average age in the mid-60s, an average body mass index above 29 kg/m2, and were also mostly male (four patients with PsA were female) and mostly white.
Patients with PsA had an average ASCVD risk score of 21.2, compared with a score of 16.5 in the control arm (P less than .0001).
Of patients with psoriatic arthritis, 23 experienced an ASCVD event, compared with 10 patients in the control arm (OR, 1.93; 95% confidence interval, 0.80-4.66). Patients with PsA appeared to experience increased risk for MI (OR, 2.5), heart failure (OR, 7.09), and cerebrovascular accident (OR, 1.96). These increased risks had confidence intervals that approached statistical significance, but did not achieve it.
“We believe that because these data approach [statistical] significance, that further studies are needed,” Dr. Ridolfi said. “Our own validation studies are currently underway.”
In part two of the study, yearly ASCVD risk outcomes were averaged over 5 years and then evaluated based on the frequency of primary care visits, lab checks for HbA1c and lipid profile, and the use of cardioprotective ancillary referrals. The researchers also investigated statin and aspirin use in primary and secondary atherosclerotic cardiovascular disease per guidelines from the American College of Cardiology/American Heart Association and the United States Preventive Services Task Force.
Patients in both groups had similar primary care visits, HbA1c and lipid profiles, and use of cardioprotective supplements (such as fish oil and niacin). However, 36% of patients in the control arm received a nonpharmacologic ancillary referral, compared with just 11% of patients with PsA. Those referrals were in regard to weight loss and diabetes and dietary education, according to the research.
Dr. Truong and Dr. Ridolfi reported that, for primary prevention of ASCVD, PsA patients received treatment less often than did the controls with aspirin (0% vs. 26%, respectively) and statins (40% vs. 50%); there was also less use of statins for secondary prevention (73% vs. 85%).
“These data show that there is an educational opportunity in the primary care setting,” Dr. Truong said. “Or that there is an argument to be made that PsA should be managed by a rheumatology specialist.”
Dr. Truong and Dr. Ridolfi reported no disclosures.
CAMBRIDGE, MASS. – Patients with psoriatic arthritis (PsA) receive risk factor management and follow-up for atherosclerotic cardiovascular disease (ASCVD) that is similar to the population at large, according to research presented in a poster session at the annual meeting of the Spondyloarthritis Research and Treatment Network (SPARTAN).
The researchers found that patients with PsA in their single-center study did not receive more intensive management and follow-up despite having a higher risk score for ASCVD. This may be caused in part by the fact that there are no specific guidelines for ASCVD management in patients with PsA, according to study authors Linh Truong, MD, and Nicole Ridolfi, DO, internal medicine residents at the University of California, Irvine.
Even though this study and others have shown that patients with psoriatic arthritis have a higher risk for ASCVD, “currently, patients with PsA are being managed exactly the same way as everyone else,” Dr. Truong said.
In addition to assessing ASCVD morbidity in patients with PsA, Dr. Truong and Dr. Ridolfi also wanted to investigate how management of the known ASCVD risk in these patients in a primary care settings compared with national guidelines. Their research came in two parts. In the first part, they calculated 10-year ASCVD risk using a risk score estimator from the American Heart Association/American College of Cardiology. They then calculated an odds ratio for relevant atherosclerotic cardiovascular disease events, such as MI, heart failure, and cerebrovascular accidents. In the second part, they evaluated how the risk factors for ASCVD in patients with PsA were managed in the primary care setting.
The researchers compared data from a single center for 103 patients – 61 with PsA and 42 matching controls. Patients in both groups had an average age in the mid-60s, an average body mass index above 29 kg/m2, and were also mostly male (four patients with PsA were female) and mostly white.
Patients with PsA had an average ASCVD risk score of 21.2, compared with a score of 16.5 in the control arm (P less than .0001).
Of patients with psoriatic arthritis, 23 experienced an ASCVD event, compared with 10 patients in the control arm (OR, 1.93; 95% confidence interval, 0.80-4.66). Patients with PsA appeared to experience increased risk for MI (OR, 2.5), heart failure (OR, 7.09), and cerebrovascular accident (OR, 1.96). These increased risks had confidence intervals that approached statistical significance, but did not achieve it.
“We believe that because these data approach [statistical] significance, that further studies are needed,” Dr. Ridolfi said. “Our own validation studies are currently underway.”
In part two of the study, yearly ASCVD risk outcomes were averaged over 5 years and then evaluated based on the frequency of primary care visits, lab checks for HbA1c and lipid profile, and the use of cardioprotective ancillary referrals. The researchers also investigated statin and aspirin use in primary and secondary atherosclerotic cardiovascular disease per guidelines from the American College of Cardiology/American Heart Association and the United States Preventive Services Task Force.
Patients in both groups had similar primary care visits, HbA1c and lipid profiles, and use of cardioprotective supplements (such as fish oil and niacin). However, 36% of patients in the control arm received a nonpharmacologic ancillary referral, compared with just 11% of patients with PsA. Those referrals were in regard to weight loss and diabetes and dietary education, according to the research.
Dr. Truong and Dr. Ridolfi reported that, for primary prevention of ASCVD, PsA patients received treatment less often than did the controls with aspirin (0% vs. 26%, respectively) and statins (40% vs. 50%); there was also less use of statins for secondary prevention (73% vs. 85%).
“These data show that there is an educational opportunity in the primary care setting,” Dr. Truong said. “Or that there is an argument to be made that PsA should be managed by a rheumatology specialist.”
Dr. Truong and Dr. Ridolfi reported no disclosures.
CAMBRIDGE, MASS. – Patients with psoriatic arthritis (PsA) receive risk factor management and follow-up for atherosclerotic cardiovascular disease (ASCVD) that is similar to the population at large, according to research presented in a poster session at the annual meeting of the Spondyloarthritis Research and Treatment Network (SPARTAN).
The researchers found that patients with PsA in their single-center study did not receive more intensive management and follow-up despite having a higher risk score for ASCVD. This may be caused in part by the fact that there are no specific guidelines for ASCVD management in patients with PsA, according to study authors Linh Truong, MD, and Nicole Ridolfi, DO, internal medicine residents at the University of California, Irvine.
Even though this study and others have shown that patients with psoriatic arthritis have a higher risk for ASCVD, “currently, patients with PsA are being managed exactly the same way as everyone else,” Dr. Truong said.
In addition to assessing ASCVD morbidity in patients with PsA, Dr. Truong and Dr. Ridolfi also wanted to investigate how management of the known ASCVD risk in these patients in a primary care settings compared with national guidelines. Their research came in two parts. In the first part, they calculated 10-year ASCVD risk using a risk score estimator from the American Heart Association/American College of Cardiology. They then calculated an odds ratio for relevant atherosclerotic cardiovascular disease events, such as MI, heart failure, and cerebrovascular accidents. In the second part, they evaluated how the risk factors for ASCVD in patients with PsA were managed in the primary care setting.
The researchers compared data from a single center for 103 patients – 61 with PsA and 42 matching controls. Patients in both groups had an average age in the mid-60s, an average body mass index above 29 kg/m2, and were also mostly male (four patients with PsA were female) and mostly white.
Patients with PsA had an average ASCVD risk score of 21.2, compared with a score of 16.5 in the control arm (P less than .0001).
Of patients with psoriatic arthritis, 23 experienced an ASCVD event, compared with 10 patients in the control arm (OR, 1.93; 95% confidence interval, 0.80-4.66). Patients with PsA appeared to experience increased risk for MI (OR, 2.5), heart failure (OR, 7.09), and cerebrovascular accident (OR, 1.96). These increased risks had confidence intervals that approached statistical significance, but did not achieve it.
“We believe that because these data approach [statistical] significance, that further studies are needed,” Dr. Ridolfi said. “Our own validation studies are currently underway.”
In part two of the study, yearly ASCVD risk outcomes were averaged over 5 years and then evaluated based on the frequency of primary care visits, lab checks for HbA1c and lipid profile, and the use of cardioprotective ancillary referrals. The researchers also investigated statin and aspirin use in primary and secondary atherosclerotic cardiovascular disease per guidelines from the American College of Cardiology/American Heart Association and the United States Preventive Services Task Force.
Patients in both groups had similar primary care visits, HbA1c and lipid profiles, and use of cardioprotective supplements (such as fish oil and niacin). However, 36% of patients in the control arm received a nonpharmacologic ancillary referral, compared with just 11% of patients with PsA. Those referrals were in regard to weight loss and diabetes and dietary education, according to the research.
Dr. Truong and Dr. Ridolfi reported that, for primary prevention of ASCVD, PsA patients received treatment less often than did the controls with aspirin (0% vs. 26%, respectively) and statins (40% vs. 50%); there was also less use of statins for secondary prevention (73% vs. 85%).
“These data show that there is an educational opportunity in the primary care setting,” Dr. Truong said. “Or that there is an argument to be made that PsA should be managed by a rheumatology specialist.”
Dr. Truong and Dr. Ridolfi reported no disclosures.
REPORTING FROM SPARTAN 2018
Key clinical point: PsA patients have increased risk for ASCVD but are managed with the same intensity as the general population.
Major finding: Patients with PsA had an average ASCVD risk score of 21.2, compared with a score of 16.5 in the control arm.
Study details: A single-center study of 61 patients with PsA and 42 matching controls.
Disclosures: The presenters reported having no disclosures.
The FDA’s novel drugs approved in 2017
Novel drugs are innovative new products that have never before been used in clinical practice. Among the 46 that the Food and Drug Administration approved in 2017, 45 could be used in pregnancy. One, cerliponase alfa (Brineura), is indicated for pediatric patients 3 years of age or older, for treatment of late infantile neuronal ceroid lipofuscinosis type 2. It is doubtful that this drug would be used in pregnancy or during breastfeeding.
With the two exceptions noted below, there are no human pregnancy data for these drugs. It is important to consider that although high molecular weight (MW) drugs (for example, greater than 1,000) probably do not usually cross the placenta in the first half of pregnancy, they may do so in late pregnancy. The cited MWs are shown as the nearest whole number. Animal reproductive data are also cited because, although not definitive, they can provide some measure of the human embryo-fetal risk.
Anti-infectives
Benznidazole (same trade name) (MW 441), given orally, is indicated for pediatric patients aged 2-12 years for treatment of Chagas disease (American trypanosomiasis) caused by Trypanosoma cruzi. However, there are international reports describing its use in pregnancy and breastfeeding. No fetal harm from these exposures were noted. Nevertheless, because of the low MW and the reported animal risk, avoiding the drug during the first half of pregnancy appears to be the best choice. Delafloxacin (Baxdela) (MW 441), a fluoroquinolone antimicrobial given intravenously or orally, is indicated for acute bacterial skin infections. The animal data suggest low risk. However, like other fluoroquinolones, it is contraindicated in pregnancy and should be used only if there are no other alternatives.
Sofosbuvir/velpatasvir /voxilaprevir (Vosevi) (MWs 529, 883, 869), a fixed oral dose combination of three antivirals, is indicated for the treatment of hepatitis C virus infection. The MWs suggest that all three will cross the human placenta. The animal data suggest low risk. Secnidazole (Solosec) (MW 185), given orally, is indicated for the treatment of bacterial vaginosis. It is closely related to metronidazole. No evidence of embryo-fetal toxicity was observed in rats and rabbits, suggesting that the human risk is low. In a report from Brazil, 134 pregnant women with bacterial vaginosis were treated with secnidazole, metronidazole, or tinidazole in the second and third trimesters. Treatment significantly decreased the incidence of premature rupture of membranes, preterm labor, preterm birth, and low birth weight. No fetal harm was reported.
Antineoplastics
[Note: All of the drugs in this category are best avoided, if possible, in pregnancy and breastfeeding.]
Abemaciclib (Verzenio) (MW 507), an oral inhibitor of cyclin-dependent kinases, is indicated for the treatment of breast cancer. The drug is teratogenic in rats. Acalabrutinib (Calquence) (MW 466) is an oral kinase inhibitor indicated for mantle cell lymphoma. The drug had no effect on the rat embryo-fetus but caused decreased fetal body weights and delayed skeletal ossification in rabbits. Avelumab (Bavencio) (MW 147,000) is given intravenously for the treatment of metastatic Merkel cell carcinoma and metastatic urothelial carcinoma. Animal reproduction studies have not been conducted. However, based on its mechanism of action, fetal exposure may increase the risk of developing immune-related disorders or altering the normal immune response.
Brigatinib (Alunbrig) (MW 584) is given orally for the treatment of metastatic non–small-cell lung cancer. In rats, doses less than or slightly above the human exposure caused multiple anomalies in the fetuses of pregnant rats. Copanlisib (Aliqopa) (MW 553) is a kinase inhibitor that is given intravenously for relapsed follicular lymphoma. In rats during organogenesis, doses based on body surface area that were a fraction of the human dose caused embryo-fetal death and fetal defects. Durvalumab (Imfinzi) (MW 146,000), given intravenously, is indicated for the treatment of metastatic urothelial carcinoma and non–small-cell lung cancer. Monkeys given the drug from organogenesis through delivery experienced increased premature birth, fetal loss, and premature neonatal death. Women of reproductive potential should use effective contraception during treatment and for at least 3 months after the last dose.
Enasidenib (Idhifa) (MW 569), given orally, is indicated for the treatment of myeloid leukemia. The drug caused maternal toxicity and adverse embryo-fetal effects (postimplantation loss, resorptions, decrease viable fetuses, lower fetal birth weights, and skeletal variations) in rats and spontaneous abortions in rabbits. Inotuzumab ozogamicin (Besponsa) (MW 160,000), given intravenously, is indicated for relapsed or refractory B-cell precursor acute lymphoblastic leukemia. The drug caused fetal harm in rats but not in rabbits. Midostaurin (Rydapt) (MW 571) is an oral kinase inhibitor indicated for myeloid leukemia. In rats, a dose given during the first week of pregnancy that was a small fraction of the human exposure caused pre- and postimplantation loss. When very small doses were given during organogenesis to rats and rabbits there was significant maternal and fetal toxicity.
Neratinib (Nerlynx) (MW 673) is an oral kinase inhibitor for breast cancer. Although the drug did not cause embryo-fetal toxicity in rats, it did cause this toxicity in rabbits. Doses that resulted in exposures that were less than the human exposure caused maternal toxicity, abortions, and embryo-fetal death. Lower doses caused multiple fetal anomalies. Niraparib (Zejula) (MW 511) is indicated for treatment of epithelial ovarian, fallopian, or peritoneal cancer. Because of the potential human embryo-fetal risk based on its mechanism of action, pregnant animal studies were not conducted. Women with reproductive potential should use effective contraception during treatment and for 6 months after the last dose. Ribociclib (Kisqali) (MW 553) is an oral kinase inhibitor indicated for postmenopausal women with breast cancer. In rats, the drug cause reduced fetal weights and skeletal changes. Increased incidences of fetal abnormalities and lower fetal weights were observed in rabbits.
Cardiovascular
Angiotensin II (Giapreza) (MW 1,046) is a naturally occurring peptide hormone given as an intravenous infusion. It is indicated as a vasoconstrictor to increase blood pressure in adults with septic or other distributive shock. Animal reproduction studies have not been conducted. Because septic or other distributive shock is a medical emergency that can be fatal, the use of this agent in pregnancy should not be withheld.
Central nervous system
Deutetrabenazine (Austedo) (MW 324) is an oral drug indicated for the treatment of chorea associated with Huntington’s disease and for tardive dyskinesia. When given to rats during organogenesis there was no clear effect on embryo-fetal development.
Edaravone (Radicava) (MW 174), given as an intravenous infusion, is indicated for the treatment of amyotrophic lateral sclerosis. Doses that were not maternal toxic did not cause embryo-fetal toxicity in rats and rabbits. However, the no-effect dose for developmental toxicity was less than the recommended human dose. Naldemedine (Symproic) (MW 743) is an opioid antagonist indicated for the treatment of opioid-induced constipation. The drug crosses the human placenta and may precipitate opioid withdrawal in the fetus. The drug caused no embryo-fetal adverse effects, even at high doses, in pregnant rats and rabbits.
Ocrelizumab (Ocrevus) (MW 145,000), an intravenous agent, is used to treat patients with multiple sclerosis. The MW is high but immunoglobulins are known to cross the placenta. When given to monkeys at doses similar to or greater than the human dose, there was increased perinatal mortality, depletion of B-cell populations, and renal, bone marrow, and testicular toxicity in the offspring in the absence of maternal toxicity. Safinamide (Xadago) (MW 399) is an oral drug indicated as adjunctive treatment to levodopa/carbidopa in Parkinson’s disease. In rats, the drug was teratogenic (mainly urogenital defects) at all doses. When it was combined with levodopa/carbidopa or used alone, increased rates of fetal visceral and skeletal defects occurred at all doses studied. In rabbits, given the combination throughout organogenesis, there was an increased incidence of embryo-fetal death and cardiac and skeletal defects. Based on these data, avoiding the drug in pregnancy appears to be the best course.
Valbenazine (Ingrezza) (MW 419) is indicated for the treatment of tardive dyskinesia. The drug caused no malformations in rats and rabbits. However, in rats given the drug during organogenesis through lactation, an increase in the number of stillborn pups and postnatal pup mortalities was observed.
Dermatologic
Brodalumab (Siliq) (MW 144,000), given subcutaneously, is indicated for the treatment of moderate to severe plaque psoriasis. It is a human monoclonal IgG antibody and, even though the MW is high, IgG antibodies are known to cross the placenta. In monkeys, no drug-related effects on embryo-fetal toxicity or malformations, or on morphological, functional, or immunological development were observed in infants from mothers given weekly subcutaneous doses of the drug. Dupilumab (Dupixent) (MW 144,000) is given subcutaneously for the treatment of atopic dermatitis. It is a human monoclonal IgG antibody and, even though the MW is high, IgG antibodies are known to cross the placenta. In pregnant monkeys given subcutaneous doses of the drug, no drug-related effects on embryo-fetal toxicity or malformations, or on morphological, functional, or immunological development were observed in infants from birth to 6 months of age.
Guselkumab (Tremfya) (MW 143,600) is given subcutaneously for the treatment of moderate to severe plaque psoriasis. It is a human monoclonal IgG antibody and, even though the MW is high, IgG antibodies are known to cross the placenta. In pregnant monkeys given subcutaneous doses of the drug, no drug-related effects on embryo-fetal toxicity or malformations, or on morphological, functional, or immunological development were observed in infants from birth to 6 months of age. However, neonatal deaths were observed in three monkeys given six times the maximum recommended human dose.
Endocrine/metabolic
Deflazacort (Emflaza) (MW 442) is an oral corticosteroid prodrug indicated for the treatment of Duchenne muscular dystrophy. The drug is converted in vivo to an active metabolite. The drug readily crosses the placenta. Although animal reproduction studies have not been conducted, such studies with other corticosteroids in various animal species have shown an increased incidence of cleft palate. In some species, there was an increase in embryo-fetal death, intrauterine growth restriction, and constriction of the ductus arteriosus.
Ertugliflozin (Steglatro) (MW 566) is an oral drug indicated to improve glycemic control in adults with type 2 diabetes mellitus. In juvenile rats, doses that were about 13 times the human dose caused increased kidney weight, renal tubule and renal pelvis dilatation, and renal mineralization. These effects occurred during periods of rat renal development that correspond to the late second and third trimester of human renal development, and did not fully reverse within a 1-month recovery period. Etelcalcetide (Parsabiv) (MW 1,048), an intravenous calcium-sensing receptor agonist, is indicated for patients on hemodialysis who have secondary hyperparathyroidism. In rats and rabbits given the drug during organogenesis, there was reduced fetal growth. In rats given the drug during organogenesis through birth and weaning, there was a slight increase in pup mortality, delay in parturition, and transient effects on pup growth, but there were no effects on sexual maturation, neurobehavioral, or reproductive function. Macimorelin (Macrilen) (MW 535) is an oral growth hormone secretagogue receptor agonist. It is indicated for adult growth hormone deficiency. Animal reproduction studies have not been conducted.
Semaglutide (Ozempic) (MW 4,114), given subcutaneously, is a glucagon-like peptide indicated to improve glycemic control in type 2 diabetes mellitus. In rats given the drug during organogenesis, embryo-fetal death, structural defects, and alterations in growth were observed. In rabbits and monkeys given the drug during organogenesis, there were early pregnancy losses and structural abnormalities. In addition, there was marked maternal body weight loss in both animal species. Vestronidase alfa-vjbk (Mepsevii) is given intravenously. It is indicated for the treatment of Mucopolysaccharidosis VII (Sly syndrome). The calculated average MW of each nonglycosylated peptide chain is 72,562. In rats and rabbits given the drug during organogenesis, there was no maternal toxicity or adverse developmental outcomes.
Gastrointestinal
Plecanatide (Trulance) (MW 1,682) is an oral drug indicated for the treatment of constipation. The drug and its active metabolite are negligibly absorbed systemically and fetal exposure to the drug is not expected. In mice and rabbits given the oral drug during organogenesis, no effects on embryo-fetal development were observed. Telotristat ethyl (Xermelo) (MW 754) is an oral drug indicated for the treatment of carcinoid syndrome diarrhea in combination with somatostatin analog (MW not specified) therapy in adults not controlled by somatostatin analog. When given during organogenesis in rats, there was no effect on embryo-fetal development at doses that were about nine times the recommended human dose. However, an increased incidence of mortality in rat offspring was observed when the drug was given from organogenesis through lactation. During organogenesis in rabbits, the drug had no embryo-fetal effects at doses that were 10 or more times the human dose.
Hematologics
Betrixaban (Bevyxxa) (MW 568) is an oral factor Xa inhibitor indicated for the prophylaxis of venous thromboembolism. The drug was not associated with adverse developmental fetal outcomes in rats and rabbits. However, maternal hemorrhage did occur. In humans, there is an increased risk of hemorrhage during pregnancy and delivery. Emicizumab (Hemlibra) (MW 145,600), given subcutaneously, is indicated for routine prophylaxis to prevent or reduce the frequency of bleeding in patients with hemophilia A with factor VIII inhibitors. Animal reproduction studies have not been conducted. It is a human monoclonal IgG antibody and, though the MW is high, IgG antibodies are known to cross the placenta.
Immunologic
Sarilumab (Kevzara) (MW 150,000) is given subcutaneously. It is indicated for patients with moderate to severe rheumatoid arthritis. Reproduction studies were conducted in pregnant monkeys. There was no evidence of embryo toxicity or fetal malformations. Based on this data, the human pregnancy risk is low.
Ophthalmic
Latanoprostene bunod (Vyzulta) (MW 508) is a prostaglandin analog that is indicated to reduce intraocular pressure. No quantifiable plasma concentrations of latanoprostene bunod were detected in nonpregnant patients. However, very low levels of latanoprost acid (51-59 pg/mL), the active metabolite, were detected with the maximal plasma concentration occurring 5 minutes after administration. When given intravenously to pregnant rabbits, the drug was shown to be abortifacient and teratogenic, but these effects were not observed in pregnant rats. Netarsudil (Rhopressa) (MW 454) is a kinase inhibitor indicated to reduce intraocular pressure in patients with open-angle glaucoma or ocular hypertension. No quantifiable plasma concentrations of netarsudil were detected in 18 subjects. For the active metabolite, a plasma level of 0.11 ng/mL was found in one subject. Intravenous doses to pregnant rats and rabbits during organogenesis did not cause embryo-fetal adverse effects at clinically relevant systemic exposures.
Parathyroid hormone
Abaloparatide (Tymlos) (MW 3,961), given subcutaneously, is a human parathyroid hormone related peptide analog that is indicated for postmenopausal women with osteoporosis at high risk for fracture. Reproduction studies in animals have not been conducted. Because of the indication, it is doubtful if the agent will be used in pregnancy or during breastfeeding.
Respiratory
Benralizumab (Fasenra) (MW 150,000), given subcutaneously, is indicated for the add-on maintenance treatment of severe eosinophilic asthma. It is a human monoclonal IgG antibody and, though the MW is high, IgG antibodies are known to cross the placenta. Studies in monkeys found no evidence of fetal harm with intravenous doses throughout pregnancy that produced exposures up to about 310 times the exposure at the maximum recommended human dose.
The potential adverse effects in an infant when the mother is taking one of the above drugs while breastfeeding will be covered in my next column.
Mr. Briggs is clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, as well as at Washington State University, Spokane. He coauthored “Drugs in Pregnancy and Lactation” and coedited “Diseases, Complications, and Drug Therapy in Obstetrics.” He reported having no relevant financial disclosures.
Novel drugs are innovative new products that have never before been used in clinical practice. Among the 46 that the Food and Drug Administration approved in 2017, 45 could be used in pregnancy. One, cerliponase alfa (Brineura), is indicated for pediatric patients 3 years of age or older, for treatment of late infantile neuronal ceroid lipofuscinosis type 2. It is doubtful that this drug would be used in pregnancy or during breastfeeding.
With the two exceptions noted below, there are no human pregnancy data for these drugs. It is important to consider that although high molecular weight (MW) drugs (for example, greater than 1,000) probably do not usually cross the placenta in the first half of pregnancy, they may do so in late pregnancy. The cited MWs are shown as the nearest whole number. Animal reproductive data are also cited because, although not definitive, they can provide some measure of the human embryo-fetal risk.
Anti-infectives
Benznidazole (same trade name) (MW 441), given orally, is indicated for pediatric patients aged 2-12 years for treatment of Chagas disease (American trypanosomiasis) caused by Trypanosoma cruzi. However, there are international reports describing its use in pregnancy and breastfeeding. No fetal harm from these exposures were noted. Nevertheless, because of the low MW and the reported animal risk, avoiding the drug during the first half of pregnancy appears to be the best choice. Delafloxacin (Baxdela) (MW 441), a fluoroquinolone antimicrobial given intravenously or orally, is indicated for acute bacterial skin infections. The animal data suggest low risk. However, like other fluoroquinolones, it is contraindicated in pregnancy and should be used only if there are no other alternatives.
Sofosbuvir/velpatasvir /voxilaprevir (Vosevi) (MWs 529, 883, 869), a fixed oral dose combination of three antivirals, is indicated for the treatment of hepatitis C virus infection. The MWs suggest that all three will cross the human placenta. The animal data suggest low risk. Secnidazole (Solosec) (MW 185), given orally, is indicated for the treatment of bacterial vaginosis. It is closely related to metronidazole. No evidence of embryo-fetal toxicity was observed in rats and rabbits, suggesting that the human risk is low. In a report from Brazil, 134 pregnant women with bacterial vaginosis were treated with secnidazole, metronidazole, or tinidazole in the second and third trimesters. Treatment significantly decreased the incidence of premature rupture of membranes, preterm labor, preterm birth, and low birth weight. No fetal harm was reported.
Antineoplastics
[Note: All of the drugs in this category are best avoided, if possible, in pregnancy and breastfeeding.]
Abemaciclib (Verzenio) (MW 507), an oral inhibitor of cyclin-dependent kinases, is indicated for the treatment of breast cancer. The drug is teratogenic in rats. Acalabrutinib (Calquence) (MW 466) is an oral kinase inhibitor indicated for mantle cell lymphoma. The drug had no effect on the rat embryo-fetus but caused decreased fetal body weights and delayed skeletal ossification in rabbits. Avelumab (Bavencio) (MW 147,000) is given intravenously for the treatment of metastatic Merkel cell carcinoma and metastatic urothelial carcinoma. Animal reproduction studies have not been conducted. However, based on its mechanism of action, fetal exposure may increase the risk of developing immune-related disorders or altering the normal immune response.
Brigatinib (Alunbrig) (MW 584) is given orally for the treatment of metastatic non–small-cell lung cancer. In rats, doses less than or slightly above the human exposure caused multiple anomalies in the fetuses of pregnant rats. Copanlisib (Aliqopa) (MW 553) is a kinase inhibitor that is given intravenously for relapsed follicular lymphoma. In rats during organogenesis, doses based on body surface area that were a fraction of the human dose caused embryo-fetal death and fetal defects. Durvalumab (Imfinzi) (MW 146,000), given intravenously, is indicated for the treatment of metastatic urothelial carcinoma and non–small-cell lung cancer. Monkeys given the drug from organogenesis through delivery experienced increased premature birth, fetal loss, and premature neonatal death. Women of reproductive potential should use effective contraception during treatment and for at least 3 months after the last dose.
Enasidenib (Idhifa) (MW 569), given orally, is indicated for the treatment of myeloid leukemia. The drug caused maternal toxicity and adverse embryo-fetal effects (postimplantation loss, resorptions, decrease viable fetuses, lower fetal birth weights, and skeletal variations) in rats and spontaneous abortions in rabbits. Inotuzumab ozogamicin (Besponsa) (MW 160,000), given intravenously, is indicated for relapsed or refractory B-cell precursor acute lymphoblastic leukemia. The drug caused fetal harm in rats but not in rabbits. Midostaurin (Rydapt) (MW 571) is an oral kinase inhibitor indicated for myeloid leukemia. In rats, a dose given during the first week of pregnancy that was a small fraction of the human exposure caused pre- and postimplantation loss. When very small doses were given during organogenesis to rats and rabbits there was significant maternal and fetal toxicity.
Neratinib (Nerlynx) (MW 673) is an oral kinase inhibitor for breast cancer. Although the drug did not cause embryo-fetal toxicity in rats, it did cause this toxicity in rabbits. Doses that resulted in exposures that were less than the human exposure caused maternal toxicity, abortions, and embryo-fetal death. Lower doses caused multiple fetal anomalies. Niraparib (Zejula) (MW 511) is indicated for treatment of epithelial ovarian, fallopian, or peritoneal cancer. Because of the potential human embryo-fetal risk based on its mechanism of action, pregnant animal studies were not conducted. Women with reproductive potential should use effective contraception during treatment and for 6 months after the last dose. Ribociclib (Kisqali) (MW 553) is an oral kinase inhibitor indicated for postmenopausal women with breast cancer. In rats, the drug cause reduced fetal weights and skeletal changes. Increased incidences of fetal abnormalities and lower fetal weights were observed in rabbits.
Cardiovascular
Angiotensin II (Giapreza) (MW 1,046) is a naturally occurring peptide hormone given as an intravenous infusion. It is indicated as a vasoconstrictor to increase blood pressure in adults with septic or other distributive shock. Animal reproduction studies have not been conducted. Because septic or other distributive shock is a medical emergency that can be fatal, the use of this agent in pregnancy should not be withheld.
Central nervous system
Deutetrabenazine (Austedo) (MW 324) is an oral drug indicated for the treatment of chorea associated with Huntington’s disease and for tardive dyskinesia. When given to rats during organogenesis there was no clear effect on embryo-fetal development.
Edaravone (Radicava) (MW 174), given as an intravenous infusion, is indicated for the treatment of amyotrophic lateral sclerosis. Doses that were not maternal toxic did not cause embryo-fetal toxicity in rats and rabbits. However, the no-effect dose for developmental toxicity was less than the recommended human dose. Naldemedine (Symproic) (MW 743) is an opioid antagonist indicated for the treatment of opioid-induced constipation. The drug crosses the human placenta and may precipitate opioid withdrawal in the fetus. The drug caused no embryo-fetal adverse effects, even at high doses, in pregnant rats and rabbits.
Ocrelizumab (Ocrevus) (MW 145,000), an intravenous agent, is used to treat patients with multiple sclerosis. The MW is high but immunoglobulins are known to cross the placenta. When given to monkeys at doses similar to or greater than the human dose, there was increased perinatal mortality, depletion of B-cell populations, and renal, bone marrow, and testicular toxicity in the offspring in the absence of maternal toxicity. Safinamide (Xadago) (MW 399) is an oral drug indicated as adjunctive treatment to levodopa/carbidopa in Parkinson’s disease. In rats, the drug was teratogenic (mainly urogenital defects) at all doses. When it was combined with levodopa/carbidopa or used alone, increased rates of fetal visceral and skeletal defects occurred at all doses studied. In rabbits, given the combination throughout organogenesis, there was an increased incidence of embryo-fetal death and cardiac and skeletal defects. Based on these data, avoiding the drug in pregnancy appears to be the best course.
Valbenazine (Ingrezza) (MW 419) is indicated for the treatment of tardive dyskinesia. The drug caused no malformations in rats and rabbits. However, in rats given the drug during organogenesis through lactation, an increase in the number of stillborn pups and postnatal pup mortalities was observed.
Dermatologic
Brodalumab (Siliq) (MW 144,000), given subcutaneously, is indicated for the treatment of moderate to severe plaque psoriasis. It is a human monoclonal IgG antibody and, even though the MW is high, IgG antibodies are known to cross the placenta. In monkeys, no drug-related effects on embryo-fetal toxicity or malformations, or on morphological, functional, or immunological development were observed in infants from mothers given weekly subcutaneous doses of the drug. Dupilumab (Dupixent) (MW 144,000) is given subcutaneously for the treatment of atopic dermatitis. It is a human monoclonal IgG antibody and, even though the MW is high, IgG antibodies are known to cross the placenta. In pregnant monkeys given subcutaneous doses of the drug, no drug-related effects on embryo-fetal toxicity or malformations, or on morphological, functional, or immunological development were observed in infants from birth to 6 months of age.
Guselkumab (Tremfya) (MW 143,600) is given subcutaneously for the treatment of moderate to severe plaque psoriasis. It is a human monoclonal IgG antibody and, even though the MW is high, IgG antibodies are known to cross the placenta. In pregnant monkeys given subcutaneous doses of the drug, no drug-related effects on embryo-fetal toxicity or malformations, or on morphological, functional, or immunological development were observed in infants from birth to 6 months of age. However, neonatal deaths were observed in three monkeys given six times the maximum recommended human dose.
Endocrine/metabolic
Deflazacort (Emflaza) (MW 442) is an oral corticosteroid prodrug indicated for the treatment of Duchenne muscular dystrophy. The drug is converted in vivo to an active metabolite. The drug readily crosses the placenta. Although animal reproduction studies have not been conducted, such studies with other corticosteroids in various animal species have shown an increased incidence of cleft palate. In some species, there was an increase in embryo-fetal death, intrauterine growth restriction, and constriction of the ductus arteriosus.
Ertugliflozin (Steglatro) (MW 566) is an oral drug indicated to improve glycemic control in adults with type 2 diabetes mellitus. In juvenile rats, doses that were about 13 times the human dose caused increased kidney weight, renal tubule and renal pelvis dilatation, and renal mineralization. These effects occurred during periods of rat renal development that correspond to the late second and third trimester of human renal development, and did not fully reverse within a 1-month recovery period. Etelcalcetide (Parsabiv) (MW 1,048), an intravenous calcium-sensing receptor agonist, is indicated for patients on hemodialysis who have secondary hyperparathyroidism. In rats and rabbits given the drug during organogenesis, there was reduced fetal growth. In rats given the drug during organogenesis through birth and weaning, there was a slight increase in pup mortality, delay in parturition, and transient effects on pup growth, but there were no effects on sexual maturation, neurobehavioral, or reproductive function. Macimorelin (Macrilen) (MW 535) is an oral growth hormone secretagogue receptor agonist. It is indicated for adult growth hormone deficiency. Animal reproduction studies have not been conducted.
Semaglutide (Ozempic) (MW 4,114), given subcutaneously, is a glucagon-like peptide indicated to improve glycemic control in type 2 diabetes mellitus. In rats given the drug during organogenesis, embryo-fetal death, structural defects, and alterations in growth were observed. In rabbits and monkeys given the drug during organogenesis, there were early pregnancy losses and structural abnormalities. In addition, there was marked maternal body weight loss in both animal species. Vestronidase alfa-vjbk (Mepsevii) is given intravenously. It is indicated for the treatment of Mucopolysaccharidosis VII (Sly syndrome). The calculated average MW of each nonglycosylated peptide chain is 72,562. In rats and rabbits given the drug during organogenesis, there was no maternal toxicity or adverse developmental outcomes.
Gastrointestinal
Plecanatide (Trulance) (MW 1,682) is an oral drug indicated for the treatment of constipation. The drug and its active metabolite are negligibly absorbed systemically and fetal exposure to the drug is not expected. In mice and rabbits given the oral drug during organogenesis, no effects on embryo-fetal development were observed. Telotristat ethyl (Xermelo) (MW 754) is an oral drug indicated for the treatment of carcinoid syndrome diarrhea in combination with somatostatin analog (MW not specified) therapy in adults not controlled by somatostatin analog. When given during organogenesis in rats, there was no effect on embryo-fetal development at doses that were about nine times the recommended human dose. However, an increased incidence of mortality in rat offspring was observed when the drug was given from organogenesis through lactation. During organogenesis in rabbits, the drug had no embryo-fetal effects at doses that were 10 or more times the human dose.
Hematologics
Betrixaban (Bevyxxa) (MW 568) is an oral factor Xa inhibitor indicated for the prophylaxis of venous thromboembolism. The drug was not associated with adverse developmental fetal outcomes in rats and rabbits. However, maternal hemorrhage did occur. In humans, there is an increased risk of hemorrhage during pregnancy and delivery. Emicizumab (Hemlibra) (MW 145,600), given subcutaneously, is indicated for routine prophylaxis to prevent or reduce the frequency of bleeding in patients with hemophilia A with factor VIII inhibitors. Animal reproduction studies have not been conducted. It is a human monoclonal IgG antibody and, though the MW is high, IgG antibodies are known to cross the placenta.
Immunologic
Sarilumab (Kevzara) (MW 150,000) is given subcutaneously. It is indicated for patients with moderate to severe rheumatoid arthritis. Reproduction studies were conducted in pregnant monkeys. There was no evidence of embryo toxicity or fetal malformations. Based on this data, the human pregnancy risk is low.
Ophthalmic
Latanoprostene bunod (Vyzulta) (MW 508) is a prostaglandin analog that is indicated to reduce intraocular pressure. No quantifiable plasma concentrations of latanoprostene bunod were detected in nonpregnant patients. However, very low levels of latanoprost acid (51-59 pg/mL), the active metabolite, were detected with the maximal plasma concentration occurring 5 minutes after administration. When given intravenously to pregnant rabbits, the drug was shown to be abortifacient and teratogenic, but these effects were not observed in pregnant rats. Netarsudil (Rhopressa) (MW 454) is a kinase inhibitor indicated to reduce intraocular pressure in patients with open-angle glaucoma or ocular hypertension. No quantifiable plasma concentrations of netarsudil were detected in 18 subjects. For the active metabolite, a plasma level of 0.11 ng/mL was found in one subject. Intravenous doses to pregnant rats and rabbits during organogenesis did not cause embryo-fetal adverse effects at clinically relevant systemic exposures.
Parathyroid hormone
Abaloparatide (Tymlos) (MW 3,961), given subcutaneously, is a human parathyroid hormone related peptide analog that is indicated for postmenopausal women with osteoporosis at high risk for fracture. Reproduction studies in animals have not been conducted. Because of the indication, it is doubtful if the agent will be used in pregnancy or during breastfeeding.
Respiratory
Benralizumab (Fasenra) (MW 150,000), given subcutaneously, is indicated for the add-on maintenance treatment of severe eosinophilic asthma. It is a human monoclonal IgG antibody and, though the MW is high, IgG antibodies are known to cross the placenta. Studies in monkeys found no evidence of fetal harm with intravenous doses throughout pregnancy that produced exposures up to about 310 times the exposure at the maximum recommended human dose.
The potential adverse effects in an infant when the mother is taking one of the above drugs while breastfeeding will be covered in my next column.
Mr. Briggs is clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, as well as at Washington State University, Spokane. He coauthored “Drugs in Pregnancy and Lactation” and coedited “Diseases, Complications, and Drug Therapy in Obstetrics.” He reported having no relevant financial disclosures.
Novel drugs are innovative new products that have never before been used in clinical practice. Among the 46 that the Food and Drug Administration approved in 2017, 45 could be used in pregnancy. One, cerliponase alfa (Brineura), is indicated for pediatric patients 3 years of age or older, for treatment of late infantile neuronal ceroid lipofuscinosis type 2. It is doubtful that this drug would be used in pregnancy or during breastfeeding.
With the two exceptions noted below, there are no human pregnancy data for these drugs. It is important to consider that although high molecular weight (MW) drugs (for example, greater than 1,000) probably do not usually cross the placenta in the first half of pregnancy, they may do so in late pregnancy. The cited MWs are shown as the nearest whole number. Animal reproductive data are also cited because, although not definitive, they can provide some measure of the human embryo-fetal risk.
Anti-infectives
Benznidazole (same trade name) (MW 441), given orally, is indicated for pediatric patients aged 2-12 years for treatment of Chagas disease (American trypanosomiasis) caused by Trypanosoma cruzi. However, there are international reports describing its use in pregnancy and breastfeeding. No fetal harm from these exposures were noted. Nevertheless, because of the low MW and the reported animal risk, avoiding the drug during the first half of pregnancy appears to be the best choice. Delafloxacin (Baxdela) (MW 441), a fluoroquinolone antimicrobial given intravenously or orally, is indicated for acute bacterial skin infections. The animal data suggest low risk. However, like other fluoroquinolones, it is contraindicated in pregnancy and should be used only if there are no other alternatives.
Sofosbuvir/velpatasvir /voxilaprevir (Vosevi) (MWs 529, 883, 869), a fixed oral dose combination of three antivirals, is indicated for the treatment of hepatitis C virus infection. The MWs suggest that all three will cross the human placenta. The animal data suggest low risk. Secnidazole (Solosec) (MW 185), given orally, is indicated for the treatment of bacterial vaginosis. It is closely related to metronidazole. No evidence of embryo-fetal toxicity was observed in rats and rabbits, suggesting that the human risk is low. In a report from Brazil, 134 pregnant women with bacterial vaginosis were treated with secnidazole, metronidazole, or tinidazole in the second and third trimesters. Treatment significantly decreased the incidence of premature rupture of membranes, preterm labor, preterm birth, and low birth weight. No fetal harm was reported.
Antineoplastics
[Note: All of the drugs in this category are best avoided, if possible, in pregnancy and breastfeeding.]
Abemaciclib (Verzenio) (MW 507), an oral inhibitor of cyclin-dependent kinases, is indicated for the treatment of breast cancer. The drug is teratogenic in rats. Acalabrutinib (Calquence) (MW 466) is an oral kinase inhibitor indicated for mantle cell lymphoma. The drug had no effect on the rat embryo-fetus but caused decreased fetal body weights and delayed skeletal ossification in rabbits. Avelumab (Bavencio) (MW 147,000) is given intravenously for the treatment of metastatic Merkel cell carcinoma and metastatic urothelial carcinoma. Animal reproduction studies have not been conducted. However, based on its mechanism of action, fetal exposure may increase the risk of developing immune-related disorders or altering the normal immune response.
Brigatinib (Alunbrig) (MW 584) is given orally for the treatment of metastatic non–small-cell lung cancer. In rats, doses less than or slightly above the human exposure caused multiple anomalies in the fetuses of pregnant rats. Copanlisib (Aliqopa) (MW 553) is a kinase inhibitor that is given intravenously for relapsed follicular lymphoma. In rats during organogenesis, doses based on body surface area that were a fraction of the human dose caused embryo-fetal death and fetal defects. Durvalumab (Imfinzi) (MW 146,000), given intravenously, is indicated for the treatment of metastatic urothelial carcinoma and non–small-cell lung cancer. Monkeys given the drug from organogenesis through delivery experienced increased premature birth, fetal loss, and premature neonatal death. Women of reproductive potential should use effective contraception during treatment and for at least 3 months after the last dose.
Enasidenib (Idhifa) (MW 569), given orally, is indicated for the treatment of myeloid leukemia. The drug caused maternal toxicity and adverse embryo-fetal effects (postimplantation loss, resorptions, decrease viable fetuses, lower fetal birth weights, and skeletal variations) in rats and spontaneous abortions in rabbits. Inotuzumab ozogamicin (Besponsa) (MW 160,000), given intravenously, is indicated for relapsed or refractory B-cell precursor acute lymphoblastic leukemia. The drug caused fetal harm in rats but not in rabbits. Midostaurin (Rydapt) (MW 571) is an oral kinase inhibitor indicated for myeloid leukemia. In rats, a dose given during the first week of pregnancy that was a small fraction of the human exposure caused pre- and postimplantation loss. When very small doses were given during organogenesis to rats and rabbits there was significant maternal and fetal toxicity.
Neratinib (Nerlynx) (MW 673) is an oral kinase inhibitor for breast cancer. Although the drug did not cause embryo-fetal toxicity in rats, it did cause this toxicity in rabbits. Doses that resulted in exposures that were less than the human exposure caused maternal toxicity, abortions, and embryo-fetal death. Lower doses caused multiple fetal anomalies. Niraparib (Zejula) (MW 511) is indicated for treatment of epithelial ovarian, fallopian, or peritoneal cancer. Because of the potential human embryo-fetal risk based on its mechanism of action, pregnant animal studies were not conducted. Women with reproductive potential should use effective contraception during treatment and for 6 months after the last dose. Ribociclib (Kisqali) (MW 553) is an oral kinase inhibitor indicated for postmenopausal women with breast cancer. In rats, the drug cause reduced fetal weights and skeletal changes. Increased incidences of fetal abnormalities and lower fetal weights were observed in rabbits.
Cardiovascular
Angiotensin II (Giapreza) (MW 1,046) is a naturally occurring peptide hormone given as an intravenous infusion. It is indicated as a vasoconstrictor to increase blood pressure in adults with septic or other distributive shock. Animal reproduction studies have not been conducted. Because septic or other distributive shock is a medical emergency that can be fatal, the use of this agent in pregnancy should not be withheld.
Central nervous system
Deutetrabenazine (Austedo) (MW 324) is an oral drug indicated for the treatment of chorea associated with Huntington’s disease and for tardive dyskinesia. When given to rats during organogenesis there was no clear effect on embryo-fetal development.
Edaravone (Radicava) (MW 174), given as an intravenous infusion, is indicated for the treatment of amyotrophic lateral sclerosis. Doses that were not maternal toxic did not cause embryo-fetal toxicity in rats and rabbits. However, the no-effect dose for developmental toxicity was less than the recommended human dose. Naldemedine (Symproic) (MW 743) is an opioid antagonist indicated for the treatment of opioid-induced constipation. The drug crosses the human placenta and may precipitate opioid withdrawal in the fetus. The drug caused no embryo-fetal adverse effects, even at high doses, in pregnant rats and rabbits.
Ocrelizumab (Ocrevus) (MW 145,000), an intravenous agent, is used to treat patients with multiple sclerosis. The MW is high but immunoglobulins are known to cross the placenta. When given to monkeys at doses similar to or greater than the human dose, there was increased perinatal mortality, depletion of B-cell populations, and renal, bone marrow, and testicular toxicity in the offspring in the absence of maternal toxicity. Safinamide (Xadago) (MW 399) is an oral drug indicated as adjunctive treatment to levodopa/carbidopa in Parkinson’s disease. In rats, the drug was teratogenic (mainly urogenital defects) at all doses. When it was combined with levodopa/carbidopa or used alone, increased rates of fetal visceral and skeletal defects occurred at all doses studied. In rabbits, given the combination throughout organogenesis, there was an increased incidence of embryo-fetal death and cardiac and skeletal defects. Based on these data, avoiding the drug in pregnancy appears to be the best course.
Valbenazine (Ingrezza) (MW 419) is indicated for the treatment of tardive dyskinesia. The drug caused no malformations in rats and rabbits. However, in rats given the drug during organogenesis through lactation, an increase in the number of stillborn pups and postnatal pup mortalities was observed.
Dermatologic
Brodalumab (Siliq) (MW 144,000), given subcutaneously, is indicated for the treatment of moderate to severe plaque psoriasis. It is a human monoclonal IgG antibody and, even though the MW is high, IgG antibodies are known to cross the placenta. In monkeys, no drug-related effects on embryo-fetal toxicity or malformations, or on morphological, functional, or immunological development were observed in infants from mothers given weekly subcutaneous doses of the drug. Dupilumab (Dupixent) (MW 144,000) is given subcutaneously for the treatment of atopic dermatitis. It is a human monoclonal IgG antibody and, even though the MW is high, IgG antibodies are known to cross the placenta. In pregnant monkeys given subcutaneous doses of the drug, no drug-related effects on embryo-fetal toxicity or malformations, or on morphological, functional, or immunological development were observed in infants from birth to 6 months of age.
Guselkumab (Tremfya) (MW 143,600) is given subcutaneously for the treatment of moderate to severe plaque psoriasis. It is a human monoclonal IgG antibody and, even though the MW is high, IgG antibodies are known to cross the placenta. In pregnant monkeys given subcutaneous doses of the drug, no drug-related effects on embryo-fetal toxicity or malformations, or on morphological, functional, or immunological development were observed in infants from birth to 6 months of age. However, neonatal deaths were observed in three monkeys given six times the maximum recommended human dose.
Endocrine/metabolic
Deflazacort (Emflaza) (MW 442) is an oral corticosteroid prodrug indicated for the treatment of Duchenne muscular dystrophy. The drug is converted in vivo to an active metabolite. The drug readily crosses the placenta. Although animal reproduction studies have not been conducted, such studies with other corticosteroids in various animal species have shown an increased incidence of cleft palate. In some species, there was an increase in embryo-fetal death, intrauterine growth restriction, and constriction of the ductus arteriosus.
Ertugliflozin (Steglatro) (MW 566) is an oral drug indicated to improve glycemic control in adults with type 2 diabetes mellitus. In juvenile rats, doses that were about 13 times the human dose caused increased kidney weight, renal tubule and renal pelvis dilatation, and renal mineralization. These effects occurred during periods of rat renal development that correspond to the late second and third trimester of human renal development, and did not fully reverse within a 1-month recovery period. Etelcalcetide (Parsabiv) (MW 1,048), an intravenous calcium-sensing receptor agonist, is indicated for patients on hemodialysis who have secondary hyperparathyroidism. In rats and rabbits given the drug during organogenesis, there was reduced fetal growth. In rats given the drug during organogenesis through birth and weaning, there was a slight increase in pup mortality, delay in parturition, and transient effects on pup growth, but there were no effects on sexual maturation, neurobehavioral, or reproductive function. Macimorelin (Macrilen) (MW 535) is an oral growth hormone secretagogue receptor agonist. It is indicated for adult growth hormone deficiency. Animal reproduction studies have not been conducted.
Semaglutide (Ozempic) (MW 4,114), given subcutaneously, is a glucagon-like peptide indicated to improve glycemic control in type 2 diabetes mellitus. In rats given the drug during organogenesis, embryo-fetal death, structural defects, and alterations in growth were observed. In rabbits and monkeys given the drug during organogenesis, there were early pregnancy losses and structural abnormalities. In addition, there was marked maternal body weight loss in both animal species. Vestronidase alfa-vjbk (Mepsevii) is given intravenously. It is indicated for the treatment of Mucopolysaccharidosis VII (Sly syndrome). The calculated average MW of each nonglycosylated peptide chain is 72,562. In rats and rabbits given the drug during organogenesis, there was no maternal toxicity or adverse developmental outcomes.
Gastrointestinal
Plecanatide (Trulance) (MW 1,682) is an oral drug indicated for the treatment of constipation. The drug and its active metabolite are negligibly absorbed systemically and fetal exposure to the drug is not expected. In mice and rabbits given the oral drug during organogenesis, no effects on embryo-fetal development were observed. Telotristat ethyl (Xermelo) (MW 754) is an oral drug indicated for the treatment of carcinoid syndrome diarrhea in combination with somatostatin analog (MW not specified) therapy in adults not controlled by somatostatin analog. When given during organogenesis in rats, there was no effect on embryo-fetal development at doses that were about nine times the recommended human dose. However, an increased incidence of mortality in rat offspring was observed when the drug was given from organogenesis through lactation. During organogenesis in rabbits, the drug had no embryo-fetal effects at doses that were 10 or more times the human dose.
Hematologics
Betrixaban (Bevyxxa) (MW 568) is an oral factor Xa inhibitor indicated for the prophylaxis of venous thromboembolism. The drug was not associated with adverse developmental fetal outcomes in rats and rabbits. However, maternal hemorrhage did occur. In humans, there is an increased risk of hemorrhage during pregnancy and delivery. Emicizumab (Hemlibra) (MW 145,600), given subcutaneously, is indicated for routine prophylaxis to prevent or reduce the frequency of bleeding in patients with hemophilia A with factor VIII inhibitors. Animal reproduction studies have not been conducted. It is a human monoclonal IgG antibody and, though the MW is high, IgG antibodies are known to cross the placenta.
Immunologic
Sarilumab (Kevzara) (MW 150,000) is given subcutaneously. It is indicated for patients with moderate to severe rheumatoid arthritis. Reproduction studies were conducted in pregnant monkeys. There was no evidence of embryo toxicity or fetal malformations. Based on this data, the human pregnancy risk is low.
Ophthalmic
Latanoprostene bunod (Vyzulta) (MW 508) is a prostaglandin analog that is indicated to reduce intraocular pressure. No quantifiable plasma concentrations of latanoprostene bunod were detected in nonpregnant patients. However, very low levels of latanoprost acid (51-59 pg/mL), the active metabolite, were detected with the maximal plasma concentration occurring 5 minutes after administration. When given intravenously to pregnant rabbits, the drug was shown to be abortifacient and teratogenic, but these effects were not observed in pregnant rats. Netarsudil (Rhopressa) (MW 454) is a kinase inhibitor indicated to reduce intraocular pressure in patients with open-angle glaucoma or ocular hypertension. No quantifiable plasma concentrations of netarsudil were detected in 18 subjects. For the active metabolite, a plasma level of 0.11 ng/mL was found in one subject. Intravenous doses to pregnant rats and rabbits during organogenesis did not cause embryo-fetal adverse effects at clinically relevant systemic exposures.
Parathyroid hormone
Abaloparatide (Tymlos) (MW 3,961), given subcutaneously, is a human parathyroid hormone related peptide analog that is indicated for postmenopausal women with osteoporosis at high risk for fracture. Reproduction studies in animals have not been conducted. Because of the indication, it is doubtful if the agent will be used in pregnancy or during breastfeeding.
Respiratory
Benralizumab (Fasenra) (MW 150,000), given subcutaneously, is indicated for the add-on maintenance treatment of severe eosinophilic asthma. It is a human monoclonal IgG antibody and, though the MW is high, IgG antibodies are known to cross the placenta. Studies in monkeys found no evidence of fetal harm with intravenous doses throughout pregnancy that produced exposures up to about 310 times the exposure at the maximum recommended human dose.
The potential adverse effects in an infant when the mother is taking one of the above drugs while breastfeeding will be covered in my next column.
Mr. Briggs is clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, as well as at Washington State University, Spokane. He coauthored “Drugs in Pregnancy and Lactation” and coedited “Diseases, Complications, and Drug Therapy in Obstetrics.” He reported having no relevant financial disclosures.
Dr. Dinah Miller, Dr. Annette Hanson on involuntary psychiatric care
Dr. Miller is a private practice psychiatrist and professor at Johns Hopkins University. Dr. Hanson is the director of the forensic psychiatry fellowship at the University of Maryland. Dr. Miller and Dr. Hanson are board members for Clinical Psychiatry News.
Dr. Miller is a private practice psychiatrist and professor at Johns Hopkins University. Dr. Hanson is the director of the forensic psychiatry fellowship at the University of Maryland. Dr. Miller and Dr. Hanson are board members for Clinical Psychiatry News.
Dr. Miller is a private practice psychiatrist and professor at Johns Hopkins University. Dr. Hanson is the director of the forensic psychiatry fellowship at the University of Maryland. Dr. Miller and Dr. Hanson are board members for Clinical Psychiatry News.
Radiographic Study of Humeral Stem in Shoulder Arthroplasty After Lesser Tuberosity Osteotomy or Subscapularis Tenotomy
ABSTRACT
Lesser tuberosity osteotomy (LTO) and subscapularis tenotomy (ST) are used for takedown of the subscapularis during shoulder arthroplasty. LTO offers the theoretical but unproven benefit of improved healing and function of the subscapularis. However, humeral stem subsidence and loosening may be greater when osteotomy is performed, which may compromise functional outcomes. Our hypothesis is that no difference in proximal collar press-fit humeral stem subsidence or loosening exists, with no impairment of functional outcomes using the LTO technique.
During the surgical approach for total shoulder arthroplasty (TSA), the subscapularis is taken down for adequate exposure to the glenohumeral joint. Various methods are available for taking down the subscapularis, including lesser tuberosity osteotomy (LTO) and a subscapularis tenotomy (ST). LTO offers the theoretical but unproven benefit of improved healing and function of the subscapularis secondary to bone-to-bone healing. One concern, however, is that humeral stem subsidence may be greater when an osteotomy is performed owing to compromise of metaphyseal cortical bone, which may compromise functional outcomes. The humeral stem design may also influence subsidence when metaphyseal bone proximally is compromised. This is a concern in both metaphyseal and diaphyseal fitting stems. Metaphyseal collars on diaphyseal fitting stems rely on adequate bone stock in the metaphysis to provide the additional support needed. Also, posterior subluxation remains a challenge in shoulder arthroplasty. The integrity of the subscapularis is important in prevention of posterior subluxation.1 To our knowledge, no study to date has directly compared differences in humeral stem subsidence, loosening, or posterior subluxation between LTO and ST techniques with any humeral stem design. Our hypothesis is that no difference in proximal collar press-fit humeral stem subsidence or loosening exists, with no impairment of functional outcomes using the LTO technique. We also hypothesize that no difference in posterior subluxation exists between LTO and ST techniques.
MATERIALS AND METHODS
INCLUSION CRITERIA
Consecutive patients with a minimum of 12 months of radiographic follow-up were selected from 2007 to 2010 after TSA was performed by 1 of the senior authors (Dr. Miller and Dr. Voloshin). Study patients underwent primary TSA for primary osteoarthritis or rheumatoid arthritis.
EXCLUSION CRITERIA
Patients were excluded if they underwent TSA for posttraumatic glenohumeral arthritis, hemiarthroplasty, or osteonecrosis. Patients were also excluded if a rotator cuff tear was discovered intraoperatively or if they had a history of a rotator cuff repair. Additional exclusion criteria included postoperative trauma to the operative shoulder, postoperative infection, extensive documentation of chronic pain, and underlying neurologic disorder (eg, Parkinson disease, dystonia). Patients with a history of diabetes mellitus were not excluded.
SURGICAL TECHNIQUE
All patients underwent TSA via a deltopectoral approach in a modified beach chair position. Biceps tendons were tenodesed at the level of the pectoralis major. All patients received the same proximal collar press-fit implant (Bigliani-Flatow; Zimmer Biomet). These stems provide rotational stability in the metaphyseal segment via fins, vertical stability with the proximal collar, and distal fixation via an interference fit. All parts of the procedure were performed in similar fashion with the exception of ST vs LTO (Figures 1A-1D).
Continue to: LTO was performed as the primary...
LESSER TUBEROSITY OSTEOTOMY
LTO was performed as the primary or preferred technique of 1 surgeon. After completion of the biceps tenodesis, the lesser tuberosity is reflected off with the subscapularis intact using an osteotome. After placement of the press-fit humeral stem, the LTO is repaired using No. 5 Ethibond Excel sutures (Ethicon) passed through previously created bone tunnels in the greater tuberosity. These sutures are tied over metal buttons over the lateral cortex of the greater tuberosity. Last, the lateral corner of the rotator interval is repaired using a single No. 2 FiberWire (Arthrex).2
SUBSCAPULARIS TENOTOMY
ST is the preferred surgical technique of the second surgeon. After a biceps tenodesis, the subscapularis tendon is released from the lesser tuberosity at the margin of the bicipital groove. Through careful dissection, a single flap including the underlying capsule is created and reflected medially to the level of the coracoid. After placement of the press-fit humeral stem and humeral head, the subscapularis is repaired back in place through previous bone tunnels and with a No. 5 Ethibond Excel suture under the appropriate tension. Then, the lateral corner of the rotator interval is closed using a single No. 2 Ethibond Excel suture in a figure-of-eight fashion.2
RADIOGRAPHIC ANALYSIS
The primary variables analyzed were subsidence and loosening. Additional variables, including humeral-acromial distance (HAD) and subluxation index, were also analyzed to assess for any additional impact caused by subsidence or loosening.3 All radiographic measurements were taken from the Grashey (true anteroposterior) view, except subluxation index, which was calculated using the axillary view. All radiographic measurements were completed by 3 independent reviewers. All radiographs were completed in a consistent manner according to postoperative protocols.
HAD was measured preoperatively, immediately postoperatively, and at final follow-up at a minimum of 1 year. The HAD was measured from the lowest point on the acromion to the humerus using a perpendicular line (Figure 2).
Subsidence of the prosthesis was calculated by determining the difference between immediate postoperative heights of the prosthesis in comparison to the value of the final follow-up films. To calculate the height, 2 lines were drawn, 1 line was drawn perpendicular to the top of the prosthetic head and 1 perpendicular to the top of the greater tuberosity (Figure 3).
Continue to: Posterior subluxation is indicated...
Posterior subluxation is indicated by a value >65%, a centered head is between 35% and 65%, and anterior subluxation is indicated by a value <35% (Figure 4).3
The humeral stems were evaluated for loosening by assessing for lucency on final radiographic follow-up films. These were evaluated in a zonal fashion as demonstrated by Sanchez-Sotelo and colleagues4 and in Figure 5.
FUNCTIONAL OUTCOME EVALUATION
Before clinical evaluation, each study patient completed the Western Ontario Osteoarthritis of the Shoulder (WOOS) index; the Disabilities of the Hand, Arm and Shoulder (DASH) questionnaire, and the pain and function sections of the Constant score. The functional outcomes scores were captured postoperatively from October to November 2011. The WOOS is a validated outcome measure specific to osteoarthritis of the shoulder and has been used in prior studies evaluating outcomes of TSA.5-7 Previous studies have determined that the minimal clinically important difference for the WOOS score is 15 on a normalized 0 to 100 scale (100 being the best). The DASH score is a validated outcome measure for disorders of the upper extremity but is not specific to osteoarthritis of the shoulder.8 The Constant score is a validated outcome measure for a number of shoulder disorders, including TSA.9,10
STATISTICAL ANALYSIS
Statistical analyses were completed by a trained biostatistician. A power analysis was calculated using the noninferiority test to determine if adequate data had been obtained for this study. This was calculated by using previously accepted data demonstrating a statistically significant difference for subsidence and HAD. The data from these studies were used to make assumptions regarding accepted standard deviations and noninferiority margins, as calculated from the mean values of the 2 groups analyzed in each respective study.4,11 This analysis demonstrated power of 0.97 and 0.85 for the subsidence and HAD, respectively, given the current sample sizes. Intraclass coefficients were calculated to evaluate the measurements obtained during the radiographic analysis to determine the interrater agreement. Two samples’ t tests were calculated for the variables analyzed, along with P values and means.
RESULTS
DEMOGRAPHICS
A total of 51 consecutive patients were retrospectively selected for analysis. Of these, 16 patients were excluded from the study because they had <9 months of radiographic follow-up and were unavailable for further follow-up evaluation. Of the remaining 35 patients available for analysis, 4 patients had bilateral TSA, providing 39 shoulders for evaluation. Demographic characteristics of the study cohort are reported in Table 1.
| Table 1. Demographic Characteristics | |||
| Tenotomy (n = 24) | Osteotomy (n = 15) | P-value | |
| Age | 68.2 [7.4] | 70.2 [7.1] | 0.46 |
| Follow-up | 20.6 [11.5] | 18.5 [6.25] | 0.94 |
| Females | 7 (29%) | 6 (40%) | 0.58 |
| Dominant shoulder | 14 (58%) | 8 (53%) | 0.81 |
| Primary Diagnosis | |||
| Osteoarthritis | 22 (92%) | 15 (100%) | |
| Rheumatoid arthritis | 2 (8%) | 0 (0%) |
Fifteen patients underwent LTO, and 24 underwent ST. One patient underwent a tenotomy of the right shoulder and LTO of the left shoulder. Three LTOs were performed by the surgeon who primarily performed ST, owing to potential benefits of LTO. He eventually returned to his preferred technique of ST because of surgeon preference. Three ST procedures were completed by the surgeon who typically performed LTO at the start of the series prior to establishing LTO as his preferred technique. There was no significant difference between the study populations in terms of age, follow-up, male-to-female ratio, hand dominance, and primary diagnosis of osteoarthritis vs rheumatoid arthritis.
Continue to: There was no significant difference...
RADIOGRAPHIC DATA
There was no significant difference in preoperative HAD between the LTO and ST groups (9.5 ± 2.4 mm vs 10.9 ± 2.7 mm, P = .11). The immediate postoperative HAD was statistically significant between the LTO and ST groups (11.9 ± 3.7 mm vs 15.9 ± 4.5 mm, P = .005). There was as statistically significant difference noted in the final follow-up films between the LTO and ST groups (11.8 ± 3.2 mm vs 14.5 ± 3.9 mm, P = .025) (Table 2).
Table 2. Radiographic Data | |||||
Humeral Acromial Distance | |||||
| LTO | ST | P-Value | ||
Preoperative, mm | 9.5 | [2.4] | 10.9 | [2.7] | 0.11 |
Postoperative, mm | 11.9 | [3.7] | 15.9 | [4.5] | 0.005 |
Final follow-up, mm | 11.8 | [3.2] | 14.5 | [3.9] | 0.025 |
Subsidence | |||||
| LTO | ST | P-Value | ||
Subsidence, mm | 2.8 | [3.1] | 2.5 | [3.1] | 0.72 |
Subluxation Index | |||||
| LTO | ST | P-Value | ||
Preoperative, % | 0.55 | [0.06] | 0.54 | [0.07] | 0.45 |
Postoperative, % | 0.55 | [0.09] | 0.48 | [0.05] | 0.015 |
Lucent Lines | |||||
| LTO | ST | P-Value | ||
Lines >2 mm, % | 0.00 | 0.08 | 0.51 | ||
Abbreviations: LTO, lesser tuberosity osteotomy; ST, subscapularis tenotomy.
There were no statistically significant differences found in subsidence between LTO and ST groups at final follow-up (2.8 mm ± 3.1 mm vs 2.5 mm ± 3.1 mm, P = .72) (Table 2). No statistically significant difference was noted in the subluxation index between the LTO and ST groups (0.55% ± .06% vs 0.54% ± 0.07%, P = .45), but there was a statistically significant difference noted postoperatively between the LTO and ST groups (0.55% ± 0.09% vs .48% ± 0.05%, P = .015) (Table 2).
Two stems were noted to have lucent lines >2 mm, both within the ST cohort. Each had 1 stem zone >2 mm, 1 in zone 7, and 1 in zone 4. No statistically significant difference was identified between the LTO and ST groups (0/15 vs 2/24, P = .51) (Table 2).
FUNCTIONAL OUTCOMES
Study patients were evaluated using functional outcome scores, including the Constant, WOOS, and DASH scores (Table 3).
| Table 3. Functional Data | |||||
| LTO | ST | P-Value | |||
| WOOS index | 93.3 | [5.3] | 81.5 | [20.8] | 0.013 |
| DASH score | 8.4 | [6.6] | 13.8 | [4.9] | 0.13 |
| Constant score | 83.3 | [9.1] | 81.8 | [10.1] | 0.64 |
Abbreviations: DASH, disabilities of the arm, shoulder and hand; WOOS, Western Ontario Osteoarthritis of the Shoulder.
No statistically significant differences were noted in the DASH scores (8.4 ± 6.6 vs 13.8 ± 4.9, P = .13) or Constant scores (83.3 ± 9.1 vs 81.8 ± 10.1, P = .64) between the LTO and ST cohorts. There was a statistically significant difference between the WOOS scores (93.3 ± 5.3 vs 81.5 ± 20.8, P = .013). Because separate radiographic reviews were done by 3 independent personnel at 3 different times, it was important to ensure agreement among the reviewers. This was compared using the intraclass correlation coefficients. In the statistical analysis completed, the intraclass coefficients showed the 3 reviewers agreed with each other throughout the radiographic analysis (Table 4).
| Table 4. Testing Agreement: ICC | ||||
| ICC | CI, 2.5% | CI, 97.5% | ||
| HAD | Preoperative | 0.4451 | 0.2202 | 0.6443 |
| Postoperative | 0.6997 | 0.4836 | 0.834 | |
| Final follow-up | 0.5575 | 0.3592 | 0.7218 | |
| Subsidence | 0.6863 | 0.5349 | 0.807 | |
| SI | Preoperative | 0.3087 | 0.1061 | 0.5213 |
| Final follow-up | 0.5364 | 0.299 | 0.7186 |
Abbreviations: CI, confidence interval; HAD, humeral acromial distance; ICC, intraclass correlation coefficient; SI, subluxation index.
DISCUSSION
At final follow-up, we identified no statistically significant difference between the LTO and ST patients in subsidence, lucent lines >2 mm, or functional outcomes (Constant and DASH scores) in patients who underwent TSA with the same proximal collar press-fit humeral stem. In regard to the functional outcome scores, although the WOOS score was statistically significant (P = .013) between the LTO and ST cohorts, we do not feel that this is clinically relevant because it does not reach the minimal clinically important difference threshold of 15 points.8
A statistically significant difference was noted in postoperative subluxation index but was not clinically relevant, because the values between the LTO and ST groups (0.55 vs 0.48) still showed a centered humeral head. Gerber and colleagues3 discussed using a value of 0.65 as a measure of posterior humeral head subluxation, whereas Walch and colleagues12 defined posterior humeral head subluxation as a value >0.55. On the basis of these numbers, the values obtained in this study demonstrated that the postoperative values were still centered on the glenoid, and therefore were not clinically significant.3,12
Continue to: In regard to HAD, there...
In regard to HAD, there was a statistically significant difference noted postoperatively (P = .005) and at final follow-up (P = .025) between the LTO and ST cohorts. Saupe and colleagues13 demonstrated that a HAD <7 mm was considered abnormal and reflected subacromial space narrowing. The values noted in the LTO and ST patients on postoperative and final follow-up radiographs were statistically significant (Table 2), but not clinically relevant because both were >7 mm. A potential source for the variation in HAD may be due to X-ray position and angle.
Studies have shown a concern regarding the integrity of the subscapularis after tenotomy or peel used in TSA with abnormal subscapularis function.14,15 Miller and colleagues15 reported 41 patients, nearly two-thirds, of whom described subscapularis dysfunction. Those authors’ response to the poor clinical outcomes was to remove a fleck of bone with the tendon to achieve “bone-to-bone” healing.14 Gerber and colleagues16 reported on a series of patients using LTO and repair in TSA with 75% and 89% intact subscapularis function on clinical testing.16 Studies by Qureshi and colleagues17 and Scalise and colleagues18 showed similar results after LTO. Biomechanical studies have shown mixed results. Ponce and colleagues19 showed biomechanically superior results for LTO in comparison to the various repair techniques for ST. In another study, Giuseffi and colleagues20 showed no difference in LTO vs ST during biomechanical testing. In response to the increased concern regarding subscapularis integrity, Caplan and colleagues21 reported on 45 arthroplasties in 43 patients with improved postoperative testing with intact subscapularis testing in 90% to 100% of patients. A level 1 randomized control trial conducted by Lapner and colleagues22 did not demonstrate any clear clinical advantage of LTO vs ST. Controversy still exists regarding which is the preferred technique for TSA.
Sanchez-Sotelo and colleagues4 evaluated uncemented humeral components in 72 patients who underwent TSA. They found a humeral component was at risk for loosening if a radiolucent line ≥2 mm was present in at least 3 radiographic zones. They also evaluated tilt or subsidence by measurement and whether the components were observed to have changed. Their measured values correlated with their observed values. That study provided a benchmark for evaluation of loosening and subsidence used during this study.4 Although radiographic follow-up is limited in this study, we feel that any potential subsidence secondary to use of the LTO technique would be radiographically apparent at 1 year. There were 16 patients without adequate radiographic follow-up included in the study. However, we feel that this was not a large concern, because the study was adequately powered with the patients available to determine a difference based on subsidence.
CONCLUSION
We found no difference in subsidence, lucent lines >2 mm, posterior subluxation, and the Constant and DASH functional outcome scores when we compared TSA performed by a LTO with an ST technique with proximal collar press-fit humeral stem. These data cannot be extrapolated to metaphyseal fit stems, which may exhibit different settling characteristics in the setting of the LTO technique.
This paper will be judged for the Resident Writer’s Award.
1. Blasier R, Soslowsky L, Malicky D, Palmer M. Posterior glenohumeral subluxation: Active and passive stabilization in a biomechanical model. J Bone Joint Surg Am. 1997;79-A(3):433-440.
2. Buckley T, Miller R, Nicandri G, Lewis R, Voloshin I. Analysis of subscapularis integrity and function after lesser tuberosity osteotomy versus subscapularis tenotomy in total shoulder arthroplasty using ultrasound and validated clinical outcome measures. J Shoulder Elbow Surg. 2014;23(9):1309-1317. doi:10.1016/j.jse.2013.12.009.
3. Gerber C, Costouros JG, Sukthankar A, Fucentese SF. Static posterior humeral head subluxation and total shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18(4):505-510. doi:10.1016/j.jse.2009.03.003.
4. Sanchez-Sotelo J, Wright TW, O'Driscoll SW, Cofield RH, Rowland CM. Radiographic assessment of uncemented humeral components in total shoulder arthroplasty. J Arthroplasty. 2001;16(2):180-187.
5. Litchfield RB, McKee MD, Balyk R, et al. Cemented versus uncemented fixation of humeral components in total shoulder arthroplasty for osteoarthrtitis of the shoulder: A prospective, randomized, double-blind clinical trial-A JOINTs Canada Project. J Shoulder Elbow Surg. 2013;20(4):529-536. doi:10.1016/j.jse.2011.01.041.
6. Lo IK, Griffin S, Kirkley A. The development of a disease specific quality of life measurement tool for osteoarthritis of the shoulder: The Western Ontario Osteoarthritis of the Shoulder (WOOS) index. Osteoarthritis Cartilage. 2001;9(8):771-778. doi:10.1053/joca.2001.0474
7. Lo IK, Litchfield RB, Griffin S, Faber K, Patterson SD, Kirkley A. Quality of life outcome following hemiarthroplasty or total shoulder arthroplasty in patients with osteoarthritis. A prospective, randomized trial. J Bone Joint Surg Am. 2005;87(10):2178-2185. doi:10.2106/JBJS.D.02198
8. Hudak PL, Amadio PC, Bombardier C. Development of an upper extremity outcome measure: the DASH (disabilities of the arm, shoulder and hand) [corrected]. The Upper Extremity Collaborative Group (UECG). Am J Ind Med. 1996;29(6):602-608. doi:10.1002/(SICI)1097-0274(199606)29:6<602::AID-AJIM4>3.0.CO;2-L.
9. Constant CR, Gerber C, Emery RJ, Sojbjerg JO, Gohlke F, Boileau P. A review of the constant score: Modifications and guidelines for its use. J Shoulder Elbow Surg. 2008;17(2):355-361. doi:10.1016/j.jse.2007.06.022.
10. Constant CR, Murley AH. A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res. 1987;(214):160-164.
11. Mayerhoefer ME, Breitenseher MJ, Wurnig C, Roposch A. Shoulder impingement: Relationship of clinical symptoms and imaging criteria. Clin J Sport Med. 2009;19(2):83-89. doi:10.1097/JSM.0b013e318198e2e3.
12. Walch G, Badet R, Boulahia A, Khoury A. Morphologic study of the glenoid in primary glenohumeral osteoarthritis. J Arthroplasy. 1999;14(6):756-760.
13. Saupe N, Pfirmann CW, Schmid MR, et al. Association between rotator cuff abnormalities and reduced acromiohumeral distance. AJR Am J Roentgenol. 2006;187(2):376-382. doi:10.2214/AJR.05.0435.
14. Jackson J, Cil A, Smith J, Steinmann SP. Integrity and function of the subscapularis after total shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(7):1085-1090. doi:10.1016/j.jse.2010.04.001.
15. Miller SL, Hazrati Y, Klepps S, Chiang A, Flatow EL. Loss of subscapularis function after total shoulder replacement: a seldom recognized problem. J Shoulder Elbow Surg. 2003;12(1):29-34. doi:10.1067/mse.2003.128195.
16. Gerber C, Yian EH, Pfirrmann AW, Zumstein MA, Werner CM. Subscapularis muscle function and structure after total shoulder replacement with lesser tuberosity osteotomy and repair. J Bone Joint Surg Am. 2005;87(8):1739-1745. doi:10.2106/JBJS.D.02788.
17. Qureshi S, Hsiao A, Klug RA, Lee E, Braman J, Flatow EL. Subscapularis function after total shoulder replacement: results with lesser tuberosity osteotomy. J Shoulder Elbow Surg. 2008;17(1): 68-72. doi:10.1016/j.jse.2007.04.018.
18. Scalise JJ, Ciccone J, Iannotti JP. Clinical, radiographic and ultrasonographic comparison of subscapularis tenotomy and lesser tuberosity osteotomy for total shoulder arthroplasty. J Bone Joint Surg Am. 2010;92(7):1627-1634. doi:10.2106/JBJS.G.01461.
19. Ponce BA, Ahluwalia RS, Mazzocca AD, Gobezie RG, Warner JJ, Millett PJ. Biomechanical and clinical evaluation of a novel lesser tuberosity in total shoulder arthroplasty. J Bone Joint Surg Am. 2005;87 Suppl 2:1-8.
20. Giuseffi SA, Wongtriratanachai P, Omae H, et al. Biomechanical comparison of lesser tuberosity osteotomy versus subscapularis tenotomy in total shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(8):1087-1095. doi:10.1016/j.jse.2011.07.008.
21. Caplan JL, Whitfield W, Nevasier RJ. Subscapularis function after primary tendon to tendon repair in patients after replacement arthroplasty of the shoulder. J Shoulder Elbow Surg. 2009;18(2):193-196. doi:10.1016/j.jse.2008.10.019.
22. Lapner PLC, Sabri E, Rakhra K, Bell K, Athwal GS. Comparison of LTO to subscapularis peel in shoulder arthroplasty. J Bone Joint Surg Am. 2012;94(24):2239-2246. doi:10.2106/JBJS.K.01365.
ABSTRACT
Lesser tuberosity osteotomy (LTO) and subscapularis tenotomy (ST) are used for takedown of the subscapularis during shoulder arthroplasty. LTO offers the theoretical but unproven benefit of improved healing and function of the subscapularis. However, humeral stem subsidence and loosening may be greater when osteotomy is performed, which may compromise functional outcomes. Our hypothesis is that no difference in proximal collar press-fit humeral stem subsidence or loosening exists, with no impairment of functional outcomes using the LTO technique.
During the surgical approach for total shoulder arthroplasty (TSA), the subscapularis is taken down for adequate exposure to the glenohumeral joint. Various methods are available for taking down the subscapularis, including lesser tuberosity osteotomy (LTO) and a subscapularis tenotomy (ST). LTO offers the theoretical but unproven benefit of improved healing and function of the subscapularis secondary to bone-to-bone healing. One concern, however, is that humeral stem subsidence may be greater when an osteotomy is performed owing to compromise of metaphyseal cortical bone, which may compromise functional outcomes. The humeral stem design may also influence subsidence when metaphyseal bone proximally is compromised. This is a concern in both metaphyseal and diaphyseal fitting stems. Metaphyseal collars on diaphyseal fitting stems rely on adequate bone stock in the metaphysis to provide the additional support needed. Also, posterior subluxation remains a challenge in shoulder arthroplasty. The integrity of the subscapularis is important in prevention of posterior subluxation.1 To our knowledge, no study to date has directly compared differences in humeral stem subsidence, loosening, or posterior subluxation between LTO and ST techniques with any humeral stem design. Our hypothesis is that no difference in proximal collar press-fit humeral stem subsidence or loosening exists, with no impairment of functional outcomes using the LTO technique. We also hypothesize that no difference in posterior subluxation exists between LTO and ST techniques.
MATERIALS AND METHODS
INCLUSION CRITERIA
Consecutive patients with a minimum of 12 months of radiographic follow-up were selected from 2007 to 2010 after TSA was performed by 1 of the senior authors (Dr. Miller and Dr. Voloshin). Study patients underwent primary TSA for primary osteoarthritis or rheumatoid arthritis.
EXCLUSION CRITERIA
Patients were excluded if they underwent TSA for posttraumatic glenohumeral arthritis, hemiarthroplasty, or osteonecrosis. Patients were also excluded if a rotator cuff tear was discovered intraoperatively or if they had a history of a rotator cuff repair. Additional exclusion criteria included postoperative trauma to the operative shoulder, postoperative infection, extensive documentation of chronic pain, and underlying neurologic disorder (eg, Parkinson disease, dystonia). Patients with a history of diabetes mellitus were not excluded.
SURGICAL TECHNIQUE
All patients underwent TSA via a deltopectoral approach in a modified beach chair position. Biceps tendons were tenodesed at the level of the pectoralis major. All patients received the same proximal collar press-fit implant (Bigliani-Flatow; Zimmer Biomet). These stems provide rotational stability in the metaphyseal segment via fins, vertical stability with the proximal collar, and distal fixation via an interference fit. All parts of the procedure were performed in similar fashion with the exception of ST vs LTO (Figures 1A-1D).
Continue to: LTO was performed as the primary...
LESSER TUBEROSITY OSTEOTOMY
LTO was performed as the primary or preferred technique of 1 surgeon. After completion of the biceps tenodesis, the lesser tuberosity is reflected off with the subscapularis intact using an osteotome. After placement of the press-fit humeral stem, the LTO is repaired using No. 5 Ethibond Excel sutures (Ethicon) passed through previously created bone tunnels in the greater tuberosity. These sutures are tied over metal buttons over the lateral cortex of the greater tuberosity. Last, the lateral corner of the rotator interval is repaired using a single No. 2 FiberWire (Arthrex).2
SUBSCAPULARIS TENOTOMY
ST is the preferred surgical technique of the second surgeon. After a biceps tenodesis, the subscapularis tendon is released from the lesser tuberosity at the margin of the bicipital groove. Through careful dissection, a single flap including the underlying capsule is created and reflected medially to the level of the coracoid. After placement of the press-fit humeral stem and humeral head, the subscapularis is repaired back in place through previous bone tunnels and with a No. 5 Ethibond Excel suture under the appropriate tension. Then, the lateral corner of the rotator interval is closed using a single No. 2 Ethibond Excel suture in a figure-of-eight fashion.2
RADIOGRAPHIC ANALYSIS
The primary variables analyzed were subsidence and loosening. Additional variables, including humeral-acromial distance (HAD) and subluxation index, were also analyzed to assess for any additional impact caused by subsidence or loosening.3 All radiographic measurements were taken from the Grashey (true anteroposterior) view, except subluxation index, which was calculated using the axillary view. All radiographic measurements were completed by 3 independent reviewers. All radiographs were completed in a consistent manner according to postoperative protocols.
HAD was measured preoperatively, immediately postoperatively, and at final follow-up at a minimum of 1 year. The HAD was measured from the lowest point on the acromion to the humerus using a perpendicular line (Figure 2).
Subsidence of the prosthesis was calculated by determining the difference between immediate postoperative heights of the prosthesis in comparison to the value of the final follow-up films. To calculate the height, 2 lines were drawn, 1 line was drawn perpendicular to the top of the prosthetic head and 1 perpendicular to the top of the greater tuberosity (Figure 3).
Continue to: Posterior subluxation is indicated...
Posterior subluxation is indicated by a value >65%, a centered head is between 35% and 65%, and anterior subluxation is indicated by a value <35% (Figure 4).3
The humeral stems were evaluated for loosening by assessing for lucency on final radiographic follow-up films. These were evaluated in a zonal fashion as demonstrated by Sanchez-Sotelo and colleagues4 and in Figure 5.
FUNCTIONAL OUTCOME EVALUATION
Before clinical evaluation, each study patient completed the Western Ontario Osteoarthritis of the Shoulder (WOOS) index; the Disabilities of the Hand, Arm and Shoulder (DASH) questionnaire, and the pain and function sections of the Constant score. The functional outcomes scores were captured postoperatively from October to November 2011. The WOOS is a validated outcome measure specific to osteoarthritis of the shoulder and has been used in prior studies evaluating outcomes of TSA.5-7 Previous studies have determined that the minimal clinically important difference for the WOOS score is 15 on a normalized 0 to 100 scale (100 being the best). The DASH score is a validated outcome measure for disorders of the upper extremity but is not specific to osteoarthritis of the shoulder.8 The Constant score is a validated outcome measure for a number of shoulder disorders, including TSA.9,10
STATISTICAL ANALYSIS
Statistical analyses were completed by a trained biostatistician. A power analysis was calculated using the noninferiority test to determine if adequate data had been obtained for this study. This was calculated by using previously accepted data demonstrating a statistically significant difference for subsidence and HAD. The data from these studies were used to make assumptions regarding accepted standard deviations and noninferiority margins, as calculated from the mean values of the 2 groups analyzed in each respective study.4,11 This analysis demonstrated power of 0.97 and 0.85 for the subsidence and HAD, respectively, given the current sample sizes. Intraclass coefficients were calculated to evaluate the measurements obtained during the radiographic analysis to determine the interrater agreement. Two samples’ t tests were calculated for the variables analyzed, along with P values and means.
RESULTS
DEMOGRAPHICS
A total of 51 consecutive patients were retrospectively selected for analysis. Of these, 16 patients were excluded from the study because they had <9 months of radiographic follow-up and were unavailable for further follow-up evaluation. Of the remaining 35 patients available for analysis, 4 patients had bilateral TSA, providing 39 shoulders for evaluation. Demographic characteristics of the study cohort are reported in Table 1.
| Table 1. Demographic Characteristics | |||
| Tenotomy (n = 24) | Osteotomy (n = 15) | P-value | |
| Age | 68.2 [7.4] | 70.2 [7.1] | 0.46 |
| Follow-up | 20.6 [11.5] | 18.5 [6.25] | 0.94 |
| Females | 7 (29%) | 6 (40%) | 0.58 |
| Dominant shoulder | 14 (58%) | 8 (53%) | 0.81 |
| Primary Diagnosis | |||
| Osteoarthritis | 22 (92%) | 15 (100%) | |
| Rheumatoid arthritis | 2 (8%) | 0 (0%) |
Fifteen patients underwent LTO, and 24 underwent ST. One patient underwent a tenotomy of the right shoulder and LTO of the left shoulder. Three LTOs were performed by the surgeon who primarily performed ST, owing to potential benefits of LTO. He eventually returned to his preferred technique of ST because of surgeon preference. Three ST procedures were completed by the surgeon who typically performed LTO at the start of the series prior to establishing LTO as his preferred technique. There was no significant difference between the study populations in terms of age, follow-up, male-to-female ratio, hand dominance, and primary diagnosis of osteoarthritis vs rheumatoid arthritis.
Continue to: There was no significant difference...
RADIOGRAPHIC DATA
There was no significant difference in preoperative HAD between the LTO and ST groups (9.5 ± 2.4 mm vs 10.9 ± 2.7 mm, P = .11). The immediate postoperative HAD was statistically significant between the LTO and ST groups (11.9 ± 3.7 mm vs 15.9 ± 4.5 mm, P = .005). There was as statistically significant difference noted in the final follow-up films between the LTO and ST groups (11.8 ± 3.2 mm vs 14.5 ± 3.9 mm, P = .025) (Table 2).
Table 2. Radiographic Data | |||||
Humeral Acromial Distance | |||||
| LTO | ST | P-Value | ||
Preoperative, mm | 9.5 | [2.4] | 10.9 | [2.7] | 0.11 |
Postoperative, mm | 11.9 | [3.7] | 15.9 | [4.5] | 0.005 |
Final follow-up, mm | 11.8 | [3.2] | 14.5 | [3.9] | 0.025 |
Subsidence | |||||
| LTO | ST | P-Value | ||
Subsidence, mm | 2.8 | [3.1] | 2.5 | [3.1] | 0.72 |
Subluxation Index | |||||
| LTO | ST | P-Value | ||
Preoperative, % | 0.55 | [0.06] | 0.54 | [0.07] | 0.45 |
Postoperative, % | 0.55 | [0.09] | 0.48 | [0.05] | 0.015 |
Lucent Lines | |||||
| LTO | ST | P-Value | ||
Lines >2 mm, % | 0.00 | 0.08 | 0.51 | ||
Abbreviations: LTO, lesser tuberosity osteotomy; ST, subscapularis tenotomy.
There were no statistically significant differences found in subsidence between LTO and ST groups at final follow-up (2.8 mm ± 3.1 mm vs 2.5 mm ± 3.1 mm, P = .72) (Table 2). No statistically significant difference was noted in the subluxation index between the LTO and ST groups (0.55% ± .06% vs 0.54% ± 0.07%, P = .45), but there was a statistically significant difference noted postoperatively between the LTO and ST groups (0.55% ± 0.09% vs .48% ± 0.05%, P = .015) (Table 2).
Two stems were noted to have lucent lines >2 mm, both within the ST cohort. Each had 1 stem zone >2 mm, 1 in zone 7, and 1 in zone 4. No statistically significant difference was identified between the LTO and ST groups (0/15 vs 2/24, P = .51) (Table 2).
FUNCTIONAL OUTCOMES
Study patients were evaluated using functional outcome scores, including the Constant, WOOS, and DASH scores (Table 3).
| Table 3. Functional Data | |||||
| LTO | ST | P-Value | |||
| WOOS index | 93.3 | [5.3] | 81.5 | [20.8] | 0.013 |
| DASH score | 8.4 | [6.6] | 13.8 | [4.9] | 0.13 |
| Constant score | 83.3 | [9.1] | 81.8 | [10.1] | 0.64 |
Abbreviations: DASH, disabilities of the arm, shoulder and hand; WOOS, Western Ontario Osteoarthritis of the Shoulder.
No statistically significant differences were noted in the DASH scores (8.4 ± 6.6 vs 13.8 ± 4.9, P = .13) or Constant scores (83.3 ± 9.1 vs 81.8 ± 10.1, P = .64) between the LTO and ST cohorts. There was a statistically significant difference between the WOOS scores (93.3 ± 5.3 vs 81.5 ± 20.8, P = .013). Because separate radiographic reviews were done by 3 independent personnel at 3 different times, it was important to ensure agreement among the reviewers. This was compared using the intraclass correlation coefficients. In the statistical analysis completed, the intraclass coefficients showed the 3 reviewers agreed with each other throughout the radiographic analysis (Table 4).
| Table 4. Testing Agreement: ICC | ||||
| ICC | CI, 2.5% | CI, 97.5% | ||
| HAD | Preoperative | 0.4451 | 0.2202 | 0.6443 |
| Postoperative | 0.6997 | 0.4836 | 0.834 | |
| Final follow-up | 0.5575 | 0.3592 | 0.7218 | |
| Subsidence | 0.6863 | 0.5349 | 0.807 | |
| SI | Preoperative | 0.3087 | 0.1061 | 0.5213 |
| Final follow-up | 0.5364 | 0.299 | 0.7186 |
Abbreviations: CI, confidence interval; HAD, humeral acromial distance; ICC, intraclass correlation coefficient; SI, subluxation index.
DISCUSSION
At final follow-up, we identified no statistically significant difference between the LTO and ST patients in subsidence, lucent lines >2 mm, or functional outcomes (Constant and DASH scores) in patients who underwent TSA with the same proximal collar press-fit humeral stem. In regard to the functional outcome scores, although the WOOS score was statistically significant (P = .013) between the LTO and ST cohorts, we do not feel that this is clinically relevant because it does not reach the minimal clinically important difference threshold of 15 points.8
A statistically significant difference was noted in postoperative subluxation index but was not clinically relevant, because the values between the LTO and ST groups (0.55 vs 0.48) still showed a centered humeral head. Gerber and colleagues3 discussed using a value of 0.65 as a measure of posterior humeral head subluxation, whereas Walch and colleagues12 defined posterior humeral head subluxation as a value >0.55. On the basis of these numbers, the values obtained in this study demonstrated that the postoperative values were still centered on the glenoid, and therefore were not clinically significant.3,12
Continue to: In regard to HAD, there...
In regard to HAD, there was a statistically significant difference noted postoperatively (P = .005) and at final follow-up (P = .025) between the LTO and ST cohorts. Saupe and colleagues13 demonstrated that a HAD <7 mm was considered abnormal and reflected subacromial space narrowing. The values noted in the LTO and ST patients on postoperative and final follow-up radiographs were statistically significant (Table 2), but not clinically relevant because both were >7 mm. A potential source for the variation in HAD may be due to X-ray position and angle.
Studies have shown a concern regarding the integrity of the subscapularis after tenotomy or peel used in TSA with abnormal subscapularis function.14,15 Miller and colleagues15 reported 41 patients, nearly two-thirds, of whom described subscapularis dysfunction. Those authors’ response to the poor clinical outcomes was to remove a fleck of bone with the tendon to achieve “bone-to-bone” healing.14 Gerber and colleagues16 reported on a series of patients using LTO and repair in TSA with 75% and 89% intact subscapularis function on clinical testing.16 Studies by Qureshi and colleagues17 and Scalise and colleagues18 showed similar results after LTO. Biomechanical studies have shown mixed results. Ponce and colleagues19 showed biomechanically superior results for LTO in comparison to the various repair techniques for ST. In another study, Giuseffi and colleagues20 showed no difference in LTO vs ST during biomechanical testing. In response to the increased concern regarding subscapularis integrity, Caplan and colleagues21 reported on 45 arthroplasties in 43 patients with improved postoperative testing with intact subscapularis testing in 90% to 100% of patients. A level 1 randomized control trial conducted by Lapner and colleagues22 did not demonstrate any clear clinical advantage of LTO vs ST. Controversy still exists regarding which is the preferred technique for TSA.
Sanchez-Sotelo and colleagues4 evaluated uncemented humeral components in 72 patients who underwent TSA. They found a humeral component was at risk for loosening if a radiolucent line ≥2 mm was present in at least 3 radiographic zones. They also evaluated tilt or subsidence by measurement and whether the components were observed to have changed. Their measured values correlated with their observed values. That study provided a benchmark for evaluation of loosening and subsidence used during this study.4 Although radiographic follow-up is limited in this study, we feel that any potential subsidence secondary to use of the LTO technique would be radiographically apparent at 1 year. There were 16 patients without adequate radiographic follow-up included in the study. However, we feel that this was not a large concern, because the study was adequately powered with the patients available to determine a difference based on subsidence.
CONCLUSION
We found no difference in subsidence, lucent lines >2 mm, posterior subluxation, and the Constant and DASH functional outcome scores when we compared TSA performed by a LTO with an ST technique with proximal collar press-fit humeral stem. These data cannot be extrapolated to metaphyseal fit stems, which may exhibit different settling characteristics in the setting of the LTO technique.
This paper will be judged for the Resident Writer’s Award.
ABSTRACT
Lesser tuberosity osteotomy (LTO) and subscapularis tenotomy (ST) are used for takedown of the subscapularis during shoulder arthroplasty. LTO offers the theoretical but unproven benefit of improved healing and function of the subscapularis. However, humeral stem subsidence and loosening may be greater when osteotomy is performed, which may compromise functional outcomes. Our hypothesis is that no difference in proximal collar press-fit humeral stem subsidence or loosening exists, with no impairment of functional outcomes using the LTO technique.
During the surgical approach for total shoulder arthroplasty (TSA), the subscapularis is taken down for adequate exposure to the glenohumeral joint. Various methods are available for taking down the subscapularis, including lesser tuberosity osteotomy (LTO) and a subscapularis tenotomy (ST). LTO offers the theoretical but unproven benefit of improved healing and function of the subscapularis secondary to bone-to-bone healing. One concern, however, is that humeral stem subsidence may be greater when an osteotomy is performed owing to compromise of metaphyseal cortical bone, which may compromise functional outcomes. The humeral stem design may also influence subsidence when metaphyseal bone proximally is compromised. This is a concern in both metaphyseal and diaphyseal fitting stems. Metaphyseal collars on diaphyseal fitting stems rely on adequate bone stock in the metaphysis to provide the additional support needed. Also, posterior subluxation remains a challenge in shoulder arthroplasty. The integrity of the subscapularis is important in prevention of posterior subluxation.1 To our knowledge, no study to date has directly compared differences in humeral stem subsidence, loosening, or posterior subluxation between LTO and ST techniques with any humeral stem design. Our hypothesis is that no difference in proximal collar press-fit humeral stem subsidence or loosening exists, with no impairment of functional outcomes using the LTO technique. We also hypothesize that no difference in posterior subluxation exists between LTO and ST techniques.
MATERIALS AND METHODS
INCLUSION CRITERIA
Consecutive patients with a minimum of 12 months of radiographic follow-up were selected from 2007 to 2010 after TSA was performed by 1 of the senior authors (Dr. Miller and Dr. Voloshin). Study patients underwent primary TSA for primary osteoarthritis or rheumatoid arthritis.
EXCLUSION CRITERIA
Patients were excluded if they underwent TSA for posttraumatic glenohumeral arthritis, hemiarthroplasty, or osteonecrosis. Patients were also excluded if a rotator cuff tear was discovered intraoperatively or if they had a history of a rotator cuff repair. Additional exclusion criteria included postoperative trauma to the operative shoulder, postoperative infection, extensive documentation of chronic pain, and underlying neurologic disorder (eg, Parkinson disease, dystonia). Patients with a history of diabetes mellitus were not excluded.
SURGICAL TECHNIQUE
All patients underwent TSA via a deltopectoral approach in a modified beach chair position. Biceps tendons were tenodesed at the level of the pectoralis major. All patients received the same proximal collar press-fit implant (Bigliani-Flatow; Zimmer Biomet). These stems provide rotational stability in the metaphyseal segment via fins, vertical stability with the proximal collar, and distal fixation via an interference fit. All parts of the procedure were performed in similar fashion with the exception of ST vs LTO (Figures 1A-1D).
Continue to: LTO was performed as the primary...
LESSER TUBEROSITY OSTEOTOMY
LTO was performed as the primary or preferred technique of 1 surgeon. After completion of the biceps tenodesis, the lesser tuberosity is reflected off with the subscapularis intact using an osteotome. After placement of the press-fit humeral stem, the LTO is repaired using No. 5 Ethibond Excel sutures (Ethicon) passed through previously created bone tunnels in the greater tuberosity. These sutures are tied over metal buttons over the lateral cortex of the greater tuberosity. Last, the lateral corner of the rotator interval is repaired using a single No. 2 FiberWire (Arthrex).2
SUBSCAPULARIS TENOTOMY
ST is the preferred surgical technique of the second surgeon. After a biceps tenodesis, the subscapularis tendon is released from the lesser tuberosity at the margin of the bicipital groove. Through careful dissection, a single flap including the underlying capsule is created and reflected medially to the level of the coracoid. After placement of the press-fit humeral stem and humeral head, the subscapularis is repaired back in place through previous bone tunnels and with a No. 5 Ethibond Excel suture under the appropriate tension. Then, the lateral corner of the rotator interval is closed using a single No. 2 Ethibond Excel suture in a figure-of-eight fashion.2
RADIOGRAPHIC ANALYSIS
The primary variables analyzed were subsidence and loosening. Additional variables, including humeral-acromial distance (HAD) and subluxation index, were also analyzed to assess for any additional impact caused by subsidence or loosening.3 All radiographic measurements were taken from the Grashey (true anteroposterior) view, except subluxation index, which was calculated using the axillary view. All radiographic measurements were completed by 3 independent reviewers. All radiographs were completed in a consistent manner according to postoperative protocols.
HAD was measured preoperatively, immediately postoperatively, and at final follow-up at a minimum of 1 year. The HAD was measured from the lowest point on the acromion to the humerus using a perpendicular line (Figure 2).
Subsidence of the prosthesis was calculated by determining the difference between immediate postoperative heights of the prosthesis in comparison to the value of the final follow-up films. To calculate the height, 2 lines were drawn, 1 line was drawn perpendicular to the top of the prosthetic head and 1 perpendicular to the top of the greater tuberosity (Figure 3).
Continue to: Posterior subluxation is indicated...
Posterior subluxation is indicated by a value >65%, a centered head is between 35% and 65%, and anterior subluxation is indicated by a value <35% (Figure 4).3
The humeral stems were evaluated for loosening by assessing for lucency on final radiographic follow-up films. These were evaluated in a zonal fashion as demonstrated by Sanchez-Sotelo and colleagues4 and in Figure 5.
FUNCTIONAL OUTCOME EVALUATION
Before clinical evaluation, each study patient completed the Western Ontario Osteoarthritis of the Shoulder (WOOS) index; the Disabilities of the Hand, Arm and Shoulder (DASH) questionnaire, and the pain and function sections of the Constant score. The functional outcomes scores were captured postoperatively from October to November 2011. The WOOS is a validated outcome measure specific to osteoarthritis of the shoulder and has been used in prior studies evaluating outcomes of TSA.5-7 Previous studies have determined that the minimal clinically important difference for the WOOS score is 15 on a normalized 0 to 100 scale (100 being the best). The DASH score is a validated outcome measure for disorders of the upper extremity but is not specific to osteoarthritis of the shoulder.8 The Constant score is a validated outcome measure for a number of shoulder disorders, including TSA.9,10
STATISTICAL ANALYSIS
Statistical analyses were completed by a trained biostatistician. A power analysis was calculated using the noninferiority test to determine if adequate data had been obtained for this study. This was calculated by using previously accepted data demonstrating a statistically significant difference for subsidence and HAD. The data from these studies were used to make assumptions regarding accepted standard deviations and noninferiority margins, as calculated from the mean values of the 2 groups analyzed in each respective study.4,11 This analysis demonstrated power of 0.97 and 0.85 for the subsidence and HAD, respectively, given the current sample sizes. Intraclass coefficients were calculated to evaluate the measurements obtained during the radiographic analysis to determine the interrater agreement. Two samples’ t tests were calculated for the variables analyzed, along with P values and means.
RESULTS
DEMOGRAPHICS
A total of 51 consecutive patients were retrospectively selected for analysis. Of these, 16 patients were excluded from the study because they had <9 months of radiographic follow-up and were unavailable for further follow-up evaluation. Of the remaining 35 patients available for analysis, 4 patients had bilateral TSA, providing 39 shoulders for evaluation. Demographic characteristics of the study cohort are reported in Table 1.
| Table 1. Demographic Characteristics | |||
| Tenotomy (n = 24) | Osteotomy (n = 15) | P-value | |
| Age | 68.2 [7.4] | 70.2 [7.1] | 0.46 |
| Follow-up | 20.6 [11.5] | 18.5 [6.25] | 0.94 |
| Females | 7 (29%) | 6 (40%) | 0.58 |
| Dominant shoulder | 14 (58%) | 8 (53%) | 0.81 |
| Primary Diagnosis | |||
| Osteoarthritis | 22 (92%) | 15 (100%) | |
| Rheumatoid arthritis | 2 (8%) | 0 (0%) |
Fifteen patients underwent LTO, and 24 underwent ST. One patient underwent a tenotomy of the right shoulder and LTO of the left shoulder. Three LTOs were performed by the surgeon who primarily performed ST, owing to potential benefits of LTO. He eventually returned to his preferred technique of ST because of surgeon preference. Three ST procedures were completed by the surgeon who typically performed LTO at the start of the series prior to establishing LTO as his preferred technique. There was no significant difference between the study populations in terms of age, follow-up, male-to-female ratio, hand dominance, and primary diagnosis of osteoarthritis vs rheumatoid arthritis.
Continue to: There was no significant difference...
RADIOGRAPHIC DATA
There was no significant difference in preoperative HAD between the LTO and ST groups (9.5 ± 2.4 mm vs 10.9 ± 2.7 mm, P = .11). The immediate postoperative HAD was statistically significant between the LTO and ST groups (11.9 ± 3.7 mm vs 15.9 ± 4.5 mm, P = .005). There was as statistically significant difference noted in the final follow-up films between the LTO and ST groups (11.8 ± 3.2 mm vs 14.5 ± 3.9 mm, P = .025) (Table 2).
Table 2. Radiographic Data | |||||
Humeral Acromial Distance | |||||
| LTO | ST | P-Value | ||
Preoperative, mm | 9.5 | [2.4] | 10.9 | [2.7] | 0.11 |
Postoperative, mm | 11.9 | [3.7] | 15.9 | [4.5] | 0.005 |
Final follow-up, mm | 11.8 | [3.2] | 14.5 | [3.9] | 0.025 |
Subsidence | |||||
| LTO | ST | P-Value | ||
Subsidence, mm | 2.8 | [3.1] | 2.5 | [3.1] | 0.72 |
Subluxation Index | |||||
| LTO | ST | P-Value | ||
Preoperative, % | 0.55 | [0.06] | 0.54 | [0.07] | 0.45 |
Postoperative, % | 0.55 | [0.09] | 0.48 | [0.05] | 0.015 |
Lucent Lines | |||||
| LTO | ST | P-Value | ||
Lines >2 mm, % | 0.00 | 0.08 | 0.51 | ||
Abbreviations: LTO, lesser tuberosity osteotomy; ST, subscapularis tenotomy.
There were no statistically significant differences found in subsidence between LTO and ST groups at final follow-up (2.8 mm ± 3.1 mm vs 2.5 mm ± 3.1 mm, P = .72) (Table 2). No statistically significant difference was noted in the subluxation index between the LTO and ST groups (0.55% ± .06% vs 0.54% ± 0.07%, P = .45), but there was a statistically significant difference noted postoperatively between the LTO and ST groups (0.55% ± 0.09% vs .48% ± 0.05%, P = .015) (Table 2).
Two stems were noted to have lucent lines >2 mm, both within the ST cohort. Each had 1 stem zone >2 mm, 1 in zone 7, and 1 in zone 4. No statistically significant difference was identified between the LTO and ST groups (0/15 vs 2/24, P = .51) (Table 2).
FUNCTIONAL OUTCOMES
Study patients were evaluated using functional outcome scores, including the Constant, WOOS, and DASH scores (Table 3).
| Table 3. Functional Data | |||||
| LTO | ST | P-Value | |||
| WOOS index | 93.3 | [5.3] | 81.5 | [20.8] | 0.013 |
| DASH score | 8.4 | [6.6] | 13.8 | [4.9] | 0.13 |
| Constant score | 83.3 | [9.1] | 81.8 | [10.1] | 0.64 |
Abbreviations: DASH, disabilities of the arm, shoulder and hand; WOOS, Western Ontario Osteoarthritis of the Shoulder.
No statistically significant differences were noted in the DASH scores (8.4 ± 6.6 vs 13.8 ± 4.9, P = .13) or Constant scores (83.3 ± 9.1 vs 81.8 ± 10.1, P = .64) between the LTO and ST cohorts. There was a statistically significant difference between the WOOS scores (93.3 ± 5.3 vs 81.5 ± 20.8, P = .013). Because separate radiographic reviews were done by 3 independent personnel at 3 different times, it was important to ensure agreement among the reviewers. This was compared using the intraclass correlation coefficients. In the statistical analysis completed, the intraclass coefficients showed the 3 reviewers agreed with each other throughout the radiographic analysis (Table 4).
| Table 4. Testing Agreement: ICC | ||||
| ICC | CI, 2.5% | CI, 97.5% | ||
| HAD | Preoperative | 0.4451 | 0.2202 | 0.6443 |
| Postoperative | 0.6997 | 0.4836 | 0.834 | |
| Final follow-up | 0.5575 | 0.3592 | 0.7218 | |
| Subsidence | 0.6863 | 0.5349 | 0.807 | |
| SI | Preoperative | 0.3087 | 0.1061 | 0.5213 |
| Final follow-up | 0.5364 | 0.299 | 0.7186 |
Abbreviations: CI, confidence interval; HAD, humeral acromial distance; ICC, intraclass correlation coefficient; SI, subluxation index.
DISCUSSION
At final follow-up, we identified no statistically significant difference between the LTO and ST patients in subsidence, lucent lines >2 mm, or functional outcomes (Constant and DASH scores) in patients who underwent TSA with the same proximal collar press-fit humeral stem. In regard to the functional outcome scores, although the WOOS score was statistically significant (P = .013) between the LTO and ST cohorts, we do not feel that this is clinically relevant because it does not reach the minimal clinically important difference threshold of 15 points.8
A statistically significant difference was noted in postoperative subluxation index but was not clinically relevant, because the values between the LTO and ST groups (0.55 vs 0.48) still showed a centered humeral head. Gerber and colleagues3 discussed using a value of 0.65 as a measure of posterior humeral head subluxation, whereas Walch and colleagues12 defined posterior humeral head subluxation as a value >0.55. On the basis of these numbers, the values obtained in this study demonstrated that the postoperative values were still centered on the glenoid, and therefore were not clinically significant.3,12
Continue to: In regard to HAD, there...
In regard to HAD, there was a statistically significant difference noted postoperatively (P = .005) and at final follow-up (P = .025) between the LTO and ST cohorts. Saupe and colleagues13 demonstrated that a HAD <7 mm was considered abnormal and reflected subacromial space narrowing. The values noted in the LTO and ST patients on postoperative and final follow-up radiographs were statistically significant (Table 2), but not clinically relevant because both were >7 mm. A potential source for the variation in HAD may be due to X-ray position and angle.
Studies have shown a concern regarding the integrity of the subscapularis after tenotomy or peel used in TSA with abnormal subscapularis function.14,15 Miller and colleagues15 reported 41 patients, nearly two-thirds, of whom described subscapularis dysfunction. Those authors’ response to the poor clinical outcomes was to remove a fleck of bone with the tendon to achieve “bone-to-bone” healing.14 Gerber and colleagues16 reported on a series of patients using LTO and repair in TSA with 75% and 89% intact subscapularis function on clinical testing.16 Studies by Qureshi and colleagues17 and Scalise and colleagues18 showed similar results after LTO. Biomechanical studies have shown mixed results. Ponce and colleagues19 showed biomechanically superior results for LTO in comparison to the various repair techniques for ST. In another study, Giuseffi and colleagues20 showed no difference in LTO vs ST during biomechanical testing. In response to the increased concern regarding subscapularis integrity, Caplan and colleagues21 reported on 45 arthroplasties in 43 patients with improved postoperative testing with intact subscapularis testing in 90% to 100% of patients. A level 1 randomized control trial conducted by Lapner and colleagues22 did not demonstrate any clear clinical advantage of LTO vs ST. Controversy still exists regarding which is the preferred technique for TSA.
Sanchez-Sotelo and colleagues4 evaluated uncemented humeral components in 72 patients who underwent TSA. They found a humeral component was at risk for loosening if a radiolucent line ≥2 mm was present in at least 3 radiographic zones. They also evaluated tilt or subsidence by measurement and whether the components were observed to have changed. Their measured values correlated with their observed values. That study provided a benchmark for evaluation of loosening and subsidence used during this study.4 Although radiographic follow-up is limited in this study, we feel that any potential subsidence secondary to use of the LTO technique would be radiographically apparent at 1 year. There were 16 patients without adequate radiographic follow-up included in the study. However, we feel that this was not a large concern, because the study was adequately powered with the patients available to determine a difference based on subsidence.
CONCLUSION
We found no difference in subsidence, lucent lines >2 mm, posterior subluxation, and the Constant and DASH functional outcome scores when we compared TSA performed by a LTO with an ST technique with proximal collar press-fit humeral stem. These data cannot be extrapolated to metaphyseal fit stems, which may exhibit different settling characteristics in the setting of the LTO technique.
This paper will be judged for the Resident Writer’s Award.
1. Blasier R, Soslowsky L, Malicky D, Palmer M. Posterior glenohumeral subluxation: Active and passive stabilization in a biomechanical model. J Bone Joint Surg Am. 1997;79-A(3):433-440.
2. Buckley T, Miller R, Nicandri G, Lewis R, Voloshin I. Analysis of subscapularis integrity and function after lesser tuberosity osteotomy versus subscapularis tenotomy in total shoulder arthroplasty using ultrasound and validated clinical outcome measures. J Shoulder Elbow Surg. 2014;23(9):1309-1317. doi:10.1016/j.jse.2013.12.009.
3. Gerber C, Costouros JG, Sukthankar A, Fucentese SF. Static posterior humeral head subluxation and total shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18(4):505-510. doi:10.1016/j.jse.2009.03.003.
4. Sanchez-Sotelo J, Wright TW, O'Driscoll SW, Cofield RH, Rowland CM. Radiographic assessment of uncemented humeral components in total shoulder arthroplasty. J Arthroplasty. 2001;16(2):180-187.
5. Litchfield RB, McKee MD, Balyk R, et al. Cemented versus uncemented fixation of humeral components in total shoulder arthroplasty for osteoarthrtitis of the shoulder: A prospective, randomized, double-blind clinical trial-A JOINTs Canada Project. J Shoulder Elbow Surg. 2013;20(4):529-536. doi:10.1016/j.jse.2011.01.041.
6. Lo IK, Griffin S, Kirkley A. The development of a disease specific quality of life measurement tool for osteoarthritis of the shoulder: The Western Ontario Osteoarthritis of the Shoulder (WOOS) index. Osteoarthritis Cartilage. 2001;9(8):771-778. doi:10.1053/joca.2001.0474
7. Lo IK, Litchfield RB, Griffin S, Faber K, Patterson SD, Kirkley A. Quality of life outcome following hemiarthroplasty or total shoulder arthroplasty in patients with osteoarthritis. A prospective, randomized trial. J Bone Joint Surg Am. 2005;87(10):2178-2185. doi:10.2106/JBJS.D.02198
8. Hudak PL, Amadio PC, Bombardier C. Development of an upper extremity outcome measure: the DASH (disabilities of the arm, shoulder and hand) [corrected]. The Upper Extremity Collaborative Group (UECG). Am J Ind Med. 1996;29(6):602-608. doi:10.1002/(SICI)1097-0274(199606)29:6<602::AID-AJIM4>3.0.CO;2-L.
9. Constant CR, Gerber C, Emery RJ, Sojbjerg JO, Gohlke F, Boileau P. A review of the constant score: Modifications and guidelines for its use. J Shoulder Elbow Surg. 2008;17(2):355-361. doi:10.1016/j.jse.2007.06.022.
10. Constant CR, Murley AH. A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res. 1987;(214):160-164.
11. Mayerhoefer ME, Breitenseher MJ, Wurnig C, Roposch A. Shoulder impingement: Relationship of clinical symptoms and imaging criteria. Clin J Sport Med. 2009;19(2):83-89. doi:10.1097/JSM.0b013e318198e2e3.
12. Walch G, Badet R, Boulahia A, Khoury A. Morphologic study of the glenoid in primary glenohumeral osteoarthritis. J Arthroplasy. 1999;14(6):756-760.
13. Saupe N, Pfirmann CW, Schmid MR, et al. Association between rotator cuff abnormalities and reduced acromiohumeral distance. AJR Am J Roentgenol. 2006;187(2):376-382. doi:10.2214/AJR.05.0435.
14. Jackson J, Cil A, Smith J, Steinmann SP. Integrity and function of the subscapularis after total shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(7):1085-1090. doi:10.1016/j.jse.2010.04.001.
15. Miller SL, Hazrati Y, Klepps S, Chiang A, Flatow EL. Loss of subscapularis function after total shoulder replacement: a seldom recognized problem. J Shoulder Elbow Surg. 2003;12(1):29-34. doi:10.1067/mse.2003.128195.
16. Gerber C, Yian EH, Pfirrmann AW, Zumstein MA, Werner CM. Subscapularis muscle function and structure after total shoulder replacement with lesser tuberosity osteotomy and repair. J Bone Joint Surg Am. 2005;87(8):1739-1745. doi:10.2106/JBJS.D.02788.
17. Qureshi S, Hsiao A, Klug RA, Lee E, Braman J, Flatow EL. Subscapularis function after total shoulder replacement: results with lesser tuberosity osteotomy. J Shoulder Elbow Surg. 2008;17(1): 68-72. doi:10.1016/j.jse.2007.04.018.
18. Scalise JJ, Ciccone J, Iannotti JP. Clinical, radiographic and ultrasonographic comparison of subscapularis tenotomy and lesser tuberosity osteotomy for total shoulder arthroplasty. J Bone Joint Surg Am. 2010;92(7):1627-1634. doi:10.2106/JBJS.G.01461.
19. Ponce BA, Ahluwalia RS, Mazzocca AD, Gobezie RG, Warner JJ, Millett PJ. Biomechanical and clinical evaluation of a novel lesser tuberosity in total shoulder arthroplasty. J Bone Joint Surg Am. 2005;87 Suppl 2:1-8.
20. Giuseffi SA, Wongtriratanachai P, Omae H, et al. Biomechanical comparison of lesser tuberosity osteotomy versus subscapularis tenotomy in total shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(8):1087-1095. doi:10.1016/j.jse.2011.07.008.
21. Caplan JL, Whitfield W, Nevasier RJ. Subscapularis function after primary tendon to tendon repair in patients after replacement arthroplasty of the shoulder. J Shoulder Elbow Surg. 2009;18(2):193-196. doi:10.1016/j.jse.2008.10.019.
22. Lapner PLC, Sabri E, Rakhra K, Bell K, Athwal GS. Comparison of LTO to subscapularis peel in shoulder arthroplasty. J Bone Joint Surg Am. 2012;94(24):2239-2246. doi:10.2106/JBJS.K.01365.
1. Blasier R, Soslowsky L, Malicky D, Palmer M. Posterior glenohumeral subluxation: Active and passive stabilization in a biomechanical model. J Bone Joint Surg Am. 1997;79-A(3):433-440.
2. Buckley T, Miller R, Nicandri G, Lewis R, Voloshin I. Analysis of subscapularis integrity and function after lesser tuberosity osteotomy versus subscapularis tenotomy in total shoulder arthroplasty using ultrasound and validated clinical outcome measures. J Shoulder Elbow Surg. 2014;23(9):1309-1317. doi:10.1016/j.jse.2013.12.009.
3. Gerber C, Costouros JG, Sukthankar A, Fucentese SF. Static posterior humeral head subluxation and total shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18(4):505-510. doi:10.1016/j.jse.2009.03.003.
4. Sanchez-Sotelo J, Wright TW, O'Driscoll SW, Cofield RH, Rowland CM. Radiographic assessment of uncemented humeral components in total shoulder arthroplasty. J Arthroplasty. 2001;16(2):180-187.
5. Litchfield RB, McKee MD, Balyk R, et al. Cemented versus uncemented fixation of humeral components in total shoulder arthroplasty for osteoarthrtitis of the shoulder: A prospective, randomized, double-blind clinical trial-A JOINTs Canada Project. J Shoulder Elbow Surg. 2013;20(4):529-536. doi:10.1016/j.jse.2011.01.041.
6. Lo IK, Griffin S, Kirkley A. The development of a disease specific quality of life measurement tool for osteoarthritis of the shoulder: The Western Ontario Osteoarthritis of the Shoulder (WOOS) index. Osteoarthritis Cartilage. 2001;9(8):771-778. doi:10.1053/joca.2001.0474
7. Lo IK, Litchfield RB, Griffin S, Faber K, Patterson SD, Kirkley A. Quality of life outcome following hemiarthroplasty or total shoulder arthroplasty in patients with osteoarthritis. A prospective, randomized trial. J Bone Joint Surg Am. 2005;87(10):2178-2185. doi:10.2106/JBJS.D.02198
8. Hudak PL, Amadio PC, Bombardier C. Development of an upper extremity outcome measure: the DASH (disabilities of the arm, shoulder and hand) [corrected]. The Upper Extremity Collaborative Group (UECG). Am J Ind Med. 1996;29(6):602-608. doi:10.1002/(SICI)1097-0274(199606)29:6<602::AID-AJIM4>3.0.CO;2-L.
9. Constant CR, Gerber C, Emery RJ, Sojbjerg JO, Gohlke F, Boileau P. A review of the constant score: Modifications and guidelines for its use. J Shoulder Elbow Surg. 2008;17(2):355-361. doi:10.1016/j.jse.2007.06.022.
10. Constant CR, Murley AH. A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res. 1987;(214):160-164.
11. Mayerhoefer ME, Breitenseher MJ, Wurnig C, Roposch A. Shoulder impingement: Relationship of clinical symptoms and imaging criteria. Clin J Sport Med. 2009;19(2):83-89. doi:10.1097/JSM.0b013e318198e2e3.
12. Walch G, Badet R, Boulahia A, Khoury A. Morphologic study of the glenoid in primary glenohumeral osteoarthritis. J Arthroplasy. 1999;14(6):756-760.
13. Saupe N, Pfirmann CW, Schmid MR, et al. Association between rotator cuff abnormalities and reduced acromiohumeral distance. AJR Am J Roentgenol. 2006;187(2):376-382. doi:10.2214/AJR.05.0435.
14. Jackson J, Cil A, Smith J, Steinmann SP. Integrity and function of the subscapularis after total shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(7):1085-1090. doi:10.1016/j.jse.2010.04.001.
15. Miller SL, Hazrati Y, Klepps S, Chiang A, Flatow EL. Loss of subscapularis function after total shoulder replacement: a seldom recognized problem. J Shoulder Elbow Surg. 2003;12(1):29-34. doi:10.1067/mse.2003.128195.
16. Gerber C, Yian EH, Pfirrmann AW, Zumstein MA, Werner CM. Subscapularis muscle function and structure after total shoulder replacement with lesser tuberosity osteotomy and repair. J Bone Joint Surg Am. 2005;87(8):1739-1745. doi:10.2106/JBJS.D.02788.
17. Qureshi S, Hsiao A, Klug RA, Lee E, Braman J, Flatow EL. Subscapularis function after total shoulder replacement: results with lesser tuberosity osteotomy. J Shoulder Elbow Surg. 2008;17(1): 68-72. doi:10.1016/j.jse.2007.04.018.
18. Scalise JJ, Ciccone J, Iannotti JP. Clinical, radiographic and ultrasonographic comparison of subscapularis tenotomy and lesser tuberosity osteotomy for total shoulder arthroplasty. J Bone Joint Surg Am. 2010;92(7):1627-1634. doi:10.2106/JBJS.G.01461.
19. Ponce BA, Ahluwalia RS, Mazzocca AD, Gobezie RG, Warner JJ, Millett PJ. Biomechanical and clinical evaluation of a novel lesser tuberosity in total shoulder arthroplasty. J Bone Joint Surg Am. 2005;87 Suppl 2:1-8.
20. Giuseffi SA, Wongtriratanachai P, Omae H, et al. Biomechanical comparison of lesser tuberosity osteotomy versus subscapularis tenotomy in total shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(8):1087-1095. doi:10.1016/j.jse.2011.07.008.
21. Caplan JL, Whitfield W, Nevasier RJ. Subscapularis function after primary tendon to tendon repair in patients after replacement arthroplasty of the shoulder. J Shoulder Elbow Surg. 2009;18(2):193-196. doi:10.1016/j.jse.2008.10.019.
22. Lapner PLC, Sabri E, Rakhra K, Bell K, Athwal GS. Comparison of LTO to subscapularis peel in shoulder arthroplasty. J Bone Joint Surg Am. 2012;94(24):2239-2246. doi:10.2106/JBJS.K.01365.
TAKE-HOME POINTS
- LTO and ST remain viable options for takedown of the subscapularis.
- No difference exists in subsidence, lucent lines, and posterior subluxation on radiographic evaluation between LTO and ST.
- No clinically significant difference exists between outcome scores of patients with either technique.
- HAD was statistically significant but not clinically relevant between the 2 techniques.
- Results from the study do not apply to metaphyseal fitting stems, only diaphyseal fitting stems.