User login
Study Reaffirms Tenofovir’s Safety for Pregnant Women
A reanalysis of 2 National Institutes of Health (NIH)-funded studies refutes the conclusions of PROMISE (Promoting Maternal and Infant Survival Everywhere), which found tenofovir disoproxil fumarate (TDF) combination treatment raised the risk of adverse outcomes.
The PROMISE study compared several treatments in pregnant women with HIV in India and Africa. According to those researchers, women on TDF regimens were twice as likely as those on zidovudine to have a very preterm infant, and their infants were more likely to die within the first 2 weeks.
Those results surprised the current researchers, given that other, earlier studies had found TDF combinations safe for use during pregnancy. In fact, the World Health Organization recommends that all adults with HIV, including pregnant women, receive TDF combination therapy.
In the current study, NIH researchers compared outcomes from 2 US studies, reviewing records on more than 4,600 infants born to 3,847 women. The women were on either a combination regimen including zidovudine or 1 containing TDF.
The researchers found no significant differences in the risk of preterm birth or low birth weight, nor did they find any significant difference in severe birth outcomes, including infant death, in the first 2 weeks. “Notably,” they add, comparing women on zidovudine combination treatment with TDF/emtricitabine/atazanavir/ritonavir, the researchers found the TDF group had a 10% lower chance of preterm birth, low birth weight, and infant death.
Source:
National Institutes of Health. https://www.nih.gov/news-events/news-releases/anti-hiv-drug-combination-does-not-increase-preterm-birth-risk-study-suggests. Published April 25, 2018. Accessed May 24, 2018.
A reanalysis of 2 National Institutes of Health (NIH)-funded studies refutes the conclusions of PROMISE (Promoting Maternal and Infant Survival Everywhere), which found tenofovir disoproxil fumarate (TDF) combination treatment raised the risk of adverse outcomes.
The PROMISE study compared several treatments in pregnant women with HIV in India and Africa. According to those researchers, women on TDF regimens were twice as likely as those on zidovudine to have a very preterm infant, and their infants were more likely to die within the first 2 weeks.
Those results surprised the current researchers, given that other, earlier studies had found TDF combinations safe for use during pregnancy. In fact, the World Health Organization recommends that all adults with HIV, including pregnant women, receive TDF combination therapy.
In the current study, NIH researchers compared outcomes from 2 US studies, reviewing records on more than 4,600 infants born to 3,847 women. The women were on either a combination regimen including zidovudine or 1 containing TDF.
The researchers found no significant differences in the risk of preterm birth or low birth weight, nor did they find any significant difference in severe birth outcomes, including infant death, in the first 2 weeks. “Notably,” they add, comparing women on zidovudine combination treatment with TDF/emtricitabine/atazanavir/ritonavir, the researchers found the TDF group had a 10% lower chance of preterm birth, low birth weight, and infant death.
Source:
National Institutes of Health. https://www.nih.gov/news-events/news-releases/anti-hiv-drug-combination-does-not-increase-preterm-birth-risk-study-suggests. Published April 25, 2018. Accessed May 24, 2018.
A reanalysis of 2 National Institutes of Health (NIH)-funded studies refutes the conclusions of PROMISE (Promoting Maternal and Infant Survival Everywhere), which found tenofovir disoproxil fumarate (TDF) combination treatment raised the risk of adverse outcomes.
The PROMISE study compared several treatments in pregnant women with HIV in India and Africa. According to those researchers, women on TDF regimens were twice as likely as those on zidovudine to have a very preterm infant, and their infants were more likely to die within the first 2 weeks.
Those results surprised the current researchers, given that other, earlier studies had found TDF combinations safe for use during pregnancy. In fact, the World Health Organization recommends that all adults with HIV, including pregnant women, receive TDF combination therapy.
In the current study, NIH researchers compared outcomes from 2 US studies, reviewing records on more than 4,600 infants born to 3,847 women. The women were on either a combination regimen including zidovudine or 1 containing TDF.
The researchers found no significant differences in the risk of preterm birth or low birth weight, nor did they find any significant difference in severe birth outcomes, including infant death, in the first 2 weeks. “Notably,” they add, comparing women on zidovudine combination treatment with TDF/emtricitabine/atazanavir/ritonavir, the researchers found the TDF group had a 10% lower chance of preterm birth, low birth weight, and infant death.
Source:
National Institutes of Health. https://www.nih.gov/news-events/news-releases/anti-hiv-drug-combination-does-not-increase-preterm-birth-risk-study-suggests. Published April 25, 2018. Accessed May 24, 2018.
Erenumab May Control Migraines When Other Preventives Have Failed
LOS ANGELES—Patients who have tried and failed other preventive therapies may find that erenumab can prevent their migraines, according to a study presented at the 70th Annual Meeting of the American Academy of Neurology. Erenumab is a fully human monoclonal antibody that inhibits the calcitonin gene-related peptide (CGRP) receptor.
Current oral preventive therapies are associated with low adherence rates due to their lack of efficacy or poor tolerability. Therefore, researchers thought it important to assess the safety and efficacy of erenumab in patients who had failed multiple therapies. A previous post hoc analysis of the STRIVE study showed that patients who had failed two prior preventives responded to erenumab. “Their odds ratio was far greater than [that of] those who were previous preventive naïve,” said Peter J. Goadsby, MD, PhD, who presented the newest erenumab data in the Emerging Science plenary session. Dr. Goadsby is a Professor of Neurology at Kings College London and the University of California, San Francisco.
“The people we included in our study were considered more difficult to treat, meaning that up to four other preventive treatments had not worked for them,” said lead study author
The LIBERTY Study
The LIBERTY study was a phase IIIb, randomized, double-blind, placebo-controlled trial thta assessed erenumab in patients who had failed at least two and not more than four prior preventives. A total of 246 patients with episodic migraine were randomized one to one to an injection of erenumab (140 mg) or placebo once per month for three months. There were 125 patients in the placebo arm and 121 in the erenumab arm. The primary end point was the proportion of patients achieving a 50% or greater reduction in mean monthly migraine days (MMDs) during weeks nine to 12 (ie, month three) of the study. Secondary end points included change from baseline to month three in MMDs and monthly acute migraine-specific medication days (MSMDs) and safety and tolerability.
At baseline, 39% of participants had been treated unsuccessfully with two other medications, 38% with three medications, and 23% with four medications. On average, participants had nine migraine days per month and used an acute migraine drug to stop an attack five times per month.
At week 12, the proportion of patients achieving a 50% or greater reduction in MMDs was higher in those treated with erenumab versus placebo (30.3% vs 13.7%). At week 12, there were greater reductions in MMDs and MSMDs with erenumab versus placebo. The safety and tolerability of erenumab were comparable to those of placebo. No patients in the erenumab group discontinued treatment due to adverse events.
Hope for Refractory Patients?
LIBERTY is the first dedicated study to address a problem that is all too common in neurology, said Dr. Goadsby. “It is hard to deal with patients who have failed prior preventives.” Erenumab, he said, is the first of the CGRP monoclonal antibodies to “control migraine and prevent migraine in patients who are so disabled and whom we cannot treat with current medicine.”
“Our results show that people who thought their migraines were difficult to prevent may actually have hope of finding pain relief,” said Dr. Reuter. “More research is now needed to understand who is most likely to benefit from this new treatment.”
The study was supported by Novartis Pharma
—Glenn S. Williams
LOS ANGELES—Patients who have tried and failed other preventive therapies may find that erenumab can prevent their migraines, according to a study presented at the 70th Annual Meeting of the American Academy of Neurology. Erenumab is a fully human monoclonal antibody that inhibits the calcitonin gene-related peptide (CGRP) receptor.
Current oral preventive therapies are associated with low adherence rates due to their lack of efficacy or poor tolerability. Therefore, researchers thought it important to assess the safety and efficacy of erenumab in patients who had failed multiple therapies. A previous post hoc analysis of the STRIVE study showed that patients who had failed two prior preventives responded to erenumab. “Their odds ratio was far greater than [that of] those who were previous preventive naïve,” said Peter J. Goadsby, MD, PhD, who presented the newest erenumab data in the Emerging Science plenary session. Dr. Goadsby is a Professor of Neurology at Kings College London and the University of California, San Francisco.
“The people we included in our study were considered more difficult to treat, meaning that up to four other preventive treatments had not worked for them,” said lead study author
The LIBERTY Study
The LIBERTY study was a phase IIIb, randomized, double-blind, placebo-controlled trial thta assessed erenumab in patients who had failed at least two and not more than four prior preventives. A total of 246 patients with episodic migraine were randomized one to one to an injection of erenumab (140 mg) or placebo once per month for three months. There were 125 patients in the placebo arm and 121 in the erenumab arm. The primary end point was the proportion of patients achieving a 50% or greater reduction in mean monthly migraine days (MMDs) during weeks nine to 12 (ie, month three) of the study. Secondary end points included change from baseline to month three in MMDs and monthly acute migraine-specific medication days (MSMDs) and safety and tolerability.
At baseline, 39% of participants had been treated unsuccessfully with two other medications, 38% with three medications, and 23% with four medications. On average, participants had nine migraine days per month and used an acute migraine drug to stop an attack five times per month.
At week 12, the proportion of patients achieving a 50% or greater reduction in MMDs was higher in those treated with erenumab versus placebo (30.3% vs 13.7%). At week 12, there were greater reductions in MMDs and MSMDs with erenumab versus placebo. The safety and tolerability of erenumab were comparable to those of placebo. No patients in the erenumab group discontinued treatment due to adverse events.
Hope for Refractory Patients?
LIBERTY is the first dedicated study to address a problem that is all too common in neurology, said Dr. Goadsby. “It is hard to deal with patients who have failed prior preventives.” Erenumab, he said, is the first of the CGRP monoclonal antibodies to “control migraine and prevent migraine in patients who are so disabled and whom we cannot treat with current medicine.”
“Our results show that people who thought their migraines were difficult to prevent may actually have hope of finding pain relief,” said Dr. Reuter. “More research is now needed to understand who is most likely to benefit from this new treatment.”
The study was supported by Novartis Pharma
—Glenn S. Williams
LOS ANGELES—Patients who have tried and failed other preventive therapies may find that erenumab can prevent their migraines, according to a study presented at the 70th Annual Meeting of the American Academy of Neurology. Erenumab is a fully human monoclonal antibody that inhibits the calcitonin gene-related peptide (CGRP) receptor.
Current oral preventive therapies are associated with low adherence rates due to their lack of efficacy or poor tolerability. Therefore, researchers thought it important to assess the safety and efficacy of erenumab in patients who had failed multiple therapies. A previous post hoc analysis of the STRIVE study showed that patients who had failed two prior preventives responded to erenumab. “Their odds ratio was far greater than [that of] those who were previous preventive naïve,” said Peter J. Goadsby, MD, PhD, who presented the newest erenumab data in the Emerging Science plenary session. Dr. Goadsby is a Professor of Neurology at Kings College London and the University of California, San Francisco.
“The people we included in our study were considered more difficult to treat, meaning that up to four other preventive treatments had not worked for them,” said lead study author
The LIBERTY Study
The LIBERTY study was a phase IIIb, randomized, double-blind, placebo-controlled trial thta assessed erenumab in patients who had failed at least two and not more than four prior preventives. A total of 246 patients with episodic migraine were randomized one to one to an injection of erenumab (140 mg) or placebo once per month for three months. There were 125 patients in the placebo arm and 121 in the erenumab arm. The primary end point was the proportion of patients achieving a 50% or greater reduction in mean monthly migraine days (MMDs) during weeks nine to 12 (ie, month three) of the study. Secondary end points included change from baseline to month three in MMDs and monthly acute migraine-specific medication days (MSMDs) and safety and tolerability.
At baseline, 39% of participants had been treated unsuccessfully with two other medications, 38% with three medications, and 23% with four medications. On average, participants had nine migraine days per month and used an acute migraine drug to stop an attack five times per month.
At week 12, the proportion of patients achieving a 50% or greater reduction in MMDs was higher in those treated with erenumab versus placebo (30.3% vs 13.7%). At week 12, there were greater reductions in MMDs and MSMDs with erenumab versus placebo. The safety and tolerability of erenumab were comparable to those of placebo. No patients in the erenumab group discontinued treatment due to adverse events.
Hope for Refractory Patients?
LIBERTY is the first dedicated study to address a problem that is all too common in neurology, said Dr. Goadsby. “It is hard to deal with patients who have failed prior preventives.” Erenumab, he said, is the first of the CGRP monoclonal antibodies to “control migraine and prevent migraine in patients who are so disabled and whom we cannot treat with current medicine.”
“Our results show that people who thought their migraines were difficult to prevent may actually have hope of finding pain relief,” said Dr. Reuter. “More research is now needed to understand who is most likely to benefit from this new treatment.”
The study was supported by Novartis Pharma
—Glenn S. Williams
Genes, not adiposity, may be driving appetite differences in obesity
BOSTON – Evidence from a twin study points to genes, rather than just adiposity, as the underlying factor in differences in appetite and satiety that have been observed in obesity.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The work adds a new dimension – and some questions – to previous research, which suggested individuals with obesity show heightened brain activation to food cues, especially calorically dense food.
“We thought it was fat mass…but when we controlled for everything that monozygotic pairs have in common, that relationship went away, implicating something that the monozygotic twins have in common, i.e., genetics,” said first author Jennifer Rosenbaum, MD, in a video interview at the annual meeting of the American Academy of Clinical Endocrinologists.
Dr. Rosenbaum, a fellow in the department of metabolism, endocrinology, and nutrition at the University of Washington, Seattle, and her collaborators made use of a statewide twin registry to conduct an extensive investigation of subjective and objective measures of appetite and satiety in the 42 twin pairs.
Twins had a mean age of 31 years; 27 of the twin pairs were monozygotic, Dr. Rosenbaum said. At least one member of each twin pair met criteria for obesity, and participants had a mean body mass index of 32.8 kg/m2.
On the study day, participants arrived in fasting state, and had a fixed-calorie breakfast equivalent to 10% of their daily caloric needs. They then underwent dual-energy x-ray absorptiometry scanning to determine adiposity, and also filled out a behavioral questionnaire.
Then, participants received the first of two functional MRI scans; during the scan, they were shown images of high calorie foods, low calorie foods, and nonfood objects, completing ratings of how appealing they found each image. After consuming another standardized meal equivalent to 20% of daily caloric needs, the fMRI scan was repeated.
Finally, participants were given access to a buffet meal and allowed to eat as much as they chose; consumption was measured. Before and after each meal and scan, and at various points during the day, the investigators also obtained blood samples and asked participants to rate their hunger on a visual analog scale.
“When compared with how much fat mass they had, there was no relationship between how hungry or full they were when they were fasting, how hungry or full they were with a snack, or when they ate the buffet. It just didn’t matter how much fat mass they had” for subjective reporting of hunger and fullness, said Dr. Rosenbaum.
However, there was a direct correlation between fat mass and amount consumed at the ad libitum buffet. Additionally, the fMRI analysis showed that “the brain activation that we would expect to go down, didn’t seem to go down as much if you had more adiposity,” she said.
As fat mass went up, areas of the brain implicated in appetite and reward showed more activity when participants were presented with the tempting images of high calorie foods, regardless of the calories consumed. These areas include the ventral and dorsal striata, the amygdala, the insula, the ventral tegmental area, and the medial orbitofrontal cortex.
Next, the researchers looked for differences within the monozygotic twin pairs, who essentially share a genome. They compared the brain activation of the twin with the higher fat mass with that of the twin with lower fat mass. Instead of seeing the same correlation between higher adiposity and greater brain activation with tempting stimuli, “Suddenly, we lost that relationship between how many calories they would eat and how their brain activated with the food,” said Dr. Rosenbaum. This is a clue, she said, that genetics, rather than simple adiposity, is driving the different responses to food cues.
The study was funded by the National Institutes of Health. Dr. Rosenbaum reported no financial disclosures.
BOSTON – Evidence from a twin study points to genes, rather than just adiposity, as the underlying factor in differences in appetite and satiety that have been observed in obesity.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The work adds a new dimension – and some questions – to previous research, which suggested individuals with obesity show heightened brain activation to food cues, especially calorically dense food.
“We thought it was fat mass…but when we controlled for everything that monozygotic pairs have in common, that relationship went away, implicating something that the monozygotic twins have in common, i.e., genetics,” said first author Jennifer Rosenbaum, MD, in a video interview at the annual meeting of the American Academy of Clinical Endocrinologists.
Dr. Rosenbaum, a fellow in the department of metabolism, endocrinology, and nutrition at the University of Washington, Seattle, and her collaborators made use of a statewide twin registry to conduct an extensive investigation of subjective and objective measures of appetite and satiety in the 42 twin pairs.
Twins had a mean age of 31 years; 27 of the twin pairs were monozygotic, Dr. Rosenbaum said. At least one member of each twin pair met criteria for obesity, and participants had a mean body mass index of 32.8 kg/m2.
On the study day, participants arrived in fasting state, and had a fixed-calorie breakfast equivalent to 10% of their daily caloric needs. They then underwent dual-energy x-ray absorptiometry scanning to determine adiposity, and also filled out a behavioral questionnaire.
Then, participants received the first of two functional MRI scans; during the scan, they were shown images of high calorie foods, low calorie foods, and nonfood objects, completing ratings of how appealing they found each image. After consuming another standardized meal equivalent to 20% of daily caloric needs, the fMRI scan was repeated.
Finally, participants were given access to a buffet meal and allowed to eat as much as they chose; consumption was measured. Before and after each meal and scan, and at various points during the day, the investigators also obtained blood samples and asked participants to rate their hunger on a visual analog scale.
“When compared with how much fat mass they had, there was no relationship between how hungry or full they were when they were fasting, how hungry or full they were with a snack, or when they ate the buffet. It just didn’t matter how much fat mass they had” for subjective reporting of hunger and fullness, said Dr. Rosenbaum.
However, there was a direct correlation between fat mass and amount consumed at the ad libitum buffet. Additionally, the fMRI analysis showed that “the brain activation that we would expect to go down, didn’t seem to go down as much if you had more adiposity,” she said.
As fat mass went up, areas of the brain implicated in appetite and reward showed more activity when participants were presented with the tempting images of high calorie foods, regardless of the calories consumed. These areas include the ventral and dorsal striata, the amygdala, the insula, the ventral tegmental area, and the medial orbitofrontal cortex.
Next, the researchers looked for differences within the monozygotic twin pairs, who essentially share a genome. They compared the brain activation of the twin with the higher fat mass with that of the twin with lower fat mass. Instead of seeing the same correlation between higher adiposity and greater brain activation with tempting stimuli, “Suddenly, we lost that relationship between how many calories they would eat and how their brain activated with the food,” said Dr. Rosenbaum. This is a clue, she said, that genetics, rather than simple adiposity, is driving the different responses to food cues.
The study was funded by the National Institutes of Health. Dr. Rosenbaum reported no financial disclosures.
BOSTON – Evidence from a twin study points to genes, rather than just adiposity, as the underlying factor in differences in appetite and satiety that have been observed in obesity.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The work adds a new dimension – and some questions – to previous research, which suggested individuals with obesity show heightened brain activation to food cues, especially calorically dense food.
“We thought it was fat mass…but when we controlled for everything that monozygotic pairs have in common, that relationship went away, implicating something that the monozygotic twins have in common, i.e., genetics,” said first author Jennifer Rosenbaum, MD, in a video interview at the annual meeting of the American Academy of Clinical Endocrinologists.
Dr. Rosenbaum, a fellow in the department of metabolism, endocrinology, and nutrition at the University of Washington, Seattle, and her collaborators made use of a statewide twin registry to conduct an extensive investigation of subjective and objective measures of appetite and satiety in the 42 twin pairs.
Twins had a mean age of 31 years; 27 of the twin pairs were monozygotic, Dr. Rosenbaum said. At least one member of each twin pair met criteria for obesity, and participants had a mean body mass index of 32.8 kg/m2.
On the study day, participants arrived in fasting state, and had a fixed-calorie breakfast equivalent to 10% of their daily caloric needs. They then underwent dual-energy x-ray absorptiometry scanning to determine adiposity, and also filled out a behavioral questionnaire.
Then, participants received the first of two functional MRI scans; during the scan, they were shown images of high calorie foods, low calorie foods, and nonfood objects, completing ratings of how appealing they found each image. After consuming another standardized meal equivalent to 20% of daily caloric needs, the fMRI scan was repeated.
Finally, participants were given access to a buffet meal and allowed to eat as much as they chose; consumption was measured. Before and after each meal and scan, and at various points during the day, the investigators also obtained blood samples and asked participants to rate their hunger on a visual analog scale.
“When compared with how much fat mass they had, there was no relationship between how hungry or full they were when they were fasting, how hungry or full they were with a snack, or when they ate the buffet. It just didn’t matter how much fat mass they had” for subjective reporting of hunger and fullness, said Dr. Rosenbaum.
However, there was a direct correlation between fat mass and amount consumed at the ad libitum buffet. Additionally, the fMRI analysis showed that “the brain activation that we would expect to go down, didn’t seem to go down as much if you had more adiposity,” she said.
As fat mass went up, areas of the brain implicated in appetite and reward showed more activity when participants were presented with the tempting images of high calorie foods, regardless of the calories consumed. These areas include the ventral and dorsal striata, the amygdala, the insula, the ventral tegmental area, and the medial orbitofrontal cortex.
Next, the researchers looked for differences within the monozygotic twin pairs, who essentially share a genome. They compared the brain activation of the twin with the higher fat mass with that of the twin with lower fat mass. Instead of seeing the same correlation between higher adiposity and greater brain activation with tempting stimuli, “Suddenly, we lost that relationship between how many calories they would eat and how their brain activated with the food,” said Dr. Rosenbaum. This is a clue, she said, that genetics, rather than simple adiposity, is driving the different responses to food cues.
The study was funded by the National Institutes of Health. Dr. Rosenbaum reported no financial disclosures.
REPORTING FROM AACE 2018
Experimental drug may help those living with celiac disease
“Contamination with gluten happens during food processing, food packaging, cooking, and [because of] inadequate labeling,” Francisco Leon, MD, a consultant for Amgen and former CEO of Celimmune, said in a media briefing in advance of the annual Digestive Disease Week® (DDW). “Gluten is [also] present in unsuspected places like lipstick, toothpaste, even medicines. It is virtually impossible for celiac patients to completely avoid gluten.”
The new drug, AMG 714, works by blocking interleukin 15, a known intestinal inflammatory mediator, and lowers inflammation, resulting in fewer symptoms for patients.
In a 12-week study, patients received either 150- or 300-mg doses of subcutaneous AMG 714, or placebo. All doses of AMG 714 were administered six times over the course of the study. From weeks 2 to 12, patients were challenged with a daily dose of 2.5 grams of gluten, which is considered a high dose.
The results showed that AMG 714 significantly reduced Celiac Disease Patient Reported Outcomes at the 300-mg dose (P = .02) compared with placebo. Similarly, AMG 714 decreased Celiac Disease Gastrointestinal Symptom Rating Scale scores at the 150-mg (P = .17) and 300-mg (P = .07) doses, compared with placebo.
Dr. Leon noted that there was another subset of patients who did not receive the gluten challenge because they were shown to have intestinal atrophy – marked by the presence of flat mucosa – because of contamination in their gluten-free diets. This was measured by way of a novel stool and urine detection test. This information will be presented at DDW.
AMG 714 also improved gluten-triggered diarrhea and physician assessments, according to Dr. Leon. In fact, using the Bristol Stool Form Scale, patients who received both the 150-mg (P = .01) and 300-mg (P = .0002) doses had substantially lower rates of diarrhea, compared with placebo.
These positive results were not isolated to the Bristol Stool Form Scale scores, but extended to the Physician Global Assessment as well.
“The proportion of subjects whom physicians evaluated as clinically active at week 12 was 0 in the high dose of AMG 714 while it was 33% in the placebo group,” stated Dr. Leon.
While AMG 714 has shown promise in alleviating celiac disease symptoms for patients inadvertently exposed to gluten, it is not intended to treat exposure to high amounts of gluten.
“It is important to note that AMG 714 is intended to protect against modest contamination [with] gluten and not against the effects of regularly eaten large amounts of gluten, like bread or pasta,” Dr. Leon cautioned. “But we know that people with celiac disease are inadvertently exposed to gluten. So it is our hope that AMG 714 will allow these patients to experience fewer gluten-triggered events.”
The information presented by Dr. Leon was only a taste of what will be presented at DDW®.
“At DDW, we will show you a reduction in inflammation, and we will also show you the safety profile. There were no serious adverse events.
“We are hopeful that this will become one of the tools to combat celiac disease.”
“Contamination with gluten happens during food processing, food packaging, cooking, and [because of] inadequate labeling,” Francisco Leon, MD, a consultant for Amgen and former CEO of Celimmune, said in a media briefing in advance of the annual Digestive Disease Week® (DDW). “Gluten is [also] present in unsuspected places like lipstick, toothpaste, even medicines. It is virtually impossible for celiac patients to completely avoid gluten.”
The new drug, AMG 714, works by blocking interleukin 15, a known intestinal inflammatory mediator, and lowers inflammation, resulting in fewer symptoms for patients.
In a 12-week study, patients received either 150- or 300-mg doses of subcutaneous AMG 714, or placebo. All doses of AMG 714 were administered six times over the course of the study. From weeks 2 to 12, patients were challenged with a daily dose of 2.5 grams of gluten, which is considered a high dose.
The results showed that AMG 714 significantly reduced Celiac Disease Patient Reported Outcomes at the 300-mg dose (P = .02) compared with placebo. Similarly, AMG 714 decreased Celiac Disease Gastrointestinal Symptom Rating Scale scores at the 150-mg (P = .17) and 300-mg (P = .07) doses, compared with placebo.
Dr. Leon noted that there was another subset of patients who did not receive the gluten challenge because they were shown to have intestinal atrophy – marked by the presence of flat mucosa – because of contamination in their gluten-free diets. This was measured by way of a novel stool and urine detection test. This information will be presented at DDW.
AMG 714 also improved gluten-triggered diarrhea and physician assessments, according to Dr. Leon. In fact, using the Bristol Stool Form Scale, patients who received both the 150-mg (P = .01) and 300-mg (P = .0002) doses had substantially lower rates of diarrhea, compared with placebo.
These positive results were not isolated to the Bristol Stool Form Scale scores, but extended to the Physician Global Assessment as well.
“The proportion of subjects whom physicians evaluated as clinically active at week 12 was 0 in the high dose of AMG 714 while it was 33% in the placebo group,” stated Dr. Leon.
While AMG 714 has shown promise in alleviating celiac disease symptoms for patients inadvertently exposed to gluten, it is not intended to treat exposure to high amounts of gluten.
“It is important to note that AMG 714 is intended to protect against modest contamination [with] gluten and not against the effects of regularly eaten large amounts of gluten, like bread or pasta,” Dr. Leon cautioned. “But we know that people with celiac disease are inadvertently exposed to gluten. So it is our hope that AMG 714 will allow these patients to experience fewer gluten-triggered events.”
The information presented by Dr. Leon was only a taste of what will be presented at DDW®.
“At DDW, we will show you a reduction in inflammation, and we will also show you the safety profile. There were no serious adverse events.
“We are hopeful that this will become one of the tools to combat celiac disease.”
“Contamination with gluten happens during food processing, food packaging, cooking, and [because of] inadequate labeling,” Francisco Leon, MD, a consultant for Amgen and former CEO of Celimmune, said in a media briefing in advance of the annual Digestive Disease Week® (DDW). “Gluten is [also] present in unsuspected places like lipstick, toothpaste, even medicines. It is virtually impossible for celiac patients to completely avoid gluten.”
The new drug, AMG 714, works by blocking interleukin 15, a known intestinal inflammatory mediator, and lowers inflammation, resulting in fewer symptoms for patients.
In a 12-week study, patients received either 150- or 300-mg doses of subcutaneous AMG 714, or placebo. All doses of AMG 714 were administered six times over the course of the study. From weeks 2 to 12, patients were challenged with a daily dose of 2.5 grams of gluten, which is considered a high dose.
The results showed that AMG 714 significantly reduced Celiac Disease Patient Reported Outcomes at the 300-mg dose (P = .02) compared with placebo. Similarly, AMG 714 decreased Celiac Disease Gastrointestinal Symptom Rating Scale scores at the 150-mg (P = .17) and 300-mg (P = .07) doses, compared with placebo.
Dr. Leon noted that there was another subset of patients who did not receive the gluten challenge because they were shown to have intestinal atrophy – marked by the presence of flat mucosa – because of contamination in their gluten-free diets. This was measured by way of a novel stool and urine detection test. This information will be presented at DDW.
AMG 714 also improved gluten-triggered diarrhea and physician assessments, according to Dr. Leon. In fact, using the Bristol Stool Form Scale, patients who received both the 150-mg (P = .01) and 300-mg (P = .0002) doses had substantially lower rates of diarrhea, compared with placebo.
These positive results were not isolated to the Bristol Stool Form Scale scores, but extended to the Physician Global Assessment as well.
“The proportion of subjects whom physicians evaluated as clinically active at week 12 was 0 in the high dose of AMG 714 while it was 33% in the placebo group,” stated Dr. Leon.
While AMG 714 has shown promise in alleviating celiac disease symptoms for patients inadvertently exposed to gluten, it is not intended to treat exposure to high amounts of gluten.
“It is important to note that AMG 714 is intended to protect against modest contamination [with] gluten and not against the effects of regularly eaten large amounts of gluten, like bread or pasta,” Dr. Leon cautioned. “But we know that people with celiac disease are inadvertently exposed to gluten. So it is our hope that AMG 714 will allow these patients to experience fewer gluten-triggered events.”
The information presented by Dr. Leon was only a taste of what will be presented at DDW®.
“At DDW, we will show you a reduction in inflammation, and we will also show you the safety profile. There were no serious adverse events.
“We are hopeful that this will become one of the tools to combat celiac disease.”
REPORTING FROM DDW 2018
Ureteral reimplantation for injuries not easily managed with stenting
The risk of lower urinary tract injury (bladder or ureters) at the time of benign gynecologic surgery is estimated to be between 0.3% and 4%. The majority are bladder injuries, with ureteral injuries occurring in 0.3%-1.8% of hysterectomies. While urologic procedures account for the majority of iatrogenic ureteral injuries, gynecologic surgery is the second leading cause, followed by general surgery and colorectal surgery.
With respect to hysterectomy in particular, the risk of ureteral injury is less than 1%. In a large prospective cohort of women undergoing hysterectomy for benign indications in 53 hospitals in Finland, rates of ureteral injury varied based on the route of hysterectomy, with laparoscopic and abdominal routes having an injury rate of 0.3% and the vaginal route having an injury rate of 0.04% (Human Reprod. 2011;26[7]:1741-51). The risk generally is higher with procedures for endometriosis, large fibroids, cancer, or pelvic organ prolapse.
During hysterectomy and with gynecologic surgery overall, ureteral injuries occur most commonly at three locations: at the level of the infundibulopelvic ligament and ovarian vessels, at the level of the uterine artery, and near the vaginal cuff. Identification and knowledge of the course of the ureter at these three locations is essential in preventing ureteral injury during pelvic surgery.

SOURCE: DR. MUELLER AND DR. KENTON
Perioperative ureteral stenting has been proposed as a method of preventing iatrogenic injury by allowing surgeons to more easily identify the ureters during surgery. Available reports suggest, however, that the actual risk of injury is not decreased and may even be increased by placing prophylactic ureteral stents, and most surgeons have moved away from this practice. The use of lighted ureteral stents during complex laparoscopic endometriosis resections may be helpful.
Many health care systems recommend intraoperative cystoscopy with bladder and ureteral survey to evaluate the integrity of the lower urinary tract at the time of all hysterectomies. A recent study of nearly 3,000 women undergoing benign hysterectomies at the University of Michigan, Ann Arbor, showed a significant decrease in the rate of delayed diagnosis of urinary tract injuries with implementation of a universal cystoscopy policy. While the rate of lower urinary tract injury was fairly consistent before and after implementation of the policy (2.6% and 1.8%, respectively), the rate of delayed detection of a lower urinary tract injury decreased from 0.7% before the policy to 0.1% after implementation (Obstet Gynecol. 2016;127[2]:369-75). The study also showed that hospital costs nearly doubled with a delayed detection of a lower urinary tract injury.
Unfortunately, even a normal postoperative cystoscopy does not ensure there is no lower urinary tract injury, especially considering that thermal injuries resulting from the use of energy devices typically do not present until 7-14 days after surgery. Overall, however, ureteral injury detection rates with universal cystoscopy approach 97% (Obstet Gynecol. 2009;113:6-10).
Identifying injuries
Intraoperative recognition and repair always is preferred, and when ureteral injuries are discovered or suspected in the operating room, cystoscopy and retrograde pyelography is the most helpful imaging tool. Contrast dye is injected during cystoscopy directly into the renal collecting system through the ureteral orifices; with fluoroscopy, the surgeon can visualize the integrity of the ureter from the bladder to the renal pelvis to diagnosis a ureteral injury, including ureteral transection, kinking, or ligation caused by a suture or sealing device.
If retrograde pyelography shows a transection or injury from a crushing clamp or sealing device, we recommend ureteroureteral anastomosis or urethral neocystostomy depending on the extent and location of the injury. If the ureter just appears kinked, sometime simply releasing the cuff sutures or uterosacral ligament sutures will resolve the obstruction. If there is extravasation of contrast suggesting a partial tear, placing a double-J ureteral stent for 6-8 weeks is frequently sufficient.
Patients with delayed iatrogenic ureteral injuries present with symptoms that often are nonspecific and that include abdominal or flank pain, fever, nausea, vomiting, back pain, and leukocytosis.
We recommend that patients with a history of surgery and symptoms suggestive of a ureteral injury be initially evaluated with CT urography that images the renal collecting system both as contrast dye is instilled and again several minutes later as it has progressed through the entire urinary tract. Alternatively, if CT urography is unavailable, a retrograde pyelogram can be performed as an emergency procedure to determine the location of renal injury.
Surgical management
Delayed ureteral injuries resulting in partial ureteral obstruction or extravasation of urine into the pelvis can sometimes be managed conservatively though placement of an internalized double J stent. The stent can be placed in a retrograde fashion via cystoscopy by a
We typically manage delayed ureteral injuries that are not amenable to, or do not heal with, ureteral stenting with ureteroneocystotomy, or ureteral reimplantation, into the bladder. This technique is effective for distal ureteral injuries that result in obstruction or fistula and are in close proximity to the bladder (most iatrogenic gynecologic injuries). We perform ureteroneocystotomy via an open, robotic, or laparoscopic route of access depending on the circumstance.
Our preferred route of access is minimally invasive with the da Vinci robot. A camera port is placed at the umbilicus, two robotic ports are placed on the patient’s left side at the level of the umbilicus, and one is placed on the patient’s right side at the level of the umbilicus with an additional assistant port on the right side. It is helpful if each port is at least 8 cm apart. If obstruction or transection is suspected to be more proximal, the ports may have been shifted above the umbilicus to optimally mobilize the ureter.
First, the ureter is identified and dissected. Regardless of the site of injury, which is usually identifiable with inflammation and scar tissue, it is always easiest to identify the ureter at the bifurcation of the common iliac vessels. The isolated ureter is inspected proximally and above the area of injury, and we find it helpful to place a vessel loop around the ureter for easy manipulation and counter traction. Care must be taken not to disturb the adventitia and blood supply. We do not transect the ureter until we’re ready to reimplant it in the bladder.
To mobilize the bladder and prepare for a tension-free anastomosis, an adequate retropubic dissection is performed, starting with an incision in the anterior abdominal wall peritoneum and taking it down to the level of the pubic bone and into the retropubic space. It is important to be mindful of the location of the obturator neurovascular bundle when performing this dissection.
Achieving a tension-free and water-tight anastomosis of the ureter to the bladder is critical. The bladder should be mobilized such that it reaches to above the injured portion of the ureter. The bladder is retrograde filled with approximately 300 mL and a reimplantation site of the posterior bladder is identified. When there is concern about tension, a psoas hitch suture can be placed to keep the bladder in a superior position with reduced tension. Because of high rates of the congenital absence of the psoas tendon minor, we advocate direct visualization of the genitofemoral nerve by incising the peritoneum; this will avoid nerve entrapment.
Once the bladder is mobilized and the ureter isolated, we perform an intentional cystotomy in the posterior lateral aspect of the bladder. The ureter, which is on the vessel loop, must be transected proximal to the site of injury. To facilitate this, we spatulate the ureter, making a vertical incision of often about 5 mm in length to increase our surface area for anastomosis. Placement of a suture at the apex of the spatulated ureter helps us maintain orientation.
Anastomosis of the ureter and bladder is achieved in a mucosa-to-mucosa fashion using a series of interrupted monofilament fine absorbable sutures; we use a 3-0 monocryl suture. The most posterior anastomotic sutures are placed first to allow for optimal visualization, and prior to completing the anastomosis, a guide wire is placed through the open ureter and a double-J stent is introduced into the renal pelvis. The wire is then removed and the distal end of the stent coiled in the bladder. This stent will protect the ureter for about 6 weeks while it heals. The anastomosis is then completed on the anterior aspect, with a watertight closure ensured.
Postoperatively, we routinely perform an x-ray to ensure proper placement of the stent in the reimplanted ureter. To determine correct stent placement, the last rib is identified at T12 vertebrae. The renal pelvis is located at the level of the L2-L3 with the left being slightly higher than the right. A Foley catheter is maintained in the bladder for approximately 2 weeks, and the stent is maintained for approximately 6 weeks. Both the catheter and the stent can be removed in the office with cystoscopic guidance.
Imaging at 4-6 weeks after removal of the stent is performed to rule out development of an obstruction or a stricture. In patients who did not have a dilated ureter and renal collecting system prior to reimplantation, a renal ultrasound is sufficient to identify hydroureter/hydronephrosis or a urinoma. Many patients with a markedly dilated renal-collecting system prior to ureteral reimplantation will have persistent hydroureter/hydronephrosis (similar to a latex balloon that does not return to its original size after it is blown up) after reimplantation. A Lasix renal scan is a better imaging modality in these patients because it can differentiate a ureter that is dilated from one that is dilated and obstructed.
It is important to note that prompt ureteroneocystotomy is feasible only when the delayed ureteral injury presents within approximately 7 days of surgery. If the patient presents more than a week after surgery, inflammation is so significant that conservative management is necessary with reevaluation for reimplantation in another 6 weeks. Decompression of the system prior to reimplantation can be achieved through either stent placement or placement of a percutaneous nephrostomy tube. We prefer the latter because it reduces inflammation around the ureter that may make subsequent dissection and surgery more difficult.
Dr. Kenton is chief of urogynecology, Northwestern University, Chicago, and Dr. Mueller also is in the division of female pelvic medicine and reconstructive surgery–urogynecology at Northwestern. Dr. Kenton discloses grant funding from Boston Scientific.
The risk of lower urinary tract injury (bladder or ureters) at the time of benign gynecologic surgery is estimated to be between 0.3% and 4%. The majority are bladder injuries, with ureteral injuries occurring in 0.3%-1.8% of hysterectomies. While urologic procedures account for the majority of iatrogenic ureteral injuries, gynecologic surgery is the second leading cause, followed by general surgery and colorectal surgery.
With respect to hysterectomy in particular, the risk of ureteral injury is less than 1%. In a large prospective cohort of women undergoing hysterectomy for benign indications in 53 hospitals in Finland, rates of ureteral injury varied based on the route of hysterectomy, with laparoscopic and abdominal routes having an injury rate of 0.3% and the vaginal route having an injury rate of 0.04% (Human Reprod. 2011;26[7]:1741-51). The risk generally is higher with procedures for endometriosis, large fibroids, cancer, or pelvic organ prolapse.
During hysterectomy and with gynecologic surgery overall, ureteral injuries occur most commonly at three locations: at the level of the infundibulopelvic ligament and ovarian vessels, at the level of the uterine artery, and near the vaginal cuff. Identification and knowledge of the course of the ureter at these three locations is essential in preventing ureteral injury during pelvic surgery.

SOURCE: DR. MUELLER AND DR. KENTON
Perioperative ureteral stenting has been proposed as a method of preventing iatrogenic injury by allowing surgeons to more easily identify the ureters during surgery. Available reports suggest, however, that the actual risk of injury is not decreased and may even be increased by placing prophylactic ureteral stents, and most surgeons have moved away from this practice. The use of lighted ureteral stents during complex laparoscopic endometriosis resections may be helpful.
Many health care systems recommend intraoperative cystoscopy with bladder and ureteral survey to evaluate the integrity of the lower urinary tract at the time of all hysterectomies. A recent study of nearly 3,000 women undergoing benign hysterectomies at the University of Michigan, Ann Arbor, showed a significant decrease in the rate of delayed diagnosis of urinary tract injuries with implementation of a universal cystoscopy policy. While the rate of lower urinary tract injury was fairly consistent before and after implementation of the policy (2.6% and 1.8%, respectively), the rate of delayed detection of a lower urinary tract injury decreased from 0.7% before the policy to 0.1% after implementation (Obstet Gynecol. 2016;127[2]:369-75). The study also showed that hospital costs nearly doubled with a delayed detection of a lower urinary tract injury.
Unfortunately, even a normal postoperative cystoscopy does not ensure there is no lower urinary tract injury, especially considering that thermal injuries resulting from the use of energy devices typically do not present until 7-14 days after surgery. Overall, however, ureteral injury detection rates with universal cystoscopy approach 97% (Obstet Gynecol. 2009;113:6-10).
Identifying injuries
Intraoperative recognition and repair always is preferred, and when ureteral injuries are discovered or suspected in the operating room, cystoscopy and retrograde pyelography is the most helpful imaging tool. Contrast dye is injected during cystoscopy directly into the renal collecting system through the ureteral orifices; with fluoroscopy, the surgeon can visualize the integrity of the ureter from the bladder to the renal pelvis to diagnosis a ureteral injury, including ureteral transection, kinking, or ligation caused by a suture or sealing device.
If retrograde pyelography shows a transection or injury from a crushing clamp or sealing device, we recommend ureteroureteral anastomosis or urethral neocystostomy depending on the extent and location of the injury. If the ureter just appears kinked, sometime simply releasing the cuff sutures or uterosacral ligament sutures will resolve the obstruction. If there is extravasation of contrast suggesting a partial tear, placing a double-J ureteral stent for 6-8 weeks is frequently sufficient.
Patients with delayed iatrogenic ureteral injuries present with symptoms that often are nonspecific and that include abdominal or flank pain, fever, nausea, vomiting, back pain, and leukocytosis.
We recommend that patients with a history of surgery and symptoms suggestive of a ureteral injury be initially evaluated with CT urography that images the renal collecting system both as contrast dye is instilled and again several minutes later as it has progressed through the entire urinary tract. Alternatively, if CT urography is unavailable, a retrograde pyelogram can be performed as an emergency procedure to determine the location of renal injury.
Surgical management
Delayed ureteral injuries resulting in partial ureteral obstruction or extravasation of urine into the pelvis can sometimes be managed conservatively though placement of an internalized double J stent. The stent can be placed in a retrograde fashion via cystoscopy by a
We typically manage delayed ureteral injuries that are not amenable to, or do not heal with, ureteral stenting with ureteroneocystotomy, or ureteral reimplantation, into the bladder. This technique is effective for distal ureteral injuries that result in obstruction or fistula and are in close proximity to the bladder (most iatrogenic gynecologic injuries). We perform ureteroneocystotomy via an open, robotic, or laparoscopic route of access depending on the circumstance.
Our preferred route of access is minimally invasive with the da Vinci robot. A camera port is placed at the umbilicus, two robotic ports are placed on the patient’s left side at the level of the umbilicus, and one is placed on the patient’s right side at the level of the umbilicus with an additional assistant port on the right side. It is helpful if each port is at least 8 cm apart. If obstruction or transection is suspected to be more proximal, the ports may have been shifted above the umbilicus to optimally mobilize the ureter.
First, the ureter is identified and dissected. Regardless of the site of injury, which is usually identifiable with inflammation and scar tissue, it is always easiest to identify the ureter at the bifurcation of the common iliac vessels. The isolated ureter is inspected proximally and above the area of injury, and we find it helpful to place a vessel loop around the ureter for easy manipulation and counter traction. Care must be taken not to disturb the adventitia and blood supply. We do not transect the ureter until we’re ready to reimplant it in the bladder.
To mobilize the bladder and prepare for a tension-free anastomosis, an adequate retropubic dissection is performed, starting with an incision in the anterior abdominal wall peritoneum and taking it down to the level of the pubic bone and into the retropubic space. It is important to be mindful of the location of the obturator neurovascular bundle when performing this dissection.
Achieving a tension-free and water-tight anastomosis of the ureter to the bladder is critical. The bladder should be mobilized such that it reaches to above the injured portion of the ureter. The bladder is retrograde filled with approximately 300 mL and a reimplantation site of the posterior bladder is identified. When there is concern about tension, a psoas hitch suture can be placed to keep the bladder in a superior position with reduced tension. Because of high rates of the congenital absence of the psoas tendon minor, we advocate direct visualization of the genitofemoral nerve by incising the peritoneum; this will avoid nerve entrapment.
Once the bladder is mobilized and the ureter isolated, we perform an intentional cystotomy in the posterior lateral aspect of the bladder. The ureter, which is on the vessel loop, must be transected proximal to the site of injury. To facilitate this, we spatulate the ureter, making a vertical incision of often about 5 mm in length to increase our surface area for anastomosis. Placement of a suture at the apex of the spatulated ureter helps us maintain orientation.
Anastomosis of the ureter and bladder is achieved in a mucosa-to-mucosa fashion using a series of interrupted monofilament fine absorbable sutures; we use a 3-0 monocryl suture. The most posterior anastomotic sutures are placed first to allow for optimal visualization, and prior to completing the anastomosis, a guide wire is placed through the open ureter and a double-J stent is introduced into the renal pelvis. The wire is then removed and the distal end of the stent coiled in the bladder. This stent will protect the ureter for about 6 weeks while it heals. The anastomosis is then completed on the anterior aspect, with a watertight closure ensured.
Postoperatively, we routinely perform an x-ray to ensure proper placement of the stent in the reimplanted ureter. To determine correct stent placement, the last rib is identified at T12 vertebrae. The renal pelvis is located at the level of the L2-L3 with the left being slightly higher than the right. A Foley catheter is maintained in the bladder for approximately 2 weeks, and the stent is maintained for approximately 6 weeks. Both the catheter and the stent can be removed in the office with cystoscopic guidance.
Imaging at 4-6 weeks after removal of the stent is performed to rule out development of an obstruction or a stricture. In patients who did not have a dilated ureter and renal collecting system prior to reimplantation, a renal ultrasound is sufficient to identify hydroureter/hydronephrosis or a urinoma. Many patients with a markedly dilated renal-collecting system prior to ureteral reimplantation will have persistent hydroureter/hydronephrosis (similar to a latex balloon that does not return to its original size after it is blown up) after reimplantation. A Lasix renal scan is a better imaging modality in these patients because it can differentiate a ureter that is dilated from one that is dilated and obstructed.
It is important to note that prompt ureteroneocystotomy is feasible only when the delayed ureteral injury presents within approximately 7 days of surgery. If the patient presents more than a week after surgery, inflammation is so significant that conservative management is necessary with reevaluation for reimplantation in another 6 weeks. Decompression of the system prior to reimplantation can be achieved through either stent placement or placement of a percutaneous nephrostomy tube. We prefer the latter because it reduces inflammation around the ureter that may make subsequent dissection and surgery more difficult.
Dr. Kenton is chief of urogynecology, Northwestern University, Chicago, and Dr. Mueller also is in the division of female pelvic medicine and reconstructive surgery–urogynecology at Northwestern. Dr. Kenton discloses grant funding from Boston Scientific.
The risk of lower urinary tract injury (bladder or ureters) at the time of benign gynecologic surgery is estimated to be between 0.3% and 4%. The majority are bladder injuries, with ureteral injuries occurring in 0.3%-1.8% of hysterectomies. While urologic procedures account for the majority of iatrogenic ureteral injuries, gynecologic surgery is the second leading cause, followed by general surgery and colorectal surgery.
With respect to hysterectomy in particular, the risk of ureteral injury is less than 1%. In a large prospective cohort of women undergoing hysterectomy for benign indications in 53 hospitals in Finland, rates of ureteral injury varied based on the route of hysterectomy, with laparoscopic and abdominal routes having an injury rate of 0.3% and the vaginal route having an injury rate of 0.04% (Human Reprod. 2011;26[7]:1741-51). The risk generally is higher with procedures for endometriosis, large fibroids, cancer, or pelvic organ prolapse.
During hysterectomy and with gynecologic surgery overall, ureteral injuries occur most commonly at three locations: at the level of the infundibulopelvic ligament and ovarian vessels, at the level of the uterine artery, and near the vaginal cuff. Identification and knowledge of the course of the ureter at these three locations is essential in preventing ureteral injury during pelvic surgery.

SOURCE: DR. MUELLER AND DR. KENTON
Perioperative ureteral stenting has been proposed as a method of preventing iatrogenic injury by allowing surgeons to more easily identify the ureters during surgery. Available reports suggest, however, that the actual risk of injury is not decreased and may even be increased by placing prophylactic ureteral stents, and most surgeons have moved away from this practice. The use of lighted ureteral stents during complex laparoscopic endometriosis resections may be helpful.
Many health care systems recommend intraoperative cystoscopy with bladder and ureteral survey to evaluate the integrity of the lower urinary tract at the time of all hysterectomies. A recent study of nearly 3,000 women undergoing benign hysterectomies at the University of Michigan, Ann Arbor, showed a significant decrease in the rate of delayed diagnosis of urinary tract injuries with implementation of a universal cystoscopy policy. While the rate of lower urinary tract injury was fairly consistent before and after implementation of the policy (2.6% and 1.8%, respectively), the rate of delayed detection of a lower urinary tract injury decreased from 0.7% before the policy to 0.1% after implementation (Obstet Gynecol. 2016;127[2]:369-75). The study also showed that hospital costs nearly doubled with a delayed detection of a lower urinary tract injury.
Unfortunately, even a normal postoperative cystoscopy does not ensure there is no lower urinary tract injury, especially considering that thermal injuries resulting from the use of energy devices typically do not present until 7-14 days after surgery. Overall, however, ureteral injury detection rates with universal cystoscopy approach 97% (Obstet Gynecol. 2009;113:6-10).
Identifying injuries
Intraoperative recognition and repair always is preferred, and when ureteral injuries are discovered or suspected in the operating room, cystoscopy and retrograde pyelography is the most helpful imaging tool. Contrast dye is injected during cystoscopy directly into the renal collecting system through the ureteral orifices; with fluoroscopy, the surgeon can visualize the integrity of the ureter from the bladder to the renal pelvis to diagnosis a ureteral injury, including ureteral transection, kinking, or ligation caused by a suture or sealing device.
If retrograde pyelography shows a transection or injury from a crushing clamp or sealing device, we recommend ureteroureteral anastomosis or urethral neocystostomy depending on the extent and location of the injury. If the ureter just appears kinked, sometime simply releasing the cuff sutures or uterosacral ligament sutures will resolve the obstruction. If there is extravasation of contrast suggesting a partial tear, placing a double-J ureteral stent for 6-8 weeks is frequently sufficient.
Patients with delayed iatrogenic ureteral injuries present with symptoms that often are nonspecific and that include abdominal or flank pain, fever, nausea, vomiting, back pain, and leukocytosis.
We recommend that patients with a history of surgery and symptoms suggestive of a ureteral injury be initially evaluated with CT urography that images the renal collecting system both as contrast dye is instilled and again several minutes later as it has progressed through the entire urinary tract. Alternatively, if CT urography is unavailable, a retrograde pyelogram can be performed as an emergency procedure to determine the location of renal injury.
Surgical management
Delayed ureteral injuries resulting in partial ureteral obstruction or extravasation of urine into the pelvis can sometimes be managed conservatively though placement of an internalized double J stent. The stent can be placed in a retrograde fashion via cystoscopy by a
We typically manage delayed ureteral injuries that are not amenable to, or do not heal with, ureteral stenting with ureteroneocystotomy, or ureteral reimplantation, into the bladder. This technique is effective for distal ureteral injuries that result in obstruction or fistula and are in close proximity to the bladder (most iatrogenic gynecologic injuries). We perform ureteroneocystotomy via an open, robotic, or laparoscopic route of access depending on the circumstance.
Our preferred route of access is minimally invasive with the da Vinci robot. A camera port is placed at the umbilicus, two robotic ports are placed on the patient’s left side at the level of the umbilicus, and one is placed on the patient’s right side at the level of the umbilicus with an additional assistant port on the right side. It is helpful if each port is at least 8 cm apart. If obstruction or transection is suspected to be more proximal, the ports may have been shifted above the umbilicus to optimally mobilize the ureter.
First, the ureter is identified and dissected. Regardless of the site of injury, which is usually identifiable with inflammation and scar tissue, it is always easiest to identify the ureter at the bifurcation of the common iliac vessels. The isolated ureter is inspected proximally and above the area of injury, and we find it helpful to place a vessel loop around the ureter for easy manipulation and counter traction. Care must be taken not to disturb the adventitia and blood supply. We do not transect the ureter until we’re ready to reimplant it in the bladder.
To mobilize the bladder and prepare for a tension-free anastomosis, an adequate retropubic dissection is performed, starting with an incision in the anterior abdominal wall peritoneum and taking it down to the level of the pubic bone and into the retropubic space. It is important to be mindful of the location of the obturator neurovascular bundle when performing this dissection.
Achieving a tension-free and water-tight anastomosis of the ureter to the bladder is critical. The bladder should be mobilized such that it reaches to above the injured portion of the ureter. The bladder is retrograde filled with approximately 300 mL and a reimplantation site of the posterior bladder is identified. When there is concern about tension, a psoas hitch suture can be placed to keep the bladder in a superior position with reduced tension. Because of high rates of the congenital absence of the psoas tendon minor, we advocate direct visualization of the genitofemoral nerve by incising the peritoneum; this will avoid nerve entrapment.
Once the bladder is mobilized and the ureter isolated, we perform an intentional cystotomy in the posterior lateral aspect of the bladder. The ureter, which is on the vessel loop, must be transected proximal to the site of injury. To facilitate this, we spatulate the ureter, making a vertical incision of often about 5 mm in length to increase our surface area for anastomosis. Placement of a suture at the apex of the spatulated ureter helps us maintain orientation.
Anastomosis of the ureter and bladder is achieved in a mucosa-to-mucosa fashion using a series of interrupted monofilament fine absorbable sutures; we use a 3-0 monocryl suture. The most posterior anastomotic sutures are placed first to allow for optimal visualization, and prior to completing the anastomosis, a guide wire is placed through the open ureter and a double-J stent is introduced into the renal pelvis. The wire is then removed and the distal end of the stent coiled in the bladder. This stent will protect the ureter for about 6 weeks while it heals. The anastomosis is then completed on the anterior aspect, with a watertight closure ensured.
Postoperatively, we routinely perform an x-ray to ensure proper placement of the stent in the reimplanted ureter. To determine correct stent placement, the last rib is identified at T12 vertebrae. The renal pelvis is located at the level of the L2-L3 with the left being slightly higher than the right. A Foley catheter is maintained in the bladder for approximately 2 weeks, and the stent is maintained for approximately 6 weeks. Both the catheter and the stent can be removed in the office with cystoscopic guidance.
Imaging at 4-6 weeks after removal of the stent is performed to rule out development of an obstruction or a stricture. In patients who did not have a dilated ureter and renal collecting system prior to reimplantation, a renal ultrasound is sufficient to identify hydroureter/hydronephrosis or a urinoma. Many patients with a markedly dilated renal-collecting system prior to ureteral reimplantation will have persistent hydroureter/hydronephrosis (similar to a latex balloon that does not return to its original size after it is blown up) after reimplantation. A Lasix renal scan is a better imaging modality in these patients because it can differentiate a ureter that is dilated from one that is dilated and obstructed.
It is important to note that prompt ureteroneocystotomy is feasible only when the delayed ureteral injury presents within approximately 7 days of surgery. If the patient presents more than a week after surgery, inflammation is so significant that conservative management is necessary with reevaluation for reimplantation in another 6 weeks. Decompression of the system prior to reimplantation can be achieved through either stent placement or placement of a percutaneous nephrostomy tube. We prefer the latter because it reduces inflammation around the ureter that may make subsequent dissection and surgery more difficult.
Dr. Kenton is chief of urogynecology, Northwestern University, Chicago, and Dr. Mueller also is in the division of female pelvic medicine and reconstructive surgery–urogynecology at Northwestern. Dr. Kenton discloses grant funding from Boston Scientific.
The diagnosis and treatment of ureteral injury
A gynecologic surgeon learns very early in his/her career to respect the ureter. Whether from the procedure being performed (endometriosis surgery, hysterectomy, myomectomy for ligamentous fibroids, salpingo-oophorectomy, excision of ovarian remnants, adhesiolysis), blood loss that obscures visualization and must be controlled, or use of energy for cutting, desiccation, and coagulation leading to potential lateral tissue damage, ureteral injury is a well-known complication. Even normal anatomic variations may put some women at greater risk; according to Hurd et al. (Am J Obstet Gynecol. 2001;184:336-9). In a small subset of women, the distance between the cervix and the ureter may be less than 0.5 cm.
As a practicing minimally invasive gynecologic surgeon for the past 30 years, and an early adapter to laparoscopic hysterectomy, I remember quite well the recommendation to always dissect out ureters at time of the procedure. At present, most will agree that selective dissection is safe and thus, more desirable, as bleeding, damage secondary to desiccation, and ureter devascularization with subsequent necrosis are all increased with ureterolysis.
I agree with Dr. Kenton and Dr. Mueller that ureteral stenting has not been shown to significantly decrease ureteral injury rates. Often times, with loss of peristalsis secondary to stent placement, locating the ureter may be even more difficult. Recent advances using lighted stents or indocyanine green, which fluoresces in response to near-infrared laser and can be injected into the ureter via the ureteral catheter tip, are still in the feasibility phase of evaluation and can be costly.
As most urogenital fistulae are secondary to unrecognized injuries at time of surgery, and due to the fact that intraoperative recognition of the injury allows for primary repair, thus, decreasing the rate of secondary surgery and the associated increased morbidity, I recommend cystoscopy to check for ureteral jets (ureteral efflux) be performed when there is concern regarding ureter compromise.
Currently, I utilize a 70° cystoscope to visualize the ureters. While in the past, I have used intravenous indigo carmine, methylene blue, or fluorescein sodium, I currently use Pyridium (phenazopyridine) 200 mg taken by mouth 1 hour prior to the procedure.
Unfortunately, ureteral jetting still may be noted despite partial ligation, laceration, or desiccation of the ureter.
If ureteral injury is not recognized at time of surgery, it can lead to various postoperative symptoms. If there is a ureteral defect, the patient may note profuse wound leakage, increased abdominal fluid, or a urinoma, ileus, fever, peritonitis, or hematuria. With ureteral obstruction, flank or abdominal pain or anuria can be noted; while, with fistula formation, the patient will likely present with urinary incontinence or watery vaginal discharge.
Dr. Miller is a minimally invasive gynecologic surgeon in Naperville, Ill., and a past president of the AAGL.
A gynecologic surgeon learns very early in his/her career to respect the ureter. Whether from the procedure being performed (endometriosis surgery, hysterectomy, myomectomy for ligamentous fibroids, salpingo-oophorectomy, excision of ovarian remnants, adhesiolysis), blood loss that obscures visualization and must be controlled, or use of energy for cutting, desiccation, and coagulation leading to potential lateral tissue damage, ureteral injury is a well-known complication. Even normal anatomic variations may put some women at greater risk; according to Hurd et al. (Am J Obstet Gynecol. 2001;184:336-9). In a small subset of women, the distance between the cervix and the ureter may be less than 0.5 cm.
As a practicing minimally invasive gynecologic surgeon for the past 30 years, and an early adapter to laparoscopic hysterectomy, I remember quite well the recommendation to always dissect out ureters at time of the procedure. At present, most will agree that selective dissection is safe and thus, more desirable, as bleeding, damage secondary to desiccation, and ureter devascularization with subsequent necrosis are all increased with ureterolysis.
I agree with Dr. Kenton and Dr. Mueller that ureteral stenting has not been shown to significantly decrease ureteral injury rates. Often times, with loss of peristalsis secondary to stent placement, locating the ureter may be even more difficult. Recent advances using lighted stents or indocyanine green, which fluoresces in response to near-infrared laser and can be injected into the ureter via the ureteral catheter tip, are still in the feasibility phase of evaluation and can be costly.
As most urogenital fistulae are secondary to unrecognized injuries at time of surgery, and due to the fact that intraoperative recognition of the injury allows for primary repair, thus, decreasing the rate of secondary surgery and the associated increased morbidity, I recommend cystoscopy to check for ureteral jets (ureteral efflux) be performed when there is concern regarding ureter compromise.
Currently, I utilize a 70° cystoscope to visualize the ureters. While in the past, I have used intravenous indigo carmine, methylene blue, or fluorescein sodium, I currently use Pyridium (phenazopyridine) 200 mg taken by mouth 1 hour prior to the procedure.
Unfortunately, ureteral jetting still may be noted despite partial ligation, laceration, or desiccation of the ureter.
If ureteral injury is not recognized at time of surgery, it can lead to various postoperative symptoms. If there is a ureteral defect, the patient may note profuse wound leakage, increased abdominal fluid, or a urinoma, ileus, fever, peritonitis, or hematuria. With ureteral obstruction, flank or abdominal pain or anuria can be noted; while, with fistula formation, the patient will likely present with urinary incontinence or watery vaginal discharge.
Dr. Miller is a minimally invasive gynecologic surgeon in Naperville, Ill., and a past president of the AAGL.
A gynecologic surgeon learns very early in his/her career to respect the ureter. Whether from the procedure being performed (endometriosis surgery, hysterectomy, myomectomy for ligamentous fibroids, salpingo-oophorectomy, excision of ovarian remnants, adhesiolysis), blood loss that obscures visualization and must be controlled, or use of energy for cutting, desiccation, and coagulation leading to potential lateral tissue damage, ureteral injury is a well-known complication. Even normal anatomic variations may put some women at greater risk; according to Hurd et al. (Am J Obstet Gynecol. 2001;184:336-9). In a small subset of women, the distance between the cervix and the ureter may be less than 0.5 cm.
As a practicing minimally invasive gynecologic surgeon for the past 30 years, and an early adapter to laparoscopic hysterectomy, I remember quite well the recommendation to always dissect out ureters at time of the procedure. At present, most will agree that selective dissection is safe and thus, more desirable, as bleeding, damage secondary to desiccation, and ureter devascularization with subsequent necrosis are all increased with ureterolysis.
I agree with Dr. Kenton and Dr. Mueller that ureteral stenting has not been shown to significantly decrease ureteral injury rates. Often times, with loss of peristalsis secondary to stent placement, locating the ureter may be even more difficult. Recent advances using lighted stents or indocyanine green, which fluoresces in response to near-infrared laser and can be injected into the ureter via the ureteral catheter tip, are still in the feasibility phase of evaluation and can be costly.
As most urogenital fistulae are secondary to unrecognized injuries at time of surgery, and due to the fact that intraoperative recognition of the injury allows for primary repair, thus, decreasing the rate of secondary surgery and the associated increased morbidity, I recommend cystoscopy to check for ureteral jets (ureteral efflux) be performed when there is concern regarding ureter compromise.
Currently, I utilize a 70° cystoscope to visualize the ureters. While in the past, I have used intravenous indigo carmine, methylene blue, or fluorescein sodium, I currently use Pyridium (phenazopyridine) 200 mg taken by mouth 1 hour prior to the procedure.
Unfortunately, ureteral jetting still may be noted despite partial ligation, laceration, or desiccation of the ureter.
If ureteral injury is not recognized at time of surgery, it can lead to various postoperative symptoms. If there is a ureteral defect, the patient may note profuse wound leakage, increased abdominal fluid, or a urinoma, ileus, fever, peritonitis, or hematuria. With ureteral obstruction, flank or abdominal pain or anuria can be noted; while, with fistula formation, the patient will likely present with urinary incontinence or watery vaginal discharge.
Dr. Miller is a minimally invasive gynecologic surgeon in Naperville, Ill., and a past president of the AAGL.
Conservative early approach likely best, RA expert says
SANDESTIN, FLA. – A conservative approach to early rheumatoid arthritis treatment has carried the day in the practice of Gerd R. Burmester, MD.
In a talk at the annual Congress of Clinical Rheumatology, Dr. Burmester said that, although there is room to argue for a more aggressive approach, with more intense treatment early, a less aggressive philosophy has worked well in his clinic.
Dr. Burmester, director of rheumatology and clinical immunology at Charite-University in Berlin and a past president of the European League Against Rheumatism (EULAR), said he drew inspiration from the results of the 2015 study CARE-RA, in which patients were treated with initial therapy of methotrexate plus sulfasalazine and a fairly high dose of 60 mg of prednisolone; methotrexate plus leflunomide plus 30 mg of prednisolone; or just methotrexate plus 30 mg of prednisolone that is quickly tapered down (Ann Rheum Dis. 2015 Jan;74[1]:27-34).
“Everyone would say, ‘Okay, this is quite easy – the more intensive drug regimen should give you better results,’ ” Dr. Burmester said. “But if you look at the data, there’s no difference.” And after just 8 weeks, the patients’ corticosteroid dose was down to 5 mg.
This, he said, “has changed my daily typical practice, quite a bit.”
“I start with, usually, 15 mg of methotrexate subcutaneously,” because of better efficacy and less liver toxicity than oral administration, he said, or an oral dose if a patient resists the subcutaneous administration or there is another reason to avoid it. “And I add 30 mg of prednisone and taper it down – 30, 20, 12.5 mg, and then down to 5 and eventually discontinued altogether.”
“This is an interesting scheme,” he said. “And this is exactly what I do with my patients.”
His approach might be worth noting not only for his leadership roles, but because of his fastidious approach to being a clinician – he said he still, personally, takes every patient’s 28-joint Disease Activity Score and Simple Disease Activity Index at every visit.
In a recent paper, he argued, along with prominent Canadian rheumatologist Janet Pope, both sides of the debate, for and against more aggressive treatment – methotrexate combined with conventional synthetic or biologic DMARDs – very early in the disease course (Lancet. 2017 Jun 10;389[10086]:2338-48).
“If you use a combination treatment with a biologic right away, what might be the advantages?” he said. “More patients would achieve rapid remission. It might result in long-term benefits, less joint damage, higher chance of reducing therapy in the future.”
On the other hand, he said, there are disadvantages.
“This is, of course, more expensive, if you use a biologic up front in early RA,” he said. “Not all patients of course need it, and some have also side effects.” He added that little time is lost if a treat-to-target principle is followed. Plus, patients tend to be more accepting of monotherapy than combination therapy at the start of treatment, and combination therapy might require more time spent in the clinic.
Data from German databases, dating back to 1997, show that far more patients are reaching remission today after several years of treatment (Z Rheumatol. 2017 Feb;76[1]:50-7). But, he added, “It’s not yet perfect. ... We still have quite a few patients who are in moderate disease activity” despite the availability of so many treatment options.
“There’s still, of course, a huge unmet need in this devastating disease if you don’t treat it correctly.”
Dr. Burmester reports receiving clinical trial support and/or honoraria for lectures and consulting from AbbVie, Bristol-Myers Squibb, Lilly, Roche, MedImmune, Merck Sharpe & Dohme, Pfizer, Sanofi, and UCB.
SANDESTIN, FLA. – A conservative approach to early rheumatoid arthritis treatment has carried the day in the practice of Gerd R. Burmester, MD.
In a talk at the annual Congress of Clinical Rheumatology, Dr. Burmester said that, although there is room to argue for a more aggressive approach, with more intense treatment early, a less aggressive philosophy has worked well in his clinic.
Dr. Burmester, director of rheumatology and clinical immunology at Charite-University in Berlin and a past president of the European League Against Rheumatism (EULAR), said he drew inspiration from the results of the 2015 study CARE-RA, in which patients were treated with initial therapy of methotrexate plus sulfasalazine and a fairly high dose of 60 mg of prednisolone; methotrexate plus leflunomide plus 30 mg of prednisolone; or just methotrexate plus 30 mg of prednisolone that is quickly tapered down (Ann Rheum Dis. 2015 Jan;74[1]:27-34).
“Everyone would say, ‘Okay, this is quite easy – the more intensive drug regimen should give you better results,’ ” Dr. Burmester said. “But if you look at the data, there’s no difference.” And after just 8 weeks, the patients’ corticosteroid dose was down to 5 mg.
This, he said, “has changed my daily typical practice, quite a bit.”
“I start with, usually, 15 mg of methotrexate subcutaneously,” because of better efficacy and less liver toxicity than oral administration, he said, or an oral dose if a patient resists the subcutaneous administration or there is another reason to avoid it. “And I add 30 mg of prednisone and taper it down – 30, 20, 12.5 mg, and then down to 5 and eventually discontinued altogether.”
“This is an interesting scheme,” he said. “And this is exactly what I do with my patients.”
His approach might be worth noting not only for his leadership roles, but because of his fastidious approach to being a clinician – he said he still, personally, takes every patient’s 28-joint Disease Activity Score and Simple Disease Activity Index at every visit.
In a recent paper, he argued, along with prominent Canadian rheumatologist Janet Pope, both sides of the debate, for and against more aggressive treatment – methotrexate combined with conventional synthetic or biologic DMARDs – very early in the disease course (Lancet. 2017 Jun 10;389[10086]:2338-48).
“If you use a combination treatment with a biologic right away, what might be the advantages?” he said. “More patients would achieve rapid remission. It might result in long-term benefits, less joint damage, higher chance of reducing therapy in the future.”
On the other hand, he said, there are disadvantages.
“This is, of course, more expensive, if you use a biologic up front in early RA,” he said. “Not all patients of course need it, and some have also side effects.” He added that little time is lost if a treat-to-target principle is followed. Plus, patients tend to be more accepting of monotherapy than combination therapy at the start of treatment, and combination therapy might require more time spent in the clinic.
Data from German databases, dating back to 1997, show that far more patients are reaching remission today after several years of treatment (Z Rheumatol. 2017 Feb;76[1]:50-7). But, he added, “It’s not yet perfect. ... We still have quite a few patients who are in moderate disease activity” despite the availability of so many treatment options.
“There’s still, of course, a huge unmet need in this devastating disease if you don’t treat it correctly.”
Dr. Burmester reports receiving clinical trial support and/or honoraria for lectures and consulting from AbbVie, Bristol-Myers Squibb, Lilly, Roche, MedImmune, Merck Sharpe & Dohme, Pfizer, Sanofi, and UCB.
SANDESTIN, FLA. – A conservative approach to early rheumatoid arthritis treatment has carried the day in the practice of Gerd R. Burmester, MD.
In a talk at the annual Congress of Clinical Rheumatology, Dr. Burmester said that, although there is room to argue for a more aggressive approach, with more intense treatment early, a less aggressive philosophy has worked well in his clinic.
Dr. Burmester, director of rheumatology and clinical immunology at Charite-University in Berlin and a past president of the European League Against Rheumatism (EULAR), said he drew inspiration from the results of the 2015 study CARE-RA, in which patients were treated with initial therapy of methotrexate plus sulfasalazine and a fairly high dose of 60 mg of prednisolone; methotrexate plus leflunomide plus 30 mg of prednisolone; or just methotrexate plus 30 mg of prednisolone that is quickly tapered down (Ann Rheum Dis. 2015 Jan;74[1]:27-34).
“Everyone would say, ‘Okay, this is quite easy – the more intensive drug regimen should give you better results,’ ” Dr. Burmester said. “But if you look at the data, there’s no difference.” And after just 8 weeks, the patients’ corticosteroid dose was down to 5 mg.
This, he said, “has changed my daily typical practice, quite a bit.”
“I start with, usually, 15 mg of methotrexate subcutaneously,” because of better efficacy and less liver toxicity than oral administration, he said, or an oral dose if a patient resists the subcutaneous administration or there is another reason to avoid it. “And I add 30 mg of prednisone and taper it down – 30, 20, 12.5 mg, and then down to 5 and eventually discontinued altogether.”
“This is an interesting scheme,” he said. “And this is exactly what I do with my patients.”
His approach might be worth noting not only for his leadership roles, but because of his fastidious approach to being a clinician – he said he still, personally, takes every patient’s 28-joint Disease Activity Score and Simple Disease Activity Index at every visit.
In a recent paper, he argued, along with prominent Canadian rheumatologist Janet Pope, both sides of the debate, for and against more aggressive treatment – methotrexate combined with conventional synthetic or biologic DMARDs – very early in the disease course (Lancet. 2017 Jun 10;389[10086]:2338-48).
“If you use a combination treatment with a biologic right away, what might be the advantages?” he said. “More patients would achieve rapid remission. It might result in long-term benefits, less joint damage, higher chance of reducing therapy in the future.”
On the other hand, he said, there are disadvantages.
“This is, of course, more expensive, if you use a biologic up front in early RA,” he said. “Not all patients of course need it, and some have also side effects.” He added that little time is lost if a treat-to-target principle is followed. Plus, patients tend to be more accepting of monotherapy than combination therapy at the start of treatment, and combination therapy might require more time spent in the clinic.
Data from German databases, dating back to 1997, show that far more patients are reaching remission today after several years of treatment (Z Rheumatol. 2017 Feb;76[1]:50-7). But, he added, “It’s not yet perfect. ... We still have quite a few patients who are in moderate disease activity” despite the availability of so many treatment options.
“There’s still, of course, a huge unmet need in this devastating disease if you don’t treat it correctly.”
Dr. Burmester reports receiving clinical trial support and/or honoraria for lectures and consulting from AbbVie, Bristol-Myers Squibb, Lilly, Roche, MedImmune, Merck Sharpe & Dohme, Pfizer, Sanofi, and UCB.
EXPERT ANALYSIS FROM CCR 18
NIH launches early Ebola treatment trial
A study of a potential new Ebola treatment has begun at the National Institutes of Health Clinical Center in Bethesda, Md. The small phase 1 clinical trial will examine the safety and tolerability of a single monoclonal antibody (mAb114), which was developed by scientists at the National Institute of Allergy and Infectious Diseases (NIAID) and their collaborators. Investigators plan to enroll between 18 and 30 healthy volunteers aged 18-60. The trial will not expose participants to Ebola virus, according to the NIH announcement.
MAb114 is a monoclonal antibody – a protein that binds to a single target on a pathogen — isolated from a human survivor of the 1995 Ebola outbreak in Kikwit, Democratic Republic of the Congo. Nancy Sullivan, PhD, chief of the Biodefense Research Section in NIAID’s Vaccine Research Center, and her team, in collaboration with researchers from the National Institute of Biomedical Research in the Democratic Republic of the Congo and the Institute for Biomedical Research in Switzerland, discovered that the survivor retained antibodies against Ebola 11 years after infection. They isolated and tested the antibodies and selected mAb114 as the most promising.
Although rVSV-ZEBOV, an experimental vaccine, is now available and in use in Africa during the current outbreak, specific treatment modalities are lacking.
More information can be found at www.clinicaltrials.gov, trial # NCT03478891.
A study of a potential new Ebola treatment has begun at the National Institutes of Health Clinical Center in Bethesda, Md. The small phase 1 clinical trial will examine the safety and tolerability of a single monoclonal antibody (mAb114), which was developed by scientists at the National Institute of Allergy and Infectious Diseases (NIAID) and their collaborators. Investigators plan to enroll between 18 and 30 healthy volunteers aged 18-60. The trial will not expose participants to Ebola virus, according to the NIH announcement.
MAb114 is a monoclonal antibody – a protein that binds to a single target on a pathogen — isolated from a human survivor of the 1995 Ebola outbreak in Kikwit, Democratic Republic of the Congo. Nancy Sullivan, PhD, chief of the Biodefense Research Section in NIAID’s Vaccine Research Center, and her team, in collaboration with researchers from the National Institute of Biomedical Research in the Democratic Republic of the Congo and the Institute for Biomedical Research in Switzerland, discovered that the survivor retained antibodies against Ebola 11 years after infection. They isolated and tested the antibodies and selected mAb114 as the most promising.
Although rVSV-ZEBOV, an experimental vaccine, is now available and in use in Africa during the current outbreak, specific treatment modalities are lacking.
More information can be found at www.clinicaltrials.gov, trial # NCT03478891.
A study of a potential new Ebola treatment has begun at the National Institutes of Health Clinical Center in Bethesda, Md. The small phase 1 clinical trial will examine the safety and tolerability of a single monoclonal antibody (mAb114), which was developed by scientists at the National Institute of Allergy and Infectious Diseases (NIAID) and their collaborators. Investigators plan to enroll between 18 and 30 healthy volunteers aged 18-60. The trial will not expose participants to Ebola virus, according to the NIH announcement.
MAb114 is a monoclonal antibody – a protein that binds to a single target on a pathogen — isolated from a human survivor of the 1995 Ebola outbreak in Kikwit, Democratic Republic of the Congo. Nancy Sullivan, PhD, chief of the Biodefense Research Section in NIAID’s Vaccine Research Center, and her team, in collaboration with researchers from the National Institute of Biomedical Research in the Democratic Republic of the Congo and the Institute for Biomedical Research in Switzerland, discovered that the survivor retained antibodies against Ebola 11 years after infection. They isolated and tested the antibodies and selected mAb114 as the most promising.
Although rVSV-ZEBOV, an experimental vaccine, is now available and in use in Africa during the current outbreak, specific treatment modalities are lacking.
More information can be found at www.clinicaltrials.gov, trial # NCT03478891.
MDR Candida auris is on the move
MADRID – The anticipated global emergence of multidrug resistant Candida auris is now an established fact, but a case study presented at the European Society of Clinical Microbiology and Infectious Diseases annual congress demonstrates just how devastating an outbreak can be to a medical facility and its surgical ICU patients.
The dangerous invasive infection is spreading through Asia, Europe, and the Americas, causing potentially fatal candidemias and proving devilishly difficult to eradicate in health care facilities once it becomes established.
Several multidrug resistant (MDR) C. auris outbreaks were reported at the ECCMID meeting. Most troubling: a continuing outbreak in a hospital in Valencia, Spain, in which 17 patients have died – a 41% fatality rate among those who developed a fulminant C. auris candidemia, Javier Pemán, MD, said at the meeting. The strain appeared to be a clonal population not previously identified in published reports.
“C. auris is hard to remove from the hospital environment,” once it becomes established, said Dr. Pemán of La Fe University and Polytechnic Hospital, Valencia. “When an outbreak lasts for months, as ours has, it is difficult, but necessary, to maintain control measures, identify it early in the lab, and isolate and treat patients early with combination therapy.”
He and his team have relied primarily on a combination of amphotericin B and echinocandin (AMB+ECN), although, he added, the optimal dosing and treatment time aren’t known, and many C. auris isolates are echinocandin resistant.
MDR C. auris first appearedin Tokyo in 2009. It then spread to South Korea around 2011, and then appeared across Asia and Western Europe. Its first appearance in Spain was the 2016 Le Fe outbreak.
According to the Centers for Disease Control and Prevention, single cases have appeared in Austria, Belgium, Malaysia, Norway, and the United Arab Emirates. Canada, Colombia, France, Germany, India, Israel, Japan, Kenya, Kuwait, Oman, Pakistan, Panama, South Korea, South Africa, Spain, the United Arab Emirates, the United Kingdom, and Venezuela have experienced multiple outbreaks.
The CDC has recorded 257 confirmed and 30 probable cases of MDR C. auris in the United States as of March 31, 2018. Most of these occurred in New York City and New Jersey; a number of patients had recent stays in hospitals in India, Pakistan, South Africa, the UAE, and Venezuela.
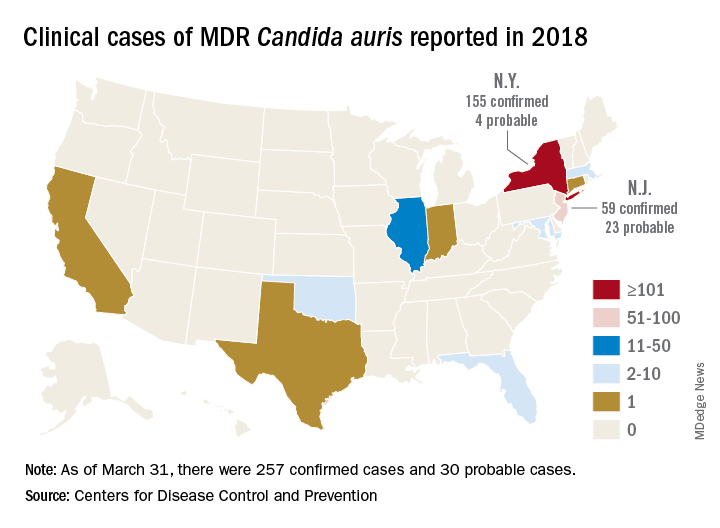
Jacques Meis, MD, of the department of medical microbiology and infectious diseases at Canisius Wilhelmina Hospital, Nijmegen, the Netherlands, set the stage for an extended discussion of C. auris at the meeting.
“This is a multidrug resistant yeast that has emerged in the last decade. Some rare isolates are resistant to all three major antifungal classes. Unlike other Candida species, it seems to persist for prolonged periods in health care environments and to colonize patients’ skin. It behaves rather like resistant bacteria.”
Once established in a health care setting – often an intensive care ward – C. auris poses major infection controls challenges and can be very hard to identify and eradicate, said Dr. Meis.
The identification problem is well known. The 2016 CDC alert noted that “commercially available biochemical-based tests, including API strips and VITEK-2, used in many U.S. laboratories to identify fungi, cannot differentiate C. auris from related species. Because of these challenges, clinical laboratories have misidentified the organism as C. haemulonii and Saccharomyces cerevisiae.”
“It’s often misidentified as other Candida species or as Saccharomyces when we investigate with biochemical methods. C. auris is best identified using Matrix Assisted Laser Desorption/Ionization time of flight mass spectrometry (MALDI-TOF),” said Dr. Meis.
Among the presentations at ECCMID were a report of a U.K. outbreak that affected 70 patients in a neuroscience ICU. It was traced to axillary skin-surface temperature probes, and eradicated only after those probes were removed. More than 90% of the isolates were resistant to fluconazole, voriconazole, and posaconazole; 18% were amphotericin resistant.
A poster described the microbiological characteristics of 50 C. auris isolates taken from 11 hospitals in Korea.
Dr. Pemán described the outbreak in Valencia, which began in April 2016; the report was simultaneously published in the online journal Mycoses (2018 Apr 14. doi: 10.1111/myc.12781).
The index case was a 66-year-old man with hepatocellular carcinoma who underwent a liver resection at Hospital Le Fe in April 2016. During his stay in the surgical ICU (SICU), he developed a fungal infection from an unknown, highly fluconazole-resistant yeast. The pathogen was twice misidentified, first as C. haemulonii and then as S. cerevisiae.
Three weeks later, the patient in the adjacent bed developed a similar infection. Sequencing of the internal transcribed spacer confirmed both as a Candida isolate – an organism previously unknown in Spain.
The SICU setup was apparently very conducive to the C. auris life cycle, Dr. Pemán said. It’s a relatively open ward divided into three rooms with 12 beds in each. There are no isolation beds, and dozens of workers have access to the ward every day, including clinical and cleaning staff.
After identifying the second isolate, Dr. Pemán said, infection control staff went into action. They instituted contact precautions in the SICU, and took regular cultures from newly admitted patients and cultures of every SICU patient every 7 days.
“We also started an intense search for more cases throughout the hospital and in 101 SICU workers. Of 305 samples from hands and ears, we found nothing.” They reviewed all the prior fluconazole-resistant Candida isolates; C. auris was not present in the hospital before the index case.
Three weeks after case 2, six new SICU patients tested positive for C. auris (two blood cultures, one vascular line, one respiratory specimen, two rectal swabs, and one urinary tract sample).
“We reinforced contact precautions in colonized and infected patients and started a twice-daily environmental cleaning practice with quaternary ammonium around them,” said Dr. Pemán. They instituted a proactive hospital-wide hand hygiene campaign and spread the word about the outbreak.
By July, there were 11 new colonized patients, 3 of whom developed candidemia. These patients were grouped in the same SICU ward and underwent daily skin treatments with 4% aqueous chlorhexidine wipes.
The environmental inspection found C. auris on beds, tables, walls, and the floor all around infected patients. The pathogen also was living on IV pumps, computer keyboards, and bedside tables. Blood pressure cuffs were a favorite haunt: 19 of 36 samples in the adjacent ICU were positive. These data were separately reported at ECCMID.
Despite all of these efforts at eradication, infections continued to rise. By November, there were 24 newly colonized patients and nine new candidemia episodes in SICU and regular ICU patients. In December, a new infection control bundle began: A surveillance nurse in the C. auris SICU ward was in charge of compliance; any patient with any yeast growth in culture was isolated, and staff used 2% alcohol chlorhexidine wipes before and after IV catheter handling. Staff also washed down all surfaces three times daily with a disinfectant.
Patients could leave isolation after three consecutive C. auris–negative cultures. After discharge, an ultraviolet light decontamination procedure disinfected each patient room.
The pathogen was almost unbelievably resilient, Dr. Pemán noted in the Mycoses article. “In some cases, C. auris was recovered from walls after cleaning with cationic surface–active products ... it was not known until very recently that these products, as well as quaternary ammonium disinfectants, cannot effectively remove C. auris from surfaces.”
As a result of the previous measures, the outbreak slowed down during December 2016, with two new candidemia cases, but by February, the outbreak resumed with 50 new cases and 18 candidemias detected. Cases continued to emerge throughout 2017.
By September 2017, 250 patients had been colonized; 116 of these were included in the Mycoses report. There were 30 episodes of candidemia (26%); of these, 17 died by 30 days (41.4%). Spondylodiscitis and endocarditis each developed in two patients and one developed ventriculitis.
A separate poster by Dr. Pemán and his colleagues gave more details:
• A 52-year-old woman with C. auris–induced endocarditis died after 4 weeks of treatment with AMB+ECN and flucytosine. She had undergone a prosthetic heart valve placement for Ebstein’s anomaly.
• A 71-year-old man with hydrocephalus developed a C. auris–induced infection of his ventriculoperitoneal shunt; he also had undergone cardiovascular surgery and had an ischemic cardiomyopathy. He died despite shunt removal and 8 weeks of AMB+ECN.
• A 71-year-old man who underwent cardiovascular surgery and received a prosthetic heart valve developed endocarditis. He is alive and at last report, on week 26 of AMB+ECN, flucytosine, and isavuconazole.
• A 68-year-old man who underwent abdominal surgery for hepatocellular carcinoma developed spondylodiscitis and is alive after 24 weeks of AMB+ECN.
• A 48-year old female multiple trauma patient developed spondylodiscitis and is alive after 48 weeks of treatment with AMB+ECN.
A multivariate analysis determined that antibacterial treatment increased the risk of candidemia by almost 30 times (odds ratio, 29.59). The next highest risk was neutropenia (OR, 20.7) and then simply being a hospital and SICU patient. Dr. Pemán’s poster said, “In the 16 months before the index case, La Fe recorded 89 candidemias, none caused by C. auris. In the 16 months afterward, there were 154 candidemias, largely C. auris. Before April 2016, C. parapsilosis accounted for the largest portion of candidemias (46%) followed by C. albicans. After the index case, C. auris accounted for 42%, followed by C. parapsilosis (21%) and C. albicans (18%).”
Because of its fluconazole resistance, patients with C. auris received a combined antifungal treatment of liposomal amphotericin B 3 mg/kg per day for 5 days, and a standard dose of echinocandin for 3 weeks. Many C. auris strains are echinocandin resistant, Dr. Pemán noted. This particular strain was clonal, different from any other previously reported, he said.
“Our results confirm those previously reported by other authors, that C. auris is grouped in different independent clusters according to its geographical origin. Although all Spanish isolates were genotypically distinct from Indian, Omani, U.K., and Venezuelan isolates, there seems to be some connection with South African isolates.”
Hospital Le Fe continues to struggle with C. auris. As of March, 335 patients have tested positive for the pathogen, and 80 have developed candidemias.
“We feel we may be approaching the end of this episode, but it’s really not possible to be sure,” he said.
Dr. Pemán had no relevant financial disclosures.
SOURCE: ECCMID 2018 Peman et al. S0067.
MADRID – The anticipated global emergence of multidrug resistant Candida auris is now an established fact, but a case study presented at the European Society of Clinical Microbiology and Infectious Diseases annual congress demonstrates just how devastating an outbreak can be to a medical facility and its surgical ICU patients.
The dangerous invasive infection is spreading through Asia, Europe, and the Americas, causing potentially fatal candidemias and proving devilishly difficult to eradicate in health care facilities once it becomes established.
Several multidrug resistant (MDR) C. auris outbreaks were reported at the ECCMID meeting. Most troubling: a continuing outbreak in a hospital in Valencia, Spain, in which 17 patients have died – a 41% fatality rate among those who developed a fulminant C. auris candidemia, Javier Pemán, MD, said at the meeting. The strain appeared to be a clonal population not previously identified in published reports.
“C. auris is hard to remove from the hospital environment,” once it becomes established, said Dr. Pemán of La Fe University and Polytechnic Hospital, Valencia. “When an outbreak lasts for months, as ours has, it is difficult, but necessary, to maintain control measures, identify it early in the lab, and isolate and treat patients early with combination therapy.”
He and his team have relied primarily on a combination of amphotericin B and echinocandin (AMB+ECN), although, he added, the optimal dosing and treatment time aren’t known, and many C. auris isolates are echinocandin resistant.
MDR C. auris first appearedin Tokyo in 2009. It then spread to South Korea around 2011, and then appeared across Asia and Western Europe. Its first appearance in Spain was the 2016 Le Fe outbreak.
According to the Centers for Disease Control and Prevention, single cases have appeared in Austria, Belgium, Malaysia, Norway, and the United Arab Emirates. Canada, Colombia, France, Germany, India, Israel, Japan, Kenya, Kuwait, Oman, Pakistan, Panama, South Korea, South Africa, Spain, the United Arab Emirates, the United Kingdom, and Venezuela have experienced multiple outbreaks.
The CDC has recorded 257 confirmed and 30 probable cases of MDR C. auris in the United States as of March 31, 2018. Most of these occurred in New York City and New Jersey; a number of patients had recent stays in hospitals in India, Pakistan, South Africa, the UAE, and Venezuela.

Jacques Meis, MD, of the department of medical microbiology and infectious diseases at Canisius Wilhelmina Hospital, Nijmegen, the Netherlands, set the stage for an extended discussion of C. auris at the meeting.
“This is a multidrug resistant yeast that has emerged in the last decade. Some rare isolates are resistant to all three major antifungal classes. Unlike other Candida species, it seems to persist for prolonged periods in health care environments and to colonize patients’ skin. It behaves rather like resistant bacteria.”
Once established in a health care setting – often an intensive care ward – C. auris poses major infection controls challenges and can be very hard to identify and eradicate, said Dr. Meis.
The identification problem is well known. The 2016 CDC alert noted that “commercially available biochemical-based tests, including API strips and VITEK-2, used in many U.S. laboratories to identify fungi, cannot differentiate C. auris from related species. Because of these challenges, clinical laboratories have misidentified the organism as C. haemulonii and Saccharomyces cerevisiae.”
“It’s often misidentified as other Candida species or as Saccharomyces when we investigate with biochemical methods. C. auris is best identified using Matrix Assisted Laser Desorption/Ionization time of flight mass spectrometry (MALDI-TOF),” said Dr. Meis.
Among the presentations at ECCMID were a report of a U.K. outbreak that affected 70 patients in a neuroscience ICU. It was traced to axillary skin-surface temperature probes, and eradicated only after those probes were removed. More than 90% of the isolates were resistant to fluconazole, voriconazole, and posaconazole; 18% were amphotericin resistant.
A poster described the microbiological characteristics of 50 C. auris isolates taken from 11 hospitals in Korea.
Dr. Pemán described the outbreak in Valencia, which began in April 2016; the report was simultaneously published in the online journal Mycoses (2018 Apr 14. doi: 10.1111/myc.12781).
The index case was a 66-year-old man with hepatocellular carcinoma who underwent a liver resection at Hospital Le Fe in April 2016. During his stay in the surgical ICU (SICU), he developed a fungal infection from an unknown, highly fluconazole-resistant yeast. The pathogen was twice misidentified, first as C. haemulonii and then as S. cerevisiae.
Three weeks later, the patient in the adjacent bed developed a similar infection. Sequencing of the internal transcribed spacer confirmed both as a Candida isolate – an organism previously unknown in Spain.
The SICU setup was apparently very conducive to the C. auris life cycle, Dr. Pemán said. It’s a relatively open ward divided into three rooms with 12 beds in each. There are no isolation beds, and dozens of workers have access to the ward every day, including clinical and cleaning staff.
After identifying the second isolate, Dr. Pemán said, infection control staff went into action. They instituted contact precautions in the SICU, and took regular cultures from newly admitted patients and cultures of every SICU patient every 7 days.
“We also started an intense search for more cases throughout the hospital and in 101 SICU workers. Of 305 samples from hands and ears, we found nothing.” They reviewed all the prior fluconazole-resistant Candida isolates; C. auris was not present in the hospital before the index case.
Three weeks after case 2, six new SICU patients tested positive for C. auris (two blood cultures, one vascular line, one respiratory specimen, two rectal swabs, and one urinary tract sample).
“We reinforced contact precautions in colonized and infected patients and started a twice-daily environmental cleaning practice with quaternary ammonium around them,” said Dr. Pemán. They instituted a proactive hospital-wide hand hygiene campaign and spread the word about the outbreak.
By July, there were 11 new colonized patients, 3 of whom developed candidemia. These patients were grouped in the same SICU ward and underwent daily skin treatments with 4% aqueous chlorhexidine wipes.
The environmental inspection found C. auris on beds, tables, walls, and the floor all around infected patients. The pathogen also was living on IV pumps, computer keyboards, and bedside tables. Blood pressure cuffs were a favorite haunt: 19 of 36 samples in the adjacent ICU were positive. These data were separately reported at ECCMID.
Despite all of these efforts at eradication, infections continued to rise. By November, there were 24 newly colonized patients and nine new candidemia episodes in SICU and regular ICU patients. In December, a new infection control bundle began: A surveillance nurse in the C. auris SICU ward was in charge of compliance; any patient with any yeast growth in culture was isolated, and staff used 2% alcohol chlorhexidine wipes before and after IV catheter handling. Staff also washed down all surfaces three times daily with a disinfectant.
Patients could leave isolation after three consecutive C. auris–negative cultures. After discharge, an ultraviolet light decontamination procedure disinfected each patient room.
The pathogen was almost unbelievably resilient, Dr. Pemán noted in the Mycoses article. “In some cases, C. auris was recovered from walls after cleaning with cationic surface–active products ... it was not known until very recently that these products, as well as quaternary ammonium disinfectants, cannot effectively remove C. auris from surfaces.”
As a result of the previous measures, the outbreak slowed down during December 2016, with two new candidemia cases, but by February, the outbreak resumed with 50 new cases and 18 candidemias detected. Cases continued to emerge throughout 2017.
By September 2017, 250 patients had been colonized; 116 of these were included in the Mycoses report. There were 30 episodes of candidemia (26%); of these, 17 died by 30 days (41.4%). Spondylodiscitis and endocarditis each developed in two patients and one developed ventriculitis.
A separate poster by Dr. Pemán and his colleagues gave more details:
• A 52-year-old woman with C. auris–induced endocarditis died after 4 weeks of treatment with AMB+ECN and flucytosine. She had undergone a prosthetic heart valve placement for Ebstein’s anomaly.
• A 71-year-old man with hydrocephalus developed a C. auris–induced infection of his ventriculoperitoneal shunt; he also had undergone cardiovascular surgery and had an ischemic cardiomyopathy. He died despite shunt removal and 8 weeks of AMB+ECN.
• A 71-year-old man who underwent cardiovascular surgery and received a prosthetic heart valve developed endocarditis. He is alive and at last report, on week 26 of AMB+ECN, flucytosine, and isavuconazole.
• A 68-year-old man who underwent abdominal surgery for hepatocellular carcinoma developed spondylodiscitis and is alive after 24 weeks of AMB+ECN.
• A 48-year old female multiple trauma patient developed spondylodiscitis and is alive after 48 weeks of treatment with AMB+ECN.
A multivariate analysis determined that antibacterial treatment increased the risk of candidemia by almost 30 times (odds ratio, 29.59). The next highest risk was neutropenia (OR, 20.7) and then simply being a hospital and SICU patient. Dr. Pemán’s poster said, “In the 16 months before the index case, La Fe recorded 89 candidemias, none caused by C. auris. In the 16 months afterward, there were 154 candidemias, largely C. auris. Before April 2016, C. parapsilosis accounted for the largest portion of candidemias (46%) followed by C. albicans. After the index case, C. auris accounted for 42%, followed by C. parapsilosis (21%) and C. albicans (18%).”
Because of its fluconazole resistance, patients with C. auris received a combined antifungal treatment of liposomal amphotericin B 3 mg/kg per day for 5 days, and a standard dose of echinocandin for 3 weeks. Many C. auris strains are echinocandin resistant, Dr. Pemán noted. This particular strain was clonal, different from any other previously reported, he said.
“Our results confirm those previously reported by other authors, that C. auris is grouped in different independent clusters according to its geographical origin. Although all Spanish isolates were genotypically distinct from Indian, Omani, U.K., and Venezuelan isolates, there seems to be some connection with South African isolates.”
Hospital Le Fe continues to struggle with C. auris. As of March, 335 patients have tested positive for the pathogen, and 80 have developed candidemias.
“We feel we may be approaching the end of this episode, but it’s really not possible to be sure,” he said.
Dr. Pemán had no relevant financial disclosures.
SOURCE: ECCMID 2018 Peman et al. S0067.
MADRID – The anticipated global emergence of multidrug resistant Candida auris is now an established fact, but a case study presented at the European Society of Clinical Microbiology and Infectious Diseases annual congress demonstrates just how devastating an outbreak can be to a medical facility and its surgical ICU patients.
The dangerous invasive infection is spreading through Asia, Europe, and the Americas, causing potentially fatal candidemias and proving devilishly difficult to eradicate in health care facilities once it becomes established.
Several multidrug resistant (MDR) C. auris outbreaks were reported at the ECCMID meeting. Most troubling: a continuing outbreak in a hospital in Valencia, Spain, in which 17 patients have died – a 41% fatality rate among those who developed a fulminant C. auris candidemia, Javier Pemán, MD, said at the meeting. The strain appeared to be a clonal population not previously identified in published reports.
“C. auris is hard to remove from the hospital environment,” once it becomes established, said Dr. Pemán of La Fe University and Polytechnic Hospital, Valencia. “When an outbreak lasts for months, as ours has, it is difficult, but necessary, to maintain control measures, identify it early in the lab, and isolate and treat patients early with combination therapy.”
He and his team have relied primarily on a combination of amphotericin B and echinocandin (AMB+ECN), although, he added, the optimal dosing and treatment time aren’t known, and many C. auris isolates are echinocandin resistant.
MDR C. auris first appearedin Tokyo in 2009. It then spread to South Korea around 2011, and then appeared across Asia and Western Europe. Its first appearance in Spain was the 2016 Le Fe outbreak.
According to the Centers for Disease Control and Prevention, single cases have appeared in Austria, Belgium, Malaysia, Norway, and the United Arab Emirates. Canada, Colombia, France, Germany, India, Israel, Japan, Kenya, Kuwait, Oman, Pakistan, Panama, South Korea, South Africa, Spain, the United Arab Emirates, the United Kingdom, and Venezuela have experienced multiple outbreaks.
The CDC has recorded 257 confirmed and 30 probable cases of MDR C. auris in the United States as of March 31, 2018. Most of these occurred in New York City and New Jersey; a number of patients had recent stays in hospitals in India, Pakistan, South Africa, the UAE, and Venezuela.

Jacques Meis, MD, of the department of medical microbiology and infectious diseases at Canisius Wilhelmina Hospital, Nijmegen, the Netherlands, set the stage for an extended discussion of C. auris at the meeting.
“This is a multidrug resistant yeast that has emerged in the last decade. Some rare isolates are resistant to all three major antifungal classes. Unlike other Candida species, it seems to persist for prolonged periods in health care environments and to colonize patients’ skin. It behaves rather like resistant bacteria.”
Once established in a health care setting – often an intensive care ward – C. auris poses major infection controls challenges and can be very hard to identify and eradicate, said Dr. Meis.
The identification problem is well known. The 2016 CDC alert noted that “commercially available biochemical-based tests, including API strips and VITEK-2, used in many U.S. laboratories to identify fungi, cannot differentiate C. auris from related species. Because of these challenges, clinical laboratories have misidentified the organism as C. haemulonii and Saccharomyces cerevisiae.”
“It’s often misidentified as other Candida species or as Saccharomyces when we investigate with biochemical methods. C. auris is best identified using Matrix Assisted Laser Desorption/Ionization time of flight mass spectrometry (MALDI-TOF),” said Dr. Meis.
Among the presentations at ECCMID were a report of a U.K. outbreak that affected 70 patients in a neuroscience ICU. It was traced to axillary skin-surface temperature probes, and eradicated only after those probes were removed. More than 90% of the isolates were resistant to fluconazole, voriconazole, and posaconazole; 18% were amphotericin resistant.
A poster described the microbiological characteristics of 50 C. auris isolates taken from 11 hospitals in Korea.
Dr. Pemán described the outbreak in Valencia, which began in April 2016; the report was simultaneously published in the online journal Mycoses (2018 Apr 14. doi: 10.1111/myc.12781).
The index case was a 66-year-old man with hepatocellular carcinoma who underwent a liver resection at Hospital Le Fe in April 2016. During his stay in the surgical ICU (SICU), he developed a fungal infection from an unknown, highly fluconazole-resistant yeast. The pathogen was twice misidentified, first as C. haemulonii and then as S. cerevisiae.
Three weeks later, the patient in the adjacent bed developed a similar infection. Sequencing of the internal transcribed spacer confirmed both as a Candida isolate – an organism previously unknown in Spain.
The SICU setup was apparently very conducive to the C. auris life cycle, Dr. Pemán said. It’s a relatively open ward divided into three rooms with 12 beds in each. There are no isolation beds, and dozens of workers have access to the ward every day, including clinical and cleaning staff.
After identifying the second isolate, Dr. Pemán said, infection control staff went into action. They instituted contact precautions in the SICU, and took regular cultures from newly admitted patients and cultures of every SICU patient every 7 days.
“We also started an intense search for more cases throughout the hospital and in 101 SICU workers. Of 305 samples from hands and ears, we found nothing.” They reviewed all the prior fluconazole-resistant Candida isolates; C. auris was not present in the hospital before the index case.
Three weeks after case 2, six new SICU patients tested positive for C. auris (two blood cultures, one vascular line, one respiratory specimen, two rectal swabs, and one urinary tract sample).
“We reinforced contact precautions in colonized and infected patients and started a twice-daily environmental cleaning practice with quaternary ammonium around them,” said Dr. Pemán. They instituted a proactive hospital-wide hand hygiene campaign and spread the word about the outbreak.
By July, there were 11 new colonized patients, 3 of whom developed candidemia. These patients were grouped in the same SICU ward and underwent daily skin treatments with 4% aqueous chlorhexidine wipes.
The environmental inspection found C. auris on beds, tables, walls, and the floor all around infected patients. The pathogen also was living on IV pumps, computer keyboards, and bedside tables. Blood pressure cuffs were a favorite haunt: 19 of 36 samples in the adjacent ICU were positive. These data were separately reported at ECCMID.
Despite all of these efforts at eradication, infections continued to rise. By November, there were 24 newly colonized patients and nine new candidemia episodes in SICU and regular ICU patients. In December, a new infection control bundle began: A surveillance nurse in the C. auris SICU ward was in charge of compliance; any patient with any yeast growth in culture was isolated, and staff used 2% alcohol chlorhexidine wipes before and after IV catheter handling. Staff also washed down all surfaces three times daily with a disinfectant.
Patients could leave isolation after three consecutive C. auris–negative cultures. After discharge, an ultraviolet light decontamination procedure disinfected each patient room.
The pathogen was almost unbelievably resilient, Dr. Pemán noted in the Mycoses article. “In some cases, C. auris was recovered from walls after cleaning with cationic surface–active products ... it was not known until very recently that these products, as well as quaternary ammonium disinfectants, cannot effectively remove C. auris from surfaces.”
As a result of the previous measures, the outbreak slowed down during December 2016, with two new candidemia cases, but by February, the outbreak resumed with 50 new cases and 18 candidemias detected. Cases continued to emerge throughout 2017.
By September 2017, 250 patients had been colonized; 116 of these were included in the Mycoses report. There were 30 episodes of candidemia (26%); of these, 17 died by 30 days (41.4%). Spondylodiscitis and endocarditis each developed in two patients and one developed ventriculitis.
A separate poster by Dr. Pemán and his colleagues gave more details:
• A 52-year-old woman with C. auris–induced endocarditis died after 4 weeks of treatment with AMB+ECN and flucytosine. She had undergone a prosthetic heart valve placement for Ebstein’s anomaly.
• A 71-year-old man with hydrocephalus developed a C. auris–induced infection of his ventriculoperitoneal shunt; he also had undergone cardiovascular surgery and had an ischemic cardiomyopathy. He died despite shunt removal and 8 weeks of AMB+ECN.
• A 71-year-old man who underwent cardiovascular surgery and received a prosthetic heart valve developed endocarditis. He is alive and at last report, on week 26 of AMB+ECN, flucytosine, and isavuconazole.
• A 68-year-old man who underwent abdominal surgery for hepatocellular carcinoma developed spondylodiscitis and is alive after 24 weeks of AMB+ECN.
• A 48-year old female multiple trauma patient developed spondylodiscitis and is alive after 48 weeks of treatment with AMB+ECN.
A multivariate analysis determined that antibacterial treatment increased the risk of candidemia by almost 30 times (odds ratio, 29.59). The next highest risk was neutropenia (OR, 20.7) and then simply being a hospital and SICU patient. Dr. Pemán’s poster said, “In the 16 months before the index case, La Fe recorded 89 candidemias, none caused by C. auris. In the 16 months afterward, there were 154 candidemias, largely C. auris. Before April 2016, C. parapsilosis accounted for the largest portion of candidemias (46%) followed by C. albicans. After the index case, C. auris accounted for 42%, followed by C. parapsilosis (21%) and C. albicans (18%).”
Because of its fluconazole resistance, patients with C. auris received a combined antifungal treatment of liposomal amphotericin B 3 mg/kg per day for 5 days, and a standard dose of echinocandin for 3 weeks. Many C. auris strains are echinocandin resistant, Dr. Pemán noted. This particular strain was clonal, different from any other previously reported, he said.
“Our results confirm those previously reported by other authors, that C. auris is grouped in different independent clusters according to its geographical origin. Although all Spanish isolates were genotypically distinct from Indian, Omani, U.K., and Venezuelan isolates, there seems to be some connection with South African isolates.”
Hospital Le Fe continues to struggle with C. auris. As of March, 335 patients have tested positive for the pathogen, and 80 have developed candidemias.
“We feel we may be approaching the end of this episode, but it’s really not possible to be sure,” he said.
Dr. Pemán had no relevant financial disclosures.
SOURCE: ECCMID 2018 Peman et al. S0067.
REPORTING FROM ECCMID 2018
Time Won't Heal This Wound
An 85-year-old black man presents with a nonhealing, asymptomatic lesion on his cheek. He says the problem began several months ago, during a fishing trip, when some fishing line got caught in his beard, pulling out a few hairs in the process.
An accompanying relative, however, is quite certain that the lesion predates the fishing incident (for which he was present). He believes the lesion has been there for two years. He also advises that the patient’s memory is “not what it used to be.”
The patient has a significant history of sun exposure from his job as a stonemason, which kept him outdoors most of the time. He has been seen by a variety of providers and diagnosed with several infections, including pyoderma—but antibiotics have had no effect on the lesion.
EXAMINATION
Located on the right lateral cheek is a 2.4-cm, full-thickness ulceration that penetrates well into adipose tissue. Little if any redness can be seen around the lesion, and no adjacent nodes are palpable. A shave biopsy of the lesion is obtained.
What is the diagnosis?
DISCUSSION
The pathology report showed evidence of a basosquamous cell carcinoma.
This case effectively illustrates a key message: Nonhealing lesions should be considered cancerous until proven otherwise (via biopsy). This remains true even in individuals with darker skin; they may have lower risk for skin cancer than do fair-skinned individuals, but they do not have no risk—especially if there is a lifetime history of sun exposure.
The depth and width of the lesion suggest it had been present for many years, slowing growing. This timeframe, along with the lack of response to antibiotics, made infection unlikely. Furthermore, an infection serious enough to cause ulceration would be red and painful.
The mixed picture on the pathology report is unusual but not at all unknown; it just means the lesion had features of both basal and squamous cell carcinoma. Unfortunately, the biopsy results, in conjunction with the lesion’s dimensions, indicate an increased risk for metastasis (or at least spread to local nodes). There could also be perineural involvement if the cancer cells spread to deeper structures through the penetrating nerves.
The entire clinical picture in this case made the patient a candidate for Mohs surgery, which would ensure two things: clear excision margins and optimal wound closure. Should the surgeon find perineural involvement, he or she might advise postoperative radiation therapy to guarantee complete eradication of the cancer.
TAKE-HOME LEARNING POINTS
- Nonhealing lesions should be considered cancerous until proven otherwise by biopsy.
- Even though dark-skinned individuals have far less risk for skin cancer than those with fair skin, a lifetime of sun exposure can overcome the odds.
- Size and depth of the lesion increases risk for metastasis or perineural involvement (spreading to deeper structures through the nerves).
- Mohs surgery, as well as postoperative radiation therapy, can be used to completely eradicate the cancer.
An 85-year-old black man presents with a nonhealing, asymptomatic lesion on his cheek. He says the problem began several months ago, during a fishing trip, when some fishing line got caught in his beard, pulling out a few hairs in the process.
An accompanying relative, however, is quite certain that the lesion predates the fishing incident (for which he was present). He believes the lesion has been there for two years. He also advises that the patient’s memory is “not what it used to be.”
The patient has a significant history of sun exposure from his job as a stonemason, which kept him outdoors most of the time. He has been seen by a variety of providers and diagnosed with several infections, including pyoderma—but antibiotics have had no effect on the lesion.
EXAMINATION
Located on the right lateral cheek is a 2.4-cm, full-thickness ulceration that penetrates well into adipose tissue. Little if any redness can be seen around the lesion, and no adjacent nodes are palpable. A shave biopsy of the lesion is obtained.

What is the diagnosis?
DISCUSSION
The pathology report showed evidence of a basosquamous cell carcinoma.
This case effectively illustrates a key message: Nonhealing lesions should be considered cancerous until proven otherwise (via biopsy). This remains true even in individuals with darker skin; they may have lower risk for skin cancer than do fair-skinned individuals, but they do not have no risk—especially if there is a lifetime history of sun exposure.
The depth and width of the lesion suggest it had been present for many years, slowing growing. This timeframe, along with the lack of response to antibiotics, made infection unlikely. Furthermore, an infection serious enough to cause ulceration would be red and painful.
The mixed picture on the pathology report is unusual but not at all unknown; it just means the lesion had features of both basal and squamous cell carcinoma. Unfortunately, the biopsy results, in conjunction with the lesion’s dimensions, indicate an increased risk for metastasis (or at least spread to local nodes). There could also be perineural involvement if the cancer cells spread to deeper structures through the penetrating nerves.
The entire clinical picture in this case made the patient a candidate for Mohs surgery, which would ensure two things: clear excision margins and optimal wound closure. Should the surgeon find perineural involvement, he or she might advise postoperative radiation therapy to guarantee complete eradication of the cancer.
TAKE-HOME LEARNING POINTS
- Nonhealing lesions should be considered cancerous until proven otherwise by biopsy.
- Even though dark-skinned individuals have far less risk for skin cancer than those with fair skin, a lifetime of sun exposure can overcome the odds.
- Size and depth of the lesion increases risk for metastasis or perineural involvement (spreading to deeper structures through the nerves).
- Mohs surgery, as well as postoperative radiation therapy, can be used to completely eradicate the cancer.
An 85-year-old black man presents with a nonhealing, asymptomatic lesion on his cheek. He says the problem began several months ago, during a fishing trip, when some fishing line got caught in his beard, pulling out a few hairs in the process.
An accompanying relative, however, is quite certain that the lesion predates the fishing incident (for which he was present). He believes the lesion has been there for two years. He also advises that the patient’s memory is “not what it used to be.”
The patient has a significant history of sun exposure from his job as a stonemason, which kept him outdoors most of the time. He has been seen by a variety of providers and diagnosed with several infections, including pyoderma—but antibiotics have had no effect on the lesion.
EXAMINATION
Located on the right lateral cheek is a 2.4-cm, full-thickness ulceration that penetrates well into adipose tissue. Little if any redness can be seen around the lesion, and no adjacent nodes are palpable. A shave biopsy of the lesion is obtained.

What is the diagnosis?
DISCUSSION
The pathology report showed evidence of a basosquamous cell carcinoma.
This case effectively illustrates a key message: Nonhealing lesions should be considered cancerous until proven otherwise (via biopsy). This remains true even in individuals with darker skin; they may have lower risk for skin cancer than do fair-skinned individuals, but they do not have no risk—especially if there is a lifetime history of sun exposure.
The depth and width of the lesion suggest it had been present for many years, slowing growing. This timeframe, along with the lack of response to antibiotics, made infection unlikely. Furthermore, an infection serious enough to cause ulceration would be red and painful.
The mixed picture on the pathology report is unusual but not at all unknown; it just means the lesion had features of both basal and squamous cell carcinoma. Unfortunately, the biopsy results, in conjunction with the lesion’s dimensions, indicate an increased risk for metastasis (or at least spread to local nodes). There could also be perineural involvement if the cancer cells spread to deeper structures through the penetrating nerves.
The entire clinical picture in this case made the patient a candidate for Mohs surgery, which would ensure two things: clear excision margins and optimal wound closure. Should the surgeon find perineural involvement, he or she might advise postoperative radiation therapy to guarantee complete eradication of the cancer.
TAKE-HOME LEARNING POINTS
- Nonhealing lesions should be considered cancerous until proven otherwise by biopsy.
- Even though dark-skinned individuals have far less risk for skin cancer than those with fair skin, a lifetime of sun exposure can overcome the odds.
- Size and depth of the lesion increases risk for metastasis or perineural involvement (spreading to deeper structures through the nerves).
- Mohs surgery, as well as postoperative radiation therapy, can be used to completely eradicate the cancer.