User login
MDedge Daily News: Genes, not adiposity, may be driving appetite differences in obesity
Also today, conservative early approach likely best, the true burden of nonmelanoma skin cancer and atopic eczema is linked to cardiovascular disease.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
Also today, conservative early approach likely best, the true burden of nonmelanoma skin cancer and atopic eczema is linked to cardiovascular disease.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
Also today, conservative early approach likely best, the true burden of nonmelanoma skin cancer and atopic eczema is linked to cardiovascular disease.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
VA Nurses Address Critical Needs
For more than 35 years, the Nurses Organization of Veterans Affairs (NOVA) has been the voice of more than 3,000 Department of Veterans Affairs (VA) nurses caring for veterans. Speaking on behalf of its members, NOVA leaders provide an annual list of its legislative priority goals, which identifies concerns that require either legislation, funding, or implementation at the regulatory level within the VA.
At the top of this list of priorities is the ability to retain, recruit, and hire critical staff. The VA has had difficulty hiring essential staff at many levels within its health care facilities. A VA internal audit found that the need for additional doctors, nurses, and other specialty care was the highest barrier or challenge to providing access to care for veterans. Both congressional VA oversight committees have discussed this issue and included hiring provisions in their respective Choice/Community Care bills that await final action in both chambers.
In its recruitment/staffing goals, NOVA identified the 5 following areas:
- Hire additional human resources (HR) staff and review and streamline policies and procedures to improve the hiring process;
- Review thoroughly downgrades and reclassification of critical positions across the VA;
- Increase training of HR personnel on use of locality pay process in hiring;
- Revise the cap on nurse pay structures and registered nurse pay schedules and reclassification of critical positions so that VA can ensure competitive salaries; and
- Address USAJOBS website problems, including the complexity and excessive time required to complete application and inadequate applications response/feedback.
Addressing Choice/Community Integrated Health Care—Choice 2.0—is another NOVA goal. When the VA cannot provide timely care to veterans, NOVA supports the use of outside provide
Although NOVA supports the addition of community providers as a crucial part of an integrated network designed to provide care where there are shortages, the change has called attention to myriad problems created by outside providers, such as delays in care, the wrong care, or the veteran not being seen at all. Any final Choice/Community Care legislation must include mandatory training for both VA personnel and community providers to improve coordination and timeliness of care and services. The legislation also must hold community providers to the same high standards and quality metrics already in place at the VA.
Last, NOVA addresses information technology (IT) across VHA, which includes supporting an electronic health record for seamless transition of care between DoD and VA, proper funding for all IT stations to improve patient safety, software usability, and standardization of patient health care records across the system. As the VA continues to modernize, NOVA asks that nursing leadership be at the forefront of all strategic decision making.
As an advocate for its members and the patients they serve, NOVA will continue to share its views with Congress, the administration, and VA leadership on how they can work together toward common goals—educating the next generation of nurses, providing innovative health care solutions, or learning how veterans envision their health care. For more information about NOVA, a list of its 2018 Legislative Priority Goals, or to become a member, visit vanurse.org.
For more than 35 years, the Nurses Organization of Veterans Affairs (NOVA) has been the voice of more than 3,000 Department of Veterans Affairs (VA) nurses caring for veterans. Speaking on behalf of its members, NOVA leaders provide an annual list of its legislative priority goals, which identifies concerns that require either legislation, funding, or implementation at the regulatory level within the VA.
At the top of this list of priorities is the ability to retain, recruit, and hire critical staff. The VA has had difficulty hiring essential staff at many levels within its health care facilities. A VA internal audit found that the need for additional doctors, nurses, and other specialty care was the highest barrier or challenge to providing access to care for veterans. Both congressional VA oversight committees have discussed this issue and included hiring provisions in their respective Choice/Community Care bills that await final action in both chambers.
In its recruitment/staffing goals, NOVA identified the 5 following areas:
- Hire additional human resources (HR) staff and review and streamline policies and procedures to improve the hiring process;
- Review thoroughly downgrades and reclassification of critical positions across the VA;
- Increase training of HR personnel on use of locality pay process in hiring;
- Revise the cap on nurse pay structures and registered nurse pay schedules and reclassification of critical positions so that VA can ensure competitive salaries; and
- Address USAJOBS website problems, including the complexity and excessive time required to complete application and inadequate applications response/feedback.
Addressing Choice/Community Integrated Health Care—Choice 2.0—is another NOVA goal. When the VA cannot provide timely care to veterans, NOVA supports the use of outside provide
Although NOVA supports the addition of community providers as a crucial part of an integrated network designed to provide care where there are shortages, the change has called attention to myriad problems created by outside providers, such as delays in care, the wrong care, or the veteran not being seen at all. Any final Choice/Community Care legislation must include mandatory training for both VA personnel and community providers to improve coordination and timeliness of care and services. The legislation also must hold community providers to the same high standards and quality metrics already in place at the VA.
Last, NOVA addresses information technology (IT) across VHA, which includes supporting an electronic health record for seamless transition of care between DoD and VA, proper funding for all IT stations to improve patient safety, software usability, and standardization of patient health care records across the system. As the VA continues to modernize, NOVA asks that nursing leadership be at the forefront of all strategic decision making.
As an advocate for its members and the patients they serve, NOVA will continue to share its views with Congress, the administration, and VA leadership on how they can work together toward common goals—educating the next generation of nurses, providing innovative health care solutions, or learning how veterans envision their health care. For more information about NOVA, a list of its 2018 Legislative Priority Goals, or to become a member, visit vanurse.org.
For more than 35 years, the Nurses Organization of Veterans Affairs (NOVA) has been the voice of more than 3,000 Department of Veterans Affairs (VA) nurses caring for veterans. Speaking on behalf of its members, NOVA leaders provide an annual list of its legislative priority goals, which identifies concerns that require either legislation, funding, or implementation at the regulatory level within the VA.
At the top of this list of priorities is the ability to retain, recruit, and hire critical staff. The VA has had difficulty hiring essential staff at many levels within its health care facilities. A VA internal audit found that the need for additional doctors, nurses, and other specialty care was the highest barrier or challenge to providing access to care for veterans. Both congressional VA oversight committees have discussed this issue and included hiring provisions in their respective Choice/Community Care bills that await final action in both chambers.
In its recruitment/staffing goals, NOVA identified the 5 following areas:
- Hire additional human resources (HR) staff and review and streamline policies and procedures to improve the hiring process;
- Review thoroughly downgrades and reclassification of critical positions across the VA;
- Increase training of HR personnel on use of locality pay process in hiring;
- Revise the cap on nurse pay structures and registered nurse pay schedules and reclassification of critical positions so that VA can ensure competitive salaries; and
- Address USAJOBS website problems, including the complexity and excessive time required to complete application and inadequate applications response/feedback.
Addressing Choice/Community Integrated Health Care—Choice 2.0—is another NOVA goal. When the VA cannot provide timely care to veterans, NOVA supports the use of outside provide
Although NOVA supports the addition of community providers as a crucial part of an integrated network designed to provide care where there are shortages, the change has called attention to myriad problems created by outside providers, such as delays in care, the wrong care, or the veteran not being seen at all. Any final Choice/Community Care legislation must include mandatory training for both VA personnel and community providers to improve coordination and timeliness of care and services. The legislation also must hold community providers to the same high standards and quality metrics already in place at the VA.
Last, NOVA addresses information technology (IT) across VHA, which includes supporting an electronic health record for seamless transition of care between DoD and VA, proper funding for all IT stations to improve patient safety, software usability, and standardization of patient health care records across the system. As the VA continues to modernize, NOVA asks that nursing leadership be at the forefront of all strategic decision making.
As an advocate for its members and the patients they serve, NOVA will continue to share its views with Congress, the administration, and VA leadership on how they can work together toward common goals—educating the next generation of nurses, providing innovative health care solutions, or learning how veterans envision their health care. For more information about NOVA, a list of its 2018 Legislative Priority Goals, or to become a member, visit vanurse.org.
New and Noteworthy Information—May 2018
Starting School Later Improves Sleep Time
Delaying school start time can provide sustained benefits for sleep duration, daytime alertness, and mental well-being, according to a study published online ahead of print April 10 in Sleep. The study included 375 girls in grades 7 to 10 from a secondary school for girls. The school delayed its start time from 7:30 to 8:15 in the morning. Self-reports of sleep timing, sleepiness, and well-being were obtained at baseline before the delay and at approximately one and nine months after the delay. After one month, bedtimes on school nights were delayed by nine minutes, while rise times were delayed by 31.6 minutes, resulting in an increase in time in bed of 23.2 minutes. After nine months, the increase in time in bed was sustained, and total sleep time increased by 10 minutes.
Lo JC, Lee SM, Lee XK, et al. Sustained benefits of delaying school start time on adolescent sleep and well-being. Sleep. 2018 Apr 10 [Epub ahead of print].
Stroke Affects Social, Cognitive, and Psychologic Outcomes
Patients with ischemic stroke report symptoms in multiple domains that increase to variable degrees at higher levels of disability, according to a study published online ahead of print March 28 in Neurology. The observational cohort included 1,195 patients who completed Quality of Life in Neurological Disorders or the Patient-Reported Outcomes Measurement Information System scales as part of routine care. Participants were questioned about their physical function, satisfaction with social roles, fatigue, anxiety, depression, pain interference, and sleep disturbance. Researchers also measured participants’ level of disability. Among people with stroke, scores were considerably worse than those in the general population in every area except sleep and depression. About 58% of people with stroke had scores related to satisfaction with social roles that were meaningfully worse than those of the general population.
Katzan IL, Thompson NR, Uchino K, Lapin B. The most affected health domains after ischemic stroke. Neurology. 2018 Mar 28 [Epub ahead of print].
ALS Genetic Variant Also a Risk Factor for Frontotemporal Dementia
One of the newly identified genetic variants associated with amyotrophic lateral sclerosis (ALS) also is a risk factor for frontotemporal dementia (FTD), according to a study published online ahead of print April 9 in JAMA Neurology. Researchers pooled data from previous genome-wide association studies that included genetic data from 124,876 participants. The studies included healthy controls and participants with ALS, Alzheimer’s disease, Parkinson’s disease, FTD, corticobasal degeneration, and progressive supranuclear palsy. Investigators found that a variation in a region of DNA containing the gene for tau protein was associated with elevated risk for ALS. In addition, study authors found significant genetic overlap between ALS and FTD at known ALS loci rs13302855, rs3849942, and rs4239633. They also found a genetic variation at rs538622 that is associated with ALS and FTD and affects BNIP1 production in the brain.
Karch CM, Wen N, Fan CC, et al. Selective genetic overlap between amyotrophic lateral sclerosis and diseases of the frontotemporal dementia spectrum. JAMA Neurol. 2018 Apr 9 [Epub ahead of print].
Intervention Promotes Stroke Preparedness
Hip-Hop Stroke (HHS) is an effective, intergenerational model for increasing stroke preparedness among economically disadvantaged minorities, according to a study published in the April issue of Stroke. HHS is a three-hour, culturally tailored, theory-based, multimedia stroke literacy intervention that empowers schoolchildren to share stroke information with parents. Researchers recruited 3,070 fourth- through sixth-graders and 1,144 parents from 22 schools into a cluster-randomized trial. Schools were randomized to the HHS intervention or attentional control (ie, nutrition classes). Main outcome measures were stroke knowledge and preparedness of children and parents using validated surrogates. Among children, it was estimated that 1% of controls and 2% of the intervention group demonstrated optimal stroke preparedness at baseline, increasing to 57% immediately after the program in the intervention group.
Williams O, Leighton-Herrmann Quinn E, Teresi J, et al. Improving community stroke preparedness in the HHS (Hip-Hop Stroke) randomized clinical trial. Stroke. 2018;49(4):972-979.
Risk of Unnatural Death Is Increased in Epilepsy
People with epilepsy are at increased risk of mortality from suicide and accidents, according to a study published online ahead of print April 9 in JAMA Neurology. Researchers examined the Clinical Practice Research Datalink (CPRD) and the Secure Anonymized Information Linkage (SAIL) databank, which are linked to hospitalization and mortality records. They matched people with epilepsy on age, sex, and general practice with as many as 20 controls. In all, 44,678 people in the CPRD and 14,051 individuals in the SAIL databank were identified in the prevalent epilepsy cohorts. Furthermore, 891,429 participants from the CPRD and 279,365 people from the SAIL databank were identified as controls. People with epilepsy were significantly more likely to die of an unnatural cause, unintentional injury, poisoning, or suicide, compared with controls.
Gorton HC, Webb RT, Carr MJ, et al. Risk of unnatural mortality in people with epilepsy. JAMA Neurol. 2018 Apr 9 [Epub ahead of print].
Method to Assess Consciousness May Improve Care
The Glasgow Coma Scale-Pupil (GCS-P) score provides a way to rapidly assess levels of consciousness in people with head injuries and could improve patient care, according to a study published online ahead of print April 10 in the Journal of Neurosurgery. Investigators obtained patient-level information about early GCS scores, pupil responses, late outcomes on the Glasgow Outcome Scale, and mortality by reviewing data from the Corticosteroid Randomization After Significant Head Injury study and the International Mission for Prognosis and Clinical Trials database. GCS score and pupil response were independently related to patient outcome. Adding information about pupil response to the GCS score improved the information. The performance of the GCS-P was similar to that of methods for evaluating traumatic brain damage.
Brennan PM, Murray GD, Teasdale GM. Simplifying the use of prognostic information in traumatic brain injury. Part 1: The GCS-Pupils score: an extended index of clinical severity. J Neurosurg. 2018 Apr 10 [Epub ahead of print].
Biomarkers of Dementia Risk Identified
Researchers have identified novel biomarkers of risk for future dementia, according to a study published online ahead of print February 28 in Alzheimer’s & Dementia. Investigators analyzed metabolites in blood samples drawn from 22,623 people enrolled in eight prospective cohorts. Over 246,698 person-years, they detected 995 cases of incident dementia and 745 cases of incident Alzheimer’s disease. Isoleucine, leucine, valine, creatinine, and two VLDL-specific lipoprotein lipid subclasses were associated with lower dementia risk. One HDL and one VLDL lipoprotein lipid subclass were associated with increased dementia risk. Branched-chain amino acids also were associated with decreased Alzheimer’s disease risk, and the concentration of cholesterol esters relative to total lipids in large HDL was associated with increased Alzheimer’s disease risk.
Tynkkynen J, Chouraki V, van der Lee SJ, et al. Association of branched-chain amino acids and other circulating metabolites with risk of incident dementia and Alzheimer’s disease: a prospective study in eight cohorts. Alzheimers Dement. 2018 Feb 28 [Epub ahead of print].
Smartphone App Helps Doctors Track Parkinson’s Disease Symptoms
A smartphone app generates a score that reflects symptom severity in patients with Parkinson’s disease, according to a study published online ahead of print March 26 in JAMA Neurology. This observational study assessed people with Parkinson’s disease who remotely completed voice, finger tapping, gait, balance, and reaction time tasks on the app. Researchers generated a mobile Parkinson’s disease score (mPDS) of 0 to 100 that objectively weighed features derived from each smartphone activity. The mPDS was based on 6,148 smartphone activity assessments from 129 individuals. Gait features contributed most to the total mPDS (33.4%). The mPDS detected symptom fluctuations with a mean intraday change of 13.9 points. The mPDS improved by a mean of 16.3 points in response to dopaminergic therapy.
Zhan A, Mohan S, Tarolli C, et al. Using smartphones and machine learning to quantify Parkinson disease severity: the mobile Parkinson disease score. JAMA Neurol. 2018 Mar 26 [Epub ahead of print].
Age at Natural Menopause Linked to Memory
Entering menopause at a later age may benefit memory in women years later, according to a study published online ahead of print April 11 in Neurology. Researchers used data from 1,315 participants in the Medical Research Council National Survey of Health and Development with known age at period cessation and as many as four assessments of verbal memory and processing speed at ages 43, 53, between 60 and 64, and 69. Investigators fitted multilevel models with linear and quadratic age terms, stratified by natural or surgical menopause, and adjusted for hormone replacement therapy, BMI, smoking, occupational class, education, and childhood cognitive ability. Verbal memory increased with later age at natural menopause and with later age at surgical menopause. The association between age at natural menopause and verbal memory remained significant after data adjustment.
Kuh D, Cooper R, Moore A, et al. Age at menopause and lifetime cognition: findings from a British birth cohort study. Neurology. 2018 Apr 11 [Epub ahead of print].
Single-Pulse TMS Decreases Migraine Frequency
Single-pulse transcranial magnetic stimulation (sTMS) may be an effective, well-tolerated treatment option for migraine prevention, according to a study published online ahead of print March 4 in Cephalalgia. Researchers examined data from the eNeura SpringTMS Post-Market Observational US Study of Migraine, a multicenter, prospective, open-label, observational study. A total of 263 patients with migraine completed a one-month baseline headache diary, followed by three months of preventive (four pulses bid) and acute treatment (three pulses repeated as many as three times per attack). In all, 220 participants were eligible based on their number of headache days. The device was assigned to 217 subjects, and 132 were included in the intention-to-treat full-analysis set. Treatment reduced mean monthly headache days by 2.75. The most common adverse events were tingling, lightheadedness, and tinnitus.
Starling AJ, Tepper SJ, Marmura MJ, et al. A multicenter, prospective, single arm, open label, observational study of sTMS for migraine prevention (ESPOUSE Study). Cephalalgia. 2018 Mar 4 [Epub ahead of print].
Short Sleep Increases Risk of Obesity in the Young
Short sleep duration is a risk factor for obesity in infants, children, and adolescents, according to a study published online ahead of print February 1 in Sleep. Researchers reviewed the results of 42 population studies that included 75,499 infants, children, and adolescents ages 0 to 18. Sleep duration was assessed through methods such as questionnaires and wearable technology. The investigators classified participants as short sleepers or regular sleepers. Short sleepers were defined as having less sleep than the reference category for their age. Participants were followed up for a median of three years, and changes in BMI and incidence of overweight status and obesity were recorded. At all ages, short sleepers gained more weight and were 58% more likely to become overweight or obese.
Miller MA, Kruisbrink M, Wallace J, et al. Sleep duration and incidence of obesity in infants, children and adolescents: a systematic review and meta-analysis of prospective studies. Sleep. 2018 Feb 1 [Epub ahead of print].
—Kimberly Williams
Starting School Later Improves Sleep Time
Delaying school start time can provide sustained benefits for sleep duration, daytime alertness, and mental well-being, according to a study published online ahead of print April 10 in Sleep. The study included 375 girls in grades 7 to 10 from a secondary school for girls. The school delayed its start time from 7:30 to 8:15 in the morning. Self-reports of sleep timing, sleepiness, and well-being were obtained at baseline before the delay and at approximately one and nine months after the delay. After one month, bedtimes on school nights were delayed by nine minutes, while rise times were delayed by 31.6 minutes, resulting in an increase in time in bed of 23.2 minutes. After nine months, the increase in time in bed was sustained, and total sleep time increased by 10 minutes.
Lo JC, Lee SM, Lee XK, et al. Sustained benefits of delaying school start time on adolescent sleep and well-being. Sleep. 2018 Apr 10 [Epub ahead of print].
Stroke Affects Social, Cognitive, and Psychologic Outcomes
Patients with ischemic stroke report symptoms in multiple domains that increase to variable degrees at higher levels of disability, according to a study published online ahead of print March 28 in Neurology. The observational cohort included 1,195 patients who completed Quality of Life in Neurological Disorders or the Patient-Reported Outcomes Measurement Information System scales as part of routine care. Participants were questioned about their physical function, satisfaction with social roles, fatigue, anxiety, depression, pain interference, and sleep disturbance. Researchers also measured participants’ level of disability. Among people with stroke, scores were considerably worse than those in the general population in every area except sleep and depression. About 58% of people with stroke had scores related to satisfaction with social roles that were meaningfully worse than those of the general population.
Katzan IL, Thompson NR, Uchino K, Lapin B. The most affected health domains after ischemic stroke. Neurology. 2018 Mar 28 [Epub ahead of print].
ALS Genetic Variant Also a Risk Factor for Frontotemporal Dementia
One of the newly identified genetic variants associated with amyotrophic lateral sclerosis (ALS) also is a risk factor for frontotemporal dementia (FTD), according to a study published online ahead of print April 9 in JAMA Neurology. Researchers pooled data from previous genome-wide association studies that included genetic data from 124,876 participants. The studies included healthy controls and participants with ALS, Alzheimer’s disease, Parkinson’s disease, FTD, corticobasal degeneration, and progressive supranuclear palsy. Investigators found that a variation in a region of DNA containing the gene for tau protein was associated with elevated risk for ALS. In addition, study authors found significant genetic overlap between ALS and FTD at known ALS loci rs13302855, rs3849942, and rs4239633. They also found a genetic variation at rs538622 that is associated with ALS and FTD and affects BNIP1 production in the brain.
Karch CM, Wen N, Fan CC, et al. Selective genetic overlap between amyotrophic lateral sclerosis and diseases of the frontotemporal dementia spectrum. JAMA Neurol. 2018 Apr 9 [Epub ahead of print].
Intervention Promotes Stroke Preparedness
Hip-Hop Stroke (HHS) is an effective, intergenerational model for increasing stroke preparedness among economically disadvantaged minorities, according to a study published in the April issue of Stroke. HHS is a three-hour, culturally tailored, theory-based, multimedia stroke literacy intervention that empowers schoolchildren to share stroke information with parents. Researchers recruited 3,070 fourth- through sixth-graders and 1,144 parents from 22 schools into a cluster-randomized trial. Schools were randomized to the HHS intervention or attentional control (ie, nutrition classes). Main outcome measures were stroke knowledge and preparedness of children and parents using validated surrogates. Among children, it was estimated that 1% of controls and 2% of the intervention group demonstrated optimal stroke preparedness at baseline, increasing to 57% immediately after the program in the intervention group.
Williams O, Leighton-Herrmann Quinn E, Teresi J, et al. Improving community stroke preparedness in the HHS (Hip-Hop Stroke) randomized clinical trial. Stroke. 2018;49(4):972-979.
Risk of Unnatural Death Is Increased in Epilepsy
People with epilepsy are at increased risk of mortality from suicide and accidents, according to a study published online ahead of print April 9 in JAMA Neurology. Researchers examined the Clinical Practice Research Datalink (CPRD) and the Secure Anonymized Information Linkage (SAIL) databank, which are linked to hospitalization and mortality records. They matched people with epilepsy on age, sex, and general practice with as many as 20 controls. In all, 44,678 people in the CPRD and 14,051 individuals in the SAIL databank were identified in the prevalent epilepsy cohorts. Furthermore, 891,429 participants from the CPRD and 279,365 people from the SAIL databank were identified as controls. People with epilepsy were significantly more likely to die of an unnatural cause, unintentional injury, poisoning, or suicide, compared with controls.
Gorton HC, Webb RT, Carr MJ, et al. Risk of unnatural mortality in people with epilepsy. JAMA Neurol. 2018 Apr 9 [Epub ahead of print].
Method to Assess Consciousness May Improve Care
The Glasgow Coma Scale-Pupil (GCS-P) score provides a way to rapidly assess levels of consciousness in people with head injuries and could improve patient care, according to a study published online ahead of print April 10 in the Journal of Neurosurgery. Investigators obtained patient-level information about early GCS scores, pupil responses, late outcomes on the Glasgow Outcome Scale, and mortality by reviewing data from the Corticosteroid Randomization After Significant Head Injury study and the International Mission for Prognosis and Clinical Trials database. GCS score and pupil response were independently related to patient outcome. Adding information about pupil response to the GCS score improved the information. The performance of the GCS-P was similar to that of methods for evaluating traumatic brain damage.
Brennan PM, Murray GD, Teasdale GM. Simplifying the use of prognostic information in traumatic brain injury. Part 1: The GCS-Pupils score: an extended index of clinical severity. J Neurosurg. 2018 Apr 10 [Epub ahead of print].
Biomarkers of Dementia Risk Identified
Researchers have identified novel biomarkers of risk for future dementia, according to a study published online ahead of print February 28 in Alzheimer’s & Dementia. Investigators analyzed metabolites in blood samples drawn from 22,623 people enrolled in eight prospective cohorts. Over 246,698 person-years, they detected 995 cases of incident dementia and 745 cases of incident Alzheimer’s disease. Isoleucine, leucine, valine, creatinine, and two VLDL-specific lipoprotein lipid subclasses were associated with lower dementia risk. One HDL and one VLDL lipoprotein lipid subclass were associated with increased dementia risk. Branched-chain amino acids also were associated with decreased Alzheimer’s disease risk, and the concentration of cholesterol esters relative to total lipids in large HDL was associated with increased Alzheimer’s disease risk.
Tynkkynen J, Chouraki V, van der Lee SJ, et al. Association of branched-chain amino acids and other circulating metabolites with risk of incident dementia and Alzheimer’s disease: a prospective study in eight cohorts. Alzheimers Dement. 2018 Feb 28 [Epub ahead of print].
Smartphone App Helps Doctors Track Parkinson’s Disease Symptoms
A smartphone app generates a score that reflects symptom severity in patients with Parkinson’s disease, according to a study published online ahead of print March 26 in JAMA Neurology. This observational study assessed people with Parkinson’s disease who remotely completed voice, finger tapping, gait, balance, and reaction time tasks on the app. Researchers generated a mobile Parkinson’s disease score (mPDS) of 0 to 100 that objectively weighed features derived from each smartphone activity. The mPDS was based on 6,148 smartphone activity assessments from 129 individuals. Gait features contributed most to the total mPDS (33.4%). The mPDS detected symptom fluctuations with a mean intraday change of 13.9 points. The mPDS improved by a mean of 16.3 points in response to dopaminergic therapy.
Zhan A, Mohan S, Tarolli C, et al. Using smartphones and machine learning to quantify Parkinson disease severity: the mobile Parkinson disease score. JAMA Neurol. 2018 Mar 26 [Epub ahead of print].
Age at Natural Menopause Linked to Memory
Entering menopause at a later age may benefit memory in women years later, according to a study published online ahead of print April 11 in Neurology. Researchers used data from 1,315 participants in the Medical Research Council National Survey of Health and Development with known age at period cessation and as many as four assessments of verbal memory and processing speed at ages 43, 53, between 60 and 64, and 69. Investigators fitted multilevel models with linear and quadratic age terms, stratified by natural or surgical menopause, and adjusted for hormone replacement therapy, BMI, smoking, occupational class, education, and childhood cognitive ability. Verbal memory increased with later age at natural menopause and with later age at surgical menopause. The association between age at natural menopause and verbal memory remained significant after data adjustment.
Kuh D, Cooper R, Moore A, et al. Age at menopause and lifetime cognition: findings from a British birth cohort study. Neurology. 2018 Apr 11 [Epub ahead of print].
Single-Pulse TMS Decreases Migraine Frequency
Single-pulse transcranial magnetic stimulation (sTMS) may be an effective, well-tolerated treatment option for migraine prevention, according to a study published online ahead of print March 4 in Cephalalgia. Researchers examined data from the eNeura SpringTMS Post-Market Observational US Study of Migraine, a multicenter, prospective, open-label, observational study. A total of 263 patients with migraine completed a one-month baseline headache diary, followed by three months of preventive (four pulses bid) and acute treatment (three pulses repeated as many as three times per attack). In all, 220 participants were eligible based on their number of headache days. The device was assigned to 217 subjects, and 132 were included in the intention-to-treat full-analysis set. Treatment reduced mean monthly headache days by 2.75. The most common adverse events were tingling, lightheadedness, and tinnitus.
Starling AJ, Tepper SJ, Marmura MJ, et al. A multicenter, prospective, single arm, open label, observational study of sTMS for migraine prevention (ESPOUSE Study). Cephalalgia. 2018 Mar 4 [Epub ahead of print].
Short Sleep Increases Risk of Obesity in the Young
Short sleep duration is a risk factor for obesity in infants, children, and adolescents, according to a study published online ahead of print February 1 in Sleep. Researchers reviewed the results of 42 population studies that included 75,499 infants, children, and adolescents ages 0 to 18. Sleep duration was assessed through methods such as questionnaires and wearable technology. The investigators classified participants as short sleepers or regular sleepers. Short sleepers were defined as having less sleep than the reference category for their age. Participants were followed up for a median of three years, and changes in BMI and incidence of overweight status and obesity were recorded. At all ages, short sleepers gained more weight and were 58% more likely to become overweight or obese.
Miller MA, Kruisbrink M, Wallace J, et al. Sleep duration and incidence of obesity in infants, children and adolescents: a systematic review and meta-analysis of prospective studies. Sleep. 2018 Feb 1 [Epub ahead of print].
—Kimberly Williams
Starting School Later Improves Sleep Time
Delaying school start time can provide sustained benefits for sleep duration, daytime alertness, and mental well-being, according to a study published online ahead of print April 10 in Sleep. The study included 375 girls in grades 7 to 10 from a secondary school for girls. The school delayed its start time from 7:30 to 8:15 in the morning. Self-reports of sleep timing, sleepiness, and well-being were obtained at baseline before the delay and at approximately one and nine months after the delay. After one month, bedtimes on school nights were delayed by nine minutes, while rise times were delayed by 31.6 minutes, resulting in an increase in time in bed of 23.2 minutes. After nine months, the increase in time in bed was sustained, and total sleep time increased by 10 minutes.
Lo JC, Lee SM, Lee XK, et al. Sustained benefits of delaying school start time on adolescent sleep and well-being. Sleep. 2018 Apr 10 [Epub ahead of print].
Stroke Affects Social, Cognitive, and Psychologic Outcomes
Patients with ischemic stroke report symptoms in multiple domains that increase to variable degrees at higher levels of disability, according to a study published online ahead of print March 28 in Neurology. The observational cohort included 1,195 patients who completed Quality of Life in Neurological Disorders or the Patient-Reported Outcomes Measurement Information System scales as part of routine care. Participants were questioned about their physical function, satisfaction with social roles, fatigue, anxiety, depression, pain interference, and sleep disturbance. Researchers also measured participants’ level of disability. Among people with stroke, scores were considerably worse than those in the general population in every area except sleep and depression. About 58% of people with stroke had scores related to satisfaction with social roles that were meaningfully worse than those of the general population.
Katzan IL, Thompson NR, Uchino K, Lapin B. The most affected health domains after ischemic stroke. Neurology. 2018 Mar 28 [Epub ahead of print].
ALS Genetic Variant Also a Risk Factor for Frontotemporal Dementia
One of the newly identified genetic variants associated with amyotrophic lateral sclerosis (ALS) also is a risk factor for frontotemporal dementia (FTD), according to a study published online ahead of print April 9 in JAMA Neurology. Researchers pooled data from previous genome-wide association studies that included genetic data from 124,876 participants. The studies included healthy controls and participants with ALS, Alzheimer’s disease, Parkinson’s disease, FTD, corticobasal degeneration, and progressive supranuclear palsy. Investigators found that a variation in a region of DNA containing the gene for tau protein was associated with elevated risk for ALS. In addition, study authors found significant genetic overlap between ALS and FTD at known ALS loci rs13302855, rs3849942, and rs4239633. They also found a genetic variation at rs538622 that is associated with ALS and FTD and affects BNIP1 production in the brain.
Karch CM, Wen N, Fan CC, et al. Selective genetic overlap between amyotrophic lateral sclerosis and diseases of the frontotemporal dementia spectrum. JAMA Neurol. 2018 Apr 9 [Epub ahead of print].
Intervention Promotes Stroke Preparedness
Hip-Hop Stroke (HHS) is an effective, intergenerational model for increasing stroke preparedness among economically disadvantaged minorities, according to a study published in the April issue of Stroke. HHS is a three-hour, culturally tailored, theory-based, multimedia stroke literacy intervention that empowers schoolchildren to share stroke information with parents. Researchers recruited 3,070 fourth- through sixth-graders and 1,144 parents from 22 schools into a cluster-randomized trial. Schools were randomized to the HHS intervention or attentional control (ie, nutrition classes). Main outcome measures were stroke knowledge and preparedness of children and parents using validated surrogates. Among children, it was estimated that 1% of controls and 2% of the intervention group demonstrated optimal stroke preparedness at baseline, increasing to 57% immediately after the program in the intervention group.
Williams O, Leighton-Herrmann Quinn E, Teresi J, et al. Improving community stroke preparedness in the HHS (Hip-Hop Stroke) randomized clinical trial. Stroke. 2018;49(4):972-979.
Risk of Unnatural Death Is Increased in Epilepsy
People with epilepsy are at increased risk of mortality from suicide and accidents, according to a study published online ahead of print April 9 in JAMA Neurology. Researchers examined the Clinical Practice Research Datalink (CPRD) and the Secure Anonymized Information Linkage (SAIL) databank, which are linked to hospitalization and mortality records. They matched people with epilepsy on age, sex, and general practice with as many as 20 controls. In all, 44,678 people in the CPRD and 14,051 individuals in the SAIL databank were identified in the prevalent epilepsy cohorts. Furthermore, 891,429 participants from the CPRD and 279,365 people from the SAIL databank were identified as controls. People with epilepsy were significantly more likely to die of an unnatural cause, unintentional injury, poisoning, or suicide, compared with controls.
Gorton HC, Webb RT, Carr MJ, et al. Risk of unnatural mortality in people with epilepsy. JAMA Neurol. 2018 Apr 9 [Epub ahead of print].
Method to Assess Consciousness May Improve Care
The Glasgow Coma Scale-Pupil (GCS-P) score provides a way to rapidly assess levels of consciousness in people with head injuries and could improve patient care, according to a study published online ahead of print April 10 in the Journal of Neurosurgery. Investigators obtained patient-level information about early GCS scores, pupil responses, late outcomes on the Glasgow Outcome Scale, and mortality by reviewing data from the Corticosteroid Randomization After Significant Head Injury study and the International Mission for Prognosis and Clinical Trials database. GCS score and pupil response were independently related to patient outcome. Adding information about pupil response to the GCS score improved the information. The performance of the GCS-P was similar to that of methods for evaluating traumatic brain damage.
Brennan PM, Murray GD, Teasdale GM. Simplifying the use of prognostic information in traumatic brain injury. Part 1: The GCS-Pupils score: an extended index of clinical severity. J Neurosurg. 2018 Apr 10 [Epub ahead of print].
Biomarkers of Dementia Risk Identified
Researchers have identified novel biomarkers of risk for future dementia, according to a study published online ahead of print February 28 in Alzheimer’s & Dementia. Investigators analyzed metabolites in blood samples drawn from 22,623 people enrolled in eight prospective cohorts. Over 246,698 person-years, they detected 995 cases of incident dementia and 745 cases of incident Alzheimer’s disease. Isoleucine, leucine, valine, creatinine, and two VLDL-specific lipoprotein lipid subclasses were associated with lower dementia risk. One HDL and one VLDL lipoprotein lipid subclass were associated with increased dementia risk. Branched-chain amino acids also were associated with decreased Alzheimer’s disease risk, and the concentration of cholesterol esters relative to total lipids in large HDL was associated with increased Alzheimer’s disease risk.
Tynkkynen J, Chouraki V, van der Lee SJ, et al. Association of branched-chain amino acids and other circulating metabolites with risk of incident dementia and Alzheimer’s disease: a prospective study in eight cohorts. Alzheimers Dement. 2018 Feb 28 [Epub ahead of print].
Smartphone App Helps Doctors Track Parkinson’s Disease Symptoms
A smartphone app generates a score that reflects symptom severity in patients with Parkinson’s disease, according to a study published online ahead of print March 26 in JAMA Neurology. This observational study assessed people with Parkinson’s disease who remotely completed voice, finger tapping, gait, balance, and reaction time tasks on the app. Researchers generated a mobile Parkinson’s disease score (mPDS) of 0 to 100 that objectively weighed features derived from each smartphone activity. The mPDS was based on 6,148 smartphone activity assessments from 129 individuals. Gait features contributed most to the total mPDS (33.4%). The mPDS detected symptom fluctuations with a mean intraday change of 13.9 points. The mPDS improved by a mean of 16.3 points in response to dopaminergic therapy.
Zhan A, Mohan S, Tarolli C, et al. Using smartphones and machine learning to quantify Parkinson disease severity: the mobile Parkinson disease score. JAMA Neurol. 2018 Mar 26 [Epub ahead of print].
Age at Natural Menopause Linked to Memory
Entering menopause at a later age may benefit memory in women years later, according to a study published online ahead of print April 11 in Neurology. Researchers used data from 1,315 participants in the Medical Research Council National Survey of Health and Development with known age at period cessation and as many as four assessments of verbal memory and processing speed at ages 43, 53, between 60 and 64, and 69. Investigators fitted multilevel models with linear and quadratic age terms, stratified by natural or surgical menopause, and adjusted for hormone replacement therapy, BMI, smoking, occupational class, education, and childhood cognitive ability. Verbal memory increased with later age at natural menopause and with later age at surgical menopause. The association between age at natural menopause and verbal memory remained significant after data adjustment.
Kuh D, Cooper R, Moore A, et al. Age at menopause and lifetime cognition: findings from a British birth cohort study. Neurology. 2018 Apr 11 [Epub ahead of print].
Single-Pulse TMS Decreases Migraine Frequency
Single-pulse transcranial magnetic stimulation (sTMS) may be an effective, well-tolerated treatment option for migraine prevention, according to a study published online ahead of print March 4 in Cephalalgia. Researchers examined data from the eNeura SpringTMS Post-Market Observational US Study of Migraine, a multicenter, prospective, open-label, observational study. A total of 263 patients with migraine completed a one-month baseline headache diary, followed by three months of preventive (four pulses bid) and acute treatment (three pulses repeated as many as three times per attack). In all, 220 participants were eligible based on their number of headache days. The device was assigned to 217 subjects, and 132 were included in the intention-to-treat full-analysis set. Treatment reduced mean monthly headache days by 2.75. The most common adverse events were tingling, lightheadedness, and tinnitus.
Starling AJ, Tepper SJ, Marmura MJ, et al. A multicenter, prospective, single arm, open label, observational study of sTMS for migraine prevention (ESPOUSE Study). Cephalalgia. 2018 Mar 4 [Epub ahead of print].
Short Sleep Increases Risk of Obesity in the Young
Short sleep duration is a risk factor for obesity in infants, children, and adolescents, according to a study published online ahead of print February 1 in Sleep. Researchers reviewed the results of 42 population studies that included 75,499 infants, children, and adolescents ages 0 to 18. Sleep duration was assessed through methods such as questionnaires and wearable technology. The investigators classified participants as short sleepers or regular sleepers. Short sleepers were defined as having less sleep than the reference category for their age. Participants were followed up for a median of three years, and changes in BMI and incidence of overweight status and obesity were recorded. At all ages, short sleepers gained more weight and were 58% more likely to become overweight or obese.
Miller MA, Kruisbrink M, Wallace J, et al. Sleep duration and incidence of obesity in infants, children and adolescents: a systematic review and meta-analysis of prospective studies. Sleep. 2018 Feb 1 [Epub ahead of print].
—Kimberly Williams
Why Iron Can Make Malaria Worse
A National Institute of Health (NIH) research team that may have found the explanation for why iron supplements can sometimes worsen malaria infection. “Our study helps solve a long-standing mystery,” says Tracey Rouault, MD, a member of the team.
The researchers say the answer may lie with ferroportin, a protein that prevents iron from building to toxic levels in red blood cells and helps prevent malaria infection. Red blood cells use ferroportin to remove excess iron (a food source for malaria parasites). When the researchers fed mice a high-iron diet, they found that a hormone called hepcidin regulates ferroportin in erythroid cells. The hormone, which is more abundant in high-iron environments, not only lowered ferroportin levels in erythroblasts and red blood cells, but physically bound to ferroportin, preventing iron from being removed from cells.
Findings from 2 malaria studies suggest that Q248H, a ferroportin mutation often found in malaria-endemic regions, protects against malaria. In 1 study, nearly 20% of 66 children hospitalized for malaria in Zambia had the mutation; these children also tended to have fewer malarial parasites in the blood and tolerated their fevers for a longer period before coming to the hospital.
In the other study, of 290 pregnant women in Ghana, nearly 9% had the mutation. Those women were significantly less likely to have pregnancy-associated malaria.
Rouault says the findings may help explain why iron supplements can sometimes worsen malaria infection and why, conversely, iron deficiency can sometimes be protective. The findings also may help researchers develop strategies to prevent and treat malarial infections, which numbered nearly 216 million in 2016.
A National Institute of Health (NIH) research team that may have found the explanation for why iron supplements can sometimes worsen malaria infection. “Our study helps solve a long-standing mystery,” says Tracey Rouault, MD, a member of the team.
The researchers say the answer may lie with ferroportin, a protein that prevents iron from building to toxic levels in red blood cells and helps prevent malaria infection. Red blood cells use ferroportin to remove excess iron (a food source for malaria parasites). When the researchers fed mice a high-iron diet, they found that a hormone called hepcidin regulates ferroportin in erythroid cells. The hormone, which is more abundant in high-iron environments, not only lowered ferroportin levels in erythroblasts and red blood cells, but physically bound to ferroportin, preventing iron from being removed from cells.
Findings from 2 malaria studies suggest that Q248H, a ferroportin mutation often found in malaria-endemic regions, protects against malaria. In 1 study, nearly 20% of 66 children hospitalized for malaria in Zambia had the mutation; these children also tended to have fewer malarial parasites in the blood and tolerated their fevers for a longer period before coming to the hospital.
In the other study, of 290 pregnant women in Ghana, nearly 9% had the mutation. Those women were significantly less likely to have pregnancy-associated malaria.
Rouault says the findings may help explain why iron supplements can sometimes worsen malaria infection and why, conversely, iron deficiency can sometimes be protective. The findings also may help researchers develop strategies to prevent and treat malarial infections, which numbered nearly 216 million in 2016.
A National Institute of Health (NIH) research team that may have found the explanation for why iron supplements can sometimes worsen malaria infection. “Our study helps solve a long-standing mystery,” says Tracey Rouault, MD, a member of the team.
The researchers say the answer may lie with ferroportin, a protein that prevents iron from building to toxic levels in red blood cells and helps prevent malaria infection. Red blood cells use ferroportin to remove excess iron (a food source for malaria parasites). When the researchers fed mice a high-iron diet, they found that a hormone called hepcidin regulates ferroportin in erythroid cells. The hormone, which is more abundant in high-iron environments, not only lowered ferroportin levels in erythroblasts and red blood cells, but physically bound to ferroportin, preventing iron from being removed from cells.
Findings from 2 malaria studies suggest that Q248H, a ferroportin mutation often found in malaria-endemic regions, protects against malaria. In 1 study, nearly 20% of 66 children hospitalized for malaria in Zambia had the mutation; these children also tended to have fewer malarial parasites in the blood and tolerated their fevers for a longer period before coming to the hospital.
In the other study, of 290 pregnant women in Ghana, nearly 9% had the mutation. Those women were significantly less likely to have pregnancy-associated malaria.
Rouault says the findings may help explain why iron supplements can sometimes worsen malaria infection and why, conversely, iron deficiency can sometimes be protective. The findings also may help researchers develop strategies to prevent and treat malarial infections, which numbered nearly 216 million in 2016.
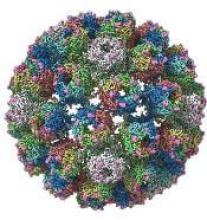
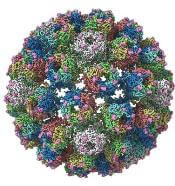
A closer look at the BK polyomavirus
A detailed image at near-atomic levels of the BK polyomavirus (BKV), which affects kidney and bone marrow transplant patients, is now available for the first time.
This molecular-level structural visualization could allow scientists to study potential targets for antiviral therapies or drugs.
Approximately 80% to 90% of the general population are seropositive for the most prevalent BKV genotypes. Yet the virus rarely causes illness in people with healthy immune systems.
But the BKV causes nephropathy and hemorrhagic cystitis in immunosuppressed patients. Currently, no effective antiviral agents are available specifically targeting BKV.
Using high-resolution cryoelectron microscopy (cryo-EM), the research team at the University of Leeds Astbury Centre for Structural Molecular Biology determined the structure of BKV at 3.8 Å resolution.
At this resolution, they could highlight differences between human BKV and BKV pathogens that infect simian and murine hosts.
The investigators created the structures by freezing infectious BKV particles and taking thousands of images using the microscopes. The two-dimensional images were then combined computationally to produce a high-resolution, three-dimensional view of the virus.
They reported their findings in the journal Structure.
Senior investigator Neil A. Ranson, PhD, of the University of Leeds , noted that cryo-EM has been around for 30 years.
Although it’s been useful, the technology lacked the ability to routinely look at molecules at the level of detail needed, he said.
"However, the Titan Krios microscopes we have installed in Leeds are absolutely state-of-the-art and mean that these limitations have been shattered. Researchers and industry users who work with us can now image biological molecules with an incredible resolution. “
The investigators believe the quality and completeness of the cryo-EM structures they captured allows researchers to describe the various conformations of the C termini of the major capsid protein VP1, which play a fundamental role in the assembly and stability of a polyomavirus capsid.
The investigators say the images also present a more complete picture of disulfide bonding in the capsid.
Thus, the images provide insights into the structure of the human pathogen at near native conditions and give scientists a better-quality research tool to use.
“Crucially, we'll also be able to see how these molecules interact with each other," Dr Ransom said.
The BK Polyomavirus structure research was funded by Wellcome, Kidney Research UK, and Kidney Research Yorkshire.
A detailed image at near-atomic levels of the BK polyomavirus (BKV), which affects kidney and bone marrow transplant patients, is now available for the first time.
This molecular-level structural visualization could allow scientists to study potential targets for antiviral therapies or drugs.
Approximately 80% to 90% of the general population are seropositive for the most prevalent BKV genotypes. Yet the virus rarely causes illness in people with healthy immune systems.
But the BKV causes nephropathy and hemorrhagic cystitis in immunosuppressed patients. Currently, no effective antiviral agents are available specifically targeting BKV.
Using high-resolution cryoelectron microscopy (cryo-EM), the research team at the University of Leeds Astbury Centre for Structural Molecular Biology determined the structure of BKV at 3.8 Å resolution.
At this resolution, they could highlight differences between human BKV and BKV pathogens that infect simian and murine hosts.
The investigators created the structures by freezing infectious BKV particles and taking thousands of images using the microscopes. The two-dimensional images were then combined computationally to produce a high-resolution, three-dimensional view of the virus.
They reported their findings in the journal Structure.
Senior investigator Neil A. Ranson, PhD, of the University of Leeds , noted that cryo-EM has been around for 30 years.
Although it’s been useful, the technology lacked the ability to routinely look at molecules at the level of detail needed, he said.
"However, the Titan Krios microscopes we have installed in Leeds are absolutely state-of-the-art and mean that these limitations have been shattered. Researchers and industry users who work with us can now image biological molecules with an incredible resolution. “
The investigators believe the quality and completeness of the cryo-EM structures they captured allows researchers to describe the various conformations of the C termini of the major capsid protein VP1, which play a fundamental role in the assembly and stability of a polyomavirus capsid.
The investigators say the images also present a more complete picture of disulfide bonding in the capsid.
Thus, the images provide insights into the structure of the human pathogen at near native conditions and give scientists a better-quality research tool to use.
“Crucially, we'll also be able to see how these molecules interact with each other," Dr Ransom said.
The BK Polyomavirus structure research was funded by Wellcome, Kidney Research UK, and Kidney Research Yorkshire.
A detailed image at near-atomic levels of the BK polyomavirus (BKV), which affects kidney and bone marrow transplant patients, is now available for the first time.
This molecular-level structural visualization could allow scientists to study potential targets for antiviral therapies or drugs.
Approximately 80% to 90% of the general population are seropositive for the most prevalent BKV genotypes. Yet the virus rarely causes illness in people with healthy immune systems.
But the BKV causes nephropathy and hemorrhagic cystitis in immunosuppressed patients. Currently, no effective antiviral agents are available specifically targeting BKV.
Using high-resolution cryoelectron microscopy (cryo-EM), the research team at the University of Leeds Astbury Centre for Structural Molecular Biology determined the structure of BKV at 3.8 Å resolution.
At this resolution, they could highlight differences between human BKV and BKV pathogens that infect simian and murine hosts.
The investigators created the structures by freezing infectious BKV particles and taking thousands of images using the microscopes. The two-dimensional images were then combined computationally to produce a high-resolution, three-dimensional view of the virus.
They reported their findings in the journal Structure.
Senior investigator Neil A. Ranson, PhD, of the University of Leeds , noted that cryo-EM has been around for 30 years.
Although it’s been useful, the technology lacked the ability to routinely look at molecules at the level of detail needed, he said.
"However, the Titan Krios microscopes we have installed in Leeds are absolutely state-of-the-art and mean that these limitations have been shattered. Researchers and industry users who work with us can now image biological molecules with an incredible resolution. “
The investigators believe the quality and completeness of the cryo-EM structures they captured allows researchers to describe the various conformations of the C termini of the major capsid protein VP1, which play a fundamental role in the assembly and stability of a polyomavirus capsid.
The investigators say the images also present a more complete picture of disulfide bonding in the capsid.
Thus, the images provide insights into the structure of the human pathogen at near native conditions and give scientists a better-quality research tool to use.
“Crucially, we'll also be able to see how these molecules interact with each other," Dr Ransom said.
The BK Polyomavirus structure research was funded by Wellcome, Kidney Research UK, and Kidney Research Yorkshire.
Neurology Board Review: Multiple Sclerosis
Click here to read Neurology Board Review: Multiple Sclerosis
Neurology Board Review: Multiple Sclerosis is a resource developed by leading clinical educators for studying for board certification and maintenance of certification exams.
After reading the article, Click Here to Access the Board Review Questions
About the Authors
Michael J. Bradshaw, MD
Clinical Fellow in Neurology
Partners Multiple Sclerosis Center
Brigham and Women’s Hospital
Massachusetts General Hospital
Harvard Medical School
Boston, Massachusetts
Maria K. Houtchens, MD, MMSC
Director, Women’s Health Program
Partners Multiple Sclerosis Center
Brigham and Women’s Hospital
Harvard Medical School
Boston, Massachusetts
Click here to read Neurology Board Review: Multiple Sclerosis
After reading the article, Click Here to Access the Board Review Questions
Click here to read Neurology Board Review: Multiple Sclerosis
Neurology Board Review: Multiple Sclerosis is a resource developed by leading clinical educators for studying for board certification and maintenance of certification exams.
After reading the article, Click Here to Access the Board Review Questions
About the Authors
Michael J. Bradshaw, MD
Clinical Fellow in Neurology
Partners Multiple Sclerosis Center
Brigham and Women’s Hospital
Massachusetts General Hospital
Harvard Medical School
Boston, Massachusetts
Maria K. Houtchens, MD, MMSC
Director, Women’s Health Program
Partners Multiple Sclerosis Center
Brigham and Women’s Hospital
Harvard Medical School
Boston, Massachusetts
Click here to read Neurology Board Review: Multiple Sclerosis
After reading the article, Click Here to Access the Board Review Questions
Click here to read Neurology Board Review: Multiple Sclerosis
Neurology Board Review: Multiple Sclerosis is a resource developed by leading clinical educators for studying for board certification and maintenance of certification exams.
After reading the article, Click Here to Access the Board Review Questions
About the Authors
Michael J. Bradshaw, MD
Clinical Fellow in Neurology
Partners Multiple Sclerosis Center
Brigham and Women’s Hospital
Massachusetts General Hospital
Harvard Medical School
Boston, Massachusetts
Maria K. Houtchens, MD, MMSC
Director, Women’s Health Program
Partners Multiple Sclerosis Center
Brigham and Women’s Hospital
Harvard Medical School
Boston, Massachusetts
Click here to read Neurology Board Review: Multiple Sclerosis
After reading the article, Click Here to Access the Board Review Questions
Newer IgG4 testing proving effective in assessing patients
SANDESTIN, FLA. – New forms of IgG4 testing could be more helpful in making diagnoses of immunoglobulin G4-related disease, an expert said at the annual Congress of Clinical Rheumatology, while cautioning that the diagnosis is more about histology and pattern of involvement than antibody testing.
Arezou Khosroshahi, MD, of Emory University, Atlanta, said that the IgG4 levels found in serum using nephelometry can often be low in patients who otherwise show signs of the disease, which can affect a wide array of organs and typically involves elevated IgG4. Newer forms of testing – enzyme-linked ImmunoSpot (ELISPOT) and quantitative reverse transcription polymerase chain reaction (RT-qPCR) – could be more telling, she said.
But she found that, on flow cytometry, 88% of the woman’s circulating B cells were positive for IgG4, so the woman was treated with rituximab to deplete these cells.
“When the B cells were gone, we had release of the IgG4 in the serum and now we could pick it up with nephelometry,” she said.
This missed IgG4 with nephelometry prompted researchers to turn to ELISPOT, a sensitive method to count antibody-secreting cells. The test works well by capturing the antibodies’ presence right after they’re secreted, before they can become lost to receptor binding or in other ways.
“This was a better assay to measure the IgG4 antibodies rather than nephelometry,” she said.
Perhaps even better, studies in Europe have found that RT-qPCR testing for IgG4 RNA can be effective. This type of testing is easier than ELISPOT, and “they are finding the sensitivity to be much superior to nephelometry for immunoglobulin levels,” Dr. Khosroshahi said.
The higher the levels of IgG4, the more likely an IgG4-related disease diagnosis is warranted, and higher levels tend to lead to worse outcomes, she said.
She waved a caution flag, though: Other diseases can involve elevated IgG4, and even a normal IgG4 level does not necessarily rule out the disease. Other evaluations really form the cornerstone of the diagnosis of IgG4-related disease, she said.
“There should be characteristic histology and of course IgG4-staining, but more importantly, pattern of organ involvement. It’s very important,” Dr. Khosroshahi said. “If there is a mass in the pancreas and there is salivary gland and parotid gland swellings and other features of that going on, you are more concerned that that is a process going on.”
Dr. Khosroshahi had no relevant disclosures.
SANDESTIN, FLA. – New forms of IgG4 testing could be more helpful in making diagnoses of immunoglobulin G4-related disease, an expert said at the annual Congress of Clinical Rheumatology, while cautioning that the diagnosis is more about histology and pattern of involvement than antibody testing.
Arezou Khosroshahi, MD, of Emory University, Atlanta, said that the IgG4 levels found in serum using nephelometry can often be low in patients who otherwise show signs of the disease, which can affect a wide array of organs and typically involves elevated IgG4. Newer forms of testing – enzyme-linked ImmunoSpot (ELISPOT) and quantitative reverse transcription polymerase chain reaction (RT-qPCR) – could be more telling, she said.
But she found that, on flow cytometry, 88% of the woman’s circulating B cells were positive for IgG4, so the woman was treated with rituximab to deplete these cells.
“When the B cells were gone, we had release of the IgG4 in the serum and now we could pick it up with nephelometry,” she said.
This missed IgG4 with nephelometry prompted researchers to turn to ELISPOT, a sensitive method to count antibody-secreting cells. The test works well by capturing the antibodies’ presence right after they’re secreted, before they can become lost to receptor binding or in other ways.
“This was a better assay to measure the IgG4 antibodies rather than nephelometry,” she said.
Perhaps even better, studies in Europe have found that RT-qPCR testing for IgG4 RNA can be effective. This type of testing is easier than ELISPOT, and “they are finding the sensitivity to be much superior to nephelometry for immunoglobulin levels,” Dr. Khosroshahi said.
The higher the levels of IgG4, the more likely an IgG4-related disease diagnosis is warranted, and higher levels tend to lead to worse outcomes, she said.
She waved a caution flag, though: Other diseases can involve elevated IgG4, and even a normal IgG4 level does not necessarily rule out the disease. Other evaluations really form the cornerstone of the diagnosis of IgG4-related disease, she said.
“There should be characteristic histology and of course IgG4-staining, but more importantly, pattern of organ involvement. It’s very important,” Dr. Khosroshahi said. “If there is a mass in the pancreas and there is salivary gland and parotid gland swellings and other features of that going on, you are more concerned that that is a process going on.”
Dr. Khosroshahi had no relevant disclosures.
SANDESTIN, FLA. – New forms of IgG4 testing could be more helpful in making diagnoses of immunoglobulin G4-related disease, an expert said at the annual Congress of Clinical Rheumatology, while cautioning that the diagnosis is more about histology and pattern of involvement than antibody testing.
Arezou Khosroshahi, MD, of Emory University, Atlanta, said that the IgG4 levels found in serum using nephelometry can often be low in patients who otherwise show signs of the disease, which can affect a wide array of organs and typically involves elevated IgG4. Newer forms of testing – enzyme-linked ImmunoSpot (ELISPOT) and quantitative reverse transcription polymerase chain reaction (RT-qPCR) – could be more telling, she said.
But she found that, on flow cytometry, 88% of the woman’s circulating B cells were positive for IgG4, so the woman was treated with rituximab to deplete these cells.
“When the B cells were gone, we had release of the IgG4 in the serum and now we could pick it up with nephelometry,” she said.
This missed IgG4 with nephelometry prompted researchers to turn to ELISPOT, a sensitive method to count antibody-secreting cells. The test works well by capturing the antibodies’ presence right after they’re secreted, before they can become lost to receptor binding or in other ways.
“This was a better assay to measure the IgG4 antibodies rather than nephelometry,” she said.
Perhaps even better, studies in Europe have found that RT-qPCR testing for IgG4 RNA can be effective. This type of testing is easier than ELISPOT, and “they are finding the sensitivity to be much superior to nephelometry for immunoglobulin levels,” Dr. Khosroshahi said.
The higher the levels of IgG4, the more likely an IgG4-related disease diagnosis is warranted, and higher levels tend to lead to worse outcomes, she said.
She waved a caution flag, though: Other diseases can involve elevated IgG4, and even a normal IgG4 level does not necessarily rule out the disease. Other evaluations really form the cornerstone of the diagnosis of IgG4-related disease, she said.
“There should be characteristic histology and of course IgG4-staining, but more importantly, pattern of organ involvement. It’s very important,” Dr. Khosroshahi said. “If there is a mass in the pancreas and there is salivary gland and parotid gland swellings and other features of that going on, you are more concerned that that is a process going on.”
Dr. Khosroshahi had no relevant disclosures.
EXPERT ANALYSIS FROM CCR 18
I’M NOT A PROVIDER
I am not sure when it occurred. I don’t know how it happened. I don’t think anyone took a vote on it. It happened gradually over the last decade. I think it happened when the administrative staff became larger than the medical staff. In order to include everyone under the same umbrella, everyone became a provider.
All those patients in our waiting room suddenly became consumers or clients. My grandfather ran a grocery store and had a lot of customers, and my father was a lawyer and had a lot of clients. None of those customers or clients would come to see me as their doctor with their illnesses today if I were a provider. The use of the terminology of “providers” and “customers” lowers all health care staff to the lowest common denominator and demeans the concerns of my patients.
I do not mean to diminish the role of the auto mechanic and salesperson, but they know and I know that our roles and are different and we are not just providers of a medical commodity. They do not expect me to deal with them as though they were coming to buy a car. They understand that we are actually trying to cure and treat worried patients and not to sell to customers in a show room.
This change in nomenclature that has permeated health care has had significant effects on how medical care is provided. Hospital care has been depersonalized in order to expedite hospital stays and maximize reimbursement. Gone is the hospital visit of your doctors when you need them the most.
Part of it is the complexity of contemporary care that requires the input from varying levels of expertise. Patients are often shuttled from one doctor to another. Communication is carried out through the web and rarely doctor to doctor. Often doctors are dealt with both at the patient level and the administrative level as commodities off the shelf, like buying a pair of shoes. And doctors in the hospital and in the clinic can be replaced by another one as the shift changes or the schedule dictates with little regard to the patient’s – or customer’s – choice.
Can we return to the days of yore? Probably not. All we can do now is try to inject some level of humanity and empathy as we see our patients in today’s world of mechanized medicine.
Dr. Goldstein, medical editor of Cardiology News, is a professor of medicine at Wayne State University and the division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies.
I am not sure when it occurred. I don’t know how it happened. I don’t think anyone took a vote on it. It happened gradually over the last decade. I think it happened when the administrative staff became larger than the medical staff. In order to include everyone under the same umbrella, everyone became a provider.
All those patients in our waiting room suddenly became consumers or clients. My grandfather ran a grocery store and had a lot of customers, and my father was a lawyer and had a lot of clients. None of those customers or clients would come to see me as their doctor with their illnesses today if I were a provider. The use of the terminology of “providers” and “customers” lowers all health care staff to the lowest common denominator and demeans the concerns of my patients.
I do not mean to diminish the role of the auto mechanic and salesperson, but they know and I know that our roles and are different and we are not just providers of a medical commodity. They do not expect me to deal with them as though they were coming to buy a car. They understand that we are actually trying to cure and treat worried patients and not to sell to customers in a show room.
This change in nomenclature that has permeated health care has had significant effects on how medical care is provided. Hospital care has been depersonalized in order to expedite hospital stays and maximize reimbursement. Gone is the hospital visit of your doctors when you need them the most.
Part of it is the complexity of contemporary care that requires the input from varying levels of expertise. Patients are often shuttled from one doctor to another. Communication is carried out through the web and rarely doctor to doctor. Often doctors are dealt with both at the patient level and the administrative level as commodities off the shelf, like buying a pair of shoes. And doctors in the hospital and in the clinic can be replaced by another one as the shift changes or the schedule dictates with little regard to the patient’s – or customer’s – choice.
Can we return to the days of yore? Probably not. All we can do now is try to inject some level of humanity and empathy as we see our patients in today’s world of mechanized medicine.
Dr. Goldstein, medical editor of Cardiology News, is a professor of medicine at Wayne State University and the division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies.
I am not sure when it occurred. I don’t know how it happened. I don’t think anyone took a vote on it. It happened gradually over the last decade. I think it happened when the administrative staff became larger than the medical staff. In order to include everyone under the same umbrella, everyone became a provider.
All those patients in our waiting room suddenly became consumers or clients. My grandfather ran a grocery store and had a lot of customers, and my father was a lawyer and had a lot of clients. None of those customers or clients would come to see me as their doctor with their illnesses today if I were a provider. The use of the terminology of “providers” and “customers” lowers all health care staff to the lowest common denominator and demeans the concerns of my patients.
I do not mean to diminish the role of the auto mechanic and salesperson, but they know and I know that our roles and are different and we are not just providers of a medical commodity. They do not expect me to deal with them as though they were coming to buy a car. They understand that we are actually trying to cure and treat worried patients and not to sell to customers in a show room.
This change in nomenclature that has permeated health care has had significant effects on how medical care is provided. Hospital care has been depersonalized in order to expedite hospital stays and maximize reimbursement. Gone is the hospital visit of your doctors when you need them the most.
Part of it is the complexity of contemporary care that requires the input from varying levels of expertise. Patients are often shuttled from one doctor to another. Communication is carried out through the web and rarely doctor to doctor. Often doctors are dealt with both at the patient level and the administrative level as commodities off the shelf, like buying a pair of shoes. And doctors in the hospital and in the clinic can be replaced by another one as the shift changes or the schedule dictates with little regard to the patient’s – or customer’s – choice.
Can we return to the days of yore? Probably not. All we can do now is try to inject some level of humanity and empathy as we see our patients in today’s world of mechanized medicine.
Dr. Goldstein, medical editor of Cardiology News, is a professor of medicine at Wayne State University and the division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies.
Link between alcohol consumption, neuroinflammation has possible treatment implications
NEW YORK – Recent discoveries regarding the relationship between alcohol consumption and neuroinflammation suggest a possible role for adjunctive treatments and supplements in addiction treatment, according to Shram Shukla, MD.
For example, a qualitative review of the literature over the past 2-3 years showed that the “neuroinflammatory process in and of itself drives epigenetic changes, which ultimately upregulate neuroinflammation of the brain,” according to Dr. Shukla of Walter Reed National Military Medical Center, Bethesda, Md., who reported the findings in a poster at the annual meeting of the American Psychiatric Association.
In this video interview, he explained that this finding is important because “neuroinflammation leads to neurotoxicity, which leads to neuronal degeneration.”
“As we know, with patients who chronically abuse alcohol, they do have a level of cortical degeneration that we often see on imaging, so there is, perhaps, a role that this may play in that,” he added.
Dr. Shukla said he also found that the neuroinflammatory process, when it drives the epigenetic changes, affects the amygdala, which is known as a “high stress part of the brain.”
“What we found in animal models so far is that if we can impact where that epigenetic change occurs, we can prevent the anxiogenic behaviors we often see in alcohol withdrawal and abstinence; we associate that, in humans, to be the high-stress state we often see in patients when they ... are withdrawing from alcohol.”
This raised questions about whether certain medications and supplements, including vitamin C, pioglitazone, infliximab, and omega-3 fatty acids, could be of benefit, and it was shown that these do affect neuroinflammation and stop neurotoxicity from occurring – and also, in turn, prevent the epigenetic changes, he said.
The take-away is that
“Now we have something, potentially ... that can do some of the ancillary stuff, working on the withdrawal effects, helping with the behavioral response that we see in patients that are suffering from addiction,” he said.
Dr. Shukla reported having no disclosures.
SOURCE: Shukla S et al. APA Poster Session 4, Poster 3.
NEW YORK – Recent discoveries regarding the relationship between alcohol consumption and neuroinflammation suggest a possible role for adjunctive treatments and supplements in addiction treatment, according to Shram Shukla, MD.
For example, a qualitative review of the literature over the past 2-3 years showed that the “neuroinflammatory process in and of itself drives epigenetic changes, which ultimately upregulate neuroinflammation of the brain,” according to Dr. Shukla of Walter Reed National Military Medical Center, Bethesda, Md., who reported the findings in a poster at the annual meeting of the American Psychiatric Association.
In this video interview, he explained that this finding is important because “neuroinflammation leads to neurotoxicity, which leads to neuronal degeneration.”
“As we know, with patients who chronically abuse alcohol, they do have a level of cortical degeneration that we often see on imaging, so there is, perhaps, a role that this may play in that,” he added.
Dr. Shukla said he also found that the neuroinflammatory process, when it drives the epigenetic changes, affects the amygdala, which is known as a “high stress part of the brain.”
“What we found in animal models so far is that if we can impact where that epigenetic change occurs, we can prevent the anxiogenic behaviors we often see in alcohol withdrawal and abstinence; we associate that, in humans, to be the high-stress state we often see in patients when they ... are withdrawing from alcohol.”
This raised questions about whether certain medications and supplements, including vitamin C, pioglitazone, infliximab, and omega-3 fatty acids, could be of benefit, and it was shown that these do affect neuroinflammation and stop neurotoxicity from occurring – and also, in turn, prevent the epigenetic changes, he said.
The take-away is that
“Now we have something, potentially ... that can do some of the ancillary stuff, working on the withdrawal effects, helping with the behavioral response that we see in patients that are suffering from addiction,” he said.
Dr. Shukla reported having no disclosures.
SOURCE: Shukla S et al. APA Poster Session 4, Poster 3.
NEW YORK – Recent discoveries regarding the relationship between alcohol consumption and neuroinflammation suggest a possible role for adjunctive treatments and supplements in addiction treatment, according to Shram Shukla, MD.
For example, a qualitative review of the literature over the past 2-3 years showed that the “neuroinflammatory process in and of itself drives epigenetic changes, which ultimately upregulate neuroinflammation of the brain,” according to Dr. Shukla of Walter Reed National Military Medical Center, Bethesda, Md., who reported the findings in a poster at the annual meeting of the American Psychiatric Association.
In this video interview, he explained that this finding is important because “neuroinflammation leads to neurotoxicity, which leads to neuronal degeneration.”
“As we know, with patients who chronically abuse alcohol, they do have a level of cortical degeneration that we often see on imaging, so there is, perhaps, a role that this may play in that,” he added.
Dr. Shukla said he also found that the neuroinflammatory process, when it drives the epigenetic changes, affects the amygdala, which is known as a “high stress part of the brain.”
“What we found in animal models so far is that if we can impact where that epigenetic change occurs, we can prevent the anxiogenic behaviors we often see in alcohol withdrawal and abstinence; we associate that, in humans, to be the high-stress state we often see in patients when they ... are withdrawing from alcohol.”
This raised questions about whether certain medications and supplements, including vitamin C, pioglitazone, infliximab, and omega-3 fatty acids, could be of benefit, and it was shown that these do affect neuroinflammation and stop neurotoxicity from occurring – and also, in turn, prevent the epigenetic changes, he said.
The take-away is that
“Now we have something, potentially ... that can do some of the ancillary stuff, working on the withdrawal effects, helping with the behavioral response that we see in patients that are suffering from addiction,” he said.
Dr. Shukla reported having no disclosures.
SOURCE: Shukla S et al. APA Poster Session 4, Poster 3.
REPORTING FROM APA
New ‘immune checkpoint’ vaccine shows promise in treating colorectal cancer
A novel vaccine may provide a breakthrough treatment option in colorectal and other cancers.
“We [researchers] at the Peter MacCallum Cancer Centre in Melbourne, Australia, have developed a new, DNA-based vaccine, which we call TetMYB, for the treatment of MYB-overexpressing cancers such as colorectal, adenoid cystic carcinoma, and breast cancer, to name a few,” Toan Pham, MD, of the Peter MacCallum Cancer Centre, said in a media briefing in advance of the annual Digestive Disease Week® conference.
The immunotherapy approach described by Dr. Pham involves combining the TetMYB vaccine, which boosts the immune system, and an antibody, BGB-A317, that helps enhance the effectiveness of the vaccine.
“The research we are presenting at DDW involves testing this approach in mouse models, which we had previously published. In our studies, tumors in the mice responded very well to the treatment and cancer was cured in about half of them,” stated Dr. Pham.
The study is really composed of two smaller studies that looked at colonic adenoma, induced using tamoxifen-enriched feed, in a total of 22 mice. The mice were split into two study groups: 15 in a prophylactic study and 7 in a therapeutic pilot study.
In the prophylactic study, eight treatment mice received three TetMYB vaccinations at weeks 7, 9, and 11. Tamoxifen was given to the treatment and control mice at week 13.
The seven mice in the therapeutic pilot study were given tamoxifen at week 9 and subsequently monitored via colonoscopy weekly. Once the presence of adenoma was identified, mice received six doses of TetMYB and four doses of anti-PD1 antibody.
In both the prophylactic and therapeutic study, the mice survival rates were higher than expected. In the prophylactic study, the treatment groups’ median survival time was 356 days, nearly double the control group (183 days). More impressively, all mice in the therapeutic group were alive at 235 days.
“The mice were only expected to live for a couple of days or weeks. But, about 50% of them, they lived for more than 2 years,” said Dr. Pham. “Additionally, when the cured mice from the original study were later rechallenged with the same treatment, it was immediately rejected, thus proving there is an immune memory induced by the vaccine.”
With the positive results of the mouse trial, Dr. Pham spoke to the ongoing human clinical trial and the future of this vaccine.
“Currently, we are conducting a first-in-human phase 1 clinical trial,” stated Dr. Pham. “The next phase of evolution for our vaccine, which I will also be presenting at DDW, is testing our vaccine as an anti-adenoma vaccine.” Adenomas account for nearly 80% of bowel cancers, according to Dr. Pham.
Should the safety data from the phase 1 trial prove the vaccine to be safe, a follow-up clinical trial looking at high-risk populations would be the next step.
A novel vaccine may provide a breakthrough treatment option in colorectal and other cancers.
“We [researchers] at the Peter MacCallum Cancer Centre in Melbourne, Australia, have developed a new, DNA-based vaccine, which we call TetMYB, for the treatment of MYB-overexpressing cancers such as colorectal, adenoid cystic carcinoma, and breast cancer, to name a few,” Toan Pham, MD, of the Peter MacCallum Cancer Centre, said in a media briefing in advance of the annual Digestive Disease Week® conference.
The immunotherapy approach described by Dr. Pham involves combining the TetMYB vaccine, which boosts the immune system, and an antibody, BGB-A317, that helps enhance the effectiveness of the vaccine.
“The research we are presenting at DDW involves testing this approach in mouse models, which we had previously published. In our studies, tumors in the mice responded very well to the treatment and cancer was cured in about half of them,” stated Dr. Pham.
The study is really composed of two smaller studies that looked at colonic adenoma, induced using tamoxifen-enriched feed, in a total of 22 mice. The mice were split into two study groups: 15 in a prophylactic study and 7 in a therapeutic pilot study.
In the prophylactic study, eight treatment mice received three TetMYB vaccinations at weeks 7, 9, and 11. Tamoxifen was given to the treatment and control mice at week 13.
The seven mice in the therapeutic pilot study were given tamoxifen at week 9 and subsequently monitored via colonoscopy weekly. Once the presence of adenoma was identified, mice received six doses of TetMYB and four doses of anti-PD1 antibody.
In both the prophylactic and therapeutic study, the mice survival rates were higher than expected. In the prophylactic study, the treatment groups’ median survival time was 356 days, nearly double the control group (183 days). More impressively, all mice in the therapeutic group were alive at 235 days.
“The mice were only expected to live for a couple of days or weeks. But, about 50% of them, they lived for more than 2 years,” said Dr. Pham. “Additionally, when the cured mice from the original study were later rechallenged with the same treatment, it was immediately rejected, thus proving there is an immune memory induced by the vaccine.”
With the positive results of the mouse trial, Dr. Pham spoke to the ongoing human clinical trial and the future of this vaccine.
“Currently, we are conducting a first-in-human phase 1 clinical trial,” stated Dr. Pham. “The next phase of evolution for our vaccine, which I will also be presenting at DDW, is testing our vaccine as an anti-adenoma vaccine.” Adenomas account for nearly 80% of bowel cancers, according to Dr. Pham.
Should the safety data from the phase 1 trial prove the vaccine to be safe, a follow-up clinical trial looking at high-risk populations would be the next step.
A novel vaccine may provide a breakthrough treatment option in colorectal and other cancers.
“We [researchers] at the Peter MacCallum Cancer Centre in Melbourne, Australia, have developed a new, DNA-based vaccine, which we call TetMYB, for the treatment of MYB-overexpressing cancers such as colorectal, adenoid cystic carcinoma, and breast cancer, to name a few,” Toan Pham, MD, of the Peter MacCallum Cancer Centre, said in a media briefing in advance of the annual Digestive Disease Week® conference.
The immunotherapy approach described by Dr. Pham involves combining the TetMYB vaccine, which boosts the immune system, and an antibody, BGB-A317, that helps enhance the effectiveness of the vaccine.
“The research we are presenting at DDW involves testing this approach in mouse models, which we had previously published. In our studies, tumors in the mice responded very well to the treatment and cancer was cured in about half of them,” stated Dr. Pham.
The study is really composed of two smaller studies that looked at colonic adenoma, induced using tamoxifen-enriched feed, in a total of 22 mice. The mice were split into two study groups: 15 in a prophylactic study and 7 in a therapeutic pilot study.
In the prophylactic study, eight treatment mice received three TetMYB vaccinations at weeks 7, 9, and 11. Tamoxifen was given to the treatment and control mice at week 13.
The seven mice in the therapeutic pilot study were given tamoxifen at week 9 and subsequently monitored via colonoscopy weekly. Once the presence of adenoma was identified, mice received six doses of TetMYB and four doses of anti-PD1 antibody.
In both the prophylactic and therapeutic study, the mice survival rates were higher than expected. In the prophylactic study, the treatment groups’ median survival time was 356 days, nearly double the control group (183 days). More impressively, all mice in the therapeutic group were alive at 235 days.
“The mice were only expected to live for a couple of days or weeks. But, about 50% of them, they lived for more than 2 years,” said Dr. Pham. “Additionally, when the cured mice from the original study were later rechallenged with the same treatment, it was immediately rejected, thus proving there is an immune memory induced by the vaccine.”
With the positive results of the mouse trial, Dr. Pham spoke to the ongoing human clinical trial and the future of this vaccine.
“Currently, we are conducting a first-in-human phase 1 clinical trial,” stated Dr. Pham. “The next phase of evolution for our vaccine, which I will also be presenting at DDW, is testing our vaccine as an anti-adenoma vaccine.” Adenomas account for nearly 80% of bowel cancers, according to Dr. Pham.
Should the safety data from the phase 1 trial prove the vaccine to be safe, a follow-up clinical trial looking at high-risk populations would be the next step.
REPORTING FROM DDW