User login
Was this your first one? Lessons in empathy and mourning
“Was this your first one?”
You might be asked this question in several circumstances. Was this your first 5K run? Was this your first time taking the MCAT? For me, I was asked if this was the first time a patient had died while in my care as a resident.
As it turns out, this was the first patient who died under my care. This seemed obvious to everyone around me because when I received the news, offhandedly, days after my patient’s discharge, I cried. My colleagues’ responses to my tears were kind and supportive. No one was callous or judgmental. I was given time to compose myself before continuing my rounds for the day. Yet, the most common question asked of me was, “Was this your first one?”
The implication of this question was that these situations would become easier and less emotional over time. Everyone believed my tears were a special response, privileged only to my first experience. This was conveyed to me as if it was a chance to explain my emotions. As if grieving alone was not sufficient to explain tears, I began to run through the reasons for my behavior, and my mind rapidly searched for answers. I thought:
- “I saw this patient daily for weeks, and I was close to him.”
- “There was an element of suicidality in this case, so it was different.”
- “After all, this was my first experience like this.”
In reality, these reasons were irrelevant and not needed to explain my tears. I was mourning the loss of a life—someone I had come to know well—and this was a life that ultimately could not be saved by the health care system.
These were my feelings during the July of my intern year. It is now a year later; I have since experienced an incredible number of moments that warranted mourning. There were oncology patients with advanced diseases who needed help disclosing their prognosis to family members. There were days when I was tasked with altering treatment courses from aggressive treatment to comfort measures only. There was the Christmas Eve when a pair of brothers dropped off their elderly father in the emergency department because no one was able or willing to care for him at home.
These moments can come fast, and they occur more frequently than one might imagine. Each is worthy of mourning. Each is worthy of tears, whether it is my first loss or my 50th. But the reality is that I could not function in my job and care for all my patients equally if I stopped every time to acknowledge my emotions. As much as it causes my stomach to turn, I understand the “Was this your first one?” phenomenon. To outwardly express your emotions and openly mourn in the moment, you need to have the time and allow yourself the vulnerability to do so. After your “first one,” you realize that mournful moments can occur regularly and you must choose to process emotions on your own time because you don’t have the luxury of processing a loss of life in the present moment.
Continue to: This is not to diminish...
This is not to diminish the importance of mourning or belittle the experience of processing one’s emotions. It simply highlights the importance of self-care. Emotions need to be processed outside of the hustle and bustle of the work day. There are other patients who require and deserve their clinician’s undivided attention. To truly provide patients with the highest quality of care, I find caring for myself and processing my reactions to daily events to be essential. Writing down my thoughts or talking to loved ones are ways that help me process my experiences. Taking a trip out of town, going for a hike, or watching a movie also are ways to help create balance in an emotionally charged career. I hope to use my new understanding of the “first one” phenomenon as a reminder to maintain my own well-being. In doing so, I can be attuned to my patients’ needs and provide them with empathic care. Even though my outward expression may change as I learn to process these moments differently, I want to treat each patient with the same level of empathy, value, and compassion as my first one.
“Was this your first one?”
You might be asked this question in several circumstances. Was this your first 5K run? Was this your first time taking the MCAT? For me, I was asked if this was the first time a patient had died while in my care as a resident.
As it turns out, this was the first patient who died under my care. This seemed obvious to everyone around me because when I received the news, offhandedly, days after my patient’s discharge, I cried. My colleagues’ responses to my tears were kind and supportive. No one was callous or judgmental. I was given time to compose myself before continuing my rounds for the day. Yet, the most common question asked of me was, “Was this your first one?”
The implication of this question was that these situations would become easier and less emotional over time. Everyone believed my tears were a special response, privileged only to my first experience. This was conveyed to me as if it was a chance to explain my emotions. As if grieving alone was not sufficient to explain tears, I began to run through the reasons for my behavior, and my mind rapidly searched for answers. I thought:
- “I saw this patient daily for weeks, and I was close to him.”
- “There was an element of suicidality in this case, so it was different.”
- “After all, this was my first experience like this.”
In reality, these reasons were irrelevant and not needed to explain my tears. I was mourning the loss of a life—someone I had come to know well—and this was a life that ultimately could not be saved by the health care system.
These were my feelings during the July of my intern year. It is now a year later; I have since experienced an incredible number of moments that warranted mourning. There were oncology patients with advanced diseases who needed help disclosing their prognosis to family members. There were days when I was tasked with altering treatment courses from aggressive treatment to comfort measures only. There was the Christmas Eve when a pair of brothers dropped off their elderly father in the emergency department because no one was able or willing to care for him at home.
These moments can come fast, and they occur more frequently than one might imagine. Each is worthy of mourning. Each is worthy of tears, whether it is my first loss or my 50th. But the reality is that I could not function in my job and care for all my patients equally if I stopped every time to acknowledge my emotions. As much as it causes my stomach to turn, I understand the “Was this your first one?” phenomenon. To outwardly express your emotions and openly mourn in the moment, you need to have the time and allow yourself the vulnerability to do so. After your “first one,” you realize that mournful moments can occur regularly and you must choose to process emotions on your own time because you don’t have the luxury of processing a loss of life in the present moment.
Continue to: This is not to diminish...
This is not to diminish the importance of mourning or belittle the experience of processing one’s emotions. It simply highlights the importance of self-care. Emotions need to be processed outside of the hustle and bustle of the work day. There are other patients who require and deserve their clinician’s undivided attention. To truly provide patients with the highest quality of care, I find caring for myself and processing my reactions to daily events to be essential. Writing down my thoughts or talking to loved ones are ways that help me process my experiences. Taking a trip out of town, going for a hike, or watching a movie also are ways to help create balance in an emotionally charged career. I hope to use my new understanding of the “first one” phenomenon as a reminder to maintain my own well-being. In doing so, I can be attuned to my patients’ needs and provide them with empathic care. Even though my outward expression may change as I learn to process these moments differently, I want to treat each patient with the same level of empathy, value, and compassion as my first one.
“Was this your first one?”
You might be asked this question in several circumstances. Was this your first 5K run? Was this your first time taking the MCAT? For me, I was asked if this was the first time a patient had died while in my care as a resident.
As it turns out, this was the first patient who died under my care. This seemed obvious to everyone around me because when I received the news, offhandedly, days after my patient’s discharge, I cried. My colleagues’ responses to my tears were kind and supportive. No one was callous or judgmental. I was given time to compose myself before continuing my rounds for the day. Yet, the most common question asked of me was, “Was this your first one?”
The implication of this question was that these situations would become easier and less emotional over time. Everyone believed my tears were a special response, privileged only to my first experience. This was conveyed to me as if it was a chance to explain my emotions. As if grieving alone was not sufficient to explain tears, I began to run through the reasons for my behavior, and my mind rapidly searched for answers. I thought:
- “I saw this patient daily for weeks, and I was close to him.”
- “There was an element of suicidality in this case, so it was different.”
- “After all, this was my first experience like this.”
In reality, these reasons were irrelevant and not needed to explain my tears. I was mourning the loss of a life—someone I had come to know well—and this was a life that ultimately could not be saved by the health care system.
These were my feelings during the July of my intern year. It is now a year later; I have since experienced an incredible number of moments that warranted mourning. There were oncology patients with advanced diseases who needed help disclosing their prognosis to family members. There were days when I was tasked with altering treatment courses from aggressive treatment to comfort measures only. There was the Christmas Eve when a pair of brothers dropped off their elderly father in the emergency department because no one was able or willing to care for him at home.
These moments can come fast, and they occur more frequently than one might imagine. Each is worthy of mourning. Each is worthy of tears, whether it is my first loss or my 50th. But the reality is that I could not function in my job and care for all my patients equally if I stopped every time to acknowledge my emotions. As much as it causes my stomach to turn, I understand the “Was this your first one?” phenomenon. To outwardly express your emotions and openly mourn in the moment, you need to have the time and allow yourself the vulnerability to do so. After your “first one,” you realize that mournful moments can occur regularly and you must choose to process emotions on your own time because you don’t have the luxury of processing a loss of life in the present moment.
Continue to: This is not to diminish...
This is not to diminish the importance of mourning or belittle the experience of processing one’s emotions. It simply highlights the importance of self-care. Emotions need to be processed outside of the hustle and bustle of the work day. There are other patients who require and deserve their clinician’s undivided attention. To truly provide patients with the highest quality of care, I find caring for myself and processing my reactions to daily events to be essential. Writing down my thoughts or talking to loved ones are ways that help me process my experiences. Taking a trip out of town, going for a hike, or watching a movie also are ways to help create balance in an emotionally charged career. I hope to use my new understanding of the “first one” phenomenon as a reminder to maintain my own well-being. In doing so, I can be attuned to my patients’ needs and provide them with empathic care. Even though my outward expression may change as I learn to process these moments differently, I want to treat each patient with the same level of empathy, value, and compassion as my first one.
Yoga’s lesson for a young psychiatrist
I have often turned to yoga for my own reprieve; I find the heat, breath, and movement exhilarating. Training to become a yoga teacher has taught me that medicine, not unlike yoga, requires patience and resiliency.
It is 3
4
Let me tell you, when you’re getting certified in Advanced Cardiac Life Support, you go to a class on a Saturday, there are snacks, and your instructor will probably be a paramedic who is earning side cash teaching CPR. You will watch funny videos—we even danced to the Bee Gees’ Stayin’ Alive. But this is not funny, nor is it fun. And my head is spinning so fast, the sound of the Bee Gees smears to silence.
I take off my white coat and trot to the center of the room. The lights are bright, I am hot, and people are moving really fast. I feel like I’m in a vignette. It seems like we’re in the fourth movement of a Shostakovich symphony, the attending is cueing, up and down, like Bernstein conducting the Philharmonic, her arms are flailing; this production is hers and everyone is in sync. The man’s skin is pale, almost gray, he smells like sweat and urine and vomit. His irises are blue, blue like the ocean. His beard is thick and opaque, speckled with premature dots of gray. He looks calm. Listless. Dead. He is looking at me, like the Mona Lisa, as if beckoning me to save him, to give him another chance. At life. I put my hands gently on his sternum and I start my round of chest compressions. His skin is rubbery. I feel like I’m breaking his ribs, am I? This is not like the class. The cardiac monitor flatlines. Was it like that before? I think so, I’m not really sure. The attending stops conducting and runs over. Someone taps me and says it’s time to switch. Shortly thereafter, time of death is pronounced. “Damn it,” I hear the attending exclaim quietly but deliberately. I am hot, and I have a headache. I take off my gloves. Where’s my cell phone? I’m going to check Facebook, maybe ESPN.com. I feel heavy. And then I’m sitting at the computer screen again, after the rain. The attending comes back to her seat. She has a green smoothie, she takes a sip, and is slow to return the oversized cup to the table.
This night 3 years ago remains vivid. I am looking at her now. The unabashed attending. We are all looking at her.
She pulls out a petite makeup case and opens an oval mirror. She applies 2 thin lines of lustrous lip gloss, smacks her lips, grounding herself, then places the mirror back in her bag. She takes one deep breath, pauses briefly, and, letting go, she sits up tall, her dignity restored, then looks at me and claps, “Come on, doctor, we’ve got more patients to see.”
That night in the ER, I experienced how troubling it is losing a life with the burden of responsibility, but also the beauty of Aparigraha, letting go, and moving forward. I learned this lesson, unspoken, from an admirable attending, and was reminded of it 3 years later as I pursued a deeper understanding of yoga.
I have often turned to yoga for my own reprieve; I find the heat, breath, and movement exhilarating. Training to become a yoga teacher has taught me that medicine, not unlike yoga, requires patience and resiliency.
It is 3
4
Let me tell you, when you’re getting certified in Advanced Cardiac Life Support, you go to a class on a Saturday, there are snacks, and your instructor will probably be a paramedic who is earning side cash teaching CPR. You will watch funny videos—we even danced to the Bee Gees’ Stayin’ Alive. But this is not funny, nor is it fun. And my head is spinning so fast, the sound of the Bee Gees smears to silence.
I take off my white coat and trot to the center of the room. The lights are bright, I am hot, and people are moving really fast. I feel like I’m in a vignette. It seems like we’re in the fourth movement of a Shostakovich symphony, the attending is cueing, up and down, like Bernstein conducting the Philharmonic, her arms are flailing; this production is hers and everyone is in sync. The man’s skin is pale, almost gray, he smells like sweat and urine and vomit. His irises are blue, blue like the ocean. His beard is thick and opaque, speckled with premature dots of gray. He looks calm. Listless. Dead. He is looking at me, like the Mona Lisa, as if beckoning me to save him, to give him another chance. At life. I put my hands gently on his sternum and I start my round of chest compressions. His skin is rubbery. I feel like I’m breaking his ribs, am I? This is not like the class. The cardiac monitor flatlines. Was it like that before? I think so, I’m not really sure. The attending stops conducting and runs over. Someone taps me and says it’s time to switch. Shortly thereafter, time of death is pronounced. “Damn it,” I hear the attending exclaim quietly but deliberately. I am hot, and I have a headache. I take off my gloves. Where’s my cell phone? I’m going to check Facebook, maybe ESPN.com. I feel heavy. And then I’m sitting at the computer screen again, after the rain. The attending comes back to her seat. She has a green smoothie, she takes a sip, and is slow to return the oversized cup to the table.
This night 3 years ago remains vivid. I am looking at her now. The unabashed attending. We are all looking at her.
She pulls out a petite makeup case and opens an oval mirror. She applies 2 thin lines of lustrous lip gloss, smacks her lips, grounding herself, then places the mirror back in her bag. She takes one deep breath, pauses briefly, and, letting go, she sits up tall, her dignity restored, then looks at me and claps, “Come on, doctor, we’ve got more patients to see.”
That night in the ER, I experienced how troubling it is losing a life with the burden of responsibility, but also the beauty of Aparigraha, letting go, and moving forward. I learned this lesson, unspoken, from an admirable attending, and was reminded of it 3 years later as I pursued a deeper understanding of yoga.
I have often turned to yoga for my own reprieve; I find the heat, breath, and movement exhilarating. Training to become a yoga teacher has taught me that medicine, not unlike yoga, requires patience and resiliency.
It is 3
4
Let me tell you, when you’re getting certified in Advanced Cardiac Life Support, you go to a class on a Saturday, there are snacks, and your instructor will probably be a paramedic who is earning side cash teaching CPR. You will watch funny videos—we even danced to the Bee Gees’ Stayin’ Alive. But this is not funny, nor is it fun. And my head is spinning so fast, the sound of the Bee Gees smears to silence.
I take off my white coat and trot to the center of the room. The lights are bright, I am hot, and people are moving really fast. I feel like I’m in a vignette. It seems like we’re in the fourth movement of a Shostakovich symphony, the attending is cueing, up and down, like Bernstein conducting the Philharmonic, her arms are flailing; this production is hers and everyone is in sync. The man’s skin is pale, almost gray, he smells like sweat and urine and vomit. His irises are blue, blue like the ocean. His beard is thick and opaque, speckled with premature dots of gray. He looks calm. Listless. Dead. He is looking at me, like the Mona Lisa, as if beckoning me to save him, to give him another chance. At life. I put my hands gently on his sternum and I start my round of chest compressions. His skin is rubbery. I feel like I’m breaking his ribs, am I? This is not like the class. The cardiac monitor flatlines. Was it like that before? I think so, I’m not really sure. The attending stops conducting and runs over. Someone taps me and says it’s time to switch. Shortly thereafter, time of death is pronounced. “Damn it,” I hear the attending exclaim quietly but deliberately. I am hot, and I have a headache. I take off my gloves. Where’s my cell phone? I’m going to check Facebook, maybe ESPN.com. I feel heavy. And then I’m sitting at the computer screen again, after the rain. The attending comes back to her seat. She has a green smoothie, she takes a sip, and is slow to return the oversized cup to the table.
This night 3 years ago remains vivid. I am looking at her now. The unabashed attending. We are all looking at her.
She pulls out a petite makeup case and opens an oval mirror. She applies 2 thin lines of lustrous lip gloss, smacks her lips, grounding herself, then places the mirror back in her bag. She takes one deep breath, pauses briefly, and, letting go, she sits up tall, her dignity restored, then looks at me and claps, “Come on, doctor, we’ve got more patients to see.”
That night in the ER, I experienced how troubling it is losing a life with the burden of responsibility, but also the beauty of Aparigraha, letting go, and moving forward. I learned this lesson, unspoken, from an admirable attending, and was reminded of it 3 years later as I pursued a deeper understanding of yoga.
Making sense of CYP2D6 and CYP1A2 genotype vs phenotype
The clinical response to the same dose of a drug may vary among individuals. Cytochrome P450 (CYP) 2D6 and 1A2 are enzymes that metabolize many psychotropic medications. Genetic variations in these enzymes may cause changes in their activity and result in differences in effectiveness and adverse effects. Although pharmacogenetic testing is available for CYP2D6 and CYP1A2, interpretation and clinical application of the results may not be straightforward.
Genetic variations in CYP450 enzymes determine enzymatic activity, which can have a large effect on drug levels, efficacy, and toxicity. However, there are many other important factors that clinicians should consider when trying to predict the effects of medications. While clinicians often focus on a patient’s genotype, this only provides information on a chromosomal level, and this information never changes. In contrast, a patient’s phenotype, or status of metabolism, is subject to change throughout the patient’s life.
Many circumstances influence phenotype, including the use of medications that induce or inhibit CYP450 enzymes, environmental factors, and comorbidities. Phenoconversion occurs when these factors result in a phenotype that is different from that predicted by genotype. Because of the possibility of phenoconversion, knowing a patient’s genotype may be of limited value in making clinical decisions. This article provides guidance on interpreting both the genotype and phenotype of CYP2D6 and CYP1A2. Case 1 and Case 2 illustrate these concepts.
CYP2D6
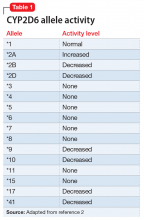
The enzyme activity of CYP2D6 varies among individuals and may include no activity, decreased activity, normal activity, or increased activity. After obtaining the genotype, the activity level of the CYP2D6 alleles may be determined. The frequency with which certain alleles occur varies with ancestry. More than 100 allelic variants and subvariants have been discovered, and new alleles are continuing to be discovered.1Table 12 lists some of the most common CYP2D6 alleles.
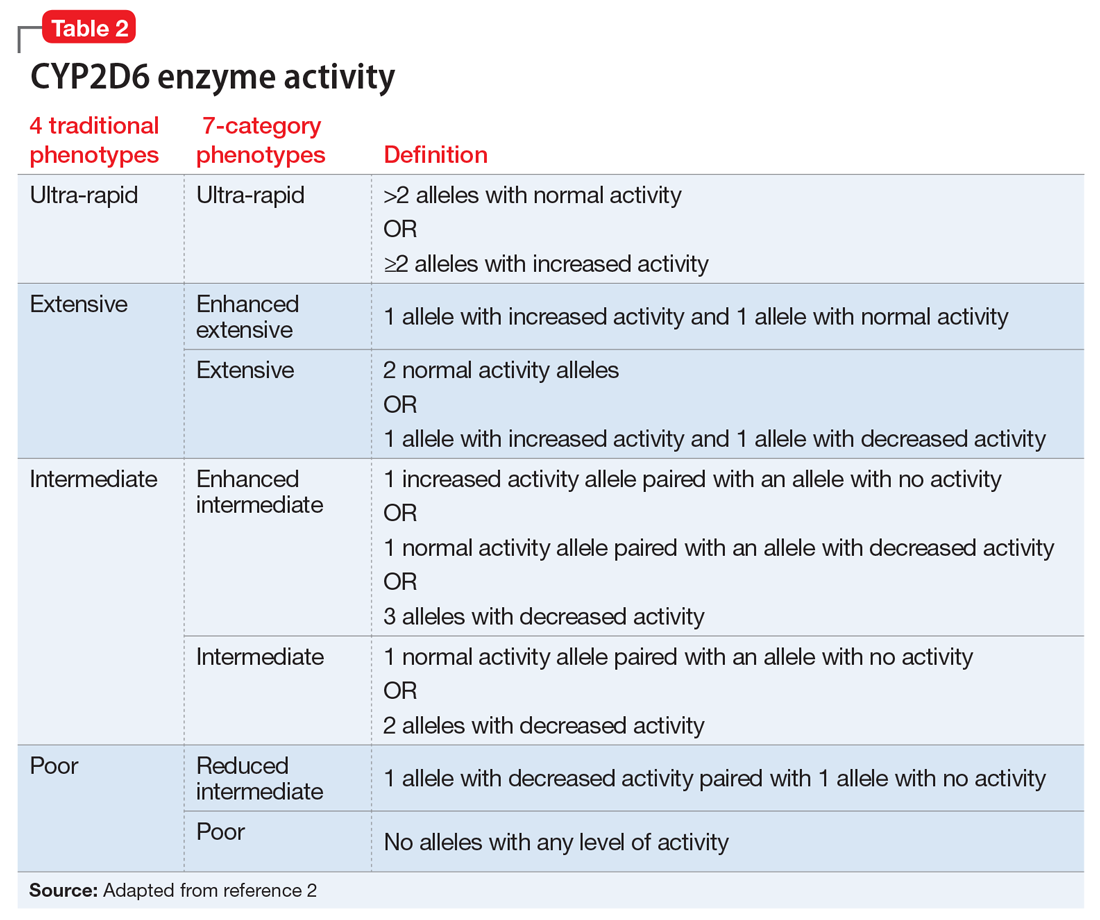
Based on the CYP2D6 enzyme activity determined from the alleles, 4 “traditional” phenotypes can be predicted from the genotype (Table 22). The 7-category phenotypes reported by some laboratory companies provide a more explicit method for reporting phenotypes.
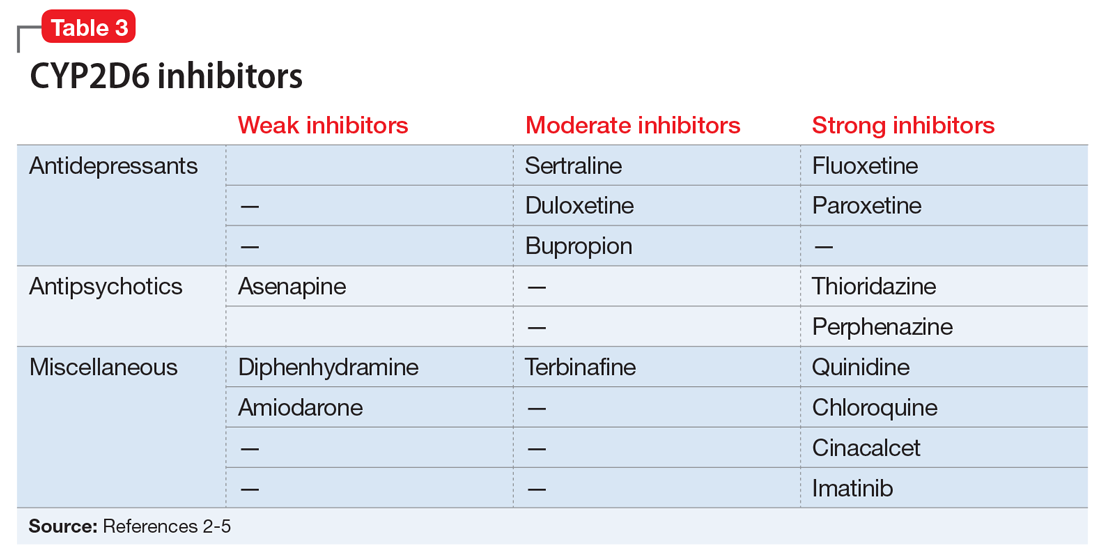
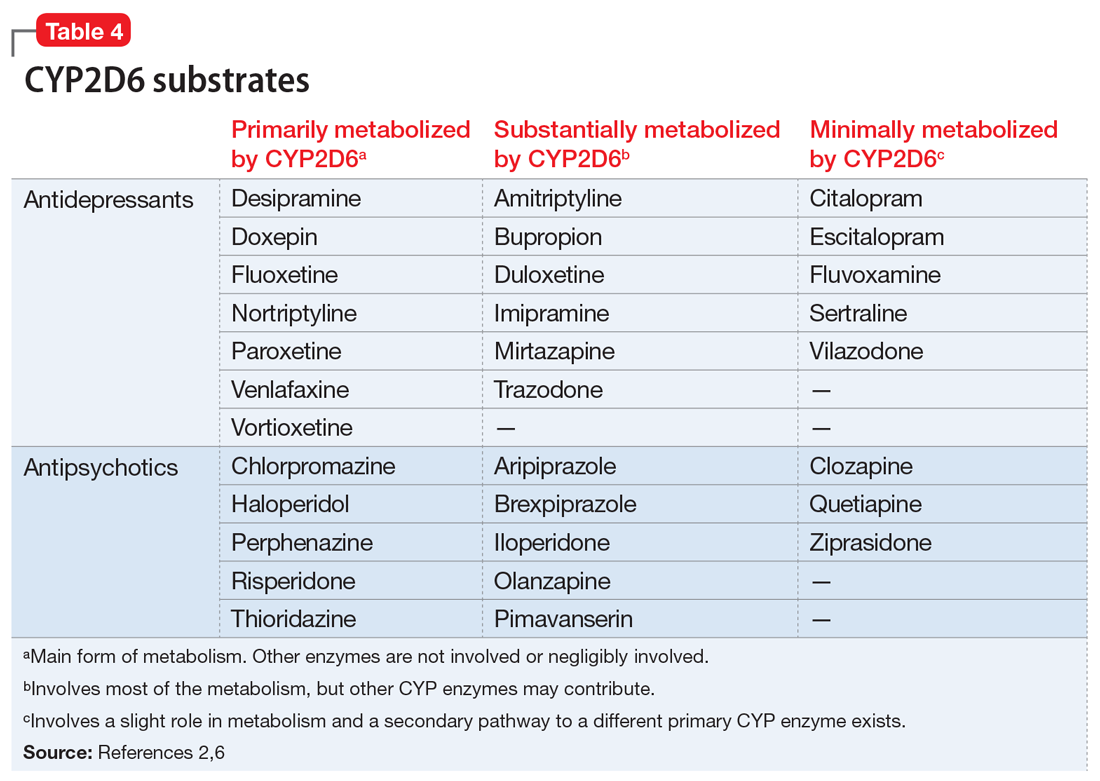
Evidence suggests that, unlike most other CYP450 enzymes, CYP2D6 is not very susceptible to enzyme induction.2 Thus, genetics, rather than drug therapy, accounts for most ultra-rapid CYP2D6 metabolizers. CYP2D6 can be inhibited by the use of medications (Table 32-5) and/or substrates (Table 42,6). Similar to inhibitors, substrates may be saturating high affinity-low capacity enzymes such as CYP2D6, resulting in phenoconversion to poor metabolizers. However, this is unlikely to be the case for substrates of low affinity-high capacity enzymes such as CYP3A4.7 Ultimately, substrates and/or inhibitors of CYP2D6 may result in a phenotype that does not correspond to genotype.
Phenoconversion
Genotyping may not reflect the true prevalence of the CYP2D6 poor metabolizer phenotype when using multiple medications that are substrates and/or inhibitors of CYP2D6.8 In the presence of strong CYP2D6 inhibitors, up to 80% of individuals with a non-poor metabolizer genotype are converted to a poor metabolizer phenotype.8 While the phenotype provides a clearer representation of metabolism status than genotype, this information may not always be available.
Continue to: Determining CYP2D6 phenotype
Determining CYP2D6 phenotype
Risperidone and venlafaxine levels are useful tools for predicting CYP2D6 phenotype.3,8 When a risperidone level is ordered, the results include a risperidone level and a 9-hydroxyrisperidone level. The active metabolite of risperidone is 9-hydroxyrisperidone (
- Ultra-rapid metabolizer: 0.03 (0.02 to 0.06)
- Extensive metabolizer: 0.08 (0.04 to 0.17)
- Intermediate metabolizer: 0.56 (0.30 to 1.0)
- Poor metabolizer: 2.5 (1.8 to 4.1).
Although a R-to-9-OHR concentration ratio >1 generally indicates a poor metabolizer, it could also indicate the presence of a powerful CYP2D6 inhibitor.9
When a venlafaxine level is ordered, the results include a venlafaxine level and an O-desmethylvenlafaxine level. O-desmethylvenlafaxine (
CYP1A2
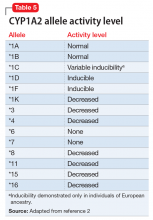
While the activity of CYP2D6 alleles is determined primarily by genetic factors and medications, the activity of CYP1A2 alleles is largely determined by environmental factors (diet, medications, disease) and genetic variability.2 Consequently, CYP1A2 genotyping may be less clinically useful than CYP2D6 genotyping. The CYP1A2 genotype–phenotype relationship incorporates the degree of allele activity (Table 52), and inducibility in the presence of environmental factors.
Continue to: CYP1A2 inhibiton
CYP1A2 inhibition
A variety of medications and environmental factors may inhibit CYP1A2.
Medications. Medications that may inhibit CYP1A2 include a
Caffeine. A significant increase in caffeine consumption can result in inhibition.3 Among non-tobacco smokers, an increase of 1 cup/d of coffee or 2 cans/d of caffeinated soda would be considered significant.3 However, tobacco smokers would require an increase of 3 cups/d of coffee or 6 cans/d of soda.
Diet. An increase in the daily dietary intake of certain vegetables for 6 days has been shown to result in inhibition.10 Apiaceous (Apiaceae or Umbelliferae) vegetables such as carrots (3/4 cup), celery (1/2 cup), dill (1 teaspoon), parsley (3 tablespoons), and parsnips (1¼ cup) can decrease CYP1A2 activity by approximately 13% to 25%. Allium (Liliaceae) vegetables, such as garlic, leeks, and onions, have no effect on CYP1A2 activity.
Infection. Pneumonia, upper respiratory infections with fever, pyelonephritis or appendicitis, or inflammation are suspected to decrease CYP1A2 activity.8
Continue to: CYP1A2 induction
CYP1A2 induction
A variety of medications and environmental factors may induce CYP1A2.
Medications. Certain medications may induce CYP1A2, including c
Cigarette smoking. A significant increase in smoking after 1 to 3 weeks may decrease drug levels, whereas a significant decrease in smoking after 1 to 3 weeks may result in elevated drug levels.3 Nicotine is not the causative agent of induction, but rather hydrocarbons found in cigarette smoke.11
Diet. An increase in daily dietary intake of certain vegetables for 6 days has been shown to result in induction.3 Brassica (Cruciferae) vegetables such as broccoli (2 cups), cauliflower (1 cup), cabbage (1 cup), and radish sprouts (1/2 cup) have been found to increase CYP1A2 activity by 18% to 37%.10 Grilled meat also plays a role in induction.10
Related Resource
- Ellingrod VL, Ward KM. Using pharmacogenetics guidelines when prescribing: What's available. Current Psychiatry. 2018;17(1):43-46.
Drug Brand Names
Amiodarone • Cordarone, Pacerone
Amitriptyline • Elavil, Endep
Aripiprazole • Abilify
Asenapine • Saphris
Atazanavir • Reyataz
Brexpiprazole • Rexulti
Bupropion • Wellbutrin, Zyban
Carbamazepine • Carbatrol, Tegretol
Chlorpromazine • Thorazine
Chloroquine • Aralen
Cinacalcet • Sensipar
Ciprofloxacin • Cipro
Citalopram • Celexa
Clozapine • Clozaril
Desipramine • Norpramin
Desvenlafaxine • Pristiq
Diphenhydramine • Benadryl
Doxepin • Silenor
Duloxetine • Cymbalta
Escitalopram • Lexapro
Ethinyl estradiol • Estinyl
Fluoxetine • Prozac
Fluvoxamine • Luvox
Haloperidol • Haldol
Iloperidone • Fanapt
Imatinib • Gleevec
Imipramine • Tofranil
Mirtazapine • Remeron
Nortriptyline • Pamelor
Olanzapine • Zyprexa
Paliperidone • Invega
Paroxetine • Paxil
Perphenazine • Trilafon
Phenytoin • Dilantin
Pimavanserin • Nuplazid
Primidone • Mysoline
Quetiapine • Seroquel
Quinidine • Cardioquin
Rifampin • Rifadin
Risperidone • Risperdal
Sertraline • Zoloft
Terbinafine • Lamisil
Thioridazine • Mellaril
Trazodone • Desyrel, Oleptro
Venlafaxine • Effexor
Vilazodone • Viibryd
Vortioxetine • Trintellix
Ziprasidone • Geodon
1. Pharmacogene Variation Consoritum. CYP2D6 allele nomenclature. https://www.pharmvar.org/gene/CYP2D6. Updated May 22, 2018. Accessed June 11, 2018.
2. Mrazek D. Psychiatric pharmacogenomics. New York, NY: Oxford University Press; 2010:33,42,44,45,85.
3. Spina E, de Leon J. Clinical applications of CYP genotyping in psychiatry. J Neural Transm (Vienna). 2015;122(1):5-28.
4. Adedoyin A, Frye RF, Mauro K, et al. Chloroquine modulation of specific metabolizing enzymes activities: investigation with selective five drug cocktail. Br J Clin Pharmacol. 1998;46(3):215-219.
5. Filppula AM, Laitila J, Neuvonen PJ, et al. Potent mechanism-based inhibition of CYP3A4 by imatinib explains its liability to interact with CYP3A4 substrates. Br J Pharmacol. 2012;165(8):2787-2798.
6. U.S. National Library of Medicine. DailyMed. http://dailymed.nlm.nih.gov/dailymed/about.cfm. Accessed April 26, 2018.
7. Monte AA, Heard KJ, Campbell J, et al. The effect of CYP2D6 drug-drug interactions on hydrocodone effectiveness. Acad Emerg Med. 2014;21(8):879-885.
8. Preskorn SH, Kane CP, Lobello K, et al. Cytochrome P450 2D6 phenoconversion is common in patients being treated for depression: implications for personalized medicine. J Clin Psychiatry. 2013;74(6):614-621.
9. de Leon, J, Susce, MT, Johnson, M, et al. DNA microarray technology in the clinical environment: the AmpliChip CYP450 test for CYP2D6 and CYP2C19 genotyping. CNS Spectr. 2009;14(1):19-34.
10. Lampe JW, King IB, Li S, et al. Brassica vegetables increase and apiaceous vegetables decrease cytochrome P450 1A2 activity in humans: changes in caffeine metabolite ratios in response to controlled vegetable diets. Carcinogenesis. 2000;21(6):1157-1162.
11. Zevin S, Benowitz NL. Drug interaction with tobacco smoking. An update. Clin Pharmacokinet. 1999;36(3):425-438.
The clinical response to the same dose of a drug may vary among individuals. Cytochrome P450 (CYP) 2D6 and 1A2 are enzymes that metabolize many psychotropic medications. Genetic variations in these enzymes may cause changes in their activity and result in differences in effectiveness and adverse effects. Although pharmacogenetic testing is available for CYP2D6 and CYP1A2, interpretation and clinical application of the results may not be straightforward.
Genetic variations in CYP450 enzymes determine enzymatic activity, which can have a large effect on drug levels, efficacy, and toxicity. However, there are many other important factors that clinicians should consider when trying to predict the effects of medications. While clinicians often focus on a patient’s genotype, this only provides information on a chromosomal level, and this information never changes. In contrast, a patient’s phenotype, or status of metabolism, is subject to change throughout the patient’s life.
Many circumstances influence phenotype, including the use of medications that induce or inhibit CYP450 enzymes, environmental factors, and comorbidities. Phenoconversion occurs when these factors result in a phenotype that is different from that predicted by genotype. Because of the possibility of phenoconversion, knowing a patient’s genotype may be of limited value in making clinical decisions. This article provides guidance on interpreting both the genotype and phenotype of CYP2D6 and CYP1A2. Case 1 and Case 2 illustrate these concepts.
CYP2D6
The enzyme activity of CYP2D6 varies among individuals and may include no activity, decreased activity, normal activity, or increased activity. After obtaining the genotype, the activity level of the CYP2D6 alleles may be determined. The frequency with which certain alleles occur varies with ancestry. More than 100 allelic variants and subvariants have been discovered, and new alleles are continuing to be discovered.1Table 12 lists some of the most common CYP2D6 alleles.

Based on the CYP2D6 enzyme activity determined from the alleles, 4 “traditional” phenotypes can be predicted from the genotype (Table 22). The 7-category phenotypes reported by some laboratory companies provide a more explicit method for reporting phenotypes.

Evidence suggests that, unlike most other CYP450 enzymes, CYP2D6 is not very susceptible to enzyme induction.2 Thus, genetics, rather than drug therapy, accounts for most ultra-rapid CYP2D6 metabolizers. CYP2D6 can be inhibited by the use of medications (Table 32-5) and/or substrates (Table 42,6). Similar to inhibitors, substrates may be saturating high affinity-low capacity enzymes such as CYP2D6, resulting in phenoconversion to poor metabolizers. However, this is unlikely to be the case for substrates of low affinity-high capacity enzymes such as CYP3A4.7 Ultimately, substrates and/or inhibitors of CYP2D6 may result in a phenotype that does not correspond to genotype.

Phenoconversion
Genotyping may not reflect the true prevalence of the CYP2D6 poor metabolizer phenotype when using multiple medications that are substrates and/or inhibitors of CYP2D6.8 In the presence of strong CYP2D6 inhibitors, up to 80% of individuals with a non-poor metabolizer genotype are converted to a poor metabolizer phenotype.8 While the phenotype provides a clearer representation of metabolism status than genotype, this information may not always be available.
Continue to: Determining CYP2D6 phenotype
Determining CYP2D6 phenotype
Risperidone and venlafaxine levels are useful tools for predicting CYP2D6 phenotype.3,8 When a risperidone level is ordered, the results include a risperidone level and a 9-hydroxyrisperidone level. The active metabolite of risperidone is 9-hydroxyrisperidone (
- Ultra-rapid metabolizer: 0.03 (0.02 to 0.06)
- Extensive metabolizer: 0.08 (0.04 to 0.17)
- Intermediate metabolizer: 0.56 (0.30 to 1.0)
- Poor metabolizer: 2.5 (1.8 to 4.1).
Although a R-to-9-OHR concentration ratio >1 generally indicates a poor metabolizer, it could also indicate the presence of a powerful CYP2D6 inhibitor.9
When a venlafaxine level is ordered, the results include a venlafaxine level and an O-desmethylvenlafaxine level. O-desmethylvenlafaxine (
CYP1A2
While the activity of CYP2D6 alleles is determined primarily by genetic factors and medications, the activity of CYP1A2 alleles is largely determined by environmental factors (diet, medications, disease) and genetic variability.2 Consequently, CYP1A2 genotyping may be less clinically useful than CYP2D6 genotyping. The CYP1A2 genotype–phenotype relationship incorporates the degree of allele activity (Table 52), and inducibility in the presence of environmental factors.
Continue to: CYP1A2 inhibiton
CYP1A2 inhibition
A variety of medications and environmental factors may inhibit CYP1A2.
Medications. Medications that may inhibit CYP1A2 include a
Caffeine. A significant increase in caffeine consumption can result in inhibition.3 Among non-tobacco smokers, an increase of 1 cup/d of coffee or 2 cans/d of caffeinated soda would be considered significant.3 However, tobacco smokers would require an increase of 3 cups/d of coffee or 6 cans/d of soda.
Diet. An increase in the daily dietary intake of certain vegetables for 6 days has been shown to result in inhibition.10 Apiaceous (Apiaceae or Umbelliferae) vegetables such as carrots (3/4 cup), celery (1/2 cup), dill (1 teaspoon), parsley (3 tablespoons), and parsnips (1¼ cup) can decrease CYP1A2 activity by approximately 13% to 25%. Allium (Liliaceae) vegetables, such as garlic, leeks, and onions, have no effect on CYP1A2 activity.
Infection. Pneumonia, upper respiratory infections with fever, pyelonephritis or appendicitis, or inflammation are suspected to decrease CYP1A2 activity.8
Continue to: CYP1A2 induction
CYP1A2 induction
A variety of medications and environmental factors may induce CYP1A2.
Medications. Certain medications may induce CYP1A2, including c
Cigarette smoking. A significant increase in smoking after 1 to 3 weeks may decrease drug levels, whereas a significant decrease in smoking after 1 to 3 weeks may result in elevated drug levels.3 Nicotine is not the causative agent of induction, but rather hydrocarbons found in cigarette smoke.11
Diet. An increase in daily dietary intake of certain vegetables for 6 days has been shown to result in induction.3 Brassica (Cruciferae) vegetables such as broccoli (2 cups), cauliflower (1 cup), cabbage (1 cup), and radish sprouts (1/2 cup) have been found to increase CYP1A2 activity by 18% to 37%.10 Grilled meat also plays a role in induction.10
Related Resource
- Ellingrod VL, Ward KM. Using pharmacogenetics guidelines when prescribing: What's available. Current Psychiatry. 2018;17(1):43-46.
Drug Brand Names
Amiodarone • Cordarone, Pacerone
Amitriptyline • Elavil, Endep
Aripiprazole • Abilify
Asenapine • Saphris
Atazanavir • Reyataz
Brexpiprazole • Rexulti
Bupropion • Wellbutrin, Zyban
Carbamazepine • Carbatrol, Tegretol
Chlorpromazine • Thorazine
Chloroquine • Aralen
Cinacalcet • Sensipar
Ciprofloxacin • Cipro
Citalopram • Celexa
Clozapine • Clozaril
Desipramine • Norpramin
Desvenlafaxine • Pristiq
Diphenhydramine • Benadryl
Doxepin • Silenor
Duloxetine • Cymbalta
Escitalopram • Lexapro
Ethinyl estradiol • Estinyl
Fluoxetine • Prozac
Fluvoxamine • Luvox
Haloperidol • Haldol
Iloperidone • Fanapt
Imatinib • Gleevec
Imipramine • Tofranil
Mirtazapine • Remeron
Nortriptyline • Pamelor
Olanzapine • Zyprexa
Paliperidone • Invega
Paroxetine • Paxil
Perphenazine • Trilafon
Phenytoin • Dilantin
Pimavanserin • Nuplazid
Primidone • Mysoline
Quetiapine • Seroquel
Quinidine • Cardioquin
Rifampin • Rifadin
Risperidone • Risperdal
Sertraline • Zoloft
Terbinafine • Lamisil
Thioridazine • Mellaril
Trazodone • Desyrel, Oleptro
Venlafaxine • Effexor
Vilazodone • Viibryd
Vortioxetine • Trintellix
Ziprasidone • Geodon
The clinical response to the same dose of a drug may vary among individuals. Cytochrome P450 (CYP) 2D6 and 1A2 are enzymes that metabolize many psychotropic medications. Genetic variations in these enzymes may cause changes in their activity and result in differences in effectiveness and adverse effects. Although pharmacogenetic testing is available for CYP2D6 and CYP1A2, interpretation and clinical application of the results may not be straightforward.
Genetic variations in CYP450 enzymes determine enzymatic activity, which can have a large effect on drug levels, efficacy, and toxicity. However, there are many other important factors that clinicians should consider when trying to predict the effects of medications. While clinicians often focus on a patient’s genotype, this only provides information on a chromosomal level, and this information never changes. In contrast, a patient’s phenotype, or status of metabolism, is subject to change throughout the patient’s life.
Many circumstances influence phenotype, including the use of medications that induce or inhibit CYP450 enzymes, environmental factors, and comorbidities. Phenoconversion occurs when these factors result in a phenotype that is different from that predicted by genotype. Because of the possibility of phenoconversion, knowing a patient’s genotype may be of limited value in making clinical decisions. This article provides guidance on interpreting both the genotype and phenotype of CYP2D6 and CYP1A2. Case 1 and Case 2 illustrate these concepts.
CYP2D6
The enzyme activity of CYP2D6 varies among individuals and may include no activity, decreased activity, normal activity, or increased activity. After obtaining the genotype, the activity level of the CYP2D6 alleles may be determined. The frequency with which certain alleles occur varies with ancestry. More than 100 allelic variants and subvariants have been discovered, and new alleles are continuing to be discovered.1Table 12 lists some of the most common CYP2D6 alleles.

Based on the CYP2D6 enzyme activity determined from the alleles, 4 “traditional” phenotypes can be predicted from the genotype (Table 22). The 7-category phenotypes reported by some laboratory companies provide a more explicit method for reporting phenotypes.

Evidence suggests that, unlike most other CYP450 enzymes, CYP2D6 is not very susceptible to enzyme induction.2 Thus, genetics, rather than drug therapy, accounts for most ultra-rapid CYP2D6 metabolizers. CYP2D6 can be inhibited by the use of medications (Table 32-5) and/or substrates (Table 42,6). Similar to inhibitors, substrates may be saturating high affinity-low capacity enzymes such as CYP2D6, resulting in phenoconversion to poor metabolizers. However, this is unlikely to be the case for substrates of low affinity-high capacity enzymes such as CYP3A4.7 Ultimately, substrates and/or inhibitors of CYP2D6 may result in a phenotype that does not correspond to genotype.

Phenoconversion
Genotyping may not reflect the true prevalence of the CYP2D6 poor metabolizer phenotype when using multiple medications that are substrates and/or inhibitors of CYP2D6.8 In the presence of strong CYP2D6 inhibitors, up to 80% of individuals with a non-poor metabolizer genotype are converted to a poor metabolizer phenotype.8 While the phenotype provides a clearer representation of metabolism status than genotype, this information may not always be available.
Continue to: Determining CYP2D6 phenotype
Determining CYP2D6 phenotype
Risperidone and venlafaxine levels are useful tools for predicting CYP2D6 phenotype.3,8 When a risperidone level is ordered, the results include a risperidone level and a 9-hydroxyrisperidone level. The active metabolite of risperidone is 9-hydroxyrisperidone (
- Ultra-rapid metabolizer: 0.03 (0.02 to 0.06)
- Extensive metabolizer: 0.08 (0.04 to 0.17)
- Intermediate metabolizer: 0.56 (0.30 to 1.0)
- Poor metabolizer: 2.5 (1.8 to 4.1).
Although a R-to-9-OHR concentration ratio >1 generally indicates a poor metabolizer, it could also indicate the presence of a powerful CYP2D6 inhibitor.9
When a venlafaxine level is ordered, the results include a venlafaxine level and an O-desmethylvenlafaxine level. O-desmethylvenlafaxine (
CYP1A2
While the activity of CYP2D6 alleles is determined primarily by genetic factors and medications, the activity of CYP1A2 alleles is largely determined by environmental factors (diet, medications, disease) and genetic variability.2 Consequently, CYP1A2 genotyping may be less clinically useful than CYP2D6 genotyping. The CYP1A2 genotype–phenotype relationship incorporates the degree of allele activity (Table 52), and inducibility in the presence of environmental factors.
Continue to: CYP1A2 inhibiton
CYP1A2 inhibition
A variety of medications and environmental factors may inhibit CYP1A2.
Medications. Medications that may inhibit CYP1A2 include a
Caffeine. A significant increase in caffeine consumption can result in inhibition.3 Among non-tobacco smokers, an increase of 1 cup/d of coffee or 2 cans/d of caffeinated soda would be considered significant.3 However, tobacco smokers would require an increase of 3 cups/d of coffee or 6 cans/d of soda.
Diet. An increase in the daily dietary intake of certain vegetables for 6 days has been shown to result in inhibition.10 Apiaceous (Apiaceae or Umbelliferae) vegetables such as carrots (3/4 cup), celery (1/2 cup), dill (1 teaspoon), parsley (3 tablespoons), and parsnips (1¼ cup) can decrease CYP1A2 activity by approximately 13% to 25%. Allium (Liliaceae) vegetables, such as garlic, leeks, and onions, have no effect on CYP1A2 activity.
Infection. Pneumonia, upper respiratory infections with fever, pyelonephritis or appendicitis, or inflammation are suspected to decrease CYP1A2 activity.8
Continue to: CYP1A2 induction
CYP1A2 induction
A variety of medications and environmental factors may induce CYP1A2.
Medications. Certain medications may induce CYP1A2, including c
Cigarette smoking. A significant increase in smoking after 1 to 3 weeks may decrease drug levels, whereas a significant decrease in smoking after 1 to 3 weeks may result in elevated drug levels.3 Nicotine is not the causative agent of induction, but rather hydrocarbons found in cigarette smoke.11
Diet. An increase in daily dietary intake of certain vegetables for 6 days has been shown to result in induction.3 Brassica (Cruciferae) vegetables such as broccoli (2 cups), cauliflower (1 cup), cabbage (1 cup), and radish sprouts (1/2 cup) have been found to increase CYP1A2 activity by 18% to 37%.10 Grilled meat also plays a role in induction.10
Related Resource
- Ellingrod VL, Ward KM. Using pharmacogenetics guidelines when prescribing: What's available. Current Psychiatry. 2018;17(1):43-46.
Drug Brand Names
Amiodarone • Cordarone, Pacerone
Amitriptyline • Elavil, Endep
Aripiprazole • Abilify
Asenapine • Saphris
Atazanavir • Reyataz
Brexpiprazole • Rexulti
Bupropion • Wellbutrin, Zyban
Carbamazepine • Carbatrol, Tegretol
Chlorpromazine • Thorazine
Chloroquine • Aralen
Cinacalcet • Sensipar
Ciprofloxacin • Cipro
Citalopram • Celexa
Clozapine • Clozaril
Desipramine • Norpramin
Desvenlafaxine • Pristiq
Diphenhydramine • Benadryl
Doxepin • Silenor
Duloxetine • Cymbalta
Escitalopram • Lexapro
Ethinyl estradiol • Estinyl
Fluoxetine • Prozac
Fluvoxamine • Luvox
Haloperidol • Haldol
Iloperidone • Fanapt
Imatinib • Gleevec
Imipramine • Tofranil
Mirtazapine • Remeron
Nortriptyline • Pamelor
Olanzapine • Zyprexa
Paliperidone • Invega
Paroxetine • Paxil
Perphenazine • Trilafon
Phenytoin • Dilantin
Pimavanserin • Nuplazid
Primidone • Mysoline
Quetiapine • Seroquel
Quinidine • Cardioquin
Rifampin • Rifadin
Risperidone • Risperdal
Sertraline • Zoloft
Terbinafine • Lamisil
Thioridazine • Mellaril
Trazodone • Desyrel, Oleptro
Venlafaxine • Effexor
Vilazodone • Viibryd
Vortioxetine • Trintellix
Ziprasidone • Geodon
1. Pharmacogene Variation Consoritum. CYP2D6 allele nomenclature. https://www.pharmvar.org/gene/CYP2D6. Updated May 22, 2018. Accessed June 11, 2018.
2. Mrazek D. Psychiatric pharmacogenomics. New York, NY: Oxford University Press; 2010:33,42,44,45,85.
3. Spina E, de Leon J. Clinical applications of CYP genotyping in psychiatry. J Neural Transm (Vienna). 2015;122(1):5-28.
4. Adedoyin A, Frye RF, Mauro K, et al. Chloroquine modulation of specific metabolizing enzymes activities: investigation with selective five drug cocktail. Br J Clin Pharmacol. 1998;46(3):215-219.
5. Filppula AM, Laitila J, Neuvonen PJ, et al. Potent mechanism-based inhibition of CYP3A4 by imatinib explains its liability to interact with CYP3A4 substrates. Br J Pharmacol. 2012;165(8):2787-2798.
6. U.S. National Library of Medicine. DailyMed. http://dailymed.nlm.nih.gov/dailymed/about.cfm. Accessed April 26, 2018.
7. Monte AA, Heard KJ, Campbell J, et al. The effect of CYP2D6 drug-drug interactions on hydrocodone effectiveness. Acad Emerg Med. 2014;21(8):879-885.
8. Preskorn SH, Kane CP, Lobello K, et al. Cytochrome P450 2D6 phenoconversion is common in patients being treated for depression: implications for personalized medicine. J Clin Psychiatry. 2013;74(6):614-621.
9. de Leon, J, Susce, MT, Johnson, M, et al. DNA microarray technology in the clinical environment: the AmpliChip CYP450 test for CYP2D6 and CYP2C19 genotyping. CNS Spectr. 2009;14(1):19-34.
10. Lampe JW, King IB, Li S, et al. Brassica vegetables increase and apiaceous vegetables decrease cytochrome P450 1A2 activity in humans: changes in caffeine metabolite ratios in response to controlled vegetable diets. Carcinogenesis. 2000;21(6):1157-1162.
11. Zevin S, Benowitz NL. Drug interaction with tobacco smoking. An update. Clin Pharmacokinet. 1999;36(3):425-438.
1. Pharmacogene Variation Consoritum. CYP2D6 allele nomenclature. https://www.pharmvar.org/gene/CYP2D6. Updated May 22, 2018. Accessed June 11, 2018.
2. Mrazek D. Psychiatric pharmacogenomics. New York, NY: Oxford University Press; 2010:33,42,44,45,85.
3. Spina E, de Leon J. Clinical applications of CYP genotyping in psychiatry. J Neural Transm (Vienna). 2015;122(1):5-28.
4. Adedoyin A, Frye RF, Mauro K, et al. Chloroquine modulation of specific metabolizing enzymes activities: investigation with selective five drug cocktail. Br J Clin Pharmacol. 1998;46(3):215-219.
5. Filppula AM, Laitila J, Neuvonen PJ, et al. Potent mechanism-based inhibition of CYP3A4 by imatinib explains its liability to interact with CYP3A4 substrates. Br J Pharmacol. 2012;165(8):2787-2798.
6. U.S. National Library of Medicine. DailyMed. http://dailymed.nlm.nih.gov/dailymed/about.cfm. Accessed April 26, 2018.
7. Monte AA, Heard KJ, Campbell J, et al. The effect of CYP2D6 drug-drug interactions on hydrocodone effectiveness. Acad Emerg Med. 2014;21(8):879-885.
8. Preskorn SH, Kane CP, Lobello K, et al. Cytochrome P450 2D6 phenoconversion is common in patients being treated for depression: implications for personalized medicine. J Clin Psychiatry. 2013;74(6):614-621.
9. de Leon, J, Susce, MT, Johnson, M, et al. DNA microarray technology in the clinical environment: the AmpliChip CYP450 test for CYP2D6 and CYP2C19 genotyping. CNS Spectr. 2009;14(1):19-34.
10. Lampe JW, King IB, Li S, et al. Brassica vegetables increase and apiaceous vegetables decrease cytochrome P450 1A2 activity in humans: changes in caffeine metabolite ratios in response to controlled vegetable diets. Carcinogenesis. 2000;21(6):1157-1162.
11. Zevin S, Benowitz NL. Drug interaction with tobacco smoking. An update. Clin Pharmacokinet. 1999;36(3):425-438.
Is this adolescent suicidal? Challenges in pediatric inpatient consultation-liaison
CASE Attempted suicide?
Ms. S, a 16-year-old Yemeni-American girl, is brought to the emergency department (ED) by her mother and brother after ingesting an overdose of painkillers and fainting. During the initial evaluation, Ms. S says she had in the past attempted suicide by knife. The medical team suspects that the current overdose is a suicide attempt, and they call the consultation-liaison (C-L) psychiatry/psychology team. Ms. S’s brother strongly denies that his sister had previously attempted suicide, stating, “She’s from a good family, and she is smart. She cannot feel that way.” He also requests the name of the clinician who documented this information in the medical record.
During the consultation, Ms. S reports that the previous morning, she developed strong abdominal pain and discovered that she was menstruating for the first time. She explains that she did not understand what was happening to her and that no one had discussed menstruation with her before. Ms. S took her mother’s opioid pain medication. Ms. S reports she took one pill, but when it did not immediately alleviate her pain, she ingested several more. After this, Ms. S says she went to play with her siblings, but gradually became dizzy and confused, and informed her sister and mother of this. The family was fasting in observance of Ramadan, and as they walked toward the mosque, Ms. S fainted, which prompted her family to bring her to the ED.
During the C-L consultation, Ms. S’s brother, who speaks English, is present, as is her mother, who speaks only Arabic and thus needs a phone interpreter. As the C-L team asks Ms. S a question, it is translated to her mother, and then Ms. S’s response is also translated, and then finally, the mother shares her own response. At times, her brother provides translation. Ms. S speaks in English, but often asks for the translation of words or questions.
Ms. S reports that she and her family emigrated from Yemen to the United States 9 months ago. Ms. S says that she enjoys school and is doing well academically. She denies experiencing any anxiety, worry, or stress related to her life in Yemen, her move to a new country, her parents’ health, school, or other domains. Ms. S also denies any history of depressive episodes or previous suicidal ideation, intention, or attempt, which contradicts her endorsement of a previous suicide attempt to one clinician when she was initially evaluated.
[polldaddy:10040204]
Continue to: The authors' observation
The authors’ observation
The C-L team determined that Ms. S did not meet criteria for major depressive disorder. She did not endorse current feelings of depression and denied anhedonia and other associated symptoms included in DSM-5 criteria for major depressive disorder or adjustment disorder with depressed mood (Table 11).
Although Ms. S and her family recently emigrated from Yemen, she did not report any symptoms consistent with an adjustment disorder with depression. Further,
Accurate case conceptualization and diagnosis is particularly crucial in C-L services, where there is an urgency for clinical decision-making after an initial evaluation without the luxury of amending conceptualization in follow-up sessions. Providing a diagnosis for which a patient does not fully or accurately meet the criteria can have deleterious effects. An inaccurate diagnosis for Ms. S would have unnecessarily added the perceived stigma of a mental disorder to her medical record. Additionally, misdiagnosing or pathologizing a natural process of acculturation could have led to inappropriate or even harmful treatment.
The C-L team evaluated alternative explanations for Ms. S’s statements that suggested she was suicidal. First, they considered her mental status at the time she presented to the ED. An overdose of opioids alters mental status. Complicating reversal of opioid overdose is that some opioids have longer half-lives than naloxone, an opioid antagonist, so the individual can become reintoxicated. Similarly, some opioids are more potent and difficult to reverse.2 An altered mental status may have limited Ms. S’s ability to comprehend and answer questions accurately when she first presented to the ED.
Continue to: Cultural factors and the clinical evaluation
Cultural factors and the clinical evaluation
Next, the C-L team considered Ms. S’s clinical picture as it related to her cultural background. Cultural factors interact with the clinical evaluation in a complex manner, influencing the way patients approach the encounter, the symptoms they report, and the language they use to describe their experiences. While these variables are thoroughly evaluated during comprehensive psychological assessments, within the inpatient consultation service, the goal for pediatric C-L clinicians is to conduct a focused assessment to answer specific and critically important questions about a youth’s psychological functioning. Thus, the fundamental challenge of inpatient consultation is to answer the referral question in a brief period and in a culturally informed manner, to appraise the referring medical team about the relevant clinical and cultural issues, with the goal of ethical and clinically sound decision-making.
The C-L team considered key cultural factors in its assessment of Ms. S (Table 31). Several issues were of concern. First, language is often cited as the top barrier to health care access by Arab Americans, even by those with competency in English.3
Experts in culture argue that even with access to interpreters, many words and phrases lack direct translation, and their implicit meaning may be difficult to reveal. Additionally, at times more significance is placed on nonverbal cues and unspoken expectations.4 This can create barriers to communication with clinicians, especially in the context of an inpatient psychiatric consultation, when thorough understanding of an adolescent and family often needs to occur in a single encounter, and clinicians may not appreciate the subtle nuances of nonverbal communication.
The language barrier also may have influenced Ms. S’s initial endorsement of a previous suicide attempt by knife because the medical staff first interviewed Ms. S without an interpreter. For instance, many medical and psychosocial providers probe patients regarding suicidality with questions such as “Have you ever hurt yourself?” or “Have you ever tried to hurt yourself?” It is possible that in another language, an individual might interpret that question as, “Have you ever gotten hurt?” This interpretation completely alters the meaning of the question and eliminates intention or motivation to harm oneself. Language ambiguity and lack of shared cultural understanding may have influenced Ms. S’s interpretation of and response to such questions. Ms. S and her family were perplexed by the C-L team’s reference to the knife and continued to deny the incident.
Continue to: Cultural attitudes to puberty
Cultural attitudes to puberty
Cultures vary with respect to education of sensitive topics such as puberty. The medical providers assumed that Ms. S was informed about the onset of menses. Therefore, they could not consider the strong impact of such an event on an unsuspecting adolescent. Many adolescent girls in Yemen have poor health and lack menstruation-related knowledge, and many are “prescribed” medications by their mothers without contacting a physician.5 Ms. S reported to the C-L team that no one from her family had discussed menstruation with her. She reported that since arriving at the hospital, nurses had educated her about menstruation, and that she was no longer afraid. She also noted that if she experienced such pain again, she would go to the hospital or “just deal with it.”
Family identification and attitudes toward mental health
Ms. S’s strong identification with her family and attitudes toward mental health may have limited what she chose to disclose regarding her experiences of loss related to leaving her country of origin, adjustment, and acculturation to the new environment, as well as feelings of sadness. Family has a central and critical role in Arab cultures. Commitment to a family’s well-being and enhancement of honor and status is highly valued and encouraged.4 Conversely, being concerned with individual needs may be a source of guilt and feelings of betraying the family.6 Arab Americans tend not to discuss personal problems with people outside their extended family, including counselors and therapists, partly because of cultural stigma against mental illness7,8 and partly because revealing family problems to strangers (ie, clinicians) may be considered a cultural taboo9 and a threat to family honor.10 Although Ms. S was interviewed privately when she first came to the ED and also during the psychiatric consultation, the stigma of psychiatric problems11 and possible concerns about protecting her family’s name may have influenced her readiness to reveal intimate information to “strangers.”
Additionally, family statements that appeared to imply negative beliefs about mental health would have strongly deterred Ms. S from expressing any psychological concerns. For example, Ms. S’s brother took offense when the C-L team said it was evaluating his sister because she had said she had previously attempted suicide.
The tenets of Islam may have provided a framework through which Ms. S interprets emotional concerns and may have defined her explanatory models of psychological stress. For instance, it is not uncommon among American Muslims to view mental health problems as rising from “loss of faith in God,”9 and suicidal ideation may not be disclosed because suicide is forbidden in Islam.12 Therefore, it might be particularly difficult to assess suicidal ideation in a patient who is Muslim, especially those who are less acculturated to Western culture.13
Continue to: Directly asking Ms. S...
Directly asking Ms. S if she had thoughts of harming herself may have been too frightening or guilt-provoking for an adolescent with her background. Asking about passive expression of suicidal ideation would have been more culturally appropriate. For example, asking, “Do you wish that God would let you die?”12 may have elicited more meaningful clinical information about Ms. S’s emotional state and possibly suicide risk.
Furthermore, Ms. S’s identification of coping strategies (ie, “just deal with it”) may have sounded limited to a Western clinician, but this may have been consistent with cultural norms of emotional expression of limiting complaints.4 Also, among Arab Americans, psychiatric symptoms often are expressed through somatization.7,14 Expressing psychological pain through physical symptoms appears protective against public stigma. Public image and opinion is important, and behaviors that would reflect well to others are dictated by the family. These attitudes, beliefs, and values likely impact how Ms. S presented her psychological concerns.
[polldaddy:10040206]
The authors’ observations
Although inpatient hospitalization was initially considered, it was not pursued due to denial of past and current suicidal ideation or suicide attempts, the lack of comorbidity, age-appropriate functioning, and a supportive family environment. Similarly, due to the absence of acute psychiatric symptoms, partial hospitalization was not pursued. The C-L team evaluated treatment options with extreme caution and sensitivity because recommending the wrong treatment option could have deleterious effects on Ms. S and her family’s life. If inpatient hospitalization had been pursued, it could have likely caused the family unnecessary suffering and could have negatively affected familial relationships. Strong feelings of shame, betrayal, and guilt would be intensified, impairing the family’s cohesion, removing environmental and family supports, and putting Ms. S at further risk of developing more severe symptoms of low mood.
Although there were significant concerns about making the wrong recommendation to the family, the C-L team’s highest priority was Ms. S’s safety. Despite cultural concerns, the team would have recommended hospitalization if Ms. S’s clinical picture had warranted this decision.
Continue to: OUTCOME Culturally-appropriate outpatient therapy
OUTCOME Culturally-appropriate outpatient therapy
Due to the lack of substantial evidence of apparent risk for self-harm, the presence of a supportive family, and Ms. S’s high academic performance and future orientation, the C-L team concludes that Ms. S’s concerns were most likely the result of the challenges of acculturation related to the language barrier and a lack of health knowledge. However, the C-L team remains cautious that Ms. S may have minimized or denied her mental health concerns due to various cultural factors. The team recommends that Ms. S seek outpatient psychotherapy from a clinician who specializes in working with Arab American individuals and families in their native language. The C-L team communicates these conclusions to the medical team verbally and in writing.
The authors’ observations
Cultural issues experienced during this consultation may not generalize to other Arab American adolescents and their families because there is diversity even within groups that share common cultural characteristics. Nevertheless, this case underscores the challenge of accurately assessing suicide risk, and making a differential diagnosis in the presence of complex cultural data and the dilemmas clinicians may encounter when attempting to answer important referral questions such as, “Is this adolescent suicidal and in need of psychiatric hospitalization?”
Bottom Line
Cultural factors and attitudes toward mental health and language barriers may play a large role in how patients answer clinical questions. Cultural issues may add a level of intricacy not easily resolved within the restrictions of an inpatient setting, and this complexity may influence clinical judgment, recommendations, and possibly health outcomes. Culturally appropriate psychotherapy is key for patients experiencing difficulty with acculturation.
Related Resources
- Adam B. Caring for Muslim patients: Understanding cultural and religious factors. Current Psychiatry. 2017;16(12):56-57.
- Nassar-McMillan SC, Hakim-Larson J. Counseling considerations among Arab Americans. Journal of Counseling & Development. 2003;81(2):150-159.
- Sue DW. Multidimensional facets of cultural competence. The Counseling Psychologist. 2001;29(6):790-821.
Drug Brand Name
Naloxone • Narcan
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Meehan TJ, Bryant SM, Aks SE. Drugs of abuse: the highs and lows of altered mental states in the emergency department. Emerg Med Clin North Am. 2010;28(3):663-682.
3. Shah SM, Ayash C, Pharaon NA, et al. Arab American immigrants in New York: health care and cancer knowledge, attitudes, and beliefs. J Immigr Minor Health. 2008;10(5):429-436.
4. Budman CL, Lipson JG, Meleis AI. The cultural consultant in mental health care: the case of an Arab adolescent. Am J Orthopsychiatry. 1992; 62(3):359-370.
5. Mohamed EM, Mohamed AG, Al-Ajeal Ly. Knowledge, beliefs and practices regarding menstruation among adolescent schoolgirls in Seiyun City, Yemen. Al-Azhar Assiut Medical Journal. 2011;9(3):67-86.
6. Gorkin M, Masalha S, Yatziv G. Psychotherapy of Israeli-Arab patients: some cultural considerations. Journal of Psychoanalytic Anthropology. 1985;8(4);215-230.
7. Gearing RE, MacKenzie MJ, Ibrahim RW, et al. Stigma and mental health treatment of adolescents with depression in Jordan. Community Ment Health J. 2015;51(1):111-117.
8. Timimi SB. Adolescence in immigrant Arab families. Psychotherapy: theory, research, practice, training. 1995;32(1):141-149.
9. Ahmed S and Reddy LA. Understanding the mental health needs of American Muslims: recommendations and considerations for practice. Journal of Multicultural Counseling and Development. 2007;35(4):207-218.
10. Abudabbeh N, Nydell MK. Transcultural counseling and Arab Americans. In: McFadden J, ed. Transcultural counseling: bilateral and international perspectives. Alexandria, VA. American Counseling Association. 1993:261-284.
11. Erickson CD, al-Timimi NR. Providing mental health services to Arab Americans: recommendations and considerations. Cultur Divers Ethnic Minor Psychol. 2001;7(4):308-327.
12. Ali SR, Liu WM, Humedian M. Islam 101: understanding the religion and therapy implications. Prof Psychol Res Pr. 2004;35(6):635-642.
13. Hedayat-Diba Z. Psychotherapy with Muslims. In: Richards PS, Bergin AE, eds. Handbook of psychotherapy and religious diversity, 2nd ed. Washington, DC: Amercian Psychological Association. 2000:289-314.
14. Al-Krenawi A. Mental health practice in Arab countries. Curr Opin in Psychiatry. 2005;18(5):560-564.
CASE Attempted suicide?
Ms. S, a 16-year-old Yemeni-American girl, is brought to the emergency department (ED) by her mother and brother after ingesting an overdose of painkillers and fainting. During the initial evaluation, Ms. S says she had in the past attempted suicide by knife. The medical team suspects that the current overdose is a suicide attempt, and they call the consultation-liaison (C-L) psychiatry/psychology team. Ms. S’s brother strongly denies that his sister had previously attempted suicide, stating, “She’s from a good family, and she is smart. She cannot feel that way.” He also requests the name of the clinician who documented this information in the medical record.
During the consultation, Ms. S reports that the previous morning, she developed strong abdominal pain and discovered that she was menstruating for the first time. She explains that she did not understand what was happening to her and that no one had discussed menstruation with her before. Ms. S took her mother’s opioid pain medication. Ms. S reports she took one pill, but when it did not immediately alleviate her pain, she ingested several more. After this, Ms. S says she went to play with her siblings, but gradually became dizzy and confused, and informed her sister and mother of this. The family was fasting in observance of Ramadan, and as they walked toward the mosque, Ms. S fainted, which prompted her family to bring her to the ED.
During the C-L consultation, Ms. S’s brother, who speaks English, is present, as is her mother, who speaks only Arabic and thus needs a phone interpreter. As the C-L team asks Ms. S a question, it is translated to her mother, and then Ms. S’s response is also translated, and then finally, the mother shares her own response. At times, her brother provides translation. Ms. S speaks in English, but often asks for the translation of words or questions.
Ms. S reports that she and her family emigrated from Yemen to the United States 9 months ago. Ms. S says that she enjoys school and is doing well academically. She denies experiencing any anxiety, worry, or stress related to her life in Yemen, her move to a new country, her parents’ health, school, or other domains. Ms. S also denies any history of depressive episodes or previous suicidal ideation, intention, or attempt, which contradicts her endorsement of a previous suicide attempt to one clinician when she was initially evaluated.
[polldaddy:10040204]
Continue to: The authors' observation
The authors’ observation
The C-L team determined that Ms. S did not meet criteria for major depressive disorder. She did not endorse current feelings of depression and denied anhedonia and other associated symptoms included in DSM-5 criteria for major depressive disorder or adjustment disorder with depressed mood (Table 11).
Although Ms. S and her family recently emigrated from Yemen, she did not report any symptoms consistent with an adjustment disorder with depression. Further,
Accurate case conceptualization and diagnosis is particularly crucial in C-L services, where there is an urgency for clinical decision-making after an initial evaluation without the luxury of amending conceptualization in follow-up sessions. Providing a diagnosis for which a patient does not fully or accurately meet the criteria can have deleterious effects. An inaccurate diagnosis for Ms. S would have unnecessarily added the perceived stigma of a mental disorder to her medical record. Additionally, misdiagnosing or pathologizing a natural process of acculturation could have led to inappropriate or even harmful treatment.
The C-L team evaluated alternative explanations for Ms. S’s statements that suggested she was suicidal. First, they considered her mental status at the time she presented to the ED. An overdose of opioids alters mental status. Complicating reversal of opioid overdose is that some opioids have longer half-lives than naloxone, an opioid antagonist, so the individual can become reintoxicated. Similarly, some opioids are more potent and difficult to reverse.2 An altered mental status may have limited Ms. S’s ability to comprehend and answer questions accurately when she first presented to the ED.
Continue to: Cultural factors and the clinical evaluation
Cultural factors and the clinical evaluation
Next, the C-L team considered Ms. S’s clinical picture as it related to her cultural background. Cultural factors interact with the clinical evaluation in a complex manner, influencing the way patients approach the encounter, the symptoms they report, and the language they use to describe their experiences. While these variables are thoroughly evaluated during comprehensive psychological assessments, within the inpatient consultation service, the goal for pediatric C-L clinicians is to conduct a focused assessment to answer specific and critically important questions about a youth’s psychological functioning. Thus, the fundamental challenge of inpatient consultation is to answer the referral question in a brief period and in a culturally informed manner, to appraise the referring medical team about the relevant clinical and cultural issues, with the goal of ethical and clinically sound decision-making.
The C-L team considered key cultural factors in its assessment of Ms. S (Table 31). Several issues were of concern. First, language is often cited as the top barrier to health care access by Arab Americans, even by those with competency in English.3
Experts in culture argue that even with access to interpreters, many words and phrases lack direct translation, and their implicit meaning may be difficult to reveal. Additionally, at times more significance is placed on nonverbal cues and unspoken expectations.4 This can create barriers to communication with clinicians, especially in the context of an inpatient psychiatric consultation, when thorough understanding of an adolescent and family often needs to occur in a single encounter, and clinicians may not appreciate the subtle nuances of nonverbal communication.
The language barrier also may have influenced Ms. S’s initial endorsement of a previous suicide attempt by knife because the medical staff first interviewed Ms. S without an interpreter. For instance, many medical and psychosocial providers probe patients regarding suicidality with questions such as “Have you ever hurt yourself?” or “Have you ever tried to hurt yourself?” It is possible that in another language, an individual might interpret that question as, “Have you ever gotten hurt?” This interpretation completely alters the meaning of the question and eliminates intention or motivation to harm oneself. Language ambiguity and lack of shared cultural understanding may have influenced Ms. S’s interpretation of and response to such questions. Ms. S and her family were perplexed by the C-L team’s reference to the knife and continued to deny the incident.
Continue to: Cultural attitudes to puberty
Cultural attitudes to puberty
Cultures vary with respect to education of sensitive topics such as puberty. The medical providers assumed that Ms. S was informed about the onset of menses. Therefore, they could not consider the strong impact of such an event on an unsuspecting adolescent. Many adolescent girls in Yemen have poor health and lack menstruation-related knowledge, and many are “prescribed” medications by their mothers without contacting a physician.5 Ms. S reported to the C-L team that no one from her family had discussed menstruation with her. She reported that since arriving at the hospital, nurses had educated her about menstruation, and that she was no longer afraid. She also noted that if she experienced such pain again, she would go to the hospital or “just deal with it.”
Family identification and attitudes toward mental health
Ms. S’s strong identification with her family and attitudes toward mental health may have limited what she chose to disclose regarding her experiences of loss related to leaving her country of origin, adjustment, and acculturation to the new environment, as well as feelings of sadness. Family has a central and critical role in Arab cultures. Commitment to a family’s well-being and enhancement of honor and status is highly valued and encouraged.4 Conversely, being concerned with individual needs may be a source of guilt and feelings of betraying the family.6 Arab Americans tend not to discuss personal problems with people outside their extended family, including counselors and therapists, partly because of cultural stigma against mental illness7,8 and partly because revealing family problems to strangers (ie, clinicians) may be considered a cultural taboo9 and a threat to family honor.10 Although Ms. S was interviewed privately when she first came to the ED and also during the psychiatric consultation, the stigma of psychiatric problems11 and possible concerns about protecting her family’s name may have influenced her readiness to reveal intimate information to “strangers.”
Additionally, family statements that appeared to imply negative beliefs about mental health would have strongly deterred Ms. S from expressing any psychological concerns. For example, Ms. S’s brother took offense when the C-L team said it was evaluating his sister because she had said she had previously attempted suicide.
The tenets of Islam may have provided a framework through which Ms. S interprets emotional concerns and may have defined her explanatory models of psychological stress. For instance, it is not uncommon among American Muslims to view mental health problems as rising from “loss of faith in God,”9 and suicidal ideation may not be disclosed because suicide is forbidden in Islam.12 Therefore, it might be particularly difficult to assess suicidal ideation in a patient who is Muslim, especially those who are less acculturated to Western culture.13
Continue to: Directly asking Ms. S...
Directly asking Ms. S if she had thoughts of harming herself may have been too frightening or guilt-provoking for an adolescent with her background. Asking about passive expression of suicidal ideation would have been more culturally appropriate. For example, asking, “Do you wish that God would let you die?”12 may have elicited more meaningful clinical information about Ms. S’s emotional state and possibly suicide risk.
Furthermore, Ms. S’s identification of coping strategies (ie, “just deal with it”) may have sounded limited to a Western clinician, but this may have been consistent with cultural norms of emotional expression of limiting complaints.4 Also, among Arab Americans, psychiatric symptoms often are expressed through somatization.7,14 Expressing psychological pain through physical symptoms appears protective against public stigma. Public image and opinion is important, and behaviors that would reflect well to others are dictated by the family. These attitudes, beliefs, and values likely impact how Ms. S presented her psychological concerns.
[polldaddy:10040206]
The authors’ observations
Although inpatient hospitalization was initially considered, it was not pursued due to denial of past and current suicidal ideation or suicide attempts, the lack of comorbidity, age-appropriate functioning, and a supportive family environment. Similarly, due to the absence of acute psychiatric symptoms, partial hospitalization was not pursued. The C-L team evaluated treatment options with extreme caution and sensitivity because recommending the wrong treatment option could have deleterious effects on Ms. S and her family’s life. If inpatient hospitalization had been pursued, it could have likely caused the family unnecessary suffering and could have negatively affected familial relationships. Strong feelings of shame, betrayal, and guilt would be intensified, impairing the family’s cohesion, removing environmental and family supports, and putting Ms. S at further risk of developing more severe symptoms of low mood.
Although there were significant concerns about making the wrong recommendation to the family, the C-L team’s highest priority was Ms. S’s safety. Despite cultural concerns, the team would have recommended hospitalization if Ms. S’s clinical picture had warranted this decision.
Continue to: OUTCOME Culturally-appropriate outpatient therapy
OUTCOME Culturally-appropriate outpatient therapy
Due to the lack of substantial evidence of apparent risk for self-harm, the presence of a supportive family, and Ms. S’s high academic performance and future orientation, the C-L team concludes that Ms. S’s concerns were most likely the result of the challenges of acculturation related to the language barrier and a lack of health knowledge. However, the C-L team remains cautious that Ms. S may have minimized or denied her mental health concerns due to various cultural factors. The team recommends that Ms. S seek outpatient psychotherapy from a clinician who specializes in working with Arab American individuals and families in their native language. The C-L team communicates these conclusions to the medical team verbally and in writing.
The authors’ observations
Cultural issues experienced during this consultation may not generalize to other Arab American adolescents and their families because there is diversity even within groups that share common cultural characteristics. Nevertheless, this case underscores the challenge of accurately assessing suicide risk, and making a differential diagnosis in the presence of complex cultural data and the dilemmas clinicians may encounter when attempting to answer important referral questions such as, “Is this adolescent suicidal and in need of psychiatric hospitalization?”
Bottom Line
Cultural factors and attitudes toward mental health and language barriers may play a large role in how patients answer clinical questions. Cultural issues may add a level of intricacy not easily resolved within the restrictions of an inpatient setting, and this complexity may influence clinical judgment, recommendations, and possibly health outcomes. Culturally appropriate psychotherapy is key for patients experiencing difficulty with acculturation.
Related Resources
- Adam B. Caring for Muslim patients: Understanding cultural and religious factors. Current Psychiatry. 2017;16(12):56-57.
- Nassar-McMillan SC, Hakim-Larson J. Counseling considerations among Arab Americans. Journal of Counseling & Development. 2003;81(2):150-159.
- Sue DW. Multidimensional facets of cultural competence. The Counseling Psychologist. 2001;29(6):790-821.
Drug Brand Name
Naloxone • Narcan
CASE Attempted suicide?
Ms. S, a 16-year-old Yemeni-American girl, is brought to the emergency department (ED) by her mother and brother after ingesting an overdose of painkillers and fainting. During the initial evaluation, Ms. S says she had in the past attempted suicide by knife. The medical team suspects that the current overdose is a suicide attempt, and they call the consultation-liaison (C-L) psychiatry/psychology team. Ms. S’s brother strongly denies that his sister had previously attempted suicide, stating, “She’s from a good family, and she is smart. She cannot feel that way.” He also requests the name of the clinician who documented this information in the medical record.
During the consultation, Ms. S reports that the previous morning, she developed strong abdominal pain and discovered that she was menstruating for the first time. She explains that she did not understand what was happening to her and that no one had discussed menstruation with her before. Ms. S took her mother’s opioid pain medication. Ms. S reports she took one pill, but when it did not immediately alleviate her pain, she ingested several more. After this, Ms. S says she went to play with her siblings, but gradually became dizzy and confused, and informed her sister and mother of this. The family was fasting in observance of Ramadan, and as they walked toward the mosque, Ms. S fainted, which prompted her family to bring her to the ED.
During the C-L consultation, Ms. S’s brother, who speaks English, is present, as is her mother, who speaks only Arabic and thus needs a phone interpreter. As the C-L team asks Ms. S a question, it is translated to her mother, and then Ms. S’s response is also translated, and then finally, the mother shares her own response. At times, her brother provides translation. Ms. S speaks in English, but often asks for the translation of words or questions.
Ms. S reports that she and her family emigrated from Yemen to the United States 9 months ago. Ms. S says that she enjoys school and is doing well academically. She denies experiencing any anxiety, worry, or stress related to her life in Yemen, her move to a new country, her parents’ health, school, or other domains. Ms. S also denies any history of depressive episodes or previous suicidal ideation, intention, or attempt, which contradicts her endorsement of a previous suicide attempt to one clinician when she was initially evaluated.
[polldaddy:10040204]
Continue to: The authors' observation
The authors’ observation
The C-L team determined that Ms. S did not meet criteria for major depressive disorder. She did not endorse current feelings of depression and denied anhedonia and other associated symptoms included in DSM-5 criteria for major depressive disorder or adjustment disorder with depressed mood (Table 11).
Although Ms. S and her family recently emigrated from Yemen, she did not report any symptoms consistent with an adjustment disorder with depression. Further,
Accurate case conceptualization and diagnosis is particularly crucial in C-L services, where there is an urgency for clinical decision-making after an initial evaluation without the luxury of amending conceptualization in follow-up sessions. Providing a diagnosis for which a patient does not fully or accurately meet the criteria can have deleterious effects. An inaccurate diagnosis for Ms. S would have unnecessarily added the perceived stigma of a mental disorder to her medical record. Additionally, misdiagnosing or pathologizing a natural process of acculturation could have led to inappropriate or even harmful treatment.
The C-L team evaluated alternative explanations for Ms. S’s statements that suggested she was suicidal. First, they considered her mental status at the time she presented to the ED. An overdose of opioids alters mental status. Complicating reversal of opioid overdose is that some opioids have longer half-lives than naloxone, an opioid antagonist, so the individual can become reintoxicated. Similarly, some opioids are more potent and difficult to reverse.2 An altered mental status may have limited Ms. S’s ability to comprehend and answer questions accurately when she first presented to the ED.
Continue to: Cultural factors and the clinical evaluation
Cultural factors and the clinical evaluation
Next, the C-L team considered Ms. S’s clinical picture as it related to her cultural background. Cultural factors interact with the clinical evaluation in a complex manner, influencing the way patients approach the encounter, the symptoms they report, and the language they use to describe their experiences. While these variables are thoroughly evaluated during comprehensive psychological assessments, within the inpatient consultation service, the goal for pediatric C-L clinicians is to conduct a focused assessment to answer specific and critically important questions about a youth’s psychological functioning. Thus, the fundamental challenge of inpatient consultation is to answer the referral question in a brief period and in a culturally informed manner, to appraise the referring medical team about the relevant clinical and cultural issues, with the goal of ethical and clinically sound decision-making.
The C-L team considered key cultural factors in its assessment of Ms. S (Table 31). Several issues were of concern. First, language is often cited as the top barrier to health care access by Arab Americans, even by those with competency in English.3
Experts in culture argue that even with access to interpreters, many words and phrases lack direct translation, and their implicit meaning may be difficult to reveal. Additionally, at times more significance is placed on nonverbal cues and unspoken expectations.4 This can create barriers to communication with clinicians, especially in the context of an inpatient psychiatric consultation, when thorough understanding of an adolescent and family often needs to occur in a single encounter, and clinicians may not appreciate the subtle nuances of nonverbal communication.
The language barrier also may have influenced Ms. S’s initial endorsement of a previous suicide attempt by knife because the medical staff first interviewed Ms. S without an interpreter. For instance, many medical and psychosocial providers probe patients regarding suicidality with questions such as “Have you ever hurt yourself?” or “Have you ever tried to hurt yourself?” It is possible that in another language, an individual might interpret that question as, “Have you ever gotten hurt?” This interpretation completely alters the meaning of the question and eliminates intention or motivation to harm oneself. Language ambiguity and lack of shared cultural understanding may have influenced Ms. S’s interpretation of and response to such questions. Ms. S and her family were perplexed by the C-L team’s reference to the knife and continued to deny the incident.
Continue to: Cultural attitudes to puberty
Cultural attitudes to puberty
Cultures vary with respect to education of sensitive topics such as puberty. The medical providers assumed that Ms. S was informed about the onset of menses. Therefore, they could not consider the strong impact of such an event on an unsuspecting adolescent. Many adolescent girls in Yemen have poor health and lack menstruation-related knowledge, and many are “prescribed” medications by their mothers without contacting a physician.5 Ms. S reported to the C-L team that no one from her family had discussed menstruation with her. She reported that since arriving at the hospital, nurses had educated her about menstruation, and that she was no longer afraid. She also noted that if she experienced such pain again, she would go to the hospital or “just deal with it.”
Family identification and attitudes toward mental health
Ms. S’s strong identification with her family and attitudes toward mental health may have limited what she chose to disclose regarding her experiences of loss related to leaving her country of origin, adjustment, and acculturation to the new environment, as well as feelings of sadness. Family has a central and critical role in Arab cultures. Commitment to a family’s well-being and enhancement of honor and status is highly valued and encouraged.4 Conversely, being concerned with individual needs may be a source of guilt and feelings of betraying the family.6 Arab Americans tend not to discuss personal problems with people outside their extended family, including counselors and therapists, partly because of cultural stigma against mental illness7,8 and partly because revealing family problems to strangers (ie, clinicians) may be considered a cultural taboo9 and a threat to family honor.10 Although Ms. S was interviewed privately when she first came to the ED and also during the psychiatric consultation, the stigma of psychiatric problems11 and possible concerns about protecting her family’s name may have influenced her readiness to reveal intimate information to “strangers.”
Additionally, family statements that appeared to imply negative beliefs about mental health would have strongly deterred Ms. S from expressing any psychological concerns. For example, Ms. S’s brother took offense when the C-L team said it was evaluating his sister because she had said she had previously attempted suicide.
The tenets of Islam may have provided a framework through which Ms. S interprets emotional concerns and may have defined her explanatory models of psychological stress. For instance, it is not uncommon among American Muslims to view mental health problems as rising from “loss of faith in God,”9 and suicidal ideation may not be disclosed because suicide is forbidden in Islam.12 Therefore, it might be particularly difficult to assess suicidal ideation in a patient who is Muslim, especially those who are less acculturated to Western culture.13
Continue to: Directly asking Ms. S...
Directly asking Ms. S if she had thoughts of harming herself may have been too frightening or guilt-provoking for an adolescent with her background. Asking about passive expression of suicidal ideation would have been more culturally appropriate. For example, asking, “Do you wish that God would let you die?”12 may have elicited more meaningful clinical information about Ms. S’s emotional state and possibly suicide risk.
Furthermore, Ms. S’s identification of coping strategies (ie, “just deal with it”) may have sounded limited to a Western clinician, but this may have been consistent with cultural norms of emotional expression of limiting complaints.4 Also, among Arab Americans, psychiatric symptoms often are expressed through somatization.7,14 Expressing psychological pain through physical symptoms appears protective against public stigma. Public image and opinion is important, and behaviors that would reflect well to others are dictated by the family. These attitudes, beliefs, and values likely impact how Ms. S presented her psychological concerns.
[polldaddy:10040206]
The authors’ observations
Although inpatient hospitalization was initially considered, it was not pursued due to denial of past and current suicidal ideation or suicide attempts, the lack of comorbidity, age-appropriate functioning, and a supportive family environment. Similarly, due to the absence of acute psychiatric symptoms, partial hospitalization was not pursued. The C-L team evaluated treatment options with extreme caution and sensitivity because recommending the wrong treatment option could have deleterious effects on Ms. S and her family’s life. If inpatient hospitalization had been pursued, it could have likely caused the family unnecessary suffering and could have negatively affected familial relationships. Strong feelings of shame, betrayal, and guilt would be intensified, impairing the family’s cohesion, removing environmental and family supports, and putting Ms. S at further risk of developing more severe symptoms of low mood.
Although there were significant concerns about making the wrong recommendation to the family, the C-L team’s highest priority was Ms. S’s safety. Despite cultural concerns, the team would have recommended hospitalization if Ms. S’s clinical picture had warranted this decision.
Continue to: OUTCOME Culturally-appropriate outpatient therapy
OUTCOME Culturally-appropriate outpatient therapy
Due to the lack of substantial evidence of apparent risk for self-harm, the presence of a supportive family, and Ms. S’s high academic performance and future orientation, the C-L team concludes that Ms. S’s concerns were most likely the result of the challenges of acculturation related to the language barrier and a lack of health knowledge. However, the C-L team remains cautious that Ms. S may have minimized or denied her mental health concerns due to various cultural factors. The team recommends that Ms. S seek outpatient psychotherapy from a clinician who specializes in working with Arab American individuals and families in their native language. The C-L team communicates these conclusions to the medical team verbally and in writing.
The authors’ observations
Cultural issues experienced during this consultation may not generalize to other Arab American adolescents and their families because there is diversity even within groups that share common cultural characteristics. Nevertheless, this case underscores the challenge of accurately assessing suicide risk, and making a differential diagnosis in the presence of complex cultural data and the dilemmas clinicians may encounter when attempting to answer important referral questions such as, “Is this adolescent suicidal and in need of psychiatric hospitalization?”
Bottom Line
Cultural factors and attitudes toward mental health and language barriers may play a large role in how patients answer clinical questions. Cultural issues may add a level of intricacy not easily resolved within the restrictions of an inpatient setting, and this complexity may influence clinical judgment, recommendations, and possibly health outcomes. Culturally appropriate psychotherapy is key for patients experiencing difficulty with acculturation.
Related Resources
- Adam B. Caring for Muslim patients: Understanding cultural and religious factors. Current Psychiatry. 2017;16(12):56-57.
- Nassar-McMillan SC, Hakim-Larson J. Counseling considerations among Arab Americans. Journal of Counseling & Development. 2003;81(2):150-159.
- Sue DW. Multidimensional facets of cultural competence. The Counseling Psychologist. 2001;29(6):790-821.
Drug Brand Name
Naloxone • Narcan
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Meehan TJ, Bryant SM, Aks SE. Drugs of abuse: the highs and lows of altered mental states in the emergency department. Emerg Med Clin North Am. 2010;28(3):663-682.
3. Shah SM, Ayash C, Pharaon NA, et al. Arab American immigrants in New York: health care and cancer knowledge, attitudes, and beliefs. J Immigr Minor Health. 2008;10(5):429-436.
4. Budman CL, Lipson JG, Meleis AI. The cultural consultant in mental health care: the case of an Arab adolescent. Am J Orthopsychiatry. 1992; 62(3):359-370.
5. Mohamed EM, Mohamed AG, Al-Ajeal Ly. Knowledge, beliefs and practices regarding menstruation among adolescent schoolgirls in Seiyun City, Yemen. Al-Azhar Assiut Medical Journal. 2011;9(3):67-86.
6. Gorkin M, Masalha S, Yatziv G. Psychotherapy of Israeli-Arab patients: some cultural considerations. Journal of Psychoanalytic Anthropology. 1985;8(4);215-230.
7. Gearing RE, MacKenzie MJ, Ibrahim RW, et al. Stigma and mental health treatment of adolescents with depression in Jordan. Community Ment Health J. 2015;51(1):111-117.
8. Timimi SB. Adolescence in immigrant Arab families. Psychotherapy: theory, research, practice, training. 1995;32(1):141-149.
9. Ahmed S and Reddy LA. Understanding the mental health needs of American Muslims: recommendations and considerations for practice. Journal of Multicultural Counseling and Development. 2007;35(4):207-218.
10. Abudabbeh N, Nydell MK. Transcultural counseling and Arab Americans. In: McFadden J, ed. Transcultural counseling: bilateral and international perspectives. Alexandria, VA. American Counseling Association. 1993:261-284.
11. Erickson CD, al-Timimi NR. Providing mental health services to Arab Americans: recommendations and considerations. Cultur Divers Ethnic Minor Psychol. 2001;7(4):308-327.
12. Ali SR, Liu WM, Humedian M. Islam 101: understanding the religion and therapy implications. Prof Psychol Res Pr. 2004;35(6):635-642.
13. Hedayat-Diba Z. Psychotherapy with Muslims. In: Richards PS, Bergin AE, eds. Handbook of psychotherapy and religious diversity, 2nd ed. Washington, DC: Amercian Psychological Association. 2000:289-314.
14. Al-Krenawi A. Mental health practice in Arab countries. Curr Opin in Psychiatry. 2005;18(5):560-564.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Meehan TJ, Bryant SM, Aks SE. Drugs of abuse: the highs and lows of altered mental states in the emergency department. Emerg Med Clin North Am. 2010;28(3):663-682.
3. Shah SM, Ayash C, Pharaon NA, et al. Arab American immigrants in New York: health care and cancer knowledge, attitudes, and beliefs. J Immigr Minor Health. 2008;10(5):429-436.
4. Budman CL, Lipson JG, Meleis AI. The cultural consultant in mental health care: the case of an Arab adolescent. Am J Orthopsychiatry. 1992; 62(3):359-370.
5. Mohamed EM, Mohamed AG, Al-Ajeal Ly. Knowledge, beliefs and practices regarding menstruation among adolescent schoolgirls in Seiyun City, Yemen. Al-Azhar Assiut Medical Journal. 2011;9(3):67-86.
6. Gorkin M, Masalha S, Yatziv G. Psychotherapy of Israeli-Arab patients: some cultural considerations. Journal of Psychoanalytic Anthropology. 1985;8(4);215-230.
7. Gearing RE, MacKenzie MJ, Ibrahim RW, et al. Stigma and mental health treatment of adolescents with depression in Jordan. Community Ment Health J. 2015;51(1):111-117.
8. Timimi SB. Adolescence in immigrant Arab families. Psychotherapy: theory, research, practice, training. 1995;32(1):141-149.
9. Ahmed S and Reddy LA. Understanding the mental health needs of American Muslims: recommendations and considerations for practice. Journal of Multicultural Counseling and Development. 2007;35(4):207-218.
10. Abudabbeh N, Nydell MK. Transcultural counseling and Arab Americans. In: McFadden J, ed. Transcultural counseling: bilateral and international perspectives. Alexandria, VA. American Counseling Association. 1993:261-284.
11. Erickson CD, al-Timimi NR. Providing mental health services to Arab Americans: recommendations and considerations. Cultur Divers Ethnic Minor Psychol. 2001;7(4):308-327.
12. Ali SR, Liu WM, Humedian M. Islam 101: understanding the religion and therapy implications. Prof Psychol Res Pr. 2004;35(6):635-642.
13. Hedayat-Diba Z. Psychotherapy with Muslims. In: Richards PS, Bergin AE, eds. Handbook of psychotherapy and religious diversity, 2nd ed. Washington, DC: Amercian Psychological Association. 2000:289-314.
14. Al-Krenawi A. Mental health practice in Arab countries. Curr Opin in Psychiatry. 2005;18(5):560-564.
No Guns, No Glory?
I read your editorial with appreciation this morning. In my practice, I have worked with adolescents and young adults for more than 30 years, so the fact that many mass shooters are so young has caught my attention.
Of course, there is never just one cause for such horrific actions. But it seems to me that there are two recurring themes in these situations: (1) many shooters are socially isolated and (2) in our culture, the use of guns is glorified as a representation of power and a source of satisfaction. Here are my thoughts on each:
1. Shooters are often loners who exhibit behavior that is deviant or in some way socially unacceptable; this varies from public masturbation or the voicing of scary thoughts and ideas to simply exhibiting social awkwardness. When the lonely, shunned child has access to powerful, multiple-shot weapons, this is a setup for a shooting.
Perhaps society inadvertently causes harm by forcing classroom integration. About 30 years ago, with the hope to normalize abnormal social interactions and to help students understand and accept those who are different from them, schools began to mainstream students with serious learning difficulties, including students with mental illnesses. These children lost their “group,” their classroom community of those similar to themselves. They are still assigned personalized curricula and work one-on-one with special education teachers, but they do not have much of a peer group anymore. These students are put with a group of students—normal kids—that frequently rejects them.
Part of the problem has to do with the demonization of the term “normal”; it is now politically incorrect to designate a student as normal or not, despite the persistence of the bell-shaped curve. If normal, nonviolent behavior is desired, we need to be able to name abnormal behaviors as such.
Parents, understandably, want this type of social integration for their child. But when these students are bullied throughout the day, it takes a toll. We, as parents or providers, may not fully recognize how children experience this bullying, and those affected may not be able to describe it. They then withdraw, not finding anyone to share their feelings and experiences with, to cope. Some of these kids live as loners in school, and around ages 16 to 25, this lifestyle can become intolerable. They may become depressed from the emotional pain, seek revenge, and wish to die.
The desire to die—and to get lots of attention from it—is recognized in many adolescents. Lonely, troubled teens imagine what others will say about them when they are dead, and think of themselves looking down from heaven or somewhere, watching the grief and surprise overtake their peers.
Continue to: To take out others with themselves...
To take out others with themselves makes dying less of a lonely act. Killing themselves and either people they hate or someone they love, to keep them company in the afterlife or to make sure they both suffer together, both make some sense. And both are tragic.
We need to better identify these lonely teens and help them find a friend. They need regular social interaction and monitoring by the same people daily. Red flags often exist. If they are depressed, more intense care and monitoring are needed.
2. The glorification of guns stems from the need of every civilization for warriors to protect them from invaders. Soldiers, war, and weapons of destruction thus become known—to young men, in particular—because there is a need for youth to join the military and train, even if just for a few years. We honor brave soldiers who die in the line of duty.
Some of these men develop expertise in the use of military machine guns—rapid-fire weapons that can kill many people from afar in minutes. They may wish to continue using their skills upon separating from the military; they want military-grade guns at home. This makes sense, for they mastered and practiced this skill for years and can handle guns safely.
Gun shows allow the purchase of military-grade weapons fairly easily in many states. In the right hands, no problem. But our laws about guns are minimal and do not check to ensure that the buyer is one of these healthy, normal, skilled men.
Continue to: This accessibility is compounded with...
This accessibility is compounded with the popularity of violent video games among teens and young adults, which often involve shooting animated characters and animals in realistic scenarios. There is apparently a sense of adventure, satisfaction, and power that comes from this.
The desire to hunt in the real world, with real weapons, for a heightened sense of accomplishment or power as a follow-up to this fantastical activity seems a normal progression of behavior. Shooting ranges, wild animal hunting farms, and deer hunting in autumn are usual and customary ways to fulfill this desire. Families in western New York, where I live, often teach their sons and daughters to use a gun starting at age 12 and take them on annual hunting trips. This is not a problem.
When a teen or young person becomes despondent and lonely, the feelings of power and satisfaction from hunting or killing something can act as an antidote to the negative feelings. If anger or jealousy are part of the problem, the weapon can be turned on the perceived source of those feelings. The shooters in these devastating massacres seem to be able to use their chosen weapon competently. Where does this familiarity come from? Often, the weapon was accessible in their own home.
Teens and young adults with depression or feelings of loneliness, anger, or jealousy should be evaluated for access to weapons, and for their knowledge of use of these weapons, as part of the care plan. Parents and other adults should be queried and warned to keep weapons away from them until their mood has stabilized. There is reluctance to do this in our society. Health care and law enforcement professionals need to put out public announcements that give this warning to everyone.
Alone, these two ideas I’ve outlined will not prevent all mass shootings. But a more rapid reaction to the red flags of loneliness, mood changes, and social changes in a young person, and seeking to change that dynamic, might reduce the prevalence. Public health is at stake, and prevention will require a willingness to see and react to troubled people—before the police need to be called for a crime.
Sandra Glantz, MSN, MPA, FNP-BC
Pittsford, NY
I read your editorial with appreciation this morning. In my practice, I have worked with adolescents and young adults for more than 30 years, so the fact that many mass shooters are so young has caught my attention.
Of course, there is never just one cause for such horrific actions. But it seems to me that there are two recurring themes in these situations: (1) many shooters are socially isolated and (2) in our culture, the use of guns is glorified as a representation of power and a source of satisfaction. Here are my thoughts on each:
1. Shooters are often loners who exhibit behavior that is deviant or in some way socially unacceptable; this varies from public masturbation or the voicing of scary thoughts and ideas to simply exhibiting social awkwardness. When the lonely, shunned child has access to powerful, multiple-shot weapons, this is a setup for a shooting.
Perhaps society inadvertently causes harm by forcing classroom integration. About 30 years ago, with the hope to normalize abnormal social interactions and to help students understand and accept those who are different from them, schools began to mainstream students with serious learning difficulties, including students with mental illnesses. These children lost their “group,” their classroom community of those similar to themselves. They are still assigned personalized curricula and work one-on-one with special education teachers, but they do not have much of a peer group anymore. These students are put with a group of students—normal kids—that frequently rejects them.
Part of the problem has to do with the demonization of the term “normal”; it is now politically incorrect to designate a student as normal or not, despite the persistence of the bell-shaped curve. If normal, nonviolent behavior is desired, we need to be able to name abnormal behaviors as such.
Parents, understandably, want this type of social integration for their child. But when these students are bullied throughout the day, it takes a toll. We, as parents or providers, may not fully recognize how children experience this bullying, and those affected may not be able to describe it. They then withdraw, not finding anyone to share their feelings and experiences with, to cope. Some of these kids live as loners in school, and around ages 16 to 25, this lifestyle can become intolerable. They may become depressed from the emotional pain, seek revenge, and wish to die.
The desire to die—and to get lots of attention from it—is recognized in many adolescents. Lonely, troubled teens imagine what others will say about them when they are dead, and think of themselves looking down from heaven or somewhere, watching the grief and surprise overtake their peers.
Continue to: To take out others with themselves...
To take out others with themselves makes dying less of a lonely act. Killing themselves and either people they hate or someone they love, to keep them company in the afterlife or to make sure they both suffer together, both make some sense. And both are tragic.
We need to better identify these lonely teens and help them find a friend. They need regular social interaction and monitoring by the same people daily. Red flags often exist. If they are depressed, more intense care and monitoring are needed.
2. The glorification of guns stems from the need of every civilization for warriors to protect them from invaders. Soldiers, war, and weapons of destruction thus become known—to young men, in particular—because there is a need for youth to join the military and train, even if just for a few years. We honor brave soldiers who die in the line of duty.
Some of these men develop expertise in the use of military machine guns—rapid-fire weapons that can kill many people from afar in minutes. They may wish to continue using their skills upon separating from the military; they want military-grade guns at home. This makes sense, for they mastered and practiced this skill for years and can handle guns safely.
Gun shows allow the purchase of military-grade weapons fairly easily in many states. In the right hands, no problem. But our laws about guns are minimal and do not check to ensure that the buyer is one of these healthy, normal, skilled men.
Continue to: This accessibility is compounded with...
This accessibility is compounded with the popularity of violent video games among teens and young adults, which often involve shooting animated characters and animals in realistic scenarios. There is apparently a sense of adventure, satisfaction, and power that comes from this.
The desire to hunt in the real world, with real weapons, for a heightened sense of accomplishment or power as a follow-up to this fantastical activity seems a normal progression of behavior. Shooting ranges, wild animal hunting farms, and deer hunting in autumn are usual and customary ways to fulfill this desire. Families in western New York, where I live, often teach their sons and daughters to use a gun starting at age 12 and take them on annual hunting trips. This is not a problem.
When a teen or young person becomes despondent and lonely, the feelings of power and satisfaction from hunting or killing something can act as an antidote to the negative feelings. If anger or jealousy are part of the problem, the weapon can be turned on the perceived source of those feelings. The shooters in these devastating massacres seem to be able to use their chosen weapon competently. Where does this familiarity come from? Often, the weapon was accessible in their own home.
Teens and young adults with depression or feelings of loneliness, anger, or jealousy should be evaluated for access to weapons, and for their knowledge of use of these weapons, as part of the care plan. Parents and other adults should be queried and warned to keep weapons away from them until their mood has stabilized. There is reluctance to do this in our society. Health care and law enforcement professionals need to put out public announcements that give this warning to everyone.
Alone, these two ideas I’ve outlined will not prevent all mass shootings. But a more rapid reaction to the red flags of loneliness, mood changes, and social changes in a young person, and seeking to change that dynamic, might reduce the prevalence. Public health is at stake, and prevention will require a willingness to see and react to troubled people—before the police need to be called for a crime.
Sandra Glantz, MSN, MPA, FNP-BC
Pittsford, NY
I read your editorial with appreciation this morning. In my practice, I have worked with adolescents and young adults for more than 30 years, so the fact that many mass shooters are so young has caught my attention.
Of course, there is never just one cause for such horrific actions. But it seems to me that there are two recurring themes in these situations: (1) many shooters are socially isolated and (2) in our culture, the use of guns is glorified as a representation of power and a source of satisfaction. Here are my thoughts on each:
1. Shooters are often loners who exhibit behavior that is deviant or in some way socially unacceptable; this varies from public masturbation or the voicing of scary thoughts and ideas to simply exhibiting social awkwardness. When the lonely, shunned child has access to powerful, multiple-shot weapons, this is a setup for a shooting.
Perhaps society inadvertently causes harm by forcing classroom integration. About 30 years ago, with the hope to normalize abnormal social interactions and to help students understand and accept those who are different from them, schools began to mainstream students with serious learning difficulties, including students with mental illnesses. These children lost their “group,” their classroom community of those similar to themselves. They are still assigned personalized curricula and work one-on-one with special education teachers, but they do not have much of a peer group anymore. These students are put with a group of students—normal kids—that frequently rejects them.
Part of the problem has to do with the demonization of the term “normal”; it is now politically incorrect to designate a student as normal or not, despite the persistence of the bell-shaped curve. If normal, nonviolent behavior is desired, we need to be able to name abnormal behaviors as such.
Parents, understandably, want this type of social integration for their child. But when these students are bullied throughout the day, it takes a toll. We, as parents or providers, may not fully recognize how children experience this bullying, and those affected may not be able to describe it. They then withdraw, not finding anyone to share their feelings and experiences with, to cope. Some of these kids live as loners in school, and around ages 16 to 25, this lifestyle can become intolerable. They may become depressed from the emotional pain, seek revenge, and wish to die.
The desire to die—and to get lots of attention from it—is recognized in many adolescents. Lonely, troubled teens imagine what others will say about them when they are dead, and think of themselves looking down from heaven or somewhere, watching the grief and surprise overtake their peers.
Continue to: To take out others with themselves...
To take out others with themselves makes dying less of a lonely act. Killing themselves and either people they hate or someone they love, to keep them company in the afterlife or to make sure they both suffer together, both make some sense. And both are tragic.
We need to better identify these lonely teens and help them find a friend. They need regular social interaction and monitoring by the same people daily. Red flags often exist. If they are depressed, more intense care and monitoring are needed.
2. The glorification of guns stems from the need of every civilization for warriors to protect them from invaders. Soldiers, war, and weapons of destruction thus become known—to young men, in particular—because there is a need for youth to join the military and train, even if just for a few years. We honor brave soldiers who die in the line of duty.
Some of these men develop expertise in the use of military machine guns—rapid-fire weapons that can kill many people from afar in minutes. They may wish to continue using their skills upon separating from the military; they want military-grade guns at home. This makes sense, for they mastered and practiced this skill for years and can handle guns safely.
Gun shows allow the purchase of military-grade weapons fairly easily in many states. In the right hands, no problem. But our laws about guns are minimal and do not check to ensure that the buyer is one of these healthy, normal, skilled men.
Continue to: This accessibility is compounded with...
This accessibility is compounded with the popularity of violent video games among teens and young adults, which often involve shooting animated characters and animals in realistic scenarios. There is apparently a sense of adventure, satisfaction, and power that comes from this.
The desire to hunt in the real world, with real weapons, for a heightened sense of accomplishment or power as a follow-up to this fantastical activity seems a normal progression of behavior. Shooting ranges, wild animal hunting farms, and deer hunting in autumn are usual and customary ways to fulfill this desire. Families in western New York, where I live, often teach their sons and daughters to use a gun starting at age 12 and take them on annual hunting trips. This is not a problem.
When a teen or young person becomes despondent and lonely, the feelings of power and satisfaction from hunting or killing something can act as an antidote to the negative feelings. If anger or jealousy are part of the problem, the weapon can be turned on the perceived source of those feelings. The shooters in these devastating massacres seem to be able to use their chosen weapon competently. Where does this familiarity come from? Often, the weapon was accessible in their own home.
Teens and young adults with depression or feelings of loneliness, anger, or jealousy should be evaluated for access to weapons, and for their knowledge of use of these weapons, as part of the care plan. Parents and other adults should be queried and warned to keep weapons away from them until their mood has stabilized. There is reluctance to do this in our society. Health care and law enforcement professionals need to put out public announcements that give this warning to everyone.
Alone, these two ideas I’ve outlined will not prevent all mass shootings. But a more rapid reaction to the red flags of loneliness, mood changes, and social changes in a young person, and seeking to change that dynamic, might reduce the prevalence. Public health is at stake, and prevention will require a willingness to see and react to troubled people—before the police need to be called for a crime.
Sandra Glantz, MSN, MPA, FNP-BC
Pittsford, NY
What are the benefits/risks of giving betamethasone to women at risk of late preterm labor?
EVIDENCE SUMMARY
A 2016 systematic review and meta-analysis of 3 RCTs that included 3200 women with late preterm labor (between 34 weeks 0 days and 36 weeks 6 days) found that women who were given betamethasone had a significantly lower incidence of transient tachypnea of the newborn (number needed to treat [NNT]=37; relative risk [RR]=0.72; 95% confidence interval [CI], 0.56-0.92), severe respiratory distress syndrome (NNT=114; RR=0.60; 95% CI, 0.33-0.94), and use of surfactant (NNT=92; RR=0.61; 95% CI, 0.38-0.99).1
A composite outcome measure also favors betamethasone
In addition to these 3 outcomes, the largest RCT in the meta-analysis evaluated a composite outcome and found that betamethasone improved it by 20%. The RCT, comparing 1427 women in the experimental arm with 1400 controls, found benefit to administering 12 mg betamethasone intramuscularly every 24 hours for 2 days for women at high risk of late preterm delivery.2 Enrollment criteria included women with 3 cm dilation or 75% effacement, preterm premature rupture of membranes, or a planned delivery scheduled in the late preterm period.
The primary outcome was a composite score based on one or more of the following within 72 hours after birth: continuous positive airway pressure or high-flow nasal cannula for at least 2 continuous hours, supplemental oxygen with a fraction of inspired oxygen of 0.30 or more for at least 4 continuous hours, mechanical ventilation, stillbirth or neonatal death, or the need for extracorporeal circulation membrane oxygenation. The betamethasone group had 165 women (11.6%) with the primary outcome compared with 202 (14.4%) in the control arm (NNT=34; RR=0.80; 95% CI, 0.66–0.97; P<.02).
Neonatal hypoglycemia may increase, but not dangerously
The same RCT explored the risks of late preterm betamethasone. There was no increase in chorioamnionitis nor neonatal sepsis in the betamethasone group.2 Although neonatal hypoglycemia increased (24% vs 15%; number needed to harm=11.1; RR=1.60; 95% CI, 1.37-1.87; P<.001), no increase was seen in intermediate care nursery or ICU stays (41.8% vs 44.9%; RR=0.93; 95% CI, 0.85-1.01; P=.09) nor length of hospital stay (7 vs 8 days; P=.20).
Three letters to the editor questioned whether hypoglycemia from late-term corticosteroids may lead to long-term neurocognitive delays.3 The authors responded that meta-analyses of RCTs haven’t found an association between antenatal steroid use and neurocognitive delay and that studies that have found an association between hypoglycemia and neurocognitive delay looked at profound and prolonged hypoglycemia, which wasn’t seen in the late preterm betamethasone study.
Continue to: RECOMMENDATIONS
RECOMMENDATIONS
Both the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine have published recommendations supporting corticosteroids for threatened late preterm delivery with certain caveats.4-6 Because of a lack of evidence, maternity care providers shouldn’t give corticosteroids for threatened late preterm delivery to women with multiple gestation, diabetes, previous exposure to steroids during pregnancy, or pregnancies with major nonlethal fetal malformations.4-6 Evidence doesn’t support tocolysis when steroids are given in the late preterm period.4,5
1. Saccone G, Berghella V. Antenatal corticosteroids for maturity of term or near term fetuses: systematic review and meta-analysis of randomized controlled trials. BMJ. 2016;355:5044.
2. Gyamfi-Bannerman C, Thom E, Blackwell S, et al. Antenatal betamethasone for women at risk for late preterm delivery. N Engl J Med. 2016;374:1311-1320.
3. Gyamfi-Bannerman C, Thom E. Antenatal betamethasone for women at risk for late preterm delivery. N Engl J Med. 2016;375:486-487.
4. American College of Obstetricians and Gynecologists. Committee Opinion No. 677: Antenatal Corticosteroid Therapy for Fetal Maturation. Obstet Gynecol. 2016;128:e187-e194.
5. Society for Maternal-Fetal Medicine (SMFM) Publications Committee. Implementation of the use of antenatal corticosteroids in the late preterm birth period in women at risk for preterm delivery. Am J Obstet Gynecol. 2016;215:B13-B15.
6. American College of Obstetricians and Gynecologists. Practice Bulletin No. 159: Management of Preterm Labor. Obstet Gynecol. 2016;127:e29-e38.
EVIDENCE SUMMARY
A 2016 systematic review and meta-analysis of 3 RCTs that included 3200 women with late preterm labor (between 34 weeks 0 days and 36 weeks 6 days) found that women who were given betamethasone had a significantly lower incidence of transient tachypnea of the newborn (number needed to treat [NNT]=37; relative risk [RR]=0.72; 95% confidence interval [CI], 0.56-0.92), severe respiratory distress syndrome (NNT=114; RR=0.60; 95% CI, 0.33-0.94), and use of surfactant (NNT=92; RR=0.61; 95% CI, 0.38-0.99).1
A composite outcome measure also favors betamethasone
In addition to these 3 outcomes, the largest RCT in the meta-analysis evaluated a composite outcome and found that betamethasone improved it by 20%. The RCT, comparing 1427 women in the experimental arm with 1400 controls, found benefit to administering 12 mg betamethasone intramuscularly every 24 hours for 2 days for women at high risk of late preterm delivery.2 Enrollment criteria included women with 3 cm dilation or 75% effacement, preterm premature rupture of membranes, or a planned delivery scheduled in the late preterm period.
The primary outcome was a composite score based on one or more of the following within 72 hours after birth: continuous positive airway pressure or high-flow nasal cannula for at least 2 continuous hours, supplemental oxygen with a fraction of inspired oxygen of 0.30 or more for at least 4 continuous hours, mechanical ventilation, stillbirth or neonatal death, or the need for extracorporeal circulation membrane oxygenation. The betamethasone group had 165 women (11.6%) with the primary outcome compared with 202 (14.4%) in the control arm (NNT=34; RR=0.80; 95% CI, 0.66–0.97; P<.02).
Neonatal hypoglycemia may increase, but not dangerously
The same RCT explored the risks of late preterm betamethasone. There was no increase in chorioamnionitis nor neonatal sepsis in the betamethasone group.2 Although neonatal hypoglycemia increased (24% vs 15%; number needed to harm=11.1; RR=1.60; 95% CI, 1.37-1.87; P<.001), no increase was seen in intermediate care nursery or ICU stays (41.8% vs 44.9%; RR=0.93; 95% CI, 0.85-1.01; P=.09) nor length of hospital stay (7 vs 8 days; P=.20).
Three letters to the editor questioned whether hypoglycemia from late-term corticosteroids may lead to long-term neurocognitive delays.3 The authors responded that meta-analyses of RCTs haven’t found an association between antenatal steroid use and neurocognitive delay and that studies that have found an association between hypoglycemia and neurocognitive delay looked at profound and prolonged hypoglycemia, which wasn’t seen in the late preterm betamethasone study.
Continue to: RECOMMENDATIONS
RECOMMENDATIONS
Both the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine have published recommendations supporting corticosteroids for threatened late preterm delivery with certain caveats.4-6 Because of a lack of evidence, maternity care providers shouldn’t give corticosteroids for threatened late preterm delivery to women with multiple gestation, diabetes, previous exposure to steroids during pregnancy, or pregnancies with major nonlethal fetal malformations.4-6 Evidence doesn’t support tocolysis when steroids are given in the late preterm period.4,5
EVIDENCE SUMMARY
A 2016 systematic review and meta-analysis of 3 RCTs that included 3200 women with late preterm labor (between 34 weeks 0 days and 36 weeks 6 days) found that women who were given betamethasone had a significantly lower incidence of transient tachypnea of the newborn (number needed to treat [NNT]=37; relative risk [RR]=0.72; 95% confidence interval [CI], 0.56-0.92), severe respiratory distress syndrome (NNT=114; RR=0.60; 95% CI, 0.33-0.94), and use of surfactant (NNT=92; RR=0.61; 95% CI, 0.38-0.99).1
A composite outcome measure also favors betamethasone
In addition to these 3 outcomes, the largest RCT in the meta-analysis evaluated a composite outcome and found that betamethasone improved it by 20%. The RCT, comparing 1427 women in the experimental arm with 1400 controls, found benefit to administering 12 mg betamethasone intramuscularly every 24 hours for 2 days for women at high risk of late preterm delivery.2 Enrollment criteria included women with 3 cm dilation or 75% effacement, preterm premature rupture of membranes, or a planned delivery scheduled in the late preterm period.
The primary outcome was a composite score based on one or more of the following within 72 hours after birth: continuous positive airway pressure or high-flow nasal cannula for at least 2 continuous hours, supplemental oxygen with a fraction of inspired oxygen of 0.30 or more for at least 4 continuous hours, mechanical ventilation, stillbirth or neonatal death, or the need for extracorporeal circulation membrane oxygenation. The betamethasone group had 165 women (11.6%) with the primary outcome compared with 202 (14.4%) in the control arm (NNT=34; RR=0.80; 95% CI, 0.66–0.97; P<.02).
Neonatal hypoglycemia may increase, but not dangerously
The same RCT explored the risks of late preterm betamethasone. There was no increase in chorioamnionitis nor neonatal sepsis in the betamethasone group.2 Although neonatal hypoglycemia increased (24% vs 15%; number needed to harm=11.1; RR=1.60; 95% CI, 1.37-1.87; P<.001), no increase was seen in intermediate care nursery or ICU stays (41.8% vs 44.9%; RR=0.93; 95% CI, 0.85-1.01; P=.09) nor length of hospital stay (7 vs 8 days; P=.20).
Three letters to the editor questioned whether hypoglycemia from late-term corticosteroids may lead to long-term neurocognitive delays.3 The authors responded that meta-analyses of RCTs haven’t found an association between antenatal steroid use and neurocognitive delay and that studies that have found an association between hypoglycemia and neurocognitive delay looked at profound and prolonged hypoglycemia, which wasn’t seen in the late preterm betamethasone study.
Continue to: RECOMMENDATIONS
RECOMMENDATIONS
Both the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine have published recommendations supporting corticosteroids for threatened late preterm delivery with certain caveats.4-6 Because of a lack of evidence, maternity care providers shouldn’t give corticosteroids for threatened late preterm delivery to women with multiple gestation, diabetes, previous exposure to steroids during pregnancy, or pregnancies with major nonlethal fetal malformations.4-6 Evidence doesn’t support tocolysis when steroids are given in the late preterm period.4,5
1. Saccone G, Berghella V. Antenatal corticosteroids for maturity of term or near term fetuses: systematic review and meta-analysis of randomized controlled trials. BMJ. 2016;355:5044.
2. Gyamfi-Bannerman C, Thom E, Blackwell S, et al. Antenatal betamethasone for women at risk for late preterm delivery. N Engl J Med. 2016;374:1311-1320.
3. Gyamfi-Bannerman C, Thom E. Antenatal betamethasone for women at risk for late preterm delivery. N Engl J Med. 2016;375:486-487.
4. American College of Obstetricians and Gynecologists. Committee Opinion No. 677: Antenatal Corticosteroid Therapy for Fetal Maturation. Obstet Gynecol. 2016;128:e187-e194.
5. Society for Maternal-Fetal Medicine (SMFM) Publications Committee. Implementation of the use of antenatal corticosteroids in the late preterm birth period in women at risk for preterm delivery. Am J Obstet Gynecol. 2016;215:B13-B15.
6. American College of Obstetricians and Gynecologists. Practice Bulletin No. 159: Management of Preterm Labor. Obstet Gynecol. 2016;127:e29-e38.
1. Saccone G, Berghella V. Antenatal corticosteroids for maturity of term or near term fetuses: systematic review and meta-analysis of randomized controlled trials. BMJ. 2016;355:5044.
2. Gyamfi-Bannerman C, Thom E, Blackwell S, et al. Antenatal betamethasone for women at risk for late preterm delivery. N Engl J Med. 2016;374:1311-1320.
3. Gyamfi-Bannerman C, Thom E. Antenatal betamethasone for women at risk for late preterm delivery. N Engl J Med. 2016;375:486-487.
4. American College of Obstetricians and Gynecologists. Committee Opinion No. 677: Antenatal Corticosteroid Therapy for Fetal Maturation. Obstet Gynecol. 2016;128:e187-e194.
5. Society for Maternal-Fetal Medicine (SMFM) Publications Committee. Implementation of the use of antenatal corticosteroids in the late preterm birth period in women at risk for preterm delivery. Am J Obstet Gynecol. 2016;215:B13-B15.
6. American College of Obstetricians and Gynecologists. Practice Bulletin No. 159: Management of Preterm Labor. Obstet Gynecol. 2016;127:e29-e38.
EVIDENCE-BASED ANSWER:
Giving betamethasone to women at risk for delivery between 34 weeks 0 days and 36 weeks 6 days can lower by almost 40% the incidence of transient tachypnea of the newborn, severe respiratory distress syndrome, and the use of surfactant (strength of recommendation [SOR]: A, systematic review of randomized controlled trials [RCTs]).
Betamethasone may increase neonatal hypoglycemia, but the hypoglycemia isn’t associated with a prolonged hospital stay or other negative outcomes.
Can CBT effectively treat adult insomnia disorder?
EVIDENCE SUMMARY
Three meta-analyses that included only randomized controlled trials (RCTs) compared various CBT delivery methods with controls (wait-listed for treatment or general sleep hygiene education) to assess sleep outcomes for adults with insomnia.1-3 TABLE 11-3 summarizes the results.

CBT is comparable to pharmacotherapy
A 2002 comparative meta-analysis of 21 RCTs with a total of 470 patients examined the effectiveness of CBT (stimulus control and/or sleep restriction) compared with pharmacotherapy (benzodiazepines or benzodiazepine agonists) for treating primary insomnia of longer than one month’s duration in adults with no comorbid medical or psychiatric diagnoses.4 The CBT group received intervention over an average of 5 weeks, and the pharmacotherapy group received intervention over an average of 2 weeks.
CBT produced greater reductions in sleep onset latency than pharmacotherapy based on mean weighted effect size (1.05 vs 0.45 weighted effect size; 95% confidence interval, 0.17-1.04; P=.01). Although both CBT and pharmacotherapy improved sleep outcomes, no statistical differences were found in wake after sleep onset time, total sleep time, number of awakenings, or sleep quality ratings (TABLE 24).

Continue to: CBT has significant benefit for comorbid insomnia
CBT has significant benefit for comorbid insomnia
A 2015 meta-analysis of 23 studies enrolling a total of 1379 adults with a number of illnesses (chronic pain, alcohol dependence, breast cancer, psychiatric disorders, chronic obstructive pulmonary disease, fibromyalgia) and comorbid insomnia investigated the qualitative effectiveness of individual or group CBT therapy.5 Subjects received at least 4 face-to-face sessions and at least 2 components of CBT.
The primary outcome showed that sleep quality improved, as measured by a 6.36-point reduction in the Insomnia Severity Index (ISI; a 7-question scale on which 0=no insomnia and 28=severe insomnia) and a 3.3-point reduction in the Pittsburgh Sleep Quality Index (PSQI; a 7-category assessment tool on which 0=perfect quality and 21=poor quality). The effect size was large for both ISI and PSQI, as indicated by standard mean differences greater than 0.8 (1.22 and 0.88, respectively) and was sustained for as long as 18 months.
RECOMMENDATIONS
The American College of Physicians strongly recommends that all adult patients receive CBT as initial treatment for chronic insomnia disorder. It can be performed in multiple settings, including the primary care setting. Compared with hypnotics, CBT is unlikely to have any adverse effects.6
1. Trauer J, Qian M, Doyle J, et al. Cognitive behavioral therapy for chronic insomnia: a systematic review and meta-analysis. Ann Intern Med. 2015;163:191-204.
2. Koffel E, Koffel J, Gehrman P. A meta-analysis of group cognitive behavioral therapy for insomnia. Sleep Med Rev. 2015;19:6-16.
3. Ye Y, Chen N, Chen J, et al. Internet-based cognitive-behavioral therapy for insomnia (ICBT-i): a meta-analysis of randomized controlled trials. BMJ Open. 2016;6:e010707.
4. Smith M, Perlis M, Park S, et al. Comparative meta-analysis of pharmacotherapy and behavior therapy for persistent insomnia. Am J Psychiatry. 2002;159:5-11.
5. Geiger-Brown J, Rogers V, Liu W, et al. Cognitive behavioral therapy in persons with comorbid insomnia: a meta-analysis. Sleep Med Rev. 2015;23:54-67.
6. Qaseem A, Kansagara D, Forciea M, et al. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165:125-133.
EVIDENCE SUMMARY
Three meta-analyses that included only randomized controlled trials (RCTs) compared various CBT delivery methods with controls (wait-listed for treatment or general sleep hygiene education) to assess sleep outcomes for adults with insomnia.1-3 TABLE 11-3 summarizes the results.

CBT is comparable to pharmacotherapy
A 2002 comparative meta-analysis of 21 RCTs with a total of 470 patients examined the effectiveness of CBT (stimulus control and/or sleep restriction) compared with pharmacotherapy (benzodiazepines or benzodiazepine agonists) for treating primary insomnia of longer than one month’s duration in adults with no comorbid medical or psychiatric diagnoses.4 The CBT group received intervention over an average of 5 weeks, and the pharmacotherapy group received intervention over an average of 2 weeks.
CBT produced greater reductions in sleep onset latency than pharmacotherapy based on mean weighted effect size (1.05 vs 0.45 weighted effect size; 95% confidence interval, 0.17-1.04; P=.01). Although both CBT and pharmacotherapy improved sleep outcomes, no statistical differences were found in wake after sleep onset time, total sleep time, number of awakenings, or sleep quality ratings (TABLE 24).

Continue to: CBT has significant benefit for comorbid insomnia
CBT has significant benefit for comorbid insomnia
A 2015 meta-analysis of 23 studies enrolling a total of 1379 adults with a number of illnesses (chronic pain, alcohol dependence, breast cancer, psychiatric disorders, chronic obstructive pulmonary disease, fibromyalgia) and comorbid insomnia investigated the qualitative effectiveness of individual or group CBT therapy.5 Subjects received at least 4 face-to-face sessions and at least 2 components of CBT.
The primary outcome showed that sleep quality improved, as measured by a 6.36-point reduction in the Insomnia Severity Index (ISI; a 7-question scale on which 0=no insomnia and 28=severe insomnia) and a 3.3-point reduction in the Pittsburgh Sleep Quality Index (PSQI; a 7-category assessment tool on which 0=perfect quality and 21=poor quality). The effect size was large for both ISI and PSQI, as indicated by standard mean differences greater than 0.8 (1.22 and 0.88, respectively) and was sustained for as long as 18 months.
RECOMMENDATIONS
The American College of Physicians strongly recommends that all adult patients receive CBT as initial treatment for chronic insomnia disorder. It can be performed in multiple settings, including the primary care setting. Compared with hypnotics, CBT is unlikely to have any adverse effects.6
EVIDENCE SUMMARY
Three meta-analyses that included only randomized controlled trials (RCTs) compared various CBT delivery methods with controls (wait-listed for treatment or general sleep hygiene education) to assess sleep outcomes for adults with insomnia.1-3 TABLE 11-3 summarizes the results.

CBT is comparable to pharmacotherapy
A 2002 comparative meta-analysis of 21 RCTs with a total of 470 patients examined the effectiveness of CBT (stimulus control and/or sleep restriction) compared with pharmacotherapy (benzodiazepines or benzodiazepine agonists) for treating primary insomnia of longer than one month’s duration in adults with no comorbid medical or psychiatric diagnoses.4 The CBT group received intervention over an average of 5 weeks, and the pharmacotherapy group received intervention over an average of 2 weeks.
CBT produced greater reductions in sleep onset latency than pharmacotherapy based on mean weighted effect size (1.05 vs 0.45 weighted effect size; 95% confidence interval, 0.17-1.04; P=.01). Although both CBT and pharmacotherapy improved sleep outcomes, no statistical differences were found in wake after sleep onset time, total sleep time, number of awakenings, or sleep quality ratings (TABLE 24).

Continue to: CBT has significant benefit for comorbid insomnia
CBT has significant benefit for comorbid insomnia
A 2015 meta-analysis of 23 studies enrolling a total of 1379 adults with a number of illnesses (chronic pain, alcohol dependence, breast cancer, psychiatric disorders, chronic obstructive pulmonary disease, fibromyalgia) and comorbid insomnia investigated the qualitative effectiveness of individual or group CBT therapy.5 Subjects received at least 4 face-to-face sessions and at least 2 components of CBT.
The primary outcome showed that sleep quality improved, as measured by a 6.36-point reduction in the Insomnia Severity Index (ISI; a 7-question scale on which 0=no insomnia and 28=severe insomnia) and a 3.3-point reduction in the Pittsburgh Sleep Quality Index (PSQI; a 7-category assessment tool on which 0=perfect quality and 21=poor quality). The effect size was large for both ISI and PSQI, as indicated by standard mean differences greater than 0.8 (1.22 and 0.88, respectively) and was sustained for as long as 18 months.
RECOMMENDATIONS
The American College of Physicians strongly recommends that all adult patients receive CBT as initial treatment for chronic insomnia disorder. It can be performed in multiple settings, including the primary care setting. Compared with hypnotics, CBT is unlikely to have any adverse effects.6
1. Trauer J, Qian M, Doyle J, et al. Cognitive behavioral therapy for chronic insomnia: a systematic review and meta-analysis. Ann Intern Med. 2015;163:191-204.
2. Koffel E, Koffel J, Gehrman P. A meta-analysis of group cognitive behavioral therapy for insomnia. Sleep Med Rev. 2015;19:6-16.
3. Ye Y, Chen N, Chen J, et al. Internet-based cognitive-behavioral therapy for insomnia (ICBT-i): a meta-analysis of randomized controlled trials. BMJ Open. 2016;6:e010707.
4. Smith M, Perlis M, Park S, et al. Comparative meta-analysis of pharmacotherapy and behavior therapy for persistent insomnia. Am J Psychiatry. 2002;159:5-11.
5. Geiger-Brown J, Rogers V, Liu W, et al. Cognitive behavioral therapy in persons with comorbid insomnia: a meta-analysis. Sleep Med Rev. 2015;23:54-67.
6. Qaseem A, Kansagara D, Forciea M, et al. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165:125-133.
1. Trauer J, Qian M, Doyle J, et al. Cognitive behavioral therapy for chronic insomnia: a systematic review and meta-analysis. Ann Intern Med. 2015;163:191-204.
2. Koffel E, Koffel J, Gehrman P. A meta-analysis of group cognitive behavioral therapy for insomnia. Sleep Med Rev. 2015;19:6-16.
3. Ye Y, Chen N, Chen J, et al. Internet-based cognitive-behavioral therapy for insomnia (ICBT-i): a meta-analysis of randomized controlled trials. BMJ Open. 2016;6:e010707.
4. Smith M, Perlis M, Park S, et al. Comparative meta-analysis of pharmacotherapy and behavior therapy for persistent insomnia. Am J Psychiatry. 2002;159:5-11.
5. Geiger-Brown J, Rogers V, Liu W, et al. Cognitive behavioral therapy in persons with comorbid insomnia: a meta-analysis. Sleep Med Rev. 2015;23:54-67.
6. Qaseem A, Kansagara D, Forciea M, et al. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165:125-133.
EVIDENCE-BASED ANSWER:
Yes. Cognitive behavioral therapy (CBT) administered individually, in a group setting, or on the internet is effective for treating insomnia in adults compared with control (strength of recommendation [SOR]: A, meta-analyses).
CBT is comparable to pharmacotherapy for improving measures of sleep (SOR: A, comparative meta-analysis).
CBT produces sustainable improvements in subjective sleep quality for adults with comorbid insomnia (SOR: A, meta-analysis).
Pain in right shoulder
A 44-year-old African-American woman with sickle cell trait presented to the clinic to establish care. She complained of polyarthralgias that she’d had since adolescence; the pain was worst in her right shoulder. She reported morning stiffness that lasted up to 8 hours, an intermittent facial rash, oral ulcers, joint edema (of which she had pictures on her phone), and photosensitivity. She took ibuprofen and acetaminophen as needed for pain. She once worked as a medical assistant but hadn’t been able to work since 2014 due to pain. She reported having been told as a teenager that she might have juvenile arthritis, but she didn’t recall ever having diagnostic tests performed or receiving treatment other than anti-inflammatories.
A couple of weeks after an initial visit with a rheumatologist, the patient returned to the family medicine clinic. She said she was upset that the specialist had x-rayed her hands, but had not checked her shoulder, which was the primary source of her pain. She also had pain in her hands, hips, feet, and knees.
On physical exam, the patient looked fatigued. A musculoskeletal exam revealed no joint effusions or edema, but was significant for right shoulder pain with reduced abduction to 90°. Gross motor strength was 5/5 in all 4 extremities. Laboratory testing revealed an antinuclear antibody titer of 1:160 and was negative for double-stranded DNA. Bilateral hand and foot x-rays showed no joint erosions. An x-ray of the right shoulder was obtained, which showed evidence of osteopenia and an erosion in the humeral head (FIGURES 1A and 1B).
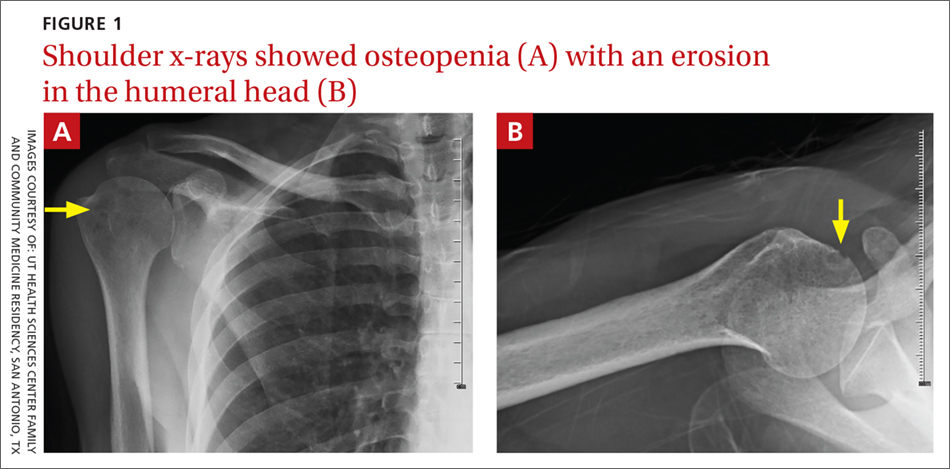
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Rheumatoid arthritis
The patient’s history of morning stiffness and the joint erosion observed on x-ray were highly suggestive of rheumatoid arthritis (RA).
RA is a symmetric, inflammatory, peripheral polyarthritis of unknown etiology. It is the most common form of inflammatory arthritis, affecting 1% of the population worldwide.1 It causes cartilage and bone to erode, leading to the deformation and destruction of joints. If RA is left untreated or is unresponsive to therapy, it can eventually lead to loss of physical function.
Making the diagnosis
The distinctive signs of RA are joint erosions and rheumatoid nodules, which are often absent on initial presentation.
Classification criteria. The 2010 American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) classification criteria for RA2 are based on the presence of synovitis in at least one joint, the absence of an alternative diagnosis that better explains the synovitis, and a cumulative score of at least 6/10 from the following 4 domains:
- Number and site of involved joints
- 2 to 10 large joints (shoulders, elbows, hips, knees, ankles)=1 point
- 1 to 3 small joints (metacarpophalangeal [MCP] joints, proximal interphalangeal [PIP] joints, 2nd-5th metatarsophalangeal joints, thumb interphalangeal joints, wrists)=2 points
- 4 to 10 small joints=3 points
- More than 10 joints (including at least 1 small joint)=5 points
- Serologic abnormality (rheumatoid factor [RF] and anti-cyclic citrullinated peptide)
- Low positive=2 points
- High positive=3 points
- Elevated acute phase response (erythrocyte sedimentation rate [ESR] or C-reactive protein [CRP])=1 point
- Symptom duration of at least 6 weeks=1 point.
These criteria are best suited for early disease. For patients with longstanding symptoms, diagnosis is based on an erosive disease with a history of criteria fulfillment, or a currently inactive longstanding disease, with or without treatment, that has previously fulfilled the criteria.3
Continue to: The differential Dx is extensive
The differential Dx is extensive
The differential includes polyarthralgias such as viral polyarthritis, systemic rheumatic diseases, and osteoarthritis.
Viral polyarthritis is caused by rubella, parvovirus B194, alphaviruses, and hepatitis B. Symptoms can last from 3 days to several weeks, but rarely persist beyond 6 weeks; alphaviruses, however, can last 3 to 6 months.5 The common symptom triad includes fever, arthritis, and rash. Chikungunya is an example of an alphavirus that has become a global disease. Alphavirus arthritis can mimic seronegative RA and even satisfy the classification criteria for RA if the initial symptoms of fever and rash and history of travel to endemic regions are not appreciated.5
Systemic rheumatic diseases. Early RA may mimic the arthritis of systemic lupus erythematosus (SLE), Sjögren’s syndrome, dermatomyositis, or mixed connective tissue disease.6 In contrast to RA, these disorders generally have systemic features, such as rashes, dry mouth and eyes, myositis, or nephritis, and generate autobodies, which are not seen with RA. The CRP is often normal in patients with active SLE, even when the ESR is elevated.
Osteoarthritis (OA) can be confused with RA, particularly when small joints are involved. A thorough history helps elucidate the diagnosis. For example, OA of the fingers affects distal interphalangeal joints and is associated with Heberden’s nodes, while RA more commonly affects MCP and PIP joints. Swelling from OA is typically firm, while swelling due to RA is warm, boggy, and tender. Joint stiffness due to OA is worse with activity and generally lasts only a few minutes, while joint stiffness due to RA is worse at rest and lasts 30 minutes or more. X-rays show joint-space narrowing with OA, but no erosions or cysts. RF may be present at low levels in older patients with OA, while it is usually associated with high levels in patients with seropositive RA.
Continue to: Treat with disease-modifying antirheumatic drugs
Treat with disease-modifying antirheumatic drugs
Immediate treatment of RA with disease-modifying antirheumatic drugs (DMARDs) is important to achieve control of the disease and prevent disability.
Our patient. We ordered a purified protein derivative skin test in preparation for the patient to start therapy with a DMARD. Our patient followed up with Rheumatology and was started on indomethacin, with an initial dose of 25 mg bid. (DMARDs are first-line therapy; indomethacin is a second-line choice. In this case, the patient declined DMARDs after hearing they lowered the body’s ability to fight infection.) An RF level measured 6.74 IU/ml, which is within normal limits. The patient was subsequently lost to follow-up.
CORRESPONDENCE
Barbara Kiersz, DO, 6835 Austin Center Blvd., Austin, TX 78731; [email protected].
1. Rothschild BM, Turner KR, DeLuca MA. Symmetrical erosive peripheral polyarthritis in the Late Archaic Period of Alabama. Science. 1988;241:1498-1501.
2. Aletaha D, Neogi T, Silman AJ, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis. 2010;69:1580-1588.
3. Pincus T, Callahan LF. How many types of patients meet classification criteria for rheumatoid arthritis? J Rheumatol. 1994;21:1385-1389.
4. Smith CA, Woolf AD, Lenci M. Parvoviruses: infections and arthropathies. Rheum Dis Clin North Am. 1987;13:249-263.
5. Miner JJ, Aw-Yeang HX, Fox JM, et al. Chikungunya viral arthritis in the United States: a mimic of seronegative rheumatoid arthritis. Arthritis Rheumatol. 2015;67:1214-1220.
6. Cronin ME. Musculoskeletal manifestations of systemic lupus erythematosus. Rheum Dis Clin North Am. 1988;14:99-116.
A 44-year-old African-American woman with sickle cell trait presented to the clinic to establish care. She complained of polyarthralgias that she’d had since adolescence; the pain was worst in her right shoulder. She reported morning stiffness that lasted up to 8 hours, an intermittent facial rash, oral ulcers, joint edema (of which she had pictures on her phone), and photosensitivity. She took ibuprofen and acetaminophen as needed for pain. She once worked as a medical assistant but hadn’t been able to work since 2014 due to pain. She reported having been told as a teenager that she might have juvenile arthritis, but she didn’t recall ever having diagnostic tests performed or receiving treatment other than anti-inflammatories.
A couple of weeks after an initial visit with a rheumatologist, the patient returned to the family medicine clinic. She said she was upset that the specialist had x-rayed her hands, but had not checked her shoulder, which was the primary source of her pain. She also had pain in her hands, hips, feet, and knees.
On physical exam, the patient looked fatigued. A musculoskeletal exam revealed no joint effusions or edema, but was significant for right shoulder pain with reduced abduction to 90°. Gross motor strength was 5/5 in all 4 extremities. Laboratory testing revealed an antinuclear antibody titer of 1:160 and was negative for double-stranded DNA. Bilateral hand and foot x-rays showed no joint erosions. An x-ray of the right shoulder was obtained, which showed evidence of osteopenia and an erosion in the humeral head (FIGURES 1A and 1B).

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Rheumatoid arthritis
The patient’s history of morning stiffness and the joint erosion observed on x-ray were highly suggestive of rheumatoid arthritis (RA).
RA is a symmetric, inflammatory, peripheral polyarthritis of unknown etiology. It is the most common form of inflammatory arthritis, affecting 1% of the population worldwide.1 It causes cartilage and bone to erode, leading to the deformation and destruction of joints. If RA is left untreated or is unresponsive to therapy, it can eventually lead to loss of physical function.
Making the diagnosis
The distinctive signs of RA are joint erosions and rheumatoid nodules, which are often absent on initial presentation.
Classification criteria. The 2010 American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) classification criteria for RA2 are based on the presence of synovitis in at least one joint, the absence of an alternative diagnosis that better explains the synovitis, and a cumulative score of at least 6/10 from the following 4 domains:
- Number and site of involved joints
- 2 to 10 large joints (shoulders, elbows, hips, knees, ankles)=1 point
- 1 to 3 small joints (metacarpophalangeal [MCP] joints, proximal interphalangeal [PIP] joints, 2nd-5th metatarsophalangeal joints, thumb interphalangeal joints, wrists)=2 points
- 4 to 10 small joints=3 points
- More than 10 joints (including at least 1 small joint)=5 points
- Serologic abnormality (rheumatoid factor [RF] and anti-cyclic citrullinated peptide)
- Low positive=2 points
- High positive=3 points
- Elevated acute phase response (erythrocyte sedimentation rate [ESR] or C-reactive protein [CRP])=1 point
- Symptom duration of at least 6 weeks=1 point.
These criteria are best suited for early disease. For patients with longstanding symptoms, diagnosis is based on an erosive disease with a history of criteria fulfillment, or a currently inactive longstanding disease, with or without treatment, that has previously fulfilled the criteria.3
Continue to: The differential Dx is extensive
The differential Dx is extensive
The differential includes polyarthralgias such as viral polyarthritis, systemic rheumatic diseases, and osteoarthritis.
Viral polyarthritis is caused by rubella, parvovirus B194, alphaviruses, and hepatitis B. Symptoms can last from 3 days to several weeks, but rarely persist beyond 6 weeks; alphaviruses, however, can last 3 to 6 months.5 The common symptom triad includes fever, arthritis, and rash. Chikungunya is an example of an alphavirus that has become a global disease. Alphavirus arthritis can mimic seronegative RA and even satisfy the classification criteria for RA if the initial symptoms of fever and rash and history of travel to endemic regions are not appreciated.5
Systemic rheumatic diseases. Early RA may mimic the arthritis of systemic lupus erythematosus (SLE), Sjögren’s syndrome, dermatomyositis, or mixed connective tissue disease.6 In contrast to RA, these disorders generally have systemic features, such as rashes, dry mouth and eyes, myositis, or nephritis, and generate autobodies, which are not seen with RA. The CRP is often normal in patients with active SLE, even when the ESR is elevated.
Osteoarthritis (OA) can be confused with RA, particularly when small joints are involved. A thorough history helps elucidate the diagnosis. For example, OA of the fingers affects distal interphalangeal joints and is associated with Heberden’s nodes, while RA more commonly affects MCP and PIP joints. Swelling from OA is typically firm, while swelling due to RA is warm, boggy, and tender. Joint stiffness due to OA is worse with activity and generally lasts only a few minutes, while joint stiffness due to RA is worse at rest and lasts 30 minutes or more. X-rays show joint-space narrowing with OA, but no erosions or cysts. RF may be present at low levels in older patients with OA, while it is usually associated with high levels in patients with seropositive RA.
Continue to: Treat with disease-modifying antirheumatic drugs
Treat with disease-modifying antirheumatic drugs
Immediate treatment of RA with disease-modifying antirheumatic drugs (DMARDs) is important to achieve control of the disease and prevent disability.
Our patient. We ordered a purified protein derivative skin test in preparation for the patient to start therapy with a DMARD. Our patient followed up with Rheumatology and was started on indomethacin, with an initial dose of 25 mg bid. (DMARDs are first-line therapy; indomethacin is a second-line choice. In this case, the patient declined DMARDs after hearing they lowered the body’s ability to fight infection.) An RF level measured 6.74 IU/ml, which is within normal limits. The patient was subsequently lost to follow-up.
CORRESPONDENCE
Barbara Kiersz, DO, 6835 Austin Center Blvd., Austin, TX 78731; [email protected].
A 44-year-old African-American woman with sickle cell trait presented to the clinic to establish care. She complained of polyarthralgias that she’d had since adolescence; the pain was worst in her right shoulder. She reported morning stiffness that lasted up to 8 hours, an intermittent facial rash, oral ulcers, joint edema (of which she had pictures on her phone), and photosensitivity. She took ibuprofen and acetaminophen as needed for pain. She once worked as a medical assistant but hadn’t been able to work since 2014 due to pain. She reported having been told as a teenager that she might have juvenile arthritis, but she didn’t recall ever having diagnostic tests performed or receiving treatment other than anti-inflammatories.
A couple of weeks after an initial visit with a rheumatologist, the patient returned to the family medicine clinic. She said she was upset that the specialist had x-rayed her hands, but had not checked her shoulder, which was the primary source of her pain. She also had pain in her hands, hips, feet, and knees.
On physical exam, the patient looked fatigued. A musculoskeletal exam revealed no joint effusions or edema, but was significant for right shoulder pain with reduced abduction to 90°. Gross motor strength was 5/5 in all 4 extremities. Laboratory testing revealed an antinuclear antibody titer of 1:160 and was negative for double-stranded DNA. Bilateral hand and foot x-rays showed no joint erosions. An x-ray of the right shoulder was obtained, which showed evidence of osteopenia and an erosion in the humeral head (FIGURES 1A and 1B).

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Rheumatoid arthritis
The patient’s history of morning stiffness and the joint erosion observed on x-ray were highly suggestive of rheumatoid arthritis (RA).
RA is a symmetric, inflammatory, peripheral polyarthritis of unknown etiology. It is the most common form of inflammatory arthritis, affecting 1% of the population worldwide.1 It causes cartilage and bone to erode, leading to the deformation and destruction of joints. If RA is left untreated or is unresponsive to therapy, it can eventually lead to loss of physical function.
Making the diagnosis
The distinctive signs of RA are joint erosions and rheumatoid nodules, which are often absent on initial presentation.
Classification criteria. The 2010 American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) classification criteria for RA2 are based on the presence of synovitis in at least one joint, the absence of an alternative diagnosis that better explains the synovitis, and a cumulative score of at least 6/10 from the following 4 domains:
- Number and site of involved joints
- 2 to 10 large joints (shoulders, elbows, hips, knees, ankles)=1 point
- 1 to 3 small joints (metacarpophalangeal [MCP] joints, proximal interphalangeal [PIP] joints, 2nd-5th metatarsophalangeal joints, thumb interphalangeal joints, wrists)=2 points
- 4 to 10 small joints=3 points
- More than 10 joints (including at least 1 small joint)=5 points
- Serologic abnormality (rheumatoid factor [RF] and anti-cyclic citrullinated peptide)
- Low positive=2 points
- High positive=3 points
- Elevated acute phase response (erythrocyte sedimentation rate [ESR] or C-reactive protein [CRP])=1 point
- Symptom duration of at least 6 weeks=1 point.
These criteria are best suited for early disease. For patients with longstanding symptoms, diagnosis is based on an erosive disease with a history of criteria fulfillment, or a currently inactive longstanding disease, with or without treatment, that has previously fulfilled the criteria.3
Continue to: The differential Dx is extensive
The differential Dx is extensive
The differential includes polyarthralgias such as viral polyarthritis, systemic rheumatic diseases, and osteoarthritis.
Viral polyarthritis is caused by rubella, parvovirus B194, alphaviruses, and hepatitis B. Symptoms can last from 3 days to several weeks, but rarely persist beyond 6 weeks; alphaviruses, however, can last 3 to 6 months.5 The common symptom triad includes fever, arthritis, and rash. Chikungunya is an example of an alphavirus that has become a global disease. Alphavirus arthritis can mimic seronegative RA and even satisfy the classification criteria for RA if the initial symptoms of fever and rash and history of travel to endemic regions are not appreciated.5
Systemic rheumatic diseases. Early RA may mimic the arthritis of systemic lupus erythematosus (SLE), Sjögren’s syndrome, dermatomyositis, or mixed connective tissue disease.6 In contrast to RA, these disorders generally have systemic features, such as rashes, dry mouth and eyes, myositis, or nephritis, and generate autobodies, which are not seen with RA. The CRP is often normal in patients with active SLE, even when the ESR is elevated.
Osteoarthritis (OA) can be confused with RA, particularly when small joints are involved. A thorough history helps elucidate the diagnosis. For example, OA of the fingers affects distal interphalangeal joints and is associated with Heberden’s nodes, while RA more commonly affects MCP and PIP joints. Swelling from OA is typically firm, while swelling due to RA is warm, boggy, and tender. Joint stiffness due to OA is worse with activity and generally lasts only a few minutes, while joint stiffness due to RA is worse at rest and lasts 30 minutes or more. X-rays show joint-space narrowing with OA, but no erosions or cysts. RF may be present at low levels in older patients with OA, while it is usually associated with high levels in patients with seropositive RA.
Continue to: Treat with disease-modifying antirheumatic drugs
Treat with disease-modifying antirheumatic drugs
Immediate treatment of RA with disease-modifying antirheumatic drugs (DMARDs) is important to achieve control of the disease and prevent disability.
Our patient. We ordered a purified protein derivative skin test in preparation for the patient to start therapy with a DMARD. Our patient followed up with Rheumatology and was started on indomethacin, with an initial dose of 25 mg bid. (DMARDs are first-line therapy; indomethacin is a second-line choice. In this case, the patient declined DMARDs after hearing they lowered the body’s ability to fight infection.) An RF level measured 6.74 IU/ml, which is within normal limits. The patient was subsequently lost to follow-up.
CORRESPONDENCE
Barbara Kiersz, DO, 6835 Austin Center Blvd., Austin, TX 78731; [email protected].
1. Rothschild BM, Turner KR, DeLuca MA. Symmetrical erosive peripheral polyarthritis in the Late Archaic Period of Alabama. Science. 1988;241:1498-1501.
2. Aletaha D, Neogi T, Silman AJ, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis. 2010;69:1580-1588.
3. Pincus T, Callahan LF. How many types of patients meet classification criteria for rheumatoid arthritis? J Rheumatol. 1994;21:1385-1389.
4. Smith CA, Woolf AD, Lenci M. Parvoviruses: infections and arthropathies. Rheum Dis Clin North Am. 1987;13:249-263.
5. Miner JJ, Aw-Yeang HX, Fox JM, et al. Chikungunya viral arthritis in the United States: a mimic of seronegative rheumatoid arthritis. Arthritis Rheumatol. 2015;67:1214-1220.
6. Cronin ME. Musculoskeletal manifestations of systemic lupus erythematosus. Rheum Dis Clin North Am. 1988;14:99-116.
1. Rothschild BM, Turner KR, DeLuca MA. Symmetrical erosive peripheral polyarthritis in the Late Archaic Period of Alabama. Science. 1988;241:1498-1501.
2. Aletaha D, Neogi T, Silman AJ, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis. 2010;69:1580-1588.
3. Pincus T, Callahan LF. How many types of patients meet classification criteria for rheumatoid arthritis? J Rheumatol. 1994;21:1385-1389.
4. Smith CA, Woolf AD, Lenci M. Parvoviruses: infections and arthropathies. Rheum Dis Clin North Am. 1987;13:249-263.
5. Miner JJ, Aw-Yeang HX, Fox JM, et al. Chikungunya viral arthritis in the United States: a mimic of seronegative rheumatoid arthritis. Arthritis Rheumatol. 2015;67:1214-1220.
6. Cronin ME. Musculoskeletal manifestations of systemic lupus erythematosus. Rheum Dis Clin North Am. 1988;14:99-116.
Thrombocytopenia and neutropenia: A structured approach to evaluation
Thrombocytopenia and neutropenia are commonly encountered laboratory abnormalities. The presence of either requires that you promptly evaluate for life-threatening causes and identify the appropriate etiology. This article identifies key questions to ask. It also includes algorithms and tables that will facilitate your evaluation of patients with isolated thrombocytopenia or isolated neutropenia and speed the way toward appropriate treatment.
Thrombocytopenia: A look at the numbers
Thrombocytopenia is defined as a platelet count <150,000/mcL.1 The blood abnormality is either suspected based on the patient’s signs or symptoms, such as ecchymoses, petechiae, purpura, epistaxis, gingival bleeding, or melena, or it is incidentally discovered during review of a complete blood count (CBC).
The development of clinical symptoms is closely related to the severity of the thrombocytopenia, with platelet counts <30,000/mcL more likely to result in clinical symptoms with minor trauma and counts <5,000/mcL potentially resulting in spontaneous bleeding. While most patients will have asymptomatic, incidentally-found thrombocytopenia, and likely a benign etiology, those with the signs/symptoms just described, evidence of infection, or thrombosis are more likely to have a serious etiology and require an expedited work-up. Although pregnancy may be associated with thrombocytopenia, this review confines itself to the causes of thrombocytopenia in non-pregnant adults.
Rule out pseudothrombocytopenia
When isolated thrombocytopenia is discovered incidentally in an asymptomatic person, the first step is to perform a repeat CBC with a peripheral smear to confirm the presence of thrombocytopenia, rule out laboratory error, and assess for platelet clumping. If thrombocytopenia is confirmed and platelet clumping is present, it may be due to the calcium chelator in the ethylenediaminetetraacetic anticoagulant contained within the laboratory transport tube; this cause of pseudothrombocytopenia occurs in up to 0.29% of the population.1 Obtaining a platelet count from a citrated or heparinized tube avoids this phenomenon.
Is the patient’s thrombocytopenia drug induced?
Once true thrombocytopenia is confirmed, the next step is to review the patient’s prescribed medications, as well as any illicit drugs used, for potential causes of drug-induced thrombocytopenia. DITP can be either immune-mediated or nonimmune-mediated.
Immune-mediated DITP typically occurs within 1 to 2 weeks of medication exposure and begins to improve within 1 to 2 days of stopping the offending drug.2 (See TABLE 13 for a list of medications that can induce thrombocytopenia.) It should be noted that most patients who take the medications listed in TABLE 1 do not experience thrombocytopenia; nonetheless, it is a potential risk associated with their use.
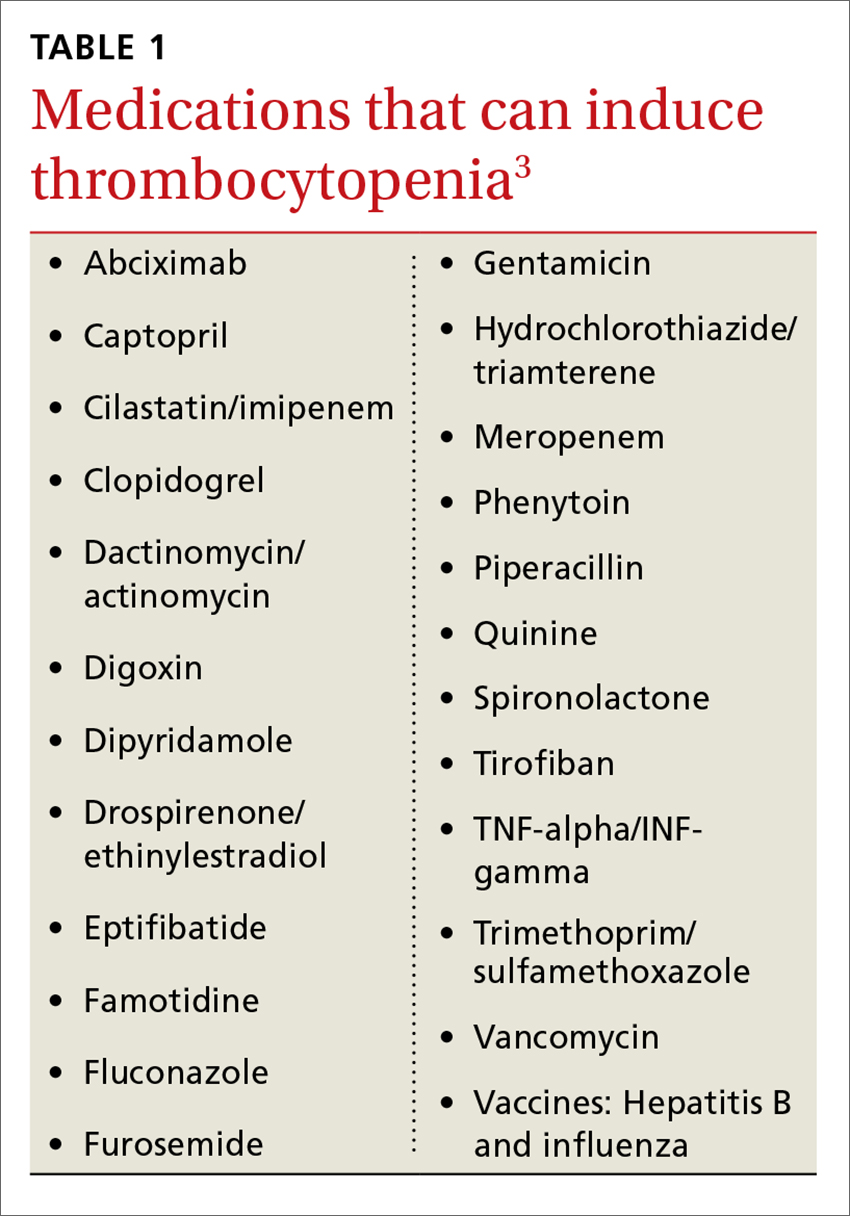
Heparin-induced thrombocytopenia (HIT) is a unique form of immune-mediated DITP in that it is caused by antibody complexes, resulting in platelet activation, clumping, and thrombotic events.4 HIT occurs <1% of patients in intensive care units, but can occur in any patient on long-term heparin therapy. It manifests as a >50% drop in platelet count within 5 to 14 days of the introduction of heparin; however, in those previously exposed to heparin, it can occur within 24 hours.4,5
Continue to: Non-immune-mediated DITP
Non-immune-mediated DITP, resulting from myelosuppression, chemotherapeutic agents, or valproic acid, is less common.1,2
Acute and chronic alcohol use. Although alcohol is not a drug per se, it can also result in thrombocytopenia. The mechanism is the direct suppression of bone marrow, although alcohol also causes B12 and folate deficiency, further contributing to the development of the blood abnormality.1
Is there thrombosis?
In addition to exploring a connection between thrombocytopenia and the drugs a patient is taking, it’s also important to look for evidence of thrombosis. The causes of thrombocytopenia that paradoxically result in thrombosis are: disseminated intravascular coagulation, hemolytic uremic syndrome, thrombotic thrombocytopenic purpura, catastrophic antiphospholipid antibody syndrome, and the previously mentioned HIT. TABLE 24,6-9 outlines the clinical settings, laboratory findings, and treatments of thrombocytopenia associated with thrombosis.
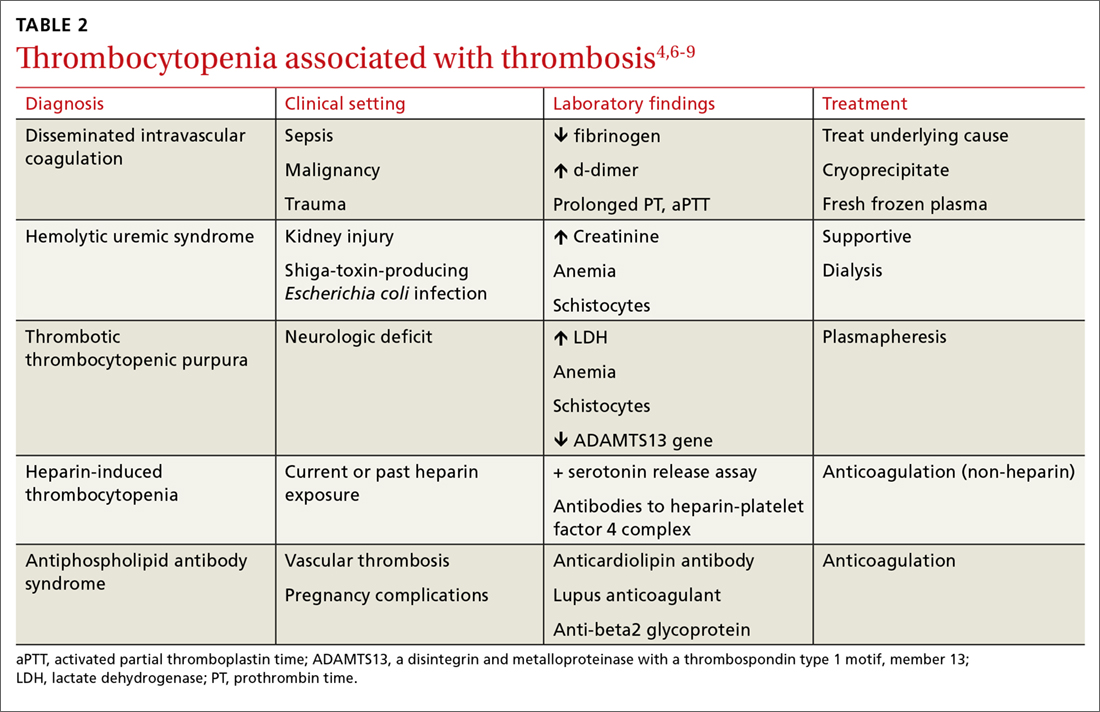
Is an infectious cause to blame?
If the patient is ill, consider infectious causes of thrombocytopenia. Thrombocytopenia associated with infection may result from an immune-mediated response to an illness itself, to treatment of an illness, to splenic sequestration, or to bone marrow suppression. TABLE 31,9-11 lists common infections that may cause thrombocytopenia.
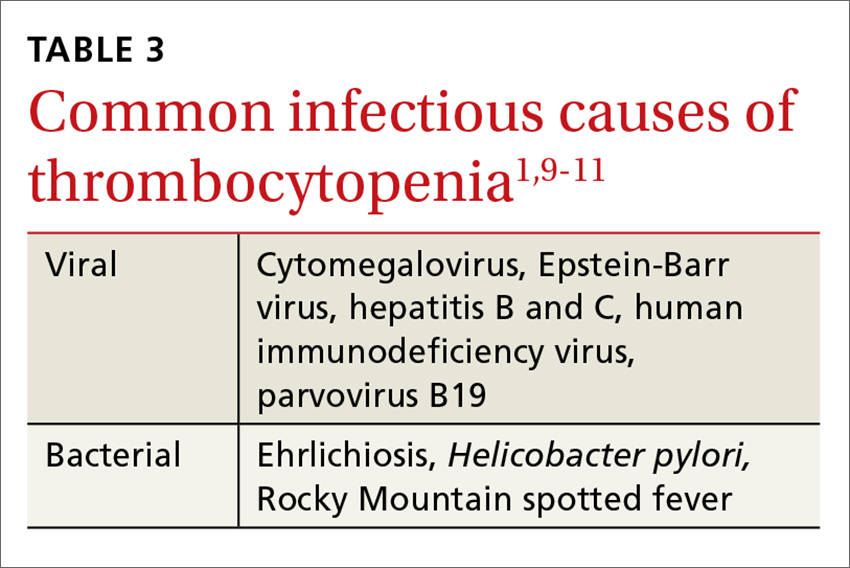
Of note, infection with Helicobacter pylori can cause asymptomatic thrombocytopenia via an immune-mediated mechanism.12 Eradication of H pylori results in a variable elevation in platelets, on average 30,000/mcL in 50% of patients with the infection.13
Is there pancytopenia?
A review of the peripheral smear, with attention to abnormalities in other cell lines, may assist in arriving at a diagnosis. If the peripheral smear reveals pancytopenia, then, in addition to many of the etiologies described earlier, one should also consider vitamin B12 or folate deficiency, copper deficiency, drug- and viral-induced aplastic anemia, paroxysmal nocturnal hemoglobinuria, leukemias, myelodysplastic disorders, and systemic lupus erythematosis.14 Pancytopenia is also seen with hypersplenism, which is often associated with cirrhosis.15 If the etiology isn’t readily apparent, a bone marrow biopsy may be required.
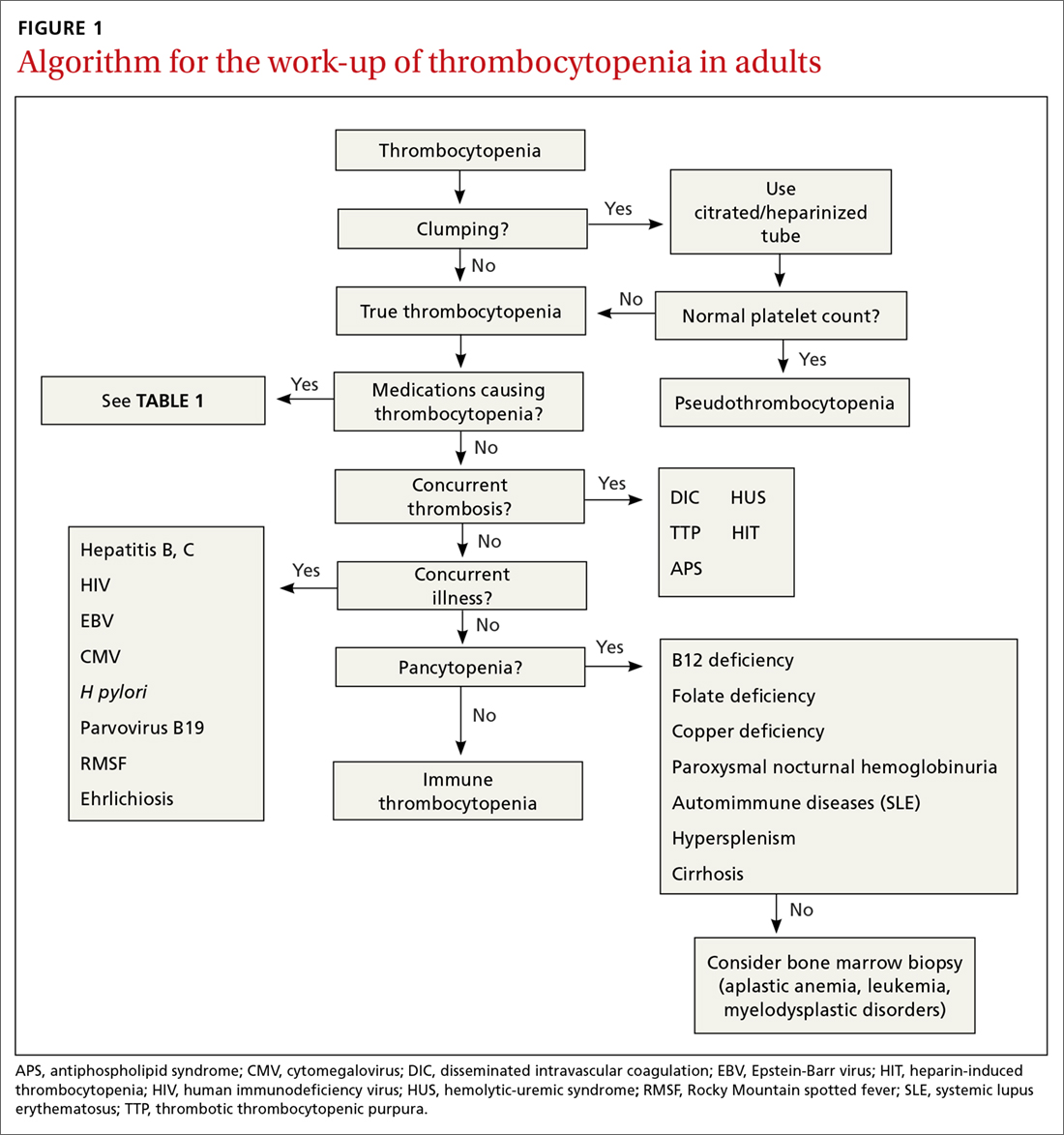
Continue to: Is immune thrombocytopenia to blame?
Is immune thrombocytopenia to blame?
Immune thrombocytopenia (ITP) is an autoimmune disorder resulting in the destruction of normal platelets and may be primary or secondary to processes described previously (HIT, H pylori infection, etc). Consider ITP if, after a thorough work-up, a cause of isolated thrombocytopenia is not identified.16 Treatment for ITP is outlined in TABLE 4.16 FIGURE 1 is an algorithm for the complete evaluation of thrombocytopenia in adults.
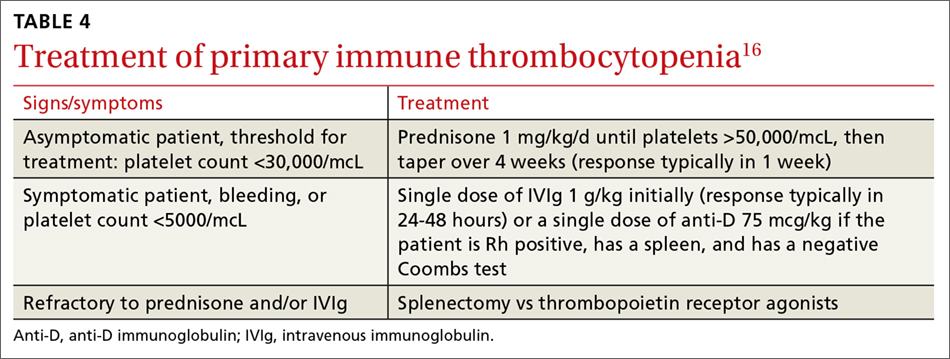
Treatment: Platelet transfusions
In general, patients who are not actively bleeding are considered stable and do not require platelet transfusions to minimize their risk of bleeding or prevent bleeding during a planned procedure unless their platelet count falls below the levels specified in TABLE 5.17 For patients who are actively bleeding, a more aggressive approach may be required. Locally-derived transfusion protocols typically guide transfusions for the actively hemorrhaging patient. The American Association of Blood Banks has put forth evidence-based guidelines for platelet transfusions when a patient is given a diagnosis of thrombocytopenia (see TABLE 5).17 Single-donor platelets have a shelf life of 3 to 5 days, and one unit will raise platelets 30,000 to 50,000/mcL.
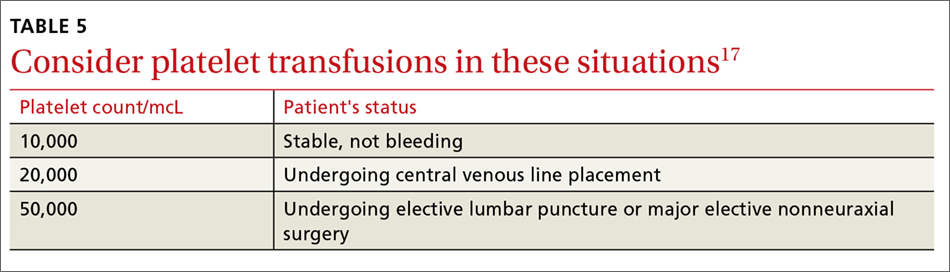
Neutropenia: Prevalence varies by ethnicity
An absolute neutrophil count (ANC) of <1500 cells/mcL traditionally defines neutropenia, with an ANC of 1000 to 1500 cells/mcL constituting mild neutropenia; 500 to 999 cells/mcL, moderate; and <500 cells/mcL, severe.18 Similar to the evaluation of thrombocytopenia, it is important to repeat the CBC prior to initiating a work-up in order to confirm that the neutropenia is not a laboratory error. Additionally, patients with signs or symptoms of infection should be worked up expeditiously.
The prevalence of neutropenia varies by ethnicity. According to the National Health and Nutrition Examination Survey 1999 to 2004, the prevalence was 4.5%, 0.79%, and 0.38% in black, white, and Mexican-American participants, respectively.19 FIGURE 2 outlines the outpatient work-up of adult patients with neutropenia not related to chemotherapy.
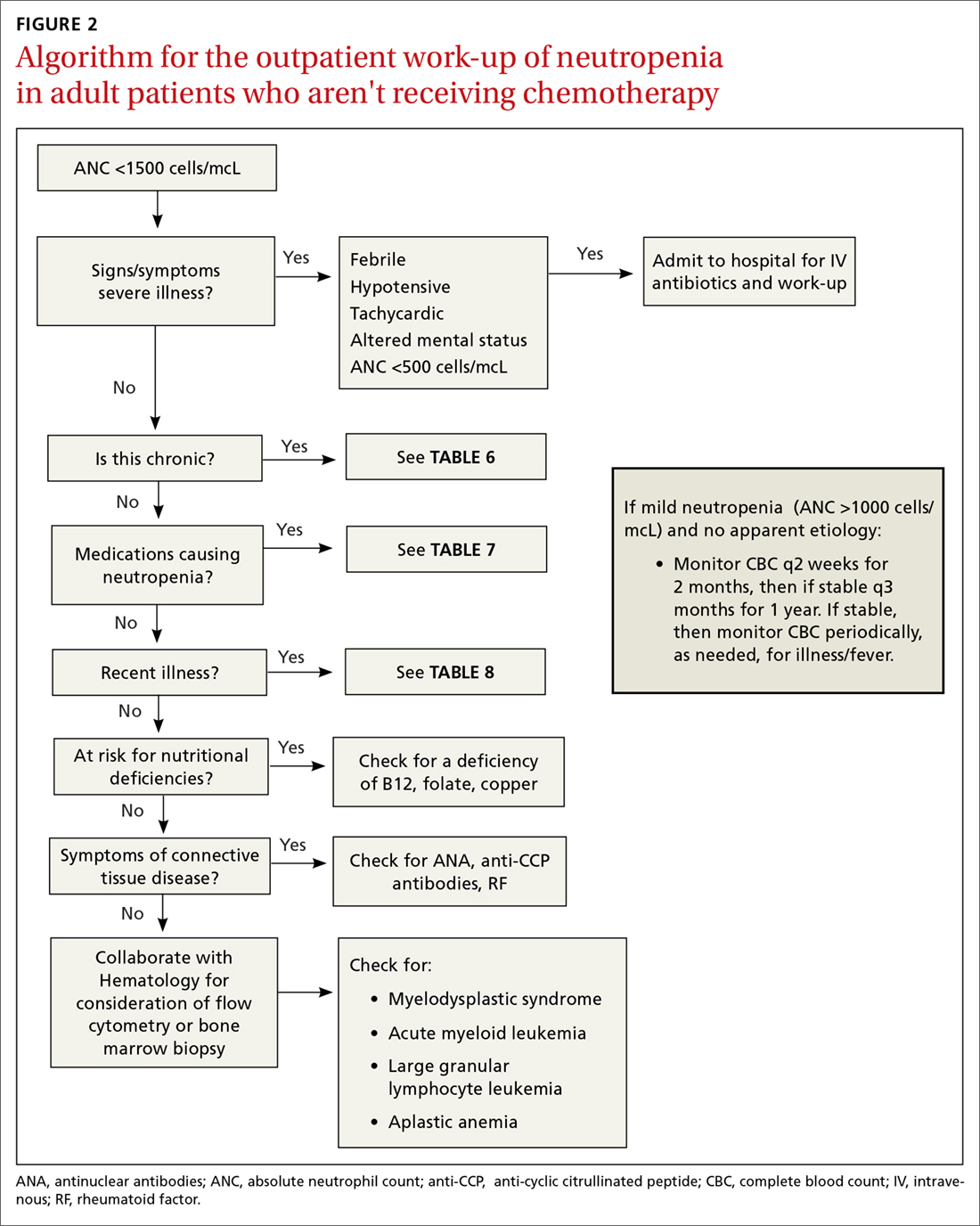
Continue to: Is the patient severely ill?
Is the patient severely ill?
The prognosis of the patient is related both to the etiology of the neutropenia, as well as to the nadir of the neutrophil count. Patients who have an ANC <500 cells/mcL or who have inadequate bone marrow reserves are at highest risk for an overwhelming infection.20,21 The absence of oral ulcers and gingivitis and/or the presence of purulent material at the site of an infection are signs of adequate bone marrow reserves.
Additionally, neutropenia may be the source—or the result—of a serious life-threatening illness. This distinction may not be readily apparent at the time of the patient’s presentation. If signs or symptoms of a severe illness are apparent (fever, hypotension, tachycardia, ANC <500 cells/mcL), admit the patient to the hospital for evaluation and initiation of antibiotics.
Is the neutropenia chronic?
A review of previous CBCs will identify whether this condition is new or chronic. A persistent, mild neutropenia (ANC 1000-1500 cells/mcL) in a healthy individual is consistent with benign familial or ethnic neutropenia (see TABLE 6).20 If prior CBCs are unavailable, then a diagnosis of chronic neutropenia may be established by verifying the persistence of mild neutropenia over time.

Cyclic neutropenia is a periodic neutropenia (occurring every 2-5 weeks) associated with mild illnesses that are related to the nadir of the neutrophil count. The diagnosis is established by obtaining serial CBCs twice weekly for 4 to 6 weeks, which reflect cycling of the neutrophil count.20,22
Are any medications contributing to the neutropenia?
Medications that suppress bone marrow or that interfere with other immune-mediated processes are the most common cause of acquired neutropenia.23 Drug-induced agranulocytosis is defined as an ANC <500 cells/mcL due to exposure to a drug that results in immunologic or cytotoxic destruction of neutrophils.24
A systematic review of case reports of drug-induced agranulocytosis (a decrease in peripheral neutrophil count to <500 cells/mcL) revealed that although at least 125 drugs were probably related to agranulocytosis, only 11 drugs were responsible for 50% of cases (carbimazole, clozapine, dapsone, dipyrone, methimazole, penicillin G, procainamide, propylthiouracil, rituximab, sulfasalazine, and ticlopidine), and fatality rates were higher (10% vs 3%) among those patients with a nadir <100 cells/mcL.25 TABLE 725 lists medications that can be associated with agranulocytosis. Depending on prior exposure to a drug, neutropenia/agranulocytosis can occur within hours to months of exposure to the causal drug and can take a few days to 3 weeks to resolve after cessation.25,26
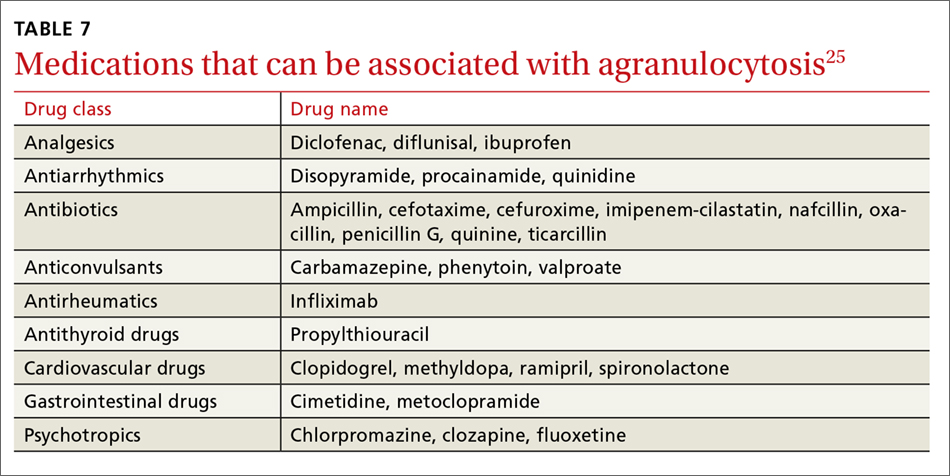
Continue to: Has the patient had any recent illnesses?
Has the patient had any recent illnesses?
The usual response to an infection is an increase in neutrophil count. However, certain bacterial, rickettsial, parasitic, and viral infections can result in neutropenia (see TABLE 823,27-29). Viral infections may cause transient neutropenia because of either bone marrow suppression or increased peripheral destruction, while neutropenia related to an overwhelming bacterial infection results from the depletion of bone marrow reserves.23,27

Do you suspect a nutritional deficiency?
Patients with a nutritional deficiency of B12, folate, or copper are likely to exhibit a deficiency in more than just neutrophils.23,27 In developed countries, people with neutropenia may have a history of malnutrition due to a disease (eg, anorexia nervosa) or surgery (eg, gastric bypass) that causes severe calorie restriction.20
Does your patient have symptoms of a connective tissue disease?
Neutropenia, in association with arthralgias, joint swelling, splenomegaly, or rash may be a manifestation of an underlying collagen vascular disorder, such as rheumatoid arthritis (RA) or systemic lupus erythematosus (SLE).20 If the clinical scenario supports one of these diagnoses, undertake or refer the patient for a rheumatologic evaluation. This may include studies of anti-cyclic citrullinated peptide antibodies, rheumatoid factor to evaluate for RA, and/or antinuclear antibodies to evaluate for SLE.30,31 While most neutropenias associated with autoimmune disease are mild, neutropenia associated with Felty syndrome (RA, splenomegaly, and neutropenia) may be severe (ANC <100 cells/mcL).20,23
Is the etiology unclear?
Patients with moderate to severe neutropenia without an apparent etiology, in the setting of aplastic anemia, or in the presence of splenomegaly and/or lymphadenopathy, should undergo a hematologic evaluation and/or bone marrow biopsy, given that hematologic malignancy is a potential cause.20,27
The treatment of neutropenia hinges on correctly identifying the etiology of the diminished neutrophil count. If the cause is a medication, infection, underlying rheumatologic condition, or nutritional deficiency, then either treating the entity or withdrawing the offending medication should result in resolution of the neutropenia. If the cause is determined to be familial or ethnic, then patient reassurance is all that is required.
CORRESPONDENCE
Richard W. Temple, MD, FAAFP, CDR MC USN, Camp Lejeune Family Medicine Residency, Naval Medical Center Camp Lejeune, 100 Brewster Blvd, Camp Lejeune, NC 28547-2538; [email protected].
1. Wong EY, Rose MG. Why does my patient have thrombocytopenia? Hematol Oncol Clin North Am. 2012;26:231-252.
2. Aster RH, Bougie DW. Drug-induced immune thrombocytopenia. N Engl J Med. 2007;357:580-587.
3. University of Oklahoma Health Sciences Center. Database for Drug–induced thrombocytopenia from group patient reports: an update. Available at: http://www.ouhsc.edu/platelets/InternetPostingGroupFrames2014.htm. Accessed May 7, 2018.
4. Sniecinski RM, Hursting MJ, Paidas MJ, et al. Etiology and assessment of hypercoagulability with lessons from heparin-induced thrombocytopenia. Anesth Analg. 2011;112:46-58.
5. Warkentin TE. Heparin-induced thrombocytopenia in critically ill patients. Crit Care Clin. 2011;27:805-823.
6. Connell NT, Sweeney JD. Does my patient have life- or limb-threatening thrombocytopenia? Hematol Oncol Clin North Am. 2012;26:369-382.
7. George JN, Nester CM. Syndromes of thrombotic microangiopathy. N Engl J Med. 2014;371:654-666.
8. Hanly JG. Antiphospholipid syndrome: an overview. CMAJ. 2003;168:1675-1682.
9. Sekhon SS, Roy V. Thrombocytopenia in adults: a practical approach to evaluation and management. South Med J. 2006;99:491-498.
10. Gauer RL, Braun MM. Thrombocytopenia. Am Fam Physician. 2012;85:612-622.
11. Bratton RL, Corey R. Tick-borne disease. Am Fam Physician. 2005;71:2323-2330.
12. Yeh JJ, Tsai S, Wu DC, et al. P-selectin-dependent platelet aggregation and apoptosis may explain the decrease in platelet count during Helicobacter pylori infection. Blood. 2010;115:4247-4253.
13. Stasi R, Sarpatwari A, Segal JB, et al. Effects of eradication of Helicobacter pylori infection in patients with immune thrombocytopenic purpura: a systemic review. Blood. 2009;113:1231-1240.
14. Weinzierl EP, Arber DA. The differential diagnosis and bone marrow evaluation of new-onset pancytopenia. Am J Clin Pathol. 2013;139:9-29.
15. Peck-Radosavljevic M. Hypersplenism. Eur J Gastroenterol Hepatol. 2001;13:317-323.
16. Neunert C, Lim W, Crowther M, et al. The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood. 2011;117:4190-4207.
17. Kaufman RM, Djulbegovic B, Gernsheimer T, et al. Platelet transfusion: a clinical practice guideline from the AABB. Ann Int Med. 2015;162:205-213.
18. Palmblad J, Dufour C, Papadaki HA. How we diagnose neutropenia in the adult and elderly patient. Haematologica. 2014;99:1130-1133.
19. Hsieh MM, Everhart JE, Byrd-Holt DD, et al. Prevalence of neutropenia in the U.S. population: age, sex, smoking status, and ethnic differences. Ann Intern Med. 2007;146:486-492.
20. Gibson C, Berliner N. How we evaluate and treat neutropenia in adults. Blood. 2014;124:1251-1258.
21. Urabe A. Clinical features of the neutropenic host: definitions and initial evaluation. CID. 2004;39(suppl 1):S53-S55.
22. Dale DC, Hammond WP 4th. Cyclic neutropenia: a clinical review. Blood Rev. 1988;2:178-185.
23. Munshi HG, Montgomery RB. Severe neutropenia: a diagnostic approach. West J Med. 2000;172:248-252.
24. Pisciotta AV. Drug-induced agranulocytosis peripheral destruction of polymorphonuclear leukocytes and their marrow precursors. Blood Rev. 1990;4:226-237.
25. Andersohn F, Konzen C, Garbe E. Systematic review: agranulocytosis induced by nonchemotherapy drugs. Ann Intern Med. 2007;146:657-665.
26. Bhatt V, Saleem A. Review: drug-induced neutropenia – pathophysiology, clinical features, and management. Ann Clin Lab Sci. 2004;34:131-137.
27. Newburger PE, Dale DC. Evaluation and management of patients with isolated neutropenia. Semin Hematol. 2013;50:198-206.
28. Bakken JS, Krueth J, Wilson-Nordskog C, et al. Clinical and laboratory characteristics of human granulcytic ehrlichiosis. JAMA. 1996;275:199-205.
29. Hall GW, Schwartz RP. White blood cell count and differential in Rocky Mountain spotted fever. NC Med J. 1979;40:212-214.
30. Nishimura K, Sugiyama D, Kogata Y, et al. Meta-analysis: diagnostic accuracy of anti-cyclic citrullinated peptide antibody and rheumatoid factor for rheumatoid arthritis. Ann Intern Med. 2007;146:797-808.
31. Petri M, Orbai AM, Alarcón GS, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012; 64:2677-2686.
Thrombocytopenia and neutropenia are commonly encountered laboratory abnormalities. The presence of either requires that you promptly evaluate for life-threatening causes and identify the appropriate etiology. This article identifies key questions to ask. It also includes algorithms and tables that will facilitate your evaluation of patients with isolated thrombocytopenia or isolated neutropenia and speed the way toward appropriate treatment.
Thrombocytopenia: A look at the numbers
Thrombocytopenia is defined as a platelet count <150,000/mcL.1 The blood abnormality is either suspected based on the patient’s signs or symptoms, such as ecchymoses, petechiae, purpura, epistaxis, gingival bleeding, or melena, or it is incidentally discovered during review of a complete blood count (CBC).
The development of clinical symptoms is closely related to the severity of the thrombocytopenia, with platelet counts <30,000/mcL more likely to result in clinical symptoms with minor trauma and counts <5,000/mcL potentially resulting in spontaneous bleeding. While most patients will have asymptomatic, incidentally-found thrombocytopenia, and likely a benign etiology, those with the signs/symptoms just described, evidence of infection, or thrombosis are more likely to have a serious etiology and require an expedited work-up. Although pregnancy may be associated with thrombocytopenia, this review confines itself to the causes of thrombocytopenia in non-pregnant adults.
Rule out pseudothrombocytopenia
When isolated thrombocytopenia is discovered incidentally in an asymptomatic person, the first step is to perform a repeat CBC with a peripheral smear to confirm the presence of thrombocytopenia, rule out laboratory error, and assess for platelet clumping. If thrombocytopenia is confirmed and platelet clumping is present, it may be due to the calcium chelator in the ethylenediaminetetraacetic anticoagulant contained within the laboratory transport tube; this cause of pseudothrombocytopenia occurs in up to 0.29% of the population.1 Obtaining a platelet count from a citrated or heparinized tube avoids this phenomenon.
Is the patient’s thrombocytopenia drug induced?
Once true thrombocytopenia is confirmed, the next step is to review the patient’s prescribed medications, as well as any illicit drugs used, for potential causes of drug-induced thrombocytopenia. DITP can be either immune-mediated or nonimmune-mediated.
Immune-mediated DITP typically occurs within 1 to 2 weeks of medication exposure and begins to improve within 1 to 2 days of stopping the offending drug.2 (See TABLE 13 for a list of medications that can induce thrombocytopenia.) It should be noted that most patients who take the medications listed in TABLE 1 do not experience thrombocytopenia; nonetheless, it is a potential risk associated with their use.

Heparin-induced thrombocytopenia (HIT) is a unique form of immune-mediated DITP in that it is caused by antibody complexes, resulting in platelet activation, clumping, and thrombotic events.4 HIT occurs <1% of patients in intensive care units, but can occur in any patient on long-term heparin therapy. It manifests as a >50% drop in platelet count within 5 to 14 days of the introduction of heparin; however, in those previously exposed to heparin, it can occur within 24 hours.4,5
Continue to: Non-immune-mediated DITP
Non-immune-mediated DITP, resulting from myelosuppression, chemotherapeutic agents, or valproic acid, is less common.1,2
Acute and chronic alcohol use. Although alcohol is not a drug per se, it can also result in thrombocytopenia. The mechanism is the direct suppression of bone marrow, although alcohol also causes B12 and folate deficiency, further contributing to the development of the blood abnormality.1
Is there thrombosis?
In addition to exploring a connection between thrombocytopenia and the drugs a patient is taking, it’s also important to look for evidence of thrombosis. The causes of thrombocytopenia that paradoxically result in thrombosis are: disseminated intravascular coagulation, hemolytic uremic syndrome, thrombotic thrombocytopenic purpura, catastrophic antiphospholipid antibody syndrome, and the previously mentioned HIT. TABLE 24,6-9 outlines the clinical settings, laboratory findings, and treatments of thrombocytopenia associated with thrombosis.

Is an infectious cause to blame?
If the patient is ill, consider infectious causes of thrombocytopenia. Thrombocytopenia associated with infection may result from an immune-mediated response to an illness itself, to treatment of an illness, to splenic sequestration, or to bone marrow suppression. TABLE 31,9-11 lists common infections that may cause thrombocytopenia.

Of note, infection with Helicobacter pylori can cause asymptomatic thrombocytopenia via an immune-mediated mechanism.12 Eradication of H pylori results in a variable elevation in platelets, on average 30,000/mcL in 50% of patients with the infection.13
Is there pancytopenia?
A review of the peripheral smear, with attention to abnormalities in other cell lines, may assist in arriving at a diagnosis. If the peripheral smear reveals pancytopenia, then, in addition to many of the etiologies described earlier, one should also consider vitamin B12 or folate deficiency, copper deficiency, drug- and viral-induced aplastic anemia, paroxysmal nocturnal hemoglobinuria, leukemias, myelodysplastic disorders, and systemic lupus erythematosis.14 Pancytopenia is also seen with hypersplenism, which is often associated with cirrhosis.15 If the etiology isn’t readily apparent, a bone marrow biopsy may be required.

Continue to: Is immune thrombocytopenia to blame?
Is immune thrombocytopenia to blame?
Immune thrombocytopenia (ITP) is an autoimmune disorder resulting in the destruction of normal platelets and may be primary or secondary to processes described previously (HIT, H pylori infection, etc). Consider ITP if, after a thorough work-up, a cause of isolated thrombocytopenia is not identified.16 Treatment for ITP is outlined in TABLE 4.16 FIGURE 1 is an algorithm for the complete evaluation of thrombocytopenia in adults.

Treatment: Platelet transfusions
In general, patients who are not actively bleeding are considered stable and do not require platelet transfusions to minimize their risk of bleeding or prevent bleeding during a planned procedure unless their platelet count falls below the levels specified in TABLE 5.17 For patients who are actively bleeding, a more aggressive approach may be required. Locally-derived transfusion protocols typically guide transfusions for the actively hemorrhaging patient. The American Association of Blood Banks has put forth evidence-based guidelines for platelet transfusions when a patient is given a diagnosis of thrombocytopenia (see TABLE 5).17 Single-donor platelets have a shelf life of 3 to 5 days, and one unit will raise platelets 30,000 to 50,000/mcL.

Neutropenia: Prevalence varies by ethnicity
An absolute neutrophil count (ANC) of <1500 cells/mcL traditionally defines neutropenia, with an ANC of 1000 to 1500 cells/mcL constituting mild neutropenia; 500 to 999 cells/mcL, moderate; and <500 cells/mcL, severe.18 Similar to the evaluation of thrombocytopenia, it is important to repeat the CBC prior to initiating a work-up in order to confirm that the neutropenia is not a laboratory error. Additionally, patients with signs or symptoms of infection should be worked up expeditiously.
The prevalence of neutropenia varies by ethnicity. According to the National Health and Nutrition Examination Survey 1999 to 2004, the prevalence was 4.5%, 0.79%, and 0.38% in black, white, and Mexican-American participants, respectively.19 FIGURE 2 outlines the outpatient work-up of adult patients with neutropenia not related to chemotherapy.

Continue to: Is the patient severely ill?
Is the patient severely ill?
The prognosis of the patient is related both to the etiology of the neutropenia, as well as to the nadir of the neutrophil count. Patients who have an ANC <500 cells/mcL or who have inadequate bone marrow reserves are at highest risk for an overwhelming infection.20,21 The absence of oral ulcers and gingivitis and/or the presence of purulent material at the site of an infection are signs of adequate bone marrow reserves.
Additionally, neutropenia may be the source—or the result—of a serious life-threatening illness. This distinction may not be readily apparent at the time of the patient’s presentation. If signs or symptoms of a severe illness are apparent (fever, hypotension, tachycardia, ANC <500 cells/mcL), admit the patient to the hospital for evaluation and initiation of antibiotics.
Is the neutropenia chronic?
A review of previous CBCs will identify whether this condition is new or chronic. A persistent, mild neutropenia (ANC 1000-1500 cells/mcL) in a healthy individual is consistent with benign familial or ethnic neutropenia (see TABLE 6).20 If prior CBCs are unavailable, then a diagnosis of chronic neutropenia may be established by verifying the persistence of mild neutropenia over time.

Cyclic neutropenia is a periodic neutropenia (occurring every 2-5 weeks) associated with mild illnesses that are related to the nadir of the neutrophil count. The diagnosis is established by obtaining serial CBCs twice weekly for 4 to 6 weeks, which reflect cycling of the neutrophil count.20,22
Are any medications contributing to the neutropenia?
Medications that suppress bone marrow or that interfere with other immune-mediated processes are the most common cause of acquired neutropenia.23 Drug-induced agranulocytosis is defined as an ANC <500 cells/mcL due to exposure to a drug that results in immunologic or cytotoxic destruction of neutrophils.24
A systematic review of case reports of drug-induced agranulocytosis (a decrease in peripheral neutrophil count to <500 cells/mcL) revealed that although at least 125 drugs were probably related to agranulocytosis, only 11 drugs were responsible for 50% of cases (carbimazole, clozapine, dapsone, dipyrone, methimazole, penicillin G, procainamide, propylthiouracil, rituximab, sulfasalazine, and ticlopidine), and fatality rates were higher (10% vs 3%) among those patients with a nadir <100 cells/mcL.25 TABLE 725 lists medications that can be associated with agranulocytosis. Depending on prior exposure to a drug, neutropenia/agranulocytosis can occur within hours to months of exposure to the causal drug and can take a few days to 3 weeks to resolve after cessation.25,26

Continue to: Has the patient had any recent illnesses?
Has the patient had any recent illnesses?
The usual response to an infection is an increase in neutrophil count. However, certain bacterial, rickettsial, parasitic, and viral infections can result in neutropenia (see TABLE 823,27-29). Viral infections may cause transient neutropenia because of either bone marrow suppression or increased peripheral destruction, while neutropenia related to an overwhelming bacterial infection results from the depletion of bone marrow reserves.23,27

Do you suspect a nutritional deficiency?
Patients with a nutritional deficiency of B12, folate, or copper are likely to exhibit a deficiency in more than just neutrophils.23,27 In developed countries, people with neutropenia may have a history of malnutrition due to a disease (eg, anorexia nervosa) or surgery (eg, gastric bypass) that causes severe calorie restriction.20
Does your patient have symptoms of a connective tissue disease?
Neutropenia, in association with arthralgias, joint swelling, splenomegaly, or rash may be a manifestation of an underlying collagen vascular disorder, such as rheumatoid arthritis (RA) or systemic lupus erythematosus (SLE).20 If the clinical scenario supports one of these diagnoses, undertake or refer the patient for a rheumatologic evaluation. This may include studies of anti-cyclic citrullinated peptide antibodies, rheumatoid factor to evaluate for RA, and/or antinuclear antibodies to evaluate for SLE.30,31 While most neutropenias associated with autoimmune disease are mild, neutropenia associated with Felty syndrome (RA, splenomegaly, and neutropenia) may be severe (ANC <100 cells/mcL).20,23
Is the etiology unclear?
Patients with moderate to severe neutropenia without an apparent etiology, in the setting of aplastic anemia, or in the presence of splenomegaly and/or lymphadenopathy, should undergo a hematologic evaluation and/or bone marrow biopsy, given that hematologic malignancy is a potential cause.20,27
The treatment of neutropenia hinges on correctly identifying the etiology of the diminished neutrophil count. If the cause is a medication, infection, underlying rheumatologic condition, or nutritional deficiency, then either treating the entity or withdrawing the offending medication should result in resolution of the neutropenia. If the cause is determined to be familial or ethnic, then patient reassurance is all that is required.
CORRESPONDENCE
Richard W. Temple, MD, FAAFP, CDR MC USN, Camp Lejeune Family Medicine Residency, Naval Medical Center Camp Lejeune, 100 Brewster Blvd, Camp Lejeune, NC 28547-2538; [email protected].
Thrombocytopenia and neutropenia are commonly encountered laboratory abnormalities. The presence of either requires that you promptly evaluate for life-threatening causes and identify the appropriate etiology. This article identifies key questions to ask. It also includes algorithms and tables that will facilitate your evaluation of patients with isolated thrombocytopenia or isolated neutropenia and speed the way toward appropriate treatment.
Thrombocytopenia: A look at the numbers
Thrombocytopenia is defined as a platelet count <150,000/mcL.1 The blood abnormality is either suspected based on the patient’s signs or symptoms, such as ecchymoses, petechiae, purpura, epistaxis, gingival bleeding, or melena, or it is incidentally discovered during review of a complete blood count (CBC).
The development of clinical symptoms is closely related to the severity of the thrombocytopenia, with platelet counts <30,000/mcL more likely to result in clinical symptoms with minor trauma and counts <5,000/mcL potentially resulting in spontaneous bleeding. While most patients will have asymptomatic, incidentally-found thrombocytopenia, and likely a benign etiology, those with the signs/symptoms just described, evidence of infection, or thrombosis are more likely to have a serious etiology and require an expedited work-up. Although pregnancy may be associated with thrombocytopenia, this review confines itself to the causes of thrombocytopenia in non-pregnant adults.
Rule out pseudothrombocytopenia
When isolated thrombocytopenia is discovered incidentally in an asymptomatic person, the first step is to perform a repeat CBC with a peripheral smear to confirm the presence of thrombocytopenia, rule out laboratory error, and assess for platelet clumping. If thrombocytopenia is confirmed and platelet clumping is present, it may be due to the calcium chelator in the ethylenediaminetetraacetic anticoagulant contained within the laboratory transport tube; this cause of pseudothrombocytopenia occurs in up to 0.29% of the population.1 Obtaining a platelet count from a citrated or heparinized tube avoids this phenomenon.
Is the patient’s thrombocytopenia drug induced?
Once true thrombocytopenia is confirmed, the next step is to review the patient’s prescribed medications, as well as any illicit drugs used, for potential causes of drug-induced thrombocytopenia. DITP can be either immune-mediated or nonimmune-mediated.
Immune-mediated DITP typically occurs within 1 to 2 weeks of medication exposure and begins to improve within 1 to 2 days of stopping the offending drug.2 (See TABLE 13 for a list of medications that can induce thrombocytopenia.) It should be noted that most patients who take the medications listed in TABLE 1 do not experience thrombocytopenia; nonetheless, it is a potential risk associated with their use.

Heparin-induced thrombocytopenia (HIT) is a unique form of immune-mediated DITP in that it is caused by antibody complexes, resulting in platelet activation, clumping, and thrombotic events.4 HIT occurs <1% of patients in intensive care units, but can occur in any patient on long-term heparin therapy. It manifests as a >50% drop in platelet count within 5 to 14 days of the introduction of heparin; however, in those previously exposed to heparin, it can occur within 24 hours.4,5
Continue to: Non-immune-mediated DITP
Non-immune-mediated DITP, resulting from myelosuppression, chemotherapeutic agents, or valproic acid, is less common.1,2
Acute and chronic alcohol use. Although alcohol is not a drug per se, it can also result in thrombocytopenia. The mechanism is the direct suppression of bone marrow, although alcohol also causes B12 and folate deficiency, further contributing to the development of the blood abnormality.1
Is there thrombosis?
In addition to exploring a connection between thrombocytopenia and the drugs a patient is taking, it’s also important to look for evidence of thrombosis. The causes of thrombocytopenia that paradoxically result in thrombosis are: disseminated intravascular coagulation, hemolytic uremic syndrome, thrombotic thrombocytopenic purpura, catastrophic antiphospholipid antibody syndrome, and the previously mentioned HIT. TABLE 24,6-9 outlines the clinical settings, laboratory findings, and treatments of thrombocytopenia associated with thrombosis.

Is an infectious cause to blame?
If the patient is ill, consider infectious causes of thrombocytopenia. Thrombocytopenia associated with infection may result from an immune-mediated response to an illness itself, to treatment of an illness, to splenic sequestration, or to bone marrow suppression. TABLE 31,9-11 lists common infections that may cause thrombocytopenia.

Of note, infection with Helicobacter pylori can cause asymptomatic thrombocytopenia via an immune-mediated mechanism.12 Eradication of H pylori results in a variable elevation in platelets, on average 30,000/mcL in 50% of patients with the infection.13
Is there pancytopenia?
A review of the peripheral smear, with attention to abnormalities in other cell lines, may assist in arriving at a diagnosis. If the peripheral smear reveals pancytopenia, then, in addition to many of the etiologies described earlier, one should also consider vitamin B12 or folate deficiency, copper deficiency, drug- and viral-induced aplastic anemia, paroxysmal nocturnal hemoglobinuria, leukemias, myelodysplastic disorders, and systemic lupus erythematosis.14 Pancytopenia is also seen with hypersplenism, which is often associated with cirrhosis.15 If the etiology isn’t readily apparent, a bone marrow biopsy may be required.

Continue to: Is immune thrombocytopenia to blame?
Is immune thrombocytopenia to blame?
Immune thrombocytopenia (ITP) is an autoimmune disorder resulting in the destruction of normal platelets and may be primary or secondary to processes described previously (HIT, H pylori infection, etc). Consider ITP if, after a thorough work-up, a cause of isolated thrombocytopenia is not identified.16 Treatment for ITP is outlined in TABLE 4.16 FIGURE 1 is an algorithm for the complete evaluation of thrombocytopenia in adults.

Treatment: Platelet transfusions
In general, patients who are not actively bleeding are considered stable and do not require platelet transfusions to minimize their risk of bleeding or prevent bleeding during a planned procedure unless their platelet count falls below the levels specified in TABLE 5.17 For patients who are actively bleeding, a more aggressive approach may be required. Locally-derived transfusion protocols typically guide transfusions for the actively hemorrhaging patient. The American Association of Blood Banks has put forth evidence-based guidelines for platelet transfusions when a patient is given a diagnosis of thrombocytopenia (see TABLE 5).17 Single-donor platelets have a shelf life of 3 to 5 days, and one unit will raise platelets 30,000 to 50,000/mcL.

Neutropenia: Prevalence varies by ethnicity
An absolute neutrophil count (ANC) of <1500 cells/mcL traditionally defines neutropenia, with an ANC of 1000 to 1500 cells/mcL constituting mild neutropenia; 500 to 999 cells/mcL, moderate; and <500 cells/mcL, severe.18 Similar to the evaluation of thrombocytopenia, it is important to repeat the CBC prior to initiating a work-up in order to confirm that the neutropenia is not a laboratory error. Additionally, patients with signs or symptoms of infection should be worked up expeditiously.
The prevalence of neutropenia varies by ethnicity. According to the National Health and Nutrition Examination Survey 1999 to 2004, the prevalence was 4.5%, 0.79%, and 0.38% in black, white, and Mexican-American participants, respectively.19 FIGURE 2 outlines the outpatient work-up of adult patients with neutropenia not related to chemotherapy.

Continue to: Is the patient severely ill?
Is the patient severely ill?
The prognosis of the patient is related both to the etiology of the neutropenia, as well as to the nadir of the neutrophil count. Patients who have an ANC <500 cells/mcL or who have inadequate bone marrow reserves are at highest risk for an overwhelming infection.20,21 The absence of oral ulcers and gingivitis and/or the presence of purulent material at the site of an infection are signs of adequate bone marrow reserves.
Additionally, neutropenia may be the source—or the result—of a serious life-threatening illness. This distinction may not be readily apparent at the time of the patient’s presentation. If signs or symptoms of a severe illness are apparent (fever, hypotension, tachycardia, ANC <500 cells/mcL), admit the patient to the hospital for evaluation and initiation of antibiotics.
Is the neutropenia chronic?
A review of previous CBCs will identify whether this condition is new or chronic. A persistent, mild neutropenia (ANC 1000-1500 cells/mcL) in a healthy individual is consistent with benign familial or ethnic neutropenia (see TABLE 6).20 If prior CBCs are unavailable, then a diagnosis of chronic neutropenia may be established by verifying the persistence of mild neutropenia over time.

Cyclic neutropenia is a periodic neutropenia (occurring every 2-5 weeks) associated with mild illnesses that are related to the nadir of the neutrophil count. The diagnosis is established by obtaining serial CBCs twice weekly for 4 to 6 weeks, which reflect cycling of the neutrophil count.20,22
Are any medications contributing to the neutropenia?
Medications that suppress bone marrow or that interfere with other immune-mediated processes are the most common cause of acquired neutropenia.23 Drug-induced agranulocytosis is defined as an ANC <500 cells/mcL due to exposure to a drug that results in immunologic or cytotoxic destruction of neutrophils.24
A systematic review of case reports of drug-induced agranulocytosis (a decrease in peripheral neutrophil count to <500 cells/mcL) revealed that although at least 125 drugs were probably related to agranulocytosis, only 11 drugs were responsible for 50% of cases (carbimazole, clozapine, dapsone, dipyrone, methimazole, penicillin G, procainamide, propylthiouracil, rituximab, sulfasalazine, and ticlopidine), and fatality rates were higher (10% vs 3%) among those patients with a nadir <100 cells/mcL.25 TABLE 725 lists medications that can be associated with agranulocytosis. Depending on prior exposure to a drug, neutropenia/agranulocytosis can occur within hours to months of exposure to the causal drug and can take a few days to 3 weeks to resolve after cessation.25,26

Continue to: Has the patient had any recent illnesses?
Has the patient had any recent illnesses?
The usual response to an infection is an increase in neutrophil count. However, certain bacterial, rickettsial, parasitic, and viral infections can result in neutropenia (see TABLE 823,27-29). Viral infections may cause transient neutropenia because of either bone marrow suppression or increased peripheral destruction, while neutropenia related to an overwhelming bacterial infection results from the depletion of bone marrow reserves.23,27

Do you suspect a nutritional deficiency?
Patients with a nutritional deficiency of B12, folate, or copper are likely to exhibit a deficiency in more than just neutrophils.23,27 In developed countries, people with neutropenia may have a history of malnutrition due to a disease (eg, anorexia nervosa) or surgery (eg, gastric bypass) that causes severe calorie restriction.20
Does your patient have symptoms of a connective tissue disease?
Neutropenia, in association with arthralgias, joint swelling, splenomegaly, or rash may be a manifestation of an underlying collagen vascular disorder, such as rheumatoid arthritis (RA) or systemic lupus erythematosus (SLE).20 If the clinical scenario supports one of these diagnoses, undertake or refer the patient for a rheumatologic evaluation. This may include studies of anti-cyclic citrullinated peptide antibodies, rheumatoid factor to evaluate for RA, and/or antinuclear antibodies to evaluate for SLE.30,31 While most neutropenias associated with autoimmune disease are mild, neutropenia associated with Felty syndrome (RA, splenomegaly, and neutropenia) may be severe (ANC <100 cells/mcL).20,23
Is the etiology unclear?
Patients with moderate to severe neutropenia without an apparent etiology, in the setting of aplastic anemia, or in the presence of splenomegaly and/or lymphadenopathy, should undergo a hematologic evaluation and/or bone marrow biopsy, given that hematologic malignancy is a potential cause.20,27
The treatment of neutropenia hinges on correctly identifying the etiology of the diminished neutrophil count. If the cause is a medication, infection, underlying rheumatologic condition, or nutritional deficiency, then either treating the entity or withdrawing the offending medication should result in resolution of the neutropenia. If the cause is determined to be familial or ethnic, then patient reassurance is all that is required.
CORRESPONDENCE
Richard W. Temple, MD, FAAFP, CDR MC USN, Camp Lejeune Family Medicine Residency, Naval Medical Center Camp Lejeune, 100 Brewster Blvd, Camp Lejeune, NC 28547-2538; [email protected].
1. Wong EY, Rose MG. Why does my patient have thrombocytopenia? Hematol Oncol Clin North Am. 2012;26:231-252.
2. Aster RH, Bougie DW. Drug-induced immune thrombocytopenia. N Engl J Med. 2007;357:580-587.
3. University of Oklahoma Health Sciences Center. Database for Drug–induced thrombocytopenia from group patient reports: an update. Available at: http://www.ouhsc.edu/platelets/InternetPostingGroupFrames2014.htm. Accessed May 7, 2018.
4. Sniecinski RM, Hursting MJ, Paidas MJ, et al. Etiology and assessment of hypercoagulability with lessons from heparin-induced thrombocytopenia. Anesth Analg. 2011;112:46-58.
5. Warkentin TE. Heparin-induced thrombocytopenia in critically ill patients. Crit Care Clin. 2011;27:805-823.
6. Connell NT, Sweeney JD. Does my patient have life- or limb-threatening thrombocytopenia? Hematol Oncol Clin North Am. 2012;26:369-382.
7. George JN, Nester CM. Syndromes of thrombotic microangiopathy. N Engl J Med. 2014;371:654-666.
8. Hanly JG. Antiphospholipid syndrome: an overview. CMAJ. 2003;168:1675-1682.
9. Sekhon SS, Roy V. Thrombocytopenia in adults: a practical approach to evaluation and management. South Med J. 2006;99:491-498.
10. Gauer RL, Braun MM. Thrombocytopenia. Am Fam Physician. 2012;85:612-622.
11. Bratton RL, Corey R. Tick-borne disease. Am Fam Physician. 2005;71:2323-2330.
12. Yeh JJ, Tsai S, Wu DC, et al. P-selectin-dependent platelet aggregation and apoptosis may explain the decrease in platelet count during Helicobacter pylori infection. Blood. 2010;115:4247-4253.
13. Stasi R, Sarpatwari A, Segal JB, et al. Effects of eradication of Helicobacter pylori infection in patients with immune thrombocytopenic purpura: a systemic review. Blood. 2009;113:1231-1240.
14. Weinzierl EP, Arber DA. The differential diagnosis and bone marrow evaluation of new-onset pancytopenia. Am J Clin Pathol. 2013;139:9-29.
15. Peck-Radosavljevic M. Hypersplenism. Eur J Gastroenterol Hepatol. 2001;13:317-323.
16. Neunert C, Lim W, Crowther M, et al. The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood. 2011;117:4190-4207.
17. Kaufman RM, Djulbegovic B, Gernsheimer T, et al. Platelet transfusion: a clinical practice guideline from the AABB. Ann Int Med. 2015;162:205-213.
18. Palmblad J, Dufour C, Papadaki HA. How we diagnose neutropenia in the adult and elderly patient. Haematologica. 2014;99:1130-1133.
19. Hsieh MM, Everhart JE, Byrd-Holt DD, et al. Prevalence of neutropenia in the U.S. population: age, sex, smoking status, and ethnic differences. Ann Intern Med. 2007;146:486-492.
20. Gibson C, Berliner N. How we evaluate and treat neutropenia in adults. Blood. 2014;124:1251-1258.
21. Urabe A. Clinical features of the neutropenic host: definitions and initial evaluation. CID. 2004;39(suppl 1):S53-S55.
22. Dale DC, Hammond WP 4th. Cyclic neutropenia: a clinical review. Blood Rev. 1988;2:178-185.
23. Munshi HG, Montgomery RB. Severe neutropenia: a diagnostic approach. West J Med. 2000;172:248-252.
24. Pisciotta AV. Drug-induced agranulocytosis peripheral destruction of polymorphonuclear leukocytes and their marrow precursors. Blood Rev. 1990;4:226-237.
25. Andersohn F, Konzen C, Garbe E. Systematic review: agranulocytosis induced by nonchemotherapy drugs. Ann Intern Med. 2007;146:657-665.
26. Bhatt V, Saleem A. Review: drug-induced neutropenia – pathophysiology, clinical features, and management. Ann Clin Lab Sci. 2004;34:131-137.
27. Newburger PE, Dale DC. Evaluation and management of patients with isolated neutropenia. Semin Hematol. 2013;50:198-206.
28. Bakken JS, Krueth J, Wilson-Nordskog C, et al. Clinical and laboratory characteristics of human granulcytic ehrlichiosis. JAMA. 1996;275:199-205.
29. Hall GW, Schwartz RP. White blood cell count and differential in Rocky Mountain spotted fever. NC Med J. 1979;40:212-214.
30. Nishimura K, Sugiyama D, Kogata Y, et al. Meta-analysis: diagnostic accuracy of anti-cyclic citrullinated peptide antibody and rheumatoid factor for rheumatoid arthritis. Ann Intern Med. 2007;146:797-808.
31. Petri M, Orbai AM, Alarcón GS, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012; 64:2677-2686.
1. Wong EY, Rose MG. Why does my patient have thrombocytopenia? Hematol Oncol Clin North Am. 2012;26:231-252.
2. Aster RH, Bougie DW. Drug-induced immune thrombocytopenia. N Engl J Med. 2007;357:580-587.
3. University of Oklahoma Health Sciences Center. Database for Drug–induced thrombocytopenia from group patient reports: an update. Available at: http://www.ouhsc.edu/platelets/InternetPostingGroupFrames2014.htm. Accessed May 7, 2018.
4. Sniecinski RM, Hursting MJ, Paidas MJ, et al. Etiology and assessment of hypercoagulability with lessons from heparin-induced thrombocytopenia. Anesth Analg. 2011;112:46-58.
5. Warkentin TE. Heparin-induced thrombocytopenia in critically ill patients. Crit Care Clin. 2011;27:805-823.
6. Connell NT, Sweeney JD. Does my patient have life- or limb-threatening thrombocytopenia? Hematol Oncol Clin North Am. 2012;26:369-382.
7. George JN, Nester CM. Syndromes of thrombotic microangiopathy. N Engl J Med. 2014;371:654-666.
8. Hanly JG. Antiphospholipid syndrome: an overview. CMAJ. 2003;168:1675-1682.
9. Sekhon SS, Roy V. Thrombocytopenia in adults: a practical approach to evaluation and management. South Med J. 2006;99:491-498.
10. Gauer RL, Braun MM. Thrombocytopenia. Am Fam Physician. 2012;85:612-622.
11. Bratton RL, Corey R. Tick-borne disease. Am Fam Physician. 2005;71:2323-2330.
12. Yeh JJ, Tsai S, Wu DC, et al. P-selectin-dependent platelet aggregation and apoptosis may explain the decrease in platelet count during Helicobacter pylori infection. Blood. 2010;115:4247-4253.
13. Stasi R, Sarpatwari A, Segal JB, et al. Effects of eradication of Helicobacter pylori infection in patients with immune thrombocytopenic purpura: a systemic review. Blood. 2009;113:1231-1240.
14. Weinzierl EP, Arber DA. The differential diagnosis and bone marrow evaluation of new-onset pancytopenia. Am J Clin Pathol. 2013;139:9-29.
15. Peck-Radosavljevic M. Hypersplenism. Eur J Gastroenterol Hepatol. 2001;13:317-323.
16. Neunert C, Lim W, Crowther M, et al. The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood. 2011;117:4190-4207.
17. Kaufman RM, Djulbegovic B, Gernsheimer T, et al. Platelet transfusion: a clinical practice guideline from the AABB. Ann Int Med. 2015;162:205-213.
18. Palmblad J, Dufour C, Papadaki HA. How we diagnose neutropenia in the adult and elderly patient. Haematologica. 2014;99:1130-1133.
19. Hsieh MM, Everhart JE, Byrd-Holt DD, et al. Prevalence of neutropenia in the U.S. population: age, sex, smoking status, and ethnic differences. Ann Intern Med. 2007;146:486-492.
20. Gibson C, Berliner N. How we evaluate and treat neutropenia in adults. Blood. 2014;124:1251-1258.
21. Urabe A. Clinical features of the neutropenic host: definitions and initial evaluation. CID. 2004;39(suppl 1):S53-S55.
22. Dale DC, Hammond WP 4th. Cyclic neutropenia: a clinical review. Blood Rev. 1988;2:178-185.
23. Munshi HG, Montgomery RB. Severe neutropenia: a diagnostic approach. West J Med. 2000;172:248-252.
24. Pisciotta AV. Drug-induced agranulocytosis peripheral destruction of polymorphonuclear leukocytes and their marrow precursors. Blood Rev. 1990;4:226-237.
25. Andersohn F, Konzen C, Garbe E. Systematic review: agranulocytosis induced by nonchemotherapy drugs. Ann Intern Med. 2007;146:657-665.
26. Bhatt V, Saleem A. Review: drug-induced neutropenia – pathophysiology, clinical features, and management. Ann Clin Lab Sci. 2004;34:131-137.
27. Newburger PE, Dale DC. Evaluation and management of patients with isolated neutropenia. Semin Hematol. 2013;50:198-206.
28. Bakken JS, Krueth J, Wilson-Nordskog C, et al. Clinical and laboratory characteristics of human granulcytic ehrlichiosis. JAMA. 1996;275:199-205.
29. Hall GW, Schwartz RP. White blood cell count and differential in Rocky Mountain spotted fever. NC Med J. 1979;40:212-214.
30. Nishimura K, Sugiyama D, Kogata Y, et al. Meta-analysis: diagnostic accuracy of anti-cyclic citrullinated peptide antibody and rheumatoid factor for rheumatoid arthritis. Ann Intern Med. 2007;146:797-808.
31. Petri M, Orbai AM, Alarcón GS, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012; 64:2677-2686.
From The Journal of Family Practice | 2018;67(7):E1-E8.
PRACTICE RECOMMENDATIONS
› Employ a systematic approach to the diagnosis and treatment of thrombocytopenia and neutropenia. C
› Do not transfuse platelets in patients with platelet counts >10,000/mcL who are stable and are not undergoing an invasive procedure. C
› Monitor patients on heparin therapy for >4 days for heparin-induced thrombocytopenia. C
› Monitor (for life) patients with a history of gastric bypass for the development of nutritional neutropenias. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
How best to manage chronic cholestasis
CASE
A 44-year-old nurse describes persistent fatigue and itching over the last 2 months. She is taking ramipril 5 mg/d for hypertension and has a family history of rheumatic disease. Lab tests reveal a recurrent moderate elevation of gamma glutamyl-transpeptidase (gGT; 75 U/L) associated with, on some occasions, mild elevation of alanine aminotransferase (ALT) levels (100 U/L) of unknown origin. She has no history of hepatitis virus infection, hepatotoxic medications, or alcohol intake. She is overweight with a body mass index of 28.5 kg/m2 and a waist circumference of 99 cm (39 inches). Liver ultrasonography detects an enlarged liver with diffuse echostructure dishomogeneity, but no signs of cirrhosis or portal hypertension. The patient’s biliary tree is not dilated.
How would you proceed with the care of this patient?
Cholestasis is characterized by the alteration of bile flow through any part of the biliary system, from the hepatocyte basocellular membrane to the duodenum. The condition is classified as intrahepatic when the cause is a defect of hepatocellular function or obstruction of the biliary tree within the liver. The extrahepatic form includes all conditions obstructing bile flow in the main biliary tract (choledochus, common bile duct).
The key to successfully managing cholestasis lies in the early identification of subtle signs and symptoms before serious complications can arise. In the review that follows, we provide guidance for evaluating laboratory and imaging results that are vital to the accurate diagnosis of intrahepatic and extrahepatic cholestasis. We also detail treatment recommendations.
Clues—subtle and otherwise—of cholestasis
Clinical features of cholestasis include fatigue and itching all over the skin. The latter likely is caused by induction of the enzyme autotaxin, which produces the neuronal activator lysophosphatidic acid. Retention of pruritogenic substances that normally are excreted into bile might contribute to pruritus as well.1 Jaundice, dark urine, and pale and fatty stools occur with advanced disease. However, a cholestatic condition can be detected in asymptomatic patients with elevated biochemical markers.
Continue to: Mildly elevated gGT and/or alkaline phosphatase (ALP)
Mildly elevated gGT and/or alkaline phosphatase (ALP) (0.5-2.5 times the upper normal limit [UNL] or 19-95 U/L and 60-300 U/L, respectively2) in the presence of normal transaminase levels (<20 U/L) in an asymptomatic patient can indicate chronic liver disease. Signs suggestive of significant liver disease have been reported in many patients with gGT or ALP elevation with good sensitivity (65%) and specificity (83%) for a diagnosis of intrahepatic cholestasis.3 However, because abnormal gGT values are common and often resolve spontaneously, family physicians (FPs) may pay little attention to this finding, thus missing an opportunity for early identification and treatment.
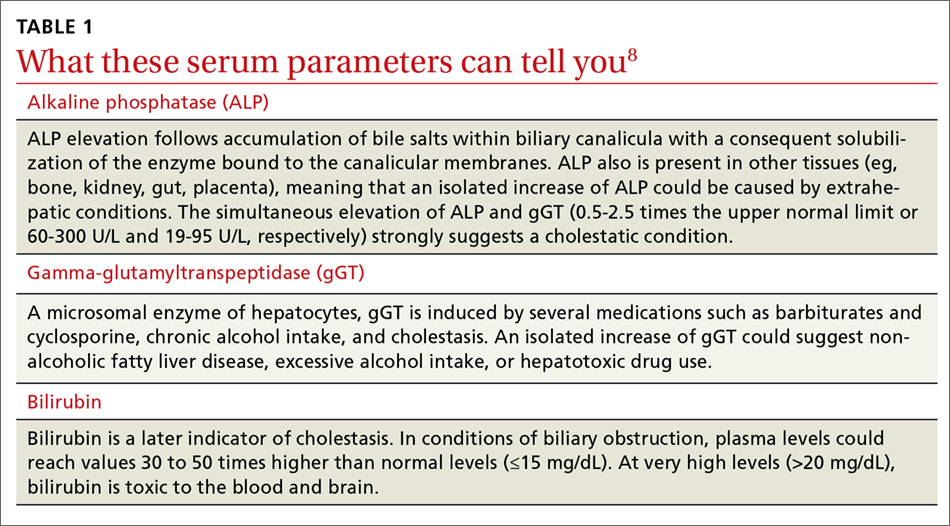
That’s why it’s important to schedule follow-up testing within 6 months for asymptomatic patients with abnormal laboratory findings. Persistent elevation of gGT alone or accompanied by ALP and ALT elevation (ALT >0.5 times the UNL or >18 U/L) is the most common feature of a chronic (>6 months) cholestatic condition.4 (In particular, elevated ALP levels appear to be associated with more aggressive disease and predict risk of liver transplantation or death in patients with primary biliary cholangitis (PBC).5,6 Lowering ALP levels is associated with improved disease outcomes, including transplant-free survival rates.5,7)
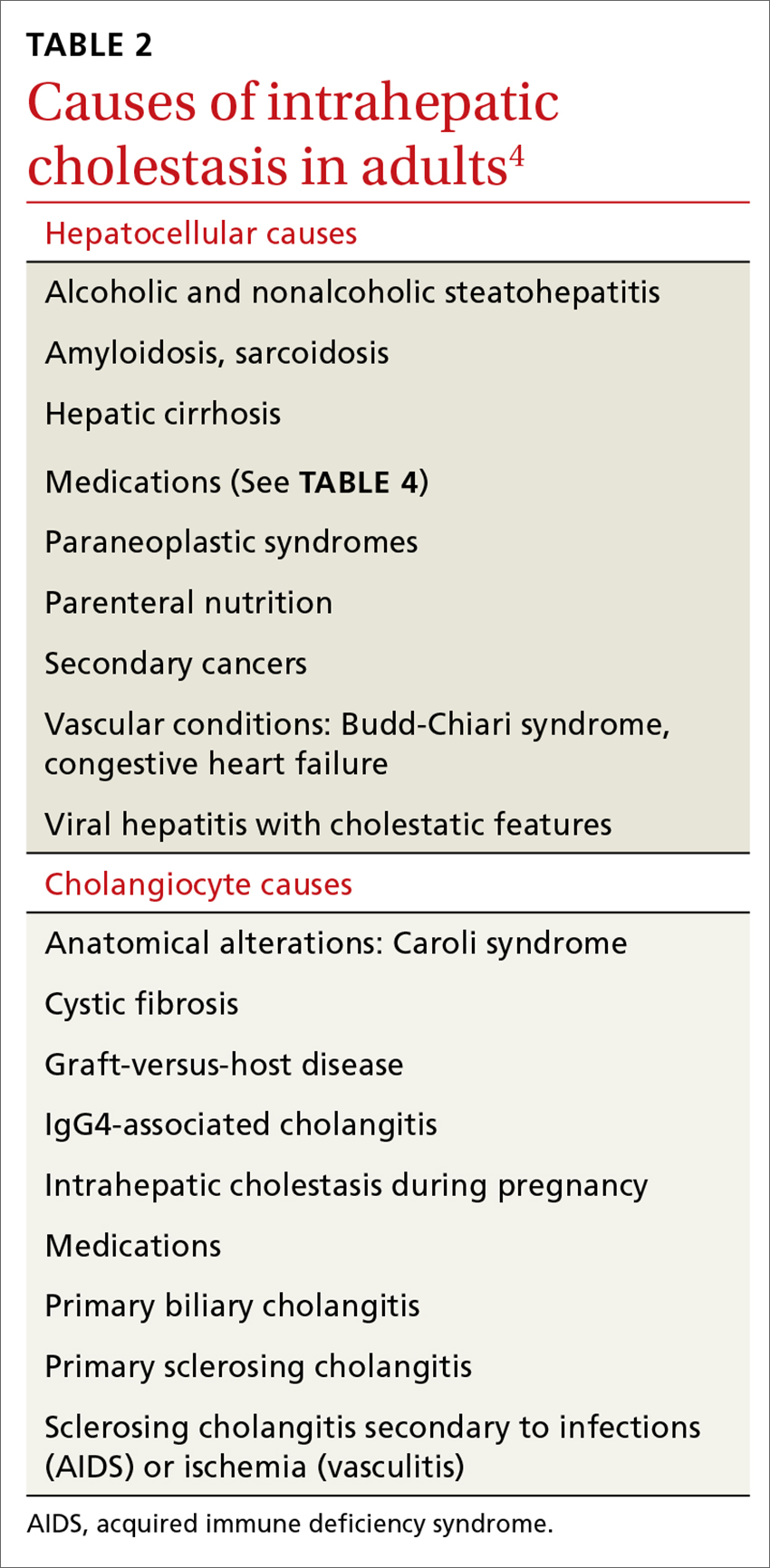
Elevated serum aminotransferase levels (aspartate aminotransferase [AST] >0.5 times the UNL or 17.5 U/L; ALT >0.5 times the UNL or >18 U/L) and bilirubin (>1.1 mg/dL), with predominance of the conjugated form (TABLE 18), suggest possible cholestasis. In light of such findings, a clinician’s next step should be to distinguish intrahepatic from extrahepatic conditions. (For a detailed list of the causes of intra- and extrahepatic cholestasis, see TABLES 24 and 3.9)

Patient’s history can provide important clues
A thorough patient history is especially important when cholestasis is suspected. Details about the patient’s occupation, environment, and lifestyle are key, as are the specifics of prescribed or over-the-counter medications and supplements that could be hepatotoxic (TABLE 410). A number of exogenous substances can cause liver injury, and the use of some herbal products (senna, black cohosh, greater celandine, kava) have been linked to hepatitis and cholestasis.11 Ask patients about alcohol use and history of conditions associated with liver disease, such as diabetes, hyperlipidemia, and thyroid disorders.

Continue to: Indicators pointing to cholestasis? It's time for ultrasonography
Indicators pointing to cholestasis? It’s time for ultrasonography
While biopsy is considered the gold standard for diagnosing and staging chronic cholestatic liver disease and can exclude an extrahepatic obstruction, it should be employed only if blood tests have been confirmed, second-level tests have been performed, and ultrasound is inconclusive.12 (More on biopsy in a bit.)
Ultrasonography is a low-cost, widely available, noninvasive test that allows easy identification of extrahepatic dilatation of the biliary tree and sometimes the underlying cause, as well. Ultrasonography identifies extrahepatic cholestasis by allowing visualization of an enlarged choledochus (>7 mm) or common hepatic duct (>5 mm) and an intrahepatic bile duct diameter that is more than 40% larger than adjacent branches of the portal vein.13 However, ultrasonography has a low diagnostic sensitivity for many conditions (eg, 15% to 89% for detecting common bile duct stones),14 requiring other diagnostic procedures, such as endoscopic retrograde cholangiopancreatography (ERCP) or magnetic resonance cholangiopancreatography (MRCP), before reaching a diagnosis.
For asymptomatic patients with cirrhosis or those at an early stage of liver disease, ultrasound at 6-month intervals combined with serum liver function tests can be useful to track disease progression and screen for hepatocellular carcinoma or cholangiocarcinoma.15,16
New noninvasive methods. Noninvasive tools for evaluating the presence and severity of liver fibrosis and for differentiating cirrhosis from noncirrhotic conditions have positive predictive values >85% to 90% for some chronic liver diseases.17 Transient elastography, which assesses liver stiffness, is one such method. Although it is often used successfully, morbid obesity, small intercostal spaces, and ascites limit its diagnostic capability.18 Recently, some questions about the validity of elastography to assess the extent of fibrosis in patients with chronic cholestatic conditions have been reported.19,20
Suspect intrahepatic cholestasis? Your next steps
If imaging techniques do not show bile duct obstruction and you suspect the intrahepatic form, second-level tests could have strategic importance. This is where antimitochondrial antibodies (AMAs) come in. AMAs are immunoglobulins (IgG and IgM) directed against mitochondrial antigens. They are important markers for PBC, which is a T-lymphocyte-mediated attack on small intralobular bile ducts resulting in their gradual destruction and eventual disappearance. The sustained loss of intralobular bile ducts leads to signs and symptoms of cholestasis and eventually results in cirrhosis and liver failure.
AMA serum levels show high sensitivity and specificity (90% and 95%, respectively) for PBC.21 Some PBC patients (<5%) show histologic confirmation of the disease, but have negative AMA tests (AMA negative PBC or autoimmune cholangitis).22 Therefore, according to the American Association for the Study of Liver Diseases, diagnosis of PBC is guided by the combination of serologic, biochemical, and histologic criteria.23 Many PBC patients with or without a positive AMA (≥1:40) also have positive circulating antinuclear antibodies (ANA; ≥1:80). The recent availability of lab tests for antibodies (anti-M2, anti-gp120, anti-sp100) has allowed identification of subgroups of patients who have a more aggressive form of PBC. Patients with PBC often have elevated levels of circulating IgM (>280 mg/dL).
Continue to: Other circulating antibodies
Other circulating antibodies can help discriminate among cholestatic disorders. In particular, positive tests for perinuclear anti-neutrophil cytoplasmic antibodies (pANCA) are found in 25% to 95% of patients with primary sclerosing cholangitis (PSC), a chronic progressive disorder of unknown etiology that is characterized by inflammation, fibrosis, and stricturing of medium and large ducts of the intrahepatic and extrahepatic biliary tree.24 Anti-smooth muscle antibodies (SMA) can be observed in both PSC and autoimmune hepatitis.
Finally, there are syndromes with serologic and histologic overlap that are characterized by the simultaneous presence of PBC with autoimmune hepatitis or PSC or overlap of PSC with autoimmune hepatitis.
Liver biopsy fills in the rest of the diagnostic picture
Unfortunately, blood tests reveal little about organ integrity and are not useful for disease staging. The decision to perform a liver biopsy should be based on several factors, including the patient’s age, serum parameters, the need to stage the disease, therapy choices, and prognosis.12 One should also consider that biopsy is a costly procedure with potentially serious adverse effects; it should not be repeated frequently. However, when a biopsy is done, it provides critical information, including damage to medium-sized intrahepatic bile ducts with neoductular formation or bile duct scars and strictures.
Treating intrahepatic cholestasis
Although FPs often can provide most—or even all—of the care for patients with stable conditions, a specialist consultation might recommend further testing to identify the underlying disease, which is essential to establish the most appropriate treatment.
Treatment of patients with PBC is based on administering hydrophilic secondary bile salt ursodeoxycholic acid (UDCA) 15 mg/kg/d, which is used to equilibrate the ratio between hydrophilic and hydrophobic bile salts in the liver and bile,25 and is the only treatment approved by the US Food and Drug Administration (FDA) for PBC.4 Tauroursodeoxycholate is better absorbed than UDCA, and, although partially deconjugated and reconjugated with glycine, it undergoes reduced biotransformation to more hydrophobic metabolites and has benefits, including antioxidant, immunomodulation, and neuroprotective effects over UDCA—especially for long-term therapy in PBC.26 However, it is not used often in clinical practice.
Continue to: Bile acid administration counters the cytotoxic effect...
Bile acid administration counters the cytotoxic effect of hydrophobic bile salts. Although it seems that UDCA might improve biochemical and histologic features of the disease at earlier stages (I-II), it fails in patients with more advanced disease.27 In addition, monitoring and defining response to UDCA is inconsistent, partly because of variations in guideline criteria.28,29
Recently a new molecule, obeticholic acid (OCA), has been approved by the FDA. A farnesoid X receptor agonist, OCA is indicated for treating patients who do not tolerate UDCA or as an adjunct to UDCA in those with a partial response to UDCA, defined as lowering ALP levels by <1.5 times the baseline value after 12 months of treatment.
Treating PSC is more complex. Combination therapy with prednisone and azathioprine is recommended only when there is an overlap syndrome between PSC and autoimmune hepatitis.4 UDCA at a high dosage (15-20 mg/kg/d) is used to facilitate long-lasting biochemical remission. These patients also need to be monitored for inflammatory bowel diseases, which affect up to 75% of patients,30 and for cholangiocarcinoma, which is a life-limiting complication because of a lack of therapy options. Finally, these patients might need endoscopic-guided dilatation of the biliary tree when they have evidence of dominant fibrotic strictures of the greater bile ducts.14,31
Addressing the systemic effects of intrahepatic cholestasis
Pruritus. A number of potential pruritogens, including bile salts, endogenous opioids, histamine, serotonin, and lisophosphatidic acid (LPA), can be targeted to relieve pruritus.
- Bile acid resin binders such as cholestyramine are the first step for treating pruritus. UDCA also can be useful, mainly for intrahepatic cholestasis during pregnancy. Rifampicin, 300 mg/d, improves cholestatic pruritus, but is associated with hepatotoxicity and a number of severe reactions, such as nausea, loss of appetite, hemolytic anemia, and thrombocytopenia.31
- Most evidence favors a role for opioids in relieving itch, and micro-opioid receptor antagonists (naltrexone, naloxone, nalmefene) that exert an antipruritic effect can be effective.
- Sertraline (a selective serotonin reuptake inhibitor), 50 to 75 mg/d, usually is well tolerated in patients with chronic cholestasis and exerts a beneficial effect on pruritus in approximately 40% of patients.32
- Extracorporeal albumin dialysis removes albumin-bound pruritogens and has been found to be effective in patients with liver failure. Steroids and UV light also can be used in select patients.
- The potent neuronal activator LPA and its converting enzyme autotaxin have been identified in the serum of patients with cholestatic pruritus; experimental modalities using LPA antagonists are ongoing for treating pruritus in patients who do not respond to other medications.33
Continue to: Malnutrition
Malnutrition. Many patients with cholestasis are at risk for malnutrition, which can be exacerbated in those with cirrhosis. Causes of malnutrition include poor oral intake, malabsorption, or dental problems that prevent the patient from chewing. Assess the nutritional status of every patient with chronic cholestasis, and stress the importance of multivitamin supplementation to reverse systemic alterations caused by malnutrition.34
When the patient has advanced disease
Despite progress in diagnostic techniques, life expectancy and quality of life for patients with advanced cholestatic conditions remain poor. Patients routinely experience fatigue, pruritus, and complications of cirrhosis including ascites, encephalopathy, and bleeding. Cholestasis also carries the risk of life-threatening complications, partly because of comorbidities such as osteoporosis and malabsorption.
Liver transplantation can improve the life expectancy of patients with advanced disease, but because of long waiting lists, candidates for transplant often die before an organ becomes available. For many patients who are not in end-stage condition, targeted therapy is crucial to slow disease progression and is recommended along with hepatitis A and B vaccinations and nutritional counseling.35
Extrahepatic cholestasis is suspected? How to proceed
Computer tomography (CT) is recommended for better identification of neoplastic causes of biliary obstruction and for staging purposes. MRCP is an excellent noninvasive imaging technique for evaluating biliary ducts.36
MRCP has 92% to 93% sensitivity and 97% to 98% specificity for diagnosing biliary duct stones.37 MRCP also is the first-choice modality for evaluating bile ducts in patients with suspected PSC. If performed in expert centers, the diagnostic accuracy reaches that of ERCP. A meta-analysis of studies from 2000 to 2006 has shown a sensitivity of 86% and specificity of 94% for diagnosing PSC.38
Endoscopic ultrasonography, which uses an ultrasonographic probe, allows clinicians to evaluate the integrity of the biliary and pancreatic ducts and is effective for diagnosing and staging cancer of the ampulla of Vater (sensitivity 93% vs 7% for abdominal ultrasonography and 29% for CT), and identifying biliary stones and biliary tree strictures.
Continue to: ERCP
ERCP is widely employed for diagnosing and treating pancreatobiliary diseases; however, its use has dropped over the last 10 years because of the risk of complications. ERCP is nearly exclusively used as a therapeutic procedure for pancreatic sphincterotomy, biliary dilatations, and removing biliary stones. It also has a diagnostic role in dominant stenosis or suspected biliary malignancy using brushing cytology and sampling biopsies of the bile ducts.
Treating extrahepatic cholestasis
Treatment of the different underlying conditions that cause extrahepatic cholestasis is surgical. Thus, the potential surgical techniques that can resolve or improve an extrahepatic cholestatic condition are guided by the surgeon and beyond the scope of this article.
Treating osteopenia: A concern for intra- and extrahepatic cholestasis
Vitamin D deficiency as a consequence of reduced intestinal absorption (poor availability of bile salts) or decreased hepatic activation to 25,OH-cholecalcipherol in both intrahepatic and extrahepatic cholestasis can lead to reduced bone formation.39 However, osteopenia can occur even in early stages of the disease. Prescribing bisphosphonates, in combination with calcium and vitamin D3, to improve bone mineral density is a good practice.40
CASE
Blood tests and ultrasound imaging suggest the presence of a chronic liver disease. Other lab tests indicate that the patient has an ALP level 3 times normal. This finding, together with the other tests, points to a likely diagnosis of intrahepatic cholestatic liver disease. Serology confirms positivity for ANA (1:160) and AMA (1:640). The clinician suspects PBC, so the patient is referred to a liver specialist for further evaluation and to determine whether a liver biopsy is needed.
The liver specialist confirms the diagnosis of PBC, performs a transient elastographym, which indicates a low-grade liver fibrosis (F1 out of 4), and starts therapy with UDCA.
CORRESPONDENCE
Ignazio Grattagliano, MD, Italian College of General Practitioners and Primary Care, Via del Sansovino 179, 50142, Florence, Italy; [email protected].
1. Kremer AE, Namer B, Bolier R, et al. Pathogenesis and management of pruritus in PBC and PSC. Dig Dis. 2015;33(suppl 2):164-175.
2. Deska Pagana K, Pagana TJ. Mosby’s Diagnostic and Laboratory Test Reference. 13th ed. St. Louis, MO: Elsevier; 2017.
3. Sapey T, Mendler MH, Guyader D, et al. Respective value of alkaline phosphatase, gamma-glutamyl transpeptidase and 5’ nucleotidase serum activity in the diagnosis of cholestasis: a prospective study of 80 patients. J Clin Gastroenterol. 2000;30:259-263.
4. European Association for the Study of the Liver. EASL Clinical practice guidelines: management of cholestatic liver diseases. J Hepatol. 2009;51:237-267.
5. Lammers WJ, van Buuren HR, Hirschfield GM, et al; Global PBC Study Group. Levels of alkaline phosphatase and bilirubin are surrogate end points of outcomes of patients with primary biliary cirrhosis: an international follow-up study. Gastroenterology. 2014;147:1338-1349.
6. Trivedi PJ, Corpechot C, Pares A, et al. Risk stratification in autoimmune cholestatic liver diseases: opportunities for clinicians and trialists. Hepatology. 2016;63:644-659.
7. Lammers WJ, Hirschfield GM, Corpechot C, et al. Development and validation of a scoring system to predict outcomes of patients with primary biliary cirrhosis receiving ursodeoxycholic acid therapy. Gastroenterology. 2015;149:1804-1812.
8. Johnston DE. Special considerations in interpreting liver function tests. Am Fam Physician. 1999;59:2223-2230.
9. Assy N, Jacob G, Spira G, et al. Diagnostic approach to patients with cholestatic jaundice. World J Gastroenterol. 1999;5:252-262.
10. Padda MS, Sanchez M, Akhtar AJ, et al. Drug-induced cholestasis. Hepatology. 2011;53:1377-1387.
11. US Food and Drug Administration. Food. Consumer advisory: kava-containing dietary supplements may be associated with severe liver injury. March 25, 2002. Available at: http://wayback.archive-it.org/7993/20171114232640/https://www.fda.gov/Food/RecallsOutbreaksEmergencies/SafetyAlertsAdvisories/ucm085482.htm. Accessed June 19, 2018.
12. Green RM, Flamm S. AGA technical review on the evaluation of liver chemistry tests. Gastroenterology. 2002;123:1367-1384.
13. Rogoveanu I, Gheonea DI, Saftoiu A, et al. The role of imaging methods in identifying the causes of extrahepatic cholestasis. J Gastrointestin Liver Dis. 2006;15:265-271.
14. Gotthardt DN, Rudolph G, Klöters-Plachky P, et al. Endoscopic dilation of dominant stenoses in primary sclerosing cholangitis: outcome after long-term treatment. Gastrointest Endosc. 2010;71:527-534.
15. Fitzmorris P, Singal AK. Surveillance and diagnosis of hepatocellular carcinoma. Gastroenterol Hepatol (NY). 2015;11:38-46.
16. Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022.
17. Pinzani M, Vizzutti F, Arena U, et al. Technology insight: noninvasive assessment of liver fibrosis by biochemical scores and elastography. Nat Clin Pract Gastroenterol Hepatol. 2008;5:95-106.
18. Castéra L, Vergniol J, Foucher J, et al. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343-350.
19. Van Gossum A, Pironi L, Messing B, et al. Transient elastography (FibroScan) is not correlated with liver fibrosis but with cholestasis in patients with long-term home parenteral nutrition. JPEN. 2015;39:719-724.
20. Millonig G, Reimann FM, Friedrich S, et al. Extrahepatic cholestasis increases liver stiffness (FibroScan) irrespective of fibrosis. Hepatology. 2008;48:1718-1723.
21. European Association for the Study of the Liver. EASL clinical practice guidelines: the diagnosis and management of patients with primary biliary cholangitis. J Hepatol. 2017;67:145-172.
22. Ozaslan E, Efe C, Gokbulut Ozaslan N. The diagnosis of antimitochondrial antibody-negative primary biliary cholangitis. Clin Res Hepatol Gastroenterol. 2016;40:553-561.
23. Lindor KD, Gershwin ME, Poupon R, et al; American Association for Study of Liver Diseases. Primary biliary cirrhosis. Hepatology. 2009;50:291-308.
24. Hov JR, Boberg KM, Karlsen TH. Autoantibodies in primary sclerosing cholangitis. World J Gastroenterol. 2008;14:3781-3791.
25. Dilger K, Hohenester S, Winkler-Budenhofer U, et al. Effect of ursodeoxycholic acid on bile acid profiles and intestinal detoxification machinery in primary biliary cirrhosis and health. J Hepatol. 2012;57:133-140.
26. Invernizzi P, Setchell KD, Crosignani A, et al. Differences in the metabolism and disposition of ursodeoxycholic acid and of its taurine-conjugated species in patients with primary biliary cirrhosis. Hepatology. 1999;29:320-327.
27. Jorgensen R, Angulo P, Dickson ER, et al. Results of long-term ursodiol treatment for patients with primary biliary cirrhosis. Am J Gastroenterol. 2002;97:2647-2650.
28. Parés A, Caballería L, Rodés J. Excellent long-term survival in patients with primary biliary cirrhosis and biochemical response to ursodeoxycholic acid. Gastroenterology. 2006;130:715-720.
29. Corpechot C, Abenavoli L, Rabahi N, et al. Biochemical response to ursodeoxycholic acid and long-term prognosis in primary biliary cirrhosis. Hepatology. 2008;48:871-877.
30. Levine JS, Burakoff R. Extraintestinal manifestations of inflammatory bowel disease. Gastroenterol Hepatol (NY). 2011;7:235-241.
31. Rodriguez HJ, Bass NM. Primary sclerosing cholangitis. Semin Gastrointest Dis. 2003;14:189-198.
32. Tajiri K, Shimizu Y. Recent advances in the management of pruritus in chronic liver diseases. World J Gastroenterol. 2017;23:3418-3426.
33. Kremer AE, Namer B, Bolier R, et al. Pathogenesis and management of pruritus in PBC and PSC. Dig Dis. 2015;33(suppl 2):164-175.
34. Buyse S, Durand F, Joly F. Nutritional assessment in cirrhosis. Gastroenterol Clin Biol. 2008;32:265-273.
35. Fagiuoli S, Colli A, Bruno R, et al; 2011 AISF Single Topic Group. Management of infections pre- and post-liver transplantation: report of an AISF consensus conference. J Hepatol. 2014;60:1075-1089.
36. Kanaan Z, Antaki F. Magnetic resonance cholangiopancreatography still plays a role in the preoperative evaluation of choledocholithiasis and biliary pathology. J Am Coll Surg. 2016;222:325-326.
37. McMahon CJ. The relative roles of magnetic resonance cholangiopancreatography (MRCP) and endoscopic ultrasound in diagnosis of common bile duct calculi: a critically appraised topic. Abdom Imaging. 2008;33:6-9.
38. Njei B, McCarty TR, Varadarajulu S, et al. Systematic review with meta-analysis: endoscopic retrograde cholangiopancreatography-based modalities for the diagnosis of cholangiocarcinoma in primary sclerosing cholangitis. Aliment Pharmacol Ther. 2016;44:1139-1151.
39. Wimalawansa SJ, Razzaque DMS, Al-Daghri NM. Calcium and vitamin D in human health: hype or real? J Steroid Biochem Mol Biol. 2017. doi: 10.1016/j.jsbmb.2017.12.009.
40. Yadav A, Carey EJ. Osteoporosis in chronic liver disease. Nutr Clin Pract. 2013;28:52-64.
CASE
A 44-year-old nurse describes persistent fatigue and itching over the last 2 months. She is taking ramipril 5 mg/d for hypertension and has a family history of rheumatic disease. Lab tests reveal a recurrent moderate elevation of gamma glutamyl-transpeptidase (gGT; 75 U/L) associated with, on some occasions, mild elevation of alanine aminotransferase (ALT) levels (100 U/L) of unknown origin. She has no history of hepatitis virus infection, hepatotoxic medications, or alcohol intake. She is overweight with a body mass index of 28.5 kg/m2 and a waist circumference of 99 cm (39 inches). Liver ultrasonography detects an enlarged liver with diffuse echostructure dishomogeneity, but no signs of cirrhosis or portal hypertension. The patient’s biliary tree is not dilated.
How would you proceed with the care of this patient?
Cholestasis is characterized by the alteration of bile flow through any part of the biliary system, from the hepatocyte basocellular membrane to the duodenum. The condition is classified as intrahepatic when the cause is a defect of hepatocellular function or obstruction of the biliary tree within the liver. The extrahepatic form includes all conditions obstructing bile flow in the main biliary tract (choledochus, common bile duct).
The key to successfully managing cholestasis lies in the early identification of subtle signs and symptoms before serious complications can arise. In the review that follows, we provide guidance for evaluating laboratory and imaging results that are vital to the accurate diagnosis of intrahepatic and extrahepatic cholestasis. We also detail treatment recommendations.
Clues—subtle and otherwise—of cholestasis
Clinical features of cholestasis include fatigue and itching all over the skin. The latter likely is caused by induction of the enzyme autotaxin, which produces the neuronal activator lysophosphatidic acid. Retention of pruritogenic substances that normally are excreted into bile might contribute to pruritus as well.1 Jaundice, dark urine, and pale and fatty stools occur with advanced disease. However, a cholestatic condition can be detected in asymptomatic patients with elevated biochemical markers.
Continue to: Mildly elevated gGT and/or alkaline phosphatase (ALP)
Mildly elevated gGT and/or alkaline phosphatase (ALP) (0.5-2.5 times the upper normal limit [UNL] or 19-95 U/L and 60-300 U/L, respectively2) in the presence of normal transaminase levels (<20 U/L) in an asymptomatic patient can indicate chronic liver disease. Signs suggestive of significant liver disease have been reported in many patients with gGT or ALP elevation with good sensitivity (65%) and specificity (83%) for a diagnosis of intrahepatic cholestasis.3 However, because abnormal gGT values are common and often resolve spontaneously, family physicians (FPs) may pay little attention to this finding, thus missing an opportunity for early identification and treatment.

That’s why it’s important to schedule follow-up testing within 6 months for asymptomatic patients with abnormal laboratory findings. Persistent elevation of gGT alone or accompanied by ALP and ALT elevation (ALT >0.5 times the UNL or >18 U/L) is the most common feature of a chronic (>6 months) cholestatic condition.4 (In particular, elevated ALP levels appear to be associated with more aggressive disease and predict risk of liver transplantation or death in patients with primary biliary cholangitis (PBC).5,6 Lowering ALP levels is associated with improved disease outcomes, including transplant-free survival rates.5,7)

Elevated serum aminotransferase levels (aspartate aminotransferase [AST] >0.5 times the UNL or 17.5 U/L; ALT >0.5 times the UNL or >18 U/L) and bilirubin (>1.1 mg/dL), with predominance of the conjugated form (TABLE 18), suggest possible cholestasis. In light of such findings, a clinician’s next step should be to distinguish intrahepatic from extrahepatic conditions. (For a detailed list of the causes of intra- and extrahepatic cholestasis, see TABLES 24 and 3.9)

Patient’s history can provide important clues
A thorough patient history is especially important when cholestasis is suspected. Details about the patient’s occupation, environment, and lifestyle are key, as are the specifics of prescribed or over-the-counter medications and supplements that could be hepatotoxic (TABLE 410). A number of exogenous substances can cause liver injury, and the use of some herbal products (senna, black cohosh, greater celandine, kava) have been linked to hepatitis and cholestasis.11 Ask patients about alcohol use and history of conditions associated with liver disease, such as diabetes, hyperlipidemia, and thyroid disorders.

Continue to: Indicators pointing to cholestasis? It's time for ultrasonography
Indicators pointing to cholestasis? It’s time for ultrasonography
While biopsy is considered the gold standard for diagnosing and staging chronic cholestatic liver disease and can exclude an extrahepatic obstruction, it should be employed only if blood tests have been confirmed, second-level tests have been performed, and ultrasound is inconclusive.12 (More on biopsy in a bit.)
Ultrasonography is a low-cost, widely available, noninvasive test that allows easy identification of extrahepatic dilatation of the biliary tree and sometimes the underlying cause, as well. Ultrasonography identifies extrahepatic cholestasis by allowing visualization of an enlarged choledochus (>7 mm) or common hepatic duct (>5 mm) and an intrahepatic bile duct diameter that is more than 40% larger than adjacent branches of the portal vein.13 However, ultrasonography has a low diagnostic sensitivity for many conditions (eg, 15% to 89% for detecting common bile duct stones),14 requiring other diagnostic procedures, such as endoscopic retrograde cholangiopancreatography (ERCP) or magnetic resonance cholangiopancreatography (MRCP), before reaching a diagnosis.
For asymptomatic patients with cirrhosis or those at an early stage of liver disease, ultrasound at 6-month intervals combined with serum liver function tests can be useful to track disease progression and screen for hepatocellular carcinoma or cholangiocarcinoma.15,16
New noninvasive methods. Noninvasive tools for evaluating the presence and severity of liver fibrosis and for differentiating cirrhosis from noncirrhotic conditions have positive predictive values >85% to 90% for some chronic liver diseases.17 Transient elastography, which assesses liver stiffness, is one such method. Although it is often used successfully, morbid obesity, small intercostal spaces, and ascites limit its diagnostic capability.18 Recently, some questions about the validity of elastography to assess the extent of fibrosis in patients with chronic cholestatic conditions have been reported.19,20
Suspect intrahepatic cholestasis? Your next steps
If imaging techniques do not show bile duct obstruction and you suspect the intrahepatic form, second-level tests could have strategic importance. This is where antimitochondrial antibodies (AMAs) come in. AMAs are immunoglobulins (IgG and IgM) directed against mitochondrial antigens. They are important markers for PBC, which is a T-lymphocyte-mediated attack on small intralobular bile ducts resulting in their gradual destruction and eventual disappearance. The sustained loss of intralobular bile ducts leads to signs and symptoms of cholestasis and eventually results in cirrhosis and liver failure.
AMA serum levels show high sensitivity and specificity (90% and 95%, respectively) for PBC.21 Some PBC patients (<5%) show histologic confirmation of the disease, but have negative AMA tests (AMA negative PBC or autoimmune cholangitis).22 Therefore, according to the American Association for the Study of Liver Diseases, diagnosis of PBC is guided by the combination of serologic, biochemical, and histologic criteria.23 Many PBC patients with or without a positive AMA (≥1:40) also have positive circulating antinuclear antibodies (ANA; ≥1:80). The recent availability of lab tests for antibodies (anti-M2, anti-gp120, anti-sp100) has allowed identification of subgroups of patients who have a more aggressive form of PBC. Patients with PBC often have elevated levels of circulating IgM (>280 mg/dL).
Continue to: Other circulating antibodies
Other circulating antibodies can help discriminate among cholestatic disorders. In particular, positive tests for perinuclear anti-neutrophil cytoplasmic antibodies (pANCA) are found in 25% to 95% of patients with primary sclerosing cholangitis (PSC), a chronic progressive disorder of unknown etiology that is characterized by inflammation, fibrosis, and stricturing of medium and large ducts of the intrahepatic and extrahepatic biliary tree.24 Anti-smooth muscle antibodies (SMA) can be observed in both PSC and autoimmune hepatitis.
Finally, there are syndromes with serologic and histologic overlap that are characterized by the simultaneous presence of PBC with autoimmune hepatitis or PSC or overlap of PSC with autoimmune hepatitis.
Liver biopsy fills in the rest of the diagnostic picture
Unfortunately, blood tests reveal little about organ integrity and are not useful for disease staging. The decision to perform a liver biopsy should be based on several factors, including the patient’s age, serum parameters, the need to stage the disease, therapy choices, and prognosis.12 One should also consider that biopsy is a costly procedure with potentially serious adverse effects; it should not be repeated frequently. However, when a biopsy is done, it provides critical information, including damage to medium-sized intrahepatic bile ducts with neoductular formation or bile duct scars and strictures.
Treating intrahepatic cholestasis
Although FPs often can provide most—or even all—of the care for patients with stable conditions, a specialist consultation might recommend further testing to identify the underlying disease, which is essential to establish the most appropriate treatment.
Treatment of patients with PBC is based on administering hydrophilic secondary bile salt ursodeoxycholic acid (UDCA) 15 mg/kg/d, which is used to equilibrate the ratio between hydrophilic and hydrophobic bile salts in the liver and bile,25 and is the only treatment approved by the US Food and Drug Administration (FDA) for PBC.4 Tauroursodeoxycholate is better absorbed than UDCA, and, although partially deconjugated and reconjugated with glycine, it undergoes reduced biotransformation to more hydrophobic metabolites and has benefits, including antioxidant, immunomodulation, and neuroprotective effects over UDCA—especially for long-term therapy in PBC.26 However, it is not used often in clinical practice.
Continue to: Bile acid administration counters the cytotoxic effect...
Bile acid administration counters the cytotoxic effect of hydrophobic bile salts. Although it seems that UDCA might improve biochemical and histologic features of the disease at earlier stages (I-II), it fails in patients with more advanced disease.27 In addition, monitoring and defining response to UDCA is inconsistent, partly because of variations in guideline criteria.28,29
Recently a new molecule, obeticholic acid (OCA), has been approved by the FDA. A farnesoid X receptor agonist, OCA is indicated for treating patients who do not tolerate UDCA or as an adjunct to UDCA in those with a partial response to UDCA, defined as lowering ALP levels by <1.5 times the baseline value after 12 months of treatment.
Treating PSC is more complex. Combination therapy with prednisone and azathioprine is recommended only when there is an overlap syndrome between PSC and autoimmune hepatitis.4 UDCA at a high dosage (15-20 mg/kg/d) is used to facilitate long-lasting biochemical remission. These patients also need to be monitored for inflammatory bowel diseases, which affect up to 75% of patients,30 and for cholangiocarcinoma, which is a life-limiting complication because of a lack of therapy options. Finally, these patients might need endoscopic-guided dilatation of the biliary tree when they have evidence of dominant fibrotic strictures of the greater bile ducts.14,31
Addressing the systemic effects of intrahepatic cholestasis
Pruritus. A number of potential pruritogens, including bile salts, endogenous opioids, histamine, serotonin, and lisophosphatidic acid (LPA), can be targeted to relieve pruritus.
- Bile acid resin binders such as cholestyramine are the first step for treating pruritus. UDCA also can be useful, mainly for intrahepatic cholestasis during pregnancy. Rifampicin, 300 mg/d, improves cholestatic pruritus, but is associated with hepatotoxicity and a number of severe reactions, such as nausea, loss of appetite, hemolytic anemia, and thrombocytopenia.31
- Most evidence favors a role for opioids in relieving itch, and micro-opioid receptor antagonists (naltrexone, naloxone, nalmefene) that exert an antipruritic effect can be effective.
- Sertraline (a selective serotonin reuptake inhibitor), 50 to 75 mg/d, usually is well tolerated in patients with chronic cholestasis and exerts a beneficial effect on pruritus in approximately 40% of patients.32
- Extracorporeal albumin dialysis removes albumin-bound pruritogens and has been found to be effective in patients with liver failure. Steroids and UV light also can be used in select patients.
- The potent neuronal activator LPA and its converting enzyme autotaxin have been identified in the serum of patients with cholestatic pruritus; experimental modalities using LPA antagonists are ongoing for treating pruritus in patients who do not respond to other medications.33
Continue to: Malnutrition
Malnutrition. Many patients with cholestasis are at risk for malnutrition, which can be exacerbated in those with cirrhosis. Causes of malnutrition include poor oral intake, malabsorption, or dental problems that prevent the patient from chewing. Assess the nutritional status of every patient with chronic cholestasis, and stress the importance of multivitamin supplementation to reverse systemic alterations caused by malnutrition.34
When the patient has advanced disease
Despite progress in diagnostic techniques, life expectancy and quality of life for patients with advanced cholestatic conditions remain poor. Patients routinely experience fatigue, pruritus, and complications of cirrhosis including ascites, encephalopathy, and bleeding. Cholestasis also carries the risk of life-threatening complications, partly because of comorbidities such as osteoporosis and malabsorption.
Liver transplantation can improve the life expectancy of patients with advanced disease, but because of long waiting lists, candidates for transplant often die before an organ becomes available. For many patients who are not in end-stage condition, targeted therapy is crucial to slow disease progression and is recommended along with hepatitis A and B vaccinations and nutritional counseling.35
Extrahepatic cholestasis is suspected? How to proceed
Computer tomography (CT) is recommended for better identification of neoplastic causes of biliary obstruction and for staging purposes. MRCP is an excellent noninvasive imaging technique for evaluating biliary ducts.36
MRCP has 92% to 93% sensitivity and 97% to 98% specificity for diagnosing biliary duct stones.37 MRCP also is the first-choice modality for evaluating bile ducts in patients with suspected PSC. If performed in expert centers, the diagnostic accuracy reaches that of ERCP. A meta-analysis of studies from 2000 to 2006 has shown a sensitivity of 86% and specificity of 94% for diagnosing PSC.38
Endoscopic ultrasonography, which uses an ultrasonographic probe, allows clinicians to evaluate the integrity of the biliary and pancreatic ducts and is effective for diagnosing and staging cancer of the ampulla of Vater (sensitivity 93% vs 7% for abdominal ultrasonography and 29% for CT), and identifying biliary stones and biliary tree strictures.
Continue to: ERCP
ERCP is widely employed for diagnosing and treating pancreatobiliary diseases; however, its use has dropped over the last 10 years because of the risk of complications. ERCP is nearly exclusively used as a therapeutic procedure for pancreatic sphincterotomy, biliary dilatations, and removing biliary stones. It also has a diagnostic role in dominant stenosis or suspected biliary malignancy using brushing cytology and sampling biopsies of the bile ducts.
Treating extrahepatic cholestasis
Treatment of the different underlying conditions that cause extrahepatic cholestasis is surgical. Thus, the potential surgical techniques that can resolve or improve an extrahepatic cholestatic condition are guided by the surgeon and beyond the scope of this article.
Treating osteopenia: A concern for intra- and extrahepatic cholestasis
Vitamin D deficiency as a consequence of reduced intestinal absorption (poor availability of bile salts) or decreased hepatic activation to 25,OH-cholecalcipherol in both intrahepatic and extrahepatic cholestasis can lead to reduced bone formation.39 However, osteopenia can occur even in early stages of the disease. Prescribing bisphosphonates, in combination with calcium and vitamin D3, to improve bone mineral density is a good practice.40
CASE
Blood tests and ultrasound imaging suggest the presence of a chronic liver disease. Other lab tests indicate that the patient has an ALP level 3 times normal. This finding, together with the other tests, points to a likely diagnosis of intrahepatic cholestatic liver disease. Serology confirms positivity for ANA (1:160) and AMA (1:640). The clinician suspects PBC, so the patient is referred to a liver specialist for further evaluation and to determine whether a liver biopsy is needed.
The liver specialist confirms the diagnosis of PBC, performs a transient elastographym, which indicates a low-grade liver fibrosis (F1 out of 4), and starts therapy with UDCA.
CORRESPONDENCE
Ignazio Grattagliano, MD, Italian College of General Practitioners and Primary Care, Via del Sansovino 179, 50142, Florence, Italy; [email protected].
CASE
A 44-year-old nurse describes persistent fatigue and itching over the last 2 months. She is taking ramipril 5 mg/d for hypertension and has a family history of rheumatic disease. Lab tests reveal a recurrent moderate elevation of gamma glutamyl-transpeptidase (gGT; 75 U/L) associated with, on some occasions, mild elevation of alanine aminotransferase (ALT) levels (100 U/L) of unknown origin. She has no history of hepatitis virus infection, hepatotoxic medications, or alcohol intake. She is overweight with a body mass index of 28.5 kg/m2 and a waist circumference of 99 cm (39 inches). Liver ultrasonography detects an enlarged liver with diffuse echostructure dishomogeneity, but no signs of cirrhosis or portal hypertension. The patient’s biliary tree is not dilated.
How would you proceed with the care of this patient?
Cholestasis is characterized by the alteration of bile flow through any part of the biliary system, from the hepatocyte basocellular membrane to the duodenum. The condition is classified as intrahepatic when the cause is a defect of hepatocellular function or obstruction of the biliary tree within the liver. The extrahepatic form includes all conditions obstructing bile flow in the main biliary tract (choledochus, common bile duct).
The key to successfully managing cholestasis lies in the early identification of subtle signs and symptoms before serious complications can arise. In the review that follows, we provide guidance for evaluating laboratory and imaging results that are vital to the accurate diagnosis of intrahepatic and extrahepatic cholestasis. We also detail treatment recommendations.
Clues—subtle and otherwise—of cholestasis
Clinical features of cholestasis include fatigue and itching all over the skin. The latter likely is caused by induction of the enzyme autotaxin, which produces the neuronal activator lysophosphatidic acid. Retention of pruritogenic substances that normally are excreted into bile might contribute to pruritus as well.1 Jaundice, dark urine, and pale and fatty stools occur with advanced disease. However, a cholestatic condition can be detected in asymptomatic patients with elevated biochemical markers.
Continue to: Mildly elevated gGT and/or alkaline phosphatase (ALP)
Mildly elevated gGT and/or alkaline phosphatase (ALP) (0.5-2.5 times the upper normal limit [UNL] or 19-95 U/L and 60-300 U/L, respectively2) in the presence of normal transaminase levels (<20 U/L) in an asymptomatic patient can indicate chronic liver disease. Signs suggestive of significant liver disease have been reported in many patients with gGT or ALP elevation with good sensitivity (65%) and specificity (83%) for a diagnosis of intrahepatic cholestasis.3 However, because abnormal gGT values are common and often resolve spontaneously, family physicians (FPs) may pay little attention to this finding, thus missing an opportunity for early identification and treatment.

That’s why it’s important to schedule follow-up testing within 6 months for asymptomatic patients with abnormal laboratory findings. Persistent elevation of gGT alone or accompanied by ALP and ALT elevation (ALT >0.5 times the UNL or >18 U/L) is the most common feature of a chronic (>6 months) cholestatic condition.4 (In particular, elevated ALP levels appear to be associated with more aggressive disease and predict risk of liver transplantation or death in patients with primary biliary cholangitis (PBC).5,6 Lowering ALP levels is associated with improved disease outcomes, including transplant-free survival rates.5,7)

Elevated serum aminotransferase levels (aspartate aminotransferase [AST] >0.5 times the UNL or 17.5 U/L; ALT >0.5 times the UNL or >18 U/L) and bilirubin (>1.1 mg/dL), with predominance of the conjugated form (TABLE 18), suggest possible cholestasis. In light of such findings, a clinician’s next step should be to distinguish intrahepatic from extrahepatic conditions. (For a detailed list of the causes of intra- and extrahepatic cholestasis, see TABLES 24 and 3.9)

Patient’s history can provide important clues
A thorough patient history is especially important when cholestasis is suspected. Details about the patient’s occupation, environment, and lifestyle are key, as are the specifics of prescribed or over-the-counter medications and supplements that could be hepatotoxic (TABLE 410). A number of exogenous substances can cause liver injury, and the use of some herbal products (senna, black cohosh, greater celandine, kava) have been linked to hepatitis and cholestasis.11 Ask patients about alcohol use and history of conditions associated with liver disease, such as diabetes, hyperlipidemia, and thyroid disorders.

Continue to: Indicators pointing to cholestasis? It's time for ultrasonography
Indicators pointing to cholestasis? It’s time for ultrasonography
While biopsy is considered the gold standard for diagnosing and staging chronic cholestatic liver disease and can exclude an extrahepatic obstruction, it should be employed only if blood tests have been confirmed, second-level tests have been performed, and ultrasound is inconclusive.12 (More on biopsy in a bit.)
Ultrasonography is a low-cost, widely available, noninvasive test that allows easy identification of extrahepatic dilatation of the biliary tree and sometimes the underlying cause, as well. Ultrasonography identifies extrahepatic cholestasis by allowing visualization of an enlarged choledochus (>7 mm) or common hepatic duct (>5 mm) and an intrahepatic bile duct diameter that is more than 40% larger than adjacent branches of the portal vein.13 However, ultrasonography has a low diagnostic sensitivity for many conditions (eg, 15% to 89% for detecting common bile duct stones),14 requiring other diagnostic procedures, such as endoscopic retrograde cholangiopancreatography (ERCP) or magnetic resonance cholangiopancreatography (MRCP), before reaching a diagnosis.
For asymptomatic patients with cirrhosis or those at an early stage of liver disease, ultrasound at 6-month intervals combined with serum liver function tests can be useful to track disease progression and screen for hepatocellular carcinoma or cholangiocarcinoma.15,16
New noninvasive methods. Noninvasive tools for evaluating the presence and severity of liver fibrosis and for differentiating cirrhosis from noncirrhotic conditions have positive predictive values >85% to 90% for some chronic liver diseases.17 Transient elastography, which assesses liver stiffness, is one such method. Although it is often used successfully, morbid obesity, small intercostal spaces, and ascites limit its diagnostic capability.18 Recently, some questions about the validity of elastography to assess the extent of fibrosis in patients with chronic cholestatic conditions have been reported.19,20
Suspect intrahepatic cholestasis? Your next steps
If imaging techniques do not show bile duct obstruction and you suspect the intrahepatic form, second-level tests could have strategic importance. This is where antimitochondrial antibodies (AMAs) come in. AMAs are immunoglobulins (IgG and IgM) directed against mitochondrial antigens. They are important markers for PBC, which is a T-lymphocyte-mediated attack on small intralobular bile ducts resulting in their gradual destruction and eventual disappearance. The sustained loss of intralobular bile ducts leads to signs and symptoms of cholestasis and eventually results in cirrhosis and liver failure.
AMA serum levels show high sensitivity and specificity (90% and 95%, respectively) for PBC.21 Some PBC patients (<5%) show histologic confirmation of the disease, but have negative AMA tests (AMA negative PBC or autoimmune cholangitis).22 Therefore, according to the American Association for the Study of Liver Diseases, diagnosis of PBC is guided by the combination of serologic, biochemical, and histologic criteria.23 Many PBC patients with or without a positive AMA (≥1:40) also have positive circulating antinuclear antibodies (ANA; ≥1:80). The recent availability of lab tests for antibodies (anti-M2, anti-gp120, anti-sp100) has allowed identification of subgroups of patients who have a more aggressive form of PBC. Patients with PBC often have elevated levels of circulating IgM (>280 mg/dL).
Continue to: Other circulating antibodies
Other circulating antibodies can help discriminate among cholestatic disorders. In particular, positive tests for perinuclear anti-neutrophil cytoplasmic antibodies (pANCA) are found in 25% to 95% of patients with primary sclerosing cholangitis (PSC), a chronic progressive disorder of unknown etiology that is characterized by inflammation, fibrosis, and stricturing of medium and large ducts of the intrahepatic and extrahepatic biliary tree.24 Anti-smooth muscle antibodies (SMA) can be observed in both PSC and autoimmune hepatitis.
Finally, there are syndromes with serologic and histologic overlap that are characterized by the simultaneous presence of PBC with autoimmune hepatitis or PSC or overlap of PSC with autoimmune hepatitis.
Liver biopsy fills in the rest of the diagnostic picture
Unfortunately, blood tests reveal little about organ integrity and are not useful for disease staging. The decision to perform a liver biopsy should be based on several factors, including the patient’s age, serum parameters, the need to stage the disease, therapy choices, and prognosis.12 One should also consider that biopsy is a costly procedure with potentially serious adverse effects; it should not be repeated frequently. However, when a biopsy is done, it provides critical information, including damage to medium-sized intrahepatic bile ducts with neoductular formation or bile duct scars and strictures.
Treating intrahepatic cholestasis
Although FPs often can provide most—or even all—of the care for patients with stable conditions, a specialist consultation might recommend further testing to identify the underlying disease, which is essential to establish the most appropriate treatment.
Treatment of patients with PBC is based on administering hydrophilic secondary bile salt ursodeoxycholic acid (UDCA) 15 mg/kg/d, which is used to equilibrate the ratio between hydrophilic and hydrophobic bile salts in the liver and bile,25 and is the only treatment approved by the US Food and Drug Administration (FDA) for PBC.4 Tauroursodeoxycholate is better absorbed than UDCA, and, although partially deconjugated and reconjugated with glycine, it undergoes reduced biotransformation to more hydrophobic metabolites and has benefits, including antioxidant, immunomodulation, and neuroprotective effects over UDCA—especially for long-term therapy in PBC.26 However, it is not used often in clinical practice.
Continue to: Bile acid administration counters the cytotoxic effect...
Bile acid administration counters the cytotoxic effect of hydrophobic bile salts. Although it seems that UDCA might improve biochemical and histologic features of the disease at earlier stages (I-II), it fails in patients with more advanced disease.27 In addition, monitoring and defining response to UDCA is inconsistent, partly because of variations in guideline criteria.28,29
Recently a new molecule, obeticholic acid (OCA), has been approved by the FDA. A farnesoid X receptor agonist, OCA is indicated for treating patients who do not tolerate UDCA or as an adjunct to UDCA in those with a partial response to UDCA, defined as lowering ALP levels by <1.5 times the baseline value after 12 months of treatment.
Treating PSC is more complex. Combination therapy with prednisone and azathioprine is recommended only when there is an overlap syndrome between PSC and autoimmune hepatitis.4 UDCA at a high dosage (15-20 mg/kg/d) is used to facilitate long-lasting biochemical remission. These patients also need to be monitored for inflammatory bowel diseases, which affect up to 75% of patients,30 and for cholangiocarcinoma, which is a life-limiting complication because of a lack of therapy options. Finally, these patients might need endoscopic-guided dilatation of the biliary tree when they have evidence of dominant fibrotic strictures of the greater bile ducts.14,31
Addressing the systemic effects of intrahepatic cholestasis
Pruritus. A number of potential pruritogens, including bile salts, endogenous opioids, histamine, serotonin, and lisophosphatidic acid (LPA), can be targeted to relieve pruritus.
- Bile acid resin binders such as cholestyramine are the first step for treating pruritus. UDCA also can be useful, mainly for intrahepatic cholestasis during pregnancy. Rifampicin, 300 mg/d, improves cholestatic pruritus, but is associated with hepatotoxicity and a number of severe reactions, such as nausea, loss of appetite, hemolytic anemia, and thrombocytopenia.31
- Most evidence favors a role for opioids in relieving itch, and micro-opioid receptor antagonists (naltrexone, naloxone, nalmefene) that exert an antipruritic effect can be effective.
- Sertraline (a selective serotonin reuptake inhibitor), 50 to 75 mg/d, usually is well tolerated in patients with chronic cholestasis and exerts a beneficial effect on pruritus in approximately 40% of patients.32
- Extracorporeal albumin dialysis removes albumin-bound pruritogens and has been found to be effective in patients with liver failure. Steroids and UV light also can be used in select patients.
- The potent neuronal activator LPA and its converting enzyme autotaxin have been identified in the serum of patients with cholestatic pruritus; experimental modalities using LPA antagonists are ongoing for treating pruritus in patients who do not respond to other medications.33
Continue to: Malnutrition
Malnutrition. Many patients with cholestasis are at risk for malnutrition, which can be exacerbated in those with cirrhosis. Causes of malnutrition include poor oral intake, malabsorption, or dental problems that prevent the patient from chewing. Assess the nutritional status of every patient with chronic cholestasis, and stress the importance of multivitamin supplementation to reverse systemic alterations caused by malnutrition.34
When the patient has advanced disease
Despite progress in diagnostic techniques, life expectancy and quality of life for patients with advanced cholestatic conditions remain poor. Patients routinely experience fatigue, pruritus, and complications of cirrhosis including ascites, encephalopathy, and bleeding. Cholestasis also carries the risk of life-threatening complications, partly because of comorbidities such as osteoporosis and malabsorption.
Liver transplantation can improve the life expectancy of patients with advanced disease, but because of long waiting lists, candidates for transplant often die before an organ becomes available. For many patients who are not in end-stage condition, targeted therapy is crucial to slow disease progression and is recommended along with hepatitis A and B vaccinations and nutritional counseling.35
Extrahepatic cholestasis is suspected? How to proceed
Computer tomography (CT) is recommended for better identification of neoplastic causes of biliary obstruction and for staging purposes. MRCP is an excellent noninvasive imaging technique for evaluating biliary ducts.36
MRCP has 92% to 93% sensitivity and 97% to 98% specificity for diagnosing biliary duct stones.37 MRCP also is the first-choice modality for evaluating bile ducts in patients with suspected PSC. If performed in expert centers, the diagnostic accuracy reaches that of ERCP. A meta-analysis of studies from 2000 to 2006 has shown a sensitivity of 86% and specificity of 94% for diagnosing PSC.38
Endoscopic ultrasonography, which uses an ultrasonographic probe, allows clinicians to evaluate the integrity of the biliary and pancreatic ducts and is effective for diagnosing and staging cancer of the ampulla of Vater (sensitivity 93% vs 7% for abdominal ultrasonography and 29% for CT), and identifying biliary stones and biliary tree strictures.
Continue to: ERCP
ERCP is widely employed for diagnosing and treating pancreatobiliary diseases; however, its use has dropped over the last 10 years because of the risk of complications. ERCP is nearly exclusively used as a therapeutic procedure for pancreatic sphincterotomy, biliary dilatations, and removing biliary stones. It also has a diagnostic role in dominant stenosis or suspected biliary malignancy using brushing cytology and sampling biopsies of the bile ducts.
Treating extrahepatic cholestasis
Treatment of the different underlying conditions that cause extrahepatic cholestasis is surgical. Thus, the potential surgical techniques that can resolve or improve an extrahepatic cholestatic condition are guided by the surgeon and beyond the scope of this article.
Treating osteopenia: A concern for intra- and extrahepatic cholestasis
Vitamin D deficiency as a consequence of reduced intestinal absorption (poor availability of bile salts) or decreased hepatic activation to 25,OH-cholecalcipherol in both intrahepatic and extrahepatic cholestasis can lead to reduced bone formation.39 However, osteopenia can occur even in early stages of the disease. Prescribing bisphosphonates, in combination with calcium and vitamin D3, to improve bone mineral density is a good practice.40
CASE
Blood tests and ultrasound imaging suggest the presence of a chronic liver disease. Other lab tests indicate that the patient has an ALP level 3 times normal. This finding, together with the other tests, points to a likely diagnosis of intrahepatic cholestatic liver disease. Serology confirms positivity for ANA (1:160) and AMA (1:640). The clinician suspects PBC, so the patient is referred to a liver specialist for further evaluation and to determine whether a liver biopsy is needed.
The liver specialist confirms the diagnosis of PBC, performs a transient elastographym, which indicates a low-grade liver fibrosis (F1 out of 4), and starts therapy with UDCA.
CORRESPONDENCE
Ignazio Grattagliano, MD, Italian College of General Practitioners and Primary Care, Via del Sansovino 179, 50142, Florence, Italy; [email protected].
1. Kremer AE, Namer B, Bolier R, et al. Pathogenesis and management of pruritus in PBC and PSC. Dig Dis. 2015;33(suppl 2):164-175.
2. Deska Pagana K, Pagana TJ. Mosby’s Diagnostic and Laboratory Test Reference. 13th ed. St. Louis, MO: Elsevier; 2017.
3. Sapey T, Mendler MH, Guyader D, et al. Respective value of alkaline phosphatase, gamma-glutamyl transpeptidase and 5’ nucleotidase serum activity in the diagnosis of cholestasis: a prospective study of 80 patients. J Clin Gastroenterol. 2000;30:259-263.
4. European Association for the Study of the Liver. EASL Clinical practice guidelines: management of cholestatic liver diseases. J Hepatol. 2009;51:237-267.
5. Lammers WJ, van Buuren HR, Hirschfield GM, et al; Global PBC Study Group. Levels of alkaline phosphatase and bilirubin are surrogate end points of outcomes of patients with primary biliary cirrhosis: an international follow-up study. Gastroenterology. 2014;147:1338-1349.
6. Trivedi PJ, Corpechot C, Pares A, et al. Risk stratification in autoimmune cholestatic liver diseases: opportunities for clinicians and trialists. Hepatology. 2016;63:644-659.
7. Lammers WJ, Hirschfield GM, Corpechot C, et al. Development and validation of a scoring system to predict outcomes of patients with primary biliary cirrhosis receiving ursodeoxycholic acid therapy. Gastroenterology. 2015;149:1804-1812.
8. Johnston DE. Special considerations in interpreting liver function tests. Am Fam Physician. 1999;59:2223-2230.
9. Assy N, Jacob G, Spira G, et al. Diagnostic approach to patients with cholestatic jaundice. World J Gastroenterol. 1999;5:252-262.
10. Padda MS, Sanchez M, Akhtar AJ, et al. Drug-induced cholestasis. Hepatology. 2011;53:1377-1387.
11. US Food and Drug Administration. Food. Consumer advisory: kava-containing dietary supplements may be associated with severe liver injury. March 25, 2002. Available at: http://wayback.archive-it.org/7993/20171114232640/https://www.fda.gov/Food/RecallsOutbreaksEmergencies/SafetyAlertsAdvisories/ucm085482.htm. Accessed June 19, 2018.
12. Green RM, Flamm S. AGA technical review on the evaluation of liver chemistry tests. Gastroenterology. 2002;123:1367-1384.
13. Rogoveanu I, Gheonea DI, Saftoiu A, et al. The role of imaging methods in identifying the causes of extrahepatic cholestasis. J Gastrointestin Liver Dis. 2006;15:265-271.
14. Gotthardt DN, Rudolph G, Klöters-Plachky P, et al. Endoscopic dilation of dominant stenoses in primary sclerosing cholangitis: outcome after long-term treatment. Gastrointest Endosc. 2010;71:527-534.
15. Fitzmorris P, Singal AK. Surveillance and diagnosis of hepatocellular carcinoma. Gastroenterol Hepatol (NY). 2015;11:38-46.
16. Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022.
17. Pinzani M, Vizzutti F, Arena U, et al. Technology insight: noninvasive assessment of liver fibrosis by biochemical scores and elastography. Nat Clin Pract Gastroenterol Hepatol. 2008;5:95-106.
18. Castéra L, Vergniol J, Foucher J, et al. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343-350.
19. Van Gossum A, Pironi L, Messing B, et al. Transient elastography (FibroScan) is not correlated with liver fibrosis but with cholestasis in patients with long-term home parenteral nutrition. JPEN. 2015;39:719-724.
20. Millonig G, Reimann FM, Friedrich S, et al. Extrahepatic cholestasis increases liver stiffness (FibroScan) irrespective of fibrosis. Hepatology. 2008;48:1718-1723.
21. European Association for the Study of the Liver. EASL clinical practice guidelines: the diagnosis and management of patients with primary biliary cholangitis. J Hepatol. 2017;67:145-172.
22. Ozaslan E, Efe C, Gokbulut Ozaslan N. The diagnosis of antimitochondrial antibody-negative primary biliary cholangitis. Clin Res Hepatol Gastroenterol. 2016;40:553-561.
23. Lindor KD, Gershwin ME, Poupon R, et al; American Association for Study of Liver Diseases. Primary biliary cirrhosis. Hepatology. 2009;50:291-308.
24. Hov JR, Boberg KM, Karlsen TH. Autoantibodies in primary sclerosing cholangitis. World J Gastroenterol. 2008;14:3781-3791.
25. Dilger K, Hohenester S, Winkler-Budenhofer U, et al. Effect of ursodeoxycholic acid on bile acid profiles and intestinal detoxification machinery in primary biliary cirrhosis and health. J Hepatol. 2012;57:133-140.
26. Invernizzi P, Setchell KD, Crosignani A, et al. Differences in the metabolism and disposition of ursodeoxycholic acid and of its taurine-conjugated species in patients with primary biliary cirrhosis. Hepatology. 1999;29:320-327.
27. Jorgensen R, Angulo P, Dickson ER, et al. Results of long-term ursodiol treatment for patients with primary biliary cirrhosis. Am J Gastroenterol. 2002;97:2647-2650.
28. Parés A, Caballería L, Rodés J. Excellent long-term survival in patients with primary biliary cirrhosis and biochemical response to ursodeoxycholic acid. Gastroenterology. 2006;130:715-720.
29. Corpechot C, Abenavoli L, Rabahi N, et al. Biochemical response to ursodeoxycholic acid and long-term prognosis in primary biliary cirrhosis. Hepatology. 2008;48:871-877.
30. Levine JS, Burakoff R. Extraintestinal manifestations of inflammatory bowel disease. Gastroenterol Hepatol (NY). 2011;7:235-241.
31. Rodriguez HJ, Bass NM. Primary sclerosing cholangitis. Semin Gastrointest Dis. 2003;14:189-198.
32. Tajiri K, Shimizu Y. Recent advances in the management of pruritus in chronic liver diseases. World J Gastroenterol. 2017;23:3418-3426.
33. Kremer AE, Namer B, Bolier R, et al. Pathogenesis and management of pruritus in PBC and PSC. Dig Dis. 2015;33(suppl 2):164-175.
34. Buyse S, Durand F, Joly F. Nutritional assessment in cirrhosis. Gastroenterol Clin Biol. 2008;32:265-273.
35. Fagiuoli S, Colli A, Bruno R, et al; 2011 AISF Single Topic Group. Management of infections pre- and post-liver transplantation: report of an AISF consensus conference. J Hepatol. 2014;60:1075-1089.
36. Kanaan Z, Antaki F. Magnetic resonance cholangiopancreatography still plays a role in the preoperative evaluation of choledocholithiasis and biliary pathology. J Am Coll Surg. 2016;222:325-326.
37. McMahon CJ. The relative roles of magnetic resonance cholangiopancreatography (MRCP) and endoscopic ultrasound in diagnosis of common bile duct calculi: a critically appraised topic. Abdom Imaging. 2008;33:6-9.
38. Njei B, McCarty TR, Varadarajulu S, et al. Systematic review with meta-analysis: endoscopic retrograde cholangiopancreatography-based modalities for the diagnosis of cholangiocarcinoma in primary sclerosing cholangitis. Aliment Pharmacol Ther. 2016;44:1139-1151.
39. Wimalawansa SJ, Razzaque DMS, Al-Daghri NM. Calcium and vitamin D in human health: hype or real? J Steroid Biochem Mol Biol. 2017. doi: 10.1016/j.jsbmb.2017.12.009.
40. Yadav A, Carey EJ. Osteoporosis in chronic liver disease. Nutr Clin Pract. 2013;28:52-64.
1. Kremer AE, Namer B, Bolier R, et al. Pathogenesis and management of pruritus in PBC and PSC. Dig Dis. 2015;33(suppl 2):164-175.
2. Deska Pagana K, Pagana TJ. Mosby’s Diagnostic and Laboratory Test Reference. 13th ed. St. Louis, MO: Elsevier; 2017.
3. Sapey T, Mendler MH, Guyader D, et al. Respective value of alkaline phosphatase, gamma-glutamyl transpeptidase and 5’ nucleotidase serum activity in the diagnosis of cholestasis: a prospective study of 80 patients. J Clin Gastroenterol. 2000;30:259-263.
4. European Association for the Study of the Liver. EASL Clinical practice guidelines: management of cholestatic liver diseases. J Hepatol. 2009;51:237-267.
5. Lammers WJ, van Buuren HR, Hirschfield GM, et al; Global PBC Study Group. Levels of alkaline phosphatase and bilirubin are surrogate end points of outcomes of patients with primary biliary cirrhosis: an international follow-up study. Gastroenterology. 2014;147:1338-1349.
6. Trivedi PJ, Corpechot C, Pares A, et al. Risk stratification in autoimmune cholestatic liver diseases: opportunities for clinicians and trialists. Hepatology. 2016;63:644-659.
7. Lammers WJ, Hirschfield GM, Corpechot C, et al. Development and validation of a scoring system to predict outcomes of patients with primary biliary cirrhosis receiving ursodeoxycholic acid therapy. Gastroenterology. 2015;149:1804-1812.
8. Johnston DE. Special considerations in interpreting liver function tests. Am Fam Physician. 1999;59:2223-2230.
9. Assy N, Jacob G, Spira G, et al. Diagnostic approach to patients with cholestatic jaundice. World J Gastroenterol. 1999;5:252-262.
10. Padda MS, Sanchez M, Akhtar AJ, et al. Drug-induced cholestasis. Hepatology. 2011;53:1377-1387.
11. US Food and Drug Administration. Food. Consumer advisory: kava-containing dietary supplements may be associated with severe liver injury. March 25, 2002. Available at: http://wayback.archive-it.org/7993/20171114232640/https://www.fda.gov/Food/RecallsOutbreaksEmergencies/SafetyAlertsAdvisories/ucm085482.htm. Accessed June 19, 2018.
12. Green RM, Flamm S. AGA technical review on the evaluation of liver chemistry tests. Gastroenterology. 2002;123:1367-1384.
13. Rogoveanu I, Gheonea DI, Saftoiu A, et al. The role of imaging methods in identifying the causes of extrahepatic cholestasis. J Gastrointestin Liver Dis. 2006;15:265-271.
14. Gotthardt DN, Rudolph G, Klöters-Plachky P, et al. Endoscopic dilation of dominant stenoses in primary sclerosing cholangitis: outcome after long-term treatment. Gastrointest Endosc. 2010;71:527-534.
15. Fitzmorris P, Singal AK. Surveillance and diagnosis of hepatocellular carcinoma. Gastroenterol Hepatol (NY). 2015;11:38-46.
16. Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022.
17. Pinzani M, Vizzutti F, Arena U, et al. Technology insight: noninvasive assessment of liver fibrosis by biochemical scores and elastography. Nat Clin Pract Gastroenterol Hepatol. 2008;5:95-106.
18. Castéra L, Vergniol J, Foucher J, et al. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343-350.
19. Van Gossum A, Pironi L, Messing B, et al. Transient elastography (FibroScan) is not correlated with liver fibrosis but with cholestasis in patients with long-term home parenteral nutrition. JPEN. 2015;39:719-724.
20. Millonig G, Reimann FM, Friedrich S, et al. Extrahepatic cholestasis increases liver stiffness (FibroScan) irrespective of fibrosis. Hepatology. 2008;48:1718-1723.
21. European Association for the Study of the Liver. EASL clinical practice guidelines: the diagnosis and management of patients with primary biliary cholangitis. J Hepatol. 2017;67:145-172.
22. Ozaslan E, Efe C, Gokbulut Ozaslan N. The diagnosis of antimitochondrial antibody-negative primary biliary cholangitis. Clin Res Hepatol Gastroenterol. 2016;40:553-561.
23. Lindor KD, Gershwin ME, Poupon R, et al; American Association for Study of Liver Diseases. Primary biliary cirrhosis. Hepatology. 2009;50:291-308.
24. Hov JR, Boberg KM, Karlsen TH. Autoantibodies in primary sclerosing cholangitis. World J Gastroenterol. 2008;14:3781-3791.
25. Dilger K, Hohenester S, Winkler-Budenhofer U, et al. Effect of ursodeoxycholic acid on bile acid profiles and intestinal detoxification machinery in primary biliary cirrhosis and health. J Hepatol. 2012;57:133-140.
26. Invernizzi P, Setchell KD, Crosignani A, et al. Differences in the metabolism and disposition of ursodeoxycholic acid and of its taurine-conjugated species in patients with primary biliary cirrhosis. Hepatology. 1999;29:320-327.
27. Jorgensen R, Angulo P, Dickson ER, et al. Results of long-term ursodiol treatment for patients with primary biliary cirrhosis. Am J Gastroenterol. 2002;97:2647-2650.
28. Parés A, Caballería L, Rodés J. Excellent long-term survival in patients with primary biliary cirrhosis and biochemical response to ursodeoxycholic acid. Gastroenterology. 2006;130:715-720.
29. Corpechot C, Abenavoli L, Rabahi N, et al. Biochemical response to ursodeoxycholic acid and long-term prognosis in primary biliary cirrhosis. Hepatology. 2008;48:871-877.
30. Levine JS, Burakoff R. Extraintestinal manifestations of inflammatory bowel disease. Gastroenterol Hepatol (NY). 2011;7:235-241.
31. Rodriguez HJ, Bass NM. Primary sclerosing cholangitis. Semin Gastrointest Dis. 2003;14:189-198.
32. Tajiri K, Shimizu Y. Recent advances in the management of pruritus in chronic liver diseases. World J Gastroenterol. 2017;23:3418-3426.
33. Kremer AE, Namer B, Bolier R, et al. Pathogenesis and management of pruritus in PBC and PSC. Dig Dis. 2015;33(suppl 2):164-175.
34. Buyse S, Durand F, Joly F. Nutritional assessment in cirrhosis. Gastroenterol Clin Biol. 2008;32:265-273.
35. Fagiuoli S, Colli A, Bruno R, et al; 2011 AISF Single Topic Group. Management of infections pre- and post-liver transplantation: report of an AISF consensus conference. J Hepatol. 2014;60:1075-1089.
36. Kanaan Z, Antaki F. Magnetic resonance cholangiopancreatography still plays a role in the preoperative evaluation of choledocholithiasis and biliary pathology. J Am Coll Surg. 2016;222:325-326.
37. McMahon CJ. The relative roles of magnetic resonance cholangiopancreatography (MRCP) and endoscopic ultrasound in diagnosis of common bile duct calculi: a critically appraised topic. Abdom Imaging. 2008;33:6-9.
38. Njei B, McCarty TR, Varadarajulu S, et al. Systematic review with meta-analysis: endoscopic retrograde cholangiopancreatography-based modalities for the diagnosis of cholangiocarcinoma in primary sclerosing cholangitis. Aliment Pharmacol Ther. 2016;44:1139-1151.
39. Wimalawansa SJ, Razzaque DMS, Al-Daghri NM. Calcium and vitamin D in human health: hype or real? J Steroid Biochem Mol Biol. 2017. doi: 10.1016/j.jsbmb.2017.12.009.
40. Yadav A, Carey EJ. Osteoporosis in chronic liver disease. Nutr Clin Pract. 2013;28:52-64.
From The Journal of Family Practice | 2018;67(7):E9-E15.
PRACTICE RECOMMENDATIONS
› Suspect intrahepatic cholestasis in a patient with pruritus, normal transaminases, and mildly elevated gamma glutamyl-transpeptidase and alkaline phosphatase levels. A
› Use ultrasonography as a first-line diagnostic tool for cholestasis. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series