User login
July 2018 Question 2
Q2. Correct Answer: A
Rationale
Anti-TNF therapy is relatively safe and well-tolerated. However, there are a few important issues to consider prior to initiation of therapy. There is a risk of reactivation of both Mycobacterium tuberculosis and hepatitis B. In this patient’s case, her PPD positivity is likely a false positive from remote BCG vaccination. An interferon gamma release assay (e.g. QuantiFERON®) can be checked to confirm this; even if that is positive, in the absence of active tuberculosis (TB), she can be treated for latent TB for several weeks prior to initiation of anti-TNF therapy. Her hepatitis B serologies do not suggest chronic infection but rather prior infection with resolution. In this case, anti-TNF therapy is not precluded; rather, the AGA recommends considering concurrent antiviral prophylaxis while on anti-TNF therapy. Anti-TNF agents are not known to significantly increase the risk of progressive multifocal leukoencephalopathy like the nonselective anti-integrin natalizumab, so JC virus antibody positivity does not preclude their use. There is a slight increased risk of melanoma in those on anti-TNF therapy; non-melanoma skin cancers are of greater concern in those on thiopurine therapy. Finally, anti-TNF therapy should be avoided in those with demyelinating diseases or those at high risk for such diseases.
References
1. Reddy K.R., Beavers K.L., Hammond S.P., et al. American Gastroenterological Association Institute Guideline on the prevention and treatment of Hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology. 2014;148[1]:215-9.
2. Long M.D., Martin C.F., Pipkin C.A., et al. Risk of melanoma and nonmelanoma skin cancer among patients with inflammatory bowel disease. Gastroenterology. 2012;143[2]:390-9.
3. Ariyaratnam J., Subramanian V. Association between thiopurine use and nonmelanoma skin cancers in patients with inflammatory bowel disease: A meta-analysis. Am J Gastroenterol. 2014;109:163-9.
Q2. Correct Answer: A
Rationale
Anti-TNF therapy is relatively safe and well-tolerated. However, there are a few important issues to consider prior to initiation of therapy. There is a risk of reactivation of both Mycobacterium tuberculosis and hepatitis B. In this patient’s case, her PPD positivity is likely a false positive from remote BCG vaccination. An interferon gamma release assay (e.g. QuantiFERON®) can be checked to confirm this; even if that is positive, in the absence of active tuberculosis (TB), she can be treated for latent TB for several weeks prior to initiation of anti-TNF therapy. Her hepatitis B serologies do not suggest chronic infection but rather prior infection with resolution. In this case, anti-TNF therapy is not precluded; rather, the AGA recommends considering concurrent antiviral prophylaxis while on anti-TNF therapy. Anti-TNF agents are not known to significantly increase the risk of progressive multifocal leukoencephalopathy like the nonselective anti-integrin natalizumab, so JC virus antibody positivity does not preclude their use. There is a slight increased risk of melanoma in those on anti-TNF therapy; non-melanoma skin cancers are of greater concern in those on thiopurine therapy. Finally, anti-TNF therapy should be avoided in those with demyelinating diseases or those at high risk for such diseases.
References
1. Reddy K.R., Beavers K.L., Hammond S.P., et al. American Gastroenterological Association Institute Guideline on the prevention and treatment of Hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology. 2014;148[1]:215-9.
2. Long M.D., Martin C.F., Pipkin C.A., et al. Risk of melanoma and nonmelanoma skin cancer among patients with inflammatory bowel disease. Gastroenterology. 2012;143[2]:390-9.
3. Ariyaratnam J., Subramanian V. Association between thiopurine use and nonmelanoma skin cancers in patients with inflammatory bowel disease: A meta-analysis. Am J Gastroenterol. 2014;109:163-9.
Q2. Correct Answer: A
Rationale
Anti-TNF therapy is relatively safe and well-tolerated. However, there are a few important issues to consider prior to initiation of therapy. There is a risk of reactivation of both Mycobacterium tuberculosis and hepatitis B. In this patient’s case, her PPD positivity is likely a false positive from remote BCG vaccination. An interferon gamma release assay (e.g. QuantiFERON®) can be checked to confirm this; even if that is positive, in the absence of active tuberculosis (TB), she can be treated for latent TB for several weeks prior to initiation of anti-TNF therapy. Her hepatitis B serologies do not suggest chronic infection but rather prior infection with resolution. In this case, anti-TNF therapy is not precluded; rather, the AGA recommends considering concurrent antiviral prophylaxis while on anti-TNF therapy. Anti-TNF agents are not known to significantly increase the risk of progressive multifocal leukoencephalopathy like the nonselective anti-integrin natalizumab, so JC virus antibody positivity does not preclude their use. There is a slight increased risk of melanoma in those on anti-TNF therapy; non-melanoma skin cancers are of greater concern in those on thiopurine therapy. Finally, anti-TNF therapy should be avoided in those with demyelinating diseases or those at high risk for such diseases.
References
1. Reddy K.R., Beavers K.L., Hammond S.P., et al. American Gastroenterological Association Institute Guideline on the prevention and treatment of Hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology. 2014;148[1]:215-9.
2. Long M.D., Martin C.F., Pipkin C.A., et al. Risk of melanoma and nonmelanoma skin cancer among patients with inflammatory bowel disease. Gastroenterology. 2012;143[2]:390-9.
3. Ariyaratnam J., Subramanian V. Association between thiopurine use and nonmelanoma skin cancers in patients with inflammatory bowel disease: A meta-analysis. Am J Gastroenterol. 2014;109:163-9.
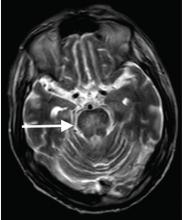
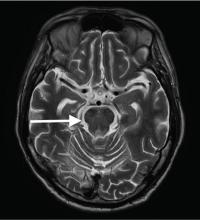
A 54-year-old woman presents for management of moderately-severe ileocolonic Crohn’s disease. She has a strong family history of multiple sclerosis and recently noted some tingling in her toes for which she is undergoing neurologic evaluation. She has had two small basal cell carcinomas removed from her cheek in the last year. She received the BCG vaccine as a child and had a positive PPD skin test within the last year. Laboratory evaluation reveals HBsAg negative, anti-HBs positive, and anti-HBc positive; JC virus antibody is positive.
July 2018 Question 1
Q1. Correct Answer: A
Rationale
This patient has an idiopathic, non-NSAID, non-H. pylori-associated ulcer and should be on daily PPI indefinitely. These patients have a high rate of recurrent bleeding (42%) and mortality when followed prospectively without being on antisecretory therapy. Although no randomized trials have assessed the benefit of medical cotherapy in this population, antiulcer therapy seems to reduce recurrent idiopathic ulcers.
References
1. Wong G.L.H., Wong V.W.S. Chan Y., et al. High incidence of mortality and recurrent bleeding in patients with Helicobacter pylori-negative idiopathic bleeding ulcers. Gastroenterology. 2009;137:525-31.
2. Laine L. Jensen D.M. Management of patients with ulcer bleeding. Am J Gastroenterol. 2012;107[3]:345-60.
Q1. Correct Answer: A
Rationale
This patient has an idiopathic, non-NSAID, non-H. pylori-associated ulcer and should be on daily PPI indefinitely. These patients have a high rate of recurrent bleeding (42%) and mortality when followed prospectively without being on antisecretory therapy. Although no randomized trials have assessed the benefit of medical cotherapy in this population, antiulcer therapy seems to reduce recurrent idiopathic ulcers.
References
1. Wong G.L.H., Wong V.W.S. Chan Y., et al. High incidence of mortality and recurrent bleeding in patients with Helicobacter pylori-negative idiopathic bleeding ulcers. Gastroenterology. 2009;137:525-31.
2. Laine L. Jensen D.M. Management of patients with ulcer bleeding. Am J Gastroenterol. 2012;107[3]:345-60.
Q1. Correct Answer: A
Rationale
This patient has an idiopathic, non-NSAID, non-H. pylori-associated ulcer and should be on daily PPI indefinitely. These patients have a high rate of recurrent bleeding (42%) and mortality when followed prospectively without being on antisecretory therapy. Although no randomized trials have assessed the benefit of medical cotherapy in this population, antiulcer therapy seems to reduce recurrent idiopathic ulcers.
References
1. Wong G.L.H., Wong V.W.S. Chan Y., et al. High incidence of mortality and recurrent bleeding in patients with Helicobacter pylori-negative idiopathic bleeding ulcers. Gastroenterology. 2009;137:525-31.
2. Laine L. Jensen D.M. Management of patients with ulcer bleeding. Am J Gastroenterol. 2012;107[3]:345-60.
A 60-year-old woman is admitted to the hospital with an upper GI bleed and found to have a gastric ulcer. Biopsies from the ulcer show no malignancy. Gastric biopsies reveal no Helicobacter pylori and stool antigen for H. pylori is also negative. The patient denies any NSAID use. She is discharged home on twice-daily PPI. Two months later, she returns for a follow-up endoscopy, and the ulcer has healed.
Navigating travel with diabetes
Travel, once reserved for wealthy vacationers and high-level executives, has become a regular experience for many people. The US Travel and Tourism Overview reported that US domestic travel climbed to more than 2.25 billion person-trips in 2017.1 The US Centers for Disease Control and Prevention (CDC) and the US Travel Association suggest that, based on this frequency and the known rate of diabetes, 17 million people with diabetes travel annually for leisure and 5.6 million for business, and these numbers are expected to increase.2
It stands to reason that as the number of people who travel continues to increase, so too will the number of patients with diabetes seeking medical travel advice. Despite resources available to travelers with diabetes, researchers at the 2016 meeting of the American Diabetes Association noted that only 30% of patients with diabetes who responded to a survey reported being satisfied with the resources available to help them manage their diabetes while traveling.2 This article discusses how clinicians can help patients manage their diabetes while traveling, address common travel questions, and prepare patients for emergencies that may arise while traveling.
PRE-TRIP PREPARATION
Provider visit before travel: Checking the bases
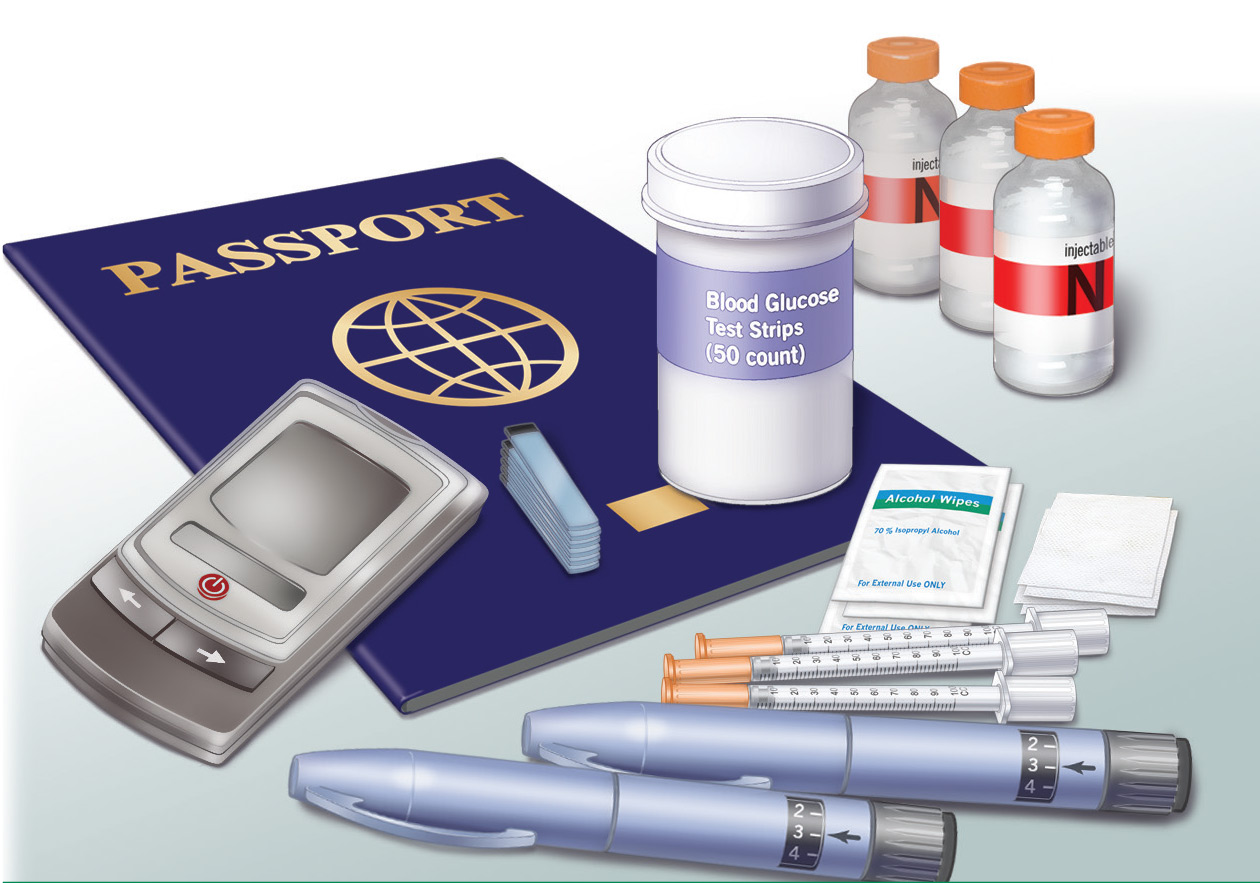
Advise patients to schedule an appointment 4 to 6 weeks before their trip.3 At this appointment, give the patient a healthcare provider travel letter (Figure 1) and prescriptions that the patient can hand-carry en route.3 The provider letter should state that the patient has diabetes and should list all supplies the patient needs. The letter should also include specific medications used by the patient and the devices that deliver these medications, eg, Humalog insulin and U-100 syringes4 to administer insulin, as well as any food and medication allergies.
Prescriptions should be written for patients to use in the event of an emergency during travel. Prescriptions for diabetes medications should be written with generic names to minimize confusion for those traveling internationally. Additionally, all prescriptions should provide enough medication to last throughout the trip.4
Advise patients that rules for filling prescriptions may vary between states and countries.3 Also, the strength of insulin may vary between the United States and other countries. Patients should understand that if they fill their insulin prescription in a foreign country, they may need to purchase new syringes to match the insulin dose. For example, if patients use U-100 syringes and purchase U-40 insulin, they will need to buy U-40 syringes or risk taking too little of a dose.
Remind patients that prescriptions are not necessary for all diabetes supplies but are essential for coverage by insurance companies. Blood glucose testing supplies, ketone strips, and glucose tablets may be purchased in a pharmacy without a prescription. Human insulin may also be purchased over the counter. However, oral medications, glucagon, and analog insulins require a prescription. We suggest that patients who travel have their prescriptions on file at a chain pharmacy rather than an independent one. If they are in the United States, they can go to any branch of the chain pharmacy and easily fill a prescription.
Work with the patient to compile a separate document that details the medication dosing, correction-scale instructions, carbohydrate-to-insulin ratios, and pump settings (basal rates, insulin sensitivity, active insulin time).4 Patients who use an insulin pump should record all pump settings in the event that they need to convert to insulin injections during travel.4 We suggest that all patients with an insulin pump have an alternate insulin method (eg, pens, vials) and that they carry this with them along with basal insulin in case the pump fails. This level of preparation empowers the patient to assume responsibility for his or her own care if a healthcare provider is not available during travel.
Like all travelers, patients with diabetes should confirm that their immunizations are up to date. Encourage patients to the CDC’s page (wwwnc.cdc.gov/travel) to check the list of vaccines necessary for their region of travel.4,5 Many special immunizations can be acquired only from a public health department and not from a clinician’s office.
Additionally, depending on the region of travel, prescribing antibiotics or antidiarrheal medications may be necessary to ensure patient safety and comfort. We also recommend that patients with type 1 diabetes obtain a supply of antibiotics and antidiarrheals because they can become sick quickly.
Packing with diabetes: Double is better
The American Diabetes Association recommends that patients pack at least twice the medication and blood-testing supplies they anticipate needing.3 Reinforce to patients the need to pack all medications and supplies in their carry-on bag and to keep this bag in their possession at all times to avoid damage, loss, and extreme changes in temperature and air pressure, which can adversely affect the activity and stability of insulin.
Ask patients about the activities they plan to participate in and how many days they will be traveling, and then recommend shoes that will encourage appropriate foot care.4 Patients with diabetes should choose comfort over style when selecting footwear. All new shoes should be purchased and “broken in” 2 to 3 weeks before the trip. Alternating shoes decreases the risk of blisters and calluses.4
Emergency abroad: Planning to be prepared
It is crucial to counsel patients on how to respond in an emergency.
Encourage patients with diabetes, especially those who use insulin, to obtain a medical identification bracelet, necklace, or in some cases, a tattoo, that states they use insulin and discloses any allergies.3 This ensures that emergency medical personnel will be aware of the patient’s condition when providing care. Also suggest that your patients have emergency contact information available on their person and their cell phone to expedite assistance in an emergency (Table 2).
Urge patients to determine prior to their departure if their health coverage will change once they leave the state or the country. Some insurance companies require patients to go to a specific healthcare system while others regulate the amount of time a patient can be in the hospital before being transferred home. It is important for patients to be aware of these terms in the event of hospitalization.4 Travel insurance should be considered for international travel.
AIRPORT SECURITY: WHAT TO EXPECT WITH DIABETES
The American Diabetes Association works with the US Transportation Security Administration (TSA) to ensure that passengers with diabetes have access to supplies. Travelers with diabetes are allowed to apply for an optional disability notification card, which discreetly informs officers that the passenger has a condition or device that may affect screening procedures.6
The TSA suggests that, before going through airport screening, patients with diabetes separate their diabetes supplies from their luggage and declare all items.6 Including prescription labels for medications and medical devices helps speed up the security process. Advise patients to carry glucose tablets and other solid foods for treating hypoglycemia when passing through airport security checkpoints.7
Since 2016, the TSA has allowed all diabetes-related supplies, medications, and equipment, including liquids and devices, through security after they have been screened by the x-ray scanner or by hand.7 People with diabetes are allowed to carry insulin and other liquid medications in amounts greater than 3.4 ounces (100 mLs) through airport security checkpoints.
Insulin can pass safely through x-ray scanners, but if patients are concerned, they may request that their insulin be inspected by hand.7 Patients must inform airport security of this decision before the screening process begins. A hand inspection may include swabbing for explosives.
Patients with an insulin pump and a continuous glucose monitoring device may feel uncomfortable during x-ray screening and special security screenings. Remind patients that it is TSA policy that patients do not need to disconnect their devices and can request screening by pat-down rather than x-ray scanner.6 It is the responsibility of the patient to research whether the pump can pass through x-ray scanners.
All patients have the right to request a pat-down and can opt out of passing through the x-ray scanner.6 However, patients need to inform officers about a pump before screening and must understand that the pump may be subject to further inspection. Usually, this additional inspection includes swabbing the patient’s hands to check for explosive material and a simple pat-down of the insulin pump.7
IN-FLIGHT TIPS
Time zones and insulin dosing
Diabetes management is often based on a 24-hour medication schedule. Travel can disrupt this schedule, making it challenging for patients to determine the appropriate medication adjustments. With some assistance, the patient can determine the best course of action based on the direction of travel and the number of time zones crossed.
According to Chandran and Edelman,7 medication adjustments are needed only when the patient is traveling east or west, not north or south. As time zones change, day length changes and, consequently, so does the 24-hour regimen many patients follow. As a general rule, traveling east results in a shortened day, requiring a potential reduction in insulin, while traveling west results in a longer day, possibly requiring an increase in insulin dose.7 However, this is a guideline and may not be applicable to all patients.7
Advise patients to follow local time to administer medications beginning the morning after arrival.7 It is not uncommon, due to changes in meal schedules and dosing, for patients to experience hyperglycemia during travel. They should be prepared to correct this if necessary.
Patients using insulin injections should plan to adjust to the new time zone as soon as possible. If the time change is only 1 or 2 hours, they should take their medications before departure according to their normal home time.7 Upon arrival, they should resume their insulin regimen based on the local time.
Westward travel. If the patient is traveling west with a time change of 3 or more hours, additional changes may be necessary. Advise patients to take their insulin according to their normal home time before departure. The change in dosing and schedule will depend largely on current glucose control, time of travel, and availability of food and glucose during travel. Encourage patients to discuss these matters with you in advance of any long travel.
Eastward travel. When the patient is traveling east with a time change greater than 3 hours, the day will be consequently shortened. On the day of travel, patients should take their morning dose according to home time. If they are concerned about hypoglycemia, suggest that they decrease the dose by 10%.6 On arrival, they should adhere to the new time zone and base insulin dosing on local time.
Advice for insulin pump users. Patients with an insulin pump need make only minimal changes to their dosing schedule. They should continue their routine of basal and bolus doses and change the time on their insulin pump to local time when they arrive. Insulin pump users should bring insulin and syringes as backup; in the event of pump malfunction, the patient should continue to use the same amount of bolus insulin to correct glucose readings and to cover meals.7 As for the basal dose, patients can administer a once-daily injection of long-acting insulin, which can be calculated from their pump or accessed from the list they created as part of their pre-travel preparation.7
Advice for patients on oral diabetes medications
If a patient is taking an oral medication, it is less crucial to adhere to a time schedule. In fact, in some cases it may be preferable to skip a dose and risk slight hyperglycemia for a few hours rather than take medication too close in time and risk hypoglycemia.7
Remind patients to anticipate a change in their oral medication regimen if they travel farther than 5 time zones.7 Encourage patients to research time changes and discuss the necessary changes in medication dosage on the day of travel as well as the specific aspects of their trip. A time-zone converter can be found at www.timeanddate.com.8
WHAT TO EXPECT WHILE ON LAND
Insulin 101
Storing insulin at the appropriate temperature may be a concern. Insulin should be kept between 40°F and 86°F (4°C–30°C).4 Remind patients to carry their insulin with them at all times and to not store it in a car glove compartment or backpack where it can be exposed to excessive sun. The Frio cold pack (ReadyCare, Walnut Creek, CA) is a helpful alternative to refrigeration and can be used to cool insulin when hiking or participating in activities where insulin can overheat. These cooling gel packs are activated when exposed to cold water for 5 to 7 minutes5 and are reusable.
Alert patients that insulin names and concentrations may vary among countries. Most insulins are U-100 concentration, which means that for every 1 mL of liquid there are 100 units of insulin. This is the standard insulin concentration used in the United States. There are U-200, U-300, and U-500 insulins as well. In Europe, the standard concentration is U-40 insulin. Syringe sizes are designed to accommodate either U-100 or U-40 insulin. Review these differences with patients and explain the consequences of mixing insulin concentration with syringes of different sizes. Figure 2 shows how to calculate equivalent doses.
Resort tips: Food, drinks, and excursions
A large component of travel is indulging in local cuisine. Patients with diabetes need to be aware of how different foods can affect their diabetes control. Encourage them to research the foods common to the local cuisine. Websites such as Calorie King, MyFitnessPal, Lose it!, and Nutrition Data can help identify the caloric and nutritional makeup of foods.9
Advise patients to actively monitor how their blood glucose is affected by new foods by checking blood glucose levels before and after each meal.9 Opting for vegetables and protein sources minimizes glucose fluctuations. Remind patients that drinks at resorts may contain more sugar than advertised. Patients should continue to manage their blood glucose by checking levels and by making appropriate insulin adjustments based on the readings. We often advise patients to pack a jar of peanut butter when traveling to ensure a ready source of protein.
Patients who plan to participate in physically challenging activities while travelling should inform all relevant members of the activity staff of their condition. In case of an emergency, hotel staff and guides will be better equipped to help with situations such as hypoglycemia. As noted above, patients should always carry snacks and supplies to treat hypoglycemia in case no alternative food options are available during an excursion. Also, warn patients to avoid walking barefoot. Water shoes are a good alternative to protect feet from cuts and sores.
Patients should inquire about the safety of high-elevation activities. With many glucose meters, every 1,000 feet of elevation results in a 1% to 2% underestimation of blood glucose,10 which could result in an inaccurate reading. If high-altitude activities are planned, advise patients to bring multiple meters to cross-check glucose readings in cases where inaccuracies (due to elevation) are possible.
- US Travel Association. US travel and tourism overview. www.ustravel.org/system/files/media_root/document/Research_Fact-Sheet_US-Travel-and-Tourism-Overview.pdf. Accessed June 14, 2018.
- Brunk D. Long haul travel turbulent for many with type 1 diabetes. Clinical Endocrinology News 2016. www.mdedge.com/clinicalendocrinologynews/article/109866/diabetes/long-haul-travel-turbulent-many-type-1-diabetes. Accessed June 14, 2018.
- American Diabetes Association. When you travel. www.diabetes.org/living-with-diabetes/treatment-and-care/when-you-travel.html?utm_source=DSH_BLOG&utm_medium=BlogPost&utm_content=051514-travel&utm_campaign=CON. Accessed June 14, 2018.
- Kruger DF. The Diabetes Travel Guide. How to travel with diabetes-anywhere in the world. Arlington, VA: American Diabetes Association; 2000.
- Centers for Disease Control and Prevention. Travelers’ health. wwwnc.cdc.gov/travel/. Accessed June 14, 2018.
- American Diabetes Association. What special concerns may arise? www.diabetes.org/living-with-diabetes/know-your-rights/discrimination/public-accommodations/air-travel-and-diabetes/what-special-concerns-may.html. Accessed June 14, 2018.
- Chandran M, Edelman SV. Have insulin, will fly: diabetes management during air travel and time zone adjustment strategies. Clinical Diabetes 2003; 21(2):82–85. doi:10.2337/diaclin.21.2.82
- Time and Date AS. Time zone converter. timeanddate.com. Accessed March 19, 2018.
- Joslin Diabetes Center. Diabetes and travel—10 tips for a safe trip. www.joslin.org/info/diabetes_and_travel_10_tips_for_a_safe_trip.html. Accessed June 14, 2018.
- Jendle J, Adolfsson P. Impact of high altitudes on glucose control. J Diabetes Sci Technol 2011; 5(6):1621–1622. doi:10.1177/193229681100500642
Travel, once reserved for wealthy vacationers and high-level executives, has become a regular experience for many people. The US Travel and Tourism Overview reported that US domestic travel climbed to more than 2.25 billion person-trips in 2017.1 The US Centers for Disease Control and Prevention (CDC) and the US Travel Association suggest that, based on this frequency and the known rate of diabetes, 17 million people with diabetes travel annually for leisure and 5.6 million for business, and these numbers are expected to increase.2
It stands to reason that as the number of people who travel continues to increase, so too will the number of patients with diabetes seeking medical travel advice. Despite resources available to travelers with diabetes, researchers at the 2016 meeting of the American Diabetes Association noted that only 30% of patients with diabetes who responded to a survey reported being satisfied with the resources available to help them manage their diabetes while traveling.2 This article discusses how clinicians can help patients manage their diabetes while traveling, address common travel questions, and prepare patients for emergencies that may arise while traveling.
PRE-TRIP PREPARATION
Provider visit before travel: Checking the bases
Advise patients to schedule an appointment 4 to 6 weeks before their trip.3 At this appointment, give the patient a healthcare provider travel letter (Figure 1) and prescriptions that the patient can hand-carry en route.3 The provider letter should state that the patient has diabetes and should list all supplies the patient needs. The letter should also include specific medications used by the patient and the devices that deliver these medications, eg, Humalog insulin and U-100 syringes4 to administer insulin, as well as any food and medication allergies.
Prescriptions should be written for patients to use in the event of an emergency during travel. Prescriptions for diabetes medications should be written with generic names to minimize confusion for those traveling internationally. Additionally, all prescriptions should provide enough medication to last throughout the trip.4
Advise patients that rules for filling prescriptions may vary between states and countries.3 Also, the strength of insulin may vary between the United States and other countries. Patients should understand that if they fill their insulin prescription in a foreign country, they may need to purchase new syringes to match the insulin dose. For example, if patients use U-100 syringes and purchase U-40 insulin, they will need to buy U-40 syringes or risk taking too little of a dose.
Remind patients that prescriptions are not necessary for all diabetes supplies but are essential for coverage by insurance companies. Blood glucose testing supplies, ketone strips, and glucose tablets may be purchased in a pharmacy without a prescription. Human insulin may also be purchased over the counter. However, oral medications, glucagon, and analog insulins require a prescription. We suggest that patients who travel have their prescriptions on file at a chain pharmacy rather than an independent one. If they are in the United States, they can go to any branch of the chain pharmacy and easily fill a prescription.
Work with the patient to compile a separate document that details the medication dosing, correction-scale instructions, carbohydrate-to-insulin ratios, and pump settings (basal rates, insulin sensitivity, active insulin time).4 Patients who use an insulin pump should record all pump settings in the event that they need to convert to insulin injections during travel.4 We suggest that all patients with an insulin pump have an alternate insulin method (eg, pens, vials) and that they carry this with them along with basal insulin in case the pump fails. This level of preparation empowers the patient to assume responsibility for his or her own care if a healthcare provider is not available during travel.
Like all travelers, patients with diabetes should confirm that their immunizations are up to date. Encourage patients to the CDC’s page (wwwnc.cdc.gov/travel) to check the list of vaccines necessary for their region of travel.4,5 Many special immunizations can be acquired only from a public health department and not from a clinician’s office.
Additionally, depending on the region of travel, prescribing antibiotics or antidiarrheal medications may be necessary to ensure patient safety and comfort. We also recommend that patients with type 1 diabetes obtain a supply of antibiotics and antidiarrheals because they can become sick quickly.
Packing with diabetes: Double is better
The American Diabetes Association recommends that patients pack at least twice the medication and blood-testing supplies they anticipate needing.3 Reinforce to patients the need to pack all medications and supplies in their carry-on bag and to keep this bag in their possession at all times to avoid damage, loss, and extreme changes in temperature and air pressure, which can adversely affect the activity and stability of insulin.
Ask patients about the activities they plan to participate in and how many days they will be traveling, and then recommend shoes that will encourage appropriate foot care.4 Patients with diabetes should choose comfort over style when selecting footwear. All new shoes should be purchased and “broken in” 2 to 3 weeks before the trip. Alternating shoes decreases the risk of blisters and calluses.4
Emergency abroad: Planning to be prepared
It is crucial to counsel patients on how to respond in an emergency.
Encourage patients with diabetes, especially those who use insulin, to obtain a medical identification bracelet, necklace, or in some cases, a tattoo, that states they use insulin and discloses any allergies.3 This ensures that emergency medical personnel will be aware of the patient’s condition when providing care. Also suggest that your patients have emergency contact information available on their person and their cell phone to expedite assistance in an emergency (Table 2).
Urge patients to determine prior to their departure if their health coverage will change once they leave the state or the country. Some insurance companies require patients to go to a specific healthcare system while others regulate the amount of time a patient can be in the hospital before being transferred home. It is important for patients to be aware of these terms in the event of hospitalization.4 Travel insurance should be considered for international travel.
AIRPORT SECURITY: WHAT TO EXPECT WITH DIABETES
The American Diabetes Association works with the US Transportation Security Administration (TSA) to ensure that passengers with diabetes have access to supplies. Travelers with diabetes are allowed to apply for an optional disability notification card, which discreetly informs officers that the passenger has a condition or device that may affect screening procedures.6
The TSA suggests that, before going through airport screening, patients with diabetes separate their diabetes supplies from their luggage and declare all items.6 Including prescription labels for medications and medical devices helps speed up the security process. Advise patients to carry glucose tablets and other solid foods for treating hypoglycemia when passing through airport security checkpoints.7
Since 2016, the TSA has allowed all diabetes-related supplies, medications, and equipment, including liquids and devices, through security after they have been screened by the x-ray scanner or by hand.7 People with diabetes are allowed to carry insulin and other liquid medications in amounts greater than 3.4 ounces (100 mLs) through airport security checkpoints.
Insulin can pass safely through x-ray scanners, but if patients are concerned, they may request that their insulin be inspected by hand.7 Patients must inform airport security of this decision before the screening process begins. A hand inspection may include swabbing for explosives.
Patients with an insulin pump and a continuous glucose monitoring device may feel uncomfortable during x-ray screening and special security screenings. Remind patients that it is TSA policy that patients do not need to disconnect their devices and can request screening by pat-down rather than x-ray scanner.6 It is the responsibility of the patient to research whether the pump can pass through x-ray scanners.
All patients have the right to request a pat-down and can opt out of passing through the x-ray scanner.6 However, patients need to inform officers about a pump before screening and must understand that the pump may be subject to further inspection. Usually, this additional inspection includes swabbing the patient’s hands to check for explosive material and a simple pat-down of the insulin pump.7
IN-FLIGHT TIPS
Time zones and insulin dosing
Diabetes management is often based on a 24-hour medication schedule. Travel can disrupt this schedule, making it challenging for patients to determine the appropriate medication adjustments. With some assistance, the patient can determine the best course of action based on the direction of travel and the number of time zones crossed.
According to Chandran and Edelman,7 medication adjustments are needed only when the patient is traveling east or west, not north or south. As time zones change, day length changes and, consequently, so does the 24-hour regimen many patients follow. As a general rule, traveling east results in a shortened day, requiring a potential reduction in insulin, while traveling west results in a longer day, possibly requiring an increase in insulin dose.7 However, this is a guideline and may not be applicable to all patients.7
Advise patients to follow local time to administer medications beginning the morning after arrival.7 It is not uncommon, due to changes in meal schedules and dosing, for patients to experience hyperglycemia during travel. They should be prepared to correct this if necessary.
Patients using insulin injections should plan to adjust to the new time zone as soon as possible. If the time change is only 1 or 2 hours, they should take their medications before departure according to their normal home time.7 Upon arrival, they should resume their insulin regimen based on the local time.
Westward travel. If the patient is traveling west with a time change of 3 or more hours, additional changes may be necessary. Advise patients to take their insulin according to their normal home time before departure. The change in dosing and schedule will depend largely on current glucose control, time of travel, and availability of food and glucose during travel. Encourage patients to discuss these matters with you in advance of any long travel.
Eastward travel. When the patient is traveling east with a time change greater than 3 hours, the day will be consequently shortened. On the day of travel, patients should take their morning dose according to home time. If they are concerned about hypoglycemia, suggest that they decrease the dose by 10%.6 On arrival, they should adhere to the new time zone and base insulin dosing on local time.
Advice for insulin pump users. Patients with an insulin pump need make only minimal changes to their dosing schedule. They should continue their routine of basal and bolus doses and change the time on their insulin pump to local time when they arrive. Insulin pump users should bring insulin and syringes as backup; in the event of pump malfunction, the patient should continue to use the same amount of bolus insulin to correct glucose readings and to cover meals.7 As for the basal dose, patients can administer a once-daily injection of long-acting insulin, which can be calculated from their pump or accessed from the list they created as part of their pre-travel preparation.7
Advice for patients on oral diabetes medications
If a patient is taking an oral medication, it is less crucial to adhere to a time schedule. In fact, in some cases it may be preferable to skip a dose and risk slight hyperglycemia for a few hours rather than take medication too close in time and risk hypoglycemia.7
Remind patients to anticipate a change in their oral medication regimen if they travel farther than 5 time zones.7 Encourage patients to research time changes and discuss the necessary changes in medication dosage on the day of travel as well as the specific aspects of their trip. A time-zone converter can be found at www.timeanddate.com.8
WHAT TO EXPECT WHILE ON LAND
Insulin 101
Storing insulin at the appropriate temperature may be a concern. Insulin should be kept between 40°F and 86°F (4°C–30°C).4 Remind patients to carry their insulin with them at all times and to not store it in a car glove compartment or backpack where it can be exposed to excessive sun. The Frio cold pack (ReadyCare, Walnut Creek, CA) is a helpful alternative to refrigeration and can be used to cool insulin when hiking or participating in activities where insulin can overheat. These cooling gel packs are activated when exposed to cold water for 5 to 7 minutes5 and are reusable.
Alert patients that insulin names and concentrations may vary among countries. Most insulins are U-100 concentration, which means that for every 1 mL of liquid there are 100 units of insulin. This is the standard insulin concentration used in the United States. There are U-200, U-300, and U-500 insulins as well. In Europe, the standard concentration is U-40 insulin. Syringe sizes are designed to accommodate either U-100 or U-40 insulin. Review these differences with patients and explain the consequences of mixing insulin concentration with syringes of different sizes. Figure 2 shows how to calculate equivalent doses.
Resort tips: Food, drinks, and excursions
A large component of travel is indulging in local cuisine. Patients with diabetes need to be aware of how different foods can affect their diabetes control. Encourage them to research the foods common to the local cuisine. Websites such as Calorie King, MyFitnessPal, Lose it!, and Nutrition Data can help identify the caloric and nutritional makeup of foods.9
Advise patients to actively monitor how their blood glucose is affected by new foods by checking blood glucose levels before and after each meal.9 Opting for vegetables and protein sources minimizes glucose fluctuations. Remind patients that drinks at resorts may contain more sugar than advertised. Patients should continue to manage their blood glucose by checking levels and by making appropriate insulin adjustments based on the readings. We often advise patients to pack a jar of peanut butter when traveling to ensure a ready source of protein.
Patients who plan to participate in physically challenging activities while travelling should inform all relevant members of the activity staff of their condition. In case of an emergency, hotel staff and guides will be better equipped to help with situations such as hypoglycemia. As noted above, patients should always carry snacks and supplies to treat hypoglycemia in case no alternative food options are available during an excursion. Also, warn patients to avoid walking barefoot. Water shoes are a good alternative to protect feet from cuts and sores.
Patients should inquire about the safety of high-elevation activities. With many glucose meters, every 1,000 feet of elevation results in a 1% to 2% underestimation of blood glucose,10 which could result in an inaccurate reading. If high-altitude activities are planned, advise patients to bring multiple meters to cross-check glucose readings in cases where inaccuracies (due to elevation) are possible.
Travel, once reserved for wealthy vacationers and high-level executives, has become a regular experience for many people. The US Travel and Tourism Overview reported that US domestic travel climbed to more than 2.25 billion person-trips in 2017.1 The US Centers for Disease Control and Prevention (CDC) and the US Travel Association suggest that, based on this frequency and the known rate of diabetes, 17 million people with diabetes travel annually for leisure and 5.6 million for business, and these numbers are expected to increase.2
It stands to reason that as the number of people who travel continues to increase, so too will the number of patients with diabetes seeking medical travel advice. Despite resources available to travelers with diabetes, researchers at the 2016 meeting of the American Diabetes Association noted that only 30% of patients with diabetes who responded to a survey reported being satisfied with the resources available to help them manage their diabetes while traveling.2 This article discusses how clinicians can help patients manage their diabetes while traveling, address common travel questions, and prepare patients for emergencies that may arise while traveling.
PRE-TRIP PREPARATION
Provider visit before travel: Checking the bases
Advise patients to schedule an appointment 4 to 6 weeks before their trip.3 At this appointment, give the patient a healthcare provider travel letter (Figure 1) and prescriptions that the patient can hand-carry en route.3 The provider letter should state that the patient has diabetes and should list all supplies the patient needs. The letter should also include specific medications used by the patient and the devices that deliver these medications, eg, Humalog insulin and U-100 syringes4 to administer insulin, as well as any food and medication allergies.
Prescriptions should be written for patients to use in the event of an emergency during travel. Prescriptions for diabetes medications should be written with generic names to minimize confusion for those traveling internationally. Additionally, all prescriptions should provide enough medication to last throughout the trip.4
Advise patients that rules for filling prescriptions may vary between states and countries.3 Also, the strength of insulin may vary between the United States and other countries. Patients should understand that if they fill their insulin prescription in a foreign country, they may need to purchase new syringes to match the insulin dose. For example, if patients use U-100 syringes and purchase U-40 insulin, they will need to buy U-40 syringes or risk taking too little of a dose.
Remind patients that prescriptions are not necessary for all diabetes supplies but are essential for coverage by insurance companies. Blood glucose testing supplies, ketone strips, and glucose tablets may be purchased in a pharmacy without a prescription. Human insulin may also be purchased over the counter. However, oral medications, glucagon, and analog insulins require a prescription. We suggest that patients who travel have their prescriptions on file at a chain pharmacy rather than an independent one. If they are in the United States, they can go to any branch of the chain pharmacy and easily fill a prescription.
Work with the patient to compile a separate document that details the medication dosing, correction-scale instructions, carbohydrate-to-insulin ratios, and pump settings (basal rates, insulin sensitivity, active insulin time).4 Patients who use an insulin pump should record all pump settings in the event that they need to convert to insulin injections during travel.4 We suggest that all patients with an insulin pump have an alternate insulin method (eg, pens, vials) and that they carry this with them along with basal insulin in case the pump fails. This level of preparation empowers the patient to assume responsibility for his or her own care if a healthcare provider is not available during travel.
Like all travelers, patients with diabetes should confirm that their immunizations are up to date. Encourage patients to the CDC’s page (wwwnc.cdc.gov/travel) to check the list of vaccines necessary for their region of travel.4,5 Many special immunizations can be acquired only from a public health department and not from a clinician’s office.
Additionally, depending on the region of travel, prescribing antibiotics or antidiarrheal medications may be necessary to ensure patient safety and comfort. We also recommend that patients with type 1 diabetes obtain a supply of antibiotics and antidiarrheals because they can become sick quickly.
Packing with diabetes: Double is better
The American Diabetes Association recommends that patients pack at least twice the medication and blood-testing supplies they anticipate needing.3 Reinforce to patients the need to pack all medications and supplies in their carry-on bag and to keep this bag in their possession at all times to avoid damage, loss, and extreme changes in temperature and air pressure, which can adversely affect the activity and stability of insulin.
Ask patients about the activities they plan to participate in and how many days they will be traveling, and then recommend shoes that will encourage appropriate foot care.4 Patients with diabetes should choose comfort over style when selecting footwear. All new shoes should be purchased and “broken in” 2 to 3 weeks before the trip. Alternating shoes decreases the risk of blisters and calluses.4
Emergency abroad: Planning to be prepared
It is crucial to counsel patients on how to respond in an emergency.
Encourage patients with diabetes, especially those who use insulin, to obtain a medical identification bracelet, necklace, or in some cases, a tattoo, that states they use insulin and discloses any allergies.3 This ensures that emergency medical personnel will be aware of the patient’s condition when providing care. Also suggest that your patients have emergency contact information available on their person and their cell phone to expedite assistance in an emergency (Table 2).
Urge patients to determine prior to their departure if their health coverage will change once they leave the state or the country. Some insurance companies require patients to go to a specific healthcare system while others regulate the amount of time a patient can be in the hospital before being transferred home. It is important for patients to be aware of these terms in the event of hospitalization.4 Travel insurance should be considered for international travel.
AIRPORT SECURITY: WHAT TO EXPECT WITH DIABETES
The American Diabetes Association works with the US Transportation Security Administration (TSA) to ensure that passengers with diabetes have access to supplies. Travelers with diabetes are allowed to apply for an optional disability notification card, which discreetly informs officers that the passenger has a condition or device that may affect screening procedures.6
The TSA suggests that, before going through airport screening, patients with diabetes separate their diabetes supplies from their luggage and declare all items.6 Including prescription labels for medications and medical devices helps speed up the security process. Advise patients to carry glucose tablets and other solid foods for treating hypoglycemia when passing through airport security checkpoints.7
Since 2016, the TSA has allowed all diabetes-related supplies, medications, and equipment, including liquids and devices, through security after they have been screened by the x-ray scanner or by hand.7 People with diabetes are allowed to carry insulin and other liquid medications in amounts greater than 3.4 ounces (100 mLs) through airport security checkpoints.
Insulin can pass safely through x-ray scanners, but if patients are concerned, they may request that their insulin be inspected by hand.7 Patients must inform airport security of this decision before the screening process begins. A hand inspection may include swabbing for explosives.
Patients with an insulin pump and a continuous glucose monitoring device may feel uncomfortable during x-ray screening and special security screenings. Remind patients that it is TSA policy that patients do not need to disconnect their devices and can request screening by pat-down rather than x-ray scanner.6 It is the responsibility of the patient to research whether the pump can pass through x-ray scanners.
All patients have the right to request a pat-down and can opt out of passing through the x-ray scanner.6 However, patients need to inform officers about a pump before screening and must understand that the pump may be subject to further inspection. Usually, this additional inspection includes swabbing the patient’s hands to check for explosive material and a simple pat-down of the insulin pump.7
IN-FLIGHT TIPS
Time zones and insulin dosing
Diabetes management is often based on a 24-hour medication schedule. Travel can disrupt this schedule, making it challenging for patients to determine the appropriate medication adjustments. With some assistance, the patient can determine the best course of action based on the direction of travel and the number of time zones crossed.
According to Chandran and Edelman,7 medication adjustments are needed only when the patient is traveling east or west, not north or south. As time zones change, day length changes and, consequently, so does the 24-hour regimen many patients follow. As a general rule, traveling east results in a shortened day, requiring a potential reduction in insulin, while traveling west results in a longer day, possibly requiring an increase in insulin dose.7 However, this is a guideline and may not be applicable to all patients.7
Advise patients to follow local time to administer medications beginning the morning after arrival.7 It is not uncommon, due to changes in meal schedules and dosing, for patients to experience hyperglycemia during travel. They should be prepared to correct this if necessary.
Patients using insulin injections should plan to adjust to the new time zone as soon as possible. If the time change is only 1 or 2 hours, they should take their medications before departure according to their normal home time.7 Upon arrival, they should resume their insulin regimen based on the local time.
Westward travel. If the patient is traveling west with a time change of 3 or more hours, additional changes may be necessary. Advise patients to take their insulin according to their normal home time before departure. The change in dosing and schedule will depend largely on current glucose control, time of travel, and availability of food and glucose during travel. Encourage patients to discuss these matters with you in advance of any long travel.
Eastward travel. When the patient is traveling east with a time change greater than 3 hours, the day will be consequently shortened. On the day of travel, patients should take their morning dose according to home time. If they are concerned about hypoglycemia, suggest that they decrease the dose by 10%.6 On arrival, they should adhere to the new time zone and base insulin dosing on local time.
Advice for insulin pump users. Patients with an insulin pump need make only minimal changes to their dosing schedule. They should continue their routine of basal and bolus doses and change the time on their insulin pump to local time when they arrive. Insulin pump users should bring insulin and syringes as backup; in the event of pump malfunction, the patient should continue to use the same amount of bolus insulin to correct glucose readings and to cover meals.7 As for the basal dose, patients can administer a once-daily injection of long-acting insulin, which can be calculated from their pump or accessed from the list they created as part of their pre-travel preparation.7
Advice for patients on oral diabetes medications
If a patient is taking an oral medication, it is less crucial to adhere to a time schedule. In fact, in some cases it may be preferable to skip a dose and risk slight hyperglycemia for a few hours rather than take medication too close in time and risk hypoglycemia.7
Remind patients to anticipate a change in their oral medication regimen if they travel farther than 5 time zones.7 Encourage patients to research time changes and discuss the necessary changes in medication dosage on the day of travel as well as the specific aspects of their trip. A time-zone converter can be found at www.timeanddate.com.8
WHAT TO EXPECT WHILE ON LAND
Insulin 101
Storing insulin at the appropriate temperature may be a concern. Insulin should be kept between 40°F and 86°F (4°C–30°C).4 Remind patients to carry their insulin with them at all times and to not store it in a car glove compartment or backpack where it can be exposed to excessive sun. The Frio cold pack (ReadyCare, Walnut Creek, CA) is a helpful alternative to refrigeration and can be used to cool insulin when hiking or participating in activities where insulin can overheat. These cooling gel packs are activated when exposed to cold water for 5 to 7 minutes5 and are reusable.
Alert patients that insulin names and concentrations may vary among countries. Most insulins are U-100 concentration, which means that for every 1 mL of liquid there are 100 units of insulin. This is the standard insulin concentration used in the United States. There are U-200, U-300, and U-500 insulins as well. In Europe, the standard concentration is U-40 insulin. Syringe sizes are designed to accommodate either U-100 or U-40 insulin. Review these differences with patients and explain the consequences of mixing insulin concentration with syringes of different sizes. Figure 2 shows how to calculate equivalent doses.
Resort tips: Food, drinks, and excursions
A large component of travel is indulging in local cuisine. Patients with diabetes need to be aware of how different foods can affect their diabetes control. Encourage them to research the foods common to the local cuisine. Websites such as Calorie King, MyFitnessPal, Lose it!, and Nutrition Data can help identify the caloric and nutritional makeup of foods.9
Advise patients to actively monitor how their blood glucose is affected by new foods by checking blood glucose levels before and after each meal.9 Opting for vegetables and protein sources minimizes glucose fluctuations. Remind patients that drinks at resorts may contain more sugar than advertised. Patients should continue to manage their blood glucose by checking levels and by making appropriate insulin adjustments based on the readings. We often advise patients to pack a jar of peanut butter when traveling to ensure a ready source of protein.
Patients who plan to participate in physically challenging activities while travelling should inform all relevant members of the activity staff of their condition. In case of an emergency, hotel staff and guides will be better equipped to help with situations such as hypoglycemia. As noted above, patients should always carry snacks and supplies to treat hypoglycemia in case no alternative food options are available during an excursion. Also, warn patients to avoid walking barefoot. Water shoes are a good alternative to protect feet from cuts and sores.
Patients should inquire about the safety of high-elevation activities. With many glucose meters, every 1,000 feet of elevation results in a 1% to 2% underestimation of blood glucose,10 which could result in an inaccurate reading. If high-altitude activities are planned, advise patients to bring multiple meters to cross-check glucose readings in cases where inaccuracies (due to elevation) are possible.
- US Travel Association. US travel and tourism overview. www.ustravel.org/system/files/media_root/document/Research_Fact-Sheet_US-Travel-and-Tourism-Overview.pdf. Accessed June 14, 2018.
- Brunk D. Long haul travel turbulent for many with type 1 diabetes. Clinical Endocrinology News 2016. www.mdedge.com/clinicalendocrinologynews/article/109866/diabetes/long-haul-travel-turbulent-many-type-1-diabetes. Accessed June 14, 2018.
- American Diabetes Association. When you travel. www.diabetes.org/living-with-diabetes/treatment-and-care/when-you-travel.html?utm_source=DSH_BLOG&utm_medium=BlogPost&utm_content=051514-travel&utm_campaign=CON. Accessed June 14, 2018.
- Kruger DF. The Diabetes Travel Guide. How to travel with diabetes-anywhere in the world. Arlington, VA: American Diabetes Association; 2000.
- Centers for Disease Control and Prevention. Travelers’ health. wwwnc.cdc.gov/travel/. Accessed June 14, 2018.
- American Diabetes Association. What special concerns may arise? www.diabetes.org/living-with-diabetes/know-your-rights/discrimination/public-accommodations/air-travel-and-diabetes/what-special-concerns-may.html. Accessed June 14, 2018.
- Chandran M, Edelman SV. Have insulin, will fly: diabetes management during air travel and time zone adjustment strategies. Clinical Diabetes 2003; 21(2):82–85. doi:10.2337/diaclin.21.2.82
- Time and Date AS. Time zone converter. timeanddate.com. Accessed March 19, 2018.
- Joslin Diabetes Center. Diabetes and travel—10 tips for a safe trip. www.joslin.org/info/diabetes_and_travel_10_tips_for_a_safe_trip.html. Accessed June 14, 2018.
- Jendle J, Adolfsson P. Impact of high altitudes on glucose control. J Diabetes Sci Technol 2011; 5(6):1621–1622. doi:10.1177/193229681100500642
- US Travel Association. US travel and tourism overview. www.ustravel.org/system/files/media_root/document/Research_Fact-Sheet_US-Travel-and-Tourism-Overview.pdf. Accessed June 14, 2018.
- Brunk D. Long haul travel turbulent for many with type 1 diabetes. Clinical Endocrinology News 2016. www.mdedge.com/clinicalendocrinologynews/article/109866/diabetes/long-haul-travel-turbulent-many-type-1-diabetes. Accessed June 14, 2018.
- American Diabetes Association. When you travel. www.diabetes.org/living-with-diabetes/treatment-and-care/when-you-travel.html?utm_source=DSH_BLOG&utm_medium=BlogPost&utm_content=051514-travel&utm_campaign=CON. Accessed June 14, 2018.
- Kruger DF. The Diabetes Travel Guide. How to travel with diabetes-anywhere in the world. Arlington, VA: American Diabetes Association; 2000.
- Centers for Disease Control and Prevention. Travelers’ health. wwwnc.cdc.gov/travel/. Accessed June 14, 2018.
- American Diabetes Association. What special concerns may arise? www.diabetes.org/living-with-diabetes/know-your-rights/discrimination/public-accommodations/air-travel-and-diabetes/what-special-concerns-may.html. Accessed June 14, 2018.
- Chandran M, Edelman SV. Have insulin, will fly: diabetes management during air travel and time zone adjustment strategies. Clinical Diabetes 2003; 21(2):82–85. doi:10.2337/diaclin.21.2.82
- Time and Date AS. Time zone converter. timeanddate.com. Accessed March 19, 2018.
- Joslin Diabetes Center. Diabetes and travel—10 tips for a safe trip. www.joslin.org/info/diabetes_and_travel_10_tips_for_a_safe_trip.html. Accessed June 14, 2018.
- Jendle J, Adolfsson P. Impact of high altitudes on glucose control. J Diabetes Sci Technol 2011; 5(6):1621–1622. doi:10.1177/193229681100500642
KEY POINTS
- Patients should pack all diabetes medications and supplies in a carry-on bag and keep it in their possession at all times.
- A travel letter will facilitate easy transfer through security and customs.
- Patients should always take more supplies than needed to accommodate changes in travel plans.
- If patients will cross multiple time zones during their travel, they will likely need to adjust their medication and food schedules.
‘Dry drowning’ and other myths
In June 2017, a 4-year-old boy died 1 week after being knocked over and briefly submerged while playing in knee-deep water. This story was widely reported as a case of a rare occurrence called “dry” or “secondary” drowning, depending on the source.1 The media accounts went viral, spreading fear in parents and others learning about these alleged conditions from the news and social media.
Many alleged cases of dry drowning are reported every year, but each has been found to have a recognized medical source that has a legitimate medically recognized diagnosis (which dry and secondary drowning are not).
Drowning is one of the most common causes of death in children, and so we ought to make sure that the information we share about it is accurate, as it is vital to effective prevention, rescue, and treatment.
Unfortunately, medical providers, medical journals, and the mass media continue to disseminate misinformation on drowning.2 These reports often prevail over updated information and hinder accurate understanding of the drowning problem and its solutions.
Every death is tragic, especially the death of a child, and our heartfelt sympathies go out to the family in this alleged drowning case, as well as to all families suffering the loss of a loved one to drowning. However, in the 2017 case, the cause of death was found on autopsy to be myocarditis not related in any way to drowning. As often happens in such situations, this clarification did not receive any media attention, despite the wide reporting and penetration of the original, erroneous story.
We hope our review will reduce misunderstanding among the public and healthcare providers, contribute to improved data collection, and help to promote interventions aimed at prevention, rescue, and mitigation of drowning incidents.
WHAT IS DROWNING?
A consensus committee of the World Health Organization defined drowning as “the process of experiencing respiratory impairment from submersion/immersion in liquid.”3 The process begins when the victim’s airway goes below the surface of the liquid (submersion) or when water splashes over the face (immersion). If the victim is rescued at any time, the process is interrupted, and this is termed a nonfatal drowning. If the victim dies at any time, this is a fatal drowning. Any water-distress incident without evidence of respiratory impairment (ie, without aspiration) should be considered a water rescue and not a drowning.
Rarely do minimally symptomatic cases progress to death, just as most cases of chest pain do not progress to cardiac arrest.4 Nonetheless, rescued drowning victims can deteriorate, which is why we encourage people to seek medical care immediately upon warning signs, as we do with chest pain. For drowning, such warning signs are any water distress followed by difficulty breathing, excessive coughing, foam in the mouth, or abnormal behavior.
A SERIOUS PUBLIC HEALTH ISSUE
Drowning is a serious and neglected public health issue, claiming the lives of 372,000 people a year worldwide.5 It is a leading cause of death in children ages 1 to 14. The toll continues largely unabated, and in low- and middle-income nations it does not attract the levels of funding that go to other forms of injury prevention, such as road safety.
Nonfatal drowning—with symptoms ranging from mild cough to severe pulmonary edema, and complications ranging from none to severe neurologic impairment—is far more common than fatal drowning.6 For every fatal drowning, there are at least 5 nonfatal drowning incidents in which medical care is needed, and 200 rescues are performed.7–10
In the United States, drowning accounts for almost 13,000 emergency department visits per year and about 3,500 deaths.7,8
In Brazil, with two-thirds the population of the United States, drowning accounts for far fewer hospital visits but about twice as many deaths. In Rio de Janeiro, where a highly effective and specialized prehospital service is provided at 3 drowning resuscitation centers staffed by medical doctors, an analysis of the 46,060 cases of rescue in 10 years from 1991 to 2000 showed that medical assistance was needed in only 930 cases (2%).10 The preventive and rescue actions of parents, bystanders, lifeguards, and prehospital rescue services significantly reduce the number of drowning deaths, but these groups do not consistently gather data on nonfatal drowning that can be included in a comprehensive database.
DROWNING IS A PROCESS
When a person in the water can no longer keep the airway clear, water that enters the mouth is voluntarily spit out or swallowed. Within a few seconds to minutes, the person can no longer clear the airways and water is aspirated, stimulating the cough reflex. Laryngospasm, another myth concerning drowning, is presumed to protect the airways but does not, as it is rare, occurring in less than 2% of cases.11,12
If the person is not rescued, aspiration of water continues, and hypoxemia leads to loss of consciousness and apnea within seconds to a few minutes, followed by cardiac arrest. As a consequence, hypoxemic cardiac arrest generally occurs after a period of tachycardia followed by bradycardia and pulseless electrical activity, usually leading to asystole.13,14
The entire drowning process, from water distress to cardiac arrest, usually takes a few minutes, but in rare situations, such as rapid hypothermia, it can go on for up to an hour.15 Most drowning patients have an otherwise healthy heart, and the apnea and hypoxemia precede the cardiac arrest by only a few seconds to minutes; thus, cardiac arrest is caused by the hypoxemic insult and not by ventricular dysrhythmias.6,16
Drowning can be interrupted at any point between distress and death. If the person is rescued early, the clinical picture is determined by the reactivity of the airway and the amount of water that has been aspirated, but not by the type of water (salt or fresh).
Another myth is that drowning in salt water is different from drowning in fresh water. Both salt water and fresh water cause similar surfactant destruction and washout and disrupt the alveolar-capillary membrane. Disruption of the alveolar-capillary membrane increases its permeability and exacerbates shifting of fluid, plasma, and electrolytes into the alveoli.13 The clinical picture of the damage is one of regional or generalized pulmonary edema, which interferes with gas exchange in the lungs.6,13,17
Animal studies by Modell et al showed that aspiration of just 2.2 mL of water per kilogram of body weight is sufficient to cause severe disturbances in oxygen exchange,17 reflected in a rise in arterial pH and a drop in partial pressure of oxygen. The situation must be similar in humans. In a 70-kg person, this is only about 154 mL of water—about two-thirds of a cup.
The combined effects of fluid in the lungs, the loss of surfactant, and the increase in capillary-alveolar permeability can result in decreased lung compliance, increased right-to-left shunting in the lungs, atelectasis, alveolitis, hypoxemia, and cerebral hypoxia.13
If the victim needs cardiopulmonary resuscitation, the possibility of neurologic damage is similar to that in other cardiac arrest situations, but exceptions exist. For example, in rare cases, hypothermia provides a protective mechanism that allows victims to survive prolonged submersion.4,15
The duration of submersion is the best predictor of death.18 Underwater, people are not taking in oxygen, and cerebral hypoxia causes both morbidity and death. For this reason, reversing cerebral hypoxia with effective ventilation, oxygen, and chest compression is the priority of treatment.
MYTHS AND SLOPPY TERMINOLOGY
“Near drowning,” “dry drowning,” “wet drowning,” “delayed drowning,” and “secondary drowning” are not medically accepted diagnoses,3,4,19 and many organizations and lifesaving institutions around the world discourage the use of these terms.19,20 Unfortunately, these terms still slip past the editors of medical journals and are thus perpetuated. The terms are most pervasive in the nonmedical media, where drowning seems to be synonymous with death.3,19,21 We urge all authors and stakeholders to abandon these terms in favor of understanding and communicating drowning as a process that can vary in severity and have a fatal or nonfatal outcome.
Near-drowning
Historically, drowning meant death, while near-drowning meant the victim survived, at least initially (usually for at least 24 hours).
Before 2002, there were 13 different published definitions of near-drowning.21,22 This variability has caused a great deal of confusion when trying to describe and monitor drowning.
A person can drown and survive, just as a person can have cardiac arrest and survive.4,21 Just as there is no recognized condition of “near-cardiac arrest,” there is also no condition of near-drowning. Using near-drowning as a medical diagnosis hides the true burden of drowning and consequently amplifies difficulties in developing effective prevention, rescue, and treatment programs.
Dry drowning
Dry drowning has never been an accepted medical term, although it has been used to describe different parts of the drowning process. While many authors use it as a synonym for secondary drowning (described below), in the past it was usually used in cases in which no water was found in the lungs at autopsy in persons who were found dead in the water.2–4,21 This occurred in about 10% to 15% of cases and was also called drowning “without water aspiration.”
Perhaps some victims suffer sudden cardiac death. It happens on land—why not in the water? Modell et al stated, “In the absence of the common finding of significant pulmonary edema in the victim’s respiratory system, to conclude his or her death was caused by ‘drowning without aspiration’ is unwise.”23
Laryngospasm is another proposed explanation. It could play a role in the fewer than 2% of cases in which no other cause of death is found on clinical examination or autopsy,11,12,19,23 but it does not occur in most cases of drowning, or it is brief and is terminated by the respiratory movements that allow the air in the lung to escape and water to be inhaled.
The problem with the term dry drowning is the harm caused by misdiagnosing cases of sudden death as drowning, when an alternative cause is present. Most importantly, the management is the same if small amounts of water are present or not; therefore, no clinical distinction is made between wet and dry drowning.
Secondary drowning
Secondary drowning, sometimes called delayed drowning, is another term that is not medically accepted. The historical use of this term reflects the reality that some patients may worsen due to pulmonary edema after aspirating small amounts of water.
Drowning starts with aspiration, and few or only mild symptoms may be present as soon as the person is removed from the water. Either the small amount of water in the lungs is absorbed and causes no complications or, rarely, the patient’s condition becomes progressively worse over the next few hours as the alveoli become inflamed and the alveolar-capillary membrane is disrupted. But people do not unexpectedly die of drowning days or weeks later with no preceding symptoms. The lungs and heart do not “fill up with water,” and water does not need to be pumped out of the lungs.
There has never been a case published in the medical literature of a patient who underwent clinical evaluation, was initially without symptoms, and later deteriorated and died more than 8 hours after the incident.6,10,21 People who have drowned and have minimal symptoms get better (usually) or worse (rarely) within 4 to 8 hours. In a study of more than 41,000 lifeguard rescues, only 0.5% of symptomatic patients died.6
Drowning secondary to injury or sudden illness
Any injury, trauma, or sudden illness that can cause loss of consciousness or mental or physical weakness can lead to drowning. Physicians need to recognize these situations to treat them appropriately. Drowning that is secondary to other primary insults can be classified as24:
- Drowning caused by injury or trauma (eg, a surfing, boating, or a hang-gliding accident)
- Drowning caused by a sudden illness such as cardiac disease (eg, myocardial ischemia, arrhythmias, prolonged QT syndrome, hypertrophic cardiomyopathy) or neurologic disease (eg, epilepsy, stroke)
- Diving disease (eg, decompression sickness, pulmonary overpressurization syndrome, compression barotrauma, narcosis [“rapture of the deep”], shallow water blackout, immersion pulmonary edema).
PREVENTION IS BEST
Drowning is a leading and preventable cause of death worldwide and for people of all ages. The danger is real, not esoteric or rare, and healthcare providers should use any opportunity to discuss with patients, parents, and the media the most important tool for treating drowning: primary prevention.
For example, small children should be continuously and uninterruptedly supervised within arm’s reach while in the water, even if a lifeguard is present. Other preventive measures are lifejackets, fences completely enclosing pools or ponds, and swimming and water safety lessons. Drowning often occurs in a deceptively pleasant environment that may not seem dangerous.
RECOGNIZE DISTRESS
When preventive measures fail, responders (usually a health professional is involved) need to be able to perform the necessary steps to interrupt the drowning process.
The first challenge is to recognize when someone in the water is at risk of drowning and needs to be rescued.25 Early self-rescue or rescue by others may stop the drowning process and prevent most cases of initial and subsequent water aspiration, respiratory distress, and medical complications.
DON’T BECOME A VICTIM
Rescuers must take care not to become victims themselves. Panicked swimmers can thrash about and injure the rescuer or clutch at anything they encounter, dragging the rescuer under. And the rescuer can succumb to the same hazards that got the victim into trouble, such as strong currents, deep water, or underwater hazards.
Certified lifeguards are trained to get victims out of the water safely. The American Red Cross slogan “Reach or throw, don’t go” means “Reach out with a pole or other object or throw something that floats; don’t get in the water yourself.”
WHAT TO TELL THE PUBLIC
While some journalists acknowledge that the terms dry drowning and secondary drowning are medically discredited, they still use them in their reports. The novelty of this story—and its appeal to media outlets—is precisely the unfamiliarity of these terms to the general public and the perceived mysterious, looming threat.
We often hear that these terms are more familiar to the public, which is likely true. More concerning, some physicians continue to use them (and older definitions of drowning that equate it with death) in media interviews, clinical care, and publications. The paradox is that we, the medical community, invented these terms, not patients or the media.
As clinicians and researchers, we should drive popular culture definitions, not the other way around. Rather than dismiss these terms as “semantics” or “technicalities,” we should take the opportunity to highlight the dangers of drowning and the importance of prevention, and to promote simpler language that is easier for us and our patients to understand.19,21
Healthcare providers should understand and share modern drowning science and best practices, which will reduce fear, improve resource utilization, and prevent potentially deadly consequences due to misunderstanding or misinterpretation of incorrect terminology.
WHEN PATIENTS SHOULD SEEK CARE
Anyone who experiences cough, breathlessness, or other worrisome symptoms such as abnormal mentation within 8 hours of a drowning incident (using the modern definition above) should seek medical advice immediately.
We tell people to seek care if symptoms seem any worse than the experience of a drink “going down the wrong pipe” at the dinner table.21 But symptoms can be minimal. Careful attention should be given to mild symptoms that get progressively worse during that time. These cases can rarely progress to acute respiratory distress syndrome.
Table 1 explores who needs further medical help after being rescued from the water.26
In most of these cases, it is most appropriate to call an ambulance, but care may involve seeing a doctor depending on the severity of the symptoms.6,21 Usually, drowning patients are observed for 4 to 8 hours in an emergency department and are discharged if normal. Symptoms that are more significant include persistent cough, foam at the mouth or nose, confusion, or abnormal behavior, and these require further medical evaluation.
Patients should also seek medical care even if they are 100% normal upon exiting the water but develop worrisome symptoms more than 8 hours later, and providers should consider diagnoses other than primary drowning. Spontaneous pneumothorax, chemical pneumonitis, bacterial or viral pneumonia, head injury, asthma, chest trauma, and acute respiratory distress syndrome have been mislabeled as delayed, dry, or secondary drowning.3,4,19,21
- Buffington B. Texas boy dies from ‘dry drowning’ days after swimming. USA Today, June 8, 2017. www.usatoday.com/story/news/nation-now/2017/06/08/texas-boy-dies-dry-drowning-days-after-swimming/379944001.
- Schmidt AC, Sempsrott JR, Szpilman D, et al. The use of non-uniform drowning terminology: a follow-up study. Scand J Trauma Resusc Emerg Med 2017; 25(1):72. doi:10.1186/s13049-017-0405-x
- van Beeck EF, Branche CM, Szpilman D, Modell JH, Bierens JJ. A new definition of drowning: towards documentation and prevention of a global public health problem. Bull World Health Organ 2005; 83(11):853–856. pmid:16302042
- Szpilman D, Bierens JJ, Handley AJ, Orlowski JP. Drowning. N Engl J Med 2012; 366(22):2102–2110. doi:10.1056/NEJMra1013317
- World Health Organization. Global report on drowning: preventing a leading killer. www.who.int/violence_injury_prevention/global_report_drowning/en. Accessed June 13, 2018.
- Szpilman D. Near-drowning and drowning classification: a proposal to stratify mortality based on the analysis of 1,831 cases. Chest 1997; 112(3):660–665. pmid:9315798
- Centers for Disease Control and Prevention. Welcome to WISQARS. www.cdc.gov/injury/wisqars. Accessed June 13, 2018.
- Centers for Disease Control and Prevention. WONDER. https://wonder.cdc.gov. Accessed June 13, 2018.
- Cummings P, Quan L. Trends in unintentional drowning: the role of alcohol and medical care. JAMA 1999; 281(23):2198–2202. pmid:10376572
- Szpilman D, Elmann J, Cruz-Filho FES. Drowning classification: a revalidation study based on the analysis of 930 cases over 10 years. World Congress on Drowning, Netherlands 2002. www.researchgate.net/publication/267981062_DROWNING_CLASSIFICATION_a_revalidation_study_based_on_the_analysis_of_930_cases_over_10_years. Accessed June 13, 2018.
- Szpilman D, Elmann J, Cruz-Filho FES. Dry-drowning—fact or myth? World Congress on Drowning. Netherlands, 2002. www.researchgate.net/publication/267981164_Dry-drowning_-Fact_or_Myth. Accessed June 13, 2018.
- Lunetta P, Modell JH, Sajantila A. What is the incidence and significance of "dry-lungs" in bodies found in water? Am J Forensic Med Pathol 2004; 25(4):291–301. pmid:15577518
- Orlowski JP, Abulleil MM, Phillips JM. The hemodynamic and cardiovascular effects of near-drowning in hypotonic, isotonic, or hypertonic solutions. Ann Emerg Med 1989; 18:1044–1049. pmid:2802278
- Grmec S, Strnad M, Podgorsek D. Comparison of the characteristics and outcome among patients suffering from out-of-hospital primary cardiac arrest and drowning victims in cardiac arrest. Int J Emerg Med 2009; 2(1):7–12. doi:10.1007/s12245-009-0084-0
- Tipton MJ, Golden FS. A proposed decision-making guide for the search, rescue and resuscitation of submersion (head under) victims based on expert opinion. Resuscitation 2011; 82(7):819–824. doi:10.1016/j.resuscitation.2011.02.021
- Orlowski JP, Szpilman D. Drowning. Rescue, resuscitation, and reanimation. Pediatr Clin North Am 2001; 48(3):627–646. pmid:11411297
- Modell JH, Moya F, Newby EJ, Ruiz BC, Showers AV. The effects of fluid volume in seawater drowning. Ann Intern Med 1967; 67(1):68–80. pmid:6028660
- Quan L, Wentz KR, Gore EJ, Copass MK. Outcome and predictors of outcome in pediatric submersion victims receiving prehospital care in King County, Washington. Pediatrics 1990; 86(4):586–593. pmid:2216625
- Szpilman D, Orlowski JP, Cruz-Filho FES. Hey “Near-drowning,” you’ve been messing up our minds! World Congress on Drowning. Amsterdam, 2002. www.researchgate.net/publication/267981173_HEY_Near-drowning_YOU%27VE_BEEN_MESSING_UP_OUR_MINDS. Accessed June 13, 2018.
- American College of Emergency Physicians. Death after swimming is extremely rare—and is not “dry drowning.” http://newsroom.acep.org/2017-07-11-Death-After-Swimming-Is-Extremely-Rare-And-Is-NOT-Dry-Drowning. Accessed June 13, 2018.
- Hawkins SC, Sempsrott J, Schmidt A. “Drowning” in a sea of misinformation. Emergency Medicine News 2017; 39*8):1. http://journals.lww.com/em-news/blog/BreakingNews/pages/post.aspx?PostID=377. Accessed June 5, 2018.
- Szpilman D, Tipton M, Sempsrott J, et al. Drowning timeline: a new systematic model of the drowning process. Am J Emerg Med 2016; 34(11):2224–2226. doi:10.1016/j.ajem.2016.07.063
- Modell JH, Bellefleur M, Davis JH. Drowning without aspiration: is this an appropriate diagnosis? J Forensic Sci 1999; 44(6):1119–1123. pmid:10582353
- Szpilman D, Orlowski JP. Sports related to drowning. Eur Respir Rev 2016; 25(141):348–359. doi:10.1183/16000617.0038-2016
- Szpilman D, Webber J, Quan L, et al. Creating a drowning chain of survival. Resuscitation 2014; 85(9):1149–1152. doi:10.1016/j.resuscitation.2014.05.034
- International Life Saving Federation. Who needs further medical help after rescue from the water. Medical Position Statement - MPS 06, 2016. www.ilsf.org/file/3916/download?token=pDnPDCrk. Accessed June 13, 2018.
In June 2017, a 4-year-old boy died 1 week after being knocked over and briefly submerged while playing in knee-deep water. This story was widely reported as a case of a rare occurrence called “dry” or “secondary” drowning, depending on the source.1 The media accounts went viral, spreading fear in parents and others learning about these alleged conditions from the news and social media.
Many alleged cases of dry drowning are reported every year, but each has been found to have a recognized medical source that has a legitimate medically recognized diagnosis (which dry and secondary drowning are not).
Drowning is one of the most common causes of death in children, and so we ought to make sure that the information we share about it is accurate, as it is vital to effective prevention, rescue, and treatment.
Unfortunately, medical providers, medical journals, and the mass media continue to disseminate misinformation on drowning.2 These reports often prevail over updated information and hinder accurate understanding of the drowning problem and its solutions.
Every death is tragic, especially the death of a child, and our heartfelt sympathies go out to the family in this alleged drowning case, as well as to all families suffering the loss of a loved one to drowning. However, in the 2017 case, the cause of death was found on autopsy to be myocarditis not related in any way to drowning. As often happens in such situations, this clarification did not receive any media attention, despite the wide reporting and penetration of the original, erroneous story.
We hope our review will reduce misunderstanding among the public and healthcare providers, contribute to improved data collection, and help to promote interventions aimed at prevention, rescue, and mitigation of drowning incidents.
WHAT IS DROWNING?
A consensus committee of the World Health Organization defined drowning as “the process of experiencing respiratory impairment from submersion/immersion in liquid.”3 The process begins when the victim’s airway goes below the surface of the liquid (submersion) or when water splashes over the face (immersion). If the victim is rescued at any time, the process is interrupted, and this is termed a nonfatal drowning. If the victim dies at any time, this is a fatal drowning. Any water-distress incident without evidence of respiratory impairment (ie, without aspiration) should be considered a water rescue and not a drowning.
Rarely do minimally symptomatic cases progress to death, just as most cases of chest pain do not progress to cardiac arrest.4 Nonetheless, rescued drowning victims can deteriorate, which is why we encourage people to seek medical care immediately upon warning signs, as we do with chest pain. For drowning, such warning signs are any water distress followed by difficulty breathing, excessive coughing, foam in the mouth, or abnormal behavior.
A SERIOUS PUBLIC HEALTH ISSUE
Drowning is a serious and neglected public health issue, claiming the lives of 372,000 people a year worldwide.5 It is a leading cause of death in children ages 1 to 14. The toll continues largely unabated, and in low- and middle-income nations it does not attract the levels of funding that go to other forms of injury prevention, such as road safety.
Nonfatal drowning—with symptoms ranging from mild cough to severe pulmonary edema, and complications ranging from none to severe neurologic impairment—is far more common than fatal drowning.6 For every fatal drowning, there are at least 5 nonfatal drowning incidents in which medical care is needed, and 200 rescues are performed.7–10
In the United States, drowning accounts for almost 13,000 emergency department visits per year and about 3,500 deaths.7,8
In Brazil, with two-thirds the population of the United States, drowning accounts for far fewer hospital visits but about twice as many deaths. In Rio de Janeiro, where a highly effective and specialized prehospital service is provided at 3 drowning resuscitation centers staffed by medical doctors, an analysis of the 46,060 cases of rescue in 10 years from 1991 to 2000 showed that medical assistance was needed in only 930 cases (2%).10 The preventive and rescue actions of parents, bystanders, lifeguards, and prehospital rescue services significantly reduce the number of drowning deaths, but these groups do not consistently gather data on nonfatal drowning that can be included in a comprehensive database.
DROWNING IS A PROCESS
When a person in the water can no longer keep the airway clear, water that enters the mouth is voluntarily spit out or swallowed. Within a few seconds to minutes, the person can no longer clear the airways and water is aspirated, stimulating the cough reflex. Laryngospasm, another myth concerning drowning, is presumed to protect the airways but does not, as it is rare, occurring in less than 2% of cases.11,12
If the person is not rescued, aspiration of water continues, and hypoxemia leads to loss of consciousness and apnea within seconds to a few minutes, followed by cardiac arrest. As a consequence, hypoxemic cardiac arrest generally occurs after a period of tachycardia followed by bradycardia and pulseless electrical activity, usually leading to asystole.13,14
The entire drowning process, from water distress to cardiac arrest, usually takes a few minutes, but in rare situations, such as rapid hypothermia, it can go on for up to an hour.15 Most drowning patients have an otherwise healthy heart, and the apnea and hypoxemia precede the cardiac arrest by only a few seconds to minutes; thus, cardiac arrest is caused by the hypoxemic insult and not by ventricular dysrhythmias.6,16
Drowning can be interrupted at any point between distress and death. If the person is rescued early, the clinical picture is determined by the reactivity of the airway and the amount of water that has been aspirated, but not by the type of water (salt or fresh).
Another myth is that drowning in salt water is different from drowning in fresh water. Both salt water and fresh water cause similar surfactant destruction and washout and disrupt the alveolar-capillary membrane. Disruption of the alveolar-capillary membrane increases its permeability and exacerbates shifting of fluid, plasma, and electrolytes into the alveoli.13 The clinical picture of the damage is one of regional or generalized pulmonary edema, which interferes with gas exchange in the lungs.6,13,17
Animal studies by Modell et al showed that aspiration of just 2.2 mL of water per kilogram of body weight is sufficient to cause severe disturbances in oxygen exchange,17 reflected in a rise in arterial pH and a drop in partial pressure of oxygen. The situation must be similar in humans. In a 70-kg person, this is only about 154 mL of water—about two-thirds of a cup.
The combined effects of fluid in the lungs, the loss of surfactant, and the increase in capillary-alveolar permeability can result in decreased lung compliance, increased right-to-left shunting in the lungs, atelectasis, alveolitis, hypoxemia, and cerebral hypoxia.13
If the victim needs cardiopulmonary resuscitation, the possibility of neurologic damage is similar to that in other cardiac arrest situations, but exceptions exist. For example, in rare cases, hypothermia provides a protective mechanism that allows victims to survive prolonged submersion.4,15
The duration of submersion is the best predictor of death.18 Underwater, people are not taking in oxygen, and cerebral hypoxia causes both morbidity and death. For this reason, reversing cerebral hypoxia with effective ventilation, oxygen, and chest compression is the priority of treatment.
MYTHS AND SLOPPY TERMINOLOGY
“Near drowning,” “dry drowning,” “wet drowning,” “delayed drowning,” and “secondary drowning” are not medically accepted diagnoses,3,4,19 and many organizations and lifesaving institutions around the world discourage the use of these terms.19,20 Unfortunately, these terms still slip past the editors of medical journals and are thus perpetuated. The terms are most pervasive in the nonmedical media, where drowning seems to be synonymous with death.3,19,21 We urge all authors and stakeholders to abandon these terms in favor of understanding and communicating drowning as a process that can vary in severity and have a fatal or nonfatal outcome.
Near-drowning
Historically, drowning meant death, while near-drowning meant the victim survived, at least initially (usually for at least 24 hours).
Before 2002, there were 13 different published definitions of near-drowning.21,22 This variability has caused a great deal of confusion when trying to describe and monitor drowning.
A person can drown and survive, just as a person can have cardiac arrest and survive.4,21 Just as there is no recognized condition of “near-cardiac arrest,” there is also no condition of near-drowning. Using near-drowning as a medical diagnosis hides the true burden of drowning and consequently amplifies difficulties in developing effective prevention, rescue, and treatment programs.
Dry drowning
Dry drowning has never been an accepted medical term, although it has been used to describe different parts of the drowning process. While many authors use it as a synonym for secondary drowning (described below), in the past it was usually used in cases in which no water was found in the lungs at autopsy in persons who were found dead in the water.2–4,21 This occurred in about 10% to 15% of cases and was also called drowning “without water aspiration.”
Perhaps some victims suffer sudden cardiac death. It happens on land—why not in the water? Modell et al stated, “In the absence of the common finding of significant pulmonary edema in the victim’s respiratory system, to conclude his or her death was caused by ‘drowning without aspiration’ is unwise.”23
Laryngospasm is another proposed explanation. It could play a role in the fewer than 2% of cases in which no other cause of death is found on clinical examination or autopsy,11,12,19,23 but it does not occur in most cases of drowning, or it is brief and is terminated by the respiratory movements that allow the air in the lung to escape and water to be inhaled.
The problem with the term dry drowning is the harm caused by misdiagnosing cases of sudden death as drowning, when an alternative cause is present. Most importantly, the management is the same if small amounts of water are present or not; therefore, no clinical distinction is made between wet and dry drowning.
Secondary drowning
Secondary drowning, sometimes called delayed drowning, is another term that is not medically accepted. The historical use of this term reflects the reality that some patients may worsen due to pulmonary edema after aspirating small amounts of water.
Drowning starts with aspiration, and few or only mild symptoms may be present as soon as the person is removed from the water. Either the small amount of water in the lungs is absorbed and causes no complications or, rarely, the patient’s condition becomes progressively worse over the next few hours as the alveoli become inflamed and the alveolar-capillary membrane is disrupted. But people do not unexpectedly die of drowning days or weeks later with no preceding symptoms. The lungs and heart do not “fill up with water,” and water does not need to be pumped out of the lungs.
There has never been a case published in the medical literature of a patient who underwent clinical evaluation, was initially without symptoms, and later deteriorated and died more than 8 hours after the incident.6,10,21 People who have drowned and have minimal symptoms get better (usually) or worse (rarely) within 4 to 8 hours. In a study of more than 41,000 lifeguard rescues, only 0.5% of symptomatic patients died.6
Drowning secondary to injury or sudden illness
Any injury, trauma, or sudden illness that can cause loss of consciousness or mental or physical weakness can lead to drowning. Physicians need to recognize these situations to treat them appropriately. Drowning that is secondary to other primary insults can be classified as24:
- Drowning caused by injury or trauma (eg, a surfing, boating, or a hang-gliding accident)
- Drowning caused by a sudden illness such as cardiac disease (eg, myocardial ischemia, arrhythmias, prolonged QT syndrome, hypertrophic cardiomyopathy) or neurologic disease (eg, epilepsy, stroke)
- Diving disease (eg, decompression sickness, pulmonary overpressurization syndrome, compression barotrauma, narcosis [“rapture of the deep”], shallow water blackout, immersion pulmonary edema).
PREVENTION IS BEST
Drowning is a leading and preventable cause of death worldwide and for people of all ages. The danger is real, not esoteric or rare, and healthcare providers should use any opportunity to discuss with patients, parents, and the media the most important tool for treating drowning: primary prevention.
For example, small children should be continuously and uninterruptedly supervised within arm’s reach while in the water, even if a lifeguard is present. Other preventive measures are lifejackets, fences completely enclosing pools or ponds, and swimming and water safety lessons. Drowning often occurs in a deceptively pleasant environment that may not seem dangerous.
RECOGNIZE DISTRESS
When preventive measures fail, responders (usually a health professional is involved) need to be able to perform the necessary steps to interrupt the drowning process.
The first challenge is to recognize when someone in the water is at risk of drowning and needs to be rescued.25 Early self-rescue or rescue by others may stop the drowning process and prevent most cases of initial and subsequent water aspiration, respiratory distress, and medical complications.
DON’T BECOME A VICTIM
Rescuers must take care not to become victims themselves. Panicked swimmers can thrash about and injure the rescuer or clutch at anything they encounter, dragging the rescuer under. And the rescuer can succumb to the same hazards that got the victim into trouble, such as strong currents, deep water, or underwater hazards.
Certified lifeguards are trained to get victims out of the water safely. The American Red Cross slogan “Reach or throw, don’t go” means “Reach out with a pole or other object or throw something that floats; don’t get in the water yourself.”
WHAT TO TELL THE PUBLIC
While some journalists acknowledge that the terms dry drowning and secondary drowning are medically discredited, they still use them in their reports. The novelty of this story—and its appeal to media outlets—is precisely the unfamiliarity of these terms to the general public and the perceived mysterious, looming threat.
We often hear that these terms are more familiar to the public, which is likely true. More concerning, some physicians continue to use them (and older definitions of drowning that equate it with death) in media interviews, clinical care, and publications. The paradox is that we, the medical community, invented these terms, not patients or the media.
As clinicians and researchers, we should drive popular culture definitions, not the other way around. Rather than dismiss these terms as “semantics” or “technicalities,” we should take the opportunity to highlight the dangers of drowning and the importance of prevention, and to promote simpler language that is easier for us and our patients to understand.19,21
Healthcare providers should understand and share modern drowning science and best practices, which will reduce fear, improve resource utilization, and prevent potentially deadly consequences due to misunderstanding or misinterpretation of incorrect terminology.
WHEN PATIENTS SHOULD SEEK CARE
Anyone who experiences cough, breathlessness, or other worrisome symptoms such as abnormal mentation within 8 hours of a drowning incident (using the modern definition above) should seek medical advice immediately.
We tell people to seek care if symptoms seem any worse than the experience of a drink “going down the wrong pipe” at the dinner table.21 But symptoms can be minimal. Careful attention should be given to mild symptoms that get progressively worse during that time. These cases can rarely progress to acute respiratory distress syndrome.
Table 1 explores who needs further medical help after being rescued from the water.26
In most of these cases, it is most appropriate to call an ambulance, but care may involve seeing a doctor depending on the severity of the symptoms.6,21 Usually, drowning patients are observed for 4 to 8 hours in an emergency department and are discharged if normal. Symptoms that are more significant include persistent cough, foam at the mouth or nose, confusion, or abnormal behavior, and these require further medical evaluation.
Patients should also seek medical care even if they are 100% normal upon exiting the water but develop worrisome symptoms more than 8 hours later, and providers should consider diagnoses other than primary drowning. Spontaneous pneumothorax, chemical pneumonitis, bacterial or viral pneumonia, head injury, asthma, chest trauma, and acute respiratory distress syndrome have been mislabeled as delayed, dry, or secondary drowning.3,4,19,21
In June 2017, a 4-year-old boy died 1 week after being knocked over and briefly submerged while playing in knee-deep water. This story was widely reported as a case of a rare occurrence called “dry” or “secondary” drowning, depending on the source.1 The media accounts went viral, spreading fear in parents and others learning about these alleged conditions from the news and social media.
Many alleged cases of dry drowning are reported every year, but each has been found to have a recognized medical source that has a legitimate medically recognized diagnosis (which dry and secondary drowning are not).
Drowning is one of the most common causes of death in children, and so we ought to make sure that the information we share about it is accurate, as it is vital to effective prevention, rescue, and treatment.
Unfortunately, medical providers, medical journals, and the mass media continue to disseminate misinformation on drowning.2 These reports often prevail over updated information and hinder accurate understanding of the drowning problem and its solutions.
Every death is tragic, especially the death of a child, and our heartfelt sympathies go out to the family in this alleged drowning case, as well as to all families suffering the loss of a loved one to drowning. However, in the 2017 case, the cause of death was found on autopsy to be myocarditis not related in any way to drowning. As often happens in such situations, this clarification did not receive any media attention, despite the wide reporting and penetration of the original, erroneous story.
We hope our review will reduce misunderstanding among the public and healthcare providers, contribute to improved data collection, and help to promote interventions aimed at prevention, rescue, and mitigation of drowning incidents.
WHAT IS DROWNING?
A consensus committee of the World Health Organization defined drowning as “the process of experiencing respiratory impairment from submersion/immersion in liquid.”3 The process begins when the victim’s airway goes below the surface of the liquid (submersion) or when water splashes over the face (immersion). If the victim is rescued at any time, the process is interrupted, and this is termed a nonfatal drowning. If the victim dies at any time, this is a fatal drowning. Any water-distress incident without evidence of respiratory impairment (ie, without aspiration) should be considered a water rescue and not a drowning.
Rarely do minimally symptomatic cases progress to death, just as most cases of chest pain do not progress to cardiac arrest.4 Nonetheless, rescued drowning victims can deteriorate, which is why we encourage people to seek medical care immediately upon warning signs, as we do with chest pain. For drowning, such warning signs are any water distress followed by difficulty breathing, excessive coughing, foam in the mouth, or abnormal behavior.
A SERIOUS PUBLIC HEALTH ISSUE
Drowning is a serious and neglected public health issue, claiming the lives of 372,000 people a year worldwide.5 It is a leading cause of death in children ages 1 to 14. The toll continues largely unabated, and in low- and middle-income nations it does not attract the levels of funding that go to other forms of injury prevention, such as road safety.
Nonfatal drowning—with symptoms ranging from mild cough to severe pulmonary edema, and complications ranging from none to severe neurologic impairment—is far more common than fatal drowning.6 For every fatal drowning, there are at least 5 nonfatal drowning incidents in which medical care is needed, and 200 rescues are performed.7–10
In the United States, drowning accounts for almost 13,000 emergency department visits per year and about 3,500 deaths.7,8
In Brazil, with two-thirds the population of the United States, drowning accounts for far fewer hospital visits but about twice as many deaths. In Rio de Janeiro, where a highly effective and specialized prehospital service is provided at 3 drowning resuscitation centers staffed by medical doctors, an analysis of the 46,060 cases of rescue in 10 years from 1991 to 2000 showed that medical assistance was needed in only 930 cases (2%).10 The preventive and rescue actions of parents, bystanders, lifeguards, and prehospital rescue services significantly reduce the number of drowning deaths, but these groups do not consistently gather data on nonfatal drowning that can be included in a comprehensive database.
DROWNING IS A PROCESS
When a person in the water can no longer keep the airway clear, water that enters the mouth is voluntarily spit out or swallowed. Within a few seconds to minutes, the person can no longer clear the airways and water is aspirated, stimulating the cough reflex. Laryngospasm, another myth concerning drowning, is presumed to protect the airways but does not, as it is rare, occurring in less than 2% of cases.11,12
If the person is not rescued, aspiration of water continues, and hypoxemia leads to loss of consciousness and apnea within seconds to a few minutes, followed by cardiac arrest. As a consequence, hypoxemic cardiac arrest generally occurs after a period of tachycardia followed by bradycardia and pulseless electrical activity, usually leading to asystole.13,14
The entire drowning process, from water distress to cardiac arrest, usually takes a few minutes, but in rare situations, such as rapid hypothermia, it can go on for up to an hour.15 Most drowning patients have an otherwise healthy heart, and the apnea and hypoxemia precede the cardiac arrest by only a few seconds to minutes; thus, cardiac arrest is caused by the hypoxemic insult and not by ventricular dysrhythmias.6,16
Drowning can be interrupted at any point between distress and death. If the person is rescued early, the clinical picture is determined by the reactivity of the airway and the amount of water that has been aspirated, but not by the type of water (salt or fresh).
Another myth is that drowning in salt water is different from drowning in fresh water. Both salt water and fresh water cause similar surfactant destruction and washout and disrupt the alveolar-capillary membrane. Disruption of the alveolar-capillary membrane increases its permeability and exacerbates shifting of fluid, plasma, and electrolytes into the alveoli.13 The clinical picture of the damage is one of regional or generalized pulmonary edema, which interferes with gas exchange in the lungs.6,13,17
Animal studies by Modell et al showed that aspiration of just 2.2 mL of water per kilogram of body weight is sufficient to cause severe disturbances in oxygen exchange,17 reflected in a rise in arterial pH and a drop in partial pressure of oxygen. The situation must be similar in humans. In a 70-kg person, this is only about 154 mL of water—about two-thirds of a cup.
The combined effects of fluid in the lungs, the loss of surfactant, and the increase in capillary-alveolar permeability can result in decreased lung compliance, increased right-to-left shunting in the lungs, atelectasis, alveolitis, hypoxemia, and cerebral hypoxia.13
If the victim needs cardiopulmonary resuscitation, the possibility of neurologic damage is similar to that in other cardiac arrest situations, but exceptions exist. For example, in rare cases, hypothermia provides a protective mechanism that allows victims to survive prolonged submersion.4,15
The duration of submersion is the best predictor of death.18 Underwater, people are not taking in oxygen, and cerebral hypoxia causes both morbidity and death. For this reason, reversing cerebral hypoxia with effective ventilation, oxygen, and chest compression is the priority of treatment.
MYTHS AND SLOPPY TERMINOLOGY
“Near drowning,” “dry drowning,” “wet drowning,” “delayed drowning,” and “secondary drowning” are not medically accepted diagnoses,3,4,19 and many organizations and lifesaving institutions around the world discourage the use of these terms.19,20 Unfortunately, these terms still slip past the editors of medical journals and are thus perpetuated. The terms are most pervasive in the nonmedical media, where drowning seems to be synonymous with death.3,19,21 We urge all authors and stakeholders to abandon these terms in favor of understanding and communicating drowning as a process that can vary in severity and have a fatal or nonfatal outcome.
Near-drowning
Historically, drowning meant death, while near-drowning meant the victim survived, at least initially (usually for at least 24 hours).
Before 2002, there were 13 different published definitions of near-drowning.21,22 This variability has caused a great deal of confusion when trying to describe and monitor drowning.
A person can drown and survive, just as a person can have cardiac arrest and survive.4,21 Just as there is no recognized condition of “near-cardiac arrest,” there is also no condition of near-drowning. Using near-drowning as a medical diagnosis hides the true burden of drowning and consequently amplifies difficulties in developing effective prevention, rescue, and treatment programs.
Dry drowning
Dry drowning has never been an accepted medical term, although it has been used to describe different parts of the drowning process. While many authors use it as a synonym for secondary drowning (described below), in the past it was usually used in cases in which no water was found in the lungs at autopsy in persons who were found dead in the water.2–4,21 This occurred in about 10% to 15% of cases and was also called drowning “without water aspiration.”
Perhaps some victims suffer sudden cardiac death. It happens on land—why not in the water? Modell et al stated, “In the absence of the common finding of significant pulmonary edema in the victim’s respiratory system, to conclude his or her death was caused by ‘drowning without aspiration’ is unwise.”23
Laryngospasm is another proposed explanation. It could play a role in the fewer than 2% of cases in which no other cause of death is found on clinical examination or autopsy,11,12,19,23 but it does not occur in most cases of drowning, or it is brief and is terminated by the respiratory movements that allow the air in the lung to escape and water to be inhaled.
The problem with the term dry drowning is the harm caused by misdiagnosing cases of sudden death as drowning, when an alternative cause is present. Most importantly, the management is the same if small amounts of water are present or not; therefore, no clinical distinction is made between wet and dry drowning.
Secondary drowning
Secondary drowning, sometimes called delayed drowning, is another term that is not medically accepted. The historical use of this term reflects the reality that some patients may worsen due to pulmonary edema after aspirating small amounts of water.
Drowning starts with aspiration, and few or only mild symptoms may be present as soon as the person is removed from the water. Either the small amount of water in the lungs is absorbed and causes no complications or, rarely, the patient’s condition becomes progressively worse over the next few hours as the alveoli become inflamed and the alveolar-capillary membrane is disrupted. But people do not unexpectedly die of drowning days or weeks later with no preceding symptoms. The lungs and heart do not “fill up with water,” and water does not need to be pumped out of the lungs.
There has never been a case published in the medical literature of a patient who underwent clinical evaluation, was initially without symptoms, and later deteriorated and died more than 8 hours after the incident.6,10,21 People who have drowned and have minimal symptoms get better (usually) or worse (rarely) within 4 to 8 hours. In a study of more than 41,000 lifeguard rescues, only 0.5% of symptomatic patients died.6
Drowning secondary to injury or sudden illness
Any injury, trauma, or sudden illness that can cause loss of consciousness or mental or physical weakness can lead to drowning. Physicians need to recognize these situations to treat them appropriately. Drowning that is secondary to other primary insults can be classified as24:
- Drowning caused by injury or trauma (eg, a surfing, boating, or a hang-gliding accident)
- Drowning caused by a sudden illness such as cardiac disease (eg, myocardial ischemia, arrhythmias, prolonged QT syndrome, hypertrophic cardiomyopathy) or neurologic disease (eg, epilepsy, stroke)
- Diving disease (eg, decompression sickness, pulmonary overpressurization syndrome, compression barotrauma, narcosis [“rapture of the deep”], shallow water blackout, immersion pulmonary edema).
PREVENTION IS BEST
Drowning is a leading and preventable cause of death worldwide and for people of all ages. The danger is real, not esoteric or rare, and healthcare providers should use any opportunity to discuss with patients, parents, and the media the most important tool for treating drowning: primary prevention.
For example, small children should be continuously and uninterruptedly supervised within arm’s reach while in the water, even if a lifeguard is present. Other preventive measures are lifejackets, fences completely enclosing pools or ponds, and swimming and water safety lessons. Drowning often occurs in a deceptively pleasant environment that may not seem dangerous.
RECOGNIZE DISTRESS
When preventive measures fail, responders (usually a health professional is involved) need to be able to perform the necessary steps to interrupt the drowning process.
The first challenge is to recognize when someone in the water is at risk of drowning and needs to be rescued.25 Early self-rescue or rescue by others may stop the drowning process and prevent most cases of initial and subsequent water aspiration, respiratory distress, and medical complications.
DON’T BECOME A VICTIM
Rescuers must take care not to become victims themselves. Panicked swimmers can thrash about and injure the rescuer or clutch at anything they encounter, dragging the rescuer under. And the rescuer can succumb to the same hazards that got the victim into trouble, such as strong currents, deep water, or underwater hazards.
Certified lifeguards are trained to get victims out of the water safely. The American Red Cross slogan “Reach or throw, don’t go” means “Reach out with a pole or other object or throw something that floats; don’t get in the water yourself.”
WHAT TO TELL THE PUBLIC
While some journalists acknowledge that the terms dry drowning and secondary drowning are medically discredited, they still use them in their reports. The novelty of this story—and its appeal to media outlets—is precisely the unfamiliarity of these terms to the general public and the perceived mysterious, looming threat.
We often hear that these terms are more familiar to the public, which is likely true. More concerning, some physicians continue to use them (and older definitions of drowning that equate it with death) in media interviews, clinical care, and publications. The paradox is that we, the medical community, invented these terms, not patients or the media.
As clinicians and researchers, we should drive popular culture definitions, not the other way around. Rather than dismiss these terms as “semantics” or “technicalities,” we should take the opportunity to highlight the dangers of drowning and the importance of prevention, and to promote simpler language that is easier for us and our patients to understand.19,21
Healthcare providers should understand and share modern drowning science and best practices, which will reduce fear, improve resource utilization, and prevent potentially deadly consequences due to misunderstanding or misinterpretation of incorrect terminology.
WHEN PATIENTS SHOULD SEEK CARE
Anyone who experiences cough, breathlessness, or other worrisome symptoms such as abnormal mentation within 8 hours of a drowning incident (using the modern definition above) should seek medical advice immediately.
We tell people to seek care if symptoms seem any worse than the experience of a drink “going down the wrong pipe” at the dinner table.21 But symptoms can be minimal. Careful attention should be given to mild symptoms that get progressively worse during that time. These cases can rarely progress to acute respiratory distress syndrome.
Table 1 explores who needs further medical help after being rescued from the water.26
In most of these cases, it is most appropriate to call an ambulance, but care may involve seeing a doctor depending on the severity of the symptoms.6,21 Usually, drowning patients are observed for 4 to 8 hours in an emergency department and are discharged if normal. Symptoms that are more significant include persistent cough, foam at the mouth or nose, confusion, or abnormal behavior, and these require further medical evaluation.
Patients should also seek medical care even if they are 100% normal upon exiting the water but develop worrisome symptoms more than 8 hours later, and providers should consider diagnoses other than primary drowning. Spontaneous pneumothorax, chemical pneumonitis, bacterial or viral pneumonia, head injury, asthma, chest trauma, and acute respiratory distress syndrome have been mislabeled as delayed, dry, or secondary drowning.3,4,19,21
- Buffington B. Texas boy dies from ‘dry drowning’ days after swimming. USA Today, June 8, 2017. www.usatoday.com/story/news/nation-now/2017/06/08/texas-boy-dies-dry-drowning-days-after-swimming/379944001.
- Schmidt AC, Sempsrott JR, Szpilman D, et al. The use of non-uniform drowning terminology: a follow-up study. Scand J Trauma Resusc Emerg Med 2017; 25(1):72. doi:10.1186/s13049-017-0405-x
- van Beeck EF, Branche CM, Szpilman D, Modell JH, Bierens JJ. A new definition of drowning: towards documentation and prevention of a global public health problem. Bull World Health Organ 2005; 83(11):853–856. pmid:16302042
- Szpilman D, Bierens JJ, Handley AJ, Orlowski JP. Drowning. N Engl J Med 2012; 366(22):2102–2110. doi:10.1056/NEJMra1013317
- World Health Organization. Global report on drowning: preventing a leading killer. www.who.int/violence_injury_prevention/global_report_drowning/en. Accessed June 13, 2018.
- Szpilman D. Near-drowning and drowning classification: a proposal to stratify mortality based on the analysis of 1,831 cases. Chest 1997; 112(3):660–665. pmid:9315798
- Centers for Disease Control and Prevention. Welcome to WISQARS. www.cdc.gov/injury/wisqars. Accessed June 13, 2018.
- Centers for Disease Control and Prevention. WONDER. https://wonder.cdc.gov. Accessed June 13, 2018.
- Cummings P, Quan L. Trends in unintentional drowning: the role of alcohol and medical care. JAMA 1999; 281(23):2198–2202. pmid:10376572
- Szpilman D, Elmann J, Cruz-Filho FES. Drowning classification: a revalidation study based on the analysis of 930 cases over 10 years. World Congress on Drowning, Netherlands 2002. www.researchgate.net/publication/267981062_DROWNING_CLASSIFICATION_a_revalidation_study_based_on_the_analysis_of_930_cases_over_10_years. Accessed June 13, 2018.
- Szpilman D, Elmann J, Cruz-Filho FES. Dry-drowning—fact or myth? World Congress on Drowning. Netherlands, 2002. www.researchgate.net/publication/267981164_Dry-drowning_-Fact_or_Myth. Accessed June 13, 2018.
- Lunetta P, Modell JH, Sajantila A. What is the incidence and significance of "dry-lungs" in bodies found in water? Am J Forensic Med Pathol 2004; 25(4):291–301. pmid:15577518
- Orlowski JP, Abulleil MM, Phillips JM. The hemodynamic and cardiovascular effects of near-drowning in hypotonic, isotonic, or hypertonic solutions. Ann Emerg Med 1989; 18:1044–1049. pmid:2802278
- Grmec S, Strnad M, Podgorsek D. Comparison of the characteristics and outcome among patients suffering from out-of-hospital primary cardiac arrest and drowning victims in cardiac arrest. Int J Emerg Med 2009; 2(1):7–12. doi:10.1007/s12245-009-0084-0
- Tipton MJ, Golden FS. A proposed decision-making guide for the search, rescue and resuscitation of submersion (head under) victims based on expert opinion. Resuscitation 2011; 82(7):819–824. doi:10.1016/j.resuscitation.2011.02.021
- Orlowski JP, Szpilman D. Drowning. Rescue, resuscitation, and reanimation. Pediatr Clin North Am 2001; 48(3):627–646. pmid:11411297
- Modell JH, Moya F, Newby EJ, Ruiz BC, Showers AV. The effects of fluid volume in seawater drowning. Ann Intern Med 1967; 67(1):68–80. pmid:6028660
- Quan L, Wentz KR, Gore EJ, Copass MK. Outcome and predictors of outcome in pediatric submersion victims receiving prehospital care in King County, Washington. Pediatrics 1990; 86(4):586–593. pmid:2216625
- Szpilman D, Orlowski JP, Cruz-Filho FES. Hey “Near-drowning,” you’ve been messing up our minds! World Congress on Drowning. Amsterdam, 2002. www.researchgate.net/publication/267981173_HEY_Near-drowning_YOU%27VE_BEEN_MESSING_UP_OUR_MINDS. Accessed June 13, 2018.
- American College of Emergency Physicians. Death after swimming is extremely rare—and is not “dry drowning.” http://newsroom.acep.org/2017-07-11-Death-After-Swimming-Is-Extremely-Rare-And-Is-NOT-Dry-Drowning. Accessed June 13, 2018.
- Hawkins SC, Sempsrott J, Schmidt A. “Drowning” in a sea of misinformation. Emergency Medicine News 2017; 39*8):1. http://journals.lww.com/em-news/blog/BreakingNews/pages/post.aspx?PostID=377. Accessed June 5, 2018.
- Szpilman D, Tipton M, Sempsrott J, et al. Drowning timeline: a new systematic model of the drowning process. Am J Emerg Med 2016; 34(11):2224–2226. doi:10.1016/j.ajem.2016.07.063
- Modell JH, Bellefleur M, Davis JH. Drowning without aspiration: is this an appropriate diagnosis? J Forensic Sci 1999; 44(6):1119–1123. pmid:10582353
- Szpilman D, Orlowski JP. Sports related to drowning. Eur Respir Rev 2016; 25(141):348–359. doi:10.1183/16000617.0038-2016
- Szpilman D, Webber J, Quan L, et al. Creating a drowning chain of survival. Resuscitation 2014; 85(9):1149–1152. doi:10.1016/j.resuscitation.2014.05.034
- International Life Saving Federation. Who needs further medical help after rescue from the water. Medical Position Statement - MPS 06, 2016. www.ilsf.org/file/3916/download?token=pDnPDCrk. Accessed June 13, 2018.
- Buffington B. Texas boy dies from ‘dry drowning’ days after swimming. USA Today, June 8, 2017. www.usatoday.com/story/news/nation-now/2017/06/08/texas-boy-dies-dry-drowning-days-after-swimming/379944001.
- Schmidt AC, Sempsrott JR, Szpilman D, et al. The use of non-uniform drowning terminology: a follow-up study. Scand J Trauma Resusc Emerg Med 2017; 25(1):72. doi:10.1186/s13049-017-0405-x
- van Beeck EF, Branche CM, Szpilman D, Modell JH, Bierens JJ. A new definition of drowning: towards documentation and prevention of a global public health problem. Bull World Health Organ 2005; 83(11):853–856. pmid:16302042
- Szpilman D, Bierens JJ, Handley AJ, Orlowski JP. Drowning. N Engl J Med 2012; 366(22):2102–2110. doi:10.1056/NEJMra1013317
- World Health Organization. Global report on drowning: preventing a leading killer. www.who.int/violence_injury_prevention/global_report_drowning/en. Accessed June 13, 2018.
- Szpilman D. Near-drowning and drowning classification: a proposal to stratify mortality based on the analysis of 1,831 cases. Chest 1997; 112(3):660–665. pmid:9315798
- Centers for Disease Control and Prevention. Welcome to WISQARS. www.cdc.gov/injury/wisqars. Accessed June 13, 2018.
- Centers for Disease Control and Prevention. WONDER. https://wonder.cdc.gov. Accessed June 13, 2018.
- Cummings P, Quan L. Trends in unintentional drowning: the role of alcohol and medical care. JAMA 1999; 281(23):2198–2202. pmid:10376572
- Szpilman D, Elmann J, Cruz-Filho FES. Drowning classification: a revalidation study based on the analysis of 930 cases over 10 years. World Congress on Drowning, Netherlands 2002. www.researchgate.net/publication/267981062_DROWNING_CLASSIFICATION_a_revalidation_study_based_on_the_analysis_of_930_cases_over_10_years. Accessed June 13, 2018.
- Szpilman D, Elmann J, Cruz-Filho FES. Dry-drowning—fact or myth? World Congress on Drowning. Netherlands, 2002. www.researchgate.net/publication/267981164_Dry-drowning_-Fact_or_Myth. Accessed June 13, 2018.
- Lunetta P, Modell JH, Sajantila A. What is the incidence and significance of "dry-lungs" in bodies found in water? Am J Forensic Med Pathol 2004; 25(4):291–301. pmid:15577518
- Orlowski JP, Abulleil MM, Phillips JM. The hemodynamic and cardiovascular effects of near-drowning in hypotonic, isotonic, or hypertonic solutions. Ann Emerg Med 1989; 18:1044–1049. pmid:2802278
- Grmec S, Strnad M, Podgorsek D. Comparison of the characteristics and outcome among patients suffering from out-of-hospital primary cardiac arrest and drowning victims in cardiac arrest. Int J Emerg Med 2009; 2(1):7–12. doi:10.1007/s12245-009-0084-0
- Tipton MJ, Golden FS. A proposed decision-making guide for the search, rescue and resuscitation of submersion (head under) victims based on expert opinion. Resuscitation 2011; 82(7):819–824. doi:10.1016/j.resuscitation.2011.02.021
- Orlowski JP, Szpilman D. Drowning. Rescue, resuscitation, and reanimation. Pediatr Clin North Am 2001; 48(3):627–646. pmid:11411297
- Modell JH, Moya F, Newby EJ, Ruiz BC, Showers AV. The effects of fluid volume in seawater drowning. Ann Intern Med 1967; 67(1):68–80. pmid:6028660
- Quan L, Wentz KR, Gore EJ, Copass MK. Outcome and predictors of outcome in pediatric submersion victims receiving prehospital care in King County, Washington. Pediatrics 1990; 86(4):586–593. pmid:2216625
- Szpilman D, Orlowski JP, Cruz-Filho FES. Hey “Near-drowning,” you’ve been messing up our minds! World Congress on Drowning. Amsterdam, 2002. www.researchgate.net/publication/267981173_HEY_Near-drowning_YOU%27VE_BEEN_MESSING_UP_OUR_MINDS. Accessed June 13, 2018.
- American College of Emergency Physicians. Death after swimming is extremely rare—and is not “dry drowning.” http://newsroom.acep.org/2017-07-11-Death-After-Swimming-Is-Extremely-Rare-And-Is-NOT-Dry-Drowning. Accessed June 13, 2018.
- Hawkins SC, Sempsrott J, Schmidt A. “Drowning” in a sea of misinformation. Emergency Medicine News 2017; 39*8):1. http://journals.lww.com/em-news/blog/BreakingNews/pages/post.aspx?PostID=377. Accessed June 5, 2018.
- Szpilman D, Tipton M, Sempsrott J, et al. Drowning timeline: a new systematic model of the drowning process. Am J Emerg Med 2016; 34(11):2224–2226. doi:10.1016/j.ajem.2016.07.063
- Modell JH, Bellefleur M, Davis JH. Drowning without aspiration: is this an appropriate diagnosis? J Forensic Sci 1999; 44(6):1119–1123. pmid:10582353
- Szpilman D, Orlowski JP. Sports related to drowning. Eur Respir Rev 2016; 25(141):348–359. doi:10.1183/16000617.0038-2016
- Szpilman D, Webber J, Quan L, et al. Creating a drowning chain of survival. Resuscitation 2014; 85(9):1149–1152. doi:10.1016/j.resuscitation.2014.05.034
- International Life Saving Federation. Who needs further medical help after rescue from the water. Medical Position Statement - MPS 06, 2016. www.ilsf.org/file/3916/download?token=pDnPDCrk. Accessed June 13, 2018.
KEY POINTS
- Drowning is a process of aspiration leading to hypoxia and eventually cardiac arrest. However, it is not synonymous with death: it can be interrupted.
- Patients who have been rescued from drowning and who have minimal symptoms generally get better within 4 to 8 hours of the event.
- Rescued victims should be warned that, although a rare condition, if they develop cough, breathlessness, or any other worrisome symptom within 8 hours of being in the water, they should seek medical attention immediately.
Wolff-Parkinson-White pattern unmasked by severe musculoskeletal pain
A 55-year-old man with no significant medical history presented to the emergency department with left-sided flank pain that had begun 3 days earlier. He described the pain as continuous, sharp, and aggravated by movement. He worked in construction, and before the pain started he had moved 8 sheets of drywall and lifted 5-gallon buckets of spackling compound. He denied any associated chest pain, palpitations, dyspnea, cough, or lightheadedness. His family history included sudden cardiac death in 2 second-degree relatives.
On arrival in the emergency department, his vital signs were normal, as were the rest of the findings on physical examination except for reproducible point tenderness below the left scapula.
Laboratory workup revealed normal blood cell counts, liver enzymes, and kidney function. His initial troponin test was negative.
The patient was referred to an electrophysiologist for further evaluation, but he returned to his home country (Haiti) after discharge and was lost to follow-up.
WOLFF-PARKINSON-WHITE PATTERN VS SYNDROME
WPW syndrome is a disorder of the conduction system leading to preexcitation of the ventricles by an accessory pathway between the atria and ventricles. It is characterized by preexcitation manifested on electrocardiography and by symptomatic arrhythmias.
In contrast, the WPW pattern is defined only by preexcitation findings on electrocardiography without symptomatic arrhythmias. Patients with WPW syndrome can present with palpitation, dizziness, and syncope resulting from underlying arrhythmia.1 This is not seen in patients with the WPW pattern.
A short PR interval with or without delta waves can also be seen in the absence of an accessory pathway, eg, in hypoplastic left heart syndrome, atrioventricular canal defect, and Ebstein anomaly. These conditions are termed pseudopreexcitation syndrome.2
Our patient presented with severe musculoskeletal pain that precipitated the electrocardiographic changes of the WPW pattern and resolved with adequate pain control. The WPW pattern can be unmasked under different scenarios, including anesthesia, sympathomimetic drugs, and postoperatively.3–5
Catecholamine challenge has been used to unmask high-risk features in WPW syndrome.3 Our patient may have had a transient spike in catecholamine levels because of severe musculoskeletal pain, leading to unmasking of accessory pathways and resulting in the WPW pattern on electrocardiography.
Most patients with the WPW pattern experience no symptoms, but a small percentage develop arrhythmias.
In rare cases, sudden cardiac death can be the presenting feature of WPW syndrome. The estimated risk of sudden cardiac death in patients with the WPW pattern is 1.25 per 1,000 person-years; ventricular fibrillation is the underlying mechanism.6 As our patient had a family history of sudden cardiac death, he was considered at high risk and was therefore referred to an electrophysiologist.
- Munger TM, Packer DL, Hammill SC, et al. A population study of the natural history of Wolff-Parkinson-White syndrome in Olmsted County, Minnesota, 1953–1989. Circulation 1993; 87(3):866–873. pmid:8443907
- Carlson AM, Turek JW, Law IH, Von Bergen NH. Pseudo-preexcitation is prevalent among patients with repaired complex congenital heart disease. Pediatr Cardiol.2015; 36(1):8–13. doi:10.1007/s00246-014-0955-x
- Aleong RG, Singh SM, Levinson JR, Milan DJ. Catecholamine challenge unmasking high-risk features in the Wolff-Parkinson-White syndrome. Europace 2009; 11(10):1396–1398. doi:10.1093/europace/eup211
- Sahu S, Karna ST, Karna A, Lata I, Kapoor D. Anaesthetic management of Wolff-Parkinson-White syndrome for hysterectomy. Indian J Anaesth 2011; 55(4):378–380. doi:10.4103/0019-5049.84866
- Tseng ZH, Yadav AV, Scheinman MM. Catecholamine dependent accessory pathway automaticity. Pacing Clin Electrophysiol 2004; 27(7):1005–1007. doi:10.1111/j.1540-8159.2004.00574.x
- Obeyesekere MN, Leong-Sit P, Massel D, et al. Risk of arrhythmia and sudden death in patients with asymptomatic preexcitation: a meta-analysis. Circulation 2012; 125(19):2308–2315. doi:10.1161/CIRCULATIONAHA.111.055350
A 55-year-old man with no significant medical history presented to the emergency department with left-sided flank pain that had begun 3 days earlier. He described the pain as continuous, sharp, and aggravated by movement. He worked in construction, and before the pain started he had moved 8 sheets of drywall and lifted 5-gallon buckets of spackling compound. He denied any associated chest pain, palpitations, dyspnea, cough, or lightheadedness. His family history included sudden cardiac death in 2 second-degree relatives.
On arrival in the emergency department, his vital signs were normal, as were the rest of the findings on physical examination except for reproducible point tenderness below the left scapula.
Laboratory workup revealed normal blood cell counts, liver enzymes, and kidney function. His initial troponin test was negative.
The patient was referred to an electrophysiologist for further evaluation, but he returned to his home country (Haiti) after discharge and was lost to follow-up.
WOLFF-PARKINSON-WHITE PATTERN VS SYNDROME
WPW syndrome is a disorder of the conduction system leading to preexcitation of the ventricles by an accessory pathway between the atria and ventricles. It is characterized by preexcitation manifested on electrocardiography and by symptomatic arrhythmias.
In contrast, the WPW pattern is defined only by preexcitation findings on electrocardiography without symptomatic arrhythmias. Patients with WPW syndrome can present with palpitation, dizziness, and syncope resulting from underlying arrhythmia.1 This is not seen in patients with the WPW pattern.
A short PR interval with or without delta waves can also be seen in the absence of an accessory pathway, eg, in hypoplastic left heart syndrome, atrioventricular canal defect, and Ebstein anomaly. These conditions are termed pseudopreexcitation syndrome.2
Our patient presented with severe musculoskeletal pain that precipitated the electrocardiographic changes of the WPW pattern and resolved with adequate pain control. The WPW pattern can be unmasked under different scenarios, including anesthesia, sympathomimetic drugs, and postoperatively.3–5
Catecholamine challenge has been used to unmask high-risk features in WPW syndrome.3 Our patient may have had a transient spike in catecholamine levels because of severe musculoskeletal pain, leading to unmasking of accessory pathways and resulting in the WPW pattern on electrocardiography.
Most patients with the WPW pattern experience no symptoms, but a small percentage develop arrhythmias.
In rare cases, sudden cardiac death can be the presenting feature of WPW syndrome. The estimated risk of sudden cardiac death in patients with the WPW pattern is 1.25 per 1,000 person-years; ventricular fibrillation is the underlying mechanism.6 As our patient had a family history of sudden cardiac death, he was considered at high risk and was therefore referred to an electrophysiologist.
A 55-year-old man with no significant medical history presented to the emergency department with left-sided flank pain that had begun 3 days earlier. He described the pain as continuous, sharp, and aggravated by movement. He worked in construction, and before the pain started he had moved 8 sheets of drywall and lifted 5-gallon buckets of spackling compound. He denied any associated chest pain, palpitations, dyspnea, cough, or lightheadedness. His family history included sudden cardiac death in 2 second-degree relatives.
On arrival in the emergency department, his vital signs were normal, as were the rest of the findings on physical examination except for reproducible point tenderness below the left scapula.
Laboratory workup revealed normal blood cell counts, liver enzymes, and kidney function. His initial troponin test was negative.
The patient was referred to an electrophysiologist for further evaluation, but he returned to his home country (Haiti) after discharge and was lost to follow-up.
WOLFF-PARKINSON-WHITE PATTERN VS SYNDROME
WPW syndrome is a disorder of the conduction system leading to preexcitation of the ventricles by an accessory pathway between the atria and ventricles. It is characterized by preexcitation manifested on electrocardiography and by symptomatic arrhythmias.
In contrast, the WPW pattern is defined only by preexcitation findings on electrocardiography without symptomatic arrhythmias. Patients with WPW syndrome can present with palpitation, dizziness, and syncope resulting from underlying arrhythmia.1 This is not seen in patients with the WPW pattern.
A short PR interval with or without delta waves can also be seen in the absence of an accessory pathway, eg, in hypoplastic left heart syndrome, atrioventricular canal defect, and Ebstein anomaly. These conditions are termed pseudopreexcitation syndrome.2
Our patient presented with severe musculoskeletal pain that precipitated the electrocardiographic changes of the WPW pattern and resolved with adequate pain control. The WPW pattern can be unmasked under different scenarios, including anesthesia, sympathomimetic drugs, and postoperatively.3–5
Catecholamine challenge has been used to unmask high-risk features in WPW syndrome.3 Our patient may have had a transient spike in catecholamine levels because of severe musculoskeletal pain, leading to unmasking of accessory pathways and resulting in the WPW pattern on electrocardiography.
Most patients with the WPW pattern experience no symptoms, but a small percentage develop arrhythmias.
In rare cases, sudden cardiac death can be the presenting feature of WPW syndrome. The estimated risk of sudden cardiac death in patients with the WPW pattern is 1.25 per 1,000 person-years; ventricular fibrillation is the underlying mechanism.6 As our patient had a family history of sudden cardiac death, he was considered at high risk and was therefore referred to an electrophysiologist.
- Munger TM, Packer DL, Hammill SC, et al. A population study of the natural history of Wolff-Parkinson-White syndrome in Olmsted County, Minnesota, 1953–1989. Circulation 1993; 87(3):866–873. pmid:8443907
- Carlson AM, Turek JW, Law IH, Von Bergen NH. Pseudo-preexcitation is prevalent among patients with repaired complex congenital heart disease. Pediatr Cardiol.2015; 36(1):8–13. doi:10.1007/s00246-014-0955-x
- Aleong RG, Singh SM, Levinson JR, Milan DJ. Catecholamine challenge unmasking high-risk features in the Wolff-Parkinson-White syndrome. Europace 2009; 11(10):1396–1398. doi:10.1093/europace/eup211
- Sahu S, Karna ST, Karna A, Lata I, Kapoor D. Anaesthetic management of Wolff-Parkinson-White syndrome for hysterectomy. Indian J Anaesth 2011; 55(4):378–380. doi:10.4103/0019-5049.84866
- Tseng ZH, Yadav AV, Scheinman MM. Catecholamine dependent accessory pathway automaticity. Pacing Clin Electrophysiol 2004; 27(7):1005–1007. doi:10.1111/j.1540-8159.2004.00574.x
- Obeyesekere MN, Leong-Sit P, Massel D, et al. Risk of arrhythmia and sudden death in patients with asymptomatic preexcitation: a meta-analysis. Circulation 2012; 125(19):2308–2315. doi:10.1161/CIRCULATIONAHA.111.055350
- Munger TM, Packer DL, Hammill SC, et al. A population study of the natural history of Wolff-Parkinson-White syndrome in Olmsted County, Minnesota, 1953–1989. Circulation 1993; 87(3):866–873. pmid:8443907
- Carlson AM, Turek JW, Law IH, Von Bergen NH. Pseudo-preexcitation is prevalent among patients with repaired complex congenital heart disease. Pediatr Cardiol.2015; 36(1):8–13. doi:10.1007/s00246-014-0955-x
- Aleong RG, Singh SM, Levinson JR, Milan DJ. Catecholamine challenge unmasking high-risk features in the Wolff-Parkinson-White syndrome. Europace 2009; 11(10):1396–1398. doi:10.1093/europace/eup211
- Sahu S, Karna ST, Karna A, Lata I, Kapoor D. Anaesthetic management of Wolff-Parkinson-White syndrome for hysterectomy. Indian J Anaesth 2011; 55(4):378–380. doi:10.4103/0019-5049.84866
- Tseng ZH, Yadav AV, Scheinman MM. Catecholamine dependent accessory pathway automaticity. Pacing Clin Electrophysiol 2004; 27(7):1005–1007. doi:10.1111/j.1540-8159.2004.00574.x
- Obeyesekere MN, Leong-Sit P, Massel D, et al. Risk of arrhythmia and sudden death in patients with asymptomatic preexcitation: a meta-analysis. Circulation 2012; 125(19):2308–2315. doi:10.1161/CIRCULATIONAHA.111.055350
Osmotic demyelination syndrome due to hyperosmolar hyperglycemia
A 55-year-old man with a 20-year history of type 2 diabetes mellitus was admitted to the hospital after presenting to the emergency department with an acute change in mental status. Three days earlier, he had begun to feel abdominal discomfort and dizziness, which gradually worsened.
On presentation, his Glasgow Coma Scale score was 13 out of 15 (eye-opening response 3 of 4, verbal response 4 of 5, motor response 6 of 6), his blood pressure was 221/114 mm Hg, and other vital signs were normal. Physical examination including a neurologic examination was normal. No gait abnormality or ataxia was noted.
When asked about current medications, he said that 2 years earlier he had missed an appointment with his primary care physician and so had never obtained refills of his diabetes medications.
Results of laboratory testing were as follows:
- Blood glucose 1,011 mg/dL (reference range 65–110)
- Hemoglobin A1c 17.8% (4%–5.6%)
- Sodium 126 mmol/L (135–145)
- Sodium corrected for serum glucose 141 mmol/L
- Potassium 3.2 mmol/L (3.5–5.0)
- Blood urea nitrogen 43.8 mg/dL (8–21)
- Calculated serum osmolality 324 mosm/kg (275–295).
Blood gas analysis showed no acidosis. Tests for urinary and serum ketones were negative. Computed tomography (CT) of the head without contrast was normal.
Based on the results of the evaluation, the patient’s condition was diagnosed as a hyperosmolar hyperglycemic state, presumably from dehydration and noncompliance with diabetes medications. His altered mental status was also attributed to this diagnosis. He was started on aggressive hydration and insulin infusion to correct the blood glucose level. Repeat laboratory testing 7 hours after admission revealed a blood glucose of 49 mg/dL, sodium 148 mmol/L, blood urea nitrogen 43 mg/dL, and calculated serum osmolality 290 mosm/kg.
The insulin infusion was suspended, and glucose infusion was started. With this treatment, his blood glucose level stabilized, but his Glasgow Coma Scale score was unchanged from the time of presentation. A neurologic examination at this time showed bilateral dysmetria. Cranial nerves were normal. Motor examination showed normal tone with a Medical Research Council score of 5 of 5 in all extremities. Sensory examination revealed a glove-and-stocking pattern of loss of vibratory sensation. Tendon reflexes were normal except for diminished bilateral knee-jerk and ankle-jerk responses.
OSMOTIC DEMYELINATION SYNDROME
Central pontine myelinolysis is a pivotal manifestation of the syndrome and is characterized by progressive lethargy, quadriparesis, dysarthria, ophthalmoplegia, dysphasia, ataxia, and reflex changes. Clinical symptoms of extrapontine myelinolysis are variable.4
Although CT may underestimate osmotic demyelination syndrome, the typical radiologic findings on brain MRI are hyperintense lesions in the central pons or associated extrapontine structures on T2-weighted and fluid-attenuated inversion recovery sequences.4
A precise definition of hyperosmolar hyperglycemia does not exist. The Joint British Diabetes Societies for Inpatient Care suggested the following features: a measured osmolality of 320 mosm/kg or higher, a blood glucose level of 541 mg/dL or higher, severe dehydration, and feeling unwell.5
Our patient’s clinical course and high hemoglobin A1c suggested prolonged hyperglycemia and high serum osmolality before his admission. After his admission, aggressive hydration and insulin therapy corrected the hyperglycemia and serum osmolality too rapidly for his brain cells to adjust to the change. It was reasonable to suspect a hyperosmolar hyperglycemic state as one of the main causes of his mental status change and ataxia. This, along with lack of improvement in his impaired metal status and new-onset ataxia despite treatment, led to suspicion of osmotic demyelination syndrome. His diminished bilateral knee-jerk and ankle-jerk responses more likely represented diabetic neuropathy rather than osmotic demyelination syndrome.
Osmotic demyelination syndrome has seldom been reported as a complication of hyperosmolar hyperglycemia.6–13 And extrapontine myelinolysis with hyperosmolar hyperglycemia is extremely rare, with only 2 reports to date to the best of our knowledge.6,10
There is no specific treatment for osmotic demyelination syndrome except for supportive care and treatment of coexisting conditions. Once an osmotic derangement is identified, we recommend correcting chronically elevated serum glucose values gradually to avoid overtreatment, just as we would do with elevated serum sodium levels. Changes in neurologic findings, serum blood glucose level, and serum osmolality should be followed closely. A review showed that a favorable recovery from osmotic demyelination syndrome is possible even with severe neurologic deficits.4
TAKE-AWAY POINTS
- Osmotic demyelination syndrome is a rare but severe complication of a hyperosmolar hyperglycemic state.
- Physicians should be aware not only of changes in serum sodium, but also of changes in serum osmolality and serum glucose.
- When a new-onset neurologic deficit is found during treatment of a hyperosmolar hyperglycemic state, suspect osmotic demyelination syndrome, monitor changes in serum osmolality, and consider brain MRI.
- Brown WD. Osmotic demyelination disorders: central pontine and extrapontine myelinolysis. Curr Opin Neurol 2000; 13(6):691–697. pmid:11148672
- Laureno R, Karp BI. Myelinolysis after correction of hyponatraemia. Ann Intern Med 1997; 126(1):57–62. pmid:8992924
- Adams RD, Victor M, Mancall EL. Central pontine myelinolysis: a hitherto undescribed disease occurring in alcoholic and malnourished patients. AMA Arch Neurol Psychiatry 1959; 81(2):154–172. pmid:13616772
- Singh TD, Fugate JE, Rabinstein AA. Central pontine and extrapontine myelinolysis: a systematic review. Eur J Neurol 2014; 21(12):1443–1450. doi:10.1111/ene.12571
- Scott AR; Joint British Diabetes Societies (JBDS) for Inpatient Care; JBDS Hyperosmolar Hyperglycaemic Guidelines Group. Management of hyperosmolar hyperglycaemic state in adults with diabetes. Diabet Med 2015; 32(6):714–724. doi:10.1111/dme.12757
- McComb RD, Pfeiffer RF, Casey JH, Wolcott G, Till DJ. Lateral pontine and extrapontine myelinolysis associated with hypernatremia and hyperglycemia. Clin Neuropathol 1989; 8(6):284–288. pmid:2695277
- O’Malley G, Moran C, Draman MS, et al. Central pontine myelinolysis complicating treatment of the hyperglycaemic hyperosmolar state. Ann Clin Biochem 2008; 45(pt 4):440–443. doi:10.1258/acb.2008.007171
- Burns JD, Kosa SC, Wijdicks EF. Central pontine myelinolysis in a patient with hyperosmolar hyperglycemia and consistently normal serum sodium. Neurocrit Care 2009; 11(2):251–254. doi:10.1007/s12028-009-9241-9
- Mao S, Liu Z, Ding M. Central pontine myelinolysis in a patient with epilepsia partialis continua and hyperglycaemic hyperosmolar state. Ann Clin Biochem 2011; 48(pt 1):79–82. doi:10.1258/acb.2010.010152. Epub 2010 Nov 23.
- Guerrero WR, Dababneh H, Nadeau SE. Hemiparesis, encephalopathy, and extrapontine osmotic myelinolysis in the setting of hyperosmolar hyperglycemia. J Clin Neurosci 2013; 20(6):894–896. doi:10.1016/j.jocn.2012.05.045
- Hegazi MO, Mashankar A. Central pontine myelinolysis in the hyperosmolar hyperglycaemic state. Med Princ Pract 2013; 22(1):96–99. doi:10.1159/000341718
- Rodríguez-Velver KV, Soto-Garcia AJ, Zapata-Rivera MA, Montes-Villarreal J, Villarreal-Pérez JZ, Rodríguez-Gutiérrez R. Osmotic demyelination syndrome as the initial manifestation of a hyperosmolar hyperglycemic state. Case Rep Neurol Med 2014; 2014:652523. doi:10.1155/2014/652523
- Chang YM. Central pontine myelinolysis associated with diabetic hyperglycemia. JSM Clin Case Rep 2014; 2(6):1059.
A 55-year-old man with a 20-year history of type 2 diabetes mellitus was admitted to the hospital after presenting to the emergency department with an acute change in mental status. Three days earlier, he had begun to feel abdominal discomfort and dizziness, which gradually worsened.
On presentation, his Glasgow Coma Scale score was 13 out of 15 (eye-opening response 3 of 4, verbal response 4 of 5, motor response 6 of 6), his blood pressure was 221/114 mm Hg, and other vital signs were normal. Physical examination including a neurologic examination was normal. No gait abnormality or ataxia was noted.
When asked about current medications, he said that 2 years earlier he had missed an appointment with his primary care physician and so had never obtained refills of his diabetes medications.
Results of laboratory testing were as follows:
- Blood glucose 1,011 mg/dL (reference range 65–110)
- Hemoglobin A1c 17.8% (4%–5.6%)
- Sodium 126 mmol/L (135–145)
- Sodium corrected for serum glucose 141 mmol/L
- Potassium 3.2 mmol/L (3.5–5.0)
- Blood urea nitrogen 43.8 mg/dL (8–21)
- Calculated serum osmolality 324 mosm/kg (275–295).
Blood gas analysis showed no acidosis. Tests for urinary and serum ketones were negative. Computed tomography (CT) of the head without contrast was normal.
Based on the results of the evaluation, the patient’s condition was diagnosed as a hyperosmolar hyperglycemic state, presumably from dehydration and noncompliance with diabetes medications. His altered mental status was also attributed to this diagnosis. He was started on aggressive hydration and insulin infusion to correct the blood glucose level. Repeat laboratory testing 7 hours after admission revealed a blood glucose of 49 mg/dL, sodium 148 mmol/L, blood urea nitrogen 43 mg/dL, and calculated serum osmolality 290 mosm/kg.
The insulin infusion was suspended, and glucose infusion was started. With this treatment, his blood glucose level stabilized, but his Glasgow Coma Scale score was unchanged from the time of presentation. A neurologic examination at this time showed bilateral dysmetria. Cranial nerves were normal. Motor examination showed normal tone with a Medical Research Council score of 5 of 5 in all extremities. Sensory examination revealed a glove-and-stocking pattern of loss of vibratory sensation. Tendon reflexes were normal except for diminished bilateral knee-jerk and ankle-jerk responses.
OSMOTIC DEMYELINATION SYNDROME
Central pontine myelinolysis is a pivotal manifestation of the syndrome and is characterized by progressive lethargy, quadriparesis, dysarthria, ophthalmoplegia, dysphasia, ataxia, and reflex changes. Clinical symptoms of extrapontine myelinolysis are variable.4
Although CT may underestimate osmotic demyelination syndrome, the typical radiologic findings on brain MRI are hyperintense lesions in the central pons or associated extrapontine structures on T2-weighted and fluid-attenuated inversion recovery sequences.4
A precise definition of hyperosmolar hyperglycemia does not exist. The Joint British Diabetes Societies for Inpatient Care suggested the following features: a measured osmolality of 320 mosm/kg or higher, a blood glucose level of 541 mg/dL or higher, severe dehydration, and feeling unwell.5
Our patient’s clinical course and high hemoglobin A1c suggested prolonged hyperglycemia and high serum osmolality before his admission. After his admission, aggressive hydration and insulin therapy corrected the hyperglycemia and serum osmolality too rapidly for his brain cells to adjust to the change. It was reasonable to suspect a hyperosmolar hyperglycemic state as one of the main causes of his mental status change and ataxia. This, along with lack of improvement in his impaired metal status and new-onset ataxia despite treatment, led to suspicion of osmotic demyelination syndrome. His diminished bilateral knee-jerk and ankle-jerk responses more likely represented diabetic neuropathy rather than osmotic demyelination syndrome.
Osmotic demyelination syndrome has seldom been reported as a complication of hyperosmolar hyperglycemia.6–13 And extrapontine myelinolysis with hyperosmolar hyperglycemia is extremely rare, with only 2 reports to date to the best of our knowledge.6,10
There is no specific treatment for osmotic demyelination syndrome except for supportive care and treatment of coexisting conditions. Once an osmotic derangement is identified, we recommend correcting chronically elevated serum glucose values gradually to avoid overtreatment, just as we would do with elevated serum sodium levels. Changes in neurologic findings, serum blood glucose level, and serum osmolality should be followed closely. A review showed that a favorable recovery from osmotic demyelination syndrome is possible even with severe neurologic deficits.4
TAKE-AWAY POINTS
- Osmotic demyelination syndrome is a rare but severe complication of a hyperosmolar hyperglycemic state.
- Physicians should be aware not only of changes in serum sodium, but also of changes in serum osmolality and serum glucose.
- When a new-onset neurologic deficit is found during treatment of a hyperosmolar hyperglycemic state, suspect osmotic demyelination syndrome, monitor changes in serum osmolality, and consider brain MRI.
A 55-year-old man with a 20-year history of type 2 diabetes mellitus was admitted to the hospital after presenting to the emergency department with an acute change in mental status. Three days earlier, he had begun to feel abdominal discomfort and dizziness, which gradually worsened.
On presentation, his Glasgow Coma Scale score was 13 out of 15 (eye-opening response 3 of 4, verbal response 4 of 5, motor response 6 of 6), his blood pressure was 221/114 mm Hg, and other vital signs were normal. Physical examination including a neurologic examination was normal. No gait abnormality or ataxia was noted.
When asked about current medications, he said that 2 years earlier he had missed an appointment with his primary care physician and so had never obtained refills of his diabetes medications.
Results of laboratory testing were as follows:
- Blood glucose 1,011 mg/dL (reference range 65–110)
- Hemoglobin A1c 17.8% (4%–5.6%)
- Sodium 126 mmol/L (135–145)
- Sodium corrected for serum glucose 141 mmol/L
- Potassium 3.2 mmol/L (3.5–5.0)
- Blood urea nitrogen 43.8 mg/dL (8–21)
- Calculated serum osmolality 324 mosm/kg (275–295).
Blood gas analysis showed no acidosis. Tests for urinary and serum ketones were negative. Computed tomography (CT) of the head without contrast was normal.
Based on the results of the evaluation, the patient’s condition was diagnosed as a hyperosmolar hyperglycemic state, presumably from dehydration and noncompliance with diabetes medications. His altered mental status was also attributed to this diagnosis. He was started on aggressive hydration and insulin infusion to correct the blood glucose level. Repeat laboratory testing 7 hours after admission revealed a blood glucose of 49 mg/dL, sodium 148 mmol/L, blood urea nitrogen 43 mg/dL, and calculated serum osmolality 290 mosm/kg.
The insulin infusion was suspended, and glucose infusion was started. With this treatment, his blood glucose level stabilized, but his Glasgow Coma Scale score was unchanged from the time of presentation. A neurologic examination at this time showed bilateral dysmetria. Cranial nerves were normal. Motor examination showed normal tone with a Medical Research Council score of 5 of 5 in all extremities. Sensory examination revealed a glove-and-stocking pattern of loss of vibratory sensation. Tendon reflexes were normal except for diminished bilateral knee-jerk and ankle-jerk responses.
OSMOTIC DEMYELINATION SYNDROME
Central pontine myelinolysis is a pivotal manifestation of the syndrome and is characterized by progressive lethargy, quadriparesis, dysarthria, ophthalmoplegia, dysphasia, ataxia, and reflex changes. Clinical symptoms of extrapontine myelinolysis are variable.4
Although CT may underestimate osmotic demyelination syndrome, the typical radiologic findings on brain MRI are hyperintense lesions in the central pons or associated extrapontine structures on T2-weighted and fluid-attenuated inversion recovery sequences.4
A precise definition of hyperosmolar hyperglycemia does not exist. The Joint British Diabetes Societies for Inpatient Care suggested the following features: a measured osmolality of 320 mosm/kg or higher, a blood glucose level of 541 mg/dL or higher, severe dehydration, and feeling unwell.5
Our patient’s clinical course and high hemoglobin A1c suggested prolonged hyperglycemia and high serum osmolality before his admission. After his admission, aggressive hydration and insulin therapy corrected the hyperglycemia and serum osmolality too rapidly for his brain cells to adjust to the change. It was reasonable to suspect a hyperosmolar hyperglycemic state as one of the main causes of his mental status change and ataxia. This, along with lack of improvement in his impaired metal status and new-onset ataxia despite treatment, led to suspicion of osmotic demyelination syndrome. His diminished bilateral knee-jerk and ankle-jerk responses more likely represented diabetic neuropathy rather than osmotic demyelination syndrome.
Osmotic demyelination syndrome has seldom been reported as a complication of hyperosmolar hyperglycemia.6–13 And extrapontine myelinolysis with hyperosmolar hyperglycemia is extremely rare, with only 2 reports to date to the best of our knowledge.6,10
There is no specific treatment for osmotic demyelination syndrome except for supportive care and treatment of coexisting conditions. Once an osmotic derangement is identified, we recommend correcting chronically elevated serum glucose values gradually to avoid overtreatment, just as we would do with elevated serum sodium levels. Changes in neurologic findings, serum blood glucose level, and serum osmolality should be followed closely. A review showed that a favorable recovery from osmotic demyelination syndrome is possible even with severe neurologic deficits.4
TAKE-AWAY POINTS
- Osmotic demyelination syndrome is a rare but severe complication of a hyperosmolar hyperglycemic state.
- Physicians should be aware not only of changes in serum sodium, but also of changes in serum osmolality and serum glucose.
- When a new-onset neurologic deficit is found during treatment of a hyperosmolar hyperglycemic state, suspect osmotic demyelination syndrome, monitor changes in serum osmolality, and consider brain MRI.
- Brown WD. Osmotic demyelination disorders: central pontine and extrapontine myelinolysis. Curr Opin Neurol 2000; 13(6):691–697. pmid:11148672
- Laureno R, Karp BI. Myelinolysis after correction of hyponatraemia. Ann Intern Med 1997; 126(1):57–62. pmid:8992924
- Adams RD, Victor M, Mancall EL. Central pontine myelinolysis: a hitherto undescribed disease occurring in alcoholic and malnourished patients. AMA Arch Neurol Psychiatry 1959; 81(2):154–172. pmid:13616772
- Singh TD, Fugate JE, Rabinstein AA. Central pontine and extrapontine myelinolysis: a systematic review. Eur J Neurol 2014; 21(12):1443–1450. doi:10.1111/ene.12571
- Scott AR; Joint British Diabetes Societies (JBDS) for Inpatient Care; JBDS Hyperosmolar Hyperglycaemic Guidelines Group. Management of hyperosmolar hyperglycaemic state in adults with diabetes. Diabet Med 2015; 32(6):714–724. doi:10.1111/dme.12757
- McComb RD, Pfeiffer RF, Casey JH, Wolcott G, Till DJ. Lateral pontine and extrapontine myelinolysis associated with hypernatremia and hyperglycemia. Clin Neuropathol 1989; 8(6):284–288. pmid:2695277
- O’Malley G, Moran C, Draman MS, et al. Central pontine myelinolysis complicating treatment of the hyperglycaemic hyperosmolar state. Ann Clin Biochem 2008; 45(pt 4):440–443. doi:10.1258/acb.2008.007171
- Burns JD, Kosa SC, Wijdicks EF. Central pontine myelinolysis in a patient with hyperosmolar hyperglycemia and consistently normal serum sodium. Neurocrit Care 2009; 11(2):251–254. doi:10.1007/s12028-009-9241-9
- Mao S, Liu Z, Ding M. Central pontine myelinolysis in a patient with epilepsia partialis continua and hyperglycaemic hyperosmolar state. Ann Clin Biochem 2011; 48(pt 1):79–82. doi:10.1258/acb.2010.010152. Epub 2010 Nov 23.
- Guerrero WR, Dababneh H, Nadeau SE. Hemiparesis, encephalopathy, and extrapontine osmotic myelinolysis in the setting of hyperosmolar hyperglycemia. J Clin Neurosci 2013; 20(6):894–896. doi:10.1016/j.jocn.2012.05.045
- Hegazi MO, Mashankar A. Central pontine myelinolysis in the hyperosmolar hyperglycaemic state. Med Princ Pract 2013; 22(1):96–99. doi:10.1159/000341718
- Rodríguez-Velver KV, Soto-Garcia AJ, Zapata-Rivera MA, Montes-Villarreal J, Villarreal-Pérez JZ, Rodríguez-Gutiérrez R. Osmotic demyelination syndrome as the initial manifestation of a hyperosmolar hyperglycemic state. Case Rep Neurol Med 2014; 2014:652523. doi:10.1155/2014/652523
- Chang YM. Central pontine myelinolysis associated with diabetic hyperglycemia. JSM Clin Case Rep 2014; 2(6):1059.
- Brown WD. Osmotic demyelination disorders: central pontine and extrapontine myelinolysis. Curr Opin Neurol 2000; 13(6):691–697. pmid:11148672
- Laureno R, Karp BI. Myelinolysis after correction of hyponatraemia. Ann Intern Med 1997; 126(1):57–62. pmid:8992924
- Adams RD, Victor M, Mancall EL. Central pontine myelinolysis: a hitherto undescribed disease occurring in alcoholic and malnourished patients. AMA Arch Neurol Psychiatry 1959; 81(2):154–172. pmid:13616772
- Singh TD, Fugate JE, Rabinstein AA. Central pontine and extrapontine myelinolysis: a systematic review. Eur J Neurol 2014; 21(12):1443–1450. doi:10.1111/ene.12571
- Scott AR; Joint British Diabetes Societies (JBDS) for Inpatient Care; JBDS Hyperosmolar Hyperglycaemic Guidelines Group. Management of hyperosmolar hyperglycaemic state in adults with diabetes. Diabet Med 2015; 32(6):714–724. doi:10.1111/dme.12757
- McComb RD, Pfeiffer RF, Casey JH, Wolcott G, Till DJ. Lateral pontine and extrapontine myelinolysis associated with hypernatremia and hyperglycemia. Clin Neuropathol 1989; 8(6):284–288. pmid:2695277
- O’Malley G, Moran C, Draman MS, et al. Central pontine myelinolysis complicating treatment of the hyperglycaemic hyperosmolar state. Ann Clin Biochem 2008; 45(pt 4):440–443. doi:10.1258/acb.2008.007171
- Burns JD, Kosa SC, Wijdicks EF. Central pontine myelinolysis in a patient with hyperosmolar hyperglycemia and consistently normal serum sodium. Neurocrit Care 2009; 11(2):251–254. doi:10.1007/s12028-009-9241-9
- Mao S, Liu Z, Ding M. Central pontine myelinolysis in a patient with epilepsia partialis continua and hyperglycaemic hyperosmolar state. Ann Clin Biochem 2011; 48(pt 1):79–82. doi:10.1258/acb.2010.010152. Epub 2010 Nov 23.
- Guerrero WR, Dababneh H, Nadeau SE. Hemiparesis, encephalopathy, and extrapontine osmotic myelinolysis in the setting of hyperosmolar hyperglycemia. J Clin Neurosci 2013; 20(6):894–896. doi:10.1016/j.jocn.2012.05.045
- Hegazi MO, Mashankar A. Central pontine myelinolysis in the hyperosmolar hyperglycaemic state. Med Princ Pract 2013; 22(1):96–99. doi:10.1159/000341718
- Rodríguez-Velver KV, Soto-Garcia AJ, Zapata-Rivera MA, Montes-Villarreal J, Villarreal-Pérez JZ, Rodríguez-Gutiérrez R. Osmotic demyelination syndrome as the initial manifestation of a hyperosmolar hyperglycemic state. Case Rep Neurol Med 2014; 2014:652523. doi:10.1155/2014/652523
- Chang YM. Central pontine myelinolysis associated with diabetic hyperglycemia. JSM Clin Case Rep 2014; 2(6):1059.
What inpatient treatments do we have for acute intractable migraine?
We recommend the following combination treatment:
Normal saline (0.9% NaCl) 1 to 2 L by intravenous (IV) infusion over 2 to 4 hours. This can be repeated every 6 to 12 hours.
Ketorolac 30-mg IV bolus, which can be repeated every 6 hours. However, patients with coronary artery disease, uncontrolled hypertension, acute renal failure, or cerebrovascular disease should instead receive acetaminophen 1,000 mg, naproxen sodium 550 mg, or aspirin 325 mg by mouth.
Prochlorperazine or metoclopramide 10-mg IV infusion. This can be repeated every 6 hours. However, to reduce the extrapyramidal adverse effects of these drugs, patients should first receive diphenhydramine 25- to 50-mg IV bolus, which can be repeated every 6 to 8 hours.
Sodium valproate 500 to 1,000 mg by IV infusion over 20 minutes. This can be repeated after 8 hours.
Dexamethasone 4-mg IV bolus every 6 hours, or 10-mg IV bolus once in 24 hours.
Magnesium sulfate 500 to 1,000 mg by IV infusion over 1 hour. This can be repeated every 6 to 12 hours.
If the migraine has not improved after 3 cycles of this regimen, a neurologic consultation should be considered. Other options include dihydroergotamine and occipital nerve blocks1 performed at the bedside.
GENERAL PRINCIPLES
Managing acute intractable migraine can be frustrating for both the practitioner and the patient. Some general principles are helpful.
Use a combination of drugs. Aborting a severe migraine attack often requires a combination of medications that work synergistically.
Use IV and intramuscular formulations rather than oral formulations: they are more rapidly absorbed, provide faster pain relief, and can be given when the nausea that often accompanies migraine precludes oral treatments.
Rule out secondary causes. The mnemonic SNOOP—systemic signs, neurologic signs, onset, older age, progression of existing headache disorder—is useful for assessing underlying causes.2 Any patient presenting with intractable migraine should also have a thorough neurologic examination.
Screening electrocardiography may be helpful, as the pretreatment QTc interval may direct the choice of intravenous treatment. If the patient has a prolonged QTc or is taking another drug that could prolong the QTc, certain medications, specifically dopamine receptor antagonists and diphenhydramine, should be avoided.
Ask the patient what has worked previously. A particular agent may have been effective in aborting the migraine; thus, a single dose of it could abort the headache, expediting discharge.
Establish if a triptan or ergot derivative has been used during the 24 hours before presentation, as repeated dosing within this interval is not recommended.3
Establish the baseline headache severity. Complete headache relief is difficult to achieve in a patient with chronic daily headache, and establishing a more realistic goal (eg, 50% relief) from the outset is useful.
OPTIONS FOR DRUG THERAPY
Antiemetics
Dopamine receptor antagonists are assumed to merely treat nausea in patients with migraine; however, they act independently to abort migraine and thus should be considered, irrespective of the presence of nausea.
The two most commonly used agents are prochlorperazine and metoclopramide. The American Academy of Neurology guidelines recommend prochlorperazine as first-line therapy for acute migraine. Metoclopramide is rated slightly lower and is considered to have moderate benefit.4 The Canadian Headache Society cites a high level of evidence supporting prochlorperazine and a moderate level of evidence supporting metoclopramide.5 The American Headache Society assessment of parenteral pharmacotherapies gives prochlorperazine and metoclopramide a level B recommendation of “should offer” (a recommendation only additionally assigned to subcutaneous sumatriptan).3 Hence, either agent can be used.
To reduce the risk of posttreatment akathisia, diphenhydramine or benztropine may be given before starting a dopamine receptor antagonist. Diphenhydramine may be independently effective in migraine treatment,6,7 but data on this are limited.
Ketorolac, ibuprofen
Ketorolac and ibuprofen are the only available nonsteroidal antiinflammatory drugs (NSAIDs) for IV administration. The Canadian Headache Society guidelines strongly recommend ketorolac for the treatment of migraine in emergency settings.5 Doses range from 30 mg to 60 mg.1 Ibuprofen 400 to 800 mg by IV infusion is an acceptable alternative. These medications should be avoided in patients with renal failure or severe coronary artery disease.
Oral naproxen sodium is a possible alternative in patients with cardiovascular disease, as it has been shown to carry a lower cardiovascular risk than other NSAIDs.8
The same concerns in patients with renal dysfunction apply to any NSAID, as the enzyme cyclooxygenase plays a constitutive role in glomerular function.
Antiepileptic drugs
The antiepileptic drugs sodium valproate and levetiracetam are available in IV formulations that have demonstrated efficacy in the treatment of status migrainosus1 (ie, migraine lasting more than 72 hours). Valproate has the strongest track record, is well tolerated, and is effective in rapidly aborting migraine.9
Volume repletion
Although its use is anecdotal and to date no trial has measured its efficacy, IV volume repletion is often used in acute migraine, as most headache experts surmise it to be highly effective, especially in patients with prolonged nausea or vomiting.1
Magnesium
IV magnesium is effective, particularly for migraine with aura.10 Hypotension is a common side effect, and pretreatment or concurrent treatment with IV fluids is advised. Doses from 500 mg to 1,000 mg have demonstrated efficacy.10
Corticosteroids
Corticosteroids can be used in the treatment of status migrainosus. Most studies have shown benefit in preventing recurrences rather than merely aborting migraine.11 A systematic review suggested that recurrent headaches are milder with corticosteroid treatment; 19 of 25 studies indicated favorable benefit, and 6 of 19 studies indicated noninferior outcomes.12
Both IV methylprednisolone and IV dexamethasone may be considered.12 Dexamethasone appears to be particularly effective in preventing headache recurrence when combined with other IV therapies.13 It can be given as a single dose of 10 mg, or as repeated doses of 4 mg up to 16 mg/day.1 Patients with active psychosis or uncontrolled diabetes should be closely monitored for these conditions, which corticosteroids can worsen.
Serotoninergic agents
Serotonin agonists including subcutaneous sumatriptan and IV dihydroergotamine are highly effective, with proven statistical and clinical benefit.4 They should be considered in patients with no known history of coronary artery disease or other vaso-occlusive vascular disorder.1
Ideally, IV dihydroergotamine should be administered after consultation with a neurologist or headache specialist, given the pretreatment and cotreatment requirements often necessary to suppress its side effects. Careful titration is important to prevent transient headache exacerbations during infusion, as well as abdominal cramping, nausea, and diarrhea.
Avoid opioids
Opioids should be avoided. Evidence supporting their use in acute migraine is extremely limited,3 and the risks of migraine becoming chronic and of addiction are high.14 Safer, more effective alternatives have been detailed above.
A detailed algorithm for the management of patients with acute migraine has been published14 and is aimed at decreasing acute treatment with opioids and barbiturates.
- Rozen TD. Emergency department and inpatient management of status migrainosus and intractable headache. Continuum (Minneap Minn) 2015; 21(4):1004–1017. doi:10.1212/CON.0000000000000191
- Dodick D. Headache as a symptom of ominous disease. What are the warning signals? Postgrad Med 1997; 101(5):46–50, 55–56, 62–64. doi:10.3810/pgm.1997.05.217
- Orr SL, Friedman BW, Christie S, et al. Management of adults with acute migraine in the emergency department: the American Headache Society evidence assessment of parenteral pharmacotherapies. Headache 2016; 56(6):911–940. doi:10.1111/head.12835
- Silberstein SD. Practice parameter: evidence-based guidelines for migraine headache (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2000; 55(6):754–762. doi:10.1212/WNL.55.6.754
- Orr SL, Aubé M, Becker WJ, et al. Canadian Headache Society systematic review and recommendations on the treatment of migraine pain in emergency settings. Cephalalgia 2015; 35(3):271–284. doi:10.1177/0333102414535997
- Swidan SZ, Lake AE 3rd, Saper JR. Efficacy of intravenous diphenhydramine versus intravenous DHE-45 in the treatment of severe migraine headache. Curr Pain Headache Rep 2005; 9(1):65–70. doi:10.1007/s11916-005-0077-5
- Marmura MJ, Goldberg SW. Inpatient management of migraine. Curr Neurol Neurosci Rep 2015; 15(4):13. doi:10.1007/s11910-015-0539-z
- Farkouh ME, Greenberg BP. An evidence-based review of the cardiovascular risks of nonsteroidal anti-inflammatory drugs. Am J Cardiol 2009; 103(9):1227–1237. doi:10.1016/j.amjcard.2009.01.014
- Stillman MJ, Zajac D, Rybicki LA. Treatment of primary headache disorders with intravenous valproate: initial outpatient experience. Headache 2004; 44(1):65–69. doi:10.1111/j.1526-4610.2004.04010.x
- Marmura MJ, Silberstein SD, Schwedt TJ. The acute treatment of migraine in adults: the American Headache Society evidence assessment of migraine pharmacotherapies. Headache 2015; 55(1):3–20. doi:10.1111/head.12499
- Colman I, Friedman BW, Brown MD, et al. Parenteral dexamethasone for acute severe migraine headache: meta-analysis of randomised controlled trials for preventing recurrence. BMJ 2008; 336(7657):1359–1361. doi:10.1136/bmj.39566.806725.BE
- Woldeamanuel YW, Rapoport AM, Cowan RP. The place of corticosteroids in migraine attack management: a 65-year systematic review with pooled analysis and critical appraisal. Cephalalgia 2015; 35(1):996–1024. doi:10.1177/0333102414566200
- Singh A, Alter HJ, Zaia B. Does the addition of dexamethasone to standard therapy for acute migraine headache decrease the incidence of recurrent headache for patients treated in the emergency department? A meta-analysis and systematic review of the literature. Acad Emerg Med 2008; 15(12):1223–1233. doi:10.1111/j.1553-2712.2008.00283.x
- Ahmed ZA, Nacopoulos DA, John S, Papesh N, Levine D, Bamford CC. An algorithm for opioid and barbiturate reduction in the acute management of headache in the emergency department. Headache 2017; 57(1):71–79. doi:10.1111/head.12961
We recommend the following combination treatment:
Normal saline (0.9% NaCl) 1 to 2 L by intravenous (IV) infusion over 2 to 4 hours. This can be repeated every 6 to 12 hours.
Ketorolac 30-mg IV bolus, which can be repeated every 6 hours. However, patients with coronary artery disease, uncontrolled hypertension, acute renal failure, or cerebrovascular disease should instead receive acetaminophen 1,000 mg, naproxen sodium 550 mg, or aspirin 325 mg by mouth.
Prochlorperazine or metoclopramide 10-mg IV infusion. This can be repeated every 6 hours. However, to reduce the extrapyramidal adverse effects of these drugs, patients should first receive diphenhydramine 25- to 50-mg IV bolus, which can be repeated every 6 to 8 hours.
Sodium valproate 500 to 1,000 mg by IV infusion over 20 minutes. This can be repeated after 8 hours.
Dexamethasone 4-mg IV bolus every 6 hours, or 10-mg IV bolus once in 24 hours.
Magnesium sulfate 500 to 1,000 mg by IV infusion over 1 hour. This can be repeated every 6 to 12 hours.
If the migraine has not improved after 3 cycles of this regimen, a neurologic consultation should be considered. Other options include dihydroergotamine and occipital nerve blocks1 performed at the bedside.
GENERAL PRINCIPLES
Managing acute intractable migraine can be frustrating for both the practitioner and the patient. Some general principles are helpful.
Use a combination of drugs. Aborting a severe migraine attack often requires a combination of medications that work synergistically.
Use IV and intramuscular formulations rather than oral formulations: they are more rapidly absorbed, provide faster pain relief, and can be given when the nausea that often accompanies migraine precludes oral treatments.
Rule out secondary causes. The mnemonic SNOOP—systemic signs, neurologic signs, onset, older age, progression of existing headache disorder—is useful for assessing underlying causes.2 Any patient presenting with intractable migraine should also have a thorough neurologic examination.
Screening electrocardiography may be helpful, as the pretreatment QTc interval may direct the choice of intravenous treatment. If the patient has a prolonged QTc or is taking another drug that could prolong the QTc, certain medications, specifically dopamine receptor antagonists and diphenhydramine, should be avoided.
Ask the patient what has worked previously. A particular agent may have been effective in aborting the migraine; thus, a single dose of it could abort the headache, expediting discharge.
Establish if a triptan or ergot derivative has been used during the 24 hours before presentation, as repeated dosing within this interval is not recommended.3
Establish the baseline headache severity. Complete headache relief is difficult to achieve in a patient with chronic daily headache, and establishing a more realistic goal (eg, 50% relief) from the outset is useful.
OPTIONS FOR DRUG THERAPY
Antiemetics
Dopamine receptor antagonists are assumed to merely treat nausea in patients with migraine; however, they act independently to abort migraine and thus should be considered, irrespective of the presence of nausea.
The two most commonly used agents are prochlorperazine and metoclopramide. The American Academy of Neurology guidelines recommend prochlorperazine as first-line therapy for acute migraine. Metoclopramide is rated slightly lower and is considered to have moderate benefit.4 The Canadian Headache Society cites a high level of evidence supporting prochlorperazine and a moderate level of evidence supporting metoclopramide.5 The American Headache Society assessment of parenteral pharmacotherapies gives prochlorperazine and metoclopramide a level B recommendation of “should offer” (a recommendation only additionally assigned to subcutaneous sumatriptan).3 Hence, either agent can be used.
To reduce the risk of posttreatment akathisia, diphenhydramine or benztropine may be given before starting a dopamine receptor antagonist. Diphenhydramine may be independently effective in migraine treatment,6,7 but data on this are limited.
Ketorolac, ibuprofen
Ketorolac and ibuprofen are the only available nonsteroidal antiinflammatory drugs (NSAIDs) for IV administration. The Canadian Headache Society guidelines strongly recommend ketorolac for the treatment of migraine in emergency settings.5 Doses range from 30 mg to 60 mg.1 Ibuprofen 400 to 800 mg by IV infusion is an acceptable alternative. These medications should be avoided in patients with renal failure or severe coronary artery disease.
Oral naproxen sodium is a possible alternative in patients with cardiovascular disease, as it has been shown to carry a lower cardiovascular risk than other NSAIDs.8
The same concerns in patients with renal dysfunction apply to any NSAID, as the enzyme cyclooxygenase plays a constitutive role in glomerular function.
Antiepileptic drugs
The antiepileptic drugs sodium valproate and levetiracetam are available in IV formulations that have demonstrated efficacy in the treatment of status migrainosus1 (ie, migraine lasting more than 72 hours). Valproate has the strongest track record, is well tolerated, and is effective in rapidly aborting migraine.9
Volume repletion
Although its use is anecdotal and to date no trial has measured its efficacy, IV volume repletion is often used in acute migraine, as most headache experts surmise it to be highly effective, especially in patients with prolonged nausea or vomiting.1
Magnesium
IV magnesium is effective, particularly for migraine with aura.10 Hypotension is a common side effect, and pretreatment or concurrent treatment with IV fluids is advised. Doses from 500 mg to 1,000 mg have demonstrated efficacy.10
Corticosteroids
Corticosteroids can be used in the treatment of status migrainosus. Most studies have shown benefit in preventing recurrences rather than merely aborting migraine.11 A systematic review suggested that recurrent headaches are milder with corticosteroid treatment; 19 of 25 studies indicated favorable benefit, and 6 of 19 studies indicated noninferior outcomes.12
Both IV methylprednisolone and IV dexamethasone may be considered.12 Dexamethasone appears to be particularly effective in preventing headache recurrence when combined with other IV therapies.13 It can be given as a single dose of 10 mg, or as repeated doses of 4 mg up to 16 mg/day.1 Patients with active psychosis or uncontrolled diabetes should be closely monitored for these conditions, which corticosteroids can worsen.
Serotoninergic agents
Serotonin agonists including subcutaneous sumatriptan and IV dihydroergotamine are highly effective, with proven statistical and clinical benefit.4 They should be considered in patients with no known history of coronary artery disease or other vaso-occlusive vascular disorder.1
Ideally, IV dihydroergotamine should be administered after consultation with a neurologist or headache specialist, given the pretreatment and cotreatment requirements often necessary to suppress its side effects. Careful titration is important to prevent transient headache exacerbations during infusion, as well as abdominal cramping, nausea, and diarrhea.
Avoid opioids
Opioids should be avoided. Evidence supporting their use in acute migraine is extremely limited,3 and the risks of migraine becoming chronic and of addiction are high.14 Safer, more effective alternatives have been detailed above.
A detailed algorithm for the management of patients with acute migraine has been published14 and is aimed at decreasing acute treatment with opioids and barbiturates.
We recommend the following combination treatment:
Normal saline (0.9% NaCl) 1 to 2 L by intravenous (IV) infusion over 2 to 4 hours. This can be repeated every 6 to 12 hours.
Ketorolac 30-mg IV bolus, which can be repeated every 6 hours. However, patients with coronary artery disease, uncontrolled hypertension, acute renal failure, or cerebrovascular disease should instead receive acetaminophen 1,000 mg, naproxen sodium 550 mg, or aspirin 325 mg by mouth.
Prochlorperazine or metoclopramide 10-mg IV infusion. This can be repeated every 6 hours. However, to reduce the extrapyramidal adverse effects of these drugs, patients should first receive diphenhydramine 25- to 50-mg IV bolus, which can be repeated every 6 to 8 hours.
Sodium valproate 500 to 1,000 mg by IV infusion over 20 minutes. This can be repeated after 8 hours.
Dexamethasone 4-mg IV bolus every 6 hours, or 10-mg IV bolus once in 24 hours.
Magnesium sulfate 500 to 1,000 mg by IV infusion over 1 hour. This can be repeated every 6 to 12 hours.
If the migraine has not improved after 3 cycles of this regimen, a neurologic consultation should be considered. Other options include dihydroergotamine and occipital nerve blocks1 performed at the bedside.
GENERAL PRINCIPLES
Managing acute intractable migraine can be frustrating for both the practitioner and the patient. Some general principles are helpful.
Use a combination of drugs. Aborting a severe migraine attack often requires a combination of medications that work synergistically.
Use IV and intramuscular formulations rather than oral formulations: they are more rapidly absorbed, provide faster pain relief, and can be given when the nausea that often accompanies migraine precludes oral treatments.
Rule out secondary causes. The mnemonic SNOOP—systemic signs, neurologic signs, onset, older age, progression of existing headache disorder—is useful for assessing underlying causes.2 Any patient presenting with intractable migraine should also have a thorough neurologic examination.
Screening electrocardiography may be helpful, as the pretreatment QTc interval may direct the choice of intravenous treatment. If the patient has a prolonged QTc or is taking another drug that could prolong the QTc, certain medications, specifically dopamine receptor antagonists and diphenhydramine, should be avoided.
Ask the patient what has worked previously. A particular agent may have been effective in aborting the migraine; thus, a single dose of it could abort the headache, expediting discharge.
Establish if a triptan or ergot derivative has been used during the 24 hours before presentation, as repeated dosing within this interval is not recommended.3
Establish the baseline headache severity. Complete headache relief is difficult to achieve in a patient with chronic daily headache, and establishing a more realistic goal (eg, 50% relief) from the outset is useful.
OPTIONS FOR DRUG THERAPY
Antiemetics
Dopamine receptor antagonists are assumed to merely treat nausea in patients with migraine; however, they act independently to abort migraine and thus should be considered, irrespective of the presence of nausea.
The two most commonly used agents are prochlorperazine and metoclopramide. The American Academy of Neurology guidelines recommend prochlorperazine as first-line therapy for acute migraine. Metoclopramide is rated slightly lower and is considered to have moderate benefit.4 The Canadian Headache Society cites a high level of evidence supporting prochlorperazine and a moderate level of evidence supporting metoclopramide.5 The American Headache Society assessment of parenteral pharmacotherapies gives prochlorperazine and metoclopramide a level B recommendation of “should offer” (a recommendation only additionally assigned to subcutaneous sumatriptan).3 Hence, either agent can be used.
To reduce the risk of posttreatment akathisia, diphenhydramine or benztropine may be given before starting a dopamine receptor antagonist. Diphenhydramine may be independently effective in migraine treatment,6,7 but data on this are limited.
Ketorolac, ibuprofen
Ketorolac and ibuprofen are the only available nonsteroidal antiinflammatory drugs (NSAIDs) for IV administration. The Canadian Headache Society guidelines strongly recommend ketorolac for the treatment of migraine in emergency settings.5 Doses range from 30 mg to 60 mg.1 Ibuprofen 400 to 800 mg by IV infusion is an acceptable alternative. These medications should be avoided in patients with renal failure or severe coronary artery disease.
Oral naproxen sodium is a possible alternative in patients with cardiovascular disease, as it has been shown to carry a lower cardiovascular risk than other NSAIDs.8
The same concerns in patients with renal dysfunction apply to any NSAID, as the enzyme cyclooxygenase plays a constitutive role in glomerular function.
Antiepileptic drugs
The antiepileptic drugs sodium valproate and levetiracetam are available in IV formulations that have demonstrated efficacy in the treatment of status migrainosus1 (ie, migraine lasting more than 72 hours). Valproate has the strongest track record, is well tolerated, and is effective in rapidly aborting migraine.9
Volume repletion
Although its use is anecdotal and to date no trial has measured its efficacy, IV volume repletion is often used in acute migraine, as most headache experts surmise it to be highly effective, especially in patients with prolonged nausea or vomiting.1
Magnesium
IV magnesium is effective, particularly for migraine with aura.10 Hypotension is a common side effect, and pretreatment or concurrent treatment with IV fluids is advised. Doses from 500 mg to 1,000 mg have demonstrated efficacy.10
Corticosteroids
Corticosteroids can be used in the treatment of status migrainosus. Most studies have shown benefit in preventing recurrences rather than merely aborting migraine.11 A systematic review suggested that recurrent headaches are milder with corticosteroid treatment; 19 of 25 studies indicated favorable benefit, and 6 of 19 studies indicated noninferior outcomes.12
Both IV methylprednisolone and IV dexamethasone may be considered.12 Dexamethasone appears to be particularly effective in preventing headache recurrence when combined with other IV therapies.13 It can be given as a single dose of 10 mg, or as repeated doses of 4 mg up to 16 mg/day.1 Patients with active psychosis or uncontrolled diabetes should be closely monitored for these conditions, which corticosteroids can worsen.
Serotoninergic agents
Serotonin agonists including subcutaneous sumatriptan and IV dihydroergotamine are highly effective, with proven statistical and clinical benefit.4 They should be considered in patients with no known history of coronary artery disease or other vaso-occlusive vascular disorder.1
Ideally, IV dihydroergotamine should be administered after consultation with a neurologist or headache specialist, given the pretreatment and cotreatment requirements often necessary to suppress its side effects. Careful titration is important to prevent transient headache exacerbations during infusion, as well as abdominal cramping, nausea, and diarrhea.
Avoid opioids
Opioids should be avoided. Evidence supporting their use in acute migraine is extremely limited,3 and the risks of migraine becoming chronic and of addiction are high.14 Safer, more effective alternatives have been detailed above.
A detailed algorithm for the management of patients with acute migraine has been published14 and is aimed at decreasing acute treatment with opioids and barbiturates.
- Rozen TD. Emergency department and inpatient management of status migrainosus and intractable headache. Continuum (Minneap Minn) 2015; 21(4):1004–1017. doi:10.1212/CON.0000000000000191
- Dodick D. Headache as a symptom of ominous disease. What are the warning signals? Postgrad Med 1997; 101(5):46–50, 55–56, 62–64. doi:10.3810/pgm.1997.05.217
- Orr SL, Friedman BW, Christie S, et al. Management of adults with acute migraine in the emergency department: the American Headache Society evidence assessment of parenteral pharmacotherapies. Headache 2016; 56(6):911–940. doi:10.1111/head.12835
- Silberstein SD. Practice parameter: evidence-based guidelines for migraine headache (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2000; 55(6):754–762. doi:10.1212/WNL.55.6.754
- Orr SL, Aubé M, Becker WJ, et al. Canadian Headache Society systematic review and recommendations on the treatment of migraine pain in emergency settings. Cephalalgia 2015; 35(3):271–284. doi:10.1177/0333102414535997
- Swidan SZ, Lake AE 3rd, Saper JR. Efficacy of intravenous diphenhydramine versus intravenous DHE-45 in the treatment of severe migraine headache. Curr Pain Headache Rep 2005; 9(1):65–70. doi:10.1007/s11916-005-0077-5
- Marmura MJ, Goldberg SW. Inpatient management of migraine. Curr Neurol Neurosci Rep 2015; 15(4):13. doi:10.1007/s11910-015-0539-z
- Farkouh ME, Greenberg BP. An evidence-based review of the cardiovascular risks of nonsteroidal anti-inflammatory drugs. Am J Cardiol 2009; 103(9):1227–1237. doi:10.1016/j.amjcard.2009.01.014
- Stillman MJ, Zajac D, Rybicki LA. Treatment of primary headache disorders with intravenous valproate: initial outpatient experience. Headache 2004; 44(1):65–69. doi:10.1111/j.1526-4610.2004.04010.x
- Marmura MJ, Silberstein SD, Schwedt TJ. The acute treatment of migraine in adults: the American Headache Society evidence assessment of migraine pharmacotherapies. Headache 2015; 55(1):3–20. doi:10.1111/head.12499
- Colman I, Friedman BW, Brown MD, et al. Parenteral dexamethasone for acute severe migraine headache: meta-analysis of randomised controlled trials for preventing recurrence. BMJ 2008; 336(7657):1359–1361. doi:10.1136/bmj.39566.806725.BE
- Woldeamanuel YW, Rapoport AM, Cowan RP. The place of corticosteroids in migraine attack management: a 65-year systematic review with pooled analysis and critical appraisal. Cephalalgia 2015; 35(1):996–1024. doi:10.1177/0333102414566200
- Singh A, Alter HJ, Zaia B. Does the addition of dexamethasone to standard therapy for acute migraine headache decrease the incidence of recurrent headache for patients treated in the emergency department? A meta-analysis and systematic review of the literature. Acad Emerg Med 2008; 15(12):1223–1233. doi:10.1111/j.1553-2712.2008.00283.x
- Ahmed ZA, Nacopoulos DA, John S, Papesh N, Levine D, Bamford CC. An algorithm for opioid and barbiturate reduction in the acute management of headache in the emergency department. Headache 2017; 57(1):71–79. doi:10.1111/head.12961
- Rozen TD. Emergency department and inpatient management of status migrainosus and intractable headache. Continuum (Minneap Minn) 2015; 21(4):1004–1017. doi:10.1212/CON.0000000000000191
- Dodick D. Headache as a symptom of ominous disease. What are the warning signals? Postgrad Med 1997; 101(5):46–50, 55–56, 62–64. doi:10.3810/pgm.1997.05.217
- Orr SL, Friedman BW, Christie S, et al. Management of adults with acute migraine in the emergency department: the American Headache Society evidence assessment of parenteral pharmacotherapies. Headache 2016; 56(6):911–940. doi:10.1111/head.12835
- Silberstein SD. Practice parameter: evidence-based guidelines for migraine headache (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2000; 55(6):754–762. doi:10.1212/WNL.55.6.754
- Orr SL, Aubé M, Becker WJ, et al. Canadian Headache Society systematic review and recommendations on the treatment of migraine pain in emergency settings. Cephalalgia 2015; 35(3):271–284. doi:10.1177/0333102414535997
- Swidan SZ, Lake AE 3rd, Saper JR. Efficacy of intravenous diphenhydramine versus intravenous DHE-45 in the treatment of severe migraine headache. Curr Pain Headache Rep 2005; 9(1):65–70. doi:10.1007/s11916-005-0077-5
- Marmura MJ, Goldberg SW. Inpatient management of migraine. Curr Neurol Neurosci Rep 2015; 15(4):13. doi:10.1007/s11910-015-0539-z
- Farkouh ME, Greenberg BP. An evidence-based review of the cardiovascular risks of nonsteroidal anti-inflammatory drugs. Am J Cardiol 2009; 103(9):1227–1237. doi:10.1016/j.amjcard.2009.01.014
- Stillman MJ, Zajac D, Rybicki LA. Treatment of primary headache disorders with intravenous valproate: initial outpatient experience. Headache 2004; 44(1):65–69. doi:10.1111/j.1526-4610.2004.04010.x
- Marmura MJ, Silberstein SD, Schwedt TJ. The acute treatment of migraine in adults: the American Headache Society evidence assessment of migraine pharmacotherapies. Headache 2015; 55(1):3–20. doi:10.1111/head.12499
- Colman I, Friedman BW, Brown MD, et al. Parenteral dexamethasone for acute severe migraine headache: meta-analysis of randomised controlled trials for preventing recurrence. BMJ 2008; 336(7657):1359–1361. doi:10.1136/bmj.39566.806725.BE
- Woldeamanuel YW, Rapoport AM, Cowan RP. The place of corticosteroids in migraine attack management: a 65-year systematic review with pooled analysis and critical appraisal. Cephalalgia 2015; 35(1):996–1024. doi:10.1177/0333102414566200
- Singh A, Alter HJ, Zaia B. Does the addition of dexamethasone to standard therapy for acute migraine headache decrease the incidence of recurrent headache for patients treated in the emergency department? A meta-analysis and systematic review of the literature. Acad Emerg Med 2008; 15(12):1223–1233. doi:10.1111/j.1553-2712.2008.00283.x
- Ahmed ZA, Nacopoulos DA, John S, Papesh N, Levine D, Bamford CC. An algorithm for opioid and barbiturate reduction in the acute management of headache in the emergency department. Headache 2017; 57(1):71–79. doi:10.1111/head.12961
When does S aureus bacteremia require transesophageal echocardiography?
Staphylococcus aureus is the most common infective agent in native and prosthetic valve endocarditis, and 13% to 22% of patients with S aureus bacteremia have infective endocarditis.1
Transthoracic echocardiography (TTE) is a good starting point in the workup of suspected infective endocarditis, but transesophageal echocardiography (TEE) plays a key role in diagnosis and is indicated in patients with a high pretest probability of infective endocarditis, as in the following scenarios:
- Clinical picture consistent with infective endocarditis
- Presence of previously placed port or other indwelling vascular device
- Presence of a prosthetic valve or other prosthetic material
- Presence of a pacemaker
- History of valve disease
- Injection drug use
- Positive blood cultures after 72 hours despite appropriate antibiotic treatment
- Abnormal TTE result requiring better visualization of valvular anatomy and function and confirmation of local complications
- Absence of another reasonable explanation for S aureus bacteremia.
Forgoing TEE is reasonable in patients with normal results on TTE, no predisposing risk factors, a reasonable alternative explanation for S aureus bacteremia, and a low pretest probability of infective endocarditis.1 TEE may also be unnecessary if there is another disease focus requiring extended treatment (eg, vertebral infection) and there are no findings suggesting complicated infective endocarditis, eg, persistent bacteremia, symptoms of heart failure, and conduction abnormality.1
TEE also may be unnecessary in patients at low risk who have identifiable foci of bacteremia due to soft-tissue infection or a newly placed vascular catheter and whose bacteremia clears within 72 hours of the start of antibiotic therapy. These patients may be followed clinically for the development of new findings such as metastatic foci of infection (eg, septic pulmonary emboli, renal infarction, splenic abscess or infarction), the new onset of heart failure or cardiac conduction abnormality, or recurrence of previously cleared S aureus bacteremia. If these should develop, then a more invasive study such as TEE may be warranted.
INFECTIVE ENDOCARDITIS: EPIDEMIOLOGY AND MICROBIOLOGY
The US incidence rate of infective endocarditis has steadily increased, with an estimated 457,052 hospitalizations from 2000 to 2011. During that period, from 2000 to 2007, there was a marked increase in valve replacement surgeries.2 This trend is likely explained by an increase in the at-risk population—eg, elderly patients, patients with opiate dependence or diabetes, and patients on hemodialysis.
Although S aureus is the predominant pathogen in infective endocarditis,2–5 S aureus bacteremia is often observed in patients with skin or soft-tissue infection, prosthetic device infection, vascular graft or catheter infection, and bone and joint infections. S aureus bacteremia necessitates a search for the source of infection.
S aureus is a major pathogen in bloodstream infections, and up to 14% of patients with S aureus bacteremia have infective endocarditis as the primary source of infection.3 The pathogenesis of S aureus infective endocarditis is thought to be mediated by cell-wall factors that promote adhesion to the extracellular matrix of intravascular structures.3
A new localizing symptom such as back pain, joint pain, or swelling in a patient with S aureus bacteremia should trigger an investigation for metastatic infection.
Infectious disease consultation in patients with S aureus bacteremia is associated with improved outcomes and, thus, should be pursued.3
A cardiac surgery consult is recommended early on in cases of infective endocarditis caused by vancomycin-resistant enterococci, Pseudomonas aeruginosa, and fungi, as well as in patients with complications such as valvular insufficiency, perivalvular abscess, conduction abnormalities, persistent bacteremia, and metastatic foci of infection.6
RISK FACTORS
Risk factors for infective endocarditis include injection drug abuse, valvular heart disease, congenital heart disease (unrepaired, repaired with residual defects, or fully repaired within the past 6 months), previous infective endocarditis, prosthetic heart valve, and cardiac transplant.2–4,6 Other risk factors are poor dentition, hemodialysis, ventriculoatrial shunts, intravascular devices including vascular grafts, and pacemakers.2,3 Many risk factors for infective endocarditis and S aureus bacteremia overlap.3
DIAGNOSTIC PRINCIPLES
The clinical presentation of infective endocarditis can vary from a nonspecific infectious syndrome, to overt organ failure (heart failure, kidney failure), to an acute vascular catastrophe (arterial ischemia, cerebrovascular accidents, myocardial infarction). Patients may present with indolent symptoms such as fever, fatigue, and weight loss,6 or they may present at an advanced stage, with fulminant acute heart failure due to valvular insufficiency or with arrhythmias due to a perivalvular abscess infiltrating the conduction system. Extracardiac clinical manifestations may be related to direct infective metastatic foci such as septic emboli or to immunologic phenomena such as glomerulonephritis or Osler nodes.
ECHOCARDIOGRAPHY’S ROLE IN DIAGNOSIS
TTE plays an important role in diagnosis and risk stratification of infective endocarditis.6 TTE is usually done first because of its low cost, wide availability, and safety; it has a sensitivity of 70% and a specificity over 95%.8 While a normal result on TTE does not completely rule out infective endocarditis, completely normal valvular morphology and function on TTE make the diagnosis less likely.8,9
If suspicion remains high despite a normal study, repeating TTE at a later time may result in a higher diagnostic yield because of growth of the suspected vegetation. Otherwise, TEE should be considered.
TEE provides a higher spatial resolution and diagnostic yield than TTE, especially for detecting complex pathology such as pseudoaneurysm, valve perforation, or valvular abscess. TEE has a sensitivity and specificity of approximately 95% for infective endocarditis.8 It should be performed early in patients with preexisting valve disease, prosthetic cardiac material (eg, valves), or a pacemaker or implantable cardioverter-defibrillator.6,7
Detecting valve vegetation provides answers about the cause of S aureus bacteremia with its complications (eg, septic emboli, mycotic aneurysm) and informs decisions about the duration of antibiotic therapy and the need for surgery.3,6
As with any diagnostic test, it is important to compare the results of any recent study with those of previous studies whenever possible to differentiate new from old findings.
WHEN TO FORGO TEE IN S AUREUS BACTEREMIA
Because TEE is invasive and requires the patient to swallow an endoscopic probe,10 it is important to screen patients for esophageal disease, cervical spine conditions, and baseline respiratory insufficiency. Complications are rare but include esophageal perforation, esophageal bleeding, pharyngeal hematoma, and reactions to anesthesia.10
As with any diagnostic test, the clinician first needs to consider the patient’s pretest probability of the disease, the diagnostic accuracy, the associated risks and costs, and the implications of the results.
While TEE provides better diagnostic images than TTE, a normal TEE study does not exclude the diagnosis of infective endocarditis: small lesions and complications such as paravalvular abscess of a prosthetic aortic valve may still be missed. In such patients, a repeat TEE examination or additional imaging study (eg, gated computed tomographic angiography) should be considered.6
Noninfective sterile echodensities, valvular tumors such as papillary fibroelastomas, Lambl excrescences, and suture lines of prosthetic valves are among the conditions and factors that can cause a false-positive result on TEE.
- Young H, Knepper BC, Price CS, Heard S, Jenkins TC. Clinical reasoning of infectious diseases physicians behind the use or nonuse of transesophageal echocardiography in Staphylococcus aureus bacteremia. Open Forum Infect Dis 2016; 3(4):ofw204. doi:10.1093/ofid/ofw204
- Pant S, Patel NJ, Deshmukh A, et al. Trends in infective endocarditis incidence, microbiology, and valve replacement in the United States from 2000 to 2011. J Am Coll Cardiol 2015; 65(19):2070–2076. doi:10.1016/j.jacc.2015.03.518
- Tong SY, Davis JS, Eichenberger E, Holland TL, Fowler VG Jr. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev 2015; 28(3):603–661. doi:10.1128/CMR.00134-14
- Palraj BR, Baddour LM, Hess EP, et al. Predicting risk of endocarditis using a clinical tool (PREDICT): scoring system to guide use of echocardiography in the management of Staphylococcus aureus bacteremia. Clin Infect Dis 2015; 61(1):18–28. doi:10.1093/cid/civ235
- Barton T, Moir S, Rehmani H, Woolley I, Korman TM, Stuart RL. Low rates of endocarditis in healthcare-associated Staphylococcus aureus bacteremia suggest that echocardiography might not always be required. Eur J Clin Microbiol Infect Dis 2016; 35(1):49–55. doi:10.1007/s10096-015-2505-8
- Baddour LM, Wilson WR, Bayer AS, et al; American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015; 132(15):1435–1486. doi10.1161/CIR.0000000000000296
- Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 2000; 30(4):633–638. doi:10.1086/313753
- Habib G, Badano L, Tribouilloy C, et al; European Association of Echocardiography. Recommendations for the practice of echocardiography in infective endocarditis. Eur J Echocardiogr 2010; 11(2):202–219. doi:10.1093/ejechocard/jeq004
- Irani WN, Grayburn PA, Afridi I. A negative transthoracic echocardiogram obviates the need for transesophageal echocardiography in patients with suspected native valve active infective endocarditis. Am J Cardiol 1996; 78(1):101–103. pmid:8712097
- Hahn RT, Abraham T, Adams MS, et al. Guidelines for performing a comprehensive transesophageal echocardiographic examination: recommendations from the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. J Am Soc Echocardiogr 2013; 26(9):921–964. doi:10.1016/j.echo.2013.07.009
Staphylococcus aureus is the most common infective agent in native and prosthetic valve endocarditis, and 13% to 22% of patients with S aureus bacteremia have infective endocarditis.1
Transthoracic echocardiography (TTE) is a good starting point in the workup of suspected infective endocarditis, but transesophageal echocardiography (TEE) plays a key role in diagnosis and is indicated in patients with a high pretest probability of infective endocarditis, as in the following scenarios:
- Clinical picture consistent with infective endocarditis
- Presence of previously placed port or other indwelling vascular device
- Presence of a prosthetic valve or other prosthetic material
- Presence of a pacemaker
- History of valve disease
- Injection drug use
- Positive blood cultures after 72 hours despite appropriate antibiotic treatment
- Abnormal TTE result requiring better visualization of valvular anatomy and function and confirmation of local complications
- Absence of another reasonable explanation for S aureus bacteremia.
Forgoing TEE is reasonable in patients with normal results on TTE, no predisposing risk factors, a reasonable alternative explanation for S aureus bacteremia, and a low pretest probability of infective endocarditis.1 TEE may also be unnecessary if there is another disease focus requiring extended treatment (eg, vertebral infection) and there are no findings suggesting complicated infective endocarditis, eg, persistent bacteremia, symptoms of heart failure, and conduction abnormality.1
TEE also may be unnecessary in patients at low risk who have identifiable foci of bacteremia due to soft-tissue infection or a newly placed vascular catheter and whose bacteremia clears within 72 hours of the start of antibiotic therapy. These patients may be followed clinically for the development of new findings such as metastatic foci of infection (eg, septic pulmonary emboli, renal infarction, splenic abscess or infarction), the new onset of heart failure or cardiac conduction abnormality, or recurrence of previously cleared S aureus bacteremia. If these should develop, then a more invasive study such as TEE may be warranted.
INFECTIVE ENDOCARDITIS: EPIDEMIOLOGY AND MICROBIOLOGY
The US incidence rate of infective endocarditis has steadily increased, with an estimated 457,052 hospitalizations from 2000 to 2011. During that period, from 2000 to 2007, there was a marked increase in valve replacement surgeries.2 This trend is likely explained by an increase in the at-risk population—eg, elderly patients, patients with opiate dependence or diabetes, and patients on hemodialysis.
Although S aureus is the predominant pathogen in infective endocarditis,2–5 S aureus bacteremia is often observed in patients with skin or soft-tissue infection, prosthetic device infection, vascular graft or catheter infection, and bone and joint infections. S aureus bacteremia necessitates a search for the source of infection.
S aureus is a major pathogen in bloodstream infections, and up to 14% of patients with S aureus bacteremia have infective endocarditis as the primary source of infection.3 The pathogenesis of S aureus infective endocarditis is thought to be mediated by cell-wall factors that promote adhesion to the extracellular matrix of intravascular structures.3
A new localizing symptom such as back pain, joint pain, or swelling in a patient with S aureus bacteremia should trigger an investigation for metastatic infection.
Infectious disease consultation in patients with S aureus bacteremia is associated with improved outcomes and, thus, should be pursued.3
A cardiac surgery consult is recommended early on in cases of infective endocarditis caused by vancomycin-resistant enterococci, Pseudomonas aeruginosa, and fungi, as well as in patients with complications such as valvular insufficiency, perivalvular abscess, conduction abnormalities, persistent bacteremia, and metastatic foci of infection.6
RISK FACTORS
Risk factors for infective endocarditis include injection drug abuse, valvular heart disease, congenital heart disease (unrepaired, repaired with residual defects, or fully repaired within the past 6 months), previous infective endocarditis, prosthetic heart valve, and cardiac transplant.2–4,6 Other risk factors are poor dentition, hemodialysis, ventriculoatrial shunts, intravascular devices including vascular grafts, and pacemakers.2,3 Many risk factors for infective endocarditis and S aureus bacteremia overlap.3
DIAGNOSTIC PRINCIPLES
The clinical presentation of infective endocarditis can vary from a nonspecific infectious syndrome, to overt organ failure (heart failure, kidney failure), to an acute vascular catastrophe (arterial ischemia, cerebrovascular accidents, myocardial infarction). Patients may present with indolent symptoms such as fever, fatigue, and weight loss,6 or they may present at an advanced stage, with fulminant acute heart failure due to valvular insufficiency or with arrhythmias due to a perivalvular abscess infiltrating the conduction system. Extracardiac clinical manifestations may be related to direct infective metastatic foci such as septic emboli or to immunologic phenomena such as glomerulonephritis or Osler nodes.
ECHOCARDIOGRAPHY’S ROLE IN DIAGNOSIS
TTE plays an important role in diagnosis and risk stratification of infective endocarditis.6 TTE is usually done first because of its low cost, wide availability, and safety; it has a sensitivity of 70% and a specificity over 95%.8 While a normal result on TTE does not completely rule out infective endocarditis, completely normal valvular morphology and function on TTE make the diagnosis less likely.8,9
If suspicion remains high despite a normal study, repeating TTE at a later time may result in a higher diagnostic yield because of growth of the suspected vegetation. Otherwise, TEE should be considered.
TEE provides a higher spatial resolution and diagnostic yield than TTE, especially for detecting complex pathology such as pseudoaneurysm, valve perforation, or valvular abscess. TEE has a sensitivity and specificity of approximately 95% for infective endocarditis.8 It should be performed early in patients with preexisting valve disease, prosthetic cardiac material (eg, valves), or a pacemaker or implantable cardioverter-defibrillator.6,7
Detecting valve vegetation provides answers about the cause of S aureus bacteremia with its complications (eg, septic emboli, mycotic aneurysm) and informs decisions about the duration of antibiotic therapy and the need for surgery.3,6
As with any diagnostic test, it is important to compare the results of any recent study with those of previous studies whenever possible to differentiate new from old findings.
WHEN TO FORGO TEE IN S AUREUS BACTEREMIA
Because TEE is invasive and requires the patient to swallow an endoscopic probe,10 it is important to screen patients for esophageal disease, cervical spine conditions, and baseline respiratory insufficiency. Complications are rare but include esophageal perforation, esophageal bleeding, pharyngeal hematoma, and reactions to anesthesia.10
As with any diagnostic test, the clinician first needs to consider the patient’s pretest probability of the disease, the diagnostic accuracy, the associated risks and costs, and the implications of the results.
While TEE provides better diagnostic images than TTE, a normal TEE study does not exclude the diagnosis of infective endocarditis: small lesions and complications such as paravalvular abscess of a prosthetic aortic valve may still be missed. In such patients, a repeat TEE examination or additional imaging study (eg, gated computed tomographic angiography) should be considered.6
Noninfective sterile echodensities, valvular tumors such as papillary fibroelastomas, Lambl excrescences, and suture lines of prosthetic valves are among the conditions and factors that can cause a false-positive result on TEE.
Staphylococcus aureus is the most common infective agent in native and prosthetic valve endocarditis, and 13% to 22% of patients with S aureus bacteremia have infective endocarditis.1
Transthoracic echocardiography (TTE) is a good starting point in the workup of suspected infective endocarditis, but transesophageal echocardiography (TEE) plays a key role in diagnosis and is indicated in patients with a high pretest probability of infective endocarditis, as in the following scenarios:
- Clinical picture consistent with infective endocarditis
- Presence of previously placed port or other indwelling vascular device
- Presence of a prosthetic valve or other prosthetic material
- Presence of a pacemaker
- History of valve disease
- Injection drug use
- Positive blood cultures after 72 hours despite appropriate antibiotic treatment
- Abnormal TTE result requiring better visualization of valvular anatomy and function and confirmation of local complications
- Absence of another reasonable explanation for S aureus bacteremia.
Forgoing TEE is reasonable in patients with normal results on TTE, no predisposing risk factors, a reasonable alternative explanation for S aureus bacteremia, and a low pretest probability of infective endocarditis.1 TEE may also be unnecessary if there is another disease focus requiring extended treatment (eg, vertebral infection) and there are no findings suggesting complicated infective endocarditis, eg, persistent bacteremia, symptoms of heart failure, and conduction abnormality.1
TEE also may be unnecessary in patients at low risk who have identifiable foci of bacteremia due to soft-tissue infection or a newly placed vascular catheter and whose bacteremia clears within 72 hours of the start of antibiotic therapy. These patients may be followed clinically for the development of new findings such as metastatic foci of infection (eg, septic pulmonary emboli, renal infarction, splenic abscess or infarction), the new onset of heart failure or cardiac conduction abnormality, or recurrence of previously cleared S aureus bacteremia. If these should develop, then a more invasive study such as TEE may be warranted.
INFECTIVE ENDOCARDITIS: EPIDEMIOLOGY AND MICROBIOLOGY
The US incidence rate of infective endocarditis has steadily increased, with an estimated 457,052 hospitalizations from 2000 to 2011. During that period, from 2000 to 2007, there was a marked increase in valve replacement surgeries.2 This trend is likely explained by an increase in the at-risk population—eg, elderly patients, patients with opiate dependence or diabetes, and patients on hemodialysis.
Although S aureus is the predominant pathogen in infective endocarditis,2–5 S aureus bacteremia is often observed in patients with skin or soft-tissue infection, prosthetic device infection, vascular graft or catheter infection, and bone and joint infections. S aureus bacteremia necessitates a search for the source of infection.
S aureus is a major pathogen in bloodstream infections, and up to 14% of patients with S aureus bacteremia have infective endocarditis as the primary source of infection.3 The pathogenesis of S aureus infective endocarditis is thought to be mediated by cell-wall factors that promote adhesion to the extracellular matrix of intravascular structures.3
A new localizing symptom such as back pain, joint pain, or swelling in a patient with S aureus bacteremia should trigger an investigation for metastatic infection.
Infectious disease consultation in patients with S aureus bacteremia is associated with improved outcomes and, thus, should be pursued.3
A cardiac surgery consult is recommended early on in cases of infective endocarditis caused by vancomycin-resistant enterococci, Pseudomonas aeruginosa, and fungi, as well as in patients with complications such as valvular insufficiency, perivalvular abscess, conduction abnormalities, persistent bacteremia, and metastatic foci of infection.6
RISK FACTORS
Risk factors for infective endocarditis include injection drug abuse, valvular heart disease, congenital heart disease (unrepaired, repaired with residual defects, or fully repaired within the past 6 months), previous infective endocarditis, prosthetic heart valve, and cardiac transplant.2–4,6 Other risk factors are poor dentition, hemodialysis, ventriculoatrial shunts, intravascular devices including vascular grafts, and pacemakers.2,3 Many risk factors for infective endocarditis and S aureus bacteremia overlap.3
DIAGNOSTIC PRINCIPLES
The clinical presentation of infective endocarditis can vary from a nonspecific infectious syndrome, to overt organ failure (heart failure, kidney failure), to an acute vascular catastrophe (arterial ischemia, cerebrovascular accidents, myocardial infarction). Patients may present with indolent symptoms such as fever, fatigue, and weight loss,6 or they may present at an advanced stage, with fulminant acute heart failure due to valvular insufficiency or with arrhythmias due to a perivalvular abscess infiltrating the conduction system. Extracardiac clinical manifestations may be related to direct infective metastatic foci such as septic emboli or to immunologic phenomena such as glomerulonephritis or Osler nodes.
ECHOCARDIOGRAPHY’S ROLE IN DIAGNOSIS
TTE plays an important role in diagnosis and risk stratification of infective endocarditis.6 TTE is usually done first because of its low cost, wide availability, and safety; it has a sensitivity of 70% and a specificity over 95%.8 While a normal result on TTE does not completely rule out infective endocarditis, completely normal valvular morphology and function on TTE make the diagnosis less likely.8,9
If suspicion remains high despite a normal study, repeating TTE at a later time may result in a higher diagnostic yield because of growth of the suspected vegetation. Otherwise, TEE should be considered.
TEE provides a higher spatial resolution and diagnostic yield than TTE, especially for detecting complex pathology such as pseudoaneurysm, valve perforation, or valvular abscess. TEE has a sensitivity and specificity of approximately 95% for infective endocarditis.8 It should be performed early in patients with preexisting valve disease, prosthetic cardiac material (eg, valves), or a pacemaker or implantable cardioverter-defibrillator.6,7
Detecting valve vegetation provides answers about the cause of S aureus bacteremia with its complications (eg, septic emboli, mycotic aneurysm) and informs decisions about the duration of antibiotic therapy and the need for surgery.3,6
As with any diagnostic test, it is important to compare the results of any recent study with those of previous studies whenever possible to differentiate new from old findings.
WHEN TO FORGO TEE IN S AUREUS BACTEREMIA
Because TEE is invasive and requires the patient to swallow an endoscopic probe,10 it is important to screen patients for esophageal disease, cervical spine conditions, and baseline respiratory insufficiency. Complications are rare but include esophageal perforation, esophageal bleeding, pharyngeal hematoma, and reactions to anesthesia.10
As with any diagnostic test, the clinician first needs to consider the patient’s pretest probability of the disease, the diagnostic accuracy, the associated risks and costs, and the implications of the results.
While TEE provides better diagnostic images than TTE, a normal TEE study does not exclude the diagnosis of infective endocarditis: small lesions and complications such as paravalvular abscess of a prosthetic aortic valve may still be missed. In such patients, a repeat TEE examination or additional imaging study (eg, gated computed tomographic angiography) should be considered.6
Noninfective sterile echodensities, valvular tumors such as papillary fibroelastomas, Lambl excrescences, and suture lines of prosthetic valves are among the conditions and factors that can cause a false-positive result on TEE.
- Young H, Knepper BC, Price CS, Heard S, Jenkins TC. Clinical reasoning of infectious diseases physicians behind the use or nonuse of transesophageal echocardiography in Staphylococcus aureus bacteremia. Open Forum Infect Dis 2016; 3(4):ofw204. doi:10.1093/ofid/ofw204
- Pant S, Patel NJ, Deshmukh A, et al. Trends in infective endocarditis incidence, microbiology, and valve replacement in the United States from 2000 to 2011. J Am Coll Cardiol 2015; 65(19):2070–2076. doi:10.1016/j.jacc.2015.03.518
- Tong SY, Davis JS, Eichenberger E, Holland TL, Fowler VG Jr. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev 2015; 28(3):603–661. doi:10.1128/CMR.00134-14
- Palraj BR, Baddour LM, Hess EP, et al. Predicting risk of endocarditis using a clinical tool (PREDICT): scoring system to guide use of echocardiography in the management of Staphylococcus aureus bacteremia. Clin Infect Dis 2015; 61(1):18–28. doi:10.1093/cid/civ235
- Barton T, Moir S, Rehmani H, Woolley I, Korman TM, Stuart RL. Low rates of endocarditis in healthcare-associated Staphylococcus aureus bacteremia suggest that echocardiography might not always be required. Eur J Clin Microbiol Infect Dis 2016; 35(1):49–55. doi:10.1007/s10096-015-2505-8
- Baddour LM, Wilson WR, Bayer AS, et al; American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015; 132(15):1435–1486. doi10.1161/CIR.0000000000000296
- Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 2000; 30(4):633–638. doi:10.1086/313753
- Habib G, Badano L, Tribouilloy C, et al; European Association of Echocardiography. Recommendations for the practice of echocardiography in infective endocarditis. Eur J Echocardiogr 2010; 11(2):202–219. doi:10.1093/ejechocard/jeq004
- Irani WN, Grayburn PA, Afridi I. A negative transthoracic echocardiogram obviates the need for transesophageal echocardiography in patients with suspected native valve active infective endocarditis. Am J Cardiol 1996; 78(1):101–103. pmid:8712097
- Hahn RT, Abraham T, Adams MS, et al. Guidelines for performing a comprehensive transesophageal echocardiographic examination: recommendations from the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. J Am Soc Echocardiogr 2013; 26(9):921–964. doi:10.1016/j.echo.2013.07.009
- Young H, Knepper BC, Price CS, Heard S, Jenkins TC. Clinical reasoning of infectious diseases physicians behind the use or nonuse of transesophageal echocardiography in Staphylococcus aureus bacteremia. Open Forum Infect Dis 2016; 3(4):ofw204. doi:10.1093/ofid/ofw204
- Pant S, Patel NJ, Deshmukh A, et al. Trends in infective endocarditis incidence, microbiology, and valve replacement in the United States from 2000 to 2011. J Am Coll Cardiol 2015; 65(19):2070–2076. doi:10.1016/j.jacc.2015.03.518
- Tong SY, Davis JS, Eichenberger E, Holland TL, Fowler VG Jr. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev 2015; 28(3):603–661. doi:10.1128/CMR.00134-14
- Palraj BR, Baddour LM, Hess EP, et al. Predicting risk of endocarditis using a clinical tool (PREDICT): scoring system to guide use of echocardiography in the management of Staphylococcus aureus bacteremia. Clin Infect Dis 2015; 61(1):18–28. doi:10.1093/cid/civ235
- Barton T, Moir S, Rehmani H, Woolley I, Korman TM, Stuart RL. Low rates of endocarditis in healthcare-associated Staphylococcus aureus bacteremia suggest that echocardiography might not always be required. Eur J Clin Microbiol Infect Dis 2016; 35(1):49–55. doi:10.1007/s10096-015-2505-8
- Baddour LM, Wilson WR, Bayer AS, et al; American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015; 132(15):1435–1486. doi10.1161/CIR.0000000000000296
- Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 2000; 30(4):633–638. doi:10.1086/313753
- Habib G, Badano L, Tribouilloy C, et al; European Association of Echocardiography. Recommendations for the practice of echocardiography in infective endocarditis. Eur J Echocardiogr 2010; 11(2):202–219. doi:10.1093/ejechocard/jeq004
- Irani WN, Grayburn PA, Afridi I. A negative transthoracic echocardiogram obviates the need for transesophageal echocardiography in patients with suspected native valve active infective endocarditis. Am J Cardiol 1996; 78(1):101–103. pmid:8712097
- Hahn RT, Abraham T, Adams MS, et al. Guidelines for performing a comprehensive transesophageal echocardiographic examination: recommendations from the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. J Am Soc Echocardiogr 2013; 26(9):921–964. doi:10.1016/j.echo.2013.07.009
S aureus bacteremia: TEE and infectious disease consultation
Morbidity and mortality rates in patients with Staphylococcus aureus bacteremia remain high even though diagnostic tests have improved and antibiotic therapy is effective. Diagnosis and management are made more complex by difficulties in finding the source of bacteremia and sites of metastatic infection.
S aureus bacteremia is a finding that demands further investigation, since up to 25% of people who have it may have endocarditis, a condition with even worse consequences.1 The ability of S aureus to infect normal valves2,3 adds to the challenge. In the mid-20th century, Wilson and Hamburger4 demonstrated that 64% of patients with S aureus bacteremia had evidence of valvular infection at autopsy. In a more recent case series of patients with S aureus endocarditis, the diagnosis was established at autopsy in 32%.5
Specific clinical findings in patients with complicated S aureus bacteremia—those who have a site of infection remote from or extended beyond the primary focus—may be useful in determining the need for additional diagnostic and therapeutic measures.
In a prospective cohort study, Fowler et al6 identified several factors that predicted complicated S aureus bacteremia (including but not limited to endocarditis):
- Prolonged bacteremia (> 48–72 hours after initiation of therapy)
- Community onset
- Fever persisting more than 72 hours
- Skin findings suggesting systemic infection.
THE ROLE OF ECHOCARDIOGRAPHY
Infective endocarditis may be difficult to detect in patients with S aureus bacteremia; experts recommend routine use of echocardiography in this process.7,8 Transesophageal echocardiography (TEE) detects more cases of endocarditis than transthoracic echocardiography (TTE),9,10 but access, cost, and risks lead to questions about its utility.
Guidance for the use of echocardiography in S aureus bacteremia1,10–14 continues to evolve. Consensus seems to be emerging that the risk of endocarditis is lower in patients with S aureus bacteremia who:
- Do not have a prosthetic valve or other permanent intracardiac device
- Have sterile blood cultures within 96 hours after the initial set
- Are not hemodialysis-dependent
- Developed the bacteremia in a healthcare setting
- Have no secondary focus of infection
- Have no clinical signs of infective endocarditis.
Heriot et al14 point out that studies of risk-stratification approaches to echocardiography in patients with S aureus bacteremia are difficult to interpret, as there are questions regarding the validity of the studies and the balance of the risks and benefits.1 The question of timing of TEE remains largely unexplored, both in initial screening and in follow-up of previously undiagnosed cases of S aureus endocarditis.
In this issue of the Journal, Mirrakhimov et al15 weigh in on use of a risk-stratification model to guide use of TEE in patients with S aureus bacteremia. Their comments about avoiding TEE in patients who have an alternative explanation for S aureus bacteremia and a low pretest probability for infectious endocarditis and in patients with a disease focus that requires extended treatment are derived from a survey of infectious disease physicians.16
ROLE OF INFECTIOUS DISEASE CONSULTATION
Infectious disease consultation reduces mortality rates and healthcare costs for a variety of infections, with endocarditis as a prime example.17 For S aureus bacteremia, a large and growing body of literature demonstrates the impact of infectious disease consultation, including improved adherence to guidelines and quality measures,18–20 lower in-hospital mortality rates18–21 and earlier hospital discharge.18 In the era of “curbside consults” and “e-consultation,” it is interesting to note the enduring value of bedside, in-person consultation in the management of S aureus bacteremia.20
Many people with S aureus bacteremia should undergo TEE. Until the evidence becomes more robust, the decision to forgo TEE must be made with caution. The expertise of infectious disease physicians in the diagnosis and management of endocarditis can assist clinicians working with the often-complex patients who develop S aureus bacteremia. If the goal is to improve outcomes, infectious disease consultation may be at least as important as appropriate selection of patients for TEE.
- Rasmussen RV, Høst U, Arpi M, et al. Prevalence of infective endocarditis in patients with Staphylococcus aureus bacteraemia: the value of screening with echocardiography. Eur J Echocardiogr 2011; 12(6):414–420. doi:10.1093/ejechocard/jer023
- Vogler, WR, Dorney ER. Bacterial endocarditis in normal heart. Bull Emory Univ Clin 1961; 1:21–31.
- Thayer WS. Bacterial or infective endocarditis. Edinburgh Med J 1931; 38:237–265, 307–334.
- Wilson R, Hamburger M. Fifteen years’ experience with staphylococcus septicemia in large city hospital: analysis of fifty-five cases in Cincinnati General Hospital 1940 to 1954. Am J Med 1957; 22(3):437–457. pmid:13402795
- Røder BL, Wandall DA, Frimodt-Møllar N, Espersen F, Skinhøj P, Rosdahl VT. Clinical features of Staphylococcus aureus endocarditis: a 10-year experience in Denmark. Arch Intern Med 1999; 159(5):462–469. pmid:10074954
- Fowler VG Jr, Olsen MK, Corey GR, et al. Clinical identifiers of complicated Staphylococcus aureus bacteremia. Arch Intern Med 2003; 163(17):2066–2072. doi:10.1001/archinte.163.17.2066
- Baddour LM, Wilson WR, Bayer AS, et al; American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015; 132(15):1435–1486. doi:10.1161/CIR.0000000000000296
- Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis 2011; 52(3):285–292. doi:10.1093/cid/cir034
- Reynolds HR, Jagen MA, Tunick PA, Kronzon I. Sensitivity of transthoracic versus transesophageal echocardiography for the detection of native valve vegetations in the modern era. J Am Soc Echocardiogr 2003; 16(1):67–70. doi:10.1067/mje.2003.43
- Holland TL, Arnold C, Fowler VG Jr. Clinical management of Staphylococcus aureus bacteremia: a review. JAMA 2014; 312(13):1330–1341. doi:10.1001/jama.2014.9743
- Kaasch AJ, Folwler VG Jr, Rieg S, et al. Use of a simple criteria set for guiding echocardiography in nosocomial Staphylococcus aureus bacteremia. Clin Infect Dis 2011; 53(1):1–9. doi:10.1093/cid/cir320
- Palraj BR, Baddour LM, Hess EP, et al. Predicting risk of endocarditis using a clinical tool (PREDICT): scoring system to guide use of echocardiography in the management of Staphylococcus aureus bacteremia. Clin Infect Dis 2015; 61(1):18–28. doi:10.1093/cid/civ235
- Bai AD, Agarawal A, Steinberg M, et al. Clinical predictors and clinical prediction rules to estimate initial patient risk for infective endocarditis in Staphylococcus aureus bacteremia: a systematic review and meta-analysis. Clin Microbiol Infect 2017; 23(12):900-906. doi:10.1016/j.cmi.2017.04.025
- Heriot GS, Cronin K, Tong SYC, Cheng AC, Liew D. Criteria for identifying patients with Staphylococcus aureus bacteremia who are at low risk of endocarditis: a systematic review. Open Forum Infect Dis 2017; 4(4):ofx261. doi:10.1093/ofid/ofx261
- Mirrakhimov AE, Jesinger ME, Ayach T, Gray A. When does S aureus bacteremia require transesophageal echocardiography? Cleve Clin J Med 2018; 85(7):517–520. doi:10.3949/ccjm.85a.16095
- Young H, Knepper BC, Price CS, Heard S, Jenkins TC. Clinical reasoning of infectious diseases physicians behind the use or nonuse of transesophageal echocardiography in Staphylococcus aureus bacteremia. Open Forum Infect Dis 2016; 3(4):ofw204. doi:10.1093/ofid/ofw204
- Schmitt S, McQuillen DP, Nahass R, et al. Infectious diseases specialty intervention is associated with decreased mortality and lower healthcare costs. Clin Infect Dis 2014; 58(1):22–28. doi:10.1093/cid/cit610
- Bai AD, Showler A, Burry L, et al. Impact of infectious disease consultation on quality of care, mortality, and length of stay in Staphylococcus aureus bacteremia: results from a large multicenter cohort study. Clin Infect Dis. 2015; 60(10):1451–1461. doi:10.1093/cid/civ120
- Buehrle K, Pisano J, Han Z, Pettit NN. Guideline compliance and clinical outcomes among patients with Staphylococcus aureus bacteremia with infectious diseases consultation in addition to antimicrobial stewardship-directed review. Am J Infect Control 2017; 45(7):713–716. doi:10.1016/j.ajic.2017.02.030
- Saunderson RB, Gouliouris T, Nickerson EK, et al. Impact of routine bedside infectious disease consultation on clinical management and outcome of Staphylococcus aureus bacteremia in adults. Clin Microbiol Infect 2015; 21(8):779–785. doi:10.1016/j.cmi.2015.05.026
- Lahey T, Shah R, Gittzus J, Schwartzman J, Kirkland K. Infectious diseases consultation lowers mortality from Staphylococcus aureus bacteremia. Medicine (Baltimore). 2009; 88(5):263–267. doi:10.1097/MD.0b013e3181b8fccb
Morbidity and mortality rates in patients with Staphylococcus aureus bacteremia remain high even though diagnostic tests have improved and antibiotic therapy is effective. Diagnosis and management are made more complex by difficulties in finding the source of bacteremia and sites of metastatic infection.
S aureus bacteremia is a finding that demands further investigation, since up to 25% of people who have it may have endocarditis, a condition with even worse consequences.1 The ability of S aureus to infect normal valves2,3 adds to the challenge. In the mid-20th century, Wilson and Hamburger4 demonstrated that 64% of patients with S aureus bacteremia had evidence of valvular infection at autopsy. In a more recent case series of patients with S aureus endocarditis, the diagnosis was established at autopsy in 32%.5
Specific clinical findings in patients with complicated S aureus bacteremia—those who have a site of infection remote from or extended beyond the primary focus—may be useful in determining the need for additional diagnostic and therapeutic measures.
In a prospective cohort study, Fowler et al6 identified several factors that predicted complicated S aureus bacteremia (including but not limited to endocarditis):
- Prolonged bacteremia (> 48–72 hours after initiation of therapy)
- Community onset
- Fever persisting more than 72 hours
- Skin findings suggesting systemic infection.
THE ROLE OF ECHOCARDIOGRAPHY
Infective endocarditis may be difficult to detect in patients with S aureus bacteremia; experts recommend routine use of echocardiography in this process.7,8 Transesophageal echocardiography (TEE) detects more cases of endocarditis than transthoracic echocardiography (TTE),9,10 but access, cost, and risks lead to questions about its utility.
Guidance for the use of echocardiography in S aureus bacteremia1,10–14 continues to evolve. Consensus seems to be emerging that the risk of endocarditis is lower in patients with S aureus bacteremia who:
- Do not have a prosthetic valve or other permanent intracardiac device
- Have sterile blood cultures within 96 hours after the initial set
- Are not hemodialysis-dependent
- Developed the bacteremia in a healthcare setting
- Have no secondary focus of infection
- Have no clinical signs of infective endocarditis.
Heriot et al14 point out that studies of risk-stratification approaches to echocardiography in patients with S aureus bacteremia are difficult to interpret, as there are questions regarding the validity of the studies and the balance of the risks and benefits.1 The question of timing of TEE remains largely unexplored, both in initial screening and in follow-up of previously undiagnosed cases of S aureus endocarditis.
In this issue of the Journal, Mirrakhimov et al15 weigh in on use of a risk-stratification model to guide use of TEE in patients with S aureus bacteremia. Their comments about avoiding TEE in patients who have an alternative explanation for S aureus bacteremia and a low pretest probability for infectious endocarditis and in patients with a disease focus that requires extended treatment are derived from a survey of infectious disease physicians.16
ROLE OF INFECTIOUS DISEASE CONSULTATION
Infectious disease consultation reduces mortality rates and healthcare costs for a variety of infections, with endocarditis as a prime example.17 For S aureus bacteremia, a large and growing body of literature demonstrates the impact of infectious disease consultation, including improved adherence to guidelines and quality measures,18–20 lower in-hospital mortality rates18–21 and earlier hospital discharge.18 In the era of “curbside consults” and “e-consultation,” it is interesting to note the enduring value of bedside, in-person consultation in the management of S aureus bacteremia.20
Many people with S aureus bacteremia should undergo TEE. Until the evidence becomes more robust, the decision to forgo TEE must be made with caution. The expertise of infectious disease physicians in the diagnosis and management of endocarditis can assist clinicians working with the often-complex patients who develop S aureus bacteremia. If the goal is to improve outcomes, infectious disease consultation may be at least as important as appropriate selection of patients for TEE.
Morbidity and mortality rates in patients with Staphylococcus aureus bacteremia remain high even though diagnostic tests have improved and antibiotic therapy is effective. Diagnosis and management are made more complex by difficulties in finding the source of bacteremia and sites of metastatic infection.
S aureus bacteremia is a finding that demands further investigation, since up to 25% of people who have it may have endocarditis, a condition with even worse consequences.1 The ability of S aureus to infect normal valves2,3 adds to the challenge. In the mid-20th century, Wilson and Hamburger4 demonstrated that 64% of patients with S aureus bacteremia had evidence of valvular infection at autopsy. In a more recent case series of patients with S aureus endocarditis, the diagnosis was established at autopsy in 32%.5
Specific clinical findings in patients with complicated S aureus bacteremia—those who have a site of infection remote from or extended beyond the primary focus—may be useful in determining the need for additional diagnostic and therapeutic measures.
In a prospective cohort study, Fowler et al6 identified several factors that predicted complicated S aureus bacteremia (including but not limited to endocarditis):
- Prolonged bacteremia (> 48–72 hours after initiation of therapy)
- Community onset
- Fever persisting more than 72 hours
- Skin findings suggesting systemic infection.
THE ROLE OF ECHOCARDIOGRAPHY
Infective endocarditis may be difficult to detect in patients with S aureus bacteremia; experts recommend routine use of echocardiography in this process.7,8 Transesophageal echocardiography (TEE) detects more cases of endocarditis than transthoracic echocardiography (TTE),9,10 but access, cost, and risks lead to questions about its utility.
Guidance for the use of echocardiography in S aureus bacteremia1,10–14 continues to evolve. Consensus seems to be emerging that the risk of endocarditis is lower in patients with S aureus bacteremia who:
- Do not have a prosthetic valve or other permanent intracardiac device
- Have sterile blood cultures within 96 hours after the initial set
- Are not hemodialysis-dependent
- Developed the bacteremia in a healthcare setting
- Have no secondary focus of infection
- Have no clinical signs of infective endocarditis.
Heriot et al14 point out that studies of risk-stratification approaches to echocardiography in patients with S aureus bacteremia are difficult to interpret, as there are questions regarding the validity of the studies and the balance of the risks and benefits.1 The question of timing of TEE remains largely unexplored, both in initial screening and in follow-up of previously undiagnosed cases of S aureus endocarditis.
In this issue of the Journal, Mirrakhimov et al15 weigh in on use of a risk-stratification model to guide use of TEE in patients with S aureus bacteremia. Their comments about avoiding TEE in patients who have an alternative explanation for S aureus bacteremia and a low pretest probability for infectious endocarditis and in patients with a disease focus that requires extended treatment are derived from a survey of infectious disease physicians.16
ROLE OF INFECTIOUS DISEASE CONSULTATION
Infectious disease consultation reduces mortality rates and healthcare costs for a variety of infections, with endocarditis as a prime example.17 For S aureus bacteremia, a large and growing body of literature demonstrates the impact of infectious disease consultation, including improved adherence to guidelines and quality measures,18–20 lower in-hospital mortality rates18–21 and earlier hospital discharge.18 In the era of “curbside consults” and “e-consultation,” it is interesting to note the enduring value of bedside, in-person consultation in the management of S aureus bacteremia.20
Many people with S aureus bacteremia should undergo TEE. Until the evidence becomes more robust, the decision to forgo TEE must be made with caution. The expertise of infectious disease physicians in the diagnosis and management of endocarditis can assist clinicians working with the often-complex patients who develop S aureus bacteremia. If the goal is to improve outcomes, infectious disease consultation may be at least as important as appropriate selection of patients for TEE.
- Rasmussen RV, Høst U, Arpi M, et al. Prevalence of infective endocarditis in patients with Staphylococcus aureus bacteraemia: the value of screening with echocardiography. Eur J Echocardiogr 2011; 12(6):414–420. doi:10.1093/ejechocard/jer023
- Vogler, WR, Dorney ER. Bacterial endocarditis in normal heart. Bull Emory Univ Clin 1961; 1:21–31.
- Thayer WS. Bacterial or infective endocarditis. Edinburgh Med J 1931; 38:237–265, 307–334.
- Wilson R, Hamburger M. Fifteen years’ experience with staphylococcus septicemia in large city hospital: analysis of fifty-five cases in Cincinnati General Hospital 1940 to 1954. Am J Med 1957; 22(3):437–457. pmid:13402795
- Røder BL, Wandall DA, Frimodt-Møllar N, Espersen F, Skinhøj P, Rosdahl VT. Clinical features of Staphylococcus aureus endocarditis: a 10-year experience in Denmark. Arch Intern Med 1999; 159(5):462–469. pmid:10074954
- Fowler VG Jr, Olsen MK, Corey GR, et al. Clinical identifiers of complicated Staphylococcus aureus bacteremia. Arch Intern Med 2003; 163(17):2066–2072. doi:10.1001/archinte.163.17.2066
- Baddour LM, Wilson WR, Bayer AS, et al; American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015; 132(15):1435–1486. doi:10.1161/CIR.0000000000000296
- Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis 2011; 52(3):285–292. doi:10.1093/cid/cir034
- Reynolds HR, Jagen MA, Tunick PA, Kronzon I. Sensitivity of transthoracic versus transesophageal echocardiography for the detection of native valve vegetations in the modern era. J Am Soc Echocardiogr 2003; 16(1):67–70. doi:10.1067/mje.2003.43
- Holland TL, Arnold C, Fowler VG Jr. Clinical management of Staphylococcus aureus bacteremia: a review. JAMA 2014; 312(13):1330–1341. doi:10.1001/jama.2014.9743
- Kaasch AJ, Folwler VG Jr, Rieg S, et al. Use of a simple criteria set for guiding echocardiography in nosocomial Staphylococcus aureus bacteremia. Clin Infect Dis 2011; 53(1):1–9. doi:10.1093/cid/cir320
- Palraj BR, Baddour LM, Hess EP, et al. Predicting risk of endocarditis using a clinical tool (PREDICT): scoring system to guide use of echocardiography in the management of Staphylococcus aureus bacteremia. Clin Infect Dis 2015; 61(1):18–28. doi:10.1093/cid/civ235
- Bai AD, Agarawal A, Steinberg M, et al. Clinical predictors and clinical prediction rules to estimate initial patient risk for infective endocarditis in Staphylococcus aureus bacteremia: a systematic review and meta-analysis. Clin Microbiol Infect 2017; 23(12):900-906. doi:10.1016/j.cmi.2017.04.025
- Heriot GS, Cronin K, Tong SYC, Cheng AC, Liew D. Criteria for identifying patients with Staphylococcus aureus bacteremia who are at low risk of endocarditis: a systematic review. Open Forum Infect Dis 2017; 4(4):ofx261. doi:10.1093/ofid/ofx261
- Mirrakhimov AE, Jesinger ME, Ayach T, Gray A. When does S aureus bacteremia require transesophageal echocardiography? Cleve Clin J Med 2018; 85(7):517–520. doi:10.3949/ccjm.85a.16095
- Young H, Knepper BC, Price CS, Heard S, Jenkins TC. Clinical reasoning of infectious diseases physicians behind the use or nonuse of transesophageal echocardiography in Staphylococcus aureus bacteremia. Open Forum Infect Dis 2016; 3(4):ofw204. doi:10.1093/ofid/ofw204
- Schmitt S, McQuillen DP, Nahass R, et al. Infectious diseases specialty intervention is associated with decreased mortality and lower healthcare costs. Clin Infect Dis 2014; 58(1):22–28. doi:10.1093/cid/cit610
- Bai AD, Showler A, Burry L, et al. Impact of infectious disease consultation on quality of care, mortality, and length of stay in Staphylococcus aureus bacteremia: results from a large multicenter cohort study. Clin Infect Dis. 2015; 60(10):1451–1461. doi:10.1093/cid/civ120
- Buehrle K, Pisano J, Han Z, Pettit NN. Guideline compliance and clinical outcomes among patients with Staphylococcus aureus bacteremia with infectious diseases consultation in addition to antimicrobial stewardship-directed review. Am J Infect Control 2017; 45(7):713–716. doi:10.1016/j.ajic.2017.02.030
- Saunderson RB, Gouliouris T, Nickerson EK, et al. Impact of routine bedside infectious disease consultation on clinical management and outcome of Staphylococcus aureus bacteremia in adults. Clin Microbiol Infect 2015; 21(8):779–785. doi:10.1016/j.cmi.2015.05.026
- Lahey T, Shah R, Gittzus J, Schwartzman J, Kirkland K. Infectious diseases consultation lowers mortality from Staphylococcus aureus bacteremia. Medicine (Baltimore). 2009; 88(5):263–267. doi:10.1097/MD.0b013e3181b8fccb
- Rasmussen RV, Høst U, Arpi M, et al. Prevalence of infective endocarditis in patients with Staphylococcus aureus bacteraemia: the value of screening with echocardiography. Eur J Echocardiogr 2011; 12(6):414–420. doi:10.1093/ejechocard/jer023
- Vogler, WR, Dorney ER. Bacterial endocarditis in normal heart. Bull Emory Univ Clin 1961; 1:21–31.
- Thayer WS. Bacterial or infective endocarditis. Edinburgh Med J 1931; 38:237–265, 307–334.
- Wilson R, Hamburger M. Fifteen years’ experience with staphylococcus septicemia in large city hospital: analysis of fifty-five cases in Cincinnati General Hospital 1940 to 1954. Am J Med 1957; 22(3):437–457. pmid:13402795
- Røder BL, Wandall DA, Frimodt-Møllar N, Espersen F, Skinhøj P, Rosdahl VT. Clinical features of Staphylococcus aureus endocarditis: a 10-year experience in Denmark. Arch Intern Med 1999; 159(5):462–469. pmid:10074954
- Fowler VG Jr, Olsen MK, Corey GR, et al. Clinical identifiers of complicated Staphylococcus aureus bacteremia. Arch Intern Med 2003; 163(17):2066–2072. doi:10.1001/archinte.163.17.2066
- Baddour LM, Wilson WR, Bayer AS, et al; American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015; 132(15):1435–1486. doi:10.1161/CIR.0000000000000296
- Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis 2011; 52(3):285–292. doi:10.1093/cid/cir034
- Reynolds HR, Jagen MA, Tunick PA, Kronzon I. Sensitivity of transthoracic versus transesophageal echocardiography for the detection of native valve vegetations in the modern era. J Am Soc Echocardiogr 2003; 16(1):67–70. doi:10.1067/mje.2003.43
- Holland TL, Arnold C, Fowler VG Jr. Clinical management of Staphylococcus aureus bacteremia: a review. JAMA 2014; 312(13):1330–1341. doi:10.1001/jama.2014.9743
- Kaasch AJ, Folwler VG Jr, Rieg S, et al. Use of a simple criteria set for guiding echocardiography in nosocomial Staphylococcus aureus bacteremia. Clin Infect Dis 2011; 53(1):1–9. doi:10.1093/cid/cir320
- Palraj BR, Baddour LM, Hess EP, et al. Predicting risk of endocarditis using a clinical tool (PREDICT): scoring system to guide use of echocardiography in the management of Staphylococcus aureus bacteremia. Clin Infect Dis 2015; 61(1):18–28. doi:10.1093/cid/civ235
- Bai AD, Agarawal A, Steinberg M, et al. Clinical predictors and clinical prediction rules to estimate initial patient risk for infective endocarditis in Staphylococcus aureus bacteremia: a systematic review and meta-analysis. Clin Microbiol Infect 2017; 23(12):900-906. doi:10.1016/j.cmi.2017.04.025
- Heriot GS, Cronin K, Tong SYC, Cheng AC, Liew D. Criteria for identifying patients with Staphylococcus aureus bacteremia who are at low risk of endocarditis: a systematic review. Open Forum Infect Dis 2017; 4(4):ofx261. doi:10.1093/ofid/ofx261
- Mirrakhimov AE, Jesinger ME, Ayach T, Gray A. When does S aureus bacteremia require transesophageal echocardiography? Cleve Clin J Med 2018; 85(7):517–520. doi:10.3949/ccjm.85a.16095
- Young H, Knepper BC, Price CS, Heard S, Jenkins TC. Clinical reasoning of infectious diseases physicians behind the use or nonuse of transesophageal echocardiography in Staphylococcus aureus bacteremia. Open Forum Infect Dis 2016; 3(4):ofw204. doi:10.1093/ofid/ofw204
- Schmitt S, McQuillen DP, Nahass R, et al. Infectious diseases specialty intervention is associated with decreased mortality and lower healthcare costs. Clin Infect Dis 2014; 58(1):22–28. doi:10.1093/cid/cit610
- Bai AD, Showler A, Burry L, et al. Impact of infectious disease consultation on quality of care, mortality, and length of stay in Staphylococcus aureus bacteremia: results from a large multicenter cohort study. Clin Infect Dis. 2015; 60(10):1451–1461. doi:10.1093/cid/civ120
- Buehrle K, Pisano J, Han Z, Pettit NN. Guideline compliance and clinical outcomes among patients with Staphylococcus aureus bacteremia with infectious diseases consultation in addition to antimicrobial stewardship-directed review. Am J Infect Control 2017; 45(7):713–716. doi:10.1016/j.ajic.2017.02.030
- Saunderson RB, Gouliouris T, Nickerson EK, et al. Impact of routine bedside infectious disease consultation on clinical management and outcome of Staphylococcus aureus bacteremia in adults. Clin Microbiol Infect 2015; 21(8):779–785. doi:10.1016/j.cmi.2015.05.026
- Lahey T, Shah R, Gittzus J, Schwartzman J, Kirkland K. Infectious diseases consultation lowers mortality from Staphylococcus aureus bacteremia. Medicine (Baltimore). 2009; 88(5):263–267. doi:10.1097/MD.0b013e3181b8fccb
What should I address at follow-up of patients who survive critical illness?
Patients who survive critical illness such as shock or respiratory failure warranting admission to an intensive care unit (ICU) often develop a constellation of chronic symptoms including cognitive decline, psychiatric disturbances, and physical weakness. These changes can prevent patients from returning to their former level of function and often necessitate significant support for patients and their caregivers.1
With growing awareness of the unique needs of ICU survivors, multidisciplinary PICS clinics have emerged. However, access to these clinics is limited, and most patients discharged from the ICU eventually follow up with their primary care provider. Primary care physicians who recognize PICS, understand its prognosis and its burden on caregivers, and are aware of tools that have shown promise in its management will be well prepared to address the needs of these patients.
COGNITIVE DECLINE
Several studies have shown that survivors of critical illness suffer from long-term impairment of multiple domains of cognition, including executive function. In one study, 40% of ICU survivors had global cognition scores at 1 year after discharge that were worse than those seen in moderate traumatic brain injury, and over 25% had scores similar to those seen in Alzheimer dementia.2 Age had poor correlation with the incidence of long-term cognitive impairment. Cognitive impairment may not be recognized in younger patients without a high index of suspicion and directed cognitive screening. Well-known cognitive impairment screening tests such as the Montreal Cognitive Assessment may help in the evaluation of PICS.
No treatment has been shown to improve long-term cognitive impairment from any cause. The most important intervention is to recognize it and to consider how impaired executive function may interfere with other aspects of treatment, such as participation in physical therapy and adherence to medication regimens.
Evidence is also emerging that patients are often inappropriately discharged on psychoactive medications (including atypical antipsychotic drugs and sedatives) that were started in the inpatient setting.7 These medications increase the risk of accidents, arrhythmia, and infection, as well as add to the overall cost of postdischarge care, and they do not improve the prolonged confusion and cognitive impairment associated with PICS.8 Psychoactive medications should be discontinued once delirium-associated behavior has resolved, as recommended in the American Geriatrics Society guideline on postoperative delirium.9 Further, patients and caregivers should be counseled so that they have reasonable expectations regarding the timing of cognitive recovery, which may be prolonged and incomplete.
PHYSICAL WEAKNESS
Prolonged physical weakness may affect up to one-third of patients who survive critical illness, and it may persist for years, severely compromising quality of life.10 In addition to deconditioning due to bedrest and illness, ICU patients often develop critical illness myopathy and critical illness polyneuropathy.
Although the mechanisms and risk factors for injury to muscles and peripheral nerves are not completely understood, the severity has been well described and ranges from proximal muscle weakness to complete quadriparesis, with inability to wean from mechanical ventilation. There is also an association with the severity of sepsis and the use of glucocorticoids and paralytics.10
Physical weakness can be readily apparent on routine history and physical examination. Differentiating critical illness myopathy from critical illness polyneuropathy requires invasive testing, including electromyography, but the results may not change management in the outpatient setting, making it unnecessary for most patients.
Physical weakness places a heavy burden on patients and their family and caregivers. As a result, most ICU patients suffer loss of employment and require supportive services on discharge, including home health aides and even institutionalization.
Physical therapy and occupational therapy are effective in reducing weakness and improving physical functioning; starting physical therapy in the outpatient setting may be as effective as early intervention in the ICU.11 Given the high prevalence of respiratory and cardiovascular disease in patients after ICU discharge, referral for pulmonary or cardiovascular rehabilitation is recommended. Because of the possible link between glucocorticoids and critical illness myopathy, these drugs should be decreased or discontinued as soon as possible.
PSYCHIATRIC DISTURBANCES
Mental health impairments in ICU survivors are common, severe, debilitating, and unfortunately, commonly overlooked. A recent study found a 37% incidence of depression and a 40% incidence of anxiety; further, 22% of patients met criteria for posttraumatic stress disorder.12 Patients with critical illness are also more likely to have had untreated mental health illness before hospitalization. Anxiety may present with poor sleep, irritability, and fatigue. Posttraumatic stress disorder may manifest as flashbacks or as a severe cognitive or behavioral response to provocation. All of these may be assessed using standard screening questionnaires, including the Posttraumatic Stress Disorder Checklist, the 2-item Patient Health Questionnaire (PHQ-2) for depression, and the 7-item Generalized Anxiety Disorder Screen (GAD-7).
Many primary care physicians are comfortable treating some of the psychiatric disturbances associated with PICS, such as depression, but may be challenged by the spectrum and complexity of mental illness of ICU survivors. Early referral to a mental health professional ensures optimal psychiatric care and allows more time to focus on the patient’s medical comorbidities.
SOCIAL SUPPORT
The cognitive, physical, and mental health complications coupled with other medical and psychiatric comorbidities result in serious social and financial stress on patients and their families. Long-term follow-up studies show that only half of patients return to work within 1 year of critical illness and that nearly one-fourth require continued assistance with activities of daily living.13 Reassuringly, however, most patients in 1 study had returned to work by 2 years from discharge.3
The immense burden on caregivers, the decrease in income, and increased expenditures in providing care result in increased stress on families. The incidence of depression, anxiety, and posttraumatic stress disorder is similar among patients and their caregivers.11 The frequency of emotional morbidity and the severity of the caregiver burden associated with caring for ICU survivors led to the description of a new entity: post-intensive care syndrome-family, or PICS-F.
Because of these stresses, patients often benefit from referral to a social worker. Patients should also be encouraged to bring their caregivers to physician appointments, and family members should be encouraged to discuss their perspectives in the context of a dedicated appointment. Family members should also be screened and treated for their own medical and mental health challenges. A dedicated ICU survivorship clinic may help facilitate this holistic approach and provide complementary services to the primary care provider.
CRITICAL CARE RECOVERY
As survival rates after critical illness continue to improve and clinicians encounter more patients with PICS, it is essential to appreciate the extent of associated physical, emotional, and financial hardship and to recognize when cognitive impairment may interfere with treatment. Early and accurate recognition of these challenges can help the primary care physician arrange and coordinate recovery services that ICU survivors require. Including family members in follow-up appointments can help overcome challenges in adherence to treatment plans, uncover gaps in social support, and identify signs of caregiver distress.
A thorough physical assessment and a thoughtful reconciliation of medications are critical, as is engaging the assistance of physical and occupational therapists, mental health professionals, and social workers.
Risk factors for the illness that necessitated the ICU stay such as uncontrolled diabetes, chronic obstructive pulmonary disease, and substance abuse, as well as medical sequelae such as chronic respiratory failure and heart failure, must be considered and addressed by the primary care physician, with referral to medical specialists if necessary.
Referral to an ICU survivorship center, if locally available, could help the physician manage the patient’s complex and multidisciplinary physical and neuropsychiatric needs. The Society of Critical Care Medicine maintains a resource for survivors and families at www.myicucare.org/thrive/pages/find-in-person-support-groups.aspx.
- Needham DM, Davidson J, Cohen H, et al. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders’ conference. Crit Care Med 2012; 40(2):502–509. doi:10.1097/CCM.0b013e318232da75
- Pandharipande PP, Girard TD, Jackson JC, et al; BRAIN-ICU Study Investigators. Long-term cognitive impairment after critical illness. N Engl J Med 2013; 369(14):1306–1316. doi:10.1056/NEJMoa1301372
- Herridge MS, Tansey CM, Matte A, et al; Canadian Critical Care Trials Group. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med 2011; 364(14):1293–1304. doi:10.1056/NEJMoa1011802
- Rothenhäusler H-B, Ehrentraut S, Stoll C, Schelling G, Kapfhammer H-P. The relationship between cognitive performance and employment and health status in long-term survivors of the acute respiratory distress syndrome: results of an exploratory study. Gen Hosp Psychiatry 2001; 23(2):90–96. pmid:11313077
- Nikayin S, Rabiee A, Hashem MD, et al. Anxiety symptoms in survivors of critical illness: a systematic review and meta-analysis. Gen Hosp Psychiatry 2016; 43:23–29. doi:10.1016/j.genhosppsych.2016.08.005
- Jackson JC, Pandharipande PP, Girard TD, et al; Bringing to light the Risk Factors And Incidence of Neuropsychological dysfunction in ICU survivors (BRAIN-ICU) study investigators. Depression, post-traumatic stress disorder, and functional disability in survivors of critical illness in the BRAIN-ICU study: a longitudinal cohort study. Lancet Respir Med 2014; 2(5):369–379. doi:10.1016/S2213-2600(14)70051-7
- Morandi A, Vasilevskis E, Pandharipande PP, et al. Inappropriate medication prescriptions in elderly adults surviving an intensive care unit hospitalization. J Am Geriatr Soc 2013; 61(7):1128–1134. doi:10.1111/jgs.12329
- Johnson KG, Fashoyin A, Madden-Fuentes R, Muzyk AJ, Gagliardi JP, Yanamadala M. Discharge plans for geriatric inpatients with delirium: a plan to stop antipsychotics? J Am Geriatr Soc 2017; 65(10):2278–2281. doi:10.1111/jgs.15026
- American Geriatrics Society Expert Panel on Postoperative Delirium in Older Adults. American Geriatrics Society abstracted clinical practice guideline for postoperative delirium in older adults. J Am Geriatr Soc 2015; 63(1):142–150. doi:10.1111/jgs.13281
- Hermans G, Van den Berghe G. Clinical review: intensive care unit acquired weakness. Crit Care 2015; 19:274. doi:10.1186/s13054-015-0993-7
- Calvo-Ayala E, Khan BA, Farber MO, Ely EW, Boustani MA. Interventions to improve the physical function of ICU survivors: a systematic review. Chest 2013; 144(5):1469–1480. doi:10.1378/chest.13-0779
- Wang S, Allen D, Kheir YN, Campbell N, Khan B. Aging and post-intensive care syndrome: a critical need for geriatric psychiatry. Am J Geriatr Psychiatry 2018; 26(2):212–221. doi:10.1016/j.jagp.2017.05.016
- Myhren H, Ekeberg O, Stokland O. Health-related quality of life and return to work after critical illness in general intensive care unit patients: a 1-year follow-up study. Crit Care Med 2010; 38(7):1554–1561. doi:10.1097/CCM.0b013e3181e2c8b1
- van Beusekom I, Bakhshi-Raiez F, de Keizer NF, Dongelmans DA, van der Schaaf M. Reported burden on informal caregivers of ICU survivors: a literature review. Crit Care 2016; 20:16. doi:10.1186/s13054-016-1185-9
Patients who survive critical illness such as shock or respiratory failure warranting admission to an intensive care unit (ICU) often develop a constellation of chronic symptoms including cognitive decline, psychiatric disturbances, and physical weakness. These changes can prevent patients from returning to their former level of function and often necessitate significant support for patients and their caregivers.1
With growing awareness of the unique needs of ICU survivors, multidisciplinary PICS clinics have emerged. However, access to these clinics is limited, and most patients discharged from the ICU eventually follow up with their primary care provider. Primary care physicians who recognize PICS, understand its prognosis and its burden on caregivers, and are aware of tools that have shown promise in its management will be well prepared to address the needs of these patients.
COGNITIVE DECLINE
Several studies have shown that survivors of critical illness suffer from long-term impairment of multiple domains of cognition, including executive function. In one study, 40% of ICU survivors had global cognition scores at 1 year after discharge that were worse than those seen in moderate traumatic brain injury, and over 25% had scores similar to those seen in Alzheimer dementia.2 Age had poor correlation with the incidence of long-term cognitive impairment. Cognitive impairment may not be recognized in younger patients without a high index of suspicion and directed cognitive screening. Well-known cognitive impairment screening tests such as the Montreal Cognitive Assessment may help in the evaluation of PICS.
No treatment has been shown to improve long-term cognitive impairment from any cause. The most important intervention is to recognize it and to consider how impaired executive function may interfere with other aspects of treatment, such as participation in physical therapy and adherence to medication regimens.
Evidence is also emerging that patients are often inappropriately discharged on psychoactive medications (including atypical antipsychotic drugs and sedatives) that were started in the inpatient setting.7 These medications increase the risk of accidents, arrhythmia, and infection, as well as add to the overall cost of postdischarge care, and they do not improve the prolonged confusion and cognitive impairment associated with PICS.8 Psychoactive medications should be discontinued once delirium-associated behavior has resolved, as recommended in the American Geriatrics Society guideline on postoperative delirium.9 Further, patients and caregivers should be counseled so that they have reasonable expectations regarding the timing of cognitive recovery, which may be prolonged and incomplete.
PHYSICAL WEAKNESS
Prolonged physical weakness may affect up to one-third of patients who survive critical illness, and it may persist for years, severely compromising quality of life.10 In addition to deconditioning due to bedrest and illness, ICU patients often develop critical illness myopathy and critical illness polyneuropathy.
Although the mechanisms and risk factors for injury to muscles and peripheral nerves are not completely understood, the severity has been well described and ranges from proximal muscle weakness to complete quadriparesis, with inability to wean from mechanical ventilation. There is also an association with the severity of sepsis and the use of glucocorticoids and paralytics.10
Physical weakness can be readily apparent on routine history and physical examination. Differentiating critical illness myopathy from critical illness polyneuropathy requires invasive testing, including electromyography, but the results may not change management in the outpatient setting, making it unnecessary for most patients.
Physical weakness places a heavy burden on patients and their family and caregivers. As a result, most ICU patients suffer loss of employment and require supportive services on discharge, including home health aides and even institutionalization.
Physical therapy and occupational therapy are effective in reducing weakness and improving physical functioning; starting physical therapy in the outpatient setting may be as effective as early intervention in the ICU.11 Given the high prevalence of respiratory and cardiovascular disease in patients after ICU discharge, referral for pulmonary or cardiovascular rehabilitation is recommended. Because of the possible link between glucocorticoids and critical illness myopathy, these drugs should be decreased or discontinued as soon as possible.
PSYCHIATRIC DISTURBANCES
Mental health impairments in ICU survivors are common, severe, debilitating, and unfortunately, commonly overlooked. A recent study found a 37% incidence of depression and a 40% incidence of anxiety; further, 22% of patients met criteria for posttraumatic stress disorder.12 Patients with critical illness are also more likely to have had untreated mental health illness before hospitalization. Anxiety may present with poor sleep, irritability, and fatigue. Posttraumatic stress disorder may manifest as flashbacks or as a severe cognitive or behavioral response to provocation. All of these may be assessed using standard screening questionnaires, including the Posttraumatic Stress Disorder Checklist, the 2-item Patient Health Questionnaire (PHQ-2) for depression, and the 7-item Generalized Anxiety Disorder Screen (GAD-7).
Many primary care physicians are comfortable treating some of the psychiatric disturbances associated with PICS, such as depression, but may be challenged by the spectrum and complexity of mental illness of ICU survivors. Early referral to a mental health professional ensures optimal psychiatric care and allows more time to focus on the patient’s medical comorbidities.
SOCIAL SUPPORT
The cognitive, physical, and mental health complications coupled with other medical and psychiatric comorbidities result in serious social and financial stress on patients and their families. Long-term follow-up studies show that only half of patients return to work within 1 year of critical illness and that nearly one-fourth require continued assistance with activities of daily living.13 Reassuringly, however, most patients in 1 study had returned to work by 2 years from discharge.3
The immense burden on caregivers, the decrease in income, and increased expenditures in providing care result in increased stress on families. The incidence of depression, anxiety, and posttraumatic stress disorder is similar among patients and their caregivers.11 The frequency of emotional morbidity and the severity of the caregiver burden associated with caring for ICU survivors led to the description of a new entity: post-intensive care syndrome-family, or PICS-F.
Because of these stresses, patients often benefit from referral to a social worker. Patients should also be encouraged to bring their caregivers to physician appointments, and family members should be encouraged to discuss their perspectives in the context of a dedicated appointment. Family members should also be screened and treated for their own medical and mental health challenges. A dedicated ICU survivorship clinic may help facilitate this holistic approach and provide complementary services to the primary care provider.
CRITICAL CARE RECOVERY
As survival rates after critical illness continue to improve and clinicians encounter more patients with PICS, it is essential to appreciate the extent of associated physical, emotional, and financial hardship and to recognize when cognitive impairment may interfere with treatment. Early and accurate recognition of these challenges can help the primary care physician arrange and coordinate recovery services that ICU survivors require. Including family members in follow-up appointments can help overcome challenges in adherence to treatment plans, uncover gaps in social support, and identify signs of caregiver distress.
A thorough physical assessment and a thoughtful reconciliation of medications are critical, as is engaging the assistance of physical and occupational therapists, mental health professionals, and social workers.
Risk factors for the illness that necessitated the ICU stay such as uncontrolled diabetes, chronic obstructive pulmonary disease, and substance abuse, as well as medical sequelae such as chronic respiratory failure and heart failure, must be considered and addressed by the primary care physician, with referral to medical specialists if necessary.
Referral to an ICU survivorship center, if locally available, could help the physician manage the patient’s complex and multidisciplinary physical and neuropsychiatric needs. The Society of Critical Care Medicine maintains a resource for survivors and families at www.myicucare.org/thrive/pages/find-in-person-support-groups.aspx.
Patients who survive critical illness such as shock or respiratory failure warranting admission to an intensive care unit (ICU) often develop a constellation of chronic symptoms including cognitive decline, psychiatric disturbances, and physical weakness. These changes can prevent patients from returning to their former level of function and often necessitate significant support for patients and their caregivers.1
With growing awareness of the unique needs of ICU survivors, multidisciplinary PICS clinics have emerged. However, access to these clinics is limited, and most patients discharged from the ICU eventually follow up with their primary care provider. Primary care physicians who recognize PICS, understand its prognosis and its burden on caregivers, and are aware of tools that have shown promise in its management will be well prepared to address the needs of these patients.
COGNITIVE DECLINE
Several studies have shown that survivors of critical illness suffer from long-term impairment of multiple domains of cognition, including executive function. In one study, 40% of ICU survivors had global cognition scores at 1 year after discharge that were worse than those seen in moderate traumatic brain injury, and over 25% had scores similar to those seen in Alzheimer dementia.2 Age had poor correlation with the incidence of long-term cognitive impairment. Cognitive impairment may not be recognized in younger patients without a high index of suspicion and directed cognitive screening. Well-known cognitive impairment screening tests such as the Montreal Cognitive Assessment may help in the evaluation of PICS.
No treatment has been shown to improve long-term cognitive impairment from any cause. The most important intervention is to recognize it and to consider how impaired executive function may interfere with other aspects of treatment, such as participation in physical therapy and adherence to medication regimens.
Evidence is also emerging that patients are often inappropriately discharged on psychoactive medications (including atypical antipsychotic drugs and sedatives) that were started in the inpatient setting.7 These medications increase the risk of accidents, arrhythmia, and infection, as well as add to the overall cost of postdischarge care, and they do not improve the prolonged confusion and cognitive impairment associated with PICS.8 Psychoactive medications should be discontinued once delirium-associated behavior has resolved, as recommended in the American Geriatrics Society guideline on postoperative delirium.9 Further, patients and caregivers should be counseled so that they have reasonable expectations regarding the timing of cognitive recovery, which may be prolonged and incomplete.
PHYSICAL WEAKNESS
Prolonged physical weakness may affect up to one-third of patients who survive critical illness, and it may persist for years, severely compromising quality of life.10 In addition to deconditioning due to bedrest and illness, ICU patients often develop critical illness myopathy and critical illness polyneuropathy.
Although the mechanisms and risk factors for injury to muscles and peripheral nerves are not completely understood, the severity has been well described and ranges from proximal muscle weakness to complete quadriparesis, with inability to wean from mechanical ventilation. There is also an association with the severity of sepsis and the use of glucocorticoids and paralytics.10
Physical weakness can be readily apparent on routine history and physical examination. Differentiating critical illness myopathy from critical illness polyneuropathy requires invasive testing, including electromyography, but the results may not change management in the outpatient setting, making it unnecessary for most patients.
Physical weakness places a heavy burden on patients and their family and caregivers. As a result, most ICU patients suffer loss of employment and require supportive services on discharge, including home health aides and even institutionalization.
Physical therapy and occupational therapy are effective in reducing weakness and improving physical functioning; starting physical therapy in the outpatient setting may be as effective as early intervention in the ICU.11 Given the high prevalence of respiratory and cardiovascular disease in patients after ICU discharge, referral for pulmonary or cardiovascular rehabilitation is recommended. Because of the possible link between glucocorticoids and critical illness myopathy, these drugs should be decreased or discontinued as soon as possible.
PSYCHIATRIC DISTURBANCES
Mental health impairments in ICU survivors are common, severe, debilitating, and unfortunately, commonly overlooked. A recent study found a 37% incidence of depression and a 40% incidence of anxiety; further, 22% of patients met criteria for posttraumatic stress disorder.12 Patients with critical illness are also more likely to have had untreated mental health illness before hospitalization. Anxiety may present with poor sleep, irritability, and fatigue. Posttraumatic stress disorder may manifest as flashbacks or as a severe cognitive or behavioral response to provocation. All of these may be assessed using standard screening questionnaires, including the Posttraumatic Stress Disorder Checklist, the 2-item Patient Health Questionnaire (PHQ-2) for depression, and the 7-item Generalized Anxiety Disorder Screen (GAD-7).
Many primary care physicians are comfortable treating some of the psychiatric disturbances associated with PICS, such as depression, but may be challenged by the spectrum and complexity of mental illness of ICU survivors. Early referral to a mental health professional ensures optimal psychiatric care and allows more time to focus on the patient’s medical comorbidities.
SOCIAL SUPPORT
The cognitive, physical, and mental health complications coupled with other medical and psychiatric comorbidities result in serious social and financial stress on patients and their families. Long-term follow-up studies show that only half of patients return to work within 1 year of critical illness and that nearly one-fourth require continued assistance with activities of daily living.13 Reassuringly, however, most patients in 1 study had returned to work by 2 years from discharge.3
The immense burden on caregivers, the decrease in income, and increased expenditures in providing care result in increased stress on families. The incidence of depression, anxiety, and posttraumatic stress disorder is similar among patients and their caregivers.11 The frequency of emotional morbidity and the severity of the caregiver burden associated with caring for ICU survivors led to the description of a new entity: post-intensive care syndrome-family, or PICS-F.
Because of these stresses, patients often benefit from referral to a social worker. Patients should also be encouraged to bring their caregivers to physician appointments, and family members should be encouraged to discuss their perspectives in the context of a dedicated appointment. Family members should also be screened and treated for their own medical and mental health challenges. A dedicated ICU survivorship clinic may help facilitate this holistic approach and provide complementary services to the primary care provider.
CRITICAL CARE RECOVERY
As survival rates after critical illness continue to improve and clinicians encounter more patients with PICS, it is essential to appreciate the extent of associated physical, emotional, and financial hardship and to recognize when cognitive impairment may interfere with treatment. Early and accurate recognition of these challenges can help the primary care physician arrange and coordinate recovery services that ICU survivors require. Including family members in follow-up appointments can help overcome challenges in adherence to treatment plans, uncover gaps in social support, and identify signs of caregiver distress.
A thorough physical assessment and a thoughtful reconciliation of medications are critical, as is engaging the assistance of physical and occupational therapists, mental health professionals, and social workers.
Risk factors for the illness that necessitated the ICU stay such as uncontrolled diabetes, chronic obstructive pulmonary disease, and substance abuse, as well as medical sequelae such as chronic respiratory failure and heart failure, must be considered and addressed by the primary care physician, with referral to medical specialists if necessary.
Referral to an ICU survivorship center, if locally available, could help the physician manage the patient’s complex and multidisciplinary physical and neuropsychiatric needs. The Society of Critical Care Medicine maintains a resource for survivors and families at www.myicucare.org/thrive/pages/find-in-person-support-groups.aspx.
- Needham DM, Davidson J, Cohen H, et al. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders’ conference. Crit Care Med 2012; 40(2):502–509. doi:10.1097/CCM.0b013e318232da75
- Pandharipande PP, Girard TD, Jackson JC, et al; BRAIN-ICU Study Investigators. Long-term cognitive impairment after critical illness. N Engl J Med 2013; 369(14):1306–1316. doi:10.1056/NEJMoa1301372
- Herridge MS, Tansey CM, Matte A, et al; Canadian Critical Care Trials Group. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med 2011; 364(14):1293–1304. doi:10.1056/NEJMoa1011802
- Rothenhäusler H-B, Ehrentraut S, Stoll C, Schelling G, Kapfhammer H-P. The relationship between cognitive performance and employment and health status in long-term survivors of the acute respiratory distress syndrome: results of an exploratory study. Gen Hosp Psychiatry 2001; 23(2):90–96. pmid:11313077
- Nikayin S, Rabiee A, Hashem MD, et al. Anxiety symptoms in survivors of critical illness: a systematic review and meta-analysis. Gen Hosp Psychiatry 2016; 43:23–29. doi:10.1016/j.genhosppsych.2016.08.005
- Jackson JC, Pandharipande PP, Girard TD, et al; Bringing to light the Risk Factors And Incidence of Neuropsychological dysfunction in ICU survivors (BRAIN-ICU) study investigators. Depression, post-traumatic stress disorder, and functional disability in survivors of critical illness in the BRAIN-ICU study: a longitudinal cohort study. Lancet Respir Med 2014; 2(5):369–379. doi:10.1016/S2213-2600(14)70051-7
- Morandi A, Vasilevskis E, Pandharipande PP, et al. Inappropriate medication prescriptions in elderly adults surviving an intensive care unit hospitalization. J Am Geriatr Soc 2013; 61(7):1128–1134. doi:10.1111/jgs.12329
- Johnson KG, Fashoyin A, Madden-Fuentes R, Muzyk AJ, Gagliardi JP, Yanamadala M. Discharge plans for geriatric inpatients with delirium: a plan to stop antipsychotics? J Am Geriatr Soc 2017; 65(10):2278–2281. doi:10.1111/jgs.15026
- American Geriatrics Society Expert Panel on Postoperative Delirium in Older Adults. American Geriatrics Society abstracted clinical practice guideline for postoperative delirium in older adults. J Am Geriatr Soc 2015; 63(1):142–150. doi:10.1111/jgs.13281
- Hermans G, Van den Berghe G. Clinical review: intensive care unit acquired weakness. Crit Care 2015; 19:274. doi:10.1186/s13054-015-0993-7
- Calvo-Ayala E, Khan BA, Farber MO, Ely EW, Boustani MA. Interventions to improve the physical function of ICU survivors: a systematic review. Chest 2013; 144(5):1469–1480. doi:10.1378/chest.13-0779
- Wang S, Allen D, Kheir YN, Campbell N, Khan B. Aging and post-intensive care syndrome: a critical need for geriatric psychiatry. Am J Geriatr Psychiatry 2018; 26(2):212–221. doi:10.1016/j.jagp.2017.05.016
- Myhren H, Ekeberg O, Stokland O. Health-related quality of life and return to work after critical illness in general intensive care unit patients: a 1-year follow-up study. Crit Care Med 2010; 38(7):1554–1561. doi:10.1097/CCM.0b013e3181e2c8b1
- van Beusekom I, Bakhshi-Raiez F, de Keizer NF, Dongelmans DA, van der Schaaf M. Reported burden on informal caregivers of ICU survivors: a literature review. Crit Care 2016; 20:16. doi:10.1186/s13054-016-1185-9
- Needham DM, Davidson J, Cohen H, et al. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders’ conference. Crit Care Med 2012; 40(2):502–509. doi:10.1097/CCM.0b013e318232da75
- Pandharipande PP, Girard TD, Jackson JC, et al; BRAIN-ICU Study Investigators. Long-term cognitive impairment after critical illness. N Engl J Med 2013; 369(14):1306–1316. doi:10.1056/NEJMoa1301372
- Herridge MS, Tansey CM, Matte A, et al; Canadian Critical Care Trials Group. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med 2011; 364(14):1293–1304. doi:10.1056/NEJMoa1011802
- Rothenhäusler H-B, Ehrentraut S, Stoll C, Schelling G, Kapfhammer H-P. The relationship between cognitive performance and employment and health status in long-term survivors of the acute respiratory distress syndrome: results of an exploratory study. Gen Hosp Psychiatry 2001; 23(2):90–96. pmid:11313077
- Nikayin S, Rabiee A, Hashem MD, et al. Anxiety symptoms in survivors of critical illness: a systematic review and meta-analysis. Gen Hosp Psychiatry 2016; 43:23–29. doi:10.1016/j.genhosppsych.2016.08.005
- Jackson JC, Pandharipande PP, Girard TD, et al; Bringing to light the Risk Factors And Incidence of Neuropsychological dysfunction in ICU survivors (BRAIN-ICU) study investigators. Depression, post-traumatic stress disorder, and functional disability in survivors of critical illness in the BRAIN-ICU study: a longitudinal cohort study. Lancet Respir Med 2014; 2(5):369–379. doi:10.1016/S2213-2600(14)70051-7
- Morandi A, Vasilevskis E, Pandharipande PP, et al. Inappropriate medication prescriptions in elderly adults surviving an intensive care unit hospitalization. J Am Geriatr Soc 2013; 61(7):1128–1134. doi:10.1111/jgs.12329
- Johnson KG, Fashoyin A, Madden-Fuentes R, Muzyk AJ, Gagliardi JP, Yanamadala M. Discharge plans for geriatric inpatients with delirium: a plan to stop antipsychotics? J Am Geriatr Soc 2017; 65(10):2278–2281. doi:10.1111/jgs.15026
- American Geriatrics Society Expert Panel on Postoperative Delirium in Older Adults. American Geriatrics Society abstracted clinical practice guideline for postoperative delirium in older adults. J Am Geriatr Soc 2015; 63(1):142–150. doi:10.1111/jgs.13281
- Hermans G, Van den Berghe G. Clinical review: intensive care unit acquired weakness. Crit Care 2015; 19:274. doi:10.1186/s13054-015-0993-7
- Calvo-Ayala E, Khan BA, Farber MO, Ely EW, Boustani MA. Interventions to improve the physical function of ICU survivors: a systematic review. Chest 2013; 144(5):1469–1480. doi:10.1378/chest.13-0779
- Wang S, Allen D, Kheir YN, Campbell N, Khan B. Aging and post-intensive care syndrome: a critical need for geriatric psychiatry. Am J Geriatr Psychiatry 2018; 26(2):212–221. doi:10.1016/j.jagp.2017.05.016
- Myhren H, Ekeberg O, Stokland O. Health-related quality of life and return to work after critical illness in general intensive care unit patients: a 1-year follow-up study. Crit Care Med 2010; 38(7):1554–1561. doi:10.1097/CCM.0b013e3181e2c8b1
- van Beusekom I, Bakhshi-Raiez F, de Keizer NF, Dongelmans DA, van der Schaaf M. Reported burden on informal caregivers of ICU survivors: a literature review. Crit Care 2016; 20:16. doi:10.1186/s13054-016-1185-9