User login
Rare Cancer Misdiagnosed As Orchitis
A 70-year-old man underwent salvage therapy for multiple myeloma (MM). While on maintenance immunotherapy he developed a sternal plasmacytoma. After the fifth cycle of treatment, he developed swelling, erythema, and pain in his right testis.
The main differential diagnoses for those symptoms are infections and tumors; infection is more common, so his clinicians at Indiana University School of Medicine presumed orchitis and started him on IV antibiotics. The pain resolved, but the swelling persisted after the antibiotic course. The clinicians turned to biochemical marker screening for germ cell tumors, but those were negative. Serial ultrasound imaging, which they had begun during his admission, remained unchanged.
Meanwhile, the patient’s chemotherapy was being held back, and he developed another sternal mass, prompting a fluorodeoxyglucose-positron emission tomography–computed tomography (PET/CT) scan to evaluate for relapse of myeloma. The scan revealed an enlarged, diffusely hypermetabolic right testicle. Believing the symptoms were related to the myeloma and not orchitis, the clinicians advised a radical orchiectomy.
A biopsy after the surgery showed tumor cells consistent with testicular plasmacytoma.
While rare, testicular plasmacytoma is commonly associated with MM, especially in the later stages, when cancer cells are more aggressive and not relying on bone marrow for survival, the clinicians say. Unlike myeloma, which typically spreads via blood to bone sites, testicular plasmacytoma may spread via lymphatic channels to the regional lymph nodes and subsequently to distant sites, the clinicians add, similarly to lymphoma or germ cell tumor.
It is hard to diagnose, though. The clinicians say the patient’s case illustrates the challenges. Imaging studies such as ultrasound and CT scans are not specific. And although FDG-PET/CT imaging is a standard staging tool for myeloma and helpful in identifying plasmacytoma when evidenced as intramedullary or extramedullary hypermetabolic lesions, hypermetabolic lesions are not always malignant, they note. FDG-PET/CT can’t differentiate between orchitis and testicular plasmacytoma. Biopsy remains the diagnostic gold standard.
Source:
Schiavo C, Mann SA, Mer J, Suvannasankha A. BMJ Case Rep. 2018;pii:bcr-2017-222046.
doi: 10.1136/bcr-2017-222046.
A 70-year-old man underwent salvage therapy for multiple myeloma (MM). While on maintenance immunotherapy he developed a sternal plasmacytoma. After the fifth cycle of treatment, he developed swelling, erythema, and pain in his right testis.
The main differential diagnoses for those symptoms are infections and tumors; infection is more common, so his clinicians at Indiana University School of Medicine presumed orchitis and started him on IV antibiotics. The pain resolved, but the swelling persisted after the antibiotic course. The clinicians turned to biochemical marker screening for germ cell tumors, but those were negative. Serial ultrasound imaging, which they had begun during his admission, remained unchanged.
Meanwhile, the patient’s chemotherapy was being held back, and he developed another sternal mass, prompting a fluorodeoxyglucose-positron emission tomography–computed tomography (PET/CT) scan to evaluate for relapse of myeloma. The scan revealed an enlarged, diffusely hypermetabolic right testicle. Believing the symptoms were related to the myeloma and not orchitis, the clinicians advised a radical orchiectomy.
A biopsy after the surgery showed tumor cells consistent with testicular plasmacytoma.
While rare, testicular plasmacytoma is commonly associated with MM, especially in the later stages, when cancer cells are more aggressive and not relying on bone marrow for survival, the clinicians say. Unlike myeloma, which typically spreads via blood to bone sites, testicular plasmacytoma may spread via lymphatic channels to the regional lymph nodes and subsequently to distant sites, the clinicians add, similarly to lymphoma or germ cell tumor.
It is hard to diagnose, though. The clinicians say the patient’s case illustrates the challenges. Imaging studies such as ultrasound and CT scans are not specific. And although FDG-PET/CT imaging is a standard staging tool for myeloma and helpful in identifying plasmacytoma when evidenced as intramedullary or extramedullary hypermetabolic lesions, hypermetabolic lesions are not always malignant, they note. FDG-PET/CT can’t differentiate between orchitis and testicular plasmacytoma. Biopsy remains the diagnostic gold standard.
Source:
Schiavo C, Mann SA, Mer J, Suvannasankha A. BMJ Case Rep. 2018;pii:bcr-2017-222046.
doi: 10.1136/bcr-2017-222046.
A 70-year-old man underwent salvage therapy for multiple myeloma (MM). While on maintenance immunotherapy he developed a sternal plasmacytoma. After the fifth cycle of treatment, he developed swelling, erythema, and pain in his right testis.
The main differential diagnoses for those symptoms are infections and tumors; infection is more common, so his clinicians at Indiana University School of Medicine presumed orchitis and started him on IV antibiotics. The pain resolved, but the swelling persisted after the antibiotic course. The clinicians turned to biochemical marker screening for germ cell tumors, but those were negative. Serial ultrasound imaging, which they had begun during his admission, remained unchanged.
Meanwhile, the patient’s chemotherapy was being held back, and he developed another sternal mass, prompting a fluorodeoxyglucose-positron emission tomography–computed tomography (PET/CT) scan to evaluate for relapse of myeloma. The scan revealed an enlarged, diffusely hypermetabolic right testicle. Believing the symptoms were related to the myeloma and not orchitis, the clinicians advised a radical orchiectomy.
A biopsy after the surgery showed tumor cells consistent with testicular plasmacytoma.
While rare, testicular plasmacytoma is commonly associated with MM, especially in the later stages, when cancer cells are more aggressive and not relying on bone marrow for survival, the clinicians say. Unlike myeloma, which typically spreads via blood to bone sites, testicular plasmacytoma may spread via lymphatic channels to the regional lymph nodes and subsequently to distant sites, the clinicians add, similarly to lymphoma or germ cell tumor.
It is hard to diagnose, though. The clinicians say the patient’s case illustrates the challenges. Imaging studies such as ultrasound and CT scans are not specific. And although FDG-PET/CT imaging is a standard staging tool for myeloma and helpful in identifying plasmacytoma when evidenced as intramedullary or extramedullary hypermetabolic lesions, hypermetabolic lesions are not always malignant, they note. FDG-PET/CT can’t differentiate between orchitis and testicular plasmacytoma. Biopsy remains the diagnostic gold standard.
Source:
Schiavo C, Mann SA, Mer J, Suvannasankha A. BMJ Case Rep. 2018;pii:bcr-2017-222046.
doi: 10.1136/bcr-2017-222046.
‘Very encouraging’ results in BPDCN
STOCKHOLM—Tagraxofusp (SL-401) has produced “very encouraging” results in a phase 2 trial of patients with blastic plasmacytoid dendritic cell neoplasm (BPDCN), according to an investigator.
Tagraxofusp, a targeted therapy directed to CD123, produced an overall response rate (ORR) of 83% and a complete response (CR) rate of 62% in patients with previously untreated or relapsed/refractory BPDCN.
Common adverse events (AEs) related to tagraxofusp include hypoalbuminemia, transaminitis, and thrombocytopenia. There was 1 grade 5 AE—a case of capillary leak syndrome (CLS).
Study investigator Naveen Pemmaraju, MD, of The University of Texas MD Anderson Cancer Center in Houston, presented these results at the 23rd Congress of the European Hematology Association (EHA) as abstract S116.
The trial was sponsored by Stemline Therapeutics.
Dr Pemmaraju noted that there are no approved therapies for BPDCN, so patients may be treated with therapies intended for acute myeloid leukemia (AML), acute lymphoblastic leukemia, or lymphomas.
“These are usually quite intense cytotoxic chemotherapy regimens,” he said. “But even with these regimens, most groups report median overall survival times of 8 to 14 months.”
And although stem cell transplants can be effective in BPDCN, a “vast majority” of patients are not fit for transplant, according to Dr Pemmaraju.
With this in mind, he and his colleagues are conducting this trial of tagraxofusp in BPDCN.
The trial has 4 stages. In stage 1, patients received tagraxofusp at 7, 9, 12, or 16 μg/kg on days 1 to 5 of a 21-day cycle. In stages 2 and 3, patients received the drug at 12 μg/kg on days 1 to 5 of a 21-day cycle. Stage 4 is still enrolling.
Efficacy
Dr Pemmaraju presented results in 45 patients—32 with previously untreated BPDCN and 13 with relapsed/refractory BPDCN. The patients’ median age at baseline was 70 (range, 22-84), and 82% were male.
Three patients received tagraxofusp at 7 μg/kg/day, and the rest received the 12 μg/kg/day dose.
Among patients who received the 12 μg/kg/day dose, the ORR was 83% (35/42). The ORR was 90% (26/29) in previously untreated patients and 69% (9/13) in relapsed/refractory patients.
“These are very encouraging results—a 90% overall response rate in the frontline setting,” Dr Pemmaraju noted.
The composite CR rate was 62% (n=26) overall, 72% (n=21) in previously untreated patients, and 38% (n=5) in relapsed/refractory patients.
This included 13 patients with a CR (1 relapsed/refractory), 10 with a clinical CR (3 relapsed/refractory), and 3 with a CR with incomplete hematologic recovery (1 relapsed/refractory). A clinical CR was defined as absence of gross disease with minimal residual skin abnormality.
Fourteen patients went on to stem cell transplant, 1 of whom had relapsed/refractory disease at baseline.
Overall survival results were only available for the 29 previously untreated patients who received tagraxofusp at 12 μg/kg/day. In this group, the median overall survival has not been reached at a median follow-up of 13.8 months (range, 0.2 to 37.4 months).
Dr Pemmaraju said this result is important because it contrasts with the historical expectation of a median overall survival of 8 to 14 months.
Safety
Dr Pemmaraju presented safety results in 114 patients who have received tagraxofusp at 12 μg/kg/day on all trials of the drug. These data include patients with AML, myelofibrosis, and chronic myelomonocytic leukemia in addition to the 45 patients with BPDCN. However, AEs were similar regardless of disease.
Common treatment-related AEs (of any grade, occurring in at least 15% of patients) included hypoalbuminemia (49%), ALT increase (48%), AST increase (48%), thrombocytopenia (29%), nausea (27%), pyrexia (25%), chills (23%), fatigue (23%), weight increase (19%), hypotension (18%), peripheral edema (17%), and vomiting (15%).
CLS of any grade was also a common AE, occurring in 20% of patients (n=23). Most cases of CLS were grade 1 or 2, but there were grade 3 (n=5) and 4 (n=2) cases, as well as a single case of grade 5 CLS that occurred in a BPDCN patient.
Dr Pemmaraju did note that CLS has proven manageable with monitoring and pre-emptive measures. Specifically, inclusion criteria were changed so that patients must have normal cardiac function, adequate kidney function, and albumin of at least 3.2 g/dl. Investigators also began monitoring patients’ weight, albumin levels, and kidney function.
“With the combination of greater understanding of CLS, actual definitive protocol adjustments made by investigators, and monitoring, this has been a highly manageable phenomenon,” Dr Pemmaraju said.
Next steps
The investigators plan to continue enrolling patients in this study and collect additional safety and survival data, but Dr Pemmaraju and his colleagues also want to evaluate tagraxofusp in combination with other therapies.
Tagraxofusp is already under investigation in combination with azacitidine in a phase 1/2 trial of patients with high-risk myelodysplastic syndromes and AML.
Dr Pemmaraju is interested in combining hypomethylating agents with tagraxofusp for BPDCN patients as well, to build upon the encouraging results with tagraxofusp alone.
“An extraordinarily rare disease that used to not have any therapies at all now has at least one ongoing clinical trial with some encouraging activity,” he said. “I hope that gives hope to people with rare diseases, to let them know they’re not alone. There may be someone out there who’s researching their disease, no matter how rare it is.”
STOCKHOLM—Tagraxofusp (SL-401) has produced “very encouraging” results in a phase 2 trial of patients with blastic plasmacytoid dendritic cell neoplasm (BPDCN), according to an investigator.
Tagraxofusp, a targeted therapy directed to CD123, produced an overall response rate (ORR) of 83% and a complete response (CR) rate of 62% in patients with previously untreated or relapsed/refractory BPDCN.
Common adverse events (AEs) related to tagraxofusp include hypoalbuminemia, transaminitis, and thrombocytopenia. There was 1 grade 5 AE—a case of capillary leak syndrome (CLS).
Study investigator Naveen Pemmaraju, MD, of The University of Texas MD Anderson Cancer Center in Houston, presented these results at the 23rd Congress of the European Hematology Association (EHA) as abstract S116.
The trial was sponsored by Stemline Therapeutics.
Dr Pemmaraju noted that there are no approved therapies for BPDCN, so patients may be treated with therapies intended for acute myeloid leukemia (AML), acute lymphoblastic leukemia, or lymphomas.
“These are usually quite intense cytotoxic chemotherapy regimens,” he said. “But even with these regimens, most groups report median overall survival times of 8 to 14 months.”
And although stem cell transplants can be effective in BPDCN, a “vast majority” of patients are not fit for transplant, according to Dr Pemmaraju.
With this in mind, he and his colleagues are conducting this trial of tagraxofusp in BPDCN.
The trial has 4 stages. In stage 1, patients received tagraxofusp at 7, 9, 12, or 16 μg/kg on days 1 to 5 of a 21-day cycle. In stages 2 and 3, patients received the drug at 12 μg/kg on days 1 to 5 of a 21-day cycle. Stage 4 is still enrolling.
Efficacy
Dr Pemmaraju presented results in 45 patients—32 with previously untreated BPDCN and 13 with relapsed/refractory BPDCN. The patients’ median age at baseline was 70 (range, 22-84), and 82% were male.
Three patients received tagraxofusp at 7 μg/kg/day, and the rest received the 12 μg/kg/day dose.
Among patients who received the 12 μg/kg/day dose, the ORR was 83% (35/42). The ORR was 90% (26/29) in previously untreated patients and 69% (9/13) in relapsed/refractory patients.
“These are very encouraging results—a 90% overall response rate in the frontline setting,” Dr Pemmaraju noted.
The composite CR rate was 62% (n=26) overall, 72% (n=21) in previously untreated patients, and 38% (n=5) in relapsed/refractory patients.
This included 13 patients with a CR (1 relapsed/refractory), 10 with a clinical CR (3 relapsed/refractory), and 3 with a CR with incomplete hematologic recovery (1 relapsed/refractory). A clinical CR was defined as absence of gross disease with minimal residual skin abnormality.
Fourteen patients went on to stem cell transplant, 1 of whom had relapsed/refractory disease at baseline.
Overall survival results were only available for the 29 previously untreated patients who received tagraxofusp at 12 μg/kg/day. In this group, the median overall survival has not been reached at a median follow-up of 13.8 months (range, 0.2 to 37.4 months).
Dr Pemmaraju said this result is important because it contrasts with the historical expectation of a median overall survival of 8 to 14 months.
Safety
Dr Pemmaraju presented safety results in 114 patients who have received tagraxofusp at 12 μg/kg/day on all trials of the drug. These data include patients with AML, myelofibrosis, and chronic myelomonocytic leukemia in addition to the 45 patients with BPDCN. However, AEs were similar regardless of disease.
Common treatment-related AEs (of any grade, occurring in at least 15% of patients) included hypoalbuminemia (49%), ALT increase (48%), AST increase (48%), thrombocytopenia (29%), nausea (27%), pyrexia (25%), chills (23%), fatigue (23%), weight increase (19%), hypotension (18%), peripheral edema (17%), and vomiting (15%).
CLS of any grade was also a common AE, occurring in 20% of patients (n=23). Most cases of CLS were grade 1 or 2, but there were grade 3 (n=5) and 4 (n=2) cases, as well as a single case of grade 5 CLS that occurred in a BPDCN patient.
Dr Pemmaraju did note that CLS has proven manageable with monitoring and pre-emptive measures. Specifically, inclusion criteria were changed so that patients must have normal cardiac function, adequate kidney function, and albumin of at least 3.2 g/dl. Investigators also began monitoring patients’ weight, albumin levels, and kidney function.
“With the combination of greater understanding of CLS, actual definitive protocol adjustments made by investigators, and monitoring, this has been a highly manageable phenomenon,” Dr Pemmaraju said.
Next steps
The investigators plan to continue enrolling patients in this study and collect additional safety and survival data, but Dr Pemmaraju and his colleagues also want to evaluate tagraxofusp in combination with other therapies.
Tagraxofusp is already under investigation in combination with azacitidine in a phase 1/2 trial of patients with high-risk myelodysplastic syndromes and AML.
Dr Pemmaraju is interested in combining hypomethylating agents with tagraxofusp for BPDCN patients as well, to build upon the encouraging results with tagraxofusp alone.
“An extraordinarily rare disease that used to not have any therapies at all now has at least one ongoing clinical trial with some encouraging activity,” he said. “I hope that gives hope to people with rare diseases, to let them know they’re not alone. There may be someone out there who’s researching their disease, no matter how rare it is.”
STOCKHOLM—Tagraxofusp (SL-401) has produced “very encouraging” results in a phase 2 trial of patients with blastic plasmacytoid dendritic cell neoplasm (BPDCN), according to an investigator.
Tagraxofusp, a targeted therapy directed to CD123, produced an overall response rate (ORR) of 83% and a complete response (CR) rate of 62% in patients with previously untreated or relapsed/refractory BPDCN.
Common adverse events (AEs) related to tagraxofusp include hypoalbuminemia, transaminitis, and thrombocytopenia. There was 1 grade 5 AE—a case of capillary leak syndrome (CLS).
Study investigator Naveen Pemmaraju, MD, of The University of Texas MD Anderson Cancer Center in Houston, presented these results at the 23rd Congress of the European Hematology Association (EHA) as abstract S116.
The trial was sponsored by Stemline Therapeutics.
Dr Pemmaraju noted that there are no approved therapies for BPDCN, so patients may be treated with therapies intended for acute myeloid leukemia (AML), acute lymphoblastic leukemia, or lymphomas.
“These are usually quite intense cytotoxic chemotherapy regimens,” he said. “But even with these regimens, most groups report median overall survival times of 8 to 14 months.”
And although stem cell transplants can be effective in BPDCN, a “vast majority” of patients are not fit for transplant, according to Dr Pemmaraju.
With this in mind, he and his colleagues are conducting this trial of tagraxofusp in BPDCN.
The trial has 4 stages. In stage 1, patients received tagraxofusp at 7, 9, 12, or 16 μg/kg on days 1 to 5 of a 21-day cycle. In stages 2 and 3, patients received the drug at 12 μg/kg on days 1 to 5 of a 21-day cycle. Stage 4 is still enrolling.
Efficacy
Dr Pemmaraju presented results in 45 patients—32 with previously untreated BPDCN and 13 with relapsed/refractory BPDCN. The patients’ median age at baseline was 70 (range, 22-84), and 82% were male.
Three patients received tagraxofusp at 7 μg/kg/day, and the rest received the 12 μg/kg/day dose.
Among patients who received the 12 μg/kg/day dose, the ORR was 83% (35/42). The ORR was 90% (26/29) in previously untreated patients and 69% (9/13) in relapsed/refractory patients.
“These are very encouraging results—a 90% overall response rate in the frontline setting,” Dr Pemmaraju noted.
The composite CR rate was 62% (n=26) overall, 72% (n=21) in previously untreated patients, and 38% (n=5) in relapsed/refractory patients.
This included 13 patients with a CR (1 relapsed/refractory), 10 with a clinical CR (3 relapsed/refractory), and 3 with a CR with incomplete hematologic recovery (1 relapsed/refractory). A clinical CR was defined as absence of gross disease with minimal residual skin abnormality.
Fourteen patients went on to stem cell transplant, 1 of whom had relapsed/refractory disease at baseline.
Overall survival results were only available for the 29 previously untreated patients who received tagraxofusp at 12 μg/kg/day. In this group, the median overall survival has not been reached at a median follow-up of 13.8 months (range, 0.2 to 37.4 months).
Dr Pemmaraju said this result is important because it contrasts with the historical expectation of a median overall survival of 8 to 14 months.
Safety
Dr Pemmaraju presented safety results in 114 patients who have received tagraxofusp at 12 μg/kg/day on all trials of the drug. These data include patients with AML, myelofibrosis, and chronic myelomonocytic leukemia in addition to the 45 patients with BPDCN. However, AEs were similar regardless of disease.
Common treatment-related AEs (of any grade, occurring in at least 15% of patients) included hypoalbuminemia (49%), ALT increase (48%), AST increase (48%), thrombocytopenia (29%), nausea (27%), pyrexia (25%), chills (23%), fatigue (23%), weight increase (19%), hypotension (18%), peripheral edema (17%), and vomiting (15%).
CLS of any grade was also a common AE, occurring in 20% of patients (n=23). Most cases of CLS were grade 1 or 2, but there were grade 3 (n=5) and 4 (n=2) cases, as well as a single case of grade 5 CLS that occurred in a BPDCN patient.
Dr Pemmaraju did note that CLS has proven manageable with monitoring and pre-emptive measures. Specifically, inclusion criteria were changed so that patients must have normal cardiac function, adequate kidney function, and albumin of at least 3.2 g/dl. Investigators also began monitoring patients’ weight, albumin levels, and kidney function.
“With the combination of greater understanding of CLS, actual definitive protocol adjustments made by investigators, and monitoring, this has been a highly manageable phenomenon,” Dr Pemmaraju said.
Next steps
The investigators plan to continue enrolling patients in this study and collect additional safety and survival data, but Dr Pemmaraju and his colleagues also want to evaluate tagraxofusp in combination with other therapies.
Tagraxofusp is already under investigation in combination with azacitidine in a phase 1/2 trial of patients with high-risk myelodysplastic syndromes and AML.
Dr Pemmaraju is interested in combining hypomethylating agents with tagraxofusp for BPDCN patients as well, to build upon the encouraging results with tagraxofusp alone.
“An extraordinarily rare disease that used to not have any therapies at all now has at least one ongoing clinical trial with some encouraging activity,” he said. “I hope that gives hope to people with rare diseases, to let them know they’re not alone. There may be someone out there who’s researching their disease, no matter how rare it is.”
CHMP recommends rVWF for VWD
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended marketing authorization for vonicog alfa (Veyvondi), a recombinant von Willebrand factor (rVWF) product.
The CHMP is recommending vonicog alfa for the treatment of bleeding events and treatment/prevention of surgical bleeding in adults (age 18 and older) with von Willebrand disease (VWD) when desmopressin treatment alone is ineffective or not indicated.
The CHMP’s recommendation will be reviewed by the European Commission, which has the authority to approve medicines for use in the European Union, Norway, Iceland, and Liechtenstein.
The European Commission usually makes a decision within 67 days of the CHMP’s recommendation.
The CHMP’s opinion of vonicog alfa is supported by a pair of phase 3 trials.
Non-surgical setting
Results from a phase 3 trial of vonicog alfa in a non-surgical setting were published in Blood in 2015.
The study included 49 patients with VWD who received vonicog alfa with or without recombinant factor VIII (FVIII).
All participants had successful treatment of bleeding episodes. Most (96.9%) treated bleeds (n=192 bleeds in 22 patients) were given an “excellent” efficacy rating (as good as or better than expected).
Most bleeds (81.8%) were resolved with a single infusion of vonicog alfa, and the treatment had a mean half-life of 21.9 hours.
There were 8 adverse events (AEs) considered related to vonicog alfa, and 2 were serious. One patient experienced 2 simultaneous serious AEs—chest discomfort and increased heart rate—but these were resolved.
There were no thrombotic events in this trial, no treatment-related binding or neutralizing antibodies against VWF, and no neutralizing antibodies against FVIII.
Surgical setting
Results from the phase 3 trial in a surgical setting were presented at the WFH 2018 World Congress.
The trial enrolled 15 adults with severe VWD who were undergoing elective surgical procedures (10 of them major procedures).
Patients received vonicog alfa at 40 to 60 IU per kg of body weight 12 to 24 hours before surgery. Within 3 hours of surgery, each patient’s FVIII level (FVIII:C) was assessed, with a target of 30 IU/dL for minor surgeries and 60 IU/dL for major surgeries.
Within an hour of surgery, patients received a dose of vonicog alfa, with or without recombinant factor VIII, depending on the target FVIII:C levels at the 3-hour assessment.
Ten patients received rVWF alone, 12 did not receive any preoperative FVIII, and 2 did not receive rVWF postoperatively.
The study’s primary endpoint was met. Vonicog alfa demonstrated overall hemostatic efficacy, as assessed 24 hours after the last perioperative infusion or the completion of the study visit, whichever occurred earlier.
Intra- and post-operative hemostasis was rated as “excellent” (as good as or better than expected) in 60% of patients and “good” (probably as good as expected) in 40% of patients.
One patient developed deep vein thrombosis 3 days after undergoing hip replacement surgery.
One patient tested positive for binding antibodies to VWF. None of the patients developed binding antibodies against potential impurities such as rFurin, CHO-protein, or mouse IgG.
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended marketing authorization for vonicog alfa (Veyvondi), a recombinant von Willebrand factor (rVWF) product.
The CHMP is recommending vonicog alfa for the treatment of bleeding events and treatment/prevention of surgical bleeding in adults (age 18 and older) with von Willebrand disease (VWD) when desmopressin treatment alone is ineffective or not indicated.
The CHMP’s recommendation will be reviewed by the European Commission, which has the authority to approve medicines for use in the European Union, Norway, Iceland, and Liechtenstein.
The European Commission usually makes a decision within 67 days of the CHMP’s recommendation.
The CHMP’s opinion of vonicog alfa is supported by a pair of phase 3 trials.
Non-surgical setting
Results from a phase 3 trial of vonicog alfa in a non-surgical setting were published in Blood in 2015.
The study included 49 patients with VWD who received vonicog alfa with or without recombinant factor VIII (FVIII).
All participants had successful treatment of bleeding episodes. Most (96.9%) treated bleeds (n=192 bleeds in 22 patients) were given an “excellent” efficacy rating (as good as or better than expected).
Most bleeds (81.8%) were resolved with a single infusion of vonicog alfa, and the treatment had a mean half-life of 21.9 hours.
There were 8 adverse events (AEs) considered related to vonicog alfa, and 2 were serious. One patient experienced 2 simultaneous serious AEs—chest discomfort and increased heart rate—but these were resolved.
There were no thrombotic events in this trial, no treatment-related binding or neutralizing antibodies against VWF, and no neutralizing antibodies against FVIII.
Surgical setting
Results from the phase 3 trial in a surgical setting were presented at the WFH 2018 World Congress.
The trial enrolled 15 adults with severe VWD who were undergoing elective surgical procedures (10 of them major procedures).
Patients received vonicog alfa at 40 to 60 IU per kg of body weight 12 to 24 hours before surgery. Within 3 hours of surgery, each patient’s FVIII level (FVIII:C) was assessed, with a target of 30 IU/dL for minor surgeries and 60 IU/dL for major surgeries.
Within an hour of surgery, patients received a dose of vonicog alfa, with or without recombinant factor VIII, depending on the target FVIII:C levels at the 3-hour assessment.
Ten patients received rVWF alone, 12 did not receive any preoperative FVIII, and 2 did not receive rVWF postoperatively.
The study’s primary endpoint was met. Vonicog alfa demonstrated overall hemostatic efficacy, as assessed 24 hours after the last perioperative infusion or the completion of the study visit, whichever occurred earlier.
Intra- and post-operative hemostasis was rated as “excellent” (as good as or better than expected) in 60% of patients and “good” (probably as good as expected) in 40% of patients.
One patient developed deep vein thrombosis 3 days after undergoing hip replacement surgery.
One patient tested positive for binding antibodies to VWF. None of the patients developed binding antibodies against potential impurities such as rFurin, CHO-protein, or mouse IgG.
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended marketing authorization for vonicog alfa (Veyvondi), a recombinant von Willebrand factor (rVWF) product.
The CHMP is recommending vonicog alfa for the treatment of bleeding events and treatment/prevention of surgical bleeding in adults (age 18 and older) with von Willebrand disease (VWD) when desmopressin treatment alone is ineffective or not indicated.
The CHMP’s recommendation will be reviewed by the European Commission, which has the authority to approve medicines for use in the European Union, Norway, Iceland, and Liechtenstein.
The European Commission usually makes a decision within 67 days of the CHMP’s recommendation.
The CHMP’s opinion of vonicog alfa is supported by a pair of phase 3 trials.
Non-surgical setting
Results from a phase 3 trial of vonicog alfa in a non-surgical setting were published in Blood in 2015.
The study included 49 patients with VWD who received vonicog alfa with or without recombinant factor VIII (FVIII).
All participants had successful treatment of bleeding episodes. Most (96.9%) treated bleeds (n=192 bleeds in 22 patients) were given an “excellent” efficacy rating (as good as or better than expected).
Most bleeds (81.8%) were resolved with a single infusion of vonicog alfa, and the treatment had a mean half-life of 21.9 hours.
There were 8 adverse events (AEs) considered related to vonicog alfa, and 2 were serious. One patient experienced 2 simultaneous serious AEs—chest discomfort and increased heart rate—but these were resolved.
There were no thrombotic events in this trial, no treatment-related binding or neutralizing antibodies against VWF, and no neutralizing antibodies against FVIII.
Surgical setting
Results from the phase 3 trial in a surgical setting were presented at the WFH 2018 World Congress.
The trial enrolled 15 adults with severe VWD who were undergoing elective surgical procedures (10 of them major procedures).
Patients received vonicog alfa at 40 to 60 IU per kg of body weight 12 to 24 hours before surgery. Within 3 hours of surgery, each patient’s FVIII level (FVIII:C) was assessed, with a target of 30 IU/dL for minor surgeries and 60 IU/dL for major surgeries.
Within an hour of surgery, patients received a dose of vonicog alfa, with or without recombinant factor VIII, depending on the target FVIII:C levels at the 3-hour assessment.
Ten patients received rVWF alone, 12 did not receive any preoperative FVIII, and 2 did not receive rVWF postoperatively.
The study’s primary endpoint was met. Vonicog alfa demonstrated overall hemostatic efficacy, as assessed 24 hours after the last perioperative infusion or the completion of the study visit, whichever occurred earlier.
Intra- and post-operative hemostasis was rated as “excellent” (as good as or better than expected) in 60% of patients and “good” (probably as good as expected) in 40% of patients.
One patient developed deep vein thrombosis 3 days after undergoing hip replacement surgery.
One patient tested positive for binding antibodies to VWF. None of the patients developed binding antibodies against potential impurities such as rFurin, CHO-protein, or mouse IgG.
CHMP supports authorization of drug for AML
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended marketing authorization for CPX-351 (Vyxeos™), a liposomal formulation that delivers a fixed ratio (1:5) of daunorubicin and cytarabine.
The CHMP is recommending approval of CPX-351 (44 mg/100 mg) for the treatment of adults with newly diagnosed, therapy-related acute myeloid leukemia or AML with myelodysplasia-related changes.
The CHMP’s recommendation will be reviewed by the European Commission, which has the authority to approve medicines for use in the European Union, Norway, Iceland, and Liechtenstein.
The European Commission usually makes a decision within 67 days of the CHMP’s recommendation.
The marketing authorization application for CPX-351 is supported by data from 5 studies, including a phase 3 study.
Data from the phase 3 study were presented at the 2016 ASCO Annual Meeting and are available in the US prescribing information for CPX-351. (The following data are taken from the prescribing information.)
This trial enrolled 309 patients, ages 60 to 75, with newly diagnosed, therapy-related AML or AML with myelodysplasia-related changes.
They received CPX-351 (n=153) or cytarabine and daunorubicin (7+3; n=156).
The complete response rate was 38% in the CPX-351 arm and 26% in the 7+3 arm (P=0.036).
The rate of hematopoietic stem cell transplant was 34% in the CPX-351 arm and 25% in the 7+3 arm.
The median overall survival was 9.6 months in the CPX-351 arm and 5.9 months in the 7+3 arm (P=0.005).
All-cause 30-day mortality was 6% in the CPX-351 arm and 11% in the 7+3 arm. Sixty-day mortality was 14% and 21%, respectively.
Six percent of patients in both arms had a fatal adverse event (AE) on treatment or within 30 days of therapy that was not in the setting of progressive disease.
The rate of AEs that led to discontinuation was 18% in the CPX-351 arm and 13% in the 7+3 arm. AEs leading to discontinuation in the CPX-351 arm included prolonged cytopenias, infection, cardiotoxicity, respiratory failure, hemorrhage, renal insufficiency, colitis, and generalized medical deterioration.
The most common AEs (incidence ≥ 25%) in the CPX-351 arm were hemorrhagic events, febrile neutropenia, rash, edema, nausea, mucositis, diarrhea, constipation, musculoskeletal pain, fatigue, abdominal pain, dyspnea, headache, cough, decreased appetite, arrhythmia, pneumonia, bacteremia, chills, sleep disorders, and vomiting.
The most common serious AEs (incidence ≥ 5%) in the CPX-351 arm were dyspnea, myocardial toxicity, sepsis, pneumonia, febrile neutropenia, bacteremia, and hemorrhage.
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended marketing authorization for CPX-351 (Vyxeos™), a liposomal formulation that delivers a fixed ratio (1:5) of daunorubicin and cytarabine.
The CHMP is recommending approval of CPX-351 (44 mg/100 mg) for the treatment of adults with newly diagnosed, therapy-related acute myeloid leukemia or AML with myelodysplasia-related changes.
The CHMP’s recommendation will be reviewed by the European Commission, which has the authority to approve medicines for use in the European Union, Norway, Iceland, and Liechtenstein.
The European Commission usually makes a decision within 67 days of the CHMP’s recommendation.
The marketing authorization application for CPX-351 is supported by data from 5 studies, including a phase 3 study.
Data from the phase 3 study were presented at the 2016 ASCO Annual Meeting and are available in the US prescribing information for CPX-351. (The following data are taken from the prescribing information.)
This trial enrolled 309 patients, ages 60 to 75, with newly diagnosed, therapy-related AML or AML with myelodysplasia-related changes.
They received CPX-351 (n=153) or cytarabine and daunorubicin (7+3; n=156).
The complete response rate was 38% in the CPX-351 arm and 26% in the 7+3 arm (P=0.036).
The rate of hematopoietic stem cell transplant was 34% in the CPX-351 arm and 25% in the 7+3 arm.
The median overall survival was 9.6 months in the CPX-351 arm and 5.9 months in the 7+3 arm (P=0.005).
All-cause 30-day mortality was 6% in the CPX-351 arm and 11% in the 7+3 arm. Sixty-day mortality was 14% and 21%, respectively.
Six percent of patients in both arms had a fatal adverse event (AE) on treatment or within 30 days of therapy that was not in the setting of progressive disease.
The rate of AEs that led to discontinuation was 18% in the CPX-351 arm and 13% in the 7+3 arm. AEs leading to discontinuation in the CPX-351 arm included prolonged cytopenias, infection, cardiotoxicity, respiratory failure, hemorrhage, renal insufficiency, colitis, and generalized medical deterioration.
The most common AEs (incidence ≥ 25%) in the CPX-351 arm were hemorrhagic events, febrile neutropenia, rash, edema, nausea, mucositis, diarrhea, constipation, musculoskeletal pain, fatigue, abdominal pain, dyspnea, headache, cough, decreased appetite, arrhythmia, pneumonia, bacteremia, chills, sleep disorders, and vomiting.
The most common serious AEs (incidence ≥ 5%) in the CPX-351 arm were dyspnea, myocardial toxicity, sepsis, pneumonia, febrile neutropenia, bacteremia, and hemorrhage.
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended marketing authorization for CPX-351 (Vyxeos™), a liposomal formulation that delivers a fixed ratio (1:5) of daunorubicin and cytarabine.
The CHMP is recommending approval of CPX-351 (44 mg/100 mg) for the treatment of adults with newly diagnosed, therapy-related acute myeloid leukemia or AML with myelodysplasia-related changes.
The CHMP’s recommendation will be reviewed by the European Commission, which has the authority to approve medicines for use in the European Union, Norway, Iceland, and Liechtenstein.
The European Commission usually makes a decision within 67 days of the CHMP’s recommendation.
The marketing authorization application for CPX-351 is supported by data from 5 studies, including a phase 3 study.
Data from the phase 3 study were presented at the 2016 ASCO Annual Meeting and are available in the US prescribing information for CPX-351. (The following data are taken from the prescribing information.)
This trial enrolled 309 patients, ages 60 to 75, with newly diagnosed, therapy-related AML or AML with myelodysplasia-related changes.
They received CPX-351 (n=153) or cytarabine and daunorubicin (7+3; n=156).
The complete response rate was 38% in the CPX-351 arm and 26% in the 7+3 arm (P=0.036).
The rate of hematopoietic stem cell transplant was 34% in the CPX-351 arm and 25% in the 7+3 arm.
The median overall survival was 9.6 months in the CPX-351 arm and 5.9 months in the 7+3 arm (P=0.005).
All-cause 30-day mortality was 6% in the CPX-351 arm and 11% in the 7+3 arm. Sixty-day mortality was 14% and 21%, respectively.
Six percent of patients in both arms had a fatal adverse event (AE) on treatment or within 30 days of therapy that was not in the setting of progressive disease.
The rate of AEs that led to discontinuation was 18% in the CPX-351 arm and 13% in the 7+3 arm. AEs leading to discontinuation in the CPX-351 arm included prolonged cytopenias, infection, cardiotoxicity, respiratory failure, hemorrhage, renal insufficiency, colitis, and generalized medical deterioration.
The most common AEs (incidence ≥ 25%) in the CPX-351 arm were hemorrhagic events, febrile neutropenia, rash, edema, nausea, mucositis, diarrhea, constipation, musculoskeletal pain, fatigue, abdominal pain, dyspnea, headache, cough, decreased appetite, arrhythmia, pneumonia, bacteremia, chills, sleep disorders, and vomiting.
The most common serious AEs (incidence ≥ 5%) in the CPX-351 arm were dyspnea, myocardial toxicity, sepsis, pneumonia, febrile neutropenia, bacteremia, and hemorrhage.
CHMP backs approval of caplacizumab
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended approval of caplacizumab (Cablivi) for the treatment of adults with acquired thrombotic thrombocytopenic purpura (aTTP).
Caplacizumab is a humanized bivalent nanobody that inhibits the interaction between von Willebrand factor and platelets.
The CHMP’s recommendation regarding caplacizumab will be reviewed by the European Commission, which has the authority to approve medicines for use in the European Union, Norway, Iceland, and Liechtenstein.
The European Commission usually makes a decision within 67 days of the CHMP’s recommendation.
The CHMP’s opinion of caplacizumab is supported by data from the phase 2 TITAN study and the phase 3 HERCULES study.
TITAN trial
Results from TITAN were published in NEJM in 2016. TITAN included 75 aTTP patients who were randomized to caplacizumab (n=36) or placebo (n=39), with all patients receiving the current standard of care (daily plasma exchange and immunosuppressive therapy).
The study’s primary endpoint was time to response (platelet count normalization). Patients in the caplacizumab arm had a 39% reduction in the median time to response compared to patients in the placebo arm (P=0.005).
Among the 69 patients who had not undergone a plasma-exchange session before enrollment, the median time to response was 3.0 days in the caplacizumab arm and 4.9 days in the placebo arm.
Among the 6 patients who did undergo a plasma-exchange session before enrollment, the median time to a response was 2.4 days in the caplacizumab arm and 4.3 days in the placebo arm.
The rate of confirmed response was 86.1% (n=31) in the caplacizumab arm and 71.8% (n=28) in the placebo arm.
There were 541 adverse events (AEs) in 34 of the 35 evaluable patients receiving caplacizumab (97%) and 522 AEs in all 37 evaluable patients receiving placebo (100%). TTP exacerbations and relapses were not included as AEs.
The rate of AEs thought to be related to the study drug was 17% in the caplacizumab arm and 11% in the placebo arm. The rate of AEs that were possibly related was 54% and 8%, respectively. And the rate of serious AEs was 37% and 32%, respectively.
There were no deaths in the caplacizumab arm and 2 in the placebo arm. One death was due to severe, refractory TTP, and the other was due to cerebral hemorrhage.
HERCULES trial
Results from HERCULES were presented at the 2017 ASH Annual Meeting.
The study enrolled patients with an acute episode of aTTP. They were randomized to receive either caplacizumab (n=72) or placebo (n=73) in addition to standard care, which consisted of plasma exchange and immunosuppression.
The study’s primary endpoint was the time to normalization of platelet count response, which was defined as an initial platelet count of at least 150 x 109/L with subsequent stop of daily plasma exchange within 5 days.
There was a significant reduction in time to platelet count response in the caplacizumab arm compared to the placebo arm. The platelet normalization rate ratio was 1.55 (P<0.01).
A secondary endpoint was the combination of aTTP-related death, aTTP recurrence, and at least 1 major thromboembolic event during study treatment. The incidence of this combined endpoint was 12.7% (n=9) in the caplacizumab arm and 49.3% (n=36) in the placebo arm (P<0.0001).
The incidence of aTTP-related death was 0% (n=0) in the caplacizumab arm and 4.1% (n=3) in the placebo arm. The incidence of aTTP recurrence was 4.2% (n=3) and 38.4% (n=28), respectively. And the incidence of at least 1 major thromboembolic event was 8.5% (n=6) and 8.2% (n=6), respectively.
The proportion of patients with at least 1 study-drug-related AE was 57.7% in the caplacizumab arm and 43.8% in the placebo arm. The proportion of patients with at least 1 study-drug-related serious AE was 14.1% (n=10) and 5.5% (n=4), respectively. The rate of discontinuation due to at least 1 AE was 7.0% and 12.3%, respectively.
During the treatment period, there were no deaths in the caplacizumab arm and 3 deaths in the placebo arm. There was 1 death in the caplacizumab arm during the follow-up period, but it was considered unrelated to caplacizumab.
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended approval of caplacizumab (Cablivi) for the treatment of adults with acquired thrombotic thrombocytopenic purpura (aTTP).
Caplacizumab is a humanized bivalent nanobody that inhibits the interaction between von Willebrand factor and platelets.
The CHMP’s recommendation regarding caplacizumab will be reviewed by the European Commission, which has the authority to approve medicines for use in the European Union, Norway, Iceland, and Liechtenstein.
The European Commission usually makes a decision within 67 days of the CHMP’s recommendation.
The CHMP’s opinion of caplacizumab is supported by data from the phase 2 TITAN study and the phase 3 HERCULES study.
TITAN trial
Results from TITAN were published in NEJM in 2016. TITAN included 75 aTTP patients who were randomized to caplacizumab (n=36) or placebo (n=39), with all patients receiving the current standard of care (daily plasma exchange and immunosuppressive therapy).
The study’s primary endpoint was time to response (platelet count normalization). Patients in the caplacizumab arm had a 39% reduction in the median time to response compared to patients in the placebo arm (P=0.005).
Among the 69 patients who had not undergone a plasma-exchange session before enrollment, the median time to response was 3.0 days in the caplacizumab arm and 4.9 days in the placebo arm.
Among the 6 patients who did undergo a plasma-exchange session before enrollment, the median time to a response was 2.4 days in the caplacizumab arm and 4.3 days in the placebo arm.
The rate of confirmed response was 86.1% (n=31) in the caplacizumab arm and 71.8% (n=28) in the placebo arm.
There were 541 adverse events (AEs) in 34 of the 35 evaluable patients receiving caplacizumab (97%) and 522 AEs in all 37 evaluable patients receiving placebo (100%). TTP exacerbations and relapses were not included as AEs.
The rate of AEs thought to be related to the study drug was 17% in the caplacizumab arm and 11% in the placebo arm. The rate of AEs that were possibly related was 54% and 8%, respectively. And the rate of serious AEs was 37% and 32%, respectively.
There were no deaths in the caplacizumab arm and 2 in the placebo arm. One death was due to severe, refractory TTP, and the other was due to cerebral hemorrhage.
HERCULES trial
Results from HERCULES were presented at the 2017 ASH Annual Meeting.
The study enrolled patients with an acute episode of aTTP. They were randomized to receive either caplacizumab (n=72) or placebo (n=73) in addition to standard care, which consisted of plasma exchange and immunosuppression.
The study’s primary endpoint was the time to normalization of platelet count response, which was defined as an initial platelet count of at least 150 x 109/L with subsequent stop of daily plasma exchange within 5 days.
There was a significant reduction in time to platelet count response in the caplacizumab arm compared to the placebo arm. The platelet normalization rate ratio was 1.55 (P<0.01).
A secondary endpoint was the combination of aTTP-related death, aTTP recurrence, and at least 1 major thromboembolic event during study treatment. The incidence of this combined endpoint was 12.7% (n=9) in the caplacizumab arm and 49.3% (n=36) in the placebo arm (P<0.0001).
The incidence of aTTP-related death was 0% (n=0) in the caplacizumab arm and 4.1% (n=3) in the placebo arm. The incidence of aTTP recurrence was 4.2% (n=3) and 38.4% (n=28), respectively. And the incidence of at least 1 major thromboembolic event was 8.5% (n=6) and 8.2% (n=6), respectively.
The proportion of patients with at least 1 study-drug-related AE was 57.7% in the caplacizumab arm and 43.8% in the placebo arm. The proportion of patients with at least 1 study-drug-related serious AE was 14.1% (n=10) and 5.5% (n=4), respectively. The rate of discontinuation due to at least 1 AE was 7.0% and 12.3%, respectively.
During the treatment period, there were no deaths in the caplacizumab arm and 3 deaths in the placebo arm. There was 1 death in the caplacizumab arm during the follow-up period, but it was considered unrelated to caplacizumab.
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended approval of caplacizumab (Cablivi) for the treatment of adults with acquired thrombotic thrombocytopenic purpura (aTTP).
Caplacizumab is a humanized bivalent nanobody that inhibits the interaction between von Willebrand factor and platelets.
The CHMP’s recommendation regarding caplacizumab will be reviewed by the European Commission, which has the authority to approve medicines for use in the European Union, Norway, Iceland, and Liechtenstein.
The European Commission usually makes a decision within 67 days of the CHMP’s recommendation.
The CHMP’s opinion of caplacizumab is supported by data from the phase 2 TITAN study and the phase 3 HERCULES study.
TITAN trial
Results from TITAN were published in NEJM in 2016. TITAN included 75 aTTP patients who were randomized to caplacizumab (n=36) or placebo (n=39), with all patients receiving the current standard of care (daily plasma exchange and immunosuppressive therapy).
The study’s primary endpoint was time to response (platelet count normalization). Patients in the caplacizumab arm had a 39% reduction in the median time to response compared to patients in the placebo arm (P=0.005).
Among the 69 patients who had not undergone a plasma-exchange session before enrollment, the median time to response was 3.0 days in the caplacizumab arm and 4.9 days in the placebo arm.
Among the 6 patients who did undergo a plasma-exchange session before enrollment, the median time to a response was 2.4 days in the caplacizumab arm and 4.3 days in the placebo arm.
The rate of confirmed response was 86.1% (n=31) in the caplacizumab arm and 71.8% (n=28) in the placebo arm.
There were 541 adverse events (AEs) in 34 of the 35 evaluable patients receiving caplacizumab (97%) and 522 AEs in all 37 evaluable patients receiving placebo (100%). TTP exacerbations and relapses were not included as AEs.
The rate of AEs thought to be related to the study drug was 17% in the caplacizumab arm and 11% in the placebo arm. The rate of AEs that were possibly related was 54% and 8%, respectively. And the rate of serious AEs was 37% and 32%, respectively.
There were no deaths in the caplacizumab arm and 2 in the placebo arm. One death was due to severe, refractory TTP, and the other was due to cerebral hemorrhage.
HERCULES trial
Results from HERCULES were presented at the 2017 ASH Annual Meeting.
The study enrolled patients with an acute episode of aTTP. They were randomized to receive either caplacizumab (n=72) or placebo (n=73) in addition to standard care, which consisted of plasma exchange and immunosuppression.
The study’s primary endpoint was the time to normalization of platelet count response, which was defined as an initial platelet count of at least 150 x 109/L with subsequent stop of daily plasma exchange within 5 days.
There was a significant reduction in time to platelet count response in the caplacizumab arm compared to the placebo arm. The platelet normalization rate ratio was 1.55 (P<0.01).
A secondary endpoint was the combination of aTTP-related death, aTTP recurrence, and at least 1 major thromboembolic event during study treatment. The incidence of this combined endpoint was 12.7% (n=9) in the caplacizumab arm and 49.3% (n=36) in the placebo arm (P<0.0001).
The incidence of aTTP-related death was 0% (n=0) in the caplacizumab arm and 4.1% (n=3) in the placebo arm. The incidence of aTTP recurrence was 4.2% (n=3) and 38.4% (n=28), respectively. And the incidence of at least 1 major thromboembolic event was 8.5% (n=6) and 8.2% (n=6), respectively.
The proportion of patients with at least 1 study-drug-related AE was 57.7% in the caplacizumab arm and 43.8% in the placebo arm. The proportion of patients with at least 1 study-drug-related serious AE was 14.1% (n=10) and 5.5% (n=4), respectively. The rate of discontinuation due to at least 1 AE was 7.0% and 12.3%, respectively.
During the treatment period, there were no deaths in the caplacizumab arm and 3 deaths in the placebo arm. There was 1 death in the caplacizumab arm during the follow-up period, but it was considered unrelated to caplacizumab.
Risk of adverse birth outcomes for singleton infants born to ART-treated or subfertile women
Singleton infants born to mothers who are subfertile or treated with assisted reproductive technology (ART) are at higher risk for multiple adverse health outcomes beyond prematurity, a recent retrospective study shows.
Risks of chromosomal abnormalities, infectious diseases, and cardiovascular and respiratory conditions were all increased, compared with infants born to fertile mothers, in analyses of neonatal outcomes stratified by gestational age.
This population-based study is among the first to show differences in adverse birth outcomes beyond preterm birth and, more specifically, by organ system conditions across gestational age categories, according to Sunah S. Hwang, MD, MPH, of the University of Colorado at Denver, Aurora, and her coinvestigators.
“With this approach, we offer more detailed associations between maternal fertility and the receipt of treatment along the continuum of fetal organ development and subsequent infant health conditions,” Dr. Hwang and her coauthors wrote in Pediatrics.
The study, which included singleton infants of at least 23 weeks’ gestational age born during 2004-2010, was based on data from a Massachusetts clinical ART database (MOSART) that was linked with state vital records.
Out of 350,123 infants with birth hospitalization records in the study cohort, 336,705 were born to fertile women, while 8,375 were born to women treated with ART, and 5,403 were born to subfertile women.
After adjustment for key maternal and infant characteristics, infants born to subfertile or ART-treated women were more often preterm as compared with infants to fertile mothers. Adjusted odds ratios were 1.39 (95% confidence interval, 1.26-1.54) and 1.72 (95% CI, 1.60-1.85) for infants of subfertile and ART-treated women, respectively, Dr. Hwang and her coinvestigators reported.
Infants born to subfertile or ART-treated women were also more likely to have adverse respiratory, gastrointestinal, or nutritional outcomes, with adjusted ORs ranging from 1.12 to 1.18, they added in the report.
Looking specifically at outcomes stratified by gestational age, they found an increased risk of congenital malformations, infectious diseases, and cardiovascular or respiratory outcomes, with adjusted ORs from 1.30 to 2.61, in the data published in the journal.
By contrast, there were no differences in risks of neonatal mortality, length of hospitalization, low birth weight, or neurologic and hematologic abnormalities for infants of subfertile and ART-treated women, compared with fertile women, according to Dr. Hwang and her coauthors.
These results confirm results of some previous studies that suggested a higher risk of adverse birth outcomes among infants born as singletons, according to the study authors.
“Although it is clearly accepted that multiple gestation is a significant predictor of preterm birth and low birth weight, recent studies have also revealed that, even among singleton births, mothers with infertility without ART treatment along with those who do undergo ART treatment are at higher risk for preterm delivery,” they wrote.
The study was funded by a grant from the National Institutes of Health. Authors said they had no financial relationships relevant to the study.
SOURCE: Hwang SS et al. Pediatrics. 2018 Aug;142(2):e20174069.
Singleton infants born to mothers who are subfertile or treated with assisted reproductive technology (ART) are at higher risk for multiple adverse health outcomes beyond prematurity, a recent retrospective study shows.
Risks of chromosomal abnormalities, infectious diseases, and cardiovascular and respiratory conditions were all increased, compared with infants born to fertile mothers, in analyses of neonatal outcomes stratified by gestational age.
This population-based study is among the first to show differences in adverse birth outcomes beyond preterm birth and, more specifically, by organ system conditions across gestational age categories, according to Sunah S. Hwang, MD, MPH, of the University of Colorado at Denver, Aurora, and her coinvestigators.
“With this approach, we offer more detailed associations between maternal fertility and the receipt of treatment along the continuum of fetal organ development and subsequent infant health conditions,” Dr. Hwang and her coauthors wrote in Pediatrics.
The study, which included singleton infants of at least 23 weeks’ gestational age born during 2004-2010, was based on data from a Massachusetts clinical ART database (MOSART) that was linked with state vital records.
Out of 350,123 infants with birth hospitalization records in the study cohort, 336,705 were born to fertile women, while 8,375 were born to women treated with ART, and 5,403 were born to subfertile women.
After adjustment for key maternal and infant characteristics, infants born to subfertile or ART-treated women were more often preterm as compared with infants to fertile mothers. Adjusted odds ratios were 1.39 (95% confidence interval, 1.26-1.54) and 1.72 (95% CI, 1.60-1.85) for infants of subfertile and ART-treated women, respectively, Dr. Hwang and her coinvestigators reported.
Infants born to subfertile or ART-treated women were also more likely to have adverse respiratory, gastrointestinal, or nutritional outcomes, with adjusted ORs ranging from 1.12 to 1.18, they added in the report.
Looking specifically at outcomes stratified by gestational age, they found an increased risk of congenital malformations, infectious diseases, and cardiovascular or respiratory outcomes, with adjusted ORs from 1.30 to 2.61, in the data published in the journal.
By contrast, there were no differences in risks of neonatal mortality, length of hospitalization, low birth weight, or neurologic and hematologic abnormalities for infants of subfertile and ART-treated women, compared with fertile women, according to Dr. Hwang and her coauthors.
These results confirm results of some previous studies that suggested a higher risk of adverse birth outcomes among infants born as singletons, according to the study authors.
“Although it is clearly accepted that multiple gestation is a significant predictor of preterm birth and low birth weight, recent studies have also revealed that, even among singleton births, mothers with infertility without ART treatment along with those who do undergo ART treatment are at higher risk for preterm delivery,” they wrote.
The study was funded by a grant from the National Institutes of Health. Authors said they had no financial relationships relevant to the study.
SOURCE: Hwang SS et al. Pediatrics. 2018 Aug;142(2):e20174069.
Singleton infants born to mothers who are subfertile or treated with assisted reproductive technology (ART) are at higher risk for multiple adverse health outcomes beyond prematurity, a recent retrospective study shows.
Risks of chromosomal abnormalities, infectious diseases, and cardiovascular and respiratory conditions were all increased, compared with infants born to fertile mothers, in analyses of neonatal outcomes stratified by gestational age.
This population-based study is among the first to show differences in adverse birth outcomes beyond preterm birth and, more specifically, by organ system conditions across gestational age categories, according to Sunah S. Hwang, MD, MPH, of the University of Colorado at Denver, Aurora, and her coinvestigators.
“With this approach, we offer more detailed associations between maternal fertility and the receipt of treatment along the continuum of fetal organ development and subsequent infant health conditions,” Dr. Hwang and her coauthors wrote in Pediatrics.
The study, which included singleton infants of at least 23 weeks’ gestational age born during 2004-2010, was based on data from a Massachusetts clinical ART database (MOSART) that was linked with state vital records.
Out of 350,123 infants with birth hospitalization records in the study cohort, 336,705 were born to fertile women, while 8,375 were born to women treated with ART, and 5,403 were born to subfertile women.
After adjustment for key maternal and infant characteristics, infants born to subfertile or ART-treated women were more often preterm as compared with infants to fertile mothers. Adjusted odds ratios were 1.39 (95% confidence interval, 1.26-1.54) and 1.72 (95% CI, 1.60-1.85) for infants of subfertile and ART-treated women, respectively, Dr. Hwang and her coinvestigators reported.
Infants born to subfertile or ART-treated women were also more likely to have adverse respiratory, gastrointestinal, or nutritional outcomes, with adjusted ORs ranging from 1.12 to 1.18, they added in the report.
Looking specifically at outcomes stratified by gestational age, they found an increased risk of congenital malformations, infectious diseases, and cardiovascular or respiratory outcomes, with adjusted ORs from 1.30 to 2.61, in the data published in the journal.
By contrast, there were no differences in risks of neonatal mortality, length of hospitalization, low birth weight, or neurologic and hematologic abnormalities for infants of subfertile and ART-treated women, compared with fertile women, according to Dr. Hwang and her coauthors.
These results confirm results of some previous studies that suggested a higher risk of adverse birth outcomes among infants born as singletons, according to the study authors.
“Although it is clearly accepted that multiple gestation is a significant predictor of preterm birth and low birth weight, recent studies have also revealed that, even among singleton births, mothers with infertility without ART treatment along with those who do undergo ART treatment are at higher risk for preterm delivery,” they wrote.
The study was funded by a grant from the National Institutes of Health. Authors said they had no financial relationships relevant to the study.
SOURCE: Hwang SS et al. Pediatrics. 2018 Aug;142(2):e20174069.
FROM PEDIATRICS
Key clinical point: Subfertility, whether treated by ART or not, is associated with adverse health outcomes for infants.
Major finding: Infants of subfertile and ART-treated women were more likely to be born preterm (odds ratios, 1.39 and 1.72, respectively) than were the infants of fertile women.
Study details: Population-based study of 350,123 infants from a Massachusetts clinical database.
Disclosures: The study was funded by a grant from the National Institutes of Health. The authors said they had no financial relationships relevant to the study.
Source: Hwang SS et al. Pediatrics. 2018 Aug;142(2):e20174069.
Thrown Off Track
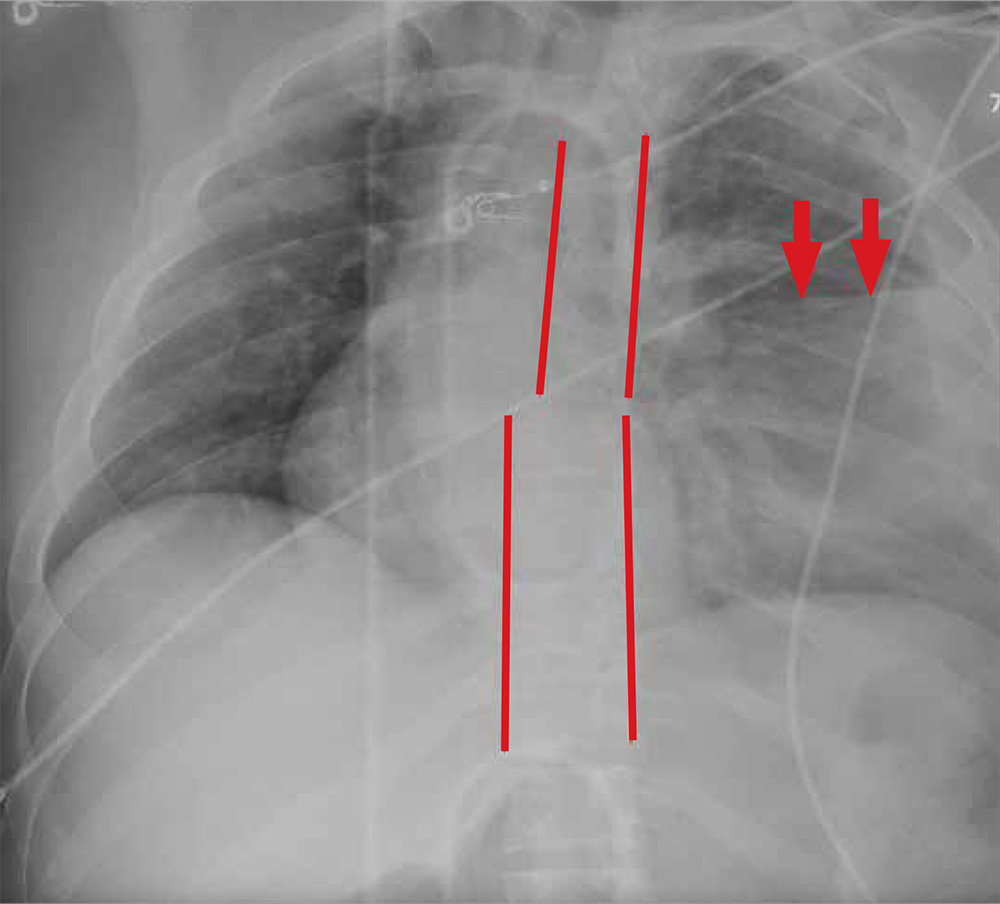
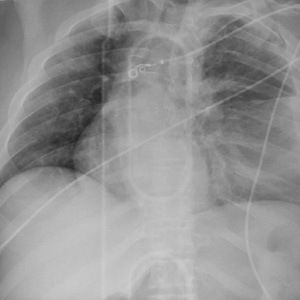
ANSWER
The radiograph shows rib fractures on the left side (arrows); on the same side, there is a moderate-sized pleural effusion—presumably a hemothorax from the trauma.
A closer look at the mid-thoracic spine reveals some irregularity and possible deformity—note the slight offset. This finding is strongly suspicious for a fracture.
A subsequent CT revealed a thoracic burst fracture with retropulsion into the spinal canal.

ANSWER
The radiograph shows rib fractures on the left side (arrows); on the same side, there is a moderate-sized pleural effusion—presumably a hemothorax from the trauma.
A closer look at the mid-thoracic spine reveals some irregularity and possible deformity—note the slight offset. This finding is strongly suspicious for a fracture.
A subsequent CT revealed a thoracic burst fracture with retropulsion into the spinal canal.

ANSWER
The radiograph shows rib fractures on the left side (arrows); on the same side, there is a moderate-sized pleural effusion—presumably a hemothorax from the trauma.
A closer look at the mid-thoracic spine reveals some irregularity and possible deformity—note the slight offset. This finding is strongly suspicious for a fracture.
A subsequent CT revealed a thoracic burst fracture with retropulsion into the spinal canal.
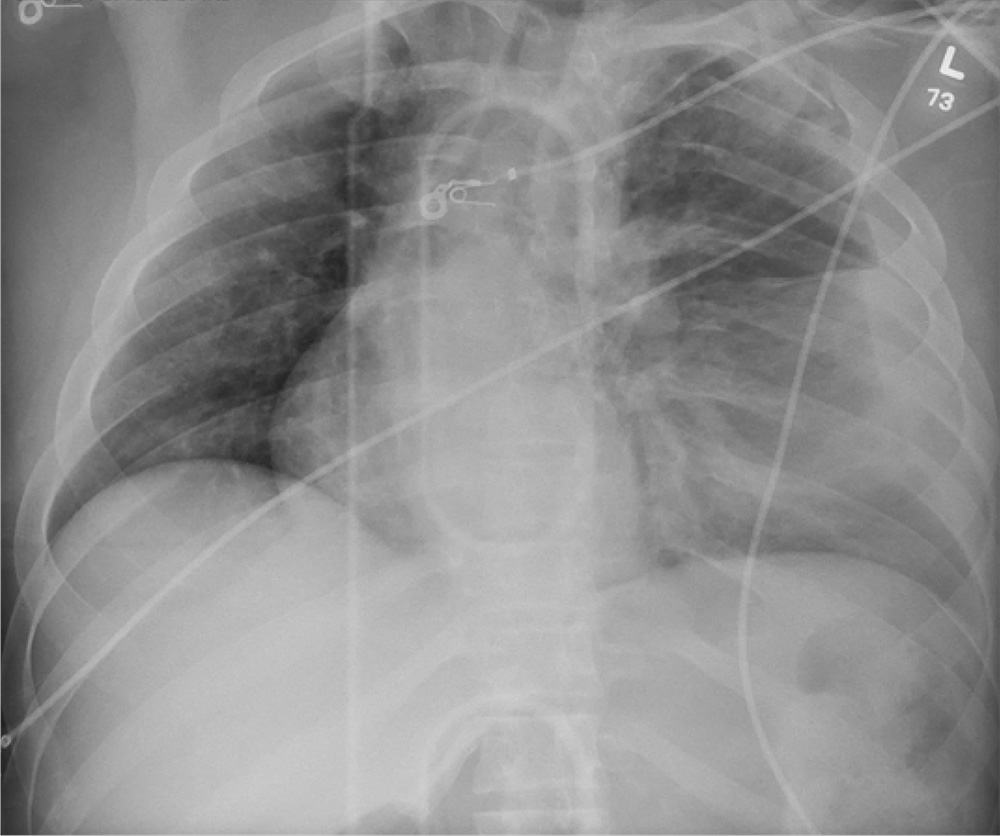
A 20-year-old man is riding a four-wheel all-terrain vehicle at a high rate of speed when he loses control and is thrown off. He is not wearin
As you begin your primary survey, you note a young male who is anxious but awake and able to converse. He is receiving 100% oxygen via a non-rebreather mask. His heart rate is 130 beats/min and his blood pressure, 80/40 mm Hg. Breath sounds are somewhat decreased on the left side. The patient can move both arms, and his strength is normal. However, he is insensate from his mid-chest down and is unable to move his legs at all.
Portable radiographs are obtained, including a chest radiograph (shown). What is your impression?
From the EVP/CEO
As we wrap up CHEST’s fiscal year 2017-18 (our fiscal year runs July 1 – June 30), it has been an incredibly positive and productive year, on all fronts. We have educated more learners than ever before, expanded our educational offerings, increased our collaboration with other organizations, grown our CHEST Foundation activities, and are in excellent financial shape to continue our commitment to clinical chest medicine education.
As we prepare for fiscal year 2018-19, I want to highlight some of the key programs, events, and projects we will be undertaking that will support our strategic plan (http://www.chestnet.org/About/Overview/Strategic-Plan) and achieve our mission to champion the prevention, diagnosis, and treatment of chest diseases through education, communication, and research.
Our organization goals are primarily focused on (but are not limited to) the following broad achievements:
a. Increasing the number of learners that CHEST engages (and increasing their engagement with our content) and assessing the results of our educational interactions
b. Keeping our journal CHEST® among the top Pulmonary, Critical Care, and Sleep peer review journals in the world
c. Expanding domestic and global access to CHEST guidelines and other relevant clinical content
d. Continuing to offer a positive and inclusive culture and work environment at CHEST, for our volunteers, world-class faculty, members, and staff
e. Meeting or exceeding our budget, reserve policy, and grant funding targets to ensure delivery of our mission-based educational efforts and programs
Because our mission as a 501(c)3 not-for-profit is education, I’ll start with those key programs that are driving our budget for FY2018-19 and will also cover publishing and membership.
Education – Clinical
• Increased global activity due to global partnerships with several key international educational providers
• Holding April 2019 CHEST World Congress in Bangkok
• Planning June 2019 Board Review conference in Athens, Greece
• 21 total international courses planned
• Increased Live Learning courses, simulation, and hands-on skills training
• 21 courses planned (including 3 new courses)
• Holding two Fellows courses at CHEST HQ (up to 80 fellows)
• Annual Meeting includes 11 postgraduate programs and 24 simulation courses (including more cadaver courses)
• Includes more Fellows courses (up to 240 Fellows)
• Board Courses include two half-day simulation courses; more sponsorship/exhibits, games, and virtual patient tours (VPTs)
• Continuing to build Board Review on-demand and e-learning content packages for those who cannot attend live events
• Launching inaugural e-Learning program with Elsevier
Education – Patient
• Developing multiple CHEST Foundation disease awareness campaigns and patient education resources
• New patient education guides
• Increased visual content (infographics, graphically based materials)
• Increased use of multimedia and video content
• Increased funding for clinical research grants, community service programs and lung health events, and fund-raising through cause marketing (i.e., Feldman Family Poker Night, NYC events, and other local fund raising events)
• Expanding awareness and access of our patient education materials
• Institutions, large group practices
• International reach
• Digital distribution via social media and online campaigns
Education – Industry
• Projecting seven new live clinical immersion courses
• Two new proposed PREP courses with CTS
• Expansion of educational games, VPTs, and e-learning
• Expanded CHEST Analytics Product Lines
• View Points (3 focus groups, 4-5 KOL panels, 4 pulse surveys)
• Deep Dives (3 advanced analytics projects, 5 premium research projects, 2 ethnography studies, and 4-6 Clinical Perspectives)
• Data Lab (looking to launch beta partner)
• Booth IQ (increasing capacity for booth flow and booth intel reports)
Publications, Guidelines and Digital Content
• CHEST® Journal
• Elsevier partnership remains strong; leveraging key data and Elsevier offerings, will be announcing the next Editor in Chief
• CHEST Physician
• New content and delivery mechanisms
• Supplements
• Electronic features
• CHEST SEEK
• Publish Volume 28 (Critical Care)
• Continue development of SEEK online library
• Guidelines
• Completions: Antithrombotic therapy, cough, ILD diagnosis, hypersensitivity pneumonitis, lung cancer, and PAH
• Updates: Antithrombotic therapy, lung cancer, cough, neuromuscular weakness, EBUS needle sampling, and blood transfusions in critical care setting (doing more in critical care)
• Piloting use of DoctorEvidence methodology services and platform for “living guidelines”
Membership
• Focusing on adding value to CHEST membership for key segments
• Bundling e-learning packages with membership
• Exploring international group/society memberships and group practice/institutional memberships
• Working to attract advanced practice providers
• Performing member market research, including member satisfaction, net promoter scores, and other key metrics
Supporting Divisions (Finance, Marketing, IT, Capital expenses)
• Have more visibility (booth presence) at more meetings (AACN, AARC (new), ALAT, APSR, ATS, CTS, ERS, SCCM, and more)
• Develop and execute comprehensive marketing and branding strategies for all business units
• Clinical Education (CHEST annual meeting, Board Reviews, all int’l meetings and live learning, simulation)
• Industry Education (PREP, CHEST Analytics)
• Patient Education
• Foundation Fundraising
• Publishing and Content Strategy
• Membership
• Support new IT platforms and bolster security (HR, Finance, Board Effect, Tableau, CHEST analytics, LMS, CMS, NetForum AMS), as well as marketing and social interaction tools (HubSpot)
• Maintain Capital Budget for building, infrastructure, technology, etc
All in all, CHEST has a very active fiscal year planned, with a number of new educational programs and e-learning opportunities showcasing CHEST’s unique brand of innovative clinical education. We look forward to connecting with you and impacting health-care delivery and patient outcomes. It is an honor and a privilege to be able to lead this organization, and all of this news is directly attributable to our dedicated volunteer leadership, faculty, content expertise, staff, and valuable time that you all contribute to make this organization great. Thank you for your ongoing support of CHEST.
.
As we wrap up CHEST’s fiscal year 2017-18 (our fiscal year runs July 1 – June 30), it has been an incredibly positive and productive year, on all fronts. We have educated more learners than ever before, expanded our educational offerings, increased our collaboration with other organizations, grown our CHEST Foundation activities, and are in excellent financial shape to continue our commitment to clinical chest medicine education.
As we prepare for fiscal year 2018-19, I want to highlight some of the key programs, events, and projects we will be undertaking that will support our strategic plan (http://www.chestnet.org/About/Overview/Strategic-Plan) and achieve our mission to champion the prevention, diagnosis, and treatment of chest diseases through education, communication, and research.
Our organization goals are primarily focused on (but are not limited to) the following broad achievements:
a. Increasing the number of learners that CHEST engages (and increasing their engagement with our content) and assessing the results of our educational interactions
b. Keeping our journal CHEST® among the top Pulmonary, Critical Care, and Sleep peer review journals in the world
c. Expanding domestic and global access to CHEST guidelines and other relevant clinical content
d. Continuing to offer a positive and inclusive culture and work environment at CHEST, for our volunteers, world-class faculty, members, and staff
e. Meeting or exceeding our budget, reserve policy, and grant funding targets to ensure delivery of our mission-based educational efforts and programs
Because our mission as a 501(c)3 not-for-profit is education, I’ll start with those key programs that are driving our budget for FY2018-19 and will also cover publishing and membership.
Education – Clinical
• Increased global activity due to global partnerships with several key international educational providers
• Holding April 2019 CHEST World Congress in Bangkok
• Planning June 2019 Board Review conference in Athens, Greece
• 21 total international courses planned
• Increased Live Learning courses, simulation, and hands-on skills training
• 21 courses planned (including 3 new courses)
• Holding two Fellows courses at CHEST HQ (up to 80 fellows)
• Annual Meeting includes 11 postgraduate programs and 24 simulation courses (including more cadaver courses)
• Includes more Fellows courses (up to 240 Fellows)
• Board Courses include two half-day simulation courses; more sponsorship/exhibits, games, and virtual patient tours (VPTs)
• Continuing to build Board Review on-demand and e-learning content packages for those who cannot attend live events
• Launching inaugural e-Learning program with Elsevier
Education – Patient
• Developing multiple CHEST Foundation disease awareness campaigns and patient education resources
• New patient education guides
• Increased visual content (infographics, graphically based materials)
• Increased use of multimedia and video content
• Increased funding for clinical research grants, community service programs and lung health events, and fund-raising through cause marketing (i.e., Feldman Family Poker Night, NYC events, and other local fund raising events)
• Expanding awareness and access of our patient education materials
• Institutions, large group practices
• International reach
• Digital distribution via social media and online campaigns
Education – Industry
• Projecting seven new live clinical immersion courses
• Two new proposed PREP courses with CTS
• Expansion of educational games, VPTs, and e-learning
• Expanded CHEST Analytics Product Lines
• View Points (3 focus groups, 4-5 KOL panels, 4 pulse surveys)
• Deep Dives (3 advanced analytics projects, 5 premium research projects, 2 ethnography studies, and 4-6 Clinical Perspectives)
• Data Lab (looking to launch beta partner)
• Booth IQ (increasing capacity for booth flow and booth intel reports)
Publications, Guidelines and Digital Content
• CHEST® Journal
• Elsevier partnership remains strong; leveraging key data and Elsevier offerings, will be announcing the next Editor in Chief
• CHEST Physician
• New content and delivery mechanisms
• Supplements
• Electronic features
• CHEST SEEK
• Publish Volume 28 (Critical Care)
• Continue development of SEEK online library
• Guidelines
• Completions: Antithrombotic therapy, cough, ILD diagnosis, hypersensitivity pneumonitis, lung cancer, and PAH
• Updates: Antithrombotic therapy, lung cancer, cough, neuromuscular weakness, EBUS needle sampling, and blood transfusions in critical care setting (doing more in critical care)
• Piloting use of DoctorEvidence methodology services and platform for “living guidelines”
Membership
• Focusing on adding value to CHEST membership for key segments
• Bundling e-learning packages with membership
• Exploring international group/society memberships and group practice/institutional memberships
• Working to attract advanced practice providers
• Performing member market research, including member satisfaction, net promoter scores, and other key metrics
Supporting Divisions (Finance, Marketing, IT, Capital expenses)
• Have more visibility (booth presence) at more meetings (AACN, AARC (new), ALAT, APSR, ATS, CTS, ERS, SCCM, and more)
• Develop and execute comprehensive marketing and branding strategies for all business units
• Clinical Education (CHEST annual meeting, Board Reviews, all int’l meetings and live learning, simulation)
• Industry Education (PREP, CHEST Analytics)
• Patient Education
• Foundation Fundraising
• Publishing and Content Strategy
• Membership
• Support new IT platforms and bolster security (HR, Finance, Board Effect, Tableau, CHEST analytics, LMS, CMS, NetForum AMS), as well as marketing and social interaction tools (HubSpot)
• Maintain Capital Budget for building, infrastructure, technology, etc
All in all, CHEST has a very active fiscal year planned, with a number of new educational programs and e-learning opportunities showcasing CHEST’s unique brand of innovative clinical education. We look forward to connecting with you and impacting health-care delivery and patient outcomes. It is an honor and a privilege to be able to lead this organization, and all of this news is directly attributable to our dedicated volunteer leadership, faculty, content expertise, staff, and valuable time that you all contribute to make this organization great. Thank you for your ongoing support of CHEST.
.
As we wrap up CHEST’s fiscal year 2017-18 (our fiscal year runs July 1 – June 30), it has been an incredibly positive and productive year, on all fronts. We have educated more learners than ever before, expanded our educational offerings, increased our collaboration with other organizations, grown our CHEST Foundation activities, and are in excellent financial shape to continue our commitment to clinical chest medicine education.
As we prepare for fiscal year 2018-19, I want to highlight some of the key programs, events, and projects we will be undertaking that will support our strategic plan (http://www.chestnet.org/About/Overview/Strategic-Plan) and achieve our mission to champion the prevention, diagnosis, and treatment of chest diseases through education, communication, and research.
Our organization goals are primarily focused on (but are not limited to) the following broad achievements:
a. Increasing the number of learners that CHEST engages (and increasing their engagement with our content) and assessing the results of our educational interactions
b. Keeping our journal CHEST® among the top Pulmonary, Critical Care, and Sleep peer review journals in the world
c. Expanding domestic and global access to CHEST guidelines and other relevant clinical content
d. Continuing to offer a positive and inclusive culture and work environment at CHEST, for our volunteers, world-class faculty, members, and staff
e. Meeting or exceeding our budget, reserve policy, and grant funding targets to ensure delivery of our mission-based educational efforts and programs
Because our mission as a 501(c)3 not-for-profit is education, I’ll start with those key programs that are driving our budget for FY2018-19 and will also cover publishing and membership.
Education – Clinical
• Increased global activity due to global partnerships with several key international educational providers
• Holding April 2019 CHEST World Congress in Bangkok
• Planning June 2019 Board Review conference in Athens, Greece
• 21 total international courses planned
• Increased Live Learning courses, simulation, and hands-on skills training
• 21 courses planned (including 3 new courses)
• Holding two Fellows courses at CHEST HQ (up to 80 fellows)
• Annual Meeting includes 11 postgraduate programs and 24 simulation courses (including more cadaver courses)
• Includes more Fellows courses (up to 240 Fellows)
• Board Courses include two half-day simulation courses; more sponsorship/exhibits, games, and virtual patient tours (VPTs)
• Continuing to build Board Review on-demand and e-learning content packages for those who cannot attend live events
• Launching inaugural e-Learning program with Elsevier
Education – Patient
• Developing multiple CHEST Foundation disease awareness campaigns and patient education resources
• New patient education guides
• Increased visual content (infographics, graphically based materials)
• Increased use of multimedia and video content
• Increased funding for clinical research grants, community service programs and lung health events, and fund-raising through cause marketing (i.e., Feldman Family Poker Night, NYC events, and other local fund raising events)
• Expanding awareness and access of our patient education materials
• Institutions, large group practices
• International reach
• Digital distribution via social media and online campaigns
Education – Industry
• Projecting seven new live clinical immersion courses
• Two new proposed PREP courses with CTS
• Expansion of educational games, VPTs, and e-learning
• Expanded CHEST Analytics Product Lines
• View Points (3 focus groups, 4-5 KOL panels, 4 pulse surveys)
• Deep Dives (3 advanced analytics projects, 5 premium research projects, 2 ethnography studies, and 4-6 Clinical Perspectives)
• Data Lab (looking to launch beta partner)
• Booth IQ (increasing capacity for booth flow and booth intel reports)
Publications, Guidelines and Digital Content
• CHEST® Journal
• Elsevier partnership remains strong; leveraging key data and Elsevier offerings, will be announcing the next Editor in Chief
• CHEST Physician
• New content and delivery mechanisms
• Supplements
• Electronic features
• CHEST SEEK
• Publish Volume 28 (Critical Care)
• Continue development of SEEK online library
• Guidelines
• Completions: Antithrombotic therapy, cough, ILD diagnosis, hypersensitivity pneumonitis, lung cancer, and PAH
• Updates: Antithrombotic therapy, lung cancer, cough, neuromuscular weakness, EBUS needle sampling, and blood transfusions in critical care setting (doing more in critical care)
• Piloting use of DoctorEvidence methodology services and platform for “living guidelines”
Membership
• Focusing on adding value to CHEST membership for key segments
• Bundling e-learning packages with membership
• Exploring international group/society memberships and group practice/institutional memberships
• Working to attract advanced practice providers
• Performing member market research, including member satisfaction, net promoter scores, and other key metrics
Supporting Divisions (Finance, Marketing, IT, Capital expenses)
• Have more visibility (booth presence) at more meetings (AACN, AARC (new), ALAT, APSR, ATS, CTS, ERS, SCCM, and more)
• Develop and execute comprehensive marketing and branding strategies for all business units
• Clinical Education (CHEST annual meeting, Board Reviews, all int’l meetings and live learning, simulation)
• Industry Education (PREP, CHEST Analytics)
• Patient Education
• Foundation Fundraising
• Publishing and Content Strategy
• Membership
• Support new IT platforms and bolster security (HR, Finance, Board Effect, Tableau, CHEST analytics, LMS, CMS, NetForum AMS), as well as marketing and social interaction tools (HubSpot)
• Maintain Capital Budget for building, infrastructure, technology, etc
All in all, CHEST has a very active fiscal year planned, with a number of new educational programs and e-learning opportunities showcasing CHEST’s unique brand of innovative clinical education. We look forward to connecting with you and impacting health-care delivery and patient outcomes. It is an honor and a privilege to be able to lead this organization, and all of this news is directly attributable to our dedicated volunteer leadership, faculty, content expertise, staff, and valuable time that you all contribute to make this organization great. Thank you for your ongoing support of CHEST.
.
Airways, Consent, Fluid Resuscitation, Home Ventilation
Airways Disorders
Quadrupling the inhaled glucocorticoid dose in those with deteriorating asthma control: Zone 2 asthma
Asthma exacerbations account for most asthma-associated health-care costs and are a key outcome for successful asthma management programs. Inhaled corticosteroid (ICS) forms the cornerstone of asthma maintenance therapy.
Previously published data show that:
Most therapeutic benefit of budesonide was achieved at dose range of 400-1000 µg/day (Masoli et al. Eur Respir J. 2004;23:552).
Doubling ICS dose was ineffective in preventing acute asthma exacerbations (Harrison et al. Lancet. 2004;363:271. FitzGerald et al. Thorax. 2004;59: 550).
Increasing ICS dose was unlikely to reduce systemic glucocorticoid use or hospitalization for asthma exacerbations(Kew et al. Cochrane Database Syst Rev. 2016;6:CD007524).
A recent open-label pragmatic study, published in the New England Journal of Medicine, included 1,922 adolescents and adults with asthma. The authors observed a small reduction in severe asthma exacerbations (Hazard ratio 0.81 for time to first severe exacerbation) by quadrupling the dose of ICS during periods of worsening asthma control (McKeever et al. N Engl J Med. 2018;378:902).
This study does create opportunities for cost-benefit by decreasing health-care utilization, decrease in systemic steroid exposure in some patients, and increase in patient awareness of asthma control allowing self-management. Although statistically significant, the treatment effect was small, with 45% of subjects in the ‘quadrupling dose’ arm still experiencing severe exacerbations. Intervention arm also experienced increased rate of adverse effects.
Additional studies are needed before this strategy can be broadly applied. In the same issue of NEJM, quintupling the dose of ICS in children was not associated with decrease in exacerbations (Jackson et al. N Engl J Med. 2018;378:891). The fact that nearly half of asthmatics who quadrupled ICS dose had exacerbations is disconcerting. This highlights an urgent need to understand treatment-responsive phenotypes, mechanisms of steroid sensitivity, and modalities to improve them, if we are to reduce asthma morbidity in the community.
Navitha Ramesh, MD
Steering Committee Member
Mahesh Padukudru Anand, MBBS, FCCP
Steering Committee Member
Clinical Research
Informed consent: Do we need to change our practice?
Informed consent is the keystone of clinical research and helps respect and protect the rights of the participants/subjects. While the informed consent process has been standardized, some challenges still remain, such as pieces of information that should be disclosed, how to disclose information and document understanding of participants, and how detailed that disclosure should be (Grady, N Engl J Med. 2015;372:855). Digital technology can and has been used to improve the process of obtaining informed consent.
Substituting long and complex written forms with electronic consent (e-consent), however, has issues. Few people read through online agreements before clicking “agree,” which may lead to participants consenting without a clear understanding of what they are consenting to. On the other hand, it is also possible to use e-consent to improve comprehension by including videos and graphics. Interactive quizzes can assess the understanding of the participants, embedded links to audios or videos can further enhance the grasp of information. With e-consents, queries from participants can be answered via phone call or email. When e-consent is obtained remotely, the identity can be confirmed by electronic signatures, username, password, or biometrics.
E-consent has advantages, can be done remotely, no paper is needed, etc. It has potential disadvantages like being costly, videos can add time to the process, and multicenter international trials can be difficult (Grady, et al. N Engl J Med, 2017; 376:e43). Studying e-consents to identify gaps in communication between the researcher and the participant in the digitalized world may help improve the process and allow research to proceed with better understanding of the risks and benefits of involvement in clinical research.
Mohsin Ijaz, MD, FCCP
Steering Committee Member
Critical Care
Fluid Resuscitation in ICU Patients With Sepsis
Appropriate fluid resuscitation is a major goal in sepsis management. Debate remains regarding fluid choice and the impact on acute kidney injury (AKI), renal replacement therapy (RRT), and mortality. Normal saline solution (NS) may be associated with hyperchloremic metabolic acidosis, AKI, and death, but study results have been inconsistent. A large before-after study revealed that balanced crystalloids (BC) were associated with lower rates of AKI and RRT but did not impact mortality (Yunos et al. JAMA. 2012;308:1566). A meta-analysis specifically examining patients with sepsis failed to find a significant difference in RRT or mortality, although this conclusion was of low certainty (Rochwerg, et al., Intensive Care Med. 2015;41:1561).
Earlier this year, a large RCT comparing NS vs BC demonstrated a reduction in major adverse kidney events using BC. Independent rates of new RRT, mortality, and persistent renal dysfunction were not significant, but when combined as a composite outcome, the difference was significant. A 30-day mortality reduction was significant in patients with sepsis (25.2% BC vs 29.4% NS) and in patients with large infusions of NS (Semler et al., N Engl J Med. 2018;378:829). Given these results, a move toward a “balanced approach” to fluid resuscitation seems prudent and may be the next step toward improving outcomes in sepsis. These results are likely related to the large infusions of fluid in patients with sepsis or to the inflammatory effects of the disease. Finally, the applicability of these outcomes to the overall critically ill population is still open to debate.
Margaret Disselkamp, MD
Steering Committee Member
Home-Based Mechanical Ventilation and Neuromuscular Disease
Transcutaneous Carbon Dioxide Monitoring: New Era for Home Ventilation
A primary objective of noninvasive home ventilation is normalization of arterial blood gas tensions, night and day. Pulse oximetry has long enabled estimation of arterial oxygen saturation (SpO2) in outpatient offices and overnight at home; however, until recently, measurement of the partial pressure of carbon dioxide (PCO2) has been limited to invasive arterial blood gas testing (PaCO2) or end-tidal CO2 (PetCO2) measurements. Assessment of PetCO2 has been limited by challenges in accessing true end-tidal exhaled gas under a face mask during noninvasive ventilation, particularly for patients with parenchymal lung diseases such as COPD.
Thanks to recent technological advances, transcutaneous measurement of carbon dioxide (PtcCO2) is emerging as the method of choice for assessing the adequacy of noninvasive ventilation. PtcCO2 monitoring is a standard assessment for pediatric patients in the sleep lab, and it is increasingly being utilized in adults to complement diagnostic and treatment purposes. The transcutaneous CO2 sensors work by heating underlying skin to approximately 43° C, increasing blood flow through the underlying dermal capillary bed. Within 2 to 5 minutes, the “arteriolized” capillary PtcCO2 approximates PaCO2. Commercially available devices for measuring PtcCO2 reliably estimate PaCO2 in patients undergoing noninvasive ventilation to within 5 mm Hg (95% CI) (Storre et al. Respir Med. 2010;105:143).
PtcCO2 measurement has limitations. Measured PtcCO2 can drift upward (i.e., technical drift) during continuous monitoring; however, currently available devices adequately adjust for this phenomenon. Arterialization may be limited by thickened skin, edema, or hypoperfusion.
Currently, U.S. insurance companies do not accept PtcCO2 for documentation of hypercapnia, and the cost of measuring PtcCO2 is not reimbursed. Nevertheless, PtcCO2 technology promises a new era for home mechanical ventilation guided by accurate and practical assessment of PCO2, in particular for chronic respiratory failure syndromes. In this setting, home PtcCO2 monitoring potentially can be utilized in place of in-laboratory sleep studies for assessment of nocturnal hypoventilation and optimizing home mechanical ventilation.
Jason Ackrivo, MD
Steering Committee Member
Interstitial and Diffuse Lung Disease
Electronic Patient Education
The management of patients with an interstitial lung disease (ILD) is challenging. A provider must examine the fine details about current and prior medication history, explore various occupational and environmental exposures, perform a thorough physical examination that includes a careful dermatologic and rheumatologic review, and peruse the objective data, such as the high-resolution CT scan of the chest and pulmonary function tests. Then, the pulmonologist and the patient (plus often multiple family members) discuss diagnostic possibilities, any future testing for confirmation, and prognostic implications. Understandably, the patient may leave the office bewildered, overwhelmed, and in search of clarification.
Bewilderment may lead to the internet. In 2001, 4.5% of all internet searches were determined to be health-care-related (Eysenbach et al. AMIA Annu Symp Proc. 2003;225). It is reasonable to presume the percentage is higher today. Just as with any nonmedical website, the choices for digital health-care information are sometimes not contemporaneous and vary in quality. By exploring the most common “hits” on popular search engines when searching for idiopathic pulmonary fibrosis, a 2016 study found that not only is information presented at a high reading level – 12th grade – but often outdated or simply wrong (Fisher, et al. Am J Respir Crit Care Med. 2016;194[2)]:218). Adding to a patient’s possible confusion is that websites expected to be the most helpful, foundation or advocacy websites, were more likely to suggest disproven and even harmful therapies years after those conclusions were published.
CHEST and the Interstitial and Diffuse Lung Disease NetWork are committed to patient education both in and out of the clinical setting. An ongoing redesign of ILD patient education on the CHEST Foundation website is nearing completion and will ensure patients have the most accurate and understandable information available.
Corey Kershaw, MD
Steering Committee Member
Airways Disorders
Quadrupling the inhaled glucocorticoid dose in those with deteriorating asthma control: Zone 2 asthma
Asthma exacerbations account for most asthma-associated health-care costs and are a key outcome for successful asthma management programs. Inhaled corticosteroid (ICS) forms the cornerstone of asthma maintenance therapy.
Previously published data show that:
Most therapeutic benefit of budesonide was achieved at dose range of 400-1000 µg/day (Masoli et al. Eur Respir J. 2004;23:552).
Doubling ICS dose was ineffective in preventing acute asthma exacerbations (Harrison et al. Lancet. 2004;363:271. FitzGerald et al. Thorax. 2004;59: 550).
Increasing ICS dose was unlikely to reduce systemic glucocorticoid use or hospitalization for asthma exacerbations(Kew et al. Cochrane Database Syst Rev. 2016;6:CD007524).
A recent open-label pragmatic study, published in the New England Journal of Medicine, included 1,922 adolescents and adults with asthma. The authors observed a small reduction in severe asthma exacerbations (Hazard ratio 0.81 for time to first severe exacerbation) by quadrupling the dose of ICS during periods of worsening asthma control (McKeever et al. N Engl J Med. 2018;378:902).
This study does create opportunities for cost-benefit by decreasing health-care utilization, decrease in systemic steroid exposure in some patients, and increase in patient awareness of asthma control allowing self-management. Although statistically significant, the treatment effect was small, with 45% of subjects in the ‘quadrupling dose’ arm still experiencing severe exacerbations. Intervention arm also experienced increased rate of adverse effects.
Additional studies are needed before this strategy can be broadly applied. In the same issue of NEJM, quintupling the dose of ICS in children was not associated with decrease in exacerbations (Jackson et al. N Engl J Med. 2018;378:891). The fact that nearly half of asthmatics who quadrupled ICS dose had exacerbations is disconcerting. This highlights an urgent need to understand treatment-responsive phenotypes, mechanisms of steroid sensitivity, and modalities to improve them, if we are to reduce asthma morbidity in the community.
Navitha Ramesh, MD
Steering Committee Member
Mahesh Padukudru Anand, MBBS, FCCP
Steering Committee Member
Clinical Research
Informed consent: Do we need to change our practice?
Informed consent is the keystone of clinical research and helps respect and protect the rights of the participants/subjects. While the informed consent process has been standardized, some challenges still remain, such as pieces of information that should be disclosed, how to disclose information and document understanding of participants, and how detailed that disclosure should be (Grady, N Engl J Med. 2015;372:855). Digital technology can and has been used to improve the process of obtaining informed consent.
Substituting long and complex written forms with electronic consent (e-consent), however, has issues. Few people read through online agreements before clicking “agree,” which may lead to participants consenting without a clear understanding of what they are consenting to. On the other hand, it is also possible to use e-consent to improve comprehension by including videos and graphics. Interactive quizzes can assess the understanding of the participants, embedded links to audios or videos can further enhance the grasp of information. With e-consents, queries from participants can be answered via phone call or email. When e-consent is obtained remotely, the identity can be confirmed by electronic signatures, username, password, or biometrics.
E-consent has advantages, can be done remotely, no paper is needed, etc. It has potential disadvantages like being costly, videos can add time to the process, and multicenter international trials can be difficult (Grady, et al. N Engl J Med, 2017; 376:e43). Studying e-consents to identify gaps in communication between the researcher and the participant in the digitalized world may help improve the process and allow research to proceed with better understanding of the risks and benefits of involvement in clinical research.
Mohsin Ijaz, MD, FCCP
Steering Committee Member
Critical Care
Fluid Resuscitation in ICU Patients With Sepsis
Appropriate fluid resuscitation is a major goal in sepsis management. Debate remains regarding fluid choice and the impact on acute kidney injury (AKI), renal replacement therapy (RRT), and mortality. Normal saline solution (NS) may be associated with hyperchloremic metabolic acidosis, AKI, and death, but study results have been inconsistent. A large before-after study revealed that balanced crystalloids (BC) were associated with lower rates of AKI and RRT but did not impact mortality (Yunos et al. JAMA. 2012;308:1566). A meta-analysis specifically examining patients with sepsis failed to find a significant difference in RRT or mortality, although this conclusion was of low certainty (Rochwerg, et al., Intensive Care Med. 2015;41:1561).
Earlier this year, a large RCT comparing NS vs BC demonstrated a reduction in major adverse kidney events using BC. Independent rates of new RRT, mortality, and persistent renal dysfunction were not significant, but when combined as a composite outcome, the difference was significant. A 30-day mortality reduction was significant in patients with sepsis (25.2% BC vs 29.4% NS) and in patients with large infusions of NS (Semler et al., N Engl J Med. 2018;378:829). Given these results, a move toward a “balanced approach” to fluid resuscitation seems prudent and may be the next step toward improving outcomes in sepsis. These results are likely related to the large infusions of fluid in patients with sepsis or to the inflammatory effects of the disease. Finally, the applicability of these outcomes to the overall critically ill population is still open to debate.
Margaret Disselkamp, MD
Steering Committee Member
Home-Based Mechanical Ventilation and Neuromuscular Disease
Transcutaneous Carbon Dioxide Monitoring: New Era for Home Ventilation
A primary objective of noninvasive home ventilation is normalization of arterial blood gas tensions, night and day. Pulse oximetry has long enabled estimation of arterial oxygen saturation (SpO2) in outpatient offices and overnight at home; however, until recently, measurement of the partial pressure of carbon dioxide (PCO2) has been limited to invasive arterial blood gas testing (PaCO2) or end-tidal CO2 (PetCO2) measurements. Assessment of PetCO2 has been limited by challenges in accessing true end-tidal exhaled gas under a face mask during noninvasive ventilation, particularly for patients with parenchymal lung diseases such as COPD.
Thanks to recent technological advances, transcutaneous measurement of carbon dioxide (PtcCO2) is emerging as the method of choice for assessing the adequacy of noninvasive ventilation. PtcCO2 monitoring is a standard assessment for pediatric patients in the sleep lab, and it is increasingly being utilized in adults to complement diagnostic and treatment purposes. The transcutaneous CO2 sensors work by heating underlying skin to approximately 43° C, increasing blood flow through the underlying dermal capillary bed. Within 2 to 5 minutes, the “arteriolized” capillary PtcCO2 approximates PaCO2. Commercially available devices for measuring PtcCO2 reliably estimate PaCO2 in patients undergoing noninvasive ventilation to within 5 mm Hg (95% CI) (Storre et al. Respir Med. 2010;105:143).
PtcCO2 measurement has limitations. Measured PtcCO2 can drift upward (i.e., technical drift) during continuous monitoring; however, currently available devices adequately adjust for this phenomenon. Arterialization may be limited by thickened skin, edema, or hypoperfusion.
Currently, U.S. insurance companies do not accept PtcCO2 for documentation of hypercapnia, and the cost of measuring PtcCO2 is not reimbursed. Nevertheless, PtcCO2 technology promises a new era for home mechanical ventilation guided by accurate and practical assessment of PCO2, in particular for chronic respiratory failure syndromes. In this setting, home PtcCO2 monitoring potentially can be utilized in place of in-laboratory sleep studies for assessment of nocturnal hypoventilation and optimizing home mechanical ventilation.
Jason Ackrivo, MD
Steering Committee Member
Interstitial and Diffuse Lung Disease
Electronic Patient Education
The management of patients with an interstitial lung disease (ILD) is challenging. A provider must examine the fine details about current and prior medication history, explore various occupational and environmental exposures, perform a thorough physical examination that includes a careful dermatologic and rheumatologic review, and peruse the objective data, such as the high-resolution CT scan of the chest and pulmonary function tests. Then, the pulmonologist and the patient (plus often multiple family members) discuss diagnostic possibilities, any future testing for confirmation, and prognostic implications. Understandably, the patient may leave the office bewildered, overwhelmed, and in search of clarification.
Bewilderment may lead to the internet. In 2001, 4.5% of all internet searches were determined to be health-care-related (Eysenbach et al. AMIA Annu Symp Proc. 2003;225). It is reasonable to presume the percentage is higher today. Just as with any nonmedical website, the choices for digital health-care information are sometimes not contemporaneous and vary in quality. By exploring the most common “hits” on popular search engines when searching for idiopathic pulmonary fibrosis, a 2016 study found that not only is information presented at a high reading level – 12th grade – but often outdated or simply wrong (Fisher, et al. Am J Respir Crit Care Med. 2016;194[2)]:218). Adding to a patient’s possible confusion is that websites expected to be the most helpful, foundation or advocacy websites, were more likely to suggest disproven and even harmful therapies years after those conclusions were published.
CHEST and the Interstitial and Diffuse Lung Disease NetWork are committed to patient education both in and out of the clinical setting. An ongoing redesign of ILD patient education on the CHEST Foundation website is nearing completion and will ensure patients have the most accurate and understandable information available.
Corey Kershaw, MD
Steering Committee Member
Airways Disorders
Quadrupling the inhaled glucocorticoid dose in those with deteriorating asthma control: Zone 2 asthma
Asthma exacerbations account for most asthma-associated health-care costs and are a key outcome for successful asthma management programs. Inhaled corticosteroid (ICS) forms the cornerstone of asthma maintenance therapy.
Previously published data show that:
Most therapeutic benefit of budesonide was achieved at dose range of 400-1000 µg/day (Masoli et al. Eur Respir J. 2004;23:552).
Doubling ICS dose was ineffective in preventing acute asthma exacerbations (Harrison et al. Lancet. 2004;363:271. FitzGerald et al. Thorax. 2004;59: 550).
Increasing ICS dose was unlikely to reduce systemic glucocorticoid use or hospitalization for asthma exacerbations(Kew et al. Cochrane Database Syst Rev. 2016;6:CD007524).
A recent open-label pragmatic study, published in the New England Journal of Medicine, included 1,922 adolescents and adults with asthma. The authors observed a small reduction in severe asthma exacerbations (Hazard ratio 0.81 for time to first severe exacerbation) by quadrupling the dose of ICS during periods of worsening asthma control (McKeever et al. N Engl J Med. 2018;378:902).
This study does create opportunities for cost-benefit by decreasing health-care utilization, decrease in systemic steroid exposure in some patients, and increase in patient awareness of asthma control allowing self-management. Although statistically significant, the treatment effect was small, with 45% of subjects in the ‘quadrupling dose’ arm still experiencing severe exacerbations. Intervention arm also experienced increased rate of adverse effects.
Additional studies are needed before this strategy can be broadly applied. In the same issue of NEJM, quintupling the dose of ICS in children was not associated with decrease in exacerbations (Jackson et al. N Engl J Med. 2018;378:891). The fact that nearly half of asthmatics who quadrupled ICS dose had exacerbations is disconcerting. This highlights an urgent need to understand treatment-responsive phenotypes, mechanisms of steroid sensitivity, and modalities to improve them, if we are to reduce asthma morbidity in the community.
Navitha Ramesh, MD
Steering Committee Member
Mahesh Padukudru Anand, MBBS, FCCP
Steering Committee Member
Clinical Research
Informed consent: Do we need to change our practice?
Informed consent is the keystone of clinical research and helps respect and protect the rights of the participants/subjects. While the informed consent process has been standardized, some challenges still remain, such as pieces of information that should be disclosed, how to disclose information and document understanding of participants, and how detailed that disclosure should be (Grady, N Engl J Med. 2015;372:855). Digital technology can and has been used to improve the process of obtaining informed consent.
Substituting long and complex written forms with electronic consent (e-consent), however, has issues. Few people read through online agreements before clicking “agree,” which may lead to participants consenting without a clear understanding of what they are consenting to. On the other hand, it is also possible to use e-consent to improve comprehension by including videos and graphics. Interactive quizzes can assess the understanding of the participants, embedded links to audios or videos can further enhance the grasp of information. With e-consents, queries from participants can be answered via phone call or email. When e-consent is obtained remotely, the identity can be confirmed by electronic signatures, username, password, or biometrics.
E-consent has advantages, can be done remotely, no paper is needed, etc. It has potential disadvantages like being costly, videos can add time to the process, and multicenter international trials can be difficult (Grady, et al. N Engl J Med, 2017; 376:e43). Studying e-consents to identify gaps in communication between the researcher and the participant in the digitalized world may help improve the process and allow research to proceed with better understanding of the risks and benefits of involvement in clinical research.
Mohsin Ijaz, MD, FCCP
Steering Committee Member
Critical Care
Fluid Resuscitation in ICU Patients With Sepsis
Appropriate fluid resuscitation is a major goal in sepsis management. Debate remains regarding fluid choice and the impact on acute kidney injury (AKI), renal replacement therapy (RRT), and mortality. Normal saline solution (NS) may be associated with hyperchloremic metabolic acidosis, AKI, and death, but study results have been inconsistent. A large before-after study revealed that balanced crystalloids (BC) were associated with lower rates of AKI and RRT but did not impact mortality (Yunos et al. JAMA. 2012;308:1566). A meta-analysis specifically examining patients with sepsis failed to find a significant difference in RRT or mortality, although this conclusion was of low certainty (Rochwerg, et al., Intensive Care Med. 2015;41:1561).
Earlier this year, a large RCT comparing NS vs BC demonstrated a reduction in major adverse kidney events using BC. Independent rates of new RRT, mortality, and persistent renal dysfunction were not significant, but when combined as a composite outcome, the difference was significant. A 30-day mortality reduction was significant in patients with sepsis (25.2% BC vs 29.4% NS) and in patients with large infusions of NS (Semler et al., N Engl J Med. 2018;378:829). Given these results, a move toward a “balanced approach” to fluid resuscitation seems prudent and may be the next step toward improving outcomes in sepsis. These results are likely related to the large infusions of fluid in patients with sepsis or to the inflammatory effects of the disease. Finally, the applicability of these outcomes to the overall critically ill population is still open to debate.
Margaret Disselkamp, MD
Steering Committee Member
Home-Based Mechanical Ventilation and Neuromuscular Disease
Transcutaneous Carbon Dioxide Monitoring: New Era for Home Ventilation
A primary objective of noninvasive home ventilation is normalization of arterial blood gas tensions, night and day. Pulse oximetry has long enabled estimation of arterial oxygen saturation (SpO2) in outpatient offices and overnight at home; however, until recently, measurement of the partial pressure of carbon dioxide (PCO2) has been limited to invasive arterial blood gas testing (PaCO2) or end-tidal CO2 (PetCO2) measurements. Assessment of PetCO2 has been limited by challenges in accessing true end-tidal exhaled gas under a face mask during noninvasive ventilation, particularly for patients with parenchymal lung diseases such as COPD.
Thanks to recent technological advances, transcutaneous measurement of carbon dioxide (PtcCO2) is emerging as the method of choice for assessing the adequacy of noninvasive ventilation. PtcCO2 monitoring is a standard assessment for pediatric patients in the sleep lab, and it is increasingly being utilized in adults to complement diagnostic and treatment purposes. The transcutaneous CO2 sensors work by heating underlying skin to approximately 43° C, increasing blood flow through the underlying dermal capillary bed. Within 2 to 5 minutes, the “arteriolized” capillary PtcCO2 approximates PaCO2. Commercially available devices for measuring PtcCO2 reliably estimate PaCO2 in patients undergoing noninvasive ventilation to within 5 mm Hg (95% CI) (Storre et al. Respir Med. 2010;105:143).
PtcCO2 measurement has limitations. Measured PtcCO2 can drift upward (i.e., technical drift) during continuous monitoring; however, currently available devices adequately adjust for this phenomenon. Arterialization may be limited by thickened skin, edema, or hypoperfusion.
Currently, U.S. insurance companies do not accept PtcCO2 for documentation of hypercapnia, and the cost of measuring PtcCO2 is not reimbursed. Nevertheless, PtcCO2 technology promises a new era for home mechanical ventilation guided by accurate and practical assessment of PCO2, in particular for chronic respiratory failure syndromes. In this setting, home PtcCO2 monitoring potentially can be utilized in place of in-laboratory sleep studies for assessment of nocturnal hypoventilation and optimizing home mechanical ventilation.
Jason Ackrivo, MD
Steering Committee Member
Interstitial and Diffuse Lung Disease
Electronic Patient Education
The management of patients with an interstitial lung disease (ILD) is challenging. A provider must examine the fine details about current and prior medication history, explore various occupational and environmental exposures, perform a thorough physical examination that includes a careful dermatologic and rheumatologic review, and peruse the objective data, such as the high-resolution CT scan of the chest and pulmonary function tests. Then, the pulmonologist and the patient (plus often multiple family members) discuss diagnostic possibilities, any future testing for confirmation, and prognostic implications. Understandably, the patient may leave the office bewildered, overwhelmed, and in search of clarification.
Bewilderment may lead to the internet. In 2001, 4.5% of all internet searches were determined to be health-care-related (Eysenbach et al. AMIA Annu Symp Proc. 2003;225). It is reasonable to presume the percentage is higher today. Just as with any nonmedical website, the choices for digital health-care information are sometimes not contemporaneous and vary in quality. By exploring the most common “hits” on popular search engines when searching for idiopathic pulmonary fibrosis, a 2016 study found that not only is information presented at a high reading level – 12th grade – but often outdated or simply wrong (Fisher, et al. Am J Respir Crit Care Med. 2016;194[2)]:218). Adding to a patient’s possible confusion is that websites expected to be the most helpful, foundation or advocacy websites, were more likely to suggest disproven and even harmful therapies years after those conclusions were published.
CHEST and the Interstitial and Diffuse Lung Disease NetWork are committed to patient education both in and out of the clinical setting. An ongoing redesign of ILD patient education on the CHEST Foundation website is nearing completion and will ensure patients have the most accurate and understandable information available.
Corey Kershaw, MD
Steering Committee Member
Mentors Creating Mentors
Upon wrapping up a successful 2018 NetWorks Challenge Giving campaign – supporting travel grants to CHEST 2018 for early career and diverse clinicians, CHEST Foundation staff sat down with one of our champions, Demondes Haynes, MD, FCCP. Our conversation focused heavily on the role of mentorship in the development of early career clinicians and his own experience as both a mentor and mentee.
Dr. Haynes has had several mentors over the course of his career, but one stands out to him in particular: Doug Campbell, MD, FCCP. Dr. Campbell is a pulmonary and critical care physician who was the division chief at the University of Mississippi Medical Center in Jackson. “When I was finishing my chief residency, the entire pulmonary division imploded. All of the faculty left, except one or two professors, and all those who were going to become fellows here started looking for other places to go. I was actively looking as well…planning to leave my home state, which was not my initial plan. Dr. Campbell came in about that time and promised me that if I gave him some time, we could rebuild the division. He told me if I stayed for my fellowship, I could really help rebuild it. From that day forward, he was my mentor. I stayed for my fellowship under Dr. Campbell.
He delivered on all of those promises. He taught pulmonary medicine extremely well. Not only was a he a great clinician, but he built up the faculty – started a telemedicine program for the ICU and brought in a diverse set of faculty who had all trained at other institutions. He really helped build the program up to be a strong program. I was very happy I chose to stay and learn under his leadership.”
Doug Campbell not only had an impact on Dr. Haynes’ professional life, but also his personal life. “When I agreed to stay for my fellowship, he sent a beautiful handwritten note to my mother, thanking her for raising me to be respectful. She was amazed.” Dr. Haynes mother passed 10 years ago. The night before the funeral at the visitation, Dr. Campbell brought the card his mother sent back – an exchange that Dr. Haynes never knew took place. “It really meant the world to me, not only had he mentored me in my academic career, but he made those personal touches. Those moments are very special to me.”
Dr. Haynes is now mentoring residents and feels it is even more rewarding being a mentor. “You actually get to invest in others, and when you invest in others, the best comes out in them. Sometimes, in this mentoring role, you’re helping people uncover what their qualities are. Sometimes they don’t even know what they are capable of until you push them just a little bit. That’s been so rewarding. I have been blessed, my mentors have invested so much in me, and I am able to pay it forward and give back.”
Dr. Haynes chose to honor Dr. Campbell through giving during the NetWorks Challenge Giving Month. “The NetWork Challenge is great because part of our mission as an organization is philanthropy. We are an education organization, and, in medicine in general, we should support philanthropy. We talk a lot about empathy for our patients… and giving back is just a small part of that. There is a scripture that says, ‘To whom much is given, much is required.’ I truly believe that. I believe that it should just be an ingrained part of our calling as physicians.”
Your generosity funds young clinicians’ learning opportunities that will change the future of patient outcomes and lung diseases. Thank you for making these opportunities possible.
Your continued support will support the next generation of mentees launching their careers (with the proper hands-on training). You can be a Champion for Lung Health and DONATE today through a new gift to the CHEST Foundation by going to chestfoundation.org/donate or calling 224/521-9527.
Again, thank you for all you do to improve patient outcomes. You are the lung health champions who patients and families count on to positively impact lung health.
Upon wrapping up a successful 2018 NetWorks Challenge Giving campaign – supporting travel grants to CHEST 2018 for early career and diverse clinicians, CHEST Foundation staff sat down with one of our champions, Demondes Haynes, MD, FCCP. Our conversation focused heavily on the role of mentorship in the development of early career clinicians and his own experience as both a mentor and mentee.
Dr. Haynes has had several mentors over the course of his career, but one stands out to him in particular: Doug Campbell, MD, FCCP. Dr. Campbell is a pulmonary and critical care physician who was the division chief at the University of Mississippi Medical Center in Jackson. “When I was finishing my chief residency, the entire pulmonary division imploded. All of the faculty left, except one or two professors, and all those who were going to become fellows here started looking for other places to go. I was actively looking as well…planning to leave my home state, which was not my initial plan. Dr. Campbell came in about that time and promised me that if I gave him some time, we could rebuild the division. He told me if I stayed for my fellowship, I could really help rebuild it. From that day forward, he was my mentor. I stayed for my fellowship under Dr. Campbell.
He delivered on all of those promises. He taught pulmonary medicine extremely well. Not only was a he a great clinician, but he built up the faculty – started a telemedicine program for the ICU and brought in a diverse set of faculty who had all trained at other institutions. He really helped build the program up to be a strong program. I was very happy I chose to stay and learn under his leadership.”
Doug Campbell not only had an impact on Dr. Haynes’ professional life, but also his personal life. “When I agreed to stay for my fellowship, he sent a beautiful handwritten note to my mother, thanking her for raising me to be respectful. She was amazed.” Dr. Haynes mother passed 10 years ago. The night before the funeral at the visitation, Dr. Campbell brought the card his mother sent back – an exchange that Dr. Haynes never knew took place. “It really meant the world to me, not only had he mentored me in my academic career, but he made those personal touches. Those moments are very special to me.”
Dr. Haynes is now mentoring residents and feels it is even more rewarding being a mentor. “You actually get to invest in others, and when you invest in others, the best comes out in them. Sometimes, in this mentoring role, you’re helping people uncover what their qualities are. Sometimes they don’t even know what they are capable of until you push them just a little bit. That’s been so rewarding. I have been blessed, my mentors have invested so much in me, and I am able to pay it forward and give back.”
Dr. Haynes chose to honor Dr. Campbell through giving during the NetWorks Challenge Giving Month. “The NetWork Challenge is great because part of our mission as an organization is philanthropy. We are an education organization, and, in medicine in general, we should support philanthropy. We talk a lot about empathy for our patients… and giving back is just a small part of that. There is a scripture that says, ‘To whom much is given, much is required.’ I truly believe that. I believe that it should just be an ingrained part of our calling as physicians.”
Your generosity funds young clinicians’ learning opportunities that will change the future of patient outcomes and lung diseases. Thank you for making these opportunities possible.
Your continued support will support the next generation of mentees launching their careers (with the proper hands-on training). You can be a Champion for Lung Health and DONATE today through a new gift to the CHEST Foundation by going to chestfoundation.org/donate or calling 224/521-9527.
Again, thank you for all you do to improve patient outcomes. You are the lung health champions who patients and families count on to positively impact lung health.
Upon wrapping up a successful 2018 NetWorks Challenge Giving campaign – supporting travel grants to CHEST 2018 for early career and diverse clinicians, CHEST Foundation staff sat down with one of our champions, Demondes Haynes, MD, FCCP. Our conversation focused heavily on the role of mentorship in the development of early career clinicians and his own experience as both a mentor and mentee.
Dr. Haynes has had several mentors over the course of his career, but one stands out to him in particular: Doug Campbell, MD, FCCP. Dr. Campbell is a pulmonary and critical care physician who was the division chief at the University of Mississippi Medical Center in Jackson. “When I was finishing my chief residency, the entire pulmonary division imploded. All of the faculty left, except one or two professors, and all those who were going to become fellows here started looking for other places to go. I was actively looking as well…planning to leave my home state, which was not my initial plan. Dr. Campbell came in about that time and promised me that if I gave him some time, we could rebuild the division. He told me if I stayed for my fellowship, I could really help rebuild it. From that day forward, he was my mentor. I stayed for my fellowship under Dr. Campbell.
He delivered on all of those promises. He taught pulmonary medicine extremely well. Not only was a he a great clinician, but he built up the faculty – started a telemedicine program for the ICU and brought in a diverse set of faculty who had all trained at other institutions. He really helped build the program up to be a strong program. I was very happy I chose to stay and learn under his leadership.”
Doug Campbell not only had an impact on Dr. Haynes’ professional life, but also his personal life. “When I agreed to stay for my fellowship, he sent a beautiful handwritten note to my mother, thanking her for raising me to be respectful. She was amazed.” Dr. Haynes mother passed 10 years ago. The night before the funeral at the visitation, Dr. Campbell brought the card his mother sent back – an exchange that Dr. Haynes never knew took place. “It really meant the world to me, not only had he mentored me in my academic career, but he made those personal touches. Those moments are very special to me.”
Dr. Haynes is now mentoring residents and feels it is even more rewarding being a mentor. “You actually get to invest in others, and when you invest in others, the best comes out in them. Sometimes, in this mentoring role, you’re helping people uncover what their qualities are. Sometimes they don’t even know what they are capable of until you push them just a little bit. That’s been so rewarding. I have been blessed, my mentors have invested so much in me, and I am able to pay it forward and give back.”
Dr. Haynes chose to honor Dr. Campbell through giving during the NetWorks Challenge Giving Month. “The NetWork Challenge is great because part of our mission as an organization is philanthropy. We are an education organization, and, in medicine in general, we should support philanthropy. We talk a lot about empathy for our patients… and giving back is just a small part of that. There is a scripture that says, ‘To whom much is given, much is required.’ I truly believe that. I believe that it should just be an ingrained part of our calling as physicians.”
Your generosity funds young clinicians’ learning opportunities that will change the future of patient outcomes and lung diseases. Thank you for making these opportunities possible.
Your continued support will support the next generation of mentees launching their careers (with the proper hands-on training). You can be a Champion for Lung Health and DONATE today through a new gift to the CHEST Foundation by going to chestfoundation.org/donate or calling 224/521-9527.
Again, thank you for all you do to improve patient outcomes. You are the lung health champions who patients and families count on to positively impact lung health.